
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
FENSULFOTHION
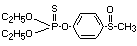 Explanation
Fensulfothion was evaluated for ADI by the Joint FAO/WHO Meeting
in 1972 (FAO/WHO 1973) 1. The ADI was allocated on the basis of the
no-effect level observed in a 2-year study in dogs.
Teratogenicity studies at higher levels and studies on human
exposure were considered desirable. Mutagenicity and teratogenicity
studies were from Industrial Bio-Test Laboratories.
Since the previous evaluation, a new embryotoxicity/
teratogenicity study, mutagenicity studies and other studies have
been presented and are summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on Enzymes
A study was undertaken to evaluate the interaction potential of
the widely used organophosphorous insecticide Dasanit (fensulfothion)
with the local anaesthetic ester, procaine, and the analgesic and
antipyretic amide, acetanilide, in the mouse and rat. Groups of 5 or 6
male mice were treated with 1-10 mg Dasanit/kg and sacrificed without
challenge 1 h later. A dose of 5 mg/kg did not inhibit brain
cholinesterase activity, yet caused greater than 50% inhibition of
liver hydrolsysis of diethylsuccinate, triacetin and procaine. A
time-course study indicated that liver hydrolysis of these substrates
was maximally inhibited at 0.5-1 h after a dose of 7.5 mg/kg. Several
1 See Annex 2 for WHO and FAO documentation.
additional substrates were used to study the interaction of Dasanit
with procaine and acetanilide in the rat. It is suggested that
commonly used insecticidal organophosphates, at apparently non-toxic
doses, may alter the metabolism and toxicity of ester- and amide-
containing drugs, which are dependent upon carboxylesterases for their
biotransformation (Ouellette 1979).
TOXICOLOGICAL STUDIES
Special Studies on Embryotoxicity and Teratogenicity
Groups of rabbits (12 pregnant Himalayan rabbits/group) were
administered fensulfothion by gavage at dosages of 0, 0.1, 0.3 and
1.0 mg/kg bw from day 6 to 18 of gestation. The administered dosages
were set on the basis of results of a tolerance test performed on non-
pregnant rabbits, in which 3 mg/kg/day had a lethal effect.
Himalayan rabbits are indicated to react sensitively to the
embryotoxic effect of thalidomide. On day 29 of gestation, all
pregnant animals were sacrificed and foetuses were delivered by
caesarean section.
At a dosage up to and including 1 mg/kg bw/day, administration of
fensulfothion did not have any adverse effect on the physical
appearance and behaviour patterns of the does or on their weight gain.
Only one rabbit of the 1 mg/kg group died after 11 applications; the
cause of its death could not be established. All other treated rabbits
survived. There was no significant differences between the control
and treated groups with respect to pregnancy quota, number of
implantations, foetuses delivered, resorptions plus abortions, sex
ratio of foetuses, average foetal weight and average placenta weight.
The average number of stunted foetuses (i.e. weighing less than 25
grams) showed a slight increase in the 1 mg/kg group as compared with
the controls. This result was brought about by one single mother
delivering foetuses all with a reduced weight. Hence, this was an
isolated case that was not characteristic of the group and, therefore,
was not treatment-related. No foetuses with retardations of the
skeletal system were seen in any of the groups. The only malformation
observed in this study was indicated to be arthrogryposis of the left
fore extremity. This finding was considered a typical spontaneous
malformation of the rabbit breed. The no-effect dosage of
fensulfothion on embryonic development was 1 mg/kg/day in this study
on rabbits. There were no indications of any teratogenic effect
(Machemer 1978).
Special Study on a Mixture of Fensulfothion and its Metabolites
Chicken
Groups of laying hens (4 Breed Babcock 300, 32-week old
hens/group), were fed fensulfothion and its major metabolites in the
diet at a dosage level of 0, 0.25, 0.75 2.5 and 10.0 ppm (calculated
in fensulfothion equivalents) for 28 days. In the diet, fensulfothion
and its major metabolites (i.e. fensulfothion sulphone, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. A decrease (15-25%) in feed consumption was observed
in birds of the 10.0 ppm group after the first seven days to the end
of the study. Two of the four birds in the 10.0ppm group showed a
decrease in egg production of 20%. Significant blood cholinesterase
depression began with the 2.5 ppm group and was at 79% of normal in
the 0.75 ppm group. No significant residues were found in any of the
tissues or eggs from birds fed 2.5 and 10.0 ppm. The group fed 2.5 and
10.0 ppm showed average weight gains of only 50-60% of those of the
control birds. Owing to the large variability of weight gains by
individual chickens, this fact was not considered relevant. It was
considered significant that none of the individual birds showed any
weight loss over the observation period. No significant residues (less
than 0.01 ppm) of fensulfothion and metabolites were found in tissues
analysed at the end of the study.
The no-effect level may be assumed to be 0.75 ppm, equal to
0.03 mg/kg/day of fensulfothion equivalent (Thornton 1975a).
Dairy cattle
Groups of cows (3 Holstein dairy cows/treated group; 1 in the
control) were fed fensulfothion and its major metabolites in the
diet at dosage levels of 0, 1.8, 3.6 and 7.2 ppm (calculated as
fensulfothion equivalents) for 28 days. In the diet, fensulfothion and
its major metabolites (i.e. fensulfothion sulphon, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. At the end of test period, the animals were
sacrificed and analysed for residues of fensulfothion and metabolites
in tissues and milk. A decrease of blood cholinesterase activity, feed
consumption, milk production and body weight were noted in the animals
fed the highest (10.8 ppm) dosage level. Blood cholinesterase was
depressed 30% at the end of the test for cows fed 3.6 ppm. Animals
fed the lowest (1.8 ppm) dosage level were not significantly affected.
No significant residues (less than 0.01 ppm) of fensulfothion and
metabolites were found in tissues analyses at the end of test period.
The no-effect level is 1.8 ppm, equal to 0.07 mg/kg/day of
fensulfothion equivalents (Thornton 1975b).
Special Studies on Fensulfothion and its Major Metabolites
Maize was sprayed in the field with Dasanit (fensulfothion) at 0,
0.56, 1.12 and 2.24 kg/ha and ensiled one day later. After 76 days of
ensiling, maize treated as above contained residues of 16.5, 27.8 and
50.6 mg/kg. Resulting silages were fed to 4 cows/treatment. Silage
intakes, milk production, body weight gains and blood cholinesterase
activities of cows were severely and rapidly depressed by ingestion of
Dasanit residues. These measures of performance were inversely related
to the amount of treatment and recovery was slow. One cow fed silage
from 2.24 kg/ha treatment (i.e. containing 50.6 ppm) died after
ingesting Dasanit residues amounting to a total of 2.75 mg/kg bw
during the first seven days of experimental feeding. Dasanit and/or
its metabolites were in milk, urine and faeces from cows fed silage
from 0.56, 1.12 and 2.24 kg/ha treatments.
Total residues in milk, urine and faeces were free of residues
within one week after the cows were withdrawn from treated silage
(Johnson et al 1973),
Special Studies on Potentiation
Phenamiphos was administered orally to male Wistar II albino rats
in combination with either fensulfothion or isofenphos or phoxim.
Mixtures of phenamiphos with each of the other compounds were
prepared with percentage ratios proportional to the LD50 of each
(equitoxic amounts of the two compounds in the mixture). Increasing
doses were administered to determine the LD50 of each combination,
which was then compared to the expected LD50 of the respective
combination. There was no evidence of greater than additive acute
effects (Thyssen 1976).
Special Studies on Mutagenicity
Dominant lethal test
In a dominant lethal test, groups of 50 NMRI/W77 male mice
(8-12 weeks old) were given a single oral dose of 0 or 2 mg/kg bw of
fensulfothion (95.1% purity) in 0.5% Cremophor emulsion. The dose was
chosen after a range finding test in which five animals were given
orally 1, 2 or 4 mg/kg bw, the intermediate dose being without
symptoms.
The mice strain was known to be sensitive to known mutagens, such
as cyclophosphamide, MMS and Trenimon.
Beginning on the day of treatment, each male of each group was
mated with one un-treated virgin female for a period of 4 days, after
which the female was removed and replaced by another female. This
procedure continued for 12 consecutive mating periods. The females
were sacrificed around the 14th day from each midperiod and uteri were
removed for examination. Data were collected on the number of corpora
lutea, total implantations, live implants and dead implants.
Salmonella/microsome test
An evaluation of the mutagenic potential of fensulfothion (95.1%
purity) was carried out using the standard Ames assay. Four strains of
Salmonella typhimurium (TA 1535, TA 100, TA 1537 and TA 98), with
and without a metabolic activation system (S-9 mix) derived from
liver of male Sprague-Dawley rats treated intraperitoneally with
Arochlor 1254, were treated at concentrations up to and including
12 500 µg/plate. Positive controls were 2-amino anthracene (2-AA) for
the four strains, cyclophosphamide only for TA 1535 and TA 100 and
Tryplafavin only for TA 1537 and TA 98.
Four plates for each strain, and for each compound and dose were
used as were also for the negative controls. Two plates/group were
used to determine the total number of bacteria. Negative controls were
the respective solution media. Doses up to and including 500 µg/plate
were not toxic to the bacteria. A dose of 2 500 µg had slight toxic
effect to the bacteria and was useful for the test. The highest dose
resulted in precipitation of the compound and could not be used.
Neither dose-related nor significant (more than double of negative
controls) increments of the number of mutants were observed with any
of the strains at the concentrations tested. Positive controls
displayed a clear mutagenic effect. Thus, at concentrations up to
2500 µg/plate, fensulfothion failed to show mutagenic potential in the
Salmonella/microsome test (Herbold 1980).
Short-Term Studies
Rat - Inhalation
Groups of rats (10 male and 10 female SPF albino rats/group,
Wistar II strain) were exposed to fensulfothion in a dynamic flow
inhalation apparatus for 6 hours daily over a 12-week period (total of
60 6-hour exposures).
Average concentrations of fensulfothion in the aerosol
(determined by gas chromatographic analysis) were 0, 0.097, 0.818 and
7.21 mg/m3 air. Intake of the aerosol by the rats was possible only
together with inhaled air and not by another other route; 95% of the
aerosol droplets had a diameter of 1.0 ± 0.5 µm, with the rest being
less than 5.0 µm.
Poisoning symptoms, mortalities, poor health conditions and lower
body weight gains occurred in the rats exposed to 7.21 mg/m3 air.
Lower body weight gains in male rats and significant differences in
absolute and relative organ weights in male and female rats (heart,
spleen, lung) were noted only for male rats exposed to a concentration
of 7.21 mg/m3 air. Significant depression of plasma -, erythrocyte-,
and brain-cholinesterase activity was noted in rats exposed to a
concentration of 7,21 mg/m3 air. The threshold value (80%) of
cholinesterase activity occurred at 0.818 mg/m3 air. Haematological,
clinical-chemical and urinalysis values were all within the normal
range for exposed rats and controls. Macroscopic and histopathological
examinations of tissues showed no noteworthy alterations. The no-
effect concentration with respect to cholinesterase activity was
0.097 mg/m3 air (Kimmerle 1976).
The treated males exhibited no signs of toxicity. There were no
compound-related mortalities in the treated group, one female in
mating period 7 (due to bladder tumour) and one female in the mating
period 9 (.due to autolysis) were not considered for the test.
There were no statistically significant differences between the
control and treated groups with respect to fertility rate and pre-
implantations loss (estimated both directly as difference between
corpora lutea and implantations, and indirectly through the comparison
of the average number of implants per fertilized female in the control
and treated group). The post-implantation loss was estimated on the
basis of live and dead implants. More than the average of one dead
implant per animal in the treated group was observed in mating periods
5 and 10, but a similar finding was also observed in mating periods 10
and 12 for the control group. These values for dead implants were
considered within the normal fluctuation of the strain.
Statistical analysis (comparison of the distribution of
frequency) showed that there were no significant differences between
the control and treated groups with respect to the ratio of dead
implants to total implants or the number of dead implants to living
implants, both within the single mating periods and in the whole test.
In the dominant-lethal test on male mice, fensulfothion failed to
show mutagenic potential at the acute oral dose of 2 mg/kg bw (Herbold
1981).
COMMENTS
Following the proposals of the 1972 JMPR, a new embryotoxicity/
teratogenicity study at increased dose levels has been submitted and
evaluated. The new teratogenicity study confirmed that fensulfothion
is not teratogenic to rabbit at dosages of up to 1.0 mg/kg bw.
The new mutagenicity studies ( Salmonella/microsome test;
dominant lethal test on male mice) failed to show a mutagenic
potential of fensulfothion.
The Meeting considered that a no-effect level in long-term
studies had not been established. However, according to the 1972
Evaluations, a theoretical no-effect level in rats was estimated by
plotting effect (serum, RBC, brain cholinesterase depression) against
dose. A no-effect level has been demonstrated in the dog. The Meeting
agreed to retain the present ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog : 1 ppm in the diet, equivalent to 0.025 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES
Herbold, B. Fensulfothion. Salmonella/Microsomen. Test zur
1980 Untersuchung auf Punkmutagene Wirkung. Report (No. 9481)
from Institut Für Toxicologie, Bayer, F.R.G. submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1981 Fensulfothion. Dominant-lethal Test an der mänlichen Maus
zur Prufüng auf mutagene Wirkung. Report (No. 10263) from
Institut für Toxicologie, Bayer, F.R.G., submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Johnson, J.C. Jr., Bowman, M.C., Lenk, D.B. and Knox, F.E. Persistence
1973 of Dasanit in corn silage and effects of feeding dairy cows
the treated silage. J. Dairy Sci. 56 (6): 775-782.
Kimmerle, G. Subchronic inhalation toxicity study on rats. Report (No.
1976 6101) from Institut für Toxicologie, Bayer, F.R.G. submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Machemer, L. Evaluation for embryotoxic and teratogenic effects on
1978 orally dosed rabbits. Report (No. 7745) from Institut für
Toxicologie, Bayer, F.R.G., submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Oullette, R.E. Dasanit inhibition of tissue esterases and amidases and
1979 interaction with ester and amide drugs. Diss. Abstract.
Int.B. 40:(2):698.
Thornton, J.S. Effect of feeding Dasanit (R) and metabolites to
1975a chickens, Report (No. 45868, ref. 74-2, 75-69) from Chemagro
Agricultural Division of Mobay Chemical Corp., submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1975b The effect of feeding Dasanit (R) and metabolites to dairy
cattle. Report (No. 46415, ref. 72-2, 75-137, 75-10) from
Chemagro Agricultural Division of Mobay Chemical Corp.,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isophenphos or phoxim. Report (No. 3958) from
Institut für Toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Explanation
Fensulfothion was evaluated for ADI by the Joint FAO/WHO Meeting
in 1972 (FAO/WHO 1973) 1. The ADI was allocated on the basis of the
no-effect level observed in a 2-year study in dogs.
Teratogenicity studies at higher levels and studies on human
exposure were considered desirable. Mutagenicity and teratogenicity
studies were from Industrial Bio-Test Laboratories.
Since the previous evaluation, a new embryotoxicity/
teratogenicity study, mutagenicity studies and other studies have
been presented and are summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on Enzymes
A study was undertaken to evaluate the interaction potential of
the widely used organophosphorous insecticide Dasanit (fensulfothion)
with the local anaesthetic ester, procaine, and the analgesic and
antipyretic amide, acetanilide, in the mouse and rat. Groups of 5 or 6
male mice were treated with 1-10 mg Dasanit/kg and sacrificed without
challenge 1 h later. A dose of 5 mg/kg did not inhibit brain
cholinesterase activity, yet caused greater than 50% inhibition of
liver hydrolsysis of diethylsuccinate, triacetin and procaine. A
time-course study indicated that liver hydrolysis of these substrates
was maximally inhibited at 0.5-1 h after a dose of 7.5 mg/kg. Several
1 See Annex 2 for WHO and FAO documentation.
additional substrates were used to study the interaction of Dasanit
with procaine and acetanilide in the rat. It is suggested that
commonly used insecticidal organophosphates, at apparently non-toxic
doses, may alter the metabolism and toxicity of ester- and amide-
containing drugs, which are dependent upon carboxylesterases for their
biotransformation (Ouellette 1979).
TOXICOLOGICAL STUDIES
Special Studies on Embryotoxicity and Teratogenicity
Groups of rabbits (12 pregnant Himalayan rabbits/group) were
administered fensulfothion by gavage at dosages of 0, 0.1, 0.3 and
1.0 mg/kg bw from day 6 to 18 of gestation. The administered dosages
were set on the basis of results of a tolerance test performed on non-
pregnant rabbits, in which 3 mg/kg/day had a lethal effect.
Himalayan rabbits are indicated to react sensitively to the
embryotoxic effect of thalidomide. On day 29 of gestation, all
pregnant animals were sacrificed and foetuses were delivered by
caesarean section.
At a dosage up to and including 1 mg/kg bw/day, administration of
fensulfothion did not have any adverse effect on the physical
appearance and behaviour patterns of the does or on their weight gain.
Only one rabbit of the 1 mg/kg group died after 11 applications; the
cause of its death could not be established. All other treated rabbits
survived. There was no significant differences between the control
and treated groups with respect to pregnancy quota, number of
implantations, foetuses delivered, resorptions plus abortions, sex
ratio of foetuses, average foetal weight and average placenta weight.
The average number of stunted foetuses (i.e. weighing less than 25
grams) showed a slight increase in the 1 mg/kg group as compared with
the controls. This result was brought about by one single mother
delivering foetuses all with a reduced weight. Hence, this was an
isolated case that was not characteristic of the group and, therefore,
was not treatment-related. No foetuses with retardations of the
skeletal system were seen in any of the groups. The only malformation
observed in this study was indicated to be arthrogryposis of the left
fore extremity. This finding was considered a typical spontaneous
malformation of the rabbit breed. The no-effect dosage of
fensulfothion on embryonic development was 1 mg/kg/day in this study
on rabbits. There were no indications of any teratogenic effect
(Machemer 1978).
Special Study on a Mixture of Fensulfothion and its Metabolites
Chicken
Groups of laying hens (4 Breed Babcock 300, 32-week old
hens/group), were fed fensulfothion and its major metabolites in the
diet at a dosage level of 0, 0.25, 0.75 2.5 and 10.0 ppm (calculated
in fensulfothion equivalents) for 28 days. In the diet, fensulfothion
and its major metabolites (i.e. fensulfothion sulphone, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. A decrease (15-25%) in feed consumption was observed
in birds of the 10.0 ppm group after the first seven days to the end
of the study. Two of the four birds in the 10.0ppm group showed a
decrease in egg production of 20%. Significant blood cholinesterase
depression began with the 2.5 ppm group and was at 79% of normal in
the 0.75 ppm group. No significant residues were found in any of the
tissues or eggs from birds fed 2.5 and 10.0 ppm. The group fed 2.5 and
10.0 ppm showed average weight gains of only 50-60% of those of the
control birds. Owing to the large variability of weight gains by
individual chickens, this fact was not considered relevant. It was
considered significant that none of the individual birds showed any
weight loss over the observation period. No significant residues (less
than 0.01 ppm) of fensulfothion and metabolites were found in tissues
analysed at the end of the study.
The no-effect level may be assumed to be 0.75 ppm, equal to
0.03 mg/kg/day of fensulfothion equivalent (Thornton 1975a).
Dairy cattle
Groups of cows (3 Holstein dairy cows/treated group; 1 in the
control) were fed fensulfothion and its major metabolites in the
diet at dosage levels of 0, 1.8, 3.6 and 7.2 ppm (calculated as
fensulfothion equivalents) for 28 days. In the diet, fensulfothion and
its major metabolites (i.e. fensulfothion sulphon, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. At the end of test period, the animals were
sacrificed and analysed for residues of fensulfothion and metabolites
in tissues and milk. A decrease of blood cholinesterase activity, feed
consumption, milk production and body weight were noted in the animals
fed the highest (10.8 ppm) dosage level. Blood cholinesterase was
depressed 30% at the end of the test for cows fed 3.6 ppm. Animals
fed the lowest (1.8 ppm) dosage level were not significantly affected.
No significant residues (less than 0.01 ppm) of fensulfothion and
metabolites were found in tissues analyses at the end of test period.
The no-effect level is 1.8 ppm, equal to 0.07 mg/kg/day of
fensulfothion equivalents (Thornton 1975b).
Special Studies on Fensulfothion and its Major Metabolites
Maize was sprayed in the field with Dasanit (fensulfothion) at 0,
0.56, 1.12 and 2.24 kg/ha and ensiled one day later. After 76 days of
ensiling, maize treated as above contained residues of 16.5, 27.8 and
50.6 mg/kg. Resulting silages were fed to 4 cows/treatment. Silage
intakes, milk production, body weight gains and blood cholinesterase
activities of cows were severely and rapidly depressed by ingestion of
Dasanit residues. These measures of performance were inversely related
to the amount of treatment and recovery was slow. One cow fed silage
from 2.24 kg/ha treatment (i.e. containing 50.6 ppm) died after
ingesting Dasanit residues amounting to a total of 2.75 mg/kg bw
during the first seven days of experimental feeding. Dasanit and/or
its metabolites were in milk, urine and faeces from cows fed silage
from 0.56, 1.12 and 2.24 kg/ha treatments.
Total residues in milk, urine and faeces were free of residues
within one week after the cows were withdrawn from treated silage
(Johnson et al 1973),
Special Studies on Potentiation
Phenamiphos was administered orally to male Wistar II albino rats
in combination with either fensulfothion or isofenphos or phoxim.
Mixtures of phenamiphos with each of the other compounds were
prepared with percentage ratios proportional to the LD50 of each
(equitoxic amounts of the two compounds in the mixture). Increasing
doses were administered to determine the LD50 of each combination,
which was then compared to the expected LD50 of the respective
combination. There was no evidence of greater than additive acute
effects (Thyssen 1976).
Special Studies on Mutagenicity
Dominant lethal test
In a dominant lethal test, groups of 50 NMRI/W77 male mice
(8-12 weeks old) were given a single oral dose of 0 or 2 mg/kg bw of
fensulfothion (95.1% purity) in 0.5% Cremophor emulsion. The dose was
chosen after a range finding test in which five animals were given
orally 1, 2 or 4 mg/kg bw, the intermediate dose being without
symptoms.
The mice strain was known to be sensitive to known mutagens, such
as cyclophosphamide, MMS and Trenimon.
Beginning on the day of treatment, each male of each group was
mated with one un-treated virgin female for a period of 4 days, after
which the female was removed and replaced by another female. This
procedure continued for 12 consecutive mating periods. The females
were sacrificed around the 14th day from each midperiod and uteri were
removed for examination. Data were collected on the number of corpora
lutea, total implantations, live implants and dead implants.
Salmonella/microsome test
An evaluation of the mutagenic potential of fensulfothion (95.1%
purity) was carried out using the standard Ames assay. Four strains of
Salmonella typhimurium (TA 1535, TA 100, TA 1537 and TA 98), with
and without a metabolic activation system (S-9 mix) derived from
liver of male Sprague-Dawley rats treated intraperitoneally with
Arochlor 1254, were treated at concentrations up to and including
12 500 µg/plate. Positive controls were 2-amino anthracene (2-AA) for
the four strains, cyclophosphamide only for TA 1535 and TA 100 and
Tryplafavin only for TA 1537 and TA 98.
Four plates for each strain, and for each compound and dose were
used as were also for the negative controls. Two plates/group were
used to determine the total number of bacteria. Negative controls were
the respective solution media. Doses up to and including 500 µg/plate
were not toxic to the bacteria. A dose of 2 500 µg had slight toxic
effect to the bacteria and was useful for the test. The highest dose
resulted in precipitation of the compound and could not be used.
Neither dose-related nor significant (more than double of negative
controls) increments of the number of mutants were observed with any
of the strains at the concentrations tested. Positive controls
displayed a clear mutagenic effect. Thus, at concentrations up to
2500 µg/plate, fensulfothion failed to show mutagenic potential in the
Salmonella/microsome test (Herbold 1980).
Short-Term Studies
Rat - Inhalation
Groups of rats (10 male and 10 female SPF albino rats/group,
Wistar II strain) were exposed to fensulfothion in a dynamic flow
inhalation apparatus for 6 hours daily over a 12-week period (total of
60 6-hour exposures).
Average concentrations of fensulfothion in the aerosol
(determined by gas chromatographic analysis) were 0, 0.097, 0.818 and
7.21 mg/m3 air. Intake of the aerosol by the rats was possible only
together with inhaled air and not by another other route; 95% of the
aerosol droplets had a diameter of 1.0 ± 0.5 µm, with the rest being
less than 5.0 µm.
Poisoning symptoms, mortalities, poor health conditions and lower
body weight gains occurred in the rats exposed to 7.21 mg/m3 air.
Lower body weight gains in male rats and significant differences in
absolute and relative organ weights in male and female rats (heart,
spleen, lung) were noted only for male rats exposed to a concentration
of 7.21 mg/m3 air. Significant depression of plasma -, erythrocyte-,
and brain-cholinesterase activity was noted in rats exposed to a
concentration of 7,21 mg/m3 air. The threshold value (80%) of
cholinesterase activity occurred at 0.818 mg/m3 air. Haematological,
clinical-chemical and urinalysis values were all within the normal
range for exposed rats and controls. Macroscopic and histopathological
examinations of tissues showed no noteworthy alterations. The no-
effect concentration with respect to cholinesterase activity was
0.097 mg/m3 air (Kimmerle 1976).
The treated males exhibited no signs of toxicity. There were no
compound-related mortalities in the treated group, one female in
mating period 7 (due to bladder tumour) and one female in the mating
period 9 (.due to autolysis) were not considered for the test.
There were no statistically significant differences between the
control and treated groups with respect to fertility rate and pre-
implantations loss (estimated both directly as difference between
corpora lutea and implantations, and indirectly through the comparison
of the average number of implants per fertilized female in the control
and treated group). The post-implantation loss was estimated on the
basis of live and dead implants. More than the average of one dead
implant per animal in the treated group was observed in mating periods
5 and 10, but a similar finding was also observed in mating periods 10
and 12 for the control group. These values for dead implants were
considered within the normal fluctuation of the strain.
Statistical analysis (comparison of the distribution of
frequency) showed that there were no significant differences between
the control and treated groups with respect to the ratio of dead
implants to total implants or the number of dead implants to living
implants, both within the single mating periods and in the whole test.
In the dominant-lethal test on male mice, fensulfothion failed to
show mutagenic potential at the acute oral dose of 2 mg/kg bw (Herbold
1981).
COMMENTS
Following the proposals of the 1972 JMPR, a new embryotoxicity/
teratogenicity study at increased dose levels has been submitted and
evaluated. The new teratogenicity study confirmed that fensulfothion
is not teratogenic to rabbit at dosages of up to 1.0 mg/kg bw.
The new mutagenicity studies ( Salmonella/microsome test;
dominant lethal test on male mice) failed to show a mutagenic
potential of fensulfothion.
The Meeting considered that a no-effect level in long-term
studies had not been established. However, according to the 1972
Evaluations, a theoretical no-effect level in rats was estimated by
plotting effect (serum, RBC, brain cholinesterase depression) against
dose. A no-effect level has been demonstrated in the dog. The Meeting
agreed to retain the present ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog : 1 ppm in the diet, equivalent to 0.025 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES
Herbold, B. Fensulfothion. Salmonella/Microsomen. Test zur
1980 Untersuchung auf Punkmutagene Wirkung. Report (No. 9481)
from Institut Für Toxicologie, Bayer, F.R.G. submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1981 Fensulfothion. Dominant-lethal Test an der mänlichen Maus
zur Prufüng auf mutagene Wirkung. Report (No. 10263) from
Institut für Toxicologie, Bayer, F.R.G., submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Johnson, J.C. Jr., Bowman, M.C., Lenk, D.B. and Knox, F.E. Persistence
1973 of Dasanit in corn silage and effects of feeding dairy cows
the treated silage. J. Dairy Sci. 56 (6): 775-782.
Kimmerle, G. Subchronic inhalation toxicity study on rats. Report (No.
1976 6101) from Institut für Toxicologie, Bayer, F.R.G. submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Machemer, L. Evaluation for embryotoxic and teratogenic effects on
1978 orally dosed rabbits. Report (No. 7745) from Institut für
Toxicologie, Bayer, F.R.G., submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Oullette, R.E. Dasanit inhibition of tissue esterases and amidases and
1979 interaction with ester and amide drugs. Diss. Abstract.
Int.B. 40:(2):698.
Thornton, J.S. Effect of feeding Dasanit (R) and metabolites to
1975a chickens, Report (No. 45868, ref. 74-2, 75-69) from Chemagro
Agricultural Division of Mobay Chemical Corp., submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1975b The effect of feeding Dasanit (R) and metabolites to dairy
cattle. Report (No. 46415, ref. 72-2, 75-137, 75-10) from
Chemagro Agricultural Division of Mobay Chemical Corp.,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isophenphos or phoxim. Report (No. 3958) from
Institut für Toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
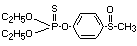 Explanation
Fensulfothion was evaluated for ADI by the Joint FAO/WHO Meeting
in 1972 (FAO/WHO 1973) 1. The ADI was allocated on the basis of the
no-effect level observed in a 2-year study in dogs.
Teratogenicity studies at higher levels and studies on human
exposure were considered desirable. Mutagenicity and teratogenicity
studies were from Industrial Bio-Test Laboratories.
Since the previous evaluation, a new embryotoxicity/
teratogenicity study, mutagenicity studies and other studies have
been presented and are summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on Enzymes
A study was undertaken to evaluate the interaction potential of
the widely used organophosphorous insecticide Dasanit (fensulfothion)
with the local anaesthetic ester, procaine, and the analgesic and
antipyretic amide, acetanilide, in the mouse and rat. Groups of 5 or 6
male mice were treated with 1-10 mg Dasanit/kg and sacrificed without
challenge 1 h later. A dose of 5 mg/kg did not inhibit brain
cholinesterase activity, yet caused greater than 50% inhibition of
liver hydrolsysis of diethylsuccinate, triacetin and procaine. A
time-course study indicated that liver hydrolysis of these substrates
was maximally inhibited at 0.5-1 h after a dose of 7.5 mg/kg. Several
1 See Annex 2 for WHO and FAO documentation.
additional substrates were used to study the interaction of Dasanit
with procaine and acetanilide in the rat. It is suggested that
commonly used insecticidal organophosphates, at apparently non-toxic
doses, may alter the metabolism and toxicity of ester- and amide-
containing drugs, which are dependent upon carboxylesterases for their
biotransformation (Ouellette 1979).
TOXICOLOGICAL STUDIES
Special Studies on Embryotoxicity and Teratogenicity
Groups of rabbits (12 pregnant Himalayan rabbits/group) were
administered fensulfothion by gavage at dosages of 0, 0.1, 0.3 and
1.0 mg/kg bw from day 6 to 18 of gestation. The administered dosages
were set on the basis of results of a tolerance test performed on non-
pregnant rabbits, in which 3 mg/kg/day had a lethal effect.
Himalayan rabbits are indicated to react sensitively to the
embryotoxic effect of thalidomide. On day 29 of gestation, all
pregnant animals were sacrificed and foetuses were delivered by
caesarean section.
At a dosage up to and including 1 mg/kg bw/day, administration of
fensulfothion did not have any adverse effect on the physical
appearance and behaviour patterns of the does or on their weight gain.
Only one rabbit of the 1 mg/kg group died after 11 applications; the
cause of its death could not be established. All other treated rabbits
survived. There was no significant differences between the control
and treated groups with respect to pregnancy quota, number of
implantations, foetuses delivered, resorptions plus abortions, sex
ratio of foetuses, average foetal weight and average placenta weight.
The average number of stunted foetuses (i.e. weighing less than 25
grams) showed a slight increase in the 1 mg/kg group as compared with
the controls. This result was brought about by one single mother
delivering foetuses all with a reduced weight. Hence, this was an
isolated case that was not characteristic of the group and, therefore,
was not treatment-related. No foetuses with retardations of the
skeletal system were seen in any of the groups. The only malformation
observed in this study was indicated to be arthrogryposis of the left
fore extremity. This finding was considered a typical spontaneous
malformation of the rabbit breed. The no-effect dosage of
fensulfothion on embryonic development was 1 mg/kg/day in this study
on rabbits. There were no indications of any teratogenic effect
(Machemer 1978).
Special Study on a Mixture of Fensulfothion and its Metabolites
Chicken
Groups of laying hens (4 Breed Babcock 300, 32-week old
hens/group), were fed fensulfothion and its major metabolites in the
diet at a dosage level of 0, 0.25, 0.75 2.5 and 10.0 ppm (calculated
in fensulfothion equivalents) for 28 days. In the diet, fensulfothion
and its major metabolites (i.e. fensulfothion sulphone, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. A decrease (15-25%) in feed consumption was observed
in birds of the 10.0 ppm group after the first seven days to the end
of the study. Two of the four birds in the 10.0ppm group showed a
decrease in egg production of 20%. Significant blood cholinesterase
depression began with the 2.5 ppm group and was at 79% of normal in
the 0.75 ppm group. No significant residues were found in any of the
tissues or eggs from birds fed 2.5 and 10.0 ppm. The group fed 2.5 and
10.0 ppm showed average weight gains of only 50-60% of those of the
control birds. Owing to the large variability of weight gains by
individual chickens, this fact was not considered relevant. It was
considered significant that none of the individual birds showed any
weight loss over the observation period. No significant residues (less
than 0.01 ppm) of fensulfothion and metabolites were found in tissues
analysed at the end of the study.
The no-effect level may be assumed to be 0.75 ppm, equal to
0.03 mg/kg/day of fensulfothion equivalent (Thornton 1975a).
Dairy cattle
Groups of cows (3 Holstein dairy cows/treated group; 1 in the
control) were fed fensulfothion and its major metabolites in the
diet at dosage levels of 0, 1.8, 3.6 and 7.2 ppm (calculated as
fensulfothion equivalents) for 28 days. In the diet, fensulfothion and
its major metabolites (i.e. fensulfothion sulphon, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. At the end of test period, the animals were
sacrificed and analysed for residues of fensulfothion and metabolites
in tissues and milk. A decrease of blood cholinesterase activity, feed
consumption, milk production and body weight were noted in the animals
fed the highest (10.8 ppm) dosage level. Blood cholinesterase was
depressed 30% at the end of the test for cows fed 3.6 ppm. Animals
fed the lowest (1.8 ppm) dosage level were not significantly affected.
No significant residues (less than 0.01 ppm) of fensulfothion and
metabolites were found in tissues analyses at the end of test period.
The no-effect level is 1.8 ppm, equal to 0.07 mg/kg/day of
fensulfothion equivalents (Thornton 1975b).
Special Studies on Fensulfothion and its Major Metabolites
Maize was sprayed in the field with Dasanit (fensulfothion) at 0,
0.56, 1.12 and 2.24 kg/ha and ensiled one day later. After 76 days of
ensiling, maize treated as above contained residues of 16.5, 27.8 and
50.6 mg/kg. Resulting silages were fed to 4 cows/treatment. Silage
intakes, milk production, body weight gains and blood cholinesterase
activities of cows were severely and rapidly depressed by ingestion of
Dasanit residues. These measures of performance were inversely related
to the amount of treatment and recovery was slow. One cow fed silage
from 2.24 kg/ha treatment (i.e. containing 50.6 ppm) died after
ingesting Dasanit residues amounting to a total of 2.75 mg/kg bw
during the first seven days of experimental feeding. Dasanit and/or
its metabolites were in milk, urine and faeces from cows fed silage
from 0.56, 1.12 and 2.24 kg/ha treatments.
Total residues in milk, urine and faeces were free of residues
within one week after the cows were withdrawn from treated silage
(Johnson et al 1973),
Special Studies on Potentiation
Phenamiphos was administered orally to male Wistar II albino rats
in combination with either fensulfothion or isofenphos or phoxim.
Mixtures of phenamiphos with each of the other compounds were
prepared with percentage ratios proportional to the LD50 of each
(equitoxic amounts of the two compounds in the mixture). Increasing
doses were administered to determine the LD50 of each combination,
which was then compared to the expected LD50 of the respective
combination. There was no evidence of greater than additive acute
effects (Thyssen 1976).
Special Studies on Mutagenicity
Dominant lethal test
In a dominant lethal test, groups of 50 NMRI/W77 male mice
(8-12 weeks old) were given a single oral dose of 0 or 2 mg/kg bw of
fensulfothion (95.1% purity) in 0.5% Cremophor emulsion. The dose was
chosen after a range finding test in which five animals were given
orally 1, 2 or 4 mg/kg bw, the intermediate dose being without
symptoms.
The mice strain was known to be sensitive to known mutagens, such
as cyclophosphamide, MMS and Trenimon.
Beginning on the day of treatment, each male of each group was
mated with one un-treated virgin female for a period of 4 days, after
which the female was removed and replaced by another female. This
procedure continued for 12 consecutive mating periods. The females
were sacrificed around the 14th day from each midperiod and uteri were
removed for examination. Data were collected on the number of corpora
lutea, total implantations, live implants and dead implants.
Salmonella/microsome test
An evaluation of the mutagenic potential of fensulfothion (95.1%
purity) was carried out using the standard Ames assay. Four strains of
Salmonella typhimurium (TA 1535, TA 100, TA 1537 and TA 98), with
and without a metabolic activation system (S-9 mix) derived from
liver of male Sprague-Dawley rats treated intraperitoneally with
Arochlor 1254, were treated at concentrations up to and including
12 500 µg/plate. Positive controls were 2-amino anthracene (2-AA) for
the four strains, cyclophosphamide only for TA 1535 and TA 100 and
Tryplafavin only for TA 1537 and TA 98.
Four plates for each strain, and for each compound and dose were
used as were also for the negative controls. Two plates/group were
used to determine the total number of bacteria. Negative controls were
the respective solution media. Doses up to and including 500 µg/plate
were not toxic to the bacteria. A dose of 2 500 µg had slight toxic
effect to the bacteria and was useful for the test. The highest dose
resulted in precipitation of the compound and could not be used.
Neither dose-related nor significant (more than double of negative
controls) increments of the number of mutants were observed with any
of the strains at the concentrations tested. Positive controls
displayed a clear mutagenic effect. Thus, at concentrations up to
2500 µg/plate, fensulfothion failed to show mutagenic potential in the
Salmonella/microsome test (Herbold 1980).
Short-Term Studies
Rat - Inhalation
Groups of rats (10 male and 10 female SPF albino rats/group,
Wistar II strain) were exposed to fensulfothion in a dynamic flow
inhalation apparatus for 6 hours daily over a 12-week period (total of
60 6-hour exposures).
Average concentrations of fensulfothion in the aerosol
(determined by gas chromatographic analysis) were 0, 0.097, 0.818 and
7.21 mg/m3 air. Intake of the aerosol by the rats was possible only
together with inhaled air and not by another other route; 95% of the
aerosol droplets had a diameter of 1.0 ± 0.5 µm, with the rest being
less than 5.0 µm.
Poisoning symptoms, mortalities, poor health conditions and lower
body weight gains occurred in the rats exposed to 7.21 mg/m3 air.
Lower body weight gains in male rats and significant differences in
absolute and relative organ weights in male and female rats (heart,
spleen, lung) were noted only for male rats exposed to a concentration
of 7.21 mg/m3 air. Significant depression of plasma -, erythrocyte-,
and brain-cholinesterase activity was noted in rats exposed to a
concentration of 7,21 mg/m3 air. The threshold value (80%) of
cholinesterase activity occurred at 0.818 mg/m3 air. Haematological,
clinical-chemical and urinalysis values were all within the normal
range for exposed rats and controls. Macroscopic and histopathological
examinations of tissues showed no noteworthy alterations. The no-
effect concentration with respect to cholinesterase activity was
0.097 mg/m3 air (Kimmerle 1976).
The treated males exhibited no signs of toxicity. There were no
compound-related mortalities in the treated group, one female in
mating period 7 (due to bladder tumour) and one female in the mating
period 9 (.due to autolysis) were not considered for the test.
There were no statistically significant differences between the
control and treated groups with respect to fertility rate and pre-
implantations loss (estimated both directly as difference between
corpora lutea and implantations, and indirectly through the comparison
of the average number of implants per fertilized female in the control
and treated group). The post-implantation loss was estimated on the
basis of live and dead implants. More than the average of one dead
implant per animal in the treated group was observed in mating periods
5 and 10, but a similar finding was also observed in mating periods 10
and 12 for the control group. These values for dead implants were
considered within the normal fluctuation of the strain.
Statistical analysis (comparison of the distribution of
frequency) showed that there were no significant differences between
the control and treated groups with respect to the ratio of dead
implants to total implants or the number of dead implants to living
implants, both within the single mating periods and in the whole test.
In the dominant-lethal test on male mice, fensulfothion failed to
show mutagenic potential at the acute oral dose of 2 mg/kg bw (Herbold
1981).
COMMENTS
Following the proposals of the 1972 JMPR, a new embryotoxicity/
teratogenicity study at increased dose levels has been submitted and
evaluated. The new teratogenicity study confirmed that fensulfothion
is not teratogenic to rabbit at dosages of up to 1.0 mg/kg bw.
The new mutagenicity studies ( Salmonella/microsome test;
dominant lethal test on male mice) failed to show a mutagenic
potential of fensulfothion.
The Meeting considered that a no-effect level in long-term
studies had not been established. However, according to the 1972
Evaluations, a theoretical no-effect level in rats was estimated by
plotting effect (serum, RBC, brain cholinesterase depression) against
dose. A no-effect level has been demonstrated in the dog. The Meeting
agreed to retain the present ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog : 1 ppm in the diet, equivalent to 0.025 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES
Herbold, B. Fensulfothion. Salmonella/Microsomen. Test zur
1980 Untersuchung auf Punkmutagene Wirkung. Report (No. 9481)
from Institut Für Toxicologie, Bayer, F.R.G. submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1981 Fensulfothion. Dominant-lethal Test an der mänlichen Maus
zur Prufüng auf mutagene Wirkung. Report (No. 10263) from
Institut für Toxicologie, Bayer, F.R.G., submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Johnson, J.C. Jr., Bowman, M.C., Lenk, D.B. and Knox, F.E. Persistence
1973 of Dasanit in corn silage and effects of feeding dairy cows
the treated silage. J. Dairy Sci. 56 (6): 775-782.
Kimmerle, G. Subchronic inhalation toxicity study on rats. Report (No.
1976 6101) from Institut für Toxicologie, Bayer, F.R.G. submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Machemer, L. Evaluation for embryotoxic and teratogenic effects on
1978 orally dosed rabbits. Report (No. 7745) from Institut für
Toxicologie, Bayer, F.R.G., submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Oullette, R.E. Dasanit inhibition of tissue esterases and amidases and
1979 interaction with ester and amide drugs. Diss. Abstract.
Int.B. 40:(2):698.
Thornton, J.S. Effect of feeding Dasanit (R) and metabolites to
1975a chickens, Report (No. 45868, ref. 74-2, 75-69) from Chemagro
Agricultural Division of Mobay Chemical Corp., submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1975b The effect of feeding Dasanit (R) and metabolites to dairy
cattle. Report (No. 46415, ref. 72-2, 75-137, 75-10) from
Chemagro Agricultural Division of Mobay Chemical Corp.,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isophenphos or phoxim. Report (No. 3958) from
Institut für Toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
Explanation
Fensulfothion was evaluated for ADI by the Joint FAO/WHO Meeting
in 1972 (FAO/WHO 1973) 1. The ADI was allocated on the basis of the
no-effect level observed in a 2-year study in dogs.
Teratogenicity studies at higher levels and studies on human
exposure were considered desirable. Mutagenicity and teratogenicity
studies were from Industrial Bio-Test Laboratories.
Since the previous evaluation, a new embryotoxicity/
teratogenicity study, mutagenicity studies and other studies have
been presented and are summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Effects on Enzymes
A study was undertaken to evaluate the interaction potential of
the widely used organophosphorous insecticide Dasanit (fensulfothion)
with the local anaesthetic ester, procaine, and the analgesic and
antipyretic amide, acetanilide, in the mouse and rat. Groups of 5 or 6
male mice were treated with 1-10 mg Dasanit/kg and sacrificed without
challenge 1 h later. A dose of 5 mg/kg did not inhibit brain
cholinesterase activity, yet caused greater than 50% inhibition of
liver hydrolsysis of diethylsuccinate, triacetin and procaine. A
time-course study indicated that liver hydrolysis of these substrates
was maximally inhibited at 0.5-1 h after a dose of 7.5 mg/kg. Several
1 See Annex 2 for WHO and FAO documentation.
additional substrates were used to study the interaction of Dasanit
with procaine and acetanilide in the rat. It is suggested that
commonly used insecticidal organophosphates, at apparently non-toxic
doses, may alter the metabolism and toxicity of ester- and amide-
containing drugs, which are dependent upon carboxylesterases for their
biotransformation (Ouellette 1979).
TOXICOLOGICAL STUDIES
Special Studies on Embryotoxicity and Teratogenicity
Groups of rabbits (12 pregnant Himalayan rabbits/group) were
administered fensulfothion by gavage at dosages of 0, 0.1, 0.3 and
1.0 mg/kg bw from day 6 to 18 of gestation. The administered dosages
were set on the basis of results of a tolerance test performed on non-
pregnant rabbits, in which 3 mg/kg/day had a lethal effect.
Himalayan rabbits are indicated to react sensitively to the
embryotoxic effect of thalidomide. On day 29 of gestation, all
pregnant animals were sacrificed and foetuses were delivered by
caesarean section.
At a dosage up to and including 1 mg/kg bw/day, administration of
fensulfothion did not have any adverse effect on the physical
appearance and behaviour patterns of the does or on their weight gain.
Only one rabbit of the 1 mg/kg group died after 11 applications; the
cause of its death could not be established. All other treated rabbits
survived. There was no significant differences between the control
and treated groups with respect to pregnancy quota, number of
implantations, foetuses delivered, resorptions plus abortions, sex
ratio of foetuses, average foetal weight and average placenta weight.
The average number of stunted foetuses (i.e. weighing less than 25
grams) showed a slight increase in the 1 mg/kg group as compared with
the controls. This result was brought about by one single mother
delivering foetuses all with a reduced weight. Hence, this was an
isolated case that was not characteristic of the group and, therefore,
was not treatment-related. No foetuses with retardations of the
skeletal system were seen in any of the groups. The only malformation
observed in this study was indicated to be arthrogryposis of the left
fore extremity. This finding was considered a typical spontaneous
malformation of the rabbit breed. The no-effect dosage of
fensulfothion on embryonic development was 1 mg/kg/day in this study
on rabbits. There were no indications of any teratogenic effect
(Machemer 1978).
Special Study on a Mixture of Fensulfothion and its Metabolites
Chicken
Groups of laying hens (4 Breed Babcock 300, 32-week old
hens/group), were fed fensulfothion and its major metabolites in the
diet at a dosage level of 0, 0.25, 0.75 2.5 and 10.0 ppm (calculated
in fensulfothion equivalents) for 28 days. In the diet, fensulfothion
and its major metabolites (i.e. fensulfothion sulphone, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. A decrease (15-25%) in feed consumption was observed
in birds of the 10.0 ppm group after the first seven days to the end
of the study. Two of the four birds in the 10.0ppm group showed a
decrease in egg production of 20%. Significant blood cholinesterase
depression began with the 2.5 ppm group and was at 79% of normal in
the 0.75 ppm group. No significant residues were found in any of the
tissues or eggs from birds fed 2.5 and 10.0 ppm. The group fed 2.5 and
10.0 ppm showed average weight gains of only 50-60% of those of the
control birds. Owing to the large variability of weight gains by
individual chickens, this fact was not considered relevant. It was
considered significant that none of the individual birds showed any
weight loss over the observation period. No significant residues (less
than 0.01 ppm) of fensulfothion and metabolites were found in tissues
analysed at the end of the study.
The no-effect level may be assumed to be 0.75 ppm, equal to
0.03 mg/kg/day of fensulfothion equivalent (Thornton 1975a).
Dairy cattle
Groups of cows (3 Holstein dairy cows/treated group; 1 in the
control) were fed fensulfothion and its major metabolites in the
diet at dosage levels of 0, 1.8, 3.6 and 7.2 ppm (calculated as
fensulfothion equivalents) for 28 days. In the diet, fensulfothion and
its major metabolites (i.e. fensulfothion sulphon, fensulfothion
oxygen analogue, fensulfothion oxygen analogue sulphone) were in the
amount and ratio (1:1:2:2), representative of that found in field-
weathered crops. At the end of test period, the animals were
sacrificed and analysed for residues of fensulfothion and metabolites
in tissues and milk. A decrease of blood cholinesterase activity, feed
consumption, milk production and body weight were noted in the animals
fed the highest (10.8 ppm) dosage level. Blood cholinesterase was
depressed 30% at the end of the test for cows fed 3.6 ppm. Animals
fed the lowest (1.8 ppm) dosage level were not significantly affected.
No significant residues (less than 0.01 ppm) of fensulfothion and
metabolites were found in tissues analyses at the end of test period.
The no-effect level is 1.8 ppm, equal to 0.07 mg/kg/day of
fensulfothion equivalents (Thornton 1975b).
Special Studies on Fensulfothion and its Major Metabolites
Maize was sprayed in the field with Dasanit (fensulfothion) at 0,
0.56, 1.12 and 2.24 kg/ha and ensiled one day later. After 76 days of
ensiling, maize treated as above contained residues of 16.5, 27.8 and
50.6 mg/kg. Resulting silages were fed to 4 cows/treatment. Silage
intakes, milk production, body weight gains and blood cholinesterase
activities of cows were severely and rapidly depressed by ingestion of
Dasanit residues. These measures of performance were inversely related
to the amount of treatment and recovery was slow. One cow fed silage
from 2.24 kg/ha treatment (i.e. containing 50.6 ppm) died after
ingesting Dasanit residues amounting to a total of 2.75 mg/kg bw
during the first seven days of experimental feeding. Dasanit and/or
its metabolites were in milk, urine and faeces from cows fed silage
from 0.56, 1.12 and 2.24 kg/ha treatments.
Total residues in milk, urine and faeces were free of residues
within one week after the cows were withdrawn from treated silage
(Johnson et al 1973),
Special Studies on Potentiation
Phenamiphos was administered orally to male Wistar II albino rats
in combination with either fensulfothion or isofenphos or phoxim.
Mixtures of phenamiphos with each of the other compounds were
prepared with percentage ratios proportional to the LD50 of each
(equitoxic amounts of the two compounds in the mixture). Increasing
doses were administered to determine the LD50 of each combination,
which was then compared to the expected LD50 of the respective
combination. There was no evidence of greater than additive acute
effects (Thyssen 1976).
Special Studies on Mutagenicity
Dominant lethal test
In a dominant lethal test, groups of 50 NMRI/W77 male mice
(8-12 weeks old) were given a single oral dose of 0 or 2 mg/kg bw of
fensulfothion (95.1% purity) in 0.5% Cremophor emulsion. The dose was
chosen after a range finding test in which five animals were given
orally 1, 2 or 4 mg/kg bw, the intermediate dose being without
symptoms.
The mice strain was known to be sensitive to known mutagens, such
as cyclophosphamide, MMS and Trenimon.
Beginning on the day of treatment, each male of each group was
mated with one un-treated virgin female for a period of 4 days, after
which the female was removed and replaced by another female. This
procedure continued for 12 consecutive mating periods. The females
were sacrificed around the 14th day from each midperiod and uteri were
removed for examination. Data were collected on the number of corpora
lutea, total implantations, live implants and dead implants.
Salmonella/microsome test
An evaluation of the mutagenic potential of fensulfothion (95.1%
purity) was carried out using the standard Ames assay. Four strains of
Salmonella typhimurium (TA 1535, TA 100, TA 1537 and TA 98), with
and without a metabolic activation system (S-9 mix) derived from
liver of male Sprague-Dawley rats treated intraperitoneally with
Arochlor 1254, were treated at concentrations up to and including
12 500 µg/plate. Positive controls were 2-amino anthracene (2-AA) for
the four strains, cyclophosphamide only for TA 1535 and TA 100 and
Tryplafavin only for TA 1537 and TA 98.
Four plates for each strain, and for each compound and dose were
used as were also for the negative controls. Two plates/group were
used to determine the total number of bacteria. Negative controls were
the respective solution media. Doses up to and including 500 µg/plate
were not toxic to the bacteria. A dose of 2 500 µg had slight toxic
effect to the bacteria and was useful for the test. The highest dose
resulted in precipitation of the compound and could not be used.
Neither dose-related nor significant (more than double of negative
controls) increments of the number of mutants were observed with any
of the strains at the concentrations tested. Positive controls
displayed a clear mutagenic effect. Thus, at concentrations up to
2500 µg/plate, fensulfothion failed to show mutagenic potential in the
Salmonella/microsome test (Herbold 1980).
Short-Term Studies
Rat - Inhalation
Groups of rats (10 male and 10 female SPF albino rats/group,
Wistar II strain) were exposed to fensulfothion in a dynamic flow
inhalation apparatus for 6 hours daily over a 12-week period (total of
60 6-hour exposures).
Average concentrations of fensulfothion in the aerosol
(determined by gas chromatographic analysis) were 0, 0.097, 0.818 and
7.21 mg/m3 air. Intake of the aerosol by the rats was possible only
together with inhaled air and not by another other route; 95% of the
aerosol droplets had a diameter of 1.0 ± 0.5 µm, with the rest being
less than 5.0 µm.
Poisoning symptoms, mortalities, poor health conditions and lower
body weight gains occurred in the rats exposed to 7.21 mg/m3 air.
Lower body weight gains in male rats and significant differences in
absolute and relative organ weights in male and female rats (heart,
spleen, lung) were noted only for male rats exposed to a concentration
of 7.21 mg/m3 air. Significant depression of plasma -, erythrocyte-,
and brain-cholinesterase activity was noted in rats exposed to a
concentration of 7,21 mg/m3 air. The threshold value (80%) of
cholinesterase activity occurred at 0.818 mg/m3 air. Haematological,
clinical-chemical and urinalysis values were all within the normal
range for exposed rats and controls. Macroscopic and histopathological
examinations of tissues showed no noteworthy alterations. The no-
effect concentration with respect to cholinesterase activity was
0.097 mg/m3 air (Kimmerle 1976).
The treated males exhibited no signs of toxicity. There were no
compound-related mortalities in the treated group, one female in
mating period 7 (due to bladder tumour) and one female in the mating
period 9 (.due to autolysis) were not considered for the test.
There were no statistically significant differences between the
control and treated groups with respect to fertility rate and pre-
implantations loss (estimated both directly as difference between
corpora lutea and implantations, and indirectly through the comparison
of the average number of implants per fertilized female in the control
and treated group). The post-implantation loss was estimated on the
basis of live and dead implants. More than the average of one dead
implant per animal in the treated group was observed in mating periods
5 and 10, but a similar finding was also observed in mating periods 10
and 12 for the control group. These values for dead implants were
considered within the normal fluctuation of the strain.
Statistical analysis (comparison of the distribution of
frequency) showed that there were no significant differences between
the control and treated groups with respect to the ratio of dead
implants to total implants or the number of dead implants to living
implants, both within the single mating periods and in the whole test.
In the dominant-lethal test on male mice, fensulfothion failed to
show mutagenic potential at the acute oral dose of 2 mg/kg bw (Herbold
1981).
COMMENTS
Following the proposals of the 1972 JMPR, a new embryotoxicity/
teratogenicity study at increased dose levels has been submitted and
evaluated. The new teratogenicity study confirmed that fensulfothion
is not teratogenic to rabbit at dosages of up to 1.0 mg/kg bw.
The new mutagenicity studies ( Salmonella/microsome test;
dominant lethal test on male mice) failed to show a mutagenic
potential of fensulfothion.
The Meeting considered that a no-effect level in long-term
studies had not been established. However, according to the 1972
Evaluations, a theoretical no-effect level in rats was estimated by
plotting effect (serum, RBC, brain cholinesterase depression) against
dose. A no-effect level has been demonstrated in the dog. The Meeting
agreed to retain the present ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Dog : 1 ppm in the diet, equivalent to 0.025 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Observations in humans.
REFERENCES
Herbold, B. Fensulfothion. Salmonella/Microsomen. Test zur
1980 Untersuchung auf Punkmutagene Wirkung. Report (No. 9481)
from Institut Für Toxicologie, Bayer, F.R.G. submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1981 Fensulfothion. Dominant-lethal Test an der mänlichen Maus
zur Prufüng auf mutagene Wirkung. Report (No. 10263) from
Institut für Toxicologie, Bayer, F.R.G., submitted to the
World Health Organization by Bayer, F.R.G. (Unpublished)
Johnson, J.C. Jr., Bowman, M.C., Lenk, D.B. and Knox, F.E. Persistence
1973 of Dasanit in corn silage and effects of feeding dairy cows
the treated silage. J. Dairy Sci. 56 (6): 775-782.
Kimmerle, G. Subchronic inhalation toxicity study on rats. Report (No.
1976 6101) from Institut für Toxicologie, Bayer, F.R.G. submitted
to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Machemer, L. Evaluation for embryotoxic and teratogenic effects on
1978 orally dosed rabbits. Report (No. 7745) from Institut für
Toxicologie, Bayer, F.R.G., submitted to the World Health
Organization by Bayer, F.R.G. (Unpublished)
Oullette, R.E. Dasanit inhibition of tissue esterases and amidases and
1979 interaction with ester and amide drugs. Diss. Abstract.
Int.B. 40:(2):698.
Thornton, J.S. Effect of feeding Dasanit (R) and metabolites to
1975a chickens, Report (No. 45868, ref. 74-2, 75-69) from Chemagro
Agricultural Division of Mobay Chemical Corp., submitted to
the World Health Organization by Bayer, F.R.G. (Unpublished)
1975b The effect of feeding Dasanit (R) and metabolites to dairy
cattle. Report (No. 46415, ref. 72-2, 75-137, 75-10) from
Chemagro Agricultural Division of Mobay Chemical Corp.,
submitted to the World Health Organization by Bayer, F.R.G.
(Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isophenphos or phoxim. Report (No. 3958) from
Institut für Toxicologie, Bayer, submitted to the World
Health Organization by Bayer, F.R.G. (Unpublished)
