
FLUCYTHRINATE
EXPLANATION
Flucythrinate is an insecticide related to synthetic pyrethroids.
It was considered for the first time by the present Meeting.
IDENTITY
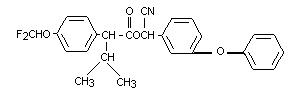
CHEMICAL NAME: (RS)-alpha-cyano-3-phenoxybenzyl-(S)-2-
(IUPAC) [4-(difluoromethoxy)phenyl]-3-methylbutyrate
SYNONYMS: OMS 2007 (WHO); A13-29391 (USDA); AC 222,750; CL
222,705; PAY-OFF(R); FUCHING JUJR(R); CYBOLT(R)
EMPIRICAL FORMULA: C26H23F2NO4
 OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 451.4
PHYSICAL FORM: Technical material is a viscous, dark amber liquid
with a very slight, ester-like odour, 80-88% pure.
VAPOR PRESSURE
(vapor saturation
technique, mm Hg): 8.7 × 10-9 at 25°C
SOLUBILITY
(g/100 ml solvent
at 21°C): Acetone 82
Corn oil 56
Cottonseed oil 30
Hexane 9
Propanol 78
Soybean oil 30
Water 0.00005
Xylene 181
PARTITION COEFFICIENT
(n-octanol/water): 120
HYDROLYSIS RATES
(half-life in days) at: 27°C 35°C
pH3 ca. 40 ca. 40
pH6 52 31
pH9 6.3 2.6
Distilled water 40 29
FORMULATIONS AVAILABLE
COMMERCIALLY: 3, 5, 10, and 30% emulsifiable concentrate;
5% wettable powder
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and metabolism
When C13/C14-alcohol labelled flucythrinate (19.7 mg/kg) was
dosed orally to rats, about 30% of administered radioactivity was
excreted in the urine and 70% in the faeces after 8 days. With
C13/C14-acid labelled compound similar results were obtained, 22% and
73%, respectively. Highest tissue residues were found in adipose
tissue, but these declined with time (Table 1).
Table 1. Adipose tissue residues of flucythrinate (ppm)
24 hr. 192 hr
C13/C14-alcohol label 4.47 1.91
C13/C14-acid label 0.69 0.26
Metabolites were qualitatively identified by thin layer
chromatography and combined gas liquid chromatography/mass
spectrometry. Principal metabolic pathways involved ester cleavage and
oxidation at the para position of the alcohol moiety and at the gem-
dimethyl groups of the acid moiety. The metabolite with the hydroxyl
substitutent in the alcohol moiety was excreted as the sulphate
conjugate in urine. A glycine conjugate was also formed. Alcohol
radiolabelled flucythrinate yielded at least 29 urinary metabolites,
the principal one being 3-(phenoxy)-hippuric acid. With the acid
radiolabel the major urinary metabolite was 2-[4-(difluoromethoxy)
phenyl]-3-methylbutyric acid, accounting for 60% of urinary radio
activity (Zulalian, 1979).
Toxicological studies
Special studies on reproduction
Rat
Groups of 10 male COBS/CD rats (Sprague-Dawley) received
technical flucythrinate (80% pure) at doses of 0, 2, 5, and 10 mg/kg
in corn oil by gavage for 5 consecutive days and then were
subjected to a standard dominant-lethal assay breeding program.
Triethylenemelamine (0.05 mg/kg i.p.) was administered as a positive
control. One male receiving 10 mg/kg flucythrinate died after the 3rd
dose. Treatment with flucythrinate had no effect on pregnancy rate,
number of implantation sites, number of live foetuses or the number of
post implantation deaths. Flucythrinate was found to have no effect in
the dominant-lethal assay (Harnois, 1979).
In a three generation reproduction study, groups of 12 male and
24 female COBS/CD (Sprague-Dawley derived) weanling rats received
technical flucythrinate (80% pure) in the diet at concentrations of 0,
30, 60, or 120 ppm for 10 weeks before mating (2 females/male) and
then continuously. A similar dosing regimen was maintained throughout
two successive generations resulting from the matings of the F1
parents selected from the F1b generation and F2 parents selected
from the F2b generation.
Skin irritation, observed as hair loss, scabbing, and open
lesions, was observed with low incidence in the F1 and F2 parental
high-dose groups. No data were given for the F0 generation. In the
F1 and F2 generations, weight gains were reduced at 60 and 120 ppm
of both males and females for the duration of the study beyond
weaning. There were no treatment-related effects on food consumption,
fertility rate, gestation length or numbers of liveborn pups. Four-day
survival was decreased at 120 ppm for the 1st and 3rd generations in
both litters. Twenty-one day survival was decreased in both high-dose
litters of the 1st generation.
Pup body weights were reduced at 120 ppm and marginally reduced
at 60 ppm for both sexes and in all generations. Alteration of the
21-day sex ratio in favour of males was observed in the F2a litter at
120 ppm. As this effect was not observed in any other litter, its
toxicological significance can be discounted. A slight increase in the
number of stillborn pups per litter was observed for the 1st litter of
each generation at 60 and 120 ppm. Based on the findings of reduced
weight gain and increased number of stillborn pups per litter, the no
effect level established by this study was 30 ppm (Lang, 1981a).
Special studies on teratogenicity
Rat
Groups of 30 mated Charles-River CD rats received technical
flucythrinate (80% pure) in corn oil by gavage at 0, 2, 4, or 8 mg/kg
on days 6-15 (inclusive) of gestation and were maintained without
treatment until sacrificed at day 20.
In an apparently treatment-related manner, 1 dam treated at
4 mg/kg died, while 19 died at 8 mg/kg, but the cause of death was not
determined. Absolute body weights were reduced at 4 and 8 mg/kg as
were body-weight gains. Incomplete recovery of body weight had
occurred by day 20 (termination).
No treatment-related effects were observed for numbers of
corpora lutea or live foetuses per dam, pre- or post-implantation
losses, pregnancy rate, mean foetal body weight, foetal crown-rump
length, or sex ratio.
Increases in the incidence of 14th rudimentary ribs and
incomplete ossification of sternebrae and other bones were observed at
8 mg/kg. The results of this study indicate that daily treatment up to
4 mg/kg was without effect, while daily treatment up to 8 mg/kg, which
produced severe maternal toxicity in the rat, produced no teratogenic
effect (Rodwell et al., 1979).
Rabbit
Groups of twenty female New Zealand white rabbits were
artifically inseminated after induction of ovulation with chorionic
gonadotropin on 2 consecutive days. Technical flucythrinate (80% pure)
was administered in corn oil at 0, 10, 30, or 60 mg/kg daily from days
6-18 (inclusive) of gestation and were maintained without treatment
until sacrificed at day 29.
One animal in the control group died prior to dosing, one rebbit
fed 30 mg/kg died on day 17, and two fed 60 mg/kg died on days 16 and
18. Mortality was probably not compound-related; it was attributed to
peumonia.
Depression of maternal body weight and faecal scouring were
observed in the high-dose (60 mg/kg) group up to day 18. Maternal
weight gain was significantly reduced in the mid- and high-dose groups
and its recovery in the high-dose group was incomplete at day 29
(termination).
At autopsy one "dam" of the low-dose (10 mg/kg) group was found
to be male. Mean uterine weight was decreased for the 60 mg/kg group.
No treatment-related effects were seen for rates of pregnancy,
corpora lutea or viable foetuses per dam, resorptions, sex ratio or
foetal body weight. A slight decrease in the mumber of implants per
dam was observed at 60 mg/kg. Post-implantation losses were decreased
(50%) in all treated groups. The latter effect was not dose-related
and was considered to be not compound-related. Increases in the
incidence of bent hyoid arch and major-vessel variations were found in
the high-dose group (60 mg/kg). No treatment-related terata were found
at any dosage.
The results of this study indicate a no-effect level at 30 mg/kg
based on implantation rates and minor variations and no teratogenic
effects at 60 mg/kg, although treatment at this level caused maternal
toxicity (Janes et al., 1980).
Special studies on mutagenicity
Flucythrinate was without mutagenic activity in a number of
assays with microorganisms and mammalian cells (Table 2).
Acute toxicity
Several acute toxicity studies are available on flucythrinate
(Table 3). Signs of flucythrinate toxicity included decreased
activity, salivation and tremors. Death usually occurred within two
days and recovery among survivors was generally complete by six days.
Table 2. Results of mutagenicity assays on flucythrinate
Test System Test Object Concentrations Reference
used Purity(%) Result
Reverse mutation S. typhimurium* plate 84.8 - Allen, 1979
incorporation
TA1535 0, 0.01, -
TA1537 0.1, 1 mg -
TA98 spot test -
TA100 0, 2 mg
Reverse mutation E coli** spot test 84.8 - Allen, 1979
wp2/uvr A- 0, 1 mg
plate
incorporation
0, 0.01,
0.1, 1 mg
Unscheduled primary-culture 0.001, 0.005, 85.4 - Dulak, 1982
DNA synthesis rat-hepatocytes 0.01, 0.05,
0.1, 1.0 mg/ml
CHO/HGPRT locus Chinese 0, 0.001, 0.01, 85.4 - Johnson &
ribosyl transferase Hamster 0.1, 0.25, Allen, 1982
(Chinese Ovary Cells 0.5, 1, 5 mg/ml
Hamster Ovary/ (CHO-K1
hypoxanthineguanine -BH4)**
phosphoribosyl
transferase
locus mutation
induction)
* + S-9 metabolic activation
** ± S-9 metabolic activation
Table 3. Results of acute toxicity assays on flucythrinate
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse F oral 76 Fischer, 1978a
Rat M oral 81 " "
F oral 67 " "
Rat M,F inhalation LC50 = 65 µg/1 Daly, 1985
Rabbit M dermal > 1000 Fischer, 1978a
F dermal > 1000 " "
Short-term studies
Rat
Groups of CD (Sprague-Dawley derived) rats received technical
flucythrinate (86% pure) in the diet daily for 28 days at 0, 6, 30 ppm
(8 male and 8 female rats per group) or at 150 or 300 ppm (12 male and
12 female rats per group). Some survivors from the 150 and 300 ppm
groups were used to study reversibility of toxicity. Animals receiving
300 ppm exhibited severe hind limb ataxia, diuresis, hypersensitivity
and salivation typical of pyrethroid intoxication. Animals receiving
150 ppm were much less affected, while females generally exhibited
greater sensitivity to flucythrinate than males. Five females of the
300 ppm group died without apparent cause. Absolute weight and weight
gain were markedly depressed for males and females in the groups
receiving 150 and 300 ppm. All symptoms in the two highest-dose groups
were reversed in 48 hours, while the weight loss, relative to
controls, was regained within 4 weeks after cessation of exposure.
Weight loss in the two highest-dosage groups was attributable to
decreased food intake. Plasma urea nitrogen was elevated for females
receiving 300 ppm. Absolute and relative liver weights were elevated
in females receiving 300 ppm. Based on the findings of weight loss, a
no-effect level of 30 ppm was determined (Fischer, 1979).
Groups of 20 male and 20 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% purity) in the diet at 0, 15,
30, 60, or 120 ppm daily for 90 days.
No symptoms or mortality were observed and body weights in males
and females receiving 120 ppm were only marginally depressed. No
compound-related effect was noted on food intake, haematology,
clinical chemistry or urinalysis.
At autopsy, an increase in the total incidence of alopecia was
noted. Females receiving 120 ppm had a slightly higher incidence of
hydronephrosis than controls (4/20 vs 1/20 for 120 and 0 ppm,
respectively). No compound-related effects on organ weights were
found. Histology showed a slight increase in the incidence of skin
disorders as attributable to irritation at 120 ppm. No pathological
basis for the hydronephrosis was found, suggesting that this finding
is without toxicological significance.
Based on decreased body weight, a no-effect level of 60 ppm was
determined (Jefferson & Jessup, 1979).
Groups of 20 male and 20 female SPF (Sprague-Dawley derived) rats
received 0, 30, 60, 120, or 240 ppm technical flucythrinate (85.4%
pure) in the diet for 6 months.
Males and females that received 240 ppm flucythrinate exhibited
symptoms of decreased motor activity and ataxia, characterized by
weakness in the extremities and gait disturbances. One control and 1
male and 4 females of the 240-ppm group died during the study. Males
receiving 240 ppm and females receiving 240 and 120 ppm flucythrinate
exhibited weight loss attributable to decreased food consumption.
Water intake was depressed in males and females receiving 240 ppm.
Leucocyte counts were slightly depressed in males receiving 120 and
240 ppm. Organ weights were in accord with body weights. An increased
incidence of brown pigmentation in the spleen was observed in high-
dose males and females.
Based on the depression in body weight, the no-effect level for
this study was 60 ppm (Shirasu, 1983).
Dog
Groups of 2 male and 2 female Beagle dogs received technical (80%
pure) flucythrinate in the diet at 0, 30, 150, or 300 ppm daily for 14
days. Symptoms were primarily vomiting at 150 and 300 ppm with some
evidence of diarrhoea at the same dosages. After 14 days weight gain
was markedly depressed at 300 ppm due to decreased food consumption.
No other toxicological parameters were studied (Fischer, 1978b).
Groups of 4 male and 4 female Beagle dogs received technical
flucythrinate (80% pure) in the diet at 0, 30, 150, or 300 ppm daily
for 90 days. Emesis occurred in the 150 and 300 ppm dose groups. Body
weight was depressed in both males and females of the high-dose group
and no unscheduled deaths occurred. Weight gain was slightly depressed
at 150 ppm and markedly depressed at 300 ppm, although food
consumption at this dose was not remarkably affected.
Slight anaemia was observed in dogs of each sex receiving
300 ppm, while males receiving this dosage also exhibited a decreased
leucocyte count. Clinical chemistry showed no treatment-related
effects while urinary pH was lower in males and females receiving
300 ppm.
At autopsy only slight changes in organ weights were observed,
with males receiving 300 ppm having slightly lower relative heart
weights than controls. Female heart weight, although depressed, was
consistent with the decreased body weight at 300 ppm.
At autopsy, 2/8 males receiving 300 ppm and 1/8 females receiving
30 ppm flucythrinate were found to have oral papillomas. Only the
latter lesion was confirmed histologically. There were no other
significant histological findings (Mehring & Jessup, 1979).
The results of this study indicate a no-effect level of 30 ppm
based upon reduced weight gain.
Long-term studies
Mouse
Groups of 50 male and 50 female CD-1 mice received technical
flucythrinate (80% pure) in the diet at 0, 30, 60, or 120 ppm daily
for 18 months.
Skin lesions (abrasions, ulceration and scabs) were observed in
high-dose males and females. No treatment-related symptoms or
treatment-related changes in survival were found. No haematology,
clinical chemistry or urinalysis were undertaken.
At necropsy, hepatocellular adenomas were found in all control
and treated groups. The incidence was variable and statistically-
significant only in high-dose males. Hepatocellular adenocarcinoma and
hepatocellular carcinoma were found in low incidence in all male
groups, but only in control and low-dose female mice. The incidences
of these neoplasms were similar to those previously found in mice and
were apparently unrelated to treatment.
Mild sciatic nerve degeneration occurred in all groups, but at
slightly increased incidence in treated groups, especially high-dose
males. There was no apparent dose-response relationship. The incidence
of mild axonal degeneration was similar in all groups. The no-effect
level for this study was therefore set at 30 ppm (Lang, 1981b).
Rat
Groups of 50 male and 50 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% pure) in the diet at 0, 30, 60
or 120 ppm daily for 24 months.
Skin lesions consistent with scratching of the head, neck and
thorax were observed in all mid- and high-dose groups throughout the
study. Mortality was decreased in high-dose males. Terminal body
weights were decreased in treated animals, but especially in high-dose
males and females. This was possibly related to a depression of food
intake. A very mild anaemia was observed in the 3rd months for both
high-dose males and females. Blood glucose was slightly, though
consistently, depressed for high-dose males and females at months 3
and 6. Urinalysis was normal at 3 and 6 months.
At sacrifice, blood urea nitrogen was elevated in mid- and high-
dose males. All other haematology, clinical chemistry and urinalysis
parameters were unchanged by treatment. At autopsy, high-dose females
exhibited an increased incidence of cystic uterus. Absolute and
relative kidney weights were significantly elevated in mid- and high-
dose males while only the relative kidney weight was elevated in high-
dose females. A slight increase in relative heart weight was found in
high-dose males.
No increase in abnormal histopathology or neoplasia was observed
in any group of treated males. The uterine cysts found at autopsy in
the high-dose females were characterized as endometrial cysts. In the
high-dose females, further slight increases in uterine pathology were
described histologically, namely etritis/endometritis, cystic
endometrial hyperplasia and uterine fibrovascular polyps.
Mammary fibroadenomas occurred at similar incidences in all
female groups. The incidence of mammary adenomas in treated females
exceeded that of controls, but not in a dose-related manner. Mammary
adenocarcinomas occurred at higher incidence at 60 and 120 ppm, but
not in a dose-related manner. The latter incidences remained within
the range of historical control data. The variable incidences and lack
of dose-response relationships contraindicate a neoplastic response,
but in view of the observed weight losses, especially in high-dose
groups, these findings cannot be discounted entirely. Based on these
findings, the increased blood urea nitrogen found in males treated at
60 and 120 ppm and slight changes in organ weights, the no-effect
level of this study was set at 30 ppm (Brewer et al., 1981).
Dog
Groups of 6 male and 6 female Beagle dogs received technical
flucythrinate (87.3% pure) in the diet at 0, 30, 100, or 300 ppm daily
for 24 months.
Male dogs receiving 300 ppm flucythrinate in the diet appeared
thin and exhibited alopecia and dermal scaling; one dog died. Food
consumption was reduced in both males and females fed at this level
and there was a marked decrease in the body weights of both sexes.
These changes may have related to increased emesis observed in these
dogs. A statistically-significant but transient anaemia was observed
in high-dose males at month 18. This was attributable to a persistent
but fluctuating anaemia in one of the high-dose male dogs present
throughout the study. This same animal also had depression of serum
calcium and albumin and was later diagnosed at autopsy as one of two
males suffering nematode infestation. Urinalysis and gross pathology
exhibited no treatment-related changes.
At sacrifice, the relative liver, kidney, and pituitary weights
were increased in both high-dose males and females, while increases in
relative spleen, testis and lung weights were noted for high-dose
males only. Upon histological examination, mid- and high-dose groups
exhibited increased evidence of interstitial pneumonia, compared to
controls. As it is likely that the nutritional status of the dogs was
compromised by compound-induced emesis, these findings were considered
not directly attributable to treatment.
The results of this study indicate a no-effect level of 100 ppm
based upon changes in relative organ weights and body weight (Spicer
et al., 1984).
COMMENTS
The principal mechanism of flucythrinate metabolism involves
ester cleavage and oxidation at the para-position of the alcohol
moiety and at the gem-dimethyl group of the acid moiety. Flucythrinate
does not bioaccumulate and the metabolites are mostly excreted in the
urine and do not accumulate in the tissues.
The toxicological profile of flucythrinate is similar to that of
related pyrethroids, although the acute oral toxicity is relatively
high. The production of hepatocellular tumours in the mouse is not
considered to be of biological significance, considering the known
susceptibility of the mouse to this effect.
Flucythrinate is not teratogenic in the rat or rabbit. The
compound was observed to cause mild maternal weight reduction in a
reproduction study, but not at levels causing concern.
Flucythrinate has no dominant lethal effect in rats and was not
mutagenic in several assays.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 30 ppm in the diet, equal to 4.0 mg/kg b.w.
Rat: 30 ppm in the diet, equal to 1.6 mg/kg b.w.
Dog: 100 ppm in the diet, equal to 2.5 mg/kg b.w.
ESTIMATE OF ACCEPTABLE DAILY TAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
DESIRED
1. Further studies on the biological activity of flucythrinate
relevant to the mild nerve demyelination observed in the mouse
and on possible effects on neurotransmitters.
2. Observations in man.
REFERENCES
Allen, J.S. Mutagenicity testing of CL 222,705; (+) Butyric acid, 2-
(1979) (phi-(difluromethoxy)phenyl)-cyano-m-phenoxybenzyl ester, in
the Ames bacterial test. Unpublished report Project No. 0-
796 by the Agricultural Research Division, American Cyanamid
Co. Submitted to WHO by the American Cyanamid Co.,
Princeton, NJ, USA.
Brewer, L., Jefferson, N.D., & Blair, M. 24-Month feeding study in
(1981) rats. Unpublished report No.141-005 from International
Research and Development Corporation, submitted to WHO by
the American Cyanamid Co., Princeton, NJ, USA.
Daly, I.W. An acute inhalation study of AC 222,705 in the rat.
(1985) Unpublished report No. 78-7226 from Bio/dynamics Inc.,
submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Dulak, L. The unscheduled DNA synthesis (UDS) test on CL 222,705 using
(1982) primary rat hepatocytes in culture. Unpublished report No.
81319 by the Experimental Pathology Department, American
Cyanamid Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Toxicity data report. Unpublished report No. A78-88 by
(1978a) the Agricultural Division, American Cyanamid Co., submitted
to WHO by the American Cyanimid Co., Princeton, NJ, USA.
Fischer, J.E. Experiment L-1715: 14 Day feeding to dogs of CL 222,705.
(1978b) Unpublished report No. A78-77 from the American Cyanamid
Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Experiment L-1705: 28-day feeding to rats of CL 222,705.
(1979) Unpublished report No. AX-79-1 by the American Cyanamid Co.,
submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Harnois, M. A dominant lethal test in male rats treated with CL
(1979) 222,705 by gavage for 5 days. Unpublished report No. 79072
by the Medical Research Division, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study with AC
(1980) 222,705 in rabbits. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Jefferson, N.D. & Jessup, D.C. 90-Day feeding study in rats.
(1979) Unpublished report No. 141-003 from International Research
and Development Corporation. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Johnson, E. & Allen, J.S. Mutagenicity testing of CL 222,705 in the
(1982) in vitro CHO/HGPRT mutation assay. Unpublished report No.
0414 by the Discovery Department, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Lang, P. Three generation reproduction study of AC 222,705 to rats.
(1981a) Unpublished report No. 141-008 from International Research
and Development Corporation, submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Lang, P. 18-Month feeding study of AC 222,705 to mice. Unpublished
(1981b) report No. 141-009 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Co., Princeton, NJ, USA.
Mehring, J. & Jessup, D.C. 90-Day feeding study in dogs. Unpublished
(1979) report No. 141-004 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Go., Princeton, NJ, USA.
Rodwell, D.E., Benson, B.W., & Jessup, D.C. Teratology study of AC
(1979) 222,705 in rats. Unpublished report from International
Research and Development corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Shirasu, Y. AC 222,705: 6-month oral sub-chronic toxicity study in
(1983) rats. Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Spicer, E.J.F., Jefferson, N.D., & Blair, M. Chronic dietary toxicity
(1984) study in dogs. Unpublished report No. 141-025 from
International Research and Development Corporation.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Zulalian, J. Pyrethroids, I. A study of the disposition of
(1979) carbon-14 labelled CL 222,705 [butyric acid, 2-(4-
difluoromethoxy)phenyl)-3-methyl-alpha-cyano-m-phenoxybenzyl
ester] in rats. Unpublished report by the American Cyanamid
Co., Agricultural Research Division. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 451.4
PHYSICAL FORM: Technical material is a viscous, dark amber liquid
with a very slight, ester-like odour, 80-88% pure.
VAPOR PRESSURE
(vapor saturation
technique, mm Hg): 8.7 × 10-9 at 25°C
SOLUBILITY
(g/100 ml solvent
at 21°C): Acetone 82
Corn oil 56
Cottonseed oil 30
Hexane 9
Propanol 78
Soybean oil 30
Water 0.00005
Xylene 181
PARTITION COEFFICIENT
(n-octanol/water): 120
HYDROLYSIS RATES
(half-life in days) at: 27°C 35°C
pH3 ca. 40 ca. 40
pH6 52 31
pH9 6.3 2.6
Distilled water 40 29
FORMULATIONS AVAILABLE
COMMERCIALLY: 3, 5, 10, and 30% emulsifiable concentrate;
5% wettable powder
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and metabolism
When C13/C14-alcohol labelled flucythrinate (19.7 mg/kg) was
dosed orally to rats, about 30% of administered radioactivity was
excreted in the urine and 70% in the faeces after 8 days. With
C13/C14-acid labelled compound similar results were obtained, 22% and
73%, respectively. Highest tissue residues were found in adipose
tissue, but these declined with time (Table 1).
Table 1. Adipose tissue residues of flucythrinate (ppm)
24 hr. 192 hr
C13/C14-alcohol label 4.47 1.91
C13/C14-acid label 0.69 0.26
Metabolites were qualitatively identified by thin layer
chromatography and combined gas liquid chromatography/mass
spectrometry. Principal metabolic pathways involved ester cleavage and
oxidation at the para position of the alcohol moiety and at the gem-
dimethyl groups of the acid moiety. The metabolite with the hydroxyl
substitutent in the alcohol moiety was excreted as the sulphate
conjugate in urine. A glycine conjugate was also formed. Alcohol
radiolabelled flucythrinate yielded at least 29 urinary metabolites,
the principal one being 3-(phenoxy)-hippuric acid. With the acid
radiolabel the major urinary metabolite was 2-[4-(difluoromethoxy)
phenyl]-3-methylbutyric acid, accounting for 60% of urinary radio
activity (Zulalian, 1979).
Toxicological studies
Special studies on reproduction
Rat
Groups of 10 male COBS/CD rats (Sprague-Dawley) received
technical flucythrinate (80% pure) at doses of 0, 2, 5, and 10 mg/kg
in corn oil by gavage for 5 consecutive days and then were
subjected to a standard dominant-lethal assay breeding program.
Triethylenemelamine (0.05 mg/kg i.p.) was administered as a positive
control. One male receiving 10 mg/kg flucythrinate died after the 3rd
dose. Treatment with flucythrinate had no effect on pregnancy rate,
number of implantation sites, number of live foetuses or the number of
post implantation deaths. Flucythrinate was found to have no effect in
the dominant-lethal assay (Harnois, 1979).
In a three generation reproduction study, groups of 12 male and
24 female COBS/CD (Sprague-Dawley derived) weanling rats received
technical flucythrinate (80% pure) in the diet at concentrations of 0,
30, 60, or 120 ppm for 10 weeks before mating (2 females/male) and
then continuously. A similar dosing regimen was maintained throughout
two successive generations resulting from the matings of the F1
parents selected from the F1b generation and F2 parents selected
from the F2b generation.
Skin irritation, observed as hair loss, scabbing, and open
lesions, was observed with low incidence in the F1 and F2 parental
high-dose groups. No data were given for the F0 generation. In the
F1 and F2 generations, weight gains were reduced at 60 and 120 ppm
of both males and females for the duration of the study beyond
weaning. There were no treatment-related effects on food consumption,
fertility rate, gestation length or numbers of liveborn pups. Four-day
survival was decreased at 120 ppm for the 1st and 3rd generations in
both litters. Twenty-one day survival was decreased in both high-dose
litters of the 1st generation.
Pup body weights were reduced at 120 ppm and marginally reduced
at 60 ppm for both sexes and in all generations. Alteration of the
21-day sex ratio in favour of males was observed in the F2a litter at
120 ppm. As this effect was not observed in any other litter, its
toxicological significance can be discounted. A slight increase in the
number of stillborn pups per litter was observed for the 1st litter of
each generation at 60 and 120 ppm. Based on the findings of reduced
weight gain and increased number of stillborn pups per litter, the no
effect level established by this study was 30 ppm (Lang, 1981a).
Special studies on teratogenicity
Rat
Groups of 30 mated Charles-River CD rats received technical
flucythrinate (80% pure) in corn oil by gavage at 0, 2, 4, or 8 mg/kg
on days 6-15 (inclusive) of gestation and were maintained without
treatment until sacrificed at day 20.
In an apparently treatment-related manner, 1 dam treated at
4 mg/kg died, while 19 died at 8 mg/kg, but the cause of death was not
determined. Absolute body weights were reduced at 4 and 8 mg/kg as
were body-weight gains. Incomplete recovery of body weight had
occurred by day 20 (termination).
No treatment-related effects were observed for numbers of
corpora lutea or live foetuses per dam, pre- or post-implantation
losses, pregnancy rate, mean foetal body weight, foetal crown-rump
length, or sex ratio.
Increases in the incidence of 14th rudimentary ribs and
incomplete ossification of sternebrae and other bones were observed at
8 mg/kg. The results of this study indicate that daily treatment up to
4 mg/kg was without effect, while daily treatment up to 8 mg/kg, which
produced severe maternal toxicity in the rat, produced no teratogenic
effect (Rodwell et al., 1979).
Rabbit
Groups of twenty female New Zealand white rabbits were
artifically inseminated after induction of ovulation with chorionic
gonadotropin on 2 consecutive days. Technical flucythrinate (80% pure)
was administered in corn oil at 0, 10, 30, or 60 mg/kg daily from days
6-18 (inclusive) of gestation and were maintained without treatment
until sacrificed at day 29.
One animal in the control group died prior to dosing, one rebbit
fed 30 mg/kg died on day 17, and two fed 60 mg/kg died on days 16 and
18. Mortality was probably not compound-related; it was attributed to
peumonia.
Depression of maternal body weight and faecal scouring were
observed in the high-dose (60 mg/kg) group up to day 18. Maternal
weight gain was significantly reduced in the mid- and high-dose groups
and its recovery in the high-dose group was incomplete at day 29
(termination).
At autopsy one "dam" of the low-dose (10 mg/kg) group was found
to be male. Mean uterine weight was decreased for the 60 mg/kg group.
No treatment-related effects were seen for rates of pregnancy,
corpora lutea or viable foetuses per dam, resorptions, sex ratio or
foetal body weight. A slight decrease in the mumber of implants per
dam was observed at 60 mg/kg. Post-implantation losses were decreased
(50%) in all treated groups. The latter effect was not dose-related
and was considered to be not compound-related. Increases in the
incidence of bent hyoid arch and major-vessel variations were found in
the high-dose group (60 mg/kg). No treatment-related terata were found
at any dosage.
The results of this study indicate a no-effect level at 30 mg/kg
based on implantation rates and minor variations and no teratogenic
effects at 60 mg/kg, although treatment at this level caused maternal
toxicity (Janes et al., 1980).
Special studies on mutagenicity
Flucythrinate was without mutagenic activity in a number of
assays with microorganisms and mammalian cells (Table 2).
Acute toxicity
Several acute toxicity studies are available on flucythrinate
(Table 3). Signs of flucythrinate toxicity included decreased
activity, salivation and tremors. Death usually occurred within two
days and recovery among survivors was generally complete by six days.
Table 2. Results of mutagenicity assays on flucythrinate
Test System Test Object Concentrations Reference
used Purity(%) Result
Reverse mutation S. typhimurium* plate 84.8 - Allen, 1979
incorporation
TA1535 0, 0.01, -
TA1537 0.1, 1 mg -
TA98 spot test -
TA100 0, 2 mg
Reverse mutation E coli** spot test 84.8 - Allen, 1979
wp2/uvr A- 0, 1 mg
plate
incorporation
0, 0.01,
0.1, 1 mg
Unscheduled primary-culture 0.001, 0.005, 85.4 - Dulak, 1982
DNA synthesis rat-hepatocytes 0.01, 0.05,
0.1, 1.0 mg/ml
CHO/HGPRT locus Chinese 0, 0.001, 0.01, 85.4 - Johnson &
ribosyl transferase Hamster 0.1, 0.25, Allen, 1982
(Chinese Ovary Cells 0.5, 1, 5 mg/ml
Hamster Ovary/ (CHO-K1
hypoxanthineguanine -BH4)**
phosphoribosyl
transferase
locus mutation
induction)
* + S-9 metabolic activation
** ± S-9 metabolic activation
Table 3. Results of acute toxicity assays on flucythrinate
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse F oral 76 Fischer, 1978a
Rat M oral 81 " "
F oral 67 " "
Rat M,F inhalation LC50 = 65 µg/1 Daly, 1985
Rabbit M dermal > 1000 Fischer, 1978a
F dermal > 1000 " "
Short-term studies
Rat
Groups of CD (Sprague-Dawley derived) rats received technical
flucythrinate (86% pure) in the diet daily for 28 days at 0, 6, 30 ppm
(8 male and 8 female rats per group) or at 150 or 300 ppm (12 male and
12 female rats per group). Some survivors from the 150 and 300 ppm
groups were used to study reversibility of toxicity. Animals receiving
300 ppm exhibited severe hind limb ataxia, diuresis, hypersensitivity
and salivation typical of pyrethroid intoxication. Animals receiving
150 ppm were much less affected, while females generally exhibited
greater sensitivity to flucythrinate than males. Five females of the
300 ppm group died without apparent cause. Absolute weight and weight
gain were markedly depressed for males and females in the groups
receiving 150 and 300 ppm. All symptoms in the two highest-dose groups
were reversed in 48 hours, while the weight loss, relative to
controls, was regained within 4 weeks after cessation of exposure.
Weight loss in the two highest-dosage groups was attributable to
decreased food intake. Plasma urea nitrogen was elevated for females
receiving 300 ppm. Absolute and relative liver weights were elevated
in females receiving 300 ppm. Based on the findings of weight loss, a
no-effect level of 30 ppm was determined (Fischer, 1979).
Groups of 20 male and 20 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% purity) in the diet at 0, 15,
30, 60, or 120 ppm daily for 90 days.
No symptoms or mortality were observed and body weights in males
and females receiving 120 ppm were only marginally depressed. No
compound-related effect was noted on food intake, haematology,
clinical chemistry or urinalysis.
At autopsy, an increase in the total incidence of alopecia was
noted. Females receiving 120 ppm had a slightly higher incidence of
hydronephrosis than controls (4/20 vs 1/20 for 120 and 0 ppm,
respectively). No compound-related effects on organ weights were
found. Histology showed a slight increase in the incidence of skin
disorders as attributable to irritation at 120 ppm. No pathological
basis for the hydronephrosis was found, suggesting that this finding
is without toxicological significance.
Based on decreased body weight, a no-effect level of 60 ppm was
determined (Jefferson & Jessup, 1979).
Groups of 20 male and 20 female SPF (Sprague-Dawley derived) rats
received 0, 30, 60, 120, or 240 ppm technical flucythrinate (85.4%
pure) in the diet for 6 months.
Males and females that received 240 ppm flucythrinate exhibited
symptoms of decreased motor activity and ataxia, characterized by
weakness in the extremities and gait disturbances. One control and 1
male and 4 females of the 240-ppm group died during the study. Males
receiving 240 ppm and females receiving 240 and 120 ppm flucythrinate
exhibited weight loss attributable to decreased food consumption.
Water intake was depressed in males and females receiving 240 ppm.
Leucocyte counts were slightly depressed in males receiving 120 and
240 ppm. Organ weights were in accord with body weights. An increased
incidence of brown pigmentation in the spleen was observed in high-
dose males and females.
Based on the depression in body weight, the no-effect level for
this study was 60 ppm (Shirasu, 1983).
Dog
Groups of 2 male and 2 female Beagle dogs received technical (80%
pure) flucythrinate in the diet at 0, 30, 150, or 300 ppm daily for 14
days. Symptoms were primarily vomiting at 150 and 300 ppm with some
evidence of diarrhoea at the same dosages. After 14 days weight gain
was markedly depressed at 300 ppm due to decreased food consumption.
No other toxicological parameters were studied (Fischer, 1978b).
Groups of 4 male and 4 female Beagle dogs received technical
flucythrinate (80% pure) in the diet at 0, 30, 150, or 300 ppm daily
for 90 days. Emesis occurred in the 150 and 300 ppm dose groups. Body
weight was depressed in both males and females of the high-dose group
and no unscheduled deaths occurred. Weight gain was slightly depressed
at 150 ppm and markedly depressed at 300 ppm, although food
consumption at this dose was not remarkably affected.
Slight anaemia was observed in dogs of each sex receiving
300 ppm, while males receiving this dosage also exhibited a decreased
leucocyte count. Clinical chemistry showed no treatment-related
effects while urinary pH was lower in males and females receiving
300 ppm.
At autopsy only slight changes in organ weights were observed,
with males receiving 300 ppm having slightly lower relative heart
weights than controls. Female heart weight, although depressed, was
consistent with the decreased body weight at 300 ppm.
At autopsy, 2/8 males receiving 300 ppm and 1/8 females receiving
30 ppm flucythrinate were found to have oral papillomas. Only the
latter lesion was confirmed histologically. There were no other
significant histological findings (Mehring & Jessup, 1979).
The results of this study indicate a no-effect level of 30 ppm
based upon reduced weight gain.
Long-term studies
Mouse
Groups of 50 male and 50 female CD-1 mice received technical
flucythrinate (80% pure) in the diet at 0, 30, 60, or 120 ppm daily
for 18 months.
Skin lesions (abrasions, ulceration and scabs) were observed in
high-dose males and females. No treatment-related symptoms or
treatment-related changes in survival were found. No haematology,
clinical chemistry or urinalysis were undertaken.
At necropsy, hepatocellular adenomas were found in all control
and treated groups. The incidence was variable and statistically-
significant only in high-dose males. Hepatocellular adenocarcinoma and
hepatocellular carcinoma were found in low incidence in all male
groups, but only in control and low-dose female mice. The incidences
of these neoplasms were similar to those previously found in mice and
were apparently unrelated to treatment.
Mild sciatic nerve degeneration occurred in all groups, but at
slightly increased incidence in treated groups, especially high-dose
males. There was no apparent dose-response relationship. The incidence
of mild axonal degeneration was similar in all groups. The no-effect
level for this study was therefore set at 30 ppm (Lang, 1981b).
Rat
Groups of 50 male and 50 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% pure) in the diet at 0, 30, 60
or 120 ppm daily for 24 months.
Skin lesions consistent with scratching of the head, neck and
thorax were observed in all mid- and high-dose groups throughout the
study. Mortality was decreased in high-dose males. Terminal body
weights were decreased in treated animals, but especially in high-dose
males and females. This was possibly related to a depression of food
intake. A very mild anaemia was observed in the 3rd months for both
high-dose males and females. Blood glucose was slightly, though
consistently, depressed for high-dose males and females at months 3
and 6. Urinalysis was normal at 3 and 6 months.
At sacrifice, blood urea nitrogen was elevated in mid- and high-
dose males. All other haematology, clinical chemistry and urinalysis
parameters were unchanged by treatment. At autopsy, high-dose females
exhibited an increased incidence of cystic uterus. Absolute and
relative kidney weights were significantly elevated in mid- and high-
dose males while only the relative kidney weight was elevated in high-
dose females. A slight increase in relative heart weight was found in
high-dose males.
No increase in abnormal histopathology or neoplasia was observed
in any group of treated males. The uterine cysts found at autopsy in
the high-dose females were characterized as endometrial cysts. In the
high-dose females, further slight increases in uterine pathology were
described histologically, namely etritis/endometritis, cystic
endometrial hyperplasia and uterine fibrovascular polyps.
Mammary fibroadenomas occurred at similar incidences in all
female groups. The incidence of mammary adenomas in treated females
exceeded that of controls, but not in a dose-related manner. Mammary
adenocarcinomas occurred at higher incidence at 60 and 120 ppm, but
not in a dose-related manner. The latter incidences remained within
the range of historical control data. The variable incidences and lack
of dose-response relationships contraindicate a neoplastic response,
but in view of the observed weight losses, especially in high-dose
groups, these findings cannot be discounted entirely. Based on these
findings, the increased blood urea nitrogen found in males treated at
60 and 120 ppm and slight changes in organ weights, the no-effect
level of this study was set at 30 ppm (Brewer et al., 1981).
Dog
Groups of 6 male and 6 female Beagle dogs received technical
flucythrinate (87.3% pure) in the diet at 0, 30, 100, or 300 ppm daily
for 24 months.
Male dogs receiving 300 ppm flucythrinate in the diet appeared
thin and exhibited alopecia and dermal scaling; one dog died. Food
consumption was reduced in both males and females fed at this level
and there was a marked decrease in the body weights of both sexes.
These changes may have related to increased emesis observed in these
dogs. A statistically-significant but transient anaemia was observed
in high-dose males at month 18. This was attributable to a persistent
but fluctuating anaemia in one of the high-dose male dogs present
throughout the study. This same animal also had depression of serum
calcium and albumin and was later diagnosed at autopsy as one of two
males suffering nematode infestation. Urinalysis and gross pathology
exhibited no treatment-related changes.
At sacrifice, the relative liver, kidney, and pituitary weights
were increased in both high-dose males and females, while increases in
relative spleen, testis and lung weights were noted for high-dose
males only. Upon histological examination, mid- and high-dose groups
exhibited increased evidence of interstitial pneumonia, compared to
controls. As it is likely that the nutritional status of the dogs was
compromised by compound-induced emesis, these findings were considered
not directly attributable to treatment.
The results of this study indicate a no-effect level of 100 ppm
based upon changes in relative organ weights and body weight (Spicer
et al., 1984).
COMMENTS
The principal mechanism of flucythrinate metabolism involves
ester cleavage and oxidation at the para-position of the alcohol
moiety and at the gem-dimethyl group of the acid moiety. Flucythrinate
does not bioaccumulate and the metabolites are mostly excreted in the
urine and do not accumulate in the tissues.
The toxicological profile of flucythrinate is similar to that of
related pyrethroids, although the acute oral toxicity is relatively
high. The production of hepatocellular tumours in the mouse is not
considered to be of biological significance, considering the known
susceptibility of the mouse to this effect.
Flucythrinate is not teratogenic in the rat or rabbit. The
compound was observed to cause mild maternal weight reduction in a
reproduction study, but not at levels causing concern.
Flucythrinate has no dominant lethal effect in rats and was not
mutagenic in several assays.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 30 ppm in the diet, equal to 4.0 mg/kg b.w.
Rat: 30 ppm in the diet, equal to 1.6 mg/kg b.w.
Dog: 100 ppm in the diet, equal to 2.5 mg/kg b.w.
ESTIMATE OF ACCEPTABLE DAILY TAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
DESIRED
1. Further studies on the biological activity of flucythrinate
relevant to the mild nerve demyelination observed in the mouse
and on possible effects on neurotransmitters.
2. Observations in man.
REFERENCES
Allen, J.S. Mutagenicity testing of CL 222,705; (+) Butyric acid, 2-
(1979) (phi-(difluromethoxy)phenyl)-cyano-m-phenoxybenzyl ester, in
the Ames bacterial test. Unpublished report Project No. 0-
796 by the Agricultural Research Division, American Cyanamid
Co. Submitted to WHO by the American Cyanamid Co.,
Princeton, NJ, USA.
Brewer, L., Jefferson, N.D., & Blair, M. 24-Month feeding study in
(1981) rats. Unpublished report No.141-005 from International
Research and Development Corporation, submitted to WHO by
the American Cyanamid Co., Princeton, NJ, USA.
Daly, I.W. An acute inhalation study of AC 222,705 in the rat.
(1985) Unpublished report No. 78-7226 from Bio/dynamics Inc.,
submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Dulak, L. The unscheduled DNA synthesis (UDS) test on CL 222,705 using
(1982) primary rat hepatocytes in culture. Unpublished report No.
81319 by the Experimental Pathology Department, American
Cyanamid Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Toxicity data report. Unpublished report No. A78-88 by
(1978a) the Agricultural Division, American Cyanamid Co., submitted
to WHO by the American Cyanimid Co., Princeton, NJ, USA.
Fischer, J.E. Experiment L-1715: 14 Day feeding to dogs of CL 222,705.
(1978b) Unpublished report No. A78-77 from the American Cyanamid
Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Experiment L-1705: 28-day feeding to rats of CL 222,705.
(1979) Unpublished report No. AX-79-1 by the American Cyanamid Co.,
submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Harnois, M. A dominant lethal test in male rats treated with CL
(1979) 222,705 by gavage for 5 days. Unpublished report No. 79072
by the Medical Research Division, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study with AC
(1980) 222,705 in rabbits. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Jefferson, N.D. & Jessup, D.C. 90-Day feeding study in rats.
(1979) Unpublished report No. 141-003 from International Research
and Development Corporation. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Johnson, E. & Allen, J.S. Mutagenicity testing of CL 222,705 in the
(1982) in vitro CHO/HGPRT mutation assay. Unpublished report No.
0414 by the Discovery Department, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Lang, P. Three generation reproduction study of AC 222,705 to rats.
(1981a) Unpublished report No. 141-008 from International Research
and Development Corporation, submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Lang, P. 18-Month feeding study of AC 222,705 to mice. Unpublished
(1981b) report No. 141-009 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Co., Princeton, NJ, USA.
Mehring, J. & Jessup, D.C. 90-Day feeding study in dogs. Unpublished
(1979) report No. 141-004 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Go., Princeton, NJ, USA.
Rodwell, D.E., Benson, B.W., & Jessup, D.C. Teratology study of AC
(1979) 222,705 in rats. Unpublished report from International
Research and Development corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Shirasu, Y. AC 222,705: 6-month oral sub-chronic toxicity study in
(1983) rats. Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Spicer, E.J.F., Jefferson, N.D., & Blair, M. Chronic dietary toxicity
(1984) study in dogs. Unpublished report No. 141-025 from
International Research and Development Corporation.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Zulalian, J. Pyrethroids, I. A study of the disposition of
(1979) carbon-14 labelled CL 222,705 [butyric acid, 2-(4-
difluoromethoxy)phenyl)-3-methyl-alpha-cyano-m-phenoxybenzyl
ester] in rats. Unpublished report by the American Cyanamid
Co., Agricultural Research Division. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
 OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 451.4
PHYSICAL FORM: Technical material is a viscous, dark amber liquid
with a very slight, ester-like odour, 80-88% pure.
VAPOR PRESSURE
(vapor saturation
technique, mm Hg): 8.7 × 10-9 at 25°C
SOLUBILITY
(g/100 ml solvent
at 21°C): Acetone 82
Corn oil 56
Cottonseed oil 30
Hexane 9
Propanol 78
Soybean oil 30
Water 0.00005
Xylene 181
PARTITION COEFFICIENT
(n-octanol/water): 120
HYDROLYSIS RATES
(half-life in days) at: 27°C 35°C
pH3 ca. 40 ca. 40
pH6 52 31
pH9 6.3 2.6
Distilled water 40 29
FORMULATIONS AVAILABLE
COMMERCIALLY: 3, 5, 10, and 30% emulsifiable concentrate;
5% wettable powder
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and metabolism
When C13/C14-alcohol labelled flucythrinate (19.7 mg/kg) was
dosed orally to rats, about 30% of administered radioactivity was
excreted in the urine and 70% in the faeces after 8 days. With
C13/C14-acid labelled compound similar results were obtained, 22% and
73%, respectively. Highest tissue residues were found in adipose
tissue, but these declined with time (Table 1).
Table 1. Adipose tissue residues of flucythrinate (ppm)
24 hr. 192 hr
C13/C14-alcohol label 4.47 1.91
C13/C14-acid label 0.69 0.26
Metabolites were qualitatively identified by thin layer
chromatography and combined gas liquid chromatography/mass
spectrometry. Principal metabolic pathways involved ester cleavage and
oxidation at the para position of the alcohol moiety and at the gem-
dimethyl groups of the acid moiety. The metabolite with the hydroxyl
substitutent in the alcohol moiety was excreted as the sulphate
conjugate in urine. A glycine conjugate was also formed. Alcohol
radiolabelled flucythrinate yielded at least 29 urinary metabolites,
the principal one being 3-(phenoxy)-hippuric acid. With the acid
radiolabel the major urinary metabolite was 2-[4-(difluoromethoxy)
phenyl]-3-methylbutyric acid, accounting for 60% of urinary radio
activity (Zulalian, 1979).
Toxicological studies
Special studies on reproduction
Rat
Groups of 10 male COBS/CD rats (Sprague-Dawley) received
technical flucythrinate (80% pure) at doses of 0, 2, 5, and 10 mg/kg
in corn oil by gavage for 5 consecutive days and then were
subjected to a standard dominant-lethal assay breeding program.
Triethylenemelamine (0.05 mg/kg i.p.) was administered as a positive
control. One male receiving 10 mg/kg flucythrinate died after the 3rd
dose. Treatment with flucythrinate had no effect on pregnancy rate,
number of implantation sites, number of live foetuses or the number of
post implantation deaths. Flucythrinate was found to have no effect in
the dominant-lethal assay (Harnois, 1979).
In a three generation reproduction study, groups of 12 male and
24 female COBS/CD (Sprague-Dawley derived) weanling rats received
technical flucythrinate (80% pure) in the diet at concentrations of 0,
30, 60, or 120 ppm for 10 weeks before mating (2 females/male) and
then continuously. A similar dosing regimen was maintained throughout
two successive generations resulting from the matings of the F1
parents selected from the F1b generation and F2 parents selected
from the F2b generation.
Skin irritation, observed as hair loss, scabbing, and open
lesions, was observed with low incidence in the F1 and F2 parental
high-dose groups. No data were given for the F0 generation. In the
F1 and F2 generations, weight gains were reduced at 60 and 120 ppm
of both males and females for the duration of the study beyond
weaning. There were no treatment-related effects on food consumption,
fertility rate, gestation length or numbers of liveborn pups. Four-day
survival was decreased at 120 ppm for the 1st and 3rd generations in
both litters. Twenty-one day survival was decreased in both high-dose
litters of the 1st generation.
Pup body weights were reduced at 120 ppm and marginally reduced
at 60 ppm for both sexes and in all generations. Alteration of the
21-day sex ratio in favour of males was observed in the F2a litter at
120 ppm. As this effect was not observed in any other litter, its
toxicological significance can be discounted. A slight increase in the
number of stillborn pups per litter was observed for the 1st litter of
each generation at 60 and 120 ppm. Based on the findings of reduced
weight gain and increased number of stillborn pups per litter, the no
effect level established by this study was 30 ppm (Lang, 1981a).
Special studies on teratogenicity
Rat
Groups of 30 mated Charles-River CD rats received technical
flucythrinate (80% pure) in corn oil by gavage at 0, 2, 4, or 8 mg/kg
on days 6-15 (inclusive) of gestation and were maintained without
treatment until sacrificed at day 20.
In an apparently treatment-related manner, 1 dam treated at
4 mg/kg died, while 19 died at 8 mg/kg, but the cause of death was not
determined. Absolute body weights were reduced at 4 and 8 mg/kg as
were body-weight gains. Incomplete recovery of body weight had
occurred by day 20 (termination).
No treatment-related effects were observed for numbers of
corpora lutea or live foetuses per dam, pre- or post-implantation
losses, pregnancy rate, mean foetal body weight, foetal crown-rump
length, or sex ratio.
Increases in the incidence of 14th rudimentary ribs and
incomplete ossification of sternebrae and other bones were observed at
8 mg/kg. The results of this study indicate that daily treatment up to
4 mg/kg was without effect, while daily treatment up to 8 mg/kg, which
produced severe maternal toxicity in the rat, produced no teratogenic
effect (Rodwell et al., 1979).
Rabbit
Groups of twenty female New Zealand white rabbits were
artifically inseminated after induction of ovulation with chorionic
gonadotropin on 2 consecutive days. Technical flucythrinate (80% pure)
was administered in corn oil at 0, 10, 30, or 60 mg/kg daily from days
6-18 (inclusive) of gestation and were maintained without treatment
until sacrificed at day 29.
One animal in the control group died prior to dosing, one rebbit
fed 30 mg/kg died on day 17, and two fed 60 mg/kg died on days 16 and
18. Mortality was probably not compound-related; it was attributed to
peumonia.
Depression of maternal body weight and faecal scouring were
observed in the high-dose (60 mg/kg) group up to day 18. Maternal
weight gain was significantly reduced in the mid- and high-dose groups
and its recovery in the high-dose group was incomplete at day 29
(termination).
At autopsy one "dam" of the low-dose (10 mg/kg) group was found
to be male. Mean uterine weight was decreased for the 60 mg/kg group.
No treatment-related effects were seen for rates of pregnancy,
corpora lutea or viable foetuses per dam, resorptions, sex ratio or
foetal body weight. A slight decrease in the mumber of implants per
dam was observed at 60 mg/kg. Post-implantation losses were decreased
(50%) in all treated groups. The latter effect was not dose-related
and was considered to be not compound-related. Increases in the
incidence of bent hyoid arch and major-vessel variations were found in
the high-dose group (60 mg/kg). No treatment-related terata were found
at any dosage.
The results of this study indicate a no-effect level at 30 mg/kg
based on implantation rates and minor variations and no teratogenic
effects at 60 mg/kg, although treatment at this level caused maternal
toxicity (Janes et al., 1980).
Special studies on mutagenicity
Flucythrinate was without mutagenic activity in a number of
assays with microorganisms and mammalian cells (Table 2).
Acute toxicity
Several acute toxicity studies are available on flucythrinate
(Table 3). Signs of flucythrinate toxicity included decreased
activity, salivation and tremors. Death usually occurred within two
days and recovery among survivors was generally complete by six days.
Table 2. Results of mutagenicity assays on flucythrinate
Test System Test Object Concentrations Reference
used Purity(%) Result
Reverse mutation S. typhimurium* plate 84.8 - Allen, 1979
incorporation
TA1535 0, 0.01, -
TA1537 0.1, 1 mg -
TA98 spot test -
TA100 0, 2 mg
Reverse mutation E coli** spot test 84.8 - Allen, 1979
wp2/uvr A- 0, 1 mg
plate
incorporation
0, 0.01,
0.1, 1 mg
Unscheduled primary-culture 0.001, 0.005, 85.4 - Dulak, 1982
DNA synthesis rat-hepatocytes 0.01, 0.05,
0.1, 1.0 mg/ml
CHO/HGPRT locus Chinese 0, 0.001, 0.01, 85.4 - Johnson &
ribosyl transferase Hamster 0.1, 0.25, Allen, 1982
(Chinese Ovary Cells 0.5, 1, 5 mg/ml
Hamster Ovary/ (CHO-K1
hypoxanthineguanine -BH4)**
phosphoribosyl
transferase
locus mutation
induction)
* + S-9 metabolic activation
** ± S-9 metabolic activation
Table 3. Results of acute toxicity assays on flucythrinate
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse F oral 76 Fischer, 1978a
Rat M oral 81 " "
F oral 67 " "
Rat M,F inhalation LC50 = 65 µg/1 Daly, 1985
Rabbit M dermal > 1000 Fischer, 1978a
F dermal > 1000 " "
Short-term studies
Rat
Groups of CD (Sprague-Dawley derived) rats received technical
flucythrinate (86% pure) in the diet daily for 28 days at 0, 6, 30 ppm
(8 male and 8 female rats per group) or at 150 or 300 ppm (12 male and
12 female rats per group). Some survivors from the 150 and 300 ppm
groups were used to study reversibility of toxicity. Animals receiving
300 ppm exhibited severe hind limb ataxia, diuresis, hypersensitivity
and salivation typical of pyrethroid intoxication. Animals receiving
150 ppm were much less affected, while females generally exhibited
greater sensitivity to flucythrinate than males. Five females of the
300 ppm group died without apparent cause. Absolute weight and weight
gain were markedly depressed for males and females in the groups
receiving 150 and 300 ppm. All symptoms in the two highest-dose groups
were reversed in 48 hours, while the weight loss, relative to
controls, was regained within 4 weeks after cessation of exposure.
Weight loss in the two highest-dosage groups was attributable to
decreased food intake. Plasma urea nitrogen was elevated for females
receiving 300 ppm. Absolute and relative liver weights were elevated
in females receiving 300 ppm. Based on the findings of weight loss, a
no-effect level of 30 ppm was determined (Fischer, 1979).
Groups of 20 male and 20 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% purity) in the diet at 0, 15,
30, 60, or 120 ppm daily for 90 days.
No symptoms or mortality were observed and body weights in males
and females receiving 120 ppm were only marginally depressed. No
compound-related effect was noted on food intake, haematology,
clinical chemistry or urinalysis.
At autopsy, an increase in the total incidence of alopecia was
noted. Females receiving 120 ppm had a slightly higher incidence of
hydronephrosis than controls (4/20 vs 1/20 for 120 and 0 ppm,
respectively). No compound-related effects on organ weights were
found. Histology showed a slight increase in the incidence of skin
disorders as attributable to irritation at 120 ppm. No pathological
basis for the hydronephrosis was found, suggesting that this finding
is without toxicological significance.
Based on decreased body weight, a no-effect level of 60 ppm was
determined (Jefferson & Jessup, 1979).
Groups of 20 male and 20 female SPF (Sprague-Dawley derived) rats
received 0, 30, 60, 120, or 240 ppm technical flucythrinate (85.4%
pure) in the diet for 6 months.
Males and females that received 240 ppm flucythrinate exhibited
symptoms of decreased motor activity and ataxia, characterized by
weakness in the extremities and gait disturbances. One control and 1
male and 4 females of the 240-ppm group died during the study. Males
receiving 240 ppm and females receiving 240 and 120 ppm flucythrinate
exhibited weight loss attributable to decreased food consumption.
Water intake was depressed in males and females receiving 240 ppm.
Leucocyte counts were slightly depressed in males receiving 120 and
240 ppm. Organ weights were in accord with body weights. An increased
incidence of brown pigmentation in the spleen was observed in high-
dose males and females.
Based on the depression in body weight, the no-effect level for
this study was 60 ppm (Shirasu, 1983).
Dog
Groups of 2 male and 2 female Beagle dogs received technical (80%
pure) flucythrinate in the diet at 0, 30, 150, or 300 ppm daily for 14
days. Symptoms were primarily vomiting at 150 and 300 ppm with some
evidence of diarrhoea at the same dosages. After 14 days weight gain
was markedly depressed at 300 ppm due to decreased food consumption.
No other toxicological parameters were studied (Fischer, 1978b).
Groups of 4 male and 4 female Beagle dogs received technical
flucythrinate (80% pure) in the diet at 0, 30, 150, or 300 ppm daily
for 90 days. Emesis occurred in the 150 and 300 ppm dose groups. Body
weight was depressed in both males and females of the high-dose group
and no unscheduled deaths occurred. Weight gain was slightly depressed
at 150 ppm and markedly depressed at 300 ppm, although food
consumption at this dose was not remarkably affected.
Slight anaemia was observed in dogs of each sex receiving
300 ppm, while males receiving this dosage also exhibited a decreased
leucocyte count. Clinical chemistry showed no treatment-related
effects while urinary pH was lower in males and females receiving
300 ppm.
At autopsy only slight changes in organ weights were observed,
with males receiving 300 ppm having slightly lower relative heart
weights than controls. Female heart weight, although depressed, was
consistent with the decreased body weight at 300 ppm.
At autopsy, 2/8 males receiving 300 ppm and 1/8 females receiving
30 ppm flucythrinate were found to have oral papillomas. Only the
latter lesion was confirmed histologically. There were no other
significant histological findings (Mehring & Jessup, 1979).
The results of this study indicate a no-effect level of 30 ppm
based upon reduced weight gain.
Long-term studies
Mouse
Groups of 50 male and 50 female CD-1 mice received technical
flucythrinate (80% pure) in the diet at 0, 30, 60, or 120 ppm daily
for 18 months.
Skin lesions (abrasions, ulceration and scabs) were observed in
high-dose males and females. No treatment-related symptoms or
treatment-related changes in survival were found. No haematology,
clinical chemistry or urinalysis were undertaken.
At necropsy, hepatocellular adenomas were found in all control
and treated groups. The incidence was variable and statistically-
significant only in high-dose males. Hepatocellular adenocarcinoma and
hepatocellular carcinoma were found in low incidence in all male
groups, but only in control and low-dose female mice. The incidences
of these neoplasms were similar to those previously found in mice and
were apparently unrelated to treatment.
Mild sciatic nerve degeneration occurred in all groups, but at
slightly increased incidence in treated groups, especially high-dose
males. There was no apparent dose-response relationship. The incidence
of mild axonal degeneration was similar in all groups. The no-effect
level for this study was therefore set at 30 ppm (Lang, 1981b).
Rat
Groups of 50 male and 50 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% pure) in the diet at 0, 30, 60
or 120 ppm daily for 24 months.
Skin lesions consistent with scratching of the head, neck and
thorax were observed in all mid- and high-dose groups throughout the
study. Mortality was decreased in high-dose males. Terminal body
weights were decreased in treated animals, but especially in high-dose
males and females. This was possibly related to a depression of food
intake. A very mild anaemia was observed in the 3rd months for both
high-dose males and females. Blood glucose was slightly, though
consistently, depressed for high-dose males and females at months 3
and 6. Urinalysis was normal at 3 and 6 months.
At sacrifice, blood urea nitrogen was elevated in mid- and high-
dose males. All other haematology, clinical chemistry and urinalysis
parameters were unchanged by treatment. At autopsy, high-dose females
exhibited an increased incidence of cystic uterus. Absolute and
relative kidney weights were significantly elevated in mid- and high-
dose males while only the relative kidney weight was elevated in high-
dose females. A slight increase in relative heart weight was found in
high-dose males.
No increase in abnormal histopathology or neoplasia was observed
in any group of treated males. The uterine cysts found at autopsy in
the high-dose females were characterized as endometrial cysts. In the
high-dose females, further slight increases in uterine pathology were
described histologically, namely etritis/endometritis, cystic
endometrial hyperplasia and uterine fibrovascular polyps.
Mammary fibroadenomas occurred at similar incidences in all
female groups. The incidence of mammary adenomas in treated females
exceeded that of controls, but not in a dose-related manner. Mammary
adenocarcinomas occurred at higher incidence at 60 and 120 ppm, but
not in a dose-related manner. The latter incidences remained within
the range of historical control data. The variable incidences and lack
of dose-response relationships contraindicate a neoplastic response,
but in view of the observed weight losses, especially in high-dose
groups, these findings cannot be discounted entirely. Based on these
findings, the increased blood urea nitrogen found in males treated at
60 and 120 ppm and slight changes in organ weights, the no-effect
level of this study was set at 30 ppm (Brewer et al., 1981).
Dog
Groups of 6 male and 6 female Beagle dogs received technical
flucythrinate (87.3% pure) in the diet at 0, 30, 100, or 300 ppm daily
for 24 months.
Male dogs receiving 300 ppm flucythrinate in the diet appeared
thin and exhibited alopecia and dermal scaling; one dog died. Food
consumption was reduced in both males and females fed at this level
and there was a marked decrease in the body weights of both sexes.
These changes may have related to increased emesis observed in these
dogs. A statistically-significant but transient anaemia was observed
in high-dose males at month 18. This was attributable to a persistent
but fluctuating anaemia in one of the high-dose male dogs present
throughout the study. This same animal also had depression of serum
calcium and albumin and was later diagnosed at autopsy as one of two
males suffering nematode infestation. Urinalysis and gross pathology
exhibited no treatment-related changes.
At sacrifice, the relative liver, kidney, and pituitary weights
were increased in both high-dose males and females, while increases in
relative spleen, testis and lung weights were noted for high-dose
males only. Upon histological examination, mid- and high-dose groups
exhibited increased evidence of interstitial pneumonia, compared to
controls. As it is likely that the nutritional status of the dogs was
compromised by compound-induced emesis, these findings were considered
not directly attributable to treatment.
The results of this study indicate a no-effect level of 100 ppm
based upon changes in relative organ weights and body weight (Spicer
et al., 1984).
COMMENTS
The principal mechanism of flucythrinate metabolism involves
ester cleavage and oxidation at the para-position of the alcohol
moiety and at the gem-dimethyl group of the acid moiety. Flucythrinate
does not bioaccumulate and the metabolites are mostly excreted in the
urine and do not accumulate in the tissues.
The toxicological profile of flucythrinate is similar to that of
related pyrethroids, although the acute oral toxicity is relatively
high. The production of hepatocellular tumours in the mouse is not
considered to be of biological significance, considering the known
susceptibility of the mouse to this effect.
Flucythrinate is not teratogenic in the rat or rabbit. The
compound was observed to cause mild maternal weight reduction in a
reproduction study, but not at levels causing concern.
Flucythrinate has no dominant lethal effect in rats and was not
mutagenic in several assays.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 30 ppm in the diet, equal to 4.0 mg/kg b.w.
Rat: 30 ppm in the diet, equal to 1.6 mg/kg b.w.
Dog: 100 ppm in the diet, equal to 2.5 mg/kg b.w.
ESTIMATE OF ACCEPTABLE DAILY TAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
DESIRED
1. Further studies on the biological activity of flucythrinate
relevant to the mild nerve demyelination observed in the mouse
and on possible effects on neurotransmitters.
2. Observations in man.
REFERENCES
Allen, J.S. Mutagenicity testing of CL 222,705; (+) Butyric acid, 2-
(1979) (phi-(difluromethoxy)phenyl)-cyano-m-phenoxybenzyl ester, in
the Ames bacterial test. Unpublished report Project No. 0-
796 by the Agricultural Research Division, American Cyanamid
Co. Submitted to WHO by the American Cyanamid Co.,
Princeton, NJ, USA.
Brewer, L., Jefferson, N.D., & Blair, M. 24-Month feeding study in
(1981) rats. Unpublished report No.141-005 from International
Research and Development Corporation, submitted to WHO by
the American Cyanamid Co., Princeton, NJ, USA.
Daly, I.W. An acute inhalation study of AC 222,705 in the rat.
(1985) Unpublished report No. 78-7226 from Bio/dynamics Inc.,
submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Dulak, L. The unscheduled DNA synthesis (UDS) test on CL 222,705 using
(1982) primary rat hepatocytes in culture. Unpublished report No.
81319 by the Experimental Pathology Department, American
Cyanamid Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Toxicity data report. Unpublished report No. A78-88 by
(1978a) the Agricultural Division, American Cyanamid Co., submitted
to WHO by the American Cyanimid Co., Princeton, NJ, USA.
Fischer, J.E. Experiment L-1715: 14 Day feeding to dogs of CL 222,705.
(1978b) Unpublished report No. A78-77 from the American Cyanamid
Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Experiment L-1705: 28-day feeding to rats of CL 222,705.
(1979) Unpublished report No. AX-79-1 by the American Cyanamid Co.,
submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Harnois, M. A dominant lethal test in male rats treated with CL
(1979) 222,705 by gavage for 5 days. Unpublished report No. 79072
by the Medical Research Division, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study with AC
(1980) 222,705 in rabbits. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Jefferson, N.D. & Jessup, D.C. 90-Day feeding study in rats.
(1979) Unpublished report No. 141-003 from International Research
and Development Corporation. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Johnson, E. & Allen, J.S. Mutagenicity testing of CL 222,705 in the
(1982) in vitro CHO/HGPRT mutation assay. Unpublished report No.
0414 by the Discovery Department, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Lang, P. Three generation reproduction study of AC 222,705 to rats.
(1981a) Unpublished report No. 141-008 from International Research
and Development Corporation, submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Lang, P. 18-Month feeding study of AC 222,705 to mice. Unpublished
(1981b) report No. 141-009 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Co., Princeton, NJ, USA.
Mehring, J. & Jessup, D.C. 90-Day feeding study in dogs. Unpublished
(1979) report No. 141-004 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Go., Princeton, NJ, USA.
Rodwell, D.E., Benson, B.W., & Jessup, D.C. Teratology study of AC
(1979) 222,705 in rats. Unpublished report from International
Research and Development corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Shirasu, Y. AC 222,705: 6-month oral sub-chronic toxicity study in
(1983) rats. Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Spicer, E.J.F., Jefferson, N.D., & Blair, M. Chronic dietary toxicity
(1984) study in dogs. Unpublished report No. 141-025 from
International Research and Development Corporation.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Zulalian, J. Pyrethroids, I. A study of the disposition of
(1979) carbon-14 labelled CL 222,705 [butyric acid, 2-(4-
difluoromethoxy)phenyl)-3-methyl-alpha-cyano-m-phenoxybenzyl
ester] in rats. Unpublished report by the American Cyanamid
Co., Agricultural Research Division. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 451.4
PHYSICAL FORM: Technical material is a viscous, dark amber liquid
with a very slight, ester-like odour, 80-88% pure.
VAPOR PRESSURE
(vapor saturation
technique, mm Hg): 8.7 × 10-9 at 25°C
SOLUBILITY
(g/100 ml solvent
at 21°C): Acetone 82
Corn oil 56
Cottonseed oil 30
Hexane 9
Propanol 78
Soybean oil 30
Water 0.00005
Xylene 181
PARTITION COEFFICIENT
(n-octanol/water): 120
HYDROLYSIS RATES
(half-life in days) at: 27°C 35°C
pH3 ca. 40 ca. 40
pH6 52 31
pH9 6.3 2.6
Distilled water 40 29
FORMULATIONS AVAILABLE
COMMERCIALLY: 3, 5, 10, and 30% emulsifiable concentrate;
5% wettable powder
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and metabolism
When C13/C14-alcohol labelled flucythrinate (19.7 mg/kg) was
dosed orally to rats, about 30% of administered radioactivity was
excreted in the urine and 70% in the faeces after 8 days. With
C13/C14-acid labelled compound similar results were obtained, 22% and
73%, respectively. Highest tissue residues were found in adipose
tissue, but these declined with time (Table 1).
Table 1. Adipose tissue residues of flucythrinate (ppm)
24 hr. 192 hr
C13/C14-alcohol label 4.47 1.91
C13/C14-acid label 0.69 0.26
Metabolites were qualitatively identified by thin layer
chromatography and combined gas liquid chromatography/mass
spectrometry. Principal metabolic pathways involved ester cleavage and
oxidation at the para position of the alcohol moiety and at the gem-
dimethyl groups of the acid moiety. The metabolite with the hydroxyl
substitutent in the alcohol moiety was excreted as the sulphate
conjugate in urine. A glycine conjugate was also formed. Alcohol
radiolabelled flucythrinate yielded at least 29 urinary metabolites,
the principal one being 3-(phenoxy)-hippuric acid. With the acid
radiolabel the major urinary metabolite was 2-[4-(difluoromethoxy)
phenyl]-3-methylbutyric acid, accounting for 60% of urinary radio
activity (Zulalian, 1979).
Toxicological studies
Special studies on reproduction
Rat
Groups of 10 male COBS/CD rats (Sprague-Dawley) received
technical flucythrinate (80% pure) at doses of 0, 2, 5, and 10 mg/kg
in corn oil by gavage for 5 consecutive days and then were
subjected to a standard dominant-lethal assay breeding program.
Triethylenemelamine (0.05 mg/kg i.p.) was administered as a positive
control. One male receiving 10 mg/kg flucythrinate died after the 3rd
dose. Treatment with flucythrinate had no effect on pregnancy rate,
number of implantation sites, number of live foetuses or the number of
post implantation deaths. Flucythrinate was found to have no effect in
the dominant-lethal assay (Harnois, 1979).
In a three generation reproduction study, groups of 12 male and
24 female COBS/CD (Sprague-Dawley derived) weanling rats received
technical flucythrinate (80% pure) in the diet at concentrations of 0,
30, 60, or 120 ppm for 10 weeks before mating (2 females/male) and
then continuously. A similar dosing regimen was maintained throughout
two successive generations resulting from the matings of the F1
parents selected from the F1b generation and F2 parents selected
from the F2b generation.
Skin irritation, observed as hair loss, scabbing, and open
lesions, was observed with low incidence in the F1 and F2 parental
high-dose groups. No data were given for the F0 generation. In the
F1 and F2 generations, weight gains were reduced at 60 and 120 ppm
of both males and females for the duration of the study beyond
weaning. There were no treatment-related effects on food consumption,
fertility rate, gestation length or numbers of liveborn pups. Four-day
survival was decreased at 120 ppm for the 1st and 3rd generations in
both litters. Twenty-one day survival was decreased in both high-dose
litters of the 1st generation.
Pup body weights were reduced at 120 ppm and marginally reduced
at 60 ppm for both sexes and in all generations. Alteration of the
21-day sex ratio in favour of males was observed in the F2a litter at
120 ppm. As this effect was not observed in any other litter, its
toxicological significance can be discounted. A slight increase in the
number of stillborn pups per litter was observed for the 1st litter of
each generation at 60 and 120 ppm. Based on the findings of reduced
weight gain and increased number of stillborn pups per litter, the no
effect level established by this study was 30 ppm (Lang, 1981a).
Special studies on teratogenicity
Rat
Groups of 30 mated Charles-River CD rats received technical
flucythrinate (80% pure) in corn oil by gavage at 0, 2, 4, or 8 mg/kg
on days 6-15 (inclusive) of gestation and were maintained without
treatment until sacrificed at day 20.
In an apparently treatment-related manner, 1 dam treated at
4 mg/kg died, while 19 died at 8 mg/kg, but the cause of death was not
determined. Absolute body weights were reduced at 4 and 8 mg/kg as
were body-weight gains. Incomplete recovery of body weight had
occurred by day 20 (termination).
No treatment-related effects were observed for numbers of
corpora lutea or live foetuses per dam, pre- or post-implantation
losses, pregnancy rate, mean foetal body weight, foetal crown-rump
length, or sex ratio.
Increases in the incidence of 14th rudimentary ribs and
incomplete ossification of sternebrae and other bones were observed at
8 mg/kg. The results of this study indicate that daily treatment up to
4 mg/kg was without effect, while daily treatment up to 8 mg/kg, which
produced severe maternal toxicity in the rat, produced no teratogenic
effect (Rodwell et al., 1979).
Rabbit
Groups of twenty female New Zealand white rabbits were
artifically inseminated after induction of ovulation with chorionic
gonadotropin on 2 consecutive days. Technical flucythrinate (80% pure)
was administered in corn oil at 0, 10, 30, or 60 mg/kg daily from days
6-18 (inclusive) of gestation and were maintained without treatment
until sacrificed at day 29.
One animal in the control group died prior to dosing, one rebbit
fed 30 mg/kg died on day 17, and two fed 60 mg/kg died on days 16 and
18. Mortality was probably not compound-related; it was attributed to
peumonia.
Depression of maternal body weight and faecal scouring were
observed in the high-dose (60 mg/kg) group up to day 18. Maternal
weight gain was significantly reduced in the mid- and high-dose groups
and its recovery in the high-dose group was incomplete at day 29
(termination).
At autopsy one "dam" of the low-dose (10 mg/kg) group was found
to be male. Mean uterine weight was decreased for the 60 mg/kg group.
No treatment-related effects were seen for rates of pregnancy,
corpora lutea or viable foetuses per dam, resorptions, sex ratio or
foetal body weight. A slight decrease in the mumber of implants per
dam was observed at 60 mg/kg. Post-implantation losses were decreased
(50%) in all treated groups. The latter effect was not dose-related
and was considered to be not compound-related. Increases in the
incidence of bent hyoid arch and major-vessel variations were found in
the high-dose group (60 mg/kg). No treatment-related terata were found
at any dosage.
The results of this study indicate a no-effect level at 30 mg/kg
based on implantation rates and minor variations and no teratogenic
effects at 60 mg/kg, although treatment at this level caused maternal
toxicity (Janes et al., 1980).
Special studies on mutagenicity
Flucythrinate was without mutagenic activity in a number of
assays with microorganisms and mammalian cells (Table 2).
Acute toxicity
Several acute toxicity studies are available on flucythrinate
(Table 3). Signs of flucythrinate toxicity included decreased
activity, salivation and tremors. Death usually occurred within two
days and recovery among survivors was generally complete by six days.
Table 2. Results of mutagenicity assays on flucythrinate
Test System Test Object Concentrations Reference
used Purity(%) Result
Reverse mutation S. typhimurium* plate 84.8 - Allen, 1979
incorporation
TA1535 0, 0.01, -
TA1537 0.1, 1 mg -
TA98 spot test -
TA100 0, 2 mg
Reverse mutation E coli** spot test 84.8 - Allen, 1979
wp2/uvr A- 0, 1 mg
plate
incorporation
0, 0.01,
0.1, 1 mg
Unscheduled primary-culture 0.001, 0.005, 85.4 - Dulak, 1982
DNA synthesis rat-hepatocytes 0.01, 0.05,
0.1, 1.0 mg/ml
CHO/HGPRT locus Chinese 0, 0.001, 0.01, 85.4 - Johnson &
ribosyl transferase Hamster 0.1, 0.25, Allen, 1982
(Chinese Ovary Cells 0.5, 1, 5 mg/ml
Hamster Ovary/ (CHO-K1
hypoxanthineguanine -BH4)**
phosphoribosyl
transferase
locus mutation
induction)
* + S-9 metabolic activation
** ± S-9 metabolic activation
Table 3. Results of acute toxicity assays on flucythrinate
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse F oral 76 Fischer, 1978a
Rat M oral 81 " "
F oral 67 " "
Rat M,F inhalation LC50 = 65 µg/1 Daly, 1985
Rabbit M dermal > 1000 Fischer, 1978a
F dermal > 1000 " "
Short-term studies
Rat
Groups of CD (Sprague-Dawley derived) rats received technical
flucythrinate (86% pure) in the diet daily for 28 days at 0, 6, 30 ppm
(8 male and 8 female rats per group) or at 150 or 300 ppm (12 male and
12 female rats per group). Some survivors from the 150 and 300 ppm
groups were used to study reversibility of toxicity. Animals receiving
300 ppm exhibited severe hind limb ataxia, diuresis, hypersensitivity
and salivation typical of pyrethroid intoxication. Animals receiving
150 ppm were much less affected, while females generally exhibited
greater sensitivity to flucythrinate than males. Five females of the
300 ppm group died without apparent cause. Absolute weight and weight
gain were markedly depressed for males and females in the groups
receiving 150 and 300 ppm. All symptoms in the two highest-dose groups
were reversed in 48 hours, while the weight loss, relative to
controls, was regained within 4 weeks after cessation of exposure.
Weight loss in the two highest-dosage groups was attributable to
decreased food intake. Plasma urea nitrogen was elevated for females
receiving 300 ppm. Absolute and relative liver weights were elevated
in females receiving 300 ppm. Based on the findings of weight loss, a
no-effect level of 30 ppm was determined (Fischer, 1979).
Groups of 20 male and 20 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% purity) in the diet at 0, 15,
30, 60, or 120 ppm daily for 90 days.
No symptoms or mortality were observed and body weights in males
and females receiving 120 ppm were only marginally depressed. No
compound-related effect was noted on food intake, haematology,
clinical chemistry or urinalysis.
At autopsy, an increase in the total incidence of alopecia was
noted. Females receiving 120 ppm had a slightly higher incidence of
hydronephrosis than controls (4/20 vs 1/20 for 120 and 0 ppm,
respectively). No compound-related effects on organ weights were
found. Histology showed a slight increase in the incidence of skin
disorders as attributable to irritation at 120 ppm. No pathological
basis for the hydronephrosis was found, suggesting that this finding
is without toxicological significance.
Based on decreased body weight, a no-effect level of 60 ppm was
determined (Jefferson & Jessup, 1979).
Groups of 20 male and 20 female SPF (Sprague-Dawley derived) rats
received 0, 30, 60, 120, or 240 ppm technical flucythrinate (85.4%
pure) in the diet for 6 months.
Males and females that received 240 ppm flucythrinate exhibited
symptoms of decreased motor activity and ataxia, characterized by
weakness in the extremities and gait disturbances. One control and 1
male and 4 females of the 240-ppm group died during the study. Males
receiving 240 ppm and females receiving 240 and 120 ppm flucythrinate
exhibited weight loss attributable to decreased food consumption.
Water intake was depressed in males and females receiving 240 ppm.
Leucocyte counts were slightly depressed in males receiving 120 and
240 ppm. Organ weights were in accord with body weights. An increased
incidence of brown pigmentation in the spleen was observed in high-
dose males and females.
Based on the depression in body weight, the no-effect level for
this study was 60 ppm (Shirasu, 1983).
Dog
Groups of 2 male and 2 female Beagle dogs received technical (80%
pure) flucythrinate in the diet at 0, 30, 150, or 300 ppm daily for 14
days. Symptoms were primarily vomiting at 150 and 300 ppm with some
evidence of diarrhoea at the same dosages. After 14 days weight gain
was markedly depressed at 300 ppm due to decreased food consumption.
No other toxicological parameters were studied (Fischer, 1978b).
Groups of 4 male and 4 female Beagle dogs received technical
flucythrinate (80% pure) in the diet at 0, 30, 150, or 300 ppm daily
for 90 days. Emesis occurred in the 150 and 300 ppm dose groups. Body
weight was depressed in both males and females of the high-dose group
and no unscheduled deaths occurred. Weight gain was slightly depressed
at 150 ppm and markedly depressed at 300 ppm, although food
consumption at this dose was not remarkably affected.
Slight anaemia was observed in dogs of each sex receiving
300 ppm, while males receiving this dosage also exhibited a decreased
leucocyte count. Clinical chemistry showed no treatment-related
effects while urinary pH was lower in males and females receiving
300 ppm.
At autopsy only slight changes in organ weights were observed,
with males receiving 300 ppm having slightly lower relative heart
weights than controls. Female heart weight, although depressed, was
consistent with the decreased body weight at 300 ppm.
At autopsy, 2/8 males receiving 300 ppm and 1/8 females receiving
30 ppm flucythrinate were found to have oral papillomas. Only the
latter lesion was confirmed histologically. There were no other
significant histological findings (Mehring & Jessup, 1979).
The results of this study indicate a no-effect level of 30 ppm
based upon reduced weight gain.
Long-term studies
Mouse
Groups of 50 male and 50 female CD-1 mice received technical
flucythrinate (80% pure) in the diet at 0, 30, 60, or 120 ppm daily
for 18 months.
Skin lesions (abrasions, ulceration and scabs) were observed in
high-dose males and females. No treatment-related symptoms or
treatment-related changes in survival were found. No haematology,
clinical chemistry or urinalysis were undertaken.
At necropsy, hepatocellular adenomas were found in all control
and treated groups. The incidence was variable and statistically-
significant only in high-dose males. Hepatocellular adenocarcinoma and
hepatocellular carcinoma were found in low incidence in all male
groups, but only in control and low-dose female mice. The incidences
of these neoplasms were similar to those previously found in mice and
were apparently unrelated to treatment.
Mild sciatic nerve degeneration occurred in all groups, but at
slightly increased incidence in treated groups, especially high-dose
males. There was no apparent dose-response relationship. The incidence
of mild axonal degeneration was similar in all groups. The no-effect
level for this study was therefore set at 30 ppm (Lang, 1981b).
Rat
Groups of 50 male and 50 female CD (Sprague-Dawley derived) rats
received technical flucythrinate (80% pure) in the diet at 0, 30, 60
or 120 ppm daily for 24 months.
Skin lesions consistent with scratching of the head, neck and
thorax were observed in all mid- and high-dose groups throughout the
study. Mortality was decreased in high-dose males. Terminal body
weights were decreased in treated animals, but especially in high-dose
males and females. This was possibly related to a depression of food
intake. A very mild anaemia was observed in the 3rd months for both
high-dose males and females. Blood glucose was slightly, though
consistently, depressed for high-dose males and females at months 3
and 6. Urinalysis was normal at 3 and 6 months.
At sacrifice, blood urea nitrogen was elevated in mid- and high-
dose males. All other haematology, clinical chemistry and urinalysis
parameters were unchanged by treatment. At autopsy, high-dose females
exhibited an increased incidence of cystic uterus. Absolute and
relative kidney weights were significantly elevated in mid- and high-
dose males while only the relative kidney weight was elevated in high-
dose females. A slight increase in relative heart weight was found in
high-dose males.
No increase in abnormal histopathology or neoplasia was observed
in any group of treated males. The uterine cysts found at autopsy in
the high-dose females were characterized as endometrial cysts. In the
high-dose females, further slight increases in uterine pathology were
described histologically, namely etritis/endometritis, cystic
endometrial hyperplasia and uterine fibrovascular polyps.
Mammary fibroadenomas occurred at similar incidences in all
female groups. The incidence of mammary adenomas in treated females
exceeded that of controls, but not in a dose-related manner. Mammary
adenocarcinomas occurred at higher incidence at 60 and 120 ppm, but
not in a dose-related manner. The latter incidences remained within
the range of historical control data. The variable incidences and lack
of dose-response relationships contraindicate a neoplastic response,
but in view of the observed weight losses, especially in high-dose
groups, these findings cannot be discounted entirely. Based on these
findings, the increased blood urea nitrogen found in males treated at
60 and 120 ppm and slight changes in organ weights, the no-effect
level of this study was set at 30 ppm (Brewer et al., 1981).
Dog
Groups of 6 male and 6 female Beagle dogs received technical
flucythrinate (87.3% pure) in the diet at 0, 30, 100, or 300 ppm daily
for 24 months.
Male dogs receiving 300 ppm flucythrinate in the diet appeared
thin and exhibited alopecia and dermal scaling; one dog died. Food
consumption was reduced in both males and females fed at this level
and there was a marked decrease in the body weights of both sexes.
These changes may have related to increased emesis observed in these
dogs. A statistically-significant but transient anaemia was observed
in high-dose males at month 18. This was attributable to a persistent
but fluctuating anaemia in one of the high-dose male dogs present
throughout the study. This same animal also had depression of serum
calcium and albumin and was later diagnosed at autopsy as one of two
males suffering nematode infestation. Urinalysis and gross pathology
exhibited no treatment-related changes.
At sacrifice, the relative liver, kidney, and pituitary weights
were increased in both high-dose males and females, while increases in
relative spleen, testis and lung weights were noted for high-dose
males only. Upon histological examination, mid- and high-dose groups
exhibited increased evidence of interstitial pneumonia, compared to
controls. As it is likely that the nutritional status of the dogs was
compromised by compound-induced emesis, these findings were considered
not directly attributable to treatment.
The results of this study indicate a no-effect level of 100 ppm
based upon changes in relative organ weights and body weight (Spicer
et al., 1984).
COMMENTS
The principal mechanism of flucythrinate metabolism involves
ester cleavage and oxidation at the para-position of the alcohol
moiety and at the gem-dimethyl group of the acid moiety. Flucythrinate
does not bioaccumulate and the metabolites are mostly excreted in the
urine and do not accumulate in the tissues.
The toxicological profile of flucythrinate is similar to that of
related pyrethroids, although the acute oral toxicity is relatively
high. The production of hepatocellular tumours in the mouse is not
considered to be of biological significance, considering the known
susceptibility of the mouse to this effect.
Flucythrinate is not teratogenic in the rat or rabbit. The
compound was observed to cause mild maternal weight reduction in a
reproduction study, but not at levels causing concern.
Flucythrinate has no dominant lethal effect in rats and was not
mutagenic in several assays.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 30 ppm in the diet, equal to 4.0 mg/kg b.w.
Rat: 30 ppm in the diet, equal to 1.6 mg/kg b.w.
Dog: 100 ppm in the diet, equal to 2.5 mg/kg b.w.
ESTIMATE OF ACCEPTABLE DAILY TAKE FOR MAN
0 - 0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION
DESIRED
1. Further studies on the biological activity of flucythrinate
relevant to the mild nerve demyelination observed in the mouse
and on possible effects on neurotransmitters.
2. Observations in man.
REFERENCES
Allen, J.S. Mutagenicity testing of CL 222,705; (+) Butyric acid, 2-
(1979) (phi-(difluromethoxy)phenyl)-cyano-m-phenoxybenzyl ester, in
the Ames bacterial test. Unpublished report Project No. 0-
796 by the Agricultural Research Division, American Cyanamid
Co. Submitted to WHO by the American Cyanamid Co.,
Princeton, NJ, USA.
Brewer, L., Jefferson, N.D., & Blair, M. 24-Month feeding study in
(1981) rats. Unpublished report No.141-005 from International
Research and Development Corporation, submitted to WHO by
the American Cyanamid Co., Princeton, NJ, USA.
Daly, I.W. An acute inhalation study of AC 222,705 in the rat.
(1985) Unpublished report No. 78-7226 from Bio/dynamics Inc.,
submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Dulak, L. The unscheduled DNA synthesis (UDS) test on CL 222,705 using
(1982) primary rat hepatocytes in culture. Unpublished report No.
81319 by the Experimental Pathology Department, American
Cyanamid Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Toxicity data report. Unpublished report No. A78-88 by
(1978a) the Agricultural Division, American Cyanamid Co., submitted
to WHO by the American Cyanimid Co., Princeton, NJ, USA.
Fischer, J.E. Experiment L-1715: 14 Day feeding to dogs of CL 222,705.
(1978b) Unpublished report No. A78-77 from the American Cyanamid
Co., submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Fischer, J.E. Experiment L-1705: 28-day feeding to rats of CL 222,705.
(1979) Unpublished report No. AX-79-1 by the American Cyanamid Co.,
submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Harnois, M. A dominant lethal test in male rats treated with CL
(1979) 222,705 by gavage for 5 days. Unpublished report No. 79072
by the Medical Research Division, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study with AC
(1980) 222,705 in rabbits. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Jefferson, N.D. & Jessup, D.C. 90-Day feeding study in rats.
(1979) Unpublished report No. 141-003 from International Research
and Development Corporation. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Johnson, E. & Allen, J.S. Mutagenicity testing of CL 222,705 in the
(1982) in vitro CHO/HGPRT mutation assay. Unpublished report No.
0414 by the Discovery Department, American Cyanamid Co.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Lang, P. Three generation reproduction study of AC 222,705 to rats.
(1981a) Unpublished report No. 141-008 from International Research
and Development Corporation, submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
Lang, P. 18-Month feeding study of AC 222,705 to mice. Unpublished
(1981b) report No. 141-009 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Co., Princeton, NJ, USA.
Mehring, J. & Jessup, D.C. 90-Day feeding study in dogs. Unpublished
(1979) report No. 141-004 from International Research and
Development Corporation. Submitted to WHO by the American
Cyanimid Go., Princeton, NJ, USA.
Rodwell, D.E., Benson, B.W., & Jessup, D.C. Teratology study of AC
(1979) 222,705 in rats. Unpublished report from International
Research and Development corporation. Submitted to WHO by
the American Cyanimid Co., Princeton, NJ, USA.
Shirasu, Y. AC 222,705: 6-month oral sub-chronic toxicity study in
(1983) rats. Unpublished report from the Institute of Environmental
Toxicology. Submitted to WHO by the American Cyanimid Co.,
Princeton, NJ, USA.
Spicer, E.J.F., Jefferson, N.D., & Blair, M. Chronic dietary toxicity
(1984) study in dogs. Unpublished report No. 141-025 from
International Research and Development Corporation.
Submitted to WHO by the American Cyanimid Co., Princeton,
NJ, USA.
Zulalian, J. Pyrethroids, I. A study of the disposition of
(1979) carbon-14 labelled CL 222,705 [butyric acid, 2-(4-
difluoromethoxy)phenyl)-3-methyl-alpha-cyano-m-phenoxybenzyl
ester] in rats. Unpublished report by the American Cyanamid
Co., Agricultural Research Division. Submitted to WHO by the
American Cyanimid Co., Princeton, NJ, USA.
