
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 83
POLYBROMINATED BIPHENYLS (PBBs)
HEALTH AND SAFETY GUIDE
UNITED NATIONS INTERNATIONAL
ENVIRONMENT PROGRAMME LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1993
This is a companion volume to Environmental Health Criteria 152:
Polybrominated biphenyls (PBBs)
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Polybrominated biphenyls : health and safety guide.
(Health and safety guide ; no. 83)
1.Biphenyl compounds - standards I.Series
ISBN 92 4 151083 8 (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analysis
1.4. Production and uses
1.5. Thermal decomposition
2. SUMMARY AND EVALUATION
2.1. Sources of human and environmental exposure
2.2. Environmental transport, distribution, and transformation
2.3. Environmental levels and human exposure
2.4. Kinetics and metabolism
2.5. Effects on organisms in the environment
2.6. Effects on experimental animals and in vitro test systems
2.7. Effects on humans
2.8. Overall evaluation of the toxicity
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Human health hazards, prevention and protection, first aid
4.1.1. Advice to physicians
4.1.1.1 Symptoms of poisoning30
4.1.1.2 Medical advice
4.1.2. Health surveillance advice
4.2. Explosion and fire hazards
4.2.1. Explosion hazards
4.2.2. Fire hazards
4.3. Storage
4.4. Transport
4.5. Spillage
4.6. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Previous evaluation by international bodies
6.2. Exposure limit values
6.3. Specific restrictions
6.4. Labelling, packaging, and transport
6.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. The section on regulatory information has been extracted
from the legal file of the International Register of Potentially Toxic
Chemicals (IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical formula: C12 H(10-x-y)Brx+y
(both x and y = 1 to 5)
Chemical structure:
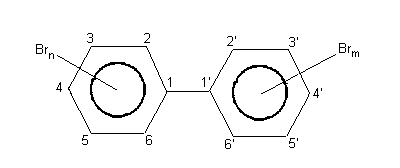 Molecular weight: 154.21 + 78.90 (x+y)
Common trade names: Adine 0102; Berkflam B10; Bromkal 80-9D;
FireMaster BP-6; FireMaster FF-1;
Flammex B10; HFO 101; Octabromobiphenyl
FR 250 BA; Octabromobiphenyl XN-1902.
Composition of technical products
PBBs synthesized for commercial use as fire retardants contain a
mixture of various brominated biphenyls, chiefly the hexa-, octa-,
nona-, and decabromobiphenyls, as well as small amounts of related
brominated substances.
1.2 Physical and Chemical Properties
PBBs are solids with a low volatility that decreases with an increase
in the number of bromine atoms. PBBs are virtually insoluble in water,
soluble in fat and slightly to highly soluble in various organic
solvents, the solubility also decreasing with an increase in the
number of bromine atoms. These compounds are relatively stable and
chemically unreactive, though highly brominated PBB mixtures degrade
rather rapidly on exposure to ultraviolet radiation.
The technical mixtures are typically white, off-white, or beige
powders.
1.3 Analysis
Gas chromatography with electron-capture or mass-spectrometric
detection are useful techniques for determining PBB levels.
High-resolution gas chromatography is preferred as this makes it
possible to determine specific congeners, but reference compounds are
available for only a few of the 209 possible PBB congeners.
1.4 Production and Uses
The commercial production of PBBs (as FireMaster(R)) was started in
the USA in 1970. After the production of about 6000 tonnes, further
production was virtually discontinued in 1974 after the inadvertent
addition of PBBs to animal feed. This resulted in the contamination of
farm animals and their subsequent massive destruction. Approximately
400 tonnes of octa- and decabromobiphenyl were produced in the USA
until 1979. Bromkal 80-9D was produced in Germany until 1985; a few
hundred tonnes per year of Adine 0102 are currently being produced in
France; in 1977, production of decabromobiphenyl ceased in the United
Kingdom.
PBBs are mainly used as flame retardants in moulded thermoplastics,
mostly in small appliances and automotive applications. Earlier
applications in synthetic fibres have been discontinued.
1.5 Thermal Decomposition
The products of the thermal decomposition of PBBs depend on the
temperature, the amount of oxygen present, and a number of other
factors. Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen (600-900 °C) have shown that bromobenzenes and lower
brominated biphenyls are formed, but no polybrominated furans. In
contrast, pyrolysis in the presence of oxygen (700-900 °C) yielded
some di- to heptabromodibenzofurans. In the presence of polystyrene
and polyethylene, higher levels were found. Pyrolysis of FireMaster
BP-6 with PVC at 800 °C yielded mixed bromochlorobiphenyls. There is
no information on the nature of the products of incineration of
PBB-containing compounds. Little is known about the toxicity of
brominated and brominated/chlorinated dioxins and furans, but their
toxicity is estimated to be of about the same order as that of
chlorinated dioxins and furans.
2. SUMMARY AND EVALUATION
2.1 Sources of Human and Environmental Exposure
PBBs were introduced as flame retardants in the early 1970s. Prior to
November 1974, hexabromobiphenyl was the most commercially significant
PBB in the USA and was incorporated into ABS (acrylonitrile-butadiene-
styrene) plastics (PBB content 10% - mainly small appliance and
automotive applications), coatings and lacquers, and polyurethane
foam. The other PBB flame retardants have similar uses.
Losses of PBB into the environment during normal production can occur
through emission into the air and waste waters, and through losses
into the soil and into landfills, but these quantities have been found
to be generally low. These chemicals can also enter the environment
during shipping and handling, and following accidents, as occurred in
Michigan, USA, in 1973.
PBBs can also enter the environment as a result of the incineration of
materials containing PBB, as well as during accidental fires, with the
formation of other toxic chemicals, such as polybromodibenzofurans or
mixed bromochloro derivatives.
Most of the total production of these compounds will ultimately enter
the environment, either unchanged or as breakdown products.
2.2 Environmental Transport, Distribution, and Transformation
Long-range transport of PBB in the atmosphere has not been proven, but
the presence of these compounds in Arctic seal samples indicates a
wide geographical distribution.
The main route by which PBBs enter the aquatic environment is in
industrial waste discharges and leachates from industrial dumping
sites, as well as by erosion of polluted soils. PBBs are almost
insoluble in water and are primarily found in the sediments of
polluted lakes and rivers.
Pollution of the soil can originate from point sources such as a PBB
production plant or a waste dump. Once introduced into the soil, PBBs
do not appear to be translocated readily. PBBs have been found to be
200 times more soluble in a landfill leachate than in distilled water;
this may result in a wider distribution in the environment. The
hydrophobic properties of PBBs make them easily adsorbed, from aqueous
solutions, on to soil particles. Preferential adsorption of PBB
congeners has been noted depending on the characteristics of the soil
(e.g., organic matter content) as well as on the degree and position
of the bromine substitution.
PBBs are stable and persistent, lipophilic, and only slightly soluble
in water; some of the congeners are poorly metabolized and therefore
accumulate in the lipid compartments of biota. Once they have been
released into the environment, they can reach the food chain, where
they are accumulated.
PBBs have been detected in fish from several regions. The ingestion
of fish is a means of PBB transfer to mammals and birds. PBBs have
also been detected in ducks, a turtle, in the eggs of water birds, as
well as in the fat of deer, rabbits, coyote, ravens, reindeer, seals,
and osprey.
The degradation of PBBs by purely abiotic chemical reactions
(excluding photochemical reactions) is considered unlikely. PBBs have
been reported to be persistent under field conditions. Soil samples
from a former PBB manufacturing site, analysed several years after the
Michigan incident, still contained PBBs, though the congener
composition was different, indicating partial degradation of the PBB
residue in the soil sample.
Under laboratory conditions, PBBs are easily degraded by ultraviolet
radiation. Photodegradation of the commercial FireMaster(R) mixture
led to decreased concentrations of the more highly substituted PBB
congeners. The rates and extent of the photolytic reactions of PBBs in
the environment have not been determined in detail, though field
observations indicate high persistence of the original PBBs or partial
degradation to less brominated compounds.
In laboratory investigations, mixtures of PBB appear to be fairly
resistant to microbial degradation.
No uptake or degradation of PBBs by plants has been recorded. In
contrast, PBBs are easily absorbed by animals, and although they were
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs. No investigations of sulfur-containing
metabolites analogous to those of PCBs have been reported.
The bioaccumulation of PBBs in fish has been investigated.
Bioaccumulation of PBBs in terrestrial animals has been investigated
for avian and mammalian species. Data were obtained through field
observations, evaluation of the Michigan disaster, and through
controlled feeding studies. Generally, the accumulation of PBBs in
body fat depended on dosage and duration of exposure.
For the individual congeners of PBB, bioaccumulation has been found to
increase with degree of bromination, up to at least the
tetrabromobiphenyls. Higher brominated congeners can be expected to
accumulate to an even higher degree. However, no such information is
available for decabromobiphenyl (DeBB). It is possible that DeBB is
poorly absorbed.
2.3 Environmental Levels and Human Exposure
There is only one report on PBB levels in air, the concentrations
being measured in the vicinity of three PBB-manufacturing or
PBB-processing plants in the USA.
PBB levels in surface waters were monitored in the same vicinity, as
well in the area of the Gratiot County landfill (Michigan, USA), which
received more than 100 tonnes of waste between 1971 and 1973,
containing 60-70% PBB.
Groundwater monitoring data from Gratiot County have shown trace
levels of PBBs even outside the landfill area; however, PBBs were not
detected in drinking-water wells in the area.
Data on soil pollution by PBBs are available for areas of manufacture,
use, or disposal, and for soils from fields of the PBB-contaminated
Michigan farms.
In the Michigan disaster, FireMaster(R) was inadvertently added to
animal feed. It was almost a year later that the mixing error was
discovered and the analyses indicated that PBBs were responsible.
During this time (summer 1973-May 1974), contaminated animals and
their produce entered the human food supply, and the environment of
the State of Michigan. Hundreds of farms were affected and thousands
of animals had to be slaughtered and buried, as well as thousands of
tonnes of farm produce.
Most data available on the PBB contamination of wildlife refer to fish
and birds, primarily water-fowl, in the vicinity of industrial sites,
and to marine mammals.
Recent reports on the PBB contamination of fish, terrestrial and
marine mammals, and birds in Europe and USA indicate a wide
distribution of these compounds. The congener pattern found in fish
samples is quite different from that found in commercial products.
Many of the main compounds found could well be the result of
photochemical debromination of decabromobiphenyl (BB 209), but this
has not been confirmed.
Occupational exposure has been found in employees of US chemical
plants, and in farm workers, as a result of the Michigan PBB incident.
Median levels of PBB in serum and adipose tissue were higher among
chemical workers. Information is not available on occupational
exposure associated with manufacturing, formulation, and commercial
uses from other companies or countries.
For most human populations, the exposure to PBBs from various sources
has not been measured directly. Widespread human exposure has been
reported from Michigan, USA, as a result of direct contact with
contaminated feed, but mainly from consumption of PBBs in meat, eggs,
and dairy products. At least 2000 families (mainly farmers and their
neighbours) were exposed to high levels of PBBs. Recently, PBBs have
been detected in cow's and human milk in Germany.
The congener patterns in these human samples were different from those
found in fish. The concentration of BB 153 was higher in the human
milk than in fish.
The routes by which the general population is exposed to PBBs are not
well known. Present knowledge indicates that the ambient air and
water do not contain high levels. Lipid-rich food, especially from
contaminated waters, is probably of great importance. There is no
information on the level of exposure from indoor air or on dermal
exposure from materials containing PBB flame retardants.
The PBB congener pattern found in human milk samples collected in
Germany resembles that found in cow's milk from the same region, but
the levels in the human samples were substantially higher.
An estimate of daily intake of PBB for the general population from
food has to be based on very few data. If fish is assumed to contain
20 µg PBB/kg lipid and 5% lipid and that a 60-kg person eats 100 g
fish/day, the intake will be 0.002 µg/kg body weight per day. A
PBB concentration of 0.05 µg/kg lipid in milk and a milk consumption
of 500 ml/day will give the same person a PBB intake of about
0.00002 µg/kg body weight per day.
An infant of 6 kg body weight consuming 800 ml breast milk (3.5%
lipid) per day will ingest 0.01 µg PBB/kg body weight per day, if the
milk contains 2 µg PBB/kg lipid.
2.4 Kinetics and Metabolism
Gastrointestinal absorption of PBBs varies according to the degree of
bromination, the lower brominated compounds being more easily
absorbed. There is inadequate information concerning the absorption
of DeBB and OcBB.
PBBs are distributed throughout the entire body of animal species and
human beings, with highest equilibrium concentrations being found in
the adipose tissues. Relatively high levels are also found in the
liver; in particular, the more toxic congeners appear to be
concentrated in the liver. The partitioning of the various PBB
congeners appears to differ in different tissues. Generally, there is
a marked tendency for bioaccumulation. In mammals, transfer of PBBs to
offspring occurs through the transplacental and milk routes. Human
breast milk was found to have levels of 2,2',4,4',5,5'-
hexabromobiphenyl, more than 100 times higher than the maternal serum.
During a multigeneration study on rats, administration of PBBs to one
generation resulted in detectable residues in more than two subsequent
generations. Eggs of avian species are also affected by the maternal
PBB body burden.
Many PBB congeners are persistent in biological systems. There was no
evidence of significant metabolism or excretion of the more abundant
components of the FireMaster(R) mixture, or of octa- or
decabromobiphenyl. In vitro studies showed that the metabolism of
PBBs is affected by the structure-activity relationship. PBBs were
metabolized by phenobarbital-induced microsomes only if they possessed
adjacent non-brominated carbons meta and para to the biphenyl bridge
on at least one ring. Metabolism by 3-methylcholanthrene-induced
microsomes required adjacent non-brominated ortho and meta positions
on at least one ring of lower substituted congeners; a higher degree
of bromination appeared to prevent metabolism. Hydroxylated
derivatives have been identified in vertebrates as major in vitro
and in vivo metabolism products of lower brominated biphenyls. The
metabolic yield was relatively low. The hydroxylation reaction
probably proceeds via both arene oxide intermediates and by direct
hydroxylation.
Humans, rats, rhesus monkeys, pigs, cows, and chickens eliminate PBBs
mainly in the faeces. In most cases, elimination rates seem to be
slow. Concentrations of 2,2',4,4',5,5'-hexabromobiphenyl observed in
the bile and faeces of humans were about 1/2 to 7/10 of the serum
levels, or approximately 0.5% of the adipose levels. Treatment to
increase elimination of PBB in animals or humans was usually
unsuccessful. Another pathway of elimination could be excretion in
milk.
Complex and varied relationships were found among PBB tissue
concentrations with time after PBB administration to rats or other
animals. They are described by several compartmental models. A
half-life of approximately 69 weeks was calculated for the elimination
of 2,2',4,4',5,5'-hexabromobiphenyl from body fat of the rats; this
period increased to more than 4 years for rhesus monkeys. The average
half-life of 2,2',4,4',5,5'-hexabromobiphenyl in humans has been
estimated to be between 7.8 and 12 years; periods ranging from 4.6 to
94.7 years have been suggested in the literature. Some differences in
retention and turnover have been demonstrated between individual PBB
congeners. The results of analyses of serum from farmers and chemical
workers for 2,3',4,4',5-pentabromobiphenyl have been inconsistent.
This inconsistency is probably due to the different sources of
exposure. The workers were exposed to all compounds of FireMaster(R),
while the Michigan population was exposed to contaminated meat and
milk containing a different mixture of PBBs as a result of metabolic
processes in farm animals. Bromine levels did not decrease in the
adipose tissue of rats when they were given technical grade
octabromobiphenyl. No information is available on the retention of
decabromobiphenyl.
Humans may have a greater tendency to retain certain PBB congeners
than experimental animals. This factor should be taken into
consideration in evaluating the human health hazards associated with
these chemicals.
In conclusion, all the available data indicate that PBBs have a marked
tendency to bioaccumulate and persist. Metabolism is poor and the
half-life in man is in the order of 8-12 years, or longer.
2.5 Effects on Organisms in the Environment
Only few data are available on the effects of PBBs on organisms in the
environment. They refer to microorganisms, water fleas, water birds,
and farm animals.
Water birds nesting on islands in northwestern Lake Michigan were
studied to determine whether environmental contaminants were affecting
their reproduction. Seventeen contaminants, including PBB, were
measured, but none seemed to have a pronounced effect on reproduction.
Farm animals that ingested feed inadvertently containing Firemaster(R)
FF-1, in place of magnesium oxide, became sick. The estimated average
exposure of cows on the first identified, highly contaminated farm was
250 mg/kg body weight. The clinical signs of toxicity were a 50%
reduction in feed consumption (anorexia) and a 40% decrease in milk
production a few weeks after ingestion of the contaminated feed.
Although the supplemented feed was discontinued within 16 days, milk
production did not recover. Some animals showed an increased frequency
of urination and increased lacrimation, and developed haematomas,
abscesses, abnormal hoof growth, lameness, alopecia, hyperkeratosis,
and cachexia, and several died within 6 months of exposure.
Altogether, the death rate on this farm was 24 out of 400. The death
rate of 6-18-month-old calves was much higher. About 50% died within 6
weeks, only 2 out of 12 surviving after 5 months. They developed
hyperkeratosis over their entire bodies. There were also a variety of
reproductive problems.
Necropsy findings have been reported for some of the mature cows that
died in the 6 months after exposure. Histopathological studies
revealed variable liver and kidney changes.
The clinical signs and pathological changes noted above were later
confirmed in controlled feeding studies (anorexia, dehydration,
excessive lacrimation, emaciation, hyperkeratosis, reproductive
difficulties, some clinical chemistry changes, and renal damage).
A drop in production and sterility were reported from herds with a low
level of contamination. This was in contrast to the results from
controlled studies which did not show significant differences between
herds with low-level contamination and control herds.
Although it was cattle feed that was originally contaminated, other
animal feeds became involved by cross-contamination, e.g., in the
machinery used to prepare other feeds. It is likely that the exposure
from these sources was not as high as that of the cattle. Although
other animals (poultry, pigs, horses, rabbits, goats, and sheep) were
reported as being exposed and were killed, details of ill effects were
not recorded.
No information is available on the effect of PBBs on ecosystems.
2.6 Effects on Experimental Animals and In Vitro Test Systems
Commercial mixtures show relatively low acute toxicity (LD50
>1 g/kg body weight) in rats, rabbits, and quails following oral or
dermal administration. Deaths and acute manifestations of toxicity
are delayed after administration of PBB. The total dose administered
determines the degree of toxicity, whether given as a single dose or
as repeated doses over short periods (up to 50 days). The toxicity of
PBBs is higher with multiple-dose rather than with single-dose
administration.
The few studies performed with commercial mixtures of octa- and
decabromobiphenyl resulted in no mortality of rats or fish. Of the
individual PBB congeners, only 3 hexa isomers have been tested,
3,3',4,4',5,5'-HxBB and 2,3',4,4',5,5'-HxBB being more toxic to rats
than 2,2',4,4',5,5'-HxBB. On the basis of limited data, OcBB and DeBB
appear to be less toxic than the PBB mixtures, and less well absorbed.
In many acute and short-term studies, signs of PBB (mostly FireMaster)
toxicity include reductions in feed consumption. At lethal doses, the
cause of death cannot be ascribed to pathology in a particular organ,
but rather the animals develop a "wasting syndrome" as the first
indication of toxicity. At death, the loss in body weight can be as
great as 30-40%. The few studies with technical OcBB and DeBB show no
such effects.
The morphological and histopathological changes due to PBB are most
prominent in the liver. Enlargement of the liver frequently occurs at
doses lower than those required to produce body weight changes. The
principal histopathological alterations in rodent species may consist
of extensive swelling and vacuolation of hepatocytes, proliferation of
the smooth endoplasmic reticulum, and single-cell necrosis. The
severity of the lesions depends on the dose and composition of the PBB
material given.
Decreases in thymus weights were observed in rats, mice, and cattle
after doses of FireMaster(R), but not after OcBB or DeBB.
After exposure of rats to low concentrations of PBBs, there have been
some reports of increase in thyroid weight and histological changes in
the thyroid.
It is evident that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological changes
in the liver and thymus. The halogenated biphenyls have been
categorized on the basis of structure. Category 1 comprises isomers
and congeners lacking orthosubstituents (coplanar PBBs).
Mono-ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more orthobromines) form the
third category. The congeners in the first category tend to elicit the
most severe effects, those in the second and third categories showing
less severe toxicological effects. Within the category, the degree of
bromination may also influence toxicity.
Of all the combinations tested, 3,3',4,4',5,5'-HxBB was found to be
the most toxic PBB. This congener is present in low concentrations as
a constituent of FireMaster(R). Of the major FireMaster(R)
constituents, 2,3,3',4,4',5-HxBB appeared to be the most toxic,
followed by 2,3',4,4',5,5'-HxBB and 2,3',4,4',5-PeBB. The main
component of the FireMaster(R) mixture, 2,2',4,4',5,5'-HxBB was
relatively non-toxic, as was 2,2',3,4,4',5,5'-HpBB, the second most
abundant constituent.
Technical OcBB and DeBB contain various amounts of other brominated
biphenyls. Information on other contaminants is lacking.
The common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions to the technical PBB
mixtures tested (OcBB and DeBB). However, hyperkeratosis and hair loss
were seen in cattle, and lesions resembling chloracne were seen in
rhesus monkeys following the ingestion of a FireMaster(R) mixture.
Hyperkeratosis of the inner surface of the rabbit ear was produced by
FireMaster, but not by its main components (2,2',4,4',5,5'-HxBB and
2,2',3,4,4',5,5'-HpBB). Fractionation of FireMaster(R) revealed that
most activity was associated with the more polar fractions containing
minor components. Treatment with sunlight-irradiated HxBB caused
severe hyperkeratosis in rabbit ears.
In rats, low-dose, long-term feeding of technical OcBB did not affect
food consumption and body weight, but an increase in the relative
liver weights of exposed rats was found at 2.5 mg/kg body weight for
7 months. Long-term feeding of FireMaster(R) to rats at doses of
10 mg/kg body weight for 6 months did not affect food consumption.
Doses of 1 mg/kg body weight for a 6-month period affected liver
weight. The thymus weight was decreased by a dose of 0.3 mg/kg body
weight in female rats. Histopathological changes were also noted.
Controlled long-term feeding studies in cattle exposed to low doses of
FireMaster(R) showed no adverse effects, as indicated by food intake,
clinical signs, clinicopathological changes, or performance. Minks,
guinea-pigs, and monkeys appeared to be more susceptible to PBB
toxicity.
Long-term effects have been recorded in rats related to pronounced
retention of the administered PBBs following pre- or perinatal
exposure to high doses of FireMaster(R).
The most common adverse effects on reproduction were fetal wastage and
a decrease in viability of the offspring. Some effects were still
noted in mink at concentrations of 1 mg/kg diet. Following a
12.5-month exposure to FireMaster(R) (0.3 mg/kg diet), a minor
decrease was observed in the viability of the offspring of rhesus
monkeys. The monkeys received a daily dose of 0.01 mg/kg body weight
and a total dose of 3.8 mg/kg body weight. Reproduction and
neurobehavioural studies in monkeys and rats with a low level of
exposure could not be appropriately evaluated since insufficient
information was given in the published papers on the experimental
design of the study. A weak teratogenic potential was seen in rodents
at high doses that may have caused some maternal toxicity.
PBBs interact with the endocrine system. Rats and pigs were found to
have dose-related decreases in serum thyroxine and triiodothyronine.
PBBs have also been reported to affect the levels of steroid hormones;
the extent depends on the species as well as the dose and the period
of administration.
PBBs produce porphyria in rats and male mice at doses as low as
0.3 mg/kg body weight per day; the no-effect level was 0.1 mg/kg per
day. PBBs had a pronounced influence on vitamin A storage, as well as
effects on the intermediary metabolism.
Atrophy of the thymus was a frequent observation following PBB
exposure. Other lymphoid tissues have also been affected.
FireMaster(R) also induced further indications of a suppressed immune
function. Data is not available concerning the effects of OcBB, DeBB,
or individual PBB congeners.
One of the most intensively studied effects of PBBs is their induction
of mixed function oxidase (MFO) enzymes. Consistently, FireMaster(R)
was found to be a mixed-type inducer of hepatic microsomal enzymes in
rats, as well as in all other animal species tested. Induction was
also found to a lesser extent in other tissues. The ability to induce
hepatic microsomal enzymes differed for individual PBB congeners.
Correlations between structure and microsomal enzyme-inducing activity
have been demonstrated.
Several studies have shown that PBBs are able to alter the biological
activity of a variety of drugs and toxicants. This may be due partly
to the ability of PBBs to induce the microsomal enzymes involved in
activation or deactivation of xenobiotics.
The FireMaster(R) mixture, and some of its major components, were
found to be capable of inhibiting intercellular communication in
vitro. This inhibition occurs at non-cytotoxic concentrations. Both
the cytotoxicity and the metabolic cooperation-inhibiting properties
of PBB congeners seem to be related to their structure, i.e., presence
or lack of ortho-substitution. In vitro and in vivo assays
(microbial and mammalian cell mutagenesis, mammalian cell chromosomal
damage, mammalian cell transformation, and DNA damage and repair
(UDS)) have failed to indicate any mutagenicity or genotoxicity of
individual PBB congeners or commercial mixtures.
Long-term carcinogenicity studies with PBBs have shown that the
principal site of tumours is the liver. The incidences of
hepatocellular carcinoma were significantly increased in both sexes of
mice and rats receiving oral doses of the FireMaster(R) mixture.
Bromkal 80-9 (technical nonabromobiphenyl) induced carcinogenic
effects in the liver in mice receiving diets containing 100 mg/kg diet
(5 mg/kg per day), or more, for 18 months. The lowest dose of PBB
that produced tumours (mostly adenomas) in rodents was 0.5 mg/kg body
weight per day for 2 years. The rats receiving 0.15 mg/kg in addition
to pre- and perinatal exposure did not show any adverse effects. The
carcinogenicity of technical octabromobiphenyl and decabromobiphenyl
has not been studied.
Neither FireMaster BP-6 nor 2,2',4,4',5,5'-hexabromobiphenyl showed
tumour-initiating (using TPA as promoter) or tumour-promoting (using
DMBA as initiator) activity in a mouse skin bioassay. However, in
other mouse skin models (using DMBA or MNNG as initiators), both
FireMaster FF-1 and 3,3',4,4',5,5'-hexabromobiphenyl, but not
2,2',4,4',5,5'-hexabromobiphenyl, showed tumour-promoting activity.
In a two-stage rat liver bioassay using phenobarbital as promoter,
3,3',4,4'-tetrabromobiphenyl showed a weak initiating activity. In
the two-stage rat liver model, using diethylnitrosamine and partial
hepatectomy, FireMaster, 3,3',4,4'-tetrabromobiphenyl, and
2,2',4,4',5,5'-hexabromobiphenyl showed tumour-promoting activity, but
3,3,',4,4',5,5'-hexabromobiphenyl did not.
The results of the studies on cell communication, the negative results
on genotoxicity and mutagenicity, and the results of tumour-promotion
assays indicate that the mixtures and congeners studied cause cancer
by epigenetic mechanisms. No information is available on technical
octa-, nona-, or decabromobiphenyl.
The mechanisms of action underlying the many manifestations of the
toxicity of PBBs and related compounds are not known. However, some of
the effects, such as the wasting syndrome, thymus atrophy,
hepatotoxicity, skin disorders, and the reproductive toxicity, may be
related to interaction with the so-called Ah- or TCDD-receptors
causing alteration in the expression of a number of genes. Different
PBB congeners vary in their interaction with the receptor, the
coplanar congeners being more active.
Many of the PBB effects are seen after long-lasting exposure. The
reason for this observation may be the pronounced accumulation of some
PBB congeners and the poor ability of the body to metabolize and
eliminate them. This results in a build-up of the chemical in the
body which overcomes the compensatory mechanisms and leads to adverse
effects.
Some polybrominated naphthalenes (PBNs), known contaminants of the
FireMaster(R) mixture, are potent toxicants and teratogens. Although
PBNs are only present at low levels in the FireMaster(R) mixture, it
is possible they may contribute to its toxicity.
Studies of the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-HxBB, showed that the photolysis products were more
toxic than the original PBB. Under some conditions of pyrolysis of
FireMaster(R), polybrominated dibenzofurans are formed. The pyrolysis
products of technical OcBB caused liver enlargement.
2.7 Effects on Humans
There have been no examples of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures following
the poisoning incident in Michigan, USA, in 1973. The main
epidemiological studies were conducted by the Michigan Department of
Public Health (MDPH) and the Environmental Science Laboratory, Mount
Sinai School of Medicine, New York (ESL).
It was estimated that the most highly exposed people consumed 5-15 g
PBB in milk over a 230-day period. Some additional exposure may have
occurred by consuming meat. The exposure in some of the farmers, and
most of the general population, in Michigan was much lower. For them
the total exposure was 9-10 mg. However, some people in this group
may have received a total exposure of about 800-900 mg. A total dose
of 9 mg corresponds to 0.15 mg/kg body weight, and 900 mg to 15 mg/kg
body weight for a 60-kg average adult; the dose/kg body weight will be
higher for children.
In 1974, the first MDPH study compared the health status of people on
quarantined farms with people on non-quarantined farms in the same
area. Although a variety of symptoms were reported by both groups,
there was no pattern to the differences between the groups. The
heart, liver, spleen, nervous system, urinalysis, blood counts, and
all other medical conditions examined were found to be normal. In a
later, comprehensive, MDPH study including groups with different
levels of exposure, there was no positive association between serum
concentrations of PBB and reported symptoms or disease frequencies.
The ESL studies involved about 990 farm residents, 55 chemical
workers, and a group of Wisconsin dairy farmers who were used as a
control group. The prevalence of symptoms in Michigan farmers was
greater than the prevalence in Wisconsin farmers. The greatest
differences were in the broad classification of neurological and
musculoskeletal symptoms. Elevated serum concentrations of some liver
enzymes and carcinoembryonic antigen were more prevalent in Michigan
farmers than in Wisconsin farmers. Chemical workers had a higher
prevalence of chest and skin symptoms and a lower prevalence of
musculoskeletal symptoms than farmers. Although the results of the ESL
studies were at times interpreted differently from the results of
comparable studies, there was one area of consistent agreement. None
of the sets of studies demonstrated a positive dose-response
relationship between PBB levels in serum or in adipose tissue and the
prevalence of symptoms or abnormal clinical measurements. Several
clinical areas were investigated by more intensive special studies.
Examination of the neurological aspects by means of objective
performance tests revealed, in one study, a negative correlation
between serum PBB levels and performance test scores, particularly in
males in the older age groups. The other studies showed no association
between the concentration of PBB in serum or fat and performance in a
battery of tests measuring memory, motor strength, coordination,
cortical-sensory perception, personality, higher cognitive
functioning, and other functions. The paediatric aspects of PBB
exposure were examined in the families in the ESL studies. Although
many symptoms were reported, physical examination failed to reveal any
objective alteration that could be attributed to PBB. There were
different views about the more subtle neuropsychological effects in
the offspring, and the results of investigations of developmental
abilities also remain controversial. The same is true for the
investigation of lymphocyte and immune function. One set of authors
found no differences in lymphocyte count or functions between groups
with high and low serum PBB levels, the other found a significant
decrease in T and B lymphocyte subpopulations in about 40% of an
exposed Michigan group compared with unexposed groups, and also
impaired lymphocyte function, i.e., decreased response to mitogens.
The epidemiological studies reviewed were planned to evaluate the
relationship between PBB exposure and a large number of adverse
effects, including behavioural effects and subjective complaints.
However, most of the studies suffer from major faults in design that
introduced confounders which make it difficult or impossible to draw
valid conclusions. The follow-up time has not been long enough to
evaluate a possible carcinogenic effect.
Two small groups of workers were identified with occupational exposure
to a mixture of PBBs, or to DeBB and DBBO. Lesions resembling
chloracne were found in 13% of the workers exposed to the PBB mixture,
but such lesions were not seen in the workers exposed to DeBB.
However, a significantly higher prevalence of hypothyroidism was seen
in the latter group.
2.8 Overall Evaluation of the Toxicity
The only lifetime and carcinogenicity study with a PBB mixture was
conducted in rats and mice in a recent NTP bioassay. The lowest dose
tested that still produced carcinogenic effects was 0.5 mg/kg body
weight per day (liver tumours in rodents). In other carcinogenicity
studies, 3 mg/kg body weight given for 6 months resulted in a
carcinogenic response. The 6-month study demonstrates that a less
than lifetime exposure at similar doses will also result in similar
adverse effects. Effects on reproduction in subhuman primates and
mink may occur at somewhat lower doses.
In addition, in the 2-year NTP study, a daily dose of 0.15 mg/kg body
weight per day and prenatal and perinatal exposure of the dam to
0.05 mg/kg body weight per day did not result in any adverse effects.
Thus, the total daily intake from food, water, air, and soil should be
less than 0.15 µg/kg body weight per day, extrapolating from the NOAEL
(no-observed-adverse-effect level) of a positive carcinogenicity
study, using an uncertainty (and safety) factor of 1000, since these
compounds probably induce cancer by an epigenetic mechanism.
The total dose received by the subpopulation in Michigan was estimated
to have ranged from 0.15 to 15 mg/kg body weight over a 230-day
period. For this population, dividing the doses over a lifetime for
the average human being would be equivalent to a daily dose ranging
from 6 ng to 0.6 µg/kg body weight per day.
Estimates for the general population indicate a total intake by adults
of 2 ng PBB/kg body weight per day from known sources, and an intake
of 10 ng/kg body weight per day for infants receiving breast milk. It
should be kept in mind that these estimates are based on a very
limited and regional data base.
These calculations assume that a steady state for PBBs would not be
reached over a lifetime, and that short-term, higher exposures can be
substituted for long-term, lower exposures, since these compounds are
extremely poorly metabolized and excreted.
Insufficient information is available for OcBB, NoBB, and DeBB to
calculate a total daily intake that would not result in adverse
effects.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Most of the PBB congeners found in commercial flame retardants are
lipophilic, persistent, and bioaccumulating. These compounds are
biomagnified in environmental food-webs and pose a particular threat
to organisms in the higher levels of these webs. Furthermore, some
PBB products form toxic polybrominated dibenzofurans during combustion
processes.
In addition to emissions during manufacture and use, PBB will enter
the environment from the widespread use of flame-retarded products. A
considerable part of the PBB produced will probably reach the
environment sooner or later because of the high stability of these
compounds.
PBBs are also found in environmental and human samples also from
places far from known point sources. The congener patterns in the
environmental samples do not match those found in the technical
products indicating environmental alteration, possibly photochemical
debromination.
Very little information is currently available on the degree of
exposure of the general population to PBBs. However, in the few
instances where measurements have been made, trace amounts of PBBs
were identified. At present, this exposure does not give rise to
concern but further build-up should be avoided. Human data from the
Michigan episode (see sections 2.3 and 2.7) suggest that exposures in
Michigan were several orders of magnitude greater than the exposure of
the general population. No definitive health effects could be
correlated with PBB exposure in the Michigan population, though the
follow-up period has not been long enough for the occurrence of cancer
to be evaluated. Since the PBB levels remain high in adipose tissue
and serum in the Michigan population, their internal exposure
continues. In contrast, in Michigan cattle toxic effects were
observed. This discrepancy is explained by differences in the degree
of exposure of the cattle.
Occupational exposure has been examined in only two plants in the USA.
It appears that chloracne-like lesions may develop in workers
producing PBB, and hypothyroidism in workers exposed to DBB. No
studies have been conducted in workers incorporating deca-, octa-, or
nonabromobiphenyl into commercial products.
PBBs are extremely persistent in living organisms and have been shown
to produce chronic toxic effects and cancer in animals. Though the
acute toxicity was low, cancer was induced at a dose of 0.5 mg/kg body
weight per day and the no-observed-effect level was 0.15 mg/kg body
weight per day. A number of chronic toxic effects have been observed
in experimental animals at doses around 1 mg/kg body weight per day
following long-term exposure.
3.2 Recommendations
General
* The Task Group is of the opinion that human beings and the
environment should not be exposed to PBBs, in view of their high
persistency and bioaccumulation and the potential adverse effects
at very low levels after long-term exposure. Therefore, PBBs
should no longer be used commercially.
* Because of the limited data on the toxicity of DeBB and OcBB,
their extreme persistence, and their potential for breakdown in
the environment and through combustion to more toxic persistent
compounds, they should also not be used commercially unless their
safety has been demonstrated.
Future work
* It is known that observations on the Michigan cohort are still
continuing. Publication of these data is required.
* Future human and environmental PBB monitoring should be expanded
and be congener specific, and include the OcBB, NoBB, and DeBB.
These compounds should be included in monitoring programmes for
other halogenated compounds.
* The time trends and geographical distribution of PBB levels in
the environment should continue to be monitored. The release of
PBBs into the environment from waste disposal sites should be
surveyed.
* Thermolysis experiments should be conducted to simulate the
conditions in accidental fires and municipal incinerators.
* Research should be continued on the mechanisms of toxicity and
carcinogenicity of PBBs and related compounds. PBBs may serve as
model compounds for mechanistic research. Purified congeners
should be used in these studies.
* The effects on reproduction have not been not well elucidated.
Therefore, well designed long-term reproductive studies should be
performed, at low doses, using a sensitive species.
* There is also a need for more information on the bioavailability
and toxicokinetics of OcBB/NoBB, DeBB and selected congeners.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
PBBs are highly brominated organic substances. They are very
persistent and can be hazardous for human beings if incorrectly or
carelessly handled. It is therefore essential that the correct
precautions are observed during handling and use.
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The acute oral and dermal toxicity is low. In occupational
conditions, skin itching, peeling, scaling and (halo) acne are found
after exposure to PBBs. Besides these dermal signs of intoxication,
liver disturbances, chest irritation, and neurological and unspecific
effects, such as depression, memory disturbances and nervousness, have
been reported, but not confirmed.
4.1.1.2 Medical advice
Medical treatment is symptomatic and supportive.
4.1.2 Health surveillance advice
A complete medical history of regularly exposed workers should be
taken, and physical examination made, on an annual basis, and
monitoring of PBB levels in blood serum should be done (frequency
depending on the results).
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
PBBs are not flammable; on the contrary, they are flame retardants.
However, on heating, toxic fumes, such as hydrogen bromide and
brominated dibenzofurans may be formed.
4.2.2 Fire hazards
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. Fire-fighters should be equipped with self-contained
breathing apparatus, eye protection, and full protective clothing.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding the accumulation of polluted run-off from the
site.
4.3 Storage
Products should be stored in locked buildings, out of reach of
animals, children, and unauthorized personnel. Do not store near
foodstuffs or animal feed.
4.4 Transport
Comply with any local requirements regarding movements of hazardous
goods. Do not transport in the same compartment as foodstuffs. Check
that containers are sound and labels undamaged before despatch.
4.5 Spillage
Before dealing with any spillage, precautions should be taken as
required, and appropriate personal protection should be used. Prevent
material from spreading or contaminating other cargo, vegetation, or
waterways, by making a barrier of the most suitable available
material, e.g., earth or sand.
Empty any product remaining in damaged/leaking containers into a clean
empty drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
Absorb spilled liquid with sawdust, sand, or earth, sweep up and place
the sweepings in a closeable container for later transfer to a safe
place for disposal.
As soon as possible after the spillage and before reuse, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place the sweepings in a closeable container for later transfer to a
safe place for disposal. Care should be taken to avoid run-off into
watercourses.
4.6 Disposal
Any surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Waste material should be burned in
a proper incinerator designed for organohalogen waste disposal with
effluent-gas scrubbing. For PBB wastes, incineration must be for more
than 2 seconds at 1200 °C or higher. Combustion of PBBs at lower
temperatures can produce brominated dibenzofurans. If high
temperature incineration is not possible, bury waste in an approved
dump or landfill where there is no risk of contamination of surface or
groundwater. Decomposition of PBBs will be extremely slow.
Comply with any local legislation regarding disposal of toxic wastes.
Puncture container to prevent reuse.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
PBBs are very resistant to degradation and very persistent in the
environment. Because of their high solubility in fat, they
bioaccumulate, especially in the fatty tissues of all living
organisms, and biomagnify in the higher trophic levels of the food
chain. Although their acute toxicity is relatively low, this
bioaccumulation and biomagnification may lead to toxic effects.
Industrial discharges occurring during manufacture, formulation, or
technical applications should be treated properly and should not be
allowed to pollute the environment. Any spillage or unused product
should be treated and disposed of properly (see section 4.5 and 4.6)
and should be prevented from spreading to the environment.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. A full reference to the
original national document from which the information was extracted
can be obtained from IRPTC.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluation by International Bodies
The International Agency for Research on Cancer (IARC) evaluated
polybrominated biphenyls in 1986 and 1987 and concluded that there was
inadequate evidence of their carcinogenicity in humans, but sufficient
evidence of their carcinogenicity in experimental animals (Group 2B).
6.2 Exposure Limit Values
No exposure limit values for PBBs were found.
6.3 Specific Restrictions
In the USA, all use of hexabromobiphenyls, the main PBB isomer used in
industrial processes, was discontinued in 1974, because of the hazard
to human health discovered after its accidental misuse in Michigan in
1973. The US Environmental Protection Agency has since required
notification regarding any manufacture or importation of PBBs. The
purpose of this requirement is to confirm that there are no
significant sources of these substances and to ensure that EPA has the
opportunity to investigate the circumstances of any resumption of
production.
In Canada, all commercial, manufacturing, and processing uses are
banned.
In the countries of the EEC, PBBs may not be used in textile articles,
such as garments, undergarments and linen intended to come into
contact with the skin.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies PBBs in:
Hazard Class 9: miscellaneous dangerous substance
Packing Group II: substances presenting medium danger
The European Community legislation requires the labelling of PBBs as
harmful substances using the symbol.
Molecular weight: 154.21 + 78.90 (x+y)
Common trade names: Adine 0102; Berkflam B10; Bromkal 80-9D;
FireMaster BP-6; FireMaster FF-1;
Flammex B10; HFO 101; Octabromobiphenyl
FR 250 BA; Octabromobiphenyl XN-1902.
Composition of technical products
PBBs synthesized for commercial use as fire retardants contain a
mixture of various brominated biphenyls, chiefly the hexa-, octa-,
nona-, and decabromobiphenyls, as well as small amounts of related
brominated substances.
1.2 Physical and Chemical Properties
PBBs are solids with a low volatility that decreases with an increase
in the number of bromine atoms. PBBs are virtually insoluble in water,
soluble in fat and slightly to highly soluble in various organic
solvents, the solubility also decreasing with an increase in the
number of bromine atoms. These compounds are relatively stable and
chemically unreactive, though highly brominated PBB mixtures degrade
rather rapidly on exposure to ultraviolet radiation.
The technical mixtures are typically white, off-white, or beige
powders.
1.3 Analysis
Gas chromatography with electron-capture or mass-spectrometric
detection are useful techniques for determining PBB levels.
High-resolution gas chromatography is preferred as this makes it
possible to determine specific congeners, but reference compounds are
available for only a few of the 209 possible PBB congeners.
1.4 Production and Uses
The commercial production of PBBs (as FireMaster(R)) was started in
the USA in 1970. After the production of about 6000 tonnes, further
production was virtually discontinued in 1974 after the inadvertent
addition of PBBs to animal feed. This resulted in the contamination of
farm animals and their subsequent massive destruction. Approximately
400 tonnes of octa- and decabromobiphenyl were produced in the USA
until 1979. Bromkal 80-9D was produced in Germany until 1985; a few
hundred tonnes per year of Adine 0102 are currently being produced in
France; in 1977, production of decabromobiphenyl ceased in the United
Kingdom.
PBBs are mainly used as flame retardants in moulded thermoplastics,
mostly in small appliances and automotive applications. Earlier
applications in synthetic fibres have been discontinued.
1.5 Thermal Decomposition
The products of the thermal decomposition of PBBs depend on the
temperature, the amount of oxygen present, and a number of other
factors. Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen (600-900 °C) have shown that bromobenzenes and lower
brominated biphenyls are formed, but no polybrominated furans. In
contrast, pyrolysis in the presence of oxygen (700-900 °C) yielded
some di- to heptabromodibenzofurans. In the presence of polystyrene
and polyethylene, higher levels were found. Pyrolysis of FireMaster
BP-6 with PVC at 800 °C yielded mixed bromochlorobiphenyls. There is
no information on the nature of the products of incineration of
PBB-containing compounds. Little is known about the toxicity of
brominated and brominated/chlorinated dioxins and furans, but their
toxicity is estimated to be of about the same order as that of
chlorinated dioxins and furans.
2. SUMMARY AND EVALUATION
2.1 Sources of Human and Environmental Exposure
PBBs were introduced as flame retardants in the early 1970s. Prior to
November 1974, hexabromobiphenyl was the most commercially significant
PBB in the USA and was incorporated into ABS (acrylonitrile-butadiene-
styrene) plastics (PBB content 10% - mainly small appliance and
automotive applications), coatings and lacquers, and polyurethane
foam. The other PBB flame retardants have similar uses.
Losses of PBB into the environment during normal production can occur
through emission into the air and waste waters, and through losses
into the soil and into landfills, but these quantities have been found
to be generally low. These chemicals can also enter the environment
during shipping and handling, and following accidents, as occurred in
Michigan, USA, in 1973.
PBBs can also enter the environment as a result of the incineration of
materials containing PBB, as well as during accidental fires, with the
formation of other toxic chemicals, such as polybromodibenzofurans or
mixed bromochloro derivatives.
Most of the total production of these compounds will ultimately enter
the environment, either unchanged or as breakdown products.
2.2 Environmental Transport, Distribution, and Transformation
Long-range transport of PBB in the atmosphere has not been proven, but
the presence of these compounds in Arctic seal samples indicates a
wide geographical distribution.
The main route by which PBBs enter the aquatic environment is in
industrial waste discharges and leachates from industrial dumping
sites, as well as by erosion of polluted soils. PBBs are almost
insoluble in water and are primarily found in the sediments of
polluted lakes and rivers.
Pollution of the soil can originate from point sources such as a PBB
production plant or a waste dump. Once introduced into the soil, PBBs
do not appear to be translocated readily. PBBs have been found to be
200 times more soluble in a landfill leachate than in distilled water;
this may result in a wider distribution in the environment. The
hydrophobic properties of PBBs make them easily adsorbed, from aqueous
solutions, on to soil particles. Preferential adsorption of PBB
congeners has been noted depending on the characteristics of the soil
(e.g., organic matter content) as well as on the degree and position
of the bromine substitution.
PBBs are stable and persistent, lipophilic, and only slightly soluble
in water; some of the congeners are poorly metabolized and therefore
accumulate in the lipid compartments of biota. Once they have been
released into the environment, they can reach the food chain, where
they are accumulated.
PBBs have been detected in fish from several regions. The ingestion
of fish is a means of PBB transfer to mammals and birds. PBBs have
also been detected in ducks, a turtle, in the eggs of water birds, as
well as in the fat of deer, rabbits, coyote, ravens, reindeer, seals,
and osprey.
The degradation of PBBs by purely abiotic chemical reactions
(excluding photochemical reactions) is considered unlikely. PBBs have
been reported to be persistent under field conditions. Soil samples
from a former PBB manufacturing site, analysed several years after the
Michigan incident, still contained PBBs, though the congener
composition was different, indicating partial degradation of the PBB
residue in the soil sample.
Under laboratory conditions, PBBs are easily degraded by ultraviolet
radiation. Photodegradation of the commercial FireMaster(R) mixture
led to decreased concentrations of the more highly substituted PBB
congeners. The rates and extent of the photolytic reactions of PBBs in
the environment have not been determined in detail, though field
observations indicate high persistence of the original PBBs or partial
degradation to less brominated compounds.
In laboratory investigations, mixtures of PBB appear to be fairly
resistant to microbial degradation.
No uptake or degradation of PBBs by plants has been recorded. In
contrast, PBBs are easily absorbed by animals, and although they were
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs. No investigations of sulfur-containing
metabolites analogous to those of PCBs have been reported.
The bioaccumulation of PBBs in fish has been investigated.
Bioaccumulation of PBBs in terrestrial animals has been investigated
for avian and mammalian species. Data were obtained through field
observations, evaluation of the Michigan disaster, and through
controlled feeding studies. Generally, the accumulation of PBBs in
body fat depended on dosage and duration of exposure.
For the individual congeners of PBB, bioaccumulation has been found to
increase with degree of bromination, up to at least the
tetrabromobiphenyls. Higher brominated congeners can be expected to
accumulate to an even higher degree. However, no such information is
available for decabromobiphenyl (DeBB). It is possible that DeBB is
poorly absorbed.
2.3 Environmental Levels and Human Exposure
There is only one report on PBB levels in air, the concentrations
being measured in the vicinity of three PBB-manufacturing or
PBB-processing plants in the USA.
PBB levels in surface waters were monitored in the same vicinity, as
well in the area of the Gratiot County landfill (Michigan, USA), which
received more than 100 tonnes of waste between 1971 and 1973,
containing 60-70% PBB.
Groundwater monitoring data from Gratiot County have shown trace
levels of PBBs even outside the landfill area; however, PBBs were not
detected in drinking-water wells in the area.
Data on soil pollution by PBBs are available for areas of manufacture,
use, or disposal, and for soils from fields of the PBB-contaminated
Michigan farms.
In the Michigan disaster, FireMaster(R) was inadvertently added to
animal feed. It was almost a year later that the mixing error was
discovered and the analyses indicated that PBBs were responsible.
During this time (summer 1973-May 1974), contaminated animals and
their produce entered the human food supply, and the environment of
the State of Michigan. Hundreds of farms were affected and thousands
of animals had to be slaughtered and buried, as well as thousands of
tonnes of farm produce.
Most data available on the PBB contamination of wildlife refer to fish
and birds, primarily water-fowl, in the vicinity of industrial sites,
and to marine mammals.
Recent reports on the PBB contamination of fish, terrestrial and
marine mammals, and birds in Europe and USA indicate a wide
distribution of these compounds. The congener pattern found in fish
samples is quite different from that found in commercial products.
Many of the main compounds found could well be the result of
photochemical debromination of decabromobiphenyl (BB 209), but this
has not been confirmed.
Occupational exposure has been found in employees of US chemical
plants, and in farm workers, as a result of the Michigan PBB incident.
Median levels of PBB in serum and adipose tissue were higher among
chemical workers. Information is not available on occupational
exposure associated with manufacturing, formulation, and commercial
uses from other companies or countries.
For most human populations, the exposure to PBBs from various sources
has not been measured directly. Widespread human exposure has been
reported from Michigan, USA, as a result of direct contact with
contaminated feed, but mainly from consumption of PBBs in meat, eggs,
and dairy products. At least 2000 families (mainly farmers and their
neighbours) were exposed to high levels of PBBs. Recently, PBBs have
been detected in cow's and human milk in Germany.
The congener patterns in these human samples were different from those
found in fish. The concentration of BB 153 was higher in the human
milk than in fish.
The routes by which the general population is exposed to PBBs are not
well known. Present knowledge indicates that the ambient air and
water do not contain high levels. Lipid-rich food, especially from
contaminated waters, is probably of great importance. There is no
information on the level of exposure from indoor air or on dermal
exposure from materials containing PBB flame retardants.
The PBB congener pattern found in human milk samples collected in
Germany resembles that found in cow's milk from the same region, but
the levels in the human samples were substantially higher.
An estimate of daily intake of PBB for the general population from
food has to be based on very few data. If fish is assumed to contain
20 µg PBB/kg lipid and 5% lipid and that a 60-kg person eats 100 g
fish/day, the intake will be 0.002 µg/kg body weight per day. A
PBB concentration of 0.05 µg/kg lipid in milk and a milk consumption
of 500 ml/day will give the same person a PBB intake of about
0.00002 µg/kg body weight per day.
An infant of 6 kg body weight consuming 800 ml breast milk (3.5%
lipid) per day will ingest 0.01 µg PBB/kg body weight per day, if the
milk contains 2 µg PBB/kg lipid.
2.4 Kinetics and Metabolism
Gastrointestinal absorption of PBBs varies according to the degree of
bromination, the lower brominated compounds being more easily
absorbed. There is inadequate information concerning the absorption
of DeBB and OcBB.
PBBs are distributed throughout the entire body of animal species and
human beings, with highest equilibrium concentrations being found in
the adipose tissues. Relatively high levels are also found in the
liver; in particular, the more toxic congeners appear to be
concentrated in the liver. The partitioning of the various PBB
congeners appears to differ in different tissues. Generally, there is
a marked tendency for bioaccumulation. In mammals, transfer of PBBs to
offspring occurs through the transplacental and milk routes. Human
breast milk was found to have levels of 2,2',4,4',5,5'-
hexabromobiphenyl, more than 100 times higher than the maternal serum.
During a multigeneration study on rats, administration of PBBs to one
generation resulted in detectable residues in more than two subsequent
generations. Eggs of avian species are also affected by the maternal
PBB body burden.
Many PBB congeners are persistent in biological systems. There was no
evidence of significant metabolism or excretion of the more abundant
components of the FireMaster(R) mixture, or of octa- or
decabromobiphenyl. In vitro studies showed that the metabolism of
PBBs is affected by the structure-activity relationship. PBBs were
metabolized by phenobarbital-induced microsomes only if they possessed
adjacent non-brominated carbons meta and para to the biphenyl bridge
on at least one ring. Metabolism by 3-methylcholanthrene-induced
microsomes required adjacent non-brominated ortho and meta positions
on at least one ring of lower substituted congeners; a higher degree
of bromination appeared to prevent metabolism. Hydroxylated
derivatives have been identified in vertebrates as major in vitro
and in vivo metabolism products of lower brominated biphenyls. The
metabolic yield was relatively low. The hydroxylation reaction
probably proceeds via both arene oxide intermediates and by direct
hydroxylation.
Humans, rats, rhesus monkeys, pigs, cows, and chickens eliminate PBBs
mainly in the faeces. In most cases, elimination rates seem to be
slow. Concentrations of 2,2',4,4',5,5'-hexabromobiphenyl observed in
the bile and faeces of humans were about 1/2 to 7/10 of the serum
levels, or approximately 0.5% of the adipose levels. Treatment to
increase elimination of PBB in animals or humans was usually
unsuccessful. Another pathway of elimination could be excretion in
milk.
Complex and varied relationships were found among PBB tissue
concentrations with time after PBB administration to rats or other
animals. They are described by several compartmental models. A
half-life of approximately 69 weeks was calculated for the elimination
of 2,2',4,4',5,5'-hexabromobiphenyl from body fat of the rats; this
period increased to more than 4 years for rhesus monkeys. The average
half-life of 2,2',4,4',5,5'-hexabromobiphenyl in humans has been
estimated to be between 7.8 and 12 years; periods ranging from 4.6 to
94.7 years have been suggested in the literature. Some differences in
retention and turnover have been demonstrated between individual PBB
congeners. The results of analyses of serum from farmers and chemical
workers for 2,3',4,4',5-pentabromobiphenyl have been inconsistent.
This inconsistency is probably due to the different sources of
exposure. The workers were exposed to all compounds of FireMaster(R),
while the Michigan population was exposed to contaminated meat and
milk containing a different mixture of PBBs as a result of metabolic
processes in farm animals. Bromine levels did not decrease in the
adipose tissue of rats when they were given technical grade
octabromobiphenyl. No information is available on the retention of
decabromobiphenyl.
Humans may have a greater tendency to retain certain PBB congeners
than experimental animals. This factor should be taken into
consideration in evaluating the human health hazards associated with
these chemicals.
In conclusion, all the available data indicate that PBBs have a marked
tendency to bioaccumulate and persist. Metabolism is poor and the
half-life in man is in the order of 8-12 years, or longer.
2.5 Effects on Organisms in the Environment
Only few data are available on the effects of PBBs on organisms in the
environment. They refer to microorganisms, water fleas, water birds,
and farm animals.
Water birds nesting on islands in northwestern Lake Michigan were
studied to determine whether environmental contaminants were affecting
their reproduction. Seventeen contaminants, including PBB, were
measured, but none seemed to have a pronounced effect on reproduction.
Farm animals that ingested feed inadvertently containing Firemaster(R)
FF-1, in place of magnesium oxide, became sick. The estimated average
exposure of cows on the first identified, highly contaminated farm was
250 mg/kg body weight. The clinical signs of toxicity were a 50%
reduction in feed consumption (anorexia) and a 40% decrease in milk
production a few weeks after ingestion of the contaminated feed.
Although the supplemented feed was discontinued within 16 days, milk
production did not recover. Some animals showed an increased frequency
of urination and increased lacrimation, and developed haematomas,
abscesses, abnormal hoof growth, lameness, alopecia, hyperkeratosis,
and cachexia, and several died within 6 months of exposure.
Altogether, the death rate on this farm was 24 out of 400. The death
rate of 6-18-month-old calves was much higher. About 50% died within 6
weeks, only 2 out of 12 surviving after 5 months. They developed
hyperkeratosis over their entire bodies. There were also a variety of
reproductive problems.
Necropsy findings have been reported for some of the mature cows that
died in the 6 months after exposure. Histopathological studies
revealed variable liver and kidney changes.
The clinical signs and pathological changes noted above were later
confirmed in controlled feeding studies (anorexia, dehydration,
excessive lacrimation, emaciation, hyperkeratosis, reproductive
difficulties, some clinical chemistry changes, and renal damage).
A drop in production and sterility were reported from herds with a low
level of contamination. This was in contrast to the results from
controlled studies which did not show significant differences between
herds with low-level contamination and control herds.
Although it was cattle feed that was originally contaminated, other
animal feeds became involved by cross-contamination, e.g., in the
machinery used to prepare other feeds. It is likely that the exposure
from these sources was not as high as that of the cattle. Although
other animals (poultry, pigs, horses, rabbits, goats, and sheep) were
reported as being exposed and were killed, details of ill effects were
not recorded.
No information is available on the effect of PBBs on ecosystems.
2.6 Effects on Experimental Animals and In Vitro Test Systems
Commercial mixtures show relatively low acute toxicity (LD50
>1 g/kg body weight) in rats, rabbits, and quails following oral or
dermal administration. Deaths and acute manifestations of toxicity
are delayed after administration of PBB. The total dose administered
determines the degree of toxicity, whether given as a single dose or
as repeated doses over short periods (up to 50 days). The toxicity of
PBBs is higher with multiple-dose rather than with single-dose
administration.
The few studies performed with commercial mixtures of octa- and
decabromobiphenyl resulted in no mortality of rats or fish. Of the
individual PBB congeners, only 3 hexa isomers have been tested,
3,3',4,4',5,5'-HxBB and 2,3',4,4',5,5'-HxBB being more toxic to rats
than 2,2',4,4',5,5'-HxBB. On the basis of limited data, OcBB and DeBB
appear to be less toxic than the PBB mixtures, and less well absorbed.
In many acute and short-term studies, signs of PBB (mostly FireMaster)
toxicity include reductions in feed consumption. At lethal doses, the
cause of death cannot be ascribed to pathology in a particular organ,
but rather the animals develop a "wasting syndrome" as the first
indication of toxicity. At death, the loss in body weight can be as
great as 30-40%. The few studies with technical OcBB and DeBB show no
such effects.
The morphological and histopathological changes due to PBB are most
prominent in the liver. Enlargement of the liver frequently occurs at
doses lower than those required to produce body weight changes. The
principal histopathological alterations in rodent species may consist
of extensive swelling and vacuolation of hepatocytes, proliferation of
the smooth endoplasmic reticulum, and single-cell necrosis. The
severity of the lesions depends on the dose and composition of the PBB
material given.
Decreases in thymus weights were observed in rats, mice, and cattle
after doses of FireMaster(R), but not after OcBB or DeBB.
After exposure of rats to low concentrations of PBBs, there have been
some reports of increase in thyroid weight and histological changes in
the thyroid.
It is evident that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological changes
in the liver and thymus. The halogenated biphenyls have been
categorized on the basis of structure. Category 1 comprises isomers
and congeners lacking orthosubstituents (coplanar PBBs).
Mono-ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more orthobromines) form the
third category. The congeners in the first category tend to elicit the
most severe effects, those in the second and third categories showing
less severe toxicological effects. Within the category, the degree of
bromination may also influence toxicity.
Of all the combinations tested, 3,3',4,4',5,5'-HxBB was found to be
the most toxic PBB. This congener is present in low concentrations as
a constituent of FireMaster(R). Of the major FireMaster(R)
constituents, 2,3,3',4,4',5-HxBB appeared to be the most toxic,
followed by 2,3',4,4',5,5'-HxBB and 2,3',4,4',5-PeBB. The main
component of the FireMaster(R) mixture, 2,2',4,4',5,5'-HxBB was
relatively non-toxic, as was 2,2',3,4,4',5,5'-HpBB, the second most
abundant constituent.
Technical OcBB and DeBB contain various amounts of other brominated
biphenyls. Information on other contaminants is lacking.
The common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions to the technical PBB
mixtures tested (OcBB and DeBB). However, hyperkeratosis and hair loss
were seen in cattle, and lesions resembling chloracne were seen in
rhesus monkeys following the ingestion of a FireMaster(R) mixture.
Hyperkeratosis of the inner surface of the rabbit ear was produced by
FireMaster, but not by its main components (2,2',4,4',5,5'-HxBB and
2,2',3,4,4',5,5'-HpBB). Fractionation of FireMaster(R) revealed that
most activity was associated with the more polar fractions containing
minor components. Treatment with sunlight-irradiated HxBB caused
severe hyperkeratosis in rabbit ears.
In rats, low-dose, long-term feeding of technical OcBB did not affect
food consumption and body weight, but an increase in the relative
liver weights of exposed rats was found at 2.5 mg/kg body weight for
7 months. Long-term feeding of FireMaster(R) to rats at doses of
10 mg/kg body weight for 6 months did not affect food consumption.
Doses of 1 mg/kg body weight for a 6-month period affected liver
weight. The thymus weight was decreased by a dose of 0.3 mg/kg body
weight in female rats. Histopathological changes were also noted.
Controlled long-term feeding studies in cattle exposed to low doses of
FireMaster(R) showed no adverse effects, as indicated by food intake,
clinical signs, clinicopathological changes, or performance. Minks,
guinea-pigs, and monkeys appeared to be more susceptible to PBB
toxicity.
Long-term effects have been recorded in rats related to pronounced
retention of the administered PBBs following pre- or perinatal
exposure to high doses of FireMaster(R).
The most common adverse effects on reproduction were fetal wastage and
a decrease in viability of the offspring. Some effects were still
noted in mink at concentrations of 1 mg/kg diet. Following a
12.5-month exposure to FireMaster(R) (0.3 mg/kg diet), a minor
decrease was observed in the viability of the offspring of rhesus
monkeys. The monkeys received a daily dose of 0.01 mg/kg body weight
and a total dose of 3.8 mg/kg body weight. Reproduction and
neurobehavioural studies in monkeys and rats with a low level of
exposure could not be appropriately evaluated since insufficient
information was given in the published papers on the experimental
design of the study. A weak teratogenic potential was seen in rodents
at high doses that may have caused some maternal toxicity.
PBBs interact with the endocrine system. Rats and pigs were found to
have dose-related decreases in serum thyroxine and triiodothyronine.
PBBs have also been reported to affect the levels of steroid hormones;
the extent depends on the species as well as the dose and the period
of administration.
PBBs produce porphyria in rats and male mice at doses as low as
0.3 mg/kg body weight per day; the no-effect level was 0.1 mg/kg per
day. PBBs had a pronounced influence on vitamin A storage, as well as
effects on the intermediary metabolism.
Atrophy of the thymus was a frequent observation following PBB
exposure. Other lymphoid tissues have also been affected.
FireMaster(R) also induced further indications of a suppressed immune
function. Data is not available concerning the effects of OcBB, DeBB,
or individual PBB congeners.
One of the most intensively studied effects of PBBs is their induction
of mixed function oxidase (MFO) enzymes. Consistently, FireMaster(R)
was found to be a mixed-type inducer of hepatic microsomal enzymes in
rats, as well as in all other animal species tested. Induction was
also found to a lesser extent in other tissues. The ability to induce
hepatic microsomal enzymes differed for individual PBB congeners.
Correlations between structure and microsomal enzyme-inducing activity
have been demonstrated.
Several studies have shown that PBBs are able to alter the biological
activity of a variety of drugs and toxicants. This may be due partly
to the ability of PBBs to induce the microsomal enzymes involved in
activation or deactivation of xenobiotics.
The FireMaster(R) mixture, and some of its major components, were
found to be capable of inhibiting intercellular communication in
vitro. This inhibition occurs at non-cytotoxic concentrations. Both
the cytotoxicity and the metabolic cooperation-inhibiting properties
of PBB congeners seem to be related to their structure, i.e., presence
or lack of ortho-substitution. In vitro and in vivo assays
(microbial and mammalian cell mutagenesis, mammalian cell chromosomal
damage, mammalian cell transformation, and DNA damage and repair
(UDS)) have failed to indicate any mutagenicity or genotoxicity of
individual PBB congeners or commercial mixtures.
Long-term carcinogenicity studies with PBBs have shown that the
principal site of tumours is the liver. The incidences of
hepatocellular carcinoma were significantly increased in both sexes of
mice and rats receiving oral doses of the FireMaster(R) mixture.
Bromkal 80-9 (technical nonabromobiphenyl) induced carcinogenic
effects in the liver in mice receiving diets containing 100 mg/kg diet
(5 mg/kg per day), or more, for 18 months. The lowest dose of PBB
that produced tumours (mostly adenomas) in rodents was 0.5 mg/kg body
weight per day for 2 years. The rats receiving 0.15 mg/kg in addition
to pre- and perinatal exposure did not show any adverse effects. The
carcinogenicity of technical octabromobiphenyl and decabromobiphenyl
has not been studied.
Neither FireMaster BP-6 nor 2,2',4,4',5,5'-hexabromobiphenyl showed
tumour-initiating (using TPA as promoter) or tumour-promoting (using
DMBA as initiator) activity in a mouse skin bioassay. However, in
other mouse skin models (using DMBA or MNNG as initiators), both
FireMaster FF-1 and 3,3',4,4',5,5'-hexabromobiphenyl, but not
2,2',4,4',5,5'-hexabromobiphenyl, showed tumour-promoting activity.
In a two-stage rat liver bioassay using phenobarbital as promoter,
3,3',4,4'-tetrabromobiphenyl showed a weak initiating activity. In
the two-stage rat liver model, using diethylnitrosamine and partial
hepatectomy, FireMaster, 3,3',4,4'-tetrabromobiphenyl, and
2,2',4,4',5,5'-hexabromobiphenyl showed tumour-promoting activity, but
3,3,',4,4',5,5'-hexabromobiphenyl did not.
The results of the studies on cell communication, the negative results
on genotoxicity and mutagenicity, and the results of tumour-promotion
assays indicate that the mixtures and congeners studied cause cancer
by epigenetic mechanisms. No information is available on technical
octa-, nona-, or decabromobiphenyl.
The mechanisms of action underlying the many manifestations of the
toxicity of PBBs and related compounds are not known. However, some of
the effects, such as the wasting syndrome, thymus atrophy,
hepatotoxicity, skin disorders, and the reproductive toxicity, may be
related to interaction with the so-called Ah- or TCDD-receptors
causing alteration in the expression of a number of genes. Different
PBB congeners vary in their interaction with the receptor, the
coplanar congeners being more active.
Many of the PBB effects are seen after long-lasting exposure. The
reason for this observation may be the pronounced accumulation of some
PBB congeners and the poor ability of the body to metabolize and
eliminate them. This results in a build-up of the chemical in the
body which overcomes the compensatory mechanisms and leads to adverse
effects.
Some polybrominated naphthalenes (PBNs), known contaminants of the
FireMaster(R) mixture, are potent toxicants and teratogens. Although
PBNs are only present at low levels in the FireMaster(R) mixture, it
is possible they may contribute to its toxicity.
Studies of the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-HxBB, showed that the photolysis products were more
toxic than the original PBB. Under some conditions of pyrolysis of
FireMaster(R), polybrominated dibenzofurans are formed. The pyrolysis
products of technical OcBB caused liver enlargement.
2.7 Effects on Humans
There have been no examples of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures following
the poisoning incident in Michigan, USA, in 1973. The main
epidemiological studies were conducted by the Michigan Department of
Public Health (MDPH) and the Environmental Science Laboratory, Mount
Sinai School of Medicine, New York (ESL).
It was estimated that the most highly exposed people consumed 5-15 g
PBB in milk over a 230-day period. Some additional exposure may have
occurred by consuming meat. The exposure in some of the farmers, and
most of the general population, in Michigan was much lower. For them
the total exposure was 9-10 mg. However, some people in this group
may have received a total exposure of about 800-900 mg. A total dose
of 9 mg corresponds to 0.15 mg/kg body weight, and 900 mg to 15 mg/kg
body weight for a 60-kg average adult; the dose/kg body weight will be
higher for children.
In 1974, the first MDPH study compared the health status of people on
quarantined farms with people on non-quarantined farms in the same
area. Although a variety of symptoms were reported by both groups,
there was no pattern to the differences between the groups. The
heart, liver, spleen, nervous system, urinalysis, blood counts, and
all other medical conditions examined were found to be normal. In a
later, comprehensive, MDPH study including groups with different
levels of exposure, there was no positive association between serum
concentrations of PBB and reported symptoms or disease frequencies.
The ESL studies involved about 990 farm residents, 55 chemical
workers, and a group of Wisconsin dairy farmers who were used as a
control group. The prevalence of symptoms in Michigan farmers was
greater than the prevalence in Wisconsin farmers. The greatest
differences were in the broad classification of neurological and
musculoskeletal symptoms. Elevated serum concentrations of some liver
enzymes and carcinoembryonic antigen were more prevalent in Michigan
farmers than in Wisconsin farmers. Chemical workers had a higher
prevalence of chest and skin symptoms and a lower prevalence of
musculoskeletal symptoms than farmers. Although the results of the ESL
studies were at times interpreted differently from the results of
comparable studies, there was one area of consistent agreement. None
of the sets of studies demonstrated a positive dose-response
relationship between PBB levels in serum or in adipose tissue and the
prevalence of symptoms or abnormal clinical measurements. Several
clinical areas were investigated by more intensive special studies.
Examination of the neurological aspects by means of objective
performance tests revealed, in one study, a negative correlation
between serum PBB levels and performance test scores, particularly in
males in the older age groups. The other studies showed no association
between the concentration of PBB in serum or fat and performance in a
battery of tests measuring memory, motor strength, coordination,
cortical-sensory perception, personality, higher cognitive
functioning, and other functions. The paediatric aspects of PBB
exposure were examined in the families in the ESL studies. Although
many symptoms were reported, physical examination failed to reveal any
objective alteration that could be attributed to PBB. There were
different views about the more subtle neuropsychological effects in
the offspring, and the results of investigations of developmental
abilities also remain controversial. The same is true for the
investigation of lymphocyte and immune function. One set of authors
found no differences in lymphocyte count or functions between groups
with high and low serum PBB levels, the other found a significant
decrease in T and B lymphocyte subpopulations in about 40% of an
exposed Michigan group compared with unexposed groups, and also
impaired lymphocyte function, i.e., decreased response to mitogens.
The epidemiological studies reviewed were planned to evaluate the
relationship between PBB exposure and a large number of adverse
effects, including behavioural effects and subjective complaints.
However, most of the studies suffer from major faults in design that
introduced confounders which make it difficult or impossible to draw
valid conclusions. The follow-up time has not been long enough to
evaluate a possible carcinogenic effect.
Two small groups of workers were identified with occupational exposure
to a mixture of PBBs, or to DeBB and DBBO. Lesions resembling
chloracne were found in 13% of the workers exposed to the PBB mixture,
but such lesions were not seen in the workers exposed to DeBB.
However, a significantly higher prevalence of hypothyroidism was seen
in the latter group.
2.8 Overall Evaluation of the Toxicity
The only lifetime and carcinogenicity study with a PBB mixture was
conducted in rats and mice in a recent NTP bioassay. The lowest dose
tested that still produced carcinogenic effects was 0.5 mg/kg body
weight per day (liver tumours in rodents). In other carcinogenicity
studies, 3 mg/kg body weight given for 6 months resulted in a
carcinogenic response. The 6-month study demonstrates that a less
than lifetime exposure at similar doses will also result in similar
adverse effects. Effects on reproduction in subhuman primates and
mink may occur at somewhat lower doses.
In addition, in the 2-year NTP study, a daily dose of 0.15 mg/kg body
weight per day and prenatal and perinatal exposure of the dam to
0.05 mg/kg body weight per day did not result in any adverse effects.
Thus, the total daily intake from food, water, air, and soil should be
less than 0.15 µg/kg body weight per day, extrapolating from the NOAEL
(no-observed-adverse-effect level) of a positive carcinogenicity
study, using an uncertainty (and safety) factor of 1000, since these
compounds probably induce cancer by an epigenetic mechanism.
The total dose received by the subpopulation in Michigan was estimated
to have ranged from 0.15 to 15 mg/kg body weight over a 230-day
period. For this population, dividing the doses over a lifetime for
the average human being would be equivalent to a daily dose ranging
from 6 ng to 0.6 µg/kg body weight per day.
Estimates for the general population indicate a total intake by adults
of 2 ng PBB/kg body weight per day from known sources, and an intake
of 10 ng/kg body weight per day for infants receiving breast milk. It
should be kept in mind that these estimates are based on a very
limited and regional data base.
These calculations assume that a steady state for PBBs would not be
reached over a lifetime, and that short-term, higher exposures can be
substituted for long-term, lower exposures, since these compounds are
extremely poorly metabolized and excreted.
Insufficient information is available for OcBB, NoBB, and DeBB to
calculate a total daily intake that would not result in adverse
effects.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Most of the PBB congeners found in commercial flame retardants are
lipophilic, persistent, and bioaccumulating. These compounds are
biomagnified in environmental food-webs and pose a particular threat
to organisms in the higher levels of these webs. Furthermore, some
PBB products form toxic polybrominated dibenzofurans during combustion
processes.
In addition to emissions during manufacture and use, PBB will enter
the environment from the widespread use of flame-retarded products. A
considerable part of the PBB produced will probably reach the
environment sooner or later because of the high stability of these
compounds.
PBBs are also found in environmental and human samples also from
places far from known point sources. The congener patterns in the
environmental samples do not match those found in the technical
products indicating environmental alteration, possibly photochemical
debromination.
Very little information is currently available on the degree of
exposure of the general population to PBBs. However, in the few
instances where measurements have been made, trace amounts of PBBs
were identified. At present, this exposure does not give rise to
concern but further build-up should be avoided. Human data from the
Michigan episode (see sections 2.3 and 2.7) suggest that exposures in
Michigan were several orders of magnitude greater than the exposure of
the general population. No definitive health effects could be
correlated with PBB exposure in the Michigan population, though the
follow-up period has not been long enough for the occurrence of cancer
to be evaluated. Since the PBB levels remain high in adipose tissue
and serum in the Michigan population, their internal exposure
continues. In contrast, in Michigan cattle toxic effects were
observed. This discrepancy is explained by differences in the degree
of exposure of the cattle.
Occupational exposure has been examined in only two plants in the USA.
It appears that chloracne-like lesions may develop in workers
producing PBB, and hypothyroidism in workers exposed to DBB. No
studies have been conducted in workers incorporating deca-, octa-, or
nonabromobiphenyl into commercial products.
PBBs are extremely persistent in living organisms and have been shown
to produce chronic toxic effects and cancer in animals. Though the
acute toxicity was low, cancer was induced at a dose of 0.5 mg/kg body
weight per day and the no-observed-effect level was 0.15 mg/kg body
weight per day. A number of chronic toxic effects have been observed
in experimental animals at doses around 1 mg/kg body weight per day
following long-term exposure.
3.2 Recommendations
General
* The Task Group is of the opinion that human beings and the
environment should not be exposed to PBBs, in view of their high
persistency and bioaccumulation and the potential adverse effects
at very low levels after long-term exposure. Therefore, PBBs
should no longer be used commercially.
* Because of the limited data on the toxicity of DeBB and OcBB,
their extreme persistence, and their potential for breakdown in
the environment and through combustion to more toxic persistent
compounds, they should also not be used commercially unless their
safety has been demonstrated.
Future work
* It is known that observations on the Michigan cohort are still
continuing. Publication of these data is required.
* Future human and environmental PBB monitoring should be expanded
and be congener specific, and include the OcBB, NoBB, and DeBB.
These compounds should be included in monitoring programmes for
other halogenated compounds.
* The time trends and geographical distribution of PBB levels in
the environment should continue to be monitored. The release of
PBBs into the environment from waste disposal sites should be
surveyed.
* Thermolysis experiments should be conducted to simulate the
conditions in accidental fires and municipal incinerators.
* Research should be continued on the mechanisms of toxicity and
carcinogenicity of PBBs and related compounds. PBBs may serve as
model compounds for mechanistic research. Purified congeners
should be used in these studies.
* The effects on reproduction have not been not well elucidated.
Therefore, well designed long-term reproductive studies should be
performed, at low doses, using a sensitive species.
* There is also a need for more information on the bioavailability
and toxicokinetics of OcBB/NoBB, DeBB and selected congeners.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
PBBs are highly brominated organic substances. They are very
persistent and can be hazardous for human beings if incorrectly or
carelessly handled. It is therefore essential that the correct
precautions are observed during handling and use.
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The acute oral and dermal toxicity is low. In occupational
conditions, skin itching, peeling, scaling and (halo) acne are found
after exposure to PBBs. Besides these dermal signs of intoxication,
liver disturbances, chest irritation, and neurological and unspecific
effects, such as depression, memory disturbances and nervousness, have
been reported, but not confirmed.
4.1.1.2 Medical advice
Medical treatment is symptomatic and supportive.
4.1.2 Health surveillance advice
A complete medical history of regularly exposed workers should be
taken, and physical examination made, on an annual basis, and
monitoring of PBB levels in blood serum should be done (frequency
depending on the results).
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
PBBs are not flammable; on the contrary, they are flame retardants.
However, on heating, toxic fumes, such as hydrogen bromide and
brominated dibenzofurans may be formed.
4.2.2 Fire hazards
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. Fire-fighters should be equipped with self-contained
breathing apparatus, eye protection, and full protective clothing.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding the accumulation of polluted run-off from the
site.
4.3 Storage
Products should be stored in locked buildings, out of reach of
animals, children, and unauthorized personnel. Do not store near
foodstuffs or animal feed.
4.4 Transport
Comply with any local requirements regarding movements of hazardous
goods. Do not transport in the same compartment as foodstuffs. Check
that containers are sound and labels undamaged before despatch.
4.5 Spillage
Before dealing with any spillage, precautions should be taken as
required, and appropriate personal protection should be used. Prevent
material from spreading or contaminating other cargo, vegetation, or
waterways, by making a barrier of the most suitable available
material, e.g., earth or sand.
Empty any product remaining in damaged/leaking containers into a clean
empty drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
Absorb spilled liquid with sawdust, sand, or earth, sweep up and place
the sweepings in a closeable container for later transfer to a safe
place for disposal.
As soon as possible after the spillage and before reuse, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place the sweepings in a closeable container for later transfer to a
safe place for disposal. Care should be taken to avoid run-off into
watercourses.
4.6 Disposal
Any surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Waste material should be burned in
a proper incinerator designed for organohalogen waste disposal with
effluent-gas scrubbing. For PBB wastes, incineration must be for more
than 2 seconds at 1200 °C or higher. Combustion of PBBs at lower
temperatures can produce brominated dibenzofurans. If high
temperature incineration is not possible, bury waste in an approved
dump or landfill where there is no risk of contamination of surface or
groundwater. Decomposition of PBBs will be extremely slow.
Comply with any local legislation regarding disposal of toxic wastes.
Puncture container to prevent reuse.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
PBBs are very resistant to degradation and very persistent in the
environment. Because of their high solubility in fat, they
bioaccumulate, especially in the fatty tissues of all living
organisms, and biomagnify in the higher trophic levels of the food
chain. Although their acute toxicity is relatively low, this
bioaccumulation and biomagnification may lead to toxic effects.
Industrial discharges occurring during manufacture, formulation, or
technical applications should be treated properly and should not be
allowed to pollute the environment. Any spillage or unused product
should be treated and disposed of properly (see section 4.5 and 4.6)
and should be prevented from spreading to the environment.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. A full reference to the
original national document from which the information was extracted
can be obtained from IRPTC.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluation by International Bodies
The International Agency for Research on Cancer (IARC) evaluated
polybrominated biphenyls in 1986 and 1987 and concluded that there was
inadequate evidence of their carcinogenicity in humans, but sufficient
evidence of their carcinogenicity in experimental animals (Group 2B).
6.2 Exposure Limit Values
No exposure limit values for PBBs were found.
6.3 Specific Restrictions
In the USA, all use of hexabromobiphenyls, the main PBB isomer used in
industrial processes, was discontinued in 1974, because of the hazard
to human health discovered after its accidental misuse in Michigan in
1973. The US Environmental Protection Agency has since required
notification regarding any manufacture or importation of PBBs. The
purpose of this requirement is to confirm that there are no
significant sources of these substances and to ensure that EPA has the
opportunity to investigate the circumstances of any resumption of
production.
In Canada, all commercial, manufacturing, and processing uses are
banned.
In the countries of the EEC, PBBs may not be used in textile articles,
such as garments, undergarments and linen intended to come into
contact with the skin.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies PBBs in:
Hazard Class 9: miscellaneous dangerous substance
Packing Group II: substances presenting medium danger
The European Community legislation requires the labelling of PBBs as
harmful substances using the symbol.
 The label must read:
Danger of cumulative effects. This material and its container
must be disposed of in a safe way. It should be stated on the
label whether the substance is a specific isomer or a mixture of
isomers.
6.5 Waste Disposal
No waste disposal regulations for PBBs were found.
BIBLIOGRAPHY
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E. (1984)
Clinical toxicology of commercial products. 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyon, International Agency
for Research on Cancer.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances (unpublished
file). Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1991) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by Governments. 4th Ed. New York, United
Nations.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
WHO (In Press, 1993) Environmental health criteria 152.
Polybrominated biphenyls (PBBs). Geneva, World Health Organization.
The label must read:
Danger of cumulative effects. This material and its container
must be disposed of in a safe way. It should be stated on the
label whether the substance is a specific isomer or a mixture of
isomers.
6.5 Waste Disposal
No waste disposal regulations for PBBs were found.
BIBLIOGRAPHY
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E. (1984)
Clinical toxicology of commercial products. 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyon, International Agency
for Research on Cancer.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances (unpublished
file). Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1991) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by Governments. 4th Ed. New York, United
Nations.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
WHO (In Press, 1993) Environmental health criteria 152.
Polybrominated biphenyls (PBBs). Geneva, World Health Organization.
 Molecular weight: 154.21 + 78.90 (x+y)
Common trade names: Adine 0102; Berkflam B10; Bromkal 80-9D;
FireMaster BP-6; FireMaster FF-1;
Flammex B10; HFO 101; Octabromobiphenyl
FR 250 BA; Octabromobiphenyl XN-1902.
Composition of technical products
PBBs synthesized for commercial use as fire retardants contain a
mixture of various brominated biphenyls, chiefly the hexa-, octa-,
nona-, and decabromobiphenyls, as well as small amounts of related
brominated substances.
1.2 Physical and Chemical Properties
PBBs are solids with a low volatility that decreases with an increase
in the number of bromine atoms. PBBs are virtually insoluble in water,
soluble in fat and slightly to highly soluble in various organic
solvents, the solubility also decreasing with an increase in the
number of bromine atoms. These compounds are relatively stable and
chemically unreactive, though highly brominated PBB mixtures degrade
rather rapidly on exposure to ultraviolet radiation.
The technical mixtures are typically white, off-white, or beige
powders.
1.3 Analysis
Gas chromatography with electron-capture or mass-spectrometric
detection are useful techniques for determining PBB levels.
High-resolution gas chromatography is preferred as this makes it
possible to determine specific congeners, but reference compounds are
available for only a few of the 209 possible PBB congeners.
1.4 Production and Uses
The commercial production of PBBs (as FireMaster(R)) was started in
the USA in 1970. After the production of about 6000 tonnes, further
production was virtually discontinued in 1974 after the inadvertent
addition of PBBs to animal feed. This resulted in the contamination of
farm animals and their subsequent massive destruction. Approximately
400 tonnes of octa- and decabromobiphenyl were produced in the USA
until 1979. Bromkal 80-9D was produced in Germany until 1985; a few
hundred tonnes per year of Adine 0102 are currently being produced in
France; in 1977, production of decabromobiphenyl ceased in the United
Kingdom.
PBBs are mainly used as flame retardants in moulded thermoplastics,
mostly in small appliances and automotive applications. Earlier
applications in synthetic fibres have been discontinued.
1.5 Thermal Decomposition
The products of the thermal decomposition of PBBs depend on the
temperature, the amount of oxygen present, and a number of other
factors. Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen (600-900 °C) have shown that bromobenzenes and lower
brominated biphenyls are formed, but no polybrominated furans. In
contrast, pyrolysis in the presence of oxygen (700-900 °C) yielded
some di- to heptabromodibenzofurans. In the presence of polystyrene
and polyethylene, higher levels were found. Pyrolysis of FireMaster
BP-6 with PVC at 800 °C yielded mixed bromochlorobiphenyls. There is
no information on the nature of the products of incineration of
PBB-containing compounds. Little is known about the toxicity of
brominated and brominated/chlorinated dioxins and furans, but their
toxicity is estimated to be of about the same order as that of
chlorinated dioxins and furans.
2. SUMMARY AND EVALUATION
2.1 Sources of Human and Environmental Exposure
PBBs were introduced as flame retardants in the early 1970s. Prior to
November 1974, hexabromobiphenyl was the most commercially significant
PBB in the USA and was incorporated into ABS (acrylonitrile-butadiene-
styrene) plastics (PBB content 10% - mainly small appliance and
automotive applications), coatings and lacquers, and polyurethane
foam. The other PBB flame retardants have similar uses.
Losses of PBB into the environment during normal production can occur
through emission into the air and waste waters, and through losses
into the soil and into landfills, but these quantities have been found
to be generally low. These chemicals can also enter the environment
during shipping and handling, and following accidents, as occurred in
Michigan, USA, in 1973.
PBBs can also enter the environment as a result of the incineration of
materials containing PBB, as well as during accidental fires, with the
formation of other toxic chemicals, such as polybromodibenzofurans or
mixed bromochloro derivatives.
Most of the total production of these compounds will ultimately enter
the environment, either unchanged or as breakdown products.
2.2 Environmental Transport, Distribution, and Transformation
Long-range transport of PBB in the atmosphere has not been proven, but
the presence of these compounds in Arctic seal samples indicates a
wide geographical distribution.
The main route by which PBBs enter the aquatic environment is in
industrial waste discharges and leachates from industrial dumping
sites, as well as by erosion of polluted soils. PBBs are almost
insoluble in water and are primarily found in the sediments of
polluted lakes and rivers.
Pollution of the soil can originate from point sources such as a PBB
production plant or a waste dump. Once introduced into the soil, PBBs
do not appear to be translocated readily. PBBs have been found to be
200 times more soluble in a landfill leachate than in distilled water;
this may result in a wider distribution in the environment. The
hydrophobic properties of PBBs make them easily adsorbed, from aqueous
solutions, on to soil particles. Preferential adsorption of PBB
congeners has been noted depending on the characteristics of the soil
(e.g., organic matter content) as well as on the degree and position
of the bromine substitution.
PBBs are stable and persistent, lipophilic, and only slightly soluble
in water; some of the congeners are poorly metabolized and therefore
accumulate in the lipid compartments of biota. Once they have been
released into the environment, they can reach the food chain, where
they are accumulated.
PBBs have been detected in fish from several regions. The ingestion
of fish is a means of PBB transfer to mammals and birds. PBBs have
also been detected in ducks, a turtle, in the eggs of water birds, as
well as in the fat of deer, rabbits, coyote, ravens, reindeer, seals,
and osprey.
The degradation of PBBs by purely abiotic chemical reactions
(excluding photochemical reactions) is considered unlikely. PBBs have
been reported to be persistent under field conditions. Soil samples
from a former PBB manufacturing site, analysed several years after the
Michigan incident, still contained PBBs, though the congener
composition was different, indicating partial degradation of the PBB
residue in the soil sample.
Under laboratory conditions, PBBs are easily degraded by ultraviolet
radiation. Photodegradation of the commercial FireMaster(R) mixture
led to decreased concentrations of the more highly substituted PBB
congeners. The rates and extent of the photolytic reactions of PBBs in
the environment have not been determined in detail, though field
observations indicate high persistence of the original PBBs or partial
degradation to less brominated compounds.
In laboratory investigations, mixtures of PBB appear to be fairly
resistant to microbial degradation.
No uptake or degradation of PBBs by plants has been recorded. In
contrast, PBBs are easily absorbed by animals, and although they were
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs. No investigations of sulfur-containing
metabolites analogous to those of PCBs have been reported.
The bioaccumulation of PBBs in fish has been investigated.
Bioaccumulation of PBBs in terrestrial animals has been investigated
for avian and mammalian species. Data were obtained through field
observations, evaluation of the Michigan disaster, and through
controlled feeding studies. Generally, the accumulation of PBBs in
body fat depended on dosage and duration of exposure.
For the individual congeners of PBB, bioaccumulation has been found to
increase with degree of bromination, up to at least the
tetrabromobiphenyls. Higher brominated congeners can be expected to
accumulate to an even higher degree. However, no such information is
available for decabromobiphenyl (DeBB). It is possible that DeBB is
poorly absorbed.
2.3 Environmental Levels and Human Exposure
There is only one report on PBB levels in air, the concentrations
being measured in the vicinity of three PBB-manufacturing or
PBB-processing plants in the USA.
PBB levels in surface waters were monitored in the same vicinity, as
well in the area of the Gratiot County landfill (Michigan, USA), which
received more than 100 tonnes of waste between 1971 and 1973,
containing 60-70% PBB.
Groundwater monitoring data from Gratiot County have shown trace
levels of PBBs even outside the landfill area; however, PBBs were not
detected in drinking-water wells in the area.
Data on soil pollution by PBBs are available for areas of manufacture,
use, or disposal, and for soils from fields of the PBB-contaminated
Michigan farms.
In the Michigan disaster, FireMaster(R) was inadvertently added to
animal feed. It was almost a year later that the mixing error was
discovered and the analyses indicated that PBBs were responsible.
During this time (summer 1973-May 1974), contaminated animals and
their produce entered the human food supply, and the environment of
the State of Michigan. Hundreds of farms were affected and thousands
of animals had to be slaughtered and buried, as well as thousands of
tonnes of farm produce.
Most data available on the PBB contamination of wildlife refer to fish
and birds, primarily water-fowl, in the vicinity of industrial sites,
and to marine mammals.
Recent reports on the PBB contamination of fish, terrestrial and
marine mammals, and birds in Europe and USA indicate a wide
distribution of these compounds. The congener pattern found in fish
samples is quite different from that found in commercial products.
Many of the main compounds found could well be the result of
photochemical debromination of decabromobiphenyl (BB 209), but this
has not been confirmed.
Occupational exposure has been found in employees of US chemical
plants, and in farm workers, as a result of the Michigan PBB incident.
Median levels of PBB in serum and adipose tissue were higher among
chemical workers. Information is not available on occupational
exposure associated with manufacturing, formulation, and commercial
uses from other companies or countries.
For most human populations, the exposure to PBBs from various sources
has not been measured directly. Widespread human exposure has been
reported from Michigan, USA, as a result of direct contact with
contaminated feed, but mainly from consumption of PBBs in meat, eggs,
and dairy products. At least 2000 families (mainly farmers and their
neighbours) were exposed to high levels of PBBs. Recently, PBBs have
been detected in cow's and human milk in Germany.
The congener patterns in these human samples were different from those
found in fish. The concentration of BB 153 was higher in the human
milk than in fish.
The routes by which the general population is exposed to PBBs are not
well known. Present knowledge indicates that the ambient air and
water do not contain high levels. Lipid-rich food, especially from
contaminated waters, is probably of great importance. There is no
information on the level of exposure from indoor air or on dermal
exposure from materials containing PBB flame retardants.
The PBB congener pattern found in human milk samples collected in
Germany resembles that found in cow's milk from the same region, but
the levels in the human samples were substantially higher.
An estimate of daily intake of PBB for the general population from
food has to be based on very few data. If fish is assumed to contain
20 µg PBB/kg lipid and 5% lipid and that a 60-kg person eats 100 g
fish/day, the intake will be 0.002 µg/kg body weight per day. A
PBB concentration of 0.05 µg/kg lipid in milk and a milk consumption
of 500 ml/day will give the same person a PBB intake of about
0.00002 µg/kg body weight per day.
An infant of 6 kg body weight consuming 800 ml breast milk (3.5%
lipid) per day will ingest 0.01 µg PBB/kg body weight per day, if the
milk contains 2 µg PBB/kg lipid.
2.4 Kinetics and Metabolism
Gastrointestinal absorption of PBBs varies according to the degree of
bromination, the lower brominated compounds being more easily
absorbed. There is inadequate information concerning the absorption
of DeBB and OcBB.
PBBs are distributed throughout the entire body of animal species and
human beings, with highest equilibrium concentrations being found in
the adipose tissues. Relatively high levels are also found in the
liver; in particular, the more toxic congeners appear to be
concentrated in the liver. The partitioning of the various PBB
congeners appears to differ in different tissues. Generally, there is
a marked tendency for bioaccumulation. In mammals, transfer of PBBs to
offspring occurs through the transplacental and milk routes. Human
breast milk was found to have levels of 2,2',4,4',5,5'-
hexabromobiphenyl, more than 100 times higher than the maternal serum.
During a multigeneration study on rats, administration of PBBs to one
generation resulted in detectable residues in more than two subsequent
generations. Eggs of avian species are also affected by the maternal
PBB body burden.
Many PBB congeners are persistent in biological systems. There was no
evidence of significant metabolism or excretion of the more abundant
components of the FireMaster(R) mixture, or of octa- or
decabromobiphenyl. In vitro studies showed that the metabolism of
PBBs is affected by the structure-activity relationship. PBBs were
metabolized by phenobarbital-induced microsomes only if they possessed
adjacent non-brominated carbons meta and para to the biphenyl bridge
on at least one ring. Metabolism by 3-methylcholanthrene-induced
microsomes required adjacent non-brominated ortho and meta positions
on at least one ring of lower substituted congeners; a higher degree
of bromination appeared to prevent metabolism. Hydroxylated
derivatives have been identified in vertebrates as major in vitro
and in vivo metabolism products of lower brominated biphenyls. The
metabolic yield was relatively low. The hydroxylation reaction
probably proceeds via both arene oxide intermediates and by direct
hydroxylation.
Humans, rats, rhesus monkeys, pigs, cows, and chickens eliminate PBBs
mainly in the faeces. In most cases, elimination rates seem to be
slow. Concentrations of 2,2',4,4',5,5'-hexabromobiphenyl observed in
the bile and faeces of humans were about 1/2 to 7/10 of the serum
levels, or approximately 0.5% of the adipose levels. Treatment to
increase elimination of PBB in animals or humans was usually
unsuccessful. Another pathway of elimination could be excretion in
milk.
Complex and varied relationships were found among PBB tissue
concentrations with time after PBB administration to rats or other
animals. They are described by several compartmental models. A
half-life of approximately 69 weeks was calculated for the elimination
of 2,2',4,4',5,5'-hexabromobiphenyl from body fat of the rats; this
period increased to more than 4 years for rhesus monkeys. The average
half-life of 2,2',4,4',5,5'-hexabromobiphenyl in humans has been
estimated to be between 7.8 and 12 years; periods ranging from 4.6 to
94.7 years have been suggested in the literature. Some differences in
retention and turnover have been demonstrated between individual PBB
congeners. The results of analyses of serum from farmers and chemical
workers for 2,3',4,4',5-pentabromobiphenyl have been inconsistent.
This inconsistency is probably due to the different sources of
exposure. The workers were exposed to all compounds of FireMaster(R),
while the Michigan population was exposed to contaminated meat and
milk containing a different mixture of PBBs as a result of metabolic
processes in farm animals. Bromine levels did not decrease in the
adipose tissue of rats when they were given technical grade
octabromobiphenyl. No information is available on the retention of
decabromobiphenyl.
Humans may have a greater tendency to retain certain PBB congeners
than experimental animals. This factor should be taken into
consideration in evaluating the human health hazards associated with
these chemicals.
In conclusion, all the available data indicate that PBBs have a marked
tendency to bioaccumulate and persist. Metabolism is poor and the
half-life in man is in the order of 8-12 years, or longer.
2.5 Effects on Organisms in the Environment
Only few data are available on the effects of PBBs on organisms in the
environment. They refer to microorganisms, water fleas, water birds,
and farm animals.
Water birds nesting on islands in northwestern Lake Michigan were
studied to determine whether environmental contaminants were affecting
their reproduction. Seventeen contaminants, including PBB, were
measured, but none seemed to have a pronounced effect on reproduction.
Farm animals that ingested feed inadvertently containing Firemaster(R)
FF-1, in place of magnesium oxide, became sick. The estimated average
exposure of cows on the first identified, highly contaminated farm was
250 mg/kg body weight. The clinical signs of toxicity were a 50%
reduction in feed consumption (anorexia) and a 40% decrease in milk
production a few weeks after ingestion of the contaminated feed.
Although the supplemented feed was discontinued within 16 days, milk
production did not recover. Some animals showed an increased frequency
of urination and increased lacrimation, and developed haematomas,
abscesses, abnormal hoof growth, lameness, alopecia, hyperkeratosis,
and cachexia, and several died within 6 months of exposure.
Altogether, the death rate on this farm was 24 out of 400. The death
rate of 6-18-month-old calves was much higher. About 50% died within 6
weeks, only 2 out of 12 surviving after 5 months. They developed
hyperkeratosis over their entire bodies. There were also a variety of
reproductive problems.
Necropsy findings have been reported for some of the mature cows that
died in the 6 months after exposure. Histopathological studies
revealed variable liver and kidney changes.
The clinical signs and pathological changes noted above were later
confirmed in controlled feeding studies (anorexia, dehydration,
excessive lacrimation, emaciation, hyperkeratosis, reproductive
difficulties, some clinical chemistry changes, and renal damage).
A drop in production and sterility were reported from herds with a low
level of contamination. This was in contrast to the results from
controlled studies which did not show significant differences between
herds with low-level contamination and control herds.
Although it was cattle feed that was originally contaminated, other
animal feeds became involved by cross-contamination, e.g., in the
machinery used to prepare other feeds. It is likely that the exposure
from these sources was not as high as that of the cattle. Although
other animals (poultry, pigs, horses, rabbits, goats, and sheep) were
reported as being exposed and were killed, details of ill effects were
not recorded.
No information is available on the effect of PBBs on ecosystems.
2.6 Effects on Experimental Animals and In Vitro Test Systems
Commercial mixtures show relatively low acute toxicity (LD50
>1 g/kg body weight) in rats, rabbits, and quails following oral or
dermal administration. Deaths and acute manifestations of toxicity
are delayed after administration of PBB. The total dose administered
determines the degree of toxicity, whether given as a single dose or
as repeated doses over short periods (up to 50 days). The toxicity of
PBBs is higher with multiple-dose rather than with single-dose
administration.
The few studies performed with commercial mixtures of octa- and
decabromobiphenyl resulted in no mortality of rats or fish. Of the
individual PBB congeners, only 3 hexa isomers have been tested,
3,3',4,4',5,5'-HxBB and 2,3',4,4',5,5'-HxBB being more toxic to rats
than 2,2',4,4',5,5'-HxBB. On the basis of limited data, OcBB and DeBB
appear to be less toxic than the PBB mixtures, and less well absorbed.
In many acute and short-term studies, signs of PBB (mostly FireMaster)
toxicity include reductions in feed consumption. At lethal doses, the
cause of death cannot be ascribed to pathology in a particular organ,
but rather the animals develop a "wasting syndrome" as the first
indication of toxicity. At death, the loss in body weight can be as
great as 30-40%. The few studies with technical OcBB and DeBB show no
such effects.
The morphological and histopathological changes due to PBB are most
prominent in the liver. Enlargement of the liver frequently occurs at
doses lower than those required to produce body weight changes. The
principal histopathological alterations in rodent species may consist
of extensive swelling and vacuolation of hepatocytes, proliferation of
the smooth endoplasmic reticulum, and single-cell necrosis. The
severity of the lesions depends on the dose and composition of the PBB
material given.
Decreases in thymus weights were observed in rats, mice, and cattle
after doses of FireMaster(R), but not after OcBB or DeBB.
After exposure of rats to low concentrations of PBBs, there have been
some reports of increase in thyroid weight and histological changes in
the thyroid.
It is evident that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological changes
in the liver and thymus. The halogenated biphenyls have been
categorized on the basis of structure. Category 1 comprises isomers
and congeners lacking orthosubstituents (coplanar PBBs).
Mono-ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more orthobromines) form the
third category. The congeners in the first category tend to elicit the
most severe effects, those in the second and third categories showing
less severe toxicological effects. Within the category, the degree of
bromination may also influence toxicity.
Of all the combinations tested, 3,3',4,4',5,5'-HxBB was found to be
the most toxic PBB. This congener is present in low concentrations as
a constituent of FireMaster(R). Of the major FireMaster(R)
constituents, 2,3,3',4,4',5-HxBB appeared to be the most toxic,
followed by 2,3',4,4',5,5'-HxBB and 2,3',4,4',5-PeBB. The main
component of the FireMaster(R) mixture, 2,2',4,4',5,5'-HxBB was
relatively non-toxic, as was 2,2',3,4,4',5,5'-HpBB, the second most
abundant constituent.
Technical OcBB and DeBB contain various amounts of other brominated
biphenyls. Information on other contaminants is lacking.
The common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions to the technical PBB
mixtures tested (OcBB and DeBB). However, hyperkeratosis and hair loss
were seen in cattle, and lesions resembling chloracne were seen in
rhesus monkeys following the ingestion of a FireMaster(R) mixture.
Hyperkeratosis of the inner surface of the rabbit ear was produced by
FireMaster, but not by its main components (2,2',4,4',5,5'-HxBB and
2,2',3,4,4',5,5'-HpBB). Fractionation of FireMaster(R) revealed that
most activity was associated with the more polar fractions containing
minor components. Treatment with sunlight-irradiated HxBB caused
severe hyperkeratosis in rabbit ears.
In rats, low-dose, long-term feeding of technical OcBB did not affect
food consumption and body weight, but an increase in the relative
liver weights of exposed rats was found at 2.5 mg/kg body weight for
7 months. Long-term feeding of FireMaster(R) to rats at doses of
10 mg/kg body weight for 6 months did not affect food consumption.
Doses of 1 mg/kg body weight for a 6-month period affected liver
weight. The thymus weight was decreased by a dose of 0.3 mg/kg body
weight in female rats. Histopathological changes were also noted.
Controlled long-term feeding studies in cattle exposed to low doses of
FireMaster(R) showed no adverse effects, as indicated by food intake,
clinical signs, clinicopathological changes, or performance. Minks,
guinea-pigs, and monkeys appeared to be more susceptible to PBB
toxicity.
Long-term effects have been recorded in rats related to pronounced
retention of the administered PBBs following pre- or perinatal
exposure to high doses of FireMaster(R).
The most common adverse effects on reproduction were fetal wastage and
a decrease in viability of the offspring. Some effects were still
noted in mink at concentrations of 1 mg/kg diet. Following a
12.5-month exposure to FireMaster(R) (0.3 mg/kg diet), a minor
decrease was observed in the viability of the offspring of rhesus
monkeys. The monkeys received a daily dose of 0.01 mg/kg body weight
and a total dose of 3.8 mg/kg body weight. Reproduction and
neurobehavioural studies in monkeys and rats with a low level of
exposure could not be appropriately evaluated since insufficient
information was given in the published papers on the experimental
design of the study. A weak teratogenic potential was seen in rodents
at high doses that may have caused some maternal toxicity.
PBBs interact with the endocrine system. Rats and pigs were found to
have dose-related decreases in serum thyroxine and triiodothyronine.
PBBs have also been reported to affect the levels of steroid hormones;
the extent depends on the species as well as the dose and the period
of administration.
PBBs produce porphyria in rats and male mice at doses as low as
0.3 mg/kg body weight per day; the no-effect level was 0.1 mg/kg per
day. PBBs had a pronounced influence on vitamin A storage, as well as
effects on the intermediary metabolism.
Atrophy of the thymus was a frequent observation following PBB
exposure. Other lymphoid tissues have also been affected.
FireMaster(R) also induced further indications of a suppressed immune
function. Data is not available concerning the effects of OcBB, DeBB,
or individual PBB congeners.
One of the most intensively studied effects of PBBs is their induction
of mixed function oxidase (MFO) enzymes. Consistently, FireMaster(R)
was found to be a mixed-type inducer of hepatic microsomal enzymes in
rats, as well as in all other animal species tested. Induction was
also found to a lesser extent in other tissues. The ability to induce
hepatic microsomal enzymes differed for individual PBB congeners.
Correlations between structure and microsomal enzyme-inducing activity
have been demonstrated.
Several studies have shown that PBBs are able to alter the biological
activity of a variety of drugs and toxicants. This may be due partly
to the ability of PBBs to induce the microsomal enzymes involved in
activation or deactivation of xenobiotics.
The FireMaster(R) mixture, and some of its major components, were
found to be capable of inhibiting intercellular communication in
vitro. This inhibition occurs at non-cytotoxic concentrations. Both
the cytotoxicity and the metabolic cooperation-inhibiting properties
of PBB congeners seem to be related to their structure, i.e., presence
or lack of ortho-substitution. In vitro and in vivo assays
(microbial and mammalian cell mutagenesis, mammalian cell chromosomal
damage, mammalian cell transformation, and DNA damage and repair
(UDS)) have failed to indicate any mutagenicity or genotoxicity of
individual PBB congeners or commercial mixtures.
Long-term carcinogenicity studies with PBBs have shown that the
principal site of tumours is the liver. The incidences of
hepatocellular carcinoma were significantly increased in both sexes of
mice and rats receiving oral doses of the FireMaster(R) mixture.
Bromkal 80-9 (technical nonabromobiphenyl) induced carcinogenic
effects in the liver in mice receiving diets containing 100 mg/kg diet
(5 mg/kg per day), or more, for 18 months. The lowest dose of PBB
that produced tumours (mostly adenomas) in rodents was 0.5 mg/kg body
weight per day for 2 years. The rats receiving 0.15 mg/kg in addition
to pre- and perinatal exposure did not show any adverse effects. The
carcinogenicity of technical octabromobiphenyl and decabromobiphenyl
has not been studied.
Neither FireMaster BP-6 nor 2,2',4,4',5,5'-hexabromobiphenyl showed
tumour-initiating (using TPA as promoter) or tumour-promoting (using
DMBA as initiator) activity in a mouse skin bioassay. However, in
other mouse skin models (using DMBA or MNNG as initiators), both
FireMaster FF-1 and 3,3',4,4',5,5'-hexabromobiphenyl, but not
2,2',4,4',5,5'-hexabromobiphenyl, showed tumour-promoting activity.
In a two-stage rat liver bioassay using phenobarbital as promoter,
3,3',4,4'-tetrabromobiphenyl showed a weak initiating activity. In
the two-stage rat liver model, using diethylnitrosamine and partial
hepatectomy, FireMaster, 3,3',4,4'-tetrabromobiphenyl, and
2,2',4,4',5,5'-hexabromobiphenyl showed tumour-promoting activity, but
3,3,',4,4',5,5'-hexabromobiphenyl did not.
The results of the studies on cell communication, the negative results
on genotoxicity and mutagenicity, and the results of tumour-promotion
assays indicate that the mixtures and congeners studied cause cancer
by epigenetic mechanisms. No information is available on technical
octa-, nona-, or decabromobiphenyl.
The mechanisms of action underlying the many manifestations of the
toxicity of PBBs and related compounds are not known. However, some of
the effects, such as the wasting syndrome, thymus atrophy,
hepatotoxicity, skin disorders, and the reproductive toxicity, may be
related to interaction with the so-called Ah- or TCDD-receptors
causing alteration in the expression of a number of genes. Different
PBB congeners vary in their interaction with the receptor, the
coplanar congeners being more active.
Many of the PBB effects are seen after long-lasting exposure. The
reason for this observation may be the pronounced accumulation of some
PBB congeners and the poor ability of the body to metabolize and
eliminate them. This results in a build-up of the chemical in the
body which overcomes the compensatory mechanisms and leads to adverse
effects.
Some polybrominated naphthalenes (PBNs), known contaminants of the
FireMaster(R) mixture, are potent toxicants and teratogens. Although
PBNs are only present at low levels in the FireMaster(R) mixture, it
is possible they may contribute to its toxicity.
Studies of the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-HxBB, showed that the photolysis products were more
toxic than the original PBB. Under some conditions of pyrolysis of
FireMaster(R), polybrominated dibenzofurans are formed. The pyrolysis
products of technical OcBB caused liver enlargement.
2.7 Effects on Humans
There have been no examples of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures following
the poisoning incident in Michigan, USA, in 1973. The main
epidemiological studies were conducted by the Michigan Department of
Public Health (MDPH) and the Environmental Science Laboratory, Mount
Sinai School of Medicine, New York (ESL).
It was estimated that the most highly exposed people consumed 5-15 g
PBB in milk over a 230-day period. Some additional exposure may have
occurred by consuming meat. The exposure in some of the farmers, and
most of the general population, in Michigan was much lower. For them
the total exposure was 9-10 mg. However, some people in this group
may have received a total exposure of about 800-900 mg. A total dose
of 9 mg corresponds to 0.15 mg/kg body weight, and 900 mg to 15 mg/kg
body weight for a 60-kg average adult; the dose/kg body weight will be
higher for children.
In 1974, the first MDPH study compared the health status of people on
quarantined farms with people on non-quarantined farms in the same
area. Although a variety of symptoms were reported by both groups,
there was no pattern to the differences between the groups. The
heart, liver, spleen, nervous system, urinalysis, blood counts, and
all other medical conditions examined were found to be normal. In a
later, comprehensive, MDPH study including groups with different
levels of exposure, there was no positive association between serum
concentrations of PBB and reported symptoms or disease frequencies.
The ESL studies involved about 990 farm residents, 55 chemical
workers, and a group of Wisconsin dairy farmers who were used as a
control group. The prevalence of symptoms in Michigan farmers was
greater than the prevalence in Wisconsin farmers. The greatest
differences were in the broad classification of neurological and
musculoskeletal symptoms. Elevated serum concentrations of some liver
enzymes and carcinoembryonic antigen were more prevalent in Michigan
farmers than in Wisconsin farmers. Chemical workers had a higher
prevalence of chest and skin symptoms and a lower prevalence of
musculoskeletal symptoms than farmers. Although the results of the ESL
studies were at times interpreted differently from the results of
comparable studies, there was one area of consistent agreement. None
of the sets of studies demonstrated a positive dose-response
relationship between PBB levels in serum or in adipose tissue and the
prevalence of symptoms or abnormal clinical measurements. Several
clinical areas were investigated by more intensive special studies.
Examination of the neurological aspects by means of objective
performance tests revealed, in one study, a negative correlation
between serum PBB levels and performance test scores, particularly in
males in the older age groups. The other studies showed no association
between the concentration of PBB in serum or fat and performance in a
battery of tests measuring memory, motor strength, coordination,
cortical-sensory perception, personality, higher cognitive
functioning, and other functions. The paediatric aspects of PBB
exposure were examined in the families in the ESL studies. Although
many symptoms were reported, physical examination failed to reveal any
objective alteration that could be attributed to PBB. There were
different views about the more subtle neuropsychological effects in
the offspring, and the results of investigations of developmental
abilities also remain controversial. The same is true for the
investigation of lymphocyte and immune function. One set of authors
found no differences in lymphocyte count or functions between groups
with high and low serum PBB levels, the other found a significant
decrease in T and B lymphocyte subpopulations in about 40% of an
exposed Michigan group compared with unexposed groups, and also
impaired lymphocyte function, i.e., decreased response to mitogens.
The epidemiological studies reviewed were planned to evaluate the
relationship between PBB exposure and a large number of adverse
effects, including behavioural effects and subjective complaints.
However, most of the studies suffer from major faults in design that
introduced confounders which make it difficult or impossible to draw
valid conclusions. The follow-up time has not been long enough to
evaluate a possible carcinogenic effect.
Two small groups of workers were identified with occupational exposure
to a mixture of PBBs, or to DeBB and DBBO. Lesions resembling
chloracne were found in 13% of the workers exposed to the PBB mixture,
but such lesions were not seen in the workers exposed to DeBB.
However, a significantly higher prevalence of hypothyroidism was seen
in the latter group.
2.8 Overall Evaluation of the Toxicity
The only lifetime and carcinogenicity study with a PBB mixture was
conducted in rats and mice in a recent NTP bioassay. The lowest dose
tested that still produced carcinogenic effects was 0.5 mg/kg body
weight per day (liver tumours in rodents). In other carcinogenicity
studies, 3 mg/kg body weight given for 6 months resulted in a
carcinogenic response. The 6-month study demonstrates that a less
than lifetime exposure at similar doses will also result in similar
adverse effects. Effects on reproduction in subhuman primates and
mink may occur at somewhat lower doses.
In addition, in the 2-year NTP study, a daily dose of 0.15 mg/kg body
weight per day and prenatal and perinatal exposure of the dam to
0.05 mg/kg body weight per day did not result in any adverse effects.
Thus, the total daily intake from food, water, air, and soil should be
less than 0.15 µg/kg body weight per day, extrapolating from the NOAEL
(no-observed-adverse-effect level) of a positive carcinogenicity
study, using an uncertainty (and safety) factor of 1000, since these
compounds probably induce cancer by an epigenetic mechanism.
The total dose received by the subpopulation in Michigan was estimated
to have ranged from 0.15 to 15 mg/kg body weight over a 230-day
period. For this population, dividing the doses over a lifetime for
the average human being would be equivalent to a daily dose ranging
from 6 ng to 0.6 µg/kg body weight per day.
Estimates for the general population indicate a total intake by adults
of 2 ng PBB/kg body weight per day from known sources, and an intake
of 10 ng/kg body weight per day for infants receiving breast milk. It
should be kept in mind that these estimates are based on a very
limited and regional data base.
These calculations assume that a steady state for PBBs would not be
reached over a lifetime, and that short-term, higher exposures can be
substituted for long-term, lower exposures, since these compounds are
extremely poorly metabolized and excreted.
Insufficient information is available for OcBB, NoBB, and DeBB to
calculate a total daily intake that would not result in adverse
effects.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Most of the PBB congeners found in commercial flame retardants are
lipophilic, persistent, and bioaccumulating. These compounds are
biomagnified in environmental food-webs and pose a particular threat
to organisms in the higher levels of these webs. Furthermore, some
PBB products form toxic polybrominated dibenzofurans during combustion
processes.
In addition to emissions during manufacture and use, PBB will enter
the environment from the widespread use of flame-retarded products. A
considerable part of the PBB produced will probably reach the
environment sooner or later because of the high stability of these
compounds.
PBBs are also found in environmental and human samples also from
places far from known point sources. The congener patterns in the
environmental samples do not match those found in the technical
products indicating environmental alteration, possibly photochemical
debromination.
Very little information is currently available on the degree of
exposure of the general population to PBBs. However, in the few
instances where measurements have been made, trace amounts of PBBs
were identified. At present, this exposure does not give rise to
concern but further build-up should be avoided. Human data from the
Michigan episode (see sections 2.3 and 2.7) suggest that exposures in
Michigan were several orders of magnitude greater than the exposure of
the general population. No definitive health effects could be
correlated with PBB exposure in the Michigan population, though the
follow-up period has not been long enough for the occurrence of cancer
to be evaluated. Since the PBB levels remain high in adipose tissue
and serum in the Michigan population, their internal exposure
continues. In contrast, in Michigan cattle toxic effects were
observed. This discrepancy is explained by differences in the degree
of exposure of the cattle.
Occupational exposure has been examined in only two plants in the USA.
It appears that chloracne-like lesions may develop in workers
producing PBB, and hypothyroidism in workers exposed to DBB. No
studies have been conducted in workers incorporating deca-, octa-, or
nonabromobiphenyl into commercial products.
PBBs are extremely persistent in living organisms and have been shown
to produce chronic toxic effects and cancer in animals. Though the
acute toxicity was low, cancer was induced at a dose of 0.5 mg/kg body
weight per day and the no-observed-effect level was 0.15 mg/kg body
weight per day. A number of chronic toxic effects have been observed
in experimental animals at doses around 1 mg/kg body weight per day
following long-term exposure.
3.2 Recommendations
General
* The Task Group is of the opinion that human beings and the
environment should not be exposed to PBBs, in view of their high
persistency and bioaccumulation and the potential adverse effects
at very low levels after long-term exposure. Therefore, PBBs
should no longer be used commercially.
* Because of the limited data on the toxicity of DeBB and OcBB,
their extreme persistence, and their potential for breakdown in
the environment and through combustion to more toxic persistent
compounds, they should also not be used commercially unless their
safety has been demonstrated.
Future work
* It is known that observations on the Michigan cohort are still
continuing. Publication of these data is required.
* Future human and environmental PBB monitoring should be expanded
and be congener specific, and include the OcBB, NoBB, and DeBB.
These compounds should be included in monitoring programmes for
other halogenated compounds.
* The time trends and geographical distribution of PBB levels in
the environment should continue to be monitored. The release of
PBBs into the environment from waste disposal sites should be
surveyed.
* Thermolysis experiments should be conducted to simulate the
conditions in accidental fires and municipal incinerators.
* Research should be continued on the mechanisms of toxicity and
carcinogenicity of PBBs and related compounds. PBBs may serve as
model compounds for mechanistic research. Purified congeners
should be used in these studies.
* The effects on reproduction have not been not well elucidated.
Therefore, well designed long-term reproductive studies should be
performed, at low doses, using a sensitive species.
* There is also a need for more information on the bioavailability
and toxicokinetics of OcBB/NoBB, DeBB and selected congeners.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
PBBs are highly brominated organic substances. They are very
persistent and can be hazardous for human beings if incorrectly or
carelessly handled. It is therefore essential that the correct
precautions are observed during handling and use.
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The acute oral and dermal toxicity is low. In occupational
conditions, skin itching, peeling, scaling and (halo) acne are found
after exposure to PBBs. Besides these dermal signs of intoxication,
liver disturbances, chest irritation, and neurological and unspecific
effects, such as depression, memory disturbances and nervousness, have
been reported, but not confirmed.
4.1.1.2 Medical advice
Medical treatment is symptomatic and supportive.
4.1.2 Health surveillance advice
A complete medical history of regularly exposed workers should be
taken, and physical examination made, on an annual basis, and
monitoring of PBB levels in blood serum should be done (frequency
depending on the results).
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
PBBs are not flammable; on the contrary, they are flame retardants.
However, on heating, toxic fumes, such as hydrogen bromide and
brominated dibenzofurans may be formed.
4.2.2 Fire hazards
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. Fire-fighters should be equipped with self-contained
breathing apparatus, eye protection, and full protective clothing.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding the accumulation of polluted run-off from the
site.
4.3 Storage
Products should be stored in locked buildings, out of reach of
animals, children, and unauthorized personnel. Do not store near
foodstuffs or animal feed.
4.4 Transport
Comply with any local requirements regarding movements of hazardous
goods. Do not transport in the same compartment as foodstuffs. Check
that containers are sound and labels undamaged before despatch.
4.5 Spillage
Before dealing with any spillage, precautions should be taken as
required, and appropriate personal protection should be used. Prevent
material from spreading or contaminating other cargo, vegetation, or
waterways, by making a barrier of the most suitable available
material, e.g., earth or sand.
Empty any product remaining in damaged/leaking containers into a clean
empty drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
Absorb spilled liquid with sawdust, sand, or earth, sweep up and place
the sweepings in a closeable container for later transfer to a safe
place for disposal.
As soon as possible after the spillage and before reuse, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place the sweepings in a closeable container for later transfer to a
safe place for disposal. Care should be taken to avoid run-off into
watercourses.
4.6 Disposal
Any surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Waste material should be burned in
a proper incinerator designed for organohalogen waste disposal with
effluent-gas scrubbing. For PBB wastes, incineration must be for more
than 2 seconds at 1200 °C or higher. Combustion of PBBs at lower
temperatures can produce brominated dibenzofurans. If high
temperature incineration is not possible, bury waste in an approved
dump or landfill where there is no risk of contamination of surface or
groundwater. Decomposition of PBBs will be extremely slow.
Comply with any local legislation regarding disposal of toxic wastes.
Puncture container to prevent reuse.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
PBBs are very resistant to degradation and very persistent in the
environment. Because of their high solubility in fat, they
bioaccumulate, especially in the fatty tissues of all living
organisms, and biomagnify in the higher trophic levels of the food
chain. Although their acute toxicity is relatively low, this
bioaccumulation and biomagnification may lead to toxic effects.
Industrial discharges occurring during manufacture, formulation, or
technical applications should be treated properly and should not be
allowed to pollute the environment. Any spillage or unused product
should be treated and disposed of properly (see section 4.5 and 4.6)
and should be prevented from spreading to the environment.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. A full reference to the
original national document from which the information was extracted
can be obtained from IRPTC.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluation by International Bodies
The International Agency for Research on Cancer (IARC) evaluated
polybrominated biphenyls in 1986 and 1987 and concluded that there was
inadequate evidence of their carcinogenicity in humans, but sufficient
evidence of their carcinogenicity in experimental animals (Group 2B).
6.2 Exposure Limit Values
No exposure limit values for PBBs were found.
6.3 Specific Restrictions
In the USA, all use of hexabromobiphenyls, the main PBB isomer used in
industrial processes, was discontinued in 1974, because of the hazard
to human health discovered after its accidental misuse in Michigan in
1973. The US Environmental Protection Agency has since required
notification regarding any manufacture or importation of PBBs. The
purpose of this requirement is to confirm that there are no
significant sources of these substances and to ensure that EPA has the
opportunity to investigate the circumstances of any resumption of
production.
In Canada, all commercial, manufacturing, and processing uses are
banned.
In the countries of the EEC, PBBs may not be used in textile articles,
such as garments, undergarments and linen intended to come into
contact with the skin.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies PBBs in:
Hazard Class 9: miscellaneous dangerous substance
Packing Group II: substances presenting medium danger
The European Community legislation requires the labelling of PBBs as
harmful substances using the symbol.
Molecular weight: 154.21 + 78.90 (x+y)
Common trade names: Adine 0102; Berkflam B10; Bromkal 80-9D;
FireMaster BP-6; FireMaster FF-1;
Flammex B10; HFO 101; Octabromobiphenyl
FR 250 BA; Octabromobiphenyl XN-1902.
Composition of technical products
PBBs synthesized for commercial use as fire retardants contain a
mixture of various brominated biphenyls, chiefly the hexa-, octa-,
nona-, and decabromobiphenyls, as well as small amounts of related
brominated substances.
1.2 Physical and Chemical Properties
PBBs are solids with a low volatility that decreases with an increase
in the number of bromine atoms. PBBs are virtually insoluble in water,
soluble in fat and slightly to highly soluble in various organic
solvents, the solubility also decreasing with an increase in the
number of bromine atoms. These compounds are relatively stable and
chemically unreactive, though highly brominated PBB mixtures degrade
rather rapidly on exposure to ultraviolet radiation.
The technical mixtures are typically white, off-white, or beige
powders.
1.3 Analysis
Gas chromatography with electron-capture or mass-spectrometric
detection are useful techniques for determining PBB levels.
High-resolution gas chromatography is preferred as this makes it
possible to determine specific congeners, but reference compounds are
available for only a few of the 209 possible PBB congeners.
1.4 Production and Uses
The commercial production of PBBs (as FireMaster(R)) was started in
the USA in 1970. After the production of about 6000 tonnes, further
production was virtually discontinued in 1974 after the inadvertent
addition of PBBs to animal feed. This resulted in the contamination of
farm animals and their subsequent massive destruction. Approximately
400 tonnes of octa- and decabromobiphenyl were produced in the USA
until 1979. Bromkal 80-9D was produced in Germany until 1985; a few
hundred tonnes per year of Adine 0102 are currently being produced in
France; in 1977, production of decabromobiphenyl ceased in the United
Kingdom.
PBBs are mainly used as flame retardants in moulded thermoplastics,
mostly in small appliances and automotive applications. Earlier
applications in synthetic fibres have been discontinued.
1.5 Thermal Decomposition
The products of the thermal decomposition of PBBs depend on the
temperature, the amount of oxygen present, and a number of other
factors. Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen (600-900 °C) have shown that bromobenzenes and lower
brominated biphenyls are formed, but no polybrominated furans. In
contrast, pyrolysis in the presence of oxygen (700-900 °C) yielded
some di- to heptabromodibenzofurans. In the presence of polystyrene
and polyethylene, higher levels were found. Pyrolysis of FireMaster
BP-6 with PVC at 800 °C yielded mixed bromochlorobiphenyls. There is
no information on the nature of the products of incineration of
PBB-containing compounds. Little is known about the toxicity of
brominated and brominated/chlorinated dioxins and furans, but their
toxicity is estimated to be of about the same order as that of
chlorinated dioxins and furans.
2. SUMMARY AND EVALUATION
2.1 Sources of Human and Environmental Exposure
PBBs were introduced as flame retardants in the early 1970s. Prior to
November 1974, hexabromobiphenyl was the most commercially significant
PBB in the USA and was incorporated into ABS (acrylonitrile-butadiene-
styrene) plastics (PBB content 10% - mainly small appliance and
automotive applications), coatings and lacquers, and polyurethane
foam. The other PBB flame retardants have similar uses.
Losses of PBB into the environment during normal production can occur
through emission into the air and waste waters, and through losses
into the soil and into landfills, but these quantities have been found
to be generally low. These chemicals can also enter the environment
during shipping and handling, and following accidents, as occurred in
Michigan, USA, in 1973.
PBBs can also enter the environment as a result of the incineration of
materials containing PBB, as well as during accidental fires, with the
formation of other toxic chemicals, such as polybromodibenzofurans or
mixed bromochloro derivatives.
Most of the total production of these compounds will ultimately enter
the environment, either unchanged or as breakdown products.
2.2 Environmental Transport, Distribution, and Transformation
Long-range transport of PBB in the atmosphere has not been proven, but
the presence of these compounds in Arctic seal samples indicates a
wide geographical distribution.
The main route by which PBBs enter the aquatic environment is in
industrial waste discharges and leachates from industrial dumping
sites, as well as by erosion of polluted soils. PBBs are almost
insoluble in water and are primarily found in the sediments of
polluted lakes and rivers.
Pollution of the soil can originate from point sources such as a PBB
production plant or a waste dump. Once introduced into the soil, PBBs
do not appear to be translocated readily. PBBs have been found to be
200 times more soluble in a landfill leachate than in distilled water;
this may result in a wider distribution in the environment. The
hydrophobic properties of PBBs make them easily adsorbed, from aqueous
solutions, on to soil particles. Preferential adsorption of PBB
congeners has been noted depending on the characteristics of the soil
(e.g., organic matter content) as well as on the degree and position
of the bromine substitution.
PBBs are stable and persistent, lipophilic, and only slightly soluble
in water; some of the congeners are poorly metabolized and therefore
accumulate in the lipid compartments of biota. Once they have been
released into the environment, they can reach the food chain, where
they are accumulated.
PBBs have been detected in fish from several regions. The ingestion
of fish is a means of PBB transfer to mammals and birds. PBBs have
also been detected in ducks, a turtle, in the eggs of water birds, as
well as in the fat of deer, rabbits, coyote, ravens, reindeer, seals,
and osprey.
The degradation of PBBs by purely abiotic chemical reactions
(excluding photochemical reactions) is considered unlikely. PBBs have
been reported to be persistent under field conditions. Soil samples
from a former PBB manufacturing site, analysed several years after the
Michigan incident, still contained PBBs, though the congener
composition was different, indicating partial degradation of the PBB
residue in the soil sample.
Under laboratory conditions, PBBs are easily degraded by ultraviolet
radiation. Photodegradation of the commercial FireMaster(R) mixture
led to decreased concentrations of the more highly substituted PBB
congeners. The rates and extent of the photolytic reactions of PBBs in
the environment have not been determined in detail, though field
observations indicate high persistence of the original PBBs or partial
degradation to less brominated compounds.
In laboratory investigations, mixtures of PBB appear to be fairly
resistant to microbial degradation.
No uptake or degradation of PBBs by plants has been recorded. In
contrast, PBBs are easily absorbed by animals, and although they were
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs. No investigations of sulfur-containing
metabolites analogous to those of PCBs have been reported.
The bioaccumulation of PBBs in fish has been investigated.
Bioaccumulation of PBBs in terrestrial animals has been investigated
for avian and mammalian species. Data were obtained through field
observations, evaluation of the Michigan disaster, and through
controlled feeding studies. Generally, the accumulation of PBBs in
body fat depended on dosage and duration of exposure.
For the individual congeners of PBB, bioaccumulation has been found to
increase with degree of bromination, up to at least the
tetrabromobiphenyls. Higher brominated congeners can be expected to
accumulate to an even higher degree. However, no such information is
available for decabromobiphenyl (DeBB). It is possible that DeBB is
poorly absorbed.
2.3 Environmental Levels and Human Exposure
There is only one report on PBB levels in air, the concentrations
being measured in the vicinity of three PBB-manufacturing or
PBB-processing plants in the USA.
PBB levels in surface waters were monitored in the same vicinity, as
well in the area of the Gratiot County landfill (Michigan, USA), which
received more than 100 tonnes of waste between 1971 and 1973,
containing 60-70% PBB.
Groundwater monitoring data from Gratiot County have shown trace
levels of PBBs even outside the landfill area; however, PBBs were not
detected in drinking-water wells in the area.
Data on soil pollution by PBBs are available for areas of manufacture,
use, or disposal, and for soils from fields of the PBB-contaminated
Michigan farms.
In the Michigan disaster, FireMaster(R) was inadvertently added to
animal feed. It was almost a year later that the mixing error was
discovered and the analyses indicated that PBBs were responsible.
During this time (summer 1973-May 1974), contaminated animals and
their produce entered the human food supply, and the environment of
the State of Michigan. Hundreds of farms were affected and thousands
of animals had to be slaughtered and buried, as well as thousands of
tonnes of farm produce.
Most data available on the PBB contamination of wildlife refer to fish
and birds, primarily water-fowl, in the vicinity of industrial sites,
and to marine mammals.
Recent reports on the PBB contamination of fish, terrestrial and
marine mammals, and birds in Europe and USA indicate a wide
distribution of these compounds. The congener pattern found in fish
samples is quite different from that found in commercial products.
Many of the main compounds found could well be the result of
photochemical debromination of decabromobiphenyl (BB 209), but this
has not been confirmed.
Occupational exposure has been found in employees of US chemical
plants, and in farm workers, as a result of the Michigan PBB incident.
Median levels of PBB in serum and adipose tissue were higher among
chemical workers. Information is not available on occupational
exposure associated with manufacturing, formulation, and commercial
uses from other companies or countries.
For most human populations, the exposure to PBBs from various sources
has not been measured directly. Widespread human exposure has been
reported from Michigan, USA, as a result of direct contact with
contaminated feed, but mainly from consumption of PBBs in meat, eggs,
and dairy products. At least 2000 families (mainly farmers and their
neighbours) were exposed to high levels of PBBs. Recently, PBBs have
been detected in cow's and human milk in Germany.
The congener patterns in these human samples were different from those
found in fish. The concentration of BB 153 was higher in the human
milk than in fish.
The routes by which the general population is exposed to PBBs are not
well known. Present knowledge indicates that the ambient air and
water do not contain high levels. Lipid-rich food, especially from
contaminated waters, is probably of great importance. There is no
information on the level of exposure from indoor air or on dermal
exposure from materials containing PBB flame retardants.
The PBB congener pattern found in human milk samples collected in
Germany resembles that found in cow's milk from the same region, but
the levels in the human samples were substantially higher.
An estimate of daily intake of PBB for the general population from
food has to be based on very few data. If fish is assumed to contain
20 µg PBB/kg lipid and 5% lipid and that a 60-kg person eats 100 g
fish/day, the intake will be 0.002 µg/kg body weight per day. A
PBB concentration of 0.05 µg/kg lipid in milk and a milk consumption
of 500 ml/day will give the same person a PBB intake of about
0.00002 µg/kg body weight per day.
An infant of 6 kg body weight consuming 800 ml breast milk (3.5%
lipid) per day will ingest 0.01 µg PBB/kg body weight per day, if the
milk contains 2 µg PBB/kg lipid.
2.4 Kinetics and Metabolism
Gastrointestinal absorption of PBBs varies according to the degree of
bromination, the lower brominated compounds being more easily
absorbed. There is inadequate information concerning the absorption
of DeBB and OcBB.
PBBs are distributed throughout the entire body of animal species and
human beings, with highest equilibrium concentrations being found in
the adipose tissues. Relatively high levels are also found in the
liver; in particular, the more toxic congeners appear to be
concentrated in the liver. The partitioning of the various PBB
congeners appears to differ in different tissues. Generally, there is
a marked tendency for bioaccumulation. In mammals, transfer of PBBs to
offspring occurs through the transplacental and milk routes. Human
breast milk was found to have levels of 2,2',4,4',5,5'-
hexabromobiphenyl, more than 100 times higher than the maternal serum.
During a multigeneration study on rats, administration of PBBs to one
generation resulted in detectable residues in more than two subsequent
generations. Eggs of avian species are also affected by the maternal
PBB body burden.
Many PBB congeners are persistent in biological systems. There was no
evidence of significant metabolism or excretion of the more abundant
components of the FireMaster(R) mixture, or of octa- or
decabromobiphenyl. In vitro studies showed that the metabolism of
PBBs is affected by the structure-activity relationship. PBBs were
metabolized by phenobarbital-induced microsomes only if they possessed
adjacent non-brominated carbons meta and para to the biphenyl bridge
on at least one ring. Metabolism by 3-methylcholanthrene-induced
microsomes required adjacent non-brominated ortho and meta positions
on at least one ring of lower substituted congeners; a higher degree
of bromination appeared to prevent metabolism. Hydroxylated
derivatives have been identified in vertebrates as major in vitro
and in vivo metabolism products of lower brominated biphenyls. The
metabolic yield was relatively low. The hydroxylation reaction
probably proceeds via both arene oxide intermediates and by direct
hydroxylation.
Humans, rats, rhesus monkeys, pigs, cows, and chickens eliminate PBBs
mainly in the faeces. In most cases, elimination rates seem to be
slow. Concentrations of 2,2',4,4',5,5'-hexabromobiphenyl observed in
the bile and faeces of humans were about 1/2 to 7/10 of the serum
levels, or approximately 0.5% of the adipose levels. Treatment to
increase elimination of PBB in animals or humans was usually
unsuccessful. Another pathway of elimination could be excretion in
milk.
Complex and varied relationships were found among PBB tissue
concentrations with time after PBB administration to rats or other
animals. They are described by several compartmental models. A
half-life of approximately 69 weeks was calculated for the elimination
of 2,2',4,4',5,5'-hexabromobiphenyl from body fat of the rats; this
period increased to more than 4 years for rhesus monkeys. The average
half-life of 2,2',4,4',5,5'-hexabromobiphenyl in humans has been
estimated to be between 7.8 and 12 years; periods ranging from 4.6 to
94.7 years have been suggested in the literature. Some differences in
retention and turnover have been demonstrated between individual PBB
congeners. The results of analyses of serum from farmers and chemical
workers for 2,3',4,4',5-pentabromobiphenyl have been inconsistent.
This inconsistency is probably due to the different sources of
exposure. The workers were exposed to all compounds of FireMaster(R),
while the Michigan population was exposed to contaminated meat and
milk containing a different mixture of PBBs as a result of metabolic
processes in farm animals. Bromine levels did not decrease in the
adipose tissue of rats when they were given technical grade
octabromobiphenyl. No information is available on the retention of
decabromobiphenyl.
Humans may have a greater tendency to retain certain PBB congeners
than experimental animals. This factor should be taken into
consideration in evaluating the human health hazards associated with
these chemicals.
In conclusion, all the available data indicate that PBBs have a marked
tendency to bioaccumulate and persist. Metabolism is poor and the
half-life in man is in the order of 8-12 years, or longer.
2.5 Effects on Organisms in the Environment
Only few data are available on the effects of PBBs on organisms in the
environment. They refer to microorganisms, water fleas, water birds,
and farm animals.
Water birds nesting on islands in northwestern Lake Michigan were
studied to determine whether environmental contaminants were affecting
their reproduction. Seventeen contaminants, including PBB, were
measured, but none seemed to have a pronounced effect on reproduction.
Farm animals that ingested feed inadvertently containing Firemaster(R)
FF-1, in place of magnesium oxide, became sick. The estimated average
exposure of cows on the first identified, highly contaminated farm was
250 mg/kg body weight. The clinical signs of toxicity were a 50%
reduction in feed consumption (anorexia) and a 40% decrease in milk
production a few weeks after ingestion of the contaminated feed.
Although the supplemented feed was discontinued within 16 days, milk
production did not recover. Some animals showed an increased frequency
of urination and increased lacrimation, and developed haematomas,
abscesses, abnormal hoof growth, lameness, alopecia, hyperkeratosis,
and cachexia, and several died within 6 months of exposure.
Altogether, the death rate on this farm was 24 out of 400. The death
rate of 6-18-month-old calves was much higher. About 50% died within 6
weeks, only 2 out of 12 surviving after 5 months. They developed
hyperkeratosis over their entire bodies. There were also a variety of
reproductive problems.
Necropsy findings have been reported for some of the mature cows that
died in the 6 months after exposure. Histopathological studies
revealed variable liver and kidney changes.
The clinical signs and pathological changes noted above were later
confirmed in controlled feeding studies (anorexia, dehydration,
excessive lacrimation, emaciation, hyperkeratosis, reproductive
difficulties, some clinical chemistry changes, and renal damage).
A drop in production and sterility were reported from herds with a low
level of contamination. This was in contrast to the results from
controlled studies which did not show significant differences between
herds with low-level contamination and control herds.
Although it was cattle feed that was originally contaminated, other
animal feeds became involved by cross-contamination, e.g., in the
machinery used to prepare other feeds. It is likely that the exposure
from these sources was not as high as that of the cattle. Although
other animals (poultry, pigs, horses, rabbits, goats, and sheep) were
reported as being exposed and were killed, details of ill effects were
not recorded.
No information is available on the effect of PBBs on ecosystems.
2.6 Effects on Experimental Animals and In Vitro Test Systems
Commercial mixtures show relatively low acute toxicity (LD50
>1 g/kg body weight) in rats, rabbits, and quails following oral or
dermal administration. Deaths and acute manifestations of toxicity
are delayed after administration of PBB. The total dose administered
determines the degree of toxicity, whether given as a single dose or
as repeated doses over short periods (up to 50 days). The toxicity of
PBBs is higher with multiple-dose rather than with single-dose
administration.
The few studies performed with commercial mixtures of octa- and
decabromobiphenyl resulted in no mortality of rats or fish. Of the
individual PBB congeners, only 3 hexa isomers have been tested,
3,3',4,4',5,5'-HxBB and 2,3',4,4',5,5'-HxBB being more toxic to rats
than 2,2',4,4',5,5'-HxBB. On the basis of limited data, OcBB and DeBB
appear to be less toxic than the PBB mixtures, and less well absorbed.
In many acute and short-term studies, signs of PBB (mostly FireMaster)
toxicity include reductions in feed consumption. At lethal doses, the
cause of death cannot be ascribed to pathology in a particular organ,
but rather the animals develop a "wasting syndrome" as the first
indication of toxicity. At death, the loss in body weight can be as
great as 30-40%. The few studies with technical OcBB and DeBB show no
such effects.
The morphological and histopathological changes due to PBB are most
prominent in the liver. Enlargement of the liver frequently occurs at
doses lower than those required to produce body weight changes. The
principal histopathological alterations in rodent species may consist
of extensive swelling and vacuolation of hepatocytes, proliferation of
the smooth endoplasmic reticulum, and single-cell necrosis. The
severity of the lesions depends on the dose and composition of the PBB
material given.
Decreases in thymus weights were observed in rats, mice, and cattle
after doses of FireMaster(R), but not after OcBB or DeBB.
After exposure of rats to low concentrations of PBBs, there have been
some reports of increase in thyroid weight and histological changes in
the thyroid.
It is evident that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological changes
in the liver and thymus. The halogenated biphenyls have been
categorized on the basis of structure. Category 1 comprises isomers
and congeners lacking orthosubstituents (coplanar PBBs).
Mono-ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more orthobromines) form the
third category. The congeners in the first category tend to elicit the
most severe effects, those in the second and third categories showing
less severe toxicological effects. Within the category, the degree of
bromination may also influence toxicity.
Of all the combinations tested, 3,3',4,4',5,5'-HxBB was found to be
the most toxic PBB. This congener is present in low concentrations as
a constituent of FireMaster(R). Of the major FireMaster(R)
constituents, 2,3,3',4,4',5-HxBB appeared to be the most toxic,
followed by 2,3',4,4',5,5'-HxBB and 2,3',4,4',5-PeBB. The main
component of the FireMaster(R) mixture, 2,2',4,4',5,5'-HxBB was
relatively non-toxic, as was 2,2',3,4,4',5,5'-HpBB, the second most
abundant constituent.
Technical OcBB and DeBB contain various amounts of other brominated
biphenyls. Information on other contaminants is lacking.
The common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions to the technical PBB
mixtures tested (OcBB and DeBB). However, hyperkeratosis and hair loss
were seen in cattle, and lesions resembling chloracne were seen in
rhesus monkeys following the ingestion of a FireMaster(R) mixture.
Hyperkeratosis of the inner surface of the rabbit ear was produced by
FireMaster, but not by its main components (2,2',4,4',5,5'-HxBB and
2,2',3,4,4',5,5'-HpBB). Fractionation of FireMaster(R) revealed that
most activity was associated with the more polar fractions containing
minor components. Treatment with sunlight-irradiated HxBB caused
severe hyperkeratosis in rabbit ears.
In rats, low-dose, long-term feeding of technical OcBB did not affect
food consumption and body weight, but an increase in the relative
liver weights of exposed rats was found at 2.5 mg/kg body weight for
7 months. Long-term feeding of FireMaster(R) to rats at doses of
10 mg/kg body weight for 6 months did not affect food consumption.
Doses of 1 mg/kg body weight for a 6-month period affected liver
weight. The thymus weight was decreased by a dose of 0.3 mg/kg body
weight in female rats. Histopathological changes were also noted.
Controlled long-term feeding studies in cattle exposed to low doses of
FireMaster(R) showed no adverse effects, as indicated by food intake,
clinical signs, clinicopathological changes, or performance. Minks,
guinea-pigs, and monkeys appeared to be more susceptible to PBB
toxicity.
Long-term effects have been recorded in rats related to pronounced
retention of the administered PBBs following pre- or perinatal
exposure to high doses of FireMaster(R).
The most common adverse effects on reproduction were fetal wastage and
a decrease in viability of the offspring. Some effects were still
noted in mink at concentrations of 1 mg/kg diet. Following a
12.5-month exposure to FireMaster(R) (0.3 mg/kg diet), a minor
decrease was observed in the viability of the offspring of rhesus
monkeys. The monkeys received a daily dose of 0.01 mg/kg body weight
and a total dose of 3.8 mg/kg body weight. Reproduction and
neurobehavioural studies in monkeys and rats with a low level of
exposure could not be appropriately evaluated since insufficient
information was given in the published papers on the experimental
design of the study. A weak teratogenic potential was seen in rodents
at high doses that may have caused some maternal toxicity.
PBBs interact with the endocrine system. Rats and pigs were found to
have dose-related decreases in serum thyroxine and triiodothyronine.
PBBs have also been reported to affect the levels of steroid hormones;
the extent depends on the species as well as the dose and the period
of administration.
PBBs produce porphyria in rats and male mice at doses as low as
0.3 mg/kg body weight per day; the no-effect level was 0.1 mg/kg per
day. PBBs had a pronounced influence on vitamin A storage, as well as
effects on the intermediary metabolism.
Atrophy of the thymus was a frequent observation following PBB
exposure. Other lymphoid tissues have also been affected.
FireMaster(R) also induced further indications of a suppressed immune
function. Data is not available concerning the effects of OcBB, DeBB,
or individual PBB congeners.
One of the most intensively studied effects of PBBs is their induction
of mixed function oxidase (MFO) enzymes. Consistently, FireMaster(R)
was found to be a mixed-type inducer of hepatic microsomal enzymes in
rats, as well as in all other animal species tested. Induction was
also found to a lesser extent in other tissues. The ability to induce
hepatic microsomal enzymes differed for individual PBB congeners.
Correlations between structure and microsomal enzyme-inducing activity
have been demonstrated.
Several studies have shown that PBBs are able to alter the biological
activity of a variety of drugs and toxicants. This may be due partly
to the ability of PBBs to induce the microsomal enzymes involved in
activation or deactivation of xenobiotics.
The FireMaster(R) mixture, and some of its major components, were
found to be capable of inhibiting intercellular communication in
vitro. This inhibition occurs at non-cytotoxic concentrations. Both
the cytotoxicity and the metabolic cooperation-inhibiting properties
of PBB congeners seem to be related to their structure, i.e., presence
or lack of ortho-substitution. In vitro and in vivo assays
(microbial and mammalian cell mutagenesis, mammalian cell chromosomal
damage, mammalian cell transformation, and DNA damage and repair
(UDS)) have failed to indicate any mutagenicity or genotoxicity of
individual PBB congeners or commercial mixtures.
Long-term carcinogenicity studies with PBBs have shown that the
principal site of tumours is the liver. The incidences of
hepatocellular carcinoma were significantly increased in both sexes of
mice and rats receiving oral doses of the FireMaster(R) mixture.
Bromkal 80-9 (technical nonabromobiphenyl) induced carcinogenic
effects in the liver in mice receiving diets containing 100 mg/kg diet
(5 mg/kg per day), or more, for 18 months. The lowest dose of PBB
that produced tumours (mostly adenomas) in rodents was 0.5 mg/kg body
weight per day for 2 years. The rats receiving 0.15 mg/kg in addition
to pre- and perinatal exposure did not show any adverse effects. The
carcinogenicity of technical octabromobiphenyl and decabromobiphenyl
has not been studied.
Neither FireMaster BP-6 nor 2,2',4,4',5,5'-hexabromobiphenyl showed
tumour-initiating (using TPA as promoter) or tumour-promoting (using
DMBA as initiator) activity in a mouse skin bioassay. However, in
other mouse skin models (using DMBA or MNNG as initiators), both
FireMaster FF-1 and 3,3',4,4',5,5'-hexabromobiphenyl, but not
2,2',4,4',5,5'-hexabromobiphenyl, showed tumour-promoting activity.
In a two-stage rat liver bioassay using phenobarbital as promoter,
3,3',4,4'-tetrabromobiphenyl showed a weak initiating activity. In
the two-stage rat liver model, using diethylnitrosamine and partial
hepatectomy, FireMaster, 3,3',4,4'-tetrabromobiphenyl, and
2,2',4,4',5,5'-hexabromobiphenyl showed tumour-promoting activity, but
3,3,',4,4',5,5'-hexabromobiphenyl did not.
The results of the studies on cell communication, the negative results
on genotoxicity and mutagenicity, and the results of tumour-promotion
assays indicate that the mixtures and congeners studied cause cancer
by epigenetic mechanisms. No information is available on technical
octa-, nona-, or decabromobiphenyl.
The mechanisms of action underlying the many manifestations of the
toxicity of PBBs and related compounds are not known. However, some of
the effects, such as the wasting syndrome, thymus atrophy,
hepatotoxicity, skin disorders, and the reproductive toxicity, may be
related to interaction with the so-called Ah- or TCDD-receptors
causing alteration in the expression of a number of genes. Different
PBB congeners vary in their interaction with the receptor, the
coplanar congeners being more active.
Many of the PBB effects are seen after long-lasting exposure. The
reason for this observation may be the pronounced accumulation of some
PBB congeners and the poor ability of the body to metabolize and
eliminate them. This results in a build-up of the chemical in the
body which overcomes the compensatory mechanisms and leads to adverse
effects.
Some polybrominated naphthalenes (PBNs), known contaminants of the
FireMaster(R) mixture, are potent toxicants and teratogens. Although
PBNs are only present at low levels in the FireMaster(R) mixture, it
is possible they may contribute to its toxicity.
Studies of the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-HxBB, showed that the photolysis products were more
toxic than the original PBB. Under some conditions of pyrolysis of
FireMaster(R), polybrominated dibenzofurans are formed. The pyrolysis
products of technical OcBB caused liver enlargement.
2.7 Effects on Humans
There have been no examples of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures following
the poisoning incident in Michigan, USA, in 1973. The main
epidemiological studies were conducted by the Michigan Department of
Public Health (MDPH) and the Environmental Science Laboratory, Mount
Sinai School of Medicine, New York (ESL).
It was estimated that the most highly exposed people consumed 5-15 g
PBB in milk over a 230-day period. Some additional exposure may have
occurred by consuming meat. The exposure in some of the farmers, and
most of the general population, in Michigan was much lower. For them
the total exposure was 9-10 mg. However, some people in this group
may have received a total exposure of about 800-900 mg. A total dose
of 9 mg corresponds to 0.15 mg/kg body weight, and 900 mg to 15 mg/kg
body weight for a 60-kg average adult; the dose/kg body weight will be
higher for children.
In 1974, the first MDPH study compared the health status of people on
quarantined farms with people on non-quarantined farms in the same
area. Although a variety of symptoms were reported by both groups,
there was no pattern to the differences between the groups. The
heart, liver, spleen, nervous system, urinalysis, blood counts, and
all other medical conditions examined were found to be normal. In a
later, comprehensive, MDPH study including groups with different
levels of exposure, there was no positive association between serum
concentrations of PBB and reported symptoms or disease frequencies.
The ESL studies involved about 990 farm residents, 55 chemical
workers, and a group of Wisconsin dairy farmers who were used as a
control group. The prevalence of symptoms in Michigan farmers was
greater than the prevalence in Wisconsin farmers. The greatest
differences were in the broad classification of neurological and
musculoskeletal symptoms. Elevated serum concentrations of some liver
enzymes and carcinoembryonic antigen were more prevalent in Michigan
farmers than in Wisconsin farmers. Chemical workers had a higher
prevalence of chest and skin symptoms and a lower prevalence of
musculoskeletal symptoms than farmers. Although the results of the ESL
studies were at times interpreted differently from the results of
comparable studies, there was one area of consistent agreement. None
of the sets of studies demonstrated a positive dose-response
relationship between PBB levels in serum or in adipose tissue and the
prevalence of symptoms or abnormal clinical measurements. Several
clinical areas were investigated by more intensive special studies.
Examination of the neurological aspects by means of objective
performance tests revealed, in one study, a negative correlation
between serum PBB levels and performance test scores, particularly in
males in the older age groups. The other studies showed no association
between the concentration of PBB in serum or fat and performance in a
battery of tests measuring memory, motor strength, coordination,
cortical-sensory perception, personality, higher cognitive
functioning, and other functions. The paediatric aspects of PBB
exposure were examined in the families in the ESL studies. Although
many symptoms were reported, physical examination failed to reveal any
objective alteration that could be attributed to PBB. There were
different views about the more subtle neuropsychological effects in
the offspring, and the results of investigations of developmental
abilities also remain controversial. The same is true for the
investigation of lymphocyte and immune function. One set of authors
found no differences in lymphocyte count or functions between groups
with high and low serum PBB levels, the other found a significant
decrease in T and B lymphocyte subpopulations in about 40% of an
exposed Michigan group compared with unexposed groups, and also
impaired lymphocyte function, i.e., decreased response to mitogens.
The epidemiological studies reviewed were planned to evaluate the
relationship between PBB exposure and a large number of adverse
effects, including behavioural effects and subjective complaints.
However, most of the studies suffer from major faults in design that
introduced confounders which make it difficult or impossible to draw
valid conclusions. The follow-up time has not been long enough to
evaluate a possible carcinogenic effect.
Two small groups of workers were identified with occupational exposure
to a mixture of PBBs, or to DeBB and DBBO. Lesions resembling
chloracne were found in 13% of the workers exposed to the PBB mixture,
but such lesions were not seen in the workers exposed to DeBB.
However, a significantly higher prevalence of hypothyroidism was seen
in the latter group.
2.8 Overall Evaluation of the Toxicity
The only lifetime and carcinogenicity study with a PBB mixture was
conducted in rats and mice in a recent NTP bioassay. The lowest dose
tested that still produced carcinogenic effects was 0.5 mg/kg body
weight per day (liver tumours in rodents). In other carcinogenicity
studies, 3 mg/kg body weight given for 6 months resulted in a
carcinogenic response. The 6-month study demonstrates that a less
than lifetime exposure at similar doses will also result in similar
adverse effects. Effects on reproduction in subhuman primates and
mink may occur at somewhat lower doses.
In addition, in the 2-year NTP study, a daily dose of 0.15 mg/kg body
weight per day and prenatal and perinatal exposure of the dam to
0.05 mg/kg body weight per day did not result in any adverse effects.
Thus, the total daily intake from food, water, air, and soil should be
less than 0.15 µg/kg body weight per day, extrapolating from the NOAEL
(no-observed-adverse-effect level) of a positive carcinogenicity
study, using an uncertainty (and safety) factor of 1000, since these
compounds probably induce cancer by an epigenetic mechanism.
The total dose received by the subpopulation in Michigan was estimated
to have ranged from 0.15 to 15 mg/kg body weight over a 230-day
period. For this population, dividing the doses over a lifetime for
the average human being would be equivalent to a daily dose ranging
from 6 ng to 0.6 µg/kg body weight per day.
Estimates for the general population indicate a total intake by adults
of 2 ng PBB/kg body weight per day from known sources, and an intake
of 10 ng/kg body weight per day for infants receiving breast milk. It
should be kept in mind that these estimates are based on a very
limited and regional data base.
These calculations assume that a steady state for PBBs would not be
reached over a lifetime, and that short-term, higher exposures can be
substituted for long-term, lower exposures, since these compounds are
extremely poorly metabolized and excreted.
Insufficient information is available for OcBB, NoBB, and DeBB to
calculate a total daily intake that would not result in adverse
effects.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Most of the PBB congeners found in commercial flame retardants are
lipophilic, persistent, and bioaccumulating. These compounds are
biomagnified in environmental food-webs and pose a particular threat
to organisms in the higher levels of these webs. Furthermore, some
PBB products form toxic polybrominated dibenzofurans during combustion
processes.
In addition to emissions during manufacture and use, PBB will enter
the environment from the widespread use of flame-retarded products. A
considerable part of the PBB produced will probably reach the
environment sooner or later because of the high stability of these
compounds.
PBBs are also found in environmental and human samples also from
places far from known point sources. The congener patterns in the
environmental samples do not match those found in the technical
products indicating environmental alteration, possibly photochemical
debromination.
Very little information is currently available on the degree of
exposure of the general population to PBBs. However, in the few
instances where measurements have been made, trace amounts of PBBs
were identified. At present, this exposure does not give rise to
concern but further build-up should be avoided. Human data from the
Michigan episode (see sections 2.3 and 2.7) suggest that exposures in
Michigan were several orders of magnitude greater than the exposure of
the general population. No definitive health effects could be
correlated with PBB exposure in the Michigan population, though the
follow-up period has not been long enough for the occurrence of cancer
to be evaluated. Since the PBB levels remain high in adipose tissue
and serum in the Michigan population, their internal exposure
continues. In contrast, in Michigan cattle toxic effects were
observed. This discrepancy is explained by differences in the degree
of exposure of the cattle.
Occupational exposure has been examined in only two plants in the USA.
It appears that chloracne-like lesions may develop in workers
producing PBB, and hypothyroidism in workers exposed to DBB. No
studies have been conducted in workers incorporating deca-, octa-, or
nonabromobiphenyl into commercial products.
PBBs are extremely persistent in living organisms and have been shown
to produce chronic toxic effects and cancer in animals. Though the
acute toxicity was low, cancer was induced at a dose of 0.5 mg/kg body
weight per day and the no-observed-effect level was 0.15 mg/kg body
weight per day. A number of chronic toxic effects have been observed
in experimental animals at doses around 1 mg/kg body weight per day
following long-term exposure.
3.2 Recommendations
General
* The Task Group is of the opinion that human beings and the
environment should not be exposed to PBBs, in view of their high
persistency and bioaccumulation and the potential adverse effects
at very low levels after long-term exposure. Therefore, PBBs
should no longer be used commercially.
* Because of the limited data on the toxicity of DeBB and OcBB,
their extreme persistence, and their potential for breakdown in
the environment and through combustion to more toxic persistent
compounds, they should also not be used commercially unless their
safety has been demonstrated.
Future work
* It is known that observations on the Michigan cohort are still
continuing. Publication of these data is required.
* Future human and environmental PBB monitoring should be expanded
and be congener specific, and include the OcBB, NoBB, and DeBB.
These compounds should be included in monitoring programmes for
other halogenated compounds.
* The time trends and geographical distribution of PBB levels in
the environment should continue to be monitored. The release of
PBBs into the environment from waste disposal sites should be
surveyed.
* Thermolysis experiments should be conducted to simulate the
conditions in accidental fires and municipal incinerators.
* Research should be continued on the mechanisms of toxicity and
carcinogenicity of PBBs and related compounds. PBBs may serve as
model compounds for mechanistic research. Purified congeners
should be used in these studies.
* The effects on reproduction have not been not well elucidated.
Therefore, well designed long-term reproductive studies should be
performed, at low doses, using a sensitive species.
* There is also a need for more information on the bioavailability
and toxicokinetics of OcBB/NoBB, DeBB and selected congeners.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
PBBs are highly brominated organic substances. They are very
persistent and can be hazardous for human beings if incorrectly or
carelessly handled. It is therefore essential that the correct
precautions are observed during handling and use.
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The acute oral and dermal toxicity is low. In occupational
conditions, skin itching, peeling, scaling and (halo) acne are found
after exposure to PBBs. Besides these dermal signs of intoxication,
liver disturbances, chest irritation, and neurological and unspecific
effects, such as depression, memory disturbances and nervousness, have
been reported, but not confirmed.
4.1.1.2 Medical advice
Medical treatment is symptomatic and supportive.
4.1.2 Health surveillance advice
A complete medical history of regularly exposed workers should be
taken, and physical examination made, on an annual basis, and
monitoring of PBB levels in blood serum should be done (frequency
depending on the results).
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
PBBs are not flammable; on the contrary, they are flame retardants.
However, on heating, toxic fumes, such as hydrogen bromide and
brominated dibenzofurans may be formed.
4.2.2 Fire hazards
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. Fire-fighters should be equipped with self-contained
breathing apparatus, eye protection, and full protective clothing.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding the accumulation of polluted run-off from the
site.
4.3 Storage
Products should be stored in locked buildings, out of reach of
animals, children, and unauthorized personnel. Do not store near
foodstuffs or animal feed.
4.4 Transport
Comply with any local requirements regarding movements of hazardous
goods. Do not transport in the same compartment as foodstuffs. Check
that containers are sound and labels undamaged before despatch.
4.5 Spillage
Before dealing with any spillage, precautions should be taken as
required, and appropriate personal protection should be used. Prevent
material from spreading or contaminating other cargo, vegetation, or
waterways, by making a barrier of the most suitable available
material, e.g., earth or sand.
Empty any product remaining in damaged/leaking containers into a clean
empty drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
Absorb spilled liquid with sawdust, sand, or earth, sweep up and place
the sweepings in a closeable container for later transfer to a safe
place for disposal.
As soon as possible after the spillage and before reuse, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place the sweepings in a closeable container for later transfer to a
safe place for disposal. Care should be taken to avoid run-off into
watercourses.
4.6 Disposal
Any surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Waste material should be burned in
a proper incinerator designed for organohalogen waste disposal with
effluent-gas scrubbing. For PBB wastes, incineration must be for more
than 2 seconds at 1200 °C or higher. Combustion of PBBs at lower
temperatures can produce brominated dibenzofurans. If high
temperature incineration is not possible, bury waste in an approved
dump or landfill where there is no risk of contamination of surface or
groundwater. Decomposition of PBBs will be extremely slow.
Comply with any local legislation regarding disposal of toxic wastes.
Puncture container to prevent reuse.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
PBBs are very resistant to degradation and very persistent in the
environment. Because of their high solubility in fat, they
bioaccumulate, especially in the fatty tissues of all living
organisms, and biomagnify in the higher trophic levels of the food
chain. Although their acute toxicity is relatively low, this
bioaccumulation and biomagnification may lead to toxic effects.
Industrial discharges occurring during manufacture, formulation, or
technical applications should be treated properly and should not be
allowed to pollute the environment. Any spillage or unused product
should be treated and disposed of properly (see section 4.5 and 4.6)
and should be prevented from spreading to the environment.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. A full reference to the
original national document from which the information was extracted
can be obtained from IRPTC.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluation by International Bodies
The International Agency for Research on Cancer (IARC) evaluated
polybrominated biphenyls in 1986 and 1987 and concluded that there was
inadequate evidence of their carcinogenicity in humans, but sufficient
evidence of their carcinogenicity in experimental animals (Group 2B).
6.2 Exposure Limit Values
No exposure limit values for PBBs were found.
6.3 Specific Restrictions
In the USA, all use of hexabromobiphenyls, the main PBB isomer used in
industrial processes, was discontinued in 1974, because of the hazard
to human health discovered after its accidental misuse in Michigan in
1973. The US Environmental Protection Agency has since required
notification regarding any manufacture or importation of PBBs. The
purpose of this requirement is to confirm that there are no
significant sources of these substances and to ensure that EPA has the
opportunity to investigate the circumstances of any resumption of
production.
In Canada, all commercial, manufacturing, and processing uses are
banned.
In the countries of the EEC, PBBs may not be used in textile articles,
such as garments, undergarments and linen intended to come into
contact with the skin.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies PBBs in:
Hazard Class 9: miscellaneous dangerous substance
Packing Group II: substances presenting medium danger
The European Community legislation requires the labelling of PBBs as
harmful substances using the symbol.
 The label must read:
Danger of cumulative effects. This material and its container
must be disposed of in a safe way. It should be stated on the
label whether the substance is a specific isomer or a mixture of
isomers.
6.5 Waste Disposal
No waste disposal regulations for PBBs were found.
BIBLIOGRAPHY
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E. (1984)
Clinical toxicology of commercial products. 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyon, International Agency
for Research on Cancer.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances (unpublished
file). Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1991) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by Governments. 4th Ed. New York, United
Nations.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
WHO (In Press, 1993) Environmental health criteria 152.
Polybrominated biphenyls (PBBs). Geneva, World Health Organization.
The label must read:
Danger of cumulative effects. This material and its container
must be disposed of in a safe way. It should be stated on the
label whether the substance is a specific isomer or a mixture of
isomers.
6.5 Waste Disposal
No waste disposal regulations for PBBs were found.
BIBLIOGRAPHY
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E. (1984)
Clinical toxicology of commercial products. 5th ed. Baltimore,
Maryland, Williams and Wilkins Company.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyon, International Agency
for Research on Cancer.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC Data profile on individual chemical substances (unpublished
file). Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1991) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by Governments. 4th Ed. New York, United
Nations.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
WHO (In Press, 1993) Environmental health criteria 152.
Polybrominated biphenyls (PBBs). Geneva, World Health Organization.
