
BENZYLPENICILLIN
1. EXPLANATION
Benzylpenicillin is used to treat or prevent local and systemic
infections caused by susceptible bacteria. Intramammary
administration to treat or prevent bovine mastitis is widespread.
Subtherapeutic concentrations in feed have been used for decades in
some countries to improve growth and to prevent infections.
Benzylpenicillin had been previously evaluated at the twelfth
meeting of the FAO/WHO Joint Expert Committee on Food Additives (Annex
1, Reference 17).
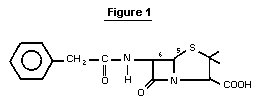
Benzylpenicillin is a compound belonging to the class of ß-lactam
antibiotics. Its structure is shown in Figure 1. The free acid (CAS
registry number 61-33-6) is relatively unstable. Therefore, the mono-
sodium salt (CAS registry number 113-98-4), or other salts are
normally used. Benzylpenicillin is obtained from penicillium molds by
fermentation.
The international unit of penicillin is the specific penicillin
activity contained in 0.5988 µg of the international master standard.
 2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution, and excretion
In humans, about one third of an orally administered dose of
benzylpenicillin is absorbed from the intestinal tract under favorable
conditions. Only a small portion is absorbed from the stomach.
Absorption occurs mainly in the duodenum. Ingestion of food
interferes with enteric absorption. Benzylpenicillin is widely
distributed throughout the body. Its apparent volume of distribution
is in about 50% of total body water. Benzylpenicillin is rapidly
eliminated from the body (mainly by the kidney) (Mandell & Sande,
1985).
2.1.2 Biotransformation
Gastric acid hydrolyzes the amide side chain and opens the lactam
ring, with concomitant loss of antibacterial activity. The
biotransformation of benzylpenicillin is not well understood. The
drug is only partially metabolized and the major fraction is excreted
unchanged (Huber, 1988).
2.1.3 Effects on enzymes
Benzylpenicillin selectively inhibits bacterial cell wall
biosynthesis. The linear polysaccharide ("glycan") of the cell wall
is cross-linked by branched peptide chains to form a structure termed
"peptidoglycan". The primary transpeptidase reaction leading to
covalent linkage of new chains to the pre-existing peptidoglycan
network is highly sensitive to penicillin. Several proteins,
including transpeptidases, located in the bacterial cell membrane bind
penicillin covalently in the form of a penicilloyl moiety via an ester
linkage (cleavage of the lactam ring). It appears that the antibiotic
is recognized as a pseudo-substrate which subsequently acts as a
strong acylating agent to form a covalent enzyme-inhibitor complex at
the active site (Bycroft & Shute, 1985).
2.1.4 Immunogenicity and antigenicity
Many antigenic determinants may derive from benzylpenicillin.
The biochemical routes to the formation of some of these determinants
may be multiple. The benzylpenicilloyl (BPO) determinant has been
designated the "major" antigenic determinant of penicillin allergy.
BPO haptenic groups can be formed by direct acylation of proteins or
from benzylpenicillenic acid, either by direct reaction or via a
postulated reactive intermediate, thiazolidinyl-oxazolidone. The
formation of BPO determinants from benzylpenicillin acid itself, the
major product of hydrolysis of benzylpenicillin, has not yet been
definitely established.
All other determinants resulting from the binding of other
metabolites of benzylpenicillin are collectively referred to as the
"minor" determinants. Many questions pertain to both. The mechanisms
of hapten formation and the immunogenicity of certain "minor"
determinants remain open. Antibodies specifically directed to one or
another of these determinants have, however, been found in patients
(e.g., antibodies with penicillamine or penicillemyl specificity) or
have been induced in experimental animals. It cannot be ruled out
that such determinants may be frequently involved in penicillin
allergy. Polymerization of benzylpenicillin, which can readily occur
under certain conditions, has been described. It is, however, not yet
fully clear whether and under which circumstances such polymers are
also capable of reacting as immunogens.
Protein impurities in benzylpenicillin preparations, which are
formed during the fermentation process and which could be sufficiently
highly substituted to efficiently raise antibodies, could contribute
to the overall immunogenicity. The critical number of drug-related
epitopes on carriers may depend on the nature of the carrier. Low-
substituted benzylpencilloyl-conjugates with human serum albumin were
of very poor immunogenicity. On the other hand, penicillin-autologous
carrier protein conjugates have been shown to be immunogenic in a
guinea pig model (De Weck, 1983).
Penicilloylated proteins from milk or tissue as they may appear
after high-dose treatment of animals may possess antigenic properties
if absorbed through the mucosa of the gut (Wal & Boris 1975; Wal,
1980).
Antibodies raised against benzylpenicillin-derived determinants
may cross-react to some extent with certain antigens derived from
other ß-lactams including cephalosporins. Similarly, antigens
carrying benzylpenicillin-derived haptenic groups may cross-react with
antibodies to other ß-lactams (DeSwarte, 1985).
2.1.5 Inhibitory activity on microorganisms used in industrial
food-processing
A variety of microorganisms (e.g., lactobacilli, streptococci,
certain yeasts and fungi) are used as "starter cultures" in the
manufacturing processes of milk products, such as sour milk, sour
cream, yoghurt, butter, kefir, and many different kinds of cheese.
It is well known that penicillin residues in milk can negatively
influence both the quality and yield of such milk-products (Jakimov,
1970; Cogan, 1972; Abo-Elnage et al., 1973; Dolezalek & Behavkova,
1974; Bayer et al., 1978; Kondratenko et al., 1978; Loussouran,
1982).
Microbiologically active residues of benzylpenicillin can cause
severe economic losses by inhibiting such microorganisms and thus
interfering with biotechnological food processing. Streptococcus
thermophilus, for example, is partially inhibited at concentrations of
penicillin as low as 0.0017 I.U./ml. Total inhibition occurs at
0.025-0.05 I.U./ml (Terplan & Zaadhoff, 1967).
2.2 Toxicological studies
No experimental studies were available for review. Information
contained in the open literature does not meet minimum requirements
for an evaluation of the toxicological properties of the drug.
2.3 Observations in humans
Benzylpenicillin may induce practically all possible clinical
forms of allergic reactions depending on dose, route, frequency of
exposure, genetic predisposition and other factors. Penicillin will
induce an immune response in practically every person who receives the
drug. Low titers of benzylpenicilloyl-specific IgM antibodies can be
detected in virtually everyone. However, the mere presence of
antipencillin-antibodies of any class does not necessarily denote
clinical sensitivity. Hypersensitivity reactions are by far the most
common adverse effects noted with benzylpenicillin (DeSwarte, 1985).
The proportion of the general population that may be susceptible
to the development of allergy is unknown. Available data did not
allow conclusions as to the true prevalence of penicillin-sensitized
individuals in the population since all available studies were carried
out in selected subpopulations using tests of limited diagnostic
value. The frequency of allergic side reactions has been reported to
vary from 0.7% to 10% in different studies (Idso et al., 1968).
The overall prevalence of penicillin allergy has been estimated
to be between 3% and 10%, indicating that a substantial proportion of
the population is at risk (Anderson & Adkinson, 1987).
Frequencies of skin reactions to commonly used drugs were
estimated from the records of the Boston Collaborative Drug
Surveillance Program (data base from 1966 through May, 1975). Fifty-
one individuals out of a total of 3286 recipients showed
benzylpenicillin-induced skin reactions corresponding to a rate of
16/1000) (Arndt & Jick, 1976).
In a second evaluation (data base: June 1975 through June 1982)
17 recipients out of a total number of 918 gave a cutaneous reaction
with benzylpenicillin (rate: 18.5/1000) (Bigby et al., 1986).
2.3.1 Sensitizing capacity
2.3.1.1 Qualitative aspects
Using techniques such as skin testing and RAST (radio-
allergosorbent test), antibodies with reactivities to the following
determinants have been found in humans: benzylpenicilloyl-,
benzylpenicillenate-, benzylopenicillenyl- and penicillamine-
determinant (De Weck, 1983).
2.3.1.2 Quantitative aspects
It remains impossible to describe in quantitative terms in
humans:
- the major pathways and kinetics (e.g., concentrations, rates,
catalysts) of the in vivo formation of antigenic
determinants,
- the nature of the biological carrier (e.g., soluble proteins,
membranes of lymphoid cells).
- relative abundances and biological activities of the various
haptenic groups/determinants derived from benzylpenicillin.
There were no useful data available to calculate the minimum
required dose of any potential hapten to produce the minimum required
amount of complete immunogen with the appropriate number of haptenic
epitopes per molecule to induce an immune response following oral
administration.
In a detailed analysis of data on penicillin-sensitive reactions
in Taiwan, it has been reported that a 50-year old man who had taken
a penicillin tablet of 50,000 units one year before the injection of
a combination of benzylpenicillin and streptomycin died 20 minutes
after the injection (Idso & Wang, 1958). If one assumes that this was
the patient's only exposure to the drug, this would suggest that a
single dose of approximately 30 mg of benzylpenicillin could sensitize
a human.
However, this information is of very limited value since it is
known that repeated administration of low doses of immunogen is the
most effective way to produce an IgE antibody response (Levine & Vaz,
1970; Marsh, 1975). Virtually nothing about the immunogenicity of
chronic low-level administration of benzylpenicillin in humans is
known.
2.3.2 Eliciting capacity
2.3.2.1 Qualitative aspects
The initial event in IgE-mediated reactions is the interaction of
bivalent or polyvalent antigen with antibody bound to high affinity
Fc-receptors for IgE on tissue mast cells and blood basophils followed
by aggregation (at least dimerization) of Fc-receptors (Metzger,
1988).
The elicitation of reaction in already-sensitized individuals
requires no macromolecular antigen. The low molecular weight N6-N6-
bis-benzylpencilloyl-diaminohexane, for example, can elicit
anaphylactic reactions (Schneider, 1983).
It is still not completely understood how benzylpenicillin itself
and its active low molecular weight metabolites could so rapidly react
in vivo to form such divalent or polyvalent antigens. Positive
skin tests have been obtained with the following haptens in patients
allergic to penicillin: benzylpenicillin, benzylpenicilloic acid,
benzylpenicillin-oligomer, benzylpenicillin-polymers, and carrier-
conjugates exhibiting the following haptenic groups/determinants:
benzylpencilloyl, benzylpenicillenyl, and penicillamine (De Weck,
1983).
In consequence, the above substances, and at least all divalent
antigens carrying benzylpenicilloyl- and/or benzylpenicillenyl- and/or
penicillamine-determinants formed upon covalent binding of reactive
haptens to tissue and/or milk proteins of target animals should be
considered as potential eliciting substances. It cannot be ruled out
that some of these molecules (if they existed as residues) could reach
at least the mast cells of the gut mucosa following oral ingestion.
2.3.2.2 Quantitative aspects
The overwhelming majority of penicillin preparations causing
reactions were administered parenterally. Severe reactions in
sensitive individuals after skin tests with less than one unit of
benzylpenicillin are documented including one exceptional case where
only 3 x 10-7 units had been applied (Bierlein, 1956). Such data,
however, are inappropriate to evaluate the risk of orally ingested
residues.
In an analysis of 151 fatalities from anaphylactic penicillin
reactions, it was reported that one patient died after an oral dose in
the range of 0.1 to 0.5 mega-units of benzylpenicillin (Idso et
al., 1968).
Severe reactions following the use of penicillin tablets have
also been reported. A fatality occurred after administration of one
tablet of 1000 units (Guthe et al., 1958).
Siegel has described experiments in which sera obtained from
patients with high IgE titers were used to sensitize skin sites on
normal subjects maintained on a milk free diet. Forty-eight to 72
hours later, the recipients were given oral doses of benzylpenicillin
in water. In one experiment, the smallest oral dose of
benzylpenicillin which could induce a whealing reaction was found to
be 40 units with a time to occurrence of 50 minutes. With penicillin
administered in milk as a diluent, threshold levels were slightly
elevated and time to occurrence was slightly prolonged. The needed
doses to elicit a similar reaction in allergic donors of such sera
would probably be lower (by a factor of 100 to 1000 or even more)
(Siegel, 1959).
An acute allergic reaction in a patient who had ingested milk
from a commercial supply which contained approximately 10 units/ml has
been reported (Wicher et al., 1969).
In a highly sensitized 25-year-old woman, less than 1 unit of
daily orally administered penicillin was sufficient to provoke
allergic symptoms. This patient suffered from a moderately severe
subacute eczematous eruption, which was traceable to penicillin-
contaminated milk. The patient's symptoms were relieved by addition
of penicillinase to the milk she consumed (Borrie & Barret, 1961).
A report documented a single case of acute angioedema and
pruritus in a penicillin-allergic patient who ingested freshly
processed meat from a pig injected with penicillin three days before
slaughter. The patient noted symptoms after the first bite of ground
pork. Analysis revealed a penicillin content of 0.3-0.45 units/gram
of meat (Tscheuschner, 1972).
Lindemayr and co-workers challenged nine penicillin-allergic
volunteers with 150g of raw pork meat (content: 0.024-0.04 ug/g) from
an animal treated with procaine-benzylpenicilline. Two subjects
reported itchy or local anesthetic sensations during the first 2
hours. However no objective symptoms of allergy could be observed
(Lindemayr et al., 1981).
Other cases (mainly anecdotal observations) have been reported.
There was, however, insufficient or even no evidence provided to
support the view that penicillin was the causative agent (Dewdney &
Edwards, 1984).
3. COMMENTS AND EVALUATION
No toxicological studies were available for review. Among the
adverse reactions which had been reported in people consuming food
containing benzylpenicillin residues, hypersensitivity reactions were
the most common. The overall prevalence of allergy to penicillin,
taking into account various reports of allergic reactions in different
populations and using a variety of test procedures, was estimated to
be 3 - 10%. There was no evidence of sensitization caused by
benzylpenicillin residues in food. The Committee evaluated the
available data on allergic reactions caused by penicillin residues.
Only four cases were considered to be adequately documented to
demonstrate that hypersensitivity reactions could be caused by
ingestion of less than 40 µg of the drug.
Residues of benzylpenicillin can also inhibit starter cultures
used in the production of yoghurt, cheese and other milk products.
The Committee concluded that allergy was the determinating factor
in the safety evaluation of residues of benzylpenicillin. In the
absence of adequate data to establish a no-effect level, the Committee
recommended that the daily intake from food be kept as low as
practicable, and in any case below 30 µg of the parent drug. The risk
associated with the occurrence of mild hypersensitivity reactions at
this level was considered to be insignificant.
5. REFERENCES
ABO-ELNAGE, I.G. ABDEL-MOTTALEB, L., & MAHMOUD, M. (1973).
Characteristics of Gouda cheese and yoghurt made from milk containing
penicillin. Scienza e Tecnica Lattiro-Casearia 24, 25-32.
ANDERSON, J.A. & ADKINSON, N.F. (1987). Allergic reactions to drugs
and biologic agents. JAMA 258, 2891-2899.
ARNDT, K.A. & JICK, H. (1976). Rates of cutaneous reactions to drugs.
A Report From the Boston Collaborative Drug Surveillance Program.
JAMA, 235, 918-922.
BAYER, A.S., CHOW, A.W., CONCEPTION, N., & GUZE, L.B. (1978).
Susceptibility of 40 lactobacilli to six antimicrobial agents with
broad Grampositive anaerobic spectra. Antimicrobial Agents and
Chemotherapy, 14, 720-722.
BIGBY, M., JICK, S., JICK, H., & ARNDT, K. (1986). Drug induced
cutaneous reactions. A report from the Boston Collaborative Drug
Surveillance Program on 15,438 consecutive inpatients. JAMA, 256,
3358-3363.
BIERLEIN, K.J. (1956). Repeated anaphylactic reactions in a patient
highly sensitized to penicillin. A case report. Ann. Allergy, 14,
35-40.
BORRIE. P., & BARRET, J. (1961). Dermatitis caused by penicillin in
bulked milk supplies. Br. Med. J., 2, 1267.
BYCROFT, B.W. & SHUTE, R.E. (1985). The molecular basis for the mode
of action of beta-lactam antibiotics and mechanisms of resistance.
Pharma. Res., 1985,3-14.
COGAN, T.M. (1972). Susceptibility of cheese and yoghurt starter
bacteria to antibiotics. Appl. Microbiol., 23, 960-965.
DESWARTE, R.D. (1985). Drug Allergy. in: Patterson, R. (ed.) Allergic
Diseases. Diagnosis and Management. 3rd edition, J.P. Lippincott,
Philadelphia, Chapter 19, pp. 595-614.
DEWDNEY, J.M. & EDWARDS, R.G. (1984). Penicillin hypersensitivity-is
milk a significant hazard? J. Roy. Soc. Med., 77, 866-877.
DE WECK, A.L. (1983). Penicillins and Cephalosporins. in: Allergic
reactions to drugs, De Weck, A.L. & Bundgaard, H. (eds.), Springer
Verlag, Berlin, pp. 423-482.
DOLEZALEK, J. & BEHAVKOVA, A. (1974). Effect of penicillin on the
formation of some flavour constituents in ripening of pure dairy
cultures. XIX International Dairy Congress, 1E, 442.
GUTHE, T., IDSO, O., & WILLCOX, R.R. (1958). Untoward penicillin
reactions. Bull. Wld. Hlth Org., 19, 427-501.
HUBER, W.G. (1988). Penicillins. in: Booth, N.H. & McDonald, L.E.
(eds.) Veterinary Pharmacology and Therapeutics, 6th edition, Iowa
State University Press, Ames, Iowa, Chapter 49, pp.796-812.
IDSO, 0. & WANG, P.N. (1958). Penicillin-sensitive reactions in
Taiwan. Bull. Wld. Hlth. Org., 18, 323-344.
IDSO, O., GUTHE, T., WILLCOX, R.R., & DE WECK, A.L. (1968). Nature
and extent of penicillin side-reactions, with particular reference to
fatalities from anaphylactic shock. Bull. Wld. Hlth. Org., 38,
159-188.
JAKIMOV, N. (1970). Antibiotics in milk and their effect on lactic
acid bacteria. Mikrobiologija, 7, 99-109.
KONDRATENKO, M., SHISHKOVA, I., TSANEVA, K. & G'OSHEV, B. (1978).
Inhibitory effects of antibiotics on yoghurt production. XX Inter.
Dairy Congress, E, 834-835.
LEVINE, B.B. & VAZ, N.M. (1970). Effect of combination of inbred
strain, antigen and antigen dose on immune responsiveness and reagin
production in mice. Int. Arch. Allergy App. Immunol. 39, 156-171.
LINDEMAYR, H., KNOBLER, R., KRAFT, D., & BAUMGARTNER, W. (1981).
Challenge of penicillin-allergic volunteers with
penicillin-contaminated meat. Allergy, 36, 471-478.
LOUSSOUARN, S. (1982). Sensitivity of lactic cultures to certain
antibiotics. Technique Taitiere 965, 49-50.
MANDELL, G.L. & SANDE, M.A. (1985). Antimicrobial agents. penicillins,
cephalosporins, and other beta-lactam antibiotics. in: Goodman and
Gilman's The Pharmaceutical Basis of Therapeutics. Eds. Gilman, A.G.,
Goodman, L.S., Rall, T.W. & Murad, F. 7th edition, Macmillan
Publishing Company, New York, Chapter 50, pp. 1115-1149.
MARSH, D.G. (1975). Allergens and the genetics of allergy. in: Sela,
M. (ed.) The antigens, Vol III, Academic Press, New York, San
Francisco, and London; pp. 271-361.
METZGER, H. (1988). Molecular aspects of receptors and binding
factors for IgE. Adv. in Immunol., 43, 277-312.
OLSON, J.C., JR. & SANDERS, A.C. (1975). Penicillin in milk and milk
products: Some regulatory and public health considerations. J. Milk
Food Technol., 38, 630-633.
SCHNEIDER, C.H. (1983). Immunochemical basis of allergic reactions to
drugs. in: Allergic reactions to drugs, De Weck, A.L. & Bundgaard, H.
(eds.), Springer-Verlag, Berlin, pp. 3-35.
SIEGEL, B.B. (1959). Hidden contacts with penicillin. Bull. Wld.
Hlth Org. 21, 703-713.
TERPLAN, G., & ZAADHOFF, K.J. (1967). On the incidence and detection
of inhibitory substances in milk - a short review (orig. in German).
Milchwissenschaft, 22, 761-771.
TSCHEUSCHNER, I. (1972). [translation from German:] Anaphylactic
reaction to penicillin after ingestion of pork. Z. Haut
Geschleantskr., 47, 591-592.
WAL, J.M. (1980). Enzymatic unmasking for antibodies of penicilloyl
residues bound to albumin. Biochem. Pharmacol., 29, 195-199.
WAL, J.M. & BORIS, G. (1975). Elimination of free penicillin and
penicilloyl-protein conjugates in the milk of cows following
intramammary administration of penicillin G. Annales biol. animale
biochim. biophys., 15, 615-617.
WICHER, K., REISMAN, R.E., & ARBESMAN, C. E. (1969). Allergic
reaction to penicillin present in milk. JAMA, 208, 143-145
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution, and excretion
In humans, about one third of an orally administered dose of
benzylpenicillin is absorbed from the intestinal tract under favorable
conditions. Only a small portion is absorbed from the stomach.
Absorption occurs mainly in the duodenum. Ingestion of food
interferes with enteric absorption. Benzylpenicillin is widely
distributed throughout the body. Its apparent volume of distribution
is in about 50% of total body water. Benzylpenicillin is rapidly
eliminated from the body (mainly by the kidney) (Mandell & Sande,
1985).
2.1.2 Biotransformation
Gastric acid hydrolyzes the amide side chain and opens the lactam
ring, with concomitant loss of antibacterial activity. The
biotransformation of benzylpenicillin is not well understood. The
drug is only partially metabolized and the major fraction is excreted
unchanged (Huber, 1988).
2.1.3 Effects on enzymes
Benzylpenicillin selectively inhibits bacterial cell wall
biosynthesis. The linear polysaccharide ("glycan") of the cell wall
is cross-linked by branched peptide chains to form a structure termed
"peptidoglycan". The primary transpeptidase reaction leading to
covalent linkage of new chains to the pre-existing peptidoglycan
network is highly sensitive to penicillin. Several proteins,
including transpeptidases, located in the bacterial cell membrane bind
penicillin covalently in the form of a penicilloyl moiety via an ester
linkage (cleavage of the lactam ring). It appears that the antibiotic
is recognized as a pseudo-substrate which subsequently acts as a
strong acylating agent to form a covalent enzyme-inhibitor complex at
the active site (Bycroft & Shute, 1985).
2.1.4 Immunogenicity and antigenicity
Many antigenic determinants may derive from benzylpenicillin.
The biochemical routes to the formation of some of these determinants
may be multiple. The benzylpenicilloyl (BPO) determinant has been
designated the "major" antigenic determinant of penicillin allergy.
BPO haptenic groups can be formed by direct acylation of proteins or
from benzylpenicillenic acid, either by direct reaction or via a
postulated reactive intermediate, thiazolidinyl-oxazolidone. The
formation of BPO determinants from benzylpenicillin acid itself, the
major product of hydrolysis of benzylpenicillin, has not yet been
definitely established.
All other determinants resulting from the binding of other
metabolites of benzylpenicillin are collectively referred to as the
"minor" determinants. Many questions pertain to both. The mechanisms
of hapten formation and the immunogenicity of certain "minor"
determinants remain open. Antibodies specifically directed to one or
another of these determinants have, however, been found in patients
(e.g., antibodies with penicillamine or penicillemyl specificity) or
have been induced in experimental animals. It cannot be ruled out
that such determinants may be frequently involved in penicillin
allergy. Polymerization of benzylpenicillin, which can readily occur
under certain conditions, has been described. It is, however, not yet
fully clear whether and under which circumstances such polymers are
also capable of reacting as immunogens.
Protein impurities in benzylpenicillin preparations, which are
formed during the fermentation process and which could be sufficiently
highly substituted to efficiently raise antibodies, could contribute
to the overall immunogenicity. The critical number of drug-related
epitopes on carriers may depend on the nature of the carrier. Low-
substituted benzylpencilloyl-conjugates with human serum albumin were
of very poor immunogenicity. On the other hand, penicillin-autologous
carrier protein conjugates have been shown to be immunogenic in a
guinea pig model (De Weck, 1983).
Penicilloylated proteins from milk or tissue as they may appear
after high-dose treatment of animals may possess antigenic properties
if absorbed through the mucosa of the gut (Wal & Boris 1975; Wal,
1980).
Antibodies raised against benzylpenicillin-derived determinants
may cross-react to some extent with certain antigens derived from
other ß-lactams including cephalosporins. Similarly, antigens
carrying benzylpenicillin-derived haptenic groups may cross-react with
antibodies to other ß-lactams (DeSwarte, 1985).
2.1.5 Inhibitory activity on microorganisms used in industrial
food-processing
A variety of microorganisms (e.g., lactobacilli, streptococci,
certain yeasts and fungi) are used as "starter cultures" in the
manufacturing processes of milk products, such as sour milk, sour
cream, yoghurt, butter, kefir, and many different kinds of cheese.
It is well known that penicillin residues in milk can negatively
influence both the quality and yield of such milk-products (Jakimov,
1970; Cogan, 1972; Abo-Elnage et al., 1973; Dolezalek & Behavkova,
1974; Bayer et al., 1978; Kondratenko et al., 1978; Loussouran,
1982).
Microbiologically active residues of benzylpenicillin can cause
severe economic losses by inhibiting such microorganisms and thus
interfering with biotechnological food processing. Streptococcus
thermophilus, for example, is partially inhibited at concentrations of
penicillin as low as 0.0017 I.U./ml. Total inhibition occurs at
0.025-0.05 I.U./ml (Terplan & Zaadhoff, 1967).
2.2 Toxicological studies
No experimental studies were available for review. Information
contained in the open literature does not meet minimum requirements
for an evaluation of the toxicological properties of the drug.
2.3 Observations in humans
Benzylpenicillin may induce practically all possible clinical
forms of allergic reactions depending on dose, route, frequency of
exposure, genetic predisposition and other factors. Penicillin will
induce an immune response in practically every person who receives the
drug. Low titers of benzylpenicilloyl-specific IgM antibodies can be
detected in virtually everyone. However, the mere presence of
antipencillin-antibodies of any class does not necessarily denote
clinical sensitivity. Hypersensitivity reactions are by far the most
common adverse effects noted with benzylpenicillin (DeSwarte, 1985).
The proportion of the general population that may be susceptible
to the development of allergy is unknown. Available data did not
allow conclusions as to the true prevalence of penicillin-sensitized
individuals in the population since all available studies were carried
out in selected subpopulations using tests of limited diagnostic
value. The frequency of allergic side reactions has been reported to
vary from 0.7% to 10% in different studies (Idso et al., 1968).
The overall prevalence of penicillin allergy has been estimated
to be between 3% and 10%, indicating that a substantial proportion of
the population is at risk (Anderson & Adkinson, 1987).
Frequencies of skin reactions to commonly used drugs were
estimated from the records of the Boston Collaborative Drug
Surveillance Program (data base from 1966 through May, 1975). Fifty-
one individuals out of a total of 3286 recipients showed
benzylpenicillin-induced skin reactions corresponding to a rate of
16/1000) (Arndt & Jick, 1976).
In a second evaluation (data base: June 1975 through June 1982)
17 recipients out of a total number of 918 gave a cutaneous reaction
with benzylpenicillin (rate: 18.5/1000) (Bigby et al., 1986).
2.3.1 Sensitizing capacity
2.3.1.1 Qualitative aspects
Using techniques such as skin testing and RAST (radio-
allergosorbent test), antibodies with reactivities to the following
determinants have been found in humans: benzylpenicilloyl-,
benzylpenicillenate-, benzylopenicillenyl- and penicillamine-
determinant (De Weck, 1983).
2.3.1.2 Quantitative aspects
It remains impossible to describe in quantitative terms in
humans:
- the major pathways and kinetics (e.g., concentrations, rates,
catalysts) of the in vivo formation of antigenic
determinants,
- the nature of the biological carrier (e.g., soluble proteins,
membranes of lymphoid cells).
- relative abundances and biological activities of the various
haptenic groups/determinants derived from benzylpenicillin.
There were no useful data available to calculate the minimum
required dose of any potential hapten to produce the minimum required
amount of complete immunogen with the appropriate number of haptenic
epitopes per molecule to induce an immune response following oral
administration.
In a detailed analysis of data on penicillin-sensitive reactions
in Taiwan, it has been reported that a 50-year old man who had taken
a penicillin tablet of 50,000 units one year before the injection of
a combination of benzylpenicillin and streptomycin died 20 minutes
after the injection (Idso & Wang, 1958). If one assumes that this was
the patient's only exposure to the drug, this would suggest that a
single dose of approximately 30 mg of benzylpenicillin could sensitize
a human.
However, this information is of very limited value since it is
known that repeated administration of low doses of immunogen is the
most effective way to produce an IgE antibody response (Levine & Vaz,
1970; Marsh, 1975). Virtually nothing about the immunogenicity of
chronic low-level administration of benzylpenicillin in humans is
known.
2.3.2 Eliciting capacity
2.3.2.1 Qualitative aspects
The initial event in IgE-mediated reactions is the interaction of
bivalent or polyvalent antigen with antibody bound to high affinity
Fc-receptors for IgE on tissue mast cells and blood basophils followed
by aggregation (at least dimerization) of Fc-receptors (Metzger,
1988).
The elicitation of reaction in already-sensitized individuals
requires no macromolecular antigen. The low molecular weight N6-N6-
bis-benzylpencilloyl-diaminohexane, for example, can elicit
anaphylactic reactions (Schneider, 1983).
It is still not completely understood how benzylpenicillin itself
and its active low molecular weight metabolites could so rapidly react
in vivo to form such divalent or polyvalent antigens. Positive
skin tests have been obtained with the following haptens in patients
allergic to penicillin: benzylpenicillin, benzylpenicilloic acid,
benzylpenicillin-oligomer, benzylpenicillin-polymers, and carrier-
conjugates exhibiting the following haptenic groups/determinants:
benzylpencilloyl, benzylpenicillenyl, and penicillamine (De Weck,
1983).
In consequence, the above substances, and at least all divalent
antigens carrying benzylpenicilloyl- and/or benzylpenicillenyl- and/or
penicillamine-determinants formed upon covalent binding of reactive
haptens to tissue and/or milk proteins of target animals should be
considered as potential eliciting substances. It cannot be ruled out
that some of these molecules (if they existed as residues) could reach
at least the mast cells of the gut mucosa following oral ingestion.
2.3.2.2 Quantitative aspects
The overwhelming majority of penicillin preparations causing
reactions were administered parenterally. Severe reactions in
sensitive individuals after skin tests with less than one unit of
benzylpenicillin are documented including one exceptional case where
only 3 x 10-7 units had been applied (Bierlein, 1956). Such data,
however, are inappropriate to evaluate the risk of orally ingested
residues.
In an analysis of 151 fatalities from anaphylactic penicillin
reactions, it was reported that one patient died after an oral dose in
the range of 0.1 to 0.5 mega-units of benzylpenicillin (Idso et
al., 1968).
Severe reactions following the use of penicillin tablets have
also been reported. A fatality occurred after administration of one
tablet of 1000 units (Guthe et al., 1958).
Siegel has described experiments in which sera obtained from
patients with high IgE titers were used to sensitize skin sites on
normal subjects maintained on a milk free diet. Forty-eight to 72
hours later, the recipients were given oral doses of benzylpenicillin
in water. In one experiment, the smallest oral dose of
benzylpenicillin which could induce a whealing reaction was found to
be 40 units with a time to occurrence of 50 minutes. With penicillin
administered in milk as a diluent, threshold levels were slightly
elevated and time to occurrence was slightly prolonged. The needed
doses to elicit a similar reaction in allergic donors of such sera
would probably be lower (by a factor of 100 to 1000 or even more)
(Siegel, 1959).
An acute allergic reaction in a patient who had ingested milk
from a commercial supply which contained approximately 10 units/ml has
been reported (Wicher et al., 1969).
In a highly sensitized 25-year-old woman, less than 1 unit of
daily orally administered penicillin was sufficient to provoke
allergic symptoms. This patient suffered from a moderately severe
subacute eczematous eruption, which was traceable to penicillin-
contaminated milk. The patient's symptoms were relieved by addition
of penicillinase to the milk she consumed (Borrie & Barret, 1961).
A report documented a single case of acute angioedema and
pruritus in a penicillin-allergic patient who ingested freshly
processed meat from a pig injected with penicillin three days before
slaughter. The patient noted symptoms after the first bite of ground
pork. Analysis revealed a penicillin content of 0.3-0.45 units/gram
of meat (Tscheuschner, 1972).
Lindemayr and co-workers challenged nine penicillin-allergic
volunteers with 150g of raw pork meat (content: 0.024-0.04 ug/g) from
an animal treated with procaine-benzylpenicilline. Two subjects
reported itchy or local anesthetic sensations during the first 2
hours. However no objective symptoms of allergy could be observed
(Lindemayr et al., 1981).
Other cases (mainly anecdotal observations) have been reported.
There was, however, insufficient or even no evidence provided to
support the view that penicillin was the causative agent (Dewdney &
Edwards, 1984).
3. COMMENTS AND EVALUATION
No toxicological studies were available for review. Among the
adverse reactions which had been reported in people consuming food
containing benzylpenicillin residues, hypersensitivity reactions were
the most common. The overall prevalence of allergy to penicillin,
taking into account various reports of allergic reactions in different
populations and using a variety of test procedures, was estimated to
be 3 - 10%. There was no evidence of sensitization caused by
benzylpenicillin residues in food. The Committee evaluated the
available data on allergic reactions caused by penicillin residues.
Only four cases were considered to be adequately documented to
demonstrate that hypersensitivity reactions could be caused by
ingestion of less than 40 µg of the drug.
Residues of benzylpenicillin can also inhibit starter cultures
used in the production of yoghurt, cheese and other milk products.
The Committee concluded that allergy was the determinating factor
in the safety evaluation of residues of benzylpenicillin. In the
absence of adequate data to establish a no-effect level, the Committee
recommended that the daily intake from food be kept as low as
practicable, and in any case below 30 µg of the parent drug. The risk
associated with the occurrence of mild hypersensitivity reactions at
this level was considered to be insignificant.
5. REFERENCES
ABO-ELNAGE, I.G. ABDEL-MOTTALEB, L., & MAHMOUD, M. (1973).
Characteristics of Gouda cheese and yoghurt made from milk containing
penicillin. Scienza e Tecnica Lattiro-Casearia 24, 25-32.
ANDERSON, J.A. & ADKINSON, N.F. (1987). Allergic reactions to drugs
and biologic agents. JAMA 258, 2891-2899.
ARNDT, K.A. & JICK, H. (1976). Rates of cutaneous reactions to drugs.
A Report From the Boston Collaborative Drug Surveillance Program.
JAMA, 235, 918-922.
BAYER, A.S., CHOW, A.W., CONCEPTION, N., & GUZE, L.B. (1978).
Susceptibility of 40 lactobacilli to six antimicrobial agents with
broad Grampositive anaerobic spectra. Antimicrobial Agents and
Chemotherapy, 14, 720-722.
BIGBY, M., JICK, S., JICK, H., & ARNDT, K. (1986). Drug induced
cutaneous reactions. A report from the Boston Collaborative Drug
Surveillance Program on 15,438 consecutive inpatients. JAMA, 256,
3358-3363.
BIERLEIN, K.J. (1956). Repeated anaphylactic reactions in a patient
highly sensitized to penicillin. A case report. Ann. Allergy, 14,
35-40.
BORRIE. P., & BARRET, J. (1961). Dermatitis caused by penicillin in
bulked milk supplies. Br. Med. J., 2, 1267.
BYCROFT, B.W. & SHUTE, R.E. (1985). The molecular basis for the mode
of action of beta-lactam antibiotics and mechanisms of resistance.
Pharma. Res., 1985,3-14.
COGAN, T.M. (1972). Susceptibility of cheese and yoghurt starter
bacteria to antibiotics. Appl. Microbiol., 23, 960-965.
DESWARTE, R.D. (1985). Drug Allergy. in: Patterson, R. (ed.) Allergic
Diseases. Diagnosis and Management. 3rd edition, J.P. Lippincott,
Philadelphia, Chapter 19, pp. 595-614.
DEWDNEY, J.M. & EDWARDS, R.G. (1984). Penicillin hypersensitivity-is
milk a significant hazard? J. Roy. Soc. Med., 77, 866-877.
DE WECK, A.L. (1983). Penicillins and Cephalosporins. in: Allergic
reactions to drugs, De Weck, A.L. & Bundgaard, H. (eds.), Springer
Verlag, Berlin, pp. 423-482.
DOLEZALEK, J. & BEHAVKOVA, A. (1974). Effect of penicillin on the
formation of some flavour constituents in ripening of pure dairy
cultures. XIX International Dairy Congress, 1E, 442.
GUTHE, T., IDSO, O., & WILLCOX, R.R. (1958). Untoward penicillin
reactions. Bull. Wld. Hlth Org., 19, 427-501.
HUBER, W.G. (1988). Penicillins. in: Booth, N.H. & McDonald, L.E.
(eds.) Veterinary Pharmacology and Therapeutics, 6th edition, Iowa
State University Press, Ames, Iowa, Chapter 49, pp.796-812.
IDSO, 0. & WANG, P.N. (1958). Penicillin-sensitive reactions in
Taiwan. Bull. Wld. Hlth. Org., 18, 323-344.
IDSO, O., GUTHE, T., WILLCOX, R.R., & DE WECK, A.L. (1968). Nature
and extent of penicillin side-reactions, with particular reference to
fatalities from anaphylactic shock. Bull. Wld. Hlth. Org., 38,
159-188.
JAKIMOV, N. (1970). Antibiotics in milk and their effect on lactic
acid bacteria. Mikrobiologija, 7, 99-109.
KONDRATENKO, M., SHISHKOVA, I., TSANEVA, K. & G'OSHEV, B. (1978).
Inhibitory effects of antibiotics on yoghurt production. XX Inter.
Dairy Congress, E, 834-835.
LEVINE, B.B. & VAZ, N.M. (1970). Effect of combination of inbred
strain, antigen and antigen dose on immune responsiveness and reagin
production in mice. Int. Arch. Allergy App. Immunol. 39, 156-171.
LINDEMAYR, H., KNOBLER, R., KRAFT, D., & BAUMGARTNER, W. (1981).
Challenge of penicillin-allergic volunteers with
penicillin-contaminated meat. Allergy, 36, 471-478.
LOUSSOUARN, S. (1982). Sensitivity of lactic cultures to certain
antibiotics. Technique Taitiere 965, 49-50.
MANDELL, G.L. & SANDE, M.A. (1985). Antimicrobial agents. penicillins,
cephalosporins, and other beta-lactam antibiotics. in: Goodman and
Gilman's The Pharmaceutical Basis of Therapeutics. Eds. Gilman, A.G.,
Goodman, L.S., Rall, T.W. & Murad, F. 7th edition, Macmillan
Publishing Company, New York, Chapter 50, pp. 1115-1149.
MARSH, D.G. (1975). Allergens and the genetics of allergy. in: Sela,
M. (ed.) The antigens, Vol III, Academic Press, New York, San
Francisco, and London; pp. 271-361.
METZGER, H. (1988). Molecular aspects of receptors and binding
factors for IgE. Adv. in Immunol., 43, 277-312.
OLSON, J.C., JR. & SANDERS, A.C. (1975). Penicillin in milk and milk
products: Some regulatory and public health considerations. J. Milk
Food Technol., 38, 630-633.
SCHNEIDER, C.H. (1983). Immunochemical basis of allergic reactions to
drugs. in: Allergic reactions to drugs, De Weck, A.L. & Bundgaard, H.
(eds.), Springer-Verlag, Berlin, pp. 3-35.
SIEGEL, B.B. (1959). Hidden contacts with penicillin. Bull. Wld.
Hlth Org. 21, 703-713.
TERPLAN, G., & ZAADHOFF, K.J. (1967). On the incidence and detection
of inhibitory substances in milk - a short review (orig. in German).
Milchwissenschaft, 22, 761-771.
TSCHEUSCHNER, I. (1972). [translation from German:] Anaphylactic
reaction to penicillin after ingestion of pork. Z. Haut
Geschleantskr., 47, 591-592.
WAL, J.M. (1980). Enzymatic unmasking for antibodies of penicilloyl
residues bound to albumin. Biochem. Pharmacol., 29, 195-199.
WAL, J.M. & BORIS, G. (1975). Elimination of free penicillin and
penicilloyl-protein conjugates in the milk of cows following
intramammary administration of penicillin G. Annales biol. animale
biochim. biophys., 15, 615-617.
WICHER, K., REISMAN, R.E., & ARBESMAN, C. E. (1969). Allergic
reaction to penicillin present in milk. JAMA, 208, 143-145
 2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution, and excretion
In humans, about one third of an orally administered dose of
benzylpenicillin is absorbed from the intestinal tract under favorable
conditions. Only a small portion is absorbed from the stomach.
Absorption occurs mainly in the duodenum. Ingestion of food
interferes with enteric absorption. Benzylpenicillin is widely
distributed throughout the body. Its apparent volume of distribution
is in about 50% of total body water. Benzylpenicillin is rapidly
eliminated from the body (mainly by the kidney) (Mandell & Sande,
1985).
2.1.2 Biotransformation
Gastric acid hydrolyzes the amide side chain and opens the lactam
ring, with concomitant loss of antibacterial activity. The
biotransformation of benzylpenicillin is not well understood. The
drug is only partially metabolized and the major fraction is excreted
unchanged (Huber, 1988).
2.1.3 Effects on enzymes
Benzylpenicillin selectively inhibits bacterial cell wall
biosynthesis. The linear polysaccharide ("glycan") of the cell wall
is cross-linked by branched peptide chains to form a structure termed
"peptidoglycan". The primary transpeptidase reaction leading to
covalent linkage of new chains to the pre-existing peptidoglycan
network is highly sensitive to penicillin. Several proteins,
including transpeptidases, located in the bacterial cell membrane bind
penicillin covalently in the form of a penicilloyl moiety via an ester
linkage (cleavage of the lactam ring). It appears that the antibiotic
is recognized as a pseudo-substrate which subsequently acts as a
strong acylating agent to form a covalent enzyme-inhibitor complex at
the active site (Bycroft & Shute, 1985).
2.1.4 Immunogenicity and antigenicity
Many antigenic determinants may derive from benzylpenicillin.
The biochemical routes to the formation of some of these determinants
may be multiple. The benzylpenicilloyl (BPO) determinant has been
designated the "major" antigenic determinant of penicillin allergy.
BPO haptenic groups can be formed by direct acylation of proteins or
from benzylpenicillenic acid, either by direct reaction or via a
postulated reactive intermediate, thiazolidinyl-oxazolidone. The
formation of BPO determinants from benzylpenicillin acid itself, the
major product of hydrolysis of benzylpenicillin, has not yet been
definitely established.
All other determinants resulting from the binding of other
metabolites of benzylpenicillin are collectively referred to as the
"minor" determinants. Many questions pertain to both. The mechanisms
of hapten formation and the immunogenicity of certain "minor"
determinants remain open. Antibodies specifically directed to one or
another of these determinants have, however, been found in patients
(e.g., antibodies with penicillamine or penicillemyl specificity) or
have been induced in experimental animals. It cannot be ruled out
that such determinants may be frequently involved in penicillin
allergy. Polymerization of benzylpenicillin, which can readily occur
under certain conditions, has been described. It is, however, not yet
fully clear whether and under which circumstances such polymers are
also capable of reacting as immunogens.
Protein impurities in benzylpenicillin preparations, which are
formed during the fermentation process and which could be sufficiently
highly substituted to efficiently raise antibodies, could contribute
to the overall immunogenicity. The critical number of drug-related
epitopes on carriers may depend on the nature of the carrier. Low-
substituted benzylpencilloyl-conjugates with human serum albumin were
of very poor immunogenicity. On the other hand, penicillin-autologous
carrier protein conjugates have been shown to be immunogenic in a
guinea pig model (De Weck, 1983).
Penicilloylated proteins from milk or tissue as they may appear
after high-dose treatment of animals may possess antigenic properties
if absorbed through the mucosa of the gut (Wal & Boris 1975; Wal,
1980).
Antibodies raised against benzylpenicillin-derived determinants
may cross-react to some extent with certain antigens derived from
other ß-lactams including cephalosporins. Similarly, antigens
carrying benzylpenicillin-derived haptenic groups may cross-react with
antibodies to other ß-lactams (DeSwarte, 1985).
2.1.5 Inhibitory activity on microorganisms used in industrial
food-processing
A variety of microorganisms (e.g., lactobacilli, streptococci,
certain yeasts and fungi) are used as "starter cultures" in the
manufacturing processes of milk products, such as sour milk, sour
cream, yoghurt, butter, kefir, and many different kinds of cheese.
It is well known that penicillin residues in milk can negatively
influence both the quality and yield of such milk-products (Jakimov,
1970; Cogan, 1972; Abo-Elnage et al., 1973; Dolezalek & Behavkova,
1974; Bayer et al., 1978; Kondratenko et al., 1978; Loussouran,
1982).
Microbiologically active residues of benzylpenicillin can cause
severe economic losses by inhibiting such microorganisms and thus
interfering with biotechnological food processing. Streptococcus
thermophilus, for example, is partially inhibited at concentrations of
penicillin as low as 0.0017 I.U./ml. Total inhibition occurs at
0.025-0.05 I.U./ml (Terplan & Zaadhoff, 1967).
2.2 Toxicological studies
No experimental studies were available for review. Information
contained in the open literature does not meet minimum requirements
for an evaluation of the toxicological properties of the drug.
2.3 Observations in humans
Benzylpenicillin may induce practically all possible clinical
forms of allergic reactions depending on dose, route, frequency of
exposure, genetic predisposition and other factors. Penicillin will
induce an immune response in practically every person who receives the
drug. Low titers of benzylpenicilloyl-specific IgM antibodies can be
detected in virtually everyone. However, the mere presence of
antipencillin-antibodies of any class does not necessarily denote
clinical sensitivity. Hypersensitivity reactions are by far the most
common adverse effects noted with benzylpenicillin (DeSwarte, 1985).
The proportion of the general population that may be susceptible
to the development of allergy is unknown. Available data did not
allow conclusions as to the true prevalence of penicillin-sensitized
individuals in the population since all available studies were carried
out in selected subpopulations using tests of limited diagnostic
value. The frequency of allergic side reactions has been reported to
vary from 0.7% to 10% in different studies (Idso et al., 1968).
The overall prevalence of penicillin allergy has been estimated
to be between 3% and 10%, indicating that a substantial proportion of
the population is at risk (Anderson & Adkinson, 1987).
Frequencies of skin reactions to commonly used drugs were
estimated from the records of the Boston Collaborative Drug
Surveillance Program (data base from 1966 through May, 1975). Fifty-
one individuals out of a total of 3286 recipients showed
benzylpenicillin-induced skin reactions corresponding to a rate of
16/1000) (Arndt & Jick, 1976).
In a second evaluation (data base: June 1975 through June 1982)
17 recipients out of a total number of 918 gave a cutaneous reaction
with benzylpenicillin (rate: 18.5/1000) (Bigby et al., 1986).
2.3.1 Sensitizing capacity
2.3.1.1 Qualitative aspects
Using techniques such as skin testing and RAST (radio-
allergosorbent test), antibodies with reactivities to the following
determinants have been found in humans: benzylpenicilloyl-,
benzylpenicillenate-, benzylopenicillenyl- and penicillamine-
determinant (De Weck, 1983).
2.3.1.2 Quantitative aspects
It remains impossible to describe in quantitative terms in
humans:
- the major pathways and kinetics (e.g., concentrations, rates,
catalysts) of the in vivo formation of antigenic
determinants,
- the nature of the biological carrier (e.g., soluble proteins,
membranes of lymphoid cells).
- relative abundances and biological activities of the various
haptenic groups/determinants derived from benzylpenicillin.
There were no useful data available to calculate the minimum
required dose of any potential hapten to produce the minimum required
amount of complete immunogen with the appropriate number of haptenic
epitopes per molecule to induce an immune response following oral
administration.
In a detailed analysis of data on penicillin-sensitive reactions
in Taiwan, it has been reported that a 50-year old man who had taken
a penicillin tablet of 50,000 units one year before the injection of
a combination of benzylpenicillin and streptomycin died 20 minutes
after the injection (Idso & Wang, 1958). If one assumes that this was
the patient's only exposure to the drug, this would suggest that a
single dose of approximately 30 mg of benzylpenicillin could sensitize
a human.
However, this information is of very limited value since it is
known that repeated administration of low doses of immunogen is the
most effective way to produce an IgE antibody response (Levine & Vaz,
1970; Marsh, 1975). Virtually nothing about the immunogenicity of
chronic low-level administration of benzylpenicillin in humans is
known.
2.3.2 Eliciting capacity
2.3.2.1 Qualitative aspects
The initial event in IgE-mediated reactions is the interaction of
bivalent or polyvalent antigen with antibody bound to high affinity
Fc-receptors for IgE on tissue mast cells and blood basophils followed
by aggregation (at least dimerization) of Fc-receptors (Metzger,
1988).
The elicitation of reaction in already-sensitized individuals
requires no macromolecular antigen. The low molecular weight N6-N6-
bis-benzylpencilloyl-diaminohexane, for example, can elicit
anaphylactic reactions (Schneider, 1983).
It is still not completely understood how benzylpenicillin itself
and its active low molecular weight metabolites could so rapidly react
in vivo to form such divalent or polyvalent antigens. Positive
skin tests have been obtained with the following haptens in patients
allergic to penicillin: benzylpenicillin, benzylpenicilloic acid,
benzylpenicillin-oligomer, benzylpenicillin-polymers, and carrier-
conjugates exhibiting the following haptenic groups/determinants:
benzylpencilloyl, benzylpenicillenyl, and penicillamine (De Weck,
1983).
In consequence, the above substances, and at least all divalent
antigens carrying benzylpenicilloyl- and/or benzylpenicillenyl- and/or
penicillamine-determinants formed upon covalent binding of reactive
haptens to tissue and/or milk proteins of target animals should be
considered as potential eliciting substances. It cannot be ruled out
that some of these molecules (if they existed as residues) could reach
at least the mast cells of the gut mucosa following oral ingestion.
2.3.2.2 Quantitative aspects
The overwhelming majority of penicillin preparations causing
reactions were administered parenterally. Severe reactions in
sensitive individuals after skin tests with less than one unit of
benzylpenicillin are documented including one exceptional case where
only 3 x 10-7 units had been applied (Bierlein, 1956). Such data,
however, are inappropriate to evaluate the risk of orally ingested
residues.
In an analysis of 151 fatalities from anaphylactic penicillin
reactions, it was reported that one patient died after an oral dose in
the range of 0.1 to 0.5 mega-units of benzylpenicillin (Idso et
al., 1968).
Severe reactions following the use of penicillin tablets have
also been reported. A fatality occurred after administration of one
tablet of 1000 units (Guthe et al., 1958).
Siegel has described experiments in which sera obtained from
patients with high IgE titers were used to sensitize skin sites on
normal subjects maintained on a milk free diet. Forty-eight to 72
hours later, the recipients were given oral doses of benzylpenicillin
in water. In one experiment, the smallest oral dose of
benzylpenicillin which could induce a whealing reaction was found to
be 40 units with a time to occurrence of 50 minutes. With penicillin
administered in milk as a diluent, threshold levels were slightly
elevated and time to occurrence was slightly prolonged. The needed
doses to elicit a similar reaction in allergic donors of such sera
would probably be lower (by a factor of 100 to 1000 or even more)
(Siegel, 1959).
An acute allergic reaction in a patient who had ingested milk
from a commercial supply which contained approximately 10 units/ml has
been reported (Wicher et al., 1969).
In a highly sensitized 25-year-old woman, less than 1 unit of
daily orally administered penicillin was sufficient to provoke
allergic symptoms. This patient suffered from a moderately severe
subacute eczematous eruption, which was traceable to penicillin-
contaminated milk. The patient's symptoms were relieved by addition
of penicillinase to the milk she consumed (Borrie & Barret, 1961).
A report documented a single case of acute angioedema and
pruritus in a penicillin-allergic patient who ingested freshly
processed meat from a pig injected with penicillin three days before
slaughter. The patient noted symptoms after the first bite of ground
pork. Analysis revealed a penicillin content of 0.3-0.45 units/gram
of meat (Tscheuschner, 1972).
Lindemayr and co-workers challenged nine penicillin-allergic
volunteers with 150g of raw pork meat (content: 0.024-0.04 ug/g) from
an animal treated with procaine-benzylpenicilline. Two subjects
reported itchy or local anesthetic sensations during the first 2
hours. However no objective symptoms of allergy could be observed
(Lindemayr et al., 1981).
Other cases (mainly anecdotal observations) have been reported.
There was, however, insufficient or even no evidence provided to
support the view that penicillin was the causative agent (Dewdney &
Edwards, 1984).
3. COMMENTS AND EVALUATION
No toxicological studies were available for review. Among the
adverse reactions which had been reported in people consuming food
containing benzylpenicillin residues, hypersensitivity reactions were
the most common. The overall prevalence of allergy to penicillin,
taking into account various reports of allergic reactions in different
populations and using a variety of test procedures, was estimated to
be 3 - 10%. There was no evidence of sensitization caused by
benzylpenicillin residues in food. The Committee evaluated the
available data on allergic reactions caused by penicillin residues.
Only four cases were considered to be adequately documented to
demonstrate that hypersensitivity reactions could be caused by
ingestion of less than 40 µg of the drug.
Residues of benzylpenicillin can also inhibit starter cultures
used in the production of yoghurt, cheese and other milk products.
The Committee concluded that allergy was the determinating factor
in the safety evaluation of residues of benzylpenicillin. In the
absence of adequate data to establish a no-effect level, the Committee
recommended that the daily intake from food be kept as low as
practicable, and in any case below 30 µg of the parent drug. The risk
associated with the occurrence of mild hypersensitivity reactions at
this level was considered to be insignificant.
5. REFERENCES
ABO-ELNAGE, I.G. ABDEL-MOTTALEB, L., & MAHMOUD, M. (1973).
Characteristics of Gouda cheese and yoghurt made from milk containing
penicillin. Scienza e Tecnica Lattiro-Casearia 24, 25-32.
ANDERSON, J.A. & ADKINSON, N.F. (1987). Allergic reactions to drugs
and biologic agents. JAMA 258, 2891-2899.
ARNDT, K.A. & JICK, H. (1976). Rates of cutaneous reactions to drugs.
A Report From the Boston Collaborative Drug Surveillance Program.
JAMA, 235, 918-922.
BAYER, A.S., CHOW, A.W., CONCEPTION, N., & GUZE, L.B. (1978).
Susceptibility of 40 lactobacilli to six antimicrobial agents with
broad Grampositive anaerobic spectra. Antimicrobial Agents and
Chemotherapy, 14, 720-722.
BIGBY, M., JICK, S., JICK, H., & ARNDT, K. (1986). Drug induced
cutaneous reactions. A report from the Boston Collaborative Drug
Surveillance Program on 15,438 consecutive inpatients. JAMA, 256,
3358-3363.
BIERLEIN, K.J. (1956). Repeated anaphylactic reactions in a patient
highly sensitized to penicillin. A case report. Ann. Allergy, 14,
35-40.
BORRIE. P., & BARRET, J. (1961). Dermatitis caused by penicillin in
bulked milk supplies. Br. Med. J., 2, 1267.
BYCROFT, B.W. & SHUTE, R.E. (1985). The molecular basis for the mode
of action of beta-lactam antibiotics and mechanisms of resistance.
Pharma. Res., 1985,3-14.
COGAN, T.M. (1972). Susceptibility of cheese and yoghurt starter
bacteria to antibiotics. Appl. Microbiol., 23, 960-965.
DESWARTE, R.D. (1985). Drug Allergy. in: Patterson, R. (ed.) Allergic
Diseases. Diagnosis and Management. 3rd edition, J.P. Lippincott,
Philadelphia, Chapter 19, pp. 595-614.
DEWDNEY, J.M. & EDWARDS, R.G. (1984). Penicillin hypersensitivity-is
milk a significant hazard? J. Roy. Soc. Med., 77, 866-877.
DE WECK, A.L. (1983). Penicillins and Cephalosporins. in: Allergic
reactions to drugs, De Weck, A.L. & Bundgaard, H. (eds.), Springer
Verlag, Berlin, pp. 423-482.
DOLEZALEK, J. & BEHAVKOVA, A. (1974). Effect of penicillin on the
formation of some flavour constituents in ripening of pure dairy
cultures. XIX International Dairy Congress, 1E, 442.
GUTHE, T., IDSO, O., & WILLCOX, R.R. (1958). Untoward penicillin
reactions. Bull. Wld. Hlth Org., 19, 427-501.
HUBER, W.G. (1988). Penicillins. in: Booth, N.H. & McDonald, L.E.
(eds.) Veterinary Pharmacology and Therapeutics, 6th edition, Iowa
State University Press, Ames, Iowa, Chapter 49, pp.796-812.
IDSO, 0. & WANG, P.N. (1958). Penicillin-sensitive reactions in
Taiwan. Bull. Wld. Hlth. Org., 18, 323-344.
IDSO, O., GUTHE, T., WILLCOX, R.R., & DE WECK, A.L. (1968). Nature
and extent of penicillin side-reactions, with particular reference to
fatalities from anaphylactic shock. Bull. Wld. Hlth. Org., 38,
159-188.
JAKIMOV, N. (1970). Antibiotics in milk and their effect on lactic
acid bacteria. Mikrobiologija, 7, 99-109.
KONDRATENKO, M., SHISHKOVA, I., TSANEVA, K. & G'OSHEV, B. (1978).
Inhibitory effects of antibiotics on yoghurt production. XX Inter.
Dairy Congress, E, 834-835.
LEVINE, B.B. & VAZ, N.M. (1970). Effect of combination of inbred
strain, antigen and antigen dose on immune responsiveness and reagin
production in mice. Int. Arch. Allergy App. Immunol. 39, 156-171.
LINDEMAYR, H., KNOBLER, R., KRAFT, D., & BAUMGARTNER, W. (1981).
Challenge of penicillin-allergic volunteers with
penicillin-contaminated meat. Allergy, 36, 471-478.
LOUSSOUARN, S. (1982). Sensitivity of lactic cultures to certain
antibiotics. Technique Taitiere 965, 49-50.
MANDELL, G.L. & SANDE, M.A. (1985). Antimicrobial agents. penicillins,
cephalosporins, and other beta-lactam antibiotics. in: Goodman and
Gilman's The Pharmaceutical Basis of Therapeutics. Eds. Gilman, A.G.,
Goodman, L.S., Rall, T.W. & Murad, F. 7th edition, Macmillan
Publishing Company, New York, Chapter 50, pp. 1115-1149.
MARSH, D.G. (1975). Allergens and the genetics of allergy. in: Sela,
M. (ed.) The antigens, Vol III, Academic Press, New York, San
Francisco, and London; pp. 271-361.
METZGER, H. (1988). Molecular aspects of receptors and binding
factors for IgE. Adv. in Immunol., 43, 277-312.
OLSON, J.C., JR. & SANDERS, A.C. (1975). Penicillin in milk and milk
products: Some regulatory and public health considerations. J. Milk
Food Technol., 38, 630-633.
SCHNEIDER, C.H. (1983). Immunochemical basis of allergic reactions to
drugs. in: Allergic reactions to drugs, De Weck, A.L. & Bundgaard, H.
(eds.), Springer-Verlag, Berlin, pp. 3-35.
SIEGEL, B.B. (1959). Hidden contacts with penicillin. Bull. Wld.
Hlth Org. 21, 703-713.
TERPLAN, G., & ZAADHOFF, K.J. (1967). On the incidence and detection
of inhibitory substances in milk - a short review (orig. in German).
Milchwissenschaft, 22, 761-771.
TSCHEUSCHNER, I. (1972). [translation from German:] Anaphylactic
reaction to penicillin after ingestion of pork. Z. Haut
Geschleantskr., 47, 591-592.
WAL, J.M. (1980). Enzymatic unmasking for antibodies of penicilloyl
residues bound to albumin. Biochem. Pharmacol., 29, 195-199.
WAL, J.M. & BORIS, G. (1975). Elimination of free penicillin and
penicilloyl-protein conjugates in the milk of cows following
intramammary administration of penicillin G. Annales biol. animale
biochim. biophys., 15, 615-617.
WICHER, K., REISMAN, R.E., & ARBESMAN, C. E. (1969). Allergic
reaction to penicillin present in milk. JAMA, 208, 143-145
2. BIOLOGICAL DATA
2.1 Biochemical Aspects
2.1.1 Absorption, distribution, and excretion
In humans, about one third of an orally administered dose of
benzylpenicillin is absorbed from the intestinal tract under favorable
conditions. Only a small portion is absorbed from the stomach.
Absorption occurs mainly in the duodenum. Ingestion of food
interferes with enteric absorption. Benzylpenicillin is widely
distributed throughout the body. Its apparent volume of distribution
is in about 50% of total body water. Benzylpenicillin is rapidly
eliminated from the body (mainly by the kidney) (Mandell & Sande,
1985).
2.1.2 Biotransformation
Gastric acid hydrolyzes the amide side chain and opens the lactam
ring, with concomitant loss of antibacterial activity. The
biotransformation of benzylpenicillin is not well understood. The
drug is only partially metabolized and the major fraction is excreted
unchanged (Huber, 1988).
2.1.3 Effects on enzymes
Benzylpenicillin selectively inhibits bacterial cell wall
biosynthesis. The linear polysaccharide ("glycan") of the cell wall
is cross-linked by branched peptide chains to form a structure termed
"peptidoglycan". The primary transpeptidase reaction leading to
covalent linkage of new chains to the pre-existing peptidoglycan
network is highly sensitive to penicillin. Several proteins,
including transpeptidases, located in the bacterial cell membrane bind
penicillin covalently in the form of a penicilloyl moiety via an ester
linkage (cleavage of the lactam ring). It appears that the antibiotic
is recognized as a pseudo-substrate which subsequently acts as a
strong acylating agent to form a covalent enzyme-inhibitor complex at
the active site (Bycroft & Shute, 1985).
2.1.4 Immunogenicity and antigenicity
Many antigenic determinants may derive from benzylpenicillin.
The biochemical routes to the formation of some of these determinants
may be multiple. The benzylpenicilloyl (BPO) determinant has been
designated the "major" antigenic determinant of penicillin allergy.
BPO haptenic groups can be formed by direct acylation of proteins or
from benzylpenicillenic acid, either by direct reaction or via a
postulated reactive intermediate, thiazolidinyl-oxazolidone. The
formation of BPO determinants from benzylpenicillin acid itself, the
major product of hydrolysis of benzylpenicillin, has not yet been
definitely established.
All other determinants resulting from the binding of other
metabolites of benzylpenicillin are collectively referred to as the
"minor" determinants. Many questions pertain to both. The mechanisms
of hapten formation and the immunogenicity of certain "minor"
determinants remain open. Antibodies specifically directed to one or
another of these determinants have, however, been found in patients
(e.g., antibodies with penicillamine or penicillemyl specificity) or
have been induced in experimental animals. It cannot be ruled out
that such determinants may be frequently involved in penicillin
allergy. Polymerization of benzylpenicillin, which can readily occur
under certain conditions, has been described. It is, however, not yet
fully clear whether and under which circumstances such polymers are
also capable of reacting as immunogens.
Protein impurities in benzylpenicillin preparations, which are
formed during the fermentation process and which could be sufficiently
highly substituted to efficiently raise antibodies, could contribute
to the overall immunogenicity. The critical number of drug-related
epitopes on carriers may depend on the nature of the carrier. Low-
substituted benzylpencilloyl-conjugates with human serum albumin were
of very poor immunogenicity. On the other hand, penicillin-autologous
carrier protein conjugates have been shown to be immunogenic in a
guinea pig model (De Weck, 1983).
Penicilloylated proteins from milk or tissue as they may appear
after high-dose treatment of animals may possess antigenic properties
if absorbed through the mucosa of the gut (Wal & Boris 1975; Wal,
1980).
Antibodies raised against benzylpenicillin-derived determinants
may cross-react to some extent with certain antigens derived from
other ß-lactams including cephalosporins. Similarly, antigens
carrying benzylpenicillin-derived haptenic groups may cross-react with
antibodies to other ß-lactams (DeSwarte, 1985).
2.1.5 Inhibitory activity on microorganisms used in industrial
food-processing
A variety of microorganisms (e.g., lactobacilli, streptococci,
certain yeasts and fungi) are used as "starter cultures" in the
manufacturing processes of milk products, such as sour milk, sour
cream, yoghurt, butter, kefir, and many different kinds of cheese.
It is well known that penicillin residues in milk can negatively
influence both the quality and yield of such milk-products (Jakimov,
1970; Cogan, 1972; Abo-Elnage et al., 1973; Dolezalek & Behavkova,
1974; Bayer et al., 1978; Kondratenko et al., 1978; Loussouran,
1982).
Microbiologically active residues of benzylpenicillin can cause
severe economic losses by inhibiting such microorganisms and thus
interfering with biotechnological food processing. Streptococcus
thermophilus, for example, is partially inhibited at concentrations of
penicillin as low as 0.0017 I.U./ml. Total inhibition occurs at
0.025-0.05 I.U./ml (Terplan & Zaadhoff, 1967).
2.2 Toxicological studies
No experimental studies were available for review. Information
contained in the open literature does not meet minimum requirements
for an evaluation of the toxicological properties of the drug.
2.3 Observations in humans
Benzylpenicillin may induce practically all possible clinical
forms of allergic reactions depending on dose, route, frequency of
exposure, genetic predisposition and other factors. Penicillin will
induce an immune response in practically every person who receives the
drug. Low titers of benzylpenicilloyl-specific IgM antibodies can be
detected in virtually everyone. However, the mere presence of
antipencillin-antibodies of any class does not necessarily denote
clinical sensitivity. Hypersensitivity reactions are by far the most
common adverse effects noted with benzylpenicillin (DeSwarte, 1985).
The proportion of the general population that may be susceptible
to the development of allergy is unknown. Available data did not
allow conclusions as to the true prevalence of penicillin-sensitized
individuals in the population since all available studies were carried
out in selected subpopulations using tests of limited diagnostic
value. The frequency of allergic side reactions has been reported to
vary from 0.7% to 10% in different studies (Idso et al., 1968).
The overall prevalence of penicillin allergy has been estimated
to be between 3% and 10%, indicating that a substantial proportion of
the population is at risk (Anderson & Adkinson, 1987).
Frequencies of skin reactions to commonly used drugs were
estimated from the records of the Boston Collaborative Drug
Surveillance Program (data base from 1966 through May, 1975). Fifty-
one individuals out of a total of 3286 recipients showed
benzylpenicillin-induced skin reactions corresponding to a rate of
16/1000) (Arndt & Jick, 1976).
In a second evaluation (data base: June 1975 through June 1982)
17 recipients out of a total number of 918 gave a cutaneous reaction
with benzylpenicillin (rate: 18.5/1000) (Bigby et al., 1986).
2.3.1 Sensitizing capacity
2.3.1.1 Qualitative aspects
Using techniques such as skin testing and RAST (radio-
allergosorbent test), antibodies with reactivities to the following
determinants have been found in humans: benzylpenicilloyl-,
benzylpenicillenate-, benzylopenicillenyl- and penicillamine-
determinant (De Weck, 1983).
2.3.1.2 Quantitative aspects
It remains impossible to describe in quantitative terms in
humans:
- the major pathways and kinetics (e.g., concentrations, rates,
catalysts) of the in vivo formation of antigenic
determinants,
- the nature of the biological carrier (e.g., soluble proteins,
membranes of lymphoid cells).
- relative abundances and biological activities of the various
haptenic groups/determinants derived from benzylpenicillin.
There were no useful data available to calculate the minimum
required dose of any potential hapten to produce the minimum required
amount of complete immunogen with the appropriate number of haptenic
epitopes per molecule to induce an immune response following oral
administration.
In a detailed analysis of data on penicillin-sensitive reactions
in Taiwan, it has been reported that a 50-year old man who had taken
a penicillin tablet of 50,000 units one year before the injection of
a combination of benzylpenicillin and streptomycin died 20 minutes
after the injection (Idso & Wang, 1958). If one assumes that this was
the patient's only exposure to the drug, this would suggest that a
single dose of approximately 30 mg of benzylpenicillin could sensitize
a human.
However, this information is of very limited value since it is
known that repeated administration of low doses of immunogen is the
most effective way to produce an IgE antibody response (Levine & Vaz,
1970; Marsh, 1975). Virtually nothing about the immunogenicity of
chronic low-level administration of benzylpenicillin in humans is
known.
2.3.2 Eliciting capacity
2.3.2.1 Qualitative aspects
The initial event in IgE-mediated reactions is the interaction of
bivalent or polyvalent antigen with antibody bound to high affinity
Fc-receptors for IgE on tissue mast cells and blood basophils followed
by aggregation (at least dimerization) of Fc-receptors (Metzger,
1988).
The elicitation of reaction in already-sensitized individuals
requires no macromolecular antigen. The low molecular weight N6-N6-
bis-benzylpencilloyl-diaminohexane, for example, can elicit
anaphylactic reactions (Schneider, 1983).
It is still not completely understood how benzylpenicillin itself
and its active low molecular weight metabolites could so rapidly react
in vivo to form such divalent or polyvalent antigens. Positive
skin tests have been obtained with the following haptens in patients
allergic to penicillin: benzylpenicillin, benzylpenicilloic acid,
benzylpenicillin-oligomer, benzylpenicillin-polymers, and carrier-
conjugates exhibiting the following haptenic groups/determinants:
benzylpencilloyl, benzylpenicillenyl, and penicillamine (De Weck,
1983).
In consequence, the above substances, and at least all divalent
antigens carrying benzylpenicilloyl- and/or benzylpenicillenyl- and/or
penicillamine-determinants formed upon covalent binding of reactive
haptens to tissue and/or milk proteins of target animals should be
considered as potential eliciting substances. It cannot be ruled out
that some of these molecules (if they existed as residues) could reach
at least the mast cells of the gut mucosa following oral ingestion.
2.3.2.2 Quantitative aspects
The overwhelming majority of penicillin preparations causing
reactions were administered parenterally. Severe reactions in
sensitive individuals after skin tests with less than one unit of
benzylpenicillin are documented including one exceptional case where
only 3 x 10-7 units had been applied (Bierlein, 1956). Such data,
however, are inappropriate to evaluate the risk of orally ingested
residues.
In an analysis of 151 fatalities from anaphylactic penicillin
reactions, it was reported that one patient died after an oral dose in
the range of 0.1 to 0.5 mega-units of benzylpenicillin (Idso et
al., 1968).
Severe reactions following the use of penicillin tablets have
also been reported. A fatality occurred after administration of one
tablet of 1000 units (Guthe et al., 1958).
Siegel has described experiments in which sera obtained from
patients with high IgE titers were used to sensitize skin sites on
normal subjects maintained on a milk free diet. Forty-eight to 72
hours later, the recipients were given oral doses of benzylpenicillin
in water. In one experiment, the smallest oral dose of
benzylpenicillin which could induce a whealing reaction was found to
be 40 units with a time to occurrence of 50 minutes. With penicillin
administered in milk as a diluent, threshold levels were slightly
elevated and time to occurrence was slightly prolonged. The needed
doses to elicit a similar reaction in allergic donors of such sera
would probably be lower (by a factor of 100 to 1000 or even more)
(Siegel, 1959).
An acute allergic reaction in a patient who had ingested milk
from a commercial supply which contained approximately 10 units/ml has
been reported (Wicher et al., 1969).
In a highly sensitized 25-year-old woman, less than 1 unit of
daily orally administered penicillin was sufficient to provoke
allergic symptoms. This patient suffered from a moderately severe
subacute eczematous eruption, which was traceable to penicillin-
contaminated milk. The patient's symptoms were relieved by addition
of penicillinase to the milk she consumed (Borrie & Barret, 1961).
A report documented a single case of acute angioedema and
pruritus in a penicillin-allergic patient who ingested freshly
processed meat from a pig injected with penicillin three days before
slaughter. The patient noted symptoms after the first bite of ground
pork. Analysis revealed a penicillin content of 0.3-0.45 units/gram
of meat (Tscheuschner, 1972).
Lindemayr and co-workers challenged nine penicillin-allergic
volunteers with 150g of raw pork meat (content: 0.024-0.04 ug/g) from
an animal treated with procaine-benzylpenicilline. Two subjects
reported itchy or local anesthetic sensations during the first 2
hours. However no objective symptoms of allergy could be observed
(Lindemayr et al., 1981).
Other cases (mainly anecdotal observations) have been reported.
There was, however, insufficient or even no evidence provided to
support the view that penicillin was the causative agent (Dewdney &
Edwards, 1984).
3. COMMENTS AND EVALUATION
No toxicological studies were available for review. Among the
adverse reactions which had been reported in people consuming food
containing benzylpenicillin residues, hypersensitivity reactions were
the most common. The overall prevalence of allergy to penicillin,
taking into account various reports of allergic reactions in different
populations and using a variety of test procedures, was estimated to
be 3 - 10%. There was no evidence of sensitization caused by
benzylpenicillin residues in food. The Committee evaluated the
available data on allergic reactions caused by penicillin residues.
Only four cases were considered to be adequately documented to
demonstrate that hypersensitivity reactions could be caused by
ingestion of less than 40 µg of the drug.
Residues of benzylpenicillin can also inhibit starter cultures
used in the production of yoghurt, cheese and other milk products.
The Committee concluded that allergy was the determinating factor
in the safety evaluation of residues of benzylpenicillin. In the
absence of adequate data to establish a no-effect level, the Committee
recommended that the daily intake from food be kept as low as
practicable, and in any case below 30 µg of the parent drug. The risk
associated with the occurrence of mild hypersensitivity reactions at
this level was considered to be insignificant.
5. REFERENCES
ABO-ELNAGE, I.G. ABDEL-MOTTALEB, L., & MAHMOUD, M. (1973).
Characteristics of Gouda cheese and yoghurt made from milk containing
penicillin. Scienza e Tecnica Lattiro-Casearia 24, 25-32.
ANDERSON, J.A. & ADKINSON, N.F. (1987). Allergic reactions to drugs
and biologic agents. JAMA 258, 2891-2899.
ARNDT, K.A. & JICK, H. (1976). Rates of cutaneous reactions to drugs.
A Report From the Boston Collaborative Drug Surveillance Program.
JAMA, 235, 918-922.
BAYER, A.S., CHOW, A.W., CONCEPTION, N., & GUZE, L.B. (1978).
Susceptibility of 40 lactobacilli to six antimicrobial agents with
broad Grampositive anaerobic spectra. Antimicrobial Agents and
Chemotherapy, 14, 720-722.
BIGBY, M., JICK, S., JICK, H., & ARNDT, K. (1986). Drug induced
cutaneous reactions. A report from the Boston Collaborative Drug
Surveillance Program on 15,438 consecutive inpatients. JAMA, 256,
3358-3363.
BIERLEIN, K.J. (1956). Repeated anaphylactic reactions in a patient
highly sensitized to penicillin. A case report. Ann. Allergy, 14,
35-40.
BORRIE. P., & BARRET, J. (1961). Dermatitis caused by penicillin in
bulked milk supplies. Br. Med. J., 2, 1267.
BYCROFT, B.W. & SHUTE, R.E. (1985). The molecular basis for the mode
of action of beta-lactam antibiotics and mechanisms of resistance.
Pharma. Res., 1985,3-14.
COGAN, T.M. (1972). Susceptibility of cheese and yoghurt starter
bacteria to antibiotics. Appl. Microbiol., 23, 960-965.
DESWARTE, R.D. (1985). Drug Allergy. in: Patterson, R. (ed.) Allergic
Diseases. Diagnosis and Management. 3rd edition, J.P. Lippincott,
Philadelphia, Chapter 19, pp. 595-614.
DEWDNEY, J.M. & EDWARDS, R.G. (1984). Penicillin hypersensitivity-is
milk a significant hazard? J. Roy. Soc. Med., 77, 866-877.
DE WECK, A.L. (1983). Penicillins and Cephalosporins. in: Allergic
reactions to drugs, De Weck, A.L. & Bundgaard, H. (eds.), Springer
Verlag, Berlin, pp. 423-482.
DOLEZALEK, J. & BEHAVKOVA, A. (1974). Effect of penicillin on the
formation of some flavour constituents in ripening of pure dairy
cultures. XIX International Dairy Congress, 1E, 442.
GUTHE, T., IDSO, O., & WILLCOX, R.R. (1958). Untoward penicillin
reactions. Bull. Wld. Hlth Org., 19, 427-501.
HUBER, W.G. (1988). Penicillins. in: Booth, N.H. & McDonald, L.E.
(eds.) Veterinary Pharmacology and Therapeutics, 6th edition, Iowa
State University Press, Ames, Iowa, Chapter 49, pp.796-812.
IDSO, 0. & WANG, P.N. (1958). Penicillin-sensitive reactions in
Taiwan. Bull. Wld. Hlth. Org., 18, 323-344.
IDSO, O., GUTHE, T., WILLCOX, R.R., & DE WECK, A.L. (1968). Nature
and extent of penicillin side-reactions, with particular reference to
fatalities from anaphylactic shock. Bull. Wld. Hlth. Org., 38,
159-188.
JAKIMOV, N. (1970). Antibiotics in milk and their effect on lactic
acid bacteria. Mikrobiologija, 7, 99-109.
KONDRATENKO, M., SHISHKOVA, I., TSANEVA, K. & G'OSHEV, B. (1978).
Inhibitory effects of antibiotics on yoghurt production. XX Inter.
Dairy Congress, E, 834-835.
LEVINE, B.B. & VAZ, N.M. (1970). Effect of combination of inbred
strain, antigen and antigen dose on immune responsiveness and reagin
production in mice. Int. Arch. Allergy App. Immunol. 39, 156-171.
LINDEMAYR, H., KNOBLER, R., KRAFT, D., & BAUMGARTNER, W. (1981).
Challenge of penicillin-allergic volunteers with
penicillin-contaminated meat. Allergy, 36, 471-478.
LOUSSOUARN, S. (1982). Sensitivity of lactic cultures to certain
antibiotics. Technique Taitiere 965, 49-50.
MANDELL, G.L. & SANDE, M.A. (1985). Antimicrobial agents. penicillins,
cephalosporins, and other beta-lactam antibiotics. in: Goodman and
Gilman's The Pharmaceutical Basis of Therapeutics. Eds. Gilman, A.G.,
Goodman, L.S., Rall, T.W. & Murad, F. 7th edition, Macmillan
Publishing Company, New York, Chapter 50, pp. 1115-1149.
MARSH, D.G. (1975). Allergens and the genetics of allergy. in: Sela,
M. (ed.) The antigens, Vol III, Academic Press, New York, San
Francisco, and London; pp. 271-361.
METZGER, H. (1988). Molecular aspects of receptors and binding
factors for IgE. Adv. in Immunol., 43, 277-312.
OLSON, J.C., JR. & SANDERS, A.C. (1975). Penicillin in milk and milk
products: Some regulatory and public health considerations. J. Milk
Food Technol., 38, 630-633.
SCHNEIDER, C.H. (1983). Immunochemical basis of allergic reactions to
drugs. in: Allergic reactions to drugs, De Weck, A.L. & Bundgaard, H.
(eds.), Springer-Verlag, Berlin, pp. 3-35.
SIEGEL, B.B. (1959). Hidden contacts with penicillin. Bull. Wld.
Hlth Org. 21, 703-713.
TERPLAN, G., & ZAADHOFF, K.J. (1967). On the incidence and detection
of inhibitory substances in milk - a short review (orig. in German).
Milchwissenschaft, 22, 761-771.
TSCHEUSCHNER, I. (1972). [translation from German:] Anaphylactic
reaction to penicillin after ingestion of pork. Z. Haut
Geschleantskr., 47, 591-592.
WAL, J.M. (1980). Enzymatic unmasking for antibodies of penicilloyl
residues bound to albumin. Biochem. Pharmacol., 29, 195-199.
WAL, J.M. & BORIS, G. (1975). Elimination of free penicillin and
penicilloyl-protein conjugates in the milk of cows following
intramammary administration of penicillin G. Annales biol. animale
biochim. biophys., 15, 615-617.
WICHER, K., REISMAN, R.E., & ARBESMAN, C. E. (1969). Allergic
reaction to penicillin present in milk. JAMA, 208, 143-145
