
PESTICIDE RESIDUES IN FOOD - 1983
Sponsored jointly by FAO and WHO
EVALUATIONS 1983
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Geneva, 5 - 14 December 1983
Food and Agriculture Organization of the United Nations
Rome 1985
PHOXIM
RESIDUES
IDENTITY
Chemical Name
(diethoxy-thiophosphoryloxyimino)-phenylacetonitrile
Synonyms
VolatonR
BaythionR
SebacilR
SamacuranR
SRA 7502
BAYER 77488
Molecular Formula
C12H15N2O3PS
Structural Formula
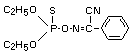 Other Information on Identity and Properties
Molecular weight 298.3
Appearance light yellow oil liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20° C(pure active ingredient)
4°
Vapour pressure approximately 10-4 mm Hg at 20° C
Solubility in water 0.7
(g a.i./100 g in cyclohexanone > 60
solvent at 20°C) in isopropyl alcohol > 60
in methylene chloride > 60
in toluene > 60
Minimum degree of purity 82.0 percent (pre-solution for reasons
of stability in 9-11 percent butanol).
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phoxim is an insecticide of the group of phosphoric ester
compounds. It is a stomach and contact poison and has a depth effect
but no systemic effect. The initial effect is rapid, with a short to
moderate duration, depending on the application. The active ingredient
has a broad spectrum of activity and it is most effective against
biting insects. Phoxim is used as foliage and soil-applied insecticide
and as seed dressing, and is also used for application on livestock
against mites and other ectoparasites.
Phoxim used as insecticide on crops is registered and marketed in
several countries, including European and Central American ones,
Australia, South Africa, Egypt, Turkey and Taiwan (province of China).
Phoxim is registered in six European countries, four South
American countries and two African countries for veterinary use. An
earlier recommendation for the use of phoxim in stored cereals has
been withdrawn by the manufacturer, because of its long persistence in
storage.
Preharvest Uses
Phoxim is formulated as emulsifiable concentrate, granules, dust
or bait. As a foliage-applied insecticide it is mainly used on cotton,
maize and sugarbeet for the control of lepidopterous larvae.
Soil application of phoxim is based on a row or over-all
treatment before, with or directly after sowing or planting.
Therefore, the safety interval includes the entire period of
vegetative growth before harvest. It is mainly used on potatoes. Good
effects against locusts have been reported.
Phoxim is used as a seed dressing at a rate of 40 g a.i./kg seed.
Use in Animals
The 50 percent E.C. formulation is used in 0.025-0.050 percent
a.i. concentrations for spraying or dipping of animals against mites
and other ectoparasites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of phoxim were determined following foliar application
of emulsifiable concentrates to cotton and vegetables and of dust
powder on coffee. With the exception of tomatoes, in which residues
ranging from 0.10 to 0.15 mg/kg were found, the residue levels in the
other crops at the end of the safety interval were either nil or about
the limit of determination.
Table 1 Some Uses of Phoxim Formulations
Crop Recommended dosage Formulation
Application to foliage
Cotton 1.5-2.0 kg/ha 3-10 treatments E.C.
2.3 kg/ha Max. 2 treatments ULV
Vegetables 0.75-1.5 kg/ha. 2-3 treatments E.C.
Maize 1-2.5 kg/ha. 1-3 treatments E.C.
0.25-0.65 kg/ha. 1-2 treatments GR
Application to soil
Vegetables 3.75-5.0 kg/ha E.C.
25-50 mg/plant E.C.-GR
2.0-5.0 kg/ha GR
0.8-1.2 kg/ha bait
Potatoes 1-2.5 kg/ha row treatment GR
1-7.5 kg/ha over-all treatment GR
Maize 0.65-1.25 kg/ha row treatment GR
2.0-7.5 kg/ha over-all treatment GR
Cereals 5 kg/ha over-all treatment GR
Following application of granular phoxim to soil, residue
levels determined in vegetables, potatoes and maize were below
or about the limit of detection in most crops with the exception
of cauliflower (0.1 mg/kg).
Following application of phoxim as a bait formulation to
beans, cabbage, lettuce and spinach, no residues appeared in the
crops in most of the trials; measurable residues were present in
only a few samples.
The results of supervised residue trials are reported in
Table 2.
In animal treatments
Phoxim is used against ectoparasites on sheep, cattle and
pigs at recommended concentrations of 250-500 mg a.i./l.
Experiments have been performed on sheep, pigs and cattle by
spraying or dipping with phoxim in concentrations ranging from
500 to 3 000 mg a.i./l. Fat, muscle, liver and kidney were
analysed 14 to 45 days after application. In all samples of liver,
kidney and muscle of sheep, cattle and pigs, residues were below
0.01 mg/kg. The highest residue, 2.8 mg/kg, was found in fat
from sheep after a 3 000 mg/l plunge dip treatment for 1 min.
30 days before slaughtering. The residues in fat from sheep,
cattle and pig ranged from non-detectable to 0.66 mg/kg. The
results are reported in Table 3.
FATE OF RESIDUES
Degradation pathways and metabolite patterns of phoxim in
soil and mammals have been subject to many investigations. The
findings of these are summarized in Figure 1 and Table 4.
Compounds identified in metabolism studies are referred to by
Roman numerals shown in Figure 1 and Table 4.
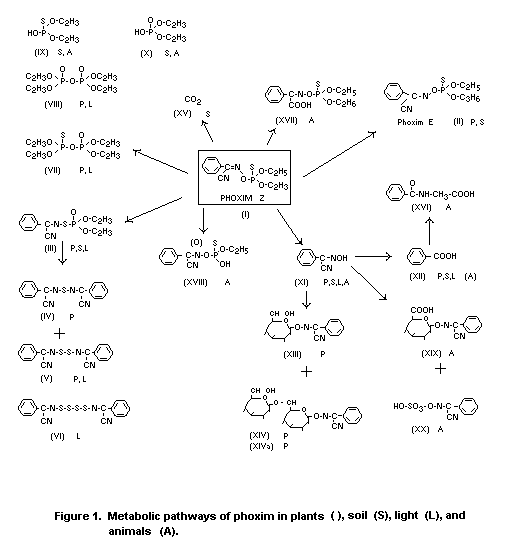
In soil the diethyl phosphoric acid fragments (IX, X) and
the aromatic compounds (XI and XII), formed by hydrolysis of
phoxim (I), were detected. The phenyl ring of (I) is further
oxidized to CO2 (XV) in soil.
Metabolism of phoxim (I) in plants is governed largely by
its photochemical degradation and further reactions of the
degradation products. Basically, three groups of metabolites are
detectable:
- compounds formed due to exposure of (I) to irradiation of the
leaf surface (II, III, IV, V, VI, VIII); these include the
isomeric compounds (II, III) of (I) and the metabolites
resulting from them (IV, V),
- hydrolysis products (XI, XII),
Table 2 Residues of Phoxim From Supervised Trials
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Cotton (11-13 x 1.67-2.0 7 n.d. (2) United States
(seed) kg/ha E.C. (ULV)
Carrots 3.75 kg/ha GR 112-158 n.d.-0.02 (0.2) Fed. Rep. Germany1
Beans 4x1.5-3.0 kg/ha E.C. 14 n.d. (2) Egypt
Beans 0.8 kg/ha bait 30 n.d.-<0.05(2) Italy
(pod) 3.0 kg/ha 30 n.d. (2) Italy
Red cabbage 50 mg/plant GR 69 n.d. (1) Fed. Rep. Germany1
1.2 kg/ha bait 20-100 n.d. (2) Fed. Rep, Germany1
White cabbage 1.2 kg/ha bait 11-95 n.d.-0.03 (4) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cabbage 100 mg/plant 49-63 n.d.-<0.03 (3) Finland
Savoy cabbage 0.6-1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cauliflower 2 kg/ha GR 64 0.1 (1) Fed. Rep. Germany1
25-50 mg/plant GR 42-59 n.d. (5) Fed. Rep. Germany1
100 mg/plant 42-63 n.d.-<0.05 (4) Finland
Eggplant 4x0.6-1.2 kg/ha 14 n.d. (2) Egypt
Lettuce 0.8-2.0 kg/ha bait 30-110 n.d.-0.1(6) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (1) Fed. Rep. Germany1
Spinach 1.2 kg/ha bait 19-36 n.d.-0.20(8) Fed. Rep. Germany1
Table 2 (continued)
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Maize 0.65-1.25 kg/ha GR 109-165 n.d. (3) France
7.5 kg/ha GR 148-169 n.d. (3) Fed. Rep. Germany1
Potatoes 1.5-2.8 kg/ha 78-154 n.d.-0.02(24) United Kingdom1
GR France
Sweden
4.2-7.5 kg/ha GR 78-156 n.d.-<0.01(22) Fed. Rep. Germany1
United Kingdom1
Sweden
Onions 3.75 kg/ha GR 154 n.d.(1) Netherlands
5.0 kg/ha E.C. 130 n.d.(1) Netherlands
Peppers 5x0.77-1.54 kg/ha E.C. 14 n.d.-<0.05(2) Egypt
Tomatoes 4x1.5-3.0 kg/ha E.C. 14 C.10-0.15(2) Egypt
Barley 4.2-5.0 kg/ha GR 95-134 n.d.(4) Fed. Rep. Germany1
(grains) United Kingdom
Oats 5.0 kg/ha GR 97 n.d.(1) Fed. Rep. Germany1
(grains)
Wheat 5.0 kg/ha GR 119 n.d.(1) Fed. Rep. Germany1
(grains)
Coffee 0.94 kg/ha DP 8 n.d.(1) Ivory Coast
green
1 Phoxim is neither registered nor marketed.
Table 3 Residues of Phoxim from Animal Treatments
Interval
Animal Application Conc. between Residues (mg/kg) No. of samples () Country
(mg/kg) last treatment Muscle Fat Liver Kidney
and
slaughter
(days)
Sheep Dip 1 000 30 n.d.1 n.d.-0.7 n.d. n.d.(3) Australia
45 n.d. n.d. n.d. n.d.(3)
2 000 30 n.d. 1.2-1.8 n.d. n.d.(3)
45 n.d. 0.5-0.6 n.d. n.d.(3)
3 000 30 n.d. 1.6-2.8 n.d. n.d.(3)
45 n.d. 0.1-1.0 n.d. n.d.(3)
2x spray 500 21 <0.01 0.03-0.17 <0.01 <0.01(3) South Africa
500 14 <0.01 0.17-0.66 <0.01 <0.01(3)
1 000 21 <0.01 0.20-0.52 <0.01 <0.01(3)
Cattle 2x spray 1 000 28 <0.01 0.02 <0.01 <0.01(2)
1 000 14 <0.01 0.32-0.37 <0.01 <0.01(2)
Pig 2x spray 1 000 28 <0.01 <0.01-0.13 <0.01 <0.01(2)
1 n. d. = not detected.
Table 4 Chemical Names and Occurrence of Compounds Identified in Metabolism Studies on Phoxim
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
I (Diethoxy-thiophosphoryloxyimino)-phenyl= Z-3,5-Dioxa-6-aza-4-phosphaoct- X X X X
acetonitrile 6-ene-8-nitrile, 4-ethoxy-7-
= Phoxim Z phenyl-4-sulfide
II ditto E-3,5-Dioxa-6-aza-4-phosphaoct- X X X
= Phoxim E 6-ene-8-nitrile, 4-ethoxy-7-
phenyl-4-sulfide
III Diethoxy-phosphoylthioimino-phenylaceto= 3-Oxa-5-thia-6-aza-4-phospha- X X X
nitrile oct-6-ene-8-nitrile, 4-ethoxy-
= Photoisomeres 7-phenyl-4-oxide
IV N,N'-[Thio-bis(alpha- alpha,alpha'(Thiodinitrilo)-bis- X
iminophenylacetonitrile)] benzeneacetonitrile
V N,N'-[Dithio-bis(alpha- alpha,alpha'-(Dithiodinitrilo)- X X
iminophenylacetonitrile)] bis benzeneacetonitrile
VI Bis-alpha-dithioiminophenylacetonitrile X
VII O,O,O,O-Tetraethyldiphosphate Diphosphoric acid tetraethyl- X X
ester
VIII O,O,O,O-Tetraethylmonothiodiphosphate Thiodiphosphoric acid tetra= X X
ethylester
IX O,O-Diethyl thiophosphoric acid Phosphorothioic acid O,O,di= X X
ethylester
Table 4 (continued)
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
X Diethyl phosphoric acid Phosphoric acid diethylester X X
XI alpha-Hydroxy-imino-phenylacetonitrile Benzeneacetonitrile, X X X X
-(hydroxy-imino)-
XII Benzoic acid Benzoic acid X X X
XIII alpha-Hydroxy-imino-phenylacetonitrile
glucoside X
XIV alpha-Hydroxy-imino-phenylacetonitrile X
gentio=bioside
XIVa alpha-Hydroxy-imino-phenylacetonitrile X
glycoside
XV Carbon dioxide Carbon dioxide X
XVI Hippuric acid X
XVII "phoxim carbonic acid" X
XVIII Desethylphoxim X
(Desethyl-PO-phoxim)
XIX alpha-Hydroxy-imino-phenylacetonitrile X
glucuronide
XX alpha-Hydroxy-imino-phenylacetonitrile X
sulfate
Other Information on Identity and Properties
Molecular weight 298.3
Appearance light yellow oil liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20° C(pure active ingredient)
4°
Vapour pressure approximately 10-4 mm Hg at 20° C
Solubility in water 0.7
(g a.i./100 g in cyclohexanone > 60
solvent at 20°C) in isopropyl alcohol > 60
in methylene chloride > 60
in toluene > 60
Minimum degree of purity 82.0 percent (pre-solution for reasons
of stability in 9-11 percent butanol).
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phoxim is an insecticide of the group of phosphoric ester
compounds. It is a stomach and contact poison and has a depth effect
but no systemic effect. The initial effect is rapid, with a short to
moderate duration, depending on the application. The active ingredient
has a broad spectrum of activity and it is most effective against
biting insects. Phoxim is used as foliage and soil-applied insecticide
and as seed dressing, and is also used for application on livestock
against mites and other ectoparasites.
Phoxim used as insecticide on crops is registered and marketed in
several countries, including European and Central American ones,
Australia, South Africa, Egypt, Turkey and Taiwan (province of China).
Phoxim is registered in six European countries, four South
American countries and two African countries for veterinary use. An
earlier recommendation for the use of phoxim in stored cereals has
been withdrawn by the manufacturer, because of its long persistence in
storage.
Preharvest Uses
Phoxim is formulated as emulsifiable concentrate, granules, dust
or bait. As a foliage-applied insecticide it is mainly used on cotton,
maize and sugarbeet for the control of lepidopterous larvae.
Soil application of phoxim is based on a row or over-all
treatment before, with or directly after sowing or planting.
Therefore, the safety interval includes the entire period of
vegetative growth before harvest. It is mainly used on potatoes. Good
effects against locusts have been reported.
Phoxim is used as a seed dressing at a rate of 40 g a.i./kg seed.
Use in Animals
The 50 percent E.C. formulation is used in 0.025-0.050 percent
a.i. concentrations for spraying or dipping of animals against mites
and other ectoparasites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of phoxim were determined following foliar application
of emulsifiable concentrates to cotton and vegetables and of dust
powder on coffee. With the exception of tomatoes, in which residues
ranging from 0.10 to 0.15 mg/kg were found, the residue levels in the
other crops at the end of the safety interval were either nil or about
the limit of determination.
Table 1 Some Uses of Phoxim Formulations
Crop Recommended dosage Formulation
Application to foliage
Cotton 1.5-2.0 kg/ha 3-10 treatments E.C.
2.3 kg/ha Max. 2 treatments ULV
Vegetables 0.75-1.5 kg/ha. 2-3 treatments E.C.
Maize 1-2.5 kg/ha. 1-3 treatments E.C.
0.25-0.65 kg/ha. 1-2 treatments GR
Application to soil
Vegetables 3.75-5.0 kg/ha E.C.
25-50 mg/plant E.C.-GR
2.0-5.0 kg/ha GR
0.8-1.2 kg/ha bait
Potatoes 1-2.5 kg/ha row treatment GR
1-7.5 kg/ha over-all treatment GR
Maize 0.65-1.25 kg/ha row treatment GR
2.0-7.5 kg/ha over-all treatment GR
Cereals 5 kg/ha over-all treatment GR
Following application of granular phoxim to soil, residue
levels determined in vegetables, potatoes and maize were below
or about the limit of detection in most crops with the exception
of cauliflower (0.1 mg/kg).
Following application of phoxim as a bait formulation to
beans, cabbage, lettuce and spinach, no residues appeared in the
crops in most of the trials; measurable residues were present in
only a few samples.
The results of supervised residue trials are reported in
Table 2.
In animal treatments
Phoxim is used against ectoparasites on sheep, cattle and
pigs at recommended concentrations of 250-500 mg a.i./l.
Experiments have been performed on sheep, pigs and cattle by
spraying or dipping with phoxim in concentrations ranging from
500 to 3 000 mg a.i./l. Fat, muscle, liver and kidney were
analysed 14 to 45 days after application. In all samples of liver,
kidney and muscle of sheep, cattle and pigs, residues were below
0.01 mg/kg. The highest residue, 2.8 mg/kg, was found in fat
from sheep after a 3 000 mg/l plunge dip treatment for 1 min.
30 days before slaughtering. The residues in fat from sheep,
cattle and pig ranged from non-detectable to 0.66 mg/kg. The
results are reported in Table 3.
FATE OF RESIDUES
Degradation pathways and metabolite patterns of phoxim in
soil and mammals have been subject to many investigations. The
findings of these are summarized in Figure 1 and Table 4.
Compounds identified in metabolism studies are referred to by
Roman numerals shown in Figure 1 and Table 4.
In soil the diethyl phosphoric acid fragments (IX, X) and
the aromatic compounds (XI and XII), formed by hydrolysis of
phoxim (I), were detected. The phenyl ring of (I) is further
oxidized to CO2 (XV) in soil.
Metabolism of phoxim (I) in plants is governed largely by
its photochemical degradation and further reactions of the
degradation products. Basically, three groups of metabolites are
detectable:
- compounds formed due to exposure of (I) to irradiation of the
leaf surface (II, III, IV, V, VI, VIII); these include the
isomeric compounds (II, III) of (I) and the metabolites
resulting from them (IV, V),
- hydrolysis products (XI, XII),
Table 2 Residues of Phoxim From Supervised Trials
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Cotton (11-13 x 1.67-2.0 7 n.d. (2) United States
(seed) kg/ha E.C. (ULV)
Carrots 3.75 kg/ha GR 112-158 n.d.-0.02 (0.2) Fed. Rep. Germany1
Beans 4x1.5-3.0 kg/ha E.C. 14 n.d. (2) Egypt
Beans 0.8 kg/ha bait 30 n.d.-<0.05(2) Italy
(pod) 3.0 kg/ha 30 n.d. (2) Italy
Red cabbage 50 mg/plant GR 69 n.d. (1) Fed. Rep. Germany1
1.2 kg/ha bait 20-100 n.d. (2) Fed. Rep, Germany1
White cabbage 1.2 kg/ha bait 11-95 n.d.-0.03 (4) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cabbage 100 mg/plant 49-63 n.d.-<0.03 (3) Finland
Savoy cabbage 0.6-1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cauliflower 2 kg/ha GR 64 0.1 (1) Fed. Rep. Germany1
25-50 mg/plant GR 42-59 n.d. (5) Fed. Rep. Germany1
100 mg/plant 42-63 n.d.-<0.05 (4) Finland
Eggplant 4x0.6-1.2 kg/ha 14 n.d. (2) Egypt
Lettuce 0.8-2.0 kg/ha bait 30-110 n.d.-0.1(6) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (1) Fed. Rep. Germany1
Spinach 1.2 kg/ha bait 19-36 n.d.-0.20(8) Fed. Rep. Germany1
Table 2 (continued)
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Maize 0.65-1.25 kg/ha GR 109-165 n.d. (3) France
7.5 kg/ha GR 148-169 n.d. (3) Fed. Rep. Germany1
Potatoes 1.5-2.8 kg/ha 78-154 n.d.-0.02(24) United Kingdom1
GR France
Sweden
4.2-7.5 kg/ha GR 78-156 n.d.-<0.01(22) Fed. Rep. Germany1
United Kingdom1
Sweden
Onions 3.75 kg/ha GR 154 n.d.(1) Netherlands
5.0 kg/ha E.C. 130 n.d.(1) Netherlands
Peppers 5x0.77-1.54 kg/ha E.C. 14 n.d.-<0.05(2) Egypt
Tomatoes 4x1.5-3.0 kg/ha E.C. 14 C.10-0.15(2) Egypt
Barley 4.2-5.0 kg/ha GR 95-134 n.d.(4) Fed. Rep. Germany1
(grains) United Kingdom
Oats 5.0 kg/ha GR 97 n.d.(1) Fed. Rep. Germany1
(grains)
Wheat 5.0 kg/ha GR 119 n.d.(1) Fed. Rep. Germany1
(grains)
Coffee 0.94 kg/ha DP 8 n.d.(1) Ivory Coast
green
1 Phoxim is neither registered nor marketed.
Table 3 Residues of Phoxim from Animal Treatments
Interval
Animal Application Conc. between Residues (mg/kg) No. of samples () Country
(mg/kg) last treatment Muscle Fat Liver Kidney
and
slaughter
(days)
Sheep Dip 1 000 30 n.d.1 n.d.-0.7 n.d. n.d.(3) Australia
45 n.d. n.d. n.d. n.d.(3)
2 000 30 n.d. 1.2-1.8 n.d. n.d.(3)
45 n.d. 0.5-0.6 n.d. n.d.(3)
3 000 30 n.d. 1.6-2.8 n.d. n.d.(3)
45 n.d. 0.1-1.0 n.d. n.d.(3)
2x spray 500 21 <0.01 0.03-0.17 <0.01 <0.01(3) South Africa
500 14 <0.01 0.17-0.66 <0.01 <0.01(3)
1 000 21 <0.01 0.20-0.52 <0.01 <0.01(3)
Cattle 2x spray 1 000 28 <0.01 0.02 <0.01 <0.01(2)
1 000 14 <0.01 0.32-0.37 <0.01 <0.01(2)
Pig 2x spray 1 000 28 <0.01 <0.01-0.13 <0.01 <0.01(2)
1 n. d. = not detected.
Table 4 Chemical Names and Occurrence of Compounds Identified in Metabolism Studies on Phoxim
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
I (Diethoxy-thiophosphoryloxyimino)-phenyl= Z-3,5-Dioxa-6-aza-4-phosphaoct- X X X X
acetonitrile 6-ene-8-nitrile, 4-ethoxy-7-
= Phoxim Z phenyl-4-sulfide
II ditto E-3,5-Dioxa-6-aza-4-phosphaoct- X X X
= Phoxim E 6-ene-8-nitrile, 4-ethoxy-7-
phenyl-4-sulfide
III Diethoxy-phosphoylthioimino-phenylaceto= 3-Oxa-5-thia-6-aza-4-phospha- X X X
nitrile oct-6-ene-8-nitrile, 4-ethoxy-
= Photoisomeres 7-phenyl-4-oxide
IV N,N'-[Thio-bis(alpha- alpha,alpha'(Thiodinitrilo)-bis- X
iminophenylacetonitrile)] benzeneacetonitrile
V N,N'-[Dithio-bis(alpha- alpha,alpha'-(Dithiodinitrilo)- X X
iminophenylacetonitrile)] bis benzeneacetonitrile
VI Bis-alpha-dithioiminophenylacetonitrile X
VII O,O,O,O-Tetraethyldiphosphate Diphosphoric acid tetraethyl- X X
ester
VIII O,O,O,O-Tetraethylmonothiodiphosphate Thiodiphosphoric acid tetra= X X
ethylester
IX O,O-Diethyl thiophosphoric acid Phosphorothioic acid O,O,di= X X
ethylester
Table 4 (continued)
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
X Diethyl phosphoric acid Phosphoric acid diethylester X X
XI alpha-Hydroxy-imino-phenylacetonitrile Benzeneacetonitrile, X X X X
-(hydroxy-imino)-
XII Benzoic acid Benzoic acid X X X
XIII alpha-Hydroxy-imino-phenylacetonitrile
glucoside X
XIV alpha-Hydroxy-imino-phenylacetonitrile X
gentio=bioside
XIVa alpha-Hydroxy-imino-phenylacetonitrile X
glycoside
XV Carbon dioxide Carbon dioxide X
XVI Hippuric acid X
XVII "phoxim carbonic acid" X
XVIII Desethylphoxim X
(Desethyl-PO-phoxim)
XIX alpha-Hydroxy-imino-phenylacetonitrile X
glucuronide
XX alpha-Hydroxy-imino-phenylacetonitrile X
sulfate
 - carbohydrate conjugates (XIII, XIV, XIVa) of the hydrolysis
product (XI); as carbohydrate components, glucose and
gentiobiose, as well as another disaccharide with an unknown
carbohydrate structure, were detected.
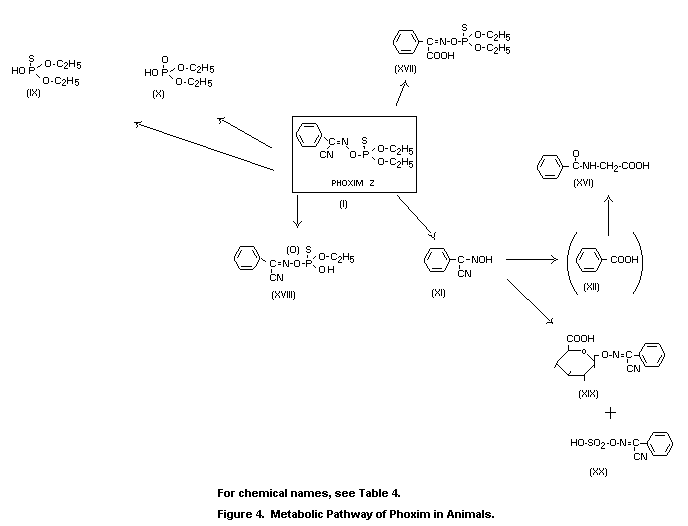
The metabolic fate in mammals was governed by rapid and
almost complete absorption after oral dosing. Owing to rapid
elimination, essentially renal, no accumulation in organs or
tissues was observed. The metabolites (IX, X, XI, XVII, XVIII)
were formed by hydrolysis. The hydrolysis product (XI) was
conjugated to (XIX) and (XX) and further hydrolysed to benzoic
acid (XII), which was excreted as hippuric acid (XVI).
In Soil
The behaviour of phoxim in soil was investigated by studies
on leaching of unlabelled and ring-14C-phoxim in greenhouse,
field and laboratory experiments (soil columns and soil thin-layer
plates); degradation under different conditions in the laboratory
and in the field; and metabolism of unlabelled and
ring-14C-phoxim in the field, in the greenhouse and in a
closed system.
Leaching
Phoxim (I) undergoes only slight leaching in soil, according
to both metabolism studies and specially conducted leaching
studies. In a greenhouse experiment, a 10 percent granular
formulation was applied to maize at sowing, using
ring-14C-phoxim; no increase in 14C radioactivity was
observed in the 5 to 20-cm layer (Dräger 1978b). In a
corresponding field experiment, 14C radioactivity of the
parent compound found only in the upper 10 cm of soil (Steffens
1978). From the results of these studies, it can therefore be
concluded that the metabolites also do not leach.
Gas-chromatographic analyses of different soil layers,
performed after field application of a 5 percent granular
formulation at a rate of 100 kg/ha, yielded similar results for
phoxim (II) and the metabolites (III, IX and X) (Dräger 1977).
Laboratory experiments on leaching of phoxim (I) and its
photoisomer (III) revealed that there were no residues in the
leachate after 60 h of simulated rainfall of 210 to 230 mm
(Dräger 1977). Analysis of the different layers of the soil column
showed that (I) and (III) remained in the upper 0 to 5 cm. During
this period, (III) underwent degradation to a residue of about 1
percent. The oxon of phoxim (I) (not included in the scheme of
formulae because its formation was not shown by the reported
metabolic studies) was not detectable in the soil layers or in the
leachate, as it underwent complete hydrolysis.
In further laboratory leaching experiments conducted with the
parent compound and different formulations (500 E.C. 5 percent
granular, 4 percent bait) by the method specified in BBA Leaflet
No. 37 (Biologische Bundesanstalt für Land- und Forstwirtschaft
1980), using standard soils, no residues were found in the
leachate (Bayer 1972, 1974, 1977).
In a study undertaken to characterize leaching behaviour,
spots of phoxim (I) and 23 other pesticides were applied on
thin-layer plates coated with different types of soils of varying
textures, ranging from non-adsorptive sand to fine textured clay.
The plates were developed with distilled water and the leaching
behaviour was determined by comparing Rf values. According to
leaching behaviour, the compounds were grouped into five
categories, ranging from immobile to highly mobile. Phoxim (I)
was categorized as having low mobility (Thornton et al. 1976).
Degradation
The half-life of phoxim (I) in soil was determined in
laboratory and field experiments. After application of a 5 percent
granular formulation to bare field soils at a rate of 100 kg/ha,
half-lives of about two weeks were determined (Bayer 1970). In
laboratory experiments, addition of 3 mg/kg of phoxim to soil
resulted in half-lives of 1 to 11 days (Nitokuno 1977). The
results of the reported studies are presented in Table 5.
Metabolism
The studies to investigate metabolism of phoxim (I) in soil
were conducted with unlabelled and ring-14C-labelled
compounds. After incorporation of a 5 percent granular formulation
in fallow soils with no plant cover, degradation was studied by
gas chromatography (Dräger 1977). With initial parent compound
concentrations of 4.0 mg/kg in the 0 to 15 cm layer, less than
0.1 mg/kg was found after two months; by day 150, the parent
compound concentration was less than 0.05 mg/kg. The photoisomer
(III) was detectable only on day 0.
The levels of diethyl phosphoric acids (IX) and (X) declined
to 0.09 mg/kg (IX) and 0.05 mg/kg (X) by day 26. Details are given
in Table 6.
After application of a 10 percent formulation, using
ring-14C-phoxim (I), to maize at sowing, the compounds (XI)
and (XII) were detected (Dräger 1978a). Residues of both compounds
were less than 0.1 mg/kg at 59 to 128 days after application.
During this period, phoxim (I) decreased in the 0 to 5-cm layer
from 0.33 mg/kg on day 59 to 0.10 mg/kg on day 128 (Table 7).
Table 5 Half-lives of Phoxim in Different Soils
Soil Application Half-life
Laboratory studies
sandy loam soil 0.8 % org. matter; 13.6% clay; pH 6.5 3 mg/kg a.i. ca. 1 day
clay loam soil 12.7 % org. matter; 1.5% clay; pH 5.6 3 mg/kg a.i. ca. 11 days
sandy loam soil 5.85% org. matter; 14.0% clay; pH 5.7 3 mg/kg a.i. ca. 9 days
Greenhouse study
loamy sand 0.89% C; 31.4% fines; pH 6.0 10% granular; ca. 8 weeks
0.5 g/m row
Field studies
light humus 3.2% C; 9.8% clay; 10.3% silt; pH 5.2 5% granular; ca. 2 weeks
100 kg/ha
loamy sand 3.8% C; 15.0% clay; 14.4% silt; pH 6.1 5% granular; ca. 2.5 weeks
100 kg/ha
loam soil 3% microgranular;
2 × 90 kg/ha ca. 10 days
clay loam soil 12.7% org. matter; 1.5% clay 3% microgranular
2 × 90 kg/ha ca. 2 weeks
Table 6 Metabolism of Phoxim in Soil, Field Study1
Day 0 Day 14 Day 26 Day 60
Analysed
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-10 10-15 0-15 0-5 5-10 10-25 0-15 0-5 5-10 10-15 0-15 0-5 5-10 10-15 0-15
(I) 9.4 2.3 0.39 4.0 4.9 1.34 0.35 2.2 1.42 0.42 0.06 0.6 0.08 0.04 0.04 0.05
(III) 1.4 1.1 n.d. 0.8 0.24 n.d. n.d. 0.08 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d.
(IX) 2.46 0.30 n.d. 0.92 1.09 0.06 n.d. 0.38 0.09 0.02 n.d. 0.04 n.d. n.d. n.d. n.d.
(X) 0.69 0.26 n.d. 0.32 0.10 0.06 n.d. 0.05 0.05 n.d. n.d. 0.02 n.d. n.d. n.d. n.d.
1 Soil type: light humus; pH 5.2; 20.1% fines; 9.8% clay; 10.3% silt; 3.2% C. Application rate - 5 kg a.i./ha. Residue levels
reported in mg/kg in relation to dry material.
2 For Roman numerals see Table 4.
n.d. = not detected.
Table 7 Metabolism of Ring-14C-Phoxim (I) in Soil1
Analysed Day 59 Day 91 Day 128
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-20 0-5 5-20 0-5 5-20
(I) 0.33 0.10 0.22 0.09 0.10 0.04
(XI) 0.06 0.01 0.05 0.01 0.04 0.01
(XII) 0.02 0.001 0.006 0.001 0.004 n.d.
1 pH 6.0; 31.4% fines; 0.89%C. Greenhouse study. Residue levels in mg/kg.
2 For Roman numerals see Table 4.
Results of studies conducted in a closed system, using
ring-14C-phoxim (I) incorporated as a granular formulation into the
soil, showed that the aromatic remainder of the molecule was
mineralized substantially to CO2 (XV) (Steffens 1978). Within 56 days
after application, 25 to 28 percent of the applied 14C activity was
released into the atmosphere as 14CO2.
The metabolic pathway of phoxim in soil is illustrated in Figure
2.
In Plants
The behaviour of phoxim in plants was investigated by studies of
absorption, translocation and accumulation of ring-14C-phoxim in
maize plants, in greenhouse and field studies, and of the metabolism
of 32P and ring-14C-phoxim after foliar application to cotton plants
and after injection of ring-14C-(XI) into tomato plants.
Absorption, translocation and accumulation
The behaviour of phoxim (I) after application as a soil
insecticide was investigated in a greenhouse study in which a 10
percent granular formulation was incorporated in the soil at the time
of sowing maize. The distribution of plant-absorbed 14C activity was
monitored in green maize and at the milk-ripe and harvest-ripe stages
(Dräger 1978a). The results are given in Table 8.
After application of the 10 percent granular formulation of
phoxim to maize in a field experiment (Steffens 1978), less 14C
activity was absorbed by the plant than in the aforementioned
greenhouse study; for example, the harvest-ripe kernels contained 0.02
percent, compared with 0.08 percent in the greenhouse experiment, of
the 14C dose applied to the soil (Table 9).
Metabolism
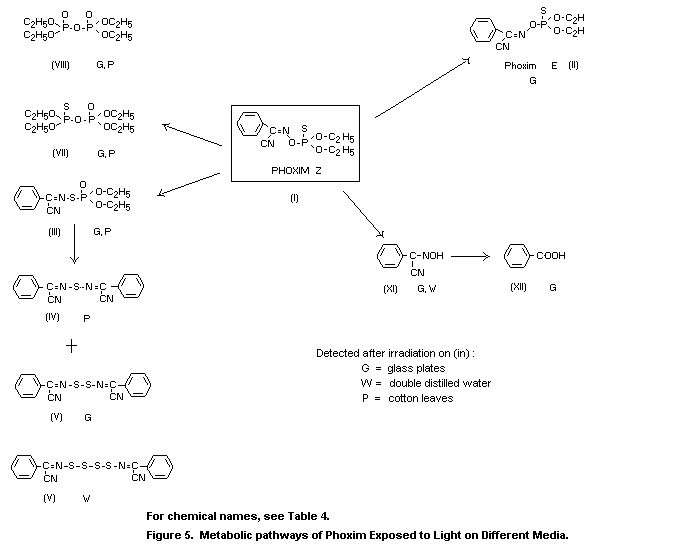
The effect of light on the metabolism of phoxim (I) was evident
in greenhouse studies conducted with 32P-labelled phoxim on cotton
plants (Dräger 1971). After application of the spray solution to
cotton leaves, the isomeric compound (III) was detected, which was
formed solely on irradiation of the treated test plants. The
simultaneously formed diphosphates (VII) and (VIII) were present only
at very low levels.
Metabolism studies with ring-14C parent compound applied to
foliage of cotton plants are based on model experiments that provided
further information on photolytically formed metabolites and on the
incorporation of metabolites in plant constituents. After exposure for
96 h to irradiation on glass plates, 1 percent (XII), 2 percent (XI),
11 percent (II), 5 percent (III) and 5 percent of the phosphorus-free
metabolite (V) were detected (Dräger, 1978b).
- carbohydrate conjugates (XIII, XIV, XIVa) of the hydrolysis
product (XI); as carbohydrate components, glucose and
gentiobiose, as well as another disaccharide with an unknown
carbohydrate structure, were detected.
The metabolic fate in mammals was governed by rapid and
almost complete absorption after oral dosing. Owing to rapid
elimination, essentially renal, no accumulation in organs or
tissues was observed. The metabolites (IX, X, XI, XVII, XVIII)
were formed by hydrolysis. The hydrolysis product (XI) was
conjugated to (XIX) and (XX) and further hydrolysed to benzoic
acid (XII), which was excreted as hippuric acid (XVI).
In Soil
The behaviour of phoxim in soil was investigated by studies
on leaching of unlabelled and ring-14C-phoxim in greenhouse,
field and laboratory experiments (soil columns and soil thin-layer
plates); degradation under different conditions in the laboratory
and in the field; and metabolism of unlabelled and
ring-14C-phoxim in the field, in the greenhouse and in a
closed system.
Leaching
Phoxim (I) undergoes only slight leaching in soil, according
to both metabolism studies and specially conducted leaching
studies. In a greenhouse experiment, a 10 percent granular
formulation was applied to maize at sowing, using
ring-14C-phoxim; no increase in 14C radioactivity was
observed in the 5 to 20-cm layer (Dräger 1978b). In a
corresponding field experiment, 14C radioactivity of the
parent compound found only in the upper 10 cm of soil (Steffens
1978). From the results of these studies, it can therefore be
concluded that the metabolites also do not leach.
Gas-chromatographic analyses of different soil layers,
performed after field application of a 5 percent granular
formulation at a rate of 100 kg/ha, yielded similar results for
phoxim (II) and the metabolites (III, IX and X) (Dräger 1977).
Laboratory experiments on leaching of phoxim (I) and its
photoisomer (III) revealed that there were no residues in the
leachate after 60 h of simulated rainfall of 210 to 230 mm
(Dräger 1977). Analysis of the different layers of the soil column
showed that (I) and (III) remained in the upper 0 to 5 cm. During
this period, (III) underwent degradation to a residue of about 1
percent. The oxon of phoxim (I) (not included in the scheme of
formulae because its formation was not shown by the reported
metabolic studies) was not detectable in the soil layers or in the
leachate, as it underwent complete hydrolysis.
In further laboratory leaching experiments conducted with the
parent compound and different formulations (500 E.C. 5 percent
granular, 4 percent bait) by the method specified in BBA Leaflet
No. 37 (Biologische Bundesanstalt für Land- und Forstwirtschaft
1980), using standard soils, no residues were found in the
leachate (Bayer 1972, 1974, 1977).
In a study undertaken to characterize leaching behaviour,
spots of phoxim (I) and 23 other pesticides were applied on
thin-layer plates coated with different types of soils of varying
textures, ranging from non-adsorptive sand to fine textured clay.
The plates were developed with distilled water and the leaching
behaviour was determined by comparing Rf values. According to
leaching behaviour, the compounds were grouped into five
categories, ranging from immobile to highly mobile. Phoxim (I)
was categorized as having low mobility (Thornton et al. 1976).
Degradation
The half-life of phoxim (I) in soil was determined in
laboratory and field experiments. After application of a 5 percent
granular formulation to bare field soils at a rate of 100 kg/ha,
half-lives of about two weeks were determined (Bayer 1970). In
laboratory experiments, addition of 3 mg/kg of phoxim to soil
resulted in half-lives of 1 to 11 days (Nitokuno 1977). The
results of the reported studies are presented in Table 5.
Metabolism
The studies to investigate metabolism of phoxim (I) in soil
were conducted with unlabelled and ring-14C-labelled
compounds. After incorporation of a 5 percent granular formulation
in fallow soils with no plant cover, degradation was studied by
gas chromatography (Dräger 1977). With initial parent compound
concentrations of 4.0 mg/kg in the 0 to 15 cm layer, less than
0.1 mg/kg was found after two months; by day 150, the parent
compound concentration was less than 0.05 mg/kg. The photoisomer
(III) was detectable only on day 0.
The levels of diethyl phosphoric acids (IX) and (X) declined
to 0.09 mg/kg (IX) and 0.05 mg/kg (X) by day 26. Details are given
in Table 6.
After application of a 10 percent formulation, using
ring-14C-phoxim (I), to maize at sowing, the compounds (XI)
and (XII) were detected (Dräger 1978a). Residues of both compounds
were less than 0.1 mg/kg at 59 to 128 days after application.
During this period, phoxim (I) decreased in the 0 to 5-cm layer
from 0.33 mg/kg on day 59 to 0.10 mg/kg on day 128 (Table 7).
Table 5 Half-lives of Phoxim in Different Soils
Soil Application Half-life
Laboratory studies
sandy loam soil 0.8 % org. matter; 13.6% clay; pH 6.5 3 mg/kg a.i. ca. 1 day
clay loam soil 12.7 % org. matter; 1.5% clay; pH 5.6 3 mg/kg a.i. ca. 11 days
sandy loam soil 5.85% org. matter; 14.0% clay; pH 5.7 3 mg/kg a.i. ca. 9 days
Greenhouse study
loamy sand 0.89% C; 31.4% fines; pH 6.0 10% granular; ca. 8 weeks
0.5 g/m row
Field studies
light humus 3.2% C; 9.8% clay; 10.3% silt; pH 5.2 5% granular; ca. 2 weeks
100 kg/ha
loamy sand 3.8% C; 15.0% clay; 14.4% silt; pH 6.1 5% granular; ca. 2.5 weeks
100 kg/ha
loam soil 3% microgranular;
2 × 90 kg/ha ca. 10 days
clay loam soil 12.7% org. matter; 1.5% clay 3% microgranular
2 × 90 kg/ha ca. 2 weeks
Table 6 Metabolism of Phoxim in Soil, Field Study1
Day 0 Day 14 Day 26 Day 60
Analysed
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-10 10-15 0-15 0-5 5-10 10-25 0-15 0-5 5-10 10-15 0-15 0-5 5-10 10-15 0-15
(I) 9.4 2.3 0.39 4.0 4.9 1.34 0.35 2.2 1.42 0.42 0.06 0.6 0.08 0.04 0.04 0.05
(III) 1.4 1.1 n.d. 0.8 0.24 n.d. n.d. 0.08 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d.
(IX) 2.46 0.30 n.d. 0.92 1.09 0.06 n.d. 0.38 0.09 0.02 n.d. 0.04 n.d. n.d. n.d. n.d.
(X) 0.69 0.26 n.d. 0.32 0.10 0.06 n.d. 0.05 0.05 n.d. n.d. 0.02 n.d. n.d. n.d. n.d.
1 Soil type: light humus; pH 5.2; 20.1% fines; 9.8% clay; 10.3% silt; 3.2% C. Application rate - 5 kg a.i./ha. Residue levels
reported in mg/kg in relation to dry material.
2 For Roman numerals see Table 4.
n.d. = not detected.
Table 7 Metabolism of Ring-14C-Phoxim (I) in Soil1
Analysed Day 59 Day 91 Day 128
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-20 0-5 5-20 0-5 5-20
(I) 0.33 0.10 0.22 0.09 0.10 0.04
(XI) 0.06 0.01 0.05 0.01 0.04 0.01
(XII) 0.02 0.001 0.006 0.001 0.004 n.d.
1 pH 6.0; 31.4% fines; 0.89%C. Greenhouse study. Residue levels in mg/kg.
2 For Roman numerals see Table 4.
Results of studies conducted in a closed system, using
ring-14C-phoxim (I) incorporated as a granular formulation into the
soil, showed that the aromatic remainder of the molecule was
mineralized substantially to CO2 (XV) (Steffens 1978). Within 56 days
after application, 25 to 28 percent of the applied 14C activity was
released into the atmosphere as 14CO2.
The metabolic pathway of phoxim in soil is illustrated in Figure
2.
In Plants
The behaviour of phoxim in plants was investigated by studies of
absorption, translocation and accumulation of ring-14C-phoxim in
maize plants, in greenhouse and field studies, and of the metabolism
of 32P and ring-14C-phoxim after foliar application to cotton plants
and after injection of ring-14C-(XI) into tomato plants.
Absorption, translocation and accumulation
The behaviour of phoxim (I) after application as a soil
insecticide was investigated in a greenhouse study in which a 10
percent granular formulation was incorporated in the soil at the time
of sowing maize. The distribution of plant-absorbed 14C activity was
monitored in green maize and at the milk-ripe and harvest-ripe stages
(Dräger 1978a). The results are given in Table 8.
After application of the 10 percent granular formulation of
phoxim to maize in a field experiment (Steffens 1978), less 14C
activity was absorbed by the plant than in the aforementioned
greenhouse study; for example, the harvest-ripe kernels contained 0.02
percent, compared with 0.08 percent in the greenhouse experiment, of
the 14C dose applied to the soil (Table 9).
Metabolism
The effect of light on the metabolism of phoxim (I) was evident
in greenhouse studies conducted with 32P-labelled phoxim on cotton
plants (Dräger 1971). After application of the spray solution to
cotton leaves, the isomeric compound (III) was detected, which was
formed solely on irradiation of the treated test plants. The
simultaneously formed diphosphates (VII) and (VIII) were present only
at very low levels.
Metabolism studies with ring-14C parent compound applied to
foliage of cotton plants are based on model experiments that provided
further information on photolytically formed metabolites and on the
incorporation of metabolites in plant constituents. After exposure for
96 h to irradiation on glass plates, 1 percent (XII), 2 percent (XI),
11 percent (II), 5 percent (III) and 5 percent of the phosphorus-free
metabolite (V) were detected (Dräger, 1978b).
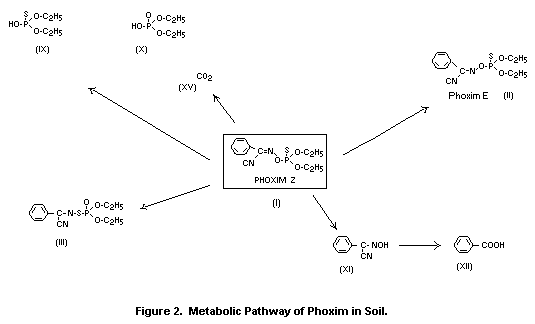 Table 8 14C Activity in Maize (Greenhouse Experiment)
% of applied 14C activity
Plant part Green maize Milk-ripe stage Harvest-ripe stage
(day 59) (Day 91) (Day 128)
Root 0.97 3.3 1.6
Stem + leaves 0.27 0.47 0.68
Cob 0.0097
Spike 0.025
Kernels 0.082
Table 9 14C Activity in Maize (Field Study)
% of applied 14C activity
Plant part Flowering Milk-ripe stage Harvest-ripe stage
(Day 101) (Day 129) (Day 157)
Stem + leaves 0.1 0.1 0.2
Flowers + 0.01 - -
infructescence
Spike - 0.01 0.01
Kernels - 0.01 0.02
Compound (XI), the "acyl" component of phoxim, which was formed
in both irradiation studies and soil studies, was investigated for its
reaction with plant constituents (Dräger 1980a). Following injection
of ring-14C-(XI) into tomato plants, three glycosides were isolated
and identified. At 37 days after application, 13 percent of the
applied 14C activity was present as glucoside (XIII), 4 percent as
gentiobioside (XIV) and 16 percent as a glycoside with an unknown
disaccharide remainder; 39 percent was accounted for by non-reacted
(XI). Of the applied 14C activity, 23 percent was not extractable.
Incorporation into cellulose did not take place; owing to the
stability of the compound to hydrolysis, it is assumed to have been
incorporated into the lignin constituents of the plant.
Metabolism studies with ring-14C-phoxim (I) on cotton leaves in
a greenhouse (Dräger 1980b) resulted in a metabolite pattern similar
to that obtained in the two aforementioned model experiments. In
addition to the compounds found in those two model experiments, a
further phosphorus-free metabolite (IV) was detected. The bulk (85 to
95 percent) of the metabolite mixture was present on the leaf surface
and could be washed off with chloroform. Carbohydrate conjugates of
(XI) were mainly present in the interior of the leaf.
This experiment also provided confirmation of the rapid
degradation of phoxim (I). Residues of the parent compound decreased
with a half-life of about three days. Seven days after application,
phoxim accounted for only 11 percent of the total activity. The
nonextractable portion of 14C activity had increased up to 21 percent
by day 7. It can be assumed that this portion was also incorporated in
plant constituents (lignin).
The metabolic pathways of phoxim in plants are illustrated in
Figure 3.
In Animals
The behaviour of phoxim in animals was investigated in mice and
rats. Absorption, distribution, excretion and metabolism of
ring-UL-14C-phoxim and 32P-phoxim, respectively, were studied. The
biokinetic behaviour of ring-UL-14C-phoxim was investigated in rats
after oral administration at dose levels of 1 mg/kg and 10 mg/kg
(Daniel et el. 1978a). Some studies were conducted with 32P-phoxim
on mice dosed orally with 10.5; 114 and 955 mg/kg (Vinopal & Fukuto
1971).
Absorption
The compound was readily absorbed from the gastrointestinal tract
of male rats, with average maximal plasma levels equivalent to 0.35
and 2.44 µg phoxim/ml being achieved within 30 min. after dosing at 1
and 10 mg/kg, respectively. No radioactivity was detected at 24 h in
the plasma of rats dosed with 1 mg/kg, whereas a value of 0.04 µg
phoxim equivalents/ml was recorded for rats dosed with 10 mg/kg.
Table 8 14C Activity in Maize (Greenhouse Experiment)
% of applied 14C activity
Plant part Green maize Milk-ripe stage Harvest-ripe stage
(day 59) (Day 91) (Day 128)
Root 0.97 3.3 1.6
Stem + leaves 0.27 0.47 0.68
Cob 0.0097
Spike 0.025
Kernels 0.082
Table 9 14C Activity in Maize (Field Study)
% of applied 14C activity
Plant part Flowering Milk-ripe stage Harvest-ripe stage
(Day 101) (Day 129) (Day 157)
Stem + leaves 0.1 0.1 0.2
Flowers + 0.01 - -
infructescence
Spike - 0.01 0.01
Kernels - 0.01 0.02
Compound (XI), the "acyl" component of phoxim, which was formed
in both irradiation studies and soil studies, was investigated for its
reaction with plant constituents (Dräger 1980a). Following injection
of ring-14C-(XI) into tomato plants, three glycosides were isolated
and identified. At 37 days after application, 13 percent of the
applied 14C activity was present as glucoside (XIII), 4 percent as
gentiobioside (XIV) and 16 percent as a glycoside with an unknown
disaccharide remainder; 39 percent was accounted for by non-reacted
(XI). Of the applied 14C activity, 23 percent was not extractable.
Incorporation into cellulose did not take place; owing to the
stability of the compound to hydrolysis, it is assumed to have been
incorporated into the lignin constituents of the plant.
Metabolism studies with ring-14C-phoxim (I) on cotton leaves in
a greenhouse (Dräger 1980b) resulted in a metabolite pattern similar
to that obtained in the two aforementioned model experiments. In
addition to the compounds found in those two model experiments, a
further phosphorus-free metabolite (IV) was detected. The bulk (85 to
95 percent) of the metabolite mixture was present on the leaf surface
and could be washed off with chloroform. Carbohydrate conjugates of
(XI) were mainly present in the interior of the leaf.
This experiment also provided confirmation of the rapid
degradation of phoxim (I). Residues of the parent compound decreased
with a half-life of about three days. Seven days after application,
phoxim accounted for only 11 percent of the total activity. The
nonextractable portion of 14C activity had increased up to 21 percent
by day 7. It can be assumed that this portion was also incorporated in
plant constituents (lignin).
The metabolic pathways of phoxim in plants are illustrated in
Figure 3.
In Animals
The behaviour of phoxim in animals was investigated in mice and
rats. Absorption, distribution, excretion and metabolism of
ring-UL-14C-phoxim and 32P-phoxim, respectively, were studied. The
biokinetic behaviour of ring-UL-14C-phoxim was investigated in rats
after oral administration at dose levels of 1 mg/kg and 10 mg/kg
(Daniel et el. 1978a). Some studies were conducted with 32P-phoxim
on mice dosed orally with 10.5; 114 and 955 mg/kg (Vinopal & Fukuto
1971).
Absorption
The compound was readily absorbed from the gastrointestinal tract
of male rats, with average maximal plasma levels equivalent to 0.35
and 2.44 µg phoxim/ml being achieved within 30 min. after dosing at 1
and 10 mg/kg, respectively. No radioactivity was detected at 24 h in
the plasma of rats dosed with 1 mg/kg, whereas a value of 0.04 µg
phoxim equivalents/ml was recorded for rats dosed with 10 mg/kg.
 Distribution
The distribution of radioactivity in the organs and tissues of
male rats intubated with 14C-phoxim at 10 mg/kg has been
investigated. Apart from the large intestine and its contents, the
distribution of radioactivity was essentially similar to that for
plasma (Daniel et al. 1978a). A study has also been made of
radioactivity in the gastrointestinal tract of male rats up to 7.5 h
after dosing with phoxim at 1 mg/kg. In these studies, no accumulation
of radioactivity in any organ or tissue was found.
The studies of Vinopal & Fukuto (1971) on white mice were
negative for the period 0 to 48 h after oral application of 114 mg/kg
32P-phoxim (Table 10).
The amounts of organosoluble material (which might include the
strong anticholinesterase PO phoxim) were essentially insignificant.
The reason for the apparent uptake of radioactivity in the urinary
bladder of the mouse is not clear.
Excretion
A study was made on excretion of radioactivity in the urine and
faeces of male and female rats given a single oral dose of 14C-phoxim
at 10 mg/kg (Daniel et al 1978a). Male rats excreted an average of
92.2 percent of the radioactivity in the urine and 4.9 percent in the
faeces in ten days, while females excreted 86.1 percent of the dose in
the urine and 6.9 percent in the faeces in the same period.
Male rats intubated with 14C-phoxim at a dose of 1 mg/kg
excreted 82 percent of the radioactivity in the urine and 7.9 percent
in the faeces in ten days. The results indicate that most of the
radioactivity was eliminated in 24 h and excretion was virtually
complete within two days. No evidence was obtained for the presence of
14CO2 in expired air.
An average of 4.1 percent S.D. 2.3) of the radioactivity was
excreted within 0-24 h in the bile of male rats intubated with
14C-phoxim at 10 mg/kg (Daniel et al. 1978a).
Following oral application of 10.5, 114 and 955 mg/kg 32P-phoxim
to mice, the ultimate recovery of administered radioactivity in the
urine and faeces was in the range of 73 to 84 percent (Vinopal &
Fukuto 1971). However, the radioactivity appeared in the urine and
faeces at a much lower rate than expected. For example, 24 h after
oral treatment of mice at 10.5 and 114 mg/kg with radioactive phoxim,
only 43 percent and 22 percent, respectively, of the administered
radioactivity was excreted in the urine. At 955 mg/kg, only 17 percent
of the administered radioactivity was excreted in the urine after
30 h.
Metabolism
Studies by Daniel et al. (1978b) with ring-UL-14C-phoxim on
rats produced the metabolic pathway shown in Figure 4. Phoxim was
largely absorbed and was detected in plasma as a dealkylated compound
in the P=S and P=O form. The radioactive metabolites in the urine of
rats at 0 to 24 h after oral application of 10 mg/kg consisted of
about 90 percent sulphate and glucuronide conjugates that were
hydrolysed enzymatically to alpha-hydroxy-imono-phenylacetonitrile
(XI), as demonstrated by thin-layer chromatography and mass
spectrometry. Hippuric acid was detected as a metabolite by inverse
isotope dilution analysis; it represented about 5 percent of the
radioactivity eliminated through the kidneys.
Radioactive urine from white mice after oral treatment with
32P-phoxim was analysed by ion-exchange and thin-layer chromatography
(Vinopal & Fukuto 1971). Five metabolites were recovered after
treatment with 32P-phoxim at 114 mg/kg and 955 mg/kg. The identity of
the metabolites was established by thin-layer chromatography with
specific staining reagents and/or IR-spectroscopy. The metabolites
were identified as diethylphosphoric acid (X), phoxim (I), phoxim
carboxylic acid (XVIII), O,O-diethyl phosphorothioic acid (IX) and
either desethyl phoxim or desethyl PO phoxim (XVIII). The amounts of
radioactivity relative to total radioactivity renally eliminated are
summarized in Table 11.
Photodegradation
The U.V. spectrum of phoxim (in methanol) shows a relatively
long-wave peak at 282 nm (… = 11.180); its long wavelength absorption
edge overlaps with the sunlight emission in the troposphere (lambda
> 290 nm). In the environment, direct interaction of the molecule
with sunlight is, therefore, possible (Wilmes 1981).
Experiments using double-distilled water (5 ng/kg, 0.5 percent
acetonitrile) yielded very short half-lives. The half-life was less
than 10 min with TQ 150 high-pressure mercury lamps (Duran filter,
laubda > 290 nm) and about 2 h with sunlight-simulating fluorescent
tubes (TRU LITE) (Wilmes 1981).
The results of studies on cotton leaves and glass plates with
differently labelled phoxim (Dräger 1971, 1978a) are indicative of the
significance of photodegradation in this compound. Among others, a
photoisomerization product (III) was isolated and identified, which
was also detected in chlorphoxim (Dräger 1972) and methylphoxim
(Dräger 1975) derivatives. After exposure of phoxim to irradiation on
glass plates, the metabolites III, V, VII, VIII, XI and XII were
isolated and identified. Partial isomerization of the Z-isomer (I),
prevalent in the technical grade compound, to the E-isomer (II) was
also observed.
Table 10 Summary of Autopsy Data on a White Mouse
after Oral Treatment with 32P-phoxim1
% Recovered internal radioactivity
Organ Organo- Water- Total
soluble soluble
Brain 0.07 0.14 0.21
Thymus gland 0.01 0.05 0.06
Hind leg muscle n.d. 0.54 0.54
Heart 0.01 0.23 0.24
Kidney 0.03 0.13 0.16
Liver 0.05 1.60 1.65
Gut 0.17 8.60 8.77
Urinary bladder 2.10 86.30 88.40
Total 2.44 97.59 100.0
1 Postmortem examination 48 h after dosing with
114 mg/kg 32P-phoxim.
Table 11 Metabolites in Urine from White Mice after Treatment
with Phoxim
Metabolite1 % of radioactivity in urine
114 mg/kg dose 955 mg/kg dose
(after 24 h) (after 30 h)
X 58.9 43.1
I 1.1 2.1
XVII 2.8 23.6
IX 20.0 17.7
XVIII 6.2 5.0
RA not in peaks 4.3 5.4
unrecovered2 6.7 3.1
1 For chemical names, see Table 4.
2 Unrecovered after ion-exchange column chromatography.
Distribution
The distribution of radioactivity in the organs and tissues of
male rats intubated with 14C-phoxim at 10 mg/kg has been
investigated. Apart from the large intestine and its contents, the
distribution of radioactivity was essentially similar to that for
plasma (Daniel et al. 1978a). A study has also been made of
radioactivity in the gastrointestinal tract of male rats up to 7.5 h
after dosing with phoxim at 1 mg/kg. In these studies, no accumulation
of radioactivity in any organ or tissue was found.
The studies of Vinopal & Fukuto (1971) on white mice were
negative for the period 0 to 48 h after oral application of 114 mg/kg
32P-phoxim (Table 10).
The amounts of organosoluble material (which might include the
strong anticholinesterase PO phoxim) were essentially insignificant.
The reason for the apparent uptake of radioactivity in the urinary
bladder of the mouse is not clear.
Excretion
A study was made on excretion of radioactivity in the urine and
faeces of male and female rats given a single oral dose of 14C-phoxim
at 10 mg/kg (Daniel et al 1978a). Male rats excreted an average of
92.2 percent of the radioactivity in the urine and 4.9 percent in the
faeces in ten days, while females excreted 86.1 percent of the dose in
the urine and 6.9 percent in the faeces in the same period.
Male rats intubated with 14C-phoxim at a dose of 1 mg/kg
excreted 82 percent of the radioactivity in the urine and 7.9 percent
in the faeces in ten days. The results indicate that most of the
radioactivity was eliminated in 24 h and excretion was virtually
complete within two days. No evidence was obtained for the presence of
14CO2 in expired air.
An average of 4.1 percent S.D. 2.3) of the radioactivity was
excreted within 0-24 h in the bile of male rats intubated with
14C-phoxim at 10 mg/kg (Daniel et al. 1978a).
Following oral application of 10.5, 114 and 955 mg/kg 32P-phoxim
to mice, the ultimate recovery of administered radioactivity in the
urine and faeces was in the range of 73 to 84 percent (Vinopal &
Fukuto 1971). However, the radioactivity appeared in the urine and
faeces at a much lower rate than expected. For example, 24 h after
oral treatment of mice at 10.5 and 114 mg/kg with radioactive phoxim,
only 43 percent and 22 percent, respectively, of the administered
radioactivity was excreted in the urine. At 955 mg/kg, only 17 percent
of the administered radioactivity was excreted in the urine after
30 h.
Metabolism
Studies by Daniel et al. (1978b) with ring-UL-14C-phoxim on
rats produced the metabolic pathway shown in Figure 4. Phoxim was
largely absorbed and was detected in plasma as a dealkylated compound
in the P=S and P=O form. The radioactive metabolites in the urine of
rats at 0 to 24 h after oral application of 10 mg/kg consisted of
about 90 percent sulphate and glucuronide conjugates that were
hydrolysed enzymatically to alpha-hydroxy-imono-phenylacetonitrile
(XI), as demonstrated by thin-layer chromatography and mass
spectrometry. Hippuric acid was detected as a metabolite by inverse
isotope dilution analysis; it represented about 5 percent of the
radioactivity eliminated through the kidneys.
Radioactive urine from white mice after oral treatment with
32P-phoxim was analysed by ion-exchange and thin-layer chromatography
(Vinopal & Fukuto 1971). Five metabolites were recovered after
treatment with 32P-phoxim at 114 mg/kg and 955 mg/kg. The identity of
the metabolites was established by thin-layer chromatography with
specific staining reagents and/or IR-spectroscopy. The metabolites
were identified as diethylphosphoric acid (X), phoxim (I), phoxim
carboxylic acid (XVIII), O,O-diethyl phosphorothioic acid (IX) and
either desethyl phoxim or desethyl PO phoxim (XVIII). The amounts of
radioactivity relative to total radioactivity renally eliminated are
summarized in Table 11.
Photodegradation
The U.V. spectrum of phoxim (in methanol) shows a relatively
long-wave peak at 282 nm (… = 11.180); its long wavelength absorption
edge overlaps with the sunlight emission in the troposphere (lambda
> 290 nm). In the environment, direct interaction of the molecule
with sunlight is, therefore, possible (Wilmes 1981).
Experiments using double-distilled water (5 ng/kg, 0.5 percent
acetonitrile) yielded very short half-lives. The half-life was less
than 10 min with TQ 150 high-pressure mercury lamps (Duran filter,
laubda > 290 nm) and about 2 h with sunlight-simulating fluorescent
tubes (TRU LITE) (Wilmes 1981).
The results of studies on cotton leaves and glass plates with
differently labelled phoxim (Dräger 1971, 1978a) are indicative of the
significance of photodegradation in this compound. Among others, a
photoisomerization product (III) was isolated and identified, which
was also detected in chlorphoxim (Dräger 1972) and methylphoxim
(Dräger 1975) derivatives. After exposure of phoxim to irradiation on
glass plates, the metabolites III, V, VII, VIII, XI and XII were
isolated and identified. Partial isomerization of the Z-isomer (I),
prevalent in the technical grade compound, to the E-isomer (II) was
also observed.
Table 10 Summary of Autopsy Data on a White Mouse
after Oral Treatment with 32P-phoxim1
% Recovered internal radioactivity
Organ Organo- Water- Total
soluble soluble
Brain 0.07 0.14 0.21
Thymus gland 0.01 0.05 0.06
Hind leg muscle n.d. 0.54 0.54
Heart 0.01 0.23 0.24
Kidney 0.03 0.13 0.16
Liver 0.05 1.60 1.65
Gut 0.17 8.60 8.77
Urinary bladder 2.10 86.30 88.40
Total 2.44 97.59 100.0
1 Postmortem examination 48 h after dosing with
114 mg/kg 32P-phoxim.
Table 11 Metabolites in Urine from White Mice after Treatment
with Phoxim
Metabolite1 % of radioactivity in urine
114 mg/kg dose 955 mg/kg dose
(after 24 h) (after 30 h)
X 58.9 43.1
I 1.1 2.1
XVII 2.8 23.6
IX 20.0 17.7
XVIII 6.2 5.0
RA not in peaks 4.3 5.4
unrecovered2 6.7 3.1
1 For chemical names, see Table 4.
2 Unrecovered after ion-exchange column chromatography.
 Photoproducts detected in aqueous solution (50 percent
acetonitrile), after complete degradation of the parent compound, were
alpha-cyanobenzaldoxime (XI) and bis-alpha-
dithioiminophenylacetronitrile (VI) (Wilmes 1981).
Pao (1975) reported studies conducted to investigate degradation
of phoxim on tea leaves under natural conditions (sunlight) and in the
laboratory (UV light). In both cases, distinct photodegradation was
observed. The same results were obtained in studies on glass plates
and tomato leaves (Makari et al. 1981).
In Storage and Processing
The stability of phoxim residues during storage at -20°C was
monitored on lettuce over a period of 2.5 years. From regression
curves plotted for the determined residue values in relation to time,
assuming a first-order reaction, it was evident that no significant
decrease in the phoxim residues occurred during low-temperature
storage (Dräger 1982).
The stability of phoxim residues in grain stored at 26.7°C was
monitored for 12 months after application of phoxim at 5, 10 and 20
mg/kg to maize, wheat and sorghum. Only minor losses were recorded
during this period (LaHue & Dicke 1971).
Wheat grains fortified with 8 mg/kg phoxim were milled in an
experimental study. The flour from the first and second milling
operations, which accounted for 73 percent of the total material
milled, contained 13 percent of the applied phoxim dose; a phoxim
content of 1.3 mg/kg was determined by measuring 32P activity. The
bulk of phoxim was present in the middlings and semolina, accounting
for 35 percent and 43 percent, respectively, of the applied phoxim
dose. These milled fractions represented only 11 percent and 7
percent, respectively, of the total milled material; therefore, their
content of phoxim amounting to 23 mg/kg and 44 mg/kg, respectively,
was substantially higher than that in flour (Dräger 1968). Extraction
of wheat bread made from the milled fractions 1 and 2 showed that the
major portion of the degradation products that arise from phoxim
during the baking process, either due to chemical decomposition or as
a result of enzymatic degradation, were present in the form of highly
polar, water-soluble compounds. Further quantitative analyses were
performed by gas-chromatography on whole-meal bread made by baking the
whole of the milled material; 65 percent of the applied phoxim was
split into diethyl thiophosphoric acid during the baking process. It
was also observed that no more unchanged parent compound was present
(Dräger 1968).
The effect of boiling on residues of phoxim and phoxim-oxon was
studied in potatoes. The phoxim residues were reduced by 86 percent
during boiling; of the remaining residue, one fifth was present in the
water used for boiling. The oxon of phoxim was reduced by 91 percent
during boiling; of the compound still present, two thirds was found in
the water used for boiling (Dräger 1978c).
METHODS OF RESIDUE ANALYSIS
Residues of phoxim in plant and soil samples can be determined by
gas chromatography using a phosphorus-specific flame-photometric
detector or a thermionic phosphorus detector. These methods
simultaneously determine the P=O compound of phoxim (Bowman & Leuck
1971; Dräger 1969 a,b; Thornton 1969). Thin-layer chromatography has
also been used for determining phoxim residues (Bykhovets 1974; Pao
1975).
For the extraction of residues from plant material, acetone,
acetonitrile, chloroform or benzene was used. For Soxhlet extraction
of plant material, a mixture of 10 percent methanol in benzene was
used (Bowman & Leuck 1971). Soil samples were extracted with methanol.
Isolation of the residues from the extracts were achieved by
partitioning with N-hexane or chloroform. Separation of co-extracted
plant constituents was achieved by precipitating with a solution of
ammonium chloride/phosphoric acid or by partitioning between
acetonitrile and N-hexane. For column-chromatographic cleanup of the
extracts, silica gel or activated charcoal/aluminium oxide was used.
Owing to the termal instability of phoxim, it is necessary, for
gas-chromatographic determination of the parent compound, to use
supports coated with, for example, 5 percent OV-101 on Gas Chrom Q or
2 percent DC-200 + 1 percent (or 2 percent) QF-1 on Gas Chrom Q.
Injection port and column temperatures were set at 160° - 170° (Dräger
1969a,b; Bowman & Leuck 1971; Thornton 1969).
For thin-layer chromatographic detection of residues, silica gel
plates were used with the following solvents and reagents:
N-hexane/acetone 4:1 - bromophenol blue/silver nitrate (Bykhovets
1974) and benzene/butane 1:1 - Congo Red (Pao 1975). The lower limit
of determination for gas-chromatographic detection of residues in
plant and soil samples was usually 0.05 mg/kg; in special cases,
residues were determined down to a level of 0.004 mg/kg. For
thin-layer chromatographic determination in tea, the lower limit of
determination was found to be 0.5 mg/kg (Pao 1975).
Residues on phoxim in animal tissues can be determined by
gas-liquid chromatography, using a thermionic detector or, preferably,
a flame photometric detector. Residues are extracted from tissues with
hexane in a Waring blender and by soaking after addition of anhydrous
sulphate. The extract is cleaned up by partitioning with acetonitrile
and residues are transferred to hexane for the GLC-determination. A
2 percent OV-101 glass column may be used. The limit of determination
has been reported as 0.05 mg/kg (Hopkins 1980).
Residues of phoxim and its oxygen analogue in milk have been
determined by thin-layer chromatography after extraction with ethyl
acetate and cleanup of the extract by partitioning with acetonitrile
and hexane. A homogenate of bee-heads is used for determination of the
cholinesterase inhibition effect. The limit of determination has been
reported as 0.01 mg/kg (Ernst 1980).
In an interference study, performed to determine whether any of
the organophosphorus compounds registered for use on bananas to
control insect pests would interfere with the method for
gas-chromatographic determination of phoxim, no interferences were
observed from any of the compounds and metabolites examined (Olson
1974).
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) and associated preharvest
intervals reported to the Meeting are shown in Table 12.
APPRAISAL
Phoxim is a nonsystemic contact and stomach organophosphorus
insecticide. It is registered and used in a number of countries and is
especially effective against biting insects. It is applied both to
foliage and soil and is also used as a seed dressing. Phoxim is
registered for veterinary use against ectoparasites.
When applied to vegetable foliage, the dosage rates are 0.75 kg
to 1.5 kg a.i./ha and on cotton the rate is 1.5 to 2.3 kg a.i./ha.
Phoxim is applied as a soil treatment to vegetables, using
emulsifiable concentrates, baits or granules at dosage rates of 0.8 to
5.0 kg a.i./ha. Phoxim is applied as granules for soil treatment to
potatoes at a dosage rate of 1.5 to 7.5 kg a.i./ha as a soil treatment
for grains using 5.0 kg a.i./ha of granular formulation.
Residues in crops following foliar and soil treatment are
generally below 0.2 mg/kg and average values are generally below the
limit of determination (0.004 to 0.05 mg/kg). Residues in grains (oat,
barley, wheat and maize) with soil treatment before sowing were all
below the limit of determination. Also, residues with soil treatment
before or at sowing or planting of beans, cauliflower, cabbage and
onions were below the limit of determination. Average residues in
potatoes after soil treatment were below the limit of determination,
with the highest single value being 0.02 mg/kg. Residue after foliar
treatment in vegetables were low, with the highest average value being
0.13 mg/kg (tomatoes) and the highest single value 0.20 mg/kg
(spinach).
Table 12 National Maximum Residue Limits and Preharvest Intervals
Reported to the Meeting
Country Crop MRL Preharvest interval
(mg/kg) (days)
Australia Potatoes 0.05
Austria All food crops 0.05
Czechoslovakia Potatoes 14
Fed. Rep. Germany All food crops 0.05
Italy Fruit 0.05 42
Vegetables 0.05 42
Luxembourg All food crops 0.05
The Netherlands Vegetables 0.05
Milk & milk products 0.01
Meat & meat products 0.01 -> 0.05
Other products 0 -> 0.05
South Africa Cereals 0.2
Peanuts 0.2
Spain General 15
Thailand Maize 15
Cotton 15
Potatoes 15
Sorghum 15
Vegetables 15
Soviet Union Brassicas 20
Eggplant 20
Perennial grasses 20
Potatoes 20
Sugarbeet 20
Tomatoes 20
Yugoslavia All crops 0.05
In experimental treatment of sheep, cattle and pigs with phoxim,
by dipping or spraying, dosages ranged from 500 to 3 000 mg/l. At
slaughter, 14 to 45 days after treatment, no residues were observed in
samples of liver, kidney or muscle. However, residues were found in
fat from all animals, ranging from the limit of determination to 2.8
mg/kg, depending on treatment and waiting period.
In laboratory and field experiments phoxim degradation was
observed in different soil types, with half-lives from one day to
about two weeks, but in a greenhouse study the half-life was about
eight weeks. The main metabolites of phoxim in soil were
alpha-hydroxy-imino-phenylacetonitrile and benzoic acid, both formed
by hydrolysis, but diethyl phosphoric acids were also found in soil.
Laboratory and field leaching experiments in soil showed that
phoxim did not reach a depth below 5 cm and 10 cm, respectively, in
the laboratory and field.
Phoxim or its metabolites were found in roots, stems and leaves
of maize after soil treatment. The cobs, spikes and kernels contained
very little phoxim or metabolites. After foliar treatment, the leaves
contained phoxim and metabolites, but most of these were present on
the surface of the leaves and could be removed with chloroform.
The metabolites formed in or on plants consisted of an isoner:
diethoxy-phosphoryl-thioimino-phenyl-acetonitrile, which was formed
mainly by irradation of the plant, together with minor amounts of
tetraethyl diphosphate and tetraethyl mono-thio-diphosphate. The
"acyl"-compounds (phosphorous-free metabolites) formed by hydrolysis
reacted with plant constituents to three glycosides. The parent
compound degraded rapidly; after seven days only 11 percent of the
total activity was attributable to phoxim.
Investigations on animal metabolism were made in rats and mice.
No information was available on metabolism in cattle, pigs and poultry
for evaluation of residues of phoxim and its metabolites in animal
products.
Experiments were carried out on the stability of phoxim during
storage. Lettuce stored at -20° C for 2.5 years showed no significant
decrease in phoxim content.
Flour milled from grain fortified with phoxim contained 13
percent of the applied phoxim, while middlings and semolina accounted
for 35 percent and 43 percent, respectively, of the applied dose.
Bread made from flour containing phoxim did not contain unchanged
parent compound. Phoxim residues in potatoes were reduced by 91
percent during boiling.
Residues of phoxim in plant and soil samples can be determined by
gas-liquid chromatography with a flame photometric detector or a
thermonic detector. Thin-layer chromatography has also been used for
determining phoxim residues. Extraction can be made from plant
material with acetone, acetonitrile, chloroform or benzene. For
Soxhlet extraction, 10 percent methanol in benzene has been used. Soil
is extracted with methanol. Residues of phoxim in animal tissues are
extracted with hexane and residues in milk with ethylacetate. Cleanup
of extracts has been achieved by partitioning, precipitation with
ammonium chloride/phosphoric acid and/or column chromatography.
RECOMMENDATIONS
The following levels are recommended as MRLs, which need not be
exceeded when phoxim is used according to good agriculture practice.
The limits refer to the parent compound only.
Commodity MRL Preharvest intervals
on which recommendations
are based
Cereal grains 0.05** 1)
Lettuce 0.1 14 days
Beans 0.05** 14 lays
Cauliflower 0.05** 42 days
Potatoes 0.05** 1)
Tomatoes 0.2 14 days
Cotton seed 0.05** 14 days
Interval between treatment
and slaughtering
Cattle, carcase 0.02
meat (in carcase fat) 28 days
Sheep, carcase meat 1 (in carcase 28 days
fat)
1) Soil treatment at sowing or planting.
** Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Residue data in milk from treatment of cattle and residue data in
fat from treatment of pigs.
2. Studies on metabolism of phoxim from treatment of cattle, pigs
and sheep.
3. Information on good agriculture practice in the use of phoxim on
vegetables.
REFERENCES - RESIDUES
Mobay reports are unpublished reports prepared by the R & D Dept.
of Mobay Chemical Corporation, Agricultural Chemicals Division, Kansas
City, Mo. 64120, USA.
Nitokuno reports are unpublished reports prepared by Nihon
Tokushu Noyaku Seizo, K.K., No. 8, 2-chome, Nihonbashi Muro-machi,
Chuo-ku, Tokyo, Japan.
Bayer. Pflanzenschutzmittel-Rückstände. Reports Nos. 165-166/70.
1970 (Unpublished)
Bayer. Pflanzenschutzmittel-Rückstände im Sickerwasser. Reports Nos.
1972 283-288/72; Reports Nos. 3003-3004/74; Reports Nos. 3020-
1974 3022/77. (Unpublished)
1977
Biologische Bundesanstalt für Land- und Forstwirtschaft. Prüfung des
1980 Versickerung-sverhaltens von Pflanzenschutzmitteln.
Merkblatt Nr. 37, 2. Auflage.
Bowman, M.C. & Leuck, D.B. Determination and persistence of phoxim and
1971 its oxygen analog in forage corn and grass. J. Agric. Food
Chem., 19: 1215-1218.
Bykhovets, A.J. Thin-layer chromatographic method to determine Basudin
1974 and Valexon in a plant sample. Aktual. Vopr. Zashch. Rast.
BSSR, Mater, Resp. Konf, Molodykh Uch., 1: 81-82.
Daniel, J.W., Swanson, S. & McLean, J. Phoxim - Pharmacokinetics and
1978a biotransformation in the rat. Life Science Research, U.K.
Report 78/BAG5/194. (Unpublished)
Daniel, J.W., McLean, J. & Pringuer, M. Phoxim - Biotransformation in
1978b the rat. Life Science Research, U.K. Report 78/BAG5/317.
(Unpublished)
Dräger, G. Studies on the metabolism of BAY 77 488. Bayer AG,
1968 Pflanzenschutz-Anwendungstechnik, Report. (Unpublished)
Dräger, G. Gaschromatographische Methode zur Bestimmung von RValexon
1969a Rückständen in Pflanzen. Pflanzenschutz-Nachr. Bayer,
22:311-317 (1969a)
Dräger, G. Rückstandsanalytik von Pflanzenschutzmitteln. DFG-
1969b Methodensammlung, 1. Lieferung 1969; methode Nr. 307.
Dräger, G. Studies on the metabolism of phoxim (BAY 77 488).
1971 Pflanzenschutz-Nachr. Bayer, 24: 239-251.
Dräger, G. Reaction products of chlorphoxim (BAY 78 182) formed by
1972 exposure of the parent compound to irradiation.
Pflanzenschutz-Nachr. Bayer, 25: 32-42.
Dräger, G. Metabolisierung von Phoxim-methyl durch Belichtung. Bayer
1975 AG, Pflanzenschutz-Anwendungstechnik report RA-57.
(Unpublished)
Dräger, G. Studies on the fate of phoxim (BAY 77 488) in soil.
1977 Pflanzenschutz-Nachr. Bayer, 30:28-41.
Dräger, G. Untersuchung des Metabolismus von 14C-ringmarkiertem
Phoxim
1978a in Mais und Boden nach Anwendung enies Granulates. 1. 14C-
Bilanz und Metabolismus im Boden. Bayer AG, Pflanzenschutz-
Anwendungstechnik report RA-278. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim; Belichtung von
1978b 14C-ringmarkiertem Wirkstoff auf Glassplatten. Bayer AG,
Pflanzenschutz-Anwendungstechnik report RA-618.
(Unpublished)
Dräger, G. Stability of phoxim residues during boiling of potatoes.
1978c Bayer AG, Pflanzenschutz-Anwendungstechnik report RA-843.
(Unpublished)
Dräger, G. Untersuchung zum Metabolismus von Phoxim; Metabolismus
1980a des Abbauproduktes alpha-Hydroxyiminophenolacetonitril in
Tomatenpflanzen. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-106. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim auf
1980b Baumwollblättern. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-208. (Unpublished)
Dräger, G. Stability of phoxim residues in lettuce during low
1982 temperature storage. Bayer AG, Pflanzenschutz-
Anwendungstechnik, report RA-69. (Unpublished)
Ernst, G. F., Brinkman, M.B.C. & Mattern, E.M. Methode van onderzoek
1981 voor het bepalen van residuen van foxim en foxon im melk.
KvW Utrecht, IR/71/80/R79-Okt.
Hopkins, T., Lemprecht, C. & Lindsay, G. Bay 9053 - 50% w/v E.C. -
1980 residues - sheep. Bayer Doc. No. 80/9955.
LaHue, D.W. & Dicke, E.B. Phoxim as an insect protectant for stored
1971 grains. J. Econ. Entomol., 64: 1530-1533.
Makari, M.H., Riskallah, M.R., Hegazy, M.E. & Belal, M.H. Photolysis
1981 of phoxim on glass and on tomato leaves. Bull. Environ.
Contam. Toxicol. 26: 413-419 (1981).
Nitokuno. Residual analysis results of VolatonR (phoxim) in upland
1977 soil. Report No. 1058 (RA). (Unpublished)
Olson, T.J. An interference study for the residue method for Volaton
1974 determination on bananas. Mobay Report No. 40 095.
(Unpublished)
Pao, K.C.H. Residue studies of phoxim (Baythion) on the tea bushes.
1975 Acta Entomol. Sin., 18: 133-139.
Steffens, W. Bilanzversuche mit (benzolring-U-14C) Phoxim nach
1978 Verwendung als Bodeninsektizid zu Mais in einem
Freilandlysimeter und einem geschlossenen System im
Gewächshaus. Kernforschungsnalage Jülich, Institut für
Radioagronomie, report ARA 14/78. (Unpublished)
Thornton, J.S. Determination of residues of BAY 77 488 in stored
1969 grains. Mobay report No. 24 575. (Unpublished)
Thornton, J.S., Hurley, J.B. & Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticides chemicals. Mobay report No. 51 016.
(Unpublished)
Wilmes, R. Orientierende Lichtstabilität (Phoxim). Bayer AG,
1981 Pflanzenschutz-Anwendungstechnik report. (Unpublished)
Vinopal, J.H.A. & Fukuto, T.R. Selective toxicity of phoxim
1971 (phenylglyoxylonitrile oxime 0,0-diethyl phosphorothioate).
Pestic. Biochem. Physiol., 1: 44-60.
Photoproducts detected in aqueous solution (50 percent
acetonitrile), after complete degradation of the parent compound, were
alpha-cyanobenzaldoxime (XI) and bis-alpha-
dithioiminophenylacetronitrile (VI) (Wilmes 1981).
Pao (1975) reported studies conducted to investigate degradation
of phoxim on tea leaves under natural conditions (sunlight) and in the
laboratory (UV light). In both cases, distinct photodegradation was
observed. The same results were obtained in studies on glass plates
and tomato leaves (Makari et al. 1981).
In Storage and Processing
The stability of phoxim residues during storage at -20°C was
monitored on lettuce over a period of 2.5 years. From regression
curves plotted for the determined residue values in relation to time,
assuming a first-order reaction, it was evident that no significant
decrease in the phoxim residues occurred during low-temperature
storage (Dräger 1982).
The stability of phoxim residues in grain stored at 26.7°C was
monitored for 12 months after application of phoxim at 5, 10 and 20
mg/kg to maize, wheat and sorghum. Only minor losses were recorded
during this period (LaHue & Dicke 1971).
Wheat grains fortified with 8 mg/kg phoxim were milled in an
experimental study. The flour from the first and second milling
operations, which accounted for 73 percent of the total material
milled, contained 13 percent of the applied phoxim dose; a phoxim
content of 1.3 mg/kg was determined by measuring 32P activity. The
bulk of phoxim was present in the middlings and semolina, accounting
for 35 percent and 43 percent, respectively, of the applied phoxim
dose. These milled fractions represented only 11 percent and 7
percent, respectively, of the total milled material; therefore, their
content of phoxim amounting to 23 mg/kg and 44 mg/kg, respectively,
was substantially higher than that in flour (Dräger 1968). Extraction
of wheat bread made from the milled fractions 1 and 2 showed that the
major portion of the degradation products that arise from phoxim
during the baking process, either due to chemical decomposition or as
a result of enzymatic degradation, were present in the form of highly
polar, water-soluble compounds. Further quantitative analyses were
performed by gas-chromatography on whole-meal bread made by baking the
whole of the milled material; 65 percent of the applied phoxim was
split into diethyl thiophosphoric acid during the baking process. It
was also observed that no more unchanged parent compound was present
(Dräger 1968).
The effect of boiling on residues of phoxim and phoxim-oxon was
studied in potatoes. The phoxim residues were reduced by 86 percent
during boiling; of the remaining residue, one fifth was present in the
water used for boiling. The oxon of phoxim was reduced by 91 percent
during boiling; of the compound still present, two thirds was found in
the water used for boiling (Dräger 1978c).
METHODS OF RESIDUE ANALYSIS
Residues of phoxim in plant and soil samples can be determined by
gas chromatography using a phosphorus-specific flame-photometric
detector or a thermionic phosphorus detector. These methods
simultaneously determine the P=O compound of phoxim (Bowman & Leuck
1971; Dräger 1969 a,b; Thornton 1969). Thin-layer chromatography has
also been used for determining phoxim residues (Bykhovets 1974; Pao
1975).
For the extraction of residues from plant material, acetone,
acetonitrile, chloroform or benzene was used. For Soxhlet extraction
of plant material, a mixture of 10 percent methanol in benzene was
used (Bowman & Leuck 1971). Soil samples were extracted with methanol.
Isolation of the residues from the extracts were achieved by
partitioning with N-hexane or chloroform. Separation of co-extracted
plant constituents was achieved by precipitating with a solution of
ammonium chloride/phosphoric acid or by partitioning between
acetonitrile and N-hexane. For column-chromatographic cleanup of the
extracts, silica gel or activated charcoal/aluminium oxide was used.
Owing to the termal instability of phoxim, it is necessary, for
gas-chromatographic determination of the parent compound, to use
supports coated with, for example, 5 percent OV-101 on Gas Chrom Q or
2 percent DC-200 + 1 percent (or 2 percent) QF-1 on Gas Chrom Q.
Injection port and column temperatures were set at 160° - 170° (Dräger
1969a,b; Bowman & Leuck 1971; Thornton 1969).
For thin-layer chromatographic detection of residues, silica gel
plates were used with the following solvents and reagents:
N-hexane/acetone 4:1 - bromophenol blue/silver nitrate (Bykhovets
1974) and benzene/butane 1:1 - Congo Red (Pao 1975). The lower limit
of determination for gas-chromatographic detection of residues in
plant and soil samples was usually 0.05 mg/kg; in special cases,
residues were determined down to a level of 0.004 mg/kg. For
thin-layer chromatographic determination in tea, the lower limit of
determination was found to be 0.5 mg/kg (Pao 1975).
Residues on phoxim in animal tissues can be determined by
gas-liquid chromatography, using a thermionic detector or, preferably,
a flame photometric detector. Residues are extracted from tissues with
hexane in a Waring blender and by soaking after addition of anhydrous
sulphate. The extract is cleaned up by partitioning with acetonitrile
and residues are transferred to hexane for the GLC-determination. A
2 percent OV-101 glass column may be used. The limit of determination
has been reported as 0.05 mg/kg (Hopkins 1980).
Residues of phoxim and its oxygen analogue in milk have been
determined by thin-layer chromatography after extraction with ethyl
acetate and cleanup of the extract by partitioning with acetonitrile
and hexane. A homogenate of bee-heads is used for determination of the
cholinesterase inhibition effect. The limit of determination has been
reported as 0.01 mg/kg (Ernst 1980).
In an interference study, performed to determine whether any of
the organophosphorus compounds registered for use on bananas to
control insect pests would interfere with the method for
gas-chromatographic determination of phoxim, no interferences were
observed from any of the compounds and metabolites examined (Olson
1974).
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) and associated preharvest
intervals reported to the Meeting are shown in Table 12.
APPRAISAL
Phoxim is a nonsystemic contact and stomach organophosphorus
insecticide. It is registered and used in a number of countries and is
especially effective against biting insects. It is applied both to
foliage and soil and is also used as a seed dressing. Phoxim is
registered for veterinary use against ectoparasites.
When applied to vegetable foliage, the dosage rates are 0.75 kg
to 1.5 kg a.i./ha and on cotton the rate is 1.5 to 2.3 kg a.i./ha.
Phoxim is applied as a soil treatment to vegetables, using
emulsifiable concentrates, baits or granules at dosage rates of 0.8 to
5.0 kg a.i./ha. Phoxim is applied as granules for soil treatment to
potatoes at a dosage rate of 1.5 to 7.5 kg a.i./ha as a soil treatment
for grains using 5.0 kg a.i./ha of granular formulation.
Residues in crops following foliar and soil treatment are
generally below 0.2 mg/kg and average values are generally below the
limit of determination (0.004 to 0.05 mg/kg). Residues in grains (oat,
barley, wheat and maize) with soil treatment before sowing were all
below the limit of determination. Also, residues with soil treatment
before or at sowing or planting of beans, cauliflower, cabbage and
onions were below the limit of determination. Average residues in
potatoes after soil treatment were below the limit of determination,
with the highest single value being 0.02 mg/kg. Residue after foliar
treatment in vegetables were low, with the highest average value being
0.13 mg/kg (tomatoes) and the highest single value 0.20 mg/kg
(spinach).
Table 12 National Maximum Residue Limits and Preharvest Intervals
Reported to the Meeting
Country Crop MRL Preharvest interval
(mg/kg) (days)
Australia Potatoes 0.05
Austria All food crops 0.05
Czechoslovakia Potatoes 14
Fed. Rep. Germany All food crops 0.05
Italy Fruit 0.05 42
Vegetables 0.05 42
Luxembourg All food crops 0.05
The Netherlands Vegetables 0.05
Milk & milk products 0.01
Meat & meat products 0.01 -> 0.05
Other products 0 -> 0.05
South Africa Cereals 0.2
Peanuts 0.2
Spain General 15
Thailand Maize 15
Cotton 15
Potatoes 15
Sorghum 15
Vegetables 15
Soviet Union Brassicas 20
Eggplant 20
Perennial grasses 20
Potatoes 20
Sugarbeet 20
Tomatoes 20
Yugoslavia All crops 0.05
In experimental treatment of sheep, cattle and pigs with phoxim,
by dipping or spraying, dosages ranged from 500 to 3 000 mg/l. At
slaughter, 14 to 45 days after treatment, no residues were observed in
samples of liver, kidney or muscle. However, residues were found in
fat from all animals, ranging from the limit of determination to 2.8
mg/kg, depending on treatment and waiting period.
In laboratory and field experiments phoxim degradation was
observed in different soil types, with half-lives from one day to
about two weeks, but in a greenhouse study the half-life was about
eight weeks. The main metabolites of phoxim in soil were
alpha-hydroxy-imino-phenylacetonitrile and benzoic acid, both formed
by hydrolysis, but diethyl phosphoric acids were also found in soil.
Laboratory and field leaching experiments in soil showed that
phoxim did not reach a depth below 5 cm and 10 cm, respectively, in
the laboratory and field.
Phoxim or its metabolites were found in roots, stems and leaves
of maize after soil treatment. The cobs, spikes and kernels contained
very little phoxim or metabolites. After foliar treatment, the leaves
contained phoxim and metabolites, but most of these were present on
the surface of the leaves and could be removed with chloroform.
The metabolites formed in or on plants consisted of an isoner:
diethoxy-phosphoryl-thioimino-phenyl-acetonitrile, which was formed
mainly by irradation of the plant, together with minor amounts of
tetraethyl diphosphate and tetraethyl mono-thio-diphosphate. The
"acyl"-compounds (phosphorous-free metabolites) formed by hydrolysis
reacted with plant constituents to three glycosides. The parent
compound degraded rapidly; after seven days only 11 percent of the
total activity was attributable to phoxim.
Investigations on animal metabolism were made in rats and mice.
No information was available on metabolism in cattle, pigs and poultry
for evaluation of residues of phoxim and its metabolites in animal
products.
Experiments were carried out on the stability of phoxim during
storage. Lettuce stored at -20° C for 2.5 years showed no significant
decrease in phoxim content.
Flour milled from grain fortified with phoxim contained 13
percent of the applied phoxim, while middlings and semolina accounted
for 35 percent and 43 percent, respectively, of the applied dose.
Bread made from flour containing phoxim did not contain unchanged
parent compound. Phoxim residues in potatoes were reduced by 91
percent during boiling.
Residues of phoxim in plant and soil samples can be determined by
gas-liquid chromatography with a flame photometric detector or a
thermonic detector. Thin-layer chromatography has also been used for
determining phoxim residues. Extraction can be made from plant
material with acetone, acetonitrile, chloroform or benzene. For
Soxhlet extraction, 10 percent methanol in benzene has been used. Soil
is extracted with methanol. Residues of phoxim in animal tissues are
extracted with hexane and residues in milk with ethylacetate. Cleanup
of extracts has been achieved by partitioning, precipitation with
ammonium chloride/phosphoric acid and/or column chromatography.
RECOMMENDATIONS
The following levels are recommended as MRLs, which need not be
exceeded when phoxim is used according to good agriculture practice.
The limits refer to the parent compound only.
Commodity MRL Preharvest intervals
on which recommendations
are based
Cereal grains 0.05** 1)
Lettuce 0.1 14 days
Beans 0.05** 14 lays
Cauliflower 0.05** 42 days
Potatoes 0.05** 1)
Tomatoes 0.2 14 days
Cotton seed 0.05** 14 days
Interval between treatment
and slaughtering
Cattle, carcase 0.02
meat (in carcase fat) 28 days
Sheep, carcase meat 1 (in carcase 28 days
fat)
1) Soil treatment at sowing or planting.
** Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Residue data in milk from treatment of cattle and residue data in
fat from treatment of pigs.
2. Studies on metabolism of phoxim from treatment of cattle, pigs
and sheep.
3. Information on good agriculture practice in the use of phoxim on
vegetables.
REFERENCES - RESIDUES
Mobay reports are unpublished reports prepared by the R & D Dept.
of Mobay Chemical Corporation, Agricultural Chemicals Division, Kansas
City, Mo. 64120, USA.
Nitokuno reports are unpublished reports prepared by Nihon
Tokushu Noyaku Seizo, K.K., No. 8, 2-chome, Nihonbashi Muro-machi,
Chuo-ku, Tokyo, Japan.
Bayer. Pflanzenschutzmittel-Rückstände. Reports Nos. 165-166/70.
1970 (Unpublished)
Bayer. Pflanzenschutzmittel-Rückstände im Sickerwasser. Reports Nos.
1972 283-288/72; Reports Nos. 3003-3004/74; Reports Nos. 3020-
1974 3022/77. (Unpublished)
1977
Biologische Bundesanstalt für Land- und Forstwirtschaft. Prüfung des
1980 Versickerung-sverhaltens von Pflanzenschutzmitteln.
Merkblatt Nr. 37, 2. Auflage.
Bowman, M.C. & Leuck, D.B. Determination and persistence of phoxim and
1971 its oxygen analog in forage corn and grass. J. Agric. Food
Chem., 19: 1215-1218.
Bykhovets, A.J. Thin-layer chromatographic method to determine Basudin
1974 and Valexon in a plant sample. Aktual. Vopr. Zashch. Rast.
BSSR, Mater, Resp. Konf, Molodykh Uch., 1: 81-82.
Daniel, J.W., Swanson, S. & McLean, J. Phoxim - Pharmacokinetics and
1978a biotransformation in the rat. Life Science Research, U.K.
Report 78/BAG5/194. (Unpublished)
Daniel, J.W., McLean, J. & Pringuer, M. Phoxim - Biotransformation in
1978b the rat. Life Science Research, U.K. Report 78/BAG5/317.
(Unpublished)
Dräger, G. Studies on the metabolism of BAY 77 488. Bayer AG,
1968 Pflanzenschutz-Anwendungstechnik, Report. (Unpublished)
Dräger, G. Gaschromatographische Methode zur Bestimmung von RValexon
1969a Rückständen in Pflanzen. Pflanzenschutz-Nachr. Bayer,
22:311-317 (1969a)
Dräger, G. Rückstandsanalytik von Pflanzenschutzmitteln. DFG-
1969b Methodensammlung, 1. Lieferung 1969; methode Nr. 307.
Dräger, G. Studies on the metabolism of phoxim (BAY 77 488).
1971 Pflanzenschutz-Nachr. Bayer, 24: 239-251.
Dräger, G. Reaction products of chlorphoxim (BAY 78 182) formed by
1972 exposure of the parent compound to irradiation.
Pflanzenschutz-Nachr. Bayer, 25: 32-42.
Dräger, G. Metabolisierung von Phoxim-methyl durch Belichtung. Bayer
1975 AG, Pflanzenschutz-Anwendungstechnik report RA-57.
(Unpublished)
Dräger, G. Studies on the fate of phoxim (BAY 77 488) in soil.
1977 Pflanzenschutz-Nachr. Bayer, 30:28-41.
Dräger, G. Untersuchung des Metabolismus von 14C-ringmarkiertem
Phoxim
1978a in Mais und Boden nach Anwendung enies Granulates. 1. 14C-
Bilanz und Metabolismus im Boden. Bayer AG, Pflanzenschutz-
Anwendungstechnik report RA-278. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim; Belichtung von
1978b 14C-ringmarkiertem Wirkstoff auf Glassplatten. Bayer AG,
Pflanzenschutz-Anwendungstechnik report RA-618.
(Unpublished)
Dräger, G. Stability of phoxim residues during boiling of potatoes.
1978c Bayer AG, Pflanzenschutz-Anwendungstechnik report RA-843.
(Unpublished)
Dräger, G. Untersuchung zum Metabolismus von Phoxim; Metabolismus
1980a des Abbauproduktes alpha-Hydroxyiminophenolacetonitril in
Tomatenpflanzen. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-106. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim auf
1980b Baumwollblättern. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-208. (Unpublished)
Dräger, G. Stability of phoxim residues in lettuce during low
1982 temperature storage. Bayer AG, Pflanzenschutz-
Anwendungstechnik, report RA-69. (Unpublished)
Ernst, G. F., Brinkman, M.B.C. & Mattern, E.M. Methode van onderzoek
1981 voor het bepalen van residuen van foxim en foxon im melk.
KvW Utrecht, IR/71/80/R79-Okt.
Hopkins, T., Lemprecht, C. & Lindsay, G. Bay 9053 - 50% w/v E.C. -
1980 residues - sheep. Bayer Doc. No. 80/9955.
LaHue, D.W. & Dicke, E.B. Phoxim as an insect protectant for stored
1971 grains. J. Econ. Entomol., 64: 1530-1533.
Makari, M.H., Riskallah, M.R., Hegazy, M.E. & Belal, M.H. Photolysis
1981 of phoxim on glass and on tomato leaves. Bull. Environ.
Contam. Toxicol. 26: 413-419 (1981).
Nitokuno. Residual analysis results of VolatonR (phoxim) in upland
1977 soil. Report No. 1058 (RA). (Unpublished)
Olson, T.J. An interference study for the residue method for Volaton
1974 determination on bananas. Mobay Report No. 40 095.
(Unpublished)
Pao, K.C.H. Residue studies of phoxim (Baythion) on the tea bushes.
1975 Acta Entomol. Sin., 18: 133-139.
Steffens, W. Bilanzversuche mit (benzolring-U-14C) Phoxim nach
1978 Verwendung als Bodeninsektizid zu Mais in einem
Freilandlysimeter und einem geschlossenen System im
Gewächshaus. Kernforschungsnalage Jülich, Institut für
Radioagronomie, report ARA 14/78. (Unpublished)
Thornton, J.S. Determination of residues of BAY 77 488 in stored
1969 grains. Mobay report No. 24 575. (Unpublished)
Thornton, J.S., Hurley, J.B. & Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticides chemicals. Mobay report No. 51 016.
(Unpublished)
Wilmes, R. Orientierende Lichtstabilität (Phoxim). Bayer AG,
1981 Pflanzenschutz-Anwendungstechnik report. (Unpublished)
Vinopal, J.H.A. & Fukuto, T.R. Selective toxicity of phoxim
1971 (phenylglyoxylonitrile oxime 0,0-diethyl phosphorothioate).
Pestic. Biochem. Physiol., 1: 44-60.
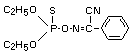 Other Information on Identity and Properties
Molecular weight 298.3
Appearance light yellow oil liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20° C(pure active ingredient)
4°
Vapour pressure approximately 10-4 mm Hg at 20° C
Solubility in water 0.7
(g a.i./100 g in cyclohexanone > 60
solvent at 20°C) in isopropyl alcohol > 60
in methylene chloride > 60
in toluene > 60
Minimum degree of purity 82.0 percent (pre-solution for reasons
of stability in 9-11 percent butanol).
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phoxim is an insecticide of the group of phosphoric ester
compounds. It is a stomach and contact poison and has a depth effect
but no systemic effect. The initial effect is rapid, with a short to
moderate duration, depending on the application. The active ingredient
has a broad spectrum of activity and it is most effective against
biting insects. Phoxim is used as foliage and soil-applied insecticide
and as seed dressing, and is also used for application on livestock
against mites and other ectoparasites.
Phoxim used as insecticide on crops is registered and marketed in
several countries, including European and Central American ones,
Australia, South Africa, Egypt, Turkey and Taiwan (province of China).
Phoxim is registered in six European countries, four South
American countries and two African countries for veterinary use. An
earlier recommendation for the use of phoxim in stored cereals has
been withdrawn by the manufacturer, because of its long persistence in
storage.
Preharvest Uses
Phoxim is formulated as emulsifiable concentrate, granules, dust
or bait. As a foliage-applied insecticide it is mainly used on cotton,
maize and sugarbeet for the control of lepidopterous larvae.
Soil application of phoxim is based on a row or over-all
treatment before, with or directly after sowing or planting.
Therefore, the safety interval includes the entire period of
vegetative growth before harvest. It is mainly used on potatoes. Good
effects against locusts have been reported.
Phoxim is used as a seed dressing at a rate of 40 g a.i./kg seed.
Use in Animals
The 50 percent E.C. formulation is used in 0.025-0.050 percent
a.i. concentrations for spraying or dipping of animals against mites
and other ectoparasites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of phoxim were determined following foliar application
of emulsifiable concentrates to cotton and vegetables and of dust
powder on coffee. With the exception of tomatoes, in which residues
ranging from 0.10 to 0.15 mg/kg were found, the residue levels in the
other crops at the end of the safety interval were either nil or about
the limit of determination.
Table 1 Some Uses of Phoxim Formulations
Crop Recommended dosage Formulation
Application to foliage
Cotton 1.5-2.0 kg/ha 3-10 treatments E.C.
2.3 kg/ha Max. 2 treatments ULV
Vegetables 0.75-1.5 kg/ha. 2-3 treatments E.C.
Maize 1-2.5 kg/ha. 1-3 treatments E.C.
0.25-0.65 kg/ha. 1-2 treatments GR
Application to soil
Vegetables 3.75-5.0 kg/ha E.C.
25-50 mg/plant E.C.-GR
2.0-5.0 kg/ha GR
0.8-1.2 kg/ha bait
Potatoes 1-2.5 kg/ha row treatment GR
1-7.5 kg/ha over-all treatment GR
Maize 0.65-1.25 kg/ha row treatment GR
2.0-7.5 kg/ha over-all treatment GR
Cereals 5 kg/ha over-all treatment GR
Following application of granular phoxim to soil, residue
levels determined in vegetables, potatoes and maize were below
or about the limit of detection in most crops with the exception
of cauliflower (0.1 mg/kg).
Following application of phoxim as a bait formulation to
beans, cabbage, lettuce and spinach, no residues appeared in the
crops in most of the trials; measurable residues were present in
only a few samples.
The results of supervised residue trials are reported in
Table 2.
In animal treatments
Phoxim is used against ectoparasites on sheep, cattle and
pigs at recommended concentrations of 250-500 mg a.i./l.
Experiments have been performed on sheep, pigs and cattle by
spraying or dipping with phoxim in concentrations ranging from
500 to 3 000 mg a.i./l. Fat, muscle, liver and kidney were
analysed 14 to 45 days after application. In all samples of liver,
kidney and muscle of sheep, cattle and pigs, residues were below
0.01 mg/kg. The highest residue, 2.8 mg/kg, was found in fat
from sheep after a 3 000 mg/l plunge dip treatment for 1 min.
30 days before slaughtering. The residues in fat from sheep,
cattle and pig ranged from non-detectable to 0.66 mg/kg. The
results are reported in Table 3.
FATE OF RESIDUES
Degradation pathways and metabolite patterns of phoxim in
soil and mammals have been subject to many investigations. The
findings of these are summarized in Figure 1 and Table 4.
Compounds identified in metabolism studies are referred to by
Roman numerals shown in Figure 1 and Table 4.
In soil the diethyl phosphoric acid fragments (IX, X) and
the aromatic compounds (XI and XII), formed by hydrolysis of
phoxim (I), were detected. The phenyl ring of (I) is further
oxidized to CO2 (XV) in soil.
Metabolism of phoxim (I) in plants is governed largely by
its photochemical degradation and further reactions of the
degradation products. Basically, three groups of metabolites are
detectable:
- compounds formed due to exposure of (I) to irradiation of the
leaf surface (II, III, IV, V, VI, VIII); these include the
isomeric compounds (II, III) of (I) and the metabolites
resulting from them (IV, V),
- hydrolysis products (XI, XII),
Table 2 Residues of Phoxim From Supervised Trials
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Cotton (11-13 x 1.67-2.0 7 n.d. (2) United States
(seed) kg/ha E.C. (ULV)
Carrots 3.75 kg/ha GR 112-158 n.d.-0.02 (0.2) Fed. Rep. Germany1
Beans 4x1.5-3.0 kg/ha E.C. 14 n.d. (2) Egypt
Beans 0.8 kg/ha bait 30 n.d.-<0.05(2) Italy
(pod) 3.0 kg/ha 30 n.d. (2) Italy
Red cabbage 50 mg/plant GR 69 n.d. (1) Fed. Rep. Germany1
1.2 kg/ha bait 20-100 n.d. (2) Fed. Rep, Germany1
White cabbage 1.2 kg/ha bait 11-95 n.d.-0.03 (4) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cabbage 100 mg/plant 49-63 n.d.-<0.03 (3) Finland
Savoy cabbage 0.6-1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cauliflower 2 kg/ha GR 64 0.1 (1) Fed. Rep. Germany1
25-50 mg/plant GR 42-59 n.d. (5) Fed. Rep. Germany1
100 mg/plant 42-63 n.d.-<0.05 (4) Finland
Eggplant 4x0.6-1.2 kg/ha 14 n.d. (2) Egypt
Lettuce 0.8-2.0 kg/ha bait 30-110 n.d.-0.1(6) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (1) Fed. Rep. Germany1
Spinach 1.2 kg/ha bait 19-36 n.d.-0.20(8) Fed. Rep. Germany1
Table 2 (continued)
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Maize 0.65-1.25 kg/ha GR 109-165 n.d. (3) France
7.5 kg/ha GR 148-169 n.d. (3) Fed. Rep. Germany1
Potatoes 1.5-2.8 kg/ha 78-154 n.d.-0.02(24) United Kingdom1
GR France
Sweden
4.2-7.5 kg/ha GR 78-156 n.d.-<0.01(22) Fed. Rep. Germany1
United Kingdom1
Sweden
Onions 3.75 kg/ha GR 154 n.d.(1) Netherlands
5.0 kg/ha E.C. 130 n.d.(1) Netherlands
Peppers 5x0.77-1.54 kg/ha E.C. 14 n.d.-<0.05(2) Egypt
Tomatoes 4x1.5-3.0 kg/ha E.C. 14 C.10-0.15(2) Egypt
Barley 4.2-5.0 kg/ha GR 95-134 n.d.(4) Fed. Rep. Germany1
(grains) United Kingdom
Oats 5.0 kg/ha GR 97 n.d.(1) Fed. Rep. Germany1
(grains)
Wheat 5.0 kg/ha GR 119 n.d.(1) Fed. Rep. Germany1
(grains)
Coffee 0.94 kg/ha DP 8 n.d.(1) Ivory Coast
green
1 Phoxim is neither registered nor marketed.
Table 3 Residues of Phoxim from Animal Treatments
Interval
Animal Application Conc. between Residues (mg/kg) No. of samples () Country
(mg/kg) last treatment Muscle Fat Liver Kidney
and
slaughter
(days)
Sheep Dip 1 000 30 n.d.1 n.d.-0.7 n.d. n.d.(3) Australia
45 n.d. n.d. n.d. n.d.(3)
2 000 30 n.d. 1.2-1.8 n.d. n.d.(3)
45 n.d. 0.5-0.6 n.d. n.d.(3)
3 000 30 n.d. 1.6-2.8 n.d. n.d.(3)
45 n.d. 0.1-1.0 n.d. n.d.(3)
2x spray 500 21 <0.01 0.03-0.17 <0.01 <0.01(3) South Africa
500 14 <0.01 0.17-0.66 <0.01 <0.01(3)
1 000 21 <0.01 0.20-0.52 <0.01 <0.01(3)
Cattle 2x spray 1 000 28 <0.01 0.02 <0.01 <0.01(2)
1 000 14 <0.01 0.32-0.37 <0.01 <0.01(2)
Pig 2x spray 1 000 28 <0.01 <0.01-0.13 <0.01 <0.01(2)
1 n. d. = not detected.
Table 4 Chemical Names and Occurrence of Compounds Identified in Metabolism Studies on Phoxim
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
I (Diethoxy-thiophosphoryloxyimino)-phenyl= Z-3,5-Dioxa-6-aza-4-phosphaoct- X X X X
acetonitrile 6-ene-8-nitrile, 4-ethoxy-7-
= Phoxim Z phenyl-4-sulfide
II ditto E-3,5-Dioxa-6-aza-4-phosphaoct- X X X
= Phoxim E 6-ene-8-nitrile, 4-ethoxy-7-
phenyl-4-sulfide
III Diethoxy-phosphoylthioimino-phenylaceto= 3-Oxa-5-thia-6-aza-4-phospha- X X X
nitrile oct-6-ene-8-nitrile, 4-ethoxy-
= Photoisomeres 7-phenyl-4-oxide
IV N,N'-[Thio-bis(alpha- alpha,alpha'(Thiodinitrilo)-bis- X
iminophenylacetonitrile)] benzeneacetonitrile
V N,N'-[Dithio-bis(alpha- alpha,alpha'-(Dithiodinitrilo)- X X
iminophenylacetonitrile)] bis benzeneacetonitrile
VI Bis-alpha-dithioiminophenylacetonitrile X
VII O,O,O,O-Tetraethyldiphosphate Diphosphoric acid tetraethyl- X X
ester
VIII O,O,O,O-Tetraethylmonothiodiphosphate Thiodiphosphoric acid tetra= X X
ethylester
IX O,O-Diethyl thiophosphoric acid Phosphorothioic acid O,O,di= X X
ethylester
Table 4 (continued)
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
X Diethyl phosphoric acid Phosphoric acid diethylester X X
XI alpha-Hydroxy-imino-phenylacetonitrile Benzeneacetonitrile, X X X X
-(hydroxy-imino)-
XII Benzoic acid Benzoic acid X X X
XIII alpha-Hydroxy-imino-phenylacetonitrile
glucoside X
XIV alpha-Hydroxy-imino-phenylacetonitrile X
gentio=bioside
XIVa alpha-Hydroxy-imino-phenylacetonitrile X
glycoside
XV Carbon dioxide Carbon dioxide X
XVI Hippuric acid X
XVII "phoxim carbonic acid" X
XVIII Desethylphoxim X
(Desethyl-PO-phoxim)
XIX alpha-Hydroxy-imino-phenylacetonitrile X
glucuronide
XX alpha-Hydroxy-imino-phenylacetonitrile X
sulfate
Other Information on Identity and Properties
Molecular weight 298.3
Appearance light yellow oil liquid (pure active
ingredient)
Melting point 5-6°C (pure active ingredient)
Specific gravity 1.176 at 20° C(pure active ingredient)
4°
Vapour pressure approximately 10-4 mm Hg at 20° C
Solubility in water 0.7
(g a.i./100 g in cyclohexanone > 60
solvent at 20°C) in isopropyl alcohol > 60
in methylene chloride > 60
in toluene > 60
Minimum degree of purity 82.0 percent (pre-solution for reasons
of stability in 9-11 percent butanol).
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phoxim is an insecticide of the group of phosphoric ester
compounds. It is a stomach and contact poison and has a depth effect
but no systemic effect. The initial effect is rapid, with a short to
moderate duration, depending on the application. The active ingredient
has a broad spectrum of activity and it is most effective against
biting insects. Phoxim is used as foliage and soil-applied insecticide
and as seed dressing, and is also used for application on livestock
against mites and other ectoparasites.
Phoxim used as insecticide on crops is registered and marketed in
several countries, including European and Central American ones,
Australia, South Africa, Egypt, Turkey and Taiwan (province of China).
Phoxim is registered in six European countries, four South
American countries and two African countries for veterinary use. An
earlier recommendation for the use of phoxim in stored cereals has
been withdrawn by the manufacturer, because of its long persistence in
storage.
Preharvest Uses
Phoxim is formulated as emulsifiable concentrate, granules, dust
or bait. As a foliage-applied insecticide it is mainly used on cotton,
maize and sugarbeet for the control of lepidopterous larvae.
Soil application of phoxim is based on a row or over-all
treatment before, with or directly after sowing or planting.
Therefore, the safety interval includes the entire period of
vegetative growth before harvest. It is mainly used on potatoes. Good
effects against locusts have been reported.
Phoxim is used as a seed dressing at a rate of 40 g a.i./kg seed.
Use in Animals
The 50 percent E.C. formulation is used in 0.025-0.050 percent
a.i. concentrations for spraying or dipping of animals against mites
and other ectoparasites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of phoxim were determined following foliar application
of emulsifiable concentrates to cotton and vegetables and of dust
powder on coffee. With the exception of tomatoes, in which residues
ranging from 0.10 to 0.15 mg/kg were found, the residue levels in the
other crops at the end of the safety interval were either nil or about
the limit of determination.
Table 1 Some Uses of Phoxim Formulations
Crop Recommended dosage Formulation
Application to foliage
Cotton 1.5-2.0 kg/ha 3-10 treatments E.C.
2.3 kg/ha Max. 2 treatments ULV
Vegetables 0.75-1.5 kg/ha. 2-3 treatments E.C.
Maize 1-2.5 kg/ha. 1-3 treatments E.C.
0.25-0.65 kg/ha. 1-2 treatments GR
Application to soil
Vegetables 3.75-5.0 kg/ha E.C.
25-50 mg/plant E.C.-GR
2.0-5.0 kg/ha GR
0.8-1.2 kg/ha bait
Potatoes 1-2.5 kg/ha row treatment GR
1-7.5 kg/ha over-all treatment GR
Maize 0.65-1.25 kg/ha row treatment GR
2.0-7.5 kg/ha over-all treatment GR
Cereals 5 kg/ha over-all treatment GR
Following application of granular phoxim to soil, residue
levels determined in vegetables, potatoes and maize were below
or about the limit of detection in most crops with the exception
of cauliflower (0.1 mg/kg).
Following application of phoxim as a bait formulation to
beans, cabbage, lettuce and spinach, no residues appeared in the
crops in most of the trials; measurable residues were present in
only a few samples.
The results of supervised residue trials are reported in
Table 2.
In animal treatments
Phoxim is used against ectoparasites on sheep, cattle and
pigs at recommended concentrations of 250-500 mg a.i./l.
Experiments have been performed on sheep, pigs and cattle by
spraying or dipping with phoxim in concentrations ranging from
500 to 3 000 mg a.i./l. Fat, muscle, liver and kidney were
analysed 14 to 45 days after application. In all samples of liver,
kidney and muscle of sheep, cattle and pigs, residues were below
0.01 mg/kg. The highest residue, 2.8 mg/kg, was found in fat
from sheep after a 3 000 mg/l plunge dip treatment for 1 min.
30 days before slaughtering. The residues in fat from sheep,
cattle and pig ranged from non-detectable to 0.66 mg/kg. The
results are reported in Table 3.
FATE OF RESIDUES
Degradation pathways and metabolite patterns of phoxim in
soil and mammals have been subject to many investigations. The
findings of these are summarized in Figure 1 and Table 4.
Compounds identified in metabolism studies are referred to by
Roman numerals shown in Figure 1 and Table 4.
In soil the diethyl phosphoric acid fragments (IX, X) and
the aromatic compounds (XI and XII), formed by hydrolysis of
phoxim (I), were detected. The phenyl ring of (I) is further
oxidized to CO2 (XV) in soil.
Metabolism of phoxim (I) in plants is governed largely by
its photochemical degradation and further reactions of the
degradation products. Basically, three groups of metabolites are
detectable:
- compounds formed due to exposure of (I) to irradiation of the
leaf surface (II, III, IV, V, VI, VIII); these include the
isomeric compounds (II, III) of (I) and the metabolites
resulting from them (IV, V),
- hydrolysis products (XI, XII),
Table 2 Residues of Phoxim From Supervised Trials
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Cotton (11-13 x 1.67-2.0 7 n.d. (2) United States
(seed) kg/ha E.C. (ULV)
Carrots 3.75 kg/ha GR 112-158 n.d.-0.02 (0.2) Fed. Rep. Germany1
Beans 4x1.5-3.0 kg/ha E.C. 14 n.d. (2) Egypt
Beans 0.8 kg/ha bait 30 n.d.-<0.05(2) Italy
(pod) 3.0 kg/ha 30 n.d. (2) Italy
Red cabbage 50 mg/plant GR 69 n.d. (1) Fed. Rep. Germany1
1.2 kg/ha bait 20-100 n.d. (2) Fed. Rep, Germany1
White cabbage 1.2 kg/ha bait 11-95 n.d.-0.03 (4) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cabbage 100 mg/plant 49-63 n.d.-<0.03 (3) Finland
Savoy cabbage 0.6-1.0 kg/ha E.C. 14 n.d. (2) Fed. Rep. Germany1
Cauliflower 2 kg/ha GR 64 0.1 (1) Fed. Rep. Germany1
25-50 mg/plant GR 42-59 n.d. (5) Fed. Rep. Germany1
100 mg/plant 42-63 n.d.-<0.05 (4) Finland
Eggplant 4x0.6-1.2 kg/ha 14 n.d. (2) Egypt
Lettuce 0.8-2.0 kg/ha bait 30-110 n.d.-0.1(6) Fed. Rep. Germany1
1.0 kg/ha E.C. 14 n.d. (1) Fed. Rep. Germany1
Spinach 1.2 kg/ha bait 19-36 n.d.-0.20(8) Fed. Rep. Germany1
Table 2 (continued)
Application/
Crop Formulation Interval after Residues
last treatment (mg/kg) Country
(days) () = No. of samples
Maize 0.65-1.25 kg/ha GR 109-165 n.d. (3) France
7.5 kg/ha GR 148-169 n.d. (3) Fed. Rep. Germany1
Potatoes 1.5-2.8 kg/ha 78-154 n.d.-0.02(24) United Kingdom1
GR France
Sweden
4.2-7.5 kg/ha GR 78-156 n.d.-<0.01(22) Fed. Rep. Germany1
United Kingdom1
Sweden
Onions 3.75 kg/ha GR 154 n.d.(1) Netherlands
5.0 kg/ha E.C. 130 n.d.(1) Netherlands
Peppers 5x0.77-1.54 kg/ha E.C. 14 n.d.-<0.05(2) Egypt
Tomatoes 4x1.5-3.0 kg/ha E.C. 14 C.10-0.15(2) Egypt
Barley 4.2-5.0 kg/ha GR 95-134 n.d.(4) Fed. Rep. Germany1
(grains) United Kingdom
Oats 5.0 kg/ha GR 97 n.d.(1) Fed. Rep. Germany1
(grains)
Wheat 5.0 kg/ha GR 119 n.d.(1) Fed. Rep. Germany1
(grains)
Coffee 0.94 kg/ha DP 8 n.d.(1) Ivory Coast
green
1 Phoxim is neither registered nor marketed.
Table 3 Residues of Phoxim from Animal Treatments
Interval
Animal Application Conc. between Residues (mg/kg) No. of samples () Country
(mg/kg) last treatment Muscle Fat Liver Kidney
and
slaughter
(days)
Sheep Dip 1 000 30 n.d.1 n.d.-0.7 n.d. n.d.(3) Australia
45 n.d. n.d. n.d. n.d.(3)
2 000 30 n.d. 1.2-1.8 n.d. n.d.(3)
45 n.d. 0.5-0.6 n.d. n.d.(3)
3 000 30 n.d. 1.6-2.8 n.d. n.d.(3)
45 n.d. 0.1-1.0 n.d. n.d.(3)
2x spray 500 21 <0.01 0.03-0.17 <0.01 <0.01(3) South Africa
500 14 <0.01 0.17-0.66 <0.01 <0.01(3)
1 000 21 <0.01 0.20-0.52 <0.01 <0.01(3)
Cattle 2x spray 1 000 28 <0.01 0.02 <0.01 <0.01(2)
1 000 14 <0.01 0.32-0.37 <0.01 <0.01(2)
Pig 2x spray 1 000 28 <0.01 <0.01-0.13 <0.01 <0.01(2)
1 n. d. = not detected.
Table 4 Chemical Names and Occurrence of Compounds Identified in Metabolism Studies on Phoxim
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
I (Diethoxy-thiophosphoryloxyimino)-phenyl= Z-3,5-Dioxa-6-aza-4-phosphaoct- X X X X
acetonitrile 6-ene-8-nitrile, 4-ethoxy-7-
= Phoxim Z phenyl-4-sulfide
II ditto E-3,5-Dioxa-6-aza-4-phosphaoct- X X X
= Phoxim E 6-ene-8-nitrile, 4-ethoxy-7-
phenyl-4-sulfide
III Diethoxy-phosphoylthioimino-phenylaceto= 3-Oxa-5-thia-6-aza-4-phospha- X X X
nitrile oct-6-ene-8-nitrile, 4-ethoxy-
= Photoisomeres 7-phenyl-4-oxide
IV N,N'-[Thio-bis(alpha- alpha,alpha'(Thiodinitrilo)-bis- X
iminophenylacetonitrile)] benzeneacetonitrile
V N,N'-[Dithio-bis(alpha- alpha,alpha'-(Dithiodinitrilo)- X X
iminophenylacetonitrile)] bis benzeneacetonitrile
VI Bis-alpha-dithioiminophenylacetonitrile X
VII O,O,O,O-Tetraethyldiphosphate Diphosphoric acid tetraethyl- X X
ester
VIII O,O,O,O-Tetraethylmonothiodiphosphate Thiodiphosphoric acid tetra= X X
ethylester
IX O,O-Diethyl thiophosphoric acid Phosphorothioic acid O,O,di= X X
ethylester
Table 4 (continued)
No. Chemical names Occurrence
According to Soil Plant Animal Light
Chemical Abstract Service
X Diethyl phosphoric acid Phosphoric acid diethylester X X
XI alpha-Hydroxy-imino-phenylacetonitrile Benzeneacetonitrile, X X X X
-(hydroxy-imino)-
XII Benzoic acid Benzoic acid X X X
XIII alpha-Hydroxy-imino-phenylacetonitrile
glucoside X
XIV alpha-Hydroxy-imino-phenylacetonitrile X
gentio=bioside
XIVa alpha-Hydroxy-imino-phenylacetonitrile X
glycoside
XV Carbon dioxide Carbon dioxide X
XVI Hippuric acid X
XVII "phoxim carbonic acid" X
XVIII Desethylphoxim X
(Desethyl-PO-phoxim)
XIX alpha-Hydroxy-imino-phenylacetonitrile X
glucuronide
XX alpha-Hydroxy-imino-phenylacetonitrile X
sulfate
 - carbohydrate conjugates (XIII, XIV, XIVa) of the hydrolysis
product (XI); as carbohydrate components, glucose and
gentiobiose, as well as another disaccharide with an unknown
carbohydrate structure, were detected.
The metabolic fate in mammals was governed by rapid and
almost complete absorption after oral dosing. Owing to rapid
elimination, essentially renal, no accumulation in organs or
tissues was observed. The metabolites (IX, X, XI, XVII, XVIII)
were formed by hydrolysis. The hydrolysis product (XI) was
conjugated to (XIX) and (XX) and further hydrolysed to benzoic
acid (XII), which was excreted as hippuric acid (XVI).
In Soil
The behaviour of phoxim in soil was investigated by studies
on leaching of unlabelled and ring-14C-phoxim in greenhouse,
field and laboratory experiments (soil columns and soil thin-layer
plates); degradation under different conditions in the laboratory
and in the field; and metabolism of unlabelled and
ring-14C-phoxim in the field, in the greenhouse and in a
closed system.
Leaching
Phoxim (I) undergoes only slight leaching in soil, according
to both metabolism studies and specially conducted leaching
studies. In a greenhouse experiment, a 10 percent granular
formulation was applied to maize at sowing, using
ring-14C-phoxim; no increase in 14C radioactivity was
observed in the 5 to 20-cm layer (Dräger 1978b). In a
corresponding field experiment, 14C radioactivity of the
parent compound found only in the upper 10 cm of soil (Steffens
1978). From the results of these studies, it can therefore be
concluded that the metabolites also do not leach.
Gas-chromatographic analyses of different soil layers,
performed after field application of a 5 percent granular
formulation at a rate of 100 kg/ha, yielded similar results for
phoxim (II) and the metabolites (III, IX and X) (Dräger 1977).
Laboratory experiments on leaching of phoxim (I) and its
photoisomer (III) revealed that there were no residues in the
leachate after 60 h of simulated rainfall of 210 to 230 mm
(Dräger 1977). Analysis of the different layers of the soil column
showed that (I) and (III) remained in the upper 0 to 5 cm. During
this period, (III) underwent degradation to a residue of about 1
percent. The oxon of phoxim (I) (not included in the scheme of
formulae because its formation was not shown by the reported
metabolic studies) was not detectable in the soil layers or in the
leachate, as it underwent complete hydrolysis.
In further laboratory leaching experiments conducted with the
parent compound and different formulations (500 E.C. 5 percent
granular, 4 percent bait) by the method specified in BBA Leaflet
No. 37 (Biologische Bundesanstalt für Land- und Forstwirtschaft
1980), using standard soils, no residues were found in the
leachate (Bayer 1972, 1974, 1977).
In a study undertaken to characterize leaching behaviour,
spots of phoxim (I) and 23 other pesticides were applied on
thin-layer plates coated with different types of soils of varying
textures, ranging from non-adsorptive sand to fine textured clay.
The plates were developed with distilled water and the leaching
behaviour was determined by comparing Rf values. According to
leaching behaviour, the compounds were grouped into five
categories, ranging from immobile to highly mobile. Phoxim (I)
was categorized as having low mobility (Thornton et al. 1976).
Degradation
The half-life of phoxim (I) in soil was determined in
laboratory and field experiments. After application of a 5 percent
granular formulation to bare field soils at a rate of 100 kg/ha,
half-lives of about two weeks were determined (Bayer 1970). In
laboratory experiments, addition of 3 mg/kg of phoxim to soil
resulted in half-lives of 1 to 11 days (Nitokuno 1977). The
results of the reported studies are presented in Table 5.
Metabolism
The studies to investigate metabolism of phoxim (I) in soil
were conducted with unlabelled and ring-14C-labelled
compounds. After incorporation of a 5 percent granular formulation
in fallow soils with no plant cover, degradation was studied by
gas chromatography (Dräger 1977). With initial parent compound
concentrations of 4.0 mg/kg in the 0 to 15 cm layer, less than
0.1 mg/kg was found after two months; by day 150, the parent
compound concentration was less than 0.05 mg/kg. The photoisomer
(III) was detectable only on day 0.
The levels of diethyl phosphoric acids (IX) and (X) declined
to 0.09 mg/kg (IX) and 0.05 mg/kg (X) by day 26. Details are given
in Table 6.
After application of a 10 percent formulation, using
ring-14C-phoxim (I), to maize at sowing, the compounds (XI)
and (XII) were detected (Dräger 1978a). Residues of both compounds
were less than 0.1 mg/kg at 59 to 128 days after application.
During this period, phoxim (I) decreased in the 0 to 5-cm layer
from 0.33 mg/kg on day 59 to 0.10 mg/kg on day 128 (Table 7).
Table 5 Half-lives of Phoxim in Different Soils
Soil Application Half-life
Laboratory studies
sandy loam soil 0.8 % org. matter; 13.6% clay; pH 6.5 3 mg/kg a.i. ca. 1 day
clay loam soil 12.7 % org. matter; 1.5% clay; pH 5.6 3 mg/kg a.i. ca. 11 days
sandy loam soil 5.85% org. matter; 14.0% clay; pH 5.7 3 mg/kg a.i. ca. 9 days
Greenhouse study
loamy sand 0.89% C; 31.4% fines; pH 6.0 10% granular; ca. 8 weeks
0.5 g/m row
Field studies
light humus 3.2% C; 9.8% clay; 10.3% silt; pH 5.2 5% granular; ca. 2 weeks
100 kg/ha
loamy sand 3.8% C; 15.0% clay; 14.4% silt; pH 6.1 5% granular; ca. 2.5 weeks
100 kg/ha
loam soil 3% microgranular;
2 × 90 kg/ha ca. 10 days
clay loam soil 12.7% org. matter; 1.5% clay 3% microgranular
2 × 90 kg/ha ca. 2 weeks
Table 6 Metabolism of Phoxim in Soil, Field Study1
Day 0 Day 14 Day 26 Day 60
Analysed
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-10 10-15 0-15 0-5 5-10 10-25 0-15 0-5 5-10 10-15 0-15 0-5 5-10 10-15 0-15
(I) 9.4 2.3 0.39 4.0 4.9 1.34 0.35 2.2 1.42 0.42 0.06 0.6 0.08 0.04 0.04 0.05
(III) 1.4 1.1 n.d. 0.8 0.24 n.d. n.d. 0.08 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d.
(IX) 2.46 0.30 n.d. 0.92 1.09 0.06 n.d. 0.38 0.09 0.02 n.d. 0.04 n.d. n.d. n.d. n.d.
(X) 0.69 0.26 n.d. 0.32 0.10 0.06 n.d. 0.05 0.05 n.d. n.d. 0.02 n.d. n.d. n.d. n.d.
1 Soil type: light humus; pH 5.2; 20.1% fines; 9.8% clay; 10.3% silt; 3.2% C. Application rate - 5 kg a.i./ha. Residue levels
reported in mg/kg in relation to dry material.
2 For Roman numerals see Table 4.
n.d. = not detected.
Table 7 Metabolism of Ring-14C-Phoxim (I) in Soil1
Analysed Day 59 Day 91 Day 128
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-20 0-5 5-20 0-5 5-20
(I) 0.33 0.10 0.22 0.09 0.10 0.04
(XI) 0.06 0.01 0.05 0.01 0.04 0.01
(XII) 0.02 0.001 0.006 0.001 0.004 n.d.
1 pH 6.0; 31.4% fines; 0.89%C. Greenhouse study. Residue levels in mg/kg.
2 For Roman numerals see Table 4.
Results of studies conducted in a closed system, using
ring-14C-phoxim (I) incorporated as a granular formulation into the
soil, showed that the aromatic remainder of the molecule was
mineralized substantially to CO2 (XV) (Steffens 1978). Within 56 days
after application, 25 to 28 percent of the applied 14C activity was
released into the atmosphere as 14CO2.
The metabolic pathway of phoxim in soil is illustrated in Figure
2.
In Plants
The behaviour of phoxim in plants was investigated by studies of
absorption, translocation and accumulation of ring-14C-phoxim in
maize plants, in greenhouse and field studies, and of the metabolism
of 32P and ring-14C-phoxim after foliar application to cotton plants
and after injection of ring-14C-(XI) into tomato plants.
Absorption, translocation and accumulation
The behaviour of phoxim (I) after application as a soil
insecticide was investigated in a greenhouse study in which a 10
percent granular formulation was incorporated in the soil at the time
of sowing maize. The distribution of plant-absorbed 14C activity was
monitored in green maize and at the milk-ripe and harvest-ripe stages
(Dräger 1978a). The results are given in Table 8.
After application of the 10 percent granular formulation of
phoxim to maize in a field experiment (Steffens 1978), less 14C
activity was absorbed by the plant than in the aforementioned
greenhouse study; for example, the harvest-ripe kernels contained 0.02
percent, compared with 0.08 percent in the greenhouse experiment, of
the 14C dose applied to the soil (Table 9).
Metabolism
The effect of light on the metabolism of phoxim (I) was evident
in greenhouse studies conducted with 32P-labelled phoxim on cotton
plants (Dräger 1971). After application of the spray solution to
cotton leaves, the isomeric compound (III) was detected, which was
formed solely on irradiation of the treated test plants. The
simultaneously formed diphosphates (VII) and (VIII) were present only
at very low levels.
Metabolism studies with ring-14C parent compound applied to
foliage of cotton plants are based on model experiments that provided
further information on photolytically formed metabolites and on the
incorporation of metabolites in plant constituents. After exposure for
96 h to irradiation on glass plates, 1 percent (XII), 2 percent (XI),
11 percent (II), 5 percent (III) and 5 percent of the phosphorus-free
metabolite (V) were detected (Dräger, 1978b).
- carbohydrate conjugates (XIII, XIV, XIVa) of the hydrolysis
product (XI); as carbohydrate components, glucose and
gentiobiose, as well as another disaccharide with an unknown
carbohydrate structure, were detected.
The metabolic fate in mammals was governed by rapid and
almost complete absorption after oral dosing. Owing to rapid
elimination, essentially renal, no accumulation in organs or
tissues was observed. The metabolites (IX, X, XI, XVII, XVIII)
were formed by hydrolysis. The hydrolysis product (XI) was
conjugated to (XIX) and (XX) and further hydrolysed to benzoic
acid (XII), which was excreted as hippuric acid (XVI).
In Soil
The behaviour of phoxim in soil was investigated by studies
on leaching of unlabelled and ring-14C-phoxim in greenhouse,
field and laboratory experiments (soil columns and soil thin-layer
plates); degradation under different conditions in the laboratory
and in the field; and metabolism of unlabelled and
ring-14C-phoxim in the field, in the greenhouse and in a
closed system.
Leaching
Phoxim (I) undergoes only slight leaching in soil, according
to both metabolism studies and specially conducted leaching
studies. In a greenhouse experiment, a 10 percent granular
formulation was applied to maize at sowing, using
ring-14C-phoxim; no increase in 14C radioactivity was
observed in the 5 to 20-cm layer (Dräger 1978b). In a
corresponding field experiment, 14C radioactivity of the
parent compound found only in the upper 10 cm of soil (Steffens
1978). From the results of these studies, it can therefore be
concluded that the metabolites also do not leach.
Gas-chromatographic analyses of different soil layers,
performed after field application of a 5 percent granular
formulation at a rate of 100 kg/ha, yielded similar results for
phoxim (II) and the metabolites (III, IX and X) (Dräger 1977).
Laboratory experiments on leaching of phoxim (I) and its
photoisomer (III) revealed that there were no residues in the
leachate after 60 h of simulated rainfall of 210 to 230 mm
(Dräger 1977). Analysis of the different layers of the soil column
showed that (I) and (III) remained in the upper 0 to 5 cm. During
this period, (III) underwent degradation to a residue of about 1
percent. The oxon of phoxim (I) (not included in the scheme of
formulae because its formation was not shown by the reported
metabolic studies) was not detectable in the soil layers or in the
leachate, as it underwent complete hydrolysis.
In further laboratory leaching experiments conducted with the
parent compound and different formulations (500 E.C. 5 percent
granular, 4 percent bait) by the method specified in BBA Leaflet
No. 37 (Biologische Bundesanstalt für Land- und Forstwirtschaft
1980), using standard soils, no residues were found in the
leachate (Bayer 1972, 1974, 1977).
In a study undertaken to characterize leaching behaviour,
spots of phoxim (I) and 23 other pesticides were applied on
thin-layer plates coated with different types of soils of varying
textures, ranging from non-adsorptive sand to fine textured clay.
The plates were developed with distilled water and the leaching
behaviour was determined by comparing Rf values. According to
leaching behaviour, the compounds were grouped into five
categories, ranging from immobile to highly mobile. Phoxim (I)
was categorized as having low mobility (Thornton et al. 1976).
Degradation
The half-life of phoxim (I) in soil was determined in
laboratory and field experiments. After application of a 5 percent
granular formulation to bare field soils at a rate of 100 kg/ha,
half-lives of about two weeks were determined (Bayer 1970). In
laboratory experiments, addition of 3 mg/kg of phoxim to soil
resulted in half-lives of 1 to 11 days (Nitokuno 1977). The
results of the reported studies are presented in Table 5.
Metabolism
The studies to investigate metabolism of phoxim (I) in soil
were conducted with unlabelled and ring-14C-labelled
compounds. After incorporation of a 5 percent granular formulation
in fallow soils with no plant cover, degradation was studied by
gas chromatography (Dräger 1977). With initial parent compound
concentrations of 4.0 mg/kg in the 0 to 15 cm layer, less than
0.1 mg/kg was found after two months; by day 150, the parent
compound concentration was less than 0.05 mg/kg. The photoisomer
(III) was detectable only on day 0.
The levels of diethyl phosphoric acids (IX) and (X) declined
to 0.09 mg/kg (IX) and 0.05 mg/kg (X) by day 26. Details are given
in Table 6.
After application of a 10 percent formulation, using
ring-14C-phoxim (I), to maize at sowing, the compounds (XI)
and (XII) were detected (Dräger 1978a). Residues of both compounds
were less than 0.1 mg/kg at 59 to 128 days after application.
During this period, phoxim (I) decreased in the 0 to 5-cm layer
from 0.33 mg/kg on day 59 to 0.10 mg/kg on day 128 (Table 7).
Table 5 Half-lives of Phoxim in Different Soils
Soil Application Half-life
Laboratory studies
sandy loam soil 0.8 % org. matter; 13.6% clay; pH 6.5 3 mg/kg a.i. ca. 1 day
clay loam soil 12.7 % org. matter; 1.5% clay; pH 5.6 3 mg/kg a.i. ca. 11 days
sandy loam soil 5.85% org. matter; 14.0% clay; pH 5.7 3 mg/kg a.i. ca. 9 days
Greenhouse study
loamy sand 0.89% C; 31.4% fines; pH 6.0 10% granular; ca. 8 weeks
0.5 g/m row
Field studies
light humus 3.2% C; 9.8% clay; 10.3% silt; pH 5.2 5% granular; ca. 2 weeks
100 kg/ha
loamy sand 3.8% C; 15.0% clay; 14.4% silt; pH 6.1 5% granular; ca. 2.5 weeks
100 kg/ha
loam soil 3% microgranular;
2 × 90 kg/ha ca. 10 days
clay loam soil 12.7% org. matter; 1.5% clay 3% microgranular
2 × 90 kg/ha ca. 2 weeks
Table 6 Metabolism of Phoxim in Soil, Field Study1
Day 0 Day 14 Day 26 Day 60
Analysed
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-10 10-15 0-15 0-5 5-10 10-25 0-15 0-5 5-10 10-15 0-15 0-5 5-10 10-15 0-15
(I) 9.4 2.3 0.39 4.0 4.9 1.34 0.35 2.2 1.42 0.42 0.06 0.6 0.08 0.04 0.04 0.05
(III) 1.4 1.1 n.d. 0.8 0.24 n.d. n.d. 0.08 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d.
(IX) 2.46 0.30 n.d. 0.92 1.09 0.06 n.d. 0.38 0.09 0.02 n.d. 0.04 n.d. n.d. n.d. n.d.
(X) 0.69 0.26 n.d. 0.32 0.10 0.06 n.d. 0.05 0.05 n.d. n.d. 0.02 n.d. n.d. n.d. n.d.
1 Soil type: light humus; pH 5.2; 20.1% fines; 9.8% clay; 10.3% silt; 3.2% C. Application rate - 5 kg a.i./ha. Residue levels
reported in mg/kg in relation to dry material.
2 For Roman numerals see Table 4.
n.d. = not detected.
Table 7 Metabolism of Ring-14C-Phoxim (I) in Soil1
Analysed Day 59 Day 91 Day 128
component2 Soil layer (cm) Soil layer (cm) Soil layer (cm)
0-5 5-20 0-5 5-20 0-5 5-20
(I) 0.33 0.10 0.22 0.09 0.10 0.04
(XI) 0.06 0.01 0.05 0.01 0.04 0.01
(XII) 0.02 0.001 0.006 0.001 0.004 n.d.
1 pH 6.0; 31.4% fines; 0.89%C. Greenhouse study. Residue levels in mg/kg.
2 For Roman numerals see Table 4.
Results of studies conducted in a closed system, using
ring-14C-phoxim (I) incorporated as a granular formulation into the
soil, showed that the aromatic remainder of the molecule was
mineralized substantially to CO2 (XV) (Steffens 1978). Within 56 days
after application, 25 to 28 percent of the applied 14C activity was
released into the atmosphere as 14CO2.
The metabolic pathway of phoxim in soil is illustrated in Figure
2.
In Plants
The behaviour of phoxim in plants was investigated by studies of
absorption, translocation and accumulation of ring-14C-phoxim in
maize plants, in greenhouse and field studies, and of the metabolism
of 32P and ring-14C-phoxim after foliar application to cotton plants
and after injection of ring-14C-(XI) into tomato plants.
Absorption, translocation and accumulation
The behaviour of phoxim (I) after application as a soil
insecticide was investigated in a greenhouse study in which a 10
percent granular formulation was incorporated in the soil at the time
of sowing maize. The distribution of plant-absorbed 14C activity was
monitored in green maize and at the milk-ripe and harvest-ripe stages
(Dräger 1978a). The results are given in Table 8.
After application of the 10 percent granular formulation of
phoxim to maize in a field experiment (Steffens 1978), less 14C
activity was absorbed by the plant than in the aforementioned
greenhouse study; for example, the harvest-ripe kernels contained 0.02
percent, compared with 0.08 percent in the greenhouse experiment, of
the 14C dose applied to the soil (Table 9).
Metabolism
The effect of light on the metabolism of phoxim (I) was evident
in greenhouse studies conducted with 32P-labelled phoxim on cotton
plants (Dräger 1971). After application of the spray solution to
cotton leaves, the isomeric compound (III) was detected, which was
formed solely on irradiation of the treated test plants. The
simultaneously formed diphosphates (VII) and (VIII) were present only
at very low levels.
Metabolism studies with ring-14C parent compound applied to
foliage of cotton plants are based on model experiments that provided
further information on photolytically formed metabolites and on the
incorporation of metabolites in plant constituents. After exposure for
96 h to irradiation on glass plates, 1 percent (XII), 2 percent (XI),
11 percent (II), 5 percent (III) and 5 percent of the phosphorus-free
metabolite (V) were detected (Dräger, 1978b).
 Table 8 14C Activity in Maize (Greenhouse Experiment)
% of applied 14C activity
Plant part Green maize Milk-ripe stage Harvest-ripe stage
(day 59) (Day 91) (Day 128)
Root 0.97 3.3 1.6
Stem + leaves 0.27 0.47 0.68
Cob 0.0097
Spike 0.025
Kernels 0.082
Table 9 14C Activity in Maize (Field Study)
% of applied 14C activity
Plant part Flowering Milk-ripe stage Harvest-ripe stage
(Day 101) (Day 129) (Day 157)
Stem + leaves 0.1 0.1 0.2
Flowers + 0.01 - -
infructescence
Spike - 0.01 0.01
Kernels - 0.01 0.02
Compound (XI), the "acyl" component of phoxim, which was formed
in both irradiation studies and soil studies, was investigated for its
reaction with plant constituents (Dräger 1980a). Following injection
of ring-14C-(XI) into tomato plants, three glycosides were isolated
and identified. At 37 days after application, 13 percent of the
applied 14C activity was present as glucoside (XIII), 4 percent as
gentiobioside (XIV) and 16 percent as a glycoside with an unknown
disaccharide remainder; 39 percent was accounted for by non-reacted
(XI). Of the applied 14C activity, 23 percent was not extractable.
Incorporation into cellulose did not take place; owing to the
stability of the compound to hydrolysis, it is assumed to have been
incorporated into the lignin constituents of the plant.
Metabolism studies with ring-14C-phoxim (I) on cotton leaves in
a greenhouse (Dräger 1980b) resulted in a metabolite pattern similar
to that obtained in the two aforementioned model experiments. In
addition to the compounds found in those two model experiments, a
further phosphorus-free metabolite (IV) was detected. The bulk (85 to
95 percent) of the metabolite mixture was present on the leaf surface
and could be washed off with chloroform. Carbohydrate conjugates of
(XI) were mainly present in the interior of the leaf.
This experiment also provided confirmation of the rapid
degradation of phoxim (I). Residues of the parent compound decreased
with a half-life of about three days. Seven days after application,
phoxim accounted for only 11 percent of the total activity. The
nonextractable portion of 14C activity had increased up to 21 percent
by day 7. It can be assumed that this portion was also incorporated in
plant constituents (lignin).
The metabolic pathways of phoxim in plants are illustrated in
Figure 3.
In Animals
The behaviour of phoxim in animals was investigated in mice and
rats. Absorption, distribution, excretion and metabolism of
ring-UL-14C-phoxim and 32P-phoxim, respectively, were studied. The
biokinetic behaviour of ring-UL-14C-phoxim was investigated in rats
after oral administration at dose levels of 1 mg/kg and 10 mg/kg
(Daniel et el. 1978a). Some studies were conducted with 32P-phoxim
on mice dosed orally with 10.5; 114 and 955 mg/kg (Vinopal & Fukuto
1971).
Absorption
The compound was readily absorbed from the gastrointestinal tract
of male rats, with average maximal plasma levels equivalent to 0.35
and 2.44 µg phoxim/ml being achieved within 30 min. after dosing at 1
and 10 mg/kg, respectively. No radioactivity was detected at 24 h in
the plasma of rats dosed with 1 mg/kg, whereas a value of 0.04 µg
phoxim equivalents/ml was recorded for rats dosed with 10 mg/kg.
Table 8 14C Activity in Maize (Greenhouse Experiment)
% of applied 14C activity
Plant part Green maize Milk-ripe stage Harvest-ripe stage
(day 59) (Day 91) (Day 128)
Root 0.97 3.3 1.6
Stem + leaves 0.27 0.47 0.68
Cob 0.0097
Spike 0.025
Kernels 0.082
Table 9 14C Activity in Maize (Field Study)
% of applied 14C activity
Plant part Flowering Milk-ripe stage Harvest-ripe stage
(Day 101) (Day 129) (Day 157)
Stem + leaves 0.1 0.1 0.2
Flowers + 0.01 - -
infructescence
Spike - 0.01 0.01
Kernels - 0.01 0.02
Compound (XI), the "acyl" component of phoxim, which was formed
in both irradiation studies and soil studies, was investigated for its
reaction with plant constituents (Dräger 1980a). Following injection
of ring-14C-(XI) into tomato plants, three glycosides were isolated
and identified. At 37 days after application, 13 percent of the
applied 14C activity was present as glucoside (XIII), 4 percent as
gentiobioside (XIV) and 16 percent as a glycoside with an unknown
disaccharide remainder; 39 percent was accounted for by non-reacted
(XI). Of the applied 14C activity, 23 percent was not extractable.
Incorporation into cellulose did not take place; owing to the
stability of the compound to hydrolysis, it is assumed to have been
incorporated into the lignin constituents of the plant.
Metabolism studies with ring-14C-phoxim (I) on cotton leaves in
a greenhouse (Dräger 1980b) resulted in a metabolite pattern similar
to that obtained in the two aforementioned model experiments. In
addition to the compounds found in those two model experiments, a
further phosphorus-free metabolite (IV) was detected. The bulk (85 to
95 percent) of the metabolite mixture was present on the leaf surface
and could be washed off with chloroform. Carbohydrate conjugates of
(XI) were mainly present in the interior of the leaf.
This experiment also provided confirmation of the rapid
degradation of phoxim (I). Residues of the parent compound decreased
with a half-life of about three days. Seven days after application,
phoxim accounted for only 11 percent of the total activity. The
nonextractable portion of 14C activity had increased up to 21 percent
by day 7. It can be assumed that this portion was also incorporated in
plant constituents (lignin).
The metabolic pathways of phoxim in plants are illustrated in
Figure 3.
In Animals
The behaviour of phoxim in animals was investigated in mice and
rats. Absorption, distribution, excretion and metabolism of
ring-UL-14C-phoxim and 32P-phoxim, respectively, were studied. The
biokinetic behaviour of ring-UL-14C-phoxim was investigated in rats
after oral administration at dose levels of 1 mg/kg and 10 mg/kg
(Daniel et el. 1978a). Some studies were conducted with 32P-phoxim
on mice dosed orally with 10.5; 114 and 955 mg/kg (Vinopal & Fukuto
1971).
Absorption
The compound was readily absorbed from the gastrointestinal tract
of male rats, with average maximal plasma levels equivalent to 0.35
and 2.44 µg phoxim/ml being achieved within 30 min. after dosing at 1
and 10 mg/kg, respectively. No radioactivity was detected at 24 h in
the plasma of rats dosed with 1 mg/kg, whereas a value of 0.04 µg
phoxim equivalents/ml was recorded for rats dosed with 10 mg/kg.
 Distribution
The distribution of radioactivity in the organs and tissues of
male rats intubated with 14C-phoxim at 10 mg/kg has been
investigated. Apart from the large intestine and its contents, the
distribution of radioactivity was essentially similar to that for
plasma (Daniel et al. 1978a). A study has also been made of
radioactivity in the gastrointestinal tract of male rats up to 7.5 h
after dosing with phoxim at 1 mg/kg. In these studies, no accumulation
of radioactivity in any organ or tissue was found.
The studies of Vinopal & Fukuto (1971) on white mice were
negative for the period 0 to 48 h after oral application of 114 mg/kg
32P-phoxim (Table 10).
The amounts of organosoluble material (which might include the
strong anticholinesterase PO phoxim) were essentially insignificant.
The reason for the apparent uptake of radioactivity in the urinary
bladder of the mouse is not clear.
Excretion
A study was made on excretion of radioactivity in the urine and
faeces of male and female rats given a single oral dose of 14C-phoxim
at 10 mg/kg (Daniel et al 1978a). Male rats excreted an average of
92.2 percent of the radioactivity in the urine and 4.9 percent in the
faeces in ten days, while females excreted 86.1 percent of the dose in
the urine and 6.9 percent in the faeces in the same period.
Male rats intubated with 14C-phoxim at a dose of 1 mg/kg
excreted 82 percent of the radioactivity in the urine and 7.9 percent
in the faeces in ten days. The results indicate that most of the
radioactivity was eliminated in 24 h and excretion was virtually
complete within two days. No evidence was obtained for the presence of
14CO2 in expired air.
An average of 4.1 percent S.D. 2.3) of the radioactivity was
excreted within 0-24 h in the bile of male rats intubated with
14C-phoxim at 10 mg/kg (Daniel et al. 1978a).
Following oral application of 10.5, 114 and 955 mg/kg 32P-phoxim
to mice, the ultimate recovery of administered radioactivity in the
urine and faeces was in the range of 73 to 84 percent (Vinopal &
Fukuto 1971). However, the radioactivity appeared in the urine and
faeces at a much lower rate than expected. For example, 24 h after
oral treatment of mice at 10.5 and 114 mg/kg with radioactive phoxim,
only 43 percent and 22 percent, respectively, of the administered
radioactivity was excreted in the urine. At 955 mg/kg, only 17 percent
of the administered radioactivity was excreted in the urine after
30 h.
Metabolism
Studies by Daniel et al. (1978b) with ring-UL-14C-phoxim on
rats produced the metabolic pathway shown in Figure 4. Phoxim was
largely absorbed and was detected in plasma as a dealkylated compound
in the P=S and P=O form. The radioactive metabolites in the urine of
rats at 0 to 24 h after oral application of 10 mg/kg consisted of
about 90 percent sulphate and glucuronide conjugates that were
hydrolysed enzymatically to alpha-hydroxy-imono-phenylacetonitrile
(XI), as demonstrated by thin-layer chromatography and mass
spectrometry. Hippuric acid was detected as a metabolite by inverse
isotope dilution analysis; it represented about 5 percent of the
radioactivity eliminated through the kidneys.
Radioactive urine from white mice after oral treatment with
32P-phoxim was analysed by ion-exchange and thin-layer chromatography
(Vinopal & Fukuto 1971). Five metabolites were recovered after
treatment with 32P-phoxim at 114 mg/kg and 955 mg/kg. The identity of
the metabolites was established by thin-layer chromatography with
specific staining reagents and/or IR-spectroscopy. The metabolites
were identified as diethylphosphoric acid (X), phoxim (I), phoxim
carboxylic acid (XVIII), O,O-diethyl phosphorothioic acid (IX) and
either desethyl phoxim or desethyl PO phoxim (XVIII). The amounts of
radioactivity relative to total radioactivity renally eliminated are
summarized in Table 11.
Photodegradation
The U.V. spectrum of phoxim (in methanol) shows a relatively
long-wave peak at 282 nm (… = 11.180); its long wavelength absorption
edge overlaps with the sunlight emission in the troposphere (lambda
> 290 nm). In the environment, direct interaction of the molecule
with sunlight is, therefore, possible (Wilmes 1981).
Experiments using double-distilled water (5 ng/kg, 0.5 percent
acetonitrile) yielded very short half-lives. The half-life was less
than 10 min with TQ 150 high-pressure mercury lamps (Duran filter,
laubda > 290 nm) and about 2 h with sunlight-simulating fluorescent
tubes (TRU LITE) (Wilmes 1981).
The results of studies on cotton leaves and glass plates with
differently labelled phoxim (Dräger 1971, 1978a) are indicative of the
significance of photodegradation in this compound. Among others, a
photoisomerization product (III) was isolated and identified, which
was also detected in chlorphoxim (Dräger 1972) and methylphoxim
(Dräger 1975) derivatives. After exposure of phoxim to irradiation on
glass plates, the metabolites III, V, VII, VIII, XI and XII were
isolated and identified. Partial isomerization of the Z-isomer (I),
prevalent in the technical grade compound, to the E-isomer (II) was
also observed.
Table 10 Summary of Autopsy Data on a White Mouse
after Oral Treatment with 32P-phoxim1
% Recovered internal radioactivity
Organ Organo- Water- Total
soluble soluble
Brain 0.07 0.14 0.21
Thymus gland 0.01 0.05 0.06
Hind leg muscle n.d. 0.54 0.54
Heart 0.01 0.23 0.24
Kidney 0.03 0.13 0.16
Liver 0.05 1.60 1.65
Gut 0.17 8.60 8.77
Urinary bladder 2.10 86.30 88.40
Total 2.44 97.59 100.0
1 Postmortem examination 48 h after dosing with
114 mg/kg 32P-phoxim.
Table 11 Metabolites in Urine from White Mice after Treatment
with Phoxim
Metabolite1 % of radioactivity in urine
114 mg/kg dose 955 mg/kg dose
(after 24 h) (after 30 h)
X 58.9 43.1
I 1.1 2.1
XVII 2.8 23.6
IX 20.0 17.7
XVIII 6.2 5.0
RA not in peaks 4.3 5.4
unrecovered2 6.7 3.1
1 For chemical names, see Table 4.
2 Unrecovered after ion-exchange column chromatography.
Distribution
The distribution of radioactivity in the organs and tissues of
male rats intubated with 14C-phoxim at 10 mg/kg has been
investigated. Apart from the large intestine and its contents, the
distribution of radioactivity was essentially similar to that for
plasma (Daniel et al. 1978a). A study has also been made of
radioactivity in the gastrointestinal tract of male rats up to 7.5 h
after dosing with phoxim at 1 mg/kg. In these studies, no accumulation
of radioactivity in any organ or tissue was found.
The studies of Vinopal & Fukuto (1971) on white mice were
negative for the period 0 to 48 h after oral application of 114 mg/kg
32P-phoxim (Table 10).
The amounts of organosoluble material (which might include the
strong anticholinesterase PO phoxim) were essentially insignificant.
The reason for the apparent uptake of radioactivity in the urinary
bladder of the mouse is not clear.
Excretion
A study was made on excretion of radioactivity in the urine and
faeces of male and female rats given a single oral dose of 14C-phoxim
at 10 mg/kg (Daniel et al 1978a). Male rats excreted an average of
92.2 percent of the radioactivity in the urine and 4.9 percent in the
faeces in ten days, while females excreted 86.1 percent of the dose in
the urine and 6.9 percent in the faeces in the same period.
Male rats intubated with 14C-phoxim at a dose of 1 mg/kg
excreted 82 percent of the radioactivity in the urine and 7.9 percent
in the faeces in ten days. The results indicate that most of the
radioactivity was eliminated in 24 h and excretion was virtually
complete within two days. No evidence was obtained for the presence of
14CO2 in expired air.
An average of 4.1 percent S.D. 2.3) of the radioactivity was
excreted within 0-24 h in the bile of male rats intubated with
14C-phoxim at 10 mg/kg (Daniel et al. 1978a).
Following oral application of 10.5, 114 and 955 mg/kg 32P-phoxim
to mice, the ultimate recovery of administered radioactivity in the
urine and faeces was in the range of 73 to 84 percent (Vinopal &
Fukuto 1971). However, the radioactivity appeared in the urine and
faeces at a much lower rate than expected. For example, 24 h after
oral treatment of mice at 10.5 and 114 mg/kg with radioactive phoxim,
only 43 percent and 22 percent, respectively, of the administered
radioactivity was excreted in the urine. At 955 mg/kg, only 17 percent
of the administered radioactivity was excreted in the urine after
30 h.
Metabolism
Studies by Daniel et al. (1978b) with ring-UL-14C-phoxim on
rats produced the metabolic pathway shown in Figure 4. Phoxim was
largely absorbed and was detected in plasma as a dealkylated compound
in the P=S and P=O form. The radioactive metabolites in the urine of
rats at 0 to 24 h after oral application of 10 mg/kg consisted of
about 90 percent sulphate and glucuronide conjugates that were
hydrolysed enzymatically to alpha-hydroxy-imono-phenylacetonitrile
(XI), as demonstrated by thin-layer chromatography and mass
spectrometry. Hippuric acid was detected as a metabolite by inverse
isotope dilution analysis; it represented about 5 percent of the
radioactivity eliminated through the kidneys.
Radioactive urine from white mice after oral treatment with
32P-phoxim was analysed by ion-exchange and thin-layer chromatography
(Vinopal & Fukuto 1971). Five metabolites were recovered after
treatment with 32P-phoxim at 114 mg/kg and 955 mg/kg. The identity of
the metabolites was established by thin-layer chromatography with
specific staining reagents and/or IR-spectroscopy. The metabolites
were identified as diethylphosphoric acid (X), phoxim (I), phoxim
carboxylic acid (XVIII), O,O-diethyl phosphorothioic acid (IX) and
either desethyl phoxim or desethyl PO phoxim (XVIII). The amounts of
radioactivity relative to total radioactivity renally eliminated are
summarized in Table 11.
Photodegradation
The U.V. spectrum of phoxim (in methanol) shows a relatively
long-wave peak at 282 nm (… = 11.180); its long wavelength absorption
edge overlaps with the sunlight emission in the troposphere (lambda
> 290 nm). In the environment, direct interaction of the molecule
with sunlight is, therefore, possible (Wilmes 1981).
Experiments using double-distilled water (5 ng/kg, 0.5 percent
acetonitrile) yielded very short half-lives. The half-life was less
than 10 min with TQ 150 high-pressure mercury lamps (Duran filter,
laubda > 290 nm) and about 2 h with sunlight-simulating fluorescent
tubes (TRU LITE) (Wilmes 1981).
The results of studies on cotton leaves and glass plates with
differently labelled phoxim (Dräger 1971, 1978a) are indicative of the
significance of photodegradation in this compound. Among others, a
photoisomerization product (III) was isolated and identified, which
was also detected in chlorphoxim (Dräger 1972) and methylphoxim
(Dräger 1975) derivatives. After exposure of phoxim to irradiation on
glass plates, the metabolites III, V, VII, VIII, XI and XII were
isolated and identified. Partial isomerization of the Z-isomer (I),
prevalent in the technical grade compound, to the E-isomer (II) was
also observed.
Table 10 Summary of Autopsy Data on a White Mouse
after Oral Treatment with 32P-phoxim1
% Recovered internal radioactivity
Organ Organo- Water- Total
soluble soluble
Brain 0.07 0.14 0.21
Thymus gland 0.01 0.05 0.06
Hind leg muscle n.d. 0.54 0.54
Heart 0.01 0.23 0.24
Kidney 0.03 0.13 0.16
Liver 0.05 1.60 1.65
Gut 0.17 8.60 8.77
Urinary bladder 2.10 86.30 88.40
Total 2.44 97.59 100.0
1 Postmortem examination 48 h after dosing with
114 mg/kg 32P-phoxim.
Table 11 Metabolites in Urine from White Mice after Treatment
with Phoxim
Metabolite1 % of radioactivity in urine
114 mg/kg dose 955 mg/kg dose
(after 24 h) (after 30 h)
X 58.9 43.1
I 1.1 2.1
XVII 2.8 23.6
IX 20.0 17.7
XVIII 6.2 5.0
RA not in peaks 4.3 5.4
unrecovered2 6.7 3.1
1 For chemical names, see Table 4.
2 Unrecovered after ion-exchange column chromatography.

 Photoproducts detected in aqueous solution (50 percent
acetonitrile), after complete degradation of the parent compound, were
alpha-cyanobenzaldoxime (XI) and bis-alpha-
dithioiminophenylacetronitrile (VI) (Wilmes 1981).
Pao (1975) reported studies conducted to investigate degradation
of phoxim on tea leaves under natural conditions (sunlight) and in the
laboratory (UV light). In both cases, distinct photodegradation was
observed. The same results were obtained in studies on glass plates
and tomato leaves (Makari et al. 1981).
In Storage and Processing
The stability of phoxim residues during storage at -20°C was
monitored on lettuce over a period of 2.5 years. From regression
curves plotted for the determined residue values in relation to time,
assuming a first-order reaction, it was evident that no significant
decrease in the phoxim residues occurred during low-temperature
storage (Dräger 1982).
The stability of phoxim residues in grain stored at 26.7°C was
monitored for 12 months after application of phoxim at 5, 10 and 20
mg/kg to maize, wheat and sorghum. Only minor losses were recorded
during this period (LaHue & Dicke 1971).
Wheat grains fortified with 8 mg/kg phoxim were milled in an
experimental study. The flour from the first and second milling
operations, which accounted for 73 percent of the total material
milled, contained 13 percent of the applied phoxim dose; a phoxim
content of 1.3 mg/kg was determined by measuring 32P activity. The
bulk of phoxim was present in the middlings and semolina, accounting
for 35 percent and 43 percent, respectively, of the applied phoxim
dose. These milled fractions represented only 11 percent and 7
percent, respectively, of the total milled material; therefore, their
content of phoxim amounting to 23 mg/kg and 44 mg/kg, respectively,
was substantially higher than that in flour (Dräger 1968). Extraction
of wheat bread made from the milled fractions 1 and 2 showed that the
major portion of the degradation products that arise from phoxim
during the baking process, either due to chemical decomposition or as
a result of enzymatic degradation, were present in the form of highly
polar, water-soluble compounds. Further quantitative analyses were
performed by gas-chromatography on whole-meal bread made by baking the
whole of the milled material; 65 percent of the applied phoxim was
split into diethyl thiophosphoric acid during the baking process. It
was also observed that no more unchanged parent compound was present
(Dräger 1968).
The effect of boiling on residues of phoxim and phoxim-oxon was
studied in potatoes. The phoxim residues were reduced by 86 percent
during boiling; of the remaining residue, one fifth was present in the
water used for boiling. The oxon of phoxim was reduced by 91 percent
during boiling; of the compound still present, two thirds was found in
the water used for boiling (Dräger 1978c).
METHODS OF RESIDUE ANALYSIS
Residues of phoxim in plant and soil samples can be determined by
gas chromatography using a phosphorus-specific flame-photometric
detector or a thermionic phosphorus detector. These methods
simultaneously determine the P=O compound of phoxim (Bowman & Leuck
1971; Dräger 1969 a,b; Thornton 1969). Thin-layer chromatography has
also been used for determining phoxim residues (Bykhovets 1974; Pao
1975).
For the extraction of residues from plant material, acetone,
acetonitrile, chloroform or benzene was used. For Soxhlet extraction
of plant material, a mixture of 10 percent methanol in benzene was
used (Bowman & Leuck 1971). Soil samples were extracted with methanol.
Isolation of the residues from the extracts were achieved by
partitioning with N-hexane or chloroform. Separation of co-extracted
plant constituents was achieved by precipitating with a solution of
ammonium chloride/phosphoric acid or by partitioning between
acetonitrile and N-hexane. For column-chromatographic cleanup of the
extracts, silica gel or activated charcoal/aluminium oxide was used.
Owing to the termal instability of phoxim, it is necessary, for
gas-chromatographic determination of the parent compound, to use
supports coated with, for example, 5 percent OV-101 on Gas Chrom Q or
2 percent DC-200 + 1 percent (or 2 percent) QF-1 on Gas Chrom Q.
Injection port and column temperatures were set at 160° - 170° (Dräger
1969a,b; Bowman & Leuck 1971; Thornton 1969).
For thin-layer chromatographic detection of residues, silica gel
plates were used with the following solvents and reagents:
N-hexane/acetone 4:1 - bromophenol blue/silver nitrate (Bykhovets
1974) and benzene/butane 1:1 - Congo Red (Pao 1975). The lower limit
of determination for gas-chromatographic detection of residues in
plant and soil samples was usually 0.05 mg/kg; in special cases,
residues were determined down to a level of 0.004 mg/kg. For
thin-layer chromatographic determination in tea, the lower limit of
determination was found to be 0.5 mg/kg (Pao 1975).
Residues on phoxim in animal tissues can be determined by
gas-liquid chromatography, using a thermionic detector or, preferably,
a flame photometric detector. Residues are extracted from tissues with
hexane in a Waring blender and by soaking after addition of anhydrous
sulphate. The extract is cleaned up by partitioning with acetonitrile
and residues are transferred to hexane for the GLC-determination. A
2 percent OV-101 glass column may be used. The limit of determination
has been reported as 0.05 mg/kg (Hopkins 1980).
Residues of phoxim and its oxygen analogue in milk have been
determined by thin-layer chromatography after extraction with ethyl
acetate and cleanup of the extract by partitioning with acetonitrile
and hexane. A homogenate of bee-heads is used for determination of the
cholinesterase inhibition effect. The limit of determination has been
reported as 0.01 mg/kg (Ernst 1980).
In an interference study, performed to determine whether any of
the organophosphorus compounds registered for use on bananas to
control insect pests would interfere with the method for
gas-chromatographic determination of phoxim, no interferences were
observed from any of the compounds and metabolites examined (Olson
1974).
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) and associated preharvest
intervals reported to the Meeting are shown in Table 12.
APPRAISAL
Phoxim is a nonsystemic contact and stomach organophosphorus
insecticide. It is registered and used in a number of countries and is
especially effective against biting insects. It is applied both to
foliage and soil and is also used as a seed dressing. Phoxim is
registered for veterinary use against ectoparasites.
When applied to vegetable foliage, the dosage rates are 0.75 kg
to 1.5 kg a.i./ha and on cotton the rate is 1.5 to 2.3 kg a.i./ha.
Phoxim is applied as a soil treatment to vegetables, using
emulsifiable concentrates, baits or granules at dosage rates of 0.8 to
5.0 kg a.i./ha. Phoxim is applied as granules for soil treatment to
potatoes at a dosage rate of 1.5 to 7.5 kg a.i./ha as a soil treatment
for grains using 5.0 kg a.i./ha of granular formulation.
Residues in crops following foliar and soil treatment are
generally below 0.2 mg/kg and average values are generally below the
limit of determination (0.004 to 0.05 mg/kg). Residues in grains (oat,
barley, wheat and maize) with soil treatment before sowing were all
below the limit of determination. Also, residues with soil treatment
before or at sowing or planting of beans, cauliflower, cabbage and
onions were below the limit of determination. Average residues in
potatoes after soil treatment were below the limit of determination,
with the highest single value being 0.02 mg/kg. Residue after foliar
treatment in vegetables were low, with the highest average value being
0.13 mg/kg (tomatoes) and the highest single value 0.20 mg/kg
(spinach).
Table 12 National Maximum Residue Limits and Preharvest Intervals
Reported to the Meeting
Country Crop MRL Preharvest interval
(mg/kg) (days)
Australia Potatoes 0.05
Austria All food crops 0.05
Czechoslovakia Potatoes 14
Fed. Rep. Germany All food crops 0.05
Italy Fruit 0.05 42
Vegetables 0.05 42
Luxembourg All food crops 0.05
The Netherlands Vegetables 0.05
Milk & milk products 0.01
Meat & meat products 0.01 -> 0.05
Other products 0 -> 0.05
South Africa Cereals 0.2
Peanuts 0.2
Spain General 15
Thailand Maize 15
Cotton 15
Potatoes 15
Sorghum 15
Vegetables 15
Soviet Union Brassicas 20
Eggplant 20
Perennial grasses 20
Potatoes 20
Sugarbeet 20
Tomatoes 20
Yugoslavia All crops 0.05
In experimental treatment of sheep, cattle and pigs with phoxim,
by dipping or spraying, dosages ranged from 500 to 3 000 mg/l. At
slaughter, 14 to 45 days after treatment, no residues were observed in
samples of liver, kidney or muscle. However, residues were found in
fat from all animals, ranging from the limit of determination to 2.8
mg/kg, depending on treatment and waiting period.
In laboratory and field experiments phoxim degradation was
observed in different soil types, with half-lives from one day to
about two weeks, but in a greenhouse study the half-life was about
eight weeks. The main metabolites of phoxim in soil were
alpha-hydroxy-imino-phenylacetonitrile and benzoic acid, both formed
by hydrolysis, but diethyl phosphoric acids were also found in soil.
Laboratory and field leaching experiments in soil showed that
phoxim did not reach a depth below 5 cm and 10 cm, respectively, in
the laboratory and field.
Phoxim or its metabolites were found in roots, stems and leaves
of maize after soil treatment. The cobs, spikes and kernels contained
very little phoxim or metabolites. After foliar treatment, the leaves
contained phoxim and metabolites, but most of these were present on
the surface of the leaves and could be removed with chloroform.
The metabolites formed in or on plants consisted of an isoner:
diethoxy-phosphoryl-thioimino-phenyl-acetonitrile, which was formed
mainly by irradation of the plant, together with minor amounts of
tetraethyl diphosphate and tetraethyl mono-thio-diphosphate. The
"acyl"-compounds (phosphorous-free metabolites) formed by hydrolysis
reacted with plant constituents to three glycosides. The parent
compound degraded rapidly; after seven days only 11 percent of the
total activity was attributable to phoxim.
Investigations on animal metabolism were made in rats and mice.
No information was available on metabolism in cattle, pigs and poultry
for evaluation of residues of phoxim and its metabolites in animal
products.
Experiments were carried out on the stability of phoxim during
storage. Lettuce stored at -20° C for 2.5 years showed no significant
decrease in phoxim content.
Flour milled from grain fortified with phoxim contained 13
percent of the applied phoxim, while middlings and semolina accounted
for 35 percent and 43 percent, respectively, of the applied dose.
Bread made from flour containing phoxim did not contain unchanged
parent compound. Phoxim residues in potatoes were reduced by 91
percent during boiling.
Residues of phoxim in plant and soil samples can be determined by
gas-liquid chromatography with a flame photometric detector or a
thermonic detector. Thin-layer chromatography has also been used for
determining phoxim residues. Extraction can be made from plant
material with acetone, acetonitrile, chloroform or benzene. For
Soxhlet extraction, 10 percent methanol in benzene has been used. Soil
is extracted with methanol. Residues of phoxim in animal tissues are
extracted with hexane and residues in milk with ethylacetate. Cleanup
of extracts has been achieved by partitioning, precipitation with
ammonium chloride/phosphoric acid and/or column chromatography.
RECOMMENDATIONS
The following levels are recommended as MRLs, which need not be
exceeded when phoxim is used according to good agriculture practice.
The limits refer to the parent compound only.
Commodity MRL Preharvest intervals
on which recommendations
are based
Cereal grains 0.05** 1)
Lettuce 0.1 14 days
Beans 0.05** 14 lays
Cauliflower 0.05** 42 days
Potatoes 0.05** 1)
Tomatoes 0.2 14 days
Cotton seed 0.05** 14 days
Interval between treatment
and slaughtering
Cattle, carcase 0.02
meat (in carcase fat) 28 days
Sheep, carcase meat 1 (in carcase 28 days
fat)
1) Soil treatment at sowing or planting.
** Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Residue data in milk from treatment of cattle and residue data in
fat from treatment of pigs.
2. Studies on metabolism of phoxim from treatment of cattle, pigs
and sheep.
3. Information on good agriculture practice in the use of phoxim on
vegetables.
REFERENCES - RESIDUES
Mobay reports are unpublished reports prepared by the R & D Dept.
of Mobay Chemical Corporation, Agricultural Chemicals Division, Kansas
City, Mo. 64120, USA.
Nitokuno reports are unpublished reports prepared by Nihon
Tokushu Noyaku Seizo, K.K., No. 8, 2-chome, Nihonbashi Muro-machi,
Chuo-ku, Tokyo, Japan.
Bayer. Pflanzenschutzmittel-Rückstände. Reports Nos. 165-166/70.
1970 (Unpublished)
Bayer. Pflanzenschutzmittel-Rückstände im Sickerwasser. Reports Nos.
1972 283-288/72; Reports Nos. 3003-3004/74; Reports Nos. 3020-
1974 3022/77. (Unpublished)
1977
Biologische Bundesanstalt für Land- und Forstwirtschaft. Prüfung des
1980 Versickerung-sverhaltens von Pflanzenschutzmitteln.
Merkblatt Nr. 37, 2. Auflage.
Bowman, M.C. & Leuck, D.B. Determination and persistence of phoxim and
1971 its oxygen analog in forage corn and grass. J. Agric. Food
Chem., 19: 1215-1218.
Bykhovets, A.J. Thin-layer chromatographic method to determine Basudin
1974 and Valexon in a plant sample. Aktual. Vopr. Zashch. Rast.
BSSR, Mater, Resp. Konf, Molodykh Uch., 1: 81-82.
Daniel, J.W., Swanson, S. & McLean, J. Phoxim - Pharmacokinetics and
1978a biotransformation in the rat. Life Science Research, U.K.
Report 78/BAG5/194. (Unpublished)
Daniel, J.W., McLean, J. & Pringuer, M. Phoxim - Biotransformation in
1978b the rat. Life Science Research, U.K. Report 78/BAG5/317.
(Unpublished)
Dräger, G. Studies on the metabolism of BAY 77 488. Bayer AG,
1968 Pflanzenschutz-Anwendungstechnik, Report. (Unpublished)
Dräger, G. Gaschromatographische Methode zur Bestimmung von RValexon
1969a Rückständen in Pflanzen. Pflanzenschutz-Nachr. Bayer,
22:311-317 (1969a)
Dräger, G. Rückstandsanalytik von Pflanzenschutzmitteln. DFG-
1969b Methodensammlung, 1. Lieferung 1969; methode Nr. 307.
Dräger, G. Studies on the metabolism of phoxim (BAY 77 488).
1971 Pflanzenschutz-Nachr. Bayer, 24: 239-251.
Dräger, G. Reaction products of chlorphoxim (BAY 78 182) formed by
1972 exposure of the parent compound to irradiation.
Pflanzenschutz-Nachr. Bayer, 25: 32-42.
Dräger, G. Metabolisierung von Phoxim-methyl durch Belichtung. Bayer
1975 AG, Pflanzenschutz-Anwendungstechnik report RA-57.
(Unpublished)
Dräger, G. Studies on the fate of phoxim (BAY 77 488) in soil.
1977 Pflanzenschutz-Nachr. Bayer, 30:28-41.
Dräger, G. Untersuchung des Metabolismus von 14C-ringmarkiertem
Phoxim
1978a in Mais und Boden nach Anwendung enies Granulates. 1. 14C-
Bilanz und Metabolismus im Boden. Bayer AG, Pflanzenschutz-
Anwendungstechnik report RA-278. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim; Belichtung von
1978b 14C-ringmarkiertem Wirkstoff auf Glassplatten. Bayer AG,
Pflanzenschutz-Anwendungstechnik report RA-618.
(Unpublished)
Dräger, G. Stability of phoxim residues during boiling of potatoes.
1978c Bayer AG, Pflanzenschutz-Anwendungstechnik report RA-843.
(Unpublished)
Dräger, G. Untersuchung zum Metabolismus von Phoxim; Metabolismus
1980a des Abbauproduktes alpha-Hydroxyiminophenolacetonitril in
Tomatenpflanzen. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-106. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim auf
1980b Baumwollblättern. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-208. (Unpublished)
Dräger, G. Stability of phoxim residues in lettuce during low
1982 temperature storage. Bayer AG, Pflanzenschutz-
Anwendungstechnik, report RA-69. (Unpublished)
Ernst, G. F., Brinkman, M.B.C. & Mattern, E.M. Methode van onderzoek
1981 voor het bepalen van residuen van foxim en foxon im melk.
KvW Utrecht, IR/71/80/R79-Okt.
Hopkins, T., Lemprecht, C. & Lindsay, G. Bay 9053 - 50% w/v E.C. -
1980 residues - sheep. Bayer Doc. No. 80/9955.
LaHue, D.W. & Dicke, E.B. Phoxim as an insect protectant for stored
1971 grains. J. Econ. Entomol., 64: 1530-1533.
Makari, M.H., Riskallah, M.R., Hegazy, M.E. & Belal, M.H. Photolysis
1981 of phoxim on glass and on tomato leaves. Bull. Environ.
Contam. Toxicol. 26: 413-419 (1981).
Nitokuno. Residual analysis results of VolatonR (phoxim) in upland
1977 soil. Report No. 1058 (RA). (Unpublished)
Olson, T.J. An interference study for the residue method for Volaton
1974 determination on bananas. Mobay Report No. 40 095.
(Unpublished)
Pao, K.C.H. Residue studies of phoxim (Baythion) on the tea bushes.
1975 Acta Entomol. Sin., 18: 133-139.
Steffens, W. Bilanzversuche mit (benzolring-U-14C) Phoxim nach
1978 Verwendung als Bodeninsektizid zu Mais in einem
Freilandlysimeter und einem geschlossenen System im
Gewächshaus. Kernforschungsnalage Jülich, Institut für
Radioagronomie, report ARA 14/78. (Unpublished)
Thornton, J.S. Determination of residues of BAY 77 488 in stored
1969 grains. Mobay report No. 24 575. (Unpublished)
Thornton, J.S., Hurley, J.B. & Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticides chemicals. Mobay report No. 51 016.
(Unpublished)
Wilmes, R. Orientierende Lichtstabilität (Phoxim). Bayer AG,
1981 Pflanzenschutz-Anwendungstechnik report. (Unpublished)
Vinopal, J.H.A. & Fukuto, T.R. Selective toxicity of phoxim
1971 (phenylglyoxylonitrile oxime 0,0-diethyl phosphorothioate).
Pestic. Biochem. Physiol., 1: 44-60.
Photoproducts detected in aqueous solution (50 percent
acetonitrile), after complete degradation of the parent compound, were
alpha-cyanobenzaldoxime (XI) and bis-alpha-
dithioiminophenylacetronitrile (VI) (Wilmes 1981).
Pao (1975) reported studies conducted to investigate degradation
of phoxim on tea leaves under natural conditions (sunlight) and in the
laboratory (UV light). In both cases, distinct photodegradation was
observed. The same results were obtained in studies on glass plates
and tomato leaves (Makari et al. 1981).
In Storage and Processing
The stability of phoxim residues during storage at -20°C was
monitored on lettuce over a period of 2.5 years. From regression
curves plotted for the determined residue values in relation to time,
assuming a first-order reaction, it was evident that no significant
decrease in the phoxim residues occurred during low-temperature
storage (Dräger 1982).
The stability of phoxim residues in grain stored at 26.7°C was
monitored for 12 months after application of phoxim at 5, 10 and 20
mg/kg to maize, wheat and sorghum. Only minor losses were recorded
during this period (LaHue & Dicke 1971).
Wheat grains fortified with 8 mg/kg phoxim were milled in an
experimental study. The flour from the first and second milling
operations, which accounted for 73 percent of the total material
milled, contained 13 percent of the applied phoxim dose; a phoxim
content of 1.3 mg/kg was determined by measuring 32P activity. The
bulk of phoxim was present in the middlings and semolina, accounting
for 35 percent and 43 percent, respectively, of the applied phoxim
dose. These milled fractions represented only 11 percent and 7
percent, respectively, of the total milled material; therefore, their
content of phoxim amounting to 23 mg/kg and 44 mg/kg, respectively,
was substantially higher than that in flour (Dräger 1968). Extraction
of wheat bread made from the milled fractions 1 and 2 showed that the
major portion of the degradation products that arise from phoxim
during the baking process, either due to chemical decomposition or as
a result of enzymatic degradation, were present in the form of highly
polar, water-soluble compounds. Further quantitative analyses were
performed by gas-chromatography on whole-meal bread made by baking the
whole of the milled material; 65 percent of the applied phoxim was
split into diethyl thiophosphoric acid during the baking process. It
was also observed that no more unchanged parent compound was present
(Dräger 1968).
The effect of boiling on residues of phoxim and phoxim-oxon was
studied in potatoes. The phoxim residues were reduced by 86 percent
during boiling; of the remaining residue, one fifth was present in the
water used for boiling. The oxon of phoxim was reduced by 91 percent
during boiling; of the compound still present, two thirds was found in
the water used for boiling (Dräger 1978c).
METHODS OF RESIDUE ANALYSIS
Residues of phoxim in plant and soil samples can be determined by
gas chromatography using a phosphorus-specific flame-photometric
detector or a thermionic phosphorus detector. These methods
simultaneously determine the P=O compound of phoxim (Bowman & Leuck
1971; Dräger 1969 a,b; Thornton 1969). Thin-layer chromatography has
also been used for determining phoxim residues (Bykhovets 1974; Pao
1975).
For the extraction of residues from plant material, acetone,
acetonitrile, chloroform or benzene was used. For Soxhlet extraction
of plant material, a mixture of 10 percent methanol in benzene was
used (Bowman & Leuck 1971). Soil samples were extracted with methanol.
Isolation of the residues from the extracts were achieved by
partitioning with N-hexane or chloroform. Separation of co-extracted
plant constituents was achieved by precipitating with a solution of
ammonium chloride/phosphoric acid or by partitioning between
acetonitrile and N-hexane. For column-chromatographic cleanup of the
extracts, silica gel or activated charcoal/aluminium oxide was used.
Owing to the termal instability of phoxim, it is necessary, for
gas-chromatographic determination of the parent compound, to use
supports coated with, for example, 5 percent OV-101 on Gas Chrom Q or
2 percent DC-200 + 1 percent (or 2 percent) QF-1 on Gas Chrom Q.
Injection port and column temperatures were set at 160° - 170° (Dräger
1969a,b; Bowman & Leuck 1971; Thornton 1969).
For thin-layer chromatographic detection of residues, silica gel
plates were used with the following solvents and reagents:
N-hexane/acetone 4:1 - bromophenol blue/silver nitrate (Bykhovets
1974) and benzene/butane 1:1 - Congo Red (Pao 1975). The lower limit
of determination for gas-chromatographic detection of residues in
plant and soil samples was usually 0.05 mg/kg; in special cases,
residues were determined down to a level of 0.004 mg/kg. For
thin-layer chromatographic determination in tea, the lower limit of
determination was found to be 0.5 mg/kg (Pao 1975).
Residues on phoxim in animal tissues can be determined by
gas-liquid chromatography, using a thermionic detector or, preferably,
a flame photometric detector. Residues are extracted from tissues with
hexane in a Waring blender and by soaking after addition of anhydrous
sulphate. The extract is cleaned up by partitioning with acetonitrile
and residues are transferred to hexane for the GLC-determination. A
2 percent OV-101 glass column may be used. The limit of determination
has been reported as 0.05 mg/kg (Hopkins 1980).
Residues of phoxim and its oxygen analogue in milk have been
determined by thin-layer chromatography after extraction with ethyl
acetate and cleanup of the extract by partitioning with acetonitrile
and hexane. A homogenate of bee-heads is used for determination of the
cholinesterase inhibition effect. The limit of determination has been
reported as 0.01 mg/kg (Ernst 1980).
In an interference study, performed to determine whether any of
the organophosphorus compounds registered for use on bananas to
control insect pests would interfere with the method for
gas-chromatographic determination of phoxim, no interferences were
observed from any of the compounds and metabolites examined (Olson
1974).
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) and associated preharvest
intervals reported to the Meeting are shown in Table 12.
APPRAISAL
Phoxim is a nonsystemic contact and stomach organophosphorus
insecticide. It is registered and used in a number of countries and is
especially effective against biting insects. It is applied both to
foliage and soil and is also used as a seed dressing. Phoxim is
registered for veterinary use against ectoparasites.
When applied to vegetable foliage, the dosage rates are 0.75 kg
to 1.5 kg a.i./ha and on cotton the rate is 1.5 to 2.3 kg a.i./ha.
Phoxim is applied as a soil treatment to vegetables, using
emulsifiable concentrates, baits or granules at dosage rates of 0.8 to
5.0 kg a.i./ha. Phoxim is applied as granules for soil treatment to
potatoes at a dosage rate of 1.5 to 7.5 kg a.i./ha as a soil treatment
for grains using 5.0 kg a.i./ha of granular formulation.
Residues in crops following foliar and soil treatment are
generally below 0.2 mg/kg and average values are generally below the
limit of determination (0.004 to 0.05 mg/kg). Residues in grains (oat,
barley, wheat and maize) with soil treatment before sowing were all
below the limit of determination. Also, residues with soil treatment
before or at sowing or planting of beans, cauliflower, cabbage and
onions were below the limit of determination. Average residues in
potatoes after soil treatment were below the limit of determination,
with the highest single value being 0.02 mg/kg. Residue after foliar
treatment in vegetables were low, with the highest average value being
0.13 mg/kg (tomatoes) and the highest single value 0.20 mg/kg
(spinach).
Table 12 National Maximum Residue Limits and Preharvest Intervals
Reported to the Meeting
Country Crop MRL Preharvest interval
(mg/kg) (days)
Australia Potatoes 0.05
Austria All food crops 0.05
Czechoslovakia Potatoes 14
Fed. Rep. Germany All food crops 0.05
Italy Fruit 0.05 42
Vegetables 0.05 42
Luxembourg All food crops 0.05
The Netherlands Vegetables 0.05
Milk & milk products 0.01
Meat & meat products 0.01 -> 0.05
Other products 0 -> 0.05
South Africa Cereals 0.2
Peanuts 0.2
Spain General 15
Thailand Maize 15
Cotton 15
Potatoes 15
Sorghum 15
Vegetables 15
Soviet Union Brassicas 20
Eggplant 20
Perennial grasses 20
Potatoes 20
Sugarbeet 20
Tomatoes 20
Yugoslavia All crops 0.05
In experimental treatment of sheep, cattle and pigs with phoxim,
by dipping or spraying, dosages ranged from 500 to 3 000 mg/l. At
slaughter, 14 to 45 days after treatment, no residues were observed in
samples of liver, kidney or muscle. However, residues were found in
fat from all animals, ranging from the limit of determination to 2.8
mg/kg, depending on treatment and waiting period.
In laboratory and field experiments phoxim degradation was
observed in different soil types, with half-lives from one day to
about two weeks, but in a greenhouse study the half-life was about
eight weeks. The main metabolites of phoxim in soil were
alpha-hydroxy-imino-phenylacetonitrile and benzoic acid, both formed
by hydrolysis, but diethyl phosphoric acids were also found in soil.
Laboratory and field leaching experiments in soil showed that
phoxim did not reach a depth below 5 cm and 10 cm, respectively, in
the laboratory and field.
Phoxim or its metabolites were found in roots, stems and leaves
of maize after soil treatment. The cobs, spikes and kernels contained
very little phoxim or metabolites. After foliar treatment, the leaves
contained phoxim and metabolites, but most of these were present on
the surface of the leaves and could be removed with chloroform.
The metabolites formed in or on plants consisted of an isoner:
diethoxy-phosphoryl-thioimino-phenyl-acetonitrile, which was formed
mainly by irradation of the plant, together with minor amounts of
tetraethyl diphosphate and tetraethyl mono-thio-diphosphate. The
"acyl"-compounds (phosphorous-free metabolites) formed by hydrolysis
reacted with plant constituents to three glycosides. The parent
compound degraded rapidly; after seven days only 11 percent of the
total activity was attributable to phoxim.
Investigations on animal metabolism were made in rats and mice.
No information was available on metabolism in cattle, pigs and poultry
for evaluation of residues of phoxim and its metabolites in animal
products.
Experiments were carried out on the stability of phoxim during
storage. Lettuce stored at -20° C for 2.5 years showed no significant
decrease in phoxim content.
Flour milled from grain fortified with phoxim contained 13
percent of the applied phoxim, while middlings and semolina accounted
for 35 percent and 43 percent, respectively, of the applied dose.
Bread made from flour containing phoxim did not contain unchanged
parent compound. Phoxim residues in potatoes were reduced by 91
percent during boiling.
Residues of phoxim in plant and soil samples can be determined by
gas-liquid chromatography with a flame photometric detector or a
thermonic detector. Thin-layer chromatography has also been used for
determining phoxim residues. Extraction can be made from plant
material with acetone, acetonitrile, chloroform or benzene. For
Soxhlet extraction, 10 percent methanol in benzene has been used. Soil
is extracted with methanol. Residues of phoxim in animal tissues are
extracted with hexane and residues in milk with ethylacetate. Cleanup
of extracts has been achieved by partitioning, precipitation with
ammonium chloride/phosphoric acid and/or column chromatography.
RECOMMENDATIONS
The following levels are recommended as MRLs, which need not be
exceeded when phoxim is used according to good agriculture practice.
The limits refer to the parent compound only.
Commodity MRL Preharvest intervals
on which recommendations
are based
Cereal grains 0.05** 1)
Lettuce 0.1 14 days
Beans 0.05** 14 lays
Cauliflower 0.05** 42 days
Potatoes 0.05** 1)
Tomatoes 0.2 14 days
Cotton seed 0.05** 14 days
Interval between treatment
and slaughtering
Cattle, carcase 0.02
meat (in carcase fat) 28 days
Sheep, carcase meat 1 (in carcase 28 days
fat)
1) Soil treatment at sowing or planting.
** Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Residue data in milk from treatment of cattle and residue data in
fat from treatment of pigs.
2. Studies on metabolism of phoxim from treatment of cattle, pigs
and sheep.
3. Information on good agriculture practice in the use of phoxim on
vegetables.
REFERENCES - RESIDUES
Mobay reports are unpublished reports prepared by the R & D Dept.
of Mobay Chemical Corporation, Agricultural Chemicals Division, Kansas
City, Mo. 64120, USA.
Nitokuno reports are unpublished reports prepared by Nihon
Tokushu Noyaku Seizo, K.K., No. 8, 2-chome, Nihonbashi Muro-machi,
Chuo-ku, Tokyo, Japan.
Bayer. Pflanzenschutzmittel-Rückstände. Reports Nos. 165-166/70.
1970 (Unpublished)
Bayer. Pflanzenschutzmittel-Rückstände im Sickerwasser. Reports Nos.
1972 283-288/72; Reports Nos. 3003-3004/74; Reports Nos. 3020-
1974 3022/77. (Unpublished)
1977
Biologische Bundesanstalt für Land- und Forstwirtschaft. Prüfung des
1980 Versickerung-sverhaltens von Pflanzenschutzmitteln.
Merkblatt Nr. 37, 2. Auflage.
Bowman, M.C. & Leuck, D.B. Determination and persistence of phoxim and
1971 its oxygen analog in forage corn and grass. J. Agric. Food
Chem., 19: 1215-1218.
Bykhovets, A.J. Thin-layer chromatographic method to determine Basudin
1974 and Valexon in a plant sample. Aktual. Vopr. Zashch. Rast.
BSSR, Mater, Resp. Konf, Molodykh Uch., 1: 81-82.
Daniel, J.W., Swanson, S. & McLean, J. Phoxim - Pharmacokinetics and
1978a biotransformation in the rat. Life Science Research, U.K.
Report 78/BAG5/194. (Unpublished)
Daniel, J.W., McLean, J. & Pringuer, M. Phoxim - Biotransformation in
1978b the rat. Life Science Research, U.K. Report 78/BAG5/317.
(Unpublished)
Dräger, G. Studies on the metabolism of BAY 77 488. Bayer AG,
1968 Pflanzenschutz-Anwendungstechnik, Report. (Unpublished)
Dräger, G. Gaschromatographische Methode zur Bestimmung von RValexon
1969a Rückständen in Pflanzen. Pflanzenschutz-Nachr. Bayer,
22:311-317 (1969a)
Dräger, G. Rückstandsanalytik von Pflanzenschutzmitteln. DFG-
1969b Methodensammlung, 1. Lieferung 1969; methode Nr. 307.
Dräger, G. Studies on the metabolism of phoxim (BAY 77 488).
1971 Pflanzenschutz-Nachr. Bayer, 24: 239-251.
Dräger, G. Reaction products of chlorphoxim (BAY 78 182) formed by
1972 exposure of the parent compound to irradiation.
Pflanzenschutz-Nachr. Bayer, 25: 32-42.
Dräger, G. Metabolisierung von Phoxim-methyl durch Belichtung. Bayer
1975 AG, Pflanzenschutz-Anwendungstechnik report RA-57.
(Unpublished)
Dräger, G. Studies on the fate of phoxim (BAY 77 488) in soil.
1977 Pflanzenschutz-Nachr. Bayer, 30:28-41.
Dräger, G. Untersuchung des Metabolismus von 14C-ringmarkiertem
Phoxim
1978a in Mais und Boden nach Anwendung enies Granulates. 1. 14C-
Bilanz und Metabolismus im Boden. Bayer AG, Pflanzenschutz-
Anwendungstechnik report RA-278. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim; Belichtung von
1978b 14C-ringmarkiertem Wirkstoff auf Glassplatten. Bayer AG,
Pflanzenschutz-Anwendungstechnik report RA-618.
(Unpublished)
Dräger, G. Stability of phoxim residues during boiling of potatoes.
1978c Bayer AG, Pflanzenschutz-Anwendungstechnik report RA-843.
(Unpublished)
Dräger, G. Untersuchung zum Metabolismus von Phoxim; Metabolismus
1980a des Abbauproduktes alpha-Hydroxyiminophenolacetonitril in
Tomatenpflanzen. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-106. (Unpublished)
Dräger, G. Untersuchung des Metabolismus von Phoxim auf
1980b Baumwollblättern. Bayer AG, Pflanzenschutz-Anwendungstechnik
report RA-208. (Unpublished)
Dräger, G. Stability of phoxim residues in lettuce during low
1982 temperature storage. Bayer AG, Pflanzenschutz-
Anwendungstechnik, report RA-69. (Unpublished)
Ernst, G. F., Brinkman, M.B.C. & Mattern, E.M. Methode van onderzoek
1981 voor het bepalen van residuen van foxim en foxon im melk.
KvW Utrecht, IR/71/80/R79-Okt.
Hopkins, T., Lemprecht, C. & Lindsay, G. Bay 9053 - 50% w/v E.C. -
1980 residues - sheep. Bayer Doc. No. 80/9955.
LaHue, D.W. & Dicke, E.B. Phoxim as an insect protectant for stored
1971 grains. J. Econ. Entomol., 64: 1530-1533.
Makari, M.H., Riskallah, M.R., Hegazy, M.E. & Belal, M.H. Photolysis
1981 of phoxim on glass and on tomato leaves. Bull. Environ.
Contam. Toxicol. 26: 413-419 (1981).
Nitokuno. Residual analysis results of VolatonR (phoxim) in upland
1977 soil. Report No. 1058 (RA). (Unpublished)
Olson, T.J. An interference study for the residue method for Volaton
1974 determination on bananas. Mobay Report No. 40 095.
(Unpublished)
Pao, K.C.H. Residue studies of phoxim (Baythion) on the tea bushes.
1975 Acta Entomol. Sin., 18: 133-139.
Steffens, W. Bilanzversuche mit (benzolring-U-14C) Phoxim nach
1978 Verwendung als Bodeninsektizid zu Mais in einem
Freilandlysimeter und einem geschlossenen System im
Gewächshaus. Kernforschungsnalage Jülich, Institut für
Radioagronomie, report ARA 14/78. (Unpublished)
Thornton, J.S. Determination of residues of BAY 77 488 in stored
1969 grains. Mobay report No. 24 575. (Unpublished)
Thornton, J.S., Hurley, J.B. & Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticides chemicals. Mobay report No. 51 016.
(Unpublished)
Wilmes, R. Orientierende Lichtstabilität (Phoxim). Bayer AG,
1981 Pflanzenschutz-Anwendungstechnik report. (Unpublished)
Vinopal, J.H.A. & Fukuto, T.R. Selective toxicity of phoxim
1971 (phenylglyoxylonitrile oxime 0,0-diethyl phosphorothioate).
Pestic. Biochem. Physiol., 1: 44-60.
