
GENTAMICIN
First draft prepared by
Dr G. Roberts
Commonwealth Department of Human Services and Health
Canberra, Australia
1. EXPLANATION
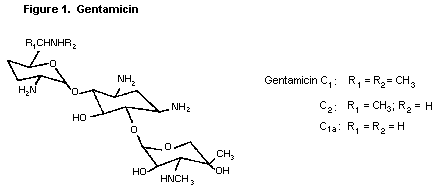
Gentamicin is an aminoglycoside antibiotic effective against a
wide variety of microorganisms. Gentamicin had not been previously
reviewed by the Committee. The structure of gentamicin is shown in
Figure 1.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism and excretion
Few specific studies on the pharmacokinetics of gentamicin were
available, however, the aminoglycosides as a class have been
extensively reviewed. In view of their polar nature and high aqueous
solubility, aminoglycosides are essentially not absorbed after oral
administration. Absorption after intramuscular or subcutaneous
administration is good with peak blood levels occurring within 30 to
90 minutes of an intramuscular injection (Riviere et al., 1991;
Sande & Mandell, 1990).
The aminoglycosides distribute into the extracellular space with
minimal penetration into tissues other than the kidneys and the inner
ear. Plasma protein binding is reported to be less than 20%. Levels in
amniotic fluid and fetal tissue are very low in most species.
Aminoglycosides as a class are not metabolized and are eliminated
unchanged in the urine by glomerular filtration. Recovery of 80% to
90% of the administered dose can occur within 24 hours (Fraser
et al., 1991).
One beagle dog was given a single intravenous dose of radio-
labelled gentamicin sulfate. The apparent half-life of elimination
from serum was approximately 1 hour initially and approximately 2´
hours 8 and 10 hours after dosing. Distribution to organs and tissues
was extensive but exceptionally high levels were found in the kidney,
mainly in the cortex. A comparison of the levels of serum gentamicin
using radioassay, radioimmunoassay and microbiological assay were
similar, suggesting that there was little metabolism of the drug.
Excretion was primarily renal with some 70% recovered in the urine but
very little (1%) in the faeces. Approximately 2% of the dose remained
in tissues 8 days after dosing; the remainder was unaccounted for
(Miller et al., 1976).
2.2 Toxicological studies
Unless specified otherwise, toxicological studies were carried
out using the gentamicin sulfate salt and dose levels are expressed as
gentamicin base.
2.2.1 Acute toxicity studies
The results of acute toxicity studies with gentamicin are
summarized in Table 1.
Clinical signs of intoxication in rodents included convulsions,
prostration, hypoactivity, polydipsia, dyspnoea and ataxia. Dogs
exhibited muscle tremors, salivation, and anorexia. Histopathological
examination of kidneys from dogs that died up to 13 days after dosing
revealed necrosis of the proximal convoluted tubule.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 0 or 40 mg/kg bw/day gentamicin in saline were
administered i.p. to groups of 5 male albino rats. Treatment was for
10 consecutive days and animals were killed 48 hours after the last
dose. There were no reported overt signs of toxicity. Serum
chemistries at termination showed an increase in creatinine, BUN, and
SGPT. Pathology was restricted to kidneys and liver and included
degeneration and necrosis of renal tubular epithelium and moderate
vacuolization of hepatocytes. The author also noted that enhancement
of nephrotoxicity and hepatotoxicity occurs in alloxan-induced
diabetic rats administered gentamicin (Atef et al., 1992).
Groups of Charles River CD1 rats (15/sex/group) received i.m.
injections of gentamicin daily for 14 days. The drug was administered
in saline at dose levels of 0, 10, 25, 50, or 100 mg/kg bw/day. Overt
signs of toxicity were noted only in the highest-dose group, which
included reduced body-weight gain, polydipsia, hypoactivity, ataxia,
lethargy, dyspnoea, emaciation and prostration. A total of 7 rats in
this group died or were killed because of their moribund condition.
Clinical laboratory investigations showed indications of
treatment-related effects on renal function. Serum creatinine and BUN
were increased. Glucose and occult blood were present in the urine at
doses of 50 and 100 mg/kg bw/day. Decreased urine specific gravity and
increased urine volume were noted at 25 mg/kg bw/day and above. At
necropsy, renal cortical pallor was observed in all treated groups
with dose-related increases in incidence and severity. At 25 mg/kg and
above, kidney weights were increased as was the severity of renal
tubular nephrosis (Robbins et al., 1973d).
Neonatal Carworth CFE rats (10 litters per group, 10 pups/litter)
were administered i.m. injections of gentamicin for 14 days. The
doses were 0, 10, 25, 50 or 100 mg/kg bw/day in a water vehicle.
There were no clinical signs of toxicity, but body-weight gains were
depressed at all doses. Laboratory investigations were not
undertaken. At the end of the study, kidney weights were increased at
all doses. Dilation of renal pelves was observed in treated groups
but with no clear dose-response relationship. At 25 mg/kg bw/day and
above, pale renal cortices and dark red medullas were noted. Cloudy
swelling of renal tubules, the presence of proteinaceous material in
tubules, desquamation of epithelial cells and tubular necrosis
occurred in all dose groups, which were considered to be treatment-
related (Eggert et al., 1975).
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 10 000 Hara et al., 1977a
M i.v. 40 Robbins et al., 1971
M&F i.v. 51-57 Hara et al., 1977a
M&F i.v. 37-40 McCormick, 1983
M i.m. 213 Robbins et al., 1971
M&F i.m. 223-233 Hara et al., 1977a
M i.p. 232 Robbins et al., 1971
M&F i.p. 279-305 Hara et al., 1977a
M s.c. 228 Robbins et al., 1971
M&F s.c. 292-312 Hara et al., 1977a
Rat M oral 8000-10 000 Hara et al., 1977a
M i.v. 67 Robbins et al., 1971
M i.m. 384 Eggert et al., 1965
M i.m. 371 Robbins et al., 1971
M&F i.m. 489-511 Hara et al., 1977a
M i.p. 674 Robbins et al., 1971
M&F i.p. 478-559 Hara et al., 1977a
M&F s.c. 817-893 Hara et al., 1977a
Rat M&F i.m. 500 Eggert et al., 1975
(neonate) i.p. 508 Eggert et al., 1975
Guinea-pig M&F i.m. 320 Robbins et al., 1973c
Dog M&F i.m. 300-400 Eggert et al., 1975
Gentamicin B in an aqueous vehicle was administered i.m. to
groups of Carworth CFE rats (10/sex/group) for 14 days. Doses were 0,
50, 100, or 250 mg/kg bw/day. Polydipsia and ptosis were observed in
all treated rats. Body-weight gain was reduced at 250 mg/kg bw/day.
Haematology and urinalysis parameters and BUN of treated rats were
comparable to control values. At necropsy, haemorrhage and necrosis
at the injection site was noted in all rats in the high-dose group.
Renal cortical pallor and increased kidney weights were seen in all
treated groups. At 250 mg/kg bw/day degenerative changes in proximal
convoluted tubules were noted including swelling, granularity of
epithelial cells, casts, loss of brush borders and fat droplets in the
epithelium. Liver weights were decreased at 100 and 250 mg/kg bw/day
(Robbins et al., 1972a).
Carworth CFE rats (22/sex/group) were administered i.m.
injections of either gentamicin or gentamicin C1 at doses of 0, 10,
25 or 50 mg/kg bw/day. In each dose group, 2 males and 2 females were
killed on days 2, 4, 6, 8, 10, and 12, and 10 males and 10 females
were killed after 14 days. There was no treatment-related mortality in
any dose group. The only adverse effects noted in clinical parameters
were a slight increase in BUN and reduced body-weight gain in the 50
mg/kg bw/day group. Histopathological examinations revealed
haemorrhage and lymphocytic infiltration at the injection site, which
was seen in all treatment groups. Pale renal cortices were observed in
all dosed groups, which were more pronounced with increasing doses of
gentamicin. In the gentamicin-treated groups, lymphocytic infiltration
in the renal cortex was first noted in the 50 mg/kg bw/day dose group
after 5 doses and at lower doses thereafter. Proximal convoluted
tubule degeneration required 7 doses of 50 mg/kg bw/day. Gentamicin
C1 induced lymphocytic infiltration at 25 and 50 mg/kg after 14 days
only, but with no tubular degeneration, suggesting a lower potential
for renal toxicity than gentamicin (Robbins et al., 1972b).
Carworth CFE rats (10/sex/group) were administered i.m.
injections of gentamicin in saline, 6 days/week for 4 weeks. The doses
were 0, 25, 50, or 200 mg/kg bw/day. Overt signs of toxicity occurred
at 200 mg/kg bw/day, which included hypoactivity, bradypnoea,
bradycardia, and increased mortality. Body-weight gain was depressed
at 50 and 200 mg/kg bw/day. Haematological examinations revealed
slight depressions in haemoglobin and haematocrit at 200 mg/kg bw/day.
Serum chemistry and urinalyses were not performed. A limited necropsy
revealed pale renal cortices, increased kidney weights, renal tubular
necrosis, haemorrhage and inflammatory changes at the injection site,
and increased parenchymatous degeneration of the liver. These lesions
were found only at 200 mg/kg bw/day. The NOEL was 25 mg/kg bw/day
(Eggert et al., 1963a).
Charles River CD rats (5 males/group) received daily subcutaneous
injections of gentamicin in an aqueous vehicle. Doses of 0, 50, or
150 mg/kg bw/day were administered for 14, 21 or 28 days. At 150
mg/kg bw/day, food and water intake were reduced and rats exhibited
prostration, dyspnoea, and "staring coat". All animals at this dose
died during week 2. Body-weight gain was reduced in both dose groups,
and vestibular function was unimpaired. Serum chemistry analyses and
urinalysis revealed evidence of altered renal function at 50 mg/kg
bw/day in animals killed after 14 days of treatment, but not in those
killed at later times. The findings included increased serum
creatinine and BUN, decreased specific gravity and pH of urine,
glucosuria and a reduced clearance rate of endogenous creatinine.
Haematology parameters were not evaluated. At necropsy, pallor of the
renal cortex was increased in treated groups. Histological
examination, which was restricted to the kidneys, showed proximal
tubular necrosis in all gentamicin-treated rats, the severity being
related to dose (Robbins et al., 1976a).
Wistar rats (20/sex/group) were administered i.m. injections of
0, 25, 63, or 156 mg/kg bw/day gentamicin for 30 days. Groups of 5
rats/sex/dose were allowed a recovery (no treatment) period of 30
days. In the highest-dose group, decreased motor activity,
prostration, ptosis, piloerection, congested conjunctiva, lacrymation,
ataxia, and soft faeces were noted. Approximately half the high-dose
animals died within 12 days, the remainder were killed on day 12.
Body-weight gain and food consumption were depressed at all dose
levels.
Decreases in erythrocytes, haemoglobin, and haematocrit were
observed in the 63 mg/kg bw/day group. At 25 mg/kg bw/day and above,
leucocytes, BUN, cholesterol, albumin/globulin ratio and SGOT were
increased. At necropsy, swelling and discoloration of the kidney and
inflation of the caecum were noted at 63 mg/kg bw/day and above.
Degeneration of renal cortical tubules, tubular dilatation, protein
casts and infiltration of round cells in the interstitium were found
in all treated groups. At 156 mg/kg bw/day, necrosis of renal tubules,
hepatocyte vacuolation, and bone marrow hypoplasia were also observed.
Inflammatory cell infiltration, haemorrhage and edema at the injection
site were dose-related. All changes tended to show some reversal
following the 30-day recovery period (Hara et al., 1977a).
Groups of Charles River CD rats (20/sex/group) received daily
i.m. injections of gentamicin in an aqueous vehicle. Doses of 0,
12.5, or 50 mg/kg bw/day were administered for up to 13 weeks. After
4 weeks of treatment, 5 males and 5 females were killed and examined.
There were no clinical signs of toxicity apart from thinness and
locomotor ataxia in a 50 mg/kg bw/day male which was killed because of
its moribund condition on day 15. The same animal had severely
depressed haematocrit, haemoglobin and leucocyte counts. It is
unlikely that the effects seen in this animal were due to treatment
with the test article.
Food intake and body-weight gain were decreased in all treated
groups. Results of vestibular function tests and ophthalmological
evaluations were unremarkable. In the 50 mg/kg bw/day group,
haemoglobin and haematocrit were slightly decreased and BUN was
slightly increased. Urine specific gravity and pH were slightly
decreased at both treatment levels. Increases in kidney weights,
renal tubular necrosis, basophilia and leucocytic infiltration were
observed in both dose groups. Pale kidneys were reported in the 50
mg/kg bw/day group only. Injection site haemorrhages were also seen in
both dose groups (Robbins et al., 1976b).
Groups of Sprague-Dawley CD rats (10/sex/group) were fed
gentamicin in the diet for 13 weeks. The concentration in the diet was
adjusted as needed to maintain doses of 0, 4, 19 or 116 mg/kg bw/day.
The high-dose group was increased to 233 mg/kg bw/day from week 10.
No overt signs of toxicity were observed in any dose group.
Ophthalmologic evaluations, body-weight gain, food intake, and
mortality in treated groups were comparable to controls. Soft stools
were observed in the highest-dose group. Haematological parameters,
BUN, blood glucose and serum iron were unaffected by treatment.
Urinalyses showed an increased number of high-dose males with ketone
bodies in urine. At the end of the study, kidneys, liver, heart and
brain were weighed and gross and histopathological examinations were
performed on a wide range of tissues. No treatment-related effects
were noted. The NOEL was 19 mg/kg bw/day (Woodard et al., 1970).
Carworth CFE rats (controls: 20/sex; treated: 30/sex/group) were
administered i.m. injections of gentamicin in an aqueous vehicle, 5
days per week for up to 52 weeks. The doses were 0, 5, 10, or 20
mg/kg bw/day. At 26 weeks, 10/sex from the treatment groups and 5/sex
from the control group were killed and examined. The remainder were
killed at termination.
There were no clinical signs of toxicity or mortality. Body-
weight gain was depressed in males in the 20 mg/kg bw/day group from
week 10, although feed intake was comparable to controls. Haemoglobin
and haematocrits were slightly decreased in the mid- and high-dose
groups during the last half of the study. Leucocytes, blood glucose
and urinalysis parameters remained within normal limits.
At necropsy, liver, heart and brain weights were unaffected.
Adverse effects on the kidney included increased organ weight and
pitted or mottled surfaces at 10 and 20 mg/kg bw/day. The onset of
interstitial nephritis (seen commonly in aging rats) occurred earlier
in treated groups compared to controls. At 6 months, only one rat in
the control group was affected, while almost all rats in the high-dose
group exhibited this effect. At 12-months, the incidence was
statistically significant in the high-dose group only, with the
incidence in other groups not differing significantly from controls.
Injection-site reactions were observed in all dose groups with a dose-
related increase in severity. Muscle fibrosis was seen at the higher
treatment levels. The NOEL was 5 mg/kg bw/day (Robbins et al.,
1970a).
2.2.2.2 Rabbits
Two dermal studies were conducted in New Zealand white rabbits at
doses of 0, 0.5, 1, or 2 mg/kg bw/day gentamicin. Animals were
treated 6 hours/day, 5 days/week on intact and abraded skin for either
21 days (3 females per group) or 90 days (2 males and 1 female per
group). A different vehicle (unidentified) was used in each study.
Clinical signs, body weight, serum chemistry, haematology, urinalysis,
and gross and microscopic pathology were unaffected. Erythema and
edema at the application sites were slight in one study and moderate
in the other. These effects were considered to be vehicle-related
(Eggert et al., 1963b; 1964).
2.2.2.3 Dogs
Groups of beagle dogs (2/sex/group) were administered i.m.
injections of gentamicin or gentamicin C1 in an aqueous vehicle
(sterile water) daily for 14 consecutive days. The doses were 0, 15,
30, or 45 mg/kg bw/day.
There were no deaths or clinical signs of toxicity, nor were body
weight or ophthalmological parameters affected. Clinical chemistry
analyses showed that both drugs caused an increase in BUN at 45 mg/kg
bw/day. Other biochemical and haematological parameters remained
within normal limits. Urine specific gravity was lower and urine
volume increased in the 45 mg/kg bw/day gentamicin group. Occult blood
and albumin in urine were increased in treated groups, particularly in
the 45 mg gentamicin/kg bw/day group. One dog in this group also
showed glucosuria.
Gross examination of organs revealed pale and congested renal
cortices at 45 mg/kg bw/day. Kidney weights were increased in a dose-
related manner in all treated groups. Renal proximal convoluted
tubule degeneration or necrosis was increased with 30 and 45 mg/kg
bw/day gentamicin and 45 mg/kg bw/day gentamicin C1. Haemorrhage,
inflammatory changes and/or necrosis were observed at all injection
sites, including vehicle controls (Robbins et al., 1972c).
In another 14-day study, groups of beagle dogs (2/sex/group)
recieved i.m. injections of 0, 10, or 45 mg/kg bw/day gentamicin or
45, 90, or 120 mg/kg bw/day gentamicin B in an aqueous vehicle.
There were no clinical signs of toxicity. Food intake and body
weights were depressed at 45 mg/kg bw/day gentamicin and 120 mg/kg
bw/day gentamicin B. Three dogs given 45 mg gentamicin/kg bw/day were
killed on day 14 because of their moribund condition. Gentamicin-
induced changes in clinical laboratory investigations were recorded in
the 45 mg/kg bw/day group, which included increases in neutrophils,
BUN and SGPT; a slight decrease in urine specific gravity; and glucose
and occult blood in urine. Increases in SGPT were seen at all dose
levels of gentamicin B.
At necropsy, kidney and liver weights were unaffected by either
drug at any dose level. Pale renal cortices and increased lymphocytic
infiltration and coalescence of mitochondria in proximal convoluted
tubule epithelial cells were seen in all drug-treated groups.
Proximal tubular necrosis and excessive fat deposition were associated
with administration of 45 mg/kg bw/day gentamicin (Robbins et al.,
1973a).
One-day-old beagle puppies (4 litters per dose, 6-9 pups/litter)
were injected i.m. with gentamicin in an aqueous vehicle daily for 14
consecutive days. Doses were 0, 2, 6, or 10 mg/kg bw/day. Two
litters per dose were killed after 5 days and 2 pups per litter were
maintained for a drug-free recovery period of 6 weeks following the
last treatment. There was no mortality and no clinical signs of
toxicity. Body weight, righting reflex, haematology and serum
biochemistry parameters remained within normal limits. Post-mortem
examination of kidneys and liver showed no effects on organ weight or
histo-pathology. Histolopathological evaluations of the vestibular
apparatus of the ear were unremarkable (Vymetal et al., 1969).
Daily i.m. injections of gentamicin in a saline vehicle were
administered to groups of beagle dogs (2/sex/group) for up to 3 weeks.
The doses were 0, 15, 30 or 60 mg/kg bw/day. Vomiting was reported in
3/4 dogs in the high-dose group between days 9 and 12 of treatment and
in 3/4 dogs in the mid-dose group between days 16 and 20 of treatment.
Vomiting occurred within one or two hours of treatment. All dogs in
the 60 mg/kg bw/day group were killed between days 9 and 12 because of
their extreme moribund condition characterized by weakness, lethargy,
recumbency and irregular respiration. Slight body-weight loss was
noted during the second week in the 30 mg/kg bw/day group.
Ophthalmological findings were unremarkable.
Haematological parameters remained within normal limits. Serum
creatinine and BUN were increased at 30 and 60 mg/kg bw/day. At the
end of the study, SGPT was elevated and serum sodium and chloride ions
were decreased at 30 mg/kg bw/day. At 30 mg/kg bw/day and above,
urine specific gravity was decreased compared to controls and
glucosuria and occult blood were detected. Urine volume was increased
at 60 mg/kg bw/day. Kidney weights were increased in all treated
groups. Gross and histopathological examination revealed renal
cortical pallor and renal tubular necrosis in all treated animals.
Liver congestion was observed at 30 and 60 mg/kg bw/day. Muscle
degeneration and fibrosis of injection sites were seen at 60 mg/kg
bw/day (Robbins et al., 1973b).
In two separate studies, beagle dogs (2/sex/group) were
administered i.m. injections of gentamicin consisting of two different
ratios of the components C1 and C2, viz. 58:42 and 70:30. Groups
were treated with 0, 6, 10, or 50 mg/kg bw/day for 7 weeks. The
findings were essentially the same for each ratio. Clinical signs were
reported as anorexia, ataxia, prostration, vomiting and convulsions,
but the group incidences were not provided. Dogs in the highest-dose
group died or were killed during weeks 2 and 3. Body weights were
unaffected.
Haemoglobin and haematocrits fell sharply just prior to
intercurrent death or terminal kill in all treated animals. Increased
BUN, glucosuria, albuminuria, and urinary casts were observed in the
10 and 50 mg/kg bw/day groups. Pale renal corticies were observed in
all treated groups. This finding was accompanied by a gelatinous
transudate in animals dosed at 50 mg/kg bw/day. Toxic nephrosis was
noted in treated animals, which was characterized as cloudy swelling
at 6 mg/kg bw/day to severe tubular necrosis at 50 mg/kg bw/day.
Muscle damage was seen at injection sites in all groups including
controls. (Eggert et al., 1963c; 1973).
Beagle dogs (2/sex/group) received i.m. injections of 0, 63, or
100 mg gentamicin/kg bw/day. The duration was intended to be 30 days,
but all treated animals died within 18 days. Signs of gentamicin
toxicosis included vomiting, salivation, gait disturbances, difficulty
in standing, decreases in heart and respiration rates, and reduced
body weight and food intake. Gross and histopathological examinations
revealed swelling, discoloration and increased weight of the kidney,
degeneration and necrosis of renal tubular epithelium, infiltration of
round cells, protein casts and narrowing and dilatation of tubules.
These findings were observed at both treatment levels. Inflammation
and muscle fibre degeneration at the injection sites were also
observed in both dose groups with a dose-related increase in severity
(Hara et al., 1977b).
Groups of beagle dogs (4/sex/group) were administered i.m. doses
of 0, 12.5, or 25 mg/kg bw/day gentamicin in an aqueous vehicle, for
12 weeks. There were no treatment-related clinical signs of toxicity.
Food consumption was unaffected. Body-weight loss occurred in all
groups, which was more severe in the 25 mg/kg bw/day group and the
12.5 mg/kg bw/day females.
Ophthalmological and haematological parameters remained within
normal limits. There were slight elevations in SGPT, SGOT and
creatine phosphokinase at both treatment levels. At 25 mg/kg bw/day,
urine specific gravity was decreased and urine volume was increased.
Glucosuria and occult blood were present in one 12.5 and two 25 mg/kg
bw/day animals. Gross and histopathological findings included
increased kidney and liver weights, pale renal cortices, and proximal
renal tubular necrosis in all treated groups (Robbins et al.,
1976c).
Groups of beagle dogs (4/sex/group) were administered oral doses
of 0, 2, 10, or 60 mg/kg bw/day gentamicin in capsules for 14 weeks.
The highest level was increased to 120 mg/kg bw/day after 2 months.
Emesis and diarrhoea were observed occasionally in treated dogs.
There were no effects on body weight, ophthalmology, haematology,
blood chemistry or urinalysis. The only postmortem change was
interstitial nephritis observed in 2 animals in the high-dose group.
The NOEL was 10 mg/kg bw/day (Vymetal et al., 1970).
Beagle dogs (controls: 2/sex; treated: 4/sex/dose) were injected
i.m. with doses of 0, 3, 5, or 8 mg/kg bw/day gentamicin in an aqueous
vehicle. Animals were treated 5 days per week for up to 12 months.
One dog per sex per group was scheduled for an interim kill after 6
months. Localized pain and transient lameness of the injected leg
were seen in all groups. Ophthalmological and physical examination
findings were within normal limits. Vomiting and profuse salivation
were noted in one animal in the 8 mg/kg bw/day group in weeks 23 and
24. This animal lost considerable body weight during the last 8 weeks
of treatment and had a slightly increased BUN, glucosuria, decreased
urine specific gravity and increased urine volume. Body weight,
haematology, blood chemistry and urinalysis were within normal limits
in all other dogs.
Pale renal corticies and interstitial nephritis were seen after
gross and histopathological examinations in all treatment groups with
a dose-response relationship. Organ weights were similar in all
groups. Inflammatory changes, haemorrhage and fibrosis at the
injection sites were present in all groups, with an increase in
severity in treated animals (Robbins et al., 1970b).
2.2.2.4 Monkeys
Groups of 4 squirrel monkeys (sex not specified) were
administered daily s.c. injections of 0, 25, 50 or 75 mg/kg bw
gentamicin in saline, for up to 3 weeks. All monkeys receiving
gentamicin (except one animal in the low dose group) had to be killed
intercurrently because of their moribund condition. The average
survival times were 15.3, 10.3 and 7.5 days at 25, 50 and 75 mg/kg
bw/day, respectively. Clinical signs included ataxia, weakness,
hypoactivity, irregular respiration and eye scratching at each dose
level. Sporadic convulsions occurred at 50 and 75 mg/kg bw/day.
Body-weight loss was recorded in all groups, including controls, which
appeared to be enhanced with gentamicin treatment. However, there was
no clear, dose-response relationship.
Post-injection acoustic reflex measurements could not be obtained
from the 75 mg/kg bw/day group due to their rapid decline in
condition. In addition, instrument failure resulted in the loss of 3
post-injection data points. Therefore, evaluations were performed on
a total of 14 monkeys out of 28. Results indicated that hearing
deficits occurred in both the low- and mid-dose groups. Apart from the
single low-dose survivor, all animals had increased serum creatinine
and BUN. The author stated that the majority of dosed animals showed
severe renal tubular necrosis. Individual animal data were not
provided (Igarashi et al., undated).
Groups of 3 female Rhesus monkeys were injected i.m. with doses
of 0, 6 or 30 mg/kg bw/day gentamicin in an aqueous vehicle for 3
weeks. Adverse clinical signs were limited to the 30 mg/kg bw/day
group, which included pronounced facial paling and ptosis, markedly
disturbed equilibrium from day 20, and depressed food intake and body-
weight gain from week 2 onwards. Two monkeys in this group were
killed because of their moribund condition on day 21.
Haematological examinations showed decreases in haemoglobin,
haematocrit, and erythrocytes at 30 mg/kg bw/day. Serum chemistry
findings included increases in SGPT, SGOT and LDH, and decreases in
serum potassium, sodium, chloride and calcium ions in the 30 mg/kg
bw/day dose group. Serum creatinine and BUN were increased in both
dose groups. Urinalyses revealed proteinuria, glucosuria and an
increased presence of casts and epithelial cells at 30 mg/kg bw/day.
At necropsy, kidney weights were increased and had a yellow-grey
appearance. Histologically, cloudy swelling, cortical striation,
degeneration and necrosis of proximal convoluted tubules were
reported. The renal lesions which were seen in both dose groups, were
considered to be treatment-related. Vacuolar degeneration of
hepatocytes was noted at 30 mg/kg bw/day. Injection sites showed
dose-related muscle degeneration and necrosis accompanied by
haemorrhage and cellular infiltration. Electron microscopy of renal
tubules from the 30 mg/kg bw/day monkeys revealed myeloid bodies
present in both tubular cells and lumen, increased phagosomes,
disappearance of brush borders and sloughing of epithelial cells from
the basement membrane (Tanaka et al., 1977).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
Groups of Carworth CFE rats (30/sex/group) were injected 6 days/
week with i.m. doses of 0, 5, or 20 mg/kg bw/day gentamicin in saline.
Males were injected from 70 days prior to mating until 7 days before
mating. Females in the 5 mg/kg bw/day group were injected from 14
days prior to pairing with a male until post partum day 21. Females
in the 20 mg/kg bw/day group were treated from the time of
impregnation (determined by vaginal smear) until post partum day 21.
Ten females per group were killed for litter and dam evaluations on
gestation day 21, the remainder were allowed to deliver normally. F1
offspring were not treated. Groups of 10 males and 10 females from
randomly selected litters were paired within treatment levels
(avoiding brother-sister mating) to produce an F2 generation.
F1 Dams were allowed to deliver normally.
After 13 doses, penile bleeding was observed in high-dose F0
males. Because of this finding, the dose was reduced to 15 mg/kg
bw/day after which bleeding ceased. In F0 males of the high dose,
body-weight gain was slightly lower during the second half of the
treatment period. There were no overt effects in females.
Pregnancy rates were not affected. There was no prenatal
mortality and litter sizes and weights were similar between groups.
Early postnatal mortality was increased at both doses, which was
attributed to maternal neglect and failure to lactate, neither of
which were considered treatment-related. Fetal abnormalities were not
observed in delivered pups or caesarian-derived fetuses. Gentamicin
was present in the amniotic fluid in 5/10 low-dose dams, and 8/10
high-dose dams but was not detected in homogenized fetuses. No
treatment-related changes in pregnancy rate, litter size and weight,
prenatal mortality or fetal abnormalities were reported in this study
(Robbins et al., 1966).
2.2.4 Special studies on embryotoxicity and teratogenicity
2.2.4.1 Mice
Female JCL-ICR mice (15-33/group) received s.c. injections of
gentamicin in saline during gestation days 6 to 10. The doses
administered were 0, 1, 10 or 100 mg/kg bw/day. In the high-dose
group, body-weight gain was depressed during treatment and kidney
weight was increased. Gentamicin was measured in amniotic fluid in
the high-dose group. It was not clear whether attempts were made to
measure gentamicin in amniotic fluid in the other two dose groups. In
five control and five 100 mg/kg bw/day dams, delivery of fetuses was
allowed to occur normally. The development of eye and ear openings
was unaffected and kidney and liver weights were similar between
groups at post partum day 35. The remaining females were killed on
gestation day 17 for dam and litter evaluations. Prenatal loss was
increased at 10 and 100 mg/kg bw/day. There were no treatment-related
increases in fetal abnormalities (Nishio et al., 1987).
2.2.4.2 Rats
Female Carworth CFE rats (40/group) were injected i.m. with
gentamicin in saline on gestation days 3 to 16. The doses
administered were 0, 25, or 50 mg/kg bw/day, 6 days per week. Females
were allowed to deliver normally. At least 10 days after weaning the
first litter, dams were bred again and the above procedures were
repeated. Adult females showed no clinical signs of toxicity. Body-
weight gain was lower in the 50 mg/kg bw/day group during the
treatment period. At necropsy, pale renal cortices were observed in
both dose groups. Resorption rates were comparable to control values.
The number of stillborn pups was slightly increased at 50 mg/kg
bw/day following the second series of matings. Offspring were killed
and examined 21 days post partum. No treatment-related abnormalities
were reported (Eggert & Haring, 1964).
Groups of 17 female Sprague-Dawley rats were given intra-muscular
injections of 0 or 75 mg/kg bw/day gentamicin in saline. Treatment
was on gestation days 10 to parturition. Dams were allowed to deliver
normally. Parturition was delayed in gentamicin-treated dams by an
average of 15 hours and birth weights were lower. In pups showing the
lowest birth weights, kidney weight and the number of nephrons were
reduced at birth and at 14 and 21 days post partum. Pups with higher
birth weight showed similar kidney weight and numbers of nephrons to
controls; however, a relative decrease in the number of nephrons was
reported in these animals at 14 and 21 days post partum. Necrosis was
not reported but vacuoles in proximal tubular epithelium were
increased with loss of brush border microvilli and the presence of
debris in the tubular lumen. Gentamicin was detected in the kidneys
of all pups born to treated mothers, the levels being higher in pups
showing the lowest birth weights.
In 7 litters per group, half the pups received subcutaneous doses
of 0 or 75 mg/kg bw/day of gentamicin on post partum days 1 to 13,
then killed on post partum day 14. Drug treatment caused an increase
in kidney weight but the number of glomeruli were similar in both
groups (Gilbert et al., 1987).
In an abstract, it was reported that rats were given s.c. doses
of 0 or 110 mg/kg bw/day gentamicin on either gestation days 10 to 15
or 15 to 20. Females were allowed to deliver normally. Measurements
of arterial blood pressure in offspring, one year after birth,
revealed significantly elevated blood pressure in females but not in
males (Chahoud et al., 1986).
2.2.4.3 Guinea-pigs
Guinea-pigs (6 females per group) were administered i.m.
injections of 0 or 4 mg/kg bw/day gentamicin in saline on gestation
days 48 to 54. Females were allowed to deliver normally. Litter size
and weight and body and kidney weights of offspring from the treated
group were comparable to controls to day 20 post partum. Studies of
kidney function in offspring showed a higher excretion of phosphate in
3-day-old pups but not at 10 days of age. Excretion of sodium,
potassium, magnesium and calcium, urinary flow rate, glomerular
filtration rate and the numbers of glomeruli were unaffected. The
length of proximal tubules and glomerular volume were reduced at 3 and
10 days post partum but not at 20 days. Gentamicin was present in pup
renal cortex at higher levels that in the medulla. Levels were
highest 3 days after birth and decreased thereafter (Le Lievre
et al., 1987).
2.2.4.4 Rabbits
Groups of 13 female New Zealand white rabbits were administered
i.m. doses of 0, 0.8, or 4 mg/kg bw/day gentamicin in saline. The
high dose was given as two divided doses, 7 hours apart. Treatments
were administered on gestation days 6 to 16. Eight females per group
were killed for dam and litter evaluations on gestation day 30. The
remainder were allowed to deliver normally. Pups were killed and
examined on post partum day 21. Adult animals were in good health
throughout the study. No treatment-related effects were noted on
prenatal loss, litter size or weight, fetal morphology, or postnatal
survival or body-weight gain. (Robbins & Klein, 1966).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies on gentamicin are summarized
in Table 2.
2.2.6 Special studies on kidney function
2.2.6.1 Rats
A group of 6 male Sprague-Dawley rats was injected i.m. with
doses of 80 to 160 mg/kg bw/day gentamicin for 7 days. At
termination, animals were anaesthetized with thiobarbital and kidney
function was assessed. The glomerular filtration rate, as measured by
inulin clearance, was reduced in a dose-related manner in all
treatment groups. Aciduria and glucosuria (determined by Labstix)
were induced at 160 mg/kg bw/day, but not at the lower doses,
indicating that reabsorption of glucose in the proximal renal tubule
had been impaired (Chiu et al., 1977).
Sprague-Dawley rats (group sizes not given) were injected s.c.
with 0 or 100 mg/kg bw/day gentamicin for up to 7 days. After four
doses, serum creatinine and BUN were increased and urine osmolality
was decreased, suggesting impairment of urine concentrating capacity.
Tubular reabsorption of solute free water was unaffected. Tubular
secretion of para-aminohippurate (PAH) was increased, although
glomerular filtration rate was decreased, after 4 days. Kidney
cortical slices, isolated from rats treated with gentamicin,
accumulated PAH in vitro at a faster rate than slices from control
animals; this effect was inhibited in the presence of probenecid. This
provided evidence that an alteration in the organic acid transport
system was responsible for the increased rate of PAH accumulation
(Cohen et al., 1974).
Female Sprague-Dawley rats (group sizes not given) were injected
s.c. with 0, 50, 100, or 150 mg/kg bw/day gentamicin for up to 14
days. During the treatment period, urine osmolality was decreased
followed by a rise in urine volume and water intake. Proteinuria was
seen from day 3 and serum creatinine and BUN were increased at 100 and
150 mg/kg bw/day. At termination, microscopic examination of kidneys
revealed dose-related injury to proximal tubules in all dose groups,
consisting of increased lysosomal bodies, disruption of brush borders,
cellular swelling and necrosis. Kidney cortical slices, from rats
treated with gentamicin, showed increased uptake of PAH in vitro
(Kaloyanides, 1977).
Sprague-Dawley rats (group sizes not given) were injected i.p.
with 0 or 10 mg/kg bw/day gentamicin for up to 14 days. Kidney
function was unaffected throughout the treatment period. Animals were
killed after 1, 4, 7 and 14 doses and kidneys removed for examination.
Microscopic examinations found proliferation of lysosomes in proximal
tubular cells on day 4. This was associated with decreases in
lysosomal sphingomyelinase and phospholipase activities, suggesting
lysosomal dysfunction. At day 9, protrusion of cells and/or nuclei
into tubular lumens was observed and several cells had lost part of
their brush borders. Focal necrosis was evident on day 14 (Carlier
et al., 1982).
2.2.6.2 Dogs
Male beagle dogs (group sizes not given) were injected i.m. with
0 or 30 mg/kg bw/day gentamicin for up to 11 days. Serum creatinine,
BUN, and urine protein were increased, and creatinine clearance and
urine osmolality were decreased in gentamicin-treated animals. In
addition, urinary levels of beta-glucuronidase, N-acetyl-beta-
glucosaminidase and muramidase were elevated. Microscopic renal
lesions ranged from diffuse moderate hyaline droplet degeneration to
frank tubular necrosis. The pathological and enzyme changes appeared
to precede the functional alterations (Adelman et al., undated).
2.2.7 Special study on ototoxicity
2.2.7.1 Monkeys
Groups of 3 female Cynomolgus monkeys were injected i.m. with
doses of 0, 25, or 50 mg/kg bw/day gentamicin for 35 days. All monkeys
dosed at 50 mg/kg bw/day died: one on day 7 and the other two on day
22. Prior to death, the pinna reflex to high frequencies in these
monkeys was slightly reduced, but there was no observable ataxia
indicative of vestibular impairment. Histopathological examination of
the crista ampullaris showed vacuole formation at both doses and, at
the higher dose, hair cell loss and a reduction in the thickness of
sensory epithelium. In the organ of Corti, cochlear hair cell loss was
noted at 50 mg/kg bw/day while the histology of the macula was not
significantly affected (Yakota et al., 1984).
2.2.8 Special study on neurotoxicity
Gentamicin was administered parenterally to Charles River CD
rats, cats and beagle dogs, to assess its neurotoxicity potential.
The treatment regimes were as follows:
Rats - 0, 6.6, 16.6, 41.5 or 104 mg/kg bw/day, s.c., for
41 days.
Cats - 0, 2.5, 5.0 or 10.0 mg/kg bw/day, s.c., for 42 days.
Dogs - 0, 5.0, 8.3 or 41.5 mg/kg bw/day, i.m., for 49 days.
In rats, righting reflex was impaired at 104 mg/kg bw/day and
ataxia was observed in dogs given 8.3 and 41.5 mg/kg bw/day. Ataxia
was not produced in cats at any dose (Taber et al., 1963).
2.2.9 Special studies on microbiological effects
Results of investigations into the effects of gentamicin on human
intestinal flora were not available. However, two published reports
were available on the inhibitory activity of gentamicin against a wide
range of bacterial strains in vitro.
Table 2. Results of genotoxicity studies on gentamicin
Test system Test object Results Reference
Reverse mutation1 S. typhimurium negative2 Houk et al., 1989
Reverse mutation1 S. typhimurium negative3 Koeda & Hirano, 1979
TA98, TA100,
TA1535, TA1536,
TA1537, TA1538
Reverse mutation1 S. typhimurium negative2 Mitchell et al., 1980
TA98, TA100,
E. coli WP2
Reverse mutation1 E. coli CM891 negative3 Mitchell & Gilbert, 1984
Forward mutation1 E. coli CM891 positive3 at a Mitchell & Gilbert, 1984
cytotoxic level
(0.25 µg/ml)
Forward mutation1 E. coli WP2 positive2 at a Mitchell et al., 1980
cytotoxic level
(0.49 µg/ml)
Mitotic crossing- S. cerevisiae D5 negative3 Koeda & Hirano, 1979
over1
Gene conversion1 S. cerevisiae D4 negative3 Mitchell et al., 1980
Petite inducation1 S. cerevisiae D4 positive3 (500 Mitchell et al., 1980
µg/ml) at pH4.4,
not pH 7.2
DNA repair1 E. coli WP2, negative2 Mitchell et al., 1980
WP100
Rec-assay1 B. subtilis negative3 Kada et al., 1980
M45, H17
SOS function1 E. coli PQ37 negative2 Mamber et al., 1986
DNA-cell binding E. coli Q13 positive2 Kubinski et al., 1981
(20 µg/ml) test
not validated
Table 2 (contd)
Test system Test object Results Reference
Chromosome Mouse L-cells positive3 but Leonard & Botis, 1975
aberrations mitosis inhibited
(500 µg/ml x 4 d
plus 100 µg/ml x
20 d)
SCE Human fibroblasts positive3 but McDaniel & Schultz, 1993
GM00847 less than doubling
(75-250 µg/ml)
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
3 Without metabolic activation.
One article reported the minimum inhibitory concentrations (MICs)
for gentamicin in 601 clinical isolates of anaerobic bacteria. The
experiments were conducted using the agar-dilution technique at 37°C
for 48 hours in an anaerobic incubator, an inoculum of 103 to 104
colony-forming units and serial concentrations of gentamicin in the
range of 0.1 to 25 µg/ml (Martin et al., 1972). In a second
publication, results were reported for Clostridium difficile
strains, but there was no information on the culture conditions
employed (Lorian, undated). All results are presented in Table 3.
The susceptibility to gentamicin of most anaerobic bacteria was
low, the most sensitive being Eubacterium sp., with a modal MIC
value of 0.8 µg/ml for the 20 strains tested.
2.3 Observations in humans
In a prospective, randomized, blinded, comparative trial, 54
patients treated with gentamicin were evaluated for nephrotoxicity and
ototoxicity. The dosage regime was usually 1.7 mg/kg bw i.v. every 8
hours (5.1 mg/kg bw/day) for 7 to 54 days. Desired serum drug levels
were maintained by reducing the dose in patients with impaired renal
function. Nephrotoxicity, defined as a 50% increase in serum
creatinine, was produced in 8 (15%) patients. Auditory toxicity,
defined as a decrease in the sensorineural threshold of at least 15
decibels in two or more frequencies, was produced in 3 (6%) patients.
Vestibular toxicity, defined as at least a 50% decrease in maximum
average slow-phase eye speed following water stimulation of the ear,
was induced in 3 of 33 (9%) patients. Auditory and vestibular effects
were not noted in the same individuals (Lerner et al., 1986).
Kidney biopsies were obtained from 5 patients with renal cancer
following surgical removal of both kidneys. Prior to surgery,
gentamicin was administered at a dosage of 1.5 mg/kg bw three times a
day (4.5 mg/kg bw/day) for 4 days. Microscopic evaluation of the
kidney specimens revealed increases in lysosome numbers with moderate
to marked decreases in the activity of sphingomyelinase and acid
phospholipase A1 (Carlier et al., 1982).
In a retrospective study involving patients on chronic
haemodialysis therapy, 23 patients were treated with gentamicin.
Dosages were not identified. Of the drug-treated subjects, 7 (30%)
developed clinically detectable vestibular toxicity. The principal
risk factors for the development of ototoxicity were age, duration of
treatment and the total dose administered. There was no positive
association with peak serum levels of gentamicin or the total absence
of kidneys (Gailiunas Jr. et al., 1978).
A review, focusing mainly on prospective trials, reported on a
total of more than 10 000 patients treated with aminoglycoside
antibiotics. The incidence of cochlear toxicity associated with
gentamicin administration ranged from 5 to 10% in adults. The
incidence was claimed to be lower in infants (Matz, 1993).
Adverse reactions to gentamicin during the years 1963 to 1973
were reviewed. Of the 3500 patients studied, the main non-specific
reactions were increases in serum transaminases (16), skin rashes (16)
and granulocytopaenia (8). Also presented were results from clinical
trials with gentamicin. Possible or probable nephrotoxicity was found
in 22 of 285 (7.7%) patients in 1965 to 1966, 70 of 1450 (4.8%)
patients in 1967 to 1969 and 48 of 1635 (2.9%) patients in 1970 to
1973. Possible or probable ototoxicity was found in 16 of 565 (2.8%)
patients in 1963 to 1966, 27 of 1450 (1.8%) patients in 1967 to 1969
and 15 of 1635 (1%) patients in 1970 to 1973. Critical doses or
duration of treatment were not given, but ototoxicity usually occurred
in association with impaired renal function (Hewitt, 1974).
The rates of allergic cutaneous reactions were determined in 15
438 consecutive in-patients monitored by the Boston Collaborative Drug
Surveillance Program from June 1975 to June 1982. Of the 670 patients
receiving gentamicin, there were 3 (0.5%) skin reactions.
Table 3. Minimum inhibitory concentration (MIC) values for gentamicin against anaerobic bacteria
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Bacteroides fragilis (195) 1 6
B. incommunio (10) 10 40
B. variabilis (5) 60
B. oralis (2) 50 100
B. terebrans (5) 40 60
B. melaninogenicus (29) 24 31 34 45 59 76
Bacteroides F1,F2,F3 (11) 27 36 45 73
Fusobacterium fusiforme (18) 5 11 56
Fusobacterium sp. (2) 50
Clostridium perfringens (34) 6
C. difficile (15) 20 40
Clostridium sp. (17) 6 18
Peptococcus sp. (145) 2 3 5 7 11 30 62 95
Peptostreptococcus sp. (72) 1 9 13 18 20 33 58 78
Veillonella sp. (13) 8 31 46 85
Table 3 (contd)
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Propionibacterium acnes (16) 25 56 75 81
Eubacterium lentum (14) 43 57 71 85 93 100
E. alactolyticum (2) 50
Eubacterium sp. (5) 20 40
Bifidobacterium sp. (5) 20 60 80 100
Catenabacterium (3) 66
filamentosum
Catenabacterium sp. (2) 50 100
3. COMMENTS
A range of studies on gentamicin was submitted for assessment,
including information on pharmacokinetics and metabolism, acute
toxicity, short-term toxicity, reproductive toxicity and
teratogenicity, genotoxicity, antimicrobial activity, a variety of
special studies and observations in humans.
Gentamicin, in common with other aminoglycosidic drugs, is poorly
absorbed from the gastrointestinal tract. Parenteral doses distribute
mainly into extracellular space, but there is significant penetration
into the kidney cortex and the inner ear. There is negligible
metabolism of the drug and unchanged gentamicin is excreted rapidly,
mostly in the urine.
Single oral doses of gentamicin were slightly toxic in rodents
(LD50 = 8000 to 10 000 mg/kg bw), which supports the view that the
drug is largely unabsorbed when administered by this route. Parenteral
dosing of mice, rats, guinea-pigs and dogs demonstrated high
intravenous toxicity (LD50 = 37 to 67 mg/kg bw) and moderate toxicity
by i.m., s.c. and i.p. routes (LD50 = 213 to 893 mg/kg bw).
Gentamicin was extensively tested by the i.m. and s.c. routes in
rats administered the compound for periods of up to 12 months (doses
up to 200 mg/kg bw/day) in dogs for up to 12 months (doses up to 100
mg/kg bw/day) and in monkeys for 3 weeks (doses up to 75 mg/kg
bw/day). The kidney was the primary target organ in each species with
effects observed predominantly in the renal cortex. The major
findings were an increase in interstitial nephritis and toxic
nephrosis in the proximal convoluted tubules. The latter was
characterized by a dose- and time-dependent progression from loss of
brush borders, cloudy swelling, the presence of casts and
proteinaceous material in the tubules, desquamation of epithelial
cells and necrosis. These changes were associated with a profound
impairment of renal function, and in extreme cases, death of severely
affected animals. Special studies in rats suggested that the
nephrotoxicity may be associated with disruption of lysosomal
function. Nephrotoxicity has been observed in humans treated with
gentamicin.
Toxicity to the inner ear may occur after exposure to
aminoglycosides, including gentamicin. There were no clear
indications of ototoxicity in the general toxicity studies; however,
a special study in monkeys, at doses up to 50 mg/kg bw/day, showed
hair cell loss and reduced sensory epithelium in structures of the ear
as well as a functional loss in hearing. Observations in humans have
revealed auditory or vestibular toxicity after therapeutic
administration of gentamicin.
Oral dosing of rats and dogs for a period of 3 months, at doses
up to 116 and 60 mg/kg bw/day, respectively, was virtually without
systemic effects. Soft stools or diarrhoea were observed in both
species and dogs showed interstitial nephritis at high doses. The
NOELs were 19 mg/kg bw/day in rats and 10 mg/kg bw/day in dogs.
A multigeneration reproductive toxicity study in the rat showed
no adverse effects on reproduction parameters after intramuscular
injections of 5 or 20 mg gentamicin/kg bw/day. Teratogenicity studies
in mice, rats, guinea-pigs and rabbits did not identify any potential
for the production of fetal abnormalities. Fetotoxicity, in the form
of fetal death in mice (10 mg/kg bw/day) and rats (50 mg/kg bw/day)
and reduced birth weight in rats (75 mg/kg bw/day), was observed after
parenteral dosing.
Positive results were obtained in some in vitro genotoxicity
studies, but none of these was adequate to evaluate the genotoxicity
of gentamicin. There were no carcinogenicity studies available on
gentamicin. In toxicity studies in animals of up to one year
duration, there were no pre-neoplastic or neoplastic lesions. The
Committee considered that the data were inadequate to fully assess the
carcinogenicity of gentamicin.
Data on the effects of gentamicin on human gut flora were not
available. However, in vitro data were presented on MICs for over
600 clinical isolates of anaerobic bacteria including some species
representative of the microbial flora in the human gastrointestinal
tract. Many of the organisms were found to be resistant to gentamicin;
Eubacterium species were the most sensitive. Using an inoculum
density of 103 to 104 colony forming units per plate, MIC values for
Eubacterium species were in the range 0.1 to 25 µg/ml with an MIC50
of 0.8 µg/ml.
In view of the poor oral absorption of gentamicin and the
reported occurrence of diarrhoea in experimental animals, it was
considered that effects on the gastrointestinal microflora would be
the most sensitive effect of residues. In calculating the ADI, the
formula developed by the thirty-eighth meeting of the Committee (Annex
1, reference 97) was employed:
Concentration without effect x Daily faecal bolus (g)
on human gut flsor (µg/ml)a
Upper limit of ADI =
Fraction of oral x Safety x Human body
dose availableb factorc weight
(60 kg)
(0.8 x 2) x 150
=
1 x 1 x 60
= 4 µg/kg bw
a This value accounts for the range of MICs in appropriate
bacterial species and the use of the most sensitive anaerobic
organism. A two-fold adjustment was deemed to be necessary to
account for the relatively low inoculum density.
b Absorption of gentamicin after oral dosing is very poor, therefore
a factor of 1 was used to represent 100% availability of ingested
drug in the gastrointestinal tract.
c Data on more than 600 clinical isolates of anaerobic bacteria were
available for determination of the MIC. Because the colonic flora
is relatively stable and variability within a particular individual
may be as great as variability between individuals, and because it
was recognized that other values selected for this calculation were
conservative and already incorporated an adequate margin of safety,
a safety factor of 1 was selected to cover fully the variability
between people of all extrapolated parameters.
4. EVALUATION
In view of the absence of information on the effects of
gentamicin on microorganisms obtained from the human intestine and the
need for further genotoxicity studies, the Committee established a
temporary ADI of 0-4 µg/kg bw based on the results of micro-biological
testing of clinical isolates, with a request for further information.
An increased safety factor was not used to account for the temporary
status of the ADI because conservative factors were used in deriving
the temporary ADI. The lowest toxicological NOEL was 10 mg/kg bw/day
in dogs, which is more than 2000-fold higher than the
microbiologically based ADI.
5. REFERENCES
ADELMAN, R.D., CONZELMAN, G., SPANGLER, W. & ISHIZAKI, G. (undated).
Comparative nephrotoxicity of gentamicin and netilmicin : functional
and morphological correlations with urinary enzyme activities.
Unpublished Report No. D 11403. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ATEF, M., ARBID, M.S. & HANAFY, M.S.M. (1992). Comparison of
gentamicin toxicity in normal and diabetic rats. Acta Veterinaria
Hungarica, 40: 107-111.
BIGBY, M., JICK, S., JICK, H. & ARNDT, K. (1986). Drug-induced
cutaneous reactions. JAMA, 256: 3358-3363.
CARLIER, M.B., PAULUS, G., MALDAGUE, P., GIURGEA, L., WILMOTTE, E., DE
BROC, M.E. & TULKENS, P. (1982). Early toxic events in kidney of rat
and man following administration of gentamicin at low doses.
Arch. Toxicol. Suppl. 5: 287-290.
CHAHOUD, I., STAHLMANN, R. & NEUBERT, D. (1986). Blood pressure
elevation in one-year-old female rats after prenatal exposure to
gentamicin. Teratology, 33: 13A.
CHIU, P.J.S., LONG, J.F., BARNETT, A., TABER, R.I., MILLER, G. &
WAITZ, J.A. (1977). Kidney function in rats chronically treated with
aminoglycosides. Unpublished Report No. P 4485. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
COHEN, L., LAPKIN, R. & KALOYANIDES, G.J. (1974). Effect of gentamicin
on renal function in the rat. Unpublished Report No. D 6338. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
EGGERT, M.J., DAVENPORT, L.R. & WILLIAMSON, N.K. (1973). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3379. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., EVANS, M. & WILLIAMSON, N.K. (1963a). SCH 9724 in rats.
Subacute (4 weeks) intramuscular toxicity study. Unpublished Report
No: P 3355. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J. & HARING, G.D. (1964). Intramuscular teratogenic study of
SCH 9724 (gentamicin sulfate 70:30) in rats. Unpublished Report No: P
3397. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., HARING, G.D. & VYMETAL, F. (1965). Acute toxicity
studies of gentamicin sulfate intramuscularly in rats and dogs.
Unpublished Report No: P 3502. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., HARING, G.D., WILLIAMSON, N.K. & WEINSTEIN, M.J. (1975).
Acute and subacute toxicity studies of SCH 9724 (gentamicin sulfate)
in rat neonates. Unpublished Report No: P 3497. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1963b).
Gentamicin sulfate-90 day dermal toxicity in rabbits. Unpublished
Report No: P 3410. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1964).
Dermal toxicity of SCH 9724 in rabbits. Unpublished Report No: P 3425.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., VYMETAL, F. & WILLIAMSON, N.K. (1963c). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3350. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
FRASER, C.M., BERGERON, J.A., MAYS, A. & AIELLO, S.E. (1991).
Aminoglycosides. In: The Merck Veterinary Manual, 7th Edition ,
Merck & Co., Rahway, pp 1429-1435.
GAILIUNAS JR., P., DOMINGUEZ-MORENO, M., LAZARUS, J.M., LOWRIE, G.,
GOTTLIEB, M.N. & MERRILL, P. (1978). Vestibular toxicity of
gentamicin. Arch. Intern. Med., 138: 1621-1624.
GILBERT, T., LELIEVRE-PEGORIER, M., MALIENOU, R., MEULEMANS, A. &
MERLET-BENICHOU, C. (1987). Effects of prenatal and postnatal exposure
to gentamicin on renal differentiation in the rat. Toxicology, 43:
301-313.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977a).
Safety evaluation of KW-1062. I. Acute toxicity in mice, rats and
dogs, subacute and chronic toxicity in rats. Jap. J. Antibiotics ,
30: 386-407.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977b).
Safety evaluation of KW-1062. II. Subacute and chronic toxicity
studies in dogs. Jap. J. Antibiotics, 30: 408-422.
HEWITT, W.L. (1974). Gentamicin: toxicity in perspective. Postgrad.
Med. J., (Suppl. 7): 55-59.
HOUK, V.S., SCHALKOWSKY, S. & CLAXTON, L.D. (1989). Development and
validation of the spiral Salmonella assay: an automated approach to
bacterial mutagenicity testing. Mutation Res., 223: 49-64.
IGARASHI, M., LEVY, J.K., JERGER, J., WAITZ, J.A. & ARCIERI, G.
(undated). Comparative toxicity of netilmicin and gentamicin in
squirrel monkeys. Unpublished Draft Report No: D 10312. Submitted to
WHO by Inveresk Research International, Trenant, Scotland, on behalf
of Schering Plough Animal Health.
KADA, T., HIRANO, K. & SHIRASU, Y. (1980). Screening of environmental
chemical mutagens by the Rec-assay with Bacillus subtilis. In:
Chemical Mutagens and Principles for their Detection. Chap. 6: pp
149-173.
KALOYANIDES, G.J. (1977). Untitled. Unpublished Report No: D 10069.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
KOEDA, T. & HIRANO, F. (1979). Evaluation of the mutagenicity of
aminoglycoside antibiotics in Salmonella typhimurium and
Saccharomyces cerevisiae. J. Antibiotics, 32: 607-609.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA cell-binding
assay for suspected carcinogens and mutagens. Mutation Res., 89:
95-136.
LELIEVRE, M., GILBERT, T., SAKLY, R., MEULEMANS, A. & MERLET-BENICHOU,
C. (1987). Effect of fetal exposure to gentamicin on kidneys of young
guinea pigs. Antimicrob. Agents Chemother., 31: 88-92.
LEONARD, A. & BOTIS, S. (1975). Chromosome damage induced by
gentamicin in mouse L-cells. Experientia, 31: 341-343.
LERNER, S.A., SCHMITT, B.A., SELIGSOHN, R. & MATZ, G.J. (1986).
Comparative study of ototoxicity and nephrotoxicity in patients
randomly assigned to treatment with amikacin or gentamicin.
Am. J. Med., 80(6B): 98-104.
LORIAN, V. (undated). Tables 28.13 and 28.15. In: Antibiotics in
Laboratory Medicine. 2nd Edition. Williams & Wilkins, Baltimore-
London-Los Angeles-Sydney, pp 1087-1089 and 1142-1146.
MAMBER, S.W., OKASINSKI, W.G., PINTER, C.D. & TUNAC, J.B. (1986). The
Escherichia coli K-12 SOS chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutation Res., 171: 83-90.
MARTIN, W.J., GARDNER, M. & WASHINGTON II, J.A. (1972). In vitro
antimicrobial susceptibility of anaerobic bacteria isolated from
clinical specimens. Antimicrob. Agents Chemother., 1: 148-158.
MATZ, G. (1993). Aminoglycoside cochlear ototoxicity. Otolaryngologic
Clinics of North America, 26: 705-712.
McCORMICK, G.C. (1983). Acute intravenous toxicity comparison of
gentamicin sulfate and gentamicin C2 sulfate in mice (SN 83272).
Unpublished Report No: D17472. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
McDANIEL, L.D. & SCHULTZ, R.A. (1993). Elevation of sister chromatid
exchange frequency in transformed human fibroblasts following exposure
to widely used aminoglycosides. Environ. Molec. Mutagenesis, 21:
67-72.
MILLER, G.H., WAITZ, J.A. & WEINSTEIN, M.J. (1976). Pharmacokinetics
of C14-gentamicin in dogs. Unpublished Report No: P 4440. Submitted
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
MITCHELL, I DEG., DIXON, P.A., GILBERT, P.J. & WHITE, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Mutation Res., 79:
91-105.
MITCHELL, I DEG. & GILBERT, P.J. (1984). The effect of pretreatment of
Escherichia coli CM 891 with ethylenediaminetetraacetate on
sensitivity to a variety of standard mutagens. Mutation Res., 140:
13-19.
NISHIO, A., RYO, S. & MIYAO, N. (1987). Effects of gentamicin,
exotoxin and their combination on pregnant mice. Bull. Fac. Agric.
Kagoshima Univ., 37: 129-136.
RIVIERE, J.E., CRAIGMILL, A.L. & SUNDLOF, S.F. (1991).
Aminoglycosides. In: Handbook of Comparative Pharmacokinetics and
Residues of Veterinary Antimicrobials, CRC Press, Boca Roton, Florida,
pp 263-335.
ROBBINS, G.R., CASTELLANO, R.F., TORNING, F.E., FABRY, A. & FIELDER,
F.G. (1971).- Acute toxicity in rats (intramuscular, intravenous and
intraperitoneal) and mice (intramuscular, intravenous, intraperitoneal
and subcutaneous). Unpublished Report No : P 3967. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., FABRY, A., MORGAN, W.A. & EGGERT, M.J. (1972a).
Subacute (two-week) intramuscular toxicity study of SCH 14342 in rats.
Unpublished Report No: P 4074. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1970a).
Chronic toxicity study of SCH 9724 by intramuscular injection in rats.
Unpublished Report No: P 3884. Submitted by Inveresk Research
International, Trenant, Scotland, to WHO on behalf of Schering Plough
Animal Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1972b).
Subacute (two-week) toxicity study of SCH 13706 by intramuscular
injection in rats. Unpublished Report No: P 4072. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R. & KLEIN, M.F. (1966). Reproduction study of gentamicin
sulfate in rabbits. Unpublished Report No : P 3595. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., KLEIN, M.F. & EGGERT, M.J. (1966). Reproduction study
using gentamicin sulfate intramuscularly in rats. Unpublished Report
No : P 3598. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970b).
Chronic (1-year) intramuscular toxicity study in dogs using gentamicin
sulfate (SCH 9724). Unpublished Report No: P 3848. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1972c).
Subacute (14-day) intramuscular toxicity study of SCH 13706 Gentamicin
C1 ) in dogs. Unpublished Report No: P 4065. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1973a).
Subacute intramuscular toxicity study of SCH 14342 as compared with
SCH 9724 in dogs. Unpublished Report No: P 4138. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H. & GRAY, W.D. (1976a). The subacute
toxicity of SCH 20569 sulfate, (vs gentamicin, tobramycin and
kanamycin) administered subcutaneously to rats. Unpublished Report No:
P 4424. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D. & FIELD, W.E. (1976b).
Subacute intramuscular toxicity of SCH 20569 in rats. Unpublished
Report No: P 4393. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D., FIELD, W.E. & SCHIAVO, D.M.
(1976c). Subacute intramuscular toxicity of SCH 20569 in dogs.
Unpublished Report No: P 4392. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R., FIELD, W.E. & OWEN, G.
(1973b). Subacute intramuscular toxicity in dogs using SCH 15666 and
SCH 9724. Unpublished Report No: P 4196. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R. & OWEN, G. (1973c). Acute
intramuscular toxicity study in guinea pigs. Unpublished Report No: P
4174. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., OWEN, G., MARCHESO, R. & FIELD, W.E.
(1973d). Subacute intramuscular toxicity in rats using SCH 15666 and
SCH 9724. Unpublished Report No: P 4212. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
SANDE, M.A. & MANDELL, G.L. (1990). Antimicrobial agents. The
aminoglycosides. In: GILMAN, A.G., RALL, T.W., NIES, A.S. & TAYLOR, P.
(eds), Goodman & Gilman's The Pharmacological Basis of Therapeutics,
8th Edition, Pergamon Press, New York-Oxford-Beijing-Sao Paulo-Sydney-
Tokyo-Toronto, pp 1098-1116.
TABER, R., IRWIN, S. & PERLMAN, P.L. (1963). The effect of subchronic
administration of gentamicin sulfate, kanamycin sulfate and
streptomycin sulfate in the rat, cat and dog. Unpublished Report No:
P 3316. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
TANAKA, H., WATANABE, M. & HATTORI, H. (1977). Subacute toxicity of
sisomycin administered intramuscularly in monkeys. Comparison with
gentamicin and tobramycin. Unpublished Report No: D 10555. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970). Subacute (14 week)
oral toxicity study of SCH 14894 (gentamicin sulfate, veterinary
grade) in dogs. Unpublished Report No: P 3923. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
VYMETAL, F., EGGERT, M.J. & ROBBINS, G.R. (1969). Toxicity study of
gentamicin sulfate by injection in neonatal dogs. Unpublished Report
No: P 3818. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
WOODARD, M.W., HOWARD, D.J., WOODARD, G. & EGGERT, M.J. (1970).
Gentamicin sulfate safety evaluation by repeated oral administration
to rats for 13 weeks. Unpublished Report No: P 3924. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
YAKOTA, M., TAKEDA, U., SAKAMOTO, K., SETO, N. & IGARASHI, M. (1984).
The comparative ototoxicities of panimycin and gentamicin in
cynomolgus monkeys (Maccaca fascicularis). Chemotherapy, 30:
248-254.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism and excretion
Few specific studies on the pharmacokinetics of gentamicin were
available, however, the aminoglycosides as a class have been
extensively reviewed. In view of their polar nature and high aqueous
solubility, aminoglycosides are essentially not absorbed after oral
administration. Absorption after intramuscular or subcutaneous
administration is good with peak blood levels occurring within 30 to
90 minutes of an intramuscular injection (Riviere et al., 1991;
Sande & Mandell, 1990).
The aminoglycosides distribute into the extracellular space with
minimal penetration into tissues other than the kidneys and the inner
ear. Plasma protein binding is reported to be less than 20%. Levels in
amniotic fluid and fetal tissue are very low in most species.
Aminoglycosides as a class are not metabolized and are eliminated
unchanged in the urine by glomerular filtration. Recovery of 80% to
90% of the administered dose can occur within 24 hours (Fraser
et al., 1991).
One beagle dog was given a single intravenous dose of radio-
labelled gentamicin sulfate. The apparent half-life of elimination
from serum was approximately 1 hour initially and approximately 2´
hours 8 and 10 hours after dosing. Distribution to organs and tissues
was extensive but exceptionally high levels were found in the kidney,
mainly in the cortex. A comparison of the levels of serum gentamicin
using radioassay, radioimmunoassay and microbiological assay were
similar, suggesting that there was little metabolism of the drug.
Excretion was primarily renal with some 70% recovered in the urine but
very little (1%) in the faeces. Approximately 2% of the dose remained
in tissues 8 days after dosing; the remainder was unaccounted for
(Miller et al., 1976).
2.2 Toxicological studies
Unless specified otherwise, toxicological studies were carried
out using the gentamicin sulfate salt and dose levels are expressed as
gentamicin base.
2.2.1 Acute toxicity studies
The results of acute toxicity studies with gentamicin are
summarized in Table 1.
Clinical signs of intoxication in rodents included convulsions,
prostration, hypoactivity, polydipsia, dyspnoea and ataxia. Dogs
exhibited muscle tremors, salivation, and anorexia. Histopathological
examination of kidneys from dogs that died up to 13 days after dosing
revealed necrosis of the proximal convoluted tubule.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 0 or 40 mg/kg bw/day gentamicin in saline were
administered i.p. to groups of 5 male albino rats. Treatment was for
10 consecutive days and animals were killed 48 hours after the last
dose. There were no reported overt signs of toxicity. Serum
chemistries at termination showed an increase in creatinine, BUN, and
SGPT. Pathology was restricted to kidneys and liver and included
degeneration and necrosis of renal tubular epithelium and moderate
vacuolization of hepatocytes. The author also noted that enhancement
of nephrotoxicity and hepatotoxicity occurs in alloxan-induced
diabetic rats administered gentamicin (Atef et al., 1992).
Groups of Charles River CD1 rats (15/sex/group) received i.m.
injections of gentamicin daily for 14 days. The drug was administered
in saline at dose levels of 0, 10, 25, 50, or 100 mg/kg bw/day. Overt
signs of toxicity were noted only in the highest-dose group, which
included reduced body-weight gain, polydipsia, hypoactivity, ataxia,
lethargy, dyspnoea, emaciation and prostration. A total of 7 rats in
this group died or were killed because of their moribund condition.
Clinical laboratory investigations showed indications of
treatment-related effects on renal function. Serum creatinine and BUN
were increased. Glucose and occult blood were present in the urine at
doses of 50 and 100 mg/kg bw/day. Decreased urine specific gravity and
increased urine volume were noted at 25 mg/kg bw/day and above. At
necropsy, renal cortical pallor was observed in all treated groups
with dose-related increases in incidence and severity. At 25 mg/kg and
above, kidney weights were increased as was the severity of renal
tubular nephrosis (Robbins et al., 1973d).
Neonatal Carworth CFE rats (10 litters per group, 10 pups/litter)
were administered i.m. injections of gentamicin for 14 days. The
doses were 0, 10, 25, 50 or 100 mg/kg bw/day in a water vehicle.
There were no clinical signs of toxicity, but body-weight gains were
depressed at all doses. Laboratory investigations were not
undertaken. At the end of the study, kidney weights were increased at
all doses. Dilation of renal pelves was observed in treated groups
but with no clear dose-response relationship. At 25 mg/kg bw/day and
above, pale renal cortices and dark red medullas were noted. Cloudy
swelling of renal tubules, the presence of proteinaceous material in
tubules, desquamation of epithelial cells and tubular necrosis
occurred in all dose groups, which were considered to be treatment-
related (Eggert et al., 1975).
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 10 000 Hara et al., 1977a
M i.v. 40 Robbins et al., 1971
M&F i.v. 51-57 Hara et al., 1977a
M&F i.v. 37-40 McCormick, 1983
M i.m. 213 Robbins et al., 1971
M&F i.m. 223-233 Hara et al., 1977a
M i.p. 232 Robbins et al., 1971
M&F i.p. 279-305 Hara et al., 1977a
M s.c. 228 Robbins et al., 1971
M&F s.c. 292-312 Hara et al., 1977a
Rat M oral 8000-10 000 Hara et al., 1977a
M i.v. 67 Robbins et al., 1971
M i.m. 384 Eggert et al., 1965
M i.m. 371 Robbins et al., 1971
M&F i.m. 489-511 Hara et al., 1977a
M i.p. 674 Robbins et al., 1971
M&F i.p. 478-559 Hara et al., 1977a
M&F s.c. 817-893 Hara et al., 1977a
Rat M&F i.m. 500 Eggert et al., 1975
(neonate) i.p. 508 Eggert et al., 1975
Guinea-pig M&F i.m. 320 Robbins et al., 1973c
Dog M&F i.m. 300-400 Eggert et al., 1975
Gentamicin B in an aqueous vehicle was administered i.m. to
groups of Carworth CFE rats (10/sex/group) for 14 days. Doses were 0,
50, 100, or 250 mg/kg bw/day. Polydipsia and ptosis were observed in
all treated rats. Body-weight gain was reduced at 250 mg/kg bw/day.
Haematology and urinalysis parameters and BUN of treated rats were
comparable to control values. At necropsy, haemorrhage and necrosis
at the injection site was noted in all rats in the high-dose group.
Renal cortical pallor and increased kidney weights were seen in all
treated groups. At 250 mg/kg bw/day degenerative changes in proximal
convoluted tubules were noted including swelling, granularity of
epithelial cells, casts, loss of brush borders and fat droplets in the
epithelium. Liver weights were decreased at 100 and 250 mg/kg bw/day
(Robbins et al., 1972a).
Carworth CFE rats (22/sex/group) were administered i.m.
injections of either gentamicin or gentamicin C1 at doses of 0, 10,
25 or 50 mg/kg bw/day. In each dose group, 2 males and 2 females were
killed on days 2, 4, 6, 8, 10, and 12, and 10 males and 10 females
were killed after 14 days. There was no treatment-related mortality in
any dose group. The only adverse effects noted in clinical parameters
were a slight increase in BUN and reduced body-weight gain in the 50
mg/kg bw/day group. Histopathological examinations revealed
haemorrhage and lymphocytic infiltration at the injection site, which
was seen in all treatment groups. Pale renal cortices were observed in
all dosed groups, which were more pronounced with increasing doses of
gentamicin. In the gentamicin-treated groups, lymphocytic infiltration
in the renal cortex was first noted in the 50 mg/kg bw/day dose group
after 5 doses and at lower doses thereafter. Proximal convoluted
tubule degeneration required 7 doses of 50 mg/kg bw/day. Gentamicin
C1 induced lymphocytic infiltration at 25 and 50 mg/kg after 14 days
only, but with no tubular degeneration, suggesting a lower potential
for renal toxicity than gentamicin (Robbins et al., 1972b).
Carworth CFE rats (10/sex/group) were administered i.m.
injections of gentamicin in saline, 6 days/week for 4 weeks. The doses
were 0, 25, 50, or 200 mg/kg bw/day. Overt signs of toxicity occurred
at 200 mg/kg bw/day, which included hypoactivity, bradypnoea,
bradycardia, and increased mortality. Body-weight gain was depressed
at 50 and 200 mg/kg bw/day. Haematological examinations revealed
slight depressions in haemoglobin and haematocrit at 200 mg/kg bw/day.
Serum chemistry and urinalyses were not performed. A limited necropsy
revealed pale renal cortices, increased kidney weights, renal tubular
necrosis, haemorrhage and inflammatory changes at the injection site,
and increased parenchymatous degeneration of the liver. These lesions
were found only at 200 mg/kg bw/day. The NOEL was 25 mg/kg bw/day
(Eggert et al., 1963a).
Charles River CD rats (5 males/group) received daily subcutaneous
injections of gentamicin in an aqueous vehicle. Doses of 0, 50, or
150 mg/kg bw/day were administered for 14, 21 or 28 days. At 150
mg/kg bw/day, food and water intake were reduced and rats exhibited
prostration, dyspnoea, and "staring coat". All animals at this dose
died during week 2. Body-weight gain was reduced in both dose groups,
and vestibular function was unimpaired. Serum chemistry analyses and
urinalysis revealed evidence of altered renal function at 50 mg/kg
bw/day in animals killed after 14 days of treatment, but not in those
killed at later times. The findings included increased serum
creatinine and BUN, decreased specific gravity and pH of urine,
glucosuria and a reduced clearance rate of endogenous creatinine.
Haematology parameters were not evaluated. At necropsy, pallor of the
renal cortex was increased in treated groups. Histological
examination, which was restricted to the kidneys, showed proximal
tubular necrosis in all gentamicin-treated rats, the severity being
related to dose (Robbins et al., 1976a).
Wistar rats (20/sex/group) were administered i.m. injections of
0, 25, 63, or 156 mg/kg bw/day gentamicin for 30 days. Groups of 5
rats/sex/dose were allowed a recovery (no treatment) period of 30
days. In the highest-dose group, decreased motor activity,
prostration, ptosis, piloerection, congested conjunctiva, lacrymation,
ataxia, and soft faeces were noted. Approximately half the high-dose
animals died within 12 days, the remainder were killed on day 12.
Body-weight gain and food consumption were depressed at all dose
levels.
Decreases in erythrocytes, haemoglobin, and haematocrit were
observed in the 63 mg/kg bw/day group. At 25 mg/kg bw/day and above,
leucocytes, BUN, cholesterol, albumin/globulin ratio and SGOT were
increased. At necropsy, swelling and discoloration of the kidney and
inflation of the caecum were noted at 63 mg/kg bw/day and above.
Degeneration of renal cortical tubules, tubular dilatation, protein
casts and infiltration of round cells in the interstitium were found
in all treated groups. At 156 mg/kg bw/day, necrosis of renal tubules,
hepatocyte vacuolation, and bone marrow hypoplasia were also observed.
Inflammatory cell infiltration, haemorrhage and edema at the injection
site were dose-related. All changes tended to show some reversal
following the 30-day recovery period (Hara et al., 1977a).
Groups of Charles River CD rats (20/sex/group) received daily
i.m. injections of gentamicin in an aqueous vehicle. Doses of 0,
12.5, or 50 mg/kg bw/day were administered for up to 13 weeks. After
4 weeks of treatment, 5 males and 5 females were killed and examined.
There were no clinical signs of toxicity apart from thinness and
locomotor ataxia in a 50 mg/kg bw/day male which was killed because of
its moribund condition on day 15. The same animal had severely
depressed haematocrit, haemoglobin and leucocyte counts. It is
unlikely that the effects seen in this animal were due to treatment
with the test article.
Food intake and body-weight gain were decreased in all treated
groups. Results of vestibular function tests and ophthalmological
evaluations were unremarkable. In the 50 mg/kg bw/day group,
haemoglobin and haematocrit were slightly decreased and BUN was
slightly increased. Urine specific gravity and pH were slightly
decreased at both treatment levels. Increases in kidney weights,
renal tubular necrosis, basophilia and leucocytic infiltration were
observed in both dose groups. Pale kidneys were reported in the 50
mg/kg bw/day group only. Injection site haemorrhages were also seen in
both dose groups (Robbins et al., 1976b).
Groups of Sprague-Dawley CD rats (10/sex/group) were fed
gentamicin in the diet for 13 weeks. The concentration in the diet was
adjusted as needed to maintain doses of 0, 4, 19 or 116 mg/kg bw/day.
The high-dose group was increased to 233 mg/kg bw/day from week 10.
No overt signs of toxicity were observed in any dose group.
Ophthalmologic evaluations, body-weight gain, food intake, and
mortality in treated groups were comparable to controls. Soft stools
were observed in the highest-dose group. Haematological parameters,
BUN, blood glucose and serum iron were unaffected by treatment.
Urinalyses showed an increased number of high-dose males with ketone
bodies in urine. At the end of the study, kidneys, liver, heart and
brain were weighed and gross and histopathological examinations were
performed on a wide range of tissues. No treatment-related effects
were noted. The NOEL was 19 mg/kg bw/day (Woodard et al., 1970).
Carworth CFE rats (controls: 20/sex; treated: 30/sex/group) were
administered i.m. injections of gentamicin in an aqueous vehicle, 5
days per week for up to 52 weeks. The doses were 0, 5, 10, or 20
mg/kg bw/day. At 26 weeks, 10/sex from the treatment groups and 5/sex
from the control group were killed and examined. The remainder were
killed at termination.
There were no clinical signs of toxicity or mortality. Body-
weight gain was depressed in males in the 20 mg/kg bw/day group from
week 10, although feed intake was comparable to controls. Haemoglobin
and haematocrits were slightly decreased in the mid- and high-dose
groups during the last half of the study. Leucocytes, blood glucose
and urinalysis parameters remained within normal limits.
At necropsy, liver, heart and brain weights were unaffected.
Adverse effects on the kidney included increased organ weight and
pitted or mottled surfaces at 10 and 20 mg/kg bw/day. The onset of
interstitial nephritis (seen commonly in aging rats) occurred earlier
in treated groups compared to controls. At 6 months, only one rat in
the control group was affected, while almost all rats in the high-dose
group exhibited this effect. At 12-months, the incidence was
statistically significant in the high-dose group only, with the
incidence in other groups not differing significantly from controls.
Injection-site reactions were observed in all dose groups with a dose-
related increase in severity. Muscle fibrosis was seen at the higher
treatment levels. The NOEL was 5 mg/kg bw/day (Robbins et al.,
1970a).
2.2.2.2 Rabbits
Two dermal studies were conducted in New Zealand white rabbits at
doses of 0, 0.5, 1, or 2 mg/kg bw/day gentamicin. Animals were
treated 6 hours/day, 5 days/week on intact and abraded skin for either
21 days (3 females per group) or 90 days (2 males and 1 female per
group). A different vehicle (unidentified) was used in each study.
Clinical signs, body weight, serum chemistry, haematology, urinalysis,
and gross and microscopic pathology were unaffected. Erythema and
edema at the application sites were slight in one study and moderate
in the other. These effects were considered to be vehicle-related
(Eggert et al., 1963b; 1964).
2.2.2.3 Dogs
Groups of beagle dogs (2/sex/group) were administered i.m.
injections of gentamicin or gentamicin C1 in an aqueous vehicle
(sterile water) daily for 14 consecutive days. The doses were 0, 15,
30, or 45 mg/kg bw/day.
There were no deaths or clinical signs of toxicity, nor were body
weight or ophthalmological parameters affected. Clinical chemistry
analyses showed that both drugs caused an increase in BUN at 45 mg/kg
bw/day. Other biochemical and haematological parameters remained
within normal limits. Urine specific gravity was lower and urine
volume increased in the 45 mg/kg bw/day gentamicin group. Occult blood
and albumin in urine were increased in treated groups, particularly in
the 45 mg gentamicin/kg bw/day group. One dog in this group also
showed glucosuria.
Gross examination of organs revealed pale and congested renal
cortices at 45 mg/kg bw/day. Kidney weights were increased in a dose-
related manner in all treated groups. Renal proximal convoluted
tubule degeneration or necrosis was increased with 30 and 45 mg/kg
bw/day gentamicin and 45 mg/kg bw/day gentamicin C1. Haemorrhage,
inflammatory changes and/or necrosis were observed at all injection
sites, including vehicle controls (Robbins et al., 1972c).
In another 14-day study, groups of beagle dogs (2/sex/group)
recieved i.m. injections of 0, 10, or 45 mg/kg bw/day gentamicin or
45, 90, or 120 mg/kg bw/day gentamicin B in an aqueous vehicle.
There were no clinical signs of toxicity. Food intake and body
weights were depressed at 45 mg/kg bw/day gentamicin and 120 mg/kg
bw/day gentamicin B. Three dogs given 45 mg gentamicin/kg bw/day were
killed on day 14 because of their moribund condition. Gentamicin-
induced changes in clinical laboratory investigations were recorded in
the 45 mg/kg bw/day group, which included increases in neutrophils,
BUN and SGPT; a slight decrease in urine specific gravity; and glucose
and occult blood in urine. Increases in SGPT were seen at all dose
levels of gentamicin B.
At necropsy, kidney and liver weights were unaffected by either
drug at any dose level. Pale renal cortices and increased lymphocytic
infiltration and coalescence of mitochondria in proximal convoluted
tubule epithelial cells were seen in all drug-treated groups.
Proximal tubular necrosis and excessive fat deposition were associated
with administration of 45 mg/kg bw/day gentamicin (Robbins et al.,
1973a).
One-day-old beagle puppies (4 litters per dose, 6-9 pups/litter)
were injected i.m. with gentamicin in an aqueous vehicle daily for 14
consecutive days. Doses were 0, 2, 6, or 10 mg/kg bw/day. Two
litters per dose were killed after 5 days and 2 pups per litter were
maintained for a drug-free recovery period of 6 weeks following the
last treatment. There was no mortality and no clinical signs of
toxicity. Body weight, righting reflex, haematology and serum
biochemistry parameters remained within normal limits. Post-mortem
examination of kidneys and liver showed no effects on organ weight or
histo-pathology. Histolopathological evaluations of the vestibular
apparatus of the ear were unremarkable (Vymetal et al., 1969).
Daily i.m. injections of gentamicin in a saline vehicle were
administered to groups of beagle dogs (2/sex/group) for up to 3 weeks.
The doses were 0, 15, 30 or 60 mg/kg bw/day. Vomiting was reported in
3/4 dogs in the high-dose group between days 9 and 12 of treatment and
in 3/4 dogs in the mid-dose group between days 16 and 20 of treatment.
Vomiting occurred within one or two hours of treatment. All dogs in
the 60 mg/kg bw/day group were killed between days 9 and 12 because of
their extreme moribund condition characterized by weakness, lethargy,
recumbency and irregular respiration. Slight body-weight loss was
noted during the second week in the 30 mg/kg bw/day group.
Ophthalmological findings were unremarkable.
Haematological parameters remained within normal limits. Serum
creatinine and BUN were increased at 30 and 60 mg/kg bw/day. At the
end of the study, SGPT was elevated and serum sodium and chloride ions
were decreased at 30 mg/kg bw/day. At 30 mg/kg bw/day and above,
urine specific gravity was decreased compared to controls and
glucosuria and occult blood were detected. Urine volume was increased
at 60 mg/kg bw/day. Kidney weights were increased in all treated
groups. Gross and histopathological examination revealed renal
cortical pallor and renal tubular necrosis in all treated animals.
Liver congestion was observed at 30 and 60 mg/kg bw/day. Muscle
degeneration and fibrosis of injection sites were seen at 60 mg/kg
bw/day (Robbins et al., 1973b).
In two separate studies, beagle dogs (2/sex/group) were
administered i.m. injections of gentamicin consisting of two different
ratios of the components C1 and C2, viz. 58:42 and 70:30. Groups
were treated with 0, 6, 10, or 50 mg/kg bw/day for 7 weeks. The
findings were essentially the same for each ratio. Clinical signs were
reported as anorexia, ataxia, prostration, vomiting and convulsions,
but the group incidences were not provided. Dogs in the highest-dose
group died or were killed during weeks 2 and 3. Body weights were
unaffected.
Haemoglobin and haematocrits fell sharply just prior to
intercurrent death or terminal kill in all treated animals. Increased
BUN, glucosuria, albuminuria, and urinary casts were observed in the
10 and 50 mg/kg bw/day groups. Pale renal corticies were observed in
all treated groups. This finding was accompanied by a gelatinous
transudate in animals dosed at 50 mg/kg bw/day. Toxic nephrosis was
noted in treated animals, which was characterized as cloudy swelling
at 6 mg/kg bw/day to severe tubular necrosis at 50 mg/kg bw/day.
Muscle damage was seen at injection sites in all groups including
controls. (Eggert et al., 1963c; 1973).
Beagle dogs (2/sex/group) received i.m. injections of 0, 63, or
100 mg gentamicin/kg bw/day. The duration was intended to be 30 days,
but all treated animals died within 18 days. Signs of gentamicin
toxicosis included vomiting, salivation, gait disturbances, difficulty
in standing, decreases in heart and respiration rates, and reduced
body weight and food intake. Gross and histopathological examinations
revealed swelling, discoloration and increased weight of the kidney,
degeneration and necrosis of renal tubular epithelium, infiltration of
round cells, protein casts and narrowing and dilatation of tubules.
These findings were observed at both treatment levels. Inflammation
and muscle fibre degeneration at the injection sites were also
observed in both dose groups with a dose-related increase in severity
(Hara et al., 1977b).
Groups of beagle dogs (4/sex/group) were administered i.m. doses
of 0, 12.5, or 25 mg/kg bw/day gentamicin in an aqueous vehicle, for
12 weeks. There were no treatment-related clinical signs of toxicity.
Food consumption was unaffected. Body-weight loss occurred in all
groups, which was more severe in the 25 mg/kg bw/day group and the
12.5 mg/kg bw/day females.
Ophthalmological and haematological parameters remained within
normal limits. There were slight elevations in SGPT, SGOT and
creatine phosphokinase at both treatment levels. At 25 mg/kg bw/day,
urine specific gravity was decreased and urine volume was increased.
Glucosuria and occult blood were present in one 12.5 and two 25 mg/kg
bw/day animals. Gross and histopathological findings included
increased kidney and liver weights, pale renal cortices, and proximal
renal tubular necrosis in all treated groups (Robbins et al.,
1976c).
Groups of beagle dogs (4/sex/group) were administered oral doses
of 0, 2, 10, or 60 mg/kg bw/day gentamicin in capsules for 14 weeks.
The highest level was increased to 120 mg/kg bw/day after 2 months.
Emesis and diarrhoea were observed occasionally in treated dogs.
There were no effects on body weight, ophthalmology, haematology,
blood chemistry or urinalysis. The only postmortem change was
interstitial nephritis observed in 2 animals in the high-dose group.
The NOEL was 10 mg/kg bw/day (Vymetal et al., 1970).
Beagle dogs (controls: 2/sex; treated: 4/sex/dose) were injected
i.m. with doses of 0, 3, 5, or 8 mg/kg bw/day gentamicin in an aqueous
vehicle. Animals were treated 5 days per week for up to 12 months.
One dog per sex per group was scheduled for an interim kill after 6
months. Localized pain and transient lameness of the injected leg
were seen in all groups. Ophthalmological and physical examination
findings were within normal limits. Vomiting and profuse salivation
were noted in one animal in the 8 mg/kg bw/day group in weeks 23 and
24. This animal lost considerable body weight during the last 8 weeks
of treatment and had a slightly increased BUN, glucosuria, decreased
urine specific gravity and increased urine volume. Body weight,
haematology, blood chemistry and urinalysis were within normal limits
in all other dogs.
Pale renal corticies and interstitial nephritis were seen after
gross and histopathological examinations in all treatment groups with
a dose-response relationship. Organ weights were similar in all
groups. Inflammatory changes, haemorrhage and fibrosis at the
injection sites were present in all groups, with an increase in
severity in treated animals (Robbins et al., 1970b).
2.2.2.4 Monkeys
Groups of 4 squirrel monkeys (sex not specified) were
administered daily s.c. injections of 0, 25, 50 or 75 mg/kg bw
gentamicin in saline, for up to 3 weeks. All monkeys receiving
gentamicin (except one animal in the low dose group) had to be killed
intercurrently because of their moribund condition. The average
survival times were 15.3, 10.3 and 7.5 days at 25, 50 and 75 mg/kg
bw/day, respectively. Clinical signs included ataxia, weakness,
hypoactivity, irregular respiration and eye scratching at each dose
level. Sporadic convulsions occurred at 50 and 75 mg/kg bw/day.
Body-weight loss was recorded in all groups, including controls, which
appeared to be enhanced with gentamicin treatment. However, there was
no clear, dose-response relationship.
Post-injection acoustic reflex measurements could not be obtained
from the 75 mg/kg bw/day group due to their rapid decline in
condition. In addition, instrument failure resulted in the loss of 3
post-injection data points. Therefore, evaluations were performed on
a total of 14 monkeys out of 28. Results indicated that hearing
deficits occurred in both the low- and mid-dose groups. Apart from the
single low-dose survivor, all animals had increased serum creatinine
and BUN. The author stated that the majority of dosed animals showed
severe renal tubular necrosis. Individual animal data were not
provided (Igarashi et al., undated).
Groups of 3 female Rhesus monkeys were injected i.m. with doses
of 0, 6 or 30 mg/kg bw/day gentamicin in an aqueous vehicle for 3
weeks. Adverse clinical signs were limited to the 30 mg/kg bw/day
group, which included pronounced facial paling and ptosis, markedly
disturbed equilibrium from day 20, and depressed food intake and body-
weight gain from week 2 onwards. Two monkeys in this group were
killed because of their moribund condition on day 21.
Haematological examinations showed decreases in haemoglobin,
haematocrit, and erythrocytes at 30 mg/kg bw/day. Serum chemistry
findings included increases in SGPT, SGOT and LDH, and decreases in
serum potassium, sodium, chloride and calcium ions in the 30 mg/kg
bw/day dose group. Serum creatinine and BUN were increased in both
dose groups. Urinalyses revealed proteinuria, glucosuria and an
increased presence of casts and epithelial cells at 30 mg/kg bw/day.
At necropsy, kidney weights were increased and had a yellow-grey
appearance. Histologically, cloudy swelling, cortical striation,
degeneration and necrosis of proximal convoluted tubules were
reported. The renal lesions which were seen in both dose groups, were
considered to be treatment-related. Vacuolar degeneration of
hepatocytes was noted at 30 mg/kg bw/day. Injection sites showed
dose-related muscle degeneration and necrosis accompanied by
haemorrhage and cellular infiltration. Electron microscopy of renal
tubules from the 30 mg/kg bw/day monkeys revealed myeloid bodies
present in both tubular cells and lumen, increased phagosomes,
disappearance of brush borders and sloughing of epithelial cells from
the basement membrane (Tanaka et al., 1977).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
Groups of Carworth CFE rats (30/sex/group) were injected 6 days/
week with i.m. doses of 0, 5, or 20 mg/kg bw/day gentamicin in saline.
Males were injected from 70 days prior to mating until 7 days before
mating. Females in the 5 mg/kg bw/day group were injected from 14
days prior to pairing with a male until post partum day 21. Females
in the 20 mg/kg bw/day group were treated from the time of
impregnation (determined by vaginal smear) until post partum day 21.
Ten females per group were killed for litter and dam evaluations on
gestation day 21, the remainder were allowed to deliver normally. F1
offspring were not treated. Groups of 10 males and 10 females from
randomly selected litters were paired within treatment levels
(avoiding brother-sister mating) to produce an F2 generation.
F1 Dams were allowed to deliver normally.
After 13 doses, penile bleeding was observed in high-dose F0
males. Because of this finding, the dose was reduced to 15 mg/kg
bw/day after which bleeding ceased. In F0 males of the high dose,
body-weight gain was slightly lower during the second half of the
treatment period. There were no overt effects in females.
Pregnancy rates were not affected. There was no prenatal
mortality and litter sizes and weights were similar between groups.
Early postnatal mortality was increased at both doses, which was
attributed to maternal neglect and failure to lactate, neither of
which were considered treatment-related. Fetal abnormalities were not
observed in delivered pups or caesarian-derived fetuses. Gentamicin
was present in the amniotic fluid in 5/10 low-dose dams, and 8/10
high-dose dams but was not detected in homogenized fetuses. No
treatment-related changes in pregnancy rate, litter size and weight,
prenatal mortality or fetal abnormalities were reported in this study
(Robbins et al., 1966).
2.2.4 Special studies on embryotoxicity and teratogenicity
2.2.4.1 Mice
Female JCL-ICR mice (15-33/group) received s.c. injections of
gentamicin in saline during gestation days 6 to 10. The doses
administered were 0, 1, 10 or 100 mg/kg bw/day. In the high-dose
group, body-weight gain was depressed during treatment and kidney
weight was increased. Gentamicin was measured in amniotic fluid in
the high-dose group. It was not clear whether attempts were made to
measure gentamicin in amniotic fluid in the other two dose groups. In
five control and five 100 mg/kg bw/day dams, delivery of fetuses was
allowed to occur normally. The development of eye and ear openings
was unaffected and kidney and liver weights were similar between
groups at post partum day 35. The remaining females were killed on
gestation day 17 for dam and litter evaluations. Prenatal loss was
increased at 10 and 100 mg/kg bw/day. There were no treatment-related
increases in fetal abnormalities (Nishio et al., 1987).
2.2.4.2 Rats
Female Carworth CFE rats (40/group) were injected i.m. with
gentamicin in saline on gestation days 3 to 16. The doses
administered were 0, 25, or 50 mg/kg bw/day, 6 days per week. Females
were allowed to deliver normally. At least 10 days after weaning the
first litter, dams were bred again and the above procedures were
repeated. Adult females showed no clinical signs of toxicity. Body-
weight gain was lower in the 50 mg/kg bw/day group during the
treatment period. At necropsy, pale renal cortices were observed in
both dose groups. Resorption rates were comparable to control values.
The number of stillborn pups was slightly increased at 50 mg/kg
bw/day following the second series of matings. Offspring were killed
and examined 21 days post partum. No treatment-related abnormalities
were reported (Eggert & Haring, 1964).
Groups of 17 female Sprague-Dawley rats were given intra-muscular
injections of 0 or 75 mg/kg bw/day gentamicin in saline. Treatment
was on gestation days 10 to parturition. Dams were allowed to deliver
normally. Parturition was delayed in gentamicin-treated dams by an
average of 15 hours and birth weights were lower. In pups showing the
lowest birth weights, kidney weight and the number of nephrons were
reduced at birth and at 14 and 21 days post partum. Pups with higher
birth weight showed similar kidney weight and numbers of nephrons to
controls; however, a relative decrease in the number of nephrons was
reported in these animals at 14 and 21 days post partum. Necrosis was
not reported but vacuoles in proximal tubular epithelium were
increased with loss of brush border microvilli and the presence of
debris in the tubular lumen. Gentamicin was detected in the kidneys
of all pups born to treated mothers, the levels being higher in pups
showing the lowest birth weights.
In 7 litters per group, half the pups received subcutaneous doses
of 0 or 75 mg/kg bw/day of gentamicin on post partum days 1 to 13,
then killed on post partum day 14. Drug treatment caused an increase
in kidney weight but the number of glomeruli were similar in both
groups (Gilbert et al., 1987).
In an abstract, it was reported that rats were given s.c. doses
of 0 or 110 mg/kg bw/day gentamicin on either gestation days 10 to 15
or 15 to 20. Females were allowed to deliver normally. Measurements
of arterial blood pressure in offspring, one year after birth,
revealed significantly elevated blood pressure in females but not in
males (Chahoud et al., 1986).
2.2.4.3 Guinea-pigs
Guinea-pigs (6 females per group) were administered i.m.
injections of 0 or 4 mg/kg bw/day gentamicin in saline on gestation
days 48 to 54. Females were allowed to deliver normally. Litter size
and weight and body and kidney weights of offspring from the treated
group were comparable to controls to day 20 post partum. Studies of
kidney function in offspring showed a higher excretion of phosphate in
3-day-old pups but not at 10 days of age. Excretion of sodium,
potassium, magnesium and calcium, urinary flow rate, glomerular
filtration rate and the numbers of glomeruli were unaffected. The
length of proximal tubules and glomerular volume were reduced at 3 and
10 days post partum but not at 20 days. Gentamicin was present in pup
renal cortex at higher levels that in the medulla. Levels were
highest 3 days after birth and decreased thereafter (Le Lievre
et al., 1987).
2.2.4.4 Rabbits
Groups of 13 female New Zealand white rabbits were administered
i.m. doses of 0, 0.8, or 4 mg/kg bw/day gentamicin in saline. The
high dose was given as two divided doses, 7 hours apart. Treatments
were administered on gestation days 6 to 16. Eight females per group
were killed for dam and litter evaluations on gestation day 30. The
remainder were allowed to deliver normally. Pups were killed and
examined on post partum day 21. Adult animals were in good health
throughout the study. No treatment-related effects were noted on
prenatal loss, litter size or weight, fetal morphology, or postnatal
survival or body-weight gain. (Robbins & Klein, 1966).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies on gentamicin are summarized
in Table 2.
2.2.6 Special studies on kidney function
2.2.6.1 Rats
A group of 6 male Sprague-Dawley rats was injected i.m. with
doses of 80 to 160 mg/kg bw/day gentamicin for 7 days. At
termination, animals were anaesthetized with thiobarbital and kidney
function was assessed. The glomerular filtration rate, as measured by
inulin clearance, was reduced in a dose-related manner in all
treatment groups. Aciduria and glucosuria (determined by Labstix)
were induced at 160 mg/kg bw/day, but not at the lower doses,
indicating that reabsorption of glucose in the proximal renal tubule
had been impaired (Chiu et al., 1977).
Sprague-Dawley rats (group sizes not given) were injected s.c.
with 0 or 100 mg/kg bw/day gentamicin for up to 7 days. After four
doses, serum creatinine and BUN were increased and urine osmolality
was decreased, suggesting impairment of urine concentrating capacity.
Tubular reabsorption of solute free water was unaffected. Tubular
secretion of para-aminohippurate (PAH) was increased, although
glomerular filtration rate was decreased, after 4 days. Kidney
cortical slices, isolated from rats treated with gentamicin,
accumulated PAH in vitro at a faster rate than slices from control
animals; this effect was inhibited in the presence of probenecid. This
provided evidence that an alteration in the organic acid transport
system was responsible for the increased rate of PAH accumulation
(Cohen et al., 1974).
Female Sprague-Dawley rats (group sizes not given) were injected
s.c. with 0, 50, 100, or 150 mg/kg bw/day gentamicin for up to 14
days. During the treatment period, urine osmolality was decreased
followed by a rise in urine volume and water intake. Proteinuria was
seen from day 3 and serum creatinine and BUN were increased at 100 and
150 mg/kg bw/day. At termination, microscopic examination of kidneys
revealed dose-related injury to proximal tubules in all dose groups,
consisting of increased lysosomal bodies, disruption of brush borders,
cellular swelling and necrosis. Kidney cortical slices, from rats
treated with gentamicin, showed increased uptake of PAH in vitro
(Kaloyanides, 1977).
Sprague-Dawley rats (group sizes not given) were injected i.p.
with 0 or 10 mg/kg bw/day gentamicin for up to 14 days. Kidney
function was unaffected throughout the treatment period. Animals were
killed after 1, 4, 7 and 14 doses and kidneys removed for examination.
Microscopic examinations found proliferation of lysosomes in proximal
tubular cells on day 4. This was associated with decreases in
lysosomal sphingomyelinase and phospholipase activities, suggesting
lysosomal dysfunction. At day 9, protrusion of cells and/or nuclei
into tubular lumens was observed and several cells had lost part of
their brush borders. Focal necrosis was evident on day 14 (Carlier
et al., 1982).
2.2.6.2 Dogs
Male beagle dogs (group sizes not given) were injected i.m. with
0 or 30 mg/kg bw/day gentamicin for up to 11 days. Serum creatinine,
BUN, and urine protein were increased, and creatinine clearance and
urine osmolality were decreased in gentamicin-treated animals. In
addition, urinary levels of beta-glucuronidase, N-acetyl-beta-
glucosaminidase and muramidase were elevated. Microscopic renal
lesions ranged from diffuse moderate hyaline droplet degeneration to
frank tubular necrosis. The pathological and enzyme changes appeared
to precede the functional alterations (Adelman et al., undated).
2.2.7 Special study on ototoxicity
2.2.7.1 Monkeys
Groups of 3 female Cynomolgus monkeys were injected i.m. with
doses of 0, 25, or 50 mg/kg bw/day gentamicin for 35 days. All monkeys
dosed at 50 mg/kg bw/day died: one on day 7 and the other two on day
22. Prior to death, the pinna reflex to high frequencies in these
monkeys was slightly reduced, but there was no observable ataxia
indicative of vestibular impairment. Histopathological examination of
the crista ampullaris showed vacuole formation at both doses and, at
the higher dose, hair cell loss and a reduction in the thickness of
sensory epithelium. In the organ of Corti, cochlear hair cell loss was
noted at 50 mg/kg bw/day while the histology of the macula was not
significantly affected (Yakota et al., 1984).
2.2.8 Special study on neurotoxicity
Gentamicin was administered parenterally to Charles River CD
rats, cats and beagle dogs, to assess its neurotoxicity potential.
The treatment regimes were as follows:
Rats - 0, 6.6, 16.6, 41.5 or 104 mg/kg bw/day, s.c., for
41 days.
Cats - 0, 2.5, 5.0 or 10.0 mg/kg bw/day, s.c., for 42 days.
Dogs - 0, 5.0, 8.3 or 41.5 mg/kg bw/day, i.m., for 49 days.
In rats, righting reflex was impaired at 104 mg/kg bw/day and
ataxia was observed in dogs given 8.3 and 41.5 mg/kg bw/day. Ataxia
was not produced in cats at any dose (Taber et al., 1963).
2.2.9 Special studies on microbiological effects
Results of investigations into the effects of gentamicin on human
intestinal flora were not available. However, two published reports
were available on the inhibitory activity of gentamicin against a wide
range of bacterial strains in vitro.
Table 2. Results of genotoxicity studies on gentamicin
Test system Test object Results Reference
Reverse mutation1 S. typhimurium negative2 Houk et al., 1989
Reverse mutation1 S. typhimurium negative3 Koeda & Hirano, 1979
TA98, TA100,
TA1535, TA1536,
TA1537, TA1538
Reverse mutation1 S. typhimurium negative2 Mitchell et al., 1980
TA98, TA100,
E. coli WP2
Reverse mutation1 E. coli CM891 negative3 Mitchell & Gilbert, 1984
Forward mutation1 E. coli CM891 positive3 at a Mitchell & Gilbert, 1984
cytotoxic level
(0.25 µg/ml)
Forward mutation1 E. coli WP2 positive2 at a Mitchell et al., 1980
cytotoxic level
(0.49 µg/ml)
Mitotic crossing- S. cerevisiae D5 negative3 Koeda & Hirano, 1979
over1
Gene conversion1 S. cerevisiae D4 negative3 Mitchell et al., 1980
Petite inducation1 S. cerevisiae D4 positive3 (500 Mitchell et al., 1980
µg/ml) at pH4.4,
not pH 7.2
DNA repair1 E. coli WP2, negative2 Mitchell et al., 1980
WP100
Rec-assay1 B. subtilis negative3 Kada et al., 1980
M45, H17
SOS function1 E. coli PQ37 negative2 Mamber et al., 1986
DNA-cell binding E. coli Q13 positive2 Kubinski et al., 1981
(20 µg/ml) test
not validated
Table 2 (contd)
Test system Test object Results Reference
Chromosome Mouse L-cells positive3 but Leonard & Botis, 1975
aberrations mitosis inhibited
(500 µg/ml x 4 d
plus 100 µg/ml x
20 d)
SCE Human fibroblasts positive3 but McDaniel & Schultz, 1993
GM00847 less than doubling
(75-250 µg/ml)
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
3 Without metabolic activation.
One article reported the minimum inhibitory concentrations (MICs)
for gentamicin in 601 clinical isolates of anaerobic bacteria. The
experiments were conducted using the agar-dilution technique at 37°C
for 48 hours in an anaerobic incubator, an inoculum of 103 to 104
colony-forming units and serial concentrations of gentamicin in the
range of 0.1 to 25 µg/ml (Martin et al., 1972). In a second
publication, results were reported for Clostridium difficile
strains, but there was no information on the culture conditions
employed (Lorian, undated). All results are presented in Table 3.
The susceptibility to gentamicin of most anaerobic bacteria was
low, the most sensitive being Eubacterium sp., with a modal MIC
value of 0.8 µg/ml for the 20 strains tested.
2.3 Observations in humans
In a prospective, randomized, blinded, comparative trial, 54
patients treated with gentamicin were evaluated for nephrotoxicity and
ototoxicity. The dosage regime was usually 1.7 mg/kg bw i.v. every 8
hours (5.1 mg/kg bw/day) for 7 to 54 days. Desired serum drug levels
were maintained by reducing the dose in patients with impaired renal
function. Nephrotoxicity, defined as a 50% increase in serum
creatinine, was produced in 8 (15%) patients. Auditory toxicity,
defined as a decrease in the sensorineural threshold of at least 15
decibels in two or more frequencies, was produced in 3 (6%) patients.
Vestibular toxicity, defined as at least a 50% decrease in maximum
average slow-phase eye speed following water stimulation of the ear,
was induced in 3 of 33 (9%) patients. Auditory and vestibular effects
were not noted in the same individuals (Lerner et al., 1986).
Kidney biopsies were obtained from 5 patients with renal cancer
following surgical removal of both kidneys. Prior to surgery,
gentamicin was administered at a dosage of 1.5 mg/kg bw three times a
day (4.5 mg/kg bw/day) for 4 days. Microscopic evaluation of the
kidney specimens revealed increases in lysosome numbers with moderate
to marked decreases in the activity of sphingomyelinase and acid
phospholipase A1 (Carlier et al., 1982).
In a retrospective study involving patients on chronic
haemodialysis therapy, 23 patients were treated with gentamicin.
Dosages were not identified. Of the drug-treated subjects, 7 (30%)
developed clinically detectable vestibular toxicity. The principal
risk factors for the development of ototoxicity were age, duration of
treatment and the total dose administered. There was no positive
association with peak serum levels of gentamicin or the total absence
of kidneys (Gailiunas Jr. et al., 1978).
A review, focusing mainly on prospective trials, reported on a
total of more than 10 000 patients treated with aminoglycoside
antibiotics. The incidence of cochlear toxicity associated with
gentamicin administration ranged from 5 to 10% in adults. The
incidence was claimed to be lower in infants (Matz, 1993).
Adverse reactions to gentamicin during the years 1963 to 1973
were reviewed. Of the 3500 patients studied, the main non-specific
reactions were increases in serum transaminases (16), skin rashes (16)
and granulocytopaenia (8). Also presented were results from clinical
trials with gentamicin. Possible or probable nephrotoxicity was found
in 22 of 285 (7.7%) patients in 1965 to 1966, 70 of 1450 (4.8%)
patients in 1967 to 1969 and 48 of 1635 (2.9%) patients in 1970 to
1973. Possible or probable ototoxicity was found in 16 of 565 (2.8%)
patients in 1963 to 1966, 27 of 1450 (1.8%) patients in 1967 to 1969
and 15 of 1635 (1%) patients in 1970 to 1973. Critical doses or
duration of treatment were not given, but ototoxicity usually occurred
in association with impaired renal function (Hewitt, 1974).
The rates of allergic cutaneous reactions were determined in 15
438 consecutive in-patients monitored by the Boston Collaborative Drug
Surveillance Program from June 1975 to June 1982. Of the 670 patients
receiving gentamicin, there were 3 (0.5%) skin reactions.
Table 3. Minimum inhibitory concentration (MIC) values for gentamicin against anaerobic bacteria
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Bacteroides fragilis (195) 1 6
B. incommunio (10) 10 40
B. variabilis (5) 60
B. oralis (2) 50 100
B. terebrans (5) 40 60
B. melaninogenicus (29) 24 31 34 45 59 76
Bacteroides F1,F2,F3 (11) 27 36 45 73
Fusobacterium fusiforme (18) 5 11 56
Fusobacterium sp. (2) 50
Clostridium perfringens (34) 6
C. difficile (15) 20 40
Clostridium sp. (17) 6 18
Peptococcus sp. (145) 2 3 5 7 11 30 62 95
Peptostreptococcus sp. (72) 1 9 13 18 20 33 58 78
Veillonella sp. (13) 8 31 46 85
Table 3 (contd)
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Propionibacterium acnes (16) 25 56 75 81
Eubacterium lentum (14) 43 57 71 85 93 100
E. alactolyticum (2) 50
Eubacterium sp. (5) 20 40
Bifidobacterium sp. (5) 20 60 80 100
Catenabacterium (3) 66
filamentosum
Catenabacterium sp. (2) 50 100
3. COMMENTS
A range of studies on gentamicin was submitted for assessment,
including information on pharmacokinetics and metabolism, acute
toxicity, short-term toxicity, reproductive toxicity and
teratogenicity, genotoxicity, antimicrobial activity, a variety of
special studies and observations in humans.
Gentamicin, in common with other aminoglycosidic drugs, is poorly
absorbed from the gastrointestinal tract. Parenteral doses distribute
mainly into extracellular space, but there is significant penetration
into the kidney cortex and the inner ear. There is negligible
metabolism of the drug and unchanged gentamicin is excreted rapidly,
mostly in the urine.
Single oral doses of gentamicin were slightly toxic in rodents
(LD50 = 8000 to 10 000 mg/kg bw), which supports the view that the
drug is largely unabsorbed when administered by this route. Parenteral
dosing of mice, rats, guinea-pigs and dogs demonstrated high
intravenous toxicity (LD50 = 37 to 67 mg/kg bw) and moderate toxicity
by i.m., s.c. and i.p. routes (LD50 = 213 to 893 mg/kg bw).
Gentamicin was extensively tested by the i.m. and s.c. routes in
rats administered the compound for periods of up to 12 months (doses
up to 200 mg/kg bw/day) in dogs for up to 12 months (doses up to 100
mg/kg bw/day) and in monkeys for 3 weeks (doses up to 75 mg/kg
bw/day). The kidney was the primary target organ in each species with
effects observed predominantly in the renal cortex. The major
findings were an increase in interstitial nephritis and toxic
nephrosis in the proximal convoluted tubules. The latter was
characterized by a dose- and time-dependent progression from loss of
brush borders, cloudy swelling, the presence of casts and
proteinaceous material in the tubules, desquamation of epithelial
cells and necrosis. These changes were associated with a profound
impairment of renal function, and in extreme cases, death of severely
affected animals. Special studies in rats suggested that the
nephrotoxicity may be associated with disruption of lysosomal
function. Nephrotoxicity has been observed in humans treated with
gentamicin.
Toxicity to the inner ear may occur after exposure to
aminoglycosides, including gentamicin. There were no clear
indications of ototoxicity in the general toxicity studies; however,
a special study in monkeys, at doses up to 50 mg/kg bw/day, showed
hair cell loss and reduced sensory epithelium in structures of the ear
as well as a functional loss in hearing. Observations in humans have
revealed auditory or vestibular toxicity after therapeutic
administration of gentamicin.
Oral dosing of rats and dogs for a period of 3 months, at doses
up to 116 and 60 mg/kg bw/day, respectively, was virtually without
systemic effects. Soft stools or diarrhoea were observed in both
species and dogs showed interstitial nephritis at high doses. The
NOELs were 19 mg/kg bw/day in rats and 10 mg/kg bw/day in dogs.
A multigeneration reproductive toxicity study in the rat showed
no adverse effects on reproduction parameters after intramuscular
injections of 5 or 20 mg gentamicin/kg bw/day. Teratogenicity studies
in mice, rats, guinea-pigs and rabbits did not identify any potential
for the production of fetal abnormalities. Fetotoxicity, in the form
of fetal death in mice (10 mg/kg bw/day) and rats (50 mg/kg bw/day)
and reduced birth weight in rats (75 mg/kg bw/day), was observed after
parenteral dosing.
Positive results were obtained in some in vitro genotoxicity
studies, but none of these was adequate to evaluate the genotoxicity
of gentamicin. There were no carcinogenicity studies available on
gentamicin. In toxicity studies in animals of up to one year
duration, there were no pre-neoplastic or neoplastic lesions. The
Committee considered that the data were inadequate to fully assess the
carcinogenicity of gentamicin.
Data on the effects of gentamicin on human gut flora were not
available. However, in vitro data were presented on MICs for over
600 clinical isolates of anaerobic bacteria including some species
representative of the microbial flora in the human gastrointestinal
tract. Many of the organisms were found to be resistant to gentamicin;
Eubacterium species were the most sensitive. Using an inoculum
density of 103 to 104 colony forming units per plate, MIC values for
Eubacterium species were in the range 0.1 to 25 µg/ml with an MIC50
of 0.8 µg/ml.
In view of the poor oral absorption of gentamicin and the
reported occurrence of diarrhoea in experimental animals, it was
considered that effects on the gastrointestinal microflora would be
the most sensitive effect of residues. In calculating the ADI, the
formula developed by the thirty-eighth meeting of the Committee (Annex
1, reference 97) was employed:
Concentration without effect x Daily faecal bolus (g)
on human gut flsor (µg/ml)a
Upper limit of ADI =
Fraction of oral x Safety x Human body
dose availableb factorc weight
(60 kg)
(0.8 x 2) x 150
=
1 x 1 x 60
= 4 µg/kg bw
a This value accounts for the range of MICs in appropriate
bacterial species and the use of the most sensitive anaerobic
organism. A two-fold adjustment was deemed to be necessary to
account for the relatively low inoculum density.
b Absorption of gentamicin after oral dosing is very poor, therefore
a factor of 1 was used to represent 100% availability of ingested
drug in the gastrointestinal tract.
c Data on more than 600 clinical isolates of anaerobic bacteria were
available for determination of the MIC. Because the colonic flora
is relatively stable and variability within a particular individual
may be as great as variability between individuals, and because it
was recognized that other values selected for this calculation were
conservative and already incorporated an adequate margin of safety,
a safety factor of 1 was selected to cover fully the variability
between people of all extrapolated parameters.
4. EVALUATION
In view of the absence of information on the effects of
gentamicin on microorganisms obtained from the human intestine and the
need for further genotoxicity studies, the Committee established a
temporary ADI of 0-4 µg/kg bw based on the results of micro-biological
testing of clinical isolates, with a request for further information.
An increased safety factor was not used to account for the temporary
status of the ADI because conservative factors were used in deriving
the temporary ADI. The lowest toxicological NOEL was 10 mg/kg bw/day
in dogs, which is more than 2000-fold higher than the
microbiologically based ADI.
5. REFERENCES
ADELMAN, R.D., CONZELMAN, G., SPANGLER, W. & ISHIZAKI, G. (undated).
Comparative nephrotoxicity of gentamicin and netilmicin : functional
and morphological correlations with urinary enzyme activities.
Unpublished Report No. D 11403. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ATEF, M., ARBID, M.S. & HANAFY, M.S.M. (1992). Comparison of
gentamicin toxicity in normal and diabetic rats. Acta Veterinaria
Hungarica, 40: 107-111.
BIGBY, M., JICK, S., JICK, H. & ARNDT, K. (1986). Drug-induced
cutaneous reactions. JAMA, 256: 3358-3363.
CARLIER, M.B., PAULUS, G., MALDAGUE, P., GIURGEA, L., WILMOTTE, E., DE
BROC, M.E. & TULKENS, P. (1982). Early toxic events in kidney of rat
and man following administration of gentamicin at low doses.
Arch. Toxicol. Suppl. 5: 287-290.
CHAHOUD, I., STAHLMANN, R. & NEUBERT, D. (1986). Blood pressure
elevation in one-year-old female rats after prenatal exposure to
gentamicin. Teratology, 33: 13A.
CHIU, P.J.S., LONG, J.F., BARNETT, A., TABER, R.I., MILLER, G. &
WAITZ, J.A. (1977). Kidney function in rats chronically treated with
aminoglycosides. Unpublished Report No. P 4485. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
COHEN, L., LAPKIN, R. & KALOYANIDES, G.J. (1974). Effect of gentamicin
on renal function in the rat. Unpublished Report No. D 6338. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
EGGERT, M.J., DAVENPORT, L.R. & WILLIAMSON, N.K. (1973). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3379. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., EVANS, M. & WILLIAMSON, N.K. (1963a). SCH 9724 in rats.
Subacute (4 weeks) intramuscular toxicity study. Unpublished Report
No: P 3355. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J. & HARING, G.D. (1964). Intramuscular teratogenic study of
SCH 9724 (gentamicin sulfate 70:30) in rats. Unpublished Report No: P
3397. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., HARING, G.D. & VYMETAL, F. (1965). Acute toxicity
studies of gentamicin sulfate intramuscularly in rats and dogs.
Unpublished Report No: P 3502. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., HARING, G.D., WILLIAMSON, N.K. & WEINSTEIN, M.J. (1975).
Acute and subacute toxicity studies of SCH 9724 (gentamicin sulfate)
in rat neonates. Unpublished Report No: P 3497. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1963b).
Gentamicin sulfate-90 day dermal toxicity in rabbits. Unpublished
Report No: P 3410. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1964).
Dermal toxicity of SCH 9724 in rabbits. Unpublished Report No: P 3425.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., VYMETAL, F. & WILLIAMSON, N.K. (1963c). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3350. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
FRASER, C.M., BERGERON, J.A., MAYS, A. & AIELLO, S.E. (1991).
Aminoglycosides. In: The Merck Veterinary Manual, 7th Edition ,
Merck & Co., Rahway, pp 1429-1435.
GAILIUNAS JR., P., DOMINGUEZ-MORENO, M., LAZARUS, J.M., LOWRIE, G.,
GOTTLIEB, M.N. & MERRILL, P. (1978). Vestibular toxicity of
gentamicin. Arch. Intern. Med., 138: 1621-1624.
GILBERT, T., LELIEVRE-PEGORIER, M., MALIENOU, R., MEULEMANS, A. &
MERLET-BENICHOU, C. (1987). Effects of prenatal and postnatal exposure
to gentamicin on renal differentiation in the rat. Toxicology, 43:
301-313.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977a).
Safety evaluation of KW-1062. I. Acute toxicity in mice, rats and
dogs, subacute and chronic toxicity in rats. Jap. J. Antibiotics ,
30: 386-407.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977b).
Safety evaluation of KW-1062. II. Subacute and chronic toxicity
studies in dogs. Jap. J. Antibiotics, 30: 408-422.
HEWITT, W.L. (1974). Gentamicin: toxicity in perspective. Postgrad.
Med. J., (Suppl. 7): 55-59.
HOUK, V.S., SCHALKOWSKY, S. & CLAXTON, L.D. (1989). Development and
validation of the spiral Salmonella assay: an automated approach to
bacterial mutagenicity testing. Mutation Res., 223: 49-64.
IGARASHI, M., LEVY, J.K., JERGER, J., WAITZ, J.A. & ARCIERI, G.
(undated). Comparative toxicity of netilmicin and gentamicin in
squirrel monkeys. Unpublished Draft Report No: D 10312. Submitted to
WHO by Inveresk Research International, Trenant, Scotland, on behalf
of Schering Plough Animal Health.
KADA, T., HIRANO, K. & SHIRASU, Y. (1980). Screening of environmental
chemical mutagens by the Rec-assay with Bacillus subtilis. In:
Chemical Mutagens and Principles for their Detection. Chap. 6: pp
149-173.
KALOYANIDES, G.J. (1977). Untitled. Unpublished Report No: D 10069.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
KOEDA, T. & HIRANO, F. (1979). Evaluation of the mutagenicity of
aminoglycoside antibiotics in Salmonella typhimurium and
Saccharomyces cerevisiae. J. Antibiotics, 32: 607-609.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA cell-binding
assay for suspected carcinogens and mutagens. Mutation Res., 89:
95-136.
LELIEVRE, M., GILBERT, T., SAKLY, R., MEULEMANS, A. & MERLET-BENICHOU,
C. (1987). Effect of fetal exposure to gentamicin on kidneys of young
guinea pigs. Antimicrob. Agents Chemother., 31: 88-92.
LEONARD, A. & BOTIS, S. (1975). Chromosome damage induced by
gentamicin in mouse L-cells. Experientia, 31: 341-343.
LERNER, S.A., SCHMITT, B.A., SELIGSOHN, R. & MATZ, G.J. (1986).
Comparative study of ototoxicity and nephrotoxicity in patients
randomly assigned to treatment with amikacin or gentamicin.
Am. J. Med., 80(6B): 98-104.
LORIAN, V. (undated). Tables 28.13 and 28.15. In: Antibiotics in
Laboratory Medicine. 2nd Edition. Williams & Wilkins, Baltimore-
London-Los Angeles-Sydney, pp 1087-1089 and 1142-1146.
MAMBER, S.W., OKASINSKI, W.G., PINTER, C.D. & TUNAC, J.B. (1986). The
Escherichia coli K-12 SOS chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutation Res., 171: 83-90.
MARTIN, W.J., GARDNER, M. & WASHINGTON II, J.A. (1972). In vitro
antimicrobial susceptibility of anaerobic bacteria isolated from
clinical specimens. Antimicrob. Agents Chemother., 1: 148-158.
MATZ, G. (1993). Aminoglycoside cochlear ototoxicity. Otolaryngologic
Clinics of North America, 26: 705-712.
McCORMICK, G.C. (1983). Acute intravenous toxicity comparison of
gentamicin sulfate and gentamicin C2 sulfate in mice (SN 83272).
Unpublished Report No: D17472. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
McDANIEL, L.D. & SCHULTZ, R.A. (1993). Elevation of sister chromatid
exchange frequency in transformed human fibroblasts following exposure
to widely used aminoglycosides. Environ. Molec. Mutagenesis, 21:
67-72.
MILLER, G.H., WAITZ, J.A. & WEINSTEIN, M.J. (1976). Pharmacokinetics
of C14-gentamicin in dogs. Unpublished Report No: P 4440. Submitted
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
MITCHELL, I DEG., DIXON, P.A., GILBERT, P.J. & WHITE, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Mutation Res., 79:
91-105.
MITCHELL, I DEG. & GILBERT, P.J. (1984). The effect of pretreatment of
Escherichia coli CM 891 with ethylenediaminetetraacetate on
sensitivity to a variety of standard mutagens. Mutation Res., 140:
13-19.
NISHIO, A., RYO, S. & MIYAO, N. (1987). Effects of gentamicin,
exotoxin and their combination on pregnant mice. Bull. Fac. Agric.
Kagoshima Univ., 37: 129-136.
RIVIERE, J.E., CRAIGMILL, A.L. & SUNDLOF, S.F. (1991).
Aminoglycosides. In: Handbook of Comparative Pharmacokinetics and
Residues of Veterinary Antimicrobials, CRC Press, Boca Roton, Florida,
pp 263-335.
ROBBINS, G.R., CASTELLANO, R.F., TORNING, F.E., FABRY, A. & FIELDER,
F.G. (1971).- Acute toxicity in rats (intramuscular, intravenous and
intraperitoneal) and mice (intramuscular, intravenous, intraperitoneal
and subcutaneous). Unpublished Report No : P 3967. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., FABRY, A., MORGAN, W.A. & EGGERT, M.J. (1972a).
Subacute (two-week) intramuscular toxicity study of SCH 14342 in rats.
Unpublished Report No: P 4074. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1970a).
Chronic toxicity study of SCH 9724 by intramuscular injection in rats.
Unpublished Report No: P 3884. Submitted by Inveresk Research
International, Trenant, Scotland, to WHO on behalf of Schering Plough
Animal Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1972b).
Subacute (two-week) toxicity study of SCH 13706 by intramuscular
injection in rats. Unpublished Report No: P 4072. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R. & KLEIN, M.F. (1966). Reproduction study of gentamicin
sulfate in rabbits. Unpublished Report No : P 3595. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., KLEIN, M.F. & EGGERT, M.J. (1966). Reproduction study
using gentamicin sulfate intramuscularly in rats. Unpublished Report
No : P 3598. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970b).
Chronic (1-year) intramuscular toxicity study in dogs using gentamicin
sulfate (SCH 9724). Unpublished Report No: P 3848. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1972c).
Subacute (14-day) intramuscular toxicity study of SCH 13706 Gentamicin
C1 ) in dogs. Unpublished Report No: P 4065. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1973a).
Subacute intramuscular toxicity study of SCH 14342 as compared with
SCH 9724 in dogs. Unpublished Report No: P 4138. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H. & GRAY, W.D. (1976a). The subacute
toxicity of SCH 20569 sulfate, (vs gentamicin, tobramycin and
kanamycin) administered subcutaneously to rats. Unpublished Report No:
P 4424. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D. & FIELD, W.E. (1976b).
Subacute intramuscular toxicity of SCH 20569 in rats. Unpublished
Report No: P 4393. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D., FIELD, W.E. & SCHIAVO, D.M.
(1976c). Subacute intramuscular toxicity of SCH 20569 in dogs.
Unpublished Report No: P 4392. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R., FIELD, W.E. & OWEN, G.
(1973b). Subacute intramuscular toxicity in dogs using SCH 15666 and
SCH 9724. Unpublished Report No: P 4196. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R. & OWEN, G. (1973c). Acute
intramuscular toxicity study in guinea pigs. Unpublished Report No: P
4174. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., OWEN, G., MARCHESO, R. & FIELD, W.E.
(1973d). Subacute intramuscular toxicity in rats using SCH 15666 and
SCH 9724. Unpublished Report No: P 4212. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
SANDE, M.A. & MANDELL, G.L. (1990). Antimicrobial agents. The
aminoglycosides. In: GILMAN, A.G., RALL, T.W., NIES, A.S. & TAYLOR, P.
(eds), Goodman & Gilman's The Pharmacological Basis of Therapeutics,
8th Edition, Pergamon Press, New York-Oxford-Beijing-Sao Paulo-Sydney-
Tokyo-Toronto, pp 1098-1116.
TABER, R., IRWIN, S. & PERLMAN, P.L. (1963). The effect of subchronic
administration of gentamicin sulfate, kanamycin sulfate and
streptomycin sulfate in the rat, cat and dog. Unpublished Report No:
P 3316. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
TANAKA, H., WATANABE, M. & HATTORI, H. (1977). Subacute toxicity of
sisomycin administered intramuscularly in monkeys. Comparison with
gentamicin and tobramycin. Unpublished Report No: D 10555. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970). Subacute (14 week)
oral toxicity study of SCH 14894 (gentamicin sulfate, veterinary
grade) in dogs. Unpublished Report No: P 3923. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
VYMETAL, F., EGGERT, M.J. & ROBBINS, G.R. (1969). Toxicity study of
gentamicin sulfate by injection in neonatal dogs. Unpublished Report
No: P 3818. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
WOODARD, M.W., HOWARD, D.J., WOODARD, G. & EGGERT, M.J. (1970).
Gentamicin sulfate safety evaluation by repeated oral administration
to rats for 13 weeks. Unpublished Report No: P 3924. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
YAKOTA, M., TAKEDA, U., SAKAMOTO, K., SETO, N. & IGARASHI, M. (1984).
The comparative ototoxicities of panimycin and gentamicin in
cynomolgus monkeys (Maccaca fascicularis). Chemotherapy, 30:
248-254.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism and excretion
Few specific studies on the pharmacokinetics of gentamicin were
available, however, the aminoglycosides as a class have been
extensively reviewed. In view of their polar nature and high aqueous
solubility, aminoglycosides are essentially not absorbed after oral
administration. Absorption after intramuscular or subcutaneous
administration is good with peak blood levels occurring within 30 to
90 minutes of an intramuscular injection (Riviere et al., 1991;
Sande & Mandell, 1990).
The aminoglycosides distribute into the extracellular space with
minimal penetration into tissues other than the kidneys and the inner
ear. Plasma protein binding is reported to be less than 20%. Levels in
amniotic fluid and fetal tissue are very low in most species.
Aminoglycosides as a class are not metabolized and are eliminated
unchanged in the urine by glomerular filtration. Recovery of 80% to
90% of the administered dose can occur within 24 hours (Fraser
et al., 1991).
One beagle dog was given a single intravenous dose of radio-
labelled gentamicin sulfate. The apparent half-life of elimination
from serum was approximately 1 hour initially and approximately 2´
hours 8 and 10 hours after dosing. Distribution to organs and tissues
was extensive but exceptionally high levels were found in the kidney,
mainly in the cortex. A comparison of the levels of serum gentamicin
using radioassay, radioimmunoassay and microbiological assay were
similar, suggesting that there was little metabolism of the drug.
Excretion was primarily renal with some 70% recovered in the urine but
very little (1%) in the faeces. Approximately 2% of the dose remained
in tissues 8 days after dosing; the remainder was unaccounted for
(Miller et al., 1976).
2.2 Toxicological studies
Unless specified otherwise, toxicological studies were carried
out using the gentamicin sulfate salt and dose levels are expressed as
gentamicin base.
2.2.1 Acute toxicity studies
The results of acute toxicity studies with gentamicin are
summarized in Table 1.
Clinical signs of intoxication in rodents included convulsions,
prostration, hypoactivity, polydipsia, dyspnoea and ataxia. Dogs
exhibited muscle tremors, salivation, and anorexia. Histopathological
examination of kidneys from dogs that died up to 13 days after dosing
revealed necrosis of the proximal convoluted tubule.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 0 or 40 mg/kg bw/day gentamicin in saline were
administered i.p. to groups of 5 male albino rats. Treatment was for
10 consecutive days and animals were killed 48 hours after the last
dose. There were no reported overt signs of toxicity. Serum
chemistries at termination showed an increase in creatinine, BUN, and
SGPT. Pathology was restricted to kidneys and liver and included
degeneration and necrosis of renal tubular epithelium and moderate
vacuolization of hepatocytes. The author also noted that enhancement
of nephrotoxicity and hepatotoxicity occurs in alloxan-induced
diabetic rats administered gentamicin (Atef et al., 1992).
Groups of Charles River CD1 rats (15/sex/group) received i.m.
injections of gentamicin daily for 14 days. The drug was administered
in saline at dose levels of 0, 10, 25, 50, or 100 mg/kg bw/day. Overt
signs of toxicity were noted only in the highest-dose group, which
included reduced body-weight gain, polydipsia, hypoactivity, ataxia,
lethargy, dyspnoea, emaciation and prostration. A total of 7 rats in
this group died or were killed because of their moribund condition.
Clinical laboratory investigations showed indications of
treatment-related effects on renal function. Serum creatinine and BUN
were increased. Glucose and occult blood were present in the urine at
doses of 50 and 100 mg/kg bw/day. Decreased urine specific gravity and
increased urine volume were noted at 25 mg/kg bw/day and above. At
necropsy, renal cortical pallor was observed in all treated groups
with dose-related increases in incidence and severity. At 25 mg/kg and
above, kidney weights were increased as was the severity of renal
tubular nephrosis (Robbins et al., 1973d).
Neonatal Carworth CFE rats (10 litters per group, 10 pups/litter)
were administered i.m. injections of gentamicin for 14 days. The
doses were 0, 10, 25, 50 or 100 mg/kg bw/day in a water vehicle.
There were no clinical signs of toxicity, but body-weight gains were
depressed at all doses. Laboratory investigations were not
undertaken. At the end of the study, kidney weights were increased at
all doses. Dilation of renal pelves was observed in treated groups
but with no clear dose-response relationship. At 25 mg/kg bw/day and
above, pale renal cortices and dark red medullas were noted. Cloudy
swelling of renal tubules, the presence of proteinaceous material in
tubules, desquamation of epithelial cells and tubular necrosis
occurred in all dose groups, which were considered to be treatment-
related (Eggert et al., 1975).
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 10 000 Hara et al., 1977a
M i.v. 40 Robbins et al., 1971
M&F i.v. 51-57 Hara et al., 1977a
M&F i.v. 37-40 McCormick, 1983
M i.m. 213 Robbins et al., 1971
M&F i.m. 223-233 Hara et al., 1977a
M i.p. 232 Robbins et al., 1971
M&F i.p. 279-305 Hara et al., 1977a
M s.c. 228 Robbins et al., 1971
M&F s.c. 292-312 Hara et al., 1977a
Rat M oral 8000-10 000 Hara et al., 1977a
M i.v. 67 Robbins et al., 1971
M i.m. 384 Eggert et al., 1965
M i.m. 371 Robbins et al., 1971
M&F i.m. 489-511 Hara et al., 1977a
M i.p. 674 Robbins et al., 1971
M&F i.p. 478-559 Hara et al., 1977a
M&F s.c. 817-893 Hara et al., 1977a
Rat M&F i.m. 500 Eggert et al., 1975
(neonate) i.p. 508 Eggert et al., 1975
Guinea-pig M&F i.m. 320 Robbins et al., 1973c
Dog M&F i.m. 300-400 Eggert et al., 1975
Gentamicin B in an aqueous vehicle was administered i.m. to
groups of Carworth CFE rats (10/sex/group) for 14 days. Doses were 0,
50, 100, or 250 mg/kg bw/day. Polydipsia and ptosis were observed in
all treated rats. Body-weight gain was reduced at 250 mg/kg bw/day.
Haematology and urinalysis parameters and BUN of treated rats were
comparable to control values. At necropsy, haemorrhage and necrosis
at the injection site was noted in all rats in the high-dose group.
Renal cortical pallor and increased kidney weights were seen in all
treated groups. At 250 mg/kg bw/day degenerative changes in proximal
convoluted tubules were noted including swelling, granularity of
epithelial cells, casts, loss of brush borders and fat droplets in the
epithelium. Liver weights were decreased at 100 and 250 mg/kg bw/day
(Robbins et al., 1972a).
Carworth CFE rats (22/sex/group) were administered i.m.
injections of either gentamicin or gentamicin C1 at doses of 0, 10,
25 or 50 mg/kg bw/day. In each dose group, 2 males and 2 females were
killed on days 2, 4, 6, 8, 10, and 12, and 10 males and 10 females
were killed after 14 days. There was no treatment-related mortality in
any dose group. The only adverse effects noted in clinical parameters
were a slight increase in BUN and reduced body-weight gain in the 50
mg/kg bw/day group. Histopathological examinations revealed
haemorrhage and lymphocytic infiltration at the injection site, which
was seen in all treatment groups. Pale renal cortices were observed in
all dosed groups, which were more pronounced with increasing doses of
gentamicin. In the gentamicin-treated groups, lymphocytic infiltration
in the renal cortex was first noted in the 50 mg/kg bw/day dose group
after 5 doses and at lower doses thereafter. Proximal convoluted
tubule degeneration required 7 doses of 50 mg/kg bw/day. Gentamicin
C1 induced lymphocytic infiltration at 25 and 50 mg/kg after 14 days
only, but with no tubular degeneration, suggesting a lower potential
for renal toxicity than gentamicin (Robbins et al., 1972b).
Carworth CFE rats (10/sex/group) were administered i.m.
injections of gentamicin in saline, 6 days/week for 4 weeks. The doses
were 0, 25, 50, or 200 mg/kg bw/day. Overt signs of toxicity occurred
at 200 mg/kg bw/day, which included hypoactivity, bradypnoea,
bradycardia, and increased mortality. Body-weight gain was depressed
at 50 and 200 mg/kg bw/day. Haematological examinations revealed
slight depressions in haemoglobin and haematocrit at 200 mg/kg bw/day.
Serum chemistry and urinalyses were not performed. A limited necropsy
revealed pale renal cortices, increased kidney weights, renal tubular
necrosis, haemorrhage and inflammatory changes at the injection site,
and increased parenchymatous degeneration of the liver. These lesions
were found only at 200 mg/kg bw/day. The NOEL was 25 mg/kg bw/day
(Eggert et al., 1963a).
Charles River CD rats (5 males/group) received daily subcutaneous
injections of gentamicin in an aqueous vehicle. Doses of 0, 50, or
150 mg/kg bw/day were administered for 14, 21 or 28 days. At 150
mg/kg bw/day, food and water intake were reduced and rats exhibited
prostration, dyspnoea, and "staring coat". All animals at this dose
died during week 2. Body-weight gain was reduced in both dose groups,
and vestibular function was unimpaired. Serum chemistry analyses and
urinalysis revealed evidence of altered renal function at 50 mg/kg
bw/day in animals killed after 14 days of treatment, but not in those
killed at later times. The findings included increased serum
creatinine and BUN, decreased specific gravity and pH of urine,
glucosuria and a reduced clearance rate of endogenous creatinine.
Haematology parameters were not evaluated. At necropsy, pallor of the
renal cortex was increased in treated groups. Histological
examination, which was restricted to the kidneys, showed proximal
tubular necrosis in all gentamicin-treated rats, the severity being
related to dose (Robbins et al., 1976a).
Wistar rats (20/sex/group) were administered i.m. injections of
0, 25, 63, or 156 mg/kg bw/day gentamicin for 30 days. Groups of 5
rats/sex/dose were allowed a recovery (no treatment) period of 30
days. In the highest-dose group, decreased motor activity,
prostration, ptosis, piloerection, congested conjunctiva, lacrymation,
ataxia, and soft faeces were noted. Approximately half the high-dose
animals died within 12 days, the remainder were killed on day 12.
Body-weight gain and food consumption were depressed at all dose
levels.
Decreases in erythrocytes, haemoglobin, and haematocrit were
observed in the 63 mg/kg bw/day group. At 25 mg/kg bw/day and above,
leucocytes, BUN, cholesterol, albumin/globulin ratio and SGOT were
increased. At necropsy, swelling and discoloration of the kidney and
inflation of the caecum were noted at 63 mg/kg bw/day and above.
Degeneration of renal cortical tubules, tubular dilatation, protein
casts and infiltration of round cells in the interstitium were found
in all treated groups. At 156 mg/kg bw/day, necrosis of renal tubules,
hepatocyte vacuolation, and bone marrow hypoplasia were also observed.
Inflammatory cell infiltration, haemorrhage and edema at the injection
site were dose-related. All changes tended to show some reversal
following the 30-day recovery period (Hara et al., 1977a).
Groups of Charles River CD rats (20/sex/group) received daily
i.m. injections of gentamicin in an aqueous vehicle. Doses of 0,
12.5, or 50 mg/kg bw/day were administered for up to 13 weeks. After
4 weeks of treatment, 5 males and 5 females were killed and examined.
There were no clinical signs of toxicity apart from thinness and
locomotor ataxia in a 50 mg/kg bw/day male which was killed because of
its moribund condition on day 15. The same animal had severely
depressed haematocrit, haemoglobin and leucocyte counts. It is
unlikely that the effects seen in this animal were due to treatment
with the test article.
Food intake and body-weight gain were decreased in all treated
groups. Results of vestibular function tests and ophthalmological
evaluations were unremarkable. In the 50 mg/kg bw/day group,
haemoglobin and haematocrit were slightly decreased and BUN was
slightly increased. Urine specific gravity and pH were slightly
decreased at both treatment levels. Increases in kidney weights,
renal tubular necrosis, basophilia and leucocytic infiltration were
observed in both dose groups. Pale kidneys were reported in the 50
mg/kg bw/day group only. Injection site haemorrhages were also seen in
both dose groups (Robbins et al., 1976b).
Groups of Sprague-Dawley CD rats (10/sex/group) were fed
gentamicin in the diet for 13 weeks. The concentration in the diet was
adjusted as needed to maintain doses of 0, 4, 19 or 116 mg/kg bw/day.
The high-dose group was increased to 233 mg/kg bw/day from week 10.
No overt signs of toxicity were observed in any dose group.
Ophthalmologic evaluations, body-weight gain, food intake, and
mortality in treated groups were comparable to controls. Soft stools
were observed in the highest-dose group. Haematological parameters,
BUN, blood glucose and serum iron were unaffected by treatment.
Urinalyses showed an increased number of high-dose males with ketone
bodies in urine. At the end of the study, kidneys, liver, heart and
brain were weighed and gross and histopathological examinations were
performed on a wide range of tissues. No treatment-related effects
were noted. The NOEL was 19 mg/kg bw/day (Woodard et al., 1970).
Carworth CFE rats (controls: 20/sex; treated: 30/sex/group) were
administered i.m. injections of gentamicin in an aqueous vehicle, 5
days per week for up to 52 weeks. The doses were 0, 5, 10, or 20
mg/kg bw/day. At 26 weeks, 10/sex from the treatment groups and 5/sex
from the control group were killed and examined. The remainder were
killed at termination.
There were no clinical signs of toxicity or mortality. Body-
weight gain was depressed in males in the 20 mg/kg bw/day group from
week 10, although feed intake was comparable to controls. Haemoglobin
and haematocrits were slightly decreased in the mid- and high-dose
groups during the last half of the study. Leucocytes, blood glucose
and urinalysis parameters remained within normal limits.
At necropsy, liver, heart and brain weights were unaffected.
Adverse effects on the kidney included increased organ weight and
pitted or mottled surfaces at 10 and 20 mg/kg bw/day. The onset of
interstitial nephritis (seen commonly in aging rats) occurred earlier
in treated groups compared to controls. At 6 months, only one rat in
the control group was affected, while almost all rats in the high-dose
group exhibited this effect. At 12-months, the incidence was
statistically significant in the high-dose group only, with the
incidence in other groups not differing significantly from controls.
Injection-site reactions were observed in all dose groups with a dose-
related increase in severity. Muscle fibrosis was seen at the higher
treatment levels. The NOEL was 5 mg/kg bw/day (Robbins et al.,
1970a).
2.2.2.2 Rabbits
Two dermal studies were conducted in New Zealand white rabbits at
doses of 0, 0.5, 1, or 2 mg/kg bw/day gentamicin. Animals were
treated 6 hours/day, 5 days/week on intact and abraded skin for either
21 days (3 females per group) or 90 days (2 males and 1 female per
group). A different vehicle (unidentified) was used in each study.
Clinical signs, body weight, serum chemistry, haematology, urinalysis,
and gross and microscopic pathology were unaffected. Erythema and
edema at the application sites were slight in one study and moderate
in the other. These effects were considered to be vehicle-related
(Eggert et al., 1963b; 1964).
2.2.2.3 Dogs
Groups of beagle dogs (2/sex/group) were administered i.m.
injections of gentamicin or gentamicin C1 in an aqueous vehicle
(sterile water) daily for 14 consecutive days. The doses were 0, 15,
30, or 45 mg/kg bw/day.
There were no deaths or clinical signs of toxicity, nor were body
weight or ophthalmological parameters affected. Clinical chemistry
analyses showed that both drugs caused an increase in BUN at 45 mg/kg
bw/day. Other biochemical and haematological parameters remained
within normal limits. Urine specific gravity was lower and urine
volume increased in the 45 mg/kg bw/day gentamicin group. Occult blood
and albumin in urine were increased in treated groups, particularly in
the 45 mg gentamicin/kg bw/day group. One dog in this group also
showed glucosuria.
Gross examination of organs revealed pale and congested renal
cortices at 45 mg/kg bw/day. Kidney weights were increased in a dose-
related manner in all treated groups. Renal proximal convoluted
tubule degeneration or necrosis was increased with 30 and 45 mg/kg
bw/day gentamicin and 45 mg/kg bw/day gentamicin C1. Haemorrhage,
inflammatory changes and/or necrosis were observed at all injection
sites, including vehicle controls (Robbins et al., 1972c).
In another 14-day study, groups of beagle dogs (2/sex/group)
recieved i.m. injections of 0, 10, or 45 mg/kg bw/day gentamicin or
45, 90, or 120 mg/kg bw/day gentamicin B in an aqueous vehicle.
There were no clinical signs of toxicity. Food intake and body
weights were depressed at 45 mg/kg bw/day gentamicin and 120 mg/kg
bw/day gentamicin B. Three dogs given 45 mg gentamicin/kg bw/day were
killed on day 14 because of their moribund condition. Gentamicin-
induced changes in clinical laboratory investigations were recorded in
the 45 mg/kg bw/day group, which included increases in neutrophils,
BUN and SGPT; a slight decrease in urine specific gravity; and glucose
and occult blood in urine. Increases in SGPT were seen at all dose
levels of gentamicin B.
At necropsy, kidney and liver weights were unaffected by either
drug at any dose level. Pale renal cortices and increased lymphocytic
infiltration and coalescence of mitochondria in proximal convoluted
tubule epithelial cells were seen in all drug-treated groups.
Proximal tubular necrosis and excessive fat deposition were associated
with administration of 45 mg/kg bw/day gentamicin (Robbins et al.,
1973a).
One-day-old beagle puppies (4 litters per dose, 6-9 pups/litter)
were injected i.m. with gentamicin in an aqueous vehicle daily for 14
consecutive days. Doses were 0, 2, 6, or 10 mg/kg bw/day. Two
litters per dose were killed after 5 days and 2 pups per litter were
maintained for a drug-free recovery period of 6 weeks following the
last treatment. There was no mortality and no clinical signs of
toxicity. Body weight, righting reflex, haematology and serum
biochemistry parameters remained within normal limits. Post-mortem
examination of kidneys and liver showed no effects on organ weight or
histo-pathology. Histolopathological evaluations of the vestibular
apparatus of the ear were unremarkable (Vymetal et al., 1969).
Daily i.m. injections of gentamicin in a saline vehicle were
administered to groups of beagle dogs (2/sex/group) for up to 3 weeks.
The doses were 0, 15, 30 or 60 mg/kg bw/day. Vomiting was reported in
3/4 dogs in the high-dose group between days 9 and 12 of treatment and
in 3/4 dogs in the mid-dose group between days 16 and 20 of treatment.
Vomiting occurred within one or two hours of treatment. All dogs in
the 60 mg/kg bw/day group were killed between days 9 and 12 because of
their extreme moribund condition characterized by weakness, lethargy,
recumbency and irregular respiration. Slight body-weight loss was
noted during the second week in the 30 mg/kg bw/day group.
Ophthalmological findings were unremarkable.
Haematological parameters remained within normal limits. Serum
creatinine and BUN were increased at 30 and 60 mg/kg bw/day. At the
end of the study, SGPT was elevated and serum sodium and chloride ions
were decreased at 30 mg/kg bw/day. At 30 mg/kg bw/day and above,
urine specific gravity was decreased compared to controls and
glucosuria and occult blood were detected. Urine volume was increased
at 60 mg/kg bw/day. Kidney weights were increased in all treated
groups. Gross and histopathological examination revealed renal
cortical pallor and renal tubular necrosis in all treated animals.
Liver congestion was observed at 30 and 60 mg/kg bw/day. Muscle
degeneration and fibrosis of injection sites were seen at 60 mg/kg
bw/day (Robbins et al., 1973b).
In two separate studies, beagle dogs (2/sex/group) were
administered i.m. injections of gentamicin consisting of two different
ratios of the components C1 and C2, viz. 58:42 and 70:30. Groups
were treated with 0, 6, 10, or 50 mg/kg bw/day for 7 weeks. The
findings were essentially the same for each ratio. Clinical signs were
reported as anorexia, ataxia, prostration, vomiting and convulsions,
but the group incidences were not provided. Dogs in the highest-dose
group died or were killed during weeks 2 and 3. Body weights were
unaffected.
Haemoglobin and haematocrits fell sharply just prior to
intercurrent death or terminal kill in all treated animals. Increased
BUN, glucosuria, albuminuria, and urinary casts were observed in the
10 and 50 mg/kg bw/day groups. Pale renal corticies were observed in
all treated groups. This finding was accompanied by a gelatinous
transudate in animals dosed at 50 mg/kg bw/day. Toxic nephrosis was
noted in treated animals, which was characterized as cloudy swelling
at 6 mg/kg bw/day to severe tubular necrosis at 50 mg/kg bw/day.
Muscle damage was seen at injection sites in all groups including
controls. (Eggert et al., 1963c; 1973).
Beagle dogs (2/sex/group) received i.m. injections of 0, 63, or
100 mg gentamicin/kg bw/day. The duration was intended to be 30 days,
but all treated animals died within 18 days. Signs of gentamicin
toxicosis included vomiting, salivation, gait disturbances, difficulty
in standing, decreases in heart and respiration rates, and reduced
body weight and food intake. Gross and histopathological examinations
revealed swelling, discoloration and increased weight of the kidney,
degeneration and necrosis of renal tubular epithelium, infiltration of
round cells, protein casts and narrowing and dilatation of tubules.
These findings were observed at both treatment levels. Inflammation
and muscle fibre degeneration at the injection sites were also
observed in both dose groups with a dose-related increase in severity
(Hara et al., 1977b).
Groups of beagle dogs (4/sex/group) were administered i.m. doses
of 0, 12.5, or 25 mg/kg bw/day gentamicin in an aqueous vehicle, for
12 weeks. There were no treatment-related clinical signs of toxicity.
Food consumption was unaffected. Body-weight loss occurred in all
groups, which was more severe in the 25 mg/kg bw/day group and the
12.5 mg/kg bw/day females.
Ophthalmological and haematological parameters remained within
normal limits. There were slight elevations in SGPT, SGOT and
creatine phosphokinase at both treatment levels. At 25 mg/kg bw/day,
urine specific gravity was decreased and urine volume was increased.
Glucosuria and occult blood were present in one 12.5 and two 25 mg/kg
bw/day animals. Gross and histopathological findings included
increased kidney and liver weights, pale renal cortices, and proximal
renal tubular necrosis in all treated groups (Robbins et al.,
1976c).
Groups of beagle dogs (4/sex/group) were administered oral doses
of 0, 2, 10, or 60 mg/kg bw/day gentamicin in capsules for 14 weeks.
The highest level was increased to 120 mg/kg bw/day after 2 months.
Emesis and diarrhoea were observed occasionally in treated dogs.
There were no effects on body weight, ophthalmology, haematology,
blood chemistry or urinalysis. The only postmortem change was
interstitial nephritis observed in 2 animals in the high-dose group.
The NOEL was 10 mg/kg bw/day (Vymetal et al., 1970).
Beagle dogs (controls: 2/sex; treated: 4/sex/dose) were injected
i.m. with doses of 0, 3, 5, or 8 mg/kg bw/day gentamicin in an aqueous
vehicle. Animals were treated 5 days per week for up to 12 months.
One dog per sex per group was scheduled for an interim kill after 6
months. Localized pain and transient lameness of the injected leg
were seen in all groups. Ophthalmological and physical examination
findings were within normal limits. Vomiting and profuse salivation
were noted in one animal in the 8 mg/kg bw/day group in weeks 23 and
24. This animal lost considerable body weight during the last 8 weeks
of treatment and had a slightly increased BUN, glucosuria, decreased
urine specific gravity and increased urine volume. Body weight,
haematology, blood chemistry and urinalysis were within normal limits
in all other dogs.
Pale renal corticies and interstitial nephritis were seen after
gross and histopathological examinations in all treatment groups with
a dose-response relationship. Organ weights were similar in all
groups. Inflammatory changes, haemorrhage and fibrosis at the
injection sites were present in all groups, with an increase in
severity in treated animals (Robbins et al., 1970b).
2.2.2.4 Monkeys
Groups of 4 squirrel monkeys (sex not specified) were
administered daily s.c. injections of 0, 25, 50 or 75 mg/kg bw
gentamicin in saline, for up to 3 weeks. All monkeys receiving
gentamicin (except one animal in the low dose group) had to be killed
intercurrently because of their moribund condition. The average
survival times were 15.3, 10.3 and 7.5 days at 25, 50 and 75 mg/kg
bw/day, respectively. Clinical signs included ataxia, weakness,
hypoactivity, irregular respiration and eye scratching at each dose
level. Sporadic convulsions occurred at 50 and 75 mg/kg bw/day.
Body-weight loss was recorded in all groups, including controls, which
appeared to be enhanced with gentamicin treatment. However, there was
no clear, dose-response relationship.
Post-injection acoustic reflex measurements could not be obtained
from the 75 mg/kg bw/day group due to their rapid decline in
condition. In addition, instrument failure resulted in the loss of 3
post-injection data points. Therefore, evaluations were performed on
a total of 14 monkeys out of 28. Results indicated that hearing
deficits occurred in both the low- and mid-dose groups. Apart from the
single low-dose survivor, all animals had increased serum creatinine
and BUN. The author stated that the majority of dosed animals showed
severe renal tubular necrosis. Individual animal data were not
provided (Igarashi et al., undated).
Groups of 3 female Rhesus monkeys were injected i.m. with doses
of 0, 6 or 30 mg/kg bw/day gentamicin in an aqueous vehicle for 3
weeks. Adverse clinical signs were limited to the 30 mg/kg bw/day
group, which included pronounced facial paling and ptosis, markedly
disturbed equilibrium from day 20, and depressed food intake and body-
weight gain from week 2 onwards. Two monkeys in this group were
killed because of their moribund condition on day 21.
Haematological examinations showed decreases in haemoglobin,
haematocrit, and erythrocytes at 30 mg/kg bw/day. Serum chemistry
findings included increases in SGPT, SGOT and LDH, and decreases in
serum potassium, sodium, chloride and calcium ions in the 30 mg/kg
bw/day dose group. Serum creatinine and BUN were increased in both
dose groups. Urinalyses revealed proteinuria, glucosuria and an
increased presence of casts and epithelial cells at 30 mg/kg bw/day.
At necropsy, kidney weights were increased and had a yellow-grey
appearance. Histologically, cloudy swelling, cortical striation,
degeneration and necrosis of proximal convoluted tubules were
reported. The renal lesions which were seen in both dose groups, were
considered to be treatment-related. Vacuolar degeneration of
hepatocytes was noted at 30 mg/kg bw/day. Injection sites showed
dose-related muscle degeneration and necrosis accompanied by
haemorrhage and cellular infiltration. Electron microscopy of renal
tubules from the 30 mg/kg bw/day monkeys revealed myeloid bodies
present in both tubular cells and lumen, increased phagosomes,
disappearance of brush borders and sloughing of epithelial cells from
the basement membrane (Tanaka et al., 1977).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
Groups of Carworth CFE rats (30/sex/group) were injected 6 days/
week with i.m. doses of 0, 5, or 20 mg/kg bw/day gentamicin in saline.
Males were injected from 70 days prior to mating until 7 days before
mating. Females in the 5 mg/kg bw/day group were injected from 14
days prior to pairing with a male until post partum day 21. Females
in the 20 mg/kg bw/day group were treated from the time of
impregnation (determined by vaginal smear) until post partum day 21.
Ten females per group were killed for litter and dam evaluations on
gestation day 21, the remainder were allowed to deliver normally. F1
offspring were not treated. Groups of 10 males and 10 females from
randomly selected litters were paired within treatment levels
(avoiding brother-sister mating) to produce an F2 generation.
F1 Dams were allowed to deliver normally.
After 13 doses, penile bleeding was observed in high-dose F0
males. Because of this finding, the dose was reduced to 15 mg/kg
bw/day after which bleeding ceased. In F0 males of the high dose,
body-weight gain was slightly lower during the second half of the
treatment period. There were no overt effects in females.
Pregnancy rates were not affected. There was no prenatal
mortality and litter sizes and weights were similar between groups.
Early postnatal mortality was increased at both doses, which was
attributed to maternal neglect and failure to lactate, neither of
which were considered treatment-related. Fetal abnormalities were not
observed in delivered pups or caesarian-derived fetuses. Gentamicin
was present in the amniotic fluid in 5/10 low-dose dams, and 8/10
high-dose dams but was not detected in homogenized fetuses. No
treatment-related changes in pregnancy rate, litter size and weight,
prenatal mortality or fetal abnormalities were reported in this study
(Robbins et al., 1966).
2.2.4 Special studies on embryotoxicity and teratogenicity
2.2.4.1 Mice
Female JCL-ICR mice (15-33/group) received s.c. injections of
gentamicin in saline during gestation days 6 to 10. The doses
administered were 0, 1, 10 or 100 mg/kg bw/day. In the high-dose
group, body-weight gain was depressed during treatment and kidney
weight was increased. Gentamicin was measured in amniotic fluid in
the high-dose group. It was not clear whether attempts were made to
measure gentamicin in amniotic fluid in the other two dose groups. In
five control and five 100 mg/kg bw/day dams, delivery of fetuses was
allowed to occur normally. The development of eye and ear openings
was unaffected and kidney and liver weights were similar between
groups at post partum day 35. The remaining females were killed on
gestation day 17 for dam and litter evaluations. Prenatal loss was
increased at 10 and 100 mg/kg bw/day. There were no treatment-related
increases in fetal abnormalities (Nishio et al., 1987).
2.2.4.2 Rats
Female Carworth CFE rats (40/group) were injected i.m. with
gentamicin in saline on gestation days 3 to 16. The doses
administered were 0, 25, or 50 mg/kg bw/day, 6 days per week. Females
were allowed to deliver normally. At least 10 days after weaning the
first litter, dams were bred again and the above procedures were
repeated. Adult females showed no clinical signs of toxicity. Body-
weight gain was lower in the 50 mg/kg bw/day group during the
treatment period. At necropsy, pale renal cortices were observed in
both dose groups. Resorption rates were comparable to control values.
The number of stillborn pups was slightly increased at 50 mg/kg
bw/day following the second series of matings. Offspring were killed
and examined 21 days post partum. No treatment-related abnormalities
were reported (Eggert & Haring, 1964).
Groups of 17 female Sprague-Dawley rats were given intra-muscular
injections of 0 or 75 mg/kg bw/day gentamicin in saline. Treatment
was on gestation days 10 to parturition. Dams were allowed to deliver
normally. Parturition was delayed in gentamicin-treated dams by an
average of 15 hours and birth weights were lower. In pups showing the
lowest birth weights, kidney weight and the number of nephrons were
reduced at birth and at 14 and 21 days post partum. Pups with higher
birth weight showed similar kidney weight and numbers of nephrons to
controls; however, a relative decrease in the number of nephrons was
reported in these animals at 14 and 21 days post partum. Necrosis was
not reported but vacuoles in proximal tubular epithelium were
increased with loss of brush border microvilli and the presence of
debris in the tubular lumen. Gentamicin was detected in the kidneys
of all pups born to treated mothers, the levels being higher in pups
showing the lowest birth weights.
In 7 litters per group, half the pups received subcutaneous doses
of 0 or 75 mg/kg bw/day of gentamicin on post partum days 1 to 13,
then killed on post partum day 14. Drug treatment caused an increase
in kidney weight but the number of glomeruli were similar in both
groups (Gilbert et al., 1987).
In an abstract, it was reported that rats were given s.c. doses
of 0 or 110 mg/kg bw/day gentamicin on either gestation days 10 to 15
or 15 to 20. Females were allowed to deliver normally. Measurements
of arterial blood pressure in offspring, one year after birth,
revealed significantly elevated blood pressure in females but not in
males (Chahoud et al., 1986).
2.2.4.3 Guinea-pigs
Guinea-pigs (6 females per group) were administered i.m.
injections of 0 or 4 mg/kg bw/day gentamicin in saline on gestation
days 48 to 54. Females were allowed to deliver normally. Litter size
and weight and body and kidney weights of offspring from the treated
group were comparable to controls to day 20 post partum. Studies of
kidney function in offspring showed a higher excretion of phosphate in
3-day-old pups but not at 10 days of age. Excretion of sodium,
potassium, magnesium and calcium, urinary flow rate, glomerular
filtration rate and the numbers of glomeruli were unaffected. The
length of proximal tubules and glomerular volume were reduced at 3 and
10 days post partum but not at 20 days. Gentamicin was present in pup
renal cortex at higher levels that in the medulla. Levels were
highest 3 days after birth and decreased thereafter (Le Lievre
et al., 1987).
2.2.4.4 Rabbits
Groups of 13 female New Zealand white rabbits were administered
i.m. doses of 0, 0.8, or 4 mg/kg bw/day gentamicin in saline. The
high dose was given as two divided doses, 7 hours apart. Treatments
were administered on gestation days 6 to 16. Eight females per group
were killed for dam and litter evaluations on gestation day 30. The
remainder were allowed to deliver normally. Pups were killed and
examined on post partum day 21. Adult animals were in good health
throughout the study. No treatment-related effects were noted on
prenatal loss, litter size or weight, fetal morphology, or postnatal
survival or body-weight gain. (Robbins & Klein, 1966).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies on gentamicin are summarized
in Table 2.
2.2.6 Special studies on kidney function
2.2.6.1 Rats
A group of 6 male Sprague-Dawley rats was injected i.m. with
doses of 80 to 160 mg/kg bw/day gentamicin for 7 days. At
termination, animals were anaesthetized with thiobarbital and kidney
function was assessed. The glomerular filtration rate, as measured by
inulin clearance, was reduced in a dose-related manner in all
treatment groups. Aciduria and glucosuria (determined by Labstix)
were induced at 160 mg/kg bw/day, but not at the lower doses,
indicating that reabsorption of glucose in the proximal renal tubule
had been impaired (Chiu et al., 1977).
Sprague-Dawley rats (group sizes not given) were injected s.c.
with 0 or 100 mg/kg bw/day gentamicin for up to 7 days. After four
doses, serum creatinine and BUN were increased and urine osmolality
was decreased, suggesting impairment of urine concentrating capacity.
Tubular reabsorption of solute free water was unaffected. Tubular
secretion of para-aminohippurate (PAH) was increased, although
glomerular filtration rate was decreased, after 4 days. Kidney
cortical slices, isolated from rats treated with gentamicin,
accumulated PAH in vitro at a faster rate than slices from control
animals; this effect was inhibited in the presence of probenecid. This
provided evidence that an alteration in the organic acid transport
system was responsible for the increased rate of PAH accumulation
(Cohen et al., 1974).
Female Sprague-Dawley rats (group sizes not given) were injected
s.c. with 0, 50, 100, or 150 mg/kg bw/day gentamicin for up to 14
days. During the treatment period, urine osmolality was decreased
followed by a rise in urine volume and water intake. Proteinuria was
seen from day 3 and serum creatinine and BUN were increased at 100 and
150 mg/kg bw/day. At termination, microscopic examination of kidneys
revealed dose-related injury to proximal tubules in all dose groups,
consisting of increased lysosomal bodies, disruption of brush borders,
cellular swelling and necrosis. Kidney cortical slices, from rats
treated with gentamicin, showed increased uptake of PAH in vitro
(Kaloyanides, 1977).
Sprague-Dawley rats (group sizes not given) were injected i.p.
with 0 or 10 mg/kg bw/day gentamicin for up to 14 days. Kidney
function was unaffected throughout the treatment period. Animals were
killed after 1, 4, 7 and 14 doses and kidneys removed for examination.
Microscopic examinations found proliferation of lysosomes in proximal
tubular cells on day 4. This was associated with decreases in
lysosomal sphingomyelinase and phospholipase activities, suggesting
lysosomal dysfunction. At day 9, protrusion of cells and/or nuclei
into tubular lumens was observed and several cells had lost part of
their brush borders. Focal necrosis was evident on day 14 (Carlier
et al., 1982).
2.2.6.2 Dogs
Male beagle dogs (group sizes not given) were injected i.m. with
0 or 30 mg/kg bw/day gentamicin for up to 11 days. Serum creatinine,
BUN, and urine protein were increased, and creatinine clearance and
urine osmolality were decreased in gentamicin-treated animals. In
addition, urinary levels of beta-glucuronidase, N-acetyl-beta-
glucosaminidase and muramidase were elevated. Microscopic renal
lesions ranged from diffuse moderate hyaline droplet degeneration to
frank tubular necrosis. The pathological and enzyme changes appeared
to precede the functional alterations (Adelman et al., undated).
2.2.7 Special study on ototoxicity
2.2.7.1 Monkeys
Groups of 3 female Cynomolgus monkeys were injected i.m. with
doses of 0, 25, or 50 mg/kg bw/day gentamicin for 35 days. All monkeys
dosed at 50 mg/kg bw/day died: one on day 7 and the other two on day
22. Prior to death, the pinna reflex to high frequencies in these
monkeys was slightly reduced, but there was no observable ataxia
indicative of vestibular impairment. Histopathological examination of
the crista ampullaris showed vacuole formation at both doses and, at
the higher dose, hair cell loss and a reduction in the thickness of
sensory epithelium. In the organ of Corti, cochlear hair cell loss was
noted at 50 mg/kg bw/day while the histology of the macula was not
significantly affected (Yakota et al., 1984).
2.2.8 Special study on neurotoxicity
Gentamicin was administered parenterally to Charles River CD
rats, cats and beagle dogs, to assess its neurotoxicity potential.
The treatment regimes were as follows:
Rats - 0, 6.6, 16.6, 41.5 or 104 mg/kg bw/day, s.c., for
41 days.
Cats - 0, 2.5, 5.0 or 10.0 mg/kg bw/day, s.c., for 42 days.
Dogs - 0, 5.0, 8.3 or 41.5 mg/kg bw/day, i.m., for 49 days.
In rats, righting reflex was impaired at 104 mg/kg bw/day and
ataxia was observed in dogs given 8.3 and 41.5 mg/kg bw/day. Ataxia
was not produced in cats at any dose (Taber et al., 1963).
2.2.9 Special studies on microbiological effects
Results of investigations into the effects of gentamicin on human
intestinal flora were not available. However, two published reports
were available on the inhibitory activity of gentamicin against a wide
range of bacterial strains in vitro.
Table 2. Results of genotoxicity studies on gentamicin
Test system Test object Results Reference
Reverse mutation1 S. typhimurium negative2 Houk et al., 1989
Reverse mutation1 S. typhimurium negative3 Koeda & Hirano, 1979
TA98, TA100,
TA1535, TA1536,
TA1537, TA1538
Reverse mutation1 S. typhimurium negative2 Mitchell et al., 1980
TA98, TA100,
E. coli WP2
Reverse mutation1 E. coli CM891 negative3 Mitchell & Gilbert, 1984
Forward mutation1 E. coli CM891 positive3 at a Mitchell & Gilbert, 1984
cytotoxic level
(0.25 µg/ml)
Forward mutation1 E. coli WP2 positive2 at a Mitchell et al., 1980
cytotoxic level
(0.49 µg/ml)
Mitotic crossing- S. cerevisiae D5 negative3 Koeda & Hirano, 1979
over1
Gene conversion1 S. cerevisiae D4 negative3 Mitchell et al., 1980
Petite inducation1 S. cerevisiae D4 positive3 (500 Mitchell et al., 1980
µg/ml) at pH4.4,
not pH 7.2
DNA repair1 E. coli WP2, negative2 Mitchell et al., 1980
WP100
Rec-assay1 B. subtilis negative3 Kada et al., 1980
M45, H17
SOS function1 E. coli PQ37 negative2 Mamber et al., 1986
DNA-cell binding E. coli Q13 positive2 Kubinski et al., 1981
(20 µg/ml) test
not validated
Table 2 (contd)
Test system Test object Results Reference
Chromosome Mouse L-cells positive3 but Leonard & Botis, 1975
aberrations mitosis inhibited
(500 µg/ml x 4 d
plus 100 µg/ml x
20 d)
SCE Human fibroblasts positive3 but McDaniel & Schultz, 1993
GM00847 less than doubling
(75-250 µg/ml)
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
3 Without metabolic activation.
One article reported the minimum inhibitory concentrations (MICs)
for gentamicin in 601 clinical isolates of anaerobic bacteria. The
experiments were conducted using the agar-dilution technique at 37°C
for 48 hours in an anaerobic incubator, an inoculum of 103 to 104
colony-forming units and serial concentrations of gentamicin in the
range of 0.1 to 25 µg/ml (Martin et al., 1972). In a second
publication, results were reported for Clostridium difficile
strains, but there was no information on the culture conditions
employed (Lorian, undated). All results are presented in Table 3.
The susceptibility to gentamicin of most anaerobic bacteria was
low, the most sensitive being Eubacterium sp., with a modal MIC
value of 0.8 µg/ml for the 20 strains tested.
2.3 Observations in humans
In a prospective, randomized, blinded, comparative trial, 54
patients treated with gentamicin were evaluated for nephrotoxicity and
ototoxicity. The dosage regime was usually 1.7 mg/kg bw i.v. every 8
hours (5.1 mg/kg bw/day) for 7 to 54 days. Desired serum drug levels
were maintained by reducing the dose in patients with impaired renal
function. Nephrotoxicity, defined as a 50% increase in serum
creatinine, was produced in 8 (15%) patients. Auditory toxicity,
defined as a decrease in the sensorineural threshold of at least 15
decibels in two or more frequencies, was produced in 3 (6%) patients.
Vestibular toxicity, defined as at least a 50% decrease in maximum
average slow-phase eye speed following water stimulation of the ear,
was induced in 3 of 33 (9%) patients. Auditory and vestibular effects
were not noted in the same individuals (Lerner et al., 1986).
Kidney biopsies were obtained from 5 patients with renal cancer
following surgical removal of both kidneys. Prior to surgery,
gentamicin was administered at a dosage of 1.5 mg/kg bw three times a
day (4.5 mg/kg bw/day) for 4 days. Microscopic evaluation of the
kidney specimens revealed increases in lysosome numbers with moderate
to marked decreases in the activity of sphingomyelinase and acid
phospholipase A1 (Carlier et al., 1982).
In a retrospective study involving patients on chronic
haemodialysis therapy, 23 patients were treated with gentamicin.
Dosages were not identified. Of the drug-treated subjects, 7 (30%)
developed clinically detectable vestibular toxicity. The principal
risk factors for the development of ototoxicity were age, duration of
treatment and the total dose administered. There was no positive
association with peak serum levels of gentamicin or the total absence
of kidneys (Gailiunas Jr. et al., 1978).
A review, focusing mainly on prospective trials, reported on a
total of more than 10 000 patients treated with aminoglycoside
antibiotics. The incidence of cochlear toxicity associated with
gentamicin administration ranged from 5 to 10% in adults. The
incidence was claimed to be lower in infants (Matz, 1993).
Adverse reactions to gentamicin during the years 1963 to 1973
were reviewed. Of the 3500 patients studied, the main non-specific
reactions were increases in serum transaminases (16), skin rashes (16)
and granulocytopaenia (8). Also presented were results from clinical
trials with gentamicin. Possible or probable nephrotoxicity was found
in 22 of 285 (7.7%) patients in 1965 to 1966, 70 of 1450 (4.8%)
patients in 1967 to 1969 and 48 of 1635 (2.9%) patients in 1970 to
1973. Possible or probable ototoxicity was found in 16 of 565 (2.8%)
patients in 1963 to 1966, 27 of 1450 (1.8%) patients in 1967 to 1969
and 15 of 1635 (1%) patients in 1970 to 1973. Critical doses or
duration of treatment were not given, but ototoxicity usually occurred
in association with impaired renal function (Hewitt, 1974).
The rates of allergic cutaneous reactions were determined in 15
438 consecutive in-patients monitored by the Boston Collaborative Drug
Surveillance Program from June 1975 to June 1982. Of the 670 patients
receiving gentamicin, there were 3 (0.5%) skin reactions.
Table 3. Minimum inhibitory concentration (MIC) values for gentamicin against anaerobic bacteria
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Bacteroides fragilis (195) 1 6
B. incommunio (10) 10 40
B. variabilis (5) 60
B. oralis (2) 50 100
B. terebrans (5) 40 60
B. melaninogenicus (29) 24 31 34 45 59 76
Bacteroides F1,F2,F3 (11) 27 36 45 73
Fusobacterium fusiforme (18) 5 11 56
Fusobacterium sp. (2) 50
Clostridium perfringens (34) 6
C. difficile (15) 20 40
Clostridium sp. (17) 6 18
Peptococcus sp. (145) 2 3 5 7 11 30 62 95
Peptostreptococcus sp. (72) 1 9 13 18 20 33 58 78
Veillonella sp. (13) 8 31 46 85
Table 3 (contd)
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Propionibacterium acnes (16) 25 56 75 81
Eubacterium lentum (14) 43 57 71 85 93 100
E. alactolyticum (2) 50
Eubacterium sp. (5) 20 40
Bifidobacterium sp. (5) 20 60 80 100
Catenabacterium (3) 66
filamentosum
Catenabacterium sp. (2) 50 100
3. COMMENTS
A range of studies on gentamicin was submitted for assessment,
including information on pharmacokinetics and metabolism, acute
toxicity, short-term toxicity, reproductive toxicity and
teratogenicity, genotoxicity, antimicrobial activity, a variety of
special studies and observations in humans.
Gentamicin, in common with other aminoglycosidic drugs, is poorly
absorbed from the gastrointestinal tract. Parenteral doses distribute
mainly into extracellular space, but there is significant penetration
into the kidney cortex and the inner ear. There is negligible
metabolism of the drug and unchanged gentamicin is excreted rapidly,
mostly in the urine.
Single oral doses of gentamicin were slightly toxic in rodents
(LD50 = 8000 to 10 000 mg/kg bw), which supports the view that the
drug is largely unabsorbed when administered by this route. Parenteral
dosing of mice, rats, guinea-pigs and dogs demonstrated high
intravenous toxicity (LD50 = 37 to 67 mg/kg bw) and moderate toxicity
by i.m., s.c. and i.p. routes (LD50 = 213 to 893 mg/kg bw).
Gentamicin was extensively tested by the i.m. and s.c. routes in
rats administered the compound for periods of up to 12 months (doses
up to 200 mg/kg bw/day) in dogs for up to 12 months (doses up to 100
mg/kg bw/day) and in monkeys for 3 weeks (doses up to 75 mg/kg
bw/day). The kidney was the primary target organ in each species with
effects observed predominantly in the renal cortex. The major
findings were an increase in interstitial nephritis and toxic
nephrosis in the proximal convoluted tubules. The latter was
characterized by a dose- and time-dependent progression from loss of
brush borders, cloudy swelling, the presence of casts and
proteinaceous material in the tubules, desquamation of epithelial
cells and necrosis. These changes were associated with a profound
impairment of renal function, and in extreme cases, death of severely
affected animals. Special studies in rats suggested that the
nephrotoxicity may be associated with disruption of lysosomal
function. Nephrotoxicity has been observed in humans treated with
gentamicin.
Toxicity to the inner ear may occur after exposure to
aminoglycosides, including gentamicin. There were no clear
indications of ototoxicity in the general toxicity studies; however,
a special study in monkeys, at doses up to 50 mg/kg bw/day, showed
hair cell loss and reduced sensory epithelium in structures of the ear
as well as a functional loss in hearing. Observations in humans have
revealed auditory or vestibular toxicity after therapeutic
administration of gentamicin.
Oral dosing of rats and dogs for a period of 3 months, at doses
up to 116 and 60 mg/kg bw/day, respectively, was virtually without
systemic effects. Soft stools or diarrhoea were observed in both
species and dogs showed interstitial nephritis at high doses. The
NOELs were 19 mg/kg bw/day in rats and 10 mg/kg bw/day in dogs.
A multigeneration reproductive toxicity study in the rat showed
no adverse effects on reproduction parameters after intramuscular
injections of 5 or 20 mg gentamicin/kg bw/day. Teratogenicity studies
in mice, rats, guinea-pigs and rabbits did not identify any potential
for the production of fetal abnormalities. Fetotoxicity, in the form
of fetal death in mice (10 mg/kg bw/day) and rats (50 mg/kg bw/day)
and reduced birth weight in rats (75 mg/kg bw/day), was observed after
parenteral dosing.
Positive results were obtained in some in vitro genotoxicity
studies, but none of these was adequate to evaluate the genotoxicity
of gentamicin. There were no carcinogenicity studies available on
gentamicin. In toxicity studies in animals of up to one year
duration, there were no pre-neoplastic or neoplastic lesions. The
Committee considered that the data were inadequate to fully assess the
carcinogenicity of gentamicin.
Data on the effects of gentamicin on human gut flora were not
available. However, in vitro data were presented on MICs for over
600 clinical isolates of anaerobic bacteria including some species
representative of the microbial flora in the human gastrointestinal
tract. Many of the organisms were found to be resistant to gentamicin;
Eubacterium species were the most sensitive. Using an inoculum
density of 103 to 104 colony forming units per plate, MIC values for
Eubacterium species were in the range 0.1 to 25 µg/ml with an MIC50
of 0.8 µg/ml.
In view of the poor oral absorption of gentamicin and the
reported occurrence of diarrhoea in experimental animals, it was
considered that effects on the gastrointestinal microflora would be
the most sensitive effect of residues. In calculating the ADI, the
formula developed by the thirty-eighth meeting of the Committee (Annex
1, reference 97) was employed:
Concentration without effect x Daily faecal bolus (g)
on human gut flsor (µg/ml)a
Upper limit of ADI =
Fraction of oral x Safety x Human body
dose availableb factorc weight
(60 kg)
(0.8 x 2) x 150
=
1 x 1 x 60
= 4 µg/kg bw
a This value accounts for the range of MICs in appropriate
bacterial species and the use of the most sensitive anaerobic
organism. A two-fold adjustment was deemed to be necessary to
account for the relatively low inoculum density.
b Absorption of gentamicin after oral dosing is very poor, therefore
a factor of 1 was used to represent 100% availability of ingested
drug in the gastrointestinal tract.
c Data on more than 600 clinical isolates of anaerobic bacteria were
available for determination of the MIC. Because the colonic flora
is relatively stable and variability within a particular individual
may be as great as variability between individuals, and because it
was recognized that other values selected for this calculation were
conservative and already incorporated an adequate margin of safety,
a safety factor of 1 was selected to cover fully the variability
between people of all extrapolated parameters.
4. EVALUATION
In view of the absence of information on the effects of
gentamicin on microorganisms obtained from the human intestine and the
need for further genotoxicity studies, the Committee established a
temporary ADI of 0-4 µg/kg bw based on the results of micro-biological
testing of clinical isolates, with a request for further information.
An increased safety factor was not used to account for the temporary
status of the ADI because conservative factors were used in deriving
the temporary ADI. The lowest toxicological NOEL was 10 mg/kg bw/day
in dogs, which is more than 2000-fold higher than the
microbiologically based ADI.
5. REFERENCES
ADELMAN, R.D., CONZELMAN, G., SPANGLER, W. & ISHIZAKI, G. (undated).
Comparative nephrotoxicity of gentamicin and netilmicin : functional
and morphological correlations with urinary enzyme activities.
Unpublished Report No. D 11403. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ATEF, M., ARBID, M.S. & HANAFY, M.S.M. (1992). Comparison of
gentamicin toxicity in normal and diabetic rats. Acta Veterinaria
Hungarica, 40: 107-111.
BIGBY, M., JICK, S., JICK, H. & ARNDT, K. (1986). Drug-induced
cutaneous reactions. JAMA, 256: 3358-3363.
CARLIER, M.B., PAULUS, G., MALDAGUE, P., GIURGEA, L., WILMOTTE, E., DE
BROC, M.E. & TULKENS, P. (1982). Early toxic events in kidney of rat
and man following administration of gentamicin at low doses.
Arch. Toxicol. Suppl. 5: 287-290.
CHAHOUD, I., STAHLMANN, R. & NEUBERT, D. (1986). Blood pressure
elevation in one-year-old female rats after prenatal exposure to
gentamicin. Teratology, 33: 13A.
CHIU, P.J.S., LONG, J.F., BARNETT, A., TABER, R.I., MILLER, G. &
WAITZ, J.A. (1977). Kidney function in rats chronically treated with
aminoglycosides. Unpublished Report No. P 4485. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
COHEN, L., LAPKIN, R. & KALOYANIDES, G.J. (1974). Effect of gentamicin
on renal function in the rat. Unpublished Report No. D 6338. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
EGGERT, M.J., DAVENPORT, L.R. & WILLIAMSON, N.K. (1973). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3379. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., EVANS, M. & WILLIAMSON, N.K. (1963a). SCH 9724 in rats.
Subacute (4 weeks) intramuscular toxicity study. Unpublished Report
No: P 3355. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J. & HARING, G.D. (1964). Intramuscular teratogenic study of
SCH 9724 (gentamicin sulfate 70:30) in rats. Unpublished Report No: P
3397. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., HARING, G.D. & VYMETAL, F. (1965). Acute toxicity
studies of gentamicin sulfate intramuscularly in rats and dogs.
Unpublished Report No: P 3502. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., HARING, G.D., WILLIAMSON, N.K. & WEINSTEIN, M.J. (1975).
Acute and subacute toxicity studies of SCH 9724 (gentamicin sulfate)
in rat neonates. Unpublished Report No: P 3497. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1963b).
Gentamicin sulfate-90 day dermal toxicity in rabbits. Unpublished
Report No: P 3410. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1964).
Dermal toxicity of SCH 9724 in rabbits. Unpublished Report No: P 3425.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., VYMETAL, F. & WILLIAMSON, N.K. (1963c). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3350. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
FRASER, C.M., BERGERON, J.A., MAYS, A. & AIELLO, S.E. (1991).
Aminoglycosides. In: The Merck Veterinary Manual, 7th Edition ,
Merck & Co., Rahway, pp 1429-1435.
GAILIUNAS JR., P., DOMINGUEZ-MORENO, M., LAZARUS, J.M., LOWRIE, G.,
GOTTLIEB, M.N. & MERRILL, P. (1978). Vestibular toxicity of
gentamicin. Arch. Intern. Med., 138: 1621-1624.
GILBERT, T., LELIEVRE-PEGORIER, M., MALIENOU, R., MEULEMANS, A. &
MERLET-BENICHOU, C. (1987). Effects of prenatal and postnatal exposure
to gentamicin on renal differentiation in the rat. Toxicology, 43:
301-313.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977a).
Safety evaluation of KW-1062. I. Acute toxicity in mice, rats and
dogs, subacute and chronic toxicity in rats. Jap. J. Antibiotics ,
30: 386-407.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977b).
Safety evaluation of KW-1062. II. Subacute and chronic toxicity
studies in dogs. Jap. J. Antibiotics, 30: 408-422.
HEWITT, W.L. (1974). Gentamicin: toxicity in perspective. Postgrad.
Med. J., (Suppl. 7): 55-59.
HOUK, V.S., SCHALKOWSKY, S. & CLAXTON, L.D. (1989). Development and
validation of the spiral Salmonella assay: an automated approach to
bacterial mutagenicity testing. Mutation Res., 223: 49-64.
IGARASHI, M., LEVY, J.K., JERGER, J., WAITZ, J.A. & ARCIERI, G.
(undated). Comparative toxicity of netilmicin and gentamicin in
squirrel monkeys. Unpublished Draft Report No: D 10312. Submitted to
WHO by Inveresk Research International, Trenant, Scotland, on behalf
of Schering Plough Animal Health.
KADA, T., HIRANO, K. & SHIRASU, Y. (1980). Screening of environmental
chemical mutagens by the Rec-assay with Bacillus subtilis. In:
Chemical Mutagens and Principles for their Detection. Chap. 6: pp
149-173.
KALOYANIDES, G.J. (1977). Untitled. Unpublished Report No: D 10069.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
KOEDA, T. & HIRANO, F. (1979). Evaluation of the mutagenicity of
aminoglycoside antibiotics in Salmonella typhimurium and
Saccharomyces cerevisiae. J. Antibiotics, 32: 607-609.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA cell-binding
assay for suspected carcinogens and mutagens. Mutation Res., 89:
95-136.
LELIEVRE, M., GILBERT, T., SAKLY, R., MEULEMANS, A. & MERLET-BENICHOU,
C. (1987). Effect of fetal exposure to gentamicin on kidneys of young
guinea pigs. Antimicrob. Agents Chemother., 31: 88-92.
LEONARD, A. & BOTIS, S. (1975). Chromosome damage induced by
gentamicin in mouse L-cells. Experientia, 31: 341-343.
LERNER, S.A., SCHMITT, B.A., SELIGSOHN, R. & MATZ, G.J. (1986).
Comparative study of ototoxicity and nephrotoxicity in patients
randomly assigned to treatment with amikacin or gentamicin.
Am. J. Med., 80(6B): 98-104.
LORIAN, V. (undated). Tables 28.13 and 28.15. In: Antibiotics in
Laboratory Medicine. 2nd Edition. Williams & Wilkins, Baltimore-
London-Los Angeles-Sydney, pp 1087-1089 and 1142-1146.
MAMBER, S.W., OKASINSKI, W.G., PINTER, C.D. & TUNAC, J.B. (1986). The
Escherichia coli K-12 SOS chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutation Res., 171: 83-90.
MARTIN, W.J., GARDNER, M. & WASHINGTON II, J.A. (1972). In vitro
antimicrobial susceptibility of anaerobic bacteria isolated from
clinical specimens. Antimicrob. Agents Chemother., 1: 148-158.
MATZ, G. (1993). Aminoglycoside cochlear ototoxicity. Otolaryngologic
Clinics of North America, 26: 705-712.
McCORMICK, G.C. (1983). Acute intravenous toxicity comparison of
gentamicin sulfate and gentamicin C2 sulfate in mice (SN 83272).
Unpublished Report No: D17472. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
McDANIEL, L.D. & SCHULTZ, R.A. (1993). Elevation of sister chromatid
exchange frequency in transformed human fibroblasts following exposure
to widely used aminoglycosides. Environ. Molec. Mutagenesis, 21:
67-72.
MILLER, G.H., WAITZ, J.A. & WEINSTEIN, M.J. (1976). Pharmacokinetics
of C14-gentamicin in dogs. Unpublished Report No: P 4440. Submitted
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
MITCHELL, I DEG., DIXON, P.A., GILBERT, P.J. & WHITE, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Mutation Res., 79:
91-105.
MITCHELL, I DEG. & GILBERT, P.J. (1984). The effect of pretreatment of
Escherichia coli CM 891 with ethylenediaminetetraacetate on
sensitivity to a variety of standard mutagens. Mutation Res., 140:
13-19.
NISHIO, A., RYO, S. & MIYAO, N. (1987). Effects of gentamicin,
exotoxin and their combination on pregnant mice. Bull. Fac. Agric.
Kagoshima Univ., 37: 129-136.
RIVIERE, J.E., CRAIGMILL, A.L. & SUNDLOF, S.F. (1991).
Aminoglycosides. In: Handbook of Comparative Pharmacokinetics and
Residues of Veterinary Antimicrobials, CRC Press, Boca Roton, Florida,
pp 263-335.
ROBBINS, G.R., CASTELLANO, R.F., TORNING, F.E., FABRY, A. & FIELDER,
F.G. (1971).- Acute toxicity in rats (intramuscular, intravenous and
intraperitoneal) and mice (intramuscular, intravenous, intraperitoneal
and subcutaneous). Unpublished Report No : P 3967. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., FABRY, A., MORGAN, W.A. & EGGERT, M.J. (1972a).
Subacute (two-week) intramuscular toxicity study of SCH 14342 in rats.
Unpublished Report No: P 4074. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1970a).
Chronic toxicity study of SCH 9724 by intramuscular injection in rats.
Unpublished Report No: P 3884. Submitted by Inveresk Research
International, Trenant, Scotland, to WHO on behalf of Schering Plough
Animal Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1972b).
Subacute (two-week) toxicity study of SCH 13706 by intramuscular
injection in rats. Unpublished Report No: P 4072. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R. & KLEIN, M.F. (1966). Reproduction study of gentamicin
sulfate in rabbits. Unpublished Report No : P 3595. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., KLEIN, M.F. & EGGERT, M.J. (1966). Reproduction study
using gentamicin sulfate intramuscularly in rats. Unpublished Report
No : P 3598. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970b).
Chronic (1-year) intramuscular toxicity study in dogs using gentamicin
sulfate (SCH 9724). Unpublished Report No: P 3848. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1972c).
Subacute (14-day) intramuscular toxicity study of SCH 13706 Gentamicin
C1 ) in dogs. Unpublished Report No: P 4065. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1973a).
Subacute intramuscular toxicity study of SCH 14342 as compared with
SCH 9724 in dogs. Unpublished Report No: P 4138. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H. & GRAY, W.D. (1976a). The subacute
toxicity of SCH 20569 sulfate, (vs gentamicin, tobramycin and
kanamycin) administered subcutaneously to rats. Unpublished Report No:
P 4424. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D. & FIELD, W.E. (1976b).
Subacute intramuscular toxicity of SCH 20569 in rats. Unpublished
Report No: P 4393. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D., FIELD, W.E. & SCHIAVO, D.M.
(1976c). Subacute intramuscular toxicity of SCH 20569 in dogs.
Unpublished Report No: P 4392. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R., FIELD, W.E. & OWEN, G.
(1973b). Subacute intramuscular toxicity in dogs using SCH 15666 and
SCH 9724. Unpublished Report No: P 4196. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R. & OWEN, G. (1973c). Acute
intramuscular toxicity study in guinea pigs. Unpublished Report No: P
4174. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., OWEN, G., MARCHESO, R. & FIELD, W.E.
(1973d). Subacute intramuscular toxicity in rats using SCH 15666 and
SCH 9724. Unpublished Report No: P 4212. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
SANDE, M.A. & MANDELL, G.L. (1990). Antimicrobial agents. The
aminoglycosides. In: GILMAN, A.G., RALL, T.W., NIES, A.S. & TAYLOR, P.
(eds), Goodman & Gilman's The Pharmacological Basis of Therapeutics,
8th Edition, Pergamon Press, New York-Oxford-Beijing-Sao Paulo-Sydney-
Tokyo-Toronto, pp 1098-1116.
TABER, R., IRWIN, S. & PERLMAN, P.L. (1963). The effect of subchronic
administration of gentamicin sulfate, kanamycin sulfate and
streptomycin sulfate in the rat, cat and dog. Unpublished Report No:
P 3316. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
TANAKA, H., WATANABE, M. & HATTORI, H. (1977). Subacute toxicity of
sisomycin administered intramuscularly in monkeys. Comparison with
gentamicin and tobramycin. Unpublished Report No: D 10555. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970). Subacute (14 week)
oral toxicity study of SCH 14894 (gentamicin sulfate, veterinary
grade) in dogs. Unpublished Report No: P 3923. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
VYMETAL, F., EGGERT, M.J. & ROBBINS, G.R. (1969). Toxicity study of
gentamicin sulfate by injection in neonatal dogs. Unpublished Report
No: P 3818. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
WOODARD, M.W., HOWARD, D.J., WOODARD, G. & EGGERT, M.J. (1970).
Gentamicin sulfate safety evaluation by repeated oral administration
to rats for 13 weeks. Unpublished Report No: P 3924. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
YAKOTA, M., TAKEDA, U., SAKAMOTO, K., SETO, N. & IGARASHI, M. (1984).
The comparative ototoxicities of panimycin and gentamicin in
cynomolgus monkeys (Maccaca fascicularis). Chemotherapy, 30:
248-254.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, metabolism and excretion
Few specific studies on the pharmacokinetics of gentamicin were
available, however, the aminoglycosides as a class have been
extensively reviewed. In view of their polar nature and high aqueous
solubility, aminoglycosides are essentially not absorbed after oral
administration. Absorption after intramuscular or subcutaneous
administration is good with peak blood levels occurring within 30 to
90 minutes of an intramuscular injection (Riviere et al., 1991;
Sande & Mandell, 1990).
The aminoglycosides distribute into the extracellular space with
minimal penetration into tissues other than the kidneys and the inner
ear. Plasma protein binding is reported to be less than 20%. Levels in
amniotic fluid and fetal tissue are very low in most species.
Aminoglycosides as a class are not metabolized and are eliminated
unchanged in the urine by glomerular filtration. Recovery of 80% to
90% of the administered dose can occur within 24 hours (Fraser
et al., 1991).
One beagle dog was given a single intravenous dose of radio-
labelled gentamicin sulfate. The apparent half-life of elimination
from serum was approximately 1 hour initially and approximately 2´
hours 8 and 10 hours after dosing. Distribution to organs and tissues
was extensive but exceptionally high levels were found in the kidney,
mainly in the cortex. A comparison of the levels of serum gentamicin
using radioassay, radioimmunoassay and microbiological assay were
similar, suggesting that there was little metabolism of the drug.
Excretion was primarily renal with some 70% recovered in the urine but
very little (1%) in the faeces. Approximately 2% of the dose remained
in tissues 8 days after dosing; the remainder was unaccounted for
(Miller et al., 1976).
2.2 Toxicological studies
Unless specified otherwise, toxicological studies were carried
out using the gentamicin sulfate salt and dose levels are expressed as
gentamicin base.
2.2.1 Acute toxicity studies
The results of acute toxicity studies with gentamicin are
summarized in Table 1.
Clinical signs of intoxication in rodents included convulsions,
prostration, hypoactivity, polydipsia, dyspnoea and ataxia. Dogs
exhibited muscle tremors, salivation, and anorexia. Histopathological
examination of kidneys from dogs that died up to 13 days after dosing
revealed necrosis of the proximal convoluted tubule.
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 0 or 40 mg/kg bw/day gentamicin in saline were
administered i.p. to groups of 5 male albino rats. Treatment was for
10 consecutive days and animals were killed 48 hours after the last
dose. There were no reported overt signs of toxicity. Serum
chemistries at termination showed an increase in creatinine, BUN, and
SGPT. Pathology was restricted to kidneys and liver and included
degeneration and necrosis of renal tubular epithelium and moderate
vacuolization of hepatocytes. The author also noted that enhancement
of nephrotoxicity and hepatotoxicity occurs in alloxan-induced
diabetic rats administered gentamicin (Atef et al., 1992).
Groups of Charles River CD1 rats (15/sex/group) received i.m.
injections of gentamicin daily for 14 days. The drug was administered
in saline at dose levels of 0, 10, 25, 50, or 100 mg/kg bw/day. Overt
signs of toxicity were noted only in the highest-dose group, which
included reduced body-weight gain, polydipsia, hypoactivity, ataxia,
lethargy, dyspnoea, emaciation and prostration. A total of 7 rats in
this group died or were killed because of their moribund condition.
Clinical laboratory investigations showed indications of
treatment-related effects on renal function. Serum creatinine and BUN
were increased. Glucose and occult blood were present in the urine at
doses of 50 and 100 mg/kg bw/day. Decreased urine specific gravity and
increased urine volume were noted at 25 mg/kg bw/day and above. At
necropsy, renal cortical pallor was observed in all treated groups
with dose-related increases in incidence and severity. At 25 mg/kg and
above, kidney weights were increased as was the severity of renal
tubular nephrosis (Robbins et al., 1973d).
Neonatal Carworth CFE rats (10 litters per group, 10 pups/litter)
were administered i.m. injections of gentamicin for 14 days. The
doses were 0, 10, 25, 50 or 100 mg/kg bw/day in a water vehicle.
There were no clinical signs of toxicity, but body-weight gains were
depressed at all doses. Laboratory investigations were not
undertaken. At the end of the study, kidney weights were increased at
all doses. Dilation of renal pelves was observed in treated groups
but with no clear dose-response relationship. At 25 mg/kg bw/day and
above, pale renal cortices and dark red medullas were noted. Cloudy
swelling of renal tubules, the presence of proteinaceous material in
tubules, desquamation of epithelial cells and tubular necrosis
occurred in all dose groups, which were considered to be treatment-
related (Eggert et al., 1975).
Table 1. Acute toxicity
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse M&F oral 10 000 Hara et al., 1977a
M i.v. 40 Robbins et al., 1971
M&F i.v. 51-57 Hara et al., 1977a
M&F i.v. 37-40 McCormick, 1983
M i.m. 213 Robbins et al., 1971
M&F i.m. 223-233 Hara et al., 1977a
M i.p. 232 Robbins et al., 1971
M&F i.p. 279-305 Hara et al., 1977a
M s.c. 228 Robbins et al., 1971
M&F s.c. 292-312 Hara et al., 1977a
Rat M oral 8000-10 000 Hara et al., 1977a
M i.v. 67 Robbins et al., 1971
M i.m. 384 Eggert et al., 1965
M i.m. 371 Robbins et al., 1971
M&F i.m. 489-511 Hara et al., 1977a
M i.p. 674 Robbins et al., 1971
M&F i.p. 478-559 Hara et al., 1977a
M&F s.c. 817-893 Hara et al., 1977a
Rat M&F i.m. 500 Eggert et al., 1975
(neonate) i.p. 508 Eggert et al., 1975
Guinea-pig M&F i.m. 320 Robbins et al., 1973c
Dog M&F i.m. 300-400 Eggert et al., 1975
Gentamicin B in an aqueous vehicle was administered i.m. to
groups of Carworth CFE rats (10/sex/group) for 14 days. Doses were 0,
50, 100, or 250 mg/kg bw/day. Polydipsia and ptosis were observed in
all treated rats. Body-weight gain was reduced at 250 mg/kg bw/day.
Haematology and urinalysis parameters and BUN of treated rats were
comparable to control values. At necropsy, haemorrhage and necrosis
at the injection site was noted in all rats in the high-dose group.
Renal cortical pallor and increased kidney weights were seen in all
treated groups. At 250 mg/kg bw/day degenerative changes in proximal
convoluted tubules were noted including swelling, granularity of
epithelial cells, casts, loss of brush borders and fat droplets in the
epithelium. Liver weights were decreased at 100 and 250 mg/kg bw/day
(Robbins et al., 1972a).
Carworth CFE rats (22/sex/group) were administered i.m.
injections of either gentamicin or gentamicin C1 at doses of 0, 10,
25 or 50 mg/kg bw/day. In each dose group, 2 males and 2 females were
killed on days 2, 4, 6, 8, 10, and 12, and 10 males and 10 females
were killed after 14 days. There was no treatment-related mortality in
any dose group. The only adverse effects noted in clinical parameters
were a slight increase in BUN and reduced body-weight gain in the 50
mg/kg bw/day group. Histopathological examinations revealed
haemorrhage and lymphocytic infiltration at the injection site, which
was seen in all treatment groups. Pale renal cortices were observed in
all dosed groups, which were more pronounced with increasing doses of
gentamicin. In the gentamicin-treated groups, lymphocytic infiltration
in the renal cortex was first noted in the 50 mg/kg bw/day dose group
after 5 doses and at lower doses thereafter. Proximal convoluted
tubule degeneration required 7 doses of 50 mg/kg bw/day. Gentamicin
C1 induced lymphocytic infiltration at 25 and 50 mg/kg after 14 days
only, but with no tubular degeneration, suggesting a lower potential
for renal toxicity than gentamicin (Robbins et al., 1972b).
Carworth CFE rats (10/sex/group) were administered i.m.
injections of gentamicin in saline, 6 days/week for 4 weeks. The doses
were 0, 25, 50, or 200 mg/kg bw/day. Overt signs of toxicity occurred
at 200 mg/kg bw/day, which included hypoactivity, bradypnoea,
bradycardia, and increased mortality. Body-weight gain was depressed
at 50 and 200 mg/kg bw/day. Haematological examinations revealed
slight depressions in haemoglobin and haematocrit at 200 mg/kg bw/day.
Serum chemistry and urinalyses were not performed. A limited necropsy
revealed pale renal cortices, increased kidney weights, renal tubular
necrosis, haemorrhage and inflammatory changes at the injection site,
and increased parenchymatous degeneration of the liver. These lesions
were found only at 200 mg/kg bw/day. The NOEL was 25 mg/kg bw/day
(Eggert et al., 1963a).
Charles River CD rats (5 males/group) received daily subcutaneous
injections of gentamicin in an aqueous vehicle. Doses of 0, 50, or
150 mg/kg bw/day were administered for 14, 21 or 28 days. At 150
mg/kg bw/day, food and water intake were reduced and rats exhibited
prostration, dyspnoea, and "staring coat". All animals at this dose
died during week 2. Body-weight gain was reduced in both dose groups,
and vestibular function was unimpaired. Serum chemistry analyses and
urinalysis revealed evidence of altered renal function at 50 mg/kg
bw/day in animals killed after 14 days of treatment, but not in those
killed at later times. The findings included increased serum
creatinine and BUN, decreased specific gravity and pH of urine,
glucosuria and a reduced clearance rate of endogenous creatinine.
Haematology parameters were not evaluated. At necropsy, pallor of the
renal cortex was increased in treated groups. Histological
examination, which was restricted to the kidneys, showed proximal
tubular necrosis in all gentamicin-treated rats, the severity being
related to dose (Robbins et al., 1976a).
Wistar rats (20/sex/group) were administered i.m. injections of
0, 25, 63, or 156 mg/kg bw/day gentamicin for 30 days. Groups of 5
rats/sex/dose were allowed a recovery (no treatment) period of 30
days. In the highest-dose group, decreased motor activity,
prostration, ptosis, piloerection, congested conjunctiva, lacrymation,
ataxia, and soft faeces were noted. Approximately half the high-dose
animals died within 12 days, the remainder were killed on day 12.
Body-weight gain and food consumption were depressed at all dose
levels.
Decreases in erythrocytes, haemoglobin, and haematocrit were
observed in the 63 mg/kg bw/day group. At 25 mg/kg bw/day and above,
leucocytes, BUN, cholesterol, albumin/globulin ratio and SGOT were
increased. At necropsy, swelling and discoloration of the kidney and
inflation of the caecum were noted at 63 mg/kg bw/day and above.
Degeneration of renal cortical tubules, tubular dilatation, protein
casts and infiltration of round cells in the interstitium were found
in all treated groups. At 156 mg/kg bw/day, necrosis of renal tubules,
hepatocyte vacuolation, and bone marrow hypoplasia were also observed.
Inflammatory cell infiltration, haemorrhage and edema at the injection
site were dose-related. All changes tended to show some reversal
following the 30-day recovery period (Hara et al., 1977a).
Groups of Charles River CD rats (20/sex/group) received daily
i.m. injections of gentamicin in an aqueous vehicle. Doses of 0,
12.5, or 50 mg/kg bw/day were administered for up to 13 weeks. After
4 weeks of treatment, 5 males and 5 females were killed and examined.
There were no clinical signs of toxicity apart from thinness and
locomotor ataxia in a 50 mg/kg bw/day male which was killed because of
its moribund condition on day 15. The same animal had severely
depressed haematocrit, haemoglobin and leucocyte counts. It is
unlikely that the effects seen in this animal were due to treatment
with the test article.
Food intake and body-weight gain were decreased in all treated
groups. Results of vestibular function tests and ophthalmological
evaluations were unremarkable. In the 50 mg/kg bw/day group,
haemoglobin and haematocrit were slightly decreased and BUN was
slightly increased. Urine specific gravity and pH were slightly
decreased at both treatment levels. Increases in kidney weights,
renal tubular necrosis, basophilia and leucocytic infiltration were
observed in both dose groups. Pale kidneys were reported in the 50
mg/kg bw/day group only. Injection site haemorrhages were also seen in
both dose groups (Robbins et al., 1976b).
Groups of Sprague-Dawley CD rats (10/sex/group) were fed
gentamicin in the diet for 13 weeks. The concentration in the diet was
adjusted as needed to maintain doses of 0, 4, 19 or 116 mg/kg bw/day.
The high-dose group was increased to 233 mg/kg bw/day from week 10.
No overt signs of toxicity were observed in any dose group.
Ophthalmologic evaluations, body-weight gain, food intake, and
mortality in treated groups were comparable to controls. Soft stools
were observed in the highest-dose group. Haematological parameters,
BUN, blood glucose and serum iron were unaffected by treatment.
Urinalyses showed an increased number of high-dose males with ketone
bodies in urine. At the end of the study, kidneys, liver, heart and
brain were weighed and gross and histopathological examinations were
performed on a wide range of tissues. No treatment-related effects
were noted. The NOEL was 19 mg/kg bw/day (Woodard et al., 1970).
Carworth CFE rats (controls: 20/sex; treated: 30/sex/group) were
administered i.m. injections of gentamicin in an aqueous vehicle, 5
days per week for up to 52 weeks. The doses were 0, 5, 10, or 20
mg/kg bw/day. At 26 weeks, 10/sex from the treatment groups and 5/sex
from the control group were killed and examined. The remainder were
killed at termination.
There were no clinical signs of toxicity or mortality. Body-
weight gain was depressed in males in the 20 mg/kg bw/day group from
week 10, although feed intake was comparable to controls. Haemoglobin
and haematocrits were slightly decreased in the mid- and high-dose
groups during the last half of the study. Leucocytes, blood glucose
and urinalysis parameters remained within normal limits.
At necropsy, liver, heart and brain weights were unaffected.
Adverse effects on the kidney included increased organ weight and
pitted or mottled surfaces at 10 and 20 mg/kg bw/day. The onset of
interstitial nephritis (seen commonly in aging rats) occurred earlier
in treated groups compared to controls. At 6 months, only one rat in
the control group was affected, while almost all rats in the high-dose
group exhibited this effect. At 12-months, the incidence was
statistically significant in the high-dose group only, with the
incidence in other groups not differing significantly from controls.
Injection-site reactions were observed in all dose groups with a dose-
related increase in severity. Muscle fibrosis was seen at the higher
treatment levels. The NOEL was 5 mg/kg bw/day (Robbins et al.,
1970a).
2.2.2.2 Rabbits
Two dermal studies were conducted in New Zealand white rabbits at
doses of 0, 0.5, 1, or 2 mg/kg bw/day gentamicin. Animals were
treated 6 hours/day, 5 days/week on intact and abraded skin for either
21 days (3 females per group) or 90 days (2 males and 1 female per
group). A different vehicle (unidentified) was used in each study.
Clinical signs, body weight, serum chemistry, haematology, urinalysis,
and gross and microscopic pathology were unaffected. Erythema and
edema at the application sites were slight in one study and moderate
in the other. These effects were considered to be vehicle-related
(Eggert et al., 1963b; 1964).
2.2.2.3 Dogs
Groups of beagle dogs (2/sex/group) were administered i.m.
injections of gentamicin or gentamicin C1 in an aqueous vehicle
(sterile water) daily for 14 consecutive days. The doses were 0, 15,
30, or 45 mg/kg bw/day.
There were no deaths or clinical signs of toxicity, nor were body
weight or ophthalmological parameters affected. Clinical chemistry
analyses showed that both drugs caused an increase in BUN at 45 mg/kg
bw/day. Other biochemical and haematological parameters remained
within normal limits. Urine specific gravity was lower and urine
volume increased in the 45 mg/kg bw/day gentamicin group. Occult blood
and albumin in urine were increased in treated groups, particularly in
the 45 mg gentamicin/kg bw/day group. One dog in this group also
showed glucosuria.
Gross examination of organs revealed pale and congested renal
cortices at 45 mg/kg bw/day. Kidney weights were increased in a dose-
related manner in all treated groups. Renal proximal convoluted
tubule degeneration or necrosis was increased with 30 and 45 mg/kg
bw/day gentamicin and 45 mg/kg bw/day gentamicin C1. Haemorrhage,
inflammatory changes and/or necrosis were observed at all injection
sites, including vehicle controls (Robbins et al., 1972c).
In another 14-day study, groups of beagle dogs (2/sex/group)
recieved i.m. injections of 0, 10, or 45 mg/kg bw/day gentamicin or
45, 90, or 120 mg/kg bw/day gentamicin B in an aqueous vehicle.
There were no clinical signs of toxicity. Food intake and body
weights were depressed at 45 mg/kg bw/day gentamicin and 120 mg/kg
bw/day gentamicin B. Three dogs given 45 mg gentamicin/kg bw/day were
killed on day 14 because of their moribund condition. Gentamicin-
induced changes in clinical laboratory investigations were recorded in
the 45 mg/kg bw/day group, which included increases in neutrophils,
BUN and SGPT; a slight decrease in urine specific gravity; and glucose
and occult blood in urine. Increases in SGPT were seen at all dose
levels of gentamicin B.
At necropsy, kidney and liver weights were unaffected by either
drug at any dose level. Pale renal cortices and increased lymphocytic
infiltration and coalescence of mitochondria in proximal convoluted
tubule epithelial cells were seen in all drug-treated groups.
Proximal tubular necrosis and excessive fat deposition were associated
with administration of 45 mg/kg bw/day gentamicin (Robbins et al.,
1973a).
One-day-old beagle puppies (4 litters per dose, 6-9 pups/litter)
were injected i.m. with gentamicin in an aqueous vehicle daily for 14
consecutive days. Doses were 0, 2, 6, or 10 mg/kg bw/day. Two
litters per dose were killed after 5 days and 2 pups per litter were
maintained for a drug-free recovery period of 6 weeks following the
last treatment. There was no mortality and no clinical signs of
toxicity. Body weight, righting reflex, haematology and serum
biochemistry parameters remained within normal limits. Post-mortem
examination of kidneys and liver showed no effects on organ weight or
histo-pathology. Histolopathological evaluations of the vestibular
apparatus of the ear were unremarkable (Vymetal et al., 1969).
Daily i.m. injections of gentamicin in a saline vehicle were
administered to groups of beagle dogs (2/sex/group) for up to 3 weeks.
The doses were 0, 15, 30 or 60 mg/kg bw/day. Vomiting was reported in
3/4 dogs in the high-dose group between days 9 and 12 of treatment and
in 3/4 dogs in the mid-dose group between days 16 and 20 of treatment.
Vomiting occurred within one or two hours of treatment. All dogs in
the 60 mg/kg bw/day group were killed between days 9 and 12 because of
their extreme moribund condition characterized by weakness, lethargy,
recumbency and irregular respiration. Slight body-weight loss was
noted during the second week in the 30 mg/kg bw/day group.
Ophthalmological findings were unremarkable.
Haematological parameters remained within normal limits. Serum
creatinine and BUN were increased at 30 and 60 mg/kg bw/day. At the
end of the study, SGPT was elevated and serum sodium and chloride ions
were decreased at 30 mg/kg bw/day. At 30 mg/kg bw/day and above,
urine specific gravity was decreased compared to controls and
glucosuria and occult blood were detected. Urine volume was increased
at 60 mg/kg bw/day. Kidney weights were increased in all treated
groups. Gross and histopathological examination revealed renal
cortical pallor and renal tubular necrosis in all treated animals.
Liver congestion was observed at 30 and 60 mg/kg bw/day. Muscle
degeneration and fibrosis of injection sites were seen at 60 mg/kg
bw/day (Robbins et al., 1973b).
In two separate studies, beagle dogs (2/sex/group) were
administered i.m. injections of gentamicin consisting of two different
ratios of the components C1 and C2, viz. 58:42 and 70:30. Groups
were treated with 0, 6, 10, or 50 mg/kg bw/day for 7 weeks. The
findings were essentially the same for each ratio. Clinical signs were
reported as anorexia, ataxia, prostration, vomiting and convulsions,
but the group incidences were not provided. Dogs in the highest-dose
group died or were killed during weeks 2 and 3. Body weights were
unaffected.
Haemoglobin and haematocrits fell sharply just prior to
intercurrent death or terminal kill in all treated animals. Increased
BUN, glucosuria, albuminuria, and urinary casts were observed in the
10 and 50 mg/kg bw/day groups. Pale renal corticies were observed in
all treated groups. This finding was accompanied by a gelatinous
transudate in animals dosed at 50 mg/kg bw/day. Toxic nephrosis was
noted in treated animals, which was characterized as cloudy swelling
at 6 mg/kg bw/day to severe tubular necrosis at 50 mg/kg bw/day.
Muscle damage was seen at injection sites in all groups including
controls. (Eggert et al., 1963c; 1973).
Beagle dogs (2/sex/group) received i.m. injections of 0, 63, or
100 mg gentamicin/kg bw/day. The duration was intended to be 30 days,
but all treated animals died within 18 days. Signs of gentamicin
toxicosis included vomiting, salivation, gait disturbances, difficulty
in standing, decreases in heart and respiration rates, and reduced
body weight and food intake. Gross and histopathological examinations
revealed swelling, discoloration and increased weight of the kidney,
degeneration and necrosis of renal tubular epithelium, infiltration of
round cells, protein casts and narrowing and dilatation of tubules.
These findings were observed at both treatment levels. Inflammation
and muscle fibre degeneration at the injection sites were also
observed in both dose groups with a dose-related increase in severity
(Hara et al., 1977b).
Groups of beagle dogs (4/sex/group) were administered i.m. doses
of 0, 12.5, or 25 mg/kg bw/day gentamicin in an aqueous vehicle, for
12 weeks. There were no treatment-related clinical signs of toxicity.
Food consumption was unaffected. Body-weight loss occurred in all
groups, which was more severe in the 25 mg/kg bw/day group and the
12.5 mg/kg bw/day females.
Ophthalmological and haematological parameters remained within
normal limits. There were slight elevations in SGPT, SGOT and
creatine phosphokinase at both treatment levels. At 25 mg/kg bw/day,
urine specific gravity was decreased and urine volume was increased.
Glucosuria and occult blood were present in one 12.5 and two 25 mg/kg
bw/day animals. Gross and histopathological findings included
increased kidney and liver weights, pale renal cortices, and proximal
renal tubular necrosis in all treated groups (Robbins et al.,
1976c).
Groups of beagle dogs (4/sex/group) were administered oral doses
of 0, 2, 10, or 60 mg/kg bw/day gentamicin in capsules for 14 weeks.
The highest level was increased to 120 mg/kg bw/day after 2 months.
Emesis and diarrhoea were observed occasionally in treated dogs.
There were no effects on body weight, ophthalmology, haematology,
blood chemistry or urinalysis. The only postmortem change was
interstitial nephritis observed in 2 animals in the high-dose group.
The NOEL was 10 mg/kg bw/day (Vymetal et al., 1970).
Beagle dogs (controls: 2/sex; treated: 4/sex/dose) were injected
i.m. with doses of 0, 3, 5, or 8 mg/kg bw/day gentamicin in an aqueous
vehicle. Animals were treated 5 days per week for up to 12 months.
One dog per sex per group was scheduled for an interim kill after 6
months. Localized pain and transient lameness of the injected leg
were seen in all groups. Ophthalmological and physical examination
findings were within normal limits. Vomiting and profuse salivation
were noted in one animal in the 8 mg/kg bw/day group in weeks 23 and
24. This animal lost considerable body weight during the last 8 weeks
of treatment and had a slightly increased BUN, glucosuria, decreased
urine specific gravity and increased urine volume. Body weight,
haematology, blood chemistry and urinalysis were within normal limits
in all other dogs.
Pale renal corticies and interstitial nephritis were seen after
gross and histopathological examinations in all treatment groups with
a dose-response relationship. Organ weights were similar in all
groups. Inflammatory changes, haemorrhage and fibrosis at the
injection sites were present in all groups, with an increase in
severity in treated animals (Robbins et al., 1970b).
2.2.2.4 Monkeys
Groups of 4 squirrel monkeys (sex not specified) were
administered daily s.c. injections of 0, 25, 50 or 75 mg/kg bw
gentamicin in saline, for up to 3 weeks. All monkeys receiving
gentamicin (except one animal in the low dose group) had to be killed
intercurrently because of their moribund condition. The average
survival times were 15.3, 10.3 and 7.5 days at 25, 50 and 75 mg/kg
bw/day, respectively. Clinical signs included ataxia, weakness,
hypoactivity, irregular respiration and eye scratching at each dose
level. Sporadic convulsions occurred at 50 and 75 mg/kg bw/day.
Body-weight loss was recorded in all groups, including controls, which
appeared to be enhanced with gentamicin treatment. However, there was
no clear, dose-response relationship.
Post-injection acoustic reflex measurements could not be obtained
from the 75 mg/kg bw/day group due to their rapid decline in
condition. In addition, instrument failure resulted in the loss of 3
post-injection data points. Therefore, evaluations were performed on
a total of 14 monkeys out of 28. Results indicated that hearing
deficits occurred in both the low- and mid-dose groups. Apart from the
single low-dose survivor, all animals had increased serum creatinine
and BUN. The author stated that the majority of dosed animals showed
severe renal tubular necrosis. Individual animal data were not
provided (Igarashi et al., undated).
Groups of 3 female Rhesus monkeys were injected i.m. with doses
of 0, 6 or 30 mg/kg bw/day gentamicin in an aqueous vehicle for 3
weeks. Adverse clinical signs were limited to the 30 mg/kg bw/day
group, which included pronounced facial paling and ptosis, markedly
disturbed equilibrium from day 20, and depressed food intake and body-
weight gain from week 2 onwards. Two monkeys in this group were
killed because of their moribund condition on day 21.
Haematological examinations showed decreases in haemoglobin,
haematocrit, and erythrocytes at 30 mg/kg bw/day. Serum chemistry
findings included increases in SGPT, SGOT and LDH, and decreases in
serum potassium, sodium, chloride and calcium ions in the 30 mg/kg
bw/day dose group. Serum creatinine and BUN were increased in both
dose groups. Urinalyses revealed proteinuria, glucosuria and an
increased presence of casts and epithelial cells at 30 mg/kg bw/day.
At necropsy, kidney weights were increased and had a yellow-grey
appearance. Histologically, cloudy swelling, cortical striation,
degeneration and necrosis of proximal convoluted tubules were
reported. The renal lesions which were seen in both dose groups, were
considered to be treatment-related. Vacuolar degeneration of
hepatocytes was noted at 30 mg/kg bw/day. Injection sites showed
dose-related muscle degeneration and necrosis accompanied by
haemorrhage and cellular infiltration. Electron microscopy of renal
tubules from the 30 mg/kg bw/day monkeys revealed myeloid bodies
present in both tubular cells and lumen, increased phagosomes,
disappearance of brush borders and sloughing of epithelial cells from
the basement membrane (Tanaka et al., 1977).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
Groups of Carworth CFE rats (30/sex/group) were injected 6 days/
week with i.m. doses of 0, 5, or 20 mg/kg bw/day gentamicin in saline.
Males were injected from 70 days prior to mating until 7 days before
mating. Females in the 5 mg/kg bw/day group were injected from 14
days prior to pairing with a male until post partum day 21. Females
in the 20 mg/kg bw/day group were treated from the time of
impregnation (determined by vaginal smear) until post partum day 21.
Ten females per group were killed for litter and dam evaluations on
gestation day 21, the remainder were allowed to deliver normally. F1
offspring were not treated. Groups of 10 males and 10 females from
randomly selected litters were paired within treatment levels
(avoiding brother-sister mating) to produce an F2 generation.
F1 Dams were allowed to deliver normally.
After 13 doses, penile bleeding was observed in high-dose F0
males. Because of this finding, the dose was reduced to 15 mg/kg
bw/day after which bleeding ceased. In F0 males of the high dose,
body-weight gain was slightly lower during the second half of the
treatment period. There were no overt effects in females.
Pregnancy rates were not affected. There was no prenatal
mortality and litter sizes and weights were similar between groups.
Early postnatal mortality was increased at both doses, which was
attributed to maternal neglect and failure to lactate, neither of
which were considered treatment-related. Fetal abnormalities were not
observed in delivered pups or caesarian-derived fetuses. Gentamicin
was present in the amniotic fluid in 5/10 low-dose dams, and 8/10
high-dose dams but was not detected in homogenized fetuses. No
treatment-related changes in pregnancy rate, litter size and weight,
prenatal mortality or fetal abnormalities were reported in this study
(Robbins et al., 1966).
2.2.4 Special studies on embryotoxicity and teratogenicity
2.2.4.1 Mice
Female JCL-ICR mice (15-33/group) received s.c. injections of
gentamicin in saline during gestation days 6 to 10. The doses
administered were 0, 1, 10 or 100 mg/kg bw/day. In the high-dose
group, body-weight gain was depressed during treatment and kidney
weight was increased. Gentamicin was measured in amniotic fluid in
the high-dose group. It was not clear whether attempts were made to
measure gentamicin in amniotic fluid in the other two dose groups. In
five control and five 100 mg/kg bw/day dams, delivery of fetuses was
allowed to occur normally. The development of eye and ear openings
was unaffected and kidney and liver weights were similar between
groups at post partum day 35. The remaining females were killed on
gestation day 17 for dam and litter evaluations. Prenatal loss was
increased at 10 and 100 mg/kg bw/day. There were no treatment-related
increases in fetal abnormalities (Nishio et al., 1987).
2.2.4.2 Rats
Female Carworth CFE rats (40/group) were injected i.m. with
gentamicin in saline on gestation days 3 to 16. The doses
administered were 0, 25, or 50 mg/kg bw/day, 6 days per week. Females
were allowed to deliver normally. At least 10 days after weaning the
first litter, dams were bred again and the above procedures were
repeated. Adult females showed no clinical signs of toxicity. Body-
weight gain was lower in the 50 mg/kg bw/day group during the
treatment period. At necropsy, pale renal cortices were observed in
both dose groups. Resorption rates were comparable to control values.
The number of stillborn pups was slightly increased at 50 mg/kg
bw/day following the second series of matings. Offspring were killed
and examined 21 days post partum. No treatment-related abnormalities
were reported (Eggert & Haring, 1964).
Groups of 17 female Sprague-Dawley rats were given intra-muscular
injections of 0 or 75 mg/kg bw/day gentamicin in saline. Treatment
was on gestation days 10 to parturition. Dams were allowed to deliver
normally. Parturition was delayed in gentamicin-treated dams by an
average of 15 hours and birth weights were lower. In pups showing the
lowest birth weights, kidney weight and the number of nephrons were
reduced at birth and at 14 and 21 days post partum. Pups with higher
birth weight showed similar kidney weight and numbers of nephrons to
controls; however, a relative decrease in the number of nephrons was
reported in these animals at 14 and 21 days post partum. Necrosis was
not reported but vacuoles in proximal tubular epithelium were
increased with loss of brush border microvilli and the presence of
debris in the tubular lumen. Gentamicin was detected in the kidneys
of all pups born to treated mothers, the levels being higher in pups
showing the lowest birth weights.
In 7 litters per group, half the pups received subcutaneous doses
of 0 or 75 mg/kg bw/day of gentamicin on post partum days 1 to 13,
then killed on post partum day 14. Drug treatment caused an increase
in kidney weight but the number of glomeruli were similar in both
groups (Gilbert et al., 1987).
In an abstract, it was reported that rats were given s.c. doses
of 0 or 110 mg/kg bw/day gentamicin on either gestation days 10 to 15
or 15 to 20. Females were allowed to deliver normally. Measurements
of arterial blood pressure in offspring, one year after birth,
revealed significantly elevated blood pressure in females but not in
males (Chahoud et al., 1986).
2.2.4.3 Guinea-pigs
Guinea-pigs (6 females per group) were administered i.m.
injections of 0 or 4 mg/kg bw/day gentamicin in saline on gestation
days 48 to 54. Females were allowed to deliver normally. Litter size
and weight and body and kidney weights of offspring from the treated
group were comparable to controls to day 20 post partum. Studies of
kidney function in offspring showed a higher excretion of phosphate in
3-day-old pups but not at 10 days of age. Excretion of sodium,
potassium, magnesium and calcium, urinary flow rate, glomerular
filtration rate and the numbers of glomeruli were unaffected. The
length of proximal tubules and glomerular volume were reduced at 3 and
10 days post partum but not at 20 days. Gentamicin was present in pup
renal cortex at higher levels that in the medulla. Levels were
highest 3 days after birth and decreased thereafter (Le Lievre
et al., 1987).
2.2.4.4 Rabbits
Groups of 13 female New Zealand white rabbits were administered
i.m. doses of 0, 0.8, or 4 mg/kg bw/day gentamicin in saline. The
high dose was given as two divided doses, 7 hours apart. Treatments
were administered on gestation days 6 to 16. Eight females per group
were killed for dam and litter evaluations on gestation day 30. The
remainder were allowed to deliver normally. Pups were killed and
examined on post partum day 21. Adult animals were in good health
throughout the study. No treatment-related effects were noted on
prenatal loss, litter size or weight, fetal morphology, or postnatal
survival or body-weight gain. (Robbins & Klein, 1966).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies on gentamicin are summarized
in Table 2.
2.2.6 Special studies on kidney function
2.2.6.1 Rats
A group of 6 male Sprague-Dawley rats was injected i.m. with
doses of 80 to 160 mg/kg bw/day gentamicin for 7 days. At
termination, animals were anaesthetized with thiobarbital and kidney
function was assessed. The glomerular filtration rate, as measured by
inulin clearance, was reduced in a dose-related manner in all
treatment groups. Aciduria and glucosuria (determined by Labstix)
were induced at 160 mg/kg bw/day, but not at the lower doses,
indicating that reabsorption of glucose in the proximal renal tubule
had been impaired (Chiu et al., 1977).
Sprague-Dawley rats (group sizes not given) were injected s.c.
with 0 or 100 mg/kg bw/day gentamicin for up to 7 days. After four
doses, serum creatinine and BUN were increased and urine osmolality
was decreased, suggesting impairment of urine concentrating capacity.
Tubular reabsorption of solute free water was unaffected. Tubular
secretion of para-aminohippurate (PAH) was increased, although
glomerular filtration rate was decreased, after 4 days. Kidney
cortical slices, isolated from rats treated with gentamicin,
accumulated PAH in vitro at a faster rate than slices from control
animals; this effect was inhibited in the presence of probenecid. This
provided evidence that an alteration in the organic acid transport
system was responsible for the increased rate of PAH accumulation
(Cohen et al., 1974).
Female Sprague-Dawley rats (group sizes not given) were injected
s.c. with 0, 50, 100, or 150 mg/kg bw/day gentamicin for up to 14
days. During the treatment period, urine osmolality was decreased
followed by a rise in urine volume and water intake. Proteinuria was
seen from day 3 and serum creatinine and BUN were increased at 100 and
150 mg/kg bw/day. At termination, microscopic examination of kidneys
revealed dose-related injury to proximal tubules in all dose groups,
consisting of increased lysosomal bodies, disruption of brush borders,
cellular swelling and necrosis. Kidney cortical slices, from rats
treated with gentamicin, showed increased uptake of PAH in vitro
(Kaloyanides, 1977).
Sprague-Dawley rats (group sizes not given) were injected i.p.
with 0 or 10 mg/kg bw/day gentamicin for up to 14 days. Kidney
function was unaffected throughout the treatment period. Animals were
killed after 1, 4, 7 and 14 doses and kidneys removed for examination.
Microscopic examinations found proliferation of lysosomes in proximal
tubular cells on day 4. This was associated with decreases in
lysosomal sphingomyelinase and phospholipase activities, suggesting
lysosomal dysfunction. At day 9, protrusion of cells and/or nuclei
into tubular lumens was observed and several cells had lost part of
their brush borders. Focal necrosis was evident on day 14 (Carlier
et al., 1982).
2.2.6.2 Dogs
Male beagle dogs (group sizes not given) were injected i.m. with
0 or 30 mg/kg bw/day gentamicin for up to 11 days. Serum creatinine,
BUN, and urine protein were increased, and creatinine clearance and
urine osmolality were decreased in gentamicin-treated animals. In
addition, urinary levels of beta-glucuronidase, N-acetyl-beta-
glucosaminidase and muramidase were elevated. Microscopic renal
lesions ranged from diffuse moderate hyaline droplet degeneration to
frank tubular necrosis. The pathological and enzyme changes appeared
to precede the functional alterations (Adelman et al., undated).
2.2.7 Special study on ototoxicity
2.2.7.1 Monkeys
Groups of 3 female Cynomolgus monkeys were injected i.m. with
doses of 0, 25, or 50 mg/kg bw/day gentamicin for 35 days. All monkeys
dosed at 50 mg/kg bw/day died: one on day 7 and the other two on day
22. Prior to death, the pinna reflex to high frequencies in these
monkeys was slightly reduced, but there was no observable ataxia
indicative of vestibular impairment. Histopathological examination of
the crista ampullaris showed vacuole formation at both doses and, at
the higher dose, hair cell loss and a reduction in the thickness of
sensory epithelium. In the organ of Corti, cochlear hair cell loss was
noted at 50 mg/kg bw/day while the histology of the macula was not
significantly affected (Yakota et al., 1984).
2.2.8 Special study on neurotoxicity
Gentamicin was administered parenterally to Charles River CD
rats, cats and beagle dogs, to assess its neurotoxicity potential.
The treatment regimes were as follows:
Rats - 0, 6.6, 16.6, 41.5 or 104 mg/kg bw/day, s.c., for
41 days.
Cats - 0, 2.5, 5.0 or 10.0 mg/kg bw/day, s.c., for 42 days.
Dogs - 0, 5.0, 8.3 or 41.5 mg/kg bw/day, i.m., for 49 days.
In rats, righting reflex was impaired at 104 mg/kg bw/day and
ataxia was observed in dogs given 8.3 and 41.5 mg/kg bw/day. Ataxia
was not produced in cats at any dose (Taber et al., 1963).
2.2.9 Special studies on microbiological effects
Results of investigations into the effects of gentamicin on human
intestinal flora were not available. However, two published reports
were available on the inhibitory activity of gentamicin against a wide
range of bacterial strains in vitro.
Table 2. Results of genotoxicity studies on gentamicin
Test system Test object Results Reference
Reverse mutation1 S. typhimurium negative2 Houk et al., 1989
Reverse mutation1 S. typhimurium negative3 Koeda & Hirano, 1979
TA98, TA100,
TA1535, TA1536,
TA1537, TA1538
Reverse mutation1 S. typhimurium negative2 Mitchell et al., 1980
TA98, TA100,
E. coli WP2
Reverse mutation1 E. coli CM891 negative3 Mitchell & Gilbert, 1984
Forward mutation1 E. coli CM891 positive3 at a Mitchell & Gilbert, 1984
cytotoxic level
(0.25 µg/ml)
Forward mutation1 E. coli WP2 positive2 at a Mitchell et al., 1980
cytotoxic level
(0.49 µg/ml)
Mitotic crossing- S. cerevisiae D5 negative3 Koeda & Hirano, 1979
over1
Gene conversion1 S. cerevisiae D4 negative3 Mitchell et al., 1980
Petite inducation1 S. cerevisiae D4 positive3 (500 Mitchell et al., 1980
µg/ml) at pH4.4,
not pH 7.2
DNA repair1 E. coli WP2, negative2 Mitchell et al., 1980
WP100
Rec-assay1 B. subtilis negative3 Kada et al., 1980
M45, H17
SOS function1 E. coli PQ37 negative2 Mamber et al., 1986
DNA-cell binding E. coli Q13 positive2 Kubinski et al., 1981
(20 µg/ml) test
not validated
Table 2 (contd)
Test system Test object Results Reference
Chromosome Mouse L-cells positive3 but Leonard & Botis, 1975
aberrations mitosis inhibited
(500 µg/ml x 4 d
plus 100 µg/ml x
20 d)
SCE Human fibroblasts positive3 but McDaniel & Schultz, 1993
GM00847 less than doubling
(75-250 µg/ml)
1 Appropriate positive controls were used.
2 Both with and without rat liver S9 fraction.
3 Without metabolic activation.
One article reported the minimum inhibitory concentrations (MICs)
for gentamicin in 601 clinical isolates of anaerobic bacteria. The
experiments were conducted using the agar-dilution technique at 37°C
for 48 hours in an anaerobic incubator, an inoculum of 103 to 104
colony-forming units and serial concentrations of gentamicin in the
range of 0.1 to 25 µg/ml (Martin et al., 1972). In a second
publication, results were reported for Clostridium difficile
strains, but there was no information on the culture conditions
employed (Lorian, undated). All results are presented in Table 3.
The susceptibility to gentamicin of most anaerobic bacteria was
low, the most sensitive being Eubacterium sp., with a modal MIC
value of 0.8 µg/ml for the 20 strains tested.
2.3 Observations in humans
In a prospective, randomized, blinded, comparative trial, 54
patients treated with gentamicin were evaluated for nephrotoxicity and
ototoxicity. The dosage regime was usually 1.7 mg/kg bw i.v. every 8
hours (5.1 mg/kg bw/day) for 7 to 54 days. Desired serum drug levels
were maintained by reducing the dose in patients with impaired renal
function. Nephrotoxicity, defined as a 50% increase in serum
creatinine, was produced in 8 (15%) patients. Auditory toxicity,
defined as a decrease in the sensorineural threshold of at least 15
decibels in two or more frequencies, was produced in 3 (6%) patients.
Vestibular toxicity, defined as at least a 50% decrease in maximum
average slow-phase eye speed following water stimulation of the ear,
was induced in 3 of 33 (9%) patients. Auditory and vestibular effects
were not noted in the same individuals (Lerner et al., 1986).
Kidney biopsies were obtained from 5 patients with renal cancer
following surgical removal of both kidneys. Prior to surgery,
gentamicin was administered at a dosage of 1.5 mg/kg bw three times a
day (4.5 mg/kg bw/day) for 4 days. Microscopic evaluation of the
kidney specimens revealed increases in lysosome numbers with moderate
to marked decreases in the activity of sphingomyelinase and acid
phospholipase A1 (Carlier et al., 1982).
In a retrospective study involving patients on chronic
haemodialysis therapy, 23 patients were treated with gentamicin.
Dosages were not identified. Of the drug-treated subjects, 7 (30%)
developed clinically detectable vestibular toxicity. The principal
risk factors for the development of ototoxicity were age, duration of
treatment and the total dose administered. There was no positive
association with peak serum levels of gentamicin or the total absence
of kidneys (Gailiunas Jr. et al., 1978).
A review, focusing mainly on prospective trials, reported on a
total of more than 10 000 patients treated with aminoglycoside
antibiotics. The incidence of cochlear toxicity associated with
gentamicin administration ranged from 5 to 10% in adults. The
incidence was claimed to be lower in infants (Matz, 1993).
Adverse reactions to gentamicin during the years 1963 to 1973
were reviewed. Of the 3500 patients studied, the main non-specific
reactions were increases in serum transaminases (16), skin rashes (16)
and granulocytopaenia (8). Also presented were results from clinical
trials with gentamicin. Possible or probable nephrotoxicity was found
in 22 of 285 (7.7%) patients in 1965 to 1966, 70 of 1450 (4.8%)
patients in 1967 to 1969 and 48 of 1635 (2.9%) patients in 1970 to
1973. Possible or probable ototoxicity was found in 16 of 565 (2.8%)
patients in 1963 to 1966, 27 of 1450 (1.8%) patients in 1967 to 1969
and 15 of 1635 (1%) patients in 1970 to 1973. Critical doses or
duration of treatment were not given, but ototoxicity usually occurred
in association with impaired renal function (Hewitt, 1974).
The rates of allergic cutaneous reactions were determined in 15
438 consecutive in-patients monitored by the Boston Collaborative Drug
Surveillance Program from June 1975 to June 1982. Of the 670 patients
receiving gentamicin, there were 3 (0.5%) skin reactions.
Table 3. Minimum inhibitory concentration (MIC) values for gentamicin against anaerobic bacteria
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Bacteroides fragilis (195) 1 6
B. incommunio (10) 10 40
B. variabilis (5) 60
B. oralis (2) 50 100
B. terebrans (5) 40 60
B. melaninogenicus (29) 24 31 34 45 59 76
Bacteroides F1,F2,F3 (11) 27 36 45 73
Fusobacterium fusiforme (18) 5 11 56
Fusobacterium sp. (2) 50
Clostridium perfringens (34) 6
C. difficile (15) 20 40
Clostridium sp. (17) 6 18
Peptococcus sp. (145) 2 3 5 7 11 30 62 95
Peptostreptococcus sp. (72) 1 9 13 18 20 33 58 78
Veillonella sp. (13) 8 31 46 85
Table 3 (contd)
Organism (No. of isolates) Cumulative percent inhibition at each concentration (µg/ml)
0.1 0.4 0.8 1.6 3.1 6.2 12.5 25
Propionibacterium acnes (16) 25 56 75 81
Eubacterium lentum (14) 43 57 71 85 93 100
E. alactolyticum (2) 50
Eubacterium sp. (5) 20 40
Bifidobacterium sp. (5) 20 60 80 100
Catenabacterium (3) 66
filamentosum
Catenabacterium sp. (2) 50 100
3. COMMENTS
A range of studies on gentamicin was submitted for assessment,
including information on pharmacokinetics and metabolism, acute
toxicity, short-term toxicity, reproductive toxicity and
teratogenicity, genotoxicity, antimicrobial activity, a variety of
special studies and observations in humans.
Gentamicin, in common with other aminoglycosidic drugs, is poorly
absorbed from the gastrointestinal tract. Parenteral doses distribute
mainly into extracellular space, but there is significant penetration
into the kidney cortex and the inner ear. There is negligible
metabolism of the drug and unchanged gentamicin is excreted rapidly,
mostly in the urine.
Single oral doses of gentamicin were slightly toxic in rodents
(LD50 = 8000 to 10 000 mg/kg bw), which supports the view that the
drug is largely unabsorbed when administered by this route. Parenteral
dosing of mice, rats, guinea-pigs and dogs demonstrated high
intravenous toxicity (LD50 = 37 to 67 mg/kg bw) and moderate toxicity
by i.m., s.c. and i.p. routes (LD50 = 213 to 893 mg/kg bw).
Gentamicin was extensively tested by the i.m. and s.c. routes in
rats administered the compound for periods of up to 12 months (doses
up to 200 mg/kg bw/day) in dogs for up to 12 months (doses up to 100
mg/kg bw/day) and in monkeys for 3 weeks (doses up to 75 mg/kg
bw/day). The kidney was the primary target organ in each species with
effects observed predominantly in the renal cortex. The major
findings were an increase in interstitial nephritis and toxic
nephrosis in the proximal convoluted tubules. The latter was
characterized by a dose- and time-dependent progression from loss of
brush borders, cloudy swelling, the presence of casts and
proteinaceous material in the tubules, desquamation of epithelial
cells and necrosis. These changes were associated with a profound
impairment of renal function, and in extreme cases, death of severely
affected animals. Special studies in rats suggested that the
nephrotoxicity may be associated with disruption of lysosomal
function. Nephrotoxicity has been observed in humans treated with
gentamicin.
Toxicity to the inner ear may occur after exposure to
aminoglycosides, including gentamicin. There were no clear
indications of ototoxicity in the general toxicity studies; however,
a special study in monkeys, at doses up to 50 mg/kg bw/day, showed
hair cell loss and reduced sensory epithelium in structures of the ear
as well as a functional loss in hearing. Observations in humans have
revealed auditory or vestibular toxicity after therapeutic
administration of gentamicin.
Oral dosing of rats and dogs for a period of 3 months, at doses
up to 116 and 60 mg/kg bw/day, respectively, was virtually without
systemic effects. Soft stools or diarrhoea were observed in both
species and dogs showed interstitial nephritis at high doses. The
NOELs were 19 mg/kg bw/day in rats and 10 mg/kg bw/day in dogs.
A multigeneration reproductive toxicity study in the rat showed
no adverse effects on reproduction parameters after intramuscular
injections of 5 or 20 mg gentamicin/kg bw/day. Teratogenicity studies
in mice, rats, guinea-pigs and rabbits did not identify any potential
for the production of fetal abnormalities. Fetotoxicity, in the form
of fetal death in mice (10 mg/kg bw/day) and rats (50 mg/kg bw/day)
and reduced birth weight in rats (75 mg/kg bw/day), was observed after
parenteral dosing.
Positive results were obtained in some in vitro genotoxicity
studies, but none of these was adequate to evaluate the genotoxicity
of gentamicin. There were no carcinogenicity studies available on
gentamicin. In toxicity studies in animals of up to one year
duration, there were no pre-neoplastic or neoplastic lesions. The
Committee considered that the data were inadequate to fully assess the
carcinogenicity of gentamicin.
Data on the effects of gentamicin on human gut flora were not
available. However, in vitro data were presented on MICs for over
600 clinical isolates of anaerobic bacteria including some species
representative of the microbial flora in the human gastrointestinal
tract. Many of the organisms were found to be resistant to gentamicin;
Eubacterium species were the most sensitive. Using an inoculum
density of 103 to 104 colony forming units per plate, MIC values for
Eubacterium species were in the range 0.1 to 25 µg/ml with an MIC50
of 0.8 µg/ml.
In view of the poor oral absorption of gentamicin and the
reported occurrence of diarrhoea in experimental animals, it was
considered that effects on the gastrointestinal microflora would be
the most sensitive effect of residues. In calculating the ADI, the
formula developed by the thirty-eighth meeting of the Committee (Annex
1, reference 97) was employed:
Concentration without effect x Daily faecal bolus (g)
on human gut flsor (µg/ml)a
Upper limit of ADI =
Fraction of oral x Safety x Human body
dose availableb factorc weight
(60 kg)
(0.8 x 2) x 150
=
1 x 1 x 60
= 4 µg/kg bw
a This value accounts for the range of MICs in appropriate
bacterial species and the use of the most sensitive anaerobic
organism. A two-fold adjustment was deemed to be necessary to
account for the relatively low inoculum density.
b Absorption of gentamicin after oral dosing is very poor, therefore
a factor of 1 was used to represent 100% availability of ingested
drug in the gastrointestinal tract.
c Data on more than 600 clinical isolates of anaerobic bacteria were
available for determination of the MIC. Because the colonic flora
is relatively stable and variability within a particular individual
may be as great as variability between individuals, and because it
was recognized that other values selected for this calculation were
conservative and already incorporated an adequate margin of safety,
a safety factor of 1 was selected to cover fully the variability
between people of all extrapolated parameters.
4. EVALUATION
In view of the absence of information on the effects of
gentamicin on microorganisms obtained from the human intestine and the
need for further genotoxicity studies, the Committee established a
temporary ADI of 0-4 µg/kg bw based on the results of micro-biological
testing of clinical isolates, with a request for further information.
An increased safety factor was not used to account for the temporary
status of the ADI because conservative factors were used in deriving
the temporary ADI. The lowest toxicological NOEL was 10 mg/kg bw/day
in dogs, which is more than 2000-fold higher than the
microbiologically based ADI.
5. REFERENCES
ADELMAN, R.D., CONZELMAN, G., SPANGLER, W. & ISHIZAKI, G. (undated).
Comparative nephrotoxicity of gentamicin and netilmicin : functional
and morphological correlations with urinary enzyme activities.
Unpublished Report No. D 11403. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ATEF, M., ARBID, M.S. & HANAFY, M.S.M. (1992). Comparison of
gentamicin toxicity in normal and diabetic rats. Acta Veterinaria
Hungarica, 40: 107-111.
BIGBY, M., JICK, S., JICK, H. & ARNDT, K. (1986). Drug-induced
cutaneous reactions. JAMA, 256: 3358-3363.
CARLIER, M.B., PAULUS, G., MALDAGUE, P., GIURGEA, L., WILMOTTE, E., DE
BROC, M.E. & TULKENS, P. (1982). Early toxic events in kidney of rat
and man following administration of gentamicin at low doses.
Arch. Toxicol. Suppl. 5: 287-290.
CHAHOUD, I., STAHLMANN, R. & NEUBERT, D. (1986). Blood pressure
elevation in one-year-old female rats after prenatal exposure to
gentamicin. Teratology, 33: 13A.
CHIU, P.J.S., LONG, J.F., BARNETT, A., TABER, R.I., MILLER, G. &
WAITZ, J.A. (1977). Kidney function in rats chronically treated with
aminoglycosides. Unpublished Report No. P 4485. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
COHEN, L., LAPKIN, R. & KALOYANIDES, G.J. (1974). Effect of gentamicin
on renal function in the rat. Unpublished Report No. D 6338. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
EGGERT, M.J., DAVENPORT, L.R. & WILLIAMSON, N.K. (1973). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3379. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., EVANS, M. & WILLIAMSON, N.K. (1963a). SCH 9724 in rats.
Subacute (4 weeks) intramuscular toxicity study. Unpublished Report
No: P 3355. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J. & HARING, G.D. (1964). Intramuscular teratogenic study of
SCH 9724 (gentamicin sulfate 70:30) in rats. Unpublished Report No: P
3397. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., HARING, G.D. & VYMETAL, F. (1965). Acute toxicity
studies of gentamicin sulfate intramuscularly in rats and dogs.
Unpublished Report No: P 3502. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., HARING, G.D., WILLIAMSON, N.K. & WEINSTEIN, M.J. (1975).
Acute and subacute toxicity studies of SCH 9724 (gentamicin sulfate)
in rat neonates. Unpublished Report No: P 3497. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1963b).
Gentamicin sulfate-90 day dermal toxicity in rabbits. Unpublished
Report No: P 3410. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
EGGERT, M.J., ROSS, M.R., WEINSTEIN, M.J. & WILLIAMSON, N.K. (1964).
Dermal toxicity of SCH 9724 in rabbits. Unpublished Report No: P 3425.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
EGGERT, M.J., VYMETAL, F. & WILLIAMSON, N.K. (1963c). Subacute
intramuscular toxicity study of SCH 9724 in dogs. Unpublished Report
No: P 3350. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
FRASER, C.M., BERGERON, J.A., MAYS, A. & AIELLO, S.E. (1991).
Aminoglycosides. In: The Merck Veterinary Manual, 7th Edition ,
Merck & Co., Rahway, pp 1429-1435.
GAILIUNAS JR., P., DOMINGUEZ-MORENO, M., LAZARUS, J.M., LOWRIE, G.,
GOTTLIEB, M.N. & MERRILL, P. (1978). Vestibular toxicity of
gentamicin. Arch. Intern. Med., 138: 1621-1624.
GILBERT, T., LELIEVRE-PEGORIER, M., MALIENOU, R., MEULEMANS, A. &
MERLET-BENICHOU, C. (1987). Effects of prenatal and postnatal exposure
to gentamicin on renal differentiation in the rat. Toxicology, 43:
301-313.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977a).
Safety evaluation of KW-1062. I. Acute toxicity in mice, rats and
dogs, subacute and chronic toxicity in rats. Jap. J. Antibiotics ,
30: 386-407.
HARA, T., KOYAMA, K., MIYAZAKI, H., OHGURO, Y. & SHIMIZU, M. (1977b).
Safety evaluation of KW-1062. II. Subacute and chronic toxicity
studies in dogs. Jap. J. Antibiotics, 30: 408-422.
HEWITT, W.L. (1974). Gentamicin: toxicity in perspective. Postgrad.
Med. J., (Suppl. 7): 55-59.
HOUK, V.S., SCHALKOWSKY, S. & CLAXTON, L.D. (1989). Development and
validation of the spiral Salmonella assay: an automated approach to
bacterial mutagenicity testing. Mutation Res., 223: 49-64.
IGARASHI, M., LEVY, J.K., JERGER, J., WAITZ, J.A. & ARCIERI, G.
(undated). Comparative toxicity of netilmicin and gentamicin in
squirrel monkeys. Unpublished Draft Report No: D 10312. Submitted to
WHO by Inveresk Research International, Trenant, Scotland, on behalf
of Schering Plough Animal Health.
KADA, T., HIRANO, K. & SHIRASU, Y. (1980). Screening of environmental
chemical mutagens by the Rec-assay with Bacillus subtilis. In:
Chemical Mutagens and Principles for their Detection. Chap. 6: pp
149-173.
KALOYANIDES, G.J. (1977). Untitled. Unpublished Report No: D 10069.
Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
KOEDA, T. & HIRANO, F. (1979). Evaluation of the mutagenicity of
aminoglycoside antibiotics in Salmonella typhimurium and
Saccharomyces cerevisiae. J. Antibiotics, 32: 607-609.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA cell-binding
assay for suspected carcinogens and mutagens. Mutation Res., 89:
95-136.
LELIEVRE, M., GILBERT, T., SAKLY, R., MEULEMANS, A. & MERLET-BENICHOU,
C. (1987). Effect of fetal exposure to gentamicin on kidneys of young
guinea pigs. Antimicrob. Agents Chemother., 31: 88-92.
LEONARD, A. & BOTIS, S. (1975). Chromosome damage induced by
gentamicin in mouse L-cells. Experientia, 31: 341-343.
LERNER, S.A., SCHMITT, B.A., SELIGSOHN, R. & MATZ, G.J. (1986).
Comparative study of ototoxicity and nephrotoxicity in patients
randomly assigned to treatment with amikacin or gentamicin.
Am. J. Med., 80(6B): 98-104.
LORIAN, V. (undated). Tables 28.13 and 28.15. In: Antibiotics in
Laboratory Medicine. 2nd Edition. Williams & Wilkins, Baltimore-
London-Los Angeles-Sydney, pp 1087-1089 and 1142-1146.
MAMBER, S.W., OKASINSKI, W.G., PINTER, C.D. & TUNAC, J.B. (1986). The
Escherichia coli K-12 SOS chromotest agar spot test for simple,
rapid detection of genotoxic agents. Mutation Res., 171: 83-90.
MARTIN, W.J., GARDNER, M. & WASHINGTON II, J.A. (1972). In vitro
antimicrobial susceptibility of anaerobic bacteria isolated from
clinical specimens. Antimicrob. Agents Chemother., 1: 148-158.
MATZ, G. (1993). Aminoglycoside cochlear ototoxicity. Otolaryngologic
Clinics of North America, 26: 705-712.
McCORMICK, G.C. (1983). Acute intravenous toxicity comparison of
gentamicin sulfate and gentamicin C2 sulfate in mice (SN 83272).
Unpublished Report No: D17472. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
McDANIEL, L.D. & SCHULTZ, R.A. (1993). Elevation of sister chromatid
exchange frequency in transformed human fibroblasts following exposure
to widely used aminoglycosides. Environ. Molec. Mutagenesis, 21:
67-72.
MILLER, G.H., WAITZ, J.A. & WEINSTEIN, M.J. (1976). Pharmacokinetics
of C14-gentamicin in dogs. Unpublished Report No: P 4440. Submitted
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
MITCHELL, I DEG., DIXON, P.A., GILBERT, P.J. & WHITE, D.J. (1980).
Mutagenicity of antibiotics in microbial assays. Mutation Res., 79:
91-105.
MITCHELL, I DEG. & GILBERT, P.J. (1984). The effect of pretreatment of
Escherichia coli CM 891 with ethylenediaminetetraacetate on
sensitivity to a variety of standard mutagens. Mutation Res., 140:
13-19.
NISHIO, A., RYO, S. & MIYAO, N. (1987). Effects of gentamicin,
exotoxin and their combination on pregnant mice. Bull. Fac. Agric.
Kagoshima Univ., 37: 129-136.
RIVIERE, J.E., CRAIGMILL, A.L. & SUNDLOF, S.F. (1991).
Aminoglycosides. In: Handbook of Comparative Pharmacokinetics and
Residues of Veterinary Antimicrobials, CRC Press, Boca Roton, Florida,
pp 263-335.
ROBBINS, G.R., CASTELLANO, R.F., TORNING, F.E., FABRY, A. & FIELDER,
F.G. (1971).- Acute toxicity in rats (intramuscular, intravenous and
intraperitoneal) and mice (intramuscular, intravenous, intraperitoneal
and subcutaneous). Unpublished Report No : P 3967. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., FABRY, A., MORGAN, W.A. & EGGERT, M.J. (1972a).
Subacute (two-week) intramuscular toxicity study of SCH 14342 in rats.
Unpublished Report No: P 4074. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1970a).
Chronic toxicity study of SCH 9724 by intramuscular injection in rats.
Unpublished Report No: P 3884. Submitted by Inveresk Research
International, Trenant, Scotland, to WHO on behalf of Schering Plough
Animal Health.
ROBBINS, G.R., FABRY, A., TORNING, F.E. & EGGERT, M.J. (1972b).
Subacute (two-week) toxicity study of SCH 13706 by intramuscular
injection in rats. Unpublished Report No: P 4072. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R. & KLEIN, M.F. (1966). Reproduction study of gentamicin
sulfate in rabbits. Unpublished Report No : P 3595. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., KLEIN, M.F. & EGGERT, M.J. (1966). Reproduction study
using gentamicin sulfate intramuscularly in rats. Unpublished Report
No : P 3598. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970b).
Chronic (1-year) intramuscular toxicity study in dogs using gentamicin
sulfate (SCH 9724). Unpublished Report No: P 3848. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1972c).
Subacute (14-day) intramuscular toxicity study of SCH 13706 Gentamicin
C1 ) in dogs. Unpublished Report No: P 4065. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1973a).
Subacute intramuscular toxicity study of SCH 14342 as compared with
SCH 9724 in dogs. Unpublished Report No: P 4138. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H. & GRAY, W.D. (1976a). The subacute
toxicity of SCH 20569 sulfate, (vs gentamicin, tobramycin and
kanamycin) administered subcutaneously to rats. Unpublished Report No:
P 4424. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D. & FIELD, W.E. (1976b).
Subacute intramuscular toxicity of SCH 20569 in rats. Unpublished
Report No: P 4393. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., GRAY, W.D., FIELD, W.E. & SCHIAVO, D.M.
(1976c). Subacute intramuscular toxicity of SCH 20569 in dogs.
Unpublished Report No: P 4392. Submitted to WHO by Inveresk Research
International, Trenant, Scotland, on behalf of Schering Plough Animal
Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R., FIELD, W.E. & OWEN, G.
(1973b). Subacute intramuscular toxicity in dogs using SCH 15666 and
SCH 9724. Unpublished Report No: P 4196. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., MARCHESO, R. & OWEN, G. (1973c). Acute
intramuscular toxicity study in guinea pigs. Unpublished Report No: P
4174. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
ROBBINS, G.R., WEINBERG, E.H., OWEN, G., MARCHESO, R. & FIELD, W.E.
(1973d). Subacute intramuscular toxicity in rats using SCH 15666 and
SCH 9724. Unpublished Report No: P 4212. Submitted to WHO by Inveresk
Research International, Trenant, Scotland, on behalf of Schering
Plough Animal Health.
SANDE, M.A. & MANDELL, G.L. (1990). Antimicrobial agents. The
aminoglycosides. In: GILMAN, A.G., RALL, T.W., NIES, A.S. & TAYLOR, P.
(eds), Goodman & Gilman's The Pharmacological Basis of Therapeutics,
8th Edition, Pergamon Press, New York-Oxford-Beijing-Sao Paulo-Sydney-
Tokyo-Toronto, pp 1098-1116.
TABER, R., IRWIN, S. & PERLMAN, P.L. (1963). The effect of subchronic
administration of gentamicin sulfate, kanamycin sulfate and
streptomycin sulfate in the rat, cat and dog. Unpublished Report No:
P 3316. Submitted to WHO by Inveresk Research International, Trenant,
Scotland, on behalf of Schering Plough Animal Health.
TANAKA, H., WATANABE, M. & HATTORI, H. (1977). Subacute toxicity of
sisomycin administered intramuscularly in monkeys. Comparison with
gentamicin and tobramycin. Unpublished Report No: D 10555. Submitted
to WHO by Inveresk Research International, Trenant, Scotland, on
behalf of Schering Plough Animal Health.
VYMETAL, F., BAKER, F.W. & EGGERT, M.J. (1970). Subacute (14 week)
oral toxicity study of SCH 14894 (gentamicin sulfate, veterinary
grade) in dogs. Unpublished Report No: P 3923. Submitted to WHO by
Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
VYMETAL, F., EGGERT, M.J. & ROBBINS, G.R. (1969). Toxicity study of
gentamicin sulfate by injection in neonatal dogs. Unpublished Report
No: P 3818. Submitted to WHO by Inveresk Research International,
Trenant, Scotland, on behalf of Schering Plough Animal Health.
WOODARD, M.W., HOWARD, D.J., WOODARD, G. & EGGERT, M.J. (1970).
Gentamicin sulfate safety evaluation by repeated oral administration
to rats for 13 weeks. Unpublished Report No: P 3924. Submitted to WHO
by Inveresk Research International, Trenant, Scotland, on behalf of
Schering Plough Animal Health.
YAKOTA, M., TAKEDA, U., SAKAMOTO, K., SETO, N. & IGARASHI, M. (1984).
The comparative ototoxicities of panimycin and gentamicin in
cynomolgus monkeys (Maccaca fascicularis). Chemotherapy, 30:
248-254.
