
Pesticide residues in food - 2003 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
A. Bartholomaeus
Office of Chemical Safety
Therapeutic Goods Administration, Canberra, Australia
|
Comparison with other members of the strobilurin class of fungicides |
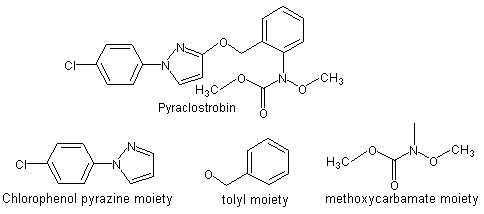
Pyraclostrobin is the provisionally approved ISO name for methyl N-{2-[1-(4-chlorophenyl)-1H-pyrazol-3-yloxymethyl]phenyl}(N-methoxy)carbamate (Figure 1). Pyraclostrobin is a member of the strobilurin group of fungicides. The strobilurin fungicides act through inhibition of mitochondrial respiration by blocking electron transfer within the respiratory chain, which in turn causes important cellular biochemical processes to be severely disrupted, and results in cessation of fungal growth. Pyraclostrobin has not been evaluated previously by the JMPR.

Figure 1. Pyraclostrobin and its principle subcomponents
The specifications for the active ingredient, pyraclostrobin, permit a maximum content of 0.0003% (3 mg/kg of feed) of the impurity dimethyl sulfate. Dimethyl sulfate is both mutagenic and carcinogenic. For a substantial proportion of the toxicological studies considered in this monograph, there is uncertainty about the presence and level of this impurity in the pyraclostrobin used, although the studies of mutagenicity were performed with material known to contain dimethyl sulfate at 1 mg/kg of feed. This uncertainty will need to be taken into account when performing risk assessments for pyraclostrobin. The available body of analytical information however, suggests that the concentration of DMS in nearly all batches was less than 0.0001%.
Unless otherwise stated, the studies evaluated in this monograph were certified as having been performed in compliance with good laboratory practice and in accordance with the relevant OECD test guidelines. As these guidelines specify the tissues normally examined and the clinical pathology tests normally performed, only the exceptions to these guidelines are reported here, to avoid repetitive listing of study parameters. For ease of reference, the standard test parameters for studies of repeated doses are provided in Tables 1, 2 and 3.
Table 1. Standard clinical chemistry parameters for studies of repeated doses
|
Haematology |
Clinical chemistry |
Urine analysis |
|
Differential blood count |
Alanine aminotransferase |
Bilirubin |
|
Erythrocyte count |
Albumin |
Blood |
|
Erythrocyte volume fraction |
Alkaline phosphatase |
Colour |
|
Haemoglobin |
Aspartate aminotransferase |
Glucose |
|
Leukocyte count |
Bilirubin |
Ketones |
|
Mean corpuscular haemoglobin (MCH) |
Brain cholinesterase |
Nitrite |
|
Calcium |
pH |
|
|
Mean corpuscular haemoglobin |
Chloride |
Protein |
|
concentration (MCHC) |
Cholesterol |
Sediment |
|
Mean corpuscular volume (MCV) |
Creatinine |
Specific gravity |
|
Platelet count |
Erythrocyte cholinesterase (ECHE) |
Turbidity |
|
Prothrombin time |
Globulin |
Urobilinogen |
|
Glucose |
Volume |
|
|
Inorganic phosphate |
||
|
Magnesium |
||
|
Potassium |
||
|
Protein (total) |
||
|
Serum cholinesterase (SCHE) |
||
|
Serum-gamma-glutamyltransferase |
||
|
Sodium |
||
|
Triglycerides |
||
|
Urea |
Table 2. Organs weighed at sacrifice in studies of repeated doses
|
Organs weighed at sacrifice |
|
Adrenal gland(s) |
|
Brain |
|
Epididymis (ides) |
|
Heart |
|
Kidney(s) |
|
Liver |
|
Ovary (ies) |
|
Spleen |
|
Testes |
|
Thymus gland |
Table 3. Tissues examined microscopically in studies of repeated doses
|
Tissues examined microscopically |
|||
|
Adrenal gland(s) |
Heart |
Pancreas |
Spleen |
|
Aorta |
Ileum |
Parathyroid gland(s) |
Sternum (and marrow) |
|
Bone marrow (femur) |
Jejunum |
Pituitary gland |
Stomach |
|
Brain |
Kidney(s) |
Prostate gland |
Testis(es) |
|
Caecum |
Liver |
Rectum |
Thymus gland |
|
Colon |
Lung(s) |
Salivary gland(s) |
Thyroid gland(s) |
|
Duodenum |
Lymph nodes |
Sciatic nerve(s) |
Trachea |
|
Epididymis(ides) |
Mammary gland |
Seminal vesicles |
Urinary bladder |
|
Eye(s) |
(females) |
Skeletal muscle |
Uterus |
|
Femur (with knee |
Oesophagus |
Skin |
Vagina |
|
joint/glenoid surface) |
Ovary(ies) |
Spinal cord |
|
|
Oviducts |
|||
The absorption, distribution, and elimination of pyraclostrobin were studied in male and female Wistar rats (aged at least 7 weeks) after oral administration of pyraclostrobin (purity, >98%) radiolabelled with 14C at either the tolyl or chlorophenyl rings.
In a preliminary test, two male and two female rats were assessed for clinical signs for at least 24 h after dosing with unlabelled pyraclostrobin at 50 mg/kg bw; the dose was well tolerated.
In a series of four experiments, the excretion of pyraclostrobin was studied in excreta collected at 6, 12 and 24 h after dosing, and at 24 h intervals thereafter for 168 h, or until 90% of the applied radioactivity had been excreted. In the first three experiments, groups of four male and four female rats were given a single oral dose of [14C]tolyl- or [14C]chlorophenyl-labelled pyraclostrobin or unlabelled pyraclostrobin at 50 mg/kg bw. In the fourth experiment, four rats of each sex were given a single oral dose of [14C]tolyl-labelled pyraclostrobin at 5 mg/kg bw. At the end of each of these experiments, the animals were sacrificed and the heart, liver, spleen, bone, skin, lung, ovaries, bone marrow, carcass, muscle, kidney, testes, brain, pancreas, uterus, adipose tissue, stomach and contents, thyroid glands, adrenal glands, blood/plasma and intestinal tract and contents were assessed for radioactivity. Exhaled air was also collected from two males in each of the two experiments using radiolabelled pyraclostrobin in order to determine exhalation of 14C-labelled gases.
Two additional experiments were conducted to examine blood concentrations of radioactivity after administration of [14C]tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw. Blood samples (100–200 µl) were taken from animals at 0.5, 1, 2, 4, 8, 24, 48, 72, 96 and 120 h after dosing, and the amount of radioactivity in whole blood and plasma was assessed. Tissue distribution was examined in animals sacrificed at 0.5, 8, 20 and 42 h after dosing at 5 mg/kg bw, and at 0.5, 24, 36 and 72 h after dosing at 50 mg/kg bw. The heart, liver, spleen, bone, skin, lung, ovaries, bone marrow, carcass, muscle, kidney, testes, brain, pancreas, uterus, adipose tissue, stomach and contents, thyroid glands, adrenal glands, blood/plasma and intestinal tract and contents were assessed for radioactivity. To examine biliary excretion of pyraclostrobin, bile ducts of the animals were cannulated and bile was collected at 3 h intervals until 48 h after administration of [14C]tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw in four animals of each sex at each dose (the duration depended on the health of the animals and the excretion rate at later time-points).
In rats given a single dose of [14C]tolyl-labelled pyraclostrobin at either 5 or 50 mg/kg bw, plasma concentrations of radioactivity initially peaked after 0.5–1 h; there was a secondary peak after 8 h in males at 5 or 50 mg/kg bw and females given 5 mg/kg bw, and after 24 h in females given 50 mg/kg bw. The magnitude of the difference in the time to peak for females, given the high dose, is likely to be at least partially artifactual owing to the absence of a sampling point between 8 and 24 h. After the second peak, plasma concentrations declined to <0.1 µg equivalent/g after 120 h. The terminal half-lives were similar in males and females, but were 50% longer at 5 mg/kg bw than at 50 mg/kg bw. The area under the curve of plasma concentration–time was approximately proportional to dose for each sex, indicating that absorption was not saturated at the higher dose. Key kinetic data are shown in Table 4.
Table 4. The kinetics of pyraclostrobin in rats
|
Parameter |
Dose (mg/kg bw) |
|||
|
5 |
50 |
|||
|
Males |
Females |
Males |
Females |
|
|
First peak blood concentration (µg equivalent/g plasma) |
0.432 |
0.537 |
1.96 |
2.62 |
|
Time to peak (h) |
1.0 |
0.5 |
0.5 |
0.5 |
|
Second peak blood concentration (µg equivalent/g plasma) |
0.458 |
0.353 |
2.04 |
1.77 |
|
Time to peak (h) |
8.0 |
8.0 |
8.0 |
24.0 |
|
Cmax (µg/g) |
0.458 |
0.537 |
2.04 |
2.62 |
|
Initial t1/2 (h) |
9.0 |
10.5 |
— |
— |
|
Terminal t1/2 (h) |
37.4 |
31.6 |
20.7 |
19.7 |
|
AUC (µg Eq*h/g) |
9.46 |
8.74 |
93.97 |
66.41 |
|
Clearance (g/min) |
8.81 |
9.54 |
8.87 |
12.4 |
From Leibold et al. (1998)
AUC, Area under curve
After a single oral dose of [14C]tolyl-labelled pyraclostrobin at 50 mg/kg bw, the highest concentrations of radioactivity were found in the gastrointestinal tract (gut, 28– 39 µg equivalent/g; gut contents, 63–92 µg equivalent/g; stomach, 325–613 µg equivalent/g; stomach contents, 1273–1696 µg equivalent/g) after 0.5 h. The liver (13–25 µg equivalent/g) had higher concentrations of radioactivity than the kidneys (4–7 µg equivalent/g) and plasma (2–6 µg equivalent/g), with lowest values being recorded in the bone (0.1–0.3 µg equivalent/g) and brain (1–2 µg equivalent/g). After 72 h, tissues and organs contained <2.6 µg equivalent/g. After a dose of 5 mg/kg bw, the highest concentrations of radioactivity were also found in the gastrointestinal tract (gut, 5 µg equivalent/g; gut contents, 7–9 µ equivalent/g; stomach, 49–89 µg equivalent/g; stomach contents, 160–205 µg equivalent/g) after 0.5 h. After 42 h, tissues and organs contained <0.7 µg equivalent/g. In rats that were pretreated with unlabelled pyraclostrobin for 14 days and given a single oral dose of [14C]tolyl-labelled pyraclostrobin at 5 mg/kg bw, the highest concentrations of radioactivity after 120 h were found in the thyroid gland (0.18–0.35 µg equivalent/g) and the liver (0.1 µg equivalent/g). In all other tissues, the concentration of radioactivity recorded was <0.1 µg equivalent/g. The rapid and essentially complete excretion of pyraclostrobin and the decline of tissue concentrations to low levels over the observation period, suggests a low potential for accumulation.
The overall recovery of radioactivity was 91–105% in all experiments. In the first 48 h after a single oral dose of [14C]tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw, 10–13% of the administered radioactivity was excreted in the urine and 74–91% was excreted in the faeces. The total amount of radioactivity excreted in the urine and faeces after 120 h was 11–15% and 81–92%, respectively. A similar pattern of excretion was observed in rats that were pre-treated with unlabelled pyraclostrobin for 14 days and given a single oral dose of [14C]tolyl-labelled pyraclostrobin at 5 mg/kg bw of (12–13% in the urine and 76–77% in the faeces after 48 h; 12–14% in the urine and 79–81% in the faeces after 120 h) and in rats given a single oral dose of chlorophenyl-labelled pyraclostrobin at 50 mg/kg bw (11–15% in the urine and 68–85% in the faeces after 48 h; 12–16% in the urine and 74–89% in the faeces after 120 h). There was no detectable radioactivity in the expired air from rats treated with [14C]tolyl- or [14C]chlorophenyl-labelled pyraclostrobin at 50 mg/kg bw. In tissues and organs, the radioactivity that remained after 120 h was <1 mg equivalent/g at 50 mg/kg bw and <0.1 mg equivalent/g at 5 mg/kg bw. Within 48 h after administration of [14C]tolyl-labelled pyraclostrobin at 5 or 50 mg/kg bw of, 35–38% of the administered radioactivity was excreted via the bile, indicating, in conjunction with observations on urinary excretion, that approximately 50% of the administered dose had been absorbed (Leibold et al., 1998).
The absorption and, to a limited extent, the distribution and excretion of 14C-labelled pyraclostrobin (in Solvesso) in groups of 16 male Wistar rats was assessed after a single dermal application at a nominal dose of 0.015, 0.075 or 0.375 mg/cm2, corresponding to 0.15, 0.75 and 3.75 mg/animal or approximately 0.8, 4 and 18 mg/kg bw. Animals were exposed to the test material for 4 (four rats per group) or 8 (12 rats per group) h and four rats per group were sacrificed at 4, 8, 24 or 72 h after the start of the exposure. An area of approximately 10 cm2 on the shoulders was clipped free of hair and was washed with acetone 24 h before dosing. A silicone ring was glued to the skin and the test substance preparation (10 µl/cm2) was administered with a syringe, which was weighed before and after application. A nylon mesh was then glued to the surface of the silicone ring and covered with a porous bandage. After the exposure period, the protective covers were removed and the exposed skin was washed with a soap solution. After sacrifice, the concentration of radioactivity in the excreta, blood cells, plasma, liver, kidneys, carcass, treated and untreated skin was assessed. Radioactivity in the cage and skin wash and the protective covering, including the silicone ring, was also assessed. In all groups, 99–110% of the radioactivity was recovered. At sacrifice at 72 h, after an 8 h exposure, 1.6–2.6% of the administered dose was absorbed, 22–26% was on the skin or in the skin wash, and 72–80% was recovered on the protective cover. Only 0.2–0.4% and 0.9–1.8% was excreted in the urine and faeces, respectively (Leibold & Hoffmann, 1999).
Table 5. Dermal absorption of pyraclostrobin in rats (mean recovery of radioactivity (%))
|
Recovery |
Exposure (h) |
Sacrifice (h) |
Dose (mg/cm2) |
||
|
0.375 |
0.075 |
0.015 |
|||
|
Absorbed* |
4 |
4 |
0.51 |
0.43 |
0.55 |
|
8 |
8 |
0.51 |
0.85 |
0.64 |
|
|
8 |
24 |
1.19 |
2.56 |
1.49 |
|
|
8 |
72 |
1.58 |
2.59 |
1.57 |
|
|
Skin (application site) |
4 |
4 |
7.11 |
7.56 |
7.78 |
|
8 |
8 |
7.85 |
10.85 |
10.60 |
|
|
8 |
24 |
9.25 |
12.61 |
6.40 |
|
|
8 |
72 |
3.37 |
13.68 |
12.10 |
|
|
Urine/faeces |
4 |
4 |
0.01/0.01 |
0.01/0.01 |
0.01/0.00 |
|
8 |
8 |
0.04/0.01 |
0.05/0.02 |
0.03/0.01 |
|
|
8 |
24 |
0.16/0.42 |
0.22/0.56 |
0.17/0.49 |
|
|
8 |
72 |
0.22/0.91 |
0.38/1.76 |
0.27/1.04 |
|
|
Total recovery |
4 |
4 |
107 |
102 |
103 |
|
8 |
8 |
99 |
105 |
109 |
|
|
8 |
24 |
100 |
110 |
105 |
|
|
8 |
72 |
105 |
104 |
100 |
|
From Leibold & Hoffmann (1999)
* Radioactivity recovered in excreta, cage wash, blood, kidney, liver and the carcass
In a second study of dermal application, 14C-labelled pyraclostrobin (in a commercial formulation, details of which were not provided) was applied at a dose of 15, 75 and 375 µg/active ingredient/cm2 to the upper surface of epidermal membranes from Wistar rats and human cadavers in vitro, and left unoccluded for 24 h. Skin samples were obtained from the dorsal/dorso-lumbar region from sacrificed rats and human cadavers and were mounted in glass diffusion cells to give a surface area of approximately 1.77 cm2. The receptor chamber contained an ethanol/water (1 : 1) mix as the receptor fluid. Ten skin preparations per species and dose were assessed. Only tissues in which the epidermal layer was intact were used in the study. On the day before application of the formulation, the integrity of the skin was assessed by measuring the penetration of tritiated water, which was applied to the epidermal surface. The test material was applied to the upper surface of the epidermal membranes and duplicate aliquots (100 ml) of receptor fluid were taken at 1, 2, 4, 6, 10 and 24 h subsequently. Residual test material was washed from the skin surface with a 10% w/v soap solution, and the washings, remaining receptor fluid, ethanol washings of the dismantled diffusion cells and solubilized skin membranes were retained for analysis of residual radioactivity. During the 24 h after application, 21–51% and 3–8% of the applied [14C]pyr-aclostrobin was absorbed across the rat and human epidermis respectively, with 13–22% (rat) and 14–17% (human) of the dose recovered on the skin. The majority of absorption by rat skin occurred in the first 6 h, whereas total absorption by human skin increased throughout the entire 24 h period. In total, 91–95% (rat) and 88–106% (human) of the applied dose was recovered (Thomley & Wood, 1999).
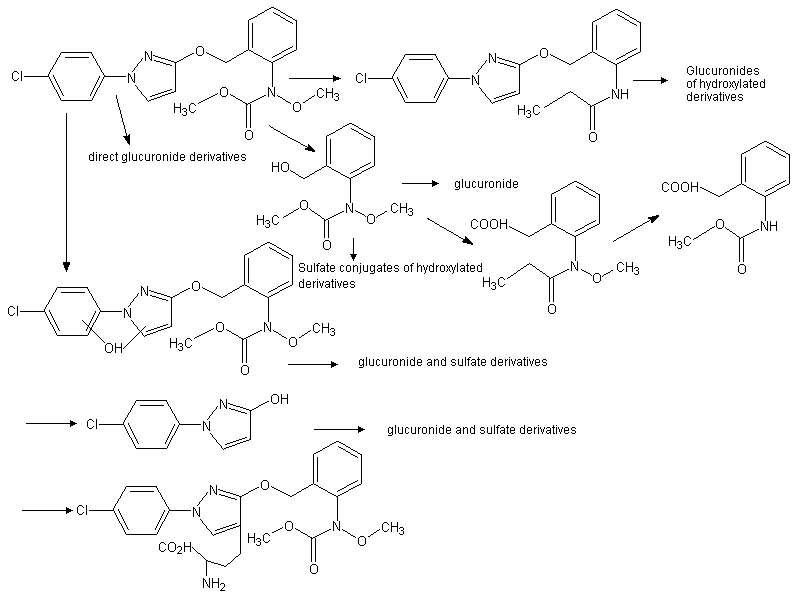
Figure 2. Proposed metabolic pathways for pyraclostrobin in rats
From Velic (1999)
Tissues, excreta and bile from animals used in the toxicokinetics studies and from additional groups given a single dose at 50 mg/kg bw per day (to provide more material for analysis) were analysed for metabolites of pyraclostrobin. In order to determine the metabolites in the plasma, liver and kidneys, additional groups were treated with a single dose of 14C]tolyl- or [14C]chlorophenol ring-labelled pyraclostrobin at 5 and 50 mg/kg bw and sacrificed 8 h later. Metabolites were identified using high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC–MS) and nuclear magnetic resonance (NMR). The metabolism of pyraclostrobin proceeded through three main pathways primarily involving alterations to the three major portions of the pyraclostrobin molecule.
The methoxy group on the tolyl-methoxycarbamate moiety was readily lost, with few major metabolites retaining this group. Hydroxylation of the aromatic and/or pyrazole rings was followed by glucuronide and occasionally sulfate conjugation, and many metabolites were derived from the chlorophenol-pyrazole or tolyl-methoxycarbamate moieties of pyraclostrobin, following cleavage of the ether linkage, with subsequent ring hydroxylation and glucuronide or sulfate conjugation. Metabolites were similar in both sexes and at all doses. No unchanged parent compound was found in the bile or urine and only small amounts in the faeces. Compounds dominating the identified metabolites recovered from the urine were: ring-hydroxylated pyraclostrobin; the chlorophenol pyrazole moiety hydroxylated on the pyrazole ring with or without a sulfate conjugate; a glucuronide of the tolyl-methoxycarbamate moiety; and a benzoic acid derivative of the tolyl-methoxycarbamate moiety. In the faeces, the dominant metabolite was a demethoxylated and pyrazole ring hydroxylated pyraclostrobin. In the bile, the primary metabolite was a glucuronide of pyraclostrobin hydroxylated on the pyrazole ring at the 4’ position and this compound, together with the demethoxylated derivative found in the faeces, was also the dominant metabolite isolated from the plasma and the liver. Demethoxylation of the methoxycarbamate moiety appeared to occur primarily in the gut, as the major metabolite in the bile retains this group intact whereas in the faeces the major metabolite is the demethoxylated derivative. Most of the radiolabel isolated from the kidneys was in the form of the unchanged parent compound and a demethoxylated derivative (Velic, 1999).
The acute toxicity of pyraclostrobin is summarized in Table 6.
Table 6. Studies of acute toxicity with pyraclostrobin
|
Species |
Strain |
Sex |
Route and vehicle |
Dose (mg/kg bw) |
Purity (%) |
LD50 (mg/kg bw) or LC50 (mg/l air) |
Reference |
|
Rat |
Wistar |
Male & female |
Orala |
2 000, 5 000 mg/kg bw, in 0.5% aqueous Tylose CB 30 000 |
>98.2 |
>5000 mg/kg bw |
Wiemann & Hellwig (1998a) |
|
Rat |
Wistar |
Male & female (five of each sex) |
Dermalb |
2 000 mg/kg bw in 0.5% aqueous Tylose CB 30 000 |
>98.2 |
>2000 mg/kg bw |
Wiemann & Hellwig (1998b) |
|
Rat |
Wistar |
Male & female (five of each sex) |
Inhalationc |
0, 0.89, 1.96, 4.07, 7.3 mg/l, 40% in Solvesso (head and nose only), 4 h |
>98.2 |
>4.07 mg/l, |
Gamer et al. (2001) |
|
Rat |
Wistar |
Male & female (five of each sex) |
Inhalationc |
0, 0.310, 1.070, 5.270 mg/l, in acetone 1 : 2 (head and nose only), 4 h |
>98.2% |
>0.310 mg/l, |
Gamer & Hoffmann (1997) |
a Dose volume = 10 and 20 ml/kg bw
b Intact skin, 24 h exposure, 50 cm2 area
c As pyraclostrobin is a viscous fluid with a "negligibly low" vapour pressure (2.6 × 10-10 hPa), it was dissolved in acetone (4-h LC50, approximately 80 mg/l) or Solvesso to facilitate aerosolization. Mean mass aerodynamic diameter was 1.0, 1.2 and 2.9 µm for the 0.310, 1.070 and 5.270 mg/l groups, respectively, for the study using acetone as the solvent, and between 2.7 and 4.3 µm for the study using Solvesso as the solvent
Clinical signs after oral administration of pyraclostrobin consisted of dyspnoea, staggering, piloerection, and diarrhoea in all animals, resolving by day 6. There were no pathology findings. In a study of acute inhalation using acetone as the solvent, all animals at 1.070 and 5.300 mg/l died on the day of exposure. At 0.310 mg/l, bloody discharge from the nose (two males), piloerection and smeared fur (10 out of 10 animals) were observed. All effects had resolved in surviving animals by day 7. Where Solvesso was used as the solvent, all males and four out of five females at 7.3 mg/l died, and one out of 10 animals died at each of the two lower doses. There were no deaths at 0.89 mg/l.
Undiluted pyraclostrobin (500 mg, purity 98.2%) was applied to the shaved, intact skin on the back/flanks of six New Zealand White rabbits under a semi occlusive bandage for 4 h. At the end of the exposure period, the test substance was removed and the treated area was rinsed with polyethylene glycol and water. There were no mortalities. Erythema was observed in all animals from 1 h after removal of the bandage and persisting in most animals until day 8, and in three animals until day 15. The maximum Draize score for erythema was 3 and the average scores at day 1 and 8 were 2 and 1.5 respectively. Oedema with a Draize score of 1 was observed in four out of six rabbits on day 1, resolving in all except two rabbits by day 8, but persisting in one rabbit until day 15. It was concluded that pyraclostrobin is a slight but prolonged skin irritant (Wiemann & Hellwig, 1998c).
Pyraclostrobin (0.1 ml; purity, 98.2%) was instilled into the conjunctival sac of the right eye of one male and five female New Zealand white rabbits. After 24 h, the test material was washed out with tap water. The left eye was not treated and served as a control. There were no deaths during the study. Conjunctival redness (score = 1–3) was observed in all animals up to 3 days after treatment, with swelling observed in five out of six rabbits at 1 h (score = 1), six out of six rabbits on day 1 (average score = 1.2), three out of six rabbits on day 2 (score = 1), and two out of six rabbits on day 3 (score = 1). Discharge (score = 1) occurred in one out of six rabbits at 1 h. There were no corneal or iridal effects and all conjunctival effects had resolved by day 8. "Loss of hair at the margins of the eyelids" occurred in six out of six rabbits from 1 day after treatment. Under the conditions of the study, pyraclostrobin was a slight ocular irritant in rabbits (Wiemann & Hellwig, 1998d).
In a Magnusson-Kligman maximization test, intradermal injections (2 × 0.1 ml) of Freund adjuvant in a 0.9% aqueous solution of sodium chloride (1 : 1), 5% pyraclostrobin in Freund adjuvant and 5% pyraclostrobin in 1% Tylose CB 30 000 in Aqua bidest (Tylose) were given to the left and right shoulders of each of 20 guinea-pigs. Sites were evaluated 24 h after injections were given. One week later, 5% pyraclostrobin in Tylose (1 ml) was applied to a gauze patch of surface area 2 × 4 cm and administered topically to the same sites, then covered with an occlusive dressing for 48 h, after which time the sites were assessed. On day 22, all animals were challenged with 0.5 ml of 1% pyraclostrobin in Tylose (right flank) and Tylose alone (left flank). A second challenge was performed on day 29, when the test substance was applied to the left flank and the vehicle applied to the right flank. All challenge sites were evaluated 24 and 48 h after removal of the occlusive dressings. There were no deaths and all animals gained body weight normally over the study. Although intradermal injections of Freund adjuvant, 5% pyraclostrobin in Freund adjuvant and 5% pyraclostrobin in Tylose caused moderate and confluent erythema (Draize score = 2) and swelling in all animals, as did an occluded topical application of 5% pyraclostrobin in Tylose, the first and second challenges with 1% pyraclostrobin in Tylose and Tylose alone caused no effect in any animal at 24 or 48 h. The sensitivity of the procedure was confirmed in an assay with the positive controls technical-grade alpha-hexyl cinnamaldehyde technical (85%) and Lutrol E 400 DAB (Lutrol). Pyraclostrobin was not a skin sensitizer in guinea-pigs in this study (Wiemann & Hellwig, 1998e).
Mice
Groups of five male and five female B6C3F1 mice were given pyraclostrobin (in 0.5% aqueous carboxymethylcellulose) at a dose of 0 or 4 mg/kg bw per day for 1 week by gavage. Mice were also given diets containing pyraclostrobin at a concentration of 0 or 18 mg/kg of feed for males and 0 or 15 mg/kg of feed for females, for 1 week. Food consumption and body weight were determined daily and animals were examined for mortality and clinical signs of toxicity at least once per day. At the end of the experiment, animals were sacrificed without further examination. Mean intakes of pyraclostrobin were 5.5 mg/kg bw per day in males at 18 mg/kg, and 7.2 mg/kg bw per day in females at 15 mg/kg. After 1 week, food consumption was 31% lower than that of controls in females at 15 mg/kg, but there was no treatment-related effect on body-weight gain and there were no other effects of treatment in any of the treated groups of mice. Data for individual animals were not supplied. This was a supplemental study initiated to address the appropriateness of using an apparently lower body-weight gain in treated animals in a study of developmental toxicity in rabbits as an end-point on which to base an the establishment of an acute reference dose (RfD). In conjunction with similar studies in rabbits and rats, this study was intended to demonstrate the species-specific variability in food intake and body-weight gains in rabbits. Because of the limited parameters examined in this study, it was not adequate for the purposes of risk assessment and a no-observed-adverse-effect level (NOAEL) could not be established (Mellert, 2002a).
Groups of 10 male and 10 female B6C3F1 mice (aged 47–49 days) were given diets containing pyraclostrobin (purity, 98.5%) at a concentration of 0, 50, 150, 500, 1000, or 1500 mg/kg of feed (equal to 0, 9.2, 30, 120, 270 and 480 mg/kg bw per day) for 3 months. Mice were checked at least once daily for mortality and signs of toxicity, and a comprehensive clinical examination was performed once per week. Body weight and food consumption were recorded once weekly and water consumption was assessed daily. Blood was collected from fasted animals and haematology (excluding prothrombin time) and clinical chemistry (excluding brain, erythrocyte and serum cholinesterases) parameters were assessed in all animals. After 3 months of treatment, all mice were fasted, sacrificed and necropsied. All animals were examined grossly. In the control group and in the group receiving pyraclostrobin at 1500 mg/kg, tissues (including gall bladder) were examined microscopically. The thymus gland, lungs, liver, kidneys, adrenal glands (females), stomach, duodenum, jejunem, ileum and mesenteric lymph nodes were examined microscopically in animals at 50, 150, 500 and 1000 mg/kg, and gross lesions were assessed in all animals affected per group. Organ weights (excluding epididymides, heart and thymus gland) were recorded.
There were no treatment-related clinical signs. Body-weight gain was reduced throughout the study period in males at all doses and in females at >500 mg/kg of feed in a clear dose-related manner, with males at the highest dose experiencing a slight loss in body weight. Reduced weight gains in females at 50 and 150 mg/kg were slight, not statistically significant except at 150 mg/kg on day 77, and were not apparent before day 28 of treatment. Nonetheless a dose-related trend was apparent in females at all doses from day 28 onwards. Although spillage of food hindered interpretation, consistently lower food conversion efficiency values at 1000 and 1500 mg/kg suggested a relationship to treatment. No changes in water consumption between the groups were noted. Reductions were seen at 1500 mg/kg in haemoglobin concentration, MCV and MCH in both sexes, and in erythrocyte volume fraction in males. Platelet counts were increased in both sexes at 1500 mg/kg and in males at 500 and 1000 mg/kg. Haemoglobin concentration was also reduced in females at 1000 mg/kg and erythrocyte volume fraction was reduced in males at 150 mg/kg and above. Leukopenia was seen in both sexes at 1000 and 1500 mg/kg and in females at 500 mg/kg and possibly also 150 mg/kg. There was a reduction in the concentration of eosinophils in males at 50 mg/kg and above, in lymphocytes in both sexes at 1000 and 1500 mg/kg and females at 500 mg/kg, and in monocytes in males at >500 mg/kg. As individual control animals had eosinophil counts ranging from 0 to 0.43 × 109/l the apparent, slight, effect at 50 mg/kg was not considered to be toxicologically significant. Increases were seen in serum cholesterol in females at 1500 mg/kg and urea concentration in both sexes at >150 mg/kg. The values for urea concentration in males at the lowest dose were within the range for historical controls, but in view of the clear increase at >150 mg/kg, a substance-related effect at 50 mg/kg could not be ruled out. Decreases were observed in total protein in both sexes at 1500 mg/kg and in females at 1000 mg/kg, in globulin concentration in both sexes at 1000 and 1500 mg/kg, and in triglyceride concentration in both sexes at >150 mg/kg and in females at 50 mg/kg. Although the reduced concentration of triglyceride in females was not statistically significant a clear dose–response relationship was apparent and examination of values for individual animal confirmed a consistent pattern of reduced values.
A number of organ weight differences between groups were observed which, in the absence of histological alterations in those organs, are likely to be secondary to reduced weight gains and food conversion efficiency in groups receiving pyraclostrobin at >150 mg/kg of feed. Increased relative liver and spleen weights in males at >500 mg/kg could not be readily attributed to altered weight gains, as the relative (to body weight) liver weight tends to remain stable or decline when weight gain is reduced through reduced food intake or reduced food conversion efficiency, and the increased relative spleen weight correlated with the anaemia and leukopenia observed at 1000 and 1500 mg/kg.
Increased incidences and/or severity was seen in the following findings: thickening of the duodenal mucosa in both sexes at >500 mg/kg; erosions or ulcers in the glandular stomach in both sexes at >500 mg/kg and in females at 150 mg/kg; atrophy of the thymus gland in both sexes at >500 mg/kg and in females at 150 mg/kg; apoptotic bodies in follicles of the mesenteric lymph node in both sexes at 1500 mg/kg and in females at 500 and 1000 mg/kg. Decreases were seen in the incidences of vacuolation in cells of the X-zone in the adrenal cortex in females at >150 mg/kg and in males at 1500 mg/kg, lipid vacuoles in the kidneys of males at >500 mg/kg, and fatty infiltration in the liver of both sexes at 1500 mg/kg. A NOAEL was not identified owing to decreased body-weight gains and altered clinical pathology parameters at all doses. (Mellert et al., 1998; Mellert et al., 1999j). Taking into consideration the study of carcinogenicity in mice (doses: 0, 10, 30, 120 mg/kg) the NOAEL for reduced weight gain at 91 days in mice was 30 mg/kg (4 mg/kg bw per day). As the study of carcinogenicity did not examine clinical chemistry parameters, a specific NOAEL for elevated blood urea concentrations could not be identified for this species. Nonetheless, consideration of the dose–response trend in the 3-month study, the observation that the value for this parameter at 50 mg/kg was within the range for historical controls and the absence of abnormal histology and gross pathology in males at 120 mg/kg (17 mg/kg bw per day) and in females at 180 mg/kg (33 mg/kg bw per day) in the study of carcinogenicity in mice suggests that the overall NOAEL in mice of 30 mg/kg (4 mg/kg bw per day) was appropriate.
Table 7. Haematology and clinical chemistry values in mice given diets containing pyraclostrobin for 3 months
|
Parameter |
Dietary concentration (mg/kg of feed) |
|||||||||||
|
0 (control) |
50 |
150 |
500 |
1000 |
1500 |
|||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Haematology |
||||||||||||
|
Haemoglobin (mmol/l) |
11.8 |
11.4 |
11.6 |
11.5 |
11.6 |
11.2 |
11.4 |
11.0 |
11.4 |
10.9** |
10.6*** |
10.4** |
|
Erythrocyte volume fraction (l/l) |
0.57 |
0.52 |
0.56 |
0.53 |
0.55* |
0.52 |
0.54** |
0.52 |
0.54** |
0.51 |
0.52** |
0.50 |
|
MCV (10-15l) |
48.3 |
46.4 |
48.0 |
46.7 |
47.3 |
46.3 |
47.0* |
46.8 |
46.7*** |
46.0 |
42.6*** |
42.6 |
|
MCH (10-15 mol/l) |
0.99 |
1.02 |
0.99 |
1.01 |
1.00 |
1.00 |
0.99 |
1.00* |
0.98* |
0.98** |
0.87*** |
0.90*** |
|
MCHC (mmol/l) |
20.6 |
22.0 |
20.7 |
21.6 |
21.1* |
21.7 |
21.1 |
21.3*** |
21.0 |
21.2*** |
20.5 |
21.0*** |
|
Platelets (×109/l) |
1120 |
1048 |
1168 |
1078 |
1199 |
1014 |
1271** |
1086 |
1205# |
1112 |
1236** |
1236** |
|
White blood cells (×109/l) |
5.9 |
6.0 |
5.6 |
5.2 |
5.2 |
4.1 |
6.4 |
3.7 |
2.7*** |
3.3 |
2.7*** |
3.2 |
|
Eosinophils (×109/l) |
0.14 |
0.04 |
0.08 |
0.05 |
0.06 |
0.02 |
0.02 |
0.00 |
0.00 |
0.00 |
0.00 |
0.01 |
|
Lymphocytes (×109/l) |
4.0 |
3.7 |
4.2 |
3.6 |
3.8 |
2.9 |
3.9 |
2.3 |
1.5 |
2.2 |
0.9 |
2.0 |
|
Monocyte (×109/l) |
0.39 |
0.14 |
0.23 |
0.14 |
0.22 |
0.11 |
0.07 |
0.04 |
0.01 |
0.04 |
0.01 |
0.09 |
|
Clinical chemistry |
||||||||||||
|
Urea (mmol/l) |
7.3 |
6.1 |
7.9* |
6.7 |
8.8*** |
9.1** |
10.6*** |
11.1*** |
12.0*** |
10.9*** |
12.0*** |
9.9*** |
|
Total protein (g/l) |
63.9 |
60.0 |
67.6** |
62.2 |
67.6** |
61.7 |
64.9 |
59.7 |
61.4* |
55.7*** |
57.0*** |
55.2** |
|
Globulin (g/l) |
26.0 |
22.0 |
27.5** |
22.8 |
27.3 |
22.4 |
26.2 |
20.6** |
23.3*** |
18.7*** |
21.0*** |
18.5*** |
|
Triglyceride (mmol/l) |
1.70 |
1.53 |
1.64 |
1.22 |
1.15** |
0.96* |
0.78*** |
0.58*** |
0.59*** |
0.59*** |
0.47*** |
0.58*** |
|
Cholesterol (mmol/l) |
3.5 |
2.5 |
4.0* |
2.9** |
4.0** |
2.8 |
3.7 |
3.0** |
3.8 |
3.2*** |
3.3 |
3.8*** |
From Mellert et al. (1998) and Mellert et al. (1999j)
M, Male; F, Female; MCV, Mean corpuscular volume; MCH, Mean corpuscular haemoglobin; MCHC, Mean corpuscular haemoglobin concentration
* p < 0.05; ** p < 0.02; *** p < 0.002, # An aberrant value of 528 was excluded from the mean to give a mean of 1205 instead of 1137
Table 8. Organ weights in mice given diets containing pyraclostrobin for 3 months
|
Dietary concentration (mg/kg of feed) |
||||||||||||
|
0 (control) |
50 |
150 |
500 |
1000 |
1500 |
|||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Organ weights |
||||||||||||
|
Absolute weights |
||||||||||||
|
Body (g) |
31.1 |
22.6 |
28.6 |
21.8 |
26.6** |
21.0 |
23.4** |
18.8** |
21.1** |
17.3** |
18.9** |
16.2** |
|
Adrenals (mg) |
5.5 |
11.6 |
5.5 |
10.8 |
5.7 |
10.1* |
6.1 |
8.1** |
5.7 |
6.5** |
6.5 |
6.7** |
|
Brain (mg) |
481 |
494 |
480 |
482 |
477 |
490 |
479 |
481 |
475 |
466** |
457** |
452** |
|
Kidneys (mg) |
488 |
346 |
432 |
332 |
446* |
324* |
405** |
300** |
344* |
274** |
308** |
255** |
|
Liver (mg) |
1137 |
1112 |
1053 |
1011 |
1052 |
988 |
995** |
840** |
948** |
848** |
869** |
832** |
|
Spleen (mg) |
63.4 |
73.9 |
61.0 |
68.8 |
59.4 |
67.2 |
56.9 |
60.8* |
50.7** |
53.5** |
44.0** |
50.2** |
|
Testes (mg) |
232 |
— |
224 |
— |
217* |
— |
227 |
— |
218 |
— |
203** |
— |
|
Relative (to body) weights |
||||||||||||
|
Adrenals |
0.018 |
0.051 |
0.020 |
0.050 |
0.022 |
0.049 |
0.026** |
0.043* |
0.027** |
0.037* |
0.034** |
0.041* |
|
Brain |
1.57 |
2.22 |
1.71 |
2.23 |
1.80** |
2.36 |
2.06** |
2.56* |
2.26** |
2.70** |
2.43** |
2.80** |
|
Kidneys |
1.58 |
1.55 |
1.52 |
1.53 |
1.68 |
1.56 |
1.73 |
1.60 |
1.63 |
1.58 |
1.63 |
1.58 |
|
Liver |
3.68 |
4.96 |
3.70 |
4.65 |
3.97* |
4.70 |
4.25** |
4.45 |
4.50** |
4.89 |
4.60** |
5.14 |
|
Spleen |
0.21 |
0.33 |
0.21 |
0.32 |
0.22* |
0.32 |
0.24** |
0.32 |
0.24* |
0.31 |
0.23 |
0.31 |
|
Testes |
0.75 |
— |
0.79 |
— |
0.82 |
— |
0.97** |
— |
1.03** |
— |
1.08** |
— |
From Mellert et al. (1998) and Mellert et al. (1999j)
Relative organ weight = organ weight (g)/body weight (g) × 100
* p < 0.05; ** p < 0.01
Table 9. Pathology findings in mice given diets containing pyraclostrobin for 3 months
|
Finding |
Dietary concentration (mg/kg of feed) |
|||||||||||
|
0 (control) |
50 |
150 |
500 |
1000 |
1500 |
|||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
No. of animals examined |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Gross findings |
||||||||||||
|
Thickening of duodenum wall |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
6 |
10 |
10 |
10 |
9 |
|
Erosion/ulcer of the glandular stomach |
1 |
2 |
0 |
2 |
1 |
4 |
2 |
7 |
2 |
4 |
4 |
1 |
|
Microscopic findings |
||||||||||||
|
Decreased vacuolation in adrenal cortex X-zone cells |
||||||||||||
|
Grade 1, 2 or 3 |
0 |
1 |
0 |
1 |
0 |
1 |
1 |
3 |
1 |
0 |
9 |
0 |
|
Grade 4 or 5 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
7 |
0 |
10 |
0 |
9 |
|
Total |
0 |
1 |
0 |
1 |
0 |
5 |
1 |
10 |
1 |
10 |
9 |
9 |
|
Thickening of duodenum mucosa |
||||||||||||
|
Grade 2 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
6 |
1 |
10 |
0 |
7 |
|
Grade 3 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
9 |
0 |
10 |
2 |
|
Total |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
6 |
10 |
10 |
10 |
9 |
|
Mean thickness of mucosa (mm) |
0.33 |
0.27 |
0.32 |
0.29 |
0.36 |
0.32* |
0.49** |
0.43** |
0.48** |
0.46** |
0.46** |
0.44** |
|
Glandular stomach erosion/ulcer |
1 |
1 |
1 |
3 |
2 |
5 |
4 |
7 |
5 |
6 |
8 |
6 |
|
Kidneys, lipid vacuoles |
10 |
10 |
10 |
10 |
10 |
9 |
2 |
7 |
1 |
7 |
0 |
7 |
|
Liver, diffuse fatty infiltration |
||||||||||||
|
Grade 2 or 3 |
2 |
3 |
2 |
3 |
3 |
2 |
8 |
3 |
7 |
7 |
4 |
4 |
|
Grade 4 |
8 |
7 |
8 |
7 |
7 |
6 |
2 |
3 |
3 |
2 |
0 |
3 |
|
Total |
10 |
10 |
10 |
10 |
10 |
8 |
10 |
6 |
10 |
9 |
4 |
7 |
|
Mesenteric lymph node apoptosis |
||||||||||||
|
Grade 1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
5 |
0 |
5 |
|
Grade 2 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
4 |
0 |
1 |
9 |
2 |
|
Total |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
4 |
1 |
6 |
9 |
7 |
|
Thymus gland atrophy |
||||||||||||
|
Grade 2 |
0 |
0 |
0 |
0 |
0 |
3 |
2 |
2 |
4 |
3 |
1 |
0 |
|
Grade 3 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
5 |
1 |
4 |
2 |
2 |
|
Grade 4 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
1 |
5 |
2 |
|
Total |
0 |
0 |
0 |
0 |
0 |
6 |
3 |
7 |
6 |
8 |
8 |
4 |
From Mellert et al. (1998) and Mellert et al. (1999j)
Grade 1 = minimal in severity/very few in number/very small in size; grade 2 = slight in severity/few in number/small in size;
grade 3 = moderate in severity and size/moderate to several in number, grade 4 = severe
Rats
Groups of five male and five female Wistar rats were given pyraclostrobin (in 0.5% aqueous carboxymethylcellulose) at a dose of 0 or 4 mg/kg bw per day by gavage for 1 week, or diets containing pyraclostrobin at a concentration of 0 or 34 mg/kg for 1 week. Food consumption and body weight were determined daily and animals were examined for mortality and clinical signs of toxicity at least once per day. At the end of the experiment, animals were sacrificed without further examination. The dietary concentration of 34 mg/kg of feed corresponded to mean intakes of pyraclostrobin of 3.5 mg/kg bw per day in males and 3.8 mg/kg bw per day in females. Lower body-weight gain (up to 33%) was observed in females receiving diet containing pyraclostrobin at 34 mg/kg, but not in females given an equivalent dose of pyraclostrobin by gavage, nor in males given pyraclostrobin either in the diet or by gavage. There were no deaths and no clinical signs of toxicity in any group. Data for individual animals was not supplied. This was a supplemental study initiated to address the appropriateness of using an apparently lower body-weight gain in treated animals in a study of developmental toxicity in rabbits as an end-point on which to base the establishment of an acute RfD. In conjunction with similar studies in rabbits and mice, this study was intended to demonstrate the species-specific variability in food intake and body-weight gains in rabbits. Because of the limited parameters examined in this study, it was not adequate for the purposes of risk assessment and a NOAEL could not be identified (Mellert, 2002b).
Groups of five male and five female Wistar rats (aged 42 days) were given diets containing pyraclostrobin (purity, 94–99%) at a concentration of 0, 20, 100, 500, or 1500 mg/kg of feed (equal to 0, 1.8, 9, 42 and 120 mg/kg bw per day) for 4 weeks. Dose selection was based on the results of a preliminary study (BASF Aktiengesellschaft Project No. 24S0376/96061) in which groups of five male and five female Wistar rats were given diets containing pyraclostrobin at a concentration of 400, 3000 or 15 000 mg/kg of feed for 2 weeks. A separate report was not provided for this study. Rats were checked at least daily for mortality and signs of toxicity, a comprehensive clinical examination was performed weekly, body weight and food consumption were recorded weekly and water consumption was recorded daily. Haematology, urine analysis and clinical chemistry parameters were assessed in all animals, as were gross pathology and organ weights. Histological examination was performed on the liver, spleen, fore-stomach, glandular stomach, duodenum, jejunem, ileum, caecum, colon and rectum of all animals, heart, kidneys, adrenal glands and testes in the control group and at 15 000 mg/kg only, and gross lesions were assessed in all animals affected.
In the preliminary study, all animals at 15 000 mg/kg were sacrificed because of excessive toxicity (no other details provided) and at 3000 mg/kg, body-weight gain and food consumption were reduced, with signs of anaemia apparent at 400 and 3000 mg/kg (no other details provided).
In the main study, there were no deaths or treatment-related clinical signs, but reductions of up to 16% in food consumption at 500 mg/kg and of up to 44% at 1500 mg/kg were observed. Concomittantly, body-weight gain was reduced by 14 to 32% over the study in both sexes at 1500 mg/kg and in males at 500 mg/kg, primarily owing to a pronounced reduction of 51–67% at 1500 mg/kg during the first week of the study. A slight anaemia characterized by reduced erythrocyte numbers and haemoglobin concentration was observed in females at 500 and 1500 mg/kg, with a slight, not statistically significant, reduction in haemoglobin concentration also seen in males at 1500 mg/kg. The anaemia correlated with evidence of extramedullary haematopoiesis in the liver and spleen and with increased relative spleen weights in both sexes at 500 and 1500 mg/kg. Slight decreases were seen in alanine aminotransferase in both sexes at 500 and 1500 mg/kg, and in serum cholinesterase in females at 1500 mg/kg. As decreased alanine aminotransferase activity was observed in the 3-month and long-term studies in rats also, this effect is likely to be treatment-related but, as the magnitude of the effect was small and a decrease is not normally associated with adverse organ or system effects, is unlikely to be toxicologically relevant. This conclusion was further supported by studies indicating that alanine aminotransferase (and alkaline phosphatase) activites can be affected by dietary status, as discussed later in this monograph. Urine volume was increased and specific gravity was decreased in both sexes at 1500 mg/kg.
Table 10. Clinical chemistry findings in rats given diets containing pyraclostrobin for 4 weeks
|
Parameter |
Dietary concentration (mg/kg of feed) |
|||||||||
|
0 (control) |
20 |
100 |
500 |
1500 |
||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Erythrocyte count (1012/l) |
8.5 |
8.1 |
8.1 |
7.9 |
8.1 |
8.1 |
8.2 |
7.6** |
8.2 |
7.4** |
|
Haemoglobin (mmol/l) |
9.6 |
9.5 |
9.4 |
9.3 |
9.6 |
9.4 |
9.3 |
8.8** |
8.9 |
8.8** |
|
MCV (10–15 l) |
53.7 |
54 |
54.3 |
54.7 |
55.1 |
54.1 |
53.9 |
54.2 |
53.1 |
57.5** |
|
MCHC (mmol/l) |
21.2 |
21.6 |
21.2 |
21.5 |
21.3 |
21.5 |
20.9 |
21.5 |
20.4* |
20.9** |
|
Prothrombin time (s) |
28 |
24.9 |
28.2 |
24.6 |
26.9 |
24.7 |
28.9** |
25.7 |
30.2* |
27.8** |
|
Platelets (×109/l) |
769 |
753 |
752 |
814 |
785 |
742 |
780 |
768 |
849 |
861 |
|
ALT (µkat/l) |
1.06 |
1.03 |
1.02 |
0.95 |
0.91 |
0.94 |
0.75** |
0.88 |
0.87 |
0.78 |
|
Total bilirubin (µmol/l) |
2.9 |
3.1 |
2.6 |
2.6 |
2.6 |
2.4 |
3.3 |
3.5 |
5.9** |
3.6 |
|
Globulin (g/l) |
28.2 |
26.7 |
27.3 |
25.9 |
28.4 |
26.3 |
26.5 |
24.6 |
24.4 |
23.1 |
|
Glucose (mmol/l) |
8.2 |
8.5 |
8.5 |
8.5 |
8.3 |
7.8 |
7.5 |
7.9 |
7.3 |
6.7** |
|
Inorganic phosphate (mmol/l) |
3.0 |
2.6 |
3.1 |
2.5 |
3.0 |
2.6 |
2.7 |
2.4 |
2.5* |
2.4 |
|
Serum cholinesterase (µkat/l) |
10.8 |
41.6 |
10.6 |
42.2 |
12.3 |
41.7 |
10.3 |
34.0 |
10.2 |
18.1* |
|
Urine volume (ml) |
3 |
2.4 |
3.5 |
1.9 |
3.7 |
2.2 |
4.6 |
2.5 |
7.2 |
5.2 |
|
Specific gravity (g/l) |
||||||||||
|
<1040 |
1 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
5* |
4* |
|
>1041 |
4 |
5 |
4 |
5 |
5 |
5 |
4 |
5 |
0 |
1 |
From Mellert et al. (1999d)
ALT, Alanine aminotransferase; F, female; M, male; MCHC, Mean corpuscular haemoglobin concentration; MCV, Mean corpuscular volume
* p < 0.05; ** p < 0.01
Absolute kidney, adrenal and thymus weights were decreased and relative brain weights were increased secondary to reduced body-weight gains at 1500 mg/kg. Histology did not reveal any alterations in these tissues. An increased relative liver weight correlated with increased hepatocellular hypertrophy in males at 1500 mg/kg, diminished fat storage at 500 and 1500 mg/kg, and altered clinical chemistry values at 1500 mg/kg (increased total bilirubin in males, decreased glucose concentration in both sexes, and decreased globulin concentration in both sexes). The incidence of mucosal hyperplasia in the duodenum was increased at 500 and 1500 mg/kg. Reduced body-weight gains and fat storage in the liver were likely to be secondary to reduced food consumption, which may be related to poor palatability of the treated feed. Consequently, undue weight was not attached to these findings. The NOAEL was 100 mg/kg (equal to 9 mg/kg bw per day) on the basis of anaemia and associated findings, and mucosal hyperplasia at 500 mg/kg (equal to 42 mg/kg bw per day) (Mellert et al., 1999d).
Groups of 10 male and 10 female Wistar rats (aged 9 weeks) received pyraclostrobin (purity, 99%; in 0.5% carboxymethylcellulose) at a dose of 0, 40, 100 or 250 mg/kg bw per day, 5 days per week, for 4 weeks, in an application to the clipped dorsal surface. The test site, approximately 10% of the body surface area, was covered with a semi-occlusive dressing for 6 h after application. Rats were checked at least once daily for mortality and signs of toxicity, detailed clinical examinations were conducted at least once weekly, body weight, food consumption and food conversion efficiency were recorded once per week, and ophthalmoscopy was performed on animals in the control group and in the group at 250 mg/kg bw per day before sacrifice. At study termination, haematology, urine analysis and clinical chemistry parameters were assessed, animals were examined for gross pathology and organs (including uterus) were weighed. In the control group and in the group at 250 mg/kg bw per day only, a standard range of tissues (including nasal cavity, larynx, pharynx and treated skin) were examined microscopically. The uterus, vagina and treated skin were examined microscopically in all animals at 40 and 100 mg/kg bw per day and gross lesions were assessed in all animals affected.
Table 11. Organ weights and histology findings in rats receiving diets containing pyraclostrobin for 4 weeks
|
Dietary concentration (mg/kg of feed) |
||||||||||
|
0 (control) |
20 |
100 |
500 |
1500 |
||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Organ weights |
||||||||||
|
Body weight (g) |
297 |
183 |
302 |
184 |
301 |
186 |
281 |
176 |
241** |
161* |
|
Adrenal glands (mg) |
80.4 |
84.4 |
80.4 |
91.4 |
79.6 |
86.4 |
78.6 |
85.2 |
73.6 |
72.6 |
|
Brain (g) |
1.82 |
1.77 |
1.87 |
1.73 |
1.88 |
1.80 |
1.86 |
1.70 |
1.77 |
1.71 |
|
Kidneys (g) |
2.47 |
1.73 |
2.53 |
1.70 |
2.37 |
1.71 |
2.36 |
1.64 |
2.06* |
1.50 |
|
Thymus gland (mg) |
424 |
307 |
444 |
297 |
427 |
273 |
357 |
270 |
330** |
244 |
|
Relative weighta of brain |
0.61 |
0.97 |
0.62 |
0.94 |
0.63 |
0.97 |
0.66 |
0.97 |
0.74** |
1.06* |
|
Relative weighta of liver |
3.51 |
3.45 |
3.28 |
3.40 |
3.32 |
3.34 |
3.48 |
3.43 |
4.05* |
4.36** |
|
Relative weighta of spleen |
0.21 |
0.25 |
0.25 |
0.24 |
0.24 |
0.28 |
0.28 |
0.33* |
0.35* |
0.38** |
|
Pathology |
||||||||||
|
No. of animals examined |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
|
Duodenum |
||||||||||
|
Mucosal hyperplasia |
0 |
1 |
0 |
0 |
0 |
0 |
4 |
2 |
4 |
4 |
|
Liver |
||||||||||
|
Fatty change |
5 |
4 |
5 |
5 |
5 |
5 |
0 |
0 |
0 |
0 |
|
Hepatocellular hypertrophy |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
|
Spleen |
||||||||||
|
Extramedullary haematopoiesis |
0 |
1 |
0 |
1 |
0 |
0 |
4 |
5 |
5 |
4 |
From Mellert et al. (1999d)
aRelative organ weight = organ weight (g)/body weight (g) × 100
* p > 0.05; ** p > 0.01 M, Male; F, Female
There were no mortalities, no treatment-related effects on ophthalmology, haematology, clinical chemistry and urine analysis parameters and no abnormal findings in the open field observations for any animal. On the treated skin, clinical signs included scale formation in most females and some males at 250 mg/kg bw per day, and in some females at 100 mg/kg bw per day. Slight erythema was also seen in most females at 250 mg/kg bw per day. Body-weight gain was reduced by 8% in males at 250 mg/kg bw per day and food conversion efficiency was lower in males at 100 and 250 mg/kg bw per day. Absolute and relative uterus weights were increased by 27% and 30%, respectively, at 250 mg/kg bw per day. Epidermal thickening seen at 100 and 250 mg/kg bw per day was characterized by cloudy swelling of epidermal cells, associated with hyperkeratosis. In the uteri of rats at 250 mg/kg bw per day, dilation of the lumen was more common (two, one, two and six rats at 0, 40, 100 and 250 mg/kg bw per day, respectively; n = 10). After dermal administration of pyraclostrobin to rats for 4 weeks, the NOAEL for systemic toxicity for both sexes was 100 mg/kg bw per day on the basis of effects on the uteri of females and reduced body-weight gains in males at 250 mg/kg bw per day. Signs of dermal irritation occurred in all treated animals (Mellert et al., 1999c).
Groups of 10 male and 10 female Wistar rats (aged 42 days) were given diets containing pyraclostrobin (purity, 98.5%) at a concentration of 0, 50, 150, 500, 1000, or 1500 mg/kg of feed (equal to 0, 3.5, 11, 35, 69 and 106 mg/kg bw per day) for 3 months. Rats were checked at least once daily for mortality and signs of toxicity, and a comprehensive clinical examination was performed once weekly. Body weight and food consumption were recorded once weekly and water consumption was assessed daily. Haematology (including reticulocytes), urine analysis and clinical chemistry (excluding brain cholinesterase) parameters were assessed in all animals. At sacrifice, all animals were examined grossly and organ weights (excluding epididymides, heart and thymus gland) were recorded. A full range of tissues was examined microscopically in the control group and at 1500 mg/kg. The lungs, liver, spleen, kidneys, stomach, duodenum, jejunem, ileum, sternum with marrow and bone marrow from the femur were examined microscopically in animals at 50, 150, 500 and 1000 mg/kg, and gross lesions were assessed in all animals affected per group.
There were no deaths or treatment-related clinical signs. At >500 mg/kg in males and at >1000 mg/kg in females, body-weight gain was reduced. Food consumption was consistently reduced in males, and intermittently in females at >500 mg/kg. Food conversion efficiency was also intermittently reduced at 1000 and 1500 mg/kg. Increased erythrocyte turnover was reflected in a marked increase in reticulocyte counts (41–94%) in males at >1000 mg/kg and in females at 1500 mg/kg, extramedullary haematopoiesis in both sexes at >150 mg/kg and in a slight anaemia in females at 1000 and 1500 mg/kg characterized by reductions of generally 10% or less in erythrocyte counts, haemoglobin concentrations and, at 1500 mg/kg, erythrocyte volume fraction. Other haematological effects consisted of increases in the values for MCV in both sexes at 1000 and 1500 mg/kg and females at 500 mg/kg (3–6%), prothrombin time in males at 1000 and 1500 mg/kg (11 and 13%), leukocyte counts in both sexes at 1500 mg/kg and females at 1000 mg/kg (14–71%), polymorphonuclear neutrophils in both sexes at 1000 and 1500 mg/kg (56–120%), and lymphocytes in females at 1000 and 1500 mg/kg (70–76%). Clinical chemistry changes consisted of increases in bilirubin in both sexes at 1500 mg/kg and males at 1000 mg/kg (30–95%), albumin in males at >500 mg/kg (5–6%) and erythrocyte cholinesterase activity in both sexes at 1500 mg/kg (23–33%), and in decreases in alanine aminotransferase activity in both sexes at >500 mg/kg (20–35%), alkaline phosphatase activity in both sexes at 1000 and 1500 mg/kg and females at 500 mg/kg (14–23%), globulin concentration in both sexes at 1000 and 1500 mg/kg (8–13%), concentration of triglycerides in males at 1000 and 1500 mg/kg (50–61%), cholesterol concentration in males at >500 mg/kg (19–29%), creatinine concentration in females at 1000 and 1500 mg/kg (10%) and serum cholinesterase activity in females at 1000 and 1500 mg/kg (41–49%). As alanine aminotransferase and alkaline phosphatase activities were also reduced in the 28-day and long-term studies in rats, the effect is clearly treatment-related but, given the small magnitude of the effect and as a slight reduction in these parameters is not normally associated with adverse organ or systemic effects, is unlikely to be toxicologically relevant. This conclusion is further supported by studies indicating that alanine aminotransferase (and alkaline phosphatase) activities can be affected by dietary status, as discussed later in this monograph. Males at 1000 and 1500 mg/kg had dark yellow to light red cloudy urine and females at 1500 mg/kg had cloudy urine. In females at 1000 and 1500 mg/kg, urine volume was increased by 48% and 130%, respectively, and specific gravity was decreased slightly. The number of crystals in the urinary sediment was slightly increased in males at 1500 mg/kg. Increased relative liver weights in both sexes at 1500 mg/kg and females at 1000 mg/kg (10–34%), kidney weights in both sexes at 1000 and 1500 mg/kg (8–15%), and spleen weights in both sexes at 1000 and 1500 mg/kg and females at 500 mg/kg (22–74%) were likely to be treatment-related as they correlated with histological and/or clinical pathology findings and/or were inconsistent with normal patterns of organ weight changes associated with reduced body-weight gain. Other organ weight differences were likely to be caused by reductions in body-weight gains (25–42%) in these groups. Thickening of the duodenal wall and discoloration of the spleen were more common at 1500 mg/kg. Microscopic examination demonstrated an increase in the incidence and/or severity of mucosal hyperplasia in the duodenum in both sexes at 1500 mg/kg and in males at 500 and 1000 mg/kg, hepatocellular hypertrophy in the liver of both sexes at 1500 mg/kg and in males at 500 and 1000 mg/kg and, in the spleen, distension of the sinusoids in both sexes at 1000 and 1500 mg/kg, extramedullary haematopoiesis in both sexes at >150 mg/kg and histiocytosis in both sexes at >150 mg/kg. Fat storage in the liver was diminished in both sexes at >150 mg/kg, which is likely to reflect the reduced weight gains observed at >500 mg/kg. In two long-term studies in rats, at intakes of pyraclostrobin of up to 200 mg/kg (9 mg/kg bw per day), no effects on splenic extramedullary haematopoiesis or histiocytosis, or on fat storage in the liver were seen. Consequently the low incidences of these findings at 150 mg/kg were considered to be incidental to treatment. The NOAEL was 150 mg/kg (10.7 mg/kg bw per day in males and 12.3 mg/kg bw per day in females) on the basis of reductions in body weight and food consumption, effects on clinical chemistry parameters, liver hypertrophy, and mucosal hypertrophy in the duodenum at >500 mg/kg (Mellert et al., 1999a, 1999b).
Table 12. Haematology and clinical chemistry findings in rats given diets containing pyraclostrobin for 3 months
|
Parameter Dietary concentration (mg/kg of feed) |
||||||||||||
|
0 (control) |
50 |
150 |
500 |
1000 |
1500 |
|||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Haematology |
||||||||||||
|
Erythrocytes (1012/l) |
8.5 |
8.0 |
8.5 |
7.9 |
8.8 |
8.0 |
8.6 |
7.7 |
8.4 |
7.4*** |
8.2 |
7.1*** |
|
Haemoglobin (mmol/l) |
9.7 |
9.2 |
9.5 |
9.3 |
9.8 |
9.3 |
9.7 |
9.3 |
9.5 |
8.7** |
9.4 |
8.6*** |
|
Erythrocyte volume fraction (l/l) |
0.43 |
0.41 |
0.43 |
0.41 |
0.44 |
0.42 |
0.44 |
0.42 |
0.44 |
0.40 |
0.43 |
0.39* |
|
MCV (10-15 l) |
50.6 |
51.5 |
50.1 |
52.0 |
50.2 |
52.1 |
51.3 |
53.9*** |
52.3* |
53.8*** |
52.9** |
54.8*** |
|
MCH (10-15 mol/l) |
1.14 |
1.16 |
1.12 |
1.18 |
1.12 |
1.17 |
1.13 |
1.20*** |
1.14 |
1.19** |
1.15 |
1.21*** |
|
MCHC (mmol/l) |
22.5 |
22.6 |
22.3 |
22.6 |
22.3 |
22.4 |
21.9** |
22.4 |
21.8*** |
22.2*** |
21.7*** |
22.2** |
|
Reticulocytes (10-3 |
17 |
14 |
17 |
17 |
16 |
14 |
19 |
13 |
24** |
15 |
33*** |
23*** |
|
erythrocytes) |
||||||||||||
|
Prothrombin time (s) |
26.0 |
25.6 |
26.5 |
24.7 |
26.4 |
25.5 |
27.1 |
26.1 |
28.9*** |
27.5*** |
29.4*** |
26.3 |
|
Leukocytes (×109/l) |
8.4 |
3.9 |
9.0 |
4.2 |
8.1 |
4.9 |
8.9 |
4.7 |
8.9 |
6.7*** |
9.6 |
6.6** |
|
Neutrophils (×109/l) |
0.66 |
0.68 |
0.71 |
0.81 |
0.60 |
0.68 |
0.78 |
0.71 |
1.25 |
1.06 |
1.45 |
1.25 |
|
Lymphocytes (×109/l) |
6.96 |
2.84 |
7.53 |
2.97 |
6.74 |
3.74 |
7.50 |
3.58 |
7.03 |
5.00 |
7.37 |
4.83 |
|
Clinical chemistry |
||||||||||||
|
ALT (µkat/l) |
1.05 |
0.98 |
1.03 |
0.77** |
0.91 |
0.89 |
0.73*** |
0.78* |
0.68*** |
0.65*** |
0.79** |
0.71** |
|
AP (µkat/l) |
5.6 |
4.5 |
5.7 |
4.1 |
5.9 |
4.4 |
5.3 |
3.8** |
4.3** |
3.8 |
4.3*** |
3.6*** |
|
Bilirubin (µmol/l) |
1.7 |
2.2 |
1.7 |
1.9 |
1.8 |
1.9 |
2.2 |
1.9 |
2.7*** |
2.7 |
3.3*** |
2.9** |
|
Albumin (g/l) |
35.5 |
38.3 |
35.8 |
39.8 |
36.7 |
38.2 |
37.3** |
38.6 |
37.8** |
36.6 |
37.8*** |
37.2 |
|
Globulin (g/l) |
31.6 |
30.6 |
33.1 |
32.5 |
32.6 |
30.6 |
31.2 |
29.4 |
29.2** |
26.7** |
27.5*** |
26.8** |
|
Glucose (mmol/l) |
7.9 |
7.9 |
8.1 |
8.1 |
7.6 |
7.7 |
7.6 |
7.2 |
7.3* |
7.0** |
7.2** |
7.3 |
|
ECHE (µkat/l) |
18.4 |
16.7 |
20.6* |
20.9 |
18.7 |
20.0 |
20.8 |
18.4 |
20.2 |
21.1 |
22.6** |
22.2 |
|
SCHE (µkat/l) |
10.0 |
47.9 |
9.8 |
57.5 |
10.8 |
49.9 |
10.6 |
41.2 |
10.4 |
28.5*** |
10.3 |
24.3*** |
|
Triglyceride (mmol/l) |
3.8 |
1.6 |
3.6 |
2.6 |
4.4 |
2.3 |
2.9 |
2.2 |
1.9** |
1.5 |
1.5*** |
2.1 |
|
Creatinine (µmol/l) |
49.8 |
55.6 |
49.5 |
57.3 |
50.3 |
56.1 |
48.9 |
52.8 |
48.9 |
50.0** |
48.4 |
50.3** |
|
Cholesterol (mmol/l) |
2.3 |
1.8 |
2.2 |
2.1 |
1.9 |
1.8 |
1.8* |
1.8 |
1.7*** |
1.7 |
1.6*** |
1.8 |
From Mellert et al. (1999a, 1999b) ALT, Alanine aminotransferase; AP, Alkaline phosphatase; ECHE, erythrocyte cholinesterase; F, Female; M, Male; MCH, Mean corpuscular haemoglobin; MCHC, Mean corpuscular haemoglobin concentration; MCV, Mean corpuscular volume; SCHE, serum cholinesterase
* p < 0.05; ** p < 0.02; *** p < 0.002
Table 13. Organ weights in rats receiving diets containing pyraclostrobin for 3 months
|
Organ |
Dietary concentration (mg/kg of feed) |
|||||||||||
|
0 (control) |
50 |
150 |
500 |
1000 |
1500 |
|||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Absolute weights |
||||||||||||
|
Body (g) |
439 |
230 |
434 |
243 |
424 |
235 |
406* |
224 |
370** |
218 |
319** |
210** |
|
Adrenals (mg) |
86.3 |
99.9 |
81.5 |
90.9 |
84.5 |
94.5 |
71.8** |
88.4* |
72.6** |
85.2** |
71.3** |
76.6** |
|
Brain (g) |
2.06 |
1.82 |
2.03 |
1.86 |
2.07 |
1.88* |
2.02 |
1.82 |
2.02 |
1.77* |
1.99 |
1.78* |
|
Kidneys (g) |
2.98 |
1.79 |
3.03 |
1.86 |
2.87 |
1.88 |
2.86 |
1.84 |
2.76 |
1.84 |
2.48** |
1.76 |
|
Liver (g) |
15.0 |
6.8 |
14.0 |
7.3 |
13.2* |
7.1 |
13.1* |
6.9 |
12.3** |
7.3 |
11.9** |
8.3** |
|
Ovaries (mg) |
— |
96.8 |
— |
107.1 |
— |
101.9 |
— |
103.6 |
— |
102.3 |
— |
112.9 |
|
Spleen (g) |
0.87 |
0.56 |
0.90 |
0.63 |
0.84 |
0.60 |
0.85 |
0.65* |
0.95 |
0.74* |
1.02* |
0.88** |
|
Testes (g) |
3.58 |
— |
3.57 |
— |
3.57 |
— |
3.52 |
— |
3.74 |
— |
3.68 |
— |
|
Relative (to body) weightsa |
||||||||||||
|
Adrenals |
0.020 |
0.044 |
0.018 |
0.038* |
0.020 |
0.040 |
0.018 |
0.039 |
0.020 |
0.039 |
0.022** |
0.037** |
|
Brain |
0.47 |
0.820 |
0.46 |
0.77 |
0.49 |
0.81 |
0.550 |
0.81 |
0.55** |
0.82 |
0.62** |
0.85 |
|
Kidneys |
0.68 |
0.78 |
0.68 |
0.77 |
0.68 |
0.80 |
0.71 |
0.82* |
0.75* |
0.85* |
0.78* |
0.84* |
|
Liver |
3.4 |
2.9 |
3.1 |
3.0 |
3.1 |
3.0 |
3.2 |
3.1* |
3.3 |
3.3** |
3.7 |
3.9** |
|
Ovaries |
— |
0.042 |
— |
0.044 |
— |
0.044 |
— |
0.046 |
— |
0.047 |
— |
0.054** |
|
Spleen |
0.20 |
0.24 |
0.20 |
0.26 |
0.20 |
0.26 |
0.21 |
0.29** |
0.26** |
0.34** |
0.32** |
0.42** |
|
Testes |
0.82 |
— |
0.81 |
— |
0.85 |
— |
0.87 |
— |
1.01** |
— |
1.15** |
— |
From Mellert et al. (1999a, 1999b)
F, Female; M, Male
a Relative organ weight = organ weight (g)/body weight (g) × 100; * p < 0.05; ** p < 0.01
Table 14. Pathology findings in rats receiving diets containing pyraclostrobin for 3 months
|
Finding |
Dietary concentration (mg/kg of feed) |
|||||||||||
|
0 (control) |
50 |
150 |
500 |
1000 |
1500 |
|||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
No. of animals examined |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Gross pathology |
||||||||||||
|
Duodenum, thickening of wall |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
10 |
10 |
|
Spleen, discoloration |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
6 |
5 |
|
Histopathology |
||||||||||||
|
Duodenum, mucosal hyperplasia |
||||||||||||
|
Grade 1a |
2 |
2 |
1 |
1 |
1 |
1 |
3 |
1 |
4 |
0 |
1 |
6 |
|
Grade 2a |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
0 |
1 |
1 |
9 |
4 |
|
Total |
2 |
2 |
1 |
1 |
1 |
2 |
4 |
1 |
5 |
1 |
10 |
10 |
|
Liver |
||||||||||||
|
Diffuse fatty change |
10 |
4 |
8 |
7 |
9 |
5 |
6 |
2 |
2 |
1 |
0 |
0 |
|
Hepatocellular hypertrophy |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
6 |
0 |
10 |
4 |
|
Spleen |
||||||||||||
|
Distension of sinusoids |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
2 |
10 |
8 |
8 |
10 |
|
Extramedullary haematopoiesis |
2 |
0 |
0 |
0 |
3 |
3 |
1 |
3 |
2 |
9 |
3 |
9 |
|
Histiocytosis |
0 |
0 |
0 |
0 |
1 |
1 |
3 |
2 |
6 |
7 |
10 |
7 |
From Mellert et al. (1999a, 1999b) F, Female; M, Male
a Grade 1 = minimal in severity/very few in number/very small in size; grade 2 = slight in severity/few in number/small in size; grade 3 = moderate in severity and size/moderate to several in number
Rabbits
Groups of five male and five female (not pregnant) Himalayan rabbits (Chbb:HM from Charles River Laboratories Germany) were given pyraclostrobin (in a 0.5% aqueous solution of carboxymethylcellulose) at a dose of 0 or 4 mg/kg bw per day for 1 week by gavage. Pyraclostrobin was also administered in the diet at concentrations calculated to provide doses of 0 or 4 mg/kg bw per day for 1 week. Animals were quarantined and acclimatized to standard laboratory conditions for approximately 1 week before the start of the study. Food and water were available ad libitum. Food consumption was determined daily and body weight was recorded on days 0, 2, 4, 6 and 7. Animals were examined for mortality and clinical signs of toxicity at least once per day. At the end of the experiment, animals were sacrificed for necropsy. There were no clinical signs of toxicity and no abnormal macroscopic changes observed at necropsy. From the first day of dosing, mean food consumption was about 15–40% lower in both groups of treated males, but the differences from values in the relevant control groups were not statistically significant. Similarly, in both groups of females mean food consumption was 20–40% lower from the beginning of dosing until day 5. Thereafter, it was similar in control and treated groups. Although there were some apparent differences between mean food consumption and body-weight gains in control and treated groups, there was considerable variation between individuals in all groups and also variation in values obtained for any one individual on different days. Table 15 provides the ranges of values for food consumption and body-weight gain measured in control and treated animals. There was no clear effect of treatment with pyraclostrobin and it is probable that the apparent variation observed was a part of the normal biological variation for this species. This was a supplementary study initiated to address the appropriateness of using an apparently lower body-weight gain in treated animals in a study of developmental toxicity in rabbits as an appropriate end-point on which to base an acute RfD. In conjunction with similar studies in mice and rats, this study was intended to demonstrate the species-specific variability in food intake and body-weight gains of rabbits. Because of the limited parameters examined in this study, it was not adequate for the purposes of risk assessment and a NOAEL could not be identified (Schneider & Hellwig, 2002).
Table 15. Variations in food consumption and body-weight changes in rabbits
|
Dose |
||||
|
Gavage control |
4 mg/kg bw per day, by gavage |
Diet control |
4 mg/kg bw per day via the diet |
|
|
Food consumption |
||||
|
Minimum, g/animal/day (M/F) |
14/36 |
26/26 |
12/12 |
21/21 |
|
Maximum, g/animal/day (M/F) |
134/144 |
126/108 |
112/112 |
82/93 |
|
Body-weight changes |
||||
|
Days 0–7 (M/F) |
57/41 |
-10/27 |
-8/-21 |
-48/-26 |
|
Extreme values: |
||||
|
Males |
||||
|
Days 0–2 (max/min) |
51/-18 |
22/-6 |
180/-165 |
1/-38 |
|
Days 2–4 (max/min) |
11/-53 |
13/-26 |
2/-137 |
17/-46 |
|
Days 4–6 (max/min) |
95/-46 |
4/-90 |
79/-17 |
34/-46 |
|
Days 6–7 (max/min) |
18/1 |
60/-24 |
43/15 |
4/-21 |
|
Females |
||||
|
Days 0–2 (max/min) |
55/6 |
75/-27 |
56/-76 |
21/-55 |
|
Days 2–4 (max/min) |
40/1 |
35/-26 |
21/-30 |
10/-65 |
|
Days 4–6 (max/min) |
30/-30 |
20/-24 |
24/-64 |
21/-27 |
|
Days 6–7 (max/min) |
20/-30 |
29/-15 |
55/-10 |
88/-6 |
From Schneider & Hellwig (2002)
F, Female; M, Male; max/min, maximum/minimum
Dogs
Groups of five male and five female beagle dogs (aged 7–8 months) were given diets containing pyraclostrobin (purity, 97.1%) at a concentration of 0, 100, 200, or 450 mg/kg of feed (equal to 0, 2.8, 5.8 and 13 mg/kg bw per day) for 3 months. Dogs were checked at least once daily for mortality, and clinical signs of toxicity were assessed at least once each working day. Body weight was recorded weekly and food consumption was recorded daily. Haematology (including activated partial thromboplastin time) and clinical chemistry parameters (excluding brain and erythrocyte cholinesterase activity) were assessed in all animals on days 41/43 and 90, and an ophthalmological examination was performed at the end of the study. Urine analysis parameters were assessed in all animals on days 37/38 and 86/87. At sacrifice, all dogs were examined grossly, their tissues (including gall bladder, but excluding seminal vesicles) were examined microscopically and gross lesions were assessed. Organ weights (including parathyroid and thyroid glands, but excluding heart, spleen and thymus gland) were recorded.
There were no deaths, but vomiting (10 out of 10 dogs at 450 mg/kg until week 2) and diarrhoea (10 out of 10 dogs at 450 mg/kg during most of the study) were observed. At 200 mg/kg, diarrhoea also occurred in two out of five males during week 5 only, and sporadically throughout the study in four out of five females. As diarrhoea was observed in a 12-month study in dogs at 400 but not at 200 mg/kg, the occurrences of diarrhoea at 200 mg/kg in this study were regarded as being of no toxicological concern, owing to their isolated occurrence (week 5 only) in almost all affected dogs, or the irregular occurrence in a single animal. All other observations, discussed in the following paragraphs, relate to the treatment at 450 mg/kg only, unless specifically indicated otherwise. Females lost 2% (200 g) of their body weight, and males at 200 and 450 mg/kg gained substantially less weight (up to 31%) than did the controls. The effects in males were greatly influenced by one animal at 200 mg/kg that gained no weight and one at 450 mg/kg that lost weight (1%) during the study. Consequently, the finding in males is of questionable toxicological significance, particularly as no effect on body-weight gains was observed at 200 mg/kg in a subsequent 12-month study in dogs. In females, food consumption (9%) and food conversion efficiency were reduced. There were no treatment-related effects on ophthalmology.
Platelet counts were increased by 47–60% in females, and concentrations of total protein, albumin, globulin (both sexes, 5–12%) and glucose (females, 9–13%) were reduced, and absolute and relative epididymides weights were slightly increased (10–14%). A reduction in absolute and relative liver weights in females is consistent with, and likely to be secondary to, reduced food intake and weight gain. Urine analysis parameters were unaffected by treatment.
In the duodenum of both sexes, thickening of the wall and mucosal hypertrophy, which was characterized by an increased ratio of cytoplasm to nuclei in the villi and a hyperplastic aspect in epithelial cells, were observed. The NOAEL was 200 mg/kg (5.8 mg/kg bw per day) on the basis of increased platelet counts in females, decreased concentrations of total protein, albumin, and globulin in both sexes, decreased concentration of glucose and liver weights in females, and thickening of the wall and mucosal hypertrophy in the duodenum of both sexes at 450 mg/kg (Menges et al., 1999).
Groups of five male and female beagle dogs (aged 5–8 months) were given diets containing pyraclostrobin (purity, 98.7%) at a concentration of 0, 100, 200, or 400 mg/kg of feed (equal to 0, 2.7, 5.4 and 11 mg/kg bw per day) for 12 months. Animals were individually housed in kennels in controlled conditions. Each day, 700 g of food ration was offered to each dog, and water was provided ad libitum. Dogs were checked at least once daily for mortality, and clinical signs of toxicity were assessed at least once each weekday. Body weight and food conversion efficiency were recorded/calculated weekly, while food consumption was recorded daily. An ophthalmological examination was performed at the end of the study. Haematology (including activated partial thromboplastin time) and clinical chemistry (excluding brain, erythrocyte and serum cholinesterase activities) and urine analysis parameters were assessed in all animals at 3, 6 and 12 months. After 12 months of treatment, all dogs were sacrificed and examined grossly. Tissues (including gall bladder, but excluding femur with joint/glenoid surface and seminal vesicles) were examined microscopically and gross lesions were assessed in all animals affected per group. Organ weights (including parathyroid and thyroid glands, but excluding heart, spleen and thymus gland) were recorded.
Table 16. Observations in dogs given diets containing pyraclostrobin for 3 months
|
Parameter |
Dietary concentration (mg/kg of feed) |
|||||||
|
0 (control) |
100 |
200 |
450 |
|||||
|
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Haematology |
||||||||
|
Platelets (×109/l) |
||||||||
|
Day 41/43 |
290 |
264 |
265 |
313 |
294 |
293 |
310 |
389 |
|
Day 90 |
272 |
258 |
265 |
290 |
296 |
300 |
303 |
412** |
|
Clinical chemistry |
||||||||
|
Total protein (g/l) |
||||||||
|
Day 41/43 |
57.9 |
56.4 |
56.3 |
56.0 |
56.2 |
55.5 |
53.2 |
52.0 |
|
Day 90 |
59.3 |
58.2 |
58.0 |
57.8 |
57.6 |
58.1 |
53.5 |
53.2** |
|
Albumin (g/l) |
||||||||
|
Day 41/43 |
34.0 |
33.1 |
33.2 |
34.3 |
33.4 |
33.5 |
31.6 |
31.4 |
|
Day 90 |
31.6 |
30.9 |
30.9 |
31.1 |
30.6 |
30.8 |
29.1 |
28.7 |
|
Globulin (g/l) |
||||||||
|
Day 41/43 |
23.9 |
23.2 |
23.1 |
21.7 |
22.8 |
22.0 |
21.6 |
20.5 |
|
Day 90 |
27.6 |
27.3 |
27.1 |
26.7 |
26.9 |
27.3 |
24.4 |
24.5 |
|
Glucose (mmol/l) |
||||||||
|
Day 41/43 |
6.3 |
6.4 |
6.3 |
6.3 |
6.4 |
6.1** |
5.9 |
5.8** |
|
Day 90 |
6.0 |
6.2 |
6.0 |
6.0 |
6.0 |
5.8* |
5.8 |
5.3** |
|
Absolute organ weights |
||||||||
|
Body (g) |
12 980 |
12 540 |
13 900 |
12 860 |
12 580 |
12 300 |
12 640 |
10 480 |
|
Epididymides (g) |
3.79 |
— |
3.54 |
— |
3.44 |
— |
4.17 |
— |
|
Liver (g) |
388 |
361 |
418 |
382 |
386 |
320 |
361 |
286 |
|
Relative (to body) organ weightsa |
||||||||
|
Epididymides |
0.029 |
— |
0.025 |
— |
0.027 |
— |
0.033 |
— |
|
Liver |
2.99 |
2.83 |
3.01 |
2.98 |
3.08 |
2.60 |
2.86 |
2.72 |
|
Pathology |
||||||||
|
No. of animals examined |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
|
Gross findings |
||||||||
|
Duodenum, thickening of wall |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
2 |
|
Microscopic findings |
||||||||
|
Duodenum, mucosal hypertrophy |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
From Menges et al. (1999)
a Relative organ weight = organ weight (g)/body weight (g) × 100
* p < 0.05, ** p < 0.01 M, Male; F, Female
There were no effects on the incidence of premature mortality, ophthalmology or pathology findings, or on urine analysis parameters, and treatment-related effects were only seen at 400 mg/kg. In all or most animals, vomiting was seen in week 1, and diarrhoea was seen throughout the study. Slight body-weight losses of 1–2% occurred in both sexes until day 7. Thereafter, body-weight gain in males was greater by 53% than that in controls, while in females, body-weight gain was reduced by 48% throughout the entire study. In females, food consumption was reduced by 11%. Leukocyte counts (males) and platelet counts (both sexes) were increased (23–53%). Isolated reductions in erythrocyte volume fraction and haemoglobin concentration were recorded in males at day 180 and females at day 90, but these were concluded to be incidental to treatment owing to the absence of similar findings at other observation times. Concentrations of total protein, albumin, globulin and cholesterol were reduced (5–35%) in both sexes.
Absolute liver weight was reduced by 18% in females, but relative liver (to body) weights were comparable to those of the controls, and no histological alterations were observed. It was therefore concluded that the lower liver weight was secondary to the reduced weight gains in these animals, rather than to a direct toxicological effect on the liver. Although absolute kidney weights were increased by 16–21% in males at all doses, the range of kidney weights for treated animals (54–76 g) was within the range for historical controls from 21 feeding studies (52–76 g); for the control animals, the range was 46–58 g with the weight in one animal being only 46 g, which is below the range for historical controls. On this basis, and in the absence of a dose–response relationship, effects on biochemical markers (creatinine and urea) and pathology findings, it was concluded that the apparent increases in kidney weights were incidental to treatment. The NOAEL was 200 mg/kg, equal to 5.4 mg/kg bw per day, on the basis of clinical signs of toxicity, reduced body-weight gains, and haematological and clinical biochemistry effects at 400 mg/kg (Schilling et al., 1999a).
Table 17. Principle observations in a 12-month study in dogs given diets containing pyraclostrobin
|
Parameter |
Dietary concentration (mg/kg of feed) |
|||||||
|
0 (control) |
100 |
200 |
400 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Haematology and clinical chemistry |
||||||||
|
White blood cells (×109/l) |
||||||||
|
Day 89/90 |
9.9 |
12.5 |
11.5 |
11.8 |
11.2 |
12.8 |
12.2 |
10.7 |
|
Day 180/181 |
9.0 |
10.7 |
10.3 |
10.9 |
9.6 |
10.3 |
12.8** |
11.7 |
|
Day 362/363 |
8.3 |
10.7 |
9.3* |
11.5 |
8.7 |
11.7 |
12.7** |
11.4 |
|
Platelets (×109/l) |
||||||||
|
Day 89/90 |
268 |
295 |
284 |
272 |
253 |
331 |
366** |
351 |
|
Day 180/181 |
283 |
297 |
290 |
306 |
266 |
335 |
348** |
386 |
|
Day 362/363 |
304 |
319 |
274 |
302 |
267 |
349 |
392** |
401 |
|
Total protein (g/l) |
||||||||
|
Day 89/90 |
58.3 |
58.0 |
59.0 |
58.6 |
56.9 |
57.1 |
52.6** |
51.4** |
|
Day 180/181 |
57.3 |
57.4 |
58.4 |
59.5 |
58.3 |
56.4 |
53.1** |
51.5** |
|
Day 362/363 |
61.8 |
62.0 |
61.6 |
63.3 |
62.0 |
59.4 |
53.5** |
55.4** |
|
Albumin (g/l) |
||||||||
|
Day 89/90 |
34.9 |
35.6 |
35.0 |
36.4 |
34.4 |
34.9 |
31.7** |
32.0 |
|
Day 180/181 |
30.2 |
30.5 |
30.3 |
31.0 |
30.6 |
30.2 |
27.9** |
27.7 |
|
Day 362/363 |
31.0 |
30.9 |
30.2 |
32.4 |
31.1 |
30.6 |
27.0** |
29.5 |
|
Globulin (g/l) |
||||||||
|
Day 89/90 |
23.4 |
22.5 |
24.1 |
22.1 |
22.4 |
22.2 |
20.9 |
19.4 |
|
Day 180/181 |
27.1 |
26.9 |
28.1 |
28.5 |
27.7 |
26.2 |
25.2 |
23.8** |
|
Day 362/363 |
30.8 |
31.1 |
31.5 |
30.9 |
30.9 |
28.8 |
26.6 |
25.9** |
|
Cholesterol (mmol/l) |
||||||||
|
Day 89/90 |
4.8 |
4.5 |
5.2 |
4.6 |
4.5 |
4.3 |
3.3** |
3.4 |
|
Day 180/181 |
4.3 |
4.8 |
4.8 |
5.5 |
4.4 |
4.1 |
3.2** |
3.1** |
|
Day 362/363 |
4.6 |
4.6 |
4.6 |
5.4 |
4.4 |
4.4 |
3.2** |
3.2** |
|
Organ weights |
||||||||
|
Body (g) |
12 960 |
12 680 |
12 661 |
13 480 |
13 360 |
13 060 |
13 101 |
11 140 |
|
Kidney (g) |
54.1 |
51.4 |
62.9* |
53.9 |
65.0* |
51.6 |
65.4* |
52.7 |
|
Liver (g) |
383 |
344 |
388 |
387 |
370 |
367 |
349 |
282 |
|
Relativea kidney weights |
0.42 |
0.41 |
0.50 |
0.40 |
0.49 |
0.40 |
0.50 |
0.48 |
|
Relativea liver weights |
2.99 |
2.73 |
3.06 |
2.87 |
2.76 |
2.82 |
2.66 |
2.55 |
From Schilling et al. (1999a)
a Relative organ weight = organ weight (g)/body weight (g) × 100
* p < 0.05; ** p < 0.02
Mice
Groups of 50 male and 50 female B6C3F1 mice (aged 47–51 days) were given diets containing pyraclostrobin (purity, 97.1%) at a concentration of 0, 10, 30, or 120 mg/kg of feed (equal to 0, 1.4, 4.1 and 17 mg/kg bw per day), and to females only at 180 mg/kg (equal to 33 mg/kg bw per day), for 18 months. Animals were individually housed in cages in controlled conditions and food and water were available ad libitum. Mice were checked at least once daily for mortality and signs of toxicity and a comprehensive clinical examination was performed once per week. Body weight, food consumption and food conversion efficiency were recorded/calculated weekly for the first 13 weeks and at 4-week intervals thereafter. Water consumption was checked daily. Blood was collected from non-fasted animals at 12 months and fasted animals at 18 months, blood smears were prepared and differential blood counts were assessed in animals in the control group, at 120 mg/kg (males only) and at 180 mg/kg (females only). At sacrifice, all animals were examined grossly, organ weights (excluding epididymides, heart, spleen and thymus gland) were recorded, and histology was performed on a comprehensive range of tissues (including gall bladder) from animals in the control group, at 120 mg/kg (males) and at 180 mg/kg (females). The thymus gland, lungs, liver, kidneys, stomach and duodenum were examined in animals at 10 and 30 mg/kg and in females at 120 mg/kg, and gross lesions were assessed in all animals affected. Mortality, clinical signs, food conversion efficiency and water consumption were unaffected by treatment. Body-weight gain was reduced (17–28%) in all treated groups over the study, but in both sexes at 10 and 30 mg/kg and in females at 120 mg/kg, the reductions in body-weight gain were largely caused by body-weight losses that occurred from day 399/427, which were associated with reduced food consumption (11–21%) in all treated animals from day 427. However, reductions in body-weight gain in males at 120 mg/kg and females at 180 mg/kg occurred from the end of the first week of treatment and were generally statistically significantly different from those in the control group throughout the study, and as such, only these effects were considered to be of toxicological significance.
Circulating monoblasts were observed in 15% of females at 180 mg/kg, but in no control animals. Differential blood counts were not assessed in females from the other treated groups. Absolute and relative liver weights were reduced in treated male groups, reflecting reduced weight gains and feed intake. Absolute liver weights were largely unaffected in females, but the relative weight was increased at 180 mg/kg; this is likely to reflect a treatment-related effect.
There were no treatment-related pathology or neoplastic findings. Although the limited range of investigations carried out at doses other than the control and highest dose for each sex would normally preclude identification of a NOAEL, given the absence of gross or histopathological findings at the highest dose, the NOAEL in this 18-month carcinogenicity study in mice was 30 mg/kg (4.1 mg/kg bw per day for males) on the basis of reduction of body weights in males at 120 mg/kg during the first year of the study. During the last 6 months of the study, food consumption, body weight and body-weight change were significantly reduced at all doses. However, these effects at the end of the treatment period, after the growth phase of the animals, were without any dose–response relationship and were not regarded as treatment-related at doses <120 mg/kg in males and 180 mg/kg in females. (Mellert et al., 1999g).
Table 18. Principal findings in an 18-month study in mice given diets containing pyraclostrobin
|
Organ weights |
Dietary concentration (mg/kg of feed) |
||||||||
|
0 (control) |
10 |
30 |
120 |
180 |
|||||
|
M |
F |
M |
F |
M |
F |
M |
F |
F |
|
|
Body (g) |
39 |
35 |
37** |
33* |
37** |
35 |
35** |
32** |
31** |
|
Liver (g) |
1.59 |
1.34 |
1.34** |
1.25 |
1.41* |
1.27 |
1.29** |
1.23 |
1.28 |
|
Liver, relativea to body, weight |
4.05 |
3.87 |
3.67** |
3.86 |
3.85 |
3.72 |
3.68 |
3.92 |
4.12** |
From Mellert et al. (1999g)
F, Female; M, Male
* p < 0.05, ** p < 0.02
a Relative organ weight = organ weight (g)/body weight (g) × 100
Rats
Groups of 20 male and female Wistar rats (aged 42 days) were given pyraclostrobin (purity, 97.1%) at a concentration of 0, 25, 75, or 200 mg/kg of feed (0, 1.1, 3.4 and 9 mg/kg bw per day for 24 months). Animals were individually housed in cages in controlled conditions and food and water were available ad libitum. Rats were checked at least once daily for mortality and signs of toxicity, and a comprehensive clinical examination was performed once weekly. Body weight, food consumption and food conversion efficiency were recorded or calculated weekly for the first 13 weeks and at 4-week intervals thereafter. An ophthalmological examination was performed at the end of the study. Haematology, clinical chemistry (excluding brain and erythrocyte cholinesterase activities), and urine analysis parameters were assessed at approximately 3, 6, 12, 18 and 24 months. After sacrifice, animals were examined for gross pathology, a full range of tissues was examined histologically, gross lesions were assessed in all animals affected per group and organ weights (excluding epididymides, heart, spleen and thymus gland) were recorded.
Treatment did not affect mortality, clinical signs, food consumption, food conversion efficiency, haematology or ophthalmology. Over approximately the first 18 months of the study, body-weight gain was reduced by 11–14% in males and females at 200 mg/kg. Alanine aminotransferase in males and alkaline phosphatase in both sexes were slightly but significantly reduced at 200 mg/kg. Although these effects were clearly treatment-related, and consistent with effects seen in the 28-day and 3-month studies in rats, given the direction and small magnitude of the changes it was concluded that they were of minimal toxicological significance. This conclusion was further supported by studies indicating that alanine aminotransferase (and alkaline phosphatase) activities can be affected by dietary status, as discussed later in this monograph. Transient variations in aspartate aminotransferase and serum cholinesterase activities in treated groups were not considered to be toxicologically significant. Concentrations of protein in the urine were slightly increased in treated rats, particularly males, but this was not associated with histopathological changes and has not been observed in other studies in rats. Consequently, the finding was discounted as being incidental to treatment. In males at 200 mg/kg, slight increases in absolute testes and adrenal gland weights were not considered to be toxicologically significant, because the effects were influenced by the organ weight values of only one to two animals. Tubular degeneration of the testes occurred in one out of 20, seven out of 20, seven out of 20 and six out of 20 males in the control group, at 25 mg/kg, 75 mg/kg and 200 mg/kg, respectively. Given the absence of a dose–response relationship or of similar effects in other studies in rats, including another 2-year study and a two-generation study of reproductive toxicity, reported below, it was concluded that this finding was incidental to treatment. There were no treatment-related neoplastic findings. The NOAEL was 75 mg/kg (3.4 mg/kg bw per day) on the basis of reduced body-weight gains at 200 mg/kg (Mellert et al., 1999e).
Table 19. Principal findings in a 2-year study in rats given diets containing pyraclostrobin
|
Clinical chemistry parameter |
Dietary concentration (mg/kg of feed) |
|||||||
|
0 (control) |
25 |
75 |
200 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
ALT (µkat/l) |
1.08 |
1.12 |
1.05 |
1.00 |
1.07 |
1.00 |
0.93** |
0.94 |
|
ALP (µkat/l) |
4.72 |
3.72 |
5.62* |
3.85 |
5.28* |
3.62 |
4.28* |
2.91** |
From Mellert et al. (1999e)
ALT, Alanine aminotransferase; ALP, Alkaline phosphatase
* p < 0.05; ** p < 0.02; *** p < 0.002
Groups of 50 male and female Wistar rats (aged 42 days) were given diets containing pyraclostrobin (purity, 97.1%) at a concentration of 0, 25, 75, or 200 mg/kg of feed (equal to 0, 1.2, 3.4 and 9 mg/kg bw per day) for 24 months. Animals were individually housed in cages in controlled conditions, and food and water were available ad libitum. Mortality and signs of toxicity were checked at least once daily, a comprehensive clinical examination was performed once weekly, and body weight, food consumption and food conversion efficiency were recorded/calculated weekly for the first 13 weeks and at 4-week intervals thereafter. Blood was collected from fasted animals at 24 months and differential blood counts were assessed in the control group and at 200 mg/kg. After 24 months of treatment, all rats were fasted, sacrificed and necropsied. All animals were examined for gross and histopathology, gross lesions were assessed in all animals affected per group and organ weights (excluding epididymides, heart, spleen and thymus gland) were recorded.
Treatment did not affect the nature or incidence of clinical signs or values for haematology parameters. Treatment-related effects were observed only at 200 mg/kg. Mortality was increased in males only, with 32 out of 50 deaths at 200 mg/kg compared with 22 out of 50 in controls. Body-weight gain was reduced by 10% in males over the first 18 months and by 22% in females over the entire study, but food consumption was only slightly reduced, by approximately 4%, in females up to day 91. In females, absolute liver weight was reduced (10%), but relative liver weight remained comparable to that of controls and no histological alterations were noted in female liver. It was therefore concluded that the decreased absolute liver weight was secondary to reduced weight gain, rather than a direct toxicological effect. The incidences of liver necrosis and liver adenomas were increased in males, but the incidence of liver carcinomas was unaffected. In the long-term study in rats, reported above, which was conducted concurrently with this study of carcinogenicity, the incidence of liver adenomas was lower in treated groups than in the controls (males: four, two, two, one; females: one, 0, 0, one; n = 20 for each group), liver carcinomas did not occur in a dose-related manner, as was also the case in the previous study (males: 0, two, two, 0; females: 0 for all groups; n = 20 for each group), and the incidence of tumours overall was similar in all groups. On this basis, undue weight was not attached to the apparent increase in liver adenomas in males at 200 mg/kg. Erosion and ulcers in the glandular stomach were increased in males. The NOAEL was 75 mg/kg (3.4 mg/kg bw per day) on the basis of reduced body-weight gain in both sexes, and altered liver and stomach histology in males at 200 mg/kg (Mellert et al., 1999f).
Table 20. Principal findings in a 2-year study of carcinogenicity in rats
|
Parameter/finding |
Dietary concentration (mg/kg of feed) |
|||||||
|
0 (control) |
25 |
75 |
200 |
|||||
|
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Organ weights |
||||||||
|
Body (g) |
658 |
394 |
656 |
379 |
668 |
368 |
628 |
339** |
|
Liver weight (g) |
19.8 |
12.9 |
19.8 |
12.7 |
19.1 |
12.5 |
19.2 |
11.5** |
|
Liver relativea (to body) weight |
3.02 |
3.28 |
3.05 |
3.36 |
2.87 |
3.40 |
3.05 |
3.41 |
|
Pathology |
||||||||
|
No. of animals examined |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
|
Liver |
||||||||
|
Necrosis |
1 |
3 |
2 |
4 |
2 |
2 |
10 |
3 |
|
Adenoma |
4 |
3 |
7 |
3 |
5 |
0 |
11 |
5 |
|
Carcinoma |
4 |
0 |
3 |
0 |
5 |
0 |
3 |
0 |
|
Stomach |
||||||||
|
Erosion |
2 |
3 |
5 |
3 |
7 |
4 |
10 |
3 |
|
Ulcers |
2 |
1 |
2 |
2 |
2 |
1 |
7 |
1 |
From Mellert et al. (1999f)
F, Female; M, Male
a Relative organ weight = organ weight (g)/body weight (g) × 100
** p < 0.01
Table 21. Results of studies of genotoxicity with pyraclostrobin
|
End-point |
Test object |
Concentration or dose (solvent/vehicle) |
Results |
Reference |
|
In vitro |
||||
|
Reverse mutation |
S. typhimurium |
20–5000 µg/plate, ±S9 |
Negative |
Engelhardt & Hoffman (1997) |
|
Forward mutation |
Chinese hamster ovary cells, Hgprt locus |
0.625–20 µg/ml, ±S9 |
Negative |
Engelhardt & Hoffman (1998a) |
|
Chromosomal aberration |
Chinese hamster V79 cells |
6.25–25 µg/ml, ±S9 |
Negative |
Engelhardt & Hoffman (1999) |
|
Unscheduled DNA synthesis |
Rat hepatocytes |
0.01–0.5 µg/ml |
Negative Negative |
Engelhardt & Hoffman (1998b), |
|
In vivo |
||||
|
Micronucleus formation |
Mouse bone-marrow cells |
75–300 mg/kg bw (olive oil) |
Negative |
Engelhardt & Hoffman (1998c) |
S9, 9000 × g supernatant fraction of rodent liver
Pyraclostrobin (purity, 98.2%) was evaluated for potential genotoxicity in vitro in tests for mutagenicity in bacterial and mammalian cells, for chromosome damage (clastogenicity) and for unscheduled DNA synthesis. The results of these studies demonstrated the absence of a genotoxic effect. In vivo, the test substance was assessed for the induction of micronucleus formation in mice. The result of this study showed that pyraclostrobin does not exhibit a chromosome-damaging potential. It was therefore concluded that pyraclostrobin has no mutagenic or genotoxic properties either in vitro or in vivo.
Rats
Groups of 25 male and female Wistar rats (aged 35 days) were given diets containing pyraclostrobin (purity, 98.7%) at a concentration of 0, 25, 75, or 300 mg/kg of feed (equal to 0, 2.7, 8.2 and 33 mg/kg bw per day) over two generations. Treatment was initiated at least 74 days before the mating of F0 rats (1 : 1) and continued through two generations. Females were allowed to litter their pups, and litters were culled on postnatal day 4 to eight pups/litter (ideally, four males and four females), where possible. Pups were weaned on postnatal day 21, after which F0 parental animals were sacrificed. Twenty-five F1 rats of each sex per group were selected (each litter was taken into account) for mating of the adult F1 generation, following the same protocol as described above for the F0 mating. Reproductive performance was assessed using the following parameters: male mating index, male fertility index, female mating index, female fertility index, gestation index, live birth index, and postimplantation loss. All pups in the F1 and F2 litters were examined as soon as possible after birth to determine the number of live and stillborn pups in each litter. Pups were examined daily for clinical signs. The number of live pups/litter was calculated on postnatal days 4, 7, 14 and 21. The sex ratio was calculated on postnatal days 0 and 21. Pup body weights were recorded on postnatal days 1, 4, 7, 14 and 21. During the study, rats were individually housed in cages in controlled conditions and food and water were available ad libitum. In F0 and F1 parental animals, body weight and food consumption were recorded weekly and throughout gestation (days 0, 7, 14 and 20) and lactation (1, 4, 7, 14 and/or 21). Estrous cycle (length/normality) was assessed daily in F0 and F1 female parental rats for a minimum of 3 weeks before mating and was continued until females exhibited evidence of mating. At necropsy, a vaginal smear was taken to determine the stage of the estrous cycle for each F0 and F1 female. The weights of the right testis and cauda epididymis from F0 and F1 males of all groups were recorded and the following sperm parameters were determined: motility, morphology, and head count (cauda epididymis and testis).
All F0 and F1 parental animals were examined macroscopically. Organs (standard test parameters plus pituitary and prostate glands, seminal vesicles with coagulating glands and fluids and uterus with cervix uteri and oviducts, but excluding the heart) were weighed, and the kidneys from rats in all groups were examined microscopically. The vagina, cervix uteri, uterus, ovaries, oviducts, pituitary gland, left testis and epididymis, prostate gland, seminal vesicles, coagulating glands, liver, thymus gland and adrenal glands were assessed in all control and 300 mg/kg animals and in all animals at 25 and 75 mg/kg suspected of impaired fertility. A quantitative assessment of primordial, growing and antral follicles in the ovaries was performed for all F0 and F1 parental animals in the control group and at the highest dose. Gross lesions were assessed in all animals affected per group. F1 and F2 pups were sexed and weighed on the day after birth and on postnatal days 4, 7, 14 and 21. Pup survival was also recorded. At necropsy, all pups were examined macroscopically, and brain, spleen and thymus gland weights were recorded in one pup of each sex per litter. Sexual maturation (day of preputial separation/vaginal opening) was assessed in all pups selected for the F1 parental generation.
In the F0 generation, one female at 300 mg/kg died on the first day of lactation, but there were no mortalities in the F1 generation. There were no treatment-related clinical signs in either generation. At 300 mg/kg, slight reductions in food consumption (up to 12% in both generations during pre-mating and F1 animals during gestation) and body-weight gain (up to 50% in F0 animals during pre-mating and lactation) occurred in adult animals at times during the respective observation periods. In both generations, there were no effects on fertility, gestation, parturition or pup survival. Body-weight gain in F1 and F2 pups was reduced (12–15%) at 300 mg/kg between birth and postnatal day 21. There were no treatment-related effects on organ weights in adult rats. Decreases in absolute thymus gland and spleen weights (13–20%) and increases in relative brain weight (12–15%) in F1 and F2 pups at 300 mg/kg were likely to be related to reductions in body-weight gain observed in these animals (14–16%) and were not considered to be toxicologically significant.
There were no treatment-related pathology findings in adult F0 and F1 rats, including estrous cycles, sperm morphology and ovarian parameters. In F1 pups at 300 mg/kg, there was a slight delay in vaginal opening (31.7, 32.1, 32.4, 33.3 days, control group to highest dose, respectively), which was statistically significant at 300 mg/kg, but this parameter was not assessed in F2 pups. For each treatment group, and the controls, the day of vaginal opening ranged between day 29/30 and day 37. The principle difference in the pups at the highest dose was a reduction in the number of animals showing early vaginal opening (by day 29–31), rather than an increase in the number of animals with late vaginal opening (day 36/37). The birth weight of female pups at 300 mg/kg was unaffected in both generations, but a significantly lower body-weight gain was observed thereafter. Consequently, the slightly delayed vaginal opening was likely to reflect the delayed maturation of these pups rather than an in utero effect on sexual development. As some pyraclostrobin may have been excreted in maternal milk, pups begin to eat small amounts of the maternal ration well before weaning, and as the lower body weights developed only some days post partum, the effect was likely to reflect toxicity resulting from exposure post partum, and was not therefore considered to be a reproductive effect per se. An apparent increase in the incidence of sloped incisors in F1 pups (on a pup and litter basis) at 75 and 300 mg/kg was not considered to be toxicologically significant since the effect was slight in nature, and values in the 2 pups were similar to those in the control group for that generation.
The NOAEL for general and pup effects was 75 mg/kg (8.2 mg/kg bw per day) on the basis of reduced body-weight gain and food consumption in adults, reduced body-weight gain in pups, and a slight delay in vaginal opening in F1 pups at 300 mg/kg. The NOAEL for reproductive effects was 300 mg/kg (33 mg/kg bw per day), the highest dose tested (Schilling et al., 1999b).
Groups of 25 female Wistar rats (aged 11–12 weeks) were given pyraclostrobin (purity, 98.9%) at a concentration of 0, 10, 25, or 50 mg/kg bw per day by gavage as a suspension in 0.5% aqueous Tylose solution on days 6–19 of gestation. Rats were housed in cages in controlled conditions and food and water were available ad libitum. All rats were checked at least once daily for mortality and clinical signs of toxicity and body weight and food consumption were recorded every 2–3 days until day 20 of gestation. After the final sacrifice, a corrected body-weight gain was calculated. On day 20 of gestation, all rats were sacrificed and examined macroscopically. The uterus and ovaries were removed and the weight of the "unopened" uterus, the number of corpora lutea and the number and distribution of implantation sites (classified as live fetuses or "dead implantations") were recorded. Postimplantation losses were further classified as early or late resorptions or dead fetuses. Conception rate, pre- and postimplantation loss were recorded. At necropsy, each fetus was weighed, sexed and examined macroscopically for external findings. The condition of the placentae, the umbilical cords, the fetal membranes and fluids were examined and individual placental weights were recorded. Approximately half of the fetuses from each litter were examined for visceral abnormalities, whilst the remainder were examined for skeletal abnormalities.
Table 22. Reproductive performance and pup survival in a two-generation study in rats given diets containing pyraclostrobin
|
Dietary concentration (mg/kg of feed) |
||||
|
0 (control) |
25 |
75 |
300 |
|
|
F0 generation |
||||
|
Mean live pups/litter |
14.2 |
13.4 |
12.3 |
12.9 |
|
Pup survival index (days 0–4) |
93 |
87 |
100 |
93 |
|
Mean pup body weight (g) |
||||
|
Males |
||||
|
Postnatal day 1 |
6.6 |
6.5 |
6.7 |
6.8 |
|
Postnatal day 7 |
15.2 |
15.0 |
15.5 |
14.9 |
|
Postnatal day 21 |
52.8 |
53.7 |
52.8 |
47.4** |
|
Females |
||||
|
Postnatal day 1 |
6.2 |
6.2 |
6.5 |
6.4 |
|
Postnatal day 7 |
14.7 |
14.5 |
15.0 |
14.3 |
|
Postnatal day 21 |
51.4 |
51.3 |
50.3 |
45.2** |
|
F1 generation |
||||
|
Mean No. of live pups delivered |
98 |
99 |
99 |
99 |
|
Mean pup body weight (g) |
||||
|
Males |
||||
|
Postnatal day 1 |
6.6 |
6.6 |
6.7 |
6.5 |
|
Postnatal day 7 |
15.2 |
14.9 |
14.7 |
13.5** |
|
Postnatal day 21 |
52.0 |
52.6 |
51.4 |
45.0** |
|
Females |
||||
|
Postnatal day 1 |
6.2 |
6.3 |
6.2 |
6.2 |
|
Postnatal day 7 |
14.5 |
14.6 |
14.2 |
13.1** |
|
Postnatal day 21 |
49.8 |
50.2 |
48.9 |
43.5** |
From Schilling et al. (1999b)
* p < 0.05; ** p < 0.01
*** 1 dam died on the 1st day of lactation
Table 23. Organ weights and pathology findings for pups in a two-generation study in rats
|
Dietary concentration (mg/kg of feed) |
||||||||
|
Control |
25 |
75 |
300 |
|||||
|
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Organ weights |
||||||||
|
F1 pups: |
||||||||
|
Brain (g) |
1.50 |
1.44 |
1.51 |
1.44 |
1.49 |
1.46 |
1.47 |
1.42 |
|
Spleen (g) |
0.26 |
0.24 |
0.26 |
0.24 |
0.25 |
0.24 |
0.21** |
0.21* |
|
Thymus gland (g) |
0.19 |
0.19 |
0.18 |
0.18 |
0.17* |
0.18 |
0.16** |
0.16** |
|
Relative brain weight |
2.73 |
2.85 |
2.79 |
2.88 |
2.85* |
2.87 |
3.14** |
3.14** |
|
Relative spleen weight |
0.46 |
0.47 |
0.47 |
0.47 |
0.47 |
0.47 |
0.45 |
0.46 |
|
Relative thymus gland weight |
0.35 |
0.38 |
0.34 |
0.35 |
0.33 |
0.36 |
0.33 |
0.35 |
|
F2 pups: |
||||||||
|
Brain (g) |
1.50 |
1.45 |
1.49 |
1.44 |
1.48 |
1.43 |
1.44** |
1.39** |
|
Spleen (g) |
0.23 |
0.23 |
0.24 |
0.24 |
0.24 |
0.23 |
0.20 |
0.18** |
|
Thymus gland (g) |
0.17 |
0.18 |
0.18 |
0.18 |
0.17 |
0.17 |
0.14** |
0.15** |
|
Relative brain weight |
2.88 |
2.92 |
2.84 |
2.85 |
2.88 |
2.91 |
3.20** |
3.26** |
|
Relative spleen weight |
0.44 |
0.46 |
0.45 |
0.47 |
0.46 |
0.47 |
0.45 |
0.43 |
|
Relative thymus gland weight |
0.33 |
0.35 |
0.33 |
0.36 |
0.33 |
0.35 |
0.31 |
0.35 |
|
Pathology |
||||||||
|
F1 pups sloped incisors, Pups/litters |
1/1 |
2/1 |
5/5 |
6/4 |
||||
|
F2 pups sloped incisors, Pups/litters |
3/3 |
5/4 |
4/4 |
7/5 |
||||
From Schilling et al. (1999b)
F, Female; M, Male
* p < 0.05; ** p < 0.01
Table 24. Maternal findings in a study of developmental toxicity in rats given pyraclostrobin by gavage
|
Parameter |
Dose (mg/kg bw per day) |
|||
|
0 |
10 |
25 |
50 |
|
|
Food consumption (g/animal per day), days 6–8 |
24.6 |
23.6 |
21.2** |
17.9** |
|
Body weight (g), day 20 |
369 |
373 |
354 |
350* |
|
Body weight change (g), days 6–19 |
104 |
107 |
96 |
88** |
|
Carcass weight (g) |
290 |
290 |
277 |
272** |
|
Corrected body-weight gain (g), from day 6 (g) |
40.7 |
40.9 |
31.9* |
22.3** |
From Schilling et al. (1999c)
Dunnett t-test * p < 0.05; ** p < 0.01
Table 25. Summary of findings on litters and fetal parameters in a study of developmental toxicity in rats given pyraclostrobin by gavage
|
Finding |
Dose (mg/kg bw per day) |
||||
|
0 (control) |
10 |
25 |
50 |
Range for historical controls |
|
|
Fetuses/litters examined |
148/22 |
140/20 |
136/21 |
165/24 |
|
|
Dilated renal pelvis |
Mean, 16.7 |
||||
|
Incidence |
8 (6) |
16 (10) |
20 (9) |
31 (15*) |
|
|
% |
5.4 (27) |
11 (50) |
15 (43) |
19 (63) |
8.8–28.8 |
|
Fetuses/litters examined |
158/22 |
151/20 |
147/21 |
178/25 |
|
|
Incomplete ossification of sternebra |
Mean, 24.6% |
||||
|
Incidence |
29 (15) |
39 (14) |
43 (16) |
61 (22) |
|
|
% |
18 (68) |
26 (70) |
29 (76) |
34 (88) |
0–34.5% |
|
Rudimentary cervical ribs |
Mean, 2% |
||||
|
Incidence |
1 (1) |
2 (2) |
2 (2) |
9 (8*) |
|
|
% |
0.6 (4.5) |
1.3(10) |
1.4 (9.5) |
5.1 (32) |
0.5–6.6% |
From Schilling et al. (1999c)
Number of affected litters is shown in parentheses; * p < 0.05
There were no deaths, no treatment-related clinical signs, no effects on litter or fetal parameters, and pathology in adults was unaffected. Food consumption was consistently and significantly reduced at 25 and 50 mg/kg bw per day from immediately after initiation of dosing until sacrifice, and the magnitude of the effect was dose-related for all except day 13 when the values were essentially the same for these two groups. At 50 mg/kg bw per day, body-weight gain was reduced by 16% and food consumption by 11% between gestation days 6 and 19. Corrected body-weight gain (i.e. minus the gravid uterus) was reduced by 22 and 45% at 25 and 50 mg/kg bw per day (40.7, 40.9, 31.9, 22.3 g, control group to highest dose, respectively) and carcass weights by 6% at 50 mg/kg bw per day. Gravid uterus weights were unaffected by treatment.
The incidences of dilated renal pelves, incomplete ossification of sternebra and rudimentary cervical ribs were increased on a fetal and litter basis at 50 mg/kg bw per day. Although the incidence of these effects were within the range of historical control data, they are concluded to be related to treatment because there was a clear dose–response relationship and/or they were at the upper limit of the range for historical controls. The NOAEL for maternal effects was 10 mg/kg bw per day on the basis of reduced body-weight gains and food consumption at 25 mg/kg bw per day. The NOAEL for developmental effects was 25 mg/kg bw per day on the basis of an increased incidence of dilated renal pelves, incomplete ossification of sternebra and rudimentary cervical ribs at 50 mg/kg bw per day (Schilling et al., 1999c).
Rabbits
Groups of 24 or 25 pregnant Himalayan rabbits (aged 24–29 weeks) were given pyraclostrobin (purity, 98.9%) at a concentration of 0, 5, 10 and 20 mg/kg bw per day as a suspension in 0.5% aqueous Tylose solution by gavage on days 7–28 of gestation. Rabbits were housed in cages in controlled conditions and food and water were available ad libitum. All rabbits were checked at least once daily for mortality and clinical signs of toxicity; body weight was recorded every 2–3 days during gestation and food consumption was recorded daily. After the final sacrifice, a corrected body-weight gain was calculated. On day 29 of gestation, all surviving rabbits were sacrificed and examined macroscopically. The uterus and ovaries were removed and the weight of the unopened uterus, the number of corpora lutea and the number and distribution of implantation sites (classified as live fetuses or dead implantations) were recorded. Dead implantations were further classified as early or late resorptions or dead fetuses. Conception rate, pre-implantation and postimplantation losses were recorded. At necropsy, each fetus was weighed and examined macroscopically for external findings. The condition of the placentae, the umbilical cords, the fetal membranes and fluids were examined and individual placental weights were recorded. The sex of the fetuses was determined by internal examination of the gonads. Approximately half of the fetuses from each litter were examined for visceral abnormalities, whilst the remainder were examined for skeletal abnormalities.
One control and one dam at 10 mg/kg bw per day died during or shortly after dosing on days 9 and 25 of gestation, respectively. At 10 and 20 mg/kg bw per day, blood was observed in the bedding of two and four does respectively between days 16 and 29 of gestation and was probably secondary to the increased resorption rate seen at these doses. A marked, clearly dose-related, but transient reduction in food consumption (by 31–77%) and body-weight gain (by 13–25%) was observed immediately after initiating dosing. Both food consumption and weight gain subsequently tracked rapidly towards control levels and were equal to those of the control group by day 4 after dosing for the groups at 5 and 10 mg/kg bw per day and by about day 5 for the group at 10 mg/kg bw per day. Despite continued treatment for a further 17 days, both food consumption and weight gain were comparable to that in the control group throughout the remainder of the study. This pattern of weight gain and food consumption variation is consistent with a transient food aversion related to local gastrointestinal tract and/or sensory disturbance caused by pyraclostrobin administered by gavage. Whilst this effect is clearly treatment- and dose-related, it is likely to be dependent on the local concentration of the test material administered, has little relevance to human dietary risk assessment and was consequently disregarded by the present reviewer for the purpose of considering an acute RfD; however, this does prevent the identification of a NOAEL for maternal toxicity in this study. The marked reduction in food intake is likely to have reduced the nutritional status of the does at a critical point in pregnancy, around the time of implantation, and may have contributed to the increased postimplantation loss observed at 10 and 20 mg/kg bw per day and possibly also the increased incidence of missing lumbar vertebrae. This conclusion is supported to some extent by studies showing similar effects on the fetuses of rabbits and rats following feed restriction, although the duration of the feed restriction was longer than that observed in this study. A 25% feed restriction in rabbits, for example, significantly reduced the number of fetuses per pregnant female (Warren & Kirkpatrick, 1978). In pregnant rabbits fed between 15 and 50% of the control dietary intake from day 6 to day 20 of gestation, fetal weights were reduced and the incidence of cleft palate and fused sternebrae were markedly increased at 15% of the maternal diet and to a lesser extent at 25% of the maternal diet (Noda et al., 1993). Similarly, in rats fed <80% of the control dietary intake throughout gestation, an increased number of resorptions and small fetuses, decreased fetus weights and retarded bone development were observed (Waalkens-Berendsen et al., 1990).
Defaecation was reduced in one and 10 does between days 10 and 14 of gestation at 10 and 20 mg/kg bw per day respectively and is likely to be secondary to reduced food intake. At termination, net maternal carcass weights (body weight minus the conceptus) of treated dams were comparable to those of controls (2608, 2504, 2580, 2539 g, control group to highest dose, respectively) and net body-weight gains/losses were also similar across groups (-136, -142, -133, -147). There were no fetal external or visceral abnormalities attributable to treatment and no treatment-related pathology findings in adults. A slight, non significant, increase in the incidence of skeletal malformations at 20 mg/kg bw per day was driven by an increase in the incidence of absent lumbar vertebrae at this dose (fetal incidence of 0.6, 0.7, 0.8 and 3.7%, control group to highest dose, respectively) which exceeded the mean (0.3%) and range (0–0.9) for historical controls and is therefore concluded to be treatment-related despite the lack of statistical significance. At 10 and 20 mg/kg bw per day, two and three does, respectively, had no live fetuses. At 10 and 20 mg/kg bw per day, total resorptions were increased in comparison to control (6.2, 10.2, 17.8, 38.6% control group to highest dose) primarily owing to increased early resorptions. Although the postimplantation loss that occurred at 10 mg/kg bw per day was within the range of data for historical controls (range, 5.2–20.1%; mean, 9.9%), it was concluded to be treatment-related both by the study author and the present reviewer on the basis of a comparison with the concurrent control group, association with total litter losses and blood in the bedding, and on the clear dose-dependency. Gravid uterus weight was reduced by 23 and 41% at 10 and 20 mg/kg bw per day, as was fetal weight. A NOAEL for maternal toxicity was not identified owing to a marked decrease in weight gain and food consumption at all doses. The NOAEL for fetal effects was 5 mg/kg bw per day on the basis of increased postimplantation losses and reduced fetal weight at >10 mg/kg bw per day. For the reasons discussed, undue weight was not attached to the absence of a lowest-observed-effect level (LOEL) for maternal toxicity in the presence of fetal resorptions, other than the markedly reduced body-weight gain, owing to the conclusion that the nutritional status of the does was likely to have been compromised by the marked transient reduction in food intake (Schilling et al., 1999d).
Groups of 25 presumed pregnant Himalayan rabbits (Chbb : HM,; weight, approximately 2570 g; from Boehringer Ingelheim Pharma KG, Germany) were given pyraclostrobin at a dose of 0, 1, 3, or 5 mg/kg bw per day by gavage on days 7–28 of gestation. The animals were observed twice daily for mortalities and once per day for clinical signs of toxicity. Body weights were measured every 2–3 days and food consumption was measured daily. The animals were killed on day 29 of gestation and the number of corpora lutea, implantations, early and late resorptions, and viable and non-viable fetuses were evaluated. At necropsy of dams, gross pathological examination was conducted and the gravid uterus, fetuses and placenta were weighed. Carcass weight (terminal body weight minus gravid uterus weight) and corrected body-weight gain (carcass weight minus day-7 body weight) were calculated.
Table 26. Maternal findings in a study of developmental toxicity in rabbits
|
Parameter |
Dose (mg/kg bw per day) |
|||
|
0 (control) |
5 |
10 |
20 |
|
|
Food consumption; days 7–8 (g/animal/day) |
98.1 |
35.7** |
20.4** |
10.5** |
|
Food consumption; days 10–11 (g/animal/day) |
96.4 |
90.4 |
74.8** |
35.1** |
|
Food consumption; days 13–14 (g/animal/day) |
90.9 |
84.0 |
87.6 |
73.2* |
|
Food consumption; days 14–15 (g/animal/day) |
86.9 |
80.2 |
83.5 |
79.2 |
|
Body weight; day 29 (g) |
2961 |
2807 |
2851 |
2748** |
|
Body-weight change; days 7–9 (g) |
-3.8 |
-43.8** |
-85.5** |
-146.3** |
|
Body-weight change; days 9–11 (g) |
0.2 |
14.8 |
21.6 |
-24.2 |
|
Body-weight change; days 11–14 (g) |
23.4 |
15.7 |
25.8 |
-46.8* |
|
Body-weight change; days 14–16 (g) |
52.1 |
44.5 |
42.7 |
54.2 |
|
Gravid uterus (g) |
352.6 |
302.6 |
271.2* |
209.6** |
|
Corrected body-weight gain; from day 7 (g) |
-135.7 |
-142.4 |
-132.9 |
-146.8 |
From Schilling et al. (1999d) Dunnett t-test
* p < 0.05; ** p < 0.01
Table 27. Summary of litter and fetal parameters in a study of developmental toxicity in rabbits
|
Parameter |
Dose (mg/kg bw per day) |
|||
|
0 (control) |
5 |
10 |
20 |
|
|
No. of does with live young |
24 |
24 |
20 |
22 |
|
Mean No. of live young |
6.9 |
6.0 |
6.2 |
4.9** |
|
Mean No. of corpora lutea |
8.0 |
7.9 |
7.8 |
7.7 |
|
Mean No. of implantations |
7.4 |
6.6 |
6.9 |
7.0 |
|
Implantation loss (%) |
||||
|
Pre-implantation |
7.0 |
15.7 |
10.6 |
10.1 |
|
Postimplantation |
6.2 |
10.2 |
17.8 |
38.6** |
|
Mean no. of resorptions |
||||
|
Early |
0.42 |
0.54 |
1.2 |
2.6** |
|
Late |
0.04 |
0.04 |
0.1 |
0.1 |
|
Early and late |
0.5 |
0.6 |
1.3 |
2.7** |
|
Placental weight (g) |
4.5 |
4.3 |
4.1 |
4.2 |
|
Fetal body weight (g) |
37.0 |
37.0 |
35.2 |
35.1 |
|
Sex ratio (% males) |
50.0 |
53.1 |
52.8 |
52.3 |
|
Gravid uterus (g) |
352.6 |
302.6 |
271.2* |
209.6** |
|
Incidence of malformations and variations |
||||
|
Malformations (% of fetuses) |
||||
|
External |
0.6 |
0.7 |
1.6 |
0 |
|
Visceral |
0.6 |
2.1 |
4.9 |
0.9 |
|
Skeletal |
3.6 |
2.8 |
4.1 |
8.4 |
|
Variations (% of fetuses) |
||||
|
External |
0.6 |
0.7 |
1.6 |
0 |
|
Visceral |
11 |
2.8 |
9.8 |
6.5 |
|
Skeletal |
64 |
62 |
67 |
66 |
From Schilling et al. (1999d)
* p < 0.05; ** p < 0.01
There were no deaths or clinical signs of toxicity in any of the treated dams. There were no effects of treatment on implantation, postimplantation loss or the number of live fetuses, no effects on gravid uterus, placenta or fetal weights and carcass weight was similar in all groups. Food consumption was reduced immediately after initiation of dosing at 3 (25%) and 5 mg/kg bw per day (40%), but it recovered during the remainder of the dosing period. The transient reduction in food consumption observed just after dosing resulted in food consumption being 15% and 20% lower across the entire dosing period at 3 and 5 mg/kg bw per day respectively, but the changes were not statistically significant. Body-weight loss was observed in all groups, including the control group before dosing began. Body-weight loss (between about 0.1 and 0.5% of body weight) also occurred immediately after dosing at 3 and 5 mg/kg bw per day, but body-weight gain had recovered to be similar in all groups before the day 4 of dosing and generally remained similar in all groups until the end of the dosing period. When results from days 7–28 were considered together, body-weight gains showed no effect of treatment at doses <5 mg/kg bw per day. Although there was an apparent dose-related decrease in mean corrected body-weight gains (-83, -103, 109, -137 g, control group to highest dose, respectively), there was considerable individual variation in all groups, with the standard deviation varying between 90 and 113 and therefore no statistically significant change was observed. The NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of reduced food consumption and body weight gain at 5 mg/kg bw per day. There were no effects on the fetuses that were considered to be treatment-related and therefore the NOAEL for developmental toxicity was 5 mg/kg bw per day, the highest dose tested (Schilling, Hellwig & van Ravenzwaay, 2001).
The neurotoxicity of a single dose of pyraclostrobin (purity, 99%) at 0, 100, 300 and 1000 mg/kg bw administered by gavage in 0.5% aqueous carboxymethylcellulose was assessed in groups of 10 male and 10 female Wistar rats (aged 49 days). Dose selection was based on the results of a range-finding study in which three rats of each sex were given pyraclostrobin at a dose of 1000 and 2000 mg/kg bw per day by gavage (clinical examinations were performed outside the home cage immediately after dosing as well as at 0.25, 0.5, 1, 2, 3, 4, 5 and 6 h and 1, 2 and 3 days after dosing. Animals were individually housed in cages in controlled conditions and food and water were available ad libitum. Clinical signs of toxicity were assessed at least once daily and body weight was assessed weekly. Functional observational batteries (FOB) and motor activity were assessed 7 days before dosing, and on days 0 (within a few hours after dosing), 7 and 14. The FOB consisted of four parts, starting with passive observations (without disturbing the animals), followed by removing the animal from its home cage and "open field observations" in a standard arena. Two weeks after dosing, five rats of each sex per dose were sacrificed by "in situ perfusion fixation" and were subjected to neuropathology examinations, including an examination for gross lesions. The remaining five rats of each sex per group were sacrificed without further examination. Dorsal and ventral root fibres and associated dorsal root ganglia were collected from the C3–C6 and L1–L4 areas of the spinal cord and the proximal sciatic nerve and tibial and sural nerves (at the knee) were examined in animals in the control group and at 1000 mg/kg bw per day. The brain (frontal lobe, parietal lobe with diencephalon, midbrain with occipital and temporal lobe, pons, cerebellum), spinal cord (cross sections of cervical swelling (C3–C6) and lumbar swelling (L1–L4)) and peripheral nervous system (Gasserian ganglia with nerve and gastrocnemius muscle) were examined microscopically in animals in the control group and at 1000 mg/kg bw per day.
There were no deaths in either the range-finding or main studies. In the range-finding study, animals at 1000 and 2000 mg/kg bw per day had diarrhoea and were apathetic on the first day only, and piloerection occurred in animals at 2000 mg/kg bw per day, lasting until day 6. In the main study, diarrhoea occurred at 300 (two males and one female) and 1000 (five males and four females) mg/kg bw per day and piloerection was observed in four females at 1000 mg/kg bw per day, resolving by day 7. Body-weight gain was reduced by 33% in males at 1000 mg/kg bw per day during the first week of the study. There were no treatment-related effects in the FOB or motor activity parameters on days 7 and 14, and no neuropathological effects were observed (Mellert et al., 1999h).
Groups of 10 male and female Wistar rats (aged 49 days) were given diets containing pyraclostrobin (purity, 97.1%) at a concentration of 0, 50, 250, or 750 mg/kg of feed (males only), equal to 0, 4, 17 and 50 mg/kg bw per day, and 1500 mg/kg (equal to 110 mg/kg bw per day, females only) for 3 months. Animals were individually housed in cages in controlled conditions, and food and water were available ad libitum. Clinical signs of toxicity were assessed at least once daily and body weight, food and water consumption and food conversion efficiency [(body-weight gain)/(food consumed) × 100] were assessed weekly. FOB and motor activity were assessed 7 days before dosing, and on days 22, 50 and 85. The FOBs consisted of four parts, starting with passive observations (without disturbing the animals), followed by removing the animal from its home cage and "open field observations" in a standard arena. At the end of the treatment period, five rats of each sex per dose were sacrificed by in situ perfusion fixation for neuropathology examinations, including an examination of other tissues for gross lesions. The remaining five rats of each sex per group were sacrificed without further examination. The brain weight (without the olfactory bulb) was recorded from all groups, prior to its preparation for microscopic examination. Dorsal and ventral root fibres and associated dorsal root ganglia were collected from the C3–C6 and L1–L4 areas of the spinal cord and the proximal sciatic nerve and tibial and sural nerves (at the knee) were examined in animals in the control group and in males at 750 mg/kg and females at 1500 mg/kg. The brain (frontal lobe, parietal lobe with diencephalon, midbrain with occipital and temporal lobe, pons, cerebellum), spinal cord (cross sections of cervical swelling (C3–C6) and lumbar swelling (L1–L4)) and peripheral nervous system (Gasserian ganglia with nerve and gastrocnemius muscle) were examined microscopically in animals in the control group and in males at 750 mg/kg and in females at 1500 mg/kg.
There were no deaths, treatment-related clinical signs or effects on motor activity or neuropathology findings. In males at 750 mg/kg and females at 1500 mg/kg, reduction were seen in body-weight gain by approximately 18%, food consumption by up to 40% and water consumption by up to 28%. Grip strength of the forelimbs was impaired by 17% in females at 1500 mg/kg on day 85. Impaired grip strength is a non-specific sign and, in isolation, does not indicate selective neurotoxicity. Consequently, the NOAEL for neurotoxicity in this study was 750 mg/kg (approximately 50 mg/kg bw per day), the highest dose tested in males. The overall NOAEL was 250 mg/kg (17 mg/kg bw per day) on the basis of reduced body-weight gain, food consumption and water consumption at the dose above (750 mg/kg in males and 1500 mg/kg in females) (Mellert et al., 1999i).
Reduced serum alanine aminotransferase and/or alkaline phosphatase activity in rats has been observed both for pyraclostrobin and for other strobilurins, such as kresoxim-methyl, for example (Annex 1, reference 85).
A series of experiments were conducted to investigate the cause of the observed decrease in serum alkaline phosphatase and alanine aminotransferase activities in rats. In the first study, blood was taken from five male and five female rats fed with a normal diet, before administration of diet containing pyraclostrobin at a concentration of 8000 mg/kg (equal to 400 mg/kg bw per day) for about 2 weeks. At the end of this period, blood was again taken from these animals and sera were analysed both for total alkaline phosphatase and for isozyme composition. In the second experiment, the effect of dietary manipulation on the enzyme and isozyme activities of female rats treated with pyraclostrobin and of controls was investigated.
Total alkaline phosphatase activities in treated animals fell to approximately 66% of pre-treatment levels. This fall in alkaline phosphatase activities was due almost entirely to a decline in the intestinal isozyme, with that derived from rat liver and bone being unaffected. In rats, the activity of intestinal alkaline phosphatase in serum is strongly influenced by ingestion of fats and this was confirmed in the dietary manipulation study where addition of olive oil in the diet increased intestinal alkaline phosphatase activities by about 50% in both control rats and in rats treated with pyraclostrobin. Although the rats treated with pyraclostrobin on the diets supplemented with olive oil had lower alkaline phosphatase activities than before the commencement of treatment with pyraclostrobin, the levels did not fall to below that seen in control rats on a normal diet. Thus, addition of olive oil to the diet increased total alkaline phosphatase activities in both treated and control animals, through an increase in the activity of the intestinal isozyme of alkaline phosphatase. Addition of sucrose to the diet did not significantly affect alkaline phosphatase activities in either control animals or animals treated with pyraclostrobin. Similarly, the activity of intestinal alkaline phosphatase in fasted, untreated, animals was 75% lower than that in fed animals. Both total and intestinal alkaline phosphatase activities in fasted animals treated with pyraclostrobin were similar to those in untreated fasted animals (intestinal isozyme activity, 33 U/l compared with 40 U/l for the fasted control animals). Consequently, the greatest effect on the activity of alkaline phosphatase in rat serum was due to the fasting state of the animals.
In humans, little intestinal alkaline phosphatase is present in the serum owing to active scavenging by the liver; consequently, pyraclostrobin would be unlikely to produce a decline in serum alkaline phosphatase activity if ingested by humans.
Alanine aminotransferase activity in fasted rats was lower by approximately 30–40% in both control rats and in rats treated with pyraclostrobin compared with normally fed rats. Supplementation of normal diets with sucrose or olive oil had little effect on alanine aminotransferase activities in untreated rats, but appeared to reduce the effect of pyraclostrobin on the activity of this enzyme. The addition of pyridoxyl-5’-phosphate to the assay mixture had a small but constant stimulating effect on alanine aminotransferase activity in both control rats and rats treated with pyraclostrobin, indicating that depletion of, or interference with, this co-factor is not involved in the mechanism of the dietary or pyraclostrobin effect on alanine aminotransferase. The study author concluded that the effects of pyraclostrobin on alkaline phosphatase and alanine aminotransferase activities in rats were secondary to the altered dietary status of these animals (Moss, 1994).
(c) Comparison with other members of the strobilurin class of fungicides
On the basis of publicly available toxicology summaries for kresoxim-methyl, azoxystrobin and trifloxystrobin1, in conjunction with the studies on pyraclostrobin reviewed in this monograph, the toxicological profile of pyraclostrobin is largely consistent with that of the strobilurin fungicides as a chemical class. Compounds in this class of fungicides are not reproductive or developmental toxins, are not genotoxic and, with the exception of kresoxin-methyl at very high doses in rats, are not carcinogenic.
In rats treated orally with radiolabelled pyraclostrobin, about 50% of the administered dose was absorbed. Concentrations of radiolabel in the blood peaked initially after 30 min, followed by a secondary peak at 8 h or 24 h. Most (74–91%) of the radiolabelled dose was eliminated in the faeces, with the remainder (10–13%) in the urine. The pattern of excretion was not affected by repeated administration. In rats, the metabolism of pyraclostrobin proceeds through three main pathways. The methoxy group on the tolylmethoxycarbamate moiety is readily lost, with few major metabolites retaining this group. Hydroxylation of the benzene and/or pyrazole rings is followed by conjugation with glucuronide and, to a lesser extent, sulfate. Many metabolites are derived from the chlorophenol-pyrazole or tolylmethoxycarbamate moieties of pyraclostrobin after cleavage of the ether linkage between these two groups, with subsequent ring hydroxylation and glucuronide or sulfate conjugation. Metabolites were similar in both sexes and at all doses. No unchanged parent compound was found in the bile or urine and only small amounts were found in the faeces. Most of the radiolabel isolated from kidney tissues was in the form of the unchanged parent compound and a demethoxylated derivative.
Pyraclostrobin has low acute toxicity when administered orally or dermally, with LD50s of >5000 and >2000 mg/kg bw, respectively, and no deaths in either case. The compound has moderate toxicity when administered by inhalation, with an LC50 of 0.31–1.07 mg/l when acetone is used as the solvent, and 4.07–7.3 mg/l when Solvesso is used as the solvent.
Pyraclostrobin is a mild dermal and ocular irritant, but is not a skin sensitizer. Clinical signs after oral administration consisted of dyspnoea, staggering, piloerection, and diarrhoea in all animals, which resolved by day 6. There were no pathology findings.
In short-term studies in mice, rats and dogs, the major toxicological findings after repeated doses of pyraclostrobin involved duodenal mucosal hypertrophy and, in some studies in rodents, erosion/ulceration of the stomach mucosa. These findings are suggestive of a local irritant action, a conclusion that is supported by the occurrence of vomiting in dogs. However, pyraclostrobin is not a severe dermal irritant, although in rabbits the irritation was somewhat prolonged.
Reductions in body-weight gain and food consumption were observed in all species, although the pattern of the response and relationship to treatment varied. To some extent, these effects suggest local disturbance to the gastrointestinal tract and taste aversion, particularly in rabbits, although a systemic effect may also be involved, especially in rodents.
In short-term studies with repeated doses of pyraclostrobin, reduced body-weight gains were accompanied by reductions in clinical chemistry parameters (including concentrations of total protein, globulin, glucose, triglycerides and creatinine) and reduced fat storage in the liver. These observations may be secondary to a disturbance of normal metabolic processes following the disruption of mitochondrial respiration, the primary biochemical mechanism by which pyraclostrobin acts. These effects may also reflect a reduced nutritional status caused by reduced food intake or food conversion. Reduced body-weight gain largely determined the minimally toxic dose in lifetime studies in rats and mice, but was not associated with toxicologically relevant alterations in clinical pathology values where these were measured in rats.
Mild anaemia associated with extramedullary haematopoiesis in the spleen was observed in rodents fed with repeated doses of approximately >400 mg/kg (equal to 120 mg/kg bw per day in mice and 42 mg/kg bw per day in rats) in short-term studies. Hepatocellular hypertrophy, in the absence of significant alterations in relevant clinical chemistry parameters or other histological evidence of liver injury, was also observed in rats at 120 mg/kg bw per day.
Pyraclostrobin gave negative results in an adequate battery of studies of genotoxicity in vitro and in an assay for micronucleus formation in bone-marrow cells of mice in vivo.
The Meeting concluded that pyraclostrobin was unlikely to be genotoxic.
The carcinogenic potential of pyraclostrobin was studied in rats and mice. While the incidence of hepatocellular adenomas was slightly increased in one study in rats fed with pyraclostrobin at 200 mg/kg (equal to 9 mg/kg bw per day), no increase was observed in a concurrent lifetime study. Moreover, the incidence of liver adenomas in controls was considerably higher (20% versus 8%), suggesting that a low value for controls contributed to the apparent effect observed in the first study. There was no evidence of carcinogenic potential in mice and rats. This conclusion is supported by the observation that other strobilurin fungicides have not shown carcinogenic activity of relevance to human risk assessment.
On the basis of the above consideration and the absence of genotoxicity, the Meeting concluded that pyraclostrobin is unlikely to pose a carcinogenic risk to humans.
In a two-generation study of reproductive toxicity in rats, body-weight gains and food consumption were reduced in adults and lower body-weight gains and slightly delayed vaginal patency were observed in pups at a dose of 300 mg/kg (equal to 33 mg/kg bw per day). The NOAEL for general and pup toxicity was 75 mg/kg (equal to 8.2 mg/kg bw per day). The NOAEL for effects on reproductive performance was 300 mg/kg, the highest dose tested.
Two studies of developmental toxicity were conducted in rabbits and one in rats. Maternal toxicity consisting of reduced body-weight gains and food consumption was observed at >25 mg/kg bw per day in rats, and at >5 mg/kg bw per day in rabbits. In rats, the reduction in food consumption persisted beyond the treatment period and the corrected body-weight gains at termination were also reduced. In rabbits, a transient but marked reduction in food intake (and consequently in body-weight gain) after initiation of dosing was observed which resolved within 3–5 days, despite continued dosing. The pattern of the reduced body-weight gains and food consumption in rabbits indicated that they are likely to be caused by local effects on the gastrointestinal tract related to high concentrations of pyraclostrobin or taste disturbance resulting from regurgitation or leakage of the gavaging solution. Consequently, the Meeting concluded that these effects did not reflect systemic toxicity caused by pyraclostrobin. Nonetheless, the reduced nutritional status of dams, which was caused by lower food intakes at a critical time in gestation at and immediately after implantation, must be taken into account when considering the significance of the observed fetal effects at doses that were not otherwise maternally toxic. The NOAEL for maternal toxicity was 10 mg/kg bw per day in rats and 3 mg/kg bw per day in rabbits.
Pyraclostrobin was not teratogenic in rats, but fetal effects consisting primarily of developmental delay (incomplete ossification of sternebra and rudimentary cervical ribs) and an increased incidence of dilated renal pelves, were observed at a dose of 50 mg/kg bw per day. In rabbits, fetal effects consisting of increased postimplantation losses were observed at >10 mg/kg bw per day. A slight, non-significant increase in the incidence of skeletal malformations observed at 20 mg/kg bw per day was driven by an increase in the incidence of absent lumbar vertebrae at this dose. Although the incidence was not statistically significant, it exceeded the mean for historical control values and the upper bound of the range, and the Meeting could not exclude the possibility that the effect was potentially treatment-related. The effects seen in rabbit fetuses were likely to be secondary to the marked nutritional deficit in the dams at a critical time in gestation. The Meeting concluded, however, that the available data did not provide a sufficient basis on which to confidently exclude other potential mechanisms; consequently, the NOAEL for developmental toxicity in the study in rabbits was 5 mg/kg bw per day on the basis of these fetal effects. The developmental NOAEL was 25 mg/kg bw per day in rats.
Pyraclostrobin was investigated for neurotoxicity in rats in a study in which a single dose was administered by gavage and in a 90-day study of pyraclostrobin in the diet. The NOAELs for neurotoxicity were 2000 mg/kg bw and 750 mg/kg (equal to 50 mg/kg bw per day) respectively, the highest doses tested. Pyraclostrobin was not found to be neurotoxic.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of pyraclostrobin to fetuses, infants and children.
The Meeting established an acceptable daily intake (ADI) of 0–0.03 mg/kg bw based on a NOAEL of 3.4 mg/kg bw per day identified in two 2-year studies in rats, on the basis of reduced body-weight gain and altered liver and stomach histology at 200 mg/kg and using a 100-fold safety factor.
Pyraclostrobin is not acutely toxic and short-term dosing produced no significant general toxicity; however, fetal resorptions were increased at a dose of 10 mg/kg bw per day in a study of developmental toxicity in rabbits. Although a transient but marked reduction in food intake, and consequently in body-weight gain, was observed at doses of 5 mg/kg bw per day and above after initiation of dosing in studies of developmental toxicity in rabbits, this effect resolved within 3–5 days, despite continued dosing. The pattern of the observations indicates they are likely to be caused by local gastrointestinal tract effects related to high concentrations of pyraclostrobin, or to taste disturbance resulting from regurgitation or leakage of the gavaging solution. Consequently, the Meeting concluded that these observations did not reflect systemic toxicity caused by pyraclostrobin and were not used to establish the acute RfD. The Meeting established an acute RfD of 0.05 mg/kg bw, based on the NOAEL of 5 mg/kg bw per day for fetal toxicity at 10 mg/kg bw per day in the study of developmental toxicity in rabbits and using a 100-fold safety factor. Further information on the relationship between irritation of the gastrointestinal tract and reduced body-weight gains in pregnant rabbits, and the effect of maternal nutritional deficit on fetal resorptions, may allow the acute RfD to be refined.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
18-month study of toxicity and carcinogenicitya |
Toxicity |
30 mg/kg, equal to |
120 mg/kg, equal to |
|
Carcinogenicity |
120 mg/kg, equal to |
— |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
75 mg/kg, equal to |
200 mg/kg, equal to |
|
Carcinogenicity |
200 mg/kg, equal to |
— |
||
|
3-month study of neurotoxicitya |
Neurotoxicity |
750 mg/kg, equal to |
— |
|
|
Toxicity |
250 mg/kg equal to |
750 mg/kg, equal to |
||
|
Two-generation study of reproductive toxicitya |
Parental and offspring toxicity |
75 mg/kg, equal to |
300 mg/kg, equal to |
|
|
Study of developmental toxicityc |
Maternal toxicity Embryo- and fetotoxicity |
10 mg/kg bw per day |
25 mg/kg bw per day |
|
|
Rabbit |
Study of developmental toxicityc |
Maternal toxicity |
3 mg/kg bw per day |
5 mg/kg bw per dayd |
|
Embryo- and fetotoxicity |
5 mg/kg bw per day |
10 mg/kg bw per day |
||
|
Dog |
1-year study of toxicitya |
Toxicity |
200 mg/kg, equal to |
400 mg/kg, equal to |
a Diet
b Highest dose tested
c Gavage
d A marked but transient reduction in maternal food intake occurred immediately after initiation of dosing at higher concentrations
Estimate of acceptable daily intake for humans
0–0.03 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Summary of critical end-points for pyraclostrobin
|
Absorption, distribution, excretion and metabolism in animals |
|
|
Rate and extent of oral absorption |
Rapid, approximately 50% |
|
Dermal absorption |
1.6–2.6% in rats in vivo, 3–8% across human skin in vitro (from an unspecified formulation) |
|
Distribution |
Rapidly and widely distributed with highest concentrations in the gastrointestinal tract, liver and kidneys |
|
Rate and extent of excretion |
Largely complete within 48 h; approximately 15% in urine and 85% in the faeces; |
|
Potential for accumulation |
No evidence of significant accumulation |
|
Metabolism in mammals |
Extensively metabolized with subsequent glucuronide and sulfate conjugation; the metabolites are unlikely to be toxicologically significant |
|
No unchanged parent compound in the bile or urine and only small amounts in the faeces. |
|
|
Toxicologically significant compounds |
Parent compound |
|
Acute toxicity |
|
|
Rat, LD50, oral |
>5000 mg/kg bw (no deaths) |
|
Rat, LD50, dermal |
>2000 mg/kg bw (no deaths) |
|
Rat, LC50 inhalation |
0.310–1.070 mg/l (4 h exposure, head and nose only) in acetone |
|
4.07–7.3 mg/l (4 h exposure, head and nose only) in Solvesso solvent |
|
|
Rabbit, dermal irritation |
Slight but prolonged |
|
Rabbit, ocular irritation |
Slight |
|
Skin sensitization |
Not sensitizing (Magnusson & Kligman) |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Ulceration of the glandular stomach in mice, hypertrophy of the duodenal mucosa in mice, rats and dogs, vomiting and diarrhoea in dogs, anaemia in mice and rats, decreased body-weight gains in mice, rats and dogs, hepatocellular hypertrophy in rats |
|
Lowest relevant oral NOAEL |
4 mg/kg bw per day (mice) |
|
Lowest relevant dermal NOAEL |
100 mg/kg bw per day (rats) |
|
Lowest relevant inhalational NOAEC |
No data |
|
Genotoxicity |
No genotoxic potential |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Reduced body-weight gains in rats and mice, elevated liver weights in mice, altered liver and stomach histology in rats |
|
Lowest relevant NOAEL |
3.4 mg/kg bw per day (two 2-year studies in rats) |
|
Carcinogenicity |
Not carcinogenic in rats or mice |
|
Reproductive toxicity |
|
|
Reproductive target/critical effect |
None |
|
Lowest relevant reproductive NOAEL |
>33 mg/kg bw per day (two-generation study in rats) |
|
Developmental target/critical effect |
Increased postimplantation losses and reduced fetal weight |
|
Lowest relevant developmental NOAEL |
5 mg/kg bw per day (rabbits) |
|
Neurotoxicity/delayed neurotoxicity |
No evidence of neurotoxicity in a 3-month study in rats at doses of up to 50 mg/kg bw per day |
|
Medical data |
No adverse effects have been reported but the data are limited as pyraclostrobin is a new substance |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.03 mg/kg bw |
Rat, 2-year |
100 |
|
Acute RfD |
0.05 mg/kg bw |
Rabbit, developmental |
100 |
Engelhardt, G. (2000a) In vitro gene mutation test with BAS 500 F in CHO cells (HPRT locus assay). Amendment No. 1 (Project No. 50M0308/964303). Unpublished report No. 2000/1000269 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 25 January 2000.
Engelhardt, G. (2000b) In vitro unscheduled DNA synthesis (UDS) assay with BAS 500 F in primary rat hepatocytes. Amendment No. 1 (Project No. 81M0308/964306). Unpublished report No. 2000/1000270 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 25 January 2000.
Engelhardt, G. & Hoffmann, H.D. (1997) BAS 500 F (ZHT test substance No. 96/308) in the Ames Salmonella/mammalian-microsome mutagenicity test and Escherichia coli/mammalian-microsome reverse mutation assay (standard plate test and preincubation test) (Project No. 40M0308/964244). Unpublished report No. 97/10973 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 11 August 1997.
Engelhardt, G. & Hoffmann, H.D. (1998a) In vitro gene mutation test with BAS 500 F in CHO cells (HPRT locus assay) (Project No. 50M0308/964303). Unpublished report No. 98/11422 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 8 December 1998.
Engelhardt, G. & Hoffmann, H.D. (1998b) In vitro unscheduled DNA synthesis (UDS) assay with BAS 500 F in primary rat hepatocytes (Project No. 81M0308/964306). Unpublished report No. 98/11421 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 19 October 1998.
Engelhardt, G. & Hoffmann, H.D. (1998c) Cytogenetic study in vivo with BAS 500 F in the mouse micronucleus test, single oral administration (Project No. 26M0308/964204). Unpublished report No. 98/10460 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 9 April 1998.
Engelhardt, G. & Hoffmann, H.D. (1999) In vitro chromosome aberration assay with BAS 500 F in V79 cells (Project No. 32M0308/964304). Unpublished report No. 1999/11403 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 8 October 1999.
Gamer, A.O. & Hoffmann, H.D. (1997) BAS 500 F—Acute inhalation toxicity study in Wistar rats 4 hour liquid aerosol exposure (Project No. 13I0308/967028). Unpublished report No. 97/11472 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 10 December 1997.
Gamer, A.O., Leibold, E. & Hoffmann, H.D. (2001) BAS 500 F 40% in Solvesso (technical active ingredient) acute inhalation toxicity study in Wistar rats 4 hour liquid aerosol exposure (Project No. 13/0283/01702). Unpublished report No. 2001/1010625 from BASF Aktiengesellschaft, Department of Toxicology, D-67056 Ludwigshafen, Germany, 21 June 2001.
Leibold, E. & Hoffmann, H.D. (1999) 14C-BAS 500 F—study of the dermal absorption in rats (Project No. 01B0363/966044). Unpublished report No. 1999/10716 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 18 June 1999.
Leibold, E., Hoffmann, H.D. & Hildebrand, B. (1998) 14C-BAS 500 F—study of the biokinetics in rats (Project No. 02B0364/966007). Unpublished report No. 98/10997 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 1 October 1998.
Mellert, W. (2002a) BAS 500 F mechanistic study to determine the "acute reference dose" in B6C3F1 mice. Administration in the diet and by gavage. Unpublished report No. 2002/1011459 from BASF Anktiengesellschaft, Experimental Toxicology and Ecology, Ludwigshafen/Rhein, Germany, 17 September 2002.
Mellert, W. (2002b) BAS 500 F Mechanistic study to determine the "acute reference dose" in Wistar rats. Administration in the diet and by gavage. Unpublished report No. 2002/1011458 from BASF Anktiengesellschaft, Experimental Toxicology and Ecology, Ludwigshafen/Rhein, Germany, 17 September 2002.
Mellert, W., Deckardt, K., Küttler, K. & Hildebrand, B. (1998) BAS 500 F—subchronic oral toxicity study in B6C3F1 Crl BR mice, administration in the diet for 3 months (Project No. 60C0183/96016). Unpublished report No. 98/11345 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 25 November 1998.
Mellert, W., Bahnemann, R. & Hildebrand, B. (1999a) BAS 500 F—subchronic oral toxicity study in Wistar rats, administration in the diet for 3 months. Amendment No. 1 to the Report (Project No. 50C0183/96015). Unpublished report No. 99/11899 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 13 December 1999.
Mellert, W., Deckardt, K., Bahnemann, R. & Hildebrand, B. (1999b) BAS 500 F—Subchronic oral toxicity study in Wistar rats, administration in the diet for 3 months (Project No. 50C0183/96015). Unpublished report No. 99/10195 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 2 July 1999.
Mellert, W., Deckardt, K., Gembardt, C. & Hildebrand, B. (1999c) BAS 500 F—repeated dose dermal toxicity study in Wistar rats, administration for 4 weeks (Project No. 33S0494/96179). Unpublished report No. 1999/11458 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 15 October 1999.
Mellert, W., Deckardt, K., Gembardt, C. & Hildebrand, B. (1999d) BAS 500 F—repeated dose oral toxicity study in Wistar rats, administration in the diet for 4 weeks (Project No. 30C0376/95083). Unpublished report No. 1999/11870 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 2 December 1999.
Mellert, W., Deckardt, K., Gembardt, C., Pappritz, G. & Hildebrand, B. (1999e) BAS 500 F—chronic toxicity study in Wistar rats, administration in the diet for 24 months (Project No. 82S0494/96085). Unpublished report No. 99/11672 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 9 November 1999.
Mellert, W., Deckardt, K., Gembardt, C., Pappritz, G. & Hildebrand, B. (1999f) BAS 500 F—carcinogenicity study in Wistar rats, administration in the diet for 24 months (Project No. 82S0494/96086). Unpublished report No. 99/11868 from BASF Aktiengesellschaft, Department of Toxicology, D-67056 Ludwigshafen, Germany, 22 November 1999.
Mellert, W., Deckardt, K., Küttler, K. & Hildebrand, B. (1999g) BAS 500 F—carcinogenicity study in B6C3F1 mice, administration in the diet for 18 months (Project No. 76C0494/96101). Unpublished report No. 99/11871 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 22 November 1999.
Mellert, W., Kaufmann, W. & Hildebrand, B. (1999h) BAS 500 F—acute oral neurotoxicity study in Wistar rats (Project No. 20C0494/96164). Unpublished report No. 1999/11111 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 18 August 1999.
Mellert, W., Kaufmann, W. & Hildebrand, B. (1999i) BAS 500 F—subchronic oral neurotoxicity study in Wistar rats, administration in the diet for 3 months (Project No. 50C0494/96174). Unpublished report No. 1999/11329 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 16 September 1999.
Mellert, W., Küttler, K. & Hildebrand, B. (1999j) BAS 500 F—subchronic oral toxicity study in B6C3F1 Crl BR mice, administration in the diet for 3 months. Amendment No. 1 to the Report (Project No. 60C0183/96016). Unpublished report No. 99/11900 from BASF Aktiengesellschaft, Department of Toxicology, D-67056 Ludwigshafen, Germany, 13 December 1999.
Menges, S., Schilling, K., Deckardt, K., Gembardt, C. & Hildebrand, B. (1999) BAS 500 F—subchronic oral toxicity study in beagle dogs, administration in the diet for 3 months (Project No. 31D0494/96089). Unpublished report No. 1999/11678 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 17 November 1999.
Moss, D.W. (1994) Effect of Reg. No. 242 009 on enzyme levels in rat serum. Unpublished report No. 1994/1001867 from Royal Postgraduate Medical School, Hammersmith Hospital, London, England.
Noda, A., Ito, M., Kon, N., Aoyama, S., Yamamoto, Y., Ito, Y., Hayama, T., Asada, M. & Kadowaki, K. (1993) Effects of dietary restriction on embryogenesis in Japanese white-NIBS rabbits. Teratology, 48(5), 532–2.
Schilling, K., Deckardt, K., Gembardt, C. & Hildebrand, B. (1999a) BAS 500 F—chronic oral toxicity study in beagle dogs, administration in the diet for 12 months (Project No. 33D0494/96144). Unpublished report No. 1999/11677 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 17 November 1999.
Schilling, K., Gembardt, C. & Hildebrand, B. (1999b) BAS 500 F—two-generation reproduction toxicity study in Wistar rats, continuous dietary administration (Project No. 70R0494/96172). Unpublished report No. 1999/11869 from BASF Aktiengesellschaft, Department of Toxicology, D-67056 Ludwigshafen, Germany, 29 November 1999.
Schilling, K., Hellwig, J. & Hildebrand, B. (1999c) BAS 500 F—prenatal developmental toxicity study in Wistar rats, oral administration (gavage) (Project No. 30R0494/96168). Unpublished report No. 1999/11511 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 25 October 1999.
Schilling, K., Hellwig, J. & Hildebrand, B. (1999d) BAS 500 F—prenatal developmental toxicity study in Himalayan rabbits, oral administration (gavage) (Project No. 40R0494/96159). Unpublished report No. 1999/11512 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany 25 October 1999.
Schilling, K., Hellwig, J. & van Ravenzwaay, ? (2001) BAS 500 F—additional maternal toxicity study in himalayan rabbits. Oral administration (gavage). Unpublished report No. 2001/1003803 from BASF Anktiengesellschaft, Experimental Toxicology and Ecology, Ludwigshafen/Rhein, Germany, 21 February 2001.
Schneider, S. & Hellwig, J. (2002) BAS 500 F—test study in male and non-pregnant female himalayan rabbits. Oral administration (gavage) and administration in the diet. Unpublished report No. 2002/1012052 from BASF Anktiengesellschaft, Experimental Toxicology and Ecology, Ludwigshafen/Rhein, Germany, 30 October 2002.
Thomley, K.F. & Wood, R.A. (1999) (14C)-BAS 500 F: rates of penetration through human and rat skin using an in vitro system (Project No. 50H0364/969131). Unpublished Report No. 1999/11867 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 30 December 1999.
Velic, I. (1999) The metabolism of 14C-BAS 500 F (14C-304428) in rats. Unpublished report No. 1999/11781 from BASF Aktiengesellschaft, Limburgerhof, Germany.
Waalkens-Berendsen, D.H., Koeter, H.B. & van Marwijk, M.W. (1990) Embryotoxicity/teratogenicity of isomalt in rats and rabbits. Food Chem. Toxicol., 28, 1–9.
Warren, R.J. & Kirkpatrick, R.L. (1978) Reproduction in female cottontail rabbits as influenced by mirex ingestion and nutritional restriction. Virginia J. Sci., 27, 51.
Wiemann, C. & Hellwig, J. (1998a) Study on the acute oral toxicity of BAS 500 F in rats (Project No. 10A0183/961058). Unpublished report No. 98/10965 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 16 September 1998.
Wiemann, C. & Hellwig, J. (1998b) Study on the acute dermal toxicity of BAS 500 F in rats (Project No. 11A0308/961120). Unpublished report No. 98/10966 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 15 September 1998.
Wiemann, C. & Hellwig, J. (1998c) BAS 500 F—acute dermal irritation/corrosion in rabbits (Project No. 14H0308/962190). Unpublished report No. 98/10959 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 15 September 1998.
Wiemann, C. & Hellwig, J. (1998d) BAS 500 F—acute eye irritation in rabbits (Project No. 13H0308/962191). Unpublished report No. 98/10963 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 15 September 1998.
Wiemann, C. & Hellwig, J. (1998e) BAS 500 F—maximisation test in guinea pigs (Project No. 30H0494/962329). Unpublished report No. 98/10964 from BASF Aktiengesellschaft, Department of Toxicology, Ludwigshafen, Germany, 11 September 1998.
ENDNOTES:
1. Published on the Internet by, for example, the Australian and USA pesticide regulatory bodies, among others. See www.apvma.gov.au (public release summaries) and www.epa.gov/fedregister/ (pesticide tolerance notices) for examples.
See Also:
Toxicological Abbreviations