
AMINOCARB JMPR 1978
IDENTITY
Chemical name
4-dimethylamino-m-tolyl methylcarbamate
Structural formula
CH 3 -NH-C00
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biodegradation
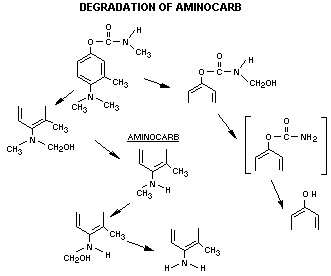
A review of the biodegradation of aminocarb has suggested that
the data on the in vivo metabolic fate has not been fully
investigated (Kuhr and Dorough, 1976). The metabolic fate of aminocarb
in animals, plants and insects has been investigated and based on
in vitro data from an extensive base and some available in vivo
studies, the following sequence of metabolism can be proposed. (This
is based on data from Krishna and Casida, 1966; Oonnithan and Casida,
1966; 1968; and Strother, 1970; 1972).
 TOXICOLOGICAL EVALUATION
The data available was insufficient and no acceptable daily
intake for man has been established.
RESIDUES IN FOOD AND THEIR EVALUATION
The limited information which was available is summarized in the
following appraisal.
APPRAISAL
Aminocarb is a non-systemic carbamate insecticide used mainly
against many lepidopterous larvae and other biting insects. It also
has some acaricidal and molluscicidal activity.
Aminocarb is available as 50% and 75% water-soluble powders and
is applied as 0.075 - 1.0%, solutions to fruits and vegetables. Use in
forestry against the budworm is becoming important and could become
the main use for aminocarb in the future.
Surface residues of aminocarb are readily changed to less
volatile products by photolyt: modification of the 4-dimethylamino
group. In the bean plant, the major metabolic product
4-methylamino-m-tolyl methylcarbamate. The other degradation
products so far identified are 4-formamido-m-tolyl methylcarbamate,
4-methylformamido-m-tolyl methylcarbamate and 4-amido-m-tolyl
methylcarbamate. Glycosides are subsequently formed. Horseradish
peroxidase attacked aminocarb quite rapidly but the tyrosinase system
did not degrade it to any extent.
Aminocarb is not persistent in soil.
Two methods of analysis, both apparently suitable for regulatory
purposes, are available. A GLC procedure has been described for
aminocarb in forest foliage and soil using the nitrogen-specific
electrical conductivity detector. The procedure still has to be
validated for fruit and vegetables. High-pressure liquid
chromatography with UV detection has also been applied to the analysis
of cabbage, corn, potatoes and wheat with satisfactory detection
limits and reproducibility.
EVALUATION
The data available were insufficient to establish an ADI or
residue limits.
FURTHER WORK OR INFORMATION
Required (before an ADI or residue limits can be established)
1. Further data on toxicology and the occurrence of residues.
Desirable
1. Data from countries on use patterns and on residues from
supervised trials on which to base residue limits.
2. Studies on the fate of residues in animals.
REFERENCES
Kuhr, R.J. and H.W. Dorough Carbamate Insecticides: Chemistry,
(1976) Biochemistry and Toxicology. CRC Press, Cleveland,
Ohio, pg. 175-177.
Krishna, J.G. and J.E. Casida, Fate in Rats of the Radiocarbon from
(1966) Ten Variously Labelled Methyl and Dimethycarbamate-14C
Insecticide Chemicals and Their Hydrolysis Products.
J. Agr. Food Chem. 14:98.
Oonnithan, E.S. and J.E. Casida Metabolites of Methyl and
(1966) Dimethylcarbamate Insecticide Chemicals as Formed by
Rat Liver Microsomes. Bull. Env. Contam. Toxicol.
1:59.
Oonnithan, E.S. and J.E. Casida Oxidation of Methyl and
(1968) Dimethylcarbamate Insecticide Chemicals by Microsomal
Enzymes and Anticholinesterase Activity of the
Metabolites. J. Agr. Food Chem. 16:28.
Strother, A. Comparative Metabolism of Selected N-Methyl Carbamates
(1970) by Human and Rat Liver Fractions. Biochem. Pharmacol.
19:2525.
Strother, A. In Vitro Metabolism of Methylcarbamate Insecticides by
Human and Rat Liver Fractions. Toxicol. Appl.
Pharmacol. 21:112.
TOXICOLOGICAL EVALUATION
The data available was insufficient and no acceptable daily
intake for man has been established.
RESIDUES IN FOOD AND THEIR EVALUATION
The limited information which was available is summarized in the
following appraisal.
APPRAISAL
Aminocarb is a non-systemic carbamate insecticide used mainly
against many lepidopterous larvae and other biting insects. It also
has some acaricidal and molluscicidal activity.
Aminocarb is available as 50% and 75% water-soluble powders and
is applied as 0.075 - 1.0%, solutions to fruits and vegetables. Use in
forestry against the budworm is becoming important and could become
the main use for aminocarb in the future.
Surface residues of aminocarb are readily changed to less
volatile products by photolyt: modification of the 4-dimethylamino
group. In the bean plant, the major metabolic product
4-methylamino-m-tolyl methylcarbamate. The other degradation
products so far identified are 4-formamido-m-tolyl methylcarbamate,
4-methylformamido-m-tolyl methylcarbamate and 4-amido-m-tolyl
methylcarbamate. Glycosides are subsequently formed. Horseradish
peroxidase attacked aminocarb quite rapidly but the tyrosinase system
did not degrade it to any extent.
Aminocarb is not persistent in soil.
Two methods of analysis, both apparently suitable for regulatory
purposes, are available. A GLC procedure has been described for
aminocarb in forest foliage and soil using the nitrogen-specific
electrical conductivity detector. The procedure still has to be
validated for fruit and vegetables. High-pressure liquid
chromatography with UV detection has also been applied to the analysis
of cabbage, corn, potatoes and wheat with satisfactory detection
limits and reproducibility.
EVALUATION
The data available were insufficient to establish an ADI or
residue limits.
FURTHER WORK OR INFORMATION
Required (before an ADI or residue limits can be established)
1. Further data on toxicology and the occurrence of residues.
Desirable
1. Data from countries on use patterns and on residues from
supervised trials on which to base residue limits.
2. Studies on the fate of residues in animals.
REFERENCES
Kuhr, R.J. and H.W. Dorough Carbamate Insecticides: Chemistry,
(1976) Biochemistry and Toxicology. CRC Press, Cleveland,
Ohio, pg. 175-177.
Krishna, J.G. and J.E. Casida, Fate in Rats of the Radiocarbon from
(1966) Ten Variously Labelled Methyl and Dimethycarbamate-14C
Insecticide Chemicals and Their Hydrolysis Products.
J. Agr. Food Chem. 14:98.
Oonnithan, E.S. and J.E. Casida Metabolites of Methyl and
(1966) Dimethylcarbamate Insecticide Chemicals as Formed by
Rat Liver Microsomes. Bull. Env. Contam. Toxicol.
1:59.
Oonnithan, E.S. and J.E. Casida Oxidation of Methyl and
(1968) Dimethylcarbamate Insecticide Chemicals by Microsomal
Enzymes and Anticholinesterase Activity of the
Metabolites. J. Agr. Food Chem. 16:28.
Strother, A. Comparative Metabolism of Selected N-Methyl Carbamates
(1970) by Human and Rat Liver Fractions. Biochem. Pharmacol.
19:2525.
Strother, A. In Vitro Metabolism of Methylcarbamate Insecticides by
Human and Rat Liver Fractions. Toxicol. Appl.
Pharmacol. 21:112.
 TOXICOLOGICAL EVALUATION
The data available was insufficient and no acceptable daily
intake for man has been established.
RESIDUES IN FOOD AND THEIR EVALUATION
The limited information which was available is summarized in the
following appraisal.
APPRAISAL
Aminocarb is a non-systemic carbamate insecticide used mainly
against many lepidopterous larvae and other biting insects. It also
has some acaricidal and molluscicidal activity.
Aminocarb is available as 50% and 75% water-soluble powders and
is applied as 0.075 - 1.0%, solutions to fruits and vegetables. Use in
forestry against the budworm is becoming important and could become
the main use for aminocarb in the future.
Surface residues of aminocarb are readily changed to less
volatile products by photolyt: modification of the 4-dimethylamino
group. In the bean plant, the major metabolic product
4-methylamino-m-tolyl methylcarbamate. The other degradation
products so far identified are 4-formamido-m-tolyl methylcarbamate,
4-methylformamido-m-tolyl methylcarbamate and 4-amido-m-tolyl
methylcarbamate. Glycosides are subsequently formed. Horseradish
peroxidase attacked aminocarb quite rapidly but the tyrosinase system
did not degrade it to any extent.
Aminocarb is not persistent in soil.
Two methods of analysis, both apparently suitable for regulatory
purposes, are available. A GLC procedure has been described for
aminocarb in forest foliage and soil using the nitrogen-specific
electrical conductivity detector. The procedure still has to be
validated for fruit and vegetables. High-pressure liquid
chromatography with UV detection has also been applied to the analysis
of cabbage, corn, potatoes and wheat with satisfactory detection
limits and reproducibility.
EVALUATION
The data available were insufficient to establish an ADI or
residue limits.
FURTHER WORK OR INFORMATION
Required (before an ADI or residue limits can be established)
1. Further data on toxicology and the occurrence of residues.
Desirable
1. Data from countries on use patterns and on residues from
supervised trials on which to base residue limits.
2. Studies on the fate of residues in animals.
REFERENCES
Kuhr, R.J. and H.W. Dorough Carbamate Insecticides: Chemistry,
(1976) Biochemistry and Toxicology. CRC Press, Cleveland,
Ohio, pg. 175-177.
Krishna, J.G. and J.E. Casida, Fate in Rats of the Radiocarbon from
(1966) Ten Variously Labelled Methyl and Dimethycarbamate-14C
Insecticide Chemicals and Their Hydrolysis Products.
J. Agr. Food Chem. 14:98.
Oonnithan, E.S. and J.E. Casida Metabolites of Methyl and
(1966) Dimethylcarbamate Insecticide Chemicals as Formed by
Rat Liver Microsomes. Bull. Env. Contam. Toxicol.
1:59.
Oonnithan, E.S. and J.E. Casida Oxidation of Methyl and
(1968) Dimethylcarbamate Insecticide Chemicals by Microsomal
Enzymes and Anticholinesterase Activity of the
Metabolites. J. Agr. Food Chem. 16:28.
Strother, A. Comparative Metabolism of Selected N-Methyl Carbamates
(1970) by Human and Rat Liver Fractions. Biochem. Pharmacol.
19:2525.
Strother, A. In Vitro Metabolism of Methylcarbamate Insecticides by
Human and Rat Liver Fractions. Toxicol. Appl.
Pharmacol. 21:112.
TOXICOLOGICAL EVALUATION
The data available was insufficient and no acceptable daily
intake for man has been established.
RESIDUES IN FOOD AND THEIR EVALUATION
The limited information which was available is summarized in the
following appraisal.
APPRAISAL
Aminocarb is a non-systemic carbamate insecticide used mainly
against many lepidopterous larvae and other biting insects. It also
has some acaricidal and molluscicidal activity.
Aminocarb is available as 50% and 75% water-soluble powders and
is applied as 0.075 - 1.0%, solutions to fruits and vegetables. Use in
forestry against the budworm is becoming important and could become
the main use for aminocarb in the future.
Surface residues of aminocarb are readily changed to less
volatile products by photolyt: modification of the 4-dimethylamino
group. In the bean plant, the major metabolic product
4-methylamino-m-tolyl methylcarbamate. The other degradation
products so far identified are 4-formamido-m-tolyl methylcarbamate,
4-methylformamido-m-tolyl methylcarbamate and 4-amido-m-tolyl
methylcarbamate. Glycosides are subsequently formed. Horseradish
peroxidase attacked aminocarb quite rapidly but the tyrosinase system
did not degrade it to any extent.
Aminocarb is not persistent in soil.
Two methods of analysis, both apparently suitable for regulatory
purposes, are available. A GLC procedure has been described for
aminocarb in forest foliage and soil using the nitrogen-specific
electrical conductivity detector. The procedure still has to be
validated for fruit and vegetables. High-pressure liquid
chromatography with UV detection has also been applied to the analysis
of cabbage, corn, potatoes and wheat with satisfactory detection
limits and reproducibility.
EVALUATION
The data available were insufficient to establish an ADI or
residue limits.
FURTHER WORK OR INFORMATION
Required (before an ADI or residue limits can be established)
1. Further data on toxicology and the occurrence of residues.
Desirable
1. Data from countries on use patterns and on residues from
supervised trials on which to base residue limits.
2. Studies on the fate of residues in animals.
REFERENCES
Kuhr, R.J. and H.W. Dorough Carbamate Insecticides: Chemistry,
(1976) Biochemistry and Toxicology. CRC Press, Cleveland,
Ohio, pg. 175-177.
Krishna, J.G. and J.E. Casida, Fate in Rats of the Radiocarbon from
(1966) Ten Variously Labelled Methyl and Dimethycarbamate-14C
Insecticide Chemicals and Their Hydrolysis Products.
J. Agr. Food Chem. 14:98.
Oonnithan, E.S. and J.E. Casida Metabolites of Methyl and
(1966) Dimethylcarbamate Insecticide Chemicals as Formed by
Rat Liver Microsomes. Bull. Env. Contam. Toxicol.
1:59.
Oonnithan, E.S. and J.E. Casida Oxidation of Methyl and
(1968) Dimethylcarbamate Insecticide Chemicals by Microsomal
Enzymes and Anticholinesterase Activity of the
Metabolites. J. Agr. Food Chem. 16:28.
Strother, A. Comparative Metabolism of Selected N-Methyl Carbamates
(1970) by Human and Rat Liver Fractions. Biochem. Pharmacol.
19:2525.
Strother, A. In Vitro Metabolism of Methylcarbamate Insecticides by
Human and Rat Liver Fractions. Toxicol. Appl.
Pharmacol. 21:112.
