
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
VANILLIN
Chemical name 4-Hydroxy-3-methoxybenzaldehyde
Empirical formula C8H8O3
Structural formula
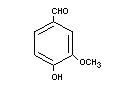 Molecular weight 152.15
Definition Vanillin contains not less than 97 per
cent. and not more than the equivalent
of 103 per cent. C8H8O3.
Description Fine, white to slightly yellow crystals,
usually needle-like, having an odour and
taste suggestive of vanilla. It is
affected by light.
Biological Data
Biochemical aspects
In rabbits, vanillin is metabolized by the following pathway:
vanillin undergoes rapid conjugation and slow oxidation to conjugated
vanillic acid, 5 per cent. of which is demethylated to conjugated
protocatechuic acid, which is then decarboxylated to conjugated
catechol. Further complete oxidation, opening the aromatic ring
between C3 and C4, then ensues (Sammons & Williams, 1941). Early
observers noted conversion to vanillic acid which was mainly excreted
as free acid or conjugated ethereal sulfate or glucurovanillic acid
(Preusse, 1880). Later work confirmed that if the rabbit 69 per cent.
of orally given vanillin is oxidized to 44 per cent. free vanillic
acid and 25 per cent. conjugated vanillic acid (10 per cent. as
ethereal sulfate and 15, per cent as p-glucuronide), all excreted in
the urine. Fourteen per cent. of orally administered vanillin is
excreted in conjugated form (8 per cent. as ethereal sulfate, 31 per
cent. as glucuronide), but none as free vanillin. Conjugated vanillic
acid and conjugated protocatechuic acid are in equilibrium with the
corresponding free acids which also appear in the urine (Sammons &
Williams, 1941).
In man and rat, vanillin is broken down by the liver to vanillic
acid which is excreted in the urine. Rat and human liver homogenates
readily convert vanillin to vanillic acid in vitro (Dirscherl &
Brisse, 1966). Quantitative conversion of dietary vanillin to urinary
vanillic acid in man has been confirmed. Endogenous vanillic acid
production and excretion from body catechol amines amounts to <0.5 mg
per day, compared with the normal contribution from dietary sources of
about 9 mg/day (Dirscherl & Wirtzfeld, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 780 Caujolle & Meynier, 1954
475 Frazer, 1967
Rat oral 1580 Jenner et al., 1964
approx. 2800 Hodge & Downs, 1961
s.c. 1800(LD) Deichmann & Kitzmiller,
1940
i.p. 1160 Caujolle et al., 1956
Guinea-pig oral 1400 Jenner et al., 1964
i.p. 1190 Caujolle et al., 1953
Dog i.v. 1320(LD) Caujolle et al., 1953
Rabbit oral 3000(LD) Deichmann & Kitzmiller,
1940
Rat s.c. 1500 Binet, 1896
Short-term studies
Rat. Intragastric administration to rats of 300 mg/kg
body-weight biweekly for 14 weeks had no deleterious effect. Groups of
16 rats were fed diets containing vanillin at the rate of 20 mg/kg
body-weight/day for 18 weeks without any adverse effects while 64
mg/kg body-weight/day for 10 weeks produced growth depression and
damage to myocardium, liver, kidney, lung, spleen and stomach. In
another experiment, 10 male and 10 female rats were fed diets
containing 0.3, 1.0 and 5.0 per cent. vanillin for 13 weeks. At the
highest level there was growth depression and enlargement of liver,
kidney and spleen; mild charges occurred at 1.0 per cent. and none at
0.3 per cent. (Deichmann & Kitzmiller, 1940). When groups of 5 males
and 5 females were fed 0, 0.1 and 1.0 per cent. vanillin in their diet
for 16-28 weeks, there were no adverse effects detectable. Groups of 5
males were given 0, 2.0 and 5.0 per cent. vanillin in their diet for 1
year without deleterious effects (Hagan et al., 1967).
Long-term studies
Rat. Groups of 12 males and 12 females were fed diets
containing 0, 0.5, 1.0 and 2.0 per cent. vanillin for 2 years without
any adverse effects (Hagan et al., 1967).
Comments
The metabolic fate of this flavouring is well established in man
and animals. The short-term studies give conflicting results, but the
long-term study is used for the evaluation.
EVALUATION
Level causing no toxicological effect
Rat. 2 per cent. (= 20 000 ppm) in the diet, equivalent to 1000
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-10
REFERENCES
Binet, P. (1896) Rev. méd. Suisse rom.,16, 449
Caujolle, F. & Meynier, D. (1954) C. R. Hebd. Séan. Acad. Sci.,
238, 2576
Caujolle, F. Meynier, D. & Forthouat, J. M. (1956) C.R. Hebd. Séan.
Acad. Sci., 243, 609
Caujolle, F., Meynier, D. & Moscarella, C. (1953) C.R. Hebd. Séan.
Acad. Sci., 236, 2549
Deichmann, W. & Kitzmiller, K. V. (1940) J. amer. Pharm ass. (Sci.
ed.), 29, 425
Dirscherl, W. & Brisse, B. (1966) Hoppe-Seyler's Z. physiol. Chem.,
346, 155
Dirscherl, W. & Wirtzfeldt, A. (1964) Hoppe-Seyler's Z. physiol.
Chem., 336 8M1
Frazer, A. C. (1967) Letter to WHO
Hagan, E. C. Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I.Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Hodge, H. C. & Downs, W. L. (1961) Toxic. appl. Pharm., 3, 689
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Preusse, C. (1880) Hoppe-Seyler's Z. physiol. Chem., 4, 213
Sammons, H. G. & Williams, R. T. (1941) Biochem. J., 35, 1175
Shillinger, J. I. (1950) Gig. i San., 3, 37
Molecular weight 152.15
Definition Vanillin contains not less than 97 per
cent. and not more than the equivalent
of 103 per cent. C8H8O3.
Description Fine, white to slightly yellow crystals,
usually needle-like, having an odour and
taste suggestive of vanilla. It is
affected by light.
Biological Data
Biochemical aspects
In rabbits, vanillin is metabolized by the following pathway:
vanillin undergoes rapid conjugation and slow oxidation to conjugated
vanillic acid, 5 per cent. of which is demethylated to conjugated
protocatechuic acid, which is then decarboxylated to conjugated
catechol. Further complete oxidation, opening the aromatic ring
between C3 and C4, then ensues (Sammons & Williams, 1941). Early
observers noted conversion to vanillic acid which was mainly excreted
as free acid or conjugated ethereal sulfate or glucurovanillic acid
(Preusse, 1880). Later work confirmed that if the rabbit 69 per cent.
of orally given vanillin is oxidized to 44 per cent. free vanillic
acid and 25 per cent. conjugated vanillic acid (10 per cent. as
ethereal sulfate and 15, per cent as p-glucuronide), all excreted in
the urine. Fourteen per cent. of orally administered vanillin is
excreted in conjugated form (8 per cent. as ethereal sulfate, 31 per
cent. as glucuronide), but none as free vanillin. Conjugated vanillic
acid and conjugated protocatechuic acid are in equilibrium with the
corresponding free acids which also appear in the urine (Sammons &
Williams, 1941).
In man and rat, vanillin is broken down by the liver to vanillic
acid which is excreted in the urine. Rat and human liver homogenates
readily convert vanillin to vanillic acid in vitro (Dirscherl &
Brisse, 1966). Quantitative conversion of dietary vanillin to urinary
vanillic acid in man has been confirmed. Endogenous vanillic acid
production and excretion from body catechol amines amounts to <0.5 mg
per day, compared with the normal contribution from dietary sources of
about 9 mg/day (Dirscherl & Wirtzfeld, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 780 Caujolle & Meynier, 1954
475 Frazer, 1967
Rat oral 1580 Jenner et al., 1964
approx. 2800 Hodge & Downs, 1961
s.c. 1800(LD) Deichmann & Kitzmiller,
1940
i.p. 1160 Caujolle et al., 1956
Guinea-pig oral 1400 Jenner et al., 1964
i.p. 1190 Caujolle et al., 1953
Dog i.v. 1320(LD) Caujolle et al., 1953
Rabbit oral 3000(LD) Deichmann & Kitzmiller,
1940
Rat s.c. 1500 Binet, 1896
Short-term studies
Rat. Intragastric administration to rats of 300 mg/kg
body-weight biweekly for 14 weeks had no deleterious effect. Groups of
16 rats were fed diets containing vanillin at the rate of 20 mg/kg
body-weight/day for 18 weeks without any adverse effects while 64
mg/kg body-weight/day for 10 weeks produced growth depression and
damage to myocardium, liver, kidney, lung, spleen and stomach. In
another experiment, 10 male and 10 female rats were fed diets
containing 0.3, 1.0 and 5.0 per cent. vanillin for 13 weeks. At the
highest level there was growth depression and enlargement of liver,
kidney and spleen; mild charges occurred at 1.0 per cent. and none at
0.3 per cent. (Deichmann & Kitzmiller, 1940). When groups of 5 males
and 5 females were fed 0, 0.1 and 1.0 per cent. vanillin in their diet
for 16-28 weeks, there were no adverse effects detectable. Groups of 5
males were given 0, 2.0 and 5.0 per cent. vanillin in their diet for 1
year without deleterious effects (Hagan et al., 1967).
Long-term studies
Rat. Groups of 12 males and 12 females were fed diets
containing 0, 0.5, 1.0 and 2.0 per cent. vanillin for 2 years without
any adverse effects (Hagan et al., 1967).
Comments
The metabolic fate of this flavouring is well established in man
and animals. The short-term studies give conflicting results, but the
long-term study is used for the evaluation.
EVALUATION
Level causing no toxicological effect
Rat. 2 per cent. (= 20 000 ppm) in the diet, equivalent to 1000
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-10
REFERENCES
Binet, P. (1896) Rev. méd. Suisse rom.,16, 449
Caujolle, F. & Meynier, D. (1954) C. R. Hebd. Séan. Acad. Sci.,
238, 2576
Caujolle, F. Meynier, D. & Forthouat, J. M. (1956) C.R. Hebd. Séan.
Acad. Sci., 243, 609
Caujolle, F., Meynier, D. & Moscarella, C. (1953) C.R. Hebd. Séan.
Acad. Sci., 236, 2549
Deichmann, W. & Kitzmiller, K. V. (1940) J. amer. Pharm ass. (Sci.
ed.), 29, 425
Dirscherl, W. & Brisse, B. (1966) Hoppe-Seyler's Z. physiol. Chem.,
346, 155
Dirscherl, W. & Wirtzfeldt, A. (1964) Hoppe-Seyler's Z. physiol.
Chem., 336 8M1
Frazer, A. C. (1967) Letter to WHO
Hagan, E. C. Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I.Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Hodge, H. C. & Downs, W. L. (1961) Toxic. appl. Pharm., 3, 689
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Preusse, C. (1880) Hoppe-Seyler's Z. physiol. Chem., 4, 213
Sammons, H. G. & Williams, R. T. (1941) Biochem. J., 35, 1175
Shillinger, J. I. (1950) Gig. i San., 3, 37
 Molecular weight 152.15
Definition Vanillin contains not less than 97 per
cent. and not more than the equivalent
of 103 per cent. C8H8O3.
Description Fine, white to slightly yellow crystals,
usually needle-like, having an odour and
taste suggestive of vanilla. It is
affected by light.
Biological Data
Biochemical aspects
In rabbits, vanillin is metabolized by the following pathway:
vanillin undergoes rapid conjugation and slow oxidation to conjugated
vanillic acid, 5 per cent. of which is demethylated to conjugated
protocatechuic acid, which is then decarboxylated to conjugated
catechol. Further complete oxidation, opening the aromatic ring
between C3 and C4, then ensues (Sammons & Williams, 1941). Early
observers noted conversion to vanillic acid which was mainly excreted
as free acid or conjugated ethereal sulfate or glucurovanillic acid
(Preusse, 1880). Later work confirmed that if the rabbit 69 per cent.
of orally given vanillin is oxidized to 44 per cent. free vanillic
acid and 25 per cent. conjugated vanillic acid (10 per cent. as
ethereal sulfate and 15, per cent as p-glucuronide), all excreted in
the urine. Fourteen per cent. of orally administered vanillin is
excreted in conjugated form (8 per cent. as ethereal sulfate, 31 per
cent. as glucuronide), but none as free vanillin. Conjugated vanillic
acid and conjugated protocatechuic acid are in equilibrium with the
corresponding free acids which also appear in the urine (Sammons &
Williams, 1941).
In man and rat, vanillin is broken down by the liver to vanillic
acid which is excreted in the urine. Rat and human liver homogenates
readily convert vanillin to vanillic acid in vitro (Dirscherl &
Brisse, 1966). Quantitative conversion of dietary vanillin to urinary
vanillic acid in man has been confirmed. Endogenous vanillic acid
production and excretion from body catechol amines amounts to <0.5 mg
per day, compared with the normal contribution from dietary sources of
about 9 mg/day (Dirscherl & Wirtzfeld, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 780 Caujolle & Meynier, 1954
475 Frazer, 1967
Rat oral 1580 Jenner et al., 1964
approx. 2800 Hodge & Downs, 1961
s.c. 1800(LD) Deichmann & Kitzmiller,
1940
i.p. 1160 Caujolle et al., 1956
Guinea-pig oral 1400 Jenner et al., 1964
i.p. 1190 Caujolle et al., 1953
Dog i.v. 1320(LD) Caujolle et al., 1953
Rabbit oral 3000(LD) Deichmann & Kitzmiller,
1940
Rat s.c. 1500 Binet, 1896
Short-term studies
Rat. Intragastric administration to rats of 300 mg/kg
body-weight biweekly for 14 weeks had no deleterious effect. Groups of
16 rats were fed diets containing vanillin at the rate of 20 mg/kg
body-weight/day for 18 weeks without any adverse effects while 64
mg/kg body-weight/day for 10 weeks produced growth depression and
damage to myocardium, liver, kidney, lung, spleen and stomach. In
another experiment, 10 male and 10 female rats were fed diets
containing 0.3, 1.0 and 5.0 per cent. vanillin for 13 weeks. At the
highest level there was growth depression and enlargement of liver,
kidney and spleen; mild charges occurred at 1.0 per cent. and none at
0.3 per cent. (Deichmann & Kitzmiller, 1940). When groups of 5 males
and 5 females were fed 0, 0.1 and 1.0 per cent. vanillin in their diet
for 16-28 weeks, there were no adverse effects detectable. Groups of 5
males were given 0, 2.0 and 5.0 per cent. vanillin in their diet for 1
year without deleterious effects (Hagan et al., 1967).
Long-term studies
Rat. Groups of 12 males and 12 females were fed diets
containing 0, 0.5, 1.0 and 2.0 per cent. vanillin for 2 years without
any adverse effects (Hagan et al., 1967).
Comments
The metabolic fate of this flavouring is well established in man
and animals. The short-term studies give conflicting results, but the
long-term study is used for the evaluation.
EVALUATION
Level causing no toxicological effect
Rat. 2 per cent. (= 20 000 ppm) in the diet, equivalent to 1000
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-10
REFERENCES
Binet, P. (1896) Rev. méd. Suisse rom.,16, 449
Caujolle, F. & Meynier, D. (1954) C. R. Hebd. Séan. Acad. Sci.,
238, 2576
Caujolle, F. Meynier, D. & Forthouat, J. M. (1956) C.R. Hebd. Séan.
Acad. Sci., 243, 609
Caujolle, F., Meynier, D. & Moscarella, C. (1953) C.R. Hebd. Séan.
Acad. Sci., 236, 2549
Deichmann, W. & Kitzmiller, K. V. (1940) J. amer. Pharm ass. (Sci.
ed.), 29, 425
Dirscherl, W. & Brisse, B. (1966) Hoppe-Seyler's Z. physiol. Chem.,
346, 155
Dirscherl, W. & Wirtzfeldt, A. (1964) Hoppe-Seyler's Z. physiol.
Chem., 336 8M1
Frazer, A. C. (1967) Letter to WHO
Hagan, E. C. Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I.Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Hodge, H. C. & Downs, W. L. (1961) Toxic. appl. Pharm., 3, 689
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Preusse, C. (1880) Hoppe-Seyler's Z. physiol. Chem., 4, 213
Sammons, H. G. & Williams, R. T. (1941) Biochem. J., 35, 1175
Shillinger, J. I. (1950) Gig. i San., 3, 37
Molecular weight 152.15
Definition Vanillin contains not less than 97 per
cent. and not more than the equivalent
of 103 per cent. C8H8O3.
Description Fine, white to slightly yellow crystals,
usually needle-like, having an odour and
taste suggestive of vanilla. It is
affected by light.
Biological Data
Biochemical aspects
In rabbits, vanillin is metabolized by the following pathway:
vanillin undergoes rapid conjugation and slow oxidation to conjugated
vanillic acid, 5 per cent. of which is demethylated to conjugated
protocatechuic acid, which is then decarboxylated to conjugated
catechol. Further complete oxidation, opening the aromatic ring
between C3 and C4, then ensues (Sammons & Williams, 1941). Early
observers noted conversion to vanillic acid which was mainly excreted
as free acid or conjugated ethereal sulfate or glucurovanillic acid
(Preusse, 1880). Later work confirmed that if the rabbit 69 per cent.
of orally given vanillin is oxidized to 44 per cent. free vanillic
acid and 25 per cent. conjugated vanillic acid (10 per cent. as
ethereal sulfate and 15, per cent as p-glucuronide), all excreted in
the urine. Fourteen per cent. of orally administered vanillin is
excreted in conjugated form (8 per cent. as ethereal sulfate, 31 per
cent. as glucuronide), but none as free vanillin. Conjugated vanillic
acid and conjugated protocatechuic acid are in equilibrium with the
corresponding free acids which also appear in the urine (Sammons &
Williams, 1941).
In man and rat, vanillin is broken down by the liver to vanillic
acid which is excreted in the urine. Rat and human liver homogenates
readily convert vanillin to vanillic acid in vitro (Dirscherl &
Brisse, 1966). Quantitative conversion of dietary vanillin to urinary
vanillic acid in man has been confirmed. Endogenous vanillic acid
production and excretion from body catechol amines amounts to <0.5 mg
per day, compared with the normal contribution from dietary sources of
about 9 mg/day (Dirscherl & Wirtzfeld, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 780 Caujolle & Meynier, 1954
475 Frazer, 1967
Rat oral 1580 Jenner et al., 1964
approx. 2800 Hodge & Downs, 1961
s.c. 1800(LD) Deichmann & Kitzmiller,
1940
i.p. 1160 Caujolle et al., 1956
Guinea-pig oral 1400 Jenner et al., 1964
i.p. 1190 Caujolle et al., 1953
Dog i.v. 1320(LD) Caujolle et al., 1953
Rabbit oral 3000(LD) Deichmann & Kitzmiller,
1940
Rat s.c. 1500 Binet, 1896
Short-term studies
Rat. Intragastric administration to rats of 300 mg/kg
body-weight biweekly for 14 weeks had no deleterious effect. Groups of
16 rats were fed diets containing vanillin at the rate of 20 mg/kg
body-weight/day for 18 weeks without any adverse effects while 64
mg/kg body-weight/day for 10 weeks produced growth depression and
damage to myocardium, liver, kidney, lung, spleen and stomach. In
another experiment, 10 male and 10 female rats were fed diets
containing 0.3, 1.0 and 5.0 per cent. vanillin for 13 weeks. At the
highest level there was growth depression and enlargement of liver,
kidney and spleen; mild charges occurred at 1.0 per cent. and none at
0.3 per cent. (Deichmann & Kitzmiller, 1940). When groups of 5 males
and 5 females were fed 0, 0.1 and 1.0 per cent. vanillin in their diet
for 16-28 weeks, there were no adverse effects detectable. Groups of 5
males were given 0, 2.0 and 5.0 per cent. vanillin in their diet for 1
year without deleterious effects (Hagan et al., 1967).
Long-term studies
Rat. Groups of 12 males and 12 females were fed diets
containing 0, 0.5, 1.0 and 2.0 per cent. vanillin for 2 years without
any adverse effects (Hagan et al., 1967).
Comments
The metabolic fate of this flavouring is well established in man
and animals. The short-term studies give conflicting results, but the
long-term study is used for the evaluation.
EVALUATION
Level causing no toxicological effect
Rat. 2 per cent. (= 20 000 ppm) in the diet, equivalent to 1000
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-10
REFERENCES
Binet, P. (1896) Rev. méd. Suisse rom.,16, 449
Caujolle, F. & Meynier, D. (1954) C. R. Hebd. Séan. Acad. Sci.,
238, 2576
Caujolle, F. Meynier, D. & Forthouat, J. M. (1956) C.R. Hebd. Séan.
Acad. Sci., 243, 609
Caujolle, F., Meynier, D. & Moscarella, C. (1953) C.R. Hebd. Séan.
Acad. Sci., 236, 2549
Deichmann, W. & Kitzmiller, K. V. (1940) J. amer. Pharm ass. (Sci.
ed.), 29, 425
Dirscherl, W. & Brisse, B. (1966) Hoppe-Seyler's Z. physiol. Chem.,
346, 155
Dirscherl, W. & Wirtzfeldt, A. (1964) Hoppe-Seyler's Z. physiol.
Chem., 336 8M1
Frazer, A. C. (1967) Letter to WHO
Hagan, E. C. Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I.Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Hodge, H. C. & Downs, W. L. (1961) Toxic. appl. Pharm., 3, 689
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Patty, F. A. (1963) Industrial Hygiene and Toxicology, Vol. II,
Interscience
Preusse, C. (1880) Hoppe-Seyler's Z. physiol. Chem., 4, 213
Sammons, H. G. & Williams, R. T. (1941) Biochem. J., 35, 1175
Shillinger, J. I. (1950) Gig. i San., 3, 37
