
ETHYLENETHIOUREA (ETU)
First draft prepared by
A. Kocialski, Office of Pesticide Programs,
US Environmental Protection Agency, Washington, DC, USA
EXPLANATION
ETU was reviewed in conjunction with the ethylene bis
dithiocarbamates (EBDCs) by the Joint Meeting in 1963, 1965, 1967,
1970, 1974, 1977, 1980, 1986, and 1988 (Annex I, references 2, 4, 8,
14, 22, 28, 34, 47 and 53). In 1988, the Joint Meeting extended the
temporary ADI of 0-0.002 mg/kg bw pending the submission of
additional data. ETU is also of interest because it forms part of
the terminal residue to which consumers of produce treated with the
EBDCs are exposed and because the levels of ETU in treated produce
generally increase during food processing as the levels of the EBDC
parent compounds decrease.
This monograph summarizes new or not previously-reviewed data
on ETU as well as relevant data on this substance from previous
monographs and monograph addenda on the EBDCs.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and biotransformation
Mice
Twenty-one adult male ND/4(S)BR mice were divided into groups
that received oral doses of 0.05 or 0.25 mmol/kg bw of either 14C-
ETU (99% pure), ethylenebis(isothiocyanate) sulfide (14C-EBIS, 99%
pure), or 14C-labelled maneb or zineb (purity not stated). EBIS,
ETU, EU (ethyleneurea) and other products were absent from maneb and
zineb. Pooled 0-24 and 24-48 hour urine samples were analyzed for
radioactive products. None of the administered compounds was
excreted as radiolabelled CO2. Essentially all of the ETU was
recovered in excreta within 48 hours. Approximately 10% of the
radioactivity from maneb and zineb was excreted in the urine whereas
between 40 and 70% of the EBIS radioactivity and about 50% of the
ETU radioactivity was excreted in the urine, approximately half of
which was unchanged ETU. Approximately 12% of the radioactivity
excreted in the urine following ETU administration was EU, with the
remainder being unidentified polar products. The administration of
EBIS at the lower dose produced 99.7% unidentified polar products
while at the higher dose, ETU and EU were each present at 10%, and,
polar products were reduced by 25%-76%. ETU in urine amounted to
0.5% and 1.3% of the administered high- and low-dose of maneb,
respectively. Following the 0.25 mmol/kg bw dose of zineb, 1% of
the radioactivity was present in the urine as ETU. The majority of
the radioactivity in the urine of mice given maneb or zineb was
present as unidentified polar products. No EBIS was detected in the
urine of mice given maneb or zineb (Jordan & Neal, 1979).
Two weeks prior to breeding, four female C57BL/6N mice were
administered ETU (96.5% purity) in the diet at dose levels of 0, 33,
100, 333, or 1000 ppm. During the gestation period the level of ETU
equivalents in amniotic fluid, placenta and fetal carcass correlated
with maternal blood levels; however, levels were increased in
maternal livers (3 times). No differences between dosed dam and
fetuses were observed. In the post-partum period, accumulation of
ETU equivalents was much more apparent, with ETU equivalents in
maternal liver approximately 10 times greater than maternal blood.
Levels of ETU equivalents were also increased 2 times in maternal
milk compared to maternal blood. Levels in maternal milk were 13
times neonatal blood levels. Neonatal liver and blood significantly
correlated with regard to ETU equivalents. Pretreatment did not
alter the pharmacokinetics of ETU in post-partum dams or their
neonates (Peters et al., 1982).
Mice/rats
A dose of 240 mg/kg bw of ETU (> 98% purity) was administered
via stomach intubation to pregnant mice and rats on day 15 of
gestation. Radiolabel concentrations peaked in mice and rats at
approximately the same time, 1.3 and 1.4 hours after dosing,
respectively, and maternal and fetal tissue levels were similar 3
hours after treatment. Thereafter levels in mouse tissues (maternal
and fetal) declined more rapidly. The half-lives for ETU
elimination from maternal blood were 5.5 and 9.4 hours in mice and
rats, respectively. The main route of excretion was via the urine
with 74% and 70% of the applied dose excreted by the mouse and rat,
respectively, in 48 hours (Ruddick et al., 1977). In mice, ETU
comprised 40% of labelled metabolites in urine versus 95% in rat.
This suggested more rapid metabolism in the mouse than in the rat.
The major urinary metabolite identified in the mouse was 2-
imidazolin-2-yl sulfemic acid from the oxidation of ETU (Savolainen
& Pyysalo, 1979).
Rats
Male Sprague-Dawley rats (4/group) received single dermal
applications of 2.6, 26, or 260 µg 14C-ETU/rat. Ten hours after
application the treatment area was wiped, excreta (urine/faeces)
were collected and animals were sacrificed. Application sites
(skin) were removed and analyzed for 14C-label. Whole blood,
plasma, thyroid, liver along with additional organs/tissues and the
remaining carcass were collected and analyzed. Another set of male
Sprague-Dawley rats (8/group) received single applications of 0 or
2.6 µg 14C-ETU/rat by either the oral, dermal, or intravenous
route. Initial blood samples were drawn at 5 and 30 minutes in
animals administered by the i.v. route and oral/dermal routes,
respectively. Initial collection of excreta began at 10 hours after
application. All samples were collected on a predetermined schedule
through termination at 7 days. The skin and carcass were examined
for 14C content. One group receiving dermal exposure had the
treatment area wiped at 10 hours, the second groups at 7 days post-
administration.
Animals receiving i.v. and oral administration showed 100 and
91% total absorption, respectively, with at least 85% (oral)
appearing in the excreta. ETU was primarily excreted in the urine
within 24 hours. The percent absorption of ETU for animals
receiving 2.6 µg/rat, swabbed (wiped) at 10 hours and terminated at
10 hours or 7 days was 17% and 26%, respectively. Animals receiving
2.6 µg/rat and left unwiped for 7 days showed 53% absorption.
Animals receiving 26 and 260 µg/rat, wiped at 10 hours and
immediately sacrificed recorded absorption values of only 5 to 6%.
Total recovery of 14C-label ranged from 80% to 100% for all routes.
The amount of applied material remaining at the skin site after 10
hours of exposure was about 40% for animals receiving 2.6 and 26 µg
and about 13% at 7 days after wiping at 10 hours.
At a single dose of 2.6 µg-ETU, 70%-90% was excreted within 24
hours, mostly in the urine (40-65%). Cumulative total 14C-
excretion in male rats following 10 hour dermal exposure with 2.6 µg
ranged between 6 and 13% at 24 hours and between 13 and 22% at 24
hours. Cumulative excretion after wiping at 10 hours indicated that
test material bound to the skin continued to be absorbed to some
degree. At 7 days, total cumulative excretion was 20-28%. The
majority of the excretion occurred in the urine. Tissue
concentration 10 hours after administration for the 12 tissues
examined ranged from 0 ppm to 0.457 ppm with the greatest amount
concentrating in the thyroid (0.457 ppm), which represented 0.01% of
the 260 µg administered. Animals receiving the lower doses of 2.6
and 26 µg/rat of ETU showed no thyroid accumulation of ETU. The
limit of detection for ETU in the thyroid was approximately 0.020
ppm. The lack of 14C accumulation in the thyroid at 26 µg/rat and
lower doses could be explained in part by the fact that the data
appeared to indicate that a finite amount (approximately 0.09 µg) of
ETU or its metabolites was preferentially bound to red blood cells
(DiDonato & Longacre, 1987).
Either 2-14C-ETU or 4,5-14C-ETU (> 98% purity) was
administered to four pregnant Wistar Imamichi rats at 100 mg/kg bw
via intra-gastric intubation on the 12th day of gestation. Whole
body radiography, TLC and GC were used to analyze the uptake of
radioactivity in tissues of both the fetus and the dam.
Radioactivity in the fetus reached maximum activity within 2 hours
and declined thereafter. Differences were observed between 2-ETU
and 4,5-ETU with respect to protein fraction incorporation.
Radioactivity was distributed homogeneously throughout all tissues
except for the thyroid, where there was an increase in activity
during the first 24 hours. Thyroid hormones are reported to play
important roles in the development of the CNS and thyroidectomy
induces malformations in the rat. There was no significant
difference in the T4 levels between treated and control maternal
serum, whereas the appearance of malformed fetuses was significant
at 100 mg/kg bw (malformations were observed in 100% of the fetuses
from treated dams) (Kato et al., 1976).
Wistar female rats were treated with single oral doses of 240
mg/kg bw ETU (purity not specified) and 25 or 50 µCi/kg bw of 14C-
ETU (99% pure) on days 11 or 12 of gestation and sacrificed at 6, 12
or 24 hours post-treatment. Radioactivity in maternal kidney,
liver, blood and urine as well as pooled embryos was determined.
Additional animals dosed on day 15 were sacrificed at 3 hours post-
dosing and in addition to the above tissues, the muscle and placenta
were analyzed for radioactivity. Blood levels were determined
at 0.5, 1, 2, 4, 6, 12, 24 and 48 hours. Urine analysis was
conducted at 12, 24, 32 and 48 hours. The binding of ETU to
maternal RBCs was also studied, as well as the binding to embryonic
tissues (DNA, RNA) in pooled day-12 embryos at 6 and 12 hours post-
treatment. Metabolites in urine were also investigated.
The distribution of radioactivity in maternal tissues (kidney,
liver, blood) was essentially the same at 6 and 12 hours post-dosing
on days 11 or 12, but the level decreased 80-90% at 24 hours. The
distribution of radioactivity in fetal tissue on days 11 or 12 at 6
and 24 hours was generally comparable. However, at 12 hours day 12
values were decreased approximately 50%, whereas the day 11 value at
12 hours was unchanged from the 6-hour reading. The distribution of
radiolabel in maternal tissues was 1.2-2.5 times greater at all time
periods at days 11 and 12. Radioactivity in urine was similar at
all times examined on days 11 and 12 of gestation. Radioactivity
levels in maternal liver, kidney, muscle and placenta and the fetus
at 3 hours post-administration on day 15 of gestation were similar.
The maternal blood half-life was calculated to be about 10 hours.
The radioactive label, which was weakly bound to metabolites,
was distributed uniformly between RBCs and plasma of maternal blood.
No radioactive label was detected in DNA, RNA or the protein
fractions of embryonic tissues. Metabolites in maternal urine
generally indicated the same pattern at all treatment times -
primarily ETU with traces of ethyleneurea and 2 unidentified
metabolites (Ruddick et al., 1976).
Two weeks prior to breeding, four female Fischer 344 rats were
administered ETU (96.7% purity) in the diet at dose levels of 0, 8,
25, 83 or 250 ppm. During the gestation period the amount of ETU
equivalents measured in maternal liver, amniotic fluid and fetal
carcass correlated with the maternal blood level, but the placental
levels did not. Transplacental transport was demonstrated. Post-
partum, there was an apparent transfer of ETU to the nursing pups
via the milk. Levels of ETU equivalents in maternal liver, maternal
milk, neonatal blood and neonatal liver were increased compared to
maternal blood levels. There were no significant differences,
however, between ETU equivalents in maternal milk and levels in
neonatal blood. No accumulation of ETU in neonatal liver or maternal
liver was observed. The level of ETU in neonatal liver correlated
with the levels in neonatal blood. Prior exposure of maternal
animals to ETU did not affect the pharmacokinetic behaviour of ETU
in post-partum animals (dam and neonate) (Peters et al., 1982).
Approximately 80-82% of a single oral 4 mg/kg bw dose of 14C-
ETU (> 99% purity) was eliminated via the urine within 24 hours by
three male Sprague-Dawley rats. A half-life of 5.6 hours in rat
blood was demonstrated. Unchanged ETU represented 62.6% of the
radioactivity in rat urine. Metabolites included EU (18.3%),
imidazolone (4.9%) and imidazoline (1.9%) (Iverson et al., 1980).
Rats/guinea-pigs
Six male adult Wistar rats and six male Hartley guinea-pigs
were fasted for 24 hours prior to the administration of 20 mg/kg bw
ETU (purity not given) by oral intubation as a single dose. Food
and water were withheld for 5 hours post-dosing. Urine and faecal
samples were collected and animals sacrificed at 96 hours post-
dosing. Liver, kidney, heart, thyroid and muscle were excised and
frozen. ETU analysis was carried out by gas-liquid chromatography.
Recovery was 90% or greater. The limit of detection was 0.005 ppm
of ETU. At 24 hours post-dosing 60% of the administered dose was
excreted unchanged in the urine of rats and 44% in the urine of
guinea-pigs. Urinary excretion of ETU was complete at 72 hours
(64%) in rats and at 48 hours (46%) in guinea-pigs. Rats eliminated
1.1% of the administered dose in faeces while guinea-pigs eliminated
0.8% in faeces within a 48-hour period. Mean residue levels in
liver, kidney, heart and muscle ranged from 0.01 ppm to 0.086 ppm.
Thyroid concentrations of ETU in rats and guinea-pigs were 0.82 and
0.75 ppm, respectively (Newsome, 1974).
Guinea-pigs
The backs of 12 male Hartley guinea-pigs were shaved and the 12
animals divided into 2 groups of 6 each. The epidermis of the backs
of one group was abraded. ETU (99% pure; 15 mg/ml) was applied to
both groups over an area of 40 X 40 mm, which was then covered with
non-woven fabric. After 24 hours three animals of each group were
sacrificed and the distribution of radioactivity as a percent of the
applied dose was determined for blood, certain internal organs,
faeces, urine, skin, the fabric covering and the rinse wash from the
application site. The remaining animals were sacrificed at 24 hours
and radioactivity was determined in a number of tissues. Another
group of 25 Hartley male guinea-pigs was dosed orally with a single
dose of 5 mg/kg bw ETU. Five animals were sacrificed 1, 3, 6, 24,
or 48 hours after dosing, and the concentration of radioactivity was
determined in a number of tissues. Additionally, urine was
withdrawn directly from the urinary bladder 2 hours after oral
administration, after which urinary metabolite determinations were
made.
Absorption of ETU from intact and abraded skin was 14 and 42%,
respectively, at 24 hours. The highest concentration of
radioactivity was found in the thyroid, which was at least 10 times
greater than in any other tissue. One hour after oral
administration, the radioactivity was distributed evenly among all
organs and tissues except adipose tissue. At 48 hours, most of the
radioactivity had cleared from all the organs and tissues except the
thyroid. The radioactive half-life was 13 hours in the liver, about
7.5 hours in the kidney and blood, and about 42 hours in the thyroid
gland. Nearly 80% of the administered radioactivity was excreted in
the urine within 48 hours and about 10% was recovered in the faeces.
Thin-layer chromatograms of urine collected from the urinary bladder
indicated that ETU was the primary metabolite (93%), with about 7%
of the parent compound converted to unidentified but strongly polar
metabolites (Teshima et al., 1982).
Cats
Approximately 80-82% of a single oral 4 mg/kg bw dose of 14C-
ETU (> 99% purity) was eliminated via the urine within 24 hours by
3 female cats. The half-life was 3.5 hours in blood. Unchanged ETU
represented 28% of the radioactivity in urine, while S-methyl ETU
comprised 64% of the radioactivity in urine (Iverson et al.,
1980).
Rats/monkeys
Four female Sprague-Dawley rats and two adult female rhesus
monkeys were given 14C-ETU (99% pure) by stomach tube at a dose of
40 mg/kg bw in a water vehicle as a single dose. Animals were
housed singly in metabolism cages and the excreta were collected
over a 48-hour period, after which time the animals were sacrificed
and all tissues, including skin, muscle and bone were weighed.
Representative samples from each tissue were oxidized and counted on
a scintillation spectrometer. Additional samples of tissue were
embedded in paraffin and sectioned followed by staining with
haematoxylin and eosin.
No gross or microscopic changes were observed in either
species. Urinary excretion at 48 hours in monkeys ranged between 47
and 64% of the total administered radioactivity, while it averaged
82% in rats. Less than 1.5% was found in the faeces of both
species. Total tissue distribution (i.e. total body burden at 48
hours) ranged between 21 and 28% of the administered dose in monkeys
and less than 1% in rats. Muscle, blood, skin and liver contained
12, 2.8, 2.4 and 1.0%, respectively, of the initial dose in monkeys
and less than 0.3% in rats. One female monkey had a slightly higher
concentration of 14C in the thyroid compared to other tissues
within the same animal. Two rats had higher 14C activity in the
thyroid gland than in other tissues (Allen et al., 1978).
Monkeys
Male Macaca mulatta (rhesus) monkeys were given 2-3 mg/kg bw
14C-ETU by oral gavage. Whole blood and excreta (urine and faeces)
were collected and examined. Radioactivity peaked in blood at 8
hours and declined rapidly at 24-48 hours. Approximately 50% of the
dose was excreted in urine within 24 hours. Less than 1% of the
dose was recovered in faeces during the first 24 hours, and none
thereafter (Emmerling, 1978a).
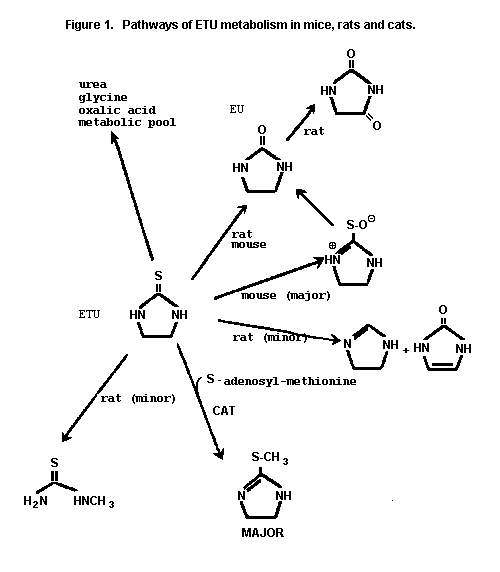
The pathways of ETU metabolism in mice, rats and cats are given
in Figure 1.
Effects on enzymes and other biochemical parameters
Mice
Liver microsomes were prepared from 3- and 30-week old male and
female Swiss-Webster mice to determine relative flavin monooxygenase
(FMO) activity and cytochrome P-450 activity via oxidation of N,N-
dimethylaniline and N-demethylation of dimethylaniline,
respectively. Enzyme activity related to ETU metabolism and binding
was also evaluated. FMO activity was significantly lower in older
males than in young males. No such differences were observed in
comparisons between females. N-Demethylase activity was not
affected by age, sex, or sensitivity to the heat denaturation
effects to FMO. ETU metabolism was similar in young and older
females, but it was significantly lower in older males than in young
males. FMO-dependent activity accounted for 75% of the total
binding in all animals, but microsomes from older males bound
significantly less radioactivity (30%) than those from young males
(Hui et al., 1988).
Mice/rats
Possible qualitative differences in the metabolism of ETU
between mice and rats have been noted on the basis of urinary
metabolites and measurement of microsomal enzymes. Microsomal
enzymes (aminopyrine N-demethylase, aniline hydroxylase, and
cytochrome P-450) were inhibited in rats, whereas in mice they were
stimulated. This suggests that ETU is metabolized by different
enzymatic pathways in the two species (Lewerenz & Plass, 1984).
 Rats
Male Sprague-Dawley rats were pre-treated with phenobarbital,
dexamethasone, beta-napthoflavone or left untreated. The in vitro
effect of ETU or EU in the presence and absence of glutathione,
NADPH and heat inactivated microsomes on P450 enzymatic activity and
on covalent binding of ETU to microsomal proteins was studied. ETU
inhibited P450 activity in pretreated and non-pretreated rats.
Inhibition was NADPH-dependent and was abolished by glutathione
(GSH). Covalent binding of 14C-ETU to microsomal protein was also
NADPH-dependent. Binding was inhibited by co-incubation with GSH.
Heat treatment of microsomes and P450 inactivation studies indicated
a prominent role of FMO in covalent binding. Addition of GSH or
dithiothreitol after incubation of microsomes resulted in release of
bound ETU. Metabolism of ETU in the presence of GSH resulted in the
formation of GSH-ETU adducts and subsequent disulfide exchange. The
results suggest that reactive metabolites from ETU generated by
either FMO or P450 are trapped by GSH. Initial oxidation of ETU to
imidazoline-2-sulfenic acid, primarily by FMO, followed by reaction
with GSH or protein sulfhydroyls under conditions of GSH depletion,
has been proposed as the route of monooxygenase-mediated metabolism
of ETU (Decker & Doerge, 1991).
Male Sprague-Dawley rats were divided into 3 groups in a study
designed to study the effect of ETU on RNA synthesis. One group
that had been fasted for 16 hours received single i.p. injections of
2.5 or 250 mg/kg bw ETU or 5.0 mg/kg bw thioacetamide, followed by
3H-orotic acid 60 minutes later. Control animals received dimethyl
sulfoxide alone. A second group received 5.0 or 250 mg/kg bw/day
ETU by gavage on 3 successive days followed by administration with
3H-orotic acid; the animals were killed on the fourth day. The
third group was administered 5.0 or 250 ppm ETU in the diet for 3
weeks. At the end of 3 weeks, a 1-hour pulse dose of 3H-orotic
acid was administered and the animals killed. Serum T4 levels in
animals given ETU were then determined by radioimmunoassay. Rats
receiving 400 ppm of acetylaminofluorene in the diet served as
positive controls.
The livers of all animals given either ETU or thioacetamide
were histologically normal at the time of sacrifice. The livers of
rats fed acetylaminofluorene showed mild hydropic change of
hepatocytes and minimal bile duct proliferation. ETU failed to
inhibit nuclear or cytoplasmic RNA synthesis under the test
conditions. However, thioacetamide and acetylaminofluorene both
reduced the incorporation of 3H-orotic acid into nuclear and
cytoplasmic RNA (Austin & Moyer, 1979).
Pigs
FMO purified from hog liver catalyzes NADPH and oxygen-
dependent sequential S-oxidation of ETU, proceeding through an
intermediate imidazolinyl sulfenic acid to the corresponding
sulfinic acid. Further oxidation to the sulfonic acid was partly
enzymic and partly due to autooxidation. The FMO-oxidative pathway
predominated over P-450 pathways in hog and hamster liver microsomes
(Poulsen et al., 1979).
The mechanism of thyroid peroxidase inhibition by ETU was
studied in vitro using purified thyroid peroxidase obtained from
hog thyroid. ETU inhibited iodination reactions catalysed by thyroid
peroxidase. Inhibition occurred only in the presence of iodide ion
and proceeded with concomitant oxidative metabolism of ETU to
imidazoline and bisulfite ion. The inhibition ceased upon
consumption of ETU, with no loss of enzymatic activity and
negligible covalent binding of ETU to the enzyme. This reversible
thyroid peroxidase inhibition contrasts with the activity of the
therapeutic antithyroid drugs such as methimazole which act as
suicide inhibitors via covalent binding to the prosthetic heme group
(Doerge & Takazawa, 1990).
Toxicological studies
Acute toxicity studies
ETU is slightly toxic after oral administration to mammalian
species with measured LD50 values ranging from 545 mg/kg bw in
pregnant rats (Teramoto, 1978) to 4000 mg/kg bw in adult mice
(Lewerenz & Plass, 1984). The acute toxicity of ETU in various
animal species is given in Table 1.
Guinea-pigs
ETU (purity not stated) is a moderate to weak sensitizer in the
Hartley strain female guinea-pig by the guinea-pig maximization
test. With induction concentrations of 5% (intradermal) or 25%
(topical) and challenge concentrations of 2% or 0.5% (topical),
females responded positively at 24 hours (1/10 at 0.5%; 7/10 at 2%)
but not at the 48-hour reading (0/10 at 0.5%; 0/10 at 2%). In the
same studies, cross sensitization responses were also seen with
maneb, mancozeb and zineb after induction with ETU. Responses
ranged from 0-40% (4/10) at 24 hours and from 0-20% (2/10) at 48
hours. Induction with ETU followed by challenge with maneb
generally gave a slightly higher overall response (10-40%) than did
induction by maneb followed by challenge with ETU (0-20%)
(Matsushita et al., 1976).
Table 1. Acute toxicity of ETU
Species Sex Route LD50 (mg/kg bw) References
Mice M&F oral 4000 Lewerenz & Plass, 1984
F oral > 3000 Teramoto et al., 1978b
F (9 days pregnant) oral > 3000 Khera, 1987
M&F oral ca 2400 Peters et al., 1980b
Rats M&F oral ca 2400 Peters et al., 1980a
M oral 1832 Graham & Hansen, 1972
M&F oral 940 Lewerenz & Plass, 1984
F (13 days pregnant) oral 600 Khera, 1987
F oral 545 Teramoto et al., 1987b
Hamsters F oral > 3000 Teramoto et al., 1987b
F (11 days pregnant) oral > 2400 Khera, 1987
Short-term toxicity studies
Mice
B6C3F1 mice (10/sex/dose), 8 to 9 weeks of age were fed
diets containing 0, 125, 250, 500, 1000, or 2000 ppm ETU (97-99%
purity), equivalent to 0, 19, 38, 75, 150 or 300 mg/kg bw/day for 13
weeks. Deaths occurred at lower doses but were not dose- or
compound-related. Body-weight gain and food consumption were
comparable to controls. Diffuse follicular cell hyperplasia of the
thyroid occurred at 500 ppm in both sexes (greater than 70%) and was
statistically significant and dose-related. No effects were observed
at lower doses. Hepatocellular cytomegaly was also observed at 500
ppm (4/10 females, 10/10 males) and above. Effects were
statistically significant and dose-related. The NOAEL in this study
was 250 ppm, equivalent to 38 mg/kg bw/day (NTP, 1992).
Groups of Charles River CD-1 mice (15/sex/dose) were
administered ETU (100% purity) in the diet at levels of 0, 1, 10,
100 or 1000 ppm for 3 months, equal to 0, 0.16, 1.7, 18 or 168 mg/kg
bw/day for males and 0, 0.22, 2.4, 24 or 230 mg/kg bw/day for
females. There were no compound-related effects on food
consumption, body weight, haematology or clinical chemistry
parameters. Mixed function oxidase activity was increased in both
sexes at 1000 ppm, but only statistically significant in males
(aniline hydroxylase, p-nitroanisole, o-demethylase). Absolute and
relative thyroid weights were increased statistically in both sexes
at 1000 ppm. Absolute and relative liver weights were significantly
increased in males at 1000 ppm; relative liver weights were
significantly increased in females at 100 and 1000 ppm ETU.
ETU produced thyroid follicular cell hyperplasia and decreased
colloid density in both sexes at > 100 ppm, with increased
follicular epithelial cytoplasmic vacuolation and interstitial
congestion in both sexes at 1000 ppm. In the liver, ETU produced
centrilobular hypertrophy, nuclear pleomorphism and increased
intranuclear inclusions in both sexes at 1000 ppm. The pigment was
believed to be similar to lipofuscin. The NOAEL was 10 ppm ETU,
equal to 1.7 and 2.4 mg/kg bw/day in males and females, respectively
(O'Hara & DiDonato, 1985).
Rats
Adult male Han:Wistar rats (6/dose group) were given ETU (>
98% pure) in drinking-water for 28 days. Drinking-water
concentrations of ETU were 0, 100, 200 or 300 mg/litre, equal to
mean daily doses of 0, 11, 18 or 23 mg/kg bw.
ETU decreased body-weight gain during the exposure. Studies of
kidney function and morphology indicated that the kidney is not a
highly sensitive target for ETU-induced toxicity. ETU did not have
a permanent physiologically significant effect on urinary sodium,
potassium, uric acid, protein or glucose excretion, or urinary
osmolality. A slight increase in urinary arginine vasopressin (AVP)
excretion was observed in ETU-treated animals on day 28. No
prominent light microscopical changes were observed in the kidneys
of ETU-exposed rats. However, at 300 mg/litre ETU induced clear
ultrastructural changes in the epithelium of renal proximal tubuli.
An increased number of lysosomes and myelin figures as well as
vacuolization and edema were observed in the cytoplasm of the
epithelial cells of proximal tubules. The proportion of the dose of
ETU excreted as ETU in urine increased with increasing dose of ETU
and were 25%, 36% and 49% (Kurrtio et al., 1991).
Using an identical protocol as above, a study was conducted to
determine the effect of ETU on thyroid gland function and
morphology. Drinking-water concentrations of ETU were 0, 100, 200
or 300 mg/litre, equal to mean daily doses of 0, 11, 18 or 23 mg/kg
bw. Blood samples for T3, T4 and TSH were taken and the levels
measured using radio immunoassay methods. Thyroid glands were
extirpated and processed for light and electron microscopy. ETU
statistically significantly decreased T4 levels at all doses while
statistically increasing TSH levels at all doses. T3 levels were
also decreased in a dose response manner but values were not
statistically significant. There were no ETU-induced morphological
changes observed under light microscopy. Conspicuous ultra
structural changes were caused by ETU since a few areas with totally
destroyed epithelial cells could be found. It was also reported that
nerves and capillaries might have been affected by ETU (Kurrtio et
al., 1986).
Sprague-Dawley rats (10/sex/group) received 0, 0.63, 1.3, 2.5,
5.0, or 25 ppm of 98% pure ETU in the diet for 8 weeks. Twenty-four
hours after the last feeding, all animals received 5 µCi of 131I
intraperitoneally. There were no treatment-related effects on
behaviour, appearance, food intake, organ or body weight or
macroscopic appearance of organs other than the thyroid. ETU had no
clinical chemistry effects at the three low doses. However at 5 and
25 ppm slight increases were observed in males and females with
respect to 131I uptake, protein bound 131I and serum thyroxine. T3
uptake power was slightly decreased. Histopathology of the thyroid
in treated animals was comparable to control group at all dose
levels. The NOAEL in this study was 25 ppm, the highest dose
tested, equal to 2.6 mg/kg bw/day (Leuschner, 1977).
F344/N rats 8 to 9 weeks of age (10/sex/group), were fed diets
containing 0, 60, 125, 250, 500 or 750 ppm of 99% pure ETU for 13
weeks. All animals survived. Final mean body weights for males were
decreased at 500 and 750 ppm by 10% and 30%, respectively. Food
consumption at the same dose levels were decreased 16% and 24%.
Final body weights and food consumption of females were decreased at
750 ppm by 30% and 25%, respectively. Females receiving 60 to 500
ppm showed a uniform 10% body weight decrease accompanied by food
consumption decreases of 13% at 250 and 500 ppm. Histopathology was
present for the thyroid and pituitary gland of both males and
females. Diffuse follicular cell hyperplasia of the thyroid was
present in all animals of both sexes at all doses. In males, focal
follicular cell hyperplasia and cellular vacuolization of the pars
distallis of the pituitary gland was statistically significantly
increased at 250 ppm. Follicular cell adenomas (3/10) were evident
at 250 ppm and statistically significant at 750 ppm. Centrilobular
cytomegaly was observed only at 750 ppm and was statistically
significant. In females, follicular cell hyperplasia (4/10) and
cellular vacuolization of the pars distallis of the pituitary gland
(10/10) were statistically significant only at 750 ppm. Follicular
cell adenomas were observed at 500 and 750 ppm (3/10) but were not
statistically significant. Centrilobular cytomegaly of the liver
was seen only at the high dose and in all animals. The NOAEL in
this study was less than 60 ppm, equal to 3.0 mg/kg bw/day for males
and 4.3 mg/kg bw/day for females, based on histopathological
findings of diffuse follicular cell hyperplasia in the thyroid (NTP,
1992).
In a 90-day study, Sprague-Dawley derived rats (60/sex/dose)
were fed ETU (96.8% pure) at 1, 5, 25, 125 or 625 ppm. Controls
(72/sex) received powdered diet with 1% corn oil. At 30-day
intervals (i.e. 30, 60, 90 days) ten rats from each test group were
sacrificed and serum T3, T4, TBG and TSH concentrations were
measured. The free thyroxine index (FTI) was also calculated. The
remaining rats (10/sex/dose/time) were used to determine 125I uptake
by the thyroid. Rats receiving 625 ppm ETU showed high mortality
and marked decrease in body-weight gain. Clinical signs were
observed at the high dose by day 8 and consisted of excessive,
salivation, loss of hair, rough and bristly hair coat and scaly skin
texture. Necropsy revealed hyperaemia of the thyroids with and
without enlargement at 125 and 625 ppm for all time intervals.
Liver congestion was also evident with dose and time. Liver changes
were distinguishable microscopically and appeared to be compound-
related but not dose-related. Thyroid to brain weight ratio was
significantly increased at 125 and 625 ppm at all time periods.
125I uptake in the thyroid was statistically significantly decreased
along with TBG, T3 and T4 values at 125 and 625 ppm. At 25 ppm,
T4 was statistically significantly decreased only at 60 days. FTI
was comparable to controls. Altered thyroid function and increased
thyroid follicular cell hyperplasia were evident at 125 and 625 ppm.
The NOAEL was 25 ppm, equal to 1.7 and 1.9 mg/kg bw/day in males and
females, respectively (Freudenthal et al., 1977).
Osborne-Mendel rats (20 males/group) were fed ETU (purity not
stated) in the diet at levels of 0, 50, 100, 500 or 750 ppm for 30,
60, 90 or 120 days. 131I activity was determined at 4 and 24 hours
post-injection (5 µCi) in 20 rats from each group at each sacrifice
period.
Body weight was decreased at > 500 ppm throughout the study.
Food consumption was reduced at 30 and 90 days at > 100 ppm and
at 60 and 120 days at > 500 ppm. Relative thyroid weights were
increased at 30 days at > 100 ppm, at 90 days at 500 ppm and at
60/120 days at > 50 ppm.
Four hours after the injection of 131I, the uptake had
decreased significantly in rats fed ETU at 500 and 750 ppm at all
feeding periods. The uptake of iodine 24 hours after injection was
decreased significantly in those animals fed ETU at 100, 500 and 750
ppm. After the 90-day feeding period, the uptake decreased
significantly in rats fed the 500 and 750 ppm levels and ranged from
6 to 13 times lower than control values.
Histologically there were no differences between the control
and 50 ppm groups. At 100 ppm there was slight hyperplasia evident
in the thyroid gland. At 500 ppm there was moderate to marked
hyperplasia, lack of colloid and heightened epithelial walls. There
was an increase in vascularization, demonstrating a response to
increased blood level TSH. At > 500 ppm, an increased incidence
of follicular adenomas was reported. One mechanism by which ETU
acts on the thyroid is via inhibition of iodide peroxidase, which
oxidizes iodide to iodine (Graham & Hansen, 1972).
Dogs
Beagle dogs (2/sex/group) received dietary concentrations of 0,
200, 980, or 4900 ppm of ETU (98% pure) for 4 weeks. Body-weight
gains and food consumption for males were comparable to controls.
Intermediate- and high-dose females gained less weight than the
controls particularly at the high dose. Haematology results were
not remarkable. T3 levels were decreased in high-dose males and
females as well as mid-dose females. T4 levels were decreased in
the mid- and high-dose males and females. Reductions were dose-
related. Enlarged thyroids were noted in all animals of the
intermediate- and high-dose groups. The NOAEL was 200 ppm, equal to
6.7-7.4 mg/kg bw/day for males and 7.4-8.5 mg/kg bw/day for females
(Morgan, 1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
10, 150 or 2000 ppm of ETU (98% pure with doses corrected to 100%
active ingredient) for 13 weeks. Two males in the high dose were
sacrificed in a moribund state with morbidity attributed to compound
administration. All other animals survived to termination.
Clinical signs in the high-dose male survivors appeared to be
unremarkable. All high-dose females showed decreased activity or
subdued behaviour for various lengths of time (1-5 weeks). A
bilobed swelling in the pharyngeal area of two females was also
reported. No treatment-related clinical signs were observed in the
low or intermediate dose groups. Body-weight changes for survivors
in treated groups were not statistically significantly different
when compared to the control group. However, a slight to severe
body-weight loss for animals killed moribund was noted. Food
consumption was statistically significantly decreased only at the
high dose for surviving males during weeks 12 and 13, and for
females during weeks 11 and 12. Ophthalmological examinations
showed no remarkable differences between treated and control groups.
At 13 weeks, males and females of the 150 and 200 ppm groups
showed statistically significant decreases in haemoglobin, packed
cell, volume and red blood cell count. Reticulocyte count was
statistically significantly increased in females, but not in males.
Values for sodium, potassium, and chloride and BUN were all within
normal limits. Phosphorous was decreased in males and females at 13
weeks in the high dose. The value was statistically significant in
males. A statistically significant increase in serum protein was
associated with an increase in serum globulin in the high-dose males
at 13 weeks. A statistically significant increase in total
cholesterol was observed in the intermediate-dose (150 ppm) males at
weeks 8 and 13 and at weeks 4, 8, and 13 in high-dose (2000 ppm)
males and females. An increase in the mean creatinine level was
noted at weeks 8 and 13 in the high-dose males and females with
statistical significance attained for males at both time periods.
ALP was statistically significantly decreased in high-dose males at
weeks 8 and 13. At week 4, there was a statistically significant
decrease in mean ASAT in males at the intermediate and high dose and
in females at all three doses. A dose response was evident in
females. However values at 4 and 8 weeks were comparable to
controls for all groups of both sexes. ALAT was statistically
significantly decreased at week 4 only in females. Results from
urinalysis were not remarkable. Urine colour was however described
as orange or dark-coloured. Thyroid hormone assays revealed no
treatment-related changes in the low- and intermediate-dose groups.
However, marked and statistically significant reductions were noted
for T3 and T4 levels in high-dose animals at weeks 8 and 13. T4
was also statistically significantly decreased in males at 4 weeks
in the high-dose group.
At week 13, females of the high-dose group showed a marked and
statistically significant increase in thyroid weights accompanied by
slight but statistically significant increases in liver and adrenal
weights. Males of the high-dose group at 13 weeks showed a marked
increase in thyroid weights concurrent with slight increase in liver
and adrenal weights. None of these organ weights were statistically
significantly increased for males. Macroscopic examinations revealed
exophthalmia in two males (the survivors) and three females of the
high-dose group. Sporadic and slight exophthalmia was also observed
in one male of the intermediate dose group. Enlargement of the
thyroid gland was noted in all surviving high-dose animals as well
as the two males sacrificed moribund. The liver and adrenal gland
both appeared unremarkable as did the remaining tissues examined
macroscopically. Salient microscopic findings were those of
hypertrophy of the basophilic cells of the pituitary with micro-
vacuolisation attended by severe follicular hyperplasia of the
thyroid gland in all surviving and sacrificed animals of the high-
dose group. The liver and adrenal gland were histologically normal.
A moderate involution of the thymus of one male and two females of
the high-dose group was reported. No treatment-related microscopic
changes were noted for the low dose (10 ppm) or the intermediate
dose (150 ppm). The salient observations related to this study are
those of the pituitary and thyroid glands of animals receiving the
highest dose of 2000 ppm (equal to a mean of 66 mg/kg bw/day for
males and 72 mg/kg bw/day for females). In the pituitary, the lesion
observed was a hypertrophy of a basophilic cell type with
microvacuolisation, while in the thyroid, the lesion observed was a
hyperplasia of the follicular cells with papillary projections of
the follicular epithelium in the lumen of the follicles. Similar
hyperplasia was observed in ectopic nodule of thyroid tissues,
scattered along the thyroglossal track. The NOAEL was 10 ppm, equal
to 0.39 mg/kg bw/day based on decreased haemoglobin, packed cell
volume and red blood cell count, and increased cholesterol at 150
ppm. Effects on the thyroid were found only at 2000 ppm (Briffaux,
1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
5, 50 or 500 ppm ETU (expressed as active ingredient taking into
account the purity index of 98% purity) for 52 weeks. Mortality was
evidenced in the high-dose group with the death of one male and the
sacrifice of one male and one female prior to study termination. No
treatment-related clinical signs were reported in either the low- or
mid-dose groups. Pale mucous membranes in four males and one female
of the high-dose group was associated with subdued behaviour and a
change in the colour of the faeces (yellow/orange). Body weight in
surviving animals at 52 weeks was decreased 15% in both males and
females at the high-dose and 8% in the mid-dose males. A dose-
related decrease was observed in body-weight gain for males of the
mid- (-43%) and high-dose (-60%) and females of the high-dose (-
60%). However, the decreases were not statistically significant.
Body-weight gain and body weights in the low-dose group were
comparable to control group. There were no statistically
significant differences in food consumption between treated and
control groups at 52 weeks. Food efficiency was generally
comparable between groups. Ophthalmological examinations revealed
comparable findings between all groups.
Haematological values between control groups and the low- and
mid-dose groups were comparable. However, in the high-dose group,
treatment-related low values (75-80% of normal) in haemoglobin, RBC,
packed cell volume were reported for all animals dying or sacrificed
moribund as well as one surviving male. Additionally the decrease
in RBC was accompanied by an increased reticulocyte count, a
decrease in mean corpuscular haemoglobin and an increase in mean
corpuscular volume. Low values in platelet count were also observed
in high-dose animals. Changes in blood clinical chemistry values
for sodium, potassium and blood urea nitrogen, cholesterol,
triglycerides, bilirubin creatinine, gamma glutamyl-transpeptidase
in surviving animals was not considered treatment-related. However,
a slight to moderate increase for total bilirubin was observed for
animals dying or sacrificed early in the high dose group. Values for
globulin were statistically significantly higher at weeks 13 and 52
for the high-dose animals (males and females combined). A decrease
in the albumin/globulin ratio was also statistically significant at
week 52 for the high-dose animals (males and females combined).
Elevated values for ASAT and ALAT were reported for both high-dose
males found dead or sacrificed moribund in the high-dose group.
Urinalysis values were unremarkable between groups.
Thyroid hormone mean values for T4 and T3 were not
statistically significantly different from control group. However,
T3 and T4 values taken shortly prior to death or sacrifice of the
three high-dose group animals revealed a mean decrease of 50% for
T3 values and 70% for T4 values. The values for decedent animals
were also below the historical range. Of the two surviving males in
the high-dose group at 52 weeks, one showed a 47% reduction of T3
from its pretest level while the other was comparable to its pretest
level. T4 values for both high-dose male survivors were generally
decreased 55% from pretest values. T3 and T4 values appeared to
be unaffected in the low-dose and mid-dose groups. A dose-related
increase in thyroid weights were observed at week 52 for the
intermediate and high-dose males and females. The increase was
statistically significant for combined males and females for
absolute, body weight and brain weight ratio (except for brain
weight ratio in the intermediate dose group). Necropsy revealed an
enlargement of the thyroid in one of the two surviving males.
High-dose animals dying on study or killed in a moribund
condition all manifested centrolobular hepatocellular necrosis of
the liver (multifocal and moderately severe in males and multifocal
and minimal in the female). Slight pigment accumulation was also
evident in Kupffer cells. Hypertrophy of follicular cells with
dilation of follicles was also seen in the thyroid of one high-dose
male that was sacrificed. Pigment accumulations in Kupffer's cells
and occasionally hepatocytes were observed in both males and females
of the intermediate and high-dose groups. Hypertrophy of the
thyroid with colloid retention was observed in the intermediate and
high-dose group and ranged in severity from slight to moderately
severe. The NOAEL was 5 ppm, equal to 0.18 mg/kg bw/day based on
reduction in body-weight gain, hypertrophy of the thyroid with
colloid retention, a slight increase in thyroid weight and pigment
accumulation in the liver at 50 ppm (Briffaux, 1992).
Monkeys
Wild-caught rhesus monkeys (5/sex/group) were administered ETU
(96.8-98.2 purity) in the diet for 5.5 or 6 months at dose levels of
0, 2, 10, 50, or 250, and 0, 50, 150 or 450 ppm, respectively.
Results of Study 1: Body weights were not affected by ETU.
Thyroid weight was increased in both sexes at 250 ppm and in females
at > 50 ppm, resulting from hyperplasia and/or hypertrophy.
Females at > 50 ppm also had enlarged pituitary glands. Ovarian
weights at 250 ppm were significantly decreased.
No changes in T3 or TBG were observed. Serum T4 was
decreased in both sexes at > 50 ppm identified from FTI analyses.
Serum TSH was increased at 250 ppm. 125I uptake also increased at
> 50 ppm in both sexes.
Lesions reportedly associated with ETU were identified in the
pituitary and thyroid gland of animals at > 50 ppm. These
included thyroid and pituitary hypertrophy, and thyroid follicular
cell hyperplasia (moderate to severe). A second study was conducted
due to the extent of tuberculosis in this first study which
necessitated the early termination at 5-5.5 months.
Results of Study 2: Body weights were not affected by ETU.
Thyroid and spleen weights were increased in males at > 150 ppm
and at all doses in females. Serum T3 decreased in males at
> 150 pm and in females at 450 ppm. Serum T4 was decreased in
both sexes at > 150 ppm. Radioactive 125I uptake was increased
in all test groups. The increased thyroid weight, thyroid iodine
uptake, decrease in T3, T4 and increase in TSH support the
evidence for hypothyroidism caused by ETU.
BUN was elevated in females at 450 ppm along with creatinine
and a decrease in calcium. Haemoglobin, haematocrit and RBC count
were decreased in both sexes at 450 ppm.
Histologic changes were identified in thyroid and pituitary
glands in both sexes, increasing in severity and incidence with
increase in dose. Thyroid follicular cell hyperplasia and pituitary
cytoplasmic vacuolation and swelling were the major changes
observed.
The NOAEL for 125I uptake was 10 ppm; the NOAEL for changes in
T3, T4 and TSH was 50 ppm. In a separate pathological
examination, 10 ppm was considered to produce compound-related
changes in the thyroid gland in 1/7 monkeys. The NOAEL was
considered by the authors to be 2 ppm in these combined studies
(Leber et al., 1978b).
However, the monkey studies were considered unreliable because
one was compromised by ill health of the animals, while little
reliance could be placed on the effects at the lowest dose used in
the second.
Long-term toxicity/carcinogenicity studies
Mice
Groups of B6C3F1 mice received perinatal (F0), adult (F1)
or both exposures to ETU at the following dietary concentrations:
(F0:F1), 0:0, 0:330, 0:1000, 33-100, 110-330, 330-0, 330-330 or
330-1000 ppm. Female C57BL/6N mice were exposed to 0, 33, 110 or
330 ppm of 99% pure of ETU in feed for one week before breeding, and
naturally inseminated by C3H/HeN males that received control feed
only. ETU exposure continued throughout pregnancy and lactation.
Weaning occurred on day 28 post-partum and dietary exposure at these
same (maternal) concentrations continued until pups were 8 weeks of
age. On post-partum day 7, litters were culled to a maximum of 8
pups, separated by sex after weaning and litter mates co-housed. At
8 weeks of age, pups were separated into groups of 60 males and 60
females to receive dietary concentrations of 0, 330 or 1000 ppm for
2 years. Groups of 34 male and 29 female mice that were fed 33 ppm
of ETU before weaning received 100 ppm for up to 2 years.
At 9 months, liver weights were increased in groups receiving
adult exposure concentrations of 330 or 1000 ppm regardless of
perinatal exposure. Increases were statistically significant with
the exception of the 0:330 group.
Thyroid weights were also reportedly increased in animals given
1000 ppm. T4 levels were statistically significantly decreased in
all animals receiving adult concentrations of 330 or 1000 ppm. TSH
levels were statistically significantly increased in males only at
330:330 and 330:1000 ppm. Follicular cell vacuolization of the
thyroid occurred in all animals receiving ETU except those only
receiving perinatal exposure (i.e. 330:0 ppm). Animals receiving a
dose of 33:100 ppm were not reported. Hyperplasia was comparable
between all groups.
Hepatocellular adenomas were present in animals receiving 1000
ppm but were not statistically significant. Centrilobular
cytomegaly was statistically significantly increased in both males
and females receiving 1000 ppm and in males receiving 110:330 and
330:330 ppm. Eosinophilic focus was statistically significantly
increased in females receiving 1000 ppm. At 2 years, there was no
survival disparity between treated and control (0:0 ppm) groups.
Clinical signs were not treatment-related. Body weights of treated
animals were statistically significantly decreased in both sexes
compared to 0:0 ppm controls, with the exception of the 330:0 ppm
dose group which was similar to controls. Statistically significant
increases in the number of animals with adenomas or carcinomas were
observed for hepatocytes, thyroid follicles and posterior pituitary
in both sexes of high-dose treated, adult only exposed groups when
compared to 0:0 ppm controls. At the next lower dose level of 0:330
ppm both sexes showed a statistically significant increase in
hepatocellular adenomas or carcinomas.
Hyperplasia of the thyroid was evident in high-dose males and
females and in 0:330 ppm females. Centrilobular cytomegaly was also
evident in males and females at both dose levels of adult only
treated animals. A comparison of animal groups receiving 0:330
versus 110:330 versus 330:330 ppm showed statistically significant
increases of thyroid follicular cell hyperplasia (males) and thyroid
adenomas (females) in perinatal treated groups at 330 ppm. Similar
comparisons for hepatocellular neoplasms and pituitary neoplasms
revealed no statistical differences. Comparison between animals
receiving 1000 ppm, with or without perinatal exposure to 330 ppm
showed no statistical differences for tumours or hyperplasia of the
thyroid or pituitary. Treatment of adult females receiving 330 ppm
with 330 ppm perinatally increased the number of tumours in the pars
distalis when compared to 0:0 ppm controls as well as those of the
thyroid. Animals receiving only a perinatal dose showed a
comparable response when measured against 0:0 ppm controls. T4
levels for both sexes were statistically significantly decreased in
both sexes at all dose levels. TSH levels were statistically
significantly increased in animals receiving 1000 ppm (i.e. adult
and perinatal/adult groups). Animals receiving 330 ppm during
adulthood showed elevated TSH levels which were statistically
significant only in females. Animals receiving perinatal exposure
only showed TSH values comparable to controls (0:0 ppm) (Chhabra et
al., 1992; NTP, 1992).
Rats
Charles River rats (60/sex/group) were fed ETU (purity not
stated) in the diet at levels of 0, 5, 25, 125, 250 or 500 ppm for 2
years. Body weights in both sexes were significantly decreased
initially at doses > 25 ppm; at 500 ppm and above (males) and 125
ppm and above (females) at 12 months; and at 500 ppm and above (both
sexes) for the remainder of the study.
Liver to body-weight ratios were significantly increased at 125
ppm and through 6 months in males, but comparable to controls for
the remainder of the study. Relative liver weights in females were
significantly increased at doses > 125 ppm at 2 months and at
doses > 250 ppm through 18 months. No differences between
control and dose groups were observed at 24 months. Thyroid to
body-weight ratio was significantly increased in males at 250 ppm
and above at 2, 6 and 18 months, and at 125 ppm and above in females
for the first 12 months. Thyroid weights were significantly
increased at 125 ppm and above in males at 12 and 24 months, and at
250 ppm and above in females at 18 and 24 months.
Uptake of 131I, expressed as counts/min/mg tissue, was
significantly decreased in males at 500 ppm throughout the study.
Thyroids of females fed 125 ppm and above were hypofunctioning at 6
months and hyperfunctioning at 12 months. At 24 months, females had
a hypofunctioning thyroid at 500 ppm.
Fewer rats survived to 24 months in the 500 ppm dose group and
there was also a significant increase in pneumonia which may have
been further complicated by obstruction of the trachea from enlarged
thyroids in the animals. Effects in the thyroid were evident at all
doses. Increased vacuolarity and hyperplasia in the thyroid were
evident at 25 ppm and above. Thyroids of treated rats were
distinguishable from controls by lobulation, follicular size and
uniformity, height of follicular epithelium, colloid staining,
keratinization of follicles, and general size.
It is possible that ETU initially reduces thyroid activity,
after which compensation occurs by an increased release of TSH and
that this increase in TSH stimulated thyroid weight in an attempt to
overcome the blocking effect of ETU. The progression to neoplasia
is believed to be a result of excessive pharmacological stimulation.
This is supported, in part, by a lack of thyroid tumours at 1 year
at 5 or 25 ppm, an increase in tumour incidence after 1 year at 125
ppm, and confirmed after 2 years in rats fed 250 and 500 ppm. The
NOAEL in this study was 5 ppm, equivalent to 0.25 mg/kg bw/day
(Graham et al., 1973, 1975).
SPF-Sprague-Dawley (30/sex/dose) received 0, 0.5, 2.5, 5 or 125
ppm of 96% pure ETU (adjusted to 100%) in feed, 7 days a week for
either 52 weeks (interim sacrifice of pre-selected animals
10/sex/dose) or 104 weeks (terminal sacrifice).
There were no compound-related deaths, clinical signs or
effects on food consumption. Body-weight gain was slightly impaired
in males at 125 ppm resulting in group mean body weights 5-6% lower
than in control males for most of the study. Female body weight was
unaffected. There were no treatment-related changes with respect to
ophthalmoscopic observations or palpable masses. Haematologic and
urinalysis finding were comparable to controls. There were no
treatment-related changes on clinical biochemistry values in males
receiving 0.5 or 2.5 ppm nor in females receiving 0.5, 2.5 or 5 ppm
of ETU. Statistically significant increases were observed in males
at 125 ppm for total protein, albumin, GGT, cholesterol, bilirubin,
TSH and T3. T4 and urea values were lower. T3 values were higher
at 5 ppm at 29 weeks. For females at 125 ppm, values for glucose
and T4 were lower, uric acid, T3 and TSH were higher. At 125 ppm
only thyroid weight was higher in males and females at the interim
and final sacrifice. Liver weight was slightly higher in both sexes
but only at the interim sacrifice. Macroscopic effects were not
observed at 52 weeks. However, at 104 weeks the incidence of
diffuse or modular enlargement of the thyroid gland was increased in
both sexes at 125 ppm.
Microscopic examination of the thyroid gland at interim
sacrifice revealed minimal to moderate diffuse follicular cell
hyperplasia in 8 animals of the control group and 9, 12, 12 and 20
animals (both sexes combined) receiving 0.5, 2.5, 5 or 125 ppm of
ETU, respectively. The incidence of this finding was significantly
increased in females at 125 ppm. The severity was increased in males
at 5 and 125 ppm and in females at 125 ppm. Slight or moderate
nodular hyperplasia and follicular adenoma were recorded in 6 and 3
males of the 125 ppm, respectively. Minimal or slight focal or
multifocal cellular hypertrophy of the anterior pituitary was
recorded in 2 control rats, 2 rats at 2.5 ppm and 7 rats at 125 ppm.
Males were predominantly affected. A significant increase in the
incidence and severity was calculated for males at 125 ppm. Other
morphological alterations observed in treated groups were considered
secondary to treatment, and affected spleen, thymus gland, auditory
sebaceous (Zymbal's) glands and lungs.
At terminal sacrifice, slight to excessive diffuse follicular
hyperplasia was recorded in 27 animals (sexes combined) receiving
125 ppm. The incidence and severity for males and females was
statistically significant for both sexes. Slight to severe nodular
hyperplasia was observed in 9 animals (sexes combined) receiving 125
ppm. The incidence and severity of this lesion was significantly
increased in males. Follicular adenomas occurred in 1 male of the
control group and in 4 males given 125 ppm. Follicular carcinomas
were observed in 2 males of the high-dose group. The combined
incidence of benign and malignant follicular neoplasms yielded a
clear dose-related trend but did not vary significantly in pairwise
comparison with the control group. Anterior pituitary gland
adenomas were recorded in 8 males and 11 females of the control
group and 15 males and 10 females at 125 ppm. There was a clear
dose-related trend for males and a marginal level of significance by
pairwise comparison with the control group. Adenomas of the
anterior pituitary recorded at the intermediate doses for males and
females were 5, 8, and 6 and 11, 12, and 11 respectively. Other
morphological alterations observed in treated groups were considered
secondary to treatment and affected the pancreas, lungs and Zymbal's
glands. The NOAEL was 5 ppm (equal to 0.37 mg/kg bw/day) based on
changes in clinical chemistry, increased T3, decreased T4,
increased thyroid and liver weights and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm
(Schmid et al., 1992).
Charles River CD rats (26/sex/group) were fed 0, 175 or 350 ppm
of technical grade ETU (97% pure) for 2 years. Follicular or
papillary carcinomas of the thyroid were observed in 17 males and 8
females at the high-dose. At 175 ppm, equivalent to 8.8 mg/kg
bw/day, 3 males and 3 females had thyroid carcinomas. Hyperplastic
goitre was observed in 17 males and 13 females of the high-dose
group and 9 males and 6 females of the low-dose group. These
lesions were not observed in control rats (Ulland et al., 1972).
Groups of F344/N rats received perinatal exposure (F0), adult
exposure (F1) or both to different concentrations (ppm) of ETU as
follows: F0, F1; 0,0; 0,83; 0,250; 9,25; 30,83; 90,0; 90,83; or
90,250. Female rats were exposed to 0, 9, 30 or 90 ppm of 99% pure
ETU in feed for 1 week before breeding. All males received control
feed. All females were naturally inseminated by males, housed
individually and continued on their previous diet. ETU exposure
continued throughout pregnancy and lactation. Weaning occurred on
day 28 post partum and dietary exposure at these same concentrations
continued until the pups were 8 weeks of age. On post partum day 4,
litters were culled to a maximum of 8 pups. Pups were separated by
sex after weaning and litter mates co-housed (5/cage). At 8 weeks
of age, pups were separated into groups of 60 males and 60 females
to receive adult dietary concentrations of 0, 25, 83 or 250 ppm for
up to 2 years.
At 9 months, liver weights were statistically significantly
increased in males receiving 0,250 or 90,250 ppm of ETU. Thyroid
follicular cell hyperplasia was greater than 50% and statistically
significantly increased for both males and females at the following
dose levels: 0,83; 0,250; 30,83; 90,83; and 90,250 ppm. Thyroid
follicular cell adenomas were observed in both males (3/10) and
females (1/10) receiving 90,250 ppm. Values were not statistically
significant. T4 values compared to 0,0 ppm controls were
statistically significantly decreased in all experimental groups of
both sexes except animals receiving 90,0 ppm. T3 values were
statistically decreased in many but not all groups. The 90,0 ppm
groups was unaffected. TSH levels were increased in all dose groups
and statistically significant only in some female groups. The 90,0
ppm groups was only very slightly increased.
At two years there were no differences in food consumption
between treated groups and controls with the exception of a decrease
in the 90,250 ppm group of males during the last month of exposure.
Final mean body weights for males and females were comparable to 0,0
control group with the exception of the 90,250 ppm male dose group
where the decrease was statistically significant. Only those
animals receiving 90,250 ppm showed a statistically significant
decrease in survival. Thyroid function values for animals receiving
90,0 or 0,83 or 9,25 ppm were not statistically different compared
to controls for males and females at 2 years. All other doses
revealed some level of statistical significance in both sexes.
There were no clinical findings that could be attributed to thyroid
dysfunction. Pathology of the thyroid for animals receiving adult
only exposures of 0,0; 0,83 or 0,250 ppm revealed statistically
significant trends and statistically significant increases in high-
dose males and females for hyperplasia, adenomas, carcinomas and
adenomas and carcinomas combined. Animals receiving 0,83 ppm of ETU
showed statistical increases in hyperplasia (males and females) and
adenomas (males). Hyperplasia of the thyroid was the only
statistically significant effect observed in both sexes when 0,0 and
90,0 ppm comparisons were made. Responses between dose groups
receiving 0,250 or 90,250 ppm revealed a statistically significant
increase in the number of adenomas in males and carcinomas in both
males and females. A comparison of the 0,83; 30,83 and 90,83 dose
groups in females showed no statistical differences in hyperplasia,
adenomas, or carcinomas, or adenomas and carcinomas combined. In
males only hyperplasia was statistically significantly increased.
ETU had no clear effects on the incidences of neoplasms or non-
neoplastic lesions at sites other than the thyroid gland. However,
some groups showed statistically significant increases relative to
controls in neoplasms of the Zymbal's gland (males and females at
90,250 ppm) and, mononuclear cell leukaemia (males and females at
90,250 ppm and males at 90,83 ppm (Chhabra et al., 1992; NTP,
1992).
Rats and Hamsters
Groups of 20 male and 20 female rats and hamsters were
administered ETU (purity not stated) in the diet for 24 and 20
months, respectively, at dose levels of 0, 5, 17, 60 or 200 ppm
(strain of animals not reported).
In rats, food consumption was reduced at 60 ppm and above and
body weight decreased at 17 ppm and above. Effects on SAP and SGPT
were not clearly demonstrated due to fluctuations in control levels.
Cholesterol was increased at 5 ppm in both sexes. Some hepatic
enzyme levels were also affected: GPT increased in males at 60 ppm;
ALP increased at 5 ppm (females) and 17 ppm (males); glucose-6-
phosphate dehydrogenase did not change. Thyroid weights were
significantly increased in both sexes at 60 ppm. No data were
available on the histologic examination.
In hamsters, food consumption and body weight were reduced at
60 ppm and above. SAP was increased in both sexes initially, then
decreased through 18 months. No effect was observed on SGPT.
Cholesterol levels were significantly increased in both sexes at all
doses compared to controls. Hepatic enzymes, GPT and ALP, were
significantly increased in both sexes at all doses. Glucose-6-
phosphate dehydrogenase was significantly decreased in both sexes at
all dose levels. Relative thyroid weights were significantly
increased at 200 ppm and above in both sexes. No data were
available on the histologic examination (Gak et al., 1976).
Reproduction studies
Rats
ETU (98% pure) was mixed in the diet and fed to Sprague-Dawley
rats (25 male and female parents per group) at concentrations of 0,
2.5, 25 or 125 ppm during a 70-day pre-pairing period and throughout
pairing, gestation and lactation of 2 generations (one litter per
generation). Body weights and mean body-weight gains were reduced
among the male parents of the F0 generation at 125 ppm. There were
otherwise no changes in the viability, clinical appearance or
behaviour, feed consumption, body weights or weight gain or
macroscopic appearance of any of the parents, F1 or F2 pups in any
of the test groups. Reproduction parameters were unaffected in any
of the dose groups in either generation. Histopathologic
examination indicated compound-related changes in the thyroid and
anterior pituitary glands at 25 and particularly at 125 ppm in both
generations. Thyroid changes in both sexes of both generations
consisted of follicular cell hypertrophy and hyperplasia which were
pronounced at 125 ppm and present to a much lesser extent at 25 ppm.
Reduced colloid was also present among F1 males and females at 125
ppm. Adenomas were observed in 3 males (not statistically
significant). Pituitary changes consisted of an increased incidence
and severity of anterior cell hypertrophy in both sexes of both
generations at 125 ppm, together with a tendency to an increase in
hypertrophy among parental generation males at 25 ppm and a slight
increase in cellular vacuolization at 125 ppm. There was no
evidence of reproductive organ toxicity up to and including 125 ppm.
The NOAEL was 2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day,
based on thyroid gland follicular cell hyperplasia and hypertrophy
at 25 ppm (Dott, 1992).
Rats/mice
In the first phase of a two-phase study, adult female rats and
mice were dosed with ETU (96.7% purity) and then bred to proven male
sires. Pregnant females delivered their pups via C-section for
tissue distribution analyses. Phase 2 consisted of weanling
rats/mice dosed for 9 weeks and then analyzed. Dose levels in the
diet were: rats: 0, 8, 25, 83 or 250 ppm; mice: 0, 33, 100, 333 or
1000 ppm (rats: Fischer 344, 3 per group; mice: C57BL/6N, 78 per
group). Two weeks after dosing began, breeding was initiated.
No rat dams or weanlings died. There was a trend toward
decreased weight gain in dams in all groups and in weanling males at
levels > 83 ppm. Food consumption was also reduced at 250 ppm
for males only. No effects on females were observed. At 250 ppm,
there was a decrease in pup survival to postnatal day 4. Thyroid
hyperplasia was observed in males at all doses and in females above
8 ppm, increasing in incidence and severity with dose. Thyroid
adenomas were reported in males at 83 ppm and above. Vacuolization
of pituitary glands in males was noted at 250 ppm.
There was a significant decrease in body weight in high-dose
female mice during the period of lactation. Weanling body weights
were decreased in males and females at 333 ppm and 1000 ppm.
Initially, insufficient pregnancies were produced in all dose
groups. A rebreeding programme, after 6.5 weeks on ETU diets,
produced sufficient numbers of litters for evaluation. However, no
pregnancies were achieved in the high-dose group, and pregnancy rate
was reduced in other dose groups in comparison to control. The
number of pups surviving to day 28 was significantly decreased in
the high-dose group. Thyroid hyperplasia and cellular alteration of
hepatocytes (cytomegaly, karyomegaly) were observed in both sexes at
1000 ppm. One male mouse at 333 ppm also had adverse effects in the
liver (Peters et al., 1982).
Special studies on embryotoxicity/teratogenicity
Rats
Pregnant Charles River Rats (ChR-CD, Sprague-Dawley) were
administered 0, 25 or 50 mg/kg bw/day of ETU (98% pure) in DMSO, or
DMSO alone (vehicle control) or water alone onto the shaved back of
each animal for 48 hours on days 10 and 11 or days 12 and 13 of
gestation.
Maternal body-weight change during the 48-hour administration
period ranged from +4 to -5%. Fetuses examined from dams
administered ETU at 50 mg/kg bw/day on days 10 and 11, showed short
tails (3/83) and fused ribs (2/83). However, dams given 50 mg/kg
bw/day on days 12 and 13 produced fetal deformities in all
offspring. Fetal defects were characterized by encephalocele, a
part or the entire tail missing, missing leg bones, hunchback
curvature to the spine, short mandible, fusion of ribs and fusion of
sternebrae.
A dose of 25 mg/kg bw/day administered only on days 10 and 11
of gestation did not result in any fetal abnormalities. A dose of 25
mg/kg bw/day was not administered on days 12 and 13 of gestation
(Stula & Krauss, 1977).
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rats
(Wistar) (10-17 pregnant dams per dose). Treatment was made for 21-
42 days before conception to pregnancy day 15, and on days 6-15 or
6-20 of pregnancy. Doses of 40 mg/kg bw/day were not toxic to rats;
however, 80 mg/kg bw/day was lethal to 9 of 11 female rats. Mean
fetal weight was reduced at 40 mg/kg bw/day. Measurements of
sterility, pre-implantation loss and post-implantation survival were
comparable to controls. The brain was the most commonly affected
organ. ETU induced meningoencephalocele, meningorrhagia,
meningorrhea, hydrocephalus, obliterated neural canal, abnormal
pelvic limb posture with equinovarus, and short or kinky tail at 10
mg/kg bw/day in all phases of the study. Although no abnormalities
were reported in rats at 5 mg/kg bw/day, there was a higher
frequency of delayed ossification of the parietal bone, compared to
controls. The NOAEL for embryo/fetotoxicity was 5 mg/kg bw/day
based on teratogenic effects observed at 10 mg/kg bw/day. The NOAEL
for maternal toxicity was 40 mg/kg bw/day (Khera, 1973).
ETU was given by gavage (distilled water, 5 ml/kg bw/day) on
days 6-20 of gestation to pregnant Sprague-Dawley rats (22/group) at
doses of 0, 15, 25, or 35 mg/kg bw/day, and dams were sacrificed on
day 21 for examination of uterine contents. Maternal appearance,
behaviour, and body-weight gain were generally unaffected, and the
incidence of pregnancy was comparable among the groups. No adverse
effect was noted on the average numbers of implantations, live
fetuses, or percentages of resorption sites per litter. Mean fetal
body weights were decreased in a dose-related manner, but were only
significantly reduced at 35 mg/kg bw/day (13-15% lower). ETU at 35
mg/kg bw/day produced external malformations including cranial
meningocele and meningorrhea, severe hindlimb talipes, and a non-
significant incidence of hydrocephaly. Short and/or kinky tails
were noted in 43.5% of the fetuses. Soft tissue examinations
revealed higher incidences of dilated brain ventricles at 25 and 35
mg/kg bw/day (33.5 and 93% of the fetuses, respectively) and of
hydroureter and dilated ureter at 35 mg/kg bw/day, and skeletal
examinations revealed a reduced ossification of skull bones and a
significantly increased incidence of dumbbell-shaped or bilobed
vertebral centra (33.5% of fetuses). There were no other treatment-
related increases in skeletal variants among any of the experimental
groups, and no treatment-related effects of any kind identified in
the 15 mg/kg bw/day group. The NOAEL for maternal toxicity was 35
mg/kg bw/day. The NOAEL for embryo/toxicity and teratogenicity was
15 mg/kg bw/day based on higher incidences of dilated brain
ventricles at 25 mg/kg bw/day (Saillenfait et al., 1991).
ETU (100% purity) was administered via oral gavage at 40 mg/kg
bw/day from days 7 to 15 of gestation to pregnant CR rats (10-12
rats/group). Rats were hypothyroid and euthyroid. There was a
problem, however, in maintaining the euthyroid state in rats given
supplement. Rats were also given thyroxine to determine if ETU
teratogenicity occurred through alterations of maternal thyroid
function. ETU was found to be teratogenic in the rat but not
through alteration of maternal thyroid status. It was also
demonstrated that ETU lowered serum T4; that hypothyroidism per se
increased the background level of malformations in the rat; that T4
alone was embryotoxic but not teratogenic; and that hypothyroidism
altered the spectrum of malformations in response to ETU both
quantitatively and qualitatively (Lu & Staples, 1978).
Virgin Sprague-Dawley rats were mated one-to-two with males
and, after pregnancy was verified, were administered ETU (unknown
purity), T3/T4 and sodium iodide via oral gavage in varying
concentrations, either singly or in combination, as well as a
control solution of water only, from day 7 to day 20 of gestation.
Dosing regimen was as follows:
Dose group Total rats per
group
Control 1 ml distilled water 14
T3 20 µg/kg bw/day + T4 100 µg/kg bw/day 10
Sodium iodide 333 µg/kg bw/day 10
ETU 20 mg/kg bw/day 10
ETU 20 mg/kg bw/day + sodium iodide 16
ETU 20 mg/kg bw/day + T3/T4 16
ETU 40 mg/kg bw/day 11
ETU 40 mg/kg bw/day + sodium iodide 14
ETU 40 mg/kg bw/day + T3/T4 15
Each pregnant dam was killed on day 20 by chloroform
asphyxiation and the fetuses removed via hysterotomy. The number of
resorptions, live/dead fetuses and fetal birth weights were
determined. Skeletal analyses were performed on 1/3 and visceral
analyses on 2/3 of the fetuses. Results indicated a possible
reduction in the teratogenic response to ETU for some malformations
when T3/T4 was administered in conjunction with ETU. For example,
20 and 40 mg/kg bw/day ETU (alone) produced 97.6 and 94.5% incidence
of hydrocephaly, respectively. In combination with T3/T4 these
same levels produced 19.6 and 74.5% incidence, respectively. These
results indicate that the teratogenic potential of ETU may in part
be secondary to the thyroid toxicity of ETU (Emmerling, 1978b).
Rats, mice and hamsters
Wistar-Imamichi rats, JCL-ICR mice, and Syrian golden hamsters
10 weeks or older were mated overnight and examined the next morning
for the presence of a vaginal plug or spermatozoa in vaginal smears.
Evidence of copulation was designated as day zero of gestation.
Pregnant females were given daily oral doses of ETU by gavage during
the period of organogenesis. Doses given to rats, mice and hamsters
were, respectively 0, 10, 20, 30, 40 or 50 mg/kg bw/day, 0, 200, 400
or 800 mg/kg bw/day and 0, 90, 270, or 810 mg/kg bw/day. Rats,
mice, and hamsters were sacrificed on days 20, 18, and 14,
respectively.
Dams did not show signs of toxicity and none died in any
species. There were no statistically significant differences between
treated and control rats for the mean number of implants and live
fetuses reported. Mean fetal weight for both males and females was
statistically significantly decreased at 30 mg/kg bw/day and higher.
The percent of fetal death was also statistically significantly
increased at 50 mg/kg bw/day. Mice showed no statistically
significant changes between treated and control values for any of
the prenatal developmental parameters (i.e mean number of implants,
mean number of live fetuses, percent fetal death and mean fetal body
weight). The mean number of implants for hamsters were comparable
to control values for all doses. There was a statistically
significant decrease in the mean number of live fetuses born
attendant to a decrease in mean fetal body weight for males and
females which was statistically significant at 810 mg/kg bw/day.
Mean fetal body weight for females was also statistically
significantly lower at 270 mg/kg bw/day without other prenatal
developmental effects. Gross external anomalies were not meaningful
between controls and treated groups for mice. Rats manifested a
short or kinky tail at 30 mg/kg and above in 80% of the offspring.
In rats, meningocele was observed in 66% of the offspring at 40
mg/kg bw/day and above and micrognathia in 30% of the offspring at
50 mg/kg bw/day. Hamsters showed multiple type anomalies at 810
mg/kg bw/day and included such signs as cleft palate, short or kinky
tail, and oligodactyly. Short or kinky tail was observed in 2% of
the animals at 270 mg/kg bw/day and 42% of the animals at 810 mg/kg
bw/day. The LOAEL in rats was 30 mg/kg and seen as curved clavicles.
There was an increased incidence of curved clavicles, fused/wavy
ribs, fused sternebrae, malformed vertebrae and scoliosis at 40 and
50 mg/kg bw/day. The LOAEL was 90 mg/kg bw/day in hamsters based on
a 2% incidence of malformed lumbar and sacral vertebrae, a 4%
incidence at 270 mg/kg bw/day and a 51% incidence at 810 mg/kg.
There were no brain or visceral anomalies observed in mice.
Dilation of the lateral 4th ventricle was observed in 5% of hamsters
at the high dose, none at lower doses and 2% in controls. Dilation
of the lateral or 4th ventricle in rats was observed in 2% and 39%
of rats at doses of 10 and 20 mg/ kg bw/day. At 30 mg/kg bw/day and
above the response was 100%. No maternal toxicity occurred at the
doses tested. The NOAEL for embryo/ fetotoxicity in the rat was 10
mg/kg bw/day based on dilation of the lateral or fourth ventricle at
20 mg/kg bw/day. The NOAEL for embryo/fetotoxicity in the hamster
was 90 mg/kg bw/day based on decrease of fetal body weight at 270
mg/kg bw/day. The NOAEL for mice was greater than 80 mg/kg bw/day
(Teramoto et al., 1978).
Hamsters
ETU (purity > 99%) was administered orally to pregnant Syrian
hamsters at doses of 600, 1200, 1800 or 2400 mg/kg bw on day 11 of
gestation. All dams were killed on day 15 of gestation for necropsy
and fetal examination. There were only 5 pregnant dams in the
control group compared to 8-10 in the treated groups.
Maternal toxicity was not reported at any dose. However, there
was an increased incidence of resorbed fetuses and fetuses dying
late in gestation with an associated decrease in the number of live
fetuses at 2400 mg/kg bw. Fetal body weights were similarly reduced
at 1800 mg/kg bw. Malformations were evident at > 1200 mg/kg bw,
with no adverse effect reported at 600 mg/kg bw. Fetal anomalies
included cleft palates ectrodactyly, hydrocephalus and hypoplastic
cerebellum. There was also an increased incidence of delayed
ossification of the calcarium and sternebrae defects. As with other
species (i.e. rat, cat), the brain was particularly sensitive to
ETU, although at higher dose levels (Khera et al., 1983).
Mice/rats/hamsters/guinea-pigs
Time-pregnant random-bred CD-1 mice, Sprague-Dawley rats golden
hamsters and Hartley strain guinea-pigs were used. Maneb (80%
pure), ETU (melting point 197-198 °C) and EBIS were administered by
gastric intubation. Control animals received vehicle alone (water
or corn oil) or remained untreated. Prenatal studies were conducted
on rats given maneb (480, 240, 120, 0 mg/kg bw/day for days 7-16) or
ETU (80, 40, 30, 20, 10, 5, 0 mg/kg bw/day for days 7-21) or EBIS
(30, 25, 7.5, 0 mg/kg/ bw/day for days 7-21). Prenatal studies were
also conducted on mice given maneb (1500, 750, 375, 0 mg/kg bw/day
for days 7-16) or ETU (200, 100, 0 mg/kg bw/day for days 7-16) or
EBIS (200, 100, 50, 0 mg/kg bw/day for days 7-16). Prenatal studies
were also conducted with hamsters and guinea-pigs but only with ETU.
Hamsters received either 100, 50, 25 or 0 mg/kg of ETU on days 5-10
of gestation. Guinea-pigs received 100, 50 or 0 mg/kg bw/day of ETU
on days 7-25. Post-natal studies were only conducted on rats. Rats
received either maneb (480, 240, 0 mg/kg bw/day) or ETU (30, 25, 20,
0 mg/kg bw/day) or EBIS (30, 15, 0 mg/kg bw/day) on days 7-15 of
gestation. Mice were killed on day 18, rats on day 21, hamsters on
day 15 and guinea-pigs on day 35. Animals designated for post-natal
study were allowed to litter normally. Litters were normalized to 4
individuals of each sex and weaned on day 22 post-partum.
In toxicity studies conducted to determine dose levels, all
compounds tested proved to be more toxic in the rat than the mouse.
Hind limb paralysis was observed in rats given maneb at the high
dose of 600 mg/kg bw/day. No toxicity was noted in mice given a
dose of 1500 mg/kg bw/day of maneb. EBIS produced death in rats at
75 mg/kg bw/day and hind limb paralysis at 50 mg/kg bw/day.
Decreased body-weight gain and death was observed in mice given 100
mg/kg bw/day. ETU produced lethality in rats at the high dose of 80
mg/kg bw/day. ETU was not toxic in mice (300 mg/kg bw/day) hamsters
(150 mg/kg bw/day) or guinea-pigs (100 mg/kg bw/day) at the high
dose tested.
Maneb administered to pregnant rats resulted in significant
dose-related decrease in maternal weight gain and increase in liver
to body-weight ratios. Fetal weight and caudal ossification were
significantly reduced only at the highest dose tested (480 mg/kg
bw/day). At the high dose (480 mg/kg bw/day), 18 fetuses from 4
litters manifested hydrocephalus. Maternal mice given maneb
manifested increased liver/body-weight ratios and decreased caudal
ossification beginning at 375 mg/kg. Dose-related trends were
evident. EBIS given to rats and mice did not result in adverse
fetal effects. Average maternal weight gain was decreased in rats
at 30 mg/kg bw/day. Maternal weight gain in mice was not affected.
Average liver to body-weight ratio was increased at the high dose
tested for rats (30 mg/kg bw/day) and mice (200 mg/kg bw/day). ETU
administered to rats at the high dose of 80 mg/kg bw/day resulted in
25% maternal mortality and reduced weight gain. Fetal toxicity was
also observed at 80 mg/kg bw/day and included mortality, decreased
weight, decreased ossification and edema. Gross defects of the
skeletal system and central nervous system were noted in a majority
of fetuses. At 40 mg/kg bw/day, fetal weight and ossification were
reduced and hydrocephalus and encephalocele were evident.
Hydrocephalus was seen at 20 mg/kg bw/day and decreased fetal body
weight at 10 mg/kg bw/day. ETU produced an increase in the liver-
body-weight ratio of mice at 100 mg/kg bw/day and 200 mg/kg bw/day
and an increase in the number of supernumerary ribs at 200 mg/kg
bw/day. No apparent effects were observed in hamsters or guinea-
pigs. Post-natal studies with maneb resulted in delayed eye opening
in males. Post-natal observations with EBIS resulted in decreased
fetal body weight at day 22 in females only as well as delayed eye
opening. ETU administration produced no observable post-natal
effects with the exception of increased total open field activity in
males. There were no apparent differences reported in open field
activity between male fetuses surviving the high dose (30 mg/kg
bw/day) with hydrocephalus and their apparently normal mates.
Hydrocephaly was not observed at lower doses (Chernoff et al.,
1979).
Rabbits
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rabbits
(New Zeeland white). There were 5-7 pregnant does per group.
Treatment was made from days 7 to 20 of pregnancy. No toxicity was
apparent in rabbits given 80 mg/kg bw/day. Fetal weights were not
affected. Measurements of sterility, pre-implantation loss and post-
implantation survival were comparable to controls. Rabbits presented
no evidence of malformations at the doses administered. However,
there was an increase in resorption sites, decreased brain weight,
and degeneration of the proximal convoluted tubules in the kidneys
of fetuses at 80 mg/kg bw/day. The NOAEL for maternal toxicity was
80 mg/kg bw/day and for embryo/fetotoxicity the NOAEL was 40 mg/kg
bw/day (Khera, 1973).
Cats
ETU (purity not stated) was administered orally (in gelatin
capsules) to pregnant European and Persian breed cats (7-14 cats per
group) at doses of 0, 5, 10, 30 or 60 mg/kg bw/day on days 16-35 of
gestation or 120 mg/kg bw/day from days 16 to 34 of gestation. No
effect was evident at 5 mg/kg bw/day. However, at > 10 mg/kg
bw/day decreased ataxia, tremors and hindlimb paralysis were
observed. No pregnant cats survived in the 30 and 60 mg/kg bw/day
dose groups. The remaining cats showed no apparent treatment-related
effect on fetal viability or fetal weight. Although this study was
inconclusive in many respects, there was an increased incidence of
toxicity to the central nervous system at 10 mg/kg bw/day. Further,
at 5 and 120 mg/kg bw/day, there were anomalous fetuses in each
group. Incidences of exencephaly, hydrocephaly, cleft palate,
kyphoscoliosis, umbilical hernia, coloboma, and spina bifida were
observed in these two treated groups. Similar anomalies were
observed in the rat (Khera & Iverson, 1978).
Special studies on genotoxicity
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induced mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic. The results of genotoxicity assays on ETU are given in
Table 2.
Table 2. Results of genotoxicity assays on ethylenethiourea
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 10-20 000 µg/plate; ? Positive Teramoto et al.,
reversion TA1535, TA1536, in DMSO? (TA1535 without 1977; Shirasu
assay TA1537, TA1538, G46 activation) et al., 1977
S. typhimurium 0.2-2000 µg/plate >98% Negative Brooks & Dean, 1981
TA1535, TA1537,
TA1538, TA92, TA98,
TA100
S. typhimurium 5-5000 µg/plate; >98% Negative Richold & Jones,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 0.1-2000 µg/plate; >98% Negative Rowland & Severn,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 10-5000 µg/plate; >98% Positive Simmon & Shepherd,
TA1535, TA1537, in DMSO (TA1535 with 1981
TA1538, TA98, TA100 & without
activation)
S. typhimurium 4-2500 µg/plate >98% Negative Trueman, 1981
TA1535, TA1537,
TA1538, TA98, TA100
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Salmonella S. typhimurium 1000-20 000 µg/plate; no ? Positive Autio et al., 1982
reversion TA1950 activation used; in DMSO
assay (cont'd)
S. typhimurium >500 µg/plate; ? Positive Moriya et al., 1983
TA1535, TA1537, in DMSO? (TA1535 with
TA1538, TA98, TA100 and without
activation)
S. typhimurium 100-10 000 µg/plate; 98.4% Positive Mortelmans et al.,
TA1535, TA1537, TA98, in DMSO (TA1535 with 1986 (SRI)
TA100 & without
activation)
Salmonella S. typhimurium 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; TA1535, TA1537, TA98 in dimethylacetamide
fluctuation test
Salmonella S. typhimurium 200-80 000 ? Positive Schupbach & Hummler,
forward TA1530 µg/plate 1977
mutation assay
E. coli E. coli WP2 hcr 10-10 000 µg/plate ? Negative Teramoto et al.,
reversion assay 1977; Shirasu
et al., 1977
E. coli 343/113/uvrB 200-4000 µg/ml; >98% Positive Mohn et al., 1981
in phosphate buffer (galR- & arg+
systems with
activation)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
E. coli E. coli WP2 uvrA 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; in dimethylacetamide
fluctuation test
Host mediated S. typhimurium 500-6000 mg/kg; ? Negative Schupbach & Hummler,
assay Swiss G46 in DMSO 1977
albino mouse
Swiss albino S. typhimurium 670-6000 mg/kg; ? Weak Schupbach & Hummler,
mouse TA1530 in DMSO Positive 1977
Male JCL-SD rat S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
G46 1977; Shirasu
et al., 1977
Male JCL-ICR S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
mouse G46 1977; Shirasu
et al., 1977
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Mouse lymphoma 140-3000 µg/ml; >98% Negative Jotz & Mitchell,
mutation assay L5178Y TK+/- in DMSO 1981
Mouse lymphoma 25-3600 µg/ml; NTP Positive McGregor et al.,
L5178Y TK +/- in DMSO chemical 1988
repository
Chinese hamster ovary 1000-2000 µg/ml; >98% Negative Carver et al., 1981
(CHO-AT3-2; several loci) in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 0.25-2.5%; ? Negative Mollet, 1975
recessive lethal in sugar water
assay
D. melanogaster 250 ppm; in DMSO >98% Negative Valencia &
Houtchens, 1981
D. melanogaster 4900 ppm injection; 97% Inconclusive Woodruff et al.,
12 500 ppm feed; in water 1985 Mason et al.,
1992
D. melanogaster 5100 ppm 98.4% Inconclusive Mason et al., 1992
1.D. Yeast and Other Fungal Assays
Forward mutation S. pombe 0.1-1 µg/ml; >98% Negative Loprieno, 1981
in DMSO
A. nidulans 0.22-116 mM; >98% Negative Crebelli et al.,
no activation used; 1986
in DMSO
Reverse mutation S. cerevisiae XV185-14C 88.9-889 µg/ml; >98% Equivocal Mehta & von Borstel,
in DMSO 1981
1.E. Plant Test
Mutation Tradescantia 9.79 X 10-5 M; ? Positive van't Hof &
clone 4430 in DMSO? Schairer, 1982
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations in Mammalian Cells
In vitro Chinese hamster cell line 1000-3200 µg/ml ? Negative Teramoto et al.,
chromosomal (Don) 1977; Shirasu
aberrations et al., 1977
Chinese hamster ovary 1670-5000 µg/ml; >98% Positive Natarajan & van
(CHO) cells in DMSO (with and Kesteren-van
without Leeuwen, 1981
activation)
Chinese hamster ovary 6000-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
2.B. In Vivo Chromosomal Alterations
Bone marrow Male & female Wistar rat 50-400 mg/kg; ? Negative Teramoto et al.,
cytogenetics in aqueous soln 1977; Shirasu
et al., 1977
Micronucleus assay Female ICR mouse 150-450 mg/kg; ? Negative Seiler, 1975
in DMSO
Male & female Swiss albino 700-6000 mg/kg; ? Negative Schupbach & Hummler,
mouse in gummi arabicum 1977
Male ICR mouse 220-880 mg/kg; >98% Negative Kirkhart, 1981
in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Micronucleus assay B6C3F1 mouse 880-1416 mg/kg; >98% Negative Salamone et al.,
(cont'd) in DMSO 1981
Male & female CD-1 mouse 220-880 mg/kg; >98% Negative Tsuchimoto & Matter,
in DMSO 1981
Dominant lethal Swiss albino mouse 500-3500 mg/kg; ? Negative Schupbach & Hummler,
assay in gummi arabicum 1977
JCL-ICR mouse 300-600 mg/kg; ? Negative Teramoto et al.,
in water with gum arabic 1977; Shirasu
et al., 1977
C3H/HeCr mouse 150 mg/kg; ? Negative Teramoto et al.,
in gum arabic soln 1978
Reciprocal D. melanogaster 500 ppm; >98% Negative NTP, 1992
translocations in 5% sucrose soln
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
Rec assay B. subtilis H17, M45 20-4000 µg/disk ? Negative Teramoto et al.,
1977; Shirasu
et al., 1977
B. subtilis H17, M45 spores 2000 µg/disk; >98% Positive Kada, 1981
in DMSO (without
activation)
E. coli WP2, WP67, CM871 Not specified >98% Negative Green, 1981
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Rec assay (cont'd) E. coli WP2, WP67, CM871 Not specified >98% Negative Tweats, 1981
E. coli several deficient 500 µg/ml; in DMSO >98% Positive Ichinotsubo et al.,
strains (with activation) 1981
S. typhimurium TA1538, 125-2000 µg/disk; ? Negative Rashid & Mumma, 1986
TA1978; in DMSO
E. coli K12 & WP2
Inhibition of DNA E. coli polA 2273 µg/ml >98% Positive Rosenkranz et al.,
polymerase I (without 1981
activation)
Lambda prophage E. coli (lysogenic) 2-20 mg/ml; only activation >98% Positive Thomson, 1981
induction used; in DMSO
E. coli E. coli PQ37 Not specified; in DMSO ? Negative Quillardet et al.,
SOS Chromotest 1985
In vitro Human fibroblasts from Not specified; in DMSO >98% Negative Agrelo & Amos, 1981
unscheduled DNA skin biopsies
synthesis (UDS)
HeLa S3 human cells Not specified; in DMSO >98% Inconclusive Martin & McDermid,
1981
WI-38 human fibroblasts 63-2000 µg/ml; in DMSO >98% Negative Robinson & Mitchell,
1981
In vitro unschuled Rat hepatocytes 9.6 X 10-9 - 3.2 X 10-3 M ? Negative Althaus et al.,
DNA synthesis (nuclei isolation) 1982
(UDS)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
In vivo/in vitro Female B6C3F1 mouse 1500 mg/kg; in corn oil 98% Negative (UDS); Frank & Muller, 1988
unscheduled DNA hepatocytes Positive (S-
synthesis (UDS)/S phase increase)
phase analysis
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 25-1000 µg/ml; >98% Negative Evans & Mitchell,
SCE assays (CHO) cells in DMSO 1981
Chinese hamster ovary 1670-5000 µg/ml; >98% Negative Natarajan & van
(CHO) cells in DMSO Kesteren-van
Leeuwan, 1981
Chinese hamster ovary 0.01-100 µg/ml >98% Negative Perry & Thomson,
(CHO) cells 1981
Chinese hamster ovary 500-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
In vivo SCE assays Male CBA/J mouse bone 1000 mg/kg; in DMSO >98% Negative Paika et al., 1981
marrow and liver
3.C. Yeast and other Fungal Assays
Mitotic aneuploidy S. cerevisiae D6 500 µg/ml; in DMSO >98% Positive Parry & Sharp, 1981
Chromosome A. nidulans 19.6-78.3 mM; no activation >98% Positive Crebelli et al.,
malsegregation used; in DMSO 1986
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Mitotic gene S. cerevisiae D4 33-333.33 µg/plate; >98% Negative Jagannath et al.,
conversion in DMSO 1981
S. cerevisiae JD1 50 µg/ml; in DMSO >98% Positive Sharp & Parry, 1981a
(without
activation)
S. cerevisiae D7 2000-4000 µg/ml >98% Negative Zimmermann & Scheel,
1981
Mitotic S. cerevisiae T1, T2 1000 µg/ml; in DMSO >98% Negative Kassinova et al.,
crossing-over 1981
A. nidulans 19.6-78.3 mM; no activation >98% Negative Crebelli et al.,
used; in DMSO 1986
Intrachromosomal S. cerevisiae RS112 5-40 mg/ml; no activation used ? Positive Schiestl et al.,
recombination 1989
Differential S. cerevisiae, T5 Not specified; >98% Negative Kassinova et al.,
killing in DMSO 1981
S. cerevisiae 197/2d, rad 300-1000 µg/ml; >98% Positive Sharp & Parry, 1981b
in DMSO (with and without
activation)
3.D. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 100-1000 µg/ml; 99.8% Negative McGlynn-Kreft &
in DMSO McCarthy, 1984
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Cell transformation Syrian hamster embryo 62-1000 µg/ml ? Negative Casto, 1975, 1976
(SHE) cells/adenovirus SA7 in: Heidelberger
et al., 1983
Syrian hamster embryo 1-24 mM ? Inconclusive Hatch et al., 1986
(SHE) cells/adenovirus SA7
Cell transformation C3H/10T 1/2 cells 33 µg/ml; in DMSO 99.8% Negative McLeod & Doolittle,
with "promotion" 1985
3.E. Germ Cell Effects
Spermhead (CBA X BALB/c) F1 mouse 250-2000 mg/kg; >98% Negative Topham, 1981
abnormalities in Tween 80
B6C3F1/CRL mouse 166-2655 mg/kg; >98% Negative Wyrobek et al., 1981
in DMSO
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report
Special studies on the thyroid
Rats
Groups of four randomly selected weanling Caesarian-delivered
Sprague-Dawley male litter-mate rats were administered 0, 75 or 150
ppm ETU (purity not stated). Within each treatment group dosing
periods and control diet periods were varied to examine the
reversibility of compound-related effects. Results suggest some
reversibility of thyroid effects which were related to time on test
and to the severity of effect on the thyroid (Arnold et al.,
1982).
Sprague-Dawley rats (50/sex/group) were fed diets containing 0,
75, 100 or 150 ppm ETU (purity not stated) mixed in corn oil for 7
weeks. Body weights decreased with increasing dose while thyroid
weights (absolute and relative) increased in both sexes. T3 levels
were somewhat variable, while T4 levels were significantly
decreased at 150 pm in both sexes. These effects partially reversed
after 4 weeks on control diets. Histopathological findings included
reduced colloid content of thyroid acini in high dose rats. Acinar
epithelial cell size and height were not different from control.
Two tumours were identified in the high dose male group: a
follicular cell adenoma and medullary carcinoma. The authors
concluded that the relationship between the duration of exposure to
ETU and the possible reversibility of various thyroid lesions
requires further study (Arnold et al., 1983).
A 22-week study was conducted in Sprague-Dawley rats
(55/sex/group) with the following dosing schedule: ETU (97% purity)
administered alone in the diet at levels of 0, 125, 250 or 625 ppm;
or with 0.2 g T3 and 1.6 g T4/rat, orally via gavage; or with
manganese and zinc. Also included were treatment groups dosed with
0, 650 or 1250 ppm mancozeb alone.
Rats receiving 625 ppm ETU alone or in combination with
manganese and zinc were removed from test diet because of alopecia,
weight loss, dermatosis and mortality. Survivors received control
diets for the remainder of the study. Serum decreased in both sexes
at all doses of ETU after 2 weeks of treatment. These levels
returned to normal when ETU was removed from the diet. Serum T3
decreased in both sexes at 625 ppm ETU after 4 weeks of dosing, and
in males at 125 and 250 ppm ETU, but by week 8 returned to normal.
In females at the same doses, T3 was normal until week 16 when it
decreased. The additions of T3/T4 by oral gavage resulted in
decreased T3 at week 8 in males and a decrease during the first 6
weeks in females at all levels. T3 returned to normal one month
after removal of ETU. TSH increased in the ETU group and less
dramatically in ETU plus T3/T4 groups. These levels returned to
normal 2 weeks after ETU was removed form the diet.
Body weights decreased in males and females after 4 weeks at
625 ppm ETU and in males after 8 weeks at 250 ppm ETU. Thyroid to
body-weight ratio increased at > 125 ppm ETU in males. When ETU
was removed from the diet, weights returned to normal. No effect
was observed on pituitary weights. Thyroid hyperplasia was
increased at 125 ppm ETU and above and reversed to normal 6-8 weeks
after ETU was removed. Approximately 1% (13/1300) of the rats
developed hyperplasia of the thyroid (focal areas of basophilic
hyperplastic follicles and follicular adenoma). A dose-related
increase in liver weight was observed at 125 ppm ETU and above for
both sexes.
Exposure to ETU resulted in a decrease in thyroid hormone
(T3/T4) levels and increased serum TSH levels in a dose-related
manner. Although TSH levels were reduced when ETU was supplemented
with T3/T4, the high dosage of ETU was apparently sufficient to
override these effects. The hormone imbalance induced by ETU
correlated with the histologic changes in the thyroid. Withdrawal
of ETU from the diet reversed the hypothyroid conditions induced to
euthyroid (Leber et al. 1978a).
In two 90-day feeding trials Sprague-Dawley rats (12/sex/group)
were given 75 or 100 ppm ETU, and thyroid function, serum T4, T3,
and TSH, T3 uptake in vitro, 131I uptake, and thyroid to body-
weight ratios were measured at days 46 and 91. Additionally, the
fate of the incorporated 131I was traced in thyroid fractions at the
100 ppm level. Groups of both sexes at the lower feeding level and
the females at 100 ppm were functionally euthyroid whereas males at
100 ppm were somewhat hypothyroid despite elevated serum T3, TSH,
and T3/T4 ratios. Results showed that the inhibitory effects of
ETU are similar to those for methimazole. ETU inhibited
monoiodotyrosine (MIT) utilization, and the coupling of
diiodotyrosine (DIT) residues to form T4, resulting in
significantly reduced active synthesis of T3 and T4 prohormones
(males 100 ppm). The capacity of serum to bind T3 was reduced;
however, there was no evidence for inhibition by ETU of T4 to T3
monodeiodination or interference with the normal feedback mechanisms
of thyroid hormones on TSH secretion (O'Neil & Marshall, 1984).
Observations in humans
A 53-year old female employed in the manufacture of products
from synthetic and natural rubber developed itching of the fingers.
Her condition improved on weekends but became worse upon returning
to work. Two months later the eruption spread to her forearms. The
individual showed positive reactions to nickel and cobalt in the
ICDRG standard patch test as well as to a component of the rubber
material from her work which was ETU. Additional patch testing with
ETU and chemically related substances showed that ETU tested
positive at dose levels at or above 0.01% (w/v). One-percent
concentrations of dibutyl-, diethyl- or diphenylthiourea all were
negative as were ethyleneurea and ethylenediaminine. Thiourea at
0.01% (w/v) was also negative. The fungicides zineb and maneb tested
negative and positive, respectively. The positive reaction to maneb
was attributed to the presence of ETU as an impurity/degradation
product and confirmed in studies using thin layer chromatography
(Bruze & Fregert, 1983).
A retrospective study of women who were employed at a rubber
manufacturer using ETU was undertaken to determine any excess of
fetal abnormalities occurring in children to women who had worked
with ETU during early pregnancy. The women were employed on hand and
machine trimming, hand cutting, coolant hose manufacture, packing
and dispatch. Women were also employed in moulding, cutting blanks
for moulding and tool and die trimming where there was a hazard from
inhalation of dust. Women born in 1918 or later who left employment
between 1963-1971 were surveyed. Of 699 women who left between
1963-1971, 255 gave birth to 420 children. Only 59 of 255 were
working at the plant at the time of early pregnancy and none of
these had abnormal children. Of the total 420 children born 11 had
abnormalities. However, figures for the group showed no excess over
the expected number of fetal abnormalities in the region surveyed.
The study did not demonstrate any risk of teratogenesis. However,
the number of women exposed during early pregnancy was small.
A total of 1929 workers engaged in the production or
manufacture of ETU were surveyed retrospectively for thyroid cancer,
and compared with the thyroid cancer list of the Birmingham
(England) Cancer Registry from 1957-1971. No thyroid cancers
occurred in these workers and the results were considered to be
preliminary (Smith, 1976).
An apparent increased incidence of miscarriages among workers
at a USA rubber products factory was investigated. Of 7 pregnancies
only 2 resulted in spontaneous abortions, one with an identified
medical problem. Before starting work at the rubber factory, the 81
women surveyed had 192 pregnancies with 16% spontaneous abortions.
There was no indication that menstrual problems increased with
duration of employment as a moulder. It was concluded that no
adverse reproductive effects could be attributed to the work
environment - although the small numbers available for study
prevented this possibility from being entirely ruled out (Wright et
al., 1981).
No hazard of clinical thyroid depression existed based on
medical evidence collected on workers exposed to ETU at a rubber
company in Michigan. Fifty-one subjects were evaluated (49 males, 2
females). Environmental sampling results demonstrated that workers
were exposed to trace amounts of ETU as airborne dust or through
direct contact of powdered ETU (Salisbury & Lybarger, 1977).
Clinical examinations and thyroid function tests were carried
out over a period of 3 years in the United Kingdom on 8 workers
involved in the manufacture of ETU and 5 workers involved in mixing
of ETU with rubber. The average length of exposure in workers
associated in the manufacturing process was 10 years with a range of
5-20 years. The exposure period for mixers was 3 years. All
subjects were males with an age range from 26-62 years. Matched
controls were also examined. In the manufacturing plant ETU levels
of 330 µg/m3 were recorded on one personal sampler. Background
levels ranged from 10-240 µg/m3. Levels of ETU recorded on
personal samplers of mixers ranged from 120-160 µg/m3. Mixers but
not process workers had significantly lower levels of T4 in their
blood compared to controls. No effects were found on TSH or thyroid
binding globulin. The authors concluded that there was no evidence
that thyroid function is severely affected by exposure to ETU at the
levels experienced by these workers nor was there any evidence of
any effect. However, the T4 results in the exposed workers were
generally lower than those in the control group with most of the
difference in distribution accounted for by the results from the
mixers (Smith, 1984).
The concentration of ETU in pesticide formulations and ambient
air were measured and exposure to maneb (80% powder) or mancozeb
(Ridomil 56% mancozeb) evaluated during the spraying of potato
fields. The mean tank mix concentrations of maneb and mancozeb were
4.0 or 7.0 g/litre, respectively. Spraying time was 0.5-7.0 hours
(mean 4.0 hours). Each single application was separated by several
weeks. Therefore only acute (single day) exposure was determined in
workers (i.e. mixer/loader/applicator).
The overall range of concentrations of ETU in air were between
0.004 and 3.3 µg/m3 in the breathing zone and 0.006 and 0.8 µg/m3
in the (closed) tractor cabin. The mean calculated concentrations
of active maneb and mancozeb in air were 7 and 20 µg/m3,
respectively. The total inhaled amount of ETU and EBDCs was 5.0 and
126.0 µg/day, respectively corresponding to a dose of 0.07 µg ETU/kg
bw and 1.8 µg EBDC/kg bw for applicators and mixer and loaders
weighing 70 kg. Patch samples on clothes and skin (back, chest,
shoulders and forearm) indicated that 1.4, 10.0, 4.0 and 1.2% of ETU
(0.07; 0.19; 0.39; 0.17 mg/cm2/hour) reached the skin respectively.
ETU in urine sample taken on days 1, 8, 15 and 22 ranged between
0.09-2.5; 0.07-1.0; 0.01-0.30 and < 0.01-0.2 µg/mmol creatinine.
Absolute concentrations of ETU in all samples ranged between < 0.2
and 11.8 µg/litre of urine. Under the exposure conditions the
urinary elimination half-life was calculated to be 100 hours
(Kurrtio et al., 1990).
COMMENTS
Following oral administration to mice essentially all of the
ETU was recovered in the excreta within 48 hours; none was recovered
as carbon dioxide. Approximately 50% of the administered dose was
found in urine as unchanged ETU.
After the oral administration of radiolabelled ETU, its
concentration in both pregnant mice and rats peaked about the same
time (1.4 hours), with concentrations in maternal and fetal tissues
similar at 3 hours. The half-life of elimination from mice and rats
was 5.5 hours and 9.4 hours, respectively. Approximately 70% of ETU
was found in urine in both species at 48 hours.
Mice metabolize ETU primarily by the flavin-monooxygenase
system and rats by the P-450 system of enzymes.
ETU is slightly toxic after acute oral administration, with the
LD50 ranging from 545 mg/kg bw in pregnant rats to 4000 mg/kg bw in
adult mice.
In a 13-week study in mice at dietary concentrations of 0, 125,
250, 500, 1000 or 2000 ppm the NOAEL was 250 ppm (equivalent to 38
mg/kg bw/day). Diffuse follicular cell hyperplasia of the thyroid
and hepatocellular cytomegaly were observed at 500 ppm.
In a three-month study in mice at dietary concentrations of 0,
1, 10, 100 or 1000 ppm the NOAEL was 10 ppm, equal to 1.7 mg/kg
bw/day. ETU produced thyroid follicular cell hyperplasia and
decreased colloid density at 100 ppm.
The NOAEL in a study in which rats were fed dietary
concentrations of ETU at 0, 0.63, 1.3, 2.5, 5.0 or 25 ppm for 8
weeks was 25 ppm (equal to 2.6 mg/kg bw/day), the highest dose
tested.
In a 13-week study in rats, ETU was administered in the diet at
concentrations of 0, 60, 125, 250, 500 or 750 ppm. The NOAEL was
less than 60 ppm (equal to 3.0 mg/kg bw/day) based on
histopathological findings of diffuse follicular cell hyperplasia in
the thyroid.
In a 90-day study in rats, ETU was administered in the diet at
concentrations of 0, 1, 5, 25, 125 or 625 ppm. The NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on hyperaemia of the thyroids,
with and without enlargement, increased thyroid to brain weight
ratio, decreased 125I thyroid uptake, decreased triiodothyronine,
decreased thyroxine and increased thyroid follicular cell
hyperplasia at 125 ppm.
In a four-week feeding study in dogs at dietary concentrations
of 0, 200, 980 or 4900 ppm, the NOAEL was 200 ppm, equal to 6.7
mg/kg bw/day. Decreased body-weight gain, decreased thyroxine and
T3 levels and enlarged thyroids were observed at 980 ppm.
In a 13-week feeding study in dogs at dietary concentrations of
0, 10, 150 or 2000 ppm the NOAEL was 10 ppm, equal to 0.39 mg/kg
bw/day. At 150 ppm haemoglobin, packed cell volume, and red blood
cell count were decreased, and cholesterol was increased. Effects
on the thyroid were found only at 2000 ppm.
In a 52-week feeding study in dogs at dietary concentrations of
0, 5, 50, or 500 ppm, the NOAEL was 5 ppm, equal to 0.18 mg/kg
bw/day. At 50 ppm a reduction in body-weight gain, hypertrophy of
the thyroid with colloid retention, a slight increase in thyroid
weight and pigment accumulation in the liver were observed.
Male and female mice received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,330; 0,1000; 330,0; 330,330; 330,1000; 110,330 or 33,100 ppm.
Mice receiving perinatal exposure only (330,0 ppm) showed no effect
on the incidences of neoplasms after 2 years. Cytoplasmic
vacuolization of follicular cells of the thyroid was evident in
males and females at 33,100 ppm, but no increases in neoplasms of
the liver, pituitary or thyroid were observed. T4 values were
significantly decreased in both sexes and thyrotropine was slightly
elevated. Animals receiving 330 ppm during adulthood showed tumours
of either the liver, pituitary or thyroid. Increasing perinatal
exposure from 0 to 330 ppm was associated with an increased
incidence of thyroid and pituitary lesions in female mice receiving
adult exposure to 330 ppm, but there were no enhancing effects of
perinatal exposure in mice receiving adult exposures of 1000 ppm
when compared to adults in the 0,1000 ppm group.
Rats were fed dietary concentrations of ETU at levels of 0, 5,
25, 125, 250 or 500 ppm for 2 years. The NOAEL was 5 ppm,
equivalent to 0.25 mg/kg bw/day. Vascularity and hyperplasia of the
thyroid were seen at 25 ppm.
In a two-year feeding study in rats using dietary
concentrations of 0, 0.5, 2.5, 5 or 125 ppm the NOAEL was 5 ppm
(equal to 0.37 mg/kg bw/day) based on changes in clinical chemistry,
increased triiodothyronine, decreased thyroxine, increased thyroid
weight, increased liver weight and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm.
In a two-year carcinogenicity study in rats using dietary
concentrations of 0, 175 or 350 ppm, thyroid carcinomas and
hyperplastic goitres were observed in both sexes at 175 ppm
(equivalent to 8.8 mg/kg bw/day).
Male and female rats received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,83; 0,250; 90,0; 90,83; 90,250; 30,83 or 9,25 ppm. Rats
receiving perinatal and adult exposure of 9,25 ppm showed no
increase in tumours and no apparent biologically meaningful changes
in thyroid hormone function at two-years when compared to 0,0 ppm
controls. Thyroid hyperplasia was evident in both sexes. At 9
months, animals given 9,25 ppm manifested decreased T3 and T4
values and increased thyrotropine without evidence of thyroid
follicular cell hyperplasia. Males and females receiving a dose of
90,0 ppm showed no hormonal changes and no tumours at 2 years.
Thyroid follicular cell hyperplasia was, however, evident. Animals
receiving adult exposure showed a significant increase in thyroid
follicular cell tumours at 83 and 250 ppm (males) and 250 ppm
(females). Males and females showed no significant differences in
the number of tumours between dose groups of 0,83; 30,83; and 90,83
ppm. Males and females receiving 90,250 ppm showed increases in
thyroid follicular cell tumours when compared to 0,250 ppm. At the
end of 2 years males and females receiving 0,83 or 0,250 manifested
increased numbers of thyroid tumours when compared to 0,0 ppm
controls.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 2.5, 25 or 125 ppm the NOAEL was 2.5 ppm, equal
to a range of 0.16-0.38 mg/kg bw/day, based on thyroid gland
follicular cell hyperplasia and hypertrophy at 25 ppm.
An oral teratogenicity study conducted in rats at dose levels
of 0, 5, 10, 20, 40 or 80 mg/kg bw/day indicated no maternal
toxicity at 40 mg/kg bw/day (NOAEL). Maternal lethality was
observed at 80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity
effects was 5 mg/kg bw/day based on teratogenic effects observed at
10 mg/kg bw/day.
An oral teratogenicity study in rats at dose levels of 0, 15,
25 or 35 mg/kg bw/day was conducted. No maternal toxicity was
observed at 35 mg/kg bw/day (NOAEL). The NOAEL for
embryo/fetotoxicity and teratogenicity was 15 mg/kg bw/day based on
higher incidences of dilated brain ventricles at 25 mg/kg bw/day.
Oral teratogenicity studies in rats (0, 10, 20, 30, 40 or 50
mg/kg bw/day), mice (0, 200, 400 or 800 mg/kg bw/day) and hamsters
(0, 90, 270 or 810 mg/kg bw/day) revealed no maternal toxicity at
the doses tested. The NOAEL for embryo/fetotoxicity in the rat was
10 mg/kg bw/day based on dilation of the lateral or fourth ventricle
at 20 mg/kg bw/day. The NOAEL for embryo/ fetotoxicity in the
hamster was 90 mg/kg bw/day based on a decrease in fetal body weight
at 270 mg/kg bw/day. The NOAEL for mice was greater than 800 mg/kg
bw/day.
In an oral teratogenicity study, rabbits received 0, 5, 10, 20,
40 or 80 mg/kg bw/day of ETU. The NOAEL for maternal toxicity was
80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was 40 mg/kg
bw/day based on an increase in resorption sites, decreased brain
weight and a degeneration of the proximal convoluted tubules in the
kidneys of fetuses at 80 mg/kg bw/day. Malformations were not
observed at the highest dose.
A study with pregnant rats administered ETU, T3/T4 and sodium
iodide in combination indicated a reduction in some of the
teratogenic responses when compared with groups administered ETU
alone. These results indicate that the teratogenic potential of ETU
may in part be secondary to the thyroid toxicity of ETU.
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induces mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic.
An ADI was allocated based upon a NOAEL of 0.39 mg/kg bw/day in
the 13-week study in dogs, since this dose level was between the
NOAEL of 5 ppm (equal to 0.18 mg/kg bw/day) and the middle dose
(effect level) of 50 ppm (equal to 1.8 mg/kg bw/day) in the 52-week
dog study. A 100-fold safety factor was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 10 ppm, equal to 1.7 mg/kg bw/day (3-month study)
Rat: 5 ppm, equal to 0.37 mg/kg bw/day (two-year study)
2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day
(reproduction study)
Dog: 10 ppm, equal to 0.39 mg/kg bw/day (13-week study)
5 ppm, equal to 0.18 mg/kg bw/day (52-week study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw.
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Agrelo, C. & Amos, H. (1981) DNA repair in human fibroblasts. Prog.
Mutation Research, 1: 528-532.
Allen, J.R., Van Miller, J.P. & Seymour, J.L. (1978). Absorption
tissue distribution and excretion of C14-ethylenethiourea by the
rhesus monkey and rat. Res. Comm. Chem. Pathol. Pharmacol. 20(1):
109-115.
Althaus, F.R., Lawrence, S.D., Sattler, G.L., Longfellow, D.G. &
Pitot, H.C. (1982). Chemical quantification of unscheduled DNA
synthesis in cultured hepatocytes as an assay for rapid screening of
potential chemical carcinogens. Cancer Res. 42: 3010-3015.
Arnold, D.L., Bickis, M.G., Nera, E.A., McGuire, P.F. & Munro, I.C.
(1982). Assessing the reversibility of thyroid lesions that result
from feeding etu. Toxicologist, 2(1): Abstract #318.
Arnold, D.L., Krewski, D.R., Junkins, D.B., McGuire, P.F., Moodie,
C.A. & Munro, I.C. (1983). Reversibility of ethylenethiourea-induced
thyroid lesions. Toxicol. Appl. Pharmacol. 67: 264-273.
Austin, G.E. & Moyer, G.H. (1979). Hepatic RNA synthesis in rats
treated with ethylenethiourea. Res. Commun. Chem. Pathol.
Pharmacol. 23(3): 639-642.
Autio, K., von Wright, A. & Pyysalo, H. (1982). The effect of
oxidation of the sulfur atom on the mutagenicity of
ethylenethiourea. Mutation Res. 106: 27-31.
Bridges, B.A., MacGregor, D. & Zeiger, E. (1981). Summary report on
the performance of bacterial mutation assays. Prog. Mutation
Research, 1: 49-67.
Briffaux, J.P. (1991). ETU: 13 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/504 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Briffaux, J.P. (1992). ETU: 52 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/505 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay with preincubation.
Prog. Mutation Research, 1: 261-270.
Bruze, M. & Fregert, S. (1983). Allergic contact dermatitis from
ethylenethiourea. Contact Dermatitis, 9: 208-212.
Carver, J.H., Salazar, E.P., Knize, M.G. & Wandres, D.L. (1981).
Mutation induction at multiple gene loci in Chinese hamster ovary
cells: the genetic activity of 15 coded carcinogens and
noncarcinogens. Prog. Mutation Research, 1: 594-601.
Casto, B.B. (1975, 1976). Progress report NIH-NCI-NOI-CP45615, pp.
1-23, 1975; pp. 1-18 (1976). (Cited in Heidelberger et al., 1983).
Chernoff, N., Kavlock, R.J., Rogers, E.H., Carver, B.D. & Murray, C.
(1979). Perinatal toxicity of maneb ethylenethiourea and
ethylenebisisothiocyanate sulfide in rodents. J. Toxicol. Environ.
Health, 5: 821-834.
Chhabra, R.S., Eustis, S., Haseman, J.K., Kurtz, P.J. & Carlton,
B.D. (1992) Comparative carcinogenicity of ethylenethiourea with or
without perinatal exposure in rats and mice. Fundamental and
Applied Toxicology, 18(3): 405-417.
Crebelli, R., Bellincampi, D., Conti, G., Conti, L., Morpurgo, G. &
Carere, A. (1986). A comparative study on selected chemical
carcinogens for chromosome malsegregation, mitotic crossing-over and
forward mutation induction in Aspergillus nidulans. Mutation
Research, 172: 139-149.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Prog. Mutation Research, 1: 626-637.
Daston, G., Ebron, M.T., Carver, B. & Stefanadis, J.G. (1987) In
vitro teratogenicity of ethylenethiourea in the rat. Teratology,
35: 239-245.
Daston, G.P., Yonker, J.E., Powers, J.F. & Heitmeyer, S.A. (1989).
Difference in teratogenic potency of ethylenethiourea in rats and
mice: relative contribution of embryonic and maternal factors.
Teratology, 40: 555-566.
Decker, C.J. & Doerge, D.R. (1991). Rat hepatic microsomal
metabolism of ethylenethiourea. Contributions of the flavin-
containing monooxygenase and cytochrome P-450 isozymes. Chem. Res.
Toxicol. 4: 482-489.
DiDonato, L.J. & Longacre, S.L. (1987). Ethylenethiourea:
dermal/oral absorbtion study in male rats. Unpublished report No,
85R-0206 from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Doerge, D. & Takazawa, R. (1990). Mechanism of thyroid peroxidase
inhibition by ethylenethiourea. Chem. Res. Toxicol. 3: 98-101.
Dotti, A. (1992). Ethylenethiourea (ETU): two-generation
reproduction study in the rat. Unpublished report No. 252360 from
Research and Consulting Company, Itingen, Switzerland. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978a). A study of the uptake and elimination of
C14 activity after oral ingestion of C14 labelled ethylenethiourea
(ETU) and mancozeb in the rhesus monkey. Revised final report,
Battelle Labs., Columbus, Ohio, USA. (July 31, 1978).
Emmerling, D.C. (1978b). The effects of thyroid hormones on the
teratogenic potential of ethylenethiourea in rats. Unpublished
report No. 78RC-1027 from Battelle Labs, Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Engst, R. & Schnaak, W. (1974). Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev. 52:
45-67.
Evans, E.L. & Mitchell, A.D. (1981). Effects of 20 coded chemicals
on sister chromatid exchange frequencies in cultured chinese hamster
cells. Prog. Mutation Research, 1: 538-550.
Frank, J. & Muller, G. (1988). ETU: In vivo/in vitro UDS/S-phase
assay in mice. Unpublished report No. 88R-47 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Freudenthal, R.I., Kerchner, G., Persing, R. & Baron, R.L. (1977).
Dietary subacute toxicity of ethylenethiourea in the laboratory rat.
J. Environ. Pathol. Toxicol. 1: 147-161.
Galloway, S.M. & Ivett, J.L. (1986). Chemically induced aneuploidy
in mammalian cells in culture. Mutation Research, 167: 89-105.
Gak, J.C., Graillot, C. & Truhaut, R. (1976). Difference in
sensitivity of the hamster and rat to the effects of long term
administration of ethylenethiourea. Eur. J. Toxicol. 9: 303-312.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the "microtiter" fluctuation test. Prog. Mutation Research, 1:
376-386.
Graham, S.L. & Hansen, W.H. (1972). Effects of short-term
administration of ethylenethiourea upon thyroid function of the rat.
Bull. Environ. Contam. Toxicol. 7(1): 19-25.
Graham, S.L., Hansen, W.H., Davis, K.J. & Perry, C.H. (1973).
Effects of one-year administration of ethylenethiourea upon the
thyroid of the rat. J. Agric. Food Chem. 21: 324-329.
Graham, S.L., Davis, K.J.Hansen, W.H. and Graham, C.H. (1975).
Effects of prolonged ethylenethiourea ingestion on the thyroid of
the rat. Food Cosmet. Toxicol. 13: 493-499.
Green, M.H.L. (1981). A differential killing test using an improved
repair-deficient strain of Escherichia coli. Prog. Mutation
Research, 1: 183-194.
Hardin, B., Schuler, R., Burg, J., Booth, G., Hazelden, K.,
MacKenzie, K., Piccirillo, V. & Smith, K. (1987). Evaluation of 60
chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen. 7: 29-48.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putnam, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W. &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagen. 8:
515-531.
Heddle, J.A., Hite, M., Kirkhart, B., Mavournin, K., MacGregor,
J.T., Newell, G.W. & Salamone, M.F. (1983). The induction of
micronuclei as a measure of genotoxicity. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 123: 61-118.
Heidelberger, C., Freeman, A.E., Pienta, R.J., Sivak, A., Bertram,
J.S., Casto, B.C., Dunkel, V.C., Francis, M.W., Kekunaga, T.,
Little, J.B. & Schechtman, L.M. (1983). Cell transformation by
chemical agents - a review and analysis of the literature. US-EPA
Gene-Tox Program. Mutation Research, 114: 283-385.
Hill, R., Erdreich, L., Paynter, O., Roberts, P., Rosenthal, S. &
Wilkinson, C. (1989). Thyroid follicular cell carcinogenesis. Fund.
Appl. Toxicol. 12: 629-697.
Hui, Q.Y., Armstrong, C., Laver, G. & Iverson, F. (1988).
Monooxygenase-mediated metabolism and binding of ethylenethiourea to
mouse liver microsomal protein. Tox. Letters. 41: 231-237.
Ichinotsubo, D., Mower, H. & Mandel, M. (1981a). Testing of a series
of paired compounds (carcinogen and noncarcinogen structural analog)
by DNA repair-deficient E. coli stains. Prog. Mutation Research,
1: 195-198.
Iverson, F., Khera, K.S. & Hierlihy, S.L. (1980). In vivo and in
vitro metabolism of ethylenethiourea in the rat and the cat.
Toxicol. Appl. Pharmacol. 52: 16-21.
Jagannath, D.R., Vultaggio, D.M. & Brusick, D.J. (1981). Genetic
activity of 42 coded compounds in the mitotic gene conversion assay
using Saccharomyces cerevisiae strain D4. Prog. Mutation
Research, 1: 456-467.
Jordan, L.W. & Neal, R.A. (1979). Examination of the in vivo
metabolism of maneb and zineb to ethylenethiourea (ETU) in mice.
Bull. Environm. Contam. Toxicol. 22: 271-277.
Jotz, M.M. & Mitchell, A.D. (1981). Effects of 20 coded chemicals on
the forward mutation frequency at the thymidine kinase locus in
L5178Y mouse lymphoma cells. Prog. Mutation Research, 1: 580-593.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Prog. Mutation Research, 1: 175-182.
Kassinova, G.V., Kovaltsova, S.V, Marfin, S.V. & Zakharov, I.A.
(1981). Activity of 42 coded compounds in differential inhibition
and mitotic crossingover assays in yeast. Prog. Mutation Research,
1: 434-455.
Kato, Y., Odanaka, Y., Teramoto, S. & Matano, O. (1976). Metabolic
fate of ethylenethiourea in pregnant rats. Bull. Environ. Contam.
Toxicol. 16: 546-555.
Khera, K. (1973a). Teratogenic effects of ethylenethiourea in rats
and rabbits, Toxicol. Appl. Pharmacol. 25: 455-456.
Khera, K.S. (1973b) Ethylenethiourea: teratogenicity study in rats
and rabbits. Teratology, 7: 243-252.
Khera, K.S. & Iverson, F. (1978). Toxicity of ethylenethiourea in
pregnant cats. Teratology, 18: 311-314.
Khera, K. (1987). Ethylenethiourea: a review of teratogenicity and
distribution studies and an assessment of reproduction risk. CRC
Crit. Rev. Toxicol. 18: 129-139.
Khera, K.S, Whalen, C. & Iverson, F. (1983). Effects of pretreatment
with SKF-525A, N-methyl-2-thioimidazole, sodium phenobarbital, or 3-
methylcholanthrene on ethylenethiourea-induced teratogenicity in
hamsters. J. Toxicol. and Environ. Health. 11: 287-300.
Kier, L.E., Brusick, D.J., Auletta, A.E., Von Halle, E.S., Brown,
M.M., Simmon, V.F., Dunkel, V., McCann, J., Mortelamans, K., Prival,
M., Rao, T.K. & Ray, V. (1986). The Salmonella
typhimurium/mammalian microsomal assay. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 168: 69-240.
Kirkhart, B. (1981). Micronucleus test on 21 compounds. Prog.
Mutation Research, 1: 698-704.
Kurrtio, P., Savolainen, K., Tuominen, R., Kosma, V.M., Naukkarinen,
A., Mannisto, P. & Collan, Y. (1986). Ethylenethiourea and nabam
induced alterations of function and morphology of thyroid glands in
rat. Arch. Toxicol., Suppl. 9: 339-344.
Kurrtio, P., Vartianinen, T. & Savolainen, K. (1990). Environmental
and biological monitoring of exposure to ethylenebisdithiocarbamate
fungicides and ethylenethiourea. British Journal of Industrial
Medicine, 47: 203-206.
Kurttio, P., Savolainen, K., Naukkarinen, A., Kosma, V., Tuomisto,
L., Penttila, I. & Jolkkonen, J. (1991). Urinary excretion of
ethylenethiourea and kidney morphology in rats after continuous oral
exposure to nabam or ethylenethiourea. Arch. Toxicol. 65: 381-385.
Leber, A.P., Wilkinson, G.E., Emmerling, D., Persing, R.L. & Thake,
D.C. (1978a). A correlation of the hormonal and pathological changes
of the thyroid as related to the treatment and withdrawal of
ethylenethiourea. Unpublished report No. 78RC-1026 from Battelle
Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA.
Leber, A.P., Wilkinson, G.E., Persing, R.L. & Holzworth, D.A.
(1978b) Effects of feeding ethylenethiourea in the rhesus monkey.
Contract No. 68-01-4717 from Battelle Labs., Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Legator, M.S., Bueding, E., Batzinger, R., Connor, T.H.,
Eisendstadt, E., Farrow, M.G., Ficsor, G., Hsie, A., Seed, J. &
Stafford, R.S. (1982). An evaluation of the host-mediated assay and
body fluid analysis. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Research, 98: 319-374.
Leuschner, F. (1977) Oral toxicity of ethylenethiourea 98% - called
for short ETU-during 8 weeks of administration in Sprague-Dawley
rats. Unpublished report No. 77RC-1159 from Laboratorium fur
Pharmakocopie und Toxicologie, Hamburg, West Germany. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lewerenz, H.J. & Plass, R. (1984). Contrasting effects of
ethylenethiourea on hepatic monooxygenase in rats and mice. Arch.
Toxicology, 56: 92-95.
Loprieno, N. (1981). Screening of coded carcinogenic/noncarcinogenic
chemicals by a forward-mutation system with yeast
Schizosaccharomyces pombe. Prog. Mutation Research 1: 424-433.
Lu, M.H. & Staples, R.E. (1978). Teratogenicity of ethylenethiourea
and thyroid function in the rat. Teratology, 17: 171-178.
Marshall, W. (1977). Thermal decomposition of
ethylenebisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. Agric. Food Chem. 25: 357-361.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Prog. Mutation Research, 1: 533-537.
Mason, J.M., Valencia, R. & Zimmering, S. (1992). Chemical
mutagenesis testing in drosophila: VIII. reexamination of
equivocal results. Environ. Mol. Mutagen. 19: 227-234.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb, and their related compounds. Int. Arch. Occup. Environ.
Hlth. 37: 169-178.
McGlynn-Kreft, A.M. & McCarthy, K.L. (1984). Ethylenethiourea
mammalian cell transformation test. Unpublished report No. 84R-56
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
McGregor, D., Brown, A., Cattanach, P., Edwards, I., McBride, D,
Riach, C. & Caspary, W.J. (1988). Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded chemicals.
Environ. Mol. Mutagen. 12: 85-154.
McLeod, P.L. & Doolittle, D.J. (1985). Ethylenethiourea mammalian
cell transformation test for promotion. Unpublished report No. 84R-
0298 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Mehta, R.D. & von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
C14. Prog. Mutation Research, 1: 414-423.
Mohn, G.R., Vogels-Bouter, S. & van der Horst-van der Zon, J.
(1981). Studies on the mutagenic activity of 20 coded compounds in
liquid tests using the multipurpose strain Escherichia coli K-
12/343/113 and derivatives. Prog. Mutation Research, 1: 396-423.
Mollett, P. (1975). Toxicity and mutagenicity of ethylenethiourea
(ETU) in drosophila. Mutat. Res. 29: 254.
Morgan, C. (1991). ETU: 4 week oral (dietary administration)
feasibility study in the beagle. Unpublished report No. 6152-616/6
from Hazleton Labs., Harrogate, North Yorkshire, England. Submitted
to WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986). Salmonella mutagenicity testing:II. Results
from the testing of 270 chemicals. Environ. Muta. 8(7): 1-119.
Natarajan, A.T. & van Kesteren-van Leeuwen, A.C. (1981). Mutagenic
activity of 20 coded compounds in chromosome aberrations/sister
chromatid exchanges assay using chinese hamster ovary (CHO) cells.
Prog. Mutation Research, 1: 551-559.
National Toxicology Program (NTP), 1992. Toxicology and
carcinogenesis studies of ethylenethiourea in F344/N rats and B6C3F1
mice (feed studies). U.S. Department of Health and Human Services,
Public Health Service, National Institutes of Health, NIH
Publication No. 92-2843, National Toxicology Program Technical
Report Series No. 388, March 1992.
Newsome, W.H. (1974). The excretion of ethylenethiourea by rat and
guinea-pig. Bull. Environ. Contamin. Toxicol. 11(2): 174-176.
National Institute for Occupational Safety and Health (NIOSH), 1981.
Ethylenethiourea, Health Hazard Evaluation Report No HETA-81-270-
1012, December 1981.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 8OR-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neil, W. & Marshal, W. (1984). Goitrogenic effects of
ethylenethiourea on rat thyroid. Pesticide Biochemistry and
Physiology, 21: 92-101.
Paika, I.J., Beauchesne, M.T., Randall, M., Schreck, R.R. & Latt,
S.A. (1981). In vivo sce analysis of 20 coded compounds. Prog.
Mutation Research, 1: 673-681.
Parry, J. (1986). The expression of recessive markers in presumptive
monosomic colonies derived from Saccharomyces cerevisiae, strain
D6. Mutagenesis, 1: 299-300.
Parry. J.M. & Sharp, D.C. (1981). Induction of mitotic aneuploidy in
the yeast strain D6 by 42 coded compounds. Prog. Mutation Research,
1: 468-480.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of the sister
chromatid exchange method in mammalian cells as a screening system
for carcinogens. Prog. Mutation Research, 1: 560-569.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980a). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in rats. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980b). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in mice. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Chin, A.E., Carlton, B.D., Chrisp, C.E.,
and Dill, G.S. (1982). Report on the maximum neonatal dose studies
with ethylenethiourea. Contract No. N01-ES8-2151. Report submitted
by Battelle Laboratories, Columbus, Ohio to NIEHS, January 29, 1982.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Pilinskaya, M. (1982) Analysis of the cytogenetic activity of
ethylenethiourea (ETU) in human peripheral lymphoblast culture in
vitro. Dopov Akad Nauk Ukr. RSR. Ser. B: Geol, Khim Biol Nauki,
10: 66-68
Poulsen, L.L., Hyslop, R.M., Ziegler, D.M. (1979). S-Oxygenation of
N-substituted thioureas catalyzed by pig liver microsomal FAD-
containing monooxygenase. Arch. Biochim. Biophys., 198(1): 78-88.
Quillardet, P., de Bellecombe, C. & Hofnung, M. (1985). The SOS
Chromotest, a colorimetric bacterial assay for genotoxins:
validation study with 83 compounds. Mutation Research, 147: 79-95.
Rashid, K.A. & Mumma, R.O. (1986). Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21(4):
319-334.
Resnick, M.A., Mayer, V.W., and Zimmermann, F.K. (1986). The
detection of chemically induced aneuploidy in Saccharomyces
cerevisiae: an assessment of mitotic and meiotic systems.
Mutation Research, 167: 47-60.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 314-322.
Robinson, D.E. & Mitchell, A.D. (1981). Unscheduled DNA synthesis
response of human fibroblasts, WI-38 cells, to 20 coded chemicals.
Prog. Mutation Research, 1: 517-527.
Rosencranz, H.S., Hyman, J. & Leifer, Z. (1981). DNA polymerase
deficient assay. Prog. Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenicity of carcinogens and
noncarcinogens in the Salmonella/microsome test. Prog. Mutation
Research, 1: 323-332.
Ruddick, J.A. & Khera, K.S. (1975). Pattern of anomalies following a
single oral dose of ethylenethiourea to pregnant rats. Teratology,
12: 277-281.
Ruddick, J.A., Williams, D.T., Hierlihy, L. & Khera, K.S. (1976).
C14-ethylenethiourea: distribution, excretion and metabolism in
pregnant rats. Teratology, 13: 35-40.
Ruddick, J.A., Newsome, W.H. & Iverson, F. (1977). A comparison of
the distribution, metabolism and excretion of ethylenethiourea in
the pregnant mouse and rat. Teratology, 16: 159-162.
Saillenfait, A.M., Sabate, J.P., Longonne, I. & De Ceaurriz, J.
(1991). Difference in the developmental toxicity of ethylene
thiourea and three N,N'-substituted thiourea derivatives in rats.
Fundamental and Applied Toxicology, 17: 686-697.
Salamone, M.F. (1981). Mutagenic activity of 41 compounds in the in
vivo micronucleus assay. Prog. Mutation Research, 1: 686-697.
Salisbury, S.A. & Lybarger, J. (1977). Health Hazard Evaluation
Determination Report No. 77-67-499, St. Clair Rubber Co,.,
Marysville, Michigan. Ethylenethiourea. US-DHEW. October 1977.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Savolainen, K. & Pyysalo, H. (1979). Identification of the main
metabolite of ethylenethiourea in mice. J. Agric. Food Chem. 27:
1177-1181.
Saxton, A.D. (1972). A C14-ethylenethiourea rat-feeding study. An
experiment to determine the excretion pattern and accumulation and
decline in thyroid tissues of C14-residues. Report 23-51 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Schiestl, R., Gietz, R.D., Mehta, R. & Hastings, P. (1989).
Carcinogens induce intrachromosomal recombination in yeast.
Carcinogenesis. 10: 1445-1455.
Schmid, H., Tennekes, H., Janiak, T., Probst, D., Leutkemeier, H.,
Pappritz, G., Marki, U., Vogel, O., Heusner, W. (1992).
Ethylenethiourea 104 week chronic toxicity (feeding) study in rats.
Unpublished study No. 256803 from Research and Consulting Company,
Itingen, Switzerland. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Schupbach, M. & Hummler, H. (1977). A comparative study of the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutation Research, 56: 111-120.
Seiler, J.P. (1974). Ethylenethiourea (ETU), a carcinogenic and
mutagenic metabolite of ethylene-bis-dithiocarbamate. Mutation
Research, 2: 189-191.
Seiler, J.P. (1975). In vivo mutagenic interaction of nitrite and
ethylenethiourea. Experientia, 31: 214-215.
Seiler, J.P. (1977a). Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short term test. Mutation Research, 46: 305-
310.
Seiler, J. (1977b). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutation
Research, 48: 225-236.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1. Prog.
Mutation Research, 1: 491-501.
Sharp, D.C. and Parry, J.M. (1981b). Use of repair-deficient strains
of yeast to assay the activity of 40 coded compounds. Prog.
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., Kato, K., Lienard, F., Tezuka, H.,
Teramoto, S. & Kada,T. (1977). Mutagenicity screening on pesticides
and modification products: a basis of carcinogenicity evaluation.
Cold Spring Harbor Conference Cell Proliferation, 4: 267-285.
Simmon, V.F. & Shepherd, G. F. (1981). Mutagenic activity of 42
coded compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 333-342.
Smith, D.M. (1976). Ethylenethiourea - a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.
26: 92-94.
Smith, D.M. (1984). Ethylenethiourea: thyroid function in two groups
of exposed workers. Brit. J. of Ind. Med. 41: 362-366.
Sram, R.J. (1975). Genetic risk from chemicals: mutagenicity studies
and evaluation. Review of Czechoslovak Medicine, 21(4): 186-193.
Stula, E.F. & Krauss, W.C. (1977). Embryotoxicity in rats and
rabbits from cutaneous application of amide-type solvents and
substituted ureas. Toxicol. Appl. Pharmacol. 41: 35.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Prog. Mutation Research, 1: 638-646.
Teramoto, S., Moriya, M., Kato, K., Tezuka, H., Nakamura, S.,
Shingu, A. & Shirasu, Y. (1977). Mutagenicity testing on
ethylenethiourea. Mutation Research, 56: 121-129.
Teramoto S., Shingu, A., Kaneda, M. & Saito, R. (1978a).
Teratogenicity studies with ethylenethiourea in rats, mice and
hamsters. Congenital Anomalies, 18: 11.
Teramoto, S., Shingu, A. & Shirasu, Y. (1978b). Induction of
dominant lethal mutations after administration of ethylenethiourea
in combination with nitrite or of N-nitro-ethylenethiourea in mice.
Mutation Research, 55: 335-340.
Teshima, R., Nagamatusu, K., Kido, Y. & Terao, T. (1981).
Absorption, distribution, excretion and metabolism of
ethylenethiourea in guinea-pigs. Eisei Kagaku, 27(2): 85-90.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Prog. Mutation Research, 1: 224-235.
Tophan, J.C. (1981). Evaluation of some chemicals by the sperm
morphology assay. Prog. Mutation Research, 1: 718-720.
Trueman, R..W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Prog. Mutation Research, 1:
343-350.
Truhaut, R., Fujita, M., Lich, N. & Chaigueau, M. (1973). Study of
metabolic transformations of zineb. C.R. Acad. Sci. Paris, Ser. D.
276: 229-233.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Prog. Mutation Research, 1: 705-711.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains WP2, WP67
(uvrA polA), and CM871 (uvA lexA recA). Prog. Mutation Research,
1: 199-209.
Ulland, B.M., Weisburgr, J.H., Weisburger, E.K., Rice, J.M. &
Cypher, R. (1972). Thyroid cancer in rats from ethylenethiourea
intake. J. Nat. Cancer Inst. 49: 583-584.
US EPA (1992) Ethylenebisdithiocarbamates (EBDCs); notice of intent
to cancel and conclusion of special review. Federal Register, 57:
7484-7530.
Valencia, R. & Houtchens, K. (1981). Mutagenic activity of 10 coded
compounds in the Drosophila sex-linked recessive lethal test.
Prog. Mutation Research, 1: 651-659.
Van't Hof, J. & Schairer, L.A. (1982). Tradescantia assay system for
gaseous mutagens. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Res. 99: 303-315.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985).
Chemical mutagenesis testing in drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutagenesis, 7: 677-702.
Wright, W.E., Peters, J.M., Plyler, E.A. & Belanger, P.L. (1981).
NIOSH Health Hazard Evaluation Report HETA 81-270-1012. December,
1981.
Wyrobek, A., Gordon, L. & Watchmaker, G. (1981). Effect of 17
chemical agents including 6 carcinogen/noncarcinogen pairs of sperm
shape abnormalities in mice. Prog. Mutation Research, 1: 712-717.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Prog. Mutation Research, 1: 481-490.
Rats
Male Sprague-Dawley rats were pre-treated with phenobarbital,
dexamethasone, beta-napthoflavone or left untreated. The in vitro
effect of ETU or EU in the presence and absence of glutathione,
NADPH and heat inactivated microsomes on P450 enzymatic activity and
on covalent binding of ETU to microsomal proteins was studied. ETU
inhibited P450 activity in pretreated and non-pretreated rats.
Inhibition was NADPH-dependent and was abolished by glutathione
(GSH). Covalent binding of 14C-ETU to microsomal protein was also
NADPH-dependent. Binding was inhibited by co-incubation with GSH.
Heat treatment of microsomes and P450 inactivation studies indicated
a prominent role of FMO in covalent binding. Addition of GSH or
dithiothreitol after incubation of microsomes resulted in release of
bound ETU. Metabolism of ETU in the presence of GSH resulted in the
formation of GSH-ETU adducts and subsequent disulfide exchange. The
results suggest that reactive metabolites from ETU generated by
either FMO or P450 are trapped by GSH. Initial oxidation of ETU to
imidazoline-2-sulfenic acid, primarily by FMO, followed by reaction
with GSH or protein sulfhydroyls under conditions of GSH depletion,
has been proposed as the route of monooxygenase-mediated metabolism
of ETU (Decker & Doerge, 1991).
Male Sprague-Dawley rats were divided into 3 groups in a study
designed to study the effect of ETU on RNA synthesis. One group
that had been fasted for 16 hours received single i.p. injections of
2.5 or 250 mg/kg bw ETU or 5.0 mg/kg bw thioacetamide, followed by
3H-orotic acid 60 minutes later. Control animals received dimethyl
sulfoxide alone. A second group received 5.0 or 250 mg/kg bw/day
ETU by gavage on 3 successive days followed by administration with
3H-orotic acid; the animals were killed on the fourth day. The
third group was administered 5.0 or 250 ppm ETU in the diet for 3
weeks. At the end of 3 weeks, a 1-hour pulse dose of 3H-orotic
acid was administered and the animals killed. Serum T4 levels in
animals given ETU were then determined by radioimmunoassay. Rats
receiving 400 ppm of acetylaminofluorene in the diet served as
positive controls.
The livers of all animals given either ETU or thioacetamide
were histologically normal at the time of sacrifice. The livers of
rats fed acetylaminofluorene showed mild hydropic change of
hepatocytes and minimal bile duct proliferation. ETU failed to
inhibit nuclear or cytoplasmic RNA synthesis under the test
conditions. However, thioacetamide and acetylaminofluorene both
reduced the incorporation of 3H-orotic acid into nuclear and
cytoplasmic RNA (Austin & Moyer, 1979).
Pigs
FMO purified from hog liver catalyzes NADPH and oxygen-
dependent sequential S-oxidation of ETU, proceeding through an
intermediate imidazolinyl sulfenic acid to the corresponding
sulfinic acid. Further oxidation to the sulfonic acid was partly
enzymic and partly due to autooxidation. The FMO-oxidative pathway
predominated over P-450 pathways in hog and hamster liver microsomes
(Poulsen et al., 1979).
The mechanism of thyroid peroxidase inhibition by ETU was
studied in vitro using purified thyroid peroxidase obtained from
hog thyroid. ETU inhibited iodination reactions catalysed by thyroid
peroxidase. Inhibition occurred only in the presence of iodide ion
and proceeded with concomitant oxidative metabolism of ETU to
imidazoline and bisulfite ion. The inhibition ceased upon
consumption of ETU, with no loss of enzymatic activity and
negligible covalent binding of ETU to the enzyme. This reversible
thyroid peroxidase inhibition contrasts with the activity of the
therapeutic antithyroid drugs such as methimazole which act as
suicide inhibitors via covalent binding to the prosthetic heme group
(Doerge & Takazawa, 1990).
Toxicological studies
Acute toxicity studies
ETU is slightly toxic after oral administration to mammalian
species with measured LD50 values ranging from 545 mg/kg bw in
pregnant rats (Teramoto, 1978) to 4000 mg/kg bw in adult mice
(Lewerenz & Plass, 1984). The acute toxicity of ETU in various
animal species is given in Table 1.
Guinea-pigs
ETU (purity not stated) is a moderate to weak sensitizer in the
Hartley strain female guinea-pig by the guinea-pig maximization
test. With induction concentrations of 5% (intradermal) or 25%
(topical) and challenge concentrations of 2% or 0.5% (topical),
females responded positively at 24 hours (1/10 at 0.5%; 7/10 at 2%)
but not at the 48-hour reading (0/10 at 0.5%; 0/10 at 2%). In the
same studies, cross sensitization responses were also seen with
maneb, mancozeb and zineb after induction with ETU. Responses
ranged from 0-40% (4/10) at 24 hours and from 0-20% (2/10) at 48
hours. Induction with ETU followed by challenge with maneb
generally gave a slightly higher overall response (10-40%) than did
induction by maneb followed by challenge with ETU (0-20%)
(Matsushita et al., 1976).
Table 1. Acute toxicity of ETU
Species Sex Route LD50 (mg/kg bw) References
Mice M&F oral 4000 Lewerenz & Plass, 1984
F oral > 3000 Teramoto et al., 1978b
F (9 days pregnant) oral > 3000 Khera, 1987
M&F oral ca 2400 Peters et al., 1980b
Rats M&F oral ca 2400 Peters et al., 1980a
M oral 1832 Graham & Hansen, 1972
M&F oral 940 Lewerenz & Plass, 1984
F (13 days pregnant) oral 600 Khera, 1987
F oral 545 Teramoto et al., 1987b
Hamsters F oral > 3000 Teramoto et al., 1987b
F (11 days pregnant) oral > 2400 Khera, 1987
Short-term toxicity studies
Mice
B6C3F1 mice (10/sex/dose), 8 to 9 weeks of age were fed
diets containing 0, 125, 250, 500, 1000, or 2000 ppm ETU (97-99%
purity), equivalent to 0, 19, 38, 75, 150 or 300 mg/kg bw/day for 13
weeks. Deaths occurred at lower doses but were not dose- or
compound-related. Body-weight gain and food consumption were
comparable to controls. Diffuse follicular cell hyperplasia of the
thyroid occurred at 500 ppm in both sexes (greater than 70%) and was
statistically significant and dose-related. No effects were observed
at lower doses. Hepatocellular cytomegaly was also observed at 500
ppm (4/10 females, 10/10 males) and above. Effects were
statistically significant and dose-related. The NOAEL in this study
was 250 ppm, equivalent to 38 mg/kg bw/day (NTP, 1992).
Groups of Charles River CD-1 mice (15/sex/dose) were
administered ETU (100% purity) in the diet at levels of 0, 1, 10,
100 or 1000 ppm for 3 months, equal to 0, 0.16, 1.7, 18 or 168 mg/kg
bw/day for males and 0, 0.22, 2.4, 24 or 230 mg/kg bw/day for
females. There were no compound-related effects on food
consumption, body weight, haematology or clinical chemistry
parameters. Mixed function oxidase activity was increased in both
sexes at 1000 ppm, but only statistically significant in males
(aniline hydroxylase, p-nitroanisole, o-demethylase). Absolute and
relative thyroid weights were increased statistically in both sexes
at 1000 ppm. Absolute and relative liver weights were significantly
increased in males at 1000 ppm; relative liver weights were
significantly increased in females at 100 and 1000 ppm ETU.
ETU produced thyroid follicular cell hyperplasia and decreased
colloid density in both sexes at > 100 ppm, with increased
follicular epithelial cytoplasmic vacuolation and interstitial
congestion in both sexes at 1000 ppm. In the liver, ETU produced
centrilobular hypertrophy, nuclear pleomorphism and increased
intranuclear inclusions in both sexes at 1000 ppm. The pigment was
believed to be similar to lipofuscin. The NOAEL was 10 ppm ETU,
equal to 1.7 and 2.4 mg/kg bw/day in males and females, respectively
(O'Hara & DiDonato, 1985).
Rats
Adult male Han:Wistar rats (6/dose group) were given ETU (>
98% pure) in drinking-water for 28 days. Drinking-water
concentrations of ETU were 0, 100, 200 or 300 mg/litre, equal to
mean daily doses of 0, 11, 18 or 23 mg/kg bw.
ETU decreased body-weight gain during the exposure. Studies of
kidney function and morphology indicated that the kidney is not a
highly sensitive target for ETU-induced toxicity. ETU did not have
a permanent physiologically significant effect on urinary sodium,
potassium, uric acid, protein or glucose excretion, or urinary
osmolality. A slight increase in urinary arginine vasopressin (AVP)
excretion was observed in ETU-treated animals on day 28. No
prominent light microscopical changes were observed in the kidneys
of ETU-exposed rats. However, at 300 mg/litre ETU induced clear
ultrastructural changes in the epithelium of renal proximal tubuli.
An increased number of lysosomes and myelin figures as well as
vacuolization and edema were observed in the cytoplasm of the
epithelial cells of proximal tubules. The proportion of the dose of
ETU excreted as ETU in urine increased with increasing dose of ETU
and were 25%, 36% and 49% (Kurrtio et al., 1991).
Using an identical protocol as above, a study was conducted to
determine the effect of ETU on thyroid gland function and
morphology. Drinking-water concentrations of ETU were 0, 100, 200
or 300 mg/litre, equal to mean daily doses of 0, 11, 18 or 23 mg/kg
bw. Blood samples for T3, T4 and TSH were taken and the levels
measured using radio immunoassay methods. Thyroid glands were
extirpated and processed for light and electron microscopy. ETU
statistically significantly decreased T4 levels at all doses while
statistically increasing TSH levels at all doses. T3 levels were
also decreased in a dose response manner but values were not
statistically significant. There were no ETU-induced morphological
changes observed under light microscopy. Conspicuous ultra
structural changes were caused by ETU since a few areas with totally
destroyed epithelial cells could be found. It was also reported that
nerves and capillaries might have been affected by ETU (Kurrtio et
al., 1986).
Sprague-Dawley rats (10/sex/group) received 0, 0.63, 1.3, 2.5,
5.0, or 25 ppm of 98% pure ETU in the diet for 8 weeks. Twenty-four
hours after the last feeding, all animals received 5 µCi of 131I
intraperitoneally. There were no treatment-related effects on
behaviour, appearance, food intake, organ or body weight or
macroscopic appearance of organs other than the thyroid. ETU had no
clinical chemistry effects at the three low doses. However at 5 and
25 ppm slight increases were observed in males and females with
respect to 131I uptake, protein bound 131I and serum thyroxine. T3
uptake power was slightly decreased. Histopathology of the thyroid
in treated animals was comparable to control group at all dose
levels. The NOAEL in this study was 25 ppm, the highest dose
tested, equal to 2.6 mg/kg bw/day (Leuschner, 1977).
F344/N rats 8 to 9 weeks of age (10/sex/group), were fed diets
containing 0, 60, 125, 250, 500 or 750 ppm of 99% pure ETU for 13
weeks. All animals survived. Final mean body weights for males were
decreased at 500 and 750 ppm by 10% and 30%, respectively. Food
consumption at the same dose levels were decreased 16% and 24%.
Final body weights and food consumption of females were decreased at
750 ppm by 30% and 25%, respectively. Females receiving 60 to 500
ppm showed a uniform 10% body weight decrease accompanied by food
consumption decreases of 13% at 250 and 500 ppm. Histopathology was
present for the thyroid and pituitary gland of both males and
females. Diffuse follicular cell hyperplasia of the thyroid was
present in all animals of both sexes at all doses. In males, focal
follicular cell hyperplasia and cellular vacuolization of the pars
distallis of the pituitary gland was statistically significantly
increased at 250 ppm. Follicular cell adenomas (3/10) were evident
at 250 ppm and statistically significant at 750 ppm. Centrilobular
cytomegaly was observed only at 750 ppm and was statistically
significant. In females, follicular cell hyperplasia (4/10) and
cellular vacuolization of the pars distallis of the pituitary gland
(10/10) were statistically significant only at 750 ppm. Follicular
cell adenomas were observed at 500 and 750 ppm (3/10) but were not
statistically significant. Centrilobular cytomegaly of the liver
was seen only at the high dose and in all animals. The NOAEL in
this study was less than 60 ppm, equal to 3.0 mg/kg bw/day for males
and 4.3 mg/kg bw/day for females, based on histopathological
findings of diffuse follicular cell hyperplasia in the thyroid (NTP,
1992).
In a 90-day study, Sprague-Dawley derived rats (60/sex/dose)
were fed ETU (96.8% pure) at 1, 5, 25, 125 or 625 ppm. Controls
(72/sex) received powdered diet with 1% corn oil. At 30-day
intervals (i.e. 30, 60, 90 days) ten rats from each test group were
sacrificed and serum T3, T4, TBG and TSH concentrations were
measured. The free thyroxine index (FTI) was also calculated. The
remaining rats (10/sex/dose/time) were used to determine 125I uptake
by the thyroid. Rats receiving 625 ppm ETU showed high mortality
and marked decrease in body-weight gain. Clinical signs were
observed at the high dose by day 8 and consisted of excessive,
salivation, loss of hair, rough and bristly hair coat and scaly skin
texture. Necropsy revealed hyperaemia of the thyroids with and
without enlargement at 125 and 625 ppm for all time intervals.
Liver congestion was also evident with dose and time. Liver changes
were distinguishable microscopically and appeared to be compound-
related but not dose-related. Thyroid to brain weight ratio was
significantly increased at 125 and 625 ppm at all time periods.
125I uptake in the thyroid was statistically significantly decreased
along with TBG, T3 and T4 values at 125 and 625 ppm. At 25 ppm,
T4 was statistically significantly decreased only at 60 days. FTI
was comparable to controls. Altered thyroid function and increased
thyroid follicular cell hyperplasia were evident at 125 and 625 ppm.
The NOAEL was 25 ppm, equal to 1.7 and 1.9 mg/kg bw/day in males and
females, respectively (Freudenthal et al., 1977).
Osborne-Mendel rats (20 males/group) were fed ETU (purity not
stated) in the diet at levels of 0, 50, 100, 500 or 750 ppm for 30,
60, 90 or 120 days. 131I activity was determined at 4 and 24 hours
post-injection (5 µCi) in 20 rats from each group at each sacrifice
period.
Body weight was decreased at > 500 ppm throughout the study.
Food consumption was reduced at 30 and 90 days at > 100 ppm and
at 60 and 120 days at > 500 ppm. Relative thyroid weights were
increased at 30 days at > 100 ppm, at 90 days at 500 ppm and at
60/120 days at > 50 ppm.
Four hours after the injection of 131I, the uptake had
decreased significantly in rats fed ETU at 500 and 750 ppm at all
feeding periods. The uptake of iodine 24 hours after injection was
decreased significantly in those animals fed ETU at 100, 500 and 750
ppm. After the 90-day feeding period, the uptake decreased
significantly in rats fed the 500 and 750 ppm levels and ranged from
6 to 13 times lower than control values.
Histologically there were no differences between the control
and 50 ppm groups. At 100 ppm there was slight hyperplasia evident
in the thyroid gland. At 500 ppm there was moderate to marked
hyperplasia, lack of colloid and heightened epithelial walls. There
was an increase in vascularization, demonstrating a response to
increased blood level TSH. At > 500 ppm, an increased incidence
of follicular adenomas was reported. One mechanism by which ETU
acts on the thyroid is via inhibition of iodide peroxidase, which
oxidizes iodide to iodine (Graham & Hansen, 1972).
Dogs
Beagle dogs (2/sex/group) received dietary concentrations of 0,
200, 980, or 4900 ppm of ETU (98% pure) for 4 weeks. Body-weight
gains and food consumption for males were comparable to controls.
Intermediate- and high-dose females gained less weight than the
controls particularly at the high dose. Haematology results were
not remarkable. T3 levels were decreased in high-dose males and
females as well as mid-dose females. T4 levels were decreased in
the mid- and high-dose males and females. Reductions were dose-
related. Enlarged thyroids were noted in all animals of the
intermediate- and high-dose groups. The NOAEL was 200 ppm, equal to
6.7-7.4 mg/kg bw/day for males and 7.4-8.5 mg/kg bw/day for females
(Morgan, 1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
10, 150 or 2000 ppm of ETU (98% pure with doses corrected to 100%
active ingredient) for 13 weeks. Two males in the high dose were
sacrificed in a moribund state with morbidity attributed to compound
administration. All other animals survived to termination.
Clinical signs in the high-dose male survivors appeared to be
unremarkable. All high-dose females showed decreased activity or
subdued behaviour for various lengths of time (1-5 weeks). A
bilobed swelling in the pharyngeal area of two females was also
reported. No treatment-related clinical signs were observed in the
low or intermediate dose groups. Body-weight changes for survivors
in treated groups were not statistically significantly different
when compared to the control group. However, a slight to severe
body-weight loss for animals killed moribund was noted. Food
consumption was statistically significantly decreased only at the
high dose for surviving males during weeks 12 and 13, and for
females during weeks 11 and 12. Ophthalmological examinations
showed no remarkable differences between treated and control groups.
At 13 weeks, males and females of the 150 and 200 ppm groups
showed statistically significant decreases in haemoglobin, packed
cell, volume and red blood cell count. Reticulocyte count was
statistically significantly increased in females, but not in males.
Values for sodium, potassium, and chloride and BUN were all within
normal limits. Phosphorous was decreased in males and females at 13
weeks in the high dose. The value was statistically significant in
males. A statistically significant increase in serum protein was
associated with an increase in serum globulin in the high-dose males
at 13 weeks. A statistically significant increase in total
cholesterol was observed in the intermediate-dose (150 ppm) males at
weeks 8 and 13 and at weeks 4, 8, and 13 in high-dose (2000 ppm)
males and females. An increase in the mean creatinine level was
noted at weeks 8 and 13 in the high-dose males and females with
statistical significance attained for males at both time periods.
ALP was statistically significantly decreased in high-dose males at
weeks 8 and 13. At week 4, there was a statistically significant
decrease in mean ASAT in males at the intermediate and high dose and
in females at all three doses. A dose response was evident in
females. However values at 4 and 8 weeks were comparable to
controls for all groups of both sexes. ALAT was statistically
significantly decreased at week 4 only in females. Results from
urinalysis were not remarkable. Urine colour was however described
as orange or dark-coloured. Thyroid hormone assays revealed no
treatment-related changes in the low- and intermediate-dose groups.
However, marked and statistically significant reductions were noted
for T3 and T4 levels in high-dose animals at weeks 8 and 13. T4
was also statistically significantly decreased in males at 4 weeks
in the high-dose group.
At week 13, females of the high-dose group showed a marked and
statistically significant increase in thyroid weights accompanied by
slight but statistically significant increases in liver and adrenal
weights. Males of the high-dose group at 13 weeks showed a marked
increase in thyroid weights concurrent with slight increase in liver
and adrenal weights. None of these organ weights were statistically
significantly increased for males. Macroscopic examinations revealed
exophthalmia in two males (the survivors) and three females of the
high-dose group. Sporadic and slight exophthalmia was also observed
in one male of the intermediate dose group. Enlargement of the
thyroid gland was noted in all surviving high-dose animals as well
as the two males sacrificed moribund. The liver and adrenal gland
both appeared unremarkable as did the remaining tissues examined
macroscopically. Salient microscopic findings were those of
hypertrophy of the basophilic cells of the pituitary with micro-
vacuolisation attended by severe follicular hyperplasia of the
thyroid gland in all surviving and sacrificed animals of the high-
dose group. The liver and adrenal gland were histologically normal.
A moderate involution of the thymus of one male and two females of
the high-dose group was reported. No treatment-related microscopic
changes were noted for the low dose (10 ppm) or the intermediate
dose (150 ppm). The salient observations related to this study are
those of the pituitary and thyroid glands of animals receiving the
highest dose of 2000 ppm (equal to a mean of 66 mg/kg bw/day for
males and 72 mg/kg bw/day for females). In the pituitary, the lesion
observed was a hypertrophy of a basophilic cell type with
microvacuolisation, while in the thyroid, the lesion observed was a
hyperplasia of the follicular cells with papillary projections of
the follicular epithelium in the lumen of the follicles. Similar
hyperplasia was observed in ectopic nodule of thyroid tissues,
scattered along the thyroglossal track. The NOAEL was 10 ppm, equal
to 0.39 mg/kg bw/day based on decreased haemoglobin, packed cell
volume and red blood cell count, and increased cholesterol at 150
ppm. Effects on the thyroid were found only at 2000 ppm (Briffaux,
1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
5, 50 or 500 ppm ETU (expressed as active ingredient taking into
account the purity index of 98% purity) for 52 weeks. Mortality was
evidenced in the high-dose group with the death of one male and the
sacrifice of one male and one female prior to study termination. No
treatment-related clinical signs were reported in either the low- or
mid-dose groups. Pale mucous membranes in four males and one female
of the high-dose group was associated with subdued behaviour and a
change in the colour of the faeces (yellow/orange). Body weight in
surviving animals at 52 weeks was decreased 15% in both males and
females at the high-dose and 8% in the mid-dose males. A dose-
related decrease was observed in body-weight gain for males of the
mid- (-43%) and high-dose (-60%) and females of the high-dose (-
60%). However, the decreases were not statistically significant.
Body-weight gain and body weights in the low-dose group were
comparable to control group. There were no statistically
significant differences in food consumption between treated and
control groups at 52 weeks. Food efficiency was generally
comparable between groups. Ophthalmological examinations revealed
comparable findings between all groups.
Haematological values between control groups and the low- and
mid-dose groups were comparable. However, in the high-dose group,
treatment-related low values (75-80% of normal) in haemoglobin, RBC,
packed cell volume were reported for all animals dying or sacrificed
moribund as well as one surviving male. Additionally the decrease
in RBC was accompanied by an increased reticulocyte count, a
decrease in mean corpuscular haemoglobin and an increase in mean
corpuscular volume. Low values in platelet count were also observed
in high-dose animals. Changes in blood clinical chemistry values
for sodium, potassium and blood urea nitrogen, cholesterol,
triglycerides, bilirubin creatinine, gamma glutamyl-transpeptidase
in surviving animals was not considered treatment-related. However,
a slight to moderate increase for total bilirubin was observed for
animals dying or sacrificed early in the high dose group. Values for
globulin were statistically significantly higher at weeks 13 and 52
for the high-dose animals (males and females combined). A decrease
in the albumin/globulin ratio was also statistically significant at
week 52 for the high-dose animals (males and females combined).
Elevated values for ASAT and ALAT were reported for both high-dose
males found dead or sacrificed moribund in the high-dose group.
Urinalysis values were unremarkable between groups.
Thyroid hormone mean values for T4 and T3 were not
statistically significantly different from control group. However,
T3 and T4 values taken shortly prior to death or sacrifice of the
three high-dose group animals revealed a mean decrease of 50% for
T3 values and 70% for T4 values. The values for decedent animals
were also below the historical range. Of the two surviving males in
the high-dose group at 52 weeks, one showed a 47% reduction of T3
from its pretest level while the other was comparable to its pretest
level. T4 values for both high-dose male survivors were generally
decreased 55% from pretest values. T3 and T4 values appeared to
be unaffected in the low-dose and mid-dose groups. A dose-related
increase in thyroid weights were observed at week 52 for the
intermediate and high-dose males and females. The increase was
statistically significant for combined males and females for
absolute, body weight and brain weight ratio (except for brain
weight ratio in the intermediate dose group). Necropsy revealed an
enlargement of the thyroid in one of the two surviving males.
High-dose animals dying on study or killed in a moribund
condition all manifested centrolobular hepatocellular necrosis of
the liver (multifocal and moderately severe in males and multifocal
and minimal in the female). Slight pigment accumulation was also
evident in Kupffer cells. Hypertrophy of follicular cells with
dilation of follicles was also seen in the thyroid of one high-dose
male that was sacrificed. Pigment accumulations in Kupffer's cells
and occasionally hepatocytes were observed in both males and females
of the intermediate and high-dose groups. Hypertrophy of the
thyroid with colloid retention was observed in the intermediate and
high-dose group and ranged in severity from slight to moderately
severe. The NOAEL was 5 ppm, equal to 0.18 mg/kg bw/day based on
reduction in body-weight gain, hypertrophy of the thyroid with
colloid retention, a slight increase in thyroid weight and pigment
accumulation in the liver at 50 ppm (Briffaux, 1992).
Monkeys
Wild-caught rhesus monkeys (5/sex/group) were administered ETU
(96.8-98.2 purity) in the diet for 5.5 or 6 months at dose levels of
0, 2, 10, 50, or 250, and 0, 50, 150 or 450 ppm, respectively.
Results of Study 1: Body weights were not affected by ETU.
Thyroid weight was increased in both sexes at 250 ppm and in females
at > 50 ppm, resulting from hyperplasia and/or hypertrophy.
Females at > 50 ppm also had enlarged pituitary glands. Ovarian
weights at 250 ppm were significantly decreased.
No changes in T3 or TBG were observed. Serum T4 was
decreased in both sexes at > 50 ppm identified from FTI analyses.
Serum TSH was increased at 250 ppm. 125I uptake also increased at
> 50 ppm in both sexes.
Lesions reportedly associated with ETU were identified in the
pituitary and thyroid gland of animals at > 50 ppm. These
included thyroid and pituitary hypertrophy, and thyroid follicular
cell hyperplasia (moderate to severe). A second study was conducted
due to the extent of tuberculosis in this first study which
necessitated the early termination at 5-5.5 months.
Results of Study 2: Body weights were not affected by ETU.
Thyroid and spleen weights were increased in males at > 150 ppm
and at all doses in females. Serum T3 decreased in males at
> 150 pm and in females at 450 ppm. Serum T4 was decreased in
both sexes at > 150 ppm. Radioactive 125I uptake was increased
in all test groups. The increased thyroid weight, thyroid iodine
uptake, decrease in T3, T4 and increase in TSH support the
evidence for hypothyroidism caused by ETU.
BUN was elevated in females at 450 ppm along with creatinine
and a decrease in calcium. Haemoglobin, haematocrit and RBC count
were decreased in both sexes at 450 ppm.
Histologic changes were identified in thyroid and pituitary
glands in both sexes, increasing in severity and incidence with
increase in dose. Thyroid follicular cell hyperplasia and pituitary
cytoplasmic vacuolation and swelling were the major changes
observed.
The NOAEL for 125I uptake was 10 ppm; the NOAEL for changes in
T3, T4 and TSH was 50 ppm. In a separate pathological
examination, 10 ppm was considered to produce compound-related
changes in the thyroid gland in 1/7 monkeys. The NOAEL was
considered by the authors to be 2 ppm in these combined studies
(Leber et al., 1978b).
However, the monkey studies were considered unreliable because
one was compromised by ill health of the animals, while little
reliance could be placed on the effects at the lowest dose used in
the second.
Long-term toxicity/carcinogenicity studies
Mice
Groups of B6C3F1 mice received perinatal (F0), adult (F1)
or both exposures to ETU at the following dietary concentrations:
(F0:F1), 0:0, 0:330, 0:1000, 33-100, 110-330, 330-0, 330-330 or
330-1000 ppm. Female C57BL/6N mice were exposed to 0, 33, 110 or
330 ppm of 99% pure of ETU in feed for one week before breeding, and
naturally inseminated by C3H/HeN males that received control feed
only. ETU exposure continued throughout pregnancy and lactation.
Weaning occurred on day 28 post-partum and dietary exposure at these
same (maternal) concentrations continued until pups were 8 weeks of
age. On post-partum day 7, litters were culled to a maximum of 8
pups, separated by sex after weaning and litter mates co-housed. At
8 weeks of age, pups were separated into groups of 60 males and 60
females to receive dietary concentrations of 0, 330 or 1000 ppm for
2 years. Groups of 34 male and 29 female mice that were fed 33 ppm
of ETU before weaning received 100 ppm for up to 2 years.
At 9 months, liver weights were increased in groups receiving
adult exposure concentrations of 330 or 1000 ppm regardless of
perinatal exposure. Increases were statistically significant with
the exception of the 0:330 group.
Thyroid weights were also reportedly increased in animals given
1000 ppm. T4 levels were statistically significantly decreased in
all animals receiving adult concentrations of 330 or 1000 ppm. TSH
levels were statistically significantly increased in males only at
330:330 and 330:1000 ppm. Follicular cell vacuolization of the
thyroid occurred in all animals receiving ETU except those only
receiving perinatal exposure (i.e. 330:0 ppm). Animals receiving a
dose of 33:100 ppm were not reported. Hyperplasia was comparable
between all groups.
Hepatocellular adenomas were present in animals receiving 1000
ppm but were not statistically significant. Centrilobular
cytomegaly was statistically significantly increased in both males
and females receiving 1000 ppm and in males receiving 110:330 and
330:330 ppm. Eosinophilic focus was statistically significantly
increased in females receiving 1000 ppm. At 2 years, there was no
survival disparity between treated and control (0:0 ppm) groups.
Clinical signs were not treatment-related. Body weights of treated
animals were statistically significantly decreased in both sexes
compared to 0:0 ppm controls, with the exception of the 330:0 ppm
dose group which was similar to controls. Statistically significant
increases in the number of animals with adenomas or carcinomas were
observed for hepatocytes, thyroid follicles and posterior pituitary
in both sexes of high-dose treated, adult only exposed groups when
compared to 0:0 ppm controls. At the next lower dose level of 0:330
ppm both sexes showed a statistically significant increase in
hepatocellular adenomas or carcinomas.
Hyperplasia of the thyroid was evident in high-dose males and
females and in 0:330 ppm females. Centrilobular cytomegaly was also
evident in males and females at both dose levels of adult only
treated animals. A comparison of animal groups receiving 0:330
versus 110:330 versus 330:330 ppm showed statistically significant
increases of thyroid follicular cell hyperplasia (males) and thyroid
adenomas (females) in perinatal treated groups at 330 ppm. Similar
comparisons for hepatocellular neoplasms and pituitary neoplasms
revealed no statistical differences. Comparison between animals
receiving 1000 ppm, with or without perinatal exposure to 330 ppm
showed no statistical differences for tumours or hyperplasia of the
thyroid or pituitary. Treatment of adult females receiving 330 ppm
with 330 ppm perinatally increased the number of tumours in the pars
distalis when compared to 0:0 ppm controls as well as those of the
thyroid. Animals receiving only a perinatal dose showed a
comparable response when measured against 0:0 ppm controls. T4
levels for both sexes were statistically significantly decreased in
both sexes at all dose levels. TSH levels were statistically
significantly increased in animals receiving 1000 ppm (i.e. adult
and perinatal/adult groups). Animals receiving 330 ppm during
adulthood showed elevated TSH levels which were statistically
significant only in females. Animals receiving perinatal exposure
only showed TSH values comparable to controls (0:0 ppm) (Chhabra et
al., 1992; NTP, 1992).
Rats
Charles River rats (60/sex/group) were fed ETU (purity not
stated) in the diet at levels of 0, 5, 25, 125, 250 or 500 ppm for 2
years. Body weights in both sexes were significantly decreased
initially at doses > 25 ppm; at 500 ppm and above (males) and 125
ppm and above (females) at 12 months; and at 500 ppm and above (both
sexes) for the remainder of the study.
Liver to body-weight ratios were significantly increased at 125
ppm and through 6 months in males, but comparable to controls for
the remainder of the study. Relative liver weights in females were
significantly increased at doses > 125 ppm at 2 months and at
doses > 250 ppm through 18 months. No differences between
control and dose groups were observed at 24 months. Thyroid to
body-weight ratio was significantly increased in males at 250 ppm
and above at 2, 6 and 18 months, and at 125 ppm and above in females
for the first 12 months. Thyroid weights were significantly
increased at 125 ppm and above in males at 12 and 24 months, and at
250 ppm and above in females at 18 and 24 months.
Uptake of 131I, expressed as counts/min/mg tissue, was
significantly decreased in males at 500 ppm throughout the study.
Thyroids of females fed 125 ppm and above were hypofunctioning at 6
months and hyperfunctioning at 12 months. At 24 months, females had
a hypofunctioning thyroid at 500 ppm.
Fewer rats survived to 24 months in the 500 ppm dose group and
there was also a significant increase in pneumonia which may have
been further complicated by obstruction of the trachea from enlarged
thyroids in the animals. Effects in the thyroid were evident at all
doses. Increased vacuolarity and hyperplasia in the thyroid were
evident at 25 ppm and above. Thyroids of treated rats were
distinguishable from controls by lobulation, follicular size and
uniformity, height of follicular epithelium, colloid staining,
keratinization of follicles, and general size.
It is possible that ETU initially reduces thyroid activity,
after which compensation occurs by an increased release of TSH and
that this increase in TSH stimulated thyroid weight in an attempt to
overcome the blocking effect of ETU. The progression to neoplasia
is believed to be a result of excessive pharmacological stimulation.
This is supported, in part, by a lack of thyroid tumours at 1 year
at 5 or 25 ppm, an increase in tumour incidence after 1 year at 125
ppm, and confirmed after 2 years in rats fed 250 and 500 ppm. The
NOAEL in this study was 5 ppm, equivalent to 0.25 mg/kg bw/day
(Graham et al., 1973, 1975).
SPF-Sprague-Dawley (30/sex/dose) received 0, 0.5, 2.5, 5 or 125
ppm of 96% pure ETU (adjusted to 100%) in feed, 7 days a week for
either 52 weeks (interim sacrifice of pre-selected animals
10/sex/dose) or 104 weeks (terminal sacrifice).
There were no compound-related deaths, clinical signs or
effects on food consumption. Body-weight gain was slightly impaired
in males at 125 ppm resulting in group mean body weights 5-6% lower
than in control males for most of the study. Female body weight was
unaffected. There were no treatment-related changes with respect to
ophthalmoscopic observations or palpable masses. Haematologic and
urinalysis finding were comparable to controls. There were no
treatment-related changes on clinical biochemistry values in males
receiving 0.5 or 2.5 ppm nor in females receiving 0.5, 2.5 or 5 ppm
of ETU. Statistically significant increases were observed in males
at 125 ppm for total protein, albumin, GGT, cholesterol, bilirubin,
TSH and T3. T4 and urea values were lower. T3 values were higher
at 5 ppm at 29 weeks. For females at 125 ppm, values for glucose
and T4 were lower, uric acid, T3 and TSH were higher. At 125 ppm
only thyroid weight was higher in males and females at the interim
and final sacrifice. Liver weight was slightly higher in both sexes
but only at the interim sacrifice. Macroscopic effects were not
observed at 52 weeks. However, at 104 weeks the incidence of
diffuse or modular enlargement of the thyroid gland was increased in
both sexes at 125 ppm.
Microscopic examination of the thyroid gland at interim
sacrifice revealed minimal to moderate diffuse follicular cell
hyperplasia in 8 animals of the control group and 9, 12, 12 and 20
animals (both sexes combined) receiving 0.5, 2.5, 5 or 125 ppm of
ETU, respectively. The incidence of this finding was significantly
increased in females at 125 ppm. The severity was increased in males
at 5 and 125 ppm and in females at 125 ppm. Slight or moderate
nodular hyperplasia and follicular adenoma were recorded in 6 and 3
males of the 125 ppm, respectively. Minimal or slight focal or
multifocal cellular hypertrophy of the anterior pituitary was
recorded in 2 control rats, 2 rats at 2.5 ppm and 7 rats at 125 ppm.
Males were predominantly affected. A significant increase in the
incidence and severity was calculated for males at 125 ppm. Other
morphological alterations observed in treated groups were considered
secondary to treatment, and affected spleen, thymus gland, auditory
sebaceous (Zymbal's) glands and lungs.
At terminal sacrifice, slight to excessive diffuse follicular
hyperplasia was recorded in 27 animals (sexes combined) receiving
125 ppm. The incidence and severity for males and females was
statistically significant for both sexes. Slight to severe nodular
hyperplasia was observed in 9 animals (sexes combined) receiving 125
ppm. The incidence and severity of this lesion was significantly
increased in males. Follicular adenomas occurred in 1 male of the
control group and in 4 males given 125 ppm. Follicular carcinomas
were observed in 2 males of the high-dose group. The combined
incidence of benign and malignant follicular neoplasms yielded a
clear dose-related trend but did not vary significantly in pairwise
comparison with the control group. Anterior pituitary gland
adenomas were recorded in 8 males and 11 females of the control
group and 15 males and 10 females at 125 ppm. There was a clear
dose-related trend for males and a marginal level of significance by
pairwise comparison with the control group. Adenomas of the
anterior pituitary recorded at the intermediate doses for males and
females were 5, 8, and 6 and 11, 12, and 11 respectively. Other
morphological alterations observed in treated groups were considered
secondary to treatment and affected the pancreas, lungs and Zymbal's
glands. The NOAEL was 5 ppm (equal to 0.37 mg/kg bw/day) based on
changes in clinical chemistry, increased T3, decreased T4,
increased thyroid and liver weights and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm
(Schmid et al., 1992).
Charles River CD rats (26/sex/group) were fed 0, 175 or 350 ppm
of technical grade ETU (97% pure) for 2 years. Follicular or
papillary carcinomas of the thyroid were observed in 17 males and 8
females at the high-dose. At 175 ppm, equivalent to 8.8 mg/kg
bw/day, 3 males and 3 females had thyroid carcinomas. Hyperplastic
goitre was observed in 17 males and 13 females of the high-dose
group and 9 males and 6 females of the low-dose group. These
lesions were not observed in control rats (Ulland et al., 1972).
Groups of F344/N rats received perinatal exposure (F0), adult
exposure (F1) or both to different concentrations (ppm) of ETU as
follows: F0, F1; 0,0; 0,83; 0,250; 9,25; 30,83; 90,0; 90,83; or
90,250. Female rats were exposed to 0, 9, 30 or 90 ppm of 99% pure
ETU in feed for 1 week before breeding. All males received control
feed. All females were naturally inseminated by males, housed
individually and continued on their previous diet. ETU exposure
continued throughout pregnancy and lactation. Weaning occurred on
day 28 post partum and dietary exposure at these same concentrations
continued until the pups were 8 weeks of age. On post partum day 4,
litters were culled to a maximum of 8 pups. Pups were separated by
sex after weaning and litter mates co-housed (5/cage). At 8 weeks
of age, pups were separated into groups of 60 males and 60 females
to receive adult dietary concentrations of 0, 25, 83 or 250 ppm for
up to 2 years.
At 9 months, liver weights were statistically significantly
increased in males receiving 0,250 or 90,250 ppm of ETU. Thyroid
follicular cell hyperplasia was greater than 50% and statistically
significantly increased for both males and females at the following
dose levels: 0,83; 0,250; 30,83; 90,83; and 90,250 ppm. Thyroid
follicular cell adenomas were observed in both males (3/10) and
females (1/10) receiving 90,250 ppm. Values were not statistically
significant. T4 values compared to 0,0 ppm controls were
statistically significantly decreased in all experimental groups of
both sexes except animals receiving 90,0 ppm. T3 values were
statistically decreased in many but not all groups. The 90,0 ppm
groups was unaffected. TSH levels were increased in all dose groups
and statistically significant only in some female groups. The 90,0
ppm groups was only very slightly increased.
At two years there were no differences in food consumption
between treated groups and controls with the exception of a decrease
in the 90,250 ppm group of males during the last month of exposure.
Final mean body weights for males and females were comparable to 0,0
control group with the exception of the 90,250 ppm male dose group
where the decrease was statistically significant. Only those
animals receiving 90,250 ppm showed a statistically significant
decrease in survival. Thyroid function values for animals receiving
90,0 or 0,83 or 9,25 ppm were not statistically different compared
to controls for males and females at 2 years. All other doses
revealed some level of statistical significance in both sexes.
There were no clinical findings that could be attributed to thyroid
dysfunction. Pathology of the thyroid for animals receiving adult
only exposures of 0,0; 0,83 or 0,250 ppm revealed statistically
significant trends and statistically significant increases in high-
dose males and females for hyperplasia, adenomas, carcinomas and
adenomas and carcinomas combined. Animals receiving 0,83 ppm of ETU
showed statistical increases in hyperplasia (males and females) and
adenomas (males). Hyperplasia of the thyroid was the only
statistically significant effect observed in both sexes when 0,0 and
90,0 ppm comparisons were made. Responses between dose groups
receiving 0,250 or 90,250 ppm revealed a statistically significant
increase in the number of adenomas in males and carcinomas in both
males and females. A comparison of the 0,83; 30,83 and 90,83 dose
groups in females showed no statistical differences in hyperplasia,
adenomas, or carcinomas, or adenomas and carcinomas combined. In
males only hyperplasia was statistically significantly increased.
ETU had no clear effects on the incidences of neoplasms or non-
neoplastic lesions at sites other than the thyroid gland. However,
some groups showed statistically significant increases relative to
controls in neoplasms of the Zymbal's gland (males and females at
90,250 ppm) and, mononuclear cell leukaemia (males and females at
90,250 ppm and males at 90,83 ppm (Chhabra et al., 1992; NTP,
1992).
Rats and Hamsters
Groups of 20 male and 20 female rats and hamsters were
administered ETU (purity not stated) in the diet for 24 and 20
months, respectively, at dose levels of 0, 5, 17, 60 or 200 ppm
(strain of animals not reported).
In rats, food consumption was reduced at 60 ppm and above and
body weight decreased at 17 ppm and above. Effects on SAP and SGPT
were not clearly demonstrated due to fluctuations in control levels.
Cholesterol was increased at 5 ppm in both sexes. Some hepatic
enzyme levels were also affected: GPT increased in males at 60 ppm;
ALP increased at 5 ppm (females) and 17 ppm (males); glucose-6-
phosphate dehydrogenase did not change. Thyroid weights were
significantly increased in both sexes at 60 ppm. No data were
available on the histologic examination.
In hamsters, food consumption and body weight were reduced at
60 ppm and above. SAP was increased in both sexes initially, then
decreased through 18 months. No effect was observed on SGPT.
Cholesterol levels were significantly increased in both sexes at all
doses compared to controls. Hepatic enzymes, GPT and ALP, were
significantly increased in both sexes at all doses. Glucose-6-
phosphate dehydrogenase was significantly decreased in both sexes at
all dose levels. Relative thyroid weights were significantly
increased at 200 ppm and above in both sexes. No data were
available on the histologic examination (Gak et al., 1976).
Reproduction studies
Rats
ETU (98% pure) was mixed in the diet and fed to Sprague-Dawley
rats (25 male and female parents per group) at concentrations of 0,
2.5, 25 or 125 ppm during a 70-day pre-pairing period and throughout
pairing, gestation and lactation of 2 generations (one litter per
generation). Body weights and mean body-weight gains were reduced
among the male parents of the F0 generation at 125 ppm. There were
otherwise no changes in the viability, clinical appearance or
behaviour, feed consumption, body weights or weight gain or
macroscopic appearance of any of the parents, F1 or F2 pups in any
of the test groups. Reproduction parameters were unaffected in any
of the dose groups in either generation. Histopathologic
examination indicated compound-related changes in the thyroid and
anterior pituitary glands at 25 and particularly at 125 ppm in both
generations. Thyroid changes in both sexes of both generations
consisted of follicular cell hypertrophy and hyperplasia which were
pronounced at 125 ppm and present to a much lesser extent at 25 ppm.
Reduced colloid was also present among F1 males and females at 125
ppm. Adenomas were observed in 3 males (not statistically
significant). Pituitary changes consisted of an increased incidence
and severity of anterior cell hypertrophy in both sexes of both
generations at 125 ppm, together with a tendency to an increase in
hypertrophy among parental generation males at 25 ppm and a slight
increase in cellular vacuolization at 125 ppm. There was no
evidence of reproductive organ toxicity up to and including 125 ppm.
The NOAEL was 2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day,
based on thyroid gland follicular cell hyperplasia and hypertrophy
at 25 ppm (Dott, 1992).
Rats/mice
In the first phase of a two-phase study, adult female rats and
mice were dosed with ETU (96.7% purity) and then bred to proven male
sires. Pregnant females delivered their pups via C-section for
tissue distribution analyses. Phase 2 consisted of weanling
rats/mice dosed for 9 weeks and then analyzed. Dose levels in the
diet were: rats: 0, 8, 25, 83 or 250 ppm; mice: 0, 33, 100, 333 or
1000 ppm (rats: Fischer 344, 3 per group; mice: C57BL/6N, 78 per
group). Two weeks after dosing began, breeding was initiated.
No rat dams or weanlings died. There was a trend toward
decreased weight gain in dams in all groups and in weanling males at
levels > 83 ppm. Food consumption was also reduced at 250 ppm
for males only. No effects on females were observed. At 250 ppm,
there was a decrease in pup survival to postnatal day 4. Thyroid
hyperplasia was observed in males at all doses and in females above
8 ppm, increasing in incidence and severity with dose. Thyroid
adenomas were reported in males at 83 ppm and above. Vacuolization
of pituitary glands in males was noted at 250 ppm.
There was a significant decrease in body weight in high-dose
female mice during the period of lactation. Weanling body weights
were decreased in males and females at 333 ppm and 1000 ppm.
Initially, insufficient pregnancies were produced in all dose
groups. A rebreeding programme, after 6.5 weeks on ETU diets,
produced sufficient numbers of litters for evaluation. However, no
pregnancies were achieved in the high-dose group, and pregnancy rate
was reduced in other dose groups in comparison to control. The
number of pups surviving to day 28 was significantly decreased in
the high-dose group. Thyroid hyperplasia and cellular alteration of
hepatocytes (cytomegaly, karyomegaly) were observed in both sexes at
1000 ppm. One male mouse at 333 ppm also had adverse effects in the
liver (Peters et al., 1982).
Special studies on embryotoxicity/teratogenicity
Rats
Pregnant Charles River Rats (ChR-CD, Sprague-Dawley) were
administered 0, 25 or 50 mg/kg bw/day of ETU (98% pure) in DMSO, or
DMSO alone (vehicle control) or water alone onto the shaved back of
each animal for 48 hours on days 10 and 11 or days 12 and 13 of
gestation.
Maternal body-weight change during the 48-hour administration
period ranged from +4 to -5%. Fetuses examined from dams
administered ETU at 50 mg/kg bw/day on days 10 and 11, showed short
tails (3/83) and fused ribs (2/83). However, dams given 50 mg/kg
bw/day on days 12 and 13 produced fetal deformities in all
offspring. Fetal defects were characterized by encephalocele, a
part or the entire tail missing, missing leg bones, hunchback
curvature to the spine, short mandible, fusion of ribs and fusion of
sternebrae.
A dose of 25 mg/kg bw/day administered only on days 10 and 11
of gestation did not result in any fetal abnormalities. A dose of 25
mg/kg bw/day was not administered on days 12 and 13 of gestation
(Stula & Krauss, 1977).
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rats
(Wistar) (10-17 pregnant dams per dose). Treatment was made for 21-
42 days before conception to pregnancy day 15, and on days 6-15 or
6-20 of pregnancy. Doses of 40 mg/kg bw/day were not toxic to rats;
however, 80 mg/kg bw/day was lethal to 9 of 11 female rats. Mean
fetal weight was reduced at 40 mg/kg bw/day. Measurements of
sterility, pre-implantation loss and post-implantation survival were
comparable to controls. The brain was the most commonly affected
organ. ETU induced meningoencephalocele, meningorrhagia,
meningorrhea, hydrocephalus, obliterated neural canal, abnormal
pelvic limb posture with equinovarus, and short or kinky tail at 10
mg/kg bw/day in all phases of the study. Although no abnormalities
were reported in rats at 5 mg/kg bw/day, there was a higher
frequency of delayed ossification of the parietal bone, compared to
controls. The NOAEL for embryo/fetotoxicity was 5 mg/kg bw/day
based on teratogenic effects observed at 10 mg/kg bw/day. The NOAEL
for maternal toxicity was 40 mg/kg bw/day (Khera, 1973).
ETU was given by gavage (distilled water, 5 ml/kg bw/day) on
days 6-20 of gestation to pregnant Sprague-Dawley rats (22/group) at
doses of 0, 15, 25, or 35 mg/kg bw/day, and dams were sacrificed on
day 21 for examination of uterine contents. Maternal appearance,
behaviour, and body-weight gain were generally unaffected, and the
incidence of pregnancy was comparable among the groups. No adverse
effect was noted on the average numbers of implantations, live
fetuses, or percentages of resorption sites per litter. Mean fetal
body weights were decreased in a dose-related manner, but were only
significantly reduced at 35 mg/kg bw/day (13-15% lower). ETU at 35
mg/kg bw/day produced external malformations including cranial
meningocele and meningorrhea, severe hindlimb talipes, and a non-
significant incidence of hydrocephaly. Short and/or kinky tails
were noted in 43.5% of the fetuses. Soft tissue examinations
revealed higher incidences of dilated brain ventricles at 25 and 35
mg/kg bw/day (33.5 and 93% of the fetuses, respectively) and of
hydroureter and dilated ureter at 35 mg/kg bw/day, and skeletal
examinations revealed a reduced ossification of skull bones and a
significantly increased incidence of dumbbell-shaped or bilobed
vertebral centra (33.5% of fetuses). There were no other treatment-
related increases in skeletal variants among any of the experimental
groups, and no treatment-related effects of any kind identified in
the 15 mg/kg bw/day group. The NOAEL for maternal toxicity was 35
mg/kg bw/day. The NOAEL for embryo/toxicity and teratogenicity was
15 mg/kg bw/day based on higher incidences of dilated brain
ventricles at 25 mg/kg bw/day (Saillenfait et al., 1991).
ETU (100% purity) was administered via oral gavage at 40 mg/kg
bw/day from days 7 to 15 of gestation to pregnant CR rats (10-12
rats/group). Rats were hypothyroid and euthyroid. There was a
problem, however, in maintaining the euthyroid state in rats given
supplement. Rats were also given thyroxine to determine if ETU
teratogenicity occurred through alterations of maternal thyroid
function. ETU was found to be teratogenic in the rat but not
through alteration of maternal thyroid status. It was also
demonstrated that ETU lowered serum T4; that hypothyroidism per se
increased the background level of malformations in the rat; that T4
alone was embryotoxic but not teratogenic; and that hypothyroidism
altered the spectrum of malformations in response to ETU both
quantitatively and qualitatively (Lu & Staples, 1978).
Virgin Sprague-Dawley rats were mated one-to-two with males
and, after pregnancy was verified, were administered ETU (unknown
purity), T3/T4 and sodium iodide via oral gavage in varying
concentrations, either singly or in combination, as well as a
control solution of water only, from day 7 to day 20 of gestation.
Dosing regimen was as follows:
Dose group Total rats per
group
Control 1 ml distilled water 14
T3 20 µg/kg bw/day + T4 100 µg/kg bw/day 10
Sodium iodide 333 µg/kg bw/day 10
ETU 20 mg/kg bw/day 10
ETU 20 mg/kg bw/day + sodium iodide 16
ETU 20 mg/kg bw/day + T3/T4 16
ETU 40 mg/kg bw/day 11
ETU 40 mg/kg bw/day + sodium iodide 14
ETU 40 mg/kg bw/day + T3/T4 15
Each pregnant dam was killed on day 20 by chloroform
asphyxiation and the fetuses removed via hysterotomy. The number of
resorptions, live/dead fetuses and fetal birth weights were
determined. Skeletal analyses were performed on 1/3 and visceral
analyses on 2/3 of the fetuses. Results indicated a possible
reduction in the teratogenic response to ETU for some malformations
when T3/T4 was administered in conjunction with ETU. For example,
20 and 40 mg/kg bw/day ETU (alone) produced 97.6 and 94.5% incidence
of hydrocephaly, respectively. In combination with T3/T4 these
same levels produced 19.6 and 74.5% incidence, respectively. These
results indicate that the teratogenic potential of ETU may in part
be secondary to the thyroid toxicity of ETU (Emmerling, 1978b).
Rats, mice and hamsters
Wistar-Imamichi rats, JCL-ICR mice, and Syrian golden hamsters
10 weeks or older were mated overnight and examined the next morning
for the presence of a vaginal plug or spermatozoa in vaginal smears.
Evidence of copulation was designated as day zero of gestation.
Pregnant females were given daily oral doses of ETU by gavage during
the period of organogenesis. Doses given to rats, mice and hamsters
were, respectively 0, 10, 20, 30, 40 or 50 mg/kg bw/day, 0, 200, 400
or 800 mg/kg bw/day and 0, 90, 270, or 810 mg/kg bw/day. Rats,
mice, and hamsters were sacrificed on days 20, 18, and 14,
respectively.
Dams did not show signs of toxicity and none died in any
species. There were no statistically significant differences between
treated and control rats for the mean number of implants and live
fetuses reported. Mean fetal weight for both males and females was
statistically significantly decreased at 30 mg/kg bw/day and higher.
The percent of fetal death was also statistically significantly
increased at 50 mg/kg bw/day. Mice showed no statistically
significant changes between treated and control values for any of
the prenatal developmental parameters (i.e mean number of implants,
mean number of live fetuses, percent fetal death and mean fetal body
weight). The mean number of implants for hamsters were comparable
to control values for all doses. There was a statistically
significant decrease in the mean number of live fetuses born
attendant to a decrease in mean fetal body weight for males and
females which was statistically significant at 810 mg/kg bw/day.
Mean fetal body weight for females was also statistically
significantly lower at 270 mg/kg bw/day without other prenatal
developmental effects. Gross external anomalies were not meaningful
between controls and treated groups for mice. Rats manifested a
short or kinky tail at 30 mg/kg and above in 80% of the offspring.
In rats, meningocele was observed in 66% of the offspring at 40
mg/kg bw/day and above and micrognathia in 30% of the offspring at
50 mg/kg bw/day. Hamsters showed multiple type anomalies at 810
mg/kg bw/day and included such signs as cleft palate, short or kinky
tail, and oligodactyly. Short or kinky tail was observed in 2% of
the animals at 270 mg/kg bw/day and 42% of the animals at 810 mg/kg
bw/day. The LOAEL in rats was 30 mg/kg and seen as curved clavicles.
There was an increased incidence of curved clavicles, fused/wavy
ribs, fused sternebrae, malformed vertebrae and scoliosis at 40 and
50 mg/kg bw/day. The LOAEL was 90 mg/kg bw/day in hamsters based on
a 2% incidence of malformed lumbar and sacral vertebrae, a 4%
incidence at 270 mg/kg bw/day and a 51% incidence at 810 mg/kg.
There were no brain or visceral anomalies observed in mice.
Dilation of the lateral 4th ventricle was observed in 5% of hamsters
at the high dose, none at lower doses and 2% in controls. Dilation
of the lateral or 4th ventricle in rats was observed in 2% and 39%
of rats at doses of 10 and 20 mg/ kg bw/day. At 30 mg/kg bw/day and
above the response was 100%. No maternal toxicity occurred at the
doses tested. The NOAEL for embryo/ fetotoxicity in the rat was 10
mg/kg bw/day based on dilation of the lateral or fourth ventricle at
20 mg/kg bw/day. The NOAEL for embryo/fetotoxicity in the hamster
was 90 mg/kg bw/day based on decrease of fetal body weight at 270
mg/kg bw/day. The NOAEL for mice was greater than 80 mg/kg bw/day
(Teramoto et al., 1978).
Hamsters
ETU (purity > 99%) was administered orally to pregnant Syrian
hamsters at doses of 600, 1200, 1800 or 2400 mg/kg bw on day 11 of
gestation. All dams were killed on day 15 of gestation for necropsy
and fetal examination. There were only 5 pregnant dams in the
control group compared to 8-10 in the treated groups.
Maternal toxicity was not reported at any dose. However, there
was an increased incidence of resorbed fetuses and fetuses dying
late in gestation with an associated decrease in the number of live
fetuses at 2400 mg/kg bw. Fetal body weights were similarly reduced
at 1800 mg/kg bw. Malformations were evident at > 1200 mg/kg bw,
with no adverse effect reported at 600 mg/kg bw. Fetal anomalies
included cleft palates ectrodactyly, hydrocephalus and hypoplastic
cerebellum. There was also an increased incidence of delayed
ossification of the calcarium and sternebrae defects. As with other
species (i.e. rat, cat), the brain was particularly sensitive to
ETU, although at higher dose levels (Khera et al., 1983).
Mice/rats/hamsters/guinea-pigs
Time-pregnant random-bred CD-1 mice, Sprague-Dawley rats golden
hamsters and Hartley strain guinea-pigs were used. Maneb (80%
pure), ETU (melting point 197-198 °C) and EBIS were administered by
gastric intubation. Control animals received vehicle alone (water
or corn oil) or remained untreated. Prenatal studies were conducted
on rats given maneb (480, 240, 120, 0 mg/kg bw/day for days 7-16) or
ETU (80, 40, 30, 20, 10, 5, 0 mg/kg bw/day for days 7-21) or EBIS
(30, 25, 7.5, 0 mg/kg/ bw/day for days 7-21). Prenatal studies were
also conducted on mice given maneb (1500, 750, 375, 0 mg/kg bw/day
for days 7-16) or ETU (200, 100, 0 mg/kg bw/day for days 7-16) or
EBIS (200, 100, 50, 0 mg/kg bw/day for days 7-16). Prenatal studies
were also conducted with hamsters and guinea-pigs but only with ETU.
Hamsters received either 100, 50, 25 or 0 mg/kg of ETU on days 5-10
of gestation. Guinea-pigs received 100, 50 or 0 mg/kg bw/day of ETU
on days 7-25. Post-natal studies were only conducted on rats. Rats
received either maneb (480, 240, 0 mg/kg bw/day) or ETU (30, 25, 20,
0 mg/kg bw/day) or EBIS (30, 15, 0 mg/kg bw/day) on days 7-15 of
gestation. Mice were killed on day 18, rats on day 21, hamsters on
day 15 and guinea-pigs on day 35. Animals designated for post-natal
study were allowed to litter normally. Litters were normalized to 4
individuals of each sex and weaned on day 22 post-partum.
In toxicity studies conducted to determine dose levels, all
compounds tested proved to be more toxic in the rat than the mouse.
Hind limb paralysis was observed in rats given maneb at the high
dose of 600 mg/kg bw/day. No toxicity was noted in mice given a
dose of 1500 mg/kg bw/day of maneb. EBIS produced death in rats at
75 mg/kg bw/day and hind limb paralysis at 50 mg/kg bw/day.
Decreased body-weight gain and death was observed in mice given 100
mg/kg bw/day. ETU produced lethality in rats at the high dose of 80
mg/kg bw/day. ETU was not toxic in mice (300 mg/kg bw/day) hamsters
(150 mg/kg bw/day) or guinea-pigs (100 mg/kg bw/day) at the high
dose tested.
Maneb administered to pregnant rats resulted in significant
dose-related decrease in maternal weight gain and increase in liver
to body-weight ratios. Fetal weight and caudal ossification were
significantly reduced only at the highest dose tested (480 mg/kg
bw/day). At the high dose (480 mg/kg bw/day), 18 fetuses from 4
litters manifested hydrocephalus. Maternal mice given maneb
manifested increased liver/body-weight ratios and decreased caudal
ossification beginning at 375 mg/kg. Dose-related trends were
evident. EBIS given to rats and mice did not result in adverse
fetal effects. Average maternal weight gain was decreased in rats
at 30 mg/kg bw/day. Maternal weight gain in mice was not affected.
Average liver to body-weight ratio was increased at the high dose
tested for rats (30 mg/kg bw/day) and mice (200 mg/kg bw/day). ETU
administered to rats at the high dose of 80 mg/kg bw/day resulted in
25% maternal mortality and reduced weight gain. Fetal toxicity was
also observed at 80 mg/kg bw/day and included mortality, decreased
weight, decreased ossification and edema. Gross defects of the
skeletal system and central nervous system were noted in a majority
of fetuses. At 40 mg/kg bw/day, fetal weight and ossification were
reduced and hydrocephalus and encephalocele were evident.
Hydrocephalus was seen at 20 mg/kg bw/day and decreased fetal body
weight at 10 mg/kg bw/day. ETU produced an increase in the liver-
body-weight ratio of mice at 100 mg/kg bw/day and 200 mg/kg bw/day
and an increase in the number of supernumerary ribs at 200 mg/kg
bw/day. No apparent effects were observed in hamsters or guinea-
pigs. Post-natal studies with maneb resulted in delayed eye opening
in males. Post-natal observations with EBIS resulted in decreased
fetal body weight at day 22 in females only as well as delayed eye
opening. ETU administration produced no observable post-natal
effects with the exception of increased total open field activity in
males. There were no apparent differences reported in open field
activity between male fetuses surviving the high dose (30 mg/kg
bw/day) with hydrocephalus and their apparently normal mates.
Hydrocephaly was not observed at lower doses (Chernoff et al.,
1979).
Rabbits
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rabbits
(New Zeeland white). There were 5-7 pregnant does per group.
Treatment was made from days 7 to 20 of pregnancy. No toxicity was
apparent in rabbits given 80 mg/kg bw/day. Fetal weights were not
affected. Measurements of sterility, pre-implantation loss and post-
implantation survival were comparable to controls. Rabbits presented
no evidence of malformations at the doses administered. However,
there was an increase in resorption sites, decreased brain weight,
and degeneration of the proximal convoluted tubules in the kidneys
of fetuses at 80 mg/kg bw/day. The NOAEL for maternal toxicity was
80 mg/kg bw/day and for embryo/fetotoxicity the NOAEL was 40 mg/kg
bw/day (Khera, 1973).
Cats
ETU (purity not stated) was administered orally (in gelatin
capsules) to pregnant European and Persian breed cats (7-14 cats per
group) at doses of 0, 5, 10, 30 or 60 mg/kg bw/day on days 16-35 of
gestation or 120 mg/kg bw/day from days 16 to 34 of gestation. No
effect was evident at 5 mg/kg bw/day. However, at > 10 mg/kg
bw/day decreased ataxia, tremors and hindlimb paralysis were
observed. No pregnant cats survived in the 30 and 60 mg/kg bw/day
dose groups. The remaining cats showed no apparent treatment-related
effect on fetal viability or fetal weight. Although this study was
inconclusive in many respects, there was an increased incidence of
toxicity to the central nervous system at 10 mg/kg bw/day. Further,
at 5 and 120 mg/kg bw/day, there were anomalous fetuses in each
group. Incidences of exencephaly, hydrocephaly, cleft palate,
kyphoscoliosis, umbilical hernia, coloboma, and spina bifida were
observed in these two treated groups. Similar anomalies were
observed in the rat (Khera & Iverson, 1978).
Special studies on genotoxicity
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induced mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic. The results of genotoxicity assays on ETU are given in
Table 2.
Table 2. Results of genotoxicity assays on ethylenethiourea
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 10-20 000 µg/plate; ? Positive Teramoto et al.,
reversion TA1535, TA1536, in DMSO? (TA1535 without 1977; Shirasu
assay TA1537, TA1538, G46 activation) et al., 1977
S. typhimurium 0.2-2000 µg/plate >98% Negative Brooks & Dean, 1981
TA1535, TA1537,
TA1538, TA92, TA98,
TA100
S. typhimurium 5-5000 µg/plate; >98% Negative Richold & Jones,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 0.1-2000 µg/plate; >98% Negative Rowland & Severn,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 10-5000 µg/plate; >98% Positive Simmon & Shepherd,
TA1535, TA1537, in DMSO (TA1535 with 1981
TA1538, TA98, TA100 & without
activation)
S. typhimurium 4-2500 µg/plate >98% Negative Trueman, 1981
TA1535, TA1537,
TA1538, TA98, TA100
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Salmonella S. typhimurium 1000-20 000 µg/plate; no ? Positive Autio et al., 1982
reversion TA1950 activation used; in DMSO
assay (cont'd)
S. typhimurium >500 µg/plate; ? Positive Moriya et al., 1983
TA1535, TA1537, in DMSO? (TA1535 with
TA1538, TA98, TA100 and without
activation)
S. typhimurium 100-10 000 µg/plate; 98.4% Positive Mortelmans et al.,
TA1535, TA1537, TA98, in DMSO (TA1535 with 1986 (SRI)
TA100 & without
activation)
Salmonella S. typhimurium 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; TA1535, TA1537, TA98 in dimethylacetamide
fluctuation test
Salmonella S. typhimurium 200-80 000 ? Positive Schupbach & Hummler,
forward TA1530 µg/plate 1977
mutation assay
E. coli E. coli WP2 hcr 10-10 000 µg/plate ? Negative Teramoto et al.,
reversion assay 1977; Shirasu
et al., 1977
E. coli 343/113/uvrB 200-4000 µg/ml; >98% Positive Mohn et al., 1981
in phosphate buffer (galR- & arg+
systems with
activation)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
E. coli E. coli WP2 uvrA 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; in dimethylacetamide
fluctuation test
Host mediated S. typhimurium 500-6000 mg/kg; ? Negative Schupbach & Hummler,
assay Swiss G46 in DMSO 1977
albino mouse
Swiss albino S. typhimurium 670-6000 mg/kg; ? Weak Schupbach & Hummler,
mouse TA1530 in DMSO Positive 1977
Male JCL-SD rat S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
G46 1977; Shirasu
et al., 1977
Male JCL-ICR S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
mouse G46 1977; Shirasu
et al., 1977
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Mouse lymphoma 140-3000 µg/ml; >98% Negative Jotz & Mitchell,
mutation assay L5178Y TK+/- in DMSO 1981
Mouse lymphoma 25-3600 µg/ml; NTP Positive McGregor et al.,
L5178Y TK +/- in DMSO chemical 1988
repository
Chinese hamster ovary 1000-2000 µg/ml; >98% Negative Carver et al., 1981
(CHO-AT3-2; several loci) in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 0.25-2.5%; ? Negative Mollet, 1975
recessive lethal in sugar water
assay
D. melanogaster 250 ppm; in DMSO >98% Negative Valencia &
Houtchens, 1981
D. melanogaster 4900 ppm injection; 97% Inconclusive Woodruff et al.,
12 500 ppm feed; in water 1985 Mason et al.,
1992
D. melanogaster 5100 ppm 98.4% Inconclusive Mason et al., 1992
1.D. Yeast and Other Fungal Assays
Forward mutation S. pombe 0.1-1 µg/ml; >98% Negative Loprieno, 1981
in DMSO
A. nidulans 0.22-116 mM; >98% Negative Crebelli et al.,
no activation used; 1986
in DMSO
Reverse mutation S. cerevisiae XV185-14C 88.9-889 µg/ml; >98% Equivocal Mehta & von Borstel,
in DMSO 1981
1.E. Plant Test
Mutation Tradescantia 9.79 X 10-5 M; ? Positive van't Hof &
clone 4430 in DMSO? Schairer, 1982
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations in Mammalian Cells
In vitro Chinese hamster cell line 1000-3200 µg/ml ? Negative Teramoto et al.,
chromosomal (Don) 1977; Shirasu
aberrations et al., 1977
Chinese hamster ovary 1670-5000 µg/ml; >98% Positive Natarajan & van
(CHO) cells in DMSO (with and Kesteren-van
without Leeuwen, 1981
activation)
Chinese hamster ovary 6000-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
2.B. In Vivo Chromosomal Alterations
Bone marrow Male & female Wistar rat 50-400 mg/kg; ? Negative Teramoto et al.,
cytogenetics in aqueous soln 1977; Shirasu
et al., 1977
Micronucleus assay Female ICR mouse 150-450 mg/kg; ? Negative Seiler, 1975
in DMSO
Male & female Swiss albino 700-6000 mg/kg; ? Negative Schupbach & Hummler,
mouse in gummi arabicum 1977
Male ICR mouse 220-880 mg/kg; >98% Negative Kirkhart, 1981
in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Micronucleus assay B6C3F1 mouse 880-1416 mg/kg; >98% Negative Salamone et al.,
(cont'd) in DMSO 1981
Male & female CD-1 mouse 220-880 mg/kg; >98% Negative Tsuchimoto & Matter,
in DMSO 1981
Dominant lethal Swiss albino mouse 500-3500 mg/kg; ? Negative Schupbach & Hummler,
assay in gummi arabicum 1977
JCL-ICR mouse 300-600 mg/kg; ? Negative Teramoto et al.,
in water with gum arabic 1977; Shirasu
et al., 1977
C3H/HeCr mouse 150 mg/kg; ? Negative Teramoto et al.,
in gum arabic soln 1978
Reciprocal D. melanogaster 500 ppm; >98% Negative NTP, 1992
translocations in 5% sucrose soln
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
Rec assay B. subtilis H17, M45 20-4000 µg/disk ? Negative Teramoto et al.,
1977; Shirasu
et al., 1977
B. subtilis H17, M45 spores 2000 µg/disk; >98% Positive Kada, 1981
in DMSO (without
activation)
E. coli WP2, WP67, CM871 Not specified >98% Negative Green, 1981
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Rec assay (cont'd) E. coli WP2, WP67, CM871 Not specified >98% Negative Tweats, 1981
E. coli several deficient 500 µg/ml; in DMSO >98% Positive Ichinotsubo et al.,
strains (with activation) 1981
S. typhimurium TA1538, 125-2000 µg/disk; ? Negative Rashid & Mumma, 1986
TA1978; in DMSO
E. coli K12 & WP2
Inhibition of DNA E. coli polA 2273 µg/ml >98% Positive Rosenkranz et al.,
polymerase I (without 1981
activation)
Lambda prophage E. coli (lysogenic) 2-20 mg/ml; only activation >98% Positive Thomson, 1981
induction used; in DMSO
E. coli E. coli PQ37 Not specified; in DMSO ? Negative Quillardet et al.,
SOS Chromotest 1985
In vitro Human fibroblasts from Not specified; in DMSO >98% Negative Agrelo & Amos, 1981
unscheduled DNA skin biopsies
synthesis (UDS)
HeLa S3 human cells Not specified; in DMSO >98% Inconclusive Martin & McDermid,
1981
WI-38 human fibroblasts 63-2000 µg/ml; in DMSO >98% Negative Robinson & Mitchell,
1981
In vitro unschuled Rat hepatocytes 9.6 X 10-9 - 3.2 X 10-3 M ? Negative Althaus et al.,
DNA synthesis (nuclei isolation) 1982
(UDS)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
In vivo/in vitro Female B6C3F1 mouse 1500 mg/kg; in corn oil 98% Negative (UDS); Frank & Muller, 1988
unscheduled DNA hepatocytes Positive (S-
synthesis (UDS)/S phase increase)
phase analysis
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 25-1000 µg/ml; >98% Negative Evans & Mitchell,
SCE assays (CHO) cells in DMSO 1981
Chinese hamster ovary 1670-5000 µg/ml; >98% Negative Natarajan & van
(CHO) cells in DMSO Kesteren-van
Leeuwan, 1981
Chinese hamster ovary 0.01-100 µg/ml >98% Negative Perry & Thomson,
(CHO) cells 1981
Chinese hamster ovary 500-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
In vivo SCE assays Male CBA/J mouse bone 1000 mg/kg; in DMSO >98% Negative Paika et al., 1981
marrow and liver
3.C. Yeast and other Fungal Assays
Mitotic aneuploidy S. cerevisiae D6 500 µg/ml; in DMSO >98% Positive Parry & Sharp, 1981
Chromosome A. nidulans 19.6-78.3 mM; no activation >98% Positive Crebelli et al.,
malsegregation used; in DMSO 1986
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Mitotic gene S. cerevisiae D4 33-333.33 µg/plate; >98% Negative Jagannath et al.,
conversion in DMSO 1981
S. cerevisiae JD1 50 µg/ml; in DMSO >98% Positive Sharp & Parry, 1981a
(without
activation)
S. cerevisiae D7 2000-4000 µg/ml >98% Negative Zimmermann & Scheel,
1981
Mitotic S. cerevisiae T1, T2 1000 µg/ml; in DMSO >98% Negative Kassinova et al.,
crossing-over 1981
A. nidulans 19.6-78.3 mM; no activation >98% Negative Crebelli et al.,
used; in DMSO 1986
Intrachromosomal S. cerevisiae RS112 5-40 mg/ml; no activation used ? Positive Schiestl et al.,
recombination 1989
Differential S. cerevisiae, T5 Not specified; >98% Negative Kassinova et al.,
killing in DMSO 1981
S. cerevisiae 197/2d, rad 300-1000 µg/ml; >98% Positive Sharp & Parry, 1981b
in DMSO (with and without
activation)
3.D. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 100-1000 µg/ml; 99.8% Negative McGlynn-Kreft &
in DMSO McCarthy, 1984
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Cell transformation Syrian hamster embryo 62-1000 µg/ml ? Negative Casto, 1975, 1976
(SHE) cells/adenovirus SA7 in: Heidelberger
et al., 1983
Syrian hamster embryo 1-24 mM ? Inconclusive Hatch et al., 1986
(SHE) cells/adenovirus SA7
Cell transformation C3H/10T 1/2 cells 33 µg/ml; in DMSO 99.8% Negative McLeod & Doolittle,
with "promotion" 1985
3.E. Germ Cell Effects
Spermhead (CBA X BALB/c) F1 mouse 250-2000 mg/kg; >98% Negative Topham, 1981
abnormalities in Tween 80
B6C3F1/CRL mouse 166-2655 mg/kg; >98% Negative Wyrobek et al., 1981
in DMSO
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report
Special studies on the thyroid
Rats
Groups of four randomly selected weanling Caesarian-delivered
Sprague-Dawley male litter-mate rats were administered 0, 75 or 150
ppm ETU (purity not stated). Within each treatment group dosing
periods and control diet periods were varied to examine the
reversibility of compound-related effects. Results suggest some
reversibility of thyroid effects which were related to time on test
and to the severity of effect on the thyroid (Arnold et al.,
1982).
Sprague-Dawley rats (50/sex/group) were fed diets containing 0,
75, 100 or 150 ppm ETU (purity not stated) mixed in corn oil for 7
weeks. Body weights decreased with increasing dose while thyroid
weights (absolute and relative) increased in both sexes. T3 levels
were somewhat variable, while T4 levels were significantly
decreased at 150 pm in both sexes. These effects partially reversed
after 4 weeks on control diets. Histopathological findings included
reduced colloid content of thyroid acini in high dose rats. Acinar
epithelial cell size and height were not different from control.
Two tumours were identified in the high dose male group: a
follicular cell adenoma and medullary carcinoma. The authors
concluded that the relationship between the duration of exposure to
ETU and the possible reversibility of various thyroid lesions
requires further study (Arnold et al., 1983).
A 22-week study was conducted in Sprague-Dawley rats
(55/sex/group) with the following dosing schedule: ETU (97% purity)
administered alone in the diet at levels of 0, 125, 250 or 625 ppm;
or with 0.2 g T3 and 1.6 g T4/rat, orally via gavage; or with
manganese and zinc. Also included were treatment groups dosed with
0, 650 or 1250 ppm mancozeb alone.
Rats receiving 625 ppm ETU alone or in combination with
manganese and zinc were removed from test diet because of alopecia,
weight loss, dermatosis and mortality. Survivors received control
diets for the remainder of the study. Serum decreased in both sexes
at all doses of ETU after 2 weeks of treatment. These levels
returned to normal when ETU was removed from the diet. Serum T3
decreased in both sexes at 625 ppm ETU after 4 weeks of dosing, and
in males at 125 and 250 ppm ETU, but by week 8 returned to normal.
In females at the same doses, T3 was normal until week 16 when it
decreased. The additions of T3/T4 by oral gavage resulted in
decreased T3 at week 8 in males and a decrease during the first 6
weeks in females at all levels. T3 returned to normal one month
after removal of ETU. TSH increased in the ETU group and less
dramatically in ETU plus T3/T4 groups. These levels returned to
normal 2 weeks after ETU was removed form the diet.
Body weights decreased in males and females after 4 weeks at
625 ppm ETU and in males after 8 weeks at 250 ppm ETU. Thyroid to
body-weight ratio increased at > 125 ppm ETU in males. When ETU
was removed from the diet, weights returned to normal. No effect
was observed on pituitary weights. Thyroid hyperplasia was
increased at 125 ppm ETU and above and reversed to normal 6-8 weeks
after ETU was removed. Approximately 1% (13/1300) of the rats
developed hyperplasia of the thyroid (focal areas of basophilic
hyperplastic follicles and follicular adenoma). A dose-related
increase in liver weight was observed at 125 ppm ETU and above for
both sexes.
Exposure to ETU resulted in a decrease in thyroid hormone
(T3/T4) levels and increased serum TSH levels in a dose-related
manner. Although TSH levels were reduced when ETU was supplemented
with T3/T4, the high dosage of ETU was apparently sufficient to
override these effects. The hormone imbalance induced by ETU
correlated with the histologic changes in the thyroid. Withdrawal
of ETU from the diet reversed the hypothyroid conditions induced to
euthyroid (Leber et al. 1978a).
In two 90-day feeding trials Sprague-Dawley rats (12/sex/group)
were given 75 or 100 ppm ETU, and thyroid function, serum T4, T3,
and TSH, T3 uptake in vitro, 131I uptake, and thyroid to body-
weight ratios were measured at days 46 and 91. Additionally, the
fate of the incorporated 131I was traced in thyroid fractions at the
100 ppm level. Groups of both sexes at the lower feeding level and
the females at 100 ppm were functionally euthyroid whereas males at
100 ppm were somewhat hypothyroid despite elevated serum T3, TSH,
and T3/T4 ratios. Results showed that the inhibitory effects of
ETU are similar to those for methimazole. ETU inhibited
monoiodotyrosine (MIT) utilization, and the coupling of
diiodotyrosine (DIT) residues to form T4, resulting in
significantly reduced active synthesis of T3 and T4 prohormones
(males 100 ppm). The capacity of serum to bind T3 was reduced;
however, there was no evidence for inhibition by ETU of T4 to T3
monodeiodination or interference with the normal feedback mechanisms
of thyroid hormones on TSH secretion (O'Neil & Marshall, 1984).
Observations in humans
A 53-year old female employed in the manufacture of products
from synthetic and natural rubber developed itching of the fingers.
Her condition improved on weekends but became worse upon returning
to work. Two months later the eruption spread to her forearms. The
individual showed positive reactions to nickel and cobalt in the
ICDRG standard patch test as well as to a component of the rubber
material from her work which was ETU. Additional patch testing with
ETU and chemically related substances showed that ETU tested
positive at dose levels at or above 0.01% (w/v). One-percent
concentrations of dibutyl-, diethyl- or diphenylthiourea all were
negative as were ethyleneurea and ethylenediaminine. Thiourea at
0.01% (w/v) was also negative. The fungicides zineb and maneb tested
negative and positive, respectively. The positive reaction to maneb
was attributed to the presence of ETU as an impurity/degradation
product and confirmed in studies using thin layer chromatography
(Bruze & Fregert, 1983).
A retrospective study of women who were employed at a rubber
manufacturer using ETU was undertaken to determine any excess of
fetal abnormalities occurring in children to women who had worked
with ETU during early pregnancy. The women were employed on hand and
machine trimming, hand cutting, coolant hose manufacture, packing
and dispatch. Women were also employed in moulding, cutting blanks
for moulding and tool and die trimming where there was a hazard from
inhalation of dust. Women born in 1918 or later who left employment
between 1963-1971 were surveyed. Of 699 women who left between
1963-1971, 255 gave birth to 420 children. Only 59 of 255 were
working at the plant at the time of early pregnancy and none of
these had abnormal children. Of the total 420 children born 11 had
abnormalities. However, figures for the group showed no excess over
the expected number of fetal abnormalities in the region surveyed.
The study did not demonstrate any risk of teratogenesis. However,
the number of women exposed during early pregnancy was small.
A total of 1929 workers engaged in the production or
manufacture of ETU were surveyed retrospectively for thyroid cancer,
and compared with the thyroid cancer list of the Birmingham
(England) Cancer Registry from 1957-1971. No thyroid cancers
occurred in these workers and the results were considered to be
preliminary (Smith, 1976).
An apparent increased incidence of miscarriages among workers
at a USA rubber products factory was investigated. Of 7 pregnancies
only 2 resulted in spontaneous abortions, one with an identified
medical problem. Before starting work at the rubber factory, the 81
women surveyed had 192 pregnancies with 16% spontaneous abortions.
There was no indication that menstrual problems increased with
duration of employment as a moulder. It was concluded that no
adverse reproductive effects could be attributed to the work
environment - although the small numbers available for study
prevented this possibility from being entirely ruled out (Wright et
al., 1981).
No hazard of clinical thyroid depression existed based on
medical evidence collected on workers exposed to ETU at a rubber
company in Michigan. Fifty-one subjects were evaluated (49 males, 2
females). Environmental sampling results demonstrated that workers
were exposed to trace amounts of ETU as airborne dust or through
direct contact of powdered ETU (Salisbury & Lybarger, 1977).
Clinical examinations and thyroid function tests were carried
out over a period of 3 years in the United Kingdom on 8 workers
involved in the manufacture of ETU and 5 workers involved in mixing
of ETU with rubber. The average length of exposure in workers
associated in the manufacturing process was 10 years with a range of
5-20 years. The exposure period for mixers was 3 years. All
subjects were males with an age range from 26-62 years. Matched
controls were also examined. In the manufacturing plant ETU levels
of 330 µg/m3 were recorded on one personal sampler. Background
levels ranged from 10-240 µg/m3. Levels of ETU recorded on
personal samplers of mixers ranged from 120-160 µg/m3. Mixers but
not process workers had significantly lower levels of T4 in their
blood compared to controls. No effects were found on TSH or thyroid
binding globulin. The authors concluded that there was no evidence
that thyroid function is severely affected by exposure to ETU at the
levels experienced by these workers nor was there any evidence of
any effect. However, the T4 results in the exposed workers were
generally lower than those in the control group with most of the
difference in distribution accounted for by the results from the
mixers (Smith, 1984).
The concentration of ETU in pesticide formulations and ambient
air were measured and exposure to maneb (80% powder) or mancozeb
(Ridomil 56% mancozeb) evaluated during the spraying of potato
fields. The mean tank mix concentrations of maneb and mancozeb were
4.0 or 7.0 g/litre, respectively. Spraying time was 0.5-7.0 hours
(mean 4.0 hours). Each single application was separated by several
weeks. Therefore only acute (single day) exposure was determined in
workers (i.e. mixer/loader/applicator).
The overall range of concentrations of ETU in air were between
0.004 and 3.3 µg/m3 in the breathing zone and 0.006 and 0.8 µg/m3
in the (closed) tractor cabin. The mean calculated concentrations
of active maneb and mancozeb in air were 7 and 20 µg/m3,
respectively. The total inhaled amount of ETU and EBDCs was 5.0 and
126.0 µg/day, respectively corresponding to a dose of 0.07 µg ETU/kg
bw and 1.8 µg EBDC/kg bw for applicators and mixer and loaders
weighing 70 kg. Patch samples on clothes and skin (back, chest,
shoulders and forearm) indicated that 1.4, 10.0, 4.0 and 1.2% of ETU
(0.07; 0.19; 0.39; 0.17 mg/cm2/hour) reached the skin respectively.
ETU in urine sample taken on days 1, 8, 15 and 22 ranged between
0.09-2.5; 0.07-1.0; 0.01-0.30 and < 0.01-0.2 µg/mmol creatinine.
Absolute concentrations of ETU in all samples ranged between < 0.2
and 11.8 µg/litre of urine. Under the exposure conditions the
urinary elimination half-life was calculated to be 100 hours
(Kurrtio et al., 1990).
COMMENTS
Following oral administration to mice essentially all of the
ETU was recovered in the excreta within 48 hours; none was recovered
as carbon dioxide. Approximately 50% of the administered dose was
found in urine as unchanged ETU.
After the oral administration of radiolabelled ETU, its
concentration in both pregnant mice and rats peaked about the same
time (1.4 hours), with concentrations in maternal and fetal tissues
similar at 3 hours. The half-life of elimination from mice and rats
was 5.5 hours and 9.4 hours, respectively. Approximately 70% of ETU
was found in urine in both species at 48 hours.
Mice metabolize ETU primarily by the flavin-monooxygenase
system and rats by the P-450 system of enzymes.
ETU is slightly toxic after acute oral administration, with the
LD50 ranging from 545 mg/kg bw in pregnant rats to 4000 mg/kg bw in
adult mice.
In a 13-week study in mice at dietary concentrations of 0, 125,
250, 500, 1000 or 2000 ppm the NOAEL was 250 ppm (equivalent to 38
mg/kg bw/day). Diffuse follicular cell hyperplasia of the thyroid
and hepatocellular cytomegaly were observed at 500 ppm.
In a three-month study in mice at dietary concentrations of 0,
1, 10, 100 or 1000 ppm the NOAEL was 10 ppm, equal to 1.7 mg/kg
bw/day. ETU produced thyroid follicular cell hyperplasia and
decreased colloid density at 100 ppm.
The NOAEL in a study in which rats were fed dietary
concentrations of ETU at 0, 0.63, 1.3, 2.5, 5.0 or 25 ppm for 8
weeks was 25 ppm (equal to 2.6 mg/kg bw/day), the highest dose
tested.
In a 13-week study in rats, ETU was administered in the diet at
concentrations of 0, 60, 125, 250, 500 or 750 ppm. The NOAEL was
less than 60 ppm (equal to 3.0 mg/kg bw/day) based on
histopathological findings of diffuse follicular cell hyperplasia in
the thyroid.
In a 90-day study in rats, ETU was administered in the diet at
concentrations of 0, 1, 5, 25, 125 or 625 ppm. The NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on hyperaemia of the thyroids,
with and without enlargement, increased thyroid to brain weight
ratio, decreased 125I thyroid uptake, decreased triiodothyronine,
decreased thyroxine and increased thyroid follicular cell
hyperplasia at 125 ppm.
In a four-week feeding study in dogs at dietary concentrations
of 0, 200, 980 or 4900 ppm, the NOAEL was 200 ppm, equal to 6.7
mg/kg bw/day. Decreased body-weight gain, decreased thyroxine and
T3 levels and enlarged thyroids were observed at 980 ppm.
In a 13-week feeding study in dogs at dietary concentrations of
0, 10, 150 or 2000 ppm the NOAEL was 10 ppm, equal to 0.39 mg/kg
bw/day. At 150 ppm haemoglobin, packed cell volume, and red blood
cell count were decreased, and cholesterol was increased. Effects
on the thyroid were found only at 2000 ppm.
In a 52-week feeding study in dogs at dietary concentrations of
0, 5, 50, or 500 ppm, the NOAEL was 5 ppm, equal to 0.18 mg/kg
bw/day. At 50 ppm a reduction in body-weight gain, hypertrophy of
the thyroid with colloid retention, a slight increase in thyroid
weight and pigment accumulation in the liver were observed.
Male and female mice received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,330; 0,1000; 330,0; 330,330; 330,1000; 110,330 or 33,100 ppm.
Mice receiving perinatal exposure only (330,0 ppm) showed no effect
on the incidences of neoplasms after 2 years. Cytoplasmic
vacuolization of follicular cells of the thyroid was evident in
males and females at 33,100 ppm, but no increases in neoplasms of
the liver, pituitary or thyroid were observed. T4 values were
significantly decreased in both sexes and thyrotropine was slightly
elevated. Animals receiving 330 ppm during adulthood showed tumours
of either the liver, pituitary or thyroid. Increasing perinatal
exposure from 0 to 330 ppm was associated with an increased
incidence of thyroid and pituitary lesions in female mice receiving
adult exposure to 330 ppm, but there were no enhancing effects of
perinatal exposure in mice receiving adult exposures of 1000 ppm
when compared to adults in the 0,1000 ppm group.
Rats were fed dietary concentrations of ETU at levels of 0, 5,
25, 125, 250 or 500 ppm for 2 years. The NOAEL was 5 ppm,
equivalent to 0.25 mg/kg bw/day. Vascularity and hyperplasia of the
thyroid were seen at 25 ppm.
In a two-year feeding study in rats using dietary
concentrations of 0, 0.5, 2.5, 5 or 125 ppm the NOAEL was 5 ppm
(equal to 0.37 mg/kg bw/day) based on changes in clinical chemistry,
increased triiodothyronine, decreased thyroxine, increased thyroid
weight, increased liver weight and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm.
In a two-year carcinogenicity study in rats using dietary
concentrations of 0, 175 or 350 ppm, thyroid carcinomas and
hyperplastic goitres were observed in both sexes at 175 ppm
(equivalent to 8.8 mg/kg bw/day).
Male and female rats received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,83; 0,250; 90,0; 90,83; 90,250; 30,83 or 9,25 ppm. Rats
receiving perinatal and adult exposure of 9,25 ppm showed no
increase in tumours and no apparent biologically meaningful changes
in thyroid hormone function at two-years when compared to 0,0 ppm
controls. Thyroid hyperplasia was evident in both sexes. At 9
months, animals given 9,25 ppm manifested decreased T3 and T4
values and increased thyrotropine without evidence of thyroid
follicular cell hyperplasia. Males and females receiving a dose of
90,0 ppm showed no hormonal changes and no tumours at 2 years.
Thyroid follicular cell hyperplasia was, however, evident. Animals
receiving adult exposure showed a significant increase in thyroid
follicular cell tumours at 83 and 250 ppm (males) and 250 ppm
(females). Males and females showed no significant differences in
the number of tumours between dose groups of 0,83; 30,83; and 90,83
ppm. Males and females receiving 90,250 ppm showed increases in
thyroid follicular cell tumours when compared to 0,250 ppm. At the
end of 2 years males and females receiving 0,83 or 0,250 manifested
increased numbers of thyroid tumours when compared to 0,0 ppm
controls.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 2.5, 25 or 125 ppm the NOAEL was 2.5 ppm, equal
to a range of 0.16-0.38 mg/kg bw/day, based on thyroid gland
follicular cell hyperplasia and hypertrophy at 25 ppm.
An oral teratogenicity study conducted in rats at dose levels
of 0, 5, 10, 20, 40 or 80 mg/kg bw/day indicated no maternal
toxicity at 40 mg/kg bw/day (NOAEL). Maternal lethality was
observed at 80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity
effects was 5 mg/kg bw/day based on teratogenic effects observed at
10 mg/kg bw/day.
An oral teratogenicity study in rats at dose levels of 0, 15,
25 or 35 mg/kg bw/day was conducted. No maternal toxicity was
observed at 35 mg/kg bw/day (NOAEL). The NOAEL for
embryo/fetotoxicity and teratogenicity was 15 mg/kg bw/day based on
higher incidences of dilated brain ventricles at 25 mg/kg bw/day.
Oral teratogenicity studies in rats (0, 10, 20, 30, 40 or 50
mg/kg bw/day), mice (0, 200, 400 or 800 mg/kg bw/day) and hamsters
(0, 90, 270 or 810 mg/kg bw/day) revealed no maternal toxicity at
the doses tested. The NOAEL for embryo/fetotoxicity in the rat was
10 mg/kg bw/day based on dilation of the lateral or fourth ventricle
at 20 mg/kg bw/day. The NOAEL for embryo/ fetotoxicity in the
hamster was 90 mg/kg bw/day based on a decrease in fetal body weight
at 270 mg/kg bw/day. The NOAEL for mice was greater than 800 mg/kg
bw/day.
In an oral teratogenicity study, rabbits received 0, 5, 10, 20,
40 or 80 mg/kg bw/day of ETU. The NOAEL for maternal toxicity was
80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was 40 mg/kg
bw/day based on an increase in resorption sites, decreased brain
weight and a degeneration of the proximal convoluted tubules in the
kidneys of fetuses at 80 mg/kg bw/day. Malformations were not
observed at the highest dose.
A study with pregnant rats administered ETU, T3/T4 and sodium
iodide in combination indicated a reduction in some of the
teratogenic responses when compared with groups administered ETU
alone. These results indicate that the teratogenic potential of ETU
may in part be secondary to the thyroid toxicity of ETU.
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induces mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic.
An ADI was allocated based upon a NOAEL of 0.39 mg/kg bw/day in
the 13-week study in dogs, since this dose level was between the
NOAEL of 5 ppm (equal to 0.18 mg/kg bw/day) and the middle dose
(effect level) of 50 ppm (equal to 1.8 mg/kg bw/day) in the 52-week
dog study. A 100-fold safety factor was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 10 ppm, equal to 1.7 mg/kg bw/day (3-month study)
Rat: 5 ppm, equal to 0.37 mg/kg bw/day (two-year study)
2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day
(reproduction study)
Dog: 10 ppm, equal to 0.39 mg/kg bw/day (13-week study)
5 ppm, equal to 0.18 mg/kg bw/day (52-week study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw.
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Agrelo, C. & Amos, H. (1981) DNA repair in human fibroblasts. Prog.
Mutation Research, 1: 528-532.
Allen, J.R., Van Miller, J.P. & Seymour, J.L. (1978). Absorption
tissue distribution and excretion of C14-ethylenethiourea by the
rhesus monkey and rat. Res. Comm. Chem. Pathol. Pharmacol. 20(1):
109-115.
Althaus, F.R., Lawrence, S.D., Sattler, G.L., Longfellow, D.G. &
Pitot, H.C. (1982). Chemical quantification of unscheduled DNA
synthesis in cultured hepatocytes as an assay for rapid screening of
potential chemical carcinogens. Cancer Res. 42: 3010-3015.
Arnold, D.L., Bickis, M.G., Nera, E.A., McGuire, P.F. & Munro, I.C.
(1982). Assessing the reversibility of thyroid lesions that result
from feeding etu. Toxicologist, 2(1): Abstract #318.
Arnold, D.L., Krewski, D.R., Junkins, D.B., McGuire, P.F., Moodie,
C.A. & Munro, I.C. (1983). Reversibility of ethylenethiourea-induced
thyroid lesions. Toxicol. Appl. Pharmacol. 67: 264-273.
Austin, G.E. & Moyer, G.H. (1979). Hepatic RNA synthesis in rats
treated with ethylenethiourea. Res. Commun. Chem. Pathol.
Pharmacol. 23(3): 639-642.
Autio, K., von Wright, A. & Pyysalo, H. (1982). The effect of
oxidation of the sulfur atom on the mutagenicity of
ethylenethiourea. Mutation Res. 106: 27-31.
Bridges, B.A., MacGregor, D. & Zeiger, E. (1981). Summary report on
the performance of bacterial mutation assays. Prog. Mutation
Research, 1: 49-67.
Briffaux, J.P. (1991). ETU: 13 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/504 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Briffaux, J.P. (1992). ETU: 52 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/505 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay with preincubation.
Prog. Mutation Research, 1: 261-270.
Bruze, M. & Fregert, S. (1983). Allergic contact dermatitis from
ethylenethiourea. Contact Dermatitis, 9: 208-212.
Carver, J.H., Salazar, E.P., Knize, M.G. & Wandres, D.L. (1981).
Mutation induction at multiple gene loci in Chinese hamster ovary
cells: the genetic activity of 15 coded carcinogens and
noncarcinogens. Prog. Mutation Research, 1: 594-601.
Casto, B.B. (1975, 1976). Progress report NIH-NCI-NOI-CP45615, pp.
1-23, 1975; pp. 1-18 (1976). (Cited in Heidelberger et al., 1983).
Chernoff, N., Kavlock, R.J., Rogers, E.H., Carver, B.D. & Murray, C.
(1979). Perinatal toxicity of maneb ethylenethiourea and
ethylenebisisothiocyanate sulfide in rodents. J. Toxicol. Environ.
Health, 5: 821-834.
Chhabra, R.S., Eustis, S., Haseman, J.K., Kurtz, P.J. & Carlton,
B.D. (1992) Comparative carcinogenicity of ethylenethiourea with or
without perinatal exposure in rats and mice. Fundamental and
Applied Toxicology, 18(3): 405-417.
Crebelli, R., Bellincampi, D., Conti, G., Conti, L., Morpurgo, G. &
Carere, A. (1986). A comparative study on selected chemical
carcinogens for chromosome malsegregation, mitotic crossing-over and
forward mutation induction in Aspergillus nidulans. Mutation
Research, 172: 139-149.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Prog. Mutation Research, 1: 626-637.
Daston, G., Ebron, M.T., Carver, B. & Stefanadis, J.G. (1987) In
vitro teratogenicity of ethylenethiourea in the rat. Teratology,
35: 239-245.
Daston, G.P., Yonker, J.E., Powers, J.F. & Heitmeyer, S.A. (1989).
Difference in teratogenic potency of ethylenethiourea in rats and
mice: relative contribution of embryonic and maternal factors.
Teratology, 40: 555-566.
Decker, C.J. & Doerge, D.R. (1991). Rat hepatic microsomal
metabolism of ethylenethiourea. Contributions of the flavin-
containing monooxygenase and cytochrome P-450 isozymes. Chem. Res.
Toxicol. 4: 482-489.
DiDonato, L.J. & Longacre, S.L. (1987). Ethylenethiourea:
dermal/oral absorbtion study in male rats. Unpublished report No,
85R-0206 from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Doerge, D. & Takazawa, R. (1990). Mechanism of thyroid peroxidase
inhibition by ethylenethiourea. Chem. Res. Toxicol. 3: 98-101.
Dotti, A. (1992). Ethylenethiourea (ETU): two-generation
reproduction study in the rat. Unpublished report No. 252360 from
Research and Consulting Company, Itingen, Switzerland. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978a). A study of the uptake and elimination of
C14 activity after oral ingestion of C14 labelled ethylenethiourea
(ETU) and mancozeb in the rhesus monkey. Revised final report,
Battelle Labs., Columbus, Ohio, USA. (July 31, 1978).
Emmerling, D.C. (1978b). The effects of thyroid hormones on the
teratogenic potential of ethylenethiourea in rats. Unpublished
report No. 78RC-1027 from Battelle Labs, Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Engst, R. & Schnaak, W. (1974). Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev. 52:
45-67.
Evans, E.L. & Mitchell, A.D. (1981). Effects of 20 coded chemicals
on sister chromatid exchange frequencies in cultured chinese hamster
cells. Prog. Mutation Research, 1: 538-550.
Frank, J. & Muller, G. (1988). ETU: In vivo/in vitro UDS/S-phase
assay in mice. Unpublished report No. 88R-47 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Freudenthal, R.I., Kerchner, G., Persing, R. & Baron, R.L. (1977).
Dietary subacute toxicity of ethylenethiourea in the laboratory rat.
J. Environ. Pathol. Toxicol. 1: 147-161.
Galloway, S.M. & Ivett, J.L. (1986). Chemically induced aneuploidy
in mammalian cells in culture. Mutation Research, 167: 89-105.
Gak, J.C., Graillot, C. & Truhaut, R. (1976). Difference in
sensitivity of the hamster and rat to the effects of long term
administration of ethylenethiourea. Eur. J. Toxicol. 9: 303-312.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the "microtiter" fluctuation test. Prog. Mutation Research, 1:
376-386.
Graham, S.L. & Hansen, W.H. (1972). Effects of short-term
administration of ethylenethiourea upon thyroid function of the rat.
Bull. Environ. Contam. Toxicol. 7(1): 19-25.
Graham, S.L., Hansen, W.H., Davis, K.J. & Perry, C.H. (1973).
Effects of one-year administration of ethylenethiourea upon the
thyroid of the rat. J. Agric. Food Chem. 21: 324-329.
Graham, S.L., Davis, K.J.Hansen, W.H. and Graham, C.H. (1975).
Effects of prolonged ethylenethiourea ingestion on the thyroid of
the rat. Food Cosmet. Toxicol. 13: 493-499.
Green, M.H.L. (1981). A differential killing test using an improved
repair-deficient strain of Escherichia coli. Prog. Mutation
Research, 1: 183-194.
Hardin, B., Schuler, R., Burg, J., Booth, G., Hazelden, K.,
MacKenzie, K., Piccirillo, V. & Smith, K. (1987). Evaluation of 60
chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen. 7: 29-48.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putnam, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W. &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagen. 8:
515-531.
Heddle, J.A., Hite, M., Kirkhart, B., Mavournin, K., MacGregor,
J.T., Newell, G.W. & Salamone, M.F. (1983). The induction of
micronuclei as a measure of genotoxicity. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 123: 61-118.
Heidelberger, C., Freeman, A.E., Pienta, R.J., Sivak, A., Bertram,
J.S., Casto, B.C., Dunkel, V.C., Francis, M.W., Kekunaga, T.,
Little, J.B. & Schechtman, L.M. (1983). Cell transformation by
chemical agents - a review and analysis of the literature. US-EPA
Gene-Tox Program. Mutation Research, 114: 283-385.
Hill, R., Erdreich, L., Paynter, O., Roberts, P., Rosenthal, S. &
Wilkinson, C. (1989). Thyroid follicular cell carcinogenesis. Fund.
Appl. Toxicol. 12: 629-697.
Hui, Q.Y., Armstrong, C., Laver, G. & Iverson, F. (1988).
Monooxygenase-mediated metabolism and binding of ethylenethiourea to
mouse liver microsomal protein. Tox. Letters. 41: 231-237.
Ichinotsubo, D., Mower, H. & Mandel, M. (1981a). Testing of a series
of paired compounds (carcinogen and noncarcinogen structural analog)
by DNA repair-deficient E. coli stains. Prog. Mutation Research,
1: 195-198.
Iverson, F., Khera, K.S. & Hierlihy, S.L. (1980). In vivo and in
vitro metabolism of ethylenethiourea in the rat and the cat.
Toxicol. Appl. Pharmacol. 52: 16-21.
Jagannath, D.R., Vultaggio, D.M. & Brusick, D.J. (1981). Genetic
activity of 42 coded compounds in the mitotic gene conversion assay
using Saccharomyces cerevisiae strain D4. Prog. Mutation
Research, 1: 456-467.
Jordan, L.W. & Neal, R.A. (1979). Examination of the in vivo
metabolism of maneb and zineb to ethylenethiourea (ETU) in mice.
Bull. Environm. Contam. Toxicol. 22: 271-277.
Jotz, M.M. & Mitchell, A.D. (1981). Effects of 20 coded chemicals on
the forward mutation frequency at the thymidine kinase locus in
L5178Y mouse lymphoma cells. Prog. Mutation Research, 1: 580-593.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Prog. Mutation Research, 1: 175-182.
Kassinova, G.V., Kovaltsova, S.V, Marfin, S.V. & Zakharov, I.A.
(1981). Activity of 42 coded compounds in differential inhibition
and mitotic crossingover assays in yeast. Prog. Mutation Research,
1: 434-455.
Kato, Y., Odanaka, Y., Teramoto, S. & Matano, O. (1976). Metabolic
fate of ethylenethiourea in pregnant rats. Bull. Environ. Contam.
Toxicol. 16: 546-555.
Khera, K. (1973a). Teratogenic effects of ethylenethiourea in rats
and rabbits, Toxicol. Appl. Pharmacol. 25: 455-456.
Khera, K.S. (1973b) Ethylenethiourea: teratogenicity study in rats
and rabbits. Teratology, 7: 243-252.
Khera, K.S. & Iverson, F. (1978). Toxicity of ethylenethiourea in
pregnant cats. Teratology, 18: 311-314.
Khera, K. (1987). Ethylenethiourea: a review of teratogenicity and
distribution studies and an assessment of reproduction risk. CRC
Crit. Rev. Toxicol. 18: 129-139.
Khera, K.S, Whalen, C. & Iverson, F. (1983). Effects of pretreatment
with SKF-525A, N-methyl-2-thioimidazole, sodium phenobarbital, or 3-
methylcholanthrene on ethylenethiourea-induced teratogenicity in
hamsters. J. Toxicol. and Environ. Health. 11: 287-300.
Kier, L.E., Brusick, D.J., Auletta, A.E., Von Halle, E.S., Brown,
M.M., Simmon, V.F., Dunkel, V., McCann, J., Mortelamans, K., Prival,
M., Rao, T.K. & Ray, V. (1986). The Salmonella
typhimurium/mammalian microsomal assay. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 168: 69-240.
Kirkhart, B. (1981). Micronucleus test on 21 compounds. Prog.
Mutation Research, 1: 698-704.
Kurrtio, P., Savolainen, K., Tuominen, R., Kosma, V.M., Naukkarinen,
A., Mannisto, P. & Collan, Y. (1986). Ethylenethiourea and nabam
induced alterations of function and morphology of thyroid glands in
rat. Arch. Toxicol., Suppl. 9: 339-344.
Kurrtio, P., Vartianinen, T. & Savolainen, K. (1990). Environmental
and biological monitoring of exposure to ethylenebisdithiocarbamate
fungicides and ethylenethiourea. British Journal of Industrial
Medicine, 47: 203-206.
Kurttio, P., Savolainen, K., Naukkarinen, A., Kosma, V., Tuomisto,
L., Penttila, I. & Jolkkonen, J. (1991). Urinary excretion of
ethylenethiourea and kidney morphology in rats after continuous oral
exposure to nabam or ethylenethiourea. Arch. Toxicol. 65: 381-385.
Leber, A.P., Wilkinson, G.E., Emmerling, D., Persing, R.L. & Thake,
D.C. (1978a). A correlation of the hormonal and pathological changes
of the thyroid as related to the treatment and withdrawal of
ethylenethiourea. Unpublished report No. 78RC-1026 from Battelle
Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA.
Leber, A.P., Wilkinson, G.E., Persing, R.L. & Holzworth, D.A.
(1978b) Effects of feeding ethylenethiourea in the rhesus monkey.
Contract No. 68-01-4717 from Battelle Labs., Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Legator, M.S., Bueding, E., Batzinger, R., Connor, T.H.,
Eisendstadt, E., Farrow, M.G., Ficsor, G., Hsie, A., Seed, J. &
Stafford, R.S. (1982). An evaluation of the host-mediated assay and
body fluid analysis. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Research, 98: 319-374.
Leuschner, F. (1977) Oral toxicity of ethylenethiourea 98% - called
for short ETU-during 8 weeks of administration in Sprague-Dawley
rats. Unpublished report No. 77RC-1159 from Laboratorium fur
Pharmakocopie und Toxicologie, Hamburg, West Germany. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lewerenz, H.J. & Plass, R. (1984). Contrasting effects of
ethylenethiourea on hepatic monooxygenase in rats and mice. Arch.
Toxicology, 56: 92-95.
Loprieno, N. (1981). Screening of coded carcinogenic/noncarcinogenic
chemicals by a forward-mutation system with yeast
Schizosaccharomyces pombe. Prog. Mutation Research 1: 424-433.
Lu, M.H. & Staples, R.E. (1978). Teratogenicity of ethylenethiourea
and thyroid function in the rat. Teratology, 17: 171-178.
Marshall, W. (1977). Thermal decomposition of
ethylenebisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. Agric. Food Chem. 25: 357-361.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Prog. Mutation Research, 1: 533-537.
Mason, J.M., Valencia, R. & Zimmering, S. (1992). Chemical
mutagenesis testing in drosophila: VIII. reexamination of
equivocal results. Environ. Mol. Mutagen. 19: 227-234.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb, and their related compounds. Int. Arch. Occup. Environ.
Hlth. 37: 169-178.
McGlynn-Kreft, A.M. & McCarthy, K.L. (1984). Ethylenethiourea
mammalian cell transformation test. Unpublished report No. 84R-56
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
McGregor, D., Brown, A., Cattanach, P., Edwards, I., McBride, D,
Riach, C. & Caspary, W.J. (1988). Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded chemicals.
Environ. Mol. Mutagen. 12: 85-154.
McLeod, P.L. & Doolittle, D.J. (1985). Ethylenethiourea mammalian
cell transformation test for promotion. Unpublished report No. 84R-
0298 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Mehta, R.D. & von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
C14. Prog. Mutation Research, 1: 414-423.
Mohn, G.R., Vogels-Bouter, S. & van der Horst-van der Zon, J.
(1981). Studies on the mutagenic activity of 20 coded compounds in
liquid tests using the multipurpose strain Escherichia coli K-
12/343/113 and derivatives. Prog. Mutation Research, 1: 396-423.
Mollett, P. (1975). Toxicity and mutagenicity of ethylenethiourea
(ETU) in drosophila. Mutat. Res. 29: 254.
Morgan, C. (1991). ETU: 4 week oral (dietary administration)
feasibility study in the beagle. Unpublished report No. 6152-616/6
from Hazleton Labs., Harrogate, North Yorkshire, England. Submitted
to WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986). Salmonella mutagenicity testing:II. Results
from the testing of 270 chemicals. Environ. Muta. 8(7): 1-119.
Natarajan, A.T. & van Kesteren-van Leeuwen, A.C. (1981). Mutagenic
activity of 20 coded compounds in chromosome aberrations/sister
chromatid exchanges assay using chinese hamster ovary (CHO) cells.
Prog. Mutation Research, 1: 551-559.
National Toxicology Program (NTP), 1992. Toxicology and
carcinogenesis studies of ethylenethiourea in F344/N rats and B6C3F1
mice (feed studies). U.S. Department of Health and Human Services,
Public Health Service, National Institutes of Health, NIH
Publication No. 92-2843, National Toxicology Program Technical
Report Series No. 388, March 1992.
Newsome, W.H. (1974). The excretion of ethylenethiourea by rat and
guinea-pig. Bull. Environ. Contamin. Toxicol. 11(2): 174-176.
National Institute for Occupational Safety and Health (NIOSH), 1981.
Ethylenethiourea, Health Hazard Evaluation Report No HETA-81-270-
1012, December 1981.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 8OR-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neil, W. & Marshal, W. (1984). Goitrogenic effects of
ethylenethiourea on rat thyroid. Pesticide Biochemistry and
Physiology, 21: 92-101.
Paika, I.J., Beauchesne, M.T., Randall, M., Schreck, R.R. & Latt,
S.A. (1981). In vivo sce analysis of 20 coded compounds. Prog.
Mutation Research, 1: 673-681.
Parry, J. (1986). The expression of recessive markers in presumptive
monosomic colonies derived from Saccharomyces cerevisiae, strain
D6. Mutagenesis, 1: 299-300.
Parry. J.M. & Sharp, D.C. (1981). Induction of mitotic aneuploidy in
the yeast strain D6 by 42 coded compounds. Prog. Mutation Research,
1: 468-480.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of the sister
chromatid exchange method in mammalian cells as a screening system
for carcinogens. Prog. Mutation Research, 1: 560-569.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980a). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in rats. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980b). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in mice. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Chin, A.E., Carlton, B.D., Chrisp, C.E.,
and Dill, G.S. (1982). Report on the maximum neonatal dose studies
with ethylenethiourea. Contract No. N01-ES8-2151. Report submitted
by Battelle Laboratories, Columbus, Ohio to NIEHS, January 29, 1982.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Pilinskaya, M. (1982) Analysis of the cytogenetic activity of
ethylenethiourea (ETU) in human peripheral lymphoblast culture in
vitro. Dopov Akad Nauk Ukr. RSR. Ser. B: Geol, Khim Biol Nauki,
10: 66-68
Poulsen, L.L., Hyslop, R.M., Ziegler, D.M. (1979). S-Oxygenation of
N-substituted thioureas catalyzed by pig liver microsomal FAD-
containing monooxygenase. Arch. Biochim. Biophys., 198(1): 78-88.
Quillardet, P., de Bellecombe, C. & Hofnung, M. (1985). The SOS
Chromotest, a colorimetric bacterial assay for genotoxins:
validation study with 83 compounds. Mutation Research, 147: 79-95.
Rashid, K.A. & Mumma, R.O. (1986). Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21(4):
319-334.
Resnick, M.A., Mayer, V.W., and Zimmermann, F.K. (1986). The
detection of chemically induced aneuploidy in Saccharomyces
cerevisiae: an assessment of mitotic and meiotic systems.
Mutation Research, 167: 47-60.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 314-322.
Robinson, D.E. & Mitchell, A.D. (1981). Unscheduled DNA synthesis
response of human fibroblasts, WI-38 cells, to 20 coded chemicals.
Prog. Mutation Research, 1: 517-527.
Rosencranz, H.S., Hyman, J. & Leifer, Z. (1981). DNA polymerase
deficient assay. Prog. Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenicity of carcinogens and
noncarcinogens in the Salmonella/microsome test. Prog. Mutation
Research, 1: 323-332.
Ruddick, J.A. & Khera, K.S. (1975). Pattern of anomalies following a
single oral dose of ethylenethiourea to pregnant rats. Teratology,
12: 277-281.
Ruddick, J.A., Williams, D.T., Hierlihy, L. & Khera, K.S. (1976).
C14-ethylenethiourea: distribution, excretion and metabolism in
pregnant rats. Teratology, 13: 35-40.
Ruddick, J.A., Newsome, W.H. & Iverson, F. (1977). A comparison of
the distribution, metabolism and excretion of ethylenethiourea in
the pregnant mouse and rat. Teratology, 16: 159-162.
Saillenfait, A.M., Sabate, J.P., Longonne, I. & De Ceaurriz, J.
(1991). Difference in the developmental toxicity of ethylene
thiourea and three N,N'-substituted thiourea derivatives in rats.
Fundamental and Applied Toxicology, 17: 686-697.
Salamone, M.F. (1981). Mutagenic activity of 41 compounds in the in
vivo micronucleus assay. Prog. Mutation Research, 1: 686-697.
Salisbury, S.A. & Lybarger, J. (1977). Health Hazard Evaluation
Determination Report No. 77-67-499, St. Clair Rubber Co,.,
Marysville, Michigan. Ethylenethiourea. US-DHEW. October 1977.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Savolainen, K. & Pyysalo, H. (1979). Identification of the main
metabolite of ethylenethiourea in mice. J. Agric. Food Chem. 27:
1177-1181.
Saxton, A.D. (1972). A C14-ethylenethiourea rat-feeding study. An
experiment to determine the excretion pattern and accumulation and
decline in thyroid tissues of C14-residues. Report 23-51 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Schiestl, R., Gietz, R.D., Mehta, R. & Hastings, P. (1989).
Carcinogens induce intrachromosomal recombination in yeast.
Carcinogenesis. 10: 1445-1455.
Schmid, H., Tennekes, H., Janiak, T., Probst, D., Leutkemeier, H.,
Pappritz, G., Marki, U., Vogel, O., Heusner, W. (1992).
Ethylenethiourea 104 week chronic toxicity (feeding) study in rats.
Unpublished study No. 256803 from Research and Consulting Company,
Itingen, Switzerland. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Schupbach, M. & Hummler, H. (1977). A comparative study of the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutation Research, 56: 111-120.
Seiler, J.P. (1974). Ethylenethiourea (ETU), a carcinogenic and
mutagenic metabolite of ethylene-bis-dithiocarbamate. Mutation
Research, 2: 189-191.
Seiler, J.P. (1975). In vivo mutagenic interaction of nitrite and
ethylenethiourea. Experientia, 31: 214-215.
Seiler, J.P. (1977a). Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short term test. Mutation Research, 46: 305-
310.
Seiler, J. (1977b). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutation
Research, 48: 225-236.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1. Prog.
Mutation Research, 1: 491-501.
Sharp, D.C. and Parry, J.M. (1981b). Use of repair-deficient strains
of yeast to assay the activity of 40 coded compounds. Prog.
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., Kato, K., Lienard, F., Tezuka, H.,
Teramoto, S. & Kada,T. (1977). Mutagenicity screening on pesticides
and modification products: a basis of carcinogenicity evaluation.
Cold Spring Harbor Conference Cell Proliferation, 4: 267-285.
Simmon, V.F. & Shepherd, G. F. (1981). Mutagenic activity of 42
coded compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 333-342.
Smith, D.M. (1976). Ethylenethiourea - a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.
26: 92-94.
Smith, D.M. (1984). Ethylenethiourea: thyroid function in two groups
of exposed workers. Brit. J. of Ind. Med. 41: 362-366.
Sram, R.J. (1975). Genetic risk from chemicals: mutagenicity studies
and evaluation. Review of Czechoslovak Medicine, 21(4): 186-193.
Stula, E.F. & Krauss, W.C. (1977). Embryotoxicity in rats and
rabbits from cutaneous application of amide-type solvents and
substituted ureas. Toxicol. Appl. Pharmacol. 41: 35.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Prog. Mutation Research, 1: 638-646.
Teramoto, S., Moriya, M., Kato, K., Tezuka, H., Nakamura, S.,
Shingu, A. & Shirasu, Y. (1977). Mutagenicity testing on
ethylenethiourea. Mutation Research, 56: 121-129.
Teramoto S., Shingu, A., Kaneda, M. & Saito, R. (1978a).
Teratogenicity studies with ethylenethiourea in rats, mice and
hamsters. Congenital Anomalies, 18: 11.
Teramoto, S., Shingu, A. & Shirasu, Y. (1978b). Induction of
dominant lethal mutations after administration of ethylenethiourea
in combination with nitrite or of N-nitro-ethylenethiourea in mice.
Mutation Research, 55: 335-340.
Teshima, R., Nagamatusu, K., Kido, Y. & Terao, T. (1981).
Absorption, distribution, excretion and metabolism of
ethylenethiourea in guinea-pigs. Eisei Kagaku, 27(2): 85-90.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Prog. Mutation Research, 1: 224-235.
Tophan, J.C. (1981). Evaluation of some chemicals by the sperm
morphology assay. Prog. Mutation Research, 1: 718-720.
Trueman, R..W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Prog. Mutation Research, 1:
343-350.
Truhaut, R., Fujita, M., Lich, N. & Chaigueau, M. (1973). Study of
metabolic transformations of zineb. C.R. Acad. Sci. Paris, Ser. D.
276: 229-233.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Prog. Mutation Research, 1: 705-711.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains WP2, WP67
(uvrA polA), and CM871 (uvA lexA recA). Prog. Mutation Research,
1: 199-209.
Ulland, B.M., Weisburgr, J.H., Weisburger, E.K., Rice, J.M. &
Cypher, R. (1972). Thyroid cancer in rats from ethylenethiourea
intake. J. Nat. Cancer Inst. 49: 583-584.
US EPA (1992) Ethylenebisdithiocarbamates (EBDCs); notice of intent
to cancel and conclusion of special review. Federal Register, 57:
7484-7530.
Valencia, R. & Houtchens, K. (1981). Mutagenic activity of 10 coded
compounds in the Drosophila sex-linked recessive lethal test.
Prog. Mutation Research, 1: 651-659.
Van't Hof, J. & Schairer, L.A. (1982). Tradescantia assay system for
gaseous mutagens. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Res. 99: 303-315.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985).
Chemical mutagenesis testing in drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutagenesis, 7: 677-702.
Wright, W.E., Peters, J.M., Plyler, E.A. & Belanger, P.L. (1981).
NIOSH Health Hazard Evaluation Report HETA 81-270-1012. December,
1981.
Wyrobek, A., Gordon, L. & Watchmaker, G. (1981). Effect of 17
chemical agents including 6 carcinogen/noncarcinogen pairs of sperm
shape abnormalities in mice. Prog. Mutation Research, 1: 712-717.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Prog. Mutation Research, 1: 481-490.
 Rats
Male Sprague-Dawley rats were pre-treated with phenobarbital,
dexamethasone, beta-napthoflavone or left untreated. The in vitro
effect of ETU or EU in the presence and absence of glutathione,
NADPH and heat inactivated microsomes on P450 enzymatic activity and
on covalent binding of ETU to microsomal proteins was studied. ETU
inhibited P450 activity in pretreated and non-pretreated rats.
Inhibition was NADPH-dependent and was abolished by glutathione
(GSH). Covalent binding of 14C-ETU to microsomal protein was also
NADPH-dependent. Binding was inhibited by co-incubation with GSH.
Heat treatment of microsomes and P450 inactivation studies indicated
a prominent role of FMO in covalent binding. Addition of GSH or
dithiothreitol after incubation of microsomes resulted in release of
bound ETU. Metabolism of ETU in the presence of GSH resulted in the
formation of GSH-ETU adducts and subsequent disulfide exchange. The
results suggest that reactive metabolites from ETU generated by
either FMO or P450 are trapped by GSH. Initial oxidation of ETU to
imidazoline-2-sulfenic acid, primarily by FMO, followed by reaction
with GSH or protein sulfhydroyls under conditions of GSH depletion,
has been proposed as the route of monooxygenase-mediated metabolism
of ETU (Decker & Doerge, 1991).
Male Sprague-Dawley rats were divided into 3 groups in a study
designed to study the effect of ETU on RNA synthesis. One group
that had been fasted for 16 hours received single i.p. injections of
2.5 or 250 mg/kg bw ETU or 5.0 mg/kg bw thioacetamide, followed by
3H-orotic acid 60 minutes later. Control animals received dimethyl
sulfoxide alone. A second group received 5.0 or 250 mg/kg bw/day
ETU by gavage on 3 successive days followed by administration with
3H-orotic acid; the animals were killed on the fourth day. The
third group was administered 5.0 or 250 ppm ETU in the diet for 3
weeks. At the end of 3 weeks, a 1-hour pulse dose of 3H-orotic
acid was administered and the animals killed. Serum T4 levels in
animals given ETU were then determined by radioimmunoassay. Rats
receiving 400 ppm of acetylaminofluorene in the diet served as
positive controls.
The livers of all animals given either ETU or thioacetamide
were histologically normal at the time of sacrifice. The livers of
rats fed acetylaminofluorene showed mild hydropic change of
hepatocytes and minimal bile duct proliferation. ETU failed to
inhibit nuclear or cytoplasmic RNA synthesis under the test
conditions. However, thioacetamide and acetylaminofluorene both
reduced the incorporation of 3H-orotic acid into nuclear and
cytoplasmic RNA (Austin & Moyer, 1979).
Pigs
FMO purified from hog liver catalyzes NADPH and oxygen-
dependent sequential S-oxidation of ETU, proceeding through an
intermediate imidazolinyl sulfenic acid to the corresponding
sulfinic acid. Further oxidation to the sulfonic acid was partly
enzymic and partly due to autooxidation. The FMO-oxidative pathway
predominated over P-450 pathways in hog and hamster liver microsomes
(Poulsen et al., 1979).
The mechanism of thyroid peroxidase inhibition by ETU was
studied in vitro using purified thyroid peroxidase obtained from
hog thyroid. ETU inhibited iodination reactions catalysed by thyroid
peroxidase. Inhibition occurred only in the presence of iodide ion
and proceeded with concomitant oxidative metabolism of ETU to
imidazoline and bisulfite ion. The inhibition ceased upon
consumption of ETU, with no loss of enzymatic activity and
negligible covalent binding of ETU to the enzyme. This reversible
thyroid peroxidase inhibition contrasts with the activity of the
therapeutic antithyroid drugs such as methimazole which act as
suicide inhibitors via covalent binding to the prosthetic heme group
(Doerge & Takazawa, 1990).
Toxicological studies
Acute toxicity studies
ETU is slightly toxic after oral administration to mammalian
species with measured LD50 values ranging from 545 mg/kg bw in
pregnant rats (Teramoto, 1978) to 4000 mg/kg bw in adult mice
(Lewerenz & Plass, 1984). The acute toxicity of ETU in various
animal species is given in Table 1.
Guinea-pigs
ETU (purity not stated) is a moderate to weak sensitizer in the
Hartley strain female guinea-pig by the guinea-pig maximization
test. With induction concentrations of 5% (intradermal) or 25%
(topical) and challenge concentrations of 2% or 0.5% (topical),
females responded positively at 24 hours (1/10 at 0.5%; 7/10 at 2%)
but not at the 48-hour reading (0/10 at 0.5%; 0/10 at 2%). In the
same studies, cross sensitization responses were also seen with
maneb, mancozeb and zineb after induction with ETU. Responses
ranged from 0-40% (4/10) at 24 hours and from 0-20% (2/10) at 48
hours. Induction with ETU followed by challenge with maneb
generally gave a slightly higher overall response (10-40%) than did
induction by maneb followed by challenge with ETU (0-20%)
(Matsushita et al., 1976).
Table 1. Acute toxicity of ETU
Species Sex Route LD50 (mg/kg bw) References
Mice M&F oral 4000 Lewerenz & Plass, 1984
F oral > 3000 Teramoto et al., 1978b
F (9 days pregnant) oral > 3000 Khera, 1987
M&F oral ca 2400 Peters et al., 1980b
Rats M&F oral ca 2400 Peters et al., 1980a
M oral 1832 Graham & Hansen, 1972
M&F oral 940 Lewerenz & Plass, 1984
F (13 days pregnant) oral 600 Khera, 1987
F oral 545 Teramoto et al., 1987b
Hamsters F oral > 3000 Teramoto et al., 1987b
F (11 days pregnant) oral > 2400 Khera, 1987
Short-term toxicity studies
Mice
B6C3F1 mice (10/sex/dose), 8 to 9 weeks of age were fed
diets containing 0, 125, 250, 500, 1000, or 2000 ppm ETU (97-99%
purity), equivalent to 0, 19, 38, 75, 150 or 300 mg/kg bw/day for 13
weeks. Deaths occurred at lower doses but were not dose- or
compound-related. Body-weight gain and food consumption were
comparable to controls. Diffuse follicular cell hyperplasia of the
thyroid occurred at 500 ppm in both sexes (greater than 70%) and was
statistically significant and dose-related. No effects were observed
at lower doses. Hepatocellular cytomegaly was also observed at 500
ppm (4/10 females, 10/10 males) and above. Effects were
statistically significant and dose-related. The NOAEL in this study
was 250 ppm, equivalent to 38 mg/kg bw/day (NTP, 1992).
Groups of Charles River CD-1 mice (15/sex/dose) were
administered ETU (100% purity) in the diet at levels of 0, 1, 10,
100 or 1000 ppm for 3 months, equal to 0, 0.16, 1.7, 18 or 168 mg/kg
bw/day for males and 0, 0.22, 2.4, 24 or 230 mg/kg bw/day for
females. There were no compound-related effects on food
consumption, body weight, haematology or clinical chemistry
parameters. Mixed function oxidase activity was increased in both
sexes at 1000 ppm, but only statistically significant in males
(aniline hydroxylase, p-nitroanisole, o-demethylase). Absolute and
relative thyroid weights were increased statistically in both sexes
at 1000 ppm. Absolute and relative liver weights were significantly
increased in males at 1000 ppm; relative liver weights were
significantly increased in females at 100 and 1000 ppm ETU.
ETU produced thyroid follicular cell hyperplasia and decreased
colloid density in both sexes at > 100 ppm, with increased
follicular epithelial cytoplasmic vacuolation and interstitial
congestion in both sexes at 1000 ppm. In the liver, ETU produced
centrilobular hypertrophy, nuclear pleomorphism and increased
intranuclear inclusions in both sexes at 1000 ppm. The pigment was
believed to be similar to lipofuscin. The NOAEL was 10 ppm ETU,
equal to 1.7 and 2.4 mg/kg bw/day in males and females, respectively
(O'Hara & DiDonato, 1985).
Rats
Adult male Han:Wistar rats (6/dose group) were given ETU (>
98% pure) in drinking-water for 28 days. Drinking-water
concentrations of ETU were 0, 100, 200 or 300 mg/litre, equal to
mean daily doses of 0, 11, 18 or 23 mg/kg bw.
ETU decreased body-weight gain during the exposure. Studies of
kidney function and morphology indicated that the kidney is not a
highly sensitive target for ETU-induced toxicity. ETU did not have
a permanent physiologically significant effect on urinary sodium,
potassium, uric acid, protein or glucose excretion, or urinary
osmolality. A slight increase in urinary arginine vasopressin (AVP)
excretion was observed in ETU-treated animals on day 28. No
prominent light microscopical changes were observed in the kidneys
of ETU-exposed rats. However, at 300 mg/litre ETU induced clear
ultrastructural changes in the epithelium of renal proximal tubuli.
An increased number of lysosomes and myelin figures as well as
vacuolization and edema were observed in the cytoplasm of the
epithelial cells of proximal tubules. The proportion of the dose of
ETU excreted as ETU in urine increased with increasing dose of ETU
and were 25%, 36% and 49% (Kurrtio et al., 1991).
Using an identical protocol as above, a study was conducted to
determine the effect of ETU on thyroid gland function and
morphology. Drinking-water concentrations of ETU were 0, 100, 200
or 300 mg/litre, equal to mean daily doses of 0, 11, 18 or 23 mg/kg
bw. Blood samples for T3, T4 and TSH were taken and the levels
measured using radio immunoassay methods. Thyroid glands were
extirpated and processed for light and electron microscopy. ETU
statistically significantly decreased T4 levels at all doses while
statistically increasing TSH levels at all doses. T3 levels were
also decreased in a dose response manner but values were not
statistically significant. There were no ETU-induced morphological
changes observed under light microscopy. Conspicuous ultra
structural changes were caused by ETU since a few areas with totally
destroyed epithelial cells could be found. It was also reported that
nerves and capillaries might have been affected by ETU (Kurrtio et
al., 1986).
Sprague-Dawley rats (10/sex/group) received 0, 0.63, 1.3, 2.5,
5.0, or 25 ppm of 98% pure ETU in the diet for 8 weeks. Twenty-four
hours after the last feeding, all animals received 5 µCi of 131I
intraperitoneally. There were no treatment-related effects on
behaviour, appearance, food intake, organ or body weight or
macroscopic appearance of organs other than the thyroid. ETU had no
clinical chemistry effects at the three low doses. However at 5 and
25 ppm slight increases were observed in males and females with
respect to 131I uptake, protein bound 131I and serum thyroxine. T3
uptake power was slightly decreased. Histopathology of the thyroid
in treated animals was comparable to control group at all dose
levels. The NOAEL in this study was 25 ppm, the highest dose
tested, equal to 2.6 mg/kg bw/day (Leuschner, 1977).
F344/N rats 8 to 9 weeks of age (10/sex/group), were fed diets
containing 0, 60, 125, 250, 500 or 750 ppm of 99% pure ETU for 13
weeks. All animals survived. Final mean body weights for males were
decreased at 500 and 750 ppm by 10% and 30%, respectively. Food
consumption at the same dose levels were decreased 16% and 24%.
Final body weights and food consumption of females were decreased at
750 ppm by 30% and 25%, respectively. Females receiving 60 to 500
ppm showed a uniform 10% body weight decrease accompanied by food
consumption decreases of 13% at 250 and 500 ppm. Histopathology was
present for the thyroid and pituitary gland of both males and
females. Diffuse follicular cell hyperplasia of the thyroid was
present in all animals of both sexes at all doses. In males, focal
follicular cell hyperplasia and cellular vacuolization of the pars
distallis of the pituitary gland was statistically significantly
increased at 250 ppm. Follicular cell adenomas (3/10) were evident
at 250 ppm and statistically significant at 750 ppm. Centrilobular
cytomegaly was observed only at 750 ppm and was statistically
significant. In females, follicular cell hyperplasia (4/10) and
cellular vacuolization of the pars distallis of the pituitary gland
(10/10) were statistically significant only at 750 ppm. Follicular
cell adenomas were observed at 500 and 750 ppm (3/10) but were not
statistically significant. Centrilobular cytomegaly of the liver
was seen only at the high dose and in all animals. The NOAEL in
this study was less than 60 ppm, equal to 3.0 mg/kg bw/day for males
and 4.3 mg/kg bw/day for females, based on histopathological
findings of diffuse follicular cell hyperplasia in the thyroid (NTP,
1992).
In a 90-day study, Sprague-Dawley derived rats (60/sex/dose)
were fed ETU (96.8% pure) at 1, 5, 25, 125 or 625 ppm. Controls
(72/sex) received powdered diet with 1% corn oil. At 30-day
intervals (i.e. 30, 60, 90 days) ten rats from each test group were
sacrificed and serum T3, T4, TBG and TSH concentrations were
measured. The free thyroxine index (FTI) was also calculated. The
remaining rats (10/sex/dose/time) were used to determine 125I uptake
by the thyroid. Rats receiving 625 ppm ETU showed high mortality
and marked decrease in body-weight gain. Clinical signs were
observed at the high dose by day 8 and consisted of excessive,
salivation, loss of hair, rough and bristly hair coat and scaly skin
texture. Necropsy revealed hyperaemia of the thyroids with and
without enlargement at 125 and 625 ppm for all time intervals.
Liver congestion was also evident with dose and time. Liver changes
were distinguishable microscopically and appeared to be compound-
related but not dose-related. Thyroid to brain weight ratio was
significantly increased at 125 and 625 ppm at all time periods.
125I uptake in the thyroid was statistically significantly decreased
along with TBG, T3 and T4 values at 125 and 625 ppm. At 25 ppm,
T4 was statistically significantly decreased only at 60 days. FTI
was comparable to controls. Altered thyroid function and increased
thyroid follicular cell hyperplasia were evident at 125 and 625 ppm.
The NOAEL was 25 ppm, equal to 1.7 and 1.9 mg/kg bw/day in males and
females, respectively (Freudenthal et al., 1977).
Osborne-Mendel rats (20 males/group) were fed ETU (purity not
stated) in the diet at levels of 0, 50, 100, 500 or 750 ppm for 30,
60, 90 or 120 days. 131I activity was determined at 4 and 24 hours
post-injection (5 µCi) in 20 rats from each group at each sacrifice
period.
Body weight was decreased at > 500 ppm throughout the study.
Food consumption was reduced at 30 and 90 days at > 100 ppm and
at 60 and 120 days at > 500 ppm. Relative thyroid weights were
increased at 30 days at > 100 ppm, at 90 days at 500 ppm and at
60/120 days at > 50 ppm.
Four hours after the injection of 131I, the uptake had
decreased significantly in rats fed ETU at 500 and 750 ppm at all
feeding periods. The uptake of iodine 24 hours after injection was
decreased significantly in those animals fed ETU at 100, 500 and 750
ppm. After the 90-day feeding period, the uptake decreased
significantly in rats fed the 500 and 750 ppm levels and ranged from
6 to 13 times lower than control values.
Histologically there were no differences between the control
and 50 ppm groups. At 100 ppm there was slight hyperplasia evident
in the thyroid gland. At 500 ppm there was moderate to marked
hyperplasia, lack of colloid and heightened epithelial walls. There
was an increase in vascularization, demonstrating a response to
increased blood level TSH. At > 500 ppm, an increased incidence
of follicular adenomas was reported. One mechanism by which ETU
acts on the thyroid is via inhibition of iodide peroxidase, which
oxidizes iodide to iodine (Graham & Hansen, 1972).
Dogs
Beagle dogs (2/sex/group) received dietary concentrations of 0,
200, 980, or 4900 ppm of ETU (98% pure) for 4 weeks. Body-weight
gains and food consumption for males were comparable to controls.
Intermediate- and high-dose females gained less weight than the
controls particularly at the high dose. Haematology results were
not remarkable. T3 levels were decreased in high-dose males and
females as well as mid-dose females. T4 levels were decreased in
the mid- and high-dose males and females. Reductions were dose-
related. Enlarged thyroids were noted in all animals of the
intermediate- and high-dose groups. The NOAEL was 200 ppm, equal to
6.7-7.4 mg/kg bw/day for males and 7.4-8.5 mg/kg bw/day for females
(Morgan, 1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
10, 150 or 2000 ppm of ETU (98% pure with doses corrected to 100%
active ingredient) for 13 weeks. Two males in the high dose were
sacrificed in a moribund state with morbidity attributed to compound
administration. All other animals survived to termination.
Clinical signs in the high-dose male survivors appeared to be
unremarkable. All high-dose females showed decreased activity or
subdued behaviour for various lengths of time (1-5 weeks). A
bilobed swelling in the pharyngeal area of two females was also
reported. No treatment-related clinical signs were observed in the
low or intermediate dose groups. Body-weight changes for survivors
in treated groups were not statistically significantly different
when compared to the control group. However, a slight to severe
body-weight loss for animals killed moribund was noted. Food
consumption was statistically significantly decreased only at the
high dose for surviving males during weeks 12 and 13, and for
females during weeks 11 and 12. Ophthalmological examinations
showed no remarkable differences between treated and control groups.
At 13 weeks, males and females of the 150 and 200 ppm groups
showed statistically significant decreases in haemoglobin, packed
cell, volume and red blood cell count. Reticulocyte count was
statistically significantly increased in females, but not in males.
Values for sodium, potassium, and chloride and BUN were all within
normal limits. Phosphorous was decreased in males and females at 13
weeks in the high dose. The value was statistically significant in
males. A statistically significant increase in serum protein was
associated with an increase in serum globulin in the high-dose males
at 13 weeks. A statistically significant increase in total
cholesterol was observed in the intermediate-dose (150 ppm) males at
weeks 8 and 13 and at weeks 4, 8, and 13 in high-dose (2000 ppm)
males and females. An increase in the mean creatinine level was
noted at weeks 8 and 13 in the high-dose males and females with
statistical significance attained for males at both time periods.
ALP was statistically significantly decreased in high-dose males at
weeks 8 and 13. At week 4, there was a statistically significant
decrease in mean ASAT in males at the intermediate and high dose and
in females at all three doses. A dose response was evident in
females. However values at 4 and 8 weeks were comparable to
controls for all groups of both sexes. ALAT was statistically
significantly decreased at week 4 only in females. Results from
urinalysis were not remarkable. Urine colour was however described
as orange or dark-coloured. Thyroid hormone assays revealed no
treatment-related changes in the low- and intermediate-dose groups.
However, marked and statistically significant reductions were noted
for T3 and T4 levels in high-dose animals at weeks 8 and 13. T4
was also statistically significantly decreased in males at 4 weeks
in the high-dose group.
At week 13, females of the high-dose group showed a marked and
statistically significant increase in thyroid weights accompanied by
slight but statistically significant increases in liver and adrenal
weights. Males of the high-dose group at 13 weeks showed a marked
increase in thyroid weights concurrent with slight increase in liver
and adrenal weights. None of these organ weights were statistically
significantly increased for males. Macroscopic examinations revealed
exophthalmia in two males (the survivors) and three females of the
high-dose group. Sporadic and slight exophthalmia was also observed
in one male of the intermediate dose group. Enlargement of the
thyroid gland was noted in all surviving high-dose animals as well
as the two males sacrificed moribund. The liver and adrenal gland
both appeared unremarkable as did the remaining tissues examined
macroscopically. Salient microscopic findings were those of
hypertrophy of the basophilic cells of the pituitary with micro-
vacuolisation attended by severe follicular hyperplasia of the
thyroid gland in all surviving and sacrificed animals of the high-
dose group. The liver and adrenal gland were histologically normal.
A moderate involution of the thymus of one male and two females of
the high-dose group was reported. No treatment-related microscopic
changes were noted for the low dose (10 ppm) or the intermediate
dose (150 ppm). The salient observations related to this study are
those of the pituitary and thyroid glands of animals receiving the
highest dose of 2000 ppm (equal to a mean of 66 mg/kg bw/day for
males and 72 mg/kg bw/day for females). In the pituitary, the lesion
observed was a hypertrophy of a basophilic cell type with
microvacuolisation, while in the thyroid, the lesion observed was a
hyperplasia of the follicular cells with papillary projections of
the follicular epithelium in the lumen of the follicles. Similar
hyperplasia was observed in ectopic nodule of thyroid tissues,
scattered along the thyroglossal track. The NOAEL was 10 ppm, equal
to 0.39 mg/kg bw/day based on decreased haemoglobin, packed cell
volume and red blood cell count, and increased cholesterol at 150
ppm. Effects on the thyroid were found only at 2000 ppm (Briffaux,
1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
5, 50 or 500 ppm ETU (expressed as active ingredient taking into
account the purity index of 98% purity) for 52 weeks. Mortality was
evidenced in the high-dose group with the death of one male and the
sacrifice of one male and one female prior to study termination. No
treatment-related clinical signs were reported in either the low- or
mid-dose groups. Pale mucous membranes in four males and one female
of the high-dose group was associated with subdued behaviour and a
change in the colour of the faeces (yellow/orange). Body weight in
surviving animals at 52 weeks was decreased 15% in both males and
females at the high-dose and 8% in the mid-dose males. A dose-
related decrease was observed in body-weight gain for males of the
mid- (-43%) and high-dose (-60%) and females of the high-dose (-
60%). However, the decreases were not statistically significant.
Body-weight gain and body weights in the low-dose group were
comparable to control group. There were no statistically
significant differences in food consumption between treated and
control groups at 52 weeks. Food efficiency was generally
comparable between groups. Ophthalmological examinations revealed
comparable findings between all groups.
Haematological values between control groups and the low- and
mid-dose groups were comparable. However, in the high-dose group,
treatment-related low values (75-80% of normal) in haemoglobin, RBC,
packed cell volume were reported for all animals dying or sacrificed
moribund as well as one surviving male. Additionally the decrease
in RBC was accompanied by an increased reticulocyte count, a
decrease in mean corpuscular haemoglobin and an increase in mean
corpuscular volume. Low values in platelet count were also observed
in high-dose animals. Changes in blood clinical chemistry values
for sodium, potassium and blood urea nitrogen, cholesterol,
triglycerides, bilirubin creatinine, gamma glutamyl-transpeptidase
in surviving animals was not considered treatment-related. However,
a slight to moderate increase for total bilirubin was observed for
animals dying or sacrificed early in the high dose group. Values for
globulin were statistically significantly higher at weeks 13 and 52
for the high-dose animals (males and females combined). A decrease
in the albumin/globulin ratio was also statistically significant at
week 52 for the high-dose animals (males and females combined).
Elevated values for ASAT and ALAT were reported for both high-dose
males found dead or sacrificed moribund in the high-dose group.
Urinalysis values were unremarkable between groups.
Thyroid hormone mean values for T4 and T3 were not
statistically significantly different from control group. However,
T3 and T4 values taken shortly prior to death or sacrifice of the
three high-dose group animals revealed a mean decrease of 50% for
T3 values and 70% for T4 values. The values for decedent animals
were also below the historical range. Of the two surviving males in
the high-dose group at 52 weeks, one showed a 47% reduction of T3
from its pretest level while the other was comparable to its pretest
level. T4 values for both high-dose male survivors were generally
decreased 55% from pretest values. T3 and T4 values appeared to
be unaffected in the low-dose and mid-dose groups. A dose-related
increase in thyroid weights were observed at week 52 for the
intermediate and high-dose males and females. The increase was
statistically significant for combined males and females for
absolute, body weight and brain weight ratio (except for brain
weight ratio in the intermediate dose group). Necropsy revealed an
enlargement of the thyroid in one of the two surviving males.
High-dose animals dying on study or killed in a moribund
condition all manifested centrolobular hepatocellular necrosis of
the liver (multifocal and moderately severe in males and multifocal
and minimal in the female). Slight pigment accumulation was also
evident in Kupffer cells. Hypertrophy of follicular cells with
dilation of follicles was also seen in the thyroid of one high-dose
male that was sacrificed. Pigment accumulations in Kupffer's cells
and occasionally hepatocytes were observed in both males and females
of the intermediate and high-dose groups. Hypertrophy of the
thyroid with colloid retention was observed in the intermediate and
high-dose group and ranged in severity from slight to moderately
severe. The NOAEL was 5 ppm, equal to 0.18 mg/kg bw/day based on
reduction in body-weight gain, hypertrophy of the thyroid with
colloid retention, a slight increase in thyroid weight and pigment
accumulation in the liver at 50 ppm (Briffaux, 1992).
Monkeys
Wild-caught rhesus monkeys (5/sex/group) were administered ETU
(96.8-98.2 purity) in the diet for 5.5 or 6 months at dose levels of
0, 2, 10, 50, or 250, and 0, 50, 150 or 450 ppm, respectively.
Results of Study 1: Body weights were not affected by ETU.
Thyroid weight was increased in both sexes at 250 ppm and in females
at > 50 ppm, resulting from hyperplasia and/or hypertrophy.
Females at > 50 ppm also had enlarged pituitary glands. Ovarian
weights at 250 ppm were significantly decreased.
No changes in T3 or TBG were observed. Serum T4 was
decreased in both sexes at > 50 ppm identified from FTI analyses.
Serum TSH was increased at 250 ppm. 125I uptake also increased at
> 50 ppm in both sexes.
Lesions reportedly associated with ETU were identified in the
pituitary and thyroid gland of animals at > 50 ppm. These
included thyroid and pituitary hypertrophy, and thyroid follicular
cell hyperplasia (moderate to severe). A second study was conducted
due to the extent of tuberculosis in this first study which
necessitated the early termination at 5-5.5 months.
Results of Study 2: Body weights were not affected by ETU.
Thyroid and spleen weights were increased in males at > 150 ppm
and at all doses in females. Serum T3 decreased in males at
> 150 pm and in females at 450 ppm. Serum T4 was decreased in
both sexes at > 150 ppm. Radioactive 125I uptake was increased
in all test groups. The increased thyroid weight, thyroid iodine
uptake, decrease in T3, T4 and increase in TSH support the
evidence for hypothyroidism caused by ETU.
BUN was elevated in females at 450 ppm along with creatinine
and a decrease in calcium. Haemoglobin, haematocrit and RBC count
were decreased in both sexes at 450 ppm.
Histologic changes were identified in thyroid and pituitary
glands in both sexes, increasing in severity and incidence with
increase in dose. Thyroid follicular cell hyperplasia and pituitary
cytoplasmic vacuolation and swelling were the major changes
observed.
The NOAEL for 125I uptake was 10 ppm; the NOAEL for changes in
T3, T4 and TSH was 50 ppm. In a separate pathological
examination, 10 ppm was considered to produce compound-related
changes in the thyroid gland in 1/7 monkeys. The NOAEL was
considered by the authors to be 2 ppm in these combined studies
(Leber et al., 1978b).
However, the monkey studies were considered unreliable because
one was compromised by ill health of the animals, while little
reliance could be placed on the effects at the lowest dose used in
the second.
Long-term toxicity/carcinogenicity studies
Mice
Groups of B6C3F1 mice received perinatal (F0), adult (F1)
or both exposures to ETU at the following dietary concentrations:
(F0:F1), 0:0, 0:330, 0:1000, 33-100, 110-330, 330-0, 330-330 or
330-1000 ppm. Female C57BL/6N mice were exposed to 0, 33, 110 or
330 ppm of 99% pure of ETU in feed for one week before breeding, and
naturally inseminated by C3H/HeN males that received control feed
only. ETU exposure continued throughout pregnancy and lactation.
Weaning occurred on day 28 post-partum and dietary exposure at these
same (maternal) concentrations continued until pups were 8 weeks of
age. On post-partum day 7, litters were culled to a maximum of 8
pups, separated by sex after weaning and litter mates co-housed. At
8 weeks of age, pups were separated into groups of 60 males and 60
females to receive dietary concentrations of 0, 330 or 1000 ppm for
2 years. Groups of 34 male and 29 female mice that were fed 33 ppm
of ETU before weaning received 100 ppm for up to 2 years.
At 9 months, liver weights were increased in groups receiving
adult exposure concentrations of 330 or 1000 ppm regardless of
perinatal exposure. Increases were statistically significant with
the exception of the 0:330 group.
Thyroid weights were also reportedly increased in animals given
1000 ppm. T4 levels were statistically significantly decreased in
all animals receiving adult concentrations of 330 or 1000 ppm. TSH
levels were statistically significantly increased in males only at
330:330 and 330:1000 ppm. Follicular cell vacuolization of the
thyroid occurred in all animals receiving ETU except those only
receiving perinatal exposure (i.e. 330:0 ppm). Animals receiving a
dose of 33:100 ppm were not reported. Hyperplasia was comparable
between all groups.
Hepatocellular adenomas were present in animals receiving 1000
ppm but were not statistically significant. Centrilobular
cytomegaly was statistically significantly increased in both males
and females receiving 1000 ppm and in males receiving 110:330 and
330:330 ppm. Eosinophilic focus was statistically significantly
increased in females receiving 1000 ppm. At 2 years, there was no
survival disparity between treated and control (0:0 ppm) groups.
Clinical signs were not treatment-related. Body weights of treated
animals were statistically significantly decreased in both sexes
compared to 0:0 ppm controls, with the exception of the 330:0 ppm
dose group which was similar to controls. Statistically significant
increases in the number of animals with adenomas or carcinomas were
observed for hepatocytes, thyroid follicles and posterior pituitary
in both sexes of high-dose treated, adult only exposed groups when
compared to 0:0 ppm controls. At the next lower dose level of 0:330
ppm both sexes showed a statistically significant increase in
hepatocellular adenomas or carcinomas.
Hyperplasia of the thyroid was evident in high-dose males and
females and in 0:330 ppm females. Centrilobular cytomegaly was also
evident in males and females at both dose levels of adult only
treated animals. A comparison of animal groups receiving 0:330
versus 110:330 versus 330:330 ppm showed statistically significant
increases of thyroid follicular cell hyperplasia (males) and thyroid
adenomas (females) in perinatal treated groups at 330 ppm. Similar
comparisons for hepatocellular neoplasms and pituitary neoplasms
revealed no statistical differences. Comparison between animals
receiving 1000 ppm, with or without perinatal exposure to 330 ppm
showed no statistical differences for tumours or hyperplasia of the
thyroid or pituitary. Treatment of adult females receiving 330 ppm
with 330 ppm perinatally increased the number of tumours in the pars
distalis when compared to 0:0 ppm controls as well as those of the
thyroid. Animals receiving only a perinatal dose showed a
comparable response when measured against 0:0 ppm controls. T4
levels for both sexes were statistically significantly decreased in
both sexes at all dose levels. TSH levels were statistically
significantly increased in animals receiving 1000 ppm (i.e. adult
and perinatal/adult groups). Animals receiving 330 ppm during
adulthood showed elevated TSH levels which were statistically
significant only in females. Animals receiving perinatal exposure
only showed TSH values comparable to controls (0:0 ppm) (Chhabra et
al., 1992; NTP, 1992).
Rats
Charles River rats (60/sex/group) were fed ETU (purity not
stated) in the diet at levels of 0, 5, 25, 125, 250 or 500 ppm for 2
years. Body weights in both sexes were significantly decreased
initially at doses > 25 ppm; at 500 ppm and above (males) and 125
ppm and above (females) at 12 months; and at 500 ppm and above (both
sexes) for the remainder of the study.
Liver to body-weight ratios were significantly increased at 125
ppm and through 6 months in males, but comparable to controls for
the remainder of the study. Relative liver weights in females were
significantly increased at doses > 125 ppm at 2 months and at
doses > 250 ppm through 18 months. No differences between
control and dose groups were observed at 24 months. Thyroid to
body-weight ratio was significantly increased in males at 250 ppm
and above at 2, 6 and 18 months, and at 125 ppm and above in females
for the first 12 months. Thyroid weights were significantly
increased at 125 ppm and above in males at 12 and 24 months, and at
250 ppm and above in females at 18 and 24 months.
Uptake of 131I, expressed as counts/min/mg tissue, was
significantly decreased in males at 500 ppm throughout the study.
Thyroids of females fed 125 ppm and above were hypofunctioning at 6
months and hyperfunctioning at 12 months. At 24 months, females had
a hypofunctioning thyroid at 500 ppm.
Fewer rats survived to 24 months in the 500 ppm dose group and
there was also a significant increase in pneumonia which may have
been further complicated by obstruction of the trachea from enlarged
thyroids in the animals. Effects in the thyroid were evident at all
doses. Increased vacuolarity and hyperplasia in the thyroid were
evident at 25 ppm and above. Thyroids of treated rats were
distinguishable from controls by lobulation, follicular size and
uniformity, height of follicular epithelium, colloid staining,
keratinization of follicles, and general size.
It is possible that ETU initially reduces thyroid activity,
after which compensation occurs by an increased release of TSH and
that this increase in TSH stimulated thyroid weight in an attempt to
overcome the blocking effect of ETU. The progression to neoplasia
is believed to be a result of excessive pharmacological stimulation.
This is supported, in part, by a lack of thyroid tumours at 1 year
at 5 or 25 ppm, an increase in tumour incidence after 1 year at 125
ppm, and confirmed after 2 years in rats fed 250 and 500 ppm. The
NOAEL in this study was 5 ppm, equivalent to 0.25 mg/kg bw/day
(Graham et al., 1973, 1975).
SPF-Sprague-Dawley (30/sex/dose) received 0, 0.5, 2.5, 5 or 125
ppm of 96% pure ETU (adjusted to 100%) in feed, 7 days a week for
either 52 weeks (interim sacrifice of pre-selected animals
10/sex/dose) or 104 weeks (terminal sacrifice).
There were no compound-related deaths, clinical signs or
effects on food consumption. Body-weight gain was slightly impaired
in males at 125 ppm resulting in group mean body weights 5-6% lower
than in control males for most of the study. Female body weight was
unaffected. There were no treatment-related changes with respect to
ophthalmoscopic observations or palpable masses. Haematologic and
urinalysis finding were comparable to controls. There were no
treatment-related changes on clinical biochemistry values in males
receiving 0.5 or 2.5 ppm nor in females receiving 0.5, 2.5 or 5 ppm
of ETU. Statistically significant increases were observed in males
at 125 ppm for total protein, albumin, GGT, cholesterol, bilirubin,
TSH and T3. T4 and urea values were lower. T3 values were higher
at 5 ppm at 29 weeks. For females at 125 ppm, values for glucose
and T4 were lower, uric acid, T3 and TSH were higher. At 125 ppm
only thyroid weight was higher in males and females at the interim
and final sacrifice. Liver weight was slightly higher in both sexes
but only at the interim sacrifice. Macroscopic effects were not
observed at 52 weeks. However, at 104 weeks the incidence of
diffuse or modular enlargement of the thyroid gland was increased in
both sexes at 125 ppm.
Microscopic examination of the thyroid gland at interim
sacrifice revealed minimal to moderate diffuse follicular cell
hyperplasia in 8 animals of the control group and 9, 12, 12 and 20
animals (both sexes combined) receiving 0.5, 2.5, 5 or 125 ppm of
ETU, respectively. The incidence of this finding was significantly
increased in females at 125 ppm. The severity was increased in males
at 5 and 125 ppm and in females at 125 ppm. Slight or moderate
nodular hyperplasia and follicular adenoma were recorded in 6 and 3
males of the 125 ppm, respectively. Minimal or slight focal or
multifocal cellular hypertrophy of the anterior pituitary was
recorded in 2 control rats, 2 rats at 2.5 ppm and 7 rats at 125 ppm.
Males were predominantly affected. A significant increase in the
incidence and severity was calculated for males at 125 ppm. Other
morphological alterations observed in treated groups were considered
secondary to treatment, and affected spleen, thymus gland, auditory
sebaceous (Zymbal's) glands and lungs.
At terminal sacrifice, slight to excessive diffuse follicular
hyperplasia was recorded in 27 animals (sexes combined) receiving
125 ppm. The incidence and severity for males and females was
statistically significant for both sexes. Slight to severe nodular
hyperplasia was observed in 9 animals (sexes combined) receiving 125
ppm. The incidence and severity of this lesion was significantly
increased in males. Follicular adenomas occurred in 1 male of the
control group and in 4 males given 125 ppm. Follicular carcinomas
were observed in 2 males of the high-dose group. The combined
incidence of benign and malignant follicular neoplasms yielded a
clear dose-related trend but did not vary significantly in pairwise
comparison with the control group. Anterior pituitary gland
adenomas were recorded in 8 males and 11 females of the control
group and 15 males and 10 females at 125 ppm. There was a clear
dose-related trend for males and a marginal level of significance by
pairwise comparison with the control group. Adenomas of the
anterior pituitary recorded at the intermediate doses for males and
females were 5, 8, and 6 and 11, 12, and 11 respectively. Other
morphological alterations observed in treated groups were considered
secondary to treatment and affected the pancreas, lungs and Zymbal's
glands. The NOAEL was 5 ppm (equal to 0.37 mg/kg bw/day) based on
changes in clinical chemistry, increased T3, decreased T4,
increased thyroid and liver weights and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm
(Schmid et al., 1992).
Charles River CD rats (26/sex/group) were fed 0, 175 or 350 ppm
of technical grade ETU (97% pure) for 2 years. Follicular or
papillary carcinomas of the thyroid were observed in 17 males and 8
females at the high-dose. At 175 ppm, equivalent to 8.8 mg/kg
bw/day, 3 males and 3 females had thyroid carcinomas. Hyperplastic
goitre was observed in 17 males and 13 females of the high-dose
group and 9 males and 6 females of the low-dose group. These
lesions were not observed in control rats (Ulland et al., 1972).
Groups of F344/N rats received perinatal exposure (F0), adult
exposure (F1) or both to different concentrations (ppm) of ETU as
follows: F0, F1; 0,0; 0,83; 0,250; 9,25; 30,83; 90,0; 90,83; or
90,250. Female rats were exposed to 0, 9, 30 or 90 ppm of 99% pure
ETU in feed for 1 week before breeding. All males received control
feed. All females were naturally inseminated by males, housed
individually and continued on their previous diet. ETU exposure
continued throughout pregnancy and lactation. Weaning occurred on
day 28 post partum and dietary exposure at these same concentrations
continued until the pups were 8 weeks of age. On post partum day 4,
litters were culled to a maximum of 8 pups. Pups were separated by
sex after weaning and litter mates co-housed (5/cage). At 8 weeks
of age, pups were separated into groups of 60 males and 60 females
to receive adult dietary concentrations of 0, 25, 83 or 250 ppm for
up to 2 years.
At 9 months, liver weights were statistically significantly
increased in males receiving 0,250 or 90,250 ppm of ETU. Thyroid
follicular cell hyperplasia was greater than 50% and statistically
significantly increased for both males and females at the following
dose levels: 0,83; 0,250; 30,83; 90,83; and 90,250 ppm. Thyroid
follicular cell adenomas were observed in both males (3/10) and
females (1/10) receiving 90,250 ppm. Values were not statistically
significant. T4 values compared to 0,0 ppm controls were
statistically significantly decreased in all experimental groups of
both sexes except animals receiving 90,0 ppm. T3 values were
statistically decreased in many but not all groups. The 90,0 ppm
groups was unaffected. TSH levels were increased in all dose groups
and statistically significant only in some female groups. The 90,0
ppm groups was only very slightly increased.
At two years there were no differences in food consumption
between treated groups and controls with the exception of a decrease
in the 90,250 ppm group of males during the last month of exposure.
Final mean body weights for males and females were comparable to 0,0
control group with the exception of the 90,250 ppm male dose group
where the decrease was statistically significant. Only those
animals receiving 90,250 ppm showed a statistically significant
decrease in survival. Thyroid function values for animals receiving
90,0 or 0,83 or 9,25 ppm were not statistically different compared
to controls for males and females at 2 years. All other doses
revealed some level of statistical significance in both sexes.
There were no clinical findings that could be attributed to thyroid
dysfunction. Pathology of the thyroid for animals receiving adult
only exposures of 0,0; 0,83 or 0,250 ppm revealed statistically
significant trends and statistically significant increases in high-
dose males and females for hyperplasia, adenomas, carcinomas and
adenomas and carcinomas combined. Animals receiving 0,83 ppm of ETU
showed statistical increases in hyperplasia (males and females) and
adenomas (males). Hyperplasia of the thyroid was the only
statistically significant effect observed in both sexes when 0,0 and
90,0 ppm comparisons were made. Responses between dose groups
receiving 0,250 or 90,250 ppm revealed a statistically significant
increase in the number of adenomas in males and carcinomas in both
males and females. A comparison of the 0,83; 30,83 and 90,83 dose
groups in females showed no statistical differences in hyperplasia,
adenomas, or carcinomas, or adenomas and carcinomas combined. In
males only hyperplasia was statistically significantly increased.
ETU had no clear effects on the incidences of neoplasms or non-
neoplastic lesions at sites other than the thyroid gland. However,
some groups showed statistically significant increases relative to
controls in neoplasms of the Zymbal's gland (males and females at
90,250 ppm) and, mononuclear cell leukaemia (males and females at
90,250 ppm and males at 90,83 ppm (Chhabra et al., 1992; NTP,
1992).
Rats and Hamsters
Groups of 20 male and 20 female rats and hamsters were
administered ETU (purity not stated) in the diet for 24 and 20
months, respectively, at dose levels of 0, 5, 17, 60 or 200 ppm
(strain of animals not reported).
In rats, food consumption was reduced at 60 ppm and above and
body weight decreased at 17 ppm and above. Effects on SAP and SGPT
were not clearly demonstrated due to fluctuations in control levels.
Cholesterol was increased at 5 ppm in both sexes. Some hepatic
enzyme levels were also affected: GPT increased in males at 60 ppm;
ALP increased at 5 ppm (females) and 17 ppm (males); glucose-6-
phosphate dehydrogenase did not change. Thyroid weights were
significantly increased in both sexes at 60 ppm. No data were
available on the histologic examination.
In hamsters, food consumption and body weight were reduced at
60 ppm and above. SAP was increased in both sexes initially, then
decreased through 18 months. No effect was observed on SGPT.
Cholesterol levels were significantly increased in both sexes at all
doses compared to controls. Hepatic enzymes, GPT and ALP, were
significantly increased in both sexes at all doses. Glucose-6-
phosphate dehydrogenase was significantly decreased in both sexes at
all dose levels. Relative thyroid weights were significantly
increased at 200 ppm and above in both sexes. No data were
available on the histologic examination (Gak et al., 1976).
Reproduction studies
Rats
ETU (98% pure) was mixed in the diet and fed to Sprague-Dawley
rats (25 male and female parents per group) at concentrations of 0,
2.5, 25 or 125 ppm during a 70-day pre-pairing period and throughout
pairing, gestation and lactation of 2 generations (one litter per
generation). Body weights and mean body-weight gains were reduced
among the male parents of the F0 generation at 125 ppm. There were
otherwise no changes in the viability, clinical appearance or
behaviour, feed consumption, body weights or weight gain or
macroscopic appearance of any of the parents, F1 or F2 pups in any
of the test groups. Reproduction parameters were unaffected in any
of the dose groups in either generation. Histopathologic
examination indicated compound-related changes in the thyroid and
anterior pituitary glands at 25 and particularly at 125 ppm in both
generations. Thyroid changes in both sexes of both generations
consisted of follicular cell hypertrophy and hyperplasia which were
pronounced at 125 ppm and present to a much lesser extent at 25 ppm.
Reduced colloid was also present among F1 males and females at 125
ppm. Adenomas were observed in 3 males (not statistically
significant). Pituitary changes consisted of an increased incidence
and severity of anterior cell hypertrophy in both sexes of both
generations at 125 ppm, together with a tendency to an increase in
hypertrophy among parental generation males at 25 ppm and a slight
increase in cellular vacuolization at 125 ppm. There was no
evidence of reproductive organ toxicity up to and including 125 ppm.
The NOAEL was 2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day,
based on thyroid gland follicular cell hyperplasia and hypertrophy
at 25 ppm (Dott, 1992).
Rats/mice
In the first phase of a two-phase study, adult female rats and
mice were dosed with ETU (96.7% purity) and then bred to proven male
sires. Pregnant females delivered their pups via C-section for
tissue distribution analyses. Phase 2 consisted of weanling
rats/mice dosed for 9 weeks and then analyzed. Dose levels in the
diet were: rats: 0, 8, 25, 83 or 250 ppm; mice: 0, 33, 100, 333 or
1000 ppm (rats: Fischer 344, 3 per group; mice: C57BL/6N, 78 per
group). Two weeks after dosing began, breeding was initiated.
No rat dams or weanlings died. There was a trend toward
decreased weight gain in dams in all groups and in weanling males at
levels > 83 ppm. Food consumption was also reduced at 250 ppm
for males only. No effects on females were observed. At 250 ppm,
there was a decrease in pup survival to postnatal day 4. Thyroid
hyperplasia was observed in males at all doses and in females above
8 ppm, increasing in incidence and severity with dose. Thyroid
adenomas were reported in males at 83 ppm and above. Vacuolization
of pituitary glands in males was noted at 250 ppm.
There was a significant decrease in body weight in high-dose
female mice during the period of lactation. Weanling body weights
were decreased in males and females at 333 ppm and 1000 ppm.
Initially, insufficient pregnancies were produced in all dose
groups. A rebreeding programme, after 6.5 weeks on ETU diets,
produced sufficient numbers of litters for evaluation. However, no
pregnancies were achieved in the high-dose group, and pregnancy rate
was reduced in other dose groups in comparison to control. The
number of pups surviving to day 28 was significantly decreased in
the high-dose group. Thyroid hyperplasia and cellular alteration of
hepatocytes (cytomegaly, karyomegaly) were observed in both sexes at
1000 ppm. One male mouse at 333 ppm also had adverse effects in the
liver (Peters et al., 1982).
Special studies on embryotoxicity/teratogenicity
Rats
Pregnant Charles River Rats (ChR-CD, Sprague-Dawley) were
administered 0, 25 or 50 mg/kg bw/day of ETU (98% pure) in DMSO, or
DMSO alone (vehicle control) or water alone onto the shaved back of
each animal for 48 hours on days 10 and 11 or days 12 and 13 of
gestation.
Maternal body-weight change during the 48-hour administration
period ranged from +4 to -5%. Fetuses examined from dams
administered ETU at 50 mg/kg bw/day on days 10 and 11, showed short
tails (3/83) and fused ribs (2/83). However, dams given 50 mg/kg
bw/day on days 12 and 13 produced fetal deformities in all
offspring. Fetal defects were characterized by encephalocele, a
part or the entire tail missing, missing leg bones, hunchback
curvature to the spine, short mandible, fusion of ribs and fusion of
sternebrae.
A dose of 25 mg/kg bw/day administered only on days 10 and 11
of gestation did not result in any fetal abnormalities. A dose of 25
mg/kg bw/day was not administered on days 12 and 13 of gestation
(Stula & Krauss, 1977).
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rats
(Wistar) (10-17 pregnant dams per dose). Treatment was made for 21-
42 days before conception to pregnancy day 15, and on days 6-15 or
6-20 of pregnancy. Doses of 40 mg/kg bw/day were not toxic to rats;
however, 80 mg/kg bw/day was lethal to 9 of 11 female rats. Mean
fetal weight was reduced at 40 mg/kg bw/day. Measurements of
sterility, pre-implantation loss and post-implantation survival were
comparable to controls. The brain was the most commonly affected
organ. ETU induced meningoencephalocele, meningorrhagia,
meningorrhea, hydrocephalus, obliterated neural canal, abnormal
pelvic limb posture with equinovarus, and short or kinky tail at 10
mg/kg bw/day in all phases of the study. Although no abnormalities
were reported in rats at 5 mg/kg bw/day, there was a higher
frequency of delayed ossification of the parietal bone, compared to
controls. The NOAEL for embryo/fetotoxicity was 5 mg/kg bw/day
based on teratogenic effects observed at 10 mg/kg bw/day. The NOAEL
for maternal toxicity was 40 mg/kg bw/day (Khera, 1973).
ETU was given by gavage (distilled water, 5 ml/kg bw/day) on
days 6-20 of gestation to pregnant Sprague-Dawley rats (22/group) at
doses of 0, 15, 25, or 35 mg/kg bw/day, and dams were sacrificed on
day 21 for examination of uterine contents. Maternal appearance,
behaviour, and body-weight gain were generally unaffected, and the
incidence of pregnancy was comparable among the groups. No adverse
effect was noted on the average numbers of implantations, live
fetuses, or percentages of resorption sites per litter. Mean fetal
body weights were decreased in a dose-related manner, but were only
significantly reduced at 35 mg/kg bw/day (13-15% lower). ETU at 35
mg/kg bw/day produced external malformations including cranial
meningocele and meningorrhea, severe hindlimb talipes, and a non-
significant incidence of hydrocephaly. Short and/or kinky tails
were noted in 43.5% of the fetuses. Soft tissue examinations
revealed higher incidences of dilated brain ventricles at 25 and 35
mg/kg bw/day (33.5 and 93% of the fetuses, respectively) and of
hydroureter and dilated ureter at 35 mg/kg bw/day, and skeletal
examinations revealed a reduced ossification of skull bones and a
significantly increased incidence of dumbbell-shaped or bilobed
vertebral centra (33.5% of fetuses). There were no other treatment-
related increases in skeletal variants among any of the experimental
groups, and no treatment-related effects of any kind identified in
the 15 mg/kg bw/day group. The NOAEL for maternal toxicity was 35
mg/kg bw/day. The NOAEL for embryo/toxicity and teratogenicity was
15 mg/kg bw/day based on higher incidences of dilated brain
ventricles at 25 mg/kg bw/day (Saillenfait et al., 1991).
ETU (100% purity) was administered via oral gavage at 40 mg/kg
bw/day from days 7 to 15 of gestation to pregnant CR rats (10-12
rats/group). Rats were hypothyroid and euthyroid. There was a
problem, however, in maintaining the euthyroid state in rats given
supplement. Rats were also given thyroxine to determine if ETU
teratogenicity occurred through alterations of maternal thyroid
function. ETU was found to be teratogenic in the rat but not
through alteration of maternal thyroid status. It was also
demonstrated that ETU lowered serum T4; that hypothyroidism per se
increased the background level of malformations in the rat; that T4
alone was embryotoxic but not teratogenic; and that hypothyroidism
altered the spectrum of malformations in response to ETU both
quantitatively and qualitatively (Lu & Staples, 1978).
Virgin Sprague-Dawley rats were mated one-to-two with males
and, after pregnancy was verified, were administered ETU (unknown
purity), T3/T4 and sodium iodide via oral gavage in varying
concentrations, either singly or in combination, as well as a
control solution of water only, from day 7 to day 20 of gestation.
Dosing regimen was as follows:
Dose group Total rats per
group
Control 1 ml distilled water 14
T3 20 µg/kg bw/day + T4 100 µg/kg bw/day 10
Sodium iodide 333 µg/kg bw/day 10
ETU 20 mg/kg bw/day 10
ETU 20 mg/kg bw/day + sodium iodide 16
ETU 20 mg/kg bw/day + T3/T4 16
ETU 40 mg/kg bw/day 11
ETU 40 mg/kg bw/day + sodium iodide 14
ETU 40 mg/kg bw/day + T3/T4 15
Each pregnant dam was killed on day 20 by chloroform
asphyxiation and the fetuses removed via hysterotomy. The number of
resorptions, live/dead fetuses and fetal birth weights were
determined. Skeletal analyses were performed on 1/3 and visceral
analyses on 2/3 of the fetuses. Results indicated a possible
reduction in the teratogenic response to ETU for some malformations
when T3/T4 was administered in conjunction with ETU. For example,
20 and 40 mg/kg bw/day ETU (alone) produced 97.6 and 94.5% incidence
of hydrocephaly, respectively. In combination with T3/T4 these
same levels produced 19.6 and 74.5% incidence, respectively. These
results indicate that the teratogenic potential of ETU may in part
be secondary to the thyroid toxicity of ETU (Emmerling, 1978b).
Rats, mice and hamsters
Wistar-Imamichi rats, JCL-ICR mice, and Syrian golden hamsters
10 weeks or older were mated overnight and examined the next morning
for the presence of a vaginal plug or spermatozoa in vaginal smears.
Evidence of copulation was designated as day zero of gestation.
Pregnant females were given daily oral doses of ETU by gavage during
the period of organogenesis. Doses given to rats, mice and hamsters
were, respectively 0, 10, 20, 30, 40 or 50 mg/kg bw/day, 0, 200, 400
or 800 mg/kg bw/day and 0, 90, 270, or 810 mg/kg bw/day. Rats,
mice, and hamsters were sacrificed on days 20, 18, and 14,
respectively.
Dams did not show signs of toxicity and none died in any
species. There were no statistically significant differences between
treated and control rats for the mean number of implants and live
fetuses reported. Mean fetal weight for both males and females was
statistically significantly decreased at 30 mg/kg bw/day and higher.
The percent of fetal death was also statistically significantly
increased at 50 mg/kg bw/day. Mice showed no statistically
significant changes between treated and control values for any of
the prenatal developmental parameters (i.e mean number of implants,
mean number of live fetuses, percent fetal death and mean fetal body
weight). The mean number of implants for hamsters were comparable
to control values for all doses. There was a statistically
significant decrease in the mean number of live fetuses born
attendant to a decrease in mean fetal body weight for males and
females which was statistically significant at 810 mg/kg bw/day.
Mean fetal body weight for females was also statistically
significantly lower at 270 mg/kg bw/day without other prenatal
developmental effects. Gross external anomalies were not meaningful
between controls and treated groups for mice. Rats manifested a
short or kinky tail at 30 mg/kg and above in 80% of the offspring.
In rats, meningocele was observed in 66% of the offspring at 40
mg/kg bw/day and above and micrognathia in 30% of the offspring at
50 mg/kg bw/day. Hamsters showed multiple type anomalies at 810
mg/kg bw/day and included such signs as cleft palate, short or kinky
tail, and oligodactyly. Short or kinky tail was observed in 2% of
the animals at 270 mg/kg bw/day and 42% of the animals at 810 mg/kg
bw/day. The LOAEL in rats was 30 mg/kg and seen as curved clavicles.
There was an increased incidence of curved clavicles, fused/wavy
ribs, fused sternebrae, malformed vertebrae and scoliosis at 40 and
50 mg/kg bw/day. The LOAEL was 90 mg/kg bw/day in hamsters based on
a 2% incidence of malformed lumbar and sacral vertebrae, a 4%
incidence at 270 mg/kg bw/day and a 51% incidence at 810 mg/kg.
There were no brain or visceral anomalies observed in mice.
Dilation of the lateral 4th ventricle was observed in 5% of hamsters
at the high dose, none at lower doses and 2% in controls. Dilation
of the lateral or 4th ventricle in rats was observed in 2% and 39%
of rats at doses of 10 and 20 mg/ kg bw/day. At 30 mg/kg bw/day and
above the response was 100%. No maternal toxicity occurred at the
doses tested. The NOAEL for embryo/ fetotoxicity in the rat was 10
mg/kg bw/day based on dilation of the lateral or fourth ventricle at
20 mg/kg bw/day. The NOAEL for embryo/fetotoxicity in the hamster
was 90 mg/kg bw/day based on decrease of fetal body weight at 270
mg/kg bw/day. The NOAEL for mice was greater than 80 mg/kg bw/day
(Teramoto et al., 1978).
Hamsters
ETU (purity > 99%) was administered orally to pregnant Syrian
hamsters at doses of 600, 1200, 1800 or 2400 mg/kg bw on day 11 of
gestation. All dams were killed on day 15 of gestation for necropsy
and fetal examination. There were only 5 pregnant dams in the
control group compared to 8-10 in the treated groups.
Maternal toxicity was not reported at any dose. However, there
was an increased incidence of resorbed fetuses and fetuses dying
late in gestation with an associated decrease in the number of live
fetuses at 2400 mg/kg bw. Fetal body weights were similarly reduced
at 1800 mg/kg bw. Malformations were evident at > 1200 mg/kg bw,
with no adverse effect reported at 600 mg/kg bw. Fetal anomalies
included cleft palates ectrodactyly, hydrocephalus and hypoplastic
cerebellum. There was also an increased incidence of delayed
ossification of the calcarium and sternebrae defects. As with other
species (i.e. rat, cat), the brain was particularly sensitive to
ETU, although at higher dose levels (Khera et al., 1983).
Mice/rats/hamsters/guinea-pigs
Time-pregnant random-bred CD-1 mice, Sprague-Dawley rats golden
hamsters and Hartley strain guinea-pigs were used. Maneb (80%
pure), ETU (melting point 197-198 °C) and EBIS were administered by
gastric intubation. Control animals received vehicle alone (water
or corn oil) or remained untreated. Prenatal studies were conducted
on rats given maneb (480, 240, 120, 0 mg/kg bw/day for days 7-16) or
ETU (80, 40, 30, 20, 10, 5, 0 mg/kg bw/day for days 7-21) or EBIS
(30, 25, 7.5, 0 mg/kg/ bw/day for days 7-21). Prenatal studies were
also conducted on mice given maneb (1500, 750, 375, 0 mg/kg bw/day
for days 7-16) or ETU (200, 100, 0 mg/kg bw/day for days 7-16) or
EBIS (200, 100, 50, 0 mg/kg bw/day for days 7-16). Prenatal studies
were also conducted with hamsters and guinea-pigs but only with ETU.
Hamsters received either 100, 50, 25 or 0 mg/kg of ETU on days 5-10
of gestation. Guinea-pigs received 100, 50 or 0 mg/kg bw/day of ETU
on days 7-25. Post-natal studies were only conducted on rats. Rats
received either maneb (480, 240, 0 mg/kg bw/day) or ETU (30, 25, 20,
0 mg/kg bw/day) or EBIS (30, 15, 0 mg/kg bw/day) on days 7-15 of
gestation. Mice were killed on day 18, rats on day 21, hamsters on
day 15 and guinea-pigs on day 35. Animals designated for post-natal
study were allowed to litter normally. Litters were normalized to 4
individuals of each sex and weaned on day 22 post-partum.
In toxicity studies conducted to determine dose levels, all
compounds tested proved to be more toxic in the rat than the mouse.
Hind limb paralysis was observed in rats given maneb at the high
dose of 600 mg/kg bw/day. No toxicity was noted in mice given a
dose of 1500 mg/kg bw/day of maneb. EBIS produced death in rats at
75 mg/kg bw/day and hind limb paralysis at 50 mg/kg bw/day.
Decreased body-weight gain and death was observed in mice given 100
mg/kg bw/day. ETU produced lethality in rats at the high dose of 80
mg/kg bw/day. ETU was not toxic in mice (300 mg/kg bw/day) hamsters
(150 mg/kg bw/day) or guinea-pigs (100 mg/kg bw/day) at the high
dose tested.
Maneb administered to pregnant rats resulted in significant
dose-related decrease in maternal weight gain and increase in liver
to body-weight ratios. Fetal weight and caudal ossification were
significantly reduced only at the highest dose tested (480 mg/kg
bw/day). At the high dose (480 mg/kg bw/day), 18 fetuses from 4
litters manifested hydrocephalus. Maternal mice given maneb
manifested increased liver/body-weight ratios and decreased caudal
ossification beginning at 375 mg/kg. Dose-related trends were
evident. EBIS given to rats and mice did not result in adverse
fetal effects. Average maternal weight gain was decreased in rats
at 30 mg/kg bw/day. Maternal weight gain in mice was not affected.
Average liver to body-weight ratio was increased at the high dose
tested for rats (30 mg/kg bw/day) and mice (200 mg/kg bw/day). ETU
administered to rats at the high dose of 80 mg/kg bw/day resulted in
25% maternal mortality and reduced weight gain. Fetal toxicity was
also observed at 80 mg/kg bw/day and included mortality, decreased
weight, decreased ossification and edema. Gross defects of the
skeletal system and central nervous system were noted in a majority
of fetuses. At 40 mg/kg bw/day, fetal weight and ossification were
reduced and hydrocephalus and encephalocele were evident.
Hydrocephalus was seen at 20 mg/kg bw/day and decreased fetal body
weight at 10 mg/kg bw/day. ETU produced an increase in the liver-
body-weight ratio of mice at 100 mg/kg bw/day and 200 mg/kg bw/day
and an increase in the number of supernumerary ribs at 200 mg/kg
bw/day. No apparent effects were observed in hamsters or guinea-
pigs. Post-natal studies with maneb resulted in delayed eye opening
in males. Post-natal observations with EBIS resulted in decreased
fetal body weight at day 22 in females only as well as delayed eye
opening. ETU administration produced no observable post-natal
effects with the exception of increased total open field activity in
males. There were no apparent differences reported in open field
activity between male fetuses surviving the high dose (30 mg/kg
bw/day) with hydrocephalus and their apparently normal mates.
Hydrocephaly was not observed at lower doses (Chernoff et al.,
1979).
Rabbits
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rabbits
(New Zeeland white). There were 5-7 pregnant does per group.
Treatment was made from days 7 to 20 of pregnancy. No toxicity was
apparent in rabbits given 80 mg/kg bw/day. Fetal weights were not
affected. Measurements of sterility, pre-implantation loss and post-
implantation survival were comparable to controls. Rabbits presented
no evidence of malformations at the doses administered. However,
there was an increase in resorption sites, decreased brain weight,
and degeneration of the proximal convoluted tubules in the kidneys
of fetuses at 80 mg/kg bw/day. The NOAEL for maternal toxicity was
80 mg/kg bw/day and for embryo/fetotoxicity the NOAEL was 40 mg/kg
bw/day (Khera, 1973).
Cats
ETU (purity not stated) was administered orally (in gelatin
capsules) to pregnant European and Persian breed cats (7-14 cats per
group) at doses of 0, 5, 10, 30 or 60 mg/kg bw/day on days 16-35 of
gestation or 120 mg/kg bw/day from days 16 to 34 of gestation. No
effect was evident at 5 mg/kg bw/day. However, at > 10 mg/kg
bw/day decreased ataxia, tremors and hindlimb paralysis were
observed. No pregnant cats survived in the 30 and 60 mg/kg bw/day
dose groups. The remaining cats showed no apparent treatment-related
effect on fetal viability or fetal weight. Although this study was
inconclusive in many respects, there was an increased incidence of
toxicity to the central nervous system at 10 mg/kg bw/day. Further,
at 5 and 120 mg/kg bw/day, there were anomalous fetuses in each
group. Incidences of exencephaly, hydrocephaly, cleft palate,
kyphoscoliosis, umbilical hernia, coloboma, and spina bifida were
observed in these two treated groups. Similar anomalies were
observed in the rat (Khera & Iverson, 1978).
Special studies on genotoxicity
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induced mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic. The results of genotoxicity assays on ETU are given in
Table 2.
Table 2. Results of genotoxicity assays on ethylenethiourea
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 10-20 000 µg/plate; ? Positive Teramoto et al.,
reversion TA1535, TA1536, in DMSO? (TA1535 without 1977; Shirasu
assay TA1537, TA1538, G46 activation) et al., 1977
S. typhimurium 0.2-2000 µg/plate >98% Negative Brooks & Dean, 1981
TA1535, TA1537,
TA1538, TA92, TA98,
TA100
S. typhimurium 5-5000 µg/plate; >98% Negative Richold & Jones,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 0.1-2000 µg/plate; >98% Negative Rowland & Severn,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 10-5000 µg/plate; >98% Positive Simmon & Shepherd,
TA1535, TA1537, in DMSO (TA1535 with 1981
TA1538, TA98, TA100 & without
activation)
S. typhimurium 4-2500 µg/plate >98% Negative Trueman, 1981
TA1535, TA1537,
TA1538, TA98, TA100
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Salmonella S. typhimurium 1000-20 000 µg/plate; no ? Positive Autio et al., 1982
reversion TA1950 activation used; in DMSO
assay (cont'd)
S. typhimurium >500 µg/plate; ? Positive Moriya et al., 1983
TA1535, TA1537, in DMSO? (TA1535 with
TA1538, TA98, TA100 and without
activation)
S. typhimurium 100-10 000 µg/plate; 98.4% Positive Mortelmans et al.,
TA1535, TA1537, TA98, in DMSO (TA1535 with 1986 (SRI)
TA100 & without
activation)
Salmonella S. typhimurium 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; TA1535, TA1537, TA98 in dimethylacetamide
fluctuation test
Salmonella S. typhimurium 200-80 000 ? Positive Schupbach & Hummler,
forward TA1530 µg/plate 1977
mutation assay
E. coli E. coli WP2 hcr 10-10 000 µg/plate ? Negative Teramoto et al.,
reversion assay 1977; Shirasu
et al., 1977
E. coli 343/113/uvrB 200-4000 µg/ml; >98% Positive Mohn et al., 1981
in phosphate buffer (galR- & arg+
systems with
activation)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
E. coli E. coli WP2 uvrA 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; in dimethylacetamide
fluctuation test
Host mediated S. typhimurium 500-6000 mg/kg; ? Negative Schupbach & Hummler,
assay Swiss G46 in DMSO 1977
albino mouse
Swiss albino S. typhimurium 670-6000 mg/kg; ? Weak Schupbach & Hummler,
mouse TA1530 in DMSO Positive 1977
Male JCL-SD rat S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
G46 1977; Shirasu
et al., 1977
Male JCL-ICR S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
mouse G46 1977; Shirasu
et al., 1977
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Mouse lymphoma 140-3000 µg/ml; >98% Negative Jotz & Mitchell,
mutation assay L5178Y TK+/- in DMSO 1981
Mouse lymphoma 25-3600 µg/ml; NTP Positive McGregor et al.,
L5178Y TK +/- in DMSO chemical 1988
repository
Chinese hamster ovary 1000-2000 µg/ml; >98% Negative Carver et al., 1981
(CHO-AT3-2; several loci) in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 0.25-2.5%; ? Negative Mollet, 1975
recessive lethal in sugar water
assay
D. melanogaster 250 ppm; in DMSO >98% Negative Valencia &
Houtchens, 1981
D. melanogaster 4900 ppm injection; 97% Inconclusive Woodruff et al.,
12 500 ppm feed; in water 1985 Mason et al.,
1992
D. melanogaster 5100 ppm 98.4% Inconclusive Mason et al., 1992
1.D. Yeast and Other Fungal Assays
Forward mutation S. pombe 0.1-1 µg/ml; >98% Negative Loprieno, 1981
in DMSO
A. nidulans 0.22-116 mM; >98% Negative Crebelli et al.,
no activation used; 1986
in DMSO
Reverse mutation S. cerevisiae XV185-14C 88.9-889 µg/ml; >98% Equivocal Mehta & von Borstel,
in DMSO 1981
1.E. Plant Test
Mutation Tradescantia 9.79 X 10-5 M; ? Positive van't Hof &
clone 4430 in DMSO? Schairer, 1982
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations in Mammalian Cells
In vitro Chinese hamster cell line 1000-3200 µg/ml ? Negative Teramoto et al.,
chromosomal (Don) 1977; Shirasu
aberrations et al., 1977
Chinese hamster ovary 1670-5000 µg/ml; >98% Positive Natarajan & van
(CHO) cells in DMSO (with and Kesteren-van
without Leeuwen, 1981
activation)
Chinese hamster ovary 6000-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
2.B. In Vivo Chromosomal Alterations
Bone marrow Male & female Wistar rat 50-400 mg/kg; ? Negative Teramoto et al.,
cytogenetics in aqueous soln 1977; Shirasu
et al., 1977
Micronucleus assay Female ICR mouse 150-450 mg/kg; ? Negative Seiler, 1975
in DMSO
Male & female Swiss albino 700-6000 mg/kg; ? Negative Schupbach & Hummler,
mouse in gummi arabicum 1977
Male ICR mouse 220-880 mg/kg; >98% Negative Kirkhart, 1981
in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Micronucleus assay B6C3F1 mouse 880-1416 mg/kg; >98% Negative Salamone et al.,
(cont'd) in DMSO 1981
Male & female CD-1 mouse 220-880 mg/kg; >98% Negative Tsuchimoto & Matter,
in DMSO 1981
Dominant lethal Swiss albino mouse 500-3500 mg/kg; ? Negative Schupbach & Hummler,
assay in gummi arabicum 1977
JCL-ICR mouse 300-600 mg/kg; ? Negative Teramoto et al.,
in water with gum arabic 1977; Shirasu
et al., 1977
C3H/HeCr mouse 150 mg/kg; ? Negative Teramoto et al.,
in gum arabic soln 1978
Reciprocal D. melanogaster 500 ppm; >98% Negative NTP, 1992
translocations in 5% sucrose soln
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
Rec assay B. subtilis H17, M45 20-4000 µg/disk ? Negative Teramoto et al.,
1977; Shirasu
et al., 1977
B. subtilis H17, M45 spores 2000 µg/disk; >98% Positive Kada, 1981
in DMSO (without
activation)
E. coli WP2, WP67, CM871 Not specified >98% Negative Green, 1981
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Rec assay (cont'd) E. coli WP2, WP67, CM871 Not specified >98% Negative Tweats, 1981
E. coli several deficient 500 µg/ml; in DMSO >98% Positive Ichinotsubo et al.,
strains (with activation) 1981
S. typhimurium TA1538, 125-2000 µg/disk; ? Negative Rashid & Mumma, 1986
TA1978; in DMSO
E. coli K12 & WP2
Inhibition of DNA E. coli polA 2273 µg/ml >98% Positive Rosenkranz et al.,
polymerase I (without 1981
activation)
Lambda prophage E. coli (lysogenic) 2-20 mg/ml; only activation >98% Positive Thomson, 1981
induction used; in DMSO
E. coli E. coli PQ37 Not specified; in DMSO ? Negative Quillardet et al.,
SOS Chromotest 1985
In vitro Human fibroblasts from Not specified; in DMSO >98% Negative Agrelo & Amos, 1981
unscheduled DNA skin biopsies
synthesis (UDS)
HeLa S3 human cells Not specified; in DMSO >98% Inconclusive Martin & McDermid,
1981
WI-38 human fibroblasts 63-2000 µg/ml; in DMSO >98% Negative Robinson & Mitchell,
1981
In vitro unschuled Rat hepatocytes 9.6 X 10-9 - 3.2 X 10-3 M ? Negative Althaus et al.,
DNA synthesis (nuclei isolation) 1982
(UDS)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
In vivo/in vitro Female B6C3F1 mouse 1500 mg/kg; in corn oil 98% Negative (UDS); Frank & Muller, 1988
unscheduled DNA hepatocytes Positive (S-
synthesis (UDS)/S phase increase)
phase analysis
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 25-1000 µg/ml; >98% Negative Evans & Mitchell,
SCE assays (CHO) cells in DMSO 1981
Chinese hamster ovary 1670-5000 µg/ml; >98% Negative Natarajan & van
(CHO) cells in DMSO Kesteren-van
Leeuwan, 1981
Chinese hamster ovary 0.01-100 µg/ml >98% Negative Perry & Thomson,
(CHO) cells 1981
Chinese hamster ovary 500-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
In vivo SCE assays Male CBA/J mouse bone 1000 mg/kg; in DMSO >98% Negative Paika et al., 1981
marrow and liver
3.C. Yeast and other Fungal Assays
Mitotic aneuploidy S. cerevisiae D6 500 µg/ml; in DMSO >98% Positive Parry & Sharp, 1981
Chromosome A. nidulans 19.6-78.3 mM; no activation >98% Positive Crebelli et al.,
malsegregation used; in DMSO 1986
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Mitotic gene S. cerevisiae D4 33-333.33 µg/plate; >98% Negative Jagannath et al.,
conversion in DMSO 1981
S. cerevisiae JD1 50 µg/ml; in DMSO >98% Positive Sharp & Parry, 1981a
(without
activation)
S. cerevisiae D7 2000-4000 µg/ml >98% Negative Zimmermann & Scheel,
1981
Mitotic S. cerevisiae T1, T2 1000 µg/ml; in DMSO >98% Negative Kassinova et al.,
crossing-over 1981
A. nidulans 19.6-78.3 mM; no activation >98% Negative Crebelli et al.,
used; in DMSO 1986
Intrachromosomal S. cerevisiae RS112 5-40 mg/ml; no activation used ? Positive Schiestl et al.,
recombination 1989
Differential S. cerevisiae, T5 Not specified; >98% Negative Kassinova et al.,
killing in DMSO 1981
S. cerevisiae 197/2d, rad 300-1000 µg/ml; >98% Positive Sharp & Parry, 1981b
in DMSO (with and without
activation)
3.D. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 100-1000 µg/ml; 99.8% Negative McGlynn-Kreft &
in DMSO McCarthy, 1984
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Cell transformation Syrian hamster embryo 62-1000 µg/ml ? Negative Casto, 1975, 1976
(SHE) cells/adenovirus SA7 in: Heidelberger
et al., 1983
Syrian hamster embryo 1-24 mM ? Inconclusive Hatch et al., 1986
(SHE) cells/adenovirus SA7
Cell transformation C3H/10T 1/2 cells 33 µg/ml; in DMSO 99.8% Negative McLeod & Doolittle,
with "promotion" 1985
3.E. Germ Cell Effects
Spermhead (CBA X BALB/c) F1 mouse 250-2000 mg/kg; >98% Negative Topham, 1981
abnormalities in Tween 80
B6C3F1/CRL mouse 166-2655 mg/kg; >98% Negative Wyrobek et al., 1981
in DMSO
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report
Special studies on the thyroid
Rats
Groups of four randomly selected weanling Caesarian-delivered
Sprague-Dawley male litter-mate rats were administered 0, 75 or 150
ppm ETU (purity not stated). Within each treatment group dosing
periods and control diet periods were varied to examine the
reversibility of compound-related effects. Results suggest some
reversibility of thyroid effects which were related to time on test
and to the severity of effect on the thyroid (Arnold et al.,
1982).
Sprague-Dawley rats (50/sex/group) were fed diets containing 0,
75, 100 or 150 ppm ETU (purity not stated) mixed in corn oil for 7
weeks. Body weights decreased with increasing dose while thyroid
weights (absolute and relative) increased in both sexes. T3 levels
were somewhat variable, while T4 levels were significantly
decreased at 150 pm in both sexes. These effects partially reversed
after 4 weeks on control diets. Histopathological findings included
reduced colloid content of thyroid acini in high dose rats. Acinar
epithelial cell size and height were not different from control.
Two tumours were identified in the high dose male group: a
follicular cell adenoma and medullary carcinoma. The authors
concluded that the relationship between the duration of exposure to
ETU and the possible reversibility of various thyroid lesions
requires further study (Arnold et al., 1983).
A 22-week study was conducted in Sprague-Dawley rats
(55/sex/group) with the following dosing schedule: ETU (97% purity)
administered alone in the diet at levels of 0, 125, 250 or 625 ppm;
or with 0.2 g T3 and 1.6 g T4/rat, orally via gavage; or with
manganese and zinc. Also included were treatment groups dosed with
0, 650 or 1250 ppm mancozeb alone.
Rats receiving 625 ppm ETU alone or in combination with
manganese and zinc were removed from test diet because of alopecia,
weight loss, dermatosis and mortality. Survivors received control
diets for the remainder of the study. Serum decreased in both sexes
at all doses of ETU after 2 weeks of treatment. These levels
returned to normal when ETU was removed from the diet. Serum T3
decreased in both sexes at 625 ppm ETU after 4 weeks of dosing, and
in males at 125 and 250 ppm ETU, but by week 8 returned to normal.
In females at the same doses, T3 was normal until week 16 when it
decreased. The additions of T3/T4 by oral gavage resulted in
decreased T3 at week 8 in males and a decrease during the first 6
weeks in females at all levels. T3 returned to normal one month
after removal of ETU. TSH increased in the ETU group and less
dramatically in ETU plus T3/T4 groups. These levels returned to
normal 2 weeks after ETU was removed form the diet.
Body weights decreased in males and females after 4 weeks at
625 ppm ETU and in males after 8 weeks at 250 ppm ETU. Thyroid to
body-weight ratio increased at > 125 ppm ETU in males. When ETU
was removed from the diet, weights returned to normal. No effect
was observed on pituitary weights. Thyroid hyperplasia was
increased at 125 ppm ETU and above and reversed to normal 6-8 weeks
after ETU was removed. Approximately 1% (13/1300) of the rats
developed hyperplasia of the thyroid (focal areas of basophilic
hyperplastic follicles and follicular adenoma). A dose-related
increase in liver weight was observed at 125 ppm ETU and above for
both sexes.
Exposure to ETU resulted in a decrease in thyroid hormone
(T3/T4) levels and increased serum TSH levels in a dose-related
manner. Although TSH levels were reduced when ETU was supplemented
with T3/T4, the high dosage of ETU was apparently sufficient to
override these effects. The hormone imbalance induced by ETU
correlated with the histologic changes in the thyroid. Withdrawal
of ETU from the diet reversed the hypothyroid conditions induced to
euthyroid (Leber et al. 1978a).
In two 90-day feeding trials Sprague-Dawley rats (12/sex/group)
were given 75 or 100 ppm ETU, and thyroid function, serum T4, T3,
and TSH, T3 uptake in vitro, 131I uptake, and thyroid to body-
weight ratios were measured at days 46 and 91. Additionally, the
fate of the incorporated 131I was traced in thyroid fractions at the
100 ppm level. Groups of both sexes at the lower feeding level and
the females at 100 ppm were functionally euthyroid whereas males at
100 ppm were somewhat hypothyroid despite elevated serum T3, TSH,
and T3/T4 ratios. Results showed that the inhibitory effects of
ETU are similar to those for methimazole. ETU inhibited
monoiodotyrosine (MIT) utilization, and the coupling of
diiodotyrosine (DIT) residues to form T4, resulting in
significantly reduced active synthesis of T3 and T4 prohormones
(males 100 ppm). The capacity of serum to bind T3 was reduced;
however, there was no evidence for inhibition by ETU of T4 to T3
monodeiodination or interference with the normal feedback mechanisms
of thyroid hormones on TSH secretion (O'Neil & Marshall, 1984).
Observations in humans
A 53-year old female employed in the manufacture of products
from synthetic and natural rubber developed itching of the fingers.
Her condition improved on weekends but became worse upon returning
to work. Two months later the eruption spread to her forearms. The
individual showed positive reactions to nickel and cobalt in the
ICDRG standard patch test as well as to a component of the rubber
material from her work which was ETU. Additional patch testing with
ETU and chemically related substances showed that ETU tested
positive at dose levels at or above 0.01% (w/v). One-percent
concentrations of dibutyl-, diethyl- or diphenylthiourea all were
negative as were ethyleneurea and ethylenediaminine. Thiourea at
0.01% (w/v) was also negative. The fungicides zineb and maneb tested
negative and positive, respectively. The positive reaction to maneb
was attributed to the presence of ETU as an impurity/degradation
product and confirmed in studies using thin layer chromatography
(Bruze & Fregert, 1983).
A retrospective study of women who were employed at a rubber
manufacturer using ETU was undertaken to determine any excess of
fetal abnormalities occurring in children to women who had worked
with ETU during early pregnancy. The women were employed on hand and
machine trimming, hand cutting, coolant hose manufacture, packing
and dispatch. Women were also employed in moulding, cutting blanks
for moulding and tool and die trimming where there was a hazard from
inhalation of dust. Women born in 1918 or later who left employment
between 1963-1971 were surveyed. Of 699 women who left between
1963-1971, 255 gave birth to 420 children. Only 59 of 255 were
working at the plant at the time of early pregnancy and none of
these had abnormal children. Of the total 420 children born 11 had
abnormalities. However, figures for the group showed no excess over
the expected number of fetal abnormalities in the region surveyed.
The study did not demonstrate any risk of teratogenesis. However,
the number of women exposed during early pregnancy was small.
A total of 1929 workers engaged in the production or
manufacture of ETU were surveyed retrospectively for thyroid cancer,
and compared with the thyroid cancer list of the Birmingham
(England) Cancer Registry from 1957-1971. No thyroid cancers
occurred in these workers and the results were considered to be
preliminary (Smith, 1976).
An apparent increased incidence of miscarriages among workers
at a USA rubber products factory was investigated. Of 7 pregnancies
only 2 resulted in spontaneous abortions, one with an identified
medical problem. Before starting work at the rubber factory, the 81
women surveyed had 192 pregnancies with 16% spontaneous abortions.
There was no indication that menstrual problems increased with
duration of employment as a moulder. It was concluded that no
adverse reproductive effects could be attributed to the work
environment - although the small numbers available for study
prevented this possibility from being entirely ruled out (Wright et
al., 1981).
No hazard of clinical thyroid depression existed based on
medical evidence collected on workers exposed to ETU at a rubber
company in Michigan. Fifty-one subjects were evaluated (49 males, 2
females). Environmental sampling results demonstrated that workers
were exposed to trace amounts of ETU as airborne dust or through
direct contact of powdered ETU (Salisbury & Lybarger, 1977).
Clinical examinations and thyroid function tests were carried
out over a period of 3 years in the United Kingdom on 8 workers
involved in the manufacture of ETU and 5 workers involved in mixing
of ETU with rubber. The average length of exposure in workers
associated in the manufacturing process was 10 years with a range of
5-20 years. The exposure period for mixers was 3 years. All
subjects were males with an age range from 26-62 years. Matched
controls were also examined. In the manufacturing plant ETU levels
of 330 µg/m3 were recorded on one personal sampler. Background
levels ranged from 10-240 µg/m3. Levels of ETU recorded on
personal samplers of mixers ranged from 120-160 µg/m3. Mixers but
not process workers had significantly lower levels of T4 in their
blood compared to controls. No effects were found on TSH or thyroid
binding globulin. The authors concluded that there was no evidence
that thyroid function is severely affected by exposure to ETU at the
levels experienced by these workers nor was there any evidence of
any effect. However, the T4 results in the exposed workers were
generally lower than those in the control group with most of the
difference in distribution accounted for by the results from the
mixers (Smith, 1984).
The concentration of ETU in pesticide formulations and ambient
air were measured and exposure to maneb (80% powder) or mancozeb
(Ridomil 56% mancozeb) evaluated during the spraying of potato
fields. The mean tank mix concentrations of maneb and mancozeb were
4.0 or 7.0 g/litre, respectively. Spraying time was 0.5-7.0 hours
(mean 4.0 hours). Each single application was separated by several
weeks. Therefore only acute (single day) exposure was determined in
workers (i.e. mixer/loader/applicator).
The overall range of concentrations of ETU in air were between
0.004 and 3.3 µg/m3 in the breathing zone and 0.006 and 0.8 µg/m3
in the (closed) tractor cabin. The mean calculated concentrations
of active maneb and mancozeb in air were 7 and 20 µg/m3,
respectively. The total inhaled amount of ETU and EBDCs was 5.0 and
126.0 µg/day, respectively corresponding to a dose of 0.07 µg ETU/kg
bw and 1.8 µg EBDC/kg bw for applicators and mixer and loaders
weighing 70 kg. Patch samples on clothes and skin (back, chest,
shoulders and forearm) indicated that 1.4, 10.0, 4.0 and 1.2% of ETU
(0.07; 0.19; 0.39; 0.17 mg/cm2/hour) reached the skin respectively.
ETU in urine sample taken on days 1, 8, 15 and 22 ranged between
0.09-2.5; 0.07-1.0; 0.01-0.30 and < 0.01-0.2 µg/mmol creatinine.
Absolute concentrations of ETU in all samples ranged between < 0.2
and 11.8 µg/litre of urine. Under the exposure conditions the
urinary elimination half-life was calculated to be 100 hours
(Kurrtio et al., 1990).
COMMENTS
Following oral administration to mice essentially all of the
ETU was recovered in the excreta within 48 hours; none was recovered
as carbon dioxide. Approximately 50% of the administered dose was
found in urine as unchanged ETU.
After the oral administration of radiolabelled ETU, its
concentration in both pregnant mice and rats peaked about the same
time (1.4 hours), with concentrations in maternal and fetal tissues
similar at 3 hours. The half-life of elimination from mice and rats
was 5.5 hours and 9.4 hours, respectively. Approximately 70% of ETU
was found in urine in both species at 48 hours.
Mice metabolize ETU primarily by the flavin-monooxygenase
system and rats by the P-450 system of enzymes.
ETU is slightly toxic after acute oral administration, with the
LD50 ranging from 545 mg/kg bw in pregnant rats to 4000 mg/kg bw in
adult mice.
In a 13-week study in mice at dietary concentrations of 0, 125,
250, 500, 1000 or 2000 ppm the NOAEL was 250 ppm (equivalent to 38
mg/kg bw/day). Diffuse follicular cell hyperplasia of the thyroid
and hepatocellular cytomegaly were observed at 500 ppm.
In a three-month study in mice at dietary concentrations of 0,
1, 10, 100 or 1000 ppm the NOAEL was 10 ppm, equal to 1.7 mg/kg
bw/day. ETU produced thyroid follicular cell hyperplasia and
decreased colloid density at 100 ppm.
The NOAEL in a study in which rats were fed dietary
concentrations of ETU at 0, 0.63, 1.3, 2.5, 5.0 or 25 ppm for 8
weeks was 25 ppm (equal to 2.6 mg/kg bw/day), the highest dose
tested.
In a 13-week study in rats, ETU was administered in the diet at
concentrations of 0, 60, 125, 250, 500 or 750 ppm. The NOAEL was
less than 60 ppm (equal to 3.0 mg/kg bw/day) based on
histopathological findings of diffuse follicular cell hyperplasia in
the thyroid.
In a 90-day study in rats, ETU was administered in the diet at
concentrations of 0, 1, 5, 25, 125 or 625 ppm. The NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on hyperaemia of the thyroids,
with and without enlargement, increased thyroid to brain weight
ratio, decreased 125I thyroid uptake, decreased triiodothyronine,
decreased thyroxine and increased thyroid follicular cell
hyperplasia at 125 ppm.
In a four-week feeding study in dogs at dietary concentrations
of 0, 200, 980 or 4900 ppm, the NOAEL was 200 ppm, equal to 6.7
mg/kg bw/day. Decreased body-weight gain, decreased thyroxine and
T3 levels and enlarged thyroids were observed at 980 ppm.
In a 13-week feeding study in dogs at dietary concentrations of
0, 10, 150 or 2000 ppm the NOAEL was 10 ppm, equal to 0.39 mg/kg
bw/day. At 150 ppm haemoglobin, packed cell volume, and red blood
cell count were decreased, and cholesterol was increased. Effects
on the thyroid were found only at 2000 ppm.
In a 52-week feeding study in dogs at dietary concentrations of
0, 5, 50, or 500 ppm, the NOAEL was 5 ppm, equal to 0.18 mg/kg
bw/day. At 50 ppm a reduction in body-weight gain, hypertrophy of
the thyroid with colloid retention, a slight increase in thyroid
weight and pigment accumulation in the liver were observed.
Male and female mice received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,330; 0,1000; 330,0; 330,330; 330,1000; 110,330 or 33,100 ppm.
Mice receiving perinatal exposure only (330,0 ppm) showed no effect
on the incidences of neoplasms after 2 years. Cytoplasmic
vacuolization of follicular cells of the thyroid was evident in
males and females at 33,100 ppm, but no increases in neoplasms of
the liver, pituitary or thyroid were observed. T4 values were
significantly decreased in both sexes and thyrotropine was slightly
elevated. Animals receiving 330 ppm during adulthood showed tumours
of either the liver, pituitary or thyroid. Increasing perinatal
exposure from 0 to 330 ppm was associated with an increased
incidence of thyroid and pituitary lesions in female mice receiving
adult exposure to 330 ppm, but there were no enhancing effects of
perinatal exposure in mice receiving adult exposures of 1000 ppm
when compared to adults in the 0,1000 ppm group.
Rats were fed dietary concentrations of ETU at levels of 0, 5,
25, 125, 250 or 500 ppm for 2 years. The NOAEL was 5 ppm,
equivalent to 0.25 mg/kg bw/day. Vascularity and hyperplasia of the
thyroid were seen at 25 ppm.
In a two-year feeding study in rats using dietary
concentrations of 0, 0.5, 2.5, 5 or 125 ppm the NOAEL was 5 ppm
(equal to 0.37 mg/kg bw/day) based on changes in clinical chemistry,
increased triiodothyronine, decreased thyroxine, increased thyroid
weight, increased liver weight and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm.
In a two-year carcinogenicity study in rats using dietary
concentrations of 0, 175 or 350 ppm, thyroid carcinomas and
hyperplastic goitres were observed in both sexes at 175 ppm
(equivalent to 8.8 mg/kg bw/day).
Male and female rats received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,83; 0,250; 90,0; 90,83; 90,250; 30,83 or 9,25 ppm. Rats
receiving perinatal and adult exposure of 9,25 ppm showed no
increase in tumours and no apparent biologically meaningful changes
in thyroid hormone function at two-years when compared to 0,0 ppm
controls. Thyroid hyperplasia was evident in both sexes. At 9
months, animals given 9,25 ppm manifested decreased T3 and T4
values and increased thyrotropine without evidence of thyroid
follicular cell hyperplasia. Males and females receiving a dose of
90,0 ppm showed no hormonal changes and no tumours at 2 years.
Thyroid follicular cell hyperplasia was, however, evident. Animals
receiving adult exposure showed a significant increase in thyroid
follicular cell tumours at 83 and 250 ppm (males) and 250 ppm
(females). Males and females showed no significant differences in
the number of tumours between dose groups of 0,83; 30,83; and 90,83
ppm. Males and females receiving 90,250 ppm showed increases in
thyroid follicular cell tumours when compared to 0,250 ppm. At the
end of 2 years males and females receiving 0,83 or 0,250 manifested
increased numbers of thyroid tumours when compared to 0,0 ppm
controls.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 2.5, 25 or 125 ppm the NOAEL was 2.5 ppm, equal
to a range of 0.16-0.38 mg/kg bw/day, based on thyroid gland
follicular cell hyperplasia and hypertrophy at 25 ppm.
An oral teratogenicity study conducted in rats at dose levels
of 0, 5, 10, 20, 40 or 80 mg/kg bw/day indicated no maternal
toxicity at 40 mg/kg bw/day (NOAEL). Maternal lethality was
observed at 80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity
effects was 5 mg/kg bw/day based on teratogenic effects observed at
10 mg/kg bw/day.
An oral teratogenicity study in rats at dose levels of 0, 15,
25 or 35 mg/kg bw/day was conducted. No maternal toxicity was
observed at 35 mg/kg bw/day (NOAEL). The NOAEL for
embryo/fetotoxicity and teratogenicity was 15 mg/kg bw/day based on
higher incidences of dilated brain ventricles at 25 mg/kg bw/day.
Oral teratogenicity studies in rats (0, 10, 20, 30, 40 or 50
mg/kg bw/day), mice (0, 200, 400 or 800 mg/kg bw/day) and hamsters
(0, 90, 270 or 810 mg/kg bw/day) revealed no maternal toxicity at
the doses tested. The NOAEL for embryo/fetotoxicity in the rat was
10 mg/kg bw/day based on dilation of the lateral or fourth ventricle
at 20 mg/kg bw/day. The NOAEL for embryo/ fetotoxicity in the
hamster was 90 mg/kg bw/day based on a decrease in fetal body weight
at 270 mg/kg bw/day. The NOAEL for mice was greater than 800 mg/kg
bw/day.
In an oral teratogenicity study, rabbits received 0, 5, 10, 20,
40 or 80 mg/kg bw/day of ETU. The NOAEL for maternal toxicity was
80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was 40 mg/kg
bw/day based on an increase in resorption sites, decreased brain
weight and a degeneration of the proximal convoluted tubules in the
kidneys of fetuses at 80 mg/kg bw/day. Malformations were not
observed at the highest dose.
A study with pregnant rats administered ETU, T3/T4 and sodium
iodide in combination indicated a reduction in some of the
teratogenic responses when compared with groups administered ETU
alone. These results indicate that the teratogenic potential of ETU
may in part be secondary to the thyroid toxicity of ETU.
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induces mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic.
An ADI was allocated based upon a NOAEL of 0.39 mg/kg bw/day in
the 13-week study in dogs, since this dose level was between the
NOAEL of 5 ppm (equal to 0.18 mg/kg bw/day) and the middle dose
(effect level) of 50 ppm (equal to 1.8 mg/kg bw/day) in the 52-week
dog study. A 100-fold safety factor was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 10 ppm, equal to 1.7 mg/kg bw/day (3-month study)
Rat: 5 ppm, equal to 0.37 mg/kg bw/day (two-year study)
2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day
(reproduction study)
Dog: 10 ppm, equal to 0.39 mg/kg bw/day (13-week study)
5 ppm, equal to 0.18 mg/kg bw/day (52-week study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw.
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Agrelo, C. & Amos, H. (1981) DNA repair in human fibroblasts. Prog.
Mutation Research, 1: 528-532.
Allen, J.R., Van Miller, J.P. & Seymour, J.L. (1978). Absorption
tissue distribution and excretion of C14-ethylenethiourea by the
rhesus monkey and rat. Res. Comm. Chem. Pathol. Pharmacol. 20(1):
109-115.
Althaus, F.R., Lawrence, S.D., Sattler, G.L., Longfellow, D.G. &
Pitot, H.C. (1982). Chemical quantification of unscheduled DNA
synthesis in cultured hepatocytes as an assay for rapid screening of
potential chemical carcinogens. Cancer Res. 42: 3010-3015.
Arnold, D.L., Bickis, M.G., Nera, E.A., McGuire, P.F. & Munro, I.C.
(1982). Assessing the reversibility of thyroid lesions that result
from feeding etu. Toxicologist, 2(1): Abstract #318.
Arnold, D.L., Krewski, D.R., Junkins, D.B., McGuire, P.F., Moodie,
C.A. & Munro, I.C. (1983). Reversibility of ethylenethiourea-induced
thyroid lesions. Toxicol. Appl. Pharmacol. 67: 264-273.
Austin, G.E. & Moyer, G.H. (1979). Hepatic RNA synthesis in rats
treated with ethylenethiourea. Res. Commun. Chem. Pathol.
Pharmacol. 23(3): 639-642.
Autio, K., von Wright, A. & Pyysalo, H. (1982). The effect of
oxidation of the sulfur atom on the mutagenicity of
ethylenethiourea. Mutation Res. 106: 27-31.
Bridges, B.A., MacGregor, D. & Zeiger, E. (1981). Summary report on
the performance of bacterial mutation assays. Prog. Mutation
Research, 1: 49-67.
Briffaux, J.P. (1991). ETU: 13 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/504 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Briffaux, J.P. (1992). ETU: 52 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/505 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay with preincubation.
Prog. Mutation Research, 1: 261-270.
Bruze, M. & Fregert, S. (1983). Allergic contact dermatitis from
ethylenethiourea. Contact Dermatitis, 9: 208-212.
Carver, J.H., Salazar, E.P., Knize, M.G. & Wandres, D.L. (1981).
Mutation induction at multiple gene loci in Chinese hamster ovary
cells: the genetic activity of 15 coded carcinogens and
noncarcinogens. Prog. Mutation Research, 1: 594-601.
Casto, B.B. (1975, 1976). Progress report NIH-NCI-NOI-CP45615, pp.
1-23, 1975; pp. 1-18 (1976). (Cited in Heidelberger et al., 1983).
Chernoff, N., Kavlock, R.J., Rogers, E.H., Carver, B.D. & Murray, C.
(1979). Perinatal toxicity of maneb ethylenethiourea and
ethylenebisisothiocyanate sulfide in rodents. J. Toxicol. Environ.
Health, 5: 821-834.
Chhabra, R.S., Eustis, S., Haseman, J.K., Kurtz, P.J. & Carlton,
B.D. (1992) Comparative carcinogenicity of ethylenethiourea with or
without perinatal exposure in rats and mice. Fundamental and
Applied Toxicology, 18(3): 405-417.
Crebelli, R., Bellincampi, D., Conti, G., Conti, L., Morpurgo, G. &
Carere, A. (1986). A comparative study on selected chemical
carcinogens for chromosome malsegregation, mitotic crossing-over and
forward mutation induction in Aspergillus nidulans. Mutation
Research, 172: 139-149.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Prog. Mutation Research, 1: 626-637.
Daston, G., Ebron, M.T., Carver, B. & Stefanadis, J.G. (1987) In
vitro teratogenicity of ethylenethiourea in the rat. Teratology,
35: 239-245.
Daston, G.P., Yonker, J.E., Powers, J.F. & Heitmeyer, S.A. (1989).
Difference in teratogenic potency of ethylenethiourea in rats and
mice: relative contribution of embryonic and maternal factors.
Teratology, 40: 555-566.
Decker, C.J. & Doerge, D.R. (1991). Rat hepatic microsomal
metabolism of ethylenethiourea. Contributions of the flavin-
containing monooxygenase and cytochrome P-450 isozymes. Chem. Res.
Toxicol. 4: 482-489.
DiDonato, L.J. & Longacre, S.L. (1987). Ethylenethiourea:
dermal/oral absorbtion study in male rats. Unpublished report No,
85R-0206 from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Doerge, D. & Takazawa, R. (1990). Mechanism of thyroid peroxidase
inhibition by ethylenethiourea. Chem. Res. Toxicol. 3: 98-101.
Dotti, A. (1992). Ethylenethiourea (ETU): two-generation
reproduction study in the rat. Unpublished report No. 252360 from
Research and Consulting Company, Itingen, Switzerland. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978a). A study of the uptake and elimination of
C14 activity after oral ingestion of C14 labelled ethylenethiourea
(ETU) and mancozeb in the rhesus monkey. Revised final report,
Battelle Labs., Columbus, Ohio, USA. (July 31, 1978).
Emmerling, D.C. (1978b). The effects of thyroid hormones on the
teratogenic potential of ethylenethiourea in rats. Unpublished
report No. 78RC-1027 from Battelle Labs, Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Engst, R. & Schnaak, W. (1974). Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev. 52:
45-67.
Evans, E.L. & Mitchell, A.D. (1981). Effects of 20 coded chemicals
on sister chromatid exchange frequencies in cultured chinese hamster
cells. Prog. Mutation Research, 1: 538-550.
Frank, J. & Muller, G. (1988). ETU: In vivo/in vitro UDS/S-phase
assay in mice. Unpublished report No. 88R-47 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Freudenthal, R.I., Kerchner, G., Persing, R. & Baron, R.L. (1977).
Dietary subacute toxicity of ethylenethiourea in the laboratory rat.
J. Environ. Pathol. Toxicol. 1: 147-161.
Galloway, S.M. & Ivett, J.L. (1986). Chemically induced aneuploidy
in mammalian cells in culture. Mutation Research, 167: 89-105.
Gak, J.C., Graillot, C. & Truhaut, R. (1976). Difference in
sensitivity of the hamster and rat to the effects of long term
administration of ethylenethiourea. Eur. J. Toxicol. 9: 303-312.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the "microtiter" fluctuation test. Prog. Mutation Research, 1:
376-386.
Graham, S.L. & Hansen, W.H. (1972). Effects of short-term
administration of ethylenethiourea upon thyroid function of the rat.
Bull. Environ. Contam. Toxicol. 7(1): 19-25.
Graham, S.L., Hansen, W.H., Davis, K.J. & Perry, C.H. (1973).
Effects of one-year administration of ethylenethiourea upon the
thyroid of the rat. J. Agric. Food Chem. 21: 324-329.
Graham, S.L., Davis, K.J.Hansen, W.H. and Graham, C.H. (1975).
Effects of prolonged ethylenethiourea ingestion on the thyroid of
the rat. Food Cosmet. Toxicol. 13: 493-499.
Green, M.H.L. (1981). A differential killing test using an improved
repair-deficient strain of Escherichia coli. Prog. Mutation
Research, 1: 183-194.
Hardin, B., Schuler, R., Burg, J., Booth, G., Hazelden, K.,
MacKenzie, K., Piccirillo, V. & Smith, K. (1987). Evaluation of 60
chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen. 7: 29-48.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putnam, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W. &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagen. 8:
515-531.
Heddle, J.A., Hite, M., Kirkhart, B., Mavournin, K., MacGregor,
J.T., Newell, G.W. & Salamone, M.F. (1983). The induction of
micronuclei as a measure of genotoxicity. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 123: 61-118.
Heidelberger, C., Freeman, A.E., Pienta, R.J., Sivak, A., Bertram,
J.S., Casto, B.C., Dunkel, V.C., Francis, M.W., Kekunaga, T.,
Little, J.B. & Schechtman, L.M. (1983). Cell transformation by
chemical agents - a review and analysis of the literature. US-EPA
Gene-Tox Program. Mutation Research, 114: 283-385.
Hill, R., Erdreich, L., Paynter, O., Roberts, P., Rosenthal, S. &
Wilkinson, C. (1989). Thyroid follicular cell carcinogenesis. Fund.
Appl. Toxicol. 12: 629-697.
Hui, Q.Y., Armstrong, C., Laver, G. & Iverson, F. (1988).
Monooxygenase-mediated metabolism and binding of ethylenethiourea to
mouse liver microsomal protein. Tox. Letters. 41: 231-237.
Ichinotsubo, D., Mower, H. & Mandel, M. (1981a). Testing of a series
of paired compounds (carcinogen and noncarcinogen structural analog)
by DNA repair-deficient E. coli stains. Prog. Mutation Research,
1: 195-198.
Iverson, F., Khera, K.S. & Hierlihy, S.L. (1980). In vivo and in
vitro metabolism of ethylenethiourea in the rat and the cat.
Toxicol. Appl. Pharmacol. 52: 16-21.
Jagannath, D.R., Vultaggio, D.M. & Brusick, D.J. (1981). Genetic
activity of 42 coded compounds in the mitotic gene conversion assay
using Saccharomyces cerevisiae strain D4. Prog. Mutation
Research, 1: 456-467.
Jordan, L.W. & Neal, R.A. (1979). Examination of the in vivo
metabolism of maneb and zineb to ethylenethiourea (ETU) in mice.
Bull. Environm. Contam. Toxicol. 22: 271-277.
Jotz, M.M. & Mitchell, A.D. (1981). Effects of 20 coded chemicals on
the forward mutation frequency at the thymidine kinase locus in
L5178Y mouse lymphoma cells. Prog. Mutation Research, 1: 580-593.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Prog. Mutation Research, 1: 175-182.
Kassinova, G.V., Kovaltsova, S.V, Marfin, S.V. & Zakharov, I.A.
(1981). Activity of 42 coded compounds in differential inhibition
and mitotic crossingover assays in yeast. Prog. Mutation Research,
1: 434-455.
Kato, Y., Odanaka, Y., Teramoto, S. & Matano, O. (1976). Metabolic
fate of ethylenethiourea in pregnant rats. Bull. Environ. Contam.
Toxicol. 16: 546-555.
Khera, K. (1973a). Teratogenic effects of ethylenethiourea in rats
and rabbits, Toxicol. Appl. Pharmacol. 25: 455-456.
Khera, K.S. (1973b) Ethylenethiourea: teratogenicity study in rats
and rabbits. Teratology, 7: 243-252.
Khera, K.S. & Iverson, F. (1978). Toxicity of ethylenethiourea in
pregnant cats. Teratology, 18: 311-314.
Khera, K. (1987). Ethylenethiourea: a review of teratogenicity and
distribution studies and an assessment of reproduction risk. CRC
Crit. Rev. Toxicol. 18: 129-139.
Khera, K.S, Whalen, C. & Iverson, F. (1983). Effects of pretreatment
with SKF-525A, N-methyl-2-thioimidazole, sodium phenobarbital, or 3-
methylcholanthrene on ethylenethiourea-induced teratogenicity in
hamsters. J. Toxicol. and Environ. Health. 11: 287-300.
Kier, L.E., Brusick, D.J., Auletta, A.E., Von Halle, E.S., Brown,
M.M., Simmon, V.F., Dunkel, V., McCann, J., Mortelamans, K., Prival,
M., Rao, T.K. & Ray, V. (1986). The Salmonella
typhimurium/mammalian microsomal assay. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 168: 69-240.
Kirkhart, B. (1981). Micronucleus test on 21 compounds. Prog.
Mutation Research, 1: 698-704.
Kurrtio, P., Savolainen, K., Tuominen, R., Kosma, V.M., Naukkarinen,
A., Mannisto, P. & Collan, Y. (1986). Ethylenethiourea and nabam
induced alterations of function and morphology of thyroid glands in
rat. Arch. Toxicol., Suppl. 9: 339-344.
Kurrtio, P., Vartianinen, T. & Savolainen, K. (1990). Environmental
and biological monitoring of exposure to ethylenebisdithiocarbamate
fungicides and ethylenethiourea. British Journal of Industrial
Medicine, 47: 203-206.
Kurttio, P., Savolainen, K., Naukkarinen, A., Kosma, V., Tuomisto,
L., Penttila, I. & Jolkkonen, J. (1991). Urinary excretion of
ethylenethiourea and kidney morphology in rats after continuous oral
exposure to nabam or ethylenethiourea. Arch. Toxicol. 65: 381-385.
Leber, A.P., Wilkinson, G.E., Emmerling, D., Persing, R.L. & Thake,
D.C. (1978a). A correlation of the hormonal and pathological changes
of the thyroid as related to the treatment and withdrawal of
ethylenethiourea. Unpublished report No. 78RC-1026 from Battelle
Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA.
Leber, A.P., Wilkinson, G.E., Persing, R.L. & Holzworth, D.A.
(1978b) Effects of feeding ethylenethiourea in the rhesus monkey.
Contract No. 68-01-4717 from Battelle Labs., Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Legator, M.S., Bueding, E., Batzinger, R., Connor, T.H.,
Eisendstadt, E., Farrow, M.G., Ficsor, G., Hsie, A., Seed, J. &
Stafford, R.S. (1982). An evaluation of the host-mediated assay and
body fluid analysis. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Research, 98: 319-374.
Leuschner, F. (1977) Oral toxicity of ethylenethiourea 98% - called
for short ETU-during 8 weeks of administration in Sprague-Dawley
rats. Unpublished report No. 77RC-1159 from Laboratorium fur
Pharmakocopie und Toxicologie, Hamburg, West Germany. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lewerenz, H.J. & Plass, R. (1984). Contrasting effects of
ethylenethiourea on hepatic monooxygenase in rats and mice. Arch.
Toxicology, 56: 92-95.
Loprieno, N. (1981). Screening of coded carcinogenic/noncarcinogenic
chemicals by a forward-mutation system with yeast
Schizosaccharomyces pombe. Prog. Mutation Research 1: 424-433.
Lu, M.H. & Staples, R.E. (1978). Teratogenicity of ethylenethiourea
and thyroid function in the rat. Teratology, 17: 171-178.
Marshall, W. (1977). Thermal decomposition of
ethylenebisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. Agric. Food Chem. 25: 357-361.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Prog. Mutation Research, 1: 533-537.
Mason, J.M., Valencia, R. & Zimmering, S. (1992). Chemical
mutagenesis testing in drosophila: VIII. reexamination of
equivocal results. Environ. Mol. Mutagen. 19: 227-234.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb, and their related compounds. Int. Arch. Occup. Environ.
Hlth. 37: 169-178.
McGlynn-Kreft, A.M. & McCarthy, K.L. (1984). Ethylenethiourea
mammalian cell transformation test. Unpublished report No. 84R-56
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
McGregor, D., Brown, A., Cattanach, P., Edwards, I., McBride, D,
Riach, C. & Caspary, W.J. (1988). Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded chemicals.
Environ. Mol. Mutagen. 12: 85-154.
McLeod, P.L. & Doolittle, D.J. (1985). Ethylenethiourea mammalian
cell transformation test for promotion. Unpublished report No. 84R-
0298 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Mehta, R.D. & von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
C14. Prog. Mutation Research, 1: 414-423.
Mohn, G.R., Vogels-Bouter, S. & van der Horst-van der Zon, J.
(1981). Studies on the mutagenic activity of 20 coded compounds in
liquid tests using the multipurpose strain Escherichia coli K-
12/343/113 and derivatives. Prog. Mutation Research, 1: 396-423.
Mollett, P. (1975). Toxicity and mutagenicity of ethylenethiourea
(ETU) in drosophila. Mutat. Res. 29: 254.
Morgan, C. (1991). ETU: 4 week oral (dietary administration)
feasibility study in the beagle. Unpublished report No. 6152-616/6
from Hazleton Labs., Harrogate, North Yorkshire, England. Submitted
to WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986). Salmonella mutagenicity testing:II. Results
from the testing of 270 chemicals. Environ. Muta. 8(7): 1-119.
Natarajan, A.T. & van Kesteren-van Leeuwen, A.C. (1981). Mutagenic
activity of 20 coded compounds in chromosome aberrations/sister
chromatid exchanges assay using chinese hamster ovary (CHO) cells.
Prog. Mutation Research, 1: 551-559.
National Toxicology Program (NTP), 1992. Toxicology and
carcinogenesis studies of ethylenethiourea in F344/N rats and B6C3F1
mice (feed studies). U.S. Department of Health and Human Services,
Public Health Service, National Institutes of Health, NIH
Publication No. 92-2843, National Toxicology Program Technical
Report Series No. 388, March 1992.
Newsome, W.H. (1974). The excretion of ethylenethiourea by rat and
guinea-pig. Bull. Environ. Contamin. Toxicol. 11(2): 174-176.
National Institute for Occupational Safety and Health (NIOSH), 1981.
Ethylenethiourea, Health Hazard Evaluation Report No HETA-81-270-
1012, December 1981.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 8OR-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neil, W. & Marshal, W. (1984). Goitrogenic effects of
ethylenethiourea on rat thyroid. Pesticide Biochemistry and
Physiology, 21: 92-101.
Paika, I.J., Beauchesne, M.T., Randall, M., Schreck, R.R. & Latt,
S.A. (1981). In vivo sce analysis of 20 coded compounds. Prog.
Mutation Research, 1: 673-681.
Parry, J. (1986). The expression of recessive markers in presumptive
monosomic colonies derived from Saccharomyces cerevisiae, strain
D6. Mutagenesis, 1: 299-300.
Parry. J.M. & Sharp, D.C. (1981). Induction of mitotic aneuploidy in
the yeast strain D6 by 42 coded compounds. Prog. Mutation Research,
1: 468-480.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of the sister
chromatid exchange method in mammalian cells as a screening system
for carcinogens. Prog. Mutation Research, 1: 560-569.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980a). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in rats. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980b). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in mice. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Chin, A.E., Carlton, B.D., Chrisp, C.E.,
and Dill, G.S. (1982). Report on the maximum neonatal dose studies
with ethylenethiourea. Contract No. N01-ES8-2151. Report submitted
by Battelle Laboratories, Columbus, Ohio to NIEHS, January 29, 1982.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Pilinskaya, M. (1982) Analysis of the cytogenetic activity of
ethylenethiourea (ETU) in human peripheral lymphoblast culture in
vitro. Dopov Akad Nauk Ukr. RSR. Ser. B: Geol, Khim Biol Nauki,
10: 66-68
Poulsen, L.L., Hyslop, R.M., Ziegler, D.M. (1979). S-Oxygenation of
N-substituted thioureas catalyzed by pig liver microsomal FAD-
containing monooxygenase. Arch. Biochim. Biophys., 198(1): 78-88.
Quillardet, P., de Bellecombe, C. & Hofnung, M. (1985). The SOS
Chromotest, a colorimetric bacterial assay for genotoxins:
validation study with 83 compounds. Mutation Research, 147: 79-95.
Rashid, K.A. & Mumma, R.O. (1986). Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21(4):
319-334.
Resnick, M.A., Mayer, V.W., and Zimmermann, F.K. (1986). The
detection of chemically induced aneuploidy in Saccharomyces
cerevisiae: an assessment of mitotic and meiotic systems.
Mutation Research, 167: 47-60.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 314-322.
Robinson, D.E. & Mitchell, A.D. (1981). Unscheduled DNA synthesis
response of human fibroblasts, WI-38 cells, to 20 coded chemicals.
Prog. Mutation Research, 1: 517-527.
Rosencranz, H.S., Hyman, J. & Leifer, Z. (1981). DNA polymerase
deficient assay. Prog. Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenicity of carcinogens and
noncarcinogens in the Salmonella/microsome test. Prog. Mutation
Research, 1: 323-332.
Ruddick, J.A. & Khera, K.S. (1975). Pattern of anomalies following a
single oral dose of ethylenethiourea to pregnant rats. Teratology,
12: 277-281.
Ruddick, J.A., Williams, D.T., Hierlihy, L. & Khera, K.S. (1976).
C14-ethylenethiourea: distribution, excretion and metabolism in
pregnant rats. Teratology, 13: 35-40.
Ruddick, J.A., Newsome, W.H. & Iverson, F. (1977). A comparison of
the distribution, metabolism and excretion of ethylenethiourea in
the pregnant mouse and rat. Teratology, 16: 159-162.
Saillenfait, A.M., Sabate, J.P., Longonne, I. & De Ceaurriz, J.
(1991). Difference in the developmental toxicity of ethylene
thiourea and three N,N'-substituted thiourea derivatives in rats.
Fundamental and Applied Toxicology, 17: 686-697.
Salamone, M.F. (1981). Mutagenic activity of 41 compounds in the in
vivo micronucleus assay. Prog. Mutation Research, 1: 686-697.
Salisbury, S.A. & Lybarger, J. (1977). Health Hazard Evaluation
Determination Report No. 77-67-499, St. Clair Rubber Co,.,
Marysville, Michigan. Ethylenethiourea. US-DHEW. October 1977.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Savolainen, K. & Pyysalo, H. (1979). Identification of the main
metabolite of ethylenethiourea in mice. J. Agric. Food Chem. 27:
1177-1181.
Saxton, A.D. (1972). A C14-ethylenethiourea rat-feeding study. An
experiment to determine the excretion pattern and accumulation and
decline in thyroid tissues of C14-residues. Report 23-51 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Schiestl, R., Gietz, R.D., Mehta, R. & Hastings, P. (1989).
Carcinogens induce intrachromosomal recombination in yeast.
Carcinogenesis. 10: 1445-1455.
Schmid, H., Tennekes, H., Janiak, T., Probst, D., Leutkemeier, H.,
Pappritz, G., Marki, U., Vogel, O., Heusner, W. (1992).
Ethylenethiourea 104 week chronic toxicity (feeding) study in rats.
Unpublished study No. 256803 from Research and Consulting Company,
Itingen, Switzerland. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Schupbach, M. & Hummler, H. (1977). A comparative study of the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutation Research, 56: 111-120.
Seiler, J.P. (1974). Ethylenethiourea (ETU), a carcinogenic and
mutagenic metabolite of ethylene-bis-dithiocarbamate. Mutation
Research, 2: 189-191.
Seiler, J.P. (1975). In vivo mutagenic interaction of nitrite and
ethylenethiourea. Experientia, 31: 214-215.
Seiler, J.P. (1977a). Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short term test. Mutation Research, 46: 305-
310.
Seiler, J. (1977b). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutation
Research, 48: 225-236.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1. Prog.
Mutation Research, 1: 491-501.
Sharp, D.C. and Parry, J.M. (1981b). Use of repair-deficient strains
of yeast to assay the activity of 40 coded compounds. Prog.
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., Kato, K., Lienard, F., Tezuka, H.,
Teramoto, S. & Kada,T. (1977). Mutagenicity screening on pesticides
and modification products: a basis of carcinogenicity evaluation.
Cold Spring Harbor Conference Cell Proliferation, 4: 267-285.
Simmon, V.F. & Shepherd, G. F. (1981). Mutagenic activity of 42
coded compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 333-342.
Smith, D.M. (1976). Ethylenethiourea - a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.
26: 92-94.
Smith, D.M. (1984). Ethylenethiourea: thyroid function in two groups
of exposed workers. Brit. J. of Ind. Med. 41: 362-366.
Sram, R.J. (1975). Genetic risk from chemicals: mutagenicity studies
and evaluation. Review of Czechoslovak Medicine, 21(4): 186-193.
Stula, E.F. & Krauss, W.C. (1977). Embryotoxicity in rats and
rabbits from cutaneous application of amide-type solvents and
substituted ureas. Toxicol. Appl. Pharmacol. 41: 35.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Prog. Mutation Research, 1: 638-646.
Teramoto, S., Moriya, M., Kato, K., Tezuka, H., Nakamura, S.,
Shingu, A. & Shirasu, Y. (1977). Mutagenicity testing on
ethylenethiourea. Mutation Research, 56: 121-129.
Teramoto S., Shingu, A., Kaneda, M. & Saito, R. (1978a).
Teratogenicity studies with ethylenethiourea in rats, mice and
hamsters. Congenital Anomalies, 18: 11.
Teramoto, S., Shingu, A. & Shirasu, Y. (1978b). Induction of
dominant lethal mutations after administration of ethylenethiourea
in combination with nitrite or of N-nitro-ethylenethiourea in mice.
Mutation Research, 55: 335-340.
Teshima, R., Nagamatusu, K., Kido, Y. & Terao, T. (1981).
Absorption, distribution, excretion and metabolism of
ethylenethiourea in guinea-pigs. Eisei Kagaku, 27(2): 85-90.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Prog. Mutation Research, 1: 224-235.
Tophan, J.C. (1981). Evaluation of some chemicals by the sperm
morphology assay. Prog. Mutation Research, 1: 718-720.
Trueman, R..W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Prog. Mutation Research, 1:
343-350.
Truhaut, R., Fujita, M., Lich, N. & Chaigueau, M. (1973). Study of
metabolic transformations of zineb. C.R. Acad. Sci. Paris, Ser. D.
276: 229-233.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Prog. Mutation Research, 1: 705-711.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains WP2, WP67
(uvrA polA), and CM871 (uvA lexA recA). Prog. Mutation Research,
1: 199-209.
Ulland, B.M., Weisburgr, J.H., Weisburger, E.K., Rice, J.M. &
Cypher, R. (1972). Thyroid cancer in rats from ethylenethiourea
intake. J. Nat. Cancer Inst. 49: 583-584.
US EPA (1992) Ethylenebisdithiocarbamates (EBDCs); notice of intent
to cancel and conclusion of special review. Federal Register, 57:
7484-7530.
Valencia, R. & Houtchens, K. (1981). Mutagenic activity of 10 coded
compounds in the Drosophila sex-linked recessive lethal test.
Prog. Mutation Research, 1: 651-659.
Van't Hof, J. & Schairer, L.A. (1982). Tradescantia assay system for
gaseous mutagens. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Res. 99: 303-315.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985).
Chemical mutagenesis testing in drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutagenesis, 7: 677-702.
Wright, W.E., Peters, J.M., Plyler, E.A. & Belanger, P.L. (1981).
NIOSH Health Hazard Evaluation Report HETA 81-270-1012. December,
1981.
Wyrobek, A., Gordon, L. & Watchmaker, G. (1981). Effect of 17
chemical agents including 6 carcinogen/noncarcinogen pairs of sperm
shape abnormalities in mice. Prog. Mutation Research, 1: 712-717.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Prog. Mutation Research, 1: 481-490.
Rats
Male Sprague-Dawley rats were pre-treated with phenobarbital,
dexamethasone, beta-napthoflavone or left untreated. The in vitro
effect of ETU or EU in the presence and absence of glutathione,
NADPH and heat inactivated microsomes on P450 enzymatic activity and
on covalent binding of ETU to microsomal proteins was studied. ETU
inhibited P450 activity in pretreated and non-pretreated rats.
Inhibition was NADPH-dependent and was abolished by glutathione
(GSH). Covalent binding of 14C-ETU to microsomal protein was also
NADPH-dependent. Binding was inhibited by co-incubation with GSH.
Heat treatment of microsomes and P450 inactivation studies indicated
a prominent role of FMO in covalent binding. Addition of GSH or
dithiothreitol after incubation of microsomes resulted in release of
bound ETU. Metabolism of ETU in the presence of GSH resulted in the
formation of GSH-ETU adducts and subsequent disulfide exchange. The
results suggest that reactive metabolites from ETU generated by
either FMO or P450 are trapped by GSH. Initial oxidation of ETU to
imidazoline-2-sulfenic acid, primarily by FMO, followed by reaction
with GSH or protein sulfhydroyls under conditions of GSH depletion,
has been proposed as the route of monooxygenase-mediated metabolism
of ETU (Decker & Doerge, 1991).
Male Sprague-Dawley rats were divided into 3 groups in a study
designed to study the effect of ETU on RNA synthesis. One group
that had been fasted for 16 hours received single i.p. injections of
2.5 or 250 mg/kg bw ETU or 5.0 mg/kg bw thioacetamide, followed by
3H-orotic acid 60 minutes later. Control animals received dimethyl
sulfoxide alone. A second group received 5.0 or 250 mg/kg bw/day
ETU by gavage on 3 successive days followed by administration with
3H-orotic acid; the animals were killed on the fourth day. The
third group was administered 5.0 or 250 ppm ETU in the diet for 3
weeks. At the end of 3 weeks, a 1-hour pulse dose of 3H-orotic
acid was administered and the animals killed. Serum T4 levels in
animals given ETU were then determined by radioimmunoassay. Rats
receiving 400 ppm of acetylaminofluorene in the diet served as
positive controls.
The livers of all animals given either ETU or thioacetamide
were histologically normal at the time of sacrifice. The livers of
rats fed acetylaminofluorene showed mild hydropic change of
hepatocytes and minimal bile duct proliferation. ETU failed to
inhibit nuclear or cytoplasmic RNA synthesis under the test
conditions. However, thioacetamide and acetylaminofluorene both
reduced the incorporation of 3H-orotic acid into nuclear and
cytoplasmic RNA (Austin & Moyer, 1979).
Pigs
FMO purified from hog liver catalyzes NADPH and oxygen-
dependent sequential S-oxidation of ETU, proceeding through an
intermediate imidazolinyl sulfenic acid to the corresponding
sulfinic acid. Further oxidation to the sulfonic acid was partly
enzymic and partly due to autooxidation. The FMO-oxidative pathway
predominated over P-450 pathways in hog and hamster liver microsomes
(Poulsen et al., 1979).
The mechanism of thyroid peroxidase inhibition by ETU was
studied in vitro using purified thyroid peroxidase obtained from
hog thyroid. ETU inhibited iodination reactions catalysed by thyroid
peroxidase. Inhibition occurred only in the presence of iodide ion
and proceeded with concomitant oxidative metabolism of ETU to
imidazoline and bisulfite ion. The inhibition ceased upon
consumption of ETU, with no loss of enzymatic activity and
negligible covalent binding of ETU to the enzyme. This reversible
thyroid peroxidase inhibition contrasts with the activity of the
therapeutic antithyroid drugs such as methimazole which act as
suicide inhibitors via covalent binding to the prosthetic heme group
(Doerge & Takazawa, 1990).
Toxicological studies
Acute toxicity studies
ETU is slightly toxic after oral administration to mammalian
species with measured LD50 values ranging from 545 mg/kg bw in
pregnant rats (Teramoto, 1978) to 4000 mg/kg bw in adult mice
(Lewerenz & Plass, 1984). The acute toxicity of ETU in various
animal species is given in Table 1.
Guinea-pigs
ETU (purity not stated) is a moderate to weak sensitizer in the
Hartley strain female guinea-pig by the guinea-pig maximization
test. With induction concentrations of 5% (intradermal) or 25%
(topical) and challenge concentrations of 2% or 0.5% (topical),
females responded positively at 24 hours (1/10 at 0.5%; 7/10 at 2%)
but not at the 48-hour reading (0/10 at 0.5%; 0/10 at 2%). In the
same studies, cross sensitization responses were also seen with
maneb, mancozeb and zineb after induction with ETU. Responses
ranged from 0-40% (4/10) at 24 hours and from 0-20% (2/10) at 48
hours. Induction with ETU followed by challenge with maneb
generally gave a slightly higher overall response (10-40%) than did
induction by maneb followed by challenge with ETU (0-20%)
(Matsushita et al., 1976).
Table 1. Acute toxicity of ETU
Species Sex Route LD50 (mg/kg bw) References
Mice M&F oral 4000 Lewerenz & Plass, 1984
F oral > 3000 Teramoto et al., 1978b
F (9 days pregnant) oral > 3000 Khera, 1987
M&F oral ca 2400 Peters et al., 1980b
Rats M&F oral ca 2400 Peters et al., 1980a
M oral 1832 Graham & Hansen, 1972
M&F oral 940 Lewerenz & Plass, 1984
F (13 days pregnant) oral 600 Khera, 1987
F oral 545 Teramoto et al., 1987b
Hamsters F oral > 3000 Teramoto et al., 1987b
F (11 days pregnant) oral > 2400 Khera, 1987
Short-term toxicity studies
Mice
B6C3F1 mice (10/sex/dose), 8 to 9 weeks of age were fed
diets containing 0, 125, 250, 500, 1000, or 2000 ppm ETU (97-99%
purity), equivalent to 0, 19, 38, 75, 150 or 300 mg/kg bw/day for 13
weeks. Deaths occurred at lower doses but were not dose- or
compound-related. Body-weight gain and food consumption were
comparable to controls. Diffuse follicular cell hyperplasia of the
thyroid occurred at 500 ppm in both sexes (greater than 70%) and was
statistically significant and dose-related. No effects were observed
at lower doses. Hepatocellular cytomegaly was also observed at 500
ppm (4/10 females, 10/10 males) and above. Effects were
statistically significant and dose-related. The NOAEL in this study
was 250 ppm, equivalent to 38 mg/kg bw/day (NTP, 1992).
Groups of Charles River CD-1 mice (15/sex/dose) were
administered ETU (100% purity) in the diet at levels of 0, 1, 10,
100 or 1000 ppm for 3 months, equal to 0, 0.16, 1.7, 18 or 168 mg/kg
bw/day for males and 0, 0.22, 2.4, 24 or 230 mg/kg bw/day for
females. There were no compound-related effects on food
consumption, body weight, haematology or clinical chemistry
parameters. Mixed function oxidase activity was increased in both
sexes at 1000 ppm, but only statistically significant in males
(aniline hydroxylase, p-nitroanisole, o-demethylase). Absolute and
relative thyroid weights were increased statistically in both sexes
at 1000 ppm. Absolute and relative liver weights were significantly
increased in males at 1000 ppm; relative liver weights were
significantly increased in females at 100 and 1000 ppm ETU.
ETU produced thyroid follicular cell hyperplasia and decreased
colloid density in both sexes at > 100 ppm, with increased
follicular epithelial cytoplasmic vacuolation and interstitial
congestion in both sexes at 1000 ppm. In the liver, ETU produced
centrilobular hypertrophy, nuclear pleomorphism and increased
intranuclear inclusions in both sexes at 1000 ppm. The pigment was
believed to be similar to lipofuscin. The NOAEL was 10 ppm ETU,
equal to 1.7 and 2.4 mg/kg bw/day in males and females, respectively
(O'Hara & DiDonato, 1985).
Rats
Adult male Han:Wistar rats (6/dose group) were given ETU (>
98% pure) in drinking-water for 28 days. Drinking-water
concentrations of ETU were 0, 100, 200 or 300 mg/litre, equal to
mean daily doses of 0, 11, 18 or 23 mg/kg bw.
ETU decreased body-weight gain during the exposure. Studies of
kidney function and morphology indicated that the kidney is not a
highly sensitive target for ETU-induced toxicity. ETU did not have
a permanent physiologically significant effect on urinary sodium,
potassium, uric acid, protein or glucose excretion, or urinary
osmolality. A slight increase in urinary arginine vasopressin (AVP)
excretion was observed in ETU-treated animals on day 28. No
prominent light microscopical changes were observed in the kidneys
of ETU-exposed rats. However, at 300 mg/litre ETU induced clear
ultrastructural changes in the epithelium of renal proximal tubuli.
An increased number of lysosomes and myelin figures as well as
vacuolization and edema were observed in the cytoplasm of the
epithelial cells of proximal tubules. The proportion of the dose of
ETU excreted as ETU in urine increased with increasing dose of ETU
and were 25%, 36% and 49% (Kurrtio et al., 1991).
Using an identical protocol as above, a study was conducted to
determine the effect of ETU on thyroid gland function and
morphology. Drinking-water concentrations of ETU were 0, 100, 200
or 300 mg/litre, equal to mean daily doses of 0, 11, 18 or 23 mg/kg
bw. Blood samples for T3, T4 and TSH were taken and the levels
measured using radio immunoassay methods. Thyroid glands were
extirpated and processed for light and electron microscopy. ETU
statistically significantly decreased T4 levels at all doses while
statistically increasing TSH levels at all doses. T3 levels were
also decreased in a dose response manner but values were not
statistically significant. There were no ETU-induced morphological
changes observed under light microscopy. Conspicuous ultra
structural changes were caused by ETU since a few areas with totally
destroyed epithelial cells could be found. It was also reported that
nerves and capillaries might have been affected by ETU (Kurrtio et
al., 1986).
Sprague-Dawley rats (10/sex/group) received 0, 0.63, 1.3, 2.5,
5.0, or 25 ppm of 98% pure ETU in the diet for 8 weeks. Twenty-four
hours after the last feeding, all animals received 5 µCi of 131I
intraperitoneally. There were no treatment-related effects on
behaviour, appearance, food intake, organ or body weight or
macroscopic appearance of organs other than the thyroid. ETU had no
clinical chemistry effects at the three low doses. However at 5 and
25 ppm slight increases were observed in males and females with
respect to 131I uptake, protein bound 131I and serum thyroxine. T3
uptake power was slightly decreased. Histopathology of the thyroid
in treated animals was comparable to control group at all dose
levels. The NOAEL in this study was 25 ppm, the highest dose
tested, equal to 2.6 mg/kg bw/day (Leuschner, 1977).
F344/N rats 8 to 9 weeks of age (10/sex/group), were fed diets
containing 0, 60, 125, 250, 500 or 750 ppm of 99% pure ETU for 13
weeks. All animals survived. Final mean body weights for males were
decreased at 500 and 750 ppm by 10% and 30%, respectively. Food
consumption at the same dose levels were decreased 16% and 24%.
Final body weights and food consumption of females were decreased at
750 ppm by 30% and 25%, respectively. Females receiving 60 to 500
ppm showed a uniform 10% body weight decrease accompanied by food
consumption decreases of 13% at 250 and 500 ppm. Histopathology was
present for the thyroid and pituitary gland of both males and
females. Diffuse follicular cell hyperplasia of the thyroid was
present in all animals of both sexes at all doses. In males, focal
follicular cell hyperplasia and cellular vacuolization of the pars
distallis of the pituitary gland was statistically significantly
increased at 250 ppm. Follicular cell adenomas (3/10) were evident
at 250 ppm and statistically significant at 750 ppm. Centrilobular
cytomegaly was observed only at 750 ppm and was statistically
significant. In females, follicular cell hyperplasia (4/10) and
cellular vacuolization of the pars distallis of the pituitary gland
(10/10) were statistically significant only at 750 ppm. Follicular
cell adenomas were observed at 500 and 750 ppm (3/10) but were not
statistically significant. Centrilobular cytomegaly of the liver
was seen only at the high dose and in all animals. The NOAEL in
this study was less than 60 ppm, equal to 3.0 mg/kg bw/day for males
and 4.3 mg/kg bw/day for females, based on histopathological
findings of diffuse follicular cell hyperplasia in the thyroid (NTP,
1992).
In a 90-day study, Sprague-Dawley derived rats (60/sex/dose)
were fed ETU (96.8% pure) at 1, 5, 25, 125 or 625 ppm. Controls
(72/sex) received powdered diet with 1% corn oil. At 30-day
intervals (i.e. 30, 60, 90 days) ten rats from each test group were
sacrificed and serum T3, T4, TBG and TSH concentrations were
measured. The free thyroxine index (FTI) was also calculated. The
remaining rats (10/sex/dose/time) were used to determine 125I uptake
by the thyroid. Rats receiving 625 ppm ETU showed high mortality
and marked decrease in body-weight gain. Clinical signs were
observed at the high dose by day 8 and consisted of excessive,
salivation, loss of hair, rough and bristly hair coat and scaly skin
texture. Necropsy revealed hyperaemia of the thyroids with and
without enlargement at 125 and 625 ppm for all time intervals.
Liver congestion was also evident with dose and time. Liver changes
were distinguishable microscopically and appeared to be compound-
related but not dose-related. Thyroid to brain weight ratio was
significantly increased at 125 and 625 ppm at all time periods.
125I uptake in the thyroid was statistically significantly decreased
along with TBG, T3 and T4 values at 125 and 625 ppm. At 25 ppm,
T4 was statistically significantly decreased only at 60 days. FTI
was comparable to controls. Altered thyroid function and increased
thyroid follicular cell hyperplasia were evident at 125 and 625 ppm.
The NOAEL was 25 ppm, equal to 1.7 and 1.9 mg/kg bw/day in males and
females, respectively (Freudenthal et al., 1977).
Osborne-Mendel rats (20 males/group) were fed ETU (purity not
stated) in the diet at levels of 0, 50, 100, 500 or 750 ppm for 30,
60, 90 or 120 days. 131I activity was determined at 4 and 24 hours
post-injection (5 µCi) in 20 rats from each group at each sacrifice
period.
Body weight was decreased at > 500 ppm throughout the study.
Food consumption was reduced at 30 and 90 days at > 100 ppm and
at 60 and 120 days at > 500 ppm. Relative thyroid weights were
increased at 30 days at > 100 ppm, at 90 days at 500 ppm and at
60/120 days at > 50 ppm.
Four hours after the injection of 131I, the uptake had
decreased significantly in rats fed ETU at 500 and 750 ppm at all
feeding periods. The uptake of iodine 24 hours after injection was
decreased significantly in those animals fed ETU at 100, 500 and 750
ppm. After the 90-day feeding period, the uptake decreased
significantly in rats fed the 500 and 750 ppm levels and ranged from
6 to 13 times lower than control values.
Histologically there were no differences between the control
and 50 ppm groups. At 100 ppm there was slight hyperplasia evident
in the thyroid gland. At 500 ppm there was moderate to marked
hyperplasia, lack of colloid and heightened epithelial walls. There
was an increase in vascularization, demonstrating a response to
increased blood level TSH. At > 500 ppm, an increased incidence
of follicular adenomas was reported. One mechanism by which ETU
acts on the thyroid is via inhibition of iodide peroxidase, which
oxidizes iodide to iodine (Graham & Hansen, 1972).
Dogs
Beagle dogs (2/sex/group) received dietary concentrations of 0,
200, 980, or 4900 ppm of ETU (98% pure) for 4 weeks. Body-weight
gains and food consumption for males were comparable to controls.
Intermediate- and high-dose females gained less weight than the
controls particularly at the high dose. Haematology results were
not remarkable. T3 levels were decreased in high-dose males and
females as well as mid-dose females. T4 levels were decreased in
the mid- and high-dose males and females. Reductions were dose-
related. Enlarged thyroids were noted in all animals of the
intermediate- and high-dose groups. The NOAEL was 200 ppm, equal to
6.7-7.4 mg/kg bw/day for males and 7.4-8.5 mg/kg bw/day for females
(Morgan, 1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
10, 150 or 2000 ppm of ETU (98% pure with doses corrected to 100%
active ingredient) for 13 weeks. Two males in the high dose were
sacrificed in a moribund state with morbidity attributed to compound
administration. All other animals survived to termination.
Clinical signs in the high-dose male survivors appeared to be
unremarkable. All high-dose females showed decreased activity or
subdued behaviour for various lengths of time (1-5 weeks). A
bilobed swelling in the pharyngeal area of two females was also
reported. No treatment-related clinical signs were observed in the
low or intermediate dose groups. Body-weight changes for survivors
in treated groups were not statistically significantly different
when compared to the control group. However, a slight to severe
body-weight loss for animals killed moribund was noted. Food
consumption was statistically significantly decreased only at the
high dose for surviving males during weeks 12 and 13, and for
females during weeks 11 and 12. Ophthalmological examinations
showed no remarkable differences between treated and control groups.
At 13 weeks, males and females of the 150 and 200 ppm groups
showed statistically significant decreases in haemoglobin, packed
cell, volume and red blood cell count. Reticulocyte count was
statistically significantly increased in females, but not in males.
Values for sodium, potassium, and chloride and BUN were all within
normal limits. Phosphorous was decreased in males and females at 13
weeks in the high dose. The value was statistically significant in
males. A statistically significant increase in serum protein was
associated with an increase in serum globulin in the high-dose males
at 13 weeks. A statistically significant increase in total
cholesterol was observed in the intermediate-dose (150 ppm) males at
weeks 8 and 13 and at weeks 4, 8, and 13 in high-dose (2000 ppm)
males and females. An increase in the mean creatinine level was
noted at weeks 8 and 13 in the high-dose males and females with
statistical significance attained for males at both time periods.
ALP was statistically significantly decreased in high-dose males at
weeks 8 and 13. At week 4, there was a statistically significant
decrease in mean ASAT in males at the intermediate and high dose and
in females at all three doses. A dose response was evident in
females. However values at 4 and 8 weeks were comparable to
controls for all groups of both sexes. ALAT was statistically
significantly decreased at week 4 only in females. Results from
urinalysis were not remarkable. Urine colour was however described
as orange or dark-coloured. Thyroid hormone assays revealed no
treatment-related changes in the low- and intermediate-dose groups.
However, marked and statistically significant reductions were noted
for T3 and T4 levels in high-dose animals at weeks 8 and 13. T4
was also statistically significantly decreased in males at 4 weeks
in the high-dose group.
At week 13, females of the high-dose group showed a marked and
statistically significant increase in thyroid weights accompanied by
slight but statistically significant increases in liver and adrenal
weights. Males of the high-dose group at 13 weeks showed a marked
increase in thyroid weights concurrent with slight increase in liver
and adrenal weights. None of these organ weights were statistically
significantly increased for males. Macroscopic examinations revealed
exophthalmia in two males (the survivors) and three females of the
high-dose group. Sporadic and slight exophthalmia was also observed
in one male of the intermediate dose group. Enlargement of the
thyroid gland was noted in all surviving high-dose animals as well
as the two males sacrificed moribund. The liver and adrenal gland
both appeared unremarkable as did the remaining tissues examined
macroscopically. Salient microscopic findings were those of
hypertrophy of the basophilic cells of the pituitary with micro-
vacuolisation attended by severe follicular hyperplasia of the
thyroid gland in all surviving and sacrificed animals of the high-
dose group. The liver and adrenal gland were histologically normal.
A moderate involution of the thymus of one male and two females of
the high-dose group was reported. No treatment-related microscopic
changes were noted for the low dose (10 ppm) or the intermediate
dose (150 ppm). The salient observations related to this study are
those of the pituitary and thyroid glands of animals receiving the
highest dose of 2000 ppm (equal to a mean of 66 mg/kg bw/day for
males and 72 mg/kg bw/day for females). In the pituitary, the lesion
observed was a hypertrophy of a basophilic cell type with
microvacuolisation, while in the thyroid, the lesion observed was a
hyperplasia of the follicular cells with papillary projections of
the follicular epithelium in the lumen of the follicles. Similar
hyperplasia was observed in ectopic nodule of thyroid tissues,
scattered along the thyroglossal track. The NOAEL was 10 ppm, equal
to 0.39 mg/kg bw/day based on decreased haemoglobin, packed cell
volume and red blood cell count, and increased cholesterol at 150
ppm. Effects on the thyroid were found only at 2000 ppm (Briffaux,
1991).
Beagle dogs (4/sex/group) received dietary concentrations of 0,
5, 50 or 500 ppm ETU (expressed as active ingredient taking into
account the purity index of 98% purity) for 52 weeks. Mortality was
evidenced in the high-dose group with the death of one male and the
sacrifice of one male and one female prior to study termination. No
treatment-related clinical signs were reported in either the low- or
mid-dose groups. Pale mucous membranes in four males and one female
of the high-dose group was associated with subdued behaviour and a
change in the colour of the faeces (yellow/orange). Body weight in
surviving animals at 52 weeks was decreased 15% in both males and
females at the high-dose and 8% in the mid-dose males. A dose-
related decrease was observed in body-weight gain for males of the
mid- (-43%) and high-dose (-60%) and females of the high-dose (-
60%). However, the decreases were not statistically significant.
Body-weight gain and body weights in the low-dose group were
comparable to control group. There were no statistically
significant differences in food consumption between treated and
control groups at 52 weeks. Food efficiency was generally
comparable between groups. Ophthalmological examinations revealed
comparable findings between all groups.
Haematological values between control groups and the low- and
mid-dose groups were comparable. However, in the high-dose group,
treatment-related low values (75-80% of normal) in haemoglobin, RBC,
packed cell volume were reported for all animals dying or sacrificed
moribund as well as one surviving male. Additionally the decrease
in RBC was accompanied by an increased reticulocyte count, a
decrease in mean corpuscular haemoglobin and an increase in mean
corpuscular volume. Low values in platelet count were also observed
in high-dose animals. Changes in blood clinical chemistry values
for sodium, potassium and blood urea nitrogen, cholesterol,
triglycerides, bilirubin creatinine, gamma glutamyl-transpeptidase
in surviving animals was not considered treatment-related. However,
a slight to moderate increase for total bilirubin was observed for
animals dying or sacrificed early in the high dose group. Values for
globulin were statistically significantly higher at weeks 13 and 52
for the high-dose animals (males and females combined). A decrease
in the albumin/globulin ratio was also statistically significant at
week 52 for the high-dose animals (males and females combined).
Elevated values for ASAT and ALAT were reported for both high-dose
males found dead or sacrificed moribund in the high-dose group.
Urinalysis values were unremarkable between groups.
Thyroid hormone mean values for T4 and T3 were not
statistically significantly different from control group. However,
T3 and T4 values taken shortly prior to death or sacrifice of the
three high-dose group animals revealed a mean decrease of 50% for
T3 values and 70% for T4 values. The values for decedent animals
were also below the historical range. Of the two surviving males in
the high-dose group at 52 weeks, one showed a 47% reduction of T3
from its pretest level while the other was comparable to its pretest
level. T4 values for both high-dose male survivors were generally
decreased 55% from pretest values. T3 and T4 values appeared to
be unaffected in the low-dose and mid-dose groups. A dose-related
increase in thyroid weights were observed at week 52 for the
intermediate and high-dose males and females. The increase was
statistically significant for combined males and females for
absolute, body weight and brain weight ratio (except for brain
weight ratio in the intermediate dose group). Necropsy revealed an
enlargement of the thyroid in one of the two surviving males.
High-dose animals dying on study or killed in a moribund
condition all manifested centrolobular hepatocellular necrosis of
the liver (multifocal and moderately severe in males and multifocal
and minimal in the female). Slight pigment accumulation was also
evident in Kupffer cells. Hypertrophy of follicular cells with
dilation of follicles was also seen in the thyroid of one high-dose
male that was sacrificed. Pigment accumulations in Kupffer's cells
and occasionally hepatocytes were observed in both males and females
of the intermediate and high-dose groups. Hypertrophy of the
thyroid with colloid retention was observed in the intermediate and
high-dose group and ranged in severity from slight to moderately
severe. The NOAEL was 5 ppm, equal to 0.18 mg/kg bw/day based on
reduction in body-weight gain, hypertrophy of the thyroid with
colloid retention, a slight increase in thyroid weight and pigment
accumulation in the liver at 50 ppm (Briffaux, 1992).
Monkeys
Wild-caught rhesus monkeys (5/sex/group) were administered ETU
(96.8-98.2 purity) in the diet for 5.5 or 6 months at dose levels of
0, 2, 10, 50, or 250, and 0, 50, 150 or 450 ppm, respectively.
Results of Study 1: Body weights were not affected by ETU.
Thyroid weight was increased in both sexes at 250 ppm and in females
at > 50 ppm, resulting from hyperplasia and/or hypertrophy.
Females at > 50 ppm also had enlarged pituitary glands. Ovarian
weights at 250 ppm were significantly decreased.
No changes in T3 or TBG were observed. Serum T4 was
decreased in both sexes at > 50 ppm identified from FTI analyses.
Serum TSH was increased at 250 ppm. 125I uptake also increased at
> 50 ppm in both sexes.
Lesions reportedly associated with ETU were identified in the
pituitary and thyroid gland of animals at > 50 ppm. These
included thyroid and pituitary hypertrophy, and thyroid follicular
cell hyperplasia (moderate to severe). A second study was conducted
due to the extent of tuberculosis in this first study which
necessitated the early termination at 5-5.5 months.
Results of Study 2: Body weights were not affected by ETU.
Thyroid and spleen weights were increased in males at > 150 ppm
and at all doses in females. Serum T3 decreased in males at
> 150 pm and in females at 450 ppm. Serum T4 was decreased in
both sexes at > 150 ppm. Radioactive 125I uptake was increased
in all test groups. The increased thyroid weight, thyroid iodine
uptake, decrease in T3, T4 and increase in TSH support the
evidence for hypothyroidism caused by ETU.
BUN was elevated in females at 450 ppm along with creatinine
and a decrease in calcium. Haemoglobin, haematocrit and RBC count
were decreased in both sexes at 450 ppm.
Histologic changes were identified in thyroid and pituitary
glands in both sexes, increasing in severity and incidence with
increase in dose. Thyroid follicular cell hyperplasia and pituitary
cytoplasmic vacuolation and swelling were the major changes
observed.
The NOAEL for 125I uptake was 10 ppm; the NOAEL for changes in
T3, T4 and TSH was 50 ppm. In a separate pathological
examination, 10 ppm was considered to produce compound-related
changes in the thyroid gland in 1/7 monkeys. The NOAEL was
considered by the authors to be 2 ppm in these combined studies
(Leber et al., 1978b).
However, the monkey studies were considered unreliable because
one was compromised by ill health of the animals, while little
reliance could be placed on the effects at the lowest dose used in
the second.
Long-term toxicity/carcinogenicity studies
Mice
Groups of B6C3F1 mice received perinatal (F0), adult (F1)
or both exposures to ETU at the following dietary concentrations:
(F0:F1), 0:0, 0:330, 0:1000, 33-100, 110-330, 330-0, 330-330 or
330-1000 ppm. Female C57BL/6N mice were exposed to 0, 33, 110 or
330 ppm of 99% pure of ETU in feed for one week before breeding, and
naturally inseminated by C3H/HeN males that received control feed
only. ETU exposure continued throughout pregnancy and lactation.
Weaning occurred on day 28 post-partum and dietary exposure at these
same (maternal) concentrations continued until pups were 8 weeks of
age. On post-partum day 7, litters were culled to a maximum of 8
pups, separated by sex after weaning and litter mates co-housed. At
8 weeks of age, pups were separated into groups of 60 males and 60
females to receive dietary concentrations of 0, 330 or 1000 ppm for
2 years. Groups of 34 male and 29 female mice that were fed 33 ppm
of ETU before weaning received 100 ppm for up to 2 years.
At 9 months, liver weights were increased in groups receiving
adult exposure concentrations of 330 or 1000 ppm regardless of
perinatal exposure. Increases were statistically significant with
the exception of the 0:330 group.
Thyroid weights were also reportedly increased in animals given
1000 ppm. T4 levels were statistically significantly decreased in
all animals receiving adult concentrations of 330 or 1000 ppm. TSH
levels were statistically significantly increased in males only at
330:330 and 330:1000 ppm. Follicular cell vacuolization of the
thyroid occurred in all animals receiving ETU except those only
receiving perinatal exposure (i.e. 330:0 ppm). Animals receiving a
dose of 33:100 ppm were not reported. Hyperplasia was comparable
between all groups.
Hepatocellular adenomas were present in animals receiving 1000
ppm but were not statistically significant. Centrilobular
cytomegaly was statistically significantly increased in both males
and females receiving 1000 ppm and in males receiving 110:330 and
330:330 ppm. Eosinophilic focus was statistically significantly
increased in females receiving 1000 ppm. At 2 years, there was no
survival disparity between treated and control (0:0 ppm) groups.
Clinical signs were not treatment-related. Body weights of treated
animals were statistically significantly decreased in both sexes
compared to 0:0 ppm controls, with the exception of the 330:0 ppm
dose group which was similar to controls. Statistically significant
increases in the number of animals with adenomas or carcinomas were
observed for hepatocytes, thyroid follicles and posterior pituitary
in both sexes of high-dose treated, adult only exposed groups when
compared to 0:0 ppm controls. At the next lower dose level of 0:330
ppm both sexes showed a statistically significant increase in
hepatocellular adenomas or carcinomas.
Hyperplasia of the thyroid was evident in high-dose males and
females and in 0:330 ppm females. Centrilobular cytomegaly was also
evident in males and females at both dose levels of adult only
treated animals. A comparison of animal groups receiving 0:330
versus 110:330 versus 330:330 ppm showed statistically significant
increases of thyroid follicular cell hyperplasia (males) and thyroid
adenomas (females) in perinatal treated groups at 330 ppm. Similar
comparisons for hepatocellular neoplasms and pituitary neoplasms
revealed no statistical differences. Comparison between animals
receiving 1000 ppm, with or without perinatal exposure to 330 ppm
showed no statistical differences for tumours or hyperplasia of the
thyroid or pituitary. Treatment of adult females receiving 330 ppm
with 330 ppm perinatally increased the number of tumours in the pars
distalis when compared to 0:0 ppm controls as well as those of the
thyroid. Animals receiving only a perinatal dose showed a
comparable response when measured against 0:0 ppm controls. T4
levels for both sexes were statistically significantly decreased in
both sexes at all dose levels. TSH levels were statistically
significantly increased in animals receiving 1000 ppm (i.e. adult
and perinatal/adult groups). Animals receiving 330 ppm during
adulthood showed elevated TSH levels which were statistically
significant only in females. Animals receiving perinatal exposure
only showed TSH values comparable to controls (0:0 ppm) (Chhabra et
al., 1992; NTP, 1992).
Rats
Charles River rats (60/sex/group) were fed ETU (purity not
stated) in the diet at levels of 0, 5, 25, 125, 250 or 500 ppm for 2
years. Body weights in both sexes were significantly decreased
initially at doses > 25 ppm; at 500 ppm and above (males) and 125
ppm and above (females) at 12 months; and at 500 ppm and above (both
sexes) for the remainder of the study.
Liver to body-weight ratios were significantly increased at 125
ppm and through 6 months in males, but comparable to controls for
the remainder of the study. Relative liver weights in females were
significantly increased at doses > 125 ppm at 2 months and at
doses > 250 ppm through 18 months. No differences between
control and dose groups were observed at 24 months. Thyroid to
body-weight ratio was significantly increased in males at 250 ppm
and above at 2, 6 and 18 months, and at 125 ppm and above in females
for the first 12 months. Thyroid weights were significantly
increased at 125 ppm and above in males at 12 and 24 months, and at
250 ppm and above in females at 18 and 24 months.
Uptake of 131I, expressed as counts/min/mg tissue, was
significantly decreased in males at 500 ppm throughout the study.
Thyroids of females fed 125 ppm and above were hypofunctioning at 6
months and hyperfunctioning at 12 months. At 24 months, females had
a hypofunctioning thyroid at 500 ppm.
Fewer rats survived to 24 months in the 500 ppm dose group and
there was also a significant increase in pneumonia which may have
been further complicated by obstruction of the trachea from enlarged
thyroids in the animals. Effects in the thyroid were evident at all
doses. Increased vacuolarity and hyperplasia in the thyroid were
evident at 25 ppm and above. Thyroids of treated rats were
distinguishable from controls by lobulation, follicular size and
uniformity, height of follicular epithelium, colloid staining,
keratinization of follicles, and general size.
It is possible that ETU initially reduces thyroid activity,
after which compensation occurs by an increased release of TSH and
that this increase in TSH stimulated thyroid weight in an attempt to
overcome the blocking effect of ETU. The progression to neoplasia
is believed to be a result of excessive pharmacological stimulation.
This is supported, in part, by a lack of thyroid tumours at 1 year
at 5 or 25 ppm, an increase in tumour incidence after 1 year at 125
ppm, and confirmed after 2 years in rats fed 250 and 500 ppm. The
NOAEL in this study was 5 ppm, equivalent to 0.25 mg/kg bw/day
(Graham et al., 1973, 1975).
SPF-Sprague-Dawley (30/sex/dose) received 0, 0.5, 2.5, 5 or 125
ppm of 96% pure ETU (adjusted to 100%) in feed, 7 days a week for
either 52 weeks (interim sacrifice of pre-selected animals
10/sex/dose) or 104 weeks (terminal sacrifice).
There were no compound-related deaths, clinical signs or
effects on food consumption. Body-weight gain was slightly impaired
in males at 125 ppm resulting in group mean body weights 5-6% lower
than in control males for most of the study. Female body weight was
unaffected. There were no treatment-related changes with respect to
ophthalmoscopic observations or palpable masses. Haematologic and
urinalysis finding were comparable to controls. There were no
treatment-related changes on clinical biochemistry values in males
receiving 0.5 or 2.5 ppm nor in females receiving 0.5, 2.5 or 5 ppm
of ETU. Statistically significant increases were observed in males
at 125 ppm for total protein, albumin, GGT, cholesterol, bilirubin,
TSH and T3. T4 and urea values were lower. T3 values were higher
at 5 ppm at 29 weeks. For females at 125 ppm, values for glucose
and T4 were lower, uric acid, T3 and TSH were higher. At 125 ppm
only thyroid weight was higher in males and females at the interim
and final sacrifice. Liver weight was slightly higher in both sexes
but only at the interim sacrifice. Macroscopic effects were not
observed at 52 weeks. However, at 104 weeks the incidence of
diffuse or modular enlargement of the thyroid gland was increased in
both sexes at 125 ppm.
Microscopic examination of the thyroid gland at interim
sacrifice revealed minimal to moderate diffuse follicular cell
hyperplasia in 8 animals of the control group and 9, 12, 12 and 20
animals (both sexes combined) receiving 0.5, 2.5, 5 or 125 ppm of
ETU, respectively. The incidence of this finding was significantly
increased in females at 125 ppm. The severity was increased in males
at 5 and 125 ppm and in females at 125 ppm. Slight or moderate
nodular hyperplasia and follicular adenoma were recorded in 6 and 3
males of the 125 ppm, respectively. Minimal or slight focal or
multifocal cellular hypertrophy of the anterior pituitary was
recorded in 2 control rats, 2 rats at 2.5 ppm and 7 rats at 125 ppm.
Males were predominantly affected. A significant increase in the
incidence and severity was calculated for males at 125 ppm. Other
morphological alterations observed in treated groups were considered
secondary to treatment, and affected spleen, thymus gland, auditory
sebaceous (Zymbal's) glands and lungs.
At terminal sacrifice, slight to excessive diffuse follicular
hyperplasia was recorded in 27 animals (sexes combined) receiving
125 ppm. The incidence and severity for males and females was
statistically significant for both sexes. Slight to severe nodular
hyperplasia was observed in 9 animals (sexes combined) receiving 125
ppm. The incidence and severity of this lesion was significantly
increased in males. Follicular adenomas occurred in 1 male of the
control group and in 4 males given 125 ppm. Follicular carcinomas
were observed in 2 males of the high-dose group. The combined
incidence of benign and malignant follicular neoplasms yielded a
clear dose-related trend but did not vary significantly in pairwise
comparison with the control group. Anterior pituitary gland
adenomas were recorded in 8 males and 11 females of the control
group and 15 males and 10 females at 125 ppm. There was a clear
dose-related trend for males and a marginal level of significance by
pairwise comparison with the control group. Adenomas of the
anterior pituitary recorded at the intermediate doses for males and
females were 5, 8, and 6 and 11, 12, and 11 respectively. Other
morphological alterations observed in treated groups were considered
secondary to treatment and affected the pancreas, lungs and Zymbal's
glands. The NOAEL was 5 ppm (equal to 0.37 mg/kg bw/day) based on
changes in clinical chemistry, increased T3, decreased T4,
increased thyroid and liver weights and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm
(Schmid et al., 1992).
Charles River CD rats (26/sex/group) were fed 0, 175 or 350 ppm
of technical grade ETU (97% pure) for 2 years. Follicular or
papillary carcinomas of the thyroid were observed in 17 males and 8
females at the high-dose. At 175 ppm, equivalent to 8.8 mg/kg
bw/day, 3 males and 3 females had thyroid carcinomas. Hyperplastic
goitre was observed in 17 males and 13 females of the high-dose
group and 9 males and 6 females of the low-dose group. These
lesions were not observed in control rats (Ulland et al., 1972).
Groups of F344/N rats received perinatal exposure (F0), adult
exposure (F1) or both to different concentrations (ppm) of ETU as
follows: F0, F1; 0,0; 0,83; 0,250; 9,25; 30,83; 90,0; 90,83; or
90,250. Female rats were exposed to 0, 9, 30 or 90 ppm of 99% pure
ETU in feed for 1 week before breeding. All males received control
feed. All females were naturally inseminated by males, housed
individually and continued on their previous diet. ETU exposure
continued throughout pregnancy and lactation. Weaning occurred on
day 28 post partum and dietary exposure at these same concentrations
continued until the pups were 8 weeks of age. On post partum day 4,
litters were culled to a maximum of 8 pups. Pups were separated by
sex after weaning and litter mates co-housed (5/cage). At 8 weeks
of age, pups were separated into groups of 60 males and 60 females
to receive adult dietary concentrations of 0, 25, 83 or 250 ppm for
up to 2 years.
At 9 months, liver weights were statistically significantly
increased in males receiving 0,250 or 90,250 ppm of ETU. Thyroid
follicular cell hyperplasia was greater than 50% and statistically
significantly increased for both males and females at the following
dose levels: 0,83; 0,250; 30,83; 90,83; and 90,250 ppm. Thyroid
follicular cell adenomas were observed in both males (3/10) and
females (1/10) receiving 90,250 ppm. Values were not statistically
significant. T4 values compared to 0,0 ppm controls were
statistically significantly decreased in all experimental groups of
both sexes except animals receiving 90,0 ppm. T3 values were
statistically decreased in many but not all groups. The 90,0 ppm
groups was unaffected. TSH levels were increased in all dose groups
and statistically significant only in some female groups. The 90,0
ppm groups was only very slightly increased.
At two years there were no differences in food consumption
between treated groups and controls with the exception of a decrease
in the 90,250 ppm group of males during the last month of exposure.
Final mean body weights for males and females were comparable to 0,0
control group with the exception of the 90,250 ppm male dose group
where the decrease was statistically significant. Only those
animals receiving 90,250 ppm showed a statistically significant
decrease in survival. Thyroid function values for animals receiving
90,0 or 0,83 or 9,25 ppm were not statistically different compared
to controls for males and females at 2 years. All other doses
revealed some level of statistical significance in both sexes.
There were no clinical findings that could be attributed to thyroid
dysfunction. Pathology of the thyroid for animals receiving adult
only exposures of 0,0; 0,83 or 0,250 ppm revealed statistically
significant trends and statistically significant increases in high-
dose males and females for hyperplasia, adenomas, carcinomas and
adenomas and carcinomas combined. Animals receiving 0,83 ppm of ETU
showed statistical increases in hyperplasia (males and females) and
adenomas (males). Hyperplasia of the thyroid was the only
statistically significant effect observed in both sexes when 0,0 and
90,0 ppm comparisons were made. Responses between dose groups
receiving 0,250 or 90,250 ppm revealed a statistically significant
increase in the number of adenomas in males and carcinomas in both
males and females. A comparison of the 0,83; 30,83 and 90,83 dose
groups in females showed no statistical differences in hyperplasia,
adenomas, or carcinomas, or adenomas and carcinomas combined. In
males only hyperplasia was statistically significantly increased.
ETU had no clear effects on the incidences of neoplasms or non-
neoplastic lesions at sites other than the thyroid gland. However,
some groups showed statistically significant increases relative to
controls in neoplasms of the Zymbal's gland (males and females at
90,250 ppm) and, mononuclear cell leukaemia (males and females at
90,250 ppm and males at 90,83 ppm (Chhabra et al., 1992; NTP,
1992).
Rats and Hamsters
Groups of 20 male and 20 female rats and hamsters were
administered ETU (purity not stated) in the diet for 24 and 20
months, respectively, at dose levels of 0, 5, 17, 60 or 200 ppm
(strain of animals not reported).
In rats, food consumption was reduced at 60 ppm and above and
body weight decreased at 17 ppm and above. Effects on SAP and SGPT
were not clearly demonstrated due to fluctuations in control levels.
Cholesterol was increased at 5 ppm in both sexes. Some hepatic
enzyme levels were also affected: GPT increased in males at 60 ppm;
ALP increased at 5 ppm (females) and 17 ppm (males); glucose-6-
phosphate dehydrogenase did not change. Thyroid weights were
significantly increased in both sexes at 60 ppm. No data were
available on the histologic examination.
In hamsters, food consumption and body weight were reduced at
60 ppm and above. SAP was increased in both sexes initially, then
decreased through 18 months. No effect was observed on SGPT.
Cholesterol levels were significantly increased in both sexes at all
doses compared to controls. Hepatic enzymes, GPT and ALP, were
significantly increased in both sexes at all doses. Glucose-6-
phosphate dehydrogenase was significantly decreased in both sexes at
all dose levels. Relative thyroid weights were significantly
increased at 200 ppm and above in both sexes. No data were
available on the histologic examination (Gak et al., 1976).
Reproduction studies
Rats
ETU (98% pure) was mixed in the diet and fed to Sprague-Dawley
rats (25 male and female parents per group) at concentrations of 0,
2.5, 25 or 125 ppm during a 70-day pre-pairing period and throughout
pairing, gestation and lactation of 2 generations (one litter per
generation). Body weights and mean body-weight gains were reduced
among the male parents of the F0 generation at 125 ppm. There were
otherwise no changes in the viability, clinical appearance or
behaviour, feed consumption, body weights or weight gain or
macroscopic appearance of any of the parents, F1 or F2 pups in any
of the test groups. Reproduction parameters were unaffected in any
of the dose groups in either generation. Histopathologic
examination indicated compound-related changes in the thyroid and
anterior pituitary glands at 25 and particularly at 125 ppm in both
generations. Thyroid changes in both sexes of both generations
consisted of follicular cell hypertrophy and hyperplasia which were
pronounced at 125 ppm and present to a much lesser extent at 25 ppm.
Reduced colloid was also present among F1 males and females at 125
ppm. Adenomas were observed in 3 males (not statistically
significant). Pituitary changes consisted of an increased incidence
and severity of anterior cell hypertrophy in both sexes of both
generations at 125 ppm, together with a tendency to an increase in
hypertrophy among parental generation males at 25 ppm and a slight
increase in cellular vacuolization at 125 ppm. There was no
evidence of reproductive organ toxicity up to and including 125 ppm.
The NOAEL was 2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day,
based on thyroid gland follicular cell hyperplasia and hypertrophy
at 25 ppm (Dott, 1992).
Rats/mice
In the first phase of a two-phase study, adult female rats and
mice were dosed with ETU (96.7% purity) and then bred to proven male
sires. Pregnant females delivered their pups via C-section for
tissue distribution analyses. Phase 2 consisted of weanling
rats/mice dosed for 9 weeks and then analyzed. Dose levels in the
diet were: rats: 0, 8, 25, 83 or 250 ppm; mice: 0, 33, 100, 333 or
1000 ppm (rats: Fischer 344, 3 per group; mice: C57BL/6N, 78 per
group). Two weeks after dosing began, breeding was initiated.
No rat dams or weanlings died. There was a trend toward
decreased weight gain in dams in all groups and in weanling males at
levels > 83 ppm. Food consumption was also reduced at 250 ppm
for males only. No effects on females were observed. At 250 ppm,
there was a decrease in pup survival to postnatal day 4. Thyroid
hyperplasia was observed in males at all doses and in females above
8 ppm, increasing in incidence and severity with dose. Thyroid
adenomas were reported in males at 83 ppm and above. Vacuolization
of pituitary glands in males was noted at 250 ppm.
There was a significant decrease in body weight in high-dose
female mice during the period of lactation. Weanling body weights
were decreased in males and females at 333 ppm and 1000 ppm.
Initially, insufficient pregnancies were produced in all dose
groups. A rebreeding programme, after 6.5 weeks on ETU diets,
produced sufficient numbers of litters for evaluation. However, no
pregnancies were achieved in the high-dose group, and pregnancy rate
was reduced in other dose groups in comparison to control. The
number of pups surviving to day 28 was significantly decreased in
the high-dose group. Thyroid hyperplasia and cellular alteration of
hepatocytes (cytomegaly, karyomegaly) were observed in both sexes at
1000 ppm. One male mouse at 333 ppm also had adverse effects in the
liver (Peters et al., 1982).
Special studies on embryotoxicity/teratogenicity
Rats
Pregnant Charles River Rats (ChR-CD, Sprague-Dawley) were
administered 0, 25 or 50 mg/kg bw/day of ETU (98% pure) in DMSO, or
DMSO alone (vehicle control) or water alone onto the shaved back of
each animal for 48 hours on days 10 and 11 or days 12 and 13 of
gestation.
Maternal body-weight change during the 48-hour administration
period ranged from +4 to -5%. Fetuses examined from dams
administered ETU at 50 mg/kg bw/day on days 10 and 11, showed short
tails (3/83) and fused ribs (2/83). However, dams given 50 mg/kg
bw/day on days 12 and 13 produced fetal deformities in all
offspring. Fetal defects were characterized by encephalocele, a
part or the entire tail missing, missing leg bones, hunchback
curvature to the spine, short mandible, fusion of ribs and fusion of
sternebrae.
A dose of 25 mg/kg bw/day administered only on days 10 and 11
of gestation did not result in any fetal abnormalities. A dose of 25
mg/kg bw/day was not administered on days 12 and 13 of gestation
(Stula & Krauss, 1977).
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rats
(Wistar) (10-17 pregnant dams per dose). Treatment was made for 21-
42 days before conception to pregnancy day 15, and on days 6-15 or
6-20 of pregnancy. Doses of 40 mg/kg bw/day were not toxic to rats;
however, 80 mg/kg bw/day was lethal to 9 of 11 female rats. Mean
fetal weight was reduced at 40 mg/kg bw/day. Measurements of
sterility, pre-implantation loss and post-implantation survival were
comparable to controls. The brain was the most commonly affected
organ. ETU induced meningoencephalocele, meningorrhagia,
meningorrhea, hydrocephalus, obliterated neural canal, abnormal
pelvic limb posture with equinovarus, and short or kinky tail at 10
mg/kg bw/day in all phases of the study. Although no abnormalities
were reported in rats at 5 mg/kg bw/day, there was a higher
frequency of delayed ossification of the parietal bone, compared to
controls. The NOAEL for embryo/fetotoxicity was 5 mg/kg bw/day
based on teratogenic effects observed at 10 mg/kg bw/day. The NOAEL
for maternal toxicity was 40 mg/kg bw/day (Khera, 1973).
ETU was given by gavage (distilled water, 5 ml/kg bw/day) on
days 6-20 of gestation to pregnant Sprague-Dawley rats (22/group) at
doses of 0, 15, 25, or 35 mg/kg bw/day, and dams were sacrificed on
day 21 for examination of uterine contents. Maternal appearance,
behaviour, and body-weight gain were generally unaffected, and the
incidence of pregnancy was comparable among the groups. No adverse
effect was noted on the average numbers of implantations, live
fetuses, or percentages of resorption sites per litter. Mean fetal
body weights were decreased in a dose-related manner, but were only
significantly reduced at 35 mg/kg bw/day (13-15% lower). ETU at 35
mg/kg bw/day produced external malformations including cranial
meningocele and meningorrhea, severe hindlimb talipes, and a non-
significant incidence of hydrocephaly. Short and/or kinky tails
were noted in 43.5% of the fetuses. Soft tissue examinations
revealed higher incidences of dilated brain ventricles at 25 and 35
mg/kg bw/day (33.5 and 93% of the fetuses, respectively) and of
hydroureter and dilated ureter at 35 mg/kg bw/day, and skeletal
examinations revealed a reduced ossification of skull bones and a
significantly increased incidence of dumbbell-shaped or bilobed
vertebral centra (33.5% of fetuses). There were no other treatment-
related increases in skeletal variants among any of the experimental
groups, and no treatment-related effects of any kind identified in
the 15 mg/kg bw/day group. The NOAEL for maternal toxicity was 35
mg/kg bw/day. The NOAEL for embryo/toxicity and teratogenicity was
15 mg/kg bw/day based on higher incidences of dilated brain
ventricles at 25 mg/kg bw/day (Saillenfait et al., 1991).
ETU (100% purity) was administered via oral gavage at 40 mg/kg
bw/day from days 7 to 15 of gestation to pregnant CR rats (10-12
rats/group). Rats were hypothyroid and euthyroid. There was a
problem, however, in maintaining the euthyroid state in rats given
supplement. Rats were also given thyroxine to determine if ETU
teratogenicity occurred through alterations of maternal thyroid
function. ETU was found to be teratogenic in the rat but not
through alteration of maternal thyroid status. It was also
demonstrated that ETU lowered serum T4; that hypothyroidism per se
increased the background level of malformations in the rat; that T4
alone was embryotoxic but not teratogenic; and that hypothyroidism
altered the spectrum of malformations in response to ETU both
quantitatively and qualitatively (Lu & Staples, 1978).
Virgin Sprague-Dawley rats were mated one-to-two with males
and, after pregnancy was verified, were administered ETU (unknown
purity), T3/T4 and sodium iodide via oral gavage in varying
concentrations, either singly or in combination, as well as a
control solution of water only, from day 7 to day 20 of gestation.
Dosing regimen was as follows:
Dose group Total rats per
group
Control 1 ml distilled water 14
T3 20 µg/kg bw/day + T4 100 µg/kg bw/day 10
Sodium iodide 333 µg/kg bw/day 10
ETU 20 mg/kg bw/day 10
ETU 20 mg/kg bw/day + sodium iodide 16
ETU 20 mg/kg bw/day + T3/T4 16
ETU 40 mg/kg bw/day 11
ETU 40 mg/kg bw/day + sodium iodide 14
ETU 40 mg/kg bw/day + T3/T4 15
Each pregnant dam was killed on day 20 by chloroform
asphyxiation and the fetuses removed via hysterotomy. The number of
resorptions, live/dead fetuses and fetal birth weights were
determined. Skeletal analyses were performed on 1/3 and visceral
analyses on 2/3 of the fetuses. Results indicated a possible
reduction in the teratogenic response to ETU for some malformations
when T3/T4 was administered in conjunction with ETU. For example,
20 and 40 mg/kg bw/day ETU (alone) produced 97.6 and 94.5% incidence
of hydrocephaly, respectively. In combination with T3/T4 these
same levels produced 19.6 and 74.5% incidence, respectively. These
results indicate that the teratogenic potential of ETU may in part
be secondary to the thyroid toxicity of ETU (Emmerling, 1978b).
Rats, mice and hamsters
Wistar-Imamichi rats, JCL-ICR mice, and Syrian golden hamsters
10 weeks or older were mated overnight and examined the next morning
for the presence of a vaginal plug or spermatozoa in vaginal smears.
Evidence of copulation was designated as day zero of gestation.
Pregnant females were given daily oral doses of ETU by gavage during
the period of organogenesis. Doses given to rats, mice and hamsters
were, respectively 0, 10, 20, 30, 40 or 50 mg/kg bw/day, 0, 200, 400
or 800 mg/kg bw/day and 0, 90, 270, or 810 mg/kg bw/day. Rats,
mice, and hamsters were sacrificed on days 20, 18, and 14,
respectively.
Dams did not show signs of toxicity and none died in any
species. There were no statistically significant differences between
treated and control rats for the mean number of implants and live
fetuses reported. Mean fetal weight for both males and females was
statistically significantly decreased at 30 mg/kg bw/day and higher.
The percent of fetal death was also statistically significantly
increased at 50 mg/kg bw/day. Mice showed no statistically
significant changes between treated and control values for any of
the prenatal developmental parameters (i.e mean number of implants,
mean number of live fetuses, percent fetal death and mean fetal body
weight). The mean number of implants for hamsters were comparable
to control values for all doses. There was a statistically
significant decrease in the mean number of live fetuses born
attendant to a decrease in mean fetal body weight for males and
females which was statistically significant at 810 mg/kg bw/day.
Mean fetal body weight for females was also statistically
significantly lower at 270 mg/kg bw/day without other prenatal
developmental effects. Gross external anomalies were not meaningful
between controls and treated groups for mice. Rats manifested a
short or kinky tail at 30 mg/kg and above in 80% of the offspring.
In rats, meningocele was observed in 66% of the offspring at 40
mg/kg bw/day and above and micrognathia in 30% of the offspring at
50 mg/kg bw/day. Hamsters showed multiple type anomalies at 810
mg/kg bw/day and included such signs as cleft palate, short or kinky
tail, and oligodactyly. Short or kinky tail was observed in 2% of
the animals at 270 mg/kg bw/day and 42% of the animals at 810 mg/kg
bw/day. The LOAEL in rats was 30 mg/kg and seen as curved clavicles.
There was an increased incidence of curved clavicles, fused/wavy
ribs, fused sternebrae, malformed vertebrae and scoliosis at 40 and
50 mg/kg bw/day. The LOAEL was 90 mg/kg bw/day in hamsters based on
a 2% incidence of malformed lumbar and sacral vertebrae, a 4%
incidence at 270 mg/kg bw/day and a 51% incidence at 810 mg/kg.
There were no brain or visceral anomalies observed in mice.
Dilation of the lateral 4th ventricle was observed in 5% of hamsters
at the high dose, none at lower doses and 2% in controls. Dilation
of the lateral or 4th ventricle in rats was observed in 2% and 39%
of rats at doses of 10 and 20 mg/ kg bw/day. At 30 mg/kg bw/day and
above the response was 100%. No maternal toxicity occurred at the
doses tested. The NOAEL for embryo/ fetotoxicity in the rat was 10
mg/kg bw/day based on dilation of the lateral or fourth ventricle at
20 mg/kg bw/day. The NOAEL for embryo/fetotoxicity in the hamster
was 90 mg/kg bw/day based on decrease of fetal body weight at 270
mg/kg bw/day. The NOAEL for mice was greater than 80 mg/kg bw/day
(Teramoto et al., 1978).
Hamsters
ETU (purity > 99%) was administered orally to pregnant Syrian
hamsters at doses of 600, 1200, 1800 or 2400 mg/kg bw on day 11 of
gestation. All dams were killed on day 15 of gestation for necropsy
and fetal examination. There were only 5 pregnant dams in the
control group compared to 8-10 in the treated groups.
Maternal toxicity was not reported at any dose. However, there
was an increased incidence of resorbed fetuses and fetuses dying
late in gestation with an associated decrease in the number of live
fetuses at 2400 mg/kg bw. Fetal body weights were similarly reduced
at 1800 mg/kg bw. Malformations were evident at > 1200 mg/kg bw,
with no adverse effect reported at 600 mg/kg bw. Fetal anomalies
included cleft palates ectrodactyly, hydrocephalus and hypoplastic
cerebellum. There was also an increased incidence of delayed
ossification of the calcarium and sternebrae defects. As with other
species (i.e. rat, cat), the brain was particularly sensitive to
ETU, although at higher dose levels (Khera et al., 1983).
Mice/rats/hamsters/guinea-pigs
Time-pregnant random-bred CD-1 mice, Sprague-Dawley rats golden
hamsters and Hartley strain guinea-pigs were used. Maneb (80%
pure), ETU (melting point 197-198 °C) and EBIS were administered by
gastric intubation. Control animals received vehicle alone (water
or corn oil) or remained untreated. Prenatal studies were conducted
on rats given maneb (480, 240, 120, 0 mg/kg bw/day for days 7-16) or
ETU (80, 40, 30, 20, 10, 5, 0 mg/kg bw/day for days 7-21) or EBIS
(30, 25, 7.5, 0 mg/kg/ bw/day for days 7-21). Prenatal studies were
also conducted on mice given maneb (1500, 750, 375, 0 mg/kg bw/day
for days 7-16) or ETU (200, 100, 0 mg/kg bw/day for days 7-16) or
EBIS (200, 100, 50, 0 mg/kg bw/day for days 7-16). Prenatal studies
were also conducted with hamsters and guinea-pigs but only with ETU.
Hamsters received either 100, 50, 25 or 0 mg/kg of ETU on days 5-10
of gestation. Guinea-pigs received 100, 50 or 0 mg/kg bw/day of ETU
on days 7-25. Post-natal studies were only conducted on rats. Rats
received either maneb (480, 240, 0 mg/kg bw/day) or ETU (30, 25, 20,
0 mg/kg bw/day) or EBIS (30, 15, 0 mg/kg bw/day) on days 7-15 of
gestation. Mice were killed on day 18, rats on day 21, hamsters on
day 15 and guinea-pigs on day 35. Animals designated for post-natal
study were allowed to litter normally. Litters were normalized to 4
individuals of each sex and weaned on day 22 post-partum.
In toxicity studies conducted to determine dose levels, all
compounds tested proved to be more toxic in the rat than the mouse.
Hind limb paralysis was observed in rats given maneb at the high
dose of 600 mg/kg bw/day. No toxicity was noted in mice given a
dose of 1500 mg/kg bw/day of maneb. EBIS produced death in rats at
75 mg/kg bw/day and hind limb paralysis at 50 mg/kg bw/day.
Decreased body-weight gain and death was observed in mice given 100
mg/kg bw/day. ETU produced lethality in rats at the high dose of 80
mg/kg bw/day. ETU was not toxic in mice (300 mg/kg bw/day) hamsters
(150 mg/kg bw/day) or guinea-pigs (100 mg/kg bw/day) at the high
dose tested.
Maneb administered to pregnant rats resulted in significant
dose-related decrease in maternal weight gain and increase in liver
to body-weight ratios. Fetal weight and caudal ossification were
significantly reduced only at the highest dose tested (480 mg/kg
bw/day). At the high dose (480 mg/kg bw/day), 18 fetuses from 4
litters manifested hydrocephalus. Maternal mice given maneb
manifested increased liver/body-weight ratios and decreased caudal
ossification beginning at 375 mg/kg. Dose-related trends were
evident. EBIS given to rats and mice did not result in adverse
fetal effects. Average maternal weight gain was decreased in rats
at 30 mg/kg bw/day. Maternal weight gain in mice was not affected.
Average liver to body-weight ratio was increased at the high dose
tested for rats (30 mg/kg bw/day) and mice (200 mg/kg bw/day). ETU
administered to rats at the high dose of 80 mg/kg bw/day resulted in
25% maternal mortality and reduced weight gain. Fetal toxicity was
also observed at 80 mg/kg bw/day and included mortality, decreased
weight, decreased ossification and edema. Gross defects of the
skeletal system and central nervous system were noted in a majority
of fetuses. At 40 mg/kg bw/day, fetal weight and ossification were
reduced and hydrocephalus and encephalocele were evident.
Hydrocephalus was seen at 20 mg/kg bw/day and decreased fetal body
weight at 10 mg/kg bw/day. ETU produced an increase in the liver-
body-weight ratio of mice at 100 mg/kg bw/day and 200 mg/kg bw/day
and an increase in the number of supernumerary ribs at 200 mg/kg
bw/day. No apparent effects were observed in hamsters or guinea-
pigs. Post-natal studies with maneb resulted in delayed eye opening
in males. Post-natal observations with EBIS resulted in decreased
fetal body weight at day 22 in females only as well as delayed eye
opening. ETU administration produced no observable post-natal
effects with the exception of increased total open field activity in
males. There were no apparent differences reported in open field
activity between male fetuses surviving the high dose (30 mg/kg
bw/day) with hydrocephalus and their apparently normal mates.
Hydrocephaly was not observed at lower doses (Chernoff et al.,
1979).
Rabbits
ETU (100% purity) was administered orally at doses of 0, 5, 10,
20, 40 or 80 mg/kg bw/day in distilled water to nulliparous rabbits
(New Zeeland white). There were 5-7 pregnant does per group.
Treatment was made from days 7 to 20 of pregnancy. No toxicity was
apparent in rabbits given 80 mg/kg bw/day. Fetal weights were not
affected. Measurements of sterility, pre-implantation loss and post-
implantation survival were comparable to controls. Rabbits presented
no evidence of malformations at the doses administered. However,
there was an increase in resorption sites, decreased brain weight,
and degeneration of the proximal convoluted tubules in the kidneys
of fetuses at 80 mg/kg bw/day. The NOAEL for maternal toxicity was
80 mg/kg bw/day and for embryo/fetotoxicity the NOAEL was 40 mg/kg
bw/day (Khera, 1973).
Cats
ETU (purity not stated) was administered orally (in gelatin
capsules) to pregnant European and Persian breed cats (7-14 cats per
group) at doses of 0, 5, 10, 30 or 60 mg/kg bw/day on days 16-35 of
gestation or 120 mg/kg bw/day from days 16 to 34 of gestation. No
effect was evident at 5 mg/kg bw/day. However, at > 10 mg/kg
bw/day decreased ataxia, tremors and hindlimb paralysis were
observed. No pregnant cats survived in the 30 and 60 mg/kg bw/day
dose groups. The remaining cats showed no apparent treatment-related
effect on fetal viability or fetal weight. Although this study was
inconclusive in many respects, there was an increased incidence of
toxicity to the central nervous system at 10 mg/kg bw/day. Further,
at 5 and 120 mg/kg bw/day, there were anomalous fetuses in each
group. Incidences of exencephaly, hydrocephaly, cleft palate,
kyphoscoliosis, umbilical hernia, coloboma, and spina bifida were
observed in these two treated groups. Similar anomalies were
observed in the rat (Khera & Iverson, 1978).
Special studies on genotoxicity
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induced mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic. The results of genotoxicity assays on ETU are given in
Table 2.
Table 2. Results of genotoxicity assays on ethylenethiourea
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium 10-20 000 µg/plate; ? Positive Teramoto et al.,
reversion TA1535, TA1536, in DMSO? (TA1535 without 1977; Shirasu
assay TA1537, TA1538, G46 activation) et al., 1977
S. typhimurium 0.2-2000 µg/plate >98% Negative Brooks & Dean, 1981
TA1535, TA1537,
TA1538, TA92, TA98,
TA100
S. typhimurium 5-5000 µg/plate; >98% Negative Richold & Jones,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 0.1-2000 µg/plate; >98% Negative Rowland & Severn,
TA1535, TA1537, in DMSO 1981
TA1538, TA98, TA100
S. typhimurium 10-5000 µg/plate; >98% Positive Simmon & Shepherd,
TA1535, TA1537, in DMSO (TA1535 with 1981
TA1538, TA98, TA100 & without
activation)
S. typhimurium 4-2500 µg/plate >98% Negative Trueman, 1981
TA1535, TA1537,
TA1538, TA98, TA100
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Salmonella S. typhimurium 1000-20 000 µg/plate; no ? Positive Autio et al., 1982
reversion TA1950 activation used; in DMSO
assay (cont'd)
S. typhimurium >500 µg/plate; ? Positive Moriya et al., 1983
TA1535, TA1537, in DMSO? (TA1535 with
TA1538, TA98, TA100 and without
activation)
S. typhimurium 100-10 000 µg/plate; 98.4% Positive Mortelmans et al.,
TA1535, TA1537, TA98, in DMSO (TA1535 with 1986 (SRI)
TA100 & without
activation)
Salmonella S. typhimurium 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; TA1535, TA1537, TA98 in dimethylacetamide
fluctuation test
Salmonella S. typhimurium 200-80 000 ? Positive Schupbach & Hummler,
forward TA1530 µg/plate 1977
mutation assay
E. coli E. coli WP2 hcr 10-10 000 µg/plate ? Negative Teramoto et al.,
reversion assay 1977; Shirasu
et al., 1977
E. coli 343/113/uvrB 200-4000 µg/ml; >98% Positive Mohn et al., 1981
in phosphate buffer (galR- & arg+
systems with
activation)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
E. coli E. coli WP2 uvrA 10-1000 µg/ml; >98% Negative Gatehouse, 1981
reversion assay; in dimethylacetamide
fluctuation test
Host mediated S. typhimurium 500-6000 mg/kg; ? Negative Schupbach & Hummler,
assay Swiss G46 in DMSO 1977
albino mouse
Swiss albino S. typhimurium 670-6000 mg/kg; ? Weak Schupbach & Hummler,
mouse TA1530 in DMSO Positive 1977
Male JCL-SD rat S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
G46 1977; Shirasu
et al., 1977
Male JCL-ICR S. typhimurium 200-400 mg/kg ? Negative Teramoto et al.,
mouse G46 1977; Shirasu
et al., 1977
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Mouse lymphoma 140-3000 µg/ml; >98% Negative Jotz & Mitchell,
mutation assay L5178Y TK+/- in DMSO 1981
Mouse lymphoma 25-3600 µg/ml; NTP Positive McGregor et al.,
L5178Y TK +/- in DMSO chemical 1988
repository
Chinese hamster ovary 1000-2000 µg/ml; >98% Negative Carver et al., 1981
(CHO-AT3-2; several loci) in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
1.C. In Vivo Gene Mutation Assays
Sex-linked D. melanogaster 0.25-2.5%; ? Negative Mollet, 1975
recessive lethal in sugar water
assay
D. melanogaster 250 ppm; in DMSO >98% Negative Valencia &
Houtchens, 1981
D. melanogaster 4900 ppm injection; 97% Inconclusive Woodruff et al.,
12 500 ppm feed; in water 1985 Mason et al.,
1992
D. melanogaster 5100 ppm 98.4% Inconclusive Mason et al., 1992
1.D. Yeast and Other Fungal Assays
Forward mutation S. pombe 0.1-1 µg/ml; >98% Negative Loprieno, 1981
in DMSO
A. nidulans 0.22-116 mM; >98% Negative Crebelli et al.,
no activation used; 1986
in DMSO
Reverse mutation S. cerevisiae XV185-14C 88.9-889 µg/ml; >98% Equivocal Mehta & von Borstel,
in DMSO 1981
1.E. Plant Test
Mutation Tradescantia 9.79 X 10-5 M; ? Positive van't Hof &
clone 4430 in DMSO? Schairer, 1982
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations in Mammalian Cells
In vitro Chinese hamster cell line 1000-3200 µg/ml ? Negative Teramoto et al.,
chromosomal (Don) 1977; Shirasu
aberrations et al., 1977
Chinese hamster ovary 1670-5000 µg/ml; >98% Positive Natarajan & van
(CHO) cells in DMSO (with and Kesteren-van
without Leeuwen, 1981
activation)
Chinese hamster ovary 6000-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
2.B. In Vivo Chromosomal Alterations
Bone marrow Male & female Wistar rat 50-400 mg/kg; ? Negative Teramoto et al.,
cytogenetics in aqueous soln 1977; Shirasu
et al., 1977
Micronucleus assay Female ICR mouse 150-450 mg/kg; ? Negative Seiler, 1975
in DMSO
Male & female Swiss albino 700-6000 mg/kg; ? Negative Schupbach & Hummler,
mouse in gummi arabicum 1977
Male ICR mouse 220-880 mg/kg; >98% Negative Kirkhart, 1981
in DMSO
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Micronucleus assay B6C3F1 mouse 880-1416 mg/kg; >98% Negative Salamone et al.,
(cont'd) in DMSO 1981
Male & female CD-1 mouse 220-880 mg/kg; >98% Negative Tsuchimoto & Matter,
in DMSO 1981
Dominant lethal Swiss albino mouse 500-3500 mg/kg; ? Negative Schupbach & Hummler,
assay in gummi arabicum 1977
JCL-ICR mouse 300-600 mg/kg; ? Negative Teramoto et al.,
in water with gum arabic 1977; Shirasu
et al., 1977
C3H/HeCr mouse 150 mg/kg; ? Negative Teramoto et al.,
in gum arabic soln 1978
Reciprocal D. melanogaster 500 ppm; >98% Negative NTP, 1992
translocations in 5% sucrose soln
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
Rec assay B. subtilis H17, M45 20-4000 µg/disk ? Negative Teramoto et al.,
1977; Shirasu
et al., 1977
B. subtilis H17, M45 spores 2000 µg/disk; >98% Positive Kada, 1981
in DMSO (without
activation)
E. coli WP2, WP67, CM871 Not specified >98% Negative Green, 1981
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Rec assay (cont'd) E. coli WP2, WP67, CM871 Not specified >98% Negative Tweats, 1981
E. coli several deficient 500 µg/ml; in DMSO >98% Positive Ichinotsubo et al.,
strains (with activation) 1981
S. typhimurium TA1538, 125-2000 µg/disk; ? Negative Rashid & Mumma, 1986
TA1978; in DMSO
E. coli K12 & WP2
Inhibition of DNA E. coli polA 2273 µg/ml >98% Positive Rosenkranz et al.,
polymerase I (without 1981
activation)
Lambda prophage E. coli (lysogenic) 2-20 mg/ml; only activation >98% Positive Thomson, 1981
induction used; in DMSO
E. coli E. coli PQ37 Not specified; in DMSO ? Negative Quillardet et al.,
SOS Chromotest 1985
In vitro Human fibroblasts from Not specified; in DMSO >98% Negative Agrelo & Amos, 1981
unscheduled DNA skin biopsies
synthesis (UDS)
HeLa S3 human cells Not specified; in DMSO >98% Inconclusive Martin & McDermid,
1981
WI-38 human fibroblasts 63-2000 µg/ml; in DMSO >98% Negative Robinson & Mitchell,
1981
In vitro unschuled Rat hepatocytes 9.6 X 10-9 - 3.2 X 10-3 M ? Negative Althaus et al.,
DNA synthesis (nuclei isolation) 1982
(UDS)
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
In vivo/in vitro Female B6C3F1 mouse 1500 mg/kg; in corn oil 98% Negative (UDS); Frank & Muller, 1988
unscheduled DNA hepatocytes Positive (S-
synthesis (UDS)/S phase increase)
phase analysis
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro Chinese hamster ovary 25-1000 µg/ml; >98% Negative Evans & Mitchell,
SCE assays (CHO) cells in DMSO 1981
Chinese hamster ovary 1670-5000 µg/ml; >98% Negative Natarajan & van
(CHO) cells in DMSO Kesteren-van
Leeuwan, 1981
Chinese hamster ovary 0.01-100 µg/ml >98% Negative Perry & Thomson,
(CHO) cells 1981
Chinese hamster ovary 500-10 000 µg/ml; >98% Negative NTP, 1992
(CHO) cells in DMSO
In vivo SCE assays Male CBA/J mouse bone 1000 mg/kg; in DMSO >98% Negative Paika et al., 1981
marrow and liver
3.C. Yeast and other Fungal Assays
Mitotic aneuploidy S. cerevisiae D6 500 µg/ml; in DMSO >98% Positive Parry & Sharp, 1981
Chromosome A. nidulans 19.6-78.3 mM; no activation >98% Positive Crebelli et al.,
malsegregation used; in DMSO 1986
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Mitotic gene S. cerevisiae D4 33-333.33 µg/plate; >98% Negative Jagannath et al.,
conversion in DMSO 1981
S. cerevisiae JD1 50 µg/ml; in DMSO >98% Positive Sharp & Parry, 1981a
(without
activation)
S. cerevisiae D7 2000-4000 µg/ml >98% Negative Zimmermann & Scheel,
1981
Mitotic S. cerevisiae T1, T2 1000 µg/ml; in DMSO >98% Negative Kassinova et al.,
crossing-over 1981
A. nidulans 19.6-78.3 mM; no activation >98% Negative Crebelli et al.,
used; in DMSO 1986
Intrachromosomal S. cerevisiae RS112 5-40 mg/ml; no activation used ? Positive Schiestl et al.,
recombination 1989
Differential S. cerevisiae, T5 Not specified; >98% Negative Kassinova et al.,
killing in DMSO 1981
S. cerevisiae 197/2d, rad 300-1000 µg/ml; >98% Positive Sharp & Parry, 1981b
in DMSO (with and without
activation)
3.D. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 100-1000 µg/ml; 99.8% Negative McGlynn-Kreft &
in DMSO McCarthy, 1984
Table 2 (contd)
Test system Test object Concentration1 Purity Results Reference
Cell transformation Syrian hamster embryo 62-1000 µg/ml ? Negative Casto, 1975, 1976
(SHE) cells/adenovirus SA7 in: Heidelberger
et al., 1983
Syrian hamster embryo 1-24 mM ? Inconclusive Hatch et al., 1986
(SHE) cells/adenovirus SA7
Cell transformation C3H/10T 1/2 cells 33 µg/ml; in DMSO 99.8% Negative McLeod & Doolittle,
with "promotion" 1985
3.E. Germ Cell Effects
Spermhead (CBA X BALB/c) F1 mouse 250-2000 mg/kg; >98% Negative Topham, 1981
abnormalities in Tween 80
B6C3F1/CRL mouse 166-2655 mg/kg; >98% Negative Wyrobek et al., 1981
in DMSO
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not normally
use such supplementation; solvent is provided if specified in the report
Special studies on the thyroid
Rats
Groups of four randomly selected weanling Caesarian-delivered
Sprague-Dawley male litter-mate rats were administered 0, 75 or 150
ppm ETU (purity not stated). Within each treatment group dosing
periods and control diet periods were varied to examine the
reversibility of compound-related effects. Results suggest some
reversibility of thyroid effects which were related to time on test
and to the severity of effect on the thyroid (Arnold et al.,
1982).
Sprague-Dawley rats (50/sex/group) were fed diets containing 0,
75, 100 or 150 ppm ETU (purity not stated) mixed in corn oil for 7
weeks. Body weights decreased with increasing dose while thyroid
weights (absolute and relative) increased in both sexes. T3 levels
were somewhat variable, while T4 levels were significantly
decreased at 150 pm in both sexes. These effects partially reversed
after 4 weeks on control diets. Histopathological findings included
reduced colloid content of thyroid acini in high dose rats. Acinar
epithelial cell size and height were not different from control.
Two tumours were identified in the high dose male group: a
follicular cell adenoma and medullary carcinoma. The authors
concluded that the relationship between the duration of exposure to
ETU and the possible reversibility of various thyroid lesions
requires further study (Arnold et al., 1983).
A 22-week study was conducted in Sprague-Dawley rats
(55/sex/group) with the following dosing schedule: ETU (97% purity)
administered alone in the diet at levels of 0, 125, 250 or 625 ppm;
or with 0.2 g T3 and 1.6 g T4/rat, orally via gavage; or with
manganese and zinc. Also included were treatment groups dosed with
0, 650 or 1250 ppm mancozeb alone.
Rats receiving 625 ppm ETU alone or in combination with
manganese and zinc were removed from test diet because of alopecia,
weight loss, dermatosis and mortality. Survivors received control
diets for the remainder of the study. Serum decreased in both sexes
at all doses of ETU after 2 weeks of treatment. These levels
returned to normal when ETU was removed from the diet. Serum T3
decreased in both sexes at 625 ppm ETU after 4 weeks of dosing, and
in males at 125 and 250 ppm ETU, but by week 8 returned to normal.
In females at the same doses, T3 was normal until week 16 when it
decreased. The additions of T3/T4 by oral gavage resulted in
decreased T3 at week 8 in males and a decrease during the first 6
weeks in females at all levels. T3 returned to normal one month
after removal of ETU. TSH increased in the ETU group and less
dramatically in ETU plus T3/T4 groups. These levels returned to
normal 2 weeks after ETU was removed form the diet.
Body weights decreased in males and females after 4 weeks at
625 ppm ETU and in males after 8 weeks at 250 ppm ETU. Thyroid to
body-weight ratio increased at > 125 ppm ETU in males. When ETU
was removed from the diet, weights returned to normal. No effect
was observed on pituitary weights. Thyroid hyperplasia was
increased at 125 ppm ETU and above and reversed to normal 6-8 weeks
after ETU was removed. Approximately 1% (13/1300) of the rats
developed hyperplasia of the thyroid (focal areas of basophilic
hyperplastic follicles and follicular adenoma). A dose-related
increase in liver weight was observed at 125 ppm ETU and above for
both sexes.
Exposure to ETU resulted in a decrease in thyroid hormone
(T3/T4) levels and increased serum TSH levels in a dose-related
manner. Although TSH levels were reduced when ETU was supplemented
with T3/T4, the high dosage of ETU was apparently sufficient to
override these effects. The hormone imbalance induced by ETU
correlated with the histologic changes in the thyroid. Withdrawal
of ETU from the diet reversed the hypothyroid conditions induced to
euthyroid (Leber et al. 1978a).
In two 90-day feeding trials Sprague-Dawley rats (12/sex/group)
were given 75 or 100 ppm ETU, and thyroid function, serum T4, T3,
and TSH, T3 uptake in vitro, 131I uptake, and thyroid to body-
weight ratios were measured at days 46 and 91. Additionally, the
fate of the incorporated 131I was traced in thyroid fractions at the
100 ppm level. Groups of both sexes at the lower feeding level and
the females at 100 ppm were functionally euthyroid whereas males at
100 ppm were somewhat hypothyroid despite elevated serum T3, TSH,
and T3/T4 ratios. Results showed that the inhibitory effects of
ETU are similar to those for methimazole. ETU inhibited
monoiodotyrosine (MIT) utilization, and the coupling of
diiodotyrosine (DIT) residues to form T4, resulting in
significantly reduced active synthesis of T3 and T4 prohormones
(males 100 ppm). The capacity of serum to bind T3 was reduced;
however, there was no evidence for inhibition by ETU of T4 to T3
monodeiodination or interference with the normal feedback mechanisms
of thyroid hormones on TSH secretion (O'Neil & Marshall, 1984).
Observations in humans
A 53-year old female employed in the manufacture of products
from synthetic and natural rubber developed itching of the fingers.
Her condition improved on weekends but became worse upon returning
to work. Two months later the eruption spread to her forearms. The
individual showed positive reactions to nickel and cobalt in the
ICDRG standard patch test as well as to a component of the rubber
material from her work which was ETU. Additional patch testing with
ETU and chemically related substances showed that ETU tested
positive at dose levels at or above 0.01% (w/v). One-percent
concentrations of dibutyl-, diethyl- or diphenylthiourea all were
negative as were ethyleneurea and ethylenediaminine. Thiourea at
0.01% (w/v) was also negative. The fungicides zineb and maneb tested
negative and positive, respectively. The positive reaction to maneb
was attributed to the presence of ETU as an impurity/degradation
product and confirmed in studies using thin layer chromatography
(Bruze & Fregert, 1983).
A retrospective study of women who were employed at a rubber
manufacturer using ETU was undertaken to determine any excess of
fetal abnormalities occurring in children to women who had worked
with ETU during early pregnancy. The women were employed on hand and
machine trimming, hand cutting, coolant hose manufacture, packing
and dispatch. Women were also employed in moulding, cutting blanks
for moulding and tool and die trimming where there was a hazard from
inhalation of dust. Women born in 1918 or later who left employment
between 1963-1971 were surveyed. Of 699 women who left between
1963-1971, 255 gave birth to 420 children. Only 59 of 255 were
working at the plant at the time of early pregnancy and none of
these had abnormal children. Of the total 420 children born 11 had
abnormalities. However, figures for the group showed no excess over
the expected number of fetal abnormalities in the region surveyed.
The study did not demonstrate any risk of teratogenesis. However,
the number of women exposed during early pregnancy was small.
A total of 1929 workers engaged in the production or
manufacture of ETU were surveyed retrospectively for thyroid cancer,
and compared with the thyroid cancer list of the Birmingham
(England) Cancer Registry from 1957-1971. No thyroid cancers
occurred in these workers and the results were considered to be
preliminary (Smith, 1976).
An apparent increased incidence of miscarriages among workers
at a USA rubber products factory was investigated. Of 7 pregnancies
only 2 resulted in spontaneous abortions, one with an identified
medical problem. Before starting work at the rubber factory, the 81
women surveyed had 192 pregnancies with 16% spontaneous abortions.
There was no indication that menstrual problems increased with
duration of employment as a moulder. It was concluded that no
adverse reproductive effects could be attributed to the work
environment - although the small numbers available for study
prevented this possibility from being entirely ruled out (Wright et
al., 1981).
No hazard of clinical thyroid depression existed based on
medical evidence collected on workers exposed to ETU at a rubber
company in Michigan. Fifty-one subjects were evaluated (49 males, 2
females). Environmental sampling results demonstrated that workers
were exposed to trace amounts of ETU as airborne dust or through
direct contact of powdered ETU (Salisbury & Lybarger, 1977).
Clinical examinations and thyroid function tests were carried
out over a period of 3 years in the United Kingdom on 8 workers
involved in the manufacture of ETU and 5 workers involved in mixing
of ETU with rubber. The average length of exposure in workers
associated in the manufacturing process was 10 years with a range of
5-20 years. The exposure period for mixers was 3 years. All
subjects were males with an age range from 26-62 years. Matched
controls were also examined. In the manufacturing plant ETU levels
of 330 µg/m3 were recorded on one personal sampler. Background
levels ranged from 10-240 µg/m3. Levels of ETU recorded on
personal samplers of mixers ranged from 120-160 µg/m3. Mixers but
not process workers had significantly lower levels of T4 in their
blood compared to controls. No effects were found on TSH or thyroid
binding globulin. The authors concluded that there was no evidence
that thyroid function is severely affected by exposure to ETU at the
levels experienced by these workers nor was there any evidence of
any effect. However, the T4 results in the exposed workers were
generally lower than those in the control group with most of the
difference in distribution accounted for by the results from the
mixers (Smith, 1984).
The concentration of ETU in pesticide formulations and ambient
air were measured and exposure to maneb (80% powder) or mancozeb
(Ridomil 56% mancozeb) evaluated during the spraying of potato
fields. The mean tank mix concentrations of maneb and mancozeb were
4.0 or 7.0 g/litre, respectively. Spraying time was 0.5-7.0 hours
(mean 4.0 hours). Each single application was separated by several
weeks. Therefore only acute (single day) exposure was determined in
workers (i.e. mixer/loader/applicator).
The overall range of concentrations of ETU in air were between
0.004 and 3.3 µg/m3 in the breathing zone and 0.006 and 0.8 µg/m3
in the (closed) tractor cabin. The mean calculated concentrations
of active maneb and mancozeb in air were 7 and 20 µg/m3,
respectively. The total inhaled amount of ETU and EBDCs was 5.0 and
126.0 µg/day, respectively corresponding to a dose of 0.07 µg ETU/kg
bw and 1.8 µg EBDC/kg bw for applicators and mixer and loaders
weighing 70 kg. Patch samples on clothes and skin (back, chest,
shoulders and forearm) indicated that 1.4, 10.0, 4.0 and 1.2% of ETU
(0.07; 0.19; 0.39; 0.17 mg/cm2/hour) reached the skin respectively.
ETU in urine sample taken on days 1, 8, 15 and 22 ranged between
0.09-2.5; 0.07-1.0; 0.01-0.30 and < 0.01-0.2 µg/mmol creatinine.
Absolute concentrations of ETU in all samples ranged between < 0.2
and 11.8 µg/litre of urine. Under the exposure conditions the
urinary elimination half-life was calculated to be 100 hours
(Kurrtio et al., 1990).
COMMENTS
Following oral administration to mice essentially all of the
ETU was recovered in the excreta within 48 hours; none was recovered
as carbon dioxide. Approximately 50% of the administered dose was
found in urine as unchanged ETU.
After the oral administration of radiolabelled ETU, its
concentration in both pregnant mice and rats peaked about the same
time (1.4 hours), with concentrations in maternal and fetal tissues
similar at 3 hours. The half-life of elimination from mice and rats
was 5.5 hours and 9.4 hours, respectively. Approximately 70% of ETU
was found in urine in both species at 48 hours.
Mice metabolize ETU primarily by the flavin-monooxygenase
system and rats by the P-450 system of enzymes.
ETU is slightly toxic after acute oral administration, with the
LD50 ranging from 545 mg/kg bw in pregnant rats to 4000 mg/kg bw in
adult mice.
In a 13-week study in mice at dietary concentrations of 0, 125,
250, 500, 1000 or 2000 ppm the NOAEL was 250 ppm (equivalent to 38
mg/kg bw/day). Diffuse follicular cell hyperplasia of the thyroid
and hepatocellular cytomegaly were observed at 500 ppm.
In a three-month study in mice at dietary concentrations of 0,
1, 10, 100 or 1000 ppm the NOAEL was 10 ppm, equal to 1.7 mg/kg
bw/day. ETU produced thyroid follicular cell hyperplasia and
decreased colloid density at 100 ppm.
The NOAEL in a study in which rats were fed dietary
concentrations of ETU at 0, 0.63, 1.3, 2.5, 5.0 or 25 ppm for 8
weeks was 25 ppm (equal to 2.6 mg/kg bw/day), the highest dose
tested.
In a 13-week study in rats, ETU was administered in the diet at
concentrations of 0, 60, 125, 250, 500 or 750 ppm. The NOAEL was
less than 60 ppm (equal to 3.0 mg/kg bw/day) based on
histopathological findings of diffuse follicular cell hyperplasia in
the thyroid.
In a 90-day study in rats, ETU was administered in the diet at
concentrations of 0, 1, 5, 25, 125 or 625 ppm. The NOAEL was 25 ppm
(equal to 1.7 mg/kg bw/day) based on hyperaemia of the thyroids,
with and without enlargement, increased thyroid to brain weight
ratio, decreased 125I thyroid uptake, decreased triiodothyronine,
decreased thyroxine and increased thyroid follicular cell
hyperplasia at 125 ppm.
In a four-week feeding study in dogs at dietary concentrations
of 0, 200, 980 or 4900 ppm, the NOAEL was 200 ppm, equal to 6.7
mg/kg bw/day. Decreased body-weight gain, decreased thyroxine and
T3 levels and enlarged thyroids were observed at 980 ppm.
In a 13-week feeding study in dogs at dietary concentrations of
0, 10, 150 or 2000 ppm the NOAEL was 10 ppm, equal to 0.39 mg/kg
bw/day. At 150 ppm haemoglobin, packed cell volume, and red blood
cell count were decreased, and cholesterol was increased. Effects
on the thyroid were found only at 2000 ppm.
In a 52-week feeding study in dogs at dietary concentrations of
0, 5, 50, or 500 ppm, the NOAEL was 5 ppm, equal to 0.18 mg/kg
bw/day. At 50 ppm a reduction in body-weight gain, hypertrophy of
the thyroid with colloid retention, a slight increase in thyroid
weight and pigment accumulation in the liver were observed.
Male and female mice received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,330; 0,1000; 330,0; 330,330; 330,1000; 110,330 or 33,100 ppm.
Mice receiving perinatal exposure only (330,0 ppm) showed no effect
on the incidences of neoplasms after 2 years. Cytoplasmic
vacuolization of follicular cells of the thyroid was evident in
males and females at 33,100 ppm, but no increases in neoplasms of
the liver, pituitary or thyroid were observed. T4 values were
significantly decreased in both sexes and thyrotropine was slightly
elevated. Animals receiving 330 ppm during adulthood showed tumours
of either the liver, pituitary or thyroid. Increasing perinatal
exposure from 0 to 330 ppm was associated with an increased
incidence of thyroid and pituitary lesions in female mice receiving
adult exposure to 330 ppm, but there were no enhancing effects of
perinatal exposure in mice receiving adult exposures of 1000 ppm
when compared to adults in the 0,1000 ppm group.
Rats were fed dietary concentrations of ETU at levels of 0, 5,
25, 125, 250 or 500 ppm for 2 years. The NOAEL was 5 ppm,
equivalent to 0.25 mg/kg bw/day. Vascularity and hyperplasia of the
thyroid were seen at 25 ppm.
In a two-year feeding study in rats using dietary
concentrations of 0, 0.5, 2.5, 5 or 125 ppm the NOAEL was 5 ppm
(equal to 0.37 mg/kg bw/day) based on changes in clinical chemistry,
increased triiodothyronine, decreased thyroxine, increased thyroid
weight, increased liver weight and an increased incidence and
severity of diffuse thyroid follicular cell hyperplasia at 125 ppm.
In a two-year carcinogenicity study in rats using dietary
concentrations of 0, 175 or 350 ppm, thyroid carcinomas and
hyperplastic goitres were observed in both sexes at 175 ppm
(equivalent to 8.8 mg/kg bw/day).
Male and female rats received perinatal (F0) and adult (F1)
exposure to ETU at the following dietary concentrations (F0,F1);
0,0; 0,83; 0,250; 90,0; 90,83; 90,250; 30,83 or 9,25 ppm. Rats
receiving perinatal and adult exposure of 9,25 ppm showed no
increase in tumours and no apparent biologically meaningful changes
in thyroid hormone function at two-years when compared to 0,0 ppm
controls. Thyroid hyperplasia was evident in both sexes. At 9
months, animals given 9,25 ppm manifested decreased T3 and T4
values and increased thyrotropine without evidence of thyroid
follicular cell hyperplasia. Males and females receiving a dose of
90,0 ppm showed no hormonal changes and no tumours at 2 years.
Thyroid follicular cell hyperplasia was, however, evident. Animals
receiving adult exposure showed a significant increase in thyroid
follicular cell tumours at 83 and 250 ppm (males) and 250 ppm
(females). Males and females showed no significant differences in
the number of tumours between dose groups of 0,83; 30,83; and 90,83
ppm. Males and females receiving 90,250 ppm showed increases in
thyroid follicular cell tumours when compared to 0,250 ppm. At the
end of 2 years males and females receiving 0,83 or 0,250 manifested
increased numbers of thyroid tumours when compared to 0,0 ppm
controls.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 2.5, 25 or 125 ppm the NOAEL was 2.5 ppm, equal
to a range of 0.16-0.38 mg/kg bw/day, based on thyroid gland
follicular cell hyperplasia and hypertrophy at 25 ppm.
An oral teratogenicity study conducted in rats at dose levels
of 0, 5, 10, 20, 40 or 80 mg/kg bw/day indicated no maternal
toxicity at 40 mg/kg bw/day (NOAEL). Maternal lethality was
observed at 80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity
effects was 5 mg/kg bw/day based on teratogenic effects observed at
10 mg/kg bw/day.
An oral teratogenicity study in rats at dose levels of 0, 15,
25 or 35 mg/kg bw/day was conducted. No maternal toxicity was
observed at 35 mg/kg bw/day (NOAEL). The NOAEL for
embryo/fetotoxicity and teratogenicity was 15 mg/kg bw/day based on
higher incidences of dilated brain ventricles at 25 mg/kg bw/day.
Oral teratogenicity studies in rats (0, 10, 20, 30, 40 or 50
mg/kg bw/day), mice (0, 200, 400 or 800 mg/kg bw/day) and hamsters
(0, 90, 270 or 810 mg/kg bw/day) revealed no maternal toxicity at
the doses tested. The NOAEL for embryo/fetotoxicity in the rat was
10 mg/kg bw/day based on dilation of the lateral or fourth ventricle
at 20 mg/kg bw/day. The NOAEL for embryo/ fetotoxicity in the
hamster was 90 mg/kg bw/day based on a decrease in fetal body weight
at 270 mg/kg bw/day. The NOAEL for mice was greater than 800 mg/kg
bw/day.
In an oral teratogenicity study, rabbits received 0, 5, 10, 20,
40 or 80 mg/kg bw/day of ETU. The NOAEL for maternal toxicity was
80 mg/kg bw/day. The NOAEL for embryo/fetotoxicity was 40 mg/kg
bw/day based on an increase in resorption sites, decreased brain
weight and a degeneration of the proximal convoluted tubules in the
kidneys of fetuses at 80 mg/kg bw/day. Malformations were not
observed at the highest dose.
A study with pregnant rats administered ETU, T3/T4 and sodium
iodide in combination indicated a reduction in some of the
teratogenic responses when compared with groups administered ETU
alone. These results indicate that the teratogenic potential of ETU
may in part be secondary to the thyroid toxicity of ETU.
ETU has been the subject of many in vitro and in vivo
studies for genotoxicity. It induces mutations in bacteria at very
high doses but variable responses have been obtained in other types
of mutation assays. Acceptable assays for other genotoxicity
endpoints in vitro were generally negative, while all in vivo
assays were negative. The Meeting concluded that ETU was not
genotoxic.
An ADI was allocated based upon a NOAEL of 0.39 mg/kg bw/day in
the 13-week study in dogs, since this dose level was between the
NOAEL of 5 ppm (equal to 0.18 mg/kg bw/day) and the middle dose
(effect level) of 50 ppm (equal to 1.8 mg/kg bw/day) in the 52-week
dog study. A 100-fold safety factor was applied.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 10 ppm, equal to 1.7 mg/kg bw/day (3-month study)
Rat: 5 ppm, equal to 0.37 mg/kg bw/day (two-year study)
2.5 ppm, equal to a range of 0.16-0.38 mg/kg bw/day
(reproduction study)
Dog: 10 ppm, equal to 0.39 mg/kg bw/day (13-week study)
5 ppm, equal to 0.18 mg/kg bw/day (52-week study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw.
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Agrelo, C. & Amos, H. (1981) DNA repair in human fibroblasts. Prog.
Mutation Research, 1: 528-532.
Allen, J.R., Van Miller, J.P. & Seymour, J.L. (1978). Absorption
tissue distribution and excretion of C14-ethylenethiourea by the
rhesus monkey and rat. Res. Comm. Chem. Pathol. Pharmacol. 20(1):
109-115.
Althaus, F.R., Lawrence, S.D., Sattler, G.L., Longfellow, D.G. &
Pitot, H.C. (1982). Chemical quantification of unscheduled DNA
synthesis in cultured hepatocytes as an assay for rapid screening of
potential chemical carcinogens. Cancer Res. 42: 3010-3015.
Arnold, D.L., Bickis, M.G., Nera, E.A., McGuire, P.F. & Munro, I.C.
(1982). Assessing the reversibility of thyroid lesions that result
from feeding etu. Toxicologist, 2(1): Abstract #318.
Arnold, D.L., Krewski, D.R., Junkins, D.B., McGuire, P.F., Moodie,
C.A. & Munro, I.C. (1983). Reversibility of ethylenethiourea-induced
thyroid lesions. Toxicol. Appl. Pharmacol. 67: 264-273.
Austin, G.E. & Moyer, G.H. (1979). Hepatic RNA synthesis in rats
treated with ethylenethiourea. Res. Commun. Chem. Pathol.
Pharmacol. 23(3): 639-642.
Autio, K., von Wright, A. & Pyysalo, H. (1982). The effect of
oxidation of the sulfur atom on the mutagenicity of
ethylenethiourea. Mutation Res. 106: 27-31.
Bridges, B.A., MacGregor, D. & Zeiger, E. (1981). Summary report on
the performance of bacterial mutation assays. Prog. Mutation
Research, 1: 49-67.
Briffaux, J.P. (1991). ETU: 13 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/504 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Briffaux, J.P. (1992). ETU: 52 week oral (dietary) toxicity study in
the beagle dog. Unpublished report No. 616/505 from Hazleton Labs.,
Lyon, France. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay with preincubation.
Prog. Mutation Research, 1: 261-270.
Bruze, M. & Fregert, S. (1983). Allergic contact dermatitis from
ethylenethiourea. Contact Dermatitis, 9: 208-212.
Carver, J.H., Salazar, E.P., Knize, M.G. & Wandres, D.L. (1981).
Mutation induction at multiple gene loci in Chinese hamster ovary
cells: the genetic activity of 15 coded carcinogens and
noncarcinogens. Prog. Mutation Research, 1: 594-601.
Casto, B.B. (1975, 1976). Progress report NIH-NCI-NOI-CP45615, pp.
1-23, 1975; pp. 1-18 (1976). (Cited in Heidelberger et al., 1983).
Chernoff, N., Kavlock, R.J., Rogers, E.H., Carver, B.D. & Murray, C.
(1979). Perinatal toxicity of maneb ethylenethiourea and
ethylenebisisothiocyanate sulfide in rodents. J. Toxicol. Environ.
Health, 5: 821-834.
Chhabra, R.S., Eustis, S., Haseman, J.K., Kurtz, P.J. & Carlton,
B.D. (1992) Comparative carcinogenicity of ethylenethiourea with or
without perinatal exposure in rats and mice. Fundamental and
Applied Toxicology, 18(3): 405-417.
Crebelli, R., Bellincampi, D., Conti, G., Conti, L., Morpurgo, G. &
Carere, A. (1986). A comparative study on selected chemical
carcinogens for chromosome malsegregation, mitotic crossing-over and
forward mutation induction in Aspergillus nidulans. Mutation
Research, 172: 139-149.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Prog. Mutation Research, 1: 626-637.
Daston, G., Ebron, M.T., Carver, B. & Stefanadis, J.G. (1987) In
vitro teratogenicity of ethylenethiourea in the rat. Teratology,
35: 239-245.
Daston, G.P., Yonker, J.E., Powers, J.F. & Heitmeyer, S.A. (1989).
Difference in teratogenic potency of ethylenethiourea in rats and
mice: relative contribution of embryonic and maternal factors.
Teratology, 40: 555-566.
Decker, C.J. & Doerge, D.R. (1991). Rat hepatic microsomal
metabolism of ethylenethiourea. Contributions of the flavin-
containing monooxygenase and cytochrome P-450 isozymes. Chem. Res.
Toxicol. 4: 482-489.
DiDonato, L.J. & Longacre, S.L. (1987). Ethylenethiourea:
dermal/oral absorbtion study in male rats. Unpublished report No,
85R-0206 from Rohm and Haas Company, Spring House, Pennsylvania,
USA. Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Doerge, D. & Takazawa, R. (1990). Mechanism of thyroid peroxidase
inhibition by ethylenethiourea. Chem. Res. Toxicol. 3: 98-101.
Dotti, A. (1992). Ethylenethiourea (ETU): two-generation
reproduction study in the rat. Unpublished report No. 252360 from
Research and Consulting Company, Itingen, Switzerland. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Emmerling, D.C. (1978a). A study of the uptake and elimination of
C14 activity after oral ingestion of C14 labelled ethylenethiourea
(ETU) and mancozeb in the rhesus monkey. Revised final report,
Battelle Labs., Columbus, Ohio, USA. (July 31, 1978).
Emmerling, D.C. (1978b). The effects of thyroid hormones on the
teratogenic potential of ethylenethiourea in rats. Unpublished
report No. 78RC-1027 from Battelle Labs, Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Engst, R. & Schnaak, W. (1974). Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev. 52:
45-67.
Evans, E.L. & Mitchell, A.D. (1981). Effects of 20 coded chemicals
on sister chromatid exchange frequencies in cultured chinese hamster
cells. Prog. Mutation Research, 1: 538-550.
Frank, J. & Muller, G. (1988). ETU: In vivo/in vitro UDS/S-phase
assay in mice. Unpublished report No. 88R-47 from Rohm and Haas
Company, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
and Haas Company, Spring House, Pennsylvania, USA.
Freudenthal, R.I., Kerchner, G., Persing, R. & Baron, R.L. (1977).
Dietary subacute toxicity of ethylenethiourea in the laboratory rat.
J. Environ. Pathol. Toxicol. 1: 147-161.
Galloway, S.M. & Ivett, J.L. (1986). Chemically induced aneuploidy
in mammalian cells in culture. Mutation Research, 167: 89-105.
Gak, J.C., Graillot, C. & Truhaut, R. (1976). Difference in
sensitivity of the hamster and rat to the effects of long term
administration of ethylenethiourea. Eur. J. Toxicol. 9: 303-312.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the "microtiter" fluctuation test. Prog. Mutation Research, 1:
376-386.
Graham, S.L. & Hansen, W.H. (1972). Effects of short-term
administration of ethylenethiourea upon thyroid function of the rat.
Bull. Environ. Contam. Toxicol. 7(1): 19-25.
Graham, S.L., Hansen, W.H., Davis, K.J. & Perry, C.H. (1973).
Effects of one-year administration of ethylenethiourea upon the
thyroid of the rat. J. Agric. Food Chem. 21: 324-329.
Graham, S.L., Davis, K.J.Hansen, W.H. and Graham, C.H. (1975).
Effects of prolonged ethylenethiourea ingestion on the thyroid of
the rat. Food Cosmet. Toxicol. 13: 493-499.
Green, M.H.L. (1981). A differential killing test using an improved
repair-deficient strain of Escherichia coli. Prog. Mutation
Research, 1: 183-194.
Hardin, B., Schuler, R., Burg, J., Booth, G., Hazelden, K.,
MacKenzie, K., Piccirillo, V. & Smith, K. (1987). Evaluation of 60
chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen. 7: 29-48.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putnam, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W. &
Schechtman, L.M. (1986). Chemical enhancement of SA7 virus
transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagen. 8:
515-531.
Heddle, J.A., Hite, M., Kirkhart, B., Mavournin, K., MacGregor,
J.T., Newell, G.W. & Salamone, M.F. (1983). The induction of
micronuclei as a measure of genotoxicity. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 123: 61-118.
Heidelberger, C., Freeman, A.E., Pienta, R.J., Sivak, A., Bertram,
J.S., Casto, B.C., Dunkel, V.C., Francis, M.W., Kekunaga, T.,
Little, J.B. & Schechtman, L.M. (1983). Cell transformation by
chemical agents - a review and analysis of the literature. US-EPA
Gene-Tox Program. Mutation Research, 114: 283-385.
Hill, R., Erdreich, L., Paynter, O., Roberts, P., Rosenthal, S. &
Wilkinson, C. (1989). Thyroid follicular cell carcinogenesis. Fund.
Appl. Toxicol. 12: 629-697.
Hui, Q.Y., Armstrong, C., Laver, G. & Iverson, F. (1988).
Monooxygenase-mediated metabolism and binding of ethylenethiourea to
mouse liver microsomal protein. Tox. Letters. 41: 231-237.
Ichinotsubo, D., Mower, H. & Mandel, M. (1981a). Testing of a series
of paired compounds (carcinogen and noncarcinogen structural analog)
by DNA repair-deficient E. coli stains. Prog. Mutation Research,
1: 195-198.
Iverson, F., Khera, K.S. & Hierlihy, S.L. (1980). In vivo and in
vitro metabolism of ethylenethiourea in the rat and the cat.
Toxicol. Appl. Pharmacol. 52: 16-21.
Jagannath, D.R., Vultaggio, D.M. & Brusick, D.J. (1981). Genetic
activity of 42 coded compounds in the mitotic gene conversion assay
using Saccharomyces cerevisiae strain D4. Prog. Mutation
Research, 1: 456-467.
Jordan, L.W. & Neal, R.A. (1979). Examination of the in vivo
metabolism of maneb and zineb to ethylenethiourea (ETU) in mice.
Bull. Environm. Contam. Toxicol. 22: 271-277.
Jotz, M.M. & Mitchell, A.D. (1981). Effects of 20 coded chemicals on
the forward mutation frequency at the thymidine kinase locus in
L5178Y mouse lymphoma cells. Prog. Mutation Research, 1: 580-593.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Prog. Mutation Research, 1: 175-182.
Kassinova, G.V., Kovaltsova, S.V, Marfin, S.V. & Zakharov, I.A.
(1981). Activity of 42 coded compounds in differential inhibition
and mitotic crossingover assays in yeast. Prog. Mutation Research,
1: 434-455.
Kato, Y., Odanaka, Y., Teramoto, S. & Matano, O. (1976). Metabolic
fate of ethylenethiourea in pregnant rats. Bull. Environ. Contam.
Toxicol. 16: 546-555.
Khera, K. (1973a). Teratogenic effects of ethylenethiourea in rats
and rabbits, Toxicol. Appl. Pharmacol. 25: 455-456.
Khera, K.S. (1973b) Ethylenethiourea: teratogenicity study in rats
and rabbits. Teratology, 7: 243-252.
Khera, K.S. & Iverson, F. (1978). Toxicity of ethylenethiourea in
pregnant cats. Teratology, 18: 311-314.
Khera, K. (1987). Ethylenethiourea: a review of teratogenicity and
distribution studies and an assessment of reproduction risk. CRC
Crit. Rev. Toxicol. 18: 129-139.
Khera, K.S, Whalen, C. & Iverson, F. (1983). Effects of pretreatment
with SKF-525A, N-methyl-2-thioimidazole, sodium phenobarbital, or 3-
methylcholanthrene on ethylenethiourea-induced teratogenicity in
hamsters. J. Toxicol. and Environ. Health. 11: 287-300.
Kier, L.E., Brusick, D.J., Auletta, A.E., Von Halle, E.S., Brown,
M.M., Simmon, V.F., Dunkel, V., McCann, J., Mortelamans, K., Prival,
M., Rao, T.K. & Ray, V. (1986). The Salmonella
typhimurium/mammalian microsomal assay. A report of the U.S.
Environmental Protection Agency Gene-Tox Program. Mutation
Research, 168: 69-240.
Kirkhart, B. (1981). Micronucleus test on 21 compounds. Prog.
Mutation Research, 1: 698-704.
Kurrtio, P., Savolainen, K., Tuominen, R., Kosma, V.M., Naukkarinen,
A., Mannisto, P. & Collan, Y. (1986). Ethylenethiourea and nabam
induced alterations of function and morphology of thyroid glands in
rat. Arch. Toxicol., Suppl. 9: 339-344.
Kurrtio, P., Vartianinen, T. & Savolainen, K. (1990). Environmental
and biological monitoring of exposure to ethylenebisdithiocarbamate
fungicides and ethylenethiourea. British Journal of Industrial
Medicine, 47: 203-206.
Kurttio, P., Savolainen, K., Naukkarinen, A., Kosma, V., Tuomisto,
L., Penttila, I. & Jolkkonen, J. (1991). Urinary excretion of
ethylenethiourea and kidney morphology in rats after continuous oral
exposure to nabam or ethylenethiourea. Arch. Toxicol. 65: 381-385.
Leber, A.P., Wilkinson, G.E., Emmerling, D., Persing, R.L. & Thake,
D.C. (1978a). A correlation of the hormonal and pathological changes
of the thyroid as related to the treatment and withdrawal of
ethylenethiourea. Unpublished report No. 78RC-1026 from Battelle
Labs., Columbus, Ohio, USA. Submitted to WHO by Rohm and Haas
Company, Spring House, Pennsylvania, USA.
Leber, A.P., Wilkinson, G.E., Persing, R.L. & Holzworth, D.A.
(1978b) Effects of feeding ethylenethiourea in the rhesus monkey.
Contract No. 68-01-4717 from Battelle Labs., Columbus, Ohio, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Legator, M.S., Bueding, E., Batzinger, R., Connor, T.H.,
Eisendstadt, E., Farrow, M.G., Ficsor, G., Hsie, A., Seed, J. &
Stafford, R.S. (1982). An evaluation of the host-mediated assay and
body fluid analysis. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Research, 98: 319-374.
Leuschner, F. (1977) Oral toxicity of ethylenethiourea 98% - called
for short ETU-during 8 weeks of administration in Sprague-Dawley
rats. Unpublished report No. 77RC-1159 from Laboratorium fur
Pharmakocopie und Toxicologie, Hamburg, West Germany. Submitted to
WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Lewerenz, H.J. & Plass, R. (1984). Contrasting effects of
ethylenethiourea on hepatic monooxygenase in rats and mice. Arch.
Toxicology, 56: 92-95.
Loprieno, N. (1981). Screening of coded carcinogenic/noncarcinogenic
chemicals by a forward-mutation system with yeast
Schizosaccharomyces pombe. Prog. Mutation Research 1: 424-433.
Lu, M.H. & Staples, R.E. (1978). Teratogenicity of ethylenethiourea
and thyroid function in the rat. Teratology, 17: 171-178.
Marshall, W. (1977). Thermal decomposition of
ethylenebisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. Agric. Food Chem. 25: 357-361.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Prog. Mutation Research, 1: 533-537.
Mason, J.M., Valencia, R. & Zimmering, S. (1992). Chemical
mutagenesis testing in drosophila: VIII. reexamination of
equivocal results. Environ. Mol. Mutagen. 19: 227-234.
Matsushita, T., Arimatsu, Y. & Nomura, S. (1976). Experimental study
on contact dermatitis caused by dithiocarbamates maneb, mancozeb,
zineb, and their related compounds. Int. Arch. Occup. Environ.
Hlth. 37: 169-178.
McGlynn-Kreft, A.M. & McCarthy, K.L. (1984). Ethylenethiourea
mammalian cell transformation test. Unpublished report No. 84R-56
from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
McGregor, D., Brown, A., Cattanach, P., Edwards, I., McBride, D,
Riach, C. & Caspary, W.J. (1988). Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded chemicals.
Environ. Mol. Mutagen. 12: 85-154.
McLeod, P.L. & Doolittle, D.J. (1985). Ethylenethiourea mammalian
cell transformation test for promotion. Unpublished report No. 84R-
0298 from Rohm and Haas Company, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Mehta, R.D. & von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
C14. Prog. Mutation Research, 1: 414-423.
Mohn, G.R., Vogels-Bouter, S. & van der Horst-van der Zon, J.
(1981). Studies on the mutagenic activity of 20 coded compounds in
liquid tests using the multipurpose strain Escherichia coli K-
12/343/113 and derivatives. Prog. Mutation Research, 1: 396-423.
Mollett, P. (1975). Toxicity and mutagenicity of ethylenethiourea
(ETU) in drosophila. Mutat. Res. 29: 254.
Morgan, C. (1991). ETU: 4 week oral (dietary administration)
feasibility study in the beagle. Unpublished report No. 6152-616/6
from Hazleton Labs., Harrogate, North Yorkshire, England. Submitted
to WHO by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986). Salmonella mutagenicity testing:II. Results
from the testing of 270 chemicals. Environ. Muta. 8(7): 1-119.
Natarajan, A.T. & van Kesteren-van Leeuwen, A.C. (1981). Mutagenic
activity of 20 coded compounds in chromosome aberrations/sister
chromatid exchanges assay using chinese hamster ovary (CHO) cells.
Prog. Mutation Research, 1: 551-559.
National Toxicology Program (NTP), 1992. Toxicology and
carcinogenesis studies of ethylenethiourea in F344/N rats and B6C3F1
mice (feed studies). U.S. Department of Health and Human Services,
Public Health Service, National Institutes of Health, NIH
Publication No. 92-2843, National Toxicology Program Technical
Report Series No. 388, March 1992.
Newsome, W.H. (1974). The excretion of ethylenethiourea by rat and
guinea-pig. Bull. Environ. Contamin. Toxicol. 11(2): 174-176.
National Institute for Occupational Safety and Health (NIOSH), 1981.
Ethylenethiourea, Health Hazard Evaluation Report No HETA-81-270-
1012, December 1981.
O'Hara, G.P. & DiDonato, L.J. (1985). Dithane M-45 and
ethylenethiourea: 3-month dietary toxicity study in mice.
Unpublished report No. 8OR-124 from Rohm and Haas Company, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
O'Neil, W. & Marshal, W. (1984). Goitrogenic effects of
ethylenethiourea on rat thyroid. Pesticide Biochemistry and
Physiology, 21: 92-101.
Paika, I.J., Beauchesne, M.T., Randall, M., Schreck, R.R. & Latt,
S.A. (1981). In vivo sce analysis of 20 coded compounds. Prog.
Mutation Research, 1: 673-681.
Parry, J. (1986). The expression of recessive markers in presumptive
monosomic colonies derived from Saccharomyces cerevisiae, strain
D6. Mutagenesis, 1: 299-300.
Parry. J.M. & Sharp, D.C. (1981). Induction of mitotic aneuploidy in
the yeast strain D6 by 42 coded compounds. Prog. Mutation Research,
1: 468-480.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of the sister
chromatid exchange method in mammalian cells as a screening system
for carcinogens. Prog. Mutation Research, 1: 560-569.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980a). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in rats. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Donorrio, D.J., Thake, D.C. & Cottrill,
D.L. (1980b). Prechronic studies of ethylenethiourea: acute,
repeated dose and subchronic in mice. Project No. G-7186. Report
submitted by Battelle Laboratories, Columbus, Ohio, USA, to NIEHS,
October 14, 1980. Submitted to WHO by Rohm and Haas Company, Spring
House, Pennsylvania, USA.
Peters, A.C., Kurtz, P.J., Chin, A.E., Carlton, B.D., Chrisp, C.E.,
and Dill, G.S. (1982). Report on the maximum neonatal dose studies
with ethylenethiourea. Contract No. N01-ES8-2151. Report submitted
by Battelle Laboratories, Columbus, Ohio to NIEHS, January 29, 1982.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Pilinskaya, M. (1982) Analysis of the cytogenetic activity of
ethylenethiourea (ETU) in human peripheral lymphoblast culture in
vitro. Dopov Akad Nauk Ukr. RSR. Ser. B: Geol, Khim Biol Nauki,
10: 66-68
Poulsen, L.L., Hyslop, R.M., Ziegler, D.M. (1979). S-Oxygenation of
N-substituted thioureas catalyzed by pig liver microsomal FAD-
containing monooxygenase. Arch. Biochim. Biophys., 198(1): 78-88.
Quillardet, P., de Bellecombe, C. & Hofnung, M. (1985). The SOS
Chromotest, a colorimetric bacterial assay for genotoxins:
validation study with 83 compounds. Mutation Research, 147: 79-95.
Rashid, K.A. & Mumma, R.O. (1986). Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21(4):
319-334.
Resnick, M.A., Mayer, V.W., and Zimmermann, F.K. (1986). The
detection of chemically induced aneuploidy in Saccharomyces
cerevisiae: an assessment of mitotic and meiotic systems.
Mutation Research, 167: 47-60.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 314-322.
Robinson, D.E. & Mitchell, A.D. (1981). Unscheduled DNA synthesis
response of human fibroblasts, WI-38 cells, to 20 coded chemicals.
Prog. Mutation Research, 1: 517-527.
Rosencranz, H.S., Hyman, J. & Leifer, Z. (1981). DNA polymerase
deficient assay. Prog. Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenicity of carcinogens and
noncarcinogens in the Salmonella/microsome test. Prog. Mutation
Research, 1: 323-332.
Ruddick, J.A. & Khera, K.S. (1975). Pattern of anomalies following a
single oral dose of ethylenethiourea to pregnant rats. Teratology,
12: 277-281.
Ruddick, J.A., Williams, D.T., Hierlihy, L. & Khera, K.S. (1976).
C14-ethylenethiourea: distribution, excretion and metabolism in
pregnant rats. Teratology, 13: 35-40.
Ruddick, J.A., Newsome, W.H. & Iverson, F. (1977). A comparison of
the distribution, metabolism and excretion of ethylenethiourea in
the pregnant mouse and rat. Teratology, 16: 159-162.
Saillenfait, A.M., Sabate, J.P., Longonne, I. & De Ceaurriz, J.
(1991). Difference in the developmental toxicity of ethylene
thiourea and three N,N'-substituted thiourea derivatives in rats.
Fundamental and Applied Toxicology, 17: 686-697.
Salamone, M.F. (1981). Mutagenic activity of 41 compounds in the in
vivo micronucleus assay. Prog. Mutation Research, 1: 686-697.
Salisbury, S.A. & Lybarger, J. (1977). Health Hazard Evaluation
Determination Report No. 77-67-499, St. Clair Rubber Co,.,
Marysville, Michigan. Ethylenethiourea. US-DHEW. October 1977.
Submitted to WHO by Rohm and Haas Company, Spring House,
Pennsylvania, USA.
Savolainen, K. & Pyysalo, H. (1979). Identification of the main
metabolite of ethylenethiourea in mice. J. Agric. Food Chem. 27:
1177-1181.
Saxton, A.D. (1972). A C14-ethylenethiourea rat-feeding study. An
experiment to determine the excretion pattern and accumulation and
decline in thyroid tissues of C14-residues. Report 23-51 from Rohm
and Haas Company, Spring House, Pennsylvania, USA. Submitted to WHO
by Rohm and Haas Company, Spring House, Pennsylvania, USA.
Schiestl, R., Gietz, R.D., Mehta, R. & Hastings, P. (1989).
Carcinogens induce intrachromosomal recombination in yeast.
Carcinogenesis. 10: 1445-1455.
Schmid, H., Tennekes, H., Janiak, T., Probst, D., Leutkemeier, H.,
Pappritz, G., Marki, U., Vogel, O., Heusner, W. (1992).
Ethylenethiourea 104 week chronic toxicity (feeding) study in rats.
Unpublished study No. 256803 from Research and Consulting Company,
Itingen, Switzerland. Submitted to WHO by Rohm and Haas Company,
Spring House, Pennsylvania, USA.
Schupbach, M. & Hummler, H. (1977). A comparative study of the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutation Research, 56: 111-120.
Seiler, J.P. (1974). Ethylenethiourea (ETU), a carcinogenic and
mutagenic metabolite of ethylene-bis-dithiocarbamate. Mutation
Research, 2: 189-191.
Seiler, J.P. (1975). In vivo mutagenic interaction of nitrite and
ethylenethiourea. Experientia, 31: 214-215.
Seiler, J.P. (1977a). Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short term test. Mutation Research, 46: 305-
310.
Seiler, J. (1977b). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutation
Research, 48: 225-236.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1. Prog.
Mutation Research, 1: 491-501.
Sharp, D.C. and Parry, J.M. (1981b). Use of repair-deficient strains
of yeast to assay the activity of 40 coded compounds. Prog.
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., Kato, K., Lienard, F., Tezuka, H.,
Teramoto, S. & Kada,T. (1977). Mutagenicity screening on pesticides
and modification products: a basis of carcinogenicity evaluation.
Cold Spring Harbor Conference Cell Proliferation, 4: 267-285.
Simmon, V.F. & Shepherd, G. F. (1981). Mutagenic activity of 42
coded compounds in the Salmonella/microsome assay. Prog. Mutation
Research, 1: 333-342.
Smith, D.M. (1976). Ethylenethiourea - a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.
26: 92-94.
Smith, D.M. (1984). Ethylenethiourea: thyroid function in two groups
of exposed workers. Brit. J. of Ind. Med. 41: 362-366.
Sram, R.J. (1975). Genetic risk from chemicals: mutagenicity studies
and evaluation. Review of Czechoslovak Medicine, 21(4): 186-193.
Stula, E.F. & Krauss, W.C. (1977). Embryotoxicity in rats and
rabbits from cutaneous application of amide-type solvents and
substituted ureas. Toxicol. Appl. Pharmacol. 41: 35.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Prog. Mutation Research, 1: 638-646.
Teramoto, S., Moriya, M., Kato, K., Tezuka, H., Nakamura, S.,
Shingu, A. & Shirasu, Y. (1977). Mutagenicity testing on
ethylenethiourea. Mutation Research, 56: 121-129.
Teramoto S., Shingu, A., Kaneda, M. & Saito, R. (1978a).
Teratogenicity studies with ethylenethiourea in rats, mice and
hamsters. Congenital Anomalies, 18: 11.
Teramoto, S., Shingu, A. & Shirasu, Y. (1978b). Induction of
dominant lethal mutations after administration of ethylenethiourea
in combination with nitrite or of N-nitro-ethylenethiourea in mice.
Mutation Research, 55: 335-340.
Teshima, R., Nagamatusu, K., Kido, Y. & Terao, T. (1981).
Absorption, distribution, excretion and metabolism of
ethylenethiourea in guinea-pigs. Eisei Kagaku, 27(2): 85-90.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Prog. Mutation Research, 1: 224-235.
Tophan, J.C. (1981). Evaluation of some chemicals by the sperm
morphology assay. Prog. Mutation Research, 1: 718-720.
Trueman, R..W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Prog. Mutation Research, 1:
343-350.
Truhaut, R., Fujita, M., Lich, N. & Chaigueau, M. (1973). Study of
metabolic transformations of zineb. C.R. Acad. Sci. Paris, Ser. D.
276: 229-233.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Prog. Mutation Research, 1: 705-711.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains WP2, WP67
(uvrA polA), and CM871 (uvA lexA recA). Prog. Mutation Research,
1: 199-209.
Ulland, B.M., Weisburgr, J.H., Weisburger, E.K., Rice, J.M. &
Cypher, R. (1972). Thyroid cancer in rats from ethylenethiourea
intake. J. Nat. Cancer Inst. 49: 583-584.
US EPA (1992) Ethylenebisdithiocarbamates (EBDCs); notice of intent
to cancel and conclusion of special review. Federal Register, 57:
7484-7530.
Valencia, R. & Houtchens, K. (1981). Mutagenic activity of 10 coded
compounds in the Drosophila sex-linked recessive lethal test.
Prog. Mutation Research, 1: 651-659.
Van't Hof, J. & Schairer, L.A. (1982). Tradescantia assay system for
gaseous mutagens. A report of the U.S. Environmental Protection
Agency Gene-Tox Program. Mutation Res. 99: 303-315.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985).
Chemical mutagenesis testing in drosophila. V. Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutagenesis, 7: 677-702.
Wright, W.E., Peters, J.M., Plyler, E.A. & Belanger, P.L. (1981).
NIOSH Health Hazard Evaluation Report HETA 81-270-1012. December,
1981.
Wyrobek, A., Gordon, L. & Watchmaker, G. (1981). Effect of 17
chemical agents including 6 carcinogen/noncarcinogen pairs of sperm
shape abnormalities in mice. Prog. Mutation Research, 1: 712-717.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Prog. Mutation Research, 1: 481-490.
