First draft prepared by
U. Mueller
Chemicals and Non-Prescription Medicines Branch
Therapeutic Goods Administration, Canberra, ACT, Australia
Fenitrothion is the ISO approved common name for O,O-dimethyl-O-(4-nitro-meta-tolyl) It is a broad-spectrum organophosphorus pesticide. Its toxicity was first evaluated by the 1969 Joint Meeting, which established a temporary ADI of 0–0.001 mg/kg bw on the basis of a NOAEL of 0.25 mg/kg bw per day in a 3-month study in rats (Annex 1, reference 12). In 1974, new data were reviewed, and the ADI was increased to 0–0.005 mg/kg bw on the basis of inhibition of plasma cholinesterase activity observed in a 92-week study in rats (Annex 1, reference 22). This ADI was reaffirmed by the 1977 Joint Meeting (Annex 1, reference 28). However, some of the pivotal studies used to establish the ADI were based on data generated by Industrial Bio-Test Laboratories and had not been validated. Replacement studies or independent validations were requested, but these were not available in time for the 1982 Joint Meeting. Consequently, a temporary, lower ADI of 0–0.001 mg/kg bw was established (Annex 1, reference 38). At the 1984 Joint Meeting, the ADI was increased to 0–0.003 mg/kg bw but was still considered temporary owing to the absence of a suitable study of developmental toxicity (Annex 1, reference 42). In 1986, an acceptable study of developmental toxicity in rats was reviewed, and an ADI of 0–0.003 mg/kg bw was established (Annex 1, reference 47). The most recent review, in 1988, which reflected the new JMPR policy of using inhibition of brain cholinesterase activity (or erythrocyte acetylcholinesterase activity as a surrogate) instead of inhibition of plasma cholinesterase activity as the toxicologically relevant end-point for cholinesterase-inhibiting compounds, included data that had been reviewed previously and increased the ADI to 0–0.005 mg/kg bw on the basis of a NOAEL of 0.5 mg/kg bw per day in a 2-year study of toxicity in rats; this value was supported by a NOAEL of 0.65 mg/kg bw per day in a study of reproductive toxicity in rats (Annex 1, reference 53). Fenitrothion was reviewed by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
(a) Absorption, distribution, and excretion
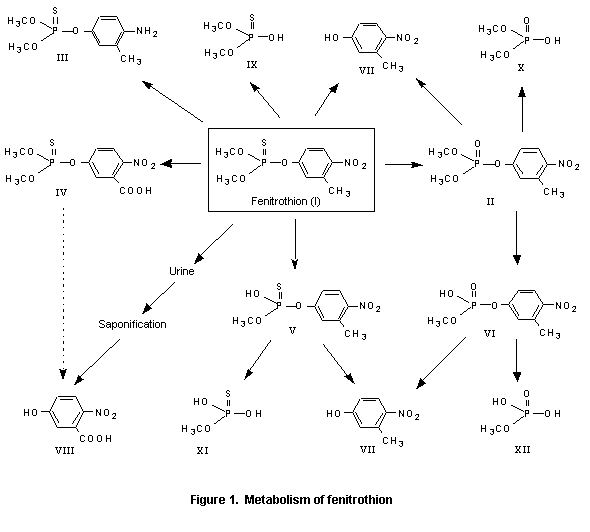
Fenitrothion is presumably rapidly absorbed from the mammalian intestinal tract, as evidenced by the appearance of radiolabel in blood from guinea-pigs and rats given [32P]fenitrothion orally. The presence of the oxygen analogue was demonstrated in all tissues examined (brain, heart, lung, liver, kidney, spleen, and muscle), and it was detectable in blood 1 min after intravenous injection of fenitrothion. This oxygen analogue (II in Figure 1) is the most important metabolite with respect to toxicity. It is formed in the microsomal fraction of the cell, the main organs responsible for the transformation being the liver and kidney. Fenitrooxon is further metabolized as indicated in Figure 1. The major excretion product found is 3-methyl-4-nitrophenol (VII) which can be oxidized further to 3-carboxyl-4-nitrophenol (VIII). Other metabolites are the demethyl derivatives (V and VI), which, with increasing dose, are excreted in increasing amounts. Nine metabolites have been isolated, most of which have also beeen identified. In vitro, formation of the oxygen analogue (II) depended on the availability of reduced nicotine adenine dinucleotide phosphate (NADPH2) and oxygen. Liver slices incubated with fenitrothion did not produce measurable amounts of fenitrooxon, while liver homogenates and the supernatant fraction of such homogenates appreciably activated added fenitrothion. No correlation between the toxicity and rate of formation of fenitrooxon could, however, be demonstrated (Miyamoto et al., 1963a; JMPR, 1969; Miyamoto, 1969). No observations were made in these studies on the distribution into fatty tissues, but studies of residues in milk, meat, and fat from cattle indicated the presence of approximately 0.001 mg/kg in these samples (JMPR, 1969; Miyamoto & Sato, 1969).
Like other organophosphorous compounds, fenitrothion acts in the organism as a cholinesterase inhibitor, probably after conversion to fenitrooxon. Some evidence indicates that the acetylcholinesterase inhibiting effect in brain depends more on the rate of penetration than on the rate of oxidation and decomposition of fenitrothion (JMPR, 1969; Miyamoto, 1969).
Female rats were given a single oral dose of 200 mg/kg bw of fenitrothion and/or malathion. The concentration of fenitrothion in the liver was lower in those animals that received both compounds than in those receiving only fenitrothion. However, after an initial decrease, the content of fenitrothion increased in blood and muscle of rats that received malathion as well. The concentration of fenitrooxon, the toxic metabolite of fenitrothion, increased markedly with time in blood and muscle of rats given fenitrothion and malathion in combination, in contrast to rats given only fenitrothion, which showed a rapid peak and decline in fenitrooxon concentrations. The liver fenitrooxon concentrations were not appreciably altered by the combined regimen. These studies suggest the possibility that fenitrothion is potentiated by malathion, but no tests for potentiation were conducted (Hladká et al., 1974; JMPR, 1974).
After oral administration of fenitrothion to mice and subcutaneous administration to rats and guinea-pigs, it was cleared quite rapidly, 90% of the administered dose being recovered within 3 days. Mice metabolized fenitrothion to fenitrooxon, 3-methyl-4-nitrophenol, and demethylated products rapidly (Douch et al., 1968).
Radiolabel from fenitrothion administered daily to goats in the diet for 7 days was detectable at high concentrations in the gastrointestinal tract and at lower concentrations in the tissues on day 8, with negligible quantities by day 25. Fenitrothion was excreted in both urine and faeces. The concentration in milk was relatively low and decreased rapidly when fenitrothion was withdrawn from the diet (Mihara et al., 1978).
[14C]Fenitrothion administered orally to rats once at 1.5 or 150 mg/kg bw or at 1.5 mg/kg bw after 14 administrations of unlabelled fenitrothion at 1.5 mg/kg bw per day over 14 days was almost completely recovered in the urine and faeces (95–102%) within 7 days. Most of the administered dose was present in urine, where it accounted for 86–101% of the total. The concentrations of residues in tissues were relatively low, the highest being found in liver. After administration of a low dose or repeated doses, 1–16 µg/kg of fenitrothion equivalents were detected, whereas after a high dose the concentration ranged from 170 to 450 µg/kg. Conjugated phenolic compounds, such as the sulfate and glucuronide of 3-methyl-4-nitrophenol, accounted for 54–66% of the administered dose after single or repeated administration of a low dose. At the high dose, the main metabolites were demethylated compounds such as demethylfenitrothion and demethylfenitrooxon (43–58% of the dose). There was no appreciable difference in metabolism between the sexes. Hence, the major biotransformations were demethylation of one of the P–O methyl groups from either the parent or the oxon, cleavage of the P–O–aryl linkage, and conjugation of the resulting phenol, mainly as sulfate but partly as glucuronide (Iba et al., 1990).
In a study of the penetration of fenitrothion and parathion-methyl into brain tissue and the effects on brain acetylcholinesterase, the concentrations of both compounds in the brain peaked 5 min after administration. Inhibition of brain acetylcholinesterase activity was more rapid and extensive after treatment with parathion-methyl than with fenitrothion (Miyamoto, 1964).
The dermal absorption of fenitrothion was examined in rats by applying a low dose (0.73 g/kg bw), a high dose (3.7 g/kg bw), or repeated doses to shaved skin. After a single application, the maximum plasma concentrations were obtained 8 h later. Whole-blood cholinesterase inhibition correlated with the fenitrothion concentration in plasma. Approximately 55% of the low dose remained unabsorbed (in and on the skin) after 24 h. No data on the absorption of the high dose were supplied. This dose produced significant clinical signs and plasma concentrations that were about 10 times higher than that observed with 0.73 g/kg bw at 48 h. Multiple applications resulted in increasing plasma concentrations of fenitrothion and increasing inhibition of cholinesterase activity (Kohli et al., 1974).
A generalized scheme for mammalian metabolism of fenitrothion is shown in Figure 1. This pathway was derived from data given in many of the studies cited in the following section. An overview of data from a number of studies indicated that fenitrothion is relatively well absorbed from the intestinal tract in most species. Activation to the active metabolite, fenitrooxon, occurs in the liver, followed by relatively rapid degradation to inactive metabolites, which are then excreted. Additionally, a direct degradation pathway is observed in rats.

A study of the metabolism of [14C-ring-methyl]fenitrothion (purity, > 99.8%) was carried out with particular attention to the fate of the phenolic moiety. Male rats were given a single oral dose of 15 mg/kg bw of labelled fenitrothion, and the concentrations were determined in tissues 1 and 24 h later. After 1 h, not more than 25% of the applied dose was present in the gastrointestinal tract, and significant amounts were found in the kidneys (12 mg of fenitrothion equivalents per kg), stomach and intestines (5.4 mg/kg), liver (2.6 mg/kg), blood (2.1 mg/kg), and lungs (1.1 mg/kg). In these tissues, > 80% of the radiolabel represented water-soluble metabolites, except in the stomach and intestines where 68% was fenitrothion. Kidneys also had a high content of 3-methyl-4-nitrophenol (VII; 2.1 mg/kg); in other tissues, this metabolite was present at < 0.2 mg/kg. Little fenitrooxon (II) was found: 0.024 mg/kg in the stomach and intestines, 0.008 mg/kg in the kidneys, and < 0.005 mg/kg in other tissues. Some fenitrothion was detected in fat and pancreas (both 0.5 mg/kg). By 24 h after administration, all tissues contained < 0.1 mg/kg of total 14C, with the highest concentrations in liver, kidneys, fat,and stomach and intestine, partly as fenitrothion and partly as metabolites. The concentrations of all metabolites in other tissues were < 0.005 mg/kg.
A similar distribution was observed on autoradiograms of mice and rats, shortly and 24 h after application of 15 mg/kg bw of labelled fenitrothion. The concentrations of fenitrothion were determined in the blood of rats, rabbits, mice, and dogs 1, 3, 9, 24, and 48 h after oral administration of 15 mg/kg bw. A maximum was observed at 1 or 3 h, and the concentrations were < 0.01 mg/kg at 24 h. After five male rabbits were fed 0.3 or 10 mg/kg bw per day of fenitrothion for 6 months, neither fenitrothion (limit of detection, 0.005 ppm) nor fenitrooxon (II; 0.001 ppm) was detectable in blood or skeletal muscle. In fat, 0.13 mg/kg of the oxygen analogue was found.
After a single oral dose of 15 mg/kg bw of labelled fenitrothion to mice, rats, rabbits, and dogs, 80–90% of the radiolabelled metabolites were excreted in the urine within 24 h and 5% with the faeces; excretion was nearly complete by 48 h after treatment. Rats did not expire radiolabel. In urine, 18 metabolites were identified; 3-methyl-4-nitrophenol (VII; as the sulfate, free or as glucuronide) represented 50–70% of the metabolites in the rodents and only 35% in dogs, while O-demethylfenitrothion (V) or O-demethylfenitrooxon (VI) represented from 10% of metabolites in rabbits to 55% in dogs. The urine of rabbits and rats also contained small quantities of other metabolites in which the nitro group was reduced to amino fenitrothion (III) (total, about 15%) or had an oxidized ring methyl group (IV, VIII; about 1%). Fenitrothion was not detectable, and traces of fenitrooxon (II; 0.4%) were determined only in rabbit urine. About 5% remained unidentified. The faeces of rats contained the same major metabolites as urine—70% 3-methyl-4-nitrophenol (VII) and 20% O-demethyfenitrothion/oxon (V, VI)—with some fenitrothion (10%) (Miyamoto et al., 1976; JMPR, 1977).
In order to investigate differences in metabolism between [32P]parathion-methyl and the structurally related fenitrothion (radiochemical purities, > 95%) in male Swiss mice, each compound was administered separately in olive oil by gavage at two different doses. The mice were housed individually in metabolism cages, and urine and faeces were collected for 72 h after dosing. Whereas parathion-methyl at 3 mg/kg bw caused slight signs of intoxication, severe cholinergic symptoms were observed at 17 mg/kg bw. No clinical signs were observed with fenitrothion at the same doses, but when it was given at 200 or 850 mg/kg bw, slight and severe cholinergic signs, respectively, were seen. Fenitrothion was excreted rapidly and relatively completely. Within 24 h, 55% of the highest dose was collected in the urine, and more than 75% of the three lower doses had been excreted. After 72 h, > 90% of all administered doses of fenitrothion had been excreted in the urine or faeces. The initial excretion of parathion-methyl followed a similar pattern, except that excretion slowed at around 24 h, and only 85% of the administered dose had been excreted by 72 h.
The metabolite profile after administration of fenitrothion at low doses differed substantially from that at high (and toxic) doses. At 3 mg/kg bw, > 30% of the urinary metabolites of fenitrothion consisted of dimethylphosphoric acid (X); desmethylphosphate (XII) made up 26% of the metabolites, while desmethylphosphorothioate (XI) made up 21%. At 850 mg/kg bw, desmethyl-phosphorothioate (XI) made up 66% of the urinary metabolites, while desmethylphosphate (XII) made up 17%. Other metabolites were present in negligible quantities after administration of this dose. In comparison, the main urinary metabolite of parathion-methyl was dimethylphosphoric acid, representing > 50% of the urinary metabolites at 3 mg/kg bw and 32% at 17 mg/kg bw. Less than 20% of the urinary metabolites was made up of desmethylphosphorothioate.
Thus, while demethylation appears to be an important step in the metabolism of fenitrothion, it is of lesser importance for parathion-methyl. Additionally, there appears to be a delay in the oxidation of the P=S bond to P=O in fenitrothion in comparison with parathion-methyl, as indicated by the higher proportion of P=O metabolites in the urine of parathion-methyl-treated animals (72% at 3 mg/kg bw) relative to fenitrothion-treated animals (64% at 3 mg/kg bw). This decrease or delay in activation may contribute both to the lower toxicity of fenitrothion in mammals and to the increase in the demethylation metabolism (Hollingworth et al., 1967).
(c) Effects on enzymes and other biochemical parameters
Fenitrothion appears to affect cytochrome P450 enzyme activity in the liver and testes of rats. In the short term (< 72 h), fenitrothion at a dose of 261 mg/kg bw caused a decrease in activity, resulting in reduced concentrations of serum testosterone (25% of normal), which returned to normal within 5 days. Treatment at 5.5 mg/kg bw for 30 days did not change the enzyme activity or the serum concentration of testosterone (Gradowska-Olszewska et al., 1984; Clos et al., 1994).
When fenitrothion was given orally to male Wistar rats for 28 days at a dose of 0, 7.25, or 14.5 mg/kg bw per day, the highest dose increased plasma corticosterone and glucose concentrations by 2.5-fold (p < 0.01) and 30% (not significant), respectively, by week 1 and preceded a significant increase in adrenal weight (35%; p < 0.05) by week 2. However, these changes were transient, and all values had returned to control levels by the end of treatment. A similar trend was observed at the lower dose, but the changes did not achieve significance (Yamamoto et al., 1982).
In order to ascertain why fenitrothion is less toxic to mammals than parathion-methyl, even though they apparently have comparable insecticidal efficacy, the two compounds were incubated with mouse liver slices or liver microsomes in vitro. After incubation, the degree of bovine erythrocyte or fly-head cholinesterase inhibition was increased with parathion-methyl and slightly reduced with fenitrothion relative to the respective parent compounds. Testing for the presence of metabolites arising from metabolism by liver supernatant revealed that the conversion to inactive metabolites was approximately three times faster with fenitrothion than with parathion-methyl. Therefore, the authors suggested that the lesser toxicity of fenitrothion is due to the greater metabolism of fenitrothion and its metabolites in mammalian liver. After incubation of the two compounds with cockroach fat, both had increased cholinesterase inhibition potency (Vardanis & Crawford, 1964).
(i) Median lethal dose
Numerous studies have been carried out with technical-grade fenitrothion and are summarized in Table 1. The lowest oral LD50 in rats was 240 mg/kg bw, and that in mice was 780 mg/kg bw. The signs of acute intoxication were consistent with cholinesterase inhibition and included inactivity, salivation, dyspnoea, flaccid paralysis, vomiting, piloerection, exophthalmia, and diarrhoea; male animals were generally more sensitive to the acute effects of fenitrothion than female animals.
Table 1. Studies of the acute toxicity of technical-grade fenitrothion
|
Species |
Strain |
Sex |
Vehicle |
Route |
LD50 |
Reference |
|
Mouse |
NR |
M/F |
Glycerol: ethanol |
Oral |
1300 (M), |
Toxicology Research Unit (1964); JMPR (1969) |
|
|
dd |
M/F |
Tween 80 |
Oral |
1000 (M), |
Kadota et al. (1972a); - JMPR (1974) |
|
|
Wistar |
M/F |
Cremophor EL |
Oral |
780 (M), |
Heimann (1982) |
|
|
dd |
M/F |
Corn oil |
Oral |
1400 (M), |
Mikami et al. (1977) |
|
|
dd |
M/F |
None |
Dermal |
> 2500 (M/F) |
Kadota et al. (1972a) |
|
|
dd |
M/F |
Tween 80 |
Subcutaneous |
1400 (M), |
Kadota et al. (1972a) |
|
Rat |
Wistar |
M/F |
NR |
Oral |
940 (M), |
Benes & Cerna (1970); JMPR (1974) |
|
|
Sherman |
M/F |
Peanut oil |
Oral |
740 (M), |
Gaines (1969); JMPR (1969) |
|
|
Sprague-Dawley |
M/F |
Ethanol: propylene glycol |
Oral |
250 (M/F) |
Dubois & Puchala (1960); JMPR (1969) |
|
|
Holtzman |
F |
Ethanol: propylene glycol |
Oral |
240 (F) (mean of four experiments) |
Dubois & Kinoshita (1970) |
|
|
NR |
M/F |
Glycerol: ethanol |
Oral |
500 (M), |
Toxicology Research Unit (1964) |
|
|
Wistar |
M |
NR |
Oral |
610 (M) |
Carmargo et al. (1970) |
|
|
Sprague-Dawley |
M/F |
Tween 80 |
Oral |
330 (M), |
Kadota et al. (1972); JMPR (1974) |
|
|
Wistar |
M |
Olive oil |
Oral |
700a (M) |
Rosival et al. (1976) |
|
|
Wistar |
M |
Olive oil |
Oral |
490 (M) |
Rosival et al. (1976) |
|
|
Sprague-Dawley |
M/F |
Corn oil |
Oral |
660 (M), |
Mikami et al. (1977) |
|
|
Wistar |
M/F |
Water |
Oral |
2000b (M), |
Hixson (1982a; GLP) |
|
|
Sprague-Dawley |
M/F |
NR |
Oral |
1700 (M), |
Kato et al. (1986; QA) |
|
|
NR |
F |
None |
Dermal |
3500 (F) |
Toxicology Research Unit (1964) |
|
|
Wistar |
F |
NR |
Dermal |
1000 (F) |
Carmargo et al. (1970) |
|
|
Sprague-Dawley |
M/F |
None |
Dermal |
890 (M), |
Kadota et al. (1972) |
|
|
Sprague-Dawley |
M/F |
Corn oil |
Dermal |
2700 (M), |
Mikami et al. (1977) |
|
|
Sprague-Dawley |
M/F |
Kerosene: xylene |
Inhalation, 8 h |
> 0.19 (M/F) |
Kohda & Kadota (1979) |
|
|
Sprague-Dawley |
M/F |
Corn oil |
Inhalation, 4 h |
> 2.2 (M/F) |
Kohda et al. (1986) |
|
|
Sprague-Dawley |
M/F |
Ethanol: propylene glycol |
Intraperitoneal |
140 (M), |
Dubois & Puchala (1960); modified from JMPR (1969) |
|
|
Sprague-Dawley |
M |
Corn oil |
Intraperitoneal |
300 |
Chevalier et al. (1982) |
|
|
Sprague-Dawley |
M/F |
Tween 80 |
Subcutaneous |
840 (M), |
Kadota et al. (1972) |
|
Guinea-pig |
NR |
M |
Glycerol: ethanol |
|
1000 (M) |
Toxicology Research Unit (1964) |
|
|
NR |
M |
Ethanol: propylene glycol |
|
500 (M) |
Dubois & Puchala (1960); modified from JMPR (1969) |
|
|
NR |
NR |
NR |
|
1800 |
Miyamoto et al. (1963b); JMPR (1969) |
|
|
NR |
M |
Ethanol: propylene glycol |
Intraperitoneal |
110 |
Dubois & Puchala (1960); modified from JMPR (1969) |
|
Rabbit |
New Zealand white |
M/F |
Water |
Dermal |
3300b (M), |
Hixson (1982b) |
|
Chicken |
NR |
F |
Tween 80 |
|
~ 500 |
Kadota et al. (1975a); JMPR (1977) |
|
Japanese quail |
NR |
M/F |
NR |
|
120 (M), |
Kadota & Miyamoto (1975); JMPR (1977) |
NR, not reported; M, male; F, female; GLP, good laboratory practice; QA, quality assurance
a
Purified fenitrothionb
Purity, 75.8%The acute toxicity of fenitrothion (purity, 96.6%) dissolved in corn oil was assessed in groups of 10 male and 10 female 5-week-old Sprague-Dawley rats who were treated by inhalation for 4 h at a mean concentration of 0 (vehicle control), 0 (air, negative control), 0.004, 0.009, 0.038, 1.0, or 2.2 mg/L in an aerosol apparatus for whole-body exposure. The median aerodynamic diameter of the aerosol particles was 0.59–0.82 µm. Clinical signs and changes in body weight were recorded during exposure and for 14 days thereafter. Plasma, erythrocyte, and brain cholinesterase activities were determined in satellite groups of 10 animals per sex at the two higher concentrations and five of each sex at the three lower concentrations. Blood was collected for monitoring of cholinesterase activity on days 1, 3, 7, and 14 from all groups and from controls and the groups at the two higher concentrations on days 21, 29, 35, and 42. Additional readings of cholinesterase activity were made for the vehicle controls and those at the highest concentration on days 48 and 56. Gross necroscopy was performed on rats that died during the study and on survivors killed at the end of exposure.
One male rat at 2.2 mg/L died on day 6, and rats at 1.0 mg/L had irregular respiration, nasal discharge, decreased spontaneous activity, lachrymation, salivation, and urinary incontinence; muscular fibrillation was observed only in male rats. At 2.2 mg/L, additional clinical signs such as hyperpnoea and intermittent tremors were observed; tonic convulsions, ataxia, and soft faeces were observed only in male rats. The clinical signs began 30 min after exposure and disappeared within 9 days after the end of exposure. A significant decrease in body-weight gain was found at the end of the observation period in males (p < 0.01) and females (p < 0.05) at 2.2 mg/L, by 58% in males and 24% in females relative to that of vehicle controls. The inhibition of cholinesterase activity relative to vehicle controls is shown in Table 2. At day 14 and thereafter, the reductions in plasma cholinesterase activity were not significant, but the significant inhibition of erythrocyte cholinesterase activity observed at 1.0 and 2.2 mg/L on days 1, 7, and 14 was still evident in animals of each sex on days 21 and 28. In brain, significant reductions in cholinesterase activity were observed at 1.0 and 2.2 mg/L on days 42 and 56, respectively. Thus, inhibition of cholinesterase activity was observed for 1–7 days after the beginning of exposure. Specifically, reduced plasma cholinesterase activity in males and females at concentrations ł 0.009 mg/L, reduced erythrocyte cholinesterase activity in males at ł 0.038 mg/L and in females at 0.009 mg/L, and reduced brain cholinesterase activity in males and females at 1.0 mg/L. The activity had generally recovered by 3–56 days after exposure, although the brain cholinesterase activity was still low on day 56 in animals at the highest concentration. As no pathomorphological findings were attributed to treatment, the LC50 was estimated to be > 2.2 mg/L for both male and female rats (Kohda et al., 1986).
Table 2. Mean percent inhibition of cholinetserase activity in rats treated with fenitrothion
|
Location |
Interval (days) |
Concentration (mg/L) |
||||
|
Male |
||||||
|
0.004 |
0.009 |
0.038 |
1.0 |
2.2 |
||
|
Plasma |
1 |
17 |
34* |
60** |
87** |
93** |
|
|
7 |
8 |
9 |
27* |
23* |
78** |
|
|
14 |
10 |
4 |
12 |
3 |
12 |
|
Erythrocyte |
1 |
[7] |
[6] |
10* |
74** |
84** |
|
|
7 |
[6] |
3 |
1 |
56** |
87** |
|
|
14 |
[3] |
[4] |
10 |
48** |
64** |
|
Brain |
7 |
1 |
7 |
5 |
65** |
85** |
|
|
14 |
2 |
7 |
14** |
40** |
57** |
Table 2 (continued)
|
Location |
Interval (days) |
Concentration (mg/L) |
||||
|
Female |
||||||
|
0.004 |
0.009 |
0.038 |
1.0 |
2.2 |
||
|
Plasma |
1 |
5 |
50** |
72** |
95** |
94** |
|
|
7 |
8 |
10 |
1 |
63* |
95** |
|
|
14 |
12 |
0 |
14 |
0 |
24* |
|
Erythrocyte |
1 |
12 |
14* |
32** |
79** |
85** |
|
|
7 |
[3] |
[4] |
10 |
48** |
64** |
|
|
14 |
7 |
7 |
15* |
53** |
60** |
|
Brain |
7 |
[9] |
[12] |
12 |
60** |
88** |
|
|
14 |
5 |
2 |
13* |
44** |
58** |
From Kohda et al. (1986)
Values in square brackets indicate the extent (%) to which the measured activity was greater than that in controls
* p
Ł 0.05; ** p Ł 0.01 (Student’s t test)(ii) Ocular and dermal irritation and dermal sensitization
Fenitrothion (purity, 75.8%) diluted to give a 24% (w/v) solution was instilled into the conjunctival sac of the left eye of seven female and two male New Zealand white rabbits in aliquots of 0.1 mL. The right eye served as a control. The treated eyes of two males and one female were flushed with water 45 s after treatment, and those of the remaining rabbits were left unflushed. The treated eyes were examined for signs of irritation 1, 2, 3, 4, and 7 days after treatment. Rabbits whose eyes showed a response on day 7 were held until day 14. Irritation was graded and scored according to the method of Draize and the criteria for eye irritants of the Federal Hazardous Substances Act, USA, 1972. The studies were conducted in accordance with GLP. The rabbits whose eyes were not flushed after treatment showed mild to moderate erythema (score, 1–2; range, 0–4) 1 day after treatment, but no erythema was observed after day 3 (two of six animals), day 4 (two of six animals), or day 7 (two of six animals). No corneal opacity or iritis was observed after treatment. Slight chemosis (score, 1; range, 0–3) was observed on day 1 only in one female rabbit. A moderate to severe discharge (score, 1–3; range, 0–3) was observed in all animals, which had cleared by day 8 after treatment. Rabbits whose eyes were flushed after treatment showed no corneal opacity or iritis. The mild to moderate erythema (score, 1–2; range, 0–4) observed in these animals had improved by day 4 (two of three animals) and day 7 (one of six animals). Slight chemosis (score, 1; range, 0–3) was observed in one female and one male. Under the conditions of this study, diluted fenitrothion was irritating to the eye, although the effect was reversed by day 8 after exposure (Hixson, 1982c).
Technical-grade fenitrothion (purity, 96.5%) was instilled into the conjunctival sac of one eye of each of nine male New Zealand white rabbits in aliquots of 0.1 mL. The treated eyes of three animals were flushed with water 30 s after treatment for 1 min. The other eye served as a control. The treated eyes were examined for signs of irritation at 1, 24, 48, 72, and 96 h and 1 week after treatment. The irritation was graded and scored according to the Draize system. No indication was given about the GLP status of the study. The only evidence of ocular irritation was slight conjunc-tival redness in the unwashed eyes 1 h after application, which had disappeared within 48 h. Under the conditions of this study, fenitrothion was not irritating to the eye (Hara & Suzuki, 1981).
Technical-grade fenitrothion (purity, 97.4%) was instilled into the conjunctival sac of one eye of each of six New Zealand white rabbits. The exact amount instilled and details of the method were not provided, but the investigator stated that the tests were performed in accordance with the protocol of the 1981 Interagency Regulatory Liaison Group, USA. The treated eyes were examined for signs of irritation at 1, 24, 48, and 72 h and at 1, 2, and 3 weeks thereafter. The irritation was graded and scored according to a scale outlined in the 1972 Federal Register of the USA. No evidence of corneal damage or eye irritation was reported other than slight redness of the conjunctivae after 1 h but not after 24 h. Under the conditions of this study, fenitrothion was not irritating to the eye (Thyssen & Lorke, 1982).
Technical-grade fenitrothion (purity, 96.5%) was applied in a volume of 1.5 ml to intact and abraded shaved skin on the backs of six New Zealand white rabbits and covered with gauze and occlusive dressings for 24 h. At that time, the test sites were wiped to remove the material. The animals were observed for skin reactions 24, 48, and 72 h and 1 week after application, and these were graded and scored according to the system of Draize. No indication was given about the GLP status of the study. No evidence of erythema or oedema was observed. Under the conditions of this study, fenitrothion was not irritating to the skin of rabbits (Hara & Suzuki, 1981).
Technical-grade fenitrothion (purity, 97.4%) was applied to skin of the backs of six male and female New Zealand white rabbits for 24 h, according to the 1973 guidelines of the US Department of Agriculture. The exact amount applied and details of the method were not given. The animals were observed for skin reactions at 24 and 72 h. No evidence of erythema or oedema was observed. Under the conditions of this study, fenitrothion was not irritating to the skin of rabbits (Thyssen & Lorke, 1982).
Technical-grade fenitrothion (purity, 75.8%) was diluted with water to a 24% (w/v) solution and applied in aliquots of 0.5 mL under a 2.5-cm gauze patch to the shaved skin of six male and six female New Zealand white rabbits. Four test sites were used on each animal: two with an abraded stratum corneum and the other two intact. The trunks of the animals were wrapped in a plastic sheet and secured with tape. After 24 h, the sites were cleaned with tissue paper soaked in water, and the skin reactions were graded and scored according to the system of Draize at 24 and 72 h. The study was conducted in accordance with GLP, and all phases of the study were reviewed by a quality assurance unit. All rabbits had slight erythema on the abraded sites 24 h after treatment; three had erythema on the two intact sites and two on one intact site. At 72 h, five rabbits had no erythema, but the remaining rabbit had erythema which cleared after an additional 120 h. Oedema was not observed at any stage. A primary irritation score of 0.46 was calculated from the erythema scores on intact and abraded skin. Under the conditions of this study, fenitrothion was mildly irritating to the skin (Hixson, 1982c).
Technical-grade fenitrothion (purity, 97.2%) was prepared as a 1% or 5% solution in corn oil and applied to the shaved dorsal skin of groups of six male Hartley guinea-pigs on alternate days, followed by a challenge 14 days after the last induction dose. Groups of three vehicle control animals received a challenge with corn oil, 0.1% or 5% fenitrothion, or 0.1% 2,4-dinitrochlorobenzene, while groups of five positive control animals were sensitized and then challenged with 2,4-dinitrochlorobenzene. Animals challenged intradermally with 1% fenitrothion had no reaction, whereas five of six of those challenged with the 5% solution had barely perceptible erythema or swelling 24 h after challenge. All three vehicle control animals had similar reactions after intradermal challenge with 5% fenitrothion and only two after challenge with 0.1%. None of the animals responded to a dermal challenge with the 1% or 5% solution. The positive control group showed the expected haemorrhage and swelling 3–4 h after challenge. Fenitrothion did not senisitize the skin of guinea-pigs under these conditions (Kohda et al., 1972).
(b) Short-term studies of toxicity
Rats
Technical-grade fenitrothion (purity, 96.7%) in sodium taurocholate was administered to male CD rats by gavage at a dose of 0, 2.5, 5, 10, or 20 mg/kg bw per day for 30 consecutive days. The animals were observed daily for signs of toxicity and morbidity. Four rats from each group were killed 8, 15, 22, and 30 days after the beginning of treatment and 8, 15, 29, 57, and 85 days after termination of treatment, and the clinical chemical parameters determined were: cholinesterase activity in plasma, erythrocytes, and brain, hepatic and renal carboxylesterase activity, serum aspartate aminotransferase, alkaline phosphatase, and amylase activity, and glucose, blood urea nitrogen, creatinine, and protein concentrations.
Eight of 36 rats at the highest dose died during the first week of treatment. The clinical signs consisted of salivation, piloerection, diarrhoea, chromodacryorrhoea, excitability, ataxia, muscle fasciculations, generalized tremors, and convulsions. No significant changes were observed in organ or body weights during the treatment period in any dose group, apart from the hghest dose. At this dose, a significant (p < 0.05) reduction in mean body weight was observed at days 30 (15%), 60 (22%) and 87 (15%). These values had returned to control levels by day 115 (i.e. 85 days after termination of treatment). A significant (p < 0.05), dose-related decrease in plasma cholinesterase activity (by 30–65%) was observed on days 8–30 of treatment in rats at doses ł 5 mg/kg bw per day, which had returned to control values by day 38 (i.e. 8 days after termination of treatment). Similarly, a significant (p < 0.05), dose-related decrease in erythrocyte cholinesterase activity (by 30–60%) was observed on days 8–30 of treatment at doses ł 5 mg/kg bw per day, but the activity did not return to control levels until day 45 (i.e. 15 days after termination of treatment). A significant (p < 0.05), dose-related decrease in brain cholinesterase activity (by 60–70%) was observed on days 8–30 of treatment at 10 or 20 mg/kg bw per day, and statistically significant (p < 0.05) reductions were observed at day 30 (by 30%) with 2.5 mg/kg bw per day and at day 22 (by 45%) with 5 mg/kg bw per day. The activities had returned to control values by day 60 (i.e. 30 days after termination of treatment).
Liver carboxylesterase activity was significantly (p < 0.05) decreased (by 50–80%) on days 8–30 at doses ł 2.5 mg/kg bw per day but had returned to control values by day 45 (15 days after termination of treatment) at all doses except 20 mg/kg bw per day, at which a decrease of 25% was still observed. At this dose, the values had returned to normal by day 87 (57 days after termination of treatment). A significant decrease in renal carboxylesterase activity (by 20–70%) was observed on days 8–30 at doses ł 5 mg/kg bw per day. Recovery of activity was rapid, and the values were comparable to those of controls by day 38 (8 days after the end of treatment). No significant treatment-related changes were observed in other clinical biochemical parameters. No NOAEL could be identified since a significant reduction in brain cholinesterase activity was observed at the lowest dose on day 30 of treatment (Trottier et al., 1980).
Technical-grade fenitrothion (purity not stated) was administered in the diet to groups of 16 or 17 male Wistar rats at a concentration of 0, 32, 63, 125, 250, or 500 ppm for 90 days. The rats were observed daily for changes in behaviour and body weight, food intake, and the presence of protein or glucose in the urine. At monthly intervals, four rats per group were killed and the following tissues were taken for examination: brain stem, brain cortex, cerebellum, thyroid, heart, lung, liver, kidney, spleen, adrenal, testis, and prostate. The cholinesterase activities in plasma, erythrocytes, brain, liver, and kidney were determined in the same satellite groups. Noradrenaline and 3-hydroxytyramine concentrations were measured in the brain stem, liver, and spleen, although the rationale was not described. The same tissues from rats killed after 90 days and from rats that died during the study were examined histologically. In addition, two groups of rats maintained on diets containing 0 or 500 ppm of fenitrothion were killed after 11 days of treatment, when the clinical cholinergic signs in the treated group were maximal.
One rat at 500 ppm died. The clinical signs at this dose included muscle fasciculations, ataxia, piloerection, and lachrymation. The ophthalmic changes included corneal opacity and corneal and conjunctival bleeding, which had disappeared by day 30. Four rats at 250 ppm showed muscle fasciculation and lachrymation on days 15–18. Animals at the highest dose appeared to have lost weight (estimated from a graph), which was most marked during the first 7 days of treatment. Food intake also appeared to be reduced over this period. No changes in body weight were observed in any other group. There were no changes in organ weights relative to body weight at 250 ppm, but at 500 ppm the weights of the testes and brain appeared to be increased. This result would be expected in view of the body-weight loss. Absolute organ weights were not reported. No significant histological changes were observed. Urinary analysis showed no treatment-related effects on protein or glucose concentrations.
The inhibition of cholinesterase activity observed during the 3 months of treatment is shown in Table 3. Significant inhibition of plasma cholinesterase activity occured at concentrations ł 125 ppm, erythrocyte cholinesterase activity was inhibited by > 20% at doses ł 63 ppm, whereas brain cholinesterase activity was inhibited only at 500 ppm. It is noteworthy that cholinergic signs probably due to inhibition of the peripheral nervous system were observed at 250 ppm, a concentration at which no appreciable inhibition of brain cholinesterase activity was seen. The noradrenaline and 3-hydroxytyramine content of the brain stem, spleen, and liver showed no treatment-related change. The NOAEL was 63 ppm, equivalent to 6.3 mg/kg bw per day, on the basis of a significant reduction in erythrocyte cholinesterase activity at 125 ppm, correlated with a corresponding significant reduction in plasma cholinesterase activity (JMPR, 1969; modified with reference to the original report of Misu et al., 1966).
Table 3. Per cent inhibition of cholinesterase activity in rats treated with fenitrothion
|
Concentration (ppm) |
Per cent reduction |
||
|
Plasma |
Erythrocytes |
Brain |
|
|
32 |
0 |
20 |
3 |
|
63 |
0 |
26 |
2 |
|
125 |
36 |
57 |
7 |
|
250 |
56 |
70 |
9 |
|
500 |
50 |
82 |
43 |
From JMPR (1969), modified after refrence to the original report of Misu et al. (1966)
Fenitrothion (purity, 97.2%), fenitrooxon (purity, 99%), and 3-methyl-4-nitrophenol (purity, 99.5%) were administered separately in the diet to groups of 15 Wistar rats of each sex for 6 months in the following dosing regimens: controls, fenitrothion at 10, 30, or 150 ppm (equal to 0.6, 1.8, or 9.2 mg/kg bw per day for males and 0.6, 2.0, or 11 mg/kg bw per day for females); fenitrooxon at 5, 15, or 50 ppm (equal to 0.3, 0.9, or 3.0 mg/kg bw per day for males and 0.3, 1.0, or 3.7 mg/kg bw per day for females); or 3-methyl-4-nitrophenol at 150, 500, or 1500 ppm (equal to 9.2, 31, or 95 mg/kg bw per day for males and 10, 33, or 100 mg/kg bw per day for females). The animals were observed daily for behavioural changes and deaths. Body weights and food and water consumption were recorded weekly. At weeks 4, 8, 12, and 24, the urine was analysed for glucose, protein, bilirubin, and urobilinogen concentrations and occult blood. At the end of the study, plasma, erythrocyte, and brain cholinesterase activity was determined. The haematological parameters measured were erythrocyte, leukocyte, and thrombocyte counts, differential leukocyte count, haemoglobin, erythrocyte volume fraction, and sedimentation rate. The clinical chemical parameters examined were sodium, potassium, chloride, total protein, albumin, glucose, bilirubin and blood urea nitrogen concentrations and alkaline phosphatase and alanine and aspartate aminotransferase activities. After sacrifice, gross autopsies were performed on all animals. The organs were weighed, and the following tissues were examined histologically: brain, eye, spinal cord, peripheral nerve, heart, lung, spleen, bone marrow, lymph nodes, thymus, oesophagus, stomach, small intestine, large intestine, liver, pancreas, kidney, urinary bladder, testis/ovary, prostate/uterus, pituitary, thyroid, adrenal, and bronchus.
During the study, there were no clinical signs of toxicity. One animal given 15 ppm of fenitrooxon died just before being killed, but the cause was not described. Only the final body weights were presented: no difference in body weights were found between groups. Although a slight decrease in body-weight gain during initial feeding of fenitrothion at 150 ppm was reported, no data were supplied to support this statement. There was no treatment-related change in food or water consumption. Haematological, clinical chemical, and urinary parameters showed no treatment-related changes.
Table 4 shows the observed inhibition of cholinesterase activity. 3-Methyl-4-nitrophenol had no intrinsic inhibitory activity on cholinesterase. With fenitrothion and fenitrooxon, females appeared to be more sensitive to inhibition: Significant (p < 0.01) inhibition of plasma cholinesterase activity was seen in females at all concentrations, although with no clear dose–response relationship, while the inhibition was significant in males only at 150 ppm. In brain and erythrocytes, significant, dose-related inhibition (p < 0.05, p < 0.01) was observed at 30 and 150 ppm in females and at 150 ppm in males. The NOAEL for fenitrothion was 10 ppm, equal to 0.6 mg/kg bw per day, on the basis of significant inhibition of brain and erythrocyte cholinesterase activity at 30 ppm (JMPR, 1974, 1977; modified with reference to the original reports of Kadota et al., 1972b, 1975b).
Table 4. Percent inhibition of cholinesterase activity in rats treated with fenitrothion or fenitrooxon
|
Concentration (ppm) |
Perecent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Fenitrothion |
||||||
|
10 |
6 |
55** |
3 |
16 |
0 |
0 |
|
30 |
33 |
50** |
17 |
29* |
10 |
31** |
|
150 |
43* |
76** |
69** |
66** |
53** |
70** |
|
Fenitrooxon |
||||||
|
5 |
26 |
13 |
0 |
0 |
0 |
3 |
|
15 |
34 |
33 |
0 |
8 |
5 |
13* |
|
30 |
33 |
58* |
33* |
61** |
2 |
24** |
From JMPR, 1974, 1977, modified after reference to the original reports of Kadota et al. (1972b, 1975b)
* p < 0.05, ** p < 0.01
In a study conducted according to GLP, five groups of 20 Crl(W1)BR rats of each sex were given whole-body exposure to an aerosol of fenitrothion (purity, 94.6%) in a chamber for 90 days. The groups consisted of controls exposed to air, controls exposed to the vehicle (acetone), and groups exposed to fenitrothion at 0.2, 1, or 10 µg/L per day for 6 h/day, 5 days/week for 90 days. The achieved concentrations of fenitrothion in respirable droplets (100% were Ł 6 µm) during exposure were close to those targeted. Each group was subdivided, and each received the same exposure: one was used for toxicological assessments during and at the end of the study, and the other was used only for determinations of plasma and erythrocyte cholinesterase activity during the study.
Animals in the first subgroups were examined twice daily for clinical signs, and abnormalities were recorded. Their body weights and food consumption were recorded weekly. All animals underwent an ophthalmoscopic examination before the study, and rats in the first subgroup were examined during week 13. Blood samples were taken from these rats during week 12 and examined to determine erythrocyte volume fraction, haemoglobin, erythrocyte count, mean corpuscular haemoglobin concentration, mean corpuscular volume, and total and differential leukocyte, platelet, and reticulocyte counts. The clinical chemical end-points determined were glucose, total protein, albumin, blood urea nitrogen, bilirubin, creatinine, sodium, potassium, calcium, phosphorus, chloride, cholesterol, and globulin concentrations, the albumin:globulin ratio, and the activities of alanine and aspartate aminotransferases, creatine phosphokinase, and alkaline phosphatase. Plasma and erythrocyte cholinesterase activity was determined in animals in the second subgroup before the study, before exposures 5, 10, 15, 20, 30, 40, and 50, and after exposure 64. Brain cholinesterase activity was determined after exposure 64.
Rats in the first subgroups were killed after 66 exposures and examined macroscopically, and the following organs were weighed; brain, pituitary, thyroids, thymus, heart, lungs, liver, spleen, kidneys, adrenals, testes with epididymides, and ovaries. The following tissues were preserved for histological examination: nasal passages, tongue, pharynx, larynx, trachea (including bifurcation), lungs, lymph nodes (tracheobronchial, mandibular, cervical, mesenteric), salivary gland, thyroids (with parathyroids), mammary gland, thymus, skin, skeletal muscle, spinal column, spinal cord (cervical, mid-thoracic, and lumbar) kidneys, urinary bladder, testes, seminal vesicles, prostate, ovaries, uterus, vagina, brain (medulla/pons, cerebellar, cortex, cerebral cortex), pituitary, eyes, liver, spleen, pancreas, adrenals, oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon, rectum, heart, aorta, sciatic nerve, sternum/ribs, femur with joint, and any gross abnormalities. The lungs from all rats were examined. All tissues from the air and vehicle controls and animals at the highest dose were examined. One female at 10 µg/L per day and 1 male at 1 µg/L per day died during blood sampling; as both animals were from the subgroup not scheduled for autopsy, the carcasses were discarded without further examination. The only clinical sign, which was seen in all treated groups but could not be quantified, was decreased responsiveness to auditory stimulation during exposure to fenitrothion in comparison with control animals. No significant changes in body weight or food consumption were seen during the study. No ophthalmic abnormalities were seen before treatment, but, at 13 weeks, a number of animals had corneal opacity (2/20, 8/20, 6/20, 5/20, and 3/20); the highest frequency was thus seen in vehicle controls, with no association to treatment with fenitrothion. This is an abnormality commonly seen in ageing rats; the increased occurrence may have been related to exposure to the vehicle. Haematological and clinical chemical examination revealed no findings attributable to treatment. The group mean cholinesterase activities relative to those of the vehicle controls after 13 weeks (i.e. 64 exposures) are shown in Table 5, the plasma activites being adjusted for those before exposure. While a clear concentration-related inhibition of plasma cholinesterase activity was observed in females, appreciable inhibition in erythrocytes was observed only at the highest concentration in animals of each sex. No appreciable inhibition of brain cholinesterase activity was observed in males, but a concentration-related inhibition was observed in females. Although the statistical significance of the reduction in cholinesterase activity in blood was tested at both the 1% and 5% levels, the activity in the brain was apparently tested only at the 1% level since a calculation shows that the degree of inhibition observed in females, namely 21%, was clearly significant at the 5% level. There were no significant macroscopic or histological findings, and the organ weights showed no significant treatment-related effects. No NOAEC could be established, on the basis of significant inhibition of cholinesterase activity in brain in females at the lowest concentration. The LOAEC was 0.2 µg/L per day (Coombs et al., 1988).
Table 5. Per cent inhibition of cholinesterase activity at week 13 in rats treated with fenitrothion
|
Concentration (µg/L per day) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
|
Males |
Females |
Males |
Females |
Males |
Females |
|
0.2 |
1 [0] |
32** [24] |
5 [8] |
1 [-1] |
7 |
21* |
|
1 |
4 [5] |
39** [33] |
10 [16] |
5 [7] |
7 |
32** |
|
10 |
20** [20] |
78** [73] |
38** [29] |
50** [49] |
16 |
57** |
From Coombs et al. (1988)
Values in square brackets represent the average inhibition seen after 1, 2, 3, 4, 6, 8, 10, and 13 weeks of treatment; negative values indicate cases in which the activity exceeded that in controls
* p < 0.05 (Student's t test; not calculated by the investigators); ** p < 0.01
Rabbits
Technical-grade fenitrothion (purity, 97.2%) was mixed into the diet and fed to groups of 15 male Japanese albino rabbits at a concentration of 0, 300, or 1000 ppm, equivalent to 0, 3, and 10 mg/kg bw per day, for 6 months. The animals were monitored daily for behaviour and weighed weekly during the first month and twice a month thereafter. Blood was collected once at the end of treatment to assess blood chemistry and haematological end-points. Plasma and erythrocyte cholinesterase activity was measured after 1, 2, 3, 4, 6, 10, 13, 18, and 24 weeks of treatment and that in brain at 24 weeks by an electrometric method (change in pH), whereas cholinesterase activity in a homogenate of medial rectus muscle was measured at 24 weeks by a colorimetric assay (Ellman method), and cholinesterase activity was localized histochemically in frozen sections of the muscle. Sections taken from brain, eye (with optic nerve and ocular muscle), heart, spinal cord, sciatic nerve, bronchus, lung, spleen, bone marrow, mesenteric lymph node, thymus, oesophagus, stomach, intestine, liver, pancreas, kidneys, urinary bladder, testes, epididymides, prostate, pituitary, thyroid, and adrenals after the final kill were examined by light microscopy. Transmission electron microscopy was performed only on the medial rectus muscles. Liver, kidney, spleen, lung, brain, heart, adrenal, testis, thyroid, and pituitary were weighed, and their weights were compared with those of controls.
There were no treatment-related deaths, although one rabbit died accidentally (group not reported) during restraint for blood collection. There were no clinical signs or significant changes in body weight and no noteworthy changes in clinical chemical or haematological end-points. The mean inhibition of cholinesterase activity after 24 weeks of treatment was 27% in plasma, 24% in erythrocytes, 9% in brain, and 9% in muscle at 3 mg/kg bw per day and 41% in plasma, 49% in erythrocytes, 32% in brain, and 8% in muscle at 10 mg/kg bw per day. The cholinesterase activity in plasma and erythrocytes was thus similarly reduced at the two doses whereas that in brain was appreciably less inhibited and almost no inhibition was seen in muscle. As might be anticipated from the absence of substantial cholinesterase inhibition in the muscle homogenate, no difference in cholinesterase activity was found in muscle sections taken from control and treated rabbits. No treatment-related histological lesions were observed in any tissue or by electron microscopy in the medial rectus muscles (Miyamoto et al., 1976).
In a study performed in compliance with GLP, technical-grade fenitrothion (purity, 93.7%) was applied to a shaved area between the shoulder and rump of groups of five male and five female New Zealand white rabbits at 0 (distilled water), 3, 10, 50, or 250 mg/kg bw per day for 21 days. At each treatment, the test material was applied for 6 h under an occlusive bandage, and the test site was then washed with non-irritating soap and water. Behaviour and general health were observed twice daily, the test sites were examined for changes before each application, and dermal reactions were scored according to the method of Draize. Body weight and food consumption were measured weekly. At the end of the study, the haematological parameters assessed were: haemoglobin, reticulocyte, erythrocyte, and leukocyte counts, erythrocyte volume fraction, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, differential blood count, thrombocyte count, coagulation time, and platelet count; and the clinical chemical parameters determined were: sodium, potassium, inorganic phosphorus, calcium, chloride, total bilirubin, total protein, blood urea nitrogen, creatinine, serum glucose, total cholesterol, albumin, and globulin concentrations, albumin:globulin ratio, and the activities of alanine and aspartate aminotransferases, alkaline phosphatase, and plasma, erythrocyte, and brain cholinesterase. Treated animals that died or were killed during the study and survivors at the final kill were necropsied. The absolute and relative weights of the heart, liver with drained gall-bladder, kidneys, testes/ovaries, epididymides, and adrenals were determined. A range of organs and tissues were preserved and examined histopathologically.
At 250 mg/kg bw per day, all males and two of five females either died or were killed when moribund. Before death, characteristic signs of poisoning with organophosphates were evident, namely hypoactivity, muscular hypotonia, tremors, bradypnoea, hypothermia, salivation, clonic convulsions, loose or mucous stools, diarrhoea, and a soiled circumanal region. At this dose, reductions in body weight were observed in males at day 7 (6%), day 14 (10%), and day 20 (21%; p < 0.05), and females showed slight reductions at day 14 (5%) and day 20 (6%), although these were not significant. Treated rabbits showed similar food intakes to controls. At 3, 10, and 50 mg/kg bw per day, very slight erythema (score, 1; range, 0–4) was observed in 13/15 males from day 5–7 onwards and in 10/15 females from day 5–9 onwards. Very slight oedema (score, 1; range, 0–4) was observed in 1/15 males on days 8–18 and in 8/15 females from day 13 onwards. The mean dermal irritation scores were 0.2–1.4 in males and 0.2–1.8 in females. Desquamation was observed in 6/15 males and 13/15 females (time of onset not stated). At 250 mg/kg bw per day, very slight erythema (score, 1) was observed in the surviving four males and three females on days 4–6 and, very slight to well defined erythema (score, 1–2) in one male (day 7–10) and two females (days 7–14). Very slight oedema (score, 1) was observed in one male on days 7 and 8 and one female on days 5–7. Very slight to moderate oedema (score, 1–3) was noted in one male on day 8. The mean dermal irritation scores were 0.2–2.7 in males and 0.2–2.4 in females. Desquamation was observed in three males and three females (time of onset not stated).
Although a significant (p < 0.05) increase in mean prothrombin time (13%) and a decrease in mean alanine aminotransferase activity (43%) was seen in males at 50 mg/kg bw per day, there was no evidence of treatment-related haematological or biochemical changes (except cholinesterase activity) at any dose. Significant (p < 0.01) decreases in mean erythrocyte cholinesterase activity were observed in males at 10 and 50 mg/kg bw per day (46% and 49%, respectively), and in females at 10, 50, and 250 mg/kg bw per day (51%, 70%, and 83%, respectively) 2 h after the last dose. After 24 h, significant (p < 0.01) decreases were observed in males at 50 mg/kg bw per day (58%) and in females at 50 and 250 mg/kg bw per day (65% and 79%, respectively). Similarly, significant (p < 0.01) reductions in mean plasma cholinesterase activity in females were observed at 10 and 50 mg/kg bw per day (73% and 89%, respectively) 2 h after final dosing. After 24 h, significant decreases were observed in males at 50 mg/kg bw per day (40%; p < 0.05) and in females at 10, 50, and 250 mg/kg bw per day (41%, 60%, and 94%, respectively; all p < 0.01). The mean brain cholinesterase activity was also significantly reduced in males at 50 mg/kg bw per day (36%; p < 0.01) and in females at 10 (21%; p < 0.05), 50 (30%; p < 0.01), and 250 mg/kg bw per day (79%; p < 0.01) at termination. Cholinesterase activity was not measured in rabbits at 250 mg/kg bw per day which died. An increase (33%; p < 0.05) in the absolute and relative (57%; not significant) weights of the adrenals was observed in females at 250 mg/kg bw per day. No other organs showed any significant difference in absolute or relative weight between treated and control groups. Histological examination of the skin revealed thickening of the epidermis, hyperkeratosis, and inflammatory infiltrates in the corium in males and females at all doses and haemorrhage in the corium at 10, 50, and 250 mg/kg bw per day. At the highest dose, one male and one female had slight necrosis of the liver, and some animals showed changes in the digestive tract and kidney that were considered to be the cause of the deterioration in general condition. The NOAEL was 3 mg/kg bw per day on the basis of inhibition of erythrocyte and brain cholinesterase activity at higher doses (Suetake et al., 1991).
Dogs
In a study performed according to GLP, technical-grade fenitrothion (purity, 96.8%) was administered in the diet to groups of six male and six female pure-bred beagle dogs at a concentration of 0, 5, 10 or 50 ppm, equal to 0, 0.17, 0.3, or 1.6 mg/kg bw per day for males and 0, 0.15, 0.3, or 1.6 mg/kg bw per day for females, for 12 months. The animals were housed individually in metabolism cages under standard laboratory conditions, and water and food were provided ad libitum. The animals were monitored for deaths and changes in appearance or behaviour twice daily. Detailed examinations were performed once weekly, and all animals were examined by a veterinarian before treatment and at 3, 6, and 12 months. Body weights and food consumption were recorded weekly. All animals underwent an ophthalmological examination before treatment and at 6 and 12 months. Haematological and clinical chemical end-points were measured before treatment and at 4, 8, 13, 17, 26, 39, and 52 weeks. The haematological parameters included erythrocyte volume fraction, haemoglobin, erythrocyte, leukocyte, differential leucocyte, platelet, and reticulocyte counts, mean corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular haemoglobin concentration. The clinical chemical parameters included the activities of lactic dehydrogenase, aspartate and alanine aminotransferases, and alkaline phosphatase, concentrations of glucose, blood urea nitrogen, total bilirubin, cholesterol, albumin, globulin, total protein, creatinine, sodium, potassium, chloride, calcium, and phosphorus, and albumin:globulin ratio. Urine was analysed before treatment and at weeks 4, 13, 26, 39, and 52 for volume, colour, appearance, pH, specific gravity, protein, glucose, ketones, urobilinogen, nitrite, and bilirubin concentrations, occult blood, and microscopic appearance. Plasma and erythrocyte cholinesterase activity was measured before treatment and at weeks 4, 8, 13, 17, 21, 26, 39, and 52. Brain cholinesterase activity was measured at the end of the study. All animals were necropsied grossly at the end of the study. The liver, kidneys, heart, testis with epididymides, brain, ovaries, pituitary, thyroid/parathyroids, and adrenal glands were weighed. Standard tissues were preserved, but a histopathological examination was not performed; the results of a histological assessment of the stored tissues were reported in a revised report (Spicer, 1986).
No deaths or treatment-related clinical signs were observed. On physical examination, some animals, mainly at the intermediate and high doses, were found to have a relatively rapid heart rate, but this was considered to be of little biological significance. There were no significant dose-related changes in body weight or food consumption during the study. Haematology, clinical chemistry, and urinary analysis showed no significant changes, although there was a slight increase in cholesterol concentration in males at the highest dose early in the study. No changes were observed on ophthalmic examination.
The mean inhibition of cholinesterase activity is shown in Table 6. Plasma cholinesterase was significantly reduced in animals of each sex at the highest dose. Erythrocyte cholinesterase activity was reduced in males at he highest dose only. Brain cholinesterase activity was unaffected by treatment. There were no treatment-related changes in organ weights and no macroscopic lesions. Although histological examination of tissues revealed several lesions, all except one appeared to be unrelated to treatment. The exception was that the incidence of haemorrhagic abdominal lymph nodes was increased, especially among females. Whereas only one male had a trace of haemorrhage in the nodes at 50 ppm, two females each at 10 and 50 ppm were affected, the the severity being scored as a trace in one bitch and mild in the other. No controls or animals at 5 ppm were affected. Although a clear dose–response relationship was not evident, this may have been due to the small group size. The investigators indicated that the spontaneous incidence of lesions including haemorrhagic lymph nodes is common among beagles and they are therefore unlikely to be of pathological significance. Although no data were provided for past controls data in the laboratory to support this contention, it is noteworthy that neither the mandibular nor the thoracic lymph nodes showed an increased incidence of haemorrhagic lesions, suggesting that the incidence of abdominal lymph-node lesions was unrelated to treatment. The NOAEL was 50 ppm on the basis of the absence of change in brain cholinesterase activity (JMPR, 1984; modified with reference to the original data of Griggs et al., 1984, and the amended report of Spicer, 1986).
Table 6. Per cent inhibition of cholinesterase activity in dogs treated with fenitrothion in the diet
|
Concentration (ppm) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
5 |
17 |
19 |
12 |
0 |
0 |
0 |
|
10 |
19 |
25 |
6 |
2 |
0 |
0 |
|
50 |
46 |
49 |
26 |
2 |
5 |
2 |
From JMPR (1984), modified after reference to the original data of Griggs et al. (1984) and an amended report by Spicer (1986)
(c) Long-term studies of toxicity and carcinogenicity
Mice
Fenitrothion (purity, 97%) was administered in the diet to groups of 50 ICR Swiss mice of each sex at a concentration of 0, 30, 100, or 200 ppm, equal to 0, 3.1, 11, or 22 mg/kg bw per day for males and 0, 3.7, 12, or 24 mg/kg bw per day for females, for 78 weeks. The mice were treated at a concentration of 0, 10, 30, or 100 ppm during the first 2 weeks of the study, before being changed to the indicated doses for the remainder. Animals were observed daily for deaths. Body weight, food consumption, behaviour, and appearance were recorded weekly during weeks 0-–10, every 2 weeks during weeks 11–26, and every 4 weeks from week 27 to the end of the study. Ophthalmoscopic examinations were performed on all mice at weeks 28 and 78. Gross necropsies were performed on mice that died during the study and on all those killed at the end of the study. The weights of the heart, liver, spleen, kidneys, testes with epididymis, and adrenals were recorded and the organ:body weight ratios determined. The following tissues were preserved for histological examination: brain, pituitary, thoracic spinal cord, eye, thyroids, oesophagus, lung, heart, liver, gall-bladder, spleen, kidneys, adrenals, stomach, pancreas, small intestine, large intestine, mesenteric lymph node, urinary bladder, testes with epididymis, seminal vesicles, prostate, ovaries, uterus, skin, bone (sternum), bone marrow (femur), nerve with muscle, and unusual lesions. Tissues collected from 10 mice of each sex in the control and highest-dose groups, and liver, kidney, and any unusual lesions from 10 mice of each sex at 30 and 100 ppm were examined microscopically. Cholinesterase activity and haematological and clinical chemical end-points were not determined.
No behavioural or clinical abnormalities were observed during the study, and no dose-related changes in body weight or food consumption occurred. The results of the ophthalmological examinations were not presented for individual animals; the summary results indicated no treatment-related effects. Gross and histological examination revealed a number of abnormalities, none of which was considered to be related to treatment. A number of neoplasms, including thyroid-cell adenoma and carcinoma, alveolar and bronchiolar adenoma and carcinoma, hepatocellular carcinoma, osteogenic sarcoma, and mammary adenocarcinoma, were found, but these tumours were either isolated or occurred with similar frequency in treated and control groups and were therefore considered unrelated to treatment. A statistically significant decrease in the absolute and relative weights of the heart was found at the highest dose, which is of questionable biological significance. The NOAEL was 200 ppm, equal to 22 mg/kg bw per day, the highest dose tested (Kundzins, 1975, 1979, 1980a).
In a study of carcinogenicity performed in accordance with Guideline 83-5 of the US Environmental Protection Agency and with GLP, technical-grade fenitrothion (purity, 96.7%) was administered to groups of 50 B6C3F1 mice of each sex in the diet at a concentration of 0, 3, 10, 100, or 1000 ppm, equal to 0.37, 1.4, 13, and 130 mg/kg bw per day for males and 0.46, 1.5, 13, and 140 mg/kg bw per day for females, for 104 weeks. The stability, homogeneity, and concentration of fenitrothion in the feed were acceptable. Subgroups of 10 mice of each sex were taken at weeks 13, 26, 52, and 78 from satellite groups of 50 mice of each sex per group that had been treated similarly to those in the main study for determination of haematological, blood chemical (including cholinesterase activity), and urinary end-points. The doses were selected on the basis of the results of a 4-week study with 0, 1, 3, 10, 30, 100, 300, or 1000 ppm in the diet, which showed significantly reduced cholinesterase activity at 10 ppm and reduced food intake and weight loss in animals of each sex at 1000 ppm. Other findings at 1000 ppm were reduced erythrocyte count, haemoglobin concentration, erythrocyte volume fraction, and alkaline phosphatase activity in females and increased cholesterol concentration in males and females.
Both the main and the satellite groups were observed for clinical signs and deaths daily, and for body weight, food and water consumption, and the results of palpation weekly for the first 14 weeks and every second week thereafter. All animals that survived to term and those that died or were killed in extremis were examined histologically. At week 104, all mice in the main group were killed for histological examination; brain, heart, liver, spleen, kidneys, adrenals, and testes or ovaries were weighed before fixation, and the thyroid and ovaries were weighed after fixation. The following tissues from mice in the satellite groups at week 52 and from all survivors in the main groups at week 104 were preserved in 10% formalin for histological examination: brain (cerebrum and cerebellum), spinal cord, sciatic nerve, eyeball, thyroid (including parathyroid), oesophagus, trachea, lung, heart, liver, gall-bladder, spleen, kidney, adrenal, stomach, pancreas, small intestine (duodenum, jejunum, ileum), large intestine (caecum, colon, rectum), mesenteric lymph node, urinary bladder, prostate, testis, seminal vesicle, epididymides, ovary, uterus, bone marrow, aorta, thymus, salivary gland, mammary gland, vagina, pituitary, muscle, bone (sternum, femur, lumbar vertebrae), skin, and any unusual lesions. Blood and urine were collected from the survivors in the main group at 104 weeks for determination of haematological end-points (erythrocyte volume fraction, haemoglobin, erythrocyte count, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, leukocyte and differential leukocyte counts), clinical chemical end-points (cholinesterase activity, glucose, blood urea nitrogen, total protein, albumin, bilirubin, and cholesterol concentrations, albumin:globulin ratio, and activities of alkaline phosphatase and alanine and aspartate aminotransferases), and urinary end-points (specific gravity, occult blood, pH, and glucose, ketone, protein, bilirubin, and urobilinogen concentrations). Ophthalmoscopic examinations were performed on mice in the main control and 1000-ppm groups at week 104.
Survival in the main and satellite groups was not significantly affected by treatment, and no appreciable differences were reported in gross changes or behaviour. No consistent treatment-related changes in haematological, urinary, or ophthalmic end-points were observed. The treatment-related effects were:
Table 7. Per cent inhibition of cholinesterase activity in mice treated with fenitrothion in the diet for 104 weeks
|
Concentration (ppm) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
3 |
0 [4] |
12 [9] |
{-9}[2] |
5 [2] |
4 [0] |
9 [2] |
|
10 |
12 [19] |
14 [15] |
3 [10] |
8 [8] |
0 [4] |
6 [3] |
|
100 |
62** [75] |
68** [73] |
80** [59] |
51** [53] |
64** [62] |
45** [51] |
|
1000 |
88** [89] |
90** [89] |
84** [85] |
80** [81] |
83** [78] |
77** [77] |
From Tamano et al. (1990)
Values in square brackets represent the average inhibition at 13, 26, 52, 78, and 104 weeks of treatment; negative values indicate values that exceeded those of controls
** p < 0.01
As shown in Table 8, no treatment-related change in the incidence of neoplasms was observed. There was thus no evidence of carcinogenicity in mice treated with fenitrothion at doses that caused a significant reduction in body weight, increased the plasma cholesterol concentration, and increased the absolute and relative weights of the brain in mice of each sex. The NOAEL was 10 ppm, equal to 1.4 mg/kg bw per day, on the basis of significant inhibition of plasma, erythrocyte, and brain cholinesterase activity and elevated plasma cholesterol concentration at 100 ppm (Tamano et al., 1990).
Table 8. Incidences of neoplasia in mice treated with fenitrothion in the diet
|
Neoplasm |
Sex |
Concentration in the diet (ppm) |
||||
|
0 |
3 |
10 |
100 |
1000 |
||
|
Benign |
Male |
40 |
47 |
32 |
45 |
24 |
|
|
Female |
23 |
14 |
18 |
19 |
16 |
|
Malignant |
Male |
24 |
25 |
27 |
40 |
17 |
|
|
Female |
26 |
30 |
25 |
31 |
29 |
|
All |
Male |
64 |
72 |
59 |
85 |
41 |
|
|
Female |
49 |
44 |
43 |
50 |
45 |
From Tamano et al. (1990)
Rats
Fenitrothion (purity, 97.2%) was administered in the diet of groups of 15 Wistar rats of each sex at a concentration of 0, 2.5, 5, or 10 ppm, equal to 0, 0.1, 0.3, or 0.5 mg/kg bw per day for males and 0, 0.2, 0.3, or 0.6 mg/kg bw per day for females, for 92 weeks. The rats were housed individually under controlled conditions. Food and water were available ad libitum. Behaviour was observed daily, and body weight, food intake, and water intake were measured weekly. Plasma and erythrocyte cholinesterase activity was measured at week 2, 4, 6, 8, 12, 16, 20, 24, 42, 68, and 92; brain cholinesterase activity was measured at the end of the study. The activity was determined by an electrometric method (change in pH). No gross pathological or histopathological examinations were performed.
No signs of toxicity were observed during the study, and deaths occurred at similar frequency in all groups. There were no significant treatment-related changes in food consumption or body weight. No appreciable inhibition of brain cholinesterase activity was observed at any concentration of fenitrothion tested (Table 9). The NOAEL was 10 ppm, equal to 0.5 mg/kg bw per day, the highest dose tested (Kadota et al., 1974b, 1977, 1980).
Table 9. Per cent inhibition of cholinesterase activity in rats treated with fenitrothion in the diet for 92 weeks
|
Concentration (ppm) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
2.5 |
1 |
2 |
1 |
1 |
1 |
0 |
|
5 |
14 |
16 |
5 |
4 |
4 |
1 |
|
10 |
18 |
28 |
10 |
13 |
6 |
4 |
From Tamano et al. (1990)
Technical-grade fenitrothion (purity, 97%) was administered to Sprague-Dawley rats in the diet at a concentration of 0, 10, 30, or 100 ppm, equal to 0, 0.5 1.5, or 5 mg/kg bw per day for males and 0, 0.6, 1.9, or 6.5 mg/kg bw per day for females, for 104 weeks. Groups of 50 weanling rats of each sex were selected at random from the F1a litters of treated rats in a three-generation study and were thus exposed to fenitrothion during gestation and lactation; 60 untreated rats of each sex were also selected. Although the rats were distributed randomly by dose, the mean body weights of those at the high dose were approximately 20% lower than those of controls. The animals were housed individually, and food and water were available ad libitum. Body weights, food consumption, appearance and behaviour were recorded weekly until week 26, every second week during weeks 27–52, and every 4 weeks during weeks 53–104, and the presence of tissue masses was recorded at these times. The animals were observed daily for clinical signs. Blood samples were taken from five rats of each sex per group at weeks 13, 52, and 104 and from five rats in the control and highest-dose groups at week 26. Erythrocyte volume fraction, haemoglobin, and erythrocyte, leukocyte, and differential leukocyte counts were determined in all bled animals. Prothrombin time was determined only for controls and rats at 100 ppm. Blood glucose, urea nitrogen, total serum protein, and total serum bilirubin concentrations, alkaline phosphatase, alanine and aspartate aminotransferase activities, and serum protein electrophoresis characteristics were determined in blood samples from fasted animals. Additionally, at weeks 52 and 104, sodium, potassium, chloride, carbon dioxide, calcium, and albumin concentrations were determined. Analysis of urine included specific gravity, pH, glucose, ketone, protein, and bilirubin concentrations and examination of the sediment. Plasma and erythrocyte cholinesterase activities were determined in 10 rats of each sex per group at weeks 0, 2, 4, 8, 13, 26, 52, 78, and 104. At weeks 52 and 104, brain cholinesterase activity was determined in 10 rats of each sex per group. Ophthalmoscopic examinations were performed on all rats at weeks 81 and 104. All animals that died during the study were necropsied grossly. At week 52, 10 rats of each sex per group were killed for interim autopsy. At week 104, all surviving rats were killed. The weights of the heart, liver, spleen, kidneys, and testes with epididymides were recorded before fixation, and the weights of the thyroid and adrenals were recorded after fixation. The brain, pituitary, thoracic spinal cord, eye, thyroid, oesophagus, lung, heart, liver, spleen, kidneys, adrenals, stomach, pancreas, small intestine, large intestine, mesenteric lymph node, urinary bladder, testis with epididymides and seminal vesicles, or ovaries and uterus, skin, costochondral junction, sternal bone marrow, nerve with muscle, and any unusual lesions were preserved in 10% formalin for histological examination.
There were no treatment-related deaths or clinical signs at any time during the study. The mean body weight of the animals at the high dose was 10–20% lower than that of control animals throughout the study, but there was no significant difference in food consumption among groups. Haematological and clinical chemical examinations showed no significant differences among groups, and there were no significant findings in the urine analysis. Dose-related inhibition of plasma, erythrocyte, and brain cholinesterase activity was observed in males and females (Table 10). No significant treatment-related macro- or microscopic findings were made post mortem. A number of neoplasms, including interstitial-cell tumours (Leydig-cell tumours), gliomas phaechromocytomas, and pituitary adenomas were seen, but the occurrences were isolated, and a statistical analysis revealed no difference in tumour incidence between control and treated groups. The NOAEL was 10 ppm, equal to 0.5 mg/kg bw per day, on the basis of marked inhibition of erythrocyte and brain cholinesterase activity at the next higher concentration (JMPR 1974; modified by reference to the original reports of Kundzins, 1974, 1980b; Patterson, 1981).
Table 10. Per cent inhibition of cholinesterase activity in rats treated with fenitrothion in the diet for 104 weeks
|
Concentration (ppm) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
10 |
19 |
35 |
9 |
11 |
7.5 |
3.5 |
|
30 |
37 |
69 |
27 |
39 |
28 |
22 |
|
100 |
54 |
82 |
54 |
75 |
38 |
55 |
From JMPR (1974), modified after reference to the original reports of Kundzins (1974, 1980b) and Patterson (1981)
The available studies of the genotoxicity of fenitrothion are summarized in Table 11.
Table 11. Results of tests for the genotoxicity of fenitrothion
|
End-point |
Test object |
Concentration / Dose |
Purity (%) |
Results |
Reference |
|
In vitro |
|
|
|
|
|
|
Reverse mutationa |
S. typhimurium TA1535, TA1537, TA1538 E. coli K12 W3102 |
10–1000 µg/plate (with and without S9 for TA1535 and TA1538) |
98.5 |
Negativea |
Suzuki & Miyamoto (1975) |
|
Reverse mutationa |
S. typhimurium TA98, TA100, TA98 (nit–)b TA100 (nit–)b |
10–1000 µg/plate |
96.8 |
Weakly positive in TA100 with S9; all others negative |
Suzuki & Miyamoto (1983) |
|
Reverse mutationa |
E. coli K12 W3623 strains |
13.2–660 µg/mL |
97 |
Negative |
Suzuki et al. (1974) |
|
Gene mutationa |
Chinese hamster lung fibroblasts (V79 cells) |
0.01–0.3 mmol/L (5 h) |
94.7 |
Negativea |
Kogiso et al. (1987); QA |
|
Chromosomal aberration |
Chinese hamster ovary cells (CHO-K1) |
3–30 µg/mL (–S9; 8, 16, or 24 h) |
96.7 |
Negativea |
Kogiso et al. (1988); QA |
|
Sister chromatid exchange |
ICR mouse embryo cells |
0.01–0.1 mmol/L (± S9; 3 h) |
98.6 |
Negative |
Suzuki & Miyamoto (1980) |
|
In vivo |
|
|
|
|
|
|
Host-mediated mutation |
Male ICR mouse S. typhimurium G46 |
100 or 200 mg/kg bw orally or intramuscularly; bacteria injected intraperitoneally |
98.5 |
Negative |
Suzuki & Miyamoto (1975) |
|
Chromosomal aberration |
Male ICR mouse bone- marrow cells |
200, 400, or 800 mg/kg bw intraperitoneally (marrow cells harvested 6, 24 or 48 h later) |
96.8 |
Negative |
Hara & Suzuki (1982a); QA |
|
Chromosomal aberration |
Male Sprague-Dawley rat bone-marrow cells |
100, 200, or 400 mg/kg bw orallyc (marrow cells harvested 6, 24 or 48 h later) 20, 40, or 80 mg/kg bw per day orallyc for 5 days (marrow cells harvested 6 h later) |
96.8 |
Negative |
Hara et al. (1983) |
|
Unscheduled DNA synthesis |
Male SD rat hepatocytes |
300 mg/kg bw orally in corn oil (3, 12, or 24 h) |
94.5 |
Negative |
Kogiso et al. (1990); GLP |
|
Dominant lethal mutation |
Male ICR mice |
20 or 200 mg/kg bw per day orally for 5 days |
98.5 |
Negative |
Kohda & Kadota (1975) |
|
Male Sprague-Dawley rats |
2, 7, or 20 mg/kg bw per day orally for 5 days |
|
Negative |
|
|
|
Dominant lethal mutation |
Male Wistar rats F1–F3 generations |
1–8 mg/kg bw in the diet for four generations |
96 |
Negative |
Benes et al. (1974a, 1975) |
|
Micronucleus formation |
Male ICR mice |
200, 400, or 800 mg/kg bw intraperitoneally in corn oil |
96.8 |
Negative |
Hara & Suzuki, 1982b); QA |
Positive control substances were used in all assays and gave the expected results.
QA, quality assurance statement; GLP, good laboratory practice; nit–, nitroreductase-defective strain
a
With and without metabolic activation (S9)b
Suspended in cottonseed oilRats
Groups of 10 male and 20 female rats were fed diets containing technical-grade fenitrothion (with a maximum of 0.5% para-nitro-meta-cresol) at a concentration of 0, 10, 40, or 80 ppm in a four-generation study of reproductive toxicity with four litters per generation. The body weight and food consumption of the parental animals and indices of fertility, gestation, live births, 24-h survival, 5-day survival, and lactation were observed; all pups were examined grossly, and for F4b weanlings the organs were weighed and examined histologically. Cholinesterase activity was measured in whole blood from males of the F2a generation (aged 15 weeks) and all F4b weanlings (aged 4 weeks).
Fertility, gestation, and live birth indices were normal in all groups, whereas the 24-h and 5-day survival indices were reduced in one or both litters of dams at 80 ppm in almost all generations. The lactation index was reduced in all generations at 40 and 80 ppm. The mean litter size was reduced in all but five dams; although the occurrence did not show a clear dose-dependence, the fewest pups were found in six of eight litters of dams at 80 ppm. The mean weight of the pups at birth and at 21 days of age was normal, whereas the growth of parental animals at 80 ppm was slightly decreased. Cholinesterase activity was decreased as a function of dose and length of exposure; the decrease was only slight in animals at 10 ppm. The weights and gross and microscopic appearance of the organs were normal (Benes et al., 1974b; JMPR, 1974).
Technical-grade fenitrothion (purity, 97%) was administered to Sprague-Dawley rats in the diet at a concentration of 0, 10, 30, or 150 ppm for 9 weeks before and during mating, gestation, parturition, and lactation for the F0 and F1a generations and at 0, 10, 30, or 100 ppm to subsequent generations in a three-generation study. The first treated generation and F1a mating consisted of 15 males and 30 females, while the controls comprised 20 males and 40 females; all other groups were reduced to 10 males and 20 females for the remainder of the study. Males and females co-habitated in the ratio of 1:2, and males were rotated weekly during the 3-week mating period. On day 2 post partum, the litters were reduced to eight by culling of randomly selected pups. F0, F1, and F2 generation parental rats were each mated twice, and the first litters (F2a, F3a) were killed at weaning, while the second litters (F1b, F2b, F3b) were used as the next generation of parents. One-third of the F2a, F2b, and F3a pups and one-half of the F3b pups were necropsied and observed grossly. The parents were killed after weaning of the F2b and F3b pups. Although the diet was not analysed regularly, it was prepared weekly, and actual food consumption was recorded at the beginning of the study and at weeks 4 and 9 before mating. The estimated group daily intake of fenitrothion was 0.5–1 mg/kg bw per day for animals receiving 10 ppm, 1.5–3.0 mg/kg bw per day for those given 30 ppm, 5–10 mg/kg bw per day for those given 100 ppm, and 7.5–15 mg/kg bw per day for those given 150 ppm for rats weighing 0.1 or 0.4 kg.
Deaths, clinical signs of toxicity, body weight, and food consumption were assessed 0, 4, and 9 weeks before mating. The maternal reproductive parameters that were determined were pregnancy and lactation indices. Offspring were assessed for litter viability, live birth rate, and pup survival. Only sections of liver, thyroid, kidney, stomach, and small intestine from five males and five of the F3b generation at 0 and 100 ppm were examined histologically, but samples of adrenals, brain, heart, eye, intestines (small and large), kidneys, liver, lung, pituitary, gonads, pancreas, spleen, stomach, bone, bone marrow, and urinary bladder were stored for possible later use. There were no deaths, clinical signs, or reductions in food consumption during the study. The mean body weight of the F0 rats given 150 ppm was reduced by 7% at week 9 for both males and females (neither significant) and by 8% for females at week 4. Although the investigators indicated that the body weights of the F1 parents at 100 ppm were also lower than those of controls, the mean body weight of these rats at week 0 (before dosing was started) was markedly lower than that of controls (by 13% for males and 12% for females). Therefore, although the absolute mean body weight of the animals at 100 ppm was lower, the body-weight gain over 0–9 weeks was greater than that of controls. The body weights of the F2 parents at 100 ppm were similar to that of controls.
The fertility indices of all treated females were comparable to those of the control groups, except for a significant reduction (p < 0.05) in the F2a generation at 10 ppm, which was not dose-related and therefore not biologically significant. Although the mean weight of pups at 150 ppm at delivery tended to be lower than in controls, those of all other treated groups appeared to be similar. The gestation index and live births ratio were comparable, but the number and weight of pups that survived to weaning were reduced at 100 ppm in all generations and at 150 ppm in the F1a generation. Thus, the survival of F1a generation pups at 150 ppm was reduced by 51% (p < 0.05), males and females being 31% and 28% lighter, respectively (both p < 0.05), than controls, and the survival of F1b, F2a, F2b, F3a, and F3b pups at 100 ppm was reduced significantly (all p < 0.05), with reductions of 28%, 22%, 12%, 25%, and 24%, respectively. Male and female pups showed weight losses of 3–17%, but the reductions were significant only for males of the F1b and males and females of the F2a generation. No histological findings that could be attributed to treatment were observed. The NOAEL for parental toxicity was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of reductions in body weight at 150 ppm. The NOAEL for reproductive effects was 30 ppm, equivalent to 1.5 mg/kg bw per day, on the basis of poor pup survival and inability to gain weight during lactation at higher doses (JMPR, 1974; modified by reference to the original reports of Olson, 1972; Rutter, 1973; Ralph & Pence, 1980).
In a study conducted according to GLP, technical-grade fenitrothion (purity, 94.6%) was administered in the diet to two generations of 45-day-old (F0) or weanling Sprague-Dawley rats (Crl:CD(SD)BR) (two litters for the first generation and one for the second) at a concentration of 0, 10, 40, or 120 ppm (corrected for impurities). The diet was prepared weekly by admixing fenitrothion suspended in corn oil with the normal dry food. The stability and homogeneity of the test substance in the diet were checked and found to be suitable for at least 21 days after preparation. The concentration of fenitrothion in the diet, verified weekly for 4 weeks and monthly thereafter, indicated that the actual concentrations were within 10% of the target doses, except for sporadic deviations of up 12% below and 61% above the target doses. The doses were selected on the basis of a one-generation study in which clinical signs and reduced food consumption and body weight were observed in rats of each sex, a reduced number of implantation sites was found in females, and reduced body weights of pups were observed at dietary concentrations in excess of 150 ppm. The doses used for the main study were calculated from actual food consumption over 202 days. Those for F0 males for days 1–82 ranged from 0.4 to 1.0 mg/kg bw per day at 10 ppm (mean, 0.65 mg/kg bw per day), 1.6–4.3 mg/kg bw per day at 40 ppm (mean, 2.7 mg/kg bw per day), and 4.8–13 mg/kg bw per day at 120 ppm (mean, 8.0 mg/kg bw per day). Those for F0 females during the 82-day premating period were 0.6–1.1 mg/kg bw per day at 10 ppm (mean, 0.7 mg/kg bw per day), 2.5–4.3 mg/kg bw per day at 40 ppm (mean, 3.1 mg/kg bw per day), and 7.8–14 mg/kg bw per day at 120 ppm (mean, 9.6 mg/kg bw per day). Owing to the increased nutritional requirements during gestation and lactation, there was an approximate 15% increase in dose during gestation and a 60–80% increase during lactation, the mean doses during the first and second lactation periods being 1.2, 4.9, and 14 mg/kg bw per day, respectively.
Rats were assigned randomly to groups of 30 of each sex and fed fenitrothion for 82 days (88 days for the F1a generation) before a 21-day mating period with one male to one female. The presence of a copulatory plug on daily examination was deemed to be day 0 of gestation. All F0 females were allowed to deliver naturally, and the litters were culled to four of each sex on day 4 of lactation. Females that had not mated within 14 days were mated a second time with a different male at the same dose. F1a generation pups were left with the dams until day 28 post partum, when 30 of each sex per group were assigned randomly for treatment at the same dose as their parents. F1b pups remained with the dams for 21 days post partum, and then the dams and pups were killed and necropsied. Weanling F1a rats were allowed to mate as before, with sibling matings avoided. All F2 litters were killed on lactation day 28, and five of each sex were necropsied. Daily assessment showed five deaths among F0 females, but none appeared to be related to treatment because they were not dose-related or did not coincide with the greatest daily intake of fenitrothion. Three dams at 10 ppm died, two during the first gestation and another during the second lactation (day 18). At 120 ppm, one dam died during the second gestation and another died during parturition (day 205). The clinical signs that preceded death were chromodacryorrhoea, alopecia, and pale mucous membranes. Reduced food consumption with concomitant weight loss was observed in all rats at 10 ppm that died but not those at 120 ppm. Necropsy revealed a ruptured blood vessel of the urinary bladder in one rat at 10 ppm and a red substance around the nose, mouth, and genitalia of another. No other gross lesions were observed in the other dead rats, although one rat at 10 ppm and another at 120 ppm were pregnant with normal fetuses at the time of death. The pregnant dam at 120 ppm died after delivery of two of four pups. One other death occurred during the study: an F1a dam at 120 ppm which died during parturition.
There were no clinical signs at any dose among rats of the F0 generation or F1a males; however, F1a females at 120 ppm had loose stools, diarrhoea, and chromorhinorrhoea during gestation and tremors during lactation. Body weight and food consumption were assessed weekly throughout the study. All F0 rats at 120 ppm and F1a rats at 40 and 120 ppm had reduced mean body-weight gains, resulting in significantly (p < 0.05–0.01) lower body weights relative to controls. Female F0 rats at 40 ppm also had significantly reduced body weights (p < 0.05) during the first lactation period. The absolute food consumption of F0 males was unaffected by treatment up to 120 ppm, but that of females was significantly (p < 0.05) decreased at 120 ppm during both the first and second lactation periods. In the F1a generation, males at 120 ppm and females at 40 and 120 ppm had significantly (p < 0.05–0.01) reduced absolute food consumption.
The pregnancy rate was reduced in all treated F0 groups during the first cohabitation. F0 males had significantly (p < 0.05) reduced fertility at 10 and 40 ppm during the first cohabitation and a significant (p < 0.05) reduction in mating performance at 120 ppm during the second cohabitation period. No significant effect on the mating performance or fertility of the F1 generation was observed at any dose. The body weights of F1a and F1b pups were either reduced or significantly (p < 0.01) reduced, and the mortality rate was significantly increased (p < 0.05–0.01) among F1a and F2 pups at 120 ppm. The viability and lactation indices were also significantly decreased in the F0 and F1a generation litters at 120 ppm. No changes were observed in the duration of gestation, implantation rate, or pup sex ratio at any dose. Similarly, no histopathological changes were observed. Fenitrothion at dietary concentrations up to 120 ppm did not impair the reproductive performance of rats in this two-generation study. The NOAEL for parental toxicity was 10 ppm, equal to 0.65 mg/kg bw per day, on the basis of dose-related reductions in food consumption and body-weight gain at higher doses. The NOAEL for reproductive toxicity was 40 ppm, equal to 3.1 mg/kg bw per day, on the basis of reduced pup weight, viability, and lactation indices at 120 ppm (Hoberman, 1990).
Mice
Groups of 25–27 pregnant ICR-JCL mice were given fenitrothion (purity, 97.2%) in maize oil at a dose of 0, 20, 70, or 200 mg/kg bw per day by oral intubation on days 7–12 of gestation. Eighteen dams from each group were killed on day 18 of gestation; their fetuses were removed surgically and examined for internal and external abnormalities and skeletal malformations. The remaining dams were allowed to deliver their young naturally; the young mice were then observed for 6 weeks for growth and development. No adverse effects on maternal body weight or physical appearance were noted. No significant difference in reproductive parameters, including numbers of implantations and dead and live fetuses, sex ratio, and mean body weight of fetuses, was observed between treated and control groups. The anomalies observed in the treated groups included cleft palate and open eyelids. No data on past control groups were provided that permitted an evaluation of the significance of these findings. The incidences of skeletal variations and ossifications were not significantly different from those in controls. The teratogenicity of fenitrothion could not be determined from this study because the dosing regimen did not cover the whole period of organogenesis (JMPR, 1984; Miyamoto et al., 1975).
Rats
Groups of 22–26 pregnant Sprague-Dawley rats were given fenitrothion (purity, 97.2%) in maize oil at a dose of 0, 2, 7, or 20 mg/kg bw per day by oral gavage on days 9–14 of gestation. Eighteen dams from each group were killed on day 20 of gestation, and their fetuses were removed surgically. The remaining dams were allowed to deliver their young naturally; the young rats were then observed for 6 weeks for growth and development. Fetuses were examined at necropsy for external and internal abnormalities and skeletal malformations. A slight reduction in maternal body-weight gain and clinical signs of toxicity were noted at the highest dose. No effects on reproductive parameters including number of implantations, incidence of dead or resorbed fetuses, fetal sex ratio, or fetal birth weight were noted in comparison with controls. Although there were no apparent compound-related skeletal or visceral anomalies, the teratogenic potential of fenitrothion could not be determined from this study because the dosing regimen did not cover the whole period of organogenesis (JMPR, 1984; Miyamoto et al., 1975).
Groups of 20 mated female Wistar rats, 12 weeks old, were given fenitrothion (purity, 97.6%) in sunflower oil by gavage at a dose of 0, 2, 8, 16, or 24 mg/kg bw per day on days 6–16 of gestation, as confirmed by a positive sperm smear. On day 20 of gestation, the rats were killed, and the numbers of viable and dead fetuses, resorptions, implantations, and corpora lutea were recorded. The fetuses were examined externally and internally (skeletal and soft tissue).
Clinical signs of maternal toxicity (tremors and chromodacryorrhoea) were observed in 19/20 dams receiving 24 mg/kg bw per day, and 11/20 dams in this group died between days 10 and 16 of gestation. Statistically significant decreases in body-weight gain were noted during both the treatment period and the entire gestation period in rats receiving 16 or 24 mg/kg bw per day, as compared with controls. The food consumption of rats at the highest dose was significantly decreased during treatment as compared with that of controls. Embryotoxicity was observed at the highest dose, with an increased total number of resorptions, a statistically significant reduction in the mean number of live fetuses, and a statistically significant reduction in the mean weight of the placenta as compared with controls. Increased numbers of resorptions were also seen at 2, 8, and 16 mg/kg bw per day, but the differences from controls were not statistically significant. There was no apparent difference between treated and control groups in fertility index, gestation index, or litter size. The sex ratio and mean body weight of fetuses were lower at the high dose than in controls, but the differences were not statistically significant. A number of skeletal variations and malformations were observed in both control and treated groups, which included fused fifth and sixth ribs and tibial aplasia in one fetus at 2 mg/kg bw per day and irregular shape of ossification centres in sternebrae in five fetuses at 8 mg/kg bw per day, in four fetuses at 24 mg/kg bw per day, and in one control fetus (Benes & Tejnorova, 1986; JMPR, 1986).
In a study performed in accordance with the US Code of Federal Chemicals Regulations, the Agricultural Chemicals Regulation Law of Japan, and testing guidelines of the US Environmental Protection Agency (section 83-3), groups of 20–24 pregnant Sprague-Dawley Crl:CD(SD)BR rats were given technical-grade fenitrothion (purity, 96.6%) at a dose of 0, 3, 8, or 25 mg/kg bw in corn oil by gavage on days 6–15 of gestation. Solutions were prepared freshly each day, and the doses were based on the body weight of animals on day 6 of gestation. The rats were observed for clinical signs, body weight, and food consumption. On day 20 of gestation, the dams were killed and examined grossly for abnormalities of the thoracic, abdominal, and pelvic viscera. The usual teratological parameters were then examined. All rats survived to day 20 of gestation.
Signs of toxicity (including tremors, rhinorrhoea, and rough coat) were seen in 18 animals at the high dose. Significantly reduced body-weight gain was observed at the high dose on days 11–19 of gestation. Food consumption was not significantly altered by treatment. The mean numbers of implantations, early and late resorptions, and live fetuses per litter and the mean fetal weight and sex ratio were similar in all groups. No dead fetuses were observed. Skeletal and visceral variations and malformations were observed in both control and treated groups. Most of the visceral variations were seen in the kidneys and ureters, consisting of small renal papillae and dilated ureters; the incidence was not dose-related. At the high dose, there was a statistically significant (p Ł 0.05) increase in the incidence (3.1%) of fetuses with one full and one rudimentary thirteenth rib, although there was no increase in the number of these ribs. Other skeletal variations included anticipated degrees of ossification delay of various bones, but these did not occur in a dose-related pattern. The only skeletal malformation, agnathia, was observed in one fetus at the high dose. The total numbers of fetuses with external, visceral, and skeletal malformations were one control, none at the low dose, one at the intermediate dose, and one at the high dose. The NOAEL for maternal toxicity was 8 mg/kg bw per day, on the basis of reduced body-weight gain and clinical signs at 25 mg/kg bw per day. The NOAEL for fetal toxicity was 25 mg/kg bw per day, the highest dose tested, in the absence of developmental effects (JMPR, 1988; modified with reference to the original report of Morseth, 1987).
Rabbits
In a study performed in accordance with the US Code of Federal Chemicals Regulations, the Agricultural Chemicals Regulation Law of Japan, and testing guidelines of the US Environmental Protection Agency (section 83-3), groups of four artificially inseminated female HRA New Zealand white rabbits were given technical-grade fenitrothion (purity, 96.6%) at a dose of 0, 3, 10, or 30 mg/kg bw per day in corn oil by gavage on days 7–19 of presumed gestation. The doses were based on the results of pilot studies in which a daily oral dose of 100 mg/kg bw caused the death of animals by day 3 but doses up to 20 mg/kg bw per day did not result in maternal or intrauterine toxicity. Solutions were prepared fresh weekly, and dosing was based on the most recently recorded body weight. The animals were observed for clinical signs, body weight, and food consumption. On day 29 of gestation, all does were killed and examined grossly for abnormalities of the thoracic, abdominal, and pelvic viscera. The usual teratological parameters were examined.
One animal in the control group and one at the intermediater dose were anorexic and were found dead on days 8 and 18 of gestation, respectively. Two animals at the low dose died due to errors in gavage. Six does at the high dose were found dead during treatment, and three does in the same group aborted or delivered prematurely after treatment had ceased. During treatment, all surviving animals at the high dose had reduced motor activity, ataxia, salivation, dyspnoea, or tremors. Only ataxia and tremors were observed after treatment. Animals at lower doses showed no clinical abnormalities, but some animals in each group were anorexic. Although the food consumption was similar in all groups, those at the high dose had a lower body-weight gain. The mean numbers of implantations and live fetuses per litter were slightly but not significantly reduced at the high dose. The incidences of fetal resorptions, the sex ratio, and mean fetal body weight were similar in all groups. The total incidences of external malformations (0–2.9%), visceral variations (1.2–4.8%), visceral malformations (0–2.4%), and skeletal malformations (0–7.2%) were not statistically significantly different between treated and control groups. No malformation was observed at the high dose. The total incidences of malformations were 2, 8, and 6% in the control, low- and intermediate-dose groups, but these incidences were considered not to be significantly different. Maternal toxicity was seen at 30 mg/kg bw per day, with no effects on fetal growth or development. The NOAEL for maternal toxicity was 10 mg/kg bw per day, and that for fetal toxicity was 30 mg/kg bw per day (JMPR, 1988; modified with reference to the original report of Morseth et al., 1986).
Chickens
In a study performed in accordance with the proposed US Environmental Protection Agency guidelines for neuromorphological testing, fenitrothion (purity, 97.2%) in Sorpol 355 (an emulsifier) was administered orally at a dose of 500 mg/kg bw to 16 adult white Leghorn hens. The anticipated clinical signs were diminished by concomitant administration of atropine at 20 mg/kg bw subcutaneously and 2-pyridine aldoxime hydrochloride at 100 mg/kg bw intraperitoneally, repeated 6, 24, 48, and 72 h later. After 3 weeks of daily observation for signs of limb paralysis, the regimen was repeated. A positive control group of three hens received tri-ortho-cresyl phosphate in 10% Tween 80 orally at 500 mg/kg bw and were monitored daily for 4 weeks for signs of delayed neurotoxicity such as limb paralysis. Four untreated hens was used as a reference for estimating changes in body weight. The hens were killed at the end of the respective observation period, and their distal sciatic nerves was excised and processed for histology. A section of the spinal cord from the lumbro-sacral region containing lumbar enlargement was also examined for possible lesions.
Five hens died 24–48 h after treatment; the survivors showed limb weakness, ataxia, loss of balance, spontaneous motor activity, and an irregular respiration rate that persisted for 5–7 days. During the second treatment cycle, no deaths occurred; similar but less severe clinical signs were observed. The mean body-weight loss after the two treatment cycles was 7%. None of the positive controls died, but limb weakness and loss of balance and leg coordination were apparent by day 12–14. By day 18, all hens had limb paralysis which persisted for the duration of the observation period. The body-weight loss in this group relative to controls was 39%.
Histological examination of 1000 transverse sections showed degeneration of the sciatic nerve and demyelination in all three positive control hens but in none of the treated hens. No swelling or degradation of nerve fibres, ganglionic cells, or Nissl granules was observed in 100 transverse spinal cord sections from treated hens or in the positive control group (JMPR, 1974, 1977; modified with reference to the original report of Kadota et al., 1974, 1975a, 1976).
Rats
In a study of behaviour and neuromorphology performed in accordance with US Environmental Protection Agency test guideline 81-8 and with GLP, technical-grade fenitrothion (purity, 94.3%) suspended in corn oil was administered by gavage to 49–52-day-old Sprague-Dawley rats (Crl:CD (SD)BR). The rats were assigned randomly to groups of 12–13 of each sex: Males received a dose of 0, 12.5, 50, or 200 mg/kg bw, and females received 0, 50, 200, or 800 mg/kg bw. Owing to dosing errors, one female at each dose and one male at the high dose had to be replaced. The treated rats were observed twice daily for clinical signs; body weight was determined at the time of dosing and then weekly thereafter. A battery of quantitative and qualitative observational tests for function (FOB) was administered before treatment, at the time of the presumed maximal effect on the first day of treatment (1–1.25 h after dosing for males and 0.75–1 h for females), and on days 7 and 14. Qualitative observations were made on rats in their home cage, during handling, and in a test arena. The observations in the home cage were body position, tremors, twitches, convulsions, and bizarre behaviour (static). The observations during handling were ease of removal from the home cage, vocalization, lachrymation, pupil size, salivation, urinary staining, diarrhoea, body and abdominal tone, extensor thrust, corneal reflex, pinna reflex, toe and tail pinch, and visual placing. The observations in the arena were rearing; ataxic, hypotonic, or impaired gait; overall gait incapacity; bizarre behaviour (movement); palpebral closure; tremors; twitches; convulsions; piloerection; respiratory rate and pattern; locomotor activity; arousal; grooming; defaecation; urination; auricular startle; olfactory response; air righting reflex; and positional passivity when placed on top of a box. The quantitative aspects entailed grip strength (fore- and hindlimb), hindlimb splay, body temperature, and motor activity (interrupting a light beam in a chamber).
At completion of the study on day 15, six rats of each sex per group were prepared for neurological assessment by whole-body perfusion with buffered paraformaldehyde-glutaraldehyde solution. Six levels of the brain—forebrain (through the septum), cerebrum (through the hypothalamus), midbrain, cerebellum and pons, midcerebellum, and medulla oblongata—and three levels of the spinal cord—cervical, thoracic, and lumbar—were examined after conventional processing with paraffin wax and haematoxylin and eosin staining. The brain weight before trimming was also recorded. The peripheral nerves examined were sciatic nerve (cross-section at mid-thigh region and longitudinal and cross-section at the sciatic notch), sural nerve (cross-section at knee), and tibial nerve (longitudinal and cross-section at knee), prepared in epoxy resin. Seven levels of the spinal cord (cross-sections of the left gasserian ganglion, the lumbar dorsal root ganglion (L4), the lumbar dorsal root (L4), the cervical dorsal root ganglion (C5), the cervical dorsal root (C5), and the cervical ventral root (C5), and longitudinal and cross-sections of the lumbar ventral root (L4)) were also prepared for examination after epoxy resin embedding. The other six rats of each sex per group underwent a complete necroscopy, and samples of brain tissue were processed and preserved for subsequent immunochemistry. Any abnormal tissues observed at necroscopy were examined histologically. Glial fibrillary acidic protein was measured in brain tissue homogenates derived from free-hand dissected cerebellum, cerebral cortex, hippocampus, striatum, and thalamus and hypothalamus regions and the rest of the brain by immunchemistry as dot blots on nitrocellulose membranes.
One male at 200 mg/kg bw and one female at 800 mg/kg bw were found dead on days 2 and 1, respectively, after treatment. The signs observed before the death of the male were decreased respiration, pallor, ocular discharge, and staining of the fur on the muzzle and ventral surface. A reduced body temperature was the only sign noted in the female. Necropsy revealed multiple dark areas on the stomach of both rats and some discolouration of the gut contents of the female. The male also had dilatation of the renal pelvis and multiple dark areas on the thymus. Surviving males at 200 mg/kg bw had reduced weight gain (20.3 g) on days 0–7 relative to the control group (45.7 g), so that the mean body weight on day 7 was significantly reduced (p < 0.01), and statistical significance (p < 0.05) was maintained for the remainder of the study. No significant differences were observed in the body weights of the females. Significant (p < 0.05–0.001) qualitative differences in the results of the FOB were found between treated and control groups only on the day of dosing. All male rats at 200 mg/kg bw and most (12/13) at 50 mg/kg bw showed tremors of the head, body, and limbs. Gait changes were observed at 50 mg/kg bw, 10/13 having moderate or more severely reduced mobility. Gait changes could not meaningfully be assessed in animals at the high dose because most (11/12) were almost completely immobile. Activities such as arousal, rearing, and grooming were also reduced in these two groups. The frequency of autonomic signs such as pupil constriction and salivation was increased at 50 and 200 mg/kg bw, most to all rats being affected. The saliva of two rats at 50 mg/kg bw and four at 200 mg/kg bw showed red discolouration. Two rats at 50 mg/kg bw had slightly but not significantly reduced extensor thrust, and at 200 mg/kg bw only three rats had a normal thrust. Other significant changes at 50 and 200 mg/kg bw were decreased pinna reflex, reduced or absent tail and toe pinch test response, impaired visual placing test response, delayed postural passivity, and increased failure in the air righting reflex test. The biological significance of the reduced urination and defaecation observed in males at 200 mg/kg bw is unclear. All 12 females at 800 mg/kg bw and four at 50 mg/kg bw had tremors. Gait changes were seen in all treated groups, with an apparent relationship to dose. At 800 mg/kg bw, 4/12 were immobile and 1/12 had severe, 6/12 had moderate, and 1/12 had slight ataxic gait. At 200 mg/kg bw, 1/12 had severe, 8/12 had moderate, and 2/12 had slight ataxic gait. At 50 mg/kg bw, 1/12 had moderate and 7/12 had slight ataxic gait. Significant reductions in arousal, rearing, and locomotor activity were observed at all doses, whereas tail and toe pinch responses and visual placing were reduced only at 200 and 800 mg/kg bw. Other significant changes were pupil constriction, salivation, failure of air righting reflex, and increased positional passivity at 200 and 800 mg/kg bw. The pinna reflex response was also reduced or absent at 800 mg/kg bw. Significant reductions (p < 0.05 or < 0.01) in grip strength (forelimb at 50 mg/kg bw and fore- and hindlimb at 200 mg/kg bw), motor activity, and body temperature were observed in males on day 0. Significant reductions in grip strength (forelimb at 200 mg/kg bw and fore- and hindlimb at 800 mg/kg bw) were recorded, and motor activity and body temperature were significantly reduced at all doses.
No gross pathological or neuropathological alterations were found in survivors at the end of the study. A significant (p < 0.05) increase in the amount of glial fibrillary acidic protein in the cerebral cortex was found in females at 800 mg/kg bw, but much of the increase was found in three rats, which had 143%, 116%, and 119% of the amount found in the control group. The biological significance of this finding in the absence of histological changes is unclear. The NOAEL was 12.5 mg/kg bw on the basis of marked changes in the FOB at 50 and 200 mg/kg bw (Beyrouty et al., 1993a).
In a behavioural and neuromorphological study performed in accordance with US Environmental Protection Agency test guidelines 82-7 and 83-1 and with GLP, groups of 12 male and 12 female 50–53-day-old Sprague-Dawley Crl:CD BR rats were fed diets containing technical-grade fenitrothion (purity, 94.3%) at a concentration of 0, 6, 20, 60, or 200 ppm, equal to 0.4, 1.3, 4.0, and 14 mg/kg bw per day for males and 0.5, 1.6, 4.8, and 18 mg/kg bw per day for females for 13 weeks. When the compound was suspended in corn oil and mixed with rodent chow, the homogeneity, stability, and concentration were found to be suitable during the week between fresh batches. Food consumption and body weight were measured weekly, and clinical monitoring was performed twice daily. An FOB was performed before treatment and during weeks 3, 7, and 12 of treatment. Brain, erythrocyte, and plasma cholinesterase activities were measured in satellite groups of five rats of each sex after 4, 8, and 13 weeks of treatment. At the end of the study, six rats of each sex per group were selected randomly for whole-body perfusion and subsequent processing for an extensive neuropathological assessment. The remaining six rats of each sex were necropsied, and the brains of control and high-dose rats were removed and dissected into six regions—cerebellum, cerebral cortex, hippocampus, striatum, thalamus and hypothalamus, and the rest—then homogenized for quantification of glial fibrillary acidic protein by the dot-immunobinding assay. The assay was repeated six times. A neuropathological assessment was conducted by light microscopy of the forebrain (through the septum), cerebrum (through the hypothalamus), midbrain, cerebellum and pons, midcerebellum and medulla oblongata and three levels of the spinal cord (cervical, thoracic, and lumbar). The brain weight before trimming was also recorded. The peripheral nerves examined by electron microscopy were sciatic nerve (cross-section at mid-thigh region and longitudinal and cross-section of the sciatic notch), sural nerve (cross-section at the knee), and tibial nerve (longitudinal and cross-section at the knee). Additionally, seven levels of the spinal cord (cross-sections of the left gasserian ganglion, lumbar dorsal root ganglion (L4), lumbar dorsal root (L4), cervical dorsal root ganglion (C5), cervical dorsal root (C5), and cervical ventral root (C5) and longitudinal and cross-sections of the lumbar ventral root (L4)) were prepared for examination after epoxy resin embedding.
Qualitative observations were performed with rats in the home cage, during handling, and in a test arena. The observations in the home cage were body position, tremors, twitches, convulsions, and bizarre behaviour (static). The observations during handling were ease of removal from the home cage, vocalization, lachrymation, pupil size, salivation, urinary staining, diarrhoea, body and abdominal tone, extensor thrust, corneal reflex, pinna reflex, toe and tail pinch, and visual placing. The observations in the arena included rearing; ataxic, hypotonic, or impaired gait; overall gait incapacity; bizarre behaviour (movement); palpebral closure; tremors; twitches; convulsions; piloerection; respiratory rate and pattern; locomotor activity; arousal; grooming; defaecation; urination; auricular startle; olfactory response; air righting reflex; and positional passivity when placed on top of a box. The quantitative aspects entailed grip strength (fore- and hindlimb), hindlimb splay, body temperature, and motor activity (interrupting a light beam in a chamber).
None of the rats died during the study. Four of 27 females at 200 ppm had tremors during the second week of treatment, 5/27 had a red–brown muzzle, and 15/27 had a brown stained tail. The group mean feed consumption was significantly (p < 0.01) lower for both males (7%) and females (14%) during the first week of treatment at 200 ppm but was significantly increased during weeks 5, 6, 7, 8, and 13 (by an average of 12%) in females only. Significant reductions (p < 0.01–0.05) in body weight were seen in males (average, 4%) and females (average, 13%) during the first 3 weeks of treatment at 200 ppm, and the effect persisted in females until week 8. At 60 ppm, only females had significantly reduced (p < 0.05) body weight during the first week of treatment. Although a statistically significant (p < 0.05) reduction in locomotor activity was seen in rats at 20 ppm at week 3 and in those at 60 ppm at week 7, there was no relationship to dose. Similarly, a significant (p < 0.05) change in positional passivity in males at week 12 was attributable to the unusual finding that the controls were more passive than treated rats. Quantitative assessments revealed no unequivocal treatment-related effects, although transient, statistically significant reductions in the grip strength of the fore- (week 3) and hindlimbs (week 7) of females were observed at 200 ppm. A significant decrease (p < 0.05) in hindlimb splay was also seen in females at 60 ppm at week 3 but not at 200 ppm. No significant changes in body temperature or motor activity were observed.
At necropsy, 1/6 females at 200 ppm had a darkened area on the meninges in the cervical region of the spinal cord. Other findings were cysts on the edge of the spleen in 1/6 males at 60 ppm and in 2/6 at 200 ppm. The pathologist’s report indicated that such cysts were common among rats in the laboratory and that these incidences were therefore unlikely to be related to treatment. Neuropathological examination revealed few abnormalities, and the incidence of those found was equal to or less than that observed in controls. The abnormalities included dilated ventricles in the brain; vacuolation of the ganglion cell body in cervical and lumbar dorsal root ganglia and in the gasserian ganglion; myelin bubbling or splitting in the lumbar dorsal and ventral roots and in nerves of the cervical and dorsal root ganglia; and axon degeneration in the lumbar ventral root and lumbar dorsal root ganglion. At 200 ppm, the mean glial fibrillary acidic protein was 79–103% in all brain regions in males and 86–98% in females. Since only values in excess of 115% are generally considered to be biologically significant, it seems reasonable to conclude that minimal injury to astrocytes occurred at 200 ppm. No significant change in brain weight was found.
Fenitrothion caused appreciable inhibition of plasma cholinesterase activity in females at the lowest concentration tested (6 ppm) (Table 12). Moreover, a clear dose–response relationship is evident. Use by the investigators of a different test (Dunn’s) to assess the statistical significance of the plasma cholinesterase inhibition in females at weeks 4 and 13 from that used to assess changes at other times during the study would appear to be an attempt to minimize the significance of the observation, and this form of data analysis is of questionable scientific validity. In contrast to the relatively good concordance between the inhibition of cholinesterase activity in plasma and brain at 60 and 200 ppm, significant inhibition of erythrocyte acetylcholinesterase activity was observed only in males at 200 ppm. The NOAEL for inhibition of brain acetylcholinesterase activity was 20 ppm, equal to 1.3 mg/kg bw per day (Beyrouty et al., 1993b).
Table 12. Per cent inhibition of cholinesterase activity in rats treated with fenitrothion in the diet for 13 weeks
|
Concentration (ppm) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
6 |
17 [13] |
52 [28] |
10 [6] |
–14 [–2] |
3 [4] |
–2 [2] |
|
20 |
13 [17] |
66 [59] |
10 [9] |
–1 [11] |
0 [0] |
5 [11] |
|
60 |
36* [40] |
77† [74] |
17 [23] |
26 [30] |
14** [16] |
58** [57] |
|
200 |
40** [49] |
87‡ [84] |
41** [44] |
38 [46] |
58** [60] |
77** [78] |
From Beyrouty et al. (1993b)
Values in square brackets represent the average inhibition measured after 4, 8, and 13 weeks of treatment; negative values indicate cases in which the activity exceeded that in controls Dunnett's test of significance was used at week 8, but Dunn's test was used at weeks 4 and 13.
* p < 0.05; ** p < 0.01 (Dunnett's test)
† p < 0.01; ‡ p < 0.001 (Dunn's test)
Rabbits
Technical-grade fenitrothion (purity, > 95%) was administered orally at a dose of 10 or 25 mg/kg bw per day to groups of six rabbits (strain and sex not reported) for 8 and 4 weeks, respectively. An untreated control group of six animals was included. No rationale was given for the dose selection. Sciatic nerve conduction velocity was determined by electrical stimulation (supramaximal stimuli of 1/s for 0.2 ms) through the intact skin of unanaesthetized rabbits and by recording the difference between the latencies of two action potentials in the gastrocnemius muscle via needle electrodes. At the end of treatment, the sciatic nerve was removed from anaesthetized animals and processed for light and electron microscopy. Neither the number of rabbits used nor the number of nerve sections examined was reported.
None of the rabbits died during the study. The sciatic nerve conduction velocity, measured at 2-week intervals, declined progressively in animals at 10 mg/kg bw per day, from 26 m/s before treatment to 24 m/s, 22 m/s, 18 m/s, and 18 m/s at each fortnightly recording, reaching statistical significance (p < 0.01) at the last two measurements. At these times, the rabbits apparently had no clinical signs, although the whole-blood cholinesterase activity was reduced by 50%. Rabbits at 25 mg/kg bw per day showed clinical signs of excitation, salivation, mild ataxia, and gait loss, but the time of onset or duration or the degree of inhibition were not reported. The authors stated that the nerve conduction velocity increased (not quantified) and rabbits responded with extensive muscle contraction after only one electrical stimulus.
The results of neuropathology at 25 mg/kg bw per day were not reported, but at 10 mg/kg bw per day, swollen, dislocated axoplasm was seen at low magnification and identified under electron microscopy as intramyelinic vacuoles containing thin lamellae of split myelin sheaths, claimed to represent evidence of fragmentation. (It is unclear exactly when histology was performed, since the text states that it was conducted after cessation of treatment, whereas the legend to a photomicrograph of a nerve bundle suggests that it was conducted after 10 days of treatment.) There was no indication of the incidence of lesions on the slides (Lehotzky & Ungváry, 1976).
Mice and rats
In a protocol developed by the National Toxicology Program to assess chemical immunotoxicity, technical-grade fenitrothion (purity, 96.7%) was administered orally in corn oil to groups of five or six male B6C3F1 mice at a dose of 0, 10, 20, or 40 mg/kg bw per day and to groups of five or six male Sprague-Dawley rats at a dose of 0, 0.3, 3, or 30 mg/kg bw per day for 14 days. The highest doses represented 1/25 of the LD50. Groups of six mice and six rats received cyclophosphamide intraperitoneally, at 45 mg/kg bw for rats and 80 mg/kg bw per day for mice, and served as positive controls. The immunological assays performed were for the plaque-forming cell response to sheep red blood cells, the Arthus reaction, delayed-type hypersensitivity, mixed lymphocyte reaction, natural killer cells, macrophage phagocytosis, flow cytometric analysis of splenocytes, and blastogenic response. Cholinesterase activity was measured in whole blood, erythrocytes, and brain in groups of five animals, except for rats at 30 mg/kg bw per day, in which it was measured in only two animals.
Four rats at 30 mg/kg bw per day died during treatment. The clinical signs observed at this dose were miosis, muscle fibrillation, salivation, soft stools, and diarrhoea; other signs of toxicity were reduced weight gain and an increased weight of the adrenals relative to body weight. At 3 mg/kg bw per day, the only clinical sign was miosis. None of the mice at 10, 20 or 40 mg/kg bw per day died or showed clinical signs, even though significant reductions (p < 0.01) were observed in brain acetylcholinesterase activity, by 50, 60, and 65%, respectively, and in plasma cholinesterase activity, by 65, 75, and 80% (all estimated from a published graph). Acetylcholinesterase activity in erthyrocytes was not reported for mice. In rats, significant, dose-related reductions (p < 0.01) in cholinesterase activity were observed at 3 and 30 mg/kg bw per day in plasma (by 33% and 55%, respectively), erythrocytes (by 46% and 88%, respectively), and brain (by 25% and 83%, respectively). The positive controls had the expected reductions in the relative weights of the thymus and spleen, with concomitant reductions in platelets, neutrophil, monocyte, eosinophil, and lymphocyte counts in blood. The number of plaque-forming cells was suppressed by 68% (positive controls, 99%) only in rats that had received 30 mg/kg bw per day. The other indicators of immune function were not significantly changed in rats at any dose. In mice, no suppression of plaque-forming cells or of the mixed lymphocyte reaction was observed, even at the highest dose. In a separate study in which corticosterone concentrations were measured in the serum of four rats after a single dose of 0, 3, or 30 mg/kg bw by gavage, a marked increase was observed at the highest dose after 3 h: 350 ng/mL as compared to < 25 ng/mL in controls and rats at 3 mg/kg bw. These findings suggest that the immune suppressive effect of fenitrothion in rats at 30 mg/kg bw per day was due to potent systemic cholinergic effects which induced corticosterone secretion and that fenitrothion causes no immune toxicity in mice or rats (Kunimatsu et al., 1996).
Rats
Fenitrothion (purity not stated) in olive oil was administered intraperitoneally at a dose of 0 or 14 mg/kg bw to male Wistar rats. Electroretinographic changes were recorded in anaesthetized animals 5 h and 2 days after dosing, and cholinesterase activity in brain and the retinochoroid was measured after 3 days. No clinical signs were reported. Cholinesterase activity was significantly reduced by 29% in brain (p < 0.05) and by 13% in the retinochoroid (not significant). Changes in the electroretinographic profile, recorded with an electrode on the cornea under local ocular anaesthesia and an electrode on the brain parietal region after successive xenon flashes (computer-generated mean profile after 10 flashes), revealed significantly increased amplitudes of the a-wave (the portion of the curve below the baseline) and b-wave (calculated from the trough of the a-wave to the peak of the curve above the baseline) after 2 days, by 19 and 21%, respectively (both p < 0.01); at 5 h, there was no change in the a-wave but a nonsignificant decrease in the amplitude of the b-wave (by 17%), with no change in latency. Other organophosphates tested (fenthion and chlorpyrifos) significantly decreased the amplitudes of the a- and b-waves with a concomitant increase in latency, whereas dichlorvos had the opposite effect on amplitude with an increased latency (Yoshikawa et al., 1990).
In view of the suggestion that some organophosphates could induce ocular lesions, a study of ocular toxicity in rats was performed according to the GLP guidelines of the OECD, Japan, and the USA. Fenitrothion (purity, 94.5%) in corn oil was administered by gavage to groups of five SPF Sprague-Dawley Crj:CD rats of each sex at a dose of 0, 20, or 200 mg/kg bw for males and 0, 40, or 400 mg/kg bw for females. The rats were observed for clinical signs 1, 3, and 6 h after dosing and daily thereafter. Body weights were measured on the day of dosing, 4 days later, at weekly intervals, and on the day of terminal kill. Before termination, the conjunctiva, cornea, iris, lens, and vitreous body were examined with an ophthalmoscope. Electroretinograms were used to determine the a-wave, b-wave, and oscillatory potential in each right eye of a satellite group after a dark adaptation period 4, 7, 14, 28, and 91 days after dosing. At the terminal kill, the eyes and their accessory organs were examined macroscopically. Blood cholinesterase activity was measured by colorimetric assay the day after electroretinography.
One male at 400 mg/kg bw died from treatment, and three males and eight females died under anaesthesia for electroretinography. Muscular fibrillation and eye discharge showing dose-related severity were observed in animals of each sex. The body weights of animals at the higher dose were reduced at 4 and 7 days after treatment. Although some females had corneal opacity, the incidence appeared to be similar in treated and control groups and was considered to be unrelated to treatment. Similarly, although the amplitude of the a-wave was significantly increased in males at 200 mg/kg bw 7 days after dosing (p < 0.05), all other electroretinographic values in this group were unaffected, and the finding was considered not to have affected the overall electroretinogram for this group. Females at 400 mg/kg bw showed a significant reduction in the latency of the b-wave and prolongation of the latency of the first peak of the oscillatory potential at 7 and 91 days. A significantly prolonged latency and decreased amplitude of the a-wave were observed in females at 40 mg/kg bw 28 days after treatment, but neither effect was dose-related, and no treatment-related ocular effects were considered to have occurred.
Erythrocyte acetylcholinesterase activity was significantly (p < 0.01) reduced in males at 200 mg/kg bw up to 15 days after treatment, some effect persisting until day 29 (Table 13). The group at 20 mg/kg bw also showed a reduction, which was more moderate, at 5 and 8 days. The females showed significant reductions in erythrocyte acetylcholinesterase activity at both doses up to day 15; however, inhibition of plasma cholinesterase activity was significant in females only at 400 mg/kg bw after 5 days. Therefore, fenitrothion at doses that caused clinical signs and significant inhibition of cholinesterase activity did not cause changes in the eye under the conditions of this study (Kosaka et al., 1989).
Table 13. Per cent inhibition of cholinesterase activity in rats treated with fenitrothion by gavage
|
Site of cholinesterase activity |
Interval |
Per cent reduction in cholinesterase activity |
|||
|
Males |
Females |
||||
|
20 mg/kg bw |
200 mg/kg bw |
40 mg/kg bw |
400 mg/kg bw |
||
|
Plasmaa |
5 |
15 |
10 |
29 |
56** |
|
|
8 |
4 |
[40]b |
24 |
21 |
|
|
15 |
17 |
[4] |
[1] |
19 |
|
Erythrocyte |
5 |
38* |
77** |
42** |
86** |
|
|
8 |
28** |
73** |
48** |
63** |
|
|
15 |
14 |
57** |
30** |
49** |
|
|
29 |
[4] |
24 |
11 |
24 |
|
|
92 |
[2] |
5 |
11 |
5 |
From Kosaka et al. (1989)
Values in square brackets indicate the extent (%) to which the measured activity was greater than that of controls
* p < 0.05; ** p < 0.01 (Dunnett’s test)
a
Three animalsb
Activities on days 29 and 92 not shown as they were similar to that of controlsIn a study performed according to the GLP guidelines of OECD, Japan, and the USA, technical-grade fenitrothion (purity, 94.5%) was administered to groups of 12 SPF Sprague-Dawley Crj:CD rats of each sex in the diet at a concentration of 0, 2.5, 5, 10, or 30 ppm, equal to 0.14, 0.28, 0.57, and 1.7 mg/kg bw per day for males and 0.17, 0.33, 0.65, and 2.0 mg/kg bw per day for females, for 13 weeks. The concentration and homogeneity of fenitrothion in the diets were found to be suitable between batches made freshly at 2-week intervals. The rats were monitored daily for clinical signs, and detailed clinical examinations of the eyes, eyelids, and conjunctiva were carried out each week. Any rats found moribund were killed for autopsy. Body weights and food consumption were recorded weekly. Blood and brain were collected from 10 rats of each sex per group at the end of treatment in order to measure cholinesterase activity by a colorimetric assay. The retina, optic nerve, and medial rectus muscle from the remaining two rats in each group were prepared for transmission electron microscopy after perfusion fixation; however, only tissues from the control and the high-dose group were examined. The conjunctiva, cornea, iris, lens, vitreous body, and retina were examined with an ophthalmoscope before treatment and again during week 13, just before termination. Electroretinography was performed in one eye for a-wave, b-wave, and oscillatory potential after a dark adaptation period during week 12. Sections were prepared from the optic nerve, medial rectus muscle, ciliary body, and iris of rats not perfused for electron microscopy and were examined by light microscopy.
No treatment-related effects on clinical signs, mortality rate, body weight, food consumption, or ophthalmoscopic or electroretinographic end-points were seen, and no treatment-related effects were seen macroscopically or microscopically. However, significant inhibition of cholinesterase activity in brain (p < 0.01) was found in females and an equivocal effect in males at 30 ppm, and significant inhibition (p < 0.01) of acetylcholinesterase activity in erythrocytes was observed in animals of each sex at 30 ppm (Table 14). The NOAEL for inhibition of brain and erythrocyte acetylcholinesterase activity was 10 ppm, equal to 0.57 mg/kg bw (Mitsumori et al., 1989).
Table 14. Per cent inhibition of cholinesterase activity in rats treated with fenitrothion in the diet for 13 weeks
|
Concentration (ppm) |
Per cent reduction in cholinesterase activity |
|||||
|
Plasma |
Erythrocytes |
Brain |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
2.5 |
23 |
28 |
0 |
0 |
[12] |
2 |
|
5 |
5 |
17 |
3 |
7 |
[16**] |
4 |
|
10 |
23 |
46 |
3 |
7 |
[11] |
4 |
|
30 |
41** |
85** |
35** |
48** |
[5] |
14** |
From Mitsumori et al. (1989)
Values in square brackets represent cases in which the activity exceeded that in controls.
** p < 0.01 (Dunnett's test)
The S-methyl isomer of fenitrothion occurs as an impurity (0.5–1.5%) in technical-grade fenitrothion. It can also be formed by thermal isomerization of fenitrothion or by isomerization catalysed by ultra-violet irradiation during prolonged storage under inappropriate conditions. The acute toxicity of the S-methyl isomer administered orally to rats and mice was approximately twice that of fenitrothion, and the signs of poisoning were typical of the muscarinic and nicotinic action of acetylcholine seen with anticholinesterase compounds. The anticholinesterase action of the isomer in vitro was compared with that of fenitrothion and its oxygen analogue in human and horse serum with fly-head cholinesterases. The negative logarithm of the molar concentration of the S-methyl isomer that caused 50% inhibition was: 7 in horse serum, 8 in human serum, and 9 in fly heads, while those for fenitrothion were: 5 in human and horse sera and 6 in fly heads. The capacity of the oxygen analogue of fenitrothion to inhibit cholinesterase directly was about equal to that of the S-methyl isomer. These results indicate that contamination of fenitrothion with this isomer can account for much of its anticholinesterase activity; this should be considered in interpreting the results of assays for anticholinesterase activity in vitro. The S-methyl isomer was degraded more rapidly than fenitrothion in rats, as shown by the rates of excretion of para-nitro-meta-cresol. Furthermore, a greater proportion of a total dose of the S-methyl isomer could be accounted for by this urinary metabolite. When groups of 10 male rats were given 0, 1, 6, or 12 mg/kg bw orally and mated with groups of 20 untreated female rats in eight sequential matings with a new group of females each week, the number of resorptions was slightly greater in the treated groups. Although there was no marked difference between the three dose groups, the investigators concluded that the S-methyl isomer has little mutagenic activity (Kovacicová et al., 1973; JMPR, 1974; Rosival et al., 1974). The LD50 values for the metabolites and impurities of technical-grade fenitrothion are shown in Table 15.
Table 15. Studies of the acute toxicity of metabolites of fenitrothion
|
Species |
Strain |
Sex |
Route |
Vehicle |
LD50 |
Reference |
||
|
Fenitrooxon (active mammalian metabolite) |
||||||||
|
Mouse |
NR |
NR |
Oral |
NR |
90 |
Miyamoto et al. (1963b); Miyamoto (1969); JMPR (1969) |
||
|
Mouse |
Swiss |
NR |
Oral |
Olive oil |
120 |
Hollingworth et al. (1967); JMPR (1969) |
||
|
Mouse |
CD1 |
Male |
Intraperitoneal |
Propylene glycol |
44.5 |
Myatt & Ecobichon (1975) |
||
|
Rat |
NR |
NR |
Oral |
NR |
24 |
Miyamoto et al. (1963b); Miyamoto (1969); JMPR (1969) |
||
|
Rat |
NR |
NR |
Intravenous |
NR |
3.3 |
Miyamoto et al. (1963b); Miyamoto (1969); JMPR (1969) |
||
|
Guinea-pig |
NR |
NR |
Oral |
NR |
221 |
Miyamoto et al. (1963b); Miyamoto (1969); JMPR (1969) |
||
|
Guinea pig |
NR |
NR |
Intravenous |
NR |
32 |
Miyamoto et al. (1963b); Miyamoto (1969); JMPR (1969) |
||
|
Hen |
NR |
Female |
Oral |
NR |
35 |
Kadota et al. (1975a); JMPR (1977) |
||
|
3-Methyl-4-nitrophenol (mammalian metabolite) |
||||||||
|
Mouse |
dd |
Male |
Oral |
Gum arabic |
250 |
Kadota & Miyamoto (1979) |
||
|
Mouse |
dd |
Male |
Dermal |
Ethanol |
> 5000 |
Kadota & Miyamoto (1979) |
||
|
Mouse |
dd |
Male |
Intraperitoneal |
Gum arabic |
136 |
Kadota & Miyamoto (1979) |
||
|
Rat |
Wistar |
Male |
Oral |
Gum arabic |
2300 |
Kadota & Miyamoto (1979) |
||
|
|
|
Female |
|
|
1200 |
|
||
|
S-Methylfenitrothion (isomer ) |
||||||||
|
Mouse |
dd |
Male |
Oral |
Corn oil |
550 |
Mikami et al. (1977) |
||
|
|
|
Female |
|
|
420 |
|
||
|
Mouse |
dd |
Male |
Dermal |
Corn oil |
1750 |
Mikami et al. (1977) |
||
|
|
|
Female |
|
|
1900 |
|
||
|
Mouse |
dd |
Male |
Intraperitoneal |
Tween 80 |
54 |
Mikami et al. (1977) |
||
|
|
|
Female |
|
|
65 |
|
||
|
Mouse |
CD1 |
Male |
Intraperitoneal |
Propylene glycol |
112 |
Myatt & Ecobichon (1975) |
||
|
Rat |
Sprague- Dawley |
Male |
Oral |
Corn oil |
460 |
Mikami et al. (1977) |
||
|
|
|
Female |
|
|
540 |
|
||
|
Rat |
Wistar |
Male |
Oral |
Olive oil |
315 |
Rosival et al. (1976); modified from JMPR (1977) |
||
|
Rat |
Sprague- |
Male |
Dermal |
Corn oil |
1450 |
Mikami et al. (1977) |
||
|
|
Dawley |
Female |
|
|
1750 |
|
||
|
Bis-fenitrothion (impurity) |
||||||||
|
Mouse |
ICR |
Male |
Oral |
Corn oil |
612 |
Suzuki (1982) |
||
|
|
|
Female |
|
|
500–750 |
|
||
NR, not reported
Fenitrothion was given to 24 volunteers as a single oral dose of 0.042–0.33 mg/kg bw or 2.5–20 mg/person. There were no signs of cholinergic activity. Excretion of a urinary metabolite, 3-methyl-4-nitrophenol, was almost complete within 24 h, representing between 70% (0.042 mg/kg bw) and 50% (0.33 mg/kg bw) of the dose. The mean plasma and erythrocyte cholinesterase activities were not depressed much below normal (Ł 10%) after 6 or 24 h, except in one person given 0.33 mg/kg bw who showed a 28% depression of of plasma cholinesterase activity. When oral doses of 0.04–0.08 mg/kg bw were given four times at 24-h intervals to five individuals, most of the 3-methyl-4-nitrophenol excreted appeared in the urine within 12 h. There were no cholinergic signs, and plasma and erythrocyte cholinesterase activity (measured by an electrometric method for change in pH) did not appear to be affected (JMPR, 1969; modified by reference to the original report of Nosál & Hladká, 1968).
Twelve adult male volunteers were given an oral dose of fenitrothion equal to 0.1 or 0.5 mg/kg bw per day followed by dermal application of 0.1 mg/kg bw per day to the arms and face for 9 days. There was no evidence of dermal irritation and no apparent difference in cholinesterase activity (JMPR, 1984; Shelanski et al., 1977).
The effects of ingesting capsules containing technical-grade fenitrothion (purity not stated) were monitored in a study in four female and eight male volunteers aged 23–50 years (mean, 33 years). The doses selected for the study were based on a pilot study performed during 1990–91 in which three men (mean age, 45) received a single dose of 0.06 mg/kg bw followed by another of 0.18 mg/kg bw and then 0.36 mg/kg bw. The interval between each dose ranged from 2 weeks to 8 months. These doses represented 12, 36, and 72 times the ADI (0–0.005 mg/kg bw per day) established by the JMPR in 1988. In the absence of any cholinergic signs or inhibition of cholinesterase activity in blood at any dose, the two highest doses, 0.18 and 0.36 mg/kg bw, were selected for the main study. The main study involved volunteers recruited from among scientific and medical staff and included two of the three who had participated in the pilot study. The exclusion criteria included being a woman of childbearing age, having diabetes, concurrent disease, or drug therapy which could not be discontinued over the duration of the study, drug addiction, alchohol abuse, heavy smoking, clinically relevant abnormalities in haematological or biochemical profiles (including plasma cholinesterase activity), and anybody who in the opinion of the investigators was likely to comply poorly with instructions. The study was approved by the appropriate institutional ethics committee, and informed consent was obtained from the participants. Only medical and scientific staff were deemed sufficiently informed about the consequences of exposure to organophosphates to give truly informed consent.
In the main study, performed during 1994–95, groups of two to four volunteers ingested capsules with food daily in divided doses at 12-h intervals. Each person in the group started with the low dose (0.18 mg/kg bw per day), followed 2 weeks to 5 months later by the high dose (0.36 mg/kg bw per day). Each daily dose was continued for 3 days, only half the daily dose being administered on day 4. During these four consecutive treatments, the volunteers were not required to remain under clinical supervision on days when no monitoring took place (i.e. on days 2 and 3) but were instructed in contingency measures. They were supplied with and instructed in the use of atropine tablets and requested to contact the unit if they felt unwell. Monitoring included self-reporting and investigator-prompted reporting of clinical symptoms and collection of blood samples for analysis of fenitrothion and fenitrooxon; clinical chemical parameters, including cholinesterase activity and haematological end-points, were measured on days 1 and 4 of the study. The fenitrothion concentration in blood was measured by chromatography on a BP-5 capillary column with a nitrogen–phosphorus detector after extraction in an organic solvent (detection limit, 0.1–0.4 ng/mL; mean recovery from 0.1–12.5 ng/mL of plasma, ~ 100%). Vital signs (pulse rate, and systolic and diastolic blood pressure) were determined before dosing and 0.5, 1, 2, 3, 4, 6, 8, 10, and 12 h after the first dose. Blood was taken for measurement of haemoglobin concentration, red blood cell, leukocyte,and platelet counts, erythrocyte sedimentation rate, blood urea nitrogen, glucose, sodium, potassium, chloride, bicarbonate, cholesterol, creatinine, triglyceride, and bilirubin concentrations, and alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, creatine kinase, and gamma-glutamyl transferase activities before dosing and on day 3 after the final dose was administered. Blood for estimation of cholinesterase activity was collected before dosing and 0, 4, and 8 h after dosing at 0.09 mg/kg bw and 0, 4, 8, and 16 h at 0.18 mg/kg bw on days 1 and 4 of each treatment regimen (i.e. after half the daily dose). Metabolites were measured in 24-h samples of urine.
No significant effects of treatment on blood pressure or pulse rate were seen in any of the volunteers in either the pilot or the main study. In the main study, 11 clinical signs were reported, four by one woman and the rest by five men. The signs were associated with four main systems: neurological (nine reports), gastrointestinal (three), respiratory (two), and skin (10). Sweating and colic were reported by one volunteer on day 1 at 0.09 mg/kg bw and then intermittantly during treatment at the higher dose, and nausea by two volunteers at 0.5 and 1 h on day 2 or 3 after dosing with 0.18 mg/kg bw. Headache was reported by one volunteer on day 4 after receiving 0.18 mg/kg bw, and gastrointestinal symptoms occurred in three volunteers. Most of these clinical signs were considered to be unrelated to treatment since they were not dose-related. The intermittent colic seen in one man after treatment with either dose and the slight sweating on day 1 after ingestion of 0.09 mg/kg bw, in the absence of consistent suppression of cholinesterase activity in blood but with a slight increase in leukocyte count (not outside the normal range), may have been related to a mild infection. It is noteworthy, however, that the only person in whom suppression of plasma cholinesterase activity (by 26%) was seen in the study was this volunteer; this occurred 8 h after ingestion of 0.18 mg/kg bw. Although another volunteer had the greatest reduction in erythrocyte cholinesterase activity (17%) after 4 h at 0.18 mg/kg bw on day 4, no clinical signs were seen or reported. Transient reductions in erythrocyte cholinesterase activity were observed in two volunteers, in whom 12% inhibition was observed, also after 4 h at 0.18 mg/kg bw on day 4.
Although some fenitrooxon was detected in the plasma in the pilot study, the values were considered too variable to be meaningful and appeared be confounded by co-elution with caffeine. The concentrations of fenitrothion could be quantified, however, and the pharmacokinetics were determined from measurements before dosing and 0.5, 1, 2, 3, 4, 6, 8, 10, and 12 h (and 16 and 24 h at 0.18 mg/kg bw) after dosing on days 1 and 4 of each treatment regimen (Table 16). The investigators suggested that the increased maximum plasma concentration (Cmax) and integrated area under the concentration–time curve (AUC) with frequency of dosing in the absence of a concomitant change in the elimination half-life may be related to the attainment of a steady state after 4 days. They dismissed the possibility of a reduction in liver cytochrome P450 enzyme activity on the basis of the absence of a change in the elimination half-life. There were no treatment-related changes in haematological or clinical chemical end-points during the study. The urinary excretion of the predominant fenitrothion metabolite, 3-methyl-4-nitrophenol, is shown in Table 17. More metabolite was excreted on day 1 than on day 4 because the volunteers received only half the daily dose on day 4. In view of the absence of cholinergic signs or inhibition of cholinesterase activity in the blood, the NOAEL was 0.36 mg/kg bw per day, the highest tested dose (Meaklim & McNeil, 1999).
Table 16. Pharmacokinetics of fenitrothion in volunteers after oral administration
|
Treatment |
Dose |
Cmax |
Tmax |
AUC |
T1/2 |
|
1 |
0.09 |
0.54 |
60 |
170 |
120 |
|
7 |
|
0.84 |
60 |
286 |
180 |
|
1 |
0.18 |
1.8 |
60 |
380 |
120 |
|
7 |
|
7.7 |
60 |
1400 |
130 |
From Meaklim & McNeil (1999)
Table 17. Urinary excretion of 3-methyl-4-nitrophenol after oral administration of fenitrothion to volunteers
|
Dose (mg/kg bw per day) |
Day |
Mean dose of fenitrothion (mg) |
Mean |
Fenitrothion equivalent (mg) |
Per cent excreted in 24 h |
|
0.18 |
1 |
13 |
6.0 |
11 |
83 |
|
0.09 |
4 |
6.8 |
3.6 |
6.6 |
97 |
|
0.36 |
1 |
26 |
9.8 |
18 |
67 |
|
0.18 |
4 |
14 |
5.7 |
10 |
76 |
From Meakim & McNeil (1999)
In a study of the clinical progress of 150 patients admitted to hospital after consuming varying quantities of insecticides, 48 had consumed fenitrothion. One died after ingesting 3 g, and paralysis was observed in two of three patients who had consumed 6 g, seven out of 20 who had consumed 3 g, two of 16 who had consumed 1.5 g, and none of nine who had consumed less than 1.5 g. In the 11 patients with paralysis, plasma cholinesterase activity was inhibited by > 80% in seven cases, by 60–80% in two cases, and by 40–60% in one case; no comment was made about the other case (Wadia et al., 1977).
‘Intermediate syndrome’ experienced after organophosphate poisoning can be distinguished from the characteristic muscarinic, nicotinic, and central nervous system effects observed soon after exposure and the delayed neurotoxicity seen 2–3 weeks later, despite apparently satisfactory clinical management. Intermediate syndrome occurs 24–96 h after exposure and is characterized by muscular weakness affecting the neck, proximal limb, and respiratory muscles. Since only some organophosphates induce this phenomenon, a study was conducted to examine 16 cases of poisoning with fenitrothion by ingestion retrospectively for the occurrence of intermediate syndrome. The patients consisted of 14 men and two women; nine had attempted suicide, and seven had consumed the product accidentally. They had consumed 50–100 mL of a 50% fenitrothion solution. Six died within 5–22 days, despite gastric lavage and atropine and oxime therapy. The concentration of fenitrothion in the plasma of the fatal cases was 0.47–8.4 µg/mL. Intermediate syndrome was observed in seven of the 10 survivors. Plasma cholinesterase activity was not detectable in survivors with intermediate syndrome (and one other) at the time of their admission, and their recovery time ranged from 5 to > 10 weeks, whereas two of three patients with no symptoms had activities of 200 and 1200 mU/mL and all three had recovered within 2–4 weeks. The concentration of fenitrothion in the plasma of survivors with intermediate syndrome was 0.18–3.0 µg/mL, while that in the others was 96–360 ng/mL (Groszek et al., 1995).
Several fenitrothion metabolites, including 3-methyl-4-nitrophenol, aminofenitrothion, S-methyl-fenitrothion, and acetylaminofenitrothion, were detected in the urine of a 23-year-old man who attempted suicide by ingesting approximately 50 mL of a 50% fenitrothion emulsion. All the metabolites except S-methylfenitrothion were detected up to 62 h after ingestion. The elimination half-life of fenitrothion was about 4.5 h, and its concentration after 3 h (first recording) was 170 ng/mL. The plasma cholinesterase activity at 3 h was approximately 13% that of the average population range, and it increased to 14% by day 1, 27% by day 2, and 34% by day 3. The patient recovered sufficiently to be discharged after 3 days (Kojima et al., 1989).
A 56-old man ingested about 60 mL of a 50% fenitrothion emulsion in an attempt to commit suicide. At admission and until his death from respiratory insufficiency on day 6, the plasma cholinesterase activity was < 10% of the normal range, despite combined haemoperfusion and haemodialysis. Urinary excretion of 3-methyl-4-nitrophenol was maximal at about 58 mg/day on day 3 after ingestion. Measurements of unchanged fenitrothion in the organs after death showed that most was in fat, followed by pancreas, muscle, and lung (Yoshida et al., 1987).
In a field-spraying operation in a village in southern Nigeria with a 5% spray of fenitrothion, 18 villagers examined 1 week later showed no clinical symptoms of toxicity or depression of plasma cholinesterase activity. The activity in the three spraymen examined on days 1, 2, and 6 after spraying was not lower than before spraying (Vandekar, 1965; JMPR, 1969).
A published paper described the extent of exposure to fenitrothion of workers involved in manual spraying of apple trees in an orchard. In a typical spray operation, groups of two operators and an assistant, protected by rubber boots and gloves, prepared, handled, transported, and sprayed a formulation daily for 3 consecutive days; the spray was prepared by the assistant. The amount of fenitrothion inhaled was monitored from a respiratory mask connected to a water trap, the volume of inhaled air being monitored with an air-flow meter. Three operators using this apparatus were exposed to clean air only, while nine others were exposed to fenitrothion. The plasma concentrations of fenitrothion were measured after work by gas chromatography (detection limit, 1 ng/mL; mean recovery of 50 ng/mL from spiked plasma, 85%). Plasma alanine aminotransferase, aspartate aminotransferase, and cholinesterase activities (by change in pH) were also determined. Urinary excretion (24-h pooled sample) of 3-methyl-4-nitrophenol was determined by gas chromatography in 19 workers, including those monitored for plasma fenitrothion.
This report does not mention any clinical signs. The mean concentration of fenitrothion inhaled by the operators was 0.011 µg/L. The operators had a lower maximum concentration of fenitrothion in plasma (16 ng/mL; median, 8 ng/mL) than the assistants (30 ng/mL); three of five operators had detectable concentrations after day 1 and one of five after day 3 of spraying. The concentrations on day 2 were not determined because rain suspended afternoon spraying. The clearance of fenitrothion from plasma appeared to be relatively rapid, since only two of seven workers (the other two in the group were not tested for unknown reasons) had detectable quantities (Cmax, 13 ng/mL) the following morning (elapsed time between sampling not reported). Determinations of aspartate and alanine aminotransferase and cholinesterase activities revealed no treatment-related changes. A urine sample collected after spraying contained a mean concentration of 3-methyl-4-nitrophenol of 130 ng/mL for operators and 230 ng/mL for the assistants, reflecting the differences in plasma concentration (JMPR, 1984; modified by reference to the original report of Usutani et al., 1978).
During a 30-day operation in southern Iran in which fenitrothion was sprayed for malaria control, 28 spraymen and 925 inhabitants were monitored for clinical signs and cholinesterase activity. Mild clinical symptoms such as nausea and dizziness were observed in eight of 20 spraymen, who also had depressed whole-blood cholinesterase activity. Very mild complaints, namely dizziness and nausea, were reported by the inhabitants (Motabar et al., 1972; JMPR, 1984).
A large-scale spray operation in Kisumu, Kenya, involving 35–40 operators spraying fenitrothion for 5 h/day, 5 days/week intermittently for 2 years was monitored by WHO. Numerous clinical parameters were measured in the operators and matched controls, and no significant difference was found between the groups.
In a trial of use of fenitrothion indoors in 978 houses in a village in central Java for 4 weeks, there were no complaints or clinical symptoms of organophosphorus toxicity among the spray operators or the inhabitants of the sprayed area. However, cholinesterase activity, determined by the tintometric method, was reduced after the third week of spraying in four of 12 spraymen to 40–60% of the values before exposure (JMPR, 1984).
After oral administration, fenitrothion is rapidly and extensively absorbed from the mammalian intestinal tract (about 90–100% of the dose) and eliminated, predominantly in the urine (up to about 93% of the dose) and faeces (6–15% of the dose), within 24 h. After dermal application, approximately 45% of an applied dose was absorbed within 24 h. Fenitrothion is rapidly metabolized by mixed-function oxidases to the highly reactive fenitrooxon by oxidative desulfuration. The oxon is then further metabolized by demethylation and hydrolysis to 3-methyl-4-nitrophenol and dimethylphosphate. A minor metabolic pathway involves further oxidation to 3-carboxyl-4-nitrophenol. After low oral doses, the urinary metabolites consisted mainly of conjugated phenolic compounds, such as the sulfate and glucuronide of 3-methyl-4-nitrophenol, whereas at higher doses demethylated compounds such as desmethyl fenitrothion and desmethyl fenitrooxon were excreted in increasing amounts. The tissue concentrations of residues of 14C-fenitrothion were very low (generally < 1 ppm) within 48 h of dosing.
In volunteers, the time to maximal concentration in plasma after oral ingestion 12 h apart of two capsules containing fenitrothion at 0.09 or 0.18 mg/kg bw for 4 days, was 1 h, and the elimination half-time ranged from 2 to 3 h, irrespective of dose. The integrated area under the curve of concentration–time and the maximum concentration, however, increased with frequency of dosing. The maximal concentration in plasma 1 day after a single dose of 0.09 mg/kg bw was 0.54 ng/ml, whereas on day 4 it was 0.84 ng/mL. At the higher dose, the maximal concentration increased from 1.8 ng/ml on day 1 to 7.7 ng/ml on day 4. In a man who attempted to commit suicide by ingesting a fenitrothion formulation, the elimination half-time of fenitrothion was 4.5 h.
The lowest oral LD50 value was 240 mg/kg bw (range, 240–1700 mg/kg bw) in rats and 780 mg/kg bw (range, 780–1400 mg/kg bw) in mice. Male rats were generally more sensitive to the acute effects of fenitrothion than females, and the vehicle used had a marked effect on the observed toxicity. The signs of acute intoxication with fenitrothion were consistent with cholinesterase inhibition. The lowest acute dermal LD50 was 890 mg/kg bw (range, 890–5000 mg/kg bw) in rats. The lowest acute LC50 in rats after whole-body exposure to a fenitrothion aerosol was 2.2 mg/L. Technical-grade fenitrothion with a purity > 95% was not irritating to the eye or skin of rabbits and did not sensitize the skin of guinea-pigs (Buehler test). WHO (1999) has classified fenitrothion as moderately hazardous.
In short-term studies of toxicity lasting less than 12 months, the NOAEL for inhibition of erythrocyte acetylcholinesterase activity was 0.6 mg/kg bw per day in rats, < 3 mg/kg bw per day in rabbits, and 0.3 mg/kg bw per day in dogs. The NOAEL for inhibition of brain cholinesterase activity was 2.5 mg/kg bw per day in rats, 3 mg/kg bw per day in rabbits, and > 1.6 mg/kg bw per day in dogs. The signs of toxicity in rats and rabbits were generally limited to cholinergic signs and decreased body weights and/or food consumption. The NOAEL for these effects in short-term studies was 4.8 mg/kg bw per day in rats and > 10 mg/kg bw per day in rabbits. When fenitrothion was applied to the skin of rabbits for 21 days, the NOAEL for inhibition of cholinesterase activity in erythrocytes and brain was 3 mg/kg bw per day. No NOAEC was identified for inhibition of brain cholinesterase in rats exposed to an aerosol of fenitrothion for 90 days. The LOAEC was 0.2 µg/L per day.
In long-term studies of toxicity, inhibition of cholinesterase activity was again the main toxicological finding in all species. In mice, erythrocyte and brain cholinesterase activities were inhibited at 13 mg/kg bw per day, with a NOAEL was 1.5 mg/kg bw per day. Reductions in body-weight gain and food consumption were reported only at the highest dietary concentration of 1000 ppm (equal to 130 mg/kg bw per day). Other treatment-related findings in mice were an elevated cholesterol concentration, with a NOAEL of 10 ppm (equal to 1.5 mg/kg bw per day), and a reduced glucose concentration, with a NOAEL of 100 ppm (equal to 13 mg/kg bw per day). Although no clinical signs were seen at doses up to 6.5 mg/kg bw per day in rats, the NOAEL was 0.5 mg/kg bw per day for inhibition of erythrocyte and brain cholinesterase activities; the NOAEL for a reduction in body-weight gain was 1.9 mg/kg bw per day. Treatment did not increase the incidence of neoplastic lesions in long-term studies in mice and rats.
On the basis of testing in an adequate range of studies in vitro and in vivo, the Meeting concluded that fenitrothion is unlikely to be genotoxic. It also concluded that fenitrothion is unlikely to pose a carcinogenic risk to humans.
In multigeneration studies of reproductive toxicity in rats, the treatment-related effects of fenitrothion were cholinergic signs at high doses and reductions in food consumption and body-weight gain. These effects were consistent with those seen in short- and long-term studies of toxicity. Pups had reduced body weight, viability, and lactation indices. The NOAEL for reduced food consumption and body-weight gain in dams was 0.65 mg/kg bw per day. The NOAEL for toxicity in offspring was 3.1 mg/kg bw per day, the effects being seen at maternally toxic doses.
In studies of developmental toxicity in rats and rabbits, the maternal effects were cholinergic signs and reduced body-weight gain (NOAEL, 8 mg/kg bw per day in rats and 10 mg/kg bw per day in rabbits). No fetal toxicity was observed at the highest dose tested (NOAEL, 25 mg/kg bw per day in rats and 30 mg/kg bw per day in rabbits); there was no evidence of treatment-induced malformations in any of the studies.
In studies of delayed neurotoxicity, fenitrothion was given to chickens as a single acutely toxic dose. There was no evidence that it caused delayed neurotoxicity, and the incidence of histopathological lesions in the nerve tissues of birds treated once at 500 mg/kg bw was not increased. In rats given single doses of fenitrothion of up to 200 mg/kg bw by gavage or as repeated doses of up to 18 mg/kg bw per day in the diet for 13 weeks, there were no treatment-related neurological lesions or effects on cognition and no inhibition of neuropathy target esterase activity, although cholinergic signs and significant inhibition of erythrocyte and brain cholinesterase activity were seen at a number of doses. In these studies, which included a functional observational battery of tests, clinical signs of intoxication were observed. However, cholinergic signs were observed only when brain cholinesterase activity was inhibited by more than 58% or when erythrocyte acetylcholinesterase activity was inhibited by more than 38%.
Fenitrothion did not induce immunotoxicity in a series of immunological tests.
Although a published report on ocular effects indicated that a single oral dose of 14 mg/kg bw administered to male rats caused significant electroretinographic changes after 2 days, this could not be confirmed in rats given either a single dose of up to 400 mg/kg bw by gavage or repeated daily doses of 2.0 mg/kg bw in the diet for 13 weeks.
When fenitrothion was given to 24 volunteers as a single oral dose of 0.042–0.33 mg/kg bw, there were no cholinergic signs and erythrocyte acetylcholinesterase activity was not significantly inhibited. However, one person given 0.33 mg/kg bw showed a reduction of 28% in plasma cholinesterase activity. With repeated doses of 0.04–0.08 mg/kg bw per day for 4 days, the cholinesterase activities in erythrocytes and plasma were unchanged. In another study, fenitrothion given to two to four volunteers as a divided daily oral dose of 0.18 or 0.36 mg/kg bw per day for 4 days did not induce cholinergic signs or changes in cholinesterase activity in erythrocytes or plasma.
In a retrospective hospital-based study of 16 cases of poisoning with fenitrothion requiring extensive, aggressive antidotal therapy, 7 of 10 survivors had symptoms consistent with ‘intermediate syndrome’, namely delayed onset (24–96 h) of muscular weakness affecting the muscles of the neck, proximal limb, and respiratory system. No plasma cholinesterase activity was detectable at the time of admission of the patients, and the recovery time ranged from 5 to more than 10 weeks.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of fenitrothion to fetuses, infants, and children. Although fenitrothion is known to be neurotoxic to adults, the Meeting did not recommend that a study of developmental neurotoxicity be conducted, since there was no evidence of increased neurotoxicity in offspring exposed pre- or postnatally, when compared with adults in the same experiment.
The Meeting affirmed the ADI of 0–0.005 mg/kg bw that was established by the 1988 Joint Meeting, which was based on a NOAEL of 0.5 mg/kg bw per day for inhibition of brain and erythrocyte cholinesterase activity in a 2-year study of toxicity in rats and a safety factor of 100. This was supported by a NOAEL of 0.57 mg/kg bw per day for inhibition of brain and erythrocyte cholinesterase activity in a 3-month study of ocular toxicity in rats and a NOAEL of 0.65 mg/kg bw per day for reduced food consumption and body-weight gain in a study of reproductive toxicity in rats. The 4-day study in volunteers was not considered suitable for establishing an ADI because of its short duration and the associated absence of steady-state kinetics.
The Meeting allocated an acute RfD of 0.04 mg/kg bw to fenitrothion on the basis of a NOAEL of 0.36 mg/kg bw for inhibition of erythrocyte acetylcholinesterase activity in a study in volunteers and a safety factor of 10.
Levels relevant for risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
104-week study of toxicity and carcinogenicitya |
Toxicity |
10 ppm, equal to 1.4 mg/kg bw per day |
100 ppm, equal to 13 mg/kg bw per day |
|
|
|
Carcinogenicity |
1000 ppm, equal to 140 mg/kg bw per dayb |
– |
|
Rat |
104-week study of toxicity and carcinogenicitya |
Toxicity |
10 ppm, equal to 0.5 mg/kg bw per day |
30 ppm, equal to 1.5 mg/kg bw per day |
|
|
|
Carcinogenicity |
100 ppm, equal to 6.5 mg/kg bw per dayb |
– |
|
|
Two-generation study of reproductive toxicitya |
Parental toxicity |
10 ppm, equal to 0.65 mg/kg bw per day |
40 ppm, equal to 3.1 mg/kg bw per day |
|
|
|
Pup toxicity |
40 ppm, equal to 3.1 mg/kg bw per day |
120 ppm, equal to 9.6 mg/kg bw per day |
|
|
Developmental toxicityc |
Maternal toxicity |
8 mg/kg bw per day |
25 mg/kg bw per day |
|
|
|
Embryo- and fetotoxicity |
25 mg/kg bw per dayb |
– |
|
|
Acute neurotoxicityc |
|
12.5 mg/kg bw |
50 mg/kg bw |
|
Rabbit |
Developmental toxicityc |
Maternal toxicity |
10 mg/kg bw per day |
30 mg/kg bw per day |
|
|
|
Embryo- and fetotoxicity |
30 mg/kg bw per dayb |
– |
|
Dog |
1-year study of toxicitya |
Toxicity |
50 ppm equal to 1.6 mg/kg bw per dayb |
– |
|
Human |
4-day study of toxicityd |
Toxicity |
0.36 mg/kg bw per dayb |
– |
a
Dietary administrationb
Highest dose testedc
Gavaged
CapsuleEstimate of acceptable daily intake for humans
0–0.005 mg/kg bw
Estimate of acute reference dose
0.04 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
• Further observations in humans
Summary of critical end-points
|
Absorption, distribution, excretion, and metabolism in mammals |
|
|
Rate and extent of oral absorption |
About 90–100% in rats within 72 h |
|
|
About 70% in humans in 96 h |
|
Dermal absorption |
About 45% after 24 h in rats |
|
Distribution |
Initially widely distributed; highest concentrations of residues in liver, kidneys, and fat at 48 h in rats |
|
Potential for accumulation |
Elimination half-time, 2–4.5 h in humans. No evidence of potential for accumulation in rats |
|
Rate and extent of excretion |
> 95% within 72 h in rats, mainly in urine (68–93%) and less in faeces (6–15%) |
|
Metabolism in animals |
Rapidly activated and deactivated |
|
Toxicologically significant compounds (animals, plants, and environment) |
Parent compound, oxon derivative, and 3-methyl-4-nitrophenol |
|
Acute toxicity |
|
|
Rat, LD 50, oral |
240 mg/kg bw (range, 240–1700 mg/kg bw) |
|
Rat, LD 50, dermal |
890 mg/kg bw (range, 890–5000 mg/kg bw) |
|
Rat, LC 50, inhalation |
2.2 mg/L (4 h; aerosol, 0.59–0.82-mm particles; whole-body exposure) |
|
Dermal irritation |
Not irritating in rabbits |
|
Ocular irritation |
Not irritating in rabbits |
|
Dermal sensitization |
Not a sensitizer in guinea-pigs |
|
Short-term toxicity |
|
|
Target/critical effect |
Inhibition of brain cholinesterase activity |
|
Lowest critical oral NOAEL |
1.3 mg/kg bw per day, rat, 13 weeks |
|
Lowest relevant dermal NOAEL |
3 mg/kg bw per day, rabbits; 21 days |
|
Lowest relevant inhalation NOAEC |
Not established; LOAEC = 0.2 µg/L per day, rat; 13 weeks |
|
Genotoxicity |
Not genotoxic |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Inhibition of brain cholinesterase activity |
|
Lowest relevant NOAEL |
0.5 mg/kg bw per day, rat, 2 years |
|
Carcinogenicity |
Not carcinogenic in rats or mice |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect |
No reproductive toxicity at the highest dose tested in rats |
|
Relevant reproductive NOAEL |
3.1 mg/kg bw per day; two-generation study in rats |
|
Developmental target/critical effect |
No fetal developmental toxicity at maternally toxic doses |
|
Lowest relevant developmental NOAEL |
25 mg/kg bw per day; rats |
|
Neurotoxicity/Delayed neurotoxicity |
Reversible neurotoxicity consistent with cholinesterase inhibition No evidence of delayed neurotoxicity or of histopathological changes in nerves of hens (500 mg/kg bw) or rats (200 mg/kg bw or 17.6 mg/kg bw per day for 13 weeks) |
|
Other toxicological studies |
No immunotoxicity or ocular toxicity |
|
Medical data |
No inhibition of erythrocyte acetylcholinesterase activity in volunteers after either a single oral dose of up to 0.33 mg/kg bw or repeated oral doses of up to 0.36 mg/kg bw per day for 4 days Poisoning cases presented with severe cholinergic effects followed by evidence of ‘intermediate syndrome’ |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.005 mg/kg bw |
Rat, 2-year, dietary |
100 |
|
Acute RfD |
0.04 mg/kg bw |
Human, repeated doses |
10 |
Benes, V. & Cerna, V. (1970) Contribution to the toxicological evaluation of fenitrothion and its residues. In: Deichmann, W.B., Radomski, J.L. & Penalver, R.A., eds, Proceedings of Pesticide Symposia. Inter-American Conferences on Toxicology and Occupational Medicine, Miami, FL, Halas & Assoc., pp. 219–222.
Benes, V. & Tejnorova, I. (1986) Fenitrothion: Teratogenicity study in rats. Unpublished report submitted to WHO by the Institute of Hygiene and Epidemiology, Prague, Czechoslovakia.
Benes, V., Srám, R.J. & Tuscany, R. (1974a) Fenitrothion. I. Study of mutagenic activity in rats. Unpublished report submitted to WHO by the Institute of Hygiene and Epidemiology, Prague, Czech Republic.
Benes, V., Nejedly, K. & Srám, R.J. (1974b) Fenitrothion. II. Reproduction study in rats. Unpublished report submitted to WHO by the Institute of Hygiene and Epidemiology, Prague, Czech Republic.
Benes, V., Srám, R.J. & Tuscany, R. (1975) Fenitrothion. I. Study of mutagenic activity in rats. J. Hyg. Epidemiol. Microbiol. Immunol., 2, 163–172.
Beyrouty, P., Benjamin, W., Robinson, K. & Noveroske, J. (1993a) An acute study of the potential effects of orally administered fenitrothion on behaviour and neuromorphology in rats. Project no. 97144. Unpublished report no. HT-21-0512 from Bio-Research Laboratories Ltd, Quebec, Canada. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Beyrouty, P., Benjamin, W., Robinson, K. & Noveroske, J. (1993b) A 3-month dietary study of the potential effects of fenitrothion on behaviour, neurochemistry, neuromorphology in rats. Project no. 97145. Unpublished report no. HT-31-0520 from Bio-Research Laboratories Ltd, Quebec, Canada. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Carmargo, W.A., Saad, W.A. & Larimi, L. (1970) Acute toxicity of the fenitrothion. Arg. Inst. Biol. (S. Paulo), 37, 219–222.
Chevalier, G., Bastie-Sigeac, I. & Cote, M.G. (1982) Morphological assessment of fenitrothion pulmonary toxicity in the rat. Toxicol. Appl. Pharmacol., 63, 91–104.
Clos, M.V., Ramoneda, M. & Garcia, G. (1994) Modification of testicular cytochrome P-450 after fenitrothion administration. Gen. Pharmacol., 25, 499–503.
Coombs, D.W., Kenny, T.J., Hardy, C.J., Clark, G.C., Chanter, D.O. & Gopinath, C. (1988) Sumithion TG. 90-day inhalation toxicity study in the rat. Unpublished report no. HT-81-0423 (SMO 300/881214) from Huntingdon Research Centre Ltd, Cambridgeshire, United Kingdom. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Douch, P.G.C., Hook, C.E.R. & Smith, J.N. (1968) Metabolism of Folithion (dimethyl 4-nitro-3-methylphenyl phosphor-thionate). Aust. J. Pharm., 29 (Suppl. 6), S70–S71.
Dubois, K.P. & Kinoshita, F.K. (1970) Acute oral toxicity of Accothion, Bay 41831 and related materials to rats. Unpublished report from the Toxicity Laboratory, University of Chicago, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Dubois, K.P. & Puchala, E. (1960) The acute toxicity and anticholinesterase action of Bayer 41831. Department of Pharmacology, University of Chicago, USA. Unpublished report submitted to WHO by Farbenfabriken Bayer AG.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl. Pharmacol., 14, 515–534.
Gradowska-Olszewska, I., Brzezinski, J. & Rusiecki, W. (1984) Excretion and peripheral metabolism of 1,2-3H-testosterone and androgens in rats following intoxication with organophosphorus insecticides. J. Appl. Toxicol., 4, 261–264.
Griggs, L.M.P., Jefferson, N.D., Blair, M., Kopplin, J.R., Richter, W.R. & Spicer, E.J.F. (1984) One year dietary toxicity study in dogs. Unpublished study No HT-41-0272. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Groszek, B., Pach, J. & Klys, M. (1995) Intermediate syndrome in acute fenitrothion poisoning. Przegl. Lek., 52, 271–274.
Hara, S. & Suzuki, T. (1981) Primary eye and skin irritation tests of Sumithion technical in rabbits. Unpublished report no. HT-10-0201 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S. & Suzuki, H. (1982a) In vivo chromosomal aberration test of Sumithion on bone marrow cells of mice. Unpublished report no. HT-20-0235 from the Biochemical Toxicology Laboratory, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S. & Suzuki, H. (1982b) The micronucleus test of Sumithion. Unpublished report no. HT-20-0234 from the Biochemical Toxicology Laboratory, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, H. & Miyamoto, J. (1983) In vivo chromosomal aberration test of Sumithion on bone marrow cells of rats. Unpublished report no. HT-30-0258 from the Biochemical Toxicology Laboratory, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Heimann, K.G. (1982) S5660 Test for acute oral toxicity on mice. Unpublished report from the Institute of Toxicology, Germany. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hixson, E.J. (1982a) Acute oral toxicity of fenitrothion concentrate use dilution. Corporate Unpublished report from the Toxicology Department, Mobay Chemical Corporation, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hixson, E.J. (1982b) Acute dermal toxicity of fenitrothion concentrate use dilution in rabbits. Unpublished report from the Toxicology Department, Mobay Chemical Corporation, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hixson, E.J. (1982c) Eye and dermal irritation of fenitrothion concentrate use dilution. Unpublished report from the Toxicology Department, Mobay Chemical Corporation, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hladka, A., Krampi, V. & Kovac, J. (1974) Effect of malathion on the content of fenitrothion and fenitrooxon in the rat. Bull. Environ. Contam. Toxicol., 12, 38–45.
Hoberman, A.M. (1990) Reproductive effects of Sumithion administered orally in feed to Crl:CD(SD)BR rats for two generations. Unpublished report no. 1119-008 from Argus Research Laboratories, Inc., Pennsylvania USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hollingworth, R.M., Metcalf, R.L. & Fukuto, T.R. (1967) The selectivity of Sumithion compared with methyl parathion. Metabolism in the white mouse. J. Agric. Food Chem., 15, 242–249.
Iba, K., Yoshitake, A., Kaneko, H., Matsunaga, H. & Yamada, H. (1990) Metabolism of fenitrothion in rats. Unpublished report no. HM-00-0112 from the Biochemistry and Toxicology Laboratory, Sumitomo Chemical Co Ltd, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T. & Miyamoto, J. (1975) Acute and sub-acute toxicity of Sumithion in Japanese quails. Botyu Kagaku, 40, 54–58.
Kadota, T. & Miyamoto, J. (1979) Acute oral, dermal and IP toxicities of p-nitro-m-cresol in rats and mice. Unpublished report from the Research Department, Pesticide Division, Sumitomo Chemical Co., Ltd, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kagoshima, M., Yamazaki, H. & Miyamoto, J. (1972a) Acute oral, subcutaneous and dermal toxicities of Sumithion technical in mice and rats. Unpublished report no. HT-20-0187 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kohda, H. & Miyamoto, J. (1972b) Six month feeding study of Sumithion, Sumioxon and p-nitrocresol in rats. Unpublished report no. HT-0538 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kagoshima, M. & Miyamoto, J. (1974a) Acute oral toxicity and delayed neurotoxicity of Sumithion in hens. Unpublished report from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kohda, H. & Miyamoto, J. (1974b) Ninety-two week feeding study of Sumithion in rats with special reference to cholinesterase activity. Unpublished report from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kagoshima, M. & Miyamoto, J. (1975a) Acute oral toxicity and delayed neurotoxicity of 5 organophosphorus compounds, Salithion, Cyanox, Surecide, Sumithion and Sumioxon in adult hens. Botyu-Kagaku, 40, 49–53.
Kadota, T., Kohda, H. & Miyamoto, J. (1975b) Subchronic toxicity studies of Sumithion, Sumioxon and p-nitrocresol in rats and 92 week feeding study of Sumithion with special reference to change of cholinesterase activity. Botyu-Kagaku, 40, 38–47.
Kadota, T., Kagoshima, M. & Miyamoto, J. (1976) (Revised report) Acute oral toxicity and delayed neurotoxicity of Sumithion in hens. Unpublished report no. HT-60 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kohda, H. & Miyamoto, J. (1977) Individual data on 92-week feeding study of Sumithion in rats with special reference to change of cholinesterase activity. Unpublished report no. HT-70-1001 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kadota, T., Kohda, H. & Miyamoto, J. (1980) Ninety-two week feeding study of Sumithion in rats with special reference to cholinesterase activity. Unpublished report no. HT-50-0001 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kato, T., Suzuki, T., Misaki, Y., Okuno, Y. & Miyamoto, J. (1986) Acute oral toxicity of Sumithion in rats. Unpublished report no. HT-60-0353 from the Takarazuka Research Center, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yoshitake, A., Hara, M., Kato, H., Kawamoto, M. & Yamada, H. (1987) In vitro gene mutation test of Sumithion in V79 Chinese hamster cells in culture. Unpublished report no. HT-70-0387 from the Biochemistry and Toxicology Laboratory, Takarazuka Research Center, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yoshitake, A., Hara, M., Yamamoto, K. & Yamada, H. (1988) In vitro chromosomal aberration test of Sumithion in Chinese hamster ovary cells (CHO-K1) in culture. Unpublished report no. HT-80-0420 from the Biochemistry and Toxicology Laboratory, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yoshitake, A., Hara, M., Ota, M. & Yamada, H. (1990) In vivo/in vitro unscheduled DNA synthesis (UDS) test of Sumithion in rat hepatocytes. Unpublished report no. HT-00-0444 (study no.1487) from the Biochemistry and Toxicology Laboratory, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kohda, H. & Kadota, T. (1975) Dominant lethal test of Sumithion in rats and mice. Unpublished report no. HT-60-0210 from the Biochemical Toxicology Laboratory, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kohda, H. & Kadota, T. (1979) Acute inhalational toxicity study of Sumithion in rats. Unpublished report from the Institute for Biological Science, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kohda, H., Kagoshima, M., Kadota, T. & Miyamoto, J. (1972) Skin sensitisation test of Sumithion technical in guinea pigs. Unpublished report no. HT-00-0181 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kohda, H., Kawagushi, S., Kamita, Y., Yamada, T., Suzuki, T., Kato, T. & Miyamoto, J. (1986) Acute inhalational toxicity of Sumithion in rats. Unpublished report no. HT-60-0352 from the Takarazuka Research Center, Sumitomo Chemical Co., Ltd, Osaka, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kohli, J.D., Hasan, M.Z. & Gupta, B.N. (1974) Dermal absorption of fenitrothion in rats. Bull. Environ. Contam. Toxicol., 11, 285–290.
Kojima, T., Yashiki, M., Miyazaki, F., Chikasue, F. & Ohtani, M. (1989) Detection of S-methylfenitrothion, aminofenitrothion and acetylaminofenitrothion in the urine of a fenitrothion intoxication case. Forensic Sci. Int., 41, 245–253.
Kosaka, T., Murakoshi, H. & Maita, K. (1989) Acute ocular toxicity study in rats. Unpublished report no. HT-91-0429 from the Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kovacicová, J., Batora, V. & Truchlik, S. (1973) Hydrolysis rate and in vitro anticholinesterase activity of fenitrothion and S-methyl fenitrothion. Pestic. Sci., 4, 759–763.
Kundzins, W. (1974) Two-year dietary administration in the rat. Unpublished report no. HT-41-0006 from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kundzins, W. (1975) 78-Week tumorigenic study in the ICR Swiss mouse using Sumithion. Project no. 343-114. Unpublished report no. HT-51-0002 from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kundzins, W. (1979) Additional histopathology and tabular data for the 78 week mouse study with Sumithion. Unpublished report from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kundzins, W. (1980a) 78-week tumorigenic study in the ICR Swiss mouse. Pathology summary groups 1 & 4. Project no. 343-114. Unpublished report from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kundzins, W. (1980b) 104-week chronic administration in rats, individual animal data Vol I & II. Unpublished report no. HT-01-0193 from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kunimatsu, T., Kamita, Y., Isobe, N. & Kawasaki, H. (1996) Immunotoxicological insignificance of fenitrothion in mice and rats. Fundam. Appl. Toxicol., 33, 246–253.
Lehotzky, K. & Ungváry, G. (1976) Experimental data on neurotoxicity of fenitrothion. Acta Pharmacol. Toxicol., 39, 374–382.
Meaklim, J.F. & McNeil, J.J. (1999) Fenitrothion ingestion in humans: Subacute effects. Unpublished report no. HT-0539 from the Department of Epidemiology and Preventative Medicine, Monash Medical School, Alfred Hospital, Victoria, Australia. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Mihara, K., Okuno, Y., Misaki, Y. & Miyamoto, J. (1978) Metabolism of fenitrothion in goats. J. Pestic. Sci., 3, 233–242.
Mikami, N., Kohda, H. & Suzuki, H. (1977) Toxicity study of S-methylfenitrothion. Unpublished report from the Institute for Biological Sciences, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Misu, Y., Segawa, T., Kuruma, T., Kojima, M. & Takagi, H. (1966) Subacute toxicity of O,O-dimethyl-O-(3-methyl-4-nitrophenyl) phosphorothioate (Sumithion) in the rat. Toxicol. Appl. Pharmacol., 9, 17–26.
Mitsumori, K., , K.. Tsuda, S., Harada, T. & Shirasu, Y. (1989) Sumithion: 13-week subchronic ocular toxicity study in rats. Unpublished report no. HT-91-0430 from the Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Miyamoto, J. (1964) Studies on the mode of action of organophosphorus compounds. Part IV. Penetration of Sumithion, methyl parathion and their oxygen analogs into guinea pig brain and inhibition of cholinesterase in vivo. Agric. Biol. Chem., 28, 422–430.
Miyamoto, J. (1969) Mechanism of low toxicity of Sumithion towards mammals. Residue Rev., 25, 251–264.
Miyamoto, J. & Sato, Y. (1969) Determination of insecticide residue in animal and plant tissues. VI. Determination of Sumithion residue in cattle tissues. Botyu Kagaku, 34, 3–6.
Miyamoto, J., Sato, Y., Kadota, T., Fujinami, A. & Endo, M. (1963a) Studies on the mode of action of organophosphorus compounds. Part I. Metabolic fate of P32 labelled Sumithion and methyl parathion in the guinea-pig and white rat. Agric. Biol. Chem., 27, 381–389.
Miyamoto, J., Sato, Y., Kadota, T. & Fujinami, A. (1963b) Studies on the mode of action of organophosphorus compounds. Part II. Inhibition of mammalian cholinesterase in vivo following administration of Sumithion and methylparathion. Agric. Biol. Chem., 27, 669–676.
Miyamoto, J., Kohda, H. & Kadota, T. (1975) Teratogenic studies with Sumithion in ICR-JCL mice and Sprague Dawley rats. Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Miyamoto, J., Hosokawa, S., Kadota, T., Kohda, H., Arai, M., Sugihara, S. & Hirao, K. (1976) Studies on cholinesterase inhibition and structural changes at neuromuscular junction in rabbits by subacute administration of Sumithion. J. Pestic. Sci., 1, 171–178.
Morseth, S.L. (1987) Teratology study in rats with Sumithion. Unpublished report no. HT-71-0382 from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Morseth, S.L., Serabian, M.A., Lichtenberger, J.M., Vargas, K.J., Thakur, A.K. & Burlew, P.L. (1986) Teratology study in rabbits with fenitrothion T.G. (Sumithion). Unpublished report no. HT-61-0367 from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Motabar, M., Sanai, G.H. & Heidari, A.A. (1972) Toxicological evaluation of Sumithion (OMS-43) on operators and inhabitants in the Mamasani area, southern Iran. Iran J. Pharm. Health, 2, 40–49.
Myatt, G.L. & Ecobichon, D.J. (1975) Fenitrooxon and S-methyl fenitrothion:acute toxicity and hydrolysis in mammals. Environ. Res., 10, 407–414.
Nosál, M. & Hladká, A. (1968) Determination of the exposure to fenitrothion [O,O-dimethyl O-(3-methyl-4-nitrophenyl) thiophosphate] on the basis of the excretion of p-nitro-m-cresol by the urne of the persons tested. Int. Arch. Gewerbepathol. Gewerbehyg., 25, 28–38.
Olson, W.A. (1972) Interim report. Three generation reproduction study—Rats: Sumithion and Neo-pynamin. Unpublished report no. 343-106 from Hazleton Laboratories, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Patterson, D.R. (1981) 104-week chronic administration in rats, project no 343-107, individual histopathology findings. Unpublished report no. HT-11-0194 from Hazleton Laboratories America, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Ralph, J.A. & Pence, D.H. (1980) Revised report—Rutter HA Jr (1973) Three generation reproduction study—Rats: Sumithion and Neo-pynamin. Unpublished report no. 343-106 from Hazleton Laboratories, Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Rosival, L., Vargová, M., Hladká, H., Bátora, V., Kovacicová, J., Szokolayová, J. & Cerey, K. (1974) On the toxic effect of the fenitrothion S-methyl isomer. Unpublished report from the Research Institute of Hygiene, Research Institute of Industrial Hygiene and Professional Diseases, and Research Institute of Agrochemical Technology, Bratislava, Slovakia, submitted to WHO by the authors.
Rosival, L., Vargová, M., Szokolayová, J. & Cerey, K. (1976) Contribution to the toxic action of S-methyl-fenitrothion. Pestic. Biochem. Physiol., 6, 280–286.
Rutter, H.A., Jr (1973) Three generation reproduction study—Rats: Sumithion and Neo-pynamin. Unpublished report no. 343-106 from Hazleton Laboratories Inc., Virginia, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Shelanski, M.V., Levenson, T. & Karras, C. (1977) Product Investigation, Inc. Conshohocken PA, USA. Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Spicer, E.J.F. (1986) One year dietary toxicity study in dogs. Sumithion technical (revised). Unpublished report no. HT-61-0374 from International Research and Development Corporation, Michigan, USA. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suetake, K., Kakino, K., Kamimura, H. & Ichiki, T. (1991) 21-day dermal toxicity study in rabbits with Sumithion TG. Unpublished report no. HT-11-0488 from Safety Assessment Laboratory, Panapharm Laboratories, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, T. (1982) Acute oral toxicity of Bis-Sumithion, O-methyl O,O-di(3-methyl-4 nitrophenyl) phosporothionate in mice. Unpublished report from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1975) Studies on mutagenicity of Sumithion with bacterial systems. Unpublished report no. HT-50-0142 from Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1980) Effects of fenitrothion on sister chromatid exchanges (SCE) in cultured mouse embryo cells. Unpublished report no. HT-00-0208 from Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1983) Mutagenicity tests of Sumithion with nitro reductase-defective bacteria. Unpublished report no. HT-30-0259 from Laboratory of Biochemistry and Toxicology, Takarazuka Research Center, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, H., Miyamoto, J. & Oka, A. (1974) Mutagenicity and radiomimeticity screening of Sumithion. Unpublished report no. HT-40-0081 from the Research Department, Pesticides Division, Sumitomo Chemical Co., Ltd, Hyogo, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Tamano, S., Shibata, M.-A., Hagiwara, A. & Suzuki, M. (1990) Chronic dietary toxicity and carcinogenicity test of Sumithion technical in mice. Unpublished report no. HT-01-0445 from Daiyu-Kai Institute of Medical Science, Aichi, Japan. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Thyssen, J. & Lorke, D. (1982) S 5660 (fenitrothion, Folithion active ingredient). Test for irritant effect on skin or mucous membrane. Unpublished report from the Institute of Toxicology, Bayer AG, Germany. Submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Toxicology Research Unit (1964) OMS 43 summary. Mammalian toxicity. Unpublished report. Carshalton, Surrey, United Kingdom.
Trottier, B., Fraser, A.R., Planet, G. & Ecobichon, D.J. (1980) Subacute toxicity of technical fenitrothion in male rats. Toxicology, 17, 29–38.
Usutani, S., Nishiyama, K., Sato, I., Matsuura, K. & Sawada, Y. (1978) Studies on the amount of exposure to pesticides and blood levels of organophosphorus pesticides of farmers engaging in joint control works over apple orchards. J. Jpn. Assoc. Rural Med., 27, 79–88.
Vandekar, M. (1965) Observations on the toxicity of carbaryl, folithion and 3-isopropylphenyl N-methylcarbamate in a village-scale trial in southern Nigeria. Bull. World Health Organ., 33, 107–115.
Vardanis, A. & Crawford, L.G. (1964) Comparative metabolism of O,O-dimethyl-nitrophenol phosphorothioate (methyl parathion) and O,O-dimethyl O-(3-methyl-4-nitrophenol) phosphorothioate (Sumithion). J. Econ. Entomol., 57, 136–139.
Wadia, R.S., Bhirud, R.H., Gulvani, A.V. & Amin, R.B. (1977) Neurological manifestations of three organophosphorus poisons. Indian J. Med. Res., 66, 460–468.
WHO (1999) Recommended classification of pesticides by hazard and guidelines to classification 1998–1999 (WHO/PCS/98.21/Rev.1), Geneva, International Programme on Chemical Safety (www.who.int/pcs/pcs-act.htm).
Yamamoto, T., Egashira, T., Yoshida, T. & Kuroiwa, Y. (1982) Increase of adrenal weight in rats by the repeated administration of fenitrothion. Toxicol. Lett., 11, 187–191.
Yoshida, M., Shimada, E., Yamanaka, S., Aoyama, H., Yamamura, Y. & Owada, S. (1987) A case of acute poisoning with fenitrothion (Sumithion). Hum. Toxicol., 6, 403–406.
Yoshikawa, H., Yoshida, M. & Hara, I. (1990) Electroretinographic changes induced by organophosphorus pesticides in rats. J. Toxicol. Sci., 15, 87–95.
See Also:
Toxicological Abbreviations
Fenitrothion (EHC 133, 1992)
Fenitrothion (HSG 65, 1991)
Fenitrothion (ICSC)
Fenitrothion (FAO/PL:1969/M/17/1)
Fenitrothion (WHO Pesticide Residues Series 4)
Fenitrothion (Pesticide residues in food: 1976 evaluations)
Fenitrothion (Pesticide residues in food: 1977 evaluations)
Fenitrothion (Pesticide residues in food: 1979 evaluations)
Fenitrothion (Pesticide residues in food: 1982 evaluations)
Fenitrothion (Pesticide residues in food: 1983 evaluations)
Fenitrothion (Pesticide residues in food: 1984 evaluations)
Fenitrothion (Pesticide residues in food: 1986 evaluations Part II Toxicology)
Fenitrothion (Pesticide residues in food: 1988 evaluations Part II Toxicology)