
MANCOZEB
First draft prepared by
A. Kocialski
Office of Pesticide Programs,
US Environmental Protection Agency, Washington, DC, USA
EXPLANATION
Mancozeb was evaluated by the Joint Meeting in 1967, 1970,
1974, 1977 and 1980 (Annex I, references 8, 14, 22, 28, 34). An ADI
of 0-0.05 mg/kg bw was established at the 1980 Meeting for mancozeb
or the sum of maneb, mancozeb and zineb, of which not more than
0.002 mg/kg bw may be present as ethylenethiourea (ETU).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biological data
Biochemical aspects
Absorption, distribution, excretion, and biotransformation
Mice
Male and female Charles River CD-1 mice were administered 14C-
ethylene-U-labelled mancozeb (98-99% pure) at single oral doses of
2.5 or 150 mg/kg bw and at repeated doses (14 daily doses) of
unlabelled mancozeb at 2.5 mg/kg bw followed by administration of a
single oral dose of labelled mancozeb at 2.5 mg/kg bw. Mancozeb was
rapidly absorbed, peaking in whole blood at 1.0 hour in males and
2.0 hours in females, extensively metabolized in both sexes, and
rapidly excreted (> 90%) in both sexes within 24 hours. Over a 7-
day period almost all (> 97%) of the compound administered was
excreted. The radioactivity recovered in urine, faeces, carbon
dioxide and carbon disulfide in exhaled air ranged between 26-44%,
48-64%, 0.4-4.2% and 0-4.0% of the administered dose, respectively.
A mean of less than 1.4% of the dose remained in the carcass and
tissues after 7 days. ETU represented < 1-3% of the administered
dose. The elimination of absorbed radioactivity by way of the bile
was less than 0.2% of the dose following a single oral
administration of mancozeb to both sexes at 2.5 mg/kg bw.
Examination of the tissue distribution of radioactivity at 2.5 mg/kg
bw (single or repeated dose) at 1.0, 8.0 or 24 hours and at 7 days
at 150 mg/kg bw indicated predominant distributions in thyroid, bone
marrow, ovaries, spleen, lungs, kidney, liver, adrenal, thymus and
whole blood. Values for whole blood were almost twice as great as
that found for plasma.
Urine and faeces were collected at 0-8 and 8-24 hours post-
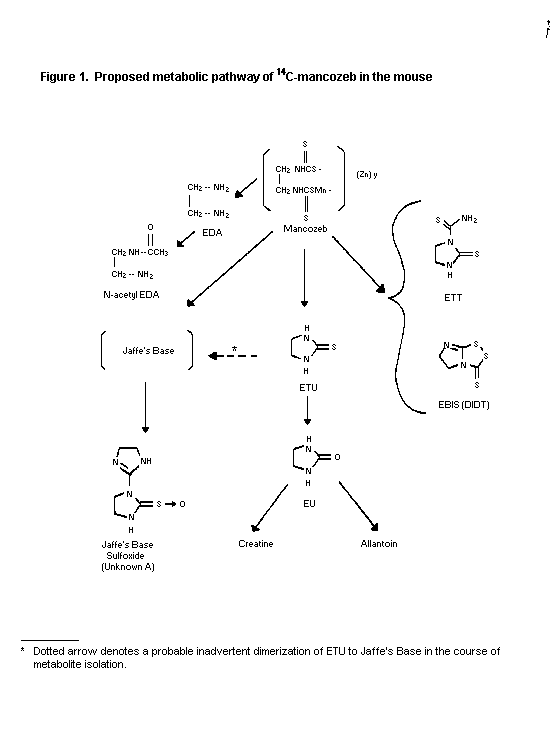
dosing for characterization of metabolites. Metabolites found in
the urine of both sexes were, in decreasing order, ETU,
ethylenethiuram monosulfide, EBIS, ethylenethiourea-N-thiocarbamide
(ETT), N-acetyl-ethylenediamine (N-acetyl-EDA), ethylenediamine
(EDA), ethyleneurea (EU), creatine and allantoin. Six unknown
metabolites were also identified in urine, one of which was
tentatively proposed as the sulfoxide of Jaffe's base. Metabolites
identified in the faeces were ETU, ethylenethiuram monosulfide,
EBIS, ETT, EDA, EU, and N-acetyl-EDA. Additional unknown
metabolites were also found in the faeces with one being
characterized as Jaffe's base sulfoxide. The proposed metabolic
pathway of 14C-mancozeb in mice is given in Figure 1 (Cameron et
al., 1990, Piccirillo et al., 1992).
Rats
Groups of female Sprague-Dawley rats (3 or 6/group) were
exposed to mancozeb (83% pure) for 6 hours and sacrificed at 2
minutes, 6 or 24 hours post-initial-administration. A 20 centimeter
square area on the back of each rat was sheared with clippers. A
single non-radiolabelled dose of 10 mg mancozeb was applied and held
in place with an elastic bandage. Following the exposure period the
bandage was removed and the area swabbed. All material (including
fringe hair) which contacted the compound was combined and analyzed
for EBDC and ETU. At termination the skin of the contact area was
removed and analyzed for both EBDC and ETU. Dermal absorbtion was
calculated from the amount of applied material remaining at the site
of application at 6 and 24 hours as well as the amount excreted at
24 hours. Absorbtion from the application site was 0.83% and 0.89%
at 6 and 24 hours post-dosing. Excretion at 24 hours was calculated
to be 1.0% (Haines, 1980).
Groups of 4 adult male Charles River rats (Crl:CD(R) BR) were
treated dermally with 0.05 ml of an aqueous suspension containing
0.1 or 1.0 mg mancozeb (80.6% pure) and applied on a 2 X 2 cm area
of the shaved back. The area was covered with a contoured glass
ring equipped with a porous top. At 0, 10 or 24 hours post-dosing
animals were anaesthetized and the application rings, covers and
site washings, together with urine and faeces were collected for
each time period. Animals were then sacrificed and the skin removed
at the site of application. All samples including the carcass, were
extracted and analyzed for mancozeb and ETU. The analysis of
mancozeb in biological matrices could not be adequately performed
due to considerable background interference encountered during the
analysis of the samples, even in control and zero hours samples. It
was hypothesized that the sulfur moieties in the biological samples
produced carbon disulfide which interfered in the analysis. The
amount of dermal absorbtion was therefore determined by the
subtraction of the amount of mancozeb recovered in the wash-off at
10 and 24 hours from the amount of mancozeb recovered in the wash-
off at zero hour (i.e. surface recovery method). Following low-dose
administration, 2 and 4% of the dose was dermally absorbed at 10 and
24 hours, respectively, and less than 1% of the high dose was
dermally absorbed at 24 hours (Tomlinson & Longacre, 1988).
Male and female Sprague-Dawley rats were administered single
oral doses of 14C-radiolabelled mancozeb (88-92% pure) at 1.5 or
100 mg/kg bw. Rats (3/sex/group) were sacrificed at 1, 6, 24 or 48
hours post-dosing and their plasma, whole blood, liver and thyroids
were extirpated for radioassay and metabolite analysis. Blood
samples were also taken at 0.5 and 3 hours from rats sacrificed at 1
and 6 hours, respectively. Urine and faeces were collected from rats
(5/sex/group) at 6, 24, 48, 72 and 96 hours post-dosing. The
remaining animals (9/sex/dose) were sacrificed at 96 hours and their
plasma, whole blood, thyroids, liver, adipose tissue, kidneys, lung,
heart, bone marrow, gonads, muscle, spleen, and brain were collected
for radioassay and metabolite analysis. An additional 5 male and 5
female rats were placed on diets of rodent chow containing
unlabelled technical mancozeb (84% pure) at 15 ppm with 0.4 ppm ETU
as contaminant. After 14 days animals were given a single oral dose
(pulse dose) of 14C-mancozeb at 1.5 mg/kg bw. Animals were then
housed in individual metabolism cages and urine and faeces collected
as noted above, and sacrificed at 96 hours post-dosing and tissues
collected as stated above. Lastly, two groups of 3 male and 3
females with cannulated bile ducts were administered single oral
doses of 14C-mancozeb at 1.5 or 100 mg/kg bw. Bile was collected
during 0-6 and 6-24 hours post-administration for radioassay and
metabolite analysis.
Most (74-94%) of the administered dose was recovered in the
excreta within 24 hours with 87-120% of the dose eliminated at 96
hours. The excreted radioactivity was approximately evenly
distributed between the urine and faeces for all three dose groups
(urine range 49-55%; faeces range 36-65%). Approximately 6-8% and 2-
4% of the dose was excreted in the bile of rats within 24 hours of
post-dosing at 1.5 and 100 mg/kg bw, respectively. The 14C plasma
concentrations for males and females given either 1.5 or 100 mg/kg
bw were similar for each dose group. The absorption half-lives (t´
absorption) was 0.7-1.0 hour for the low-dose group and 1.7 hours
for the high-dose group. Peak concentrations for the low- and high-
dose group were reached within 3 and 6 hours, respectively. The
elimination of 14C from plasma was biphasic for both sexes. The
t´ for males was 4.0 and 5.7 hours receiving the low and high
dose, respectively; for females the t´ was 4.5 and 6.0 hours for
the low and high dose, respectively. The slow elimination half-
lives for both sexes were about 25 and 36 hours in animals receiving
low and high doses, respectively. 14C levels in whole blood were
similar to corresponding plasma concentrations for each dose group;
however, the slow phase of elimination for the low-dose group was
longer in blood compared to plasma. 14C concentrations in liver
were very similar between males and females at each time point after
dosing. Peak levels were reached within 6 hours of dosing and were
2-6 fold higher than the corresponding peak 14C whole blood
concentrations after low and high-dose administration. The
elimination kinetics was biphasic with the half-lives for the rapid
and slow elimination phases being about 7.5 and 35 hours,
respectively.
 Peak concentrations of 14C in thyroid were reached within 6
and 24 hours in animals receiving the low and high doses,
respectively. Peak levels in thyroid were about 45 and 10 fold
higher than the corresponding peak levels in whole blood after low
and high-dose administration, respectively. 14C residue levels for
aforementioned tissues were comparable for males and females
receiving single dose administration except for adipose tissue and
gonads of females which were higher than those of males. Thyroid
tissue contained the highest residue levels for each group.
However, thyroid contained less than 0.10% of the dose 96 hours
post-dose. Tissue concentrations of 14C residues in rats receiving
the "pulse dose" were comparable to those in animals receiving only
the single dose of 14C-mancozeb at 1.5 mg/kg bw. The average 14C
residue levels per group remaining in the tissue at 96 hours post-
dosing ranged between 1.5-3.5% of the dose. ETU concentrations in
plasma and liver of rats 6 hours after dosing with 14C-mancozeb at
1.5 mg/kg bw were 6 and 12 times less than corresponding plasma and
liver 14C levels, respectively. ETU was not detected in the pooled
thyroids of these low-dose animals. Peak plasma levels of ETU were
reached 6 hours post-dosing in animals given 100 mg/kg bw mancozeb
and were 6-12 times less than corresponding plasma 14C levels for
both sexes. ETU was rapidly eliminated from the plasma of both male
and female rats (t´ 4.0-4.7 hours) and decreased below detectable
levels within 48-hours post-dosing. The estimated bioavailability
of ETU in rats was about 6.8 percent on a w/w basis and 20% on a
mole/mole basis. ETU concentrations in the liver of the high-dose
rats was 100 times less than liver 14C concentrations 6 hours post-
dosing. ETU was not detectable in liver 48 hours post-dosing. ETU
concentrations in thyroids were 80-100 and 30-225 times less than
corresponding thyroid 14C concentrations in males and females,
respectively, given 100 mg/kg bw mancozeb at 6 and 24 hours. ETU
was not detectable in plasma, liver, or thyroid of rats at 96 hours
after administration of a "pulse dose" (1.5 mg/kg bw of 14C-
mancozeb) preceded by a two-week dietary intake of unlabelled
technical mancozeb.
Concentrations of EBDC in the liver of rats 1, 6 and 24 hours
after dosing with 14C-mancozeb at 100 mg/kg bw were 0.25, 0.61, and
0.29 ppm for males, respectively, and 0.32, 0.35 and 0.25 ppm for
females, respectively. EBDC residues were not detectable 48 or 96
hours post-dosing. EBDC was not detected in the livers of rats
receiving the low dose (1.5 mg/kg bw) or multiple doses of mancozeb
96 hours post-dosing. Metabolite analysis revealed that mancozeb
was extensively metabolized and/or degraded. ETU was a major
metabolite found in urine, bile and faeces. EBDC was detected in
liver, faeces, and bile but not in thyroid. EBIS, ethyleneurea
(EU), N-acetyl-ethylenediamine (N-AcEDA) and ethylenediamine (EDA)
were also identified in urine, faeces and bile. Other metabolites
tentatively identified were glycine, N-acetylglycine, and N-
formylethylenediamine. Five other metabolites were also present but
were not identified (DiDonato & Longacre, 1986; Longacre, 1986;
Nelson, 1986, 1987; Kocialski, 1989).
Monkeys
Groups of male rhesus monkeys ( Macaca mulatta; 6/group) were
given single oral doses of either 14C-ETU; 14C-ETU plus manganous
sulfate and zinc sulfate or 14C-mancozeb (purity not stated) for
the determination of uptake into blood and the determination of the
major route of elimination of 14C. A sufficient dose was given to
produce 100 µCi of 14C activity per monkey. Whole blood, thyroid,
heart, lung, liver and kidney and faecal material were converted to
14C-carbon dioxide by combustion in a tissue oxidizer and analyzed
for 14C activity. Urine samples were analyzed directly. Urine and
faeces were collected separately.
14C-Labelled doses of ETU and ETU plus manganese and zinc
sulfates reached peak levels of 5% of the dose in total blood volume
at 8 hours. 14C-Mancozeb activity in the blood was less than 0.5%
at 8 hours and plateaued from 24-72 hours at slightly less than 1%
of the dose. This was in contrast to a relatively rapid decline in
14C activity in ETU-treated monkeys which at 72 hours showed 1% of
the administered dose in whole blood. ETU and ETU plus Mn:Zn-treated
monkeys showed similar rates of 14C excretion with nearly 50% of
the dose being cleared in 24 hours. The urinary clearance of 14C
activity by mancozeb-treated monkeys was slower (3.6% of the dose in
24 hours) as compared to ETU-treated groups. At 120 hours (last time
reading), the urinary route of elimination accounted for little more
than 10% of the activity in the mancozeb dose. Faecal elimination
showed less than 1% of 14C activity up to 24 hours in ETU-treated
groups. However, mancozeb-derived faecal activity ranged from 12.5-
64.0% of the dose at 144 hours, and from 0.005-12.7% at 24 hours.
14C activity was not detected in faecal samples from ETU-treated
monkeys after 24 hours. At 144 hours, blood samples were taken from
2 animals of the mancozeb-treated group, the animals sacrificed and
the following organs examined for 14C activity: thyroid, heart,
lung, liver and kidney. The remaining 4 animals were sacrificed 48
hours later (192 hours post-dosing). Comparative examination
between the groups (2 vs 4 monkeys) indicated that 14C activity
declined steadily between 144 and 192 hours in blood, whereas 14C
activity in the thyroids increased over the same 48-hour time period
(Emmerling, 1978).
Toxicological studies
Acute toxicity studies
Acute toxicity studies are summarized in Table 1. WHO has
classified mancozeb as unlikely to present acute hazard in normal
use (WHO, 1992).
Short-term toxicity studies
Mice
Charles River COBS-CD-1 mice (10/sex/group) received 0, 1, 10,
100, 1000 or 10 000 ppm of mancozeb (83% purity) adjusted to 100%
active ingredient for 4 weeks. No animals died on study and
clinical signs were absent. Females fed 10 000 ppm mancozeb showed
decreased body weights. Food consumption appeared to be comparable
for all groups on study. SGPT activity was comparable to controls
for all groups. Gross necropsy findings were not remarkable.
Thyroid weights (absolute and relative) were statistically
increased in both females at 10 000 ppm. Males appeared unaffected.
Liver weights were statistically significantly increased in males
and females given 10 000 ppm mancozeb. At 1000 ppm, absolute and
relative liver weights were significantly increased in females.
Females treated with 1000 ppm and 10 000 ppm showed thyroid
hyperplasia, congestion and decreased colloid density whereas males
showed similar effects at 10 000 ppm. No significant micropathology
was evident in the livers of animals given mancozeb (DiDonato et
al., 1985).
Mancozeb (83.1% pure) was administered to groups of Charles
River CD-1 mice (15 sex/dose) for 3 months at dietary concentrations
of 0, 10, 100, 1000, or 10 000 ppm of active ingredient. No
compound-related mortalities or clinical signs were observed.
Decreased body weights and food consumption were observed in both
sexes receiving 10 000 ppm. There were no treatment-related effects
with regard to haematology or clinical chemistry. Necropsy findings
were not remarkable. Aniline hydroxylase activity was decreased at
10 000 ppm in both sexes. Aminopyrine N-demethylase activity was
decreased in males at 1000 and 10 000 ppm. No effects were seen in
either sex at lower doses. Absolute and relative thyroid weights
were increased in both sexes receiving 10 000 ppm. Relative
increases in liver weights were seen in both sexes receiving 10 000
ppm. Absolute liver weight was increased in males administered 10
000 ppm mancozeb. Relative kidney weights were also increased in
both sexes at 10 000 ppm. Histopathologic evaluation of thyroid
revealed an increased incidence of follicular cell hyperplasia and
hypertrophy in both sexes at 1000 and 10 000 ppm. There was
Table 1. Acute toxicity of mancozeb
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse1 B6C3F1 M oral > 5000 --- Watts, 1984
Rat3 F344 M oral > 5000 --- Watts, 1984
Rat1 F344 M oral > 5000 --- Watts, 1984
Rat3 CRCD M oral > 5000 --- DeCrescente, 1980
Rat2 Wistar M/F i.p. 380 --- DeGroot, 1974
Rat 5,6 COBS-CR M/F inhalation --- 5.14 Hagan, 1982
(SD) BR 4 hour exposure
Rat 6,7 CR-SD M/F inhalation --- > 1.11 Hagan, 1980
4 hour exposure
Rabbit4 NZW M dermal > 5000 --- DeCrescente, 1980
1 Corn oil vehicle.
2 Carboxy-methyl-cellulose vehicle.
3 Aqueous dispersion.
4 Saline vehicle.
5 3/10 M and 3/10 F died during the whole body exposure/observation period (14 days). Multiple signs but no
tremors; body-weight loss and decreased weight gain. MMD 3.3 microns. Nominal concentration was
41.8 mg/litre (aerosol).
6 Whole body exposure.
7 MMD about 2.2 microns. Nominal concentration 4.46 mg/litre (dust).
increased vacuolation, interstitial congestion and decreased colloid
density in the thyroids in both sexes at 10 000 ppm. No liver
effects were observed in mancozeb-treated mice with the possible
exception of hepatocytic nuclear pleomorphism in females at 10 000
ppm. Increased deposits of (brownish) pigment were seen in the zona
reticularis of the adrenal cortex of female mice at 10 000 ppm. The
NOAEL was 100 ppm, equal to 18 mg/kg bw/day for males and 22 mg/kg
bw/day for females (O'Hara & DiDonato, 1985).
Rats
Four groups of 12 male and 12 female Crl:CD (SD)BR rats were
exposed to dust aerosols of mancozeb (84% pure) for 6 hours per day
for 10 days. The four groups were subdivided into whole-body
exposed rats and nose-only exposed rats of 5/sex/dose group. Daily
concentrations yielded total mean aerosol concentrations of 0, 23,
138 or 519 mg/m3. The mean respirable aerosol concentrations were
0, 11, 55 or 258 mg/m3 with mass median diameters of 3.5-4.9
microns.
Nose-only Exposure: There were no compound-related deaths.
No adverse clinical signs were observed in males or females exposed
to 11 or 55 mg/m3. Significant decreases in mean body weight and
mean body-weight gain were observed in the high-dose males but not
females. T3 and T4 levels were significantly reduced and the lung
to body-weight ratio increased in high-dose males but not in
females. Microscopic examination (limited to the respiratory tract)
of nasal turbinates revealed an increased incidence and degree of
multifocal mixed inflammatory cell infiltration (i.e. mononuclear
cells and neutrophils) and multifocal or focal necrosis of the
turbinate mucosa in four males and two females in the high dose.
Whole-body Exposure: There were no compound-related deaths.
No clinical signs were observed in animals receiving 11 mg/m3.
Significant reductions in male and female body weight and body-
weight gain were observed at 55 and 258 mg/m3. Male and female T4
levels and male T3 levels were significantly decreased at 55 and
258 mg/m3 of mancozeb after two weeks. TSH levels were not
significantly increased in both sexes at the high dose. Male and
female lung weights and lung to body-weight ratios were
significantly increased in both sexes at the high dose. Exposure-
related multifocal interstitial inflammation, microgranulomas,
multifocal mixed inflammatory cell infiltration, focal or multifocal
necrosis in the respiratory tract and reactive lymphoid hyperplasia
of the peribronchial lymph nodes were observed at the high dose
tested (Hagan & Baldwin, 1986).
Adult male and female Crl:CD(SD)BR rats were divided into two
nose-only exposure groups and one nose-only exposure-recovery group.
Animals received aerosol dust (84% pure mancozeb), 6 hours/day, 5
days week for 4 weeks at analytically determined mean concentrations
of 0, 22, 86 or 308 mg/m3 (equivalent to respirable concentrations
of 0, 8, 40 and 127 mg/m3) or for 13 weeks at 0, 18, 79 or 326
mg/m3 (equivalent to respirable concentrations of 0, 8, 36 or 144
mg/m3).
Results - 4 Weeks Exposure: There was no compound-related
mortality. Mean body weights and body-weight gains were decreased
for males in the high-dose group. No haematological effects were
observed in males. Data for females were inconclusive due to
insufficient amount of blood needed for the haematological
evaluations. There were no compound-related effects in either sex
for clinical chemistry, thyroid function, organ weights, or tissues
examined microscopically. Ophthalmological examinations revealed no
remarkable findings.
Results - 13 Weeks Exposure: There were no compound-related
deaths and no treatment-related signs of toxicity. Males exposed at
the high dose exhibited reduced mean body weights and body-weight
gains. Some haematological and clinical chemistry changes were
noted but were within the normal range of values and were therefore
not considered related to mancozeb exposure. T4 levels were
reduced 30% in high-dose females and considered treatment-related.
A dose-response was evident. Males showed a 9% decrease at the high
dose tested which was not statistically significant. Absolute
kidney and heart weights were reduced in males at the high dose
tested. However, organ to body weight and organ to brain weight
ratios were not statistically significantly different from controls.
Ophthalmological examination of the eyes revealed no compound-
related effects. No exposure-related histopathology was observed in
either sex at the low dose tested. Males (5/10; 8/11) and females
(10/11; 9/11) exposed to 79 or 326 mg/m3 exhibited a yellow-brown
granular pigment in the lumen of the cortical tubules of the kidney.
However, no attendant histopathological changes were reported. Mild
hyperplasia of the follicular epithelium in the thyroid glands of 3
females exposed at the high dose was observed. No exposure-related
thyroid lesions were observed at lower dose levels or in males.
Several histopathological lesions were observed in the respiratory
tract but were comparable to control incidences and not considered
treatment-related. Residue analysis of blood indicated
concentrations below the limit of detection for ETU and mancozeb for
males and females exposed to the low dose, with increasing
concentrations at higher exposures. Residue levels for ETU in the
thyroid were below the limit of detection at the low dose in both
sexes, with increasing concentrations at the higher doses. ETU and
mancozeb levels in urine were present at all doses in both sexes.
Those animals allowed to recover for 13 weeks after cessation of
exposure were comparable to a concurrent control group of non-
exposed animals for all parameters examined (Hagen et al., 1986).
Groups of Sprague-Dawley rats (Crl:CD(SD)BR); 10/sex/dose) were
administered 0, 10, 100, or 1000 mg of mancozeb (83% pure, not
adjusted for purity)/kg bw. The control group received distilled
water only. Test material was applied to the clipped dorsal area of
intact skin of each animal. An unsterile dressing sponge was placed
over the test site and held in place for 6 hours/day then removed.
All animals were treated similarly for 20 exposures over a 4-week
period. All animals were fitted with a cardboard collar to minimize
preening at the application site. The collar was only removed
during the 6 hours exposure period.
Clinical observations showed no evidence of compound-related
effects and there was no compound-related mortality. Body weight,
body-weight gain and food consumption in treated groups were
comparable to controls. Erythema was transient and slight, occurring
in not more than 2 animals/sex/dose for not longer than 2-4 days.
No other signs of dermal irritation were observed. Evaluation of
haematological and clinical chemistry parameters revealed no
statistically significant compound-related effects. Macroscopic
observations of treated skin revealed dark (yellow) area or
appearance in a dose-related increasing frequency at 100 and 1000
mg/kg bw in both sexes. Absolute and relative organ weights in
treated groups were not significantly different from control group.
Microscopic findings were limited to skin of treated and untreated
animals and characterized by increased keratin production
(hyperkeratosis) and thickening of the epidermis (acanthosis).
Severity varied from minimal to slight in all groups. These
findings were treatment-related but not compound-related. There
were no microscopic findings that were attributable to mancozeb.
Gross enlargement of the parotid salivary gland was within normal
histomorphological limits and found in all treatment groups
(Trutter, 1988b).
Groups of Crl:CD(SD) rats (14 rats/sex/group) received 0, 30,
60, 125, 250 or 1000 ppm of mancozeb (84% pure adjusted to 100%
active ingredient) for 13 weeks. Dose levels in the diet were
gradually increased with time in order to maintain approximately the
same level of compound intake throughout the feeding period.
There was no compound-related mortality or clinical signs. No
compound-related changes in body weights or food consumption were
recorded in animals fed up to and including 250 ppm mancozeb. At
1000 ppm, males showed a statistically significant decrease in body
weight from weeks 3 through 13, whereas females showed a 3-14%
decrease with statistically significant decreases only between 7 and
10 weeks. At 1000 ppm, food consumption was decreased in males for
weeks 3-13, but not in females. Males receiving 1000 ppm of
mancozeb showed statistically significant increases in BUN (84%),
creatinine (28%) and cholesterol (52%). Females receiving 1000 ppm
showed increased SAP (32%) and triglyceride (90%). However, group
increases were not considered compound-related as one male and one
female in the high-dose group were reported as having exceptionally
high values.
Clinical findings at lower mancozeb levels were not considered
treatment-related. Serum T4 levels were decreased in males and
females at 1000 ppm and TSH values increased. At 250 ppm, TSH
values were increased in males (36%) and females (50%). However,
only T4 values in females were statistically significantly
decreased at 250 ppm. MFO activity was decreased at 1000 ppm. No
mancozeb metabolite residues were detected in blood. Urine samples
of rats fed 125 to 1000 ppm of mancozeb contained 0.1 to 1.1 ppm of
parent compound. ETU metabolite of mancozeb increased in urine in a
dose-response manner from 0.3 to 10 ppm in animals given 30 to 1000
ppm mancozeb.
No mancozeb residues above the detection limit of 25 ppm were
detected in thyroids obtained from the 1000 ppm mancozeb fed rats.
ETU metabolite of mancozeb showed residues in thyroid which were
dose-related to mancozeb administration. Values ranged from less
than the detection limit of 4.0 ppm in 30 ppm mancozeb-fed animals
to 25 ppm in 1000 ppm animals. At 1000 ppm, absolute thyroid weight
was increased in males while relative weight was increased in males
and females. Relative liver weight was increased in males and
females receiving 1000 ppm. Relative spleen weight was increased
only in females receiving mancozeb at the highest dose. Compound-
related histopathology was generally confined to the liver, kidneys,
thyroid, adrenal and pituitary glands. Thyroid follicular cell
hyperplasia was seen in 90% of males and females receiving 1000 ppm;
a small, well defined basophilic focus of hyperplastic follicular
epithelial cells was seen in one male and there was also an
increased incidence and severity of hypertrophied vacuolated cells
in the pituitary of males. The kidneys of males and females
administered 125 to 1000 ppm had minimal to moderate amount of
yellow-brown pigment in the lumen of the cortical tubules. Pigment
deposits were attributed to ethylene bis(isothiocyanate) sulfide
(EBIS) a yellow coloured metabolite of mancozeb. Pigmentation
deposits were not accompanied by histopathological effects. There
was also an increased incidence of hypertrophy of cells of the zona
glomerulosa of the adrenal cortex in males given ETU and 1000 ppm
mancozeb. Hypertrophy of the centrilobular hepatocytes was seen in
males fed 1000 ppm mancozeb. The NOAEL was 125 ppm, equal to 7.4
mg/kg bw/day, based on increased serum TSH and decreased T4 values
at the next higher dose (Goldman et al., 1986).
Male H-Wistar rats (12/group) were given Dithane M-45 (80%
mancozeb) mixed in feed at doses of 0, 10,50, 75, 113, 169, 253 or
379 mg/kg bw/day for 12 weeks. One-third of the rats in the highest
dose group died within 6 weeks and showed signs of prostration,
weakness and posterior extremital paralysis. Signs were transient
in survivors and absent at 12 weeks. Body weight, body-weight gain,
total food intake and food efficiency were all depressed at 169
mg/kg bw/day and above. Effects were compound-related and
statistically significant. Blood sugar levels (after glucose
loading) and haematological parameters were comparable to control
values. Organ to body-weight ratios were increased for liver and
thyroid at 75 mg/kg bw/day and above and for kidneys, adrenals and
testes at 253 mg/kg bw/day and above. Histopathology of liver and
kidneys were comparable to controls. Thyroids, however, showed a
dose-dependent hyperplasia at 113 mg/kg bw/day and above. A 20%
decrease in the iodine content of the thyroids in rats given 10
mg/kg bw/day was not statistically significant. However at 50 mg/kg
bw/day a statistically significant decrease of 80% was recorded.
PBI was significantly decreased and serum cholinesterase
significantly increased at 169 mg/kg bw/day and above. ALP, acid
phosphatase and ASAT were statistically decreased in groups given
379, 253 or 169 mg/kg bw/day or above, respectively. Total protein
content of sera was statistically increased but still within
physiological limits. Liver triglycerides were statistically
increased at 113 mg/kg bw/day and above but serum triglyceride
levels were comparable to controls. Total serum cholesterol was
increased at 75 mg/kg bw/day and above but total liver cholesterol
was comparable to control values. Liver total lipid was similar to
controls. Aminopyrine demethylase and aniline hydroxylase activity
were both decreased at 253 mg/kg bw/day and above. The cytochrome
P-450 level remained unchanged (Szepvolgyi et al., 1989).
Dogs
Beagle dogs (6/sex/dose) received 0, 10, 100, 1000 or 5000 ppm
of 83.35% percent pure mancozeb in the diet adjusted to 100% of
active ingredient for 3 months.
Two males and one female were sacrificed in extremis in the
5000 ppm dose group as a result of compound-related anorexia and
malnutrition. Dose-related clinical signs of dehydration, thinness,
and pale mucous membranes were also noted in animals at this dose
level. Occasional instances of dehydration were seen in animals at
1000 ppm. Signs were considered secondary to anorexia and
malnutrition. Ophthalmoscopic examination did not reveal any
compound-related effects. Food consumption was decreased
approximately 10-20% at 1000 ppm and about 40% at 5000 ppm in both
sexes. Mean body weight and mean body-weight change were both
decreased at the high dose and at 1000 ppm. Decreases in
erythrocyte count, haemoglobin, and haematocrit were accompanied by
increases in mean corpuscular volume and mean corpuscular
haemoglobin. Values were statistically significant in both males
and females at 5000 ppm and females at 1000 ppm. T3 and T4 values
were decreased along with ALAT values at 5000 ppm in both sexes.
Total cholesterol was elevated in both sexes at 5000 ppm and in
females at 1000 ppm. Females at 5000 ppm also showed elevated total
bilirubin count at 5000 ppm.
At necropsy, enlarged and/or dark thyroid/parathyroids and
decreased thymus size were seen in the 1000 and 5000 ppm males and
females. A pale appearance of visceral organs was observed in the
three animals sacrificed early. Compound-related histomorphological
tissues alterations included thyroid follicular cell hyperplasia at
5000 ppm in both sexes, thymic cortical lymphoid depletion in the
1000 and 5000 ppm animals of both sexes, and hypoplastic changes in
the reproductive systems of males and females in the high-dose group
(i.e. aspermato-, hypospermato-, and hypogenesis of the testes and
hypogenesis of the ovaries), pallor of the zona fasciculata of the
adrenal gland in high-dose males and females and some sinusoidal
liver cell pigmentation in high-dose males and females. Urinalysis
indicated the presence of both parent compound and metabolite. ETU
was detected in the blood at a dose of 1000 ppm. No mancozeb was
detected in the thyroid, however ETU was detected in the thyroid of
both sexes at 100 ppm (in males 1.83 ppm and in females 0.72 ppm).
The NOAEL was 100 ppm, equal to 3.0 mg/kg bw/day, based on decreased
food consumption and body-weight gains and decreased erythrocyte
count, haematocrit, and haemoglobin at 1000 ppm (Cox, 1986).
Beagle dogs (4/sex/group) were given dietary concentrations of
0, 50, 200, 800 or 1600 ppm of mancozeb (84.5% pure) and adjusted to
100% active ingredient for 52 weeks.
Two high-dose males were sacrificed in extremis during weeks
10 and 11. One animal manifested haematuria and a hard distended
bladder prior to sacrifice. Necropsy revealed urethral calculi
lodged behind the os penis. Hydronephrosis with tubular dilation,
necrosis and congestion was noted in the kidneys. The lower urinary
tract manifested severe urethritis, prostatitis, cystitis and
ureteritis. Acute peritonitis was associated with these lesions.
The second male like the first showed a sharp drop in food
consumption and a decreased body-weight gain. A haematological
examination revealed the animal to be anaemic (decreased
haemoglobin, erythrocytes, packed cell volume and increased
reticulocytes). Haematological parameters, necropsy and
histopathological findings were consistent with a chronic
regenerative anaemia. Diffuse centrilobular necrosis with
extramedullary haemopoiesis was seen on histopathological
examination of the liver, while in the spleen and bone marrow
moderate to slight erythroid haemopoiesis with pigment was evident
and a notable reticulocytosis. All other animals survived, and
there were no apparent compound-related clinical signs or palpable
masses, nor any apparent neurological effects of treatment. Core
body temperatures were comparable between groups. With the
exception of the two animals that were terminated early there were
no consistent trends in food conversion efficiency or food
consumption. Body-weight gain for males fed 200 and 800 ppm
mancozeb showed statistically significant decreases, beginning at 24
and at 15 weeks, respectively. The two high-dose male survivors and
females showed body-weight gains comparable to controls.
Haemoglobin was significantly decreased at 800 and 1600 ppm in
females at 13 and 52 weeks along with packed cell volume at 13
weeks. Males manifested a statistically significant increase in
mean corpuscular volume at 1600 ppm at weeks 13, 26 and 52, as well
as at 800 pm at week 13. Serum cholesterol was significantly
increased in females at 1600 ppm at weeks 10, and 26. Serum
cholesterol values were dose-related in both sexes with cholesterol
values increasing at 200 ppm (7%-16% at weeks 10, 26 and 52) for
females and at 800 ppm for males.
T4 values were consistently lower than controls or pretest
values but were not statistically significant. T3 values were
comparable to controls. Urinalysis revealed no compound-related
effects. Absolute thyroid weight, and thyroid to body and brain
weight ratios were significantly increased in surviving males and
females at 1600 ppm. Absolute liver weight and liver to body-weight
ratio were increased in males but statistically significant only for
liver to brain weight ratio at 1600 ppm. Liver weight and liver
weight ratios were also increased in females but were not
statistically significant. The only apparent compound-related
effect observed was thyroid follicular distention observed in the 4
high-dose females and the two surviving high-dose males. The NOAEL
was 200 ppm, equal to 7.0 mg/kg of bw/day, based on decreases in
body-weight gain, increased cholesterol and decreased haemoglobin
and packed cell volume at 800 ppm (Shaw, 1990).
Beagle dogs (4/sex/group) were administered by gelatin capsule
0, 2.3, 23, or 113 mg/kg bw/day of mancozeb technical (88.6% pure).
The control group received gelatin capsule only. Compound was
administered once a day, seven days a week, for 52 weeks.
All animals receiving 113 mg/kg bw/day were sacrificed at 26
weeks with the exception of one male sacrificed at 13 weeks. At the
time of single sacrifice, food consumption was decreased 70% and
body weight depressed. Severe anaemia was evident. ALAT, ASAT,
urea, total bilirubin, total cholesterol were substantially
increased. T3 and T4 values were comparable to pre-test values.
Inorganic phosphorous was decreased 35%. Most organs were pale at
necropsy and histopathology was not conducted. Necropsy was not
conducted on animals terminated at 26 weeks. Faeces discoloration
(yellow/green) occurred in all groups but was most prevalent at the
high dose. Food consumption and body-weight gain were comparable
between control and low-dose groups (2.3 mg/kg bw/day) for both
sexes and for males at the mid- and high-doses. Females receiving
113 mg/kg bw/day manifested a 66% decrease in food consumption at 24
weeks and a 13% decrease at 52 weeks. Body-weight gain in females
at 52 weeks was 45% less than controls for animals receiving 23
mg/kg bw/day. At 24 weeks, haematology values for males were
comparable to controls at all doses. Females showed a frank anaemia
at 113 mg/kg bw/day and a dose-related decrease in RBCs. Blood
chemistry values for males showed dose-related trends for increased
ALP, total cholesterol, total plasma protein, phosphorous, and a
decreased trend for T4 with statistical significance attained at
113 mg/kg. Females showed decreasing trends for T3, T4, ASAT,
ALAT with an increasing trend in total cholesterol. ASAT and ALAT
values were statistically significant at 113 mg/kg bw/day. At 52
weeks, haematology, blood chemistry, urinalysis, organ weights and
histopathology were generally comparable to controls for both sexes
for animals receiving 2.3 and 23 mg/kg bw/day with the exception of
decreased T4 values in males. No dogs died at 2.3 and 23 mg/kg
bw/day. The NOAEL was 2.3 mg/kg bw/day, equal to 2.0 mg/kg bw/day
of active ingredient, based on decreased food consumption and
decreased body weight in females, and decreased T4 levels in males,
at 23 mg/kg bw/day (Broadmeadow, 1991a).
A second study in dogs of 52 weeks was conducted at a single
dose of 40 mg/kg bw/day as a result of the excessive toxicity and
termination of the high-dose experimental group (113 mg/kg bw/day)
in the preceding study. Protocols and methods were identical to the
first experiment. Clinical signs were limited in females. Two
females appeared thin looking during the course of the experiment
and one was reported as being hypothermic and of pale appearance.
No dogs died. Food consumption, body weight and body-weight gain
for males were comparable to controls for all time periods. Females
showed an immediate decrease in food consumption (20-26%) and a
statistically significant decrease in body weight and body-weight
gain throughout the experimental period. RBC count at 24 and 52
weeks for males and females was decreased (7-10%) but not
statistically significant. However, at 52 weeks MCV was
significantly increased in both sexes while MCHC was decreased
significantly in females. Other haematological parameters were
comparable to controls as were bone marrow findings. At 24 weeks ALP
and total cholesterol were substantially increased in males and
females whereas ALAT and ASAT were significantly decreased in
females. Inorganic phosphorous was significantly increased in
females. At 50 weeks, T3 and T4 values were statistically
decreased in both sexes while ALP levels were significantly
increased in females. ALAT values in females were significantly
decreased. Phosphorous levels were statistically raised in females.
Urinalysis showed a decrease in specific gravity for females
accompanied by increased urine volume. Absolute thyroid organ
weight was increased in both sexes and statistically significant in
males. Organ to body-weight ratios were comparable in males but
raised in females; however the values in females were suspect
because of severe loss of body weight. Necropsy revealed an
enlarged or swollen spleen in treated animals. Histopathology of
treated animals was generally not remarkable and comparable to
controls. However, an increased incidence of iron pigment
deposition in Kuffur cells of female livers and an increased
incidence of periacinar lipofuscinosis in males was reported in both
sexes (Broadmeadow, 1991b).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (60/sex/dose) were divided
into two experimental groups and given dietary concentrations of
mancozeb technical (88.6% pure) at 0 or 25 ppm, or 0, 100 or 1000
ppm for 78 weeks. Mancozeb was stable in the diet for at least 7
days and the diet was formulated weekly. Ten animals per sex per
dose were sacrificed at 52 weeks and all surviving animals
sacrificed at termination and necropsied. There was no compound-
related mortality or clinical signs. Body-weight gain in males was
decreased 6% at 52 weeks and 8% at 79 weeks at 1000 ppm. Values
were not, however, statistically significant. Body weight in
females at 100 ppm was decreased from weeks 1-24 and at 1000 ppm
from weeks 1-78. However, body-weight decreases in females at 100
ppm was not compound-related since there was no dose-response from
100 ppm to 1000 ppm. Body-weight gain in females was decreased 10%
at 52 weeks and 14% at 78 weeks at 1000 ppm. Body-weight gain
values at 52 and 78 weeks were not statistically decreased. Food
consumption and differential blood counts were comparable between
treated and control groups. There were no remarkable intergroup
differences for organ weights. The incidence of liver nodules in
males at 0, 100, and 1000 ppm were 4/50, 3/50 and 10/50,
respectively. The incidence of liver masses at 0, 100 and 1000 ppm
in males were 5/50, 5/50 and 9/50, respectively. Liver nodules and
liver masses were not significantly different from control values.
There were no intergroup differences in the incidence of liver
nodules or liver masses in females. There were no macroscopic or
microscopic findings that could be attributed to compound
administration. The incidence of male mice bearing liver tumours at
doses of 0, 100 and 1000 ppm was 10/50, 7/50 and 17/50,
respectively. There were no statistically significant differences or
trends. The number of male animals showing benign tumours was 8/50,
5/50 and 17/50, respectively, and the number showing hepatocellular
carcinomas was 2/50, 2/50 and 0/50. Males receiving doses of 0 or
25 ppm manifested malignant liver tumours in 3/50 and 4/50 animals,
respectively, with the total number of benign and malignant tumours
being 7/50 and 7/50 for both experimental groups. The NOAEL was 100
ppm, equal to 17 mg/kg bw/day, based on decreased body-weight gain
at 1000 ppm. There was no evidence of carcinogenicity (Everett et
al., 1992).
Groups of Charles River CD-1 mice (94/sex/group) received 0,
30, 100, or 1000 ppm of mancozeb (83% pure adjusted to 100% active
ingredient) for 78 weeks. Twenty-four animals/sex/group were
selected for an interim sacrifice at 12 months. Haematology was
conducted on 15 mice/sex/dose at 12 and 18 months. Thyroid function
assays were conducted on a minimum of eight animals/sex/dose after
12 and 18 months, with evaluations conducted on T4, T3 and TSH.
Necropsies were performed on all unscheduled deaths, on 24
animals/sex/group at interim (12-month sacrifice) and on all
surviving animals at terminal sacrifice.
There were no treatment-related increases in mortality or
clinical signs. Food consumption was similar among all groups. Body
weight for males and females of the 1000 ppm group were
statistically and consistently lower than controls through out the
78 weeks. Body-weight gains at 52 and 78 weeks for males and
females were decreased 18% and 13% and 15% and 9%, respectively.
Body weights and body-weight gains in other dose groups were similar
to controls values.
RBC counts were statistically significantly decreased in males
at 12 months at 1000 ppm. All other values for males were
comparable to controls. Females at 12 months and 1000 ppm showed
decreased RBC count, increased MCHC, and increased MCV. T3 and T4
changes were statistically significant only at the high dose tested
(1000 ppm) in both sexes. T3 and T4 levels were decreased in
females at 18 months (32% and 61% respectively). T4 levels in
females were also decreased at 12 months (76%). Males had a 56%
decrease at 12 months in T4 values and a 45% increase in T3 values
at 18 months. TSH levels were not statistically significantly
increased in either sex at any dose or time period.
Necropsy and gross examination of animals at the 12-month
interim and 18-month terminal necropsies revealed no consistent
gross lesions that could be attributed to an effect of the test
chemical. Diffuse discoloration of the lymph nodes was observed in
high-dose males (6/66) but the finding was not confirmed under
histopathological examination. There were no consistent differences
in mean organ weights, mean organ to body weight or organ to brain
weight ratios between control and compound-treated males or females
that could be attributed to the test article. Non-neoplastic
findings were comparable to controls. Hepatocellular adenomas and
carcinomas as well as pulmonary alveolar/bronchiolar adenomas and
carcinomas observed in both sexes were not statistically
significant. The NOAEL was 100 ppm, equal to 13 mg/kg bw/day in
males and 18 mg/kg bw/day in females, based on decreased body weight
and body-weight gain and decreased T3 and T4 values at 1000 ppm.
There was no evidence of carcinogenicity (Schellenberger, 1991).
Rats
Groups of Charles River Crl:CDBR rats (72/sex/dose) received 0,
20, 60, 125 or 750 ppm of mancozeb technical (83.8% pure) in the
diet for 2 years.
Mean body-weight gains in the high-dose males were
significantly lower than the control group in the first year
followed by lower but not statistically significant differences
during the second year (8.0%). Females receiving the high dose
showed a statistically significant decrease in body-weight gain at
90 days (16%) and one year (11%). Body-weight gains were comparable
to controls from 1-2 years. Food consumption was comparable for all
groups. Food efficiency was slightly but not statistically
decreased in high-dose males (5.5-7.5%) for various time intervals
and females at 90 days (10%). Erythrocytes, haemoglobin and
haematocrit were significantly decreased in male animals receiving
125 ppm at 18 months but not at other time periods. Haematological
findings were unremarkable in females. ALAT in males and
cholesterol levels in females were significantly increased at 750
ppm at 24 months. Males revealed decreased T3 values at 125 and
750 ppm at three months with decreased T4 values at 750 ppm at 6
and 18 months. TSH values were increased at 750 ppm at 12 and 18
months and at 125 ppm at 24 months. Females showed decreased T3
levels at 60, 125 and 750 ppm at 3 months. T4 values were
decreased at 750 ppm at 3, 18 and 24 months. TSH values were
increased at 750 ppm at 6 and 18 months. Urinalysis values were not
remarkable. Survival was comparable for all groups. After 24
months, absolute and relative (to body weight) thyroid/parathyroid
weights of the high-dose males and females were significantly
increased. The incidence of enlarged thyroids was also higher in
males and females at 750 ppm and the incidence of observed masses
was also higher in males. Follicular cell hypertrophy/hyperplasia of
the thyroid gland was significantly increased for males and females
receiving 750 ppm at the end of the first year.
A granular yellow-brown pigment was also present in rats fed
125 and 750 ppm at one and two years. Renal pigment remained in the
same magnitude as seen at 12 months. No attendant pathology was
evident. Bilateral retinopathy was also significant in both sexes
at the high dose at 2 years. At 2 years, in the high-dose group of
males and females, significant increases were recorded for thyroid
follicular cell hypertrophy/hyperplasia as well as nodular
hyperplasia. Thyroid follicular cell adenomas (20/61) and
carcinomas (14/61) were significant only in high-dose males.
Females showed increased incidences of thyroid follicular cell
adenomas (6/61) and carcinomas (4/61) but the increases were not
statistically significant when compared to control groups. The NOAEL
was 125 ppm, equal to 4.8 mg/kg bw/day, based on decreased body-
weight gain, decreased T3, T4 values, increased TSH values,
increased absolute and relative thyroid weight, thyroid follicular
cell hypertrophy, hyperplasia, and nodular hyperplasia, in both
sexes at 750 ppm. Carcinogenic effects were noted in both sexes in
the form of thyroid follicular cell adenomas and/or carcinomas but
only at the highest dose level (Stadler, 1990).
Sprague-Dawley (CD) rats (50 [main study] and 20 [satellite
study] animals/sex/dose) were given 0, 25, 100, or 400 ppm of
technical mancozeb in the diet (88.5% purity, adjusted to 100%
active ingredient) for 104 weeks. There were no compound-related
signs or mortality. Body-weight gain was significantly decreased in
males and females at the high dose tested for weeks 1-26 and 1-13,
respectively. Body weights were significantly decreased for males
from weeks 2-75 and for females from weeks 4-25. Food consumption
was comparable between all groups and food conversion ratios similar
for treated and control groups. There were no treatment-related
effects on haematological parameters. Reported changes were neither
dose nor time-dependent. T4 levels were significantly decreased in
males and females at 400 ppm at 26 and 52 weeks and in females alone
at 78 weeks at 100 and 400 ppm. However, the decrease at 100 ppm
was not consistent over time. T3 levels in males were decreased at
52 weeks but increased 43% at 104 weeks in the high-dose group. TSH
was increased by 86% at 78 weeks in females of the high-dose group.
The remaining statistical differences, particularly for
protein, calcium and other electrolytes, appeared to be unrelated to
treatment. Urinalysis findings were not considered to be compound-
related. Ophthalmoscopic changes were comparable between groups.
There were no compound-related organ weight changes. There was no
evidence of tumorigenicity. The incidence of thyroid follicular cell
adenomas in males was 6/50, 2/50, 2/50 and 6/50, for thyroid
follicular cell adenocarcinomas the frequency was 2/50, 0/50, 1/50,
and 3/50 for control and respective dose groups. Parafollicular
carcinomas in males was 1/50, 6/50,6/50 and 6/50. Anterior
pituitary adenomas in males occurred at a frequency of 18/50, 22/50,
28/50, and 27/50 whereas anterior pituitary adenocarcinomas were
observed at an incidence of 3/50, 2/50, 2/50 and 1/50 in controls
and respective dose groups. A statistical significance was not
achieved between groups, and values were comparable with the upper
incidence of the control range. Thyroid follicular cell adenomas and
adenocarcinomas were observed in females at frequencies of 0/50,
0/50, 2/50 and 2/50; and 0/50, 0/50, 0/50 and 1/50, respectively,
for controls and treated groups. Parafollicular malignant tumours
occurred at the same frequency (3/50) in all groups. Pituitary
adenomas in females (anterior pituitary) ranged from 28/50 to 35/50.
Pituitary adenocarcinomas ranged from 4/50 to 7/50. Non-neoplastic
findings in males were not significant for pituitary, testes,
kidneys, or other tissues with the exception of thyroid. There was a
minimal increase in the height of the follicular epithelium of the
thyroid (3/50, 1/50, 1/50, 8/50) and an increase in the number of
prominent microfollicles (0/50, 1/50, 0/50, 5/50) at the high dose.
Non-neoplastic findings for females were not significant for kidney,
pituitary or stomach. There was however a minimal increase in the
height of the follicular epithelium of the thyroid (1/50, 0/50,
1/50, 5/50) at 400 ppm. The NOAEL was 113 ppm, equal to 4.0 mg/kg
bw/day in males and 5.1 mg/kg bw/day in females, based on decreased
body-weight gain and body weight, an increase in the height of the
thyroid follicular epithelium, an increase in prominent
microfollicles, and a decrease in thyroxine levels at 450 ppm (Hooks
et al., 1992).
Reproduction studies
Rats
Sprague-Dawley Crl:CD(SD)BR rats (25/sex/dose) received 0, 25,
150, or 1100 ppm of 88.4% mancozeb technical in the diet. Compound
intake was not corrected to 100% of active ingredient in the diet.
Animals (F0 generation) were seven weeks old at the start of
treatment, acclimated to laboratory conditions and healthy. After a
premating period of 14 weeks, animals of the same dose levels were
mated for not longer than three weeks. Females were allowed to
litter and rear their offspring. Exposure to compound and diet was
continuous and ad libitum. Selected F1a pups were maintained for
14 weeks after weaning of all F1a offspring then mated for three
weeks, allowed to litter and rear their offspring (F2a generation).
There were no compound-related deaths and clinical signs were not
evident for either generation. Body weight and body-weight gain were
significantly depressed in males and females of the high-dose group
for both parental (F0, F1) generations during the 14-week pre-
treatment period. Males of the F1 generation also showed
significant decreases in body weight and body-weight gain at the
mid-dose. Body weight and body-weight gain for dams during
gestation of F0, and F1 parents, were significantly decreased at
1100 ppm and depressed at the mid-dose. Food consumption was
significantly decreased for both sexes during the premating period
of both generations at the high dose. Food consumption for F1
females was also significantly decreased at the high dose during the
periods of gestation and lactation.
Absolute organ weight at necropsy revealed statistically
significant increases for both parental sexes at the high dose.
Macroscopic examination of all tissues examined revealed no
remarkable findings. Histopathology, however, revealed thyroid
hyperplasia and hypertrophy in nearly all animals examined of F0
and F1 parents at the high dose. Thyroid follicular cell adenomas
were also found in five males of the F0 generation and in eleven
males of the F1 generation at the high dose. Indices for F1a pups
were comparable to controls. A slight delay in the opening of the
eye was however considered to be treatment-related at the high dose.
Necropsy findings were comparable between treated and control groups
for F1a and F2a litters. Viability, pups and litter weights were,
however, significantly decreased at the high dose on days 14-21.
The NOAEL in this study was 25 ppm, equal to 1.7 mg/kg bw/day, based
on decreased body weight at 150 ppm (Muller, 1992).
Charles River CRL:CDBR rats (25/sex/dose) received 0, 30, 120,
or 1200 ppm of mancozeb (84% pure) in the diet. Animals were placed
together for a period of 10 days to produce the F1a and F1b
generation. The presence of a vaginal plug was considered day zero
of gestation. On day 4, litters were culled to 5 animals/sex/dose.
Litters were weaned on day 21. One male and one female were then
selected from the F1a litter to serve as the parents for the F2
generation (F2a and F2b litters).
There were no treatment-related clinical signs or deaths in
either the F1 or F2 generation. Parental body weights for F1 and
F2 male and female rats were similar between control, 30 and 120
ppm groups throughout the 10-week treatment period prior to mating
or for F1 and F2 females during the gestation and lactation
periods. At 1200 ppm, mean body weight for male and female rats of
the F1 and F2 generation were significantly below control
throughout the pre-mating period, as well as for parental females
during gestation and lactation. Mean feed consumption of F1 and
F2 male and female rats were comparable between control, 30 and 120
ppm groups. At 1200 ppm, mean food consumption was decreased in F1
but not F2 male and female rats prior to mating. During gestation
and lactation F1 and F2 females had food consumption values
comparable to controls. Reproductive indices as measured by
fertility, gestation, viability and lactation were comparable to
controls for F1 and F2 adult and offspring at all dose levels.
Fetal body weight for F1a,b and F2a,b offspring were also comparable
to controls. There were no treatment-related effects on the
absolute and relative organ weights at 30 ppm for the F1 and F2
parents or for F1 parents at 120 ppm. F2 males showed a
statistically significant dose-related increase in relative liver
weight at 120 ppm without histopathological changes. At 1200 ppm
both F1 and F2 parents manifested significant increases in
relative liver weight and absolute and relative thyroid weights.
Relative kidney weight was increased in F1 and F2 females, but not
in males. There were no gross pathological changes considered to be
treatment-related among either F1 or F2 animals nor any which
could be substantiated by corresponding histopathology.
Treatment-related microscopic changes were observed in the
thyroid, kidney and pituitary of F1 and F2 animals. Changes
involving the follicular cells of the thyroid observed in the F1
generation were also observed in the F2 generation with generally
higher incidences or greater severity. All males of the F1 and F2
generation showed some degree of diffuse follicular cell hyperplasia
at 1200 ppm. Follicular cell adenomas and nodular/cystic follicular
cell hyperplasia were also observed at 1200 ppm. All or nearly all
females showed some degree of diffuse follicular cell hyperplasia at
1200 ppm in F1 and F2 generations. Nodular/cystic follicular cell
hyperplasia was also evident at 1200 ppm in the F2 generation.
Follicular cell adenomas were not observed in females. Brown
globular pigment was observed within the lumen of proximal tubules
in the kidneys at statistically significant incidences in both sexes
of both generations at 120 and 1200 ppm. However, no associated
pathology was observed. Hypertrophy and/or vacuolation of the
anterior pituitary was treatment-related only in males and only at
1200 ppm of the F1 and F2 generation based upon an increased
severity of response and not an increased incidence. Other
microscopic changes were not considered to be treatment-related. The
NOAEL was 120 ppm, equal to 7.0 mg/kg bw/day, based on increased
relative weights of the liver, kidney and thyroid, increased
absolute thyroid weight, decreased body weight and feed consumption
of females during gestation and lactation, and decreased pre-mating
body weight and feed consumption, at 1200 ppm (Solomon et al.,
1988).
Special study on neuropathology
Rats
Groups of Charles River Crl:CD(R) BR rats (10
animals/sex/dose) received 0, 20, 125, 750 or 5000 ppm of mancozeb
(79.3% pure and adjusted for purity) mixed in the diet for 90 days.
Two female satellite groups of 16 females each were also included
and received 5000 ppm for a two-week period only prior to sacrifice.
One male and 4 females died in the 5000 ppm group. These deaths
occurred between the second and fourth week of administration and
resulted in a cessation of test compound in females of the high dose
only and administration of control feed for the remainder of the
study. Onset of clinical signs in the second and third week of
administration consisted of generalized weakness, abnormal gait or
mobility, limited or no use of the rear legs. Loss of muscle mass
was also reported in males. However by day 60 some males in the
high-dose group appeared clinically normal and females showed
improvement after one week on control diets. Females of the
satellite group fed mancozeb for two weeks manifested limited rear
limb mobility. No clinical signs were observed below 5000 ppm.
Body weight in males was decreased 45% by day 90 in the high-
dose group. Other dose levels in males were comparable to controls.
Females receiving 5000 ppm showed initial weight loss which was
reversed after cessation of test compound and their placement on
control feed. Satellite females administered 5000 ppm of mancozeb
for two weeks showed minimal weight changes at 14 days. However, at
750 ppm and 90 days biologically significant decreases in body
weight (9%) and body-weight gain (17%) were noted. Body weights
were unaffected at lower doses. Food consumption in high-dose males
was decreased 40% compared to controls, but was similar to controls
at other dose levels. Females receiving 5000 ppm for two weeks had
decreased food consumption values ranging between 29% and 64%. In
other groups, female food consumption was similar to controls. Food
efficiency was decreased in all high-dose animals and at 750 ppm in
females (13%).
Neuropathology revealed effects in both sexes at 750 ppm and
5000 ppm. Pathology in males at 750 ppm and 5000 ppm was reported
as myelin phagocytosis, Schwann cell proliferation, myelin bubbles,
demyelination of nerve fibres associated with the posterior thigh
muscle and myelin ovoids in teased nerve fibres. Additional
observations at 5000 ppm consisted of intra-sheaths ellipsoids,
demyelination, thickening of the myelin sheath, neurofibrillary
degeneration and atrophy of the posterior thigh muscles. High-dose
females fed a recovery diet showed a thickening of the myelin
sheath, myelin bubbles, and ballooning of the myelin sheath.
Muscular atrophy was reported in one surviving female. There was
also an increased incidence of demyelination but a decrease in the
presence of myelin ovoids and debris when compared to males at this
dose level. Females fed 5000 ppm for two weeks and then sacrificed
showed myelin bubbles, sheath thickening, myelin phagocytosis and
Schwann cell proliferation. Muscle atrophy was present in 9/10
animals examined and demyelinated lengths with the presence of
myelin debris and ovoids were found upon examination of teased sural
nerve fibres. At 750 ppm in females, teased nerve fibres showed
some demyelination myelin ovoids and debris. No neuropathology was
conducted at 20 ppm dose level because of the absence of findings at
125 ppm. The NOAEL was 125 ppm, equal to 8.2 mg/kg bw/day in males
and 10.5 mg/kg bw/day in females, based on decreased body weight,
body-weight gain and food consumption in females and
neurohistopathological changes in both sexes at 750 ppm (Stadler,
1991).
Special studies on embryotoxicity/teratogenicity
Rats
Groups of mated female Crl:CD rats (27/group) were whole-body
exposed to 80% pure mancozeb dust by the inhalation route.
Administered doses were 0, 1, 17, or 55 mg/m3 for 6 hours/day on
days 6-15 of gestation. Particle size ranged from 1.4-6.4 microns.
Animals were observed for clinical signs during and after exposure
then sacrificed one day prior to natural delivery. Fetuses were
examined externally, viscerally and skeletally. No animals died.
Hind limb weakness and a slower righting reflex were observed in
6/27 high-dose dams. However, the signs disappeared during the
post-exposure observation period. High-dose animals also gained
significantly less weight than controls (40% less). There were no
differences between treated and control group as to malformations
observed. There was however an increased incidence in the number of
animals manifesting a wavy rib which was statistically significant
at the high dose. This variation was dose-related across treated
groups. There was no increase in soft tissue alterations.
Observations were typical of those commonly seen in this strain of
rat. Fetal body weights were comparable between groups (Lu &
Kennedy, 1986).
Mancozeb (83.0% active ingredient adjusted to 100% for dosing)
was administered by gavage in corn oil to groups of 26 primigravid
BLU(SD)BR rats on days 6-15 of gestation (day 0, day of
insemination) at doses of 0, 2, 8, 32, 128 or 512 mg/kg bw/day. ETU
was administered as a positive control at 50 mg/kg bw/day. All rats
were sacrificed on day 20 of gestation and caesarean sections
performed.
Food consumption and body weights were decreased at 128 mg/kg
bw/day and above in mancozeb treated dams. Body weights for all
other mancozeb-treated groups were comparable to the control group
as was the ETU-treated group. Litter data indicated significant
decreases in the average number of live fetuses, mean fetal weight
and mean gravid uteri and increased resorptions only at 512 mg/kg
bw/day for mancozeb-treated animals. Fetal weight was decreased in
ETU-treated animals, and one dam died on study. Adverse compound-
related effects were clearly manifested for mancozeb at 512 mg/kg
bw/day and ETU at 50 mg/kg bw/day for gross abnormalities (agnathia,
cleft palate, meningoencephalocele), soft tissue effects (dilated
ventricles, compressed spinal cord), and skeletal tissue (incomplete
ossification of the skull, clavicle, scapula). The effects observed
at 128 mg/kg bw/day were not biologically or statistically
significant. The NOAEL for maternal toxicity was 32 mg/kg bw/day
based on decreased body weight and decreased food consumption at 128
mg/kg bw/day. The NOAEL for teratogenic effects was 128 mg/kg bw/day
based, in part, on the presence of agnathia, cleft palate,
meningoencephalocele, dilated ventricles and incomplete ossification
of the skull at 512 mg/kg bw/day (Gallo et al., 1980).
Female Sprague-Dawley rats (25/dose/group) received 0, 10, 60,
or 360 mg/kg bw/day of mancozeb (88.6% pure) in methyl cellulose or
methyl cellulose alone (control group). Animals were dosed daily by
the oral route (gavage) from day 6 to day 15 (inclusive) of
gestation. Dams were observed on predetermined regular schedules
for clinical signs, mortality body weight, and food consumption. On
day 20 of gestation females were sacrificed with carbon dioxide and
uterine contents examined.
There were no readily apparent or statistically significant
differences between control values and values obtained in treated
groups for the parameters examined at either 10 or 60 mg/kg bw/day.
One dam was sacrificed in a moribund state at 360 mg/kg bw/day. Four
females exhibited a "reeling gait" followed by slight paralysis in
three of the dams. Body weights were decreased during the treatment
period (days 6-15) and days 16 through 20. Statistical significance
was attained on days 16 and 20. Food consumption was significantly
decreased from days 6-15 inclusive. A slight dose-related reduction
in the degree of ossification of the intraparietal bone was
statistically significant. The Incidence for this effect was 75, 80,
87 and 90% for control to high dose. The historical mean was 25% and
the range 4.90-91.0%. A marginal increase in the size of the
anterior fontanelle (12%) was also observed. The historical mean for
this effect was 2.6% with a range of 0-24%. Incomplete ossification
of the thoracic vertebrae centra was also statistically significant
at the high dose tested. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 60 mg/kg bw/day. Maternal toxicity at 360
mg/kg bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryo/fetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra (Tesh et al., 1988).
Rabbits
Groups of New Zeeland white rabbits (18 females/dose) were
treated by oral intubation with doses of 0, 5, 30 or 55 mg/kg bw/day
of Pencozeb Technical (88.4% pure) in methyl cellulose or methyl
cellulose, on days 6 through 18 of gestation. Animals were
sacrificed on day 28 post-coitum. There were no compound-related
effects at these dose levels either in-life or during examination
post-sacrifice. A second experiment was therefore conducted at a
single high dose of 100 mg/kg bw/day with a concurrent control group
following the general procedures and examinations of the prior
experiment. No treatment-related clinical effects or treatment-
related necropsy findings were detected. Two dams in the control
group and five in the 100 mg/kg bw/day dose group aborted. A clear
and substantial body-weight loss was observed in does at 100 mg/kg
bw/day for days 6-9 post-coitum (i.e. days 1-3 of compound
administration). Body-weight gain during days 9-15 was also
substantially depressed compared to controls. Food consumption was
also markedly decreased between days 6-19. A slight post-
implantation loss (5%) was also observed and may have been compound-
related. No compound-related fetal effects were observed. The NOAEL
for maternal effects was 55 mg/kg bw/day and greater than 100 mg/kg
bw/day for embryo/fetotoxic effects. An increase in abortion, body-
weight loss, suppressed body-weight gain and decreased food
consumption were observed at 100 mg/kg bw/day (Muller, 1991).
New Zeeland white female rabbits (20 animals/dose/group)
received either 0, 10, 30, or 80 mg/kg of bw/day of mancozeb (83.0%
pure) in methyl cellulose or methyl cellulose alone (control group)
by oral gavage on days 7-19 of gestation. No treatment-related
deaths occurred in control or in the 10 and 30 mg/kg bw/day dose
groups. One animal died in the 30 mg/kg bw/day group but death was
attributed to mis-dosing. Two does were sacrificed in a moribund
condition at the high dose and the deaths considered treatment-
related (one of these two does was pregnant and did not abort).
Clinical signs were comparable between controls and animals
receiving 10 and 30 mg/kg bw/day. Animals receiving 80 mg/kg bw/day
manifested significant increases in clinical signs. Alopecia,
anorexia, ataxia, scant faeces, and abortions were all observed at
the high dose tested. Body-weight gain and food consumption were not
significantly different between controls and groups receiving 10 or
30 mg/kg bw/day. At 80 mg/kg bw/day body-weight and food consumption
were significantly decreased in does that aborted and those
sacrificed moribund. Does producing at least one viable fetus had
body-weight gains and food consumption values similar to controls.
No treatment-related changes were evident in the incidence of
gross postmortem findings between does in the control and treated
group. Reproductive parameters between controls and does receiving
10 or 30 mg/kg bw/day were comparable as measured by the number of
abortions, litters produced, and the mean number per litter of
corpora lutea, implantations, resorptions, dead and live fetuses or
sex ratio. At 80 mg/kg bw/day a significant increase in does
aborting (5/15) was reported with a corresponding decrease in the
number of litters produced. All other parameters were comparable to
control group. Mean fetal body weight was similar between the
control and treated groups. There were no significant increases in
the types or incidence of malformations or developmental variations
between control or treated groups. The NOAEL for maternal toxicity
was 30 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was greater
than 80 mg/kg bw/day. Maternal toxicity at 80 mg/kg bw/day was
based on an increase in aborted fetuses, decreased number of litters
produced, decreased body-weight gain and food consumption, an
increase in clinical signs and death (Solomon & Lutz, 1987).
Special study on mancozeb stability in DMSO
14C-mancozeb (Dithane M-45; 80% wettable powder; 88% radio
purity) was suspended in DMSO (99% pure) at a nominal concentration
of 100 ppm and sampled and analyzed for mancozeb at the following
times: 0, 15, 30, 60, 90, 120, 150, 180 and 210 minutes. Maximum
concentration of mancozeb occurred at approximately 15 minutes.
Immediately after adding DMSO to mancozeb (zero time) there was
evidence of significant decomposition with continued rapid
degradation to three compounds, two of which were impurities in the
starting material. The majority of the mancozeb was degraded within
60 minutes and after 4 hours an average of 9% of the mancozeb
remained. The half-life of mancozeb in DMSO was calculated to be 37
minutes (Schweitzer, 1990).
Special studies on genotoxicity
The results of genotoxicity assays are given in Table 2.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
Special studies on irritation and sensitization
Guinea-pigs
Mancozeb has been demonstrated to be a strong sensitizer in the
Hartley strain female guinea-pig by the Guinea-Pig Maximization
Test. With induction concentrations of 5% (intradermal) and 25%
(topical) and challenge concentrations of 2% and 0.5% (topical) all
of the mancozeb-treated females (10/group) responded at both 24 and
48 hours. In the same studies, cross sensitization responses were
also seen with zineb, maneb and mancozeb. A dose-response
relationship between the incidence of skin sensitization and
induction and challenge concentration was also seen with maneb. The
purity was not stated for any of the test materials (Matsushita et
al., 1976, 1977).
Young adult Hartley guinea-pigs were divided into three
experimental groups and tested for delayed contact hypersensitivity
using a modified Buehler procedure. Animals received three 6-hour
induction doses (1 dose/week, within 3 weeks) of either 0.4 ml of
50% (w/v) mancozeb (83% pure) in distilled water, 0.1% (w/v) 1-
chloro-2,4-dinitrobenzene (DNCB) in 80% (v/v) aqueous ethanol
(positive control), or received no induction doses (control group)
but were otherwise treated similarly. All groups were challenged 12
days after the last induction dose/treatment. The sham treated
control group was challenged with test compound and DNCB on each
half of the back. Erythema and edema reactions were scored 24 and
48 hours after challenge using the method of Draize. All groups
were rechallenged 7 days after the primary challenge and a separate
additional control group was challenged as previously described. No
erythema or edema was observed in sham treated controls. Positive
controls showed positive response at 24 and 48 hours. Only one of
Table 2. Results of genotoxicity assays on mancozeb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 2.5-250 µg/plate 88% active Negative Chism, 1984a
reversion assay TA1535, TA1537, (± rat S9); ingredient (a.i.)
TA98, TA100 in distilled water
S. typhimurium 2.5-250 µg/plate (± rat S9); 88% a.i. Negative Chism, 1984b
TA1535, TA1537, in distilled water
TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.5-45 µg/ml; 88% a.i. Negative Foxall & Byers,
mutation assay (CHO)/hprt in distilled water 1984
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethal Muller-5 stock food medium Krishnamurthy, 1980
assay
Autosomal D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethals Cy/B1 L2 stock food medium Krishnamurthy, 1980
1.D. Yeast and Other Fungal Assays
Point mutation A. nidulans 0.125-12 µg/ml; Not specified Positive Martinez-Rossi &
induction strains biA1 in distilled water; Azevedo, 1987
methG1 and 118 no activation used
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vitro Chromosomal Alterations in Mammalian Cells
In vitro Cultured human 1.40 µg/ml; Not specified Positive Georgian et al.,
chromosomal lymphocytes in propylene glycol; 1983
aberrations no activation used
2.B. In Vivo Chromosomal Alterations
Bone marrow Male non-inbred 10-1000 mg/kg; Not specified Negative2 Kurinnyi et al.,
cytogenetics white mouse in milk suspension 1982
or aqueous emulsion?
Wistar rat single i.p. 2.5-10 mg/kg; Not specified Positive Georgian et al.,
in propylene glycol 1983
Wistar rat 1.7 mg/kg bw/day for Not specified Positive Georgian et al.,
280 days; mixed in feed 1983
Male Fischer 344 rat 4.4 g a.i./kg/day for 1 88% a.i. Negative Sames et al., 1983
or 5 days; in corn oil
Male albino mouse 30-300 mg/kg; Not specified Positive Gautam & Kapoor,
(Lacca strain) in distilled water? 1991
Lymphocyte Female Wistar rat 3-30 mg/kg; in saline Not specified Positive Newton, 1975
cytogenetics
Micronucleus assay Male and female 10 000 mg/kg; 88.2% Negative Allen et al., 1987
CD-1 mouse in aqueous methyl-cellulose
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2.C. Plant Tests
Chromosomal Allium cepa 500-1000 ppm spray Not specified Positive Mann, 1977
alterations peduncles
Hordeum vulgare 100-1500 ppm; Not specified Positive Murty et al., 1983
germinating seeds in phosphate buffer
(with trace DMSO?)
Allium cepa 4-500 ppm Not specified Positive Badr, 1988
root tips
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro Primary rat 0.25-10 µg/ml; 88% a.i. Suggestive Byers, 1985
unscheduled DNA hepatocytes from male in culture medium positive;
synthesis (UDS) Fischer 344 rat suggests repeat
Primary rat 0.1-10 µg/ml; 82.4% Negative O'Neill & Frank,
hepatocytes from male in culture medium (also for S 1988
Fischer 344 rat phase induction)
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 5-20 µg/ml; Not specified Positive without Ivett, 1985
SCE assays (CHO) cells in culture medium activation
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell C3H/10T 1/2 cells 0.05-0.5 µg/ml; in water 88% a.i. Negative McGlynn-Kreft &
transformation McCarthy, 1984
C3H/10T 1/2 cells 0.1 µg/ml as "promoter"; 88% a.i. Negative McLeod & Doolittle,
with "promotion" in water 1985
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report.
2 The authors judged this effect to be positive, but significance was restricted to one dose and there was no dose-related response.
twenty animals given mancozeb (1/10 females) showed slight erythema
at 48 hours. After rechallenge the original female respondent was
negative but a second female (1/10 females) showed a slight erythema
at 24 hours but not at 48 hours (Trutter, 1988a).
Rabbits
Nine rabbits of unknown strain and sex were administered 100 mg
of ground mancozeb technical (> 80% pure) into the conjunctival sac
of one eye. The treated eyes of 3 rabbits were washed with water
approximately 20-30 seconds after dosing. Animals were observed for
eye irritation at 4, 24, 48, 72 and 96 hours and on days 7, 14 and
22. Substantial irritation was evident in unwashed eyes involving
the cornea, iris and conjunctiva. Effects were reversible after 14
days in unwashed eyes and after 72 hours in washed eyes.
A second study conducted in female New Zeeland white rabbits
using the same protocol produced responses of lesser consequence and
little or no involvement of the cornea or iris. Effects in unwashed
eyes were reversible after 7 days and after 24 hours in 2 of 3
rabbits with washed eyes. However conjunctival irritation persisted
in one rabbit through 14 days and disappeared at 21 days. The
compound was considered a substantial irritant based on the duration
of irritation (DeCrescente & Chan, 1982).
Six rabbits of unknown sex and strain were administered 500 mg
of ground mancozeb technical (> 80% pure), prepared as a paste in
saline, to intact and abraded skin. The test material was held
under an impervious patch in continuous 24-hour contact with closely
clipped skin. Erythema and edema were scored at 24 and 72 hours and
7 days. The only effect observed was erythema on abraded skin. The
primary irritation score was 0.5. The compound was considered a
slight irritant (DeCrescente & Parsons, 1980).
Observations in humans
A sample population of 121 Korean orange growers was tested for
hypersensitivity to the fungicide Dithane M-45 (mancozeb). A maximum
non-irritating concentration of 10% Dithane M-45 mixed with vaseline
was previously determined on 201 Korean medical students by patch
test. Results were read 48 hours post-administration. Application
of the patch test to orange growers was read 72 hours post-
administration according to the standards of the North American
Dermatitis Research group. Dithane tested positive in 4/121
subjects (3.3%) (Lee et al., 1981).
A 37-year old male working as a florist developed erythematous
vesicular eruption on the palms of his hands resembling dyshidrotic
eczema. Symptoms worsened and spread to other body areas (face,
neck, legs) during periods of heavy work, and diminished during
holidays. Patch tests with the mancozeb products Diagent and Firma
using Finn Chambers on Scanpor Tape read at 20 minutes, 2, 3, and 4
days resulted in a strong positive reaction on day two. A weak
positive reaction to zineb was also observed. Total serum IgE was
approximately five times the normal value. Other serum
immunoglobulins, G, A, and M were normal (Crippa et al., 1990).
A 61-year old vineyard worker developed a rash on the left
forearm as well as inflammation of the eyelids after planting vine
seedlings. This was the third episode under similar circumstances.
Patch tests on two previous occasions were negative. Patch tested
with 0.002% solution of both zineb and mancozeb (1% of normal use
concentration) resulted in positive responses. Retesting a week
later with the bark of a vine stick previously treated with mancozeb
produced a strong positive reaction. It was also reported that two
other male farm workers developed papulovesicular sheeted dermatitis
of the face, hands and chest after planting mancozeb-treated
potatoes. Patch tests with 0.1% solution of mancozeb were positive
in both. Patch tests conducted on the vineyard worker with
macerated vine bark were positive with aqueous but negative with
alcoholic solution (Kleibl & Rakcova, 1980).
One-hundred-fifty-three men currently or previously exposed to
dithane for many years at a manufacturing site were compared for
thyroid function to 153 men not exposed to Dithane, its products or
ETU who also worked at the same plant. Workers and controls were
carefully matched with respect to age, race, length of employment
and type of employment. Informed consent was obtained from all
participants. It was reported that at least one abnormality was
found in 25 of the 306 men (8.2%). The findings included
abnormalities on thyroid palpitation (4.3%), serologic autoimmune
thyroiditis (4.3%), subclinical hypothyroidism (1.3%), Graves'
disease (0.3%) and miscellaneous hormonal abnormalities (1.6%).
Urinary excretion of ETU in a subgroup of 42 workers currently
exposed to Dithane was 0.02 ppm compared to 0.01 ppm in the control
group (41 men), most of whom had undetectable values. The authors
concluded that exposure to Dithane manufacture was not associated
with an increased prevalence of thyroid abnormalities and that
thyroid abnormalities were common in iodine-replete adult American
men affecting 8.2% of the population studied (Charkes et al.,
1985).
A prevalence survey of adverse reproductive outcomes was
carried out in a population of 8867 persons (2951 men and 5916
women) who had been working in the floriculture industry in the
Bogota area of Columbia for at least 6 months. These workers from
58 companies were exposed to 127 different types of pesticides. The
prevalence rates for abortion, prematurity, still births, and
malformations were estimated for pregnancies occurring among the
female workers and the wives of the male workers before and after
working in floriculture and these rates were related to various
degrees of exposure. A moderate increase in the prevalence of
abortion, prematurity and congenital malformations was detected for
pregnancies occurring after the start of work in floriculture.
However, it could not be determined whether the increase in the
prevalence of the parameters measured was real. All rates, except
those for still births were higher for pregnancies occurring after
exposure to pesticides among both the female workers and the wives
of the male workers and thus gave significantly increased odds
ratios (chi-square) for abortions, premature births and malformed
babies. However, the high odds ratio observed for induced abortions
was considered by the authors surprising and cast doubt on the
reliability of the association between the observed increased risks
and the exposure to pesticides. It was also noted that of the 10
most used pesticides in floriculture, which accounted for 67% of the
total pesticides used in this industry, mancozeb comprised 7% of the
total. However, the article addressed pesticides in general and no
one chemical was singled out for adverse reactions. The authors
recognized that multiple exposure not only posed the problem of
identifying the toxic chemical responsible in causing any adverse
effect but also the problem of interaction between chemicals in the
expression of toxic effects (Restrepo et al., 1990).
COMMENTS
In pharmacokinetic studies conducted in male and female mice,
orally administered 14C-labelled mancozeb was rapidly absorbed,
peaking in whole blood between 1 and 2 hours, extensively
metabolized, and rapidly excreted (90%) within 24 hours. ETU was
the major metabolite.
Rats given single oral doses of 14C-labelled mancozeb absorbed
about 50% of the dose within 3-6 hours. Most of the dose was
excreted in 24 hours with half eliminated in the urine and half in
the faeces. Less than 4% was found in the tissues, with the thyroid
containing the highest residue level. Most of the 14C dose in
faeces was unabsorbed, since only 2-8% of the dose was found in
bile. ETU was the major metabolite. The half-life of ETU
elimination was 4-5 hours. The estimated bioavailability of ETU in
rats was about 6.8% on a weight/weight basis and 20% on a mole/mole
basis.
The acute oral, dermal and inhalation toxicity of mancozeb
technical is low. WHO has classified mancozeb as unlikely to
present acute hazard in normal use.
In a 13-week study in rats, mancozeb was administered in
dietary concentrations of 0, 30, 60, 125, 250 or 1000 ppm. The
NOAEL was 125 ppm (equal to 7.4 mg/kg bw/day) based on increased
serum TSH and decreased T4 values at the next higher dose.
Dogs administered 0, 10, 100, 1000 or 5000 ppm of mancozeb in
the diet for three months demonstrated a NOAEL of 100 ppm, equal to
3.0 mg/kg bw/day. At the next higher dose, decreased body-weight
gains and decreased erythrocyte count, haematocrit and haemoglobin
were observed.
In a 52-week study in dogs, mancozeb was administered in the
diet at concentrations of 0, 50, 200, 800 or 1600 ppm. The NOAEL
was 200 ppm, equal to 7.0 mg/kg bw/day, based on decreases in body-
weight gain, increased cholesterol and decreased haemoglobin and
packed cell volume at 800 ppm.
The NOAEL for dogs given mancozeb technical for 52 weeks, 7
days a week, by gelatin capsule was 2.3 mg/kg bw/day based on
decreased body weight, food consumption and decreased thyroxine
levels at 23 mg/kg bw/day.
In a 78-week carcinogenicity study in mice at dietary
concentrations of 0, 25, 100 or 1000 ppm, there was no evidence of
carcinogenicity. The NOAEL was 100 ppm, equal to 17 mg/kg bw/day,
based on decreased body-weight gain at 1000 ppm.
In a second 78-week carcinogenicity study in mice at dietary
concentrations of 0, 30, 100 or 1000 ppm in the diet, there was no
evidence of carcinogenicity. The NOAEL was 100 ppm, equal to 13
mg/kg bw/day, based on decreased body weight and decreased T3 and
T4 values at 1000 ppm.
The overall NOAEL in the two 78-week studies in mice was 17
mg/kg bw/day.
In a two-year toxicity/carcinogenicity feeding study in rats at
dietary concentrations of 0, 20, 60, 125 or 750 ppm, the NOAEL was
125 ppm (equal to 4.8 mg/kg bw/day) based on decreased body-weight
gain, decreased T3 and T4 values, increased TSH values, increased
absolute and relative thyroid weight, thyroid follicular cell
hypertrophy, hyperplasia, and nodular hyperplasia at 750 ppm.
Tumorigenic effects were noted in both sexes in the form of thyroid
follicular cell adenomas and/or carcinomas at the highest dose
level.
Mancozeb technical when administered in the diet to rats for
two years at dose levels of 0, 28, 113 or 454 ppm was not
tumorigenic. The NOAEL was 113 ppm (equal to 4.0 mg/kg bw/day)
based on decreased body-weight gain, decreased thyroxine levels and
an increase in the height of the thyroid follicular epithelium and
an increase in prominent microfollicles at 450 ppm.
The overall NOAEL in the two 2-year studies in rats was 4.8
mg/kg bw/day.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 25, 150 or 1100 ppm, the NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on decreased body weight at 150
ppm.
In a second two-generation reproduction study in rats at
dietary concentrations of 0, 30, 120 or 1200 ppm, the NOAEL was 120
ppm, equal to 7.0 mg/kg bw/day based on microscopic changes in the
thyroid, kidney and pituitary, increased relative weights of the
liver, kidney and thyroid, increased absolute thyroid weight,
decreased gestation and lactation body weight and feed consumption,
decreased pre-mating body weight and feed consumption at 1200 ppm.
The overall NOAEL in both reproduction studies was 7.0 mg/kg
bw/day.
In a 90-day (neuropathology) study conducted in rats at dietary
concentrations of 0, 20, 125, 750 or 5000 ppm, the NOAEL was 125
ppm, equal to 8.2 mg/kg bw/day, based on decreased food consumption
and neuro-histopathological changes at 750 ppm.
An oral teratogenicity study in rats at dose levels of 0, 2, 8,
32, 128 or 512 mg/kg bw/day produced no maternal effects at 32 mg/kg
bw/day (NOAEL) and no teratogenic effects at 128 mg/kg bw/day
(NOAEL). Maternal effects in the form of decreased body-weight gain
and decreased food consumption were seen at 128 mg/kg bw/day.
Teratogenic effects were seen at 512 mg/kg bw/day based, in part, on
the presence of agnathia, cleft palate, meningoencephalocele and
dilated brain ventricles.
A second oral teratogenicity study in rats at dose levels of 0,
10, 60 or 360 mg/kg bw/day showed no maternal or embryo/fetotoxic
effects at 60 mg/kg bw/day (NOAEL). Maternal toxicity at 360 mg/kg
bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryofetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra.
The NOAEL in an oral teratogenicity study in rabbits given 0,
5, 30, 55, or 100 mg/kg bw/day was 55 mg/kg bw/day for maternal
effects and greater than 100 mg/kg bw/day for embryo/fetotoxic
effects. An increase in abortions, body-weight loss, and decreased
food consumption were observed at 100 mg/kg bw/day.
The NOAEL in an oral teratogenicity study in rabbits given 0,
10, 30 or 80 mg/kg bw/day was 30 mg/kg bw/day for maternal toxicity.
The NOAEL for embryo/fetotoxic effects was greater than 80 mg/kg
bw/day. Maternal toxicity at 80 mg/kg bw/day was based on an
increase in aborted fetuses, decreased number of litters produced,
decreased body-weight gain and food consumption, an increase in
clinical signs and death.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
The data on mancozeb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 4.8 mg/kg bw/day for thyroid effects in rats
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for mancozeb, alone or in combination
with maneb, metiram, and/or zineb, because of the similarity of the
chemical structure of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differentiated using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet, equal to 17 mg/kg bw/day
(78-week studies)
Rat: 125 ppm in the diet, equal to 4.8 mg/kg bw/day
(two-year studies)
120 ppm in the diet, equal to 7.0 mg/kg bw/day
(reproduction studies)
125 ppm in the diet, equal to 8.2 mg/kg bw/day
(90-day neuropathology study)
Dog: 200 ppm in the diet, equal to 7.0 mg/kg bw/day
(52-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with maneb, metiram, and zineb)
Studies which will provide valuable information in the continued
evaluation of the compound
Clarification of the genotoxic potential of mancozeb.
Observations in humans.
REFERENCES
Allen, J.A., Proudlock, R.J. & Pugh, L.C. (1987). Micronucleus test
on mancozeb technical. Unpublished report No. PWT 39/86637 from
Huntingdon Research Centre Ltd., Huntingdon, Cambridgeshire,
England. Submitted to WHO by Elf Atochem, Philadelphia,
Pennsylvania, USA (Confidential data of Elf Atochem).
Badr, A. (1988). Cytogenetic activities of some fungicides.
Cytologia, 53: 635-640.
Braverman, L.E., Lipworth, L. & Charles, D. (1978). A health survey
of workers involved in the manufacture and packaging of dithane
fungicide with special reference to thyroid function. Unpublished
report dated August 2, 1978 from the University of Massachusetts
Medical School, Worcester, Massachusetts, USA. Submitted to WHO by
Rohm and Haas Company, Spring House, Pennsylvania, USA.
Broadmeadow, A. (1991a). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
89/PTC004/0015 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Broadmeadow, A. (1991b). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
90/PTC029/0197 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Byers, M.J. (1985). Dithane M-45 in vitro unscheduled DNA
synthesis assay. Unpublished report No. 84R-280 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Cameron, B.D., Clydesdale, K. & Speirs, G.C. (1990). The disposition
of 14C mancozeb in the mouse. Unpublished report No. 4909 from
Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA (Confidential
data of Elf Atochem).
Charkes, N.D., Braverman, L.E., Penko, K.F., Gowers, D.S., Gordon,
C.F., Lipworth, L, Malmud, L.S. (1985). Thyroid function in male
workers manufacturing dithane, an agricultural fungicide, and in men
not exposed to dithane. Frontiers in Thyroidology, 2: 933-936.
Chism, E. (1984a). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced Fischer 344 rats. Unpublished
report No. 84R-059 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Chism, E. (1984b). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced B6C3F1 mice. Unpublished
report No. 84R-0060 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Cox, R.H. (1986). Three month dietary toxicity study in dogs with
mancozeb. Unpublished report No. HLA 417-416 from Hazleton Labs.,
Vienna, Virginia. USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA as Rohm and Haas Report No. 86RC-7.
Crippa, M., Misquiih, L., Lunati, A. & Pasollini, G. (1990).
Dyshidrotic eczema and sensitization to dithiocarbamates in a
florist. Contact Dermatitis, 23: 203.
DeCrescente, M.E. & Chan, P.K. (1982). Dithane M-45: rabbit eye
irritation report. Unpublished report Nos. 81R-197, 81R-176 and 82R-
10 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeCrescente, M.E. & Parsons, R.D. (1980). Dithane M-45: Acute oral
and dermal LD50 and skin and eye irritation. Unpublished report No.
79R-180, from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeGroot, A.P. (1974). Determination of acute ip toxicity of Dithane
M-45 in rats. Unpublished report from Central Institute for
Nutrition and Food Research TNO the Netherlands (Rohm and Haas
report No. 74RC-1011). Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
DiDonato, L.J., Cruzan, G. & O'Hara, G.P. (1985). Dithane M-45/ETU
four week range finding mouse study. Unpublished report No. 80R-123
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DiDonato, L.J. & Longacre, S.L. (1986). Mancozeb pharmacokinetic
study in rats. Unpublished report No. 85R-123 (Supplement/Appendix
to Nelson, S.S. [1986] report No. 31H-86-02) from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978). A study of the uptake and elimination of
14C activity after the oral ingestion of 14C-labelled
ethylenethiourea (ETU) and mancozeb in the rhesus monkey.
Unpublished final (revised) report from Battelle Labs, Columbus,
Ohio, USA. dated July 31, 1978. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA. as Rohm and Haas report
No. 78RC-1028.
Everett, D.J., Atkinson, C., Perry, C.J., Strutt, A., Millar, P.,
Hudson, P. (1992). Mancozeb 78-week dietary carcinogenicity study in
mice with 52 week interim kill. Unpublished report No. 7561 from
Inveresk Research International, Tranent, Scotland. Submitted to WHO
by Elf Atochem, Philadelphia, Pennsylvania, USA. (Confidential data
of Elf Atochem).
Foxall, S. & Byers, M.J. (1984). Dithane M-45 CHO/HGPRT gene
mutation assay. Unpublished report No. 84R-207 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Gallo, M.A., Kam, C. & Stevens, D.R. (1980). Teratologic evaluation
of dithane M-45 in the albino rat. Unpublished report No. 10065-009
from Booz, Allen, and Hamilton, Inc., Florham Park, New Jersey, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA as Rohm and Haas report No. 81RC-75.
Gautam, D.C. & Kapoor, L. (1991). Genotoxic effects of Dithane M-45
on the bone marrow cells of mice in vivo. Experimentia, 47(3):
280-2.
Georgian, L., Moraru, J., Draghicescu, T., Dinu, I. & Ghizela, G.
(1983). Cytogenetic effects of alachlor and mancozeb. Mutation Res.
116: 341-348.
Goldman, P., Bernacki, H.J. & Quinn, D.L. (1986). Mancozeb: three
month dietary toxicity study in rats. Unpublished report No. 85R-167
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1980). Acute inhalation toxicity of
dithane M-45 (TD-79-224) in rats. Unpublished report No. 79R-210
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1982). Dithane M-45: Acute inhalation
toxicology study in rats. Unpublished report No. 81R-171 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Hagan, J.V., Fisher, J.R. & Baldwin, R.C. (1986). Mancozeb:
subchronic inhalation toxicity study in rats. Unpublished report No.
86R-3 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1986). Mancozeb: two week range finding
inhalation toxicity study in rats. Unpublished report No. 85R-190
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Haines, L.D. (1980). Dithane M-45 percutaneous absorbtion in rats.
Unpublished technical report No. 34F-80-9 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 80R-1006.
Hooks, W.N., Offer, J.M., Hadley, J.C., Gibson, W.A., Gopinath, C. &
Dawe, I.S. (1992). Mancozeb technical: potential tumorigenic and
toxic effects in prolonged dietary administration to rats.
Unpublished report No. PWT 29/89669 from Huntingdon Research Centre
Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by Elf
Atochem, Philadelphia, Pennsylvania, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of Dithane M-45 in an
in vitro sister chromatid exchange assay in the Chinese hamster
ovary (CHO) cells. Unpublished reported dated March 1985 from Litton
Bionetics, Inc., Kensington, Maryland, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 84RC-60.
Kleibl, K. & Rakcova, M. (1980). Cutaneous allergic reactions to
dithiocarbamates. Contact Dermatitis, 6(5): 348-349.
Kocialski, A. (1989). Establishment of an in vitro metabolic
conversion factor of 7.5% of all EBDCs when converting EBDCs to
ethylenethiourea in vivo and recalculations of the previously
considered 20% in vivo conversion exposure factor for EBDCs to
ETU. Memorandum to the Caswell file, Health Effects Division, Office
of Pesticide Programs, United States Environmental Protection
Agency.
Kurinnyi, A., Pilinskaya, M., German, J. & L'vova, T. (1982).
Implementation of a program of cytogenetic study of pesticides:
preliminary evaluation of cytogenetic activity and potential
mutagenic hazard of 24 pesticides. Cytol. Genet. 16: 50-53.
Lee, Y.S., Cinn, Y.W., Chang, W.H. & Kim, J.S. (1981). A study on
hypersensitivity of Korean farmers to various agrochemicals.
Determination of concentration for patch tests of fruit-tree
agrochemicals and hypersensitivity of orange orchard farmers in Che-
Ju Do, Korea. The Seoul Journal of Medicine, 22: 137-142.
Longacre, S. (1986). Summary of ETU and EBDC analysis in plasma,
liver, and thyroid after mancozeb administration. Unpublished report
No. 86R-1009 from Rohm and Haas Company, (appendix/supplement to
Nelson 1986 31H-86-02) Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lu, M. & Kennedy, G. (1986). Teratogenic evaluation of mancozeb in
the rat following inhalation exposure. Toxicol. and Appl. Pharma.
84: 355-368.
Mann, S.K. (1977). Cytological and genetical effects of dithane
fungicides on Allium cepa. Environmental and Experimental Botany,
17: 7-12.
Martinez-Rossi & Azevedo, J. (1987). Detection of point mutation
mutagens in Aspergillus nidulans: comparison of methionine
suppressors and arginine resistance induction of fungicides.
Mutation Research, 176: 29-35.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb and their related compounds. Int. Arch. Occup. Environ.
Health, 37: 169-178
Matsushita, T. Yoshioka, M., Arimatsu, Y. & Nomura, S. (1977).
Experimental study on cross-contact allergy due to dithiocarbamate
fungicides. Indust. Health, 15: 87-94.
McGlynn-Kreft, A.M. & McCarthy, K.L., (1984). Dithane M-45 mammalian
cell transformation test. Unpublished report No. 84R-55 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
McLeod, P.L. & Doolittle, D.J. (1985). Dithane M-45 mammalian cell
transformation test for promotion. Unpublished report No. 84R-0297
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Muller, W. (1991). Oral (gavage) teratogenicity study in the rabbit.
Unpublished report No. HLD-853-683-002 from Hazleton Labs.,
Deutschland GmbH, Munster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Muller, W. (1992). 2-Generation oral reproduction toxicity study in
the rat. Unpublished report No. HD-852-683-001 from Hazleton Labs.,
Deutschland GmbH, Münster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Murty, K., Raju, D. & Sharma, C. (1983). Cytogenetic hazards from
agricultural chemicals. 7. Herbicides, fungicides and insecticides
screened for effects on chiasmata in Hordeum vulgare. Biol. Zb1.
102: 571-576.
Nelson, S.S. (1986). Metabolism of 14C mancozeb in rats.
Unpublished report No. 31H-86-02 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Nelson, S.S. (1987). Bioconversion of mancozeb to ETU in the rat.
Unpublished report No. 31C-87-24 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 80R-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neill, P. & Frank, J.P. (1988). Mancozeb: in vitro unscheduled
DNA synthesis assay in F/344 rat hepatocytes. Unpublished report No.
88R-079 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Piccirillo, V.J., Wu, D. & Speirs, G. (1992). Metabolism of mancozeb
in the mouse. Unpublished report No. T91-3413 from Inveresk Research
International, Ltd., Tranent, Scotland. Submitted to WHO by Elf
Atochem (Confidential data).
Restrepo, M., Munoz, N., Day, N.E., Parra, J.E., deRomero, L. &
Nguyen-Dinh, X. (1990). Prevalence of adverse reproductive outcomes
in a population occupationally exposed to pesticides in Columbia.
Scand. J. Work. Environ. Health, 16(4): 232-238.
Sames, J.L., McLeod, P.L. & Doolittle, D.J. (1984). Dithane M-45 in
vivo cytogenetic study in Fischer 344 rats. Unpublished report No.
84R-0246 Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Schweitzer, M. (1990). A study of the decomposition of mancozeb in
dimethyl sulfoxide solution. Unpublished report No. SC890012 from
Battelle Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. TR 34-90-01.
Shaw, D. (1990). Mancozeb: 52 week oral (dietary) toxicity study in
the beagle. Unpublished report No. HUK 5913-616/3 from Hazleton, UK,
Harrogate, North Yorkshire, England. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. 88RC-027.
Shellenberger, T. (1991) Mancozeb: 18-month dietary oncogenicity
study in mice. Unpublished report No. 85051 from Tegeris Labs.,
Inc., Temple Hills, Maryland, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA, as Rohm and Haas report
No. 86RC-029.
Solomon, H.M. & Lutz, M.F. (1987). Mancozeb: oral (gavage)
developmental toxicity study in rabbits. Unpublished report No. 86R-
021 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Solomon, H.M., Lutz, M.F. & Kulwich, B.A. (1988). Mancozeb: 2-
generation reproduction study in rats. Unpublished report No. 87R-
020 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Stadler, J. (1990). Combined chronic toxicity/oncogenicity 2-year
feeding study with mancozeb in rats. Unpublished report No. 259-89
from E.I. duPont de Nemours and Company, Haskell Laboratory for
Toxicology and Industrial Medicine, Newark, Delaware. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Stadler, J. (1991) Neuropathology study in rats with mancozeb.
Unpublished report No. 217-89 from E.I. duPont de Nemours and
Company, Haskell Laboratory for Toxicology and Industrial Medicine,
Newark, Delaware. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Szepvolgyi, J., Nagy, K., Sajgone-Vukan, K., Regoly-Merci, A., Soos,
K., Toth, K., Pinter, A. & Antal, M. (1989). Subacute toxicological
examination of Dithane M-45. Fd. Chem. Toxic, 27(8): 531-538.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Enticott, J., Wilby,
O.K., Tesh, S.A. (1988). Mancozeb: teratology study in the rat.
Unpublished report No. 87/PTC 007/365 from Life Science Research,
Suffolk, England. Submitted to WHO by Elf Atochem (Confidential data
of Elf Atochem).
Tomlinson, H.L. & Longacre, S.L. (1988). Mancozeb dermal absorbtion
study in male rats. Unpublished report No. 88R-218 from Rohm and
Haas, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm and
Haas, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988a). Mancozeb: delayed contact hypersensitivity
study in guinea-pigs. Unpublished report No. HLA 417-431 from
Hazleton Labs., Vienna, Virginia, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988b). Mancozeb: 4 week repeat dermal toxicity study
in rats. Unpublished report No. HLA 417-432 from Hazleton Labs.,
Vienna, Virginia, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Vasudev, V., Krishnamurthy, N.B., (1980). Non-mutagenicity of the
fungicide Dithane M-45 as inducer of recessive lethals after larval
feeding in Drosophila melanogaster. Mutation Res. 77: 189-191.
Watts, M.H. & Chan, P.K. (1984a). Dithane M-45: acute oral toxicity
study in rats and mice. Unpublished report No. 83R-213a + b from
Rohm and Haas Company, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Watts, M.H. & Chan, P.K. (1984b). Dithane M-45: acute oral toxicity
study in rats. Unpublished report No. 83R-218 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Peak concentrations of 14C in thyroid were reached within 6
and 24 hours in animals receiving the low and high doses,
respectively. Peak levels in thyroid were about 45 and 10 fold
higher than the corresponding peak levels in whole blood after low
and high-dose administration, respectively. 14C residue levels for
aforementioned tissues were comparable for males and females
receiving single dose administration except for adipose tissue and
gonads of females which were higher than those of males. Thyroid
tissue contained the highest residue levels for each group.
However, thyroid contained less than 0.10% of the dose 96 hours
post-dose. Tissue concentrations of 14C residues in rats receiving
the "pulse dose" were comparable to those in animals receiving only
the single dose of 14C-mancozeb at 1.5 mg/kg bw. The average 14C
residue levels per group remaining in the tissue at 96 hours post-
dosing ranged between 1.5-3.5% of the dose. ETU concentrations in
plasma and liver of rats 6 hours after dosing with 14C-mancozeb at
1.5 mg/kg bw were 6 and 12 times less than corresponding plasma and
liver 14C levels, respectively. ETU was not detected in the pooled
thyroids of these low-dose animals. Peak plasma levels of ETU were
reached 6 hours post-dosing in animals given 100 mg/kg bw mancozeb
and were 6-12 times less than corresponding plasma 14C levels for
both sexes. ETU was rapidly eliminated from the plasma of both male
and female rats (t´ 4.0-4.7 hours) and decreased below detectable
levels within 48-hours post-dosing. The estimated bioavailability
of ETU in rats was about 6.8 percent on a w/w basis and 20% on a
mole/mole basis. ETU concentrations in the liver of the high-dose
rats was 100 times less than liver 14C concentrations 6 hours post-
dosing. ETU was not detectable in liver 48 hours post-dosing. ETU
concentrations in thyroids were 80-100 and 30-225 times less than
corresponding thyroid 14C concentrations in males and females,
respectively, given 100 mg/kg bw mancozeb at 6 and 24 hours. ETU
was not detectable in plasma, liver, or thyroid of rats at 96 hours
after administration of a "pulse dose" (1.5 mg/kg bw of 14C-
mancozeb) preceded by a two-week dietary intake of unlabelled
technical mancozeb.
Concentrations of EBDC in the liver of rats 1, 6 and 24 hours
after dosing with 14C-mancozeb at 100 mg/kg bw were 0.25, 0.61, and
0.29 ppm for males, respectively, and 0.32, 0.35 and 0.25 ppm for
females, respectively. EBDC residues were not detectable 48 or 96
hours post-dosing. EBDC was not detected in the livers of rats
receiving the low dose (1.5 mg/kg bw) or multiple doses of mancozeb
96 hours post-dosing. Metabolite analysis revealed that mancozeb
was extensively metabolized and/or degraded. ETU was a major
metabolite found in urine, bile and faeces. EBDC was detected in
liver, faeces, and bile but not in thyroid. EBIS, ethyleneurea
(EU), N-acetyl-ethylenediamine (N-AcEDA) and ethylenediamine (EDA)
were also identified in urine, faeces and bile. Other metabolites
tentatively identified were glycine, N-acetylglycine, and N-
formylethylenediamine. Five other metabolites were also present but
were not identified (DiDonato & Longacre, 1986; Longacre, 1986;
Nelson, 1986, 1987; Kocialski, 1989).
Monkeys
Groups of male rhesus monkeys ( Macaca mulatta; 6/group) were
given single oral doses of either 14C-ETU; 14C-ETU plus manganous
sulfate and zinc sulfate or 14C-mancozeb (purity not stated) for
the determination of uptake into blood and the determination of the
major route of elimination of 14C. A sufficient dose was given to
produce 100 µCi of 14C activity per monkey. Whole blood, thyroid,
heart, lung, liver and kidney and faecal material were converted to
14C-carbon dioxide by combustion in a tissue oxidizer and analyzed
for 14C activity. Urine samples were analyzed directly. Urine and
faeces were collected separately.
14C-Labelled doses of ETU and ETU plus manganese and zinc
sulfates reached peak levels of 5% of the dose in total blood volume
at 8 hours. 14C-Mancozeb activity in the blood was less than 0.5%
at 8 hours and plateaued from 24-72 hours at slightly less than 1%
of the dose. This was in contrast to a relatively rapid decline in
14C activity in ETU-treated monkeys which at 72 hours showed 1% of
the administered dose in whole blood. ETU and ETU plus Mn:Zn-treated
monkeys showed similar rates of 14C excretion with nearly 50% of
the dose being cleared in 24 hours. The urinary clearance of 14C
activity by mancozeb-treated monkeys was slower (3.6% of the dose in
24 hours) as compared to ETU-treated groups. At 120 hours (last time
reading), the urinary route of elimination accounted for little more
than 10% of the activity in the mancozeb dose. Faecal elimination
showed less than 1% of 14C activity up to 24 hours in ETU-treated
groups. However, mancozeb-derived faecal activity ranged from 12.5-
64.0% of the dose at 144 hours, and from 0.005-12.7% at 24 hours.
14C activity was not detected in faecal samples from ETU-treated
monkeys after 24 hours. At 144 hours, blood samples were taken from
2 animals of the mancozeb-treated group, the animals sacrificed and
the following organs examined for 14C activity: thyroid, heart,
lung, liver and kidney. The remaining 4 animals were sacrificed 48
hours later (192 hours post-dosing). Comparative examination
between the groups (2 vs 4 monkeys) indicated that 14C activity
declined steadily between 144 and 192 hours in blood, whereas 14C
activity in the thyroids increased over the same 48-hour time period
(Emmerling, 1978).
Toxicological studies
Acute toxicity studies
Acute toxicity studies are summarized in Table 1. WHO has
classified mancozeb as unlikely to present acute hazard in normal
use (WHO, 1992).
Short-term toxicity studies
Mice
Charles River COBS-CD-1 mice (10/sex/group) received 0, 1, 10,
100, 1000 or 10 000 ppm of mancozeb (83% purity) adjusted to 100%
active ingredient for 4 weeks. No animals died on study and
clinical signs were absent. Females fed 10 000 ppm mancozeb showed
decreased body weights. Food consumption appeared to be comparable
for all groups on study. SGPT activity was comparable to controls
for all groups. Gross necropsy findings were not remarkable.
Thyroid weights (absolute and relative) were statistically
increased in both females at 10 000 ppm. Males appeared unaffected.
Liver weights were statistically significantly increased in males
and females given 10 000 ppm mancozeb. At 1000 ppm, absolute and
relative liver weights were significantly increased in females.
Females treated with 1000 ppm and 10 000 ppm showed thyroid
hyperplasia, congestion and decreased colloid density whereas males
showed similar effects at 10 000 ppm. No significant micropathology
was evident in the livers of animals given mancozeb (DiDonato et
al., 1985).
Mancozeb (83.1% pure) was administered to groups of Charles
River CD-1 mice (15 sex/dose) for 3 months at dietary concentrations
of 0, 10, 100, 1000, or 10 000 ppm of active ingredient. No
compound-related mortalities or clinical signs were observed.
Decreased body weights and food consumption were observed in both
sexes receiving 10 000 ppm. There were no treatment-related effects
with regard to haematology or clinical chemistry. Necropsy findings
were not remarkable. Aniline hydroxylase activity was decreased at
10 000 ppm in both sexes. Aminopyrine N-demethylase activity was
decreased in males at 1000 and 10 000 ppm. No effects were seen in
either sex at lower doses. Absolute and relative thyroid weights
were increased in both sexes receiving 10 000 ppm. Relative
increases in liver weights were seen in both sexes receiving 10 000
ppm. Absolute liver weight was increased in males administered 10
000 ppm mancozeb. Relative kidney weights were also increased in
both sexes at 10 000 ppm. Histopathologic evaluation of thyroid
revealed an increased incidence of follicular cell hyperplasia and
hypertrophy in both sexes at 1000 and 10 000 ppm. There was
Table 1. Acute toxicity of mancozeb
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse1 B6C3F1 M oral > 5000 --- Watts, 1984
Rat3 F344 M oral > 5000 --- Watts, 1984
Rat1 F344 M oral > 5000 --- Watts, 1984
Rat3 CRCD M oral > 5000 --- DeCrescente, 1980
Rat2 Wistar M/F i.p. 380 --- DeGroot, 1974
Rat 5,6 COBS-CR M/F inhalation --- 5.14 Hagan, 1982
(SD) BR 4 hour exposure
Rat 6,7 CR-SD M/F inhalation --- > 1.11 Hagan, 1980
4 hour exposure
Rabbit4 NZW M dermal > 5000 --- DeCrescente, 1980
1 Corn oil vehicle.
2 Carboxy-methyl-cellulose vehicle.
3 Aqueous dispersion.
4 Saline vehicle.
5 3/10 M and 3/10 F died during the whole body exposure/observation period (14 days). Multiple signs but no
tremors; body-weight loss and decreased weight gain. MMD 3.3 microns. Nominal concentration was
41.8 mg/litre (aerosol).
6 Whole body exposure.
7 MMD about 2.2 microns. Nominal concentration 4.46 mg/litre (dust).
increased vacuolation, interstitial congestion and decreased colloid
density in the thyroids in both sexes at 10 000 ppm. No liver
effects were observed in mancozeb-treated mice with the possible
exception of hepatocytic nuclear pleomorphism in females at 10 000
ppm. Increased deposits of (brownish) pigment were seen in the zona
reticularis of the adrenal cortex of female mice at 10 000 ppm. The
NOAEL was 100 ppm, equal to 18 mg/kg bw/day for males and 22 mg/kg
bw/day for females (O'Hara & DiDonato, 1985).
Rats
Four groups of 12 male and 12 female Crl:CD (SD)BR rats were
exposed to dust aerosols of mancozeb (84% pure) for 6 hours per day
for 10 days. The four groups were subdivided into whole-body
exposed rats and nose-only exposed rats of 5/sex/dose group. Daily
concentrations yielded total mean aerosol concentrations of 0, 23,
138 or 519 mg/m3. The mean respirable aerosol concentrations were
0, 11, 55 or 258 mg/m3 with mass median diameters of 3.5-4.9
microns.
Nose-only Exposure: There were no compound-related deaths.
No adverse clinical signs were observed in males or females exposed
to 11 or 55 mg/m3. Significant decreases in mean body weight and
mean body-weight gain were observed in the high-dose males but not
females. T3 and T4 levels were significantly reduced and the lung
to body-weight ratio increased in high-dose males but not in
females. Microscopic examination (limited to the respiratory tract)
of nasal turbinates revealed an increased incidence and degree of
multifocal mixed inflammatory cell infiltration (i.e. mononuclear
cells and neutrophils) and multifocal or focal necrosis of the
turbinate mucosa in four males and two females in the high dose.
Whole-body Exposure: There were no compound-related deaths.
No clinical signs were observed in animals receiving 11 mg/m3.
Significant reductions in male and female body weight and body-
weight gain were observed at 55 and 258 mg/m3. Male and female T4
levels and male T3 levels were significantly decreased at 55 and
258 mg/m3 of mancozeb after two weeks. TSH levels were not
significantly increased in both sexes at the high dose. Male and
female lung weights and lung to body-weight ratios were
significantly increased in both sexes at the high dose. Exposure-
related multifocal interstitial inflammation, microgranulomas,
multifocal mixed inflammatory cell infiltration, focal or multifocal
necrosis in the respiratory tract and reactive lymphoid hyperplasia
of the peribronchial lymph nodes were observed at the high dose
tested (Hagan & Baldwin, 1986).
Adult male and female Crl:CD(SD)BR rats were divided into two
nose-only exposure groups and one nose-only exposure-recovery group.
Animals received aerosol dust (84% pure mancozeb), 6 hours/day, 5
days week for 4 weeks at analytically determined mean concentrations
of 0, 22, 86 or 308 mg/m3 (equivalent to respirable concentrations
of 0, 8, 40 and 127 mg/m3) or for 13 weeks at 0, 18, 79 or 326
mg/m3 (equivalent to respirable concentrations of 0, 8, 36 or 144
mg/m3).
Results - 4 Weeks Exposure: There was no compound-related
mortality. Mean body weights and body-weight gains were decreased
for males in the high-dose group. No haematological effects were
observed in males. Data for females were inconclusive due to
insufficient amount of blood needed for the haematological
evaluations. There were no compound-related effects in either sex
for clinical chemistry, thyroid function, organ weights, or tissues
examined microscopically. Ophthalmological examinations revealed no
remarkable findings.
Results - 13 Weeks Exposure: There were no compound-related
deaths and no treatment-related signs of toxicity. Males exposed at
the high dose exhibited reduced mean body weights and body-weight
gains. Some haematological and clinical chemistry changes were
noted but were within the normal range of values and were therefore
not considered related to mancozeb exposure. T4 levels were
reduced 30% in high-dose females and considered treatment-related.
A dose-response was evident. Males showed a 9% decrease at the high
dose tested which was not statistically significant. Absolute
kidney and heart weights were reduced in males at the high dose
tested. However, organ to body weight and organ to brain weight
ratios were not statistically significantly different from controls.
Ophthalmological examination of the eyes revealed no compound-
related effects. No exposure-related histopathology was observed in
either sex at the low dose tested. Males (5/10; 8/11) and females
(10/11; 9/11) exposed to 79 or 326 mg/m3 exhibited a yellow-brown
granular pigment in the lumen of the cortical tubules of the kidney.
However, no attendant histopathological changes were reported. Mild
hyperplasia of the follicular epithelium in the thyroid glands of 3
females exposed at the high dose was observed. No exposure-related
thyroid lesions were observed at lower dose levels or in males.
Several histopathological lesions were observed in the respiratory
tract but were comparable to control incidences and not considered
treatment-related. Residue analysis of blood indicated
concentrations below the limit of detection for ETU and mancozeb for
males and females exposed to the low dose, with increasing
concentrations at higher exposures. Residue levels for ETU in the
thyroid were below the limit of detection at the low dose in both
sexes, with increasing concentrations at the higher doses. ETU and
mancozeb levels in urine were present at all doses in both sexes.
Those animals allowed to recover for 13 weeks after cessation of
exposure were comparable to a concurrent control group of non-
exposed animals for all parameters examined (Hagen et al., 1986).
Groups of Sprague-Dawley rats (Crl:CD(SD)BR); 10/sex/dose) were
administered 0, 10, 100, or 1000 mg of mancozeb (83% pure, not
adjusted for purity)/kg bw. The control group received distilled
water only. Test material was applied to the clipped dorsal area of
intact skin of each animal. An unsterile dressing sponge was placed
over the test site and held in place for 6 hours/day then removed.
All animals were treated similarly for 20 exposures over a 4-week
period. All animals were fitted with a cardboard collar to minimize
preening at the application site. The collar was only removed
during the 6 hours exposure period.
Clinical observations showed no evidence of compound-related
effects and there was no compound-related mortality. Body weight,
body-weight gain and food consumption in treated groups were
comparable to controls. Erythema was transient and slight, occurring
in not more than 2 animals/sex/dose for not longer than 2-4 days.
No other signs of dermal irritation were observed. Evaluation of
haematological and clinical chemistry parameters revealed no
statistically significant compound-related effects. Macroscopic
observations of treated skin revealed dark (yellow) area or
appearance in a dose-related increasing frequency at 100 and 1000
mg/kg bw in both sexes. Absolute and relative organ weights in
treated groups were not significantly different from control group.
Microscopic findings were limited to skin of treated and untreated
animals and characterized by increased keratin production
(hyperkeratosis) and thickening of the epidermis (acanthosis).
Severity varied from minimal to slight in all groups. These
findings were treatment-related but not compound-related. There
were no microscopic findings that were attributable to mancozeb.
Gross enlargement of the parotid salivary gland was within normal
histomorphological limits and found in all treatment groups
(Trutter, 1988b).
Groups of Crl:CD(SD) rats (14 rats/sex/group) received 0, 30,
60, 125, 250 or 1000 ppm of mancozeb (84% pure adjusted to 100%
active ingredient) for 13 weeks. Dose levels in the diet were
gradually increased with time in order to maintain approximately the
same level of compound intake throughout the feeding period.
There was no compound-related mortality or clinical signs. No
compound-related changes in body weights or food consumption were
recorded in animals fed up to and including 250 ppm mancozeb. At
1000 ppm, males showed a statistically significant decrease in body
weight from weeks 3 through 13, whereas females showed a 3-14%
decrease with statistically significant decreases only between 7 and
10 weeks. At 1000 ppm, food consumption was decreased in males for
weeks 3-13, but not in females. Males receiving 1000 ppm of
mancozeb showed statistically significant increases in BUN (84%),
creatinine (28%) and cholesterol (52%). Females receiving 1000 ppm
showed increased SAP (32%) and triglyceride (90%). However, group
increases were not considered compound-related as one male and one
female in the high-dose group were reported as having exceptionally
high values.
Clinical findings at lower mancozeb levels were not considered
treatment-related. Serum T4 levels were decreased in males and
females at 1000 ppm and TSH values increased. At 250 ppm, TSH
values were increased in males (36%) and females (50%). However,
only T4 values in females were statistically significantly
decreased at 250 ppm. MFO activity was decreased at 1000 ppm. No
mancozeb metabolite residues were detected in blood. Urine samples
of rats fed 125 to 1000 ppm of mancozeb contained 0.1 to 1.1 ppm of
parent compound. ETU metabolite of mancozeb increased in urine in a
dose-response manner from 0.3 to 10 ppm in animals given 30 to 1000
ppm mancozeb.
No mancozeb residues above the detection limit of 25 ppm were
detected in thyroids obtained from the 1000 ppm mancozeb fed rats.
ETU metabolite of mancozeb showed residues in thyroid which were
dose-related to mancozeb administration. Values ranged from less
than the detection limit of 4.0 ppm in 30 ppm mancozeb-fed animals
to 25 ppm in 1000 ppm animals. At 1000 ppm, absolute thyroid weight
was increased in males while relative weight was increased in males
and females. Relative liver weight was increased in males and
females receiving 1000 ppm. Relative spleen weight was increased
only in females receiving mancozeb at the highest dose. Compound-
related histopathology was generally confined to the liver, kidneys,
thyroid, adrenal and pituitary glands. Thyroid follicular cell
hyperplasia was seen in 90% of males and females receiving 1000 ppm;
a small, well defined basophilic focus of hyperplastic follicular
epithelial cells was seen in one male and there was also an
increased incidence and severity of hypertrophied vacuolated cells
in the pituitary of males. The kidneys of males and females
administered 125 to 1000 ppm had minimal to moderate amount of
yellow-brown pigment in the lumen of the cortical tubules. Pigment
deposits were attributed to ethylene bis(isothiocyanate) sulfide
(EBIS) a yellow coloured metabolite of mancozeb. Pigmentation
deposits were not accompanied by histopathological effects. There
was also an increased incidence of hypertrophy of cells of the zona
glomerulosa of the adrenal cortex in males given ETU and 1000 ppm
mancozeb. Hypertrophy of the centrilobular hepatocytes was seen in
males fed 1000 ppm mancozeb. The NOAEL was 125 ppm, equal to 7.4
mg/kg bw/day, based on increased serum TSH and decreased T4 values
at the next higher dose (Goldman et al., 1986).
Male H-Wistar rats (12/group) were given Dithane M-45 (80%
mancozeb) mixed in feed at doses of 0, 10,50, 75, 113, 169, 253 or
379 mg/kg bw/day for 12 weeks. One-third of the rats in the highest
dose group died within 6 weeks and showed signs of prostration,
weakness and posterior extremital paralysis. Signs were transient
in survivors and absent at 12 weeks. Body weight, body-weight gain,
total food intake and food efficiency were all depressed at 169
mg/kg bw/day and above. Effects were compound-related and
statistically significant. Blood sugar levels (after glucose
loading) and haematological parameters were comparable to control
values. Organ to body-weight ratios were increased for liver and
thyroid at 75 mg/kg bw/day and above and for kidneys, adrenals and
testes at 253 mg/kg bw/day and above. Histopathology of liver and
kidneys were comparable to controls. Thyroids, however, showed a
dose-dependent hyperplasia at 113 mg/kg bw/day and above. A 20%
decrease in the iodine content of the thyroids in rats given 10
mg/kg bw/day was not statistically significant. However at 50 mg/kg
bw/day a statistically significant decrease of 80% was recorded.
PBI was significantly decreased and serum cholinesterase
significantly increased at 169 mg/kg bw/day and above. ALP, acid
phosphatase and ASAT were statistically decreased in groups given
379, 253 or 169 mg/kg bw/day or above, respectively. Total protein
content of sera was statistically increased but still within
physiological limits. Liver triglycerides were statistically
increased at 113 mg/kg bw/day and above but serum triglyceride
levels were comparable to controls. Total serum cholesterol was
increased at 75 mg/kg bw/day and above but total liver cholesterol
was comparable to control values. Liver total lipid was similar to
controls. Aminopyrine demethylase and aniline hydroxylase activity
were both decreased at 253 mg/kg bw/day and above. The cytochrome
P-450 level remained unchanged (Szepvolgyi et al., 1989).
Dogs
Beagle dogs (6/sex/dose) received 0, 10, 100, 1000 or 5000 ppm
of 83.35% percent pure mancozeb in the diet adjusted to 100% of
active ingredient for 3 months.
Two males and one female were sacrificed in extremis in the
5000 ppm dose group as a result of compound-related anorexia and
malnutrition. Dose-related clinical signs of dehydration, thinness,
and pale mucous membranes were also noted in animals at this dose
level. Occasional instances of dehydration were seen in animals at
1000 ppm. Signs were considered secondary to anorexia and
malnutrition. Ophthalmoscopic examination did not reveal any
compound-related effects. Food consumption was decreased
approximately 10-20% at 1000 ppm and about 40% at 5000 ppm in both
sexes. Mean body weight and mean body-weight change were both
decreased at the high dose and at 1000 ppm. Decreases in
erythrocyte count, haemoglobin, and haematocrit were accompanied by
increases in mean corpuscular volume and mean corpuscular
haemoglobin. Values were statistically significant in both males
and females at 5000 ppm and females at 1000 ppm. T3 and T4 values
were decreased along with ALAT values at 5000 ppm in both sexes.
Total cholesterol was elevated in both sexes at 5000 ppm and in
females at 1000 ppm. Females at 5000 ppm also showed elevated total
bilirubin count at 5000 ppm.
At necropsy, enlarged and/or dark thyroid/parathyroids and
decreased thymus size were seen in the 1000 and 5000 ppm males and
females. A pale appearance of visceral organs was observed in the
three animals sacrificed early. Compound-related histomorphological
tissues alterations included thyroid follicular cell hyperplasia at
5000 ppm in both sexes, thymic cortical lymphoid depletion in the
1000 and 5000 ppm animals of both sexes, and hypoplastic changes in
the reproductive systems of males and females in the high-dose group
(i.e. aspermato-, hypospermato-, and hypogenesis of the testes and
hypogenesis of the ovaries), pallor of the zona fasciculata of the
adrenal gland in high-dose males and females and some sinusoidal
liver cell pigmentation in high-dose males and females. Urinalysis
indicated the presence of both parent compound and metabolite. ETU
was detected in the blood at a dose of 1000 ppm. No mancozeb was
detected in the thyroid, however ETU was detected in the thyroid of
both sexes at 100 ppm (in males 1.83 ppm and in females 0.72 ppm).
The NOAEL was 100 ppm, equal to 3.0 mg/kg bw/day, based on decreased
food consumption and body-weight gains and decreased erythrocyte
count, haematocrit, and haemoglobin at 1000 ppm (Cox, 1986).
Beagle dogs (4/sex/group) were given dietary concentrations of
0, 50, 200, 800 or 1600 ppm of mancozeb (84.5% pure) and adjusted to
100% active ingredient for 52 weeks.
Two high-dose males were sacrificed in extremis during weeks
10 and 11. One animal manifested haematuria and a hard distended
bladder prior to sacrifice. Necropsy revealed urethral calculi
lodged behind the os penis. Hydronephrosis with tubular dilation,
necrosis and congestion was noted in the kidneys. The lower urinary
tract manifested severe urethritis, prostatitis, cystitis and
ureteritis. Acute peritonitis was associated with these lesions.
The second male like the first showed a sharp drop in food
consumption and a decreased body-weight gain. A haematological
examination revealed the animal to be anaemic (decreased
haemoglobin, erythrocytes, packed cell volume and increased
reticulocytes). Haematological parameters, necropsy and
histopathological findings were consistent with a chronic
regenerative anaemia. Diffuse centrilobular necrosis with
extramedullary haemopoiesis was seen on histopathological
examination of the liver, while in the spleen and bone marrow
moderate to slight erythroid haemopoiesis with pigment was evident
and a notable reticulocytosis. All other animals survived, and
there were no apparent compound-related clinical signs or palpable
masses, nor any apparent neurological effects of treatment. Core
body temperatures were comparable between groups. With the
exception of the two animals that were terminated early there were
no consistent trends in food conversion efficiency or food
consumption. Body-weight gain for males fed 200 and 800 ppm
mancozeb showed statistically significant decreases, beginning at 24
and at 15 weeks, respectively. The two high-dose male survivors and
females showed body-weight gains comparable to controls.
Haemoglobin was significantly decreased at 800 and 1600 ppm in
females at 13 and 52 weeks along with packed cell volume at 13
weeks. Males manifested a statistically significant increase in
mean corpuscular volume at 1600 ppm at weeks 13, 26 and 52, as well
as at 800 pm at week 13. Serum cholesterol was significantly
increased in females at 1600 ppm at weeks 10, and 26. Serum
cholesterol values were dose-related in both sexes with cholesterol
values increasing at 200 ppm (7%-16% at weeks 10, 26 and 52) for
females and at 800 ppm for males.
T4 values were consistently lower than controls or pretest
values but were not statistically significant. T3 values were
comparable to controls. Urinalysis revealed no compound-related
effects. Absolute thyroid weight, and thyroid to body and brain
weight ratios were significantly increased in surviving males and
females at 1600 ppm. Absolute liver weight and liver to body-weight
ratio were increased in males but statistically significant only for
liver to brain weight ratio at 1600 ppm. Liver weight and liver
weight ratios were also increased in females but were not
statistically significant. The only apparent compound-related
effect observed was thyroid follicular distention observed in the 4
high-dose females and the two surviving high-dose males. The NOAEL
was 200 ppm, equal to 7.0 mg/kg of bw/day, based on decreases in
body-weight gain, increased cholesterol and decreased haemoglobin
and packed cell volume at 800 ppm (Shaw, 1990).
Beagle dogs (4/sex/group) were administered by gelatin capsule
0, 2.3, 23, or 113 mg/kg bw/day of mancozeb technical (88.6% pure).
The control group received gelatin capsule only. Compound was
administered once a day, seven days a week, for 52 weeks.
All animals receiving 113 mg/kg bw/day were sacrificed at 26
weeks with the exception of one male sacrificed at 13 weeks. At the
time of single sacrifice, food consumption was decreased 70% and
body weight depressed. Severe anaemia was evident. ALAT, ASAT,
urea, total bilirubin, total cholesterol were substantially
increased. T3 and T4 values were comparable to pre-test values.
Inorganic phosphorous was decreased 35%. Most organs were pale at
necropsy and histopathology was not conducted. Necropsy was not
conducted on animals terminated at 26 weeks. Faeces discoloration
(yellow/green) occurred in all groups but was most prevalent at the
high dose. Food consumption and body-weight gain were comparable
between control and low-dose groups (2.3 mg/kg bw/day) for both
sexes and for males at the mid- and high-doses. Females receiving
113 mg/kg bw/day manifested a 66% decrease in food consumption at 24
weeks and a 13% decrease at 52 weeks. Body-weight gain in females
at 52 weeks was 45% less than controls for animals receiving 23
mg/kg bw/day. At 24 weeks, haematology values for males were
comparable to controls at all doses. Females showed a frank anaemia
at 113 mg/kg bw/day and a dose-related decrease in RBCs. Blood
chemistry values for males showed dose-related trends for increased
ALP, total cholesterol, total plasma protein, phosphorous, and a
decreased trend for T4 with statistical significance attained at
113 mg/kg. Females showed decreasing trends for T3, T4, ASAT,
ALAT with an increasing trend in total cholesterol. ASAT and ALAT
values were statistically significant at 113 mg/kg bw/day. At 52
weeks, haematology, blood chemistry, urinalysis, organ weights and
histopathology were generally comparable to controls for both sexes
for animals receiving 2.3 and 23 mg/kg bw/day with the exception of
decreased T4 values in males. No dogs died at 2.3 and 23 mg/kg
bw/day. The NOAEL was 2.3 mg/kg bw/day, equal to 2.0 mg/kg bw/day
of active ingredient, based on decreased food consumption and
decreased body weight in females, and decreased T4 levels in males,
at 23 mg/kg bw/day (Broadmeadow, 1991a).
A second study in dogs of 52 weeks was conducted at a single
dose of 40 mg/kg bw/day as a result of the excessive toxicity and
termination of the high-dose experimental group (113 mg/kg bw/day)
in the preceding study. Protocols and methods were identical to the
first experiment. Clinical signs were limited in females. Two
females appeared thin looking during the course of the experiment
and one was reported as being hypothermic and of pale appearance.
No dogs died. Food consumption, body weight and body-weight gain
for males were comparable to controls for all time periods. Females
showed an immediate decrease in food consumption (20-26%) and a
statistically significant decrease in body weight and body-weight
gain throughout the experimental period. RBC count at 24 and 52
weeks for males and females was decreased (7-10%) but not
statistically significant. However, at 52 weeks MCV was
significantly increased in both sexes while MCHC was decreased
significantly in females. Other haematological parameters were
comparable to controls as were bone marrow findings. At 24 weeks ALP
and total cholesterol were substantially increased in males and
females whereas ALAT and ASAT were significantly decreased in
females. Inorganic phosphorous was significantly increased in
females. At 50 weeks, T3 and T4 values were statistically
decreased in both sexes while ALP levels were significantly
increased in females. ALAT values in females were significantly
decreased. Phosphorous levels were statistically raised in females.
Urinalysis showed a decrease in specific gravity for females
accompanied by increased urine volume. Absolute thyroid organ
weight was increased in both sexes and statistically significant in
males. Organ to body-weight ratios were comparable in males but
raised in females; however the values in females were suspect
because of severe loss of body weight. Necropsy revealed an
enlarged or swollen spleen in treated animals. Histopathology of
treated animals was generally not remarkable and comparable to
controls. However, an increased incidence of iron pigment
deposition in Kuffur cells of female livers and an increased
incidence of periacinar lipofuscinosis in males was reported in both
sexes (Broadmeadow, 1991b).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (60/sex/dose) were divided
into two experimental groups and given dietary concentrations of
mancozeb technical (88.6% pure) at 0 or 25 ppm, or 0, 100 or 1000
ppm for 78 weeks. Mancozeb was stable in the diet for at least 7
days and the diet was formulated weekly. Ten animals per sex per
dose were sacrificed at 52 weeks and all surviving animals
sacrificed at termination and necropsied. There was no compound-
related mortality or clinical signs. Body-weight gain in males was
decreased 6% at 52 weeks and 8% at 79 weeks at 1000 ppm. Values
were not, however, statistically significant. Body weight in
females at 100 ppm was decreased from weeks 1-24 and at 1000 ppm
from weeks 1-78. However, body-weight decreases in females at 100
ppm was not compound-related since there was no dose-response from
100 ppm to 1000 ppm. Body-weight gain in females was decreased 10%
at 52 weeks and 14% at 78 weeks at 1000 ppm. Body-weight gain
values at 52 and 78 weeks were not statistically decreased. Food
consumption and differential blood counts were comparable between
treated and control groups. There were no remarkable intergroup
differences for organ weights. The incidence of liver nodules in
males at 0, 100, and 1000 ppm were 4/50, 3/50 and 10/50,
respectively. The incidence of liver masses at 0, 100 and 1000 ppm
in males were 5/50, 5/50 and 9/50, respectively. Liver nodules and
liver masses were not significantly different from control values.
There were no intergroup differences in the incidence of liver
nodules or liver masses in females. There were no macroscopic or
microscopic findings that could be attributed to compound
administration. The incidence of male mice bearing liver tumours at
doses of 0, 100 and 1000 ppm was 10/50, 7/50 and 17/50,
respectively. There were no statistically significant differences or
trends. The number of male animals showing benign tumours was 8/50,
5/50 and 17/50, respectively, and the number showing hepatocellular
carcinomas was 2/50, 2/50 and 0/50. Males receiving doses of 0 or
25 ppm manifested malignant liver tumours in 3/50 and 4/50 animals,
respectively, with the total number of benign and malignant tumours
being 7/50 and 7/50 for both experimental groups. The NOAEL was 100
ppm, equal to 17 mg/kg bw/day, based on decreased body-weight gain
at 1000 ppm. There was no evidence of carcinogenicity (Everett et
al., 1992).
Groups of Charles River CD-1 mice (94/sex/group) received 0,
30, 100, or 1000 ppm of mancozeb (83% pure adjusted to 100% active
ingredient) for 78 weeks. Twenty-four animals/sex/group were
selected for an interim sacrifice at 12 months. Haematology was
conducted on 15 mice/sex/dose at 12 and 18 months. Thyroid function
assays were conducted on a minimum of eight animals/sex/dose after
12 and 18 months, with evaluations conducted on T4, T3 and TSH.
Necropsies were performed on all unscheduled deaths, on 24
animals/sex/group at interim (12-month sacrifice) and on all
surviving animals at terminal sacrifice.
There were no treatment-related increases in mortality or
clinical signs. Food consumption was similar among all groups. Body
weight for males and females of the 1000 ppm group were
statistically and consistently lower than controls through out the
78 weeks. Body-weight gains at 52 and 78 weeks for males and
females were decreased 18% and 13% and 15% and 9%, respectively.
Body weights and body-weight gains in other dose groups were similar
to controls values.
RBC counts were statistically significantly decreased in males
at 12 months at 1000 ppm. All other values for males were
comparable to controls. Females at 12 months and 1000 ppm showed
decreased RBC count, increased MCHC, and increased MCV. T3 and T4
changes were statistically significant only at the high dose tested
(1000 ppm) in both sexes. T3 and T4 levels were decreased in
females at 18 months (32% and 61% respectively). T4 levels in
females were also decreased at 12 months (76%). Males had a 56%
decrease at 12 months in T4 values and a 45% increase in T3 values
at 18 months. TSH levels were not statistically significantly
increased in either sex at any dose or time period.
Necropsy and gross examination of animals at the 12-month
interim and 18-month terminal necropsies revealed no consistent
gross lesions that could be attributed to an effect of the test
chemical. Diffuse discoloration of the lymph nodes was observed in
high-dose males (6/66) but the finding was not confirmed under
histopathological examination. There were no consistent differences
in mean organ weights, mean organ to body weight or organ to brain
weight ratios between control and compound-treated males or females
that could be attributed to the test article. Non-neoplastic
findings were comparable to controls. Hepatocellular adenomas and
carcinomas as well as pulmonary alveolar/bronchiolar adenomas and
carcinomas observed in both sexes were not statistically
significant. The NOAEL was 100 ppm, equal to 13 mg/kg bw/day in
males and 18 mg/kg bw/day in females, based on decreased body weight
and body-weight gain and decreased T3 and T4 values at 1000 ppm.
There was no evidence of carcinogenicity (Schellenberger, 1991).
Rats
Groups of Charles River Crl:CDBR rats (72/sex/dose) received 0,
20, 60, 125 or 750 ppm of mancozeb technical (83.8% pure) in the
diet for 2 years.
Mean body-weight gains in the high-dose males were
significantly lower than the control group in the first year
followed by lower but not statistically significant differences
during the second year (8.0%). Females receiving the high dose
showed a statistically significant decrease in body-weight gain at
90 days (16%) and one year (11%). Body-weight gains were comparable
to controls from 1-2 years. Food consumption was comparable for all
groups. Food efficiency was slightly but not statistically
decreased in high-dose males (5.5-7.5%) for various time intervals
and females at 90 days (10%). Erythrocytes, haemoglobin and
haematocrit were significantly decreased in male animals receiving
125 ppm at 18 months but not at other time periods. Haematological
findings were unremarkable in females. ALAT in males and
cholesterol levels in females were significantly increased at 750
ppm at 24 months. Males revealed decreased T3 values at 125 and
750 ppm at three months with decreased T4 values at 750 ppm at 6
and 18 months. TSH values were increased at 750 ppm at 12 and 18
months and at 125 ppm at 24 months. Females showed decreased T3
levels at 60, 125 and 750 ppm at 3 months. T4 values were
decreased at 750 ppm at 3, 18 and 24 months. TSH values were
increased at 750 ppm at 6 and 18 months. Urinalysis values were not
remarkable. Survival was comparable for all groups. After 24
months, absolute and relative (to body weight) thyroid/parathyroid
weights of the high-dose males and females were significantly
increased. The incidence of enlarged thyroids was also higher in
males and females at 750 ppm and the incidence of observed masses
was also higher in males. Follicular cell hypertrophy/hyperplasia of
the thyroid gland was significantly increased for males and females
receiving 750 ppm at the end of the first year.
A granular yellow-brown pigment was also present in rats fed
125 and 750 ppm at one and two years. Renal pigment remained in the
same magnitude as seen at 12 months. No attendant pathology was
evident. Bilateral retinopathy was also significant in both sexes
at the high dose at 2 years. At 2 years, in the high-dose group of
males and females, significant increases were recorded for thyroid
follicular cell hypertrophy/hyperplasia as well as nodular
hyperplasia. Thyroid follicular cell adenomas (20/61) and
carcinomas (14/61) were significant only in high-dose males.
Females showed increased incidences of thyroid follicular cell
adenomas (6/61) and carcinomas (4/61) but the increases were not
statistically significant when compared to control groups. The NOAEL
was 125 ppm, equal to 4.8 mg/kg bw/day, based on decreased body-
weight gain, decreased T3, T4 values, increased TSH values,
increased absolute and relative thyroid weight, thyroid follicular
cell hypertrophy, hyperplasia, and nodular hyperplasia, in both
sexes at 750 ppm. Carcinogenic effects were noted in both sexes in
the form of thyroid follicular cell adenomas and/or carcinomas but
only at the highest dose level (Stadler, 1990).
Sprague-Dawley (CD) rats (50 [main study] and 20 [satellite
study] animals/sex/dose) were given 0, 25, 100, or 400 ppm of
technical mancozeb in the diet (88.5% purity, adjusted to 100%
active ingredient) for 104 weeks. There were no compound-related
signs or mortality. Body-weight gain was significantly decreased in
males and females at the high dose tested for weeks 1-26 and 1-13,
respectively. Body weights were significantly decreased for males
from weeks 2-75 and for females from weeks 4-25. Food consumption
was comparable between all groups and food conversion ratios similar
for treated and control groups. There were no treatment-related
effects on haematological parameters. Reported changes were neither
dose nor time-dependent. T4 levels were significantly decreased in
males and females at 400 ppm at 26 and 52 weeks and in females alone
at 78 weeks at 100 and 400 ppm. However, the decrease at 100 ppm
was not consistent over time. T3 levels in males were decreased at
52 weeks but increased 43% at 104 weeks in the high-dose group. TSH
was increased by 86% at 78 weeks in females of the high-dose group.
The remaining statistical differences, particularly for
protein, calcium and other electrolytes, appeared to be unrelated to
treatment. Urinalysis findings were not considered to be compound-
related. Ophthalmoscopic changes were comparable between groups.
There were no compound-related organ weight changes. There was no
evidence of tumorigenicity. The incidence of thyroid follicular cell
adenomas in males was 6/50, 2/50, 2/50 and 6/50, for thyroid
follicular cell adenocarcinomas the frequency was 2/50, 0/50, 1/50,
and 3/50 for control and respective dose groups. Parafollicular
carcinomas in males was 1/50, 6/50,6/50 and 6/50. Anterior
pituitary adenomas in males occurred at a frequency of 18/50, 22/50,
28/50, and 27/50 whereas anterior pituitary adenocarcinomas were
observed at an incidence of 3/50, 2/50, 2/50 and 1/50 in controls
and respective dose groups. A statistical significance was not
achieved between groups, and values were comparable with the upper
incidence of the control range. Thyroid follicular cell adenomas and
adenocarcinomas were observed in females at frequencies of 0/50,
0/50, 2/50 and 2/50; and 0/50, 0/50, 0/50 and 1/50, respectively,
for controls and treated groups. Parafollicular malignant tumours
occurred at the same frequency (3/50) in all groups. Pituitary
adenomas in females (anterior pituitary) ranged from 28/50 to 35/50.
Pituitary adenocarcinomas ranged from 4/50 to 7/50. Non-neoplastic
findings in males were not significant for pituitary, testes,
kidneys, or other tissues with the exception of thyroid. There was a
minimal increase in the height of the follicular epithelium of the
thyroid (3/50, 1/50, 1/50, 8/50) and an increase in the number of
prominent microfollicles (0/50, 1/50, 0/50, 5/50) at the high dose.
Non-neoplastic findings for females were not significant for kidney,
pituitary or stomach. There was however a minimal increase in the
height of the follicular epithelium of the thyroid (1/50, 0/50,
1/50, 5/50) at 400 ppm. The NOAEL was 113 ppm, equal to 4.0 mg/kg
bw/day in males and 5.1 mg/kg bw/day in females, based on decreased
body-weight gain and body weight, an increase in the height of the
thyroid follicular epithelium, an increase in prominent
microfollicles, and a decrease in thyroxine levels at 450 ppm (Hooks
et al., 1992).
Reproduction studies
Rats
Sprague-Dawley Crl:CD(SD)BR rats (25/sex/dose) received 0, 25,
150, or 1100 ppm of 88.4% mancozeb technical in the diet. Compound
intake was not corrected to 100% of active ingredient in the diet.
Animals (F0 generation) were seven weeks old at the start of
treatment, acclimated to laboratory conditions and healthy. After a
premating period of 14 weeks, animals of the same dose levels were
mated for not longer than three weeks. Females were allowed to
litter and rear their offspring. Exposure to compound and diet was
continuous and ad libitum. Selected F1a pups were maintained for
14 weeks after weaning of all F1a offspring then mated for three
weeks, allowed to litter and rear their offspring (F2a generation).
There were no compound-related deaths and clinical signs were not
evident for either generation. Body weight and body-weight gain were
significantly depressed in males and females of the high-dose group
for both parental (F0, F1) generations during the 14-week pre-
treatment period. Males of the F1 generation also showed
significant decreases in body weight and body-weight gain at the
mid-dose. Body weight and body-weight gain for dams during
gestation of F0, and F1 parents, were significantly decreased at
1100 ppm and depressed at the mid-dose. Food consumption was
significantly decreased for both sexes during the premating period
of both generations at the high dose. Food consumption for F1
females was also significantly decreased at the high dose during the
periods of gestation and lactation.
Absolute organ weight at necropsy revealed statistically
significant increases for both parental sexes at the high dose.
Macroscopic examination of all tissues examined revealed no
remarkable findings. Histopathology, however, revealed thyroid
hyperplasia and hypertrophy in nearly all animals examined of F0
and F1 parents at the high dose. Thyroid follicular cell adenomas
were also found in five males of the F0 generation and in eleven
males of the F1 generation at the high dose. Indices for F1a pups
were comparable to controls. A slight delay in the opening of the
eye was however considered to be treatment-related at the high dose.
Necropsy findings were comparable between treated and control groups
for F1a and F2a litters. Viability, pups and litter weights were,
however, significantly decreased at the high dose on days 14-21.
The NOAEL in this study was 25 ppm, equal to 1.7 mg/kg bw/day, based
on decreased body weight at 150 ppm (Muller, 1992).
Charles River CRL:CDBR rats (25/sex/dose) received 0, 30, 120,
or 1200 ppm of mancozeb (84% pure) in the diet. Animals were placed
together for a period of 10 days to produce the F1a and F1b
generation. The presence of a vaginal plug was considered day zero
of gestation. On day 4, litters were culled to 5 animals/sex/dose.
Litters were weaned on day 21. One male and one female were then
selected from the F1a litter to serve as the parents for the F2
generation (F2a and F2b litters).
There were no treatment-related clinical signs or deaths in
either the F1 or F2 generation. Parental body weights for F1 and
F2 male and female rats were similar between control, 30 and 120
ppm groups throughout the 10-week treatment period prior to mating
or for F1 and F2 females during the gestation and lactation
periods. At 1200 ppm, mean body weight for male and female rats of
the F1 and F2 generation were significantly below control
throughout the pre-mating period, as well as for parental females
during gestation and lactation. Mean feed consumption of F1 and
F2 male and female rats were comparable between control, 30 and 120
ppm groups. At 1200 ppm, mean food consumption was decreased in F1
but not F2 male and female rats prior to mating. During gestation
and lactation F1 and F2 females had food consumption values
comparable to controls. Reproductive indices as measured by
fertility, gestation, viability and lactation were comparable to
controls for F1 and F2 adult and offspring at all dose levels.
Fetal body weight for F1a,b and F2a,b offspring were also comparable
to controls. There were no treatment-related effects on the
absolute and relative organ weights at 30 ppm for the F1 and F2
parents or for F1 parents at 120 ppm. F2 males showed a
statistically significant dose-related increase in relative liver
weight at 120 ppm without histopathological changes. At 1200 ppm
both F1 and F2 parents manifested significant increases in
relative liver weight and absolute and relative thyroid weights.
Relative kidney weight was increased in F1 and F2 females, but not
in males. There were no gross pathological changes considered to be
treatment-related among either F1 or F2 animals nor any which
could be substantiated by corresponding histopathology.
Treatment-related microscopic changes were observed in the
thyroid, kidney and pituitary of F1 and F2 animals. Changes
involving the follicular cells of the thyroid observed in the F1
generation were also observed in the F2 generation with generally
higher incidences or greater severity. All males of the F1 and F2
generation showed some degree of diffuse follicular cell hyperplasia
at 1200 ppm. Follicular cell adenomas and nodular/cystic follicular
cell hyperplasia were also observed at 1200 ppm. All or nearly all
females showed some degree of diffuse follicular cell hyperplasia at
1200 ppm in F1 and F2 generations. Nodular/cystic follicular cell
hyperplasia was also evident at 1200 ppm in the F2 generation.
Follicular cell adenomas were not observed in females. Brown
globular pigment was observed within the lumen of proximal tubules
in the kidneys at statistically significant incidences in both sexes
of both generations at 120 and 1200 ppm. However, no associated
pathology was observed. Hypertrophy and/or vacuolation of the
anterior pituitary was treatment-related only in males and only at
1200 ppm of the F1 and F2 generation based upon an increased
severity of response and not an increased incidence. Other
microscopic changes were not considered to be treatment-related. The
NOAEL was 120 ppm, equal to 7.0 mg/kg bw/day, based on increased
relative weights of the liver, kidney and thyroid, increased
absolute thyroid weight, decreased body weight and feed consumption
of females during gestation and lactation, and decreased pre-mating
body weight and feed consumption, at 1200 ppm (Solomon et al.,
1988).
Special study on neuropathology
Rats
Groups of Charles River Crl:CD(R) BR rats (10
animals/sex/dose) received 0, 20, 125, 750 or 5000 ppm of mancozeb
(79.3% pure and adjusted for purity) mixed in the diet for 90 days.
Two female satellite groups of 16 females each were also included
and received 5000 ppm for a two-week period only prior to sacrifice.
One male and 4 females died in the 5000 ppm group. These deaths
occurred between the second and fourth week of administration and
resulted in a cessation of test compound in females of the high dose
only and administration of control feed for the remainder of the
study. Onset of clinical signs in the second and third week of
administration consisted of generalized weakness, abnormal gait or
mobility, limited or no use of the rear legs. Loss of muscle mass
was also reported in males. However by day 60 some males in the
high-dose group appeared clinically normal and females showed
improvement after one week on control diets. Females of the
satellite group fed mancozeb for two weeks manifested limited rear
limb mobility. No clinical signs were observed below 5000 ppm.
Body weight in males was decreased 45% by day 90 in the high-
dose group. Other dose levels in males were comparable to controls.
Females receiving 5000 ppm showed initial weight loss which was
reversed after cessation of test compound and their placement on
control feed. Satellite females administered 5000 ppm of mancozeb
for two weeks showed minimal weight changes at 14 days. However, at
750 ppm and 90 days biologically significant decreases in body
weight (9%) and body-weight gain (17%) were noted. Body weights
were unaffected at lower doses. Food consumption in high-dose males
was decreased 40% compared to controls, but was similar to controls
at other dose levels. Females receiving 5000 ppm for two weeks had
decreased food consumption values ranging between 29% and 64%. In
other groups, female food consumption was similar to controls. Food
efficiency was decreased in all high-dose animals and at 750 ppm in
females (13%).
Neuropathology revealed effects in both sexes at 750 ppm and
5000 ppm. Pathology in males at 750 ppm and 5000 ppm was reported
as myelin phagocytosis, Schwann cell proliferation, myelin bubbles,
demyelination of nerve fibres associated with the posterior thigh
muscle and myelin ovoids in teased nerve fibres. Additional
observations at 5000 ppm consisted of intra-sheaths ellipsoids,
demyelination, thickening of the myelin sheath, neurofibrillary
degeneration and atrophy of the posterior thigh muscles. High-dose
females fed a recovery diet showed a thickening of the myelin
sheath, myelin bubbles, and ballooning of the myelin sheath.
Muscular atrophy was reported in one surviving female. There was
also an increased incidence of demyelination but a decrease in the
presence of myelin ovoids and debris when compared to males at this
dose level. Females fed 5000 ppm for two weeks and then sacrificed
showed myelin bubbles, sheath thickening, myelin phagocytosis and
Schwann cell proliferation. Muscle atrophy was present in 9/10
animals examined and demyelinated lengths with the presence of
myelin debris and ovoids were found upon examination of teased sural
nerve fibres. At 750 ppm in females, teased nerve fibres showed
some demyelination myelin ovoids and debris. No neuropathology was
conducted at 20 ppm dose level because of the absence of findings at
125 ppm. The NOAEL was 125 ppm, equal to 8.2 mg/kg bw/day in males
and 10.5 mg/kg bw/day in females, based on decreased body weight,
body-weight gain and food consumption in females and
neurohistopathological changes in both sexes at 750 ppm (Stadler,
1991).
Special studies on embryotoxicity/teratogenicity
Rats
Groups of mated female Crl:CD rats (27/group) were whole-body
exposed to 80% pure mancozeb dust by the inhalation route.
Administered doses were 0, 1, 17, or 55 mg/m3 for 6 hours/day on
days 6-15 of gestation. Particle size ranged from 1.4-6.4 microns.
Animals were observed for clinical signs during and after exposure
then sacrificed one day prior to natural delivery. Fetuses were
examined externally, viscerally and skeletally. No animals died.
Hind limb weakness and a slower righting reflex were observed in
6/27 high-dose dams. However, the signs disappeared during the
post-exposure observation period. High-dose animals also gained
significantly less weight than controls (40% less). There were no
differences between treated and control group as to malformations
observed. There was however an increased incidence in the number of
animals manifesting a wavy rib which was statistically significant
at the high dose. This variation was dose-related across treated
groups. There was no increase in soft tissue alterations.
Observations were typical of those commonly seen in this strain of
rat. Fetal body weights were comparable between groups (Lu &
Kennedy, 1986).
Mancozeb (83.0% active ingredient adjusted to 100% for dosing)
was administered by gavage in corn oil to groups of 26 primigravid
BLU(SD)BR rats on days 6-15 of gestation (day 0, day of
insemination) at doses of 0, 2, 8, 32, 128 or 512 mg/kg bw/day. ETU
was administered as a positive control at 50 mg/kg bw/day. All rats
were sacrificed on day 20 of gestation and caesarean sections
performed.
Food consumption and body weights were decreased at 128 mg/kg
bw/day and above in mancozeb treated dams. Body weights for all
other mancozeb-treated groups were comparable to the control group
as was the ETU-treated group. Litter data indicated significant
decreases in the average number of live fetuses, mean fetal weight
and mean gravid uteri and increased resorptions only at 512 mg/kg
bw/day for mancozeb-treated animals. Fetal weight was decreased in
ETU-treated animals, and one dam died on study. Adverse compound-
related effects were clearly manifested for mancozeb at 512 mg/kg
bw/day and ETU at 50 mg/kg bw/day for gross abnormalities (agnathia,
cleft palate, meningoencephalocele), soft tissue effects (dilated
ventricles, compressed spinal cord), and skeletal tissue (incomplete
ossification of the skull, clavicle, scapula). The effects observed
at 128 mg/kg bw/day were not biologically or statistically
significant. The NOAEL for maternal toxicity was 32 mg/kg bw/day
based on decreased body weight and decreased food consumption at 128
mg/kg bw/day. The NOAEL for teratogenic effects was 128 mg/kg bw/day
based, in part, on the presence of agnathia, cleft palate,
meningoencephalocele, dilated ventricles and incomplete ossification
of the skull at 512 mg/kg bw/day (Gallo et al., 1980).
Female Sprague-Dawley rats (25/dose/group) received 0, 10, 60,
or 360 mg/kg bw/day of mancozeb (88.6% pure) in methyl cellulose or
methyl cellulose alone (control group). Animals were dosed daily by
the oral route (gavage) from day 6 to day 15 (inclusive) of
gestation. Dams were observed on predetermined regular schedules
for clinical signs, mortality body weight, and food consumption. On
day 20 of gestation females were sacrificed with carbon dioxide and
uterine contents examined.
There were no readily apparent or statistically significant
differences between control values and values obtained in treated
groups for the parameters examined at either 10 or 60 mg/kg bw/day.
One dam was sacrificed in a moribund state at 360 mg/kg bw/day. Four
females exhibited a "reeling gait" followed by slight paralysis in
three of the dams. Body weights were decreased during the treatment
period (days 6-15) and days 16 through 20. Statistical significance
was attained on days 16 and 20. Food consumption was significantly
decreased from days 6-15 inclusive. A slight dose-related reduction
in the degree of ossification of the intraparietal bone was
statistically significant. The Incidence for this effect was 75, 80,
87 and 90% for control to high dose. The historical mean was 25% and
the range 4.90-91.0%. A marginal increase in the size of the
anterior fontanelle (12%) was also observed. The historical mean for
this effect was 2.6% with a range of 0-24%. Incomplete ossification
of the thoracic vertebrae centra was also statistically significant
at the high dose tested. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 60 mg/kg bw/day. Maternal toxicity at 360
mg/kg bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryo/fetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra (Tesh et al., 1988).
Rabbits
Groups of New Zeeland white rabbits (18 females/dose) were
treated by oral intubation with doses of 0, 5, 30 or 55 mg/kg bw/day
of Pencozeb Technical (88.4% pure) in methyl cellulose or methyl
cellulose, on days 6 through 18 of gestation. Animals were
sacrificed on day 28 post-coitum. There were no compound-related
effects at these dose levels either in-life or during examination
post-sacrifice. A second experiment was therefore conducted at a
single high dose of 100 mg/kg bw/day with a concurrent control group
following the general procedures and examinations of the prior
experiment. No treatment-related clinical effects or treatment-
related necropsy findings were detected. Two dams in the control
group and five in the 100 mg/kg bw/day dose group aborted. A clear
and substantial body-weight loss was observed in does at 100 mg/kg
bw/day for days 6-9 post-coitum (i.e. days 1-3 of compound
administration). Body-weight gain during days 9-15 was also
substantially depressed compared to controls. Food consumption was
also markedly decreased between days 6-19. A slight post-
implantation loss (5%) was also observed and may have been compound-
related. No compound-related fetal effects were observed. The NOAEL
for maternal effects was 55 mg/kg bw/day and greater than 100 mg/kg
bw/day for embryo/fetotoxic effects. An increase in abortion, body-
weight loss, suppressed body-weight gain and decreased food
consumption were observed at 100 mg/kg bw/day (Muller, 1991).
New Zeeland white female rabbits (20 animals/dose/group)
received either 0, 10, 30, or 80 mg/kg of bw/day of mancozeb (83.0%
pure) in methyl cellulose or methyl cellulose alone (control group)
by oral gavage on days 7-19 of gestation. No treatment-related
deaths occurred in control or in the 10 and 30 mg/kg bw/day dose
groups. One animal died in the 30 mg/kg bw/day group but death was
attributed to mis-dosing. Two does were sacrificed in a moribund
condition at the high dose and the deaths considered treatment-
related (one of these two does was pregnant and did not abort).
Clinical signs were comparable between controls and animals
receiving 10 and 30 mg/kg bw/day. Animals receiving 80 mg/kg bw/day
manifested significant increases in clinical signs. Alopecia,
anorexia, ataxia, scant faeces, and abortions were all observed at
the high dose tested. Body-weight gain and food consumption were not
significantly different between controls and groups receiving 10 or
30 mg/kg bw/day. At 80 mg/kg bw/day body-weight and food consumption
were significantly decreased in does that aborted and those
sacrificed moribund. Does producing at least one viable fetus had
body-weight gains and food consumption values similar to controls.
No treatment-related changes were evident in the incidence of
gross postmortem findings between does in the control and treated
group. Reproductive parameters between controls and does receiving
10 or 30 mg/kg bw/day were comparable as measured by the number of
abortions, litters produced, and the mean number per litter of
corpora lutea, implantations, resorptions, dead and live fetuses or
sex ratio. At 80 mg/kg bw/day a significant increase in does
aborting (5/15) was reported with a corresponding decrease in the
number of litters produced. All other parameters were comparable to
control group. Mean fetal body weight was similar between the
control and treated groups. There were no significant increases in
the types or incidence of malformations or developmental variations
between control or treated groups. The NOAEL for maternal toxicity
was 30 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was greater
than 80 mg/kg bw/day. Maternal toxicity at 80 mg/kg bw/day was
based on an increase in aborted fetuses, decreased number of litters
produced, decreased body-weight gain and food consumption, an
increase in clinical signs and death (Solomon & Lutz, 1987).
Special study on mancozeb stability in DMSO
14C-mancozeb (Dithane M-45; 80% wettable powder; 88% radio
purity) was suspended in DMSO (99% pure) at a nominal concentration
of 100 ppm and sampled and analyzed for mancozeb at the following
times: 0, 15, 30, 60, 90, 120, 150, 180 and 210 minutes. Maximum
concentration of mancozeb occurred at approximately 15 minutes.
Immediately after adding DMSO to mancozeb (zero time) there was
evidence of significant decomposition with continued rapid
degradation to three compounds, two of which were impurities in the
starting material. The majority of the mancozeb was degraded within
60 minutes and after 4 hours an average of 9% of the mancozeb
remained. The half-life of mancozeb in DMSO was calculated to be 37
minutes (Schweitzer, 1990).
Special studies on genotoxicity
The results of genotoxicity assays are given in Table 2.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
Special studies on irritation and sensitization
Guinea-pigs
Mancozeb has been demonstrated to be a strong sensitizer in the
Hartley strain female guinea-pig by the Guinea-Pig Maximization
Test. With induction concentrations of 5% (intradermal) and 25%
(topical) and challenge concentrations of 2% and 0.5% (topical) all
of the mancozeb-treated females (10/group) responded at both 24 and
48 hours. In the same studies, cross sensitization responses were
also seen with zineb, maneb and mancozeb. A dose-response
relationship between the incidence of skin sensitization and
induction and challenge concentration was also seen with maneb. The
purity was not stated for any of the test materials (Matsushita et
al., 1976, 1977).
Young adult Hartley guinea-pigs were divided into three
experimental groups and tested for delayed contact hypersensitivity
using a modified Buehler procedure. Animals received three 6-hour
induction doses (1 dose/week, within 3 weeks) of either 0.4 ml of
50% (w/v) mancozeb (83% pure) in distilled water, 0.1% (w/v) 1-
chloro-2,4-dinitrobenzene (DNCB) in 80% (v/v) aqueous ethanol
(positive control), or received no induction doses (control group)
but were otherwise treated similarly. All groups were challenged 12
days after the last induction dose/treatment. The sham treated
control group was challenged with test compound and DNCB on each
half of the back. Erythema and edema reactions were scored 24 and
48 hours after challenge using the method of Draize. All groups
were rechallenged 7 days after the primary challenge and a separate
additional control group was challenged as previously described. No
erythema or edema was observed in sham treated controls. Positive
controls showed positive response at 24 and 48 hours. Only one of
Table 2. Results of genotoxicity assays on mancozeb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 2.5-250 µg/plate 88% active Negative Chism, 1984a
reversion assay TA1535, TA1537, (± rat S9); ingredient (a.i.)
TA98, TA100 in distilled water
S. typhimurium 2.5-250 µg/plate (± rat S9); 88% a.i. Negative Chism, 1984b
TA1535, TA1537, in distilled water
TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.5-45 µg/ml; 88% a.i. Negative Foxall & Byers,
mutation assay (CHO)/hprt in distilled water 1984
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethal Muller-5 stock food medium Krishnamurthy, 1980
assay
Autosomal D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethals Cy/B1 L2 stock food medium Krishnamurthy, 1980
1.D. Yeast and Other Fungal Assays
Point mutation A. nidulans 0.125-12 µg/ml; Not specified Positive Martinez-Rossi &
induction strains biA1 in distilled water; Azevedo, 1987
methG1 and 118 no activation used
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vitro Chromosomal Alterations in Mammalian Cells
In vitro Cultured human 1.40 µg/ml; Not specified Positive Georgian et al.,
chromosomal lymphocytes in propylene glycol; 1983
aberrations no activation used
2.B. In Vivo Chromosomal Alterations
Bone marrow Male non-inbred 10-1000 mg/kg; Not specified Negative2 Kurinnyi et al.,
cytogenetics white mouse in milk suspension 1982
or aqueous emulsion?
Wistar rat single i.p. 2.5-10 mg/kg; Not specified Positive Georgian et al.,
in propylene glycol 1983
Wistar rat 1.7 mg/kg bw/day for Not specified Positive Georgian et al.,
280 days; mixed in feed 1983
Male Fischer 344 rat 4.4 g a.i./kg/day for 1 88% a.i. Negative Sames et al., 1983
or 5 days; in corn oil
Male albino mouse 30-300 mg/kg; Not specified Positive Gautam & Kapoor,
(Lacca strain) in distilled water? 1991
Lymphocyte Female Wistar rat 3-30 mg/kg; in saline Not specified Positive Newton, 1975
cytogenetics
Micronucleus assay Male and female 10 000 mg/kg; 88.2% Negative Allen et al., 1987
CD-1 mouse in aqueous methyl-cellulose
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2.C. Plant Tests
Chromosomal Allium cepa 500-1000 ppm spray Not specified Positive Mann, 1977
alterations peduncles
Hordeum vulgare 100-1500 ppm; Not specified Positive Murty et al., 1983
germinating seeds in phosphate buffer
(with trace DMSO?)
Allium cepa 4-500 ppm Not specified Positive Badr, 1988
root tips
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro Primary rat 0.25-10 µg/ml; 88% a.i. Suggestive Byers, 1985
unscheduled DNA hepatocytes from male in culture medium positive;
synthesis (UDS) Fischer 344 rat suggests repeat
Primary rat 0.1-10 µg/ml; 82.4% Negative O'Neill & Frank,
hepatocytes from male in culture medium (also for S 1988
Fischer 344 rat phase induction)
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 5-20 µg/ml; Not specified Positive without Ivett, 1985
SCE assays (CHO) cells in culture medium activation
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell C3H/10T 1/2 cells 0.05-0.5 µg/ml; in water 88% a.i. Negative McGlynn-Kreft &
transformation McCarthy, 1984
C3H/10T 1/2 cells 0.1 µg/ml as "promoter"; 88% a.i. Negative McLeod & Doolittle,
with "promotion" in water 1985
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report.
2 The authors judged this effect to be positive, but significance was restricted to one dose and there was no dose-related response.
twenty animals given mancozeb (1/10 females) showed slight erythema
at 48 hours. After rechallenge the original female respondent was
negative but a second female (1/10 females) showed a slight erythema
at 24 hours but not at 48 hours (Trutter, 1988a).
Rabbits
Nine rabbits of unknown strain and sex were administered 100 mg
of ground mancozeb technical (> 80% pure) into the conjunctival sac
of one eye. The treated eyes of 3 rabbits were washed with water
approximately 20-30 seconds after dosing. Animals were observed for
eye irritation at 4, 24, 48, 72 and 96 hours and on days 7, 14 and
22. Substantial irritation was evident in unwashed eyes involving
the cornea, iris and conjunctiva. Effects were reversible after 14
days in unwashed eyes and after 72 hours in washed eyes.
A second study conducted in female New Zeeland white rabbits
using the same protocol produced responses of lesser consequence and
little or no involvement of the cornea or iris. Effects in unwashed
eyes were reversible after 7 days and after 24 hours in 2 of 3
rabbits with washed eyes. However conjunctival irritation persisted
in one rabbit through 14 days and disappeared at 21 days. The
compound was considered a substantial irritant based on the duration
of irritation (DeCrescente & Chan, 1982).
Six rabbits of unknown sex and strain were administered 500 mg
of ground mancozeb technical (> 80% pure), prepared as a paste in
saline, to intact and abraded skin. The test material was held
under an impervious patch in continuous 24-hour contact with closely
clipped skin. Erythema and edema were scored at 24 and 72 hours and
7 days. The only effect observed was erythema on abraded skin. The
primary irritation score was 0.5. The compound was considered a
slight irritant (DeCrescente & Parsons, 1980).
Observations in humans
A sample population of 121 Korean orange growers was tested for
hypersensitivity to the fungicide Dithane M-45 (mancozeb). A maximum
non-irritating concentration of 10% Dithane M-45 mixed with vaseline
was previously determined on 201 Korean medical students by patch
test. Results were read 48 hours post-administration. Application
of the patch test to orange growers was read 72 hours post-
administration according to the standards of the North American
Dermatitis Research group. Dithane tested positive in 4/121
subjects (3.3%) (Lee et al., 1981).
A 37-year old male working as a florist developed erythematous
vesicular eruption on the palms of his hands resembling dyshidrotic
eczema. Symptoms worsened and spread to other body areas (face,
neck, legs) during periods of heavy work, and diminished during
holidays. Patch tests with the mancozeb products Diagent and Firma
using Finn Chambers on Scanpor Tape read at 20 minutes, 2, 3, and 4
days resulted in a strong positive reaction on day two. A weak
positive reaction to zineb was also observed. Total serum IgE was
approximately five times the normal value. Other serum
immunoglobulins, G, A, and M were normal (Crippa et al., 1990).
A 61-year old vineyard worker developed a rash on the left
forearm as well as inflammation of the eyelids after planting vine
seedlings. This was the third episode under similar circumstances.
Patch tests on two previous occasions were negative. Patch tested
with 0.002% solution of both zineb and mancozeb (1% of normal use
concentration) resulted in positive responses. Retesting a week
later with the bark of a vine stick previously treated with mancozeb
produced a strong positive reaction. It was also reported that two
other male farm workers developed papulovesicular sheeted dermatitis
of the face, hands and chest after planting mancozeb-treated
potatoes. Patch tests with 0.1% solution of mancozeb were positive
in both. Patch tests conducted on the vineyard worker with
macerated vine bark were positive with aqueous but negative with
alcoholic solution (Kleibl & Rakcova, 1980).
One-hundred-fifty-three men currently or previously exposed to
dithane for many years at a manufacturing site were compared for
thyroid function to 153 men not exposed to Dithane, its products or
ETU who also worked at the same plant. Workers and controls were
carefully matched with respect to age, race, length of employment
and type of employment. Informed consent was obtained from all
participants. It was reported that at least one abnormality was
found in 25 of the 306 men (8.2%). The findings included
abnormalities on thyroid palpitation (4.3%), serologic autoimmune
thyroiditis (4.3%), subclinical hypothyroidism (1.3%), Graves'
disease (0.3%) and miscellaneous hormonal abnormalities (1.6%).
Urinary excretion of ETU in a subgroup of 42 workers currently
exposed to Dithane was 0.02 ppm compared to 0.01 ppm in the control
group (41 men), most of whom had undetectable values. The authors
concluded that exposure to Dithane manufacture was not associated
with an increased prevalence of thyroid abnormalities and that
thyroid abnormalities were common in iodine-replete adult American
men affecting 8.2% of the population studied (Charkes et al.,
1985).
A prevalence survey of adverse reproductive outcomes was
carried out in a population of 8867 persons (2951 men and 5916
women) who had been working in the floriculture industry in the
Bogota area of Columbia for at least 6 months. These workers from
58 companies were exposed to 127 different types of pesticides. The
prevalence rates for abortion, prematurity, still births, and
malformations were estimated for pregnancies occurring among the
female workers and the wives of the male workers before and after
working in floriculture and these rates were related to various
degrees of exposure. A moderate increase in the prevalence of
abortion, prematurity and congenital malformations was detected for
pregnancies occurring after the start of work in floriculture.
However, it could not be determined whether the increase in the
prevalence of the parameters measured was real. All rates, except
those for still births were higher for pregnancies occurring after
exposure to pesticides among both the female workers and the wives
of the male workers and thus gave significantly increased odds
ratios (chi-square) for abortions, premature births and malformed
babies. However, the high odds ratio observed for induced abortions
was considered by the authors surprising and cast doubt on the
reliability of the association between the observed increased risks
and the exposure to pesticides. It was also noted that of the 10
most used pesticides in floriculture, which accounted for 67% of the
total pesticides used in this industry, mancozeb comprised 7% of the
total. However, the article addressed pesticides in general and no
one chemical was singled out for adverse reactions. The authors
recognized that multiple exposure not only posed the problem of
identifying the toxic chemical responsible in causing any adverse
effect but also the problem of interaction between chemicals in the
expression of toxic effects (Restrepo et al., 1990).
COMMENTS
In pharmacokinetic studies conducted in male and female mice,
orally administered 14C-labelled mancozeb was rapidly absorbed,
peaking in whole blood between 1 and 2 hours, extensively
metabolized, and rapidly excreted (90%) within 24 hours. ETU was
the major metabolite.
Rats given single oral doses of 14C-labelled mancozeb absorbed
about 50% of the dose within 3-6 hours. Most of the dose was
excreted in 24 hours with half eliminated in the urine and half in
the faeces. Less than 4% was found in the tissues, with the thyroid
containing the highest residue level. Most of the 14C dose in
faeces was unabsorbed, since only 2-8% of the dose was found in
bile. ETU was the major metabolite. The half-life of ETU
elimination was 4-5 hours. The estimated bioavailability of ETU in
rats was about 6.8% on a weight/weight basis and 20% on a mole/mole
basis.
The acute oral, dermal and inhalation toxicity of mancozeb
technical is low. WHO has classified mancozeb as unlikely to
present acute hazard in normal use.
In a 13-week study in rats, mancozeb was administered in
dietary concentrations of 0, 30, 60, 125, 250 or 1000 ppm. The
NOAEL was 125 ppm (equal to 7.4 mg/kg bw/day) based on increased
serum TSH and decreased T4 values at the next higher dose.
Dogs administered 0, 10, 100, 1000 or 5000 ppm of mancozeb in
the diet for three months demonstrated a NOAEL of 100 ppm, equal to
3.0 mg/kg bw/day. At the next higher dose, decreased body-weight
gains and decreased erythrocyte count, haematocrit and haemoglobin
were observed.
In a 52-week study in dogs, mancozeb was administered in the
diet at concentrations of 0, 50, 200, 800 or 1600 ppm. The NOAEL
was 200 ppm, equal to 7.0 mg/kg bw/day, based on decreases in body-
weight gain, increased cholesterol and decreased haemoglobin and
packed cell volume at 800 ppm.
The NOAEL for dogs given mancozeb technical for 52 weeks, 7
days a week, by gelatin capsule was 2.3 mg/kg bw/day based on
decreased body weight, food consumption and decreased thyroxine
levels at 23 mg/kg bw/day.
In a 78-week carcinogenicity study in mice at dietary
concentrations of 0, 25, 100 or 1000 ppm, there was no evidence of
carcinogenicity. The NOAEL was 100 ppm, equal to 17 mg/kg bw/day,
based on decreased body-weight gain at 1000 ppm.
In a second 78-week carcinogenicity study in mice at dietary
concentrations of 0, 30, 100 or 1000 ppm in the diet, there was no
evidence of carcinogenicity. The NOAEL was 100 ppm, equal to 13
mg/kg bw/day, based on decreased body weight and decreased T3 and
T4 values at 1000 ppm.
The overall NOAEL in the two 78-week studies in mice was 17
mg/kg bw/day.
In a two-year toxicity/carcinogenicity feeding study in rats at
dietary concentrations of 0, 20, 60, 125 or 750 ppm, the NOAEL was
125 ppm (equal to 4.8 mg/kg bw/day) based on decreased body-weight
gain, decreased T3 and T4 values, increased TSH values, increased
absolute and relative thyroid weight, thyroid follicular cell
hypertrophy, hyperplasia, and nodular hyperplasia at 750 ppm.
Tumorigenic effects were noted in both sexes in the form of thyroid
follicular cell adenomas and/or carcinomas at the highest dose
level.
Mancozeb technical when administered in the diet to rats for
two years at dose levels of 0, 28, 113 or 454 ppm was not
tumorigenic. The NOAEL was 113 ppm (equal to 4.0 mg/kg bw/day)
based on decreased body-weight gain, decreased thyroxine levels and
an increase in the height of the thyroid follicular epithelium and
an increase in prominent microfollicles at 450 ppm.
The overall NOAEL in the two 2-year studies in rats was 4.8
mg/kg bw/day.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 25, 150 or 1100 ppm, the NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on decreased body weight at 150
ppm.
In a second two-generation reproduction study in rats at
dietary concentrations of 0, 30, 120 or 1200 ppm, the NOAEL was 120
ppm, equal to 7.0 mg/kg bw/day based on microscopic changes in the
thyroid, kidney and pituitary, increased relative weights of the
liver, kidney and thyroid, increased absolute thyroid weight,
decreased gestation and lactation body weight and feed consumption,
decreased pre-mating body weight and feed consumption at 1200 ppm.
The overall NOAEL in both reproduction studies was 7.0 mg/kg
bw/day.
In a 90-day (neuropathology) study conducted in rats at dietary
concentrations of 0, 20, 125, 750 or 5000 ppm, the NOAEL was 125
ppm, equal to 8.2 mg/kg bw/day, based on decreased food consumption
and neuro-histopathological changes at 750 ppm.
An oral teratogenicity study in rats at dose levels of 0, 2, 8,
32, 128 or 512 mg/kg bw/day produced no maternal effects at 32 mg/kg
bw/day (NOAEL) and no teratogenic effects at 128 mg/kg bw/day
(NOAEL). Maternal effects in the form of decreased body-weight gain
and decreased food consumption were seen at 128 mg/kg bw/day.
Teratogenic effects were seen at 512 mg/kg bw/day based, in part, on
the presence of agnathia, cleft palate, meningoencephalocele and
dilated brain ventricles.
A second oral teratogenicity study in rats at dose levels of 0,
10, 60 or 360 mg/kg bw/day showed no maternal or embryo/fetotoxic
effects at 60 mg/kg bw/day (NOAEL). Maternal toxicity at 360 mg/kg
bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryofetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra.
The NOAEL in an oral teratogenicity study in rabbits given 0,
5, 30, 55, or 100 mg/kg bw/day was 55 mg/kg bw/day for maternal
effects and greater than 100 mg/kg bw/day for embryo/fetotoxic
effects. An increase in abortions, body-weight loss, and decreased
food consumption were observed at 100 mg/kg bw/day.
The NOAEL in an oral teratogenicity study in rabbits given 0,
10, 30 or 80 mg/kg bw/day was 30 mg/kg bw/day for maternal toxicity.
The NOAEL for embryo/fetotoxic effects was greater than 80 mg/kg
bw/day. Maternal toxicity at 80 mg/kg bw/day was based on an
increase in aborted fetuses, decreased number of litters produced,
decreased body-weight gain and food consumption, an increase in
clinical signs and death.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
The data on mancozeb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 4.8 mg/kg bw/day for thyroid effects in rats
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for mancozeb, alone or in combination
with maneb, metiram, and/or zineb, because of the similarity of the
chemical structure of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differentiated using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet, equal to 17 mg/kg bw/day
(78-week studies)
Rat: 125 ppm in the diet, equal to 4.8 mg/kg bw/day
(two-year studies)
120 ppm in the diet, equal to 7.0 mg/kg bw/day
(reproduction studies)
125 ppm in the diet, equal to 8.2 mg/kg bw/day
(90-day neuropathology study)
Dog: 200 ppm in the diet, equal to 7.0 mg/kg bw/day
(52-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with maneb, metiram, and zineb)
Studies which will provide valuable information in the continued
evaluation of the compound
Clarification of the genotoxic potential of mancozeb.
Observations in humans.
REFERENCES
Allen, J.A., Proudlock, R.J. & Pugh, L.C. (1987). Micronucleus test
on mancozeb technical. Unpublished report No. PWT 39/86637 from
Huntingdon Research Centre Ltd., Huntingdon, Cambridgeshire,
England. Submitted to WHO by Elf Atochem, Philadelphia,
Pennsylvania, USA (Confidential data of Elf Atochem).
Badr, A. (1988). Cytogenetic activities of some fungicides.
Cytologia, 53: 635-640.
Braverman, L.E., Lipworth, L. & Charles, D. (1978). A health survey
of workers involved in the manufacture and packaging of dithane
fungicide with special reference to thyroid function. Unpublished
report dated August 2, 1978 from the University of Massachusetts
Medical School, Worcester, Massachusetts, USA. Submitted to WHO by
Rohm and Haas Company, Spring House, Pennsylvania, USA.
Broadmeadow, A. (1991a). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
89/PTC004/0015 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Broadmeadow, A. (1991b). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
90/PTC029/0197 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Byers, M.J. (1985). Dithane M-45 in vitro unscheduled DNA
synthesis assay. Unpublished report No. 84R-280 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Cameron, B.D., Clydesdale, K. & Speirs, G.C. (1990). The disposition
of 14C mancozeb in the mouse. Unpublished report No. 4909 from
Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA (Confidential
data of Elf Atochem).
Charkes, N.D., Braverman, L.E., Penko, K.F., Gowers, D.S., Gordon,
C.F., Lipworth, L, Malmud, L.S. (1985). Thyroid function in male
workers manufacturing dithane, an agricultural fungicide, and in men
not exposed to dithane. Frontiers in Thyroidology, 2: 933-936.
Chism, E. (1984a). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced Fischer 344 rats. Unpublished
report No. 84R-059 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Chism, E. (1984b). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced B6C3F1 mice. Unpublished
report No. 84R-0060 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Cox, R.H. (1986). Three month dietary toxicity study in dogs with
mancozeb. Unpublished report No. HLA 417-416 from Hazleton Labs.,
Vienna, Virginia. USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA as Rohm and Haas Report No. 86RC-7.
Crippa, M., Misquiih, L., Lunati, A. & Pasollini, G. (1990).
Dyshidrotic eczema and sensitization to dithiocarbamates in a
florist. Contact Dermatitis, 23: 203.
DeCrescente, M.E. & Chan, P.K. (1982). Dithane M-45: rabbit eye
irritation report. Unpublished report Nos. 81R-197, 81R-176 and 82R-
10 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeCrescente, M.E. & Parsons, R.D. (1980). Dithane M-45: Acute oral
and dermal LD50 and skin and eye irritation. Unpublished report No.
79R-180, from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeGroot, A.P. (1974). Determination of acute ip toxicity of Dithane
M-45 in rats. Unpublished report from Central Institute for
Nutrition and Food Research TNO the Netherlands (Rohm and Haas
report No. 74RC-1011). Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
DiDonato, L.J., Cruzan, G. & O'Hara, G.P. (1985). Dithane M-45/ETU
four week range finding mouse study. Unpublished report No. 80R-123
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DiDonato, L.J. & Longacre, S.L. (1986). Mancozeb pharmacokinetic
study in rats. Unpublished report No. 85R-123 (Supplement/Appendix
to Nelson, S.S. [1986] report No. 31H-86-02) from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978). A study of the uptake and elimination of
14C activity after the oral ingestion of 14C-labelled
ethylenethiourea (ETU) and mancozeb in the rhesus monkey.
Unpublished final (revised) report from Battelle Labs, Columbus,
Ohio, USA. dated July 31, 1978. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA. as Rohm and Haas report
No. 78RC-1028.
Everett, D.J., Atkinson, C., Perry, C.J., Strutt, A., Millar, P.,
Hudson, P. (1992). Mancozeb 78-week dietary carcinogenicity study in
mice with 52 week interim kill. Unpublished report No. 7561 from
Inveresk Research International, Tranent, Scotland. Submitted to WHO
by Elf Atochem, Philadelphia, Pennsylvania, USA. (Confidential data
of Elf Atochem).
Foxall, S. & Byers, M.J. (1984). Dithane M-45 CHO/HGPRT gene
mutation assay. Unpublished report No. 84R-207 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Gallo, M.A., Kam, C. & Stevens, D.R. (1980). Teratologic evaluation
of dithane M-45 in the albino rat. Unpublished report No. 10065-009
from Booz, Allen, and Hamilton, Inc., Florham Park, New Jersey, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA as Rohm and Haas report No. 81RC-75.
Gautam, D.C. & Kapoor, L. (1991). Genotoxic effects of Dithane M-45
on the bone marrow cells of mice in vivo. Experimentia, 47(3):
280-2.
Georgian, L., Moraru, J., Draghicescu, T., Dinu, I. & Ghizela, G.
(1983). Cytogenetic effects of alachlor and mancozeb. Mutation Res.
116: 341-348.
Goldman, P., Bernacki, H.J. & Quinn, D.L. (1986). Mancozeb: three
month dietary toxicity study in rats. Unpublished report No. 85R-167
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1980). Acute inhalation toxicity of
dithane M-45 (TD-79-224) in rats. Unpublished report No. 79R-210
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1982). Dithane M-45: Acute inhalation
toxicology study in rats. Unpublished report No. 81R-171 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Hagan, J.V., Fisher, J.R. & Baldwin, R.C. (1986). Mancozeb:
subchronic inhalation toxicity study in rats. Unpublished report No.
86R-3 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1986). Mancozeb: two week range finding
inhalation toxicity study in rats. Unpublished report No. 85R-190
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Haines, L.D. (1980). Dithane M-45 percutaneous absorbtion in rats.
Unpublished technical report No. 34F-80-9 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 80R-1006.
Hooks, W.N., Offer, J.M., Hadley, J.C., Gibson, W.A., Gopinath, C. &
Dawe, I.S. (1992). Mancozeb technical: potential tumorigenic and
toxic effects in prolonged dietary administration to rats.
Unpublished report No. PWT 29/89669 from Huntingdon Research Centre
Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by Elf
Atochem, Philadelphia, Pennsylvania, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of Dithane M-45 in an
in vitro sister chromatid exchange assay in the Chinese hamster
ovary (CHO) cells. Unpublished reported dated March 1985 from Litton
Bionetics, Inc., Kensington, Maryland, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 84RC-60.
Kleibl, K. & Rakcova, M. (1980). Cutaneous allergic reactions to
dithiocarbamates. Contact Dermatitis, 6(5): 348-349.
Kocialski, A. (1989). Establishment of an in vitro metabolic
conversion factor of 7.5% of all EBDCs when converting EBDCs to
ethylenethiourea in vivo and recalculations of the previously
considered 20% in vivo conversion exposure factor for EBDCs to
ETU. Memorandum to the Caswell file, Health Effects Division, Office
of Pesticide Programs, United States Environmental Protection
Agency.
Kurinnyi, A., Pilinskaya, M., German, J. & L'vova, T. (1982).
Implementation of a program of cytogenetic study of pesticides:
preliminary evaluation of cytogenetic activity and potential
mutagenic hazard of 24 pesticides. Cytol. Genet. 16: 50-53.
Lee, Y.S., Cinn, Y.W., Chang, W.H. & Kim, J.S. (1981). A study on
hypersensitivity of Korean farmers to various agrochemicals.
Determination of concentration for patch tests of fruit-tree
agrochemicals and hypersensitivity of orange orchard farmers in Che-
Ju Do, Korea. The Seoul Journal of Medicine, 22: 137-142.
Longacre, S. (1986). Summary of ETU and EBDC analysis in plasma,
liver, and thyroid after mancozeb administration. Unpublished report
No. 86R-1009 from Rohm and Haas Company, (appendix/supplement to
Nelson 1986 31H-86-02) Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lu, M. & Kennedy, G. (1986). Teratogenic evaluation of mancozeb in
the rat following inhalation exposure. Toxicol. and Appl. Pharma.
84: 355-368.
Mann, S.K. (1977). Cytological and genetical effects of dithane
fungicides on Allium cepa. Environmental and Experimental Botany,
17: 7-12.
Martinez-Rossi & Azevedo, J. (1987). Detection of point mutation
mutagens in Aspergillus nidulans: comparison of methionine
suppressors and arginine resistance induction of fungicides.
Mutation Research, 176: 29-35.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb and their related compounds. Int. Arch. Occup. Environ.
Health, 37: 169-178
Matsushita, T. Yoshioka, M., Arimatsu, Y. & Nomura, S. (1977).
Experimental study on cross-contact allergy due to dithiocarbamate
fungicides. Indust. Health, 15: 87-94.
McGlynn-Kreft, A.M. & McCarthy, K.L., (1984). Dithane M-45 mammalian
cell transformation test. Unpublished report No. 84R-55 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
McLeod, P.L. & Doolittle, D.J. (1985). Dithane M-45 mammalian cell
transformation test for promotion. Unpublished report No. 84R-0297
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Muller, W. (1991). Oral (gavage) teratogenicity study in the rabbit.
Unpublished report No. HLD-853-683-002 from Hazleton Labs.,
Deutschland GmbH, Munster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Muller, W. (1992). 2-Generation oral reproduction toxicity study in
the rat. Unpublished report No. HD-852-683-001 from Hazleton Labs.,
Deutschland GmbH, Münster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Murty, K., Raju, D. & Sharma, C. (1983). Cytogenetic hazards from
agricultural chemicals. 7. Herbicides, fungicides and insecticides
screened for effects on chiasmata in Hordeum vulgare. Biol. Zb1.
102: 571-576.
Nelson, S.S. (1986). Metabolism of 14C mancozeb in rats.
Unpublished report No. 31H-86-02 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Nelson, S.S. (1987). Bioconversion of mancozeb to ETU in the rat.
Unpublished report No. 31C-87-24 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 80R-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neill, P. & Frank, J.P. (1988). Mancozeb: in vitro unscheduled
DNA synthesis assay in F/344 rat hepatocytes. Unpublished report No.
88R-079 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Piccirillo, V.J., Wu, D. & Speirs, G. (1992). Metabolism of mancozeb
in the mouse. Unpublished report No. T91-3413 from Inveresk Research
International, Ltd., Tranent, Scotland. Submitted to WHO by Elf
Atochem (Confidential data).
Restrepo, M., Munoz, N., Day, N.E., Parra, J.E., deRomero, L. &
Nguyen-Dinh, X. (1990). Prevalence of adverse reproductive outcomes
in a population occupationally exposed to pesticides in Columbia.
Scand. J. Work. Environ. Health, 16(4): 232-238.
Sames, J.L., McLeod, P.L. & Doolittle, D.J. (1984). Dithane M-45 in
vivo cytogenetic study in Fischer 344 rats. Unpublished report No.
84R-0246 Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Schweitzer, M. (1990). A study of the decomposition of mancozeb in
dimethyl sulfoxide solution. Unpublished report No. SC890012 from
Battelle Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. TR 34-90-01.
Shaw, D. (1990). Mancozeb: 52 week oral (dietary) toxicity study in
the beagle. Unpublished report No. HUK 5913-616/3 from Hazleton, UK,
Harrogate, North Yorkshire, England. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. 88RC-027.
Shellenberger, T. (1991) Mancozeb: 18-month dietary oncogenicity
study in mice. Unpublished report No. 85051 from Tegeris Labs.,
Inc., Temple Hills, Maryland, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA, as Rohm and Haas report
No. 86RC-029.
Solomon, H.M. & Lutz, M.F. (1987). Mancozeb: oral (gavage)
developmental toxicity study in rabbits. Unpublished report No. 86R-
021 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Solomon, H.M., Lutz, M.F. & Kulwich, B.A. (1988). Mancozeb: 2-
generation reproduction study in rats. Unpublished report No. 87R-
020 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Stadler, J. (1990). Combined chronic toxicity/oncogenicity 2-year
feeding study with mancozeb in rats. Unpublished report No. 259-89
from E.I. duPont de Nemours and Company, Haskell Laboratory for
Toxicology and Industrial Medicine, Newark, Delaware. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Stadler, J. (1991) Neuropathology study in rats with mancozeb.
Unpublished report No. 217-89 from E.I. duPont de Nemours and
Company, Haskell Laboratory for Toxicology and Industrial Medicine,
Newark, Delaware. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Szepvolgyi, J., Nagy, K., Sajgone-Vukan, K., Regoly-Merci, A., Soos,
K., Toth, K., Pinter, A. & Antal, M. (1989). Subacute toxicological
examination of Dithane M-45. Fd. Chem. Toxic, 27(8): 531-538.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Enticott, J., Wilby,
O.K., Tesh, S.A. (1988). Mancozeb: teratology study in the rat.
Unpublished report No. 87/PTC 007/365 from Life Science Research,
Suffolk, England. Submitted to WHO by Elf Atochem (Confidential data
of Elf Atochem).
Tomlinson, H.L. & Longacre, S.L. (1988). Mancozeb dermal absorbtion
study in male rats. Unpublished report No. 88R-218 from Rohm and
Haas, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm and
Haas, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988a). Mancozeb: delayed contact hypersensitivity
study in guinea-pigs. Unpublished report No. HLA 417-431 from
Hazleton Labs., Vienna, Virginia, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988b). Mancozeb: 4 week repeat dermal toxicity study
in rats. Unpublished report No. HLA 417-432 from Hazleton Labs.,
Vienna, Virginia, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Vasudev, V., Krishnamurthy, N.B., (1980). Non-mutagenicity of the
fungicide Dithane M-45 as inducer of recessive lethals after larval
feeding in Drosophila melanogaster. Mutation Res. 77: 189-191.
Watts, M.H. & Chan, P.K. (1984a). Dithane M-45: acute oral toxicity
study in rats and mice. Unpublished report No. 83R-213a + b from
Rohm and Haas Company, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Watts, M.H. & Chan, P.K. (1984b). Dithane M-45: acute oral toxicity
study in rats. Unpublished report No. 83R-218 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Peak concentrations of 14C in thyroid were reached within 6
and 24 hours in animals receiving the low and high doses,
respectively. Peak levels in thyroid were about 45 and 10 fold
higher than the corresponding peak levels in whole blood after low
and high-dose administration, respectively. 14C residue levels for
aforementioned tissues were comparable for males and females
receiving single dose administration except for adipose tissue and
gonads of females which were higher than those of males. Thyroid
tissue contained the highest residue levels for each group.
However, thyroid contained less than 0.10% of the dose 96 hours
post-dose. Tissue concentrations of 14C residues in rats receiving
the "pulse dose" were comparable to those in animals receiving only
the single dose of 14C-mancozeb at 1.5 mg/kg bw. The average 14C
residue levels per group remaining in the tissue at 96 hours post-
dosing ranged between 1.5-3.5% of the dose. ETU concentrations in
plasma and liver of rats 6 hours after dosing with 14C-mancozeb at
1.5 mg/kg bw were 6 and 12 times less than corresponding plasma and
liver 14C levels, respectively. ETU was not detected in the pooled
thyroids of these low-dose animals. Peak plasma levels of ETU were
reached 6 hours post-dosing in animals given 100 mg/kg bw mancozeb
and were 6-12 times less than corresponding plasma 14C levels for
both sexes. ETU was rapidly eliminated from the plasma of both male
and female rats (t´ 4.0-4.7 hours) and decreased below detectable
levels within 48-hours post-dosing. The estimated bioavailability
of ETU in rats was about 6.8 percent on a w/w basis and 20% on a
mole/mole basis. ETU concentrations in the liver of the high-dose
rats was 100 times less than liver 14C concentrations 6 hours post-
dosing. ETU was not detectable in liver 48 hours post-dosing. ETU
concentrations in thyroids were 80-100 and 30-225 times less than
corresponding thyroid 14C concentrations in males and females,
respectively, given 100 mg/kg bw mancozeb at 6 and 24 hours. ETU
was not detectable in plasma, liver, or thyroid of rats at 96 hours
after administration of a "pulse dose" (1.5 mg/kg bw of 14C-
mancozeb) preceded by a two-week dietary intake of unlabelled
technical mancozeb.
Concentrations of EBDC in the liver of rats 1, 6 and 24 hours
after dosing with 14C-mancozeb at 100 mg/kg bw were 0.25, 0.61, and
0.29 ppm for males, respectively, and 0.32, 0.35 and 0.25 ppm for
females, respectively. EBDC residues were not detectable 48 or 96
hours post-dosing. EBDC was not detected in the livers of rats
receiving the low dose (1.5 mg/kg bw) or multiple doses of mancozeb
96 hours post-dosing. Metabolite analysis revealed that mancozeb
was extensively metabolized and/or degraded. ETU was a major
metabolite found in urine, bile and faeces. EBDC was detected in
liver, faeces, and bile but not in thyroid. EBIS, ethyleneurea
(EU), N-acetyl-ethylenediamine (N-AcEDA) and ethylenediamine (EDA)
were also identified in urine, faeces and bile. Other metabolites
tentatively identified were glycine, N-acetylglycine, and N-
formylethylenediamine. Five other metabolites were also present but
were not identified (DiDonato & Longacre, 1986; Longacre, 1986;
Nelson, 1986, 1987; Kocialski, 1989).
Monkeys
Groups of male rhesus monkeys ( Macaca mulatta; 6/group) were
given single oral doses of either 14C-ETU; 14C-ETU plus manganous
sulfate and zinc sulfate or 14C-mancozeb (purity not stated) for
the determination of uptake into blood and the determination of the
major route of elimination of 14C. A sufficient dose was given to
produce 100 µCi of 14C activity per monkey. Whole blood, thyroid,
heart, lung, liver and kidney and faecal material were converted to
14C-carbon dioxide by combustion in a tissue oxidizer and analyzed
for 14C activity. Urine samples were analyzed directly. Urine and
faeces were collected separately.
14C-Labelled doses of ETU and ETU plus manganese and zinc
sulfates reached peak levels of 5% of the dose in total blood volume
at 8 hours. 14C-Mancozeb activity in the blood was less than 0.5%
at 8 hours and plateaued from 24-72 hours at slightly less than 1%
of the dose. This was in contrast to a relatively rapid decline in
14C activity in ETU-treated monkeys which at 72 hours showed 1% of
the administered dose in whole blood. ETU and ETU plus Mn:Zn-treated
monkeys showed similar rates of 14C excretion with nearly 50% of
the dose being cleared in 24 hours. The urinary clearance of 14C
activity by mancozeb-treated monkeys was slower (3.6% of the dose in
24 hours) as compared to ETU-treated groups. At 120 hours (last time
reading), the urinary route of elimination accounted for little more
than 10% of the activity in the mancozeb dose. Faecal elimination
showed less than 1% of 14C activity up to 24 hours in ETU-treated
groups. However, mancozeb-derived faecal activity ranged from 12.5-
64.0% of the dose at 144 hours, and from 0.005-12.7% at 24 hours.
14C activity was not detected in faecal samples from ETU-treated
monkeys after 24 hours. At 144 hours, blood samples were taken from
2 animals of the mancozeb-treated group, the animals sacrificed and
the following organs examined for 14C activity: thyroid, heart,
lung, liver and kidney. The remaining 4 animals were sacrificed 48
hours later (192 hours post-dosing). Comparative examination
between the groups (2 vs 4 monkeys) indicated that 14C activity
declined steadily between 144 and 192 hours in blood, whereas 14C
activity in the thyroids increased over the same 48-hour time period
(Emmerling, 1978).
Toxicological studies
Acute toxicity studies
Acute toxicity studies are summarized in Table 1. WHO has
classified mancozeb as unlikely to present acute hazard in normal
use (WHO, 1992).
Short-term toxicity studies
Mice
Charles River COBS-CD-1 mice (10/sex/group) received 0, 1, 10,
100, 1000 or 10 000 ppm of mancozeb (83% purity) adjusted to 100%
active ingredient for 4 weeks. No animals died on study and
clinical signs were absent. Females fed 10 000 ppm mancozeb showed
decreased body weights. Food consumption appeared to be comparable
for all groups on study. SGPT activity was comparable to controls
for all groups. Gross necropsy findings were not remarkable.
Thyroid weights (absolute and relative) were statistically
increased in both females at 10 000 ppm. Males appeared unaffected.
Liver weights were statistically significantly increased in males
and females given 10 000 ppm mancozeb. At 1000 ppm, absolute and
relative liver weights were significantly increased in females.
Females treated with 1000 ppm and 10 000 ppm showed thyroid
hyperplasia, congestion and decreased colloid density whereas males
showed similar effects at 10 000 ppm. No significant micropathology
was evident in the livers of animals given mancozeb (DiDonato et
al., 1985).
Mancozeb (83.1% pure) was administered to groups of Charles
River CD-1 mice (15 sex/dose) for 3 months at dietary concentrations
of 0, 10, 100, 1000, or 10 000 ppm of active ingredient. No
compound-related mortalities or clinical signs were observed.
Decreased body weights and food consumption were observed in both
sexes receiving 10 000 ppm. There were no treatment-related effects
with regard to haematology or clinical chemistry. Necropsy findings
were not remarkable. Aniline hydroxylase activity was decreased at
10 000 ppm in both sexes. Aminopyrine N-demethylase activity was
decreased in males at 1000 and 10 000 ppm. No effects were seen in
either sex at lower doses. Absolute and relative thyroid weights
were increased in both sexes receiving 10 000 ppm. Relative
increases in liver weights were seen in both sexes receiving 10 000
ppm. Absolute liver weight was increased in males administered 10
000 ppm mancozeb. Relative kidney weights were also increased in
both sexes at 10 000 ppm. Histopathologic evaluation of thyroid
revealed an increased incidence of follicular cell hyperplasia and
hypertrophy in both sexes at 1000 and 10 000 ppm. There was
Table 1. Acute toxicity of mancozeb
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse1 B6C3F1 M oral > 5000 --- Watts, 1984
Rat3 F344 M oral > 5000 --- Watts, 1984
Rat1 F344 M oral > 5000 --- Watts, 1984
Rat3 CRCD M oral > 5000 --- DeCrescente, 1980
Rat2 Wistar M/F i.p. 380 --- DeGroot, 1974
Rat 5,6 COBS-CR M/F inhalation --- 5.14 Hagan, 1982
(SD) BR 4 hour exposure
Rat 6,7 CR-SD M/F inhalation --- > 1.11 Hagan, 1980
4 hour exposure
Rabbit4 NZW M dermal > 5000 --- DeCrescente, 1980
1 Corn oil vehicle.
2 Carboxy-methyl-cellulose vehicle.
3 Aqueous dispersion.
4 Saline vehicle.
5 3/10 M and 3/10 F died during the whole body exposure/observation period (14 days). Multiple signs but no
tremors; body-weight loss and decreased weight gain. MMD 3.3 microns. Nominal concentration was
41.8 mg/litre (aerosol).
6 Whole body exposure.
7 MMD about 2.2 microns. Nominal concentration 4.46 mg/litre (dust).
increased vacuolation, interstitial congestion and decreased colloid
density in the thyroids in both sexes at 10 000 ppm. No liver
effects were observed in mancozeb-treated mice with the possible
exception of hepatocytic nuclear pleomorphism in females at 10 000
ppm. Increased deposits of (brownish) pigment were seen in the zona
reticularis of the adrenal cortex of female mice at 10 000 ppm. The
NOAEL was 100 ppm, equal to 18 mg/kg bw/day for males and 22 mg/kg
bw/day for females (O'Hara & DiDonato, 1985).
Rats
Four groups of 12 male and 12 female Crl:CD (SD)BR rats were
exposed to dust aerosols of mancozeb (84% pure) for 6 hours per day
for 10 days. The four groups were subdivided into whole-body
exposed rats and nose-only exposed rats of 5/sex/dose group. Daily
concentrations yielded total mean aerosol concentrations of 0, 23,
138 or 519 mg/m3. The mean respirable aerosol concentrations were
0, 11, 55 or 258 mg/m3 with mass median diameters of 3.5-4.9
microns.
Nose-only Exposure: There were no compound-related deaths.
No adverse clinical signs were observed in males or females exposed
to 11 or 55 mg/m3. Significant decreases in mean body weight and
mean body-weight gain were observed in the high-dose males but not
females. T3 and T4 levels were significantly reduced and the lung
to body-weight ratio increased in high-dose males but not in
females. Microscopic examination (limited to the respiratory tract)
of nasal turbinates revealed an increased incidence and degree of
multifocal mixed inflammatory cell infiltration (i.e. mononuclear
cells and neutrophils) and multifocal or focal necrosis of the
turbinate mucosa in four males and two females in the high dose.
Whole-body Exposure: There were no compound-related deaths.
No clinical signs were observed in animals receiving 11 mg/m3.
Significant reductions in male and female body weight and body-
weight gain were observed at 55 and 258 mg/m3. Male and female T4
levels and male T3 levels were significantly decreased at 55 and
258 mg/m3 of mancozeb after two weeks. TSH levels were not
significantly increased in both sexes at the high dose. Male and
female lung weights and lung to body-weight ratios were
significantly increased in both sexes at the high dose. Exposure-
related multifocal interstitial inflammation, microgranulomas,
multifocal mixed inflammatory cell infiltration, focal or multifocal
necrosis in the respiratory tract and reactive lymphoid hyperplasia
of the peribronchial lymph nodes were observed at the high dose
tested (Hagan & Baldwin, 1986).
Adult male and female Crl:CD(SD)BR rats were divided into two
nose-only exposure groups and one nose-only exposure-recovery group.
Animals received aerosol dust (84% pure mancozeb), 6 hours/day, 5
days week for 4 weeks at analytically determined mean concentrations
of 0, 22, 86 or 308 mg/m3 (equivalent to respirable concentrations
of 0, 8, 40 and 127 mg/m3) or for 13 weeks at 0, 18, 79 or 326
mg/m3 (equivalent to respirable concentrations of 0, 8, 36 or 144
mg/m3).
Results - 4 Weeks Exposure: There was no compound-related
mortality. Mean body weights and body-weight gains were decreased
for males in the high-dose group. No haematological effects were
observed in males. Data for females were inconclusive due to
insufficient amount of blood needed for the haematological
evaluations. There were no compound-related effects in either sex
for clinical chemistry, thyroid function, organ weights, or tissues
examined microscopically. Ophthalmological examinations revealed no
remarkable findings.
Results - 13 Weeks Exposure: There were no compound-related
deaths and no treatment-related signs of toxicity. Males exposed at
the high dose exhibited reduced mean body weights and body-weight
gains. Some haematological and clinical chemistry changes were
noted but were within the normal range of values and were therefore
not considered related to mancozeb exposure. T4 levels were
reduced 30% in high-dose females and considered treatment-related.
A dose-response was evident. Males showed a 9% decrease at the high
dose tested which was not statistically significant. Absolute
kidney and heart weights were reduced in males at the high dose
tested. However, organ to body weight and organ to brain weight
ratios were not statistically significantly different from controls.
Ophthalmological examination of the eyes revealed no compound-
related effects. No exposure-related histopathology was observed in
either sex at the low dose tested. Males (5/10; 8/11) and females
(10/11; 9/11) exposed to 79 or 326 mg/m3 exhibited a yellow-brown
granular pigment in the lumen of the cortical tubules of the kidney.
However, no attendant histopathological changes were reported. Mild
hyperplasia of the follicular epithelium in the thyroid glands of 3
females exposed at the high dose was observed. No exposure-related
thyroid lesions were observed at lower dose levels or in males.
Several histopathological lesions were observed in the respiratory
tract but were comparable to control incidences and not considered
treatment-related. Residue analysis of blood indicated
concentrations below the limit of detection for ETU and mancozeb for
males and females exposed to the low dose, with increasing
concentrations at higher exposures. Residue levels for ETU in the
thyroid were below the limit of detection at the low dose in both
sexes, with increasing concentrations at the higher doses. ETU and
mancozeb levels in urine were present at all doses in both sexes.
Those animals allowed to recover for 13 weeks after cessation of
exposure were comparable to a concurrent control group of non-
exposed animals for all parameters examined (Hagen et al., 1986).
Groups of Sprague-Dawley rats (Crl:CD(SD)BR); 10/sex/dose) were
administered 0, 10, 100, or 1000 mg of mancozeb (83% pure, not
adjusted for purity)/kg bw. The control group received distilled
water only. Test material was applied to the clipped dorsal area of
intact skin of each animal. An unsterile dressing sponge was placed
over the test site and held in place for 6 hours/day then removed.
All animals were treated similarly for 20 exposures over a 4-week
period. All animals were fitted with a cardboard collar to minimize
preening at the application site. The collar was only removed
during the 6 hours exposure period.
Clinical observations showed no evidence of compound-related
effects and there was no compound-related mortality. Body weight,
body-weight gain and food consumption in treated groups were
comparable to controls. Erythema was transient and slight, occurring
in not more than 2 animals/sex/dose for not longer than 2-4 days.
No other signs of dermal irritation were observed. Evaluation of
haematological and clinical chemistry parameters revealed no
statistically significant compound-related effects. Macroscopic
observations of treated skin revealed dark (yellow) area or
appearance in a dose-related increasing frequency at 100 and 1000
mg/kg bw in both sexes. Absolute and relative organ weights in
treated groups were not significantly different from control group.
Microscopic findings were limited to skin of treated and untreated
animals and characterized by increased keratin production
(hyperkeratosis) and thickening of the epidermis (acanthosis).
Severity varied from minimal to slight in all groups. These
findings were treatment-related but not compound-related. There
were no microscopic findings that were attributable to mancozeb.
Gross enlargement of the parotid salivary gland was within normal
histomorphological limits and found in all treatment groups
(Trutter, 1988b).
Groups of Crl:CD(SD) rats (14 rats/sex/group) received 0, 30,
60, 125, 250 or 1000 ppm of mancozeb (84% pure adjusted to 100%
active ingredient) for 13 weeks. Dose levels in the diet were
gradually increased with time in order to maintain approximately the
same level of compound intake throughout the feeding period.
There was no compound-related mortality or clinical signs. No
compound-related changes in body weights or food consumption were
recorded in animals fed up to and including 250 ppm mancozeb. At
1000 ppm, males showed a statistically significant decrease in body
weight from weeks 3 through 13, whereas females showed a 3-14%
decrease with statistically significant decreases only between 7 and
10 weeks. At 1000 ppm, food consumption was decreased in males for
weeks 3-13, but not in females. Males receiving 1000 ppm of
mancozeb showed statistically significant increases in BUN (84%),
creatinine (28%) and cholesterol (52%). Females receiving 1000 ppm
showed increased SAP (32%) and triglyceride (90%). However, group
increases were not considered compound-related as one male and one
female in the high-dose group were reported as having exceptionally
high values.
Clinical findings at lower mancozeb levels were not considered
treatment-related. Serum T4 levels were decreased in males and
females at 1000 ppm and TSH values increased. At 250 ppm, TSH
values were increased in males (36%) and females (50%). However,
only T4 values in females were statistically significantly
decreased at 250 ppm. MFO activity was decreased at 1000 ppm. No
mancozeb metabolite residues were detected in blood. Urine samples
of rats fed 125 to 1000 ppm of mancozeb contained 0.1 to 1.1 ppm of
parent compound. ETU metabolite of mancozeb increased in urine in a
dose-response manner from 0.3 to 10 ppm in animals given 30 to 1000
ppm mancozeb.
No mancozeb residues above the detection limit of 25 ppm were
detected in thyroids obtained from the 1000 ppm mancozeb fed rats.
ETU metabolite of mancozeb showed residues in thyroid which were
dose-related to mancozeb administration. Values ranged from less
than the detection limit of 4.0 ppm in 30 ppm mancozeb-fed animals
to 25 ppm in 1000 ppm animals. At 1000 ppm, absolute thyroid weight
was increased in males while relative weight was increased in males
and females. Relative liver weight was increased in males and
females receiving 1000 ppm. Relative spleen weight was increased
only in females receiving mancozeb at the highest dose. Compound-
related histopathology was generally confined to the liver, kidneys,
thyroid, adrenal and pituitary glands. Thyroid follicular cell
hyperplasia was seen in 90% of males and females receiving 1000 ppm;
a small, well defined basophilic focus of hyperplastic follicular
epithelial cells was seen in one male and there was also an
increased incidence and severity of hypertrophied vacuolated cells
in the pituitary of males. The kidneys of males and females
administered 125 to 1000 ppm had minimal to moderate amount of
yellow-brown pigment in the lumen of the cortical tubules. Pigment
deposits were attributed to ethylene bis(isothiocyanate) sulfide
(EBIS) a yellow coloured metabolite of mancozeb. Pigmentation
deposits were not accompanied by histopathological effects. There
was also an increased incidence of hypertrophy of cells of the zona
glomerulosa of the adrenal cortex in males given ETU and 1000 ppm
mancozeb. Hypertrophy of the centrilobular hepatocytes was seen in
males fed 1000 ppm mancozeb. The NOAEL was 125 ppm, equal to 7.4
mg/kg bw/day, based on increased serum TSH and decreased T4 values
at the next higher dose (Goldman et al., 1986).
Male H-Wistar rats (12/group) were given Dithane M-45 (80%
mancozeb) mixed in feed at doses of 0, 10,50, 75, 113, 169, 253 or
379 mg/kg bw/day for 12 weeks. One-third of the rats in the highest
dose group died within 6 weeks and showed signs of prostration,
weakness and posterior extremital paralysis. Signs were transient
in survivors and absent at 12 weeks. Body weight, body-weight gain,
total food intake and food efficiency were all depressed at 169
mg/kg bw/day and above. Effects were compound-related and
statistically significant. Blood sugar levels (after glucose
loading) and haematological parameters were comparable to control
values. Organ to body-weight ratios were increased for liver and
thyroid at 75 mg/kg bw/day and above and for kidneys, adrenals and
testes at 253 mg/kg bw/day and above. Histopathology of liver and
kidneys were comparable to controls. Thyroids, however, showed a
dose-dependent hyperplasia at 113 mg/kg bw/day and above. A 20%
decrease in the iodine content of the thyroids in rats given 10
mg/kg bw/day was not statistically significant. However at 50 mg/kg
bw/day a statistically significant decrease of 80% was recorded.
PBI was significantly decreased and serum cholinesterase
significantly increased at 169 mg/kg bw/day and above. ALP, acid
phosphatase and ASAT were statistically decreased in groups given
379, 253 or 169 mg/kg bw/day or above, respectively. Total protein
content of sera was statistically increased but still within
physiological limits. Liver triglycerides were statistically
increased at 113 mg/kg bw/day and above but serum triglyceride
levels were comparable to controls. Total serum cholesterol was
increased at 75 mg/kg bw/day and above but total liver cholesterol
was comparable to control values. Liver total lipid was similar to
controls. Aminopyrine demethylase and aniline hydroxylase activity
were both decreased at 253 mg/kg bw/day and above. The cytochrome
P-450 level remained unchanged (Szepvolgyi et al., 1989).
Dogs
Beagle dogs (6/sex/dose) received 0, 10, 100, 1000 or 5000 ppm
of 83.35% percent pure mancozeb in the diet adjusted to 100% of
active ingredient for 3 months.
Two males and one female were sacrificed in extremis in the
5000 ppm dose group as a result of compound-related anorexia and
malnutrition. Dose-related clinical signs of dehydration, thinness,
and pale mucous membranes were also noted in animals at this dose
level. Occasional instances of dehydration were seen in animals at
1000 ppm. Signs were considered secondary to anorexia and
malnutrition. Ophthalmoscopic examination did not reveal any
compound-related effects. Food consumption was decreased
approximately 10-20% at 1000 ppm and about 40% at 5000 ppm in both
sexes. Mean body weight and mean body-weight change were both
decreased at the high dose and at 1000 ppm. Decreases in
erythrocyte count, haemoglobin, and haematocrit were accompanied by
increases in mean corpuscular volume and mean corpuscular
haemoglobin. Values were statistically significant in both males
and females at 5000 ppm and females at 1000 ppm. T3 and T4 values
were decreased along with ALAT values at 5000 ppm in both sexes.
Total cholesterol was elevated in both sexes at 5000 ppm and in
females at 1000 ppm. Females at 5000 ppm also showed elevated total
bilirubin count at 5000 ppm.
At necropsy, enlarged and/or dark thyroid/parathyroids and
decreased thymus size were seen in the 1000 and 5000 ppm males and
females. A pale appearance of visceral organs was observed in the
three animals sacrificed early. Compound-related histomorphological
tissues alterations included thyroid follicular cell hyperplasia at
5000 ppm in both sexes, thymic cortical lymphoid depletion in the
1000 and 5000 ppm animals of both sexes, and hypoplastic changes in
the reproductive systems of males and females in the high-dose group
(i.e. aspermato-, hypospermato-, and hypogenesis of the testes and
hypogenesis of the ovaries), pallor of the zona fasciculata of the
adrenal gland in high-dose males and females and some sinusoidal
liver cell pigmentation in high-dose males and females. Urinalysis
indicated the presence of both parent compound and metabolite. ETU
was detected in the blood at a dose of 1000 ppm. No mancozeb was
detected in the thyroid, however ETU was detected in the thyroid of
both sexes at 100 ppm (in males 1.83 ppm and in females 0.72 ppm).
The NOAEL was 100 ppm, equal to 3.0 mg/kg bw/day, based on decreased
food consumption and body-weight gains and decreased erythrocyte
count, haematocrit, and haemoglobin at 1000 ppm (Cox, 1986).
Beagle dogs (4/sex/group) were given dietary concentrations of
0, 50, 200, 800 or 1600 ppm of mancozeb (84.5% pure) and adjusted to
100% active ingredient for 52 weeks.
Two high-dose males were sacrificed in extremis during weeks
10 and 11. One animal manifested haematuria and a hard distended
bladder prior to sacrifice. Necropsy revealed urethral calculi
lodged behind the os penis. Hydronephrosis with tubular dilation,
necrosis and congestion was noted in the kidneys. The lower urinary
tract manifested severe urethritis, prostatitis, cystitis and
ureteritis. Acute peritonitis was associated with these lesions.
The second male like the first showed a sharp drop in food
consumption and a decreased body-weight gain. A haematological
examination revealed the animal to be anaemic (decreased
haemoglobin, erythrocytes, packed cell volume and increased
reticulocytes). Haematological parameters, necropsy and
histopathological findings were consistent with a chronic
regenerative anaemia. Diffuse centrilobular necrosis with
extramedullary haemopoiesis was seen on histopathological
examination of the liver, while in the spleen and bone marrow
moderate to slight erythroid haemopoiesis with pigment was evident
and a notable reticulocytosis. All other animals survived, and
there were no apparent compound-related clinical signs or palpable
masses, nor any apparent neurological effects of treatment. Core
body temperatures were comparable between groups. With the
exception of the two animals that were terminated early there were
no consistent trends in food conversion efficiency or food
consumption. Body-weight gain for males fed 200 and 800 ppm
mancozeb showed statistically significant decreases, beginning at 24
and at 15 weeks, respectively. The two high-dose male survivors and
females showed body-weight gains comparable to controls.
Haemoglobin was significantly decreased at 800 and 1600 ppm in
females at 13 and 52 weeks along with packed cell volume at 13
weeks. Males manifested a statistically significant increase in
mean corpuscular volume at 1600 ppm at weeks 13, 26 and 52, as well
as at 800 pm at week 13. Serum cholesterol was significantly
increased in females at 1600 ppm at weeks 10, and 26. Serum
cholesterol values were dose-related in both sexes with cholesterol
values increasing at 200 ppm (7%-16% at weeks 10, 26 and 52) for
females and at 800 ppm for males.
T4 values were consistently lower than controls or pretest
values but were not statistically significant. T3 values were
comparable to controls. Urinalysis revealed no compound-related
effects. Absolute thyroid weight, and thyroid to body and brain
weight ratios were significantly increased in surviving males and
females at 1600 ppm. Absolute liver weight and liver to body-weight
ratio were increased in males but statistically significant only for
liver to brain weight ratio at 1600 ppm. Liver weight and liver
weight ratios were also increased in females but were not
statistically significant. The only apparent compound-related
effect observed was thyroid follicular distention observed in the 4
high-dose females and the two surviving high-dose males. The NOAEL
was 200 ppm, equal to 7.0 mg/kg of bw/day, based on decreases in
body-weight gain, increased cholesterol and decreased haemoglobin
and packed cell volume at 800 ppm (Shaw, 1990).
Beagle dogs (4/sex/group) were administered by gelatin capsule
0, 2.3, 23, or 113 mg/kg bw/day of mancozeb technical (88.6% pure).
The control group received gelatin capsule only. Compound was
administered once a day, seven days a week, for 52 weeks.
All animals receiving 113 mg/kg bw/day were sacrificed at 26
weeks with the exception of one male sacrificed at 13 weeks. At the
time of single sacrifice, food consumption was decreased 70% and
body weight depressed. Severe anaemia was evident. ALAT, ASAT,
urea, total bilirubin, total cholesterol were substantially
increased. T3 and T4 values were comparable to pre-test values.
Inorganic phosphorous was decreased 35%. Most organs were pale at
necropsy and histopathology was not conducted. Necropsy was not
conducted on animals terminated at 26 weeks. Faeces discoloration
(yellow/green) occurred in all groups but was most prevalent at the
high dose. Food consumption and body-weight gain were comparable
between control and low-dose groups (2.3 mg/kg bw/day) for both
sexes and for males at the mid- and high-doses. Females receiving
113 mg/kg bw/day manifested a 66% decrease in food consumption at 24
weeks and a 13% decrease at 52 weeks. Body-weight gain in females
at 52 weeks was 45% less than controls for animals receiving 23
mg/kg bw/day. At 24 weeks, haematology values for males were
comparable to controls at all doses. Females showed a frank anaemia
at 113 mg/kg bw/day and a dose-related decrease in RBCs. Blood
chemistry values for males showed dose-related trends for increased
ALP, total cholesterol, total plasma protein, phosphorous, and a
decreased trend for T4 with statistical significance attained at
113 mg/kg. Females showed decreasing trends for T3, T4, ASAT,
ALAT with an increasing trend in total cholesterol. ASAT and ALAT
values were statistically significant at 113 mg/kg bw/day. At 52
weeks, haematology, blood chemistry, urinalysis, organ weights and
histopathology were generally comparable to controls for both sexes
for animals receiving 2.3 and 23 mg/kg bw/day with the exception of
decreased T4 values in males. No dogs died at 2.3 and 23 mg/kg
bw/day. The NOAEL was 2.3 mg/kg bw/day, equal to 2.0 mg/kg bw/day
of active ingredient, based on decreased food consumption and
decreased body weight in females, and decreased T4 levels in males,
at 23 mg/kg bw/day (Broadmeadow, 1991a).
A second study in dogs of 52 weeks was conducted at a single
dose of 40 mg/kg bw/day as a result of the excessive toxicity and
termination of the high-dose experimental group (113 mg/kg bw/day)
in the preceding study. Protocols and methods were identical to the
first experiment. Clinical signs were limited in females. Two
females appeared thin looking during the course of the experiment
and one was reported as being hypothermic and of pale appearance.
No dogs died. Food consumption, body weight and body-weight gain
for males were comparable to controls for all time periods. Females
showed an immediate decrease in food consumption (20-26%) and a
statistically significant decrease in body weight and body-weight
gain throughout the experimental period. RBC count at 24 and 52
weeks for males and females was decreased (7-10%) but not
statistically significant. However, at 52 weeks MCV was
significantly increased in both sexes while MCHC was decreased
significantly in females. Other haematological parameters were
comparable to controls as were bone marrow findings. At 24 weeks ALP
and total cholesterol were substantially increased in males and
females whereas ALAT and ASAT were significantly decreased in
females. Inorganic phosphorous was significantly increased in
females. At 50 weeks, T3 and T4 values were statistically
decreased in both sexes while ALP levels were significantly
increased in females. ALAT values in females were significantly
decreased. Phosphorous levels were statistically raised in females.
Urinalysis showed a decrease in specific gravity for females
accompanied by increased urine volume. Absolute thyroid organ
weight was increased in both sexes and statistically significant in
males. Organ to body-weight ratios were comparable in males but
raised in females; however the values in females were suspect
because of severe loss of body weight. Necropsy revealed an
enlarged or swollen spleen in treated animals. Histopathology of
treated animals was generally not remarkable and comparable to
controls. However, an increased incidence of iron pigment
deposition in Kuffur cells of female livers and an increased
incidence of periacinar lipofuscinosis in males was reported in both
sexes (Broadmeadow, 1991b).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (60/sex/dose) were divided
into two experimental groups and given dietary concentrations of
mancozeb technical (88.6% pure) at 0 or 25 ppm, or 0, 100 or 1000
ppm for 78 weeks. Mancozeb was stable in the diet for at least 7
days and the diet was formulated weekly. Ten animals per sex per
dose were sacrificed at 52 weeks and all surviving animals
sacrificed at termination and necropsied. There was no compound-
related mortality or clinical signs. Body-weight gain in males was
decreased 6% at 52 weeks and 8% at 79 weeks at 1000 ppm. Values
were not, however, statistically significant. Body weight in
females at 100 ppm was decreased from weeks 1-24 and at 1000 ppm
from weeks 1-78. However, body-weight decreases in females at 100
ppm was not compound-related since there was no dose-response from
100 ppm to 1000 ppm. Body-weight gain in females was decreased 10%
at 52 weeks and 14% at 78 weeks at 1000 ppm. Body-weight gain
values at 52 and 78 weeks were not statistically decreased. Food
consumption and differential blood counts were comparable between
treated and control groups. There were no remarkable intergroup
differences for organ weights. The incidence of liver nodules in
males at 0, 100, and 1000 ppm were 4/50, 3/50 and 10/50,
respectively. The incidence of liver masses at 0, 100 and 1000 ppm
in males were 5/50, 5/50 and 9/50, respectively. Liver nodules and
liver masses were not significantly different from control values.
There were no intergroup differences in the incidence of liver
nodules or liver masses in females. There were no macroscopic or
microscopic findings that could be attributed to compound
administration. The incidence of male mice bearing liver tumours at
doses of 0, 100 and 1000 ppm was 10/50, 7/50 and 17/50,
respectively. There were no statistically significant differences or
trends. The number of male animals showing benign tumours was 8/50,
5/50 and 17/50, respectively, and the number showing hepatocellular
carcinomas was 2/50, 2/50 and 0/50. Males receiving doses of 0 or
25 ppm manifested malignant liver tumours in 3/50 and 4/50 animals,
respectively, with the total number of benign and malignant tumours
being 7/50 and 7/50 for both experimental groups. The NOAEL was 100
ppm, equal to 17 mg/kg bw/day, based on decreased body-weight gain
at 1000 ppm. There was no evidence of carcinogenicity (Everett et
al., 1992).
Groups of Charles River CD-1 mice (94/sex/group) received 0,
30, 100, or 1000 ppm of mancozeb (83% pure adjusted to 100% active
ingredient) for 78 weeks. Twenty-four animals/sex/group were
selected for an interim sacrifice at 12 months. Haematology was
conducted on 15 mice/sex/dose at 12 and 18 months. Thyroid function
assays were conducted on a minimum of eight animals/sex/dose after
12 and 18 months, with evaluations conducted on T4, T3 and TSH.
Necropsies were performed on all unscheduled deaths, on 24
animals/sex/group at interim (12-month sacrifice) and on all
surviving animals at terminal sacrifice.
There were no treatment-related increases in mortality or
clinical signs. Food consumption was similar among all groups. Body
weight for males and females of the 1000 ppm group were
statistically and consistently lower than controls through out the
78 weeks. Body-weight gains at 52 and 78 weeks for males and
females were decreased 18% and 13% and 15% and 9%, respectively.
Body weights and body-weight gains in other dose groups were similar
to controls values.
RBC counts were statistically significantly decreased in males
at 12 months at 1000 ppm. All other values for males were
comparable to controls. Females at 12 months and 1000 ppm showed
decreased RBC count, increased MCHC, and increased MCV. T3 and T4
changes were statistically significant only at the high dose tested
(1000 ppm) in both sexes. T3 and T4 levels were decreased in
females at 18 months (32% and 61% respectively). T4 levels in
females were also decreased at 12 months (76%). Males had a 56%
decrease at 12 months in T4 values and a 45% increase in T3 values
at 18 months. TSH levels were not statistically significantly
increased in either sex at any dose or time period.
Necropsy and gross examination of animals at the 12-month
interim and 18-month terminal necropsies revealed no consistent
gross lesions that could be attributed to an effect of the test
chemical. Diffuse discoloration of the lymph nodes was observed in
high-dose males (6/66) but the finding was not confirmed under
histopathological examination. There were no consistent differences
in mean organ weights, mean organ to body weight or organ to brain
weight ratios between control and compound-treated males or females
that could be attributed to the test article. Non-neoplastic
findings were comparable to controls. Hepatocellular adenomas and
carcinomas as well as pulmonary alveolar/bronchiolar adenomas and
carcinomas observed in both sexes were not statistically
significant. The NOAEL was 100 ppm, equal to 13 mg/kg bw/day in
males and 18 mg/kg bw/day in females, based on decreased body weight
and body-weight gain and decreased T3 and T4 values at 1000 ppm.
There was no evidence of carcinogenicity (Schellenberger, 1991).
Rats
Groups of Charles River Crl:CDBR rats (72/sex/dose) received 0,
20, 60, 125 or 750 ppm of mancozeb technical (83.8% pure) in the
diet for 2 years.
Mean body-weight gains in the high-dose males were
significantly lower than the control group in the first year
followed by lower but not statistically significant differences
during the second year (8.0%). Females receiving the high dose
showed a statistically significant decrease in body-weight gain at
90 days (16%) and one year (11%). Body-weight gains were comparable
to controls from 1-2 years. Food consumption was comparable for all
groups. Food efficiency was slightly but not statistically
decreased in high-dose males (5.5-7.5%) for various time intervals
and females at 90 days (10%). Erythrocytes, haemoglobin and
haematocrit were significantly decreased in male animals receiving
125 ppm at 18 months but not at other time periods. Haematological
findings were unremarkable in females. ALAT in males and
cholesterol levels in females were significantly increased at 750
ppm at 24 months. Males revealed decreased T3 values at 125 and
750 ppm at three months with decreased T4 values at 750 ppm at 6
and 18 months. TSH values were increased at 750 ppm at 12 and 18
months and at 125 ppm at 24 months. Females showed decreased T3
levels at 60, 125 and 750 ppm at 3 months. T4 values were
decreased at 750 ppm at 3, 18 and 24 months. TSH values were
increased at 750 ppm at 6 and 18 months. Urinalysis values were not
remarkable. Survival was comparable for all groups. After 24
months, absolute and relative (to body weight) thyroid/parathyroid
weights of the high-dose males and females were significantly
increased. The incidence of enlarged thyroids was also higher in
males and females at 750 ppm and the incidence of observed masses
was also higher in males. Follicular cell hypertrophy/hyperplasia of
the thyroid gland was significantly increased for males and females
receiving 750 ppm at the end of the first year.
A granular yellow-brown pigment was also present in rats fed
125 and 750 ppm at one and two years. Renal pigment remained in the
same magnitude as seen at 12 months. No attendant pathology was
evident. Bilateral retinopathy was also significant in both sexes
at the high dose at 2 years. At 2 years, in the high-dose group of
males and females, significant increases were recorded for thyroid
follicular cell hypertrophy/hyperplasia as well as nodular
hyperplasia. Thyroid follicular cell adenomas (20/61) and
carcinomas (14/61) were significant only in high-dose males.
Females showed increased incidences of thyroid follicular cell
adenomas (6/61) and carcinomas (4/61) but the increases were not
statistically significant when compared to control groups. The NOAEL
was 125 ppm, equal to 4.8 mg/kg bw/day, based on decreased body-
weight gain, decreased T3, T4 values, increased TSH values,
increased absolute and relative thyroid weight, thyroid follicular
cell hypertrophy, hyperplasia, and nodular hyperplasia, in both
sexes at 750 ppm. Carcinogenic effects were noted in both sexes in
the form of thyroid follicular cell adenomas and/or carcinomas but
only at the highest dose level (Stadler, 1990).
Sprague-Dawley (CD) rats (50 [main study] and 20 [satellite
study] animals/sex/dose) were given 0, 25, 100, or 400 ppm of
technical mancozeb in the diet (88.5% purity, adjusted to 100%
active ingredient) for 104 weeks. There were no compound-related
signs or mortality. Body-weight gain was significantly decreased in
males and females at the high dose tested for weeks 1-26 and 1-13,
respectively. Body weights were significantly decreased for males
from weeks 2-75 and for females from weeks 4-25. Food consumption
was comparable between all groups and food conversion ratios similar
for treated and control groups. There were no treatment-related
effects on haematological parameters. Reported changes were neither
dose nor time-dependent. T4 levels were significantly decreased in
males and females at 400 ppm at 26 and 52 weeks and in females alone
at 78 weeks at 100 and 400 ppm. However, the decrease at 100 ppm
was not consistent over time. T3 levels in males were decreased at
52 weeks but increased 43% at 104 weeks in the high-dose group. TSH
was increased by 86% at 78 weeks in females of the high-dose group.
The remaining statistical differences, particularly for
protein, calcium and other electrolytes, appeared to be unrelated to
treatment. Urinalysis findings were not considered to be compound-
related. Ophthalmoscopic changes were comparable between groups.
There were no compound-related organ weight changes. There was no
evidence of tumorigenicity. The incidence of thyroid follicular cell
adenomas in males was 6/50, 2/50, 2/50 and 6/50, for thyroid
follicular cell adenocarcinomas the frequency was 2/50, 0/50, 1/50,
and 3/50 for control and respective dose groups. Parafollicular
carcinomas in males was 1/50, 6/50,6/50 and 6/50. Anterior
pituitary adenomas in males occurred at a frequency of 18/50, 22/50,
28/50, and 27/50 whereas anterior pituitary adenocarcinomas were
observed at an incidence of 3/50, 2/50, 2/50 and 1/50 in controls
and respective dose groups. A statistical significance was not
achieved between groups, and values were comparable with the upper
incidence of the control range. Thyroid follicular cell adenomas and
adenocarcinomas were observed in females at frequencies of 0/50,
0/50, 2/50 and 2/50; and 0/50, 0/50, 0/50 and 1/50, respectively,
for controls and treated groups. Parafollicular malignant tumours
occurred at the same frequency (3/50) in all groups. Pituitary
adenomas in females (anterior pituitary) ranged from 28/50 to 35/50.
Pituitary adenocarcinomas ranged from 4/50 to 7/50. Non-neoplastic
findings in males were not significant for pituitary, testes,
kidneys, or other tissues with the exception of thyroid. There was a
minimal increase in the height of the follicular epithelium of the
thyroid (3/50, 1/50, 1/50, 8/50) and an increase in the number of
prominent microfollicles (0/50, 1/50, 0/50, 5/50) at the high dose.
Non-neoplastic findings for females were not significant for kidney,
pituitary or stomach. There was however a minimal increase in the
height of the follicular epithelium of the thyroid (1/50, 0/50,
1/50, 5/50) at 400 ppm. The NOAEL was 113 ppm, equal to 4.0 mg/kg
bw/day in males and 5.1 mg/kg bw/day in females, based on decreased
body-weight gain and body weight, an increase in the height of the
thyroid follicular epithelium, an increase in prominent
microfollicles, and a decrease in thyroxine levels at 450 ppm (Hooks
et al., 1992).
Reproduction studies
Rats
Sprague-Dawley Crl:CD(SD)BR rats (25/sex/dose) received 0, 25,
150, or 1100 ppm of 88.4% mancozeb technical in the diet. Compound
intake was not corrected to 100% of active ingredient in the diet.
Animals (F0 generation) were seven weeks old at the start of
treatment, acclimated to laboratory conditions and healthy. After a
premating period of 14 weeks, animals of the same dose levels were
mated for not longer than three weeks. Females were allowed to
litter and rear their offspring. Exposure to compound and diet was
continuous and ad libitum. Selected F1a pups were maintained for
14 weeks after weaning of all F1a offspring then mated for three
weeks, allowed to litter and rear their offspring (F2a generation).
There were no compound-related deaths and clinical signs were not
evident for either generation. Body weight and body-weight gain were
significantly depressed in males and females of the high-dose group
for both parental (F0, F1) generations during the 14-week pre-
treatment period. Males of the F1 generation also showed
significant decreases in body weight and body-weight gain at the
mid-dose. Body weight and body-weight gain for dams during
gestation of F0, and F1 parents, were significantly decreased at
1100 ppm and depressed at the mid-dose. Food consumption was
significantly decreased for both sexes during the premating period
of both generations at the high dose. Food consumption for F1
females was also significantly decreased at the high dose during the
periods of gestation and lactation.
Absolute organ weight at necropsy revealed statistically
significant increases for both parental sexes at the high dose.
Macroscopic examination of all tissues examined revealed no
remarkable findings. Histopathology, however, revealed thyroid
hyperplasia and hypertrophy in nearly all animals examined of F0
and F1 parents at the high dose. Thyroid follicular cell adenomas
were also found in five males of the F0 generation and in eleven
males of the F1 generation at the high dose. Indices for F1a pups
were comparable to controls. A slight delay in the opening of the
eye was however considered to be treatment-related at the high dose.
Necropsy findings were comparable between treated and control groups
for F1a and F2a litters. Viability, pups and litter weights were,
however, significantly decreased at the high dose on days 14-21.
The NOAEL in this study was 25 ppm, equal to 1.7 mg/kg bw/day, based
on decreased body weight at 150 ppm (Muller, 1992).
Charles River CRL:CDBR rats (25/sex/dose) received 0, 30, 120,
or 1200 ppm of mancozeb (84% pure) in the diet. Animals were placed
together for a period of 10 days to produce the F1a and F1b
generation. The presence of a vaginal plug was considered day zero
of gestation. On day 4, litters were culled to 5 animals/sex/dose.
Litters were weaned on day 21. One male and one female were then
selected from the F1a litter to serve as the parents for the F2
generation (F2a and F2b litters).
There were no treatment-related clinical signs or deaths in
either the F1 or F2 generation. Parental body weights for F1 and
F2 male and female rats were similar between control, 30 and 120
ppm groups throughout the 10-week treatment period prior to mating
or for F1 and F2 females during the gestation and lactation
periods. At 1200 ppm, mean body weight for male and female rats of
the F1 and F2 generation were significantly below control
throughout the pre-mating period, as well as for parental females
during gestation and lactation. Mean feed consumption of F1 and
F2 male and female rats were comparable between control, 30 and 120
ppm groups. At 1200 ppm, mean food consumption was decreased in F1
but not F2 male and female rats prior to mating. During gestation
and lactation F1 and F2 females had food consumption values
comparable to controls. Reproductive indices as measured by
fertility, gestation, viability and lactation were comparable to
controls for F1 and F2 adult and offspring at all dose levels.
Fetal body weight for F1a,b and F2a,b offspring were also comparable
to controls. There were no treatment-related effects on the
absolute and relative organ weights at 30 ppm for the F1 and F2
parents or for F1 parents at 120 ppm. F2 males showed a
statistically significant dose-related increase in relative liver
weight at 120 ppm without histopathological changes. At 1200 ppm
both F1 and F2 parents manifested significant increases in
relative liver weight and absolute and relative thyroid weights.
Relative kidney weight was increased in F1 and F2 females, but not
in males. There were no gross pathological changes considered to be
treatment-related among either F1 or F2 animals nor any which
could be substantiated by corresponding histopathology.
Treatment-related microscopic changes were observed in the
thyroid, kidney and pituitary of F1 and F2 animals. Changes
involving the follicular cells of the thyroid observed in the F1
generation were also observed in the F2 generation with generally
higher incidences or greater severity. All males of the F1 and F2
generation showed some degree of diffuse follicular cell hyperplasia
at 1200 ppm. Follicular cell adenomas and nodular/cystic follicular
cell hyperplasia were also observed at 1200 ppm. All or nearly all
females showed some degree of diffuse follicular cell hyperplasia at
1200 ppm in F1 and F2 generations. Nodular/cystic follicular cell
hyperplasia was also evident at 1200 ppm in the F2 generation.
Follicular cell adenomas were not observed in females. Brown
globular pigment was observed within the lumen of proximal tubules
in the kidneys at statistically significant incidences in both sexes
of both generations at 120 and 1200 ppm. However, no associated
pathology was observed. Hypertrophy and/or vacuolation of the
anterior pituitary was treatment-related only in males and only at
1200 ppm of the F1 and F2 generation based upon an increased
severity of response and not an increased incidence. Other
microscopic changes were not considered to be treatment-related. The
NOAEL was 120 ppm, equal to 7.0 mg/kg bw/day, based on increased
relative weights of the liver, kidney and thyroid, increased
absolute thyroid weight, decreased body weight and feed consumption
of females during gestation and lactation, and decreased pre-mating
body weight and feed consumption, at 1200 ppm (Solomon et al.,
1988).
Special study on neuropathology
Rats
Groups of Charles River Crl:CD(R) BR rats (10
animals/sex/dose) received 0, 20, 125, 750 or 5000 ppm of mancozeb
(79.3% pure and adjusted for purity) mixed in the diet for 90 days.
Two female satellite groups of 16 females each were also included
and received 5000 ppm for a two-week period only prior to sacrifice.
One male and 4 females died in the 5000 ppm group. These deaths
occurred between the second and fourth week of administration and
resulted in a cessation of test compound in females of the high dose
only and administration of control feed for the remainder of the
study. Onset of clinical signs in the second and third week of
administration consisted of generalized weakness, abnormal gait or
mobility, limited or no use of the rear legs. Loss of muscle mass
was also reported in males. However by day 60 some males in the
high-dose group appeared clinically normal and females showed
improvement after one week on control diets. Females of the
satellite group fed mancozeb for two weeks manifested limited rear
limb mobility. No clinical signs were observed below 5000 ppm.
Body weight in males was decreased 45% by day 90 in the high-
dose group. Other dose levels in males were comparable to controls.
Females receiving 5000 ppm showed initial weight loss which was
reversed after cessation of test compound and their placement on
control feed. Satellite females administered 5000 ppm of mancozeb
for two weeks showed minimal weight changes at 14 days. However, at
750 ppm and 90 days biologically significant decreases in body
weight (9%) and body-weight gain (17%) were noted. Body weights
were unaffected at lower doses. Food consumption in high-dose males
was decreased 40% compared to controls, but was similar to controls
at other dose levels. Females receiving 5000 ppm for two weeks had
decreased food consumption values ranging between 29% and 64%. In
other groups, female food consumption was similar to controls. Food
efficiency was decreased in all high-dose animals and at 750 ppm in
females (13%).
Neuropathology revealed effects in both sexes at 750 ppm and
5000 ppm. Pathology in males at 750 ppm and 5000 ppm was reported
as myelin phagocytosis, Schwann cell proliferation, myelin bubbles,
demyelination of nerve fibres associated with the posterior thigh
muscle and myelin ovoids in teased nerve fibres. Additional
observations at 5000 ppm consisted of intra-sheaths ellipsoids,
demyelination, thickening of the myelin sheath, neurofibrillary
degeneration and atrophy of the posterior thigh muscles. High-dose
females fed a recovery diet showed a thickening of the myelin
sheath, myelin bubbles, and ballooning of the myelin sheath.
Muscular atrophy was reported in one surviving female. There was
also an increased incidence of demyelination but a decrease in the
presence of myelin ovoids and debris when compared to males at this
dose level. Females fed 5000 ppm for two weeks and then sacrificed
showed myelin bubbles, sheath thickening, myelin phagocytosis and
Schwann cell proliferation. Muscle atrophy was present in 9/10
animals examined and demyelinated lengths with the presence of
myelin debris and ovoids were found upon examination of teased sural
nerve fibres. At 750 ppm in females, teased nerve fibres showed
some demyelination myelin ovoids and debris. No neuropathology was
conducted at 20 ppm dose level because of the absence of findings at
125 ppm. The NOAEL was 125 ppm, equal to 8.2 mg/kg bw/day in males
and 10.5 mg/kg bw/day in females, based on decreased body weight,
body-weight gain and food consumption in females and
neurohistopathological changes in both sexes at 750 ppm (Stadler,
1991).
Special studies on embryotoxicity/teratogenicity
Rats
Groups of mated female Crl:CD rats (27/group) were whole-body
exposed to 80% pure mancozeb dust by the inhalation route.
Administered doses were 0, 1, 17, or 55 mg/m3 for 6 hours/day on
days 6-15 of gestation. Particle size ranged from 1.4-6.4 microns.
Animals were observed for clinical signs during and after exposure
then sacrificed one day prior to natural delivery. Fetuses were
examined externally, viscerally and skeletally. No animals died.
Hind limb weakness and a slower righting reflex were observed in
6/27 high-dose dams. However, the signs disappeared during the
post-exposure observation period. High-dose animals also gained
significantly less weight than controls (40% less). There were no
differences between treated and control group as to malformations
observed. There was however an increased incidence in the number of
animals manifesting a wavy rib which was statistically significant
at the high dose. This variation was dose-related across treated
groups. There was no increase in soft tissue alterations.
Observations were typical of those commonly seen in this strain of
rat. Fetal body weights were comparable between groups (Lu &
Kennedy, 1986).
Mancozeb (83.0% active ingredient adjusted to 100% for dosing)
was administered by gavage in corn oil to groups of 26 primigravid
BLU(SD)BR rats on days 6-15 of gestation (day 0, day of
insemination) at doses of 0, 2, 8, 32, 128 or 512 mg/kg bw/day. ETU
was administered as a positive control at 50 mg/kg bw/day. All rats
were sacrificed on day 20 of gestation and caesarean sections
performed.
Food consumption and body weights were decreased at 128 mg/kg
bw/day and above in mancozeb treated dams. Body weights for all
other mancozeb-treated groups were comparable to the control group
as was the ETU-treated group. Litter data indicated significant
decreases in the average number of live fetuses, mean fetal weight
and mean gravid uteri and increased resorptions only at 512 mg/kg
bw/day for mancozeb-treated animals. Fetal weight was decreased in
ETU-treated animals, and one dam died on study. Adverse compound-
related effects were clearly manifested for mancozeb at 512 mg/kg
bw/day and ETU at 50 mg/kg bw/day for gross abnormalities (agnathia,
cleft palate, meningoencephalocele), soft tissue effects (dilated
ventricles, compressed spinal cord), and skeletal tissue (incomplete
ossification of the skull, clavicle, scapula). The effects observed
at 128 mg/kg bw/day were not biologically or statistically
significant. The NOAEL for maternal toxicity was 32 mg/kg bw/day
based on decreased body weight and decreased food consumption at 128
mg/kg bw/day. The NOAEL for teratogenic effects was 128 mg/kg bw/day
based, in part, on the presence of agnathia, cleft palate,
meningoencephalocele, dilated ventricles and incomplete ossification
of the skull at 512 mg/kg bw/day (Gallo et al., 1980).
Female Sprague-Dawley rats (25/dose/group) received 0, 10, 60,
or 360 mg/kg bw/day of mancozeb (88.6% pure) in methyl cellulose or
methyl cellulose alone (control group). Animals were dosed daily by
the oral route (gavage) from day 6 to day 15 (inclusive) of
gestation. Dams were observed on predetermined regular schedules
for clinical signs, mortality body weight, and food consumption. On
day 20 of gestation females were sacrificed with carbon dioxide and
uterine contents examined.
There were no readily apparent or statistically significant
differences between control values and values obtained in treated
groups for the parameters examined at either 10 or 60 mg/kg bw/day.
One dam was sacrificed in a moribund state at 360 mg/kg bw/day. Four
females exhibited a "reeling gait" followed by slight paralysis in
three of the dams. Body weights were decreased during the treatment
period (days 6-15) and days 16 through 20. Statistical significance
was attained on days 16 and 20. Food consumption was significantly
decreased from days 6-15 inclusive. A slight dose-related reduction
in the degree of ossification of the intraparietal bone was
statistically significant. The Incidence for this effect was 75, 80,
87 and 90% for control to high dose. The historical mean was 25% and
the range 4.90-91.0%. A marginal increase in the size of the
anterior fontanelle (12%) was also observed. The historical mean for
this effect was 2.6% with a range of 0-24%. Incomplete ossification
of the thoracic vertebrae centra was also statistically significant
at the high dose tested. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 60 mg/kg bw/day. Maternal toxicity at 360
mg/kg bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryo/fetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra (Tesh et al., 1988).
Rabbits
Groups of New Zeeland white rabbits (18 females/dose) were
treated by oral intubation with doses of 0, 5, 30 or 55 mg/kg bw/day
of Pencozeb Technical (88.4% pure) in methyl cellulose or methyl
cellulose, on days 6 through 18 of gestation. Animals were
sacrificed on day 28 post-coitum. There were no compound-related
effects at these dose levels either in-life or during examination
post-sacrifice. A second experiment was therefore conducted at a
single high dose of 100 mg/kg bw/day with a concurrent control group
following the general procedures and examinations of the prior
experiment. No treatment-related clinical effects or treatment-
related necropsy findings were detected. Two dams in the control
group and five in the 100 mg/kg bw/day dose group aborted. A clear
and substantial body-weight loss was observed in does at 100 mg/kg
bw/day for days 6-9 post-coitum (i.e. days 1-3 of compound
administration). Body-weight gain during days 9-15 was also
substantially depressed compared to controls. Food consumption was
also markedly decreased between days 6-19. A slight post-
implantation loss (5%) was also observed and may have been compound-
related. No compound-related fetal effects were observed. The NOAEL
for maternal effects was 55 mg/kg bw/day and greater than 100 mg/kg
bw/day for embryo/fetotoxic effects. An increase in abortion, body-
weight loss, suppressed body-weight gain and decreased food
consumption were observed at 100 mg/kg bw/day (Muller, 1991).
New Zeeland white female rabbits (20 animals/dose/group)
received either 0, 10, 30, or 80 mg/kg of bw/day of mancozeb (83.0%
pure) in methyl cellulose or methyl cellulose alone (control group)
by oral gavage on days 7-19 of gestation. No treatment-related
deaths occurred in control or in the 10 and 30 mg/kg bw/day dose
groups. One animal died in the 30 mg/kg bw/day group but death was
attributed to mis-dosing. Two does were sacrificed in a moribund
condition at the high dose and the deaths considered treatment-
related (one of these two does was pregnant and did not abort).
Clinical signs were comparable between controls and animals
receiving 10 and 30 mg/kg bw/day. Animals receiving 80 mg/kg bw/day
manifested significant increases in clinical signs. Alopecia,
anorexia, ataxia, scant faeces, and abortions were all observed at
the high dose tested. Body-weight gain and food consumption were not
significantly different between controls and groups receiving 10 or
30 mg/kg bw/day. At 80 mg/kg bw/day body-weight and food consumption
were significantly decreased in does that aborted and those
sacrificed moribund. Does producing at least one viable fetus had
body-weight gains and food consumption values similar to controls.
No treatment-related changes were evident in the incidence of
gross postmortem findings between does in the control and treated
group. Reproductive parameters between controls and does receiving
10 or 30 mg/kg bw/day were comparable as measured by the number of
abortions, litters produced, and the mean number per litter of
corpora lutea, implantations, resorptions, dead and live fetuses or
sex ratio. At 80 mg/kg bw/day a significant increase in does
aborting (5/15) was reported with a corresponding decrease in the
number of litters produced. All other parameters were comparable to
control group. Mean fetal body weight was similar between the
control and treated groups. There were no significant increases in
the types or incidence of malformations or developmental variations
between control or treated groups. The NOAEL for maternal toxicity
was 30 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was greater
than 80 mg/kg bw/day. Maternal toxicity at 80 mg/kg bw/day was
based on an increase in aborted fetuses, decreased number of litters
produced, decreased body-weight gain and food consumption, an
increase in clinical signs and death (Solomon & Lutz, 1987).
Special study on mancozeb stability in DMSO
14C-mancozeb (Dithane M-45; 80% wettable powder; 88% radio
purity) was suspended in DMSO (99% pure) at a nominal concentration
of 100 ppm and sampled and analyzed for mancozeb at the following
times: 0, 15, 30, 60, 90, 120, 150, 180 and 210 minutes. Maximum
concentration of mancozeb occurred at approximately 15 minutes.
Immediately after adding DMSO to mancozeb (zero time) there was
evidence of significant decomposition with continued rapid
degradation to three compounds, two of which were impurities in the
starting material. The majority of the mancozeb was degraded within
60 minutes and after 4 hours an average of 9% of the mancozeb
remained. The half-life of mancozeb in DMSO was calculated to be 37
minutes (Schweitzer, 1990).
Special studies on genotoxicity
The results of genotoxicity assays are given in Table 2.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
Special studies on irritation and sensitization
Guinea-pigs
Mancozeb has been demonstrated to be a strong sensitizer in the
Hartley strain female guinea-pig by the Guinea-Pig Maximization
Test. With induction concentrations of 5% (intradermal) and 25%
(topical) and challenge concentrations of 2% and 0.5% (topical) all
of the mancozeb-treated females (10/group) responded at both 24 and
48 hours. In the same studies, cross sensitization responses were
also seen with zineb, maneb and mancozeb. A dose-response
relationship between the incidence of skin sensitization and
induction and challenge concentration was also seen with maneb. The
purity was not stated for any of the test materials (Matsushita et
al., 1976, 1977).
Young adult Hartley guinea-pigs were divided into three
experimental groups and tested for delayed contact hypersensitivity
using a modified Buehler procedure. Animals received three 6-hour
induction doses (1 dose/week, within 3 weeks) of either 0.4 ml of
50% (w/v) mancozeb (83% pure) in distilled water, 0.1% (w/v) 1-
chloro-2,4-dinitrobenzene (DNCB) in 80% (v/v) aqueous ethanol
(positive control), or received no induction doses (control group)
but were otherwise treated similarly. All groups were challenged 12
days after the last induction dose/treatment. The sham treated
control group was challenged with test compound and DNCB on each
half of the back. Erythema and edema reactions were scored 24 and
48 hours after challenge using the method of Draize. All groups
were rechallenged 7 days after the primary challenge and a separate
additional control group was challenged as previously described. No
erythema or edema was observed in sham treated controls. Positive
controls showed positive response at 24 and 48 hours. Only one of
Table 2. Results of genotoxicity assays on mancozeb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 2.5-250 µg/plate 88% active Negative Chism, 1984a
reversion assay TA1535, TA1537, (± rat S9); ingredient (a.i.)
TA98, TA100 in distilled water
S. typhimurium 2.5-250 µg/plate (± rat S9); 88% a.i. Negative Chism, 1984b
TA1535, TA1537, in distilled water
TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.5-45 µg/ml; 88% a.i. Negative Foxall & Byers,
mutation assay (CHO)/hprt in distilled water 1984
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethal Muller-5 stock food medium Krishnamurthy, 1980
assay
Autosomal D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethals Cy/B1 L2 stock food medium Krishnamurthy, 1980
1.D. Yeast and Other Fungal Assays
Point mutation A. nidulans 0.125-12 µg/ml; Not specified Positive Martinez-Rossi &
induction strains biA1 in distilled water; Azevedo, 1987
methG1 and 118 no activation used
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vitro Chromosomal Alterations in Mammalian Cells
In vitro Cultured human 1.40 µg/ml; Not specified Positive Georgian et al.,
chromosomal lymphocytes in propylene glycol; 1983
aberrations no activation used
2.B. In Vivo Chromosomal Alterations
Bone marrow Male non-inbred 10-1000 mg/kg; Not specified Negative2 Kurinnyi et al.,
cytogenetics white mouse in milk suspension 1982
or aqueous emulsion?
Wistar rat single i.p. 2.5-10 mg/kg; Not specified Positive Georgian et al.,
in propylene glycol 1983
Wistar rat 1.7 mg/kg bw/day for Not specified Positive Georgian et al.,
280 days; mixed in feed 1983
Male Fischer 344 rat 4.4 g a.i./kg/day for 1 88% a.i. Negative Sames et al., 1983
or 5 days; in corn oil
Male albino mouse 30-300 mg/kg; Not specified Positive Gautam & Kapoor,
(Lacca strain) in distilled water? 1991
Lymphocyte Female Wistar rat 3-30 mg/kg; in saline Not specified Positive Newton, 1975
cytogenetics
Micronucleus assay Male and female 10 000 mg/kg; 88.2% Negative Allen et al., 1987
CD-1 mouse in aqueous methyl-cellulose
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2.C. Plant Tests
Chromosomal Allium cepa 500-1000 ppm spray Not specified Positive Mann, 1977
alterations peduncles
Hordeum vulgare 100-1500 ppm; Not specified Positive Murty et al., 1983
germinating seeds in phosphate buffer
(with trace DMSO?)
Allium cepa 4-500 ppm Not specified Positive Badr, 1988
root tips
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro Primary rat 0.25-10 µg/ml; 88% a.i. Suggestive Byers, 1985
unscheduled DNA hepatocytes from male in culture medium positive;
synthesis (UDS) Fischer 344 rat suggests repeat
Primary rat 0.1-10 µg/ml; 82.4% Negative O'Neill & Frank,
hepatocytes from male in culture medium (also for S 1988
Fischer 344 rat phase induction)
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 5-20 µg/ml; Not specified Positive without Ivett, 1985
SCE assays (CHO) cells in culture medium activation
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell C3H/10T 1/2 cells 0.05-0.5 µg/ml; in water 88% a.i. Negative McGlynn-Kreft &
transformation McCarthy, 1984
C3H/10T 1/2 cells 0.1 µg/ml as "promoter"; 88% a.i. Negative McLeod & Doolittle,
with "promotion" in water 1985
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report.
2 The authors judged this effect to be positive, but significance was restricted to one dose and there was no dose-related response.
twenty animals given mancozeb (1/10 females) showed slight erythema
at 48 hours. After rechallenge the original female respondent was
negative but a second female (1/10 females) showed a slight erythema
at 24 hours but not at 48 hours (Trutter, 1988a).
Rabbits
Nine rabbits of unknown strain and sex were administered 100 mg
of ground mancozeb technical (> 80% pure) into the conjunctival sac
of one eye. The treated eyes of 3 rabbits were washed with water
approximately 20-30 seconds after dosing. Animals were observed for
eye irritation at 4, 24, 48, 72 and 96 hours and on days 7, 14 and
22. Substantial irritation was evident in unwashed eyes involving
the cornea, iris and conjunctiva. Effects were reversible after 14
days in unwashed eyes and after 72 hours in washed eyes.
A second study conducted in female New Zeeland white rabbits
using the same protocol produced responses of lesser consequence and
little or no involvement of the cornea or iris. Effects in unwashed
eyes were reversible after 7 days and after 24 hours in 2 of 3
rabbits with washed eyes. However conjunctival irritation persisted
in one rabbit through 14 days and disappeared at 21 days. The
compound was considered a substantial irritant based on the duration
of irritation (DeCrescente & Chan, 1982).
Six rabbits of unknown sex and strain were administered 500 mg
of ground mancozeb technical (> 80% pure), prepared as a paste in
saline, to intact and abraded skin. The test material was held
under an impervious patch in continuous 24-hour contact with closely
clipped skin. Erythema and edema were scored at 24 and 72 hours and
7 days. The only effect observed was erythema on abraded skin. The
primary irritation score was 0.5. The compound was considered a
slight irritant (DeCrescente & Parsons, 1980).
Observations in humans
A sample population of 121 Korean orange growers was tested for
hypersensitivity to the fungicide Dithane M-45 (mancozeb). A maximum
non-irritating concentration of 10% Dithane M-45 mixed with vaseline
was previously determined on 201 Korean medical students by patch
test. Results were read 48 hours post-administration. Application
of the patch test to orange growers was read 72 hours post-
administration according to the standards of the North American
Dermatitis Research group. Dithane tested positive in 4/121
subjects (3.3%) (Lee et al., 1981).
A 37-year old male working as a florist developed erythematous
vesicular eruption on the palms of his hands resembling dyshidrotic
eczema. Symptoms worsened and spread to other body areas (face,
neck, legs) during periods of heavy work, and diminished during
holidays. Patch tests with the mancozeb products Diagent and Firma
using Finn Chambers on Scanpor Tape read at 20 minutes, 2, 3, and 4
days resulted in a strong positive reaction on day two. A weak
positive reaction to zineb was also observed. Total serum IgE was
approximately five times the normal value. Other serum
immunoglobulins, G, A, and M were normal (Crippa et al., 1990).
A 61-year old vineyard worker developed a rash on the left
forearm as well as inflammation of the eyelids after planting vine
seedlings. This was the third episode under similar circumstances.
Patch tests on two previous occasions were negative. Patch tested
with 0.002% solution of both zineb and mancozeb (1% of normal use
concentration) resulted in positive responses. Retesting a week
later with the bark of a vine stick previously treated with mancozeb
produced a strong positive reaction. It was also reported that two
other male farm workers developed papulovesicular sheeted dermatitis
of the face, hands and chest after planting mancozeb-treated
potatoes. Patch tests with 0.1% solution of mancozeb were positive
in both. Patch tests conducted on the vineyard worker with
macerated vine bark were positive with aqueous but negative with
alcoholic solution (Kleibl & Rakcova, 1980).
One-hundred-fifty-three men currently or previously exposed to
dithane for many years at a manufacturing site were compared for
thyroid function to 153 men not exposed to Dithane, its products or
ETU who also worked at the same plant. Workers and controls were
carefully matched with respect to age, race, length of employment
and type of employment. Informed consent was obtained from all
participants. It was reported that at least one abnormality was
found in 25 of the 306 men (8.2%). The findings included
abnormalities on thyroid palpitation (4.3%), serologic autoimmune
thyroiditis (4.3%), subclinical hypothyroidism (1.3%), Graves'
disease (0.3%) and miscellaneous hormonal abnormalities (1.6%).
Urinary excretion of ETU in a subgroup of 42 workers currently
exposed to Dithane was 0.02 ppm compared to 0.01 ppm in the control
group (41 men), most of whom had undetectable values. The authors
concluded that exposure to Dithane manufacture was not associated
with an increased prevalence of thyroid abnormalities and that
thyroid abnormalities were common in iodine-replete adult American
men affecting 8.2% of the population studied (Charkes et al.,
1985).
A prevalence survey of adverse reproductive outcomes was
carried out in a population of 8867 persons (2951 men and 5916
women) who had been working in the floriculture industry in the
Bogota area of Columbia for at least 6 months. These workers from
58 companies were exposed to 127 different types of pesticides. The
prevalence rates for abortion, prematurity, still births, and
malformations were estimated for pregnancies occurring among the
female workers and the wives of the male workers before and after
working in floriculture and these rates were related to various
degrees of exposure. A moderate increase in the prevalence of
abortion, prematurity and congenital malformations was detected for
pregnancies occurring after the start of work in floriculture.
However, it could not be determined whether the increase in the
prevalence of the parameters measured was real. All rates, except
those for still births were higher for pregnancies occurring after
exposure to pesticides among both the female workers and the wives
of the male workers and thus gave significantly increased odds
ratios (chi-square) for abortions, premature births and malformed
babies. However, the high odds ratio observed for induced abortions
was considered by the authors surprising and cast doubt on the
reliability of the association between the observed increased risks
and the exposure to pesticides. It was also noted that of the 10
most used pesticides in floriculture, which accounted for 67% of the
total pesticides used in this industry, mancozeb comprised 7% of the
total. However, the article addressed pesticides in general and no
one chemical was singled out for adverse reactions. The authors
recognized that multiple exposure not only posed the problem of
identifying the toxic chemical responsible in causing any adverse
effect but also the problem of interaction between chemicals in the
expression of toxic effects (Restrepo et al., 1990).
COMMENTS
In pharmacokinetic studies conducted in male and female mice,
orally administered 14C-labelled mancozeb was rapidly absorbed,
peaking in whole blood between 1 and 2 hours, extensively
metabolized, and rapidly excreted (90%) within 24 hours. ETU was
the major metabolite.
Rats given single oral doses of 14C-labelled mancozeb absorbed
about 50% of the dose within 3-6 hours. Most of the dose was
excreted in 24 hours with half eliminated in the urine and half in
the faeces. Less than 4% was found in the tissues, with the thyroid
containing the highest residue level. Most of the 14C dose in
faeces was unabsorbed, since only 2-8% of the dose was found in
bile. ETU was the major metabolite. The half-life of ETU
elimination was 4-5 hours. The estimated bioavailability of ETU in
rats was about 6.8% on a weight/weight basis and 20% on a mole/mole
basis.
The acute oral, dermal and inhalation toxicity of mancozeb
technical is low. WHO has classified mancozeb as unlikely to
present acute hazard in normal use.
In a 13-week study in rats, mancozeb was administered in
dietary concentrations of 0, 30, 60, 125, 250 or 1000 ppm. The
NOAEL was 125 ppm (equal to 7.4 mg/kg bw/day) based on increased
serum TSH and decreased T4 values at the next higher dose.
Dogs administered 0, 10, 100, 1000 or 5000 ppm of mancozeb in
the diet for three months demonstrated a NOAEL of 100 ppm, equal to
3.0 mg/kg bw/day. At the next higher dose, decreased body-weight
gains and decreased erythrocyte count, haematocrit and haemoglobin
were observed.
In a 52-week study in dogs, mancozeb was administered in the
diet at concentrations of 0, 50, 200, 800 or 1600 ppm. The NOAEL
was 200 ppm, equal to 7.0 mg/kg bw/day, based on decreases in body-
weight gain, increased cholesterol and decreased haemoglobin and
packed cell volume at 800 ppm.
The NOAEL for dogs given mancozeb technical for 52 weeks, 7
days a week, by gelatin capsule was 2.3 mg/kg bw/day based on
decreased body weight, food consumption and decreased thyroxine
levels at 23 mg/kg bw/day.
In a 78-week carcinogenicity study in mice at dietary
concentrations of 0, 25, 100 or 1000 ppm, there was no evidence of
carcinogenicity. The NOAEL was 100 ppm, equal to 17 mg/kg bw/day,
based on decreased body-weight gain at 1000 ppm.
In a second 78-week carcinogenicity study in mice at dietary
concentrations of 0, 30, 100 or 1000 ppm in the diet, there was no
evidence of carcinogenicity. The NOAEL was 100 ppm, equal to 13
mg/kg bw/day, based on decreased body weight and decreased T3 and
T4 values at 1000 ppm.
The overall NOAEL in the two 78-week studies in mice was 17
mg/kg bw/day.
In a two-year toxicity/carcinogenicity feeding study in rats at
dietary concentrations of 0, 20, 60, 125 or 750 ppm, the NOAEL was
125 ppm (equal to 4.8 mg/kg bw/day) based on decreased body-weight
gain, decreased T3 and T4 values, increased TSH values, increased
absolute and relative thyroid weight, thyroid follicular cell
hypertrophy, hyperplasia, and nodular hyperplasia at 750 ppm.
Tumorigenic effects were noted in both sexes in the form of thyroid
follicular cell adenomas and/or carcinomas at the highest dose
level.
Mancozeb technical when administered in the diet to rats for
two years at dose levels of 0, 28, 113 or 454 ppm was not
tumorigenic. The NOAEL was 113 ppm (equal to 4.0 mg/kg bw/day)
based on decreased body-weight gain, decreased thyroxine levels and
an increase in the height of the thyroid follicular epithelium and
an increase in prominent microfollicles at 450 ppm.
The overall NOAEL in the two 2-year studies in rats was 4.8
mg/kg bw/day.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 25, 150 or 1100 ppm, the NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on decreased body weight at 150
ppm.
In a second two-generation reproduction study in rats at
dietary concentrations of 0, 30, 120 or 1200 ppm, the NOAEL was 120
ppm, equal to 7.0 mg/kg bw/day based on microscopic changes in the
thyroid, kidney and pituitary, increased relative weights of the
liver, kidney and thyroid, increased absolute thyroid weight,
decreased gestation and lactation body weight and feed consumption,
decreased pre-mating body weight and feed consumption at 1200 ppm.
The overall NOAEL in both reproduction studies was 7.0 mg/kg
bw/day.
In a 90-day (neuropathology) study conducted in rats at dietary
concentrations of 0, 20, 125, 750 or 5000 ppm, the NOAEL was 125
ppm, equal to 8.2 mg/kg bw/day, based on decreased food consumption
and neuro-histopathological changes at 750 ppm.
An oral teratogenicity study in rats at dose levels of 0, 2, 8,
32, 128 or 512 mg/kg bw/day produced no maternal effects at 32 mg/kg
bw/day (NOAEL) and no teratogenic effects at 128 mg/kg bw/day
(NOAEL). Maternal effects in the form of decreased body-weight gain
and decreased food consumption were seen at 128 mg/kg bw/day.
Teratogenic effects were seen at 512 mg/kg bw/day based, in part, on
the presence of agnathia, cleft palate, meningoencephalocele and
dilated brain ventricles.
A second oral teratogenicity study in rats at dose levels of 0,
10, 60 or 360 mg/kg bw/day showed no maternal or embryo/fetotoxic
effects at 60 mg/kg bw/day (NOAEL). Maternal toxicity at 360 mg/kg
bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryofetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra.
The NOAEL in an oral teratogenicity study in rabbits given 0,
5, 30, 55, or 100 mg/kg bw/day was 55 mg/kg bw/day for maternal
effects and greater than 100 mg/kg bw/day for embryo/fetotoxic
effects. An increase in abortions, body-weight loss, and decreased
food consumption were observed at 100 mg/kg bw/day.
The NOAEL in an oral teratogenicity study in rabbits given 0,
10, 30 or 80 mg/kg bw/day was 30 mg/kg bw/day for maternal toxicity.
The NOAEL for embryo/fetotoxic effects was greater than 80 mg/kg
bw/day. Maternal toxicity at 80 mg/kg bw/day was based on an
increase in aborted fetuses, decreased number of litters produced,
decreased body-weight gain and food consumption, an increase in
clinical signs and death.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
The data on mancozeb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 4.8 mg/kg bw/day for thyroid effects in rats
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for mancozeb, alone or in combination
with maneb, metiram, and/or zineb, because of the similarity of the
chemical structure of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differentiated using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet, equal to 17 mg/kg bw/day
(78-week studies)
Rat: 125 ppm in the diet, equal to 4.8 mg/kg bw/day
(two-year studies)
120 ppm in the diet, equal to 7.0 mg/kg bw/day
(reproduction studies)
125 ppm in the diet, equal to 8.2 mg/kg bw/day
(90-day neuropathology study)
Dog: 200 ppm in the diet, equal to 7.0 mg/kg bw/day
(52-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with maneb, metiram, and zineb)
Studies which will provide valuable information in the continued
evaluation of the compound
Clarification of the genotoxic potential of mancozeb.
Observations in humans.
REFERENCES
Allen, J.A., Proudlock, R.J. & Pugh, L.C. (1987). Micronucleus test
on mancozeb technical. Unpublished report No. PWT 39/86637 from
Huntingdon Research Centre Ltd., Huntingdon, Cambridgeshire,
England. Submitted to WHO by Elf Atochem, Philadelphia,
Pennsylvania, USA (Confidential data of Elf Atochem).
Badr, A. (1988). Cytogenetic activities of some fungicides.
Cytologia, 53: 635-640.
Braverman, L.E., Lipworth, L. & Charles, D. (1978). A health survey
of workers involved in the manufacture and packaging of dithane
fungicide with special reference to thyroid function. Unpublished
report dated August 2, 1978 from the University of Massachusetts
Medical School, Worcester, Massachusetts, USA. Submitted to WHO by
Rohm and Haas Company, Spring House, Pennsylvania, USA.
Broadmeadow, A. (1991a). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
89/PTC004/0015 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Broadmeadow, A. (1991b). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
90/PTC029/0197 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Byers, M.J. (1985). Dithane M-45 in vitro unscheduled DNA
synthesis assay. Unpublished report No. 84R-280 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Cameron, B.D., Clydesdale, K. & Speirs, G.C. (1990). The disposition
of 14C mancozeb in the mouse. Unpublished report No. 4909 from
Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA (Confidential
data of Elf Atochem).
Charkes, N.D., Braverman, L.E., Penko, K.F., Gowers, D.S., Gordon,
C.F., Lipworth, L, Malmud, L.S. (1985). Thyroid function in male
workers manufacturing dithane, an agricultural fungicide, and in men
not exposed to dithane. Frontiers in Thyroidology, 2: 933-936.
Chism, E. (1984a). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced Fischer 344 rats. Unpublished
report No. 84R-059 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Chism, E. (1984b). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced B6C3F1 mice. Unpublished
report No. 84R-0060 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Cox, R.H. (1986). Three month dietary toxicity study in dogs with
mancozeb. Unpublished report No. HLA 417-416 from Hazleton Labs.,
Vienna, Virginia. USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA as Rohm and Haas Report No. 86RC-7.
Crippa, M., Misquiih, L., Lunati, A. & Pasollini, G. (1990).
Dyshidrotic eczema and sensitization to dithiocarbamates in a
florist. Contact Dermatitis, 23: 203.
DeCrescente, M.E. & Chan, P.K. (1982). Dithane M-45: rabbit eye
irritation report. Unpublished report Nos. 81R-197, 81R-176 and 82R-
10 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeCrescente, M.E. & Parsons, R.D. (1980). Dithane M-45: Acute oral
and dermal LD50 and skin and eye irritation. Unpublished report No.
79R-180, from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeGroot, A.P. (1974). Determination of acute ip toxicity of Dithane
M-45 in rats. Unpublished report from Central Institute for
Nutrition and Food Research TNO the Netherlands (Rohm and Haas
report No. 74RC-1011). Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
DiDonato, L.J., Cruzan, G. & O'Hara, G.P. (1985). Dithane M-45/ETU
four week range finding mouse study. Unpublished report No. 80R-123
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DiDonato, L.J. & Longacre, S.L. (1986). Mancozeb pharmacokinetic
study in rats. Unpublished report No. 85R-123 (Supplement/Appendix
to Nelson, S.S. [1986] report No. 31H-86-02) from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978). A study of the uptake and elimination of
14C activity after the oral ingestion of 14C-labelled
ethylenethiourea (ETU) and mancozeb in the rhesus monkey.
Unpublished final (revised) report from Battelle Labs, Columbus,
Ohio, USA. dated July 31, 1978. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA. as Rohm and Haas report
No. 78RC-1028.
Everett, D.J., Atkinson, C., Perry, C.J., Strutt, A., Millar, P.,
Hudson, P. (1992). Mancozeb 78-week dietary carcinogenicity study in
mice with 52 week interim kill. Unpublished report No. 7561 from
Inveresk Research International, Tranent, Scotland. Submitted to WHO
by Elf Atochem, Philadelphia, Pennsylvania, USA. (Confidential data
of Elf Atochem).
Foxall, S. & Byers, M.J. (1984). Dithane M-45 CHO/HGPRT gene
mutation assay. Unpublished report No. 84R-207 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Gallo, M.A., Kam, C. & Stevens, D.R. (1980). Teratologic evaluation
of dithane M-45 in the albino rat. Unpublished report No. 10065-009
from Booz, Allen, and Hamilton, Inc., Florham Park, New Jersey, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA as Rohm and Haas report No. 81RC-75.
Gautam, D.C. & Kapoor, L. (1991). Genotoxic effects of Dithane M-45
on the bone marrow cells of mice in vivo. Experimentia, 47(3):
280-2.
Georgian, L., Moraru, J., Draghicescu, T., Dinu, I. & Ghizela, G.
(1983). Cytogenetic effects of alachlor and mancozeb. Mutation Res.
116: 341-348.
Goldman, P., Bernacki, H.J. & Quinn, D.L. (1986). Mancozeb: three
month dietary toxicity study in rats. Unpublished report No. 85R-167
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1980). Acute inhalation toxicity of
dithane M-45 (TD-79-224) in rats. Unpublished report No. 79R-210
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1982). Dithane M-45: Acute inhalation
toxicology study in rats. Unpublished report No. 81R-171 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Hagan, J.V., Fisher, J.R. & Baldwin, R.C. (1986). Mancozeb:
subchronic inhalation toxicity study in rats. Unpublished report No.
86R-3 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1986). Mancozeb: two week range finding
inhalation toxicity study in rats. Unpublished report No. 85R-190
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Haines, L.D. (1980). Dithane M-45 percutaneous absorbtion in rats.
Unpublished technical report No. 34F-80-9 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 80R-1006.
Hooks, W.N., Offer, J.M., Hadley, J.C., Gibson, W.A., Gopinath, C. &
Dawe, I.S. (1992). Mancozeb technical: potential tumorigenic and
toxic effects in prolonged dietary administration to rats.
Unpublished report No. PWT 29/89669 from Huntingdon Research Centre
Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by Elf
Atochem, Philadelphia, Pennsylvania, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of Dithane M-45 in an
in vitro sister chromatid exchange assay in the Chinese hamster
ovary (CHO) cells. Unpublished reported dated March 1985 from Litton
Bionetics, Inc., Kensington, Maryland, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 84RC-60.
Kleibl, K. & Rakcova, M. (1980). Cutaneous allergic reactions to
dithiocarbamates. Contact Dermatitis, 6(5): 348-349.
Kocialski, A. (1989). Establishment of an in vitro metabolic
conversion factor of 7.5% of all EBDCs when converting EBDCs to
ethylenethiourea in vivo and recalculations of the previously
considered 20% in vivo conversion exposure factor for EBDCs to
ETU. Memorandum to the Caswell file, Health Effects Division, Office
of Pesticide Programs, United States Environmental Protection
Agency.
Kurinnyi, A., Pilinskaya, M., German, J. & L'vova, T. (1982).
Implementation of a program of cytogenetic study of pesticides:
preliminary evaluation of cytogenetic activity and potential
mutagenic hazard of 24 pesticides. Cytol. Genet. 16: 50-53.
Lee, Y.S., Cinn, Y.W., Chang, W.H. & Kim, J.S. (1981). A study on
hypersensitivity of Korean farmers to various agrochemicals.
Determination of concentration for patch tests of fruit-tree
agrochemicals and hypersensitivity of orange orchard farmers in Che-
Ju Do, Korea. The Seoul Journal of Medicine, 22: 137-142.
Longacre, S. (1986). Summary of ETU and EBDC analysis in plasma,
liver, and thyroid after mancozeb administration. Unpublished report
No. 86R-1009 from Rohm and Haas Company, (appendix/supplement to
Nelson 1986 31H-86-02) Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lu, M. & Kennedy, G. (1986). Teratogenic evaluation of mancozeb in
the rat following inhalation exposure. Toxicol. and Appl. Pharma.
84: 355-368.
Mann, S.K. (1977). Cytological and genetical effects of dithane
fungicides on Allium cepa. Environmental and Experimental Botany,
17: 7-12.
Martinez-Rossi & Azevedo, J. (1987). Detection of point mutation
mutagens in Aspergillus nidulans: comparison of methionine
suppressors and arginine resistance induction of fungicides.
Mutation Research, 176: 29-35.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb and their related compounds. Int. Arch. Occup. Environ.
Health, 37: 169-178
Matsushita, T. Yoshioka, M., Arimatsu, Y. & Nomura, S. (1977).
Experimental study on cross-contact allergy due to dithiocarbamate
fungicides. Indust. Health, 15: 87-94.
McGlynn-Kreft, A.M. & McCarthy, K.L., (1984). Dithane M-45 mammalian
cell transformation test. Unpublished report No. 84R-55 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
McLeod, P.L. & Doolittle, D.J. (1985). Dithane M-45 mammalian cell
transformation test for promotion. Unpublished report No. 84R-0297
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Muller, W. (1991). Oral (gavage) teratogenicity study in the rabbit.
Unpublished report No. HLD-853-683-002 from Hazleton Labs.,
Deutschland GmbH, Munster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Muller, W. (1992). 2-Generation oral reproduction toxicity study in
the rat. Unpublished report No. HD-852-683-001 from Hazleton Labs.,
Deutschland GmbH, Münster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Murty, K., Raju, D. & Sharma, C. (1983). Cytogenetic hazards from
agricultural chemicals. 7. Herbicides, fungicides and insecticides
screened for effects on chiasmata in Hordeum vulgare. Biol. Zb1.
102: 571-576.
Nelson, S.S. (1986). Metabolism of 14C mancozeb in rats.
Unpublished report No. 31H-86-02 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Nelson, S.S. (1987). Bioconversion of mancozeb to ETU in the rat.
Unpublished report No. 31C-87-24 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 80R-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neill, P. & Frank, J.P. (1988). Mancozeb: in vitro unscheduled
DNA synthesis assay in F/344 rat hepatocytes. Unpublished report No.
88R-079 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Piccirillo, V.J., Wu, D. & Speirs, G. (1992). Metabolism of mancozeb
in the mouse. Unpublished report No. T91-3413 from Inveresk Research
International, Ltd., Tranent, Scotland. Submitted to WHO by Elf
Atochem (Confidential data).
Restrepo, M., Munoz, N., Day, N.E., Parra, J.E., deRomero, L. &
Nguyen-Dinh, X. (1990). Prevalence of adverse reproductive outcomes
in a population occupationally exposed to pesticides in Columbia.
Scand. J. Work. Environ. Health, 16(4): 232-238.
Sames, J.L., McLeod, P.L. & Doolittle, D.J. (1984). Dithane M-45 in
vivo cytogenetic study in Fischer 344 rats. Unpublished report No.
84R-0246 Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Schweitzer, M. (1990). A study of the decomposition of mancozeb in
dimethyl sulfoxide solution. Unpublished report No. SC890012 from
Battelle Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. TR 34-90-01.
Shaw, D. (1990). Mancozeb: 52 week oral (dietary) toxicity study in
the beagle. Unpublished report No. HUK 5913-616/3 from Hazleton, UK,
Harrogate, North Yorkshire, England. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. 88RC-027.
Shellenberger, T. (1991) Mancozeb: 18-month dietary oncogenicity
study in mice. Unpublished report No. 85051 from Tegeris Labs.,
Inc., Temple Hills, Maryland, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA, as Rohm and Haas report
No. 86RC-029.
Solomon, H.M. & Lutz, M.F. (1987). Mancozeb: oral (gavage)
developmental toxicity study in rabbits. Unpublished report No. 86R-
021 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Solomon, H.M., Lutz, M.F. & Kulwich, B.A. (1988). Mancozeb: 2-
generation reproduction study in rats. Unpublished report No. 87R-
020 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Stadler, J. (1990). Combined chronic toxicity/oncogenicity 2-year
feeding study with mancozeb in rats. Unpublished report No. 259-89
from E.I. duPont de Nemours and Company, Haskell Laboratory for
Toxicology and Industrial Medicine, Newark, Delaware. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Stadler, J. (1991) Neuropathology study in rats with mancozeb.
Unpublished report No. 217-89 from E.I. duPont de Nemours and
Company, Haskell Laboratory for Toxicology and Industrial Medicine,
Newark, Delaware. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Szepvolgyi, J., Nagy, K., Sajgone-Vukan, K., Regoly-Merci, A., Soos,
K., Toth, K., Pinter, A. & Antal, M. (1989). Subacute toxicological
examination of Dithane M-45. Fd. Chem. Toxic, 27(8): 531-538.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Enticott, J., Wilby,
O.K., Tesh, S.A. (1988). Mancozeb: teratology study in the rat.
Unpublished report No. 87/PTC 007/365 from Life Science Research,
Suffolk, England. Submitted to WHO by Elf Atochem (Confidential data
of Elf Atochem).
Tomlinson, H.L. & Longacre, S.L. (1988). Mancozeb dermal absorbtion
study in male rats. Unpublished report No. 88R-218 from Rohm and
Haas, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm and
Haas, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988a). Mancozeb: delayed contact hypersensitivity
study in guinea-pigs. Unpublished report No. HLA 417-431 from
Hazleton Labs., Vienna, Virginia, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988b). Mancozeb: 4 week repeat dermal toxicity study
in rats. Unpublished report No. HLA 417-432 from Hazleton Labs.,
Vienna, Virginia, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Vasudev, V., Krishnamurthy, N.B., (1980). Non-mutagenicity of the
fungicide Dithane M-45 as inducer of recessive lethals after larval
feeding in Drosophila melanogaster. Mutation Res. 77: 189-191.
Watts, M.H. & Chan, P.K. (1984a). Dithane M-45: acute oral toxicity
study in rats and mice. Unpublished report No. 83R-213a + b from
Rohm and Haas Company, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Watts, M.H. & Chan, P.K. (1984b). Dithane M-45: acute oral toxicity
study in rats. Unpublished report No. 83R-218 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Peak concentrations of 14C in thyroid were reached within 6
and 24 hours in animals receiving the low and high doses,
respectively. Peak levels in thyroid were about 45 and 10 fold
higher than the corresponding peak levels in whole blood after low
and high-dose administration, respectively. 14C residue levels for
aforementioned tissues were comparable for males and females
receiving single dose administration except for adipose tissue and
gonads of females which were higher than those of males. Thyroid
tissue contained the highest residue levels for each group.
However, thyroid contained less than 0.10% of the dose 96 hours
post-dose. Tissue concentrations of 14C residues in rats receiving
the "pulse dose" were comparable to those in animals receiving only
the single dose of 14C-mancozeb at 1.5 mg/kg bw. The average 14C
residue levels per group remaining in the tissue at 96 hours post-
dosing ranged between 1.5-3.5% of the dose. ETU concentrations in
plasma and liver of rats 6 hours after dosing with 14C-mancozeb at
1.5 mg/kg bw were 6 and 12 times less than corresponding plasma and
liver 14C levels, respectively. ETU was not detected in the pooled
thyroids of these low-dose animals. Peak plasma levels of ETU were
reached 6 hours post-dosing in animals given 100 mg/kg bw mancozeb
and were 6-12 times less than corresponding plasma 14C levels for
both sexes. ETU was rapidly eliminated from the plasma of both male
and female rats (t´ 4.0-4.7 hours) and decreased below detectable
levels within 48-hours post-dosing. The estimated bioavailability
of ETU in rats was about 6.8 percent on a w/w basis and 20% on a
mole/mole basis. ETU concentrations in the liver of the high-dose
rats was 100 times less than liver 14C concentrations 6 hours post-
dosing. ETU was not detectable in liver 48 hours post-dosing. ETU
concentrations in thyroids were 80-100 and 30-225 times less than
corresponding thyroid 14C concentrations in males and females,
respectively, given 100 mg/kg bw mancozeb at 6 and 24 hours. ETU
was not detectable in plasma, liver, or thyroid of rats at 96 hours
after administration of a "pulse dose" (1.5 mg/kg bw of 14C-
mancozeb) preceded by a two-week dietary intake of unlabelled
technical mancozeb.
Concentrations of EBDC in the liver of rats 1, 6 and 24 hours
after dosing with 14C-mancozeb at 100 mg/kg bw were 0.25, 0.61, and
0.29 ppm for males, respectively, and 0.32, 0.35 and 0.25 ppm for
females, respectively. EBDC residues were not detectable 48 or 96
hours post-dosing. EBDC was not detected in the livers of rats
receiving the low dose (1.5 mg/kg bw) or multiple doses of mancozeb
96 hours post-dosing. Metabolite analysis revealed that mancozeb
was extensively metabolized and/or degraded. ETU was a major
metabolite found in urine, bile and faeces. EBDC was detected in
liver, faeces, and bile but not in thyroid. EBIS, ethyleneurea
(EU), N-acetyl-ethylenediamine (N-AcEDA) and ethylenediamine (EDA)
were also identified in urine, faeces and bile. Other metabolites
tentatively identified were glycine, N-acetylglycine, and N-
formylethylenediamine. Five other metabolites were also present but
were not identified (DiDonato & Longacre, 1986; Longacre, 1986;
Nelson, 1986, 1987; Kocialski, 1989).
Monkeys
Groups of male rhesus monkeys ( Macaca mulatta; 6/group) were
given single oral doses of either 14C-ETU; 14C-ETU plus manganous
sulfate and zinc sulfate or 14C-mancozeb (purity not stated) for
the determination of uptake into blood and the determination of the
major route of elimination of 14C. A sufficient dose was given to
produce 100 µCi of 14C activity per monkey. Whole blood, thyroid,
heart, lung, liver and kidney and faecal material were converted to
14C-carbon dioxide by combustion in a tissue oxidizer and analyzed
for 14C activity. Urine samples were analyzed directly. Urine and
faeces were collected separately.
14C-Labelled doses of ETU and ETU plus manganese and zinc
sulfates reached peak levels of 5% of the dose in total blood volume
at 8 hours. 14C-Mancozeb activity in the blood was less than 0.5%
at 8 hours and plateaued from 24-72 hours at slightly less than 1%
of the dose. This was in contrast to a relatively rapid decline in
14C activity in ETU-treated monkeys which at 72 hours showed 1% of
the administered dose in whole blood. ETU and ETU plus Mn:Zn-treated
monkeys showed similar rates of 14C excretion with nearly 50% of
the dose being cleared in 24 hours. The urinary clearance of 14C
activity by mancozeb-treated monkeys was slower (3.6% of the dose in
24 hours) as compared to ETU-treated groups. At 120 hours (last time
reading), the urinary route of elimination accounted for little more
than 10% of the activity in the mancozeb dose. Faecal elimination
showed less than 1% of 14C activity up to 24 hours in ETU-treated
groups. However, mancozeb-derived faecal activity ranged from 12.5-
64.0% of the dose at 144 hours, and from 0.005-12.7% at 24 hours.
14C activity was not detected in faecal samples from ETU-treated
monkeys after 24 hours. At 144 hours, blood samples were taken from
2 animals of the mancozeb-treated group, the animals sacrificed and
the following organs examined for 14C activity: thyroid, heart,
lung, liver and kidney. The remaining 4 animals were sacrificed 48
hours later (192 hours post-dosing). Comparative examination
between the groups (2 vs 4 monkeys) indicated that 14C activity
declined steadily between 144 and 192 hours in blood, whereas 14C
activity in the thyroids increased over the same 48-hour time period
(Emmerling, 1978).
Toxicological studies
Acute toxicity studies
Acute toxicity studies are summarized in Table 1. WHO has
classified mancozeb as unlikely to present acute hazard in normal
use (WHO, 1992).
Short-term toxicity studies
Mice
Charles River COBS-CD-1 mice (10/sex/group) received 0, 1, 10,
100, 1000 or 10 000 ppm of mancozeb (83% purity) adjusted to 100%
active ingredient for 4 weeks. No animals died on study and
clinical signs were absent. Females fed 10 000 ppm mancozeb showed
decreased body weights. Food consumption appeared to be comparable
for all groups on study. SGPT activity was comparable to controls
for all groups. Gross necropsy findings were not remarkable.
Thyroid weights (absolute and relative) were statistically
increased in both females at 10 000 ppm. Males appeared unaffected.
Liver weights were statistically significantly increased in males
and females given 10 000 ppm mancozeb. At 1000 ppm, absolute and
relative liver weights were significantly increased in females.
Females treated with 1000 ppm and 10 000 ppm showed thyroid
hyperplasia, congestion and decreased colloid density whereas males
showed similar effects at 10 000 ppm. No significant micropathology
was evident in the livers of animals given mancozeb (DiDonato et
al., 1985).
Mancozeb (83.1% pure) was administered to groups of Charles
River CD-1 mice (15 sex/dose) for 3 months at dietary concentrations
of 0, 10, 100, 1000, or 10 000 ppm of active ingredient. No
compound-related mortalities or clinical signs were observed.
Decreased body weights and food consumption were observed in both
sexes receiving 10 000 ppm. There were no treatment-related effects
with regard to haematology or clinical chemistry. Necropsy findings
were not remarkable. Aniline hydroxylase activity was decreased at
10 000 ppm in both sexes. Aminopyrine N-demethylase activity was
decreased in males at 1000 and 10 000 ppm. No effects were seen in
either sex at lower doses. Absolute and relative thyroid weights
were increased in both sexes receiving 10 000 ppm. Relative
increases in liver weights were seen in both sexes receiving 10 000
ppm. Absolute liver weight was increased in males administered 10
000 ppm mancozeb. Relative kidney weights were also increased in
both sexes at 10 000 ppm. Histopathologic evaluation of thyroid
revealed an increased incidence of follicular cell hyperplasia and
hypertrophy in both sexes at 1000 and 10 000 ppm. There was
Table 1. Acute toxicity of mancozeb
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse1 B6C3F1 M oral > 5000 --- Watts, 1984
Rat3 F344 M oral > 5000 --- Watts, 1984
Rat1 F344 M oral > 5000 --- Watts, 1984
Rat3 CRCD M oral > 5000 --- DeCrescente, 1980
Rat2 Wistar M/F i.p. 380 --- DeGroot, 1974
Rat 5,6 COBS-CR M/F inhalation --- 5.14 Hagan, 1982
(SD) BR 4 hour exposure
Rat 6,7 CR-SD M/F inhalation --- > 1.11 Hagan, 1980
4 hour exposure
Rabbit4 NZW M dermal > 5000 --- DeCrescente, 1980
1 Corn oil vehicle.
2 Carboxy-methyl-cellulose vehicle.
3 Aqueous dispersion.
4 Saline vehicle.
5 3/10 M and 3/10 F died during the whole body exposure/observation period (14 days). Multiple signs but no
tremors; body-weight loss and decreased weight gain. MMD 3.3 microns. Nominal concentration was
41.8 mg/litre (aerosol).
6 Whole body exposure.
7 MMD about 2.2 microns. Nominal concentration 4.46 mg/litre (dust).
increased vacuolation, interstitial congestion and decreased colloid
density in the thyroids in both sexes at 10 000 ppm. No liver
effects were observed in mancozeb-treated mice with the possible
exception of hepatocytic nuclear pleomorphism in females at 10 000
ppm. Increased deposits of (brownish) pigment were seen in the zona
reticularis of the adrenal cortex of female mice at 10 000 ppm. The
NOAEL was 100 ppm, equal to 18 mg/kg bw/day for males and 22 mg/kg
bw/day for females (O'Hara & DiDonato, 1985).
Rats
Four groups of 12 male and 12 female Crl:CD (SD)BR rats were
exposed to dust aerosols of mancozeb (84% pure) for 6 hours per day
for 10 days. The four groups were subdivided into whole-body
exposed rats and nose-only exposed rats of 5/sex/dose group. Daily
concentrations yielded total mean aerosol concentrations of 0, 23,
138 or 519 mg/m3. The mean respirable aerosol concentrations were
0, 11, 55 or 258 mg/m3 with mass median diameters of 3.5-4.9
microns.
Nose-only Exposure: There were no compound-related deaths.
No adverse clinical signs were observed in males or females exposed
to 11 or 55 mg/m3. Significant decreases in mean body weight and
mean body-weight gain were observed in the high-dose males but not
females. T3 and T4 levels were significantly reduced and the lung
to body-weight ratio increased in high-dose males but not in
females. Microscopic examination (limited to the respiratory tract)
of nasal turbinates revealed an increased incidence and degree of
multifocal mixed inflammatory cell infiltration (i.e. mononuclear
cells and neutrophils) and multifocal or focal necrosis of the
turbinate mucosa in four males and two females in the high dose.
Whole-body Exposure: There were no compound-related deaths.
No clinical signs were observed in animals receiving 11 mg/m3.
Significant reductions in male and female body weight and body-
weight gain were observed at 55 and 258 mg/m3. Male and female T4
levels and male T3 levels were significantly decreased at 55 and
258 mg/m3 of mancozeb after two weeks. TSH levels were not
significantly increased in both sexes at the high dose. Male and
female lung weights and lung to body-weight ratios were
significantly increased in both sexes at the high dose. Exposure-
related multifocal interstitial inflammation, microgranulomas,
multifocal mixed inflammatory cell infiltration, focal or multifocal
necrosis in the respiratory tract and reactive lymphoid hyperplasia
of the peribronchial lymph nodes were observed at the high dose
tested (Hagan & Baldwin, 1986).
Adult male and female Crl:CD(SD)BR rats were divided into two
nose-only exposure groups and one nose-only exposure-recovery group.
Animals received aerosol dust (84% pure mancozeb), 6 hours/day, 5
days week for 4 weeks at analytically determined mean concentrations
of 0, 22, 86 or 308 mg/m3 (equivalent to respirable concentrations
of 0, 8, 40 and 127 mg/m3) or for 13 weeks at 0, 18, 79 or 326
mg/m3 (equivalent to respirable concentrations of 0, 8, 36 or 144
mg/m3).
Results - 4 Weeks Exposure: There was no compound-related
mortality. Mean body weights and body-weight gains were decreased
for males in the high-dose group. No haematological effects were
observed in males. Data for females were inconclusive due to
insufficient amount of blood needed for the haematological
evaluations. There were no compound-related effects in either sex
for clinical chemistry, thyroid function, organ weights, or tissues
examined microscopically. Ophthalmological examinations revealed no
remarkable findings.
Results - 13 Weeks Exposure: There were no compound-related
deaths and no treatment-related signs of toxicity. Males exposed at
the high dose exhibited reduced mean body weights and body-weight
gains. Some haematological and clinical chemistry changes were
noted but were within the normal range of values and were therefore
not considered related to mancozeb exposure. T4 levels were
reduced 30% in high-dose females and considered treatment-related.
A dose-response was evident. Males showed a 9% decrease at the high
dose tested which was not statistically significant. Absolute
kidney and heart weights were reduced in males at the high dose
tested. However, organ to body weight and organ to brain weight
ratios were not statistically significantly different from controls.
Ophthalmological examination of the eyes revealed no compound-
related effects. No exposure-related histopathology was observed in
either sex at the low dose tested. Males (5/10; 8/11) and females
(10/11; 9/11) exposed to 79 or 326 mg/m3 exhibited a yellow-brown
granular pigment in the lumen of the cortical tubules of the kidney.
However, no attendant histopathological changes were reported. Mild
hyperplasia of the follicular epithelium in the thyroid glands of 3
females exposed at the high dose was observed. No exposure-related
thyroid lesions were observed at lower dose levels or in males.
Several histopathological lesions were observed in the respiratory
tract but were comparable to control incidences and not considered
treatment-related. Residue analysis of blood indicated
concentrations below the limit of detection for ETU and mancozeb for
males and females exposed to the low dose, with increasing
concentrations at higher exposures. Residue levels for ETU in the
thyroid were below the limit of detection at the low dose in both
sexes, with increasing concentrations at the higher doses. ETU and
mancozeb levels in urine were present at all doses in both sexes.
Those animals allowed to recover for 13 weeks after cessation of
exposure were comparable to a concurrent control group of non-
exposed animals for all parameters examined (Hagen et al., 1986).
Groups of Sprague-Dawley rats (Crl:CD(SD)BR); 10/sex/dose) were
administered 0, 10, 100, or 1000 mg of mancozeb (83% pure, not
adjusted for purity)/kg bw. The control group received distilled
water only. Test material was applied to the clipped dorsal area of
intact skin of each animal. An unsterile dressing sponge was placed
over the test site and held in place for 6 hours/day then removed.
All animals were treated similarly for 20 exposures over a 4-week
period. All animals were fitted with a cardboard collar to minimize
preening at the application site. The collar was only removed
during the 6 hours exposure period.
Clinical observations showed no evidence of compound-related
effects and there was no compound-related mortality. Body weight,
body-weight gain and food consumption in treated groups were
comparable to controls. Erythema was transient and slight, occurring
in not more than 2 animals/sex/dose for not longer than 2-4 days.
No other signs of dermal irritation were observed. Evaluation of
haematological and clinical chemistry parameters revealed no
statistically significant compound-related effects. Macroscopic
observations of treated skin revealed dark (yellow) area or
appearance in a dose-related increasing frequency at 100 and 1000
mg/kg bw in both sexes. Absolute and relative organ weights in
treated groups were not significantly different from control group.
Microscopic findings were limited to skin of treated and untreated
animals and characterized by increased keratin production
(hyperkeratosis) and thickening of the epidermis (acanthosis).
Severity varied from minimal to slight in all groups. These
findings were treatment-related but not compound-related. There
were no microscopic findings that were attributable to mancozeb.
Gross enlargement of the parotid salivary gland was within normal
histomorphological limits and found in all treatment groups
(Trutter, 1988b).
Groups of Crl:CD(SD) rats (14 rats/sex/group) received 0, 30,
60, 125, 250 or 1000 ppm of mancozeb (84% pure adjusted to 100%
active ingredient) for 13 weeks. Dose levels in the diet were
gradually increased with time in order to maintain approximately the
same level of compound intake throughout the feeding period.
There was no compound-related mortality or clinical signs. No
compound-related changes in body weights or food consumption were
recorded in animals fed up to and including 250 ppm mancozeb. At
1000 ppm, males showed a statistically significant decrease in body
weight from weeks 3 through 13, whereas females showed a 3-14%
decrease with statistically significant decreases only between 7 and
10 weeks. At 1000 ppm, food consumption was decreased in males for
weeks 3-13, but not in females. Males receiving 1000 ppm of
mancozeb showed statistically significant increases in BUN (84%),
creatinine (28%) and cholesterol (52%). Females receiving 1000 ppm
showed increased SAP (32%) and triglyceride (90%). However, group
increases were not considered compound-related as one male and one
female in the high-dose group were reported as having exceptionally
high values.
Clinical findings at lower mancozeb levels were not considered
treatment-related. Serum T4 levels were decreased in males and
females at 1000 ppm and TSH values increased. At 250 ppm, TSH
values were increased in males (36%) and females (50%). However,
only T4 values in females were statistically significantly
decreased at 250 ppm. MFO activity was decreased at 1000 ppm. No
mancozeb metabolite residues were detected in blood. Urine samples
of rats fed 125 to 1000 ppm of mancozeb contained 0.1 to 1.1 ppm of
parent compound. ETU metabolite of mancozeb increased in urine in a
dose-response manner from 0.3 to 10 ppm in animals given 30 to 1000
ppm mancozeb.
No mancozeb residues above the detection limit of 25 ppm were
detected in thyroids obtained from the 1000 ppm mancozeb fed rats.
ETU metabolite of mancozeb showed residues in thyroid which were
dose-related to mancozeb administration. Values ranged from less
than the detection limit of 4.0 ppm in 30 ppm mancozeb-fed animals
to 25 ppm in 1000 ppm animals. At 1000 ppm, absolute thyroid weight
was increased in males while relative weight was increased in males
and females. Relative liver weight was increased in males and
females receiving 1000 ppm. Relative spleen weight was increased
only in females receiving mancozeb at the highest dose. Compound-
related histopathology was generally confined to the liver, kidneys,
thyroid, adrenal and pituitary glands. Thyroid follicular cell
hyperplasia was seen in 90% of males and females receiving 1000 ppm;
a small, well defined basophilic focus of hyperplastic follicular
epithelial cells was seen in one male and there was also an
increased incidence and severity of hypertrophied vacuolated cells
in the pituitary of males. The kidneys of males and females
administered 125 to 1000 ppm had minimal to moderate amount of
yellow-brown pigment in the lumen of the cortical tubules. Pigment
deposits were attributed to ethylene bis(isothiocyanate) sulfide
(EBIS) a yellow coloured metabolite of mancozeb. Pigmentation
deposits were not accompanied by histopathological effects. There
was also an increased incidence of hypertrophy of cells of the zona
glomerulosa of the adrenal cortex in males given ETU and 1000 ppm
mancozeb. Hypertrophy of the centrilobular hepatocytes was seen in
males fed 1000 ppm mancozeb. The NOAEL was 125 ppm, equal to 7.4
mg/kg bw/day, based on increased serum TSH and decreased T4 values
at the next higher dose (Goldman et al., 1986).
Male H-Wistar rats (12/group) were given Dithane M-45 (80%
mancozeb) mixed in feed at doses of 0, 10,50, 75, 113, 169, 253 or
379 mg/kg bw/day for 12 weeks. One-third of the rats in the highest
dose group died within 6 weeks and showed signs of prostration,
weakness and posterior extremital paralysis. Signs were transient
in survivors and absent at 12 weeks. Body weight, body-weight gain,
total food intake and food efficiency were all depressed at 169
mg/kg bw/day and above. Effects were compound-related and
statistically significant. Blood sugar levels (after glucose
loading) and haematological parameters were comparable to control
values. Organ to body-weight ratios were increased for liver and
thyroid at 75 mg/kg bw/day and above and for kidneys, adrenals and
testes at 253 mg/kg bw/day and above. Histopathology of liver and
kidneys were comparable to controls. Thyroids, however, showed a
dose-dependent hyperplasia at 113 mg/kg bw/day and above. A 20%
decrease in the iodine content of the thyroids in rats given 10
mg/kg bw/day was not statistically significant. However at 50 mg/kg
bw/day a statistically significant decrease of 80% was recorded.
PBI was significantly decreased and serum cholinesterase
significantly increased at 169 mg/kg bw/day and above. ALP, acid
phosphatase and ASAT were statistically decreased in groups given
379, 253 or 169 mg/kg bw/day or above, respectively. Total protein
content of sera was statistically increased but still within
physiological limits. Liver triglycerides were statistically
increased at 113 mg/kg bw/day and above but serum triglyceride
levels were comparable to controls. Total serum cholesterol was
increased at 75 mg/kg bw/day and above but total liver cholesterol
was comparable to control values. Liver total lipid was similar to
controls. Aminopyrine demethylase and aniline hydroxylase activity
were both decreased at 253 mg/kg bw/day and above. The cytochrome
P-450 level remained unchanged (Szepvolgyi et al., 1989).
Dogs
Beagle dogs (6/sex/dose) received 0, 10, 100, 1000 or 5000 ppm
of 83.35% percent pure mancozeb in the diet adjusted to 100% of
active ingredient for 3 months.
Two males and one female were sacrificed in extremis in the
5000 ppm dose group as a result of compound-related anorexia and
malnutrition. Dose-related clinical signs of dehydration, thinness,
and pale mucous membranes were also noted in animals at this dose
level. Occasional instances of dehydration were seen in animals at
1000 ppm. Signs were considered secondary to anorexia and
malnutrition. Ophthalmoscopic examination did not reveal any
compound-related effects. Food consumption was decreased
approximately 10-20% at 1000 ppm and about 40% at 5000 ppm in both
sexes. Mean body weight and mean body-weight change were both
decreased at the high dose and at 1000 ppm. Decreases in
erythrocyte count, haemoglobin, and haematocrit were accompanied by
increases in mean corpuscular volume and mean corpuscular
haemoglobin. Values were statistically significant in both males
and females at 5000 ppm and females at 1000 ppm. T3 and T4 values
were decreased along with ALAT values at 5000 ppm in both sexes.
Total cholesterol was elevated in both sexes at 5000 ppm and in
females at 1000 ppm. Females at 5000 ppm also showed elevated total
bilirubin count at 5000 ppm.
At necropsy, enlarged and/or dark thyroid/parathyroids and
decreased thymus size were seen in the 1000 and 5000 ppm males and
females. A pale appearance of visceral organs was observed in the
three animals sacrificed early. Compound-related histomorphological
tissues alterations included thyroid follicular cell hyperplasia at
5000 ppm in both sexes, thymic cortical lymphoid depletion in the
1000 and 5000 ppm animals of both sexes, and hypoplastic changes in
the reproductive systems of males and females in the high-dose group
(i.e. aspermato-, hypospermato-, and hypogenesis of the testes and
hypogenesis of the ovaries), pallor of the zona fasciculata of the
adrenal gland in high-dose males and females and some sinusoidal
liver cell pigmentation in high-dose males and females. Urinalysis
indicated the presence of both parent compound and metabolite. ETU
was detected in the blood at a dose of 1000 ppm. No mancozeb was
detected in the thyroid, however ETU was detected in the thyroid of
both sexes at 100 ppm (in males 1.83 ppm and in females 0.72 ppm).
The NOAEL was 100 ppm, equal to 3.0 mg/kg bw/day, based on decreased
food consumption and body-weight gains and decreased erythrocyte
count, haematocrit, and haemoglobin at 1000 ppm (Cox, 1986).
Beagle dogs (4/sex/group) were given dietary concentrations of
0, 50, 200, 800 or 1600 ppm of mancozeb (84.5% pure) and adjusted to
100% active ingredient for 52 weeks.
Two high-dose males were sacrificed in extremis during weeks
10 and 11. One animal manifested haematuria and a hard distended
bladder prior to sacrifice. Necropsy revealed urethral calculi
lodged behind the os penis. Hydronephrosis with tubular dilation,
necrosis and congestion was noted in the kidneys. The lower urinary
tract manifested severe urethritis, prostatitis, cystitis and
ureteritis. Acute peritonitis was associated with these lesions.
The second male like the first showed a sharp drop in food
consumption and a decreased body-weight gain. A haematological
examination revealed the animal to be anaemic (decreased
haemoglobin, erythrocytes, packed cell volume and increased
reticulocytes). Haematological parameters, necropsy and
histopathological findings were consistent with a chronic
regenerative anaemia. Diffuse centrilobular necrosis with
extramedullary haemopoiesis was seen on histopathological
examination of the liver, while in the spleen and bone marrow
moderate to slight erythroid haemopoiesis with pigment was evident
and a notable reticulocytosis. All other animals survived, and
there were no apparent compound-related clinical signs or palpable
masses, nor any apparent neurological effects of treatment. Core
body temperatures were comparable between groups. With the
exception of the two animals that were terminated early there were
no consistent trends in food conversion efficiency or food
consumption. Body-weight gain for males fed 200 and 800 ppm
mancozeb showed statistically significant decreases, beginning at 24
and at 15 weeks, respectively. The two high-dose male survivors and
females showed body-weight gains comparable to controls.
Haemoglobin was significantly decreased at 800 and 1600 ppm in
females at 13 and 52 weeks along with packed cell volume at 13
weeks. Males manifested a statistically significant increase in
mean corpuscular volume at 1600 ppm at weeks 13, 26 and 52, as well
as at 800 pm at week 13. Serum cholesterol was significantly
increased in females at 1600 ppm at weeks 10, and 26. Serum
cholesterol values were dose-related in both sexes with cholesterol
values increasing at 200 ppm (7%-16% at weeks 10, 26 and 52) for
females and at 800 ppm for males.
T4 values were consistently lower than controls or pretest
values but were not statistically significant. T3 values were
comparable to controls. Urinalysis revealed no compound-related
effects. Absolute thyroid weight, and thyroid to body and brain
weight ratios were significantly increased in surviving males and
females at 1600 ppm. Absolute liver weight and liver to body-weight
ratio were increased in males but statistically significant only for
liver to brain weight ratio at 1600 ppm. Liver weight and liver
weight ratios were also increased in females but were not
statistically significant. The only apparent compound-related
effect observed was thyroid follicular distention observed in the 4
high-dose females and the two surviving high-dose males. The NOAEL
was 200 ppm, equal to 7.0 mg/kg of bw/day, based on decreases in
body-weight gain, increased cholesterol and decreased haemoglobin
and packed cell volume at 800 ppm (Shaw, 1990).
Beagle dogs (4/sex/group) were administered by gelatin capsule
0, 2.3, 23, or 113 mg/kg bw/day of mancozeb technical (88.6% pure).
The control group received gelatin capsule only. Compound was
administered once a day, seven days a week, for 52 weeks.
All animals receiving 113 mg/kg bw/day were sacrificed at 26
weeks with the exception of one male sacrificed at 13 weeks. At the
time of single sacrifice, food consumption was decreased 70% and
body weight depressed. Severe anaemia was evident. ALAT, ASAT,
urea, total bilirubin, total cholesterol were substantially
increased. T3 and T4 values were comparable to pre-test values.
Inorganic phosphorous was decreased 35%. Most organs were pale at
necropsy and histopathology was not conducted. Necropsy was not
conducted on animals terminated at 26 weeks. Faeces discoloration
(yellow/green) occurred in all groups but was most prevalent at the
high dose. Food consumption and body-weight gain were comparable
between control and low-dose groups (2.3 mg/kg bw/day) for both
sexes and for males at the mid- and high-doses. Females receiving
113 mg/kg bw/day manifested a 66% decrease in food consumption at 24
weeks and a 13% decrease at 52 weeks. Body-weight gain in females
at 52 weeks was 45% less than controls for animals receiving 23
mg/kg bw/day. At 24 weeks, haematology values for males were
comparable to controls at all doses. Females showed a frank anaemia
at 113 mg/kg bw/day and a dose-related decrease in RBCs. Blood
chemistry values for males showed dose-related trends for increased
ALP, total cholesterol, total plasma protein, phosphorous, and a
decreased trend for T4 with statistical significance attained at
113 mg/kg. Females showed decreasing trends for T3, T4, ASAT,
ALAT with an increasing trend in total cholesterol. ASAT and ALAT
values were statistically significant at 113 mg/kg bw/day. At 52
weeks, haematology, blood chemistry, urinalysis, organ weights and
histopathology were generally comparable to controls for both sexes
for animals receiving 2.3 and 23 mg/kg bw/day with the exception of
decreased T4 values in males. No dogs died at 2.3 and 23 mg/kg
bw/day. The NOAEL was 2.3 mg/kg bw/day, equal to 2.0 mg/kg bw/day
of active ingredient, based on decreased food consumption and
decreased body weight in females, and decreased T4 levels in males,
at 23 mg/kg bw/day (Broadmeadow, 1991a).
A second study in dogs of 52 weeks was conducted at a single
dose of 40 mg/kg bw/day as a result of the excessive toxicity and
termination of the high-dose experimental group (113 mg/kg bw/day)
in the preceding study. Protocols and methods were identical to the
first experiment. Clinical signs were limited in females. Two
females appeared thin looking during the course of the experiment
and one was reported as being hypothermic and of pale appearance.
No dogs died. Food consumption, body weight and body-weight gain
for males were comparable to controls for all time periods. Females
showed an immediate decrease in food consumption (20-26%) and a
statistically significant decrease in body weight and body-weight
gain throughout the experimental period. RBC count at 24 and 52
weeks for males and females was decreased (7-10%) but not
statistically significant. However, at 52 weeks MCV was
significantly increased in both sexes while MCHC was decreased
significantly in females. Other haematological parameters were
comparable to controls as were bone marrow findings. At 24 weeks ALP
and total cholesterol were substantially increased in males and
females whereas ALAT and ASAT were significantly decreased in
females. Inorganic phosphorous was significantly increased in
females. At 50 weeks, T3 and T4 values were statistically
decreased in both sexes while ALP levels were significantly
increased in females. ALAT values in females were significantly
decreased. Phosphorous levels were statistically raised in females.
Urinalysis showed a decrease in specific gravity for females
accompanied by increased urine volume. Absolute thyroid organ
weight was increased in both sexes and statistically significant in
males. Organ to body-weight ratios were comparable in males but
raised in females; however the values in females were suspect
because of severe loss of body weight. Necropsy revealed an
enlarged or swollen spleen in treated animals. Histopathology of
treated animals was generally not remarkable and comparable to
controls. However, an increased incidence of iron pigment
deposition in Kuffur cells of female livers and an increased
incidence of periacinar lipofuscinosis in males was reported in both
sexes (Broadmeadow, 1991b).
Long-term toxicity/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (60/sex/dose) were divided
into two experimental groups and given dietary concentrations of
mancozeb technical (88.6% pure) at 0 or 25 ppm, or 0, 100 or 1000
ppm for 78 weeks. Mancozeb was stable in the diet for at least 7
days and the diet was formulated weekly. Ten animals per sex per
dose were sacrificed at 52 weeks and all surviving animals
sacrificed at termination and necropsied. There was no compound-
related mortality or clinical signs. Body-weight gain in males was
decreased 6% at 52 weeks and 8% at 79 weeks at 1000 ppm. Values
were not, however, statistically significant. Body weight in
females at 100 ppm was decreased from weeks 1-24 and at 1000 ppm
from weeks 1-78. However, body-weight decreases in females at 100
ppm was not compound-related since there was no dose-response from
100 ppm to 1000 ppm. Body-weight gain in females was decreased 10%
at 52 weeks and 14% at 78 weeks at 1000 ppm. Body-weight gain
values at 52 and 78 weeks were not statistically decreased. Food
consumption and differential blood counts were comparable between
treated and control groups. There were no remarkable intergroup
differences for organ weights. The incidence of liver nodules in
males at 0, 100, and 1000 ppm were 4/50, 3/50 and 10/50,
respectively. The incidence of liver masses at 0, 100 and 1000 ppm
in males were 5/50, 5/50 and 9/50, respectively. Liver nodules and
liver masses were not significantly different from control values.
There were no intergroup differences in the incidence of liver
nodules or liver masses in females. There were no macroscopic or
microscopic findings that could be attributed to compound
administration. The incidence of male mice bearing liver tumours at
doses of 0, 100 and 1000 ppm was 10/50, 7/50 and 17/50,
respectively. There were no statistically significant differences or
trends. The number of male animals showing benign tumours was 8/50,
5/50 and 17/50, respectively, and the number showing hepatocellular
carcinomas was 2/50, 2/50 and 0/50. Males receiving doses of 0 or
25 ppm manifested malignant liver tumours in 3/50 and 4/50 animals,
respectively, with the total number of benign and malignant tumours
being 7/50 and 7/50 for both experimental groups. The NOAEL was 100
ppm, equal to 17 mg/kg bw/day, based on decreased body-weight gain
at 1000 ppm. There was no evidence of carcinogenicity (Everett et
al., 1992).
Groups of Charles River CD-1 mice (94/sex/group) received 0,
30, 100, or 1000 ppm of mancozeb (83% pure adjusted to 100% active
ingredient) for 78 weeks. Twenty-four animals/sex/group were
selected for an interim sacrifice at 12 months. Haematology was
conducted on 15 mice/sex/dose at 12 and 18 months. Thyroid function
assays were conducted on a minimum of eight animals/sex/dose after
12 and 18 months, with evaluations conducted on T4, T3 and TSH.
Necropsies were performed on all unscheduled deaths, on 24
animals/sex/group at interim (12-month sacrifice) and on all
surviving animals at terminal sacrifice.
There were no treatment-related increases in mortality or
clinical signs. Food consumption was similar among all groups. Body
weight for males and females of the 1000 ppm group were
statistically and consistently lower than controls through out the
78 weeks. Body-weight gains at 52 and 78 weeks for males and
females were decreased 18% and 13% and 15% and 9%, respectively.
Body weights and body-weight gains in other dose groups were similar
to controls values.
RBC counts were statistically significantly decreased in males
at 12 months at 1000 ppm. All other values for males were
comparable to controls. Females at 12 months and 1000 ppm showed
decreased RBC count, increased MCHC, and increased MCV. T3 and T4
changes were statistically significant only at the high dose tested
(1000 ppm) in both sexes. T3 and T4 levels were decreased in
females at 18 months (32% and 61% respectively). T4 levels in
females were also decreased at 12 months (76%). Males had a 56%
decrease at 12 months in T4 values and a 45% increase in T3 values
at 18 months. TSH levels were not statistically significantly
increased in either sex at any dose or time period.
Necropsy and gross examination of animals at the 12-month
interim and 18-month terminal necropsies revealed no consistent
gross lesions that could be attributed to an effect of the test
chemical. Diffuse discoloration of the lymph nodes was observed in
high-dose males (6/66) but the finding was not confirmed under
histopathological examination. There were no consistent differences
in mean organ weights, mean organ to body weight or organ to brain
weight ratios between control and compound-treated males or females
that could be attributed to the test article. Non-neoplastic
findings were comparable to controls. Hepatocellular adenomas and
carcinomas as well as pulmonary alveolar/bronchiolar adenomas and
carcinomas observed in both sexes were not statistically
significant. The NOAEL was 100 ppm, equal to 13 mg/kg bw/day in
males and 18 mg/kg bw/day in females, based on decreased body weight
and body-weight gain and decreased T3 and T4 values at 1000 ppm.
There was no evidence of carcinogenicity (Schellenberger, 1991).
Rats
Groups of Charles River Crl:CDBR rats (72/sex/dose) received 0,
20, 60, 125 or 750 ppm of mancozeb technical (83.8% pure) in the
diet for 2 years.
Mean body-weight gains in the high-dose males were
significantly lower than the control group in the first year
followed by lower but not statistically significant differences
during the second year (8.0%). Females receiving the high dose
showed a statistically significant decrease in body-weight gain at
90 days (16%) and one year (11%). Body-weight gains were comparable
to controls from 1-2 years. Food consumption was comparable for all
groups. Food efficiency was slightly but not statistically
decreased in high-dose males (5.5-7.5%) for various time intervals
and females at 90 days (10%). Erythrocytes, haemoglobin and
haematocrit were significantly decreased in male animals receiving
125 ppm at 18 months but not at other time periods. Haematological
findings were unremarkable in females. ALAT in males and
cholesterol levels in females were significantly increased at 750
ppm at 24 months. Males revealed decreased T3 values at 125 and
750 ppm at three months with decreased T4 values at 750 ppm at 6
and 18 months. TSH values were increased at 750 ppm at 12 and 18
months and at 125 ppm at 24 months. Females showed decreased T3
levels at 60, 125 and 750 ppm at 3 months. T4 values were
decreased at 750 ppm at 3, 18 and 24 months. TSH values were
increased at 750 ppm at 6 and 18 months. Urinalysis values were not
remarkable. Survival was comparable for all groups. After 24
months, absolute and relative (to body weight) thyroid/parathyroid
weights of the high-dose males and females were significantly
increased. The incidence of enlarged thyroids was also higher in
males and females at 750 ppm and the incidence of observed masses
was also higher in males. Follicular cell hypertrophy/hyperplasia of
the thyroid gland was significantly increased for males and females
receiving 750 ppm at the end of the first year.
A granular yellow-brown pigment was also present in rats fed
125 and 750 ppm at one and two years. Renal pigment remained in the
same magnitude as seen at 12 months. No attendant pathology was
evident. Bilateral retinopathy was also significant in both sexes
at the high dose at 2 years. At 2 years, in the high-dose group of
males and females, significant increases were recorded for thyroid
follicular cell hypertrophy/hyperplasia as well as nodular
hyperplasia. Thyroid follicular cell adenomas (20/61) and
carcinomas (14/61) were significant only in high-dose males.
Females showed increased incidences of thyroid follicular cell
adenomas (6/61) and carcinomas (4/61) but the increases were not
statistically significant when compared to control groups. The NOAEL
was 125 ppm, equal to 4.8 mg/kg bw/day, based on decreased body-
weight gain, decreased T3, T4 values, increased TSH values,
increased absolute and relative thyroid weight, thyroid follicular
cell hypertrophy, hyperplasia, and nodular hyperplasia, in both
sexes at 750 ppm. Carcinogenic effects were noted in both sexes in
the form of thyroid follicular cell adenomas and/or carcinomas but
only at the highest dose level (Stadler, 1990).
Sprague-Dawley (CD) rats (50 [main study] and 20 [satellite
study] animals/sex/dose) were given 0, 25, 100, or 400 ppm of
technical mancozeb in the diet (88.5% purity, adjusted to 100%
active ingredient) for 104 weeks. There were no compound-related
signs or mortality. Body-weight gain was significantly decreased in
males and females at the high dose tested for weeks 1-26 and 1-13,
respectively. Body weights were significantly decreased for males
from weeks 2-75 and for females from weeks 4-25. Food consumption
was comparable between all groups and food conversion ratios similar
for treated and control groups. There were no treatment-related
effects on haematological parameters. Reported changes were neither
dose nor time-dependent. T4 levels were significantly decreased in
males and females at 400 ppm at 26 and 52 weeks and in females alone
at 78 weeks at 100 and 400 ppm. However, the decrease at 100 ppm
was not consistent over time. T3 levels in males were decreased at
52 weeks but increased 43% at 104 weeks in the high-dose group. TSH
was increased by 86% at 78 weeks in females of the high-dose group.
The remaining statistical differences, particularly for
protein, calcium and other electrolytes, appeared to be unrelated to
treatment. Urinalysis findings were not considered to be compound-
related. Ophthalmoscopic changes were comparable between groups.
There were no compound-related organ weight changes. There was no
evidence of tumorigenicity. The incidence of thyroid follicular cell
adenomas in males was 6/50, 2/50, 2/50 and 6/50, for thyroid
follicular cell adenocarcinomas the frequency was 2/50, 0/50, 1/50,
and 3/50 for control and respective dose groups. Parafollicular
carcinomas in males was 1/50, 6/50,6/50 and 6/50. Anterior
pituitary adenomas in males occurred at a frequency of 18/50, 22/50,
28/50, and 27/50 whereas anterior pituitary adenocarcinomas were
observed at an incidence of 3/50, 2/50, 2/50 and 1/50 in controls
and respective dose groups. A statistical significance was not
achieved between groups, and values were comparable with the upper
incidence of the control range. Thyroid follicular cell adenomas and
adenocarcinomas were observed in females at frequencies of 0/50,
0/50, 2/50 and 2/50; and 0/50, 0/50, 0/50 and 1/50, respectively,
for controls and treated groups. Parafollicular malignant tumours
occurred at the same frequency (3/50) in all groups. Pituitary
adenomas in females (anterior pituitary) ranged from 28/50 to 35/50.
Pituitary adenocarcinomas ranged from 4/50 to 7/50. Non-neoplastic
findings in males were not significant for pituitary, testes,
kidneys, or other tissues with the exception of thyroid. There was a
minimal increase in the height of the follicular epithelium of the
thyroid (3/50, 1/50, 1/50, 8/50) and an increase in the number of
prominent microfollicles (0/50, 1/50, 0/50, 5/50) at the high dose.
Non-neoplastic findings for females were not significant for kidney,
pituitary or stomach. There was however a minimal increase in the
height of the follicular epithelium of the thyroid (1/50, 0/50,
1/50, 5/50) at 400 ppm. The NOAEL was 113 ppm, equal to 4.0 mg/kg
bw/day in males and 5.1 mg/kg bw/day in females, based on decreased
body-weight gain and body weight, an increase in the height of the
thyroid follicular epithelium, an increase in prominent
microfollicles, and a decrease in thyroxine levels at 450 ppm (Hooks
et al., 1992).
Reproduction studies
Rats
Sprague-Dawley Crl:CD(SD)BR rats (25/sex/dose) received 0, 25,
150, or 1100 ppm of 88.4% mancozeb technical in the diet. Compound
intake was not corrected to 100% of active ingredient in the diet.
Animals (F0 generation) were seven weeks old at the start of
treatment, acclimated to laboratory conditions and healthy. After a
premating period of 14 weeks, animals of the same dose levels were
mated for not longer than three weeks. Females were allowed to
litter and rear their offspring. Exposure to compound and diet was
continuous and ad libitum. Selected F1a pups were maintained for
14 weeks after weaning of all F1a offspring then mated for three
weeks, allowed to litter and rear their offspring (F2a generation).
There were no compound-related deaths and clinical signs were not
evident for either generation. Body weight and body-weight gain were
significantly depressed in males and females of the high-dose group
for both parental (F0, F1) generations during the 14-week pre-
treatment period. Males of the F1 generation also showed
significant decreases in body weight and body-weight gain at the
mid-dose. Body weight and body-weight gain for dams during
gestation of F0, and F1 parents, were significantly decreased at
1100 ppm and depressed at the mid-dose. Food consumption was
significantly decreased for both sexes during the premating period
of both generations at the high dose. Food consumption for F1
females was also significantly decreased at the high dose during the
periods of gestation and lactation.
Absolute organ weight at necropsy revealed statistically
significant increases for both parental sexes at the high dose.
Macroscopic examination of all tissues examined revealed no
remarkable findings. Histopathology, however, revealed thyroid
hyperplasia and hypertrophy in nearly all animals examined of F0
and F1 parents at the high dose. Thyroid follicular cell adenomas
were also found in five males of the F0 generation and in eleven
males of the F1 generation at the high dose. Indices for F1a pups
were comparable to controls. A slight delay in the opening of the
eye was however considered to be treatment-related at the high dose.
Necropsy findings were comparable between treated and control groups
for F1a and F2a litters. Viability, pups and litter weights were,
however, significantly decreased at the high dose on days 14-21.
The NOAEL in this study was 25 ppm, equal to 1.7 mg/kg bw/day, based
on decreased body weight at 150 ppm (Muller, 1992).
Charles River CRL:CDBR rats (25/sex/dose) received 0, 30, 120,
or 1200 ppm of mancozeb (84% pure) in the diet. Animals were placed
together for a period of 10 days to produce the F1a and F1b
generation. The presence of a vaginal plug was considered day zero
of gestation. On day 4, litters were culled to 5 animals/sex/dose.
Litters were weaned on day 21. One male and one female were then
selected from the F1a litter to serve as the parents for the F2
generation (F2a and F2b litters).
There were no treatment-related clinical signs or deaths in
either the F1 or F2 generation. Parental body weights for F1 and
F2 male and female rats were similar between control, 30 and 120
ppm groups throughout the 10-week treatment period prior to mating
or for F1 and F2 females during the gestation and lactation
periods. At 1200 ppm, mean body weight for male and female rats of
the F1 and F2 generation were significantly below control
throughout the pre-mating period, as well as for parental females
during gestation and lactation. Mean feed consumption of F1 and
F2 male and female rats were comparable between control, 30 and 120
ppm groups. At 1200 ppm, mean food consumption was decreased in F1
but not F2 male and female rats prior to mating. During gestation
and lactation F1 and F2 females had food consumption values
comparable to controls. Reproductive indices as measured by
fertility, gestation, viability and lactation were comparable to
controls for F1 and F2 adult and offspring at all dose levels.
Fetal body weight for F1a,b and F2a,b offspring were also comparable
to controls. There were no treatment-related effects on the
absolute and relative organ weights at 30 ppm for the F1 and F2
parents or for F1 parents at 120 ppm. F2 males showed a
statistically significant dose-related increase in relative liver
weight at 120 ppm without histopathological changes. At 1200 ppm
both F1 and F2 parents manifested significant increases in
relative liver weight and absolute and relative thyroid weights.
Relative kidney weight was increased in F1 and F2 females, but not
in males. There were no gross pathological changes considered to be
treatment-related among either F1 or F2 animals nor any which
could be substantiated by corresponding histopathology.
Treatment-related microscopic changes were observed in the
thyroid, kidney and pituitary of F1 and F2 animals. Changes
involving the follicular cells of the thyroid observed in the F1
generation were also observed in the F2 generation with generally
higher incidences or greater severity. All males of the F1 and F2
generation showed some degree of diffuse follicular cell hyperplasia
at 1200 ppm. Follicular cell adenomas and nodular/cystic follicular
cell hyperplasia were also observed at 1200 ppm. All or nearly all
females showed some degree of diffuse follicular cell hyperplasia at
1200 ppm in F1 and F2 generations. Nodular/cystic follicular cell
hyperplasia was also evident at 1200 ppm in the F2 generation.
Follicular cell adenomas were not observed in females. Brown
globular pigment was observed within the lumen of proximal tubules
in the kidneys at statistically significant incidences in both sexes
of both generations at 120 and 1200 ppm. However, no associated
pathology was observed. Hypertrophy and/or vacuolation of the
anterior pituitary was treatment-related only in males and only at
1200 ppm of the F1 and F2 generation based upon an increased
severity of response and not an increased incidence. Other
microscopic changes were not considered to be treatment-related. The
NOAEL was 120 ppm, equal to 7.0 mg/kg bw/day, based on increased
relative weights of the liver, kidney and thyroid, increased
absolute thyroid weight, decreased body weight and feed consumption
of females during gestation and lactation, and decreased pre-mating
body weight and feed consumption, at 1200 ppm (Solomon et al.,
1988).
Special study on neuropathology
Rats
Groups of Charles River Crl:CD(R) BR rats (10
animals/sex/dose) received 0, 20, 125, 750 or 5000 ppm of mancozeb
(79.3% pure and adjusted for purity) mixed in the diet for 90 days.
Two female satellite groups of 16 females each were also included
and received 5000 ppm for a two-week period only prior to sacrifice.
One male and 4 females died in the 5000 ppm group. These deaths
occurred between the second and fourth week of administration and
resulted in a cessation of test compound in females of the high dose
only and administration of control feed for the remainder of the
study. Onset of clinical signs in the second and third week of
administration consisted of generalized weakness, abnormal gait or
mobility, limited or no use of the rear legs. Loss of muscle mass
was also reported in males. However by day 60 some males in the
high-dose group appeared clinically normal and females showed
improvement after one week on control diets. Females of the
satellite group fed mancozeb for two weeks manifested limited rear
limb mobility. No clinical signs were observed below 5000 ppm.
Body weight in males was decreased 45% by day 90 in the high-
dose group. Other dose levels in males were comparable to controls.
Females receiving 5000 ppm showed initial weight loss which was
reversed after cessation of test compound and their placement on
control feed. Satellite females administered 5000 ppm of mancozeb
for two weeks showed minimal weight changes at 14 days. However, at
750 ppm and 90 days biologically significant decreases in body
weight (9%) and body-weight gain (17%) were noted. Body weights
were unaffected at lower doses. Food consumption in high-dose males
was decreased 40% compared to controls, but was similar to controls
at other dose levels. Females receiving 5000 ppm for two weeks had
decreased food consumption values ranging between 29% and 64%. In
other groups, female food consumption was similar to controls. Food
efficiency was decreased in all high-dose animals and at 750 ppm in
females (13%).
Neuropathology revealed effects in both sexes at 750 ppm and
5000 ppm. Pathology in males at 750 ppm and 5000 ppm was reported
as myelin phagocytosis, Schwann cell proliferation, myelin bubbles,
demyelination of nerve fibres associated with the posterior thigh
muscle and myelin ovoids in teased nerve fibres. Additional
observations at 5000 ppm consisted of intra-sheaths ellipsoids,
demyelination, thickening of the myelin sheath, neurofibrillary
degeneration and atrophy of the posterior thigh muscles. High-dose
females fed a recovery diet showed a thickening of the myelin
sheath, myelin bubbles, and ballooning of the myelin sheath.
Muscular atrophy was reported in one surviving female. There was
also an increased incidence of demyelination but a decrease in the
presence of myelin ovoids and debris when compared to males at this
dose level. Females fed 5000 ppm for two weeks and then sacrificed
showed myelin bubbles, sheath thickening, myelin phagocytosis and
Schwann cell proliferation. Muscle atrophy was present in 9/10
animals examined and demyelinated lengths with the presence of
myelin debris and ovoids were found upon examination of teased sural
nerve fibres. At 750 ppm in females, teased nerve fibres showed
some demyelination myelin ovoids and debris. No neuropathology was
conducted at 20 ppm dose level because of the absence of findings at
125 ppm. The NOAEL was 125 ppm, equal to 8.2 mg/kg bw/day in males
and 10.5 mg/kg bw/day in females, based on decreased body weight,
body-weight gain and food consumption in females and
neurohistopathological changes in both sexes at 750 ppm (Stadler,
1991).
Special studies on embryotoxicity/teratogenicity
Rats
Groups of mated female Crl:CD rats (27/group) were whole-body
exposed to 80% pure mancozeb dust by the inhalation route.
Administered doses were 0, 1, 17, or 55 mg/m3 for 6 hours/day on
days 6-15 of gestation. Particle size ranged from 1.4-6.4 microns.
Animals were observed for clinical signs during and after exposure
then sacrificed one day prior to natural delivery. Fetuses were
examined externally, viscerally and skeletally. No animals died.
Hind limb weakness and a slower righting reflex were observed in
6/27 high-dose dams. However, the signs disappeared during the
post-exposure observation period. High-dose animals also gained
significantly less weight than controls (40% less). There were no
differences between treated and control group as to malformations
observed. There was however an increased incidence in the number of
animals manifesting a wavy rib which was statistically significant
at the high dose. This variation was dose-related across treated
groups. There was no increase in soft tissue alterations.
Observations were typical of those commonly seen in this strain of
rat. Fetal body weights were comparable between groups (Lu &
Kennedy, 1986).
Mancozeb (83.0% active ingredient adjusted to 100% for dosing)
was administered by gavage in corn oil to groups of 26 primigravid
BLU(SD)BR rats on days 6-15 of gestation (day 0, day of
insemination) at doses of 0, 2, 8, 32, 128 or 512 mg/kg bw/day. ETU
was administered as a positive control at 50 mg/kg bw/day. All rats
were sacrificed on day 20 of gestation and caesarean sections
performed.
Food consumption and body weights were decreased at 128 mg/kg
bw/day and above in mancozeb treated dams. Body weights for all
other mancozeb-treated groups were comparable to the control group
as was the ETU-treated group. Litter data indicated significant
decreases in the average number of live fetuses, mean fetal weight
and mean gravid uteri and increased resorptions only at 512 mg/kg
bw/day for mancozeb-treated animals. Fetal weight was decreased in
ETU-treated animals, and one dam died on study. Adverse compound-
related effects were clearly manifested for mancozeb at 512 mg/kg
bw/day and ETU at 50 mg/kg bw/day for gross abnormalities (agnathia,
cleft palate, meningoencephalocele), soft tissue effects (dilated
ventricles, compressed spinal cord), and skeletal tissue (incomplete
ossification of the skull, clavicle, scapula). The effects observed
at 128 mg/kg bw/day were not biologically or statistically
significant. The NOAEL for maternal toxicity was 32 mg/kg bw/day
based on decreased body weight and decreased food consumption at 128
mg/kg bw/day. The NOAEL for teratogenic effects was 128 mg/kg bw/day
based, in part, on the presence of agnathia, cleft palate,
meningoencephalocele, dilated ventricles and incomplete ossification
of the skull at 512 mg/kg bw/day (Gallo et al., 1980).
Female Sprague-Dawley rats (25/dose/group) received 0, 10, 60,
or 360 mg/kg bw/day of mancozeb (88.6% pure) in methyl cellulose or
methyl cellulose alone (control group). Animals were dosed daily by
the oral route (gavage) from day 6 to day 15 (inclusive) of
gestation. Dams were observed on predetermined regular schedules
for clinical signs, mortality body weight, and food consumption. On
day 20 of gestation females were sacrificed with carbon dioxide and
uterine contents examined.
There were no readily apparent or statistically significant
differences between control values and values obtained in treated
groups for the parameters examined at either 10 or 60 mg/kg bw/day.
One dam was sacrificed in a moribund state at 360 mg/kg bw/day. Four
females exhibited a "reeling gait" followed by slight paralysis in
three of the dams. Body weights were decreased during the treatment
period (days 6-15) and days 16 through 20. Statistical significance
was attained on days 16 and 20. Food consumption was significantly
decreased from days 6-15 inclusive. A slight dose-related reduction
in the degree of ossification of the intraparietal bone was
statistically significant. The Incidence for this effect was 75, 80,
87 and 90% for control to high dose. The historical mean was 25% and
the range 4.90-91.0%. A marginal increase in the size of the
anterior fontanelle (12%) was also observed. The historical mean for
this effect was 2.6% with a range of 0-24%. Incomplete ossification
of the thoracic vertebrae centra was also statistically significant
at the high dose tested. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 60 mg/kg bw/day. Maternal toxicity at 360
mg/kg bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryo/fetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra (Tesh et al., 1988).
Rabbits
Groups of New Zeeland white rabbits (18 females/dose) were
treated by oral intubation with doses of 0, 5, 30 or 55 mg/kg bw/day
of Pencozeb Technical (88.4% pure) in methyl cellulose or methyl
cellulose, on days 6 through 18 of gestation. Animals were
sacrificed on day 28 post-coitum. There were no compound-related
effects at these dose levels either in-life or during examination
post-sacrifice. A second experiment was therefore conducted at a
single high dose of 100 mg/kg bw/day with a concurrent control group
following the general procedures and examinations of the prior
experiment. No treatment-related clinical effects or treatment-
related necropsy findings were detected. Two dams in the control
group and five in the 100 mg/kg bw/day dose group aborted. A clear
and substantial body-weight loss was observed in does at 100 mg/kg
bw/day for days 6-9 post-coitum (i.e. days 1-3 of compound
administration). Body-weight gain during days 9-15 was also
substantially depressed compared to controls. Food consumption was
also markedly decreased between days 6-19. A slight post-
implantation loss (5%) was also observed and may have been compound-
related. No compound-related fetal effects were observed. The NOAEL
for maternal effects was 55 mg/kg bw/day and greater than 100 mg/kg
bw/day for embryo/fetotoxic effects. An increase in abortion, body-
weight loss, suppressed body-weight gain and decreased food
consumption were observed at 100 mg/kg bw/day (Muller, 1991).
New Zeeland white female rabbits (20 animals/dose/group)
received either 0, 10, 30, or 80 mg/kg of bw/day of mancozeb (83.0%
pure) in methyl cellulose or methyl cellulose alone (control group)
by oral gavage on days 7-19 of gestation. No treatment-related
deaths occurred in control or in the 10 and 30 mg/kg bw/day dose
groups. One animal died in the 30 mg/kg bw/day group but death was
attributed to mis-dosing. Two does were sacrificed in a moribund
condition at the high dose and the deaths considered treatment-
related (one of these two does was pregnant and did not abort).
Clinical signs were comparable between controls and animals
receiving 10 and 30 mg/kg bw/day. Animals receiving 80 mg/kg bw/day
manifested significant increases in clinical signs. Alopecia,
anorexia, ataxia, scant faeces, and abortions were all observed at
the high dose tested. Body-weight gain and food consumption were not
significantly different between controls and groups receiving 10 or
30 mg/kg bw/day. At 80 mg/kg bw/day body-weight and food consumption
were significantly decreased in does that aborted and those
sacrificed moribund. Does producing at least one viable fetus had
body-weight gains and food consumption values similar to controls.
No treatment-related changes were evident in the incidence of
gross postmortem findings between does in the control and treated
group. Reproductive parameters between controls and does receiving
10 or 30 mg/kg bw/day were comparable as measured by the number of
abortions, litters produced, and the mean number per litter of
corpora lutea, implantations, resorptions, dead and live fetuses or
sex ratio. At 80 mg/kg bw/day a significant increase in does
aborting (5/15) was reported with a corresponding decrease in the
number of litters produced. All other parameters were comparable to
control group. Mean fetal body weight was similar between the
control and treated groups. There were no significant increases in
the types or incidence of malformations or developmental variations
between control or treated groups. The NOAEL for maternal toxicity
was 30 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was greater
than 80 mg/kg bw/day. Maternal toxicity at 80 mg/kg bw/day was
based on an increase in aborted fetuses, decreased number of litters
produced, decreased body-weight gain and food consumption, an
increase in clinical signs and death (Solomon & Lutz, 1987).
Special study on mancozeb stability in DMSO
14C-mancozeb (Dithane M-45; 80% wettable powder; 88% radio
purity) was suspended in DMSO (99% pure) at a nominal concentration
of 100 ppm and sampled and analyzed for mancozeb at the following
times: 0, 15, 30, 60, 90, 120, 150, 180 and 210 minutes. Maximum
concentration of mancozeb occurred at approximately 15 minutes.
Immediately after adding DMSO to mancozeb (zero time) there was
evidence of significant decomposition with continued rapid
degradation to three compounds, two of which were impurities in the
starting material. The majority of the mancozeb was degraded within
60 minutes and after 4 hours an average of 9% of the mancozeb
remained. The half-life of mancozeb in DMSO was calculated to be 37
minutes (Schweitzer, 1990).
Special studies on genotoxicity
The results of genotoxicity assays are given in Table 2.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
Special studies on irritation and sensitization
Guinea-pigs
Mancozeb has been demonstrated to be a strong sensitizer in the
Hartley strain female guinea-pig by the Guinea-Pig Maximization
Test. With induction concentrations of 5% (intradermal) and 25%
(topical) and challenge concentrations of 2% and 0.5% (topical) all
of the mancozeb-treated females (10/group) responded at both 24 and
48 hours. In the same studies, cross sensitization responses were
also seen with zineb, maneb and mancozeb. A dose-response
relationship between the incidence of skin sensitization and
induction and challenge concentration was also seen with maneb. The
purity was not stated for any of the test materials (Matsushita et
al., 1976, 1977).
Young adult Hartley guinea-pigs were divided into three
experimental groups and tested for delayed contact hypersensitivity
using a modified Buehler procedure. Animals received three 6-hour
induction doses (1 dose/week, within 3 weeks) of either 0.4 ml of
50% (w/v) mancozeb (83% pure) in distilled water, 0.1% (w/v) 1-
chloro-2,4-dinitrobenzene (DNCB) in 80% (v/v) aqueous ethanol
(positive control), or received no induction doses (control group)
but were otherwise treated similarly. All groups were challenged 12
days after the last induction dose/treatment. The sham treated
control group was challenged with test compound and DNCB on each
half of the back. Erythema and edema reactions were scored 24 and
48 hours after challenge using the method of Draize. All groups
were rechallenged 7 days after the primary challenge and a separate
additional control group was challenged as previously described. No
erythema or edema was observed in sham treated controls. Positive
controls showed positive response at 24 and 48 hours. Only one of
Table 2. Results of genotoxicity assays on mancozeb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 2.5-250 µg/plate 88% active Negative Chism, 1984a
reversion assay TA1535, TA1537, (± rat S9); ingredient (a.i.)
TA98, TA100 in distilled water
S. typhimurium 2.5-250 µg/plate (± rat S9); 88% a.i. Negative Chism, 1984b
TA1535, TA1537, in distilled water
TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.5-45 µg/ml; 88% a.i. Negative Foxall & Byers,
mutation assay (CHO)/hprt in distilled water 1984
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethal Muller-5 stock food medium Krishnamurthy, 1980
assay
Autosomal D. melanogaster 5-15 mg per 100 ml of Not specified Negative Vasudev &
recessive lethals Cy/B1 L2 stock food medium Krishnamurthy, 1980
1.D. Yeast and Other Fungal Assays
Point mutation A. nidulans 0.125-12 µg/ml; Not specified Positive Martinez-Rossi &
induction strains biA1 in distilled water; Azevedo, 1987
methG1 and 118 no activation used
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vitro Chromosomal Alterations in Mammalian Cells
In vitro Cultured human 1.40 µg/ml; Not specified Positive Georgian et al.,
chromosomal lymphocytes in propylene glycol; 1983
aberrations no activation used
2.B. In Vivo Chromosomal Alterations
Bone marrow Male non-inbred 10-1000 mg/kg; Not specified Negative2 Kurinnyi et al.,
cytogenetics white mouse in milk suspension 1982
or aqueous emulsion?
Wistar rat single i.p. 2.5-10 mg/kg; Not specified Positive Georgian et al.,
in propylene glycol 1983
Wistar rat 1.7 mg/kg bw/day for Not specified Positive Georgian et al.,
280 days; mixed in feed 1983
Male Fischer 344 rat 4.4 g a.i./kg/day for 1 88% a.i. Negative Sames et al., 1983
or 5 days; in corn oil
Male albino mouse 30-300 mg/kg; Not specified Positive Gautam & Kapoor,
(Lacca strain) in distilled water? 1991
Lymphocyte Female Wistar rat 3-30 mg/kg; in saline Not specified Positive Newton, 1975
cytogenetics
Micronucleus assay Male and female 10 000 mg/kg; 88.2% Negative Allen et al., 1987
CD-1 mouse in aqueous methyl-cellulose
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2.C. Plant Tests
Chromosomal Allium cepa 500-1000 ppm spray Not specified Positive Mann, 1977
alterations peduncles
Hordeum vulgare 100-1500 ppm; Not specified Positive Murty et al., 1983
germinating seeds in phosphate buffer
(with trace DMSO?)
Allium cepa 4-500 ppm Not specified Positive Badr, 1988
root tips
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro Primary rat 0.25-10 µg/ml; 88% a.i. Suggestive Byers, 1985
unscheduled DNA hepatocytes from male in culture medium positive;
synthesis (UDS) Fischer 344 rat suggests repeat
Primary rat 0.1-10 µg/ml; 82.4% Negative O'Neill & Frank,
hepatocytes from male in culture medium (also for S 1988
Fischer 344 rat phase induction)
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 5-20 µg/ml; Not specified Positive without Ivett, 1985
SCE assays (CHO) cells in culture medium activation
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell C3H/10T 1/2 cells 0.05-0.5 µg/ml; in water 88% a.i. Negative McGlynn-Kreft &
transformation McCarthy, 1984
C3H/10T 1/2 cells 0.1 µg/ml as "promoter"; 88% a.i. Negative McLeod & Doolittle,
with "promotion" in water 1985
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report.
2 The authors judged this effect to be positive, but significance was restricted to one dose and there was no dose-related response.
twenty animals given mancozeb (1/10 females) showed slight erythema
at 48 hours. After rechallenge the original female respondent was
negative but a second female (1/10 females) showed a slight erythema
at 24 hours but not at 48 hours (Trutter, 1988a).
Rabbits
Nine rabbits of unknown strain and sex were administered 100 mg
of ground mancozeb technical (> 80% pure) into the conjunctival sac
of one eye. The treated eyes of 3 rabbits were washed with water
approximately 20-30 seconds after dosing. Animals were observed for
eye irritation at 4, 24, 48, 72 and 96 hours and on days 7, 14 and
22. Substantial irritation was evident in unwashed eyes involving
the cornea, iris and conjunctiva. Effects were reversible after 14
days in unwashed eyes and after 72 hours in washed eyes.
A second study conducted in female New Zeeland white rabbits
using the same protocol produced responses of lesser consequence and
little or no involvement of the cornea or iris. Effects in unwashed
eyes were reversible after 7 days and after 24 hours in 2 of 3
rabbits with washed eyes. However conjunctival irritation persisted
in one rabbit through 14 days and disappeared at 21 days. The
compound was considered a substantial irritant based on the duration
of irritation (DeCrescente & Chan, 1982).
Six rabbits of unknown sex and strain were administered 500 mg
of ground mancozeb technical (> 80% pure), prepared as a paste in
saline, to intact and abraded skin. The test material was held
under an impervious patch in continuous 24-hour contact with closely
clipped skin. Erythema and edema were scored at 24 and 72 hours and
7 days. The only effect observed was erythema on abraded skin. The
primary irritation score was 0.5. The compound was considered a
slight irritant (DeCrescente & Parsons, 1980).
Observations in humans
A sample population of 121 Korean orange growers was tested for
hypersensitivity to the fungicide Dithane M-45 (mancozeb). A maximum
non-irritating concentration of 10% Dithane M-45 mixed with vaseline
was previously determined on 201 Korean medical students by patch
test. Results were read 48 hours post-administration. Application
of the patch test to orange growers was read 72 hours post-
administration according to the standards of the North American
Dermatitis Research group. Dithane tested positive in 4/121
subjects (3.3%) (Lee et al., 1981).
A 37-year old male working as a florist developed erythematous
vesicular eruption on the palms of his hands resembling dyshidrotic
eczema. Symptoms worsened and spread to other body areas (face,
neck, legs) during periods of heavy work, and diminished during
holidays. Patch tests with the mancozeb products Diagent and Firma
using Finn Chambers on Scanpor Tape read at 20 minutes, 2, 3, and 4
days resulted in a strong positive reaction on day two. A weak
positive reaction to zineb was also observed. Total serum IgE was
approximately five times the normal value. Other serum
immunoglobulins, G, A, and M were normal (Crippa et al., 1990).
A 61-year old vineyard worker developed a rash on the left
forearm as well as inflammation of the eyelids after planting vine
seedlings. This was the third episode under similar circumstances.
Patch tests on two previous occasions were negative. Patch tested
with 0.002% solution of both zineb and mancozeb (1% of normal use
concentration) resulted in positive responses. Retesting a week
later with the bark of a vine stick previously treated with mancozeb
produced a strong positive reaction. It was also reported that two
other male farm workers developed papulovesicular sheeted dermatitis
of the face, hands and chest after planting mancozeb-treated
potatoes. Patch tests with 0.1% solution of mancozeb were positive
in both. Patch tests conducted on the vineyard worker with
macerated vine bark were positive with aqueous but negative with
alcoholic solution (Kleibl & Rakcova, 1980).
One-hundred-fifty-three men currently or previously exposed to
dithane for many years at a manufacturing site were compared for
thyroid function to 153 men not exposed to Dithane, its products or
ETU who also worked at the same plant. Workers and controls were
carefully matched with respect to age, race, length of employment
and type of employment. Informed consent was obtained from all
participants. It was reported that at least one abnormality was
found in 25 of the 306 men (8.2%). The findings included
abnormalities on thyroid palpitation (4.3%), serologic autoimmune
thyroiditis (4.3%), subclinical hypothyroidism (1.3%), Graves'
disease (0.3%) and miscellaneous hormonal abnormalities (1.6%).
Urinary excretion of ETU in a subgroup of 42 workers currently
exposed to Dithane was 0.02 ppm compared to 0.01 ppm in the control
group (41 men), most of whom had undetectable values. The authors
concluded that exposure to Dithane manufacture was not associated
with an increased prevalence of thyroid abnormalities and that
thyroid abnormalities were common in iodine-replete adult American
men affecting 8.2% of the population studied (Charkes et al.,
1985).
A prevalence survey of adverse reproductive outcomes was
carried out in a population of 8867 persons (2951 men and 5916
women) who had been working in the floriculture industry in the
Bogota area of Columbia for at least 6 months. These workers from
58 companies were exposed to 127 different types of pesticides. The
prevalence rates for abortion, prematurity, still births, and
malformations were estimated for pregnancies occurring among the
female workers and the wives of the male workers before and after
working in floriculture and these rates were related to various
degrees of exposure. A moderate increase in the prevalence of
abortion, prematurity and congenital malformations was detected for
pregnancies occurring after the start of work in floriculture.
However, it could not be determined whether the increase in the
prevalence of the parameters measured was real. All rates, except
those for still births were higher for pregnancies occurring after
exposure to pesticides among both the female workers and the wives
of the male workers and thus gave significantly increased odds
ratios (chi-square) for abortions, premature births and malformed
babies. However, the high odds ratio observed for induced abortions
was considered by the authors surprising and cast doubt on the
reliability of the association between the observed increased risks
and the exposure to pesticides. It was also noted that of the 10
most used pesticides in floriculture, which accounted for 67% of the
total pesticides used in this industry, mancozeb comprised 7% of the
total. However, the article addressed pesticides in general and no
one chemical was singled out for adverse reactions. The authors
recognized that multiple exposure not only posed the problem of
identifying the toxic chemical responsible in causing any adverse
effect but also the problem of interaction between chemicals in the
expression of toxic effects (Restrepo et al., 1990).
COMMENTS
In pharmacokinetic studies conducted in male and female mice,
orally administered 14C-labelled mancozeb was rapidly absorbed,
peaking in whole blood between 1 and 2 hours, extensively
metabolized, and rapidly excreted (90%) within 24 hours. ETU was
the major metabolite.
Rats given single oral doses of 14C-labelled mancozeb absorbed
about 50% of the dose within 3-6 hours. Most of the dose was
excreted in 24 hours with half eliminated in the urine and half in
the faeces. Less than 4% was found in the tissues, with the thyroid
containing the highest residue level. Most of the 14C dose in
faeces was unabsorbed, since only 2-8% of the dose was found in
bile. ETU was the major metabolite. The half-life of ETU
elimination was 4-5 hours. The estimated bioavailability of ETU in
rats was about 6.8% on a weight/weight basis and 20% on a mole/mole
basis.
The acute oral, dermal and inhalation toxicity of mancozeb
technical is low. WHO has classified mancozeb as unlikely to
present acute hazard in normal use.
In a 13-week study in rats, mancozeb was administered in
dietary concentrations of 0, 30, 60, 125, 250 or 1000 ppm. The
NOAEL was 125 ppm (equal to 7.4 mg/kg bw/day) based on increased
serum TSH and decreased T4 values at the next higher dose.
Dogs administered 0, 10, 100, 1000 or 5000 ppm of mancozeb in
the diet for three months demonstrated a NOAEL of 100 ppm, equal to
3.0 mg/kg bw/day. At the next higher dose, decreased body-weight
gains and decreased erythrocyte count, haematocrit and haemoglobin
were observed.
In a 52-week study in dogs, mancozeb was administered in the
diet at concentrations of 0, 50, 200, 800 or 1600 ppm. The NOAEL
was 200 ppm, equal to 7.0 mg/kg bw/day, based on decreases in body-
weight gain, increased cholesterol and decreased haemoglobin and
packed cell volume at 800 ppm.
The NOAEL for dogs given mancozeb technical for 52 weeks, 7
days a week, by gelatin capsule was 2.3 mg/kg bw/day based on
decreased body weight, food consumption and decreased thyroxine
levels at 23 mg/kg bw/day.
In a 78-week carcinogenicity study in mice at dietary
concentrations of 0, 25, 100 or 1000 ppm, there was no evidence of
carcinogenicity. The NOAEL was 100 ppm, equal to 17 mg/kg bw/day,
based on decreased body-weight gain at 1000 ppm.
In a second 78-week carcinogenicity study in mice at dietary
concentrations of 0, 30, 100 or 1000 ppm in the diet, there was no
evidence of carcinogenicity. The NOAEL was 100 ppm, equal to 13
mg/kg bw/day, based on decreased body weight and decreased T3 and
T4 values at 1000 ppm.
The overall NOAEL in the two 78-week studies in mice was 17
mg/kg bw/day.
In a two-year toxicity/carcinogenicity feeding study in rats at
dietary concentrations of 0, 20, 60, 125 or 750 ppm, the NOAEL was
125 ppm (equal to 4.8 mg/kg bw/day) based on decreased body-weight
gain, decreased T3 and T4 values, increased TSH values, increased
absolute and relative thyroid weight, thyroid follicular cell
hypertrophy, hyperplasia, and nodular hyperplasia at 750 ppm.
Tumorigenic effects were noted in both sexes in the form of thyroid
follicular cell adenomas and/or carcinomas at the highest dose
level.
Mancozeb technical when administered in the diet to rats for
two years at dose levels of 0, 28, 113 or 454 ppm was not
tumorigenic. The NOAEL was 113 ppm (equal to 4.0 mg/kg bw/day)
based on decreased body-weight gain, decreased thyroxine levels and
an increase in the height of the thyroid follicular epithelium and
an increase in prominent microfollicles at 450 ppm.
The overall NOAEL in the two 2-year studies in rats was 4.8
mg/kg bw/day.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 25, 150 or 1100 ppm, the NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on decreased body weight at 150
ppm.
In a second two-generation reproduction study in rats at
dietary concentrations of 0, 30, 120 or 1200 ppm, the NOAEL was 120
ppm, equal to 7.0 mg/kg bw/day based on microscopic changes in the
thyroid, kidney and pituitary, increased relative weights of the
liver, kidney and thyroid, increased absolute thyroid weight,
decreased gestation and lactation body weight and feed consumption,
decreased pre-mating body weight and feed consumption at 1200 ppm.
The overall NOAEL in both reproduction studies was 7.0 mg/kg
bw/day.
In a 90-day (neuropathology) study conducted in rats at dietary
concentrations of 0, 20, 125, 750 or 5000 ppm, the NOAEL was 125
ppm, equal to 8.2 mg/kg bw/day, based on decreased food consumption
and neuro-histopathological changes at 750 ppm.
An oral teratogenicity study in rats at dose levels of 0, 2, 8,
32, 128 or 512 mg/kg bw/day produced no maternal effects at 32 mg/kg
bw/day (NOAEL) and no teratogenic effects at 128 mg/kg bw/day
(NOAEL). Maternal effects in the form of decreased body-weight gain
and decreased food consumption were seen at 128 mg/kg bw/day.
Teratogenic effects were seen at 512 mg/kg bw/day based, in part, on
the presence of agnathia, cleft palate, meningoencephalocele and
dilated brain ventricles.
A second oral teratogenicity study in rats at dose levels of 0,
10, 60 or 360 mg/kg bw/day showed no maternal or embryo/fetotoxic
effects at 60 mg/kg bw/day (NOAEL). Maternal toxicity at 360 mg/kg
bw/day was seen as "reeling gait", hind limb paralysis, and
decreased body-weight gain and food consumption. Embryofetotoxicity
at the highest dose was seen as reduction in the degree of
ossification of the intraparietal bone, a marginal increase in the
size of the anterior fontanelle and incomplete ossification of the
thoracic vertebrae centra.
The NOAEL in an oral teratogenicity study in rabbits given 0,
5, 30, 55, or 100 mg/kg bw/day was 55 mg/kg bw/day for maternal
effects and greater than 100 mg/kg bw/day for embryo/fetotoxic
effects. An increase in abortions, body-weight loss, and decreased
food consumption were observed at 100 mg/kg bw/day.
The NOAEL in an oral teratogenicity study in rabbits given 0,
10, 30 or 80 mg/kg bw/day was 30 mg/kg bw/day for maternal toxicity.
The NOAEL for embryo/fetotoxic effects was greater than 80 mg/kg
bw/day. Maternal toxicity at 80 mg/kg bw/day was based on an
increase in aborted fetuses, decreased number of litters produced,
decreased body-weight gain and food consumption, an increase in
clinical signs and death.
Mancozeb has been tested in a series of in vitro and in vivo
genotoxicity assays. Chromosomal aberrations were induced in
vitro, whereas conflicting data were obtained with in vivo
assays. There was no evidence for the induction of gene mutations
or cell transformations. The Meeting concluded that the data base
for mancozeb is equivocal for genotoxicity. A number of available
studies were not considered either because DMSO was used as a
solvent in which mancozeb is very unstable or because of important
omissions from the reports.
The data on mancozeb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 4.8 mg/kg bw/day for thyroid effects in rats
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for mancozeb, alone or in combination
with maneb, metiram, and/or zineb, because of the similarity of the
chemical structure of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differentiated using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet, equal to 17 mg/kg bw/day
(78-week studies)
Rat: 125 ppm in the diet, equal to 4.8 mg/kg bw/day
(two-year studies)
120 ppm in the diet, equal to 7.0 mg/kg bw/day
(reproduction studies)
125 ppm in the diet, equal to 8.2 mg/kg bw/day
(90-day neuropathology study)
Dog: 200 ppm in the diet, equal to 7.0 mg/kg bw/day
(52-week study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with maneb, metiram, and zineb)
Studies which will provide valuable information in the continued
evaluation of the compound
Clarification of the genotoxic potential of mancozeb.
Observations in humans.
REFERENCES
Allen, J.A., Proudlock, R.J. & Pugh, L.C. (1987). Micronucleus test
on mancozeb technical. Unpublished report No. PWT 39/86637 from
Huntingdon Research Centre Ltd., Huntingdon, Cambridgeshire,
England. Submitted to WHO by Elf Atochem, Philadelphia,
Pennsylvania, USA (Confidential data of Elf Atochem).
Badr, A. (1988). Cytogenetic activities of some fungicides.
Cytologia, 53: 635-640.
Braverman, L.E., Lipworth, L. & Charles, D. (1978). A health survey
of workers involved in the manufacture and packaging of dithane
fungicide with special reference to thyroid function. Unpublished
report dated August 2, 1978 from the University of Massachusetts
Medical School, Worcester, Massachusetts, USA. Submitted to WHO by
Rohm and Haas Company, Spring House, Pennsylvania, USA.
Broadmeadow, A. (1991a). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
89/PTC004/0015 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Broadmeadow, A. (1991b). Mancozeb technical: toxicity study by oral
administration to beagle dogs for 52 weeks. Unpublished report No.
90/PTC029/0197 from Life Science Research Ltd., Suffolk, England.
Submitted to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA
(Confidential data of Elf Atochem).
Byers, M.J. (1985). Dithane M-45 in vitro unscheduled DNA
synthesis assay. Unpublished report No. 84R-280 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Cameron, B.D., Clydesdale, K. & Speirs, G.C. (1990). The disposition
of 14C mancozeb in the mouse. Unpublished report No. 4909 from
Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Elf Atochem, Philadelphia, Pennsylvania, USA (Confidential
data of Elf Atochem).
Charkes, N.D., Braverman, L.E., Penko, K.F., Gowers, D.S., Gordon,
C.F., Lipworth, L, Malmud, L.S. (1985). Thyroid function in male
workers manufacturing dithane, an agricultural fungicide, and in men
not exposed to dithane. Frontiers in Thyroidology, 2: 933-936.
Chism, E. (1984a). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced Fischer 344 rats. Unpublished
report No. 84R-059 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Chism, E. (1984b). Dithane M-45 microbial mutagen assay: S-9
prepared from aroclor 1254 induced B6C3F1 mice. Unpublished
report No. 84R-0060 from Rohm and Haas Company, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Cox, R.H. (1986). Three month dietary toxicity study in dogs with
mancozeb. Unpublished report No. HLA 417-416 from Hazleton Labs.,
Vienna, Virginia. USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA as Rohm and Haas Report No. 86RC-7.
Crippa, M., Misquiih, L., Lunati, A. & Pasollini, G. (1990).
Dyshidrotic eczema and sensitization to dithiocarbamates in a
florist. Contact Dermatitis, 23: 203.
DeCrescente, M.E. & Chan, P.K. (1982). Dithane M-45: rabbit eye
irritation report. Unpublished report Nos. 81R-197, 81R-176 and 82R-
10 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeCrescente, M.E. & Parsons, R.D. (1980). Dithane M-45: Acute oral
and dermal LD50 and skin and eye irritation. Unpublished report No.
79R-180, from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DeGroot, A.P. (1974). Determination of acute ip toxicity of Dithane
M-45 in rats. Unpublished report from Central Institute for
Nutrition and Food Research TNO the Netherlands (Rohm and Haas
report No. 74RC-1011). Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
DiDonato, L.J., Cruzan, G. & O'Hara, G.P. (1985). Dithane M-45/ETU
four week range finding mouse study. Unpublished report No. 80R-123
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
DiDonato, L.J. & Longacre, S.L. (1986). Mancozeb pharmacokinetic
study in rats. Unpublished report No. 85R-123 (Supplement/Appendix
to Nelson, S.S. [1986] report No. 31H-86-02) from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978). A study of the uptake and elimination of
14C activity after the oral ingestion of 14C-labelled
ethylenethiourea (ETU) and mancozeb in the rhesus monkey.
Unpublished final (revised) report from Battelle Labs, Columbus,
Ohio, USA. dated July 31, 1978. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA. as Rohm and Haas report
No. 78RC-1028.
Everett, D.J., Atkinson, C., Perry, C.J., Strutt, A., Millar, P.,
Hudson, P. (1992). Mancozeb 78-week dietary carcinogenicity study in
mice with 52 week interim kill. Unpublished report No. 7561 from
Inveresk Research International, Tranent, Scotland. Submitted to WHO
by Elf Atochem, Philadelphia, Pennsylvania, USA. (Confidential data
of Elf Atochem).
Foxall, S. & Byers, M.J. (1984). Dithane M-45 CHO/HGPRT gene
mutation assay. Unpublished report No. 84R-207 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Gallo, M.A., Kam, C. & Stevens, D.R. (1980). Teratologic evaluation
of dithane M-45 in the albino rat. Unpublished report No. 10065-009
from Booz, Allen, and Hamilton, Inc., Florham Park, New Jersey, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA as Rohm and Haas report No. 81RC-75.
Gautam, D.C. & Kapoor, L. (1991). Genotoxic effects of Dithane M-45
on the bone marrow cells of mice in vivo. Experimentia, 47(3):
280-2.
Georgian, L., Moraru, J., Draghicescu, T., Dinu, I. & Ghizela, G.
(1983). Cytogenetic effects of alachlor and mancozeb. Mutation Res.
116: 341-348.
Goldman, P., Bernacki, H.J. & Quinn, D.L. (1986). Mancozeb: three
month dietary toxicity study in rats. Unpublished report No. 85R-167
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1980). Acute inhalation toxicity of
dithane M-45 (TD-79-224) in rats. Unpublished report No. 79R-210
from Rohm and Haas Company, Spring House, Pennsylvania, USA
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1982). Dithane M-45: Acute inhalation
toxicology study in rats. Unpublished report No. 81R-171 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Hagan, J.V., Fisher, J.R. & Baldwin, R.C. (1986). Mancozeb:
subchronic inhalation toxicity study in rats. Unpublished report No.
86R-3 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Hagan, J.V. & Baldwin, R.C. (1986). Mancozeb: two week range finding
inhalation toxicity study in rats. Unpublished report No. 85R-190
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Haines, L.D. (1980). Dithane M-45 percutaneous absorbtion in rats.
Unpublished technical report No. 34F-80-9 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 80R-1006.
Hooks, W.N., Offer, J.M., Hadley, J.C., Gibson, W.A., Gopinath, C. &
Dawe, I.S. (1992). Mancozeb technical: potential tumorigenic and
toxic effects in prolonged dietary administration to rats.
Unpublished report No. PWT 29/89669 from Huntingdon Research Centre
Ltd., Huntingdon, Cambridgeshire, England. Submitted to WHO by Elf
Atochem, Philadelphia, Pennsylvania, USA.
Ivett, J.L. (1985). Mutagenicity evaluation of Dithane M-45 in an
in vitro sister chromatid exchange assay in the Chinese hamster
ovary (CHO) cells. Unpublished reported dated March 1985 from Litton
Bionetics, Inc., Kensington, Maryland, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA as Rohm and Haas
report No. 84RC-60.
Kleibl, K. & Rakcova, M. (1980). Cutaneous allergic reactions to
dithiocarbamates. Contact Dermatitis, 6(5): 348-349.
Kocialski, A. (1989). Establishment of an in vitro metabolic
conversion factor of 7.5% of all EBDCs when converting EBDCs to
ethylenethiourea in vivo and recalculations of the previously
considered 20% in vivo conversion exposure factor for EBDCs to
ETU. Memorandum to the Caswell file, Health Effects Division, Office
of Pesticide Programs, United States Environmental Protection
Agency.
Kurinnyi, A., Pilinskaya, M., German, J. & L'vova, T. (1982).
Implementation of a program of cytogenetic study of pesticides:
preliminary evaluation of cytogenetic activity and potential
mutagenic hazard of 24 pesticides. Cytol. Genet. 16: 50-53.
Lee, Y.S., Cinn, Y.W., Chang, W.H. & Kim, J.S. (1981). A study on
hypersensitivity of Korean farmers to various agrochemicals.
Determination of concentration for patch tests of fruit-tree
agrochemicals and hypersensitivity of orange orchard farmers in Che-
Ju Do, Korea. The Seoul Journal of Medicine, 22: 137-142.
Longacre, S. (1986). Summary of ETU and EBDC analysis in plasma,
liver, and thyroid after mancozeb administration. Unpublished report
No. 86R-1009 from Rohm and Haas Company, (appendix/supplement to
Nelson 1986 31H-86-02) Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lu, M. & Kennedy, G. (1986). Teratogenic evaluation of mancozeb in
the rat following inhalation exposure. Toxicol. and Appl. Pharma.
84: 355-368.
Mann, S.K. (1977). Cytological and genetical effects of dithane
fungicides on Allium cepa. Environmental and Experimental Botany,
17: 7-12.
Martinez-Rossi & Azevedo, J. (1987). Detection of point mutation
mutagens in Aspergillus nidulans: comparison of methionine
suppressors and arginine resistance induction of fungicides.
Mutation Research, 176: 29-35.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb and their related compounds. Int. Arch. Occup. Environ.
Health, 37: 169-178
Matsushita, T. Yoshioka, M., Arimatsu, Y. & Nomura, S. (1977).
Experimental study on cross-contact allergy due to dithiocarbamate
fungicides. Indust. Health, 15: 87-94.
McGlynn-Kreft, A.M. & McCarthy, K.L., (1984). Dithane M-45 mammalian
cell transformation test. Unpublished report No. 84R-55 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
McLeod, P.L. & Doolittle, D.J. (1985). Dithane M-45 mammalian cell
transformation test for promotion. Unpublished report No. 84R-0297
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Muller, W. (1991). Oral (gavage) teratogenicity study in the rabbit.
Unpublished report No. HLD-853-683-002 from Hazleton Labs.,
Deutschland GmbH, Munster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Muller, W. (1992). 2-Generation oral reproduction toxicity study in
the rat. Unpublished report No. HD-852-683-001 from Hazleton Labs.,
Deutschland GmbH, Münster, Germany. Submitted to WHO by Elf Atochem.
(Confidential data).
Murty, K., Raju, D. & Sharma, C. (1983). Cytogenetic hazards from
agricultural chemicals. 7. Herbicides, fungicides and insecticides
screened for effects on chiasmata in Hordeum vulgare. Biol. Zb1.
102: 571-576.
Nelson, S.S. (1986). Metabolism of 14C mancozeb in rats.
Unpublished report No. 31H-86-02 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Nelson, S.S. (1987). Bioconversion of mancozeb to ETU in the rat.
Unpublished report No. 31C-87-24 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 80R-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neill, P. & Frank, J.P. (1988). Mancozeb: in vitro unscheduled
DNA synthesis assay in F/344 rat hepatocytes. Unpublished report No.
88R-079 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Piccirillo, V.J., Wu, D. & Speirs, G. (1992). Metabolism of mancozeb
in the mouse. Unpublished report No. T91-3413 from Inveresk Research
International, Ltd., Tranent, Scotland. Submitted to WHO by Elf
Atochem (Confidential data).
Restrepo, M., Munoz, N., Day, N.E., Parra, J.E., deRomero, L. &
Nguyen-Dinh, X. (1990). Prevalence of adverse reproductive outcomes
in a population occupationally exposed to pesticides in Columbia.
Scand. J. Work. Environ. Health, 16(4): 232-238.
Sames, J.L., McLeod, P.L. & Doolittle, D.J. (1984). Dithane M-45 in
vivo cytogenetic study in Fischer 344 rats. Unpublished report No.
84R-0246 Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Schweitzer, M. (1990). A study of the decomposition of mancozeb in
dimethyl sulfoxide solution. Unpublished report No. SC890012 from
Battelle Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. TR 34-90-01.
Shaw, D. (1990). Mancozeb: 52 week oral (dietary) toxicity study in
the beagle. Unpublished report No. HUK 5913-616/3 from Hazleton, UK,
Harrogate, North Yorkshire, England. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA, as Rohm and Haas
report No. 88RC-027.
Shellenberger, T. (1991) Mancozeb: 18-month dietary oncogenicity
study in mice. Unpublished report No. 85051 from Tegeris Labs.,
Inc., Temple Hills, Maryland, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA, as Rohm and Haas report
No. 86RC-029.
Solomon, H.M. & Lutz, M.F. (1987). Mancozeb: oral (gavage)
developmental toxicity study in rabbits. Unpublished report No. 86R-
021 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Solomon, H.M., Lutz, M.F. & Kulwich, B.A. (1988). Mancozeb: 2-
generation reproduction study in rats. Unpublished report No. 87R-
020 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Stadler, J. (1990). Combined chronic toxicity/oncogenicity 2-year
feeding study with mancozeb in rats. Unpublished report No. 259-89
from E.I. duPont de Nemours and Company, Haskell Laboratory for
Toxicology and Industrial Medicine, Newark, Delaware. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Stadler, J. (1991) Neuropathology study in rats with mancozeb.
Unpublished report No. 217-89 from E.I. duPont de Nemours and
Company, Haskell Laboratory for Toxicology and Industrial Medicine,
Newark, Delaware. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Szepvolgyi, J., Nagy, K., Sajgone-Vukan, K., Regoly-Merci, A., Soos,
K., Toth, K., Pinter, A. & Antal, M. (1989). Subacute toxicological
examination of Dithane M-45. Fd. Chem. Toxic, 27(8): 531-538.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Enticott, J., Wilby,
O.K., Tesh, S.A. (1988). Mancozeb: teratology study in the rat.
Unpublished report No. 87/PTC 007/365 from Life Science Research,
Suffolk, England. Submitted to WHO by Elf Atochem (Confidential data
of Elf Atochem).
Tomlinson, H.L. & Longacre, S.L. (1988). Mancozeb dermal absorbtion
study in male rats. Unpublished report No. 88R-218 from Rohm and
Haas, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm and
Haas, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988a). Mancozeb: delayed contact hypersensitivity
study in guinea-pigs. Unpublished report No. HLA 417-431 from
Hazleton Labs., Vienna, Virginia, USA. Submitted to WHO by Rohm and
Haas Company, Spring House, Pennsylvania, USA.
Trutter, J.A. (1988b). Mancozeb: 4 week repeat dermal toxicity study
in rats. Unpublished report No. HLA 417-432 from Hazleton Labs.,
Vienna, Virginia, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Vasudev, V., Krishnamurthy, N.B., (1980). Non-mutagenicity of the
fungicide Dithane M-45 as inducer of recessive lethals after larval
feeding in Drosophila melanogaster. Mutation Res. 77: 189-191.
Watts, M.H. & Chan, P.K. (1984a). Dithane M-45: acute oral toxicity
study in rats and mice. Unpublished report No. 83R-213a + b from
Rohm and Haas Company, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Watts, M.H. & Chan, P.K. (1984b). Dithane M-45: acute oral toxicity
study in rats. Unpublished report No. 83R-218 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
