
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
FENAMIPHOS
First draft prepared by
S. Geertsen
Health Evaluation Division, Pest Management Regulatory Agency
Health Canada, Ottawa, Ontario, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Dermal and ocular irritation and dermal
sensitization
Delayed neuropathy
Neurotoxicity
Potentiation
Comments
Toxicological evaluation
References
Explanation
Fenamiphos was first evaluated for toxicological effects by the JMPR
in 1974 (Annex 1, reference 22), at which time an ADI of 0-0.0006
mg/kg bw was established. In 1985, following a direct request for
re-evaluation by a Member State, additional data were reviewed and the
ADI was reduced to 0-0.0003 mg/kg bw and made temporary because of
concern about fetotoxicity seen in a study in rabbits (Annex 1,
reference 44). The 1985 JMPR requested submission of the results of an
on-going study of oncogenicity in rats, a full, legible report and raw
data from the study of developmental toxicity in rats, and a new study
of developmental toxicity in rabbits to clarify the observation of
fetotoxicity at low dietary levels. The results of these studies were
considered by the 1987 JMPR (Annex 1, reference 50), which established
an ADI of 0-0.0005 mg/kg bw. Fenamiphos was evaluated at the present
Meeting within the CCPR periodic review programme.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Wistar rats were given 3 mg/kg bw 14C-fenamiphos (purity, > 99%;
specific activity, 62.6 µCi/mg; labelled in the benzene ring), and one
rat was sacrificed 0.5, 2, 8, 24, and 48 h after treatment. Within 0.5
h, radiolabel was distributed throughout the body, except for compact
bone structures, the spinal marrow, and the brain, indicating minimal
ability to cross the blood-brain barrier. The highest concentrations
were seen in the stomach contents, some segments of the small
intestine, the kidney, and the urinary bladder, indicating very rapid
urinary excretion. High concentrations were also found in the liver;
this, and its presence in the intestines, indicate a biliary-faecal
route of elimination. Blood, lungs, salivary glands, parotids,
hypophysis, and tissues with large amounts of connective tissue also
had relatively high concentrations of radiolabel. Less was found in
the lymph, adrenal gland, and spleen, and only low concentrations
occurred in the musculature, fat, pancreas, and thymus. Over the
course of the study, radiolabel was also found in the region of the
hair follicles, suggesting slight elimination via this route.
Fenamiphos was rapidly cleared from the body (little remained within 8
h) with no evidence of tissue accumulation (Weber, 1988).
In a study to assess the absorption, distribution, metabolism, and
excretion of fenamiphos in Wistar rats, 14C-labelled compound
(purity, > 99%; 62.6 µCi/mg; labelled in the benzene ring) was
administered to groups of five rats of each sex in one of the
following regimens: a single intravenous dose of 0.3 mg/kg bw labelled
fenamiphos; a single oral dose of 0.3 mg/kg bw labelled fenamiphos; 14
daily oral doses of 0.3 mg/kg bw unlabelled fenamiphos followed by a
single oral dose of 0.3 mg/kg bw labelled fenamiphos; or a single oral
dose of 3 mg/kg bw labelled fenamiphos. Urinary and faecal excretion
were monitored for 48 h, when the animals were killed and tissue
residue levels determined. Elimination of labelled carbon dioxide was
monitored only in males given the high dose. The metabolites in the
excreta were identified and quantified. Fenamiphos was rapidly
excreted, 54-85% of the radiolabel being excreted renally within 4 h
of treatment. After 48 h, >96% of the recovered radiolabel had been
excreted by animals at all doses. Faecal elimination accounted for
only 1.5-3.7% of the recovered radiolabel. Small amounts were found in
the faeces after intravenous administration, indicating that a small
amount of biliary excretion occurs. Negligible amounts (< 0.1%) of
radiolabel were eliminated as carbon dioxide in the expired air. At 48
h, most of the levels in tissues were below the limit of
quantification, except in the animals given the high dose, in which
the maximum tissue residue levels were seen in the liver (8.4 ppb in
females, 3.5 ppb in males), kidney (2.1 ppb in males, 1.6 ppb in
females), and skin (3.5 ppb in males, 1.6 ppb in females). Urinary
excretion appeared to be slightly faster in males than females after
oral dosing, faster after intravenous than oral dosing, and faster
after the high than the low dose in females only. Prior treatment with
unlabelled fenamiphos had no effect on the excretion characteristics
(Ecker et al., 1989).
(b) Biotransformation
The metabolic fate of labelled fenamiphos was studied in rats
in vivo and in rat liver microsomes in vitro. The compounds were
labelled with 14C in the ethyl or isopropyl position or with 3H in
the thiomethyl position. Fenamiphos was excreted within 12-15 h after
a single oral dose of 2 mg/kg bw. In vitro a small quantity of an
unknown metabolite, possibly resulting from the N-dealkylation of
fenamiphos, was observed (Khasawinah & Flint, 1972). Apart from this
minor component, metabolism in animals and plants followed the same
pattern: oxidation of the thioether to the sulfoxide and sulfone,
dearylation to yield the methyl thioether phenol (or its sulfoxide and
sulfide), and potential dealkylation of the ethyl, isopropyl, or
isopropylamino moiety of the phosphate ester. Treatment of rats with
fenamiphos sulfoxide or sulfone produced the same excretion pattern
and almost identical urinary metabolites (Gronberg, 1969; JMPR, 1974).
Ecker et al. (1989; see above) also quantified the metabolites in the
urine of all groups and in the faeces of animals at the high dose. The
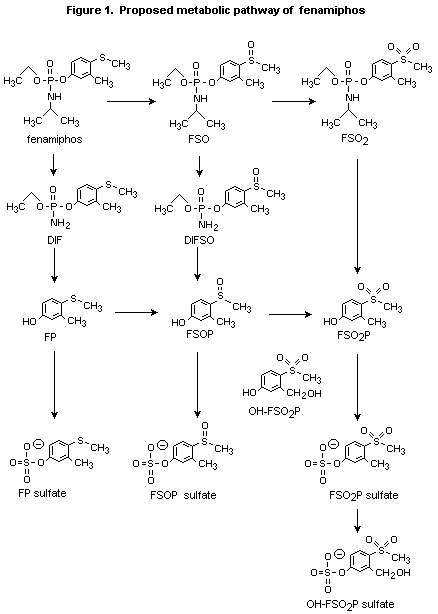
primary urinary metabolites (Figure 1) were found to be fenamiphos
sulfoxide phenol sulfate (40-54%), fenamiphos sulfoxide phenol
(4-22%), fenamiphos phenol sulfate (5-20%), and fenamiphos sulfone
phenol sulfate (4-15%). Minor metabolites included fenamiphos sulfone
phenol (2-10%), fenamiphos phenol (1-10%), fenamiphos sulfoxide
(0-12%),3-hydroxymethyl fenamiphos sulfone phenol sulfate (0-11%), and
desisopropyl fenamiphos sulfoxide (0-1.7%). About 60% of the faecal
metabolites of animals at the high dose were identified and found to
be restricted to fenamiphos sulfoxide, fenamiphos sulfoxide phenol,
fenamiphos sulfone phenol, and fenamiphos phenol sulfate. No parent
compound was identified in either urinary or faecal extracts. More
than 93% of the radiolabelled metabolites were identified.
Qualitatively, the metabolic profile was largely unaffected by sex,
dose, route, or frequency of treatment. Quantitatively, there was
greater production of fenamiphos phenol sulfate after intravenous
administration (16-19%) than after oral administration (5-8%) (Ecker
et al., 1989).
(c) Effects on enzymes and other biochemical parameters
Fenamiphos, like other organophosphate esters, inhibits cholinesterase
enzymes. The concentrations that inhibited activity by 50% in vitro
were 5.1 × 10-5 mol/L in rat serum, 6.3 × 10-4 mol/L in rat
erythrocytes, and 2.1 × 10-4 mol/L in rat brain. The maximum
inhibition of whole-blood cholinesterase in rats in vivo occurred
3 h after treatment. The sensitivity of cholinesterase in vivo
reflects the values for inhibition in vitro, plasma cholinesterase
being more sensitive than the erythrocyte enzyme (Löser & Kimmerle,
1971; JMPR, 1974). The metabolites of fenamiphos are more active
inhibitors than the parent molecule (Waggoner, 1972; JMPR, 1974). The
 inhibitory activity of fenamiphos and its metabolites in horse serum
cholinesterase in vitro was in the order fenamiphos < sulfoxide =
sulfone < unidentified metabolite.
The concentrations that inhibited the activity of chicken and monkey
liver aliesterase (triacetin hydrolysis) and monkey cholinesterase by
50% in vitro were 4.2 × 10-5 mol/L for chicken aliesterase, 1.0 ×
10-6 mol/L for monkey aliesterase, and 4.6 × 10-6 mol/L for monkey
cholinesterase (Coulston & Wills, 1974).
Male albino rats were painted dermally on the clipped dorsal area with
100-400 µg/cm2 fenamiphos, 25-800 µg/cm2 fenamiphos sulfoxide, or
200-1600 µg/cm2 fenamiphos sulfone. The test materials were applied
in acetone and allowed to dry. After 72 h, the animals were killed and
erythrocyte acetylcholinesterase activity determined. Fifty percent
inhibition was achieved with doses of 208 µg/cm2 fenamiphos, 262
µg/cm2 fenamiphos sulfoxide, and 750 µg/cm2 fenamiphos sulfone
(Knaak et al., 1981; JMPR, 1985).
Blood samples were extracted from equal numbers of male and female
Sprague-Dawley rats and then pooled for comparison of the
cholinesterase inhibition induced by fenamiphos and five of its
metabolites (fenamiphos sulfoxide, desisopropyl fenamiphos sulfoxide,
fenamiphos sulfone, desisopropyl fenamiphos sulfone, and desisopropyl
fenamiphos) in vitro. Samples were incubated for 1 h before
determination of the cholinesterase activity (Table 1). Erythrocyte
acetylcholinesterase was less sensitive to fenamiphos and its
metabolites than was plasma cholinesterase (Lamb & Landes, 1978; JMPR,
1985).
In order to assess the effects of fenamiphos on neurotoxic esterase
activity, single doses of 0 or 25 mg/kg bw technical-grade fenamiphos
(purity; 91.3%) were given by oral intubation to groups of nine
atropinized Lohmann selected Leghorn hens. Groups of three surviving
hens were killed one, two, and seven days after treatment. Even under
atropine protection, one hen died within 24 h of treatment. Fenamiphos
had no effect on neurotoxic esterase activity in the brains or spinal
cords, whereas the positive control compound, tri- ortho-cresyl
phosphate, had almost completely inhibited the activity 24 and 48 h
after treatment (Flucke & Eben, 1988).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of fenamiphos and its
metabolites are summarized in Table 2. Impurities identified as
components of the technical mixture (aryldiamide, diarylamide, diaryl
ethyl ester, diethyl ester, diethylmonamide, di-SCH3 compound, ethyl
aryl ester, ethyldiamide, and 4-methylthio- meta-cresol) were tested
for their acute toxicity to rats. At doses 1.5 times the acute LD50
of fenamiphos, none of the materials was toxic (Crawford & Anderson,
1973; JMPR, 1974).
Table 1. Inhibition of cholinesterase (%) by fenamiphos and its metabolites in vitro
Compound Plasma Erythrocytes
(Dose: ppm in whole blood) (Dose: ppm in whole blood)
6.88 68.8 688 5440 6880 6.88 68.8 688 5440 6880
Fenamiphos - 0 10 49 69 - 0 0 0 23
Fenamiphos sulfoxide 18 41 48 - 85 5 0 5 - 52
Fenamiphos sulfone 0 13 50 - 87 8 - - - -
Desisopropyl fenamiphos sulfoxide 6 40 90 - 93 4 0 47 - 50
Desisopropyl fenamiphos sulfone 0 20 69 - 90 6 0 32 - 61
Desisopropyl fenamiphos - 2 13 71 91 - 8 0 32 53
-, not determined
Table 2. Acute toxicity of fenamiphos, its metabolites, and impurities
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos
Mouse Oral M NR 22.7 Loser & Kimmerle (1971)
Intraperitoneal M/F 80 3.4 DuBois et al. (1967)
Inhalation M NR approx. 60 Kimmerle & Solmecke (1971)
(1 h static)
Rat Oral (fasted) M 88-99.7 2.4-6.0 Crawford & Anderson (1973, 1974a);
F 2.4-6.1 Lamb & Matzkanin (1975); Mihail (1980);
Heimann (1981, 1984, Krotlinger (1988)
Oral M 80-90.2 8.1-17.2 DuBois et al. (1967); Loser & Kimmerle (1971);
(not fasted) F 9.6-19.4 Kimmerle & Solmecke (1971); Kimmerle (1972a);
Heimann (1981, 1984)
Dermal M 80-92.2 72-73 Dubois et al. (1967); Flucke (1980)
84-92
Dermal M NR approx. 500 Kimmerle & Solmecke (1971)
Inhalation M 89.8 110-175 Kimmerle (1972b); Kimmerle & Solmecke
(1 h) F 130-150 Thyssen (1979a)
Inhalation M 89.8 91-100 Kimmerle & Solmecke (1971); Thyssen (1979a)
(4 h) 100
Intraperitoneal M 80 3.0-3.7 DuBois et al. (1967); Loser & Kimmerle (1971);
F 4.2-4.9 Kimmerle & Solmecke (1971)
Guinea-pig Oral M NR 56-100 DuBois et al. (1967); Loser & Kimmerle (1971);
Kimmerle & Solmecke (1971)
Intraperitoneal M NR 17.3 DuBois et al. (1967)
Rabbit Oral M NR 10-17.5 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Dermal M NR 225 Crawford & Anderson (1972)
F 179
Cat Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Dog Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Chicken Oral F NR 5.3-12 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971); DuBois et al. (1967)
Fenamiphos sulfone
Rat Oral (fasted) M NR 2.6 Crawford & Anderson (1974b)
F 2.4
Oral F NR 1-25 Thyssen (1974a)
(not fasted)
Fenamiphos sulfoxide
Rat Oral (fasted) M/F NR 2.4 Crawford & Anderson (1974b)
Oral F NR 10-25 Thyssen (1974b)
(not fasted)
Desisopropylfenamiphos
Rat Oral (fasted) M NR 1.4 Lamb & Matzkanin (1977)
F 2.1
Desisopropylfenamiphos sulfone
Rat Oral (fasted) M 95 4.1 Lamb & Matzkanin (1975)
F 3.7
Fenamiphos sulfoxide phenol
Rat Oral (fasted) M 99 1418 Crawford & Anderson (1974a)
F 1175
Oral F NR 500-1000 Thyssen (1974c)
(not fasted)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos sulfone phenol
Rat Oral (fasted) M 95 1250 Crawford & Anderson (1974a)
F 1854
Oral F NR > 1000 Thyssen (1974d)
(not fasted)
4-Methylthio-meta-cresol
Rat Oral (fasted) M 96.4 1418 Crawford & Anderson (I974a)
F 1333
Oral F NR > 2500 Thyssen (1974e)
(not fasted)
NR, not reported
Fenamiphos (purity, 97%) was given orally to fasted Wistar (Bor:WISW
(SPF-Cpb) rats at doses of 1-8 (females) or 1-100 (males) mg/kg bw.
All deaths among males at 100 mg/kg bw occurred within 10 min, and
those among animals receiving 5 mg/kg bw or more within 1.5 h.
Clinical signs of toxicity noted at doses >4 (males) or 5 (females)
mg/kg bw included apathy, palmospasms, laboured breathing, diarrhoea,
piloerection, clonic cramps, and dyspnoea. Females at does > 1
mg/kg bw had only diarrhoea (Krötlinger, 1988).
Studies in which rodents were given fenamiphos by inhalation as a
static spray at doses up to 230 µg/L for 1-4 h showed that rabbits and
guinea-pigs are more tolerant of the acute effects than rats and mice
(Kimmerle & Solmecke, 1971).
Male and female TNO/W74 albino rats were exposed to aerosolized
fenamiphos (purity, 89.8%) for 4 h per day on five consecutive days at
concentrations of 0, 0.3, 0.6, 3.3, 4, 9, or 28 µg/L. Plasma and
erythrocyte cholinesterase activities were measured before exposure,
after the first, third, and fifth exposures, and 72 h after the fifth
exposure; brain acetylcholinesterase activity was not measured. The
cholinesterase activities were depressed in a dose-related manner;
that of plasma cholinesterase was the most significantly depressed,
and females were more sensitive than males. Plasma cholinesterase
activity was 32-90% lower than before treatment in males at > 3.3
µg/L and 31-96% lower in females at > 0.3 µg/L. The activity
remained depressed by 19-44% for 72 h after the fifth exposure in
females at concentrations of > 9 µg/L. Erythrocyte
acetylcholinesterase activity in males was inhibited by < 20% at
> 9 µg/L; however, that in females was depressed by up to 28% at 9
µg/L and 33% at 28 µg/L (JMPR, 1985, slightly modified by reference to
the original report of Thyssen, 1979b).
In male rats, administration of atropine and/or 2-pralidoxime or
obidoxime after poisoning reduced the acute lethal dose by a factor of
about two. As with other organophosphorus compounds, rapid
administration of atropine and oxime reactivator after poisoning
affords some protection and alleviates the cholinergic signs of
poisoning (DuBois et al., 1967; Kimmerle, 1972c; JMPR, 1974).
(b) Short-term toxicity
Rats
Three groups of 10 male and 10 female albino Wistar TNO/W 74 rats were
exposed to technical-grade fenamiphos (purity, 92.2%) diluted with a
1:1 mixture of ethanol and polyethylene glycol 400 for aerosolization
in a dynamic flow inhalation chamber at doses of 0, 0.03, 0.25, or 3.5
µg/L for 6 h per day, five days per week for three weeks; 98% of the
particles were 3 µm or less. No toxic signs or effects on mortality,
body-weight gain, or the results of haematology, urinalysis, or
clinical chemistry were seen. A significant decrease (48-79%) in
plasma cholinesterase activity and a slight decrease (9-18%) in
erythrocyte acetylcholinesterase activity were seen in animals of each
sex at 3.5 µg/L; brain acetylcholinesterase activity was not affected.
There were no gross or histopathological changes or effects on organ
weights. The NOAEL was 3.5 µg/L (Thyssen, 1979b).
Groups of 15 male Sprague-Dawley rats were given fenamiphos orally or
by intraperitoneal injection on five days per week for 60 days. All of
the animals survived doses ranging from 1.5 mg/kg bw per day
intraperitoneally to 1.7 mg/kg bw per day orally with no cumulative
toxicological effect (Kimmerle & Solmecke, 1971; JMPR, 1974). In
another study, female rats survived daily intraperitoneal
administration of 1 mg/kg bw per day for 60 days, while 40% of those
given 2 mg/kg bw per day and all rats at 3 mg/kg bw per day died
(DuBois & Flynn, 1968; JMPR, 1974). These studies indicate little if
any cumulative toxicity.
Groups of 20 Fischer 344 rats of each sex were given diets containing
fenamiphos (purity, 89%) at 0, 0.37, 0.57, or 0.91 ppm (equal to 0,
0.03, 0.045, or 0.072 mg/kg bw per day for males and 0, 0.035, 0.053,
or 0.084 mg/kg bw per day for females) for three months. Animals were
monitored for clinical signs of toxicity, mortality, abnormality,
masses, food consumption, and body weight throughout the study.
Cholinesterase activity in plasma and erythrocytes was determined in
10 rats of each sex per group at 5, 9, 12, and 14 weeks and in brain
at termination of the study. No effects attributable to treatment were
seen on food consumption or body weight or during clinical
observation. Statistically but not toxicologically significant
decreases in both plasma and erythrocyte cholinesterase activity were
observed in treated rats of each sex when compared with controls. The
maximal inhibition of plasma cholinesterase was 17% at week 14 in
males at 0.37 ppm, with lesser inhibition at higher doses, and 24% at
week 9 in females at the high dose. Erythrocyte acetylcholinesterase
activity was inhibited by < 10% in all cases. No toxicologically
significant, treatment-related change was seen in brain
acetylcholinesterase activity. It was concluded that no biologically
significant cholinesterase activity inhibition had occurred in this
study. The NOAEL was 0.9 ppm, equal to 0.07 mg/kg bw per day, the
highest dose tested (JMPR, 1987, slightly modified by reference to the
original report of Hayes, 1986a).
Groups of Wistar SPF rats of each sex (15 per treated group, 30
controls) were fed diets containing fenamiphos (purity, 82%) at 0, 4,
8, 16, or 32 ppm (equivalent to 0.2, 0.4, 0.8, or 1.6 mg/kg bw per
day) for three months. Male and female rats at the highest dose showed
signs of cholinergic stimulation during the first two months; no
behavioural changes were seen in the other animals. Average food
consumption and growth were similar for treated and control animals.
The only effects on the haematological parameters examined were
decreased plasma cholinesterase activity (> 20%) in animals at 8 ppm
and inhibition of erythrocyte acetylcholinesterase activity (< 12%)
in those at 8 ppm throughout the study, with peak inhibition (56%) at
16 ppm by week 8. Gross and microscopic examination of tissues and
organs at the end of the experiment showed slightly increased liver
weights in males at 16 or 32 ppm, which was not reflected in the
calculated relative organ:body weight ratios or on histological
examination. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original reports of
Löser, 1968a; Mawdesley-Thomas & Urwin, 1970a). Although the NOAEL was
originally considered to be 4 ppm on the basis of inhibition of plasma
cholinesterase activity (JMPR, 1974), the present Meeting decided that
the NOAEL was 8 ppm, equivalent to 0.4 mg/kg bw per day, on the basis
of inhibition of erythrocyte acetylcholinesterase activity.
Rabbits
An aqueous formulation of technical-grade fenamiphos (purity, 89.8%)
was applied to a clipped dorsal area of groups of six male and six
female New Zealand rabbits at doses of 0, 2.5, or 10 mg/kg bw per day
for 6 h per day, five days per week for three weeks. Two additional
groups were similarly treated at 0 and 0.5 mg/kg bw per day. The skin
of half of the animals in each group was abraded. Haematology,
clinical chemistry, and urinalysis were performed before treatment and
at the end of the study. Plasma and erythrocyte cholinesterase
acetylcholinesterase activities were measured before treatment and
after the tenth and last exposures.
No signs of toxicity or mortality were observed. Although the authors
concluded that body-weight gains were decreased in animals of each sex
at 10 mg/kg bw per day (JMPR, 1985), the differences were less than
10% and were not statistically significant. Furthermore, the range of
individual weight gains seen in animals at the high dose was well
within that observed in the controls. Slight erythema was observed in
all groups only at the abraded skin sites during the initial week,
which cleared by day 7. There were no apparent differences in
haematological, urinary, or clinical chemical parameters between test
and control groups. Gross necropsy, histopathology, and organ weight
measurements showed no remarkable changes in comparison with controls.
Plasma and erythrocyte cholinesterase activity was significantly
depressed (by up to 65 and 34%, respectively) in male and female
rabbits at 10 mg/kg bw per day; however, the females were somewhat
more sensitive, with depressed plasma cholinesterase activity (by
22-31%) at 2.5 mg/kg bw per day as well. Brain acetylcholinesterase
activity was statistically significantly depressed in females at 2.5
mg/kg bw per day (by 19%) and 10 mg/kg bw per day (by 21%). The NOAEL
was 0.5 mg/kg bw per day (JMPR, 1974, slightly modified by reference
to the original report by Mihail & Schilde, 1980).
Dogs
Groups of two beagle dogs of each sex were fed fenamiphos (purity,
82%) in the diet at 0, 2, 6, or 18 ppm (equivalent to 0.05, 0.15, or
0.45 mg/kg bw per day) for three months. Behavioural abnormalities
with signs of cholinergic stimulation were evident in animals at 18
ppm, and the growth of females at this dose was reduced.
Haematological and clinical chemical parameters, including the results
of tests for liver and kidney function and urinalyses, were not
affected by treatment. The averages values for plasma and erythrocyte
cholinesterase activity were depressed in males and females at 6 ppm;
in animals at 2 ppm, no effect was found on average values for
erythrocyte acetylcholinesterase activity, while those for plasma
cholinesterase were depressed (20-30%). Brain acetylcholinesterase
activity was not measured. Gross morphological examination of tissues
and organs at the end of the study showed no abnormal effects (JMPR,
1974, slightly modified by reference to the original report by Löser,
1968b).
Groups of two male and two female beagle dogs (three of each sex as
controls) were fed fenamiphos (purity, 99.4%) in the diet at levels of
0, 1, 2, or 5 ppm (equivalent to 0.025, 0.05, or 0.13 mg/kg bw per
day) for three months. Treatment at < 5 ppm in the diet had no
effect on the average values of haematological parameters, liver
function tests, clinical chemistry, or kidney function tests or on the
gross or microscopic appearance of tissues and organs. As in other
studies, cholinesterase activity was the only parameter significantly
affected, the females being more susceptible than the males and plasma
cholinesterase activity being more sensitive to inhibition than that
of erythrocytes. The latter was unchanged at 2 ppm while plasma
cholinesterase activity was slightly, transiently depressed; 1 ppm
fenamiphos in the diet had no effect on plasma cholinesterase
activity. Brain acetylcholinesterase activity was not measured (Löser,
1969; Mawdesley-Thomas & Urwin, 1970b; JMPR, 1974).
As an extension of the previous study, an additional two dogs of each
sex were fed fenamiphos(purity, 99.4%) in the diet at 0 or 10 ppm
(equivalent to 0.25 mg/kg bw per day) for three months. No deaths
occurred during the experiment, although a very slight deviation in
the average body weight of treated animals was noted. Because of the
small number of animals, the slight weight differences cannot be fully
evaluated. There was no effect on haematological or urinary
parameters, clearance, blood sugar, or cholesterol levels.
Cholinesterase activity was significantly depressed in both plasma and
erythrocytes in male and female dogs; brain acetylcholinesterase
activity was not measured. Gross and microscopic examination of
tissues and organs showed no significant differences between treated
and control animals (Löser, 1970; Thomson et al., 1972a; JMPR, 1974).
Groups of four male and four female, four-month-old beagle dogs were
fed diets containing fenamiphos (purity, 89%) at doses of 0, 0.6, 1,
or 1.7 ppm (equivalent to 0.015, 0.025, or 0.042 mg/kg bw per day) for
three months. They were observed twice daily, and body weight and food
consumption were recorded weekly. Plasma and erythrocyte
cholinesterase activity was determined at 0, 4, 6, 8, 10, and 12
weeks; brain acetylcholinesterase was determined at termination of the
study. Haematology, clinical chemistry, and urinalysis were not
performed, and tissues and organs were not examined grossly or
histologically. No toxicological symptoms or effects on body weight
and food consumption were seen; however, plasma cholinesterase
activity was depressed by 28-35% in males given 1.7 ppm in the diet.
The author concluded that females at this dose were not similarly
sensitive (JMPR, 1985), on the basis of the < 20% inhibition relative
to control levels; however, when inhibition was calculated over the
course of the study, the reductions in plasma cholinesterase activity
in females at all doses were 2-14% in controls, 13-23% in animals at
0.6 ppm, 18-28% at 1 ppm, and 31-41% at 1.7 ppm, which were comparable
to those in males: 10-15% in controls, 17-23% in dogs at 0.6 ppm,
20-29% at 1 ppm, and 34-41% at 1.7 ppm. Erythrocyte and brain
acetylcholinesterase activities were unaffected (JMPR, 1985, slightly
modified by reference to the original report by Hayes, 1983).
Technical-grade fenamiphos (purity, 88.9%) was administered in the
diet to groups of four male and four female beagle dogs at
concentrations of 0, 1, 3, or 12 ppm (equal to 0, 0.03, 0.089, or 0.31
mg/kg bw per day in males and 0, 0.03, 0.083, and 0.35 mg/kg bw per
day in females) for one year. The animals were observed for mortality,
clinical signs, body weight, food consumption, ophthalmic alterations,
and haematological, urinary, and clinical chemical parameters. Plasma
and erythrocyte cholinesterase activity was measured before treatment
and quarterly thereafter. One-half of each brain was taken at necropsy
for measurement of acetylcholinesterase activity. At necropsy, 11
organs were taken from each dog and weighed, and about 40 tissues from
each dog were examined grossly and histopathologically.
Treatment did not affect survival, clinical signs, growth, food
consumption, ophthalmoscopic or urinary parameters, or organ weights.
Them were no gross or histopathological findings that could be
attributed to treatment. Plasma cholinesterase activity was
statistically significantly inhibited relative to the levels before
treatment in both males and females in a dose-related fashion at doses
> 1 ppm: 1 ppm, 20-32%; 3 ppm, 41-53%; 12 ppm, 54-65%; the control
values were variably reduced by up to 14% over the course of the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly decreased in both males and females at
3 ppm (by 17-36%) and 12 ppm (by 58-68%); the activity in the controls
and dogs at 1 ppm was variably, statistically nonsignificantly reduced
by 5-24% and 10-23%, respectively, during the course of the study.
Brain acetylcholinesterase activity was nonsignificantly decreased by
12% in males and sigificantly reduced by 17% in females at 12 ppm.
Males at this dose also had mild, transient anaemia characterized by
significantly decreased erythrocyte counts, haemoglobin
concentrations, and haematocrit, with a concomitant increase in mean
corpuscular volume. The authors concluded that there was no NOAEL
because of the effects on plasma cholinesterase activity at the lowest
dose (Riethet al., 1991). Owing to the variability in the activity of
plasma cholinesterase in controls seen in a six-month follow-up (Jones
& Loney, 1993; see below), the authors reconsidered the results of the
one-year study and decided that the changes seen at 1 ppm were within
the normal biological range seen in historical controls (Jones &
Greufe, 1993). The Meeting agreed that the NOAEL was 3 ppm, equal to
0.083 mg/kg bw per day, on the basis of decreased brain
acetylcholinesterase activity and anaemia at the highest dose.
In the supplemental study (Jones & Loney, 1993), technical-grade
fenamiphos (purity, 89%) was administered in the diet to groups of
four male and four female beagle dogs at concentrations of 0 or 0.5
ppm, equal to 0.011 mg/kg bw per day, for six months. The only
parameters measured were mortality, clinical and ophthalmologic signs,
body weight, food consumption, and plasma and erythrocyte
cholinesterase activity. No signs of toxicity were observed.
Groups of four male and four female pure-bred beagle dogs were fed
fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5, or 10 ppm (equal
to 0.015, 0.029, 0.06, 0.15, or 0.31 mg/kg bw per day for males and
0.014, 0.036, 0.063, 0.17, and 0.34 mg/kg bw per day for females) for
two years. There were no significant effects on growth, food
consumption, or the results of any of the standard clinical and
physiological examinations made during the course of the study. Gross
and histological examination of all tissues and organs at the
conclusion of the study showed no abnormal developments considered to
be related to treatment. The only significant physiological effect
observed was inhibition of plasma cholinesterase activity (> 20%) at
doses > 2 ppm and of erythrocyte acetylcholinesterase activity at
> 5 ppm. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original report by
Löser, 1972a; Thomson et al., 1972b).
Cattle
Groups of three dairy cows were fed fenamiphos sulfoxide in their
diets at levels of 2, 6, or 20 ppm for 29 days; one untreated cow was
used as a control. There were no apparent effects on behaviour, feed
consumption, milk production, or body-weight gain. No adverse effects
on whole-blood cholinesterase activity were seen with 2 or 6 ppm, but
a significant depression (51%) was seen at 20 ppm (Wargo, 1978; JMPR,
1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female six-week-old outbred CD1 albino mice
were given fenamiphos (purity, 89.5%) in the diet at doses of 0, 2,
10, or 50 ppm (equal to 0, 0.3, 1.4, or 7.4 mg/kg bw per day for males
and 0, 0.3, 1.8, or 8.8 mg/kg bw per day for females) for 20 months.
Toxic effects were monitored daily, and body weights and food
consumption were determined weekly. Haematological parameters were
analysed in 10 mice of each sex in each group at 6, 12, 18, and 20
months of the study. All animals underwent complete necropsy, and the
liver, kidney, heart, lungs, gonads, spleen, brain, and adrenals were
weighed. A full complement of tissues and organs from all animals were
examined histopathologically. Cholinesterase activity was not
measured. Daily observations were not reported. Survival was
comparable in all groups, with only a marginal decrease at the high
dose. Survival at 20 months was 32-45%. Body weights were
statistically significantly reduced in both males and females at 50
ppm, but the differences were less than 10%. Food consumption and
haematological parameters were unaffected by treatment.
Contrary to the conclusions of the 1985 JMPR with regard to changes in
organ weights, reexamination of the data indicates that the absolute
weights of the brains of female mice at all doses were slightly (4-6%)
but statistically significantly lower than those of controls. The
relative weights of the brains of animals at 50 ppm were, however,
increased in both males (7%, statistically nonsignificant) and females
(9%, statistically significant). The absolute and relative ovarian
weights were decreased by 16-18% in animals at 10 ppm and by 42-55% in
those at 50 ppm; and the absolute and relative splenic weights were
reduced in animals of each sex at 50 ppm. Other findings include
reductions in absolute heart, lung, liver, and kidney weights
(generally 10-15%, of variable significance) in males and females at
50 ppm, although the relative weights were comparable to those in
controls. No gross or microscopic parallel to these weight changes was
found. The most frequent pathological findings reported were chronic
multifocal interstitial nephritis, chronic peribronchiolitis,
pulmonary congestion, and acute and chronic myocarditis in all treated
animals, but no significant difference associated with dose was found.
Cystic endometrial hyperplasia was also frequent in all treated
females. A significant degree of fatty diffuse change was seen in the
liver, again with no relation to dose. In all groups, uniformly spread
amyloidosis was observed in many organ systems, including liver,
spleen, adrenals, kidneys, small intestines, thyroids, ovaries, and
submaxillary salivary glands. Fenamiphos had no oncogenic potential at
any dose. The NOAEL was 2 ppm, equal to 0.3 mg/kg bw per day, on the
basis of decreased splenic and ovarian weights at doses > 10 ppm
(JMPR, 1985, slightly modified by reference to the original report by
Hayes, 1982).
Rats
Groups of 40 male and 40 female SPF-derived Wistar rats were fed
fenamiphos in the diet at concentrations of 0, 3, 10, or 30 ppm (equal
to 0, 0.17, 0.56, or 1.7 mg/kg bw per day for males and 0, 0.23, 0.76,
or 2.2 mg/kg bw per day for females) for two years. Behavioural
abnormalities due to cholinergic stimulation were seen only during the
first six weeks of treatment at 30 ppm. The obvious effect on
cholinesterase activity disappeared after six weeks of feeding and was
not seen for the remainder of the study. Average growth, mortality,
food consumption, haematological and clinical chemical parameters, and
the results of liver and kidney function tests were unchanged. Urinary
parameters and blood sugar and cholesterol values were normal. The
thyroid weights and the thyroid:body weight ratios of females at 30
ppm were larger than those of controls but were not accompanied by
abnormal tumour development, goitre, or unusual histological findings.
All other major tissues and organs appeared normal at gross and
microscopic examination.
Feeding of 10 ppm fenamiphos in the diet inhibited plasma
cholinesterase activity by up to 55% in females but by < 20% in
males. In animals at 30 ppm, plasma cholinesterase activity was
inhibited by up to 60% and that of erythrocyte acetylcholinesterase by
up to 54% in males and 65% in females. No significant effects were
observed at 3 ppm. Although the NOAEL was originally considered to be
3 ppm, the present Meeting determined it to be 10 ppm (equal to 0.56
mg/kg bw per day) on the basis of inhibition of erythrocyte
acetylcholinestrase activity at the next highest dose (JMPR, 1974,
slightly modified by reference to the original report by Löser, 1972b;
Cherry & Newman, 1973).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenamiphos (purity, 89.3%) at a mean
concentration of 0, 1.7, 7.8, or 37 ppm (equal to 0, 0.098, 0.46, or
2.5 mg/kg bw per day for males and 0, 0.12, 0.6, or 3.4 mg/kg bw per
day for females) for two years. Clinical signs, mortality, food
consumption, haematological and blood chemical parameters (in 20 rats
of each sex per group), urinary parameters (in 10 rats of each sex per
group), and body weights were monitored throughout the study. Plasma
and erythrocyte cholinesterase, activity was monitored in 10 rats of
each sex per group at weeks 6, 10, and 15 and at 6, 12, 18, and 24
months. Additional groups of 10 rats of each sex were given 0 or 37
ppm fenamiphos for one year. Brain acetylcholinesterase activity was
determined at the end of the study in all rats in these satellite
groups and in 10 rats of each sex per group in the main study. All
rats, including those found dead or killed in extremis during the
study and those killed at 12 or 24 months, were examined grossly;
their organs were weighed, and more than 40 tissues were evaluated
microscopically. Ophthalmological examinations were performed on 10
rats of each sex per dose before and at the end of the study.
Survival at termination of the study was not related to treatment and
was 58-88% in males and 62-84% in females. Clinical observation
indicated a higher incidence of rough coat and alopecia in females at
the high dose than in controls. A statistically significant decrease
in body-weight gain was seen in male and female rats at the high dose
throughout the study, although no treatment-related effect on feed
consumption was seen. There was a dose-related, statistically
significant decrease in plasma cholinesterase activity in all treated
rats throughout the study: in mate rats, 7-38% at 1.7 ppm, 19-68% at
7.8 ppm, and 56-88% at 37 ppm; in females, 22-96% throughout the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly inhibited in treated animals: by 0-7% at
1.7 ppm, 12-25% at 7.8 ppm, and 56-81% at 37 ppm in males and 0-11% at
1.7 ppm, 21-43% at 7.8 ppm, and 62-81% at 37 ppm in females. A
statistically significant decrease in brain acetylcholinesterase
activity was observed in male rats at the high dose at termination
(-14%) and in animals of each sex (-25% in males and -24% in females)
at interim sacrifice after one year of treatment. There were
statistically significant increases in the relative weights of the
brain, heart, and lung in both males and females at the high dose at
the end of the study; females at the high dose also had significantly
increased relative kidney weights. At interim sacrifice, females had
statistically significantly increased relative weights of adrenals,
brain, heart, kidneys, and ovaries. These changes were considered to
be related to the decrease in body weight observed in rats of each sex
at 37 ppm. The only statistically significant change in absolute organ
weight at termination was a decrease in liver weight and an increase
in lung weight in animals of each sex at 37 ppm. No treatment-related
change was seen during ophthalmological examination, in food
consumption, in haematological, clinical chemical, or urinary
parameters, or on gross pathology. No treatment-related neoplastic
lesions were observed during histopathological examination, but
statistically significantly higher incidences of non-neoplastic
inflammatory lesions were observed in the nasal, laryngeal, and lung
tissues of rats receiving 37 ppm fenamiphos in the diet when compared
with controls. These changes were attributed by the author of the
study to the marked inhibition of cholinesterase activity in these
animals. No treatment-related non-neoplastic changes were noted at 1.7
or 7.8 ppm. The author concluded that fenamiphos was not oncogenic.
Contrary to the conclusions of the 1987 JMPR, the present Meeting
determined that the NOAEL was 7.8 ppm, equal to 0.46 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinestrase activity and
changes in body-weight gain, organ weights, and histoptahological
appearance at the next highest dose (JMPR, 1987, slightly modified by
reference to the original report by Hayes, 1986a).
(d) Genotoxicity
Fenamiphos has been tested adequately in a battery of tests for
mutagenicity (Table 4). It was weakly clastogenic only in vitro; no
similar response was seen in vivo in tests for micronucleus
formation and dominant lethal mutation. The Meeting concluded that
fenamiphos is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A standard three-generation (two litters per generation) study of
reproductive toxicity was performed in which groups of 10 male and 20
female FB30 rats were given fenamiphos in the diet at 0, 3, 10, or 30
ppm throughout mating, gestation, and suckling. Immediately after
birth, pups were examined for malformations and were then prepared for
another generation or killed. Five weanling rats per group from the
F3b. generation were killed, and macroscopic and microscopic
examinations were performed on the major tissues and organs. There
were no apparent differences in the limited indices of reproduction
investigated, including fertility, litter size, lactation index, or
growth of young, or in the incidence of malformations (Löser, 1972c;
Cherry et al., 1972; JMPR, 1974).
In a two-generation study of reproductive toxicity, groups of 30 male
and 30 female albino CD Sprague Dawley rats received technical-grade
fenamiphos (purity, 89%) in the diet at concentrations of 0, 2.5, 10,
or 40 ppm (equal to 0.17, 0.64, or 2.8 mg/kg bw per day for males and
0.2, 0.73, or 3.2 mg.kg bw per day for females) for 70 days before
mating. Oestrous cycles were characterized over two weeks in 10 F0
and F1 females per dose before mating After weaning, 30 F1 animals
of each sex per dose were treated for 70 days and then bred to produce
the second generation (F2); treatment was continued throughout
mating, gestation, and lactation. Groups of 10 F0 and F1 adults of
each sex per dose were used to assess cholinesterase activity in
plasma and erythrocytes in week 8 before mating and just before
sacrifice and in brain at the time of sacrifice. Plasma, erythrocyte,
and brain cholinesterase activities were measured in one pup of each
sex from each of 10 litters at the time of culling and on day 21 of
lactation. F0 and F1 females were killed after their pups had been
weaned or on day 24 of gestation. The males were killed after the last
litters were delivered. The histologic al examinations concentrated on
reproductive tissues.
There were no treatment-related deaths or clinical signs of toxicity
in the parental animals. In F0 and F1 dams at 40 ppm, statistically
significant reductions were seen in body-weight gain during lactation
(by 72 and 65%) and food consumption (by up to 11 and 19%). F1 and
F2 pups also had significant reductions in body-weight gain beginning
on day 7 of lactation. The body weights of F1 adults at 40 ppm were
significantly reduced throughout the premating period (by about 10% in
males and 7% in females), which the author attributed to their reduced
body weights at the start of the F1 premating period. Although not
reported by the author, the overall weight gain of F1 males before
mating was also reduced during the first four weeks, by 12% in gain
those at 10 ppm and by 15% in those at 40 ppm. The relative ovarian
weights of F0 females were significantly reduced, by 13% at 2.5 ppm,
11% at 10 ppm, and 20% at 40 ppm but were unaffected in F1 females.
Although the author concluded that this effect was related to
treatment at 40 ppm, no histopathological lesions were observed in the
ovaries, reproductive parameters were unchanged, and there was no
comparable effect in the F1 generation.
Plasma cholinesterase activity was significantly inhibited by > 20%
in all treated adult females of both generations, in F1 males at 10
ppm at the time of sacrifice, and in both F0 and F1 males at 40 ppm
both before mating and at sacrifice. Erythrocyte acetylcholinesterase
activity was consistently significantly inhibited in females at doses
> 10 ppm but only at 40 ppm in F0 and F1 males. At 40 ppm, brain
acetylcholinesterase activity was significantly inhibited by 21% in
F0 females, by 29% in F1 females, and by only 6% in F0 males. In
pups, plasma cholinesterase activity was significantly inhibited on
day 21 of lactation in both males and females of both generations at
doses > 10 ppm. Erythrocyte acetylcholinesterase activity was
inhibited by > 20% in males and females of both generations at 40 ppm
only. Brain acetylcholinesterase was not affected.
Table 4. Results of tests for genotoxicity with fenamiphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 4, 20, 100, 500, 2500 NR (Negative)a,b Herbold (1979)
TA1535, TA1537 µg/plate (DMSO)
TA98, TA100
Reverse mutation S. typhimurium 20, 100, 125, 250, 500, 92.4 Negativea Herbold (1985a,b)
TA98, TA100 1000, 2000, 2500
TA1535, TA1537 µg/plate (DMSO)
Forward mutation Chinese hamster Unactivated: 100, 110, 85 Negativea,c Yang et al. (1984)
ovary cells 120, 130 µg/ml (DMSO)
(CHO-K1-BH4) Activated: 170, 190,
210, 230 µg/ml (DMSO)
Unscheduled Rat hepatocytes 1.5, 5, 15, 50, 100 µg/ml 89.5 Negative Curren (1988)
DNA synthesis (DMSO)
Chromosomal Human 25, 100, 400 µg/ml 91.3 Positivea,d Herbold (1987)
aberrations lymphocytes (DMSO)
Chromosomal Human Unactivated: 91.9 Negative Herbold (1988)
aberrations lymphocytes 25, 50, 75, 100 µg/ml
(DMSO)
Activated: 100, 150, Weakly
225, 350 µg/ml (DMSO) positivee
Sister chromatid Chinese hamster Activated: 10, 20, 40, NR Negativef Chen et al. (1982)
exchange cell line (V79) 80 µg/ml
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
(%)
In vivo
Micronucleus Mouse (NMRI) 0.625, 1.25, 2.5 mg/kg 92.5 (Negative)g Herbold (1980a)
formation bone-marrow cells bw
Dominant lethal Mouse (male 5 mg/kg bw 92.5 Negative Herbold (1980b)
mutation NMRI) germ cells
NR, not reported; DMSO, dimethyl sulfoxide
a With and without metabolic activation
b The 1985 JMPR concluded that the test protocol was unacceptable. Another test was conducted in only
one strain (TA 1537), without activation, at doses of 125, 250, 500, and 1000 µg/plate.
c Positive controls (0.2 µl/ml ethylmethylsulfonate; 2 µg/ml benzo[a]pyrene) yielded the expected positive responses.
d Statistically significant increase (52% mitotic index) seen at 100 µg/ml without activation and at 400 µg/ml with
activation (< 0.1% mitotic index). Haemolysis was seen at 400 µg/ml. The author concluded that the increase in
aberrations was due exclusively to cytotoxicity.
e Statistically significant response seen only at 350 µg/ml with activation (35% mitotic index). The author concluded
that the increase in aberrations was due to cytotoxicity.
f Positive control (5 µg/ml cyclophosphamide) gave expected positive response.
g The mice were dosed twice, 24 h apart, and the bone marrow was sampled once, 6 h after the second dose. The 1985
JMPR concluded that the test protocol was unacceptable. The protocol was also considered unacceptable in the report
of the Gene-Tox Program (Mavournin et al., 1990).
Oestrous cycles, mating, fertility, and gestation indices, sex ratio,
and pup viability indices were unaffected, and no treatment-related
gross or histological lesions seen in any tissue from either parental
animals or pups. The pups showed no clinical signs of toxicity. The
NOAEL for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per
day, on the basis of decreased body-weight gain. The NOAEL for
reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per day, on
the basis of decreased pup body weights during lactation (Eigenberg,
1991).
(ii) Developmental toxicity
Rats
Four groups of 25 female FB30 rats mated overnight with untreated
males (in a ratio of one male to two females) were given fenamiphos
(purity, 92.5%) in a 0.5% aqueous Cremophor emulsion at daily doses of
0, 0.3, 1, or 3 mg/kg bw per day by gavage on days 6-15 of gestation;
the first day of gestation was that on which sperm was found in a
smear obtained the morning after mating. Control females received the
same volume (10 ml/kg bw) of the aqueous emulsion. On day 20 of
gestation, the dams were narcotized with carbon dioxide and the
fetuses were removed. Litter size, average fetus weight per litter,
sex, external and visceral abnormalities, and skeletal malformations
and development were noted. Cholinesterase activity was not measured.
Eighteen dams receiving 3 mg/kg bw per day showed signs of toxicity
(trembling and recumbency), and two died; however, the time of death
was not given and the cause of death could not be established. No
treatment-related signs of toxicity or deaths occurred in rats
receiving 0, 0.3, or 1 mg/kg bw per day. A 15% reduction in average
weight gain was seen during treatment among dams receiving 3 mg/kg bw
per day in comparison with controls, but the author reported that the
difference was not statistically significant. A total of six females
-- one control, two at 0.3 mg/kg bw per day, two at 1 mg/kg bw per
day, and one at 3 mg/kg bw per day -- were not fertilized. The average
placental weight of animals at the high dose was significantly lower
than that in controls. Treatment did not affect the number of
fertilized or pregnant females, litter size, number of resorptions,
number of fetuses, average fetal weight, sex ratio, incidence of
alterations in development, or the type or number of malformations.
The most frequent manifestations were nodulations on ribs, which were
found in two fetuses from one litter of a dam at 0.3 mg/kg bw per day
and in four fetuses of three litters of dams at 1 mg/kg bw per day.
The other malformations observed were general oedema, abdominal
fissure, and anophthalmia in one fetus at 0.3 mg/kg bw per day. No
malformations were observed in fetuses at the high dose. The lower
placental weight observed at the high dose was considered to be not
toxicologically significant because the average weight was within the
normal range and no effects were seen on embryonic or fetal
development. Thus, fenamiphos at doses < 3 mg/kg bw per day was not
embryotoxic or teratogenic; it was, however, toxic to dams at 3 mg/kg
bw per day (Schlüter, 1981; JMPR, 1987).
Groups of 33 mated female Crl:CD-BR rats were given fenamiphos
(purity, 88.7%) at doses of 0, 0.25, 0.85, or 3 mg/kg bw per day by
gavage on days 6-15 of gestation. Five dams from each group were
killed on day 16 in order to measure plasma, erythrocyte, and brain
cholinesterase activities; the remaining dams were killed on day 20 of
gestation and necropsied grossly. All dams were examined for number of
corpora lutea and implantation sites, and their uteri and placentas
were weighed. The fetuses were weighed and sexed; about half were
examined externally and in the viscera, and the other half were
processed for skeletal examination. Brain acetylcholinesterase
activity was measured in 20 fetuses per group.
Six dams at 3 mg/kg bw per day died between days 7 and 14 of
gestation, one female with convulsions on the day of its death.
Clinical signs of toxicity, such as tremors, salivation, lachrymation,
urine staining, and hypoactivity, were seen to varying extents in the
survivors. Body-weight gain and food consumption were significantly
reduced throughout treatment at this dose. Gross necropsy revealed no
treatment-related abnormalities, and gestational parameters were
unaffected. Fetal body weights were unchanged, and they had no
treatment-related variations or malformations. Plasma and erythrocyte
cholinesterase activities were statistically significantly reduced (by
50 and 42%, respectively) in dams at 3 mg/kg bw per day on day 16 of
gestation. By day 20, the plasma activity had returned to normal,
while that of erythrocyte cholinesterase was still significantly
reduced by 30%. Brain acetylcholinesterase activity was reduced by 28%
in adults at 0.85 mg/kg bw per day and by 12% in those at 3 mg/kg bw
per day; as the differences were not significant or dose-related, they
were not considered to be related to treatment. Fetal brain
acetylcholinesterase activity was unaffected. The NOAEL for maternal
toxicity was 0.85 mg/kg bw per day. Fenamiphos was not teratogenic or
fetotoxic under the conditions of this study (Clemens et al., 1989).
Rabbits
Groups of 20 double-mated female New Zealand rabbits were given
fenamiphos (purity, 88.8%) orally in corn oil at doses of 0, 0.1, 0.3,
or 1 mg/kg bw per day on days 6-18 of gestation. They were observed
daily for clinical signs of toxicity and were weighed initially,
periodically during the test, and at termination of the study. Once
the pups had been removed, the ovaries and uteri of the dams were
examined, and the fetuses were examined grossly and prepared for
evaluations of soft tissues and the skeleton. The numbers of corpora
lutea, implantations, resorptions, live and dead fetuses, and
anomalies were also determined. Cholinesterase activity was not
assessed. Dams given doses > 0.3 mg/kg bw per day showed signs of
toxicity, with decreased body-weight gain, bloody nasal discharge, and
white, mucoid ocular discharge. Treatment did not affect the number of
litters, number of pups per litter, pregnancy rate, the number of
corpora lutea, implantations, or gross abnormalities. Mean fetal
weight was slightly depressed at 1 mg/kg bw per day. One dam at 0.3
mg/kg bw per day aborted one dead pup, and two at 1 mg/kg bw per day
aborted eight dead pups and had seven late resorptions. In addition,
one dead fetus was found in each of two litters at the high dose.
The commonest developmental variation observed was the left carotid
arising from the innominate, which occurred in six to eight fetuses
(7-9%) in each of three litters (25%) at doses > 0.1 mg/kg bw per
day. This anomaly was not seen in the controls and in only one of 31
litters (3.2%) of historical controls at the laboratory where the
study was performed. More recent data (through July 1985) for
historical controls revealed incidences of 11/336 (3.3%) in fetuses
and 9/53 (17%) litters. The newer historical control data provide some
evidence that the incidence of left carotid anomalies was increased in
superovulated or artificially inseminated rabbits. Furthermore, data
for rabbits of the same strain in different labs indicate that the
anomaly is a frequent finding, occurring in about 8% of fetuses and
25% of litters; the historical data are for artificially inseminated
rabbits, while the animals used in this study were naturally bred. The
biological significance of this finding in relation to treatment is
dubious.
An increased incidence of accessory skull bones was also seen in all
treated groups, but it did not occur in a dose-related manner and was
thus not considered to be related to treatment. A significant increase
in the incidence of chain-fused sternebrae was seen in five fetuses in
three litters at 1 mg/kg bw per day, and this anomaly was also seen in
one fetus in one litter at 0.3 mg/kg bw per day. This anomaly is of
questionable biological significance. Two fetuses in one litter at the
high dose had aortic arches with a common truncus, which was
considered to be a major malformation. Other skeletal malformations
which occurred only at doses > 0.3 mg/kg bw per day included fused
ribs, scoliosis, absent vertebrae (thoracic, lumbar, sacral, and
caudal), and bipartite or malformed centra. The 1985 Meeting concluded
that these findings were related to treatment; however, these
anomalies occurred in only one or two fetuses in single litters, often
with no relation to dose. Furthermore, several of the anomalies were
clustered within a single fetus, indicating that they were not likely
to be related to treatment. The NOAEL for maternal toxicity was 0.1
mg/kg bw per day, and that for developmental toxicity was 0.3 mg/kg bw
per day. Fenamiphos had no teratogenic effects at any dose (JMPR,
1985, modified by reference to the original report by MacKenzie,
1982).
In a study to determine the doses for a study of embryotoxicity
(including teratogenicity), groups of three mated (1:1) female
Chinchilla rabbits were given single daily doses of fenamiphos
(purity, 91%) in distilled water with 0.5% Cremophor EL0 at doses of
0.1, 0.8, or 3 mg/kg bw per day by gavage on days 6-18 post coitum.
Cholinesterase activity was measured in plasma and erythrocytes before
the first dose and just after the last dose; brain
acetylcholinesterase activity was not assessed. All of the animals
were killed on day 28 post coitum, and the fetuses were removed,
sexed, weighed, and examined for gross external and internal
abnormalities.
One female at the high dose lost weight from day 10 and died on day 12
post coitum; body-weight loss and reduced food consumption were seen
throughout treatment in all animals at this dose when compared with
controls. Statistically significant reductions in both plasma (89%)
and erythrocyte cholinesterase activity (90%) relative to controls
were noted in rabbits at the high dose on day 18 post coitum. Five
preimplantation losses and one fetal resorption were observed in does
at the high dose but in no other group. Marginal effects on body
weight and food consumption were seen at 0.8 mg/kg bw per day. No
treatment-related effects were seen on the numbers of corpora lutea,
implantations, live or dead fetuses, or on fetal weight (Becker et
al., 1986; JMPR, 1987).
In the main study, four groups of 16 single-mated female Chinchilla
rabbits were given fenamiphos (purity, 91%) in distilled water
containing 0.5% Cremophor as single daily doses of 0, 0.1,0.5, or 2.5
mg/kg bw per day by gavage on days 6-18 post coitum. The does were
killed on day 28 post coitum, and the fetuses were removed. Only
does with at least one living fetus were used in calculating
body-weight gain, food consumption, and reproductive parameters. They
were examined for the position of fetuses in the uterus and numbers of
corpora lutea, implantations, resorptions, and live and dead fetuses.
The fetuses were weighed, sexed, and examined for external and
internal malformations, skeletal abnormalities, and development.
Cholinesterase activity was not assessed. Dosing solutions were
prepared daily. The concentration of fenamiphos in triplicate samples
taken for determination of homogeneity before the study was found to
vary considerably (14-140% of the nominal concentration), with mean
concentrations of 71 ± 8.3, 84 ± 64, and 70 ± 16% of the nominal
concentration at doses of 0.1, 0.5, and 2.5 mg/kg bw per day,
respectively. At the next sampling 10 days later, the mean
concentrations were all within 90% of the nominal concentration,
although the variability was still high (92 ± 37, 97 ± 45, and 99 ±
41% of the nominal concentration at the three doses, respectively).
Although it is stated that homogeneity was maintained during dosing by
use of a magnetic stirrer, it is not clear if the samples taken for
testing were subjected to the same treatment before analysis.
Four females at 2.5 mg/kg bw per day died as a result of treatment
after 3, 5, and 10 days and on day 21 post coitum. Treatment-related
signs of toxicity (salivation and dyspnoea) were observed in these
females and in five other females at the high dose between days 7 and
18 post coitum. Ataxia was seen in two females that died, and
diarrhoea was observed in another. No signs of toxicity were found in
animals at 0, 0.1, or 0.5 mg/kg bw per day. A treatment-related,
statistically significant decrease in mean food consumption (29%) and
a treatment-related reduction in body-weight gain (56%) were observed
during treatment in does at the high dose when compared with controls.
Food consumption was significantly increased in this group on days
24-28.
No treatment-related or significant difference was observed between
treated and control animals with regard to the mean numbers of
implantations, corpora lutea, live or dead fetuses, or resorptions. In
fetuses, no effect that could be attributed to treatment was seen in
sex ratio, body weight, external or internal malformations, skeletal
abnormalities, or development. One fetus at the high dose had
encephalocele with reduced brain size, but this finding was not
considered to be related to treatment. A number of skeletal changes
unrelated to treatment were seen in fetuses at all doses. The NOAEL
for maternal toxicity was 0.5 mg/kg bw per day. Although questions
remain about the doses actually administered, because of the problems
of homogeneity, the study showed no embryotoxicity or teratogenicity
at maternally toxic doses (JMPR, 1987, modified by reference to the
original report by Becker, 1986).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenamiphos painted on the skin of New Zealand white
rabbits in an acetone solution at 50 mg/kg bw resulted in slight
erythema but was not considered to be a primary irritant. Application
of technical-grade fenamiphos to the conjunctival sac of New Zealand
white rabbits as 100 mg of a crystalline material resulted in
irritation considered to be mechanical rather than physiological
(Crawford & Anderson, 1971; JMPR, 1974).
Technical-grade fenamiphos (purity, 90.7%) was melted at 35°C, and
0.5 ml was painted onto the intact or abraded skin of six
Japanese-derived albino rabbits for 24 h under occlusion. Irritation
was scored according to Draize 24, 48, 72, and 168 h after
application. Minimal irritation was observed, characterized by slight
erythema and oedema during the first 48 h. The mean primary irritation
index, based on readings at 24 and 72 h, was 0.42 (Kato 1984a).
Technical-grade fenamiphos (purity, 90.7%) was heated to liquidity
(temperature not specified) and applied at 0.1 ml to the conjunctival
sacs of nine Japanese-derived albino rabbits. The eyes of three of the
rabbits were washed with water. Irritation was scored according to
Draize 24, 48, 72, 96, 168, and 240 h after administration. In the
absence of washing, fenamiphos was a moderate irritant, with a maximum
average score of 31.3 at 24 h. The irritation scores slowly declined,
and all of the eyes were clear by 240 h. Fenamiphos was not irritating
when the eyes were washed with water after application. Midriasis,
persisting until day 2 or 3, was seen in all animals when the eyes
were not washed within 10 min of application. The translation of the
report leaves some uncertainty about other ocular effects, but signs
of systemic toxicity peaked within 3-4 h of application; these
included salivation, increased respiration, cyanosis, and slight
convulsions (number affected not specified). All animals were
reportedly normal within 6 h. No systemic toxicity was seen in the
animals when the eyes were washed (Kato, 1984b).
The potential of fenamiphos to sensitize skin was examined in a
maximization test in which 20 male Hsd Win:DH guinea-pigs were induced
intradermally with 1% fenamiphos in saline containing 2% Cremophor EL.
One week later, they were induced topically with 25% fenamiphos in
saline (with 2% Cremophor EL); they were challenged three weeks later
with 12 and 25% solutions of fenamiphos in the saline solution. Patchy
erythema was seen 24 h after removal of the patch in 22% of the
animals challenged with the 25% solution, whereas none of the 10
induced control animals showed irritation. The author considered a
response rate of > 30% to be indicative of sensitization, and
concluded that fenamiphos produced no relevant sensitization; however,
according to the original protocol of Magnusson and Kligman, these
results would indicate that fenamiphos is a mild sensitizer (Stropp,
1995).
(ii) Delayed neuropathy
Groups of eight hens were fed fenamiphos in the diet at levels of 0,
1,3, 10, or 30 ppm, equal to 0, 2, 5, 16, or 26 mg/kg bw per day, for
30 days. At the end of treatment, some birds were killed and the
remainder were observed for four weeks for neurological signs of
poisoning. Food consumption was depressed in birds at 30 ppm, and the
average body weight and growth of hens at this dose was reduced.
Whole-blood cholinesterase activity was decreased after 30 days at
doses > 1 ppm, although no signs of cholinergic poisoning were
observed. There were no indications of delayed neurotoxicity, and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with haematoxylin and eosin) did not indicate delayed
neuropathy (Kimmerle, 1970; Spicer, 1970; JMPR, 1974).
Groups of 10 hens given an LD50 a dose of fenamiphos (5.0 mg/kg bw)
orally were observed for three weeks and then killed. No evidence of
delayed neurotoxicity was observed either clinically or
histologically, whereas signs were observed with tri- ortho-cresyl
phosphate (Kimmerle, 1971; Spicer, 1971; JMPR, 1974).
Fenamiphos (purity, 91.3%) in a 2% Cremophor solution was administered
twice by intubation to 30 Lohmann selected Leghorn hens at 25 mg/kg bw
per day at a 21-day interval. Five hens serving as positive controls
received tri- ortho-cresyl phosphate at 375 mg/kg bw per day. The
hens treated with fenamiphos received atropine intramuscularly at 100
mg/kg bw before treatment and subcutaneously at 30-50 mg/kg bw 7, 24,
or 30 or 48 h after treatment. The birds were observed for body
weight, clinical signs, forced motor coordination, and gross and
histopathological changes. Although statistically significant,
treatment-related weight loss (during week 1) and signs of severe
poisoning were seen, the birds showed no impairment of motor
coordination indicative of delayed neurotoxicity. Histological
examination of tissues from the peripheral and central nervous systems
showed no changes indicative of delayed neuropathy. Birds given
tri- ortho-cresyl phosphate, however, showed clinical signs of
delayed neurotoxicity (ataxia and paresis) two weeks after treatment
and were killed in moribund condition on day 17. The histopathological
changes in these birds were typical of neuropathy (Flucke & Kaliner,
1987).
(iii) Neurotoxicity
Rats
Groups of 12 Wistar (Hsd Win:WU) rats of each sex were given single
doses of technical-grade fenamiphos (purity, 95.2%) at 0.37, 1.52, or
2.31 mg/kg bw by garage. They were observed for mortality, clinical
signs, and body weight. Functional observational and motor or
locomotor activity tests were conducted on all animals before
treatment, within 30 min of treatment, and 7 and 14 days later.
Plasma, erythrocyte, and brain cholinesterase activity was measured in
additional satellite groups of six animals of each sex at each dose,
which were killed within 1 h of treatment. One-half of the animals in
the main group were perfused, and various neural and skeletal muscle
tissues, including the brain, spinal cord and spinal ganglia, eyes and
optic nerves, peripheral nerves, gastrocnemic muscle, and trigeminal
ganglia, were processed for histological examination.
In animals at the lowest dose, plasma cholinesterase activity was
statistically significantly inhibited in females (by 55%) and
nonsignificantly decreased in males (by 23%). Erythrocyte
acetylcholinesterase activity was significantly inhibited only in
males (by 24%), but this was not considered to be an adverse effect
because no corroborating clinical signs were seen in the functional
and motor activity tests. At the next highest dose, both plasma and
erythrocyte cholinesterase activity was significantly inhibited in
both males (by 64 and 70%, respectively) and females (by 77 and 51%,
respectively). Uncoordinated gait and muscle fasciculations were also
seen in males during the functional observational battery of tests. At
the highest dose, similar signs were seen, with decreased grip
strength and deaths among both males and females and decreased motor
activity in malesś Other behavioural or physiological changes seen in
animals of each sex in the functional observational battery of tests
included piloerection, nasal, oral, and lachrymal staining,
salivation, and decreased activity and rearing in the open field.
Brain acetylcholinesterase activity was unaffected at all doses. No
treatment-related histopathological changes were seen. The NOAEL was
0.37 mg/kg bw, the lowest dose tested, on the basis of uncoordinated
gait and muscle fasciculations in males at the next highest dose
(Dreist, 1995).
In a study of similar design, technical-grade fenamiphos (purity,
95.6%) was administered in the diet to groups of 12 Wistar (Hsd
Cpb:WU) rats of each sex for 13 weeks at dietary concentrations of 0,
1, 10, or 50 ppm, equal to 0, 0.06, 0.61, or 3.1 mg/kg bw per day in
males and 0.08, 0.8, or 4 mg/kg bw per day in females. The animals
were observed for deaths, clinical signs, body weight, and food and
water consumption. Functional observational and motor or locomotor
activity tests were conducted before treatment and in weeks 4, 8, and
13. Plasma and erythrocyte cholinestemse activity was measured in six
rats of each sex at each dose on week 4 and before terminal sacrifice
at week 15, and brain acetylcholinesterase activity only at terminal
sacrifice.
All females at the highest dose had muscle fasciculations during the
first three weeks of the study. Plasma and erythrocyte cholinesterase
activities were statistically significantly reduced in both males
(> 68%) and females (> 86%). Brain acetylcholinesterase activity
was also significantly reduced (by 12%) in females, but the author
considered that this was not biologically significant. At 10 ppm,
plasma cholinesterase activity was significantly reduced in both males
(by 30-39%) and females (by 71-77%; rho < 0.01) in weeks 4 and 15.
Erythrocyte acetylcholinesterase activity was inhibited by about 25%
in week 15 in males (rho < 0.05) and in weeks 4 and 15 in females
at 10 ppm. The lowest dose resulted in nonsignificant decreases (about
30%) in plasma cholinesterase activity in females in weeks 4 and 15.
Body weights, food and water consumption, brain weights, and gross and
histopathological appearance were all unaffected by treatment. The
author extrapolated the results back to a 20% level of inhibition of
plasma cholinesterase activity and estimated that the NOAEL in females
was 0.4 ppm. The overall NOAEL was 10 ppm, equal to 0.8 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase activity
in females at the highest dose (Dreist & Popp, 1995).
(iv) Potentiation
In male rats given fenamiphos orally in combination with disulfoton or
E 154, no potentiation of the acute toxicity was seen (Kimmerle,
1972c; JMPR, 1974).
The LD50 for fenamiphos (purity, 91.8%) administered orally to male
Wistar rats was 4.6 mg/kg bw, while that of carbofuran was 8.1 mg/kg
bw. When the two compounds were given concomitantly, essentially as a
2:1 ratio of carbofuran:fenamiphos, the LD50 was 6 mg/kg bw,
indicating that the toxicity of the combination was additive but not
synergistic (Mihail, 1980 JMPR, 1985).
Comments
In rats, fenamiphos was rapidly excreted, with over 96% of the
administered dose eliminated within 48 h. Excretion was primarily in
the urine, with less than 4% of the dose eliminated in the faeces. At
48 h, the levels of tissue residues were below the limit of
quantification, except following a high dose (3 mg/kg bw), when the
maximal tissue levels observed were 3.5-8.4 µg/kg in the liver,
1.6-2.1 µg/kg in the kidney, and 1.6-3.5 µg/kg in the skin. Fenamiphos
was completely metabolized in rats. Metabolites retaining
anticholinesterase activity, such as fenamiphos sulfoxide and
desisopropyl fenamiphos sulfoxide, were seen in variable but generally
low proportions (rarely greater than 3%). Most of the products were
dephosphorylated phenol, sulfoxide phenol, or sulfone phenol
metabolites and their corresponding sulfates.
Fenamiphos is extremely hazardous after single oral doses to rats,
mice, rabbits, cats, dogs, and chickens (LD50 values = 2 4-23 mg/kg
bw) and highly hazardous after dermal administration to rats and
rabbits (LD50 values, 75-230 mg/kg bw). It is moderately hazardous
after inhalation in rats and mice (LC50 values < 100 µg/L, 4 h).
WHO has classified fenamiphos as 'extremely hazardous' (WHO, 1996).
The sulfoxide, sulfone, and desisopropylated sulfone metabolites of
fenamiphos are similarly toxic to rats after oral administration
(LD50 values = 1.4-4.1 mg/kg bw). The sulfoxide and sulfone phenol
metabolites are only slightly toxic to rats after oral administration,
with LD50 values ranging from 1200 to 1900 mg/kg bw.
Fenamiphos inhibited plasma cholinesterase more effectively than
erythrocyte acetylcholinesterase, both in vitro and in vivo.
Fenamiphos sulfoxide, fenamiphos sulfone, desisopropyl fenamiphos,
desisopropyl fenamiphos sulfoxide, and desisopropyl fenamiphos sulfone
inhibited plasma and erythrocyte cholinesterase in vitro more
effectively than fenamiphos itself.
In evaluating the following studies, inhibition of erythrocyte
acetylcholinesterase activity was not used as an indicator of adverse
effects in the nervous system when information on brain
acetylcholinesterase activity was also available. In the absence of
this information, NOAELs were determined on the basis of inhibition of
erythrocyte acetylcholinesterase (of < 20%). Statistical
significance was used as a criterion for considering depression of
brain acetylcholinesterase activity to be adverse.
In a three-week study in which rats were exposed by inhalation to
atmospheres containing fenamiphos at 0, 0.03, 0.25, or 3.5 µg/L for 6
h per day, five days per week, the only finding was inhibition of
plasma cholinesterase at the highest dose. Erythrocyte and brain
acetylcholinesterase were unaffected. The no-observed-adverse-effect
concentration (NOAEC) was 3.5 µg/L.
In a three-month study in rats, fenamiphos given at dietary
concentrations of 0, 0.37, 0.57, or 0.91 ppm inhibited plasma
cholinesterase activity only at the highest dose. No treatment-related
changes in erythrocyte or brain acetylcholinesterase activity were
seen at any dose. In a second study of short-term toxicity, rats were
fed diets containing 0, 4, 8, 16, or 32 ppm fenamiphos for three
months. Erythrocyte acetylcholinesterase activity was inhibited at
doses of 16 ppm (equivalent to 0.8 mg/kg bw per day) and above. This
was considered an adverse effect as brain acetylcholinesterase was not
measured in this study. The overall NOAEL was 8 ppm, equivalent to 0.4
mg/kg bw per day.
Rabbits received fenamiphos by dermal application at doses of 0, 0.5,
2.5, or 10 mg/kg bw per day for three weeks (6 h per day, five days
per week). Body-weight gain was slightly reduced in animals of each
sex at 10 mg/kg bw per day. In females, reductions in cholinesterase
activity in the brain (by 20%) and plasma were noted at 2.5 mg/kg bw
per day and above. Erythrocyte acetylcholinesterase activity, however,
was affected only at 10 mg/kg bw per day. In males, the only findings
were decreased plasma and erythrocyte cholinesterase activity at 10
mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day.
In a series of studies, dogs were fed diets containing 0, 0.5, 0.6, 1,
1.7, 2, 3, 5, 6, 10, 12, or 18 ppm fenamiphos for periods ranging from
three months to two years. In dogs treated at 18 ppm (equivalent to
0.45 mg/kg bw per day) for three months, muscle tremors were seen. In
dogs treated at 12 ppm (equal to 0.31 mg/kg bw per day) for one year,
brain acetylcholinesterase activity was inhibited in females (by 17%),
and males showed slight anaemia. Erythrocyte acetylcholinesterase
activity was inhibited at doses of 3 ppm (equal to 0.083 mg/kg bw per
day) and above for one year. Plasma cholinesterase activity was
inhibited at doses of 1.7 ppm (equivalent to 0.042 mg/kg bw per day)
and above in a three-month study, No other parameters were affected.
Since no information on brain acetylcholinesterase activity was
available at doses between 3 and 12 ppm, the Meeting considered 3 ppm
(equal to 0.083 mg/kg bw per day) to be the overall NOAEL in dogs.
In mice fed diets containing 0, 2, 10, or 50 ppm fenamiphos for 20
months, there were marginal decreases in survival and body-weight gain
at 50 ppm (equal to 7.4 mg/kg bw per day). The relative ovarian and
spleen weights were reduced at 10 ppm (equal to 1.4 mg/kg bw per day)
and above. There were no non-neoplastic changes that could be
attributed to treatment, and fenamiphos was not carcinogenic at any
dose. Cholinesterase activity was not measured. The NOAEL was 2 ppm,
equal to 0.3 mg/kg bw per day.
In rats fed diets containing 0, 3, 10, or 30 ppm fenamiphos for two
years, the only treatment-related effects were inhibition of
erythrocyte acetylcholinesterase activity throughout the study and
behavioural changes during the first six weeks of the study in animals
at 30 ppm. Brain acetylcholinesterase activity was not measured. The
NOAEL was 10 ppm, equal to 0.56 mg/kg bw per day.
Rats were fed diets containing 0, 1.7, 7.8, or 37 ppm fenamiphos for
two years. At 37 ppm, equal to 2.5 mg/kg bw per day, body-weight gain
in both males and females was decreased. Erythrocyte
acetylcholinesterase activity was inhibited at 7.8 ppm (equal to 0.46
mg/kg bw per day) and above. Brain acetylcholinesterase activity was
inhibited only at 37 ppm; inhibition was 25% in animals of each sex
killed after one year, and 14% in males at termination of the study.
Animals of each sex at 37 ppm also had an increased frequency of
non-neoplastic inflammatory lesions of the nasal, laryngeal, and lung
tissues and increased relative weights of the brain, heart, and lungs.
Fenamiphos was not carcinogenic at any dose. The NOAEL was 7.8 ppm,
equal to 0.46 mg/kg bw per day.
Fenamiphos was adequately tested in a battery of tests for
genotoxicity. It was found to be mildly clastogenic at cytotoxic doses
in vitro but not in vivo. It did not cause reverse or forward
mutation, unscheduled DNA synthesis, or sister chromatid exchange
in vitro. The Meeting concluded that fenamiphos is not genotoxic.
In a two-generation study of reproductive toxicity, rats were treated
with 0, 2.5, 10, or 40 ppm fenamiphos. Parental toxicity was
characterized by reduced weight gain in F0 and F1 dams at 40 ppm
(equal to 2.8 mg/kg bw per day) during lactation, and in F1 males at
10 ppm (equal to 0.64 mg/kg bw per day) and above before mating.
Pathological changes in the salivary gland were seen in F0 males and
females at 40 ppm. Erythrocyte acetylcholinesterase activity was
consistently inhibited at 10 ppm and above in females but only at 40
ppm in males. Brain acetylcholinesterase activity was inhibited at the
highest dose in adult F0 and F1 females (by 21-29%) and in F1 males
(by 6%) but not in pups of either sex. In pups at 40 ppm, erythrocyte
acetylcholinesterase activity was inhibited only on day 21 of
lactation. The only reproductive effect was decreased weight gain of
F1 and F2 pups at 40 ppm, beginning on day 7 of lactation. The NOAEL
for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per day. The
NOAEL for reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per
day.
In a study of developmental toxicity, mated rats were treated with 0,
0.3, 1, or 3 mg/kg bw per day on days 6-15 of gestation. Maternal
toxicity was seen at the highest dose, characterized by mortality,
tremors, and reduced weight gain. The fetuses were not affected at any
dose. Cholinesterase activity was not measured in this study. The
NOAELs were 1 mg/kg bw per day for maternal toxicity and 3 mg/kg bw
per day for developmental toxicity.
Fenamiphos was also administered to mated rats at doses of 0, 0.25,
0.85, or 3 mg/kg bw per day on days 6-15 of gestation. The highest
dose resulted in maternal deaths, tremors, salivation, lachrymation,
urine staining, and hypoactivity. Body-weight gain and food
consumption were also significantly reduced. Erythrocyte
acetylcholinesterase activity was reduced at this dose, but the
changes in brain acetylcholinesterase activity were not statistically
significant or dose-related. The fetuses were unaffected at 3 mg/kg bw
per day. The NOAELs were 0.85 mg/kg bw per day for maternal toxicity
and 3 mg/kg bw per day for developmental toxicity.
In a study of developmental toxicity in rabbits, animals received 0,
0.1, 0.3, or 1 mg/kg bw per day on days 6-18 of gestation. At 0.3
mg/kg bw per day and above, fenamiphos was maternally toxic, resulting
in decreased body-weight gain, bloody nasal discharge, and white
ocular discharge. Fetotoxicity, characterized by chain fusion of the
sternebrae, was seen only at 1 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.1 mg/kg bw per day, and that for developmental
toxicity was 0.3 mg/kg bw per day. Cholinesterase activity was not
measured in this study.
In a second study, mated rabbits were treated with 0, 0.1, 0.5, or 2.5
mg/kg bw per day on days 6-18 of gestation. Clear maternal toxicity
was seen at the highest dose, which included mortality, salivation,
dyspnoea, ataxia, diarrhoea, and decreased weight gain and food
consumption during treatment. Although some questions remain about the
doses that were actually administered (because of uncertain
homogeneity), no embryotoxic or teratogenic effects were seen at the
maternally toxic dose of 2.5 mg/kg bw per day.
Fenamiphos was minimally irritating to rabbit skin and moderately
irritating to rabbit eyes and was a mild skin sensitizer in the
guinea-pig.
A single dose of 25 mg/kg bw fenamiphos had no effect on neuropathy
target esterase activity in the brains or spinal cords of hens, under
atropine protection. Fenamiphos did not induce delayed neuropathy in
three studies in hens when tested at doses of 0, 2, 5, 16, or 26 mg/kg
bw per day for 30 days or when given once at doses of 0 or 25 mg/kg
bw.
In a study of acute neurotoxicity, rats were given single doses of 0,
0.37, 1.5, or 2.3 mg/kg bw fenamiphos by gavage. Erythrocyte
acetylcholinesterase activity was inhibited at the lowest dose tested
in males only, but in both males and females at higher doses. At 1.5
mg/kg bw, males showed uncoordinated gait and muscle fasciculation. At
the highest dose, decreased motor activity was also seen in males, and
clinical signs, decreased grip strength, and deaths occurred in rats
of each sex. There was no effect on brain acetylcholinesterase
activity, at any dose. The NOAEL was 0.37 mg/kg bw per day. In a
further study, rats were fed diets containing 0, 1, 10, or 50 ppm
fenamiphos for 13 weeks. At 10 ppm and above, erythrocyte
acetylcholinesterase activity was inhibited in animals of each sex. At
the highest dose, brain acetylcholinesterase activity was inhibited
(by 12%) in females only. A battery of functional observational and
motor activity tests revealed no treatment-related effects. The NOAEL
was 10 ppm, equal to 0.61 mg/kg bw per day.
An ADI of 0-0.0008 mg/kg bw was established on the basis of an overall
NOAEL of 0.083 mg/kg bw per day in the dog, and a safety factor of
100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2 ppm in the diet, equal to 0.3 mg/kg bw per day
(20-month study of toxicity and carcinogenicity)
Rat: 0.37 mg/kg (single doses, study of neurotoxicity) 10
ppm, equal to 0.61 mg/kg bw per day (three-month study
of neurotoxicity)
2.5 ppm, equal to 0.17 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
10 ppm, equal to 0.64 mg/kg bw per day (study of
reproductive toxicity)
0.85 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity)
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to fenamiphos
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral neurotoxicity, rat NOAEL = 0.37 mg/kg bw per day: effects observed
(1-7 days) during battery of functional observational tests
Oral toxicity, rat (fasted) LD50 = 2.4-6 mg/kg bw
Inhalation toxicity, 4 h, rat LC50 = 91-100 µg/L
Inhalation toxicity, 5 days, rat NOAEL = 4 µg/L
Dermal toxicity, rat LD50 = 72-92 mg/kg bw
Dermal irritation, rabbit Minimally irritating
Ocular irritation, rabbit Moderately irritating
Dermal sensitization, guinea-pig Mildly sensitizing
Medium-term Repeated inhalation toxicity, 3 weeks, rat NOAEL = 3.5 µg/L (highest dose tested)
(1-26 weeks) Repeated dermal toxicity, 3 weeks, rabbit NOAEL = 0.5 mg/kg bw per day: inhibition of brain
acetylcholinesterase activity
Repeated oral, reproductive toxicity, rat NOAEL = 0.17 mg/kg bw per day: parental toxicity
NOAEL = 0.64 mg/kg bw per day: reproductive
toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.1 mg/kg bw per day: maternal toxicity
NOAEL = 0.3 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral, 1 - 2 years, dog NOAEL = 0.083 mg/kg bw per day: inhibition of
(> 1 year) acetylcholinesterase activity, anaemia
7.8 ppm, equal to 0.46 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
0.3 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 3 ppm in the diet, equal to 0.083 mg/kg bw per day
(overall assessment)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Estimate of acute reference dose
The available data did not permit the Meeting to establish an acute
reference dose different from the ADI (0-0.0008 mg/kg bw). Although
the results of a study of neurotoxicity in rats given single doses was
available, the dog was found to be the more sensitive species.
Information on acute effects in dogs may allow the establishment of an
acute reference dose in the future.
Studies that would provide information useful for continued
evaluation of the compound
1. Effects of single doses in dogs (with appropriate evaluation of
functional changes in the cholinergic nervous system, including
brain acetylcholinesterase activity).
2. Observations in humans
References
Becker, H. (1986) Embryotoxicity (including teratogenicity) study with
SRA 3886 in the rabbit. Unpublished report No. R 3917 (project No.
065261) from Research & Consulting Co., Itingen, Switzerland.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Mueller, E., Luetkemeir, H. & Sachsse, K. (1986)
Dose-finding embryotoxicity (including teratogenicity) study with SRA
3886 in the rabbit. Unpublished report No. R3693 (project No. 065250)
from Research & Consulting Co., Itingen, Switzerland. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchange in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environ. Mutag., 4, 621624. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cherry, C. & Newman, A. (1973) Pathology report of Bayer 68138.
Chronic toxicological studies in rats. Unpublished report (addendum to
report No. 3539) from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Cherry, C., Urwin, C. & Newman, A. (1972) Pathology report of BAY
68138. Rat breeding study. Unpublished report (addendum to report No.
3424) from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1989) Teratology
study in the rat with Nemacur technical. Unpublished report No. 1145
(study no. MTD0108) from Toxicology Department, Miles Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F. & Wills, J.H. (1974) Summary of research by the Institute
of Comparative and Human Toxicology, Albany Medical College, on
phenamiphos. Report dated October 1974. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C. & Anderson, R. (1971) The skin and eye irritating
properties of BAY 68138 technical to rabbits. Unpublished report No.
29988 from Chemagro Division of Baychem Corp. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Crawford, C. & Anderson, R. (1972) The acute dermal toxicity of
Nemacur technical to rabbits. Unpublished report No. 34216 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R)
with analytical grade Nemacur(R). Unpublished report No. 34446 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974a) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Unpublished report No. 39700 from Chemagro Division of Baychem Corp.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974b) The acute oral toxicity of
Nemacur(R)-sulfone and Nemacur(R)-sulfoxide to rats. Unpublished
report No. 40215 from Chemagro Division of Baychem Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Curren, D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 991 (study no. T5724.380) from
Microbiological Associates Inc., Rockville, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Dreist, M. (1995) SPA 3886 (common name:Fenamiphos) acute oral
neurotoxicity screening study in Wistar rats. Unpublished report No.
24408 (study No. T6058166) from the Institute of Toxicology
Agrochemicals, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Dreist, M. & Popp, A. (1995) SRA 3886 (common name: Fenamiphos)
subchronic neurotoxicity screening study in Wistar rats (thirteen-week
administration in the diet). Unpublished report No. 24948 (study no.
T9058277) from Institute of Toxicology Agrochemicals, Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K. & Flynn, M. (1968) The subacute parenteral toxicity of BAY
68138 to rats. Unpublished report No. 22178 from Toxicity Laboratory,
University of Chicago, Chicago, IL, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K., Flynn, M. & Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Unpublished report No. 20810
from Toxicity Laboratory, University of Chicago, Chicago, IL, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ecker, W., Weber, H. & Brauner, A. (1989) [Phenyl-1-14C]Nemacur:
General metabolism study in the rat. Unpublished report No. PF3175
(study no. M182 0189-9) from Bayer AG, Pflanzenschutz-Zentrum Monheim,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Eigenberg, D.A. (1991) A two generation reproduction study in rats
using Fenamiphos (Nemacur(R)). Unpublished report No. 5762 (study
no. 88-671-BC) from Mobay Corp, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity. Unpublished report
dated 18 July 1980 from Bayer AG, Institute of Toxicology, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Eben, A. (1988) SPA 3886 Technical (common name:
Fenamiphos): Study of the effect on the neurotoxic esterase (NTE)
following oral administration to hens. Unpublished report No. 17388
from Fachbereich Toxikologie, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Kaliner, G. (1987) Delayed neurotoxicity studies on hens
following acute oral administration. Unpublished report No. 16187
(study no. T 1020919) from Fachbereich Toxikologie, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Gronberg, R.R. (1969) The metabolic fate of ethyl-4-(methylthio)-m-
tolyl isopropylphosphoramidate (BAY 68138), ethyl-4-(methylsulfinyl)-
m-tolyl isopropylphosphoramidate (BAY 68138 sulfoxide) and ethyl-4-
(methylsulfonyl)-m-tolyl-isopropylphosphoramidate (BAY 68138 sulfone)
in white rats. Unpublished report No. 26759 from Chemagro Division of
Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1982) Technical fenamiphos (Nemacur(R)) oncogenicity
study in mice. Unpublished report No. 241 (study No. 78CCM02) from
Stanley Research Center, Mobay Chemical Corp, Stilwell, KS, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. ( 1983 ) Ninety-day cholinesterase study on dogs with
fenamiphos in diet. Unpublished report No. 444 (study No. 83-174-01)
from Environmental Health Research, Mobay Chemical Corp, Stilwell, KS,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1986a) Ninety-day cholinesterase study on rats with
technical fenamiphos (Nemacur) in diet. Unpublished report No. 717
(study No. 83-171-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hayes, R.H. (1986b) Combined chronic toxicity/oncogenicity study of
technical fenamiphos (Nemacur) with rats. Unpublished report No. 721
(study No. 83-271-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K G (1981) Determination of acute toxicity (LD50 Unpublished
report dated 18 August 1981 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K G (1984) Determination of acute toxicity (LD) Unpublished
report dated 14 February 1984 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1979) Salmonella/microsome test for detection of
point-mutagenic effects. Unpublished report No. 8730 (study no.
Nemacur/003) from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980a) Micronucleus test on mouse to evaluate Nemacur
technical for potential mutagenic effects. Unpublished report No. 8805
(study no. SRA 3886/002) from Bayer AG Institute of Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980b) SRA 3886/dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8838 (study no. SRA
3886/001) from Institute of Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) SRA 3886: Salmonella/microsome test to evaluate
for potential point mutation. Unpublished report No. 13365 (study no.
T2017617) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985b) Addendum to report SRA 3886: Salmonella/microsome
test to evaluate for potential point mutation. Unpublished report No.
13365A (study no. T2017617) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1987) SRA 3886: Cytogenetic study of human lymphocyte
cultures in vitro to test for chromosome damage. Unpublished report
No. 15406, (study No. T3022874) from Bayer AG, Fachbereich Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1988) SRA 3886: In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report No. 16690, (study No. T1025572) from Bayer AG,
Fachbereich Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Greufe (1993) Response to US-EPA review: Chronic feeding
toxicity study with technical grade Fenamiphos (Nemacur(R)) with
dogs. Unpublished report dated 12 July 1993 (supplement to Mobay Corp.
study No. 88-274-BB) from Miles Inc., Stilwell, KS, USA. Submitted to
WHO by Bayer AG. Leverkusen, Germany.
Jones, R.D. & Loney, M.L. (1993) A subchronic feeding toxicity study
with technical grade Fenamiphos (Nemacur(R)) in dogs. Unpublished
report No. 91 - 176-KP (supplement to Mobay Corp. study No. 88-274-BB)
from Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kato, M.C. (1984a) Primary dermal irritation study of fenamiphos
technical in rabbitś Unpublished report No. B-561 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-561). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kato, M.C. (1984b) Primary eye irritation study of fenamiphos
technical in rabbitś Unpublished report No.B-562 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-562). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Khasawinah, A.M. & Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Unpublished report No. 34217 from Chemagro
Division of Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on hens.
Unpublished report No. 1831 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Unpublished
report No. 2829 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972a) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Unpublished report No. 3438 from
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Unpublished report No. 3559 from the
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972c) Antidotal experiments on rats. Unpublished report
No. 3560 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. ( 1971) BAY 68138 toxicological studies.
Unpublished report No. 2767 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Knaak, J.B., Ackerman, K.Y., Fredrickson, A.S. & Lee, P. (1981)
Reentry research: Dermal dose red cell cholinesterase-response curves
for fenamiphos, fenamiphos sulfoxide and sulfone. Unpublished report
No. 69689 from California Department of Food and Agriculture.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. ( 1988 ) Study for acute oral toxicity in rats.
Unpublished report No. 16744 (study No. T0027669) from Bayer AG,
Fachbereich Toxicologies, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Landes, A.M. (1978) In vitro inhibition of cholinesterase
by (R)Nemacur and Nemacur metabolites. Unpublished report No. 66068
(reference 77-060) from Mobay Chemical Corp., Chemagro Agricultural
Division, Kansas City, MO, US A. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of Nemacur
technical, desisopropyl Nemacur sulfoxide and desethyl Nemacur.
Unpublished report No. 44531 (reference 74-175) from Chemagro
Agricultural Division, Mobay Chemical Corp. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) The acute oral toxicity of Nemacur
desisopropyl to rats. Unpublished report No. 51597 (reference 75-144)
from Chemagro Agricultural Division, Mobay Chemical Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1968a) Subchronic toxicological studies on rats.
Unpublished report No. 745 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1968b) Subchronic toxicological studies on dogs.
Unpublished report No. 837 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser E (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Unpublished report No. 1655 from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1970) Subchronic toxicological studies on dogs. Unpublished
report No. 2008 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Unpublished report from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on rats (2 year
feeding experiment). Unpublished report No. 3539 from the Institute
for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1972c) Generation studies on rats. Unpublished report No.
3424 from the Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Löser, E. & Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachrichten Bayer,
24(1), 69-113. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MacKenzie, K.M. (1982) Teratology study with Nemacur in rabbits.
Unpublished report No. 308 (study No. 81165) from Hazelton Raltech,
Inc., Madison, WI, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mavournin, K.H., Blakey, D.H., Cimino, M.C., Salamone, M.F. & Heddle,
J.A. (1990) The in vivo micronucleus assay in mammalian bone marrow
and peripheral blood. A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 239, 29-80.
Mawdesley-Thomas, L. & Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Unpublished report
(addendum to report No, 745) from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mawdesley-Thomas, L. & Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Unpublished report (addendum
to report No. 1655) from the Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail F. (1980) SRA 3885 and Curaterr(R): Evaluation for acute
combination toxicity. Unpublished report No. 9294 from Institute of
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Mihail, F. & Schilde, B. (1980) SPA 3886 (active ingredient of
Nemacur(R)) subacute dermal toxicity study on rabbits. Unpublished
report No, 9297 from Institute of Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Rieth, J.P., Moore, K.D. & Elcock, L.E. (1991 ) Chronic feeding
toxicity study of technical grade Fenamiphos (Nemacur(R)) with dogs.
Unpublished report No. 6243 (study No. 88-274-BB) from Mobay Corp.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1981) SRA 3886 (Nemacur): Evaluation for embryotoxicity
and teratogenic effects in orally dosed rats. Unpublished report No.
9785 from Institute for Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Unpublished report (addendum to report
No. 1831 ) from the Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1971) Pathology report of BAY 68138. Hen study.
Unpublished report (addendum to report No. 2829) from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Stropp, G. (1995) Study for the skin sensitization effect in guinea
pigs (guinea pig maximization test according to Magnusson and
Kligman). Unpublished report No. 24341 (study No. T9058358) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Unpublished report (addendum
to report No. 2008) from Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Unpublished report (addendum to report No. 3561) from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted m
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974a) Nemacur(R) sulfone, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974b) Nemacur(R) sulfoxide, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974c) 3-Methyl-4-methylmercaptophenol, Acute toxicity in
rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974d) 3-Methyl-4-methane-sulfonylphenol, acute toxicity
in rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974e) 4-Methylmercapto-m-cresol, acute toxicity in rats.
Unpublished report dated 29 April 1974 from Institute for Toxicology,
Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979a) SPA 3885 (Nemacur(R) active ingredient)/acute
inhalation toxicity studies. Unpublished report No. 8210 from Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979b) Nemacur(R) active ingredient (SRA 3886)/subacute
inhalational toxicity studies. Unpublished report No. 8669 from
Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl 4-(methylthio)-N-
tolyl isopropylphosphoramidate] and identification of two metabolites
in plants. J. Agric. Food Chem., 20, 157-160. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Wargo, J.P. (1978) The effect of feeding Nemacur(R) sulfoxide to
dairy cattle. Unpublished report No. 66212 from Chemical Agricultural
Division, Mobay Chemical Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1988) [Phenyl-1-14C] Nemacur: Whole-body autoradiographic
distribution of the radioactivity in the rat. Unpublished report No.
PF3055 (study No. M181 0188-7) from Bayer AG Agrochemicals,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yang, L.L., Putnam, D.L. & Conklin, P.M. (1984) CHO/HGPRT mutation
assay in the presence and absence of exogenous metabolic activation --
Nemacur(R). Unpublished report No. 620 (study No. T2600.332) from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
inhibitory activity of fenamiphos and its metabolites in horse serum
cholinesterase in vitro was in the order fenamiphos < sulfoxide =
sulfone < unidentified metabolite.
The concentrations that inhibited the activity of chicken and monkey
liver aliesterase (triacetin hydrolysis) and monkey cholinesterase by
50% in vitro were 4.2 × 10-5 mol/L for chicken aliesterase, 1.0 ×
10-6 mol/L for monkey aliesterase, and 4.6 × 10-6 mol/L for monkey
cholinesterase (Coulston & Wills, 1974).
Male albino rats were painted dermally on the clipped dorsal area with
100-400 µg/cm2 fenamiphos, 25-800 µg/cm2 fenamiphos sulfoxide, or
200-1600 µg/cm2 fenamiphos sulfone. The test materials were applied
in acetone and allowed to dry. After 72 h, the animals were killed and
erythrocyte acetylcholinesterase activity determined. Fifty percent
inhibition was achieved with doses of 208 µg/cm2 fenamiphos, 262
µg/cm2 fenamiphos sulfoxide, and 750 µg/cm2 fenamiphos sulfone
(Knaak et al., 1981; JMPR, 1985).
Blood samples were extracted from equal numbers of male and female
Sprague-Dawley rats and then pooled for comparison of the
cholinesterase inhibition induced by fenamiphos and five of its
metabolites (fenamiphos sulfoxide, desisopropyl fenamiphos sulfoxide,
fenamiphos sulfone, desisopropyl fenamiphos sulfone, and desisopropyl
fenamiphos) in vitro. Samples were incubated for 1 h before
determination of the cholinesterase activity (Table 1). Erythrocyte
acetylcholinesterase was less sensitive to fenamiphos and its
metabolites than was plasma cholinesterase (Lamb & Landes, 1978; JMPR,
1985).
In order to assess the effects of fenamiphos on neurotoxic esterase
activity, single doses of 0 or 25 mg/kg bw technical-grade fenamiphos
(purity; 91.3%) were given by oral intubation to groups of nine
atropinized Lohmann selected Leghorn hens. Groups of three surviving
hens were killed one, two, and seven days after treatment. Even under
atropine protection, one hen died within 24 h of treatment. Fenamiphos
had no effect on neurotoxic esterase activity in the brains or spinal
cords, whereas the positive control compound, tri- ortho-cresyl
phosphate, had almost completely inhibited the activity 24 and 48 h
after treatment (Flucke & Eben, 1988).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of fenamiphos and its
metabolites are summarized in Table 2. Impurities identified as
components of the technical mixture (aryldiamide, diarylamide, diaryl
ethyl ester, diethyl ester, diethylmonamide, di-SCH3 compound, ethyl
aryl ester, ethyldiamide, and 4-methylthio- meta-cresol) were tested
for their acute toxicity to rats. At doses 1.5 times the acute LD50
of fenamiphos, none of the materials was toxic (Crawford & Anderson,
1973; JMPR, 1974).
Table 1. Inhibition of cholinesterase (%) by fenamiphos and its metabolites in vitro
Compound Plasma Erythrocytes
(Dose: ppm in whole blood) (Dose: ppm in whole blood)
6.88 68.8 688 5440 6880 6.88 68.8 688 5440 6880
Fenamiphos - 0 10 49 69 - 0 0 0 23
Fenamiphos sulfoxide 18 41 48 - 85 5 0 5 - 52
Fenamiphos sulfone 0 13 50 - 87 8 - - - -
Desisopropyl fenamiphos sulfoxide 6 40 90 - 93 4 0 47 - 50
Desisopropyl fenamiphos sulfone 0 20 69 - 90 6 0 32 - 61
Desisopropyl fenamiphos - 2 13 71 91 - 8 0 32 53
-, not determined
Table 2. Acute toxicity of fenamiphos, its metabolites, and impurities
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos
Mouse Oral M NR 22.7 Loser & Kimmerle (1971)
Intraperitoneal M/F 80 3.4 DuBois et al. (1967)
Inhalation M NR approx. 60 Kimmerle & Solmecke (1971)
(1 h static)
Rat Oral (fasted) M 88-99.7 2.4-6.0 Crawford & Anderson (1973, 1974a);
F 2.4-6.1 Lamb & Matzkanin (1975); Mihail (1980);
Heimann (1981, 1984, Krotlinger (1988)
Oral M 80-90.2 8.1-17.2 DuBois et al. (1967); Loser & Kimmerle (1971);
(not fasted) F 9.6-19.4 Kimmerle & Solmecke (1971); Kimmerle (1972a);
Heimann (1981, 1984)
Dermal M 80-92.2 72-73 Dubois et al. (1967); Flucke (1980)
84-92
Dermal M NR approx. 500 Kimmerle & Solmecke (1971)
Inhalation M 89.8 110-175 Kimmerle (1972b); Kimmerle & Solmecke
(1 h) F 130-150 Thyssen (1979a)
Inhalation M 89.8 91-100 Kimmerle & Solmecke (1971); Thyssen (1979a)
(4 h) 100
Intraperitoneal M 80 3.0-3.7 DuBois et al. (1967); Loser & Kimmerle (1971);
F 4.2-4.9 Kimmerle & Solmecke (1971)
Guinea-pig Oral M NR 56-100 DuBois et al. (1967); Loser & Kimmerle (1971);
Kimmerle & Solmecke (1971)
Intraperitoneal M NR 17.3 DuBois et al. (1967)
Rabbit Oral M NR 10-17.5 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Dermal M NR 225 Crawford & Anderson (1972)
F 179
Cat Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Dog Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Chicken Oral F NR 5.3-12 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971); DuBois et al. (1967)
Fenamiphos sulfone
Rat Oral (fasted) M NR 2.6 Crawford & Anderson (1974b)
F 2.4
Oral F NR 1-25 Thyssen (1974a)
(not fasted)
Fenamiphos sulfoxide
Rat Oral (fasted) M/F NR 2.4 Crawford & Anderson (1974b)
Oral F NR 10-25 Thyssen (1974b)
(not fasted)
Desisopropylfenamiphos
Rat Oral (fasted) M NR 1.4 Lamb & Matzkanin (1977)
F 2.1
Desisopropylfenamiphos sulfone
Rat Oral (fasted) M 95 4.1 Lamb & Matzkanin (1975)
F 3.7
Fenamiphos sulfoxide phenol
Rat Oral (fasted) M 99 1418 Crawford & Anderson (1974a)
F 1175
Oral F NR 500-1000 Thyssen (1974c)
(not fasted)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos sulfone phenol
Rat Oral (fasted) M 95 1250 Crawford & Anderson (1974a)
F 1854
Oral F NR > 1000 Thyssen (1974d)
(not fasted)
4-Methylthio-meta-cresol
Rat Oral (fasted) M 96.4 1418 Crawford & Anderson (I974a)
F 1333
Oral F NR > 2500 Thyssen (1974e)
(not fasted)
NR, not reported
Fenamiphos (purity, 97%) was given orally to fasted Wistar (Bor:WISW
(SPF-Cpb) rats at doses of 1-8 (females) or 1-100 (males) mg/kg bw.
All deaths among males at 100 mg/kg bw occurred within 10 min, and
those among animals receiving 5 mg/kg bw or more within 1.5 h.
Clinical signs of toxicity noted at doses >4 (males) or 5 (females)
mg/kg bw included apathy, palmospasms, laboured breathing, diarrhoea,
piloerection, clonic cramps, and dyspnoea. Females at does > 1
mg/kg bw had only diarrhoea (Krötlinger, 1988).
Studies in which rodents were given fenamiphos by inhalation as a
static spray at doses up to 230 µg/L for 1-4 h showed that rabbits and
guinea-pigs are more tolerant of the acute effects than rats and mice
(Kimmerle & Solmecke, 1971).
Male and female TNO/W74 albino rats were exposed to aerosolized
fenamiphos (purity, 89.8%) for 4 h per day on five consecutive days at
concentrations of 0, 0.3, 0.6, 3.3, 4, 9, or 28 µg/L. Plasma and
erythrocyte cholinesterase activities were measured before exposure,
after the first, third, and fifth exposures, and 72 h after the fifth
exposure; brain acetylcholinesterase activity was not measured. The
cholinesterase activities were depressed in a dose-related manner;
that of plasma cholinesterase was the most significantly depressed,
and females were more sensitive than males. Plasma cholinesterase
activity was 32-90% lower than before treatment in males at > 3.3
µg/L and 31-96% lower in females at > 0.3 µg/L. The activity
remained depressed by 19-44% for 72 h after the fifth exposure in
females at concentrations of > 9 µg/L. Erythrocyte
acetylcholinesterase activity in males was inhibited by < 20% at
> 9 µg/L; however, that in females was depressed by up to 28% at 9
µg/L and 33% at 28 µg/L (JMPR, 1985, slightly modified by reference to
the original report of Thyssen, 1979b).
In male rats, administration of atropine and/or 2-pralidoxime or
obidoxime after poisoning reduced the acute lethal dose by a factor of
about two. As with other organophosphorus compounds, rapid
administration of atropine and oxime reactivator after poisoning
affords some protection and alleviates the cholinergic signs of
poisoning (DuBois et al., 1967; Kimmerle, 1972c; JMPR, 1974).
(b) Short-term toxicity
Rats
Three groups of 10 male and 10 female albino Wistar TNO/W 74 rats were
exposed to technical-grade fenamiphos (purity, 92.2%) diluted with a
1:1 mixture of ethanol and polyethylene glycol 400 for aerosolization
in a dynamic flow inhalation chamber at doses of 0, 0.03, 0.25, or 3.5
µg/L for 6 h per day, five days per week for three weeks; 98% of the
particles were 3 µm or less. No toxic signs or effects on mortality,
body-weight gain, or the results of haematology, urinalysis, or
clinical chemistry were seen. A significant decrease (48-79%) in
plasma cholinesterase activity and a slight decrease (9-18%) in
erythrocyte acetylcholinesterase activity were seen in animals of each
sex at 3.5 µg/L; brain acetylcholinesterase activity was not affected.
There were no gross or histopathological changes or effects on organ
weights. The NOAEL was 3.5 µg/L (Thyssen, 1979b).
Groups of 15 male Sprague-Dawley rats were given fenamiphos orally or
by intraperitoneal injection on five days per week for 60 days. All of
the animals survived doses ranging from 1.5 mg/kg bw per day
intraperitoneally to 1.7 mg/kg bw per day orally with no cumulative
toxicological effect (Kimmerle & Solmecke, 1971; JMPR, 1974). In
another study, female rats survived daily intraperitoneal
administration of 1 mg/kg bw per day for 60 days, while 40% of those
given 2 mg/kg bw per day and all rats at 3 mg/kg bw per day died
(DuBois & Flynn, 1968; JMPR, 1974). These studies indicate little if
any cumulative toxicity.
Groups of 20 Fischer 344 rats of each sex were given diets containing
fenamiphos (purity, 89%) at 0, 0.37, 0.57, or 0.91 ppm (equal to 0,
0.03, 0.045, or 0.072 mg/kg bw per day for males and 0, 0.035, 0.053,
or 0.084 mg/kg bw per day for females) for three months. Animals were
monitored for clinical signs of toxicity, mortality, abnormality,
masses, food consumption, and body weight throughout the study.
Cholinesterase activity in plasma and erythrocytes was determined in
10 rats of each sex per group at 5, 9, 12, and 14 weeks and in brain
at termination of the study. No effects attributable to treatment were
seen on food consumption or body weight or during clinical
observation. Statistically but not toxicologically significant
decreases in both plasma and erythrocyte cholinesterase activity were
observed in treated rats of each sex when compared with controls. The
maximal inhibition of plasma cholinesterase was 17% at week 14 in
males at 0.37 ppm, with lesser inhibition at higher doses, and 24% at
week 9 in females at the high dose. Erythrocyte acetylcholinesterase
activity was inhibited by < 10% in all cases. No toxicologically
significant, treatment-related change was seen in brain
acetylcholinesterase activity. It was concluded that no biologically
significant cholinesterase activity inhibition had occurred in this
study. The NOAEL was 0.9 ppm, equal to 0.07 mg/kg bw per day, the
highest dose tested (JMPR, 1987, slightly modified by reference to the
original report of Hayes, 1986a).
Groups of Wistar SPF rats of each sex (15 per treated group, 30
controls) were fed diets containing fenamiphos (purity, 82%) at 0, 4,
8, 16, or 32 ppm (equivalent to 0.2, 0.4, 0.8, or 1.6 mg/kg bw per
day) for three months. Male and female rats at the highest dose showed
signs of cholinergic stimulation during the first two months; no
behavioural changes were seen in the other animals. Average food
consumption and growth were similar for treated and control animals.
The only effects on the haematological parameters examined were
decreased plasma cholinesterase activity (> 20%) in animals at 8 ppm
and inhibition of erythrocyte acetylcholinesterase activity (< 12%)
in those at 8 ppm throughout the study, with peak inhibition (56%) at
16 ppm by week 8. Gross and microscopic examination of tissues and
organs at the end of the experiment showed slightly increased liver
weights in males at 16 or 32 ppm, which was not reflected in the
calculated relative organ:body weight ratios or on histological
examination. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original reports of
Löser, 1968a; Mawdesley-Thomas & Urwin, 1970a). Although the NOAEL was
originally considered to be 4 ppm on the basis of inhibition of plasma
cholinesterase activity (JMPR, 1974), the present Meeting decided that
the NOAEL was 8 ppm, equivalent to 0.4 mg/kg bw per day, on the basis
of inhibition of erythrocyte acetylcholinesterase activity.
Rabbits
An aqueous formulation of technical-grade fenamiphos (purity, 89.8%)
was applied to a clipped dorsal area of groups of six male and six
female New Zealand rabbits at doses of 0, 2.5, or 10 mg/kg bw per day
for 6 h per day, five days per week for three weeks. Two additional
groups were similarly treated at 0 and 0.5 mg/kg bw per day. The skin
of half of the animals in each group was abraded. Haematology,
clinical chemistry, and urinalysis were performed before treatment and
at the end of the study. Plasma and erythrocyte cholinesterase
acetylcholinesterase activities were measured before treatment and
after the tenth and last exposures.
No signs of toxicity or mortality were observed. Although the authors
concluded that body-weight gains were decreased in animals of each sex
at 10 mg/kg bw per day (JMPR, 1985), the differences were less than
10% and were not statistically significant. Furthermore, the range of
individual weight gains seen in animals at the high dose was well
within that observed in the controls. Slight erythema was observed in
all groups only at the abraded skin sites during the initial week,
which cleared by day 7. There were no apparent differences in
haematological, urinary, or clinical chemical parameters between test
and control groups. Gross necropsy, histopathology, and organ weight
measurements showed no remarkable changes in comparison with controls.
Plasma and erythrocyte cholinesterase activity was significantly
depressed (by up to 65 and 34%, respectively) in male and female
rabbits at 10 mg/kg bw per day; however, the females were somewhat
more sensitive, with depressed plasma cholinesterase activity (by
22-31%) at 2.5 mg/kg bw per day as well. Brain acetylcholinesterase
activity was statistically significantly depressed in females at 2.5
mg/kg bw per day (by 19%) and 10 mg/kg bw per day (by 21%). The NOAEL
was 0.5 mg/kg bw per day (JMPR, 1974, slightly modified by reference
to the original report by Mihail & Schilde, 1980).
Dogs
Groups of two beagle dogs of each sex were fed fenamiphos (purity,
82%) in the diet at 0, 2, 6, or 18 ppm (equivalent to 0.05, 0.15, or
0.45 mg/kg bw per day) for three months. Behavioural abnormalities
with signs of cholinergic stimulation were evident in animals at 18
ppm, and the growth of females at this dose was reduced.
Haematological and clinical chemical parameters, including the results
of tests for liver and kidney function and urinalyses, were not
affected by treatment. The averages values for plasma and erythrocyte
cholinesterase activity were depressed in males and females at 6 ppm;
in animals at 2 ppm, no effect was found on average values for
erythrocyte acetylcholinesterase activity, while those for plasma
cholinesterase were depressed (20-30%). Brain acetylcholinesterase
activity was not measured. Gross morphological examination of tissues
and organs at the end of the study showed no abnormal effects (JMPR,
1974, slightly modified by reference to the original report by Löser,
1968b).
Groups of two male and two female beagle dogs (three of each sex as
controls) were fed fenamiphos (purity, 99.4%) in the diet at levels of
0, 1, 2, or 5 ppm (equivalent to 0.025, 0.05, or 0.13 mg/kg bw per
day) for three months. Treatment at < 5 ppm in the diet had no
effect on the average values of haematological parameters, liver
function tests, clinical chemistry, or kidney function tests or on the
gross or microscopic appearance of tissues and organs. As in other
studies, cholinesterase activity was the only parameter significantly
affected, the females being more susceptible than the males and plasma
cholinesterase activity being more sensitive to inhibition than that
of erythrocytes. The latter was unchanged at 2 ppm while plasma
cholinesterase activity was slightly, transiently depressed; 1 ppm
fenamiphos in the diet had no effect on plasma cholinesterase
activity. Brain acetylcholinesterase activity was not measured (Löser,
1969; Mawdesley-Thomas & Urwin, 1970b; JMPR, 1974).
As an extension of the previous study, an additional two dogs of each
sex were fed fenamiphos(purity, 99.4%) in the diet at 0 or 10 ppm
(equivalent to 0.25 mg/kg bw per day) for three months. No deaths
occurred during the experiment, although a very slight deviation in
the average body weight of treated animals was noted. Because of the
small number of animals, the slight weight differences cannot be fully
evaluated. There was no effect on haematological or urinary
parameters, clearance, blood sugar, or cholesterol levels.
Cholinesterase activity was significantly depressed in both plasma and
erythrocytes in male and female dogs; brain acetylcholinesterase
activity was not measured. Gross and microscopic examination of
tissues and organs showed no significant differences between treated
and control animals (Löser, 1970; Thomson et al., 1972a; JMPR, 1974).
Groups of four male and four female, four-month-old beagle dogs were
fed diets containing fenamiphos (purity, 89%) at doses of 0, 0.6, 1,
or 1.7 ppm (equivalent to 0.015, 0.025, or 0.042 mg/kg bw per day) for
three months. They were observed twice daily, and body weight and food
consumption were recorded weekly. Plasma and erythrocyte
cholinesterase activity was determined at 0, 4, 6, 8, 10, and 12
weeks; brain acetylcholinesterase was determined at termination of the
study. Haematology, clinical chemistry, and urinalysis were not
performed, and tissues and organs were not examined grossly or
histologically. No toxicological symptoms or effects on body weight
and food consumption were seen; however, plasma cholinesterase
activity was depressed by 28-35% in males given 1.7 ppm in the diet.
The author concluded that females at this dose were not similarly
sensitive (JMPR, 1985), on the basis of the < 20% inhibition relative
to control levels; however, when inhibition was calculated over the
course of the study, the reductions in plasma cholinesterase activity
in females at all doses were 2-14% in controls, 13-23% in animals at
0.6 ppm, 18-28% at 1 ppm, and 31-41% at 1.7 ppm, which were comparable
to those in males: 10-15% in controls, 17-23% in dogs at 0.6 ppm,
20-29% at 1 ppm, and 34-41% at 1.7 ppm. Erythrocyte and brain
acetylcholinesterase activities were unaffected (JMPR, 1985, slightly
modified by reference to the original report by Hayes, 1983).
Technical-grade fenamiphos (purity, 88.9%) was administered in the
diet to groups of four male and four female beagle dogs at
concentrations of 0, 1, 3, or 12 ppm (equal to 0, 0.03, 0.089, or 0.31
mg/kg bw per day in males and 0, 0.03, 0.083, and 0.35 mg/kg bw per
day in females) for one year. The animals were observed for mortality,
clinical signs, body weight, food consumption, ophthalmic alterations,
and haematological, urinary, and clinical chemical parameters. Plasma
and erythrocyte cholinesterase activity was measured before treatment
and quarterly thereafter. One-half of each brain was taken at necropsy
for measurement of acetylcholinesterase activity. At necropsy, 11
organs were taken from each dog and weighed, and about 40 tissues from
each dog were examined grossly and histopathologically.
Treatment did not affect survival, clinical signs, growth, food
consumption, ophthalmoscopic or urinary parameters, or organ weights.
Them were no gross or histopathological findings that could be
attributed to treatment. Plasma cholinesterase activity was
statistically significantly inhibited relative to the levels before
treatment in both males and females in a dose-related fashion at doses
> 1 ppm: 1 ppm, 20-32%; 3 ppm, 41-53%; 12 ppm, 54-65%; the control
values were variably reduced by up to 14% over the course of the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly decreased in both males and females at
3 ppm (by 17-36%) and 12 ppm (by 58-68%); the activity in the controls
and dogs at 1 ppm was variably, statistically nonsignificantly reduced
by 5-24% and 10-23%, respectively, during the course of the study.
Brain acetylcholinesterase activity was nonsignificantly decreased by
12% in males and sigificantly reduced by 17% in females at 12 ppm.
Males at this dose also had mild, transient anaemia characterized by
significantly decreased erythrocyte counts, haemoglobin
concentrations, and haematocrit, with a concomitant increase in mean
corpuscular volume. The authors concluded that there was no NOAEL
because of the effects on plasma cholinesterase activity at the lowest
dose (Riethet al., 1991). Owing to the variability in the activity of
plasma cholinesterase in controls seen in a six-month follow-up (Jones
& Loney, 1993; see below), the authors reconsidered the results of the
one-year study and decided that the changes seen at 1 ppm were within
the normal biological range seen in historical controls (Jones &
Greufe, 1993). The Meeting agreed that the NOAEL was 3 ppm, equal to
0.083 mg/kg bw per day, on the basis of decreased brain
acetylcholinesterase activity and anaemia at the highest dose.
In the supplemental study (Jones & Loney, 1993), technical-grade
fenamiphos (purity, 89%) was administered in the diet to groups of
four male and four female beagle dogs at concentrations of 0 or 0.5
ppm, equal to 0.011 mg/kg bw per day, for six months. The only
parameters measured were mortality, clinical and ophthalmologic signs,
body weight, food consumption, and plasma and erythrocyte
cholinesterase activity. No signs of toxicity were observed.
Groups of four male and four female pure-bred beagle dogs were fed
fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5, or 10 ppm (equal
to 0.015, 0.029, 0.06, 0.15, or 0.31 mg/kg bw per day for males and
0.014, 0.036, 0.063, 0.17, and 0.34 mg/kg bw per day for females) for
two years. There were no significant effects on growth, food
consumption, or the results of any of the standard clinical and
physiological examinations made during the course of the study. Gross
and histological examination of all tissues and organs at the
conclusion of the study showed no abnormal developments considered to
be related to treatment. The only significant physiological effect
observed was inhibition of plasma cholinesterase activity (> 20%) at
doses > 2 ppm and of erythrocyte acetylcholinesterase activity at
> 5 ppm. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original report by
Löser, 1972a; Thomson et al., 1972b).
Cattle
Groups of three dairy cows were fed fenamiphos sulfoxide in their
diets at levels of 2, 6, or 20 ppm for 29 days; one untreated cow was
used as a control. There were no apparent effects on behaviour, feed
consumption, milk production, or body-weight gain. No adverse effects
on whole-blood cholinesterase activity were seen with 2 or 6 ppm, but
a significant depression (51%) was seen at 20 ppm (Wargo, 1978; JMPR,
1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female six-week-old outbred CD1 albino mice
were given fenamiphos (purity, 89.5%) in the diet at doses of 0, 2,
10, or 50 ppm (equal to 0, 0.3, 1.4, or 7.4 mg/kg bw per day for males
and 0, 0.3, 1.8, or 8.8 mg/kg bw per day for females) for 20 months.
Toxic effects were monitored daily, and body weights and food
consumption were determined weekly. Haematological parameters were
analysed in 10 mice of each sex in each group at 6, 12, 18, and 20
months of the study. All animals underwent complete necropsy, and the
liver, kidney, heart, lungs, gonads, spleen, brain, and adrenals were
weighed. A full complement of tissues and organs from all animals were
examined histopathologically. Cholinesterase activity was not
measured. Daily observations were not reported. Survival was
comparable in all groups, with only a marginal decrease at the high
dose. Survival at 20 months was 32-45%. Body weights were
statistically significantly reduced in both males and females at 50
ppm, but the differences were less than 10%. Food consumption and
haematological parameters were unaffected by treatment.
Contrary to the conclusions of the 1985 JMPR with regard to changes in
organ weights, reexamination of the data indicates that the absolute
weights of the brains of female mice at all doses were slightly (4-6%)
but statistically significantly lower than those of controls. The
relative weights of the brains of animals at 50 ppm were, however,
increased in both males (7%, statistically nonsignificant) and females
(9%, statistically significant). The absolute and relative ovarian
weights were decreased by 16-18% in animals at 10 ppm and by 42-55% in
those at 50 ppm; and the absolute and relative splenic weights were
reduced in animals of each sex at 50 ppm. Other findings include
reductions in absolute heart, lung, liver, and kidney weights
(generally 10-15%, of variable significance) in males and females at
50 ppm, although the relative weights were comparable to those in
controls. No gross or microscopic parallel to these weight changes was
found. The most frequent pathological findings reported were chronic
multifocal interstitial nephritis, chronic peribronchiolitis,
pulmonary congestion, and acute and chronic myocarditis in all treated
animals, but no significant difference associated with dose was found.
Cystic endometrial hyperplasia was also frequent in all treated
females. A significant degree of fatty diffuse change was seen in the
liver, again with no relation to dose. In all groups, uniformly spread
amyloidosis was observed in many organ systems, including liver,
spleen, adrenals, kidneys, small intestines, thyroids, ovaries, and
submaxillary salivary glands. Fenamiphos had no oncogenic potential at
any dose. The NOAEL was 2 ppm, equal to 0.3 mg/kg bw per day, on the
basis of decreased splenic and ovarian weights at doses > 10 ppm
(JMPR, 1985, slightly modified by reference to the original report by
Hayes, 1982).
Rats
Groups of 40 male and 40 female SPF-derived Wistar rats were fed
fenamiphos in the diet at concentrations of 0, 3, 10, or 30 ppm (equal
to 0, 0.17, 0.56, or 1.7 mg/kg bw per day for males and 0, 0.23, 0.76,
or 2.2 mg/kg bw per day for females) for two years. Behavioural
abnormalities due to cholinergic stimulation were seen only during the
first six weeks of treatment at 30 ppm. The obvious effect on
cholinesterase activity disappeared after six weeks of feeding and was
not seen for the remainder of the study. Average growth, mortality,
food consumption, haematological and clinical chemical parameters, and
the results of liver and kidney function tests were unchanged. Urinary
parameters and blood sugar and cholesterol values were normal. The
thyroid weights and the thyroid:body weight ratios of females at 30
ppm were larger than those of controls but were not accompanied by
abnormal tumour development, goitre, or unusual histological findings.
All other major tissues and organs appeared normal at gross and
microscopic examination.
Feeding of 10 ppm fenamiphos in the diet inhibited plasma
cholinesterase activity by up to 55% in females but by < 20% in
males. In animals at 30 ppm, plasma cholinesterase activity was
inhibited by up to 60% and that of erythrocyte acetylcholinesterase by
up to 54% in males and 65% in females. No significant effects were
observed at 3 ppm. Although the NOAEL was originally considered to be
3 ppm, the present Meeting determined it to be 10 ppm (equal to 0.56
mg/kg bw per day) on the basis of inhibition of erythrocyte
acetylcholinestrase activity at the next highest dose (JMPR, 1974,
slightly modified by reference to the original report by Löser, 1972b;
Cherry & Newman, 1973).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenamiphos (purity, 89.3%) at a mean
concentration of 0, 1.7, 7.8, or 37 ppm (equal to 0, 0.098, 0.46, or
2.5 mg/kg bw per day for males and 0, 0.12, 0.6, or 3.4 mg/kg bw per
day for females) for two years. Clinical signs, mortality, food
consumption, haematological and blood chemical parameters (in 20 rats
of each sex per group), urinary parameters (in 10 rats of each sex per
group), and body weights were monitored throughout the study. Plasma
and erythrocyte cholinesterase, activity was monitored in 10 rats of
each sex per group at weeks 6, 10, and 15 and at 6, 12, 18, and 24
months. Additional groups of 10 rats of each sex were given 0 or 37
ppm fenamiphos for one year. Brain acetylcholinesterase activity was
determined at the end of the study in all rats in these satellite
groups and in 10 rats of each sex per group in the main study. All
rats, including those found dead or killed in extremis during the
study and those killed at 12 or 24 months, were examined grossly;
their organs were weighed, and more than 40 tissues were evaluated
microscopically. Ophthalmological examinations were performed on 10
rats of each sex per dose before and at the end of the study.
Survival at termination of the study was not related to treatment and
was 58-88% in males and 62-84% in females. Clinical observation
indicated a higher incidence of rough coat and alopecia in females at
the high dose than in controls. A statistically significant decrease
in body-weight gain was seen in male and female rats at the high dose
throughout the study, although no treatment-related effect on feed
consumption was seen. There was a dose-related, statistically
significant decrease in plasma cholinesterase activity in all treated
rats throughout the study: in mate rats, 7-38% at 1.7 ppm, 19-68% at
7.8 ppm, and 56-88% at 37 ppm; in females, 22-96% throughout the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly inhibited in treated animals: by 0-7% at
1.7 ppm, 12-25% at 7.8 ppm, and 56-81% at 37 ppm in males and 0-11% at
1.7 ppm, 21-43% at 7.8 ppm, and 62-81% at 37 ppm in females. A
statistically significant decrease in brain acetylcholinesterase
activity was observed in male rats at the high dose at termination
(-14%) and in animals of each sex (-25% in males and -24% in females)
at interim sacrifice after one year of treatment. There were
statistically significant increases in the relative weights of the
brain, heart, and lung in both males and females at the high dose at
the end of the study; females at the high dose also had significantly
increased relative kidney weights. At interim sacrifice, females had
statistically significantly increased relative weights of adrenals,
brain, heart, kidneys, and ovaries. These changes were considered to
be related to the decrease in body weight observed in rats of each sex
at 37 ppm. The only statistically significant change in absolute organ
weight at termination was a decrease in liver weight and an increase
in lung weight in animals of each sex at 37 ppm. No treatment-related
change was seen during ophthalmological examination, in food
consumption, in haematological, clinical chemical, or urinary
parameters, or on gross pathology. No treatment-related neoplastic
lesions were observed during histopathological examination, but
statistically significantly higher incidences of non-neoplastic
inflammatory lesions were observed in the nasal, laryngeal, and lung
tissues of rats receiving 37 ppm fenamiphos in the diet when compared
with controls. These changes were attributed by the author of the
study to the marked inhibition of cholinesterase activity in these
animals. No treatment-related non-neoplastic changes were noted at 1.7
or 7.8 ppm. The author concluded that fenamiphos was not oncogenic.
Contrary to the conclusions of the 1987 JMPR, the present Meeting
determined that the NOAEL was 7.8 ppm, equal to 0.46 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinestrase activity and
changes in body-weight gain, organ weights, and histoptahological
appearance at the next highest dose (JMPR, 1987, slightly modified by
reference to the original report by Hayes, 1986a).
(d) Genotoxicity
Fenamiphos has been tested adequately in a battery of tests for
mutagenicity (Table 4). It was weakly clastogenic only in vitro; no
similar response was seen in vivo in tests for micronucleus
formation and dominant lethal mutation. The Meeting concluded that
fenamiphos is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A standard three-generation (two litters per generation) study of
reproductive toxicity was performed in which groups of 10 male and 20
female FB30 rats were given fenamiphos in the diet at 0, 3, 10, or 30
ppm throughout mating, gestation, and suckling. Immediately after
birth, pups were examined for malformations and were then prepared for
another generation or killed. Five weanling rats per group from the
F3b. generation were killed, and macroscopic and microscopic
examinations were performed on the major tissues and organs. There
were no apparent differences in the limited indices of reproduction
investigated, including fertility, litter size, lactation index, or
growth of young, or in the incidence of malformations (Löser, 1972c;
Cherry et al., 1972; JMPR, 1974).
In a two-generation study of reproductive toxicity, groups of 30 male
and 30 female albino CD Sprague Dawley rats received technical-grade
fenamiphos (purity, 89%) in the diet at concentrations of 0, 2.5, 10,
or 40 ppm (equal to 0.17, 0.64, or 2.8 mg/kg bw per day for males and
0.2, 0.73, or 3.2 mg.kg bw per day for females) for 70 days before
mating. Oestrous cycles were characterized over two weeks in 10 F0
and F1 females per dose before mating After weaning, 30 F1 animals
of each sex per dose were treated for 70 days and then bred to produce
the second generation (F2); treatment was continued throughout
mating, gestation, and lactation. Groups of 10 F0 and F1 adults of
each sex per dose were used to assess cholinesterase activity in
plasma and erythrocytes in week 8 before mating and just before
sacrifice and in brain at the time of sacrifice. Plasma, erythrocyte,
and brain cholinesterase activities were measured in one pup of each
sex from each of 10 litters at the time of culling and on day 21 of
lactation. F0 and F1 females were killed after their pups had been
weaned or on day 24 of gestation. The males were killed after the last
litters were delivered. The histologic al examinations concentrated on
reproductive tissues.
There were no treatment-related deaths or clinical signs of toxicity
in the parental animals. In F0 and F1 dams at 40 ppm, statistically
significant reductions were seen in body-weight gain during lactation
(by 72 and 65%) and food consumption (by up to 11 and 19%). F1 and
F2 pups also had significant reductions in body-weight gain beginning
on day 7 of lactation. The body weights of F1 adults at 40 ppm were
significantly reduced throughout the premating period (by about 10% in
males and 7% in females), which the author attributed to their reduced
body weights at the start of the F1 premating period. Although not
reported by the author, the overall weight gain of F1 males before
mating was also reduced during the first four weeks, by 12% in gain
those at 10 ppm and by 15% in those at 40 ppm. The relative ovarian
weights of F0 females were significantly reduced, by 13% at 2.5 ppm,
11% at 10 ppm, and 20% at 40 ppm but were unaffected in F1 females.
Although the author concluded that this effect was related to
treatment at 40 ppm, no histopathological lesions were observed in the
ovaries, reproductive parameters were unchanged, and there was no
comparable effect in the F1 generation.
Plasma cholinesterase activity was significantly inhibited by > 20%
in all treated adult females of both generations, in F1 males at 10
ppm at the time of sacrifice, and in both F0 and F1 males at 40 ppm
both before mating and at sacrifice. Erythrocyte acetylcholinesterase
activity was consistently significantly inhibited in females at doses
> 10 ppm but only at 40 ppm in F0 and F1 males. At 40 ppm, brain
acetylcholinesterase activity was significantly inhibited by 21% in
F0 females, by 29% in F1 females, and by only 6% in F0 males. In
pups, plasma cholinesterase activity was significantly inhibited on
day 21 of lactation in both males and females of both generations at
doses > 10 ppm. Erythrocyte acetylcholinesterase activity was
inhibited by > 20% in males and females of both generations at 40 ppm
only. Brain acetylcholinesterase was not affected.
Table 4. Results of tests for genotoxicity with fenamiphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 4, 20, 100, 500, 2500 NR (Negative)a,b Herbold (1979)
TA1535, TA1537 µg/plate (DMSO)
TA98, TA100
Reverse mutation S. typhimurium 20, 100, 125, 250, 500, 92.4 Negativea Herbold (1985a,b)
TA98, TA100 1000, 2000, 2500
TA1535, TA1537 µg/plate (DMSO)
Forward mutation Chinese hamster Unactivated: 100, 110, 85 Negativea,c Yang et al. (1984)
ovary cells 120, 130 µg/ml (DMSO)
(CHO-K1-BH4) Activated: 170, 190,
210, 230 µg/ml (DMSO)
Unscheduled Rat hepatocytes 1.5, 5, 15, 50, 100 µg/ml 89.5 Negative Curren (1988)
DNA synthesis (DMSO)
Chromosomal Human 25, 100, 400 µg/ml 91.3 Positivea,d Herbold (1987)
aberrations lymphocytes (DMSO)
Chromosomal Human Unactivated: 91.9 Negative Herbold (1988)
aberrations lymphocytes 25, 50, 75, 100 µg/ml
(DMSO)
Activated: 100, 150, Weakly
225, 350 µg/ml (DMSO) positivee
Sister chromatid Chinese hamster Activated: 10, 20, 40, NR Negativef Chen et al. (1982)
exchange cell line (V79) 80 µg/ml
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
(%)
In vivo
Micronucleus Mouse (NMRI) 0.625, 1.25, 2.5 mg/kg 92.5 (Negative)g Herbold (1980a)
formation bone-marrow cells bw
Dominant lethal Mouse (male 5 mg/kg bw 92.5 Negative Herbold (1980b)
mutation NMRI) germ cells
NR, not reported; DMSO, dimethyl sulfoxide
a With and without metabolic activation
b The 1985 JMPR concluded that the test protocol was unacceptable. Another test was conducted in only
one strain (TA 1537), without activation, at doses of 125, 250, 500, and 1000 µg/plate.
c Positive controls (0.2 µl/ml ethylmethylsulfonate; 2 µg/ml benzo[a]pyrene) yielded the expected positive responses.
d Statistically significant increase (52% mitotic index) seen at 100 µg/ml without activation and at 400 µg/ml with
activation (< 0.1% mitotic index). Haemolysis was seen at 400 µg/ml. The author concluded that the increase in
aberrations was due exclusively to cytotoxicity.
e Statistically significant response seen only at 350 µg/ml with activation (35% mitotic index). The author concluded
that the increase in aberrations was due to cytotoxicity.
f Positive control (5 µg/ml cyclophosphamide) gave expected positive response.
g The mice were dosed twice, 24 h apart, and the bone marrow was sampled once, 6 h after the second dose. The 1985
JMPR concluded that the test protocol was unacceptable. The protocol was also considered unacceptable in the report
of the Gene-Tox Program (Mavournin et al., 1990).
Oestrous cycles, mating, fertility, and gestation indices, sex ratio,
and pup viability indices were unaffected, and no treatment-related
gross or histological lesions seen in any tissue from either parental
animals or pups. The pups showed no clinical signs of toxicity. The
NOAEL for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per
day, on the basis of decreased body-weight gain. The NOAEL for
reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per day, on
the basis of decreased pup body weights during lactation (Eigenberg,
1991).
(ii) Developmental toxicity
Rats
Four groups of 25 female FB30 rats mated overnight with untreated
males (in a ratio of one male to two females) were given fenamiphos
(purity, 92.5%) in a 0.5% aqueous Cremophor emulsion at daily doses of
0, 0.3, 1, or 3 mg/kg bw per day by gavage on days 6-15 of gestation;
the first day of gestation was that on which sperm was found in a
smear obtained the morning after mating. Control females received the
same volume (10 ml/kg bw) of the aqueous emulsion. On day 20 of
gestation, the dams were narcotized with carbon dioxide and the
fetuses were removed. Litter size, average fetus weight per litter,
sex, external and visceral abnormalities, and skeletal malformations
and development were noted. Cholinesterase activity was not measured.
Eighteen dams receiving 3 mg/kg bw per day showed signs of toxicity
(trembling and recumbency), and two died; however, the time of death
was not given and the cause of death could not be established. No
treatment-related signs of toxicity or deaths occurred in rats
receiving 0, 0.3, or 1 mg/kg bw per day. A 15% reduction in average
weight gain was seen during treatment among dams receiving 3 mg/kg bw
per day in comparison with controls, but the author reported that the
difference was not statistically significant. A total of six females
-- one control, two at 0.3 mg/kg bw per day, two at 1 mg/kg bw per
day, and one at 3 mg/kg bw per day -- were not fertilized. The average
placental weight of animals at the high dose was significantly lower
than that in controls. Treatment did not affect the number of
fertilized or pregnant females, litter size, number of resorptions,
number of fetuses, average fetal weight, sex ratio, incidence of
alterations in development, or the type or number of malformations.
The most frequent manifestations were nodulations on ribs, which were
found in two fetuses from one litter of a dam at 0.3 mg/kg bw per day
and in four fetuses of three litters of dams at 1 mg/kg bw per day.
The other malformations observed were general oedema, abdominal
fissure, and anophthalmia in one fetus at 0.3 mg/kg bw per day. No
malformations were observed in fetuses at the high dose. The lower
placental weight observed at the high dose was considered to be not
toxicologically significant because the average weight was within the
normal range and no effects were seen on embryonic or fetal
development. Thus, fenamiphos at doses < 3 mg/kg bw per day was not
embryotoxic or teratogenic; it was, however, toxic to dams at 3 mg/kg
bw per day (Schlüter, 1981; JMPR, 1987).
Groups of 33 mated female Crl:CD-BR rats were given fenamiphos
(purity, 88.7%) at doses of 0, 0.25, 0.85, or 3 mg/kg bw per day by
gavage on days 6-15 of gestation. Five dams from each group were
killed on day 16 in order to measure plasma, erythrocyte, and brain
cholinesterase activities; the remaining dams were killed on day 20 of
gestation and necropsied grossly. All dams were examined for number of
corpora lutea and implantation sites, and their uteri and placentas
were weighed. The fetuses were weighed and sexed; about half were
examined externally and in the viscera, and the other half were
processed for skeletal examination. Brain acetylcholinesterase
activity was measured in 20 fetuses per group.
Six dams at 3 mg/kg bw per day died between days 7 and 14 of
gestation, one female with convulsions on the day of its death.
Clinical signs of toxicity, such as tremors, salivation, lachrymation,
urine staining, and hypoactivity, were seen to varying extents in the
survivors. Body-weight gain and food consumption were significantly
reduced throughout treatment at this dose. Gross necropsy revealed no
treatment-related abnormalities, and gestational parameters were
unaffected. Fetal body weights were unchanged, and they had no
treatment-related variations or malformations. Plasma and erythrocyte
cholinesterase activities were statistically significantly reduced (by
50 and 42%, respectively) in dams at 3 mg/kg bw per day on day 16 of
gestation. By day 20, the plasma activity had returned to normal,
while that of erythrocyte cholinesterase was still significantly
reduced by 30%. Brain acetylcholinesterase activity was reduced by 28%
in adults at 0.85 mg/kg bw per day and by 12% in those at 3 mg/kg bw
per day; as the differences were not significant or dose-related, they
were not considered to be related to treatment. Fetal brain
acetylcholinesterase activity was unaffected. The NOAEL for maternal
toxicity was 0.85 mg/kg bw per day. Fenamiphos was not teratogenic or
fetotoxic under the conditions of this study (Clemens et al., 1989).
Rabbits
Groups of 20 double-mated female New Zealand rabbits were given
fenamiphos (purity, 88.8%) orally in corn oil at doses of 0, 0.1, 0.3,
or 1 mg/kg bw per day on days 6-18 of gestation. They were observed
daily for clinical signs of toxicity and were weighed initially,
periodically during the test, and at termination of the study. Once
the pups had been removed, the ovaries and uteri of the dams were
examined, and the fetuses were examined grossly and prepared for
evaluations of soft tissues and the skeleton. The numbers of corpora
lutea, implantations, resorptions, live and dead fetuses, and
anomalies were also determined. Cholinesterase activity was not
assessed. Dams given doses > 0.3 mg/kg bw per day showed signs of
toxicity, with decreased body-weight gain, bloody nasal discharge, and
white, mucoid ocular discharge. Treatment did not affect the number of
litters, number of pups per litter, pregnancy rate, the number of
corpora lutea, implantations, or gross abnormalities. Mean fetal
weight was slightly depressed at 1 mg/kg bw per day. One dam at 0.3
mg/kg bw per day aborted one dead pup, and two at 1 mg/kg bw per day
aborted eight dead pups and had seven late resorptions. In addition,
one dead fetus was found in each of two litters at the high dose.
The commonest developmental variation observed was the left carotid
arising from the innominate, which occurred in six to eight fetuses
(7-9%) in each of three litters (25%) at doses > 0.1 mg/kg bw per
day. This anomaly was not seen in the controls and in only one of 31
litters (3.2%) of historical controls at the laboratory where the
study was performed. More recent data (through July 1985) for
historical controls revealed incidences of 11/336 (3.3%) in fetuses
and 9/53 (17%) litters. The newer historical control data provide some
evidence that the incidence of left carotid anomalies was increased in
superovulated or artificially inseminated rabbits. Furthermore, data
for rabbits of the same strain in different labs indicate that the
anomaly is a frequent finding, occurring in about 8% of fetuses and
25% of litters; the historical data are for artificially inseminated
rabbits, while the animals used in this study were naturally bred. The
biological significance of this finding in relation to treatment is
dubious.
An increased incidence of accessory skull bones was also seen in all
treated groups, but it did not occur in a dose-related manner and was
thus not considered to be related to treatment. A significant increase
in the incidence of chain-fused sternebrae was seen in five fetuses in
three litters at 1 mg/kg bw per day, and this anomaly was also seen in
one fetus in one litter at 0.3 mg/kg bw per day. This anomaly is of
questionable biological significance. Two fetuses in one litter at the
high dose had aortic arches with a common truncus, which was
considered to be a major malformation. Other skeletal malformations
which occurred only at doses > 0.3 mg/kg bw per day included fused
ribs, scoliosis, absent vertebrae (thoracic, lumbar, sacral, and
caudal), and bipartite or malformed centra. The 1985 Meeting concluded
that these findings were related to treatment; however, these
anomalies occurred in only one or two fetuses in single litters, often
with no relation to dose. Furthermore, several of the anomalies were
clustered within a single fetus, indicating that they were not likely
to be related to treatment. The NOAEL for maternal toxicity was 0.1
mg/kg bw per day, and that for developmental toxicity was 0.3 mg/kg bw
per day. Fenamiphos had no teratogenic effects at any dose (JMPR,
1985, modified by reference to the original report by MacKenzie,
1982).
In a study to determine the doses for a study of embryotoxicity
(including teratogenicity), groups of three mated (1:1) female
Chinchilla rabbits were given single daily doses of fenamiphos
(purity, 91%) in distilled water with 0.5% Cremophor EL0 at doses of
0.1, 0.8, or 3 mg/kg bw per day by gavage on days 6-18 post coitum.
Cholinesterase activity was measured in plasma and erythrocytes before
the first dose and just after the last dose; brain
acetylcholinesterase activity was not assessed. All of the animals
were killed on day 28 post coitum, and the fetuses were removed,
sexed, weighed, and examined for gross external and internal
abnormalities.
One female at the high dose lost weight from day 10 and died on day 12
post coitum; body-weight loss and reduced food consumption were seen
throughout treatment in all animals at this dose when compared with
controls. Statistically significant reductions in both plasma (89%)
and erythrocyte cholinesterase activity (90%) relative to controls
were noted in rabbits at the high dose on day 18 post coitum. Five
preimplantation losses and one fetal resorption were observed in does
at the high dose but in no other group. Marginal effects on body
weight and food consumption were seen at 0.8 mg/kg bw per day. No
treatment-related effects were seen on the numbers of corpora lutea,
implantations, live or dead fetuses, or on fetal weight (Becker et
al., 1986; JMPR, 1987).
In the main study, four groups of 16 single-mated female Chinchilla
rabbits were given fenamiphos (purity, 91%) in distilled water
containing 0.5% Cremophor as single daily doses of 0, 0.1,0.5, or 2.5
mg/kg bw per day by gavage on days 6-18 post coitum. The does were
killed on day 28 post coitum, and the fetuses were removed. Only
does with at least one living fetus were used in calculating
body-weight gain, food consumption, and reproductive parameters. They
were examined for the position of fetuses in the uterus and numbers of
corpora lutea, implantations, resorptions, and live and dead fetuses.
The fetuses were weighed, sexed, and examined for external and
internal malformations, skeletal abnormalities, and development.
Cholinesterase activity was not assessed. Dosing solutions were
prepared daily. The concentration of fenamiphos in triplicate samples
taken for determination of homogeneity before the study was found to
vary considerably (14-140% of the nominal concentration), with mean
concentrations of 71 ± 8.3, 84 ± 64, and 70 ± 16% of the nominal
concentration at doses of 0.1, 0.5, and 2.5 mg/kg bw per day,
respectively. At the next sampling 10 days later, the mean
concentrations were all within 90% of the nominal concentration,
although the variability was still high (92 ± 37, 97 ± 45, and 99 ±
41% of the nominal concentration at the three doses, respectively).
Although it is stated that homogeneity was maintained during dosing by
use of a magnetic stirrer, it is not clear if the samples taken for
testing were subjected to the same treatment before analysis.
Four females at 2.5 mg/kg bw per day died as a result of treatment
after 3, 5, and 10 days and on day 21 post coitum. Treatment-related
signs of toxicity (salivation and dyspnoea) were observed in these
females and in five other females at the high dose between days 7 and
18 post coitum. Ataxia was seen in two females that died, and
diarrhoea was observed in another. No signs of toxicity were found in
animals at 0, 0.1, or 0.5 mg/kg bw per day. A treatment-related,
statistically significant decrease in mean food consumption (29%) and
a treatment-related reduction in body-weight gain (56%) were observed
during treatment in does at the high dose when compared with controls.
Food consumption was significantly increased in this group on days
24-28.
No treatment-related or significant difference was observed between
treated and control animals with regard to the mean numbers of
implantations, corpora lutea, live or dead fetuses, or resorptions. In
fetuses, no effect that could be attributed to treatment was seen in
sex ratio, body weight, external or internal malformations, skeletal
abnormalities, or development. One fetus at the high dose had
encephalocele with reduced brain size, but this finding was not
considered to be related to treatment. A number of skeletal changes
unrelated to treatment were seen in fetuses at all doses. The NOAEL
for maternal toxicity was 0.5 mg/kg bw per day. Although questions
remain about the doses actually administered, because of the problems
of homogeneity, the study showed no embryotoxicity or teratogenicity
at maternally toxic doses (JMPR, 1987, modified by reference to the
original report by Becker, 1986).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenamiphos painted on the skin of New Zealand white
rabbits in an acetone solution at 50 mg/kg bw resulted in slight
erythema but was not considered to be a primary irritant. Application
of technical-grade fenamiphos to the conjunctival sac of New Zealand
white rabbits as 100 mg of a crystalline material resulted in
irritation considered to be mechanical rather than physiological
(Crawford & Anderson, 1971; JMPR, 1974).
Technical-grade fenamiphos (purity, 90.7%) was melted at 35°C, and
0.5 ml was painted onto the intact or abraded skin of six
Japanese-derived albino rabbits for 24 h under occlusion. Irritation
was scored according to Draize 24, 48, 72, and 168 h after
application. Minimal irritation was observed, characterized by slight
erythema and oedema during the first 48 h. The mean primary irritation
index, based on readings at 24 and 72 h, was 0.42 (Kato 1984a).
Technical-grade fenamiphos (purity, 90.7%) was heated to liquidity
(temperature not specified) and applied at 0.1 ml to the conjunctival
sacs of nine Japanese-derived albino rabbits. The eyes of three of the
rabbits were washed with water. Irritation was scored according to
Draize 24, 48, 72, 96, 168, and 240 h after administration. In the
absence of washing, fenamiphos was a moderate irritant, with a maximum
average score of 31.3 at 24 h. The irritation scores slowly declined,
and all of the eyes were clear by 240 h. Fenamiphos was not irritating
when the eyes were washed with water after application. Midriasis,
persisting until day 2 or 3, was seen in all animals when the eyes
were not washed within 10 min of application. The translation of the
report leaves some uncertainty about other ocular effects, but signs
of systemic toxicity peaked within 3-4 h of application; these
included salivation, increased respiration, cyanosis, and slight
convulsions (number affected not specified). All animals were
reportedly normal within 6 h. No systemic toxicity was seen in the
animals when the eyes were washed (Kato, 1984b).
The potential of fenamiphos to sensitize skin was examined in a
maximization test in which 20 male Hsd Win:DH guinea-pigs were induced
intradermally with 1% fenamiphos in saline containing 2% Cremophor EL.
One week later, they were induced topically with 25% fenamiphos in
saline (with 2% Cremophor EL); they were challenged three weeks later
with 12 and 25% solutions of fenamiphos in the saline solution. Patchy
erythema was seen 24 h after removal of the patch in 22% of the
animals challenged with the 25% solution, whereas none of the 10
induced control animals showed irritation. The author considered a
response rate of > 30% to be indicative of sensitization, and
concluded that fenamiphos produced no relevant sensitization; however,
according to the original protocol of Magnusson and Kligman, these
results would indicate that fenamiphos is a mild sensitizer (Stropp,
1995).
(ii) Delayed neuropathy
Groups of eight hens were fed fenamiphos in the diet at levels of 0,
1,3, 10, or 30 ppm, equal to 0, 2, 5, 16, or 26 mg/kg bw per day, for
30 days. At the end of treatment, some birds were killed and the
remainder were observed for four weeks for neurological signs of
poisoning. Food consumption was depressed in birds at 30 ppm, and the
average body weight and growth of hens at this dose was reduced.
Whole-blood cholinesterase activity was decreased after 30 days at
doses > 1 ppm, although no signs of cholinergic poisoning were
observed. There were no indications of delayed neurotoxicity, and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with haematoxylin and eosin) did not indicate delayed
neuropathy (Kimmerle, 1970; Spicer, 1970; JMPR, 1974).
Groups of 10 hens given an LD50 a dose of fenamiphos (5.0 mg/kg bw)
orally were observed for three weeks and then killed. No evidence of
delayed neurotoxicity was observed either clinically or
histologically, whereas signs were observed with tri- ortho-cresyl
phosphate (Kimmerle, 1971; Spicer, 1971; JMPR, 1974).
Fenamiphos (purity, 91.3%) in a 2% Cremophor solution was administered
twice by intubation to 30 Lohmann selected Leghorn hens at 25 mg/kg bw
per day at a 21-day interval. Five hens serving as positive controls
received tri- ortho-cresyl phosphate at 375 mg/kg bw per day. The
hens treated with fenamiphos received atropine intramuscularly at 100
mg/kg bw before treatment and subcutaneously at 30-50 mg/kg bw 7, 24,
or 30 or 48 h after treatment. The birds were observed for body
weight, clinical signs, forced motor coordination, and gross and
histopathological changes. Although statistically significant,
treatment-related weight loss (during week 1) and signs of severe
poisoning were seen, the birds showed no impairment of motor
coordination indicative of delayed neurotoxicity. Histological
examination of tissues from the peripheral and central nervous systems
showed no changes indicative of delayed neuropathy. Birds given
tri- ortho-cresyl phosphate, however, showed clinical signs of
delayed neurotoxicity (ataxia and paresis) two weeks after treatment
and were killed in moribund condition on day 17. The histopathological
changes in these birds were typical of neuropathy (Flucke & Kaliner,
1987).
(iii) Neurotoxicity
Rats
Groups of 12 Wistar (Hsd Win:WU) rats of each sex were given single
doses of technical-grade fenamiphos (purity, 95.2%) at 0.37, 1.52, or
2.31 mg/kg bw by garage. They were observed for mortality, clinical
signs, and body weight. Functional observational and motor or
locomotor activity tests were conducted on all animals before
treatment, within 30 min of treatment, and 7 and 14 days later.
Plasma, erythrocyte, and brain cholinesterase activity was measured in
additional satellite groups of six animals of each sex at each dose,
which were killed within 1 h of treatment. One-half of the animals in
the main group were perfused, and various neural and skeletal muscle
tissues, including the brain, spinal cord and spinal ganglia, eyes and
optic nerves, peripheral nerves, gastrocnemic muscle, and trigeminal
ganglia, were processed for histological examination.
In animals at the lowest dose, plasma cholinesterase activity was
statistically significantly inhibited in females (by 55%) and
nonsignificantly decreased in males (by 23%). Erythrocyte
acetylcholinesterase activity was significantly inhibited only in
males (by 24%), but this was not considered to be an adverse effect
because no corroborating clinical signs were seen in the functional
and motor activity tests. At the next highest dose, both plasma and
erythrocyte cholinesterase activity was significantly inhibited in
both males (by 64 and 70%, respectively) and females (by 77 and 51%,
respectively). Uncoordinated gait and muscle fasciculations were also
seen in males during the functional observational battery of tests. At
the highest dose, similar signs were seen, with decreased grip
strength and deaths among both males and females and decreased motor
activity in malesś Other behavioural or physiological changes seen in
animals of each sex in the functional observational battery of tests
included piloerection, nasal, oral, and lachrymal staining,
salivation, and decreased activity and rearing in the open field.
Brain acetylcholinesterase activity was unaffected at all doses. No
treatment-related histopathological changes were seen. The NOAEL was
0.37 mg/kg bw, the lowest dose tested, on the basis of uncoordinated
gait and muscle fasciculations in males at the next highest dose
(Dreist, 1995).
In a study of similar design, technical-grade fenamiphos (purity,
95.6%) was administered in the diet to groups of 12 Wistar (Hsd
Cpb:WU) rats of each sex for 13 weeks at dietary concentrations of 0,
1, 10, or 50 ppm, equal to 0, 0.06, 0.61, or 3.1 mg/kg bw per day in
males and 0.08, 0.8, or 4 mg/kg bw per day in females. The animals
were observed for deaths, clinical signs, body weight, and food and
water consumption. Functional observational and motor or locomotor
activity tests were conducted before treatment and in weeks 4, 8, and
13. Plasma and erythrocyte cholinestemse activity was measured in six
rats of each sex at each dose on week 4 and before terminal sacrifice
at week 15, and brain acetylcholinesterase activity only at terminal
sacrifice.
All females at the highest dose had muscle fasciculations during the
first three weeks of the study. Plasma and erythrocyte cholinesterase
activities were statistically significantly reduced in both males
(> 68%) and females (> 86%). Brain acetylcholinesterase activity
was also significantly reduced (by 12%) in females, but the author
considered that this was not biologically significant. At 10 ppm,
plasma cholinesterase activity was significantly reduced in both males
(by 30-39%) and females (by 71-77%; rho < 0.01) in weeks 4 and 15.
Erythrocyte acetylcholinesterase activity was inhibited by about 25%
in week 15 in males (rho < 0.05) and in weeks 4 and 15 in females
at 10 ppm. The lowest dose resulted in nonsignificant decreases (about
30%) in plasma cholinesterase activity in females in weeks 4 and 15.
Body weights, food and water consumption, brain weights, and gross and
histopathological appearance were all unaffected by treatment. The
author extrapolated the results back to a 20% level of inhibition of
plasma cholinesterase activity and estimated that the NOAEL in females
was 0.4 ppm. The overall NOAEL was 10 ppm, equal to 0.8 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase activity
in females at the highest dose (Dreist & Popp, 1995).
(iv) Potentiation
In male rats given fenamiphos orally in combination with disulfoton or
E 154, no potentiation of the acute toxicity was seen (Kimmerle,
1972c; JMPR, 1974).
The LD50 for fenamiphos (purity, 91.8%) administered orally to male
Wistar rats was 4.6 mg/kg bw, while that of carbofuran was 8.1 mg/kg
bw. When the two compounds were given concomitantly, essentially as a
2:1 ratio of carbofuran:fenamiphos, the LD50 was 6 mg/kg bw,
indicating that the toxicity of the combination was additive but not
synergistic (Mihail, 1980 JMPR, 1985).
Comments
In rats, fenamiphos was rapidly excreted, with over 96% of the
administered dose eliminated within 48 h. Excretion was primarily in
the urine, with less than 4% of the dose eliminated in the faeces. At
48 h, the levels of tissue residues were below the limit of
quantification, except following a high dose (3 mg/kg bw), when the
maximal tissue levels observed were 3.5-8.4 µg/kg in the liver,
1.6-2.1 µg/kg in the kidney, and 1.6-3.5 µg/kg in the skin. Fenamiphos
was completely metabolized in rats. Metabolites retaining
anticholinesterase activity, such as fenamiphos sulfoxide and
desisopropyl fenamiphos sulfoxide, were seen in variable but generally
low proportions (rarely greater than 3%). Most of the products were
dephosphorylated phenol, sulfoxide phenol, or sulfone phenol
metabolites and their corresponding sulfates.
Fenamiphos is extremely hazardous after single oral doses to rats,
mice, rabbits, cats, dogs, and chickens (LD50 values = 2 4-23 mg/kg
bw) and highly hazardous after dermal administration to rats and
rabbits (LD50 values, 75-230 mg/kg bw). It is moderately hazardous
after inhalation in rats and mice (LC50 values < 100 µg/L, 4 h).
WHO has classified fenamiphos as 'extremely hazardous' (WHO, 1996).
The sulfoxide, sulfone, and desisopropylated sulfone metabolites of
fenamiphos are similarly toxic to rats after oral administration
(LD50 values = 1.4-4.1 mg/kg bw). The sulfoxide and sulfone phenol
metabolites are only slightly toxic to rats after oral administration,
with LD50 values ranging from 1200 to 1900 mg/kg bw.
Fenamiphos inhibited plasma cholinesterase more effectively than
erythrocyte acetylcholinesterase, both in vitro and in vivo.
Fenamiphos sulfoxide, fenamiphos sulfone, desisopropyl fenamiphos,
desisopropyl fenamiphos sulfoxide, and desisopropyl fenamiphos sulfone
inhibited plasma and erythrocyte cholinesterase in vitro more
effectively than fenamiphos itself.
In evaluating the following studies, inhibition of erythrocyte
acetylcholinesterase activity was not used as an indicator of adverse
effects in the nervous system when information on brain
acetylcholinesterase activity was also available. In the absence of
this information, NOAELs were determined on the basis of inhibition of
erythrocyte acetylcholinesterase (of < 20%). Statistical
significance was used as a criterion for considering depression of
brain acetylcholinesterase activity to be adverse.
In a three-week study in which rats were exposed by inhalation to
atmospheres containing fenamiphos at 0, 0.03, 0.25, or 3.5 µg/L for 6
h per day, five days per week, the only finding was inhibition of
plasma cholinesterase at the highest dose. Erythrocyte and brain
acetylcholinesterase were unaffected. The no-observed-adverse-effect
concentration (NOAEC) was 3.5 µg/L.
In a three-month study in rats, fenamiphos given at dietary
concentrations of 0, 0.37, 0.57, or 0.91 ppm inhibited plasma
cholinesterase activity only at the highest dose. No treatment-related
changes in erythrocyte or brain acetylcholinesterase activity were
seen at any dose. In a second study of short-term toxicity, rats were
fed diets containing 0, 4, 8, 16, or 32 ppm fenamiphos for three
months. Erythrocyte acetylcholinesterase activity was inhibited at
doses of 16 ppm (equivalent to 0.8 mg/kg bw per day) and above. This
was considered an adverse effect as brain acetylcholinesterase was not
measured in this study. The overall NOAEL was 8 ppm, equivalent to 0.4
mg/kg bw per day.
Rabbits received fenamiphos by dermal application at doses of 0, 0.5,
2.5, or 10 mg/kg bw per day for three weeks (6 h per day, five days
per week). Body-weight gain was slightly reduced in animals of each
sex at 10 mg/kg bw per day. In females, reductions in cholinesterase
activity in the brain (by 20%) and plasma were noted at 2.5 mg/kg bw
per day and above. Erythrocyte acetylcholinesterase activity, however,
was affected only at 10 mg/kg bw per day. In males, the only findings
were decreased plasma and erythrocyte cholinesterase activity at 10
mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day.
In a series of studies, dogs were fed diets containing 0, 0.5, 0.6, 1,
1.7, 2, 3, 5, 6, 10, 12, or 18 ppm fenamiphos for periods ranging from
three months to two years. In dogs treated at 18 ppm (equivalent to
0.45 mg/kg bw per day) for three months, muscle tremors were seen. In
dogs treated at 12 ppm (equal to 0.31 mg/kg bw per day) for one year,
brain acetylcholinesterase activity was inhibited in females (by 17%),
and males showed slight anaemia. Erythrocyte acetylcholinesterase
activity was inhibited at doses of 3 ppm (equal to 0.083 mg/kg bw per
day) and above for one year. Plasma cholinesterase activity was
inhibited at doses of 1.7 ppm (equivalent to 0.042 mg/kg bw per day)
and above in a three-month study, No other parameters were affected.
Since no information on brain acetylcholinesterase activity was
available at doses between 3 and 12 ppm, the Meeting considered 3 ppm
(equal to 0.083 mg/kg bw per day) to be the overall NOAEL in dogs.
In mice fed diets containing 0, 2, 10, or 50 ppm fenamiphos for 20
months, there were marginal decreases in survival and body-weight gain
at 50 ppm (equal to 7.4 mg/kg bw per day). The relative ovarian and
spleen weights were reduced at 10 ppm (equal to 1.4 mg/kg bw per day)
and above. There were no non-neoplastic changes that could be
attributed to treatment, and fenamiphos was not carcinogenic at any
dose. Cholinesterase activity was not measured. The NOAEL was 2 ppm,
equal to 0.3 mg/kg bw per day.
In rats fed diets containing 0, 3, 10, or 30 ppm fenamiphos for two
years, the only treatment-related effects were inhibition of
erythrocyte acetylcholinesterase activity throughout the study and
behavioural changes during the first six weeks of the study in animals
at 30 ppm. Brain acetylcholinesterase activity was not measured. The
NOAEL was 10 ppm, equal to 0.56 mg/kg bw per day.
Rats were fed diets containing 0, 1.7, 7.8, or 37 ppm fenamiphos for
two years. At 37 ppm, equal to 2.5 mg/kg bw per day, body-weight gain
in both males and females was decreased. Erythrocyte
acetylcholinesterase activity was inhibited at 7.8 ppm (equal to 0.46
mg/kg bw per day) and above. Brain acetylcholinesterase activity was
inhibited only at 37 ppm; inhibition was 25% in animals of each sex
killed after one year, and 14% in males at termination of the study.
Animals of each sex at 37 ppm also had an increased frequency of
non-neoplastic inflammatory lesions of the nasal, laryngeal, and lung
tissues and increased relative weights of the brain, heart, and lungs.
Fenamiphos was not carcinogenic at any dose. The NOAEL was 7.8 ppm,
equal to 0.46 mg/kg bw per day.
Fenamiphos was adequately tested in a battery of tests for
genotoxicity. It was found to be mildly clastogenic at cytotoxic doses
in vitro but not in vivo. It did not cause reverse or forward
mutation, unscheduled DNA synthesis, or sister chromatid exchange
in vitro. The Meeting concluded that fenamiphos is not genotoxic.
In a two-generation study of reproductive toxicity, rats were treated
with 0, 2.5, 10, or 40 ppm fenamiphos. Parental toxicity was
characterized by reduced weight gain in F0 and F1 dams at 40 ppm
(equal to 2.8 mg/kg bw per day) during lactation, and in F1 males at
10 ppm (equal to 0.64 mg/kg bw per day) and above before mating.
Pathological changes in the salivary gland were seen in F0 males and
females at 40 ppm. Erythrocyte acetylcholinesterase activity was
consistently inhibited at 10 ppm and above in females but only at 40
ppm in males. Brain acetylcholinesterase activity was inhibited at the
highest dose in adult F0 and F1 females (by 21-29%) and in F1 males
(by 6%) but not in pups of either sex. In pups at 40 ppm, erythrocyte
acetylcholinesterase activity was inhibited only on day 21 of
lactation. The only reproductive effect was decreased weight gain of
F1 and F2 pups at 40 ppm, beginning on day 7 of lactation. The NOAEL
for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per day. The
NOAEL for reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per
day.
In a study of developmental toxicity, mated rats were treated with 0,
0.3, 1, or 3 mg/kg bw per day on days 6-15 of gestation. Maternal
toxicity was seen at the highest dose, characterized by mortality,
tremors, and reduced weight gain. The fetuses were not affected at any
dose. Cholinesterase activity was not measured in this study. The
NOAELs were 1 mg/kg bw per day for maternal toxicity and 3 mg/kg bw
per day for developmental toxicity.
Fenamiphos was also administered to mated rats at doses of 0, 0.25,
0.85, or 3 mg/kg bw per day on days 6-15 of gestation. The highest
dose resulted in maternal deaths, tremors, salivation, lachrymation,
urine staining, and hypoactivity. Body-weight gain and food
consumption were also significantly reduced. Erythrocyte
acetylcholinesterase activity was reduced at this dose, but the
changes in brain acetylcholinesterase activity were not statistically
significant or dose-related. The fetuses were unaffected at 3 mg/kg bw
per day. The NOAELs were 0.85 mg/kg bw per day for maternal toxicity
and 3 mg/kg bw per day for developmental toxicity.
In a study of developmental toxicity in rabbits, animals received 0,
0.1, 0.3, or 1 mg/kg bw per day on days 6-18 of gestation. At 0.3
mg/kg bw per day and above, fenamiphos was maternally toxic, resulting
in decreased body-weight gain, bloody nasal discharge, and white
ocular discharge. Fetotoxicity, characterized by chain fusion of the
sternebrae, was seen only at 1 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.1 mg/kg bw per day, and that for developmental
toxicity was 0.3 mg/kg bw per day. Cholinesterase activity was not
measured in this study.
In a second study, mated rabbits were treated with 0, 0.1, 0.5, or 2.5
mg/kg bw per day on days 6-18 of gestation. Clear maternal toxicity
was seen at the highest dose, which included mortality, salivation,
dyspnoea, ataxia, diarrhoea, and decreased weight gain and food
consumption during treatment. Although some questions remain about the
doses that were actually administered (because of uncertain
homogeneity), no embryotoxic or teratogenic effects were seen at the
maternally toxic dose of 2.5 mg/kg bw per day.
Fenamiphos was minimally irritating to rabbit skin and moderately
irritating to rabbit eyes and was a mild skin sensitizer in the
guinea-pig.
A single dose of 25 mg/kg bw fenamiphos had no effect on neuropathy
target esterase activity in the brains or spinal cords of hens, under
atropine protection. Fenamiphos did not induce delayed neuropathy in
three studies in hens when tested at doses of 0, 2, 5, 16, or 26 mg/kg
bw per day for 30 days or when given once at doses of 0 or 25 mg/kg
bw.
In a study of acute neurotoxicity, rats were given single doses of 0,
0.37, 1.5, or 2.3 mg/kg bw fenamiphos by gavage. Erythrocyte
acetylcholinesterase activity was inhibited at the lowest dose tested
in males only, but in both males and females at higher doses. At 1.5
mg/kg bw, males showed uncoordinated gait and muscle fasciculation. At
the highest dose, decreased motor activity was also seen in males, and
clinical signs, decreased grip strength, and deaths occurred in rats
of each sex. There was no effect on brain acetylcholinesterase
activity, at any dose. The NOAEL was 0.37 mg/kg bw per day. In a
further study, rats were fed diets containing 0, 1, 10, or 50 ppm
fenamiphos for 13 weeks. At 10 ppm and above, erythrocyte
acetylcholinesterase activity was inhibited in animals of each sex. At
the highest dose, brain acetylcholinesterase activity was inhibited
(by 12%) in females only. A battery of functional observational and
motor activity tests revealed no treatment-related effects. The NOAEL
was 10 ppm, equal to 0.61 mg/kg bw per day.
An ADI of 0-0.0008 mg/kg bw was established on the basis of an overall
NOAEL of 0.083 mg/kg bw per day in the dog, and a safety factor of
100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2 ppm in the diet, equal to 0.3 mg/kg bw per day
(20-month study of toxicity and carcinogenicity)
Rat: 0.37 mg/kg (single doses, study of neurotoxicity) 10
ppm, equal to 0.61 mg/kg bw per day (three-month study
of neurotoxicity)
2.5 ppm, equal to 0.17 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
10 ppm, equal to 0.64 mg/kg bw per day (study of
reproductive toxicity)
0.85 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity)
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to fenamiphos
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral neurotoxicity, rat NOAEL = 0.37 mg/kg bw per day: effects observed
(1-7 days) during battery of functional observational tests
Oral toxicity, rat (fasted) LD50 = 2.4-6 mg/kg bw
Inhalation toxicity, 4 h, rat LC50 = 91-100 µg/L
Inhalation toxicity, 5 days, rat NOAEL = 4 µg/L
Dermal toxicity, rat LD50 = 72-92 mg/kg bw
Dermal irritation, rabbit Minimally irritating
Ocular irritation, rabbit Moderately irritating
Dermal sensitization, guinea-pig Mildly sensitizing
Medium-term Repeated inhalation toxicity, 3 weeks, rat NOAEL = 3.5 µg/L (highest dose tested)
(1-26 weeks) Repeated dermal toxicity, 3 weeks, rabbit NOAEL = 0.5 mg/kg bw per day: inhibition of brain
acetylcholinesterase activity
Repeated oral, reproductive toxicity, rat NOAEL = 0.17 mg/kg bw per day: parental toxicity
NOAEL = 0.64 mg/kg bw per day: reproductive
toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.1 mg/kg bw per day: maternal toxicity
NOAEL = 0.3 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral, 1 - 2 years, dog NOAEL = 0.083 mg/kg bw per day: inhibition of
(> 1 year) acetylcholinesterase activity, anaemia
7.8 ppm, equal to 0.46 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
0.3 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 3 ppm in the diet, equal to 0.083 mg/kg bw per day
(overall assessment)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Estimate of acute reference dose
The available data did not permit the Meeting to establish an acute
reference dose different from the ADI (0-0.0008 mg/kg bw). Although
the results of a study of neurotoxicity in rats given single doses was
available, the dog was found to be the more sensitive species.
Information on acute effects in dogs may allow the establishment of an
acute reference dose in the future.
Studies that would provide information useful for continued
evaluation of the compound
1. Effects of single doses in dogs (with appropriate evaluation of
functional changes in the cholinergic nervous system, including
brain acetylcholinesterase activity).
2. Observations in humans
References
Becker, H. (1986) Embryotoxicity (including teratogenicity) study with
SRA 3886 in the rabbit. Unpublished report No. R 3917 (project No.
065261) from Research & Consulting Co., Itingen, Switzerland.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Mueller, E., Luetkemeir, H. & Sachsse, K. (1986)
Dose-finding embryotoxicity (including teratogenicity) study with SRA
3886 in the rabbit. Unpublished report No. R3693 (project No. 065250)
from Research & Consulting Co., Itingen, Switzerland. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchange in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environ. Mutag., 4, 621624. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cherry, C. & Newman, A. (1973) Pathology report of Bayer 68138.
Chronic toxicological studies in rats. Unpublished report (addendum to
report No. 3539) from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Cherry, C., Urwin, C. & Newman, A. (1972) Pathology report of BAY
68138. Rat breeding study. Unpublished report (addendum to report No.
3424) from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1989) Teratology
study in the rat with Nemacur technical. Unpublished report No. 1145
(study no. MTD0108) from Toxicology Department, Miles Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F. & Wills, J.H. (1974) Summary of research by the Institute
of Comparative and Human Toxicology, Albany Medical College, on
phenamiphos. Report dated October 1974. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C. & Anderson, R. (1971) The skin and eye irritating
properties of BAY 68138 technical to rabbits. Unpublished report No.
29988 from Chemagro Division of Baychem Corp. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Crawford, C. & Anderson, R. (1972) The acute dermal toxicity of
Nemacur technical to rabbits. Unpublished report No. 34216 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R)
with analytical grade Nemacur(R). Unpublished report No. 34446 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974a) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Unpublished report No. 39700 from Chemagro Division of Baychem Corp.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974b) The acute oral toxicity of
Nemacur(R)-sulfone and Nemacur(R)-sulfoxide to rats. Unpublished
report No. 40215 from Chemagro Division of Baychem Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Curren, D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 991 (study no. T5724.380) from
Microbiological Associates Inc., Rockville, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Dreist, M. (1995) SPA 3886 (common name:Fenamiphos) acute oral
neurotoxicity screening study in Wistar rats. Unpublished report No.
24408 (study No. T6058166) from the Institute of Toxicology
Agrochemicals, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Dreist, M. & Popp, A. (1995) SRA 3886 (common name: Fenamiphos)
subchronic neurotoxicity screening study in Wistar rats (thirteen-week
administration in the diet). Unpublished report No. 24948 (study no.
T9058277) from Institute of Toxicology Agrochemicals, Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K. & Flynn, M. (1968) The subacute parenteral toxicity of BAY
68138 to rats. Unpublished report No. 22178 from Toxicity Laboratory,
University of Chicago, Chicago, IL, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K., Flynn, M. & Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Unpublished report No. 20810
from Toxicity Laboratory, University of Chicago, Chicago, IL, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ecker, W., Weber, H. & Brauner, A. (1989) [Phenyl-1-14C]Nemacur:
General metabolism study in the rat. Unpublished report No. PF3175
(study no. M182 0189-9) from Bayer AG, Pflanzenschutz-Zentrum Monheim,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Eigenberg, D.A. (1991) A two generation reproduction study in rats
using Fenamiphos (Nemacur(R)). Unpublished report No. 5762 (study
no. 88-671-BC) from Mobay Corp, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity. Unpublished report
dated 18 July 1980 from Bayer AG, Institute of Toxicology, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Eben, A. (1988) SPA 3886 Technical (common name:
Fenamiphos): Study of the effect on the neurotoxic esterase (NTE)
following oral administration to hens. Unpublished report No. 17388
from Fachbereich Toxikologie, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Kaliner, G. (1987) Delayed neurotoxicity studies on hens
following acute oral administration. Unpublished report No. 16187
(study no. T 1020919) from Fachbereich Toxikologie, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Gronberg, R.R. (1969) The metabolic fate of ethyl-4-(methylthio)-m-
tolyl isopropylphosphoramidate (BAY 68138), ethyl-4-(methylsulfinyl)-
m-tolyl isopropylphosphoramidate (BAY 68138 sulfoxide) and ethyl-4-
(methylsulfonyl)-m-tolyl-isopropylphosphoramidate (BAY 68138 sulfone)
in white rats. Unpublished report No. 26759 from Chemagro Division of
Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1982) Technical fenamiphos (Nemacur(R)) oncogenicity
study in mice. Unpublished report No. 241 (study No. 78CCM02) from
Stanley Research Center, Mobay Chemical Corp, Stilwell, KS, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. ( 1983 ) Ninety-day cholinesterase study on dogs with
fenamiphos in diet. Unpublished report No. 444 (study No. 83-174-01)
from Environmental Health Research, Mobay Chemical Corp, Stilwell, KS,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1986a) Ninety-day cholinesterase study on rats with
technical fenamiphos (Nemacur) in diet. Unpublished report No. 717
(study No. 83-171-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hayes, R.H. (1986b) Combined chronic toxicity/oncogenicity study of
technical fenamiphos (Nemacur) with rats. Unpublished report No. 721
(study No. 83-271-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K G (1981) Determination of acute toxicity (LD50 Unpublished
report dated 18 August 1981 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K G (1984) Determination of acute toxicity (LD) Unpublished
report dated 14 February 1984 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1979) Salmonella/microsome test for detection of
point-mutagenic effects. Unpublished report No. 8730 (study no.
Nemacur/003) from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980a) Micronucleus test on mouse to evaluate Nemacur
technical for potential mutagenic effects. Unpublished report No. 8805
(study no. SRA 3886/002) from Bayer AG Institute of Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980b) SRA 3886/dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8838 (study no. SRA
3886/001) from Institute of Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) SRA 3886: Salmonella/microsome test to evaluate
for potential point mutation. Unpublished report No. 13365 (study no.
T2017617) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985b) Addendum to report SRA 3886: Salmonella/microsome
test to evaluate for potential point mutation. Unpublished report No.
13365A (study no. T2017617) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1987) SRA 3886: Cytogenetic study of human lymphocyte
cultures in vitro to test for chromosome damage. Unpublished report
No. 15406, (study No. T3022874) from Bayer AG, Fachbereich Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1988) SRA 3886: In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report No. 16690, (study No. T1025572) from Bayer AG,
Fachbereich Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Greufe (1993) Response to US-EPA review: Chronic feeding
toxicity study with technical grade Fenamiphos (Nemacur(R)) with
dogs. Unpublished report dated 12 July 1993 (supplement to Mobay Corp.
study No. 88-274-BB) from Miles Inc., Stilwell, KS, USA. Submitted to
WHO by Bayer AG. Leverkusen, Germany.
Jones, R.D. & Loney, M.L. (1993) A subchronic feeding toxicity study
with technical grade Fenamiphos (Nemacur(R)) in dogs. Unpublished
report No. 91 - 176-KP (supplement to Mobay Corp. study No. 88-274-BB)
from Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kato, M.C. (1984a) Primary dermal irritation study of fenamiphos
technical in rabbitś Unpublished report No. B-561 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-561). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kato, M.C. (1984b) Primary eye irritation study of fenamiphos
technical in rabbitś Unpublished report No.B-562 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-562). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Khasawinah, A.M. & Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Unpublished report No. 34217 from Chemagro
Division of Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on hens.
Unpublished report No. 1831 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Unpublished
report No. 2829 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972a) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Unpublished report No. 3438 from
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Unpublished report No. 3559 from the
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972c) Antidotal experiments on rats. Unpublished report
No. 3560 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. ( 1971) BAY 68138 toxicological studies.
Unpublished report No. 2767 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Knaak, J.B., Ackerman, K.Y., Fredrickson, A.S. & Lee, P. (1981)
Reentry research: Dermal dose red cell cholinesterase-response curves
for fenamiphos, fenamiphos sulfoxide and sulfone. Unpublished report
No. 69689 from California Department of Food and Agriculture.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. ( 1988 ) Study for acute oral toxicity in rats.
Unpublished report No. 16744 (study No. T0027669) from Bayer AG,
Fachbereich Toxicologies, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Landes, A.M. (1978) In vitro inhibition of cholinesterase
by (R)Nemacur and Nemacur metabolites. Unpublished report No. 66068
(reference 77-060) from Mobay Chemical Corp., Chemagro Agricultural
Division, Kansas City, MO, US A. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of Nemacur
technical, desisopropyl Nemacur sulfoxide and desethyl Nemacur.
Unpublished report No. 44531 (reference 74-175) from Chemagro
Agricultural Division, Mobay Chemical Corp. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) The acute oral toxicity of Nemacur
desisopropyl to rats. Unpublished report No. 51597 (reference 75-144)
from Chemagro Agricultural Division, Mobay Chemical Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1968a) Subchronic toxicological studies on rats.
Unpublished report No. 745 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1968b) Subchronic toxicological studies on dogs.
Unpublished report No. 837 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser E (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Unpublished report No. 1655 from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1970) Subchronic toxicological studies on dogs. Unpublished
report No. 2008 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Unpublished report from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on rats (2 year
feeding experiment). Unpublished report No. 3539 from the Institute
for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1972c) Generation studies on rats. Unpublished report No.
3424 from the Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Löser, E. & Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachrichten Bayer,
24(1), 69-113. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MacKenzie, K.M. (1982) Teratology study with Nemacur in rabbits.
Unpublished report No. 308 (study No. 81165) from Hazelton Raltech,
Inc., Madison, WI, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mavournin, K.H., Blakey, D.H., Cimino, M.C., Salamone, M.F. & Heddle,
J.A. (1990) The in vivo micronucleus assay in mammalian bone marrow
and peripheral blood. A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 239, 29-80.
Mawdesley-Thomas, L. & Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Unpublished report
(addendum to report No, 745) from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mawdesley-Thomas, L. & Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Unpublished report (addendum
to report No. 1655) from the Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail F. (1980) SRA 3885 and Curaterr(R): Evaluation for acute
combination toxicity. Unpublished report No. 9294 from Institute of
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Mihail, F. & Schilde, B. (1980) SPA 3886 (active ingredient of
Nemacur(R)) subacute dermal toxicity study on rabbits. Unpublished
report No, 9297 from Institute of Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Rieth, J.P., Moore, K.D. & Elcock, L.E. (1991 ) Chronic feeding
toxicity study of technical grade Fenamiphos (Nemacur(R)) with dogs.
Unpublished report No. 6243 (study No. 88-274-BB) from Mobay Corp.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1981) SRA 3886 (Nemacur): Evaluation for embryotoxicity
and teratogenic effects in orally dosed rats. Unpublished report No.
9785 from Institute for Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Unpublished report (addendum to report
No. 1831 ) from the Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1971) Pathology report of BAY 68138. Hen study.
Unpublished report (addendum to report No. 2829) from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Stropp, G. (1995) Study for the skin sensitization effect in guinea
pigs (guinea pig maximization test according to Magnusson and
Kligman). Unpublished report No. 24341 (study No. T9058358) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Unpublished report (addendum
to report No. 2008) from Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Unpublished report (addendum to report No. 3561) from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted m
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974a) Nemacur(R) sulfone, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974b) Nemacur(R) sulfoxide, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974c) 3-Methyl-4-methylmercaptophenol, Acute toxicity in
rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974d) 3-Methyl-4-methane-sulfonylphenol, acute toxicity
in rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974e) 4-Methylmercapto-m-cresol, acute toxicity in rats.
Unpublished report dated 29 April 1974 from Institute for Toxicology,
Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979a) SPA 3885 (Nemacur(R) active ingredient)/acute
inhalation toxicity studies. Unpublished report No. 8210 from Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979b) Nemacur(R) active ingredient (SRA 3886)/subacute
inhalational toxicity studies. Unpublished report No. 8669 from
Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl 4-(methylthio)-N-
tolyl isopropylphosphoramidate] and identification of two metabolites
in plants. J. Agric. Food Chem., 20, 157-160. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Wargo, J.P. (1978) The effect of feeding Nemacur(R) sulfoxide to
dairy cattle. Unpublished report No. 66212 from Chemical Agricultural
Division, Mobay Chemical Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1988) [Phenyl-1-14C] Nemacur: Whole-body autoradiographic
distribution of the radioactivity in the rat. Unpublished report No.
PF3055 (study No. M181 0188-7) from Bayer AG Agrochemicals,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yang, L.L., Putnam, D.L. & Conklin, P.M. (1984) CHO/HGPRT mutation
assay in the presence and absence of exogenous metabolic activation --
Nemacur(R). Unpublished report No. 620 (study No. T2600.332) from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
 inhibitory activity of fenamiphos and its metabolites in horse serum
cholinesterase in vitro was in the order fenamiphos < sulfoxide =
sulfone < unidentified metabolite.
The concentrations that inhibited the activity of chicken and monkey
liver aliesterase (triacetin hydrolysis) and monkey cholinesterase by
50% in vitro were 4.2 × 10-5 mol/L for chicken aliesterase, 1.0 ×
10-6 mol/L for monkey aliesterase, and 4.6 × 10-6 mol/L for monkey
cholinesterase (Coulston & Wills, 1974).
Male albino rats were painted dermally on the clipped dorsal area with
100-400 µg/cm2 fenamiphos, 25-800 µg/cm2 fenamiphos sulfoxide, or
200-1600 µg/cm2 fenamiphos sulfone. The test materials were applied
in acetone and allowed to dry. After 72 h, the animals were killed and
erythrocyte acetylcholinesterase activity determined. Fifty percent
inhibition was achieved with doses of 208 µg/cm2 fenamiphos, 262
µg/cm2 fenamiphos sulfoxide, and 750 µg/cm2 fenamiphos sulfone
(Knaak et al., 1981; JMPR, 1985).
Blood samples were extracted from equal numbers of male and female
Sprague-Dawley rats and then pooled for comparison of the
cholinesterase inhibition induced by fenamiphos and five of its
metabolites (fenamiphos sulfoxide, desisopropyl fenamiphos sulfoxide,
fenamiphos sulfone, desisopropyl fenamiphos sulfone, and desisopropyl
fenamiphos) in vitro. Samples were incubated for 1 h before
determination of the cholinesterase activity (Table 1). Erythrocyte
acetylcholinesterase was less sensitive to fenamiphos and its
metabolites than was plasma cholinesterase (Lamb & Landes, 1978; JMPR,
1985).
In order to assess the effects of fenamiphos on neurotoxic esterase
activity, single doses of 0 or 25 mg/kg bw technical-grade fenamiphos
(purity; 91.3%) were given by oral intubation to groups of nine
atropinized Lohmann selected Leghorn hens. Groups of three surviving
hens were killed one, two, and seven days after treatment. Even under
atropine protection, one hen died within 24 h of treatment. Fenamiphos
had no effect on neurotoxic esterase activity in the brains or spinal
cords, whereas the positive control compound, tri- ortho-cresyl
phosphate, had almost completely inhibited the activity 24 and 48 h
after treatment (Flucke & Eben, 1988).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of fenamiphos and its
metabolites are summarized in Table 2. Impurities identified as
components of the technical mixture (aryldiamide, diarylamide, diaryl
ethyl ester, diethyl ester, diethylmonamide, di-SCH3 compound, ethyl
aryl ester, ethyldiamide, and 4-methylthio- meta-cresol) were tested
for their acute toxicity to rats. At doses 1.5 times the acute LD50
of fenamiphos, none of the materials was toxic (Crawford & Anderson,
1973; JMPR, 1974).
Table 1. Inhibition of cholinesterase (%) by fenamiphos and its metabolites in vitro
Compound Plasma Erythrocytes
(Dose: ppm in whole blood) (Dose: ppm in whole blood)
6.88 68.8 688 5440 6880 6.88 68.8 688 5440 6880
Fenamiphos - 0 10 49 69 - 0 0 0 23
Fenamiphos sulfoxide 18 41 48 - 85 5 0 5 - 52
Fenamiphos sulfone 0 13 50 - 87 8 - - - -
Desisopropyl fenamiphos sulfoxide 6 40 90 - 93 4 0 47 - 50
Desisopropyl fenamiphos sulfone 0 20 69 - 90 6 0 32 - 61
Desisopropyl fenamiphos - 2 13 71 91 - 8 0 32 53
-, not determined
Table 2. Acute toxicity of fenamiphos, its metabolites, and impurities
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos
Mouse Oral M NR 22.7 Loser & Kimmerle (1971)
Intraperitoneal M/F 80 3.4 DuBois et al. (1967)
Inhalation M NR approx. 60 Kimmerle & Solmecke (1971)
(1 h static)
Rat Oral (fasted) M 88-99.7 2.4-6.0 Crawford & Anderson (1973, 1974a);
F 2.4-6.1 Lamb & Matzkanin (1975); Mihail (1980);
Heimann (1981, 1984, Krotlinger (1988)
Oral M 80-90.2 8.1-17.2 DuBois et al. (1967); Loser & Kimmerle (1971);
(not fasted) F 9.6-19.4 Kimmerle & Solmecke (1971); Kimmerle (1972a);
Heimann (1981, 1984)
Dermal M 80-92.2 72-73 Dubois et al. (1967); Flucke (1980)
84-92
Dermal M NR approx. 500 Kimmerle & Solmecke (1971)
Inhalation M 89.8 110-175 Kimmerle (1972b); Kimmerle & Solmecke
(1 h) F 130-150 Thyssen (1979a)
Inhalation M 89.8 91-100 Kimmerle & Solmecke (1971); Thyssen (1979a)
(4 h) 100
Intraperitoneal M 80 3.0-3.7 DuBois et al. (1967); Loser & Kimmerle (1971);
F 4.2-4.9 Kimmerle & Solmecke (1971)
Guinea-pig Oral M NR 56-100 DuBois et al. (1967); Loser & Kimmerle (1971);
Kimmerle & Solmecke (1971)
Intraperitoneal M NR 17.3 DuBois et al. (1967)
Rabbit Oral M NR 10-17.5 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Dermal M NR 225 Crawford & Anderson (1972)
F 179
Cat Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Dog Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Chicken Oral F NR 5.3-12 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971); DuBois et al. (1967)
Fenamiphos sulfone
Rat Oral (fasted) M NR 2.6 Crawford & Anderson (1974b)
F 2.4
Oral F NR 1-25 Thyssen (1974a)
(not fasted)
Fenamiphos sulfoxide
Rat Oral (fasted) M/F NR 2.4 Crawford & Anderson (1974b)
Oral F NR 10-25 Thyssen (1974b)
(not fasted)
Desisopropylfenamiphos
Rat Oral (fasted) M NR 1.4 Lamb & Matzkanin (1977)
F 2.1
Desisopropylfenamiphos sulfone
Rat Oral (fasted) M 95 4.1 Lamb & Matzkanin (1975)
F 3.7
Fenamiphos sulfoxide phenol
Rat Oral (fasted) M 99 1418 Crawford & Anderson (1974a)
F 1175
Oral F NR 500-1000 Thyssen (1974c)
(not fasted)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos sulfone phenol
Rat Oral (fasted) M 95 1250 Crawford & Anderson (1974a)
F 1854
Oral F NR > 1000 Thyssen (1974d)
(not fasted)
4-Methylthio-meta-cresol
Rat Oral (fasted) M 96.4 1418 Crawford & Anderson (I974a)
F 1333
Oral F NR > 2500 Thyssen (1974e)
(not fasted)
NR, not reported
Fenamiphos (purity, 97%) was given orally to fasted Wistar (Bor:WISW
(SPF-Cpb) rats at doses of 1-8 (females) or 1-100 (males) mg/kg bw.
All deaths among males at 100 mg/kg bw occurred within 10 min, and
those among animals receiving 5 mg/kg bw or more within 1.5 h.
Clinical signs of toxicity noted at doses >4 (males) or 5 (females)
mg/kg bw included apathy, palmospasms, laboured breathing, diarrhoea,
piloerection, clonic cramps, and dyspnoea. Females at does > 1
mg/kg bw had only diarrhoea (Krötlinger, 1988).
Studies in which rodents were given fenamiphos by inhalation as a
static spray at doses up to 230 µg/L for 1-4 h showed that rabbits and
guinea-pigs are more tolerant of the acute effects than rats and mice
(Kimmerle & Solmecke, 1971).
Male and female TNO/W74 albino rats were exposed to aerosolized
fenamiphos (purity, 89.8%) for 4 h per day on five consecutive days at
concentrations of 0, 0.3, 0.6, 3.3, 4, 9, or 28 µg/L. Plasma and
erythrocyte cholinesterase activities were measured before exposure,
after the first, third, and fifth exposures, and 72 h after the fifth
exposure; brain acetylcholinesterase activity was not measured. The
cholinesterase activities were depressed in a dose-related manner;
that of plasma cholinesterase was the most significantly depressed,
and females were more sensitive than males. Plasma cholinesterase
activity was 32-90% lower than before treatment in males at > 3.3
µg/L and 31-96% lower in females at > 0.3 µg/L. The activity
remained depressed by 19-44% for 72 h after the fifth exposure in
females at concentrations of > 9 µg/L. Erythrocyte
acetylcholinesterase activity in males was inhibited by < 20% at
> 9 µg/L; however, that in females was depressed by up to 28% at 9
µg/L and 33% at 28 µg/L (JMPR, 1985, slightly modified by reference to
the original report of Thyssen, 1979b).
In male rats, administration of atropine and/or 2-pralidoxime or
obidoxime after poisoning reduced the acute lethal dose by a factor of
about two. As with other organophosphorus compounds, rapid
administration of atropine and oxime reactivator after poisoning
affords some protection and alleviates the cholinergic signs of
poisoning (DuBois et al., 1967; Kimmerle, 1972c; JMPR, 1974).
(b) Short-term toxicity
Rats
Three groups of 10 male and 10 female albino Wistar TNO/W 74 rats were
exposed to technical-grade fenamiphos (purity, 92.2%) diluted with a
1:1 mixture of ethanol and polyethylene glycol 400 for aerosolization
in a dynamic flow inhalation chamber at doses of 0, 0.03, 0.25, or 3.5
µg/L for 6 h per day, five days per week for three weeks; 98% of the
particles were 3 µm or less. No toxic signs or effects on mortality,
body-weight gain, or the results of haematology, urinalysis, or
clinical chemistry were seen. A significant decrease (48-79%) in
plasma cholinesterase activity and a slight decrease (9-18%) in
erythrocyte acetylcholinesterase activity were seen in animals of each
sex at 3.5 µg/L; brain acetylcholinesterase activity was not affected.
There were no gross or histopathological changes or effects on organ
weights. The NOAEL was 3.5 µg/L (Thyssen, 1979b).
Groups of 15 male Sprague-Dawley rats were given fenamiphos orally or
by intraperitoneal injection on five days per week for 60 days. All of
the animals survived doses ranging from 1.5 mg/kg bw per day
intraperitoneally to 1.7 mg/kg bw per day orally with no cumulative
toxicological effect (Kimmerle & Solmecke, 1971; JMPR, 1974). In
another study, female rats survived daily intraperitoneal
administration of 1 mg/kg bw per day for 60 days, while 40% of those
given 2 mg/kg bw per day and all rats at 3 mg/kg bw per day died
(DuBois & Flynn, 1968; JMPR, 1974). These studies indicate little if
any cumulative toxicity.
Groups of 20 Fischer 344 rats of each sex were given diets containing
fenamiphos (purity, 89%) at 0, 0.37, 0.57, or 0.91 ppm (equal to 0,
0.03, 0.045, or 0.072 mg/kg bw per day for males and 0, 0.035, 0.053,
or 0.084 mg/kg bw per day for females) for three months. Animals were
monitored for clinical signs of toxicity, mortality, abnormality,
masses, food consumption, and body weight throughout the study.
Cholinesterase activity in plasma and erythrocytes was determined in
10 rats of each sex per group at 5, 9, 12, and 14 weeks and in brain
at termination of the study. No effects attributable to treatment were
seen on food consumption or body weight or during clinical
observation. Statistically but not toxicologically significant
decreases in both plasma and erythrocyte cholinesterase activity were
observed in treated rats of each sex when compared with controls. The
maximal inhibition of plasma cholinesterase was 17% at week 14 in
males at 0.37 ppm, with lesser inhibition at higher doses, and 24% at
week 9 in females at the high dose. Erythrocyte acetylcholinesterase
activity was inhibited by < 10% in all cases. No toxicologically
significant, treatment-related change was seen in brain
acetylcholinesterase activity. It was concluded that no biologically
significant cholinesterase activity inhibition had occurred in this
study. The NOAEL was 0.9 ppm, equal to 0.07 mg/kg bw per day, the
highest dose tested (JMPR, 1987, slightly modified by reference to the
original report of Hayes, 1986a).
Groups of Wistar SPF rats of each sex (15 per treated group, 30
controls) were fed diets containing fenamiphos (purity, 82%) at 0, 4,
8, 16, or 32 ppm (equivalent to 0.2, 0.4, 0.8, or 1.6 mg/kg bw per
day) for three months. Male and female rats at the highest dose showed
signs of cholinergic stimulation during the first two months; no
behavioural changes were seen in the other animals. Average food
consumption and growth were similar for treated and control animals.
The only effects on the haematological parameters examined were
decreased plasma cholinesterase activity (> 20%) in animals at 8 ppm
and inhibition of erythrocyte acetylcholinesterase activity (< 12%)
in those at 8 ppm throughout the study, with peak inhibition (56%) at
16 ppm by week 8. Gross and microscopic examination of tissues and
organs at the end of the experiment showed slightly increased liver
weights in males at 16 or 32 ppm, which was not reflected in the
calculated relative organ:body weight ratios or on histological
examination. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original reports of
Löser, 1968a; Mawdesley-Thomas & Urwin, 1970a). Although the NOAEL was
originally considered to be 4 ppm on the basis of inhibition of plasma
cholinesterase activity (JMPR, 1974), the present Meeting decided that
the NOAEL was 8 ppm, equivalent to 0.4 mg/kg bw per day, on the basis
of inhibition of erythrocyte acetylcholinesterase activity.
Rabbits
An aqueous formulation of technical-grade fenamiphos (purity, 89.8%)
was applied to a clipped dorsal area of groups of six male and six
female New Zealand rabbits at doses of 0, 2.5, or 10 mg/kg bw per day
for 6 h per day, five days per week for three weeks. Two additional
groups were similarly treated at 0 and 0.5 mg/kg bw per day. The skin
of half of the animals in each group was abraded. Haematology,
clinical chemistry, and urinalysis were performed before treatment and
at the end of the study. Plasma and erythrocyte cholinesterase
acetylcholinesterase activities were measured before treatment and
after the tenth and last exposures.
No signs of toxicity or mortality were observed. Although the authors
concluded that body-weight gains were decreased in animals of each sex
at 10 mg/kg bw per day (JMPR, 1985), the differences were less than
10% and were not statistically significant. Furthermore, the range of
individual weight gains seen in animals at the high dose was well
within that observed in the controls. Slight erythema was observed in
all groups only at the abraded skin sites during the initial week,
which cleared by day 7. There were no apparent differences in
haematological, urinary, or clinical chemical parameters between test
and control groups. Gross necropsy, histopathology, and organ weight
measurements showed no remarkable changes in comparison with controls.
Plasma and erythrocyte cholinesterase activity was significantly
depressed (by up to 65 and 34%, respectively) in male and female
rabbits at 10 mg/kg bw per day; however, the females were somewhat
more sensitive, with depressed plasma cholinesterase activity (by
22-31%) at 2.5 mg/kg bw per day as well. Brain acetylcholinesterase
activity was statistically significantly depressed in females at 2.5
mg/kg bw per day (by 19%) and 10 mg/kg bw per day (by 21%). The NOAEL
was 0.5 mg/kg bw per day (JMPR, 1974, slightly modified by reference
to the original report by Mihail & Schilde, 1980).
Dogs
Groups of two beagle dogs of each sex were fed fenamiphos (purity,
82%) in the diet at 0, 2, 6, or 18 ppm (equivalent to 0.05, 0.15, or
0.45 mg/kg bw per day) for three months. Behavioural abnormalities
with signs of cholinergic stimulation were evident in animals at 18
ppm, and the growth of females at this dose was reduced.
Haematological and clinical chemical parameters, including the results
of tests for liver and kidney function and urinalyses, were not
affected by treatment. The averages values for plasma and erythrocyte
cholinesterase activity were depressed in males and females at 6 ppm;
in animals at 2 ppm, no effect was found on average values for
erythrocyte acetylcholinesterase activity, while those for plasma
cholinesterase were depressed (20-30%). Brain acetylcholinesterase
activity was not measured. Gross morphological examination of tissues
and organs at the end of the study showed no abnormal effects (JMPR,
1974, slightly modified by reference to the original report by Löser,
1968b).
Groups of two male and two female beagle dogs (three of each sex as
controls) were fed fenamiphos (purity, 99.4%) in the diet at levels of
0, 1, 2, or 5 ppm (equivalent to 0.025, 0.05, or 0.13 mg/kg bw per
day) for three months. Treatment at < 5 ppm in the diet had no
effect on the average values of haematological parameters, liver
function tests, clinical chemistry, or kidney function tests or on the
gross or microscopic appearance of tissues and organs. As in other
studies, cholinesterase activity was the only parameter significantly
affected, the females being more susceptible than the males and plasma
cholinesterase activity being more sensitive to inhibition than that
of erythrocytes. The latter was unchanged at 2 ppm while plasma
cholinesterase activity was slightly, transiently depressed; 1 ppm
fenamiphos in the diet had no effect on plasma cholinesterase
activity. Brain acetylcholinesterase activity was not measured (Löser,
1969; Mawdesley-Thomas & Urwin, 1970b; JMPR, 1974).
As an extension of the previous study, an additional two dogs of each
sex were fed fenamiphos(purity, 99.4%) in the diet at 0 or 10 ppm
(equivalent to 0.25 mg/kg bw per day) for three months. No deaths
occurred during the experiment, although a very slight deviation in
the average body weight of treated animals was noted. Because of the
small number of animals, the slight weight differences cannot be fully
evaluated. There was no effect on haematological or urinary
parameters, clearance, blood sugar, or cholesterol levels.
Cholinesterase activity was significantly depressed in both plasma and
erythrocytes in male and female dogs; brain acetylcholinesterase
activity was not measured. Gross and microscopic examination of
tissues and organs showed no significant differences between treated
and control animals (Löser, 1970; Thomson et al., 1972a; JMPR, 1974).
Groups of four male and four female, four-month-old beagle dogs were
fed diets containing fenamiphos (purity, 89%) at doses of 0, 0.6, 1,
or 1.7 ppm (equivalent to 0.015, 0.025, or 0.042 mg/kg bw per day) for
three months. They were observed twice daily, and body weight and food
consumption were recorded weekly. Plasma and erythrocyte
cholinesterase activity was determined at 0, 4, 6, 8, 10, and 12
weeks; brain acetylcholinesterase was determined at termination of the
study. Haematology, clinical chemistry, and urinalysis were not
performed, and tissues and organs were not examined grossly or
histologically. No toxicological symptoms or effects on body weight
and food consumption were seen; however, plasma cholinesterase
activity was depressed by 28-35% in males given 1.7 ppm in the diet.
The author concluded that females at this dose were not similarly
sensitive (JMPR, 1985), on the basis of the < 20% inhibition relative
to control levels; however, when inhibition was calculated over the
course of the study, the reductions in plasma cholinesterase activity
in females at all doses were 2-14% in controls, 13-23% in animals at
0.6 ppm, 18-28% at 1 ppm, and 31-41% at 1.7 ppm, which were comparable
to those in males: 10-15% in controls, 17-23% in dogs at 0.6 ppm,
20-29% at 1 ppm, and 34-41% at 1.7 ppm. Erythrocyte and brain
acetylcholinesterase activities were unaffected (JMPR, 1985, slightly
modified by reference to the original report by Hayes, 1983).
Technical-grade fenamiphos (purity, 88.9%) was administered in the
diet to groups of four male and four female beagle dogs at
concentrations of 0, 1, 3, or 12 ppm (equal to 0, 0.03, 0.089, or 0.31
mg/kg bw per day in males and 0, 0.03, 0.083, and 0.35 mg/kg bw per
day in females) for one year. The animals were observed for mortality,
clinical signs, body weight, food consumption, ophthalmic alterations,
and haematological, urinary, and clinical chemical parameters. Plasma
and erythrocyte cholinesterase activity was measured before treatment
and quarterly thereafter. One-half of each brain was taken at necropsy
for measurement of acetylcholinesterase activity. At necropsy, 11
organs were taken from each dog and weighed, and about 40 tissues from
each dog were examined grossly and histopathologically.
Treatment did not affect survival, clinical signs, growth, food
consumption, ophthalmoscopic or urinary parameters, or organ weights.
Them were no gross or histopathological findings that could be
attributed to treatment. Plasma cholinesterase activity was
statistically significantly inhibited relative to the levels before
treatment in both males and females in a dose-related fashion at doses
> 1 ppm: 1 ppm, 20-32%; 3 ppm, 41-53%; 12 ppm, 54-65%; the control
values were variably reduced by up to 14% over the course of the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly decreased in both males and females at
3 ppm (by 17-36%) and 12 ppm (by 58-68%); the activity in the controls
and dogs at 1 ppm was variably, statistically nonsignificantly reduced
by 5-24% and 10-23%, respectively, during the course of the study.
Brain acetylcholinesterase activity was nonsignificantly decreased by
12% in males and sigificantly reduced by 17% in females at 12 ppm.
Males at this dose also had mild, transient anaemia characterized by
significantly decreased erythrocyte counts, haemoglobin
concentrations, and haematocrit, with a concomitant increase in mean
corpuscular volume. The authors concluded that there was no NOAEL
because of the effects on plasma cholinesterase activity at the lowest
dose (Riethet al., 1991). Owing to the variability in the activity of
plasma cholinesterase in controls seen in a six-month follow-up (Jones
& Loney, 1993; see below), the authors reconsidered the results of the
one-year study and decided that the changes seen at 1 ppm were within
the normal biological range seen in historical controls (Jones &
Greufe, 1993). The Meeting agreed that the NOAEL was 3 ppm, equal to
0.083 mg/kg bw per day, on the basis of decreased brain
acetylcholinesterase activity and anaemia at the highest dose.
In the supplemental study (Jones & Loney, 1993), technical-grade
fenamiphos (purity, 89%) was administered in the diet to groups of
four male and four female beagle dogs at concentrations of 0 or 0.5
ppm, equal to 0.011 mg/kg bw per day, for six months. The only
parameters measured were mortality, clinical and ophthalmologic signs,
body weight, food consumption, and plasma and erythrocyte
cholinesterase activity. No signs of toxicity were observed.
Groups of four male and four female pure-bred beagle dogs were fed
fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5, or 10 ppm (equal
to 0.015, 0.029, 0.06, 0.15, or 0.31 mg/kg bw per day for males and
0.014, 0.036, 0.063, 0.17, and 0.34 mg/kg bw per day for females) for
two years. There were no significant effects on growth, food
consumption, or the results of any of the standard clinical and
physiological examinations made during the course of the study. Gross
and histological examination of all tissues and organs at the
conclusion of the study showed no abnormal developments considered to
be related to treatment. The only significant physiological effect
observed was inhibition of plasma cholinesterase activity (> 20%) at
doses > 2 ppm and of erythrocyte acetylcholinesterase activity at
> 5 ppm. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original report by
Löser, 1972a; Thomson et al., 1972b).
Cattle
Groups of three dairy cows were fed fenamiphos sulfoxide in their
diets at levels of 2, 6, or 20 ppm for 29 days; one untreated cow was
used as a control. There were no apparent effects on behaviour, feed
consumption, milk production, or body-weight gain. No adverse effects
on whole-blood cholinesterase activity were seen with 2 or 6 ppm, but
a significant depression (51%) was seen at 20 ppm (Wargo, 1978; JMPR,
1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female six-week-old outbred CD1 albino mice
were given fenamiphos (purity, 89.5%) in the diet at doses of 0, 2,
10, or 50 ppm (equal to 0, 0.3, 1.4, or 7.4 mg/kg bw per day for males
and 0, 0.3, 1.8, or 8.8 mg/kg bw per day for females) for 20 months.
Toxic effects were monitored daily, and body weights and food
consumption were determined weekly. Haematological parameters were
analysed in 10 mice of each sex in each group at 6, 12, 18, and 20
months of the study. All animals underwent complete necropsy, and the
liver, kidney, heart, lungs, gonads, spleen, brain, and adrenals were
weighed. A full complement of tissues and organs from all animals were
examined histopathologically. Cholinesterase activity was not
measured. Daily observations were not reported. Survival was
comparable in all groups, with only a marginal decrease at the high
dose. Survival at 20 months was 32-45%. Body weights were
statistically significantly reduced in both males and females at 50
ppm, but the differences were less than 10%. Food consumption and
haematological parameters were unaffected by treatment.
Contrary to the conclusions of the 1985 JMPR with regard to changes in
organ weights, reexamination of the data indicates that the absolute
weights of the brains of female mice at all doses were slightly (4-6%)
but statistically significantly lower than those of controls. The
relative weights of the brains of animals at 50 ppm were, however,
increased in both males (7%, statistically nonsignificant) and females
(9%, statistically significant). The absolute and relative ovarian
weights were decreased by 16-18% in animals at 10 ppm and by 42-55% in
those at 50 ppm; and the absolute and relative splenic weights were
reduced in animals of each sex at 50 ppm. Other findings include
reductions in absolute heart, lung, liver, and kidney weights
(generally 10-15%, of variable significance) in males and females at
50 ppm, although the relative weights were comparable to those in
controls. No gross or microscopic parallel to these weight changes was
found. The most frequent pathological findings reported were chronic
multifocal interstitial nephritis, chronic peribronchiolitis,
pulmonary congestion, and acute and chronic myocarditis in all treated
animals, but no significant difference associated with dose was found.
Cystic endometrial hyperplasia was also frequent in all treated
females. A significant degree of fatty diffuse change was seen in the
liver, again with no relation to dose. In all groups, uniformly spread
amyloidosis was observed in many organ systems, including liver,
spleen, adrenals, kidneys, small intestines, thyroids, ovaries, and
submaxillary salivary glands. Fenamiphos had no oncogenic potential at
any dose. The NOAEL was 2 ppm, equal to 0.3 mg/kg bw per day, on the
basis of decreased splenic and ovarian weights at doses > 10 ppm
(JMPR, 1985, slightly modified by reference to the original report by
Hayes, 1982).
Rats
Groups of 40 male and 40 female SPF-derived Wistar rats were fed
fenamiphos in the diet at concentrations of 0, 3, 10, or 30 ppm (equal
to 0, 0.17, 0.56, or 1.7 mg/kg bw per day for males and 0, 0.23, 0.76,
or 2.2 mg/kg bw per day for females) for two years. Behavioural
abnormalities due to cholinergic stimulation were seen only during the
first six weeks of treatment at 30 ppm. The obvious effect on
cholinesterase activity disappeared after six weeks of feeding and was
not seen for the remainder of the study. Average growth, mortality,
food consumption, haematological and clinical chemical parameters, and
the results of liver and kidney function tests were unchanged. Urinary
parameters and blood sugar and cholesterol values were normal. The
thyroid weights and the thyroid:body weight ratios of females at 30
ppm were larger than those of controls but were not accompanied by
abnormal tumour development, goitre, or unusual histological findings.
All other major tissues and organs appeared normal at gross and
microscopic examination.
Feeding of 10 ppm fenamiphos in the diet inhibited plasma
cholinesterase activity by up to 55% in females but by < 20% in
males. In animals at 30 ppm, plasma cholinesterase activity was
inhibited by up to 60% and that of erythrocyte acetylcholinesterase by
up to 54% in males and 65% in females. No significant effects were
observed at 3 ppm. Although the NOAEL was originally considered to be
3 ppm, the present Meeting determined it to be 10 ppm (equal to 0.56
mg/kg bw per day) on the basis of inhibition of erythrocyte
acetylcholinestrase activity at the next highest dose (JMPR, 1974,
slightly modified by reference to the original report by Löser, 1972b;
Cherry & Newman, 1973).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenamiphos (purity, 89.3%) at a mean
concentration of 0, 1.7, 7.8, or 37 ppm (equal to 0, 0.098, 0.46, or
2.5 mg/kg bw per day for males and 0, 0.12, 0.6, or 3.4 mg/kg bw per
day for females) for two years. Clinical signs, mortality, food
consumption, haematological and blood chemical parameters (in 20 rats
of each sex per group), urinary parameters (in 10 rats of each sex per
group), and body weights were monitored throughout the study. Plasma
and erythrocyte cholinesterase, activity was monitored in 10 rats of
each sex per group at weeks 6, 10, and 15 and at 6, 12, 18, and 24
months. Additional groups of 10 rats of each sex were given 0 or 37
ppm fenamiphos for one year. Brain acetylcholinesterase activity was
determined at the end of the study in all rats in these satellite
groups and in 10 rats of each sex per group in the main study. All
rats, including those found dead or killed in extremis during the
study and those killed at 12 or 24 months, were examined grossly;
their organs were weighed, and more than 40 tissues were evaluated
microscopically. Ophthalmological examinations were performed on 10
rats of each sex per dose before and at the end of the study.
Survival at termination of the study was not related to treatment and
was 58-88% in males and 62-84% in females. Clinical observation
indicated a higher incidence of rough coat and alopecia in females at
the high dose than in controls. A statistically significant decrease
in body-weight gain was seen in male and female rats at the high dose
throughout the study, although no treatment-related effect on feed
consumption was seen. There was a dose-related, statistically
significant decrease in plasma cholinesterase activity in all treated
rats throughout the study: in mate rats, 7-38% at 1.7 ppm, 19-68% at
7.8 ppm, and 56-88% at 37 ppm; in females, 22-96% throughout the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly inhibited in treated animals: by 0-7% at
1.7 ppm, 12-25% at 7.8 ppm, and 56-81% at 37 ppm in males and 0-11% at
1.7 ppm, 21-43% at 7.8 ppm, and 62-81% at 37 ppm in females. A
statistically significant decrease in brain acetylcholinesterase
activity was observed in male rats at the high dose at termination
(-14%) and in animals of each sex (-25% in males and -24% in females)
at interim sacrifice after one year of treatment. There were
statistically significant increases in the relative weights of the
brain, heart, and lung in both males and females at the high dose at
the end of the study; females at the high dose also had significantly
increased relative kidney weights. At interim sacrifice, females had
statistically significantly increased relative weights of adrenals,
brain, heart, kidneys, and ovaries. These changes were considered to
be related to the decrease in body weight observed in rats of each sex
at 37 ppm. The only statistically significant change in absolute organ
weight at termination was a decrease in liver weight and an increase
in lung weight in animals of each sex at 37 ppm. No treatment-related
change was seen during ophthalmological examination, in food
consumption, in haematological, clinical chemical, or urinary
parameters, or on gross pathology. No treatment-related neoplastic
lesions were observed during histopathological examination, but
statistically significantly higher incidences of non-neoplastic
inflammatory lesions were observed in the nasal, laryngeal, and lung
tissues of rats receiving 37 ppm fenamiphos in the diet when compared
with controls. These changes were attributed by the author of the
study to the marked inhibition of cholinesterase activity in these
animals. No treatment-related non-neoplastic changes were noted at 1.7
or 7.8 ppm. The author concluded that fenamiphos was not oncogenic.
Contrary to the conclusions of the 1987 JMPR, the present Meeting
determined that the NOAEL was 7.8 ppm, equal to 0.46 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinestrase activity and
changes in body-weight gain, organ weights, and histoptahological
appearance at the next highest dose (JMPR, 1987, slightly modified by
reference to the original report by Hayes, 1986a).
(d) Genotoxicity
Fenamiphos has been tested adequately in a battery of tests for
mutagenicity (Table 4). It was weakly clastogenic only in vitro; no
similar response was seen in vivo in tests for micronucleus
formation and dominant lethal mutation. The Meeting concluded that
fenamiphos is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A standard three-generation (two litters per generation) study of
reproductive toxicity was performed in which groups of 10 male and 20
female FB30 rats were given fenamiphos in the diet at 0, 3, 10, or 30
ppm throughout mating, gestation, and suckling. Immediately after
birth, pups were examined for malformations and were then prepared for
another generation or killed. Five weanling rats per group from the
F3b. generation were killed, and macroscopic and microscopic
examinations were performed on the major tissues and organs. There
were no apparent differences in the limited indices of reproduction
investigated, including fertility, litter size, lactation index, or
growth of young, or in the incidence of malformations (Löser, 1972c;
Cherry et al., 1972; JMPR, 1974).
In a two-generation study of reproductive toxicity, groups of 30 male
and 30 female albino CD Sprague Dawley rats received technical-grade
fenamiphos (purity, 89%) in the diet at concentrations of 0, 2.5, 10,
or 40 ppm (equal to 0.17, 0.64, or 2.8 mg/kg bw per day for males and
0.2, 0.73, or 3.2 mg.kg bw per day for females) for 70 days before
mating. Oestrous cycles were characterized over two weeks in 10 F0
and F1 females per dose before mating After weaning, 30 F1 animals
of each sex per dose were treated for 70 days and then bred to produce
the second generation (F2); treatment was continued throughout
mating, gestation, and lactation. Groups of 10 F0 and F1 adults of
each sex per dose were used to assess cholinesterase activity in
plasma and erythrocytes in week 8 before mating and just before
sacrifice and in brain at the time of sacrifice. Plasma, erythrocyte,
and brain cholinesterase activities were measured in one pup of each
sex from each of 10 litters at the time of culling and on day 21 of
lactation. F0 and F1 females were killed after their pups had been
weaned or on day 24 of gestation. The males were killed after the last
litters were delivered. The histologic al examinations concentrated on
reproductive tissues.
There were no treatment-related deaths or clinical signs of toxicity
in the parental animals. In F0 and F1 dams at 40 ppm, statistically
significant reductions were seen in body-weight gain during lactation
(by 72 and 65%) and food consumption (by up to 11 and 19%). F1 and
F2 pups also had significant reductions in body-weight gain beginning
on day 7 of lactation. The body weights of F1 adults at 40 ppm were
significantly reduced throughout the premating period (by about 10% in
males and 7% in females), which the author attributed to their reduced
body weights at the start of the F1 premating period. Although not
reported by the author, the overall weight gain of F1 males before
mating was also reduced during the first four weeks, by 12% in gain
those at 10 ppm and by 15% in those at 40 ppm. The relative ovarian
weights of F0 females were significantly reduced, by 13% at 2.5 ppm,
11% at 10 ppm, and 20% at 40 ppm but were unaffected in F1 females.
Although the author concluded that this effect was related to
treatment at 40 ppm, no histopathological lesions were observed in the
ovaries, reproductive parameters were unchanged, and there was no
comparable effect in the F1 generation.
Plasma cholinesterase activity was significantly inhibited by > 20%
in all treated adult females of both generations, in F1 males at 10
ppm at the time of sacrifice, and in both F0 and F1 males at 40 ppm
both before mating and at sacrifice. Erythrocyte acetylcholinesterase
activity was consistently significantly inhibited in females at doses
> 10 ppm but only at 40 ppm in F0 and F1 males. At 40 ppm, brain
acetylcholinesterase activity was significantly inhibited by 21% in
F0 females, by 29% in F1 females, and by only 6% in F0 males. In
pups, plasma cholinesterase activity was significantly inhibited on
day 21 of lactation in both males and females of both generations at
doses > 10 ppm. Erythrocyte acetylcholinesterase activity was
inhibited by > 20% in males and females of both generations at 40 ppm
only. Brain acetylcholinesterase was not affected.
Table 4. Results of tests for genotoxicity with fenamiphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 4, 20, 100, 500, 2500 NR (Negative)a,b Herbold (1979)
TA1535, TA1537 µg/plate (DMSO)
TA98, TA100
Reverse mutation S. typhimurium 20, 100, 125, 250, 500, 92.4 Negativea Herbold (1985a,b)
TA98, TA100 1000, 2000, 2500
TA1535, TA1537 µg/plate (DMSO)
Forward mutation Chinese hamster Unactivated: 100, 110, 85 Negativea,c Yang et al. (1984)
ovary cells 120, 130 µg/ml (DMSO)
(CHO-K1-BH4) Activated: 170, 190,
210, 230 µg/ml (DMSO)
Unscheduled Rat hepatocytes 1.5, 5, 15, 50, 100 µg/ml 89.5 Negative Curren (1988)
DNA synthesis (DMSO)
Chromosomal Human 25, 100, 400 µg/ml 91.3 Positivea,d Herbold (1987)
aberrations lymphocytes (DMSO)
Chromosomal Human Unactivated: 91.9 Negative Herbold (1988)
aberrations lymphocytes 25, 50, 75, 100 µg/ml
(DMSO)
Activated: 100, 150, Weakly
225, 350 µg/ml (DMSO) positivee
Sister chromatid Chinese hamster Activated: 10, 20, 40, NR Negativef Chen et al. (1982)
exchange cell line (V79) 80 µg/ml
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
(%)
In vivo
Micronucleus Mouse (NMRI) 0.625, 1.25, 2.5 mg/kg 92.5 (Negative)g Herbold (1980a)
formation bone-marrow cells bw
Dominant lethal Mouse (male 5 mg/kg bw 92.5 Negative Herbold (1980b)
mutation NMRI) germ cells
NR, not reported; DMSO, dimethyl sulfoxide
a With and without metabolic activation
b The 1985 JMPR concluded that the test protocol was unacceptable. Another test was conducted in only
one strain (TA 1537), without activation, at doses of 125, 250, 500, and 1000 µg/plate.
c Positive controls (0.2 µl/ml ethylmethylsulfonate; 2 µg/ml benzo[a]pyrene) yielded the expected positive responses.
d Statistically significant increase (52% mitotic index) seen at 100 µg/ml without activation and at 400 µg/ml with
activation (< 0.1% mitotic index). Haemolysis was seen at 400 µg/ml. The author concluded that the increase in
aberrations was due exclusively to cytotoxicity.
e Statistically significant response seen only at 350 µg/ml with activation (35% mitotic index). The author concluded
that the increase in aberrations was due to cytotoxicity.
f Positive control (5 µg/ml cyclophosphamide) gave expected positive response.
g The mice were dosed twice, 24 h apart, and the bone marrow was sampled once, 6 h after the second dose. The 1985
JMPR concluded that the test protocol was unacceptable. The protocol was also considered unacceptable in the report
of the Gene-Tox Program (Mavournin et al., 1990).
Oestrous cycles, mating, fertility, and gestation indices, sex ratio,
and pup viability indices were unaffected, and no treatment-related
gross or histological lesions seen in any tissue from either parental
animals or pups. The pups showed no clinical signs of toxicity. The
NOAEL for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per
day, on the basis of decreased body-weight gain. The NOAEL for
reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per day, on
the basis of decreased pup body weights during lactation (Eigenberg,
1991).
(ii) Developmental toxicity
Rats
Four groups of 25 female FB30 rats mated overnight with untreated
males (in a ratio of one male to two females) were given fenamiphos
(purity, 92.5%) in a 0.5% aqueous Cremophor emulsion at daily doses of
0, 0.3, 1, or 3 mg/kg bw per day by gavage on days 6-15 of gestation;
the first day of gestation was that on which sperm was found in a
smear obtained the morning after mating. Control females received the
same volume (10 ml/kg bw) of the aqueous emulsion. On day 20 of
gestation, the dams were narcotized with carbon dioxide and the
fetuses were removed. Litter size, average fetus weight per litter,
sex, external and visceral abnormalities, and skeletal malformations
and development were noted. Cholinesterase activity was not measured.
Eighteen dams receiving 3 mg/kg bw per day showed signs of toxicity
(trembling and recumbency), and two died; however, the time of death
was not given and the cause of death could not be established. No
treatment-related signs of toxicity or deaths occurred in rats
receiving 0, 0.3, or 1 mg/kg bw per day. A 15% reduction in average
weight gain was seen during treatment among dams receiving 3 mg/kg bw
per day in comparison with controls, but the author reported that the
difference was not statistically significant. A total of six females
-- one control, two at 0.3 mg/kg bw per day, two at 1 mg/kg bw per
day, and one at 3 mg/kg bw per day -- were not fertilized. The average
placental weight of animals at the high dose was significantly lower
than that in controls. Treatment did not affect the number of
fertilized or pregnant females, litter size, number of resorptions,
number of fetuses, average fetal weight, sex ratio, incidence of
alterations in development, or the type or number of malformations.
The most frequent manifestations were nodulations on ribs, which were
found in two fetuses from one litter of a dam at 0.3 mg/kg bw per day
and in four fetuses of three litters of dams at 1 mg/kg bw per day.
The other malformations observed were general oedema, abdominal
fissure, and anophthalmia in one fetus at 0.3 mg/kg bw per day. No
malformations were observed in fetuses at the high dose. The lower
placental weight observed at the high dose was considered to be not
toxicologically significant because the average weight was within the
normal range and no effects were seen on embryonic or fetal
development. Thus, fenamiphos at doses < 3 mg/kg bw per day was not
embryotoxic or teratogenic; it was, however, toxic to dams at 3 mg/kg
bw per day (Schlüter, 1981; JMPR, 1987).
Groups of 33 mated female Crl:CD-BR rats were given fenamiphos
(purity, 88.7%) at doses of 0, 0.25, 0.85, or 3 mg/kg bw per day by
gavage on days 6-15 of gestation. Five dams from each group were
killed on day 16 in order to measure plasma, erythrocyte, and brain
cholinesterase activities; the remaining dams were killed on day 20 of
gestation and necropsied grossly. All dams were examined for number of
corpora lutea and implantation sites, and their uteri and placentas
were weighed. The fetuses were weighed and sexed; about half were
examined externally and in the viscera, and the other half were
processed for skeletal examination. Brain acetylcholinesterase
activity was measured in 20 fetuses per group.
Six dams at 3 mg/kg bw per day died between days 7 and 14 of
gestation, one female with convulsions on the day of its death.
Clinical signs of toxicity, such as tremors, salivation, lachrymation,
urine staining, and hypoactivity, were seen to varying extents in the
survivors. Body-weight gain and food consumption were significantly
reduced throughout treatment at this dose. Gross necropsy revealed no
treatment-related abnormalities, and gestational parameters were
unaffected. Fetal body weights were unchanged, and they had no
treatment-related variations or malformations. Plasma and erythrocyte
cholinesterase activities were statistically significantly reduced (by
50 and 42%, respectively) in dams at 3 mg/kg bw per day on day 16 of
gestation. By day 20, the plasma activity had returned to normal,
while that of erythrocyte cholinesterase was still significantly
reduced by 30%. Brain acetylcholinesterase activity was reduced by 28%
in adults at 0.85 mg/kg bw per day and by 12% in those at 3 mg/kg bw
per day; as the differences were not significant or dose-related, they
were not considered to be related to treatment. Fetal brain
acetylcholinesterase activity was unaffected. The NOAEL for maternal
toxicity was 0.85 mg/kg bw per day. Fenamiphos was not teratogenic or
fetotoxic under the conditions of this study (Clemens et al., 1989).
Rabbits
Groups of 20 double-mated female New Zealand rabbits were given
fenamiphos (purity, 88.8%) orally in corn oil at doses of 0, 0.1, 0.3,
or 1 mg/kg bw per day on days 6-18 of gestation. They were observed
daily for clinical signs of toxicity and were weighed initially,
periodically during the test, and at termination of the study. Once
the pups had been removed, the ovaries and uteri of the dams were
examined, and the fetuses were examined grossly and prepared for
evaluations of soft tissues and the skeleton. The numbers of corpora
lutea, implantations, resorptions, live and dead fetuses, and
anomalies were also determined. Cholinesterase activity was not
assessed. Dams given doses > 0.3 mg/kg bw per day showed signs of
toxicity, with decreased body-weight gain, bloody nasal discharge, and
white, mucoid ocular discharge. Treatment did not affect the number of
litters, number of pups per litter, pregnancy rate, the number of
corpora lutea, implantations, or gross abnormalities. Mean fetal
weight was slightly depressed at 1 mg/kg bw per day. One dam at 0.3
mg/kg bw per day aborted one dead pup, and two at 1 mg/kg bw per day
aborted eight dead pups and had seven late resorptions. In addition,
one dead fetus was found in each of two litters at the high dose.
The commonest developmental variation observed was the left carotid
arising from the innominate, which occurred in six to eight fetuses
(7-9%) in each of three litters (25%) at doses > 0.1 mg/kg bw per
day. This anomaly was not seen in the controls and in only one of 31
litters (3.2%) of historical controls at the laboratory where the
study was performed. More recent data (through July 1985) for
historical controls revealed incidences of 11/336 (3.3%) in fetuses
and 9/53 (17%) litters. The newer historical control data provide some
evidence that the incidence of left carotid anomalies was increased in
superovulated or artificially inseminated rabbits. Furthermore, data
for rabbits of the same strain in different labs indicate that the
anomaly is a frequent finding, occurring in about 8% of fetuses and
25% of litters; the historical data are for artificially inseminated
rabbits, while the animals used in this study were naturally bred. The
biological significance of this finding in relation to treatment is
dubious.
An increased incidence of accessory skull bones was also seen in all
treated groups, but it did not occur in a dose-related manner and was
thus not considered to be related to treatment. A significant increase
in the incidence of chain-fused sternebrae was seen in five fetuses in
three litters at 1 mg/kg bw per day, and this anomaly was also seen in
one fetus in one litter at 0.3 mg/kg bw per day. This anomaly is of
questionable biological significance. Two fetuses in one litter at the
high dose had aortic arches with a common truncus, which was
considered to be a major malformation. Other skeletal malformations
which occurred only at doses > 0.3 mg/kg bw per day included fused
ribs, scoliosis, absent vertebrae (thoracic, lumbar, sacral, and
caudal), and bipartite or malformed centra. The 1985 Meeting concluded
that these findings were related to treatment; however, these
anomalies occurred in only one or two fetuses in single litters, often
with no relation to dose. Furthermore, several of the anomalies were
clustered within a single fetus, indicating that they were not likely
to be related to treatment. The NOAEL for maternal toxicity was 0.1
mg/kg bw per day, and that for developmental toxicity was 0.3 mg/kg bw
per day. Fenamiphos had no teratogenic effects at any dose (JMPR,
1985, modified by reference to the original report by MacKenzie,
1982).
In a study to determine the doses for a study of embryotoxicity
(including teratogenicity), groups of three mated (1:1) female
Chinchilla rabbits were given single daily doses of fenamiphos
(purity, 91%) in distilled water with 0.5% Cremophor EL0 at doses of
0.1, 0.8, or 3 mg/kg bw per day by gavage on days 6-18 post coitum.
Cholinesterase activity was measured in plasma and erythrocytes before
the first dose and just after the last dose; brain
acetylcholinesterase activity was not assessed. All of the animals
were killed on day 28 post coitum, and the fetuses were removed,
sexed, weighed, and examined for gross external and internal
abnormalities.
One female at the high dose lost weight from day 10 and died on day 12
post coitum; body-weight loss and reduced food consumption were seen
throughout treatment in all animals at this dose when compared with
controls. Statistically significant reductions in both plasma (89%)
and erythrocyte cholinesterase activity (90%) relative to controls
were noted in rabbits at the high dose on day 18 post coitum. Five
preimplantation losses and one fetal resorption were observed in does
at the high dose but in no other group. Marginal effects on body
weight and food consumption were seen at 0.8 mg/kg bw per day. No
treatment-related effects were seen on the numbers of corpora lutea,
implantations, live or dead fetuses, or on fetal weight (Becker et
al., 1986; JMPR, 1987).
In the main study, four groups of 16 single-mated female Chinchilla
rabbits were given fenamiphos (purity, 91%) in distilled water
containing 0.5% Cremophor as single daily doses of 0, 0.1,0.5, or 2.5
mg/kg bw per day by gavage on days 6-18 post coitum. The does were
killed on day 28 post coitum, and the fetuses were removed. Only
does with at least one living fetus were used in calculating
body-weight gain, food consumption, and reproductive parameters. They
were examined for the position of fetuses in the uterus and numbers of
corpora lutea, implantations, resorptions, and live and dead fetuses.
The fetuses were weighed, sexed, and examined for external and
internal malformations, skeletal abnormalities, and development.
Cholinesterase activity was not assessed. Dosing solutions were
prepared daily. The concentration of fenamiphos in triplicate samples
taken for determination of homogeneity before the study was found to
vary considerably (14-140% of the nominal concentration), with mean
concentrations of 71 ± 8.3, 84 ± 64, and 70 ± 16% of the nominal
concentration at doses of 0.1, 0.5, and 2.5 mg/kg bw per day,
respectively. At the next sampling 10 days later, the mean
concentrations were all within 90% of the nominal concentration,
although the variability was still high (92 ± 37, 97 ± 45, and 99 ±
41% of the nominal concentration at the three doses, respectively).
Although it is stated that homogeneity was maintained during dosing by
use of a magnetic stirrer, it is not clear if the samples taken for
testing were subjected to the same treatment before analysis.
Four females at 2.5 mg/kg bw per day died as a result of treatment
after 3, 5, and 10 days and on day 21 post coitum. Treatment-related
signs of toxicity (salivation and dyspnoea) were observed in these
females and in five other females at the high dose between days 7 and
18 post coitum. Ataxia was seen in two females that died, and
diarrhoea was observed in another. No signs of toxicity were found in
animals at 0, 0.1, or 0.5 mg/kg bw per day. A treatment-related,
statistically significant decrease in mean food consumption (29%) and
a treatment-related reduction in body-weight gain (56%) were observed
during treatment in does at the high dose when compared with controls.
Food consumption was significantly increased in this group on days
24-28.
No treatment-related or significant difference was observed between
treated and control animals with regard to the mean numbers of
implantations, corpora lutea, live or dead fetuses, or resorptions. In
fetuses, no effect that could be attributed to treatment was seen in
sex ratio, body weight, external or internal malformations, skeletal
abnormalities, or development. One fetus at the high dose had
encephalocele with reduced brain size, but this finding was not
considered to be related to treatment. A number of skeletal changes
unrelated to treatment were seen in fetuses at all doses. The NOAEL
for maternal toxicity was 0.5 mg/kg bw per day. Although questions
remain about the doses actually administered, because of the problems
of homogeneity, the study showed no embryotoxicity or teratogenicity
at maternally toxic doses (JMPR, 1987, modified by reference to the
original report by Becker, 1986).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenamiphos painted on the skin of New Zealand white
rabbits in an acetone solution at 50 mg/kg bw resulted in slight
erythema but was not considered to be a primary irritant. Application
of technical-grade fenamiphos to the conjunctival sac of New Zealand
white rabbits as 100 mg of a crystalline material resulted in
irritation considered to be mechanical rather than physiological
(Crawford & Anderson, 1971; JMPR, 1974).
Technical-grade fenamiphos (purity, 90.7%) was melted at 35°C, and
0.5 ml was painted onto the intact or abraded skin of six
Japanese-derived albino rabbits for 24 h under occlusion. Irritation
was scored according to Draize 24, 48, 72, and 168 h after
application. Minimal irritation was observed, characterized by slight
erythema and oedema during the first 48 h. The mean primary irritation
index, based on readings at 24 and 72 h, was 0.42 (Kato 1984a).
Technical-grade fenamiphos (purity, 90.7%) was heated to liquidity
(temperature not specified) and applied at 0.1 ml to the conjunctival
sacs of nine Japanese-derived albino rabbits. The eyes of three of the
rabbits were washed with water. Irritation was scored according to
Draize 24, 48, 72, 96, 168, and 240 h after administration. In the
absence of washing, fenamiphos was a moderate irritant, with a maximum
average score of 31.3 at 24 h. The irritation scores slowly declined,
and all of the eyes were clear by 240 h. Fenamiphos was not irritating
when the eyes were washed with water after application. Midriasis,
persisting until day 2 or 3, was seen in all animals when the eyes
were not washed within 10 min of application. The translation of the
report leaves some uncertainty about other ocular effects, but signs
of systemic toxicity peaked within 3-4 h of application; these
included salivation, increased respiration, cyanosis, and slight
convulsions (number affected not specified). All animals were
reportedly normal within 6 h. No systemic toxicity was seen in the
animals when the eyes were washed (Kato, 1984b).
The potential of fenamiphos to sensitize skin was examined in a
maximization test in which 20 male Hsd Win:DH guinea-pigs were induced
intradermally with 1% fenamiphos in saline containing 2% Cremophor EL.
One week later, they were induced topically with 25% fenamiphos in
saline (with 2% Cremophor EL); they were challenged three weeks later
with 12 and 25% solutions of fenamiphos in the saline solution. Patchy
erythema was seen 24 h after removal of the patch in 22% of the
animals challenged with the 25% solution, whereas none of the 10
induced control animals showed irritation. The author considered a
response rate of > 30% to be indicative of sensitization, and
concluded that fenamiphos produced no relevant sensitization; however,
according to the original protocol of Magnusson and Kligman, these
results would indicate that fenamiphos is a mild sensitizer (Stropp,
1995).
(ii) Delayed neuropathy
Groups of eight hens were fed fenamiphos in the diet at levels of 0,
1,3, 10, or 30 ppm, equal to 0, 2, 5, 16, or 26 mg/kg bw per day, for
30 days. At the end of treatment, some birds were killed and the
remainder were observed for four weeks for neurological signs of
poisoning. Food consumption was depressed in birds at 30 ppm, and the
average body weight and growth of hens at this dose was reduced.
Whole-blood cholinesterase activity was decreased after 30 days at
doses > 1 ppm, although no signs of cholinergic poisoning were
observed. There were no indications of delayed neurotoxicity, and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with haematoxylin and eosin) did not indicate delayed
neuropathy (Kimmerle, 1970; Spicer, 1970; JMPR, 1974).
Groups of 10 hens given an LD50 a dose of fenamiphos (5.0 mg/kg bw)
orally were observed for three weeks and then killed. No evidence of
delayed neurotoxicity was observed either clinically or
histologically, whereas signs were observed with tri- ortho-cresyl
phosphate (Kimmerle, 1971; Spicer, 1971; JMPR, 1974).
Fenamiphos (purity, 91.3%) in a 2% Cremophor solution was administered
twice by intubation to 30 Lohmann selected Leghorn hens at 25 mg/kg bw
per day at a 21-day interval. Five hens serving as positive controls
received tri- ortho-cresyl phosphate at 375 mg/kg bw per day. The
hens treated with fenamiphos received atropine intramuscularly at 100
mg/kg bw before treatment and subcutaneously at 30-50 mg/kg bw 7, 24,
or 30 or 48 h after treatment. The birds were observed for body
weight, clinical signs, forced motor coordination, and gross and
histopathological changes. Although statistically significant,
treatment-related weight loss (during week 1) and signs of severe
poisoning were seen, the birds showed no impairment of motor
coordination indicative of delayed neurotoxicity. Histological
examination of tissues from the peripheral and central nervous systems
showed no changes indicative of delayed neuropathy. Birds given
tri- ortho-cresyl phosphate, however, showed clinical signs of
delayed neurotoxicity (ataxia and paresis) two weeks after treatment
and were killed in moribund condition on day 17. The histopathological
changes in these birds were typical of neuropathy (Flucke & Kaliner,
1987).
(iii) Neurotoxicity
Rats
Groups of 12 Wistar (Hsd Win:WU) rats of each sex were given single
doses of technical-grade fenamiphos (purity, 95.2%) at 0.37, 1.52, or
2.31 mg/kg bw by garage. They were observed for mortality, clinical
signs, and body weight. Functional observational and motor or
locomotor activity tests were conducted on all animals before
treatment, within 30 min of treatment, and 7 and 14 days later.
Plasma, erythrocyte, and brain cholinesterase activity was measured in
additional satellite groups of six animals of each sex at each dose,
which were killed within 1 h of treatment. One-half of the animals in
the main group were perfused, and various neural and skeletal muscle
tissues, including the brain, spinal cord and spinal ganglia, eyes and
optic nerves, peripheral nerves, gastrocnemic muscle, and trigeminal
ganglia, were processed for histological examination.
In animals at the lowest dose, plasma cholinesterase activity was
statistically significantly inhibited in females (by 55%) and
nonsignificantly decreased in males (by 23%). Erythrocyte
acetylcholinesterase activity was significantly inhibited only in
males (by 24%), but this was not considered to be an adverse effect
because no corroborating clinical signs were seen in the functional
and motor activity tests. At the next highest dose, both plasma and
erythrocyte cholinesterase activity was significantly inhibited in
both males (by 64 and 70%, respectively) and females (by 77 and 51%,
respectively). Uncoordinated gait and muscle fasciculations were also
seen in males during the functional observational battery of tests. At
the highest dose, similar signs were seen, with decreased grip
strength and deaths among both males and females and decreased motor
activity in malesś Other behavioural or physiological changes seen in
animals of each sex in the functional observational battery of tests
included piloerection, nasal, oral, and lachrymal staining,
salivation, and decreased activity and rearing in the open field.
Brain acetylcholinesterase activity was unaffected at all doses. No
treatment-related histopathological changes were seen. The NOAEL was
0.37 mg/kg bw, the lowest dose tested, on the basis of uncoordinated
gait and muscle fasciculations in males at the next highest dose
(Dreist, 1995).
In a study of similar design, technical-grade fenamiphos (purity,
95.6%) was administered in the diet to groups of 12 Wistar (Hsd
Cpb:WU) rats of each sex for 13 weeks at dietary concentrations of 0,
1, 10, or 50 ppm, equal to 0, 0.06, 0.61, or 3.1 mg/kg bw per day in
males and 0.08, 0.8, or 4 mg/kg bw per day in females. The animals
were observed for deaths, clinical signs, body weight, and food and
water consumption. Functional observational and motor or locomotor
activity tests were conducted before treatment and in weeks 4, 8, and
13. Plasma and erythrocyte cholinestemse activity was measured in six
rats of each sex at each dose on week 4 and before terminal sacrifice
at week 15, and brain acetylcholinesterase activity only at terminal
sacrifice.
All females at the highest dose had muscle fasciculations during the
first three weeks of the study. Plasma and erythrocyte cholinesterase
activities were statistically significantly reduced in both males
(> 68%) and females (> 86%). Brain acetylcholinesterase activity
was also significantly reduced (by 12%) in females, but the author
considered that this was not biologically significant. At 10 ppm,
plasma cholinesterase activity was significantly reduced in both males
(by 30-39%) and females (by 71-77%; rho < 0.01) in weeks 4 and 15.
Erythrocyte acetylcholinesterase activity was inhibited by about 25%
in week 15 in males (rho < 0.05) and in weeks 4 and 15 in females
at 10 ppm. The lowest dose resulted in nonsignificant decreases (about
30%) in plasma cholinesterase activity in females in weeks 4 and 15.
Body weights, food and water consumption, brain weights, and gross and
histopathological appearance were all unaffected by treatment. The
author extrapolated the results back to a 20% level of inhibition of
plasma cholinesterase activity and estimated that the NOAEL in females
was 0.4 ppm. The overall NOAEL was 10 ppm, equal to 0.8 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase activity
in females at the highest dose (Dreist & Popp, 1995).
(iv) Potentiation
In male rats given fenamiphos orally in combination with disulfoton or
E 154, no potentiation of the acute toxicity was seen (Kimmerle,
1972c; JMPR, 1974).
The LD50 for fenamiphos (purity, 91.8%) administered orally to male
Wistar rats was 4.6 mg/kg bw, while that of carbofuran was 8.1 mg/kg
bw. When the two compounds were given concomitantly, essentially as a
2:1 ratio of carbofuran:fenamiphos, the LD50 was 6 mg/kg bw,
indicating that the toxicity of the combination was additive but not
synergistic (Mihail, 1980 JMPR, 1985).
Comments
In rats, fenamiphos was rapidly excreted, with over 96% of the
administered dose eliminated within 48 h. Excretion was primarily in
the urine, with less than 4% of the dose eliminated in the faeces. At
48 h, the levels of tissue residues were below the limit of
quantification, except following a high dose (3 mg/kg bw), when the
maximal tissue levels observed were 3.5-8.4 µg/kg in the liver,
1.6-2.1 µg/kg in the kidney, and 1.6-3.5 µg/kg in the skin. Fenamiphos
was completely metabolized in rats. Metabolites retaining
anticholinesterase activity, such as fenamiphos sulfoxide and
desisopropyl fenamiphos sulfoxide, were seen in variable but generally
low proportions (rarely greater than 3%). Most of the products were
dephosphorylated phenol, sulfoxide phenol, or sulfone phenol
metabolites and their corresponding sulfates.
Fenamiphos is extremely hazardous after single oral doses to rats,
mice, rabbits, cats, dogs, and chickens (LD50 values = 2 4-23 mg/kg
bw) and highly hazardous after dermal administration to rats and
rabbits (LD50 values, 75-230 mg/kg bw). It is moderately hazardous
after inhalation in rats and mice (LC50 values < 100 µg/L, 4 h).
WHO has classified fenamiphos as 'extremely hazardous' (WHO, 1996).
The sulfoxide, sulfone, and desisopropylated sulfone metabolites of
fenamiphos are similarly toxic to rats after oral administration
(LD50 values = 1.4-4.1 mg/kg bw). The sulfoxide and sulfone phenol
metabolites are only slightly toxic to rats after oral administration,
with LD50 values ranging from 1200 to 1900 mg/kg bw.
Fenamiphos inhibited plasma cholinesterase more effectively than
erythrocyte acetylcholinesterase, both in vitro and in vivo.
Fenamiphos sulfoxide, fenamiphos sulfone, desisopropyl fenamiphos,
desisopropyl fenamiphos sulfoxide, and desisopropyl fenamiphos sulfone
inhibited plasma and erythrocyte cholinesterase in vitro more
effectively than fenamiphos itself.
In evaluating the following studies, inhibition of erythrocyte
acetylcholinesterase activity was not used as an indicator of adverse
effects in the nervous system when information on brain
acetylcholinesterase activity was also available. In the absence of
this information, NOAELs were determined on the basis of inhibition of
erythrocyte acetylcholinesterase (of < 20%). Statistical
significance was used as a criterion for considering depression of
brain acetylcholinesterase activity to be adverse.
In a three-week study in which rats were exposed by inhalation to
atmospheres containing fenamiphos at 0, 0.03, 0.25, or 3.5 µg/L for 6
h per day, five days per week, the only finding was inhibition of
plasma cholinesterase at the highest dose. Erythrocyte and brain
acetylcholinesterase were unaffected. The no-observed-adverse-effect
concentration (NOAEC) was 3.5 µg/L.
In a three-month study in rats, fenamiphos given at dietary
concentrations of 0, 0.37, 0.57, or 0.91 ppm inhibited plasma
cholinesterase activity only at the highest dose. No treatment-related
changes in erythrocyte or brain acetylcholinesterase activity were
seen at any dose. In a second study of short-term toxicity, rats were
fed diets containing 0, 4, 8, 16, or 32 ppm fenamiphos for three
months. Erythrocyte acetylcholinesterase activity was inhibited at
doses of 16 ppm (equivalent to 0.8 mg/kg bw per day) and above. This
was considered an adverse effect as brain acetylcholinesterase was not
measured in this study. The overall NOAEL was 8 ppm, equivalent to 0.4
mg/kg bw per day.
Rabbits received fenamiphos by dermal application at doses of 0, 0.5,
2.5, or 10 mg/kg bw per day for three weeks (6 h per day, five days
per week). Body-weight gain was slightly reduced in animals of each
sex at 10 mg/kg bw per day. In females, reductions in cholinesterase
activity in the brain (by 20%) and plasma were noted at 2.5 mg/kg bw
per day and above. Erythrocyte acetylcholinesterase activity, however,
was affected only at 10 mg/kg bw per day. In males, the only findings
were decreased plasma and erythrocyte cholinesterase activity at 10
mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day.
In a series of studies, dogs were fed diets containing 0, 0.5, 0.6, 1,
1.7, 2, 3, 5, 6, 10, 12, or 18 ppm fenamiphos for periods ranging from
three months to two years. In dogs treated at 18 ppm (equivalent to
0.45 mg/kg bw per day) for three months, muscle tremors were seen. In
dogs treated at 12 ppm (equal to 0.31 mg/kg bw per day) for one year,
brain acetylcholinesterase activity was inhibited in females (by 17%),
and males showed slight anaemia. Erythrocyte acetylcholinesterase
activity was inhibited at doses of 3 ppm (equal to 0.083 mg/kg bw per
day) and above for one year. Plasma cholinesterase activity was
inhibited at doses of 1.7 ppm (equivalent to 0.042 mg/kg bw per day)
and above in a three-month study, No other parameters were affected.
Since no information on brain acetylcholinesterase activity was
available at doses between 3 and 12 ppm, the Meeting considered 3 ppm
(equal to 0.083 mg/kg bw per day) to be the overall NOAEL in dogs.
In mice fed diets containing 0, 2, 10, or 50 ppm fenamiphos for 20
months, there were marginal decreases in survival and body-weight gain
at 50 ppm (equal to 7.4 mg/kg bw per day). The relative ovarian and
spleen weights were reduced at 10 ppm (equal to 1.4 mg/kg bw per day)
and above. There were no non-neoplastic changes that could be
attributed to treatment, and fenamiphos was not carcinogenic at any
dose. Cholinesterase activity was not measured. The NOAEL was 2 ppm,
equal to 0.3 mg/kg bw per day.
In rats fed diets containing 0, 3, 10, or 30 ppm fenamiphos for two
years, the only treatment-related effects were inhibition of
erythrocyte acetylcholinesterase activity throughout the study and
behavioural changes during the first six weeks of the study in animals
at 30 ppm. Brain acetylcholinesterase activity was not measured. The
NOAEL was 10 ppm, equal to 0.56 mg/kg bw per day.
Rats were fed diets containing 0, 1.7, 7.8, or 37 ppm fenamiphos for
two years. At 37 ppm, equal to 2.5 mg/kg bw per day, body-weight gain
in both males and females was decreased. Erythrocyte
acetylcholinesterase activity was inhibited at 7.8 ppm (equal to 0.46
mg/kg bw per day) and above. Brain acetylcholinesterase activity was
inhibited only at 37 ppm; inhibition was 25% in animals of each sex
killed after one year, and 14% in males at termination of the study.
Animals of each sex at 37 ppm also had an increased frequency of
non-neoplastic inflammatory lesions of the nasal, laryngeal, and lung
tissues and increased relative weights of the brain, heart, and lungs.
Fenamiphos was not carcinogenic at any dose. The NOAEL was 7.8 ppm,
equal to 0.46 mg/kg bw per day.
Fenamiphos was adequately tested in a battery of tests for
genotoxicity. It was found to be mildly clastogenic at cytotoxic doses
in vitro but not in vivo. It did not cause reverse or forward
mutation, unscheduled DNA synthesis, or sister chromatid exchange
in vitro. The Meeting concluded that fenamiphos is not genotoxic.
In a two-generation study of reproductive toxicity, rats were treated
with 0, 2.5, 10, or 40 ppm fenamiphos. Parental toxicity was
characterized by reduced weight gain in F0 and F1 dams at 40 ppm
(equal to 2.8 mg/kg bw per day) during lactation, and in F1 males at
10 ppm (equal to 0.64 mg/kg bw per day) and above before mating.
Pathological changes in the salivary gland were seen in F0 males and
females at 40 ppm. Erythrocyte acetylcholinesterase activity was
consistently inhibited at 10 ppm and above in females but only at 40
ppm in males. Brain acetylcholinesterase activity was inhibited at the
highest dose in adult F0 and F1 females (by 21-29%) and in F1 males
(by 6%) but not in pups of either sex. In pups at 40 ppm, erythrocyte
acetylcholinesterase activity was inhibited only on day 21 of
lactation. The only reproductive effect was decreased weight gain of
F1 and F2 pups at 40 ppm, beginning on day 7 of lactation. The NOAEL
for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per day. The
NOAEL for reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per
day.
In a study of developmental toxicity, mated rats were treated with 0,
0.3, 1, or 3 mg/kg bw per day on days 6-15 of gestation. Maternal
toxicity was seen at the highest dose, characterized by mortality,
tremors, and reduced weight gain. The fetuses were not affected at any
dose. Cholinesterase activity was not measured in this study. The
NOAELs were 1 mg/kg bw per day for maternal toxicity and 3 mg/kg bw
per day for developmental toxicity.
Fenamiphos was also administered to mated rats at doses of 0, 0.25,
0.85, or 3 mg/kg bw per day on days 6-15 of gestation. The highest
dose resulted in maternal deaths, tremors, salivation, lachrymation,
urine staining, and hypoactivity. Body-weight gain and food
consumption were also significantly reduced. Erythrocyte
acetylcholinesterase activity was reduced at this dose, but the
changes in brain acetylcholinesterase activity were not statistically
significant or dose-related. The fetuses were unaffected at 3 mg/kg bw
per day. The NOAELs were 0.85 mg/kg bw per day for maternal toxicity
and 3 mg/kg bw per day for developmental toxicity.
In a study of developmental toxicity in rabbits, animals received 0,
0.1, 0.3, or 1 mg/kg bw per day on days 6-18 of gestation. At 0.3
mg/kg bw per day and above, fenamiphos was maternally toxic, resulting
in decreased body-weight gain, bloody nasal discharge, and white
ocular discharge. Fetotoxicity, characterized by chain fusion of the
sternebrae, was seen only at 1 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.1 mg/kg bw per day, and that for developmental
toxicity was 0.3 mg/kg bw per day. Cholinesterase activity was not
measured in this study.
In a second study, mated rabbits were treated with 0, 0.1, 0.5, or 2.5
mg/kg bw per day on days 6-18 of gestation. Clear maternal toxicity
was seen at the highest dose, which included mortality, salivation,
dyspnoea, ataxia, diarrhoea, and decreased weight gain and food
consumption during treatment. Although some questions remain about the
doses that were actually administered (because of uncertain
homogeneity), no embryotoxic or teratogenic effects were seen at the
maternally toxic dose of 2.5 mg/kg bw per day.
Fenamiphos was minimally irritating to rabbit skin and moderately
irritating to rabbit eyes and was a mild skin sensitizer in the
guinea-pig.
A single dose of 25 mg/kg bw fenamiphos had no effect on neuropathy
target esterase activity in the brains or spinal cords of hens, under
atropine protection. Fenamiphos did not induce delayed neuropathy in
three studies in hens when tested at doses of 0, 2, 5, 16, or 26 mg/kg
bw per day for 30 days or when given once at doses of 0 or 25 mg/kg
bw.
In a study of acute neurotoxicity, rats were given single doses of 0,
0.37, 1.5, or 2.3 mg/kg bw fenamiphos by gavage. Erythrocyte
acetylcholinesterase activity was inhibited at the lowest dose tested
in males only, but in both males and females at higher doses. At 1.5
mg/kg bw, males showed uncoordinated gait and muscle fasciculation. At
the highest dose, decreased motor activity was also seen in males, and
clinical signs, decreased grip strength, and deaths occurred in rats
of each sex. There was no effect on brain acetylcholinesterase
activity, at any dose. The NOAEL was 0.37 mg/kg bw per day. In a
further study, rats were fed diets containing 0, 1, 10, or 50 ppm
fenamiphos for 13 weeks. At 10 ppm and above, erythrocyte
acetylcholinesterase activity was inhibited in animals of each sex. At
the highest dose, brain acetylcholinesterase activity was inhibited
(by 12%) in females only. A battery of functional observational and
motor activity tests revealed no treatment-related effects. The NOAEL
was 10 ppm, equal to 0.61 mg/kg bw per day.
An ADI of 0-0.0008 mg/kg bw was established on the basis of an overall
NOAEL of 0.083 mg/kg bw per day in the dog, and a safety factor of
100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2 ppm in the diet, equal to 0.3 mg/kg bw per day
(20-month study of toxicity and carcinogenicity)
Rat: 0.37 mg/kg (single doses, study of neurotoxicity) 10
ppm, equal to 0.61 mg/kg bw per day (three-month study
of neurotoxicity)
2.5 ppm, equal to 0.17 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
10 ppm, equal to 0.64 mg/kg bw per day (study of
reproductive toxicity)
0.85 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity)
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to fenamiphos
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral neurotoxicity, rat NOAEL = 0.37 mg/kg bw per day: effects observed
(1-7 days) during battery of functional observational tests
Oral toxicity, rat (fasted) LD50 = 2.4-6 mg/kg bw
Inhalation toxicity, 4 h, rat LC50 = 91-100 µg/L
Inhalation toxicity, 5 days, rat NOAEL = 4 µg/L
Dermal toxicity, rat LD50 = 72-92 mg/kg bw
Dermal irritation, rabbit Minimally irritating
Ocular irritation, rabbit Moderately irritating
Dermal sensitization, guinea-pig Mildly sensitizing
Medium-term Repeated inhalation toxicity, 3 weeks, rat NOAEL = 3.5 µg/L (highest dose tested)
(1-26 weeks) Repeated dermal toxicity, 3 weeks, rabbit NOAEL = 0.5 mg/kg bw per day: inhibition of brain
acetylcholinesterase activity
Repeated oral, reproductive toxicity, rat NOAEL = 0.17 mg/kg bw per day: parental toxicity
NOAEL = 0.64 mg/kg bw per day: reproductive
toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.1 mg/kg bw per day: maternal toxicity
NOAEL = 0.3 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral, 1 - 2 years, dog NOAEL = 0.083 mg/kg bw per day: inhibition of
(> 1 year) acetylcholinesterase activity, anaemia
7.8 ppm, equal to 0.46 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
0.3 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 3 ppm in the diet, equal to 0.083 mg/kg bw per day
(overall assessment)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Estimate of acute reference dose
The available data did not permit the Meeting to establish an acute
reference dose different from the ADI (0-0.0008 mg/kg bw). Although
the results of a study of neurotoxicity in rats given single doses was
available, the dog was found to be the more sensitive species.
Information on acute effects in dogs may allow the establishment of an
acute reference dose in the future.
Studies that would provide information useful for continued
evaluation of the compound
1. Effects of single doses in dogs (with appropriate evaluation of
functional changes in the cholinergic nervous system, including
brain acetylcholinesterase activity).
2. Observations in humans
References
Becker, H. (1986) Embryotoxicity (including teratogenicity) study with
SRA 3886 in the rabbit. Unpublished report No. R 3917 (project No.
065261) from Research & Consulting Co., Itingen, Switzerland.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Mueller, E., Luetkemeir, H. & Sachsse, K. (1986)
Dose-finding embryotoxicity (including teratogenicity) study with SRA
3886 in the rabbit. Unpublished report No. R3693 (project No. 065250)
from Research & Consulting Co., Itingen, Switzerland. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchange in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environ. Mutag., 4, 621624. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cherry, C. & Newman, A. (1973) Pathology report of Bayer 68138.
Chronic toxicological studies in rats. Unpublished report (addendum to
report No. 3539) from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Cherry, C., Urwin, C. & Newman, A. (1972) Pathology report of BAY
68138. Rat breeding study. Unpublished report (addendum to report No.
3424) from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1989) Teratology
study in the rat with Nemacur technical. Unpublished report No. 1145
(study no. MTD0108) from Toxicology Department, Miles Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F. & Wills, J.H. (1974) Summary of research by the Institute
of Comparative and Human Toxicology, Albany Medical College, on
phenamiphos. Report dated October 1974. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C. & Anderson, R. (1971) The skin and eye irritating
properties of BAY 68138 technical to rabbits. Unpublished report No.
29988 from Chemagro Division of Baychem Corp. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Crawford, C. & Anderson, R. (1972) The acute dermal toxicity of
Nemacur technical to rabbits. Unpublished report No. 34216 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R)
with analytical grade Nemacur(R). Unpublished report No. 34446 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974a) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Unpublished report No. 39700 from Chemagro Division of Baychem Corp.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974b) The acute oral toxicity of
Nemacur(R)-sulfone and Nemacur(R)-sulfoxide to rats. Unpublished
report No. 40215 from Chemagro Division of Baychem Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Curren, D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 991 (study no. T5724.380) from
Microbiological Associates Inc., Rockville, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Dreist, M. (1995) SPA 3886 (common name:Fenamiphos) acute oral
neurotoxicity screening study in Wistar rats. Unpublished report No.
24408 (study No. T6058166) from the Institute of Toxicology
Agrochemicals, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Dreist, M. & Popp, A. (1995) SRA 3886 (common name: Fenamiphos)
subchronic neurotoxicity screening study in Wistar rats (thirteen-week
administration in the diet). Unpublished report No. 24948 (study no.
T9058277) from Institute of Toxicology Agrochemicals, Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K. & Flynn, M. (1968) The subacute parenteral toxicity of BAY
68138 to rats. Unpublished report No. 22178 from Toxicity Laboratory,
University of Chicago, Chicago, IL, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K., Flynn, M. & Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Unpublished report No. 20810
from Toxicity Laboratory, University of Chicago, Chicago, IL, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ecker, W., Weber, H. & Brauner, A. (1989) [Phenyl-1-14C]Nemacur:
General metabolism study in the rat. Unpublished report No. PF3175
(study no. M182 0189-9) from Bayer AG, Pflanzenschutz-Zentrum Monheim,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Eigenberg, D.A. (1991) A two generation reproduction study in rats
using Fenamiphos (Nemacur(R)). Unpublished report No. 5762 (study
no. 88-671-BC) from Mobay Corp, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity. Unpublished report
dated 18 July 1980 from Bayer AG, Institute of Toxicology, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Eben, A. (1988) SPA 3886 Technical (common name:
Fenamiphos): Study of the effect on the neurotoxic esterase (NTE)
following oral administration to hens. Unpublished report No. 17388
from Fachbereich Toxikologie, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Kaliner, G. (1987) Delayed neurotoxicity studies on hens
following acute oral administration. Unpublished report No. 16187
(study no. T 1020919) from Fachbereich Toxikologie, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Gronberg, R.R. (1969) The metabolic fate of ethyl-4-(methylthio)-m-
tolyl isopropylphosphoramidate (BAY 68138), ethyl-4-(methylsulfinyl)-
m-tolyl isopropylphosphoramidate (BAY 68138 sulfoxide) and ethyl-4-
(methylsulfonyl)-m-tolyl-isopropylphosphoramidate (BAY 68138 sulfone)
in white rats. Unpublished report No. 26759 from Chemagro Division of
Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1982) Technical fenamiphos (Nemacur(R)) oncogenicity
study in mice. Unpublished report No. 241 (study No. 78CCM02) from
Stanley Research Center, Mobay Chemical Corp, Stilwell, KS, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. ( 1983 ) Ninety-day cholinesterase study on dogs with
fenamiphos in diet. Unpublished report No. 444 (study No. 83-174-01)
from Environmental Health Research, Mobay Chemical Corp, Stilwell, KS,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1986a) Ninety-day cholinesterase study on rats with
technical fenamiphos (Nemacur) in diet. Unpublished report No. 717
(study No. 83-171-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hayes, R.H. (1986b) Combined chronic toxicity/oncogenicity study of
technical fenamiphos (Nemacur) with rats. Unpublished report No. 721
(study No. 83-271-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K G (1981) Determination of acute toxicity (LD50 Unpublished
report dated 18 August 1981 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K G (1984) Determination of acute toxicity (LD) Unpublished
report dated 14 February 1984 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1979) Salmonella/microsome test for detection of
point-mutagenic effects. Unpublished report No. 8730 (study no.
Nemacur/003) from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980a) Micronucleus test on mouse to evaluate Nemacur
technical for potential mutagenic effects. Unpublished report No. 8805
(study no. SRA 3886/002) from Bayer AG Institute of Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980b) SRA 3886/dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8838 (study no. SRA
3886/001) from Institute of Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) SRA 3886: Salmonella/microsome test to evaluate
for potential point mutation. Unpublished report No. 13365 (study no.
T2017617) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985b) Addendum to report SRA 3886: Salmonella/microsome
test to evaluate for potential point mutation. Unpublished report No.
13365A (study no. T2017617) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1987) SRA 3886: Cytogenetic study of human lymphocyte
cultures in vitro to test for chromosome damage. Unpublished report
No. 15406, (study No. T3022874) from Bayer AG, Fachbereich Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1988) SRA 3886: In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report No. 16690, (study No. T1025572) from Bayer AG,
Fachbereich Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Greufe (1993) Response to US-EPA review: Chronic feeding
toxicity study with technical grade Fenamiphos (Nemacur(R)) with
dogs. Unpublished report dated 12 July 1993 (supplement to Mobay Corp.
study No. 88-274-BB) from Miles Inc., Stilwell, KS, USA. Submitted to
WHO by Bayer AG. Leverkusen, Germany.
Jones, R.D. & Loney, M.L. (1993) A subchronic feeding toxicity study
with technical grade Fenamiphos (Nemacur(R)) in dogs. Unpublished
report No. 91 - 176-KP (supplement to Mobay Corp. study No. 88-274-BB)
from Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kato, M.C. (1984a) Primary dermal irritation study of fenamiphos
technical in rabbitś Unpublished report No. B-561 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-561). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kato, M.C. (1984b) Primary eye irritation study of fenamiphos
technical in rabbitś Unpublished report No.B-562 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-562). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Khasawinah, A.M. & Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Unpublished report No. 34217 from Chemagro
Division of Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on hens.
Unpublished report No. 1831 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Unpublished
report No. 2829 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972a) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Unpublished report No. 3438 from
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Unpublished report No. 3559 from the
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972c) Antidotal experiments on rats. Unpublished report
No. 3560 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. ( 1971) BAY 68138 toxicological studies.
Unpublished report No. 2767 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Knaak, J.B., Ackerman, K.Y., Fredrickson, A.S. & Lee, P. (1981)
Reentry research: Dermal dose red cell cholinesterase-response curves
for fenamiphos, fenamiphos sulfoxide and sulfone. Unpublished report
No. 69689 from California Department of Food and Agriculture.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. ( 1988 ) Study for acute oral toxicity in rats.
Unpublished report No. 16744 (study No. T0027669) from Bayer AG,
Fachbereich Toxicologies, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Landes, A.M. (1978) In vitro inhibition of cholinesterase
by (R)Nemacur and Nemacur metabolites. Unpublished report No. 66068
(reference 77-060) from Mobay Chemical Corp., Chemagro Agricultural
Division, Kansas City, MO, US A. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of Nemacur
technical, desisopropyl Nemacur sulfoxide and desethyl Nemacur.
Unpublished report No. 44531 (reference 74-175) from Chemagro
Agricultural Division, Mobay Chemical Corp. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) The acute oral toxicity of Nemacur
desisopropyl to rats. Unpublished report No. 51597 (reference 75-144)
from Chemagro Agricultural Division, Mobay Chemical Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1968a) Subchronic toxicological studies on rats.
Unpublished report No. 745 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1968b) Subchronic toxicological studies on dogs.
Unpublished report No. 837 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser E (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Unpublished report No. 1655 from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1970) Subchronic toxicological studies on dogs. Unpublished
report No. 2008 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Unpublished report from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on rats (2 year
feeding experiment). Unpublished report No. 3539 from the Institute
for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1972c) Generation studies on rats. Unpublished report No.
3424 from the Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Löser, E. & Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachrichten Bayer,
24(1), 69-113. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MacKenzie, K.M. (1982) Teratology study with Nemacur in rabbits.
Unpublished report No. 308 (study No. 81165) from Hazelton Raltech,
Inc., Madison, WI, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mavournin, K.H., Blakey, D.H., Cimino, M.C., Salamone, M.F. & Heddle,
J.A. (1990) The in vivo micronucleus assay in mammalian bone marrow
and peripheral blood. A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 239, 29-80.
Mawdesley-Thomas, L. & Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Unpublished report
(addendum to report No, 745) from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mawdesley-Thomas, L. & Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Unpublished report (addendum
to report No. 1655) from the Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail F. (1980) SRA 3885 and Curaterr(R): Evaluation for acute
combination toxicity. Unpublished report No. 9294 from Institute of
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Mihail, F. & Schilde, B. (1980) SPA 3886 (active ingredient of
Nemacur(R)) subacute dermal toxicity study on rabbits. Unpublished
report No, 9297 from Institute of Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Rieth, J.P., Moore, K.D. & Elcock, L.E. (1991 ) Chronic feeding
toxicity study of technical grade Fenamiphos (Nemacur(R)) with dogs.
Unpublished report No. 6243 (study No. 88-274-BB) from Mobay Corp.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1981) SRA 3886 (Nemacur): Evaluation for embryotoxicity
and teratogenic effects in orally dosed rats. Unpublished report No.
9785 from Institute for Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Unpublished report (addendum to report
No. 1831 ) from the Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1971) Pathology report of BAY 68138. Hen study.
Unpublished report (addendum to report No. 2829) from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Stropp, G. (1995) Study for the skin sensitization effect in guinea
pigs (guinea pig maximization test according to Magnusson and
Kligman). Unpublished report No. 24341 (study No. T9058358) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Unpublished report (addendum
to report No. 2008) from Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Unpublished report (addendum to report No. 3561) from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted m
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974a) Nemacur(R) sulfone, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974b) Nemacur(R) sulfoxide, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974c) 3-Methyl-4-methylmercaptophenol, Acute toxicity in
rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974d) 3-Methyl-4-methane-sulfonylphenol, acute toxicity
in rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974e) 4-Methylmercapto-m-cresol, acute toxicity in rats.
Unpublished report dated 29 April 1974 from Institute for Toxicology,
Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979a) SPA 3885 (Nemacur(R) active ingredient)/acute
inhalation toxicity studies. Unpublished report No. 8210 from Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979b) Nemacur(R) active ingredient (SRA 3886)/subacute
inhalational toxicity studies. Unpublished report No. 8669 from
Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl 4-(methylthio)-N-
tolyl isopropylphosphoramidate] and identification of two metabolites
in plants. J. Agric. Food Chem., 20, 157-160. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Wargo, J.P. (1978) The effect of feeding Nemacur(R) sulfoxide to
dairy cattle. Unpublished report No. 66212 from Chemical Agricultural
Division, Mobay Chemical Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1988) [Phenyl-1-14C] Nemacur: Whole-body autoradiographic
distribution of the radioactivity in the rat. Unpublished report No.
PF3055 (study No. M181 0188-7) from Bayer AG Agrochemicals,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yang, L.L., Putnam, D.L. & Conklin, P.M. (1984) CHO/HGPRT mutation
assay in the presence and absence of exogenous metabolic activation --
Nemacur(R). Unpublished report No. 620 (study No. T2600.332) from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
inhibitory activity of fenamiphos and its metabolites in horse serum
cholinesterase in vitro was in the order fenamiphos < sulfoxide =
sulfone < unidentified metabolite.
The concentrations that inhibited the activity of chicken and monkey
liver aliesterase (triacetin hydrolysis) and monkey cholinesterase by
50% in vitro were 4.2 × 10-5 mol/L for chicken aliesterase, 1.0 ×
10-6 mol/L for monkey aliesterase, and 4.6 × 10-6 mol/L for monkey
cholinesterase (Coulston & Wills, 1974).
Male albino rats were painted dermally on the clipped dorsal area with
100-400 µg/cm2 fenamiphos, 25-800 µg/cm2 fenamiphos sulfoxide, or
200-1600 µg/cm2 fenamiphos sulfone. The test materials were applied
in acetone and allowed to dry. After 72 h, the animals were killed and
erythrocyte acetylcholinesterase activity determined. Fifty percent
inhibition was achieved with doses of 208 µg/cm2 fenamiphos, 262
µg/cm2 fenamiphos sulfoxide, and 750 µg/cm2 fenamiphos sulfone
(Knaak et al., 1981; JMPR, 1985).
Blood samples were extracted from equal numbers of male and female
Sprague-Dawley rats and then pooled for comparison of the
cholinesterase inhibition induced by fenamiphos and five of its
metabolites (fenamiphos sulfoxide, desisopropyl fenamiphos sulfoxide,
fenamiphos sulfone, desisopropyl fenamiphos sulfone, and desisopropyl
fenamiphos) in vitro. Samples were incubated for 1 h before
determination of the cholinesterase activity (Table 1). Erythrocyte
acetylcholinesterase was less sensitive to fenamiphos and its
metabolites than was plasma cholinesterase (Lamb & Landes, 1978; JMPR,
1985).
In order to assess the effects of fenamiphos on neurotoxic esterase
activity, single doses of 0 or 25 mg/kg bw technical-grade fenamiphos
(purity; 91.3%) were given by oral intubation to groups of nine
atropinized Lohmann selected Leghorn hens. Groups of three surviving
hens were killed one, two, and seven days after treatment. Even under
atropine protection, one hen died within 24 h of treatment. Fenamiphos
had no effect on neurotoxic esterase activity in the brains or spinal
cords, whereas the positive control compound, tri- ortho-cresyl
phosphate, had almost completely inhibited the activity 24 and 48 h
after treatment (Flucke & Eben, 1988).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of fenamiphos and its
metabolites are summarized in Table 2. Impurities identified as
components of the technical mixture (aryldiamide, diarylamide, diaryl
ethyl ester, diethyl ester, diethylmonamide, di-SCH3 compound, ethyl
aryl ester, ethyldiamide, and 4-methylthio- meta-cresol) were tested
for their acute toxicity to rats. At doses 1.5 times the acute LD50
of fenamiphos, none of the materials was toxic (Crawford & Anderson,
1973; JMPR, 1974).
Table 1. Inhibition of cholinesterase (%) by fenamiphos and its metabolites in vitro
Compound Plasma Erythrocytes
(Dose: ppm in whole blood) (Dose: ppm in whole blood)
6.88 68.8 688 5440 6880 6.88 68.8 688 5440 6880
Fenamiphos - 0 10 49 69 - 0 0 0 23
Fenamiphos sulfoxide 18 41 48 - 85 5 0 5 - 52
Fenamiphos sulfone 0 13 50 - 87 8 - - - -
Desisopropyl fenamiphos sulfoxide 6 40 90 - 93 4 0 47 - 50
Desisopropyl fenamiphos sulfone 0 20 69 - 90 6 0 32 - 61
Desisopropyl fenamiphos - 2 13 71 91 - 8 0 32 53
-, not determined
Table 2. Acute toxicity of fenamiphos, its metabolites, and impurities
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos
Mouse Oral M NR 22.7 Loser & Kimmerle (1971)
Intraperitoneal M/F 80 3.4 DuBois et al. (1967)
Inhalation M NR approx. 60 Kimmerle & Solmecke (1971)
(1 h static)
Rat Oral (fasted) M 88-99.7 2.4-6.0 Crawford & Anderson (1973, 1974a);
F 2.4-6.1 Lamb & Matzkanin (1975); Mihail (1980);
Heimann (1981, 1984, Krotlinger (1988)
Oral M 80-90.2 8.1-17.2 DuBois et al. (1967); Loser & Kimmerle (1971);
(not fasted) F 9.6-19.4 Kimmerle & Solmecke (1971); Kimmerle (1972a);
Heimann (1981, 1984)
Dermal M 80-92.2 72-73 Dubois et al. (1967); Flucke (1980)
84-92
Dermal M NR approx. 500 Kimmerle & Solmecke (1971)
Inhalation M 89.8 110-175 Kimmerle (1972b); Kimmerle & Solmecke
(1 h) F 130-150 Thyssen (1979a)
Inhalation M 89.8 91-100 Kimmerle & Solmecke (1971); Thyssen (1979a)
(4 h) 100
Intraperitoneal M 80 3.0-3.7 DuBois et al. (1967); Loser & Kimmerle (1971);
F 4.2-4.9 Kimmerle & Solmecke (1971)
Guinea-pig Oral M NR 56-100 DuBois et al. (1967); Loser & Kimmerle (1971);
Kimmerle & Solmecke (1971)
Intraperitoneal M NR 17.3 DuBois et al. (1967)
Rabbit Oral M NR 10-17.5 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Dermal M NR 225 Crawford & Anderson (1972)
F 179
Cat Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Dog Oral M NR approx. 10 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971)
Chicken Oral F NR 5.3-12 Loser & Kimmerle (1971); Kimmerle &
Solmecke (1971); DuBois et al. (1967)
Fenamiphos sulfone
Rat Oral (fasted) M NR 2.6 Crawford & Anderson (1974b)
F 2.4
Oral F NR 1-25 Thyssen (1974a)
(not fasted)
Fenamiphos sulfoxide
Rat Oral (fasted) M/F NR 2.4 Crawford & Anderson (1974b)
Oral F NR 10-25 Thyssen (1974b)
(not fasted)
Desisopropylfenamiphos
Rat Oral (fasted) M NR 1.4 Lamb & Matzkanin (1977)
F 2.1
Desisopropylfenamiphos sulfone
Rat Oral (fasted) M 95 4.1 Lamb & Matzkanin (1975)
F 3.7
Fenamiphos sulfoxide phenol
Rat Oral (fasted) M 99 1418 Crawford & Anderson (1974a)
F 1175
Oral F NR 500-1000 Thyssen (1974c)
(not fasted)
Table 2. (continued)
Species Route Sex Purity LD50/LC50 Reference
(%) (mg/kg bw
or µg/L)
Fenamiphos sulfone phenol
Rat Oral (fasted) M 95 1250 Crawford & Anderson (1974a)
F 1854
Oral F NR > 1000 Thyssen (1974d)
(not fasted)
4-Methylthio-meta-cresol
Rat Oral (fasted) M 96.4 1418 Crawford & Anderson (I974a)
F 1333
Oral F NR > 2500 Thyssen (1974e)
(not fasted)
NR, not reported
Fenamiphos (purity, 97%) was given orally to fasted Wistar (Bor:WISW
(SPF-Cpb) rats at doses of 1-8 (females) or 1-100 (males) mg/kg bw.
All deaths among males at 100 mg/kg bw occurred within 10 min, and
those among animals receiving 5 mg/kg bw or more within 1.5 h.
Clinical signs of toxicity noted at doses >4 (males) or 5 (females)
mg/kg bw included apathy, palmospasms, laboured breathing, diarrhoea,
piloerection, clonic cramps, and dyspnoea. Females at does > 1
mg/kg bw had only diarrhoea (Krötlinger, 1988).
Studies in which rodents were given fenamiphos by inhalation as a
static spray at doses up to 230 µg/L for 1-4 h showed that rabbits and
guinea-pigs are more tolerant of the acute effects than rats and mice
(Kimmerle & Solmecke, 1971).
Male and female TNO/W74 albino rats were exposed to aerosolized
fenamiphos (purity, 89.8%) for 4 h per day on five consecutive days at
concentrations of 0, 0.3, 0.6, 3.3, 4, 9, or 28 µg/L. Plasma and
erythrocyte cholinesterase activities were measured before exposure,
after the first, third, and fifth exposures, and 72 h after the fifth
exposure; brain acetylcholinesterase activity was not measured. The
cholinesterase activities were depressed in a dose-related manner;
that of plasma cholinesterase was the most significantly depressed,
and females were more sensitive than males. Plasma cholinesterase
activity was 32-90% lower than before treatment in males at > 3.3
µg/L and 31-96% lower in females at > 0.3 µg/L. The activity
remained depressed by 19-44% for 72 h after the fifth exposure in
females at concentrations of > 9 µg/L. Erythrocyte
acetylcholinesterase activity in males was inhibited by < 20% at
> 9 µg/L; however, that in females was depressed by up to 28% at 9
µg/L and 33% at 28 µg/L (JMPR, 1985, slightly modified by reference to
the original report of Thyssen, 1979b).
In male rats, administration of atropine and/or 2-pralidoxime or
obidoxime after poisoning reduced the acute lethal dose by a factor of
about two. As with other organophosphorus compounds, rapid
administration of atropine and oxime reactivator after poisoning
affords some protection and alleviates the cholinergic signs of
poisoning (DuBois et al., 1967; Kimmerle, 1972c; JMPR, 1974).
(b) Short-term toxicity
Rats
Three groups of 10 male and 10 female albino Wistar TNO/W 74 rats were
exposed to technical-grade fenamiphos (purity, 92.2%) diluted with a
1:1 mixture of ethanol and polyethylene glycol 400 for aerosolization
in a dynamic flow inhalation chamber at doses of 0, 0.03, 0.25, or 3.5
µg/L for 6 h per day, five days per week for three weeks; 98% of the
particles were 3 µm or less. No toxic signs or effects on mortality,
body-weight gain, or the results of haematology, urinalysis, or
clinical chemistry were seen. A significant decrease (48-79%) in
plasma cholinesterase activity and a slight decrease (9-18%) in
erythrocyte acetylcholinesterase activity were seen in animals of each
sex at 3.5 µg/L; brain acetylcholinesterase activity was not affected.
There were no gross or histopathological changes or effects on organ
weights. The NOAEL was 3.5 µg/L (Thyssen, 1979b).
Groups of 15 male Sprague-Dawley rats were given fenamiphos orally or
by intraperitoneal injection on five days per week for 60 days. All of
the animals survived doses ranging from 1.5 mg/kg bw per day
intraperitoneally to 1.7 mg/kg bw per day orally with no cumulative
toxicological effect (Kimmerle & Solmecke, 1971; JMPR, 1974). In
another study, female rats survived daily intraperitoneal
administration of 1 mg/kg bw per day for 60 days, while 40% of those
given 2 mg/kg bw per day and all rats at 3 mg/kg bw per day died
(DuBois & Flynn, 1968; JMPR, 1974). These studies indicate little if
any cumulative toxicity.
Groups of 20 Fischer 344 rats of each sex were given diets containing
fenamiphos (purity, 89%) at 0, 0.37, 0.57, or 0.91 ppm (equal to 0,
0.03, 0.045, or 0.072 mg/kg bw per day for males and 0, 0.035, 0.053,
or 0.084 mg/kg bw per day for females) for three months. Animals were
monitored for clinical signs of toxicity, mortality, abnormality,
masses, food consumption, and body weight throughout the study.
Cholinesterase activity in plasma and erythrocytes was determined in
10 rats of each sex per group at 5, 9, 12, and 14 weeks and in brain
at termination of the study. No effects attributable to treatment were
seen on food consumption or body weight or during clinical
observation. Statistically but not toxicologically significant
decreases in both plasma and erythrocyte cholinesterase activity were
observed in treated rats of each sex when compared with controls. The
maximal inhibition of plasma cholinesterase was 17% at week 14 in
males at 0.37 ppm, with lesser inhibition at higher doses, and 24% at
week 9 in females at the high dose. Erythrocyte acetylcholinesterase
activity was inhibited by < 10% in all cases. No toxicologically
significant, treatment-related change was seen in brain
acetylcholinesterase activity. It was concluded that no biologically
significant cholinesterase activity inhibition had occurred in this
study. The NOAEL was 0.9 ppm, equal to 0.07 mg/kg bw per day, the
highest dose tested (JMPR, 1987, slightly modified by reference to the
original report of Hayes, 1986a).
Groups of Wistar SPF rats of each sex (15 per treated group, 30
controls) were fed diets containing fenamiphos (purity, 82%) at 0, 4,
8, 16, or 32 ppm (equivalent to 0.2, 0.4, 0.8, or 1.6 mg/kg bw per
day) for three months. Male and female rats at the highest dose showed
signs of cholinergic stimulation during the first two months; no
behavioural changes were seen in the other animals. Average food
consumption and growth were similar for treated and control animals.
The only effects on the haematological parameters examined were
decreased plasma cholinesterase activity (> 20%) in animals at 8 ppm
and inhibition of erythrocyte acetylcholinesterase activity (< 12%)
in those at 8 ppm throughout the study, with peak inhibition (56%) at
16 ppm by week 8. Gross and microscopic examination of tissues and
organs at the end of the experiment showed slightly increased liver
weights in males at 16 or 32 ppm, which was not reflected in the
calculated relative organ:body weight ratios or on histological
examination. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original reports of
Löser, 1968a; Mawdesley-Thomas & Urwin, 1970a). Although the NOAEL was
originally considered to be 4 ppm on the basis of inhibition of plasma
cholinesterase activity (JMPR, 1974), the present Meeting decided that
the NOAEL was 8 ppm, equivalent to 0.4 mg/kg bw per day, on the basis
of inhibition of erythrocyte acetylcholinesterase activity.
Rabbits
An aqueous formulation of technical-grade fenamiphos (purity, 89.8%)
was applied to a clipped dorsal area of groups of six male and six
female New Zealand rabbits at doses of 0, 2.5, or 10 mg/kg bw per day
for 6 h per day, five days per week for three weeks. Two additional
groups were similarly treated at 0 and 0.5 mg/kg bw per day. The skin
of half of the animals in each group was abraded. Haematology,
clinical chemistry, and urinalysis were performed before treatment and
at the end of the study. Plasma and erythrocyte cholinesterase
acetylcholinesterase activities were measured before treatment and
after the tenth and last exposures.
No signs of toxicity or mortality were observed. Although the authors
concluded that body-weight gains were decreased in animals of each sex
at 10 mg/kg bw per day (JMPR, 1985), the differences were less than
10% and were not statistically significant. Furthermore, the range of
individual weight gains seen in animals at the high dose was well
within that observed in the controls. Slight erythema was observed in
all groups only at the abraded skin sites during the initial week,
which cleared by day 7. There were no apparent differences in
haematological, urinary, or clinical chemical parameters between test
and control groups. Gross necropsy, histopathology, and organ weight
measurements showed no remarkable changes in comparison with controls.
Plasma and erythrocyte cholinesterase activity was significantly
depressed (by up to 65 and 34%, respectively) in male and female
rabbits at 10 mg/kg bw per day; however, the females were somewhat
more sensitive, with depressed plasma cholinesterase activity (by
22-31%) at 2.5 mg/kg bw per day as well. Brain acetylcholinesterase
activity was statistically significantly depressed in females at 2.5
mg/kg bw per day (by 19%) and 10 mg/kg bw per day (by 21%). The NOAEL
was 0.5 mg/kg bw per day (JMPR, 1974, slightly modified by reference
to the original report by Mihail & Schilde, 1980).
Dogs
Groups of two beagle dogs of each sex were fed fenamiphos (purity,
82%) in the diet at 0, 2, 6, or 18 ppm (equivalent to 0.05, 0.15, or
0.45 mg/kg bw per day) for three months. Behavioural abnormalities
with signs of cholinergic stimulation were evident in animals at 18
ppm, and the growth of females at this dose was reduced.
Haematological and clinical chemical parameters, including the results
of tests for liver and kidney function and urinalyses, were not
affected by treatment. The averages values for plasma and erythrocyte
cholinesterase activity were depressed in males and females at 6 ppm;
in animals at 2 ppm, no effect was found on average values for
erythrocyte acetylcholinesterase activity, while those for plasma
cholinesterase were depressed (20-30%). Brain acetylcholinesterase
activity was not measured. Gross morphological examination of tissues
and organs at the end of the study showed no abnormal effects (JMPR,
1974, slightly modified by reference to the original report by Löser,
1968b).
Groups of two male and two female beagle dogs (three of each sex as
controls) were fed fenamiphos (purity, 99.4%) in the diet at levels of
0, 1, 2, or 5 ppm (equivalent to 0.025, 0.05, or 0.13 mg/kg bw per
day) for three months. Treatment at < 5 ppm in the diet had no
effect on the average values of haematological parameters, liver
function tests, clinical chemistry, or kidney function tests or on the
gross or microscopic appearance of tissues and organs. As in other
studies, cholinesterase activity was the only parameter significantly
affected, the females being more susceptible than the males and plasma
cholinesterase activity being more sensitive to inhibition than that
of erythrocytes. The latter was unchanged at 2 ppm while plasma
cholinesterase activity was slightly, transiently depressed; 1 ppm
fenamiphos in the diet had no effect on plasma cholinesterase
activity. Brain acetylcholinesterase activity was not measured (Löser,
1969; Mawdesley-Thomas & Urwin, 1970b; JMPR, 1974).
As an extension of the previous study, an additional two dogs of each
sex were fed fenamiphos(purity, 99.4%) in the diet at 0 or 10 ppm
(equivalent to 0.25 mg/kg bw per day) for three months. No deaths
occurred during the experiment, although a very slight deviation in
the average body weight of treated animals was noted. Because of the
small number of animals, the slight weight differences cannot be fully
evaluated. There was no effect on haematological or urinary
parameters, clearance, blood sugar, or cholesterol levels.
Cholinesterase activity was significantly depressed in both plasma and
erythrocytes in male and female dogs; brain acetylcholinesterase
activity was not measured. Gross and microscopic examination of
tissues and organs showed no significant differences between treated
and control animals (Löser, 1970; Thomson et al., 1972a; JMPR, 1974).
Groups of four male and four female, four-month-old beagle dogs were
fed diets containing fenamiphos (purity, 89%) at doses of 0, 0.6, 1,
or 1.7 ppm (equivalent to 0.015, 0.025, or 0.042 mg/kg bw per day) for
three months. They were observed twice daily, and body weight and food
consumption were recorded weekly. Plasma and erythrocyte
cholinesterase activity was determined at 0, 4, 6, 8, 10, and 12
weeks; brain acetylcholinesterase was determined at termination of the
study. Haematology, clinical chemistry, and urinalysis were not
performed, and tissues and organs were not examined grossly or
histologically. No toxicological symptoms or effects on body weight
and food consumption were seen; however, plasma cholinesterase
activity was depressed by 28-35% in males given 1.7 ppm in the diet.
The author concluded that females at this dose were not similarly
sensitive (JMPR, 1985), on the basis of the < 20% inhibition relative
to control levels; however, when inhibition was calculated over the
course of the study, the reductions in plasma cholinesterase activity
in females at all doses were 2-14% in controls, 13-23% in animals at
0.6 ppm, 18-28% at 1 ppm, and 31-41% at 1.7 ppm, which were comparable
to those in males: 10-15% in controls, 17-23% in dogs at 0.6 ppm,
20-29% at 1 ppm, and 34-41% at 1.7 ppm. Erythrocyte and brain
acetylcholinesterase activities were unaffected (JMPR, 1985, slightly
modified by reference to the original report by Hayes, 1983).
Technical-grade fenamiphos (purity, 88.9%) was administered in the
diet to groups of four male and four female beagle dogs at
concentrations of 0, 1, 3, or 12 ppm (equal to 0, 0.03, 0.089, or 0.31
mg/kg bw per day in males and 0, 0.03, 0.083, and 0.35 mg/kg bw per
day in females) for one year. The animals were observed for mortality,
clinical signs, body weight, food consumption, ophthalmic alterations,
and haematological, urinary, and clinical chemical parameters. Plasma
and erythrocyte cholinesterase activity was measured before treatment
and quarterly thereafter. One-half of each brain was taken at necropsy
for measurement of acetylcholinesterase activity. At necropsy, 11
organs were taken from each dog and weighed, and about 40 tissues from
each dog were examined grossly and histopathologically.
Treatment did not affect survival, clinical signs, growth, food
consumption, ophthalmoscopic or urinary parameters, or organ weights.
Them were no gross or histopathological findings that could be
attributed to treatment. Plasma cholinesterase activity was
statistically significantly inhibited relative to the levels before
treatment in both males and females in a dose-related fashion at doses
> 1 ppm: 1 ppm, 20-32%; 3 ppm, 41-53%; 12 ppm, 54-65%; the control
values were variably reduced by up to 14% over the course of the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly decreased in both males and females at
3 ppm (by 17-36%) and 12 ppm (by 58-68%); the activity in the controls
and dogs at 1 ppm was variably, statistically nonsignificantly reduced
by 5-24% and 10-23%, respectively, during the course of the study.
Brain acetylcholinesterase activity was nonsignificantly decreased by
12% in males and sigificantly reduced by 17% in females at 12 ppm.
Males at this dose also had mild, transient anaemia characterized by
significantly decreased erythrocyte counts, haemoglobin
concentrations, and haematocrit, with a concomitant increase in mean
corpuscular volume. The authors concluded that there was no NOAEL
because of the effects on plasma cholinesterase activity at the lowest
dose (Riethet al., 1991). Owing to the variability in the activity of
plasma cholinesterase in controls seen in a six-month follow-up (Jones
& Loney, 1993; see below), the authors reconsidered the results of the
one-year study and decided that the changes seen at 1 ppm were within
the normal biological range seen in historical controls (Jones &
Greufe, 1993). The Meeting agreed that the NOAEL was 3 ppm, equal to
0.083 mg/kg bw per day, on the basis of decreased brain
acetylcholinesterase activity and anaemia at the highest dose.
In the supplemental study (Jones & Loney, 1993), technical-grade
fenamiphos (purity, 89%) was administered in the diet to groups of
four male and four female beagle dogs at concentrations of 0 or 0.5
ppm, equal to 0.011 mg/kg bw per day, for six months. The only
parameters measured were mortality, clinical and ophthalmologic signs,
body weight, food consumption, and plasma and erythrocyte
cholinesterase activity. No signs of toxicity were observed.
Groups of four male and four female pure-bred beagle dogs were fed
fenamiphos in the diet at levels of 0, 0.5, 1, 2, 5, or 10 ppm (equal
to 0.015, 0.029, 0.06, 0.15, or 0.31 mg/kg bw per day for males and
0.014, 0.036, 0.063, 0.17, and 0.34 mg/kg bw per day for females) for
two years. There were no significant effects on growth, food
consumption, or the results of any of the standard clinical and
physiological examinations made during the course of the study. Gross
and histological examination of all tissues and organs at the
conclusion of the study showed no abnormal developments considered to
be related to treatment. The only significant physiological effect
observed was inhibition of plasma cholinesterase activity (> 20%) at
doses > 2 ppm and of erythrocyte acetylcholinesterase activity at
> 5 ppm. Brain acetylcholinesterase activity was not measured
(JMPR, 1974, slightly modified by reference to the original report by
Löser, 1972a; Thomson et al., 1972b).
Cattle
Groups of three dairy cows were fed fenamiphos sulfoxide in their
diets at levels of 2, 6, or 20 ppm for 29 days; one untreated cow was
used as a control. There were no apparent effects on behaviour, feed
consumption, milk production, or body-weight gain. No adverse effects
on whole-blood cholinesterase activity were seen with 2 or 6 ppm, but
a significant depression (51%) was seen at 20 ppm (Wargo, 1978; JMPR,
1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female six-week-old outbred CD1 albino mice
were given fenamiphos (purity, 89.5%) in the diet at doses of 0, 2,
10, or 50 ppm (equal to 0, 0.3, 1.4, or 7.4 mg/kg bw per day for males
and 0, 0.3, 1.8, or 8.8 mg/kg bw per day for females) for 20 months.
Toxic effects were monitored daily, and body weights and food
consumption were determined weekly. Haematological parameters were
analysed in 10 mice of each sex in each group at 6, 12, 18, and 20
months of the study. All animals underwent complete necropsy, and the
liver, kidney, heart, lungs, gonads, spleen, brain, and adrenals were
weighed. A full complement of tissues and organs from all animals were
examined histopathologically. Cholinesterase activity was not
measured. Daily observations were not reported. Survival was
comparable in all groups, with only a marginal decrease at the high
dose. Survival at 20 months was 32-45%. Body weights were
statistically significantly reduced in both males and females at 50
ppm, but the differences were less than 10%. Food consumption and
haematological parameters were unaffected by treatment.
Contrary to the conclusions of the 1985 JMPR with regard to changes in
organ weights, reexamination of the data indicates that the absolute
weights of the brains of female mice at all doses were slightly (4-6%)
but statistically significantly lower than those of controls. The
relative weights of the brains of animals at 50 ppm were, however,
increased in both males (7%, statistically nonsignificant) and females
(9%, statistically significant). The absolute and relative ovarian
weights were decreased by 16-18% in animals at 10 ppm and by 42-55% in
those at 50 ppm; and the absolute and relative splenic weights were
reduced in animals of each sex at 50 ppm. Other findings include
reductions in absolute heart, lung, liver, and kidney weights
(generally 10-15%, of variable significance) in males and females at
50 ppm, although the relative weights were comparable to those in
controls. No gross or microscopic parallel to these weight changes was
found. The most frequent pathological findings reported were chronic
multifocal interstitial nephritis, chronic peribronchiolitis,
pulmonary congestion, and acute and chronic myocarditis in all treated
animals, but no significant difference associated with dose was found.
Cystic endometrial hyperplasia was also frequent in all treated
females. A significant degree of fatty diffuse change was seen in the
liver, again with no relation to dose. In all groups, uniformly spread
amyloidosis was observed in many organ systems, including liver,
spleen, adrenals, kidneys, small intestines, thyroids, ovaries, and
submaxillary salivary glands. Fenamiphos had no oncogenic potential at
any dose. The NOAEL was 2 ppm, equal to 0.3 mg/kg bw per day, on the
basis of decreased splenic and ovarian weights at doses > 10 ppm
(JMPR, 1985, slightly modified by reference to the original report by
Hayes, 1982).
Rats
Groups of 40 male and 40 female SPF-derived Wistar rats were fed
fenamiphos in the diet at concentrations of 0, 3, 10, or 30 ppm (equal
to 0, 0.17, 0.56, or 1.7 mg/kg bw per day for males and 0, 0.23, 0.76,
or 2.2 mg/kg bw per day for females) for two years. Behavioural
abnormalities due to cholinergic stimulation were seen only during the
first six weeks of treatment at 30 ppm. The obvious effect on
cholinesterase activity disappeared after six weeks of feeding and was
not seen for the remainder of the study. Average growth, mortality,
food consumption, haematological and clinical chemical parameters, and
the results of liver and kidney function tests were unchanged. Urinary
parameters and blood sugar and cholesterol values were normal. The
thyroid weights and the thyroid:body weight ratios of females at 30
ppm were larger than those of controls but were not accompanied by
abnormal tumour development, goitre, or unusual histological findings.
All other major tissues and organs appeared normal at gross and
microscopic examination.
Feeding of 10 ppm fenamiphos in the diet inhibited plasma
cholinesterase activity by up to 55% in females but by < 20% in
males. In animals at 30 ppm, plasma cholinesterase activity was
inhibited by up to 60% and that of erythrocyte acetylcholinesterase by
up to 54% in males and 65% in females. No significant effects were
observed at 3 ppm. Although the NOAEL was originally considered to be
3 ppm, the present Meeting determined it to be 10 ppm (equal to 0.56
mg/kg bw per day) on the basis of inhibition of erythrocyte
acetylcholinestrase activity at the next highest dose (JMPR, 1974,
slightly modified by reference to the original report by Löser, 1972b;
Cherry & Newman, 1973).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenamiphos (purity, 89.3%) at a mean
concentration of 0, 1.7, 7.8, or 37 ppm (equal to 0, 0.098, 0.46, or
2.5 mg/kg bw per day for males and 0, 0.12, 0.6, or 3.4 mg/kg bw per
day for females) for two years. Clinical signs, mortality, food
consumption, haematological and blood chemical parameters (in 20 rats
of each sex per group), urinary parameters (in 10 rats of each sex per
group), and body weights were monitored throughout the study. Plasma
and erythrocyte cholinesterase, activity was monitored in 10 rats of
each sex per group at weeks 6, 10, and 15 and at 6, 12, 18, and 24
months. Additional groups of 10 rats of each sex were given 0 or 37
ppm fenamiphos for one year. Brain acetylcholinesterase activity was
determined at the end of the study in all rats in these satellite
groups and in 10 rats of each sex per group in the main study. All
rats, including those found dead or killed in extremis during the
study and those killed at 12 or 24 months, were examined grossly;
their organs were weighed, and more than 40 tissues were evaluated
microscopically. Ophthalmological examinations were performed on 10
rats of each sex per dose before and at the end of the study.
Survival at termination of the study was not related to treatment and
was 58-88% in males and 62-84% in females. Clinical observation
indicated a higher incidence of rough coat and alopecia in females at
the high dose than in controls. A statistically significant decrease
in body-weight gain was seen in male and female rats at the high dose
throughout the study, although no treatment-related effect on feed
consumption was seen. There was a dose-related, statistically
significant decrease in plasma cholinesterase activity in all treated
rats throughout the study: in mate rats, 7-38% at 1.7 ppm, 19-68% at
7.8 ppm, and 56-88% at 37 ppm; in females, 22-96% throughout the
study. Erythrocyte acetylcholinesterase activity was also
statistically significantly inhibited in treated animals: by 0-7% at
1.7 ppm, 12-25% at 7.8 ppm, and 56-81% at 37 ppm in males and 0-11% at
1.7 ppm, 21-43% at 7.8 ppm, and 62-81% at 37 ppm in females. A
statistically significant decrease in brain acetylcholinesterase
activity was observed in male rats at the high dose at termination
(-14%) and in animals of each sex (-25% in males and -24% in females)
at interim sacrifice after one year of treatment. There were
statistically significant increases in the relative weights of the
brain, heart, and lung in both males and females at the high dose at
the end of the study; females at the high dose also had significantly
increased relative kidney weights. At interim sacrifice, females had
statistically significantly increased relative weights of adrenals,
brain, heart, kidneys, and ovaries. These changes were considered to
be related to the decrease in body weight observed in rats of each sex
at 37 ppm. The only statistically significant change in absolute organ
weight at termination was a decrease in liver weight and an increase
in lung weight in animals of each sex at 37 ppm. No treatment-related
change was seen during ophthalmological examination, in food
consumption, in haematological, clinical chemical, or urinary
parameters, or on gross pathology. No treatment-related neoplastic
lesions were observed during histopathological examination, but
statistically significantly higher incidences of non-neoplastic
inflammatory lesions were observed in the nasal, laryngeal, and lung
tissues of rats receiving 37 ppm fenamiphos in the diet when compared
with controls. These changes were attributed by the author of the
study to the marked inhibition of cholinesterase activity in these
animals. No treatment-related non-neoplastic changes were noted at 1.7
or 7.8 ppm. The author concluded that fenamiphos was not oncogenic.
Contrary to the conclusions of the 1987 JMPR, the present Meeting
determined that the NOAEL was 7.8 ppm, equal to 0.46 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinestrase activity and
changes in body-weight gain, organ weights, and histoptahological
appearance at the next highest dose (JMPR, 1987, slightly modified by
reference to the original report by Hayes, 1986a).
(d) Genotoxicity
Fenamiphos has been tested adequately in a battery of tests for
mutagenicity (Table 4). It was weakly clastogenic only in vitro; no
similar response was seen in vivo in tests for micronucleus
formation and dominant lethal mutation. The Meeting concluded that
fenamiphos is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
A standard three-generation (two litters per generation) study of
reproductive toxicity was performed in which groups of 10 male and 20
female FB30 rats were given fenamiphos in the diet at 0, 3, 10, or 30
ppm throughout mating, gestation, and suckling. Immediately after
birth, pups were examined for malformations and were then prepared for
another generation or killed. Five weanling rats per group from the
F3b. generation were killed, and macroscopic and microscopic
examinations were performed on the major tissues and organs. There
were no apparent differences in the limited indices of reproduction
investigated, including fertility, litter size, lactation index, or
growth of young, or in the incidence of malformations (Löser, 1972c;
Cherry et al., 1972; JMPR, 1974).
In a two-generation study of reproductive toxicity, groups of 30 male
and 30 female albino CD Sprague Dawley rats received technical-grade
fenamiphos (purity, 89%) in the diet at concentrations of 0, 2.5, 10,
or 40 ppm (equal to 0.17, 0.64, or 2.8 mg/kg bw per day for males and
0.2, 0.73, or 3.2 mg.kg bw per day for females) for 70 days before
mating. Oestrous cycles were characterized over two weeks in 10 F0
and F1 females per dose before mating After weaning, 30 F1 animals
of each sex per dose were treated for 70 days and then bred to produce
the second generation (F2); treatment was continued throughout
mating, gestation, and lactation. Groups of 10 F0 and F1 adults of
each sex per dose were used to assess cholinesterase activity in
plasma and erythrocytes in week 8 before mating and just before
sacrifice and in brain at the time of sacrifice. Plasma, erythrocyte,
and brain cholinesterase activities were measured in one pup of each
sex from each of 10 litters at the time of culling and on day 21 of
lactation. F0 and F1 females were killed after their pups had been
weaned or on day 24 of gestation. The males were killed after the last
litters were delivered. The histologic al examinations concentrated on
reproductive tissues.
There were no treatment-related deaths or clinical signs of toxicity
in the parental animals. In F0 and F1 dams at 40 ppm, statistically
significant reductions were seen in body-weight gain during lactation
(by 72 and 65%) and food consumption (by up to 11 and 19%). F1 and
F2 pups also had significant reductions in body-weight gain beginning
on day 7 of lactation. The body weights of F1 adults at 40 ppm were
significantly reduced throughout the premating period (by about 10% in
males and 7% in females), which the author attributed to their reduced
body weights at the start of the F1 premating period. Although not
reported by the author, the overall weight gain of F1 males before
mating was also reduced during the first four weeks, by 12% in gain
those at 10 ppm and by 15% in those at 40 ppm. The relative ovarian
weights of F0 females were significantly reduced, by 13% at 2.5 ppm,
11% at 10 ppm, and 20% at 40 ppm but were unaffected in F1 females.
Although the author concluded that this effect was related to
treatment at 40 ppm, no histopathological lesions were observed in the
ovaries, reproductive parameters were unchanged, and there was no
comparable effect in the F1 generation.
Plasma cholinesterase activity was significantly inhibited by > 20%
in all treated adult females of both generations, in F1 males at 10
ppm at the time of sacrifice, and in both F0 and F1 males at 40 ppm
both before mating and at sacrifice. Erythrocyte acetylcholinesterase
activity was consistently significantly inhibited in females at doses
> 10 ppm but only at 40 ppm in F0 and F1 males. At 40 ppm, brain
acetylcholinesterase activity was significantly inhibited by 21% in
F0 females, by 29% in F1 females, and by only 6% in F0 males. In
pups, plasma cholinesterase activity was significantly inhibited on
day 21 of lactation in both males and females of both generations at
doses > 10 ppm. Erythrocyte acetylcholinesterase activity was
inhibited by > 20% in males and females of both generations at 40 ppm
only. Brain acetylcholinesterase was not affected.
Table 4. Results of tests for genotoxicity with fenamiphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 4, 20, 100, 500, 2500 NR (Negative)a,b Herbold (1979)
TA1535, TA1537 µg/plate (DMSO)
TA98, TA100
Reverse mutation S. typhimurium 20, 100, 125, 250, 500, 92.4 Negativea Herbold (1985a,b)
TA98, TA100 1000, 2000, 2500
TA1535, TA1537 µg/plate (DMSO)
Forward mutation Chinese hamster Unactivated: 100, 110, 85 Negativea,c Yang et al. (1984)
ovary cells 120, 130 µg/ml (DMSO)
(CHO-K1-BH4) Activated: 170, 190,
210, 230 µg/ml (DMSO)
Unscheduled Rat hepatocytes 1.5, 5, 15, 50, 100 µg/ml 89.5 Negative Curren (1988)
DNA synthesis (DMSO)
Chromosomal Human 25, 100, 400 µg/ml 91.3 Positivea,d Herbold (1987)
aberrations lymphocytes (DMSO)
Chromosomal Human Unactivated: 91.9 Negative Herbold (1988)
aberrations lymphocytes 25, 50, 75, 100 µg/ml
(DMSO)
Activated: 100, 150, Weakly
225, 350 µg/ml (DMSO) positivee
Sister chromatid Chinese hamster Activated: 10, 20, 40, NR Negativef Chen et al. (1982)
exchange cell line (V79) 80 µg/ml
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
(%)
In vivo
Micronucleus Mouse (NMRI) 0.625, 1.25, 2.5 mg/kg 92.5 (Negative)g Herbold (1980a)
formation bone-marrow cells bw
Dominant lethal Mouse (male 5 mg/kg bw 92.5 Negative Herbold (1980b)
mutation NMRI) germ cells
NR, not reported; DMSO, dimethyl sulfoxide
a With and without metabolic activation
b The 1985 JMPR concluded that the test protocol was unacceptable. Another test was conducted in only
one strain (TA 1537), without activation, at doses of 125, 250, 500, and 1000 µg/plate.
c Positive controls (0.2 µl/ml ethylmethylsulfonate; 2 µg/ml benzo[a]pyrene) yielded the expected positive responses.
d Statistically significant increase (52% mitotic index) seen at 100 µg/ml without activation and at 400 µg/ml with
activation (< 0.1% mitotic index). Haemolysis was seen at 400 µg/ml. The author concluded that the increase in
aberrations was due exclusively to cytotoxicity.
e Statistically significant response seen only at 350 µg/ml with activation (35% mitotic index). The author concluded
that the increase in aberrations was due to cytotoxicity.
f Positive control (5 µg/ml cyclophosphamide) gave expected positive response.
g The mice were dosed twice, 24 h apart, and the bone marrow was sampled once, 6 h after the second dose. The 1985
JMPR concluded that the test protocol was unacceptable. The protocol was also considered unacceptable in the report
of the Gene-Tox Program (Mavournin et al., 1990).
Oestrous cycles, mating, fertility, and gestation indices, sex ratio,
and pup viability indices were unaffected, and no treatment-related
gross or histological lesions seen in any tissue from either parental
animals or pups. The pups showed no clinical signs of toxicity. The
NOAEL for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per
day, on the basis of decreased body-weight gain. The NOAEL for
reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per day, on
the basis of decreased pup body weights during lactation (Eigenberg,
1991).
(ii) Developmental toxicity
Rats
Four groups of 25 female FB30 rats mated overnight with untreated
males (in a ratio of one male to two females) were given fenamiphos
(purity, 92.5%) in a 0.5% aqueous Cremophor emulsion at daily doses of
0, 0.3, 1, or 3 mg/kg bw per day by gavage on days 6-15 of gestation;
the first day of gestation was that on which sperm was found in a
smear obtained the morning after mating. Control females received the
same volume (10 ml/kg bw) of the aqueous emulsion. On day 20 of
gestation, the dams were narcotized with carbon dioxide and the
fetuses were removed. Litter size, average fetus weight per litter,
sex, external and visceral abnormalities, and skeletal malformations
and development were noted. Cholinesterase activity was not measured.
Eighteen dams receiving 3 mg/kg bw per day showed signs of toxicity
(trembling and recumbency), and two died; however, the time of death
was not given and the cause of death could not be established. No
treatment-related signs of toxicity or deaths occurred in rats
receiving 0, 0.3, or 1 mg/kg bw per day. A 15% reduction in average
weight gain was seen during treatment among dams receiving 3 mg/kg bw
per day in comparison with controls, but the author reported that the
difference was not statistically significant. A total of six females
-- one control, two at 0.3 mg/kg bw per day, two at 1 mg/kg bw per
day, and one at 3 mg/kg bw per day -- were not fertilized. The average
placental weight of animals at the high dose was significantly lower
than that in controls. Treatment did not affect the number of
fertilized or pregnant females, litter size, number of resorptions,
number of fetuses, average fetal weight, sex ratio, incidence of
alterations in development, or the type or number of malformations.
The most frequent manifestations were nodulations on ribs, which were
found in two fetuses from one litter of a dam at 0.3 mg/kg bw per day
and in four fetuses of three litters of dams at 1 mg/kg bw per day.
The other malformations observed were general oedema, abdominal
fissure, and anophthalmia in one fetus at 0.3 mg/kg bw per day. No
malformations were observed in fetuses at the high dose. The lower
placental weight observed at the high dose was considered to be not
toxicologically significant because the average weight was within the
normal range and no effects were seen on embryonic or fetal
development. Thus, fenamiphos at doses < 3 mg/kg bw per day was not
embryotoxic or teratogenic; it was, however, toxic to dams at 3 mg/kg
bw per day (Schlüter, 1981; JMPR, 1987).
Groups of 33 mated female Crl:CD-BR rats were given fenamiphos
(purity, 88.7%) at doses of 0, 0.25, 0.85, or 3 mg/kg bw per day by
gavage on days 6-15 of gestation. Five dams from each group were
killed on day 16 in order to measure plasma, erythrocyte, and brain
cholinesterase activities; the remaining dams were killed on day 20 of
gestation and necropsied grossly. All dams were examined for number of
corpora lutea and implantation sites, and their uteri and placentas
were weighed. The fetuses were weighed and sexed; about half were
examined externally and in the viscera, and the other half were
processed for skeletal examination. Brain acetylcholinesterase
activity was measured in 20 fetuses per group.
Six dams at 3 mg/kg bw per day died between days 7 and 14 of
gestation, one female with convulsions on the day of its death.
Clinical signs of toxicity, such as tremors, salivation, lachrymation,
urine staining, and hypoactivity, were seen to varying extents in the
survivors. Body-weight gain and food consumption were significantly
reduced throughout treatment at this dose. Gross necropsy revealed no
treatment-related abnormalities, and gestational parameters were
unaffected. Fetal body weights were unchanged, and they had no
treatment-related variations or malformations. Plasma and erythrocyte
cholinesterase activities were statistically significantly reduced (by
50 and 42%, respectively) in dams at 3 mg/kg bw per day on day 16 of
gestation. By day 20, the plasma activity had returned to normal,
while that of erythrocyte cholinesterase was still significantly
reduced by 30%. Brain acetylcholinesterase activity was reduced by 28%
in adults at 0.85 mg/kg bw per day and by 12% in those at 3 mg/kg bw
per day; as the differences were not significant or dose-related, they
were not considered to be related to treatment. Fetal brain
acetylcholinesterase activity was unaffected. The NOAEL for maternal
toxicity was 0.85 mg/kg bw per day. Fenamiphos was not teratogenic or
fetotoxic under the conditions of this study (Clemens et al., 1989).
Rabbits
Groups of 20 double-mated female New Zealand rabbits were given
fenamiphos (purity, 88.8%) orally in corn oil at doses of 0, 0.1, 0.3,
or 1 mg/kg bw per day on days 6-18 of gestation. They were observed
daily for clinical signs of toxicity and were weighed initially,
periodically during the test, and at termination of the study. Once
the pups had been removed, the ovaries and uteri of the dams were
examined, and the fetuses were examined grossly and prepared for
evaluations of soft tissues and the skeleton. The numbers of corpora
lutea, implantations, resorptions, live and dead fetuses, and
anomalies were also determined. Cholinesterase activity was not
assessed. Dams given doses > 0.3 mg/kg bw per day showed signs of
toxicity, with decreased body-weight gain, bloody nasal discharge, and
white, mucoid ocular discharge. Treatment did not affect the number of
litters, number of pups per litter, pregnancy rate, the number of
corpora lutea, implantations, or gross abnormalities. Mean fetal
weight was slightly depressed at 1 mg/kg bw per day. One dam at 0.3
mg/kg bw per day aborted one dead pup, and two at 1 mg/kg bw per day
aborted eight dead pups and had seven late resorptions. In addition,
one dead fetus was found in each of two litters at the high dose.
The commonest developmental variation observed was the left carotid
arising from the innominate, which occurred in six to eight fetuses
(7-9%) in each of three litters (25%) at doses > 0.1 mg/kg bw per
day. This anomaly was not seen in the controls and in only one of 31
litters (3.2%) of historical controls at the laboratory where the
study was performed. More recent data (through July 1985) for
historical controls revealed incidences of 11/336 (3.3%) in fetuses
and 9/53 (17%) litters. The newer historical control data provide some
evidence that the incidence of left carotid anomalies was increased in
superovulated or artificially inseminated rabbits. Furthermore, data
for rabbits of the same strain in different labs indicate that the
anomaly is a frequent finding, occurring in about 8% of fetuses and
25% of litters; the historical data are for artificially inseminated
rabbits, while the animals used in this study were naturally bred. The
biological significance of this finding in relation to treatment is
dubious.
An increased incidence of accessory skull bones was also seen in all
treated groups, but it did not occur in a dose-related manner and was
thus not considered to be related to treatment. A significant increase
in the incidence of chain-fused sternebrae was seen in five fetuses in
three litters at 1 mg/kg bw per day, and this anomaly was also seen in
one fetus in one litter at 0.3 mg/kg bw per day. This anomaly is of
questionable biological significance. Two fetuses in one litter at the
high dose had aortic arches with a common truncus, which was
considered to be a major malformation. Other skeletal malformations
which occurred only at doses > 0.3 mg/kg bw per day included fused
ribs, scoliosis, absent vertebrae (thoracic, lumbar, sacral, and
caudal), and bipartite or malformed centra. The 1985 Meeting concluded
that these findings were related to treatment; however, these
anomalies occurred in only one or two fetuses in single litters, often
with no relation to dose. Furthermore, several of the anomalies were
clustered within a single fetus, indicating that they were not likely
to be related to treatment. The NOAEL for maternal toxicity was 0.1
mg/kg bw per day, and that for developmental toxicity was 0.3 mg/kg bw
per day. Fenamiphos had no teratogenic effects at any dose (JMPR,
1985, modified by reference to the original report by MacKenzie,
1982).
In a study to determine the doses for a study of embryotoxicity
(including teratogenicity), groups of three mated (1:1) female
Chinchilla rabbits were given single daily doses of fenamiphos
(purity, 91%) in distilled water with 0.5% Cremophor EL0 at doses of
0.1, 0.8, or 3 mg/kg bw per day by gavage on days 6-18 post coitum.
Cholinesterase activity was measured in plasma and erythrocytes before
the first dose and just after the last dose; brain
acetylcholinesterase activity was not assessed. All of the animals
were killed on day 28 post coitum, and the fetuses were removed,
sexed, weighed, and examined for gross external and internal
abnormalities.
One female at the high dose lost weight from day 10 and died on day 12
post coitum; body-weight loss and reduced food consumption were seen
throughout treatment in all animals at this dose when compared with
controls. Statistically significant reductions in both plasma (89%)
and erythrocyte cholinesterase activity (90%) relative to controls
were noted in rabbits at the high dose on day 18 post coitum. Five
preimplantation losses and one fetal resorption were observed in does
at the high dose but in no other group. Marginal effects on body
weight and food consumption were seen at 0.8 mg/kg bw per day. No
treatment-related effects were seen on the numbers of corpora lutea,
implantations, live or dead fetuses, or on fetal weight (Becker et
al., 1986; JMPR, 1987).
In the main study, four groups of 16 single-mated female Chinchilla
rabbits were given fenamiphos (purity, 91%) in distilled water
containing 0.5% Cremophor as single daily doses of 0, 0.1,0.5, or 2.5
mg/kg bw per day by gavage on days 6-18 post coitum. The does were
killed on day 28 post coitum, and the fetuses were removed. Only
does with at least one living fetus were used in calculating
body-weight gain, food consumption, and reproductive parameters. They
were examined for the position of fetuses in the uterus and numbers of
corpora lutea, implantations, resorptions, and live and dead fetuses.
The fetuses were weighed, sexed, and examined for external and
internal malformations, skeletal abnormalities, and development.
Cholinesterase activity was not assessed. Dosing solutions were
prepared daily. The concentration of fenamiphos in triplicate samples
taken for determination of homogeneity before the study was found to
vary considerably (14-140% of the nominal concentration), with mean
concentrations of 71 ± 8.3, 84 ± 64, and 70 ± 16% of the nominal
concentration at doses of 0.1, 0.5, and 2.5 mg/kg bw per day,
respectively. At the next sampling 10 days later, the mean
concentrations were all within 90% of the nominal concentration,
although the variability was still high (92 ± 37, 97 ± 45, and 99 ±
41% of the nominal concentration at the three doses, respectively).
Although it is stated that homogeneity was maintained during dosing by
use of a magnetic stirrer, it is not clear if the samples taken for
testing were subjected to the same treatment before analysis.
Four females at 2.5 mg/kg bw per day died as a result of treatment
after 3, 5, and 10 days and on day 21 post coitum. Treatment-related
signs of toxicity (salivation and dyspnoea) were observed in these
females and in five other females at the high dose between days 7 and
18 post coitum. Ataxia was seen in two females that died, and
diarrhoea was observed in another. No signs of toxicity were found in
animals at 0, 0.1, or 0.5 mg/kg bw per day. A treatment-related,
statistically significant decrease in mean food consumption (29%) and
a treatment-related reduction in body-weight gain (56%) were observed
during treatment in does at the high dose when compared with controls.
Food consumption was significantly increased in this group on days
24-28.
No treatment-related or significant difference was observed between
treated and control animals with regard to the mean numbers of
implantations, corpora lutea, live or dead fetuses, or resorptions. In
fetuses, no effect that could be attributed to treatment was seen in
sex ratio, body weight, external or internal malformations, skeletal
abnormalities, or development. One fetus at the high dose had
encephalocele with reduced brain size, but this finding was not
considered to be related to treatment. A number of skeletal changes
unrelated to treatment were seen in fetuses at all doses. The NOAEL
for maternal toxicity was 0.5 mg/kg bw per day. Although questions
remain about the doses actually administered, because of the problems
of homogeneity, the study showed no embryotoxicity or teratogenicity
at maternally toxic doses (JMPR, 1987, modified by reference to the
original report by Becker, 1986).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenamiphos painted on the skin of New Zealand white
rabbits in an acetone solution at 50 mg/kg bw resulted in slight
erythema but was not considered to be a primary irritant. Application
of technical-grade fenamiphos to the conjunctival sac of New Zealand
white rabbits as 100 mg of a crystalline material resulted in
irritation considered to be mechanical rather than physiological
(Crawford & Anderson, 1971; JMPR, 1974).
Technical-grade fenamiphos (purity, 90.7%) was melted at 35°C, and
0.5 ml was painted onto the intact or abraded skin of six
Japanese-derived albino rabbits for 24 h under occlusion. Irritation
was scored according to Draize 24, 48, 72, and 168 h after
application. Minimal irritation was observed, characterized by slight
erythema and oedema during the first 48 h. The mean primary irritation
index, based on readings at 24 and 72 h, was 0.42 (Kato 1984a).
Technical-grade fenamiphos (purity, 90.7%) was heated to liquidity
(temperature not specified) and applied at 0.1 ml to the conjunctival
sacs of nine Japanese-derived albino rabbits. The eyes of three of the
rabbits were washed with water. Irritation was scored according to
Draize 24, 48, 72, 96, 168, and 240 h after administration. In the
absence of washing, fenamiphos was a moderate irritant, with a maximum
average score of 31.3 at 24 h. The irritation scores slowly declined,
and all of the eyes were clear by 240 h. Fenamiphos was not irritating
when the eyes were washed with water after application. Midriasis,
persisting until day 2 or 3, was seen in all animals when the eyes
were not washed within 10 min of application. The translation of the
report leaves some uncertainty about other ocular effects, but signs
of systemic toxicity peaked within 3-4 h of application; these
included salivation, increased respiration, cyanosis, and slight
convulsions (number affected not specified). All animals were
reportedly normal within 6 h. No systemic toxicity was seen in the
animals when the eyes were washed (Kato, 1984b).
The potential of fenamiphos to sensitize skin was examined in a
maximization test in which 20 male Hsd Win:DH guinea-pigs were induced
intradermally with 1% fenamiphos in saline containing 2% Cremophor EL.
One week later, they were induced topically with 25% fenamiphos in
saline (with 2% Cremophor EL); they were challenged three weeks later
with 12 and 25% solutions of fenamiphos in the saline solution. Patchy
erythema was seen 24 h after removal of the patch in 22% of the
animals challenged with the 25% solution, whereas none of the 10
induced control animals showed irritation. The author considered a
response rate of > 30% to be indicative of sensitization, and
concluded that fenamiphos produced no relevant sensitization; however,
according to the original protocol of Magnusson and Kligman, these
results would indicate that fenamiphos is a mild sensitizer (Stropp,
1995).
(ii) Delayed neuropathy
Groups of eight hens were fed fenamiphos in the diet at levels of 0,
1,3, 10, or 30 ppm, equal to 0, 2, 5, 16, or 26 mg/kg bw per day, for
30 days. At the end of treatment, some birds were killed and the
remainder were observed for four weeks for neurological signs of
poisoning. Food consumption was depressed in birds at 30 ppm, and the
average body weight and growth of hens at this dose was reduced.
Whole-blood cholinesterase activity was decreased after 30 days at
doses > 1 ppm, although no signs of cholinergic poisoning were
observed. There were no indications of delayed neurotoxicity, and
microscopic examination of brain, spinal cord, and sciatic nerve
(stained with haematoxylin and eosin) did not indicate delayed
neuropathy (Kimmerle, 1970; Spicer, 1970; JMPR, 1974).
Groups of 10 hens given an LD50 a dose of fenamiphos (5.0 mg/kg bw)
orally were observed for three weeks and then killed. No evidence of
delayed neurotoxicity was observed either clinically or
histologically, whereas signs were observed with tri- ortho-cresyl
phosphate (Kimmerle, 1971; Spicer, 1971; JMPR, 1974).
Fenamiphos (purity, 91.3%) in a 2% Cremophor solution was administered
twice by intubation to 30 Lohmann selected Leghorn hens at 25 mg/kg bw
per day at a 21-day interval. Five hens serving as positive controls
received tri- ortho-cresyl phosphate at 375 mg/kg bw per day. The
hens treated with fenamiphos received atropine intramuscularly at 100
mg/kg bw before treatment and subcutaneously at 30-50 mg/kg bw 7, 24,
or 30 or 48 h after treatment. The birds were observed for body
weight, clinical signs, forced motor coordination, and gross and
histopathological changes. Although statistically significant,
treatment-related weight loss (during week 1) and signs of severe
poisoning were seen, the birds showed no impairment of motor
coordination indicative of delayed neurotoxicity. Histological
examination of tissues from the peripheral and central nervous systems
showed no changes indicative of delayed neuropathy. Birds given
tri- ortho-cresyl phosphate, however, showed clinical signs of
delayed neurotoxicity (ataxia and paresis) two weeks after treatment
and were killed in moribund condition on day 17. The histopathological
changes in these birds were typical of neuropathy (Flucke & Kaliner,
1987).
(iii) Neurotoxicity
Rats
Groups of 12 Wistar (Hsd Win:WU) rats of each sex were given single
doses of technical-grade fenamiphos (purity, 95.2%) at 0.37, 1.52, or
2.31 mg/kg bw by garage. They were observed for mortality, clinical
signs, and body weight. Functional observational and motor or
locomotor activity tests were conducted on all animals before
treatment, within 30 min of treatment, and 7 and 14 days later.
Plasma, erythrocyte, and brain cholinesterase activity was measured in
additional satellite groups of six animals of each sex at each dose,
which were killed within 1 h of treatment. One-half of the animals in
the main group were perfused, and various neural and skeletal muscle
tissues, including the brain, spinal cord and spinal ganglia, eyes and
optic nerves, peripheral nerves, gastrocnemic muscle, and trigeminal
ganglia, were processed for histological examination.
In animals at the lowest dose, plasma cholinesterase activity was
statistically significantly inhibited in females (by 55%) and
nonsignificantly decreased in males (by 23%). Erythrocyte
acetylcholinesterase activity was significantly inhibited only in
males (by 24%), but this was not considered to be an adverse effect
because no corroborating clinical signs were seen in the functional
and motor activity tests. At the next highest dose, both plasma and
erythrocyte cholinesterase activity was significantly inhibited in
both males (by 64 and 70%, respectively) and females (by 77 and 51%,
respectively). Uncoordinated gait and muscle fasciculations were also
seen in males during the functional observational battery of tests. At
the highest dose, similar signs were seen, with decreased grip
strength and deaths among both males and females and decreased motor
activity in malesś Other behavioural or physiological changes seen in
animals of each sex in the functional observational battery of tests
included piloerection, nasal, oral, and lachrymal staining,
salivation, and decreased activity and rearing in the open field.
Brain acetylcholinesterase activity was unaffected at all doses. No
treatment-related histopathological changes were seen. The NOAEL was
0.37 mg/kg bw, the lowest dose tested, on the basis of uncoordinated
gait and muscle fasciculations in males at the next highest dose
(Dreist, 1995).
In a study of similar design, technical-grade fenamiphos (purity,
95.6%) was administered in the diet to groups of 12 Wistar (Hsd
Cpb:WU) rats of each sex for 13 weeks at dietary concentrations of 0,
1, 10, or 50 ppm, equal to 0, 0.06, 0.61, or 3.1 mg/kg bw per day in
males and 0.08, 0.8, or 4 mg/kg bw per day in females. The animals
were observed for deaths, clinical signs, body weight, and food and
water consumption. Functional observational and motor or locomotor
activity tests were conducted before treatment and in weeks 4, 8, and
13. Plasma and erythrocyte cholinestemse activity was measured in six
rats of each sex at each dose on week 4 and before terminal sacrifice
at week 15, and brain acetylcholinesterase activity only at terminal
sacrifice.
All females at the highest dose had muscle fasciculations during the
first three weeks of the study. Plasma and erythrocyte cholinesterase
activities were statistically significantly reduced in both males
(> 68%) and females (> 86%). Brain acetylcholinesterase activity
was also significantly reduced (by 12%) in females, but the author
considered that this was not biologically significant. At 10 ppm,
plasma cholinesterase activity was significantly reduced in both males
(by 30-39%) and females (by 71-77%; rho < 0.01) in weeks 4 and 15.
Erythrocyte acetylcholinesterase activity was inhibited by about 25%
in week 15 in males (rho < 0.05) and in weeks 4 and 15 in females
at 10 ppm. The lowest dose resulted in nonsignificant decreases (about
30%) in plasma cholinesterase activity in females in weeks 4 and 15.
Body weights, food and water consumption, brain weights, and gross and
histopathological appearance were all unaffected by treatment. The
author extrapolated the results back to a 20% level of inhibition of
plasma cholinesterase activity and estimated that the NOAEL in females
was 0.4 ppm. The overall NOAEL was 10 ppm, equal to 0.8 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase activity
in females at the highest dose (Dreist & Popp, 1995).
(iv) Potentiation
In male rats given fenamiphos orally in combination with disulfoton or
E 154, no potentiation of the acute toxicity was seen (Kimmerle,
1972c; JMPR, 1974).
The LD50 for fenamiphos (purity, 91.8%) administered orally to male
Wistar rats was 4.6 mg/kg bw, while that of carbofuran was 8.1 mg/kg
bw. When the two compounds were given concomitantly, essentially as a
2:1 ratio of carbofuran:fenamiphos, the LD50 was 6 mg/kg bw,
indicating that the toxicity of the combination was additive but not
synergistic (Mihail, 1980 JMPR, 1985).
Comments
In rats, fenamiphos was rapidly excreted, with over 96% of the
administered dose eliminated within 48 h. Excretion was primarily in
the urine, with less than 4% of the dose eliminated in the faeces. At
48 h, the levels of tissue residues were below the limit of
quantification, except following a high dose (3 mg/kg bw), when the
maximal tissue levels observed were 3.5-8.4 µg/kg in the liver,
1.6-2.1 µg/kg in the kidney, and 1.6-3.5 µg/kg in the skin. Fenamiphos
was completely metabolized in rats. Metabolites retaining
anticholinesterase activity, such as fenamiphos sulfoxide and
desisopropyl fenamiphos sulfoxide, were seen in variable but generally
low proportions (rarely greater than 3%). Most of the products were
dephosphorylated phenol, sulfoxide phenol, or sulfone phenol
metabolites and their corresponding sulfates.
Fenamiphos is extremely hazardous after single oral doses to rats,
mice, rabbits, cats, dogs, and chickens (LD50 values = 2 4-23 mg/kg
bw) and highly hazardous after dermal administration to rats and
rabbits (LD50 values, 75-230 mg/kg bw). It is moderately hazardous
after inhalation in rats and mice (LC50 values < 100 µg/L, 4 h).
WHO has classified fenamiphos as 'extremely hazardous' (WHO, 1996).
The sulfoxide, sulfone, and desisopropylated sulfone metabolites of
fenamiphos are similarly toxic to rats after oral administration
(LD50 values = 1.4-4.1 mg/kg bw). The sulfoxide and sulfone phenol
metabolites are only slightly toxic to rats after oral administration,
with LD50 values ranging from 1200 to 1900 mg/kg bw.
Fenamiphos inhibited plasma cholinesterase more effectively than
erythrocyte acetylcholinesterase, both in vitro and in vivo.
Fenamiphos sulfoxide, fenamiphos sulfone, desisopropyl fenamiphos,
desisopropyl fenamiphos sulfoxide, and desisopropyl fenamiphos sulfone
inhibited plasma and erythrocyte cholinesterase in vitro more
effectively than fenamiphos itself.
In evaluating the following studies, inhibition of erythrocyte
acetylcholinesterase activity was not used as an indicator of adverse
effects in the nervous system when information on brain
acetylcholinesterase activity was also available. In the absence of
this information, NOAELs were determined on the basis of inhibition of
erythrocyte acetylcholinesterase (of < 20%). Statistical
significance was used as a criterion for considering depression of
brain acetylcholinesterase activity to be adverse.
In a three-week study in which rats were exposed by inhalation to
atmospheres containing fenamiphos at 0, 0.03, 0.25, or 3.5 µg/L for 6
h per day, five days per week, the only finding was inhibition of
plasma cholinesterase at the highest dose. Erythrocyte and brain
acetylcholinesterase were unaffected. The no-observed-adverse-effect
concentration (NOAEC) was 3.5 µg/L.
In a three-month study in rats, fenamiphos given at dietary
concentrations of 0, 0.37, 0.57, or 0.91 ppm inhibited plasma
cholinesterase activity only at the highest dose. No treatment-related
changes in erythrocyte or brain acetylcholinesterase activity were
seen at any dose. In a second study of short-term toxicity, rats were
fed diets containing 0, 4, 8, 16, or 32 ppm fenamiphos for three
months. Erythrocyte acetylcholinesterase activity was inhibited at
doses of 16 ppm (equivalent to 0.8 mg/kg bw per day) and above. This
was considered an adverse effect as brain acetylcholinesterase was not
measured in this study. The overall NOAEL was 8 ppm, equivalent to 0.4
mg/kg bw per day.
Rabbits received fenamiphos by dermal application at doses of 0, 0.5,
2.5, or 10 mg/kg bw per day for three weeks (6 h per day, five days
per week). Body-weight gain was slightly reduced in animals of each
sex at 10 mg/kg bw per day. In females, reductions in cholinesterase
activity in the brain (by 20%) and plasma were noted at 2.5 mg/kg bw
per day and above. Erythrocyte acetylcholinesterase activity, however,
was affected only at 10 mg/kg bw per day. In males, the only findings
were decreased plasma and erythrocyte cholinesterase activity at 10
mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day.
In a series of studies, dogs were fed diets containing 0, 0.5, 0.6, 1,
1.7, 2, 3, 5, 6, 10, 12, or 18 ppm fenamiphos for periods ranging from
three months to two years. In dogs treated at 18 ppm (equivalent to
0.45 mg/kg bw per day) for three months, muscle tremors were seen. In
dogs treated at 12 ppm (equal to 0.31 mg/kg bw per day) for one year,
brain acetylcholinesterase activity was inhibited in females (by 17%),
and males showed slight anaemia. Erythrocyte acetylcholinesterase
activity was inhibited at doses of 3 ppm (equal to 0.083 mg/kg bw per
day) and above for one year. Plasma cholinesterase activity was
inhibited at doses of 1.7 ppm (equivalent to 0.042 mg/kg bw per day)
and above in a three-month study, No other parameters were affected.
Since no information on brain acetylcholinesterase activity was
available at doses between 3 and 12 ppm, the Meeting considered 3 ppm
(equal to 0.083 mg/kg bw per day) to be the overall NOAEL in dogs.
In mice fed diets containing 0, 2, 10, or 50 ppm fenamiphos for 20
months, there were marginal decreases in survival and body-weight gain
at 50 ppm (equal to 7.4 mg/kg bw per day). The relative ovarian and
spleen weights were reduced at 10 ppm (equal to 1.4 mg/kg bw per day)
and above. There were no non-neoplastic changes that could be
attributed to treatment, and fenamiphos was not carcinogenic at any
dose. Cholinesterase activity was not measured. The NOAEL was 2 ppm,
equal to 0.3 mg/kg bw per day.
In rats fed diets containing 0, 3, 10, or 30 ppm fenamiphos for two
years, the only treatment-related effects were inhibition of
erythrocyte acetylcholinesterase activity throughout the study and
behavioural changes during the first six weeks of the study in animals
at 30 ppm. Brain acetylcholinesterase activity was not measured. The
NOAEL was 10 ppm, equal to 0.56 mg/kg bw per day.
Rats were fed diets containing 0, 1.7, 7.8, or 37 ppm fenamiphos for
two years. At 37 ppm, equal to 2.5 mg/kg bw per day, body-weight gain
in both males and females was decreased. Erythrocyte
acetylcholinesterase activity was inhibited at 7.8 ppm (equal to 0.46
mg/kg bw per day) and above. Brain acetylcholinesterase activity was
inhibited only at 37 ppm; inhibition was 25% in animals of each sex
killed after one year, and 14% in males at termination of the study.
Animals of each sex at 37 ppm also had an increased frequency of
non-neoplastic inflammatory lesions of the nasal, laryngeal, and lung
tissues and increased relative weights of the brain, heart, and lungs.
Fenamiphos was not carcinogenic at any dose. The NOAEL was 7.8 ppm,
equal to 0.46 mg/kg bw per day.
Fenamiphos was adequately tested in a battery of tests for
genotoxicity. It was found to be mildly clastogenic at cytotoxic doses
in vitro but not in vivo. It did not cause reverse or forward
mutation, unscheduled DNA synthesis, or sister chromatid exchange
in vitro. The Meeting concluded that fenamiphos is not genotoxic.
In a two-generation study of reproductive toxicity, rats were treated
with 0, 2.5, 10, or 40 ppm fenamiphos. Parental toxicity was
characterized by reduced weight gain in F0 and F1 dams at 40 ppm
(equal to 2.8 mg/kg bw per day) during lactation, and in F1 males at
10 ppm (equal to 0.64 mg/kg bw per day) and above before mating.
Pathological changes in the salivary gland were seen in F0 males and
females at 40 ppm. Erythrocyte acetylcholinesterase activity was
consistently inhibited at 10 ppm and above in females but only at 40
ppm in males. Brain acetylcholinesterase activity was inhibited at the
highest dose in adult F0 and F1 females (by 21-29%) and in F1 males
(by 6%) but not in pups of either sex. In pups at 40 ppm, erythrocyte
acetylcholinesterase activity was inhibited only on day 21 of
lactation. The only reproductive effect was decreased weight gain of
F1 and F2 pups at 40 ppm, beginning on day 7 of lactation. The NOAEL
for systemic toxicity was 2.5 ppm, equal to 0.17 mg/kg bw per day. The
NOAEL for reproductive toxicity was 10 ppm, equal to 0.64 mg/kg bw per
day.
In a study of developmental toxicity, mated rats were treated with 0,
0.3, 1, or 3 mg/kg bw per day on days 6-15 of gestation. Maternal
toxicity was seen at the highest dose, characterized by mortality,
tremors, and reduced weight gain. The fetuses were not affected at any
dose. Cholinesterase activity was not measured in this study. The
NOAELs were 1 mg/kg bw per day for maternal toxicity and 3 mg/kg bw
per day for developmental toxicity.
Fenamiphos was also administered to mated rats at doses of 0, 0.25,
0.85, or 3 mg/kg bw per day on days 6-15 of gestation. The highest
dose resulted in maternal deaths, tremors, salivation, lachrymation,
urine staining, and hypoactivity. Body-weight gain and food
consumption were also significantly reduced. Erythrocyte
acetylcholinesterase activity was reduced at this dose, but the
changes in brain acetylcholinesterase activity were not statistically
significant or dose-related. The fetuses were unaffected at 3 mg/kg bw
per day. The NOAELs were 0.85 mg/kg bw per day for maternal toxicity
and 3 mg/kg bw per day for developmental toxicity.
In a study of developmental toxicity in rabbits, animals received 0,
0.1, 0.3, or 1 mg/kg bw per day on days 6-18 of gestation. At 0.3
mg/kg bw per day and above, fenamiphos was maternally toxic, resulting
in decreased body-weight gain, bloody nasal discharge, and white
ocular discharge. Fetotoxicity, characterized by chain fusion of the
sternebrae, was seen only at 1 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.1 mg/kg bw per day, and that for developmental
toxicity was 0.3 mg/kg bw per day. Cholinesterase activity was not
measured in this study.
In a second study, mated rabbits were treated with 0, 0.1, 0.5, or 2.5
mg/kg bw per day on days 6-18 of gestation. Clear maternal toxicity
was seen at the highest dose, which included mortality, salivation,
dyspnoea, ataxia, diarrhoea, and decreased weight gain and food
consumption during treatment. Although some questions remain about the
doses that were actually administered (because of uncertain
homogeneity), no embryotoxic or teratogenic effects were seen at the
maternally toxic dose of 2.5 mg/kg bw per day.
Fenamiphos was minimally irritating to rabbit skin and moderately
irritating to rabbit eyes and was a mild skin sensitizer in the
guinea-pig.
A single dose of 25 mg/kg bw fenamiphos had no effect on neuropathy
target esterase activity in the brains or spinal cords of hens, under
atropine protection. Fenamiphos did not induce delayed neuropathy in
three studies in hens when tested at doses of 0, 2, 5, 16, or 26 mg/kg
bw per day for 30 days or when given once at doses of 0 or 25 mg/kg
bw.
In a study of acute neurotoxicity, rats were given single doses of 0,
0.37, 1.5, or 2.3 mg/kg bw fenamiphos by gavage. Erythrocyte
acetylcholinesterase activity was inhibited at the lowest dose tested
in males only, but in both males and females at higher doses. At 1.5
mg/kg bw, males showed uncoordinated gait and muscle fasciculation. At
the highest dose, decreased motor activity was also seen in males, and
clinical signs, decreased grip strength, and deaths occurred in rats
of each sex. There was no effect on brain acetylcholinesterase
activity, at any dose. The NOAEL was 0.37 mg/kg bw per day. In a
further study, rats were fed diets containing 0, 1, 10, or 50 ppm
fenamiphos for 13 weeks. At 10 ppm and above, erythrocyte
acetylcholinesterase activity was inhibited in animals of each sex. At
the highest dose, brain acetylcholinesterase activity was inhibited
(by 12%) in females only. A battery of functional observational and
motor activity tests revealed no treatment-related effects. The NOAEL
was 10 ppm, equal to 0.61 mg/kg bw per day.
An ADI of 0-0.0008 mg/kg bw was established on the basis of an overall
NOAEL of 0.083 mg/kg bw per day in the dog, and a safety factor of
100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2 ppm in the diet, equal to 0.3 mg/kg bw per day
(20-month study of toxicity and carcinogenicity)
Rat: 0.37 mg/kg (single doses, study of neurotoxicity) 10
ppm, equal to 0.61 mg/kg bw per day (three-month study
of neurotoxicity)
2.5 ppm, equal to 0.17 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
10 ppm, equal to 0.64 mg/kg bw per day (study of
reproductive toxicity)
0.85 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity in a study
of developmental toxicity)
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to fenamiphos
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral neurotoxicity, rat NOAEL = 0.37 mg/kg bw per day: effects observed
(1-7 days) during battery of functional observational tests
Oral toxicity, rat (fasted) LD50 = 2.4-6 mg/kg bw
Inhalation toxicity, 4 h, rat LC50 = 91-100 µg/L
Inhalation toxicity, 5 days, rat NOAEL = 4 µg/L
Dermal toxicity, rat LD50 = 72-92 mg/kg bw
Dermal irritation, rabbit Minimally irritating
Ocular irritation, rabbit Moderately irritating
Dermal sensitization, guinea-pig Mildly sensitizing
Medium-term Repeated inhalation toxicity, 3 weeks, rat NOAEL = 3.5 µg/L (highest dose tested)
(1-26 weeks) Repeated dermal toxicity, 3 weeks, rabbit NOAEL = 0.5 mg/kg bw per day: inhibition of brain
acetylcholinesterase activity
Repeated oral, reproductive toxicity, rat NOAEL = 0.17 mg/kg bw per day: parental toxicity
NOAEL = 0.64 mg/kg bw per day: reproductive
toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.1 mg/kg bw per day: maternal toxicity
NOAEL = 0.3 mg/kg bw per day: developmental
toxicity
Long-term Repeated oral, 1 - 2 years, dog NOAEL = 0.083 mg/kg bw per day: inhibition of
(> 1 year) acetylcholinesterase activity, anaemia
7.8 ppm, equal to 0.46 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
0.3 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 3 ppm in the diet, equal to 0.083 mg/kg bw per day
(overall assessment)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Estimate of acute reference dose
The available data did not permit the Meeting to establish an acute
reference dose different from the ADI (0-0.0008 mg/kg bw). Although
the results of a study of neurotoxicity in rats given single doses was
available, the dog was found to be the more sensitive species.
Information on acute effects in dogs may allow the establishment of an
acute reference dose in the future.
Studies that would provide information useful for continued
evaluation of the compound
1. Effects of single doses in dogs (with appropriate evaluation of
functional changes in the cholinergic nervous system, including
brain acetylcholinesterase activity).
2. Observations in humans
References
Becker, H. (1986) Embryotoxicity (including teratogenicity) study with
SRA 3886 in the rabbit. Unpublished report No. R 3917 (project No.
065261) from Research & Consulting Co., Itingen, Switzerland.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Mueller, E., Luetkemeir, H. & Sachsse, K. (1986)
Dose-finding embryotoxicity (including teratogenicity) study with SRA
3886 in the rabbit. Unpublished report No. R3693 (project No. 065250)
from Research & Consulting Co., Itingen, Switzerland. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982) Sister chromatid
exchange in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environ. Mutag., 4, 621624. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cherry, C. & Newman, A. (1973) Pathology report of Bayer 68138.
Chronic toxicological studies in rats. Unpublished report (addendum to
report No. 3539) from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Cherry, C., Urwin, C. & Newman, A. (1972) Pathology report of BAY
68138. Rat breeding study. Unpublished report (addendum to report No.
3424) from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R., Troup, C.M. & Hartnagel, R.E., Jr (1989) Teratology
study in the rat with Nemacur technical. Unpublished report No. 1145
(study no. MTD0108) from Toxicology Department, Miles Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Coulston, F. & Wills, J.H. (1974) Summary of research by the Institute
of Comparative and Human Toxicology, Albany Medical College, on
phenamiphos. Report dated October 1974. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C. & Anderson, R. (1971) The skin and eye irritating
properties of BAY 68138 technical to rabbits. Unpublished report No.
29988 from Chemagro Division of Baychem Corp. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Crawford, C. & Anderson, R. (1972) The acute dermal toxicity of
Nemacur technical to rabbits. Unpublished report No. 34216 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1973) Comparative oral toxicity in
rats of several impurities and a technical compound of Nemacur(R)
with analytical grade Nemacur(R). Unpublished report No. 34446 from
Chemagro Division of Baychem Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974a) The acute oral toxicity of two
Nemacur(R) phenolic metabolites and MTMC to male and female rats.
Unpublished report No. 39700 from Chemagro Division of Baychem Corp.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Crawford, C.R. & Anderson, R.H. (1974b) The acute oral toxicity of
Nemacur(R)-sulfone and Nemacur(R)-sulfoxide to rats. Unpublished
report No. 40215 from Chemagro Division of Baychem Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Curren, D. (1988) Unscheduled DNA synthesis in rat primary
hepatocytes. Unpublished report No. 991 (study no. T5724.380) from
Microbiological Associates Inc., Rockville, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Dreist, M. (1995) SPA 3886 (common name:Fenamiphos) acute oral
neurotoxicity screening study in Wistar rats. Unpublished report No.
24408 (study No. T6058166) from the Institute of Toxicology
Agrochemicals, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Dreist, M. & Popp, A. (1995) SRA 3886 (common name: Fenamiphos)
subchronic neurotoxicity screening study in Wistar rats (thirteen-week
administration in the diet). Unpublished report No. 24948 (study no.
T9058277) from Institute of Toxicology Agrochemicals, Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
DuBois, K. & Flynn, M. (1968) The subacute parenteral toxicity of BAY
68138 to rats. Unpublished report No. 22178 from Toxicity Laboratory,
University of Chicago, Chicago, IL, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
DuBois, K., Flynn, M. & Kinoshita, F. (1967) The acute toxicity and
anticholinesterase action of Bayer 68138. Unpublished report No. 20810
from Toxicity Laboratory, University of Chicago, Chicago, IL, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ecker, W., Weber, H. & Brauner, A. (1989) [Phenyl-1-14C]Nemacur:
General metabolism study in the rat. Unpublished report No. PF3175
(study no. M182 0189-9) from Bayer AG, Pflanzenschutz-Zentrum Monheim,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Eigenberg, D.A. (1991) A two generation reproduction study in rats
using Fenamiphos (Nemacur(R)). Unpublished report No. 5762 (study
no. 88-671-BC) from Mobay Corp, Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) Determination of acute toxicity. Unpublished report
dated 18 July 1980 from Bayer AG, Institute of Toxicology, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Eben, A. (1988) SPA 3886 Technical (common name:
Fenamiphos): Study of the effect on the neurotoxic esterase (NTE)
following oral administration to hens. Unpublished report No. 17388
from Fachbereich Toxikologie, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. & Kaliner, G. (1987) Delayed neurotoxicity studies on hens
following acute oral administration. Unpublished report No. 16187
(study no. T 1020919) from Fachbereich Toxikologie, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Gronberg, R.R. (1969) The metabolic fate of ethyl-4-(methylthio)-m-
tolyl isopropylphosphoramidate (BAY 68138), ethyl-4-(methylsulfinyl)-
m-tolyl isopropylphosphoramidate (BAY 68138 sulfoxide) and ethyl-4-
(methylsulfonyl)-m-tolyl-isopropylphosphoramidate (BAY 68138 sulfone)
in white rats. Unpublished report No. 26759 from Chemagro Division of
Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1982) Technical fenamiphos (Nemacur(R)) oncogenicity
study in mice. Unpublished report No. 241 (study No. 78CCM02) from
Stanley Research Center, Mobay Chemical Corp, Stilwell, KS, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. ( 1983 ) Ninety-day cholinesterase study on dogs with
fenamiphos in diet. Unpublished report No. 444 (study No. 83-174-01)
from Environmental Health Research, Mobay Chemical Corp, Stilwell, KS,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1986a) Ninety-day cholinesterase study on rats with
technical fenamiphos (Nemacur) in diet. Unpublished report No. 717
(study No. 83-171-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hayes, R.H. (1986b) Combined chronic toxicity/oncogenicity study of
technical fenamiphos (Nemacur) with rats. Unpublished report No. 721
(study No. 83-271-01) from Corporate Toxicology Dept, Mobay Chemical
Co., Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K G (1981) Determination of acute toxicity (LD50 Unpublished
report dated 18 August 1981 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K G (1984) Determination of acute toxicity (LD) Unpublished
report dated 14 February 1984 from Bayer AG/Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1979) Salmonella/microsome test for detection of
point-mutagenic effects. Unpublished report No. 8730 (study no.
Nemacur/003) from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980a) Micronucleus test on mouse to evaluate Nemacur
technical for potential mutagenic effects. Unpublished report No. 8805
(study no. SRA 3886/002) from Bayer AG Institute of Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1980b) SRA 3886/dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 8838 (study no. SRA
3886/001) from Institute of Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) SRA 3886: Salmonella/microsome test to evaluate
for potential point mutation. Unpublished report No. 13365 (study no.
T2017617) from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985b) Addendum to report SRA 3886: Salmonella/microsome
test to evaluate for potential point mutation. Unpublished report No.
13365A (study no. T2017617) from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B. (1987) SRA 3886: Cytogenetic study of human lymphocyte
cultures in vitro to test for chromosome damage. Unpublished report
No. 15406, (study No. T3022874) from Bayer AG, Fachbereich Toxicology,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1988) SRA 3886: In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report No. 16690, (study No. T1025572) from Bayer AG,
Fachbereich Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Greufe (1993) Response to US-EPA review: Chronic feeding
toxicity study with technical grade Fenamiphos (Nemacur(R)) with
dogs. Unpublished report dated 12 July 1993 (supplement to Mobay Corp.
study No. 88-274-BB) from Miles Inc., Stilwell, KS, USA. Submitted to
WHO by Bayer AG. Leverkusen, Germany.
Jones, R.D. & Loney, M.L. (1993) A subchronic feeding toxicity study
with technical grade Fenamiphos (Nemacur(R)) in dogs. Unpublished
report No. 91 - 176-KP (supplement to Mobay Corp. study No. 88-274-BB)
from Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kato, M.C. (1984a) Primary dermal irritation study of fenamiphos
technical in rabbitś Unpublished report No. B-561 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-561). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Kato, M.C. (1984b) Primary eye irritation study of fenamiphos
technical in rabbitś Unpublished report No.B-562 from Bozo Research
Center Inc., Tokyo, Japan (study No. B-562). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Khasawinah, A.M. & Flint, D.R. (1972) Metabolism of Nemacur(R)
[ethyl-4-(methylthio)-m-tolyl isopropylphosphoramidate] by rat liver
microsomes in vitro. Unpublished report No. 34217 from Chemagro
Division of Baychem Corp. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kimmerle, G. (1970) Subchronic neurotoxicity studies on hens.
Unpublished report No. 1831 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1971) Acute neurotoxicity studies on hens. Unpublished
report No. 2829 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1972a) Acute toxicity of SRA-3886 in combination with
S-276 and with E-154 to rats. Unpublished report No. 3438 from
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972b) Acute inhalation toxicity study with Nemacur(R)
active ingredient on rats. Unpublished report No. 3559 from the
Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kimmerle, G. (1972c) Antidotal experiments on rats. Unpublished report
No. 3560 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. & Solmecke, B. ( 1971) BAY 68138 toxicological studies.
Unpublished report No. 2767 from Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Knaak, J.B., Ackerman, K.Y., Fredrickson, A.S. & Lee, P. (1981)
Reentry research: Dermal dose red cell cholinesterase-response curves
for fenamiphos, fenamiphos sulfoxide and sulfone. Unpublished report
No. 69689 from California Department of Food and Agriculture.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F. ( 1988 ) Study for acute oral toxicity in rats.
Unpublished report No. 16744 (study No. T0027669) from Bayer AG,
Fachbereich Toxicologies, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Lamb, D.W. & Landes, A.M. (1978) In vitro inhibition of cholinesterase
by (R)Nemacur and Nemacur metabolites. Unpublished report No. 66068
(reference 77-060) from Mobay Chemical Corp., Chemagro Agricultural
Division, Kansas City, MO, US A. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1975) The acute oral toxicity of Nemacur
technical, desisopropyl Nemacur sulfoxide and desethyl Nemacur.
Unpublished report No. 44531 (reference 74-175) from Chemagro
Agricultural Division, Mobay Chemical Corp. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Matzkanin, C.S. (1977) The acute oral toxicity of Nemacur
desisopropyl to rats. Unpublished report No. 51597 (reference 75-144)
from Chemagro Agricultural Division, Mobay Chemical Corp. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1968a) Subchronic toxicological studies on rats.
Unpublished report No. 745 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Löser, E. (1968b) Subchronic toxicological studies on dogs.
Unpublished report No. 837 from the Institute for Toxicology, Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser E (1969) Subchronic toxicological studies on dogs (3 month
feeding test). Unpublished report No. 1655 from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1970) Subchronic toxicological studies on dogs. Unpublished
report No. 2008 from the Institute for Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on dogs (2 year
feeding experiment). Unpublished report from the Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Löser, E. (1972b) Chronic toxicological studies on rats (2 year
feeding experiment). Unpublished report No. 3539 from the Institute
for Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Löser, E. (1972c) Generation studies on rats. Unpublished report No.
3424 from the Institute for Toxicology, Bayer AG, Wuppertal-Elberfeld,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Löser, E. & Kimmerle, G. (1971) Acute and subchronic toxicity of
Nemacur(R), active ingredient. Pflanzenschutz-Nachrichten Bayer,
24(1), 69-113. Submitted to WHO by Bayer AG, Leverkusen, Germany.
MacKenzie, K.M. (1982) Teratology study with Nemacur in rabbits.
Unpublished report No. 308 (study No. 81165) from Hazelton Raltech,
Inc., Madison, WI, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mavournin, K.H., Blakey, D.H., Cimino, M.C., Salamone, M.F. & Heddle,
J.A. (1990) The in vivo micronucleus assay in mammalian bone marrow
and peripheral blood. A report of the US Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 239, 29-80.
Mawdesley-Thomas, L. & Urwin, C. (1970a) Pathology report of BAY
68138. Subchronic toxicological studies in rats. Unpublished report
(addendum to report No, 745) from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mawdesley-Thomas, L. & Urwin, C. (1970b) Pathology report of BAY
68138. Subchronic toxicity tests in dogs. Unpublished report (addendum
to report No. 1655) from the Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail F. (1980) SRA 3885 and Curaterr(R): Evaluation for acute
combination toxicity. Unpublished report No. 9294 from Institute of
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Mihail, F. & Schilde, B. (1980) SPA 3886 (active ingredient of
Nemacur(R)) subacute dermal toxicity study on rabbits. Unpublished
report No, 9297 from Institute of Toxicology, Bayer AG,
Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Rieth, J.P., Moore, K.D. & Elcock, L.E. (1991 ) Chronic feeding
toxicity study of technical grade Fenamiphos (Nemacur(R)) with dogs.
Unpublished report No. 6243 (study No. 88-274-BB) from Mobay Corp.,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1981) SRA 3886 (Nemacur): Evaluation for embryotoxicity
and teratogenic effects in orally dosed rats. Unpublished report No.
9785 from Institute for Toxicology, Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1970) Pathology report of BAY 68138. Subchronic
neurotoxicity tests on hens. Unpublished report (addendum to report
No. 1831 ) from the Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Spicer, J. (1971) Pathology report of BAY 68138. Hen study.
Unpublished report (addendum to report No. 2829) from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Stropp, G. (1995) Study for the skin sensitization effect in guinea
pigs (guinea pig maximization test according to Magnusson and
Kligman). Unpublished report No. 24341 (study No. T9058358) from Bayer
AG, Fachbereich Toxikologie, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972a) Pathology report of BAY
68138. Subchronic toxicity study in dogs. Unpublished report (addendum
to report No. 2008) from Huntingdon Research Centre, Huntingdon,
United Kingdom. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thomson, C., Newman, A. & Urwin, C. (1972b) Pathology report of BAY
68138. Chronic toxicity study in dogs (administration in diet for 2
years). Unpublished report (addendum to report No. 3561) from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted m
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974a) Nemacur(R) sulfone, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974b) Nemacur(R) sulfoxide, Acute toxicity in rats.
Unpublished report dated 11 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974c) 3-Methyl-4-methylmercaptophenol, Acute toxicity in
rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974d) 3-Methyl-4-methane-sulfonylphenol, acute toxicity
in rats. Unpublished report dated 25 February 1974 from Institute for
Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1974e) 4-Methylmercapto-m-cresol, acute toxicity in rats.
Unpublished report dated 29 April 1974 from Institute for Toxicology,
Bayer AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979a) SPA 3885 (Nemacur(R) active ingredient)/acute
inhalation toxicity studies. Unpublished report No. 8210 from Bayer
AG, Wuppertal-Elberfeld, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1979b) Nemacur(R) active ingredient (SRA 3886)/subacute
inhalational toxicity studies. Unpublished report No. 8669 from
Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Waggoner, T.B. (1972) Metabolism of Nemacur [ethyl 4-(methylthio)-N-
tolyl isopropylphosphoramidate] and identification of two metabolites
in plants. J. Agric. Food Chem., 20, 157-160. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Wargo, J.P. (1978) The effect of feeding Nemacur(R) sulfoxide to
dairy cattle. Unpublished report No. 66212 from Chemical Agricultural
Division, Mobay Chemical Corp. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1988) [Phenyl-1-14C] Nemacur: Whole-body autoradiographic
distribution of the radioactivity in the rat. Unpublished report No.
PF3055 (study No. M181 0188-7) from Bayer AG Agrochemicals,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yang, L.L., Putnam, D.L. & Conklin, P.M. (1984) CHO/HGPRT mutation
assay in the presence and absence of exogenous metabolic activation --
Nemacur(R). Unpublished report No. 620 (study No. T2600.332) from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
