
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
FENTIN COMPOUNDS
Explanation
At the 1965 Joint FAO/WHO Meeting on Pesticide Residues, the
toxicology of two fentin compounds, namely fentin hydroxide
(triphenyltin hydroxide) and fentin acetate. (triphenyltin acetate),
was considered. The deliberations are summarized in a monograph
entitled "Triphenyltin Compounds" (FAO/WHO, 1965). Since that meeting,
additional information has become available on these two fentin
compounds, as well as on fentin chloride (triphenyltin chloride).
Because all these fentin compounds appear to react similarly in plants
and in animals, they have been grouped together in this completely
revised monograph
IDENTITY
Chemical names
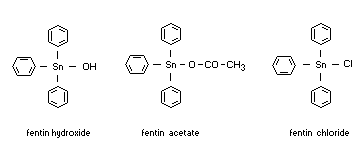
1) triphenyltin hydroxide
2) triphenyltin acetate
3) triphenyltin chloride
Synonyms
1) fentin hydroxide, Du-ter(R), (Trinicide W20)
2) fentin acetate, Brestan(R), Hoe 2824(R)
3) fentin chloride, Brestanol(R), Hoe 2872(R)
Structural formulas
 Other relevant chemical properties
The pure fentin hydroxide is a white odourless crystalline solid, with
a melting point of 118-120°C. Solubility in water at 20°C is 8 ppm,
and it is moderately soluble in most organic solvents. It is stable at
room temperature, but decomposition occurs on heating above 60°C.
The pure fentin acetate is a white, odourless, crystalline solid, with
a melting point of 124-125°C. Its solubility in water at 20°C is 28
ppm, and it is of low solubility in most organic solvents.
Fentin acetate is stable when stored under normal conditions in dry
air. At 90°C, no decomposition can be detected; at 150°C, 15 percent
decomposes within three hours. In the presence of water, the acetate
is almost completely hydrolysed within eight hours at 20°C.
The pure fentin chloride is a white crystalline solid, with melting
point at 106°C. Its solubility in water is 40 ppm. It is stable when
stored in the dark in dry air. In the presence of water it hydrolyses
into the hydroxide.
Purity
The technical products are usually of the following purities:
fentin hydroxide : 95 percent
fentin acetate : 94 percent
fentin chloride : 94 percent
The impurities consist mainly of diphenyltin compounds and
tetraphenyltin.
This report is based on the data obtained with the fentin compounds
manufactured in Germany (Hoechst) and in Holland (Philips-Duphar). It
is known that fentin compounds are also produced in the United States
and Japan, but no data were available from these sources.
Formulations
The usual formulations are:
fentin hydroxide : 20 percent and 50 percent wp
fentin acetate : 20 percent and 60 percent wp
fentin chloride : 40 percent and 45 percent wp
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Experiments on rats given 113Sn-labelled fentin chloride showed that
the absorbed fentin radical was rapidly distributed through the
tissues of the body, including the brain. The fentin radical was
eliminated relatively slowly and could be detected in the brain 38
days after a single dose (Heath, 1963).
Experimental investigations have been made on guinea pigs fed on a
diet containing 12 ppm fentin acetate. From the survival times and the
total amount consumed, it is evident that, at this dosage level,
fentin compounds are not completely cumulative. The results obtained
and those previously published (Stoner, 1966) are consistent with a
halflife of the fentin radical at the site of action of about 50 days
(Stoner and Heath, 1967).
Information on the distribution of tin after feeding fentin compounds
to farm animals is included in the section entitled "FATE OF
RESIDUES. In animals".
Effect on enzymes and other biochemical parameters
In vitro studies have shown that fentin compounds inhibit oxidative
phosphorylation by isolated liver mitochondria and the adenosine
triphosphatase (ATP) activity of brain microcomes (50 percent
inhibition by concentration of, 1 × 10-6M and 1 × 10-5M,
respectively) (W. N. Aldridge, quoted by Stoner, 1966).
The oxygen consumption of cerebral slices from rats in a moribund
condition due to fentin compounds was within normal limits (J.E.
Cremer, quoted by Stoner, 1966).
The effects of fentin hydroxide on the oxidative phosphorylation and
on permeability of rat liver mitochondria membranes for malate,
citrate and fumarate ions were investigated. In the conditions of the
investigations (concentration of 5 µ M), a strong inhibitory action on
the oxidative phosphorylation in isolated rat liver mitochondria and
deep changes in the selectively permeable mitochondrial membrane were
observed. Malate and citrate were able to penetrate mitochondria
without participation of any carrier. The altered membrane formed no
barrier for fumarate. No similar effects were observed in the case of
n-octyltin derivatives (Syrowatka, 1969).
In a further investigation, it was shown that the mitochondrial
membrane which, when intact, has a strictly limited permeability for
K+, Na+, Cl-, malate, citrate and fumarate, became permeable in a
high degree to these ions under the influence of fentin hydroxide.
Electron microscopic pictures confirmed these results (Syrowatka,
1970).
TOXICOLOGICAL STUDIES
Special studies on influence on lymphatic tissue and immune
responses
Guinea pig (fentin acetate)
These investigations were aimed at studying the influence of fentin
acetate on lymphatic tissue and immune responses in guinea pigs which
were previously (see below) shown to be much more sensitive than rats
to the decreasing effect of the compound on the lymphopoiesis. Female
guinea pigs were selected because they are more affected than males.
In a first experiment, two groups of ten female guinea pigs received
15 ppm of fentin acetate in their diet, for 49 or 77 days. Each group
was accompanied by a control group with the same number of animals.
After 77 days, a decrease in plasma cells was observed in the spleen
and the cervical, mesenteric, axillary and popliteal lymph nodes. In a
second experiment, the same dose of the compound was given in the diet
for 104 days. Two groups of ten female guinea pigs received fentin
acetate and two groups control diet only. At 21 and 7 days before the
end of the fentin acetate feeding period, half of the animals received
an injection of tetanus toxoid. Some animals were allowed to recover
for 14 days after the 104-day experimental period. Immunochemically
and serologically, a decreased immunologic response was observed in
the group fed fentin acetate after tetanus toxoid stimulation and no
signs of recovery were seen after 14 days. With the aid of the
fluorescent antibody technique, a decrease in immunologically active
cells in the popliteal lymph nodes of the animals fed fentin acetate
could be detected in the second experiment. In the other lymphatic
tissues, no differences were observed (Verschuuren at al., 1970).
Special studies on reproduction
Rat (fentin acetate)
An interim report comprising two generations of a three-generation
reproduction study in rats fed 0, 25, 80 and 250 ppm of fentin acetate
in the diet is available. No histological examination was performed,
but no abnormalities were found by macroscopic examination at the 25
ppm level (Scholz and Baeder, 1970).
Rat (fentin acetate and chloride)
Groups each of 20 sexually mature male rats (aged 35 to 40 days) were
given orally in their food, over a period of 19 days, 20 mg/kg
body-weight per day of either fentin acetate or fentin chloride. The
animals were killed on the twentieth day, and histological
preparations of the testes were made. Both compounds produced several
degenerative changes of the testicular tissue, including a decrease in
the number of cell layers per seminiferous tubule, a decrease in
tubule diameter and overall testicular size, a depletion of the more
advanced cell forms from the tubules and a closing of tubule lumina.
Effects were more pronounced in animals treated with fentin acetate
(Pate and Hays, 1968).
Groups of 15 sexually mature female rats (aged 42 to 46 days) were
given orally 20 mg/kg body-weight per day of either fentin acetate or
fentin chloride. Three animals from each group were killed after 4, 9,
14, 19 and 24 days of treatment, and histological preparations of the
ovaries were made and thoroughly examined. Both fentin compounds
produced significant changes in the ovarian tissue. Among these
changes were a decreased number of mature follicles, an increased
incidence of atresia in early follicle growth and a pronounced
decrease in the number of corpora lutea present. This last effect was
regarded as a decrease in ovulation and, thus, a decreased fertility.
This effect, as well as the others listed above, was present on or by
the first five-day sample period (Newton and Hays, 1968).
Rat (fentin hydroxide)
This experiment was started with 50 male and 100 female newly weaned
rats. The animals were divided at random over five groups and were fed
diets containing 0, 0.5, 1, 2 or 5 ppm of fentin hydroxide. Fertility
of males and females, number of young born per litter, vitality,
body-weight and mortality of the young at birth and during the
lactation period were not affected by feeding fentin hydroxide at
levels up to 5 ppm in any of the three successive generations.
Ninety-day feeding studies with F1b and F2b generation rats did not
reveal adverse effects with respect to growth, food consumption, food
efficiency and haematology. Increased relative weights of liver,
kidney and spleen were occasionally noted, but were not associated
with histological abnormalities. Testicle weight was slightly
decreased at the 5 ppm level in F1b generation rats and at the 2 and
5 ppm levels in F2b generation rats at week 13 after weaning.
Macroscopically, no changes attributable to the ingestion of fentin
hydroxide were observed in any of the successive generations, except
for small-sized testicles at feeding levels of 1 ppm and above in
generation F3b at week 2 after weaning. Microscopic examination
revealed a significant difference between treated and control rats
only in the maturing testicle. This difference found expression in the
stage of maturation of the germinal epithelium, which was less
advanced in the small-sized testicles of the rats fed fentin hydroxide
(Til et al., 1967).
Four groups of ten weanling male rats were each fed 0, 50, 100 or 200
ppm of fentin hydroxide in the diet over a period of 276 days and were
mated at intervals to nontreated females. Observations indicate that,
in comparison to the controls, there was a drop of about 27 and 18
percent, respectively, in the total number of offspring in the groups
fed 200 and 100 ppm after 64 days of exposure. However, when the males
were subsequently mated, their fertility improved and, by day 113, was
comparable to that of the controls. Leucocyte and differential counts
and haemoglobin determinations performed one week prior to the time of
sacrifice did not differ from the controls. Gross abnormalities
related to fentin hydroxide were not observed in the offspring. In the
group fed 200 ppm, one rat died and two were sacrificed when moribund.
The cause of death in this group was generalized internal haemorrhage.
In the animals that were sacrificed, no gross or microscopic
abnormalities attributable to fentin hydroxide were observed. At the
100 and 200 ppm levels, the food consumption of the diet containing
fentin hydroxide was significantly lower during the first week of the
experiment and then improved for all dosage levels. By week 7, the
animals had adapted to these concentrations of the chemical, and the
food consumption was the same as in the controls. The food intake and
weight gain were not affected in rats fed 50 ppm. Gross and
microscopic examination of rats fed fentin hydroxide at all levels did
not reveal changes in any organ that could be related to the compound
(Gaines and Kimbrough, 1968).
Three experiments on weanling male rats (10 or 20 per group), fed from
0 to 25 ppm of fentin hydroxide in two or four weeks, were conducted
to determine testicular development. Microscopic examination after two
and four weeks failed to reveal any differences between the various
test groups and the control groups (Til et al., 1968).
Acute toxicity
Symptoms of poisoning were sluggishness, unsteadiness, moderate
diarrhoea, anorexia, bloody stain around the nose and eyes and
wheezing (Gaines and Kimbrough, 1968).
In all species, the main action of these organo-tin compounds is
thought to be on the central nervous system, but in contrast to
triethyltin compounds, cerebral oedema did not occur (Cahen et al.,
1970).
Intravenous administration of fentin acetate to cats at a dose of 1
mg/kg body-weight produced an increase in blood pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of 1
to 2 mg/kg at 20 to 60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood pressure was also
found. Death took place after 6 to 14 mg/kg of fentin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Dog (fentin acetate)
Groups of dogs (four of each sex) were fed 0, 1, 5 and 20 ppm of
fentin acetate in the diet for 120 days. A level of 20 ppm resulted in
decreased food consumption, and in half of the animals, also in
decreased body-weight. No macroscopic or microscopic abnormalities
were found (Scholz and Brunk, 1968a).
Groups of six dogs, three from each sex, were fed over a period of two
years, 0, 0.5, 1 or 5 ppm of fentin acetate. Attention was paid to
behaviour, food intake, weight gain, blood picture, urine composition,
gross examination and histological characteristics of the main organs
and tissues. Except for the higher level, where a decrease in weight
gain, which might be related to a reduction of food intake, was noted
in comparison to the controls, no significant abnormalities, even in
blood picture, were observed (Scholz and Brunk, 1968b).
Dog (fentin hydroxide)
Groups of dogs consisting of three males and three females were fed 0,
10, 25 and 37 ppm of fentin hydroxide in the diet for 90 days.
TABLE I
Acute toxicities to various species: summary
Animal Fentin LD50 (mg/kg
Compound Route body-weight) Reference
Mouse (M) acetate oral 81 Ueda et al., 1961
Mouse (M) chloride oral 80 Ueda et al., 1961
Mouse (M) hydroxide oral 245 Philips-Duphar, 1964a
Mouse (F) hydroxide oral 209 Philips-Duphar, 1964a
Mouse (M) acetate ip 7.9 Stoner, 1966
Rat (M) acetate oral 136 Klimmer, 1964
Rat (F) acetate oral 491 Stoner, 1966
Rat (F) chloride oral 135 Scholz, 1963
Rat (M) hydroxide oral 240 Gaines & Kimbrough, 1968
Rat (F) hydroxide oral 360 Gaines & Kimbrough, 1968
Rat (M) acetate ip 8.5 Stoner, 1966
Rat (F) acetate ip 11.9 Stoner, 1966
Guinea pig (M) acetate oral 21 Klimmer, 1964
Guinea pig (M) hydroxide oral 27.1 Philips-Duphar, 1964a
Guinea pig (F) hydroxide oral 31.1 Philips-Duphar, 1964a
Guinea pig (M) acetate ip 3.7 Stoner, 1966
Rabbit (M) acetate oral 40 Klimmer, 1964
Rabbit (M) acetate ip 10 Klimmer, 1964
Lethargy and a darkening of the hair occurred in all groups, depending
in degree on the dosage, and an increased mortality rate occurred in
the two higher groups (Baran et al., 1966a).
Groups of dogs (three of each sex) were fed 0, 10, 25 and 37 ppm of
fentin hydroxide in the diet for 100 days. Apart from darkening of the
hair which occurred in all test groups, the only differences from the
controls were lethargy in the 37 ppm dogs and an increased quantity of
tin in the livers of five of six dogs at 37 ppm and two of six dogs at
25 ppm (Baran et al., 1966b).
Six dogs were fed, over a period of 16 weeks, a diet containing,
during the first eight weeks, 25 ppm and, afterwards, 50 ppm of fentin
hydroxide. Symptoms of toxicity, consisting principally in inhibition
of growth, alterations in the blood picture (decrease of the number of
leucocytes and erythrocytes) and in the histology of the liver, were
observed (Til and Feron, 1965).
Following this preliminary investigation, 34 dogs were fed over a
period of two years a diet containing respectively 0, 0.5, 2.5, 5 and
10 ppm. Growth, food consumption, symptomatology, blood picture, urine
analysis, liver and kidney functional exploration, serum protein
electrophoresis, water content of brain, organ weights and gross and
microscopic pathology were used as criteria to disclose signs of
injury. General appearance, behaviour, growth, food consumption,
haematological and biochemical values, urine analyses and liver and
kidney functional tests were not affected at any dietary level of the
compound. At the two highest feeding levels of 5 and 10 ppm, the
relative weights of liver and kidney and the water content of the
brain were increased in comparison to the controls, without being
associated with histological changes. Gross and microscopic
examination of organs and tissues did not reveal any abnormality which
could be attributed to the ingestion of fentin hydroxide (Til and
Feron, 1968).
Guinea pig (fentin acetate)
In one experiment, 50 ppm of fentin acetate in the diet of guinea pigs
led to the death of all of nine animals in 17 to 31 days. In other
experiments, only a group of five guinea pigs given 1 ppm for 392 days
did not show an increased mortality rate. Even at 1 ppm, food intake
was reduced. When death occurred, it was preceded by loss of weight
and generalized weakness (Stoner, 1966). Neither in these experiments,
nor in others (acute and short-term), in which guinea pigs and rats
have been treated with fentin compounds, has a significant increase in
the water content of the central nervous system been found nor the
characteristic histological lesion in the white matter produced by
triethylin compounds (Magee et al., 1957) been seen (Stoner, 1966).
In other experiments, groups of guinea pigs, 10 of each sex, were
given respectively 0, 5, 10, 20 or 50 ppm of fentin acetate for 12
weeks. In all groups, except for the males given 5 ppm, growth
inhibition was obvious.
At the end of the treatment, no animal at 50 ppm was alive; in the
other groups, the survival rates were: 20 ppm, 8 out of 10 males and 8
out of 10 females; 10 ppm, 9 out of 10 males and 10 out of 10 females;
5 ppm, 9 out of 10 males and 10 out of 10 females. In the females on
5, 10 and 20 ppm and males on 10 and 20 ppm, the number of lymphocytes
and leucocytes was significantly decreased. The leuco- and lymphopenia
was accompanied in a number of animals by histological changes in the
lymphopoietic system, e.g., atrophy of the white pulp of the spleen
was found. This change possibly induced a decrease of general
resistance, so that a mycotic infection developed in the animals. At
20 and 50 ppm, a significant increase of brain water was noticed.
Histological findings were not significantly different from those
observed in the controls (Verschuuren et al., 1966).
Groups of guinea pigs, ten of each sex, were fed 0, 1, 5, 10, 50 and
100 ppm of fentin acetate for four months. All animals on 100 ppm and
30 percent of those on 50 ppm died, and in the same groups were found
signs of disturbance of the iron metabolism. A level of 10 ppm gave a
slight reduction in weight gain, as well as some cases of decreased
haemoglobin percentage and total number of erythrocyte. The groups fed
5 ppm and 1 ppm were similar to the controls (Scholz and Weigand,
1968).
Guinea pig (fentin hydroxide)
Groups of three males and three females were given 0, 1, 2.5, 5 or 20
ppm of fentin hydroxide for three weeks. No changes in the growth
rate, the water content, or the histology of the nervous system were
observed (Verschuuren at al., 1965).
Groups of ten males and ten females were fed 0, 2.5, 5, 10, 20 or 50
ppm of fentin hydroxide in their diet for 12 weeks. Growth depression
was observed at the two highest dose levels. All animals in the 50 ppm
group died during the first half, and one each in the ten and 20 ppm
groups died later in the experiment. A decrease in the haemoglobin
content was found in all groups, except for the males in the 2.5 and 5
ppm groups there was no change in the number of erythocytes. A
decrease in the number of leucocytes (except in the males on 10 ppm)
and lymphocytes (except the males on 2.5 and 5 ppm) was found in all
the groups examined. The same changes as with fentin acetate were
found in the lymphopoietic system (see above). A decrease in relative
organ weight was found in the spleen of the females on 10 and 20 ppm
and in the thymus of both sexes and uterus and testes at 20 ppm. No
increase in the water content of the central nervous system was
observed, except in the females at 50 ppm. Apart from the changes in
the lymphopoietic system, the histological findings were not
significantly different from those observed in the controls
(Verschuuren et al., 1966).
See also Special studies on influence on lymphatic tissue and immune
responses.
Rat (fentin acetate)
Groups of 20 to 25 rats were given fentin acetate by stomach tube at
doses equivalent to 5, 10, 25 or 50 ppm for 105 to 170 days. At 50
ppm, 70 percent of the rats died within 49 days. Nervous symptoms, as
well as blood, urinary or histopathological changes, were not observed
at the lower dose levels (Klimmer, 1964).
In other experiments on rats, no deaths occurred during a ten-week
period on 200 ppm of fentin acetate. These rats were then put on a
diet containing 300 ppm, and five out of six rats died after a further
117 to 168 days (Stoner, 1966).
Groups of young rats, ten of each sex, were given respectively 0, 5,
10, 25 or 50 ppm of fentin acetate for 12 weeks. Decrease of food
intake and growth inhibition were recorded at 50 ppm, as well as
growth inhibition in males at 25 ppm. At 10 ppm and above, the number
of leucocytes in the blood was decreased in the male (leucopenia) and,
at 50 ppm, the haemoglobin was reduced. At the highest level there was
a decrease of the organ to heart weight ratio for the pituitary and
pancreas in all animals and uterus and ovary in the females. The same
ratio for the thyroid was decreased in all the females, as well as in
the males at 25 and 50 ppm. Paralysis and pronation of the hind legs,
associated with interstitial oedema of the central nervous system,
which developed in rats treated with triethyltin hydroxide at all
levels, were not observed. The water content of the spinal cord was
slightly increased at 50 ppm. Histological findings were not
significantly different from those observed in controls (Verschuuren
at al., 1966).
Groups of 20 young rats, ten of both sexes, were fed respectively, 0,
15, 40, 100, 250 or 600 ppm of fentin acetate over a period of four
months. Attention was given to behaviour, growth, blood picture, urine
composition, gross examination and histology of the main organs and
tissues. Up to 100 ppm, no abnormalities were observed in comparison
to the controls. Above 100 ppm, a dose-related inhibition of growth
was seen and 600 ppm resulted in the death of 30 percent of the
treated animals. (Scholz and Baeder, 1968).
Rat (fentin chloride)
Groups of ten rats of both sexes were given orally, over a period of
41 days, 28 doses of 0, 6.25, 12.5, 25 or 50 mg/kg body-weight. Apart
from a slight decrease in weight gain of the females at 12.5 mg/kg, no
abnormalities (growth, behaviour, blood picture and urine composition,
gross and histological characteristics of stomach, small intestine,
spleen, liver, kidney and lung) were observed at 6.25 and 12.5 mg/kg.
At higher doses, a dose-related increase in mortality compared to the
controls was observed (Scholz, 1963).
Rat (fentin hydroxide)
Groups of 10 or 20 rats of both sexes were given 0, 5, 20, 50 or 100
ppm of fentin hydroxide for 20 days. Food intake and body growth were
depressed at 20 ppm and above. Death rates were 9 out of 10 at 100
ppm; 9 out of 20 at 50 ppm, and 1 out of 20 at both 20 and 5 ppm (Van
Esch and Arnoldussen, 1962).
Groups of rats, ten of each sex, were fed 0, 1, 3.1, 5, 10 and 31 ppm
of fentin hydroxide in the diet for 90 days. Disregarding effects on
erythrocyte and leucocyte counts as neither dose related nor
chronologic, a no-effect level of 31 ppm was claimed (Wolf et al.,
1966).
Groups of ten rats of each sex were given respectively 0, 5, 10 or 25
ppm of fentin hydroxide in the diet for 12 weeks. The food intake was
comparable to the controls. The females showed growth inhibition after
six weeks, but they recovered in spite of continuing treatment. Growth
in the males was comparable to the controls. In the females, blood
leucocytes were decreased. No significant changes in water content
were found in the nervous tissues. At 25 ppm, decrease of the thyroid
weight was noticed (Verschuuren at al., 1962). In a similar
experiment, in which ten rats of each sex were given 50 ppm of the
same compound, the following features were noticed: decreased food
intake, growth inhibition in both sexes, decrease in weight of the
thyroid, pituitary, uterus, ovary, prostate and pancreas, as well as
decrease of haemoglobin and leucocytes. Paralysis and pronation of the
hindlegs, associated with interstitial oedema of the central nervous
system, which developed in rats treated with triethyltin hydroxide at
all levels, were not observed. The water content increased in the
spinal cord, but not in the brain. (In comparison, the water content
of the spinal cord and brain was increased in animals treated with
levels of 5 and 10 ppm of triethyltinhydroxide). Histological findings
in the animals treated with fentin hydroxide were not significantly
different from those observed in the controls (Verschuuren et al.,
1966).
Groups of four to five week old male rats were fed fentin hydroxide in
the diet at levels of 0, 100, 200 or 400 ppm over a period of 99 days.
The rats fed 400 ppm were apparently repelled, since they ate only
about one third as much food as the controls during the first week.
All the animals in this group died within 7 to 34 days from starvation
and extensive intra-alveolar haemorrhage in the lung. There was
partial or complete testicular atrophy and general size and weight
reduction of the organs. The rats fed 200 ppm gained less weight (231
g) than the controls (302 g). At the end of the study, the total
leucocyte number and the weights of spleen and kidneys were
significantly below those of the controls.
The rats fed 100 ppm appeared normal throughout the study except for a
significant decrease of food intake during the first week of treatment
(Gaines and Kimbrough, 1968).
Additional information on the findings following short-term
administration of fentin compounds to rats is given under Special
studies on reproduction.
Long-term studies
Guinea pig (fentin acetate)
Groups of 20 young guinea pigs, ten from each sex, were fed
respectively 0, 1, 5, 10, 50, 100 and 200 ppm of fentin acetate over a
period of two years. Attention was given to behaviour, food intake,
weight gain, blood picture, urine composition, gross examination and
histological characteristics of the main organs and tissues. At 50 ppm
and above, a dose-related increase in mortality in comparison to the
controls and an inhibition of growth were observed. At 10 ppm and
above, fatty degenerative changes were seen in liver and heart muscle.
At 1 and 5 ppm, no abnormalities, even in the blood picture, were
noted in comparison to the controls (Weigand and Kief, 1965).
Rat (fentin hydroxide)
Groups of 50 newly weaned rats, 25 from each sex, were fed over a
period of two years respectively 0, 0.5, 1, 2, 5 or 10 ppm of fentin
hydroxide. General appearance and behaviour, growth, food intake,
blood sugar and blood urea nigrogen, urine composition and SG-PT,
SG-OT and SAP activity were not adversely affected at any dietary
level of the test substance. Mortality of the females at 10 ppm was
higher than in the controls, although at the termination of the
experiment the difference was not significant. Haematological data
often showed a slight decrease in white blood cells at the highest
feeding level in the first year of the experiment only. This effect
was less often soon at 5 ppm and only once at 2 ppm in the males. The
relative thyroid weight was slightly decreased at 10 ppm in the
females only. Average relative weights of the other organs were very
uniform in all groups. Gross autopsy findings did not show evidence of
significant pathological changes. Microscopic examinations of numerous
organs and tissues, including testes, ovaries and bone marrow, did not
reveal histological alterations significantly different from those
observed in controls (Til et al., 1970).
COMMENTS
The basis for grouping the three fentin compounds for consideration
together is that fentin acetate and fentin chloride are converted in
plants and animals to the same compound, namely fentin hydroxide.
Since the previous toxicological evaluation of the fentin compounds in
1965, a considerable number of short-term and long-term feeding
studies in several species have been reported. The production of
cerebral oedema in rats and guinea pigs did not appear to be
pronounced except at a 50 ppm dose level. However, the reduction of
lymphopoiesis in both species appeared to be of some concern. With
respect to this parameter, the guinea pig appeared to be the most
sensitive species studied. A reduction in lymphocyte count was evident
in females at a dietary level of 2.5 ppm of fentin hydroxide. It was
reported that this effect was not entirely reversible after 14 days'
withdrawal from a test diet of 15 ppm of fentin acetate. In a two-year
feeding study in rats a no-effect level of 2 ppm relative to decrease
in white blood cells was evident.
In a multigeneration rat reproduction study, reduction in testicular
size was evident at 5 ppm in the F1b generation, at 2 ppm and above
in the F2b generation and at 1 ppm in the F3b generation. In a
second species, the dog, histological examination revealed no effect
on any organ at feeding levels of up to 10 ppm for two years.
There are no reports of observations of men exposed to this compound.
Bearing the abovementioned observations in mind, the Committee
considered the data adequate to establish an acceptable daily intake
for the fentin compounds.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.1 mg/kg body-weight
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 to 0.0005 mg/kg body-weight, applicable to fentin hydroxide,
fentin acetate and fentin chloride and to the sum of each if more
than one is involved
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
All the three fentin compounds are nonsystemic fungicides used in many
European countries, the United States, and several South American,
African and Asiatic countries. On rice they are used against algae.
Pre-harvest treatments
Fentin compounds are recommended to control the following pests:
Potatoes: Phytophthora infestans de Bary
of the foliage and especially
the tubers, and
Alternaria solani J. u.Gr.
Sugar beet: Cercospora beticola Sacc.
Celery and celeriac: Septoria apii Chester
Carrots: Alternaria porri f. dauci Neerg.
Pecans: Fusicladium effusum, Gnomonia spp.,
Mycosphaerella caryigena Demaree & Cole
Rice: various algae
Groundnuts: Cercospora spp.
Coffee: Colletotrichum coffeanum Noack
Cocoa: Phytophthora palmivora Butl.
The usual rates of application are between 0.15 and 0.40 kg a.i/ha in
600 to 1 000 litres of water. On coffee, the rate is 0.2 to 0.6 kg
a.i/ha in more than 1 000 litres of water. For algae control on rice,
the dosage is up to 1 kg a.i/ha in about 1 000 litres of water.
Recommended rates of application and intervals applied between last
treatment and harvest are as follows:
Potatoes: 0.18 to 0.4 kg a.i./ha. 1-10 times
(generally between 2 and 5 times).
Safety period (in Germany) 7 days.
Sugar beets: 0.18 to 0.4 kg a.i./ha. 1-3 treatments.
Celery celeriac, 0.2 to 0.32 kg a.i./ha in 600-1 000 1
carrot: water, 1-7 treatments. (In Germany
safety period for celery of 3 weeks)
Pecans: 0.22 to 0.54 kg a.i./ha, 2 to 8
treatments.
Rice: 0.75 to 1 kg a.i./ha for algae control.
Peanuts, coffee, 0.2 to 0.6 kg a.i./ha.
cocoa:
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Fentin compounds are used on ornamentals, willow and poplar. Trials on
hops are in process.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials are given in Table II.
TABLE II
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Potatoes Holland 0.20-0.46 4-7 2-98 tubers 0.01 23 17 0.04
" " 0.08-0.38 6-7 98-110 " 0.06 14 12 0.11
" England 0.22-0.56 5 21-43 " 0.01 5 5
" " 0.28 1 30-35 " 0.03 6 6
" Canada 0.15-0.25 ?-12 ?-169 " 0.03 7 7
" U.S.A. 0.17-0.34 5-11 13-15 " 0.03 17 17
" Belgium 0.30-0.36 5 22 " 0.01 1 1
Sugar beets Holland 0.30 5 43-57 beets 0.03 4 0.18
" " 0.30 5 43-57 leaves 0.06 4 1.36
" Germany 0.30 1-4 46-64 beets 0.03 5 2 0.18
" " 0.30 1-4 46-64 leaves 0.06 5 2 0.81
" Italy 0.30-0.40 ? 31 beets 0.03 10 1 0.20
" Switzerl. 0.30-0.40 2 44 leaves 0.02 4 2.45
" U.S.A. 0.22-0.34 3-6 14-45 beets 0.03 17 8 0.09
Carrots Israel 0.25-0.30 5-7 12-28 carrot 0.05 4 3 0.07
Pecans U.S.A. 0.22-0.54 2-8 46-256 nuts 0.03 17 17
Rice Italy 04-1.2 ppm 1 90 hulled rice 0.08 5 5
in irrigation
water
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Ground nuts U.S.A. 0.22-0.34 3-7 9-72 nuts 0.01 14 10 0.03
" U.S.A. 0.22-0.34 3-7 9-72 hulls 0.01 12 1 0.38
" U.S.A. 0.34 3-7 9 vines 0.01 3 2.5
" Israel 0.30 4 5-23 nuts 0.01 4 4
" " 0.30 4 5-23 vines 0.16 4 2.6
Coffee Kenya 0.22-0.60 ? 35 roasted 0.03 3 3
beans
Residues of fentin acetate
Potatoes Germany 0.216-0.324 1-5 7-21 tubers 0.03-0.04 12 8 0.07
" Ireland 0.3 3 not stated " 0.03 2 2
Sugar beets Germany 0.216-0.324 6 38 beets 0.1 6 6
Celeriac " 0.3 1-2 21-45 tubers not stated 3 3
" " 0.324 1 21-35 " 0.1 3 3
" Belgium not stated 3 21-28 stems not stated 6 6
" Germany 0.3 1-2 21-45 leaves not stated 13 2 0.19
" " 0.324 1 21-35 " 0.1 3 3
" Holland 0.24-0.30 1-6 21-28 " 0.1 2 1.0
Celeriac Holland 0.27 6 21-28 leaves 0.01 2 0.30
" Belgium not stated 3 21-28 " not stated 6 5 0.05
Carrots Germany 0.216-0.324 1-3 7-28 1/ 0.03 20 17 0.15
" " 0.216-0.324 1-3 7-28 2/ 0.02 6 5 0.06
" " 0.216-0.324 1-3 7-28 3/ 0.02 6 5 0.02
" " 0.216-0.324 1-3 7-28 4/ 0.02 6 5 0.02
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin acetate
Rice Italy 04-1.2ppm 1 appr.go hulled 0.08 3 3
in irrigation rice
water
Cocoa Nigeria not stated not stated not stated not stated 0.1 8 7 0.1
Residues of fentin chloride
Potatoes Germany 0.22-0.27 1-3 7-14 tubers 0.04 8 6 0.05
Sugar beets " 0.18-0.27 6 38 beets 0.10 4 4
Celeriac " 0.27 1 21-35 tubers 0.1 3 2 0.1
Carrots " 0.20 3 10-31 washed 0.02 4 2 0.08
carrots
1/ washed unpeeled carrots
2/ peeled/scraped carrots
3/ peels, calculated on total weight carrot
4/ scrapings, calculated on total weight carrot
The residue date of fentin hydroxide are from treatments made in the
Netherlands, Germany, Belgium, Canada, England, Italy, Israel,
Switzerland, U.S.A. and Kenya (Philips Duphar, 1963a, 1964b, 1965,
1966a-d, 1968a,b; Thompson-Hayward, 1965a,b, 1967, 1969).
The residue data of fentin acetate are from experiments made in
Germany, the Netherlands, Belgium, Ireland, Italy, Kenya and Nigeria
(Hoechst, 1963, 1964, 1965a, 1966a-d, 1967, 1968a-f,m,o,q, 1969c,d;
Hardon et al., 1962; Gembloux, 1968; Food Inspection, 1965a-b;
Kröller, 1960; Nat. Inst. Publ. Health, 1960; Philips Duphar, 1967).
The residue date of fentin chloride are from experiments made in
Germany (Hoechst, 1966e-h, 1968g-l,n,p, 1969a,b).
FATE OF RESIDUES
General comments
When triphenyltin acetate was decomposed in solution, the following
products were detected: phenol, acetone, hydrogen peroxide, degraded
organotin and benzene. There is probably also some organic or
organotin peroxide present (Hedges, 1961).
In the presence of light and humidity, fentin acetate and fentin
chloride are transformed easily into fentin hydroxide, which
metabolizes into dephenyltin and monophenyltin. Monophenyltin breaks
down very quickly into Sn salts (Kröller, 1960; Hoechst, 1961; Hedges,
1961).
At heating, triphenyltin hydroxide readily decomposes (see other
relevant chemical properties).
In animals
After ingestion of fentin compounds by cows or sheep, the compound is
largely excreted with the faeces (Herok and Götte, 1961; Brügemann et
al., 1964). Three sheep given 10 mg daily of 113Sn-labelled fentin
acetate for 25 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration: 0.0017 ppm) during the treatment, but 17 days after
withdrawal of the compound, its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than fentin acetate. During the treatment, the concentration of
113Sn in the blood and in the urine were 2.9µg/1 and 7.5µg/1,
respectively. In the sheep killed at 28 days, liver, kidney, lung,
pancreas, gall bladder and brain contained a greater amount of 113Sn
than other organs. After 218 days, the amount of 113Sn found in the
liver was still greater than in other organs (Herok and Götte, 1961).
Organs of one sheep, sacrificed at the end of the feeding period with
the 113Sn triphenyltin acetate, showed the following residues
(comprising triphenyltin and all degradation products including
inorganic tin):
Organ ppm 113Sn
liver 0.9
intestinal fat 0.01
depot fat 0.01
muscles 0.02
In plants
Studies on the effect of tin compounds on plants have been made or are
in progress in several laboratories. It has been shown that in the
presence of light and air, several byproducts of fentin acetate can be
formed, such as diphenyltin and finally insoluble tin (Kröller, 1960).
The tin content from the leaves can be transferred to the ground.
However, when 113Sn-labelled fentin acetate or hydroxide was applied
to potato foliage, no translocation of 113Sn from leaves to tubers
could be demonstrated above the limit of determination of 0.0001 ppm.
Tin could be detected in the haulm, where the percentage of the
original compound decreased for 20 days (Philips Duphar, 1964b). It
has also been shown that fentin compounds have no systemic action when
applied to celeriac and sugar beet (Herok and Götte, 1963).
Furthermore, there is no uptake of phenyltin from the soil
(Philips-Duphar, 1970; Hoechst, 1965b). This means that residues on
food or feed can only result from direct contact with the pesticide or
from contamination during harvest, transport or processing: potatoes,
carrots, sugar beets, groundnuts, coffee, cocoa, pecans and rice are
examples.
With a few crops, the plant parts carrying the residues may be used as
food or feed. Those feedstuffs are sugar beet tops and leaves,
groundnut vines and rice hulls. As reported in "Fate of residues in
animals" fentin itself and its degradation products in feed are
transferred only in very small amounts into milk and meat (Brüggemann
et al; 1964; Herok and Götte, 1963, 1964). The only crop from which
the leaves are used for human consumption is celery. This crop forms
generally only a small portion of the human diet.
In soil
Fentin compounds are degraded in soil almost completely within one
year (Hoechst, 1967).
Evidence of residues in food in commerce or at consumption
No experimental data are available on the fentin residues in food
moving in commerce or in the diets. Theoretically, it can be
postulated, however, that the chance fentin compounds would reach the
consumer is very limited. This is mainly due to the fact that the
residues of fentin compounds or their metabolites occur only on the
surfaces of those plant parts which have been subjects of direct
treatments, or of other types of contamination, and are therefore
easily removed by peeling, washing, shelling, etc., and are readily
broken down in thermal processes.
Peeling of potatoes, as well as cooking or other types of processing,
are apt to remove the residues. After sugar beets are processed into
sugar, no organic tin is found in sugar (Thompson and Hayward, 1969).
Carrots generally are washed, scraped, peeled or cooked before
consumption, which processes reduce the residues. The removal of
shells of pecans, groundnuts and cocoa removes also the residues. The
coffee beans are protected from contamination by the fruit peel and
flesh, by fermentation and by roasting. Rice is protected by husk,
which is removed by milling before the rice is cooked or baked for
consumption. Celery and celeriac if used uncooked for human
consumption may contain fentin residues at the time of consumption.
The portion of these crops is, however, generally very small in human
diets.
The foliage of treated crops may contain fentin residues which have to
be regarded as contaminants of animal feed. During silage the residues
are, however, found to be degraded and the risk of contamination of
animal products is avoided.
METHODS OF RESIDUE ANALYSIS
Fentin residues can be determined with two completely equivalent
methods which give the same results in identical samples. In the
method of Philips-Duphar, the triphenyltin compounds are determined
absorptiometrically in the extract with pyrocatechol-violet at 630 mm.
The detection limit of the method is between 0.01 and 0.12 ppm (Ross
and White, 1961; Malát, 1962). In the method of Hoechst, the
triphenyltin compound is converted into the tetrabromo compound,
separated from traces of lead and then determined polarographically.
The detection limit of the method is between 0.012 and 0.26 ppm (Bock
and Gorbach, 1958; Bock et al., 1958; Bürger, 1961a,b; Deutsche
Forschungsgemeinschaft, 1969).
Because blank values are varying according to crop variety and origin,
the detection limit is not a constant figure. None of the methods can
distinguish between the three fentin compounds; they give the sum of
these compounds. Di- and monophenyltin are not detected by these
methods. Although methods to distinguish between tri-, di- and
monophenyltin are available, some difficulties are encountered in the
presence of organic matter. Work on that subject is going on. For the
evaluation of residues in the abovementioned crops - with exception of
celery - the determination of degradation products of the three fentin
compounds does not seem relevant (see 113Sn-trials).
There are a number of industrial reports on the analytical methods
(see References).
APPRAISAL
Fentin compounds evaluated by the Working Party were fentin acetate
(triphenyltin acetate), fentin chloride (triphenyltin chloride) and
fentin hydroxide (triphenyltin hydroxide). They are used on potato,
sugar beet, celery, celeriac, leek, carrot, hops, groundnuts, pecans,
coffee and cocoa in various parts of the world to control fungus
diseases and on rice to control algae. They are usually applied to the
crops as a suspension of wettable powder.
Residue data are available from many countries: on fentin acetate from
different European and American countries, on fentin hydroxide from
U.S.A. and a number of European countries and on fentin chloride from
Germany.
The fentin compounds are subject to a gradual degradation. Fentin
acetate and fentin chloride produce at first triphenyltin hydroxide
which degrades to di- and monophenyltin hydroxide, and finally results
in the release of tin as inorganic tin salts. Among the other
degradation products, phenol, benzene and hydrogen peroxide are found.
The inorganic tin released from the organic molecule is not considered
as a residue of fentin compounds, but as a mineral element of
foodstuffs, and should be treated as such for regulatory purposes. The
degradation of fentin compounds is accelerated by sunlight, humidity,
thermal processes, etc. Due to the location of the residues on the
surface of the plants, washing has been found to cause a considerable
reduction of the residue levels. In soil, phenyltin residues degrade
almost completely within one year.
As was demonstrated with a radiolabelled (113Sn) compound in various
crops, neither phenyltin compounds nor their degradation products are
translocated within the plant after application to the leaves; neither
is there any uptake of phenyltin from soil. This means that residues
on food and feed can only result from direct contact with the
pesticide. None or negligible residues of the fentin compounds and
their metabolites can be expected at the time of consumption in the
edible parts of such crops as potatoes, carrots, groundnuts, coffee,
maize, pecans and rice. In the roots of sugar beets the residues are
normally very small and, in addition, any residues are eliminated
during processing.
The foliage of treated sugar beets has been found to contain residues.
During the silage process fentin compounds are broken down into
inorganic tin in about a month.
The fate of fentin compounds in cows has been evaluated by feeding
animals with sugar beet leaves containing about 1 ppm fentin acetate.
Only about 10 percent of the amount fed was absorbed from the
NATIONAL TOLERANCES
Compound Country Crop Tolerance
Fentin hydroxide U.S.A. potatoes 0.05 ppm
sugar beets under consideration
pecans extended
groundnuts under consideration
Fentin The Netherlands potatoes zero
celery and celeriac 1.0 ppm
Fentin hydroxide Federal Republic celery leaves 1.0 ppm calculated as tin
and fentin acetate of Germany
Fentin Yugoslavia generally 1.0 ppm calculated as tin
Belgium fruit and vegetables zero
celery and celeriac 1.0 ppm
intestinal tract. Milk contained ca. 0.004 ppm of fentin in unchanged
and changed form. Similar results have been obtained in a study on
sheep, administered 113Sn labelled fentin acetate, when it was shown
that the tin was finally excreted in inorganic form. In view of these
results, the risks that fentin would contaminate milk are negligible.
Two equivalent methods are available for the analysis of triphenyltin
residues. The sensitivity of a colorimetric method is, depending on
the type of crop, about 0.01 to 0.1 ppm and that of a polarographic
method about 0.01 to 0.3 ppm. The various triphenyltin compounds are
not distinguished by these methods: the analytical results therefore
reflect the total amount of these compounds in a particular sample
analysed.
Although methods to distinguish between tri-, di- and monophenyltin
are available (e.g. by TLC), difficulties are encountered in the
presence of organic material. Work on that subject is going on. For
the evaluation of residues in the above mentioned crops, with the
exception of celery leaves, the determination of degradation products
of fentin compounds does not seem relevant.
RECOMMENDATIONS
General provisions
Foliage of the treated crops, as well as other parts of plants which
may contain fentin residues, are not recommended to be fed fresh to
domestic animals, but they should be used only as silage. Due to the
degradation of the fentin compounds during silo storage, there is no
need, at this stage of knowledge, to establish practical residue
limits of meat and milk.
Recommendations for tolerances
Since the residues of fentins in the following crops were found to be
less than the detection limits of the analytical methods, no
tolerances are recommended: rice (detection limit: 0.08 ppm), cocoa
(0.1 ppm), pecans (0.03 ppm) and coffee (0.03 ppm).
Tolerances recommended refer to the total amount of fentin compounds
expressed in terms of triphenyltin hydroxide. (Di- and monophenyltin
and inorganic tin are not included in the figures.) The
recommendations are also made on the assumption that the crops are
treated according to good agricultural practice with one compound or,
if two or three are integrated, at a maximum total rate recommended
for any one of the three fentin compounds.
TOLERANCES RECOMMENDED
Parts Based on period
per million from treatment
to harvest
PotatoesX 0.1 7 days
Sugar beetsX 0.2 14 days
CeleryX 1 21 days
CeleriacX 0.1 21 days
CarrotsX 0.2 7 days
Groundnuts (shell-free) 0.05 7 days
XEssentially soil-free
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1972)
1. Reliable analytical methods capable of distinguishing qualitatively
between fentin compounds and tricyclohexyltin hydroxide or other
organotin compounds and, where possible, of quantitative
measurement of the separate compounds.
2. Data on the occurrence of monophenyltin and diphenyltin compounds
in the residues in celery.
DESIRABLE
1. More information on the effect of fentin compounds on the
reticuloendothelial system, especially with respect to the
reversibility of this effect.
2. Reports of toxicological observations in plant workers involved in
the manufacture of these compounds.
3. Further studies on the effect on spermatogenesis.
REFERENCES
Baran, J., Fancher, O.E. and Calandra, J.C. (1966a) 90 day subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C3964 submitted to Thompson-Hayward Chemical Co.
Baran, J., Fancher, O.E. and Calandra, J.C. (1966b) 100 days subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C4343 submitted to Thompson-Hayward Chemical Co.
Bock, R. and Gorbach, S. (1958) Die Bestimmung kleiner Mengen von
Triphenylzinnacetat in Pflanzenmaterial. Z. analyt. Chem., 163:
429-432
Bock, R., Gorbach, S. and Oeser, H. (1958) Analyse von
Triphenylzinnverbindungen. Angew. Chem., 70: 272
Brügemann, J., Barth, K. and Nieser, K.H. (1964) Experimentelle
Studien über das Auftreten von Triphenylzinnacetat Rückständen in
Rübenblättern, Rübenblattsilage, damit gefütterten Tieren und deren
Ausscheidungs-produkten. Zbl. Yet. Med., 11: 4-19
Bürger, K. (1961a) Zur Analytik von Organozinnverbindungen. Z.
Lebensmittel-Unters. u. -Forschung, 114: 1-10
Bürger, K. (1961a) Bestimmung von Mikrogramm-Mengen Zinn in tierischem
und Pflanzlichem Material nach der Dithiolmethode. Z.
Lebensmittel-Unters. u.-Forschung, 114; 10-13
Cahen, R., Boucard, M., Lalaurie, M. and Lacour, C. (1970) Production
expérimentale d'oedème cérébrale. C.R. Acad. Sci., (Paris) (in press)
Deutsche Forschungsgemeinschaft. (1969) Rückstandsanalytik von
Pflanzenschutzmitteln. Verlag Chemie GmbH, Weinheim/Bergstrasse.
Methode 55-B-1
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Food Inspection Laboratory, Amsterdam. (1962) Pesticide Report KvW 58,
dated August 1962, unpublished
Food Inspection Laboratory, Amsterdam. (1965a) Pesticide Report KvW
112, dated February 1965, unpublished
Food Inspection Laboratory, Amsterdam. (1965b) Pesticide Report KvW
115, dated May 1965, unpublished
Gaines, Th.B. and Kimbrough, R.D. (1968) Toxicity of fentin hydroxide.
Toxicol. appl. Pharmacol., 12:397-403
Gembloux Centre de Recherches de Phytopharmacie, Gembloux. (1968)
Report No. 34/68, dated June 1968
Hardon, H.J., Besemer, A.F.H. et al. (1962) Feldversuche mit
Triphenylzinn. Dtsch Lebensmitl. Rdsch, 58: 349-352
Heath, D.F. (1963) Some problems in the determination of residues in
plants and mammals in "Radiation and Radioisotopes applied to Insects
of Agricultural Importance", IAEA, Vienna, p. 185-193
Hedges (1961) Letter of the Tin Research Institute, dated 3-8-1961
with attached report
Herok, J. and Götte, H. (1961) The radiometrical estimation of the
distribution and excretion of (113Sn) triphenyltin acetate in
ruminants. Symp. radioisotopes in animal biology, Mexico City,
p.177-188
Herok, J. and Götte, H. (1963) Radiometrische Untersuchungen über das
Verhalten von Triphenylzinnacetat in Pflanze und Tier. Internat.J.
Appl. Radiation and Isotopes, 14: 461-479
Herok, J. and Götte, H. (1964) Radiometrische Stoffwechselbilanz von
Triphenylzinnazetat beim Milchschaf. Zbl. Vet. Med. A 11, 20-28
Hoechst Unpublished report (1961) Ing. Rei/kn(A), dated 22-8-1961
with attachment
Hoechst Unpublished report (1963) Dr.Cz/Bi 3543, dated 15-3-1963
Hoechst Unpublished report (1964) Dr.Cz/bi, dated 2-11-1964
Hoechst Unpublished report (1965a) Dr.Cz/bi, dated 17-9-1965
Hoechst Unpublished report (1965b) Hk/da-4565 b, dated 11-11-1965
Hoechst Unpublished report (1966a) 1/67 1 t/m 4
Hoechst Unpublished report (1966b) 94/66 1 t/m 4
Hoechst Unpublished report (1966c) 94/66 1 t/m 4
Hoechst Unpublished report (1966d) 62/661-2. 70/66 3, 82/66 4
Hoechst Unpublished report (1966e) 94/66 2 t/m 4
Hoechst Unpublished report (1966f) 95/66 1-2
Hoechst Unpublished report (1966g) 83/66 2 t/m 4
Hoechst Unpublished report (1966h) 61/66 3, 69/66 3, 83/66 41 94/66 5
Hoechst Unpublished report (1967) 84,90/66, dated 3-5-1967
Hoechst Unpublished report (1968a) A1/67 IV, dated 6-6-1968
Hoechst Unpublished report (1968b) 150/67, dated 29-5-1968
Hoechst Unpublished report (1968c) 151/67, dated 29-5-1968
Hoechst Unpublished report (1968d) 152/67, dated 29-5-1968
Hoechst Unpublished report (1968e) 160/67, dated 5-7-1968
Hoechst Unpublished report (1968f) 161/67, dated 5-7-1968
Hoechst Unpublished report (1968g) A1/67 V, dated 6-6-1968
Hoechst Unpublished report (1968h) 153/67, dated 29-5-1968
Hoechst Unpublished report (1968i) 154/67, dated 29-5-1968
Hoechst Unpublished report (1968j) 155/67, dated 29-5-1968
Hoechst Unpublished report (1968k) 1962/67, dated 5-7-1968
Hoechst Unpublished report (1968l) 163/67, dated 5-7-1968
Hoechst Unpublished report (1968m) 241/67, dated 5-3-1968
Hoechst Unpublished report (1968n) 240/67, dated 5-3-1968
Hoechst Unpublished report (1968o) 17/67, dated 1-4-1968
Hoechst Unpublished report (1968p) 16/67, dated 1-4-1968
Hoechst Unpublished report (1968q) 47/68, 99/68, 100 t/m 102/68
Hoechst Unpublished report (1969a) I-14-68, 1 zu 7/8 + 14/68, dated
7-8-1969
Hoechst Unpublished report (1969b) I-13-68, I zu 13-68, dated
20-6-1969
Hoechst Unpublished report (1969c) IV-9-68, IV zu 7-11/68, dated
7-11-1969
Hoechst Unpublished report (1969d) IV-7-68, IV zu 7-11/68, dated
7-11-1969
Klimmer, O.R. (1964) Toxikologische Untersuchungen mit
Triphenylzinnacetat. Zbl. Vet. Med., 11:29-39
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmitt-Rdsch. 7: 190-193
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmittel-Rdsch., 56: 190-193
Magee, P.N., Stoner, H.B. and Barnes, J.M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Path. Bact., 73: 107-124
Malát, M. (1962) Photometrische Zinnbestimmung mit
Brenzcatechinviolett in Anwesenheit von Gelatine; Colorimetrische
Studien (III). Anal. Chem., 187: 404-409
Nat. Inst. Public Health. (1960) Unpublished report nr. FT/17/60,
dated 23-4-1960
Newton, D.W. and Hays, R.L. (1968) Histological studies of ovaries in
rats treated with hydroxyurea, tri-phenyltin acetate and triphenyl
chloride. J. econ. Entomol., 61: 1668-1669
Pate, B.D. and Hays, R.L. (1968) Histological studies of testes in
rats treated with certain insect chemosterilants. J. econ. Entomol.,
61: 32-34
Philips Duphar Unpublished report (1963a) 56655/2A/63
Philips Duphar Unpublished report (1963b) 56655/38/63
Philips Duphar Unpublished report (1964a) 56646/5/64
Philips Duphar Unpublished report (1964b) 56655/34/63
Philips Duphar Unpublished report (1965) 56655/64/65
Philips Duphar Unpublished report (1966a) 56655/15/66
Philips Duphar Unpublished report (1966b) 56655/47A/66
Philips Duphar Unpublished report (1966c) 56655/54/66
Philips Duphar Unpublished report (1966d) 56655/61/66
Philips Duphar Unpublished report (1967) 56655/2/67
Philips Duphar Unpublished report (1968a) 56655/14/68
Philips Duphar Unpublished report (1968b) 56655/17/68
Philips Duphar Unpublished report (1970) 566461/1/70
Ross, W.J. and White, J.C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33:421-427
Scholz, D. (1963) Toxikologische Prüfung von Triphenylzinnchlorid und
Hoechst 2840-Spritzpulver. Unpublished report from Hoechst A.G., 18
December 1963
Scholz, D. and Baeder, M. (1968) Berichte über ein chronische
Toxizitätsprüfung von Triphenylzinnacetat per os an Ratten (4 Monate).
Unpublished report from Hoechst A.G.
Schulz, D. and Baeder, O. (1970) Berichte über fortpflanzungsversuche
mit triphenylzinnacetat an Ratten (Einflues auf Fertilitat,
Gravidität, postnatale Entvicklung). Unpublished report from Hoechst
AG
Scholz, D. and Brunk. (1968a) Chronische oral toxizitätsprufung von
Triphenylzinnacetat (Brestan-Wirkstoff) 120 Tage-Versuch an Hunden.
Unpublished report from Hoechst A.G.
Scholz, D. and Brunk. (1968b) Chronische oral toxizitätsprafung von
Brestan-Wirkstoff 2-Jahres-Versuch an Hunden. Unpublished report from
Hoechst A.G.
Scholz, D. and Weigand. (1968) Triphenylzinnacetat.
Wirkstoff-Technische. 4-Monate-Futterungsversuch an Meerschweinchen.
Unpublished report from Hoechst A.G.
Stoner, H.B. (1966) Toxicity of triphenyltin. Brit. J. industr. Med.,
23: 222-229
Stoner, H.B. and Heath, D.F. (1967) The cummulative action of
triphenyltin. Fd. Cosmet. Toxicol., 5: 285-286
Syrowatka, T. (1969) Investigation of the action of organotin
compounds on the oxidative phosphorylation and permeability of
membrane of rat liver mitochondria Roczn. Zak. Hig. (Warsz.), 20:
717-725
Syrowatka, T. (1970) Influence of fungicides on cellular energetic
processes. Roczn. Zak. Hig. (Warsz.), 21: 105-115
Tauberger, G. (1963) Tierexperimentelle Analyse der
Triphenylzinnwirkung nach intravenöser Injektion. Med. exp. (Basel),
9: 393-399
Thompson-Hayward Unpublished report (1965a) R-542
Thompson-Hayward Unpublished report (1965b) R-568
Thompson-Hayward Unpublished report (1967) R-650
Thompson-Hayward Unpublished report (1969) R-755
Til, H.P. and Feron, V.J. (1965) Triphenyltinhydroxide range-finding
test with dogs. Unpublished TNO report submitted by Philips-Suphar,
No. R-1994
Til, H.P., Feron, V.J. and de Groot, A.P. (1967) Reproduction study
with triphenyltinhydroxide in three generations of rats. Unpublished
TNO report submitted by Philips-Duphar, No. 2476
Til, H.P. and Feron, V.J. (1968) Chronic (two-year) toxicity study
with triphenyltinhydroxide (TPTH) in beagle dogs. Unpublished TNO
report submitted by Philips-Duphar, No. 2717
Til, H.P., Feron, V.J. and der Groot, A.P. (1968) Observations on a
possible effect of TPTH on testicular development in rats. Unpublished
TNO report No. R 2620 submitted by Philips-Duphar.
Til, H.P., Feron, V.J. and de Groot, A.P. (1970) Chronic toxicity
study with triphenyltinhydroxide in rats for two years. Unpublished
TNO report submitted by Philips-Duphar, No. R-3138
Ueda, K., Iijima, K. and Yoshida, S. (1961) Acute oral toxicity of
organotin compounds for mice. Unpublished report from Tokyo Dental
College submitted to Sankyo Chemical Co.
Van Esch, G.J. and Arnoldussen, A.M. (1962) Range-finding test as to
the toxicity of triphenyltinhydroxide during 18 days. Unpublished
report of the National Institute of Public Health, Utrecht, No. Tox
39/62
Verschuuren, H.G., Kroes, R. and van Esch, G.J. (1965) Semi-chronic
investigation as to the toxicity of triphenyltinhydroxide in guinea
pigs. Unpublished report of the National Institute of Public Health,
Utrecht, No. Tox. 33/65
Verschuuren, H.G., Kroes, R. Vink, H.H. and van Esch, G.J. (1966)
Short-term toxicity studies with triphenyltin compounds in rats and
guinea-pigs. Fd. Cosmet. Toxicol., 4: 35-45
Verschuuren, H.G., Ruitenberg, E.J., Peetoom, F., Helleman, P.W. and
van Esch, G.J. (1970) Influence of triphenyltin acetate on lymphatic
tissue and immune responses in guinea pigs. Toxicol. appl. Pharmacol.,
16: 400-410
Weigand and Kief, Brestan-Wirkstoff (1965) 96 percent ig =
Triphenylzinnacetate 96 percent ig Chronischer Futterungsversuch.
Meerschweinchen. Unpublished report from Hoechst A.G., 25 May 1965
Wolf, C., Fancher, O.E. and Calandra, J.C. (1966) 90 day sub-acute
oral toxicity of triphenyltin hydroxide - albino rats. Unpublished
report from Industrial Bio-test Laboratories Inc., submitted to
Thompson-Hayward Chemical Co.
PART II
LIST OF INDUSTRIAL REPORTS ON ANALYTICAL METHODS
Philips Duphar L3-52-16. Clean-up for the residue determination
of triphenyltin compounds in vegetable
matter.
Philips Duphar L3-52-18. Clean-up for the residue determination
of triphenyltin compounds in potatoes.
Philips Duphar L3-52-19. Absorptiometric determination of tin in
cleaned-up residues.
Philips Duphar L3-52-21. Clean-up for the residue determination
of fentin hydroxide in rice.
Philips Duphar L3-52-23. Clean-up for the residue determination
of triphenyltin compounds in tomatoes,
carrots, onions and butter-beans.
Philips Duphar L3-52-25. Clean-up for the residue determination
of triphenyltin compounds in
sugar-beets.
Philips Duphar L3-52-26. Clean-up for the residue determination
of triphenyltin compounds in celery and
sugar-beets.
Philips Duphar L3-52-29. Clean-up for the residue determination
of triphenyltin compounds in roasted
coffee-beans.
Farbwerke Hoechst. Die Bestimmung kleiner Mengen von
1966 Triphenylzinn in Pflanzenmaterial (Dr.
Gorbach 111/66 dated 1-12-1966).
Thompson-Hayward. Clean-up for the polarographic residue
1965a determination of triphenyltin compounds in
potatoes + polarographic tin determination in
pre-cleaned residues (from report 2-542).
Thompson-Hayward. Clean-up for the polarographic residue
1965b determination of triphenyltin compounds in
pecans + polarographic determination of
inorganic tin (from report R-568).
Thompson-Hayward. Clean-up procedure for the colorimetric
1967 residue determination of triphenyltin
compounds in peanuts (from report R-650).
Other relevant chemical properties
The pure fentin hydroxide is a white odourless crystalline solid, with
a melting point of 118-120°C. Solubility in water at 20°C is 8 ppm,
and it is moderately soluble in most organic solvents. It is stable at
room temperature, but decomposition occurs on heating above 60°C.
The pure fentin acetate is a white, odourless, crystalline solid, with
a melting point of 124-125°C. Its solubility in water at 20°C is 28
ppm, and it is of low solubility in most organic solvents.
Fentin acetate is stable when stored under normal conditions in dry
air. At 90°C, no decomposition can be detected; at 150°C, 15 percent
decomposes within three hours. In the presence of water, the acetate
is almost completely hydrolysed within eight hours at 20°C.
The pure fentin chloride is a white crystalline solid, with melting
point at 106°C. Its solubility in water is 40 ppm. It is stable when
stored in the dark in dry air. In the presence of water it hydrolyses
into the hydroxide.
Purity
The technical products are usually of the following purities:
fentin hydroxide : 95 percent
fentin acetate : 94 percent
fentin chloride : 94 percent
The impurities consist mainly of diphenyltin compounds and
tetraphenyltin.
This report is based on the data obtained with the fentin compounds
manufactured in Germany (Hoechst) and in Holland (Philips-Duphar). It
is known that fentin compounds are also produced in the United States
and Japan, but no data were available from these sources.
Formulations
The usual formulations are:
fentin hydroxide : 20 percent and 50 percent wp
fentin acetate : 20 percent and 60 percent wp
fentin chloride : 40 percent and 45 percent wp
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Experiments on rats given 113Sn-labelled fentin chloride showed that
the absorbed fentin radical was rapidly distributed through the
tissues of the body, including the brain. The fentin radical was
eliminated relatively slowly and could be detected in the brain 38
days after a single dose (Heath, 1963).
Experimental investigations have been made on guinea pigs fed on a
diet containing 12 ppm fentin acetate. From the survival times and the
total amount consumed, it is evident that, at this dosage level,
fentin compounds are not completely cumulative. The results obtained
and those previously published (Stoner, 1966) are consistent with a
halflife of the fentin radical at the site of action of about 50 days
(Stoner and Heath, 1967).
Information on the distribution of tin after feeding fentin compounds
to farm animals is included in the section entitled "FATE OF
RESIDUES. In animals".
Effect on enzymes and other biochemical parameters
In vitro studies have shown that fentin compounds inhibit oxidative
phosphorylation by isolated liver mitochondria and the adenosine
triphosphatase (ATP) activity of brain microcomes (50 percent
inhibition by concentration of, 1 × 10-6M and 1 × 10-5M,
respectively) (W. N. Aldridge, quoted by Stoner, 1966).
The oxygen consumption of cerebral slices from rats in a moribund
condition due to fentin compounds was within normal limits (J.E.
Cremer, quoted by Stoner, 1966).
The effects of fentin hydroxide on the oxidative phosphorylation and
on permeability of rat liver mitochondria membranes for malate,
citrate and fumarate ions were investigated. In the conditions of the
investigations (concentration of 5 µ M), a strong inhibitory action on
the oxidative phosphorylation in isolated rat liver mitochondria and
deep changes in the selectively permeable mitochondrial membrane were
observed. Malate and citrate were able to penetrate mitochondria
without participation of any carrier. The altered membrane formed no
barrier for fumarate. No similar effects were observed in the case of
n-octyltin derivatives (Syrowatka, 1969).
In a further investigation, it was shown that the mitochondrial
membrane which, when intact, has a strictly limited permeability for
K+, Na+, Cl-, malate, citrate and fumarate, became permeable in a
high degree to these ions under the influence of fentin hydroxide.
Electron microscopic pictures confirmed these results (Syrowatka,
1970).
TOXICOLOGICAL STUDIES
Special studies on influence on lymphatic tissue and immune
responses
Guinea pig (fentin acetate)
These investigations were aimed at studying the influence of fentin
acetate on lymphatic tissue and immune responses in guinea pigs which
were previously (see below) shown to be much more sensitive than rats
to the decreasing effect of the compound on the lymphopoiesis. Female
guinea pigs were selected because they are more affected than males.
In a first experiment, two groups of ten female guinea pigs received
15 ppm of fentin acetate in their diet, for 49 or 77 days. Each group
was accompanied by a control group with the same number of animals.
After 77 days, a decrease in plasma cells was observed in the spleen
and the cervical, mesenteric, axillary and popliteal lymph nodes. In a
second experiment, the same dose of the compound was given in the diet
for 104 days. Two groups of ten female guinea pigs received fentin
acetate and two groups control diet only. At 21 and 7 days before the
end of the fentin acetate feeding period, half of the animals received
an injection of tetanus toxoid. Some animals were allowed to recover
for 14 days after the 104-day experimental period. Immunochemically
and serologically, a decreased immunologic response was observed in
the group fed fentin acetate after tetanus toxoid stimulation and no
signs of recovery were seen after 14 days. With the aid of the
fluorescent antibody technique, a decrease in immunologically active
cells in the popliteal lymph nodes of the animals fed fentin acetate
could be detected in the second experiment. In the other lymphatic
tissues, no differences were observed (Verschuuren at al., 1970).
Special studies on reproduction
Rat (fentin acetate)
An interim report comprising two generations of a three-generation
reproduction study in rats fed 0, 25, 80 and 250 ppm of fentin acetate
in the diet is available. No histological examination was performed,
but no abnormalities were found by macroscopic examination at the 25
ppm level (Scholz and Baeder, 1970).
Rat (fentin acetate and chloride)
Groups each of 20 sexually mature male rats (aged 35 to 40 days) were
given orally in their food, over a period of 19 days, 20 mg/kg
body-weight per day of either fentin acetate or fentin chloride. The
animals were killed on the twentieth day, and histological
preparations of the testes were made. Both compounds produced several
degenerative changes of the testicular tissue, including a decrease in
the number of cell layers per seminiferous tubule, a decrease in
tubule diameter and overall testicular size, a depletion of the more
advanced cell forms from the tubules and a closing of tubule lumina.
Effects were more pronounced in animals treated with fentin acetate
(Pate and Hays, 1968).
Groups of 15 sexually mature female rats (aged 42 to 46 days) were
given orally 20 mg/kg body-weight per day of either fentin acetate or
fentin chloride. Three animals from each group were killed after 4, 9,
14, 19 and 24 days of treatment, and histological preparations of the
ovaries were made and thoroughly examined. Both fentin compounds
produced significant changes in the ovarian tissue. Among these
changes were a decreased number of mature follicles, an increased
incidence of atresia in early follicle growth and a pronounced
decrease in the number of corpora lutea present. This last effect was
regarded as a decrease in ovulation and, thus, a decreased fertility.
This effect, as well as the others listed above, was present on or by
the first five-day sample period (Newton and Hays, 1968).
Rat (fentin hydroxide)
This experiment was started with 50 male and 100 female newly weaned
rats. The animals were divided at random over five groups and were fed
diets containing 0, 0.5, 1, 2 or 5 ppm of fentin hydroxide. Fertility
of males and females, number of young born per litter, vitality,
body-weight and mortality of the young at birth and during the
lactation period were not affected by feeding fentin hydroxide at
levels up to 5 ppm in any of the three successive generations.
Ninety-day feeding studies with F1b and F2b generation rats did not
reveal adverse effects with respect to growth, food consumption, food
efficiency and haematology. Increased relative weights of liver,
kidney and spleen were occasionally noted, but were not associated
with histological abnormalities. Testicle weight was slightly
decreased at the 5 ppm level in F1b generation rats and at the 2 and
5 ppm levels in F2b generation rats at week 13 after weaning.
Macroscopically, no changes attributable to the ingestion of fentin
hydroxide were observed in any of the successive generations, except
for small-sized testicles at feeding levels of 1 ppm and above in
generation F3b at week 2 after weaning. Microscopic examination
revealed a significant difference between treated and control rats
only in the maturing testicle. This difference found expression in the
stage of maturation of the germinal epithelium, which was less
advanced in the small-sized testicles of the rats fed fentin hydroxide
(Til et al., 1967).
Four groups of ten weanling male rats were each fed 0, 50, 100 or 200
ppm of fentin hydroxide in the diet over a period of 276 days and were
mated at intervals to nontreated females. Observations indicate that,
in comparison to the controls, there was a drop of about 27 and 18
percent, respectively, in the total number of offspring in the groups
fed 200 and 100 ppm after 64 days of exposure. However, when the males
were subsequently mated, their fertility improved and, by day 113, was
comparable to that of the controls. Leucocyte and differential counts
and haemoglobin determinations performed one week prior to the time of
sacrifice did not differ from the controls. Gross abnormalities
related to fentin hydroxide were not observed in the offspring. In the
group fed 200 ppm, one rat died and two were sacrificed when moribund.
The cause of death in this group was generalized internal haemorrhage.
In the animals that were sacrificed, no gross or microscopic
abnormalities attributable to fentin hydroxide were observed. At the
100 and 200 ppm levels, the food consumption of the diet containing
fentin hydroxide was significantly lower during the first week of the
experiment and then improved for all dosage levels. By week 7, the
animals had adapted to these concentrations of the chemical, and the
food consumption was the same as in the controls. The food intake and
weight gain were not affected in rats fed 50 ppm. Gross and
microscopic examination of rats fed fentin hydroxide at all levels did
not reveal changes in any organ that could be related to the compound
(Gaines and Kimbrough, 1968).
Three experiments on weanling male rats (10 or 20 per group), fed from
0 to 25 ppm of fentin hydroxide in two or four weeks, were conducted
to determine testicular development. Microscopic examination after two
and four weeks failed to reveal any differences between the various
test groups and the control groups (Til et al., 1968).
Acute toxicity
Symptoms of poisoning were sluggishness, unsteadiness, moderate
diarrhoea, anorexia, bloody stain around the nose and eyes and
wheezing (Gaines and Kimbrough, 1968).
In all species, the main action of these organo-tin compounds is
thought to be on the central nervous system, but in contrast to
triethyltin compounds, cerebral oedema did not occur (Cahen et al.,
1970).
Intravenous administration of fentin acetate to cats at a dose of 1
mg/kg body-weight produced an increase in blood pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of 1
to 2 mg/kg at 20 to 60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood pressure was also
found. Death took place after 6 to 14 mg/kg of fentin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Dog (fentin acetate)
Groups of dogs (four of each sex) were fed 0, 1, 5 and 20 ppm of
fentin acetate in the diet for 120 days. A level of 20 ppm resulted in
decreased food consumption, and in half of the animals, also in
decreased body-weight. No macroscopic or microscopic abnormalities
were found (Scholz and Brunk, 1968a).
Groups of six dogs, three from each sex, were fed over a period of two
years, 0, 0.5, 1 or 5 ppm of fentin acetate. Attention was paid to
behaviour, food intake, weight gain, blood picture, urine composition,
gross examination and histological characteristics of the main organs
and tissues. Except for the higher level, where a decrease in weight
gain, which might be related to a reduction of food intake, was noted
in comparison to the controls, no significant abnormalities, even in
blood picture, were observed (Scholz and Brunk, 1968b).
Dog (fentin hydroxide)
Groups of dogs consisting of three males and three females were fed 0,
10, 25 and 37 ppm of fentin hydroxide in the diet for 90 days.
TABLE I
Acute toxicities to various species: summary
Animal Fentin LD50 (mg/kg
Compound Route body-weight) Reference
Mouse (M) acetate oral 81 Ueda et al., 1961
Mouse (M) chloride oral 80 Ueda et al., 1961
Mouse (M) hydroxide oral 245 Philips-Duphar, 1964a
Mouse (F) hydroxide oral 209 Philips-Duphar, 1964a
Mouse (M) acetate ip 7.9 Stoner, 1966
Rat (M) acetate oral 136 Klimmer, 1964
Rat (F) acetate oral 491 Stoner, 1966
Rat (F) chloride oral 135 Scholz, 1963
Rat (M) hydroxide oral 240 Gaines & Kimbrough, 1968
Rat (F) hydroxide oral 360 Gaines & Kimbrough, 1968
Rat (M) acetate ip 8.5 Stoner, 1966
Rat (F) acetate ip 11.9 Stoner, 1966
Guinea pig (M) acetate oral 21 Klimmer, 1964
Guinea pig (M) hydroxide oral 27.1 Philips-Duphar, 1964a
Guinea pig (F) hydroxide oral 31.1 Philips-Duphar, 1964a
Guinea pig (M) acetate ip 3.7 Stoner, 1966
Rabbit (M) acetate oral 40 Klimmer, 1964
Rabbit (M) acetate ip 10 Klimmer, 1964
Lethargy and a darkening of the hair occurred in all groups, depending
in degree on the dosage, and an increased mortality rate occurred in
the two higher groups (Baran et al., 1966a).
Groups of dogs (three of each sex) were fed 0, 10, 25 and 37 ppm of
fentin hydroxide in the diet for 100 days. Apart from darkening of the
hair which occurred in all test groups, the only differences from the
controls were lethargy in the 37 ppm dogs and an increased quantity of
tin in the livers of five of six dogs at 37 ppm and two of six dogs at
25 ppm (Baran et al., 1966b).
Six dogs were fed, over a period of 16 weeks, a diet containing,
during the first eight weeks, 25 ppm and, afterwards, 50 ppm of fentin
hydroxide. Symptoms of toxicity, consisting principally in inhibition
of growth, alterations in the blood picture (decrease of the number of
leucocytes and erythrocytes) and in the histology of the liver, were
observed (Til and Feron, 1965).
Following this preliminary investigation, 34 dogs were fed over a
period of two years a diet containing respectively 0, 0.5, 2.5, 5 and
10 ppm. Growth, food consumption, symptomatology, blood picture, urine
analysis, liver and kidney functional exploration, serum protein
electrophoresis, water content of brain, organ weights and gross and
microscopic pathology were used as criteria to disclose signs of
injury. General appearance, behaviour, growth, food consumption,
haematological and biochemical values, urine analyses and liver and
kidney functional tests were not affected at any dietary level of the
compound. At the two highest feeding levels of 5 and 10 ppm, the
relative weights of liver and kidney and the water content of the
brain were increased in comparison to the controls, without being
associated with histological changes. Gross and microscopic
examination of organs and tissues did not reveal any abnormality which
could be attributed to the ingestion of fentin hydroxide (Til and
Feron, 1968).
Guinea pig (fentin acetate)
In one experiment, 50 ppm of fentin acetate in the diet of guinea pigs
led to the death of all of nine animals in 17 to 31 days. In other
experiments, only a group of five guinea pigs given 1 ppm for 392 days
did not show an increased mortality rate. Even at 1 ppm, food intake
was reduced. When death occurred, it was preceded by loss of weight
and generalized weakness (Stoner, 1966). Neither in these experiments,
nor in others (acute and short-term), in which guinea pigs and rats
have been treated with fentin compounds, has a significant increase in
the water content of the central nervous system been found nor the
characteristic histological lesion in the white matter produced by
triethylin compounds (Magee et al., 1957) been seen (Stoner, 1966).
In other experiments, groups of guinea pigs, 10 of each sex, were
given respectively 0, 5, 10, 20 or 50 ppm of fentin acetate for 12
weeks. In all groups, except for the males given 5 ppm, growth
inhibition was obvious.
At the end of the treatment, no animal at 50 ppm was alive; in the
other groups, the survival rates were: 20 ppm, 8 out of 10 males and 8
out of 10 females; 10 ppm, 9 out of 10 males and 10 out of 10 females;
5 ppm, 9 out of 10 males and 10 out of 10 females. In the females on
5, 10 and 20 ppm and males on 10 and 20 ppm, the number of lymphocytes
and leucocytes was significantly decreased. The leuco- and lymphopenia
was accompanied in a number of animals by histological changes in the
lymphopoietic system, e.g., atrophy of the white pulp of the spleen
was found. This change possibly induced a decrease of general
resistance, so that a mycotic infection developed in the animals. At
20 and 50 ppm, a significant increase of brain water was noticed.
Histological findings were not significantly different from those
observed in the controls (Verschuuren et al., 1966).
Groups of guinea pigs, ten of each sex, were fed 0, 1, 5, 10, 50 and
100 ppm of fentin acetate for four months. All animals on 100 ppm and
30 percent of those on 50 ppm died, and in the same groups were found
signs of disturbance of the iron metabolism. A level of 10 ppm gave a
slight reduction in weight gain, as well as some cases of decreased
haemoglobin percentage and total number of erythrocyte. The groups fed
5 ppm and 1 ppm were similar to the controls (Scholz and Weigand,
1968).
Guinea pig (fentin hydroxide)
Groups of three males and three females were given 0, 1, 2.5, 5 or 20
ppm of fentin hydroxide for three weeks. No changes in the growth
rate, the water content, or the histology of the nervous system were
observed (Verschuuren at al., 1965).
Groups of ten males and ten females were fed 0, 2.5, 5, 10, 20 or 50
ppm of fentin hydroxide in their diet for 12 weeks. Growth depression
was observed at the two highest dose levels. All animals in the 50 ppm
group died during the first half, and one each in the ten and 20 ppm
groups died later in the experiment. A decrease in the haemoglobin
content was found in all groups, except for the males in the 2.5 and 5
ppm groups there was no change in the number of erythocytes. A
decrease in the number of leucocytes (except in the males on 10 ppm)
and lymphocytes (except the males on 2.5 and 5 ppm) was found in all
the groups examined. The same changes as with fentin acetate were
found in the lymphopoietic system (see above). A decrease in relative
organ weight was found in the spleen of the females on 10 and 20 ppm
and in the thymus of both sexes and uterus and testes at 20 ppm. No
increase in the water content of the central nervous system was
observed, except in the females at 50 ppm. Apart from the changes in
the lymphopoietic system, the histological findings were not
significantly different from those observed in the controls
(Verschuuren et al., 1966).
See also Special studies on influence on lymphatic tissue and immune
responses.
Rat (fentin acetate)
Groups of 20 to 25 rats were given fentin acetate by stomach tube at
doses equivalent to 5, 10, 25 or 50 ppm for 105 to 170 days. At 50
ppm, 70 percent of the rats died within 49 days. Nervous symptoms, as
well as blood, urinary or histopathological changes, were not observed
at the lower dose levels (Klimmer, 1964).
In other experiments on rats, no deaths occurred during a ten-week
period on 200 ppm of fentin acetate. These rats were then put on a
diet containing 300 ppm, and five out of six rats died after a further
117 to 168 days (Stoner, 1966).
Groups of young rats, ten of each sex, were given respectively 0, 5,
10, 25 or 50 ppm of fentin acetate for 12 weeks. Decrease of food
intake and growth inhibition were recorded at 50 ppm, as well as
growth inhibition in males at 25 ppm. At 10 ppm and above, the number
of leucocytes in the blood was decreased in the male (leucopenia) and,
at 50 ppm, the haemoglobin was reduced. At the highest level there was
a decrease of the organ to heart weight ratio for the pituitary and
pancreas in all animals and uterus and ovary in the females. The same
ratio for the thyroid was decreased in all the females, as well as in
the males at 25 and 50 ppm. Paralysis and pronation of the hind legs,
associated with interstitial oedema of the central nervous system,
which developed in rats treated with triethyltin hydroxide at all
levels, were not observed. The water content of the spinal cord was
slightly increased at 50 ppm. Histological findings were not
significantly different from those observed in controls (Verschuuren
at al., 1966).
Groups of 20 young rats, ten of both sexes, were fed respectively, 0,
15, 40, 100, 250 or 600 ppm of fentin acetate over a period of four
months. Attention was given to behaviour, growth, blood picture, urine
composition, gross examination and histology of the main organs and
tissues. Up to 100 ppm, no abnormalities were observed in comparison
to the controls. Above 100 ppm, a dose-related inhibition of growth
was seen and 600 ppm resulted in the death of 30 percent of the
treated animals. (Scholz and Baeder, 1968).
Rat (fentin chloride)
Groups of ten rats of both sexes were given orally, over a period of
41 days, 28 doses of 0, 6.25, 12.5, 25 or 50 mg/kg body-weight. Apart
from a slight decrease in weight gain of the females at 12.5 mg/kg, no
abnormalities (growth, behaviour, blood picture and urine composition,
gross and histological characteristics of stomach, small intestine,
spleen, liver, kidney and lung) were observed at 6.25 and 12.5 mg/kg.
At higher doses, a dose-related increase in mortality compared to the
controls was observed (Scholz, 1963).
Rat (fentin hydroxide)
Groups of 10 or 20 rats of both sexes were given 0, 5, 20, 50 or 100
ppm of fentin hydroxide for 20 days. Food intake and body growth were
depressed at 20 ppm and above. Death rates were 9 out of 10 at 100
ppm; 9 out of 20 at 50 ppm, and 1 out of 20 at both 20 and 5 ppm (Van
Esch and Arnoldussen, 1962).
Groups of rats, ten of each sex, were fed 0, 1, 3.1, 5, 10 and 31 ppm
of fentin hydroxide in the diet for 90 days. Disregarding effects on
erythrocyte and leucocyte counts as neither dose related nor
chronologic, a no-effect level of 31 ppm was claimed (Wolf et al.,
1966).
Groups of ten rats of each sex were given respectively 0, 5, 10 or 25
ppm of fentin hydroxide in the diet for 12 weeks. The food intake was
comparable to the controls. The females showed growth inhibition after
six weeks, but they recovered in spite of continuing treatment. Growth
in the males was comparable to the controls. In the females, blood
leucocytes were decreased. No significant changes in water content
were found in the nervous tissues. At 25 ppm, decrease of the thyroid
weight was noticed (Verschuuren at al., 1962). In a similar
experiment, in which ten rats of each sex were given 50 ppm of the
same compound, the following features were noticed: decreased food
intake, growth inhibition in both sexes, decrease in weight of the
thyroid, pituitary, uterus, ovary, prostate and pancreas, as well as
decrease of haemoglobin and leucocytes. Paralysis and pronation of the
hindlegs, associated with interstitial oedema of the central nervous
system, which developed in rats treated with triethyltin hydroxide at
all levels, were not observed. The water content increased in the
spinal cord, but not in the brain. (In comparison, the water content
of the spinal cord and brain was increased in animals treated with
levels of 5 and 10 ppm of triethyltinhydroxide). Histological findings
in the animals treated with fentin hydroxide were not significantly
different from those observed in the controls (Verschuuren et al.,
1966).
Groups of four to five week old male rats were fed fentin hydroxide in
the diet at levels of 0, 100, 200 or 400 ppm over a period of 99 days.
The rats fed 400 ppm were apparently repelled, since they ate only
about one third as much food as the controls during the first week.
All the animals in this group died within 7 to 34 days from starvation
and extensive intra-alveolar haemorrhage in the lung. There was
partial or complete testicular atrophy and general size and weight
reduction of the organs. The rats fed 200 ppm gained less weight (231
g) than the controls (302 g). At the end of the study, the total
leucocyte number and the weights of spleen and kidneys were
significantly below those of the controls.
The rats fed 100 ppm appeared normal throughout the study except for a
significant decrease of food intake during the first week of treatment
(Gaines and Kimbrough, 1968).
Additional information on the findings following short-term
administration of fentin compounds to rats is given under Special
studies on reproduction.
Long-term studies
Guinea pig (fentin acetate)
Groups of 20 young guinea pigs, ten from each sex, were fed
respectively 0, 1, 5, 10, 50, 100 and 200 ppm of fentin acetate over a
period of two years. Attention was given to behaviour, food intake,
weight gain, blood picture, urine composition, gross examination and
histological characteristics of the main organs and tissues. At 50 ppm
and above, a dose-related increase in mortality in comparison to the
controls and an inhibition of growth were observed. At 10 ppm and
above, fatty degenerative changes were seen in liver and heart muscle.
At 1 and 5 ppm, no abnormalities, even in the blood picture, were
noted in comparison to the controls (Weigand and Kief, 1965).
Rat (fentin hydroxide)
Groups of 50 newly weaned rats, 25 from each sex, were fed over a
period of two years respectively 0, 0.5, 1, 2, 5 or 10 ppm of fentin
hydroxide. General appearance and behaviour, growth, food intake,
blood sugar and blood urea nigrogen, urine composition and SG-PT,
SG-OT and SAP activity were not adversely affected at any dietary
level of the test substance. Mortality of the females at 10 ppm was
higher than in the controls, although at the termination of the
experiment the difference was not significant. Haematological data
often showed a slight decrease in white blood cells at the highest
feeding level in the first year of the experiment only. This effect
was less often soon at 5 ppm and only once at 2 ppm in the males. The
relative thyroid weight was slightly decreased at 10 ppm in the
females only. Average relative weights of the other organs were very
uniform in all groups. Gross autopsy findings did not show evidence of
significant pathological changes. Microscopic examinations of numerous
organs and tissues, including testes, ovaries and bone marrow, did not
reveal histological alterations significantly different from those
observed in controls (Til et al., 1970).
COMMENTS
The basis for grouping the three fentin compounds for consideration
together is that fentin acetate and fentin chloride are converted in
plants and animals to the same compound, namely fentin hydroxide.
Since the previous toxicological evaluation of the fentin compounds in
1965, a considerable number of short-term and long-term feeding
studies in several species have been reported. The production of
cerebral oedema in rats and guinea pigs did not appear to be
pronounced except at a 50 ppm dose level. However, the reduction of
lymphopoiesis in both species appeared to be of some concern. With
respect to this parameter, the guinea pig appeared to be the most
sensitive species studied. A reduction in lymphocyte count was evident
in females at a dietary level of 2.5 ppm of fentin hydroxide. It was
reported that this effect was not entirely reversible after 14 days'
withdrawal from a test diet of 15 ppm of fentin acetate. In a two-year
feeding study in rats a no-effect level of 2 ppm relative to decrease
in white blood cells was evident.
In a multigeneration rat reproduction study, reduction in testicular
size was evident at 5 ppm in the F1b generation, at 2 ppm and above
in the F2b generation and at 1 ppm in the F3b generation. In a
second species, the dog, histological examination revealed no effect
on any organ at feeding levels of up to 10 ppm for two years.
There are no reports of observations of men exposed to this compound.
Bearing the abovementioned observations in mind, the Committee
considered the data adequate to establish an acceptable daily intake
for the fentin compounds.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.1 mg/kg body-weight
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 to 0.0005 mg/kg body-weight, applicable to fentin hydroxide,
fentin acetate and fentin chloride and to the sum of each if more
than one is involved
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
All the three fentin compounds are nonsystemic fungicides used in many
European countries, the United States, and several South American,
African and Asiatic countries. On rice they are used against algae.
Pre-harvest treatments
Fentin compounds are recommended to control the following pests:
Potatoes: Phytophthora infestans de Bary
of the foliage and especially
the tubers, and
Alternaria solani J. u.Gr.
Sugar beet: Cercospora beticola Sacc.
Celery and celeriac: Septoria apii Chester
Carrots: Alternaria porri f. dauci Neerg.
Pecans: Fusicladium effusum, Gnomonia spp.,
Mycosphaerella caryigena Demaree & Cole
Rice: various algae
Groundnuts: Cercospora spp.
Coffee: Colletotrichum coffeanum Noack
Cocoa: Phytophthora palmivora Butl.
The usual rates of application are between 0.15 and 0.40 kg a.i/ha in
600 to 1 000 litres of water. On coffee, the rate is 0.2 to 0.6 kg
a.i/ha in more than 1 000 litres of water. For algae control on rice,
the dosage is up to 1 kg a.i/ha in about 1 000 litres of water.
Recommended rates of application and intervals applied between last
treatment and harvest are as follows:
Potatoes: 0.18 to 0.4 kg a.i./ha. 1-10 times
(generally between 2 and 5 times).
Safety period (in Germany) 7 days.
Sugar beets: 0.18 to 0.4 kg a.i./ha. 1-3 treatments.
Celery celeriac, 0.2 to 0.32 kg a.i./ha in 600-1 000 1
carrot: water, 1-7 treatments. (In Germany
safety period for celery of 3 weeks)
Pecans: 0.22 to 0.54 kg a.i./ha, 2 to 8
treatments.
Rice: 0.75 to 1 kg a.i./ha for algae control.
Peanuts, coffee, 0.2 to 0.6 kg a.i./ha.
cocoa:
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Fentin compounds are used on ornamentals, willow and poplar. Trials on
hops are in process.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials are given in Table II.
TABLE II
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Potatoes Holland 0.20-0.46 4-7 2-98 tubers 0.01 23 17 0.04
" " 0.08-0.38 6-7 98-110 " 0.06 14 12 0.11
" England 0.22-0.56 5 21-43 " 0.01 5 5
" " 0.28 1 30-35 " 0.03 6 6
" Canada 0.15-0.25 ?-12 ?-169 " 0.03 7 7
" U.S.A. 0.17-0.34 5-11 13-15 " 0.03 17 17
" Belgium 0.30-0.36 5 22 " 0.01 1 1
Sugar beets Holland 0.30 5 43-57 beets 0.03 4 0.18
" " 0.30 5 43-57 leaves 0.06 4 1.36
" Germany 0.30 1-4 46-64 beets 0.03 5 2 0.18
" " 0.30 1-4 46-64 leaves 0.06 5 2 0.81
" Italy 0.30-0.40 ? 31 beets 0.03 10 1 0.20
" Switzerl. 0.30-0.40 2 44 leaves 0.02 4 2.45
" U.S.A. 0.22-0.34 3-6 14-45 beets 0.03 17 8 0.09
Carrots Israel 0.25-0.30 5-7 12-28 carrot 0.05 4 3 0.07
Pecans U.S.A. 0.22-0.54 2-8 46-256 nuts 0.03 17 17
Rice Italy 04-1.2 ppm 1 90 hulled rice 0.08 5 5
in irrigation
water
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Ground nuts U.S.A. 0.22-0.34 3-7 9-72 nuts 0.01 14 10 0.03
" U.S.A. 0.22-0.34 3-7 9-72 hulls 0.01 12 1 0.38
" U.S.A. 0.34 3-7 9 vines 0.01 3 2.5
" Israel 0.30 4 5-23 nuts 0.01 4 4
" " 0.30 4 5-23 vines 0.16 4 2.6
Coffee Kenya 0.22-0.60 ? 35 roasted 0.03 3 3
beans
Residues of fentin acetate
Potatoes Germany 0.216-0.324 1-5 7-21 tubers 0.03-0.04 12 8 0.07
" Ireland 0.3 3 not stated " 0.03 2 2
Sugar beets Germany 0.216-0.324 6 38 beets 0.1 6 6
Celeriac " 0.3 1-2 21-45 tubers not stated 3 3
" " 0.324 1 21-35 " 0.1 3 3
" Belgium not stated 3 21-28 stems not stated 6 6
" Germany 0.3 1-2 21-45 leaves not stated 13 2 0.19
" " 0.324 1 21-35 " 0.1 3 3
" Holland 0.24-0.30 1-6 21-28 " 0.1 2 1.0
Celeriac Holland 0.27 6 21-28 leaves 0.01 2 0.30
" Belgium not stated 3 21-28 " not stated 6 5 0.05
Carrots Germany 0.216-0.324 1-3 7-28 1/ 0.03 20 17 0.15
" " 0.216-0.324 1-3 7-28 2/ 0.02 6 5 0.06
" " 0.216-0.324 1-3 7-28 3/ 0.02 6 5 0.02
" " 0.216-0.324 1-3 7-28 4/ 0.02 6 5 0.02
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin acetate
Rice Italy 04-1.2ppm 1 appr.go hulled 0.08 3 3
in irrigation rice
water
Cocoa Nigeria not stated not stated not stated not stated 0.1 8 7 0.1
Residues of fentin chloride
Potatoes Germany 0.22-0.27 1-3 7-14 tubers 0.04 8 6 0.05
Sugar beets " 0.18-0.27 6 38 beets 0.10 4 4
Celeriac " 0.27 1 21-35 tubers 0.1 3 2 0.1
Carrots " 0.20 3 10-31 washed 0.02 4 2 0.08
carrots
1/ washed unpeeled carrots
2/ peeled/scraped carrots
3/ peels, calculated on total weight carrot
4/ scrapings, calculated on total weight carrot
The residue date of fentin hydroxide are from treatments made in the
Netherlands, Germany, Belgium, Canada, England, Italy, Israel,
Switzerland, U.S.A. and Kenya (Philips Duphar, 1963a, 1964b, 1965,
1966a-d, 1968a,b; Thompson-Hayward, 1965a,b, 1967, 1969).
The residue data of fentin acetate are from experiments made in
Germany, the Netherlands, Belgium, Ireland, Italy, Kenya and Nigeria
(Hoechst, 1963, 1964, 1965a, 1966a-d, 1967, 1968a-f,m,o,q, 1969c,d;
Hardon et al., 1962; Gembloux, 1968; Food Inspection, 1965a-b;
Kröller, 1960; Nat. Inst. Publ. Health, 1960; Philips Duphar, 1967).
The residue date of fentin chloride are from experiments made in
Germany (Hoechst, 1966e-h, 1968g-l,n,p, 1969a,b).
FATE OF RESIDUES
General comments
When triphenyltin acetate was decomposed in solution, the following
products were detected: phenol, acetone, hydrogen peroxide, degraded
organotin and benzene. There is probably also some organic or
organotin peroxide present (Hedges, 1961).
In the presence of light and humidity, fentin acetate and fentin
chloride are transformed easily into fentin hydroxide, which
metabolizes into dephenyltin and monophenyltin. Monophenyltin breaks
down very quickly into Sn salts (Kröller, 1960; Hoechst, 1961; Hedges,
1961).
At heating, triphenyltin hydroxide readily decomposes (see other
relevant chemical properties).
In animals
After ingestion of fentin compounds by cows or sheep, the compound is
largely excreted with the faeces (Herok and Götte, 1961; Brügemann et
al., 1964). Three sheep given 10 mg daily of 113Sn-labelled fentin
acetate for 25 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration: 0.0017 ppm) during the treatment, but 17 days after
withdrawal of the compound, its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than fentin acetate. During the treatment, the concentration of
113Sn in the blood and in the urine were 2.9µg/1 and 7.5µg/1,
respectively. In the sheep killed at 28 days, liver, kidney, lung,
pancreas, gall bladder and brain contained a greater amount of 113Sn
than other organs. After 218 days, the amount of 113Sn found in the
liver was still greater than in other organs (Herok and Götte, 1961).
Organs of one sheep, sacrificed at the end of the feeding period with
the 113Sn triphenyltin acetate, showed the following residues
(comprising triphenyltin and all degradation products including
inorganic tin):
Organ ppm 113Sn
liver 0.9
intestinal fat 0.01
depot fat 0.01
muscles 0.02
In plants
Studies on the effect of tin compounds on plants have been made or are
in progress in several laboratories. It has been shown that in the
presence of light and air, several byproducts of fentin acetate can be
formed, such as diphenyltin and finally insoluble tin (Kröller, 1960).
The tin content from the leaves can be transferred to the ground.
However, when 113Sn-labelled fentin acetate or hydroxide was applied
to potato foliage, no translocation of 113Sn from leaves to tubers
could be demonstrated above the limit of determination of 0.0001 ppm.
Tin could be detected in the haulm, where the percentage of the
original compound decreased for 20 days (Philips Duphar, 1964b). It
has also been shown that fentin compounds have no systemic action when
applied to celeriac and sugar beet (Herok and Götte, 1963).
Furthermore, there is no uptake of phenyltin from the soil
(Philips-Duphar, 1970; Hoechst, 1965b). This means that residues on
food or feed can only result from direct contact with the pesticide or
from contamination during harvest, transport or processing: potatoes,
carrots, sugar beets, groundnuts, coffee, cocoa, pecans and rice are
examples.
With a few crops, the plant parts carrying the residues may be used as
food or feed. Those feedstuffs are sugar beet tops and leaves,
groundnut vines and rice hulls. As reported in "Fate of residues in
animals" fentin itself and its degradation products in feed are
transferred only in very small amounts into milk and meat (Brüggemann
et al; 1964; Herok and Götte, 1963, 1964). The only crop from which
the leaves are used for human consumption is celery. This crop forms
generally only a small portion of the human diet.
In soil
Fentin compounds are degraded in soil almost completely within one
year (Hoechst, 1967).
Evidence of residues in food in commerce or at consumption
No experimental data are available on the fentin residues in food
moving in commerce or in the diets. Theoretically, it can be
postulated, however, that the chance fentin compounds would reach the
consumer is very limited. This is mainly due to the fact that the
residues of fentin compounds or their metabolites occur only on the
surfaces of those plant parts which have been subjects of direct
treatments, or of other types of contamination, and are therefore
easily removed by peeling, washing, shelling, etc., and are readily
broken down in thermal processes.
Peeling of potatoes, as well as cooking or other types of processing,
are apt to remove the residues. After sugar beets are processed into
sugar, no organic tin is found in sugar (Thompson and Hayward, 1969).
Carrots generally are washed, scraped, peeled or cooked before
consumption, which processes reduce the residues. The removal of
shells of pecans, groundnuts and cocoa removes also the residues. The
coffee beans are protected from contamination by the fruit peel and
flesh, by fermentation and by roasting. Rice is protected by husk,
which is removed by milling before the rice is cooked or baked for
consumption. Celery and celeriac if used uncooked for human
consumption may contain fentin residues at the time of consumption.
The portion of these crops is, however, generally very small in human
diets.
The foliage of treated crops may contain fentin residues which have to
be regarded as contaminants of animal feed. During silage the residues
are, however, found to be degraded and the risk of contamination of
animal products is avoided.
METHODS OF RESIDUE ANALYSIS
Fentin residues can be determined with two completely equivalent
methods which give the same results in identical samples. In the
method of Philips-Duphar, the triphenyltin compounds are determined
absorptiometrically in the extract with pyrocatechol-violet at 630 mm.
The detection limit of the method is between 0.01 and 0.12 ppm (Ross
and White, 1961; Malát, 1962). In the method of Hoechst, the
triphenyltin compound is converted into the tetrabromo compound,
separated from traces of lead and then determined polarographically.
The detection limit of the method is between 0.012 and 0.26 ppm (Bock
and Gorbach, 1958; Bock et al., 1958; Bürger, 1961a,b; Deutsche
Forschungsgemeinschaft, 1969).
Because blank values are varying according to crop variety and origin,
the detection limit is not a constant figure. None of the methods can
distinguish between the three fentin compounds; they give the sum of
these compounds. Di- and monophenyltin are not detected by these
methods. Although methods to distinguish between tri-, di- and
monophenyltin are available, some difficulties are encountered in the
presence of organic matter. Work on that subject is going on. For the
evaluation of residues in the abovementioned crops - with exception of
celery - the determination of degradation products of the three fentin
compounds does not seem relevant (see 113Sn-trials).
There are a number of industrial reports on the analytical methods
(see References).
APPRAISAL
Fentin compounds evaluated by the Working Party were fentin acetate
(triphenyltin acetate), fentin chloride (triphenyltin chloride) and
fentin hydroxide (triphenyltin hydroxide). They are used on potato,
sugar beet, celery, celeriac, leek, carrot, hops, groundnuts, pecans,
coffee and cocoa in various parts of the world to control fungus
diseases and on rice to control algae. They are usually applied to the
crops as a suspension of wettable powder.
Residue data are available from many countries: on fentin acetate from
different European and American countries, on fentin hydroxide from
U.S.A. and a number of European countries and on fentin chloride from
Germany.
The fentin compounds are subject to a gradual degradation. Fentin
acetate and fentin chloride produce at first triphenyltin hydroxide
which degrades to di- and monophenyltin hydroxide, and finally results
in the release of tin as inorganic tin salts. Among the other
degradation products, phenol, benzene and hydrogen peroxide are found.
The inorganic tin released from the organic molecule is not considered
as a residue of fentin compounds, but as a mineral element of
foodstuffs, and should be treated as such for regulatory purposes. The
degradation of fentin compounds is accelerated by sunlight, humidity,
thermal processes, etc. Due to the location of the residues on the
surface of the plants, washing has been found to cause a considerable
reduction of the residue levels. In soil, phenyltin residues degrade
almost completely within one year.
As was demonstrated with a radiolabelled (113Sn) compound in various
crops, neither phenyltin compounds nor their degradation products are
translocated within the plant after application to the leaves; neither
is there any uptake of phenyltin from soil. This means that residues
on food and feed can only result from direct contact with the
pesticide. None or negligible residues of the fentin compounds and
their metabolites can be expected at the time of consumption in the
edible parts of such crops as potatoes, carrots, groundnuts, coffee,
maize, pecans and rice. In the roots of sugar beets the residues are
normally very small and, in addition, any residues are eliminated
during processing.
The foliage of treated sugar beets has been found to contain residues.
During the silage process fentin compounds are broken down into
inorganic tin in about a month.
The fate of fentin compounds in cows has been evaluated by feeding
animals with sugar beet leaves containing about 1 ppm fentin acetate.
Only about 10 percent of the amount fed was absorbed from the
NATIONAL TOLERANCES
Compound Country Crop Tolerance
Fentin hydroxide U.S.A. potatoes 0.05 ppm
sugar beets under consideration
pecans extended
groundnuts under consideration
Fentin The Netherlands potatoes zero
celery and celeriac 1.0 ppm
Fentin hydroxide Federal Republic celery leaves 1.0 ppm calculated as tin
and fentin acetate of Germany
Fentin Yugoslavia generally 1.0 ppm calculated as tin
Belgium fruit and vegetables zero
celery and celeriac 1.0 ppm
intestinal tract. Milk contained ca. 0.004 ppm of fentin in unchanged
and changed form. Similar results have been obtained in a study on
sheep, administered 113Sn labelled fentin acetate, when it was shown
that the tin was finally excreted in inorganic form. In view of these
results, the risks that fentin would contaminate milk are negligible.
Two equivalent methods are available for the analysis of triphenyltin
residues. The sensitivity of a colorimetric method is, depending on
the type of crop, about 0.01 to 0.1 ppm and that of a polarographic
method about 0.01 to 0.3 ppm. The various triphenyltin compounds are
not distinguished by these methods: the analytical results therefore
reflect the total amount of these compounds in a particular sample
analysed.
Although methods to distinguish between tri-, di- and monophenyltin
are available (e.g. by TLC), difficulties are encountered in the
presence of organic material. Work on that subject is going on. For
the evaluation of residues in the above mentioned crops, with the
exception of celery leaves, the determination of degradation products
of fentin compounds does not seem relevant.
RECOMMENDATIONS
General provisions
Foliage of the treated crops, as well as other parts of plants which
may contain fentin residues, are not recommended to be fed fresh to
domestic animals, but they should be used only as silage. Due to the
degradation of the fentin compounds during silo storage, there is no
need, at this stage of knowledge, to establish practical residue
limits of meat and milk.
Recommendations for tolerances
Since the residues of fentins in the following crops were found to be
less than the detection limits of the analytical methods, no
tolerances are recommended: rice (detection limit: 0.08 ppm), cocoa
(0.1 ppm), pecans (0.03 ppm) and coffee (0.03 ppm).
Tolerances recommended refer to the total amount of fentin compounds
expressed in terms of triphenyltin hydroxide. (Di- and monophenyltin
and inorganic tin are not included in the figures.) The
recommendations are also made on the assumption that the crops are
treated according to good agricultural practice with one compound or,
if two or three are integrated, at a maximum total rate recommended
for any one of the three fentin compounds.
TOLERANCES RECOMMENDED
Parts Based on period
per million from treatment
to harvest
PotatoesX 0.1 7 days
Sugar beetsX 0.2 14 days
CeleryX 1 21 days
CeleriacX 0.1 21 days
CarrotsX 0.2 7 days
Groundnuts (shell-free) 0.05 7 days
XEssentially soil-free
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1972)
1. Reliable analytical methods capable of distinguishing qualitatively
between fentin compounds and tricyclohexyltin hydroxide or other
organotin compounds and, where possible, of quantitative
measurement of the separate compounds.
2. Data on the occurrence of monophenyltin and diphenyltin compounds
in the residues in celery.
DESIRABLE
1. More information on the effect of fentin compounds on the
reticuloendothelial system, especially with respect to the
reversibility of this effect.
2. Reports of toxicological observations in plant workers involved in
the manufacture of these compounds.
3. Further studies on the effect on spermatogenesis.
REFERENCES
Baran, J., Fancher, O.E. and Calandra, J.C. (1966a) 90 day subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C3964 submitted to Thompson-Hayward Chemical Co.
Baran, J., Fancher, O.E. and Calandra, J.C. (1966b) 100 days subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C4343 submitted to Thompson-Hayward Chemical Co.
Bock, R. and Gorbach, S. (1958) Die Bestimmung kleiner Mengen von
Triphenylzinnacetat in Pflanzenmaterial. Z. analyt. Chem., 163:
429-432
Bock, R., Gorbach, S. and Oeser, H. (1958) Analyse von
Triphenylzinnverbindungen. Angew. Chem., 70: 272
Brügemann, J., Barth, K. and Nieser, K.H. (1964) Experimentelle
Studien über das Auftreten von Triphenylzinnacetat Rückständen in
Rübenblättern, Rübenblattsilage, damit gefütterten Tieren und deren
Ausscheidungs-produkten. Zbl. Yet. Med., 11: 4-19
Bürger, K. (1961a) Zur Analytik von Organozinnverbindungen. Z.
Lebensmittel-Unters. u. -Forschung, 114: 1-10
Bürger, K. (1961a) Bestimmung von Mikrogramm-Mengen Zinn in tierischem
und Pflanzlichem Material nach der Dithiolmethode. Z.
Lebensmittel-Unters. u.-Forschung, 114; 10-13
Cahen, R., Boucard, M., Lalaurie, M. and Lacour, C. (1970) Production
expérimentale d'oedème cérébrale. C.R. Acad. Sci., (Paris) (in press)
Deutsche Forschungsgemeinschaft. (1969) Rückstandsanalytik von
Pflanzenschutzmitteln. Verlag Chemie GmbH, Weinheim/Bergstrasse.
Methode 55-B-1
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Food Inspection Laboratory, Amsterdam. (1962) Pesticide Report KvW 58,
dated August 1962, unpublished
Food Inspection Laboratory, Amsterdam. (1965a) Pesticide Report KvW
112, dated February 1965, unpublished
Food Inspection Laboratory, Amsterdam. (1965b) Pesticide Report KvW
115, dated May 1965, unpublished
Gaines, Th.B. and Kimbrough, R.D. (1968) Toxicity of fentin hydroxide.
Toxicol. appl. Pharmacol., 12:397-403
Gembloux Centre de Recherches de Phytopharmacie, Gembloux. (1968)
Report No. 34/68, dated June 1968
Hardon, H.J., Besemer, A.F.H. et al. (1962) Feldversuche mit
Triphenylzinn. Dtsch Lebensmitl. Rdsch, 58: 349-352
Heath, D.F. (1963) Some problems in the determination of residues in
plants and mammals in "Radiation and Radioisotopes applied to Insects
of Agricultural Importance", IAEA, Vienna, p. 185-193
Hedges (1961) Letter of the Tin Research Institute, dated 3-8-1961
with attached report
Herok, J. and Götte, H. (1961) The radiometrical estimation of the
distribution and excretion of (113Sn) triphenyltin acetate in
ruminants. Symp. radioisotopes in animal biology, Mexico City,
p.177-188
Herok, J. and Götte, H. (1963) Radiometrische Untersuchungen über das
Verhalten von Triphenylzinnacetat in Pflanze und Tier. Internat.J.
Appl. Radiation and Isotopes, 14: 461-479
Herok, J. and Götte, H. (1964) Radiometrische Stoffwechselbilanz von
Triphenylzinnazetat beim Milchschaf. Zbl. Vet. Med. A 11, 20-28
Hoechst Unpublished report (1961) Ing. Rei/kn(A), dated 22-8-1961
with attachment
Hoechst Unpublished report (1963) Dr.Cz/Bi 3543, dated 15-3-1963
Hoechst Unpublished report (1964) Dr.Cz/bi, dated 2-11-1964
Hoechst Unpublished report (1965a) Dr.Cz/bi, dated 17-9-1965
Hoechst Unpublished report (1965b) Hk/da-4565 b, dated 11-11-1965
Hoechst Unpublished report (1966a) 1/67 1 t/m 4
Hoechst Unpublished report (1966b) 94/66 1 t/m 4
Hoechst Unpublished report (1966c) 94/66 1 t/m 4
Hoechst Unpublished report (1966d) 62/661-2. 70/66 3, 82/66 4
Hoechst Unpublished report (1966e) 94/66 2 t/m 4
Hoechst Unpublished report (1966f) 95/66 1-2
Hoechst Unpublished report (1966g) 83/66 2 t/m 4
Hoechst Unpublished report (1966h) 61/66 3, 69/66 3, 83/66 41 94/66 5
Hoechst Unpublished report (1967) 84,90/66, dated 3-5-1967
Hoechst Unpublished report (1968a) A1/67 IV, dated 6-6-1968
Hoechst Unpublished report (1968b) 150/67, dated 29-5-1968
Hoechst Unpublished report (1968c) 151/67, dated 29-5-1968
Hoechst Unpublished report (1968d) 152/67, dated 29-5-1968
Hoechst Unpublished report (1968e) 160/67, dated 5-7-1968
Hoechst Unpublished report (1968f) 161/67, dated 5-7-1968
Hoechst Unpublished report (1968g) A1/67 V, dated 6-6-1968
Hoechst Unpublished report (1968h) 153/67, dated 29-5-1968
Hoechst Unpublished report (1968i) 154/67, dated 29-5-1968
Hoechst Unpublished report (1968j) 155/67, dated 29-5-1968
Hoechst Unpublished report (1968k) 1962/67, dated 5-7-1968
Hoechst Unpublished report (1968l) 163/67, dated 5-7-1968
Hoechst Unpublished report (1968m) 241/67, dated 5-3-1968
Hoechst Unpublished report (1968n) 240/67, dated 5-3-1968
Hoechst Unpublished report (1968o) 17/67, dated 1-4-1968
Hoechst Unpublished report (1968p) 16/67, dated 1-4-1968
Hoechst Unpublished report (1968q) 47/68, 99/68, 100 t/m 102/68
Hoechst Unpublished report (1969a) I-14-68, 1 zu 7/8 + 14/68, dated
7-8-1969
Hoechst Unpublished report (1969b) I-13-68, I zu 13-68, dated
20-6-1969
Hoechst Unpublished report (1969c) IV-9-68, IV zu 7-11/68, dated
7-11-1969
Hoechst Unpublished report (1969d) IV-7-68, IV zu 7-11/68, dated
7-11-1969
Klimmer, O.R. (1964) Toxikologische Untersuchungen mit
Triphenylzinnacetat. Zbl. Vet. Med., 11:29-39
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmitt-Rdsch. 7: 190-193
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmittel-Rdsch., 56: 190-193
Magee, P.N., Stoner, H.B. and Barnes, J.M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Path. Bact., 73: 107-124
Malát, M. (1962) Photometrische Zinnbestimmung mit
Brenzcatechinviolett in Anwesenheit von Gelatine; Colorimetrische
Studien (III). Anal. Chem., 187: 404-409
Nat. Inst. Public Health. (1960) Unpublished report nr. FT/17/60,
dated 23-4-1960
Newton, D.W. and Hays, R.L. (1968) Histological studies of ovaries in
rats treated with hydroxyurea, tri-phenyltin acetate and triphenyl
chloride. J. econ. Entomol., 61: 1668-1669
Pate, B.D. and Hays, R.L. (1968) Histological studies of testes in
rats treated with certain insect chemosterilants. J. econ. Entomol.,
61: 32-34
Philips Duphar Unpublished report (1963a) 56655/2A/63
Philips Duphar Unpublished report (1963b) 56655/38/63
Philips Duphar Unpublished report (1964a) 56646/5/64
Philips Duphar Unpublished report (1964b) 56655/34/63
Philips Duphar Unpublished report (1965) 56655/64/65
Philips Duphar Unpublished report (1966a) 56655/15/66
Philips Duphar Unpublished report (1966b) 56655/47A/66
Philips Duphar Unpublished report (1966c) 56655/54/66
Philips Duphar Unpublished report (1966d) 56655/61/66
Philips Duphar Unpublished report (1967) 56655/2/67
Philips Duphar Unpublished report (1968a) 56655/14/68
Philips Duphar Unpublished report (1968b) 56655/17/68
Philips Duphar Unpublished report (1970) 566461/1/70
Ross, W.J. and White, J.C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33:421-427
Scholz, D. (1963) Toxikologische Prüfung von Triphenylzinnchlorid und
Hoechst 2840-Spritzpulver. Unpublished report from Hoechst A.G., 18
December 1963
Scholz, D. and Baeder, M. (1968) Berichte über ein chronische
Toxizitätsprüfung von Triphenylzinnacetat per os an Ratten (4 Monate).
Unpublished report from Hoechst A.G.
Schulz, D. and Baeder, O. (1970) Berichte über fortpflanzungsversuche
mit triphenylzinnacetat an Ratten (Einflues auf Fertilitat,
Gravidität, postnatale Entvicklung). Unpublished report from Hoechst
AG
Scholz, D. and Brunk. (1968a) Chronische oral toxizitätsprufung von
Triphenylzinnacetat (Brestan-Wirkstoff) 120 Tage-Versuch an Hunden.
Unpublished report from Hoechst A.G.
Scholz, D. and Brunk. (1968b) Chronische oral toxizitätsprafung von
Brestan-Wirkstoff 2-Jahres-Versuch an Hunden. Unpublished report from
Hoechst A.G.
Scholz, D. and Weigand. (1968) Triphenylzinnacetat.
Wirkstoff-Technische. 4-Monate-Futterungsversuch an Meerschweinchen.
Unpublished report from Hoechst A.G.
Stoner, H.B. (1966) Toxicity of triphenyltin. Brit. J. industr. Med.,
23: 222-229
Stoner, H.B. and Heath, D.F. (1967) The cummulative action of
triphenyltin. Fd. Cosmet. Toxicol., 5: 285-286
Syrowatka, T. (1969) Investigation of the action of organotin
compounds on the oxidative phosphorylation and permeability of
membrane of rat liver mitochondria Roczn. Zak. Hig. (Warsz.), 20:
717-725
Syrowatka, T. (1970) Influence of fungicides on cellular energetic
processes. Roczn. Zak. Hig. (Warsz.), 21: 105-115
Tauberger, G. (1963) Tierexperimentelle Analyse der
Triphenylzinnwirkung nach intravenöser Injektion. Med. exp. (Basel),
9: 393-399
Thompson-Hayward Unpublished report (1965a) R-542
Thompson-Hayward Unpublished report (1965b) R-568
Thompson-Hayward Unpublished report (1967) R-650
Thompson-Hayward Unpublished report (1969) R-755
Til, H.P. and Feron, V.J. (1965) Triphenyltinhydroxide range-finding
test with dogs. Unpublished TNO report submitted by Philips-Suphar,
No. R-1994
Til, H.P., Feron, V.J. and de Groot, A.P. (1967) Reproduction study
with triphenyltinhydroxide in three generations of rats. Unpublished
TNO report submitted by Philips-Duphar, No. 2476
Til, H.P. and Feron, V.J. (1968) Chronic (two-year) toxicity study
with triphenyltinhydroxide (TPTH) in beagle dogs. Unpublished TNO
report submitted by Philips-Duphar, No. 2717
Til, H.P., Feron, V.J. and der Groot, A.P. (1968) Observations on a
possible effect of TPTH on testicular development in rats. Unpublished
TNO report No. R 2620 submitted by Philips-Duphar.
Til, H.P., Feron, V.J. and de Groot, A.P. (1970) Chronic toxicity
study with triphenyltinhydroxide in rats for two years. Unpublished
TNO report submitted by Philips-Duphar, No. R-3138
Ueda, K., Iijima, K. and Yoshida, S. (1961) Acute oral toxicity of
organotin compounds for mice. Unpublished report from Tokyo Dental
College submitted to Sankyo Chemical Co.
Van Esch, G.J. and Arnoldussen, A.M. (1962) Range-finding test as to
the toxicity of triphenyltinhydroxide during 18 days. Unpublished
report of the National Institute of Public Health, Utrecht, No. Tox
39/62
Verschuuren, H.G., Kroes, R. and van Esch, G.J. (1965) Semi-chronic
investigation as to the toxicity of triphenyltinhydroxide in guinea
pigs. Unpublished report of the National Institute of Public Health,
Utrecht, No. Tox. 33/65
Verschuuren, H.G., Kroes, R. Vink, H.H. and van Esch, G.J. (1966)
Short-term toxicity studies with triphenyltin compounds in rats and
guinea-pigs. Fd. Cosmet. Toxicol., 4: 35-45
Verschuuren, H.G., Ruitenberg, E.J., Peetoom, F., Helleman, P.W. and
van Esch, G.J. (1970) Influence of triphenyltin acetate on lymphatic
tissue and immune responses in guinea pigs. Toxicol. appl. Pharmacol.,
16: 400-410
Weigand and Kief, Brestan-Wirkstoff (1965) 96 percent ig =
Triphenylzinnacetate 96 percent ig Chronischer Futterungsversuch.
Meerschweinchen. Unpublished report from Hoechst A.G., 25 May 1965
Wolf, C., Fancher, O.E. and Calandra, J.C. (1966) 90 day sub-acute
oral toxicity of triphenyltin hydroxide - albino rats. Unpublished
report from Industrial Bio-test Laboratories Inc., submitted to
Thompson-Hayward Chemical Co.
PART II
LIST OF INDUSTRIAL REPORTS ON ANALYTICAL METHODS
Philips Duphar L3-52-16. Clean-up for the residue determination
of triphenyltin compounds in vegetable
matter.
Philips Duphar L3-52-18. Clean-up for the residue determination
of triphenyltin compounds in potatoes.
Philips Duphar L3-52-19. Absorptiometric determination of tin in
cleaned-up residues.
Philips Duphar L3-52-21. Clean-up for the residue determination
of fentin hydroxide in rice.
Philips Duphar L3-52-23. Clean-up for the residue determination
of triphenyltin compounds in tomatoes,
carrots, onions and butter-beans.
Philips Duphar L3-52-25. Clean-up for the residue determination
of triphenyltin compounds in
sugar-beets.
Philips Duphar L3-52-26. Clean-up for the residue determination
of triphenyltin compounds in celery and
sugar-beets.
Philips Duphar L3-52-29. Clean-up for the residue determination
of triphenyltin compounds in roasted
coffee-beans.
Farbwerke Hoechst. Die Bestimmung kleiner Mengen von
1966 Triphenylzinn in Pflanzenmaterial (Dr.
Gorbach 111/66 dated 1-12-1966).
Thompson-Hayward. Clean-up for the polarographic residue
1965a determination of triphenyltin compounds in
potatoes + polarographic tin determination in
pre-cleaned residues (from report 2-542).
Thompson-Hayward. Clean-up for the polarographic residue
1965b determination of triphenyltin compounds in
pecans + polarographic determination of
inorganic tin (from report R-568).
Thompson-Hayward. Clean-up procedure for the colorimetric
1967 residue determination of triphenyltin
compounds in peanuts (from report R-650).
 Other relevant chemical properties
The pure fentin hydroxide is a white odourless crystalline solid, with
a melting point of 118-120°C. Solubility in water at 20°C is 8 ppm,
and it is moderately soluble in most organic solvents. It is stable at
room temperature, but decomposition occurs on heating above 60°C.
The pure fentin acetate is a white, odourless, crystalline solid, with
a melting point of 124-125°C. Its solubility in water at 20°C is 28
ppm, and it is of low solubility in most organic solvents.
Fentin acetate is stable when stored under normal conditions in dry
air. At 90°C, no decomposition can be detected; at 150°C, 15 percent
decomposes within three hours. In the presence of water, the acetate
is almost completely hydrolysed within eight hours at 20°C.
The pure fentin chloride is a white crystalline solid, with melting
point at 106°C. Its solubility in water is 40 ppm. It is stable when
stored in the dark in dry air. In the presence of water it hydrolyses
into the hydroxide.
Purity
The technical products are usually of the following purities:
fentin hydroxide : 95 percent
fentin acetate : 94 percent
fentin chloride : 94 percent
The impurities consist mainly of diphenyltin compounds and
tetraphenyltin.
This report is based on the data obtained with the fentin compounds
manufactured in Germany (Hoechst) and in Holland (Philips-Duphar). It
is known that fentin compounds are also produced in the United States
and Japan, but no data were available from these sources.
Formulations
The usual formulations are:
fentin hydroxide : 20 percent and 50 percent wp
fentin acetate : 20 percent and 60 percent wp
fentin chloride : 40 percent and 45 percent wp
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Experiments on rats given 113Sn-labelled fentin chloride showed that
the absorbed fentin radical was rapidly distributed through the
tissues of the body, including the brain. The fentin radical was
eliminated relatively slowly and could be detected in the brain 38
days after a single dose (Heath, 1963).
Experimental investigations have been made on guinea pigs fed on a
diet containing 12 ppm fentin acetate. From the survival times and the
total amount consumed, it is evident that, at this dosage level,
fentin compounds are not completely cumulative. The results obtained
and those previously published (Stoner, 1966) are consistent with a
halflife of the fentin radical at the site of action of about 50 days
(Stoner and Heath, 1967).
Information on the distribution of tin after feeding fentin compounds
to farm animals is included in the section entitled "FATE OF
RESIDUES. In animals".
Effect on enzymes and other biochemical parameters
In vitro studies have shown that fentin compounds inhibit oxidative
phosphorylation by isolated liver mitochondria and the adenosine
triphosphatase (ATP) activity of brain microcomes (50 percent
inhibition by concentration of, 1 × 10-6M and 1 × 10-5M,
respectively) (W. N. Aldridge, quoted by Stoner, 1966).
The oxygen consumption of cerebral slices from rats in a moribund
condition due to fentin compounds was within normal limits (J.E.
Cremer, quoted by Stoner, 1966).
The effects of fentin hydroxide on the oxidative phosphorylation and
on permeability of rat liver mitochondria membranes for malate,
citrate and fumarate ions were investigated. In the conditions of the
investigations (concentration of 5 µ M), a strong inhibitory action on
the oxidative phosphorylation in isolated rat liver mitochondria and
deep changes in the selectively permeable mitochondrial membrane were
observed. Malate and citrate were able to penetrate mitochondria
without participation of any carrier. The altered membrane formed no
barrier for fumarate. No similar effects were observed in the case of
n-octyltin derivatives (Syrowatka, 1969).
In a further investigation, it was shown that the mitochondrial
membrane which, when intact, has a strictly limited permeability for
K+, Na+, Cl-, malate, citrate and fumarate, became permeable in a
high degree to these ions under the influence of fentin hydroxide.
Electron microscopic pictures confirmed these results (Syrowatka,
1970).
TOXICOLOGICAL STUDIES
Special studies on influence on lymphatic tissue and immune
responses
Guinea pig (fentin acetate)
These investigations were aimed at studying the influence of fentin
acetate on lymphatic tissue and immune responses in guinea pigs which
were previously (see below) shown to be much more sensitive than rats
to the decreasing effect of the compound on the lymphopoiesis. Female
guinea pigs were selected because they are more affected than males.
In a first experiment, two groups of ten female guinea pigs received
15 ppm of fentin acetate in their diet, for 49 or 77 days. Each group
was accompanied by a control group with the same number of animals.
After 77 days, a decrease in plasma cells was observed in the spleen
and the cervical, mesenteric, axillary and popliteal lymph nodes. In a
second experiment, the same dose of the compound was given in the diet
for 104 days. Two groups of ten female guinea pigs received fentin
acetate and two groups control diet only. At 21 and 7 days before the
end of the fentin acetate feeding period, half of the animals received
an injection of tetanus toxoid. Some animals were allowed to recover
for 14 days after the 104-day experimental period. Immunochemically
and serologically, a decreased immunologic response was observed in
the group fed fentin acetate after tetanus toxoid stimulation and no
signs of recovery were seen after 14 days. With the aid of the
fluorescent antibody technique, a decrease in immunologically active
cells in the popliteal lymph nodes of the animals fed fentin acetate
could be detected in the second experiment. In the other lymphatic
tissues, no differences were observed (Verschuuren at al., 1970).
Special studies on reproduction
Rat (fentin acetate)
An interim report comprising two generations of a three-generation
reproduction study in rats fed 0, 25, 80 and 250 ppm of fentin acetate
in the diet is available. No histological examination was performed,
but no abnormalities were found by macroscopic examination at the 25
ppm level (Scholz and Baeder, 1970).
Rat (fentin acetate and chloride)
Groups each of 20 sexually mature male rats (aged 35 to 40 days) were
given orally in their food, over a period of 19 days, 20 mg/kg
body-weight per day of either fentin acetate or fentin chloride. The
animals were killed on the twentieth day, and histological
preparations of the testes were made. Both compounds produced several
degenerative changes of the testicular tissue, including a decrease in
the number of cell layers per seminiferous tubule, a decrease in
tubule diameter and overall testicular size, a depletion of the more
advanced cell forms from the tubules and a closing of tubule lumina.
Effects were more pronounced in animals treated with fentin acetate
(Pate and Hays, 1968).
Groups of 15 sexually mature female rats (aged 42 to 46 days) were
given orally 20 mg/kg body-weight per day of either fentin acetate or
fentin chloride. Three animals from each group were killed after 4, 9,
14, 19 and 24 days of treatment, and histological preparations of the
ovaries were made and thoroughly examined. Both fentin compounds
produced significant changes in the ovarian tissue. Among these
changes were a decreased number of mature follicles, an increased
incidence of atresia in early follicle growth and a pronounced
decrease in the number of corpora lutea present. This last effect was
regarded as a decrease in ovulation and, thus, a decreased fertility.
This effect, as well as the others listed above, was present on or by
the first five-day sample period (Newton and Hays, 1968).
Rat (fentin hydroxide)
This experiment was started with 50 male and 100 female newly weaned
rats. The animals were divided at random over five groups and were fed
diets containing 0, 0.5, 1, 2 or 5 ppm of fentin hydroxide. Fertility
of males and females, number of young born per litter, vitality,
body-weight and mortality of the young at birth and during the
lactation period were not affected by feeding fentin hydroxide at
levels up to 5 ppm in any of the three successive generations.
Ninety-day feeding studies with F1b and F2b generation rats did not
reveal adverse effects with respect to growth, food consumption, food
efficiency and haematology. Increased relative weights of liver,
kidney and spleen were occasionally noted, but were not associated
with histological abnormalities. Testicle weight was slightly
decreased at the 5 ppm level in F1b generation rats and at the 2 and
5 ppm levels in F2b generation rats at week 13 after weaning.
Macroscopically, no changes attributable to the ingestion of fentin
hydroxide were observed in any of the successive generations, except
for small-sized testicles at feeding levels of 1 ppm and above in
generation F3b at week 2 after weaning. Microscopic examination
revealed a significant difference between treated and control rats
only in the maturing testicle. This difference found expression in the
stage of maturation of the germinal epithelium, which was less
advanced in the small-sized testicles of the rats fed fentin hydroxide
(Til et al., 1967).
Four groups of ten weanling male rats were each fed 0, 50, 100 or 200
ppm of fentin hydroxide in the diet over a period of 276 days and were
mated at intervals to nontreated females. Observations indicate that,
in comparison to the controls, there was a drop of about 27 and 18
percent, respectively, in the total number of offspring in the groups
fed 200 and 100 ppm after 64 days of exposure. However, when the males
were subsequently mated, their fertility improved and, by day 113, was
comparable to that of the controls. Leucocyte and differential counts
and haemoglobin determinations performed one week prior to the time of
sacrifice did not differ from the controls. Gross abnormalities
related to fentin hydroxide were not observed in the offspring. In the
group fed 200 ppm, one rat died and two were sacrificed when moribund.
The cause of death in this group was generalized internal haemorrhage.
In the animals that were sacrificed, no gross or microscopic
abnormalities attributable to fentin hydroxide were observed. At the
100 and 200 ppm levels, the food consumption of the diet containing
fentin hydroxide was significantly lower during the first week of the
experiment and then improved for all dosage levels. By week 7, the
animals had adapted to these concentrations of the chemical, and the
food consumption was the same as in the controls. The food intake and
weight gain were not affected in rats fed 50 ppm. Gross and
microscopic examination of rats fed fentin hydroxide at all levels did
not reveal changes in any organ that could be related to the compound
(Gaines and Kimbrough, 1968).
Three experiments on weanling male rats (10 or 20 per group), fed from
0 to 25 ppm of fentin hydroxide in two or four weeks, were conducted
to determine testicular development. Microscopic examination after two
and four weeks failed to reveal any differences between the various
test groups and the control groups (Til et al., 1968).
Acute toxicity
Symptoms of poisoning were sluggishness, unsteadiness, moderate
diarrhoea, anorexia, bloody stain around the nose and eyes and
wheezing (Gaines and Kimbrough, 1968).
In all species, the main action of these organo-tin compounds is
thought to be on the central nervous system, but in contrast to
triethyltin compounds, cerebral oedema did not occur (Cahen et al.,
1970).
Intravenous administration of fentin acetate to cats at a dose of 1
mg/kg body-weight produced an increase in blood pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of 1
to 2 mg/kg at 20 to 60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood pressure was also
found. Death took place after 6 to 14 mg/kg of fentin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Dog (fentin acetate)
Groups of dogs (four of each sex) were fed 0, 1, 5 and 20 ppm of
fentin acetate in the diet for 120 days. A level of 20 ppm resulted in
decreased food consumption, and in half of the animals, also in
decreased body-weight. No macroscopic or microscopic abnormalities
were found (Scholz and Brunk, 1968a).
Groups of six dogs, three from each sex, were fed over a period of two
years, 0, 0.5, 1 or 5 ppm of fentin acetate. Attention was paid to
behaviour, food intake, weight gain, blood picture, urine composition,
gross examination and histological characteristics of the main organs
and tissues. Except for the higher level, where a decrease in weight
gain, which might be related to a reduction of food intake, was noted
in comparison to the controls, no significant abnormalities, even in
blood picture, were observed (Scholz and Brunk, 1968b).
Dog (fentin hydroxide)
Groups of dogs consisting of three males and three females were fed 0,
10, 25 and 37 ppm of fentin hydroxide in the diet for 90 days.
TABLE I
Acute toxicities to various species: summary
Animal Fentin LD50 (mg/kg
Compound Route body-weight) Reference
Mouse (M) acetate oral 81 Ueda et al., 1961
Mouse (M) chloride oral 80 Ueda et al., 1961
Mouse (M) hydroxide oral 245 Philips-Duphar, 1964a
Mouse (F) hydroxide oral 209 Philips-Duphar, 1964a
Mouse (M) acetate ip 7.9 Stoner, 1966
Rat (M) acetate oral 136 Klimmer, 1964
Rat (F) acetate oral 491 Stoner, 1966
Rat (F) chloride oral 135 Scholz, 1963
Rat (M) hydroxide oral 240 Gaines & Kimbrough, 1968
Rat (F) hydroxide oral 360 Gaines & Kimbrough, 1968
Rat (M) acetate ip 8.5 Stoner, 1966
Rat (F) acetate ip 11.9 Stoner, 1966
Guinea pig (M) acetate oral 21 Klimmer, 1964
Guinea pig (M) hydroxide oral 27.1 Philips-Duphar, 1964a
Guinea pig (F) hydroxide oral 31.1 Philips-Duphar, 1964a
Guinea pig (M) acetate ip 3.7 Stoner, 1966
Rabbit (M) acetate oral 40 Klimmer, 1964
Rabbit (M) acetate ip 10 Klimmer, 1964
Lethargy and a darkening of the hair occurred in all groups, depending
in degree on the dosage, and an increased mortality rate occurred in
the two higher groups (Baran et al., 1966a).
Groups of dogs (three of each sex) were fed 0, 10, 25 and 37 ppm of
fentin hydroxide in the diet for 100 days. Apart from darkening of the
hair which occurred in all test groups, the only differences from the
controls were lethargy in the 37 ppm dogs and an increased quantity of
tin in the livers of five of six dogs at 37 ppm and two of six dogs at
25 ppm (Baran et al., 1966b).
Six dogs were fed, over a period of 16 weeks, a diet containing,
during the first eight weeks, 25 ppm and, afterwards, 50 ppm of fentin
hydroxide. Symptoms of toxicity, consisting principally in inhibition
of growth, alterations in the blood picture (decrease of the number of
leucocytes and erythrocytes) and in the histology of the liver, were
observed (Til and Feron, 1965).
Following this preliminary investigation, 34 dogs were fed over a
period of two years a diet containing respectively 0, 0.5, 2.5, 5 and
10 ppm. Growth, food consumption, symptomatology, blood picture, urine
analysis, liver and kidney functional exploration, serum protein
electrophoresis, water content of brain, organ weights and gross and
microscopic pathology were used as criteria to disclose signs of
injury. General appearance, behaviour, growth, food consumption,
haematological and biochemical values, urine analyses and liver and
kidney functional tests were not affected at any dietary level of the
compound. At the two highest feeding levels of 5 and 10 ppm, the
relative weights of liver and kidney and the water content of the
brain were increased in comparison to the controls, without being
associated with histological changes. Gross and microscopic
examination of organs and tissues did not reveal any abnormality which
could be attributed to the ingestion of fentin hydroxide (Til and
Feron, 1968).
Guinea pig (fentin acetate)
In one experiment, 50 ppm of fentin acetate in the diet of guinea pigs
led to the death of all of nine animals in 17 to 31 days. In other
experiments, only a group of five guinea pigs given 1 ppm for 392 days
did not show an increased mortality rate. Even at 1 ppm, food intake
was reduced. When death occurred, it was preceded by loss of weight
and generalized weakness (Stoner, 1966). Neither in these experiments,
nor in others (acute and short-term), in which guinea pigs and rats
have been treated with fentin compounds, has a significant increase in
the water content of the central nervous system been found nor the
characteristic histological lesion in the white matter produced by
triethylin compounds (Magee et al., 1957) been seen (Stoner, 1966).
In other experiments, groups of guinea pigs, 10 of each sex, were
given respectively 0, 5, 10, 20 or 50 ppm of fentin acetate for 12
weeks. In all groups, except for the males given 5 ppm, growth
inhibition was obvious.
At the end of the treatment, no animal at 50 ppm was alive; in the
other groups, the survival rates were: 20 ppm, 8 out of 10 males and 8
out of 10 females; 10 ppm, 9 out of 10 males and 10 out of 10 females;
5 ppm, 9 out of 10 males and 10 out of 10 females. In the females on
5, 10 and 20 ppm and males on 10 and 20 ppm, the number of lymphocytes
and leucocytes was significantly decreased. The leuco- and lymphopenia
was accompanied in a number of animals by histological changes in the
lymphopoietic system, e.g., atrophy of the white pulp of the spleen
was found. This change possibly induced a decrease of general
resistance, so that a mycotic infection developed in the animals. At
20 and 50 ppm, a significant increase of brain water was noticed.
Histological findings were not significantly different from those
observed in the controls (Verschuuren et al., 1966).
Groups of guinea pigs, ten of each sex, were fed 0, 1, 5, 10, 50 and
100 ppm of fentin acetate for four months. All animals on 100 ppm and
30 percent of those on 50 ppm died, and in the same groups were found
signs of disturbance of the iron metabolism. A level of 10 ppm gave a
slight reduction in weight gain, as well as some cases of decreased
haemoglobin percentage and total number of erythrocyte. The groups fed
5 ppm and 1 ppm were similar to the controls (Scholz and Weigand,
1968).
Guinea pig (fentin hydroxide)
Groups of three males and three females were given 0, 1, 2.5, 5 or 20
ppm of fentin hydroxide for three weeks. No changes in the growth
rate, the water content, or the histology of the nervous system were
observed (Verschuuren at al., 1965).
Groups of ten males and ten females were fed 0, 2.5, 5, 10, 20 or 50
ppm of fentin hydroxide in their diet for 12 weeks. Growth depression
was observed at the two highest dose levels. All animals in the 50 ppm
group died during the first half, and one each in the ten and 20 ppm
groups died later in the experiment. A decrease in the haemoglobin
content was found in all groups, except for the males in the 2.5 and 5
ppm groups there was no change in the number of erythocytes. A
decrease in the number of leucocytes (except in the males on 10 ppm)
and lymphocytes (except the males on 2.5 and 5 ppm) was found in all
the groups examined. The same changes as with fentin acetate were
found in the lymphopoietic system (see above). A decrease in relative
organ weight was found in the spleen of the females on 10 and 20 ppm
and in the thymus of both sexes and uterus and testes at 20 ppm. No
increase in the water content of the central nervous system was
observed, except in the females at 50 ppm. Apart from the changes in
the lymphopoietic system, the histological findings were not
significantly different from those observed in the controls
(Verschuuren et al., 1966).
See also Special studies on influence on lymphatic tissue and immune
responses.
Rat (fentin acetate)
Groups of 20 to 25 rats were given fentin acetate by stomach tube at
doses equivalent to 5, 10, 25 or 50 ppm for 105 to 170 days. At 50
ppm, 70 percent of the rats died within 49 days. Nervous symptoms, as
well as blood, urinary or histopathological changes, were not observed
at the lower dose levels (Klimmer, 1964).
In other experiments on rats, no deaths occurred during a ten-week
period on 200 ppm of fentin acetate. These rats were then put on a
diet containing 300 ppm, and five out of six rats died after a further
117 to 168 days (Stoner, 1966).
Groups of young rats, ten of each sex, were given respectively 0, 5,
10, 25 or 50 ppm of fentin acetate for 12 weeks. Decrease of food
intake and growth inhibition were recorded at 50 ppm, as well as
growth inhibition in males at 25 ppm. At 10 ppm and above, the number
of leucocytes in the blood was decreased in the male (leucopenia) and,
at 50 ppm, the haemoglobin was reduced. At the highest level there was
a decrease of the organ to heart weight ratio for the pituitary and
pancreas in all animals and uterus and ovary in the females. The same
ratio for the thyroid was decreased in all the females, as well as in
the males at 25 and 50 ppm. Paralysis and pronation of the hind legs,
associated with interstitial oedema of the central nervous system,
which developed in rats treated with triethyltin hydroxide at all
levels, were not observed. The water content of the spinal cord was
slightly increased at 50 ppm. Histological findings were not
significantly different from those observed in controls (Verschuuren
at al., 1966).
Groups of 20 young rats, ten of both sexes, were fed respectively, 0,
15, 40, 100, 250 or 600 ppm of fentin acetate over a period of four
months. Attention was given to behaviour, growth, blood picture, urine
composition, gross examination and histology of the main organs and
tissues. Up to 100 ppm, no abnormalities were observed in comparison
to the controls. Above 100 ppm, a dose-related inhibition of growth
was seen and 600 ppm resulted in the death of 30 percent of the
treated animals. (Scholz and Baeder, 1968).
Rat (fentin chloride)
Groups of ten rats of both sexes were given orally, over a period of
41 days, 28 doses of 0, 6.25, 12.5, 25 or 50 mg/kg body-weight. Apart
from a slight decrease in weight gain of the females at 12.5 mg/kg, no
abnormalities (growth, behaviour, blood picture and urine composition,
gross and histological characteristics of stomach, small intestine,
spleen, liver, kidney and lung) were observed at 6.25 and 12.5 mg/kg.
At higher doses, a dose-related increase in mortality compared to the
controls was observed (Scholz, 1963).
Rat (fentin hydroxide)
Groups of 10 or 20 rats of both sexes were given 0, 5, 20, 50 or 100
ppm of fentin hydroxide for 20 days. Food intake and body growth were
depressed at 20 ppm and above. Death rates were 9 out of 10 at 100
ppm; 9 out of 20 at 50 ppm, and 1 out of 20 at both 20 and 5 ppm (Van
Esch and Arnoldussen, 1962).
Groups of rats, ten of each sex, were fed 0, 1, 3.1, 5, 10 and 31 ppm
of fentin hydroxide in the diet for 90 days. Disregarding effects on
erythrocyte and leucocyte counts as neither dose related nor
chronologic, a no-effect level of 31 ppm was claimed (Wolf et al.,
1966).
Groups of ten rats of each sex were given respectively 0, 5, 10 or 25
ppm of fentin hydroxide in the diet for 12 weeks. The food intake was
comparable to the controls. The females showed growth inhibition after
six weeks, but they recovered in spite of continuing treatment. Growth
in the males was comparable to the controls. In the females, blood
leucocytes were decreased. No significant changes in water content
were found in the nervous tissues. At 25 ppm, decrease of the thyroid
weight was noticed (Verschuuren at al., 1962). In a similar
experiment, in which ten rats of each sex were given 50 ppm of the
same compound, the following features were noticed: decreased food
intake, growth inhibition in both sexes, decrease in weight of the
thyroid, pituitary, uterus, ovary, prostate and pancreas, as well as
decrease of haemoglobin and leucocytes. Paralysis and pronation of the
hindlegs, associated with interstitial oedema of the central nervous
system, which developed in rats treated with triethyltin hydroxide at
all levels, were not observed. The water content increased in the
spinal cord, but not in the brain. (In comparison, the water content
of the spinal cord and brain was increased in animals treated with
levels of 5 and 10 ppm of triethyltinhydroxide). Histological findings
in the animals treated with fentin hydroxide were not significantly
different from those observed in the controls (Verschuuren et al.,
1966).
Groups of four to five week old male rats were fed fentin hydroxide in
the diet at levels of 0, 100, 200 or 400 ppm over a period of 99 days.
The rats fed 400 ppm were apparently repelled, since they ate only
about one third as much food as the controls during the first week.
All the animals in this group died within 7 to 34 days from starvation
and extensive intra-alveolar haemorrhage in the lung. There was
partial or complete testicular atrophy and general size and weight
reduction of the organs. The rats fed 200 ppm gained less weight (231
g) than the controls (302 g). At the end of the study, the total
leucocyte number and the weights of spleen and kidneys were
significantly below those of the controls.
The rats fed 100 ppm appeared normal throughout the study except for a
significant decrease of food intake during the first week of treatment
(Gaines and Kimbrough, 1968).
Additional information on the findings following short-term
administration of fentin compounds to rats is given under Special
studies on reproduction.
Long-term studies
Guinea pig (fentin acetate)
Groups of 20 young guinea pigs, ten from each sex, were fed
respectively 0, 1, 5, 10, 50, 100 and 200 ppm of fentin acetate over a
period of two years. Attention was given to behaviour, food intake,
weight gain, blood picture, urine composition, gross examination and
histological characteristics of the main organs and tissues. At 50 ppm
and above, a dose-related increase in mortality in comparison to the
controls and an inhibition of growth were observed. At 10 ppm and
above, fatty degenerative changes were seen in liver and heart muscle.
At 1 and 5 ppm, no abnormalities, even in the blood picture, were
noted in comparison to the controls (Weigand and Kief, 1965).
Rat (fentin hydroxide)
Groups of 50 newly weaned rats, 25 from each sex, were fed over a
period of two years respectively 0, 0.5, 1, 2, 5 or 10 ppm of fentin
hydroxide. General appearance and behaviour, growth, food intake,
blood sugar and blood urea nigrogen, urine composition and SG-PT,
SG-OT and SAP activity were not adversely affected at any dietary
level of the test substance. Mortality of the females at 10 ppm was
higher than in the controls, although at the termination of the
experiment the difference was not significant. Haematological data
often showed a slight decrease in white blood cells at the highest
feeding level in the first year of the experiment only. This effect
was less often soon at 5 ppm and only once at 2 ppm in the males. The
relative thyroid weight was slightly decreased at 10 ppm in the
females only. Average relative weights of the other organs were very
uniform in all groups. Gross autopsy findings did not show evidence of
significant pathological changes. Microscopic examinations of numerous
organs and tissues, including testes, ovaries and bone marrow, did not
reveal histological alterations significantly different from those
observed in controls (Til et al., 1970).
COMMENTS
The basis for grouping the three fentin compounds for consideration
together is that fentin acetate and fentin chloride are converted in
plants and animals to the same compound, namely fentin hydroxide.
Since the previous toxicological evaluation of the fentin compounds in
1965, a considerable number of short-term and long-term feeding
studies in several species have been reported. The production of
cerebral oedema in rats and guinea pigs did not appear to be
pronounced except at a 50 ppm dose level. However, the reduction of
lymphopoiesis in both species appeared to be of some concern. With
respect to this parameter, the guinea pig appeared to be the most
sensitive species studied. A reduction in lymphocyte count was evident
in females at a dietary level of 2.5 ppm of fentin hydroxide. It was
reported that this effect was not entirely reversible after 14 days'
withdrawal from a test diet of 15 ppm of fentin acetate. In a two-year
feeding study in rats a no-effect level of 2 ppm relative to decrease
in white blood cells was evident.
In a multigeneration rat reproduction study, reduction in testicular
size was evident at 5 ppm in the F1b generation, at 2 ppm and above
in the F2b generation and at 1 ppm in the F3b generation. In a
second species, the dog, histological examination revealed no effect
on any organ at feeding levels of up to 10 ppm for two years.
There are no reports of observations of men exposed to this compound.
Bearing the abovementioned observations in mind, the Committee
considered the data adequate to establish an acceptable daily intake
for the fentin compounds.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.1 mg/kg body-weight
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 to 0.0005 mg/kg body-weight, applicable to fentin hydroxide,
fentin acetate and fentin chloride and to the sum of each if more
than one is involved
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
All the three fentin compounds are nonsystemic fungicides used in many
European countries, the United States, and several South American,
African and Asiatic countries. On rice they are used against algae.
Pre-harvest treatments
Fentin compounds are recommended to control the following pests:
Potatoes: Phytophthora infestans de Bary
of the foliage and especially
the tubers, and
Alternaria solani J. u.Gr.
Sugar beet: Cercospora beticola Sacc.
Celery and celeriac: Septoria apii Chester
Carrots: Alternaria porri f. dauci Neerg.
Pecans: Fusicladium effusum, Gnomonia spp.,
Mycosphaerella caryigena Demaree & Cole
Rice: various algae
Groundnuts: Cercospora spp.
Coffee: Colletotrichum coffeanum Noack
Cocoa: Phytophthora palmivora Butl.
The usual rates of application are between 0.15 and 0.40 kg a.i/ha in
600 to 1 000 litres of water. On coffee, the rate is 0.2 to 0.6 kg
a.i/ha in more than 1 000 litres of water. For algae control on rice,
the dosage is up to 1 kg a.i/ha in about 1 000 litres of water.
Recommended rates of application and intervals applied between last
treatment and harvest are as follows:
Potatoes: 0.18 to 0.4 kg a.i./ha. 1-10 times
(generally between 2 and 5 times).
Safety period (in Germany) 7 days.
Sugar beets: 0.18 to 0.4 kg a.i./ha. 1-3 treatments.
Celery celeriac, 0.2 to 0.32 kg a.i./ha in 600-1 000 1
carrot: water, 1-7 treatments. (In Germany
safety period for celery of 3 weeks)
Pecans: 0.22 to 0.54 kg a.i./ha, 2 to 8
treatments.
Rice: 0.75 to 1 kg a.i./ha for algae control.
Peanuts, coffee, 0.2 to 0.6 kg a.i./ha.
cocoa:
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Fentin compounds are used on ornamentals, willow and poplar. Trials on
hops are in process.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials are given in Table II.
TABLE II
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Potatoes Holland 0.20-0.46 4-7 2-98 tubers 0.01 23 17 0.04
" " 0.08-0.38 6-7 98-110 " 0.06 14 12 0.11
" England 0.22-0.56 5 21-43 " 0.01 5 5
" " 0.28 1 30-35 " 0.03 6 6
" Canada 0.15-0.25 ?-12 ?-169 " 0.03 7 7
" U.S.A. 0.17-0.34 5-11 13-15 " 0.03 17 17
" Belgium 0.30-0.36 5 22 " 0.01 1 1
Sugar beets Holland 0.30 5 43-57 beets 0.03 4 0.18
" " 0.30 5 43-57 leaves 0.06 4 1.36
" Germany 0.30 1-4 46-64 beets 0.03 5 2 0.18
" " 0.30 1-4 46-64 leaves 0.06 5 2 0.81
" Italy 0.30-0.40 ? 31 beets 0.03 10 1 0.20
" Switzerl. 0.30-0.40 2 44 leaves 0.02 4 2.45
" U.S.A. 0.22-0.34 3-6 14-45 beets 0.03 17 8 0.09
Carrots Israel 0.25-0.30 5-7 12-28 carrot 0.05 4 3 0.07
Pecans U.S.A. 0.22-0.54 2-8 46-256 nuts 0.03 17 17
Rice Italy 04-1.2 ppm 1 90 hulled rice 0.08 5 5
in irrigation
water
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Ground nuts U.S.A. 0.22-0.34 3-7 9-72 nuts 0.01 14 10 0.03
" U.S.A. 0.22-0.34 3-7 9-72 hulls 0.01 12 1 0.38
" U.S.A. 0.34 3-7 9 vines 0.01 3 2.5
" Israel 0.30 4 5-23 nuts 0.01 4 4
" " 0.30 4 5-23 vines 0.16 4 2.6
Coffee Kenya 0.22-0.60 ? 35 roasted 0.03 3 3
beans
Residues of fentin acetate
Potatoes Germany 0.216-0.324 1-5 7-21 tubers 0.03-0.04 12 8 0.07
" Ireland 0.3 3 not stated " 0.03 2 2
Sugar beets Germany 0.216-0.324 6 38 beets 0.1 6 6
Celeriac " 0.3 1-2 21-45 tubers not stated 3 3
" " 0.324 1 21-35 " 0.1 3 3
" Belgium not stated 3 21-28 stems not stated 6 6
" Germany 0.3 1-2 21-45 leaves not stated 13 2 0.19
" " 0.324 1 21-35 " 0.1 3 3
" Holland 0.24-0.30 1-6 21-28 " 0.1 2 1.0
Celeriac Holland 0.27 6 21-28 leaves 0.01 2 0.30
" Belgium not stated 3 21-28 " not stated 6 5 0.05
Carrots Germany 0.216-0.324 1-3 7-28 1/ 0.03 20 17 0.15
" " 0.216-0.324 1-3 7-28 2/ 0.02 6 5 0.06
" " 0.216-0.324 1-3 7-28 3/ 0.02 6 5 0.02
" " 0.216-0.324 1-3 7-28 4/ 0.02 6 5 0.02
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin acetate
Rice Italy 04-1.2ppm 1 appr.go hulled 0.08 3 3
in irrigation rice
water
Cocoa Nigeria not stated not stated not stated not stated 0.1 8 7 0.1
Residues of fentin chloride
Potatoes Germany 0.22-0.27 1-3 7-14 tubers 0.04 8 6 0.05
Sugar beets " 0.18-0.27 6 38 beets 0.10 4 4
Celeriac " 0.27 1 21-35 tubers 0.1 3 2 0.1
Carrots " 0.20 3 10-31 washed 0.02 4 2 0.08
carrots
1/ washed unpeeled carrots
2/ peeled/scraped carrots
3/ peels, calculated on total weight carrot
4/ scrapings, calculated on total weight carrot
The residue date of fentin hydroxide are from treatments made in the
Netherlands, Germany, Belgium, Canada, England, Italy, Israel,
Switzerland, U.S.A. and Kenya (Philips Duphar, 1963a, 1964b, 1965,
1966a-d, 1968a,b; Thompson-Hayward, 1965a,b, 1967, 1969).
The residue data of fentin acetate are from experiments made in
Germany, the Netherlands, Belgium, Ireland, Italy, Kenya and Nigeria
(Hoechst, 1963, 1964, 1965a, 1966a-d, 1967, 1968a-f,m,o,q, 1969c,d;
Hardon et al., 1962; Gembloux, 1968; Food Inspection, 1965a-b;
Kröller, 1960; Nat. Inst. Publ. Health, 1960; Philips Duphar, 1967).
The residue date of fentin chloride are from experiments made in
Germany (Hoechst, 1966e-h, 1968g-l,n,p, 1969a,b).
FATE OF RESIDUES
General comments
When triphenyltin acetate was decomposed in solution, the following
products were detected: phenol, acetone, hydrogen peroxide, degraded
organotin and benzene. There is probably also some organic or
organotin peroxide present (Hedges, 1961).
In the presence of light and humidity, fentin acetate and fentin
chloride are transformed easily into fentin hydroxide, which
metabolizes into dephenyltin and monophenyltin. Monophenyltin breaks
down very quickly into Sn salts (Kröller, 1960; Hoechst, 1961; Hedges,
1961).
At heating, triphenyltin hydroxide readily decomposes (see other
relevant chemical properties).
In animals
After ingestion of fentin compounds by cows or sheep, the compound is
largely excreted with the faeces (Herok and Götte, 1961; Brügemann et
al., 1964). Three sheep given 10 mg daily of 113Sn-labelled fentin
acetate for 25 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration: 0.0017 ppm) during the treatment, but 17 days after
withdrawal of the compound, its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than fentin acetate. During the treatment, the concentration of
113Sn in the blood and in the urine were 2.9µg/1 and 7.5µg/1,
respectively. In the sheep killed at 28 days, liver, kidney, lung,
pancreas, gall bladder and brain contained a greater amount of 113Sn
than other organs. After 218 days, the amount of 113Sn found in the
liver was still greater than in other organs (Herok and Götte, 1961).
Organs of one sheep, sacrificed at the end of the feeding period with
the 113Sn triphenyltin acetate, showed the following residues
(comprising triphenyltin and all degradation products including
inorganic tin):
Organ ppm 113Sn
liver 0.9
intestinal fat 0.01
depot fat 0.01
muscles 0.02
In plants
Studies on the effect of tin compounds on plants have been made or are
in progress in several laboratories. It has been shown that in the
presence of light and air, several byproducts of fentin acetate can be
formed, such as diphenyltin and finally insoluble tin (Kröller, 1960).
The tin content from the leaves can be transferred to the ground.
However, when 113Sn-labelled fentin acetate or hydroxide was applied
to potato foliage, no translocation of 113Sn from leaves to tubers
could be demonstrated above the limit of determination of 0.0001 ppm.
Tin could be detected in the haulm, where the percentage of the
original compound decreased for 20 days (Philips Duphar, 1964b). It
has also been shown that fentin compounds have no systemic action when
applied to celeriac and sugar beet (Herok and Götte, 1963).
Furthermore, there is no uptake of phenyltin from the soil
(Philips-Duphar, 1970; Hoechst, 1965b). This means that residues on
food or feed can only result from direct contact with the pesticide or
from contamination during harvest, transport or processing: potatoes,
carrots, sugar beets, groundnuts, coffee, cocoa, pecans and rice are
examples.
With a few crops, the plant parts carrying the residues may be used as
food or feed. Those feedstuffs are sugar beet tops and leaves,
groundnut vines and rice hulls. As reported in "Fate of residues in
animals" fentin itself and its degradation products in feed are
transferred only in very small amounts into milk and meat (Brüggemann
et al; 1964; Herok and Götte, 1963, 1964). The only crop from which
the leaves are used for human consumption is celery. This crop forms
generally only a small portion of the human diet.
In soil
Fentin compounds are degraded in soil almost completely within one
year (Hoechst, 1967).
Evidence of residues in food in commerce or at consumption
No experimental data are available on the fentin residues in food
moving in commerce or in the diets. Theoretically, it can be
postulated, however, that the chance fentin compounds would reach the
consumer is very limited. This is mainly due to the fact that the
residues of fentin compounds or their metabolites occur only on the
surfaces of those plant parts which have been subjects of direct
treatments, or of other types of contamination, and are therefore
easily removed by peeling, washing, shelling, etc., and are readily
broken down in thermal processes.
Peeling of potatoes, as well as cooking or other types of processing,
are apt to remove the residues. After sugar beets are processed into
sugar, no organic tin is found in sugar (Thompson and Hayward, 1969).
Carrots generally are washed, scraped, peeled or cooked before
consumption, which processes reduce the residues. The removal of
shells of pecans, groundnuts and cocoa removes also the residues. The
coffee beans are protected from contamination by the fruit peel and
flesh, by fermentation and by roasting. Rice is protected by husk,
which is removed by milling before the rice is cooked or baked for
consumption. Celery and celeriac if used uncooked for human
consumption may contain fentin residues at the time of consumption.
The portion of these crops is, however, generally very small in human
diets.
The foliage of treated crops may contain fentin residues which have to
be regarded as contaminants of animal feed. During silage the residues
are, however, found to be degraded and the risk of contamination of
animal products is avoided.
METHODS OF RESIDUE ANALYSIS
Fentin residues can be determined with two completely equivalent
methods which give the same results in identical samples. In the
method of Philips-Duphar, the triphenyltin compounds are determined
absorptiometrically in the extract with pyrocatechol-violet at 630 mm.
The detection limit of the method is between 0.01 and 0.12 ppm (Ross
and White, 1961; Malát, 1962). In the method of Hoechst, the
triphenyltin compound is converted into the tetrabromo compound,
separated from traces of lead and then determined polarographically.
The detection limit of the method is between 0.012 and 0.26 ppm (Bock
and Gorbach, 1958; Bock et al., 1958; Bürger, 1961a,b; Deutsche
Forschungsgemeinschaft, 1969).
Because blank values are varying according to crop variety and origin,
the detection limit is not a constant figure. None of the methods can
distinguish between the three fentin compounds; they give the sum of
these compounds. Di- and monophenyltin are not detected by these
methods. Although methods to distinguish between tri-, di- and
monophenyltin are available, some difficulties are encountered in the
presence of organic matter. Work on that subject is going on. For the
evaluation of residues in the abovementioned crops - with exception of
celery - the determination of degradation products of the three fentin
compounds does not seem relevant (see 113Sn-trials).
There are a number of industrial reports on the analytical methods
(see References).
APPRAISAL
Fentin compounds evaluated by the Working Party were fentin acetate
(triphenyltin acetate), fentin chloride (triphenyltin chloride) and
fentin hydroxide (triphenyltin hydroxide). They are used on potato,
sugar beet, celery, celeriac, leek, carrot, hops, groundnuts, pecans,
coffee and cocoa in various parts of the world to control fungus
diseases and on rice to control algae. They are usually applied to the
crops as a suspension of wettable powder.
Residue data are available from many countries: on fentin acetate from
different European and American countries, on fentin hydroxide from
U.S.A. and a number of European countries and on fentin chloride from
Germany.
The fentin compounds are subject to a gradual degradation. Fentin
acetate and fentin chloride produce at first triphenyltin hydroxide
which degrades to di- and monophenyltin hydroxide, and finally results
in the release of tin as inorganic tin salts. Among the other
degradation products, phenol, benzene and hydrogen peroxide are found.
The inorganic tin released from the organic molecule is not considered
as a residue of fentin compounds, but as a mineral element of
foodstuffs, and should be treated as such for regulatory purposes. The
degradation of fentin compounds is accelerated by sunlight, humidity,
thermal processes, etc. Due to the location of the residues on the
surface of the plants, washing has been found to cause a considerable
reduction of the residue levels. In soil, phenyltin residues degrade
almost completely within one year.
As was demonstrated with a radiolabelled (113Sn) compound in various
crops, neither phenyltin compounds nor their degradation products are
translocated within the plant after application to the leaves; neither
is there any uptake of phenyltin from soil. This means that residues
on food and feed can only result from direct contact with the
pesticide. None or negligible residues of the fentin compounds and
their metabolites can be expected at the time of consumption in the
edible parts of such crops as potatoes, carrots, groundnuts, coffee,
maize, pecans and rice. In the roots of sugar beets the residues are
normally very small and, in addition, any residues are eliminated
during processing.
The foliage of treated sugar beets has been found to contain residues.
During the silage process fentin compounds are broken down into
inorganic tin in about a month.
The fate of fentin compounds in cows has been evaluated by feeding
animals with sugar beet leaves containing about 1 ppm fentin acetate.
Only about 10 percent of the amount fed was absorbed from the
NATIONAL TOLERANCES
Compound Country Crop Tolerance
Fentin hydroxide U.S.A. potatoes 0.05 ppm
sugar beets under consideration
pecans extended
groundnuts under consideration
Fentin The Netherlands potatoes zero
celery and celeriac 1.0 ppm
Fentin hydroxide Federal Republic celery leaves 1.0 ppm calculated as tin
and fentin acetate of Germany
Fentin Yugoslavia generally 1.0 ppm calculated as tin
Belgium fruit and vegetables zero
celery and celeriac 1.0 ppm
intestinal tract. Milk contained ca. 0.004 ppm of fentin in unchanged
and changed form. Similar results have been obtained in a study on
sheep, administered 113Sn labelled fentin acetate, when it was shown
that the tin was finally excreted in inorganic form. In view of these
results, the risks that fentin would contaminate milk are negligible.
Two equivalent methods are available for the analysis of triphenyltin
residues. The sensitivity of a colorimetric method is, depending on
the type of crop, about 0.01 to 0.1 ppm and that of a polarographic
method about 0.01 to 0.3 ppm. The various triphenyltin compounds are
not distinguished by these methods: the analytical results therefore
reflect the total amount of these compounds in a particular sample
analysed.
Although methods to distinguish between tri-, di- and monophenyltin
are available (e.g. by TLC), difficulties are encountered in the
presence of organic material. Work on that subject is going on. For
the evaluation of residues in the above mentioned crops, with the
exception of celery leaves, the determination of degradation products
of fentin compounds does not seem relevant.
RECOMMENDATIONS
General provisions
Foliage of the treated crops, as well as other parts of plants which
may contain fentin residues, are not recommended to be fed fresh to
domestic animals, but they should be used only as silage. Due to the
degradation of the fentin compounds during silo storage, there is no
need, at this stage of knowledge, to establish practical residue
limits of meat and milk.
Recommendations for tolerances
Since the residues of fentins in the following crops were found to be
less than the detection limits of the analytical methods, no
tolerances are recommended: rice (detection limit: 0.08 ppm), cocoa
(0.1 ppm), pecans (0.03 ppm) and coffee (0.03 ppm).
Tolerances recommended refer to the total amount of fentin compounds
expressed in terms of triphenyltin hydroxide. (Di- and monophenyltin
and inorganic tin are not included in the figures.) The
recommendations are also made on the assumption that the crops are
treated according to good agricultural practice with one compound or,
if two or three are integrated, at a maximum total rate recommended
for any one of the three fentin compounds.
TOLERANCES RECOMMENDED
Parts Based on period
per million from treatment
to harvest
PotatoesX 0.1 7 days
Sugar beetsX 0.2 14 days
CeleryX 1 21 days
CeleriacX 0.1 21 days
CarrotsX 0.2 7 days
Groundnuts (shell-free) 0.05 7 days
XEssentially soil-free
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1972)
1. Reliable analytical methods capable of distinguishing qualitatively
between fentin compounds and tricyclohexyltin hydroxide or other
organotin compounds and, where possible, of quantitative
measurement of the separate compounds.
2. Data on the occurrence of monophenyltin and diphenyltin compounds
in the residues in celery.
DESIRABLE
1. More information on the effect of fentin compounds on the
reticuloendothelial system, especially with respect to the
reversibility of this effect.
2. Reports of toxicological observations in plant workers involved in
the manufacture of these compounds.
3. Further studies on the effect on spermatogenesis.
REFERENCES
Baran, J., Fancher, O.E. and Calandra, J.C. (1966a) 90 day subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C3964 submitted to Thompson-Hayward Chemical Co.
Baran, J., Fancher, O.E. and Calandra, J.C. (1966b) 100 days subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C4343 submitted to Thompson-Hayward Chemical Co.
Bock, R. and Gorbach, S. (1958) Die Bestimmung kleiner Mengen von
Triphenylzinnacetat in Pflanzenmaterial. Z. analyt. Chem., 163:
429-432
Bock, R., Gorbach, S. and Oeser, H. (1958) Analyse von
Triphenylzinnverbindungen. Angew. Chem., 70: 272
Brügemann, J., Barth, K. and Nieser, K.H. (1964) Experimentelle
Studien über das Auftreten von Triphenylzinnacetat Rückständen in
Rübenblättern, Rübenblattsilage, damit gefütterten Tieren und deren
Ausscheidungs-produkten. Zbl. Yet. Med., 11: 4-19
Bürger, K. (1961a) Zur Analytik von Organozinnverbindungen. Z.
Lebensmittel-Unters. u. -Forschung, 114: 1-10
Bürger, K. (1961a) Bestimmung von Mikrogramm-Mengen Zinn in tierischem
und Pflanzlichem Material nach der Dithiolmethode. Z.
Lebensmittel-Unters. u.-Forschung, 114; 10-13
Cahen, R., Boucard, M., Lalaurie, M. and Lacour, C. (1970) Production
expérimentale d'oedème cérébrale. C.R. Acad. Sci., (Paris) (in press)
Deutsche Forschungsgemeinschaft. (1969) Rückstandsanalytik von
Pflanzenschutzmitteln. Verlag Chemie GmbH, Weinheim/Bergstrasse.
Methode 55-B-1
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Food Inspection Laboratory, Amsterdam. (1962) Pesticide Report KvW 58,
dated August 1962, unpublished
Food Inspection Laboratory, Amsterdam. (1965a) Pesticide Report KvW
112, dated February 1965, unpublished
Food Inspection Laboratory, Amsterdam. (1965b) Pesticide Report KvW
115, dated May 1965, unpublished
Gaines, Th.B. and Kimbrough, R.D. (1968) Toxicity of fentin hydroxide.
Toxicol. appl. Pharmacol., 12:397-403
Gembloux Centre de Recherches de Phytopharmacie, Gembloux. (1968)
Report No. 34/68, dated June 1968
Hardon, H.J., Besemer, A.F.H. et al. (1962) Feldversuche mit
Triphenylzinn. Dtsch Lebensmitl. Rdsch, 58: 349-352
Heath, D.F. (1963) Some problems in the determination of residues in
plants and mammals in "Radiation and Radioisotopes applied to Insects
of Agricultural Importance", IAEA, Vienna, p. 185-193
Hedges (1961) Letter of the Tin Research Institute, dated 3-8-1961
with attached report
Herok, J. and Götte, H. (1961) The radiometrical estimation of the
distribution and excretion of (113Sn) triphenyltin acetate in
ruminants. Symp. radioisotopes in animal biology, Mexico City,
p.177-188
Herok, J. and Götte, H. (1963) Radiometrische Untersuchungen über das
Verhalten von Triphenylzinnacetat in Pflanze und Tier. Internat.J.
Appl. Radiation and Isotopes, 14: 461-479
Herok, J. and Götte, H. (1964) Radiometrische Stoffwechselbilanz von
Triphenylzinnazetat beim Milchschaf. Zbl. Vet. Med. A 11, 20-28
Hoechst Unpublished report (1961) Ing. Rei/kn(A), dated 22-8-1961
with attachment
Hoechst Unpublished report (1963) Dr.Cz/Bi 3543, dated 15-3-1963
Hoechst Unpublished report (1964) Dr.Cz/bi, dated 2-11-1964
Hoechst Unpublished report (1965a) Dr.Cz/bi, dated 17-9-1965
Hoechst Unpublished report (1965b) Hk/da-4565 b, dated 11-11-1965
Hoechst Unpublished report (1966a) 1/67 1 t/m 4
Hoechst Unpublished report (1966b) 94/66 1 t/m 4
Hoechst Unpublished report (1966c) 94/66 1 t/m 4
Hoechst Unpublished report (1966d) 62/661-2. 70/66 3, 82/66 4
Hoechst Unpublished report (1966e) 94/66 2 t/m 4
Hoechst Unpublished report (1966f) 95/66 1-2
Hoechst Unpublished report (1966g) 83/66 2 t/m 4
Hoechst Unpublished report (1966h) 61/66 3, 69/66 3, 83/66 41 94/66 5
Hoechst Unpublished report (1967) 84,90/66, dated 3-5-1967
Hoechst Unpublished report (1968a) A1/67 IV, dated 6-6-1968
Hoechst Unpublished report (1968b) 150/67, dated 29-5-1968
Hoechst Unpublished report (1968c) 151/67, dated 29-5-1968
Hoechst Unpublished report (1968d) 152/67, dated 29-5-1968
Hoechst Unpublished report (1968e) 160/67, dated 5-7-1968
Hoechst Unpublished report (1968f) 161/67, dated 5-7-1968
Hoechst Unpublished report (1968g) A1/67 V, dated 6-6-1968
Hoechst Unpublished report (1968h) 153/67, dated 29-5-1968
Hoechst Unpublished report (1968i) 154/67, dated 29-5-1968
Hoechst Unpublished report (1968j) 155/67, dated 29-5-1968
Hoechst Unpublished report (1968k) 1962/67, dated 5-7-1968
Hoechst Unpublished report (1968l) 163/67, dated 5-7-1968
Hoechst Unpublished report (1968m) 241/67, dated 5-3-1968
Hoechst Unpublished report (1968n) 240/67, dated 5-3-1968
Hoechst Unpublished report (1968o) 17/67, dated 1-4-1968
Hoechst Unpublished report (1968p) 16/67, dated 1-4-1968
Hoechst Unpublished report (1968q) 47/68, 99/68, 100 t/m 102/68
Hoechst Unpublished report (1969a) I-14-68, 1 zu 7/8 + 14/68, dated
7-8-1969
Hoechst Unpublished report (1969b) I-13-68, I zu 13-68, dated
20-6-1969
Hoechst Unpublished report (1969c) IV-9-68, IV zu 7-11/68, dated
7-11-1969
Hoechst Unpublished report (1969d) IV-7-68, IV zu 7-11/68, dated
7-11-1969
Klimmer, O.R. (1964) Toxikologische Untersuchungen mit
Triphenylzinnacetat. Zbl. Vet. Med., 11:29-39
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmitt-Rdsch. 7: 190-193
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmittel-Rdsch., 56: 190-193
Magee, P.N., Stoner, H.B. and Barnes, J.M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Path. Bact., 73: 107-124
Malát, M. (1962) Photometrische Zinnbestimmung mit
Brenzcatechinviolett in Anwesenheit von Gelatine; Colorimetrische
Studien (III). Anal. Chem., 187: 404-409
Nat. Inst. Public Health. (1960) Unpublished report nr. FT/17/60,
dated 23-4-1960
Newton, D.W. and Hays, R.L. (1968) Histological studies of ovaries in
rats treated with hydroxyurea, tri-phenyltin acetate and triphenyl
chloride. J. econ. Entomol., 61: 1668-1669
Pate, B.D. and Hays, R.L. (1968) Histological studies of testes in
rats treated with certain insect chemosterilants. J. econ. Entomol.,
61: 32-34
Philips Duphar Unpublished report (1963a) 56655/2A/63
Philips Duphar Unpublished report (1963b) 56655/38/63
Philips Duphar Unpublished report (1964a) 56646/5/64
Philips Duphar Unpublished report (1964b) 56655/34/63
Philips Duphar Unpublished report (1965) 56655/64/65
Philips Duphar Unpublished report (1966a) 56655/15/66
Philips Duphar Unpublished report (1966b) 56655/47A/66
Philips Duphar Unpublished report (1966c) 56655/54/66
Philips Duphar Unpublished report (1966d) 56655/61/66
Philips Duphar Unpublished report (1967) 56655/2/67
Philips Duphar Unpublished report (1968a) 56655/14/68
Philips Duphar Unpublished report (1968b) 56655/17/68
Philips Duphar Unpublished report (1970) 566461/1/70
Ross, W.J. and White, J.C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33:421-427
Scholz, D. (1963) Toxikologische Prüfung von Triphenylzinnchlorid und
Hoechst 2840-Spritzpulver. Unpublished report from Hoechst A.G., 18
December 1963
Scholz, D. and Baeder, M. (1968) Berichte über ein chronische
Toxizitätsprüfung von Triphenylzinnacetat per os an Ratten (4 Monate).
Unpublished report from Hoechst A.G.
Schulz, D. and Baeder, O. (1970) Berichte über fortpflanzungsversuche
mit triphenylzinnacetat an Ratten (Einflues auf Fertilitat,
Gravidität, postnatale Entvicklung). Unpublished report from Hoechst
AG
Scholz, D. and Brunk. (1968a) Chronische oral toxizitätsprufung von
Triphenylzinnacetat (Brestan-Wirkstoff) 120 Tage-Versuch an Hunden.
Unpublished report from Hoechst A.G.
Scholz, D. and Brunk. (1968b) Chronische oral toxizitätsprafung von
Brestan-Wirkstoff 2-Jahres-Versuch an Hunden. Unpublished report from
Hoechst A.G.
Scholz, D. and Weigand. (1968) Triphenylzinnacetat.
Wirkstoff-Technische. 4-Monate-Futterungsversuch an Meerschweinchen.
Unpublished report from Hoechst A.G.
Stoner, H.B. (1966) Toxicity of triphenyltin. Brit. J. industr. Med.,
23: 222-229
Stoner, H.B. and Heath, D.F. (1967) The cummulative action of
triphenyltin. Fd. Cosmet. Toxicol., 5: 285-286
Syrowatka, T. (1969) Investigation of the action of organotin
compounds on the oxidative phosphorylation and permeability of
membrane of rat liver mitochondria Roczn. Zak. Hig. (Warsz.), 20:
717-725
Syrowatka, T. (1970) Influence of fungicides on cellular energetic
processes. Roczn. Zak. Hig. (Warsz.), 21: 105-115
Tauberger, G. (1963) Tierexperimentelle Analyse der
Triphenylzinnwirkung nach intravenöser Injektion. Med. exp. (Basel),
9: 393-399
Thompson-Hayward Unpublished report (1965a) R-542
Thompson-Hayward Unpublished report (1965b) R-568
Thompson-Hayward Unpublished report (1967) R-650
Thompson-Hayward Unpublished report (1969) R-755
Til, H.P. and Feron, V.J. (1965) Triphenyltinhydroxide range-finding
test with dogs. Unpublished TNO report submitted by Philips-Suphar,
No. R-1994
Til, H.P., Feron, V.J. and de Groot, A.P. (1967) Reproduction study
with triphenyltinhydroxide in three generations of rats. Unpublished
TNO report submitted by Philips-Duphar, No. 2476
Til, H.P. and Feron, V.J. (1968) Chronic (two-year) toxicity study
with triphenyltinhydroxide (TPTH) in beagle dogs. Unpublished TNO
report submitted by Philips-Duphar, No. 2717
Til, H.P., Feron, V.J. and der Groot, A.P. (1968) Observations on a
possible effect of TPTH on testicular development in rats. Unpublished
TNO report No. R 2620 submitted by Philips-Duphar.
Til, H.P., Feron, V.J. and de Groot, A.P. (1970) Chronic toxicity
study with triphenyltinhydroxide in rats for two years. Unpublished
TNO report submitted by Philips-Duphar, No. R-3138
Ueda, K., Iijima, K. and Yoshida, S. (1961) Acute oral toxicity of
organotin compounds for mice. Unpublished report from Tokyo Dental
College submitted to Sankyo Chemical Co.
Van Esch, G.J. and Arnoldussen, A.M. (1962) Range-finding test as to
the toxicity of triphenyltinhydroxide during 18 days. Unpublished
report of the National Institute of Public Health, Utrecht, No. Tox
39/62
Verschuuren, H.G., Kroes, R. and van Esch, G.J. (1965) Semi-chronic
investigation as to the toxicity of triphenyltinhydroxide in guinea
pigs. Unpublished report of the National Institute of Public Health,
Utrecht, No. Tox. 33/65
Verschuuren, H.G., Kroes, R. Vink, H.H. and van Esch, G.J. (1966)
Short-term toxicity studies with triphenyltin compounds in rats and
guinea-pigs. Fd. Cosmet. Toxicol., 4: 35-45
Verschuuren, H.G., Ruitenberg, E.J., Peetoom, F., Helleman, P.W. and
van Esch, G.J. (1970) Influence of triphenyltin acetate on lymphatic
tissue and immune responses in guinea pigs. Toxicol. appl. Pharmacol.,
16: 400-410
Weigand and Kief, Brestan-Wirkstoff (1965) 96 percent ig =
Triphenylzinnacetate 96 percent ig Chronischer Futterungsversuch.
Meerschweinchen. Unpublished report from Hoechst A.G., 25 May 1965
Wolf, C., Fancher, O.E. and Calandra, J.C. (1966) 90 day sub-acute
oral toxicity of triphenyltin hydroxide - albino rats. Unpublished
report from Industrial Bio-test Laboratories Inc., submitted to
Thompson-Hayward Chemical Co.
PART II
LIST OF INDUSTRIAL REPORTS ON ANALYTICAL METHODS
Philips Duphar L3-52-16. Clean-up for the residue determination
of triphenyltin compounds in vegetable
matter.
Philips Duphar L3-52-18. Clean-up for the residue determination
of triphenyltin compounds in potatoes.
Philips Duphar L3-52-19. Absorptiometric determination of tin in
cleaned-up residues.
Philips Duphar L3-52-21. Clean-up for the residue determination
of fentin hydroxide in rice.
Philips Duphar L3-52-23. Clean-up for the residue determination
of triphenyltin compounds in tomatoes,
carrots, onions and butter-beans.
Philips Duphar L3-52-25. Clean-up for the residue determination
of triphenyltin compounds in
sugar-beets.
Philips Duphar L3-52-26. Clean-up for the residue determination
of triphenyltin compounds in celery and
sugar-beets.
Philips Duphar L3-52-29. Clean-up for the residue determination
of triphenyltin compounds in roasted
coffee-beans.
Farbwerke Hoechst. Die Bestimmung kleiner Mengen von
1966 Triphenylzinn in Pflanzenmaterial (Dr.
Gorbach 111/66 dated 1-12-1966).
Thompson-Hayward. Clean-up for the polarographic residue
1965a determination of triphenyltin compounds in
potatoes + polarographic tin determination in
pre-cleaned residues (from report 2-542).
Thompson-Hayward. Clean-up for the polarographic residue
1965b determination of triphenyltin compounds in
pecans + polarographic determination of
inorganic tin (from report R-568).
Thompson-Hayward. Clean-up procedure for the colorimetric
1967 residue determination of triphenyltin
compounds in peanuts (from report R-650).
Other relevant chemical properties
The pure fentin hydroxide is a white odourless crystalline solid, with
a melting point of 118-120°C. Solubility in water at 20°C is 8 ppm,
and it is moderately soluble in most organic solvents. It is stable at
room temperature, but decomposition occurs on heating above 60°C.
The pure fentin acetate is a white, odourless, crystalline solid, with
a melting point of 124-125°C. Its solubility in water at 20°C is 28
ppm, and it is of low solubility in most organic solvents.
Fentin acetate is stable when stored under normal conditions in dry
air. At 90°C, no decomposition can be detected; at 150°C, 15 percent
decomposes within three hours. In the presence of water, the acetate
is almost completely hydrolysed within eight hours at 20°C.
The pure fentin chloride is a white crystalline solid, with melting
point at 106°C. Its solubility in water is 40 ppm. It is stable when
stored in the dark in dry air. In the presence of water it hydrolyses
into the hydroxide.
Purity
The technical products are usually of the following purities:
fentin hydroxide : 95 percent
fentin acetate : 94 percent
fentin chloride : 94 percent
The impurities consist mainly of diphenyltin compounds and
tetraphenyltin.
This report is based on the data obtained with the fentin compounds
manufactured in Germany (Hoechst) and in Holland (Philips-Duphar). It
is known that fentin compounds are also produced in the United States
and Japan, but no data were available from these sources.
Formulations
The usual formulations are:
fentin hydroxide : 20 percent and 50 percent wp
fentin acetate : 20 percent and 60 percent wp
fentin chloride : 40 percent and 45 percent wp
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Experiments on rats given 113Sn-labelled fentin chloride showed that
the absorbed fentin radical was rapidly distributed through the
tissues of the body, including the brain. The fentin radical was
eliminated relatively slowly and could be detected in the brain 38
days after a single dose (Heath, 1963).
Experimental investigations have been made on guinea pigs fed on a
diet containing 12 ppm fentin acetate. From the survival times and the
total amount consumed, it is evident that, at this dosage level,
fentin compounds are not completely cumulative. The results obtained
and those previously published (Stoner, 1966) are consistent with a
halflife of the fentin radical at the site of action of about 50 days
(Stoner and Heath, 1967).
Information on the distribution of tin after feeding fentin compounds
to farm animals is included in the section entitled "FATE OF
RESIDUES. In animals".
Effect on enzymes and other biochemical parameters
In vitro studies have shown that fentin compounds inhibit oxidative
phosphorylation by isolated liver mitochondria and the adenosine
triphosphatase (ATP) activity of brain microcomes (50 percent
inhibition by concentration of, 1 × 10-6M and 1 × 10-5M,
respectively) (W. N. Aldridge, quoted by Stoner, 1966).
The oxygen consumption of cerebral slices from rats in a moribund
condition due to fentin compounds was within normal limits (J.E.
Cremer, quoted by Stoner, 1966).
The effects of fentin hydroxide on the oxidative phosphorylation and
on permeability of rat liver mitochondria membranes for malate,
citrate and fumarate ions were investigated. In the conditions of the
investigations (concentration of 5 µ M), a strong inhibitory action on
the oxidative phosphorylation in isolated rat liver mitochondria and
deep changes in the selectively permeable mitochondrial membrane were
observed. Malate and citrate were able to penetrate mitochondria
without participation of any carrier. The altered membrane formed no
barrier for fumarate. No similar effects were observed in the case of
n-octyltin derivatives (Syrowatka, 1969).
In a further investigation, it was shown that the mitochondrial
membrane which, when intact, has a strictly limited permeability for
K+, Na+, Cl-, malate, citrate and fumarate, became permeable in a
high degree to these ions under the influence of fentin hydroxide.
Electron microscopic pictures confirmed these results (Syrowatka,
1970).
TOXICOLOGICAL STUDIES
Special studies on influence on lymphatic tissue and immune
responses
Guinea pig (fentin acetate)
These investigations were aimed at studying the influence of fentin
acetate on lymphatic tissue and immune responses in guinea pigs which
were previously (see below) shown to be much more sensitive than rats
to the decreasing effect of the compound on the lymphopoiesis. Female
guinea pigs were selected because they are more affected than males.
In a first experiment, two groups of ten female guinea pigs received
15 ppm of fentin acetate in their diet, for 49 or 77 days. Each group
was accompanied by a control group with the same number of animals.
After 77 days, a decrease in plasma cells was observed in the spleen
and the cervical, mesenteric, axillary and popliteal lymph nodes. In a
second experiment, the same dose of the compound was given in the diet
for 104 days. Two groups of ten female guinea pigs received fentin
acetate and two groups control diet only. At 21 and 7 days before the
end of the fentin acetate feeding period, half of the animals received
an injection of tetanus toxoid. Some animals were allowed to recover
for 14 days after the 104-day experimental period. Immunochemically
and serologically, a decreased immunologic response was observed in
the group fed fentin acetate after tetanus toxoid stimulation and no
signs of recovery were seen after 14 days. With the aid of the
fluorescent antibody technique, a decrease in immunologically active
cells in the popliteal lymph nodes of the animals fed fentin acetate
could be detected in the second experiment. In the other lymphatic
tissues, no differences were observed (Verschuuren at al., 1970).
Special studies on reproduction
Rat (fentin acetate)
An interim report comprising two generations of a three-generation
reproduction study in rats fed 0, 25, 80 and 250 ppm of fentin acetate
in the diet is available. No histological examination was performed,
but no abnormalities were found by macroscopic examination at the 25
ppm level (Scholz and Baeder, 1970).
Rat (fentin acetate and chloride)
Groups each of 20 sexually mature male rats (aged 35 to 40 days) were
given orally in their food, over a period of 19 days, 20 mg/kg
body-weight per day of either fentin acetate or fentin chloride. The
animals were killed on the twentieth day, and histological
preparations of the testes were made. Both compounds produced several
degenerative changes of the testicular tissue, including a decrease in
the number of cell layers per seminiferous tubule, a decrease in
tubule diameter and overall testicular size, a depletion of the more
advanced cell forms from the tubules and a closing of tubule lumina.
Effects were more pronounced in animals treated with fentin acetate
(Pate and Hays, 1968).
Groups of 15 sexually mature female rats (aged 42 to 46 days) were
given orally 20 mg/kg body-weight per day of either fentin acetate or
fentin chloride. Three animals from each group were killed after 4, 9,
14, 19 and 24 days of treatment, and histological preparations of the
ovaries were made and thoroughly examined. Both fentin compounds
produced significant changes in the ovarian tissue. Among these
changes were a decreased number of mature follicles, an increased
incidence of atresia in early follicle growth and a pronounced
decrease in the number of corpora lutea present. This last effect was
regarded as a decrease in ovulation and, thus, a decreased fertility.
This effect, as well as the others listed above, was present on or by
the first five-day sample period (Newton and Hays, 1968).
Rat (fentin hydroxide)
This experiment was started with 50 male and 100 female newly weaned
rats. The animals were divided at random over five groups and were fed
diets containing 0, 0.5, 1, 2 or 5 ppm of fentin hydroxide. Fertility
of males and females, number of young born per litter, vitality,
body-weight and mortality of the young at birth and during the
lactation period were not affected by feeding fentin hydroxide at
levels up to 5 ppm in any of the three successive generations.
Ninety-day feeding studies with F1b and F2b generation rats did not
reveal adverse effects with respect to growth, food consumption, food
efficiency and haematology. Increased relative weights of liver,
kidney and spleen were occasionally noted, but were not associated
with histological abnormalities. Testicle weight was slightly
decreased at the 5 ppm level in F1b generation rats and at the 2 and
5 ppm levels in F2b generation rats at week 13 after weaning.
Macroscopically, no changes attributable to the ingestion of fentin
hydroxide were observed in any of the successive generations, except
for small-sized testicles at feeding levels of 1 ppm and above in
generation F3b at week 2 after weaning. Microscopic examination
revealed a significant difference between treated and control rats
only in the maturing testicle. This difference found expression in the
stage of maturation of the germinal epithelium, which was less
advanced in the small-sized testicles of the rats fed fentin hydroxide
(Til et al., 1967).
Four groups of ten weanling male rats were each fed 0, 50, 100 or 200
ppm of fentin hydroxide in the diet over a period of 276 days and were
mated at intervals to nontreated females. Observations indicate that,
in comparison to the controls, there was a drop of about 27 and 18
percent, respectively, in the total number of offspring in the groups
fed 200 and 100 ppm after 64 days of exposure. However, when the males
were subsequently mated, their fertility improved and, by day 113, was
comparable to that of the controls. Leucocyte and differential counts
and haemoglobin determinations performed one week prior to the time of
sacrifice did not differ from the controls. Gross abnormalities
related to fentin hydroxide were not observed in the offspring. In the
group fed 200 ppm, one rat died and two were sacrificed when moribund.
The cause of death in this group was generalized internal haemorrhage.
In the animals that were sacrificed, no gross or microscopic
abnormalities attributable to fentin hydroxide were observed. At the
100 and 200 ppm levels, the food consumption of the diet containing
fentin hydroxide was significantly lower during the first week of the
experiment and then improved for all dosage levels. By week 7, the
animals had adapted to these concentrations of the chemical, and the
food consumption was the same as in the controls. The food intake and
weight gain were not affected in rats fed 50 ppm. Gross and
microscopic examination of rats fed fentin hydroxide at all levels did
not reveal changes in any organ that could be related to the compound
(Gaines and Kimbrough, 1968).
Three experiments on weanling male rats (10 or 20 per group), fed from
0 to 25 ppm of fentin hydroxide in two or four weeks, were conducted
to determine testicular development. Microscopic examination after two
and four weeks failed to reveal any differences between the various
test groups and the control groups (Til et al., 1968).
Acute toxicity
Symptoms of poisoning were sluggishness, unsteadiness, moderate
diarrhoea, anorexia, bloody stain around the nose and eyes and
wheezing (Gaines and Kimbrough, 1968).
In all species, the main action of these organo-tin compounds is
thought to be on the central nervous system, but in contrast to
triethyltin compounds, cerebral oedema did not occur (Cahen et al.,
1970).
Intravenous administration of fentin acetate to cats at a dose of 1
mg/kg body-weight produced an increase in blood pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of 1
to 2 mg/kg at 20 to 60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood pressure was also
found. Death took place after 6 to 14 mg/kg of fentin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Dog (fentin acetate)
Groups of dogs (four of each sex) were fed 0, 1, 5 and 20 ppm of
fentin acetate in the diet for 120 days. A level of 20 ppm resulted in
decreased food consumption, and in half of the animals, also in
decreased body-weight. No macroscopic or microscopic abnormalities
were found (Scholz and Brunk, 1968a).
Groups of six dogs, three from each sex, were fed over a period of two
years, 0, 0.5, 1 or 5 ppm of fentin acetate. Attention was paid to
behaviour, food intake, weight gain, blood picture, urine composition,
gross examination and histological characteristics of the main organs
and tissues. Except for the higher level, where a decrease in weight
gain, which might be related to a reduction of food intake, was noted
in comparison to the controls, no significant abnormalities, even in
blood picture, were observed (Scholz and Brunk, 1968b).
Dog (fentin hydroxide)
Groups of dogs consisting of three males and three females were fed 0,
10, 25 and 37 ppm of fentin hydroxide in the diet for 90 days.
TABLE I
Acute toxicities to various species: summary
Animal Fentin LD50 (mg/kg
Compound Route body-weight) Reference
Mouse (M) acetate oral 81 Ueda et al., 1961
Mouse (M) chloride oral 80 Ueda et al., 1961
Mouse (M) hydroxide oral 245 Philips-Duphar, 1964a
Mouse (F) hydroxide oral 209 Philips-Duphar, 1964a
Mouse (M) acetate ip 7.9 Stoner, 1966
Rat (M) acetate oral 136 Klimmer, 1964
Rat (F) acetate oral 491 Stoner, 1966
Rat (F) chloride oral 135 Scholz, 1963
Rat (M) hydroxide oral 240 Gaines & Kimbrough, 1968
Rat (F) hydroxide oral 360 Gaines & Kimbrough, 1968
Rat (M) acetate ip 8.5 Stoner, 1966
Rat (F) acetate ip 11.9 Stoner, 1966
Guinea pig (M) acetate oral 21 Klimmer, 1964
Guinea pig (M) hydroxide oral 27.1 Philips-Duphar, 1964a
Guinea pig (F) hydroxide oral 31.1 Philips-Duphar, 1964a
Guinea pig (M) acetate ip 3.7 Stoner, 1966
Rabbit (M) acetate oral 40 Klimmer, 1964
Rabbit (M) acetate ip 10 Klimmer, 1964
Lethargy and a darkening of the hair occurred in all groups, depending
in degree on the dosage, and an increased mortality rate occurred in
the two higher groups (Baran et al., 1966a).
Groups of dogs (three of each sex) were fed 0, 10, 25 and 37 ppm of
fentin hydroxide in the diet for 100 days. Apart from darkening of the
hair which occurred in all test groups, the only differences from the
controls were lethargy in the 37 ppm dogs and an increased quantity of
tin in the livers of five of six dogs at 37 ppm and two of six dogs at
25 ppm (Baran et al., 1966b).
Six dogs were fed, over a period of 16 weeks, a diet containing,
during the first eight weeks, 25 ppm and, afterwards, 50 ppm of fentin
hydroxide. Symptoms of toxicity, consisting principally in inhibition
of growth, alterations in the blood picture (decrease of the number of
leucocytes and erythrocytes) and in the histology of the liver, were
observed (Til and Feron, 1965).
Following this preliminary investigation, 34 dogs were fed over a
period of two years a diet containing respectively 0, 0.5, 2.5, 5 and
10 ppm. Growth, food consumption, symptomatology, blood picture, urine
analysis, liver and kidney functional exploration, serum protein
electrophoresis, water content of brain, organ weights and gross and
microscopic pathology were used as criteria to disclose signs of
injury. General appearance, behaviour, growth, food consumption,
haematological and biochemical values, urine analyses and liver and
kidney functional tests were not affected at any dietary level of the
compound. At the two highest feeding levels of 5 and 10 ppm, the
relative weights of liver and kidney and the water content of the
brain were increased in comparison to the controls, without being
associated with histological changes. Gross and microscopic
examination of organs and tissues did not reveal any abnormality which
could be attributed to the ingestion of fentin hydroxide (Til and
Feron, 1968).
Guinea pig (fentin acetate)
In one experiment, 50 ppm of fentin acetate in the diet of guinea pigs
led to the death of all of nine animals in 17 to 31 days. In other
experiments, only a group of five guinea pigs given 1 ppm for 392 days
did not show an increased mortality rate. Even at 1 ppm, food intake
was reduced. When death occurred, it was preceded by loss of weight
and generalized weakness (Stoner, 1966). Neither in these experiments,
nor in others (acute and short-term), in which guinea pigs and rats
have been treated with fentin compounds, has a significant increase in
the water content of the central nervous system been found nor the
characteristic histological lesion in the white matter produced by
triethylin compounds (Magee et al., 1957) been seen (Stoner, 1966).
In other experiments, groups of guinea pigs, 10 of each sex, were
given respectively 0, 5, 10, 20 or 50 ppm of fentin acetate for 12
weeks. In all groups, except for the males given 5 ppm, growth
inhibition was obvious.
At the end of the treatment, no animal at 50 ppm was alive; in the
other groups, the survival rates were: 20 ppm, 8 out of 10 males and 8
out of 10 females; 10 ppm, 9 out of 10 males and 10 out of 10 females;
5 ppm, 9 out of 10 males and 10 out of 10 females. In the females on
5, 10 and 20 ppm and males on 10 and 20 ppm, the number of lymphocytes
and leucocytes was significantly decreased. The leuco- and lymphopenia
was accompanied in a number of animals by histological changes in the
lymphopoietic system, e.g., atrophy of the white pulp of the spleen
was found. This change possibly induced a decrease of general
resistance, so that a mycotic infection developed in the animals. At
20 and 50 ppm, a significant increase of brain water was noticed.
Histological findings were not significantly different from those
observed in the controls (Verschuuren et al., 1966).
Groups of guinea pigs, ten of each sex, were fed 0, 1, 5, 10, 50 and
100 ppm of fentin acetate for four months. All animals on 100 ppm and
30 percent of those on 50 ppm died, and in the same groups were found
signs of disturbance of the iron metabolism. A level of 10 ppm gave a
slight reduction in weight gain, as well as some cases of decreased
haemoglobin percentage and total number of erythrocyte. The groups fed
5 ppm and 1 ppm were similar to the controls (Scholz and Weigand,
1968).
Guinea pig (fentin hydroxide)
Groups of three males and three females were given 0, 1, 2.5, 5 or 20
ppm of fentin hydroxide for three weeks. No changes in the growth
rate, the water content, or the histology of the nervous system were
observed (Verschuuren at al., 1965).
Groups of ten males and ten females were fed 0, 2.5, 5, 10, 20 or 50
ppm of fentin hydroxide in their diet for 12 weeks. Growth depression
was observed at the two highest dose levels. All animals in the 50 ppm
group died during the first half, and one each in the ten and 20 ppm
groups died later in the experiment. A decrease in the haemoglobin
content was found in all groups, except for the males in the 2.5 and 5
ppm groups there was no change in the number of erythocytes. A
decrease in the number of leucocytes (except in the males on 10 ppm)
and lymphocytes (except the males on 2.5 and 5 ppm) was found in all
the groups examined. The same changes as with fentin acetate were
found in the lymphopoietic system (see above). A decrease in relative
organ weight was found in the spleen of the females on 10 and 20 ppm
and in the thymus of both sexes and uterus and testes at 20 ppm. No
increase in the water content of the central nervous system was
observed, except in the females at 50 ppm. Apart from the changes in
the lymphopoietic system, the histological findings were not
significantly different from those observed in the controls
(Verschuuren et al., 1966).
See also Special studies on influence on lymphatic tissue and immune
responses.
Rat (fentin acetate)
Groups of 20 to 25 rats were given fentin acetate by stomach tube at
doses equivalent to 5, 10, 25 or 50 ppm for 105 to 170 days. At 50
ppm, 70 percent of the rats died within 49 days. Nervous symptoms, as
well as blood, urinary or histopathological changes, were not observed
at the lower dose levels (Klimmer, 1964).
In other experiments on rats, no deaths occurred during a ten-week
period on 200 ppm of fentin acetate. These rats were then put on a
diet containing 300 ppm, and five out of six rats died after a further
117 to 168 days (Stoner, 1966).
Groups of young rats, ten of each sex, were given respectively 0, 5,
10, 25 or 50 ppm of fentin acetate for 12 weeks. Decrease of food
intake and growth inhibition were recorded at 50 ppm, as well as
growth inhibition in males at 25 ppm. At 10 ppm and above, the number
of leucocytes in the blood was decreased in the male (leucopenia) and,
at 50 ppm, the haemoglobin was reduced. At the highest level there was
a decrease of the organ to heart weight ratio for the pituitary and
pancreas in all animals and uterus and ovary in the females. The same
ratio for the thyroid was decreased in all the females, as well as in
the males at 25 and 50 ppm. Paralysis and pronation of the hind legs,
associated with interstitial oedema of the central nervous system,
which developed in rats treated with triethyltin hydroxide at all
levels, were not observed. The water content of the spinal cord was
slightly increased at 50 ppm. Histological findings were not
significantly different from those observed in controls (Verschuuren
at al., 1966).
Groups of 20 young rats, ten of both sexes, were fed respectively, 0,
15, 40, 100, 250 or 600 ppm of fentin acetate over a period of four
months. Attention was given to behaviour, growth, blood picture, urine
composition, gross examination and histology of the main organs and
tissues. Up to 100 ppm, no abnormalities were observed in comparison
to the controls. Above 100 ppm, a dose-related inhibition of growth
was seen and 600 ppm resulted in the death of 30 percent of the
treated animals. (Scholz and Baeder, 1968).
Rat (fentin chloride)
Groups of ten rats of both sexes were given orally, over a period of
41 days, 28 doses of 0, 6.25, 12.5, 25 or 50 mg/kg body-weight. Apart
from a slight decrease in weight gain of the females at 12.5 mg/kg, no
abnormalities (growth, behaviour, blood picture and urine composition,
gross and histological characteristics of stomach, small intestine,
spleen, liver, kidney and lung) were observed at 6.25 and 12.5 mg/kg.
At higher doses, a dose-related increase in mortality compared to the
controls was observed (Scholz, 1963).
Rat (fentin hydroxide)
Groups of 10 or 20 rats of both sexes were given 0, 5, 20, 50 or 100
ppm of fentin hydroxide for 20 days. Food intake and body growth were
depressed at 20 ppm and above. Death rates were 9 out of 10 at 100
ppm; 9 out of 20 at 50 ppm, and 1 out of 20 at both 20 and 5 ppm (Van
Esch and Arnoldussen, 1962).
Groups of rats, ten of each sex, were fed 0, 1, 3.1, 5, 10 and 31 ppm
of fentin hydroxide in the diet for 90 days. Disregarding effects on
erythrocyte and leucocyte counts as neither dose related nor
chronologic, a no-effect level of 31 ppm was claimed (Wolf et al.,
1966).
Groups of ten rats of each sex were given respectively 0, 5, 10 or 25
ppm of fentin hydroxide in the diet for 12 weeks. The food intake was
comparable to the controls. The females showed growth inhibition after
six weeks, but they recovered in spite of continuing treatment. Growth
in the males was comparable to the controls. In the females, blood
leucocytes were decreased. No significant changes in water content
were found in the nervous tissues. At 25 ppm, decrease of the thyroid
weight was noticed (Verschuuren at al., 1962). In a similar
experiment, in which ten rats of each sex were given 50 ppm of the
same compound, the following features were noticed: decreased food
intake, growth inhibition in both sexes, decrease in weight of the
thyroid, pituitary, uterus, ovary, prostate and pancreas, as well as
decrease of haemoglobin and leucocytes. Paralysis and pronation of the
hindlegs, associated with interstitial oedema of the central nervous
system, which developed in rats treated with triethyltin hydroxide at
all levels, were not observed. The water content increased in the
spinal cord, but not in the brain. (In comparison, the water content
of the spinal cord and brain was increased in animals treated with
levels of 5 and 10 ppm of triethyltinhydroxide). Histological findings
in the animals treated with fentin hydroxide were not significantly
different from those observed in the controls (Verschuuren et al.,
1966).
Groups of four to five week old male rats were fed fentin hydroxide in
the diet at levels of 0, 100, 200 or 400 ppm over a period of 99 days.
The rats fed 400 ppm were apparently repelled, since they ate only
about one third as much food as the controls during the first week.
All the animals in this group died within 7 to 34 days from starvation
and extensive intra-alveolar haemorrhage in the lung. There was
partial or complete testicular atrophy and general size and weight
reduction of the organs. The rats fed 200 ppm gained less weight (231
g) than the controls (302 g). At the end of the study, the total
leucocyte number and the weights of spleen and kidneys were
significantly below those of the controls.
The rats fed 100 ppm appeared normal throughout the study except for a
significant decrease of food intake during the first week of treatment
(Gaines and Kimbrough, 1968).
Additional information on the findings following short-term
administration of fentin compounds to rats is given under Special
studies on reproduction.
Long-term studies
Guinea pig (fentin acetate)
Groups of 20 young guinea pigs, ten from each sex, were fed
respectively 0, 1, 5, 10, 50, 100 and 200 ppm of fentin acetate over a
period of two years. Attention was given to behaviour, food intake,
weight gain, blood picture, urine composition, gross examination and
histological characteristics of the main organs and tissues. At 50 ppm
and above, a dose-related increase in mortality in comparison to the
controls and an inhibition of growth were observed. At 10 ppm and
above, fatty degenerative changes were seen in liver and heart muscle.
At 1 and 5 ppm, no abnormalities, even in the blood picture, were
noted in comparison to the controls (Weigand and Kief, 1965).
Rat (fentin hydroxide)
Groups of 50 newly weaned rats, 25 from each sex, were fed over a
period of two years respectively 0, 0.5, 1, 2, 5 or 10 ppm of fentin
hydroxide. General appearance and behaviour, growth, food intake,
blood sugar and blood urea nigrogen, urine composition and SG-PT,
SG-OT and SAP activity were not adversely affected at any dietary
level of the test substance. Mortality of the females at 10 ppm was
higher than in the controls, although at the termination of the
experiment the difference was not significant. Haematological data
often showed a slight decrease in white blood cells at the highest
feeding level in the first year of the experiment only. This effect
was less often soon at 5 ppm and only once at 2 ppm in the males. The
relative thyroid weight was slightly decreased at 10 ppm in the
females only. Average relative weights of the other organs were very
uniform in all groups. Gross autopsy findings did not show evidence of
significant pathological changes. Microscopic examinations of numerous
organs and tissues, including testes, ovaries and bone marrow, did not
reveal histological alterations significantly different from those
observed in controls (Til et al., 1970).
COMMENTS
The basis for grouping the three fentin compounds for consideration
together is that fentin acetate and fentin chloride are converted in
plants and animals to the same compound, namely fentin hydroxide.
Since the previous toxicological evaluation of the fentin compounds in
1965, a considerable number of short-term and long-term feeding
studies in several species have been reported. The production of
cerebral oedema in rats and guinea pigs did not appear to be
pronounced except at a 50 ppm dose level. However, the reduction of
lymphopoiesis in both species appeared to be of some concern. With
respect to this parameter, the guinea pig appeared to be the most
sensitive species studied. A reduction in lymphocyte count was evident
in females at a dietary level of 2.5 ppm of fentin hydroxide. It was
reported that this effect was not entirely reversible after 14 days'
withdrawal from a test diet of 15 ppm of fentin acetate. In a two-year
feeding study in rats a no-effect level of 2 ppm relative to decrease
in white blood cells was evident.
In a multigeneration rat reproduction study, reduction in testicular
size was evident at 5 ppm in the F1b generation, at 2 ppm and above
in the F2b generation and at 1 ppm in the F3b generation. In a
second species, the dog, histological examination revealed no effect
on any organ at feeding levels of up to 10 ppm for two years.
There are no reports of observations of men exposed to this compound.
Bearing the abovementioned observations in mind, the Committee
considered the data adequate to establish an acceptable daily intake
for the fentin compounds.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.1 mg/kg body-weight
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 to 0.0005 mg/kg body-weight, applicable to fentin hydroxide,
fentin acetate and fentin chloride and to the sum of each if more
than one is involved
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
All the three fentin compounds are nonsystemic fungicides used in many
European countries, the United States, and several South American,
African and Asiatic countries. On rice they are used against algae.
Pre-harvest treatments
Fentin compounds are recommended to control the following pests:
Potatoes: Phytophthora infestans de Bary
of the foliage and especially
the tubers, and
Alternaria solani J. u.Gr.
Sugar beet: Cercospora beticola Sacc.
Celery and celeriac: Septoria apii Chester
Carrots: Alternaria porri f. dauci Neerg.
Pecans: Fusicladium effusum, Gnomonia spp.,
Mycosphaerella caryigena Demaree & Cole
Rice: various algae
Groundnuts: Cercospora spp.
Coffee: Colletotrichum coffeanum Noack
Cocoa: Phytophthora palmivora Butl.
The usual rates of application are between 0.15 and 0.40 kg a.i/ha in
600 to 1 000 litres of water. On coffee, the rate is 0.2 to 0.6 kg
a.i/ha in more than 1 000 litres of water. For algae control on rice,
the dosage is up to 1 kg a.i/ha in about 1 000 litres of water.
Recommended rates of application and intervals applied between last
treatment and harvest are as follows:
Potatoes: 0.18 to 0.4 kg a.i./ha. 1-10 times
(generally between 2 and 5 times).
Safety period (in Germany) 7 days.
Sugar beets: 0.18 to 0.4 kg a.i./ha. 1-3 treatments.
Celery celeriac, 0.2 to 0.32 kg a.i./ha in 600-1 000 1
carrot: water, 1-7 treatments. (In Germany
safety period for celery of 3 weeks)
Pecans: 0.22 to 0.54 kg a.i./ha, 2 to 8
treatments.
Rice: 0.75 to 1 kg a.i./ha for algae control.
Peanuts, coffee, 0.2 to 0.6 kg a.i./ha.
cocoa:
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Fentin compounds are used on ornamentals, willow and poplar. Trials on
hops are in process.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data from supervised trials are given in Table II.
TABLE II
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Potatoes Holland 0.20-0.46 4-7 2-98 tubers 0.01 23 17 0.04
" " 0.08-0.38 6-7 98-110 " 0.06 14 12 0.11
" England 0.22-0.56 5 21-43 " 0.01 5 5
" " 0.28 1 30-35 " 0.03 6 6
" Canada 0.15-0.25 ?-12 ?-169 " 0.03 7 7
" U.S.A. 0.17-0.34 5-11 13-15 " 0.03 17 17
" Belgium 0.30-0.36 5 22 " 0.01 1 1
Sugar beets Holland 0.30 5 43-57 beets 0.03 4 0.18
" " 0.30 5 43-57 leaves 0.06 4 1.36
" Germany 0.30 1-4 46-64 beets 0.03 5 2 0.18
" " 0.30 1-4 46-64 leaves 0.06 5 2 0.81
" Italy 0.30-0.40 ? 31 beets 0.03 10 1 0.20
" Switzerl. 0.30-0.40 2 44 leaves 0.02 4 2.45
" U.S.A. 0.22-0.34 3-6 14-45 beets 0.03 17 8 0.09
Carrots Israel 0.25-0.30 5-7 12-28 carrot 0.05 4 3 0.07
Pecans U.S.A. 0.22-0.54 2-8 46-256 nuts 0.03 17 17
Rice Italy 04-1.2 ppm 1 90 hulled rice 0.08 5 5
in irrigation
water
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin hydroxide
Ground nuts U.S.A. 0.22-0.34 3-7 9-72 nuts 0.01 14 10 0.03
" U.S.A. 0.22-0.34 3-7 9-72 hulls 0.01 12 1 0.38
" U.S.A. 0.34 3-7 9 vines 0.01 3 2.5
" Israel 0.30 4 5-23 nuts 0.01 4 4
" " 0.30 4 5-23 vines 0.16 4 2.6
Coffee Kenya 0.22-0.60 ? 35 roasted 0.03 3 3
beans
Residues of fentin acetate
Potatoes Germany 0.216-0.324 1-5 7-21 tubers 0.03-0.04 12 8 0.07
" Ireland 0.3 3 not stated " 0.03 2 2
Sugar beets Germany 0.216-0.324 6 38 beets 0.1 6 6
Celeriac " 0.3 1-2 21-45 tubers not stated 3 3
" " 0.324 1 21-35 " 0.1 3 3
" Belgium not stated 3 21-28 stems not stated 6 6
" Germany 0.3 1-2 21-45 leaves not stated 13 2 0.19
" " 0.324 1 21-35 " 0.1 3 3
" Holland 0.24-0.30 1-6 21-28 " 0.1 2 1.0
Celeriac Holland 0.27 6 21-28 leaves 0.01 2 0.30
" Belgium not stated 3 21-28 " not stated 6 5 0.05
Carrots Germany 0.216-0.324 1-3 7-28 1/ 0.03 20 17 0.15
" " 0.216-0.324 1-3 7-28 2/ 0.02 6 5 0.06
" " 0.216-0.324 1-3 7-28 3/ 0.02 6 5 0.02
" " 0.216-0.324 1-3 7-28 4/ 0.02 6 5 0.02
TABLE II (cont'd)
Summary of fentin compound residues in supervised trials
Crop Country Rate of Number Preharvest Plant Detection Number Number of Highest
application of interval part limit of residues residues
(kg a.i/ha) treatments (days) analyzed (ppm) analyses under found
detection (ppm)
limit
Residues of fentin acetate
Rice Italy 04-1.2ppm 1 appr.go hulled 0.08 3 3
in irrigation rice
water
Cocoa Nigeria not stated not stated not stated not stated 0.1 8 7 0.1
Residues of fentin chloride
Potatoes Germany 0.22-0.27 1-3 7-14 tubers 0.04 8 6 0.05
Sugar beets " 0.18-0.27 6 38 beets 0.10 4 4
Celeriac " 0.27 1 21-35 tubers 0.1 3 2 0.1
Carrots " 0.20 3 10-31 washed 0.02 4 2 0.08
carrots
1/ washed unpeeled carrots
2/ peeled/scraped carrots
3/ peels, calculated on total weight carrot
4/ scrapings, calculated on total weight carrot
The residue date of fentin hydroxide are from treatments made in the
Netherlands, Germany, Belgium, Canada, England, Italy, Israel,
Switzerland, U.S.A. and Kenya (Philips Duphar, 1963a, 1964b, 1965,
1966a-d, 1968a,b; Thompson-Hayward, 1965a,b, 1967, 1969).
The residue data of fentin acetate are from experiments made in
Germany, the Netherlands, Belgium, Ireland, Italy, Kenya and Nigeria
(Hoechst, 1963, 1964, 1965a, 1966a-d, 1967, 1968a-f,m,o,q, 1969c,d;
Hardon et al., 1962; Gembloux, 1968; Food Inspection, 1965a-b;
Kröller, 1960; Nat. Inst. Publ. Health, 1960; Philips Duphar, 1967).
The residue date of fentin chloride are from experiments made in
Germany (Hoechst, 1966e-h, 1968g-l,n,p, 1969a,b).
FATE OF RESIDUES
General comments
When triphenyltin acetate was decomposed in solution, the following
products were detected: phenol, acetone, hydrogen peroxide, degraded
organotin and benzene. There is probably also some organic or
organotin peroxide present (Hedges, 1961).
In the presence of light and humidity, fentin acetate and fentin
chloride are transformed easily into fentin hydroxide, which
metabolizes into dephenyltin and monophenyltin. Monophenyltin breaks
down very quickly into Sn salts (Kröller, 1960; Hoechst, 1961; Hedges,
1961).
At heating, triphenyltin hydroxide readily decomposes (see other
relevant chemical properties).
In animals
After ingestion of fentin compounds by cows or sheep, the compound is
largely excreted with the faeces (Herok and Götte, 1961; Brügemann et
al., 1964). Three sheep given 10 mg daily of 113Sn-labelled fentin
acetate for 25 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration: 0.0017 ppm) during the treatment, but 17 days after
withdrawal of the compound, its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than fentin acetate. During the treatment, the concentration of
113Sn in the blood and in the urine were 2.9µg/1 and 7.5µg/1,
respectively. In the sheep killed at 28 days, liver, kidney, lung,
pancreas, gall bladder and brain contained a greater amount of 113Sn
than other organs. After 218 days, the amount of 113Sn found in the
liver was still greater than in other organs (Herok and Götte, 1961).
Organs of one sheep, sacrificed at the end of the feeding period with
the 113Sn triphenyltin acetate, showed the following residues
(comprising triphenyltin and all degradation products including
inorganic tin):
Organ ppm 113Sn
liver 0.9
intestinal fat 0.01
depot fat 0.01
muscles 0.02
In plants
Studies on the effect of tin compounds on plants have been made or are
in progress in several laboratories. It has been shown that in the
presence of light and air, several byproducts of fentin acetate can be
formed, such as diphenyltin and finally insoluble tin (Kröller, 1960).
The tin content from the leaves can be transferred to the ground.
However, when 113Sn-labelled fentin acetate or hydroxide was applied
to potato foliage, no translocation of 113Sn from leaves to tubers
could be demonstrated above the limit of determination of 0.0001 ppm.
Tin could be detected in the haulm, where the percentage of the
original compound decreased for 20 days (Philips Duphar, 1964b). It
has also been shown that fentin compounds have no systemic action when
applied to celeriac and sugar beet (Herok and Götte, 1963).
Furthermore, there is no uptake of phenyltin from the soil
(Philips-Duphar, 1970; Hoechst, 1965b). This means that residues on
food or feed can only result from direct contact with the pesticide or
from contamination during harvest, transport or processing: potatoes,
carrots, sugar beets, groundnuts, coffee, cocoa, pecans and rice are
examples.
With a few crops, the plant parts carrying the residues may be used as
food or feed. Those feedstuffs are sugar beet tops and leaves,
groundnut vines and rice hulls. As reported in "Fate of residues in
animals" fentin itself and its degradation products in feed are
transferred only in very small amounts into milk and meat (Brüggemann
et al; 1964; Herok and Götte, 1963, 1964). The only crop from which
the leaves are used for human consumption is celery. This crop forms
generally only a small portion of the human diet.
In soil
Fentin compounds are degraded in soil almost completely within one
year (Hoechst, 1967).
Evidence of residues in food in commerce or at consumption
No experimental data are available on the fentin residues in food
moving in commerce or in the diets. Theoretically, it can be
postulated, however, that the chance fentin compounds would reach the
consumer is very limited. This is mainly due to the fact that the
residues of fentin compounds or their metabolites occur only on the
surfaces of those plant parts which have been subjects of direct
treatments, or of other types of contamination, and are therefore
easily removed by peeling, washing, shelling, etc., and are readily
broken down in thermal processes.
Peeling of potatoes, as well as cooking or other types of processing,
are apt to remove the residues. After sugar beets are processed into
sugar, no organic tin is found in sugar (Thompson and Hayward, 1969).
Carrots generally are washed, scraped, peeled or cooked before
consumption, which processes reduce the residues. The removal of
shells of pecans, groundnuts and cocoa removes also the residues. The
coffee beans are protected from contamination by the fruit peel and
flesh, by fermentation and by roasting. Rice is protected by husk,
which is removed by milling before the rice is cooked or baked for
consumption. Celery and celeriac if used uncooked for human
consumption may contain fentin residues at the time of consumption.
The portion of these crops is, however, generally very small in human
diets.
The foliage of treated crops may contain fentin residues which have to
be regarded as contaminants of animal feed. During silage the residues
are, however, found to be degraded and the risk of contamination of
animal products is avoided.
METHODS OF RESIDUE ANALYSIS
Fentin residues can be determined with two completely equivalent
methods which give the same results in identical samples. In the
method of Philips-Duphar, the triphenyltin compounds are determined
absorptiometrically in the extract with pyrocatechol-violet at 630 mm.
The detection limit of the method is between 0.01 and 0.12 ppm (Ross
and White, 1961; Malát, 1962). In the method of Hoechst, the
triphenyltin compound is converted into the tetrabromo compound,
separated from traces of lead and then determined polarographically.
The detection limit of the method is between 0.012 and 0.26 ppm (Bock
and Gorbach, 1958; Bock et al., 1958; Bürger, 1961a,b; Deutsche
Forschungsgemeinschaft, 1969).
Because blank values are varying according to crop variety and origin,
the detection limit is not a constant figure. None of the methods can
distinguish between the three fentin compounds; they give the sum of
these compounds. Di- and monophenyltin are not detected by these
methods. Although methods to distinguish between tri-, di- and
monophenyltin are available, some difficulties are encountered in the
presence of organic matter. Work on that subject is going on. For the
evaluation of residues in the abovementioned crops - with exception of
celery - the determination of degradation products of the three fentin
compounds does not seem relevant (see 113Sn-trials).
There are a number of industrial reports on the analytical methods
(see References).
APPRAISAL
Fentin compounds evaluated by the Working Party were fentin acetate
(triphenyltin acetate), fentin chloride (triphenyltin chloride) and
fentin hydroxide (triphenyltin hydroxide). They are used on potato,
sugar beet, celery, celeriac, leek, carrot, hops, groundnuts, pecans,
coffee and cocoa in various parts of the world to control fungus
diseases and on rice to control algae. They are usually applied to the
crops as a suspension of wettable powder.
Residue data are available from many countries: on fentin acetate from
different European and American countries, on fentin hydroxide from
U.S.A. and a number of European countries and on fentin chloride from
Germany.
The fentin compounds are subject to a gradual degradation. Fentin
acetate and fentin chloride produce at first triphenyltin hydroxide
which degrades to di- and monophenyltin hydroxide, and finally results
in the release of tin as inorganic tin salts. Among the other
degradation products, phenol, benzene and hydrogen peroxide are found.
The inorganic tin released from the organic molecule is not considered
as a residue of fentin compounds, but as a mineral element of
foodstuffs, and should be treated as such for regulatory purposes. The
degradation of fentin compounds is accelerated by sunlight, humidity,
thermal processes, etc. Due to the location of the residues on the
surface of the plants, washing has been found to cause a considerable
reduction of the residue levels. In soil, phenyltin residues degrade
almost completely within one year.
As was demonstrated with a radiolabelled (113Sn) compound in various
crops, neither phenyltin compounds nor their degradation products are
translocated within the plant after application to the leaves; neither
is there any uptake of phenyltin from soil. This means that residues
on food and feed can only result from direct contact with the
pesticide. None or negligible residues of the fentin compounds and
their metabolites can be expected at the time of consumption in the
edible parts of such crops as potatoes, carrots, groundnuts, coffee,
maize, pecans and rice. In the roots of sugar beets the residues are
normally very small and, in addition, any residues are eliminated
during processing.
The foliage of treated sugar beets has been found to contain residues.
During the silage process fentin compounds are broken down into
inorganic tin in about a month.
The fate of fentin compounds in cows has been evaluated by feeding
animals with sugar beet leaves containing about 1 ppm fentin acetate.
Only about 10 percent of the amount fed was absorbed from the
NATIONAL TOLERANCES
Compound Country Crop Tolerance
Fentin hydroxide U.S.A. potatoes 0.05 ppm
sugar beets under consideration
pecans extended
groundnuts under consideration
Fentin The Netherlands potatoes zero
celery and celeriac 1.0 ppm
Fentin hydroxide Federal Republic celery leaves 1.0 ppm calculated as tin
and fentin acetate of Germany
Fentin Yugoslavia generally 1.0 ppm calculated as tin
Belgium fruit and vegetables zero
celery and celeriac 1.0 ppm
intestinal tract. Milk contained ca. 0.004 ppm of fentin in unchanged
and changed form. Similar results have been obtained in a study on
sheep, administered 113Sn labelled fentin acetate, when it was shown
that the tin was finally excreted in inorganic form. In view of these
results, the risks that fentin would contaminate milk are negligible.
Two equivalent methods are available for the analysis of triphenyltin
residues. The sensitivity of a colorimetric method is, depending on
the type of crop, about 0.01 to 0.1 ppm and that of a polarographic
method about 0.01 to 0.3 ppm. The various triphenyltin compounds are
not distinguished by these methods: the analytical results therefore
reflect the total amount of these compounds in a particular sample
analysed.
Although methods to distinguish between tri-, di- and monophenyltin
are available (e.g. by TLC), difficulties are encountered in the
presence of organic material. Work on that subject is going on. For
the evaluation of residues in the above mentioned crops, with the
exception of celery leaves, the determination of degradation products
of fentin compounds does not seem relevant.
RECOMMENDATIONS
General provisions
Foliage of the treated crops, as well as other parts of plants which
may contain fentin residues, are not recommended to be fed fresh to
domestic animals, but they should be used only as silage. Due to the
degradation of the fentin compounds during silo storage, there is no
need, at this stage of knowledge, to establish practical residue
limits of meat and milk.
Recommendations for tolerances
Since the residues of fentins in the following crops were found to be
less than the detection limits of the analytical methods, no
tolerances are recommended: rice (detection limit: 0.08 ppm), cocoa
(0.1 ppm), pecans (0.03 ppm) and coffee (0.03 ppm).
Tolerances recommended refer to the total amount of fentin compounds
expressed in terms of triphenyltin hydroxide. (Di- and monophenyltin
and inorganic tin are not included in the figures.) The
recommendations are also made on the assumption that the crops are
treated according to good agricultural practice with one compound or,
if two or three are integrated, at a maximum total rate recommended
for any one of the three fentin compounds.
TOLERANCES RECOMMENDED
Parts Based on period
per million from treatment
to harvest
PotatoesX 0.1 7 days
Sugar beetsX 0.2 14 days
CeleryX 1 21 days
CeleriacX 0.1 21 days
CarrotsX 0.2 7 days
Groundnuts (shell-free) 0.05 7 days
XEssentially soil-free
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1972)
1. Reliable analytical methods capable of distinguishing qualitatively
between fentin compounds and tricyclohexyltin hydroxide or other
organotin compounds and, where possible, of quantitative
measurement of the separate compounds.
2. Data on the occurrence of monophenyltin and diphenyltin compounds
in the residues in celery.
DESIRABLE
1. More information on the effect of fentin compounds on the
reticuloendothelial system, especially with respect to the
reversibility of this effect.
2. Reports of toxicological observations in plant workers involved in
the manufacture of these compounds.
3. Further studies on the effect on spermatogenesis.
REFERENCES
Baran, J., Fancher, O.E. and Calandra, J.C. (1966a) 90 day subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C3964 submitted to Thompson-Hayward Chemical Co.
Baran, J., Fancher, O.E. and Calandra, J.C. (1966b) 100 days subacute
oral toxicity of technical triphenyltin hydroxide - beagle dogs.
Unpublished report from Industrial Bio-test Laboratories Inc., no IBT
C4343 submitted to Thompson-Hayward Chemical Co.
Bock, R. and Gorbach, S. (1958) Die Bestimmung kleiner Mengen von
Triphenylzinnacetat in Pflanzenmaterial. Z. analyt. Chem., 163:
429-432
Bock, R., Gorbach, S. and Oeser, H. (1958) Analyse von
Triphenylzinnverbindungen. Angew. Chem., 70: 272
Brügemann, J., Barth, K. and Nieser, K.H. (1964) Experimentelle
Studien über das Auftreten von Triphenylzinnacetat Rückständen in
Rübenblättern, Rübenblattsilage, damit gefütterten Tieren und deren
Ausscheidungs-produkten. Zbl. Yet. Med., 11: 4-19
Bürger, K. (1961a) Zur Analytik von Organozinnverbindungen. Z.
Lebensmittel-Unters. u. -Forschung, 114: 1-10
Bürger, K. (1961a) Bestimmung von Mikrogramm-Mengen Zinn in tierischem
und Pflanzlichem Material nach der Dithiolmethode. Z.
Lebensmittel-Unters. u.-Forschung, 114; 10-13
Cahen, R., Boucard, M., Lalaurie, M. and Lacour, C. (1970) Production
expérimentale d'oedème cérébrale. C.R. Acad. Sci., (Paris) (in press)
Deutsche Forschungsgemeinschaft. (1969) Rückstandsanalytik von
Pflanzenschutzmitteln. Verlag Chemie GmbH, Weinheim/Bergstrasse.
Methode 55-B-1
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Food Inspection Laboratory, Amsterdam. (1962) Pesticide Report KvW 58,
dated August 1962, unpublished
Food Inspection Laboratory, Amsterdam. (1965a) Pesticide Report KvW
112, dated February 1965, unpublished
Food Inspection Laboratory, Amsterdam. (1965b) Pesticide Report KvW
115, dated May 1965, unpublished
Gaines, Th.B. and Kimbrough, R.D. (1968) Toxicity of fentin hydroxide.
Toxicol. appl. Pharmacol., 12:397-403
Gembloux Centre de Recherches de Phytopharmacie, Gembloux. (1968)
Report No. 34/68, dated June 1968
Hardon, H.J., Besemer, A.F.H. et al. (1962) Feldversuche mit
Triphenylzinn. Dtsch Lebensmitl. Rdsch, 58: 349-352
Heath, D.F. (1963) Some problems in the determination of residues in
plants and mammals in "Radiation and Radioisotopes applied to Insects
of Agricultural Importance", IAEA, Vienna, p. 185-193
Hedges (1961) Letter of the Tin Research Institute, dated 3-8-1961
with attached report
Herok, J. and Götte, H. (1961) The radiometrical estimation of the
distribution and excretion of (113Sn) triphenyltin acetate in
ruminants. Symp. radioisotopes in animal biology, Mexico City,
p.177-188
Herok, J. and Götte, H. (1963) Radiometrische Untersuchungen über das
Verhalten von Triphenylzinnacetat in Pflanze und Tier. Internat.J.
Appl. Radiation and Isotopes, 14: 461-479
Herok, J. and Götte, H. (1964) Radiometrische Stoffwechselbilanz von
Triphenylzinnazetat beim Milchschaf. Zbl. Vet. Med. A 11, 20-28
Hoechst Unpublished report (1961) Ing. Rei/kn(A), dated 22-8-1961
with attachment
Hoechst Unpublished report (1963) Dr.Cz/Bi 3543, dated 15-3-1963
Hoechst Unpublished report (1964) Dr.Cz/bi, dated 2-11-1964
Hoechst Unpublished report (1965a) Dr.Cz/bi, dated 17-9-1965
Hoechst Unpublished report (1965b) Hk/da-4565 b, dated 11-11-1965
Hoechst Unpublished report (1966a) 1/67 1 t/m 4
Hoechst Unpublished report (1966b) 94/66 1 t/m 4
Hoechst Unpublished report (1966c) 94/66 1 t/m 4
Hoechst Unpublished report (1966d) 62/661-2. 70/66 3, 82/66 4
Hoechst Unpublished report (1966e) 94/66 2 t/m 4
Hoechst Unpublished report (1966f) 95/66 1-2
Hoechst Unpublished report (1966g) 83/66 2 t/m 4
Hoechst Unpublished report (1966h) 61/66 3, 69/66 3, 83/66 41 94/66 5
Hoechst Unpublished report (1967) 84,90/66, dated 3-5-1967
Hoechst Unpublished report (1968a) A1/67 IV, dated 6-6-1968
Hoechst Unpublished report (1968b) 150/67, dated 29-5-1968
Hoechst Unpublished report (1968c) 151/67, dated 29-5-1968
Hoechst Unpublished report (1968d) 152/67, dated 29-5-1968
Hoechst Unpublished report (1968e) 160/67, dated 5-7-1968
Hoechst Unpublished report (1968f) 161/67, dated 5-7-1968
Hoechst Unpublished report (1968g) A1/67 V, dated 6-6-1968
Hoechst Unpublished report (1968h) 153/67, dated 29-5-1968
Hoechst Unpublished report (1968i) 154/67, dated 29-5-1968
Hoechst Unpublished report (1968j) 155/67, dated 29-5-1968
Hoechst Unpublished report (1968k) 1962/67, dated 5-7-1968
Hoechst Unpublished report (1968l) 163/67, dated 5-7-1968
Hoechst Unpublished report (1968m) 241/67, dated 5-3-1968
Hoechst Unpublished report (1968n) 240/67, dated 5-3-1968
Hoechst Unpublished report (1968o) 17/67, dated 1-4-1968
Hoechst Unpublished report (1968p) 16/67, dated 1-4-1968
Hoechst Unpublished report (1968q) 47/68, 99/68, 100 t/m 102/68
Hoechst Unpublished report (1969a) I-14-68, 1 zu 7/8 + 14/68, dated
7-8-1969
Hoechst Unpublished report (1969b) I-13-68, I zu 13-68, dated
20-6-1969
Hoechst Unpublished report (1969c) IV-9-68, IV zu 7-11/68, dated
7-11-1969
Hoechst Unpublished report (1969d) IV-7-68, IV zu 7-11/68, dated
7-11-1969
Klimmer, O.R. (1964) Toxikologische Untersuchungen mit
Triphenylzinnacetat. Zbl. Vet. Med., 11:29-39
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmitt-Rdsch. 7: 190-193
Kröller, E. (1960) Triphenylzinnverbindungen im Pflanzenschutz und
ihre Rückstandsbestimmung. Dtsch. Lebensmittel-Rdsch., 56: 190-193
Magee, P.N., Stoner, H.B. and Barnes, J.M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Path. Bact., 73: 107-124
Malát, M. (1962) Photometrische Zinnbestimmung mit
Brenzcatechinviolett in Anwesenheit von Gelatine; Colorimetrische
Studien (III). Anal. Chem., 187: 404-409
Nat. Inst. Public Health. (1960) Unpublished report nr. FT/17/60,
dated 23-4-1960
Newton, D.W. and Hays, R.L. (1968) Histological studies of ovaries in
rats treated with hydroxyurea, tri-phenyltin acetate and triphenyl
chloride. J. econ. Entomol., 61: 1668-1669
Pate, B.D. and Hays, R.L. (1968) Histological studies of testes in
rats treated with certain insect chemosterilants. J. econ. Entomol.,
61: 32-34
Philips Duphar Unpublished report (1963a) 56655/2A/63
Philips Duphar Unpublished report (1963b) 56655/38/63
Philips Duphar Unpublished report (1964a) 56646/5/64
Philips Duphar Unpublished report (1964b) 56655/34/63
Philips Duphar Unpublished report (1965) 56655/64/65
Philips Duphar Unpublished report (1966a) 56655/15/66
Philips Duphar Unpublished report (1966b) 56655/47A/66
Philips Duphar Unpublished report (1966c) 56655/54/66
Philips Duphar Unpublished report (1966d) 56655/61/66
Philips Duphar Unpublished report (1967) 56655/2/67
Philips Duphar Unpublished report (1968a) 56655/14/68
Philips Duphar Unpublished report (1968b) 56655/17/68
Philips Duphar Unpublished report (1970) 566461/1/70
Ross, W.J. and White, J.C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33:421-427
Scholz, D. (1963) Toxikologische Prüfung von Triphenylzinnchlorid und
Hoechst 2840-Spritzpulver. Unpublished report from Hoechst A.G., 18
December 1963
Scholz, D. and Baeder, M. (1968) Berichte über ein chronische
Toxizitätsprüfung von Triphenylzinnacetat per os an Ratten (4 Monate).
Unpublished report from Hoechst A.G.
Schulz, D. and Baeder, O. (1970) Berichte über fortpflanzungsversuche
mit triphenylzinnacetat an Ratten (Einflues auf Fertilitat,
Gravidität, postnatale Entvicklung). Unpublished report from Hoechst
AG
Scholz, D. and Brunk. (1968a) Chronische oral toxizitätsprufung von
Triphenylzinnacetat (Brestan-Wirkstoff) 120 Tage-Versuch an Hunden.
Unpublished report from Hoechst A.G.
Scholz, D. and Brunk. (1968b) Chronische oral toxizitätsprafung von
Brestan-Wirkstoff 2-Jahres-Versuch an Hunden. Unpublished report from
Hoechst A.G.
Scholz, D. and Weigand. (1968) Triphenylzinnacetat.
Wirkstoff-Technische. 4-Monate-Futterungsversuch an Meerschweinchen.
Unpublished report from Hoechst A.G.
Stoner, H.B. (1966) Toxicity of triphenyltin. Brit. J. industr. Med.,
23: 222-229
Stoner, H.B. and Heath, D.F. (1967) The cummulative action of
triphenyltin. Fd. Cosmet. Toxicol., 5: 285-286
Syrowatka, T. (1969) Investigation of the action of organotin
compounds on the oxidative phosphorylation and permeability of
membrane of rat liver mitochondria Roczn. Zak. Hig. (Warsz.), 20:
717-725
Syrowatka, T. (1970) Influence of fungicides on cellular energetic
processes. Roczn. Zak. Hig. (Warsz.), 21: 105-115
Tauberger, G. (1963) Tierexperimentelle Analyse der
Triphenylzinnwirkung nach intravenöser Injektion. Med. exp. (Basel),
9: 393-399
Thompson-Hayward Unpublished report (1965a) R-542
Thompson-Hayward Unpublished report (1965b) R-568
Thompson-Hayward Unpublished report (1967) R-650
Thompson-Hayward Unpublished report (1969) R-755
Til, H.P. and Feron, V.J. (1965) Triphenyltinhydroxide range-finding
test with dogs. Unpublished TNO report submitted by Philips-Suphar,
No. R-1994
Til, H.P., Feron, V.J. and de Groot, A.P. (1967) Reproduction study
with triphenyltinhydroxide in three generations of rats. Unpublished
TNO report submitted by Philips-Duphar, No. 2476
Til, H.P. and Feron, V.J. (1968) Chronic (two-year) toxicity study
with triphenyltinhydroxide (TPTH) in beagle dogs. Unpublished TNO
report submitted by Philips-Duphar, No. 2717
Til, H.P., Feron, V.J. and der Groot, A.P. (1968) Observations on a
possible effect of TPTH on testicular development in rats. Unpublished
TNO report No. R 2620 submitted by Philips-Duphar.
Til, H.P., Feron, V.J. and de Groot, A.P. (1970) Chronic toxicity
study with triphenyltinhydroxide in rats for two years. Unpublished
TNO report submitted by Philips-Duphar, No. R-3138
Ueda, K., Iijima, K. and Yoshida, S. (1961) Acute oral toxicity of
organotin compounds for mice. Unpublished report from Tokyo Dental
College submitted to Sankyo Chemical Co.
Van Esch, G.J. and Arnoldussen, A.M. (1962) Range-finding test as to
the toxicity of triphenyltinhydroxide during 18 days. Unpublished
report of the National Institute of Public Health, Utrecht, No. Tox
39/62
Verschuuren, H.G., Kroes, R. and van Esch, G.J. (1965) Semi-chronic
investigation as to the toxicity of triphenyltinhydroxide in guinea
pigs. Unpublished report of the National Institute of Public Health,
Utrecht, No. Tox. 33/65
Verschuuren, H.G., Kroes, R. Vink, H.H. and van Esch, G.J. (1966)
Short-term toxicity studies with triphenyltin compounds in rats and
guinea-pigs. Fd. Cosmet. Toxicol., 4: 35-45
Verschuuren, H.G., Ruitenberg, E.J., Peetoom, F., Helleman, P.W. and
van Esch, G.J. (1970) Influence of triphenyltin acetate on lymphatic
tissue and immune responses in guinea pigs. Toxicol. appl. Pharmacol.,
16: 400-410
Weigand and Kief, Brestan-Wirkstoff (1965) 96 percent ig =
Triphenylzinnacetate 96 percent ig Chronischer Futterungsversuch.
Meerschweinchen. Unpublished report from Hoechst A.G., 25 May 1965
Wolf, C., Fancher, O.E. and Calandra, J.C. (1966) 90 day sub-acute
oral toxicity of triphenyltin hydroxide - albino rats. Unpublished
report from Industrial Bio-test Laboratories Inc., submitted to
Thompson-Hayward Chemical Co.
PART II
LIST OF INDUSTRIAL REPORTS ON ANALYTICAL METHODS
Philips Duphar L3-52-16. Clean-up for the residue determination
of triphenyltin compounds in vegetable
matter.
Philips Duphar L3-52-18. Clean-up for the residue determination
of triphenyltin compounds in potatoes.
Philips Duphar L3-52-19. Absorptiometric determination of tin in
cleaned-up residues.
Philips Duphar L3-52-21. Clean-up for the residue determination
of fentin hydroxide in rice.
Philips Duphar L3-52-23. Clean-up for the residue determination
of triphenyltin compounds in tomatoes,
carrots, onions and butter-beans.
Philips Duphar L3-52-25. Clean-up for the residue determination
of triphenyltin compounds in
sugar-beets.
Philips Duphar L3-52-26. Clean-up for the residue determination
of triphenyltin compounds in celery and
sugar-beets.
Philips Duphar L3-52-29. Clean-up for the residue determination
of triphenyltin compounds in roasted
coffee-beans.
Farbwerke Hoechst. Die Bestimmung kleiner Mengen von
1966 Triphenylzinn in Pflanzenmaterial (Dr.
Gorbach 111/66 dated 1-12-1966).
Thompson-Hayward. Clean-up for the polarographic residue
1965a determination of triphenyltin compounds in
potatoes + polarographic tin determination in
pre-cleaned residues (from report 2-542).
Thompson-Hayward. Clean-up for the polarographic residue
1965b determination of triphenyltin compounds in
pecans + polarographic determination of
inorganic tin (from report R-568).
Thompson-Hayward. Clean-up procedure for the colorimetric
1967 residue determination of triphenyltin
compounds in peanuts (from report R-650).
