
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 19
PENTACHLOROPHENOL
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1989
This is a companion volume to Environmental Health Criteria
71: Pentachlorophenol
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
ISBN 92 4 154341 8
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.1.1. Pentachlorophenol (PCP)
1.1.2. Sodium pentachlorophenate (Na-PCP)
1.1.3. Pentachlorophenyl laurate
1.1.4. Impurities in pentachlorophenol
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Kinetics and metabolism
2.2. Effects on experimental animals and in vitro test
systems
2.3. Evaluation of human health risks
2.3.1. Occupational exposure
2.3.1.1 Exposure levels and routes
2.3.1.2 Toxic effects
2.3.1.3 Risk evaluation
2.3.2. Non-occupational exposure
2.3.2.1 Exposure levels and routes
2.3.2.2 Risk evaluation
2.3.3. General population exposure
2.3.3.1 Exposure levels and routes
2.3.3.2 Risk evaluation
2.4. Evaluation of effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Advice to physicians
4.1.1.1 Clinical features
4.1.1.2 Medical advice
4.1.2. Health surveillance advice
4.2. Explosion and fire hazards
4.2.1. Explosion hazards
4.2.2. Fire hazards
4.3. Storage
4.4. Transport
4.5. Spillage and disposal
4.5.1. Spillage
4.5.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. INTERNATIONAL CHEMICAL SAFETY CARD
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is an International Chemical Safety Card
which should be readily available, and should be clearly explained, to
all who could come into contact with the chemical. The section on
regulatory information has been extracted from the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC) and from
other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
1.1.1 Pentachlorophenol (PCP)
Chemical structure: 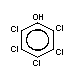 Molecular formula: C6Cl5OH
CAS chemical name: pentachlorophenol
Common synonyms: chlorophen; PCP; penchlorol; penta;
pentachlorofenol; pentachlorofenolo;
pentachlorphenol; 2,3,4,5,6-pentachlorophenol
CAS registry
number: 87-86-5
1.1.2 Sodium pentachlorophenate (Na-PCP)
Chemical structure:
Molecular formula: C6Cl5OH
CAS chemical name: pentachlorophenol
Common synonyms: chlorophen; PCP; penchlorol; penta;
pentachlorofenol; pentachlorofenolo;
pentachlorphenol; 2,3,4,5,6-pentachlorophenol
CAS registry
number: 87-86-5
1.1.2 Sodium pentachlorophenate (Na-PCP)
Chemical structure: 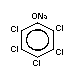 Molecular formula: C6Cl5ONa
C6Cl5ONa.H2O (as monohydrate)
Common synonyms: penta-ate; pentachlorophenate sodium;
pentachlorophenol, sodium salt;
pentachlorophenoxy sodium; pentaphenate;
phenol, pentachloro-, sodium derivative
monohydrate; sodium PCP; sodium
pentachlorophenate; sodium
pentachlorophenolate; sodium
pentachlorophenoxide
CAS registry 131-52-2 (Na-PCP);
number: 27735-64-4 (Na-PCP monohydrate)
1.1.3 Pentachlorophenyl laurate
The molecular formula of pentachlorophenyl laurate is C6Cl5OCOR; R
is the fatty acid moiety, which consists of a mixture of fatty acids
ranging in carbon chain length from C6 to C20, the predominant
fatty acid being lauric acid (C12).
1.1.4 Impurities in pentachlorophenol
Technical PCP has been shown to contain a large number of impurities,
depending on the manufacturing method. These consist of other
chlorophenols, particularly isomeric tetrachlorophenols, and several
microcontaminants, mainly polychlorodibenzodioxins (PCDDs),
polychlorodibenzofurans (PCDFs), polychlorodiphenyl ethers,
polychlorophenoxyphenols, chlorinated cyclohexenons and
cyclohexadienons, hexachlorobenzene, and polychlorinated biphenyls
(PCBs).
1.2 Physical and Chemical Properties
Pure pentachlorophenol consists of light tan to white, needle-like
crystals and is relatively volatile. It is soluble in most organic
solvents, but practically insoluble in water at the slightly acidic pH
generated by its dissociation (pKa 4.7). However, its salts, such as
sodium pentachlorophenate (Na-PCP), are readily soluble in water. At
the approximately neutral pH of most natural waters, PCP is more than
99% ionized.
Some physical and chemical properties of PCP and Na-PCP are given in
the International Chemical Safety Card.
1.3 Analytical Methods
Most of the analytical methods used today involve acidification of the
sample to convert PCP to its non-ionized form, extraction into an
organic solvent, possible cleaning by back-extraction into a basic
solution, and determination by gas chromatography with
electron-capture detector or other chromatographic methods as ester or
ether derivatives (e.g., acetyl-PCP). Depending on sampling procedures
and matrices, detection limits as low as 0.05 µg/m3 in air or
0.01 µg/litre in water can be achieved.
1.4 Production and Uses
World production of PCP is estimated to be of the order of 30 000
tonnes per year. Because of their efficiency, broad spectrum, and low
cost, PCP and its salts have been used as algicides, bactericides,
fungicides, herbicides, insecticides, and molluscicides with a variety
of applications in the industrial, agricultural, and domestic fields.
However, in recent years, most developed countries have restricted the
use of PCP, especially for agricultural and domestic applications (see
section 7.3).
PCP is mainly used as a wood preservative, particularly on a
commercial scale. The domestic use of PCP is of minor importance in
the overall PCP market, but has been of particular concern because of
possible health hazards associated with the indoor application of wood
preservatives containing PCP.
2. SUMMARY AND EVALUATION
2.1 Kinetics and Metabolism
PCP is readily absorbed through the intact skin and the respiratory
and gastrointestinal tracts, and is distributed in the tissues.
Highest levels are observed in liver and kidney, and lower levels are
found in body fat, brain, and muscle tissue. There is only a slight
tendency to bioaccumulate, and so relatively low PCP concentrations
are found in tissues. In rodent species, detoxification occurs through
the oxidative conversion of PCP to tetrachlorohydroquinone and, to a
lesser extent, to trichlorohydroquinone, as well as through
conjugation with glucuronic acid. In rhesus monkeys, no specific
metabolites have been detected. In man, metabolism of PCP to
tetrachlorohydroquinone seems to occur only to a small extent.
Rats, mice, and monkeys eliminate PCP and their metabolites, either
free or conjugated with glucuronic acid, mainly in the urine and to a
lesser extent with the faeces.
Some animal data indicate that there may be long-term accumulation and
storage of small amounts of PCP in human beings. The fact that urine-
or blood-PCP levels do not completely disappear in some occupationally
exposed people, even after a long absence of exposure, seems to
confirm this, though the biotransformation of hexachlorobenzene and
related compounds provides an alternative explanation of this
phenomenon. However, there is a lack of data concerning the long-term
fate of low PCP levels in animals as well as in man. Furthermore, no
data are available on the accumulation and effects of
microcontaminants taken up by man together with PCP.
2.2 Effects on Experimental Animals and In Vitro Test Systems
In the main, mammalian studies have been relatively consistent in
their demonstration of the effects of exposure to PCP. In rats, lethal
doses induce an increased respiratory rate, a marked rise in
temperature, tremors, and a loss of righting reflex. Asphyxial spasms
and cessation of breathing occur just before cardiac arrest, which is
in turn followed by a rapid, intense rigor morris.
PCP is highly toxic, regardless of the route, length, and frequency of
exposure. Oral LD50 values for a variety of species range between 27
and 205 mg/kg body weight according to the different solvent vehicles
and grades of PCP. There is limited evidence that the most dangerous
route of exposure to PCP is through inhalation.
PCP is also an irritant for exposed epithelial tissue, especially the
mucosal tissues of the eyes, nose, and throat. Other localized acute
effects include swelling, skin damage, and hair loss, as well as
flushed skin areas where PCP affects surface blood vessels. Exposure
to technical formulations of PCP may produce chloracne. Comparative
studies indicate that this is a response to microcontaminants,
principally PCDDs, present in the commercial product. The parent
molecule appears to be responsible for the immediate acute effects,
including irritation and the uncoupling of oxidative phosphorylation,
with a resultant elevated temperature.
The results of short- and long-term studies indicate that purified PCP
has a fairly limited range of effects in test organisms, primarily
rats. Exposure to fairly high concentrations of PCP may reduce growth
rates and serum-thyroid hormone levels, and increase liver weights
and/or the activity of some liver enzymes. In contrast, technical
formulations of PCP, usually at much lower concentrations, can
decrease growth rates, increase the weights of liver, lungs, kidneys,
and adrenals, increase the activity of a number of liver enzymes,
interfere with porphyrin metabolism, alter haematological and
biochemical parameters, and interfere with renal function. Apparently,
microcontaminants are the principal active moieties in the non-acute
toxicity of commercial PCP.
PCP is fetotoxic, delaying the development of rat embryos and reducing
litter size, neonatal body weight, neonatal survival, and the growth
of weanlings. The no-observed-adverse-effect level for technical PEP
is a maternal dose of 5 mg/kg body weight per day during
organogenesis. In one study, it was reported that purified PCP was
slightly more embryo/fetotoxic than technical PCP, presumably because
contaminants induced enzymes that detoxified the parent compound.
PCP is not considered teratogenic, though, in one instance, birth
defects arose as an indirect result of maternal hyperthermia. The
no-observed-adverse-effect level in rat reproduction studies was
3 mg/kg body weight per day. This value is remarkably close to the
value mentioned in the previous paragraph, but there are no
corroborating studies in other mammalian species.
PCP has also proved to be immunotoxic for mice, rats, chickens, and
cattle; at least part of this effect is caused by the parent molecule.
Neurotoxic effects have also been reported, but the possibility that
these are due to microcontaminants has not been excluded.
PCP is not considered carcinogenic for rats. Mutagenicity studies
support this conclusion in as much as pure PCP has not been found to
be highly mutagenic. However, its carcinogenicity remains questionable
because of shortcomings in these studies. The presence of at least one
carcinogenic microcontaminant (H6CDD) suggests that the potential
for technical PCP to cause cancer in laboratory animals cannot be
completely ruled out.
2.3 Evaluation of Human Health Risks
In this subsection, PCP and Na-PCP are referred to as PCP.
2.3.1 Occupational Exposure
2.3.1.1 Exposure levels and routes
Occupational exposure to technical PCP mainly occurs through
inhalation and dermal contact. Virtually all workers exposed to
airborne concentrations take up PCP through the lungs and skin. In
addition, workers handling treated lumber or maintaining
PCP-contaminated equipment would be exposed dermally to PCP in
solution, and may take up from one-half (based on urinary-PCP
concentrations) to two-thirds (using serum levels) of their total PCP
burden through the skin.
The actual concentrations to which workers have been exposed are
seldom measured but, where they have been monitored, they have been
predictably high. Airborne levels at PCP-production and
wood-preservation facilities have ranged from several mg/m3 to more
than 500 mg/m3 in some work areas. The outer layer of treated wood
can contain up to several hundred mg/kg, though levels are usually
less than 100 mg/kg.
These exposures result in concentrations of PCP in the serum and urine
that are 1-2 orders of magnitude higher than those found in the
general population without known exposure. Mean/median urinary-PCP
concentrations of approximately 1 mg/litre are typical for workers in
contact with PCP, compared with urinary concentrations of
approximately 0.01 mg/litre for the general population.
Automated processes and the use of closed systems have greatly reduced
worker exposure in large-scale manufacturing and modern wood-treatment
factories and sawmills. Other improvements in industrial hygiene can
significantly reduce exposure, as measured by lower urinary-PCP
concentrations.
2.3.1.2 Toxic effects
Past use of PCP has affected workers producing or using this chemical.
Chloracne, skin irritation and rashes, respiratory disorders,
neurological changes, headaches, nausea, weakness, irritability, and
drowsiness have been documented in exposed workers. Work-place
exposures are to technical PCP, which usually contains mg/kg
quantities of microcontaminants, particularly H6CDD. Subacute
effects, such as chloracne, and potential subchronic and chronic
effects, such as hepatotoxicity, fetotoxicity, and immunotoxicity (as
reported in animal studies), are probably mainly caused by
microcontaminants. However, the PCP molecule itself appears to play a
role in the pathology of the last three effects and is likely to be
wholly responsible for the reports of skin and mucous membrane
irritation, hyperpyrexia and, in severe cases, coma and death. The
toxicity of pure or purified PCP has not been evaluated for human
beings, because human exposure has usually been to technical PCP.
Investigations of biochemical changes in woodworkers with long-term
exposure to PCP have failed to detect consistently significant effects
on major organs, nerves, blood, reproduction, or the immune system.
However, the statistical power of these studies has been limited as a
result of the small sample sizes used. Overall, the body of research
suggests that long-term exposure to levels of PCP encountered in the
work-place is likely to cause borderline effects on some organ systems
and biochemical processes.
Some epidemiological studies from Sweden and the USA have revealed an
association between exposure to mixtures of chlorophenols, especially
2,4,5-T3CP, and the incidences of soft-tissue sarcomas, lymphomas,
and nasal and nasopharyngeal cancers. Other studies have failed to
detect such a relationship. It was not possible to address the effects
of exposure to PCP itself in any of these studies.
The results of animal studies, designed to assess the carcinogenicity
of PCP and reported to date, have been negative. Carcinogenicity
bioassays with one other chlorophenol (2,4,6-T3CP) and a mixture of
two H6CDD congeners found in PCP have been positive. Hence, the
carcinogenic effects of long-term exposure of animals to technical PCP
are not clear.
2.3.1.3 Risk evaluation
It is clear that the levels of PCP found in work-places have adversely
affected some aspects of the health of exposed workers. Potentially
the most deleterious effect of technical PCP is on the fetus, and
pregnant women should avoid exposure, whenever possible. There is
limited evidence that PCP may cause hepatotoxic effects, neurological
disorders, and effects on the immune system. No convincing data for or
against a carcinogenic link exist.
The US National Academy of Sciences (1977) calculated an acceptable
daily intake (ADI) for PCP of 3 µg/kg body weight per day. This ADI is
based on data from a feeding study on rats and a 1000-fold safety
factor. The results of long-term studies indicate that the
no-observed-adverse-effect level for rats is below 3 mg/kg body weight
per day. A recent human study has shown that the steady-state body
burden is 10-20 times higher than the value extrapolated from rat
pharmacokinetic data, suggesting that caution should be applied when
extrapolating directly from the rat model to man. Furthermore, the ADI
in the USA was not based on an inhalation study, and does not account
for the possibly greater toxicity of PCP via inhalation, as indicated
by animal studies. Hence, the safety factor of 1000 used to derive
this ADI value is by no means too conservative. The intake for a 60-kg
adult exposed to concentrations of PCP at the ADI level would be
180 µg/person per day.
A rough estimate of occupational exposure alone can be calculated,
assuming a moderate breathing rate of 1.8 m3/h for a 60-kg worker,
100% uptake of all inhaled PCP (which takes some account of the often
significant dermal uptake), and an 8-h working shift per day, 5 days
per week. Hence, an exposure to 500 µg PCP/m3 per shift would result
in an average daily PCP intake of approximately 5000 µg/person per
day, averaged over the entire week. Under these circumstances, the ADI
level proposed by the National Academy of Sciences is significantly
exceeded, even when consideration is given to the effects of
intermittent exposures during the working week and the high health
status assumed for workers.
There is a clear need for a reduction in occupational exposure to PCP.
Emphasis must be placed on reducing airborne concentrations at
production and wood-treatment facilities, as well as dermal contact
with solutions containing PCP. In addition, reductions in the
concentrations of microcontaminants in technical PCP, particularly
PCDDs and PCDFs, would reduce the potential for expression of several
effects and would better protect the health of workers in these
industries.
2.3.2 Non-occupational exposure
2.3.2.1 Exposure levels and routes
Domestic use of products containing technical PCP, especially the
indoor application of wood preservatives and paints based on PCP, has
led to elevated concentrations of PCP in indoor air. Indoor exposures
have been well documented in houses constructed with PCP-treated wood,
or in which interior wood panels or boards have been treated with PCP.
PCP concentrations in indoor air can be expected to reach 30 µg/m3
during the first month after treatment. Considerably higher levels, up
to 160 µg/m3, have been reported in houses with concomitant poor
indoor ventilation. Even higher concentrations can be encountered
immediately after do-it-yourself applications of PCP-containing wood
preservatives.
In the long term, values of between 1 and 10 µg/m3 are typical,
though higher levels, up to 25 µg/m3, have been found in rooms
treated one to several years earlier. Indoor air concentrations are
influenced by a variety of factors, e.g., intensity of treatment,
solvents and additives involved, species of wood treated,
environmental conditions, and time elapsed since treatment.
In many cases, levels of PCP in the serum and urine of people exposed
in the home overlap those for occupationally exposed persons; but, on
average, urine-PCP levels are approximately 0.04 mg/litre for
non-occupationally exposed persons.
Exposure to PCP in treated buildings continuously decreases with time,
owing to the high volatility of PCP. Because of their lower vapour
pressure, the volatilization of PCDDs and PCDFs from the wood surface
is much slower than that of PCP. Hence, these microcontaminants are
emitted at a low rate, but over a longer period of time. Long-term
exposure to these lipophilic contaminants is likely to lead to
accumulation of PCDDs and PCDFs in fatty body tissues.
As a result of regulations restricting the use of PCP, and also
changing use patterns, indoor exposure to PCP is probably declining in
most developed countries.
2.3.2.2 Risk evaluation
Assuming a daily respiratory volume of 20 m3/adult and 100% uptake
of all inhaled PCP (a worst case that takes some account of dermal
uptake), the exposure of persons living in PCP-treated buildings,
shortly after treatment, or, in some cases, after a long period of
time, could be expected to range between 600 and 3200 µg/person per
day. Long-term exposure to concentrations of 1-25 µg PCP/m could
result in a daily PCP intake of 20-500 µg/person per day. The median
value of 5 µg/m reported from a survey of 104 homes corresponds to a
daily PCP uptake of 100 µg/person per day. Other potential sources of
exposure to PCP including food, drinking-water, and consumer products
contribute further to PCP uptake.
The indoor air data suggest that, at least during the first weeks
following indoor treatment, and occasionally for quite prolonged
periods of time, the ADI level of 180 µg/person per day is
significantly exceeded. Under these circumstances, there is a
potential health risk. This conclusion is supported, in part, by
reports of signs and symptoms similar to those in persons
occupationally exposed to PCP (dermatosis, nausea, headache,
dizziness, fatigue). These signs and symptoms are most likely to be
associated with the effects of the PCP molecule and, in some cases,
the solvents associated with the wood treatment chemicals used. The
long-term significance of exposure to low levels of PCDDs and PCDFs
and their accumulation in human tissues is not entirely clear;
however, at least two isomeric groups of the PCDDs family are
carcinogenic for animals.
Animal data indicate that low concentrations of PCP in biological
tissues or body fluids do not signify an absence of biologically
active PCDDs and PCDFs.
It is worth noting that exposure in the home is frequently for longer
periods of time than exposures in the work-place and can affect
subpopulations potentially at greater risk than workers, for example,
children, the elderly, pregnant women, or those with an existing
adverse health condition.
2.3.3 General population exposure
2.3.3.1 Exposure levels and routes
Exposure of the general population to low levels of PCP is common. PCP
has been found in air, food, water, and other consumer products.
Biotransformation of some chlorinated hydrocarbons (e.g., lindane,
hexachlorobenzene) to PCP also contributes to the human body burden.
The ambient air in urban areas typically contains several ng/m3,
while concentrations in less developed areas are roughly an order of
magnitude lower.
Drinking-water concentrations of PCP rarely exceed several µg/litre,
even in highly industrialized regions, and most are less than
1 µg/litre.
Fruits, vegetables, and other produce usually contain much less than
10 µg/kg, but may on occasion exceed this level. Most meats contain
similar concentrations of PCP (10 µg/kg) but, a few samples,
particularly liver, can contain over 100 µg/kg. Fish skeletal muscle
typically contains PCP levels of 4 µg/kg or less. Overall estimates of
PCP intake from all foods, based on total diet samples in the USA and
the Federal Republic of Germany, are remarkably similar, i.e., up to
6 µg/person per day.
PCP is also present in a wide variety of consumer products, including
veterinary supplies, disinfectants, photographic solutions, fabrics,
home-care products, and pharmaceutical products. No calculated
estimates of the contribution made by consumer products to overall
exposure to PCP are available.
2.3.3.2 Risk evaluation
On the basis of the PCP levels in the various compartments, the
overall exposure of an average person without known specific exposure
can be estimated to be approximately 6 µg/person per day from food,
2 µg/person per day from drinking-water, and 2 µg/person per day from
the ambient air. Thus, the total exposure of the general population
could be approximately 10 µg/person per day (exclusive of exposure to
consumer products), which is far below the intake based on the ADI
proposed by the US National Academy of Science of 180 µg/person per
day. On the basis of available data, this exposure is not likely to
constitute a health hazard.
However, the diffuse contamination of the environment with technical
PCP must be considered as an important source of environmental PCDDs
and PCDFs.
2.4 Evaluation of Effects on the Environment
The widespread use of technical PCP and its physical and chemical
properties (water solubility, n-octanol/water partition coefficient,
volatility) lead to ubiquitous contamination of air, soil, water,
sediments, and organisms in the environment.
Depending on the soil type, PCP can be very mobile, potentially
leading to contamination of ground water and hence, of drinking-water.
Because applications in agriculture have been reduced, soil
contamination will, for the most part, be confined to treatment areas.
Photodecomposition and biodegradation processes may not be adequate to
eliminate PCP from the different compartments. Unfavourable
temperature, pH, and other environmental conditions may retard
degradation of PCP allowing it to persist in the environment.
Biological decomposition may also be limited in waste-treatment
factories resulting in high concentrations in the final effluents. PCP
has also been used in aquatic environments as a molluscicide and an
algicide.
PCP concentrations in surface waters are usually in the range of
0.1-1 µg/litre, though much higher levels can be found near point
sources or after accidental spills.
PCP is highly toxic for aquatic organisms. Apart from very sensitive
or resistant species, there is apparently no difference in the
sensitivity to PCP of the different taxonomic groups. Invertebrates
(annelids, molluscs, crustaceans) and fish are adversely affected by
PCP concentrations below 1 mg/litre in acute toxicity tests. Sublethal
concentrations are in the low µg/litre range.
As little as 1 µg PCP/litre can have adverse effects on very sensitive
algal species. Moreover, low concentrations (µg/litre) may lead to
substantial alterations in community structures, as seen in model
ecosystem studies.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
In this section, PCP and Na-PCP are referred to as PCP.
( a) Human exposure to PCP is usually from technical products that
contain several toxic microcontaminants, including PCDDs and PCDFs.
( b) The acute health effects of exposure to high concentrations of
technical PCP are generally the result of the biological action of the
PCP molecule itself. Sub-chronic effects and the effects of long-term
exposure to technical PCP are most probably largely related to the
biological action of the PCDDs and PCDFs.
( c) A dose-effect relationship for the acute or chronic toxicity of
technical PCP for human beings cannot be derived from available data.
Derivation of this relationship is confounded by variations in
individual susceptibility, social and environmental influences,
concomitant exposure to other chemical substances, a lack of accurate
exposure estimates, and inadequate toxicity data.
( d) Occupational exposure to technical PCP can lead to adverse
health effects.
( e) Non-occupationally exposed persons (users of products containing
technical PCP and/or those living in buildings treated with wood
preservatives or paints containing PCP) may be exposed to
concentrations of PCP in air that can have adverse health effects.
( f) The exposure of the general population to diffuse sources of PCP
(via food, drinking-water, ambient air, consumer products, chlorinated
compounds that can be metabolized to PCP) is very low and, on the
basis of available data, it is not likely to constitute a health
hazard.
( g) Epidemiological investigations and animal studies, conducted to
date, are insufficient for an evaluation of the carcinogenicity of
technical PCP. Uncertainties also exist over the genotoxic and
fetotoxic effects of technical PCP.
( h) PCP is rather persistent, quite mobile, and found in all
environmental compartments. At the higher concentrations found in the
surface water near point sources or discharges (mg/litre), aquatic
life is adversely affected. Ambient concentrations of PCP commonly
found in surface waters (0.1-1 µg/litre) may adversely affect very
sensitive organisms and may lead to alterations in the ecosystem.
( i) The use of technical PCP and its improper disposal (landfill and
low-temperature combustion) can contribute significantly to the
contamination of the environment with PCP, PCDDs, and PCDFs.
3.2 Recommendations
In this section, PCP and Na-PCP are referred to as PCP.
( a) Concentrations of microcontaminants in technical PCP, especially
PCDDs and PCDFs, must be reduced by improving the quality in
production processes.
( b) There is a need for specification of a technical PCP.
( c) The disposal of technical PCP and associated waste should
preferably involve high-temperature combustion or, where this is not
possible, the use of secure landfill sites.
( d) In order to reduce contamination of surface waters and the
hazards for the aquatic ecosystem, manufacturers and users of
technical PCP should prevent releases into the environment.
( e) Protective measures should be provided for non-target aquatic
organisms in cases where PCP is used as a molluscicide or algicide.
( f) Occupational exposure to technical PCP must be reduced to a
minimum. Reduction in exposure can be achieved by:
- explicit product labelling;
- employee instruction on product handling;
- lowering airborne concentrations; and
- use of effective protective equipment.
( g) Industries handling technical PCP should ensure adequate routine
monitoring and health surveillance of all potentially exposed
employees.
( h) The indoor application of PCP-based wood preservatives and wood
stains and the use of PCP-treated wood products in the interior of
buildings should cease.
( i) The availability and use of consumer products containing PCP
should be reduced and controlled.
( j) The following commercial uses of PCP-based products should be
eliminated, in order to reduce contamination of food and the
environment:
(i) application as wood preservatives on wooden food containers,
horticultural lumber, wood and tools in mushroom houses, and
above-ground interior wood of farm buildings;
(ii) application during the curing of hides;
(iii) application as a herbicide or soil sterilant;
(iv) application as a slimicide in wood pulp and paper
operations; and
(v) application as a molluscicide in surface water, if another
control chemical or measure is available that is less toxic for
man and the aquatic ecosystem.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
PCP is highly toxic and it is irritant to the skin, eyes and mucous
membrane. It can be highly hazardous to human beings if incorrectly
handled. For details, see the International Chemical Safety Card.
4.1.1 Advice to physicians
4.1.1.1 Clinical features
PCP uncouples oxidative phosphorylation processes thus increasing the
metabolic rate and causing hyperpyrexia. Early signs and symptoms are:
nausea, fatigue, unusual and excessive sweating, and thirst. Insomnia,
oliguria, and loss of body weight (dehydration) may occur in more
protracted cases. Anxiety and restlessness, increased rate and depth
of respiration, palpitations, tachycardia, fever, and eventually
convulsion and coma may occur in more severe cases. Laboratory
examination may reveal a rise in white blood cells and hypoglycaemia.
Pentachlorophenol can be detected in the urine.
4.1.1.2 Medical advice
Absolute rest is essential. Gastric lavage may be necessary in cases
of ingestion, followed by administration of activated charcoal. No
specific antidote or treatment is known. Poisoning cases should be
treated by continuous administration of oxygen, and fever should be
controlled by physical means, such as sponging with alcohol solutions.
Fluid losses should be replaced and urine should be kept alkaline by
the administration of sodium bicarbonate.
In very severe cases, intravenous infusion of chlorpromazine to reduce
the rate of metabolism and the body temperature may be helpful.
However, this should be done very cautiously.
Atropine and barbiturates are strictly contraindicated.
The symptoms of lung oedema often do not become manifest until after a
few hours and they are aggravated by physical effort. Rest and
hospitalization are therefore essential. As a preventive measure,
administration of a corticosteroid-containing spray should be
considered.
4.1.2 Health surveillance advice
A complete medical history and physical examination should be made, on
an annual basis, in workers regularly exposed to PCP. Special
attention should be paid to the cardiovascular system, upper
respiratory tract, liver, kidneys, and skin. Regular measurement of
PCP exposure should be undertaken, preferably in the breathing zone.
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation, or on the characteristics of the dust.
4.2.2 Fire hazards
Technical PCP will not burn. Formulated products and oil solutions are
likely to be highly flammable. All products will decompose and produce
harmful fumes, if involved in a fire, and the fire service must be
advised accordingly.
Fires should be controlled with alcohol-resistant foam, dry powder, or
carbon dioxide. The use of water should be confined to the cooling of
unaffected stock, thus avoiding the accumulation of polluted run-off.
4.3 Storage
Technical PCP is a solid. The formulated product is usually a solution
in oil or organic solvent, or an emulsifiable concentrate.
All PCP products should be stored in secure, well ventilated buildings
under cool and dry conditions, and out of reach of children and
unauthorized persons. Keep away from food, drink, and animal feed.
If any containers in the store are leaking, take precautions and use
personal protective equipment as required (see International Chemical
Safety Card). Empty any product remaining in damaged/leaking
containers into a clean, empty drum, which should then be suitably
labelled.
Sweep up spillage with sawdust, sand, or earth and dispose of safely.
Wash contaminated areas with detergent and a small amount of water,
absorbing as much as possible with sawdust, sand, or earth.
When emptied, decontaminate leaking containers several times with at
least 1 litre of water per 20-litre drum. Swirl round to rinse walls,
empty and add rinsings to the contaminated sawdust, sand, or earth.
Puncture the container to prevent re-use.
4.4 Transport
Comply with any local requirements regarding movement of hazardous
goods. Do not load with feed or foodstuffs. Check that containers are
sound and correctly labelled before despatch.
4.5 Spillage and Disposal
4.5.1 Spillage
Before dealing with any spillage, precautions should be taken as
required and appropriate personal protective equipment should be used
(see International Chemical Safety Card).
Absorb spilt liquids with sawdust, lime, sand, or earth. Prevent
liquids from spreading or contaminating other cargo, vegetation, or
waterways by building a barrier of the most readily available
material, e.g., earth or sand.
Sweep up spilt technical material and place this together with any
contaminated absorbents in a closeable container for later transfer to
a safe place for disposal.
4.5.2 Disposal
Surplus product, contaminated absorbents and containers should be
burned at a high temperature in an appropriate incinerator with
effluent gas scrubbing. When no incinerator is available, bury in an
approved dump, in an area where there is no risk of contamination of
surface or ground water. Comply with any local legislation.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Because of its action as an uncoupler of oxidative phosphorylation,
pentachlorophenol is highly hazardous for most forms of terrestrial
and aquatic life, depending on the exposure level. It is a rather
persistent and mobile pesticide and as a result it can occur in all
environmental compartments.
It is therefore essential that PCP levels in the environment be kept
as low as possible. Containers should not be emptied or washed into
ditches or waterways. Any effluent containing PCP should be properly
treated. Any PCP spillage should be prevented from contaminating
vegetation and waterways.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily, available to all health workers concerned
with, and users of, pentachlorophenol. It should be displayed at, or
near, entrances to areas where there is potential exposure to
pentachlorophenol, and on processing equipment and containers. The
card should be translated into the appropriate language(s). All
persons potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
INTERNATIONAL CHEMICAL SAFETY CARD
PENTACHLOROPHENOL CAS No. 87-86-5; C6Cl5OH
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point, decomposition 310°C Colourless to light brown flakes or crystals, with
Melting point 191°C characteristic phenolic odour; decomposes on
Relative density (water = 1) 2.0 heating in the presence of water, forming corrosive
(air = 1) 9.2 fumes (hydrochloric acid); substance may be absorbed
Vapour pressure (20°C) 2 mPa into body by inhalation, or ingestion or through skin;
Relative molecular mass 266.3 corrosive to the respiratory tract; affects the nervous
Octanol/water part. coeff. (pH 6.5) 3.56 system; not flammable and non-corrosive in unmixed
state; dissolved in oil, it causes deterioration
Solubility in water (20°C, pH 7) 2 g/litre of rubber
Solubility in organic solvents (25°C)
acetone 500 g/litre
benzene 150 g/litre
ethanol 1200 g/litre
methanol 1800 g/litre
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
SODIUM PENTACHLOROPHENATE CAS No. 131-52-2; C6Cl5ONa
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point decomposition Tan powder, pellets, or briquettes with phenolic
Melting point decomposition odour; decomposes on heating, forming toxic
Relative density (water = 1) 2.0 fumes (chlorides and sodium oxide); substance may
Relative molecular mass 288.3 be absorbed into body by inhalation, ingestion, and
Solubility in water (25°C) 330 g/litre through skin; corrosive to the respiratory tract
Solubility in organic solvents (25°C)
acetone 350 g/litre
benzene insoluble
ethanol 650 g/litre
methanol 250 g/litre
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
BOTH COMPOUNDS
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Strict hygiene; prevent dispersion
Highly toxic chemical of dust; if you feel unwell seek
medical advice (show the label where
possible); keep out of reach of
children
SKIN: toxic in contact with Avoid contact; wear PVC or neoprene Remove contaminated clothes, wash
skin; may cause white spots, gloves, neoprene apron, and rubber contaminated skin immediately with
sometimes wounds boots plenty of water and soap, and obtain
medical attention
EYES: may cause redness, pain Avoid contamination with dust or mist; First rinse with plenty of water for
use face shield or goggles 15 minutes, then transport to a doctor,
if necessary
INHALATION: toxic by inhalation; Avoid inhalation of vapour, dust, or Fresh air, rest, half-upright position, cool
may cause headache, fatigue, mist; use local exhaust ventilation down, and transport to hospital; symptoms
perspiration, thirst, faintness, or breathing protection; closed of lung oedema often do not become manifest
increased body temperature; in severe system; not recommended for until a few hours have passed, and are
cases lung oedema may occur interior use on large surface areas aggravated by physical effort; hospitalization
is therefore essential
INGESTION: toxic if swallowed; Do not eat, drink, or smoke during Do not induce vomiting; otherwise
may cause nausea, vomiting, work; keep away from food, drink, as above
abdominal spasm, diarrhoea; and animal feed
liver and kidney injury may
occur; severe cases may be fatal
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
BOTH COMPOUNDS
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
REPEATED EXPOSURE -- Take bath or shower after work and As above
SKIN, EYES, INHALATION, change clothing; otherwise as above
INGESTION: May cause
acneiform dermatitis
ENVIRONMENT: highly Avoid spillage into the
hazardous to most aquatic environment
and terrestrial organisms
SPILLAGE STORAGE FIRE AND EXPLOSION
Sweep up spilled substances, Store in secure well ventilated, Not combustible, formulated products may
and remove to safe place; cool and dry place; do not store be flammable; may give rise to harmful
carefully collect remainder near foodstuffs or animal feed decomposition products such as polychlorinated
(extra personal protection, dibenzo-p-dioxines; control fares with
particle-filter respirator); alcohol resistant foam, dry powder, or carbon
adsorb spilled liquids with dioxide
sawdust, lime, sand, or earth;
prevent liquids from spreading
or contaminating other cargo,
vegetation or waterways
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
BOTH COMPOUNDS
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
WASTE DISPOSAL
Burn at high temperature in National occupational exposure UN: 2761, 2762, 2995, 2996
incinerator with effluent gas limit:
scrubbing; if not available,
bury in an approved dump; National poison control centre:
comply with local regulations
Local trade names:
Molecular formula: C6Cl5ONa
C6Cl5ONa.H2O (as monohydrate)
Common synonyms: penta-ate; pentachlorophenate sodium;
pentachlorophenol, sodium salt;
pentachlorophenoxy sodium; pentaphenate;
phenol, pentachloro-, sodium derivative
monohydrate; sodium PCP; sodium
pentachlorophenate; sodium
pentachlorophenolate; sodium
pentachlorophenoxide
CAS registry 131-52-2 (Na-PCP);
number: 27735-64-4 (Na-PCP monohydrate)
1.1.3 Pentachlorophenyl laurate
The molecular formula of pentachlorophenyl laurate is C6Cl5OCOR; R
is the fatty acid moiety, which consists of a mixture of fatty acids
ranging in carbon chain length from C6 to C20, the predominant
fatty acid being lauric acid (C12).
1.1.4 Impurities in pentachlorophenol
Technical PCP has been shown to contain a large number of impurities,
depending on the manufacturing method. These consist of other
chlorophenols, particularly isomeric tetrachlorophenols, and several
microcontaminants, mainly polychlorodibenzodioxins (PCDDs),
polychlorodibenzofurans (PCDFs), polychlorodiphenyl ethers,
polychlorophenoxyphenols, chlorinated cyclohexenons and
cyclohexadienons, hexachlorobenzene, and polychlorinated biphenyls
(PCBs).
1.2 Physical and Chemical Properties
Pure pentachlorophenol consists of light tan to white, needle-like
crystals and is relatively volatile. It is soluble in most organic
solvents, but practically insoluble in water at the slightly acidic pH
generated by its dissociation (pKa 4.7). However, its salts, such as
sodium pentachlorophenate (Na-PCP), are readily soluble in water. At
the approximately neutral pH of most natural waters, PCP is more than
99% ionized.
Some physical and chemical properties of PCP and Na-PCP are given in
the International Chemical Safety Card.
1.3 Analytical Methods
Most of the analytical methods used today involve acidification of the
sample to convert PCP to its non-ionized form, extraction into an
organic solvent, possible cleaning by back-extraction into a basic
solution, and determination by gas chromatography with
electron-capture detector or other chromatographic methods as ester or
ether derivatives (e.g., acetyl-PCP). Depending on sampling procedures
and matrices, detection limits as low as 0.05 µg/m3 in air or
0.01 µg/litre in water can be achieved.
1.4 Production and Uses
World production of PCP is estimated to be of the order of 30 000
tonnes per year. Because of their efficiency, broad spectrum, and low
cost, PCP and its salts have been used as algicides, bactericides,
fungicides, herbicides, insecticides, and molluscicides with a variety
of applications in the industrial, agricultural, and domestic fields.
However, in recent years, most developed countries have restricted the
use of PCP, especially for agricultural and domestic applications (see
section 7.3).
PCP is mainly used as a wood preservative, particularly on a
commercial scale. The domestic use of PCP is of minor importance in
the overall PCP market, but has been of particular concern because of
possible health hazards associated with the indoor application of wood
preservatives containing PCP.
2. SUMMARY AND EVALUATION
2.1 Kinetics and Metabolism
PCP is readily absorbed through the intact skin and the respiratory
and gastrointestinal tracts, and is distributed in the tissues.
Highest levels are observed in liver and kidney, and lower levels are
found in body fat, brain, and muscle tissue. There is only a slight
tendency to bioaccumulate, and so relatively low PCP concentrations
are found in tissues. In rodent species, detoxification occurs through
the oxidative conversion of PCP to tetrachlorohydroquinone and, to a
lesser extent, to trichlorohydroquinone, as well as through
conjugation with glucuronic acid. In rhesus monkeys, no specific
metabolites have been detected. In man, metabolism of PCP to
tetrachlorohydroquinone seems to occur only to a small extent.
Rats, mice, and monkeys eliminate PCP and their metabolites, either
free or conjugated with glucuronic acid, mainly in the urine and to a
lesser extent with the faeces.
Some animal data indicate that there may be long-term accumulation and
storage of small amounts of PCP in human beings. The fact that urine-
or blood-PCP levels do not completely disappear in some occupationally
exposed people, even after a long absence of exposure, seems to
confirm this, though the biotransformation of hexachlorobenzene and
related compounds provides an alternative explanation of this
phenomenon. However, there is a lack of data concerning the long-term
fate of low PCP levels in animals as well as in man. Furthermore, no
data are available on the accumulation and effects of
microcontaminants taken up by man together with PCP.
2.2 Effects on Experimental Animals and In Vitro Test Systems
In the main, mammalian studies have been relatively consistent in
their demonstration of the effects of exposure to PCP. In rats, lethal
doses induce an increased respiratory rate, a marked rise in
temperature, tremors, and a loss of righting reflex. Asphyxial spasms
and cessation of breathing occur just before cardiac arrest, which is
in turn followed by a rapid, intense rigor morris.
PCP is highly toxic, regardless of the route, length, and frequency of
exposure. Oral LD50 values for a variety of species range between 27
and 205 mg/kg body weight according to the different solvent vehicles
and grades of PCP. There is limited evidence that the most dangerous
route of exposure to PCP is through inhalation.
PCP is also an irritant for exposed epithelial tissue, especially the
mucosal tissues of the eyes, nose, and throat. Other localized acute
effects include swelling, skin damage, and hair loss, as well as
flushed skin areas where PCP affects surface blood vessels. Exposure
to technical formulations of PCP may produce chloracne. Comparative
studies indicate that this is a response to microcontaminants,
principally PCDDs, present in the commercial product. The parent
molecule appears to be responsible for the immediate acute effects,
including irritation and the uncoupling of oxidative phosphorylation,
with a resultant elevated temperature.
The results of short- and long-term studies indicate that purified PCP
has a fairly limited range of effects in test organisms, primarily
rats. Exposure to fairly high concentrations of PCP may reduce growth
rates and serum-thyroid hormone levels, and increase liver weights
and/or the activity of some liver enzymes. In contrast, technical
formulations of PCP, usually at much lower concentrations, can
decrease growth rates, increase the weights of liver, lungs, kidneys,
and adrenals, increase the activity of a number of liver enzymes,
interfere with porphyrin metabolism, alter haematological and
biochemical parameters, and interfere with renal function. Apparently,
microcontaminants are the principal active moieties in the non-acute
toxicity of commercial PCP.
PCP is fetotoxic, delaying the development of rat embryos and reducing
litter size, neonatal body weight, neonatal survival, and the growth
of weanlings. The no-observed-adverse-effect level for technical PEP
is a maternal dose of 5 mg/kg body weight per day during
organogenesis. In one study, it was reported that purified PCP was
slightly more embryo/fetotoxic than technical PCP, presumably because
contaminants induced enzymes that detoxified the parent compound.
PCP is not considered teratogenic, though, in one instance, birth
defects arose as an indirect result of maternal hyperthermia. The
no-observed-adverse-effect level in rat reproduction studies was
3 mg/kg body weight per day. This value is remarkably close to the
value mentioned in the previous paragraph, but there are no
corroborating studies in other mammalian species.
PCP has also proved to be immunotoxic for mice, rats, chickens, and
cattle; at least part of this effect is caused by the parent molecule.
Neurotoxic effects have also been reported, but the possibility that
these are due to microcontaminants has not been excluded.
PCP is not considered carcinogenic for rats. Mutagenicity studies
support this conclusion in as much as pure PCP has not been found to
be highly mutagenic. However, its carcinogenicity remains questionable
because of shortcomings in these studies. The presence of at least one
carcinogenic microcontaminant (H6CDD) suggests that the potential
for technical PCP to cause cancer in laboratory animals cannot be
completely ruled out.
2.3 Evaluation of Human Health Risks
In this subsection, PCP and Na-PCP are referred to as PCP.
2.3.1 Occupational Exposure
2.3.1.1 Exposure levels and routes
Occupational exposure to technical PCP mainly occurs through
inhalation and dermal contact. Virtually all workers exposed to
airborne concentrations take up PCP through the lungs and skin. In
addition, workers handling treated lumber or maintaining
PCP-contaminated equipment would be exposed dermally to PCP in
solution, and may take up from one-half (based on urinary-PCP
concentrations) to two-thirds (using serum levels) of their total PCP
burden through the skin.
The actual concentrations to which workers have been exposed are
seldom measured but, where they have been monitored, they have been
predictably high. Airborne levels at PCP-production and
wood-preservation facilities have ranged from several mg/m3 to more
than 500 mg/m3 in some work areas. The outer layer of treated wood
can contain up to several hundred mg/kg, though levels are usually
less than 100 mg/kg.
These exposures result in concentrations of PCP in the serum and urine
that are 1-2 orders of magnitude higher than those found in the
general population without known exposure. Mean/median urinary-PCP
concentrations of approximately 1 mg/litre are typical for workers in
contact with PCP, compared with urinary concentrations of
approximately 0.01 mg/litre for the general population.
Automated processes and the use of closed systems have greatly reduced
worker exposure in large-scale manufacturing and modern wood-treatment
factories and sawmills. Other improvements in industrial hygiene can
significantly reduce exposure, as measured by lower urinary-PCP
concentrations.
2.3.1.2 Toxic effects
Past use of PCP has affected workers producing or using this chemical.
Chloracne, skin irritation and rashes, respiratory disorders,
neurological changes, headaches, nausea, weakness, irritability, and
drowsiness have been documented in exposed workers. Work-place
exposures are to technical PCP, which usually contains mg/kg
quantities of microcontaminants, particularly H6CDD. Subacute
effects, such as chloracne, and potential subchronic and chronic
effects, such as hepatotoxicity, fetotoxicity, and immunotoxicity (as
reported in animal studies), are probably mainly caused by
microcontaminants. However, the PCP molecule itself appears to play a
role in the pathology of the last three effects and is likely to be
wholly responsible for the reports of skin and mucous membrane
irritation, hyperpyrexia and, in severe cases, coma and death. The
toxicity of pure or purified PCP has not been evaluated for human
beings, because human exposure has usually been to technical PCP.
Investigations of biochemical changes in woodworkers with long-term
exposure to PCP have failed to detect consistently significant effects
on major organs, nerves, blood, reproduction, or the immune system.
However, the statistical power of these studies has been limited as a
result of the small sample sizes used. Overall, the body of research
suggests that long-term exposure to levels of PCP encountered in the
work-place is likely to cause borderline effects on some organ systems
and biochemical processes.
Some epidemiological studies from Sweden and the USA have revealed an
association between exposure to mixtures of chlorophenols, especially
2,4,5-T3CP, and the incidences of soft-tissue sarcomas, lymphomas,
and nasal and nasopharyngeal cancers. Other studies have failed to
detect such a relationship. It was not possible to address the effects
of exposure to PCP itself in any of these studies.
The results of animal studies, designed to assess the carcinogenicity
of PCP and reported to date, have been negative. Carcinogenicity
bioassays with one other chlorophenol (2,4,6-T3CP) and a mixture of
two H6CDD congeners found in PCP have been positive. Hence, the
carcinogenic effects of long-term exposure of animals to technical PCP
are not clear.
2.3.1.3 Risk evaluation
It is clear that the levels of PCP found in work-places have adversely
affected some aspects of the health of exposed workers. Potentially
the most deleterious effect of technical PCP is on the fetus, and
pregnant women should avoid exposure, whenever possible. There is
limited evidence that PCP may cause hepatotoxic effects, neurological
disorders, and effects on the immune system. No convincing data for or
against a carcinogenic link exist.
The US National Academy of Sciences (1977) calculated an acceptable
daily intake (ADI) for PCP of 3 µg/kg body weight per day. This ADI is
based on data from a feeding study on rats and a 1000-fold safety
factor. The results of long-term studies indicate that the
no-observed-adverse-effect level for rats is below 3 mg/kg body weight
per day. A recent human study has shown that the steady-state body
burden is 10-20 times higher than the value extrapolated from rat
pharmacokinetic data, suggesting that caution should be applied when
extrapolating directly from the rat model to man. Furthermore, the ADI
in the USA was not based on an inhalation study, and does not account
for the possibly greater toxicity of PCP via inhalation, as indicated
by animal studies. Hence, the safety factor of 1000 used to derive
this ADI value is by no means too conservative. The intake for a 60-kg
adult exposed to concentrations of PCP at the ADI level would be
180 µg/person per day.
A rough estimate of occupational exposure alone can be calculated,
assuming a moderate breathing rate of 1.8 m3/h for a 60-kg worker,
100% uptake of all inhaled PCP (which takes some account of the often
significant dermal uptake), and an 8-h working shift per day, 5 days
per week. Hence, an exposure to 500 µg PCP/m3 per shift would result
in an average daily PCP intake of approximately 5000 µg/person per
day, averaged over the entire week. Under these circumstances, the ADI
level proposed by the National Academy of Sciences is significantly
exceeded, even when consideration is given to the effects of
intermittent exposures during the working week and the high health
status assumed for workers.
There is a clear need for a reduction in occupational exposure to PCP.
Emphasis must be placed on reducing airborne concentrations at
production and wood-treatment facilities, as well as dermal contact
with solutions containing PCP. In addition, reductions in the
concentrations of microcontaminants in technical PCP, particularly
PCDDs and PCDFs, would reduce the potential for expression of several
effects and would better protect the health of workers in these
industries.
2.3.2 Non-occupational exposure
2.3.2.1 Exposure levels and routes
Domestic use of products containing technical PCP, especially the
indoor application of wood preservatives and paints based on PCP, has
led to elevated concentrations of PCP in indoor air. Indoor exposures
have been well documented in houses constructed with PCP-treated wood,
or in which interior wood panels or boards have been treated with PCP.
PCP concentrations in indoor air can be expected to reach 30 µg/m3
during the first month after treatment. Considerably higher levels, up
to 160 µg/m3, have been reported in houses with concomitant poor
indoor ventilation. Even higher concentrations can be encountered
immediately after do-it-yourself applications of PCP-containing wood
preservatives.
In the long term, values of between 1 and 10 µg/m3 are typical,
though higher levels, up to 25 µg/m3, have been found in rooms
treated one to several years earlier. Indoor air concentrations are
influenced by a variety of factors, e.g., intensity of treatment,
solvents and additives involved, species of wood treated,
environmental conditions, and time elapsed since treatment.
In many cases, levels of PCP in the serum and urine of people exposed
in the home overlap those for occupationally exposed persons; but, on
average, urine-PCP levels are approximately 0.04 mg/litre for
non-occupationally exposed persons.
Exposure to PCP in treated buildings continuously decreases with time,
owing to the high volatility of PCP. Because of their lower vapour
pressure, the volatilization of PCDDs and PCDFs from the wood surface
is much slower than that of PCP. Hence, these microcontaminants are
emitted at a low rate, but over a longer period of time. Long-term
exposure to these lipophilic contaminants is likely to lead to
accumulation of PCDDs and PCDFs in fatty body tissues.
As a result of regulations restricting the use of PCP, and also
changing use patterns, indoor exposure to PCP is probably declining in
most developed countries.
2.3.2.2 Risk evaluation
Assuming a daily respiratory volume of 20 m3/adult and 100% uptake
of all inhaled PCP (a worst case that takes some account of dermal
uptake), the exposure of persons living in PCP-treated buildings,
shortly after treatment, or, in some cases, after a long period of
time, could be expected to range between 600 and 3200 µg/person per
day. Long-term exposure to concentrations of 1-25 µg PCP/m could
result in a daily PCP intake of 20-500 µg/person per day. The median
value of 5 µg/m reported from a survey of 104 homes corresponds to a
daily PCP uptake of 100 µg/person per day. Other potential sources of
exposure to PCP including food, drinking-water, and consumer products
contribute further to PCP uptake.
The indoor air data suggest that, at least during the first weeks
following indoor treatment, and occasionally for quite prolonged
periods of time, the ADI level of 180 µg/person per day is
significantly exceeded. Under these circumstances, there is a
potential health risk. This conclusion is supported, in part, by
reports of signs and symptoms similar to those in persons
occupationally exposed to PCP (dermatosis, nausea, headache,
dizziness, fatigue). These signs and symptoms are most likely to be
associated with the effects of the PCP molecule and, in some cases,
the solvents associated with the wood treatment chemicals used. The
long-term significance of exposure to low levels of PCDDs and PCDFs
and their accumulation in human tissues is not entirely clear;
however, at least two isomeric groups of the PCDDs family are
carcinogenic for animals.
Animal data indicate that low concentrations of PCP in biological
tissues or body fluids do not signify an absence of biologically
active PCDDs and PCDFs.
It is worth noting that exposure in the home is frequently for longer
periods of time than exposures in the work-place and can affect
subpopulations potentially at greater risk than workers, for example,
children, the elderly, pregnant women, or those with an existing
adverse health condition.
2.3.3 General population exposure
2.3.3.1 Exposure levels and routes
Exposure of the general population to low levels of PCP is common. PCP
has been found in air, food, water, and other consumer products.
Biotransformation of some chlorinated hydrocarbons (e.g., lindane,
hexachlorobenzene) to PCP also contributes to the human body burden.
The ambient air in urban areas typically contains several ng/m3,
while concentrations in less developed areas are roughly an order of
magnitude lower.
Drinking-water concentrations of PCP rarely exceed several µg/litre,
even in highly industrialized regions, and most are less than
1 µg/litre.
Fruits, vegetables, and other produce usually contain much less than
10 µg/kg, but may on occasion exceed this level. Most meats contain
similar concentrations of PCP (10 µg/kg) but, a few samples,
particularly liver, can contain over 100 µg/kg. Fish skeletal muscle
typically contains PCP levels of 4 µg/kg or less. Overall estimates of
PCP intake from all foods, based on total diet samples in the USA and
the Federal Republic of Germany, are remarkably similar, i.e., up to
6 µg/person per day.
PCP is also present in a wide variety of consumer products, including
veterinary supplies, disinfectants, photographic solutions, fabrics,
home-care products, and pharmaceutical products. No calculated
estimates of the contribution made by consumer products to overall
exposure to PCP are available.
2.3.3.2 Risk evaluation
On the basis of the PCP levels in the various compartments, the
overall exposure of an average person without known specific exposure
can be estimated to be approximately 6 µg/person per day from food,
2 µg/person per day from drinking-water, and 2 µg/person per day from
the ambient air. Thus, the total exposure of the general population
could be approximately 10 µg/person per day (exclusive of exposure to
consumer products), which is far below the intake based on the ADI
proposed by the US National Academy of Science of 180 µg/person per
day. On the basis of available data, this exposure is not likely to
constitute a health hazard.
However, the diffuse contamination of the environment with technical
PCP must be considered as an important source of environmental PCDDs
and PCDFs.
2.4 Evaluation of Effects on the Environment
The widespread use of technical PCP and its physical and chemical
properties (water solubility, n-octanol/water partition coefficient,
volatility) lead to ubiquitous contamination of air, soil, water,
sediments, and organisms in the environment.
Depending on the soil type, PCP can be very mobile, potentially
leading to contamination of ground water and hence, of drinking-water.
Because applications in agriculture have been reduced, soil
contamination will, for the most part, be confined to treatment areas.
Photodecomposition and biodegradation processes may not be adequate to
eliminate PCP from the different compartments. Unfavourable
temperature, pH, and other environmental conditions may retard
degradation of PCP allowing it to persist in the environment.
Biological decomposition may also be limited in waste-treatment
factories resulting in high concentrations in the final effluents. PCP
has also been used in aquatic environments as a molluscicide and an
algicide.
PCP concentrations in surface waters are usually in the range of
0.1-1 µg/litre, though much higher levels can be found near point
sources or after accidental spills.
PCP is highly toxic for aquatic organisms. Apart from very sensitive
or resistant species, there is apparently no difference in the
sensitivity to PCP of the different taxonomic groups. Invertebrates
(annelids, molluscs, crustaceans) and fish are adversely affected by
PCP concentrations below 1 mg/litre in acute toxicity tests. Sublethal
concentrations are in the low µg/litre range.
As little as 1 µg PCP/litre can have adverse effects on very sensitive
algal species. Moreover, low concentrations (µg/litre) may lead to
substantial alterations in community structures, as seen in model
ecosystem studies.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
In this section, PCP and Na-PCP are referred to as PCP.
( a) Human exposure to PCP is usually from technical products that
contain several toxic microcontaminants, including PCDDs and PCDFs.
( b) The acute health effects of exposure to high concentrations of
technical PCP are generally the result of the biological action of the
PCP molecule itself. Sub-chronic effects and the effects of long-term
exposure to technical PCP are most probably largely related to the
biological action of the PCDDs and PCDFs.
( c) A dose-effect relationship for the acute or chronic toxicity of
technical PCP for human beings cannot be derived from available data.
Derivation of this relationship is confounded by variations in
individual susceptibility, social and environmental influences,
concomitant exposure to other chemical substances, a lack of accurate
exposure estimates, and inadequate toxicity data.
( d) Occupational exposure to technical PCP can lead to adverse
health effects.
( e) Non-occupationally exposed persons (users of products containing
technical PCP and/or those living in buildings treated with wood
preservatives or paints containing PCP) may be exposed to
concentrations of PCP in air that can have adverse health effects.
( f) The exposure of the general population to diffuse sources of PCP
(via food, drinking-water, ambient air, consumer products, chlorinated
compounds that can be metabolized to PCP) is very low and, on the
basis of available data, it is not likely to constitute a health
hazard.
( g) Epidemiological investigations and animal studies, conducted to
date, are insufficient for an evaluation of the carcinogenicity of
technical PCP. Uncertainties also exist over the genotoxic and
fetotoxic effects of technical PCP.
( h) PCP is rather persistent, quite mobile, and found in all
environmental compartments. At the higher concentrations found in the
surface water near point sources or discharges (mg/litre), aquatic
life is adversely affected. Ambient concentrations of PCP commonly
found in surface waters (0.1-1 µg/litre) may adversely affect very
sensitive organisms and may lead to alterations in the ecosystem.
( i) The use of technical PCP and its improper disposal (landfill and
low-temperature combustion) can contribute significantly to the
contamination of the environment with PCP, PCDDs, and PCDFs.
3.2 Recommendations
In this section, PCP and Na-PCP are referred to as PCP.
( a) Concentrations of microcontaminants in technical PCP, especially
PCDDs and PCDFs, must be reduced by improving the quality in
production processes.
( b) There is a need for specification of a technical PCP.
( c) The disposal of technical PCP and associated waste should
preferably involve high-temperature combustion or, where this is not
possible, the use of secure landfill sites.
( d) In order to reduce contamination of surface waters and the
hazards for the aquatic ecosystem, manufacturers and users of
technical PCP should prevent releases into the environment.
( e) Protective measures should be provided for non-target aquatic
organisms in cases where PCP is used as a molluscicide or algicide.
( f) Occupational exposure to technical PCP must be reduced to a
minimum. Reduction in exposure can be achieved by:
- explicit product labelling;
- employee instruction on product handling;
- lowering airborne concentrations; and
- use of effective protective equipment.
( g) Industries handling technical PCP should ensure adequate routine
monitoring and health surveillance of all potentially exposed
employees.
( h) The indoor application of PCP-based wood preservatives and wood
stains and the use of PCP-treated wood products in the interior of
buildings should cease.
( i) The availability and use of consumer products containing PCP
should be reduced and controlled.
( j) The following commercial uses of PCP-based products should be
eliminated, in order to reduce contamination of food and the
environment:
(i) application as wood preservatives on wooden food containers,
horticultural lumber, wood and tools in mushroom houses, and
above-ground interior wood of farm buildings;
(ii) application during the curing of hides;
(iii) application as a herbicide or soil sterilant;
(iv) application as a slimicide in wood pulp and paper
operations; and
(v) application as a molluscicide in surface water, if another
control chemical or measure is available that is less toxic for
man and the aquatic ecosystem.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
PCP is highly toxic and it is irritant to the skin, eyes and mucous
membrane. It can be highly hazardous to human beings if incorrectly
handled. For details, see the International Chemical Safety Card.
4.1.1 Advice to physicians
4.1.1.1 Clinical features
PCP uncouples oxidative phosphorylation processes thus increasing the
metabolic rate and causing hyperpyrexia. Early signs and symptoms are:
nausea, fatigue, unusual and excessive sweating, and thirst. Insomnia,
oliguria, and loss of body weight (dehydration) may occur in more
protracted cases. Anxiety and restlessness, increased rate and depth
of respiration, palpitations, tachycardia, fever, and eventually
convulsion and coma may occur in more severe cases. Laboratory
examination may reveal a rise in white blood cells and hypoglycaemia.
Pentachlorophenol can be detected in the urine.
4.1.1.2 Medical advice
Absolute rest is essential. Gastric lavage may be necessary in cases
of ingestion, followed by administration of activated charcoal. No
specific antidote or treatment is known. Poisoning cases should be
treated by continuous administration of oxygen, and fever should be
controlled by physical means, such as sponging with alcohol solutions.
Fluid losses should be replaced and urine should be kept alkaline by
the administration of sodium bicarbonate.
In very severe cases, intravenous infusion of chlorpromazine to reduce
the rate of metabolism and the body temperature may be helpful.
However, this should be done very cautiously.
Atropine and barbiturates are strictly contraindicated.
The symptoms of lung oedema often do not become manifest until after a
few hours and they are aggravated by physical effort. Rest and
hospitalization are therefore essential. As a preventive measure,
administration of a corticosteroid-containing spray should be
considered.
4.1.2 Health surveillance advice
A complete medical history and physical examination should be made, on
an annual basis, in workers regularly exposed to PCP. Special
attention should be paid to the cardiovascular system, upper
respiratory tract, liver, kidneys, and skin. Regular measurement of
PCP exposure should be undertaken, preferably in the breathing zone.
4.2 Explosion and Fire Hazards
4.2.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation, or on the characteristics of the dust.
4.2.2 Fire hazards
Technical PCP will not burn. Formulated products and oil solutions are
likely to be highly flammable. All products will decompose and produce
harmful fumes, if involved in a fire, and the fire service must be
advised accordingly.
Fires should be controlled with alcohol-resistant foam, dry powder, or
carbon dioxide. The use of water should be confined to the cooling of
unaffected stock, thus avoiding the accumulation of polluted run-off.
4.3 Storage
Technical PCP is a solid. The formulated product is usually a solution
in oil or organic solvent, or an emulsifiable concentrate.
All PCP products should be stored in secure, well ventilated buildings
under cool and dry conditions, and out of reach of children and
unauthorized persons. Keep away from food, drink, and animal feed.
If any containers in the store are leaking, take precautions and use
personal protective equipment as required (see International Chemical
Safety Card). Empty any product remaining in damaged/leaking
containers into a clean, empty drum, which should then be suitably
labelled.
Sweep up spillage with sawdust, sand, or earth and dispose of safely.
Wash contaminated areas with detergent and a small amount of water,
absorbing as much as possible with sawdust, sand, or earth.
When emptied, decontaminate leaking containers several times with at
least 1 litre of water per 20-litre drum. Swirl round to rinse walls,
empty and add rinsings to the contaminated sawdust, sand, or earth.
Puncture the container to prevent re-use.
4.4 Transport
Comply with any local requirements regarding movement of hazardous
goods. Do not load with feed or foodstuffs. Check that containers are
sound and correctly labelled before despatch.
4.5 Spillage and Disposal
4.5.1 Spillage
Before dealing with any spillage, precautions should be taken as
required and appropriate personal protective equipment should be used
(see International Chemical Safety Card).
Absorb spilt liquids with sawdust, lime, sand, or earth. Prevent
liquids from spreading or contaminating other cargo, vegetation, or
waterways by building a barrier of the most readily available
material, e.g., earth or sand.
Sweep up spilt technical material and place this together with any
contaminated absorbents in a closeable container for later transfer to
a safe place for disposal.
4.5.2 Disposal
Surplus product, contaminated absorbents and containers should be
burned at a high temperature in an appropriate incinerator with
effluent gas scrubbing. When no incinerator is available, bury in an
approved dump, in an area where there is no risk of contamination of
surface or ground water. Comply with any local legislation.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Because of its action as an uncoupler of oxidative phosphorylation,
pentachlorophenol is highly hazardous for most forms of terrestrial
and aquatic life, depending on the exposure level. It is a rather
persistent and mobile pesticide and as a result it can occur in all
environmental compartments.
It is therefore essential that PCP levels in the environment be kept
as low as possible. Containers should not be emptied or washed into
ditches or waterways. Any effluent containing PCP should be properly
treated. Any PCP spillage should be prevented from contaminating
vegetation and waterways.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily, available to all health workers concerned
with, and users of, pentachlorophenol. It should be displayed at, or
near, entrances to areas where there is potential exposure to
pentachlorophenol, and on processing equipment and containers. The
card should be translated into the appropriate language(s). All
persons potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
INTERNATIONAL CHEMICAL SAFETY CARD
PENTACHLOROPHENOL CAS No. 87-86-5; C6Cl5OH
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point, decomposition 310°C Colourless to light brown flakes or crystals, with
Melting point 191°C characteristic phenolic odour; decomposes on
Relative density (water = 1) 2.0 heating in the presence of water, forming corrosive
(air = 1) 9.2 fumes (hydrochloric acid); substance may be absorbed
Vapour pressure (20°C) 2 mPa into body by inhalation, or ingestion or through skin;
Relative molecular mass 266.3 corrosive to the respiratory tract; affects the nervous
Octanol/water part. coeff. (pH 6.5) 3.56 system; not flammable and non-corrosive in unmixed
state; dissolved in oil, it causes deterioration
Solubility in water (20°C, pH 7) 2 g/litre of rubber
Solubility in organic solvents (25°C)
acetone 500 g/litre
benzene 150 g/litre
ethanol 1200 g/litre
methanol 1800 g/litre
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
SODIUM PENTACHLOROPHENATE CAS No. 131-52-2; C6Cl5ONa
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Boiling point decomposition Tan powder, pellets, or briquettes with phenolic
Melting point decomposition odour; decomposes on heating, forming toxic
Relative density (water = 1) 2.0 fumes (chlorides and sodium oxide); substance may
Relative molecular mass 288.3 be absorbed into body by inhalation, ingestion, and
Solubility in water (25°C) 330 g/litre through skin; corrosive to the respiratory tract
Solubility in organic solvents (25°C)
acetone 350 g/litre
benzene insoluble
ethanol 650 g/litre
methanol 250 g/litre
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
BOTH COMPOUNDS
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Strict hygiene; prevent dispersion
Highly toxic chemical of dust; if you feel unwell seek
medical advice (show the label where
possible); keep out of reach of
children
SKIN: toxic in contact with Avoid contact; wear PVC or neoprene Remove contaminated clothes, wash
skin; may cause white spots, gloves, neoprene apron, and rubber contaminated skin immediately with
sometimes wounds boots plenty of water and soap, and obtain
medical attention
EYES: may cause redness, pain Avoid contamination with dust or mist; First rinse with plenty of water for
use face shield or goggles 15 minutes, then transport to a doctor,
if necessary
INHALATION: toxic by inhalation; Avoid inhalation of vapour, dust, or Fresh air, rest, half-upright position, cool
may cause headache, fatigue, mist; use local exhaust ventilation down, and transport to hospital; symptoms
perspiration, thirst, faintness, or breathing protection; closed of lung oedema often do not become manifest
increased body temperature; in severe system; not recommended for until a few hours have passed, and are
cases lung oedema may occur interior use on large surface areas aggravated by physical effort; hospitalization
is therefore essential
INGESTION: toxic if swallowed; Do not eat, drink, or smoke during Do not induce vomiting; otherwise
may cause nausea, vomiting, work; keep away from food, drink, as above
abdominal spasm, diarrhoea; and animal feed
liver and kidney injury may
occur; severe cases may be fatal
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
BOTH COMPOUNDS
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
REPEATED EXPOSURE -- Take bath or shower after work and As above
SKIN, EYES, INHALATION, change clothing; otherwise as above
INGESTION: May cause
acneiform dermatitis
ENVIRONMENT: highly Avoid spillage into the
hazardous to most aquatic environment
and terrestrial organisms
SPILLAGE STORAGE FIRE AND EXPLOSION
Sweep up spilled substances, Store in secure well ventilated, Not combustible, formulated products may
and remove to safe place; cool and dry place; do not store be flammable; may give rise to harmful
carefully collect remainder near foodstuffs or animal feed decomposition products such as polychlorinated
(extra personal protection, dibenzo-p-dioxines; control fares with
particle-filter respirator); alcohol resistant foam, dry powder, or carbon
adsorb spilled liquids with dioxide
sawdust, lime, sand, or earth;
prevent liquids from spreading
or contaminating other cargo,
vegetation or waterways
INTERNATIONAL CHEMICAL SAFETY CARD (cont'd).
BOTH COMPOUNDS
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
WASTE DISPOSAL
Burn at high temperature in National occupational exposure UN: 2761, 2762, 2995, 2996
incinerator with effluent gas limit:
scrubbing; if not available,
bury in an approved dump; National poison control centre:
comply with local regulations
Local trade names:
 7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. The intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
"The WHO Recommended Classification of Pesticides by Hazard" (WHO,
1986) distinguishes between the four hazard classes Ia, Ib, II, and
III, on the basis of the toxicity of technical products. In this
report, PCP is classified in Class Ib, being highly hazardous.
The WHO manual "Prevention, diagnosis and treatment of insecticide
poisoning" (Plestina, 1984) provides practical advice that generally
applies to nitro- and chlorophenols. In the WHO Guidelines for
drinking-water quality, a guideline value of 10 µg/litre is
recommended for PCP.
No evaluation of the carcinogenicity of PCP has been made by the
International Agency for Research on Cancer, because the available
data on the carcinogenic and mutagenic effects of PCP were considered
inadequate for a sound evaluation.
IRPTC (1984) issued a review on pentachlorophenol in its series
"Scientific reviews of Soviet literature on toxicity and hazard of
chemicals".
7.2 Exposure Limit Values
Some examples of exposure limit values are shown in the following
table. When no effective date appears in the IRPTC legal file, the
year of the reference from which the data are taken is indicated by
(r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace Japan Maximum allowable concentration (MAC) 0.5 mg/m3
- time-weighted average (TWA)
- short-term exposure limit (STEL)
Sweden Hygienic limit value (8-h TWA) 0.5 mg/m3
- short-term exposure limit 1.5 mg/m3
United Kingdom Recommended limit (RECL) (8-h TWA) 0.5 mg/m3
- short-term exposure limit (10-min TWA) 1.5 mg/m3
Germany, Maximum work-site concentration
Federal - time-weighted average (8 hours) 0.5 mg/m3
Republic of - 30-min STEL, 4 ×/shift 1 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.1 mg/m3 1977
USA Permissible exposure limit (PEL-TWA) 0.5 mg/m3
- STEL 1.5 mg/m3
AIR Workplace Italy Threshold limit value (TLV) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (1 × per day) 0.005 mg/m3
(average per day) 0.001 mg/m3
Preliminary safety limits (PSL) (1 × per day) 0.02 µg/m3
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD USA Acceptable daily intake (ADI) 3 µg/kg 1977
body weight
per day
FOOD Plant Germany, Maximum residue limits 0.01-0.03 mg/kg 1978
Federal
Republic of
WATER Surface USSR Maximum allowable concentration 0.01 mg/litre 1983
WATER Drinking WHO Maximum allowable concentration
(guideline value) 10 µg/litre 1984
7.3 Specific Restrictions
The use of pentachorophenol has been more and more restricted during
the last few years as a result of the increasing concern about the
potential health and environmental hazards of PCP and its impurities.
To mention a few:
- Sweden banned all use of PCP in 1977 and the Federal Republic of
Germany banned all use in 1987;
- the USA cancelled its registration for herbicidal and
anti-microbial use and for the preservation of wood in contact
with food, feed, domestic animals, and livestock. The sale and
use of PCP is restricted to certified applicators;
- the agricultural use of PCP has been suspended or restricted in,
among others, Canada, Denmark, German Democratic Republic, and
Japan;
- Canada and the Netherlands have suspended its use for indoor wood
treatment.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pentachlorophenol in:
- Hazard Class 6.1: poisonous substance;
- Packing Group II: a substance presenting a serious risk of
poisoning in transport.
The label should be as follows:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. The intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
"The WHO Recommended Classification of Pesticides by Hazard" (WHO,
1986) distinguishes between the four hazard classes Ia, Ib, II, and
III, on the basis of the toxicity of technical products. In this
report, PCP is classified in Class Ib, being highly hazardous.
The WHO manual "Prevention, diagnosis and treatment of insecticide
poisoning" (Plestina, 1984) provides practical advice that generally
applies to nitro- and chlorophenols. In the WHO Guidelines for
drinking-water quality, a guideline value of 10 µg/litre is
recommended for PCP.
No evaluation of the carcinogenicity of PCP has been made by the
International Agency for Research on Cancer, because the available
data on the carcinogenic and mutagenic effects of PCP were considered
inadequate for a sound evaluation.
IRPTC (1984) issued a review on pentachlorophenol in its series
"Scientific reviews of Soviet literature on toxicity and hazard of
chemicals".
7.2 Exposure Limit Values
Some examples of exposure limit values are shown in the following
table. When no effective date appears in the IRPTC legal file, the
year of the reference from which the data are taken is indicated by
(r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace Japan Maximum allowable concentration (MAC) 0.5 mg/m3
- time-weighted average (TWA)
- short-term exposure limit (STEL)
Sweden Hygienic limit value (8-h TWA) 0.5 mg/m3
- short-term exposure limit 1.5 mg/m3
United Kingdom Recommended limit (RECL) (8-h TWA) 0.5 mg/m3
- short-term exposure limit (10-min TWA) 1.5 mg/m3
Germany, Maximum work-site concentration
Federal - time-weighted average (8 hours) 0.5 mg/m3
Republic of - 30-min STEL, 4 ×/shift 1 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.1 mg/m3 1977
USA Permissible exposure limit (PEL-TWA) 0.5 mg/m3
- STEL 1.5 mg/m3
AIR Workplace Italy Threshold limit value (TLV) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (1 × per day) 0.005 mg/m3
(average per day) 0.001 mg/m3
Preliminary safety limits (PSL) (1 × per day) 0.02 µg/m3
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD USA Acceptable daily intake (ADI) 3 µg/kg 1977
body weight
per day
FOOD Plant Germany, Maximum residue limits 0.01-0.03 mg/kg 1978
Federal
Republic of
WATER Surface USSR Maximum allowable concentration 0.01 mg/litre 1983
WATER Drinking WHO Maximum allowable concentration
(guideline value) 10 µg/litre 1984
7.3 Specific Restrictions
The use of pentachorophenol has been more and more restricted during
the last few years as a result of the increasing concern about the
potential health and environmental hazards of PCP and its impurities.
To mention a few:
- Sweden banned all use of PCP in 1977 and the Federal Republic of
Germany banned all use in 1987;
- the USA cancelled its registration for herbicidal and
anti-microbial use and for the preservation of wood in contact
with food, feed, domestic animals, and livestock. The sale and
use of PCP is restricted to certified applicators;
- the agricultural use of PCP has been suspended or restricted in,
among others, Canada, Denmark, German Democratic Republic, and
Japan;
- Canada and the Netherlands have suspended its use for indoor wood
treatment.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pentachlorophenol in:
- Hazard Class 6.1: poisonous substance;
- Packing Group II: a substance presenting a serious risk of
poisoning in transport.
The label should be as follows:
 The European Community legislation requires labelling as a dangerous
substance using the symbol:
The European Community legislation requires labelling as a dangerous
substance using the symbol:
 The label must read:
Toxic by inhalation, in contact with skin and if swallowed, after
contact with skill, wash immediately with plenty of ........
(to be specified by the manufacturer); wear suitable protective
clothing and eye/face protection; if you feel unwell, seek medical
advice(show the label where possible).
The European Community legislation on the labelling of paints,
varnishes, printing inks, adhesives, and similar products requires the
following labelling.
( a) When the concentration of pentachlorophenol in these
preparations exceeds 5%, the symbol used should be:
The label must read:
Toxic by inhalation, in contact with skin and if swallowed, after
contact with skill, wash immediately with plenty of ........
(to be specified by the manufacturer); wear suitable protective
clothing and eye/face protection; if you feel unwell, seek medical
advice(show the label where possible).
The European Community legislation on the labelling of paints,
varnishes, printing inks, adhesives, and similar products requires the
following labelling.
( a) When the concentration of pentachlorophenol in these
preparations exceeds 5%, the symbol used should be:
 ( b) When the concentration is between 0.5 and 5%, the symbol should
be:
( b) When the concentration is between 0.5 and 5%, the symbol should
be:
 The European Community legislation on the labelling of pesticide
preparations classifies pentachlorophenols in Class 1/a for the
purpose of determining the label for pesticide preparations containing
this substance.
7.5 Waste Disposal
In the USA, pentachlorophenol is regarded as hazardous and restricted
for the purpose of discharge into waters. Detailed instructions are
given.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing storage and transport. Brussels, Groupement
International des Associations Nationales des Fabricants de Produits
Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyons, International Agency for Research on
Cancer.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols, Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHSS(NIOSH) 01-123).
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished WHO document VBC/86.1).
WHO (1987) EHC No. 71: Pentachlorophenol. Geneva, World Health
Organization, 236pp.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The European Community legislation on the labelling of pesticide
preparations classifies pentachlorophenols in Class 1/a for the
purpose of determining the label for pesticide preparations containing
this substance.
7.5 Waste Disposal
In the USA, pentachlorophenol is regarded as hazardous and restricted
for the purpose of discharge into waters. Detailed instructions are
given.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing storage and transport. Brussels, Groupement
International des Associations Nationales des Fabricants de Produits
Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyons, International Agency for Research on
Cancer.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols, Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHSS(NIOSH) 01-123).
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished WHO document VBC/86.1).
WHO (1987) EHC No. 71: Pentachlorophenol. Geneva, World Health
Organization, 236pp.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
 Molecular formula: C6Cl5OH
CAS chemical name: pentachlorophenol
Common synonyms: chlorophen; PCP; penchlorol; penta;
pentachlorofenol; pentachlorofenolo;
pentachlorphenol; 2,3,4,5,6-pentachlorophenol
CAS registry
number:
Molecular formula: C6Cl5OH
CAS chemical name: pentachlorophenol
Common synonyms: chlorophen; PCP; penchlorol; penta;
pentachlorofenol; pentachlorofenolo;
pentachlorphenol; 2,3,4,5,6-pentachlorophenol
CAS registry
number:  Molecular formula: C6Cl5ONa
C6Cl5ONa.H2O (as monohydrate)
Common synonyms: penta-ate; pentachlorophenate sodium;
pentachlorophenol, sodium salt;
pentachlorophenoxy sodium; pentaphenate;
phenol, pentachloro-, sodium derivative
monohydrate; sodium PCP; sodium
pentachlorophenate; sodium
pentachlorophenolate; sodium
pentachlorophenoxide
CAS registry
Molecular formula: C6Cl5ONa
C6Cl5ONa.H2O (as monohydrate)
Common synonyms: penta-ate; pentachlorophenate sodium;
pentachlorophenol, sodium salt;
pentachlorophenoxy sodium; pentaphenate;
phenol, pentachloro-, sodium derivative
monohydrate; sodium PCP; sodium
pentachlorophenate; sodium
pentachlorophenolate; sodium
pentachlorophenoxide
CAS registry  7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. The intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
"The WHO Recommended Classification of Pesticides by Hazard" (WHO,
1986) distinguishes between the four hazard classes Ia, Ib, II, and
III, on the basis of the toxicity of technical products. In this
report, PCP is classified in Class Ib, being highly hazardous.
The WHO manual "Prevention, diagnosis and treatment of insecticide
poisoning" (Plestina, 1984) provides practical advice that generally
applies to nitro- and chlorophenols. In the WHO Guidelines for
drinking-water quality, a guideline value of 10 µg/litre is
recommended for PCP.
No evaluation of the carcinogenicity of PCP has been made by the
International Agency for Research on Cancer, because the available
data on the carcinogenic and mutagenic effects of PCP were considered
inadequate for a sound evaluation.
IRPTC (1984) issued a review on pentachlorophenol in its series
"Scientific reviews of Soviet literature on toxicity and hazard of
chemicals".
7.2 Exposure Limit Values
Some examples of exposure limit values are shown in the following
table. When no effective date appears in the IRPTC legal file, the
year of the reference from which the data are taken is indicated by
(r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace Japan Maximum allowable concentration (MAC) 0.5 mg/m3
- time-weighted average (TWA)
- short-term exposure limit (STEL)
Sweden Hygienic limit value (8-h TWA) 0.5 mg/m3
- short-term exposure limit 1.5 mg/m3
United Kingdom Recommended limit (RECL) (8-h TWA) 0.5 mg/m3
- short-term exposure limit (10-min TWA) 1.5 mg/m3
Germany, Maximum work-site concentration
Federal - time-weighted average (8 hours) 0.5 mg/m3
Republic of - 30-min STEL, 4 ×/shift 1 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.1 mg/m3 1977
USA Permissible exposure limit (PEL-TWA) 0.5 mg/m3
- STEL 1.5 mg/m3
AIR Workplace Italy Threshold limit value (TLV) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (1 × per day) 0.005 mg/m3
(average per day) 0.001 mg/m3
Preliminary safety limits (PSL) (1 × per day) 0.02 µg/m3
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD USA Acceptable daily intake (ADI) 3 µg/kg 1977
body weight
per day
FOOD Plant Germany, Maximum residue limits 0.01-0.03 mg/kg 1978
Federal
Republic of
WATER Surface USSR Maximum allowable concentration 0.01 mg/litre 1983
WATER Drinking WHO Maximum allowable concentration
(guideline value) 10 µg/litre 1984
7.3 Specific Restrictions
The use of pentachorophenol has been more and more restricted during
the last few years as a result of the increasing concern about the
potential health and environmental hazards of PCP and its impurities.
To mention a few:
- Sweden banned all use of PCP in 1977 and the Federal Republic of
Germany banned all use in 1987;
- the USA cancelled its registration for herbicidal and
anti-microbial use and for the preservation of wood in contact
with food, feed, domestic animals, and livestock. The sale and
use of PCP is restricted to certified applicators;
- the agricultural use of PCP has been suspended or restricted in,
among others, Canada, Denmark, German Democratic Republic, and
Japan;
- Canada and the Netherlands have suspended its use for indoor wood
treatment.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pentachlorophenol in:
- Hazard Class 6.1: poisonous substance;
- Packing Group II: a substance presenting a serious risk of
poisoning in transport.
The label should be as follows:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. The intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
"The WHO Recommended Classification of Pesticides by Hazard" (WHO,
1986) distinguishes between the four hazard classes Ia, Ib, II, and
III, on the basis of the toxicity of technical products. In this
report, PCP is classified in Class Ib, being highly hazardous.
The WHO manual "Prevention, diagnosis and treatment of insecticide
poisoning" (Plestina, 1984) provides practical advice that generally
applies to nitro- and chlorophenols. In the WHO Guidelines for
drinking-water quality, a guideline value of 10 µg/litre is
recommended for PCP.
No evaluation of the carcinogenicity of PCP has been made by the
International Agency for Research on Cancer, because the available
data on the carcinogenic and mutagenic effects of PCP were considered
inadequate for a sound evaluation.
IRPTC (1984) issued a review on pentachlorophenol in its series
"Scientific reviews of Soviet literature on toxicity and hazard of
chemicals".
7.2 Exposure Limit Values
Some examples of exposure limit values are shown in the following
table. When no effective date appears in the IRPTC legal file, the
year of the reference from which the data are taken is indicated by
(r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace Japan Maximum allowable concentration (MAC) 0.5 mg/m3
- time-weighted average (TWA)
- short-term exposure limit (STEL)
Sweden Hygienic limit value (8-h TWA) 0.5 mg/m3
- short-term exposure limit 1.5 mg/m3
United Kingdom Recommended limit (RECL) (8-h TWA) 0.5 mg/m3
- short-term exposure limit (10-min TWA) 1.5 mg/m3
Germany, Maximum work-site concentration
Federal - time-weighted average (8 hours) 0.5 mg/m3
Republic of - 30-min STEL, 4 ×/shift 1 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.1 mg/m3 1977
USA Permissible exposure limit (PEL-TWA) 0.5 mg/m3
- STEL 1.5 mg/m3
AIR Workplace Italy Threshold limit value (TLV) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (1 × per day) 0.005 mg/m3
(average per day) 0.001 mg/m3
Preliminary safety limits (PSL) (1 × per day) 0.02 µg/m3
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD USA Acceptable daily intake (ADI) 3 µg/kg 1977
body weight
per day
FOOD Plant Germany, Maximum residue limits 0.01-0.03 mg/kg 1978
Federal
Republic of
WATER Surface USSR Maximum allowable concentration 0.01 mg/litre 1983
WATER Drinking WHO Maximum allowable concentration
(guideline value) 10 µg/litre 1984
7.3 Specific Restrictions
The use of pentachorophenol has been more and more restricted during
the last few years as a result of the increasing concern about the
potential health and environmental hazards of PCP and its impurities.
To mention a few:
- Sweden banned all use of PCP in 1977 and the Federal Republic of
Germany banned all use in 1987;
- the USA cancelled its registration for herbicidal and
anti-microbial use and for the preservation of wood in contact
with food, feed, domestic animals, and livestock. The sale and
use of PCP is restricted to certified applicators;
- the agricultural use of PCP has been suspended or restricted in,
among others, Canada, Denmark, German Democratic Republic, and
Japan;
- Canada and the Netherlands have suspended its use for indoor wood
treatment.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pentachlorophenol in:
- Hazard Class 6.1: poisonous substance;
- Packing Group II: a substance presenting a serious risk of
poisoning in transport.
The label should be as follows:
 The European Community legislation requires labelling as a dangerous
substance using the symbol:
The European Community legislation requires labelling as a dangerous
substance using the symbol:
 The label must read:
Toxic by inhalation, in contact with skin and if swallowed, after
contact with skill, wash immediately with plenty of ........
(to be specified by the manufacturer); wear suitable protective
clothing and eye/face protection; if you feel unwell, seek medical
advice(show the label where possible).
The European Community legislation on the labelling of paints,
varnishes, printing inks, adhesives, and similar products requires the
following labelling.
( a) When the concentration of pentachlorophenol in these
preparations exceeds 5%, the symbol used should be:
The label must read:
Toxic by inhalation, in contact with skin and if swallowed, after
contact with skill, wash immediately with plenty of ........
(to be specified by the manufacturer); wear suitable protective
clothing and eye/face protection; if you feel unwell, seek medical
advice(show the label where possible).
The European Community legislation on the labelling of paints,
varnishes, printing inks, adhesives, and similar products requires the
following labelling.
( a) When the concentration of pentachlorophenol in these
preparations exceeds 5%, the symbol used should be:
 ( b) When the concentration is between 0.5 and 5%, the symbol should
be:
( b) When the concentration is between 0.5 and 5%, the symbol should
be:
 The European Community legislation on the labelling of pesticide
preparations classifies pentachlorophenols in Class 1/a for the
purpose of determining the label for pesticide preparations containing
this substance.
7.5 Waste Disposal
In the USA, pentachlorophenol is regarded as hazardous and restricted
for the purpose of discharge into waters. Detailed instructions are
given.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing storage and transport. Brussels, Groupement
International des Associations Nationales des Fabricants de Produits
Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyons, International Agency for Research on
Cancer.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols, Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHSS(NIOSH) 01-123).
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished WHO document VBC/86.1).
WHO (1987) EHC No. 71: Pentachlorophenol. Geneva, World Health
Organization, 236pp.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The European Community legislation on the labelling of pesticide
preparations classifies pentachlorophenols in Class 1/a for the
purpose of determining the label for pesticide preparations containing
this substance.
7.5 Waste Disposal
In the USA, pentachlorophenol is regarded as hazardous and restricted
for the purpose of discharge into waters. Detailed instructions are
given.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing storage and transport. Brussels, Groupement
International des Associations Nationales des Fabricants de Produits
Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyons, International Agency for Research on
Cancer.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols, Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHSS(NIOSH) 01-123).
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished WHO document VBC/86.1).
WHO (1987) EHC No. 71: Pentachlorophenol. Geneva, World Health
Organization, 236pp.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
