
PHOSMET JMPR 1976
IDENTITY
Chemical name
OO-Dimethyl S-phthalimidomethyl phosphorodithioate or (in
Chemical Abstracts usage)
S-[(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)methyl]
O,O-dimethyl phosphorodithioate [732-11-6]
(Also referred to [eg in US Federal Register] as
N-(Mercaptomethyl)phthalimide
S-(O,O-dimethylphos-phorodithioate)
Synonyms
Phthalophos (USSR), PMP (Japan), IMIDANR PROLATER
Structural formula
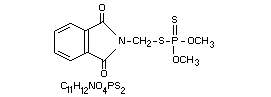 Other information on identity and properties
Molecular weight: 317.3
State: white crystalline solid
Melting point: 72.0-72.7°C
Vapour pressure: 1 x 10-3 mm Hg at 50°C
(decomposes below its boiling point)
Solubility 25 ppm in water (at 25°C), and more than
10 per cent in acetone, dichloromethane,
mesityl oxide, butanone and xylene.
Phosmet is hydrolyzed fairly rapidly in aqueous solution,
especially when the solution is neutral or alkaline. Buffered
aqueous solutions of 20µg per ml concentration at room temperature
were 50% hydrolyzed in 13 days at pH 4.5, in 5 hours at pH 7 and in
3 hours at pH 8.3.
The products of hydrolysis are phthalimide, 00-dimethyl
phosphorodithioate and formaldehyde. Oxidising agents can also
cause breakdown of phosmet. It is compatible with other pesticides
except under alkaline conditions, and is slightly corrosive.
Storage of formulations above 45°C may lead to decomposition.
The technical product is of 95 to 98 per cent purity and has
melting point 66.5 to 69.5°C. The principal impurities are
OOS-Trimethyl phosphorodithioate (1-2%) and
N-chloromethylphthalimide (1-2%). It is formulated as
emulsifiable concentrates, wettable powders, granules and: dusts at
a variety of active ingredient-concentrations.
A mixture of phosmet with carbophenothion is marketed under
the name TRIMIDANR", and the synergistic effects of styryl
phosphorothioates (Large and Pitt, 1975) and acephate (Nakatomi and
Ishibe, 1975) with phosmet have been reported recently, as has that
of 4-phenyl-1,3-dioxan (Uetani et al., 1974).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
In the absence of relevant data, toxicological evaluation of
this compound was postponed.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosmet is effective in controlling a variety of insect and
mite pests which attack livestock, pasture, fruit and field crops.
It is used as a systemic insecticide and acaricide against a
range of ectoparasites of animals, and may be applied as a dilute
aqueous spray containing up to 0.5 per cent a.i., as a ready-to-use
"pour on" formulation containing up to 4% a.i. and applied at up to
40 mg/kg body weight, as a dust containing 1 to 2 percent a.i. or
it may be administered orally.
Phosmet is non-systemic in plants, and at the concentrations
normally used is relatively harmless to a number of predators of
plant mites and insect pests. It is, therefore, used frequently in
integrated control programmes against crop pests. The crops on
which it is used include pome and stone fruits, cereals, forage
crops, pasture and pasture seed crops, nuts and green and root
vegetables.
In animals
Cattle
Phosmet is used to control cattle ticks (e.g. Boophilus
microplus and B. annulatus), cattle grubs (larvae of the bot
flies, Hypoderma lineatum and H. bovis), and the blood
sucking horn flies (Haematobia irritans), and is registered for
these uses in the U.S.A. (Anon, 1971d and 1974). Effective control
of cattle ticks has been obtained with a 0.03% spray (Drummond et
al., 1972).
It is officially recommended in Australia for use against
cattle lice and cattle ticks, and was reported to give effective
control of am acaricide-susceptible strain of B. microplus,
when used as a pour-on formulation (Loomis et al., 1862). The
maximum recommended application rate for use against lice on beef
and dairy cattle in Australia is 10 mg/kg. Sprayed onto cattle in
a finely divided dust, it was found to be effective against all
stages of a resistant strain of B. microplus (Thomas and
Taylor, 1971).
In the USSR, phosmet has been found to be effective against
ixoid ticks on cattle (Nepoklonov et al., 1969; Omarov, 1974), for
control of the mites causing cattle psoroptosis (Surorov, 1970),
and for protection of cattle against stable flies (Stomoxys
calcitrans) and other blood-sucking flies (Ruzimuradov et al.,
1973).
Sheep
Phosmet is used to control the sheep ked (Melophagus ovinus)
in the U.S.A. (Pfadt et al., 1975), and has been found effective in
the USSR against mites causing psoroptosis (Andrichuk, 1971 a,b).
Fowls
Effective control of fowl ectoparasites with phosmet in the
USSR has been reported (Frolov, 1970).
In plants, preharvest
Apples
Phosmet is used in the U.S.A. against a number of pests of
apple trees, including the plum curculio, Conotrachelus
nenuphar (Forsythe and Hall, 1972), and the codling moth
(Laspeyresia pomonella). For control of the latter, phosmet is
often used as part of an integrated control programme in which other
insecticides, sanitation measures, reliance on natural predators for
control of other pests (eg, the European red mite) and sterile moth
releases, may play a part (eg, Butt et al., 1973, Holdsworth, 1973).
In the USSR, phosmet is effective against the caterpillars of
the leaf-miner moth, Stigmella malella (Gribkova, 1973) and
against spider mites, hawthorn mites and soft scale insects.
In the U.K., phosmet has been found to be particularly
effective against the apple sucker, Psylla mali, and to give
control of other pre-blossom pests (Vernon and Gould, 1972).
Other apple pests controlled include San Jose Oystershell
scale insects, e.g. as part of an integrated control programme in
Canada (Wilde, 1973), woolly aphids, leaf roller moth (New Zealand
and Netherlands), winter moth, apple weevil in Poland (Witkowska
and Wojnarowska, 1971), and Aphis pomi in Spain.
Pears
Phosmet is used in the U.S.A. to control the pear psylla
(Psylla pyricola) as well as the codling moth. In the USSR it
gave control of Grapholitha molesta on pear trees when used as
0.1 per cent spray on six occasions at 14-day intervals (Omelyuta,
1972), and it is also used on a small scale on pears in the
Netherlands.
The recommended period between treatment and harvest is 3
weeks for both apples and pears in the Netherlands and in
Australia, and the application rate for pome fruit is usually 0.75
9 a.i/l (0.075%).
Stone fruit
In the U.S.A., phosmet is used on plums, cherries, peaches,
apricots and nectarines, and it has been found to give control of
Grapholitha molesta on peaches and apricots in the USSR. Its
use on peaches has been reported in Turkey, and it is used on
canning varieties of peaches in Australia against the oriental
fruit moth at 0.5 g a.i./l, with a pre-harvest withdrawal period of
3 weeks.
Other fruits
The use of phosmet against Patynota stultana on grapes in
the U.S.A. has been reported (Ali Niazee and Stafford, 1973). It is
also used against the grape berry moth and various gnawing pests of
grapes in the USSR. On citrus crops it has been used against the
citrus white fly; and the control of Zophodia convolutella on
gooseberries (Shapovalova et al., 1970) and of caterpillars on fig
trees (Popov, 1973) with phosmet have been reported in the USSR.
In New Zealand, phosmet is used on grapefruit, Kiwifruit and
Feijoas.
Potatoes
Phosmet is used to control the Colorado Beetle on potatoes in
Spain (Del Rivero et al., 1971), East Germany (Klunker, 1974), and
in the Netherlands, where the recommended pre-harvest interval is
4 weeks. It is recommended in Australia for control of the
red-legged earth mite in potato crops.
Other root crops
Phosmet is officially recommended in Australia for the control
of globular springtail on beet and carrots, and the red-legged
earth mite on carrots.
In the USSR it is used on sugar beet against a variety of
pests including Bothynoderes punctiventris (Nesterenko, 1970,
1973).
Green vegetables
The use of phosmet against lepidopterous pests of cabbage has
been reported in Spain, and it is officially recommended in
Australia for control of the red-legged earth mite and other pests
in beans, peas, crucifers and lettuce.
Pasture and forage crops
Phosmet is used in the U.S.A. to control the alfalfa weevil
(Hypera postica), and the clover head weevil. It has also been
found effective against the alfalfa weevil in the USSR when applied
at 0.75 kg/ha at the beginning of the egg-laying period. In
addition, it is effective against Phytonomus variabilis on
alfalfa (Marzhanyan et al., 1973). In Australia it is officially
recommended for use on cruciferous forage crops, lucerne, pasture,
and pasture seed crops to control the lucerne flea, the red-legged
earth mite and the blue oat mite, the application rate on pastures
being 0.0350.05 kg/ha with a withholding period of 7 days.
Cereal crops
In Australia satisfactory control of the blue oat mite on
wheat was achieved with the ULV application of phosmet at 0.5 oz
a.i. per acre (New South Wales Department of Agriculture, 1969 and
phosmet is officially recommended for control of this pest as well
as the lucerne flea and the redlegged earth mite in cereal crops.
Cotton
Applied at 3-5 kg/ha in cotton plantings in the USSR, phosmet
has been found very effective against cutworm moths (Nikiforov and
Mamaev, 1972). It is also reported to control other cotton pests.
Post-harvest treatments
The existence of a U.S. tolerance of 10 mg/kg for phosmet
residues on sweet potatoes from post-harvest applications (Anon,
1973), suggests that phosmet is used for the protection of this
crop in storage in the U.S.A.
Phosmet showed considerable promise in evaluation trials in
the U.S.A. against stored products insects such as the red flour
beetle, Tribolium castaneum (Herbst), (Speirs and Lang, 1970),
but it is not known whether it has been adopted for the protection
of stored grain, flour, etc.
Other uses
Phosmet has found some applications in forestry, particularly
for the protection of conifers against such pests as the pine tip
moth and the pine looper.
In the USSR, dipping sheep in 0.5 per cent emulsions of
phosmet kept the sheep free of Ixodes mites for 7 months
(Sevost'yanov and Ushakova, 1971).
Phosmet has also been found to be effective against several
pests of tobacco, including the flea beetle (Mistric and Smith,
1970). In Australia it is recommended for control of the lucerne
flea on tobacco seed beds.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
In a trial in the U.S.A. during 1964/5, five steers were
sprayed with phosmet at 0.25% a.i., and one was slaughtered on each
of days 3, 7, 14, 28 and 59 after treatment. Residues were
determined in fat, liver and muscle, with the results indicated in
Table 1 (Rogoff et al., 1967).
Several trials involving the determination of phosmet residues
in the fat of cattle at various intervals after treatment have been
carried out in Australia (Snelson, 1976). The treatments have
included spray and pour-on applications, and in one case the
animals were sprayed 3 times. The application rates and residue
results are shown in Table 1, along with the results of a trial in
the U.K. using pour-on application.
At the University of California, cattle were fed phosmet at
approximately 1 mg/kg/day for 8 weeks, and at 2 mg/kg/day for an
additional 8 weeks. The animals were killed and autopsied on days
zero, 3, 7, and 14 following the completion of feeding. The phosmet
residues in fat were all below the detection limit of 0.09 mg/kg, and
in liver, kidney and muscle they were all below the detection limit of
0.04 mg/kg with the exception of one zero day muscle sample.
There have been several studies involving the determination of
phosmet residues in the milk of dairy cows. In one of these (Johnson
and Bowman, 1968), milk from 4 dairy cows ingesting phosmet in silage
at a daily average rate of 0.22 mg/kg body weight was found to contain
less than 0.002 ppm of phosmet or its oxygen analogue. In another
(Woodard Research Corporation, 1963), 2 groups of dairy cows were
treated with phosmet by spraying 3 times at weekly intervals at 0.25%
or 0.5% a.i., whilst a further three groups were fed phosmet for 4
weeks at levels of 20, 45 and 100 ppm in the diet. Milk samples were
taken at frequent intervals from all five groups and analysed for both
phosmet and its 0-analogue. The average apparent phosmet residue for
all milk samples from treated cows was 0.016 mg/kg compared to
0.015 mg/kg for control samples.
Results for residues in milk and cream from further trials in
Australia and the U.K. are given in Table 2.
Goats
A goat given a single oral dose of phosmet (70 mg per kg,
administered in gelatine capsules) was milked dry at 8, 24 and 48
hours after treatment (Bowman and Beroza, 1967). The milk was
extracted with a 1:1 mixture of hexane and diethyl ether and the
extracts were analysed by gas chromatography using a flame photometric
detector in the sulphur mode. 0.38 mg/kg of phosmet was found in the 8
hour sample, but none was detected in the subsequent samples.
Pigs
In Bulgaria, phosmet applied externally to pigs at 1.5% a.i. was
found to penetrate through the skin in 2 to 5 days. The highest
residues (10 mg/kg) accumulated in the subcutaneous fat. The meat
accumulated approximately 8 ppm and some xylene derivatives were
detected as metabolites of phosmet (Ionova et al., 1974).
Fowls
In the USSR (Chirikashvili et al., 1970), phosmet was
administered to hens as a single oral dose of 300 mg/kg. The compound
was detectable by thin layer chromatography in various organs and
tissues, especially the lungs, heart and intestines, for 5 days after
treatment, and was present in some of the eggs at a level of about
2 mg/kg.
In plants
The available residue data from crop spraying trials are
summarised in Table 3.
Apples
In two Stauffer Chemical Company trials in the Netherlands in
1970, phosmet was applied as a spray containing 0.15% a.i. and at a
rate of 2000 l/ha. The residue disappearance curves from these trials
are reproduced as Figures 1 and 2. The oxygen analogue was measured as
well as unchanged phosmet, and residues were determined in samples of
fruit harvested at weekly intervals up to 4 weeks from treatment. The
total residue after the recommended 3 week interval from treatment to
harvest averaged 0.5 mg/kg, as against a national tolerance in the
Netherlands of 1 mg/kg, even though the concentration of the spray was
double that normally recommended.
In two similar trials in New Zealand, phosmet was applied 9 times
as a spray containing 0.15% a.i., and residues were determined over
periods of 23 and 28 days respectively. The mean residues found after
the recommended 14 day waiting period were in the range 1-3 mg/kg, as
against a New Zealand national tolerance of 10 mg/kg. The normal
application rate is 0.075-0.1% a.i.
In a trial in Australia using two application rates (1.75 and 2.0
g a.i./l) and carried out for the Stauffer Chemical Company, residues
had fallen to below 1 ppm after 3 weeks (King and Best, 1971).
In trials in the USSR (not included in Table 3), apple trees were
sprayed with phosmet at the rate of 10 litres per tree of 0.2% spray.
Residues were found to have disappeared from the apples 20 days after
treatment (Adeishvili, 1973), but the detection limit of the method
used is not known.
Stone fruit
Trials on two varieties of peaches were carried out by ICI in
Australia. In one, phosmet was applied at 1.0 g a.i./l and peaches
were sampled at 0, 7, 14 and 21 days after treatment. In the other
phosmet was applied at 2.0 or 1.0 g a.i./l and samples of peaches for
each application rate were taken at 1, 3, 5, 7 and 14 days after
treatment. For the 1.0 g a.i./l application rate, residues had fallen
to 1.1-1.5 ppm after 14 days, and to 0.5 ppm after 21 days (Snelson,
1976).
In Turkey, peaches of the Hale variety were treated 3 times in a
period of 30 days with a 0.15% spray. Samples were taken over a 3-week
period, and the residue found 21 days after the last treatment was 3.3
ppm (Otaci et al., 1972).
TABLE 1. Residues of phosmet in tissues of cattle slaughtered at various intervals after treatment
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
U.S.A. spray 2.5 1 Omental fat 3 0.40
(Rogoff et al 7 <0.2
1967) 14 <0.2
28 <0.2
59 <0.2
Peri-renal 3 0.59
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Sub-cutaneous 3 0.67
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Liver 3 0.10
7 0.09
14 0.08
28 0.06
59 0.08
U.S.A. muscle 3 0.06
7 <0.04
14 <0.04
28 <0.04
59 <0.04
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Australia Spray 0.75 3 Omental 1 1.03 (0.91-1.14)
(Snelson, 1976) fat 4 0.34 (0.28-0.40)
8 0.0
Subcutaneous 1 0.60 (0.40-0.80)
fat 4 0.06 (0.02-0.09)
1 Omental 1 0.77 (0.74-0.80)
fat 4 0.07 (0.01-0.13)
8 0.06 (0.02-0.09)
Subcutaneous 1 0.21 (0.21-0.21)
fat 4 0.01 (0-0.2)
Spray (2 0.75 1 Omental 1 0.79 (0.52-0.93)
gallons = fat 2 0.34 (0.25-0.40)
6.8 grams 3 0.24 (0.16-0.37)
technical 7 0.16 (0.11-0.21)
per beast)
Australia Peri-renal 1 0.24 (0.17-0.37)
fat 2 0.18 (0.15-0.24)
3 0.10 (0.09-0.11)
7 0.07 (0.05-0.10)
U.K. Pour-on 1 Omental 1 2.75 (1.64-3.7)
(40 mg/kg) fat 2 1.73 (1.68-1.8)
3 1.33 (0.92-1.9)
7 0.58 (0.49-0.69)
10 0.26 (0.15-0.36)
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Peri-renal 1 1.78 (1.20-2.19)
fat 2 1.47 (1.42-1.5)
3 1.05 (0.90-1.3)
7 0.73 (0.40-1.1)
10 0.37 (0.26-0.43)
TABLE 2. Residues of phosmet in the milk of lactating dairy cows at various intervals after treatment
Method Application Post-treatment Residues mg/kg mean
Country of rate No. of interval (max)
(reference) application (g. a.i./l applications (days) whole milk cream
Australia Spray 0.75 1 1/6 0.08 0.84
(King and (1 3/4 gallons 2/3 <0.02 0.10
Ferguson = 6.0 grams 1 0.02 0.10
1971) technical 2 <0.02 0.02
per beast) 3 <0.02 <0.02
4 <0.02 <0.02
2 1/6 0.03 -
2/3 0.05 -
1 <0.02 -
2 <0.02 -
3 <0.02 -
4 <0.02 -
U.K. Pour-on - 1 1/4 (0.07) (0.77)
(Snelson; (20 mg/kg)
1976
Other information on identity and properties
Molecular weight: 317.3
State: white crystalline solid
Melting point: 72.0-72.7°C
Vapour pressure: 1 x 10-3 mm Hg at 50°C
(decomposes below its boiling point)
Solubility 25 ppm in water (at 25°C), and more than
10 per cent in acetone, dichloromethane,
mesityl oxide, butanone and xylene.
Phosmet is hydrolyzed fairly rapidly in aqueous solution,
especially when the solution is neutral or alkaline. Buffered
aqueous solutions of 20µg per ml concentration at room temperature
were 50% hydrolyzed in 13 days at pH 4.5, in 5 hours at pH 7 and in
3 hours at pH 8.3.
The products of hydrolysis are phthalimide, 00-dimethyl
phosphorodithioate and formaldehyde. Oxidising agents can also
cause breakdown of phosmet. It is compatible with other pesticides
except under alkaline conditions, and is slightly corrosive.
Storage of formulations above 45°C may lead to decomposition.
The technical product is of 95 to 98 per cent purity and has
melting point 66.5 to 69.5°C. The principal impurities are
OOS-Trimethyl phosphorodithioate (1-2%) and
N-chloromethylphthalimide (1-2%). It is formulated as
emulsifiable concentrates, wettable powders, granules and: dusts at
a variety of active ingredient-concentrations.
A mixture of phosmet with carbophenothion is marketed under
the name TRIMIDANR", and the synergistic effects of styryl
phosphorothioates (Large and Pitt, 1975) and acephate (Nakatomi and
Ishibe, 1975) with phosmet have been reported recently, as has that
of 4-phenyl-1,3-dioxan (Uetani et al., 1974).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
In the absence of relevant data, toxicological evaluation of
this compound was postponed.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosmet is effective in controlling a variety of insect and
mite pests which attack livestock, pasture, fruit and field crops.
It is used as a systemic insecticide and acaricide against a
range of ectoparasites of animals, and may be applied as a dilute
aqueous spray containing up to 0.5 per cent a.i., as a ready-to-use
"pour on" formulation containing up to 4% a.i. and applied at up to
40 mg/kg body weight, as a dust containing 1 to 2 percent a.i. or
it may be administered orally.
Phosmet is non-systemic in plants, and at the concentrations
normally used is relatively harmless to a number of predators of
plant mites and insect pests. It is, therefore, used frequently in
integrated control programmes against crop pests. The crops on
which it is used include pome and stone fruits, cereals, forage
crops, pasture and pasture seed crops, nuts and green and root
vegetables.
In animals
Cattle
Phosmet is used to control cattle ticks (e.g. Boophilus
microplus and B. annulatus), cattle grubs (larvae of the bot
flies, Hypoderma lineatum and H. bovis), and the blood
sucking horn flies (Haematobia irritans), and is registered for
these uses in the U.S.A. (Anon, 1971d and 1974). Effective control
of cattle ticks has been obtained with a 0.03% spray (Drummond et
al., 1972).
It is officially recommended in Australia for use against
cattle lice and cattle ticks, and was reported to give effective
control of am acaricide-susceptible strain of B. microplus,
when used as a pour-on formulation (Loomis et al., 1862). The
maximum recommended application rate for use against lice on beef
and dairy cattle in Australia is 10 mg/kg. Sprayed onto cattle in
a finely divided dust, it was found to be effective against all
stages of a resistant strain of B. microplus (Thomas and
Taylor, 1971).
In the USSR, phosmet has been found to be effective against
ixoid ticks on cattle (Nepoklonov et al., 1969; Omarov, 1974), for
control of the mites causing cattle psoroptosis (Surorov, 1970),
and for protection of cattle against stable flies (Stomoxys
calcitrans) and other blood-sucking flies (Ruzimuradov et al.,
1973).
Sheep
Phosmet is used to control the sheep ked (Melophagus ovinus)
in the U.S.A. (Pfadt et al., 1975), and has been found effective in
the USSR against mites causing psoroptosis (Andrichuk, 1971 a,b).
Fowls
Effective control of fowl ectoparasites with phosmet in the
USSR has been reported (Frolov, 1970).
In plants, preharvest
Apples
Phosmet is used in the U.S.A. against a number of pests of
apple trees, including the plum curculio, Conotrachelus
nenuphar (Forsythe and Hall, 1972), and the codling moth
(Laspeyresia pomonella). For control of the latter, phosmet is
often used as part of an integrated control programme in which other
insecticides, sanitation measures, reliance on natural predators for
control of other pests (eg, the European red mite) and sterile moth
releases, may play a part (eg, Butt et al., 1973, Holdsworth, 1973).
In the USSR, phosmet is effective against the caterpillars of
the leaf-miner moth, Stigmella malella (Gribkova, 1973) and
against spider mites, hawthorn mites and soft scale insects.
In the U.K., phosmet has been found to be particularly
effective against the apple sucker, Psylla mali, and to give
control of other pre-blossom pests (Vernon and Gould, 1972).
Other apple pests controlled include San Jose Oystershell
scale insects, e.g. as part of an integrated control programme in
Canada (Wilde, 1973), woolly aphids, leaf roller moth (New Zealand
and Netherlands), winter moth, apple weevil in Poland (Witkowska
and Wojnarowska, 1971), and Aphis pomi in Spain.
Pears
Phosmet is used in the U.S.A. to control the pear psylla
(Psylla pyricola) as well as the codling moth. In the USSR it
gave control of Grapholitha molesta on pear trees when used as
0.1 per cent spray on six occasions at 14-day intervals (Omelyuta,
1972), and it is also used on a small scale on pears in the
Netherlands.
The recommended period between treatment and harvest is 3
weeks for both apples and pears in the Netherlands and in
Australia, and the application rate for pome fruit is usually 0.75
9 a.i/l (0.075%).
Stone fruit
In the U.S.A., phosmet is used on plums, cherries, peaches,
apricots and nectarines, and it has been found to give control of
Grapholitha molesta on peaches and apricots in the USSR. Its
use on peaches has been reported in Turkey, and it is used on
canning varieties of peaches in Australia against the oriental
fruit moth at 0.5 g a.i./l, with a pre-harvest withdrawal period of
3 weeks.
Other fruits
The use of phosmet against Patynota stultana on grapes in
the U.S.A. has been reported (Ali Niazee and Stafford, 1973). It is
also used against the grape berry moth and various gnawing pests of
grapes in the USSR. On citrus crops it has been used against the
citrus white fly; and the control of Zophodia convolutella on
gooseberries (Shapovalova et al., 1970) and of caterpillars on fig
trees (Popov, 1973) with phosmet have been reported in the USSR.
In New Zealand, phosmet is used on grapefruit, Kiwifruit and
Feijoas.
Potatoes
Phosmet is used to control the Colorado Beetle on potatoes in
Spain (Del Rivero et al., 1971), East Germany (Klunker, 1974), and
in the Netherlands, where the recommended pre-harvest interval is
4 weeks. It is recommended in Australia for control of the
red-legged earth mite in potato crops.
Other root crops
Phosmet is officially recommended in Australia for the control
of globular springtail on beet and carrots, and the red-legged
earth mite on carrots.
In the USSR it is used on sugar beet against a variety of
pests including Bothynoderes punctiventris (Nesterenko, 1970,
1973).
Green vegetables
The use of phosmet against lepidopterous pests of cabbage has
been reported in Spain, and it is officially recommended in
Australia for control of the red-legged earth mite and other pests
in beans, peas, crucifers and lettuce.
Pasture and forage crops
Phosmet is used in the U.S.A. to control the alfalfa weevil
(Hypera postica), and the clover head weevil. It has also been
found effective against the alfalfa weevil in the USSR when applied
at 0.75 kg/ha at the beginning of the egg-laying period. In
addition, it is effective against Phytonomus variabilis on
alfalfa (Marzhanyan et al., 1973). In Australia it is officially
recommended for use on cruciferous forage crops, lucerne, pasture,
and pasture seed crops to control the lucerne flea, the red-legged
earth mite and the blue oat mite, the application rate on pastures
being 0.0350.05 kg/ha with a withholding period of 7 days.
Cereal crops
In Australia satisfactory control of the blue oat mite on
wheat was achieved with the ULV application of phosmet at 0.5 oz
a.i. per acre (New South Wales Department of Agriculture, 1969 and
phosmet is officially recommended for control of this pest as well
as the lucerne flea and the redlegged earth mite in cereal crops.
Cotton
Applied at 3-5 kg/ha in cotton plantings in the USSR, phosmet
has been found very effective against cutworm moths (Nikiforov and
Mamaev, 1972). It is also reported to control other cotton pests.
Post-harvest treatments
The existence of a U.S. tolerance of 10 mg/kg for phosmet
residues on sweet potatoes from post-harvest applications (Anon,
1973), suggests that phosmet is used for the protection of this
crop in storage in the U.S.A.
Phosmet showed considerable promise in evaluation trials in
the U.S.A. against stored products insects such as the red flour
beetle, Tribolium castaneum (Herbst), (Speirs and Lang, 1970),
but it is not known whether it has been adopted for the protection
of stored grain, flour, etc.
Other uses
Phosmet has found some applications in forestry, particularly
for the protection of conifers against such pests as the pine tip
moth and the pine looper.
In the USSR, dipping sheep in 0.5 per cent emulsions of
phosmet kept the sheep free of Ixodes mites for 7 months
(Sevost'yanov and Ushakova, 1971).
Phosmet has also been found to be effective against several
pests of tobacco, including the flea beetle (Mistric and Smith,
1970). In Australia it is recommended for control of the lucerne
flea on tobacco seed beds.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
In a trial in the U.S.A. during 1964/5, five steers were
sprayed with phosmet at 0.25% a.i., and one was slaughtered on each
of days 3, 7, 14, 28 and 59 after treatment. Residues were
determined in fat, liver and muscle, with the results indicated in
Table 1 (Rogoff et al., 1967).
Several trials involving the determination of phosmet residues
in the fat of cattle at various intervals after treatment have been
carried out in Australia (Snelson, 1976). The treatments have
included spray and pour-on applications, and in one case the
animals were sprayed 3 times. The application rates and residue
results are shown in Table 1, along with the results of a trial in
the U.K. using pour-on application.
At the University of California, cattle were fed phosmet at
approximately 1 mg/kg/day for 8 weeks, and at 2 mg/kg/day for an
additional 8 weeks. The animals were killed and autopsied on days
zero, 3, 7, and 14 following the completion of feeding. The phosmet
residues in fat were all below the detection limit of 0.09 mg/kg, and
in liver, kidney and muscle they were all below the detection limit of
0.04 mg/kg with the exception of one zero day muscle sample.
There have been several studies involving the determination of
phosmet residues in the milk of dairy cows. In one of these (Johnson
and Bowman, 1968), milk from 4 dairy cows ingesting phosmet in silage
at a daily average rate of 0.22 mg/kg body weight was found to contain
less than 0.002 ppm of phosmet or its oxygen analogue. In another
(Woodard Research Corporation, 1963), 2 groups of dairy cows were
treated with phosmet by spraying 3 times at weekly intervals at 0.25%
or 0.5% a.i., whilst a further three groups were fed phosmet for 4
weeks at levels of 20, 45 and 100 ppm in the diet. Milk samples were
taken at frequent intervals from all five groups and analysed for both
phosmet and its 0-analogue. The average apparent phosmet residue for
all milk samples from treated cows was 0.016 mg/kg compared to
0.015 mg/kg for control samples.
Results for residues in milk and cream from further trials in
Australia and the U.K. are given in Table 2.
Goats
A goat given a single oral dose of phosmet (70 mg per kg,
administered in gelatine capsules) was milked dry at 8, 24 and 48
hours after treatment (Bowman and Beroza, 1967). The milk was
extracted with a 1:1 mixture of hexane and diethyl ether and the
extracts were analysed by gas chromatography using a flame photometric
detector in the sulphur mode. 0.38 mg/kg of phosmet was found in the 8
hour sample, but none was detected in the subsequent samples.
Pigs
In Bulgaria, phosmet applied externally to pigs at 1.5% a.i. was
found to penetrate through the skin in 2 to 5 days. The highest
residues (10 mg/kg) accumulated in the subcutaneous fat. The meat
accumulated approximately 8 ppm and some xylene derivatives were
detected as metabolites of phosmet (Ionova et al., 1974).
Fowls
In the USSR (Chirikashvili et al., 1970), phosmet was
administered to hens as a single oral dose of 300 mg/kg. The compound
was detectable by thin layer chromatography in various organs and
tissues, especially the lungs, heart and intestines, for 5 days after
treatment, and was present in some of the eggs at a level of about
2 mg/kg.
In plants
The available residue data from crop spraying trials are
summarised in Table 3.
Apples
In two Stauffer Chemical Company trials in the Netherlands in
1970, phosmet was applied as a spray containing 0.15% a.i. and at a
rate of 2000 l/ha. The residue disappearance curves from these trials
are reproduced as Figures 1 and 2. The oxygen analogue was measured as
well as unchanged phosmet, and residues were determined in samples of
fruit harvested at weekly intervals up to 4 weeks from treatment. The
total residue after the recommended 3 week interval from treatment to
harvest averaged 0.5 mg/kg, as against a national tolerance in the
Netherlands of 1 mg/kg, even though the concentration of the spray was
double that normally recommended.
In two similar trials in New Zealand, phosmet was applied 9 times
as a spray containing 0.15% a.i., and residues were determined over
periods of 23 and 28 days respectively. The mean residues found after
the recommended 14 day waiting period were in the range 1-3 mg/kg, as
against a New Zealand national tolerance of 10 mg/kg. The normal
application rate is 0.075-0.1% a.i.
In a trial in Australia using two application rates (1.75 and 2.0
g a.i./l) and carried out for the Stauffer Chemical Company, residues
had fallen to below 1 ppm after 3 weeks (King and Best, 1971).
In trials in the USSR (not included in Table 3), apple trees were
sprayed with phosmet at the rate of 10 litres per tree of 0.2% spray.
Residues were found to have disappeared from the apples 20 days after
treatment (Adeishvili, 1973), but the detection limit of the method
used is not known.
Stone fruit
Trials on two varieties of peaches were carried out by ICI in
Australia. In one, phosmet was applied at 1.0 g a.i./l and peaches
were sampled at 0, 7, 14 and 21 days after treatment. In the other
phosmet was applied at 2.0 or 1.0 g a.i./l and samples of peaches for
each application rate were taken at 1, 3, 5, 7 and 14 days after
treatment. For the 1.0 g a.i./l application rate, residues had fallen
to 1.1-1.5 ppm after 14 days, and to 0.5 ppm after 21 days (Snelson,
1976).
In Turkey, peaches of the Hale variety were treated 3 times in a
period of 30 days with a 0.15% spray. Samples were taken over a 3-week
period, and the residue found 21 days after the last treatment was 3.3
ppm (Otaci et al., 1972).
TABLE 1. Residues of phosmet in tissues of cattle slaughtered at various intervals after treatment
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
U.S.A. spray 2.5 1 Omental fat 3 0.40
(Rogoff et al 7 <0.2
1967) 14 <0.2
28 <0.2
59 <0.2
Peri-renal 3 0.59
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Sub-cutaneous 3 0.67
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Liver 3 0.10
7 0.09
14 0.08
28 0.06
59 0.08
U.S.A. muscle 3 0.06
7 <0.04
14 <0.04
28 <0.04
59 <0.04
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Australia Spray 0.75 3 Omental 1 1.03 (0.91-1.14)
(Snelson, 1976) fat 4 0.34 (0.28-0.40)
8 0.0
Subcutaneous 1 0.60 (0.40-0.80)
fat 4 0.06 (0.02-0.09)
1 Omental 1 0.77 (0.74-0.80)
fat 4 0.07 (0.01-0.13)
8 0.06 (0.02-0.09)
Subcutaneous 1 0.21 (0.21-0.21)
fat 4 0.01 (0-0.2)
Spray (2 0.75 1 Omental 1 0.79 (0.52-0.93)
gallons = fat 2 0.34 (0.25-0.40)
6.8 grams 3 0.24 (0.16-0.37)
technical 7 0.16 (0.11-0.21)
per beast)
Australia Peri-renal 1 0.24 (0.17-0.37)
fat 2 0.18 (0.15-0.24)
3 0.10 (0.09-0.11)
7 0.07 (0.05-0.10)
U.K. Pour-on 1 Omental 1 2.75 (1.64-3.7)
(40 mg/kg) fat 2 1.73 (1.68-1.8)
3 1.33 (0.92-1.9)
7 0.58 (0.49-0.69)
10 0.26 (0.15-0.36)
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Peri-renal 1 1.78 (1.20-2.19)
fat 2 1.47 (1.42-1.5)
3 1.05 (0.90-1.3)
7 0.73 (0.40-1.1)
10 0.37 (0.26-0.43)
TABLE 2. Residues of phosmet in the milk of lactating dairy cows at various intervals after treatment
Method Application Post-treatment Residues mg/kg mean
Country of rate No. of interval (max)
(reference) application (g. a.i./l applications (days) whole milk cream
Australia Spray 0.75 1 1/6 0.08 0.84
(King and (1 3/4 gallons 2/3 <0.02 0.10
Ferguson = 6.0 grams 1 0.02 0.10
1971) technical 2 <0.02 0.02
per beast) 3 <0.02 <0.02
4 <0.02 <0.02
2 1/6 0.03 -
2/3 0.05 -
1 <0.02 -
2 <0.02 -
3 <0.02 -
4 <0.02 -
U.K. Pour-on - 1 1/4 (0.07) (0.77)
(Snelson; (20 mg/kg)
1976
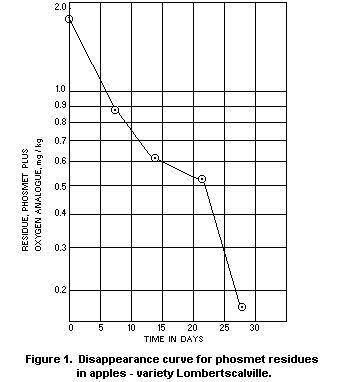
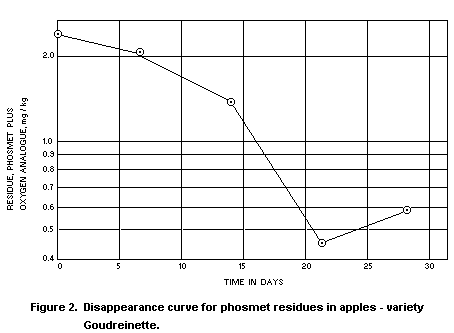 TABLE 3. Residues of phosmet in various crops from supervised spraying trials
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Apples Netherlands 1.5 1 0 1.8(1.3-2.1 0
(var. Lombertscalville) (2000 1/ha) 7 0.9(0.7-1.2) 0
14 0.6(0.3-0.9 0
21 0.5(0.3-0.6) 0
28 0.2(0.1-0.2) 0
Netherlands 1.5 1 0 2.4(1.5-3.4) 0.04(0.01-0.07)
(var. (2000 l/ha) 7 2.0(1.7-2.3) 0.06(0.04-0.09)
Goudreinette) 14 1.4(0.6-2.0) 0.06(0.03-0.07)
21 0.4(0.2-0.6) 0.03(0-0.06)
28 0.6(0.2-0.7) 0.02(0.01-0.03)
Apples New Zealand 1.5 9 2 2.0
9 1.7
15 1.1
23 0.7
1.5 9 1 4.3
3 3.6
7 4.0
14 2.9
21 3.0
28 2.1
Apples Australia 1.75 ? 0 2.2
(King & Best, 1 1.5
1971) 7 0.7
14 0.8
21 0.6
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
2.0 ? 0 2.8
1 1.5
7 1.6
14 1.0
21 0.7
Peaches Australia 1.0 1 0 3.8
(var. Golden 7 3.6
Queen) 14 1.1
21 0.5
Australia 1.0 1 1 3.4
(var.Maygold) 3 2.5
5 1.3
7 3.1
14 1.5
Peaches Australia 2.0 1 1 5.3
(var. Maygold) 3 6.2
5 4.2
7 3.9
14 6.2
Peaches Turkey 1.5 3 0 5.39
3 5.44
7 4.65
14 3.48
21 3.30
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Kiwifruit New Zealand 1.1 2 1 10.0
7 9.6
14 5.8
21 5.1
28 3.6
35 3.1
42 3.0
1.12 2 10 9.5
7.7
5.8
7.1
1.12 7 10 25.0
16.0
8.4
7.2
0.75 7 10 8.8
4.8
7.1
6.4
Grapefruit New Zealand 1.12 4 1 0.65
7 0.57
14 0.3
21 0.26
28 0.38
42 0.22
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Potatoes Netherlands 0.56 kg a.i./ 2 22 0.004 0
(var. ha(as 50% w.p.) (0.002-0.006)
Burmania)
Netherlands " 2 20 0.001 0
(var. Bintje) (0-0.003
Netherlands " 2 21 0.001 0
(var. (0.001-0.002)
Eigenheimer)
Alfalfa U.S.A. 1.1 kg a.i./ 1 1 43.5
(Shaw et al. ha(36% ec) 8 2.09
1966) 14 0.84
21 0.31
controls 0.18-0.44
Alfalfa U.S.A. 0.56 kg a.i. 1 0 33.86
(Miller et al. /ha(50% wp) 5 3.58
1969) 10 2.27
15 0.34
21 0.04
1.1 kg a.i. 1 0 57.11
/ha(50% wp) 5 10.74
10 3.42
15 1.52
21 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i. 1 0 20.95
/ha(36% ec) 5 3.87
10 2.09
15 0.94
21 0.00
Alfalfa U.S.A. 1.1 kg a.i. 1 0 44.42
/ha (36% ec) 5 9.87
10 5.06
15 2.00
21 0.37
Coastal U.S.A. 0.84 kg a.i. 1 0 37.93
bermuda (Dorough et /ha 1 32.66
grass al, 1965) 3 23.89
7 15.79
14 8.31
Coastal U.S.A. 0.28 kg a.i. 1 0 12.3 0.06
bermuda (Leuck and /ha 1 6.86 0.06
grass Bowman, 1968) 3 1.11 0.00
4 0.96 0.00
7 0.72 0.00
15 0.13 0.00
0.56 kg a.i. 1 0 27.0 0.13
/ha 1 16.4 0.13
3 2.82 0.02
4 2.30 0.02
7 1.78 0.00
15 0.36 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Coastal U.S.A. 1.1 kg a.i./ha 1 0 52.9 0.36
Bermuda 1 36.8 0.40
grass 3 7.02 0.07
4 6.34 0.06
7 3.72 0.02
15 0.57 0.00
Maize U.S.A. 0.28 kg a.i./ha 1 0 2.67 0.00
plants (Leuck and 1 1.00 0.00
(green Bowman, 1968) 2 0.39 0.00
weight 4 0.20 0.00
basis) 7 0.09 0.00
0.56 kg a.i./ha 1 0 7.07 0.01
1 Not sampled owing to rain
2 0.99 0.00
4 0.46 0.00
7 0.26 0.00
1.1 kg a.i./ha 1 0 10.9 0.02
1 Not sampled owing to rain
2 2.89 0.00
4 1.16 0.00
7 0.63 0.00
Soybean U.S.A. 0.28 kg ai./ 1 0 31.2 0.03
plants (Leuck and ha 1 21.1 0.02
(green Bowman, 1968) 2 15.0 0.01
weight 4 2.45 0.00
basis) 7 0.48 0.00
15 0.10 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i./ha 1 0 55.4 0.07
1 30.2 0.03
2 27.6 0.02
4 6.27 0.00
7 1.24 0.00
15 0.28 0.00
1.1 kg a.i./ha 1 0 82.2 0.11
1 55.4 0.07
2 43.8 0.04
4 14.7 0.01
7 2.62 0.00
15 0.76 0.00
Other fruits
Trials in New Zealand on Kiwifruit and grapefruit yielded the
residue data given in Table 3. In most cases the spray concentration
was slightly above the recommended upper limit of 0.1% a.i., but only
for the highest rate of application to Kiwifruit (7 applications of
0.112% a.i.) were residues in the whole fruit found to be above the
national tolerance of 10 mg/kg after the recommended 10-day waiting
period. Separate analyses of the skin and flesh revealed that the
residues were mainly concentrated in the skin. Four applications of
0.112% a.i. spray to grapefruit resulted in a residue of only 0.3
mg/kg in the whole fruit (0.05 mg/kg in the flesh) after the
recommended 14 day interval.
Alfalfa
In 1965, experiments were conducted in the United States to
determine the rate of disappearance of phosmet from alfalfa. An ec
formulation containing 36% a.i., w/v, was applied at the rate of 1.1
kg a.i./ha. Residues were determined by the anthranilic acid
colorimetric method over a period of 3 weeks after application. The
results are given in Table 3, and show that the phosmet residues had
declined to the levels found in untreated control samples after 21
days (Shaw et al., 1966).
In a subsequent trial on alfalfa (Miller et al., 1969), the
disappearance rates of phosmet after application in two different
formulations (the 36% ec and a 50% wp) were compared. Each formulation
was applied at the rate of 0.5 and 1 lb a.i./acre in a dilute spray
used at 100 gallons per acre. Each treatment was replicated twice, and
the crop was sampled over a 3 week period after treatment. The results
are summarised in Table 3.
In the USSR, the feeding of rabbits with alfalfa gathered 10 days
after it had been sprinkled with phosmet produced some depression of
cholinesterase activity. When the alfalfa was gathered 15 days after
treatment no depression was observed, and the residue level had
declined to 1/6 of that found 11 days after treatment and 1/15 of that
found 6 days after treatment (Semerdzhyan, 1972).
Other forage crops
Trials were conducted in the U.S.A. in which maize (for silage),
soybean plants, and coastal Bermuda grass were each sprayed at rates
of 0.28, 0.56 and 1.1 kg of phosmet per ha. Residues of phosmet and
its oxygenanalogue were determined in samples taken over a 15 day
period following treatment (Leuck and Bowman, 1968). The results are
given in Table 3, and indicate that in all cases the residues had
declined to under 1 mg/kg in 15 days or less. On the other hand, in a
trial on Coastal Bermuda grass at the Texas Agricultural Experiment
Station, in which phosmet was applied at 0.84 kg/ha, residues of about
8 mg/kg were found after 14 days (Dorough et al., 1965).
Leuck and Bowman analysed the concentrate used in their trial,
and found that it contained a trace of the oxygen-analogue equivalent
to 0.12% of the phosmet content. Thus some, at least, of the
0-analogue residues found probably originated from the spray liquor
rather than from environmental oxidation of phosmet.
FATE OF RESIDUES
General comments
The results presented in the foregoing section indicate that both
phosmet and its oxygen analogue are of low persistence in plants and
animals. The photochemistry of phosmet in diethyl ether solution was
studied by Tanabe et al. (1974), using a 450 watt UV lamp. They
isolated N-methylphthalimide and N-methoxymethylphthalimide as
photolysis products in low yield. Neither of these was insecticidally
active. No photoproduct containing P or S could be isolated from the
many minor components. Using UV radiation in the range 250-400 nm,
Klisenko and Pis'mennaya (1973) found a half-life of 70 minutes for
phosmet, and identified phthalimide, N-methoxymethylphthalimide and
phthalic acid as photolysis products, in addition to P-O analogues.
In animals
The metabolism of carbonyl-14C-labelled phosmet in rats
following oral administration was investigated by McBain et al.
(1968). Metabolites were excreted in the urine in the following
amounts (expressed as percentages of the administered dose): phthalic
acid 21.0%, phthalamic acid 40.7% and five minor metabolites
containing the phthaloyl moiety 10.9%. Not more than 0.04% of the dose
administered was excreted in the urine as unchanged phosmet or its
oxygen analogue. This study supported other evidence that phosmet is
rapidly metabolised in mammals, mainly to innocuous water soluble
compounds. For example, Chamberlain (1965) studied the fate of
14C-labelled phosmet following dermal application to a steer. It was
moderately absorbed by the skin of the animal, since 9.6% of the
applied dose was recovered in the excreta (nearly 8% in the urine)
within 7 days. It appeared that the compound was rapidly broken down
in the blood, and that the primary degradation occurred at the
nitrogen atom, resulting in the almost exclusive production of
phthalic and phthalamic acids.
Johnson and Bowman (1968) found no detectable residues of phosmet
or its oxygen analogue in the milk, urine or faeces of lactating dairy
cows fed with silage containing phosmet at a level providing 0.22
mg/kg/ day. Detection limits were 0.002 mg/kg for milk and urine, and
0.004 mg/kg for faeces.
Tests under laboratory conditions in the USSR (Vrochinskii, 1971)
showed that phosmet was stored only briefly by fish.
In plants
Trials on apples, peaches, lemons and potatoes showed that
neither phosmet nor its oxygen analogue is translocated from treated
to untreated portions of plants. Residues tend to remain on plant
surfaces and are easily brushed or washed off. They are degraded quite
rapidly by weathering.
Phosmet is hydrolysed in plant tissues and converted to phthalic
acid and/or phthalamic acid which is translocated in the plant,
decarboxylated, apparently by enzyme action, and further metabolised
to possible benzoic acid derivatives.
On apples, pears and peaches, the average half-life of phosmet
appears to be less than two weeks. The disappearance of residual
phosmet from standing corn plants was found to be rapid, but after
ensiling, the loss of phosmet was slow (about 50% loss in 92 days).
The acid conditions in the silage stack probably protected the phosmet
against spontaneous hydrolysis.
Kovac et al. (1968) reported the rapid decomposition of phosmet
to non-toxic substances in fruit and vegetables. After treatment with
a 0.25% phosmet spray, residue levels declined to 0.75 mg/kg in 1-12
days. In experiments on beet, carrots and tomatoes grown on a loamy
soil in the USSR, Boiko and Popova (1970) found no residues of phosmet
30-35 days after treatment with sprays of up to 0.6% a.i.
concentration.
In soils
Phosmet and its oxygen analogue are rapidly degraded in soils.
Degradation is favoured by increasing alkalinity and moisture content
and is less in autoclaved soil samples, indicating that hydrolysis of
phosmet is not dependent on moisture alone, but is due in some degree
to microbial action. The time for 50% degradation varied from 3 days
in a non-autoclaved loamy soil containing 10% moisture, to 19 days in
the same soil with only 2% moisture. It is stated that the phthalic
acid produced is readily metabolised and utilised by micro-organisms
so that phosmet represents a totally biodegradable pesticide leaving
no unmetabolisable residues in the environment (Snelson, 1976). The
vapour pressure of phosmet is fairly high, and under laboratory
conditions it has been shown to evaporate rapidly from peat soils, but
not from sandy soils (Zatserkovskaya, 1974).
In storage and processing
The effect of the washing, blanching and sterilisation operations
used in the canning industry on phosmet deliberately added to apples,
plums, cabbage, pears, green peas and red beet (by dipping them in a
0.4% aqueous dispersion of phosmet) has been studied in the USSR
(Gorelik et al., 1973a). Washing with 5 parts of a 0.5% NaOH solution
to one part of fruit reduced the phosmet content of apples and plums
by 50-55%. Blanching at 80°C with a liquid/fruit ratio of 5:1 removed
55% and 81% respectively of the initial phosmet loading of plums and
apples. The rate of phosmet destruction by sterilisation varied from
60% to 100% in a range of commodities, and about 38% in cabbage. No
phosmet residues were detected in canned apples and plums after
storage at 20°C for 12 months.
Similar studies were made of the effect of such processing on
phosmet residues in apples used for production of canned apple puree
as a baby food (Gorelik et al., 1973b). Blanching, crushing and
sterilisation reduced the residue level by 51%, 28% and 16-25%
respectively. The same workers studied the effects of deep freezing on
the residue dynamics of phosmet in carrots, cabbage and beet. It was
found that even prolonged deep freezing resulted in negligible
reductions of phosmet residues, and it was concluded that vegetables
containing phosmet in excess of the USSR tolerance of 0.25 mg/kg
should not be frozen prior to processing.
Evidence of residues in food in commerce or at consumption
No data were available.
METHODS OF RESIDUE ANALYSIS
Colorimetric methods
Much of the earlier work on residues resulting from supervised
trials was carried out using colorimetric procedures which determine
either the phthalimide moiety after conversion to anthranilic acid or
the phosphorodithioate moiety after conversion to phosphomolybdate.
The anthranilic acid method (Batchelder and Patchett, 1965) is the
more sensitive and specific of the two. Both methods are described in
detail by Batchelder et al.(1967), who also give typical figures for
the recovery of phosmet by these methods from various crops and animal
tissues. The anthranilic acid method is claimed to detect 2 micrograms
of phosmet reliably (giving a detection limit of 0.04 mg/kg for a 50
gram sample), and is applicable to animal or plant tissues. It will
also detect the oxygen analogue but is susceptible to interference
from folpet or azinphos-methyl. These compounds do not interfere with
the phosphomolybdate method, which includes a selective hydrolysis
step.
An alternative colorimetric procedure for determining the
phosphorodithioate moiety (Sharova and Kozhemyakin, 1973) involves the
formation of a yellow complex with copper sulphate, and has been
applied to the determination of phosmet residues in the tissues of
birds, with a detection limit of 0.5 mg/kg.
A method involving the liberation of formaldehyde by acid
hydrolysis of phosmet, and colour development with chromotropic acid,
has been applied to the measurement of phosmet residues in water, with
a detection limit of 0.01 mg/l (Novikova and Varlamova, 1973), and in
apples, with a detection limit of 0.5 mg/kg (Novikova et al., 1969).
Thin-layer chromatographic methods
Phosmet can be determined at the residue level by several of the
methods involving in situ fluorimetry on thin-layer chromatograms
which have been evolved for organophosphorus pesticides. For example,
Frei and Mallet (1971) used a chelate spray reagent consisting of a
1:2 mixture of salicyl-2-aldehyde-2-quinolylhydrazone and manganese
(II) chloride, which was applied after exposure of the plate to
bromine. They claim a visual detection limit of 0.04 µg phosmet.
Bidleman et al. (1972) used fluorescence quenched solutions of
palladium (II) - calcein and palladium(II)-calcein blue as sensitive
spray reagents for the in situ fluorometric determination of
organophosphorus pesticides including phosmet, and reported that as
little as 10.50 ng of a phosphorodithioate could be detected.
Methods have also been described in which thin-layer
chromatography is used as a clean-up procedure for phosmet residues
extracted from foods prior to determination of the eluted pesticide by
one of the colorimetric procedures referred to above. An example is
the method of Novikova and Mel'tser (1972), which uses the chromotopic
acid procedure after clean-up on silica gel plates. This method had a
detection limit of 0.6 mg/kg for phosmet residues in foods of plant
origin. Alternatively, the phosmet spot could be visualised on the
plate by spraying with bromophenol blue and silver nitrate, giving a
visual detection limit of 0.3 mg/kg.
Phosmet is also detectable on TLC plates by enzyme inhibition
methods. For example, Schutzmann and Barthel (1969) reported detection
limits of 0.5 µg and 0.05 µg for phosmet and its oxygen analogue
respectively, using horse serum cholinesterase with indoxyl acetate as
substrate. Schutzmann (1970) reported that the sensitivity of the
method could be improved by using either the acetate or butyrate of
N-methyl indoxol as substrate. Ackermann and Engst (1970) used beef
liver esterase with 1-napthyl acetate and Fast Blue B on silica gel G
plates to detect organophosphorus residues in rat foetal tissues. They
were able to detect 0.025 mg/kg of phosmet and 0.005 mg/kg of its
0-analogue by this method.
Gas chromatographic methods
Some early gas chromatographic determinations of phosmet residues
made use of the electron capture detector (ECD). For example, Bowman
and Beroza (1965) were able to determine phosmet (but not the
0-analogue) in milk and sweet corn by using the ECD, and Gutenmann et
al. (1965) applied it to the determination of phosmet (detection limit
0.1 mg/kg) and the O-analogue (0.2 mg/kg) in apples, potatoes, grapes
and alfalfa following a TLC clean-up.
Later methods have made use of either an alkali flame ionisation
detector (AFID) giving selective detection of phosphorus compounds, or
a flame photometric detector (FPD) operated in either the phosphorus
or the sulphur mode. Bowman and Beroza (1966) used the FPD with a
526 nm phosphorus filter to determine phosmet (detection limit 0.002
mg/kg) and the 0-analogue (0.004 mg/kg) in sweet corn, following
clean-up and separation on a silica gel column. The glc column packing
was 10% DC 200 on 80/100 mesh Gas Chrom Q. The column was operated at
200°C, and required conditioning with repeated injections of phosmet
and corn extract to obtain a constant response to the insecticide. The
same column packing was used by Watts and Storherr (1967) in
conjunction with an AFID for the determination of phosmet and other
organophosphorus insecticide residues in milk after a sweep
codistillation clean-up. They too found the need for thorough column
conditioning. Storherr et al. (1967) used essentially the same
procedure to determine organophosphorus pesticides including phosmet
in fortified samples of edible oils. The average recovery for phosmet
was 96%, and the method detected apparent levels in unfortified
samples ranging from less than 0.01 to 0.04 mg/kg.
Bowman and Beroza (1967) were able to determine phosmet extracted
from milk (in hexane/ether) without clean-up by using the FPD with a
394 nm sulphur filter. Bowman et al. (1968) investigated several
alternative extraction procedures for removing OP residues (including
phosmet and its O-analogue) from field treated crops, and found the
most efficient method to be soxhlet extraction for 4 hours with 10%
methanol in chloroform. Determination was by gas chromatography with
flame photometric detection using a phosphorus filter.
A gas chromatographic method employing the AFID (with rubidium
sulphate as alkali source) and a short, narrow bore column containing
10% DC 200 on 100/120 mesh Gas Chrom Q has been described by Barney et
al. (1972). For most crops, clean-up is achieved simply by shaking
with charcoal followed by filtration through a Millipore filter.
Cottonseed and cotton foliage require prior acetonitrile partition.
The lower limit of detection is given as 0.05 mg/kg and the method has
been successfully applied to a range of fruit, vegetable and fodder
crops. The authors state that the FPD in either the P or S mode may be
used instead of the AFID, but stress the importance of the small
particle size of the solid support to ensure adequate resolution of
the oxygen analogue from phosmet.
Several multi-residue gas chromatographic procedures which
include phosmet have been published recently. Some examples are:
(a) The method of Ripley et al. (1974) for OP residues in natural
waters (using the FPD).
(b) The method of Luke et al. (1975) for OP and nitrogen containing
pesticides in produce (using the AFID with a potassium chloride
source).
(c) The method of Krijgsman and Van de Kamp (1976) which utilises a
capillary column of SE-30 with a flame photometric detector.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The national tolerances shown in Table 4 have been reported to
the Meeting.
TABLE 4. National tolerances reported to the Meeting
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Australia Fat of meat of cattle 1 Snelson
Milk and milk products 1976
(fat basis) 0.2 "
Pome and stone fruit 1 "
Canada Apples, peaches and
pears 10
Plums 5
Netherlands Apples, pears 21 1
Potatoes 28 0.02
New Zealand Fruit 14 10
(Kiwifruit 10)
USA Alfalfa 40 Anon, 1969
Apples, peaches & pears 10 "
Meat & fat of cattle,
goats, hogs & sheep 0.2 Anon, 1969
Grapes 10 Anon, 1971a
Cherries 10 Anon, 1971b
Apricots, nectarines
and plums 5 " "
TABLE 4. (Cont'd.)
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Potatoes 0.1 Anon, 1971c
Sweet potatoes (from
post-harvest application 10 Anon, 1973
Almond hulls, blue berries,
corn forage and fodder
(including sweet corn,
field corn and popcorn),
cranberries, pea forage
and hay 10 Anon, 1975
Fresh corn, including sweet
corn (kernel and cob with
husk removed), corn grain
(including popcorn), and
peas 0.5 Anon, 1975
Meat, fat and meat by-products
of horses 0.2 Anon, 1975
Nuts 0.1 Anon, 1975
USSR Vegetables 0.25 Gorelik et
al, 1973b
Figs 0.25 Popov, 1973
APPRAISAL
As a systemic insecticide and acaricide against a range of
ectoparasites of animals, phosmet may be applied as a dilute aqueous
spray containing up to 0.5% a.i., as a ready-to-use "pour on"
formulation containing up to 4% a.i. and applied at up to 40 mg per kg
body weight, as a dust containing 1 to 2% a.i., or it may be
administered orally.
In plants, phosmet is used as a non-systemic pesticide and is
applied to a variety of crops to protect against insects and mites. At
the concentrations usually applied it can be effective without harming
some useful predators and this makes it of value for integrated
control programmes. On fruit, a 0.1% spray has been used on pasture
and forage crops up to 0.75 kg/ha, and on cereal crops at 35 g/ha (ULV
spray). It has been used at 3-5 kg/ha on cotton.
Data available on residues in meat of cattle is not extensive,
but would be in accord with a maximum level of 0.2 mg/kg. Applied as a
spray to apples and peaches, residues are generally less than 1 mg/kg
after a 3 weeks preharvest period. On forage crops, application of
phosmet at 1 kg a.i./ha produced residues of the order of 50 mg/kg
which declined to less than 5 mg/kg after 14 days. Because phosmet is
not excreted in the milk, cattle can graze recently treated pastures.
Both phosmet and its oxygen analogue are of low persistence in
plants and animals. In mammals phosmet is rapidly metabolised, mainly
to innocuous water-soluble compounds. The metabolites principally
excreted are phthalic acid and phthalamic acid with only minute traces
of phosmet or its oxygen analogue.
Plants treated with phosmet tend to retain it on the surface
where it is washed off or weathered. In plant tissues conversion to
phthalic or phthalamic acid again takes place and these undergo
further breakdown.
Phosmet and its oxygen analogue are rapidly degraded in soils,
degradation being favoured by increasing soil alkalinity and moisture
content. Apart from simple chemical hydrolysis, metabolism by
microorganisms also occurs and it has been stated that phosmet is
totally biodegradable.
Processing in the canning industry by washing with dilute alkali,
bleaching or sterilising will remove at least 50% of the phosmet from
fruit and vegetables.
Several analytical methods suitable for regulatory purposes are
available. These include a colorimetric method which determines the
phthalimide moiety after conversion to anthranilic acid and gas
chromatographic methods with either an alkali flame ionisation or a
flame photometric detector. There is also an enzyme inhibition.
REFERENCES
Ackermann, H., and Engst, R. Occurrence of organophosphorus
1970 insecticides in the foetus. Arch. Toxikol., 26:17.
Adeishvili, L.G. Pesticide residues in apple fruits. Tr.
1973 Nauchno-Issled. Inst. Zashch. Rast (Toflis),
25:3-5.
Ali Niazee, M.T., and Stafford, E.M. Management of grape pests in
1973 Central California vineyards. I. Cultural and
chemical control of Patynota stultana on
grapes. J. Econ. Entomol., 66 (1): 154-7
Andrichuk, B.V. Phthalophos as an acaricide for the control of
1971a sheep psoroptosis. Tr. Yses. Nauch-Issled. Inst.
Vet. Sanit., 38: 264-70
Andrichuk, B.V. Acaricidal dusts for the control of sheep
1971b psoroptosis. Tr. Vses. Nauch-Issled. Inst.Vet.
Sanit., 39: 363-8
Anon. (Environmental Protection Agency, Washington, D.C.)
1969 N-(mercaptomethyl) phthalimide S-(O,O
-dimethyl phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
34 (63): 6041.
1971a Ibid., 36(59); 5690.
1971b Ibid., 36(132): 12898-9
1971c Ibid., 36 (152): 14471-2
Anon. (Food Drug Administration, Washington, D.C.) New animal
1971d drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide S-(O,O-dimethyl
phosphorodithioate). Federal Register, 36(231):
22829.
Anon. (Environmental Protection Agency, Washington, D.C.)
1973 N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate) and
residues. Federal Register, 38(55): 7459-60.
Anon. (Food Drug Administration, Washington, D.C.) New animal
1974 drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate). Federal
Register, 39(172): 32025-6.
Anon. (Environmental Protection Agency, Washington, D.C.)
1975 N-(mercaptomethyl) phthalimide S.(O,O-
dimethyl-phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
40(48): 11352-3.
Barney, J.E., Ja, W.Y., and Shelman, D.L. Analytical method:
1972 for pesticides, plant growth regulators and food
additives. (Academic Press, Ed. G. Zweig and J.
Sherma), Vol. VI, pp. 408.134
Batchelder, G.H., and Patchett, G.G. American Chemical Society,
1965 149th Meeting, Division of Agriculture and Food
Chemistry, Paper No. 37.
Batchelder, G.H., Patchett, G.G., and Menn, J.J. Analytical
1967 methods for pesticides, plant growth regulators
and food additives (Academic Press, Ed. G. Zweig),
Vol. V, pp. 257-275.
Bidleman, T.F., Nowlan, B., and Frei, R.W. Metallofluorescent
1972 indicators as spray reagents for the in situ
determination of organophosphorus pesticides in
thin layer chromatograms. Anal. Chim. Acta, 60(1):
13-23.
Boiko, I.B., and Popova, N.G. Effect of some organophosphorus
1970 pesticides on the biological value of vegetable
crops. Med. Zh. Uzb., 9: 35-7.
Bowman, M.C., and Beroza, M. Analysis of Imidan colorimetrically
1965 and by electron-affinity gas chromatography. J.
Assoc. Offic. Anal. Chem., 48(5): 922-6.
Bowman, M.C. and Beroza, M. Determination of Imidan and Imidoxon
1966 in sweet corn by gas chromatography with flame
photometric detection. J. Assoc. Offic. Anal.
Chem., 49(6): 1154-1157.
Bowman, M.C. and Beroza, M. Note on extraction of a polar insecticide
1967 (Imidan) from milk. J. Assoc. Offic. Anal. Chem.,
50(4): 940-941.
Bowman, M.C., Beroza, M., and Leuck, D.B. Procedures for extracting
1968 residues of phosphorus insecticides and
metabolites from field treated crops. J. Agr. Food
Chem., 16(5): 796-80.
Butt, B.A., White, L.D., Moffit, H.R., Hathaway, D.O., and
1973 Schoenleber, L.G. Integration of sanitation,
insecticides, and sterile moth releases for
suppression of populations of codling moths in the
Wenas Valley of Washington. Environ. Entomol.,
2(2): 208-13.
Chamberlain, W.F. A study of the dermal treatment of a 1965 steer
with C14-labelled Imidan. J. Econ. Entomol.,
58(1):51-55.
Chirikashvili, R.V., Abbasov, T.G., and Frolov, B.A. Duration
1970 of persistence of phthalophos in organs and
tissues of hens during oral administration. Tr.
Vses. Nauch-Issled. Inst. Vet. Sanit., 37: 175-8.
Del Rivero, J.M., Lafuente, M., and Lazaro, E. New products
1971 for control of the potato beetle, Leptinotarsa
decemlineata, resistant to chlorinated
insecticides. An. Inst. Nac. Invest. Agr. (Spain),
Ser. Prot. Veg. (1): 183-7.
Dorough, H.W., Randolph, N.M., and Wimbish, G.H. Imidan, and
1965 trichlorfon residues on Coastal bermudagrass.
Texas A&M University/Texas Agricultural Experiment
Station. Progress Report PR-2385.
Drumond, R.O., Ernst, S.E., Trevino, J.L., Gladney, W.J., and
1972 Graham, O.H. Boophilus annulatus and B.
microplus. Sprays and dips of insecticides for
control on cattle. J. Econ. Entomol., 65(5):
1354-7.
Forsythe, H.Y., and Hall, F.R. Control of the plum Curculio in
1972 Ohio. J. Econ. Entomol., 65(6): 1703-6.
Frei, R.W., and Mallet, V. Quantitative thin-layer chromatography
1971 of organothiophosphorus pesticides by in situ
fluorimetry. Intern. J. Environ. Anal. Chem., 1:
99.
Frolov, B.A. Activity of some organo-phosphorus and carbamate
1970 compounds for fowl ectoparasites. (Argas
persicus and Cimex lectularius) Tr., Vses.
Nauch-Issled. Inst. Vet. Sanit., 37: 334-43
Gorelik, L.D., Kushchinskaya, In.N., and Nemets, S.M. Effect
1973a of technological conditions on the content of
phthalophos in canned food. Konserv. Ovoshchesush.
Prom. 4: 8-10.
Gorelik, L.D., Kushchinskaya, I.N., and Sazonova, A.R. Lowering
1973b the phthalophos level during the preparation of
canned apple sauce. Konserv. Ovoshchesush. Prom.
7: 21-3.
Gribkova, N.I. Effectiveness of insecticides against leaf-miner
1973 moths. Khim. Sel. Khoz., 11(8):592-4.
Gutenmann, W.H., Bache, C.A., and Lisk, D.J. Determination
1965 of Imidan and Imidoxon in crops by preparative
thin layer and by gas chromatography. J. Gas
Chromat., 3: 350-52.
Holdsworth, R.P. Realities of integrated insect control.
1973 Proc. Ohio State Hort. Soc., 126: 89-92.
Ionova, I., Zhecheva, G., and Dimitrov, K. Residual amounts
1974 of Sevin, Neguvon, and Imidan in the meat of pigs
treated against ectoparasites. Vet-Med Nauki,
11(5): 28-34.
Johnson, J.C., and Bowman, M.C. Fate of Bidrin and Imidan
1968 when fed in silage to lactating dairy cows. J.
Dairy Science, 51(8): 1225-8.
King, N.L., and Best, D.R. Unpublished report from Hawthorn Park
1971 Research Laboratories Pty-Ltd. to Stauffer Chem.
Co. (Australia) Pty. Ltd.
King, N.L., and Ferguson, A.B. Unpublished report from Hawthorn
1971 Park Research laboratories Pty. Ltd. to Stauffer
Chem. Co. (Australia) Pty. Ltd.
Klisenko, M.A., and Pis'mennaya, M.V. Effects of UV radiation
1973 on dialkyldithiophosphate pesticides. Khim. Sel.
Khoz., 11(12): 916-8.
Klunker, R. Current status of potato beetle control, especially
1974 with respect to the resistance problem.
Nachrichtenbl. Pflanzenschutz DDR 28(9). 191-6.
Kovac, J., Batora, V., Moracvik, J., and Benusova, M. Persistence
1968 of O,O-dimethyl-S-(phthalimidomethyl)
dithiophosphate residues in vegetables and fruits.
Agrochimia (Bratislava), 8(3): 77-81.
Krijgsman, W., and Van de Kamp. C.G. Analysis of organophosphorus
1976 pesticides by capillary gas chromatography with
flame photometric detection. J. Chromat.,
117(1): 201-5.
Large, G.B., and Pitt, L.S. Thiophosphate synergists. U.S. Patent
1975 3, 886, 273, 27 May 1975, Stauffer Chemical Co.
Leuck, D.B., and Bowman, M.C. Imidan insecticide residues
1968 (Imidan and Imidoxon): their persistence in corn,
grass and soybeans. J. Econ. Entomol., 61(3).
705-7.
Loomis, E.C., Noorderhaven, A., and Roulston, W.J. Control of
1972 Southern cattle tick by pour-on animal systemic
insecticides. J. Econ. Entomol., 65: 1638-41.
Luke, M.A., Froberg, J.E., and Masumoto, H.T. Extraction and
1975 clean-up of organo-chlorine, organo-phosphorus,
organo-nitrogen and hydrocarbon pesticides in
produce for determination by gas-liquid
chromatography. J. Assoc. Offic. Anal. Chem.
58(5): 1020-6.
Mardzhanyan, G.M., Semerdzhyan, O.G., Ust'yan, A.K. and Manukyan,
1973 Z.S. Possibility of controlling Phytonomus with
organo-phosphorus insecticides. Izv. Sel'skokhoz.
Nauk, 16(11-12): 36-41.
McBain, J.B., Menn, J.J., and Casida, J.E. Metabolism of
1968 carbonyl-C14 labelled Imidan,
N-(Mercapto-methyl) phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats
and cockroaches. J. Agr. Food Chem., 16(5):
813-820.
Miller, M.C., Shaw, F.R., and Smith, C.T. The comparative residual
1969 life of two formulations of Imidan on alfalfa. J.
Econ. Entomol., 62(3): 720-1.
Mistric, W.J., and Smith, F.D. Organophosphorus and carbamate
1970 insecticides as substitutes for DDT in controlling
the tobacco flea beetle on flue cured tobacco. J.
Econ. Entomol., 63: 509-11.
Nakotomi, K., and Ishibe, K. (Hokko Chemical Industry Co. Ltd.)
1975 Insecticidal synergism with acephate
phosphorothioate mixtures. Japanese Patent 75 71,
838, 14 June 1975.
Nepoklonov, A.A., Nepoklonova, M.I., and Pavlova, N.V. Acaricidal
1969 properties of phthalophos.Tr., Vses. Nauch-Issled.
Inst. Vet. Sanit., 34: 348-50.
Nesterenko, N.I. Organophosphorus preparations used to control sugar
1970 beet pests. Khim. Sel. Khoz., 8: 382-3.
1973 Ibid., 11(2): 113-6.
New South Wales Department of Agriculture. Extracts from the 1966-7
1969 annual report of the Entomology Branch. PANS, 15
(2): 225.
Nikiforov, A.M., and Mamaev, K.A. Testing of new chemical
1972 preparations (cotton pesticides). Khlopkovodstvo,
8: 31-2.
Novikova, K.F., and Mel'tser, F.R. Chromatophotometric method
1972 for determining residual amounts of phthalophos in
food products of plant origin. Probl. Anal. Khim.,
2: 95-99.
Novikova, K.F., Mel'tser, F.R., Makarova, S.V., and Navmova, V.A.
1969 Spectrophotometric determination of residual
phthalophos in apples. Gig. Primen. Toksikol.
Pestits. Klin. Otravi, 7: 595-601.
Novikova, K.F., and Varlamova, T.A. Spectrophotometric
1973 determination of microgram quantities of
phthalophos and phosalone in natural waters.
Metody Opred. Pestits. Vode, 1: 98-103.
Omarov, L.M. Phthalophos and Sevin dusts to control mites.
1974 Veterinarya. 4: 37-8.
Omelyuta, V.P. Effectiveness of insecticides against Grapholitha
1972 molesta. Khim. Sel. Khoz., 10(7): 516-7.
Otaci, C., Turhan, K., and Barkin, S. Imidan residues on peaches.
1972 Bitki Koruma Bul., 12(1): 45-8.
Pfadt, R.E., Lloyd, J.E., and Spackman, E.W. Power dusting with
1975 organo-phosphorus insecticides to control the
sheep ked. J. Econ. Entomol., 68(4): 468-70.
Popov, P.F. Effectiveness of different preparations against
1973 caterpillars of fig Glyphipterygidae. Khim. Sel.
Khoz., 11(8): 595-6.
Ripley, B.D., Wilkinson, R.J., and Chau, A.S.Y. Multi-residue
1974 analysis of 14 organo-phosphorus pesticides in
natural waters. J. Assoc. Offic. Anal. Chem.,
57(5): 1033-42.
Rogoff, W.M., Brody, G., Roth, A.R., Batchelder, G.H., Meyding,
1967 G.D., Bigley, W.S., Gretz, G.H., and Orchard, R.
Efficacy, cholinesterase inhibition and residue
persistence of Imidan for the control of cattle
grubs. J. Econ. Entomol., 60(3): 640-6.
Ruzimuradov, A., Valiev, B., and Matchanov, S. Test of insecticides
1973 against pasture ground stable flies and gnats.
Mater. Konf. Molodykh. Uch. Uzb. Sel'sk Khoz. 7th
1972: 60-2. (Published in Veterinarya, 1973, ed.
by Sh. A. Azimov et al).
Schutzmann, R.L. Note on improved spray reagents for TLC
1970 fluorogenic detection of cholinesterase
inhibitors. J.Assoc. Offic. Anal. Chem., 53(5):
1056-7.
Schutzmann, R.L., and Barthel, W.F. Indoxyl acetate spray reagent
1969 for fluorogenic detection of cholinesterase
inhibitors in environmental samples. J. Assoc.
Offic. Anal. Chem., 52(1): 151-6.
Semerdzhyan, O.G. Poisoning of rabbits fed Phthalophos treated
1972 alfalfa. Izv. Sel'skokhoz. Nauk. 15 (6): 77-9.
Sevost'yanov, A.Z., and Ushakova, N.N. Acaricidal effectiveness of
1971 some pesticides against Ixodes mites during tests
in samples of sheep wool. Tr. Stavropol
Sel'skokhoz. Inst., No. 34 (Vol 4): 74-6.
Shapovalova, G.K., Sedykh, A.S., and Abelentseva, G.M.
1970 Effectiveness of using phthalophos and
benzophosphate to combat gooseberry moth
(Zophodia convolutella), and insecticide
residues on the berries. Khim. Sel. Khoz. 8(8):
594-5.
Sharova, V.M., and Kozhemyakin, N.D. Photoelectro-colorimetric
1973 determination of metaphos and phthalophos in
tissues of experimentally poisoned birds. Khim.
Sel. Khoz, 11 (3): 229-30.
Shaw, F.R., Callahan, R.A., and Miller, M.C. Rates of disappearance
1966 of Zolone and Imidan from alfalfa. J. Econ.
Entomol., 59(6): 1524-5.
Snelson, J.T. Private communication from Department of Primary
1976 Industry, Australia.
Speirs, R.D., and Lang, J.H. Contact, residue and vapour toxicity
1970 of new insecticides to stored products insects.
II. USDA Agricultural Research Service, Marketing
Research Report No. 885.
Storherr, R.W., Murray, E.J., Klein, I., and Rosenberg, L.A.
1967 Sweep co-distillation clean-up of fortified edible
oils for determination of organo-phosphate and
chlorinated hydrocarbon pesticides. J. Assoc.
Offic. Anal. Chem., 50(3): 605-15.
Surorov, P.S. Acaricidal effect of ciodrin, phthalophos and
1970 chlorophos on cutaneous mites, cattle psoroptosis
causative agents. Tr., Vses. Nauch Issled. Inst.
Vet. Sanit., 35: 379-82.
Tanabe, M., Dehn, R.L., and Bramhall, R.R. The photochemistry
1974 of Imidan in diethyl ether. J. Agr. Food Chem.,
22(1): 54-6.
Thomas, F.J.D., and Taylor, A.S. (ICI of Australia and New Zealand
1971 Ltd.) Apparatus and compositions for controlling
cattle ticks. South African Patent 69 06, 036 22
February 1971.
Uctani, T., Hirano, T., and Tsuchitate, M. (Kumiai Chem. Industry
1974 Co. Ltd.) Acaricidal and insecticidal
compositions. Japanese Patent 74 15, 776, 17 April
1974.
Van Cleave, H.V., and Anderson, A.L., Field control tests for pecan
1971 insects. Proc. Tex. Pecan Grow. Ass.: 32-3
Vernon, J.D.R., and Gould, H.J. Further trials on alternatives
1972 to DDT for the control of pre-blossom pests on
apple and pear. Plant Pathol., 21(1). 1-9.
Vrochinskii, K.K. Accumulation of pesticides in hydrobiocts
1971 as a measure for evaluating experimental water
toxicology. Eksp. Vod. Toksikol. 149-63.
Watts, R.R., and Storherr, R.W. Sweep do-distillation clean
1967 up of milk for determination of organophosphate
and chlorinated hydrocarbon pesticides. 50(3):
581-5.
Wilde, W.H.A. Low volume concentrate spraying and integrated 1973
control in some Ontario orchards. Proc. Ohio State
Hort. Soc. 126: 85-8.
Witkowska, D., and Wojnarowska, P. Effectiveness of some chemical
1971 preparations against Anthonomus pomorum (apple
weevil). Biol. Inst. Ochr. Rosl. (48): 357-66.
Woodard Research Corporation. Unpublished report.
1963
Zatserkovskaya, G.A. Evaporation of insecticides from different
1974 types of soils. Zashch. Rast (Kiev), 20: 61-6.
TABLE 3. Residues of phosmet in various crops from supervised spraying trials
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Apples Netherlands 1.5 1 0 1.8(1.3-2.1 0
(var. Lombertscalville) (2000 1/ha) 7 0.9(0.7-1.2) 0
14 0.6(0.3-0.9 0
21 0.5(0.3-0.6) 0
28 0.2(0.1-0.2) 0
Netherlands 1.5 1 0 2.4(1.5-3.4) 0.04(0.01-0.07)
(var. (2000 l/ha) 7 2.0(1.7-2.3) 0.06(0.04-0.09)
Goudreinette) 14 1.4(0.6-2.0) 0.06(0.03-0.07)
21 0.4(0.2-0.6) 0.03(0-0.06)
28 0.6(0.2-0.7) 0.02(0.01-0.03)
Apples New Zealand 1.5 9 2 2.0
9 1.7
15 1.1
23 0.7
1.5 9 1 4.3
3 3.6
7 4.0
14 2.9
21 3.0
28 2.1
Apples Australia 1.75 ? 0 2.2
(King & Best, 1 1.5
1971) 7 0.7
14 0.8
21 0.6
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
2.0 ? 0 2.8
1 1.5
7 1.6
14 1.0
21 0.7
Peaches Australia 1.0 1 0 3.8
(var. Golden 7 3.6
Queen) 14 1.1
21 0.5
Australia 1.0 1 1 3.4
(var.Maygold) 3 2.5
5 1.3
7 3.1
14 1.5
Peaches Australia 2.0 1 1 5.3
(var. Maygold) 3 6.2
5 4.2
7 3.9
14 6.2
Peaches Turkey 1.5 3 0 5.39
3 5.44
7 4.65
14 3.48
21 3.30
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Kiwifruit New Zealand 1.1 2 1 10.0
7 9.6
14 5.8
21 5.1
28 3.6
35 3.1
42 3.0
1.12 2 10 9.5
7.7
5.8
7.1
1.12 7 10 25.0
16.0
8.4
7.2
0.75 7 10 8.8
4.8
7.1
6.4
Grapefruit New Zealand 1.12 4 1 0.65
7 0.57
14 0.3
21 0.26
28 0.38
42 0.22
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Potatoes Netherlands 0.56 kg a.i./ 2 22 0.004 0
(var. ha(as 50% w.p.) (0.002-0.006)
Burmania)
Netherlands " 2 20 0.001 0
(var. Bintje) (0-0.003
Netherlands " 2 21 0.001 0
(var. (0.001-0.002)
Eigenheimer)
Alfalfa U.S.A. 1.1 kg a.i./ 1 1 43.5
(Shaw et al. ha(36% ec) 8 2.09
1966) 14 0.84
21 0.31
controls 0.18-0.44
Alfalfa U.S.A. 0.56 kg a.i. 1 0 33.86
(Miller et al. /ha(50% wp) 5 3.58
1969) 10 2.27
15 0.34
21 0.04
1.1 kg a.i. 1 0 57.11
/ha(50% wp) 5 10.74
10 3.42
15 1.52
21 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i. 1 0 20.95
/ha(36% ec) 5 3.87
10 2.09
15 0.94
21 0.00
Alfalfa U.S.A. 1.1 kg a.i. 1 0 44.42
/ha (36% ec) 5 9.87
10 5.06
15 2.00
21 0.37
Coastal U.S.A. 0.84 kg a.i. 1 0 37.93
bermuda (Dorough et /ha 1 32.66
grass al, 1965) 3 23.89
7 15.79
14 8.31
Coastal U.S.A. 0.28 kg a.i. 1 0 12.3 0.06
bermuda (Leuck and /ha 1 6.86 0.06
grass Bowman, 1968) 3 1.11 0.00
4 0.96 0.00
7 0.72 0.00
15 0.13 0.00
0.56 kg a.i. 1 0 27.0 0.13
/ha 1 16.4 0.13
3 2.82 0.02
4 2.30 0.02
7 1.78 0.00
15 0.36 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Coastal U.S.A. 1.1 kg a.i./ha 1 0 52.9 0.36
Bermuda 1 36.8 0.40
grass 3 7.02 0.07
4 6.34 0.06
7 3.72 0.02
15 0.57 0.00
Maize U.S.A. 0.28 kg a.i./ha 1 0 2.67 0.00
plants (Leuck and 1 1.00 0.00
(green Bowman, 1968) 2 0.39 0.00
weight 4 0.20 0.00
basis) 7 0.09 0.00
0.56 kg a.i./ha 1 0 7.07 0.01
1 Not sampled owing to rain
2 0.99 0.00
4 0.46 0.00
7 0.26 0.00
1.1 kg a.i./ha 1 0 10.9 0.02
1 Not sampled owing to rain
2 2.89 0.00
4 1.16 0.00
7 0.63 0.00
Soybean U.S.A. 0.28 kg ai./ 1 0 31.2 0.03
plants (Leuck and ha 1 21.1 0.02
(green Bowman, 1968) 2 15.0 0.01
weight 4 2.45 0.00
basis) 7 0.48 0.00
15 0.10 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i./ha 1 0 55.4 0.07
1 30.2 0.03
2 27.6 0.02
4 6.27 0.00
7 1.24 0.00
15 0.28 0.00
1.1 kg a.i./ha 1 0 82.2 0.11
1 55.4 0.07
2 43.8 0.04
4 14.7 0.01
7 2.62 0.00
15 0.76 0.00
Other fruits
Trials in New Zealand on Kiwifruit and grapefruit yielded the
residue data given in Table 3. In most cases the spray concentration
was slightly above the recommended upper limit of 0.1% a.i., but only
for the highest rate of application to Kiwifruit (7 applications of
0.112% a.i.) were residues in the whole fruit found to be above the
national tolerance of 10 mg/kg after the recommended 10-day waiting
period. Separate analyses of the skin and flesh revealed that the
residues were mainly concentrated in the skin. Four applications of
0.112% a.i. spray to grapefruit resulted in a residue of only 0.3
mg/kg in the whole fruit (0.05 mg/kg in the flesh) after the
recommended 14 day interval.
Alfalfa
In 1965, experiments were conducted in the United States to
determine the rate of disappearance of phosmet from alfalfa. An ec
formulation containing 36% a.i., w/v, was applied at the rate of 1.1
kg a.i./ha. Residues were determined by the anthranilic acid
colorimetric method over a period of 3 weeks after application. The
results are given in Table 3, and show that the phosmet residues had
declined to the levels found in untreated control samples after 21
days (Shaw et al., 1966).
In a subsequent trial on alfalfa (Miller et al., 1969), the
disappearance rates of phosmet after application in two different
formulations (the 36% ec and a 50% wp) were compared. Each formulation
was applied at the rate of 0.5 and 1 lb a.i./acre in a dilute spray
used at 100 gallons per acre. Each treatment was replicated twice, and
the crop was sampled over a 3 week period after treatment. The results
are summarised in Table 3.
In the USSR, the feeding of rabbits with alfalfa gathered 10 days
after it had been sprinkled with phosmet produced some depression of
cholinesterase activity. When the alfalfa was gathered 15 days after
treatment no depression was observed, and the residue level had
declined to 1/6 of that found 11 days after treatment and 1/15 of that
found 6 days after treatment (Semerdzhyan, 1972).
Other forage crops
Trials were conducted in the U.S.A. in which maize (for silage),
soybean plants, and coastal Bermuda grass were each sprayed at rates
of 0.28, 0.56 and 1.1 kg of phosmet per ha. Residues of phosmet and
its oxygenanalogue were determined in samples taken over a 15 day
period following treatment (Leuck and Bowman, 1968). The results are
given in Table 3, and indicate that in all cases the residues had
declined to under 1 mg/kg in 15 days or less. On the other hand, in a
trial on Coastal Bermuda grass at the Texas Agricultural Experiment
Station, in which phosmet was applied at 0.84 kg/ha, residues of about
8 mg/kg were found after 14 days (Dorough et al., 1965).
Leuck and Bowman analysed the concentrate used in their trial,
and found that it contained a trace of the oxygen-analogue equivalent
to 0.12% of the phosmet content. Thus some, at least, of the
0-analogue residues found probably originated from the spray liquor
rather than from environmental oxidation of phosmet.
FATE OF RESIDUES
General comments
The results presented in the foregoing section indicate that both
phosmet and its oxygen analogue are of low persistence in plants and
animals. The photochemistry of phosmet in diethyl ether solution was
studied by Tanabe et al. (1974), using a 450 watt UV lamp. They
isolated N-methylphthalimide and N-methoxymethylphthalimide as
photolysis products in low yield. Neither of these was insecticidally
active. No photoproduct containing P or S could be isolated from the
many minor components. Using UV radiation in the range 250-400 nm,
Klisenko and Pis'mennaya (1973) found a half-life of 70 minutes for
phosmet, and identified phthalimide, N-methoxymethylphthalimide and
phthalic acid as photolysis products, in addition to P-O analogues.
In animals
The metabolism of carbonyl-14C-labelled phosmet in rats
following oral administration was investigated by McBain et al.
(1968). Metabolites were excreted in the urine in the following
amounts (expressed as percentages of the administered dose): phthalic
acid 21.0%, phthalamic acid 40.7% and five minor metabolites
containing the phthaloyl moiety 10.9%. Not more than 0.04% of the dose
administered was excreted in the urine as unchanged phosmet or its
oxygen analogue. This study supported other evidence that phosmet is
rapidly metabolised in mammals, mainly to innocuous water soluble
compounds. For example, Chamberlain (1965) studied the fate of
14C-labelled phosmet following dermal application to a steer. It was
moderately absorbed by the skin of the animal, since 9.6% of the
applied dose was recovered in the excreta (nearly 8% in the urine)
within 7 days. It appeared that the compound was rapidly broken down
in the blood, and that the primary degradation occurred at the
nitrogen atom, resulting in the almost exclusive production of
phthalic and phthalamic acids.
Johnson and Bowman (1968) found no detectable residues of phosmet
or its oxygen analogue in the milk, urine or faeces of lactating dairy
cows fed with silage containing phosmet at a level providing 0.22
mg/kg/ day. Detection limits were 0.002 mg/kg for milk and urine, and
0.004 mg/kg for faeces.
Tests under laboratory conditions in the USSR (Vrochinskii, 1971)
showed that phosmet was stored only briefly by fish.
In plants
Trials on apples, peaches, lemons and potatoes showed that
neither phosmet nor its oxygen analogue is translocated from treated
to untreated portions of plants. Residues tend to remain on plant
surfaces and are easily brushed or washed off. They are degraded quite
rapidly by weathering.
Phosmet is hydrolysed in plant tissues and converted to phthalic
acid and/or phthalamic acid which is translocated in the plant,
decarboxylated, apparently by enzyme action, and further metabolised
to possible benzoic acid derivatives.
On apples, pears and peaches, the average half-life of phosmet
appears to be less than two weeks. The disappearance of residual
phosmet from standing corn plants was found to be rapid, but after
ensiling, the loss of phosmet was slow (about 50% loss in 92 days).
The acid conditions in the silage stack probably protected the phosmet
against spontaneous hydrolysis.
Kovac et al. (1968) reported the rapid decomposition of phosmet
to non-toxic substances in fruit and vegetables. After treatment with
a 0.25% phosmet spray, residue levels declined to 0.75 mg/kg in 1-12
days. In experiments on beet, carrots and tomatoes grown on a loamy
soil in the USSR, Boiko and Popova (1970) found no residues of phosmet
30-35 days after treatment with sprays of up to 0.6% a.i.
concentration.
In soils
Phosmet and its oxygen analogue are rapidly degraded in soils.
Degradation is favoured by increasing alkalinity and moisture content
and is less in autoclaved soil samples, indicating that hydrolysis of
phosmet is not dependent on moisture alone, but is due in some degree
to microbial action. The time for 50% degradation varied from 3 days
in a non-autoclaved loamy soil containing 10% moisture, to 19 days in
the same soil with only 2% moisture. It is stated that the phthalic
acid produced is readily metabolised and utilised by micro-organisms
so that phosmet represents a totally biodegradable pesticide leaving
no unmetabolisable residues in the environment (Snelson, 1976). The
vapour pressure of phosmet is fairly high, and under laboratory
conditions it has been shown to evaporate rapidly from peat soils, but
not from sandy soils (Zatserkovskaya, 1974).
In storage and processing
The effect of the washing, blanching and sterilisation operations
used in the canning industry on phosmet deliberately added to apples,
plums, cabbage, pears, green peas and red beet (by dipping them in a
0.4% aqueous dispersion of phosmet) has been studied in the USSR
(Gorelik et al., 1973a). Washing with 5 parts of a 0.5% NaOH solution
to one part of fruit reduced the phosmet content of apples and plums
by 50-55%. Blanching at 80°C with a liquid/fruit ratio of 5:1 removed
55% and 81% respectively of the initial phosmet loading of plums and
apples. The rate of phosmet destruction by sterilisation varied from
60% to 100% in a range of commodities, and about 38% in cabbage. No
phosmet residues were detected in canned apples and plums after
storage at 20°C for 12 months.
Similar studies were made of the effect of such processing on
phosmet residues in apples used for production of canned apple puree
as a baby food (Gorelik et al., 1973b). Blanching, crushing and
sterilisation reduced the residue level by 51%, 28% and 16-25%
respectively. The same workers studied the effects of deep freezing on
the residue dynamics of phosmet in carrots, cabbage and beet. It was
found that even prolonged deep freezing resulted in negligible
reductions of phosmet residues, and it was concluded that vegetables
containing phosmet in excess of the USSR tolerance of 0.25 mg/kg
should not be frozen prior to processing.
Evidence of residues in food in commerce or at consumption
No data were available.
METHODS OF RESIDUE ANALYSIS
Colorimetric methods
Much of the earlier work on residues resulting from supervised
trials was carried out using colorimetric procedures which determine
either the phthalimide moiety after conversion to anthranilic acid or
the phosphorodithioate moiety after conversion to phosphomolybdate.
The anthranilic acid method (Batchelder and Patchett, 1965) is the
more sensitive and specific of the two. Both methods are described in
detail by Batchelder et al.(1967), who also give typical figures for
the recovery of phosmet by these methods from various crops and animal
tissues. The anthranilic acid method is claimed to detect 2 micrograms
of phosmet reliably (giving a detection limit of 0.04 mg/kg for a 50
gram sample), and is applicable to animal or plant tissues. It will
also detect the oxygen analogue but is susceptible to interference
from folpet or azinphos-methyl. These compounds do not interfere with
the phosphomolybdate method, which includes a selective hydrolysis
step.
An alternative colorimetric procedure for determining the
phosphorodithioate moiety (Sharova and Kozhemyakin, 1973) involves the
formation of a yellow complex with copper sulphate, and has been
applied to the determination of phosmet residues in the tissues of
birds, with a detection limit of 0.5 mg/kg.
A method involving the liberation of formaldehyde by acid
hydrolysis of phosmet, and colour development with chromotropic acid,
has been applied to the measurement of phosmet residues in water, with
a detection limit of 0.01 mg/l (Novikova and Varlamova, 1973), and in
apples, with a detection limit of 0.5 mg/kg (Novikova et al., 1969).
Thin-layer chromatographic methods
Phosmet can be determined at the residue level by several of the
methods involving in situ fluorimetry on thin-layer chromatograms
which have been evolved for organophosphorus pesticides. For example,
Frei and Mallet (1971) used a chelate spray reagent consisting of a
1:2 mixture of salicyl-2-aldehyde-2-quinolylhydrazone and manganese
(II) chloride, which was applied after exposure of the plate to
bromine. They claim a visual detection limit of 0.04 µg phosmet.
Bidleman et al. (1972) used fluorescence quenched solutions of
palladium (II) - calcein and palladium(II)-calcein blue as sensitive
spray reagents for the in situ fluorometric determination of
organophosphorus pesticides including phosmet, and reported that as
little as 10.50 ng of a phosphorodithioate could be detected.
Methods have also been described in which thin-layer
chromatography is used as a clean-up procedure for phosmet residues
extracted from foods prior to determination of the eluted pesticide by
one of the colorimetric procedures referred to above. An example is
the method of Novikova and Mel'tser (1972), which uses the chromotopic
acid procedure after clean-up on silica gel plates. This method had a
detection limit of 0.6 mg/kg for phosmet residues in foods of plant
origin. Alternatively, the phosmet spot could be visualised on the
plate by spraying with bromophenol blue and silver nitrate, giving a
visual detection limit of 0.3 mg/kg.
Phosmet is also detectable on TLC plates by enzyme inhibition
methods. For example, Schutzmann and Barthel (1969) reported detection
limits of 0.5 µg and 0.05 µg for phosmet and its oxygen analogue
respectively, using horse serum cholinesterase with indoxyl acetate as
substrate. Schutzmann (1970) reported that the sensitivity of the
method could be improved by using either the acetate or butyrate of
N-methyl indoxol as substrate. Ackermann and Engst (1970) used beef
liver esterase with 1-napthyl acetate and Fast Blue B on silica gel G
plates to detect organophosphorus residues in rat foetal tissues. They
were able to detect 0.025 mg/kg of phosmet and 0.005 mg/kg of its
0-analogue by this method.
Gas chromatographic methods
Some early gas chromatographic determinations of phosmet residues
made use of the electron capture detector (ECD). For example, Bowman
and Beroza (1965) were able to determine phosmet (but not the
0-analogue) in milk and sweet corn by using the ECD, and Gutenmann et
al. (1965) applied it to the determination of phosmet (detection limit
0.1 mg/kg) and the O-analogue (0.2 mg/kg) in apples, potatoes, grapes
and alfalfa following a TLC clean-up.
Later methods have made use of either an alkali flame ionisation
detector (AFID) giving selective detection of phosphorus compounds, or
a flame photometric detector (FPD) operated in either the phosphorus
or the sulphur mode. Bowman and Beroza (1966) used the FPD with a
526 nm phosphorus filter to determine phosmet (detection limit 0.002
mg/kg) and the 0-analogue (0.004 mg/kg) in sweet corn, following
clean-up and separation on a silica gel column. The glc column packing
was 10% DC 200 on 80/100 mesh Gas Chrom Q. The column was operated at
200°C, and required conditioning with repeated injections of phosmet
and corn extract to obtain a constant response to the insecticide. The
same column packing was used by Watts and Storherr (1967) in
conjunction with an AFID for the determination of phosmet and other
organophosphorus insecticide residues in milk after a sweep
codistillation clean-up. They too found the need for thorough column
conditioning. Storherr et al. (1967) used essentially the same
procedure to determine organophosphorus pesticides including phosmet
in fortified samples of edible oils. The average recovery for phosmet
was 96%, and the method detected apparent levels in unfortified
samples ranging from less than 0.01 to 0.04 mg/kg.
Bowman and Beroza (1967) were able to determine phosmet extracted
from milk (in hexane/ether) without clean-up by using the FPD with a
394 nm sulphur filter. Bowman et al. (1968) investigated several
alternative extraction procedures for removing OP residues (including
phosmet and its O-analogue) from field treated crops, and found the
most efficient method to be soxhlet extraction for 4 hours with 10%
methanol in chloroform. Determination was by gas chromatography with
flame photometric detection using a phosphorus filter.
A gas chromatographic method employing the AFID (with rubidium
sulphate as alkali source) and a short, narrow bore column containing
10% DC 200 on 100/120 mesh Gas Chrom Q has been described by Barney et
al. (1972). For most crops, clean-up is achieved simply by shaking
with charcoal followed by filtration through a Millipore filter.
Cottonseed and cotton foliage require prior acetonitrile partition.
The lower limit of detection is given as 0.05 mg/kg and the method has
been successfully applied to a range of fruit, vegetable and fodder
crops. The authors state that the FPD in either the P or S mode may be
used instead of the AFID, but stress the importance of the small
particle size of the solid support to ensure adequate resolution of
the oxygen analogue from phosmet.
Several multi-residue gas chromatographic procedures which
include phosmet have been published recently. Some examples are:
(a) The method of Ripley et al. (1974) for OP residues in natural
waters (using the FPD).
(b) The method of Luke et al. (1975) for OP and nitrogen containing
pesticides in produce (using the AFID with a potassium chloride
source).
(c) The method of Krijgsman and Van de Kamp (1976) which utilises a
capillary column of SE-30 with a flame photometric detector.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The national tolerances shown in Table 4 have been reported to
the Meeting.
TABLE 4. National tolerances reported to the Meeting
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Australia Fat of meat of cattle 1 Snelson
Milk and milk products 1976
(fat basis) 0.2 "
Pome and stone fruit 1 "
Canada Apples, peaches and
pears 10
Plums 5
Netherlands Apples, pears 21 1
Potatoes 28 0.02
New Zealand Fruit 14 10
(Kiwifruit 10)
USA Alfalfa 40 Anon, 1969
Apples, peaches & pears 10 "
Meat & fat of cattle,
goats, hogs & sheep 0.2 Anon, 1969
Grapes 10 Anon, 1971a
Cherries 10 Anon, 1971b
Apricots, nectarines
and plums 5 " "
TABLE 4. (Cont'd.)
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Potatoes 0.1 Anon, 1971c
Sweet potatoes (from
post-harvest application 10 Anon, 1973
Almond hulls, blue berries,
corn forage and fodder
(including sweet corn,
field corn and popcorn),
cranberries, pea forage
and hay 10 Anon, 1975
Fresh corn, including sweet
corn (kernel and cob with
husk removed), corn grain
(including popcorn), and
peas 0.5 Anon, 1975
Meat, fat and meat by-products
of horses 0.2 Anon, 1975
Nuts 0.1 Anon, 1975
USSR Vegetables 0.25 Gorelik et
al, 1973b
Figs 0.25 Popov, 1973
APPRAISAL
As a systemic insecticide and acaricide against a range of
ectoparasites of animals, phosmet may be applied as a dilute aqueous
spray containing up to 0.5% a.i., as a ready-to-use "pour on"
formulation containing up to 4% a.i. and applied at up to 40 mg per kg
body weight, as a dust containing 1 to 2% a.i., or it may be
administered orally.
In plants, phosmet is used as a non-systemic pesticide and is
applied to a variety of crops to protect against insects and mites. At
the concentrations usually applied it can be effective without harming
some useful predators and this makes it of value for integrated
control programmes. On fruit, a 0.1% spray has been used on pasture
and forage crops up to 0.75 kg/ha, and on cereal crops at 35 g/ha (ULV
spray). It has been used at 3-5 kg/ha on cotton.
Data available on residues in meat of cattle is not extensive,
but would be in accord with a maximum level of 0.2 mg/kg. Applied as a
spray to apples and peaches, residues are generally less than 1 mg/kg
after a 3 weeks preharvest period. On forage crops, application of
phosmet at 1 kg a.i./ha produced residues of the order of 50 mg/kg
which declined to less than 5 mg/kg after 14 days. Because phosmet is
not excreted in the milk, cattle can graze recently treated pastures.
Both phosmet and its oxygen analogue are of low persistence in
plants and animals. In mammals phosmet is rapidly metabolised, mainly
to innocuous water-soluble compounds. The metabolites principally
excreted are phthalic acid and phthalamic acid with only minute traces
of phosmet or its oxygen analogue.
Plants treated with phosmet tend to retain it on the surface
where it is washed off or weathered. In plant tissues conversion to
phthalic or phthalamic acid again takes place and these undergo
further breakdown.
Phosmet and its oxygen analogue are rapidly degraded in soils,
degradation being favoured by increasing soil alkalinity and moisture
content. Apart from simple chemical hydrolysis, metabolism by
microorganisms also occurs and it has been stated that phosmet is
totally biodegradable.
Processing in the canning industry by washing with dilute alkali,
bleaching or sterilising will remove at least 50% of the phosmet from
fruit and vegetables.
Several analytical methods suitable for regulatory purposes are
available. These include a colorimetric method which determines the
phthalimide moiety after conversion to anthranilic acid and gas
chromatographic methods with either an alkali flame ionisation or a
flame photometric detector. There is also an enzyme inhibition.
REFERENCES
Ackermann, H., and Engst, R. Occurrence of organophosphorus
1970 insecticides in the foetus. Arch. Toxikol., 26:17.
Adeishvili, L.G. Pesticide residues in apple fruits. Tr.
1973 Nauchno-Issled. Inst. Zashch. Rast (Toflis),
25:3-5.
Ali Niazee, M.T., and Stafford, E.M. Management of grape pests in
1973 Central California vineyards. I. Cultural and
chemical control of Patynota stultana on
grapes. J. Econ. Entomol., 66 (1): 154-7
Andrichuk, B.V. Phthalophos as an acaricide for the control of
1971a sheep psoroptosis. Tr. Yses. Nauch-Issled. Inst.
Vet. Sanit., 38: 264-70
Andrichuk, B.V. Acaricidal dusts for the control of sheep
1971b psoroptosis. Tr. Vses. Nauch-Issled. Inst.Vet.
Sanit., 39: 363-8
Anon. (Environmental Protection Agency, Washington, D.C.)
1969 N-(mercaptomethyl) phthalimide S-(O,O
-dimethyl phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
34 (63): 6041.
1971a Ibid., 36(59); 5690.
1971b Ibid., 36(132): 12898-9
1971c Ibid., 36 (152): 14471-2
Anon. (Food Drug Administration, Washington, D.C.) New animal
1971d drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide S-(O,O-dimethyl
phosphorodithioate). Federal Register, 36(231):
22829.
Anon. (Environmental Protection Agency, Washington, D.C.)
1973 N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate) and
residues. Federal Register, 38(55): 7459-60.
Anon. (Food Drug Administration, Washington, D.C.) New animal
1974 drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate). Federal
Register, 39(172): 32025-6.
Anon. (Environmental Protection Agency, Washington, D.C.)
1975 N-(mercaptomethyl) phthalimide S.(O,O-
dimethyl-phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
40(48): 11352-3.
Barney, J.E., Ja, W.Y., and Shelman, D.L. Analytical method:
1972 for pesticides, plant growth regulators and food
additives. (Academic Press, Ed. G. Zweig and J.
Sherma), Vol. VI, pp. 408.134
Batchelder, G.H., and Patchett, G.G. American Chemical Society,
1965 149th Meeting, Division of Agriculture and Food
Chemistry, Paper No. 37.
Batchelder, G.H., Patchett, G.G., and Menn, J.J. Analytical
1967 methods for pesticides, plant growth regulators
and food additives (Academic Press, Ed. G. Zweig),
Vol. V, pp. 257-275.
Bidleman, T.F., Nowlan, B., and Frei, R.W. Metallofluorescent
1972 indicators as spray reagents for the in situ
determination of organophosphorus pesticides in
thin layer chromatograms. Anal. Chim. Acta, 60(1):
13-23.
Boiko, I.B., and Popova, N.G. Effect of some organophosphorus
1970 pesticides on the biological value of vegetable
crops. Med. Zh. Uzb., 9: 35-7.
Bowman, M.C., and Beroza, M. Analysis of Imidan colorimetrically
1965 and by electron-affinity gas chromatography. J.
Assoc. Offic. Anal. Chem., 48(5): 922-6.
Bowman, M.C. and Beroza, M. Determination of Imidan and Imidoxon
1966 in sweet corn by gas chromatography with flame
photometric detection. J. Assoc. Offic. Anal.
Chem., 49(6): 1154-1157.
Bowman, M.C. and Beroza, M. Note on extraction of a polar insecticide
1967 (Imidan) from milk. J. Assoc. Offic. Anal. Chem.,
50(4): 940-941.
Bowman, M.C., Beroza, M., and Leuck, D.B. Procedures for extracting
1968 residues of phosphorus insecticides and
metabolites from field treated crops. J. Agr. Food
Chem., 16(5): 796-80.
Butt, B.A., White, L.D., Moffit, H.R., Hathaway, D.O., and
1973 Schoenleber, L.G. Integration of sanitation,
insecticides, and sterile moth releases for
suppression of populations of codling moths in the
Wenas Valley of Washington. Environ. Entomol.,
2(2): 208-13.
Chamberlain, W.F. A study of the dermal treatment of a 1965 steer
with C14-labelled Imidan. J. Econ. Entomol.,
58(1):51-55.
Chirikashvili, R.V., Abbasov, T.G., and Frolov, B.A. Duration
1970 of persistence of phthalophos in organs and
tissues of hens during oral administration. Tr.
Vses. Nauch-Issled. Inst. Vet. Sanit., 37: 175-8.
Del Rivero, J.M., Lafuente, M., and Lazaro, E. New products
1971 for control of the potato beetle, Leptinotarsa
decemlineata, resistant to chlorinated
insecticides. An. Inst. Nac. Invest. Agr. (Spain),
Ser. Prot. Veg. (1): 183-7.
Dorough, H.W., Randolph, N.M., and Wimbish, G.H. Imidan, and
1965 trichlorfon residues on Coastal bermudagrass.
Texas A&M University/Texas Agricultural Experiment
Station. Progress Report PR-2385.
Drumond, R.O., Ernst, S.E., Trevino, J.L., Gladney, W.J., and
1972 Graham, O.H. Boophilus annulatus and B.
microplus. Sprays and dips of insecticides for
control on cattle. J. Econ. Entomol., 65(5):
1354-7.
Forsythe, H.Y., and Hall, F.R. Control of the plum Curculio in
1972 Ohio. J. Econ. Entomol., 65(6): 1703-6.
Frei, R.W., and Mallet, V. Quantitative thin-layer chromatography
1971 of organothiophosphorus pesticides by in situ
fluorimetry. Intern. J. Environ. Anal. Chem., 1:
99.
Frolov, B.A. Activity of some organo-phosphorus and carbamate
1970 compounds for fowl ectoparasites. (Argas
persicus and Cimex lectularius) Tr., Vses.
Nauch-Issled. Inst. Vet. Sanit., 37: 334-43
Gorelik, L.D., Kushchinskaya, In.N., and Nemets, S.M. Effect
1973a of technological conditions on the content of
phthalophos in canned food. Konserv. Ovoshchesush.
Prom. 4: 8-10.
Gorelik, L.D., Kushchinskaya, I.N., and Sazonova, A.R. Lowering
1973b the phthalophos level during the preparation of
canned apple sauce. Konserv. Ovoshchesush. Prom.
7: 21-3.
Gribkova, N.I. Effectiveness of insecticides against leaf-miner
1973 moths. Khim. Sel. Khoz., 11(8):592-4.
Gutenmann, W.H., Bache, C.A., and Lisk, D.J. Determination
1965 of Imidan and Imidoxon in crops by preparative
thin layer and by gas chromatography. J. Gas
Chromat., 3: 350-52.
Holdsworth, R.P. Realities of integrated insect control.
1973 Proc. Ohio State Hort. Soc., 126: 89-92.
Ionova, I., Zhecheva, G., and Dimitrov, K. Residual amounts
1974 of Sevin, Neguvon, and Imidan in the meat of pigs
treated against ectoparasites. Vet-Med Nauki,
11(5): 28-34.
Johnson, J.C., and Bowman, M.C. Fate of Bidrin and Imidan
1968 when fed in silage to lactating dairy cows. J.
Dairy Science, 51(8): 1225-8.
King, N.L., and Best, D.R. Unpublished report from Hawthorn Park
1971 Research Laboratories Pty-Ltd. to Stauffer Chem.
Co. (Australia) Pty. Ltd.
King, N.L., and Ferguson, A.B. Unpublished report from Hawthorn
1971 Park Research laboratories Pty. Ltd. to Stauffer
Chem. Co. (Australia) Pty. Ltd.
Klisenko, M.A., and Pis'mennaya, M.V. Effects of UV radiation
1973 on dialkyldithiophosphate pesticides. Khim. Sel.
Khoz., 11(12): 916-8.
Klunker, R. Current status of potato beetle control, especially
1974 with respect to the resistance problem.
Nachrichtenbl. Pflanzenschutz DDR 28(9). 191-6.
Kovac, J., Batora, V., Moracvik, J., and Benusova, M. Persistence
1968 of O,O-dimethyl-S-(phthalimidomethyl)
dithiophosphate residues in vegetables and fruits.
Agrochimia (Bratislava), 8(3): 77-81.
Krijgsman, W., and Van de Kamp. C.G. Analysis of organophosphorus
1976 pesticides by capillary gas chromatography with
flame photometric detection. J. Chromat.,
117(1): 201-5.
Large, G.B., and Pitt, L.S. Thiophosphate synergists. U.S. Patent
1975 3, 886, 273, 27 May 1975, Stauffer Chemical Co.
Leuck, D.B., and Bowman, M.C. Imidan insecticide residues
1968 (Imidan and Imidoxon): their persistence in corn,
grass and soybeans. J. Econ. Entomol., 61(3).
705-7.
Loomis, E.C., Noorderhaven, A., and Roulston, W.J. Control of
1972 Southern cattle tick by pour-on animal systemic
insecticides. J. Econ. Entomol., 65: 1638-41.
Luke, M.A., Froberg, J.E., and Masumoto, H.T. Extraction and
1975 clean-up of organo-chlorine, organo-phosphorus,
organo-nitrogen and hydrocarbon pesticides in
produce for determination by gas-liquid
chromatography. J. Assoc. Offic. Anal. Chem.
58(5): 1020-6.
Mardzhanyan, G.M., Semerdzhyan, O.G., Ust'yan, A.K. and Manukyan,
1973 Z.S. Possibility of controlling Phytonomus with
organo-phosphorus insecticides. Izv. Sel'skokhoz.
Nauk, 16(11-12): 36-41.
McBain, J.B., Menn, J.J., and Casida, J.E. Metabolism of
1968 carbonyl-C14 labelled Imidan,
N-(Mercapto-methyl) phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats
and cockroaches. J. Agr. Food Chem., 16(5):
813-820.
Miller, M.C., Shaw, F.R., and Smith, C.T. The comparative residual
1969 life of two formulations of Imidan on alfalfa. J.
Econ. Entomol., 62(3): 720-1.
Mistric, W.J., and Smith, F.D. Organophosphorus and carbamate
1970 insecticides as substitutes for DDT in controlling
the tobacco flea beetle on flue cured tobacco. J.
Econ. Entomol., 63: 509-11.
Nakotomi, K., and Ishibe, K. (Hokko Chemical Industry Co. Ltd.)
1975 Insecticidal synergism with acephate
phosphorothioate mixtures. Japanese Patent 75 71,
838, 14 June 1975.
Nepoklonov, A.A., Nepoklonova, M.I., and Pavlova, N.V. Acaricidal
1969 properties of phthalophos.Tr., Vses. Nauch-Issled.
Inst. Vet. Sanit., 34: 348-50.
Nesterenko, N.I. Organophosphorus preparations used to control sugar
1970 beet pests. Khim. Sel. Khoz., 8: 382-3.
1973 Ibid., 11(2): 113-6.
New South Wales Department of Agriculture. Extracts from the 1966-7
1969 annual report of the Entomology Branch. PANS, 15
(2): 225.
Nikiforov, A.M., and Mamaev, K.A. Testing of new chemical
1972 preparations (cotton pesticides). Khlopkovodstvo,
8: 31-2.
Novikova, K.F., and Mel'tser, F.R. Chromatophotometric method
1972 for determining residual amounts of phthalophos in
food products of plant origin. Probl. Anal. Khim.,
2: 95-99.
Novikova, K.F., Mel'tser, F.R., Makarova, S.V., and Navmova, V.A.
1969 Spectrophotometric determination of residual
phthalophos in apples. Gig. Primen. Toksikol.
Pestits. Klin. Otravi, 7: 595-601.
Novikova, K.F., and Varlamova, T.A. Spectrophotometric
1973 determination of microgram quantities of
phthalophos and phosalone in natural waters.
Metody Opred. Pestits. Vode, 1: 98-103.
Omarov, L.M. Phthalophos and Sevin dusts to control mites.
1974 Veterinarya. 4: 37-8.
Omelyuta, V.P. Effectiveness of insecticides against Grapholitha
1972 molesta. Khim. Sel. Khoz., 10(7): 516-7.
Otaci, C., Turhan, K., and Barkin, S. Imidan residues on peaches.
1972 Bitki Koruma Bul., 12(1): 45-8.
Pfadt, R.E., Lloyd, J.E., and Spackman, E.W. Power dusting with
1975 organo-phosphorus insecticides to control the
sheep ked. J. Econ. Entomol., 68(4): 468-70.
Popov, P.F. Effectiveness of different preparations against
1973 caterpillars of fig Glyphipterygidae. Khim. Sel.
Khoz., 11(8): 595-6.
Ripley, B.D., Wilkinson, R.J., and Chau, A.S.Y. Multi-residue
1974 analysis of 14 organo-phosphorus pesticides in
natural waters. J. Assoc. Offic. Anal. Chem.,
57(5): 1033-42.
Rogoff, W.M., Brody, G., Roth, A.R., Batchelder, G.H., Meyding,
1967 G.D., Bigley, W.S., Gretz, G.H., and Orchard, R.
Efficacy, cholinesterase inhibition and residue
persistence of Imidan for the control of cattle
grubs. J. Econ. Entomol., 60(3): 640-6.
Ruzimuradov, A., Valiev, B., and Matchanov, S. Test of insecticides
1973 against pasture ground stable flies and gnats.
Mater. Konf. Molodykh. Uch. Uzb. Sel'sk Khoz. 7th
1972: 60-2. (Published in Veterinarya, 1973, ed.
by Sh. A. Azimov et al).
Schutzmann, R.L. Note on improved spray reagents for TLC
1970 fluorogenic detection of cholinesterase
inhibitors. J.Assoc. Offic. Anal. Chem., 53(5):
1056-7.
Schutzmann, R.L., and Barthel, W.F. Indoxyl acetate spray reagent
1969 for fluorogenic detection of cholinesterase
inhibitors in environmental samples. J. Assoc.
Offic. Anal. Chem., 52(1): 151-6.
Semerdzhyan, O.G. Poisoning of rabbits fed Phthalophos treated
1972 alfalfa. Izv. Sel'skokhoz. Nauk. 15 (6): 77-9.
Sevost'yanov, A.Z., and Ushakova, N.N. Acaricidal effectiveness of
1971 some pesticides against Ixodes mites during tests
in samples of sheep wool. Tr. Stavropol
Sel'skokhoz. Inst., No. 34 (Vol 4): 74-6.
Shapovalova, G.K., Sedykh, A.S., and Abelentseva, G.M.
1970 Effectiveness of using phthalophos and
benzophosphate to combat gooseberry moth
(Zophodia convolutella), and insecticide
residues on the berries. Khim. Sel. Khoz. 8(8):
594-5.
Sharova, V.M., and Kozhemyakin, N.D. Photoelectro-colorimetric
1973 determination of metaphos and phthalophos in
tissues of experimentally poisoned birds. Khim.
Sel. Khoz, 11 (3): 229-30.
Shaw, F.R., Callahan, R.A., and Miller, M.C. Rates of disappearance
1966 of Zolone and Imidan from alfalfa. J. Econ.
Entomol., 59(6): 1524-5.
Snelson, J.T. Private communication from Department of Primary
1976 Industry, Australia.
Speirs, R.D., and Lang, J.H. Contact, residue and vapour toxicity
1970 of new insecticides to stored products insects.
II. USDA Agricultural Research Service, Marketing
Research Report No. 885.
Storherr, R.W., Murray, E.J., Klein, I., and Rosenberg, L.A.
1967 Sweep co-distillation clean-up of fortified edible
oils for determination of organo-phosphate and
chlorinated hydrocarbon pesticides. J. Assoc.
Offic. Anal. Chem., 50(3): 605-15.
Surorov, P.S. Acaricidal effect of ciodrin, phthalophos and
1970 chlorophos on cutaneous mites, cattle psoroptosis
causative agents. Tr., Vses. Nauch Issled. Inst.
Vet. Sanit., 35: 379-82.
Tanabe, M., Dehn, R.L., and Bramhall, R.R. The photochemistry
1974 of Imidan in diethyl ether. J. Agr. Food Chem.,
22(1): 54-6.
Thomas, F.J.D., and Taylor, A.S. (ICI of Australia and New Zealand
1971 Ltd.) Apparatus and compositions for controlling
cattle ticks. South African Patent 69 06, 036 22
February 1971.
Uctani, T., Hirano, T., and Tsuchitate, M. (Kumiai Chem. Industry
1974 Co. Ltd.) Acaricidal and insecticidal
compositions. Japanese Patent 74 15, 776, 17 April
1974.
Van Cleave, H.V., and Anderson, A.L., Field control tests for pecan
1971 insects. Proc. Tex. Pecan Grow. Ass.: 32-3
Vernon, J.D.R., and Gould, H.J. Further trials on alternatives
1972 to DDT for the control of pre-blossom pests on
apple and pear. Plant Pathol., 21(1). 1-9.
Vrochinskii, K.K. Accumulation of pesticides in hydrobiocts
1971 as a measure for evaluating experimental water
toxicology. Eksp. Vod. Toksikol. 149-63.
Watts, R.R., and Storherr, R.W. Sweep do-distillation clean
1967 up of milk for determination of organophosphate
and chlorinated hydrocarbon pesticides. 50(3):
581-5.
Wilde, W.H.A. Low volume concentrate spraying and integrated 1973
control in some Ontario orchards. Proc. Ohio State
Hort. Soc. 126: 85-8.
Witkowska, D., and Wojnarowska, P. Effectiveness of some chemical
1971 preparations against Anthonomus pomorum (apple
weevil). Biol. Inst. Ochr. Rosl. (48): 357-66.
Woodard Research Corporation. Unpublished report.
1963
Zatserkovskaya, G.A. Evaporation of insecticides from different
1974 types of soils. Zashch. Rast (Kiev), 20: 61-6.
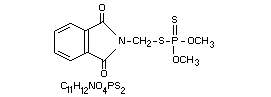 Other information on identity and properties
Molecular weight: 317.3
State: white crystalline solid
Melting point: 72.0-72.7°C
Vapour pressure: 1 x 10-3 mm Hg at 50°C
(decomposes below its boiling point)
Solubility 25 ppm in water (at 25°C), and more than
10 per cent in acetone, dichloromethane,
mesityl oxide, butanone and xylene.
Phosmet is hydrolyzed fairly rapidly in aqueous solution,
especially when the solution is neutral or alkaline. Buffered
aqueous solutions of 20µg per ml concentration at room temperature
were 50% hydrolyzed in 13 days at pH 4.5, in 5 hours at pH 7 and in
3 hours at pH 8.3.
The products of hydrolysis are phthalimide, 00-dimethyl
phosphorodithioate and formaldehyde. Oxidising agents can also
cause breakdown of phosmet. It is compatible with other pesticides
except under alkaline conditions, and is slightly corrosive.
Storage of formulations above 45°C may lead to decomposition.
The technical product is of 95 to 98 per cent purity and has
melting point 66.5 to 69.5°C. The principal impurities are
OOS-Trimethyl phosphorodithioate (1-2%) and
N-chloromethylphthalimide (1-2%). It is formulated as
emulsifiable concentrates, wettable powders, granules and: dusts at
a variety of active ingredient-concentrations.
A mixture of phosmet with carbophenothion is marketed under
the name TRIMIDANR", and the synergistic effects of styryl
phosphorothioates (Large and Pitt, 1975) and acephate (Nakatomi and
Ishibe, 1975) with phosmet have been reported recently, as has that
of 4-phenyl-1,3-dioxan (Uetani et al., 1974).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
In the absence of relevant data, toxicological evaluation of
this compound was postponed.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosmet is effective in controlling a variety of insect and
mite pests which attack livestock, pasture, fruit and field crops.
It is used as a systemic insecticide and acaricide against a
range of ectoparasites of animals, and may be applied as a dilute
aqueous spray containing up to 0.5 per cent a.i., as a ready-to-use
"pour on" formulation containing up to 4% a.i. and applied at up to
40 mg/kg body weight, as a dust containing 1 to 2 percent a.i. or
it may be administered orally.
Phosmet is non-systemic in plants, and at the concentrations
normally used is relatively harmless to a number of predators of
plant mites and insect pests. It is, therefore, used frequently in
integrated control programmes against crop pests. The crops on
which it is used include pome and stone fruits, cereals, forage
crops, pasture and pasture seed crops, nuts and green and root
vegetables.
In animals
Cattle
Phosmet is used to control cattle ticks (e.g. Boophilus
microplus and B. annulatus), cattle grubs (larvae of the bot
flies, Hypoderma lineatum and H. bovis), and the blood
sucking horn flies (Haematobia irritans), and is registered for
these uses in the U.S.A. (Anon, 1971d and 1974). Effective control
of cattle ticks has been obtained with a 0.03% spray (Drummond et
al., 1972).
It is officially recommended in Australia for use against
cattle lice and cattle ticks, and was reported to give effective
control of am acaricide-susceptible strain of B. microplus,
when used as a pour-on formulation (Loomis et al., 1862). The
maximum recommended application rate for use against lice on beef
and dairy cattle in Australia is 10 mg/kg. Sprayed onto cattle in
a finely divided dust, it was found to be effective against all
stages of a resistant strain of B. microplus (Thomas and
Taylor, 1971).
In the USSR, phosmet has been found to be effective against
ixoid ticks on cattle (Nepoklonov et al., 1969; Omarov, 1974), for
control of the mites causing cattle psoroptosis (Surorov, 1970),
and for protection of cattle against stable flies (Stomoxys
calcitrans) and other blood-sucking flies (Ruzimuradov et al.,
1973).
Sheep
Phosmet is used to control the sheep ked (Melophagus ovinus)
in the U.S.A. (Pfadt et al., 1975), and has been found effective in
the USSR against mites causing psoroptosis (Andrichuk, 1971 a,b).
Fowls
Effective control of fowl ectoparasites with phosmet in the
USSR has been reported (Frolov, 1970).
In plants, preharvest
Apples
Phosmet is used in the U.S.A. against a number of pests of
apple trees, including the plum curculio, Conotrachelus
nenuphar (Forsythe and Hall, 1972), and the codling moth
(Laspeyresia pomonella). For control of the latter, phosmet is
often used as part of an integrated control programme in which other
insecticides, sanitation measures, reliance on natural predators for
control of other pests (eg, the European red mite) and sterile moth
releases, may play a part (eg, Butt et al., 1973, Holdsworth, 1973).
In the USSR, phosmet is effective against the caterpillars of
the leaf-miner moth, Stigmella malella (Gribkova, 1973) and
against spider mites, hawthorn mites and soft scale insects.
In the U.K., phosmet has been found to be particularly
effective against the apple sucker, Psylla mali, and to give
control of other pre-blossom pests (Vernon and Gould, 1972).
Other apple pests controlled include San Jose Oystershell
scale insects, e.g. as part of an integrated control programme in
Canada (Wilde, 1973), woolly aphids, leaf roller moth (New Zealand
and Netherlands), winter moth, apple weevil in Poland (Witkowska
and Wojnarowska, 1971), and Aphis pomi in Spain.
Pears
Phosmet is used in the U.S.A. to control the pear psylla
(Psylla pyricola) as well as the codling moth. In the USSR it
gave control of Grapholitha molesta on pear trees when used as
0.1 per cent spray on six occasions at 14-day intervals (Omelyuta,
1972), and it is also used on a small scale on pears in the
Netherlands.
The recommended period between treatment and harvest is 3
weeks for both apples and pears in the Netherlands and in
Australia, and the application rate for pome fruit is usually 0.75
9 a.i/l (0.075%).
Stone fruit
In the U.S.A., phosmet is used on plums, cherries, peaches,
apricots and nectarines, and it has been found to give control of
Grapholitha molesta on peaches and apricots in the USSR. Its
use on peaches has been reported in Turkey, and it is used on
canning varieties of peaches in Australia against the oriental
fruit moth at 0.5 g a.i./l, with a pre-harvest withdrawal period of
3 weeks.
Other fruits
The use of phosmet against Patynota stultana on grapes in
the U.S.A. has been reported (Ali Niazee and Stafford, 1973). It is
also used against the grape berry moth and various gnawing pests of
grapes in the USSR. On citrus crops it has been used against the
citrus white fly; and the control of Zophodia convolutella on
gooseberries (Shapovalova et al., 1970) and of caterpillars on fig
trees (Popov, 1973) with phosmet have been reported in the USSR.
In New Zealand, phosmet is used on grapefruit, Kiwifruit and
Feijoas.
Potatoes
Phosmet is used to control the Colorado Beetle on potatoes in
Spain (Del Rivero et al., 1971), East Germany (Klunker, 1974), and
in the Netherlands, where the recommended pre-harvest interval is
4 weeks. It is recommended in Australia for control of the
red-legged earth mite in potato crops.
Other root crops
Phosmet is officially recommended in Australia for the control
of globular springtail on beet and carrots, and the red-legged
earth mite on carrots.
In the USSR it is used on sugar beet against a variety of
pests including Bothynoderes punctiventris (Nesterenko, 1970,
1973).
Green vegetables
The use of phosmet against lepidopterous pests of cabbage has
been reported in Spain, and it is officially recommended in
Australia for control of the red-legged earth mite and other pests
in beans, peas, crucifers and lettuce.
Pasture and forage crops
Phosmet is used in the U.S.A. to control the alfalfa weevil
(Hypera postica), and the clover head weevil. It has also been
found effective against the alfalfa weevil in the USSR when applied
at 0.75 kg/ha at the beginning of the egg-laying period. In
addition, it is effective against Phytonomus variabilis on
alfalfa (Marzhanyan et al., 1973). In Australia it is officially
recommended for use on cruciferous forage crops, lucerne, pasture,
and pasture seed crops to control the lucerne flea, the red-legged
earth mite and the blue oat mite, the application rate on pastures
being 0.0350.05 kg/ha with a withholding period of 7 days.
Cereal crops
In Australia satisfactory control of the blue oat mite on
wheat was achieved with the ULV application of phosmet at 0.5 oz
a.i. per acre (New South Wales Department of Agriculture, 1969 and
phosmet is officially recommended for control of this pest as well
as the lucerne flea and the redlegged earth mite in cereal crops.
Cotton
Applied at 3-5 kg/ha in cotton plantings in the USSR, phosmet
has been found very effective against cutworm moths (Nikiforov and
Mamaev, 1972). It is also reported to control other cotton pests.
Post-harvest treatments
The existence of a U.S. tolerance of 10 mg/kg for phosmet
residues on sweet potatoes from post-harvest applications (Anon,
1973), suggests that phosmet is used for the protection of this
crop in storage in the U.S.A.
Phosmet showed considerable promise in evaluation trials in
the U.S.A. against stored products insects such as the red flour
beetle, Tribolium castaneum (Herbst), (Speirs and Lang, 1970),
but it is not known whether it has been adopted for the protection
of stored grain, flour, etc.
Other uses
Phosmet has found some applications in forestry, particularly
for the protection of conifers against such pests as the pine tip
moth and the pine looper.
In the USSR, dipping sheep in 0.5 per cent emulsions of
phosmet kept the sheep free of Ixodes mites for 7 months
(Sevost'yanov and Ushakova, 1971).
Phosmet has also been found to be effective against several
pests of tobacco, including the flea beetle (Mistric and Smith,
1970). In Australia it is recommended for control of the lucerne
flea on tobacco seed beds.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
In a trial in the U.S.A. during 1964/5, five steers were
sprayed with phosmet at 0.25% a.i., and one was slaughtered on each
of days 3, 7, 14, 28 and 59 after treatment. Residues were
determined in fat, liver and muscle, with the results indicated in
Table 1 (Rogoff et al., 1967).
Several trials involving the determination of phosmet residues
in the fat of cattle at various intervals after treatment have been
carried out in Australia (Snelson, 1976). The treatments have
included spray and pour-on applications, and in one case the
animals were sprayed 3 times. The application rates and residue
results are shown in Table 1, along with the results of a trial in
the U.K. using pour-on application.
At the University of California, cattle were fed phosmet at
approximately 1 mg/kg/day for 8 weeks, and at 2 mg/kg/day for an
additional 8 weeks. The animals were killed and autopsied on days
zero, 3, 7, and 14 following the completion of feeding. The phosmet
residues in fat were all below the detection limit of 0.09 mg/kg, and
in liver, kidney and muscle they were all below the detection limit of
0.04 mg/kg with the exception of one zero day muscle sample.
There have been several studies involving the determination of
phosmet residues in the milk of dairy cows. In one of these (Johnson
and Bowman, 1968), milk from 4 dairy cows ingesting phosmet in silage
at a daily average rate of 0.22 mg/kg body weight was found to contain
less than 0.002 ppm of phosmet or its oxygen analogue. In another
(Woodard Research Corporation, 1963), 2 groups of dairy cows were
treated with phosmet by spraying 3 times at weekly intervals at 0.25%
or 0.5% a.i., whilst a further three groups were fed phosmet for 4
weeks at levels of 20, 45 and 100 ppm in the diet. Milk samples were
taken at frequent intervals from all five groups and analysed for both
phosmet and its 0-analogue. The average apparent phosmet residue for
all milk samples from treated cows was 0.016 mg/kg compared to
0.015 mg/kg for control samples.
Results for residues in milk and cream from further trials in
Australia and the U.K. are given in Table 2.
Goats
A goat given a single oral dose of phosmet (70 mg per kg,
administered in gelatine capsules) was milked dry at 8, 24 and 48
hours after treatment (Bowman and Beroza, 1967). The milk was
extracted with a 1:1 mixture of hexane and diethyl ether and the
extracts were analysed by gas chromatography using a flame photometric
detector in the sulphur mode. 0.38 mg/kg of phosmet was found in the 8
hour sample, but none was detected in the subsequent samples.
Pigs
In Bulgaria, phosmet applied externally to pigs at 1.5% a.i. was
found to penetrate through the skin in 2 to 5 days. The highest
residues (10 mg/kg) accumulated in the subcutaneous fat. The meat
accumulated approximately 8 ppm and some xylene derivatives were
detected as metabolites of phosmet (Ionova et al., 1974).
Fowls
In the USSR (Chirikashvili et al., 1970), phosmet was
administered to hens as a single oral dose of 300 mg/kg. The compound
was detectable by thin layer chromatography in various organs and
tissues, especially the lungs, heart and intestines, for 5 days after
treatment, and was present in some of the eggs at a level of about
2 mg/kg.
In plants
The available residue data from crop spraying trials are
summarised in Table 3.
Apples
In two Stauffer Chemical Company trials in the Netherlands in
1970, phosmet was applied as a spray containing 0.15% a.i. and at a
rate of 2000 l/ha. The residue disappearance curves from these trials
are reproduced as Figures 1 and 2. The oxygen analogue was measured as
well as unchanged phosmet, and residues were determined in samples of
fruit harvested at weekly intervals up to 4 weeks from treatment. The
total residue after the recommended 3 week interval from treatment to
harvest averaged 0.5 mg/kg, as against a national tolerance in the
Netherlands of 1 mg/kg, even though the concentration of the spray was
double that normally recommended.
In two similar trials in New Zealand, phosmet was applied 9 times
as a spray containing 0.15% a.i., and residues were determined over
periods of 23 and 28 days respectively. The mean residues found after
the recommended 14 day waiting period were in the range 1-3 mg/kg, as
against a New Zealand national tolerance of 10 mg/kg. The normal
application rate is 0.075-0.1% a.i.
In a trial in Australia using two application rates (1.75 and 2.0
g a.i./l) and carried out for the Stauffer Chemical Company, residues
had fallen to below 1 ppm after 3 weeks (King and Best, 1971).
In trials in the USSR (not included in Table 3), apple trees were
sprayed with phosmet at the rate of 10 litres per tree of 0.2% spray.
Residues were found to have disappeared from the apples 20 days after
treatment (Adeishvili, 1973), but the detection limit of the method
used is not known.
Stone fruit
Trials on two varieties of peaches were carried out by ICI in
Australia. In one, phosmet was applied at 1.0 g a.i./l and peaches
were sampled at 0, 7, 14 and 21 days after treatment. In the other
phosmet was applied at 2.0 or 1.0 g a.i./l and samples of peaches for
each application rate were taken at 1, 3, 5, 7 and 14 days after
treatment. For the 1.0 g a.i./l application rate, residues had fallen
to 1.1-1.5 ppm after 14 days, and to 0.5 ppm after 21 days (Snelson,
1976).
In Turkey, peaches of the Hale variety were treated 3 times in a
period of 30 days with a 0.15% spray. Samples were taken over a 3-week
period, and the residue found 21 days after the last treatment was 3.3
ppm (Otaci et al., 1972).
TABLE 1. Residues of phosmet in tissues of cattle slaughtered at various intervals after treatment
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
U.S.A. spray 2.5 1 Omental fat 3 0.40
(Rogoff et al 7 <0.2
1967) 14 <0.2
28 <0.2
59 <0.2
Peri-renal 3 0.59
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Sub-cutaneous 3 0.67
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Liver 3 0.10
7 0.09
14 0.08
28 0.06
59 0.08
U.S.A. muscle 3 0.06
7 <0.04
14 <0.04
28 <0.04
59 <0.04
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Australia Spray 0.75 3 Omental 1 1.03 (0.91-1.14)
(Snelson, 1976) fat 4 0.34 (0.28-0.40)
8 0.0
Subcutaneous 1 0.60 (0.40-0.80)
fat 4 0.06 (0.02-0.09)
1 Omental 1 0.77 (0.74-0.80)
fat 4 0.07 (0.01-0.13)
8 0.06 (0.02-0.09)
Subcutaneous 1 0.21 (0.21-0.21)
fat 4 0.01 (0-0.2)
Spray (2 0.75 1 Omental 1 0.79 (0.52-0.93)
gallons = fat 2 0.34 (0.25-0.40)
6.8 grams 3 0.24 (0.16-0.37)
technical 7 0.16 (0.11-0.21)
per beast)
Australia Peri-renal 1 0.24 (0.17-0.37)
fat 2 0.18 (0.15-0.24)
3 0.10 (0.09-0.11)
7 0.07 (0.05-0.10)
U.K. Pour-on 1 Omental 1 2.75 (1.64-3.7)
(40 mg/kg) fat 2 1.73 (1.68-1.8)
3 1.33 (0.92-1.9)
7 0.58 (0.49-0.69)
10 0.26 (0.15-0.36)
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Peri-renal 1 1.78 (1.20-2.19)
fat 2 1.47 (1.42-1.5)
3 1.05 (0.90-1.3)
7 0.73 (0.40-1.1)
10 0.37 (0.26-0.43)
TABLE 2. Residues of phosmet in the milk of lactating dairy cows at various intervals after treatment
Method Application Post-treatment Residues mg/kg mean
Country of rate No. of interval (max)
(reference) application (g. a.i./l applications (days) whole milk cream
Australia Spray 0.75 1 1/6 0.08 0.84
(King and (1 3/4 gallons 2/3 <0.02 0.10
Ferguson = 6.0 grams 1 0.02 0.10
1971) technical 2 <0.02 0.02
per beast) 3 <0.02 <0.02
4 <0.02 <0.02
2 1/6 0.03 -
2/3 0.05 -
1 <0.02 -
2 <0.02 -
3 <0.02 -
4 <0.02 -
U.K. Pour-on - 1 1/4 (0.07) (0.77)
(Snelson; (20 mg/kg)
1976
Other information on identity and properties
Molecular weight: 317.3
State: white crystalline solid
Melting point: 72.0-72.7°C
Vapour pressure: 1 x 10-3 mm Hg at 50°C
(decomposes below its boiling point)
Solubility 25 ppm in water (at 25°C), and more than
10 per cent in acetone, dichloromethane,
mesityl oxide, butanone and xylene.
Phosmet is hydrolyzed fairly rapidly in aqueous solution,
especially when the solution is neutral or alkaline. Buffered
aqueous solutions of 20µg per ml concentration at room temperature
were 50% hydrolyzed in 13 days at pH 4.5, in 5 hours at pH 7 and in
3 hours at pH 8.3.
The products of hydrolysis are phthalimide, 00-dimethyl
phosphorodithioate and formaldehyde. Oxidising agents can also
cause breakdown of phosmet. It is compatible with other pesticides
except under alkaline conditions, and is slightly corrosive.
Storage of formulations above 45°C may lead to decomposition.
The technical product is of 95 to 98 per cent purity and has
melting point 66.5 to 69.5°C. The principal impurities are
OOS-Trimethyl phosphorodithioate (1-2%) and
N-chloromethylphthalimide (1-2%). It is formulated as
emulsifiable concentrates, wettable powders, granules and: dusts at
a variety of active ingredient-concentrations.
A mixture of phosmet with carbophenothion is marketed under
the name TRIMIDANR", and the synergistic effects of styryl
phosphorothioates (Large and Pitt, 1975) and acephate (Nakatomi and
Ishibe, 1975) with phosmet have been reported recently, as has that
of 4-phenyl-1,3-dioxan (Uetani et al., 1974).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
In the absence of relevant data, toxicological evaluation of
this compound was postponed.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosmet is effective in controlling a variety of insect and
mite pests which attack livestock, pasture, fruit and field crops.
It is used as a systemic insecticide and acaricide against a
range of ectoparasites of animals, and may be applied as a dilute
aqueous spray containing up to 0.5 per cent a.i., as a ready-to-use
"pour on" formulation containing up to 4% a.i. and applied at up to
40 mg/kg body weight, as a dust containing 1 to 2 percent a.i. or
it may be administered orally.
Phosmet is non-systemic in plants, and at the concentrations
normally used is relatively harmless to a number of predators of
plant mites and insect pests. It is, therefore, used frequently in
integrated control programmes against crop pests. The crops on
which it is used include pome and stone fruits, cereals, forage
crops, pasture and pasture seed crops, nuts and green and root
vegetables.
In animals
Cattle
Phosmet is used to control cattle ticks (e.g. Boophilus
microplus and B. annulatus), cattle grubs (larvae of the bot
flies, Hypoderma lineatum and H. bovis), and the blood
sucking horn flies (Haematobia irritans), and is registered for
these uses in the U.S.A. (Anon, 1971d and 1974). Effective control
of cattle ticks has been obtained with a 0.03% spray (Drummond et
al., 1972).
It is officially recommended in Australia for use against
cattle lice and cattle ticks, and was reported to give effective
control of am acaricide-susceptible strain of B. microplus,
when used as a pour-on formulation (Loomis et al., 1862). The
maximum recommended application rate for use against lice on beef
and dairy cattle in Australia is 10 mg/kg. Sprayed onto cattle in
a finely divided dust, it was found to be effective against all
stages of a resistant strain of B. microplus (Thomas and
Taylor, 1971).
In the USSR, phosmet has been found to be effective against
ixoid ticks on cattle (Nepoklonov et al., 1969; Omarov, 1974), for
control of the mites causing cattle psoroptosis (Surorov, 1970),
and for protection of cattle against stable flies (Stomoxys
calcitrans) and other blood-sucking flies (Ruzimuradov et al.,
1973).
Sheep
Phosmet is used to control the sheep ked (Melophagus ovinus)
in the U.S.A. (Pfadt et al., 1975), and has been found effective in
the USSR against mites causing psoroptosis (Andrichuk, 1971 a,b).
Fowls
Effective control of fowl ectoparasites with phosmet in the
USSR has been reported (Frolov, 1970).
In plants, preharvest
Apples
Phosmet is used in the U.S.A. against a number of pests of
apple trees, including the plum curculio, Conotrachelus
nenuphar (Forsythe and Hall, 1972), and the codling moth
(Laspeyresia pomonella). For control of the latter, phosmet is
often used as part of an integrated control programme in which other
insecticides, sanitation measures, reliance on natural predators for
control of other pests (eg, the European red mite) and sterile moth
releases, may play a part (eg, Butt et al., 1973, Holdsworth, 1973).
In the USSR, phosmet is effective against the caterpillars of
the leaf-miner moth, Stigmella malella (Gribkova, 1973) and
against spider mites, hawthorn mites and soft scale insects.
In the U.K., phosmet has been found to be particularly
effective against the apple sucker, Psylla mali, and to give
control of other pre-blossom pests (Vernon and Gould, 1972).
Other apple pests controlled include San Jose Oystershell
scale insects, e.g. as part of an integrated control programme in
Canada (Wilde, 1973), woolly aphids, leaf roller moth (New Zealand
and Netherlands), winter moth, apple weevil in Poland (Witkowska
and Wojnarowska, 1971), and Aphis pomi in Spain.
Pears
Phosmet is used in the U.S.A. to control the pear psylla
(Psylla pyricola) as well as the codling moth. In the USSR it
gave control of Grapholitha molesta on pear trees when used as
0.1 per cent spray on six occasions at 14-day intervals (Omelyuta,
1972), and it is also used on a small scale on pears in the
Netherlands.
The recommended period between treatment and harvest is 3
weeks for both apples and pears in the Netherlands and in
Australia, and the application rate for pome fruit is usually 0.75
9 a.i/l (0.075%).
Stone fruit
In the U.S.A., phosmet is used on plums, cherries, peaches,
apricots and nectarines, and it has been found to give control of
Grapholitha molesta on peaches and apricots in the USSR. Its
use on peaches has been reported in Turkey, and it is used on
canning varieties of peaches in Australia against the oriental
fruit moth at 0.5 g a.i./l, with a pre-harvest withdrawal period of
3 weeks.
Other fruits
The use of phosmet against Patynota stultana on grapes in
the U.S.A. has been reported (Ali Niazee and Stafford, 1973). It is
also used against the grape berry moth and various gnawing pests of
grapes in the USSR. On citrus crops it has been used against the
citrus white fly; and the control of Zophodia convolutella on
gooseberries (Shapovalova et al., 1970) and of caterpillars on fig
trees (Popov, 1973) with phosmet have been reported in the USSR.
In New Zealand, phosmet is used on grapefruit, Kiwifruit and
Feijoas.
Potatoes
Phosmet is used to control the Colorado Beetle on potatoes in
Spain (Del Rivero et al., 1971), East Germany (Klunker, 1974), and
in the Netherlands, where the recommended pre-harvest interval is
4 weeks. It is recommended in Australia for control of the
red-legged earth mite in potato crops.
Other root crops
Phosmet is officially recommended in Australia for the control
of globular springtail on beet and carrots, and the red-legged
earth mite on carrots.
In the USSR it is used on sugar beet against a variety of
pests including Bothynoderes punctiventris (Nesterenko, 1970,
1973).
Green vegetables
The use of phosmet against lepidopterous pests of cabbage has
been reported in Spain, and it is officially recommended in
Australia for control of the red-legged earth mite and other pests
in beans, peas, crucifers and lettuce.
Pasture and forage crops
Phosmet is used in the U.S.A. to control the alfalfa weevil
(Hypera postica), and the clover head weevil. It has also been
found effective against the alfalfa weevil in the USSR when applied
at 0.75 kg/ha at the beginning of the egg-laying period. In
addition, it is effective against Phytonomus variabilis on
alfalfa (Marzhanyan et al., 1973). In Australia it is officially
recommended for use on cruciferous forage crops, lucerne, pasture,
and pasture seed crops to control the lucerne flea, the red-legged
earth mite and the blue oat mite, the application rate on pastures
being 0.0350.05 kg/ha with a withholding period of 7 days.
Cereal crops
In Australia satisfactory control of the blue oat mite on
wheat was achieved with the ULV application of phosmet at 0.5 oz
a.i. per acre (New South Wales Department of Agriculture, 1969 and
phosmet is officially recommended for control of this pest as well
as the lucerne flea and the redlegged earth mite in cereal crops.
Cotton
Applied at 3-5 kg/ha in cotton plantings in the USSR, phosmet
has been found very effective against cutworm moths (Nikiforov and
Mamaev, 1972). It is also reported to control other cotton pests.
Post-harvest treatments
The existence of a U.S. tolerance of 10 mg/kg for phosmet
residues on sweet potatoes from post-harvest applications (Anon,
1973), suggests that phosmet is used for the protection of this
crop in storage in the U.S.A.
Phosmet showed considerable promise in evaluation trials in
the U.S.A. against stored products insects such as the red flour
beetle, Tribolium castaneum (Herbst), (Speirs and Lang, 1970),
but it is not known whether it has been adopted for the protection
of stored grain, flour, etc.
Other uses
Phosmet has found some applications in forestry, particularly
for the protection of conifers against such pests as the pine tip
moth and the pine looper.
In the USSR, dipping sheep in 0.5 per cent emulsions of
phosmet kept the sheep free of Ixodes mites for 7 months
(Sevost'yanov and Ushakova, 1971).
Phosmet has also been found to be effective against several
pests of tobacco, including the flea beetle (Mistric and Smith,
1970). In Australia it is recommended for control of the lucerne
flea on tobacco seed beds.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
In a trial in the U.S.A. during 1964/5, five steers were
sprayed with phosmet at 0.25% a.i., and one was slaughtered on each
of days 3, 7, 14, 28 and 59 after treatment. Residues were
determined in fat, liver and muscle, with the results indicated in
Table 1 (Rogoff et al., 1967).
Several trials involving the determination of phosmet residues
in the fat of cattle at various intervals after treatment have been
carried out in Australia (Snelson, 1976). The treatments have
included spray and pour-on applications, and in one case the
animals were sprayed 3 times. The application rates and residue
results are shown in Table 1, along with the results of a trial in
the U.K. using pour-on application.
At the University of California, cattle were fed phosmet at
approximately 1 mg/kg/day for 8 weeks, and at 2 mg/kg/day for an
additional 8 weeks. The animals were killed and autopsied on days
zero, 3, 7, and 14 following the completion of feeding. The phosmet
residues in fat were all below the detection limit of 0.09 mg/kg, and
in liver, kidney and muscle they were all below the detection limit of
0.04 mg/kg with the exception of one zero day muscle sample.
There have been several studies involving the determination of
phosmet residues in the milk of dairy cows. In one of these (Johnson
and Bowman, 1968), milk from 4 dairy cows ingesting phosmet in silage
at a daily average rate of 0.22 mg/kg body weight was found to contain
less than 0.002 ppm of phosmet or its oxygen analogue. In another
(Woodard Research Corporation, 1963), 2 groups of dairy cows were
treated with phosmet by spraying 3 times at weekly intervals at 0.25%
or 0.5% a.i., whilst a further three groups were fed phosmet for 4
weeks at levels of 20, 45 and 100 ppm in the diet. Milk samples were
taken at frequent intervals from all five groups and analysed for both
phosmet and its 0-analogue. The average apparent phosmet residue for
all milk samples from treated cows was 0.016 mg/kg compared to
0.015 mg/kg for control samples.
Results for residues in milk and cream from further trials in
Australia and the U.K. are given in Table 2.
Goats
A goat given a single oral dose of phosmet (70 mg per kg,
administered in gelatine capsules) was milked dry at 8, 24 and 48
hours after treatment (Bowman and Beroza, 1967). The milk was
extracted with a 1:1 mixture of hexane and diethyl ether and the
extracts were analysed by gas chromatography using a flame photometric
detector in the sulphur mode. 0.38 mg/kg of phosmet was found in the 8
hour sample, but none was detected in the subsequent samples.
Pigs
In Bulgaria, phosmet applied externally to pigs at 1.5% a.i. was
found to penetrate through the skin in 2 to 5 days. The highest
residues (10 mg/kg) accumulated in the subcutaneous fat. The meat
accumulated approximately 8 ppm and some xylene derivatives were
detected as metabolites of phosmet (Ionova et al., 1974).
Fowls
In the USSR (Chirikashvili et al., 1970), phosmet was
administered to hens as a single oral dose of 300 mg/kg. The compound
was detectable by thin layer chromatography in various organs and
tissues, especially the lungs, heart and intestines, for 5 days after
treatment, and was present in some of the eggs at a level of about
2 mg/kg.
In plants
The available residue data from crop spraying trials are
summarised in Table 3.
Apples
In two Stauffer Chemical Company trials in the Netherlands in
1970, phosmet was applied as a spray containing 0.15% a.i. and at a
rate of 2000 l/ha. The residue disappearance curves from these trials
are reproduced as Figures 1 and 2. The oxygen analogue was measured as
well as unchanged phosmet, and residues were determined in samples of
fruit harvested at weekly intervals up to 4 weeks from treatment. The
total residue after the recommended 3 week interval from treatment to
harvest averaged 0.5 mg/kg, as against a national tolerance in the
Netherlands of 1 mg/kg, even though the concentration of the spray was
double that normally recommended.
In two similar trials in New Zealand, phosmet was applied 9 times
as a spray containing 0.15% a.i., and residues were determined over
periods of 23 and 28 days respectively. The mean residues found after
the recommended 14 day waiting period were in the range 1-3 mg/kg, as
against a New Zealand national tolerance of 10 mg/kg. The normal
application rate is 0.075-0.1% a.i.
In a trial in Australia using two application rates (1.75 and 2.0
g a.i./l) and carried out for the Stauffer Chemical Company, residues
had fallen to below 1 ppm after 3 weeks (King and Best, 1971).
In trials in the USSR (not included in Table 3), apple trees were
sprayed with phosmet at the rate of 10 litres per tree of 0.2% spray.
Residues were found to have disappeared from the apples 20 days after
treatment (Adeishvili, 1973), but the detection limit of the method
used is not known.
Stone fruit
Trials on two varieties of peaches were carried out by ICI in
Australia. In one, phosmet was applied at 1.0 g a.i./l and peaches
were sampled at 0, 7, 14 and 21 days after treatment. In the other
phosmet was applied at 2.0 or 1.0 g a.i./l and samples of peaches for
each application rate were taken at 1, 3, 5, 7 and 14 days after
treatment. For the 1.0 g a.i./l application rate, residues had fallen
to 1.1-1.5 ppm after 14 days, and to 0.5 ppm after 21 days (Snelson,
1976).
In Turkey, peaches of the Hale variety were treated 3 times in a
period of 30 days with a 0.15% spray. Samples were taken over a 3-week
period, and the residue found 21 days after the last treatment was 3.3
ppm (Otaci et al., 1972).
TABLE 1. Residues of phosmet in tissues of cattle slaughtered at various intervals after treatment
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
U.S.A. spray 2.5 1 Omental fat 3 0.40
(Rogoff et al 7 <0.2
1967) 14 <0.2
28 <0.2
59 <0.2
Peri-renal 3 0.59
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Sub-cutaneous 3 0.67
fat 7 <0.2
14 <0.2
28 <0.2
59 <0.2
Liver 3 0.10
7 0.09
14 0.08
28 0.06
59 0.08
U.S.A. muscle 3 0.06
7 <0.04
14 <0.04
28 <0.04
59 <0.04
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Australia Spray 0.75 3 Omental 1 1.03 (0.91-1.14)
(Snelson, 1976) fat 4 0.34 (0.28-0.40)
8 0.0
Subcutaneous 1 0.60 (0.40-0.80)
fat 4 0.06 (0.02-0.09)
1 Omental 1 0.77 (0.74-0.80)
fat 4 0.07 (0.01-0.13)
8 0.06 (0.02-0.09)
Subcutaneous 1 0.21 (0.21-0.21)
fat 4 0.01 (0-0.2)
Spray (2 0.75 1 Omental 1 0.79 (0.52-0.93)
gallons = fat 2 0.34 (0.25-0.40)
6.8 grams 3 0.24 (0.16-0.37)
technical 7 0.16 (0.11-0.21)
per beast)
Australia Peri-renal 1 0.24 (0.17-0.37)
fat 2 0.18 (0.15-0.24)
3 0.10 (0.09-0.11)
7 0.07 (0.05-0.10)
U.K. Pour-on 1 Omental 1 2.75 (1.64-3.7)
(40 mg/kg) fat 2 1.73 (1.68-1.8)
3 1.33 (0.92-1.9)
7 0.58 (0.49-0.69)
10 0.26 (0.15-0.36)
TABLE 1. (Cont'd.)
Application Post
treatment
Country Rate interval Residues mg/kg
(reference) Method (g a.i./l) No. Tissue (days) mean (range)
Peri-renal 1 1.78 (1.20-2.19)
fat 2 1.47 (1.42-1.5)
3 1.05 (0.90-1.3)
7 0.73 (0.40-1.1)
10 0.37 (0.26-0.43)
TABLE 2. Residues of phosmet in the milk of lactating dairy cows at various intervals after treatment
Method Application Post-treatment Residues mg/kg mean
Country of rate No. of interval (max)
(reference) application (g. a.i./l applications (days) whole milk cream
Australia Spray 0.75 1 1/6 0.08 0.84
(King and (1 3/4 gallons 2/3 <0.02 0.10
Ferguson = 6.0 grams 1 0.02 0.10
1971) technical 2 <0.02 0.02
per beast) 3 <0.02 <0.02
4 <0.02 <0.02
2 1/6 0.03 -
2/3 0.05 -
1 <0.02 -
2 <0.02 -
3 <0.02 -
4 <0.02 -
U.K. Pour-on - 1 1/4 (0.07) (0.77)
(Snelson; (20 mg/kg)
1976

 TABLE 3. Residues of phosmet in various crops from supervised spraying trials
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Apples Netherlands 1.5 1 0 1.8(1.3-2.1 0
(var. Lombertscalville) (2000 1/ha) 7 0.9(0.7-1.2) 0
14 0.6(0.3-0.9 0
21 0.5(0.3-0.6) 0
28 0.2(0.1-0.2) 0
Netherlands 1.5 1 0 2.4(1.5-3.4) 0.04(0.01-0.07)
(var. (2000 l/ha) 7 2.0(1.7-2.3) 0.06(0.04-0.09)
Goudreinette) 14 1.4(0.6-2.0) 0.06(0.03-0.07)
21 0.4(0.2-0.6) 0.03(0-0.06)
28 0.6(0.2-0.7) 0.02(0.01-0.03)
Apples New Zealand 1.5 9 2 2.0
9 1.7
15 1.1
23 0.7
1.5 9 1 4.3
3 3.6
7 4.0
14 2.9
21 3.0
28 2.1
Apples Australia 1.75 ? 0 2.2
(King & Best, 1 1.5
1971) 7 0.7
14 0.8
21 0.6
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
2.0 ? 0 2.8
1 1.5
7 1.6
14 1.0
21 0.7
Peaches Australia 1.0 1 0 3.8
(var. Golden 7 3.6
Queen) 14 1.1
21 0.5
Australia 1.0 1 1 3.4
(var.Maygold) 3 2.5
5 1.3
7 3.1
14 1.5
Peaches Australia 2.0 1 1 5.3
(var. Maygold) 3 6.2
5 4.2
7 3.9
14 6.2
Peaches Turkey 1.5 3 0 5.39
3 5.44
7 4.65
14 3.48
21 3.30
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Kiwifruit New Zealand 1.1 2 1 10.0
7 9.6
14 5.8
21 5.1
28 3.6
35 3.1
42 3.0
1.12 2 10 9.5
7.7
5.8
7.1
1.12 7 10 25.0
16.0
8.4
7.2
0.75 7 10 8.8
4.8
7.1
6.4
Grapefruit New Zealand 1.12 4 1 0.65
7 0.57
14 0.3
21 0.26
28 0.38
42 0.22
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Potatoes Netherlands 0.56 kg a.i./ 2 22 0.004 0
(var. ha(as 50% w.p.) (0.002-0.006)
Burmania)
Netherlands " 2 20 0.001 0
(var. Bintje) (0-0.003
Netherlands " 2 21 0.001 0
(var. (0.001-0.002)
Eigenheimer)
Alfalfa U.S.A. 1.1 kg a.i./ 1 1 43.5
(Shaw et al. ha(36% ec) 8 2.09
1966) 14 0.84
21 0.31
controls 0.18-0.44
Alfalfa U.S.A. 0.56 kg a.i. 1 0 33.86
(Miller et al. /ha(50% wp) 5 3.58
1969) 10 2.27
15 0.34
21 0.04
1.1 kg a.i. 1 0 57.11
/ha(50% wp) 5 10.74
10 3.42
15 1.52
21 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i. 1 0 20.95
/ha(36% ec) 5 3.87
10 2.09
15 0.94
21 0.00
Alfalfa U.S.A. 1.1 kg a.i. 1 0 44.42
/ha (36% ec) 5 9.87
10 5.06
15 2.00
21 0.37
Coastal U.S.A. 0.84 kg a.i. 1 0 37.93
bermuda (Dorough et /ha 1 32.66
grass al, 1965) 3 23.89
7 15.79
14 8.31
Coastal U.S.A. 0.28 kg a.i. 1 0 12.3 0.06
bermuda (Leuck and /ha 1 6.86 0.06
grass Bowman, 1968) 3 1.11 0.00
4 0.96 0.00
7 0.72 0.00
15 0.13 0.00
0.56 kg a.i. 1 0 27.0 0.13
/ha 1 16.4 0.13
3 2.82 0.02
4 2.30 0.02
7 1.78 0.00
15 0.36 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Coastal U.S.A. 1.1 kg a.i./ha 1 0 52.9 0.36
Bermuda 1 36.8 0.40
grass 3 7.02 0.07
4 6.34 0.06
7 3.72 0.02
15 0.57 0.00
Maize U.S.A. 0.28 kg a.i./ha 1 0 2.67 0.00
plants (Leuck and 1 1.00 0.00
(green Bowman, 1968) 2 0.39 0.00
weight 4 0.20 0.00
basis) 7 0.09 0.00
0.56 kg a.i./ha 1 0 7.07 0.01
1 Not sampled owing to rain
2 0.99 0.00
4 0.46 0.00
7 0.26 0.00
1.1 kg a.i./ha 1 0 10.9 0.02
1 Not sampled owing to rain
2 2.89 0.00
4 1.16 0.00
7 0.63 0.00
Soybean U.S.A. 0.28 kg ai./ 1 0 31.2 0.03
plants (Leuck and ha 1 21.1 0.02
(green Bowman, 1968) 2 15.0 0.01
weight 4 2.45 0.00
basis) 7 0.48 0.00
15 0.10 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i./ha 1 0 55.4 0.07
1 30.2 0.03
2 27.6 0.02
4 6.27 0.00
7 1.24 0.00
15 0.28 0.00
1.1 kg a.i./ha 1 0 82.2 0.11
1 55.4 0.07
2 43.8 0.04
4 14.7 0.01
7 2.62 0.00
15 0.76 0.00
Other fruits
Trials in New Zealand on Kiwifruit and grapefruit yielded the
residue data given in Table 3. In most cases the spray concentration
was slightly above the recommended upper limit of 0.1% a.i., but only
for the highest rate of application to Kiwifruit (7 applications of
0.112% a.i.) were residues in the whole fruit found to be above the
national tolerance of 10 mg/kg after the recommended 10-day waiting
period. Separate analyses of the skin and flesh revealed that the
residues were mainly concentrated in the skin. Four applications of
0.112% a.i. spray to grapefruit resulted in a residue of only 0.3
mg/kg in the whole fruit (0.05 mg/kg in the flesh) after the
recommended 14 day interval.
Alfalfa
In 1965, experiments were conducted in the United States to
determine the rate of disappearance of phosmet from alfalfa. An ec
formulation containing 36% a.i., w/v, was applied at the rate of 1.1
kg a.i./ha. Residues were determined by the anthranilic acid
colorimetric method over a period of 3 weeks after application. The
results are given in Table 3, and show that the phosmet residues had
declined to the levels found in untreated control samples after 21
days (Shaw et al., 1966).
In a subsequent trial on alfalfa (Miller et al., 1969), the
disappearance rates of phosmet after application in two different
formulations (the 36% ec and a 50% wp) were compared. Each formulation
was applied at the rate of 0.5 and 1 lb a.i./acre in a dilute spray
used at 100 gallons per acre. Each treatment was replicated twice, and
the crop was sampled over a 3 week period after treatment. The results
are summarised in Table 3.
In the USSR, the feeding of rabbits with alfalfa gathered 10 days
after it had been sprinkled with phosmet produced some depression of
cholinesterase activity. When the alfalfa was gathered 15 days after
treatment no depression was observed, and the residue level had
declined to 1/6 of that found 11 days after treatment and 1/15 of that
found 6 days after treatment (Semerdzhyan, 1972).
Other forage crops
Trials were conducted in the U.S.A. in which maize (for silage),
soybean plants, and coastal Bermuda grass were each sprayed at rates
of 0.28, 0.56 and 1.1 kg of phosmet per ha. Residues of phosmet and
its oxygenanalogue were determined in samples taken over a 15 day
period following treatment (Leuck and Bowman, 1968). The results are
given in Table 3, and indicate that in all cases the residues had
declined to under 1 mg/kg in 15 days or less. On the other hand, in a
trial on Coastal Bermuda grass at the Texas Agricultural Experiment
Station, in which phosmet was applied at 0.84 kg/ha, residues of about
8 mg/kg were found after 14 days (Dorough et al., 1965).
Leuck and Bowman analysed the concentrate used in their trial,
and found that it contained a trace of the oxygen-analogue equivalent
to 0.12% of the phosmet content. Thus some, at least, of the
0-analogue residues found probably originated from the spray liquor
rather than from environmental oxidation of phosmet.
FATE OF RESIDUES
General comments
The results presented in the foregoing section indicate that both
phosmet and its oxygen analogue are of low persistence in plants and
animals. The photochemistry of phosmet in diethyl ether solution was
studied by Tanabe et al. (1974), using a 450 watt UV lamp. They
isolated N-methylphthalimide and N-methoxymethylphthalimide as
photolysis products in low yield. Neither of these was insecticidally
active. No photoproduct containing P or S could be isolated from the
many minor components. Using UV radiation in the range 250-400 nm,
Klisenko and Pis'mennaya (1973) found a half-life of 70 minutes for
phosmet, and identified phthalimide, N-methoxymethylphthalimide and
phthalic acid as photolysis products, in addition to P-O analogues.
In animals
The metabolism of carbonyl-14C-labelled phosmet in rats
following oral administration was investigated by McBain et al.
(1968). Metabolites were excreted in the urine in the following
amounts (expressed as percentages of the administered dose): phthalic
acid 21.0%, phthalamic acid 40.7% and five minor metabolites
containing the phthaloyl moiety 10.9%. Not more than 0.04% of the dose
administered was excreted in the urine as unchanged phosmet or its
oxygen analogue. This study supported other evidence that phosmet is
rapidly metabolised in mammals, mainly to innocuous water soluble
compounds. For example, Chamberlain (1965) studied the fate of
14C-labelled phosmet following dermal application to a steer. It was
moderately absorbed by the skin of the animal, since 9.6% of the
applied dose was recovered in the excreta (nearly 8% in the urine)
within 7 days. It appeared that the compound was rapidly broken down
in the blood, and that the primary degradation occurred at the
nitrogen atom, resulting in the almost exclusive production of
phthalic and phthalamic acids.
Johnson and Bowman (1968) found no detectable residues of phosmet
or its oxygen analogue in the milk, urine or faeces of lactating dairy
cows fed with silage containing phosmet at a level providing 0.22
mg/kg/ day. Detection limits were 0.002 mg/kg for milk and urine, and
0.004 mg/kg for faeces.
Tests under laboratory conditions in the USSR (Vrochinskii, 1971)
showed that phosmet was stored only briefly by fish.
In plants
Trials on apples, peaches, lemons and potatoes showed that
neither phosmet nor its oxygen analogue is translocated from treated
to untreated portions of plants. Residues tend to remain on plant
surfaces and are easily brushed or washed off. They are degraded quite
rapidly by weathering.
Phosmet is hydrolysed in plant tissues and converted to phthalic
acid and/or phthalamic acid which is translocated in the plant,
decarboxylated, apparently by enzyme action, and further metabolised
to possible benzoic acid derivatives.
On apples, pears and peaches, the average half-life of phosmet
appears to be less than two weeks. The disappearance of residual
phosmet from standing corn plants was found to be rapid, but after
ensiling, the loss of phosmet was slow (about 50% loss in 92 days).
The acid conditions in the silage stack probably protected the phosmet
against spontaneous hydrolysis.
Kovac et al. (1968) reported the rapid decomposition of phosmet
to non-toxic substances in fruit and vegetables. After treatment with
a 0.25% phosmet spray, residue levels declined to 0.75 mg/kg in 1-12
days. In experiments on beet, carrots and tomatoes grown on a loamy
soil in the USSR, Boiko and Popova (1970) found no residues of phosmet
30-35 days after treatment with sprays of up to 0.6% a.i.
concentration.
In soils
Phosmet and its oxygen analogue are rapidly degraded in soils.
Degradation is favoured by increasing alkalinity and moisture content
and is less in autoclaved soil samples, indicating that hydrolysis of
phosmet is not dependent on moisture alone, but is due in some degree
to microbial action. The time for 50% degradation varied from 3 days
in a non-autoclaved loamy soil containing 10% moisture, to 19 days in
the same soil with only 2% moisture. It is stated that the phthalic
acid produced is readily metabolised and utilised by micro-organisms
so that phosmet represents a totally biodegradable pesticide leaving
no unmetabolisable residues in the environment (Snelson, 1976). The
vapour pressure of phosmet is fairly high, and under laboratory
conditions it has been shown to evaporate rapidly from peat soils, but
not from sandy soils (Zatserkovskaya, 1974).
In storage and processing
The effect of the washing, blanching and sterilisation operations
used in the canning industry on phosmet deliberately added to apples,
plums, cabbage, pears, green peas and red beet (by dipping them in a
0.4% aqueous dispersion of phosmet) has been studied in the USSR
(Gorelik et al., 1973a). Washing with 5 parts of a 0.5% NaOH solution
to one part of fruit reduced the phosmet content of apples and plums
by 50-55%. Blanching at 80°C with a liquid/fruit ratio of 5:1 removed
55% and 81% respectively of the initial phosmet loading of plums and
apples. The rate of phosmet destruction by sterilisation varied from
60% to 100% in a range of commodities, and about 38% in cabbage. No
phosmet residues were detected in canned apples and plums after
storage at 20°C for 12 months.
Similar studies were made of the effect of such processing on
phosmet residues in apples used for production of canned apple puree
as a baby food (Gorelik et al., 1973b). Blanching, crushing and
sterilisation reduced the residue level by 51%, 28% and 16-25%
respectively. The same workers studied the effects of deep freezing on
the residue dynamics of phosmet in carrots, cabbage and beet. It was
found that even prolonged deep freezing resulted in negligible
reductions of phosmet residues, and it was concluded that vegetables
containing phosmet in excess of the USSR tolerance of 0.25 mg/kg
should not be frozen prior to processing.
Evidence of residues in food in commerce or at consumption
No data were available.
METHODS OF RESIDUE ANALYSIS
Colorimetric methods
Much of the earlier work on residues resulting from supervised
trials was carried out using colorimetric procedures which determine
either the phthalimide moiety after conversion to anthranilic acid or
the phosphorodithioate moiety after conversion to phosphomolybdate.
The anthranilic acid method (Batchelder and Patchett, 1965) is the
more sensitive and specific of the two. Both methods are described in
detail by Batchelder et al.(1967), who also give typical figures for
the recovery of phosmet by these methods from various crops and animal
tissues. The anthranilic acid method is claimed to detect 2 micrograms
of phosmet reliably (giving a detection limit of 0.04 mg/kg for a 50
gram sample), and is applicable to animal or plant tissues. It will
also detect the oxygen analogue but is susceptible to interference
from folpet or azinphos-methyl. These compounds do not interfere with
the phosphomolybdate method, which includes a selective hydrolysis
step.
An alternative colorimetric procedure for determining the
phosphorodithioate moiety (Sharova and Kozhemyakin, 1973) involves the
formation of a yellow complex with copper sulphate, and has been
applied to the determination of phosmet residues in the tissues of
birds, with a detection limit of 0.5 mg/kg.
A method involving the liberation of formaldehyde by acid
hydrolysis of phosmet, and colour development with chromotropic acid,
has been applied to the measurement of phosmet residues in water, with
a detection limit of 0.01 mg/l (Novikova and Varlamova, 1973), and in
apples, with a detection limit of 0.5 mg/kg (Novikova et al., 1969).
Thin-layer chromatographic methods
Phosmet can be determined at the residue level by several of the
methods involving in situ fluorimetry on thin-layer chromatograms
which have been evolved for organophosphorus pesticides. For example,
Frei and Mallet (1971) used a chelate spray reagent consisting of a
1:2 mixture of salicyl-2-aldehyde-2-quinolylhydrazone and manganese
(II) chloride, which was applied after exposure of the plate to
bromine. They claim a visual detection limit of 0.04 µg phosmet.
Bidleman et al. (1972) used fluorescence quenched solutions of
palladium (II) - calcein and palladium(II)-calcein blue as sensitive
spray reagents for the in situ fluorometric determination of
organophosphorus pesticides including phosmet, and reported that as
little as 10.50 ng of a phosphorodithioate could be detected.
Methods have also been described in which thin-layer
chromatography is used as a clean-up procedure for phosmet residues
extracted from foods prior to determination of the eluted pesticide by
one of the colorimetric procedures referred to above. An example is
the method of Novikova and Mel'tser (1972), which uses the chromotopic
acid procedure after clean-up on silica gel plates. This method had a
detection limit of 0.6 mg/kg for phosmet residues in foods of plant
origin. Alternatively, the phosmet spot could be visualised on the
plate by spraying with bromophenol blue and silver nitrate, giving a
visual detection limit of 0.3 mg/kg.
Phosmet is also detectable on TLC plates by enzyme inhibition
methods. For example, Schutzmann and Barthel (1969) reported detection
limits of 0.5 µg and 0.05 µg for phosmet and its oxygen analogue
respectively, using horse serum cholinesterase with indoxyl acetate as
substrate. Schutzmann (1970) reported that the sensitivity of the
method could be improved by using either the acetate or butyrate of
N-methyl indoxol as substrate. Ackermann and Engst (1970) used beef
liver esterase with 1-napthyl acetate and Fast Blue B on silica gel G
plates to detect organophosphorus residues in rat foetal tissues. They
were able to detect 0.025 mg/kg of phosmet and 0.005 mg/kg of its
0-analogue by this method.
Gas chromatographic methods
Some early gas chromatographic determinations of phosmet residues
made use of the electron capture detector (ECD). For example, Bowman
and Beroza (1965) were able to determine phosmet (but not the
0-analogue) in milk and sweet corn by using the ECD, and Gutenmann et
al. (1965) applied it to the determination of phosmet (detection limit
0.1 mg/kg) and the O-analogue (0.2 mg/kg) in apples, potatoes, grapes
and alfalfa following a TLC clean-up.
Later methods have made use of either an alkali flame ionisation
detector (AFID) giving selective detection of phosphorus compounds, or
a flame photometric detector (FPD) operated in either the phosphorus
or the sulphur mode. Bowman and Beroza (1966) used the FPD with a
526 nm phosphorus filter to determine phosmet (detection limit 0.002
mg/kg) and the 0-analogue (0.004 mg/kg) in sweet corn, following
clean-up and separation on a silica gel column. The glc column packing
was 10% DC 200 on 80/100 mesh Gas Chrom Q. The column was operated at
200°C, and required conditioning with repeated injections of phosmet
and corn extract to obtain a constant response to the insecticide. The
same column packing was used by Watts and Storherr (1967) in
conjunction with an AFID for the determination of phosmet and other
organophosphorus insecticide residues in milk after a sweep
codistillation clean-up. They too found the need for thorough column
conditioning. Storherr et al. (1967) used essentially the same
procedure to determine organophosphorus pesticides including phosmet
in fortified samples of edible oils. The average recovery for phosmet
was 96%, and the method detected apparent levels in unfortified
samples ranging from less than 0.01 to 0.04 mg/kg.
Bowman and Beroza (1967) were able to determine phosmet extracted
from milk (in hexane/ether) without clean-up by using the FPD with a
394 nm sulphur filter. Bowman et al. (1968) investigated several
alternative extraction procedures for removing OP residues (including
phosmet and its O-analogue) from field treated crops, and found the
most efficient method to be soxhlet extraction for 4 hours with 10%
methanol in chloroform. Determination was by gas chromatography with
flame photometric detection using a phosphorus filter.
A gas chromatographic method employing the AFID (with rubidium
sulphate as alkali source) and a short, narrow bore column containing
10% DC 200 on 100/120 mesh Gas Chrom Q has been described by Barney et
al. (1972). For most crops, clean-up is achieved simply by shaking
with charcoal followed by filtration through a Millipore filter.
Cottonseed and cotton foliage require prior acetonitrile partition.
The lower limit of detection is given as 0.05 mg/kg and the method has
been successfully applied to a range of fruit, vegetable and fodder
crops. The authors state that the FPD in either the P or S mode may be
used instead of the AFID, but stress the importance of the small
particle size of the solid support to ensure adequate resolution of
the oxygen analogue from phosmet.
Several multi-residue gas chromatographic procedures which
include phosmet have been published recently. Some examples are:
(a) The method of Ripley et al. (1974) for OP residues in natural
waters (using the FPD).
(b) The method of Luke et al. (1975) for OP and nitrogen containing
pesticides in produce (using the AFID with a potassium chloride
source).
(c) The method of Krijgsman and Van de Kamp (1976) which utilises a
capillary column of SE-30 with a flame photometric detector.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The national tolerances shown in Table 4 have been reported to
the Meeting.
TABLE 4. National tolerances reported to the Meeting
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Australia Fat of meat of cattle 1 Snelson
Milk and milk products 1976
(fat basis) 0.2 "
Pome and stone fruit 1 "
Canada Apples, peaches and
pears 10
Plums 5
Netherlands Apples, pears 21 1
Potatoes 28 0.02
New Zealand Fruit 14 10
(Kiwifruit 10)
USA Alfalfa 40 Anon, 1969
Apples, peaches & pears 10 "
Meat & fat of cattle,
goats, hogs & sheep 0.2 Anon, 1969
Grapes 10 Anon, 1971a
Cherries 10 Anon, 1971b
Apricots, nectarines
and plums 5 " "
TABLE 4. (Cont'd.)
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Potatoes 0.1 Anon, 1971c
Sweet potatoes (from
post-harvest application 10 Anon, 1973
Almond hulls, blue berries,
corn forage and fodder
(including sweet corn,
field corn and popcorn),
cranberries, pea forage
and hay 10 Anon, 1975
Fresh corn, including sweet
corn (kernel and cob with
husk removed), corn grain
(including popcorn), and
peas 0.5 Anon, 1975
Meat, fat and meat by-products
of horses 0.2 Anon, 1975
Nuts 0.1 Anon, 1975
USSR Vegetables 0.25 Gorelik et
al, 1973b
Figs 0.25 Popov, 1973
APPRAISAL
As a systemic insecticide and acaricide against a range of
ectoparasites of animals, phosmet may be applied as a dilute aqueous
spray containing up to 0.5% a.i., as a ready-to-use "pour on"
formulation containing up to 4% a.i. and applied at up to 40 mg per kg
body weight, as a dust containing 1 to 2% a.i., or it may be
administered orally.
In plants, phosmet is used as a non-systemic pesticide and is
applied to a variety of crops to protect against insects and mites. At
the concentrations usually applied it can be effective without harming
some useful predators and this makes it of value for integrated
control programmes. On fruit, a 0.1% spray has been used on pasture
and forage crops up to 0.75 kg/ha, and on cereal crops at 35 g/ha (ULV
spray). It has been used at 3-5 kg/ha on cotton.
Data available on residues in meat of cattle is not extensive,
but would be in accord with a maximum level of 0.2 mg/kg. Applied as a
spray to apples and peaches, residues are generally less than 1 mg/kg
after a 3 weeks preharvest period. On forage crops, application of
phosmet at 1 kg a.i./ha produced residues of the order of 50 mg/kg
which declined to less than 5 mg/kg after 14 days. Because phosmet is
not excreted in the milk, cattle can graze recently treated pastures.
Both phosmet and its oxygen analogue are of low persistence in
plants and animals. In mammals phosmet is rapidly metabolised, mainly
to innocuous water-soluble compounds. The metabolites principally
excreted are phthalic acid and phthalamic acid with only minute traces
of phosmet or its oxygen analogue.
Plants treated with phosmet tend to retain it on the surface
where it is washed off or weathered. In plant tissues conversion to
phthalic or phthalamic acid again takes place and these undergo
further breakdown.
Phosmet and its oxygen analogue are rapidly degraded in soils,
degradation being favoured by increasing soil alkalinity and moisture
content. Apart from simple chemical hydrolysis, metabolism by
microorganisms also occurs and it has been stated that phosmet is
totally biodegradable.
Processing in the canning industry by washing with dilute alkali,
bleaching or sterilising will remove at least 50% of the phosmet from
fruit and vegetables.
Several analytical methods suitable for regulatory purposes are
available. These include a colorimetric method which determines the
phthalimide moiety after conversion to anthranilic acid and gas
chromatographic methods with either an alkali flame ionisation or a
flame photometric detector. There is also an enzyme inhibition.
REFERENCES
Ackermann, H., and Engst, R. Occurrence of organophosphorus
1970 insecticides in the foetus. Arch. Toxikol., 26:17.
Adeishvili, L.G. Pesticide residues in apple fruits. Tr.
1973 Nauchno-Issled. Inst. Zashch. Rast (Toflis),
25:3-5.
Ali Niazee, M.T., and Stafford, E.M. Management of grape pests in
1973 Central California vineyards. I. Cultural and
chemical control of Patynota stultana on
grapes. J. Econ. Entomol., 66 (1): 154-7
Andrichuk, B.V. Phthalophos as an acaricide for the control of
1971a sheep psoroptosis. Tr. Yses. Nauch-Issled. Inst.
Vet. Sanit., 38: 264-70
Andrichuk, B.V. Acaricidal dusts for the control of sheep
1971b psoroptosis. Tr. Vses. Nauch-Issled. Inst.Vet.
Sanit., 39: 363-8
Anon. (Environmental Protection Agency, Washington, D.C.)
1969 N-(mercaptomethyl) phthalimide S-(O,O
-dimethyl phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
34 (63): 6041.
1971a Ibid., 36(59); 5690.
1971b Ibid., 36(132): 12898-9
1971c Ibid., 36 (152): 14471-2
Anon. (Food Drug Administration, Washington, D.C.) New animal
1971d drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide S-(O,O-dimethyl
phosphorodithioate). Federal Register, 36(231):
22829.
Anon. (Environmental Protection Agency, Washington, D.C.)
1973 N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate) and
residues. Federal Register, 38(55): 7459-60.
Anon. (Food Drug Administration, Washington, D.C.) New animal
1974 drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate). Federal
Register, 39(172): 32025-6.
Anon. (Environmental Protection Agency, Washington, D.C.)
1975 N-(mercaptomethyl) phthalimide S.(O,O-
dimethyl-phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
40(48): 11352-3.
Barney, J.E., Ja, W.Y., and Shelman, D.L. Analytical method:
1972 for pesticides, plant growth regulators and food
additives. (Academic Press, Ed. G. Zweig and J.
Sherma), Vol. VI, pp. 408.134
Batchelder, G.H., and Patchett, G.G. American Chemical Society,
1965 149th Meeting, Division of Agriculture and Food
Chemistry, Paper No. 37.
Batchelder, G.H., Patchett, G.G., and Menn, J.J. Analytical
1967 methods for pesticides, plant growth regulators
and food additives (Academic Press, Ed. G. Zweig),
Vol. V, pp. 257-275.
Bidleman, T.F., Nowlan, B., and Frei, R.W. Metallofluorescent
1972 indicators as spray reagents for the in situ
determination of organophosphorus pesticides in
thin layer chromatograms. Anal. Chim. Acta, 60(1):
13-23.
Boiko, I.B., and Popova, N.G. Effect of some organophosphorus
1970 pesticides on the biological value of vegetable
crops. Med. Zh. Uzb., 9: 35-7.
Bowman, M.C., and Beroza, M. Analysis of Imidan colorimetrically
1965 and by electron-affinity gas chromatography. J.
Assoc. Offic. Anal. Chem., 48(5): 922-6.
Bowman, M.C. and Beroza, M. Determination of Imidan and Imidoxon
1966 in sweet corn by gas chromatography with flame
photometric detection. J. Assoc. Offic. Anal.
Chem., 49(6): 1154-1157.
Bowman, M.C. and Beroza, M. Note on extraction of a polar insecticide
1967 (Imidan) from milk. J. Assoc. Offic. Anal. Chem.,
50(4): 940-941.
Bowman, M.C., Beroza, M., and Leuck, D.B. Procedures for extracting
1968 residues of phosphorus insecticides and
metabolites from field treated crops. J. Agr. Food
Chem., 16(5): 796-80.
Butt, B.A., White, L.D., Moffit, H.R., Hathaway, D.O., and
1973 Schoenleber, L.G. Integration of sanitation,
insecticides, and sterile moth releases for
suppression of populations of codling moths in the
Wenas Valley of Washington. Environ. Entomol.,
2(2): 208-13.
Chamberlain, W.F. A study of the dermal treatment of a 1965 steer
with C14-labelled Imidan. J. Econ. Entomol.,
58(1):51-55.
Chirikashvili, R.V., Abbasov, T.G., and Frolov, B.A. Duration
1970 of persistence of phthalophos in organs and
tissues of hens during oral administration. Tr.
Vses. Nauch-Issled. Inst. Vet. Sanit., 37: 175-8.
Del Rivero, J.M., Lafuente, M., and Lazaro, E. New products
1971 for control of the potato beetle, Leptinotarsa
decemlineata, resistant to chlorinated
insecticides. An. Inst. Nac. Invest. Agr. (Spain),
Ser. Prot. Veg. (1): 183-7.
Dorough, H.W., Randolph, N.M., and Wimbish, G.H. Imidan, and
1965 trichlorfon residues on Coastal bermudagrass.
Texas A&M University/Texas Agricultural Experiment
Station. Progress Report PR-2385.
Drumond, R.O., Ernst, S.E., Trevino, J.L., Gladney, W.J., and
1972 Graham, O.H. Boophilus annulatus and B.
microplus. Sprays and dips of insecticides for
control on cattle. J. Econ. Entomol., 65(5):
1354-7.
Forsythe, H.Y., and Hall, F.R. Control of the plum Curculio in
1972 Ohio. J. Econ. Entomol., 65(6): 1703-6.
Frei, R.W., and Mallet, V. Quantitative thin-layer chromatography
1971 of organothiophosphorus pesticides by in situ
fluorimetry. Intern. J. Environ. Anal. Chem., 1:
99.
Frolov, B.A. Activity of some organo-phosphorus and carbamate
1970 compounds for fowl ectoparasites. (Argas
persicus and Cimex lectularius) Tr., Vses.
Nauch-Issled. Inst. Vet. Sanit., 37: 334-43
Gorelik, L.D., Kushchinskaya, In.N., and Nemets, S.M. Effect
1973a of technological conditions on the content of
phthalophos in canned food. Konserv. Ovoshchesush.
Prom. 4: 8-10.
Gorelik, L.D., Kushchinskaya, I.N., and Sazonova, A.R. Lowering
1973b the phthalophos level during the preparation of
canned apple sauce. Konserv. Ovoshchesush. Prom.
7: 21-3.
Gribkova, N.I. Effectiveness of insecticides against leaf-miner
1973 moths. Khim. Sel. Khoz., 11(8):592-4.
Gutenmann, W.H., Bache, C.A., and Lisk, D.J. Determination
1965 of Imidan and Imidoxon in crops by preparative
thin layer and by gas chromatography. J. Gas
Chromat., 3: 350-52.
Holdsworth, R.P. Realities of integrated insect control.
1973 Proc. Ohio State Hort. Soc., 126: 89-92.
Ionova, I., Zhecheva, G., and Dimitrov, K. Residual amounts
1974 of Sevin, Neguvon, and Imidan in the meat of pigs
treated against ectoparasites. Vet-Med Nauki,
11(5): 28-34.
Johnson, J.C., and Bowman, M.C. Fate of Bidrin and Imidan
1968 when fed in silage to lactating dairy cows. J.
Dairy Science, 51(8): 1225-8.
King, N.L., and Best, D.R. Unpublished report from Hawthorn Park
1971 Research Laboratories Pty-Ltd. to Stauffer Chem.
Co. (Australia) Pty. Ltd.
King, N.L., and Ferguson, A.B. Unpublished report from Hawthorn
1971 Park Research laboratories Pty. Ltd. to Stauffer
Chem. Co. (Australia) Pty. Ltd.
Klisenko, M.A., and Pis'mennaya, M.V. Effects of UV radiation
1973 on dialkyldithiophosphate pesticides. Khim. Sel.
Khoz., 11(12): 916-8.
Klunker, R. Current status of potato beetle control, especially
1974 with respect to the resistance problem.
Nachrichtenbl. Pflanzenschutz DDR 28(9). 191-6.
Kovac, J., Batora, V., Moracvik, J., and Benusova, M. Persistence
1968 of O,O-dimethyl-S-(phthalimidomethyl)
dithiophosphate residues in vegetables and fruits.
Agrochimia (Bratislava), 8(3): 77-81.
Krijgsman, W., and Van de Kamp. C.G. Analysis of organophosphorus
1976 pesticides by capillary gas chromatography with
flame photometric detection. J. Chromat.,
117(1): 201-5.
Large, G.B., and Pitt, L.S. Thiophosphate synergists. U.S. Patent
1975 3, 886, 273, 27 May 1975, Stauffer Chemical Co.
Leuck, D.B., and Bowman, M.C. Imidan insecticide residues
1968 (Imidan and Imidoxon): their persistence in corn,
grass and soybeans. J. Econ. Entomol., 61(3).
705-7.
Loomis, E.C., Noorderhaven, A., and Roulston, W.J. Control of
1972 Southern cattle tick by pour-on animal systemic
insecticides. J. Econ. Entomol., 65: 1638-41.
Luke, M.A., Froberg, J.E., and Masumoto, H.T. Extraction and
1975 clean-up of organo-chlorine, organo-phosphorus,
organo-nitrogen and hydrocarbon pesticides in
produce for determination by gas-liquid
chromatography. J. Assoc. Offic. Anal. Chem.
58(5): 1020-6.
Mardzhanyan, G.M., Semerdzhyan, O.G., Ust'yan, A.K. and Manukyan,
1973 Z.S. Possibility of controlling Phytonomus with
organo-phosphorus insecticides. Izv. Sel'skokhoz.
Nauk, 16(11-12): 36-41.
McBain, J.B., Menn, J.J., and Casida, J.E. Metabolism of
1968 carbonyl-C14 labelled Imidan,
N-(Mercapto-methyl) phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats
and cockroaches. J. Agr. Food Chem., 16(5):
813-820.
Miller, M.C., Shaw, F.R., and Smith, C.T. The comparative residual
1969 life of two formulations of Imidan on alfalfa. J.
Econ. Entomol., 62(3): 720-1.
Mistric, W.J., and Smith, F.D. Organophosphorus and carbamate
1970 insecticides as substitutes for DDT in controlling
the tobacco flea beetle on flue cured tobacco. J.
Econ. Entomol., 63: 509-11.
Nakotomi, K., and Ishibe, K. (Hokko Chemical Industry Co. Ltd.)
1975 Insecticidal synergism with acephate
phosphorothioate mixtures. Japanese Patent 75 71,
838, 14 June 1975.
Nepoklonov, A.A., Nepoklonova, M.I., and Pavlova, N.V. Acaricidal
1969 properties of phthalophos.Tr., Vses. Nauch-Issled.
Inst. Vet. Sanit., 34: 348-50.
Nesterenko, N.I. Organophosphorus preparations used to control sugar
1970 beet pests. Khim. Sel. Khoz., 8: 382-3.
1973 Ibid., 11(2): 113-6.
New South Wales Department of Agriculture. Extracts from the 1966-7
1969 annual report of the Entomology Branch. PANS, 15
(2): 225.
Nikiforov, A.M., and Mamaev, K.A. Testing of new chemical
1972 preparations (cotton pesticides). Khlopkovodstvo,
8: 31-2.
Novikova, K.F., and Mel'tser, F.R. Chromatophotometric method
1972 for determining residual amounts of phthalophos in
food products of plant origin. Probl. Anal. Khim.,
2: 95-99.
Novikova, K.F., Mel'tser, F.R., Makarova, S.V., and Navmova, V.A.
1969 Spectrophotometric determination of residual
phthalophos in apples. Gig. Primen. Toksikol.
Pestits. Klin. Otravi, 7: 595-601.
Novikova, K.F., and Varlamova, T.A. Spectrophotometric
1973 determination of microgram quantities of
phthalophos and phosalone in natural waters.
Metody Opred. Pestits. Vode, 1: 98-103.
Omarov, L.M. Phthalophos and Sevin dusts to control mites.
1974 Veterinarya. 4: 37-8.
Omelyuta, V.P. Effectiveness of insecticides against Grapholitha
1972 molesta. Khim. Sel. Khoz., 10(7): 516-7.
Otaci, C., Turhan, K., and Barkin, S. Imidan residues on peaches.
1972 Bitki Koruma Bul., 12(1): 45-8.
Pfadt, R.E., Lloyd, J.E., and Spackman, E.W. Power dusting with
1975 organo-phosphorus insecticides to control the
sheep ked. J. Econ. Entomol., 68(4): 468-70.
Popov, P.F. Effectiveness of different preparations against
1973 caterpillars of fig Glyphipterygidae. Khim. Sel.
Khoz., 11(8): 595-6.
Ripley, B.D., Wilkinson, R.J., and Chau, A.S.Y. Multi-residue
1974 analysis of 14 organo-phosphorus pesticides in
natural waters. J. Assoc. Offic. Anal. Chem.,
57(5): 1033-42.
Rogoff, W.M., Brody, G., Roth, A.R., Batchelder, G.H., Meyding,
1967 G.D., Bigley, W.S., Gretz, G.H., and Orchard, R.
Efficacy, cholinesterase inhibition and residue
persistence of Imidan for the control of cattle
grubs. J. Econ. Entomol., 60(3): 640-6.
Ruzimuradov, A., Valiev, B., and Matchanov, S. Test of insecticides
1973 against pasture ground stable flies and gnats.
Mater. Konf. Molodykh. Uch. Uzb. Sel'sk Khoz. 7th
1972: 60-2. (Published in Veterinarya, 1973, ed.
by Sh. A. Azimov et al).
Schutzmann, R.L. Note on improved spray reagents for TLC
1970 fluorogenic detection of cholinesterase
inhibitors. J.Assoc. Offic. Anal. Chem., 53(5):
1056-7.
Schutzmann, R.L., and Barthel, W.F. Indoxyl acetate spray reagent
1969 for fluorogenic detection of cholinesterase
inhibitors in environmental samples. J. Assoc.
Offic. Anal. Chem., 52(1): 151-6.
Semerdzhyan, O.G. Poisoning of rabbits fed Phthalophos treated
1972 alfalfa. Izv. Sel'skokhoz. Nauk. 15 (6): 77-9.
Sevost'yanov, A.Z., and Ushakova, N.N. Acaricidal effectiveness of
1971 some pesticides against Ixodes mites during tests
in samples of sheep wool. Tr. Stavropol
Sel'skokhoz. Inst., No. 34 (Vol 4): 74-6.
Shapovalova, G.K., Sedykh, A.S., and Abelentseva, G.M.
1970 Effectiveness of using phthalophos and
benzophosphate to combat gooseberry moth
(Zophodia convolutella), and insecticide
residues on the berries. Khim. Sel. Khoz. 8(8):
594-5.
Sharova, V.M., and Kozhemyakin, N.D. Photoelectro-colorimetric
1973 determination of metaphos and phthalophos in
tissues of experimentally poisoned birds. Khim.
Sel. Khoz, 11 (3): 229-30.
Shaw, F.R., Callahan, R.A., and Miller, M.C. Rates of disappearance
1966 of Zolone and Imidan from alfalfa. J. Econ.
Entomol., 59(6): 1524-5.
Snelson, J.T. Private communication from Department of Primary
1976 Industry, Australia.
Speirs, R.D., and Lang, J.H. Contact, residue and vapour toxicity
1970 of new insecticides to stored products insects.
II. USDA Agricultural Research Service, Marketing
Research Report No. 885.
Storherr, R.W., Murray, E.J., Klein, I., and Rosenberg, L.A.
1967 Sweep co-distillation clean-up of fortified edible
oils for determination of organo-phosphate and
chlorinated hydrocarbon pesticides. J. Assoc.
Offic. Anal. Chem., 50(3): 605-15.
Surorov, P.S. Acaricidal effect of ciodrin, phthalophos and
1970 chlorophos on cutaneous mites, cattle psoroptosis
causative agents. Tr., Vses. Nauch Issled. Inst.
Vet. Sanit., 35: 379-82.
Tanabe, M., Dehn, R.L., and Bramhall, R.R. The photochemistry
1974 of Imidan in diethyl ether. J. Agr. Food Chem.,
22(1): 54-6.
Thomas, F.J.D., and Taylor, A.S. (ICI of Australia and New Zealand
1971 Ltd.) Apparatus and compositions for controlling
cattle ticks. South African Patent 69 06, 036 22
February 1971.
Uctani, T., Hirano, T., and Tsuchitate, M. (Kumiai Chem. Industry
1974 Co. Ltd.) Acaricidal and insecticidal
compositions. Japanese Patent 74 15, 776, 17 April
1974.
Van Cleave, H.V., and Anderson, A.L., Field control tests for pecan
1971 insects. Proc. Tex. Pecan Grow. Ass.: 32-3
Vernon, J.D.R., and Gould, H.J. Further trials on alternatives
1972 to DDT for the control of pre-blossom pests on
apple and pear. Plant Pathol., 21(1). 1-9.
Vrochinskii, K.K. Accumulation of pesticides in hydrobiocts
1971 as a measure for evaluating experimental water
toxicology. Eksp. Vod. Toksikol. 149-63.
Watts, R.R., and Storherr, R.W. Sweep do-distillation clean
1967 up of milk for determination of organophosphate
and chlorinated hydrocarbon pesticides. 50(3):
581-5.
Wilde, W.H.A. Low volume concentrate spraying and integrated 1973
control in some Ontario orchards. Proc. Ohio State
Hort. Soc. 126: 85-8.
Witkowska, D., and Wojnarowska, P. Effectiveness of some chemical
1971 preparations against Anthonomus pomorum (apple
weevil). Biol. Inst. Ochr. Rosl. (48): 357-66.
Woodard Research Corporation. Unpublished report.
1963
Zatserkovskaya, G.A. Evaporation of insecticides from different
1974 types of soils. Zashch. Rast (Kiev), 20: 61-6.
TABLE 3. Residues of phosmet in various crops from supervised spraying trials
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Apples Netherlands 1.5 1 0 1.8(1.3-2.1 0
(var. Lombertscalville) (2000 1/ha) 7 0.9(0.7-1.2) 0
14 0.6(0.3-0.9 0
21 0.5(0.3-0.6) 0
28 0.2(0.1-0.2) 0
Netherlands 1.5 1 0 2.4(1.5-3.4) 0.04(0.01-0.07)
(var. (2000 l/ha) 7 2.0(1.7-2.3) 0.06(0.04-0.09)
Goudreinette) 14 1.4(0.6-2.0) 0.06(0.03-0.07)
21 0.4(0.2-0.6) 0.03(0-0.06)
28 0.6(0.2-0.7) 0.02(0.01-0.03)
Apples New Zealand 1.5 9 2 2.0
9 1.7
15 1.1
23 0.7
1.5 9 1 4.3
3 3.6
7 4.0
14 2.9
21 3.0
28 2.1
Apples Australia 1.75 ? 0 2.2
(King & Best, 1 1.5
1971) 7 0.7
14 0.8
21 0.6
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
2.0 ? 0 2.8
1 1.5
7 1.6
14 1.0
21 0.7
Peaches Australia 1.0 1 0 3.8
(var. Golden 7 3.6
Queen) 14 1.1
21 0.5
Australia 1.0 1 1 3.4
(var.Maygold) 3 2.5
5 1.3
7 3.1
14 1.5
Peaches Australia 2.0 1 1 5.3
(var. Maygold) 3 6.2
5 4.2
7 3.9
14 6.2
Peaches Turkey 1.5 3 0 5.39
3 5.44
7 4.65
14 3.48
21 3.30
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Kiwifruit New Zealand 1.1 2 1 10.0
7 9.6
14 5.8
21 5.1
28 3.6
35 3.1
42 3.0
1.12 2 10 9.5
7.7
5.8
7.1
1.12 7 10 25.0
16.0
8.4
7.2
0.75 7 10 8.8
4.8
7.1
6.4
Grapefruit New Zealand 1.12 4 1 0.65
7 0.57
14 0.3
21 0.26
28 0.38
42 0.22
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Potatoes Netherlands 0.56 kg a.i./ 2 22 0.004 0
(var. ha(as 50% w.p.) (0.002-0.006)
Burmania)
Netherlands " 2 20 0.001 0
(var. Bintje) (0-0.003
Netherlands " 2 21 0.001 0
(var. (0.001-0.002)
Eigenheimer)
Alfalfa U.S.A. 1.1 kg a.i./ 1 1 43.5
(Shaw et al. ha(36% ec) 8 2.09
1966) 14 0.84
21 0.31
controls 0.18-0.44
Alfalfa U.S.A. 0.56 kg a.i. 1 0 33.86
(Miller et al. /ha(50% wp) 5 3.58
1969) 10 2.27
15 0.34
21 0.04
1.1 kg a.i. 1 0 57.11
/ha(50% wp) 5 10.74
10 3.42
15 1.52
21 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i. 1 0 20.95
/ha(36% ec) 5 3.87
10 2.09
15 0.94
21 0.00
Alfalfa U.S.A. 1.1 kg a.i. 1 0 44.42
/ha (36% ec) 5 9.87
10 5.06
15 2.00
21 0.37
Coastal U.S.A. 0.84 kg a.i. 1 0 37.93
bermuda (Dorough et /ha 1 32.66
grass al, 1965) 3 23.89
7 15.79
14 8.31
Coastal U.S.A. 0.28 kg a.i. 1 0 12.3 0.06
bermuda (Leuck and /ha 1 6.86 0.06
grass Bowman, 1968) 3 1.11 0.00
4 0.96 0.00
7 0.72 0.00
15 0.13 0.00
0.56 kg a.i. 1 0 27.0 0.13
/ha 1 16.4 0.13
3 2.82 0.02
4 2.30 0.02
7 1.78 0.00
15 0.36 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
Coastal U.S.A. 1.1 kg a.i./ha 1 0 52.9 0.36
Bermuda 1 36.8 0.40
grass 3 7.02 0.07
4 6.34 0.06
7 3.72 0.02
15 0.57 0.00
Maize U.S.A. 0.28 kg a.i./ha 1 0 2.67 0.00
plants (Leuck and 1 1.00 0.00
(green Bowman, 1968) 2 0.39 0.00
weight 4 0.20 0.00
basis) 7 0.09 0.00
0.56 kg a.i./ha 1 0 7.07 0.01
1 Not sampled owing to rain
2 0.99 0.00
4 0.46 0.00
7 0.26 0.00
1.1 kg a.i./ha 1 0 10.9 0.02
1 Not sampled owing to rain
2 2.89 0.00
4 1.16 0.00
7 0.63 0.00
Soybean U.S.A. 0.28 kg ai./ 1 0 31.2 0.03
plants (Leuck and ha 1 21.1 0.02
(green Bowman, 1968) 2 15.0 0.01
weight 4 2.45 0.00
basis) 7 0.48 0.00
15 0.10 0.00
TABLE 3. (Cont'd.)
Application
rate
g a.i./l No. of Pre-harvest Residues at harvest
unless otherwise Applications interval mean (range) mg/kg
Crop Country stated (days) Phosmet 0-analogue
0.56 kg a.i./ha 1 0 55.4 0.07
1 30.2 0.03
2 27.6 0.02
4 6.27 0.00
7 1.24 0.00
15 0.28 0.00
1.1 kg a.i./ha 1 0 82.2 0.11
1 55.4 0.07
2 43.8 0.04
4 14.7 0.01
7 2.62 0.00
15 0.76 0.00
Other fruits
Trials in New Zealand on Kiwifruit and grapefruit yielded the
residue data given in Table 3. In most cases the spray concentration
was slightly above the recommended upper limit of 0.1% a.i., but only
for the highest rate of application to Kiwifruit (7 applications of
0.112% a.i.) were residues in the whole fruit found to be above the
national tolerance of 10 mg/kg after the recommended 10-day waiting
period. Separate analyses of the skin and flesh revealed that the
residues were mainly concentrated in the skin. Four applications of
0.112% a.i. spray to grapefruit resulted in a residue of only 0.3
mg/kg in the whole fruit (0.05 mg/kg in the flesh) after the
recommended 14 day interval.
Alfalfa
In 1965, experiments were conducted in the United States to
determine the rate of disappearance of phosmet from alfalfa. An ec
formulation containing 36% a.i., w/v, was applied at the rate of 1.1
kg a.i./ha. Residues were determined by the anthranilic acid
colorimetric method over a period of 3 weeks after application. The
results are given in Table 3, and show that the phosmet residues had
declined to the levels found in untreated control samples after 21
days (Shaw et al., 1966).
In a subsequent trial on alfalfa (Miller et al., 1969), the
disappearance rates of phosmet after application in two different
formulations (the 36% ec and a 50% wp) were compared. Each formulation
was applied at the rate of 0.5 and 1 lb a.i./acre in a dilute spray
used at 100 gallons per acre. Each treatment was replicated twice, and
the crop was sampled over a 3 week period after treatment. The results
are summarised in Table 3.
In the USSR, the feeding of rabbits with alfalfa gathered 10 days
after it had been sprinkled with phosmet produced some depression of
cholinesterase activity. When the alfalfa was gathered 15 days after
treatment no depression was observed, and the residue level had
declined to 1/6 of that found 11 days after treatment and 1/15 of that
found 6 days after treatment (Semerdzhyan, 1972).
Other forage crops
Trials were conducted in the U.S.A. in which maize (for silage),
soybean plants, and coastal Bermuda grass were each sprayed at rates
of 0.28, 0.56 and 1.1 kg of phosmet per ha. Residues of phosmet and
its oxygenanalogue were determined in samples taken over a 15 day
period following treatment (Leuck and Bowman, 1968). The results are
given in Table 3, and indicate that in all cases the residues had
declined to under 1 mg/kg in 15 days or less. On the other hand, in a
trial on Coastal Bermuda grass at the Texas Agricultural Experiment
Station, in which phosmet was applied at 0.84 kg/ha, residues of about
8 mg/kg were found after 14 days (Dorough et al., 1965).
Leuck and Bowman analysed the concentrate used in their trial,
and found that it contained a trace of the oxygen-analogue equivalent
to 0.12% of the phosmet content. Thus some, at least, of the
0-analogue residues found probably originated from the spray liquor
rather than from environmental oxidation of phosmet.
FATE OF RESIDUES
General comments
The results presented in the foregoing section indicate that both
phosmet and its oxygen analogue are of low persistence in plants and
animals. The photochemistry of phosmet in diethyl ether solution was
studied by Tanabe et al. (1974), using a 450 watt UV lamp. They
isolated N-methylphthalimide and N-methoxymethylphthalimide as
photolysis products in low yield. Neither of these was insecticidally
active. No photoproduct containing P or S could be isolated from the
many minor components. Using UV radiation in the range 250-400 nm,
Klisenko and Pis'mennaya (1973) found a half-life of 70 minutes for
phosmet, and identified phthalimide, N-methoxymethylphthalimide and
phthalic acid as photolysis products, in addition to P-O analogues.
In animals
The metabolism of carbonyl-14C-labelled phosmet in rats
following oral administration was investigated by McBain et al.
(1968). Metabolites were excreted in the urine in the following
amounts (expressed as percentages of the administered dose): phthalic
acid 21.0%, phthalamic acid 40.7% and five minor metabolites
containing the phthaloyl moiety 10.9%. Not more than 0.04% of the dose
administered was excreted in the urine as unchanged phosmet or its
oxygen analogue. This study supported other evidence that phosmet is
rapidly metabolised in mammals, mainly to innocuous water soluble
compounds. For example, Chamberlain (1965) studied the fate of
14C-labelled phosmet following dermal application to a steer. It was
moderately absorbed by the skin of the animal, since 9.6% of the
applied dose was recovered in the excreta (nearly 8% in the urine)
within 7 days. It appeared that the compound was rapidly broken down
in the blood, and that the primary degradation occurred at the
nitrogen atom, resulting in the almost exclusive production of
phthalic and phthalamic acids.
Johnson and Bowman (1968) found no detectable residues of phosmet
or its oxygen analogue in the milk, urine or faeces of lactating dairy
cows fed with silage containing phosmet at a level providing 0.22
mg/kg/ day. Detection limits were 0.002 mg/kg for milk and urine, and
0.004 mg/kg for faeces.
Tests under laboratory conditions in the USSR (Vrochinskii, 1971)
showed that phosmet was stored only briefly by fish.
In plants
Trials on apples, peaches, lemons and potatoes showed that
neither phosmet nor its oxygen analogue is translocated from treated
to untreated portions of plants. Residues tend to remain on plant
surfaces and are easily brushed or washed off. They are degraded quite
rapidly by weathering.
Phosmet is hydrolysed in plant tissues and converted to phthalic
acid and/or phthalamic acid which is translocated in the plant,
decarboxylated, apparently by enzyme action, and further metabolised
to possible benzoic acid derivatives.
On apples, pears and peaches, the average half-life of phosmet
appears to be less than two weeks. The disappearance of residual
phosmet from standing corn plants was found to be rapid, but after
ensiling, the loss of phosmet was slow (about 50% loss in 92 days).
The acid conditions in the silage stack probably protected the phosmet
against spontaneous hydrolysis.
Kovac et al. (1968) reported the rapid decomposition of phosmet
to non-toxic substances in fruit and vegetables. After treatment with
a 0.25% phosmet spray, residue levels declined to 0.75 mg/kg in 1-12
days. In experiments on beet, carrots and tomatoes grown on a loamy
soil in the USSR, Boiko and Popova (1970) found no residues of phosmet
30-35 days after treatment with sprays of up to 0.6% a.i.
concentration.
In soils
Phosmet and its oxygen analogue are rapidly degraded in soils.
Degradation is favoured by increasing alkalinity and moisture content
and is less in autoclaved soil samples, indicating that hydrolysis of
phosmet is not dependent on moisture alone, but is due in some degree
to microbial action. The time for 50% degradation varied from 3 days
in a non-autoclaved loamy soil containing 10% moisture, to 19 days in
the same soil with only 2% moisture. It is stated that the phthalic
acid produced is readily metabolised and utilised by micro-organisms
so that phosmet represents a totally biodegradable pesticide leaving
no unmetabolisable residues in the environment (Snelson, 1976). The
vapour pressure of phosmet is fairly high, and under laboratory
conditions it has been shown to evaporate rapidly from peat soils, but
not from sandy soils (Zatserkovskaya, 1974).
In storage and processing
The effect of the washing, blanching and sterilisation operations
used in the canning industry on phosmet deliberately added to apples,
plums, cabbage, pears, green peas and red beet (by dipping them in a
0.4% aqueous dispersion of phosmet) has been studied in the USSR
(Gorelik et al., 1973a). Washing with 5 parts of a 0.5% NaOH solution
to one part of fruit reduced the phosmet content of apples and plums
by 50-55%. Blanching at 80°C with a liquid/fruit ratio of 5:1 removed
55% and 81% respectively of the initial phosmet loading of plums and
apples. The rate of phosmet destruction by sterilisation varied from
60% to 100% in a range of commodities, and about 38% in cabbage. No
phosmet residues were detected in canned apples and plums after
storage at 20°C for 12 months.
Similar studies were made of the effect of such processing on
phosmet residues in apples used for production of canned apple puree
as a baby food (Gorelik et al., 1973b). Blanching, crushing and
sterilisation reduced the residue level by 51%, 28% and 16-25%
respectively. The same workers studied the effects of deep freezing on
the residue dynamics of phosmet in carrots, cabbage and beet. It was
found that even prolonged deep freezing resulted in negligible
reductions of phosmet residues, and it was concluded that vegetables
containing phosmet in excess of the USSR tolerance of 0.25 mg/kg
should not be frozen prior to processing.
Evidence of residues in food in commerce or at consumption
No data were available.
METHODS OF RESIDUE ANALYSIS
Colorimetric methods
Much of the earlier work on residues resulting from supervised
trials was carried out using colorimetric procedures which determine
either the phthalimide moiety after conversion to anthranilic acid or
the phosphorodithioate moiety after conversion to phosphomolybdate.
The anthranilic acid method (Batchelder and Patchett, 1965) is the
more sensitive and specific of the two. Both methods are described in
detail by Batchelder et al.(1967), who also give typical figures for
the recovery of phosmet by these methods from various crops and animal
tissues. The anthranilic acid method is claimed to detect 2 micrograms
of phosmet reliably (giving a detection limit of 0.04 mg/kg for a 50
gram sample), and is applicable to animal or plant tissues. It will
also detect the oxygen analogue but is susceptible to interference
from folpet or azinphos-methyl. These compounds do not interfere with
the phosphomolybdate method, which includes a selective hydrolysis
step.
An alternative colorimetric procedure for determining the
phosphorodithioate moiety (Sharova and Kozhemyakin, 1973) involves the
formation of a yellow complex with copper sulphate, and has been
applied to the determination of phosmet residues in the tissues of
birds, with a detection limit of 0.5 mg/kg.
A method involving the liberation of formaldehyde by acid
hydrolysis of phosmet, and colour development with chromotropic acid,
has been applied to the measurement of phosmet residues in water, with
a detection limit of 0.01 mg/l (Novikova and Varlamova, 1973), and in
apples, with a detection limit of 0.5 mg/kg (Novikova et al., 1969).
Thin-layer chromatographic methods
Phosmet can be determined at the residue level by several of the
methods involving in situ fluorimetry on thin-layer chromatograms
which have been evolved for organophosphorus pesticides. For example,
Frei and Mallet (1971) used a chelate spray reagent consisting of a
1:2 mixture of salicyl-2-aldehyde-2-quinolylhydrazone and manganese
(II) chloride, which was applied after exposure of the plate to
bromine. They claim a visual detection limit of 0.04 µg phosmet.
Bidleman et al. (1972) used fluorescence quenched solutions of
palladium (II) - calcein and palladium(II)-calcein blue as sensitive
spray reagents for the in situ fluorometric determination of
organophosphorus pesticides including phosmet, and reported that as
little as 10.50 ng of a phosphorodithioate could be detected.
Methods have also been described in which thin-layer
chromatography is used as a clean-up procedure for phosmet residues
extracted from foods prior to determination of the eluted pesticide by
one of the colorimetric procedures referred to above. An example is
the method of Novikova and Mel'tser (1972), which uses the chromotopic
acid procedure after clean-up on silica gel plates. This method had a
detection limit of 0.6 mg/kg for phosmet residues in foods of plant
origin. Alternatively, the phosmet spot could be visualised on the
plate by spraying with bromophenol blue and silver nitrate, giving a
visual detection limit of 0.3 mg/kg.
Phosmet is also detectable on TLC plates by enzyme inhibition
methods. For example, Schutzmann and Barthel (1969) reported detection
limits of 0.5 µg and 0.05 µg for phosmet and its oxygen analogue
respectively, using horse serum cholinesterase with indoxyl acetate as
substrate. Schutzmann (1970) reported that the sensitivity of the
method could be improved by using either the acetate or butyrate of
N-methyl indoxol as substrate. Ackermann and Engst (1970) used beef
liver esterase with 1-napthyl acetate and Fast Blue B on silica gel G
plates to detect organophosphorus residues in rat foetal tissues. They
were able to detect 0.025 mg/kg of phosmet and 0.005 mg/kg of its
0-analogue by this method.
Gas chromatographic methods
Some early gas chromatographic determinations of phosmet residues
made use of the electron capture detector (ECD). For example, Bowman
and Beroza (1965) were able to determine phosmet (but not the
0-analogue) in milk and sweet corn by using the ECD, and Gutenmann et
al. (1965) applied it to the determination of phosmet (detection limit
0.1 mg/kg) and the O-analogue (0.2 mg/kg) in apples, potatoes, grapes
and alfalfa following a TLC clean-up.
Later methods have made use of either an alkali flame ionisation
detector (AFID) giving selective detection of phosphorus compounds, or
a flame photometric detector (FPD) operated in either the phosphorus
or the sulphur mode. Bowman and Beroza (1966) used the FPD with a
526 nm phosphorus filter to determine phosmet (detection limit 0.002
mg/kg) and the 0-analogue (0.004 mg/kg) in sweet corn, following
clean-up and separation on a silica gel column. The glc column packing
was 10% DC 200 on 80/100 mesh Gas Chrom Q. The column was operated at
200°C, and required conditioning with repeated injections of phosmet
and corn extract to obtain a constant response to the insecticide. The
same column packing was used by Watts and Storherr (1967) in
conjunction with an AFID for the determination of phosmet and other
organophosphorus insecticide residues in milk after a sweep
codistillation clean-up. They too found the need for thorough column
conditioning. Storherr et al. (1967) used essentially the same
procedure to determine organophosphorus pesticides including phosmet
in fortified samples of edible oils. The average recovery for phosmet
was 96%, and the method detected apparent levels in unfortified
samples ranging from less than 0.01 to 0.04 mg/kg.
Bowman and Beroza (1967) were able to determine phosmet extracted
from milk (in hexane/ether) without clean-up by using the FPD with a
394 nm sulphur filter. Bowman et al. (1968) investigated several
alternative extraction procedures for removing OP residues (including
phosmet and its O-analogue) from field treated crops, and found the
most efficient method to be soxhlet extraction for 4 hours with 10%
methanol in chloroform. Determination was by gas chromatography with
flame photometric detection using a phosphorus filter.
A gas chromatographic method employing the AFID (with rubidium
sulphate as alkali source) and a short, narrow bore column containing
10% DC 200 on 100/120 mesh Gas Chrom Q has been described by Barney et
al. (1972). For most crops, clean-up is achieved simply by shaking
with charcoal followed by filtration through a Millipore filter.
Cottonseed and cotton foliage require prior acetonitrile partition.
The lower limit of detection is given as 0.05 mg/kg and the method has
been successfully applied to a range of fruit, vegetable and fodder
crops. The authors state that the FPD in either the P or S mode may be
used instead of the AFID, but stress the importance of the small
particle size of the solid support to ensure adequate resolution of
the oxygen analogue from phosmet.
Several multi-residue gas chromatographic procedures which
include phosmet have been published recently. Some examples are:
(a) The method of Ripley et al. (1974) for OP residues in natural
waters (using the FPD).
(b) The method of Luke et al. (1975) for OP and nitrogen containing
pesticides in produce (using the AFID with a potassium chloride
source).
(c) The method of Krijgsman and Van de Kamp (1976) which utilises a
capillary column of SE-30 with a flame photometric detector.
NATIONAL TOLERANCES REPORTED TO THE MEETING
The national tolerances shown in Table 4 have been reported to
the Meeting.
TABLE 4. National tolerances reported to the Meeting
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Australia Fat of meat of cattle 1 Snelson
Milk and milk products 1976
(fat basis) 0.2 "
Pome and stone fruit 1 "
Canada Apples, peaches and
pears 10
Plums 5
Netherlands Apples, pears 21 1
Potatoes 28 0.02
New Zealand Fruit 14 10
(Kiwifruit 10)
USA Alfalfa 40 Anon, 1969
Apples, peaches & pears 10 "
Meat & fat of cattle,
goats, hogs & sheep 0.2 Anon, 1969
Grapes 10 Anon, 1971a
Cherries 10 Anon, 1971b
Apricots, nectarines
and plums 5 " "
TABLE 4. (Cont'd.)
Pre-harvest
interval, Tolerance,
Country Crop days mg/kg Reference
Potatoes 0.1 Anon, 1971c
Sweet potatoes (from
post-harvest application 10 Anon, 1973
Almond hulls, blue berries,
corn forage and fodder
(including sweet corn,
field corn and popcorn),
cranberries, pea forage
and hay 10 Anon, 1975
Fresh corn, including sweet
corn (kernel and cob with
husk removed), corn grain
(including popcorn), and
peas 0.5 Anon, 1975
Meat, fat and meat by-products
of horses 0.2 Anon, 1975
Nuts 0.1 Anon, 1975
USSR Vegetables 0.25 Gorelik et
al, 1973b
Figs 0.25 Popov, 1973
APPRAISAL
As a systemic insecticide and acaricide against a range of
ectoparasites of animals, phosmet may be applied as a dilute aqueous
spray containing up to 0.5% a.i., as a ready-to-use "pour on"
formulation containing up to 4% a.i. and applied at up to 40 mg per kg
body weight, as a dust containing 1 to 2% a.i., or it may be
administered orally.
In plants, phosmet is used as a non-systemic pesticide and is
applied to a variety of crops to protect against insects and mites. At
the concentrations usually applied it can be effective without harming
some useful predators and this makes it of value for integrated
control programmes. On fruit, a 0.1% spray has been used on pasture
and forage crops up to 0.75 kg/ha, and on cereal crops at 35 g/ha (ULV
spray). It has been used at 3-5 kg/ha on cotton.
Data available on residues in meat of cattle is not extensive,
but would be in accord with a maximum level of 0.2 mg/kg. Applied as a
spray to apples and peaches, residues are generally less than 1 mg/kg
after a 3 weeks preharvest period. On forage crops, application of
phosmet at 1 kg a.i./ha produced residues of the order of 50 mg/kg
which declined to less than 5 mg/kg after 14 days. Because phosmet is
not excreted in the milk, cattle can graze recently treated pastures.
Both phosmet and its oxygen analogue are of low persistence in
plants and animals. In mammals phosmet is rapidly metabolised, mainly
to innocuous water-soluble compounds. The metabolites principally
excreted are phthalic acid and phthalamic acid with only minute traces
of phosmet or its oxygen analogue.
Plants treated with phosmet tend to retain it on the surface
where it is washed off or weathered. In plant tissues conversion to
phthalic or phthalamic acid again takes place and these undergo
further breakdown.
Phosmet and its oxygen analogue are rapidly degraded in soils,
degradation being favoured by increasing soil alkalinity and moisture
content. Apart from simple chemical hydrolysis, metabolism by
microorganisms also occurs and it has been stated that phosmet is
totally biodegradable.
Processing in the canning industry by washing with dilute alkali,
bleaching or sterilising will remove at least 50% of the phosmet from
fruit and vegetables.
Several analytical methods suitable for regulatory purposes are
available. These include a colorimetric method which determines the
phthalimide moiety after conversion to anthranilic acid and gas
chromatographic methods with either an alkali flame ionisation or a
flame photometric detector. There is also an enzyme inhibition.
REFERENCES
Ackermann, H., and Engst, R. Occurrence of organophosphorus
1970 insecticides in the foetus. Arch. Toxikol., 26:17.
Adeishvili, L.G. Pesticide residues in apple fruits. Tr.
1973 Nauchno-Issled. Inst. Zashch. Rast (Toflis),
25:3-5.
Ali Niazee, M.T., and Stafford, E.M. Management of grape pests in
1973 Central California vineyards. I. Cultural and
chemical control of Patynota stultana on
grapes. J. Econ. Entomol., 66 (1): 154-7
Andrichuk, B.V. Phthalophos as an acaricide for the control of
1971a sheep psoroptosis. Tr. Yses. Nauch-Issled. Inst.
Vet. Sanit., 38: 264-70
Andrichuk, B.V. Acaricidal dusts for the control of sheep
1971b psoroptosis. Tr. Vses. Nauch-Issled. Inst.Vet.
Sanit., 39: 363-8
Anon. (Environmental Protection Agency, Washington, D.C.)
1969 N-(mercaptomethyl) phthalimide S-(O,O
-dimethyl phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
34 (63): 6041.
1971a Ibid., 36(59); 5690.
1971b Ibid., 36(132): 12898-9
1971c Ibid., 36 (152): 14471-2
Anon. (Food Drug Administration, Washington, D.C.) New animal
1971d drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide S-(O,O-dimethyl
phosphorodithioate). Federal Register, 36(231):
22829.
Anon. (Environmental Protection Agency, Washington, D.C.)
1973 N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate) and
residues. Federal Register, 38(55): 7459-60.
Anon. (Food Drug Administration, Washington, D.C.) New animal
1974 drugs for ophthalmic and topical use.
N-(mercaptomethyl) phthalimide
S-(O,O-dimethylphosphorodithioate). Federal
Register, 39(172): 32025-6.
Anon. (Environmental Protection Agency, Washington, D.C.)
1975 N-(mercaptomethyl) phthalimide S.(O,O-
dimethyl-phosphorodithioate) and its oxygen
analog; tolerances for residues. Federal Register,
40(48): 11352-3.
Barney, J.E., Ja, W.Y., and Shelman, D.L. Analytical method:
1972 for pesticides, plant growth regulators and food
additives. (Academic Press, Ed. G. Zweig and J.
Sherma), Vol. VI, pp. 408.134
Batchelder, G.H., and Patchett, G.G. American Chemical Society,
1965 149th Meeting, Division of Agriculture and Food
Chemistry, Paper No. 37.
Batchelder, G.H., Patchett, G.G., and Menn, J.J. Analytical
1967 methods for pesticides, plant growth regulators
and food additives (Academic Press, Ed. G. Zweig),
Vol. V, pp. 257-275.
Bidleman, T.F., Nowlan, B., and Frei, R.W. Metallofluorescent
1972 indicators as spray reagents for the in situ
determination of organophosphorus pesticides in
thin layer chromatograms. Anal. Chim. Acta, 60(1):
13-23.
Boiko, I.B., and Popova, N.G. Effect of some organophosphorus
1970 pesticides on the biological value of vegetable
crops. Med. Zh. Uzb., 9: 35-7.
Bowman, M.C., and Beroza, M. Analysis of Imidan colorimetrically
1965 and by electron-affinity gas chromatography. J.
Assoc. Offic. Anal. Chem., 48(5): 922-6.
Bowman, M.C. and Beroza, M. Determination of Imidan and Imidoxon
1966 in sweet corn by gas chromatography with flame
photometric detection. J. Assoc. Offic. Anal.
Chem., 49(6): 1154-1157.
Bowman, M.C. and Beroza, M. Note on extraction of a polar insecticide
1967 (Imidan) from milk. J. Assoc. Offic. Anal. Chem.,
50(4): 940-941.
Bowman, M.C., Beroza, M., and Leuck, D.B. Procedures for extracting
1968 residues of phosphorus insecticides and
metabolites from field treated crops. J. Agr. Food
Chem., 16(5): 796-80.
Butt, B.A., White, L.D., Moffit, H.R., Hathaway, D.O., and
1973 Schoenleber, L.G. Integration of sanitation,
insecticides, and sterile moth releases for
suppression of populations of codling moths in the
Wenas Valley of Washington. Environ. Entomol.,
2(2): 208-13.
Chamberlain, W.F. A study of the dermal treatment of a 1965 steer
with C14-labelled Imidan. J. Econ. Entomol.,
58(1):51-55.
Chirikashvili, R.V., Abbasov, T.G., and Frolov, B.A. Duration
1970 of persistence of phthalophos in organs and
tissues of hens during oral administration. Tr.
Vses. Nauch-Issled. Inst. Vet. Sanit., 37: 175-8.
Del Rivero, J.M., Lafuente, M., and Lazaro, E. New products
1971 for control of the potato beetle, Leptinotarsa
decemlineata, resistant to chlorinated
insecticides. An. Inst. Nac. Invest. Agr. (Spain),
Ser. Prot. Veg. (1): 183-7.
Dorough, H.W., Randolph, N.M., and Wimbish, G.H. Imidan, and
1965 trichlorfon residues on Coastal bermudagrass.
Texas A&M University/Texas Agricultural Experiment
Station. Progress Report PR-2385.
Drumond, R.O., Ernst, S.E., Trevino, J.L., Gladney, W.J., and
1972 Graham, O.H. Boophilus annulatus and B.
microplus. Sprays and dips of insecticides for
control on cattle. J. Econ. Entomol., 65(5):
1354-7.
Forsythe, H.Y., and Hall, F.R. Control of the plum Curculio in
1972 Ohio. J. Econ. Entomol., 65(6): 1703-6.
Frei, R.W., and Mallet, V. Quantitative thin-layer chromatography
1971 of organothiophosphorus pesticides by in situ
fluorimetry. Intern. J. Environ. Anal. Chem., 1:
99.
Frolov, B.A. Activity of some organo-phosphorus and carbamate
1970 compounds for fowl ectoparasites. (Argas
persicus and Cimex lectularius) Tr., Vses.
Nauch-Issled. Inst. Vet. Sanit., 37: 334-43
Gorelik, L.D., Kushchinskaya, In.N., and Nemets, S.M. Effect
1973a of technological conditions on the content of
phthalophos in canned food. Konserv. Ovoshchesush.
Prom. 4: 8-10.
Gorelik, L.D., Kushchinskaya, I.N., and Sazonova, A.R. Lowering
1973b the phthalophos level during the preparation of
canned apple sauce. Konserv. Ovoshchesush. Prom.
7: 21-3.
Gribkova, N.I. Effectiveness of insecticides against leaf-miner
1973 moths. Khim. Sel. Khoz., 11(8):592-4.
Gutenmann, W.H., Bache, C.A., and Lisk, D.J. Determination
1965 of Imidan and Imidoxon in crops by preparative
thin layer and by gas chromatography. J. Gas
Chromat., 3: 350-52.
Holdsworth, R.P. Realities of integrated insect control.
1973 Proc. Ohio State Hort. Soc., 126: 89-92.
Ionova, I., Zhecheva, G., and Dimitrov, K. Residual amounts
1974 of Sevin, Neguvon, and Imidan in the meat of pigs
treated against ectoparasites. Vet-Med Nauki,
11(5): 28-34.
Johnson, J.C., and Bowman, M.C. Fate of Bidrin and Imidan
1968 when fed in silage to lactating dairy cows. J.
Dairy Science, 51(8): 1225-8.
King, N.L., and Best, D.R. Unpublished report from Hawthorn Park
1971 Research Laboratories Pty-Ltd. to Stauffer Chem.
Co. (Australia) Pty. Ltd.
King, N.L., and Ferguson, A.B. Unpublished report from Hawthorn
1971 Park Research laboratories Pty. Ltd. to Stauffer
Chem. Co. (Australia) Pty. Ltd.
Klisenko, M.A., and Pis'mennaya, M.V. Effects of UV radiation
1973 on dialkyldithiophosphate pesticides. Khim. Sel.
Khoz., 11(12): 916-8.
Klunker, R. Current status of potato beetle control, especially
1974 with respect to the resistance problem.
Nachrichtenbl. Pflanzenschutz DDR 28(9). 191-6.
Kovac, J., Batora, V., Moracvik, J., and Benusova, M. Persistence
1968 of O,O-dimethyl-S-(phthalimidomethyl)
dithiophosphate residues in vegetables and fruits.
Agrochimia (Bratislava), 8(3): 77-81.
Krijgsman, W., and Van de Kamp. C.G. Analysis of organophosphorus
1976 pesticides by capillary gas chromatography with
flame photometric detection. J. Chromat.,
117(1): 201-5.
Large, G.B., and Pitt, L.S. Thiophosphate synergists. U.S. Patent
1975 3, 886, 273, 27 May 1975, Stauffer Chemical Co.
Leuck, D.B., and Bowman, M.C. Imidan insecticide residues
1968 (Imidan and Imidoxon): their persistence in corn,
grass and soybeans. J. Econ. Entomol., 61(3).
705-7.
Loomis, E.C., Noorderhaven, A., and Roulston, W.J. Control of
1972 Southern cattle tick by pour-on animal systemic
insecticides. J. Econ. Entomol., 65: 1638-41.
Luke, M.A., Froberg, J.E., and Masumoto, H.T. Extraction and
1975 clean-up of organo-chlorine, organo-phosphorus,
organo-nitrogen and hydrocarbon pesticides in
produce for determination by gas-liquid
chromatography. J. Assoc. Offic. Anal. Chem.
58(5): 1020-6.
Mardzhanyan, G.M., Semerdzhyan, O.G., Ust'yan, A.K. and Manukyan,
1973 Z.S. Possibility of controlling Phytonomus with
organo-phosphorus insecticides. Izv. Sel'skokhoz.
Nauk, 16(11-12): 36-41.
McBain, J.B., Menn, J.J., and Casida, J.E. Metabolism of
1968 carbonyl-C14 labelled Imidan,
N-(Mercapto-methyl) phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats
and cockroaches. J. Agr. Food Chem., 16(5):
813-820.
Miller, M.C., Shaw, F.R., and Smith, C.T. The comparative residual
1969 life of two formulations of Imidan on alfalfa. J.
Econ. Entomol., 62(3): 720-1.
Mistric, W.J., and Smith, F.D. Organophosphorus and carbamate
1970 insecticides as substitutes for DDT in controlling
the tobacco flea beetle on flue cured tobacco. J.
Econ. Entomol., 63: 509-11.
Nakotomi, K., and Ishibe, K. (Hokko Chemical Industry Co. Ltd.)
1975 Insecticidal synergism with acephate
phosphorothioate mixtures. Japanese Patent 75 71,
838, 14 June 1975.
Nepoklonov, A.A., Nepoklonova, M.I., and Pavlova, N.V. Acaricidal
1969 properties of phthalophos.Tr., Vses. Nauch-Issled.
Inst. Vet. Sanit., 34: 348-50.
Nesterenko, N.I. Organophosphorus preparations used to control sugar
1970 beet pests. Khim. Sel. Khoz., 8: 382-3.
1973 Ibid., 11(2): 113-6.
New South Wales Department of Agriculture. Extracts from the 1966-7
1969 annual report of the Entomology Branch. PANS, 15
(2): 225.
Nikiforov, A.M., and Mamaev, K.A. Testing of new chemical
1972 preparations (cotton pesticides). Khlopkovodstvo,
8: 31-2.
Novikova, K.F., and Mel'tser, F.R. Chromatophotometric method
1972 for determining residual amounts of phthalophos in
food products of plant origin. Probl. Anal. Khim.,
2: 95-99.
Novikova, K.F., Mel'tser, F.R., Makarova, S.V., and Navmova, V.A.
1969 Spectrophotometric determination of residual
phthalophos in apples. Gig. Primen. Toksikol.
Pestits. Klin. Otravi, 7: 595-601.
Novikova, K.F., and Varlamova, T.A. Spectrophotometric
1973 determination of microgram quantities of
phthalophos and phosalone in natural waters.
Metody Opred. Pestits. Vode, 1: 98-103.
Omarov, L.M. Phthalophos and Sevin dusts to control mites.
1974 Veterinarya. 4: 37-8.
Omelyuta, V.P. Effectiveness of insecticides against Grapholitha
1972 molesta. Khim. Sel. Khoz., 10(7): 516-7.
Otaci, C., Turhan, K., and Barkin, S. Imidan residues on peaches.
1972 Bitki Koruma Bul., 12(1): 45-8.
Pfadt, R.E., Lloyd, J.E., and Spackman, E.W. Power dusting with
1975 organo-phosphorus insecticides to control the
sheep ked. J. Econ. Entomol., 68(4): 468-70.
Popov, P.F. Effectiveness of different preparations against
1973 caterpillars of fig Glyphipterygidae. Khim. Sel.
Khoz., 11(8): 595-6.
Ripley, B.D., Wilkinson, R.J., and Chau, A.S.Y. Multi-residue
1974 analysis of 14 organo-phosphorus pesticides in
natural waters. J. Assoc. Offic. Anal. Chem.,
57(5): 1033-42.
Rogoff, W.M., Brody, G., Roth, A.R., Batchelder, G.H., Meyding,
1967 G.D., Bigley, W.S., Gretz, G.H., and Orchard, R.
Efficacy, cholinesterase inhibition and residue
persistence of Imidan for the control of cattle
grubs. J. Econ. Entomol., 60(3): 640-6.
Ruzimuradov, A., Valiev, B., and Matchanov, S. Test of insecticides
1973 against pasture ground stable flies and gnats.
Mater. Konf. Molodykh. Uch. Uzb. Sel'sk Khoz. 7th
1972: 60-2. (Published in Veterinarya, 1973, ed.
by Sh. A. Azimov et al).
Schutzmann, R.L. Note on improved spray reagents for TLC
1970 fluorogenic detection of cholinesterase
inhibitors. J.Assoc. Offic. Anal. Chem., 53(5):
1056-7.
Schutzmann, R.L., and Barthel, W.F. Indoxyl acetate spray reagent
1969 for fluorogenic detection of cholinesterase
inhibitors in environmental samples. J. Assoc.
Offic. Anal. Chem., 52(1): 151-6.
Semerdzhyan, O.G. Poisoning of rabbits fed Phthalophos treated
1972 alfalfa. Izv. Sel'skokhoz. Nauk. 15 (6): 77-9.
Sevost'yanov, A.Z., and Ushakova, N.N. Acaricidal effectiveness of
1971 some pesticides against Ixodes mites during tests
in samples of sheep wool. Tr. Stavropol
Sel'skokhoz. Inst., No. 34 (Vol 4): 74-6.
Shapovalova, G.K., Sedykh, A.S., and Abelentseva, G.M.
1970 Effectiveness of using phthalophos and
benzophosphate to combat gooseberry moth
(Zophodia convolutella), and insecticide
residues on the berries. Khim. Sel. Khoz. 8(8):
594-5.
Sharova, V.M., and Kozhemyakin, N.D. Photoelectro-colorimetric
1973 determination of metaphos and phthalophos in
tissues of experimentally poisoned birds. Khim.
Sel. Khoz, 11 (3): 229-30.
Shaw, F.R., Callahan, R.A., and Miller, M.C. Rates of disappearance
1966 of Zolone and Imidan from alfalfa. J. Econ.
Entomol., 59(6): 1524-5.
Snelson, J.T. Private communication from Department of Primary
1976 Industry, Australia.
Speirs, R.D., and Lang, J.H. Contact, residue and vapour toxicity
1970 of new insecticides to stored products insects.
II. USDA Agricultural Research Service, Marketing
Research Report No. 885.
Storherr, R.W., Murray, E.J., Klein, I., and Rosenberg, L.A.
1967 Sweep co-distillation clean-up of fortified edible
oils for determination of organo-phosphate and
chlorinated hydrocarbon pesticides. J. Assoc.
Offic. Anal. Chem., 50(3): 605-15.
Surorov, P.S. Acaricidal effect of ciodrin, phthalophos and
1970 chlorophos on cutaneous mites, cattle psoroptosis
causative agents. Tr., Vses. Nauch Issled. Inst.
Vet. Sanit., 35: 379-82.
Tanabe, M., Dehn, R.L., and Bramhall, R.R. The photochemistry
1974 of Imidan in diethyl ether. J. Agr. Food Chem.,
22(1): 54-6.
Thomas, F.J.D., and Taylor, A.S. (ICI of Australia and New Zealand
1971 Ltd.) Apparatus and compositions for controlling
cattle ticks. South African Patent 69 06, 036 22
February 1971.
Uctani, T., Hirano, T., and Tsuchitate, M. (Kumiai Chem. Industry
1974 Co. Ltd.) Acaricidal and insecticidal
compositions. Japanese Patent 74 15, 776, 17 April
1974.
Van Cleave, H.V., and Anderson, A.L., Field control tests for pecan
1971 insects. Proc. Tex. Pecan Grow. Ass.: 32-3
Vernon, J.D.R., and Gould, H.J. Further trials on alternatives
1972 to DDT for the control of pre-blossom pests on
apple and pear. Plant Pathol., 21(1). 1-9.
Vrochinskii, K.K. Accumulation of pesticides in hydrobiocts
1971 as a measure for evaluating experimental water
toxicology. Eksp. Vod. Toksikol. 149-63.
Watts, R.R., and Storherr, R.W. Sweep do-distillation clean
1967 up of milk for determination of organophosphate
and chlorinated hydrocarbon pesticides. 50(3):
581-5.
Wilde, W.H.A. Low volume concentrate spraying and integrated 1973
control in some Ontario orchards. Proc. Ohio State
Hort. Soc. 126: 85-8.
Witkowska, D., and Wojnarowska, P. Effectiveness of some chemical
1971 preparations against Anthonomus pomorum (apple
weevil). Biol. Inst. Ochr. Rosl. (48): 357-66.
Woodard Research Corporation. Unpublished report.
1963
Zatserkovskaya, G.A. Evaporation of insecticides from different
1974 types of soils. Zashch. Rast (Kiev), 20: 61-6.
