
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
IONONES AND STRUCTURALLY RELATED SUBSTANCES
First draft prepared by
Dr P.J. Abbott
Australia New Zealand Food Authority, Canberra, Australia
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of the Procedure for the Safety
Evaluation of Flavouring Agents
Consideration of combined intakes from use as
flavouring agents
Conclusions
Relevant background information
Biological data
Absorption, metabolism, and elimination
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity
Genotoxicity
References
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 21 flavouring agents that
includes alpha- and ß-ionone and structurally related substances
(Table 1), using the procedure for the safety evaluation of flavouring
agents (Figure 1, p. 222, and Annex 1, reference 131).
Each of these substances has a cyclohexane ring with an allyl or
alkyl side-chain containing a ketone or secondary alcohol functional
group. With one exception, namely, 1,4-dimethyl-4-acetyl-1-cyclohexene
(No. 402), each contains a 2,6,6-trimethylcylcohexyl carbon skeleton
and an alkyl side chain of four to seven carbons located at the C-1
position. With the exception of gamma-ionone, each of these substances
has at least one endocyclic double bond. In the ionones, the carbonyl
or hydroxyl group is positioned gamma to the ring, while in the
damascones the carbonyl group is positioned alpha to the ring.
The Committee has previously evaluated three members of the
group. alpha-Ionone and ß-ionone were both evaluated at the
twenty-eighth meeting (Annex 1, reference 66), when an ADI of 0-0.1
mg/kg bw was established for each. Allyl-alpha-ionone was evaluated at
the twenty-fourth meeting (Annex I, reference 53), when the Committee
concluded that the data were inadequate for setting an ADI.
Table 1. Summary of results of safety evaluations of ionones and structurally related substances used as flavouring agents
Step 1: All of the substances are in structural class I.
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
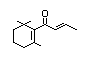
ß-Damascone 384 23726-92-3 43/10 No No Yes No safety
concern
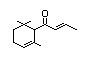
 alpha-Damascone 385 43052-87-5 8/0.4 No No Yes No safety
concern
alpha-Damascone 385 43052-87-5 8/0.4 No No Yes No safety
concern
 delta-Damascone 386 57378-68-4 0.06/0.6 No No Yes No safety
concern
delta-Damascone 386 57378-68-4 0.06/0.6 No No Yes No safety
concern
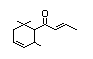 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
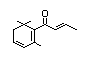
Damascenone 387 23696-85-7 86/5 No No Yes No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Damascenone 387 23696-85-7 86/5 No No Yes No safety
concern
 alpha-Ionone 388 127-41-3 310/150 Yes No - No safety
concernb
alpha-Ionone 388 127-41-3 310/150 Yes No - No safety
concernb
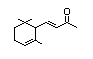 ß-lonone 389 14901-07-6 150/100 Yes No - No safety
concernb
ß-lonone 389 14901-07-6 150/100 Yes No - No safety
concernb
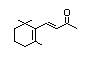 gamma-Ionone 390 79-76-5 0.01/15 No No Yes No safety
concern
gamma-Ionone 390 79-76-5 0.01/15 No No Yes No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
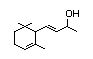
alpha-lonol 391 25312-34-9 0.7/0.06 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-lonol 391 25312-34-9 0.7/0.06 Yes No - No safety
concern
 ß-lonol 392 22029-76-1 0.9/0.1 Yes No - No safety
concern
ß-lonol 392 22029-76-1 0.9/0.1 Yes No - No safety
concern
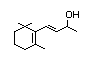 Dihydro-alpha-ionone 393 31499-72-6 0.7/0.02 Yes No - No safety
concern
Dihydro-alpha-ionone 393 31499-72-6 0.7/0.02 Yes No - No safety
concern
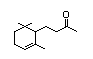 Dihydro-ß-ionone 394 17283-81-7 1/0.04 Yes No - No safety
concern
Dihydro-ß-ionone 394 17283-81-7 1/0.04 Yes No - No safety
concern
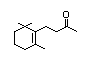 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Dihydro-ß-ionol 395 3293-47-8 0.3/0.02 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Dihydro-ß-ionol 395 3293-47-8 0.3/0.02 Yes No - No safety
concern
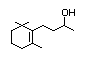 Dehydrodihydro-ionone 396 20483-36-7 0.1/0.08 No No Yes No safety
concern
Dehydrodihydro-ionone 396 20483-36-7 0.1/0.08 No No Yes No safety
concern
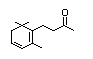 Dehydrodihydro-ionol 397 57069-86-0 8/0.01 No No Yes No safety
concern
Dehydrodihydro-ionol 397 57069-86-0 8/0.01 No No Yes No safety
concern
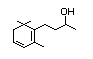 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Methyl-alpha-ionone 398 127-42-4 100/7 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Methyl-alpha-ionone 398 127-42-4 100/7 Yes No - No safety
concern
 Methyl-ß-ionone 399 127-43-5 6/0.2 Yes No - No safety
concern
Methyl-ß-ionone 399 127-43-5 6/0.2 Yes No - No safety
concern
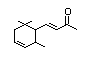 Methyl-delta-ionone 400 7748-98-7 0.4/1 Yes No - No safety
concern
Methyl-delta-ionone 400 7748-98-7 0.4/1 Yes No - No safety
concern
 Allyl-alpha-ionone 401 79-78-7 35/25 Yes No - No safety
concern
Allyl-alpha-ionone 401 79-78-7 35/25 Yes No - No safety
concern
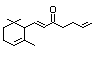 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-Irone 403 79-69-6 9/3 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-Irone 403 79-69-6 9/3 Yes No - No safety
concern
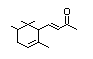 alpha-iso-Methylionone 404 127-51-5 6/1 Yes No - No safety
concern
alpha-iso-Methylionone 404 127-51-5 6/1 Yes No - No safety
concern
 a The threshold of concern for class I is 1800 µg/day.
b The ADI values previously established for alpha-ionone and ß-ionone were maintained at the present meeting.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from
surveys in Europe (International organization of the Flavor
Industry, 1994) and the United States (US Academy of Sciences,
1989) (see Table 2). The estimated total daily per capita
intake of all 21 ionones and related substances from their
use as flavouring agents is 0.76 mg/person in Europe and 0.33
mg/person in the United States. In Europe, four substances,
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (0.043
mg/person), alpha-ionone (0.31 mg/person), ß-ionone (0.15 mg/person),
and methyl-alpha-ionone (0.035 mg/person), account for approximately
85% of the total annual per capita intake of this group of
substances when used as flavouring agents. In the United States, three
substances, alpha-ionone (0.15 mg/person), ß-ionone (0.10 mg/person),
and allyl-alpha-ionone (0.025 mg/person) account for about 85% of the
total annual per capita intake of this group of substances when used
as flavouring agents.
Eleven of the substances in this group have been reported to
occur naturally in foods including raspberries, carrots, roasted
almonds, fruits, and herbs (Maarse et al., 1994). Quantitative data on
natural occurrence and consumption ratios have been reported for seven
substances (Nos 388, 389, 390, 391, 392, 393, and 394), which indicate
that they are consumed predominantly in traditional foods, i.e. the
consumption ratio is larger than 1 (Stofberg & Kirschman, 1985;
Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine.
Table 2. Most recent annual usage of ionones and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
ß-Damascone (384)
Europe 300 43 0.7
United States 50 10 0.2
alpha-Damascone (385)
Europe 57 8 0.1
United States 2 0.4 0.01
delta-Damascone (386)
Europe 0.4 0.06 0.001
United States 3 0.6 0.01
Damascenone (387)
Europe 600 86 1
United States 24 5 0.08
alpha-Ionone (388)
Europe 2200 310 5
United States 770 150 2
ß-lonone (389)
Europe 1100 150 3
United States 550 100 2
gamma-Ionone (390)
Europe 0.1 0.01 0.0002
United States 80 15 0.3
alpha-lonol (391)
Europe 5 0.7 0.01
United States 0.3 0.06 0.001
ß-lonol (392)
Europe 6 0.9 0.01
United States 0.5 0.1 0.002
Dihydro-alpha-ionone (393)
Europe 5 0.7 0.01
United States 0.1 0.02 0.0003
Dihydro-ß-ionone (394)
Europe 9 1 0.02
United States 0.2 0.04 0.0006
Dihydro-ß-ionol (395)
Europe 2.3 0.3 0.014
United States 0.1 0.02 0.0003
Dehydrodihydroionone (396)
Europe 0.7 0.1 0.002
United States 0.4 0.08 0.001
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Dehydrodihydroionol (397)
Europe 0.1 0.01 0.0002
United States 40 8 0.1
Methyl-alpha-ionone (398)
Europe 710 100 2
United States 35 7 0.1
Methyl-ß-ionone (399)
Europe 44 6 0.1
United States 0.9 0.2 0.003
Methyl-delta-ionone (400)
Europe 3 0.4 0.01
United States 6 1 0.02
Allyl-alpha-ionone (401)
Europe 250 35 0.6
United States 130 25 0.4
alpha-Irone (403)
Europe 6.3 9 0.1
United States 15 3 0.05
alpha-iso-Methylionone (404)
Europe 39 6 0.09
United States 5 1 0.02
Total
Europe 5300 760 13
United States 1700 320 5
a From US National Academy of Sciences (1989); International
Organization of the Flavor Industry (1995)
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
The structure of 1,4-dimethyl-4-acetyl-1-cyclohexene (No. 402)
was considered not to be sufficiently similar to that of the ionones
to be included in the group, and it was therefore not included in the
safety evaluation. The remaining 20 compounds were evaluated according
to the procedure.
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), all of the 20 ionones
and related substances considered to be part of this group
are in class I.
Step 2. Data were available for ß-ionone that showed that it is
metabolized by carbonyl reduction, hydroxylation of the
alicyclic ring, and glucuronic acid conjugation of
metabolites with alcohol groups. Only limited data were
available on the fate of other compounds in the group.
Although structural characteristics similar to that of
ß-ionone were found, there were differences in the number
and positions of the alicyclic double-bonds, the position of
the carbonyl group within the side-chain, and its
relationship to endocylic and exocyclic double-bonds. On the
basis of the similarities of the functional groups present
in the flavouring agents in this group, it was considered
that alpha- and ß-ionones, their alcohol analogues,
analogues with a single endocyclic double-bond, and
analogues with saturated side-chains or more extended
side-chains would be eliminated from the body by common
metabolic processes, which would lead to innocuous products.
Members of the group with two endocyclic double-bonds,
with the carboxyl group adjacent to the ring, or with an
allyl double-bond attached to the ring might be eliminated
more slowly, which might affect their toxic potency. The
decision tree would take this into account to some extent by
allocating compounds that are sterically hindered into class
II rather than the class I. In consequence, the Committee
was not able to conclude a priori that the products of
metabolism of such members of the group would be innocuous.
Substances that could be predicted to be metabolized to
innocuous products fall into the following two groups:
Group 1: alpha-Ionone (No. 388), alpha-ionol (No. 391),
dihydro-alpha-ionone (No. 393), methyl-alpha-ionone (No.
398), methyl-delta-ionone (No. 400), alpha-irone (No. 403),
2-iso-methylionone (No. 404), and allyl-alpha-ionone (No.
401) were considered likely to share a common metabolic
pathway to alpha-ionone, although the rate of metabolism for
some of these substances may be slower than for
alpha-ionone.
Group 2: ß-Ionol (No. 392), dihydro-ß-ionone (No. 394),
dihydro-ß-ionol (No. 395), methyl-ß-ionone (No. 399), and
methyl-delta-ionone (No. 400) were considered likely to
share common metabolic pathways with ß-ionone, although the
rate of metabolism for some of these substances may be
slower than for ß-ionone.
Evaluation of all of the substances in groups 1 and 2
should proceed down the 'A' side of the scheme.
Substances that cannot be predicted to be metabolized
to innocuous products are ß-damascone (No. 384),
alpha-damascone (No. 385), delta-damascone (No. 386),
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (No.
387), gamma-ionone (No. 390), dehydrodi-hydroionone (No.
396), and dehydrodihydroionol (No. 397), and their
evaluation should proceed down the 'B' side of the scheme.
Step A3. As the intakes in Europe and the United States of all 13
substances on the 'A' side of the scheme are below the
threshold of concern for class I (1800 µg/person per day),
these substances would not be expected to be of safety
concern.
Step B3. Intake of ß-damascone (No. 384), alpha-damascone (No. 385),
delta-damascone (No. 386), 4-(2,6,6-trimethylcyclohexa-1,3-
dienyl)but-2-en-4-one (No. 387), gamma-ionone (No. 390),
dehydrodihydroionone (No. 396), and dehydrodihydroionol (No.
397) in Europe and the United States is below the threshold
of concern, and the safety evaluation proceeds to step B4.
Step B4. Information on each of the compounds considered at this step
is given below:
ß -Damascone (No. 384)
The NOEL for this substance is > 2 mg/kg bw per day (90-day
study in rats), and the margin of safety between this NOEL and the
daily per capita intake under the conditions of intended use is
> 2500.
alpha-Damascone (No. 385), delta-damascone (No. 386), and
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one (No. 387)
These substances were considered likely to share common metabolic
pathways with ß-damascone (No. 384) for which the NOEL is > 2 mg/kg
bw per day, although the rate of metabolism may be slower. Therefore
the margin of safety between this NOEL and daily per capita intake
is > 1.5 × 104 for alpha-damascone, > 2 × 105 for
delta-damascone, and > 14 × 103 for
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one.
gamma -Ionone (No. 390)
This substance was considered likely to share a common metabolic
pathway with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw
per day (90-day study in rats). Therefore, the margin of safety
between this NOEL and the daily per capita intake of alpha-ionone is
> 40 × 104. It was also considered likely to share a common
metabolic pathway with carvone (No. 380), for which the NOEL is 93
mg/kg bw per day (three-month study in rats). In this case, the margin
of safety between the NOEL and the daily per capita intake of
gamma-ionone is > 4 × 105.
Dehydrodihydroionone (No. 396) and dehydrodihydroionol (No. 397)
These substances were considered likely to share common pathways
with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw per day.
The margin of safety between this NOEL and the daily per capita
intake is 6 × 106 for dehydrodihydroionone and > 7 × 105 for
dehydrodihydroionol.
Therefore, the seven substances considered on the 'B' side of the
scheme would not be expected to be of safety concern.
1.5 Consideration of combined intakes from use as flavouring agents
All of the 20 ionones and structurally related substances
considered in this evaluation would be expected to have common
metabolic pathways. In the unlikely event that all 20 substances were
consumed simultaneously on a daily basis, the estimated daily
per capita consumption in Europe and the United States would not
exceed the human intake threshold for substances in class 1.
1.6 Conclusions
In applying the procedure, the Committee concluded that use of
any of the 20 ionones and related substances as flavouring agents
would not present a safety concern at the current estimated intake
levels. The Committee noted that all of the available data on toxicity
are consistent with the results of the safety evaluation. The ADIs
previously established for alpha-ionone and ß-ionone were maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine. The data that support this conclusion are given below.
Analysis of urine collected from two rabbits fed a total of 170 g
alpha-ionone over an unspecified period revealed a hydroxylated
derivative of alpha-ionone formed from allylic ring oxidation (Prelog
& Wursch, 1951).
Urine was collected daily from one male rabbit fed a total of 23
g ß-ionone over seven days at approximately 1000 mg/kg bw per day) and
for four days after the final dose. 3-Oxo-ß-ionone, 3-oxo-ß-ionol,
dihydro-3-oxo-ß-ionol, and 3-hydroxy-ß-ionol were identified, as were
unchanged ß-ionone and the glucuronic acid conjugates of 3-oxo-ß-ionol
and dihydro-3-oxo-ß-ionol (Ide & Toki, 1970).
The urinary metabolites of two rabbits that received a total of
100 g ß-ionone by gavage over 18 days included 3-hydroxy-ß-ionone,
3-oxo-ß-ionol, and 3-hydroxy-ß-ionone. A hydroxyketone thought to be
either 3-oxo-ß-ionol or 3-hydroxy-ß-ionone was also recovered (Fujii
et al., 1972). After oral administration of ß-ionone to three rabbits
at doses of 2000-5000 mg/day for two weeks, the urine contained
ß-ionone, ß-ionol, and their exocyclic dihydro metabolites, namely,
dihydro-ß-ionol, 3-hydroxy-ß-ionol, 3-hydroxydihydro-ß-ionol,
3-hydroxy-ß-ionone, and 3-hydroxydihydro-ß-ionone (Bielig & Hayasida,
1940). (In these two studies, the urinary metabolites were identified
by IUPAC nomenclature, but the metabolites were reported by a
different naming system.)
Two dogs fed a total of 100 g ß-ionone over 18 days also excreted
3-oxo-ß-ionone and 3-hydroxy-ß-ionol in the urine (Prelog & Meier,
1950).
ß-Ionone has been found to induce biphenyl 4-hydroxylase,
glucuronyl transferase, 4-nitrobenzoate reductase, and cytochrome P450
in rats after a three-day intraperitoneal or oral administration
(Parke & Rahman, 1969).
Studies in humans of the metabolism of retinoids such as
cis-13-retinoic acid (i.e. isotretinoin) and carotenoids such as
ß-carotene, which possess ionone fragments, indicate that the
metabolism of ionones is similar to that in animals. The primary
metabolites in blood and bile after oral administration of
isotretinoin to humans included the glucuronic acid conjugates of
isotretinoin (Kraft et al., 1991) and the allylic oxidation product
(Vane et al., 1990; Kraft et al., 1991). Both metabolites were found
in the blood and bile of cynomolgus monkeys after oral administration
of isotretinoin (Kraft et al., 1991). Allylic hydroxylation of the
methyl-ring substituent and subsequent conjugation with glucuronic
acid have also been shown to occur in humans (Vane et al., 1990).
ß-Carotene is oxidized by carotenoid dioxygenase(s) and cleaved
at the 15-15' (central) double bond to yield two molecules of vitamin
A (retinal) (Simpson & Chichester, 1981), which may subsequently be
cleaved at the 9'-10' double bond to yield ß-ionone and
10'-apo-ß-carotenals. The presence of 10'-apo-ß-carotenal in rat liver
after oral administration of ß-carotene suggests that oxidative
cleavage of the 9'-10' double bond occurs in animals (Sharma et al.,
1977).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of acute toxicity with ionone and
structurally related substances are shown in Table 3.
2.1.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of ionones and structurally related substances are shown in Table 4.
Details of the studies that were critical to the safety evaluation are
given below.
ß-Damascone
Groups of 16 male and 16 female Wistar CF/Gif Carworth strain
rats were given ß-damascone in the diet at 0 or 2 mg/kg bw per day for
90 days. Individual body weights were recorded weekly, but no
difference was seen between treated and control groups. Food intake
was slightly increased in treated females over that in controls but
was associated with a slight decrease in food use. Haematological
examination and blood urea determinations carried out on half of the
rats at week 7 and on all animals at the end of the treatment period
revealed no statistically significant changes, with no major
difference between treated and control groups in haemoglobin
concentration or total or differential leukocyte counts. At necropsy,
a slight increase in the absolute and relative weights of the liver
and kidneys of females was seen in comparison with controls, but these
changes were not correlated with any histopathological lesions.
Histological examination and clinical chemistry revealed no
significant changes. Non-specific inflammatory changes were seen in
the livers and kidneys of a few animals, but these changes were not
considered to be related to treatment. The NOEL was 2 mg/kg bw per
day, the only dose tested (Posternak et al., 1975).
alpha-Ionone and ß-ionone
Studies on alpha- and ß-ionone were evaluated at the
twenty-eighth meeting (Annex 1, reference 66).
alpha-Irone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha-irone in their diets for 90 days at a daily
concentration designed to provide 5 mg/kg bw for males and 6 mg/kg bw
for females. Records of daily food and water consumption revealed no
differences between test and control groups. Haematological and blood
chemical parameters were measured in eight rats of each sex at week 6
and in all rats at week 12; they were within normal ranges. At
autopsy, no difference in liver or kidney weights was seen between
test and control groups. Histological and gross pathological
examinations revealed no statistically significant changes. Treated
females had slightly higher haemoglobin and haematocrit values, but
the findings were considered to be biologically insignificant because
the mean erythrocyte count for the group was comparable to that of
controls. Both test and control groups showed a slight degree of
reactive lymphatic hyperplasia, but the findings were not related to
treatment. The NOEL was 5 mg/kg bw per day (Oser et al., 1965).
alpha-iso-Methylionone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha- iso-methylionone in their diets for 90 days at a
daily concentration designed to provide 4 mg/kg bw. Food and water
consumption was similar for the test and control groups.
Haematological and clinical chemical measurements conducted on eight
rats of each sex at week 6 and on all rats at week 12 showed normal
values. Liver and kidney weights recorded at necropsy were similar in
test and control groups. Histological and gross pathological
examinations revealed no changes related to treatment. The male rats
had a slightly reduced haemoglobin level, but the haematocrit and
erythrocyte counts were within the control ranges. The mean blood urea
nitrogen concentration was slightly lower in the treated group than in
controls at week 12, but this change was not accompanied by changes in
kidney weight or histological appearance and was considered to be of
no biological significance. The NOEL was > 4 mg/kg bw per day (Oser
et al., 1965).
2.1.3 Genotoxicity
The results of studies of the genotoxicity of this group of
substances are shown in Table 5.
4. REFERENCES
Bielig, H. & Hayasida, A. (1940) [On the release of ß-jonone in the
animal (biochemical hydration VIII).] Hoppe-Seyler's Z. Physiol.
Chem., 266, 99-111 (in German).
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 15, 219-232.
Table 3. Studies of the acute toxicity of ionone and related substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
4-[(2,6,6)-Trimethyl-cyclo-hex-1-enyl]but-2-en-4-one 384 Rat NR Oral 2920 Posternak et al.
(1975)
(ß-Damascone) Rat M/F Gavage 2000 Firmenich (1986)
alpha-Damascone 385 Rat M/F Oral 1800 Piccirillo et al.
(1979)
delta-Damascone 386 Mouse M/F Gavage 1820 Moran et al. (1980)
4-[(2,6,6)-Trimethylcyclo-hexa-1,3-dienyl]but-2-en-4-one 387 Rat M/F Gavage > 2000 Firmenich (1986)
alpha-Ionone 388 Rat M/F Oral 4590a Jenner et al. (1964)
ß-Ionone 389 Rat M/F Oral 4590 Jenner et al. (1964)
alpha-Ionol 391 Rat NR Oral > 5000 Moreno (1980)
alpha-Ionol 391 Mouse NR Oral 7400 Pellmont (1978)
ß-Ionol 392 Rat NR Oral > 1220; Moreno (1980)
< 5000
ß-Ionol 393 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-alpha-ionone 393 Rat NR Oral > 5000 Moreno (1976)
Dihydro-ß-ionone 394 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-ß-ionol 395 Mouse NR Oral 7400 Pellmont (1977)
Methyl-alpha-ionone 398 Rat NR Oral > 5000a Moreno (1973)
Methyl-delta-ionone 400 Rat NR Oral > 5000 Moreno (1973)
Allyl alpha-ionone 401 Mouse NR Gavage 8900 Givaudan (1955)
alpha-Irone 403 Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
alpha-iso-Methylionone 404 Rat NR Oral > 5000b Moreno (1973)
NR, not reported; M/F, male and female
a Mixture of alpha- and ß-ionone
b Mixture of methyl-alpha-ionone and alpha-iso-methylionone
Table 4. Short-term and long-term studies of toxicity with ionone and related substances in rats treated orally
Substance No. Sex No. groups/ Duration NOELa Reference
no. per group (mg/kg bw
per day)
4-[(2,6,6)-Trimethylcyclohex-1-enyl]-but-2-en-4-one (ß-Damascone) 384 M/F 1/32 90 days > 2 Posternak et al.
(1975)
alpha-Ionone 388 M/F 1/30 90 days > 11 Oser et al. (1965)
alpha-Ionone 388 M/F 2/30 90 days 10 Gaunt et al. (1983)
Iononeb 388 NR 3/8-10 7-8 weeks > 10 Sporn et al. (1963)
alpha-Iononec 388 M/F 3/20 17 weeks ND Hagan et al. (1967)
ß-Ionone 389 M/F 2/15 90 days > 11 (M) Oser et al. (1965)
> 13 (F)
ß-Ionone 389 M/F 2/30 90 days 10 Gaunt et al. (1983)
alpha-Irone 403 M/F 2/15 90 days > 5 (M) Oser et al. (1965)
> 6 (F)
alpha-iso-Methylionone 404 M/F 1/30 90 days > 4 Oser et al. (1965)
NR, not reported; M, male; F, female; ND, not determined
a NOEL given as 'greater than' (>) indicates that no adverse effects were observed at the highest dose in the study and, therefore, no NOEL
was determined.
b Mixture of alpha-ionone and ß-ionone
c Mixture of 60% alpha-ionone and 40% alpha-ionone
Table 5. Results of assays for the genotoxicity of ionones and related substances
Substance No. End-point Test object Dose Result Reference
alpha-Ionone 388 Chromosomal aberration Chinese hamster B241 25 nmol/L Positivea Kasamaki et al. (1982)
cell line
Gene mutation S. typhimurium TA98, 0.01-50 mg/plate Negativea Kasamaki et al. (1982
TA100
rec assay B. subtilis H17 & M45 19 mg/disc Negativeb Oda et al. (1978)
ß-Ionone 389 Gene mutation
(preincubation) S. typhimurium TA98, 1-180 mg/plate Negativea Mortlemans et al. (1986)
TA100, TA1535, 1537
Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537
Methyl-alpha-ionone 398 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
Methyl-alpha-ionone 398 Micronucleus formation NMRI mice, male and 825-2063 mg/kg bw Negative Wild et al. (1983)
female, bone marrow
Methyl-alpha-ionone 398 Gene mutation (basc) Drosophila melanogaster 20 mmol/L Negative Wild et al. (1983)
Methyl-delta-ionone 400 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
a With and without metabolic activation
b Activation status unknown
Fujii, T., Furukawa, S. & Suzuki, S. (1972) Compounded perfumes for
toilet goods. Non-irritative compounded perfumes for soaps.
Yukagaku, 21, 904-908.
Gaunt, I.F., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hagan, E.C., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
International Organization of the Flavor Industry (1975) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Wasington DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kasamaki, A., Takahashi, H., Tsumura, N., Niwa, J., Fujita, T. &
Urasawa, S. (1982) Genotoxicity of flavoring agents. Mutat. Res.,
105, 387-392.
Kraft, J.C., Slikker, W., Jr, Bailey, J.R., Roberts, L.G., Fischer,
B., Wittfoht, W. & Nau, H. (1991) Plasma pharmacokinetics and
metabolism of 13-cis- and all-trans-retinoic acid in the cynomolgus
monkey and the identification of 13-cis- and
all-trans-retinoyl-ß-glucuronides. A comparison to one human case
study with isotretinoin. Drug Metab. Disposition, 19, 317-324.
Levenstein, I. (1955) Acute oral toxicity study of allyl-alpha-ionone
in mice. Unpublished report from Leberco Labs. Submitted to WHO by the
Food and Extract Manufacturers' Association of the United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Lab. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Lab. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Lab. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Oser, B.L., Carson, S. & Oser, M. (1965) Toxicological tests on
flavouring matters. Food Chem. Toxicol., 3, 563-569.
Parke, D.V. & Rahman, H. (1969) The effects of some terpenoids and
other dietary anutrients on hepatic drug-metabolizing enzymes.
Biochem. J., 113, 124.
Pellmont, I. (1977) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Pellmont, I. (1978) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Piccirillo, V.J., Resnick, P., Swidersky, P. & Herndon, B. (1979)
Acute oral toxicity of alpha-damascone in rats. Unpublished report.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Posternak, J.M., Dufour, J.J., Rogg, C. & Vodoz, C.A. (1975)
Toxicology tests on flavouring matters II. Pyrazines and other
compounds. Food Cosmet. Toxicol., 13, 487-490.
Prelog, V. & Meier, H.l. (1950) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 33, 1276-1284 (in
German).
Prelog, V. & Wursch, J. (1951) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 34, 859-861 (in German).
Sharma, R.V., Mathur, S.N., Dmitrovskii, A.A., Das, R.C. & Ganguly,
J.. (1977) Studies on the metabolism of ß-carotene and
apo-ß-carotenoids in rats and chickens. Biochim. Biophys. Acta, 486,
183-194.
Shelanski, M.V. & Moldovan, M. (1972) Acute oral and dermal toxicity
studies. Unpublished report. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Simpson, K.L. & Chichester, C.O. (1981) Metabolism and nutritional
significance of carotenoids. Ann. Rev. Nutr., 1, 351-374.
Sporn, A., Schobesch, O., Marin, V., Panaitescu, E. & Runcan, L.
(1963) The toxicity of butyl acetate, methyl naphthyl ketone and
ionone. Igiena, 12, 437-445.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US National Academy of Sciences (1989) 1987 Poundage and Technical
Effects Update of Substances Added to Food, Washington DC, United
States.
Vane, F.M., Bugge, C.J.L., Rodriguez, L.C., Rosenberger, M. & Doran,
T.I. (1990) Human biliary metabolites of isotretinoin: Identification,
quantification, synthesis and biological activity. Xenobiotica, 20,
193-207.
Whittaker, C.J. (1986a) Single dose oral toxicity study of
1-(2,6,6-trimethylcyclohexa-1,3-dienyl)-2-buten-1-one (damascone) in
the rat. Unpublished report from Firmenich & Co. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Whittaker, C.J. (1986b) Single dose oral toxicitystudy of
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)-but-2-en-4-one (damascenone)
in the rat. Unpublished report from Firmenich & Co. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavoring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
a The threshold of concern for class I is 1800 µg/day.
b The ADI values previously established for alpha-ionone and ß-ionone were maintained at the present meeting.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from
surveys in Europe (International organization of the Flavor
Industry, 1994) and the United States (US Academy of Sciences,
1989) (see Table 2). The estimated total daily per capita
intake of all 21 ionones and related substances from their
use as flavouring agents is 0.76 mg/person in Europe and 0.33
mg/person in the United States. In Europe, four substances,
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (0.043
mg/person), alpha-ionone (0.31 mg/person), ß-ionone (0.15 mg/person),
and methyl-alpha-ionone (0.035 mg/person), account for approximately
85% of the total annual per capita intake of this group of
substances when used as flavouring agents. In the United States, three
substances, alpha-ionone (0.15 mg/person), ß-ionone (0.10 mg/person),
and allyl-alpha-ionone (0.025 mg/person) account for about 85% of the
total annual per capita intake of this group of substances when used
as flavouring agents.
Eleven of the substances in this group have been reported to
occur naturally in foods including raspberries, carrots, roasted
almonds, fruits, and herbs (Maarse et al., 1994). Quantitative data on
natural occurrence and consumption ratios have been reported for seven
substances (Nos 388, 389, 390, 391, 392, 393, and 394), which indicate
that they are consumed predominantly in traditional foods, i.e. the
consumption ratio is larger than 1 (Stofberg & Kirschman, 1985;
Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine.
Table 2. Most recent annual usage of ionones and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
ß-Damascone (384)
Europe 300 43 0.7
United States 50 10 0.2
alpha-Damascone (385)
Europe 57 8 0.1
United States 2 0.4 0.01
delta-Damascone (386)
Europe 0.4 0.06 0.001
United States 3 0.6 0.01
Damascenone (387)
Europe 600 86 1
United States 24 5 0.08
alpha-Ionone (388)
Europe 2200 310 5
United States 770 150 2
ß-lonone (389)
Europe 1100 150 3
United States 550 100 2
gamma-Ionone (390)
Europe 0.1 0.01 0.0002
United States 80 15 0.3
alpha-lonol (391)
Europe 5 0.7 0.01
United States 0.3 0.06 0.001
ß-lonol (392)
Europe 6 0.9 0.01
United States 0.5 0.1 0.002
Dihydro-alpha-ionone (393)
Europe 5 0.7 0.01
United States 0.1 0.02 0.0003
Dihydro-ß-ionone (394)
Europe 9 1 0.02
United States 0.2 0.04 0.0006
Dihydro-ß-ionol (395)
Europe 2.3 0.3 0.014
United States 0.1 0.02 0.0003
Dehydrodihydroionone (396)
Europe 0.7 0.1 0.002
United States 0.4 0.08 0.001
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Dehydrodihydroionol (397)
Europe 0.1 0.01 0.0002
United States 40 8 0.1
Methyl-alpha-ionone (398)
Europe 710 100 2
United States 35 7 0.1
Methyl-ß-ionone (399)
Europe 44 6 0.1
United States 0.9 0.2 0.003
Methyl-delta-ionone (400)
Europe 3 0.4 0.01
United States 6 1 0.02
Allyl-alpha-ionone (401)
Europe 250 35 0.6
United States 130 25 0.4
alpha-Irone (403)
Europe 6.3 9 0.1
United States 15 3 0.05
alpha-iso-Methylionone (404)
Europe 39 6 0.09
United States 5 1 0.02
Total
Europe 5300 760 13
United States 1700 320 5
a From US National Academy of Sciences (1989); International
Organization of the Flavor Industry (1995)
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
The structure of 1,4-dimethyl-4-acetyl-1-cyclohexene (No. 402)
was considered not to be sufficiently similar to that of the ionones
to be included in the group, and it was therefore not included in the
safety evaluation. The remaining 20 compounds were evaluated according
to the procedure.
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), all of the 20 ionones
and related substances considered to be part of this group
are in class I.
Step 2. Data were available for ß-ionone that showed that it is
metabolized by carbonyl reduction, hydroxylation of the
alicyclic ring, and glucuronic acid conjugation of
metabolites with alcohol groups. Only limited data were
available on the fate of other compounds in the group.
Although structural characteristics similar to that of
ß-ionone were found, there were differences in the number
and positions of the alicyclic double-bonds, the position of
the carbonyl group within the side-chain, and its
relationship to endocylic and exocyclic double-bonds. On the
basis of the similarities of the functional groups present
in the flavouring agents in this group, it was considered
that alpha- and ß-ionones, their alcohol analogues,
analogues with a single endocyclic double-bond, and
analogues with saturated side-chains or more extended
side-chains would be eliminated from the body by common
metabolic processes, which would lead to innocuous products.
Members of the group with two endocyclic double-bonds,
with the carboxyl group adjacent to the ring, or with an
allyl double-bond attached to the ring might be eliminated
more slowly, which might affect their toxic potency. The
decision tree would take this into account to some extent by
allocating compounds that are sterically hindered into class
II rather than the class I. In consequence, the Committee
was not able to conclude a priori that the products of
metabolism of such members of the group would be innocuous.
Substances that could be predicted to be metabolized to
innocuous products fall into the following two groups:
Group 1: alpha-Ionone (No. 388), alpha-ionol (No. 391),
dihydro-alpha-ionone (No. 393), methyl-alpha-ionone (No.
398), methyl-delta-ionone (No. 400), alpha-irone (No. 403),
2-iso-methylionone (No. 404), and allyl-alpha-ionone (No.
401) were considered likely to share a common metabolic
pathway to alpha-ionone, although the rate of metabolism for
some of these substances may be slower than for
alpha-ionone.
Group 2: ß-Ionol (No. 392), dihydro-ß-ionone (No. 394),
dihydro-ß-ionol (No. 395), methyl-ß-ionone (No. 399), and
methyl-delta-ionone (No. 400) were considered likely to
share common metabolic pathways with ß-ionone, although the
rate of metabolism for some of these substances may be
slower than for ß-ionone.
Evaluation of all of the substances in groups 1 and 2
should proceed down the 'A' side of the scheme.
Substances that cannot be predicted to be metabolized
to innocuous products are ß-damascone (No. 384),
alpha-damascone (No. 385), delta-damascone (No. 386),
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (No.
387), gamma-ionone (No. 390), dehydrodi-hydroionone (No.
396), and dehydrodihydroionol (No. 397), and their
evaluation should proceed down the 'B' side of the scheme.
Step A3. As the intakes in Europe and the United States of all 13
substances on the 'A' side of the scheme are below the
threshold of concern for class I (1800 µg/person per day),
these substances would not be expected to be of safety
concern.
Step B3. Intake of ß-damascone (No. 384), alpha-damascone (No. 385),
delta-damascone (No. 386), 4-(2,6,6-trimethylcyclohexa-1,3-
dienyl)but-2-en-4-one (No. 387), gamma-ionone (No. 390),
dehydrodihydroionone (No. 396), and dehydrodihydroionol (No.
397) in Europe and the United States is below the threshold
of concern, and the safety evaluation proceeds to step B4.
Step B4. Information on each of the compounds considered at this step
is given below:
ß -Damascone (No. 384)
The NOEL for this substance is > 2 mg/kg bw per day (90-day
study in rats), and the margin of safety between this NOEL and the
daily per capita intake under the conditions of intended use is
> 2500.
alpha-Damascone (No. 385), delta-damascone (No. 386), and
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one (No. 387)
These substances were considered likely to share common metabolic
pathways with ß-damascone (No. 384) for which the NOEL is > 2 mg/kg
bw per day, although the rate of metabolism may be slower. Therefore
the margin of safety between this NOEL and daily per capita intake
is > 1.5 × 104 for alpha-damascone, > 2 × 105 for
delta-damascone, and > 14 × 103 for
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one.
gamma -Ionone (No. 390)
This substance was considered likely to share a common metabolic
pathway with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw
per day (90-day study in rats). Therefore, the margin of safety
between this NOEL and the daily per capita intake of alpha-ionone is
> 40 × 104. It was also considered likely to share a common
metabolic pathway with carvone (No. 380), for which the NOEL is 93
mg/kg bw per day (three-month study in rats). In this case, the margin
of safety between the NOEL and the daily per capita intake of
gamma-ionone is > 4 × 105.
Dehydrodihydroionone (No. 396) and dehydrodihydroionol (No. 397)
These substances were considered likely to share common pathways
with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw per day.
The margin of safety between this NOEL and the daily per capita
intake is 6 × 106 for dehydrodihydroionone and > 7 × 105 for
dehydrodihydroionol.
Therefore, the seven substances considered on the 'B' side of the
scheme would not be expected to be of safety concern.
1.5 Consideration of combined intakes from use as flavouring agents
All of the 20 ionones and structurally related substances
considered in this evaluation would be expected to have common
metabolic pathways. In the unlikely event that all 20 substances were
consumed simultaneously on a daily basis, the estimated daily
per capita consumption in Europe and the United States would not
exceed the human intake threshold for substances in class 1.
1.6 Conclusions
In applying the procedure, the Committee concluded that use of
any of the 20 ionones and related substances as flavouring agents
would not present a safety concern at the current estimated intake
levels. The Committee noted that all of the available data on toxicity
are consistent with the results of the safety evaluation. The ADIs
previously established for alpha-ionone and ß-ionone were maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine. The data that support this conclusion are given below.
Analysis of urine collected from two rabbits fed a total of 170 g
alpha-ionone over an unspecified period revealed a hydroxylated
derivative of alpha-ionone formed from allylic ring oxidation (Prelog
& Wursch, 1951).
Urine was collected daily from one male rabbit fed a total of 23
g ß-ionone over seven days at approximately 1000 mg/kg bw per day) and
for four days after the final dose. 3-Oxo-ß-ionone, 3-oxo-ß-ionol,
dihydro-3-oxo-ß-ionol, and 3-hydroxy-ß-ionol were identified, as were
unchanged ß-ionone and the glucuronic acid conjugates of 3-oxo-ß-ionol
and dihydro-3-oxo-ß-ionol (Ide & Toki, 1970).
The urinary metabolites of two rabbits that received a total of
100 g ß-ionone by gavage over 18 days included 3-hydroxy-ß-ionone,
3-oxo-ß-ionol, and 3-hydroxy-ß-ionone. A hydroxyketone thought to be
either 3-oxo-ß-ionol or 3-hydroxy-ß-ionone was also recovered (Fujii
et al., 1972). After oral administration of ß-ionone to three rabbits
at doses of 2000-5000 mg/day for two weeks, the urine contained
ß-ionone, ß-ionol, and their exocyclic dihydro metabolites, namely,
dihydro-ß-ionol, 3-hydroxy-ß-ionol, 3-hydroxydihydro-ß-ionol,
3-hydroxy-ß-ionone, and 3-hydroxydihydro-ß-ionone (Bielig & Hayasida,
1940). (In these two studies, the urinary metabolites were identified
by IUPAC nomenclature, but the metabolites were reported by a
different naming system.)
Two dogs fed a total of 100 g ß-ionone over 18 days also excreted
3-oxo-ß-ionone and 3-hydroxy-ß-ionol in the urine (Prelog & Meier,
1950).
ß-Ionone has been found to induce biphenyl 4-hydroxylase,
glucuronyl transferase, 4-nitrobenzoate reductase, and cytochrome P450
in rats after a three-day intraperitoneal or oral administration
(Parke & Rahman, 1969).
Studies in humans of the metabolism of retinoids such as
cis-13-retinoic acid (i.e. isotretinoin) and carotenoids such as
ß-carotene, which possess ionone fragments, indicate that the
metabolism of ionones is similar to that in animals. The primary
metabolites in blood and bile after oral administration of
isotretinoin to humans included the glucuronic acid conjugates of
isotretinoin (Kraft et al., 1991) and the allylic oxidation product
(Vane et al., 1990; Kraft et al., 1991). Both metabolites were found
in the blood and bile of cynomolgus monkeys after oral administration
of isotretinoin (Kraft et al., 1991). Allylic hydroxylation of the
methyl-ring substituent and subsequent conjugation with glucuronic
acid have also been shown to occur in humans (Vane et al., 1990).
ß-Carotene is oxidized by carotenoid dioxygenase(s) and cleaved
at the 15-15' (central) double bond to yield two molecules of vitamin
A (retinal) (Simpson & Chichester, 1981), which may subsequently be
cleaved at the 9'-10' double bond to yield ß-ionone and
10'-apo-ß-carotenals. The presence of 10'-apo-ß-carotenal in rat liver
after oral administration of ß-carotene suggests that oxidative
cleavage of the 9'-10' double bond occurs in animals (Sharma et al.,
1977).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of acute toxicity with ionone and
structurally related substances are shown in Table 3.
2.1.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of ionones and structurally related substances are shown in Table 4.
Details of the studies that were critical to the safety evaluation are
given below.
ß-Damascone
Groups of 16 male and 16 female Wistar CF/Gif Carworth strain
rats were given ß-damascone in the diet at 0 or 2 mg/kg bw per day for
90 days. Individual body weights were recorded weekly, but no
difference was seen between treated and control groups. Food intake
was slightly increased in treated females over that in controls but
was associated with a slight decrease in food use. Haematological
examination and blood urea determinations carried out on half of the
rats at week 7 and on all animals at the end of the treatment period
revealed no statistically significant changes, with no major
difference between treated and control groups in haemoglobin
concentration or total or differential leukocyte counts. At necropsy,
a slight increase in the absolute and relative weights of the liver
and kidneys of females was seen in comparison with controls, but these
changes were not correlated with any histopathological lesions.
Histological examination and clinical chemistry revealed no
significant changes. Non-specific inflammatory changes were seen in
the livers and kidneys of a few animals, but these changes were not
considered to be related to treatment. The NOEL was 2 mg/kg bw per
day, the only dose tested (Posternak et al., 1975).
alpha-Ionone and ß-ionone
Studies on alpha- and ß-ionone were evaluated at the
twenty-eighth meeting (Annex 1, reference 66).
alpha-Irone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha-irone in their diets for 90 days at a daily
concentration designed to provide 5 mg/kg bw for males and 6 mg/kg bw
for females. Records of daily food and water consumption revealed no
differences between test and control groups. Haematological and blood
chemical parameters were measured in eight rats of each sex at week 6
and in all rats at week 12; they were within normal ranges. At
autopsy, no difference in liver or kidney weights was seen between
test and control groups. Histological and gross pathological
examinations revealed no statistically significant changes. Treated
females had slightly higher haemoglobin and haematocrit values, but
the findings were considered to be biologically insignificant because
the mean erythrocyte count for the group was comparable to that of
controls. Both test and control groups showed a slight degree of
reactive lymphatic hyperplasia, but the findings were not related to
treatment. The NOEL was 5 mg/kg bw per day (Oser et al., 1965).
alpha-iso-Methylionone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha- iso-methylionone in their diets for 90 days at a
daily concentration designed to provide 4 mg/kg bw. Food and water
consumption was similar for the test and control groups.
Haematological and clinical chemical measurements conducted on eight
rats of each sex at week 6 and on all rats at week 12 showed normal
values. Liver and kidney weights recorded at necropsy were similar in
test and control groups. Histological and gross pathological
examinations revealed no changes related to treatment. The male rats
had a slightly reduced haemoglobin level, but the haematocrit and
erythrocyte counts were within the control ranges. The mean blood urea
nitrogen concentration was slightly lower in the treated group than in
controls at week 12, but this change was not accompanied by changes in
kidney weight or histological appearance and was considered to be of
no biological significance. The NOEL was > 4 mg/kg bw per day (Oser
et al., 1965).
2.1.3 Genotoxicity
The results of studies of the genotoxicity of this group of
substances are shown in Table 5.
4. REFERENCES
Bielig, H. & Hayasida, A. (1940) [On the release of ß-jonone in the
animal (biochemical hydration VIII).] Hoppe-Seyler's Z. Physiol.
Chem., 266, 99-111 (in German).
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 15, 219-232.
Table 3. Studies of the acute toxicity of ionone and related substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
4-[(2,6,6)-Trimethyl-cyclo-hex-1-enyl]but-2-en-4-one 384 Rat NR Oral 2920 Posternak et al.
(1975)
(ß-Damascone) Rat M/F Gavage 2000 Firmenich (1986)
alpha-Damascone 385 Rat M/F Oral 1800 Piccirillo et al.
(1979)
delta-Damascone 386 Mouse M/F Gavage 1820 Moran et al. (1980)
4-[(2,6,6)-Trimethylcyclo-hexa-1,3-dienyl]but-2-en-4-one 387 Rat M/F Gavage > 2000 Firmenich (1986)
alpha-Ionone 388 Rat M/F Oral 4590a Jenner et al. (1964)
ß-Ionone 389 Rat M/F Oral 4590 Jenner et al. (1964)
alpha-Ionol 391 Rat NR Oral > 5000 Moreno (1980)
alpha-Ionol 391 Mouse NR Oral 7400 Pellmont (1978)
ß-Ionol 392 Rat NR Oral > 1220; Moreno (1980)
< 5000
ß-Ionol 393 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-alpha-ionone 393 Rat NR Oral > 5000 Moreno (1976)
Dihydro-ß-ionone 394 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-ß-ionol 395 Mouse NR Oral 7400 Pellmont (1977)
Methyl-alpha-ionone 398 Rat NR Oral > 5000a Moreno (1973)
Methyl-delta-ionone 400 Rat NR Oral > 5000 Moreno (1973)
Allyl alpha-ionone 401 Mouse NR Gavage 8900 Givaudan (1955)
alpha-Irone 403 Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
alpha-iso-Methylionone 404 Rat NR Oral > 5000b Moreno (1973)
NR, not reported; M/F, male and female
a Mixture of alpha- and ß-ionone
b Mixture of methyl-alpha-ionone and alpha-iso-methylionone
Table 4. Short-term and long-term studies of toxicity with ionone and related substances in rats treated orally
Substance No. Sex No. groups/ Duration NOELa Reference
no. per group (mg/kg bw
per day)
4-[(2,6,6)-Trimethylcyclohex-1-enyl]-but-2-en-4-one (ß-Damascone) 384 M/F 1/32 90 days > 2 Posternak et al.
(1975)
alpha-Ionone 388 M/F 1/30 90 days > 11 Oser et al. (1965)
alpha-Ionone 388 M/F 2/30 90 days 10 Gaunt et al. (1983)
Iononeb 388 NR 3/8-10 7-8 weeks > 10 Sporn et al. (1963)
alpha-Iononec 388 M/F 3/20 17 weeks ND Hagan et al. (1967)
ß-Ionone 389 M/F 2/15 90 days > 11 (M) Oser et al. (1965)
> 13 (F)
ß-Ionone 389 M/F 2/30 90 days 10 Gaunt et al. (1983)
alpha-Irone 403 M/F 2/15 90 days > 5 (M) Oser et al. (1965)
> 6 (F)
alpha-iso-Methylionone 404 M/F 1/30 90 days > 4 Oser et al. (1965)
NR, not reported; M, male; F, female; ND, not determined
a NOEL given as 'greater than' (>) indicates that no adverse effects were observed at the highest dose in the study and, therefore, no NOEL
was determined.
b Mixture of alpha-ionone and ß-ionone
c Mixture of 60% alpha-ionone and 40% alpha-ionone
Table 5. Results of assays for the genotoxicity of ionones and related substances
Substance No. End-point Test object Dose Result Reference
alpha-Ionone 388 Chromosomal aberration Chinese hamster B241 25 nmol/L Positivea Kasamaki et al. (1982)
cell line
Gene mutation S. typhimurium TA98, 0.01-50 mg/plate Negativea Kasamaki et al. (1982
TA100
rec assay B. subtilis H17 & M45 19 mg/disc Negativeb Oda et al. (1978)
ß-Ionone 389 Gene mutation
(preincubation) S. typhimurium TA98, 1-180 mg/plate Negativea Mortlemans et al. (1986)
TA100, TA1535, 1537
Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537
Methyl-alpha-ionone 398 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
Methyl-alpha-ionone 398 Micronucleus formation NMRI mice, male and 825-2063 mg/kg bw Negative Wild et al. (1983)
female, bone marrow
Methyl-alpha-ionone 398 Gene mutation (basc) Drosophila melanogaster 20 mmol/L Negative Wild et al. (1983)
Methyl-delta-ionone 400 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
a With and without metabolic activation
b Activation status unknown
Fujii, T., Furukawa, S. & Suzuki, S. (1972) Compounded perfumes for
toilet goods. Non-irritative compounded perfumes for soaps.
Yukagaku, 21, 904-908.
Gaunt, I.F., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hagan, E.C., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
International Organization of the Flavor Industry (1975) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Wasington DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kasamaki, A., Takahashi, H., Tsumura, N., Niwa, J., Fujita, T. &
Urasawa, S. (1982) Genotoxicity of flavoring agents. Mutat. Res.,
105, 387-392.
Kraft, J.C., Slikker, W., Jr, Bailey, J.R., Roberts, L.G., Fischer,
B., Wittfoht, W. & Nau, H. (1991) Plasma pharmacokinetics and
metabolism of 13-cis- and all-trans-retinoic acid in the cynomolgus
monkey and the identification of 13-cis- and
all-trans-retinoyl-ß-glucuronides. A comparison to one human case
study with isotretinoin. Drug Metab. Disposition, 19, 317-324.
Levenstein, I. (1955) Acute oral toxicity study of allyl-alpha-ionone
in mice. Unpublished report from Leberco Labs. Submitted to WHO by the
Food and Extract Manufacturers' Association of the United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Lab. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Lab. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Lab. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Oser, B.L., Carson, S. & Oser, M. (1965) Toxicological tests on
flavouring matters. Food Chem. Toxicol., 3, 563-569.
Parke, D.V. & Rahman, H. (1969) The effects of some terpenoids and
other dietary anutrients on hepatic drug-metabolizing enzymes.
Biochem. J., 113, 124.
Pellmont, I. (1977) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Pellmont, I. (1978) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Piccirillo, V.J., Resnick, P., Swidersky, P. & Herndon, B. (1979)
Acute oral toxicity of alpha-damascone in rats. Unpublished report.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Posternak, J.M., Dufour, J.J., Rogg, C. & Vodoz, C.A. (1975)
Toxicology tests on flavouring matters II. Pyrazines and other
compounds. Food Cosmet. Toxicol., 13, 487-490.
Prelog, V. & Meier, H.l. (1950) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 33, 1276-1284 (in
German).
Prelog, V. & Wursch, J. (1951) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 34, 859-861 (in German).
Sharma, R.V., Mathur, S.N., Dmitrovskii, A.A., Das, R.C. & Ganguly,
J.. (1977) Studies on the metabolism of ß-carotene and
apo-ß-carotenoids in rats and chickens. Biochim. Biophys. Acta, 486,
183-194.
Shelanski, M.V. & Moldovan, M. (1972) Acute oral and dermal toxicity
studies. Unpublished report. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Simpson, K.L. & Chichester, C.O. (1981) Metabolism and nutritional
significance of carotenoids. Ann. Rev. Nutr., 1, 351-374.
Sporn, A., Schobesch, O., Marin, V., Panaitescu, E. & Runcan, L.
(1963) The toxicity of butyl acetate, methyl naphthyl ketone and
ionone. Igiena, 12, 437-445.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US National Academy of Sciences (1989) 1987 Poundage and Technical
Effects Update of Substances Added to Food, Washington DC, United
States.
Vane, F.M., Bugge, C.J.L., Rodriguez, L.C., Rosenberger, M. & Doran,
T.I. (1990) Human biliary metabolites of isotretinoin: Identification,
quantification, synthesis and biological activity. Xenobiotica, 20,
193-207.
Whittaker, C.J. (1986a) Single dose oral toxicity study of
1-(2,6,6-trimethylcyclohexa-1,3-dienyl)-2-buten-1-one (damascone) in
the rat. Unpublished report from Firmenich & Co. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Whittaker, C.J. (1986b) Single dose oral toxicitystudy of
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)-but-2-en-4-one (damascenone)
in the rat. Unpublished report from Firmenich & Co. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavoring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
 alpha-Damascone 385 43052-87-5 8/0.4 No No Yes No safety
concern
alpha-Damascone 385 43052-87-5 8/0.4 No No Yes No safety
concern
 delta-Damascone 386 57378-68-4 0.06/0.6 No No Yes No safety
concern
delta-Damascone 386 57378-68-4 0.06/0.6 No No Yes No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Damascenone 387 23696-85-7 86/5 No No Yes No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Damascenone 387 23696-85-7 86/5 No No Yes No safety
concern
 alpha-Ionone 388 127-41-3 310/150 Yes No - No safety
concernb
alpha-Ionone 388 127-41-3 310/150 Yes No - No safety
concernb
 ß-lonone 389 14901-07-6 150/100 Yes No - No safety
concernb
ß-lonone 389 14901-07-6 150/100 Yes No - No safety
concernb
 gamma-Ionone 390 79-76-5 0.01/15 No No Yes No safety
concern
gamma-Ionone 390 79-76-5 0.01/15 No No Yes No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-lonol 391 25312-34-9 0.7/0.06 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-lonol 391 25312-34-9 0.7/0.06 Yes No - No safety
concern
 ß-lonol 392 22029-76-1 0.9/0.1 Yes No - No safety
concern
ß-lonol 392 22029-76-1 0.9/0.1 Yes No - No safety
concern
 Dihydro-alpha-ionone 393 31499-72-6 0.7/0.02 Yes No - No safety
concern
Dihydro-alpha-ionone 393 31499-72-6 0.7/0.02 Yes No - No safety
concern
 Dihydro-ß-ionone 394 17283-81-7 1/0.04 Yes No - No safety
concern
Dihydro-ß-ionone 394 17283-81-7 1/0.04 Yes No - No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Dihydro-ß-ionol 395 3293-47-8 0.3/0.02 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Dihydro-ß-ionol 395 3293-47-8 0.3/0.02 Yes No - No safety
concern
 Dehydrodihydro-ionone 396 20483-36-7 0.1/0.08 No No Yes No safety
concern
Dehydrodihydro-ionone 396 20483-36-7 0.1/0.08 No No Yes No safety
concern
 Dehydrodihydro-ionol 397 57069-86-0 8/0.01 No No Yes No safety
concern
Dehydrodihydro-ionol 397 57069-86-0 8/0.01 No No Yes No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Methyl-alpha-ionone 398 127-42-4 100/7 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
Methyl-alpha-ionone 398 127-42-4 100/7 Yes No - No safety
concern
 Methyl-ß-ionone 399 127-43-5 6/0.2 Yes No - No safety
concern
Methyl-ß-ionone 399 127-43-5 6/0.2 Yes No - No safety
concern
 Methyl-delta-ionone 400 7748-98-7 0.4/1 Yes No - No safety
concern
Methyl-delta-ionone 400 7748-98-7 0.4/1 Yes No - No safety
concern
 Allyl-alpha-ionone 401 79-78-7 35/25 Yes No - No safety
concern
Allyl-alpha-ionone 401 79-78-7 35/25 Yes No - No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-Irone 403 79-69-6 9/3 Yes No - No safety
concern
Table 1. (continued)
Substance No. CAS No. Estmated per Step 2 Strep A3/B3 Step B4 Conclusion
capita intake, Metabolized Intake exceed Adequate NOEL based on
Europe/USA to innocuous threshold of for substance current levels
(µg/day) products? concern?a or related of intake
substance?
alpha-Irone 403 79-69-6 9/3 Yes No - No safety
concern
 alpha-iso-Methylionone 404 127-51-5 6/1 Yes No - No safety
concern
alpha-iso-Methylionone 404 127-51-5 6/1 Yes No - No safety
concern
 a The threshold of concern for class I is 1800 µg/day.
b The ADI values previously established for alpha-ionone and ß-ionone were maintained at the present meeting.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from
surveys in Europe (International organization of the Flavor
Industry, 1994) and the United States (US Academy of Sciences,
1989) (see Table 2). The estimated total daily per capita
intake of all 21 ionones and related substances from their
use as flavouring agents is 0.76 mg/person in Europe and 0.33
mg/person in the United States. In Europe, four substances,
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (0.043
mg/person), alpha-ionone (0.31 mg/person), ß-ionone (0.15 mg/person),
and methyl-alpha-ionone (0.035 mg/person), account for approximately
85% of the total annual per capita intake of this group of
substances when used as flavouring agents. In the United States, three
substances, alpha-ionone (0.15 mg/person), ß-ionone (0.10 mg/person),
and allyl-alpha-ionone (0.025 mg/person) account for about 85% of the
total annual per capita intake of this group of substances when used
as flavouring agents.
Eleven of the substances in this group have been reported to
occur naturally in foods including raspberries, carrots, roasted
almonds, fruits, and herbs (Maarse et al., 1994). Quantitative data on
natural occurrence and consumption ratios have been reported for seven
substances (Nos 388, 389, 390, 391, 392, 393, and 394), which indicate
that they are consumed predominantly in traditional foods, i.e. the
consumption ratio is larger than 1 (Stofberg & Kirschman, 1985;
Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine.
Table 2. Most recent annual usage of ionones and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
ß-Damascone (384)
Europe 300 43 0.7
United States 50 10 0.2
alpha-Damascone (385)
Europe 57 8 0.1
United States 2 0.4 0.01
delta-Damascone (386)
Europe 0.4 0.06 0.001
United States 3 0.6 0.01
Damascenone (387)
Europe 600 86 1
United States 24 5 0.08
alpha-Ionone (388)
Europe 2200 310 5
United States 770 150 2
ß-lonone (389)
Europe 1100 150 3
United States 550 100 2
gamma-Ionone (390)
Europe 0.1 0.01 0.0002
United States 80 15 0.3
alpha-lonol (391)
Europe 5 0.7 0.01
United States 0.3 0.06 0.001
ß-lonol (392)
Europe 6 0.9 0.01
United States 0.5 0.1 0.002
Dihydro-alpha-ionone (393)
Europe 5 0.7 0.01
United States 0.1 0.02 0.0003
Dihydro-ß-ionone (394)
Europe 9 1 0.02
United States 0.2 0.04 0.0006
Dihydro-ß-ionol (395)
Europe 2.3 0.3 0.014
United States 0.1 0.02 0.0003
Dehydrodihydroionone (396)
Europe 0.7 0.1 0.002
United States 0.4 0.08 0.001
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Dehydrodihydroionol (397)
Europe 0.1 0.01 0.0002
United States 40 8 0.1
Methyl-alpha-ionone (398)
Europe 710 100 2
United States 35 7 0.1
Methyl-ß-ionone (399)
Europe 44 6 0.1
United States 0.9 0.2 0.003
Methyl-delta-ionone (400)
Europe 3 0.4 0.01
United States 6 1 0.02
Allyl-alpha-ionone (401)
Europe 250 35 0.6
United States 130 25 0.4
alpha-Irone (403)
Europe 6.3 9 0.1
United States 15 3 0.05
alpha-iso-Methylionone (404)
Europe 39 6 0.09
United States 5 1 0.02
Total
Europe 5300 760 13
United States 1700 320 5
a From US National Academy of Sciences (1989); International
Organization of the Flavor Industry (1995)
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
The structure of 1,4-dimethyl-4-acetyl-1-cyclohexene (No. 402)
was considered not to be sufficiently similar to that of the ionones
to be included in the group, and it was therefore not included in the
safety evaluation. The remaining 20 compounds were evaluated according
to the procedure.
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), all of the 20 ionones
and related substances considered to be part of this group
are in class I.
Step 2. Data were available for ß-ionone that showed that it is
metabolized by carbonyl reduction, hydroxylation of the
alicyclic ring, and glucuronic acid conjugation of
metabolites with alcohol groups. Only limited data were
available on the fate of other compounds in the group.
Although structural characteristics similar to that of
ß-ionone were found, there were differences in the number
and positions of the alicyclic double-bonds, the position of
the carbonyl group within the side-chain, and its
relationship to endocylic and exocyclic double-bonds. On the
basis of the similarities of the functional groups present
in the flavouring agents in this group, it was considered
that alpha- and ß-ionones, their alcohol analogues,
analogues with a single endocyclic double-bond, and
analogues with saturated side-chains or more extended
side-chains would be eliminated from the body by common
metabolic processes, which would lead to innocuous products.
Members of the group with two endocyclic double-bonds,
with the carboxyl group adjacent to the ring, or with an
allyl double-bond attached to the ring might be eliminated
more slowly, which might affect their toxic potency. The
decision tree would take this into account to some extent by
allocating compounds that are sterically hindered into class
II rather than the class I. In consequence, the Committee
was not able to conclude a priori that the products of
metabolism of such members of the group would be innocuous.
Substances that could be predicted to be metabolized to
innocuous products fall into the following two groups:
Group 1: alpha-Ionone (No. 388), alpha-ionol (No. 391),
dihydro-alpha-ionone (No. 393), methyl-alpha-ionone (No.
398), methyl-delta-ionone (No. 400), alpha-irone (No. 403),
2-iso-methylionone (No. 404), and allyl-alpha-ionone (No.
401) were considered likely to share a common metabolic
pathway to alpha-ionone, although the rate of metabolism for
some of these substances may be slower than for
alpha-ionone.
Group 2: ß-Ionol (No. 392), dihydro-ß-ionone (No. 394),
dihydro-ß-ionol (No. 395), methyl-ß-ionone (No. 399), and
methyl-delta-ionone (No. 400) were considered likely to
share common metabolic pathways with ß-ionone, although the
rate of metabolism for some of these substances may be
slower than for ß-ionone.
Evaluation of all of the substances in groups 1 and 2
should proceed down the 'A' side of the scheme.
Substances that cannot be predicted to be metabolized
to innocuous products are ß-damascone (No. 384),
alpha-damascone (No. 385), delta-damascone (No. 386),
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (No.
387), gamma-ionone (No. 390), dehydrodi-hydroionone (No.
396), and dehydrodihydroionol (No. 397), and their
evaluation should proceed down the 'B' side of the scheme.
Step A3. As the intakes in Europe and the United States of all 13
substances on the 'A' side of the scheme are below the
threshold of concern for class I (1800 µg/person per day),
these substances would not be expected to be of safety
concern.
Step B3. Intake of ß-damascone (No. 384), alpha-damascone (No. 385),
delta-damascone (No. 386), 4-(2,6,6-trimethylcyclohexa-1,3-
dienyl)but-2-en-4-one (No. 387), gamma-ionone (No. 390),
dehydrodihydroionone (No. 396), and dehydrodihydroionol (No.
397) in Europe and the United States is below the threshold
of concern, and the safety evaluation proceeds to step B4.
Step B4. Information on each of the compounds considered at this step
is given below:
ß -Damascone (No. 384)
The NOEL for this substance is > 2 mg/kg bw per day (90-day
study in rats), and the margin of safety between this NOEL and the
daily per capita intake under the conditions of intended use is
> 2500.
alpha-Damascone (No. 385), delta-damascone (No. 386), and
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one (No. 387)
These substances were considered likely to share common metabolic
pathways with ß-damascone (No. 384) for which the NOEL is > 2 mg/kg
bw per day, although the rate of metabolism may be slower. Therefore
the margin of safety between this NOEL and daily per capita intake
is > 1.5 × 104 for alpha-damascone, > 2 × 105 for
delta-damascone, and > 14 × 103 for
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one.
gamma -Ionone (No. 390)
This substance was considered likely to share a common metabolic
pathway with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw
per day (90-day study in rats). Therefore, the margin of safety
between this NOEL and the daily per capita intake of alpha-ionone is
> 40 × 104. It was also considered likely to share a common
metabolic pathway with carvone (No. 380), for which the NOEL is 93
mg/kg bw per day (three-month study in rats). In this case, the margin
of safety between the NOEL and the daily per capita intake of
gamma-ionone is > 4 × 105.
Dehydrodihydroionone (No. 396) and dehydrodihydroionol (No. 397)
These substances were considered likely to share common pathways
with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw per day.
The margin of safety between this NOEL and the daily per capita
intake is 6 × 106 for dehydrodihydroionone and > 7 × 105 for
dehydrodihydroionol.
Therefore, the seven substances considered on the 'B' side of the
scheme would not be expected to be of safety concern.
1.5 Consideration of combined intakes from use as flavouring agents
All of the 20 ionones and structurally related substances
considered in this evaluation would be expected to have common
metabolic pathways. In the unlikely event that all 20 substances were
consumed simultaneously on a daily basis, the estimated daily
per capita consumption in Europe and the United States would not
exceed the human intake threshold for substances in class 1.
1.6 Conclusions
In applying the procedure, the Committee concluded that use of
any of the 20 ionones and related substances as flavouring agents
would not present a safety concern at the current estimated intake
levels. The Committee noted that all of the available data on toxicity
are consistent with the results of the safety evaluation. The ADIs
previously established for alpha-ionone and ß-ionone were maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine. The data that support this conclusion are given below.
Analysis of urine collected from two rabbits fed a total of 170 g
alpha-ionone over an unspecified period revealed a hydroxylated
derivative of alpha-ionone formed from allylic ring oxidation (Prelog
& Wursch, 1951).
Urine was collected daily from one male rabbit fed a total of 23
g ß-ionone over seven days at approximately 1000 mg/kg bw per day) and
for four days after the final dose. 3-Oxo-ß-ionone, 3-oxo-ß-ionol,
dihydro-3-oxo-ß-ionol, and 3-hydroxy-ß-ionol were identified, as were
unchanged ß-ionone and the glucuronic acid conjugates of 3-oxo-ß-ionol
and dihydro-3-oxo-ß-ionol (Ide & Toki, 1970).
The urinary metabolites of two rabbits that received a total of
100 g ß-ionone by gavage over 18 days included 3-hydroxy-ß-ionone,
3-oxo-ß-ionol, and 3-hydroxy-ß-ionone. A hydroxyketone thought to be
either 3-oxo-ß-ionol or 3-hydroxy-ß-ionone was also recovered (Fujii
et al., 1972). After oral administration of ß-ionone to three rabbits
at doses of 2000-5000 mg/day for two weeks, the urine contained
ß-ionone, ß-ionol, and their exocyclic dihydro metabolites, namely,
dihydro-ß-ionol, 3-hydroxy-ß-ionol, 3-hydroxydihydro-ß-ionol,
3-hydroxy-ß-ionone, and 3-hydroxydihydro-ß-ionone (Bielig & Hayasida,
1940). (In these two studies, the urinary metabolites were identified
by IUPAC nomenclature, but the metabolites were reported by a
different naming system.)
Two dogs fed a total of 100 g ß-ionone over 18 days also excreted
3-oxo-ß-ionone and 3-hydroxy-ß-ionol in the urine (Prelog & Meier,
1950).
ß-Ionone has been found to induce biphenyl 4-hydroxylase,
glucuronyl transferase, 4-nitrobenzoate reductase, and cytochrome P450
in rats after a three-day intraperitoneal or oral administration
(Parke & Rahman, 1969).
Studies in humans of the metabolism of retinoids such as
cis-13-retinoic acid (i.e. isotretinoin) and carotenoids such as
ß-carotene, which possess ionone fragments, indicate that the
metabolism of ionones is similar to that in animals. The primary
metabolites in blood and bile after oral administration of
isotretinoin to humans included the glucuronic acid conjugates of
isotretinoin (Kraft et al., 1991) and the allylic oxidation product
(Vane et al., 1990; Kraft et al., 1991). Both metabolites were found
in the blood and bile of cynomolgus monkeys after oral administration
of isotretinoin (Kraft et al., 1991). Allylic hydroxylation of the
methyl-ring substituent and subsequent conjugation with glucuronic
acid have also been shown to occur in humans (Vane et al., 1990).
ß-Carotene is oxidized by carotenoid dioxygenase(s) and cleaved
at the 15-15' (central) double bond to yield two molecules of vitamin
A (retinal) (Simpson & Chichester, 1981), which may subsequently be
cleaved at the 9'-10' double bond to yield ß-ionone and
10'-apo-ß-carotenals. The presence of 10'-apo-ß-carotenal in rat liver
after oral administration of ß-carotene suggests that oxidative
cleavage of the 9'-10' double bond occurs in animals (Sharma et al.,
1977).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of acute toxicity with ionone and
structurally related substances are shown in Table 3.
2.1.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of ionones and structurally related substances are shown in Table 4.
Details of the studies that were critical to the safety evaluation are
given below.
ß-Damascone
Groups of 16 male and 16 female Wistar CF/Gif Carworth strain
rats were given ß-damascone in the diet at 0 or 2 mg/kg bw per day for
90 days. Individual body weights were recorded weekly, but no
difference was seen between treated and control groups. Food intake
was slightly increased in treated females over that in controls but
was associated with a slight decrease in food use. Haematological
examination and blood urea determinations carried out on half of the
rats at week 7 and on all animals at the end of the treatment period
revealed no statistically significant changes, with no major
difference between treated and control groups in haemoglobin
concentration or total or differential leukocyte counts. At necropsy,
a slight increase in the absolute and relative weights of the liver
and kidneys of females was seen in comparison with controls, but these
changes were not correlated with any histopathological lesions.
Histological examination and clinical chemistry revealed no
significant changes. Non-specific inflammatory changes were seen in
the livers and kidneys of a few animals, but these changes were not
considered to be related to treatment. The NOEL was 2 mg/kg bw per
day, the only dose tested (Posternak et al., 1975).
alpha-Ionone and ß-ionone
Studies on alpha- and ß-ionone were evaluated at the
twenty-eighth meeting (Annex 1, reference 66).
alpha-Irone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha-irone in their diets for 90 days at a daily
concentration designed to provide 5 mg/kg bw for males and 6 mg/kg bw
for females. Records of daily food and water consumption revealed no
differences between test and control groups. Haematological and blood
chemical parameters were measured in eight rats of each sex at week 6
and in all rats at week 12; they were within normal ranges. At
autopsy, no difference in liver or kidney weights was seen between
test and control groups. Histological and gross pathological
examinations revealed no statistically significant changes. Treated
females had slightly higher haemoglobin and haematocrit values, but
the findings were considered to be biologically insignificant because
the mean erythrocyte count for the group was comparable to that of
controls. Both test and control groups showed a slight degree of
reactive lymphatic hyperplasia, but the findings were not related to
treatment. The NOEL was 5 mg/kg bw per day (Oser et al., 1965).
alpha-iso-Methylionone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha- iso-methylionone in their diets for 90 days at a
daily concentration designed to provide 4 mg/kg bw. Food and water
consumption was similar for the test and control groups.
Haematological and clinical chemical measurements conducted on eight
rats of each sex at week 6 and on all rats at week 12 showed normal
values. Liver and kidney weights recorded at necropsy were similar in
test and control groups. Histological and gross pathological
examinations revealed no changes related to treatment. The male rats
had a slightly reduced haemoglobin level, but the haematocrit and
erythrocyte counts were within the control ranges. The mean blood urea
nitrogen concentration was slightly lower in the treated group than in
controls at week 12, but this change was not accompanied by changes in
kidney weight or histological appearance and was considered to be of
no biological significance. The NOEL was > 4 mg/kg bw per day (Oser
et al., 1965).
2.1.3 Genotoxicity
The results of studies of the genotoxicity of this group of
substances are shown in Table 5.
4. REFERENCES
Bielig, H. & Hayasida, A. (1940) [On the release of ß-jonone in the
animal (biochemical hydration VIII).] Hoppe-Seyler's Z. Physiol.
Chem., 266, 99-111 (in German).
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 15, 219-232.
Table 3. Studies of the acute toxicity of ionone and related substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
4-[(2,6,6)-Trimethyl-cyclo-hex-1-enyl]but-2-en-4-one 384 Rat NR Oral 2920 Posternak et al.
(1975)
(ß-Damascone) Rat M/F Gavage 2000 Firmenich (1986)
alpha-Damascone 385 Rat M/F Oral 1800 Piccirillo et al.
(1979)
delta-Damascone 386 Mouse M/F Gavage 1820 Moran et al. (1980)
4-[(2,6,6)-Trimethylcyclo-hexa-1,3-dienyl]but-2-en-4-one 387 Rat M/F Gavage > 2000 Firmenich (1986)
alpha-Ionone 388 Rat M/F Oral 4590a Jenner et al. (1964)
ß-Ionone 389 Rat M/F Oral 4590 Jenner et al. (1964)
alpha-Ionol 391 Rat NR Oral > 5000 Moreno (1980)
alpha-Ionol 391 Mouse NR Oral 7400 Pellmont (1978)
ß-Ionol 392 Rat NR Oral > 1220; Moreno (1980)
< 5000
ß-Ionol 393 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-alpha-ionone 393 Rat NR Oral > 5000 Moreno (1976)
Dihydro-ß-ionone 394 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-ß-ionol 395 Mouse NR Oral 7400 Pellmont (1977)
Methyl-alpha-ionone 398 Rat NR Oral > 5000a Moreno (1973)
Methyl-delta-ionone 400 Rat NR Oral > 5000 Moreno (1973)
Allyl alpha-ionone 401 Mouse NR Gavage 8900 Givaudan (1955)
alpha-Irone 403 Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
alpha-iso-Methylionone 404 Rat NR Oral > 5000b Moreno (1973)
NR, not reported; M/F, male and female
a Mixture of alpha- and ß-ionone
b Mixture of methyl-alpha-ionone and alpha-iso-methylionone
Table 4. Short-term and long-term studies of toxicity with ionone and related substances in rats treated orally
Substance No. Sex No. groups/ Duration NOELa Reference
no. per group (mg/kg bw
per day)
4-[(2,6,6)-Trimethylcyclohex-1-enyl]-but-2-en-4-one (ß-Damascone) 384 M/F 1/32 90 days > 2 Posternak et al.
(1975)
alpha-Ionone 388 M/F 1/30 90 days > 11 Oser et al. (1965)
alpha-Ionone 388 M/F 2/30 90 days 10 Gaunt et al. (1983)
Iononeb 388 NR 3/8-10 7-8 weeks > 10 Sporn et al. (1963)
alpha-Iononec 388 M/F 3/20 17 weeks ND Hagan et al. (1967)
ß-Ionone 389 M/F 2/15 90 days > 11 (M) Oser et al. (1965)
> 13 (F)
ß-Ionone 389 M/F 2/30 90 days 10 Gaunt et al. (1983)
alpha-Irone 403 M/F 2/15 90 days > 5 (M) Oser et al. (1965)
> 6 (F)
alpha-iso-Methylionone 404 M/F 1/30 90 days > 4 Oser et al. (1965)
NR, not reported; M, male; F, female; ND, not determined
a NOEL given as 'greater than' (>) indicates that no adverse effects were observed at the highest dose in the study and, therefore, no NOEL
was determined.
b Mixture of alpha-ionone and ß-ionone
c Mixture of 60% alpha-ionone and 40% alpha-ionone
Table 5. Results of assays for the genotoxicity of ionones and related substances
Substance No. End-point Test object Dose Result Reference
alpha-Ionone 388 Chromosomal aberration Chinese hamster B241 25 nmol/L Positivea Kasamaki et al. (1982)
cell line
Gene mutation S. typhimurium TA98, 0.01-50 mg/plate Negativea Kasamaki et al. (1982
TA100
rec assay B. subtilis H17 & M45 19 mg/disc Negativeb Oda et al. (1978)
ß-Ionone 389 Gene mutation
(preincubation) S. typhimurium TA98, 1-180 mg/plate Negativea Mortlemans et al. (1986)
TA100, TA1535, 1537
Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537
Methyl-alpha-ionone 398 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
Methyl-alpha-ionone 398 Micronucleus formation NMRI mice, male and 825-2063 mg/kg bw Negative Wild et al. (1983)
female, bone marrow
Methyl-alpha-ionone 398 Gene mutation (basc) Drosophila melanogaster 20 mmol/L Negative Wild et al. (1983)
Methyl-delta-ionone 400 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
a With and without metabolic activation
b Activation status unknown
Fujii, T., Furukawa, S. & Suzuki, S. (1972) Compounded perfumes for
toilet goods. Non-irritative compounded perfumes for soaps.
Yukagaku, 21, 904-908.
Gaunt, I.F., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hagan, E.C., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
International Organization of the Flavor Industry (1975) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Wasington DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kasamaki, A., Takahashi, H., Tsumura, N., Niwa, J., Fujita, T. &
Urasawa, S. (1982) Genotoxicity of flavoring agents. Mutat. Res.,
105, 387-392.
Kraft, J.C., Slikker, W., Jr, Bailey, J.R., Roberts, L.G., Fischer,
B., Wittfoht, W. & Nau, H. (1991) Plasma pharmacokinetics and
metabolism of 13-cis- and all-trans-retinoic acid in the cynomolgus
monkey and the identification of 13-cis- and
all-trans-retinoyl-ß-glucuronides. A comparison to one human case
study with isotretinoin. Drug Metab. Disposition, 19, 317-324.
Levenstein, I. (1955) Acute oral toxicity study of allyl-alpha-ionone
in mice. Unpublished report from Leberco Labs. Submitted to WHO by the
Food and Extract Manufacturers' Association of the United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Lab. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Lab. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Lab. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Oser, B.L., Carson, S. & Oser, M. (1965) Toxicological tests on
flavouring matters. Food Chem. Toxicol., 3, 563-569.
Parke, D.V. & Rahman, H. (1969) The effects of some terpenoids and
other dietary anutrients on hepatic drug-metabolizing enzymes.
Biochem. J., 113, 124.
Pellmont, I. (1977) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Pellmont, I. (1978) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Piccirillo, V.J., Resnick, P., Swidersky, P. & Herndon, B. (1979)
Acute oral toxicity of alpha-damascone in rats. Unpublished report.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Posternak, J.M., Dufour, J.J., Rogg, C. & Vodoz, C.A. (1975)
Toxicology tests on flavouring matters II. Pyrazines and other
compounds. Food Cosmet. Toxicol., 13, 487-490.
Prelog, V. & Meier, H.l. (1950) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 33, 1276-1284 (in
German).
Prelog, V. & Wursch, J. (1951) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 34, 859-861 (in German).
Sharma, R.V., Mathur, S.N., Dmitrovskii, A.A., Das, R.C. & Ganguly,
J.. (1977) Studies on the metabolism of ß-carotene and
apo-ß-carotenoids in rats and chickens. Biochim. Biophys. Acta, 486,
183-194.
Shelanski, M.V. & Moldovan, M. (1972) Acute oral and dermal toxicity
studies. Unpublished report. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Simpson, K.L. & Chichester, C.O. (1981) Metabolism and nutritional
significance of carotenoids. Ann. Rev. Nutr., 1, 351-374.
Sporn, A., Schobesch, O., Marin, V., Panaitescu, E. & Runcan, L.
(1963) The toxicity of butyl acetate, methyl naphthyl ketone and
ionone. Igiena, 12, 437-445.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US National Academy of Sciences (1989) 1987 Poundage and Technical
Effects Update of Substances Added to Food, Washington DC, United
States.
Vane, F.M., Bugge, C.J.L., Rodriguez, L.C., Rosenberger, M. & Doran,
T.I. (1990) Human biliary metabolites of isotretinoin: Identification,
quantification, synthesis and biological activity. Xenobiotica, 20,
193-207.
Whittaker, C.J. (1986a) Single dose oral toxicity study of
1-(2,6,6-trimethylcyclohexa-1,3-dienyl)-2-buten-1-one (damascone) in
the rat. Unpublished report from Firmenich & Co. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Whittaker, C.J. (1986b) Single dose oral toxicitystudy of
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)-but-2-en-4-one (damascenone)
in the rat. Unpublished report from Firmenich & Co. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavoring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
a The threshold of concern for class I is 1800 µg/day.
b The ADI values previously established for alpha-ionone and ß-ionone were maintained at the present meeting.
1.2 Estimated daily per capita intake
Per capita intake was estimated from data derived from
surveys in Europe (International organization of the Flavor
Industry, 1994) and the United States (US Academy of Sciences,
1989) (see Table 2). The estimated total daily per capita
intake of all 21 ionones and related substances from their
use as flavouring agents is 0.76 mg/person in Europe and 0.33
mg/person in the United States. In Europe, four substances,
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (0.043
mg/person), alpha-ionone (0.31 mg/person), ß-ionone (0.15 mg/person),
and methyl-alpha-ionone (0.035 mg/person), account for approximately
85% of the total annual per capita intake of this group of
substances when used as flavouring agents. In the United States, three
substances, alpha-ionone (0.15 mg/person), ß-ionone (0.10 mg/person),
and allyl-alpha-ionone (0.025 mg/person) account for about 85% of the
total annual per capita intake of this group of substances when used
as flavouring agents.
Eleven of the substances in this group have been reported to
occur naturally in foods including raspberries, carrots, roasted
almonds, fruits, and herbs (Maarse et al., 1994). Quantitative data on
natural occurrence and consumption ratios have been reported for seven
substances (Nos 388, 389, 390, 391, 392, 393, and 394), which indicate
that they are consumed predominantly in traditional foods, i.e. the
consumption ratio is larger than 1 (Stofberg & Kirschman, 1985;
Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine.
Table 2. Most recent annual usage of ionones and structurally related
substances as flavouring agents in Europe and the United States
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
ß-Damascone (384)
Europe 300 43 0.7
United States 50 10 0.2
alpha-Damascone (385)
Europe 57 8 0.1
United States 2 0.4 0.01
delta-Damascone (386)
Europe 0.4 0.06 0.001
United States 3 0.6 0.01
Damascenone (387)
Europe 600 86 1
United States 24 5 0.08
alpha-Ionone (388)
Europe 2200 310 5
United States 770 150 2
ß-lonone (389)
Europe 1100 150 3
United States 550 100 2
gamma-Ionone (390)
Europe 0.1 0.01 0.0002
United States 80 15 0.3
alpha-lonol (391)
Europe 5 0.7 0.01
United States 0.3 0.06 0.001
ß-lonol (392)
Europe 6 0.9 0.01
United States 0.5 0.1 0.002
Dihydro-alpha-ionone (393)
Europe 5 0.7 0.01
United States 0.1 0.02 0.0003
Dihydro-ß-ionone (394)
Europe 9 1 0.02
United States 0.2 0.04 0.0006
Dihydro-ß-ionol (395)
Europe 2.3 0.3 0.014
United States 0.1 0.02 0.0003
Dehydrodihydroionone (396)
Europe 0.7 0.1 0.002
United States 0.4 0.08 0.001
Table 2. (continued)
Substance (No.) Most recent Per capita intakea
annual
volume (kg) µg/day µg/kg bw
per day
Dehydrodihydroionol (397)
Europe 0.1 0.01 0.0002
United States 40 8 0.1
Methyl-alpha-ionone (398)
Europe 710 100 2
United States 35 7 0.1
Methyl-ß-ionone (399)
Europe 44 6 0.1
United States 0.9 0.2 0.003
Methyl-delta-ionone (400)
Europe 3 0.4 0.01
United States 6 1 0.02
Allyl-alpha-ionone (401)
Europe 250 35 0.6
United States 130 25 0.4
alpha-Irone (403)
Europe 6.3 9 0.1
United States 15 3 0.05
alpha-iso-Methylionone (404)
Europe 39 6 0.09
United States 5 1 0.02
Total
Europe 5300 760 13
United States 1700 320 5
a From US National Academy of Sciences (1989); International
Organization of the Flavor Industry (1995)
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
The structure of 1,4-dimethyl-4-acetyl-1-cyclohexene (No. 402)
was considered not to be sufficiently similar to that of the ionones
to be included in the group, and it was therefore not included in the
safety evaluation. The remaining 20 compounds were evaluated according
to the procedure.
Step 1. According to the decision-tree structural class
classification (Cramer et al., 1978), all of the 20 ionones
and related substances considered to be part of this group
are in class I.
Step 2. Data were available for ß-ionone that showed that it is
metabolized by carbonyl reduction, hydroxylation of the
alicyclic ring, and glucuronic acid conjugation of
metabolites with alcohol groups. Only limited data were
available on the fate of other compounds in the group.
Although structural characteristics similar to that of
ß-ionone were found, there were differences in the number
and positions of the alicyclic double-bonds, the position of
the carbonyl group within the side-chain, and its
relationship to endocylic and exocyclic double-bonds. On the
basis of the similarities of the functional groups present
in the flavouring agents in this group, it was considered
that alpha- and ß-ionones, their alcohol analogues,
analogues with a single endocyclic double-bond, and
analogues with saturated side-chains or more extended
side-chains would be eliminated from the body by common
metabolic processes, which would lead to innocuous products.
Members of the group with two endocyclic double-bonds,
with the carboxyl group adjacent to the ring, or with an
allyl double-bond attached to the ring might be eliminated
more slowly, which might affect their toxic potency. The
decision tree would take this into account to some extent by
allocating compounds that are sterically hindered into class
II rather than the class I. In consequence, the Committee
was not able to conclude a priori that the products of
metabolism of such members of the group would be innocuous.
Substances that could be predicted to be metabolized to
innocuous products fall into the following two groups:
Group 1: alpha-Ionone (No. 388), alpha-ionol (No. 391),
dihydro-alpha-ionone (No. 393), methyl-alpha-ionone (No.
398), methyl-delta-ionone (No. 400), alpha-irone (No. 403),
2-iso-methylionone (No. 404), and allyl-alpha-ionone (No.
401) were considered likely to share a common metabolic
pathway to alpha-ionone, although the rate of metabolism for
some of these substances may be slower than for
alpha-ionone.
Group 2: ß-Ionol (No. 392), dihydro-ß-ionone (No. 394),
dihydro-ß-ionol (No. 395), methyl-ß-ionone (No. 399), and
methyl-delta-ionone (No. 400) were considered likely to
share common metabolic pathways with ß-ionone, although the
rate of metabolism for some of these substances may be
slower than for ß-ionone.
Evaluation of all of the substances in groups 1 and 2
should proceed down the 'A' side of the scheme.
Substances that cannot be predicted to be metabolized
to innocuous products are ß-damascone (No. 384),
alpha-damascone (No. 385), delta-damascone (No. 386),
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)but-2-en-4-one (No.
387), gamma-ionone (No. 390), dehydrodi-hydroionone (No.
396), and dehydrodihydroionol (No. 397), and their
evaluation should proceed down the 'B' side of the scheme.
Step A3. As the intakes in Europe and the United States of all 13
substances on the 'A' side of the scheme are below the
threshold of concern for class I (1800 µg/person per day),
these substances would not be expected to be of safety
concern.
Step B3. Intake of ß-damascone (No. 384), alpha-damascone (No. 385),
delta-damascone (No. 386), 4-(2,6,6-trimethylcyclohexa-1,3-
dienyl)but-2-en-4-one (No. 387), gamma-ionone (No. 390),
dehydrodihydroionone (No. 396), and dehydrodihydroionol (No.
397) in Europe and the United States is below the threshold
of concern, and the safety evaluation proceeds to step B4.
Step B4. Information on each of the compounds considered at this step
is given below:
ß -Damascone (No. 384)
The NOEL for this substance is > 2 mg/kg bw per day (90-day
study in rats), and the margin of safety between this NOEL and the
daily per capita intake under the conditions of intended use is
> 2500.
alpha-Damascone (No. 385), delta-damascone (No. 386), and
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one (No. 387)
These substances were considered likely to share common metabolic
pathways with ß-damascone (No. 384) for which the NOEL is > 2 mg/kg
bw per day, although the rate of metabolism may be slower. Therefore
the margin of safety between this NOEL and daily per capita intake
is > 1.5 × 104 for alpha-damascone, > 2 × 105 for
delta-damascone, and > 14 × 103 for
4-(2,6,6-trimethyl-cyclohexa-1,3-dienyl)but-2-en-4-one.
gamma -Ionone (No. 390)
This substance was considered likely to share a common metabolic
pathway with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw
per day (90-day study in rats). Therefore, the margin of safety
between this NOEL and the daily per capita intake of alpha-ionone is
> 40 × 104. It was also considered likely to share a common
metabolic pathway with carvone (No. 380), for which the NOEL is 93
mg/kg bw per day (three-month study in rats). In this case, the margin
of safety between the NOEL and the daily per capita intake of
gamma-ionone is > 4 × 105.
Dehydrodihydroionone (No. 396) and dehydrodihydroionol (No. 397)
These substances were considered likely to share common pathways
with alpha- and ß-ionone, for which the NOEL is 10 mg/kg bw per day.
The margin of safety between this NOEL and the daily per capita
intake is 6 × 106 for dehydrodihydroionone and > 7 × 105 for
dehydrodihydroionol.
Therefore, the seven substances considered on the 'B' side of the
scheme would not be expected to be of safety concern.
1.5 Consideration of combined intakes from use as flavouring agents
All of the 20 ionones and structurally related substances
considered in this evaluation would be expected to have common
metabolic pathways. In the unlikely event that all 20 substances were
consumed simultaneously on a daily basis, the estimated daily
per capita consumption in Europe and the United States would not
exceed the human intake threshold for substances in class 1.
1.6 Conclusions
In applying the procedure, the Committee concluded that use of
any of the 20 ionones and related substances as flavouring agents
would not present a safety concern at the current estimated intake
levels. The Committee noted that all of the available data on toxicity
are consistent with the results of the safety evaluation. The ADIs
previously established for alpha-ionone and ß-ionone were maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption, metabolism, and elimination
The substances in this group are structurally related, in that
each has a cyclohexane ring with an allyl side-chain containing a
ketone or secondary alcohol functional group. The available metabolic
data are derived largely from studies on ß-ionone and indicate at
least two possible detoxification pathways:
(i) allylic hydroxylation of the ring at the 3 position, followed by
oxidation of the hydroxyl group to the 3-oxo derivative, and
(ii) reduction of the ketone on the allyl side-chain to the
corresponding secondary alcohol.
A combination of these reactions results in the formation of polar
metabolites, which are excreted in the urine unchanged or conjugated
with glucuronic acid. ß-Ionone can also be excreted unchanged in the
urine. The data that support this conclusion are given below.
Analysis of urine collected from two rabbits fed a total of 170 g
alpha-ionone over an unspecified period revealed a hydroxylated
derivative of alpha-ionone formed from allylic ring oxidation (Prelog
& Wursch, 1951).
Urine was collected daily from one male rabbit fed a total of 23
g ß-ionone over seven days at approximately 1000 mg/kg bw per day) and
for four days after the final dose. 3-Oxo-ß-ionone, 3-oxo-ß-ionol,
dihydro-3-oxo-ß-ionol, and 3-hydroxy-ß-ionol were identified, as were
unchanged ß-ionone and the glucuronic acid conjugates of 3-oxo-ß-ionol
and dihydro-3-oxo-ß-ionol (Ide & Toki, 1970).
The urinary metabolites of two rabbits that received a total of
100 g ß-ionone by gavage over 18 days included 3-hydroxy-ß-ionone,
3-oxo-ß-ionol, and 3-hydroxy-ß-ionone. A hydroxyketone thought to be
either 3-oxo-ß-ionol or 3-hydroxy-ß-ionone was also recovered (Fujii
et al., 1972). After oral administration of ß-ionone to three rabbits
at doses of 2000-5000 mg/day for two weeks, the urine contained
ß-ionone, ß-ionol, and their exocyclic dihydro metabolites, namely,
dihydro-ß-ionol, 3-hydroxy-ß-ionol, 3-hydroxydihydro-ß-ionol,
3-hydroxy-ß-ionone, and 3-hydroxydihydro-ß-ionone (Bielig & Hayasida,
1940). (In these two studies, the urinary metabolites were identified
by IUPAC nomenclature, but the metabolites were reported by a
different naming system.)
Two dogs fed a total of 100 g ß-ionone over 18 days also excreted
3-oxo-ß-ionone and 3-hydroxy-ß-ionol in the urine (Prelog & Meier,
1950).
ß-Ionone has been found to induce biphenyl 4-hydroxylase,
glucuronyl transferase, 4-nitrobenzoate reductase, and cytochrome P450
in rats after a three-day intraperitoneal or oral administration
(Parke & Rahman, 1969).
Studies in humans of the metabolism of retinoids such as
cis-13-retinoic acid (i.e. isotretinoin) and carotenoids such as
ß-carotene, which possess ionone fragments, indicate that the
metabolism of ionones is similar to that in animals. The primary
metabolites in blood and bile after oral administration of
isotretinoin to humans included the glucuronic acid conjugates of
isotretinoin (Kraft et al., 1991) and the allylic oxidation product
(Vane et al., 1990; Kraft et al., 1991). Both metabolites were found
in the blood and bile of cynomolgus monkeys after oral administration
of isotretinoin (Kraft et al., 1991). Allylic hydroxylation of the
methyl-ring substituent and subsequent conjugation with glucuronic
acid have also been shown to occur in humans (Vane et al., 1990).
ß-Carotene is oxidized by carotenoid dioxygenase(s) and cleaved
at the 15-15' (central) double bond to yield two molecules of vitamin
A (retinal) (Simpson & Chichester, 1981), which may subsequently be
cleaved at the 9'-10' double bond to yield ß-ionone and
10'-apo-ß-carotenals. The presence of 10'-apo-ß-carotenal in rat liver
after oral administration of ß-carotene suggests that oxidative
cleavage of the 9'-10' double bond occurs in animals (Sharma et al.,
1977).
2.1.2 Toxicological studies
2.1.2.1 Acute toxicity
The results of studies of acute toxicity with ionone and
structurally related substances are shown in Table 3.
2.1.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of ionones and structurally related substances are shown in Table 4.
Details of the studies that were critical to the safety evaluation are
given below.
ß-Damascone
Groups of 16 male and 16 female Wistar CF/Gif Carworth strain
rats were given ß-damascone in the diet at 0 or 2 mg/kg bw per day for
90 days. Individual body weights were recorded weekly, but no
difference was seen between treated and control groups. Food intake
was slightly increased in treated females over that in controls but
was associated with a slight decrease in food use. Haematological
examination and blood urea determinations carried out on half of the
rats at week 7 and on all animals at the end of the treatment period
revealed no statistically significant changes, with no major
difference between treated and control groups in haemoglobin
concentration or total or differential leukocyte counts. At necropsy,
a slight increase in the absolute and relative weights of the liver
and kidneys of females was seen in comparison with controls, but these
changes were not correlated with any histopathological lesions.
Histological examination and clinical chemistry revealed no
significant changes. Non-specific inflammatory changes were seen in
the livers and kidneys of a few animals, but these changes were not
considered to be related to treatment. The NOEL was 2 mg/kg bw per
day, the only dose tested (Posternak et al., 1975).
alpha-Ionone and ß-ionone
Studies on alpha- and ß-ionone were evaluated at the
twenty-eighth meeting (Annex 1, reference 66).
alpha-Irone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha-irone in their diets for 90 days at a daily
concentration designed to provide 5 mg/kg bw for males and 6 mg/kg bw
for females. Records of daily food and water consumption revealed no
differences between test and control groups. Haematological and blood
chemical parameters were measured in eight rats of each sex at week 6
and in all rats at week 12; they were within normal ranges. At
autopsy, no difference in liver or kidney weights was seen between
test and control groups. Histological and gross pathological
examinations revealed no statistically significant changes. Treated
females had slightly higher haemoglobin and haematocrit values, but
the findings were considered to be biologically insignificant because
the mean erythrocyte count for the group was comparable to that of
controls. Both test and control groups showed a slight degree of
reactive lymphatic hyperplasia, but the findings were not related to
treatment. The NOEL was 5 mg/kg bw per day (Oser et al., 1965).
alpha-iso-Methylionone
As part of a study to examine the toxicity of a group of 23
flavouring substances, groups of 15 FDRL strain rats of each sex
received alpha- iso-methylionone in their diets for 90 days at a
daily concentration designed to provide 4 mg/kg bw. Food and water
consumption was similar for the test and control groups.
Haematological and clinical chemical measurements conducted on eight
rats of each sex at week 6 and on all rats at week 12 showed normal
values. Liver and kidney weights recorded at necropsy were similar in
test and control groups. Histological and gross pathological
examinations revealed no changes related to treatment. The male rats
had a slightly reduced haemoglobin level, but the haematocrit and
erythrocyte counts were within the control ranges. The mean blood urea
nitrogen concentration was slightly lower in the treated group than in
controls at week 12, but this change was not accompanied by changes in
kidney weight or histological appearance and was considered to be of
no biological significance. The NOEL was > 4 mg/kg bw per day (Oser
et al., 1965).
2.1.3 Genotoxicity
The results of studies of the genotoxicity of this group of
substances are shown in Table 5.
4. REFERENCES
Bielig, H. & Hayasida, A. (1940) [On the release of ß-jonone in the
animal (biochemical hydration VIII).] Hoppe-Seyler's Z. Physiol.
Chem., 266, 99-111 (in German).
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
Toxicology, 15, 219-232.
Table 3. Studies of the acute toxicity of ionone and related substances
Substance No. Species Sex Route LD50 Reference
(mg/kg bw)
4-[(2,6,6)-Trimethyl-cyclo-hex-1-enyl]but-2-en-4-one 384 Rat NR Oral 2920 Posternak et al.
(1975)
(ß-Damascone) Rat M/F Gavage 2000 Firmenich (1986)
alpha-Damascone 385 Rat M/F Oral 1800 Piccirillo et al.
(1979)
delta-Damascone 386 Mouse M/F Gavage 1820 Moran et al. (1980)
4-[(2,6,6)-Trimethylcyclo-hexa-1,3-dienyl]but-2-en-4-one 387 Rat M/F Gavage > 2000 Firmenich (1986)
alpha-Ionone 388 Rat M/F Oral 4590a Jenner et al. (1964)
ß-Ionone 389 Rat M/F Oral 4590 Jenner et al. (1964)
alpha-Ionol 391 Rat NR Oral > 5000 Moreno (1980)
alpha-Ionol 391 Mouse NR Oral 7400 Pellmont (1978)
ß-Ionol 392 Rat NR Oral > 1220; Moreno (1980)
< 5000
ß-Ionol 393 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-alpha-ionone 393 Rat NR Oral > 5000 Moreno (1976)
Dihydro-ß-ionone 394 Mouse NR Oral 5700 Pellmont (1977)
Dihydro-ß-ionol 395 Mouse NR Oral 7400 Pellmont (1977)
Methyl-alpha-ionone 398 Rat NR Oral > 5000a Moreno (1973)
Methyl-delta-ionone 400 Rat NR Oral > 5000 Moreno (1973)
Allyl alpha-ionone 401 Mouse NR Gavage 8900 Givaudan (1955)
alpha-Irone 403 Rat M/F Oral > 5000 Shelanski &
Moldovan (1972)
alpha-iso-Methylionone 404 Rat NR Oral > 5000b Moreno (1973)
NR, not reported; M/F, male and female
a Mixture of alpha- and ß-ionone
b Mixture of methyl-alpha-ionone and alpha-iso-methylionone
Table 4. Short-term and long-term studies of toxicity with ionone and related substances in rats treated orally
Substance No. Sex No. groups/ Duration NOELa Reference
no. per group (mg/kg bw
per day)
4-[(2,6,6)-Trimethylcyclohex-1-enyl]-but-2-en-4-one (ß-Damascone) 384 M/F 1/32 90 days > 2 Posternak et al.
(1975)
alpha-Ionone 388 M/F 1/30 90 days > 11 Oser et al. (1965)
alpha-Ionone 388 M/F 2/30 90 days 10 Gaunt et al. (1983)
Iononeb 388 NR 3/8-10 7-8 weeks > 10 Sporn et al. (1963)
alpha-Iononec 388 M/F 3/20 17 weeks ND Hagan et al. (1967)
ß-Ionone 389 M/F 2/15 90 days > 11 (M) Oser et al. (1965)
> 13 (F)
ß-Ionone 389 M/F 2/30 90 days 10 Gaunt et al. (1983)
alpha-Irone 403 M/F 2/15 90 days > 5 (M) Oser et al. (1965)
> 6 (F)
alpha-iso-Methylionone 404 M/F 1/30 90 days > 4 Oser et al. (1965)
NR, not reported; M, male; F, female; ND, not determined
a NOEL given as 'greater than' (>) indicates that no adverse effects were observed at the highest dose in the study and, therefore, no NOEL
was determined.
b Mixture of alpha-ionone and ß-ionone
c Mixture of 60% alpha-ionone and 40% alpha-ionone
Table 5. Results of assays for the genotoxicity of ionones and related substances
Substance No. End-point Test object Dose Result Reference
alpha-Ionone 388 Chromosomal aberration Chinese hamster B241 25 nmol/L Positivea Kasamaki et al. (1982)
cell line
Gene mutation S. typhimurium TA98, 0.01-50 mg/plate Negativea Kasamaki et al. (1982
TA100
rec assay B. subtilis H17 & M45 19 mg/disc Negativeb Oda et al. (1978)
ß-Ionone 389 Gene mutation
(preincubation) S. typhimurium TA98, 1-180 mg/plate Negativea Mortlemans et al. (1986)
TA100, TA1535, 1537
Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al. (1980)
TA100, TA1535, TA1537
Methyl-alpha-ionone 398 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
Methyl-alpha-ionone 398 Micronucleus formation NMRI mice, male and 825-2063 mg/kg bw Negative Wild et al. (1983)
female, bone marrow
Methyl-alpha-ionone 398 Gene mutation (basc) Drosophila melanogaster 20 mmol/L Negative Wild et al. (1983)
Methyl-delta-ionone 400 Gene mutation S. typhimurium TA1535, < 3600 mg/plate Negativea Wild et al. (1983)
TA1537, TA1538, TA98,
TA100
a With and without metabolic activation
b Activation status unknown
Fujii, T., Furukawa, S. & Suzuki, S. (1972) Compounded perfumes for
toilet goods. Non-irritative compounded perfumes for soaps.
Yukagaku, 21, 904-908.
Gaunt, I.F., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hagan, E.C., Hansen, W.H., Fitzhugh, G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Ide, H. & Toki, S. (1970) Metabolism of ß-ionone. Isolation,
characterization and identification of the metabolites in the urine of
rabbits. Biochem. J., 119, 281-287.
International Organization of the Flavor Industry (1975) European
inquiry on volume of use. Unpublished report. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Wasington DC, United States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kasamaki, A., Takahashi, H., Tsumura, N., Niwa, J., Fujita, T. &
Urasawa, S. (1982) Genotoxicity of flavoring agents. Mutat. Res.,
105, 387-392.
Kraft, J.C., Slikker, W., Jr, Bailey, J.R., Roberts, L.G., Fischer,
B., Wittfoht, W. & Nau, H. (1991) Plasma pharmacokinetics and
metabolism of 13-cis- and all-trans-retinoic acid in the cynomolgus
monkey and the identification of 13-cis- and
all-trans-retinoyl-ß-glucuronides. A comparison to one human case
study with isotretinoin. Drug Metab. Disposition, 19, 317-324.
Levenstein, I. (1955) Acute oral toxicity study of allyl-alpha-ionone
in mice. Unpublished report from Leberco Labs. Submitted to WHO by the
Food and Extract Manufacturers' Association of the United States.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Lab. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Lab. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Lab. Submitted to WHO by the Food and Extract Manufacturers'
Association of the United States, Washington DC, United States.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Oser, B.L., Carson, S. & Oser, M. (1965) Toxicological tests on
flavouring matters. Food Chem. Toxicol., 3, 563-569.
Parke, D.V. & Rahman, H. (1969) The effects of some terpenoids and
other dietary anutrients on hepatic drug-metabolizing enzymes.
Biochem. J., 113, 124.
Pellmont, I. (1977) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Pellmont, I. (1978) Unpublished report. Submitted to WHO by the Food
and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Piccirillo, V.J., Resnick, P., Swidersky, P. & Herndon, B. (1979)
Acute oral toxicity of alpha-damascone in rats. Unpublished report.
Submitted to WHO by the Food and Extract Manufacturers' Association of
the United States, Washington DC, United States.
Posternak, J.M., Dufour, J.J., Rogg, C. & Vodoz, C.A. (1975)
Toxicology tests on flavouring matters II. Pyrazines and other
compounds. Food Cosmet. Toxicol., 13, 487-490.
Prelog, V. & Meier, H.l. (1950) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 33, 1276-1284 (in
German).
Prelog, V. & Wursch, J. (1951) [On the biochemical oxidation of
ß-ionone in the animal.] Helv. Chim. Acta, 34, 859-861 (in German).
Sharma, R.V., Mathur, S.N., Dmitrovskii, A.A., Das, R.C. & Ganguly,
J.. (1977) Studies on the metabolism of ß-carotene and
apo-ß-carotenoids in rats and chickens. Biochim. Biophys. Acta, 486,
183-194.
Shelanski, M.V. & Moldovan, M. (1972) Acute oral and dermal toxicity
studies. Unpublished report. Submitted to WHO by the Food and Extract
Manufacturers' Association of the United States, Washington DC, United
States.
Simpson, K.L. & Chichester, C.O. (1981) Metabolism and nutritional
significance of carotenoids. Ann. Rev. Nutr., 1, 351-374.
Sporn, A., Schobesch, O., Marin, V., Panaitescu, E. & Runcan, L.
(1963) The toxicity of butyl acetate, methyl naphthyl ketone and
ionone. Igiena, 12, 437-445.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US National Academy of Sciences (1989) 1987 Poundage and Technical
Effects Update of Substances Added to Food, Washington DC, United
States.
Vane, F.M., Bugge, C.J.L., Rodriguez, L.C., Rosenberger, M. & Doran,
T.I. (1990) Human biliary metabolites of isotretinoin: Identification,
quantification, synthesis and biological activity. Xenobiotica, 20,
193-207.
Whittaker, C.J. (1986a) Single dose oral toxicity study of
1-(2,6,6-trimethylcyclohexa-1,3-dienyl)-2-buten-1-one (damascone) in
the rat. Unpublished report from Firmenich & Co. Submitted to WHO by
the Food and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Whittaker, C.J. (1986b) Single dose oral toxicitystudy of
4-(2,6,6-trimethylcyclohexa-1,3-dienyl)-but-2-en-4-one (damascenone)
in the rat. Unpublished report from Firmenich & Co. Submitted to WHO
by the Food and Extract Manufacturers' Association of the United
States, Washington DC, United States.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavoring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
