
CLOFENTEZINE
EXPLANATION
Clofentezine is an acaricide used for the residual control of
mites. It acts primarily as an ovicide, with some activity on early
motile stages. It was considered for the first time by the present
meeting.
IDENTITY AND PROPERTIES
CHEMICAL NAME
3,6-bis(2-chlorophenyl)-1,2,4,5-tetrazine (IUPAC)
3,6-bis(0-chlorophenyl)-1,2,4,5-tetrazine
SYNONYMS Apollo, Apolo, Acaristop; FBC code number
NO 21314
STRUCTURAL FORMULA
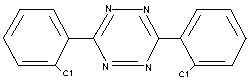 EMPIRICAL FORMULA C14 H8 Cl2 N4
MOLECULAR MASS 303.15
PHYSICAL STATE Both pure and technical materials are
magenta-coloured crystalline solids.
MELTING POINT 182 - 186°C (pure compound)
179 - 182°C (technical product)
VAPOUR PRESSURE Approximately 10-16 mm Hg = 10-14 Pa at
25°C.
SOLUBILITY Solubility (pure a.i.) at 20°C:
chloroform 5 %
dichloromethane 3.5 %
acetone 0.5 %
benzene 0.25%
ethanol < 0.1 %
hexane < 0.1 %
solvesso 200 0.25%
water < 1 µg/1
OCTANOL/WATER PARTITION
COEFFICIENT log P = 3.1
STABILITY (a.i.) The stability in aqueous solutions is pH-
and temperature-dependent; at pH 7 and
22°C, the half-life is approximately
35 hours, whereas at pH 5 and 22°C the
half-life is approximately 250 hours.
STABILITY (formulations) 50% wettable powder: stable under storage
conditions at 40°C for 6 months; 50%
aqueous suspension concentrate: stable
under storage conditions at 12 - 38°C for
12 months.
SPECIFICATION OF
TECHNICAL MATERIAL The technical material has a purity in
excess of 96% w/w, with the main
impurities being:
(a) 4-amino-3,5-bis(2-chlorophenyl)-4H-1,2,4-triazole <1%
(b) 3,6-bis(2-chlorophenyl)-1,2-dihydro-1,2,4,5-tetrazine <1%
(c) 2,5-bis(2-chlorophenyl)-1,3,4-oxadiazole <1%
(d) total volatiles (water and acetic acid) <1%.
FORMULATIONS AVAILABLE
COMMERCIALLY 50% wettable powder; 50% aqueous
suspension concentrate.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mice
14C-Labelled clofentezine was administered orally at single
doses of 10 mg/kg b.w. to 8 male and 8 female mice. Ninety-six hours
after dosing, 91 - 95% of the administered dose had been recovered in
the excreta, particularly in the faeces. Residues after 96 hours were
highest in the liver (0.11 µg/g in males and 0.18 µg/g in females).
All other tissues (including plasma) contained substantially lower
residues, in most cases below the limit of detection (Campbell &
Needham, 1982a).
Rats
Groups of 5 male and 5 female rats received single oral doses of
0.1, 10, or 1000 mg 14C-clofentezine/kg b.w. or 0.1 mg
14C-clofentezine (in ethanol)/kg b.w. i.v. Eighty-nine hours after
dosing, 22 29% of the administered 14C-clofentezine in the low-dose
groups had been recovered in the urine. Tissue residues were generally
below the detection limit (< 0.01 µg/g), with occasional samples
of liver, kidney, spleen, and heart tissues containing measurable
residues (0.01 - 0.02 µg/g). Urinary excretion accounted for 19%
(males) and 20% (females) of the administered dose after administra-
tion of 10 mg 14C-clofentezine/kg b.w., with the highest residues
in the liver (0.22 - 0.46 µg/g) and kidneys (0.12-1.28 µg/g). Plasma
levels were 0.07 - 0.22 µg/ml, and the remaining tissues contained
0.02 - 0.2 µg/g in the mid-dose group. Urinary excretion fell to only
2.0% (males) and 4.5% (females) after the administration of 1000 mg
14C-clofentezine/kg b.w. In the faeces these percentages amounted
to 99% and 96%, respectively. The highest residues were found in
plasma (9.2-19.5 µg/ml), adrenals (2.9-17.1 µg/g), liver (2.8-14.0
µg/g), and fat (3.6 - 9.4 µg/g) (Challis & Needham, 1981a; 1982a,b).
Ninety-six hours after i.v. administration of 0.1 mg 14C-clofen-
tezine/kg b.w., 25 - 29% of the administered dose had been recovered
in the urine, while in the faeces the residues were 82-85%. Tissue
residues were very low (< 0.01 µg/g) (Challis & Needham, 1983).
An examination of the distribution of radioactivity using
whole-body autography, 24 hours after the oral administration of 10 mg
14C-clofentezine/kg b.w. to rats, showed that the liver, kidneys,
and gut contain significant amounts of radioactivity. The carcass was
clear of residues 96 hours after dosing (Needham, 1982).
Rats (25/sex) received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. Residues in plasma were measured 5 minutes to 18 hours
after dosing. Total radioactivity showed maximum values of 1.3 - 1.6
µg/ml between 4 and 6 hours after dosing, which declined to 0.02 µg/ml
at 18 hours after dosing. The concentration of clofentezine reached a
maximum at 4 - 6 hours after dosing and then declined, with a half
life of 2.5 - 2.7 hours (Campbell & Challis, 1982).
Total radioactivity and unchanged clofentezine in plasma of rats
dosed with 1000 mg 14C-clofentezine/kg b.w. rose to a maximum of
14-16 mg/l and 6 - 8 mg/l, respectively, between 6 and 8 hours after
dosing. Unchanged clofentezine declined, with a half-life of 3.6 hours
(Campbell & Needham, 1985b).
When rats were fed daily oral doses of 20 mg clofentezine/kg b.w.
for 25 days, the highest residues were found in the liver (3.2-4.4
µg/g). After 5 - 10 days a plateau was reached in the liver, kidneys,
skin, and ovaries, with residues being 2 - 4 times higher than after
1 day. For residues in the adrenals, muscle, lung, and fat, no
definite plateau could be established, but residues in fat were 8
times higher after 25 days than after 1 day. No accumulation occurred
in bone, brain, spleen, testes, or blood (Challis & Needham, 1981c).
Pre-treatment with clofentezine (27 g/kg diet for 28 days) prior
to an i.v. dose of 0.1 mg 14C-clofentezine/kg b.w. resulted in an
increase in urinary excretion (34% of the radiolabelled dose in both
sexes). Tissue levels were not significantly altered (Challis &
Needham, 1981b).
Pre-treatment with clofentezine (10 mg/kg diet for 14 days) prior
to an oral dose of 10 mg/kg 14C-clofentezine/kg diet did not change
the excretion profile nor the residues disposition. The concentration
of unchanged clofentezine in plasma reached a maximum of 0.11-0.19
µg/ml and declined, with a half-life of 1.6 hours. These results are
consistent with an increase in the rate of metabolism when compared
to a single dose (Challis & Needham, 1982c) (Campbell & Needham,
1985a).
Pregnant rats received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. on day 20 post-coitum (p.c.) after being orally dosed
with 3200 mg clofentezine/kg b.w. from days 7 to 13 p.c., 320 mg/kg
b.w. on day 14 p.c., and then remaining untreated from days 15 to
19 p.c. Residue levels were much lower in the fetus (0.7-1.2 µg/g)
than in the maternal plasma (3.7 - 6.4 µg/g) 6 hours after dosing,
showing that clofentezine and/or its metabolites do not readily cross
the placenta. The clearance rate from the fetus appeared to be slower
than from the maternal tissues (Needham, 1981).
Rabbits
Rabbits (3/sex) were orally dosed with 10 mg 14C-clofen-
tezine/kg b.w. Urine and faeces were collected over 96 hours. In the
first 48 hours, 88% of the dose was excreted (35% in the urine and 53%
in the faeces). Residues after 96 hours were highest in the liver and
kidneys (0.24 µg/g and 0.90 µg/g, respectively) (Campbell & Needham,
1982b).
Dogs
After oral administration of 10 mg/kg 14C-clofentezine to dogs
(3/sex), 96% of the dose was excreted in the first 48 hours, mainly in
the faeces (94%). Nearly complete excretion (99%) was observed after
96 hours, with only 2.0% in the urine. Residues were highest in bile
(1.56 µg/ml), liver (0.21 µg/g) and thyroid (0.15 µg/g). All other
tissues contained substantially lower residues. Peak plasma
concentration (0.06 µg/ml) occurred 4 - 8 hours after dosing and by 72
hours it was reduced to 0.01 µg/ml or less (Campbell & Needham,
1982c).
Dogs (2/sex) received single i.v. doses of 0.1 mg 14C-clofen-
tezine/kg b.w. The bulk of the dose was excreted in the first 48
hours, mainly in the faeces (69%), and 21% in the urine. Tissue
residues measured 144 hours after dosing were, with few exceptions,
below the limit of detection (< 0.01 µ/g) (Campbell & Needham, 1981).
Baboons
A male and a female baboon were each given a single oral dose of
10 mg 14C-clofentezine/kg b.w. Ninety-six hours after dosing 15 -
28% of the administered dose was excreted in the urine and 42 - 44% in
the faeces. Cage debris and wash together accounted for 9%. Tissue
concentrations of radioactivity 96 hours after a second oral dose of
14C-clofentezine (following 52 daily doses of up to 400 mg
unlabelled clofentezine starting 96 hours after the first oral dose)
were similar in both baboons. The highest concentrations were detected
in fat, liver, and kidneys (0.06 - 0.23% of administered
radioactivity/kg wet tissue) while tissue-to-plasma ratios were
on the order of 3 - 10 (Sortwell et al., 1983).
Biotransformation
The metabolism of clofentezine was studied in rats. Urine and
faeces were collected 24 hours after dosing in a series of experiments
in which rats received 14C-clofentezine orally at 10 mg/kg b.w.
Absorbed clofentezine was extensively metabolized, with only 3% of the
radioactivity in the urine present as the parent compound. Two major
urinary pathways have been identified, one leading to a monochloro
sulfur-containing derivative, 3-(2'-methylthio-3'-hydroxyphenyl)-
6-(2'-chlorophenyl)-1,2,4,5-tetrazine (in free and conjugated forms
this metabolite accounted for 35% of the urinary radioactivity), and
the other involving hydroxylation of clofentezine at the 3, 4, or 5
positions (see Figure 1). At least half of the administered dose was
excreted in the faeces unchanged. The remaining clofentezine was
extensively metabolized to more than 20 minor metabolites (Challis &
Needham, 1985).
a figure that could possibly be photocopied is on p. 9 of the summary
submitted by FBC Ltd.
EMPIRICAL FORMULA C14 H8 Cl2 N4
MOLECULAR MASS 303.15
PHYSICAL STATE Both pure and technical materials are
magenta-coloured crystalline solids.
MELTING POINT 182 - 186°C (pure compound)
179 - 182°C (technical product)
VAPOUR PRESSURE Approximately 10-16 mm Hg = 10-14 Pa at
25°C.
SOLUBILITY Solubility (pure a.i.) at 20°C:
chloroform 5 %
dichloromethane 3.5 %
acetone 0.5 %
benzene 0.25%
ethanol < 0.1 %
hexane < 0.1 %
solvesso 200 0.25%
water < 1 µg/1
OCTANOL/WATER PARTITION
COEFFICIENT log P = 3.1
STABILITY (a.i.) The stability in aqueous solutions is pH-
and temperature-dependent; at pH 7 and
22°C, the half-life is approximately
35 hours, whereas at pH 5 and 22°C the
half-life is approximately 250 hours.
STABILITY (formulations) 50% wettable powder: stable under storage
conditions at 40°C for 6 months; 50%
aqueous suspension concentrate: stable
under storage conditions at 12 - 38°C for
12 months.
SPECIFICATION OF
TECHNICAL MATERIAL The technical material has a purity in
excess of 96% w/w, with the main
impurities being:
(a) 4-amino-3,5-bis(2-chlorophenyl)-4H-1,2,4-triazole <1%
(b) 3,6-bis(2-chlorophenyl)-1,2-dihydro-1,2,4,5-tetrazine <1%
(c) 2,5-bis(2-chlorophenyl)-1,3,4-oxadiazole <1%
(d) total volatiles (water and acetic acid) <1%.
FORMULATIONS AVAILABLE
COMMERCIALLY 50% wettable powder; 50% aqueous
suspension concentrate.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mice
14C-Labelled clofentezine was administered orally at single
doses of 10 mg/kg b.w. to 8 male and 8 female mice. Ninety-six hours
after dosing, 91 - 95% of the administered dose had been recovered in
the excreta, particularly in the faeces. Residues after 96 hours were
highest in the liver (0.11 µg/g in males and 0.18 µg/g in females).
All other tissues (including plasma) contained substantially lower
residues, in most cases below the limit of detection (Campbell &
Needham, 1982a).
Rats
Groups of 5 male and 5 female rats received single oral doses of
0.1, 10, or 1000 mg 14C-clofentezine/kg b.w. or 0.1 mg
14C-clofentezine (in ethanol)/kg b.w. i.v. Eighty-nine hours after
dosing, 22 29% of the administered 14C-clofentezine in the low-dose
groups had been recovered in the urine. Tissue residues were generally
below the detection limit (< 0.01 µg/g), with occasional samples
of liver, kidney, spleen, and heart tissues containing measurable
residues (0.01 - 0.02 µg/g). Urinary excretion accounted for 19%
(males) and 20% (females) of the administered dose after administra-
tion of 10 mg 14C-clofentezine/kg b.w., with the highest residues
in the liver (0.22 - 0.46 µg/g) and kidneys (0.12-1.28 µg/g). Plasma
levels were 0.07 - 0.22 µg/ml, and the remaining tissues contained
0.02 - 0.2 µg/g in the mid-dose group. Urinary excretion fell to only
2.0% (males) and 4.5% (females) after the administration of 1000 mg
14C-clofentezine/kg b.w. In the faeces these percentages amounted
to 99% and 96%, respectively. The highest residues were found in
plasma (9.2-19.5 µg/ml), adrenals (2.9-17.1 µg/g), liver (2.8-14.0
µg/g), and fat (3.6 - 9.4 µg/g) (Challis & Needham, 1981a; 1982a,b).
Ninety-six hours after i.v. administration of 0.1 mg 14C-clofen-
tezine/kg b.w., 25 - 29% of the administered dose had been recovered
in the urine, while in the faeces the residues were 82-85%. Tissue
residues were very low (< 0.01 µg/g) (Challis & Needham, 1983).
An examination of the distribution of radioactivity using
whole-body autography, 24 hours after the oral administration of 10 mg
14C-clofentezine/kg b.w. to rats, showed that the liver, kidneys,
and gut contain significant amounts of radioactivity. The carcass was
clear of residues 96 hours after dosing (Needham, 1982).
Rats (25/sex) received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. Residues in plasma were measured 5 minutes to 18 hours
after dosing. Total radioactivity showed maximum values of 1.3 - 1.6
µg/ml between 4 and 6 hours after dosing, which declined to 0.02 µg/ml
at 18 hours after dosing. The concentration of clofentezine reached a
maximum at 4 - 6 hours after dosing and then declined, with a half
life of 2.5 - 2.7 hours (Campbell & Challis, 1982).
Total radioactivity and unchanged clofentezine in plasma of rats
dosed with 1000 mg 14C-clofentezine/kg b.w. rose to a maximum of
14-16 mg/l and 6 - 8 mg/l, respectively, between 6 and 8 hours after
dosing. Unchanged clofentezine declined, with a half-life of 3.6 hours
(Campbell & Needham, 1985b).
When rats were fed daily oral doses of 20 mg clofentezine/kg b.w.
for 25 days, the highest residues were found in the liver (3.2-4.4
µg/g). After 5 - 10 days a plateau was reached in the liver, kidneys,
skin, and ovaries, with residues being 2 - 4 times higher than after
1 day. For residues in the adrenals, muscle, lung, and fat, no
definite plateau could be established, but residues in fat were 8
times higher after 25 days than after 1 day. No accumulation occurred
in bone, brain, spleen, testes, or blood (Challis & Needham, 1981c).
Pre-treatment with clofentezine (27 g/kg diet for 28 days) prior
to an i.v. dose of 0.1 mg 14C-clofentezine/kg b.w. resulted in an
increase in urinary excretion (34% of the radiolabelled dose in both
sexes). Tissue levels were not significantly altered (Challis &
Needham, 1981b).
Pre-treatment with clofentezine (10 mg/kg diet for 14 days) prior
to an oral dose of 10 mg/kg 14C-clofentezine/kg diet did not change
the excretion profile nor the residues disposition. The concentration
of unchanged clofentezine in plasma reached a maximum of 0.11-0.19
µg/ml and declined, with a half-life of 1.6 hours. These results are
consistent with an increase in the rate of metabolism when compared
to a single dose (Challis & Needham, 1982c) (Campbell & Needham,
1985a).
Pregnant rats received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. on day 20 post-coitum (p.c.) after being orally dosed
with 3200 mg clofentezine/kg b.w. from days 7 to 13 p.c., 320 mg/kg
b.w. on day 14 p.c., and then remaining untreated from days 15 to
19 p.c. Residue levels were much lower in the fetus (0.7-1.2 µg/g)
than in the maternal plasma (3.7 - 6.4 µg/g) 6 hours after dosing,
showing that clofentezine and/or its metabolites do not readily cross
the placenta. The clearance rate from the fetus appeared to be slower
than from the maternal tissues (Needham, 1981).
Rabbits
Rabbits (3/sex) were orally dosed with 10 mg 14C-clofen-
tezine/kg b.w. Urine and faeces were collected over 96 hours. In the
first 48 hours, 88% of the dose was excreted (35% in the urine and 53%
in the faeces). Residues after 96 hours were highest in the liver and
kidneys (0.24 µg/g and 0.90 µg/g, respectively) (Campbell & Needham,
1982b).
Dogs
After oral administration of 10 mg/kg 14C-clofentezine to dogs
(3/sex), 96% of the dose was excreted in the first 48 hours, mainly in
the faeces (94%). Nearly complete excretion (99%) was observed after
96 hours, with only 2.0% in the urine. Residues were highest in bile
(1.56 µg/ml), liver (0.21 µg/g) and thyroid (0.15 µg/g). All other
tissues contained substantially lower residues. Peak plasma
concentration (0.06 µg/ml) occurred 4 - 8 hours after dosing and by 72
hours it was reduced to 0.01 µg/ml or less (Campbell & Needham,
1982c).
Dogs (2/sex) received single i.v. doses of 0.1 mg 14C-clofen-
tezine/kg b.w. The bulk of the dose was excreted in the first 48
hours, mainly in the faeces (69%), and 21% in the urine. Tissue
residues measured 144 hours after dosing were, with few exceptions,
below the limit of detection (< 0.01 µ/g) (Campbell & Needham, 1981).
Baboons
A male and a female baboon were each given a single oral dose of
10 mg 14C-clofentezine/kg b.w. Ninety-six hours after dosing 15 -
28% of the administered dose was excreted in the urine and 42 - 44% in
the faeces. Cage debris and wash together accounted for 9%. Tissue
concentrations of radioactivity 96 hours after a second oral dose of
14C-clofentezine (following 52 daily doses of up to 400 mg
unlabelled clofentezine starting 96 hours after the first oral dose)
were similar in both baboons. The highest concentrations were detected
in fat, liver, and kidneys (0.06 - 0.23% of administered
radioactivity/kg wet tissue) while tissue-to-plasma ratios were
on the order of 3 - 10 (Sortwell et al., 1983).
Biotransformation
The metabolism of clofentezine was studied in rats. Urine and
faeces were collected 24 hours after dosing in a series of experiments
in which rats received 14C-clofentezine orally at 10 mg/kg b.w.
Absorbed clofentezine was extensively metabolized, with only 3% of the
radioactivity in the urine present as the parent compound. Two major
urinary pathways have been identified, one leading to a monochloro
sulfur-containing derivative, 3-(2'-methylthio-3'-hydroxyphenyl)-
6-(2'-chlorophenyl)-1,2,4,5-tetrazine (in free and conjugated forms
this metabolite accounted for 35% of the urinary radioactivity), and
the other involving hydroxylation of clofentezine at the 3, 4, or 5
positions (see Figure 1). At least half of the administered dose was
excreted in the faeces unchanged. The remaining clofentezine was
extensively metabolized to more than 20 minor metabolites (Challis &
Needham, 1985).
a figure that could possibly be photocopied is on p. 9 of the summary
submitted by FBC Ltd.
 Figure 1. The metabolism of clofentizine in animals.
Metabolites were also identified in a study with baboons.
4-Hydroxy-clofentezine in free and conjugated (beta-glucuronide
conjugate) forms accounted for over 70% of the urinary metabolites.
Other minor metabolites included 3-hydroxy-clofentezine (present as
phenol and glucuronide conjugates) and a mono-chloro sulfur-containing
compound, each accounting for less than 1% of the extractable
radioactivity (Sortwell et al., 1983).
The metabolism of clofentezine in rats, mice, rabbits, calves,
goats, dogs, and baboons was qualitatively similar, with hydroxylation
and replacement of chlorine with a methylthio group being the major
pathways (see Figure 1). In calves and baboons, hydroxylation and
subsequent conjugation were the most important pathways, while
methylthiolation was more prominent in rodents and rabbits (Challis,
1985).
The chromatographic profiles of liver extracts from rats, goats,
and calves following oral administration of 14C-clofentezine were
qualitatively similar to that of rat urine, with conjugates of 3-, 4-,
and 5-hydroxylated clofentezine and a 3-(methylthio-hydroxyphenyl)-6-
(2'-chlorophenyl)-1,2,4,5-tetrazine isomer (Needham & Challis, 1985).
Toxicological studies
Special studies on the endocrine system
After treatment of male rats with 0 or 3% clofentezine for
1 month, a slight decrease in thyroxine (T4) half-life was observed
in dosed rats compared to control animals (a slight increase occurred
over this time period in the controls). A comparison of the effect of
clofentezine treatment (0 and 3% for 4 weeks) in rats and mice (both
sexes) showed that in rats there was a significant and rapid increase
in thyroid uptake of iodine (compared to control animals) following
i.p. dosing with [131I]-sodium iodide. This effect was not observed
in mice (Challis & Creedy, 1985).
Groups of 10 rats/sex were fed diets containing 0, 400, or
30,000 ppm technical clofentezine for 6 weeks. At 30,000 ppm
clofentezine, T4, free T4 index, thyrotropin (TSH), progesterone,
dehydroepiandrosterone, and liver weights were significantly increased
in both sexes. Triiodothyronine (T3) was significantly increased in
males only. Body weights and thyroxine binding capacity were
significantly reduced in females. At 400 ppm clofentezine, T4 and
liver weight were significantly increased in males and dehydroepi-
androsterone was significantly increased in females (Saunders & Mallyon,
1986).
Special studies on enzyme induction
Mice
Groups of 9 - 10 male Charles River CD1 mice received
clofentezine at dietary levels of 0, 40, or 27,000 ppm for 8 weeks
prior to examination of their livers for aniline hydroxylase and
aminopyrine demethylase activities, for levels of cytochromes P-450
and b5, and for microsomal protein concentration. At 27,000 ppm the
levels of cytochrome P-450 and b5 were increased 3-fold. The activity
of aminopyrine demethylase rose by a factor of 2.2 and the mean liver
weights increased by 35%. There was a marginal effect on the enzyme
induction system at 40 ppm (Needham et al., 1984).
Rats
Groups of 6 male Charles River CD rats were fed diets containing
clofentezine at levels of 0, 40, or 27,000 ppm for 8 weeks.
Twenty-four hours after the final dose, their livers were examined for
aniline hydroxylase activity, for levels of cytochromes P-450 and b5,
and for microsomal protein concentration. At 27,000 ppm a 50% increase
in the level of cytochrome P-450 and 2-fold increases in the level of
cytochrome b5 and in the activity of aniline hydroxylase were found.
In a subsequent study it was discovered that the effect could be
reversed after a 2-week withdrawal period. No induction was observed
at 40 ppm (Needham et al., 1983a,b).
In a more extensive study, male and female rats were given diets
with 0, 10, 40, or 400 ppm clofentezine for 2 weeks. At 400 ppm there
was an increase in the level of liver protein (+ 12%), aldrin
epoxidase (22 - 32%), ethoxycoumarin deethylase (20 - 49%), and
cytochrome P-450 (24 - 26%) in both sexes. Liver weights and
cytochrome b5 were increased in males only. At 40 ppm, liver
ethoxycoumarin deethylase was slightly increased in males only
(Creedy et al., 1985).
Special studies on mutagenicity
Clofentezine was negative in various mutagenicity assays
(Table 1).
Table 1: Results of mutagenicity assays on clofentezine
Test Concentration
Test system Object of clofentezine Results Reference
Ames testa Salmonella 10 - 3300 negative McConville,
typhimurium µg/plate 1980
TA98, TA100,
TA1535, TA1537,
and TA1538
Yeast testa,b Saccharomyces 12.5 - 200 negative Riach &
cerevisiae D7 µg/ml McGregor, 1983
Mouse lymphoma Mouse L 5178Y 0 - 128 negative Bootman &
assaya TK +/- cells µg/ml Rees, 1982
Micronucleus Mouse 2 × 0 to negative Hounsell, 1982
assay (bone 3200 mg/kg
marrow) in 0.5%
aqueous gum,
orally
Dominant Rat (males) 0 to 400 negative Jackson, 1983
lethal test mg/kg in
the diet
for 10 weeks
a with and without metabolic activation
b gene conversion and mitotic recombination
Special study on reproduction
Rats
Groups of 6-week old Charles River rats (30 - 40/sex) were fed
diets containing 0, 4, 40, or 400 ppm clofentezine. After 74 days of
treatment the rats were paired for 20 days to start a 2-generation
(2 litters/generation) study. Diets were maintained during mating,
gestation, and lactation. Weanlings from the second litter were
selected to become parents of the next generation. The F2a litters
were grown to maturity.
In the parental generations clinical signs and food consumption
of treated rats were comparable to those of the control rats. Mating
performance, pregnancy rate, fertility, and the duration of gestation
and parturation were unaffected by clofentezine treatment. Parental
toxicity was observed at 400 ppm, which included reduced body-weight
gain of F1- and F2-generation females (maturation) and
F1-generation females (post-coitum and post-partum), as well as
significantly increased liver weights in F1- and F2-generation
males. The increased liver weight was associated with minimal
centrilobular hepatocyte enlargement and a slight reduction in
periportal fat deposition. Toxic effects on litters included
significantly reduced F1a and F2a pup weights and significantly
smaller F2b litter sizes. The only toxic effect at 40 ppm was a
significant increase in liver weights of F2a males. No adverse
effects on fertility or reproductive performance were observed at 4 or
40 ppm clofentezine (Jackson & Chambers, 1984).
Special studies on skin and eye irritation
Application of clofentezine (0.2 ml of 333 mg/ml suspension) to
the intact and abraded skin of 6 guinea pigs showed pink staining and
very slight edema (in 2 out of 12 treated areas), both receding during
observation (Crome et al., 1980b).
A dermal irritation study with 6 guinea pigs was conducted with
clofentezine using the 50% wettable powder formulation (0.2 ml of 6 or
947 mg/ml suspension). Observations were carried out at 24 hours and
then daily for one week. Pink staining and erythema (between moderate
and severe) were observed in all animals treated with 947 mg/ml
suspension, and slight erythema was observed in 3/6 guinea pigs
treated with 6 mg/ml suspension. The erythema regressed in one week.
In 2/6 guinea pigs treated with 947 mg/ml suspension, very slight
erythema was present when the animals were subjected to post-mortem
examination (no abnormalities were observed) (Crome & Sanderson,
1981b).
Treatment of the intact and abraded skin of 6 New Zealand white
rabbits with clofentezine in 50% aqueous suspension produced very
slight edema in 1/6 animals after 24 hours, with full recovery after
72 hours. Erythemal response could not be scored because of the bright
pink color of the test substance (Cuthbert et al., 1983).
Undiluted clofentezine (50% aqueous suspension) caused mild
conjunctival irritation 24 hours after installation; it had
disappeared at 48 hours (Cuthbert & Carr, 1983).
Special study on skin sensitization
Clofentezine had no sensitizing potential in guinea pigs when
tested by the Magnusson & Kligman method (Teale, 1982).
Special studies on teratogenicity
Rats
Groups of 34 - 35 pregnant female Sprague-Dawley CD rats received
0, 320, 1280, or 3200 mg clofentezine/kg b.w. by gavage from days 7 to
20 post-coitum (p.c.). The dams were examined twice daily for
mortality, appearance, and behaviour. Body weights were recorded on
days 1 and 4 and on days 7 to 21 p.c. The dams were killed on day 21
of pregnancy. The number of implantations, resorptions (early and
late), corpora lutea, and uteri were determined, as well as placental
weight, weight and histopathology of the liver of the dams, number of
live and dead fetuses, litter and pup weights, sex ratios, gross
pathology, and histopathology (skeletal and visceral) of the fetuses.
Maternal toxicity was observed at 1280 and 3200 mg/kg b.w. At the
highest dose, body weights were significantly decreased (also when
corrected for uterine content). Dose-related and significantly
increased relative liver weights were observed at all dose levels;
when corrected for the uterine contents, the increase was significant
only at 1280 and 3200 mg/kg b.w. At histopathology, slight
differential staining and enlargement of centrilobular hepatocytes
were seen at 3200 mg/kg b.w. Litter size, litter weight, mean
placental weight, and post-implantation losses were not affected at
any of the treatment levels, although mean fetal weight was increased
at 3200 mg/kg b.w.
Embryonic and fetal development were unaffected by treatment
(Jackson, 1982).
Rabbits
Groups of 14 - 15 pregnant New Zealand white rabbits were
administered daily doses of 0, 250, 1000, or 3000 mg clofentezine/kg
b.w. by gavage on days 7 to 28 of gestation. All animals were killed
on day 29 of pregnancy. The dams were examined for mortality,
appearance, behaviour, body weight, food consumption, serum
cholesterol and serum triglyceride (day 29), weight of uterus, number
of implantations, resorptions (early and late), and corpora lutea. The
fetuses, delivered by caeserean section, were examined for the number
alive and dead, litter and pup weight, gross pathology, and
histopathology (skeletal and visceral).
Maternal body-weight gain was significantly reduced at
3000 mg/kg b.w. (and to a lesser extent at 1000 mg/kg b.w.) initially
during treatment, which was associated with reduced food intake. Mean
litter and fetal weights were significantly reduced at 3000 mg/kg b.w.
No visceral or skeletal anomalies were reported (Cozens et al.,
1983).
Acute toxicity
The acute toxicity of clofentezine to several animal species is
given in Table 2. Signs of clofentezine toxicity in rats after oral
administration were confined to very slight salivation and slight
urinary incontinence. No clinical symptoms after oral administration
were observed in hamsters, mice, guinea pigs, or dogs, except for skin
reddening in dogs at the highest dose.
Short-term studies
Mice
Groups of mice (20 males and 20 females per group) were
administered clofentezine (technical grade) in the diet for 90 days at
dose levels of 0, 200, 1000, or 5000 ppm. All animals were observed
daily for mortality and signs of toxicity while body weights and food
consumption were recorded weekly. Haematology and clinical chemistry
were performed at weeks 4 and 12 on 10 mice/sex/group, and urinalysis
was performed at weeks 4 and 12 on 20 mice/sex/group. At termination
of the study all animals were killed, selected organs were weighed,
and complete gross and histopathological examinations were performed.
There were no effects on mortality, appearance, body weight, food
consumption, or haematology. Significantly increased plasma levels of
triglycerides, calcium, and phosphate were observed in mice in the
5000- and 1000-ppm groups. Urinanalysis investigations revealed red
stellar crystals in high-dose males. A dose-related urinary colour
change from yellow, through orange, to red was observed in female
mice. A treatment-related significant increase in liver weight was
observed in both sexes of the mid- and high-dose groups, which was
associated in males at 5000 ppm with centrilobular hepatocyte
enlargement (5/20). The NOEL in this study was 200 ppm (Hounsell
et al., 1982).
Table 2. Results of acute toxicity assays on clofentezine
LD501 LC502
Species Sex Route (mg/kg b.w.) (mg/l) Reference
Mouse M+F oral > 3200 - Crome et al., 1980a
Rat M+F oral > 3200 - Mallyon &
Sanderson, 1980
Rat M+F dermal > > 1332 - Crome et al., 1980c
Rat M+F i.p. > 800 - Crome et al., 1981
Rat M inhalation - > 9.08 Mallyon et al.,
1981
Hamster M+F oral > 3200 - Crome & Sanderson,
1980
Beagle dog M+F oral > 2000 - Chambers et al.,
1981
Guinea pig F oral > 1500 - Crome & Sanderson,
1981a
1 given as a 33% suspension in 0.5% gum tragacanth.
2 6 hours exposure with 80% wettable powder.
Rats
Groups of Charles River CD rats (20 males and 20 females/group)
were fed diets containing 0, 3000, 9000, or 27,000 ppm clofentezine
for 90 days. Five rats/sex/group were interim-sacrificed after 64 days
and 5 rats/sex/group were maintained untreated for a 28-day recovery
period. All animals were observed daily for mortality and physical
signs. Body weights, food consumption, and water consumption were
recorded weekly. Haematology, clinical chemistry, and urinalysis were
performed at weeks 4 and 12 on 10 rats/sex/group. Cholesterol,
triglycerides, and alanine aminotransferase were also measured after
6 weeks of treatment and at the end of the 4-week recovery period.
Detailed gross and histopathological examinations were performed and
selected organs were weighed.
Dose-related hair loss was observed in 50% and 40% of the animals
of the high- and mid-dose groups, respectively. Six animals died
throughout the study, 2/6 of the 27,000 ppm group, probably because of
the test substance. Body-weight gain was significantly reduced in
males and females of the mid- and high-dose groups. In all dose groups
some haematological and clinical chemistry values changed in a dose-
related manner (e.g., haemoglobin and packed cell volume decreased,
protein and cholesterol increased, and alkaline phosphatase and
albumin/globulin ratios decreased in treated groups compared to
controls. After the 4-week recovery period, low- and mid-dose values
were comparable to controls. Relative liver, kidney, heart, and brain
weights were significantly increased at all dose levels. Hepatocyte
enlargement was noted at all dose levels. These effects had
disappeared after the 4-week recovery period. In this experiment a
NOEL could not be established (Ginocchio & Brooks, 1981).
In a second 90-day study, groups of Charles River CD rats
(25 males and 25 females per group) were fed diets containing 0, 40,
400, or 4000 ppm clofentezine for 90 days. Five males and 5 females
per group were maintained untreated for a 6-week recovery period. All
animals were observed daily for mortality and clinical signs, while
body weights, food consumption, and water consumption were measured
weekly. Haematology, clinical chemistry, and urinalysis were performed
at 4, 8, and 12 weeks of treatment on 10 rats/sex/group, with
subsequent monitoring of the regression of treatment-related effects.
Organ weights and detailed gross and histopathological examinations
were performed on 20 male and 20 female rats after 90 days of
treatment and on the remaining animals at the end of the 6-week
regression period.
There were no effects on mortality or clinical signs. Body-weight
gain was significantly decreased in females of the 4000 ppm group. A
statistically-significant decrease in the haemoglobin concentration in
the 4000 ppm group was observed after 4 (females only), 8, and
12 weeks of treatment. This decrease was also observed in the 400 ppm
group after 12 weeks. Plasma cholesterol was significantly increased
in both sexes in the high- and mid-dose groups after 4, 8, and
12 weeks of treatment; protein and albumin levels were increased in
the same dose groups at the same times in males. All these effects had
regressed after the recovery period. A dose-related colouration from
orange to red in the urine was observed at all dose levels. Relative
liver, kidney, and spleen weights were significantly increased in both
sexes of the high-dose group and the relative liver weight was also
increased at 400 ppm. Centrilobular hepatocyte enlargement was
observed among male rats receiving 400 and 4000 ppm and among female
rats receiving 4000 ppm clofentezine. There was evidence of slight,
localized hepatocyte enlargement in males at 40 ppm. Retrospective
examination of liver tissue by electron microscopy indicated moderate
proliferation of smooth endoplasmic reticulum in centrilobular
hepatocytes of males and females treated at 4000, but not at 40 ppm,
clofentezine. These changes (but not the increase in liver weight)
were completely reversible during the 6-week recovery period. The
authors concluded that the NOEL in this study was 40 ppm clofentezine
(Brooks & Turnbull, 1983).
Dogs
Beagle dogs (4/sex/group) were fed diets containing 0, 3200,
8000, or 20,000 ppm clofentezine for 90 days. Two dogs were killed for
humane reasons in week 6 of the study (no relation to treatment:
polyarteritis). Except for pink coloration of the faeces and an
increased liver weight in all treated groups, no compound-related
effects were reported on any of the tested parameters measured: body
weight, food and water consumption, haematology, clinical chemistry,
urinalysis, ophthalmoscopy, electrocardiography, organ weights, gross
pathology, or histopathology (Hounsell et al., 1981).
Beagle dogs (6/sex/group) were fed diets containing 0, 50, 1000,
or 20,000 ppm clofentezine for 52 weeks. No mortality occurred. Apart
from reddish-pink coloured faeces in high-dose dogs, no effects of
treatment were observed on clinical signs, body weight, food and water
consumption, ophthalmoscopy, electrocardiography, blood pressure
measurements, or urinalysis. The highest dose was associated with
moderate hepatotoxicity consisting of significantly-increased relative
liver weights, enlargement of periportal hepatocytes with cytoplasmic
eosinophilia, hepatomegaly, and increased plasma cholesterol
triglycerides and alkaline phosphatase. Adrenal and thyroid weights
were also significantly increased in males and females, respectively.
At 1000 ppm clofentezine, minimal hepatotoxicity was observed, with
increased liver weights in females only. The authors concluded that
the NOEL was 50 ppm clofentezine in the diet (equal to 1.72 mg/kg
b.w./day) (Chesterman et al., 1984).
Long term studies
Mice
Groups of 52 male and 52 female CD mice were fed diets containing
0, 50, 500, or 5000 ppm clofentezine for 105 weeks. Observations
included clinical signs, mortality, body weight, food consumption,
haematological determinations (weeks 52 and 104) and organ weights and
comprehensive histopathological examinations at the end of the
experiment.
At 5000 ppm clofentezine, increased mortality attributed to
amyloidosis was observed in females. Growth was decreased during the
first 52 weeks in males at the high-dose level and after 52 weeks
decreases were also found in haemoglobin, haematocrit, and the number
of red blood cells, especially in males. Total white blood cells were
decreased in males after 52 and 105 weeks. Liver weights were
increased in both sexes (significantly in females) at the high-dose
level. Testes weights were significantly increased in males and an
increased incidence of magenta-discoloured forestomach epithelium was
noted in both sexes of the high-dose group. A higher number of benign
liver cell tumours was observed in female mice dosed with 5000 ppm
clofentezine, but the incidence was not significantly above that of
the controls. At 5000 and 500 ppm an increased incidence of focal
eosinophilic/basophilic hepatocytes was observed in females. The
authors concluded that the NOEL was 50 ppm clofentezine (equivalent to
5 mg/kg b.w./day) (Lloyd et al., 1985).
Rats
Groups of 50 male and 50 female CD rats were fed diets containing
0, 10, 40, or 400 ppm clofentezine (technical) for 118 weeks
(27 months). Additional supplementary groups of 20 male and 20 female
rats were fed diets containing clofentezine at the same dietary levels
and sacrificed after 12 months of treatment. Observations included
clinical signs, mortality, body weight, food consumption, water
consumption, haematology, clinical chemistry, urinalysis, ophthal-
moscopy, and assays for thyroid function and sex hormones. At
termination all surviving rats were killed, organs were weighed, and
comprehensive histopathological examinations were made of tissues from
each of these animals as well as from those dying during the study.
The only treatment-related effects found after haematology and
clinical chemistry analyses were decreased haemoglobin in females
after 18 and 27 months and an increase in free T4 in males at
400 ppm. However, other parameters of thyroid function were not
affected. There was no evidence of chronic toxicity in any tissues
other than the liver and the thyroid. Increased relative liver weights
and liver cell hypertrophy, characterized by centrilobular hepatocyte
enlargement and by vacuolisation and fat deposits, were observed at
400 ppm in males (in females in a few cases). There was a slight
increase in the number of follicular cell tumours in the thyroid at
400 ppm in males. This is considered of doubtful biological
significance since a comparable incidence was observed in control
males in a parallel study. The authors concluded that the NOEL was
40 ppm clofentezine in the diet (equivalent to 2 mg/kg b.w./day)
(Ginocchio & Mallyon, 1985).
Observations in humans
Information useful for evaluation is not available.
COMMENTS
Clofentezine is an acaricide with low mammalian toxicity. Its
mode of action is not understood. At high doses clofentezine exerts an
effect on the liver (enzyme induction). Pretreatment with high dietary
levels of clofentezine increases the rate of metabolism. It also
affects thyroid function and impairs steroid hormone regulation.
After oral administration of clofentezine to rats, it was
excreted predominantly in the faeces. Low levels of residues were
found in organs and tissues. The levels in plasma reached a maximum 4
to 6 hours after an oral dose of 10 mg/kg b.w. after which the level
decreased, with a half-life of 2 - 3 hours.
When clofentezine was given to rats during 25 days, a plateau was
reached after 5 - 10 days in various organs, with the highest residues
in the liver. The concentration in the liver after 25 days was 2 - 4
times higher than after 1 day, while fat residues were about 8 times
higher.
The material excreted in rat urine consisted mainly of
metabolites. The two major pathways were hydroxylation of clofentezine
and formation of a monochlorosulfur derivative. In the faeces 50% was
excreted unchanged; the rest was metabolized to more that 20 minor
metabolites.
The metabolic pattern in other species (rabbits, dogs, baboons)
was similar to the rat, with a higher excretion in the faeces than in
the urine. The same metabolites were formed in rats, mice, calves,
dogs, goats, and baboons, with methylthiolation being more prominent
in rodents. In calves and baboons, hydroxylation was more prominent.
Clofentezine has a low acute toxicity in rats, mice, hamsters,
guinea pigs, and dogs.
Clofentezine did not affect fertility or reproductive
performance. Maternal effects (decreased growth, liver enlargement)
were seen in all generations at 400 ppm. There were no indications of
a teratogenic effect in rats or rabbits.
A battery of mutagenicity tests did not indicate mutagenic
potential.
Short-term oral administration to mice, rats, and dogs produced
liver enlargement as the main toxicological effect. In rats, decreased
growth and decreased haemoglobin were observed at high-dose levels, as
well as effects on biochemical parameters. Histopathological liver
changes were in all cases associated with liver enlargement and liver
enzyme induction. In addition, changes in thyroid function and steroid
hormone regulation were observed (tested in special studies with
rats).
In long-term studies the same effects were found as in short-term
studies. In mice a non-significant increase in benign liver tumours
was found in females. In the rat study with relatively low dose levels
a slight increase of thyroid follicular cell tumours was observed.
Neither effect was considered to be an indication of carcinogenicity.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 50 ppm in the diet, equal to 5 mg/kg b.w./day.
Rat: 40 ppm in the diet, equivalent to 2 mg/kg b.w./day.
Dog: 50 ppm in the diet, equal to 1.72 mg/kg b.w./day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Observations in man.
2. Studies on the possible mode of action on the thyroid.
3. Information on possible enterohepatic recirculation.
REFERENCES
Bootman, J. & Rees, R. Technical NC 21314: Investigation of mutagenic
1982 activity in the TK +/- mouse lymphoma cell mutation system.
Unpublished report No. TOX/82/167-38 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Brooks, P.M. & Turnbull, G.J. Technical NC 21314: 90-Day dietary
1983 toxicity study in the rat - additional examination of the
liver histology. Unpublished report No. TOX/83/167-44 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Campbell, J.K. & Needham, D. Excretion and tissue residues of
1981 (14C) NC 21314 in male and female dogs following a single
intravenous dose at 0.1 mg/kg. Unpublished report
No. METAB/81/37 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Challis, I.R. Concentration of (14C) NC 21314 and
1982 its metabolites in the plasma of rats doses orally with NC
21314 at the rate of 10 mg/kg body weight. Unpublished
report No. METAB/82/39 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982a NC 21314 in male and female mice given a single oral dose of
10 mg/kg. Unpublished report No. METAB/82/11 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982b NC 21314 in male and female rabbits following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/21 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982c NC 21314 in male and female dogs following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/6 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-clofentezine and
1985a its metabolites in the plasma of rats given fourteen daily
doses of clofentezine followed by a single oral dose of
(14C)-clofentezine at 14 mg/kg bodyweight. Unpublished
report No. METAB/85/6 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-Clofentezine and
1985b its metabolites in the plasma of rats dosed orally with
clofentezine at the rate of 1000 mg/kg bodyweight.
Unpublished report No. METAB/85/2 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981a radiolabelled residues in male and female rats dosed orally
at 0.1 mg/kg with NC 21314. Unpublished report
No. METAB/81/26 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981b radiolabelled residues following a single intravenous dose
of 0.1 mg/kg (14C)-NC 21314. Unpublished report
No. METAB/81/38 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The distribution and level of
1981c radiolabelled residues in rats following repeat oral dosing
with (14C)-NC 21314 at 20 mg/kg/day. Unpublished report
No. METAB/81/32 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982a radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with NC 21314. Unpublished report No. METAB/82/1
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982b radiolabelled residues in male and female rats dosed orally
at 1000 mg/kg with NC 21314. Unpublished report
No. METAB/82/22 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982c radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with (14C)-NC 21314 following 14 days
pre-treatment by dosing with unlabelled NC 21314 at
10 mg/kg/day. Unpublished report No. METAB/82/37 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Challis, I.R. & Needham, D. The excretion and distribution of
1983 radiolabelled residues in male and female rats dosed
intravenously at 0.1 mg/kg with (14C)-NC 21314.
Unpublished report No. METAB/83/14 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. A comparison of the metabolism of clofentezine in rat,
1985 mouse, rabbit, calf, dog and baboon. Unpublished report
No. METAB/85/9 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Creedy, C.L. The effects of clofentezine on thyroid
1985 function. Unpublished report No. METAB/85/36 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The metabolism of clofentezine in the rat.
1985 Unpublished report No. METAB/85/5 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Chambers, P.R., Sanderson, D.M., & Brooks, P.N. The acute oral
1981 toxicity of technical (pilot plant) NC 21314 to the male and
female beagle dog. Unpublished report No. TOX/80/167-10 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Chesterman, H., Massey, J.E., Heywood, R., Buist, D., Street, A.E.,
1984 Gopinath, C., & Rupanagudi, S. Rao. NC 21314 oral toxicity
study in dogs (final report: repeated dietary administration
for 52 weeks). Unpublished report No. TOX/84/167-68 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire,
England, and FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Cozens, D.D., Perkin, C.J., Barton, S.J., Clark, R., Offer, J.M.,
1983 Gibson, W.A., & Street, A.E. Effect of technical NC 21314 on
pregnancy of the rabbit (teratology study). Unpublished
report No. FBC TOX/83/167-42 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, England and from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Creedy, C.L., Hemmings, P.A., & Needham, D. The effect of clofentezine
1985 on the hepatic mixed-function oxidase system of the male and
female rat following dietary administration at 10, 40, or
400 ppm diet for two weeks. Unpublished report No. METAB/
85/34 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of unformulated
1980 NC 21314 (CR 20099) to the hamster. Unpublished report
No. TOX/80/167-8 from Fisons Ltd, Saffron Walden, Essex,
England. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute oral toxicity
1980a of unformulated (CR 20099/4) to the male & female mouse.
Unpublished report No. TOX/80/167-7 from Fisons Ltd, Saffron
Walden, Essex, England. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The primary skin
1980b irritancy of unformulated NC 21314 (CR 20099/5) to the
guinea pig. Unpublished report No. TOX/80/167-6 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute dermal toxicity
1980c of unformulated NC 21314 (CR 20099/5) to the male and female
rat. Unpublished report No. TOX/80/167-4 from Fisons Ltd,
Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of technical
1981a (pilot plant) NC 21314 to the guinea pig (CR 20099).
Unpublished report No. TOX/81/167-16 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The primary skin irritancy of NC 21314
1981b 50WP to the guinea pig. Unpublished report No. TOX/81/167-13
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. Acute intraperitoneal
1981 toxicity of pilot plant technical NC 21314 to the rat.
Unpublished report No. TOX/80/167-5 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Cuthbert, J.A. & Carr, S.M.A. NC 21314 50 SC formulation primary eye
1983 irritancy study in rabbits. Unpublished report No. TOX/83/
167-46 from Inveresk Research International, Musselburgh,
Scotland, and from FBC, Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Cuthbert, J.A., Carr, S.M.A., & D'Arcy-Burt, K.J. NC 21314 50 SC
1983 formulation primary skin irritancy study in rabbits.
Unpublished report No. TOX/83/167-47 from Inveresk Research
International, Musselburgh, Scotland, and from FBC, Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Brooks, P.N. Technical NC 21314 (pilot plant, CR
1981 20099/5): the 90-day dietary toxicity study in the rat.
Unpublished report No. TOX/81/167-23/1 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Mallyon, B.A. The oncogenicity and chronic toxicity
1985 of technical NC 21314 (clofentezine) in the diet to the rat.
Unpublished report No. TOX/84/167-70 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1981 oral toxicity of technical (CR 20099/8) NC 21314 in the diet
to the dog. Unpublished report No. TOX/81/167-21/1 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Hounsell, I.A. A micronucleus study in mice using technical NC 21314.
1982 Unpublished report No. TOX/82/167-32 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1982 oral toxicity of technical (pilot plant) NC 21314 in the
diet to the mouse. Unpublished report NO. TOX/82/167-33 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Jackson, C.M. Technical NC 21314: Teratogenicity study in the rat
1982 (plus addendum). Unpublished report No. TOX/82/167-34 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. Technical NC 21314: dominant lethal mustation assay in
1983 male rats. Unpublished report No. TOX/83/167-45 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. & Chambers, P.R. Technical clofentezine: a dietary
1984 multigeneration study in the rat. Unpublished report
No. TOX/84/167-66 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Lloyd, G.K., Spencer-Briggs, D.J., Heywood, R., Gopinath, C., &
1985 Cherry, C.P. Technical NC 21314: oncogenicity in the diet to
the mouse (final report). Unpublished report No. TOX/85/
167-80 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, England, and from FBC Ltd. Submitted to WHO
by FBC Ltd., Hauxton, Cambridge, England.
Mallyon, B.A. & Sanderson, D.M. The acute oral toxicity of
1980 unformulated NC 21314 (NC 21314/4, 99% pure) to the male and
female rat. Unpublished report No. TOX/80/167-2 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Mallyon, B.A., Sanderson, D.M., & Brooks, P.N. The acute inhalational
1982 toxicity of NC 21314 80WP CR 15569, to the rat. Unpublished
report No. TOX/81/167-24 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
McConville, M. Ames test for mutagenic activity carried out with
1980 technical NC 21314 (CR 20099/4). Unpublished report
No. TOX/80/167-3 from Inveresk Research International,
Musselburgh, Scotland, and from Fisons Ltd, Saffron Walden,
Essex, England. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. The distribution of radioactivity in the maternal tissues
1981 and foetuses of rats after an oral dose of (14C)-NC 21314.
Unpublished report No. METAB/81/19 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D. The whole-body autography of (14C)-NC 21314 in rats
1982 following oral administration at 10 mg/kg body weight.
Unpublished report No. METAB/82/23 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of eight weeks
1983a dietary administration of NC 21314 at 40 and 27,000 mg/kg on
the hepatic mixed function oxidase system of the male rat.
Unpublished report No. METAB/81/31 (2nd Edn.) from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of a two week
1983b withdrawal period on the induction of hepatic microsomal
mixed function oxidases caused by dietary administration of
NC 21314 at 27,000 mg/kg diet. Unpublished report
No. METAB/82/2 (2nd Edn.) from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Campbell, J., & Challis, I. The effect of eight weeks
1984 dietary administration of NC 21314 at 27,000 and 400 mg/kg
diet on the hepatic mixed-function oxidase system of the
male mouse. Unpublished report No. METAB/82/20 (2nd Edn.)
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. & Challis, I.R. An investigation into the nature of the
1985 residues present in the liver of the rat, goat and calf
following the oral administration of clofentezine.
Unpublished report No. METAB/85/8 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Riach, C.G. & McGregor, D.B. Technical NC 21314: induction of gene
1983 conversion and mitotic recombination in yeast. Unpublished
report No. TOX/83/167-56 from Inveresk Research
International, Musselburgh, Scotland, and from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Saunders, P.C. & Mallyon, B.A. Technical clofentezine: 6 week dietary
1986 investigation of thyroid function in the rat. Unpublished
report No. TOX/85/167-77 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Sortwell, R.J., Richmond, G.P., Kirkpatrick, D., Finn, C.M., & Conway,
1983 B. Excretion and tissue distribution of radioactivity after
oral administration of (14C)-NC 21314 to a male and female
baboon. Unpublished report No. METAB/83/26 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England, and
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Teale, H.J. Delayed dermal sensitisation study in the guinea pig,
1982 NC 21314 technical. Unpublished report No. TOX/82/167-36
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Figure 1. The metabolism of clofentizine in animals.
Metabolites were also identified in a study with baboons.
4-Hydroxy-clofentezine in free and conjugated (beta-glucuronide
conjugate) forms accounted for over 70% of the urinary metabolites.
Other minor metabolites included 3-hydroxy-clofentezine (present as
phenol and glucuronide conjugates) and a mono-chloro sulfur-containing
compound, each accounting for less than 1% of the extractable
radioactivity (Sortwell et al., 1983).
The metabolism of clofentezine in rats, mice, rabbits, calves,
goats, dogs, and baboons was qualitatively similar, with hydroxylation
and replacement of chlorine with a methylthio group being the major
pathways (see Figure 1). In calves and baboons, hydroxylation and
subsequent conjugation were the most important pathways, while
methylthiolation was more prominent in rodents and rabbits (Challis,
1985).
The chromatographic profiles of liver extracts from rats, goats,
and calves following oral administration of 14C-clofentezine were
qualitatively similar to that of rat urine, with conjugates of 3-, 4-,
and 5-hydroxylated clofentezine and a 3-(methylthio-hydroxyphenyl)-6-
(2'-chlorophenyl)-1,2,4,5-tetrazine isomer (Needham & Challis, 1985).
Toxicological studies
Special studies on the endocrine system
After treatment of male rats with 0 or 3% clofentezine for
1 month, a slight decrease in thyroxine (T4) half-life was observed
in dosed rats compared to control animals (a slight increase occurred
over this time period in the controls). A comparison of the effect of
clofentezine treatment (0 and 3% for 4 weeks) in rats and mice (both
sexes) showed that in rats there was a significant and rapid increase
in thyroid uptake of iodine (compared to control animals) following
i.p. dosing with [131I]-sodium iodide. This effect was not observed
in mice (Challis & Creedy, 1985).
Groups of 10 rats/sex were fed diets containing 0, 400, or
30,000 ppm technical clofentezine for 6 weeks. At 30,000 ppm
clofentezine, T4, free T4 index, thyrotropin (TSH), progesterone,
dehydroepiandrosterone, and liver weights were significantly increased
in both sexes. Triiodothyronine (T3) was significantly increased in
males only. Body weights and thyroxine binding capacity were
significantly reduced in females. At 400 ppm clofentezine, T4 and
liver weight were significantly increased in males and dehydroepi-
androsterone was significantly increased in females (Saunders & Mallyon,
1986).
Special studies on enzyme induction
Mice
Groups of 9 - 10 male Charles River CD1 mice received
clofentezine at dietary levels of 0, 40, or 27,000 ppm for 8 weeks
prior to examination of their livers for aniline hydroxylase and
aminopyrine demethylase activities, for levels of cytochromes P-450
and b5, and for microsomal protein concentration. At 27,000 ppm the
levels of cytochrome P-450 and b5 were increased 3-fold. The activity
of aminopyrine demethylase rose by a factor of 2.2 and the mean liver
weights increased by 35%. There was a marginal effect on the enzyme
induction system at 40 ppm (Needham et al., 1984).
Rats
Groups of 6 male Charles River CD rats were fed diets containing
clofentezine at levels of 0, 40, or 27,000 ppm for 8 weeks.
Twenty-four hours after the final dose, their livers were examined for
aniline hydroxylase activity, for levels of cytochromes P-450 and b5,
and for microsomal protein concentration. At 27,000 ppm a 50% increase
in the level of cytochrome P-450 and 2-fold increases in the level of
cytochrome b5 and in the activity of aniline hydroxylase were found.
In a subsequent study it was discovered that the effect could be
reversed after a 2-week withdrawal period. No induction was observed
at 40 ppm (Needham et al., 1983a,b).
In a more extensive study, male and female rats were given diets
with 0, 10, 40, or 400 ppm clofentezine for 2 weeks. At 400 ppm there
was an increase in the level of liver protein (+ 12%), aldrin
epoxidase (22 - 32%), ethoxycoumarin deethylase (20 - 49%), and
cytochrome P-450 (24 - 26%) in both sexes. Liver weights and
cytochrome b5 were increased in males only. At 40 ppm, liver
ethoxycoumarin deethylase was slightly increased in males only
(Creedy et al., 1985).
Special studies on mutagenicity
Clofentezine was negative in various mutagenicity assays
(Table 1).
Table 1: Results of mutagenicity assays on clofentezine
Test Concentration
Test system Object of clofentezine Results Reference
Ames testa Salmonella 10 - 3300 negative McConville,
typhimurium µg/plate 1980
TA98, TA100,
TA1535, TA1537,
and TA1538
Yeast testa,b Saccharomyces 12.5 - 200 negative Riach &
cerevisiae D7 µg/ml McGregor, 1983
Mouse lymphoma Mouse L 5178Y 0 - 128 negative Bootman &
assaya TK +/- cells µg/ml Rees, 1982
Micronucleus Mouse 2 × 0 to negative Hounsell, 1982
assay (bone 3200 mg/kg
marrow) in 0.5%
aqueous gum,
orally
Dominant Rat (males) 0 to 400 negative Jackson, 1983
lethal test mg/kg in
the diet
for 10 weeks
a with and without metabolic activation
b gene conversion and mitotic recombination
Special study on reproduction
Rats
Groups of 6-week old Charles River rats (30 - 40/sex) were fed
diets containing 0, 4, 40, or 400 ppm clofentezine. After 74 days of
treatment the rats were paired for 20 days to start a 2-generation
(2 litters/generation) study. Diets were maintained during mating,
gestation, and lactation. Weanlings from the second litter were
selected to become parents of the next generation. The F2a litters
were grown to maturity.
In the parental generations clinical signs and food consumption
of treated rats were comparable to those of the control rats. Mating
performance, pregnancy rate, fertility, and the duration of gestation
and parturation were unaffected by clofentezine treatment. Parental
toxicity was observed at 400 ppm, which included reduced body-weight
gain of F1- and F2-generation females (maturation) and
F1-generation females (post-coitum and post-partum), as well as
significantly increased liver weights in F1- and F2-generation
males. The increased liver weight was associated with minimal
centrilobular hepatocyte enlargement and a slight reduction in
periportal fat deposition. Toxic effects on litters included
significantly reduced F1a and F2a pup weights and significantly
smaller F2b litter sizes. The only toxic effect at 40 ppm was a
significant increase in liver weights of F2a males. No adverse
effects on fertility or reproductive performance were observed at 4 or
40 ppm clofentezine (Jackson & Chambers, 1984).
Special studies on skin and eye irritation
Application of clofentezine (0.2 ml of 333 mg/ml suspension) to
the intact and abraded skin of 6 guinea pigs showed pink staining and
very slight edema (in 2 out of 12 treated areas), both receding during
observation (Crome et al., 1980b).
A dermal irritation study with 6 guinea pigs was conducted with
clofentezine using the 50% wettable powder formulation (0.2 ml of 6 or
947 mg/ml suspension). Observations were carried out at 24 hours and
then daily for one week. Pink staining and erythema (between moderate
and severe) were observed in all animals treated with 947 mg/ml
suspension, and slight erythema was observed in 3/6 guinea pigs
treated with 6 mg/ml suspension. The erythema regressed in one week.
In 2/6 guinea pigs treated with 947 mg/ml suspension, very slight
erythema was present when the animals were subjected to post-mortem
examination (no abnormalities were observed) (Crome & Sanderson,
1981b).
Treatment of the intact and abraded skin of 6 New Zealand white
rabbits with clofentezine in 50% aqueous suspension produced very
slight edema in 1/6 animals after 24 hours, with full recovery after
72 hours. Erythemal response could not be scored because of the bright
pink color of the test substance (Cuthbert et al., 1983).
Undiluted clofentezine (50% aqueous suspension) caused mild
conjunctival irritation 24 hours after installation; it had
disappeared at 48 hours (Cuthbert & Carr, 1983).
Special study on skin sensitization
Clofentezine had no sensitizing potential in guinea pigs when
tested by the Magnusson & Kligman method (Teale, 1982).
Special studies on teratogenicity
Rats
Groups of 34 - 35 pregnant female Sprague-Dawley CD rats received
0, 320, 1280, or 3200 mg clofentezine/kg b.w. by gavage from days 7 to
20 post-coitum (p.c.). The dams were examined twice daily for
mortality, appearance, and behaviour. Body weights were recorded on
days 1 and 4 and on days 7 to 21 p.c. The dams were killed on day 21
of pregnancy. The number of implantations, resorptions (early and
late), corpora lutea, and uteri were determined, as well as placental
weight, weight and histopathology of the liver of the dams, number of
live and dead fetuses, litter and pup weights, sex ratios, gross
pathology, and histopathology (skeletal and visceral) of the fetuses.
Maternal toxicity was observed at 1280 and 3200 mg/kg b.w. At the
highest dose, body weights were significantly decreased (also when
corrected for uterine content). Dose-related and significantly
increased relative liver weights were observed at all dose levels;
when corrected for the uterine contents, the increase was significant
only at 1280 and 3200 mg/kg b.w. At histopathology, slight
differential staining and enlargement of centrilobular hepatocytes
were seen at 3200 mg/kg b.w. Litter size, litter weight, mean
placental weight, and post-implantation losses were not affected at
any of the treatment levels, although mean fetal weight was increased
at 3200 mg/kg b.w.
Embryonic and fetal development were unaffected by treatment
(Jackson, 1982).
Rabbits
Groups of 14 - 15 pregnant New Zealand white rabbits were
administered daily doses of 0, 250, 1000, or 3000 mg clofentezine/kg
b.w. by gavage on days 7 to 28 of gestation. All animals were killed
on day 29 of pregnancy. The dams were examined for mortality,
appearance, behaviour, body weight, food consumption, serum
cholesterol and serum triglyceride (day 29), weight of uterus, number
of implantations, resorptions (early and late), and corpora lutea. The
fetuses, delivered by caeserean section, were examined for the number
alive and dead, litter and pup weight, gross pathology, and
histopathology (skeletal and visceral).
Maternal body-weight gain was significantly reduced at
3000 mg/kg b.w. (and to a lesser extent at 1000 mg/kg b.w.) initially
during treatment, which was associated with reduced food intake. Mean
litter and fetal weights were significantly reduced at 3000 mg/kg b.w.
No visceral or skeletal anomalies were reported (Cozens et al.,
1983).
Acute toxicity
The acute toxicity of clofentezine to several animal species is
given in Table 2. Signs of clofentezine toxicity in rats after oral
administration were confined to very slight salivation and slight
urinary incontinence. No clinical symptoms after oral administration
were observed in hamsters, mice, guinea pigs, or dogs, except for skin
reddening in dogs at the highest dose.
Short-term studies
Mice
Groups of mice (20 males and 20 females per group) were
administered clofentezine (technical grade) in the diet for 90 days at
dose levels of 0, 200, 1000, or 5000 ppm. All animals were observed
daily for mortality and signs of toxicity while body weights and food
consumption were recorded weekly. Haematology and clinical chemistry
were performed at weeks 4 and 12 on 10 mice/sex/group, and urinalysis
was performed at weeks 4 and 12 on 20 mice/sex/group. At termination
of the study all animals were killed, selected organs were weighed,
and complete gross and histopathological examinations were performed.
There were no effects on mortality, appearance, body weight, food
consumption, or haematology. Significantly increased plasma levels of
triglycerides, calcium, and phosphate were observed in mice in the
5000- and 1000-ppm groups. Urinanalysis investigations revealed red
stellar crystals in high-dose males. A dose-related urinary colour
change from yellow, through orange, to red was observed in female
mice. A treatment-related significant increase in liver weight was
observed in both sexes of the mid- and high-dose groups, which was
associated in males at 5000 ppm with centrilobular hepatocyte
enlargement (5/20). The NOEL in this study was 200 ppm (Hounsell
et al., 1982).
Table 2. Results of acute toxicity assays on clofentezine
LD501 LC502
Species Sex Route (mg/kg b.w.) (mg/l) Reference
Mouse M+F oral > 3200 - Crome et al., 1980a
Rat M+F oral > 3200 - Mallyon &
Sanderson, 1980
Rat M+F dermal > > 1332 - Crome et al., 1980c
Rat M+F i.p. > 800 - Crome et al., 1981
Rat M inhalation - > 9.08 Mallyon et al.,
1981
Hamster M+F oral > 3200 - Crome & Sanderson,
1980
Beagle dog M+F oral > 2000 - Chambers et al.,
1981
Guinea pig F oral > 1500 - Crome & Sanderson,
1981a
1 given as a 33% suspension in 0.5% gum tragacanth.
2 6 hours exposure with 80% wettable powder.
Rats
Groups of Charles River CD rats (20 males and 20 females/group)
were fed diets containing 0, 3000, 9000, or 27,000 ppm clofentezine
for 90 days. Five rats/sex/group were interim-sacrificed after 64 days
and 5 rats/sex/group were maintained untreated for a 28-day recovery
period. All animals were observed daily for mortality and physical
signs. Body weights, food consumption, and water consumption were
recorded weekly. Haematology, clinical chemistry, and urinalysis were
performed at weeks 4 and 12 on 10 rats/sex/group. Cholesterol,
triglycerides, and alanine aminotransferase were also measured after
6 weeks of treatment and at the end of the 4-week recovery period.
Detailed gross and histopathological examinations were performed and
selected organs were weighed.
Dose-related hair loss was observed in 50% and 40% of the animals
of the high- and mid-dose groups, respectively. Six animals died
throughout the study, 2/6 of the 27,000 ppm group, probably because of
the test substance. Body-weight gain was significantly reduced in
males and females of the mid- and high-dose groups. In all dose groups
some haematological and clinical chemistry values changed in a dose-
related manner (e.g., haemoglobin and packed cell volume decreased,
protein and cholesterol increased, and alkaline phosphatase and
albumin/globulin ratios decreased in treated groups compared to
controls. After the 4-week recovery period, low- and mid-dose values
were comparable to controls. Relative liver, kidney, heart, and brain
weights were significantly increased at all dose levels. Hepatocyte
enlargement was noted at all dose levels. These effects had
disappeared after the 4-week recovery period. In this experiment a
NOEL could not be established (Ginocchio & Brooks, 1981).
In a second 90-day study, groups of Charles River CD rats
(25 males and 25 females per group) were fed diets containing 0, 40,
400, or 4000 ppm clofentezine for 90 days. Five males and 5 females
per group were maintained untreated for a 6-week recovery period. All
animals were observed daily for mortality and clinical signs, while
body weights, food consumption, and water consumption were measured
weekly. Haematology, clinical chemistry, and urinalysis were performed
at 4, 8, and 12 weeks of treatment on 10 rats/sex/group, with
subsequent monitoring of the regression of treatment-related effects.
Organ weights and detailed gross and histopathological examinations
were performed on 20 male and 20 female rats after 90 days of
treatment and on the remaining animals at the end of the 6-week
regression period.
There were no effects on mortality or clinical signs. Body-weight
gain was significantly decreased in females of the 4000 ppm group. A
statistically-significant decrease in the haemoglobin concentration in
the 4000 ppm group was observed after 4 (females only), 8, and
12 weeks of treatment. This decrease was also observed in the 400 ppm
group after 12 weeks. Plasma cholesterol was significantly increased
in both sexes in the high- and mid-dose groups after 4, 8, and
12 weeks of treatment; protein and albumin levels were increased in
the same dose groups at the same times in males. All these effects had
regressed after the recovery period. A dose-related colouration from
orange to red in the urine was observed at all dose levels. Relative
liver, kidney, and spleen weights were significantly increased in both
sexes of the high-dose group and the relative liver weight was also
increased at 400 ppm. Centrilobular hepatocyte enlargement was
observed among male rats receiving 400 and 4000 ppm and among female
rats receiving 4000 ppm clofentezine. There was evidence of slight,
localized hepatocyte enlargement in males at 40 ppm. Retrospective
examination of liver tissue by electron microscopy indicated moderate
proliferation of smooth endoplasmic reticulum in centrilobular
hepatocytes of males and females treated at 4000, but not at 40 ppm,
clofentezine. These changes (but not the increase in liver weight)
were completely reversible during the 6-week recovery period. The
authors concluded that the NOEL in this study was 40 ppm clofentezine
(Brooks & Turnbull, 1983).
Dogs
Beagle dogs (4/sex/group) were fed diets containing 0, 3200,
8000, or 20,000 ppm clofentezine for 90 days. Two dogs were killed for
humane reasons in week 6 of the study (no relation to treatment:
polyarteritis). Except for pink coloration of the faeces and an
increased liver weight in all treated groups, no compound-related
effects were reported on any of the tested parameters measured: body
weight, food and water consumption, haematology, clinical chemistry,
urinalysis, ophthalmoscopy, electrocardiography, organ weights, gross
pathology, or histopathology (Hounsell et al., 1981).
Beagle dogs (6/sex/group) were fed diets containing 0, 50, 1000,
or 20,000 ppm clofentezine for 52 weeks. No mortality occurred. Apart
from reddish-pink coloured faeces in high-dose dogs, no effects of
treatment were observed on clinical signs, body weight, food and water
consumption, ophthalmoscopy, electrocardiography, blood pressure
measurements, or urinalysis. The highest dose was associated with
moderate hepatotoxicity consisting of significantly-increased relative
liver weights, enlargement of periportal hepatocytes with cytoplasmic
eosinophilia, hepatomegaly, and increased plasma cholesterol
triglycerides and alkaline phosphatase. Adrenal and thyroid weights
were also significantly increased in males and females, respectively.
At 1000 ppm clofentezine, minimal hepatotoxicity was observed, with
increased liver weights in females only. The authors concluded that
the NOEL was 50 ppm clofentezine in the diet (equal to 1.72 mg/kg
b.w./day) (Chesterman et al., 1984).
Long term studies
Mice
Groups of 52 male and 52 female CD mice were fed diets containing
0, 50, 500, or 5000 ppm clofentezine for 105 weeks. Observations
included clinical signs, mortality, body weight, food consumption,
haematological determinations (weeks 52 and 104) and organ weights and
comprehensive histopathological examinations at the end of the
experiment.
At 5000 ppm clofentezine, increased mortality attributed to
amyloidosis was observed in females. Growth was decreased during the
first 52 weeks in males at the high-dose level and after 52 weeks
decreases were also found in haemoglobin, haematocrit, and the number
of red blood cells, especially in males. Total white blood cells were
decreased in males after 52 and 105 weeks. Liver weights were
increased in both sexes (significantly in females) at the high-dose
level. Testes weights were significantly increased in males and an
increased incidence of magenta-discoloured forestomach epithelium was
noted in both sexes of the high-dose group. A higher number of benign
liver cell tumours was observed in female mice dosed with 5000 ppm
clofentezine, but the incidence was not significantly above that of
the controls. At 5000 and 500 ppm an increased incidence of focal
eosinophilic/basophilic hepatocytes was observed in females. The
authors concluded that the NOEL was 50 ppm clofentezine (equivalent to
5 mg/kg b.w./day) (Lloyd et al., 1985).
Rats
Groups of 50 male and 50 female CD rats were fed diets containing
0, 10, 40, or 400 ppm clofentezine (technical) for 118 weeks
(27 months). Additional supplementary groups of 20 male and 20 female
rats were fed diets containing clofentezine at the same dietary levels
and sacrificed after 12 months of treatment. Observations included
clinical signs, mortality, body weight, food consumption, water
consumption, haematology, clinical chemistry, urinalysis, ophthal-
moscopy, and assays for thyroid function and sex hormones. At
termination all surviving rats were killed, organs were weighed, and
comprehensive histopathological examinations were made of tissues from
each of these animals as well as from those dying during the study.
The only treatment-related effects found after haematology and
clinical chemistry analyses were decreased haemoglobin in females
after 18 and 27 months and an increase in free T4 in males at
400 ppm. However, other parameters of thyroid function were not
affected. There was no evidence of chronic toxicity in any tissues
other than the liver and the thyroid. Increased relative liver weights
and liver cell hypertrophy, characterized by centrilobular hepatocyte
enlargement and by vacuolisation and fat deposits, were observed at
400 ppm in males (in females in a few cases). There was a slight
increase in the number of follicular cell tumours in the thyroid at
400 ppm in males. This is considered of doubtful biological
significance since a comparable incidence was observed in control
males in a parallel study. The authors concluded that the NOEL was
40 ppm clofentezine in the diet (equivalent to 2 mg/kg b.w./day)
(Ginocchio & Mallyon, 1985).
Observations in humans
Information useful for evaluation is not available.
COMMENTS
Clofentezine is an acaricide with low mammalian toxicity. Its
mode of action is not understood. At high doses clofentezine exerts an
effect on the liver (enzyme induction). Pretreatment with high dietary
levels of clofentezine increases the rate of metabolism. It also
affects thyroid function and impairs steroid hormone regulation.
After oral administration of clofentezine to rats, it was
excreted predominantly in the faeces. Low levels of residues were
found in organs and tissues. The levels in plasma reached a maximum 4
to 6 hours after an oral dose of 10 mg/kg b.w. after which the level
decreased, with a half-life of 2 - 3 hours.
When clofentezine was given to rats during 25 days, a plateau was
reached after 5 - 10 days in various organs, with the highest residues
in the liver. The concentration in the liver after 25 days was 2 - 4
times higher than after 1 day, while fat residues were about 8 times
higher.
The material excreted in rat urine consisted mainly of
metabolites. The two major pathways were hydroxylation of clofentezine
and formation of a monochlorosulfur derivative. In the faeces 50% was
excreted unchanged; the rest was metabolized to more that 20 minor
metabolites.
The metabolic pattern in other species (rabbits, dogs, baboons)
was similar to the rat, with a higher excretion in the faeces than in
the urine. The same metabolites were formed in rats, mice, calves,
dogs, goats, and baboons, with methylthiolation being more prominent
in rodents. In calves and baboons, hydroxylation was more prominent.
Clofentezine has a low acute toxicity in rats, mice, hamsters,
guinea pigs, and dogs.
Clofentezine did not affect fertility or reproductive
performance. Maternal effects (decreased growth, liver enlargement)
were seen in all generations at 400 ppm. There were no indications of
a teratogenic effect in rats or rabbits.
A battery of mutagenicity tests did not indicate mutagenic
potential.
Short-term oral administration to mice, rats, and dogs produced
liver enlargement as the main toxicological effect. In rats, decreased
growth and decreased haemoglobin were observed at high-dose levels, as
well as effects on biochemical parameters. Histopathological liver
changes were in all cases associated with liver enlargement and liver
enzyme induction. In addition, changes in thyroid function and steroid
hormone regulation were observed (tested in special studies with
rats).
In long-term studies the same effects were found as in short-term
studies. In mice a non-significant increase in benign liver tumours
was found in females. In the rat study with relatively low dose levels
a slight increase of thyroid follicular cell tumours was observed.
Neither effect was considered to be an indication of carcinogenicity.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 50 ppm in the diet, equal to 5 mg/kg b.w./day.
Rat: 40 ppm in the diet, equivalent to 2 mg/kg b.w./day.
Dog: 50 ppm in the diet, equal to 1.72 mg/kg b.w./day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Observations in man.
2. Studies on the possible mode of action on the thyroid.
3. Information on possible enterohepatic recirculation.
REFERENCES
Bootman, J. & Rees, R. Technical NC 21314: Investigation of mutagenic
1982 activity in the TK +/- mouse lymphoma cell mutation system.
Unpublished report No. TOX/82/167-38 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Brooks, P.M. & Turnbull, G.J. Technical NC 21314: 90-Day dietary
1983 toxicity study in the rat - additional examination of the
liver histology. Unpublished report No. TOX/83/167-44 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Campbell, J.K. & Needham, D. Excretion and tissue residues of
1981 (14C) NC 21314 in male and female dogs following a single
intravenous dose at 0.1 mg/kg. Unpublished report
No. METAB/81/37 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Challis, I.R. Concentration of (14C) NC 21314 and
1982 its metabolites in the plasma of rats doses orally with NC
21314 at the rate of 10 mg/kg body weight. Unpublished
report No. METAB/82/39 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982a NC 21314 in male and female mice given a single oral dose of
10 mg/kg. Unpublished report No. METAB/82/11 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982b NC 21314 in male and female rabbits following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/21 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982c NC 21314 in male and female dogs following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/6 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-clofentezine and
1985a its metabolites in the plasma of rats given fourteen daily
doses of clofentezine followed by a single oral dose of
(14C)-clofentezine at 14 mg/kg bodyweight. Unpublished
report No. METAB/85/6 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-Clofentezine and
1985b its metabolites in the plasma of rats dosed orally with
clofentezine at the rate of 1000 mg/kg bodyweight.
Unpublished report No. METAB/85/2 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981a radiolabelled residues in male and female rats dosed orally
at 0.1 mg/kg with NC 21314. Unpublished report
No. METAB/81/26 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981b radiolabelled residues following a single intravenous dose
of 0.1 mg/kg (14C)-NC 21314. Unpublished report
No. METAB/81/38 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The distribution and level of
1981c radiolabelled residues in rats following repeat oral dosing
with (14C)-NC 21314 at 20 mg/kg/day. Unpublished report
No. METAB/81/32 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982a radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with NC 21314. Unpublished report No. METAB/82/1
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982b radiolabelled residues in male and female rats dosed orally
at 1000 mg/kg with NC 21314. Unpublished report
No. METAB/82/22 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982c radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with (14C)-NC 21314 following 14 days
pre-treatment by dosing with unlabelled NC 21314 at
10 mg/kg/day. Unpublished report No. METAB/82/37 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Challis, I.R. & Needham, D. The excretion and distribution of
1983 radiolabelled residues in male and female rats dosed
intravenously at 0.1 mg/kg with (14C)-NC 21314.
Unpublished report No. METAB/83/14 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. A comparison of the metabolism of clofentezine in rat,
1985 mouse, rabbit, calf, dog and baboon. Unpublished report
No. METAB/85/9 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Creedy, C.L. The effects of clofentezine on thyroid
1985 function. Unpublished report No. METAB/85/36 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The metabolism of clofentezine in the rat.
1985 Unpublished report No. METAB/85/5 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Chambers, P.R., Sanderson, D.M., & Brooks, P.N. The acute oral
1981 toxicity of technical (pilot plant) NC 21314 to the male and
female beagle dog. Unpublished report No. TOX/80/167-10 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Chesterman, H., Massey, J.E., Heywood, R., Buist, D., Street, A.E.,
1984 Gopinath, C., & Rupanagudi, S. Rao. NC 21314 oral toxicity
study in dogs (final report: repeated dietary administration
for 52 weeks). Unpublished report No. TOX/84/167-68 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire,
England, and FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Cozens, D.D., Perkin, C.J., Barton, S.J., Clark, R., Offer, J.M.,
1983 Gibson, W.A., & Street, A.E. Effect of technical NC 21314 on
pregnancy of the rabbit (teratology study). Unpublished
report No. FBC TOX/83/167-42 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, England and from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Creedy, C.L., Hemmings, P.A., & Needham, D. The effect of clofentezine
1985 on the hepatic mixed-function oxidase system of the male and
female rat following dietary administration at 10, 40, or
400 ppm diet for two weeks. Unpublished report No. METAB/
85/34 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of unformulated
1980 NC 21314 (CR 20099) to the hamster. Unpublished report
No. TOX/80/167-8 from Fisons Ltd, Saffron Walden, Essex,
England. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute oral toxicity
1980a of unformulated (CR 20099/4) to the male & female mouse.
Unpublished report No. TOX/80/167-7 from Fisons Ltd, Saffron
Walden, Essex, England. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The primary skin
1980b irritancy of unformulated NC 21314 (CR 20099/5) to the
guinea pig. Unpublished report No. TOX/80/167-6 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute dermal toxicity
1980c of unformulated NC 21314 (CR 20099/5) to the male and female
rat. Unpublished report No. TOX/80/167-4 from Fisons Ltd,
Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of technical
1981a (pilot plant) NC 21314 to the guinea pig (CR 20099).
Unpublished report No. TOX/81/167-16 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The primary skin irritancy of NC 21314
1981b 50WP to the guinea pig. Unpublished report No. TOX/81/167-13
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. Acute intraperitoneal
1981 toxicity of pilot plant technical NC 21314 to the rat.
Unpublished report No. TOX/80/167-5 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Cuthbert, J.A. & Carr, S.M.A. NC 21314 50 SC formulation primary eye
1983 irritancy study in rabbits. Unpublished report No. TOX/83/
167-46 from Inveresk Research International, Musselburgh,
Scotland, and from FBC, Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Cuthbert, J.A., Carr, S.M.A., & D'Arcy-Burt, K.J. NC 21314 50 SC
1983 formulation primary skin irritancy study in rabbits.
Unpublished report No. TOX/83/167-47 from Inveresk Research
International, Musselburgh, Scotland, and from FBC, Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Brooks, P.N. Technical NC 21314 (pilot plant, CR
1981 20099/5): the 90-day dietary toxicity study in the rat.
Unpublished report No. TOX/81/167-23/1 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Mallyon, B.A. The oncogenicity and chronic toxicity
1985 of technical NC 21314 (clofentezine) in the diet to the rat.
Unpublished report No. TOX/84/167-70 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1981 oral toxicity of technical (CR 20099/8) NC 21314 in the diet
to the dog. Unpublished report No. TOX/81/167-21/1 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Hounsell, I.A. A micronucleus study in mice using technical NC 21314.
1982 Unpublished report No. TOX/82/167-32 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1982 oral toxicity of technical (pilot plant) NC 21314 in the
diet to the mouse. Unpublished report NO. TOX/82/167-33 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Jackson, C.M. Technical NC 21314: Teratogenicity study in the rat
1982 (plus addendum). Unpublished report No. TOX/82/167-34 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. Technical NC 21314: dominant lethal mustation assay in
1983 male rats. Unpublished report No. TOX/83/167-45 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. & Chambers, P.R. Technical clofentezine: a dietary
1984 multigeneration study in the rat. Unpublished report
No. TOX/84/167-66 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Lloyd, G.K., Spencer-Briggs, D.J., Heywood, R., Gopinath, C., &
1985 Cherry, C.P. Technical NC 21314: oncogenicity in the diet to
the mouse (final report). Unpublished report No. TOX/85/
167-80 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, England, and from FBC Ltd. Submitted to WHO
by FBC Ltd., Hauxton, Cambridge, England.
Mallyon, B.A. & Sanderson, D.M. The acute oral toxicity of
1980 unformulated NC 21314 (NC 21314/4, 99% pure) to the male and
female rat. Unpublished report No. TOX/80/167-2 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Mallyon, B.A., Sanderson, D.M., & Brooks, P.N. The acute inhalational
1982 toxicity of NC 21314 80WP CR 15569, to the rat. Unpublished
report No. TOX/81/167-24 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
McConville, M. Ames test for mutagenic activity carried out with
1980 technical NC 21314 (CR 20099/4). Unpublished report
No. TOX/80/167-3 from Inveresk Research International,
Musselburgh, Scotland, and from Fisons Ltd, Saffron Walden,
Essex, England. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. The distribution of radioactivity in the maternal tissues
1981 and foetuses of rats after an oral dose of (14C)-NC 21314.
Unpublished report No. METAB/81/19 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D. The whole-body autography of (14C)-NC 21314 in rats
1982 following oral administration at 10 mg/kg body weight.
Unpublished report No. METAB/82/23 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of eight weeks
1983a dietary administration of NC 21314 at 40 and 27,000 mg/kg on
the hepatic mixed function oxidase system of the male rat.
Unpublished report No. METAB/81/31 (2nd Edn.) from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of a two week
1983b withdrawal period on the induction of hepatic microsomal
mixed function oxidases caused by dietary administration of
NC 21314 at 27,000 mg/kg diet. Unpublished report
No. METAB/82/2 (2nd Edn.) from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Campbell, J., & Challis, I. The effect of eight weeks
1984 dietary administration of NC 21314 at 27,000 and 400 mg/kg
diet on the hepatic mixed-function oxidase system of the
male mouse. Unpublished report No. METAB/82/20 (2nd Edn.)
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. & Challis, I.R. An investigation into the nature of the
1985 residues present in the liver of the rat, goat and calf
following the oral administration of clofentezine.
Unpublished report No. METAB/85/8 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Riach, C.G. & McGregor, D.B. Technical NC 21314: induction of gene
1983 conversion and mitotic recombination in yeast. Unpublished
report No. TOX/83/167-56 from Inveresk Research
International, Musselburgh, Scotland, and from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Saunders, P.C. & Mallyon, B.A. Technical clofentezine: 6 week dietary
1986 investigation of thyroid function in the rat. Unpublished
report No. TOX/85/167-77 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Sortwell, R.J., Richmond, G.P., Kirkpatrick, D., Finn, C.M., & Conway,
1983 B. Excretion and tissue distribution of radioactivity after
oral administration of (14C)-NC 21314 to a male and female
baboon. Unpublished report No. METAB/83/26 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England, and
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Teale, H.J. Delayed dermal sensitisation study in the guinea pig,
1982 NC 21314 technical. Unpublished report No. TOX/82/167-36
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
 EMPIRICAL FORMULA C14 H8 Cl2 N4
MOLECULAR MASS 303.15
PHYSICAL STATE Both pure and technical materials are
magenta-coloured crystalline solids.
MELTING POINT 182 - 186°C (pure compound)
179 - 182°C (technical product)
VAPOUR PRESSURE Approximately 10-16 mm Hg = 10-14 Pa at
25°C.
SOLUBILITY Solubility (pure a.i.) at 20°C:
chloroform 5 %
dichloromethane 3.5 %
acetone 0.5 %
benzene 0.25%
ethanol < 0.1 %
hexane < 0.1 %
solvesso 200 0.25%
water < 1 µg/1
OCTANOL/WATER PARTITION
COEFFICIENT log P = 3.1
STABILITY (a.i.) The stability in aqueous solutions is pH-
and temperature-dependent; at pH 7 and
22°C, the half-life is approximately
35 hours, whereas at pH 5 and 22°C the
half-life is approximately 250 hours.
STABILITY (formulations) 50% wettable powder: stable under storage
conditions at 40°C for 6 months; 50%
aqueous suspension concentrate: stable
under storage conditions at 12 - 38°C for
12 months.
SPECIFICATION OF
TECHNICAL MATERIAL The technical material has a purity in
excess of 96% w/w, with the main
impurities being:
(a) 4-amino-3,5-bis(2-chlorophenyl)-4H-1,2,4-triazole <1%
(b) 3,6-bis(2-chlorophenyl)-1,2-dihydro-1,2,4,5-tetrazine <1%
(c) 2,5-bis(2-chlorophenyl)-1,3,4-oxadiazole <1%
(d) total volatiles (water and acetic acid) <1%.
FORMULATIONS AVAILABLE
COMMERCIALLY 50% wettable powder; 50% aqueous
suspension concentrate.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mice
14C-Labelled clofentezine was administered orally at single
doses of 10 mg/kg b.w. to 8 male and 8 female mice. Ninety-six hours
after dosing, 91 - 95% of the administered dose had been recovered in
the excreta, particularly in the faeces. Residues after 96 hours were
highest in the liver (0.11 µg/g in males and 0.18 µg/g in females).
All other tissues (including plasma) contained substantially lower
residues, in most cases below the limit of detection (Campbell &
Needham, 1982a).
Rats
Groups of 5 male and 5 female rats received single oral doses of
0.1, 10, or 1000 mg 14C-clofentezine/kg b.w. or 0.1 mg
14C-clofentezine (in ethanol)/kg b.w. i.v. Eighty-nine hours after
dosing, 22 29% of the administered 14C-clofentezine in the low-dose
groups had been recovered in the urine. Tissue residues were generally
below the detection limit (< 0.01 µg/g), with occasional samples
of liver, kidney, spleen, and heart tissues containing measurable
residues (0.01 - 0.02 µg/g). Urinary excretion accounted for 19%
(males) and 20% (females) of the administered dose after administra-
tion of 10 mg 14C-clofentezine/kg b.w., with the highest residues
in the liver (0.22 - 0.46 µg/g) and kidneys (0.12-1.28 µg/g). Plasma
levels were 0.07 - 0.22 µg/ml, and the remaining tissues contained
0.02 - 0.2 µg/g in the mid-dose group. Urinary excretion fell to only
2.0% (males) and 4.5% (females) after the administration of 1000 mg
14C-clofentezine/kg b.w. In the faeces these percentages amounted
to 99% and 96%, respectively. The highest residues were found in
plasma (9.2-19.5 µg/ml), adrenals (2.9-17.1 µg/g), liver (2.8-14.0
µg/g), and fat (3.6 - 9.4 µg/g) (Challis & Needham, 1981a; 1982a,b).
Ninety-six hours after i.v. administration of 0.1 mg 14C-clofen-
tezine/kg b.w., 25 - 29% of the administered dose had been recovered
in the urine, while in the faeces the residues were 82-85%. Tissue
residues were very low (< 0.01 µg/g) (Challis & Needham, 1983).
An examination of the distribution of radioactivity using
whole-body autography, 24 hours after the oral administration of 10 mg
14C-clofentezine/kg b.w. to rats, showed that the liver, kidneys,
and gut contain significant amounts of radioactivity. The carcass was
clear of residues 96 hours after dosing (Needham, 1982).
Rats (25/sex) received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. Residues in plasma were measured 5 minutes to 18 hours
after dosing. Total radioactivity showed maximum values of 1.3 - 1.6
µg/ml between 4 and 6 hours after dosing, which declined to 0.02 µg/ml
at 18 hours after dosing. The concentration of clofentezine reached a
maximum at 4 - 6 hours after dosing and then declined, with a half
life of 2.5 - 2.7 hours (Campbell & Challis, 1982).
Total radioactivity and unchanged clofentezine in plasma of rats
dosed with 1000 mg 14C-clofentezine/kg b.w. rose to a maximum of
14-16 mg/l and 6 - 8 mg/l, respectively, between 6 and 8 hours after
dosing. Unchanged clofentezine declined, with a half-life of 3.6 hours
(Campbell & Needham, 1985b).
When rats were fed daily oral doses of 20 mg clofentezine/kg b.w.
for 25 days, the highest residues were found in the liver (3.2-4.4
µg/g). After 5 - 10 days a plateau was reached in the liver, kidneys,
skin, and ovaries, with residues being 2 - 4 times higher than after
1 day. For residues in the adrenals, muscle, lung, and fat, no
definite plateau could be established, but residues in fat were 8
times higher after 25 days than after 1 day. No accumulation occurred
in bone, brain, spleen, testes, or blood (Challis & Needham, 1981c).
Pre-treatment with clofentezine (27 g/kg diet for 28 days) prior
to an i.v. dose of 0.1 mg 14C-clofentezine/kg b.w. resulted in an
increase in urinary excretion (34% of the radiolabelled dose in both
sexes). Tissue levels were not significantly altered (Challis &
Needham, 1981b).
Pre-treatment with clofentezine (10 mg/kg diet for 14 days) prior
to an oral dose of 10 mg/kg 14C-clofentezine/kg diet did not change
the excretion profile nor the residues disposition. The concentration
of unchanged clofentezine in plasma reached a maximum of 0.11-0.19
µg/ml and declined, with a half-life of 1.6 hours. These results are
consistent with an increase in the rate of metabolism when compared
to a single dose (Challis & Needham, 1982c) (Campbell & Needham,
1985a).
Pregnant rats received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. on day 20 post-coitum (p.c.) after being orally dosed
with 3200 mg clofentezine/kg b.w. from days 7 to 13 p.c., 320 mg/kg
b.w. on day 14 p.c., and then remaining untreated from days 15 to
19 p.c. Residue levels were much lower in the fetus (0.7-1.2 µg/g)
than in the maternal plasma (3.7 - 6.4 µg/g) 6 hours after dosing,
showing that clofentezine and/or its metabolites do not readily cross
the placenta. The clearance rate from the fetus appeared to be slower
than from the maternal tissues (Needham, 1981).
Rabbits
Rabbits (3/sex) were orally dosed with 10 mg 14C-clofen-
tezine/kg b.w. Urine and faeces were collected over 96 hours. In the
first 48 hours, 88% of the dose was excreted (35% in the urine and 53%
in the faeces). Residues after 96 hours were highest in the liver and
kidneys (0.24 µg/g and 0.90 µg/g, respectively) (Campbell & Needham,
1982b).
Dogs
After oral administration of 10 mg/kg 14C-clofentezine to dogs
(3/sex), 96% of the dose was excreted in the first 48 hours, mainly in
the faeces (94%). Nearly complete excretion (99%) was observed after
96 hours, with only 2.0% in the urine. Residues were highest in bile
(1.56 µg/ml), liver (0.21 µg/g) and thyroid (0.15 µg/g). All other
tissues contained substantially lower residues. Peak plasma
concentration (0.06 µg/ml) occurred 4 - 8 hours after dosing and by 72
hours it was reduced to 0.01 µg/ml or less (Campbell & Needham,
1982c).
Dogs (2/sex) received single i.v. doses of 0.1 mg 14C-clofen-
tezine/kg b.w. The bulk of the dose was excreted in the first 48
hours, mainly in the faeces (69%), and 21% in the urine. Tissue
residues measured 144 hours after dosing were, with few exceptions,
below the limit of detection (< 0.01 µ/g) (Campbell & Needham, 1981).
Baboons
A male and a female baboon were each given a single oral dose of
10 mg 14C-clofentezine/kg b.w. Ninety-six hours after dosing 15 -
28% of the administered dose was excreted in the urine and 42 - 44% in
the faeces. Cage debris and wash together accounted for 9%. Tissue
concentrations of radioactivity 96 hours after a second oral dose of
14C-clofentezine (following 52 daily doses of up to 400 mg
unlabelled clofentezine starting 96 hours after the first oral dose)
were similar in both baboons. The highest concentrations were detected
in fat, liver, and kidneys (0.06 - 0.23% of administered
radioactivity/kg wet tissue) while tissue-to-plasma ratios were
on the order of 3 - 10 (Sortwell et al., 1983).
Biotransformation
The metabolism of clofentezine was studied in rats. Urine and
faeces were collected 24 hours after dosing in a series of experiments
in which rats received 14C-clofentezine orally at 10 mg/kg b.w.
Absorbed clofentezine was extensively metabolized, with only 3% of the
radioactivity in the urine present as the parent compound. Two major
urinary pathways have been identified, one leading to a monochloro
sulfur-containing derivative, 3-(2'-methylthio-3'-hydroxyphenyl)-
6-(2'-chlorophenyl)-1,2,4,5-tetrazine (in free and conjugated forms
this metabolite accounted for 35% of the urinary radioactivity), and
the other involving hydroxylation of clofentezine at the 3, 4, or 5
positions (see Figure 1). At least half of the administered dose was
excreted in the faeces unchanged. The remaining clofentezine was
extensively metabolized to more than 20 minor metabolites (Challis &
Needham, 1985).
a figure that could possibly be photocopied is on p. 9 of the summary
submitted by FBC Ltd.
EMPIRICAL FORMULA C14 H8 Cl2 N4
MOLECULAR MASS 303.15
PHYSICAL STATE Both pure and technical materials are
magenta-coloured crystalline solids.
MELTING POINT 182 - 186°C (pure compound)
179 - 182°C (technical product)
VAPOUR PRESSURE Approximately 10-16 mm Hg = 10-14 Pa at
25°C.
SOLUBILITY Solubility (pure a.i.) at 20°C:
chloroform 5 %
dichloromethane 3.5 %
acetone 0.5 %
benzene 0.25%
ethanol < 0.1 %
hexane < 0.1 %
solvesso 200 0.25%
water < 1 µg/1
OCTANOL/WATER PARTITION
COEFFICIENT log P = 3.1
STABILITY (a.i.) The stability in aqueous solutions is pH-
and temperature-dependent; at pH 7 and
22°C, the half-life is approximately
35 hours, whereas at pH 5 and 22°C the
half-life is approximately 250 hours.
STABILITY (formulations) 50% wettable powder: stable under storage
conditions at 40°C for 6 months; 50%
aqueous suspension concentrate: stable
under storage conditions at 12 - 38°C for
12 months.
SPECIFICATION OF
TECHNICAL MATERIAL The technical material has a purity in
excess of 96% w/w, with the main
impurities being:
(a) 4-amino-3,5-bis(2-chlorophenyl)-4H-1,2,4-triazole <1%
(b) 3,6-bis(2-chlorophenyl)-1,2-dihydro-1,2,4,5-tetrazine <1%
(c) 2,5-bis(2-chlorophenyl)-1,3,4-oxadiazole <1%
(d) total volatiles (water and acetic acid) <1%.
FORMULATIONS AVAILABLE
COMMERCIALLY 50% wettable powder; 50% aqueous
suspension concentrate.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mice
14C-Labelled clofentezine was administered orally at single
doses of 10 mg/kg b.w. to 8 male and 8 female mice. Ninety-six hours
after dosing, 91 - 95% of the administered dose had been recovered in
the excreta, particularly in the faeces. Residues after 96 hours were
highest in the liver (0.11 µg/g in males and 0.18 µg/g in females).
All other tissues (including plasma) contained substantially lower
residues, in most cases below the limit of detection (Campbell &
Needham, 1982a).
Rats
Groups of 5 male and 5 female rats received single oral doses of
0.1, 10, or 1000 mg 14C-clofentezine/kg b.w. or 0.1 mg
14C-clofentezine (in ethanol)/kg b.w. i.v. Eighty-nine hours after
dosing, 22 29% of the administered 14C-clofentezine in the low-dose
groups had been recovered in the urine. Tissue residues were generally
below the detection limit (< 0.01 µg/g), with occasional samples
of liver, kidney, spleen, and heart tissues containing measurable
residues (0.01 - 0.02 µg/g). Urinary excretion accounted for 19%
(males) and 20% (females) of the administered dose after administra-
tion of 10 mg 14C-clofentezine/kg b.w., with the highest residues
in the liver (0.22 - 0.46 µg/g) and kidneys (0.12-1.28 µg/g). Plasma
levels were 0.07 - 0.22 µg/ml, and the remaining tissues contained
0.02 - 0.2 µg/g in the mid-dose group. Urinary excretion fell to only
2.0% (males) and 4.5% (females) after the administration of 1000 mg
14C-clofentezine/kg b.w. In the faeces these percentages amounted
to 99% and 96%, respectively. The highest residues were found in
plasma (9.2-19.5 µg/ml), adrenals (2.9-17.1 µg/g), liver (2.8-14.0
µg/g), and fat (3.6 - 9.4 µg/g) (Challis & Needham, 1981a; 1982a,b).
Ninety-six hours after i.v. administration of 0.1 mg 14C-clofen-
tezine/kg b.w., 25 - 29% of the administered dose had been recovered
in the urine, while in the faeces the residues were 82-85%. Tissue
residues were very low (< 0.01 µg/g) (Challis & Needham, 1983).
An examination of the distribution of radioactivity using
whole-body autography, 24 hours after the oral administration of 10 mg
14C-clofentezine/kg b.w. to rats, showed that the liver, kidneys,
and gut contain significant amounts of radioactivity. The carcass was
clear of residues 96 hours after dosing (Needham, 1982).
Rats (25/sex) received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. Residues in plasma were measured 5 minutes to 18 hours
after dosing. Total radioactivity showed maximum values of 1.3 - 1.6
µg/ml between 4 and 6 hours after dosing, which declined to 0.02 µg/ml
at 18 hours after dosing. The concentration of clofentezine reached a
maximum at 4 - 6 hours after dosing and then declined, with a half
life of 2.5 - 2.7 hours (Campbell & Challis, 1982).
Total radioactivity and unchanged clofentezine in plasma of rats
dosed with 1000 mg 14C-clofentezine/kg b.w. rose to a maximum of
14-16 mg/l and 6 - 8 mg/l, respectively, between 6 and 8 hours after
dosing. Unchanged clofentezine declined, with a half-life of 3.6 hours
(Campbell & Needham, 1985b).
When rats were fed daily oral doses of 20 mg clofentezine/kg b.w.
for 25 days, the highest residues were found in the liver (3.2-4.4
µg/g). After 5 - 10 days a plateau was reached in the liver, kidneys,
skin, and ovaries, with residues being 2 - 4 times higher than after
1 day. For residues in the adrenals, muscle, lung, and fat, no
definite plateau could be established, but residues in fat were 8
times higher after 25 days than after 1 day. No accumulation occurred
in bone, brain, spleen, testes, or blood (Challis & Needham, 1981c).
Pre-treatment with clofentezine (27 g/kg diet for 28 days) prior
to an i.v. dose of 0.1 mg 14C-clofentezine/kg b.w. resulted in an
increase in urinary excretion (34% of the radiolabelled dose in both
sexes). Tissue levels were not significantly altered (Challis &
Needham, 1981b).
Pre-treatment with clofentezine (10 mg/kg diet for 14 days) prior
to an oral dose of 10 mg/kg 14C-clofentezine/kg diet did not change
the excretion profile nor the residues disposition. The concentration
of unchanged clofentezine in plasma reached a maximum of 0.11-0.19
µg/ml and declined, with a half-life of 1.6 hours. These results are
consistent with an increase in the rate of metabolism when compared
to a single dose (Challis & Needham, 1982c) (Campbell & Needham,
1985a).
Pregnant rats received single oral doses of 10 mg 14C-clofen-
tezine/kg b.w. on day 20 post-coitum (p.c.) after being orally dosed
with 3200 mg clofentezine/kg b.w. from days 7 to 13 p.c., 320 mg/kg
b.w. on day 14 p.c., and then remaining untreated from days 15 to
19 p.c. Residue levels were much lower in the fetus (0.7-1.2 µg/g)
than in the maternal plasma (3.7 - 6.4 µg/g) 6 hours after dosing,
showing that clofentezine and/or its metabolites do not readily cross
the placenta. The clearance rate from the fetus appeared to be slower
than from the maternal tissues (Needham, 1981).
Rabbits
Rabbits (3/sex) were orally dosed with 10 mg 14C-clofen-
tezine/kg b.w. Urine and faeces were collected over 96 hours. In the
first 48 hours, 88% of the dose was excreted (35% in the urine and 53%
in the faeces). Residues after 96 hours were highest in the liver and
kidneys (0.24 µg/g and 0.90 µg/g, respectively) (Campbell & Needham,
1982b).
Dogs
After oral administration of 10 mg/kg 14C-clofentezine to dogs
(3/sex), 96% of the dose was excreted in the first 48 hours, mainly in
the faeces (94%). Nearly complete excretion (99%) was observed after
96 hours, with only 2.0% in the urine. Residues were highest in bile
(1.56 µg/ml), liver (0.21 µg/g) and thyroid (0.15 µg/g). All other
tissues contained substantially lower residues. Peak plasma
concentration (0.06 µg/ml) occurred 4 - 8 hours after dosing and by 72
hours it was reduced to 0.01 µg/ml or less (Campbell & Needham,
1982c).
Dogs (2/sex) received single i.v. doses of 0.1 mg 14C-clofen-
tezine/kg b.w. The bulk of the dose was excreted in the first 48
hours, mainly in the faeces (69%), and 21% in the urine. Tissue
residues measured 144 hours after dosing were, with few exceptions,
below the limit of detection (< 0.01 µ/g) (Campbell & Needham, 1981).
Baboons
A male and a female baboon were each given a single oral dose of
10 mg 14C-clofentezine/kg b.w. Ninety-six hours after dosing 15 -
28% of the administered dose was excreted in the urine and 42 - 44% in
the faeces. Cage debris and wash together accounted for 9%. Tissue
concentrations of radioactivity 96 hours after a second oral dose of
14C-clofentezine (following 52 daily doses of up to 400 mg
unlabelled clofentezine starting 96 hours after the first oral dose)
were similar in both baboons. The highest concentrations were detected
in fat, liver, and kidneys (0.06 - 0.23% of administered
radioactivity/kg wet tissue) while tissue-to-plasma ratios were
on the order of 3 - 10 (Sortwell et al., 1983).
Biotransformation
The metabolism of clofentezine was studied in rats. Urine and
faeces were collected 24 hours after dosing in a series of experiments
in which rats received 14C-clofentezine orally at 10 mg/kg b.w.
Absorbed clofentezine was extensively metabolized, with only 3% of the
radioactivity in the urine present as the parent compound. Two major
urinary pathways have been identified, one leading to a monochloro
sulfur-containing derivative, 3-(2'-methylthio-3'-hydroxyphenyl)-
6-(2'-chlorophenyl)-1,2,4,5-tetrazine (in free and conjugated forms
this metabolite accounted for 35% of the urinary radioactivity), and
the other involving hydroxylation of clofentezine at the 3, 4, or 5
positions (see Figure 1). At least half of the administered dose was
excreted in the faeces unchanged. The remaining clofentezine was
extensively metabolized to more than 20 minor metabolites (Challis &
Needham, 1985).
a figure that could possibly be photocopied is on p. 9 of the summary
submitted by FBC Ltd.
 Figure 1. The metabolism of clofentizine in animals.
Metabolites were also identified in a study with baboons.
4-Hydroxy-clofentezine in free and conjugated (beta-glucuronide
conjugate) forms accounted for over 70% of the urinary metabolites.
Other minor metabolites included 3-hydroxy-clofentezine (present as
phenol and glucuronide conjugates) and a mono-chloro sulfur-containing
compound, each accounting for less than 1% of the extractable
radioactivity (Sortwell et al., 1983).
The metabolism of clofentezine in rats, mice, rabbits, calves,
goats, dogs, and baboons was qualitatively similar, with hydroxylation
and replacement of chlorine with a methylthio group being the major
pathways (see Figure 1). In calves and baboons, hydroxylation and
subsequent conjugation were the most important pathways, while
methylthiolation was more prominent in rodents and rabbits (Challis,
1985).
The chromatographic profiles of liver extracts from rats, goats,
and calves following oral administration of 14C-clofentezine were
qualitatively similar to that of rat urine, with conjugates of 3-, 4-,
and 5-hydroxylated clofentezine and a 3-(methylthio-hydroxyphenyl)-6-
(2'-chlorophenyl)-1,2,4,5-tetrazine isomer (Needham & Challis, 1985).
Toxicological studies
Special studies on the endocrine system
After treatment of male rats with 0 or 3% clofentezine for
1 month, a slight decrease in thyroxine (T4) half-life was observed
in dosed rats compared to control animals (a slight increase occurred
over this time period in the controls). A comparison of the effect of
clofentezine treatment (0 and 3% for 4 weeks) in rats and mice (both
sexes) showed that in rats there was a significant and rapid increase
in thyroid uptake of iodine (compared to control animals) following
i.p. dosing with [131I]-sodium iodide. This effect was not observed
in mice (Challis & Creedy, 1985).
Groups of 10 rats/sex were fed diets containing 0, 400, or
30,000 ppm technical clofentezine for 6 weeks. At 30,000 ppm
clofentezine, T4, free T4 index, thyrotropin (TSH), progesterone,
dehydroepiandrosterone, and liver weights were significantly increased
in both sexes. Triiodothyronine (T3) was significantly increased in
males only. Body weights and thyroxine binding capacity were
significantly reduced in females. At 400 ppm clofentezine, T4 and
liver weight were significantly increased in males and dehydroepi-
androsterone was significantly increased in females (Saunders & Mallyon,
1986).
Special studies on enzyme induction
Mice
Groups of 9 - 10 male Charles River CD1 mice received
clofentezine at dietary levels of 0, 40, or 27,000 ppm for 8 weeks
prior to examination of their livers for aniline hydroxylase and
aminopyrine demethylase activities, for levels of cytochromes P-450
and b5, and for microsomal protein concentration. At 27,000 ppm the
levels of cytochrome P-450 and b5 were increased 3-fold. The activity
of aminopyrine demethylase rose by a factor of 2.2 and the mean liver
weights increased by 35%. There was a marginal effect on the enzyme
induction system at 40 ppm (Needham et al., 1984).
Rats
Groups of 6 male Charles River CD rats were fed diets containing
clofentezine at levels of 0, 40, or 27,000 ppm for 8 weeks.
Twenty-four hours after the final dose, their livers were examined for
aniline hydroxylase activity, for levels of cytochromes P-450 and b5,
and for microsomal protein concentration. At 27,000 ppm a 50% increase
in the level of cytochrome P-450 and 2-fold increases in the level of
cytochrome b5 and in the activity of aniline hydroxylase were found.
In a subsequent study it was discovered that the effect could be
reversed after a 2-week withdrawal period. No induction was observed
at 40 ppm (Needham et al., 1983a,b).
In a more extensive study, male and female rats were given diets
with 0, 10, 40, or 400 ppm clofentezine for 2 weeks. At 400 ppm there
was an increase in the level of liver protein (+ 12%), aldrin
epoxidase (22 - 32%), ethoxycoumarin deethylase (20 - 49%), and
cytochrome P-450 (24 - 26%) in both sexes. Liver weights and
cytochrome b5 were increased in males only. At 40 ppm, liver
ethoxycoumarin deethylase was slightly increased in males only
(Creedy et al., 1985).
Special studies on mutagenicity
Clofentezine was negative in various mutagenicity assays
(Table 1).
Table 1: Results of mutagenicity assays on clofentezine
Test Concentration
Test system Object of clofentezine Results Reference
Ames testa Salmonella 10 - 3300 negative McConville,
typhimurium µg/plate 1980
TA98, TA100,
TA1535, TA1537,
and TA1538
Yeast testa,b Saccharomyces 12.5 - 200 negative Riach &
cerevisiae D7 µg/ml McGregor, 1983
Mouse lymphoma Mouse L 5178Y 0 - 128 negative Bootman &
assaya TK +/- cells µg/ml Rees, 1982
Micronucleus Mouse 2 × 0 to negative Hounsell, 1982
assay (bone 3200 mg/kg
marrow) in 0.5%
aqueous gum,
orally
Dominant Rat (males) 0 to 400 negative Jackson, 1983
lethal test mg/kg in
the diet
for 10 weeks
a with and without metabolic activation
b gene conversion and mitotic recombination
Special study on reproduction
Rats
Groups of 6-week old Charles River rats (30 - 40/sex) were fed
diets containing 0, 4, 40, or 400 ppm clofentezine. After 74 days of
treatment the rats were paired for 20 days to start a 2-generation
(2 litters/generation) study. Diets were maintained during mating,
gestation, and lactation. Weanlings from the second litter were
selected to become parents of the next generation. The F2a litters
were grown to maturity.
In the parental generations clinical signs and food consumption
of treated rats were comparable to those of the control rats. Mating
performance, pregnancy rate, fertility, and the duration of gestation
and parturation were unaffected by clofentezine treatment. Parental
toxicity was observed at 400 ppm, which included reduced body-weight
gain of F1- and F2-generation females (maturation) and
F1-generation females (post-coitum and post-partum), as well as
significantly increased liver weights in F1- and F2-generation
males. The increased liver weight was associated with minimal
centrilobular hepatocyte enlargement and a slight reduction in
periportal fat deposition. Toxic effects on litters included
significantly reduced F1a and F2a pup weights and significantly
smaller F2b litter sizes. The only toxic effect at 40 ppm was a
significant increase in liver weights of F2a males. No adverse
effects on fertility or reproductive performance were observed at 4 or
40 ppm clofentezine (Jackson & Chambers, 1984).
Special studies on skin and eye irritation
Application of clofentezine (0.2 ml of 333 mg/ml suspension) to
the intact and abraded skin of 6 guinea pigs showed pink staining and
very slight edema (in 2 out of 12 treated areas), both receding during
observation (Crome et al., 1980b).
A dermal irritation study with 6 guinea pigs was conducted with
clofentezine using the 50% wettable powder formulation (0.2 ml of 6 or
947 mg/ml suspension). Observations were carried out at 24 hours and
then daily for one week. Pink staining and erythema (between moderate
and severe) were observed in all animals treated with 947 mg/ml
suspension, and slight erythema was observed in 3/6 guinea pigs
treated with 6 mg/ml suspension. The erythema regressed in one week.
In 2/6 guinea pigs treated with 947 mg/ml suspension, very slight
erythema was present when the animals were subjected to post-mortem
examination (no abnormalities were observed) (Crome & Sanderson,
1981b).
Treatment of the intact and abraded skin of 6 New Zealand white
rabbits with clofentezine in 50% aqueous suspension produced very
slight edema in 1/6 animals after 24 hours, with full recovery after
72 hours. Erythemal response could not be scored because of the bright
pink color of the test substance (Cuthbert et al., 1983).
Undiluted clofentezine (50% aqueous suspension) caused mild
conjunctival irritation 24 hours after installation; it had
disappeared at 48 hours (Cuthbert & Carr, 1983).
Special study on skin sensitization
Clofentezine had no sensitizing potential in guinea pigs when
tested by the Magnusson & Kligman method (Teale, 1982).
Special studies on teratogenicity
Rats
Groups of 34 - 35 pregnant female Sprague-Dawley CD rats received
0, 320, 1280, or 3200 mg clofentezine/kg b.w. by gavage from days 7 to
20 post-coitum (p.c.). The dams were examined twice daily for
mortality, appearance, and behaviour. Body weights were recorded on
days 1 and 4 and on days 7 to 21 p.c. The dams were killed on day 21
of pregnancy. The number of implantations, resorptions (early and
late), corpora lutea, and uteri were determined, as well as placental
weight, weight and histopathology of the liver of the dams, number of
live and dead fetuses, litter and pup weights, sex ratios, gross
pathology, and histopathology (skeletal and visceral) of the fetuses.
Maternal toxicity was observed at 1280 and 3200 mg/kg b.w. At the
highest dose, body weights were significantly decreased (also when
corrected for uterine content). Dose-related and significantly
increased relative liver weights were observed at all dose levels;
when corrected for the uterine contents, the increase was significant
only at 1280 and 3200 mg/kg b.w. At histopathology, slight
differential staining and enlargement of centrilobular hepatocytes
were seen at 3200 mg/kg b.w. Litter size, litter weight, mean
placental weight, and post-implantation losses were not affected at
any of the treatment levels, although mean fetal weight was increased
at 3200 mg/kg b.w.
Embryonic and fetal development were unaffected by treatment
(Jackson, 1982).
Rabbits
Groups of 14 - 15 pregnant New Zealand white rabbits were
administered daily doses of 0, 250, 1000, or 3000 mg clofentezine/kg
b.w. by gavage on days 7 to 28 of gestation. All animals were killed
on day 29 of pregnancy. The dams were examined for mortality,
appearance, behaviour, body weight, food consumption, serum
cholesterol and serum triglyceride (day 29), weight of uterus, number
of implantations, resorptions (early and late), and corpora lutea. The
fetuses, delivered by caeserean section, were examined for the number
alive and dead, litter and pup weight, gross pathology, and
histopathology (skeletal and visceral).
Maternal body-weight gain was significantly reduced at
3000 mg/kg b.w. (and to a lesser extent at 1000 mg/kg b.w.) initially
during treatment, which was associated with reduced food intake. Mean
litter and fetal weights were significantly reduced at 3000 mg/kg b.w.
No visceral or skeletal anomalies were reported (Cozens et al.,
1983).
Acute toxicity
The acute toxicity of clofentezine to several animal species is
given in Table 2. Signs of clofentezine toxicity in rats after oral
administration were confined to very slight salivation and slight
urinary incontinence. No clinical symptoms after oral administration
were observed in hamsters, mice, guinea pigs, or dogs, except for skin
reddening in dogs at the highest dose.
Short-term studies
Mice
Groups of mice (20 males and 20 females per group) were
administered clofentezine (technical grade) in the diet for 90 days at
dose levels of 0, 200, 1000, or 5000 ppm. All animals were observed
daily for mortality and signs of toxicity while body weights and food
consumption were recorded weekly. Haematology and clinical chemistry
were performed at weeks 4 and 12 on 10 mice/sex/group, and urinalysis
was performed at weeks 4 and 12 on 20 mice/sex/group. At termination
of the study all animals were killed, selected organs were weighed,
and complete gross and histopathological examinations were performed.
There were no effects on mortality, appearance, body weight, food
consumption, or haematology. Significantly increased plasma levels of
triglycerides, calcium, and phosphate were observed in mice in the
5000- and 1000-ppm groups. Urinanalysis investigations revealed red
stellar crystals in high-dose males. A dose-related urinary colour
change from yellow, through orange, to red was observed in female
mice. A treatment-related significant increase in liver weight was
observed in both sexes of the mid- and high-dose groups, which was
associated in males at 5000 ppm with centrilobular hepatocyte
enlargement (5/20). The NOEL in this study was 200 ppm (Hounsell
et al., 1982).
Table 2. Results of acute toxicity assays on clofentezine
LD501 LC502
Species Sex Route (mg/kg b.w.) (mg/l) Reference
Mouse M+F oral > 3200 - Crome et al., 1980a
Rat M+F oral > 3200 - Mallyon &
Sanderson, 1980
Rat M+F dermal > > 1332 - Crome et al., 1980c
Rat M+F i.p. > 800 - Crome et al., 1981
Rat M inhalation - > 9.08 Mallyon et al.,
1981
Hamster M+F oral > 3200 - Crome & Sanderson,
1980
Beagle dog M+F oral > 2000 - Chambers et al.,
1981
Guinea pig F oral > 1500 - Crome & Sanderson,
1981a
1 given as a 33% suspension in 0.5% gum tragacanth.
2 6 hours exposure with 80% wettable powder.
Rats
Groups of Charles River CD rats (20 males and 20 females/group)
were fed diets containing 0, 3000, 9000, or 27,000 ppm clofentezine
for 90 days. Five rats/sex/group were interim-sacrificed after 64 days
and 5 rats/sex/group were maintained untreated for a 28-day recovery
period. All animals were observed daily for mortality and physical
signs. Body weights, food consumption, and water consumption were
recorded weekly. Haematology, clinical chemistry, and urinalysis were
performed at weeks 4 and 12 on 10 rats/sex/group. Cholesterol,
triglycerides, and alanine aminotransferase were also measured after
6 weeks of treatment and at the end of the 4-week recovery period.
Detailed gross and histopathological examinations were performed and
selected organs were weighed.
Dose-related hair loss was observed in 50% and 40% of the animals
of the high- and mid-dose groups, respectively. Six animals died
throughout the study, 2/6 of the 27,000 ppm group, probably because of
the test substance. Body-weight gain was significantly reduced in
males and females of the mid- and high-dose groups. In all dose groups
some haematological and clinical chemistry values changed in a dose-
related manner (e.g., haemoglobin and packed cell volume decreased,
protein and cholesterol increased, and alkaline phosphatase and
albumin/globulin ratios decreased in treated groups compared to
controls. After the 4-week recovery period, low- and mid-dose values
were comparable to controls. Relative liver, kidney, heart, and brain
weights were significantly increased at all dose levels. Hepatocyte
enlargement was noted at all dose levels. These effects had
disappeared after the 4-week recovery period. In this experiment a
NOEL could not be established (Ginocchio & Brooks, 1981).
In a second 90-day study, groups of Charles River CD rats
(25 males and 25 females per group) were fed diets containing 0, 40,
400, or 4000 ppm clofentezine for 90 days. Five males and 5 females
per group were maintained untreated for a 6-week recovery period. All
animals were observed daily for mortality and clinical signs, while
body weights, food consumption, and water consumption were measured
weekly. Haematology, clinical chemistry, and urinalysis were performed
at 4, 8, and 12 weeks of treatment on 10 rats/sex/group, with
subsequent monitoring of the regression of treatment-related effects.
Organ weights and detailed gross and histopathological examinations
were performed on 20 male and 20 female rats after 90 days of
treatment and on the remaining animals at the end of the 6-week
regression period.
There were no effects on mortality or clinical signs. Body-weight
gain was significantly decreased in females of the 4000 ppm group. A
statistically-significant decrease in the haemoglobin concentration in
the 4000 ppm group was observed after 4 (females only), 8, and
12 weeks of treatment. This decrease was also observed in the 400 ppm
group after 12 weeks. Plasma cholesterol was significantly increased
in both sexes in the high- and mid-dose groups after 4, 8, and
12 weeks of treatment; protein and albumin levels were increased in
the same dose groups at the same times in males. All these effects had
regressed after the recovery period. A dose-related colouration from
orange to red in the urine was observed at all dose levels. Relative
liver, kidney, and spleen weights were significantly increased in both
sexes of the high-dose group and the relative liver weight was also
increased at 400 ppm. Centrilobular hepatocyte enlargement was
observed among male rats receiving 400 and 4000 ppm and among female
rats receiving 4000 ppm clofentezine. There was evidence of slight,
localized hepatocyte enlargement in males at 40 ppm. Retrospective
examination of liver tissue by electron microscopy indicated moderate
proliferation of smooth endoplasmic reticulum in centrilobular
hepatocytes of males and females treated at 4000, but not at 40 ppm,
clofentezine. These changes (but not the increase in liver weight)
were completely reversible during the 6-week recovery period. The
authors concluded that the NOEL in this study was 40 ppm clofentezine
(Brooks & Turnbull, 1983).
Dogs
Beagle dogs (4/sex/group) were fed diets containing 0, 3200,
8000, or 20,000 ppm clofentezine for 90 days. Two dogs were killed for
humane reasons in week 6 of the study (no relation to treatment:
polyarteritis). Except for pink coloration of the faeces and an
increased liver weight in all treated groups, no compound-related
effects were reported on any of the tested parameters measured: body
weight, food and water consumption, haematology, clinical chemistry,
urinalysis, ophthalmoscopy, electrocardiography, organ weights, gross
pathology, or histopathology (Hounsell et al., 1981).
Beagle dogs (6/sex/group) were fed diets containing 0, 50, 1000,
or 20,000 ppm clofentezine for 52 weeks. No mortality occurred. Apart
from reddish-pink coloured faeces in high-dose dogs, no effects of
treatment were observed on clinical signs, body weight, food and water
consumption, ophthalmoscopy, electrocardiography, blood pressure
measurements, or urinalysis. The highest dose was associated with
moderate hepatotoxicity consisting of significantly-increased relative
liver weights, enlargement of periportal hepatocytes with cytoplasmic
eosinophilia, hepatomegaly, and increased plasma cholesterol
triglycerides and alkaline phosphatase. Adrenal and thyroid weights
were also significantly increased in males and females, respectively.
At 1000 ppm clofentezine, minimal hepatotoxicity was observed, with
increased liver weights in females only. The authors concluded that
the NOEL was 50 ppm clofentezine in the diet (equal to 1.72 mg/kg
b.w./day) (Chesterman et al., 1984).
Long term studies
Mice
Groups of 52 male and 52 female CD mice were fed diets containing
0, 50, 500, or 5000 ppm clofentezine for 105 weeks. Observations
included clinical signs, mortality, body weight, food consumption,
haematological determinations (weeks 52 and 104) and organ weights and
comprehensive histopathological examinations at the end of the
experiment.
At 5000 ppm clofentezine, increased mortality attributed to
amyloidosis was observed in females. Growth was decreased during the
first 52 weeks in males at the high-dose level and after 52 weeks
decreases were also found in haemoglobin, haematocrit, and the number
of red blood cells, especially in males. Total white blood cells were
decreased in males after 52 and 105 weeks. Liver weights were
increased in both sexes (significantly in females) at the high-dose
level. Testes weights were significantly increased in males and an
increased incidence of magenta-discoloured forestomach epithelium was
noted in both sexes of the high-dose group. A higher number of benign
liver cell tumours was observed in female mice dosed with 5000 ppm
clofentezine, but the incidence was not significantly above that of
the controls. At 5000 and 500 ppm an increased incidence of focal
eosinophilic/basophilic hepatocytes was observed in females. The
authors concluded that the NOEL was 50 ppm clofentezine (equivalent to
5 mg/kg b.w./day) (Lloyd et al., 1985).
Rats
Groups of 50 male and 50 female CD rats were fed diets containing
0, 10, 40, or 400 ppm clofentezine (technical) for 118 weeks
(27 months). Additional supplementary groups of 20 male and 20 female
rats were fed diets containing clofentezine at the same dietary levels
and sacrificed after 12 months of treatment. Observations included
clinical signs, mortality, body weight, food consumption, water
consumption, haematology, clinical chemistry, urinalysis, ophthal-
moscopy, and assays for thyroid function and sex hormones. At
termination all surviving rats were killed, organs were weighed, and
comprehensive histopathological examinations were made of tissues from
each of these animals as well as from those dying during the study.
The only treatment-related effects found after haematology and
clinical chemistry analyses were decreased haemoglobin in females
after 18 and 27 months and an increase in free T4 in males at
400 ppm. However, other parameters of thyroid function were not
affected. There was no evidence of chronic toxicity in any tissues
other than the liver and the thyroid. Increased relative liver weights
and liver cell hypertrophy, characterized by centrilobular hepatocyte
enlargement and by vacuolisation and fat deposits, were observed at
400 ppm in males (in females in a few cases). There was a slight
increase in the number of follicular cell tumours in the thyroid at
400 ppm in males. This is considered of doubtful biological
significance since a comparable incidence was observed in control
males in a parallel study. The authors concluded that the NOEL was
40 ppm clofentezine in the diet (equivalent to 2 mg/kg b.w./day)
(Ginocchio & Mallyon, 1985).
Observations in humans
Information useful for evaluation is not available.
COMMENTS
Clofentezine is an acaricide with low mammalian toxicity. Its
mode of action is not understood. At high doses clofentezine exerts an
effect on the liver (enzyme induction). Pretreatment with high dietary
levels of clofentezine increases the rate of metabolism. It also
affects thyroid function and impairs steroid hormone regulation.
After oral administration of clofentezine to rats, it was
excreted predominantly in the faeces. Low levels of residues were
found in organs and tissues. The levels in plasma reached a maximum 4
to 6 hours after an oral dose of 10 mg/kg b.w. after which the level
decreased, with a half-life of 2 - 3 hours.
When clofentezine was given to rats during 25 days, a plateau was
reached after 5 - 10 days in various organs, with the highest residues
in the liver. The concentration in the liver after 25 days was 2 - 4
times higher than after 1 day, while fat residues were about 8 times
higher.
The material excreted in rat urine consisted mainly of
metabolites. The two major pathways were hydroxylation of clofentezine
and formation of a monochlorosulfur derivative. In the faeces 50% was
excreted unchanged; the rest was metabolized to more that 20 minor
metabolites.
The metabolic pattern in other species (rabbits, dogs, baboons)
was similar to the rat, with a higher excretion in the faeces than in
the urine. The same metabolites were formed in rats, mice, calves,
dogs, goats, and baboons, with methylthiolation being more prominent
in rodents. In calves and baboons, hydroxylation was more prominent.
Clofentezine has a low acute toxicity in rats, mice, hamsters,
guinea pigs, and dogs.
Clofentezine did not affect fertility or reproductive
performance. Maternal effects (decreased growth, liver enlargement)
were seen in all generations at 400 ppm. There were no indications of
a teratogenic effect in rats or rabbits.
A battery of mutagenicity tests did not indicate mutagenic
potential.
Short-term oral administration to mice, rats, and dogs produced
liver enlargement as the main toxicological effect. In rats, decreased
growth and decreased haemoglobin were observed at high-dose levels, as
well as effects on biochemical parameters. Histopathological liver
changes were in all cases associated with liver enlargement and liver
enzyme induction. In addition, changes in thyroid function and steroid
hormone regulation were observed (tested in special studies with
rats).
In long-term studies the same effects were found as in short-term
studies. In mice a non-significant increase in benign liver tumours
was found in females. In the rat study with relatively low dose levels
a slight increase of thyroid follicular cell tumours was observed.
Neither effect was considered to be an indication of carcinogenicity.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 50 ppm in the diet, equal to 5 mg/kg b.w./day.
Rat: 40 ppm in the diet, equivalent to 2 mg/kg b.w./day.
Dog: 50 ppm in the diet, equal to 1.72 mg/kg b.w./day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Observations in man.
2. Studies on the possible mode of action on the thyroid.
3. Information on possible enterohepatic recirculation.
REFERENCES
Bootman, J. & Rees, R. Technical NC 21314: Investigation of mutagenic
1982 activity in the TK +/- mouse lymphoma cell mutation system.
Unpublished report No. TOX/82/167-38 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Brooks, P.M. & Turnbull, G.J. Technical NC 21314: 90-Day dietary
1983 toxicity study in the rat - additional examination of the
liver histology. Unpublished report No. TOX/83/167-44 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Campbell, J.K. & Needham, D. Excretion and tissue residues of
1981 (14C) NC 21314 in male and female dogs following a single
intravenous dose at 0.1 mg/kg. Unpublished report
No. METAB/81/37 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Challis, I.R. Concentration of (14C) NC 21314 and
1982 its metabolites in the plasma of rats doses orally with NC
21314 at the rate of 10 mg/kg body weight. Unpublished
report No. METAB/82/39 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982a NC 21314 in male and female mice given a single oral dose of
10 mg/kg. Unpublished report No. METAB/82/11 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982b NC 21314 in male and female rabbits following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/21 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982c NC 21314 in male and female dogs following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/6 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-clofentezine and
1985a its metabolites in the plasma of rats given fourteen daily
doses of clofentezine followed by a single oral dose of
(14C)-clofentezine at 14 mg/kg bodyweight. Unpublished
report No. METAB/85/6 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-Clofentezine and
1985b its metabolites in the plasma of rats dosed orally with
clofentezine at the rate of 1000 mg/kg bodyweight.
Unpublished report No. METAB/85/2 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981a radiolabelled residues in male and female rats dosed orally
at 0.1 mg/kg with NC 21314. Unpublished report
No. METAB/81/26 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981b radiolabelled residues following a single intravenous dose
of 0.1 mg/kg (14C)-NC 21314. Unpublished report
No. METAB/81/38 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The distribution and level of
1981c radiolabelled residues in rats following repeat oral dosing
with (14C)-NC 21314 at 20 mg/kg/day. Unpublished report
No. METAB/81/32 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982a radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with NC 21314. Unpublished report No. METAB/82/1
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982b radiolabelled residues in male and female rats dosed orally
at 1000 mg/kg with NC 21314. Unpublished report
No. METAB/82/22 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982c radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with (14C)-NC 21314 following 14 days
pre-treatment by dosing with unlabelled NC 21314 at
10 mg/kg/day. Unpublished report No. METAB/82/37 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Challis, I.R. & Needham, D. The excretion and distribution of
1983 radiolabelled residues in male and female rats dosed
intravenously at 0.1 mg/kg with (14C)-NC 21314.
Unpublished report No. METAB/83/14 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. A comparison of the metabolism of clofentezine in rat,
1985 mouse, rabbit, calf, dog and baboon. Unpublished report
No. METAB/85/9 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Creedy, C.L. The effects of clofentezine on thyroid
1985 function. Unpublished report No. METAB/85/36 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The metabolism of clofentezine in the rat.
1985 Unpublished report No. METAB/85/5 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Chambers, P.R., Sanderson, D.M., & Brooks, P.N. The acute oral
1981 toxicity of technical (pilot plant) NC 21314 to the male and
female beagle dog. Unpublished report No. TOX/80/167-10 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Chesterman, H., Massey, J.E., Heywood, R., Buist, D., Street, A.E.,
1984 Gopinath, C., & Rupanagudi, S. Rao. NC 21314 oral toxicity
study in dogs (final report: repeated dietary administration
for 52 weeks). Unpublished report No. TOX/84/167-68 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire,
England, and FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Cozens, D.D., Perkin, C.J., Barton, S.J., Clark, R., Offer, J.M.,
1983 Gibson, W.A., & Street, A.E. Effect of technical NC 21314 on
pregnancy of the rabbit (teratology study). Unpublished
report No. FBC TOX/83/167-42 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, England and from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Creedy, C.L., Hemmings, P.A., & Needham, D. The effect of clofentezine
1985 on the hepatic mixed-function oxidase system of the male and
female rat following dietary administration at 10, 40, or
400 ppm diet for two weeks. Unpublished report No. METAB/
85/34 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of unformulated
1980 NC 21314 (CR 20099) to the hamster. Unpublished report
No. TOX/80/167-8 from Fisons Ltd, Saffron Walden, Essex,
England. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute oral toxicity
1980a of unformulated (CR 20099/4) to the male & female mouse.
Unpublished report No. TOX/80/167-7 from Fisons Ltd, Saffron
Walden, Essex, England. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The primary skin
1980b irritancy of unformulated NC 21314 (CR 20099/5) to the
guinea pig. Unpublished report No. TOX/80/167-6 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute dermal toxicity
1980c of unformulated NC 21314 (CR 20099/5) to the male and female
rat. Unpublished report No. TOX/80/167-4 from Fisons Ltd,
Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of technical
1981a (pilot plant) NC 21314 to the guinea pig (CR 20099).
Unpublished report No. TOX/81/167-16 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The primary skin irritancy of NC 21314
1981b 50WP to the guinea pig. Unpublished report No. TOX/81/167-13
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. Acute intraperitoneal
1981 toxicity of pilot plant technical NC 21314 to the rat.
Unpublished report No. TOX/80/167-5 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Cuthbert, J.A. & Carr, S.M.A. NC 21314 50 SC formulation primary eye
1983 irritancy study in rabbits. Unpublished report No. TOX/83/
167-46 from Inveresk Research International, Musselburgh,
Scotland, and from FBC, Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Cuthbert, J.A., Carr, S.M.A., & D'Arcy-Burt, K.J. NC 21314 50 SC
1983 formulation primary skin irritancy study in rabbits.
Unpublished report No. TOX/83/167-47 from Inveresk Research
International, Musselburgh, Scotland, and from FBC, Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Brooks, P.N. Technical NC 21314 (pilot plant, CR
1981 20099/5): the 90-day dietary toxicity study in the rat.
Unpublished report No. TOX/81/167-23/1 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Mallyon, B.A. The oncogenicity and chronic toxicity
1985 of technical NC 21314 (clofentezine) in the diet to the rat.
Unpublished report No. TOX/84/167-70 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1981 oral toxicity of technical (CR 20099/8) NC 21314 in the diet
to the dog. Unpublished report No. TOX/81/167-21/1 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Hounsell, I.A. A micronucleus study in mice using technical NC 21314.
1982 Unpublished report No. TOX/82/167-32 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1982 oral toxicity of technical (pilot plant) NC 21314 in the
diet to the mouse. Unpublished report NO. TOX/82/167-33 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Jackson, C.M. Technical NC 21314: Teratogenicity study in the rat
1982 (plus addendum). Unpublished report No. TOX/82/167-34 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. Technical NC 21314: dominant lethal mustation assay in
1983 male rats. Unpublished report No. TOX/83/167-45 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. & Chambers, P.R. Technical clofentezine: a dietary
1984 multigeneration study in the rat. Unpublished report
No. TOX/84/167-66 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Lloyd, G.K., Spencer-Briggs, D.J., Heywood, R., Gopinath, C., &
1985 Cherry, C.P. Technical NC 21314: oncogenicity in the diet to
the mouse (final report). Unpublished report No. TOX/85/
167-80 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, England, and from FBC Ltd. Submitted to WHO
by FBC Ltd., Hauxton, Cambridge, England.
Mallyon, B.A. & Sanderson, D.M. The acute oral toxicity of
1980 unformulated NC 21314 (NC 21314/4, 99% pure) to the male and
female rat. Unpublished report No. TOX/80/167-2 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Mallyon, B.A., Sanderson, D.M., & Brooks, P.N. The acute inhalational
1982 toxicity of NC 21314 80WP CR 15569, to the rat. Unpublished
report No. TOX/81/167-24 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
McConville, M. Ames test for mutagenic activity carried out with
1980 technical NC 21314 (CR 20099/4). Unpublished report
No. TOX/80/167-3 from Inveresk Research International,
Musselburgh, Scotland, and from Fisons Ltd, Saffron Walden,
Essex, England. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. The distribution of radioactivity in the maternal tissues
1981 and foetuses of rats after an oral dose of (14C)-NC 21314.
Unpublished report No. METAB/81/19 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D. The whole-body autography of (14C)-NC 21314 in rats
1982 following oral administration at 10 mg/kg body weight.
Unpublished report No. METAB/82/23 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of eight weeks
1983a dietary administration of NC 21314 at 40 and 27,000 mg/kg on
the hepatic mixed function oxidase system of the male rat.
Unpublished report No. METAB/81/31 (2nd Edn.) from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of a two week
1983b withdrawal period on the induction of hepatic microsomal
mixed function oxidases caused by dietary administration of
NC 21314 at 27,000 mg/kg diet. Unpublished report
No. METAB/82/2 (2nd Edn.) from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Campbell, J., & Challis, I. The effect of eight weeks
1984 dietary administration of NC 21314 at 27,000 and 400 mg/kg
diet on the hepatic mixed-function oxidase system of the
male mouse. Unpublished report No. METAB/82/20 (2nd Edn.)
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. & Challis, I.R. An investigation into the nature of the
1985 residues present in the liver of the rat, goat and calf
following the oral administration of clofentezine.
Unpublished report No. METAB/85/8 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Riach, C.G. & McGregor, D.B. Technical NC 21314: induction of gene
1983 conversion and mitotic recombination in yeast. Unpublished
report No. TOX/83/167-56 from Inveresk Research
International, Musselburgh, Scotland, and from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Saunders, P.C. & Mallyon, B.A. Technical clofentezine: 6 week dietary
1986 investigation of thyroid function in the rat. Unpublished
report No. TOX/85/167-77 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Sortwell, R.J., Richmond, G.P., Kirkpatrick, D., Finn, C.M., & Conway,
1983 B. Excretion and tissue distribution of radioactivity after
oral administration of (14C)-NC 21314 to a male and female
baboon. Unpublished report No. METAB/83/26 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England, and
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Teale, H.J. Delayed dermal sensitisation study in the guinea pig,
1982 NC 21314 technical. Unpublished report No. TOX/82/167-36
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Figure 1. The metabolism of clofentizine in animals.
Metabolites were also identified in a study with baboons.
4-Hydroxy-clofentezine in free and conjugated (beta-glucuronide
conjugate) forms accounted for over 70% of the urinary metabolites.
Other minor metabolites included 3-hydroxy-clofentezine (present as
phenol and glucuronide conjugates) and a mono-chloro sulfur-containing
compound, each accounting for less than 1% of the extractable
radioactivity (Sortwell et al., 1983).
The metabolism of clofentezine in rats, mice, rabbits, calves,
goats, dogs, and baboons was qualitatively similar, with hydroxylation
and replacement of chlorine with a methylthio group being the major
pathways (see Figure 1). In calves and baboons, hydroxylation and
subsequent conjugation were the most important pathways, while
methylthiolation was more prominent in rodents and rabbits (Challis,
1985).
The chromatographic profiles of liver extracts from rats, goats,
and calves following oral administration of 14C-clofentezine were
qualitatively similar to that of rat urine, with conjugates of 3-, 4-,
and 5-hydroxylated clofentezine and a 3-(methylthio-hydroxyphenyl)-6-
(2'-chlorophenyl)-1,2,4,5-tetrazine isomer (Needham & Challis, 1985).
Toxicological studies
Special studies on the endocrine system
After treatment of male rats with 0 or 3% clofentezine for
1 month, a slight decrease in thyroxine (T4) half-life was observed
in dosed rats compared to control animals (a slight increase occurred
over this time period in the controls). A comparison of the effect of
clofentezine treatment (0 and 3% for 4 weeks) in rats and mice (both
sexes) showed that in rats there was a significant and rapid increase
in thyroid uptake of iodine (compared to control animals) following
i.p. dosing with [131I]-sodium iodide. This effect was not observed
in mice (Challis & Creedy, 1985).
Groups of 10 rats/sex were fed diets containing 0, 400, or
30,000 ppm technical clofentezine for 6 weeks. At 30,000 ppm
clofentezine, T4, free T4 index, thyrotropin (TSH), progesterone,
dehydroepiandrosterone, and liver weights were significantly increased
in both sexes. Triiodothyronine (T3) was significantly increased in
males only. Body weights and thyroxine binding capacity were
significantly reduced in females. At 400 ppm clofentezine, T4 and
liver weight were significantly increased in males and dehydroepi-
androsterone was significantly increased in females (Saunders & Mallyon,
1986).
Special studies on enzyme induction
Mice
Groups of 9 - 10 male Charles River CD1 mice received
clofentezine at dietary levels of 0, 40, or 27,000 ppm for 8 weeks
prior to examination of their livers for aniline hydroxylase and
aminopyrine demethylase activities, for levels of cytochromes P-450
and b5, and for microsomal protein concentration. At 27,000 ppm the
levels of cytochrome P-450 and b5 were increased 3-fold. The activity
of aminopyrine demethylase rose by a factor of 2.2 and the mean liver
weights increased by 35%. There was a marginal effect on the enzyme
induction system at 40 ppm (Needham et al., 1984).
Rats
Groups of 6 male Charles River CD rats were fed diets containing
clofentezine at levels of 0, 40, or 27,000 ppm for 8 weeks.
Twenty-four hours after the final dose, their livers were examined for
aniline hydroxylase activity, for levels of cytochromes P-450 and b5,
and for microsomal protein concentration. At 27,000 ppm a 50% increase
in the level of cytochrome P-450 and 2-fold increases in the level of
cytochrome b5 and in the activity of aniline hydroxylase were found.
In a subsequent study it was discovered that the effect could be
reversed after a 2-week withdrawal period. No induction was observed
at 40 ppm (Needham et al., 1983a,b).
In a more extensive study, male and female rats were given diets
with 0, 10, 40, or 400 ppm clofentezine for 2 weeks. At 400 ppm there
was an increase in the level of liver protein (+ 12%), aldrin
epoxidase (22 - 32%), ethoxycoumarin deethylase (20 - 49%), and
cytochrome P-450 (24 - 26%) in both sexes. Liver weights and
cytochrome b5 were increased in males only. At 40 ppm, liver
ethoxycoumarin deethylase was slightly increased in males only
(Creedy et al., 1985).
Special studies on mutagenicity
Clofentezine was negative in various mutagenicity assays
(Table 1).
Table 1: Results of mutagenicity assays on clofentezine
Test Concentration
Test system Object of clofentezine Results Reference
Ames testa Salmonella 10 - 3300 negative McConville,
typhimurium µg/plate 1980
TA98, TA100,
TA1535, TA1537,
and TA1538
Yeast testa,b Saccharomyces 12.5 - 200 negative Riach &
cerevisiae D7 µg/ml McGregor, 1983
Mouse lymphoma Mouse L 5178Y 0 - 128 negative Bootman &
assaya TK +/- cells µg/ml Rees, 1982
Micronucleus Mouse 2 × 0 to negative Hounsell, 1982
assay (bone 3200 mg/kg
marrow) in 0.5%
aqueous gum,
orally
Dominant Rat (males) 0 to 400 negative Jackson, 1983
lethal test mg/kg in
the diet
for 10 weeks
a with and without metabolic activation
b gene conversion and mitotic recombination
Special study on reproduction
Rats
Groups of 6-week old Charles River rats (30 - 40/sex) were fed
diets containing 0, 4, 40, or 400 ppm clofentezine. After 74 days of
treatment the rats were paired for 20 days to start a 2-generation
(2 litters/generation) study. Diets were maintained during mating,
gestation, and lactation. Weanlings from the second litter were
selected to become parents of the next generation. The F2a litters
were grown to maturity.
In the parental generations clinical signs and food consumption
of treated rats were comparable to those of the control rats. Mating
performance, pregnancy rate, fertility, and the duration of gestation
and parturation were unaffected by clofentezine treatment. Parental
toxicity was observed at 400 ppm, which included reduced body-weight
gain of F1- and F2-generation females (maturation) and
F1-generation females (post-coitum and post-partum), as well as
significantly increased liver weights in F1- and F2-generation
males. The increased liver weight was associated with minimal
centrilobular hepatocyte enlargement and a slight reduction in
periportal fat deposition. Toxic effects on litters included
significantly reduced F1a and F2a pup weights and significantly
smaller F2b litter sizes. The only toxic effect at 40 ppm was a
significant increase in liver weights of F2a males. No adverse
effects on fertility or reproductive performance were observed at 4 or
40 ppm clofentezine (Jackson & Chambers, 1984).
Special studies on skin and eye irritation
Application of clofentezine (0.2 ml of 333 mg/ml suspension) to
the intact and abraded skin of 6 guinea pigs showed pink staining and
very slight edema (in 2 out of 12 treated areas), both receding during
observation (Crome et al., 1980b).
A dermal irritation study with 6 guinea pigs was conducted with
clofentezine using the 50% wettable powder formulation (0.2 ml of 6 or
947 mg/ml suspension). Observations were carried out at 24 hours and
then daily for one week. Pink staining and erythema (between moderate
and severe) were observed in all animals treated with 947 mg/ml
suspension, and slight erythema was observed in 3/6 guinea pigs
treated with 6 mg/ml suspension. The erythema regressed in one week.
In 2/6 guinea pigs treated with 947 mg/ml suspension, very slight
erythema was present when the animals were subjected to post-mortem
examination (no abnormalities were observed) (Crome & Sanderson,
1981b).
Treatment of the intact and abraded skin of 6 New Zealand white
rabbits with clofentezine in 50% aqueous suspension produced very
slight edema in 1/6 animals after 24 hours, with full recovery after
72 hours. Erythemal response could not be scored because of the bright
pink color of the test substance (Cuthbert et al., 1983).
Undiluted clofentezine (50% aqueous suspension) caused mild
conjunctival irritation 24 hours after installation; it had
disappeared at 48 hours (Cuthbert & Carr, 1983).
Special study on skin sensitization
Clofentezine had no sensitizing potential in guinea pigs when
tested by the Magnusson & Kligman method (Teale, 1982).
Special studies on teratogenicity
Rats
Groups of 34 - 35 pregnant female Sprague-Dawley CD rats received
0, 320, 1280, or 3200 mg clofentezine/kg b.w. by gavage from days 7 to
20 post-coitum (p.c.). The dams were examined twice daily for
mortality, appearance, and behaviour. Body weights were recorded on
days 1 and 4 and on days 7 to 21 p.c. The dams were killed on day 21
of pregnancy. The number of implantations, resorptions (early and
late), corpora lutea, and uteri were determined, as well as placental
weight, weight and histopathology of the liver of the dams, number of
live and dead fetuses, litter and pup weights, sex ratios, gross
pathology, and histopathology (skeletal and visceral) of the fetuses.
Maternal toxicity was observed at 1280 and 3200 mg/kg b.w. At the
highest dose, body weights were significantly decreased (also when
corrected for uterine content). Dose-related and significantly
increased relative liver weights were observed at all dose levels;
when corrected for the uterine contents, the increase was significant
only at 1280 and 3200 mg/kg b.w. At histopathology, slight
differential staining and enlargement of centrilobular hepatocytes
were seen at 3200 mg/kg b.w. Litter size, litter weight, mean
placental weight, and post-implantation losses were not affected at
any of the treatment levels, although mean fetal weight was increased
at 3200 mg/kg b.w.
Embryonic and fetal development were unaffected by treatment
(Jackson, 1982).
Rabbits
Groups of 14 - 15 pregnant New Zealand white rabbits were
administered daily doses of 0, 250, 1000, or 3000 mg clofentezine/kg
b.w. by gavage on days 7 to 28 of gestation. All animals were killed
on day 29 of pregnancy. The dams were examined for mortality,
appearance, behaviour, body weight, food consumption, serum
cholesterol and serum triglyceride (day 29), weight of uterus, number
of implantations, resorptions (early and late), and corpora lutea. The
fetuses, delivered by caeserean section, were examined for the number
alive and dead, litter and pup weight, gross pathology, and
histopathology (skeletal and visceral).
Maternal body-weight gain was significantly reduced at
3000 mg/kg b.w. (and to a lesser extent at 1000 mg/kg b.w.) initially
during treatment, which was associated with reduced food intake. Mean
litter and fetal weights were significantly reduced at 3000 mg/kg b.w.
No visceral or skeletal anomalies were reported (Cozens et al.,
1983).
Acute toxicity
The acute toxicity of clofentezine to several animal species is
given in Table 2. Signs of clofentezine toxicity in rats after oral
administration were confined to very slight salivation and slight
urinary incontinence. No clinical symptoms after oral administration
were observed in hamsters, mice, guinea pigs, or dogs, except for skin
reddening in dogs at the highest dose.
Short-term studies
Mice
Groups of mice (20 males and 20 females per group) were
administered clofentezine (technical grade) in the diet for 90 days at
dose levels of 0, 200, 1000, or 5000 ppm. All animals were observed
daily for mortality and signs of toxicity while body weights and food
consumption were recorded weekly. Haematology and clinical chemistry
were performed at weeks 4 and 12 on 10 mice/sex/group, and urinalysis
was performed at weeks 4 and 12 on 20 mice/sex/group. At termination
of the study all animals were killed, selected organs were weighed,
and complete gross and histopathological examinations were performed.
There were no effects on mortality, appearance, body weight, food
consumption, or haematology. Significantly increased plasma levels of
triglycerides, calcium, and phosphate were observed in mice in the
5000- and 1000-ppm groups. Urinanalysis investigations revealed red
stellar crystals in high-dose males. A dose-related urinary colour
change from yellow, through orange, to red was observed in female
mice. A treatment-related significant increase in liver weight was
observed in both sexes of the mid- and high-dose groups, which was
associated in males at 5000 ppm with centrilobular hepatocyte
enlargement (5/20). The NOEL in this study was 200 ppm (Hounsell
et al., 1982).
Table 2. Results of acute toxicity assays on clofentezine
LD501 LC502
Species Sex Route (mg/kg b.w.) (mg/l) Reference
Mouse M+F oral > 3200 - Crome et al., 1980a
Rat M+F oral > 3200 - Mallyon &
Sanderson, 1980
Rat M+F dermal > > 1332 - Crome et al., 1980c
Rat M+F i.p. > 800 - Crome et al., 1981
Rat M inhalation - > 9.08 Mallyon et al.,
1981
Hamster M+F oral > 3200 - Crome & Sanderson,
1980
Beagle dog M+F oral > 2000 - Chambers et al.,
1981
Guinea pig F oral > 1500 - Crome & Sanderson,
1981a
1 given as a 33% suspension in 0.5% gum tragacanth.
2 6 hours exposure with 80% wettable powder.
Rats
Groups of Charles River CD rats (20 males and 20 females/group)
were fed diets containing 0, 3000, 9000, or 27,000 ppm clofentezine
for 90 days. Five rats/sex/group were interim-sacrificed after 64 days
and 5 rats/sex/group were maintained untreated for a 28-day recovery
period. All animals were observed daily for mortality and physical
signs. Body weights, food consumption, and water consumption were
recorded weekly. Haematology, clinical chemistry, and urinalysis were
performed at weeks 4 and 12 on 10 rats/sex/group. Cholesterol,
triglycerides, and alanine aminotransferase were also measured after
6 weeks of treatment and at the end of the 4-week recovery period.
Detailed gross and histopathological examinations were performed and
selected organs were weighed.
Dose-related hair loss was observed in 50% and 40% of the animals
of the high- and mid-dose groups, respectively. Six animals died
throughout the study, 2/6 of the 27,000 ppm group, probably because of
the test substance. Body-weight gain was significantly reduced in
males and females of the mid- and high-dose groups. In all dose groups
some haematological and clinical chemistry values changed in a dose-
related manner (e.g., haemoglobin and packed cell volume decreased,
protein and cholesterol increased, and alkaline phosphatase and
albumin/globulin ratios decreased in treated groups compared to
controls. After the 4-week recovery period, low- and mid-dose values
were comparable to controls. Relative liver, kidney, heart, and brain
weights were significantly increased at all dose levels. Hepatocyte
enlargement was noted at all dose levels. These effects had
disappeared after the 4-week recovery period. In this experiment a
NOEL could not be established (Ginocchio & Brooks, 1981).
In a second 90-day study, groups of Charles River CD rats
(25 males and 25 females per group) were fed diets containing 0, 40,
400, or 4000 ppm clofentezine for 90 days. Five males and 5 females
per group were maintained untreated for a 6-week recovery period. All
animals were observed daily for mortality and clinical signs, while
body weights, food consumption, and water consumption were measured
weekly. Haematology, clinical chemistry, and urinalysis were performed
at 4, 8, and 12 weeks of treatment on 10 rats/sex/group, with
subsequent monitoring of the regression of treatment-related effects.
Organ weights and detailed gross and histopathological examinations
were performed on 20 male and 20 female rats after 90 days of
treatment and on the remaining animals at the end of the 6-week
regression period.
There were no effects on mortality or clinical signs. Body-weight
gain was significantly decreased in females of the 4000 ppm group. A
statistically-significant decrease in the haemoglobin concentration in
the 4000 ppm group was observed after 4 (females only), 8, and
12 weeks of treatment. This decrease was also observed in the 400 ppm
group after 12 weeks. Plasma cholesterol was significantly increased
in both sexes in the high- and mid-dose groups after 4, 8, and
12 weeks of treatment; protein and albumin levels were increased in
the same dose groups at the same times in males. All these effects had
regressed after the recovery period. A dose-related colouration from
orange to red in the urine was observed at all dose levels. Relative
liver, kidney, and spleen weights were significantly increased in both
sexes of the high-dose group and the relative liver weight was also
increased at 400 ppm. Centrilobular hepatocyte enlargement was
observed among male rats receiving 400 and 4000 ppm and among female
rats receiving 4000 ppm clofentezine. There was evidence of slight,
localized hepatocyte enlargement in males at 40 ppm. Retrospective
examination of liver tissue by electron microscopy indicated moderate
proliferation of smooth endoplasmic reticulum in centrilobular
hepatocytes of males and females treated at 4000, but not at 40 ppm,
clofentezine. These changes (but not the increase in liver weight)
were completely reversible during the 6-week recovery period. The
authors concluded that the NOEL in this study was 40 ppm clofentezine
(Brooks & Turnbull, 1983).
Dogs
Beagle dogs (4/sex/group) were fed diets containing 0, 3200,
8000, or 20,000 ppm clofentezine for 90 days. Two dogs were killed for
humane reasons in week 6 of the study (no relation to treatment:
polyarteritis). Except for pink coloration of the faeces and an
increased liver weight in all treated groups, no compound-related
effects were reported on any of the tested parameters measured: body
weight, food and water consumption, haematology, clinical chemistry,
urinalysis, ophthalmoscopy, electrocardiography, organ weights, gross
pathology, or histopathology (Hounsell et al., 1981).
Beagle dogs (6/sex/group) were fed diets containing 0, 50, 1000,
or 20,000 ppm clofentezine for 52 weeks. No mortality occurred. Apart
from reddish-pink coloured faeces in high-dose dogs, no effects of
treatment were observed on clinical signs, body weight, food and water
consumption, ophthalmoscopy, electrocardiography, blood pressure
measurements, or urinalysis. The highest dose was associated with
moderate hepatotoxicity consisting of significantly-increased relative
liver weights, enlargement of periportal hepatocytes with cytoplasmic
eosinophilia, hepatomegaly, and increased plasma cholesterol
triglycerides and alkaline phosphatase. Adrenal and thyroid weights
were also significantly increased in males and females, respectively.
At 1000 ppm clofentezine, minimal hepatotoxicity was observed, with
increased liver weights in females only. The authors concluded that
the NOEL was 50 ppm clofentezine in the diet (equal to 1.72 mg/kg
b.w./day) (Chesterman et al., 1984).
Long term studies
Mice
Groups of 52 male and 52 female CD mice were fed diets containing
0, 50, 500, or 5000 ppm clofentezine for 105 weeks. Observations
included clinical signs, mortality, body weight, food consumption,
haematological determinations (weeks 52 and 104) and organ weights and
comprehensive histopathological examinations at the end of the
experiment.
At 5000 ppm clofentezine, increased mortality attributed to
amyloidosis was observed in females. Growth was decreased during the
first 52 weeks in males at the high-dose level and after 52 weeks
decreases were also found in haemoglobin, haematocrit, and the number
of red blood cells, especially in males. Total white blood cells were
decreased in males after 52 and 105 weeks. Liver weights were
increased in both sexes (significantly in females) at the high-dose
level. Testes weights were significantly increased in males and an
increased incidence of magenta-discoloured forestomach epithelium was
noted in both sexes of the high-dose group. A higher number of benign
liver cell tumours was observed in female mice dosed with 5000 ppm
clofentezine, but the incidence was not significantly above that of
the controls. At 5000 and 500 ppm an increased incidence of focal
eosinophilic/basophilic hepatocytes was observed in females. The
authors concluded that the NOEL was 50 ppm clofentezine (equivalent to
5 mg/kg b.w./day) (Lloyd et al., 1985).
Rats
Groups of 50 male and 50 female CD rats were fed diets containing
0, 10, 40, or 400 ppm clofentezine (technical) for 118 weeks
(27 months). Additional supplementary groups of 20 male and 20 female
rats were fed diets containing clofentezine at the same dietary levels
and sacrificed after 12 months of treatment. Observations included
clinical signs, mortality, body weight, food consumption, water
consumption, haematology, clinical chemistry, urinalysis, ophthal-
moscopy, and assays for thyroid function and sex hormones. At
termination all surviving rats were killed, organs were weighed, and
comprehensive histopathological examinations were made of tissues from
each of these animals as well as from those dying during the study.
The only treatment-related effects found after haematology and
clinical chemistry analyses were decreased haemoglobin in females
after 18 and 27 months and an increase in free T4 in males at
400 ppm. However, other parameters of thyroid function were not
affected. There was no evidence of chronic toxicity in any tissues
other than the liver and the thyroid. Increased relative liver weights
and liver cell hypertrophy, characterized by centrilobular hepatocyte
enlargement and by vacuolisation and fat deposits, were observed at
400 ppm in males (in females in a few cases). There was a slight
increase in the number of follicular cell tumours in the thyroid at
400 ppm in males. This is considered of doubtful biological
significance since a comparable incidence was observed in control
males in a parallel study. The authors concluded that the NOEL was
40 ppm clofentezine in the diet (equivalent to 2 mg/kg b.w./day)
(Ginocchio & Mallyon, 1985).
Observations in humans
Information useful for evaluation is not available.
COMMENTS
Clofentezine is an acaricide with low mammalian toxicity. Its
mode of action is not understood. At high doses clofentezine exerts an
effect on the liver (enzyme induction). Pretreatment with high dietary
levels of clofentezine increases the rate of metabolism. It also
affects thyroid function and impairs steroid hormone regulation.
After oral administration of clofentezine to rats, it was
excreted predominantly in the faeces. Low levels of residues were
found in organs and tissues. The levels in plasma reached a maximum 4
to 6 hours after an oral dose of 10 mg/kg b.w. after which the level
decreased, with a half-life of 2 - 3 hours.
When clofentezine was given to rats during 25 days, a plateau was
reached after 5 - 10 days in various organs, with the highest residues
in the liver. The concentration in the liver after 25 days was 2 - 4
times higher than after 1 day, while fat residues were about 8 times
higher.
The material excreted in rat urine consisted mainly of
metabolites. The two major pathways were hydroxylation of clofentezine
and formation of a monochlorosulfur derivative. In the faeces 50% was
excreted unchanged; the rest was metabolized to more that 20 minor
metabolites.
The metabolic pattern in other species (rabbits, dogs, baboons)
was similar to the rat, with a higher excretion in the faeces than in
the urine. The same metabolites were formed in rats, mice, calves,
dogs, goats, and baboons, with methylthiolation being more prominent
in rodents. In calves and baboons, hydroxylation was more prominent.
Clofentezine has a low acute toxicity in rats, mice, hamsters,
guinea pigs, and dogs.
Clofentezine did not affect fertility or reproductive
performance. Maternal effects (decreased growth, liver enlargement)
were seen in all generations at 400 ppm. There were no indications of
a teratogenic effect in rats or rabbits.
A battery of mutagenicity tests did not indicate mutagenic
potential.
Short-term oral administration to mice, rats, and dogs produced
liver enlargement as the main toxicological effect. In rats, decreased
growth and decreased haemoglobin were observed at high-dose levels, as
well as effects on biochemical parameters. Histopathological liver
changes were in all cases associated with liver enlargement and liver
enzyme induction. In addition, changes in thyroid function and steroid
hormone regulation were observed (tested in special studies with
rats).
In long-term studies the same effects were found as in short-term
studies. In mice a non-significant increase in benign liver tumours
was found in females. In the rat study with relatively low dose levels
a slight increase of thyroid follicular cell tumours was observed.
Neither effect was considered to be an indication of carcinogenicity.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 50 ppm in the diet, equal to 5 mg/kg b.w./day.
Rat: 40 ppm in the diet, equivalent to 2 mg/kg b.w./day.
Dog: 50 ppm in the diet, equal to 1.72 mg/kg b.w./day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Observations in man.
2. Studies on the possible mode of action on the thyroid.
3. Information on possible enterohepatic recirculation.
REFERENCES
Bootman, J. & Rees, R. Technical NC 21314: Investigation of mutagenic
1982 activity in the TK +/- mouse lymphoma cell mutation system.
Unpublished report No. TOX/82/167-38 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Brooks, P.M. & Turnbull, G.J. Technical NC 21314: 90-Day dietary
1983 toxicity study in the rat - additional examination of the
liver histology. Unpublished report No. TOX/83/167-44 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Campbell, J.K. & Needham, D. Excretion and tissue residues of
1981 (14C) NC 21314 in male and female dogs following a single
intravenous dose at 0.1 mg/kg. Unpublished report
No. METAB/81/37 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Challis, I.R. Concentration of (14C) NC 21314 and
1982 its metabolites in the plasma of rats doses orally with NC
21314 at the rate of 10 mg/kg body weight. Unpublished
report No. METAB/82/39 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982a NC 21314 in male and female mice given a single oral dose of
10 mg/kg. Unpublished report No. METAB/82/11 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982b NC 21314 in male and female rabbits following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/21 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Excretion and residues of (14C)
1982c NC 21314 in male and female dogs following a single oral
dose of 10 mg/kg. Unpublished report No. METAB/82/6 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-clofentezine and
1985a its metabolites in the plasma of rats given fourteen daily
doses of clofentezine followed by a single oral dose of
(14C)-clofentezine at 14 mg/kg bodyweight. Unpublished
report No. METAB/85/6 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Campbell, J.K. & Needham, D. Concentration of (14C)-Clofentezine and
1985b its metabolites in the plasma of rats dosed orally with
clofentezine at the rate of 1000 mg/kg bodyweight.
Unpublished report No. METAB/85/2 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981a radiolabelled residues in male and female rats dosed orally
at 0.1 mg/kg with NC 21314. Unpublished report
No. METAB/81/26 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1981b radiolabelled residues following a single intravenous dose
of 0.1 mg/kg (14C)-NC 21314. Unpublished report
No. METAB/81/38 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The distribution and level of
1981c radiolabelled residues in rats following repeat oral dosing
with (14C)-NC 21314 at 20 mg/kg/day. Unpublished report
No. METAB/81/32 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982a radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with NC 21314. Unpublished report No. METAB/82/1
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982b radiolabelled residues in male and female rats dosed orally
at 1000 mg/kg with NC 21314. Unpublished report
No. METAB/82/22 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The excretion and distribution of
1982c radiolabelled residues in male and female rats dosed orally
at 10 mg/kg with (14C)-NC 21314 following 14 days
pre-treatment by dosing with unlabelled NC 21314 at
10 mg/kg/day. Unpublished report No. METAB/82/37 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Challis, I.R. & Needham, D. The excretion and distribution of
1983 radiolabelled residues in male and female rats dosed
intravenously at 0.1 mg/kg with (14C)-NC 21314.
Unpublished report No. METAB/83/14 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. A comparison of the metabolism of clofentezine in rat,
1985 mouse, rabbit, calf, dog and baboon. Unpublished report
No. METAB/85/9 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Challis, I.R. & Creedy, C.L. The effects of clofentezine on thyroid
1985 function. Unpublished report No. METAB/85/36 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Challis, I.R. & Needham, D. The metabolism of clofentezine in the rat.
1985 Unpublished report No. METAB/85/5 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Chambers, P.R., Sanderson, D.M., & Brooks, P.N. The acute oral
1981 toxicity of technical (pilot plant) NC 21314 to the male and
female beagle dog. Unpublished report No. TOX/80/167-10 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Chesterman, H., Massey, J.E., Heywood, R., Buist, D., Street, A.E.,
1984 Gopinath, C., & Rupanagudi, S. Rao. NC 21314 oral toxicity
study in dogs (final report: repeated dietary administration
for 52 weeks). Unpublished report No. TOX/84/167-68 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire,
England, and FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Cozens, D.D., Perkin, C.J., Barton, S.J., Clark, R., Offer, J.M.,
1983 Gibson, W.A., & Street, A.E. Effect of technical NC 21314 on
pregnancy of the rabbit (teratology study). Unpublished
report No. FBC TOX/83/167-42 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, England and from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Creedy, C.L., Hemmings, P.A., & Needham, D. The effect of clofentezine
1985 on the hepatic mixed-function oxidase system of the male and
female rat following dietary administration at 10, 40, or
400 ppm diet for two weeks. Unpublished report No. METAB/
85/34 from FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of unformulated
1980 NC 21314 (CR 20099) to the hamster. Unpublished report
No. TOX/80/167-8 from Fisons Ltd, Saffron Walden, Essex,
England. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute oral toxicity
1980a of unformulated (CR 20099/4) to the male & female mouse.
Unpublished report No. TOX/80/167-7 from Fisons Ltd, Saffron
Walden, Essex, England. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The primary skin
1980b irritancy of unformulated NC 21314 (CR 20099/5) to the
guinea pig. Unpublished report No. TOX/80/167-6 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. The acute dermal toxicity
1980c of unformulated NC 21314 (CR 20099/5) to the male and female
rat. Unpublished report No. TOX/80/167-4 from Fisons Ltd,
Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The acute oral toxicity of technical
1981a (pilot plant) NC 21314 to the guinea pig (CR 20099).
Unpublished report No. TOX/81/167-16 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Crome, S.J. & Sanderson, D.M. The primary skin irritancy of NC 21314
1981b 50WP to the guinea pig. Unpublished report No. TOX/81/167-13
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Crome, S.J., Sanderson, D.M., & Brooks, P.N. Acute intraperitoneal
1981 toxicity of pilot plant technical NC 21314 to the rat.
Unpublished report No. TOX/80/167-5 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Cuthbert, J.A. & Carr, S.M.A. NC 21314 50 SC formulation primary eye
1983 irritancy study in rabbits. Unpublished report No. TOX/83/
167-46 from Inveresk Research International, Musselburgh,
Scotland, and from FBC, Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Cuthbert, J.A., Carr, S.M.A., & D'Arcy-Burt, K.J. NC 21314 50 SC
1983 formulation primary skin irritancy study in rabbits.
Unpublished report No. TOX/83/167-47 from Inveresk Research
International, Musselburgh, Scotland, and from FBC, Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Brooks, P.N. Technical NC 21314 (pilot plant, CR
1981 20099/5): the 90-day dietary toxicity study in the rat.
Unpublished report No. TOX/81/167-23/1 from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Ginocchio, A.V. & Mallyon, B.A. The oncogenicity and chronic toxicity
1985 of technical NC 21314 (clofentezine) in the diet to the rat.
Unpublished report No. TOX/84/167-70 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1981 oral toxicity of technical (CR 20099/8) NC 21314 in the diet
to the dog. Unpublished report No. TOX/81/167-21/1 from FBC
Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Hounsell, I.A. A micronucleus study in mice using technical NC 21314.
1982 Unpublished report No. TOX/82/167-32 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Hounsell, I.A., Chambers, P.R., & Brooks, P.N. The 90-day subchronic
1982 oral toxicity of technical (pilot plant) NC 21314 in the
diet to the mouse. Unpublished report NO. TOX/82/167-33 from
FBC Ltd. Submitted to WHO by FBC Ltd.,
Hauxton, Cambridge, England.
Jackson, C.M. Technical NC 21314: Teratogenicity study in the rat
1982 (plus addendum). Unpublished report No. TOX/82/167-34 from
FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. Technical NC 21314: dominant lethal mustation assay in
1983 male rats. Unpublished report No. TOX/83/167-45 from FBC
Ltd. Submitted to WHO by FBC Ltd., Hauxton, Cambridge,
England.
Jackson, C.M. & Chambers, P.R. Technical clofentezine: a dietary
1984 multigeneration study in the rat. Unpublished report
No. TOX/84/167-66 from FBC Ltd. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Lloyd, G.K., Spencer-Briggs, D.J., Heywood, R., Gopinath, C., &
1985 Cherry, C.P. Technical NC 21314: oncogenicity in the diet to
the mouse (final report). Unpublished report No. TOX/85/
167-80 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, England, and from FBC Ltd. Submitted to WHO
by FBC Ltd., Hauxton, Cambridge, England.
Mallyon, B.A. & Sanderson, D.M. The acute oral toxicity of
1980 unformulated NC 21314 (NC 21314/4, 99% pure) to the male and
female rat. Unpublished report No. TOX/80/167-2 from Fisons
Ltd, Saffron Walden, Essex, England. Submitted to WHO by FBC
Ltd., Hauxton, Cambridge, England.
Mallyon, B.A., Sanderson, D.M., & Brooks, P.N. The acute inhalational
1982 toxicity of NC 21314 80WP CR 15569, to the rat. Unpublished
report No. TOX/81/167-24 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
McConville, M. Ames test for mutagenic activity carried out with
1980 technical NC 21314 (CR 20099/4). Unpublished report
No. TOX/80/167-3 from Inveresk Research International,
Musselburgh, Scotland, and from Fisons Ltd, Saffron Walden,
Essex, England. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. The distribution of radioactivity in the maternal tissues
1981 and foetuses of rats after an oral dose of (14C)-NC 21314.
Unpublished report No. METAB/81/19 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D. The whole-body autography of (14C)-NC 21314 in rats
1982 following oral administration at 10 mg/kg body weight.
Unpublished report No. METAB/82/23 from FBC Ltd. Submitted
to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of eight weeks
1983a dietary administration of NC 21314 at 40 and 27,000 mg/kg on
the hepatic mixed function oxidase system of the male rat.
Unpublished report No. METAB/81/31 (2nd Edn.) from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Challis, I., & Campbell, J. The effect of a two week
1983b withdrawal period on the induction of hepatic microsomal
mixed function oxidases caused by dietary administration of
NC 21314 at 27,000 mg/kg diet. Unpublished report
No. METAB/82/2 (2nd Edn.) from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Needham, D., Campbell, J., & Challis, I. The effect of eight weeks
1984 dietary administration of NC 21314 at 27,000 and 400 mg/kg
diet on the hepatic mixed-function oxidase system of the
male mouse. Unpublished report No. METAB/82/20 (2nd Edn.)
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Needham, D. & Challis, I.R. An investigation into the nature of the
1985 residues present in the liver of the rat, goat and calf
following the oral administration of clofentezine.
Unpublished report No. METAB/85/8 from FBC Ltd. Submitted to
WHO by FBC Ltd., Hauxton, Cambridge, England.
Riach, C.G. & McGregor, D.B. Technical NC 21314: induction of gene
1983 conversion and mitotic recombination in yeast. Unpublished
report No. TOX/83/167-56 from Inveresk Research
International, Musselburgh, Scotland, and from FBC Ltd.
Submitted to WHO by FBC Ltd., Hauxton, Cambridge, England.
Saunders, P.C. & Mallyon, B.A. Technical clofentezine: 6 week dietary
1986 investigation of thyroid function in the rat. Unpublished
report No. TOX/85/167-77 from FBC Ltd. Submitted to WHO by
FBC Ltd., Hauxton, Cambridge, England.
Sortwell, R.J., Richmond, G.P., Kirkpatrick, D., Finn, C.M., & Conway,
1983 B. Excretion and tissue distribution of radioactivity after
oral administration of (14C)-NC 21314 to a male and female
baboon. Unpublished report No. METAB/83/26 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, England, and
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
Teale, H.J. Delayed dermal sensitisation study in the guinea pig,
1982 NC 21314 technical. Unpublished report No. TOX/82/167-36
from FBC Ltd. Submitted to WHO by FBC Ltd., Hauxton,
Cambridge, England.
