
BIORESMETHRIN
First draft prepared by Dr. W. Phang,
US Environmental Protection Agency,
Washington, D.C., United States
EXPLANATION
Bioresmethrin is a d- trans-isomer of resmethrin which consists
of four isomers (35% d- trans-isomer, 35% l- trans-isomer, 15%
d- cis-isomer, and 15% l- cis-isomer). The Joint Meeting evaluated
the available toxicological information in 1976 and concluded that
long-term studies on bioresmethrin were needed before an ADI could be
allocated (Annex I, 26). Recently, a combined long-term
feeding/oncogenicity study in rats, a two-generation reproduction
study in rats, a rat teratology study, a rat metabolism study, a
rabbit teratology study, and several acute toxicity studies have
become available. In addition, a mouse oncogenicity study and a
108-days feeding study in dogs with resmethrin which contains at least
30% of bioresmethrin were also available. These studies are evaluated
and summarized. To facilitate the evaluation of the toxicological
profile of this compound, sections of the 1977 FAO monograph on
bioresmethrin are reproduced in their entirety in this monograph
addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
The absorption, distribution, and excretion of bioresmethrin were
discussed by the 1976 Joint Meeting (Annex I, 27). Recently, a rat
metabolism study on [14C-acid]-d- trans-resmethrin has become
available; the results are summarized along with the previously
published information.
Following oral administration, bioresmethrin is rapidly absorbed
from the gut and widely distributed in the body within 3 hours. The
distribution of 3H-bioresmethrin following oral or iv administration
to rats was monitored with radio-autographic techniques. At 24 hours
following oral administration, most tissues showed greatly reduced
residual radioactivity, but concentration in adipose tissue,
mesenteric skin, testes, epididymus, lacrymal gland, and connective
tissue was high. The excretion of 3H activity into the bile duct was
demonstrated with rats surgically cannulated to collect the bile.
Shortly after iv treatment, 3H was found in the bile (50% after 24
hours and 60% after 72 hours), and a large amount of the radioactivity
was recovered in the faeces suggesting significant enterohepatic
circulation (Farebrother, 1973, as cited in Annex I, 27).
Following oral administration to rats (14C-carboxyl label, 0.87
mg/kg), bioresmethrin was slowly eliminated from the body with only
73% of the administered dose accounted for in the accounted for 32% in
faeces and 41% in urine after 6 days (means from two experiments).
After 6 days, the highest residue level was found in fat. The order of
residue levels seen in various tissues was fat > blood > lung >
kidney > liver > heart > muscle > spleen >> brain.
After two weeks excretion was not complete (Ueda et al., 1975b).
Qualitative identification of several metabolites of bioresmethrin was
performed. Intact bioresmethrin was not found in the urine or faeces.
The most slowly excreted metabolite arose from the alcohol moiety of
bioresmethrin whereas those arising from the acid moiety were rapidly
excreted.
In a recent metabolism study, groups of rats (5/sex/dose)
received either a high dose (200 mg/kg), a low dose (1 mg/kg), or
repeated dose (1 mg/kg) of [14C-acid]-d- trans-resmethrin. At 12
hours after dosing, the amount of radioactivity eliminated in the
urine was greater than that in the faeces in all test animals;
however, at day 1 or later, slightly more radioactivity was eliminated
in faeces that in the urine. By day 2 after dosing, essentially all
radioactivity was eliminated from the body. Significant amount of
radioactivity was not detected in any tissue (Ruzo, 1991).
Biotransformation
Most of the data on biotransformation of bioresmethrin in the
laboratory animals were evaluated by the 1975 and 1976 Joint Meetings
(Annex I, 25, 27). Most of the information presented in the 1977 FAO
monograph is reproduced below.
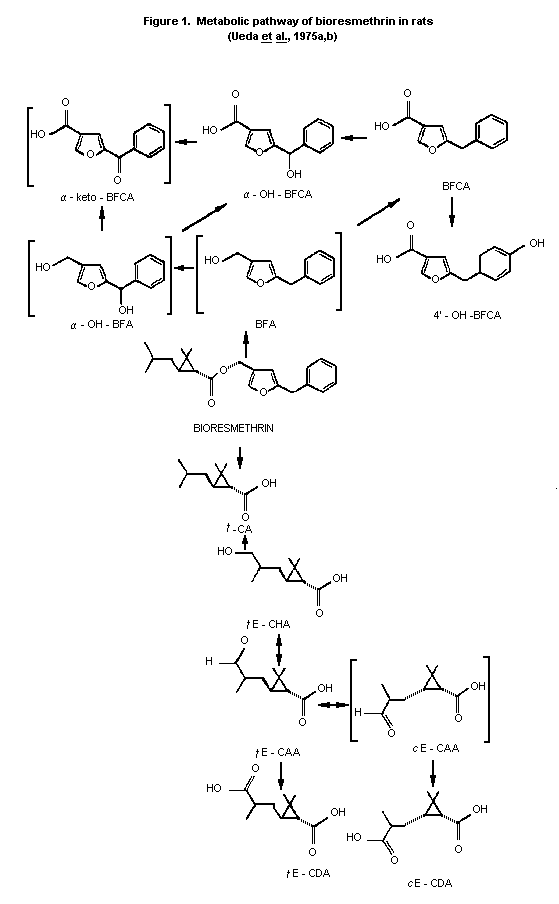
As shown in Figure 1, the biotransformation of bioresmethrin is
a complex process with several reactions occurring simultaneously at
various positions in the molecule. The initial step in the metabolism
is cleavage at the ester linkage, a reaction found to be catalyzed by
esterases localized in the liver microsome. Transisomerization was
reported with bioresmethrin but was apparently limited to the
isomerization of the metabolites. In addition, this process was seen
only when bioresmethrin was administered at low levels.
Transisomerization was not noted on administration of higher levels by
ip injection (low dose = 1 mg/kg; high dose = 3 gm/rat over a period
of 3 days administered 2X/day). Transisomerization occurred only with
the acid portion of the molecule as observed in Figure 1 (tE-CAA,
cE-CDA), which shows the probable metabolic route for both the acid
and alcohol moieties of bioresmethrin in rats.
In vitro studies with rat and mouse liver preparations
suggested mouse liver esterases hydrolyzed bioresmethrin
((d)- trans-resmethrin isomer) rapidly relative to the corresponding
cis-isomers ((d)- or (l)- cis-resmethrin) while microsomal enzymes
oxidize the (d)- trans-isomer, bioresmethrin, more slowly the
cis-isomers (Ueda et al., 1975a). When administered to rats,
bioresmethrin undergoes a complicated series of reactions involving
initial ester cleavage and subsequent oxidation with or without
conjugation of both the acid and alcohol metabolites.
Bioresmethrin is degraded by ester cleavage and the alcohol
moiety is oxidized to 5-benzyl-3-furylmethanol (BFA),
5-benzyl-3-furoic acid (BFCA), 4'-hydroxy BFCA and alpha-hydroxy BFCA
(alpha-OH-BFCA) (Figure 1). The chrysan-themate (acid) moiety
undergoes oxidation from trans-chrysanthemic acid (t-CA) to
2,2-dimethyl-3-(2'hydroxy-methyl-1'-propenyl) cyclopropane carboxylic
acid (tE-CHA) (oxidative metabolism at the methyl group of the
isobutenyl side chain trans (E) to the cyclopropane). This is
further oxidized through the formyl derivative (CAA) to the
dicarboxylic acid isomers (tE-CDA and cE-CDA). It is at the CAA
oxidation stage where isomerization may occur through the proposed
aldehyde (cE-CAA) intermediate to (cE-CDA) the cis-dicarboxylic acid
(Ueda et al., 1975b). This metabolic sequence may also account for
 the consideration of Verschoyle & Barnes (1972) that as a delay in
signs of poisoning was evident following iv administration,
bioresmethrin might be converted in vivo to a toxic metabolite. The
presence of (d)- trans-CA, BFA, and BFCA as metabolites, which are
more toxic than bioresmethrin, may account for their observation and
conclusions.
The recent bioresmethrin metabolism study indicated that
bioresmethrin was metabolized by a combination of hydrolytic,
oxidative, and conjugative processes. The results of metabolite
determinations were consistent with those of the 1975 papers of Ueda
et al. (Ruzo, 1991).
Toxicological studies
Acute toxicity
Much of the information on the acute toxicity of bioresmethrin
had been evaluated by the 1976 Joint Meeting and published in the
monograph (Annex I, 27). Some new data have become available, and
they have been summarized along with the published information in
Table 1.
Two or more hours after oral administration, the treated animals
showed signs of aggressiveness and tremors. The final stages of
poisoning consisted of convulsive twitching, prostration, coma, and
death normally between 3 and 24 hours (Annex I, 27). The recent
findings in clinical signs also include hypotonicity, slightly arched
back, and piloerection at 30 minutes after treatment (Audegond,
1989a,b).
Table 1. Acute toxicity of bioresmethrin
Species Sex Route LC50 LC50 Reference
(mg/kg b.w.) (mg/L)
Rat m/f oral >5000 Audegond, 1989a
m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chescher & Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
m/f inh. (4 h) >5.3 Hardy et al., 1989
f inh. (24 h) >872 Wallwork & Malone, 1972
f dermal >10 000 Wallwork et al., 1970
Rabbit f dermal >2000 Audegond, 1989b
Mouse f oral >10 000 Wallwork et al., 1970
m oral 3100 Ueda et al., 1975b
m ip >1500 Ueda et al., 1975b
Chicken oral >10 000 Wallwork et al., 1970
>10 000 Chester & Malone, 1970a
Acute toxicity of metabolites
The data on the acute toxicity of the metabolites of
bioresmethrin were evaluated in the 1976 Joint Meeting and published
in the monograph (FAO, 1977). No new data on the acute toxicity of
these metabolites are available; therefore, the previously published
data (Annex I, 27) are reproduced in its entirety in Table 2.
Table 2. Acute toxicity of the metabolites of bioresmethrin
(as cited in Annex 1, 27, animal species not named in
original)
Metabolite LD50 (mg/kg/bw)a
ip oral
1) (+)-trans-resmethrin (bioresmenthrin) > 1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-trans-CA (t-CA) 98 280
(+)-trans-chrysanthemic acid
3) (+)-trans-CDA (tE-CDA) 408
(+)-trans-chrysanthemundicarboxylic
acid
4) BFA 75 310
5-benzyl-3-furylmethanol
5) BFCA 46
5-benzyl-3-furoic acid
Short-term studies
New data on short-term studies are not available; however, the
1976 Joint Meeting had evaluated several short-term studies on rats
and dogs. The summaries of these study are published in the monograph
(Annex I, 27), and they are reproduced below.
Rats
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage six days per week for 3 weeks at doses of 0, 1000,
and 2000 mg/kg body weight. There was no mortality attributable to
bioresmethrin. There was a slight reduction in body weights at 2000
mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and haematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks,
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated, as cited in Annex I,
27).
Groups of rats (18/sex/group) were fed bioresmethrin at dietary
concentrations of 0, 400, 1200, and 8000 ppm (the last dose group was
fed 4000 ppm for 30 days, and the level was increased thereafter) for
91 days. There was no mortality observed in this study. Food
consumption was normal, and food conversion was unaffected by
bioresmethrin. Growth was reduced at the highest dose level which was
accompanied by changes in blood chemistry parameters indicating liver
dysfunction (ASP, SGOT, and urinary nitrogen were increased at 90
days; glucose content was decreased). Depression of red blood cell
count was observed at 1200 ppm although no consistent parallel changes
were seen in haemoglobin content or packed cell volume. Urinalyses
were normal. Gross and microscopic analyses of tissues and organs
showed an increase in the liver weight at 4000 ppm and a decrease in
several other organ weights (spleen, heart, brain, thymus, prostate,
ovary, and uterus). At 1200 and 4000/8000 ppm fatty infiltration of
liver was seen on microscopic examination. A no-effect level in this
study is 400 ppm (equivalent to an average daily intake of 32.8 to
36.1 mg/kg body weight of males and females, respectively) (Wallwork
et al., 1971, as cited in Annex I, 27).
Dogs
Groups of dogs (2/sex/group) were administered bioresmethrin by
gavage, daily at dose levels of 0 and 500 mg/kg body weight for 7 days
followed by a dose increase to 1000 mg/kg for an additional 14 days.
There were no effects noted in this test with respect to mortality,
behaviour, body weight changes, haematology, blood chemistry or
urinalysis parameters or on electrocardiograph measurements. Short
term administration for three weeks at an oral dose of 1000 mg/kg was
uneventful in the parameters measured (Malone & Chesher, 1970, as
cited in FAO, 1977).
In a continuation of the above trial, after a two-week interval
on the control diets, dogs were administered bioresmethrin by gavage
for 7 days at a dose of 2000 mg/kg body weight. Again, no significant
effects were noted in the parameters recorded above (Chesher & Malone,
1971b as cited in Annex I, 27).
Groups of dogs (3/sex/group) were administered bioresmethrin
(gelatin capsule) by gavage daily for 90 days at dose levels of 0, 25,
80, and 250 mg/kg (the high dose was increased to 500 mg/kg in week
7). There was no mortality. Growth, food consumption, and calculated
food utilization parameters were normal. Clinical biochemistry,
ophthalmological examination, and urinalysis parameters were normal at
all intervals (30, 60, and 90 days) examined. In the high dose group,
reduced RBC count, haemoglobin content, and packed cell volume were
noted. BUN was slightly increased only at the high dose after 12
weeks. There were no adverse effects noted on gross or microscopic
examination of tissues and organs (including bone marrow). The NOAEL
is 80 mg/kg (equivalent to an average of 1600 ppm in the diet) (Noel
et al., 1971 as cited in Annex I, 27).
Long-term/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (75/sex/dose) received
resmethrin at dietary concentrations of 0, 250, 500, or 1000 for 85
weeks. The survival rate of 1000 ppm females was significantly (32%)
lower (p<0.05) than that of controls from week 63 to the end of the
study. In 1000 ppm males decreased survival rate was first found at
week 81 (31% at the end of the study).
Terminal body weights of mice from the 1000 ppm groups were
significantly lower (p <0.05) than those of the controls. Although
the food consumption data indicated a slight decrease, it was not
compound-related. Haematological parameters did not show significant
changes in the treated animals relative to those of the controls.
There was a significant increase in absolute and relative adrenal
weights in 500 ppm (20% and 30% respectively) and 1000 ppm males (31%
and 50%, respectively). There were increases in the relative liver,
kidney, and brain weights of 1000 ppm males, but these increases were
mainly due to a decrease in the terminal body weights. An increase in
the incidence of amyloidosis was seen in various tissues of high-dose
animals relative to that in the controls; however, this increase was
not considered to have been compound-related since the control males
and females also had high incidence of amyloidosis. No increase in
tumour incidence was found in any treatment group. The NOAEL was 250
ppm (equivalent to 38 mg/kg bw/day or 11 mg/kg bw/day).
Rats
Groups of Sprague-Dawley rats (50/sex/dose) received
bioresmethrin (technical grade) at dietary concentrations of 0, 50,
250, or 1250 ppm for 104 weeks. These dietary concentrations were
equivalent to 3.0, 14.9, and 76.2 mg/kg/day for male rats and 4.0,
19.8, and 101.3 mg/kg/day for female rats. Two satellite groups in
each dose level were included in the study. Satellite group 1
consisted of 10 rats/sex/dose and was sacrificed on week 52 of the
study; satellite group 2 consisted of 20 rats/sex/dose and received
the test chemical for 104 weeks. An extra control group consisting of
50 animals/sex was included in the study.
Bioresmethrin did not affect clinical signs, mortality rate, body
weights, food consumption, food efficiency, haematological parameters,
or urinalysis parameters. However, a statistically significant and
compound-related decrease in cholesterol levels and an increase in
alkaline phosphatase levels were observed in 1250 ppm males at various
examination periods. An increase in the alkaline phosphatase levels
was also found in males at 250 ppm, but this increase did not always
show a statistical significance. During sacrifice at 52 and 104
weeks, a slight increase in the absolute liver weight in males and
females at 1250 ppm was found.
Gross pathology showed an increase in the incidence of paleness
of the liver in both males and females at 1250 ppm (control, 0/10;
1250 ppm males, 5/10; 1250 ppm females, 2/10) at the 52 week
sacrifice.
Histopathology data indicated an increase in the incidence of
periportal hepatic cell hypertrophy in 250 ppm females (control, 0/10;
250 ppm, 2/10) and in 1250 ppm male (3/10) and female rats (3/10) at
52 week sacrifice. At 104 week sacrifice, there was also an increase
in the incidence of periportal hepatic cell hypertrophy in 250 ppm
females (5/70) and 1250 ppm males (37/70) and females (30/70) relative
to the controls (0/70). Most animals which had periportal hepatic
cell hypertrophy also showed signs of vacuolated hepatocytes at 104
weeks. No increase in the incidence of neoplasia was found at any
site in animals receiving bioresmethrin at dose levels up to 1250 ppm.
Based upon the histopathologic findings, the NOAEL was 50 ppm (equal
to 3.0 and 4.0 mg/kg/day in males and females, respectively (Vallet,
1990).
Reproduction study
Groups of Sprague-Dawley rats (25/sex/group) received
bioresmethrin (93.5% purity) at dietary concentrations of 0, 0, 80,
250, 750, and 2250 ppm. The study began with 3 dose levels: 250, 750,
and 2250 ppm, but the 2250 ppm group had only 3 live births.
Therefore, the 80 ppm group was added along with its concurrent
control group after the birth of F1 pups. For F0 generation, the
treatment began 8 weeks prior to mating and continued through mating,
pregnancy, and lactation periods for females. For the F1 parental
animals, one male and one female per litter were selected at the
weaning and received treatment for approximately 14 weeks and then
mated. The treatment groups received biores-methrin throughout the
experiment. Each male was mated with a female of the same treatment
group until pregnancy occurred or 3 weeks had elapsed. For F1 and
F2 generations, only the first litters were produced. At day 4
post-partum, each litter was standardized to have 4 males and 4
females if possible.
For F0 parental animals, 17/20 pregnant females in the 2250 ppm
group showed clinical signs of decreased spontaneous activity and
piloerection immediately before and after parturition. There was a
slight decrease in male body weights at 250 ppm from days 22 to 64.
A significant decrease in the body weight gain was found in 2250 ppm
males from the second week of treatment to the scheduled sacrifice.
There were decreases in female body weights at 750 ppm during the
premating period, on days 14 and 21 during the pregnancy period, and
on days 1 and 4 of the lactation period. At 2250 ppm, female body
weight was decreased during the premating and pregnancy periods. Food
consumption was decreased in females at 750 and 2250 ppm during the
lactation period. No significant difference was found in the
copulation index, fertility index, or length of pregnancy between the
treated and the control animals. Macroscopic examination showed an
increase in the incidence of liver changes characterized by
accentuated lobular pattern in 2/25 females at 750 ppm. In 2250 ppm
females, 11/25 showed marked lobular pattern, and 7/25 showed paler
than normal liver. Microscopically, the hepatic changes were
associated with steatosis.
For F1 litter parameters, there was a significant decrease
(p <0.01) in birth index at 750 and 2250 ppm relative to the controls
(control, 91%; 750 ppm, 81%; 2250 ppm, 1%). In the 2250 ppm group,
only 3 pups were born alive. The viability index on day 4 post-partum
was also reduced at 750 ppm (control, 95%; 750 ppm, 55%), and there
was no survival in the the 2250 ppm group. The mean pup body weight
of the 750 ppm group was significantly decreased (p< 0.01) on days 1,
4 and 7 post-partum). No compound-related effects on physical and
behavioural developmental parameters were found in all treated pups;
the parameters examined included pinna unfolding, hair growth, incisor
eruption, eye opening, auricular duct opening, surface righting
reflex, cliff avoidance, and air righting reflexes. Gross pathology
findings revealed an increase in the incidence of discolored liver in
pups which died between days 1 and 21 postpartum at 250 ppm (2/16),
750 ppm (13/109), and 2250 ppm (3/167); at 750 ppm, similar finding
was also reported for pups, which were not selected as F1 parental
animals on day 21 post-partum.
For F1 parental animals, no compound-related clinical signs were
noted in either males or females. There was a decrease in the body
weights of 750 ppm males throughout the treatment period, and this
decrease was statistically significant between days 1 and 113. The
body weight decrease was also seen in females at 750 ppm during the
premating, pregnancy, and lactation periods. A consistent decrease in
food consumption was not seen in all treated males. In treated
females, a slight decrease in food consumption was noted at 750 ppm
from days 14 to 21 of the pregnancy, and a significant drop in this
parameter was also reported during the lactation period. As in the F0
parental animals, the compound produced no effects on copulation
index, fertility index, and length of pregnancy. Gross pathology
showed that 1/23 females at 250 ppm had pale liver associated with
hepatic steatosis. In the 750 ppm group, 1/16 males and 3/15 females
also had pale liver associated with hepatic steatosis.
For the F2 litter parameters, at 750 ppm, there was a decrease
in the birth index (control, 96%; 750 ppm, 58%), in the viability
index at birth (control, 94%; 750 ppm, 33%), and in the viability
index at weaning (control, 76%; 750 ppm, 48%). The mean pup body
weight at 750 ppm was decreased relative to that of the controls
during the lactation period, and the decrease was statistically
significant (p<0.001) on day 1 of post-partum only. Bioresmethrin
did not affect the physical and behavioural developments of the pups.
Both gross pathological and histopathological examinations on the pups
showed no abnormalities.
At 250 ppm, an increase in the incidence of pale or discolored
liver associated with hepatic steatosis was found in F1 parental
females (1/23) and in F1 pups (2/16). Therefore, the NOAEL for this
study is 80 ppm (equivalent to 4 mg/kg/day) (Savary, 1987).
Special study on developmental toxicity
Rats
Groups of pregnant Sprague-Dawley rats (40/group) received
technical grade bioresmethrin (93.5% purity) by gavage at doses of 0,
50, 100, and 200 mg/kg/day from gestation days 6 to 15. On gestation
day 20, 25 females/group were sacrificed, and the fetuses were
delivered by caesarean section. The remaining 15 females/group were
allowed to deliver normally and to nurse their offspring till weaning.
The clinical signs, mortality, and number of abortions were
comparable between the treated and the control dams. There was a
slight and statistically significant drop in body weight gain in
c-section dams at 200 mg/kg. The mean numbers of corpora lutea,
implantation, resorption, and live fetuses were comparable between the
treated and the control animals. The fetal body weights of the
treated groups were similar to those of the controls. Fetal
abnormalities were incidental and not compound-related.
The results in females which delivered normally did not show any
treatment-related effects on clinical signs, abortion, or the duration
of gestation. There was a statistically significant (p <0.001)
decrease in body weight gain in dams at 200 mg/kg. The mean live
birth, body weight of the live pups, survival rates, and the rate of
post-implantation losses were comparable to those of the controls. The
physical and behavioural development of pups from the treated groups
were similar to those of the controls. The physical and behavioural
developmental parameters examined were pinna unfolding, hair growth,
incisor eruption, eye opening, auricular duct opening, surface
righting reflex, cliff avoidance, and air righting reflexes. The
NOAEL for maternal toxicity was 200 mg/kg, and no embryotoxic,
teratogenic, and developmental toxicity effects were found in the
highest dose tested (200 mg/kg) (Savary et al., 1988).
Rabbits
Groups of artificially inseminated female rabbits (16/group)
received bioresmethrin by gavage at doses of 0, 15, 60, and 240 mg/kg
from gestation days 6 to 18. The fetuses were delivered on gestation
day 28, and dams were sacrificed at that time. Under the conditions
of the study, the compound produced no maternal nor developmental
toxicity at any dose levels. Based upon the results of this study,
the pregnant rabbits could have tolerated higher dose levels (Savary,
1990).
An older rabbit teratology study on the bioresmethrin had been
considered by the Joint Meeting in 1976 and published in 1977 FAO
monograph. The summary of the study is reproduced in its entirety
below.
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin in doses of 0, 10, 20, 40, and 80 mg/kg by oral gavage
daily from days 8-16 of gestation. The does were sacrificed on day 28
and examined for implantation, live and dead fetuses, resorption
sites, and abnormalities (after staining a representative number for
skeletal examination). There was no apparent effect on parents in the
study as growth and gestation were unaffected. There was an increase
in dead fetuses at the highest dose and a large number of resorption
sites noted at all treatment levels. There were a number of deformed
fetuses observed but the total numbers were not sufficient for
adequate statistical evaluation. The deformities included straight
tail, crossed hind limbs and unilateral union of 6th and 7th ribs at
the sternal end. An overall fetal loss was observed at all dose
levels (primarily because of the large number of resorption sites
recorded) (Waldron, 1969, as cited in Annex I, 27).
Special studies on genotoxicity
A number of mutagenicity studies have been conducted with
bioresmethrin. The results are summarized in Table 3.
Special studies on skin and eye irritation and sensitization
A volume of 0.5 ml bioresmethrin (95%) was applied under
occlusive conditions to the shaven intact skin of 3 male New Zealand
white albino rabbits for 4 hours. No evidence of skin irritation was
found up to 72 hours after application (Audegond, 1989d).
Three New Zealand white albino rabbits were administered 0.1 ml
of bioresmethrin (95%) in their conjunctival sac of the right eye.
The treated eyes did not appeared to have been washed. No eye
irritation was found under the conditions of the test (Audegond,
1989c). An older study conducted by Chesher and Malone (1970c) also
showed that bioresmethrin was not an eye irritant in rabbits (FAO,
1977).
Bioresmethrin (95%) was tested for skin sensitization in 10 male
Hartley albino guinea pigs. During the induction phase, The animals
were treated topically with 0.5 ml bioresmethrin daily for 10 days.
On day 36, the animals were challenged with 0.5 ml of the test
substance and later rechallenged with similar volume on day 43. A
positive control group of 10 males received 0.06% w/v solution of
2,4-dinitrochlorobenzene in a similar manner as those treated with
bioresmethrin. Bioresmethrin did not produce skin sensitization
reaction in guinea pigs (Kuhn, 1990).
In an older study published in the FAO Monograph (Annex I, 27),
groups of adult guinea pigs (6 males/group) were applied bioresmethrin
(0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro-benzene (DNCB)
to the ears for 4 days. On day 7, 0.2 ml of bioresmethrin or DNCB
were applied dermally. Bioresmethrin produced only traces of erythema
suggesting a low potential for sensitization and irritation (Chesher
& Malone, 1970b).
Table 3. Results of genotoxicity assays on bioresmethrin
Test system Test object Concentration Purity Results Reference
(%)
Ames test S. typhimurium 0.2. 1-5. 10. 92.2 Negativea Moore, 1981
(with and TA1535, TA1537, 50, 100, 250,
without S9) TA1538, TA98, 500, 1000,
TA100 5000 µg/plate
Ames test S. typhimurium 30, 100, 300, 97 Negative Pluijmen et
(with and TA98, TA100 1000 µg/plate al., 1984
without S9)
Gene mutation V79 Chinese 5, 10, 15, 20 97 Negative Pluijmen et
assay (with and hamster cells µg/ml al., 1984
without S9)
Micronucleus Swiss CD 1 mice 300 mg/kg (M) 93.6 Negative Vannier &
assay 450 mg/kg (F) Fournex, 1986
Metaphase human 4, 20, 40 93.6 Negative Allen, Brooker
chromosome lymphocytes µg/ml & Howell (1986)
analysis (with
and without S9)
Unscheduled human 0.125, 0.25, 93.6 Negative Allen &
DNA synthesis epithelioid 0.5, 1, 2, 4, Proudlock,
assay (with cells 8, 16, 32, 64 1986
and without S9) (HeLa S3) 128 and 256
µg/ml
a The report of this study has serious deficiencies which include lack of information on the source
of the metabolic activation system and whether the results presented in the report are the
averages or single determinations.
COMMENTS
Bioresmethrin was absorbed and distributed rapidly following oral
administration, and was quickly metabolized by oxidation and
hydrolysis at various sites in the molecule. Complete elimination of
bioresmethrin occurred slowly. The enterohepatic circulation system
was involved in the elimination. There is no indication that
isomerization of bioresmethrin to the (+)- cis-isomer occurs.
In general, bioresmethrin has low acute toxicity after oral
administration. In mammals, the cis-isomers are generally more
toxic than the corresponding trans-isomers. Some metabolites of
bioresmethrin are more toxic than the parent compound.
Short-term studies in rats show that bioresmethrin fed at 1000
ppm caused a slight increase in liver weight and a reduction in thymus
weight in rats. In a 90-day feeding study in rats, at dietary
concentrations of 0, 400, 1200 or 8000 ppm, bioresmethrin at 1200 ppm
or above induced an increase in liver weight and fatty liver which was
accompanied by changes in blood enzyme levels (serum alkaline
phosphatase and aspartate aminotransferase) indicative of liver
injury. In a 90-day gavage study in dogs, bioresmethrin at 250 mg/kg
bw/day or above reduced the erythrocyte count, haemoglobin content,
and packed cell volume.
A carcinogenicity study in mice with resmethrin (containing at
least 30% bioresmethrin) at dietary concentrations of 250, 500 and
1000 ppm for 85 weeks did not demonstrate a carcinogenic effect.
However, resmethrin decreased survival rate in both male and female
mice at 1000 ppm and adrenal weights were significantly increased in
males at 500 and 1000 ppm. The NOAEL for resmethrin was 250 ppm,
which was equal to 38 mg/kg bw/day for resmethrin and 11 mg/kg bw/day
for bioresmethrin.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 50, 250 or 1250 ppm for 104 weeks, bioresmethrin
did not produce an increase in the tumour incidence. However, it
induced an increase in alkaline phosphatase in males at 250 and 1250
ppm and a decrease in cholesterol levels in males at 1250 ppm.
Bioresmethrin caused an increase in the incidence of non-neoplastic
liver changes, including pallor and hypertrophy of hepatocytes in
males at 250 ppm and in males and females at 1250 ppm. Based upon
these findings, the NOAEL for chronic toxicity was 50 ppm, equal to
3.0 mg/kg bw/day.
In a two-generation reproduction study in rats, at dietary
concentrations of 0, 80, 250, 750 or 2250 ppm, bioresmethrin did not
affect reproductive performance at dietary concentrations of 250 ppm
or less, although reproduction was adversely affected at 750 and 2250
ppm. Based on a decrease in parental body weight and hepatotoxicity
observed at 250 ppm, the NOAEL for this study was 80 ppm, equivalent
to 4 mg/kg/day.
Studies on the developmental toxicity of bioresmethrin in rats
and rabbits failed to elicit effects at doses up to 200 and 240 mg/kg
bw/day respectively.
After reviewing all available in vitro and in vivo short-term
assays with bioresmethrin, the Meeting concluded that there was no
evidence of genotoxicity.
The ADI was based upon the long-term/carcinogenicity study in
rats utilizing a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet, equal to 3.0 mg/kg bw/day
Dog: 80 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, J.A., Brooker, P.C., & Howell, A. (1986) Bioresmethrin:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. RSL 697/851370 from Huntingdon Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Allen, J.A. & Proudlock, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after exposure to
bioresmethrin. Unpublished report No. RSL 698/851420 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989a) Bioresmethrin: Acute oral toxicity study in the
rat. Unpublished study No. 89142 from Toxicology Department, Division
Scientifique Roussel Uclaf, Romainville, France. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989b) Bioresmethrin: Acute dermal toxicity study in
the rabbit. Unpublished study No. 89143 from Toxicology Department,
Division Scientifique Roussel Uclaf, Romainville, France. Submitted to
WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989c) Bioresmethrin: Primary eye irritation study in
the male rabbit. Unpublished study No. 89144 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989d) Bioresmethrin: Primary dermal irritation study
in the male rabbit. Unpublished study No. 89145 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Casida, J.E., Fammon, D.W. & Lawrence, L.J. (1983) Mechanisms of
selective actions of pyrethroid insecticides. Ann. Rev. Pharmacol.
Toxicol., 23: 413-38.
Chesher, B.C. & Malone, J.C. (1970a) Acute toxicity test with NRDC
107 in hens. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970b) Sensitization study with NRDC
107 in guinea pigs. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971a) Acute toxicity of NRDC 107 by
the intravenous route. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971b) Toxicity to dog of NRDC 107
(continuation). Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Cox, G.E., Knickerbocker, M., & Parent, R.A. (1979) Evaluation of
dietary administration of SBP-138 in CD-1 outbred albino mice over an
85 week period. Unpublished report by Food and Drug Research
Laboratories, Inc., Study No. 5270. Sponsored by S.B. Penick & Co. and
submitted to WHO by Roussel Uclaf, Paris, France.
Farebrother, D.A. (1973) NRDC 107: Whole body radioautographical
study in albino rats. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Glomot, R. & Chevalier, B. (1969) Etude de la toxicite aigue du RU
11484 (NRDC 107). Unpublished report from Roussel Uclaf. Submitted to
WHO by the Wellcome Foundation Ltd.
Glomot, R., Undated. Three week study of RU 11484 (NRDC 107) in the
rat. Unpublished report from Roussel Uclaf. Submitted to WHO by the
Wellcome Foundation Ltd.
Hardy, C.J., Jackson, G.C., Suttie, A.W., Read, R.M., & Gopinath, C.
(1989) Bioresmethrin: Acute inhalation toxicity study in rats (4-hour
exposure). Unpublished Report No. RSL 801/89712 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Kuhn, J.O. (1990) Bioresmethrin Technical 7N1173B3: Dermal
sensitization study in guinea pigs. Unpublished Report No. 6674-89
from Stillmeadow, Inc., Houston, Texas, USA. Submitted by Roussel
Uclaf, Paris, France.
Malone, J.C. & Chesher, B.C. (1970) Toxicity to dogs of NRDC 107.
Unpublished report from the Cooper Technical Bureau, Berkhamstead, the
Wellcome Foundation Ltd., submitted to the World Health Organization
by the Wellcome Foundation Ltd.
Miyamoto, J. (1975-76) Terminal residues of bioresmethrin. Submission
to IUPAC Terminal Residues Commission, Madrid, Sept. 1975; Leverkusen,
Sept. 1976.
Moore, W.B. & Chatfield, S.N. (1981) Mutagenicity: Evaluation of
bioresmethrin using the Ames Samonella/microsome incorporation test.
Unpublished Report No. BEMU/81/1 from the Wellcome Foundation Ltd.
Submitted to WHO by Roussel Uclaf, Paris, France.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., & Spicer,
E.J.F. (1971) Oral toxicity study in beagle dogs (repeated dosage for
3 months). Unpublished report from the Huntingdon Research Centre.
Submitted to the WHO by the Wellcome Foundation Ltd.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille,
A., & Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutation Research, 137: 7-15.
Ruzo, L.O., Krautter, M.S., & Jiang, Jialiang (1991) Absorption,
distribution, elimination, and metabolism of [14C-acid]-d-
trans-resmethrin in rats. Unpublished Report, Pharmacology and
Toxicology Research Lab. East and West, Inc.; PTRL Report No.
410E/238W. Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M.H. (1987) Bioresmethrin: Two-generation reproduction
toxicity study in rats by dietary mixture administration. Unpublished
Report No. 2059 RSR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M. H. (1990) Assessment of possible embryotoxic or
teratogenic effects of oral route in rabbits. Unpublished report;
Study No. 6772 RSL from Centre International de Toxicologie, France.
Submitted to WHO Roussel Uclaf, Paris, France.
Savary, M.H., Read, M.H., & Delhomme, C. (1988) Bioresmethrin:
Assessment of possible embryotoxic, teratogenic or developmental
effects in the rats. Unpublished Report No. 2058 RSR from Centre de
Recherches Roussel Uclaf, France. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ueda, K., Gaughan, L.C., & Casida, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pesticide Biochem. Physiol.,
5: 280-294.
Ueda, K. Gaughan, L.C., & Casida, J.E. (1975b) Metabolism of
(+)- trans and (+)- cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115
Vallet, L. (1990) Combined chronic toxicity/oncogenicity study by
repeated dietary administration to rats (104 weeks). Unpublished
Report No. 2057 TCR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Vannier, B. & Fournex, R. (1986) Bioresmethrin: Detection of a
mutagenic potency, micronucleus test in the mouse. Unpublished Report
No. 85612-BB/178 from Centre de Recherches Roussel Uclaf, Romainville,
France. Submitted to WHO by Roussel Uclaf, France.
Verschoyle, R.D. & Barnes, J.M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. & Physiol., 2(3):
308-11.
Waldron, M.M. (1969) Fetal toxicity study - 95 H 56 - given orally in
the rabbit. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd., submitted to the World
Health Organization by the Wellcome Foundation Ltd.
Wallwork, L.M., Chesher, B.C., & Malone, J.C. (1970) Toxicity of NRDC
107 to laboratory animals. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M., Clampitt, R.B., & Malone, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Unpublished report
from the Cooper Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. Submitted to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1972) Bioresmethrin: Preliminary
24-hour rat inhalation study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
the consideration of Verschoyle & Barnes (1972) that as a delay in
signs of poisoning was evident following iv administration,
bioresmethrin might be converted in vivo to a toxic metabolite. The
presence of (d)- trans-CA, BFA, and BFCA as metabolites, which are
more toxic than bioresmethrin, may account for their observation and
conclusions.
The recent bioresmethrin metabolism study indicated that
bioresmethrin was metabolized by a combination of hydrolytic,
oxidative, and conjugative processes. The results of metabolite
determinations were consistent with those of the 1975 papers of Ueda
et al. (Ruzo, 1991).
Toxicological studies
Acute toxicity
Much of the information on the acute toxicity of bioresmethrin
had been evaluated by the 1976 Joint Meeting and published in the
monograph (Annex I, 27). Some new data have become available, and
they have been summarized along with the published information in
Table 1.
Two or more hours after oral administration, the treated animals
showed signs of aggressiveness and tremors. The final stages of
poisoning consisted of convulsive twitching, prostration, coma, and
death normally between 3 and 24 hours (Annex I, 27). The recent
findings in clinical signs also include hypotonicity, slightly arched
back, and piloerection at 30 minutes after treatment (Audegond,
1989a,b).
Table 1. Acute toxicity of bioresmethrin
Species Sex Route LC50 LC50 Reference
(mg/kg b.w.) (mg/L)
Rat m/f oral >5000 Audegond, 1989a
m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chescher & Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
m/f inh. (4 h) >5.3 Hardy et al., 1989
f inh. (24 h) >872 Wallwork & Malone, 1972
f dermal >10 000 Wallwork et al., 1970
Rabbit f dermal >2000 Audegond, 1989b
Mouse f oral >10 000 Wallwork et al., 1970
m oral 3100 Ueda et al., 1975b
m ip >1500 Ueda et al., 1975b
Chicken oral >10 000 Wallwork et al., 1970
>10 000 Chester & Malone, 1970a
Acute toxicity of metabolites
The data on the acute toxicity of the metabolites of
bioresmethrin were evaluated in the 1976 Joint Meeting and published
in the monograph (FAO, 1977). No new data on the acute toxicity of
these metabolites are available; therefore, the previously published
data (Annex I, 27) are reproduced in its entirety in Table 2.
Table 2. Acute toxicity of the metabolites of bioresmethrin
(as cited in Annex 1, 27, animal species not named in
original)
Metabolite LD50 (mg/kg/bw)a
ip oral
1) (+)-trans-resmethrin (bioresmenthrin) > 1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-trans-CA (t-CA) 98 280
(+)-trans-chrysanthemic acid
3) (+)-trans-CDA (tE-CDA) 408
(+)-trans-chrysanthemundicarboxylic
acid
4) BFA 75 310
5-benzyl-3-furylmethanol
5) BFCA 46
5-benzyl-3-furoic acid
Short-term studies
New data on short-term studies are not available; however, the
1976 Joint Meeting had evaluated several short-term studies on rats
and dogs. The summaries of these study are published in the monograph
(Annex I, 27), and they are reproduced below.
Rats
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage six days per week for 3 weeks at doses of 0, 1000,
and 2000 mg/kg body weight. There was no mortality attributable to
bioresmethrin. There was a slight reduction in body weights at 2000
mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and haematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks,
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated, as cited in Annex I,
27).
Groups of rats (18/sex/group) were fed bioresmethrin at dietary
concentrations of 0, 400, 1200, and 8000 ppm (the last dose group was
fed 4000 ppm for 30 days, and the level was increased thereafter) for
91 days. There was no mortality observed in this study. Food
consumption was normal, and food conversion was unaffected by
bioresmethrin. Growth was reduced at the highest dose level which was
accompanied by changes in blood chemistry parameters indicating liver
dysfunction (ASP, SGOT, and urinary nitrogen were increased at 90
days; glucose content was decreased). Depression of red blood cell
count was observed at 1200 ppm although no consistent parallel changes
were seen in haemoglobin content or packed cell volume. Urinalyses
were normal. Gross and microscopic analyses of tissues and organs
showed an increase in the liver weight at 4000 ppm and a decrease in
several other organ weights (spleen, heart, brain, thymus, prostate,
ovary, and uterus). At 1200 and 4000/8000 ppm fatty infiltration of
liver was seen on microscopic examination. A no-effect level in this
study is 400 ppm (equivalent to an average daily intake of 32.8 to
36.1 mg/kg body weight of males and females, respectively) (Wallwork
et al., 1971, as cited in Annex I, 27).
Dogs
Groups of dogs (2/sex/group) were administered bioresmethrin by
gavage, daily at dose levels of 0 and 500 mg/kg body weight for 7 days
followed by a dose increase to 1000 mg/kg for an additional 14 days.
There were no effects noted in this test with respect to mortality,
behaviour, body weight changes, haematology, blood chemistry or
urinalysis parameters or on electrocardiograph measurements. Short
term administration for three weeks at an oral dose of 1000 mg/kg was
uneventful in the parameters measured (Malone & Chesher, 1970, as
cited in FAO, 1977).
In a continuation of the above trial, after a two-week interval
on the control diets, dogs were administered bioresmethrin by gavage
for 7 days at a dose of 2000 mg/kg body weight. Again, no significant
effects were noted in the parameters recorded above (Chesher & Malone,
1971b as cited in Annex I, 27).
Groups of dogs (3/sex/group) were administered bioresmethrin
(gelatin capsule) by gavage daily for 90 days at dose levels of 0, 25,
80, and 250 mg/kg (the high dose was increased to 500 mg/kg in week
7). There was no mortality. Growth, food consumption, and calculated
food utilization parameters were normal. Clinical biochemistry,
ophthalmological examination, and urinalysis parameters were normal at
all intervals (30, 60, and 90 days) examined. In the high dose group,
reduced RBC count, haemoglobin content, and packed cell volume were
noted. BUN was slightly increased only at the high dose after 12
weeks. There were no adverse effects noted on gross or microscopic
examination of tissues and organs (including bone marrow). The NOAEL
is 80 mg/kg (equivalent to an average of 1600 ppm in the diet) (Noel
et al., 1971 as cited in Annex I, 27).
Long-term/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (75/sex/dose) received
resmethrin at dietary concentrations of 0, 250, 500, or 1000 for 85
weeks. The survival rate of 1000 ppm females was significantly (32%)
lower (p<0.05) than that of controls from week 63 to the end of the
study. In 1000 ppm males decreased survival rate was first found at
week 81 (31% at the end of the study).
Terminal body weights of mice from the 1000 ppm groups were
significantly lower (p <0.05) than those of the controls. Although
the food consumption data indicated a slight decrease, it was not
compound-related. Haematological parameters did not show significant
changes in the treated animals relative to those of the controls.
There was a significant increase in absolute and relative adrenal
weights in 500 ppm (20% and 30% respectively) and 1000 ppm males (31%
and 50%, respectively). There were increases in the relative liver,
kidney, and brain weights of 1000 ppm males, but these increases were
mainly due to a decrease in the terminal body weights. An increase in
the incidence of amyloidosis was seen in various tissues of high-dose
animals relative to that in the controls; however, this increase was
not considered to have been compound-related since the control males
and females also had high incidence of amyloidosis. No increase in
tumour incidence was found in any treatment group. The NOAEL was 250
ppm (equivalent to 38 mg/kg bw/day or 11 mg/kg bw/day).
Rats
Groups of Sprague-Dawley rats (50/sex/dose) received
bioresmethrin (technical grade) at dietary concentrations of 0, 50,
250, or 1250 ppm for 104 weeks. These dietary concentrations were
equivalent to 3.0, 14.9, and 76.2 mg/kg/day for male rats and 4.0,
19.8, and 101.3 mg/kg/day for female rats. Two satellite groups in
each dose level were included in the study. Satellite group 1
consisted of 10 rats/sex/dose and was sacrificed on week 52 of the
study; satellite group 2 consisted of 20 rats/sex/dose and received
the test chemical for 104 weeks. An extra control group consisting of
50 animals/sex was included in the study.
Bioresmethrin did not affect clinical signs, mortality rate, body
weights, food consumption, food efficiency, haematological parameters,
or urinalysis parameters. However, a statistically significant and
compound-related decrease in cholesterol levels and an increase in
alkaline phosphatase levels were observed in 1250 ppm males at various
examination periods. An increase in the alkaline phosphatase levels
was also found in males at 250 ppm, but this increase did not always
show a statistical significance. During sacrifice at 52 and 104
weeks, a slight increase in the absolute liver weight in males and
females at 1250 ppm was found.
Gross pathology showed an increase in the incidence of paleness
of the liver in both males and females at 1250 ppm (control, 0/10;
1250 ppm males, 5/10; 1250 ppm females, 2/10) at the 52 week
sacrifice.
Histopathology data indicated an increase in the incidence of
periportal hepatic cell hypertrophy in 250 ppm females (control, 0/10;
250 ppm, 2/10) and in 1250 ppm male (3/10) and female rats (3/10) at
52 week sacrifice. At 104 week sacrifice, there was also an increase
in the incidence of periportal hepatic cell hypertrophy in 250 ppm
females (5/70) and 1250 ppm males (37/70) and females (30/70) relative
to the controls (0/70). Most animals which had periportal hepatic
cell hypertrophy also showed signs of vacuolated hepatocytes at 104
weeks. No increase in the incidence of neoplasia was found at any
site in animals receiving bioresmethrin at dose levels up to 1250 ppm.
Based upon the histopathologic findings, the NOAEL was 50 ppm (equal
to 3.0 and 4.0 mg/kg/day in males and females, respectively (Vallet,
1990).
Reproduction study
Groups of Sprague-Dawley rats (25/sex/group) received
bioresmethrin (93.5% purity) at dietary concentrations of 0, 0, 80,
250, 750, and 2250 ppm. The study began with 3 dose levels: 250, 750,
and 2250 ppm, but the 2250 ppm group had only 3 live births.
Therefore, the 80 ppm group was added along with its concurrent
control group after the birth of F1 pups. For F0 generation, the
treatment began 8 weeks prior to mating and continued through mating,
pregnancy, and lactation periods for females. For the F1 parental
animals, one male and one female per litter were selected at the
weaning and received treatment for approximately 14 weeks and then
mated. The treatment groups received biores-methrin throughout the
experiment. Each male was mated with a female of the same treatment
group until pregnancy occurred or 3 weeks had elapsed. For F1 and
F2 generations, only the first litters were produced. At day 4
post-partum, each litter was standardized to have 4 males and 4
females if possible.
For F0 parental animals, 17/20 pregnant females in the 2250 ppm
group showed clinical signs of decreased spontaneous activity and
piloerection immediately before and after parturition. There was a
slight decrease in male body weights at 250 ppm from days 22 to 64.
A significant decrease in the body weight gain was found in 2250 ppm
males from the second week of treatment to the scheduled sacrifice.
There were decreases in female body weights at 750 ppm during the
premating period, on days 14 and 21 during the pregnancy period, and
on days 1 and 4 of the lactation period. At 2250 ppm, female body
weight was decreased during the premating and pregnancy periods. Food
consumption was decreased in females at 750 and 2250 ppm during the
lactation period. No significant difference was found in the
copulation index, fertility index, or length of pregnancy between the
treated and the control animals. Macroscopic examination showed an
increase in the incidence of liver changes characterized by
accentuated lobular pattern in 2/25 females at 750 ppm. In 2250 ppm
females, 11/25 showed marked lobular pattern, and 7/25 showed paler
than normal liver. Microscopically, the hepatic changes were
associated with steatosis.
For F1 litter parameters, there was a significant decrease
(p <0.01) in birth index at 750 and 2250 ppm relative to the controls
(control, 91%; 750 ppm, 81%; 2250 ppm, 1%). In the 2250 ppm group,
only 3 pups were born alive. The viability index on day 4 post-partum
was also reduced at 750 ppm (control, 95%; 750 ppm, 55%), and there
was no survival in the the 2250 ppm group. The mean pup body weight
of the 750 ppm group was significantly decreased (p< 0.01) on days 1,
4 and 7 post-partum). No compound-related effects on physical and
behavioural developmental parameters were found in all treated pups;
the parameters examined included pinna unfolding, hair growth, incisor
eruption, eye opening, auricular duct opening, surface righting
reflex, cliff avoidance, and air righting reflexes. Gross pathology
findings revealed an increase in the incidence of discolored liver in
pups which died between days 1 and 21 postpartum at 250 ppm (2/16),
750 ppm (13/109), and 2250 ppm (3/167); at 750 ppm, similar finding
was also reported for pups, which were not selected as F1 parental
animals on day 21 post-partum.
For F1 parental animals, no compound-related clinical signs were
noted in either males or females. There was a decrease in the body
weights of 750 ppm males throughout the treatment period, and this
decrease was statistically significant between days 1 and 113. The
body weight decrease was also seen in females at 750 ppm during the
premating, pregnancy, and lactation periods. A consistent decrease in
food consumption was not seen in all treated males. In treated
females, a slight decrease in food consumption was noted at 750 ppm
from days 14 to 21 of the pregnancy, and a significant drop in this
parameter was also reported during the lactation period. As in the F0
parental animals, the compound produced no effects on copulation
index, fertility index, and length of pregnancy. Gross pathology
showed that 1/23 females at 250 ppm had pale liver associated with
hepatic steatosis. In the 750 ppm group, 1/16 males and 3/15 females
also had pale liver associated with hepatic steatosis.
For the F2 litter parameters, at 750 ppm, there was a decrease
in the birth index (control, 96%; 750 ppm, 58%), in the viability
index at birth (control, 94%; 750 ppm, 33%), and in the viability
index at weaning (control, 76%; 750 ppm, 48%). The mean pup body
weight at 750 ppm was decreased relative to that of the controls
during the lactation period, and the decrease was statistically
significant (p<0.001) on day 1 of post-partum only. Bioresmethrin
did not affect the physical and behavioural developments of the pups.
Both gross pathological and histopathological examinations on the pups
showed no abnormalities.
At 250 ppm, an increase in the incidence of pale or discolored
liver associated with hepatic steatosis was found in F1 parental
females (1/23) and in F1 pups (2/16). Therefore, the NOAEL for this
study is 80 ppm (equivalent to 4 mg/kg/day) (Savary, 1987).
Special study on developmental toxicity
Rats
Groups of pregnant Sprague-Dawley rats (40/group) received
technical grade bioresmethrin (93.5% purity) by gavage at doses of 0,
50, 100, and 200 mg/kg/day from gestation days 6 to 15. On gestation
day 20, 25 females/group were sacrificed, and the fetuses were
delivered by caesarean section. The remaining 15 females/group were
allowed to deliver normally and to nurse their offspring till weaning.
The clinical signs, mortality, and number of abortions were
comparable between the treated and the control dams. There was a
slight and statistically significant drop in body weight gain in
c-section dams at 200 mg/kg. The mean numbers of corpora lutea,
implantation, resorption, and live fetuses were comparable between the
treated and the control animals. The fetal body weights of the
treated groups were similar to those of the controls. Fetal
abnormalities were incidental and not compound-related.
The results in females which delivered normally did not show any
treatment-related effects on clinical signs, abortion, or the duration
of gestation. There was a statistically significant (p <0.001)
decrease in body weight gain in dams at 200 mg/kg. The mean live
birth, body weight of the live pups, survival rates, and the rate of
post-implantation losses were comparable to those of the controls. The
physical and behavioural development of pups from the treated groups
were similar to those of the controls. The physical and behavioural
developmental parameters examined were pinna unfolding, hair growth,
incisor eruption, eye opening, auricular duct opening, surface
righting reflex, cliff avoidance, and air righting reflexes. The
NOAEL for maternal toxicity was 200 mg/kg, and no embryotoxic,
teratogenic, and developmental toxicity effects were found in the
highest dose tested (200 mg/kg) (Savary et al., 1988).
Rabbits
Groups of artificially inseminated female rabbits (16/group)
received bioresmethrin by gavage at doses of 0, 15, 60, and 240 mg/kg
from gestation days 6 to 18. The fetuses were delivered on gestation
day 28, and dams were sacrificed at that time. Under the conditions
of the study, the compound produced no maternal nor developmental
toxicity at any dose levels. Based upon the results of this study,
the pregnant rabbits could have tolerated higher dose levels (Savary,
1990).
An older rabbit teratology study on the bioresmethrin had been
considered by the Joint Meeting in 1976 and published in 1977 FAO
monograph. The summary of the study is reproduced in its entirety
below.
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin in doses of 0, 10, 20, 40, and 80 mg/kg by oral gavage
daily from days 8-16 of gestation. The does were sacrificed on day 28
and examined for implantation, live and dead fetuses, resorption
sites, and abnormalities (after staining a representative number for
skeletal examination). There was no apparent effect on parents in the
study as growth and gestation were unaffected. There was an increase
in dead fetuses at the highest dose and a large number of resorption
sites noted at all treatment levels. There were a number of deformed
fetuses observed but the total numbers were not sufficient for
adequate statistical evaluation. The deformities included straight
tail, crossed hind limbs and unilateral union of 6th and 7th ribs at
the sternal end. An overall fetal loss was observed at all dose
levels (primarily because of the large number of resorption sites
recorded) (Waldron, 1969, as cited in Annex I, 27).
Special studies on genotoxicity
A number of mutagenicity studies have been conducted with
bioresmethrin. The results are summarized in Table 3.
Special studies on skin and eye irritation and sensitization
A volume of 0.5 ml bioresmethrin (95%) was applied under
occlusive conditions to the shaven intact skin of 3 male New Zealand
white albino rabbits for 4 hours. No evidence of skin irritation was
found up to 72 hours after application (Audegond, 1989d).
Three New Zealand white albino rabbits were administered 0.1 ml
of bioresmethrin (95%) in their conjunctival sac of the right eye.
The treated eyes did not appeared to have been washed. No eye
irritation was found under the conditions of the test (Audegond,
1989c). An older study conducted by Chesher and Malone (1970c) also
showed that bioresmethrin was not an eye irritant in rabbits (FAO,
1977).
Bioresmethrin (95%) was tested for skin sensitization in 10 male
Hartley albino guinea pigs. During the induction phase, The animals
were treated topically with 0.5 ml bioresmethrin daily for 10 days.
On day 36, the animals were challenged with 0.5 ml of the test
substance and later rechallenged with similar volume on day 43. A
positive control group of 10 males received 0.06% w/v solution of
2,4-dinitrochlorobenzene in a similar manner as those treated with
bioresmethrin. Bioresmethrin did not produce skin sensitization
reaction in guinea pigs (Kuhn, 1990).
In an older study published in the FAO Monograph (Annex I, 27),
groups of adult guinea pigs (6 males/group) were applied bioresmethrin
(0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro-benzene (DNCB)
to the ears for 4 days. On day 7, 0.2 ml of bioresmethrin or DNCB
were applied dermally. Bioresmethrin produced only traces of erythema
suggesting a low potential for sensitization and irritation (Chesher
& Malone, 1970b).
Table 3. Results of genotoxicity assays on bioresmethrin
Test system Test object Concentration Purity Results Reference
(%)
Ames test S. typhimurium 0.2. 1-5. 10. 92.2 Negativea Moore, 1981
(with and TA1535, TA1537, 50, 100, 250,
without S9) TA1538, TA98, 500, 1000,
TA100 5000 µg/plate
Ames test S. typhimurium 30, 100, 300, 97 Negative Pluijmen et
(with and TA98, TA100 1000 µg/plate al., 1984
without S9)
Gene mutation V79 Chinese 5, 10, 15, 20 97 Negative Pluijmen et
assay (with and hamster cells µg/ml al., 1984
without S9)
Micronucleus Swiss CD 1 mice 300 mg/kg (M) 93.6 Negative Vannier &
assay 450 mg/kg (F) Fournex, 1986
Metaphase human 4, 20, 40 93.6 Negative Allen, Brooker
chromosome lymphocytes µg/ml & Howell (1986)
analysis (with
and without S9)
Unscheduled human 0.125, 0.25, 93.6 Negative Allen &
DNA synthesis epithelioid 0.5, 1, 2, 4, Proudlock,
assay (with cells 8, 16, 32, 64 1986
and without S9) (HeLa S3) 128 and 256
µg/ml
a The report of this study has serious deficiencies which include lack of information on the source
of the metabolic activation system and whether the results presented in the report are the
averages or single determinations.
COMMENTS
Bioresmethrin was absorbed and distributed rapidly following oral
administration, and was quickly metabolized by oxidation and
hydrolysis at various sites in the molecule. Complete elimination of
bioresmethrin occurred slowly. The enterohepatic circulation system
was involved in the elimination. There is no indication that
isomerization of bioresmethrin to the (+)- cis-isomer occurs.
In general, bioresmethrin has low acute toxicity after oral
administration. In mammals, the cis-isomers are generally more
toxic than the corresponding trans-isomers. Some metabolites of
bioresmethrin are more toxic than the parent compound.
Short-term studies in rats show that bioresmethrin fed at 1000
ppm caused a slight increase in liver weight and a reduction in thymus
weight in rats. In a 90-day feeding study in rats, at dietary
concentrations of 0, 400, 1200 or 8000 ppm, bioresmethrin at 1200 ppm
or above induced an increase in liver weight and fatty liver which was
accompanied by changes in blood enzyme levels (serum alkaline
phosphatase and aspartate aminotransferase) indicative of liver
injury. In a 90-day gavage study in dogs, bioresmethrin at 250 mg/kg
bw/day or above reduced the erythrocyte count, haemoglobin content,
and packed cell volume.
A carcinogenicity study in mice with resmethrin (containing at
least 30% bioresmethrin) at dietary concentrations of 250, 500 and
1000 ppm for 85 weeks did not demonstrate a carcinogenic effect.
However, resmethrin decreased survival rate in both male and female
mice at 1000 ppm and adrenal weights were significantly increased in
males at 500 and 1000 ppm. The NOAEL for resmethrin was 250 ppm,
which was equal to 38 mg/kg bw/day for resmethrin and 11 mg/kg bw/day
for bioresmethrin.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 50, 250 or 1250 ppm for 104 weeks, bioresmethrin
did not produce an increase in the tumour incidence. However, it
induced an increase in alkaline phosphatase in males at 250 and 1250
ppm and a decrease in cholesterol levels in males at 1250 ppm.
Bioresmethrin caused an increase in the incidence of non-neoplastic
liver changes, including pallor and hypertrophy of hepatocytes in
males at 250 ppm and in males and females at 1250 ppm. Based upon
these findings, the NOAEL for chronic toxicity was 50 ppm, equal to
3.0 mg/kg bw/day.
In a two-generation reproduction study in rats, at dietary
concentrations of 0, 80, 250, 750 or 2250 ppm, bioresmethrin did not
affect reproductive performance at dietary concentrations of 250 ppm
or less, although reproduction was adversely affected at 750 and 2250
ppm. Based on a decrease in parental body weight and hepatotoxicity
observed at 250 ppm, the NOAEL for this study was 80 ppm, equivalent
to 4 mg/kg/day.
Studies on the developmental toxicity of bioresmethrin in rats
and rabbits failed to elicit effects at doses up to 200 and 240 mg/kg
bw/day respectively.
After reviewing all available in vitro and in vivo short-term
assays with bioresmethrin, the Meeting concluded that there was no
evidence of genotoxicity.
The ADI was based upon the long-term/carcinogenicity study in
rats utilizing a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet, equal to 3.0 mg/kg bw/day
Dog: 80 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, J.A., Brooker, P.C., & Howell, A. (1986) Bioresmethrin:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. RSL 697/851370 from Huntingdon Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Allen, J.A. & Proudlock, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after exposure to
bioresmethrin. Unpublished report No. RSL 698/851420 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989a) Bioresmethrin: Acute oral toxicity study in the
rat. Unpublished study No. 89142 from Toxicology Department, Division
Scientifique Roussel Uclaf, Romainville, France. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989b) Bioresmethrin: Acute dermal toxicity study in
the rabbit. Unpublished study No. 89143 from Toxicology Department,
Division Scientifique Roussel Uclaf, Romainville, France. Submitted to
WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989c) Bioresmethrin: Primary eye irritation study in
the male rabbit. Unpublished study No. 89144 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989d) Bioresmethrin: Primary dermal irritation study
in the male rabbit. Unpublished study No. 89145 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Casida, J.E., Fammon, D.W. & Lawrence, L.J. (1983) Mechanisms of
selective actions of pyrethroid insecticides. Ann. Rev. Pharmacol.
Toxicol., 23: 413-38.
Chesher, B.C. & Malone, J.C. (1970a) Acute toxicity test with NRDC
107 in hens. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970b) Sensitization study with NRDC
107 in guinea pigs. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971a) Acute toxicity of NRDC 107 by
the intravenous route. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971b) Toxicity to dog of NRDC 107
(continuation). Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Cox, G.E., Knickerbocker, M., & Parent, R.A. (1979) Evaluation of
dietary administration of SBP-138 in CD-1 outbred albino mice over an
85 week period. Unpublished report by Food and Drug Research
Laboratories, Inc., Study No. 5270. Sponsored by S.B. Penick & Co. and
submitted to WHO by Roussel Uclaf, Paris, France.
Farebrother, D.A. (1973) NRDC 107: Whole body radioautographical
study in albino rats. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Glomot, R. & Chevalier, B. (1969) Etude de la toxicite aigue du RU
11484 (NRDC 107). Unpublished report from Roussel Uclaf. Submitted to
WHO by the Wellcome Foundation Ltd.
Glomot, R., Undated. Three week study of RU 11484 (NRDC 107) in the
rat. Unpublished report from Roussel Uclaf. Submitted to WHO by the
Wellcome Foundation Ltd.
Hardy, C.J., Jackson, G.C., Suttie, A.W., Read, R.M., & Gopinath, C.
(1989) Bioresmethrin: Acute inhalation toxicity study in rats (4-hour
exposure). Unpublished Report No. RSL 801/89712 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Kuhn, J.O. (1990) Bioresmethrin Technical 7N1173B3: Dermal
sensitization study in guinea pigs. Unpublished Report No. 6674-89
from Stillmeadow, Inc., Houston, Texas, USA. Submitted by Roussel
Uclaf, Paris, France.
Malone, J.C. & Chesher, B.C. (1970) Toxicity to dogs of NRDC 107.
Unpublished report from the Cooper Technical Bureau, Berkhamstead, the
Wellcome Foundation Ltd., submitted to the World Health Organization
by the Wellcome Foundation Ltd.
Miyamoto, J. (1975-76) Terminal residues of bioresmethrin. Submission
to IUPAC Terminal Residues Commission, Madrid, Sept. 1975; Leverkusen,
Sept. 1976.
Moore, W.B. & Chatfield, S.N. (1981) Mutagenicity: Evaluation of
bioresmethrin using the Ames Samonella/microsome incorporation test.
Unpublished Report No. BEMU/81/1 from the Wellcome Foundation Ltd.
Submitted to WHO by Roussel Uclaf, Paris, France.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., & Spicer,
E.J.F. (1971) Oral toxicity study in beagle dogs (repeated dosage for
3 months). Unpublished report from the Huntingdon Research Centre.
Submitted to the WHO by the Wellcome Foundation Ltd.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille,
A., & Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutation Research, 137: 7-15.
Ruzo, L.O., Krautter, M.S., & Jiang, Jialiang (1991) Absorption,
distribution, elimination, and metabolism of [14C-acid]-d-
trans-resmethrin in rats. Unpublished Report, Pharmacology and
Toxicology Research Lab. East and West, Inc.; PTRL Report No.
410E/238W. Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M.H. (1987) Bioresmethrin: Two-generation reproduction
toxicity study in rats by dietary mixture administration. Unpublished
Report No. 2059 RSR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M. H. (1990) Assessment of possible embryotoxic or
teratogenic effects of oral route in rabbits. Unpublished report;
Study No. 6772 RSL from Centre International de Toxicologie, France.
Submitted to WHO Roussel Uclaf, Paris, France.
Savary, M.H., Read, M.H., & Delhomme, C. (1988) Bioresmethrin:
Assessment of possible embryotoxic, teratogenic or developmental
effects in the rats. Unpublished Report No. 2058 RSR from Centre de
Recherches Roussel Uclaf, France. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ueda, K., Gaughan, L.C., & Casida, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pesticide Biochem. Physiol.,
5: 280-294.
Ueda, K. Gaughan, L.C., & Casida, J.E. (1975b) Metabolism of
(+)- trans and (+)- cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115
Vallet, L. (1990) Combined chronic toxicity/oncogenicity study by
repeated dietary administration to rats (104 weeks). Unpublished
Report No. 2057 TCR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Vannier, B. & Fournex, R. (1986) Bioresmethrin: Detection of a
mutagenic potency, micronucleus test in the mouse. Unpublished Report
No. 85612-BB/178 from Centre de Recherches Roussel Uclaf, Romainville,
France. Submitted to WHO by Roussel Uclaf, France.
Verschoyle, R.D. & Barnes, J.M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. & Physiol., 2(3):
308-11.
Waldron, M.M. (1969) Fetal toxicity study - 95 H 56 - given orally in
the rabbit. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd., submitted to the World
Health Organization by the Wellcome Foundation Ltd.
Wallwork, L.M., Chesher, B.C., & Malone, J.C. (1970) Toxicity of NRDC
107 to laboratory animals. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M., Clampitt, R.B., & Malone, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Unpublished report
from the Cooper Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. Submitted to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1972) Bioresmethrin: Preliminary
24-hour rat inhalation study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
 the consideration of Verschoyle & Barnes (1972) that as a delay in
signs of poisoning was evident following iv administration,
bioresmethrin might be converted in vivo to a toxic metabolite. The
presence of (d)- trans-CA, BFA, and BFCA as metabolites, which are
more toxic than bioresmethrin, may account for their observation and
conclusions.
The recent bioresmethrin metabolism study indicated that
bioresmethrin was metabolized by a combination of hydrolytic,
oxidative, and conjugative processes. The results of metabolite
determinations were consistent with those of the 1975 papers of Ueda
et al. (Ruzo, 1991).
Toxicological studies
Acute toxicity
Much of the information on the acute toxicity of bioresmethrin
had been evaluated by the 1976 Joint Meeting and published in the
monograph (Annex I, 27). Some new data have become available, and
they have been summarized along with the published information in
Table 1.
Two or more hours after oral administration, the treated animals
showed signs of aggressiveness and tremors. The final stages of
poisoning consisted of convulsive twitching, prostration, coma, and
death normally between 3 and 24 hours (Annex I, 27). The recent
findings in clinical signs also include hypotonicity, slightly arched
back, and piloerection at 30 minutes after treatment (Audegond,
1989a,b).
Table 1. Acute toxicity of bioresmethrin
Species Sex Route LC50 LC50 Reference
(mg/kg b.w.) (mg/L)
Rat m/f oral >5000 Audegond, 1989a
m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chescher & Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
m/f inh. (4 h) >5.3 Hardy et al., 1989
f inh. (24 h) >872 Wallwork & Malone, 1972
f dermal >10 000 Wallwork et al., 1970
Rabbit f dermal >2000 Audegond, 1989b
Mouse f oral >10 000 Wallwork et al., 1970
m oral 3100 Ueda et al., 1975b
m ip >1500 Ueda et al., 1975b
Chicken oral >10 000 Wallwork et al., 1970
>10 000 Chester & Malone, 1970a
Acute toxicity of metabolites
The data on the acute toxicity of the metabolites of
bioresmethrin were evaluated in the 1976 Joint Meeting and published
in the monograph (FAO, 1977). No new data on the acute toxicity of
these metabolites are available; therefore, the previously published
data (Annex I, 27) are reproduced in its entirety in Table 2.
Table 2. Acute toxicity of the metabolites of bioresmethrin
(as cited in Annex 1, 27, animal species not named in
original)
Metabolite LD50 (mg/kg/bw)a
ip oral
1) (+)-trans-resmethrin (bioresmenthrin) > 1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-trans-CA (t-CA) 98 280
(+)-trans-chrysanthemic acid
3) (+)-trans-CDA (tE-CDA) 408
(+)-trans-chrysanthemundicarboxylic
acid
4) BFA 75 310
5-benzyl-3-furylmethanol
5) BFCA 46
5-benzyl-3-furoic acid
Short-term studies
New data on short-term studies are not available; however, the
1976 Joint Meeting had evaluated several short-term studies on rats
and dogs. The summaries of these study are published in the monograph
(Annex I, 27), and they are reproduced below.
Rats
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage six days per week for 3 weeks at doses of 0, 1000,
and 2000 mg/kg body weight. There was no mortality attributable to
bioresmethrin. There was a slight reduction in body weights at 2000
mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and haematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks,
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated, as cited in Annex I,
27).
Groups of rats (18/sex/group) were fed bioresmethrin at dietary
concentrations of 0, 400, 1200, and 8000 ppm (the last dose group was
fed 4000 ppm for 30 days, and the level was increased thereafter) for
91 days. There was no mortality observed in this study. Food
consumption was normal, and food conversion was unaffected by
bioresmethrin. Growth was reduced at the highest dose level which was
accompanied by changes in blood chemistry parameters indicating liver
dysfunction (ASP, SGOT, and urinary nitrogen were increased at 90
days; glucose content was decreased). Depression of red blood cell
count was observed at 1200 ppm although no consistent parallel changes
were seen in haemoglobin content or packed cell volume. Urinalyses
were normal. Gross and microscopic analyses of tissues and organs
showed an increase in the liver weight at 4000 ppm and a decrease in
several other organ weights (spleen, heart, brain, thymus, prostate,
ovary, and uterus). At 1200 and 4000/8000 ppm fatty infiltration of
liver was seen on microscopic examination. A no-effect level in this
study is 400 ppm (equivalent to an average daily intake of 32.8 to
36.1 mg/kg body weight of males and females, respectively) (Wallwork
et al., 1971, as cited in Annex I, 27).
Dogs
Groups of dogs (2/sex/group) were administered bioresmethrin by
gavage, daily at dose levels of 0 and 500 mg/kg body weight for 7 days
followed by a dose increase to 1000 mg/kg for an additional 14 days.
There were no effects noted in this test with respect to mortality,
behaviour, body weight changes, haematology, blood chemistry or
urinalysis parameters or on electrocardiograph measurements. Short
term administration for three weeks at an oral dose of 1000 mg/kg was
uneventful in the parameters measured (Malone & Chesher, 1970, as
cited in FAO, 1977).
In a continuation of the above trial, after a two-week interval
on the control diets, dogs were administered bioresmethrin by gavage
for 7 days at a dose of 2000 mg/kg body weight. Again, no significant
effects were noted in the parameters recorded above (Chesher & Malone,
1971b as cited in Annex I, 27).
Groups of dogs (3/sex/group) were administered bioresmethrin
(gelatin capsule) by gavage daily for 90 days at dose levels of 0, 25,
80, and 250 mg/kg (the high dose was increased to 500 mg/kg in week
7). There was no mortality. Growth, food consumption, and calculated
food utilization parameters were normal. Clinical biochemistry,
ophthalmological examination, and urinalysis parameters were normal at
all intervals (30, 60, and 90 days) examined. In the high dose group,
reduced RBC count, haemoglobin content, and packed cell volume were
noted. BUN was slightly increased only at the high dose after 12
weeks. There were no adverse effects noted on gross or microscopic
examination of tissues and organs (including bone marrow). The NOAEL
is 80 mg/kg (equivalent to an average of 1600 ppm in the diet) (Noel
et al., 1971 as cited in Annex I, 27).
Long-term/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (75/sex/dose) received
resmethrin at dietary concentrations of 0, 250, 500, or 1000 for 85
weeks. The survival rate of 1000 ppm females was significantly (32%)
lower (p<0.05) than that of controls from week 63 to the end of the
study. In 1000 ppm males decreased survival rate was first found at
week 81 (31% at the end of the study).
Terminal body weights of mice from the 1000 ppm groups were
significantly lower (p <0.05) than those of the controls. Although
the food consumption data indicated a slight decrease, it was not
compound-related. Haematological parameters did not show significant
changes in the treated animals relative to those of the controls.
There was a significant increase in absolute and relative adrenal
weights in 500 ppm (20% and 30% respectively) and 1000 ppm males (31%
and 50%, respectively). There were increases in the relative liver,
kidney, and brain weights of 1000 ppm males, but these increases were
mainly due to a decrease in the terminal body weights. An increase in
the incidence of amyloidosis was seen in various tissues of high-dose
animals relative to that in the controls; however, this increase was
not considered to have been compound-related since the control males
and females also had high incidence of amyloidosis. No increase in
tumour incidence was found in any treatment group. The NOAEL was 250
ppm (equivalent to 38 mg/kg bw/day or 11 mg/kg bw/day).
Rats
Groups of Sprague-Dawley rats (50/sex/dose) received
bioresmethrin (technical grade) at dietary concentrations of 0, 50,
250, or 1250 ppm for 104 weeks. These dietary concentrations were
equivalent to 3.0, 14.9, and 76.2 mg/kg/day for male rats and 4.0,
19.8, and 101.3 mg/kg/day for female rats. Two satellite groups in
each dose level were included in the study. Satellite group 1
consisted of 10 rats/sex/dose and was sacrificed on week 52 of the
study; satellite group 2 consisted of 20 rats/sex/dose and received
the test chemical for 104 weeks. An extra control group consisting of
50 animals/sex was included in the study.
Bioresmethrin did not affect clinical signs, mortality rate, body
weights, food consumption, food efficiency, haematological parameters,
or urinalysis parameters. However, a statistically significant and
compound-related decrease in cholesterol levels and an increase in
alkaline phosphatase levels were observed in 1250 ppm males at various
examination periods. An increase in the alkaline phosphatase levels
was also found in males at 250 ppm, but this increase did not always
show a statistical significance. During sacrifice at 52 and 104
weeks, a slight increase in the absolute liver weight in males and
females at 1250 ppm was found.
Gross pathology showed an increase in the incidence of paleness
of the liver in both males and females at 1250 ppm (control, 0/10;
1250 ppm males, 5/10; 1250 ppm females, 2/10) at the 52 week
sacrifice.
Histopathology data indicated an increase in the incidence of
periportal hepatic cell hypertrophy in 250 ppm females (control, 0/10;
250 ppm, 2/10) and in 1250 ppm male (3/10) and female rats (3/10) at
52 week sacrifice. At 104 week sacrifice, there was also an increase
in the incidence of periportal hepatic cell hypertrophy in 250 ppm
females (5/70) and 1250 ppm males (37/70) and females (30/70) relative
to the controls (0/70). Most animals which had periportal hepatic
cell hypertrophy also showed signs of vacuolated hepatocytes at 104
weeks. No increase in the incidence of neoplasia was found at any
site in animals receiving bioresmethrin at dose levels up to 1250 ppm.
Based upon the histopathologic findings, the NOAEL was 50 ppm (equal
to 3.0 and 4.0 mg/kg/day in males and females, respectively (Vallet,
1990).
Reproduction study
Groups of Sprague-Dawley rats (25/sex/group) received
bioresmethrin (93.5% purity) at dietary concentrations of 0, 0, 80,
250, 750, and 2250 ppm. The study began with 3 dose levels: 250, 750,
and 2250 ppm, but the 2250 ppm group had only 3 live births.
Therefore, the 80 ppm group was added along with its concurrent
control group after the birth of F1 pups. For F0 generation, the
treatment began 8 weeks prior to mating and continued through mating,
pregnancy, and lactation periods for females. For the F1 parental
animals, one male and one female per litter were selected at the
weaning and received treatment for approximately 14 weeks and then
mated. The treatment groups received biores-methrin throughout the
experiment. Each male was mated with a female of the same treatment
group until pregnancy occurred or 3 weeks had elapsed. For F1 and
F2 generations, only the first litters were produced. At day 4
post-partum, each litter was standardized to have 4 males and 4
females if possible.
For F0 parental animals, 17/20 pregnant females in the 2250 ppm
group showed clinical signs of decreased spontaneous activity and
piloerection immediately before and after parturition. There was a
slight decrease in male body weights at 250 ppm from days 22 to 64.
A significant decrease in the body weight gain was found in 2250 ppm
males from the second week of treatment to the scheduled sacrifice.
There were decreases in female body weights at 750 ppm during the
premating period, on days 14 and 21 during the pregnancy period, and
on days 1 and 4 of the lactation period. At 2250 ppm, female body
weight was decreased during the premating and pregnancy periods. Food
consumption was decreased in females at 750 and 2250 ppm during the
lactation period. No significant difference was found in the
copulation index, fertility index, or length of pregnancy between the
treated and the control animals. Macroscopic examination showed an
increase in the incidence of liver changes characterized by
accentuated lobular pattern in 2/25 females at 750 ppm. In 2250 ppm
females, 11/25 showed marked lobular pattern, and 7/25 showed paler
than normal liver. Microscopically, the hepatic changes were
associated with steatosis.
For F1 litter parameters, there was a significant decrease
(p <0.01) in birth index at 750 and 2250 ppm relative to the controls
(control, 91%; 750 ppm, 81%; 2250 ppm, 1%). In the 2250 ppm group,
only 3 pups were born alive. The viability index on day 4 post-partum
was also reduced at 750 ppm (control, 95%; 750 ppm, 55%), and there
was no survival in the the 2250 ppm group. The mean pup body weight
of the 750 ppm group was significantly decreased (p< 0.01) on days 1,
4 and 7 post-partum). No compound-related effects on physical and
behavioural developmental parameters were found in all treated pups;
the parameters examined included pinna unfolding, hair growth, incisor
eruption, eye opening, auricular duct opening, surface righting
reflex, cliff avoidance, and air righting reflexes. Gross pathology
findings revealed an increase in the incidence of discolored liver in
pups which died between days 1 and 21 postpartum at 250 ppm (2/16),
750 ppm (13/109), and 2250 ppm (3/167); at 750 ppm, similar finding
was also reported for pups, which were not selected as F1 parental
animals on day 21 post-partum.
For F1 parental animals, no compound-related clinical signs were
noted in either males or females. There was a decrease in the body
weights of 750 ppm males throughout the treatment period, and this
decrease was statistically significant between days 1 and 113. The
body weight decrease was also seen in females at 750 ppm during the
premating, pregnancy, and lactation periods. A consistent decrease in
food consumption was not seen in all treated males. In treated
females, a slight decrease in food consumption was noted at 750 ppm
from days 14 to 21 of the pregnancy, and a significant drop in this
parameter was also reported during the lactation period. As in the F0
parental animals, the compound produced no effects on copulation
index, fertility index, and length of pregnancy. Gross pathology
showed that 1/23 females at 250 ppm had pale liver associated with
hepatic steatosis. In the 750 ppm group, 1/16 males and 3/15 females
also had pale liver associated with hepatic steatosis.
For the F2 litter parameters, at 750 ppm, there was a decrease
in the birth index (control, 96%; 750 ppm, 58%), in the viability
index at birth (control, 94%; 750 ppm, 33%), and in the viability
index at weaning (control, 76%; 750 ppm, 48%). The mean pup body
weight at 750 ppm was decreased relative to that of the controls
during the lactation period, and the decrease was statistically
significant (p<0.001) on day 1 of post-partum only. Bioresmethrin
did not affect the physical and behavioural developments of the pups.
Both gross pathological and histopathological examinations on the pups
showed no abnormalities.
At 250 ppm, an increase in the incidence of pale or discolored
liver associated with hepatic steatosis was found in F1 parental
females (1/23) and in F1 pups (2/16). Therefore, the NOAEL for this
study is 80 ppm (equivalent to 4 mg/kg/day) (Savary, 1987).
Special study on developmental toxicity
Rats
Groups of pregnant Sprague-Dawley rats (40/group) received
technical grade bioresmethrin (93.5% purity) by gavage at doses of 0,
50, 100, and 200 mg/kg/day from gestation days 6 to 15. On gestation
day 20, 25 females/group were sacrificed, and the fetuses were
delivered by caesarean section. The remaining 15 females/group were
allowed to deliver normally and to nurse their offspring till weaning.
The clinical signs, mortality, and number of abortions were
comparable between the treated and the control dams. There was a
slight and statistically significant drop in body weight gain in
c-section dams at 200 mg/kg. The mean numbers of corpora lutea,
implantation, resorption, and live fetuses were comparable between the
treated and the control animals. The fetal body weights of the
treated groups were similar to those of the controls. Fetal
abnormalities were incidental and not compound-related.
The results in females which delivered normally did not show any
treatment-related effects on clinical signs, abortion, or the duration
of gestation. There was a statistically significant (p <0.001)
decrease in body weight gain in dams at 200 mg/kg. The mean live
birth, body weight of the live pups, survival rates, and the rate of
post-implantation losses were comparable to those of the controls. The
physical and behavioural development of pups from the treated groups
were similar to those of the controls. The physical and behavioural
developmental parameters examined were pinna unfolding, hair growth,
incisor eruption, eye opening, auricular duct opening, surface
righting reflex, cliff avoidance, and air righting reflexes. The
NOAEL for maternal toxicity was 200 mg/kg, and no embryotoxic,
teratogenic, and developmental toxicity effects were found in the
highest dose tested (200 mg/kg) (Savary et al., 1988).
Rabbits
Groups of artificially inseminated female rabbits (16/group)
received bioresmethrin by gavage at doses of 0, 15, 60, and 240 mg/kg
from gestation days 6 to 18. The fetuses were delivered on gestation
day 28, and dams were sacrificed at that time. Under the conditions
of the study, the compound produced no maternal nor developmental
toxicity at any dose levels. Based upon the results of this study,
the pregnant rabbits could have tolerated higher dose levels (Savary,
1990).
An older rabbit teratology study on the bioresmethrin had been
considered by the Joint Meeting in 1976 and published in 1977 FAO
monograph. The summary of the study is reproduced in its entirety
below.
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin in doses of 0, 10, 20, 40, and 80 mg/kg by oral gavage
daily from days 8-16 of gestation. The does were sacrificed on day 28
and examined for implantation, live and dead fetuses, resorption
sites, and abnormalities (after staining a representative number for
skeletal examination). There was no apparent effect on parents in the
study as growth and gestation were unaffected. There was an increase
in dead fetuses at the highest dose and a large number of resorption
sites noted at all treatment levels. There were a number of deformed
fetuses observed but the total numbers were not sufficient for
adequate statistical evaluation. The deformities included straight
tail, crossed hind limbs and unilateral union of 6th and 7th ribs at
the sternal end. An overall fetal loss was observed at all dose
levels (primarily because of the large number of resorption sites
recorded) (Waldron, 1969, as cited in Annex I, 27).
Special studies on genotoxicity
A number of mutagenicity studies have been conducted with
bioresmethrin. The results are summarized in Table 3.
Special studies on skin and eye irritation and sensitization
A volume of 0.5 ml bioresmethrin (95%) was applied under
occlusive conditions to the shaven intact skin of 3 male New Zealand
white albino rabbits for 4 hours. No evidence of skin irritation was
found up to 72 hours after application (Audegond, 1989d).
Three New Zealand white albino rabbits were administered 0.1 ml
of bioresmethrin (95%) in their conjunctival sac of the right eye.
The treated eyes did not appeared to have been washed. No eye
irritation was found under the conditions of the test (Audegond,
1989c). An older study conducted by Chesher and Malone (1970c) also
showed that bioresmethrin was not an eye irritant in rabbits (FAO,
1977).
Bioresmethrin (95%) was tested for skin sensitization in 10 male
Hartley albino guinea pigs. During the induction phase, The animals
were treated topically with 0.5 ml bioresmethrin daily for 10 days.
On day 36, the animals were challenged with 0.5 ml of the test
substance and later rechallenged with similar volume on day 43. A
positive control group of 10 males received 0.06% w/v solution of
2,4-dinitrochlorobenzene in a similar manner as those treated with
bioresmethrin. Bioresmethrin did not produce skin sensitization
reaction in guinea pigs (Kuhn, 1990).
In an older study published in the FAO Monograph (Annex I, 27),
groups of adult guinea pigs (6 males/group) were applied bioresmethrin
(0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro-benzene (DNCB)
to the ears for 4 days. On day 7, 0.2 ml of bioresmethrin or DNCB
were applied dermally. Bioresmethrin produced only traces of erythema
suggesting a low potential for sensitization and irritation (Chesher
& Malone, 1970b).
Table 3. Results of genotoxicity assays on bioresmethrin
Test system Test object Concentration Purity Results Reference
(%)
Ames test S. typhimurium 0.2. 1-5. 10. 92.2 Negativea Moore, 1981
(with and TA1535, TA1537, 50, 100, 250,
without S9) TA1538, TA98, 500, 1000,
TA100 5000 µg/plate
Ames test S. typhimurium 30, 100, 300, 97 Negative Pluijmen et
(with and TA98, TA100 1000 µg/plate al., 1984
without S9)
Gene mutation V79 Chinese 5, 10, 15, 20 97 Negative Pluijmen et
assay (with and hamster cells µg/ml al., 1984
without S9)
Micronucleus Swiss CD 1 mice 300 mg/kg (M) 93.6 Negative Vannier &
assay 450 mg/kg (F) Fournex, 1986
Metaphase human 4, 20, 40 93.6 Negative Allen, Brooker
chromosome lymphocytes µg/ml & Howell (1986)
analysis (with
and without S9)
Unscheduled human 0.125, 0.25, 93.6 Negative Allen &
DNA synthesis epithelioid 0.5, 1, 2, 4, Proudlock,
assay (with cells 8, 16, 32, 64 1986
and without S9) (HeLa S3) 128 and 256
µg/ml
a The report of this study has serious deficiencies which include lack of information on the source
of the metabolic activation system and whether the results presented in the report are the
averages or single determinations.
COMMENTS
Bioresmethrin was absorbed and distributed rapidly following oral
administration, and was quickly metabolized by oxidation and
hydrolysis at various sites in the molecule. Complete elimination of
bioresmethrin occurred slowly. The enterohepatic circulation system
was involved in the elimination. There is no indication that
isomerization of bioresmethrin to the (+)- cis-isomer occurs.
In general, bioresmethrin has low acute toxicity after oral
administration. In mammals, the cis-isomers are generally more
toxic than the corresponding trans-isomers. Some metabolites of
bioresmethrin are more toxic than the parent compound.
Short-term studies in rats show that bioresmethrin fed at 1000
ppm caused a slight increase in liver weight and a reduction in thymus
weight in rats. In a 90-day feeding study in rats, at dietary
concentrations of 0, 400, 1200 or 8000 ppm, bioresmethrin at 1200 ppm
or above induced an increase in liver weight and fatty liver which was
accompanied by changes in blood enzyme levels (serum alkaline
phosphatase and aspartate aminotransferase) indicative of liver
injury. In a 90-day gavage study in dogs, bioresmethrin at 250 mg/kg
bw/day or above reduced the erythrocyte count, haemoglobin content,
and packed cell volume.
A carcinogenicity study in mice with resmethrin (containing at
least 30% bioresmethrin) at dietary concentrations of 250, 500 and
1000 ppm for 85 weeks did not demonstrate a carcinogenic effect.
However, resmethrin decreased survival rate in both male and female
mice at 1000 ppm and adrenal weights were significantly increased in
males at 500 and 1000 ppm. The NOAEL for resmethrin was 250 ppm,
which was equal to 38 mg/kg bw/day for resmethrin and 11 mg/kg bw/day
for bioresmethrin.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 50, 250 or 1250 ppm for 104 weeks, bioresmethrin
did not produce an increase in the tumour incidence. However, it
induced an increase in alkaline phosphatase in males at 250 and 1250
ppm and a decrease in cholesterol levels in males at 1250 ppm.
Bioresmethrin caused an increase in the incidence of non-neoplastic
liver changes, including pallor and hypertrophy of hepatocytes in
males at 250 ppm and in males and females at 1250 ppm. Based upon
these findings, the NOAEL for chronic toxicity was 50 ppm, equal to
3.0 mg/kg bw/day.
In a two-generation reproduction study in rats, at dietary
concentrations of 0, 80, 250, 750 or 2250 ppm, bioresmethrin did not
affect reproductive performance at dietary concentrations of 250 ppm
or less, although reproduction was adversely affected at 750 and 2250
ppm. Based on a decrease in parental body weight and hepatotoxicity
observed at 250 ppm, the NOAEL for this study was 80 ppm, equivalent
to 4 mg/kg/day.
Studies on the developmental toxicity of bioresmethrin in rats
and rabbits failed to elicit effects at doses up to 200 and 240 mg/kg
bw/day respectively.
After reviewing all available in vitro and in vivo short-term
assays with bioresmethrin, the Meeting concluded that there was no
evidence of genotoxicity.
The ADI was based upon the long-term/carcinogenicity study in
rats utilizing a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet, equal to 3.0 mg/kg bw/day
Dog: 80 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, J.A., Brooker, P.C., & Howell, A. (1986) Bioresmethrin:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. RSL 697/851370 from Huntingdon Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Allen, J.A. & Proudlock, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after exposure to
bioresmethrin. Unpublished report No. RSL 698/851420 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989a) Bioresmethrin: Acute oral toxicity study in the
rat. Unpublished study No. 89142 from Toxicology Department, Division
Scientifique Roussel Uclaf, Romainville, France. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989b) Bioresmethrin: Acute dermal toxicity study in
the rabbit. Unpublished study No. 89143 from Toxicology Department,
Division Scientifique Roussel Uclaf, Romainville, France. Submitted to
WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989c) Bioresmethrin: Primary eye irritation study in
the male rabbit. Unpublished study No. 89144 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989d) Bioresmethrin: Primary dermal irritation study
in the male rabbit. Unpublished study No. 89145 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Casida, J.E., Fammon, D.W. & Lawrence, L.J. (1983) Mechanisms of
selective actions of pyrethroid insecticides. Ann. Rev. Pharmacol.
Toxicol., 23: 413-38.
Chesher, B.C. & Malone, J.C. (1970a) Acute toxicity test with NRDC
107 in hens. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970b) Sensitization study with NRDC
107 in guinea pigs. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971a) Acute toxicity of NRDC 107 by
the intravenous route. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971b) Toxicity to dog of NRDC 107
(continuation). Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Cox, G.E., Knickerbocker, M., & Parent, R.A. (1979) Evaluation of
dietary administration of SBP-138 in CD-1 outbred albino mice over an
85 week period. Unpublished report by Food and Drug Research
Laboratories, Inc., Study No. 5270. Sponsored by S.B. Penick & Co. and
submitted to WHO by Roussel Uclaf, Paris, France.
Farebrother, D.A. (1973) NRDC 107: Whole body radioautographical
study in albino rats. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Glomot, R. & Chevalier, B. (1969) Etude de la toxicite aigue du RU
11484 (NRDC 107). Unpublished report from Roussel Uclaf. Submitted to
WHO by the Wellcome Foundation Ltd.
Glomot, R., Undated. Three week study of RU 11484 (NRDC 107) in the
rat. Unpublished report from Roussel Uclaf. Submitted to WHO by the
Wellcome Foundation Ltd.
Hardy, C.J., Jackson, G.C., Suttie, A.W., Read, R.M., & Gopinath, C.
(1989) Bioresmethrin: Acute inhalation toxicity study in rats (4-hour
exposure). Unpublished Report No. RSL 801/89712 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Kuhn, J.O. (1990) Bioresmethrin Technical 7N1173B3: Dermal
sensitization study in guinea pigs. Unpublished Report No. 6674-89
from Stillmeadow, Inc., Houston, Texas, USA. Submitted by Roussel
Uclaf, Paris, France.
Malone, J.C. & Chesher, B.C. (1970) Toxicity to dogs of NRDC 107.
Unpublished report from the Cooper Technical Bureau, Berkhamstead, the
Wellcome Foundation Ltd., submitted to the World Health Organization
by the Wellcome Foundation Ltd.
Miyamoto, J. (1975-76) Terminal residues of bioresmethrin. Submission
to IUPAC Terminal Residues Commission, Madrid, Sept. 1975; Leverkusen,
Sept. 1976.
Moore, W.B. & Chatfield, S.N. (1981) Mutagenicity: Evaluation of
bioresmethrin using the Ames Samonella/microsome incorporation test.
Unpublished Report No. BEMU/81/1 from the Wellcome Foundation Ltd.
Submitted to WHO by Roussel Uclaf, Paris, France.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., & Spicer,
E.J.F. (1971) Oral toxicity study in beagle dogs (repeated dosage for
3 months). Unpublished report from the Huntingdon Research Centre.
Submitted to the WHO by the Wellcome Foundation Ltd.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille,
A., & Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutation Research, 137: 7-15.
Ruzo, L.O., Krautter, M.S., & Jiang, Jialiang (1991) Absorption,
distribution, elimination, and metabolism of [14C-acid]-d-
trans-resmethrin in rats. Unpublished Report, Pharmacology and
Toxicology Research Lab. East and West, Inc.; PTRL Report No.
410E/238W. Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M.H. (1987) Bioresmethrin: Two-generation reproduction
toxicity study in rats by dietary mixture administration. Unpublished
Report No. 2059 RSR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M. H. (1990) Assessment of possible embryotoxic or
teratogenic effects of oral route in rabbits. Unpublished report;
Study No. 6772 RSL from Centre International de Toxicologie, France.
Submitted to WHO Roussel Uclaf, Paris, France.
Savary, M.H., Read, M.H., & Delhomme, C. (1988) Bioresmethrin:
Assessment of possible embryotoxic, teratogenic or developmental
effects in the rats. Unpublished Report No. 2058 RSR from Centre de
Recherches Roussel Uclaf, France. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ueda, K., Gaughan, L.C., & Casida, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pesticide Biochem. Physiol.,
5: 280-294.
Ueda, K. Gaughan, L.C., & Casida, J.E. (1975b) Metabolism of
(+)- trans and (+)- cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115
Vallet, L. (1990) Combined chronic toxicity/oncogenicity study by
repeated dietary administration to rats (104 weeks). Unpublished
Report No. 2057 TCR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Vannier, B. & Fournex, R. (1986) Bioresmethrin: Detection of a
mutagenic potency, micronucleus test in the mouse. Unpublished Report
No. 85612-BB/178 from Centre de Recherches Roussel Uclaf, Romainville,
France. Submitted to WHO by Roussel Uclaf, France.
Verschoyle, R.D. & Barnes, J.M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. & Physiol., 2(3):
308-11.
Waldron, M.M. (1969) Fetal toxicity study - 95 H 56 - given orally in
the rabbit. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd., submitted to the World
Health Organization by the Wellcome Foundation Ltd.
Wallwork, L.M., Chesher, B.C., & Malone, J.C. (1970) Toxicity of NRDC
107 to laboratory animals. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M., Clampitt, R.B., & Malone, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Unpublished report
from the Cooper Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. Submitted to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1972) Bioresmethrin: Preliminary
24-hour rat inhalation study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
the consideration of Verschoyle & Barnes (1972) that as a delay in
signs of poisoning was evident following iv administration,
bioresmethrin might be converted in vivo to a toxic metabolite. The
presence of (d)- trans-CA, BFA, and BFCA as metabolites, which are
more toxic than bioresmethrin, may account for their observation and
conclusions.
The recent bioresmethrin metabolism study indicated that
bioresmethrin was metabolized by a combination of hydrolytic,
oxidative, and conjugative processes. The results of metabolite
determinations were consistent with those of the 1975 papers of Ueda
et al. (Ruzo, 1991).
Toxicological studies
Acute toxicity
Much of the information on the acute toxicity of bioresmethrin
had been evaluated by the 1976 Joint Meeting and published in the
monograph (Annex I, 27). Some new data have become available, and
they have been summarized along with the published information in
Table 1.
Two or more hours after oral administration, the treated animals
showed signs of aggressiveness and tremors. The final stages of
poisoning consisted of convulsive twitching, prostration, coma, and
death normally between 3 and 24 hours (Annex I, 27). The recent
findings in clinical signs also include hypotonicity, slightly arched
back, and piloerection at 30 minutes after treatment (Audegond,
1989a,b).
Table 1. Acute toxicity of bioresmethrin
Species Sex Route LC50 LC50 Reference
(mg/kg b.w.) (mg/L)
Rat m/f oral >5000 Audegond, 1989a
m oral 8800 Glomot & Chevalier, 1969
f oral >8000 Verschoyle & Barnes, 1972
f oral 7071 Wallwork et al., 1970
f iv 340 Verschoyle & Barnes, 1972
f iv 106-133 Chescher & Malone, 1971a
f ip >8000 Wallwork & Malone, 1971
m/f inh. (4 h) >5.3 Hardy et al., 1989
f inh. (24 h) >872 Wallwork & Malone, 1972
f dermal >10 000 Wallwork et al., 1970
Rabbit f dermal >2000 Audegond, 1989b
Mouse f oral >10 000 Wallwork et al., 1970
m oral 3100 Ueda et al., 1975b
m ip >1500 Ueda et al., 1975b
Chicken oral >10 000 Wallwork et al., 1970
>10 000 Chester & Malone, 1970a
Acute toxicity of metabolites
The data on the acute toxicity of the metabolites of
bioresmethrin were evaluated in the 1976 Joint Meeting and published
in the monograph (FAO, 1977). No new data on the acute toxicity of
these metabolites are available; therefore, the previously published
data (Annex I, 27) are reproduced in its entirety in Table 2.
Table 2. Acute toxicity of the metabolites of bioresmethrin
(as cited in Annex 1, 27, animal species not named in
original)
Metabolite LD50 (mg/kg/bw)a
ip oral
1) (+)-trans-resmethrin (bioresmenthrin) > 1500 3100
(5-benzyl-3-furylmethyl (+)-trans-
chrysanthemate)
2) (+)-trans-CA (t-CA) 98 280
(+)-trans-chrysanthemic acid
3) (+)-trans-CDA (tE-CDA) 408
(+)-trans-chrysanthemundicarboxylic
acid
4) BFA 75 310
5-benzyl-3-furylmethanol
5) BFCA 46
5-benzyl-3-furoic acid
Short-term studies
New data on short-term studies are not available; however, the
1976 Joint Meeting had evaluated several short-term studies on rats
and dogs. The summaries of these study are published in the monograph
(Annex I, 27), and they are reproduced below.
Rats
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage six days per week for 3 weeks at doses of 0, 1000,
and 2000 mg/kg body weight. There was no mortality attributable to
bioresmethrin. There was a slight reduction in body weights at 2000
mg/kg. Haematology was normal with a slight reduction noted in
haemoglobin content and haematocrit value. Albumin and BUN were
increased while SGOT activity was reduced. At the end of three weeks,
gross examination of major tissues showed slight effects on liver
(increased size), reduced thymus weight (both organs affected at 1000
mg/kg), and reduced prostate (only at the high dose). Histological
examination showed only thymic involution without structural changes
with no effects noted in liver (Glomot, undated, as cited in Annex I,
27).
Groups of rats (18/sex/group) were fed bioresmethrin at dietary
concentrations of 0, 400, 1200, and 8000 ppm (the last dose group was
fed 4000 ppm for 30 days, and the level was increased thereafter) for
91 days. There was no mortality observed in this study. Food
consumption was normal, and food conversion was unaffected by
bioresmethrin. Growth was reduced at the highest dose level which was
accompanied by changes in blood chemistry parameters indicating liver
dysfunction (ASP, SGOT, and urinary nitrogen were increased at 90
days; glucose content was decreased). Depression of red blood cell
count was observed at 1200 ppm although no consistent parallel changes
were seen in haemoglobin content or packed cell volume. Urinalyses
were normal. Gross and microscopic analyses of tissues and organs
showed an increase in the liver weight at 4000 ppm and a decrease in
several other organ weights (spleen, heart, brain, thymus, prostate,
ovary, and uterus). At 1200 and 4000/8000 ppm fatty infiltration of
liver was seen on microscopic examination. A no-effect level in this
study is 400 ppm (equivalent to an average daily intake of 32.8 to
36.1 mg/kg body weight of males and females, respectively) (Wallwork
et al., 1971, as cited in Annex I, 27).
Dogs
Groups of dogs (2/sex/group) were administered bioresmethrin by
gavage, daily at dose levels of 0 and 500 mg/kg body weight for 7 days
followed by a dose increase to 1000 mg/kg for an additional 14 days.
There were no effects noted in this test with respect to mortality,
behaviour, body weight changes, haematology, blood chemistry or
urinalysis parameters or on electrocardiograph measurements. Short
term administration for three weeks at an oral dose of 1000 mg/kg was
uneventful in the parameters measured (Malone & Chesher, 1970, as
cited in FAO, 1977).
In a continuation of the above trial, after a two-week interval
on the control diets, dogs were administered bioresmethrin by gavage
for 7 days at a dose of 2000 mg/kg body weight. Again, no significant
effects were noted in the parameters recorded above (Chesher & Malone,
1971b as cited in Annex I, 27).
Groups of dogs (3/sex/group) were administered bioresmethrin
(gelatin capsule) by gavage daily for 90 days at dose levels of 0, 25,
80, and 250 mg/kg (the high dose was increased to 500 mg/kg in week
7). There was no mortality. Growth, food consumption, and calculated
food utilization parameters were normal. Clinical biochemistry,
ophthalmological examination, and urinalysis parameters were normal at
all intervals (30, 60, and 90 days) examined. In the high dose group,
reduced RBC count, haemoglobin content, and packed cell volume were
noted. BUN was slightly increased only at the high dose after 12
weeks. There were no adverse effects noted on gross or microscopic
examination of tissues and organs (including bone marrow). The NOAEL
is 80 mg/kg (equivalent to an average of 1600 ppm in the diet) (Noel
et al., 1971 as cited in Annex I, 27).
Long-term/carcinogenicity studies
Mice
Groups of Charles River CD-1 mice (75/sex/dose) received
resmethrin at dietary concentrations of 0, 250, 500, or 1000 for 85
weeks. The survival rate of 1000 ppm females was significantly (32%)
lower (p<0.05) than that of controls from week 63 to the end of the
study. In 1000 ppm males decreased survival rate was first found at
week 81 (31% at the end of the study).
Terminal body weights of mice from the 1000 ppm groups were
significantly lower (p <0.05) than those of the controls. Although
the food consumption data indicated a slight decrease, it was not
compound-related. Haematological parameters did not show significant
changes in the treated animals relative to those of the controls.
There was a significant increase in absolute and relative adrenal
weights in 500 ppm (20% and 30% respectively) and 1000 ppm males (31%
and 50%, respectively). There were increases in the relative liver,
kidney, and brain weights of 1000 ppm males, but these increases were
mainly due to a decrease in the terminal body weights. An increase in
the incidence of amyloidosis was seen in various tissues of high-dose
animals relative to that in the controls; however, this increase was
not considered to have been compound-related since the control males
and females also had high incidence of amyloidosis. No increase in
tumour incidence was found in any treatment group. The NOAEL was 250
ppm (equivalent to 38 mg/kg bw/day or 11 mg/kg bw/day).
Rats
Groups of Sprague-Dawley rats (50/sex/dose) received
bioresmethrin (technical grade) at dietary concentrations of 0, 50,
250, or 1250 ppm for 104 weeks. These dietary concentrations were
equivalent to 3.0, 14.9, and 76.2 mg/kg/day for male rats and 4.0,
19.8, and 101.3 mg/kg/day for female rats. Two satellite groups in
each dose level were included in the study. Satellite group 1
consisted of 10 rats/sex/dose and was sacrificed on week 52 of the
study; satellite group 2 consisted of 20 rats/sex/dose and received
the test chemical for 104 weeks. An extra control group consisting of
50 animals/sex was included in the study.
Bioresmethrin did not affect clinical signs, mortality rate, body
weights, food consumption, food efficiency, haematological parameters,
or urinalysis parameters. However, a statistically significant and
compound-related decrease in cholesterol levels and an increase in
alkaline phosphatase levels were observed in 1250 ppm males at various
examination periods. An increase in the alkaline phosphatase levels
was also found in males at 250 ppm, but this increase did not always
show a statistical significance. During sacrifice at 52 and 104
weeks, a slight increase in the absolute liver weight in males and
females at 1250 ppm was found.
Gross pathology showed an increase in the incidence of paleness
of the liver in both males and females at 1250 ppm (control, 0/10;
1250 ppm males, 5/10; 1250 ppm females, 2/10) at the 52 week
sacrifice.
Histopathology data indicated an increase in the incidence of
periportal hepatic cell hypertrophy in 250 ppm females (control, 0/10;
250 ppm, 2/10) and in 1250 ppm male (3/10) and female rats (3/10) at
52 week sacrifice. At 104 week sacrifice, there was also an increase
in the incidence of periportal hepatic cell hypertrophy in 250 ppm
females (5/70) and 1250 ppm males (37/70) and females (30/70) relative
to the controls (0/70). Most animals which had periportal hepatic
cell hypertrophy also showed signs of vacuolated hepatocytes at 104
weeks. No increase in the incidence of neoplasia was found at any
site in animals receiving bioresmethrin at dose levels up to 1250 ppm.
Based upon the histopathologic findings, the NOAEL was 50 ppm (equal
to 3.0 and 4.0 mg/kg/day in males and females, respectively (Vallet,
1990).
Reproduction study
Groups of Sprague-Dawley rats (25/sex/group) received
bioresmethrin (93.5% purity) at dietary concentrations of 0, 0, 80,
250, 750, and 2250 ppm. The study began with 3 dose levels: 250, 750,
and 2250 ppm, but the 2250 ppm group had only 3 live births.
Therefore, the 80 ppm group was added along with its concurrent
control group after the birth of F1 pups. For F0 generation, the
treatment began 8 weeks prior to mating and continued through mating,
pregnancy, and lactation periods for females. For the F1 parental
animals, one male and one female per litter were selected at the
weaning and received treatment for approximately 14 weeks and then
mated. The treatment groups received biores-methrin throughout the
experiment. Each male was mated with a female of the same treatment
group until pregnancy occurred or 3 weeks had elapsed. For F1 and
F2 generations, only the first litters were produced. At day 4
post-partum, each litter was standardized to have 4 males and 4
females if possible.
For F0 parental animals, 17/20 pregnant females in the 2250 ppm
group showed clinical signs of decreased spontaneous activity and
piloerection immediately before and after parturition. There was a
slight decrease in male body weights at 250 ppm from days 22 to 64.
A significant decrease in the body weight gain was found in 2250 ppm
males from the second week of treatment to the scheduled sacrifice.
There were decreases in female body weights at 750 ppm during the
premating period, on days 14 and 21 during the pregnancy period, and
on days 1 and 4 of the lactation period. At 2250 ppm, female body
weight was decreased during the premating and pregnancy periods. Food
consumption was decreased in females at 750 and 2250 ppm during the
lactation period. No significant difference was found in the
copulation index, fertility index, or length of pregnancy between the
treated and the control animals. Macroscopic examination showed an
increase in the incidence of liver changes characterized by
accentuated lobular pattern in 2/25 females at 750 ppm. In 2250 ppm
females, 11/25 showed marked lobular pattern, and 7/25 showed paler
than normal liver. Microscopically, the hepatic changes were
associated with steatosis.
For F1 litter parameters, there was a significant decrease
(p <0.01) in birth index at 750 and 2250 ppm relative to the controls
(control, 91%; 750 ppm, 81%; 2250 ppm, 1%). In the 2250 ppm group,
only 3 pups were born alive. The viability index on day 4 post-partum
was also reduced at 750 ppm (control, 95%; 750 ppm, 55%), and there
was no survival in the the 2250 ppm group. The mean pup body weight
of the 750 ppm group was significantly decreased (p< 0.01) on days 1,
4 and 7 post-partum). No compound-related effects on physical and
behavioural developmental parameters were found in all treated pups;
the parameters examined included pinna unfolding, hair growth, incisor
eruption, eye opening, auricular duct opening, surface righting
reflex, cliff avoidance, and air righting reflexes. Gross pathology
findings revealed an increase in the incidence of discolored liver in
pups which died between days 1 and 21 postpartum at 250 ppm (2/16),
750 ppm (13/109), and 2250 ppm (3/167); at 750 ppm, similar finding
was also reported for pups, which were not selected as F1 parental
animals on day 21 post-partum.
For F1 parental animals, no compound-related clinical signs were
noted in either males or females. There was a decrease in the body
weights of 750 ppm males throughout the treatment period, and this
decrease was statistically significant between days 1 and 113. The
body weight decrease was also seen in females at 750 ppm during the
premating, pregnancy, and lactation periods. A consistent decrease in
food consumption was not seen in all treated males. In treated
females, a slight decrease in food consumption was noted at 750 ppm
from days 14 to 21 of the pregnancy, and a significant drop in this
parameter was also reported during the lactation period. As in the F0
parental animals, the compound produced no effects on copulation
index, fertility index, and length of pregnancy. Gross pathology
showed that 1/23 females at 250 ppm had pale liver associated with
hepatic steatosis. In the 750 ppm group, 1/16 males and 3/15 females
also had pale liver associated with hepatic steatosis.
For the F2 litter parameters, at 750 ppm, there was a decrease
in the birth index (control, 96%; 750 ppm, 58%), in the viability
index at birth (control, 94%; 750 ppm, 33%), and in the viability
index at weaning (control, 76%; 750 ppm, 48%). The mean pup body
weight at 750 ppm was decreased relative to that of the controls
during the lactation period, and the decrease was statistically
significant (p<0.001) on day 1 of post-partum only. Bioresmethrin
did not affect the physical and behavioural developments of the pups.
Both gross pathological and histopathological examinations on the pups
showed no abnormalities.
At 250 ppm, an increase in the incidence of pale or discolored
liver associated with hepatic steatosis was found in F1 parental
females (1/23) and in F1 pups (2/16). Therefore, the NOAEL for this
study is 80 ppm (equivalent to 4 mg/kg/day) (Savary, 1987).
Special study on developmental toxicity
Rats
Groups of pregnant Sprague-Dawley rats (40/group) received
technical grade bioresmethrin (93.5% purity) by gavage at doses of 0,
50, 100, and 200 mg/kg/day from gestation days 6 to 15. On gestation
day 20, 25 females/group were sacrificed, and the fetuses were
delivered by caesarean section. The remaining 15 females/group were
allowed to deliver normally and to nurse their offspring till weaning.
The clinical signs, mortality, and number of abortions were
comparable between the treated and the control dams. There was a
slight and statistically significant drop in body weight gain in
c-section dams at 200 mg/kg. The mean numbers of corpora lutea,
implantation, resorption, and live fetuses were comparable between the
treated and the control animals. The fetal body weights of the
treated groups were similar to those of the controls. Fetal
abnormalities were incidental and not compound-related.
The results in females which delivered normally did not show any
treatment-related effects on clinical signs, abortion, or the duration
of gestation. There was a statistically significant (p <0.001)
decrease in body weight gain in dams at 200 mg/kg. The mean live
birth, body weight of the live pups, survival rates, and the rate of
post-implantation losses were comparable to those of the controls. The
physical and behavioural development of pups from the treated groups
were similar to those of the controls. The physical and behavioural
developmental parameters examined were pinna unfolding, hair growth,
incisor eruption, eye opening, auricular duct opening, surface
righting reflex, cliff avoidance, and air righting reflexes. The
NOAEL for maternal toxicity was 200 mg/kg, and no embryotoxic,
teratogenic, and developmental toxicity effects were found in the
highest dose tested (200 mg/kg) (Savary et al., 1988).
Rabbits
Groups of artificially inseminated female rabbits (16/group)
received bioresmethrin by gavage at doses of 0, 15, 60, and 240 mg/kg
from gestation days 6 to 18. The fetuses were delivered on gestation
day 28, and dams were sacrificed at that time. Under the conditions
of the study, the compound produced no maternal nor developmental
toxicity at any dose levels. Based upon the results of this study,
the pregnant rabbits could have tolerated higher dose levels (Savary,
1990).
An older rabbit teratology study on the bioresmethrin had been
considered by the Joint Meeting in 1976 and published in 1977 FAO
monograph. The summary of the study is reproduced in its entirety
below.
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin in doses of 0, 10, 20, 40, and 80 mg/kg by oral gavage
daily from days 8-16 of gestation. The does were sacrificed on day 28
and examined for implantation, live and dead fetuses, resorption
sites, and abnormalities (after staining a representative number for
skeletal examination). There was no apparent effect on parents in the
study as growth and gestation were unaffected. There was an increase
in dead fetuses at the highest dose and a large number of resorption
sites noted at all treatment levels. There were a number of deformed
fetuses observed but the total numbers were not sufficient for
adequate statistical evaluation. The deformities included straight
tail, crossed hind limbs and unilateral union of 6th and 7th ribs at
the sternal end. An overall fetal loss was observed at all dose
levels (primarily because of the large number of resorption sites
recorded) (Waldron, 1969, as cited in Annex I, 27).
Special studies on genotoxicity
A number of mutagenicity studies have been conducted with
bioresmethrin. The results are summarized in Table 3.
Special studies on skin and eye irritation and sensitization
A volume of 0.5 ml bioresmethrin (95%) was applied under
occlusive conditions to the shaven intact skin of 3 male New Zealand
white albino rabbits for 4 hours. No evidence of skin irritation was
found up to 72 hours after application (Audegond, 1989d).
Three New Zealand white albino rabbits were administered 0.1 ml
of bioresmethrin (95%) in their conjunctival sac of the right eye.
The treated eyes did not appeared to have been washed. No eye
irritation was found under the conditions of the test (Audegond,
1989c). An older study conducted by Chesher and Malone (1970c) also
showed that bioresmethrin was not an eye irritant in rabbits (FAO,
1977).
Bioresmethrin (95%) was tested for skin sensitization in 10 male
Hartley albino guinea pigs. During the induction phase, The animals
were treated topically with 0.5 ml bioresmethrin daily for 10 days.
On day 36, the animals were challenged with 0.5 ml of the test
substance and later rechallenged with similar volume on day 43. A
positive control group of 10 males received 0.06% w/v solution of
2,4-dinitrochlorobenzene in a similar manner as those treated with
bioresmethrin. Bioresmethrin did not produce skin sensitization
reaction in guinea pigs (Kuhn, 1990).
In an older study published in the FAO Monograph (Annex I, 27),
groups of adult guinea pigs (6 males/group) were applied bioresmethrin
(0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro-benzene (DNCB)
to the ears for 4 days. On day 7, 0.2 ml of bioresmethrin or DNCB
were applied dermally. Bioresmethrin produced only traces of erythema
suggesting a low potential for sensitization and irritation (Chesher
& Malone, 1970b).
Table 3. Results of genotoxicity assays on bioresmethrin
Test system Test object Concentration Purity Results Reference
(%)
Ames test S. typhimurium 0.2. 1-5. 10. 92.2 Negativea Moore, 1981
(with and TA1535, TA1537, 50, 100, 250,
without S9) TA1538, TA98, 500, 1000,
TA100 5000 µg/plate
Ames test S. typhimurium 30, 100, 300, 97 Negative Pluijmen et
(with and TA98, TA100 1000 µg/plate al., 1984
without S9)
Gene mutation V79 Chinese 5, 10, 15, 20 97 Negative Pluijmen et
assay (with and hamster cells µg/ml al., 1984
without S9)
Micronucleus Swiss CD 1 mice 300 mg/kg (M) 93.6 Negative Vannier &
assay 450 mg/kg (F) Fournex, 1986
Metaphase human 4, 20, 40 93.6 Negative Allen, Brooker
chromosome lymphocytes µg/ml & Howell (1986)
analysis (with
and without S9)
Unscheduled human 0.125, 0.25, 93.6 Negative Allen &
DNA synthesis epithelioid 0.5, 1, 2, 4, Proudlock,
assay (with cells 8, 16, 32, 64 1986
and without S9) (HeLa S3) 128 and 256
µg/ml
a The report of this study has serious deficiencies which include lack of information on the source
of the metabolic activation system and whether the results presented in the report are the
averages or single determinations.
COMMENTS
Bioresmethrin was absorbed and distributed rapidly following oral
administration, and was quickly metabolized by oxidation and
hydrolysis at various sites in the molecule. Complete elimination of
bioresmethrin occurred slowly. The enterohepatic circulation system
was involved in the elimination. There is no indication that
isomerization of bioresmethrin to the (+)- cis-isomer occurs.
In general, bioresmethrin has low acute toxicity after oral
administration. In mammals, the cis-isomers are generally more
toxic than the corresponding trans-isomers. Some metabolites of
bioresmethrin are more toxic than the parent compound.
Short-term studies in rats show that bioresmethrin fed at 1000
ppm caused a slight increase in liver weight and a reduction in thymus
weight in rats. In a 90-day feeding study in rats, at dietary
concentrations of 0, 400, 1200 or 8000 ppm, bioresmethrin at 1200 ppm
or above induced an increase in liver weight and fatty liver which was
accompanied by changes in blood enzyme levels (serum alkaline
phosphatase and aspartate aminotransferase) indicative of liver
injury. In a 90-day gavage study in dogs, bioresmethrin at 250 mg/kg
bw/day or above reduced the erythrocyte count, haemoglobin content,
and packed cell volume.
A carcinogenicity study in mice with resmethrin (containing at
least 30% bioresmethrin) at dietary concentrations of 250, 500 and
1000 ppm for 85 weeks did not demonstrate a carcinogenic effect.
However, resmethrin decreased survival rate in both male and female
mice at 1000 ppm and adrenal weights were significantly increased in
males at 500 and 1000 ppm. The NOAEL for resmethrin was 250 ppm,
which was equal to 38 mg/kg bw/day for resmethrin and 11 mg/kg bw/day
for bioresmethrin.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 50, 250 or 1250 ppm for 104 weeks, bioresmethrin
did not produce an increase in the tumour incidence. However, it
induced an increase in alkaline phosphatase in males at 250 and 1250
ppm and a decrease in cholesterol levels in males at 1250 ppm.
Bioresmethrin caused an increase in the incidence of non-neoplastic
liver changes, including pallor and hypertrophy of hepatocytes in
males at 250 ppm and in males and females at 1250 ppm. Based upon
these findings, the NOAEL for chronic toxicity was 50 ppm, equal to
3.0 mg/kg bw/day.
In a two-generation reproduction study in rats, at dietary
concentrations of 0, 80, 250, 750 or 2250 ppm, bioresmethrin did not
affect reproductive performance at dietary concentrations of 250 ppm
or less, although reproduction was adversely affected at 750 and 2250
ppm. Based on a decrease in parental body weight and hepatotoxicity
observed at 250 ppm, the NOAEL for this study was 80 ppm, equivalent
to 4 mg/kg/day.
Studies on the developmental toxicity of bioresmethrin in rats
and rabbits failed to elicit effects at doses up to 200 and 240 mg/kg
bw/day respectively.
After reviewing all available in vitro and in vivo short-term
assays with bioresmethrin, the Meeting concluded that there was no
evidence of genotoxicity.
The ADI was based upon the long-term/carcinogenicity study in
rats utilizing a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 50 ppm in the diet, equal to 3.0 mg/kg bw/day
Dog: 80 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Allen, J.A., Brooker, P.C., & Howell, A. (1986) Bioresmethrin:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. RSL 697/851370 from Huntingdon Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Roussel Uclaf,
Paris, France.
Allen, J.A. & Proudlock, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after exposure to
bioresmethrin. Unpublished report No. RSL 698/851420 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989a) Bioresmethrin: Acute oral toxicity study in the
rat. Unpublished study No. 89142 from Toxicology Department, Division
Scientifique Roussel Uclaf, Romainville, France. Submitted to WHO by
Roussel Uclaf, Paris, France.
Audegond, L. (1989b) Bioresmethrin: Acute dermal toxicity study in
the rabbit. Unpublished study No. 89143 from Toxicology Department,
Division Scientifique Roussel Uclaf, Romainville, France. Submitted to
WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989c) Bioresmethrin: Primary eye irritation study in
the male rabbit. Unpublished study No. 89144 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Audegond, L. (1989d) Bioresmethrin: Primary dermal irritation study
in the male rabbit. Unpublished study No. 89145 from Toxicology
Department, Division Scientifique Roussel Uclaf, Romainville, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Casida, J.E., Fammon, D.W. & Lawrence, L.J. (1983) Mechanisms of
selective actions of pyrethroid insecticides. Ann. Rev. Pharmacol.
Toxicol., 23: 413-38.
Chesher, B.C. & Malone, J.C. (1970a) Acute toxicity test with NRDC
107 in hens. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970b) Sensitization study with NRDC
107 in guinea pigs. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971a) Acute toxicity of NRDC 107 by
the intravenous route. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Chesher, B.C. & Malone, J.C. (1971b) Toxicity to dog of NRDC 107
(continuation). Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd. Submitted to WHO by the
Wellcome Foundation Ltd.
Cox, G.E., Knickerbocker, M., & Parent, R.A. (1979) Evaluation of
dietary administration of SBP-138 in CD-1 outbred albino mice over an
85 week period. Unpublished report by Food and Drug Research
Laboratories, Inc., Study No. 5270. Sponsored by S.B. Penick & Co. and
submitted to WHO by Roussel Uclaf, Paris, France.
Farebrother, D.A. (1973) NRDC 107: Whole body radioautographical
study in albino rats. Unpublished report from the Cooper Technical
Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted to WHO by
the Wellcome Foundation Ltd.
Glomot, R. & Chevalier, B. (1969) Etude de la toxicite aigue du RU
11484 (NRDC 107). Unpublished report from Roussel Uclaf. Submitted to
WHO by the Wellcome Foundation Ltd.
Glomot, R., Undated. Three week study of RU 11484 (NRDC 107) in the
rat. Unpublished report from Roussel Uclaf. Submitted to WHO by the
Wellcome Foundation Ltd.
Hardy, C.J., Jackson, G.C., Suttie, A.W., Read, R.M., & Gopinath, C.
(1989) Bioresmethrin: Acute inhalation toxicity study in rats (4-hour
exposure). Unpublished Report No. RSL 801/89712 from Huntingdon
Research Centre Ltd., Cambridgeshire, England. Submitted to WHO by
Roussel Uclaf, Paris, France.
Kuhn, J.O. (1990) Bioresmethrin Technical 7N1173B3: Dermal
sensitization study in guinea pigs. Unpublished Report No. 6674-89
from Stillmeadow, Inc., Houston, Texas, USA. Submitted by Roussel
Uclaf, Paris, France.
Malone, J.C. & Chesher, B.C. (1970) Toxicity to dogs of NRDC 107.
Unpublished report from the Cooper Technical Bureau, Berkhamstead, the
Wellcome Foundation Ltd., submitted to the World Health Organization
by the Wellcome Foundation Ltd.
Miyamoto, J. (1975-76) Terminal residues of bioresmethrin. Submission
to IUPAC Terminal Residues Commission, Madrid, Sept. 1975; Leverkusen,
Sept. 1976.
Moore, W.B. & Chatfield, S.N. (1981) Mutagenicity: Evaluation of
bioresmethrin using the Ames Samonella/microsome incorporation test.
Unpublished Report No. BEMU/81/1 from the Wellcome Foundation Ltd.
Submitted to WHO by Roussel Uclaf, Paris, France.
Noel, P.R.B., Rivett, K.F., Chesterman, H., Street, A.E., & Spicer,
E.J.F. (1971) Oral toxicity study in beagle dogs (repeated dosage for
3 months). Unpublished report from the Huntingdon Research Centre.
Submitted to the WHO by the Wellcome Foundation Ltd.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille,
A., & Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutation Research, 137: 7-15.
Ruzo, L.O., Krautter, M.S., & Jiang, Jialiang (1991) Absorption,
distribution, elimination, and metabolism of [14C-acid]-d-
trans-resmethrin in rats. Unpublished Report, Pharmacology and
Toxicology Research Lab. East and West, Inc.; PTRL Report No.
410E/238W. Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M.H. (1987) Bioresmethrin: Two-generation reproduction
toxicity study in rats by dietary mixture administration. Unpublished
Report No. 2059 RSR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Savary, M. H. (1990) Assessment of possible embryotoxic or
teratogenic effects of oral route in rabbits. Unpublished report;
Study No. 6772 RSL from Centre International de Toxicologie, France.
Submitted to WHO Roussel Uclaf, Paris, France.
Savary, M.H., Read, M.H., & Delhomme, C. (1988) Bioresmethrin:
Assessment of possible embryotoxic, teratogenic or developmental
effects in the rats. Unpublished Report No. 2058 RSR from Centre de
Recherches Roussel Uclaf, France. Submitted to WHO by Roussel Uclaf,
Paris, France.
Ueda, K., Gaughan, L.C., & Casida, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pesticide Biochem. Physiol.,
5: 280-294.
Ueda, K. Gaughan, L.C., & Casida, J.E. (1975b) Metabolism of
(+)- trans and (+)- cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115
Vallet, L. (1990) Combined chronic toxicity/oncogenicity study by
repeated dietary administration to rats (104 weeks). Unpublished
Report No. 2057 TCR from Centre International de Toxicologie, France.
Submitted to WHO by Roussel Uclaf, Paris, France.
Vannier, B. & Fournex, R. (1986) Bioresmethrin: Detection of a
mutagenic potency, micronucleus test in the mouse. Unpublished Report
No. 85612-BB/178 from Centre de Recherches Roussel Uclaf, Romainville,
France. Submitted to WHO by Roussel Uclaf, France.
Verschoyle, R.D. & Barnes, J.M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. & Physiol., 2(3):
308-11.
Waldron, M.M. (1969) Fetal toxicity study - 95 H 56 - given orally in
the rabbit. Unpublished report from the Cooper Technical Bureau,
Berkhamstead, the Wellcome Foundation Ltd., submitted to the World
Health Organization by the Wellcome Foundation Ltd.
Wallwork, L.M., Chesher, B.C., & Malone, J.C. (1970) Toxicity of NRDC
107 to laboratory animals. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M., Clampitt, R.B., & Malone, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Unpublished report
from the Cooper Technical Bureau, Berkhamstead, The Wellcome
Foundation Ltd. Submitted to the WHO by the Wellcome Foundation Ltd.
Wallwork, L.M. & Malone, J.C. (1972) Bioresmethrin: Preliminary
24-hour rat inhalation study. Unpublished report from the Cooper
Technical Bureau, Berkhamstead, The Wellcome Foundation Ltd. Submitted
to the WHO by the Wellcome Foundation Ltd.
