
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 98
TETRAMETHRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Tetramethrin.
(Environmental health criteria ; 98)
1.Pyrethrins I.Series
ISBN 92 4 154298 5 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TETRAMETHRIN
INTRODUCTION
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties, analytical
methods
1.1.2. Production and use
1.1.3. Human exposure
1.1.4. Environmental exposure and fate
1.1.5. Uptake, metabolism, and excretion
1.1.6. Effects on organisms in the environment
1.1.7. Effects on experimental animals and in vitro test
systems
1.1.8. Effects on human beings
1.2. Conclusions
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Industrial production
3.2. Use patterns
3.3. Residues in food
3.4. Exposure levels from household use
3.5. Environment levels
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Abiotic degradation in air and water
5. KINETICS AND METABOLISM
5.1. Metabolism in mammals
5.2. Enzymatic systems for biotransformation
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.2. Terrestrial organisms
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.2. Irritation and sensitization
7.2.1. Eye irritation
7.2.2. Skin irritation
7.2.3. Sensitization
7.3. Short-term exposure studies
7.3.1. Oral
7.3.2. Inhalation
7.4. Long-term exposures and carcinogenicity
7.5. Mutagenicity
7.6. Reproduction, embryotoxicity, and teratogenicity
7.7. Neurotoxicity - mode of action
8. EFFECTS ON HUMANS
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX I
FRENCH TRANSLATION OF SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TETRAMETHRIN
Members
Dr V. Benes, Toxicology and Reference Laboratory,
Institute of Hygiene and Epidemiology, Prague,
Czechoslovakia
Dr A.J. Browning, Toxicology Evaluation Section, Depart-
ment of Community Services and Health, Woden, ACT,
Australia
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United
Kingdom (Chairman)
Dr P. Hurley, Office of Pesticide Programme, US Environ-
mental Protection Agency, Washington DC, USA
Dr K. Imaida, Section of Tumor Pathology, Division of
Pathology, National Institute of Hygienic Sciences,
Setagaya-Ku, Tokyo, Japan
Dr S.K. Kashyap, National Institute of Occupational
Health, (I.C.M.R.) Ahmedabad, India (Vice-Chairman)
Dr Yu. I. Kundiev, Research Institute of Labour, Hygiene
and Occupational Diseases, Ul. Saksaganskogo, Kiev,
USSR
Dr J.P. Leahey, ICI Agrochemicals, Jealotts Hill Research
Station, Bracknell, United Kingdom (Rapporteur)
Dr M. Matsuo, Sumitomo Chemical Company, Biochemistry and
Toxicology Laboratory, Kasugade-naka, Konohana-Ku,
Osaka, Japan
Dr J. Sekizawa, Division of Information on Chemical
Safety, National Institute of Hygienic Sciences,
Setagaya-Ku, Tokyo, Japan (Rapporteur)
Representatives of Other Organization
Mr M. L'Hotellier, Groupement International des Associ-
ations Nationales de Fabricants de Produits Agro-
chimiques (GIFAP)
Dr N. Punja, Groupement International des Associations
Nationales de Fabricants de Produits Agrochimiques
(GIFAP)
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
(Secretary)
Dr R. Plestina, Division of Vector Biology and Control,
World Health Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria documents,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be
obtained from the International Register of Potentially
Toxic Chemicals, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 7988400 - 7985850).
* * *
The proprietary information contained in this document
cannot replace documentation for registration purposes,
because the latter has to be closely linked to the source,
the manufacturing route, and the purity/impurities of the
substance to be registered. The data should be used in
accordance with paragraphs 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation
[5].
ENVIRONMENTAL HEALTH CRITERIA FOR TETRAMETHRIN
A WHO Task Group on Environmental Health Criteria for
Tetramethrin met in Geneva from 24 to 28 October 1988.
Dr M. Mercier, Manager, IPCS, opened the meeting and wel-
comed the participants on behalf of the three IPCS cooper-
rating organizations (UNEP/ILO/WHO). The group reviewed
and revised the draft monograph and made an evaluation of
the risks for human health and the environment from
exposure to tetramethrin.
The first draft was prepared by DR J. MIYAMOTO and
DR M. MATSUO of Sumitomo Chemical Company, and
DR J. SEKIZAWA of the National Institute of Hygienic
Sciences, Tokyo, Japan.
The second draft was prepared by the IPCS secretariat,
incorporating comments received following circulation of
the first draft to the IPCS contact points for Environ-
mental Health Criteria documents. Dr K.W. Jager and Dr
P.G. Jenkins, both members of the IPCS Central Unit, were
responsible for the technical development and editing,
respectively, of this monograph.
The assistance of the Sumitomo Chemical Company in
making available to the IPCS and the Task Group its toxi-
cological proprietary information on tetramethrin is
gratefully acknowledged. This allowed the Task Group to
make its evaluation on the basis of more complete data.
* * *
The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
ABBREVIATIONS
CA chrysanthemic acid
FID-GC gas chromatography with flame ionization detector
HPI cyclohexane-1,2-dicarboximide
HPLC high performance liquid chromatography
HPTLC high performance thin-layer chromatography
ip intraperitoneal
MTI N -(hydroxymethyl)-3,4,5,6-tetrahydrophthalamide
NOEL no-observed-effect-level
TLC thin-layer chromatography
TPI 3,4,5,6-tetrahydrophthalimide
TPIA 3,4,5,6-tetrahydrophthalic acid
INTRODUCTION
SYNTHETIC PYRETHROIDS - A PROFILE
1. During investigations to modify the chemical struc-
tures of natural pyrethrins, a certain number of syn-
thetic pyrethroids were produced with improved physi-
cal and chemical properties and greater biological
activity. Several of the earlier synthetic pyrethroids
were successfully commercialized, mainly for the con-
trol of household insects. Other more recent
pyrethroids have been introduced as agricultural in-
secticides because of their excellent activity against
a wide range of insect pests and their non-persistence
in the environment.
2. The pyrethroids constitute another group of insecti-
cides in addition to organochlorine, organophosphorus,
carbamate, and other compounds. Pyrethroids commer-
cially available to date include allethrin, res-
methrin, d-phenothrin, and tetramethrin (for insects
of public health importance), and cypermethrin, delta-
methrin, fenvalerate, and permethrin (mainly for agri-
cultural insects). Other pyrethroids are also avail-
able including furamethrin, kadethrin, and tellalle-
thrin (usually for household insects), fenpropathrin,
tralomethrin, cyhalothrin, lambda-cyhalothrin, teflu-
thrin, cyfluthrin, flucythrinate, fluvalinate, and bi-
phenate (for agricultural insects).
3. Toxicological evaluations of several synthetic pyreth-
roids have been performed by the FAO/WHO Joint Meeting
on Pesticide Residues (JMPR). The acceptable daily in-
take (ADI) has been estimated by the JMPR for cyperme-
thrin, deltamethrin, fenvalerate, permethrin, d-pheno-
thrin, cyfluthrin, cyhalothrin, and flucythrinate.
4. Chemically, synthetic pyrethroids are esters of
specific acids (e.g., chrysanthemic acid, halo-substi-
tuted chrysanthemic acid, 2-(4-chlorophenyl)-3-methyl-
butyric acid) and alcohols (e.g., allethrolone, 3-
phenoxybenzyl alcohol). For certain pyrethroids, the
asymmetric centre(s) exist in the acid and/or alcohol
moiety, and the commercial products sometimes consist
of a mixture of both optical (1R/1S or d/1) and geo-
metric (cis/trans) isomers. However, most of the
insecticidal activity of such products may reside in
only one or two isomers. Some of the products (e.g.,
d-phenothrin, deltamethrin) consist only of such
active isomer(s).
5. Synthetic pyrethroids are neuropoisons acting on the
axons in the peripheral and central nervous systems by
interacting with sodium channels in mammals and/or
insects. A single dose produces toxic signs in mam-
mals, such as tremors, hyperexcitability, salivation,
choreoathetosis, and paralysis. The signs disappear
fairly rapidly, and the animals recover, generally
within a week. At near-lethal dose levels, synthetic
pyrethroids cause transient changes in the nervous
system, such as axonal swelling and/or breaks and
myelin degeneration in sciatic nerves. They are not
considered to cause delayed neurotoxicity of the kind
induced by some organophosphorus compounds. The mech-
anism of toxicity of synthetic pyrethroids and their
classification into two types are discussed in the
Appendix.
6. Some pyrethroids (e.g., deltamethrin, fenvalerate,
cyhalothrin, lambda-cyhalothrin, flucythrinate, and
cypermethrin) may cause a transient itching and/or
burning sensation in exposed human skin.
7. Synthetic pyrethroids are generally metabolized in
mammals through ester hydrolysis, oxidation, and con-
jugation, and there is no tendency to accumulate in
tissues. In the environment, synthetic pyrethroids are
fairly rapidly degraded in soil and in plants. Ester
hydrolysis and oxidation at various sites on the mol-
ecule are the major degradation processes. The
pyrethroids are strongly adsorbed on soil and sedi-
ments, and hardly eluted with water. There is little
tendency for bioaccumulation in organisms.
8. Because of low application rates and rapid degradation
in the environment, residues in food are generally
low.
9. Synthetic pyrethroids have been shown to be toxic for
fish, aquatic arthropods, and honey-bees in laboratory
tests. But, in practical usage, no serious adverse
effects have been noticed because of the low rates of
application and lack of persistence in the environ-
ment. The toxicity of synthetic pyrethroids in birds
and domestic animals is low.
10. In addition to the evaluation documents of FAO/WHO,
there are several good reviews and books on the chem-
istry, metabolism, mammalian toxicity, environmental
effects, etc. of synthetic pyrethroids, including
those by Elliott [3], Miyamoto [34], Miyamoto & Kear-
ney [35], and Leahey [26].
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and Evaluation
1.1.1 Identity, physical and chemical properties, analytical methods
Tetramethrin was first synthesized in 1964 and first
marketed in 1965. Chemically, it is an ester of chrysan-
themic acid (2,2-dimethyl-3-(2,2-dimethylvinyl)-cyclopro-
panecarboxylic acid) with 3,4,5,6-tetrahydrophthalimido-
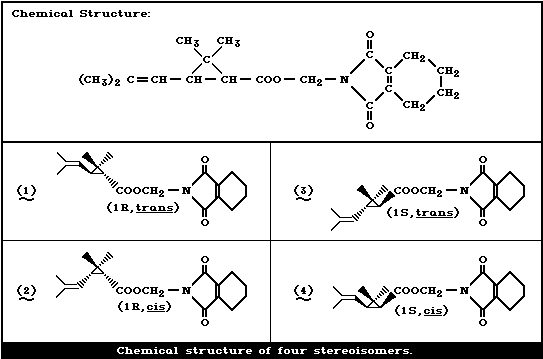
methyl alcohol. It is a mixture of four stereoisomers:
[1R,trans], [1R,cis], [1S,trans], and [1S,cis]. In techni-
cal products, the composition ratio of the isomers is
roughly 4:1:4:1. Among the isomers, the [1R,trans] isomer
is the most active biologically followed by the [1R,cis]
isomer. A mixture of the [1R,cis] and [1R,trans] isomers
(1:4) is commercialized under the trade name of `Neo-
Pynamin Forte' (designated as 1R, cis/trans-tetramethrin in
this monograph).
Technical grade tetramethrin is a colourless solid
with a melting point of 65-80°C. The specific gravity is
1.11 at 20 °C, and the vapour pressure is 0.946 mPa
(7.1 x 10-6 mmHg) at 30 °C. It is sparingly soluble in
water (4.6 mg/litre at 30 °C) but soluble in organic sol-
vents such as hexane, methanol, and xylene. It is stable
to heat but unstable to light and air. The [1R,cis/trans]
isomer of tetramethrin is a yellow viscous liquid but
otherwise has physical and chemical properties similar to
tetramethrin.
Residue analysis is carried out by quantification
using dual-wavelength densitometry (370-230 nm). Gas
chromatography with flame ionization detector is used for
technical product analysis. Formulation analysis can be
carried out by high-performance liquid chromatography with
an infra-red detector.
1.1.2 Production and use
The annual world-wide production of tetramethrin is
estimated to be a few hundred tonnes. It is mostly used
for indoor pest control, formulated as an aerosol, an
emulsifiable concentrate, or a mosquito coil. Formulations
in combination with other insecticides and synergists are
also prepared.
1.1.3 Human exposure
The general population may be exposed to tetramethrin
primarily through its use in indoor pest control. When
tetramethrin is used as recommended, the aerial levels and
those of its 1R isomer are unlikely to exceed 0.5 mg/mg3,
and the compound will degrade rapidly. Therefore, the
exposure of the general population is expected to be very
low. Tetramethrin is not used on food crops.
1.1.4 Environmental exposure and fate
Rapid degradation occurs when a thin film of tetra-
methrin is exposed to sunlight. The major photoreactions
during a 2-h exposure (30% conversion) were: epoxidation
at the isobutenyl double bond; oxidation at the trans-
methyl of the isobutenyl group to hydroxymethyl, alde-
hyde, and carboxylic acid; and hydroperoxidation to
allylic hydroperoxide.
No data are available on the exact levels of tetra-
methrin in the environment, but with the current domestic
pattern of use and when tetramethrin is used as rec-
ommended, environmental exposure is expected to be very
low. Degradation to less toxic products is rapid.
1.1.5 Uptake, metabolism, and excretion
In rats, tetramethrin radiolabelled in the acid or
alcohol moiety is readily taken up, metabolized, and
excreted after oral or subcutaneous administration. Ap-
proximately 95% is excreted in 5-7 days in the urine and
faeces in more or less equal amounts. The tissue residues
from both administration routes are very low. The meta-
bolic reactions are: ester cleavage; loss of the hydroxy-
methyl group from the alcohol moiety; reduction of the 1-2
bond of the alcohol moiety; oxidation at the isobutenyl
methyl moiety of the acid and at the 2-, 3-, and 4-pos-
itions of the alcohol moiety; conjugation of the resultant
acids and alcohols with glucuronic acid; and cis/transiso-
merization.
1.1.6 Effects on organisms in the environment
Only very limited information is available. Tetra-
methrin is highly toxic for fish, the 96-h LC50 values
for two species being 19 and 21 µg/litre. A third species
showed a 48-h LC50 of 200 µg/litre and a no-observed-
effect level of 50 µg/litre. The no-observed-effect level
for Daphnia is 50 µg/litre. Tetramethrin has very low
toxicity to birds but is toxic for honey bees. Because
tetramethrin is rapidly degraded, and provided its use is
limited to buildings, as recommended, the potential that
it has for producing effects on the environment is un-
likely to be realised.
1.1.7 Effects on experimental animals and in vitro test systems
The acute oral toxicity of tetramethrin is low. The
LD50 for rats is >5000 mg/kg with both the racemic mix-
ture and the 1R, cis/trans isomer, whereas for mice it is
about 2000 mg/kg (racemate) and 1060 mg/kg (1R, cis/
trans). The acute dermal toxicities in both rat and mouse,
as well as in the rabbit, are also low; the LD50 in rats
and mice is >5000 mg/kg, while in rabbits it is >2000
mg/kg (all studies were done with racemic mixture). In
acute inhalation studies, the LC50 in rats and mice was
2500 mg/m3 for the racemic mixture and >1180 mg/m3 for
the 1R, cis/trans isomer. The toxic signs include hyperex-
citability, tremor, ataxia, and depression (general signs
combined from all the acute studies). Mice were somewhat
more susceptible than rats, but no differences were ob-
served in susceptibility between males and females.
Tetramethrin, either as the racemic mixture or the 1R,
cis/trans isomer, is virtually non-irritating to the rab-
bit eye and is non-irritating to rabbit skin. In addition,
neither the racemic mixture nor the 1R, cis/trans isomer
is a sensitizer in guinea-pigs.
Tetramethrin is a type I pyrethroid. In mammals,
tremor (T-syndrome) is the characteristic poisoning symp-
tom.
When rats were fed tetramethrin at dietary levels of
up to 5000 mg/kg diet for 91 days, reduced body weight
gain was observed at 5000 mg/kg diet. The results from 3-
or 6-month feeding studies using the 1R, cis/trans isomer
in rats at dietary levels ranging from 25 mg/kg diet to
3000 mg/kg diet indicated that the no-observed-effect
level was 200 mg/kg diet for males and 300 mg/kg diet for
females (observations included decreases in the body
weight gain and in final body weight, and effects on the
kidney and liver). The effects on the liver were thought
to be an adaptive response to the feeding of the corn oil
vehicle.
The no-observed-effect level in a 26-week study in
dogs was 1250 mg/kg diet.
When mice and rats were exposed to aerosolized tetra-
methrin by inhalation at a concentration of 200 mg/m3
for 3-4 h/day for up to 4 weeks, no significant compound-
related changes were observed. An additional inhalation
study, in which rats were exposed to a mist (1.2-1.5 µm
diameter droplets) of 1R,cis/trans isomer in deodorized
kerosene at concentrations up to 87 mg/m3, 3 h/day,
7 days/week for 28 days, indicated a no-observed-effect
level of 49 mg/m3. Toxic signs were noted only during
the exposure period.
Neither tetramethrin nor its 1R,cis/trans isomers were
mutagenic in a variety of in vivo and in vitro test sys-
tems, which investigated gene mutations, DNA damage, DNA
repair, and chromosomal effects.
Three 104-week chronic/oncogenicity feeding studies
have been conducted on tetramethrin, two in rats and one
in mice. In mice, tetramethrin was fed at dose levels up
to 1500 mg/kg diet. No oncogenic effects were observed.
Decreased pituitary and thyroid/parathyroid weights were
observed at 60 mg/kg diet or more. The no-observed-effect
level for systemic effects was 12 mg/kg diet in mice. In
the rat studies, the test animals were exposed to tetra-
methrin at dose levels up to 5000 mg/kg diet in utero and
through long-term feeding. In both studies in rats, body
weight gains were significantly lower in animals exposed
to 3000 mg/kg diet or more. In addition, increases in
liver weight were observed at these dose levels. The no-
observed-effect level for systemic effects in both studies
in rats was 1000 mg/kg diet. The incidence of testicular
interstitial cell tumours at 3000 mg/kg diet or more was
higher than the level in the concurrent control group in
both studies. Testicular interstitial cell tumours occur
spontaneously in aged rats, and the incidence can very
greatly in control groups. This tumour is thought to be
hormonally mediated. There was no evidence of malignancy
and no evidence of this type of tumour in mice. It can be
concluded that the tumorigenic effect, if real, is most
unlikely to be relevant to human exposure.
Tetramethrin was not teratogenic or embryotoxic at
dose levels up to 1000 mg/kg body weight in rats and up to
500 mg/kg body weight in rabbits (these were the highest
dose levels tested). In a fertility study in which rats
were given tetramethrin at dose levels up to 1000 mg/kg
body weight per day, the no-observed-effect level for the
parents' reproductive ability and growth of the fetuses
was 300 mg/kg body weight per day. In a perinatal and
post-natal reproduction study in rats, the no-observed-
effect level was 100 mg/kg body weight per day (the
highest level tested).
When dose levels of 1000-6000 mg/kg diet were tested
in a one-generation reproduction study on tetramethrin in
rats, the no-observed-effect level was 1000 mg/kg diet.
Levels of the 1R,cis/trans isomer of 100-3000 mg/kg were
tested in a two-generation reproduction study, which gave
a no-observed-effect level of 500 mg/kg diet.
1.1.8 Effects on human beings
Although tetramethrin and its 1R isomer have been used
for many years, there have been no reports of poisoning or
adverse effects in human beings.
There are no indications that tetramethrin or its 1R-
isomer will have an adverse effect on human beings if it
continues to be used in low concentrations and only to
control household pests.
1.2 Conclusions
(a) General Population: The exposure of the general
population to tetramethrin, as it is currently used, is
expected to be low. It is not likely to present a hazard
if used as recommended.
(b) Occupational Exposure: When good work practices, hy-
giene measures and safety precautions are followed, tetra-
methrin is unlikely to present a hazard to those occu-
pationally exposed.
(c) Environment: It is highly unlikely that tetramethrin
or its degradation products will reach levels that could
cause adverse environmental effects.
1.3 Recommendations
Although tetramethrin and its 1R isomer have been used
for many years with no reports of adverse effects in
humans, observations of human exposure should continue.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Molecular formula: C19H25NO4
Chemical structure:
 Tetramethrin was first synthesized in 1964 by Kato
et al. [19] and is prepared by the esterificaton of
(1RS ,cis,trans)-2,2-dimethyl-3- (2,2-dimethylvinyl)-cyclo-
propanecarboxylic acid (chrysanthemic acid) with 3,4,5,6-
tetrahydrophthalimidomethyl alcohol. It is a mixture of
four stereoisomers (Fig. 1). The cis:trans ratio is re-
ported to be 1:4 and the optical ratio of 1R:1S is 1:1
(racemic). Thus its composition is roughly 4:1:4:1 for
the [IR,trans], [IR,cis],[IS,trans], and [IS,cis] isomers.
The [1R,trans] isomer is the most active biologically of
the isomers, followed by the [1R,cis] isomer. Neo-Pynamin
Forte is a mixture of the [1R,cis,] and [IR,trans] isomers
in the ratio of 1:4 (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of tetramethrin
are given in Table 2.
No data are available for boiling point and n-
octanol/water partition coefficient. Technical grade
tetramethrin is stable to heat (50 °C for 6 months) but
unstable to light and air and to alkaline condition [30,
31, 76].
2.3 Analytical Methods
Dislodgeable residues of tetramethrin can be analysed
by dual-wavelength densitometry after clean-up of the hex-
ane washings by high-performance silica gel thin-layer
chromatography (Table 3). To analyse technical grade
tetramethrin, the product and tributoxyethyl phosphate (an
internal standard) were dissolved in acetone, and the sol-
ution was injected into a gas chromatograph equipped with
flame ionization detector (FID) [37]). Analysis of tetra-
methrin formulations can also be carried out using high
performance liquid chromatography (HPLC) with an infra-red
selective detector [42].
Table 1. Chemical identity of tetramethrins of various stereoisomeric
compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry no./ Stereospecific nameb,c compositiond trade names
NIOSH Accession no.a
Tetramethrine Cyclopropanecarboxylic acid, (1):(2):(3):(4) Tetramethrine,
(racemic mixture) 2,2-dimethyl-3-(2-methyl-1-propenyl)-, = 4:1:4:1 Phthalthrin,
7696-12-0 (1,3,4,5,6,7-hexahydro-1,3-dioxo-2H - Neo-Pynamin,
GZ173000a isoindol-2-yl)methyl ester FMC-9260
3,4,5,6-Tetrahydrophthalimidomethyl
(1RS, cis,trans)-2,2-dimethyl-3-
(2,2-dimethylvinyl)cyclopropane-
carboxylate
or
3,4,5,6-Tetrahydrophthalimidomethyl-
(1RS, cis,trans)-chrysanthemate
(+)- trans-Tetramethrin Same as tetramethrin (+)- trans-
Phthalthrin
GZ1710000a 3,4,5,6-Tetrahydrophthalimidomethyl
(1R, trans)-chrysanthemate
(+)-Tetramethrin Same as tetramethrin (1):(2) = 4:1 Neo-Pynamin
3,4,5,6-Tetrahydrophthalimidomethyl Forte
GZ1720000a (1R, cis,trans)-chrysanthemate
a Registry of Toxic Effects of Chemical Substances (RTECS) (1981-82 edition).
b (1R), d, (+) or (1S), l, (-) in the acid part of tetramethrin signify the same stereospecific
conformation, respectively.
c Chrysanthemic acid is a name of the acid that forms the acid part.
d Numbers in parentheses identify the structures shown in Fig. 1.
e ISO common name: common names for pesticides and other agrochemicals approved by the Technical
Committee of the International Organization for standardization.
Table 2. Some physical and chemical properties of tetramethrin
Racemic mixture (1R) isomer
Physical state crystalline solid viscous liquid
Colour colourless yellow or brown
Odour pyrethrum-like pyrethrum-like
Relative molecular mass 331.45 331.45
Melting point (°C) 60 - 80
Water solubility 4.6 mg/litre 2 - 4 mg/litre
(30 °C) (23 °C)
Solubility in organic solublea solubleb
solvents
20 25
Density d20 1.108 (20 °C) d25 1.11
Vapour pressure (20 °C) 4.67 x 10-3 mPa 3.2 x 10-4 mPa
(3.5 x 10-8 mmHg) (2.4 x 10-9 mmHg)
(30 °C) 9.46 x 10-1 mPa
(7.1 x 10-6 mmHg)
a Methanol (53 g/kg), hexane (20 g/kg), xylene (1 kg/kg),
acetone, toluene.
b Hexane (>1 kg/kg), methanol, xylene.
Table 3. Analytical methods for tetramethrin
Sample Sample preparation Determination % Recovery Refer-
Extraction Partition Clean up Detection method (fortification ence
Solvent Column Elution and conditions level)a
Environmental
analysis
Dishb n-hexane n-hexane/ HPTLC benzene/CCl4 dual-wavelength 76-95 (0.3 mg) 61
Apple CH3CN (1/1) densitometry 88 (0.3 mg)
Spinach n-hexane/ether/ R = 370 nm; 94 (0.3 mg)
(dislodgeable formic acid S = 230 nm
residue) (70/30/1)
Rf = 0.35
Product analysis
Technical acetone FID-GC, N2 40 ml/min, 37
grade 1-m column, 2% DEGS,
200°C, 12.4 min
(retention time)
Formulations HPLC, 0.01-mm Partisil 42
column, CC14: CH2Cl2:
CHCl3: CH3CN = 42.5:
42.5: 14.85: 0.15, with
IR detection
a fortification level = concentration of tetramethrin added to control samples for the measurements of
recovery.
b Wood, glass, china, or polypropylene.
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Industrial Production
Tetramethrin was first marketed in 1964 [15]. Although
no information on production volume is publicly available,
it is estimated that a few hundred tonnes are manufactured
annually in the world, mainly in Japan.
3.2 Use Patterns
Tetramethrin is used in aerosol formulations, emulsi-
fiable concentrates, and mosquito coils for indoor pest
control. It is also formulated in combination with other
insecticides (e.g., resmethrin) and synergists (e.g.,
piperonyl butoxide).
3.3 Residues in Food
Tetramethrin is not used on food crops.
3.4 Exposure Levels from Household Use
With conventional household aerosol spraying or mos-
quito coil fumigation, the aerial levels of tetramethrin
and its 1R isomer are unlikely to exceed 0.5 mg/m3 [38].
3.5 Environmental Levels
No data are available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Abiotic Degradation in Air and Water
The photodegradation pathways for tetramethrin are
summarized in Fig. 2. Exposure of trans-[carboxyl-14C]
tetramethrin (5)a, as a thin film (0.1-0.3 mg/cm2), to
sunlight resulted in rapid degradation. During a 2-h
exposure (30% conversion), the major photoproducts were
the (1RS)-epoxides (7) (14% of the reaction mixture), the
aldehyde derivative (10) (19%) oxidized at the (E)-methyl
group in the acid moiety, the caronaldehyde derivative
(16) (6%) from cleavage upon ozonolysis, and the allylic
hydroperoxide (15) (6%) from the hydroperoxidation at the
1 -position of the isobutenyl moiety. In addition, small
amounts of cis-tetramethrin (14) (2%), the alcohol (9)
(5%) and carboxy (11) (3%) derivatives oxidized at the
(E)-methyl group, and the hydroxy derivative (6) (3%) at
the allylic methylene group in the alcohol moiety and its
epoxide (8) (2%) were detected. These identified ester
photoproducts accounted for approximately 80% in a 5% con-
version but only approximately 20% in a 50-70% conversion.
Chrysanthemic acid (12) and N-(hydroxymethyl)-tetrahydro-
phthalimide (13), formed by ester bond cleavage, were
minor products, and much of the radiocarbon remained at
the origin on the TLC plate. The cis/trans isomerization
was an inefficient reaction in an oxygen-containing atmos-
phere [50].
Tetramethrin was first synthesized in 1964 by Kato
et al. [19] and is prepared by the esterificaton of
(1RS ,cis,trans)-2,2-dimethyl-3- (2,2-dimethylvinyl)-cyclo-
propanecarboxylic acid (chrysanthemic acid) with 3,4,5,6-
tetrahydrophthalimidomethyl alcohol. It is a mixture of
four stereoisomers (Fig. 1). The cis:trans ratio is re-
ported to be 1:4 and the optical ratio of 1R:1S is 1:1
(racemic). Thus its composition is roughly 4:1:4:1 for
the [IR,trans], [IR,cis],[IS,trans], and [IS,cis] isomers.
The [1R,trans] isomer is the most active biologically of
the isomers, followed by the [1R,cis] isomer. Neo-Pynamin
Forte is a mixture of the [1R,cis,] and [IR,trans] isomers
in the ratio of 1:4 (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of tetramethrin
are given in Table 2.
No data are available for boiling point and n-
octanol/water partition coefficient. Technical grade
tetramethrin is stable to heat (50 °C for 6 months) but
unstable to light and air and to alkaline condition [30,
31, 76].
2.3 Analytical Methods
Dislodgeable residues of tetramethrin can be analysed
by dual-wavelength densitometry after clean-up of the hex-
ane washings by high-performance silica gel thin-layer
chromatography (Table 3). To analyse technical grade
tetramethrin, the product and tributoxyethyl phosphate (an
internal standard) were dissolved in acetone, and the sol-
ution was injected into a gas chromatograph equipped with
flame ionization detector (FID) [37]). Analysis of tetra-
methrin formulations can also be carried out using high
performance liquid chromatography (HPLC) with an infra-red
selective detector [42].
Table 1. Chemical identity of tetramethrins of various stereoisomeric
compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry no./ Stereospecific nameb,c compositiond trade names
NIOSH Accession no.a
Tetramethrine Cyclopropanecarboxylic acid, (1):(2):(3):(4) Tetramethrine,
(racemic mixture) 2,2-dimethyl-3-(2-methyl-1-propenyl)-, = 4:1:4:1 Phthalthrin,
7696-12-0 (1,3,4,5,6,7-hexahydro-1,3-dioxo-2H - Neo-Pynamin,
GZ173000a isoindol-2-yl)methyl ester FMC-9260
3,4,5,6-Tetrahydrophthalimidomethyl
(1RS, cis,trans)-2,2-dimethyl-3-
(2,2-dimethylvinyl)cyclopropane-
carboxylate
or
3,4,5,6-Tetrahydrophthalimidomethyl-
(1RS, cis,trans)-chrysanthemate
(+)- trans-Tetramethrin Same as tetramethrin (+)- trans-
Phthalthrin
GZ1710000a 3,4,5,6-Tetrahydrophthalimidomethyl
(1R, trans)-chrysanthemate
(+)-Tetramethrin Same as tetramethrin (1):(2) = 4:1 Neo-Pynamin
3,4,5,6-Tetrahydrophthalimidomethyl Forte
GZ1720000a (1R, cis,trans)-chrysanthemate
a Registry of Toxic Effects of Chemical Substances (RTECS) (1981-82 edition).
b (1R), d, (+) or (1S), l, (-) in the acid part of tetramethrin signify the same stereospecific
conformation, respectively.
c Chrysanthemic acid is a name of the acid that forms the acid part.
d Numbers in parentheses identify the structures shown in Fig. 1.
e ISO common name: common names for pesticides and other agrochemicals approved by the Technical
Committee of the International Organization for standardization.
Table 2. Some physical and chemical properties of tetramethrin
Racemic mixture (1R) isomer
Physical state crystalline solid viscous liquid
Colour colourless yellow or brown
Odour pyrethrum-like pyrethrum-like
Relative molecular mass 331.45 331.45
Melting point (°C) 60 - 80
Water solubility 4.6 mg/litre 2 - 4 mg/litre
(30 °C) (23 °C)
Solubility in organic solublea solubleb
solvents
20 25
Density d20 1.108 (20 °C) d25 1.11
Vapour pressure (20 °C) 4.67 x 10-3 mPa 3.2 x 10-4 mPa
(3.5 x 10-8 mmHg) (2.4 x 10-9 mmHg)
(30 °C) 9.46 x 10-1 mPa
(7.1 x 10-6 mmHg)
a Methanol (53 g/kg), hexane (20 g/kg), xylene (1 kg/kg),
acetone, toluene.
b Hexane (>1 kg/kg), methanol, xylene.
Table 3. Analytical methods for tetramethrin
Sample Sample preparation Determination % Recovery Refer-
Extraction Partition Clean up Detection method (fortification ence
Solvent Column Elution and conditions level)a
Environmental
analysis
Dishb n-hexane n-hexane/ HPTLC benzene/CCl4 dual-wavelength 76-95 (0.3 mg) 61
Apple CH3CN (1/1) densitometry 88 (0.3 mg)
Spinach n-hexane/ether/ R = 370 nm; 94 (0.3 mg)
(dislodgeable formic acid S = 230 nm
residue) (70/30/1)
Rf = 0.35
Product analysis
Technical acetone FID-GC, N2 40 ml/min, 37
grade 1-m column, 2% DEGS,
200°C, 12.4 min
(retention time)
Formulations HPLC, 0.01-mm Partisil 42
column, CC14: CH2Cl2:
CHCl3: CH3CN = 42.5:
42.5: 14.85: 0.15, with
IR detection
a fortification level = concentration of tetramethrin added to control samples for the measurements of
recovery.
b Wood, glass, china, or polypropylene.
3. SOURCES AND LEVELS OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Industrial Production
Tetramethrin was first marketed in 1964 [15]. Although
no information on production volume is publicly available,
it is estimated that a few hundred tonnes are manufactured
annually in the world, mainly in Japan.
3.2 Use Patterns
Tetramethrin is used in aerosol formulations, emulsi-
fiable concentrates, and mosquito coils for indoor pest
control. It is also formulated in combination with other
insecticides (e.g., resmethrin) and synergists (e.g.,
piperonyl butoxide).
3.3 Residues in Food
Tetramethrin is not used on food crops.
3.4 Exposure Levels from Household Use
With conventional household aerosol spraying or mos-
quito coil fumigation, the aerial levels of tetramethrin
and its 1R isomer are unlikely to exceed 0.5 mg/m3 [38].
3.5 Environmental Levels
No data are available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Abiotic Degradation in Air and Water
The photodegradation pathways for tetramethrin are
summarized in Fig. 2. Exposure of trans-[carboxyl-14C]
tetramethrin (5)a, as a thin film (0.1-0.3 mg/cm2), to
sunlight resulted in rapid degradation. During a 2-h
exposure (30% conversion), the major photoproducts were
the (1RS)-epoxides (7) (14% of the reaction mixture), the
aldehyde derivative (10) (19%) oxidized at the (E)-methyl
group in the acid moiety, the caronaldehyde derivative
(16) (6%) from cleavage upon ozonolysis, and the allylic
hydroperoxide (15) (6%) from the hydroperoxidation at the
1 -position of the isobutenyl moiety. In addition, small
amounts of cis-tetramethrin (14) (2%), the alcohol (9)
(5%) and carboxy (11) (3%) derivatives oxidized at the
(E)-methyl group, and the hydroxy derivative (6) (3%) at
the allylic methylene group in the alcohol moiety and its
epoxide (8) (2%) were detected. These identified ester
photoproducts accounted for approximately 80% in a 5% con-
version but only approximately 20% in a 50-70% conversion.
Chrysanthemic acid (12) and N-(hydroxymethyl)-tetrahydro-
phthalimide (13), formed by ester bond cleavage, were
minor products, and much of the radiocarbon remained at
the origin on the TLC plate. The cis/trans isomerization
was an inefficient reaction in an oxygen-containing atmos-
phere [50].
 a Numbers in parentheses refer to numbered chemical structures in
Figures 2 and 3.
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
The metabolic pathways of tetramethrin in mammals are
summarized in Fig. 3.
Tetramethrin is readily absorbed and excreted by rats.
Following a single oral administration of [1RS, trans]-
tetramethrin (17], labelled with 14C at the carbonyl
group of the alcohol moiety, to male Wistar rats at a con-
centration of 500 mg/kg, 47% and 42% of the radiolabel
were excreted into the urine and faeces, respectively,
during the subsequent 2 days and 95% was recovered during
the 5-day period that followed dosing. The tissue levels
during the first 2 days after administration were very low
and the tetramethrin content in tissues was less than
0.01% of the dosed radioactivity. Unmetabolized trans-
tetramethrin (17) was not excreted into the urine, and the
major urinary metabolite was 3-hydroxy-cyclohexane-1,2-di-
carboximide (19) (3-OH-HPI) in free and glucuronide forms.
N-(Hydroxymethyl)-3,4,5,6-tetrahydrophthalimide (20) (MTI),
3,4,5,6-tetrahydrophthalimide (21) (TPI), and cyclohexane-
1,2-dicarboximide (22) (HPI) were identified as minor uri-
nary and faecal metabolites [36].
Following a single oral or subcutaneous administration
to Sprague-Dawley rats of [1R, trans]- or [1R, cis]-tetra-
methrin (17,18), labelled with 14C in the acid or alcohol
moieties at concentrations of 3.2-5.3 mg/kg, the radiocar-
bon was rapidly and almost completely eliminated from the
rat body. The total recoveries 7 days after administration
were 93-97% for the trans isomer and 90-101% for the cis
isomer (approximately equal amounts being eliminated in
urine and faeces). In the case of the oral dose of acid-
labelled tetramethrin, 1-3% of the radiolabel was ex-
creted as 14CO2, whereas in other cases the amount of
14CO2 accounted for less than 1% of the dose. The tissue
residue 7 days after administration was very low. The
transisomer yielded somewhat more complete radiolabel re-
covery and lower tissue residues than the cis isomer. In
addition, labelling resulted in slightly lower tissue
residues than did alcohol labelling. However, there were
no significant differences, according to sex or adminis-
tration route, in the total radiocarbon recoveries and
tissue residue levels [18]. The major metabolic reactions
of both [1R, trans]- and [1R, cis]-tetramethrin were
ester cleavage, loss of the hydroxymethyl group from the
alcohol moiety, reduction of the 1-2 bond of the alcohol
moiety, and oxidation at the isobutenyl group of the acid
moiety and at the 2-, 3-, and 4-positions of the alcohol
moiety. The metabolites produced via these reactions were
in part conjugated with glucuronic acid. None of the trans
isomer remained unmetabolized, whereas 0.3-1.2% of the cis
isomer was found unchanged in the faeces. The major metab-
olites from the acid moiety of both isomers were chrysan-
themic acid (23, 24) (CA) and its derivatives oxidized at
the trans-methyl of the isobutenyl group. 3-(2'-E-Carboxy-
1'-propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid (25,
26) ( w t-acid- t,c-CA) accounted for 17-27% and 7-9% of
the dose of the trans and cis isomers, respectively. Other
significant metabolites were 3-(2'-E-hydroxymethyl-1'-
propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid(27,28)
( w t-alc-t,c-CA), 3-(2'-Z-carboxy-1'-propenyl)-2,2-dimethyl-
1-cyclopropanecarboxylic acid (29, 30) ( w c-acid- t,c-CA),and
3-(2'-Z-hydroxymethyl-1'-propenyl)-2,2-dimethyl-1-cyclopro-
panecarboxylic acid (31, 32) ( w c-alc- t,c-CA).
a Numbers in parentheses refer to numbered chemical structures in
Figures 2 and 3.
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
The metabolic pathways of tetramethrin in mammals are
summarized in Fig. 3.
Tetramethrin is readily absorbed and excreted by rats.
Following a single oral administration of [1RS, trans]-
tetramethrin (17], labelled with 14C at the carbonyl
group of the alcohol moiety, to male Wistar rats at a con-
centration of 500 mg/kg, 47% and 42% of the radiolabel
were excreted into the urine and faeces, respectively,
during the subsequent 2 days and 95% was recovered during
the 5-day period that followed dosing. The tissue levels
during the first 2 days after administration were very low
and the tetramethrin content in tissues was less than
0.01% of the dosed radioactivity. Unmetabolized trans-
tetramethrin (17) was not excreted into the urine, and the
major urinary metabolite was 3-hydroxy-cyclohexane-1,2-di-
carboximide (19) (3-OH-HPI) in free and glucuronide forms.
N-(Hydroxymethyl)-3,4,5,6-tetrahydrophthalimide (20) (MTI),
3,4,5,6-tetrahydrophthalimide (21) (TPI), and cyclohexane-
1,2-dicarboximide (22) (HPI) were identified as minor uri-
nary and faecal metabolites [36].
Following a single oral or subcutaneous administration
to Sprague-Dawley rats of [1R, trans]- or [1R, cis]-tetra-
methrin (17,18), labelled with 14C in the acid or alcohol
moieties at concentrations of 3.2-5.3 mg/kg, the radiocar-
bon was rapidly and almost completely eliminated from the
rat body. The total recoveries 7 days after administration
were 93-97% for the trans isomer and 90-101% for the cis
isomer (approximately equal amounts being eliminated in
urine and faeces). In the case of the oral dose of acid-
labelled tetramethrin, 1-3% of the radiolabel was ex-
creted as 14CO2, whereas in other cases the amount of
14CO2 accounted for less than 1% of the dose. The tissue
residue 7 days after administration was very low. The
transisomer yielded somewhat more complete radiolabel re-
covery and lower tissue residues than the cis isomer. In
addition, labelling resulted in slightly lower tissue
residues than did alcohol labelling. However, there were
no significant differences, according to sex or adminis-
tration route, in the total radiocarbon recoveries and
tissue residue levels [18]. The major metabolic reactions
of both [1R, trans]- and [1R, cis]-tetramethrin were
ester cleavage, loss of the hydroxymethyl group from the
alcohol moiety, reduction of the 1-2 bond of the alcohol
moiety, and oxidation at the isobutenyl group of the acid
moiety and at the 2-, 3-, and 4-positions of the alcohol
moiety. The metabolites produced via these reactions were
in part conjugated with glucuronic acid. None of the trans
isomer remained unmetabolized, whereas 0.3-1.2% of the cis
isomer was found unchanged in the faeces. The major metab-
olites from the acid moiety of both isomers were chrysan-
themic acid (23, 24) (CA) and its derivatives oxidized at
the trans-methyl of the isobutenyl group. 3-(2'-E-Carboxy-
1'-propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid (25,
26) ( w t-acid- t,c-CA) accounted for 17-27% and 7-9% of
the dose of the trans and cis isomers, respectively. Other
significant metabolites were 3-(2'-E-hydroxymethyl-1'-
propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid(27,28)
( w t-alc-t,c-CA), 3-(2'-Z-carboxy-1'-propenyl)-2,2-dimethyl-
1-cyclopropanecarboxylic acid (29, 30) ( w c-acid- t,c-CA),and
3-(2'-Z-hydroxymethyl-1'-propenyl)-2,2-dimethyl-1-cyclopro-
panecarboxylic acid (31, 32) ( w c-alc- t,c-CA).
 Judging from the metabolites derived from the acid
moiety, cis to trans isomerization of the oxidized deriva-
tives of CA occurs, as happens in resmethrin metabolism
[60].
Although both cis to trans and trans to cis isomeriza-
tions of tetramethrin were observed by Kaneko et al. [18],
cis to trans conversion seemed to be predominant. On the
other hand, the detected metabolites from the alcohol
moiety were TPI, HPI, 3-OH-TPI (33), 3,4,5,6-tetrahydro-
phthalic acid amide (34) (TPIA), 2-OH-HPI (35), 3-OH-HPI
(19), and 4-OH-HPI (36). Of these metabolites, 2-OH-HPI
was found in relatively large amounts.
Smith et al. [55] found that tetramethrin and TPI
readily underwent the Michael addition with thiols. The
tetramethrin-gluthathione (GSH) conjugate was formed under
physiological conditions in the presence of mouse liver
homogenate fractions, probably by a non-enzymatic
reaction. The soluble thiol level of mouse liver was de-
creased by intraperitoneal administration of TPI. However,
mercapturic acid and GSH conjugates of tetramethrin were
not detected in the bile or urine of rats or mice treated
intraperitoneally with tetramethrin.
5.2 Enzymatic Systems for Biotransformation
When alcohol- or acid-labelled [1RS, trans]-tetra-
methrin (1 mmol/litre) was incubated for 1 h at 37 °C with
30 mg protein of a rat liver subcellular fraction (i.e.
nuclei plus mitochondria, microsomes, and soluble frac-
tion), the microsomes and nuclei plus mitochondria frac-
tions were active in degrading tetramethrin. Rat micro-
somal fraction degraded [1RS, trans]-tetramethrin to CA,
MTI, and TPI in the absence of NADPH. In the presence of
NADPH, tetramethrin was more rapidly degraded to yield
oxidized tetramethrin ( wt-alc-, wt-ald-, and wt-acid-tetra-
methrin), oxidized CA ( wt-alc-, wt-ald-, and wt-acid-CA),
TPI, and unidentified metabolites in larger amounts. The
major metabolite TPI was shown to be produced non-enzy-
matically from MTI. The degradation rate of tetramethrin
was greatly reduced by the inhibition of ester hydrolysis
with paraoxon [57].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
As the use of tetramethrin is limited to indoor pest
control, there is a paucity of data concerning its effect
on the environment.
6.1 Aquatic Organisms
Data on the toxicity of tetramethrin to non-target
aquatic organisms are given in Table 4.
Tetramethrin is highly acutely toxic to fish in lab-
oratory tests, the 96-h LC50s for two species being ap-
proximately 20 µg/litrea. The 48-h LC50 for killifish
is about 200 µg/litre, with a no-observed-effect level
(NOEL) of 50 µg/litre [33]. The NOEL for Daphnia pulex
was reported by Miyamoto [33] to be 50 µg/litre for
racemic tetramethrin and 10 µg/litre for [1R, trans]- or
[1R, cis]-tetramethrin.
6.2 Terrestrial Organisms
Tetramethrin has low toxicity to birds. The acute oral
LD50 for Bobwhite quail is >2510 mg/kg body weight, and
the 8-day dietary LC50 to Mallard duck and Bobwhite quail
is >5620 mg/kga.
Tetramethrin is toxic to bees [14].
a written comment from US EPA to IPCS, 1987.
Table 4. Acute toxicity of tetramethrin to non-target aquatic organisms
Species Size Parameter Toxicity Formulation System Temperature Refer-
(mg/litre) (°C) ence
Fish
Killifish adult 48-h LC50 0.2 Technical Static 25 33
( Oryzias latipes) adult 48-h LC50 0.2 (+)-trans Static 25 33
adult 48-h LC50 0.15 (+)-cis Static 25 33
Bluegill sunfish 96-h LC50 0.019 a
( Lepomis macrochirur)
Rainbow trout 96-h LC50 0.021 a
( Salmo gairdneri)
Arthropods
Daphnia pulex 3-h LC50 >50 Technical Static 25 33
3-h LC50 >50 (+)-trans Static 25 33
3-h LC50 >50 (+)-cis Static 25 33
a Written comment from US EPA to IPCS, 1987.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
The acute toxicity of tetramethrin to rats and mice is
low (Table 5).
Table 5. Acute toxicity of tetramethrin to rats and mice
Compound Species Sex Route LD50 Reference
(mg/kg body
weight)
Racemic rat M,F oral 4600 41
rata M,F oralb >5000 17
rat M,F dermalb >10 000 41
mousea M oralb 1920 17
mousea F oralb 2000 17
(1R, cis/trans)- rata M,F oralb >5000 16
rat M,F subcutaneous >5000 16
rat M intraperitoneal 770 16
rat F intraperitoneal 548 16
rat M,F dermal(>24 h)b >5000 16
mousea M oral 1060 16
mousea F oral 1040 16
mouse M subcutaneous 2020 16
mouse F subcutaneous 1950 16
mouse M intraperitoneal 631 16
mouse F intraperitoneal 527 16
mouse M,F dermal (>24 h)b >5000 16
rabbit dermal (>24 h)b >2000 20
a Animals were not fasted.
b Corn oil was used as vehicle.
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1.2-
1.5 µm) of [1R, cis/trans]-tetramethrin (technical grade,
95.6% purity) in deodorized kerosene (0, 26, 131, 243,
595, and 1180 mg active ingredient per m3 air) for a
duration of 3 h. At 131 mg/m3 or more, salivation, hyper-
excitability, irregular respiration, urinary incontinence,
muscular fibrillation, limb paralysis, decrease in spon-
taneous activity, and other toxic signs were observed in
males and females. At 1180 mg/m3, 10% of female animals
died, but the body weight gain was similar to that of the
control rats. The no-observed-effect level (NOEL) for
inhalation of the compound in rats was 26 mg/m3, and the
LC50 value was >1180 mg/m3 in both sexes [58].
The toxic symptoms observed following [1R, cis/trans]-
tetramethrin administration were hyperexcitability, muscle
twitching, tremor, ataxia, irregular respiration, and
depression. Mice were invariably more susceptible than
rats. No differences in susceptibility were observed
between male and female animals [16, 17, 33].
7.2 Irritation and Sensitization
7.2.1 Eye irritation
In a study by Okuno et al. [39], 50 mg of the techni-
cal product (91.3% purity) was instilled in one eye of
Japanese albino male rabbits. The treated eye was washed
with distilled water 5 min (group I) or 24 h (group II)
thereafter. The conjunctiva, cornea, and pupil were exam-
ined, 1, 24, 72 h and 7, 14, and 21 days after appli-
cation. No particular changes were noted except that a
very slight erythema and oedema of the conjunctiva was
transiently observed in the rabbits in group II.
In a separate study, 0.1 ml [1R, cis/trans]-tetra-
methrin (technical grade, 95.6% purity) was applied to one
eye of Japanese albino rabbits. The treated eye was sub-
sequently washed in five rabbits but not in three other
rabbits. The material did not produce any lesions in the
cornea or iris of the treated eyes that were not washed,
but slight hyperemia and/or chemosis of the conjunctiva
was observed 1 h after application. In the washed eyes,
slight hyperemia of the conjunctiva was observed in all
animals 1 h after treatment. These changes, however, had
disappeared by 48 h after application in the unwashed eyes
and 24 h in the washed eyes. The irritating potency of the
material was judged to be minimal in the unwashed eyes and
negative in the washed eyes [11].
7.2.2 Skin irritation
In a study by Okuno et al. [39], 0.5 g of the techni-
cal product (91.3% purity) was applied on a lint patch
(3.8 x 3.8 cm) to the abraded or intact skin of six rab-
bits. The skin was assessed for severity of erythema and
oedema 4, 24, 48, 72 h and 7, 14, and 21 days after appli-
cation, but no particular changes were noted.
When 0.5 ml [1R, cis/trans]-tetramethrin (technical
grade, 95.6% purity) was applied on a lint patch
(2.5 x 2.5 cm) to abraded or intact skin on the back of
rabbits, again no irritating reactions such as erythema
and oedema were observed [11].
7.2.3 Sensitization
In a skin-sensitization study of tetramethrin in
guinea-pigs, Hartley male guinea-pigs (seven per group)
were sensitized ten times at intervals of one or two days
by intracutaneous injections (first injection: 0.05 ml,
subsequent ones: 0.1 ml) of a 1% solution of the technical
product (91.3% purity) in corn oil. The sensitized animals
were then challenged against the same concentration in the
same manner (0.5 ml injection) 14 days later, but no skin-
sensitization reaction was noted [40].
In another skin-sensitization test on Hartley male
guinea-pigs, 0.5 ml [1R, cis/trans]-tetramethrin (techni-
cal product, 95.6% purity) in 0.5 ml acetone was applied
topically by lint patch to the back of animals ten times
(three times per week). The animals were challenged in the
same manner 2 weeks after the last sensitizing treatment,
but no allergic reactions were observed 24 h later [12].
7.3 Short-Term Exposure Studies
7.3.1 Oral
When groups of 10 male Wistar rats were maintained for
91 days on a diet containing 0, 500, 1000, 3000, or 5000
mg tetramethrin/kg diet, there was a reduced rate of body
weight gain at 5000 mg/kg but not at 3000 mg/kg or less.
The liver glycogen level was reduced at 3000 mg/kg and
5000 mg/kg. The kidney, spleen, heart, small intestine,
and brain showed no abnormal signs, either macroscopically
or microscopically, and there were no significant changes
in blood parameters. There was no increase in the protein
or glucose levels in the urine of test animals [59].
When technical (1R, cis/trans)-tetramethrin in corn
oil was administered to Sprague-Dawley rats for 3 or 6
months at 0, 100, 300, 1000, or 3000 mg/kg diet, no treat-
ment-related changes were observed in clinical signs, or
food and water consumption, or in an ophthalmological
examination. However, the body weight gain and final body
weight of males and females in the 3000-mg/kg group were
significantly lower than those of the controls. There were
slight increases in urine protein level in the rats fed
more than 1000 mg/kg and in serum calcium level in the
male rats fed more than 300 mg/kg. During a histopatho-
logical examination, eosinophilic bodies in tubular epi-
thelial cells and hyaline droplets in kidney tubular epi-
thelium cytoplasm were observed in males fed 3000 mg/kg
diet, along with an increase in relative organ weight.
There were dose-dependent increases in absolute and rela-
tive liver weight in all treated male rats and in female
rats fed more than 1000 mg/kg diet. There were also
slightly higher serum cholesterol concentrations in rats
of both sexes fed more than 1000 mg/kg and a significant
reduction in liver lipid content among males fed 1000
mg/kg or more. However, these liver effects were not
accompanied by damage to hepatocytes and were therefore
considered to be an adaptation to the corn oil without
toxicological significance. Furthermore, there were no
marked effects, even at 200 mg/kg, when an additional sub-
chronic study was conducted at tetramethrin levels of 25,
50, 100, and 200 mg/kg diet without corn oil in order to
confirm the NOEL in male rats. The NOEL for tetramethrin
in rats in the 6-month study was concluded to be 200 mg/kg
diet for males and 300 mg/kg diet for females [16].
When technical grade tetramethrin (94.6% purity) was
administered for 26 weeks to beagle dogs (six of each sex
per group) at levels of 0, 1250, 2500, and 5000 mg/kg
diet, nervousness and tremors were observed in both males
and females at 2500 and 5000 mg/kg diet. A lack of oestrus
activity in females was also noted clinically and a lack
of corpora lutea was confirmed histologically at 5000
mg/kg diet. Absolute liver weight was increased in males
at 2500 and 5000 mg/kg and relative liver weight was sig-
nificantly increased in males and females at 5000 mg/kg.
Decreased absolute/relative ovary weights were noted for
females at 5000 mg/kg. No other treatment-related changes
were observed with respect to survival, body weight gain,
food consumption, haematology, urinalysis, ophthalmology,
gross pathology or histopathology. The NOEL was 1250 mg/kg
diet [43].
In a study by Weir & Crus (1966), groups of three male
and three female beagle dogs were fed tetramethrin dis-
solved in corn oil for 13 weeks at levels of 0, 1250,
2500, and 5000 mg/kg diet. There were no effects on haema-
tological, clinical chemistry, or urinary parameters.
Organ weights were not affected by the treatment, and
there were no significant histopathological findings.
Clinical signs were not recorded. The NOEL was >5000 mg/kg
diet.
7.3.2 Inhalation
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1-2 µm)
of tetramethrin at concentrations of 0, 26, 49, and 87 mg
active ingredient per m3 air, 3 h a day, 7 days a week,
for a period of 28 days. At 87 mg/m3, irregular respir-
ation, slight salivation, and hyperexcitability were
observed as toxic signs every day during the exposure
period, but no cumulative toxicity was noted. There were
no compound-related effects on body weight gain, food and
water consumption, urinalysis, haematology, biochemistry,
organ weight, and histopathology. The NOEL in subacute
inhalation was considered to be 49 mg/m3 [58]. This NOEL
is approximately 100 times higher than the aerial concen-
tration attained during normal use of tetramethrin [33].
7.4 Long-Term Exposures and Carcinogenicity
Appraisal
Testicular interstitial cell tumours occur spontaneously in
aged rats, and the incidence can vary greatly in control
groups. This tumour is believed to be hormonally mediated.
There was no evidence of malignancy in three rat studies and no
evidence of this type of tumour in mice. It can be concluded
that the tumorigenic effect, if real, is most unlikely to be
relevant to human exposure.
When tetramethrin (technical grade) was administered
to Sprague-Dawley CRCDR rats (50 of each sex per group,
F1A weanlings from parental animals pre-treated with the
compound at dose levels of 1000, 3000, and 6000 mg/kg
diet) at dose levels of 0, 1000, 3000, or 5000 mg/kg diet
for 104 weeks, no compound-related effects were detected
in investigations of appearance, behaviour, survival,
haematology, blood chemistry, urinalysis, eye examination,
and organ weight at up to 5000 mg/kg diet. However, the
body weight gain of male and female rats fed 3000 mg/kg or
more was significantly lower than that of controls. The
incidence for testicular interstitial cell tumours was
increased at dose levels of 3000 mg/kg or more [49].
Tetramethrin (technical grade, 90.0/93.6% purity) was
tested for long-term toxic effects and tumorigenic poten-
tial in Sprague-Dawley CRCDR and Long-Evans hooded rats
by in utero exposure and 104-week chronic exposure at dose
levels of 0, 200, 1000, and 5000 mg/kg diet. No distinct
compound-related effects were observed in either strain
with regard to fertility rate, mortality, clinical signs,
and clinical laboratory data. However, body weight gains
were significantly lower in both strains at 5000 mg/kg
diet, and absolute and relative liver weights were in-
creased in both strains at 5000 mg/kg diet. The incidence
of interstitial cell tumours in both strains at 5000 mg/kg
diet was above the level in the concurrent control groups
[44].
When tetramethrin (technical product, 93.3% purity)
was fed daily to B6C3F1 mice (dose levels of 0, 12, 60,
300, or 1500 mg/kg diet) for 104 weeks, there were no sig-
nificant dose-related changes in survival, clinical signs,
mean body weight, or food consumption. However, the mor-
tality of male mice at 300 mg/kg was significantly lower
than that of control males. The absolute and relative
weight of pituitary and thyroid/parathyroid glands was
decreased for males fed 60 mg/kg diet or more. Absolute
spleen weights were also decreased for males fed 300 mg/kg
diet or more. However, gross and microscopic examination
of these tissues did not reveal any treatment-related
histomorphological changes. There were no other histo-
pathological findings attributable to tetramethrin admin-
istration. The NOEL was considered to be 12 mg/kg diet
[45].
7.5 Mutagenicity
The results of mutagenicity tests on tetramethrin are
summarized in Table 6.
Ding et al. [2] reported the induction of unscheduled
DNA synthesis in human amnion FL cells by tetramethrin
(72% industrial grade of unknown origin). The same product
gave weakly positive results in an Ames test with Salmon-
ella typhimurium TA 97. It is not clear if the effect was
caused by tetramethrin itself or by the unidentified (28%)
portion of the industrial grade material.
7.6 Reproduction, Embryotoxicity, and Teratogenicity
In a study by Miyamoto [33], groups of 10-15 pregnant
New Zealand white rabbits received tetramethrin orally on
days 6-18 of gestation at doses of 0, 30, or 90 mg/kg per
day. Fetuses were obtained by caesarean section prior to
parturition and were examined for external and skeletal
abnormalities. Seven extra pregnant animals were allowed
to give birth naturally and the pups were examined for
several weeks to check their growth and development. No
significant adverse effects were observed.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to 6-week-old male Slc: SD rats (SPF, 20
per group) for not less than 9 weeks and to 9-week-old
females (20 per group) for 2 weeks of the non-pregnant
period and up to day 7 of pregnancy. The effects of the
material on the mating ability of male and female animals
and on the fetuses were investigated. In males, the liver
weight increased at all dose levels and a kidney weight
increase was noted at 1000 mg/kg. Salivation and a slower
body weight increase were observed during the latter half
of the administration period at 300 and 1000 mg/kg. How-
ever, no effects on the reproductive ability of males were
noted. In females, no changes were observed in the rate of
pregnancy, but there were effects on the sexual cycle and
an ovulation-inhibiting effect at 1000 mg/kg. In fetuses,
growth inhibition was suspected at 1000 mg/kg. However,
all these changes were slight. The NOEL was considered to
be 300 mg/kg body weight per day for reproductive ability
of parents and growth of fetuses [51].
Table 6. Mutagenicity studies on tetramethrin
Test System Test object Concentration Purity/ Results Reference
Compound
Ames test S. typhimurium up to 10 mg/plate 93 - 100% Negative 33
TA 1535 without activation
TA 1538
Ames test Escherichia coli up to 10 mg/plate 93 - 100% Negative 33
W 3623 without activation
W 3012
Ames test S. typhimurium 1 - 10 000 ug/plate technical Negative 56
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1538
Ames test S. typhimurium 100 - 5000 ug/plate 94.0% Negative 81
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1537
TA 97
Ames test E. coli 100 - 5000 ug/plate 94.0% Negative 81
WP2 uvr A with and without racemic
activation
Ames test S. typhimurium 10 - 5000 ug/plate 95.6%, Negative 22
TA 100 with and without 1R, cis/trans
TA 98 activation
TA 1535
TA 1537
TA 1538
Ames test E. coli 10 - 5000 ug/plate 95.6%, Negative 22
WP2 uvr A 1R, cis/trans
Ames test S. typhimurium 5 - 500 ug/plate 72% Positivea 2
TA 97 without activation industrial
grade
Rec-assay Bacillus 1 - 10 000 ug/disk technical Negative 56
subtilis racemic
M45 rec- and H17
(wild type)
Rec-assay Bacillus 50 - 5000 ug/disk 95.6%, Negative 22
subtilis 1R, cis/trans
M45 rec- and H17
(wild type)
Table 6 (contd).
Test System Test object Concentration Purity/ Results Reference
Compound
Host-mediated ICR male mice 200 - 1000 mg/kg technical Negative 56
assay S. typhimurium body weight (oral) racemic
G46
In vivo
chromosomal ICR male mice 1200, 2400, 5000 93.4% Negative 80
aberration bone marrow mg/kg body racemic
weight (ip)
In vivo
chromosomal ICR male mice 150, 300, 600 mg/kg 95.6%, Negative 13
aberration bone marrow body weight (ip) 1R, cis/trans
Unscheduled
DNA Human amnion Not recorded 72%, Positivea 2
synthesis FL cells industrial
grade
Unscheduled
DNA Rat hepatocyte 0, 0.2, 1, 94.0% Negative 21
synthesis primary cultures 5, 25, 50, racemic
and 100 ug/ml
a The test material was of unknown origin and it was unclear whether or not
positive results were due to the 28% impurities.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 30 per group) on
days 7-17 of pregnancy, and its effects on the dams,
fetuses, and growth of pups were investigated. In dams, an
inhibition of body weight increase and a decrease of food
consumption were observed at 1000 mg/kg, in addition to an
increase in liver and kidney weights at the time of
caesarean section. In fetuses, no abnormalities such as
embryo lethality, growth inhibition, or teratogenic
effects were detected. In addition, the tetramethrin had
no effect on the growth of the young after birth or on the
reproductive ability of the offspring. The NOEL was con-
sidered to be 300 mg/kg body weight per day for the dams
and >1000 mg/kg body weight per day for teratogenicity
[52].
When tetramethrin (technical product) was orally
administered at dose levels of 0, 50, 150, or 500 mg/kg
body weight per day to pregnant Japanese white rabbits,
(10 per group) on days 8-18 of pregnancy, a slight transi-
ent decline in the body weight of the dams was noted in
the middle of the treatment period at 500 mg/kg. No
adverse effects such as embryo lethality, inhibition of
fetal growth, or teratogenic action were observed at any
dose level. The NOEL for teratogenicity in rabbits was
considered to be >500 mg/kg body weight per day [53].
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 20 per group) from
day 17 of gestation to day 21 of lactation (perinatal and
postnatal period). In dams, a liver weight increase was
noted at 300 and 1000 mg/kg but there were no abnormali-
ties at delivery or during lactation. Tetramethrin had no
detectable effects on the survival rate of pups, growth
and development, sensory function, motor function, learn-
ing ability, or reproductive ability. The NOEL was
considered to be 100 mg/kg body weight per day for dams
and >1000 mg/kg body weight per day for pups [54].
In studies by Rutter [48], tetramethrin (technical
grade) was administered to Sprague-Dawley rats at dose
levels of 0, 1000, 3000, and 6000 mg/kg diet for 9 weeks
through weaning of the F1A generation. Body weight
reduction occurred at 6000 mg/kg diet in the parent rats.
The lactation index was depressed for the F1A generation
at 6000 mg/kg diet, and the weaning body weights for both
sexes of the F1A generation were reduced at doses of 3000
mg/kg diet or more. There were no other compound-related
adverse effects. The NOEL was considered to be 1000 mg/kg
diet.
(1R, cis/trans)-tetramethrin (technical product, 93.4%
purity) was administered at dose levels of 0, 100, 500,
and 3000 mg/kg diet to two successive generations of
Sprague-Dawley CDR albino rats to determine the effects on
the growth and reproductive performance. The body weights
of parental females were significantly lower during the
pre-mating growth, gestation, lactation, and post-weaning
periods, and the body weight of offsprings of both gener-
ations decreased during lactation at 3000 mg/kg diet.
Slight bile duct hyperplasia was noted in F1 females
sacrificed after a 30-day feeding period following weaning
of the F2 offspring at 3000 mg/kg diet. This was, how-
ever, a commonly observed change in old rats. Thus, tetra-
methrin did not affect the reproductive performance of
male and female rats in two successive generations at up
to 500 mg/kg diet [46].
7.7 Neurotoxicity - Mode of Action
Tetramethrin is classified as a Type I pyrethroid. The
mode of action of pyrethroids in general is described in
Appendix I.
In electrophysiological studies, tetramethrin produced
repetitive discharges in housefly muscle and uncoupling
in motor units [1, 32] and caused repetitive firing in
cockroach cercal sensory nerves at a concentration of
3 x 10-13 mol/litre [8].
The effects of tetramethrin on the sodium channel
gating mechanism were studied using the squid giant axons
under voltage clamp conditions [27, 28]. Tetramethrin pro-
longed the falling phase of sodium current during depolar-
ization and increased and prolonged the tail current
associated with repolarization. The prolongation of the
sodium current was due to the channel remaining open. The
channel returned slowly to the resting state upon repolar-
ization.
Analysis of the dose dependence of the two kinetic
phases of tail current development suggests that the ap-
parent dissociation constant for 1R, trans-tetramethrin
depends on the conformational state of the channel. Thus,
it can be concluded that tetramethrin binds to sodium
channels and modifies the state of the channel in the
resting, open, or inactivated state [28].
1R, trans-Tetramethrin markedly prolongs the open
time of single sodium channels recorded by the gigaohm-
seal voltage clamp technique in a membrane patch excised
from the N1E-115 neuroblastoma cell. Single channel con-
ductance is not altered by tetramethrin. The modification
by tetramethrin occurs in an all-or-nothing manner in a
population of sodium channels. The observed tetramethrin-
induced modification of single sodium channels is compat-
ible with previous sodium current data from axons [78].
Tetramethrin greatly prolongs the sodium current
during step depolarization and the sodium tail current
associated with step repolarization of the squid axon mem-
brane. Non-linear current-voltage relationships for the
sodium tail current were analyzed to assess the open
sodium channel properties, which included the permeation
of various cations, calcium block, and cation selectivity.
Tetramethrin had no effect on any of these properties. It
was concluded that tetramethrin modifies the sodium chan-
nel gating mechanism without affecting the pore properties
[79].
8. EFFECTS ON HUMANS
Although tetramethrin has been used for many years, no
adverse effects and no cases of human poisoning have been
reported in the published literature.
In a semi-closed patch test, an aqueous emulsion con-
taining 1.0% tetramethrin was applied to the skin of 200
human volunteers (aged 15-80, both male and female), using
cotton gauze, for 4 days. After 2 weeks, an additional ap-
plication was made in a same manner. Dermatological exam-
ination showed that tetramethrin is neither a primary
irritant nor a human skin sensitizer [73].
9. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by
Hazard, technical tetramethrin is classified as unlikely
to present an acute hazard in normal use [75].
REFERENCES
1. ADAMS, M.E. & MILLER, T.A. (1980) Neurophysiological correlation of
pyrethroid and DDT-type insecticide poisoning in the housefly Musca
domestica L. In: Insect neurobiology and pesticide action (Neutox 79),
London, Society of Chemical Industry, pp. 431-439.
2. DING, C., YU, Y., ZHANG, J., CAI, Z., & CHAN, X. (1985) Genotoxic
effects of tetramethrin on cultured mammalian cells and Salmonella
typhimurium. Zhejiang Yike Daxue Xuebao, 14(1): 1-4.
3. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
4. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
5. FAO (1982) Second Government Consultation on International Harmonization
of Pesticide Registration Requirements, Rome, 11-15 October 1982, Rome,
Food and Agriculture Organization of the United Nations.
6. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
7. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
8. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15: 181-
191.
9. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in the mouse
and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
10 GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:(6)
793-799.
11. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980a) Primary eye and skin
irritation tests of Neopynamin Forte, technical in rabbits (Technical
Report No. IT-00-0073) (Submitted to WHO by Sumitomo Chemical Co.).
12. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980b) Skin sensitization
test of Neopynamin Forte, technical in guinea-pigs (Technical Report No.
IT-00-0082) (Submitted to WHO by Sumitomo Chemical Co.).
13. HARA, M., SUZUKI, H., & MIYAMOTO, J. (1981) In vivo chromosomal
aberration test of Neopynamin Forte, on bone marrow cells of mice (Tech-
nical Report No. 81042) (Submitted to WHO by Sumitomo Chemical Co.).
14. HARTLEY, D., KIDD, H., & KENNEDY, J.M. (1987) Agrochemicals
Handbook, 2nd ed., Nottingham, United Kingdom, Royal Society of
Chemistry.
15. HAYASHI, A. (1977) [How to use insecticides safely.] Bo-satsu-kyo
News, 1: 6-11 (in Japanese).
16. HIROMORI, T., HOSOKAWA, S., OKUNO, Y., SEKI, T., SUZUKI, T.,
& MIYAMOTO, J. (1982) [Mammalian toxicity of d-isomer of phthalthrin
(Neopynamin Forte).] Pharmacometrics, 24: 179-201 (in Japanese).
17. KADOTA, T., KOHDA, H., & MIYAMOTO, J. (1977) Acute oral and
dermal toxicities of Neopynamin in mice and rats (Technical Report No.
IT-70-0003) (Submitted to WHO by Sumitomo Chemical Co.).
18. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Metabolism of
tetramethrin isomers in rats. J. Pestic. Sci., 6: 425-435.
19. KATO, T., UEDA, K., & FUJIMOTO, K. (1964) New insecticidally active
chrysanthemates. Agric. biol. Chem., 28: 914-915.
20. KATO, T., SUZUKI, T., SAKO, H., OKUNO, Y., & MIYAMOTO, J.
(1987) Acute dermal toxicity of Neopynamin in rabbits (Technical
Report No. IT-70-0207) Submitted to WHO by Sumitomo Chemical Co.).
21. KAWAMOTO, M., HARA, M., KOGISO, S., YOSHITAKE, A., &
YAMADA, H. (1988) In vitro unscheduled DNA synthesis (UDS) assay of
Neopynamin in rat hepatocytes (Technical Report No. IT-80-0213)
(Submitted to WHO by Sumitomo Chemical Co.).
22. KISHIDA, F., SUZUKI, H., & MIYAMOTO, J. (1981) Mutagenicity test of
Neopynamin Forte, in bacterial system (Technical Report No. IT-00-0101)
(Submitted to WHO by Sumitomo Chemical Co.).
23. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
24. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore
complex. Science, 221: 1399-1401.
25. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions
of pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
26. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd., p. 440.
27. LUND, A.E. & NARAHASHI, T. (1981) Kinetics of sodium channel
modification by the insecticide tetramethrin in squid axon membranes.
J. Pharmacol. exp. Ther., 219: 464-473.
28. LUND, A.E. & NARAHASHI, T. (1982) Dose-dependent interaction of the
pyrethroid isomers with sodium channels of squid axon membranes.
Neurotoxicology, 3: 11-24.
29. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane effects
of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
30. MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, p. 502.
31. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J.
(1983) Farm chemicals handbook. Section C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, p. C232.
32. MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington DC, American Chemical Society,
pp. 98-115 (ACS Symposium Series No. 42).
33. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
34. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
35. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry, human
welfare and the environment, Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September
1982, Oxford, Pergamon Press, Vol. 1-4.
36. MIYAMOTO, J., SATO, Y., & YAMAMOTO, K. (1968) Biochemical
studies on the mode of action of pyrethroidal insecticides. Part I.
Metabolic fate of phthalthrin in mammals. Agric. biol. Chem., 32:
628-640.
37. MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem., 36:
2203-2211.
38. OKUNO, Y. (1982) Exposure study with spraying of Neopynamin
aerosol (Technical Report No. IF-20-002) (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
39. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976a) Eye and skin
irritation of Neopynamin in rabbits (Technical Report No. IT-60-0014)
(Submitted to WHO by Sumitomo Chemical Co.).
40. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976b) Skin sensitization
study of Neopynamin with guinea-pigs (Technical Report No. IT-60-0013)
(Submitted to WHO by Sumitomo Chemical Co.).
41. PANSHINA, T.N. & SASINOVICH, L.M. (1983) Toxicology of synthetic
pyrethroids. Khim. Selsk. Khoz. 12: 51-53.
42. PAPADOPOULOU-MOURKIDOU, E., IWATA, Y., & GUNTHER, F.A.
(1981) Utilization of an infrared detector for selective liquid
chromatographic analysis. 2. Formulation analysis of the pyrethroid
insecticides allethrin, decamethrin, cypermethrin, phenothrin, and tetra-
methrin. J. agric. food. Chem., 29(6): 1105-1111.
43. PENCE, D.H., HAGEN, W.H., ALSAKER, R.D., DAWKINS, B.G., MARSHALL,
P.M., & TACEY, R.L. (1981a) Subchronic toxicity study in dogs.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories Inc.
(Technical Report No. IT-11-0098) (Submitted to WHO by Sumitomo Chemical
Co.).
44. PENCE, D.H., SEROTA, D.G., ALSAKER, R.D., BANAS, D.A., &
KUNDZINS, W. (1981b) Chronic toxicity study in rats. Neopynamin
Technical Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-11-0097) (Submitted to WHO by Sumitomo
Chemical Co.).
45. PENCE, D.H., COX, R.H., DUDECK, L.E., ALSAKER, R.D., JONES, S.R.,
PARKER, G.A., HEPNER, K.E., & ZOETIS, T. (1986a) Combined chronic
toxicity and oncogenicity study in mice. Neopynamin Final Report, Vienna,
Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-61-0193)
(Submitted to WHO by Sumitomo Chemical Co.).
46. PENCE, D.H., WOLFE, G.W., KULWICH, B.A., HEPNER, K.E., &
SNYDER, F.G. (1986b) Two-generation reproduction study in rats.
Neopynamin Forte, Final Report, Hazleton Laboratories, Inc. (Technical
Report No. IT-61-0201) (Submitted to WHO by Sumitomo Chemical Co.).
47. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids
on a nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
48. RUTTER, H.A., Jr (1974) One-generation reproduction study in rats.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-01-0081) (Submitted to WHO by Sumitomo
Chemical Co.).
49. RUTTER, H.A., Jr, NELSON, L.W., KUNDZINS, W., & MCHUGH (1974)
Two-year dietary administration in the rat. Neopynamin Final Report,
Vienna, Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-
41-0024) (Submitted to WHO by Sumitomo Chemical Co.).
50. RUZO, L.O., SMITH, I.H., & CASIDA, J.E. (1982) Pyrethroid
photochemistry: photooxidation reactions of the chrysanthemates,
phenothrin and tetramethrin. J. agric. food Chem., 30: 110-115.
51. SATO, T. & NARAMA, K. (1980a) Reproduction test of Neopynamin. Part 1:
Fertility study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0075) (Submitted to WHO by Sumitomo Chemical Co.).
52. SATO, T. & NARAMA, K. (1980b) Reproduction test of Neopynamin. Part 2:
Teratology study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0076) (Submitted to WHO by Sumitomo Chemical Co.).
53. SATO, T. & NARAMA, K. (1980c) Reproduction test of Neopynamin. Part 3:
Teratology study in rabbits, Shizuoka, Hamamatsu Seigiken Research (Tech-
nical Report No. IT-01-0077) (Submitted to WHO by Sumitomo Chemical Co.).
54. SATO, T., TAGAWA, G., & NARAMA, K. (1980) Reproduction test of
Neopynamin. Part 4: Perinatal and postnatal study in rats, Shizuoka,
Hamamatsu Seigiken Research (Technical Report No. IT-01-0078) (Submitted
to WHO by Sumitomo Chemical Co.).
55. SMITH, I.H., WOOD, E.J., & CASIDA, J.E. (1982) Glutathione conjugate
of the pyrethroid tetramethrin. J. agric. food Chem., 30: 598-600.
56. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of
Neopynamin with bacterial systems (Technical Report No. IT-70-0023)
(Submitted to WHO by Sumitomo Chemical Co.).
57. SUZUKI, T. & MIYAMOTO, J. (1974) Metabolism of tetramethrin in
houseflies and rats in vitro. Pestic. Biochem. Physiol., 4: 86-97.
58. SUZUKI, T., KOHDA, H., MISAKI, Y., OKUNO, Y., KOYAMA, Y.,
& MIYAMOTO, J. (1980) Acute and subacute inhalation toxicity studies of
Neopynamin Forte in rats (Technical Report No. IT-10-0144) (Submitted to
WHO by Sumitomo Chemical Co.).
59. TANAKA, H., TAKADA, S., ISEKI, M., SANO, R., AIHARA, H.,
KURODA, H., MAEBO, Y., MOTOYOSHI, I., & WAKAI, Y. (1967)
Toxicological studies of various insecticides to mammalia. 2. On the
acute and chronic toxicity of pyrethroid insecticide phthalthrin to
albino rat or guinea-pig. Med. J. Osaka City Univ., 16: 509-517.
60. UEDA, K., GAUGHAN, L.C., & CASIDA, J E. (1975) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food Chem., 23:
106-115.
61. UNO, M., OKADA, T., OHMAE, T., TERADA, I., & TANIGAWA, K.
(1982) [Determination of pyrethroid insecticides by dual-wavelength
densitometry.] Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
62. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
63. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels
in frog myelinated nerve membrane. In: Insect neurobiology and
pesticide action, London, Society of Chemical Industry, pp. 79-85.
64. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M. (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
65. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and phermones,
New York, London, Plenum Press, pp. 183-210.
66. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
67. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
68. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
69. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
70. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN,
J. (1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in myelinated nerves. Nature (Lond.), 295: 601-603.
71. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M.,
& VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
72. WEIR, R.J. & CREWS, L.M. (1966) Three month dietary administration
to dogs of Neopynamin - final report (Technical Report No. IT-61-0011)
(Submitted to WHO by Sumitomo Chemical Co.).
73. WEIR, R.J. & OSBOURN, R.A. (1966) Report of human patch test on
coded substance : 2301 D-61-1, Vienna, Virginia, Hazleton
Laboratories, Inc. (Technical Report No. IT-61-0008) (Submitted to
WHO by Sumitomo Chemical Co.).
74. WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
75. WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988/89, Geneva, World
Health Organization (Unpublished report VBC/88.953).
76. WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 520.
77. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids.
Gen. Pharmacol., 9: 387-398.
78. YAMAMOTO, D., QUANDT, F.N., & NARAHASHI, T. (1983)
Modification of single sodium channels by the insecticide
tetramethrin. Brain Res. 274: 344-349.
79. YAMAMOTO, D., YEH, J.Z., & NARAHASHI, T. (1986) Ion permeation
and selectivity of squid axon sodium channels modified by
tetramethrin. Brain Res., 372: 193-197.
80. YOSHITAKE, A., KOGISO, S., HARA, M., & MIYAMOTO, J. (1986)
In vivo chromosomal aberration test of Neopynamin in mouse bone
marrow cells (Technical Report No. IT-60-0197) (Submitted to WHO
by Sumitomo Chemical Co.).
81. YOSHITAKE, A., KOGISO, S., YAMADA, F., HARA, M., & MIYAMOTO,
J. (1987) Reverse mutation test of Neopynamin in Salmonella
typhimurium and Escherichia coli (Technical Report No. IT-70-0205)
(Submitted to WHO by Sumitomo Chemical Co.).
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs ( Xenopus laevis, Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [10, 23, 65,
66, 74]. The same distinction was found in studies on
cockroaches [8].
Based on the binding assay on the alpha-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types: the
alpha-cyano-3-phenoxybenzyl pyrethroids and the non-cyano
pyrethroids [7, 9, 24, 25].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin, cisme-
thrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [64]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [62]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [71].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [6].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [63]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [71].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans [4],
are almost identical to those of allethrin, i.e., presyn-
aptic repetitive firing, and no significant effects on
transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [64, 65, 70].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [29].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [47].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cyhalothrin, lambda-cyhalo-
thrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [69]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [67,
71].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during exci-
tation, presumably by slowing down the closing of the
activation gate of the sodium channel [67, 71]. The time
constant of closing of the activation gate in the cyper-
methrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged
sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to
cause the long-lasting repetitive firing in the lateral-
line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [29].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [9].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the toxicity
of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand,
[35S]- t-butylbicyclophosphorothionate [35S]-TBPS. Studies
with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic
cyano compounds including deltamethrin, 1R, cis-cyper-
methrin, 1R, trans-cypermethrin, and [2S, alphaS]-fenvalerate
were inhibitors, but their non-toxic stereoisomers were
not; non-cyano pyrethroids were much less potent or were
inactive [24].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [25].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S,alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex [7].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [47].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-lasting
prolongation of the transient increase in sodium per-
meability of the nerve membrane during excitation. This
results in long-lasting trains of repetitive impulses in
sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [74, 77].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [68].
1. RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1.1 Résumé et évaluation
1.1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La tétraméthrine a été synthétisée pour la première
fois en 1964 et commercialisée et en 1965. Sur la plan
chimique, c'est un ester de l'acide chrysanthémique (acide
diméthyl-2,2(diméthyl-2,2 vinyl)-3 cyclopropanecarboxylique
et de l'alcool tétrahydro-3,4,5,6 phatlimidométhylique.
Elle est constituée d'un mélange de quatre stéréoisomères
[1R,trans], [1R,cis], [1S,trans] et [1S,cis]. Les stéréo-
isomères qui entrent dans la composition des produits
techniques sont à peu près dans la proportion de 4:1:4:1.
De tous les isomères, c'est l'isomère [1R,trans] qui est
le plus actif biologiquement; vient ensuite l'isomère
1R,cis]. On commercialise sous le nom de "Neo-pynamine
Forte" (désignée dans la présente monographie sous le nom
de 1R, cis/trans-tetraméthrine), un mélange des isomères
[1R,cis] et [1R,trans] dans le proportion de 1:4.
La tétraméthrine de qualité technique est un solide
incolore dont le point de fusion est de 60-80 °C. Sa
densité est de 1,11 à 20 °C et sa tension de vapeur de
0,946 mPa (7,1 x 10-6 mm Hg) à 30 °C. Peu soluble dans
l'eau (4,6 mg/litre à 30 °C), elle est en revanche soluble
dans certains solvants organiques tels que l'hexane, le
méthanol et le xylène. Elle est stable à la chaleur mais
instable à la lumière et à l'air. L'isomère [1R,cis/trans]
est un liquide visqueux de couleur jaune dont les autres
propriétés physiques et les propriétés chimiques sont les
mêmes que celles de la tétraméthrine.
Le dosage des résidus s'effectue par densitométrie à
deux longueurs d'onde (370-230 nm). Pour l'analyse des
produits techniques, on utilise la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
Une analyse des différentes formulations peut s'effectuer
au moyen d'un chromatographe en phase liquide à haute
performance muni d'un détecteur infrarouge.
1.1.2 Production et usage
La production mondiale annuelle de tétraméthrine est
évaluée à quelques centaines de tonnes. Elle est princi-
palement utilisée pour la lutte contre les nuisibles à
l'intérieur des habitations, sous forme d'aérosols, de
concentrés émulsionnables ou de serpentins anti-moust-
iques. La tétraméthrine entre également dans la compos-
ition d'autres formulations insecticides additionnées ou
non de synergisants.
1.1.3 Exposition humaine
L'exposition de la population dans son ensemble peut
résulter de l'utilisation de ce produit pour la destruc-
tion des nuisibles dans les habitations. Lorsqu'on utilise
la tétraméthrine conformément aux recommandations, sa con-
centration atmosphérique ainsi que celle de l'isomère 1R
ne devraient pas dépasser 0,5 mg/m3; par ailleurs le
composé se dégrade rapidement. L'exposition de la popu-
lation générale est donc vraisemblablement très faible. On
n'utilise pas de tétraméthrine pour traiter les cultures
vivrières.
1.1.4 Exposition et destinée dans l'environnement
Une fine pellicule de tétraméthrine exposée à la
lumière solaire se dégrade rapidement. On a observé que
les principales réactions photochimiques qui se produis-
aient au cours d'une exposition de 2 heures (conversion de
30%) étaient: une époxydation au niveau de la double
liaison du radical isobutényle, une oxydation en hydroxy-
méthyle, en aldéhyde et en acide carboxylique du groupe
méthyle en position trans du groupement isobutényle;
enfin, une hydroperoxydation en hydroperoxyde allylique.
On ne connaît pas avec exactitude les concentrations
exactes de tétraméthrine dans l'environnement, mais compte
tenu de l'utilisation qui en est faite actuellement pour
traiter les habitations et pourvu que le produit soit
utilisé conformément aux recommandations, il est vraisem-
blable que l'exposition dans l'environnement devrait être
très faible. La tétraméthrine se décompose rapidement en
produits moins toxiques.
1.1.5 Absorption, métabolisme, et excrétion
Des rats à qui l'on avait administré par voie orale ou
sous-cutanée de la tétraméthrine radio-marquée au niveau
du reste acide ou du reste alcool ont rapidement absorbé,
métabolisé et excrété le produit. L'excrétion s'effectue
en 5 à 7 jours dans la proportion d'environ 95%, à peu
près autant par la voie urinaire que par la voie fécale.
Par ces deux voies, les résidus présents dans les tissus
sont très faibles. La métabolisation s'effectue par les
réactions suivantes: coupure de l'ester; élimination du
groupe hydroxyméthyl du reste alcool; réduction de la
double liaison 1-2 du reste alcool; oxydation du groupe-
ment méthyle de l'isobutényle au niveau du reste acide et
en 2, 3 et 4 au niveau du reste alcool; conjugaison des
acides et des alcools résultant avec l'acide glucuronique
et enfin isomérisation cis/trans.
1.1.6 Effets sur les êtres vivant dans leur milieu naturel
On ne dispose que de très peu d'informations à ce
sujet. La tétramethrine est extrêmement toxique pour les
poissons, la valeur de la CL50 à 96-h pour deux espèces
se situant respectivement à 19 et 21 µg/litre. Pour une
troisième espèce, on a obtenu une CL50 à 48 heures de
200 µg/litre et la dose sans effet observable était de
50 µg/litre. Pour la daphnie, la dose sans effet
observable est également de 50 µg/litre. La tétraméthrine
est en revanche très peu toxique pour les oiseaux mais
elle est toxique pour les abeilles. Cependant du fait que
le produit est rapidement dégradé et dans la mesure où on
ne l'utilise, conformément aux recommandations, que dans
les habitations, il est peu probable qu'il puisse excercer
des effets nocifs sur l'environnement.
1.1.7 Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in-vitro
La tétraméthrine a une faible toxicité aiguë par voie
orale. La DL50 pour le rat est >5000 mg/kg, qu'il
s'agisse du racémique ou de l'isomère (1R,cis/trans),
tandis que pour la souris elle est d'environ 2000 mg/kg
(racémique) et de 1060 mg/kg (1R,cis/trans). Chez le rat,
la souris et le lapin la toxicité aiguë par voie percu-
tanée e st également faible; la DL50 chez le rat et la
souris étant <5000 mg/kg et <2000 mg/kg chez le lapin
(toutes les études portaient sur le racémique). Les études
de toxicité aiguë par inhalation ont donné une CL50 chez
le rat et la souris de 2500 mg/m3 pour le racémique et
>1180 mg/m3 pour l'isomère (1R,cis/trans). Parmi les
signes d'intoxication on a noté une hyperexcitabilité, des
tremblements, de l'ataxie et une dépression (signes
généraux observés dans l'ensemble des études de toxicité
aiguë). Les souris se sont révélées un peu plus sensibles
que les rats mais il n'y avait pas de différences de
sensibilité entre mâles et femelles. Qu'ils s'agisse du
racémique ou de l'isomère (1R,cis/trans), la tétraméthrine
ne provoque pratiquement aucune irritation oculaire ou
cutanée chez le lapin. En outre, ni l'un ni l'autre de
ces produits n'exercent d'effet sensibilisateur chez le
cobaye.
La tétraméthrine est un pyréthroïde du type I. Chez
les mammifères ce sont les tremblements (syndrome-T) qui
constituent le symptôme d'intoxication caractéristique.
Chez des rats ayant reçu de la tétraméthrine mêlée à
leur nourriture à des concentrations allant jusqu'à 5000
mg/kg de nourriture pendant 91 jours, on a noté une réduc-
tion du gain de poids à la dose la plus forte. D'après les
résultats d'études de 3 et 6 mois, au cours desquelles des
rats ont reçu l'isomère 1R(cis/trans) dans leur nourriture
à des doses allant de 25 mg/kg à 3000 mg/kg d'aliment, la
dose sans effet observable était de 200 mg/kg de nourri-
ture pour les mâles et de 300 mg/kg pour les femelles
(parmi les anomalies observées, on notait une réduction du
gain de poids et du poids final du corps ainsi que
certains effets sur les reins et le foie). Les effets sur
le foie résultent, semble-t-il, d'une réaction d'adapt-
ation à la présence dans l'alimentation du véhicule
utilisé, à savoir l'huile de maïs.
Une étude de 26 semaines sur des chiens a fait
ressortir une dose sans effet observable de 1250 mg/kg de
nourriture.
Des souris et des rats à qui l'on avait fait inhaler
de la tétraméthrine en aérosol à une concentration de 200
mg/m3, 3 à 4 heures par jour pendant des périodes allant
jusqu'à quatre semaines, n'ont présenté aucune anomalie
imputable à ce produit. Lors d'une autre étude de ce
type, au cours de laquelle des rats ont été exposés à une
brumisation (gouttelettes de 1,2-1,5 µm de diamètre)
d'isomère (1R,cis/trans) dans du kérosène désodorisé à des
concentrations allant jusqu'à 87 mg/m3, trois heures par
jour et sept jours par semaine pendant 28 jours, on a
obtenu, pour la dose sans effet observable, une valeur de
49 mg/m3. Les signes d'intoxication n'ont été observés
qu'au cours de l'exposition.
Ni la tétraméthrine ni ses isomères (1R,cis/trans) ne
se sont révélés mutagènes dans divers systèmes d'épreuve
in vivo et in vitro utilisés pour étudier les mutations
génétiques, les lésions et les réparations de l'ADN ainsi
que les effets sur les chromosomes.
Trois études, dont deux chez le rat et une chez la
souris ont été menées pendant 104 semaines afin d'étudier
la cancérogénicité à long terme de la tétraméthrine. Les
souris ont reçu de la tétraméthrine dans leur nourriture à
des doses allant jusqu'à 1500 mg/kg de nourriture. Aucun
effet oncogène n'a été observé. A partir de 60 mg/kg de
nourriture on observait une réduction du poids de
l'hypophyse, de la thyroïde et de la parathyroïde. Chez
la souris, la dose sans effet général observable se
situait à 12 mg/kg de nourriture. Quant aux rats, ils ont
été exposés à de la tétraméthrine à des doses allant
jusqu'à 5000 mg/kg de nourriture soit in utero soit au
cours d'une période prolongée. Les deux études ont fait
ressortir un gain de poids sensiblement moindre chez les
animaux recevant 3000 mg de tétraméthrine par kg de
nourriture ou davantage. En outre, à ces concentrations,
on a observé une augmentation du poids du foie. Pour ce
qui est des effets généraux, la dose sans effet observ-
able se situait dans les deux études, à 1000 mg/kg de
nourriture. A la dose de 3000 mg/kg de nourriture,
l'incidence des tumeurs testiculaires à cellules de Leydig
était supérieure à la valeur notée dans le groupe témoin
et ce, pour les deux études. Les tumeurs à cellules de
Leydig se produisent spontanément chez les rats âgés et
leur incidence peut varier énormément dans les groupes
témoins. On pense que cette tumeur est d'origine hormon-
ale. Aucun signe de malignité et aucune tumeur de ce type
n'ont été relevés chez les souris. On peut en conclure
que cet effet oncogène, s'il existe réellement, ne peut
être pris en considération pour ce qui concerne l'homme.
La tétraméthrine ne s'est révélée ni tératogène ni
embryotoxique à des doses allant jusqu'à 1000 mg par kg de
poids corporel chez les rats et jusqu'à 500 mg/kg chez des
lapins (il s'agit des concentrations les plus fortes
étudiées). Lors d'une étude de fécondité au cours de
laquelle des rats ont reçu de la tétraméthrine à des doses
allant jusqu'à 1000 mg/kg de poids corporel par jour, la
dose sans effet observable sur la reproduction des parents
et la croissance des foetus, se situait à 300 mg/kg de
poids corporel par jour. Une étude de reproduction chez
le rat, portant sur la période périnatale et sur la
période post-natale, a permis de fixer à 100 mg/kg de
poids corporel la dose quotidienne sans effet observable
(la dose la plus forte administrée au cours de cette
étude).
Lors d'une étude de reproduction portant sur une
génération de rats, on a administré aux animaux 1000-6000
mg de tétraméthrine par kg de nourriture et constaté que
la dose sans effet observable était de 1000 mg/kg. Selon
une autre étude portant cette fois sur deux générations,
au cours de laquelle les rats ont reçu de l'isomère
(1R,cis/trans) à des doses allant de 100 à 3000 mg/kg, la
dose sans effet observable était de 500 mg/kg de
nourriture.
1.1.8 Effets sur l'homme
Bien que la tétraméthrine et son isomère 1R soient
utilisées depuis des années, on ne signale aucun cas
d'intoxication ou d'effets indésirables chez l'homme.
Rien n'indique que la tétraméthrine ou son isomère 1R
puissent avoir des effets nocifs sur l'homme si on
continue de les utiliser à faibles concentrations et
seulement pour la destruction des nuisibles à l'intérieur
des habitations.
1.2 Conclusions
a) Population générale: l'exposition de la population
générale à la tétraméthrine, dans son utilisation actu-
elle, est vraisemblablement faible. Si ce produit est
utilisé conformément aux recommandations, il ne présente
probablement aucun risque.
b) L'exposition professionnelle: moyennant de bonnes
méthodes de travail, l'application de mesures d'hygiène et
avec quelques précautions, la tétraméthrine ne devrait pas
présenter de danger pour les personnes qui y sont exposées
de par leur profession.
c) Environnement: il est tout à fait improbable que la
tétraméthrine ou ses produits de décomposition s'accum-
ulent au point d'avoir des effets nocifs sur l'environne-
ment.
1.3 Recommandations
Bien que la tétraméthrine et son isomère 1R soient
utilisés depuis des années sans qu'on ait à déplorer
d'effets nocifs chez l'homme, il est souhaitable que
l'exposition humaine continue d'être surveillée.
Judging from the metabolites derived from the acid
moiety, cis to trans isomerization of the oxidized deriva-
tives of CA occurs, as happens in resmethrin metabolism
[60].
Although both cis to trans and trans to cis isomeriza-
tions of tetramethrin were observed by Kaneko et al. [18],
cis to trans conversion seemed to be predominant. On the
other hand, the detected metabolites from the alcohol
moiety were TPI, HPI, 3-OH-TPI (33), 3,4,5,6-tetrahydro-
phthalic acid amide (34) (TPIA), 2-OH-HPI (35), 3-OH-HPI
(19), and 4-OH-HPI (36). Of these metabolites, 2-OH-HPI
was found in relatively large amounts.
Smith et al. [55] found that tetramethrin and TPI
readily underwent the Michael addition with thiols. The
tetramethrin-gluthathione (GSH) conjugate was formed under
physiological conditions in the presence of mouse liver
homogenate fractions, probably by a non-enzymatic
reaction. The soluble thiol level of mouse liver was de-
creased by intraperitoneal administration of TPI. However,
mercapturic acid and GSH conjugates of tetramethrin were
not detected in the bile or urine of rats or mice treated
intraperitoneally with tetramethrin.
5.2 Enzymatic Systems for Biotransformation
When alcohol- or acid-labelled [1RS, trans]-tetra-
methrin (1 mmol/litre) was incubated for 1 h at 37 °C with
30 mg protein of a rat liver subcellular fraction (i.e.
nuclei plus mitochondria, microsomes, and soluble frac-
tion), the microsomes and nuclei plus mitochondria frac-
tions were active in degrading tetramethrin. Rat micro-
somal fraction degraded [1RS, trans]-tetramethrin to CA,
MTI, and TPI in the absence of NADPH. In the presence of
NADPH, tetramethrin was more rapidly degraded to yield
oxidized tetramethrin ( wt-alc-, wt-ald-, and wt-acid-tetra-
methrin), oxidized CA ( wt-alc-, wt-ald-, and wt-acid-CA),
TPI, and unidentified metabolites in larger amounts. The
major metabolite TPI was shown to be produced non-enzy-
matically from MTI. The degradation rate of tetramethrin
was greatly reduced by the inhibition of ester hydrolysis
with paraoxon [57].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
As the use of tetramethrin is limited to indoor pest
control, there is a paucity of data concerning its effect
on the environment.
6.1 Aquatic Organisms
Data on the toxicity of tetramethrin to non-target
aquatic organisms are given in Table 4.
Tetramethrin is highly acutely toxic to fish in lab-
oratory tests, the 96-h LC50s for two species being ap-
proximately 20 µg/litrea. The 48-h LC50 for killifish
is about 200 µg/litre, with a no-observed-effect level
(NOEL) of 50 µg/litre [33]. The NOEL for Daphnia pulex
was reported by Miyamoto [33] to be 50 µg/litre for
racemic tetramethrin and 10 µg/litre for [1R, trans]- or
[1R, cis]-tetramethrin.
6.2 Terrestrial Organisms
Tetramethrin has low toxicity to birds. The acute oral
LD50 for Bobwhite quail is >2510 mg/kg body weight, and
the 8-day dietary LC50 to Mallard duck and Bobwhite quail
is >5620 mg/kga.
Tetramethrin is toxic to bees [14].
a written comment from US EPA to IPCS, 1987.
Table 4. Acute toxicity of tetramethrin to non-target aquatic organisms
Species Size Parameter Toxicity Formulation System Temperature Refer-
(mg/litre) (°C) ence
Fish
Killifish adult 48-h LC50 0.2 Technical Static 25 33
( Oryzias latipes) adult 48-h LC50 0.2 (+)-trans Static 25 33
adult 48-h LC50 0.15 (+)-cis Static 25 33
Bluegill sunfish 96-h LC50 0.019 a
( Lepomis macrochirur)
Rainbow trout 96-h LC50 0.021 a
( Salmo gairdneri)
Arthropods
Daphnia pulex 3-h LC50 >50 Technical Static 25 33
3-h LC50 >50 (+)-trans Static 25 33
3-h LC50 >50 (+)-cis Static 25 33
a Written comment from US EPA to IPCS, 1987.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
The acute toxicity of tetramethrin to rats and mice is
low (Table 5).
Table 5. Acute toxicity of tetramethrin to rats and mice
Compound Species Sex Route LD50 Reference
(mg/kg body
weight)
Racemic rat M,F oral 4600 41
rata M,F oralb >5000 17
rat M,F dermalb >10 000 41
mousea M oralb 1920 17
mousea F oralb 2000 17
(1R, cis/trans)- rata M,F oralb >5000 16
rat M,F subcutaneous >5000 16
rat M intraperitoneal 770 16
rat F intraperitoneal 548 16
rat M,F dermal(>24 h)b >5000 16
mousea M oral 1060 16
mousea F oral 1040 16
mouse M subcutaneous 2020 16
mouse F subcutaneous 1950 16
mouse M intraperitoneal 631 16
mouse F intraperitoneal 527 16
mouse M,F dermal (>24 h)b >5000 16
rabbit dermal (>24 h)b >2000 20
a Animals were not fasted.
b Corn oil was used as vehicle.
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1.2-
1.5 µm) of [1R, cis/trans]-tetramethrin (technical grade,
95.6% purity) in deodorized kerosene (0, 26, 131, 243,
595, and 1180 mg active ingredient per m3 air) for a
duration of 3 h. At 131 mg/m3 or more, salivation, hyper-
excitability, irregular respiration, urinary incontinence,
muscular fibrillation, limb paralysis, decrease in spon-
taneous activity, and other toxic signs were observed in
males and females. At 1180 mg/m3, 10% of female animals
died, but the body weight gain was similar to that of the
control rats. The no-observed-effect level (NOEL) for
inhalation of the compound in rats was 26 mg/m3, and the
LC50 value was >1180 mg/m3 in both sexes [58].
The toxic symptoms observed following [1R, cis/trans]-
tetramethrin administration were hyperexcitability, muscle
twitching, tremor, ataxia, irregular respiration, and
depression. Mice were invariably more susceptible than
rats. No differences in susceptibility were observed
between male and female animals [16, 17, 33].
7.2 Irritation and Sensitization
7.2.1 Eye irritation
In a study by Okuno et al. [39], 50 mg of the techni-
cal product (91.3% purity) was instilled in one eye of
Japanese albino male rabbits. The treated eye was washed
with distilled water 5 min (group I) or 24 h (group II)
thereafter. The conjunctiva, cornea, and pupil were exam-
ined, 1, 24, 72 h and 7, 14, and 21 days after appli-
cation. No particular changes were noted except that a
very slight erythema and oedema of the conjunctiva was
transiently observed in the rabbits in group II.
In a separate study, 0.1 ml [1R, cis/trans]-tetra-
methrin (technical grade, 95.6% purity) was applied to one
eye of Japanese albino rabbits. The treated eye was sub-
sequently washed in five rabbits but not in three other
rabbits. The material did not produce any lesions in the
cornea or iris of the treated eyes that were not washed,
but slight hyperemia and/or chemosis of the conjunctiva
was observed 1 h after application. In the washed eyes,
slight hyperemia of the conjunctiva was observed in all
animals 1 h after treatment. These changes, however, had
disappeared by 48 h after application in the unwashed eyes
and 24 h in the washed eyes. The irritating potency of the
material was judged to be minimal in the unwashed eyes and
negative in the washed eyes [11].
7.2.2 Skin irritation
In a study by Okuno et al. [39], 0.5 g of the techni-
cal product (91.3% purity) was applied on a lint patch
(3.8 x 3.8 cm) to the abraded or intact skin of six rab-
bits. The skin was assessed for severity of erythema and
oedema 4, 24, 48, 72 h and 7, 14, and 21 days after appli-
cation, but no particular changes were noted.
When 0.5 ml [1R, cis/trans]-tetramethrin (technical
grade, 95.6% purity) was applied on a lint patch
(2.5 x 2.5 cm) to abraded or intact skin on the back of
rabbits, again no irritating reactions such as erythema
and oedema were observed [11].
7.2.3 Sensitization
In a skin-sensitization study of tetramethrin in
guinea-pigs, Hartley male guinea-pigs (seven per group)
were sensitized ten times at intervals of one or two days
by intracutaneous injections (first injection: 0.05 ml,
subsequent ones: 0.1 ml) of a 1% solution of the technical
product (91.3% purity) in corn oil. The sensitized animals
were then challenged against the same concentration in the
same manner (0.5 ml injection) 14 days later, but no skin-
sensitization reaction was noted [40].
In another skin-sensitization test on Hartley male
guinea-pigs, 0.5 ml [1R, cis/trans]-tetramethrin (techni-
cal product, 95.6% purity) in 0.5 ml acetone was applied
topically by lint patch to the back of animals ten times
(three times per week). The animals were challenged in the
same manner 2 weeks after the last sensitizing treatment,
but no allergic reactions were observed 24 h later [12].
7.3 Short-Term Exposure Studies
7.3.1 Oral
When groups of 10 male Wistar rats were maintained for
91 days on a diet containing 0, 500, 1000, 3000, or 5000
mg tetramethrin/kg diet, there was a reduced rate of body
weight gain at 5000 mg/kg but not at 3000 mg/kg or less.
The liver glycogen level was reduced at 3000 mg/kg and
5000 mg/kg. The kidney, spleen, heart, small intestine,
and brain showed no abnormal signs, either macroscopically
or microscopically, and there were no significant changes
in blood parameters. There was no increase in the protein
or glucose levels in the urine of test animals [59].
When technical (1R, cis/trans)-tetramethrin in corn
oil was administered to Sprague-Dawley rats for 3 or 6
months at 0, 100, 300, 1000, or 3000 mg/kg diet, no treat-
ment-related changes were observed in clinical signs, or
food and water consumption, or in an ophthalmological
examination. However, the body weight gain and final body
weight of males and females in the 3000-mg/kg group were
significantly lower than those of the controls. There were
slight increases in urine protein level in the rats fed
more than 1000 mg/kg and in serum calcium level in the
male rats fed more than 300 mg/kg. During a histopatho-
logical examination, eosinophilic bodies in tubular epi-
thelial cells and hyaline droplets in kidney tubular epi-
thelium cytoplasm were observed in males fed 3000 mg/kg
diet, along with an increase in relative organ weight.
There were dose-dependent increases in absolute and rela-
tive liver weight in all treated male rats and in female
rats fed more than 1000 mg/kg diet. There were also
slightly higher serum cholesterol concentrations in rats
of both sexes fed more than 1000 mg/kg and a significant
reduction in liver lipid content among males fed 1000
mg/kg or more. However, these liver effects were not
accompanied by damage to hepatocytes and were therefore
considered to be an adaptation to the corn oil without
toxicological significance. Furthermore, there were no
marked effects, even at 200 mg/kg, when an additional sub-
chronic study was conducted at tetramethrin levels of 25,
50, 100, and 200 mg/kg diet without corn oil in order to
confirm the NOEL in male rats. The NOEL for tetramethrin
in rats in the 6-month study was concluded to be 200 mg/kg
diet for males and 300 mg/kg diet for females [16].
When technical grade tetramethrin (94.6% purity) was
administered for 26 weeks to beagle dogs (six of each sex
per group) at levels of 0, 1250, 2500, and 5000 mg/kg
diet, nervousness and tremors were observed in both males
and females at 2500 and 5000 mg/kg diet. A lack of oestrus
activity in females was also noted clinically and a lack
of corpora lutea was confirmed histologically at 5000
mg/kg diet. Absolute liver weight was increased in males
at 2500 and 5000 mg/kg and relative liver weight was sig-
nificantly increased in males and females at 5000 mg/kg.
Decreased absolute/relative ovary weights were noted for
females at 5000 mg/kg. No other treatment-related changes
were observed with respect to survival, body weight gain,
food consumption, haematology, urinalysis, ophthalmology,
gross pathology or histopathology. The NOEL was 1250 mg/kg
diet [43].
In a study by Weir & Crus (1966), groups of three male
and three female beagle dogs were fed tetramethrin dis-
solved in corn oil for 13 weeks at levels of 0, 1250,
2500, and 5000 mg/kg diet. There were no effects on haema-
tological, clinical chemistry, or urinary parameters.
Organ weights were not affected by the treatment, and
there were no significant histopathological findings.
Clinical signs were not recorded. The NOEL was >5000 mg/kg
diet.
7.3.2 Inhalation
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1-2 µm)
of tetramethrin at concentrations of 0, 26, 49, and 87 mg
active ingredient per m3 air, 3 h a day, 7 days a week,
for a period of 28 days. At 87 mg/m3, irregular respir-
ation, slight salivation, and hyperexcitability were
observed as toxic signs every day during the exposure
period, but no cumulative toxicity was noted. There were
no compound-related effects on body weight gain, food and
water consumption, urinalysis, haematology, biochemistry,
organ weight, and histopathology. The NOEL in subacute
inhalation was considered to be 49 mg/m3 [58]. This NOEL
is approximately 100 times higher than the aerial concen-
tration attained during normal use of tetramethrin [33].
7.4 Long-Term Exposures and Carcinogenicity
Appraisal
Testicular interstitial cell tumours occur spontaneously in
aged rats, and the incidence can vary greatly in control
groups. This tumour is believed to be hormonally mediated.
There was no evidence of malignancy in three rat studies and no
evidence of this type of tumour in mice. It can be concluded
that the tumorigenic effect, if real, is most unlikely to be
relevant to human exposure.
When tetramethrin (technical grade) was administered
to Sprague-Dawley CRCDR rats (50 of each sex per group,
F1A weanlings from parental animals pre-treated with the
compound at dose levels of 1000, 3000, and 6000 mg/kg
diet) at dose levels of 0, 1000, 3000, or 5000 mg/kg diet
for 104 weeks, no compound-related effects were detected
in investigations of appearance, behaviour, survival,
haematology, blood chemistry, urinalysis, eye examination,
and organ weight at up to 5000 mg/kg diet. However, the
body weight gain of male and female rats fed 3000 mg/kg or
more was significantly lower than that of controls. The
incidence for testicular interstitial cell tumours was
increased at dose levels of 3000 mg/kg or more [49].
Tetramethrin (technical grade, 90.0/93.6% purity) was
tested for long-term toxic effects and tumorigenic poten-
tial in Sprague-Dawley CRCDR and Long-Evans hooded rats
by in utero exposure and 104-week chronic exposure at dose
levels of 0, 200, 1000, and 5000 mg/kg diet. No distinct
compound-related effects were observed in either strain
with regard to fertility rate, mortality, clinical signs,
and clinical laboratory data. However, body weight gains
were significantly lower in both strains at 5000 mg/kg
diet, and absolute and relative liver weights were in-
creased in both strains at 5000 mg/kg diet. The incidence
of interstitial cell tumours in both strains at 5000 mg/kg
diet was above the level in the concurrent control groups
[44].
When tetramethrin (technical product, 93.3% purity)
was fed daily to B6C3F1 mice (dose levels of 0, 12, 60,
300, or 1500 mg/kg diet) for 104 weeks, there were no sig-
nificant dose-related changes in survival, clinical signs,
mean body weight, or food consumption. However, the mor-
tality of male mice at 300 mg/kg was significantly lower
than that of control males. The absolute and relative
weight of pituitary and thyroid/parathyroid glands was
decreased for males fed 60 mg/kg diet or more. Absolute
spleen weights were also decreased for males fed 300 mg/kg
diet or more. However, gross and microscopic examination
of these tissues did not reveal any treatment-related
histomorphological changes. There were no other histo-
pathological findings attributable to tetramethrin admin-
istration. The NOEL was considered to be 12 mg/kg diet
[45].
7.5 Mutagenicity
The results of mutagenicity tests on tetramethrin are
summarized in Table 6.
Ding et al. [2] reported the induction of unscheduled
DNA synthesis in human amnion FL cells by tetramethrin
(72% industrial grade of unknown origin). The same product
gave weakly positive results in an Ames test with Salmon-
ella typhimurium TA 97. It is not clear if the effect was
caused by tetramethrin itself or by the unidentified (28%)
portion of the industrial grade material.
7.6 Reproduction, Embryotoxicity, and Teratogenicity
In a study by Miyamoto [33], groups of 10-15 pregnant
New Zealand white rabbits received tetramethrin orally on
days 6-18 of gestation at doses of 0, 30, or 90 mg/kg per
day. Fetuses were obtained by caesarean section prior to
parturition and were examined for external and skeletal
abnormalities. Seven extra pregnant animals were allowed
to give birth naturally and the pups were examined for
several weeks to check their growth and development. No
significant adverse effects were observed.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to 6-week-old male Slc: SD rats (SPF, 20
per group) for not less than 9 weeks and to 9-week-old
females (20 per group) for 2 weeks of the non-pregnant
period and up to day 7 of pregnancy. The effects of the
material on the mating ability of male and female animals
and on the fetuses were investigated. In males, the liver
weight increased at all dose levels and a kidney weight
increase was noted at 1000 mg/kg. Salivation and a slower
body weight increase were observed during the latter half
of the administration period at 300 and 1000 mg/kg. How-
ever, no effects on the reproductive ability of males were
noted. In females, no changes were observed in the rate of
pregnancy, but there were effects on the sexual cycle and
an ovulation-inhibiting effect at 1000 mg/kg. In fetuses,
growth inhibition was suspected at 1000 mg/kg. However,
all these changes were slight. The NOEL was considered to
be 300 mg/kg body weight per day for reproductive ability
of parents and growth of fetuses [51].
Table 6. Mutagenicity studies on tetramethrin
Test System Test object Concentration Purity/ Results Reference
Compound
Ames test S. typhimurium up to 10 mg/plate 93 - 100% Negative 33
TA 1535 without activation
TA 1538
Ames test Escherichia coli up to 10 mg/plate 93 - 100% Negative 33
W 3623 without activation
W 3012
Ames test S. typhimurium 1 - 10 000 ug/plate technical Negative 56
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1538
Ames test S. typhimurium 100 - 5000 ug/plate 94.0% Negative 81
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1537
TA 97
Ames test E. coli 100 - 5000 ug/plate 94.0% Negative 81
WP2 uvr A with and without racemic
activation
Ames test S. typhimurium 10 - 5000 ug/plate 95.6%, Negative 22
TA 100 with and without 1R, cis/trans
TA 98 activation
TA 1535
TA 1537
TA 1538
Ames test E. coli 10 - 5000 ug/plate 95.6%, Negative 22
WP2 uvr A 1R, cis/trans
Ames test S. typhimurium 5 - 500 ug/plate 72% Positivea 2
TA 97 without activation industrial
grade
Rec-assay Bacillus 1 - 10 000 ug/disk technical Negative 56
subtilis racemic
M45 rec- and H17
(wild type)
Rec-assay Bacillus 50 - 5000 ug/disk 95.6%, Negative 22
subtilis 1R, cis/trans
M45 rec- and H17
(wild type)
Table 6 (contd).
Test System Test object Concentration Purity/ Results Reference
Compound
Host-mediated ICR male mice 200 - 1000 mg/kg technical Negative 56
assay S. typhimurium body weight (oral) racemic
G46
In vivo
chromosomal ICR male mice 1200, 2400, 5000 93.4% Negative 80
aberration bone marrow mg/kg body racemic
weight (ip)
In vivo
chromosomal ICR male mice 150, 300, 600 mg/kg 95.6%, Negative 13
aberration bone marrow body weight (ip) 1R, cis/trans
Unscheduled
DNA Human amnion Not recorded 72%, Positivea 2
synthesis FL cells industrial
grade
Unscheduled
DNA Rat hepatocyte 0, 0.2, 1, 94.0% Negative 21
synthesis primary cultures 5, 25, 50, racemic
and 100 ug/ml
a The test material was of unknown origin and it was unclear whether or not
positive results were due to the 28% impurities.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 30 per group) on
days 7-17 of pregnancy, and its effects on the dams,
fetuses, and growth of pups were investigated. In dams, an
inhibition of body weight increase and a decrease of food
consumption were observed at 1000 mg/kg, in addition to an
increase in liver and kidney weights at the time of
caesarean section. In fetuses, no abnormalities such as
embryo lethality, growth inhibition, or teratogenic
effects were detected. In addition, the tetramethrin had
no effect on the growth of the young after birth or on the
reproductive ability of the offspring. The NOEL was con-
sidered to be 300 mg/kg body weight per day for the dams
and >1000 mg/kg body weight per day for teratogenicity
[52].
When tetramethrin (technical product) was orally
administered at dose levels of 0, 50, 150, or 500 mg/kg
body weight per day to pregnant Japanese white rabbits,
(10 per group) on days 8-18 of pregnancy, a slight transi-
ent decline in the body weight of the dams was noted in
the middle of the treatment period at 500 mg/kg. No
adverse effects such as embryo lethality, inhibition of
fetal growth, or teratogenic action were observed at any
dose level. The NOEL for teratogenicity in rabbits was
considered to be >500 mg/kg body weight per day [53].
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 20 per group) from
day 17 of gestation to day 21 of lactation (perinatal and
postnatal period). In dams, a liver weight increase was
noted at 300 and 1000 mg/kg but there were no abnormali-
ties at delivery or during lactation. Tetramethrin had no
detectable effects on the survival rate of pups, growth
and development, sensory function, motor function, learn-
ing ability, or reproductive ability. The NOEL was
considered to be 100 mg/kg body weight per day for dams
and >1000 mg/kg body weight per day for pups [54].
In studies by Rutter [48], tetramethrin (technical
grade) was administered to Sprague-Dawley rats at dose
levels of 0, 1000, 3000, and 6000 mg/kg diet for 9 weeks
through weaning of the F1A generation. Body weight
reduction occurred at 6000 mg/kg diet in the parent rats.
The lactation index was depressed for the F1A generation
at 6000 mg/kg diet, and the weaning body weights for both
sexes of the F1A generation were reduced at doses of 3000
mg/kg diet or more. There were no other compound-related
adverse effects. The NOEL was considered to be 1000 mg/kg
diet.
(1R, cis/trans)-tetramethrin (technical product, 93.4%
purity) was administered at dose levels of 0, 100, 500,
and 3000 mg/kg diet to two successive generations of
Sprague-Dawley CDR albino rats to determine the effects on
the growth and reproductive performance. The body weights
of parental females were significantly lower during the
pre-mating growth, gestation, lactation, and post-weaning
periods, and the body weight of offsprings of both gener-
ations decreased during lactation at 3000 mg/kg diet.
Slight bile duct hyperplasia was noted in F1 females
sacrificed after a 30-day feeding period following weaning
of the F2 offspring at 3000 mg/kg diet. This was, how-
ever, a commonly observed change in old rats. Thus, tetra-
methrin did not affect the reproductive performance of
male and female rats in two successive generations at up
to 500 mg/kg diet [46].
7.7 Neurotoxicity - Mode of Action
Tetramethrin is classified as a Type I pyrethroid. The
mode of action of pyrethroids in general is described in
Appendix I.
In electrophysiological studies, tetramethrin produced
repetitive discharges in housefly muscle and uncoupling
in motor units [1, 32] and caused repetitive firing in
cockroach cercal sensory nerves at a concentration of
3 x 10-13 mol/litre [8].
The effects of tetramethrin on the sodium channel
gating mechanism were studied using the squid giant axons
under voltage clamp conditions [27, 28]. Tetramethrin pro-
longed the falling phase of sodium current during depolar-
ization and increased and prolonged the tail current
associated with repolarization. The prolongation of the
sodium current was due to the channel remaining open. The
channel returned slowly to the resting state upon repolar-
ization.
Analysis of the dose dependence of the two kinetic
phases of tail current development suggests that the ap-
parent dissociation constant for 1R, trans-tetramethrin
depends on the conformational state of the channel. Thus,
it can be concluded that tetramethrin binds to sodium
channels and modifies the state of the channel in the
resting, open, or inactivated state [28].
1R, trans-Tetramethrin markedly prolongs the open
time of single sodium channels recorded by the gigaohm-
seal voltage clamp technique in a membrane patch excised
from the N1E-115 neuroblastoma cell. Single channel con-
ductance is not altered by tetramethrin. The modification
by tetramethrin occurs in an all-or-nothing manner in a
population of sodium channels. The observed tetramethrin-
induced modification of single sodium channels is compat-
ible with previous sodium current data from axons [78].
Tetramethrin greatly prolongs the sodium current
during step depolarization and the sodium tail current
associated with step repolarization of the squid axon mem-
brane. Non-linear current-voltage relationships for the
sodium tail current were analyzed to assess the open
sodium channel properties, which included the permeation
of various cations, calcium block, and cation selectivity.
Tetramethrin had no effect on any of these properties. It
was concluded that tetramethrin modifies the sodium chan-
nel gating mechanism without affecting the pore properties
[79].
8. EFFECTS ON HUMANS
Although tetramethrin has been used for many years, no
adverse effects and no cases of human poisoning have been
reported in the published literature.
In a semi-closed patch test, an aqueous emulsion con-
taining 1.0% tetramethrin was applied to the skin of 200
human volunteers (aged 15-80, both male and female), using
cotton gauze, for 4 days. After 2 weeks, an additional ap-
plication was made in a same manner. Dermatological exam-
ination showed that tetramethrin is neither a primary
irritant nor a human skin sensitizer [73].
9. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by
Hazard, technical tetramethrin is classified as unlikely
to present an acute hazard in normal use [75].
REFERENCES
1. ADAMS, M.E. & MILLER, T.A. (1980) Neurophysiological correlation of
pyrethroid and DDT-type insecticide poisoning in the housefly Musca
domestica L. In: Insect neurobiology and pesticide action (Neutox 79),
London, Society of Chemical Industry, pp. 431-439.
2. DING, C., YU, Y., ZHANG, J., CAI, Z., & CHAN, X. (1985) Genotoxic
effects of tetramethrin on cultured mammalian cells and Salmonella
typhimurium. Zhejiang Yike Daxue Xuebao, 14(1): 1-4.
3. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
4. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
5. FAO (1982) Second Government Consultation on International Harmonization
of Pesticide Registration Requirements, Rome, 11-15 October 1982, Rome,
Food and Agriculture Organization of the United Nations.
6. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
7. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
8. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15: 181-
191.
9. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in the mouse
and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
10 GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:(6)
793-799.
11. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980a) Primary eye and skin
irritation tests of Neopynamin Forte, technical in rabbits (Technical
Report No. IT-00-0073) (Submitted to WHO by Sumitomo Chemical Co.).
12. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980b) Skin sensitization
test of Neopynamin Forte, technical in guinea-pigs (Technical Report No.
IT-00-0082) (Submitted to WHO by Sumitomo Chemical Co.).
13. HARA, M., SUZUKI, H., & MIYAMOTO, J. (1981) In vivo chromosomal
aberration test of Neopynamin Forte, on bone marrow cells of mice (Tech-
nical Report No. 81042) (Submitted to WHO by Sumitomo Chemical Co.).
14. HARTLEY, D., KIDD, H., & KENNEDY, J.M. (1987) Agrochemicals
Handbook, 2nd ed., Nottingham, United Kingdom, Royal Society of
Chemistry.
15. HAYASHI, A. (1977) [How to use insecticides safely.] Bo-satsu-kyo
News, 1: 6-11 (in Japanese).
16. HIROMORI, T., HOSOKAWA, S., OKUNO, Y., SEKI, T., SUZUKI, T.,
& MIYAMOTO, J. (1982) [Mammalian toxicity of d-isomer of phthalthrin
(Neopynamin Forte).] Pharmacometrics, 24: 179-201 (in Japanese).
17. KADOTA, T., KOHDA, H., & MIYAMOTO, J. (1977) Acute oral and
dermal toxicities of Neopynamin in mice and rats (Technical Report No.
IT-70-0003) (Submitted to WHO by Sumitomo Chemical Co.).
18. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Metabolism of
tetramethrin isomers in rats. J. Pestic. Sci., 6: 425-435.
19. KATO, T., UEDA, K., & FUJIMOTO, K. (1964) New insecticidally active
chrysanthemates. Agric. biol. Chem., 28: 914-915.
20. KATO, T., SUZUKI, T., SAKO, H., OKUNO, Y., & MIYAMOTO, J.
(1987) Acute dermal toxicity of Neopynamin in rabbits (Technical
Report No. IT-70-0207) Submitted to WHO by Sumitomo Chemical Co.).
21. KAWAMOTO, M., HARA, M., KOGISO, S., YOSHITAKE, A., &
YAMADA, H. (1988) In vitro unscheduled DNA synthesis (UDS) assay of
Neopynamin in rat hepatocytes (Technical Report No. IT-80-0213)
(Submitted to WHO by Sumitomo Chemical Co.).
22. KISHIDA, F., SUZUKI, H., & MIYAMOTO, J. (1981) Mutagenicity test of
Neopynamin Forte, in bacterial system (Technical Report No. IT-00-0101)
(Submitted to WHO by Sumitomo Chemical Co.).
23. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
24. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore
complex. Science, 221: 1399-1401.
25. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions
of pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
26. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd., p. 440.
27. LUND, A.E. & NARAHASHI, T. (1981) Kinetics of sodium channel
modification by the insecticide tetramethrin in squid axon membranes.
J. Pharmacol. exp. Ther., 219: 464-473.
28. LUND, A.E. & NARAHASHI, T. (1982) Dose-dependent interaction of the
pyrethroid isomers with sodium channels of squid axon membranes.
Neurotoxicology, 3: 11-24.
29. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane effects
of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
30. MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, p. 502.
31. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J.
(1983) Farm chemicals handbook. Section C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, p. C232.
32. MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington DC, American Chemical Society,
pp. 98-115 (ACS Symposium Series No. 42).
33. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
34. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
35. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry, human
welfare and the environment, Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September
1982, Oxford, Pergamon Press, Vol. 1-4.
36. MIYAMOTO, J., SATO, Y., & YAMAMOTO, K. (1968) Biochemical
studies on the mode of action of pyrethroidal insecticides. Part I.
Metabolic fate of phthalthrin in mammals. Agric. biol. Chem., 32:
628-640.
37. MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem., 36:
2203-2211.
38. OKUNO, Y. (1982) Exposure study with spraying of Neopynamin
aerosol (Technical Report No. IF-20-002) (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
39. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976a) Eye and skin
irritation of Neopynamin in rabbits (Technical Report No. IT-60-0014)
(Submitted to WHO by Sumitomo Chemical Co.).
40. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976b) Skin sensitization
study of Neopynamin with guinea-pigs (Technical Report No. IT-60-0013)
(Submitted to WHO by Sumitomo Chemical Co.).
41. PANSHINA, T.N. & SASINOVICH, L.M. (1983) Toxicology of synthetic
pyrethroids. Khim. Selsk. Khoz. 12: 51-53.
42. PAPADOPOULOU-MOURKIDOU, E., IWATA, Y., & GUNTHER, F.A.
(1981) Utilization of an infrared detector for selective liquid
chromatographic analysis. 2. Formulation analysis of the pyrethroid
insecticides allethrin, decamethrin, cypermethrin, phenothrin, and tetra-
methrin. J. agric. food. Chem., 29(6): 1105-1111.
43. PENCE, D.H., HAGEN, W.H., ALSAKER, R.D., DAWKINS, B.G., MARSHALL,
P.M., & TACEY, R.L. (1981a) Subchronic toxicity study in dogs.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories Inc.
(Technical Report No. IT-11-0098) (Submitted to WHO by Sumitomo Chemical
Co.).
44. PENCE, D.H., SEROTA, D.G., ALSAKER, R.D., BANAS, D.A., &
KUNDZINS, W. (1981b) Chronic toxicity study in rats. Neopynamin
Technical Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-11-0097) (Submitted to WHO by Sumitomo
Chemical Co.).
45. PENCE, D.H., COX, R.H., DUDECK, L.E., ALSAKER, R.D., JONES, S.R.,
PARKER, G.A., HEPNER, K.E., & ZOETIS, T. (1986a) Combined chronic
toxicity and oncogenicity study in mice. Neopynamin Final Report, Vienna,
Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-61-0193)
(Submitted to WHO by Sumitomo Chemical Co.).
46. PENCE, D.H., WOLFE, G.W., KULWICH, B.A., HEPNER, K.E., &
SNYDER, F.G. (1986b) Two-generation reproduction study in rats.
Neopynamin Forte, Final Report, Hazleton Laboratories, Inc. (Technical
Report No. IT-61-0201) (Submitted to WHO by Sumitomo Chemical Co.).
47. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids
on a nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
48. RUTTER, H.A., Jr (1974) One-generation reproduction study in rats.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-01-0081) (Submitted to WHO by Sumitomo
Chemical Co.).
49. RUTTER, H.A., Jr, NELSON, L.W., KUNDZINS, W., & MCHUGH (1974)
Two-year dietary administration in the rat. Neopynamin Final Report,
Vienna, Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-
41-0024) (Submitted to WHO by Sumitomo Chemical Co.).
50. RUZO, L.O., SMITH, I.H., & CASIDA, J.E. (1982) Pyrethroid
photochemistry: photooxidation reactions of the chrysanthemates,
phenothrin and tetramethrin. J. agric. food Chem., 30: 110-115.
51. SATO, T. & NARAMA, K. (1980a) Reproduction test of Neopynamin. Part 1:
Fertility study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0075) (Submitted to WHO by Sumitomo Chemical Co.).
52. SATO, T. & NARAMA, K. (1980b) Reproduction test of Neopynamin. Part 2:
Teratology study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0076) (Submitted to WHO by Sumitomo Chemical Co.).
53. SATO, T. & NARAMA, K. (1980c) Reproduction test of Neopynamin. Part 3:
Teratology study in rabbits, Shizuoka, Hamamatsu Seigiken Research (Tech-
nical Report No. IT-01-0077) (Submitted to WHO by Sumitomo Chemical Co.).
54. SATO, T., TAGAWA, G., & NARAMA, K. (1980) Reproduction test of
Neopynamin. Part 4: Perinatal and postnatal study in rats, Shizuoka,
Hamamatsu Seigiken Research (Technical Report No. IT-01-0078) (Submitted
to WHO by Sumitomo Chemical Co.).
55. SMITH, I.H., WOOD, E.J., & CASIDA, J.E. (1982) Glutathione conjugate
of the pyrethroid tetramethrin. J. agric. food Chem., 30: 598-600.
56. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of
Neopynamin with bacterial systems (Technical Report No. IT-70-0023)
(Submitted to WHO by Sumitomo Chemical Co.).
57. SUZUKI, T. & MIYAMOTO, J. (1974) Metabolism of tetramethrin in
houseflies and rats in vitro. Pestic. Biochem. Physiol., 4: 86-97.
58. SUZUKI, T., KOHDA, H., MISAKI, Y., OKUNO, Y., KOYAMA, Y.,
& MIYAMOTO, J. (1980) Acute and subacute inhalation toxicity studies of
Neopynamin Forte in rats (Technical Report No. IT-10-0144) (Submitted to
WHO by Sumitomo Chemical Co.).
59. TANAKA, H., TAKADA, S., ISEKI, M., SANO, R., AIHARA, H.,
KURODA, H., MAEBO, Y., MOTOYOSHI, I., & WAKAI, Y. (1967)
Toxicological studies of various insecticides to mammalia. 2. On the
acute and chronic toxicity of pyrethroid insecticide phthalthrin to
albino rat or guinea-pig. Med. J. Osaka City Univ., 16: 509-517.
60. UEDA, K., GAUGHAN, L.C., & CASIDA, J E. (1975) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food Chem., 23:
106-115.
61. UNO, M., OKADA, T., OHMAE, T., TERADA, I., & TANIGAWA, K.
(1982) [Determination of pyrethroid insecticides by dual-wavelength
densitometry.] Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
62. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
63. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels
in frog myelinated nerve membrane. In: Insect neurobiology and
pesticide action, London, Society of Chemical Industry, pp. 79-85.
64. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M. (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
65. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and phermones,
New York, London, Plenum Press, pp. 183-210.
66. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
67. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
68. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
69. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
70. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN,
J. (1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in myelinated nerves. Nature (Lond.), 295: 601-603.
71. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M.,
& VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
72. WEIR, R.J. & CREWS, L.M. (1966) Three month dietary administration
to dogs of Neopynamin - final report (Technical Report No. IT-61-0011)
(Submitted to WHO by Sumitomo Chemical Co.).
73. WEIR, R.J. & OSBOURN, R.A. (1966) Report of human patch test on
coded substance : 2301 D-61-1, Vienna, Virginia, Hazleton
Laboratories, Inc. (Technical Report No. IT-61-0008) (Submitted to
WHO by Sumitomo Chemical Co.).
74. WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
75. WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988/89, Geneva, World
Health Organization (Unpublished report VBC/88.953).
76. WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 520.
77. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids.
Gen. Pharmacol., 9: 387-398.
78. YAMAMOTO, D., QUANDT, F.N., & NARAHASHI, T. (1983)
Modification of single sodium channels by the insecticide
tetramethrin. Brain Res. 274: 344-349.
79. YAMAMOTO, D., YEH, J.Z., & NARAHASHI, T. (1986) Ion permeation
and selectivity of squid axon sodium channels modified by
tetramethrin. Brain Res., 372: 193-197.
80. YOSHITAKE, A., KOGISO, S., HARA, M., & MIYAMOTO, J. (1986)
In vivo chromosomal aberration test of Neopynamin in mouse bone
marrow cells (Technical Report No. IT-60-0197) (Submitted to WHO
by Sumitomo Chemical Co.).
81. YOSHITAKE, A., KOGISO, S., YAMADA, F., HARA, M., & MIYAMOTO,
J. (1987) Reverse mutation test of Neopynamin in Salmonella
typhimurium and Escherichia coli (Technical Report No. IT-70-0205)
(Submitted to WHO by Sumitomo Chemical Co.).
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs ( Xenopus laevis, Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [10, 23, 65,
66, 74]. The same distinction was found in studies on
cockroaches [8].
Based on the binding assay on the alpha-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types: the
alpha-cyano-3-phenoxybenzyl pyrethroids and the non-cyano
pyrethroids [7, 9, 24, 25].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin, cisme-
thrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [64]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [62]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [71].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [6].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [63]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [71].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans [4],
are almost identical to those of allethrin, i.e., presyn-
aptic repetitive firing, and no significant effects on
transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [64, 65, 70].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [29].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [47].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cyhalothrin, lambda-cyhalo-
thrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [69]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [67,
71].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during exci-
tation, presumably by slowing down the closing of the
activation gate of the sodium channel [67, 71]. The time
constant of closing of the activation gate in the cyper-
methrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged
sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to
cause the long-lasting repetitive firing in the lateral-
line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [29].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [9].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the toxicity
of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand,
[35S]- t-butylbicyclophosphorothionate [35S]-TBPS. Studies
with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic
cyano compounds including deltamethrin, 1R, cis-cyper-
methrin, 1R, trans-cypermethrin, and [2S, alphaS]-fenvalerate
were inhibitors, but their non-toxic stereoisomers were
not; non-cyano pyrethroids were much less potent or were
inactive [24].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [25].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S,alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex [7].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [47].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-lasting
prolongation of the transient increase in sodium per-
meability of the nerve membrane during excitation. This
results in long-lasting trains of repetitive impulses in
sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [74, 77].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [68].
1. RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1.1 Résumé et évaluation
1.1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La tétraméthrine a été synthétisée pour la première
fois en 1964 et commercialisée et en 1965. Sur la plan
chimique, c'est un ester de l'acide chrysanthémique (acide
diméthyl-2,2(diméthyl-2,2 vinyl)-3 cyclopropanecarboxylique
et de l'alcool tétrahydro-3,4,5,6 phatlimidométhylique.
Elle est constituée d'un mélange de quatre stéréoisomères
[1R,trans], [1R,cis], [1S,trans] et [1S,cis]. Les stéréo-
isomères qui entrent dans la composition des produits
techniques sont à peu près dans la proportion de 4:1:4:1.
De tous les isomères, c'est l'isomère [1R,trans] qui est
le plus actif biologiquement; vient ensuite l'isomère
1R,cis]. On commercialise sous le nom de "Neo-pynamine
Forte" (désignée dans la présente monographie sous le nom
de 1R, cis/trans-tetraméthrine), un mélange des isomères
[1R,cis] et [1R,trans] dans le proportion de 1:4.
La tétraméthrine de qualité technique est un solide
incolore dont le point de fusion est de 60-80 °C. Sa
densité est de 1,11 à 20 °C et sa tension de vapeur de
0,946 mPa (7,1 x 10-6 mm Hg) à 30 °C. Peu soluble dans
l'eau (4,6 mg/litre à 30 °C), elle est en revanche soluble
dans certains solvants organiques tels que l'hexane, le
méthanol et le xylène. Elle est stable à la chaleur mais
instable à la lumière et à l'air. L'isomère [1R,cis/trans]
est un liquide visqueux de couleur jaune dont les autres
propriétés physiques et les propriétés chimiques sont les
mêmes que celles de la tétraméthrine.
Le dosage des résidus s'effectue par densitométrie à
deux longueurs d'onde (370-230 nm). Pour l'analyse des
produits techniques, on utilise la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
Une analyse des différentes formulations peut s'effectuer
au moyen d'un chromatographe en phase liquide à haute
performance muni d'un détecteur infrarouge.
1.1.2 Production et usage
La production mondiale annuelle de tétraméthrine est
évaluée à quelques centaines de tonnes. Elle est princi-
palement utilisée pour la lutte contre les nuisibles à
l'intérieur des habitations, sous forme d'aérosols, de
concentrés émulsionnables ou de serpentins anti-moust-
iques. La tétraméthrine entre également dans la compos-
ition d'autres formulations insecticides additionnées ou
non de synergisants.
1.1.3 Exposition humaine
L'exposition de la population dans son ensemble peut
résulter de l'utilisation de ce produit pour la destruc-
tion des nuisibles dans les habitations. Lorsqu'on utilise
la tétraméthrine conformément aux recommandations, sa con-
centration atmosphérique ainsi que celle de l'isomère 1R
ne devraient pas dépasser 0,5 mg/m3; par ailleurs le
composé se dégrade rapidement. L'exposition de la popu-
lation générale est donc vraisemblablement très faible. On
n'utilise pas de tétraméthrine pour traiter les cultures
vivrières.
1.1.4 Exposition et destinée dans l'environnement
Une fine pellicule de tétraméthrine exposée à la
lumière solaire se dégrade rapidement. On a observé que
les principales réactions photochimiques qui se produis-
aient au cours d'une exposition de 2 heures (conversion de
30%) étaient: une époxydation au niveau de la double
liaison du radical isobutényle, une oxydation en hydroxy-
méthyle, en aldéhyde et en acide carboxylique du groupe
méthyle en position trans du groupement isobutényle;
enfin, une hydroperoxydation en hydroperoxyde allylique.
On ne connaît pas avec exactitude les concentrations
exactes de tétraméthrine dans l'environnement, mais compte
tenu de l'utilisation qui en est faite actuellement pour
traiter les habitations et pourvu que le produit soit
utilisé conformément aux recommandations, il est vraisem-
blable que l'exposition dans l'environnement devrait être
très faible. La tétraméthrine se décompose rapidement en
produits moins toxiques.
1.1.5 Absorption, métabolisme, et excrétion
Des rats à qui l'on avait administré par voie orale ou
sous-cutanée de la tétraméthrine radio-marquée au niveau
du reste acide ou du reste alcool ont rapidement absorbé,
métabolisé et excrété le produit. L'excrétion s'effectue
en 5 à 7 jours dans la proportion d'environ 95%, à peu
près autant par la voie urinaire que par la voie fécale.
Par ces deux voies, les résidus présents dans les tissus
sont très faibles. La métabolisation s'effectue par les
réactions suivantes: coupure de l'ester; élimination du
groupe hydroxyméthyl du reste alcool; réduction de la
double liaison 1-2 du reste alcool; oxydation du groupe-
ment méthyle de l'isobutényle au niveau du reste acide et
en 2, 3 et 4 au niveau du reste alcool; conjugaison des
acides et des alcools résultant avec l'acide glucuronique
et enfin isomérisation cis/trans.
1.1.6 Effets sur les êtres vivant dans leur milieu naturel
On ne dispose que de très peu d'informations à ce
sujet. La tétramethrine est extrêmement toxique pour les
poissons, la valeur de la CL50 à 96-h pour deux espèces
se situant respectivement à 19 et 21 µg/litre. Pour une
troisième espèce, on a obtenu une CL50 à 48 heures de
200 µg/litre et la dose sans effet observable était de
50 µg/litre. Pour la daphnie, la dose sans effet
observable est également de 50 µg/litre. La tétraméthrine
est en revanche très peu toxique pour les oiseaux mais
elle est toxique pour les abeilles. Cependant du fait que
le produit est rapidement dégradé et dans la mesure où on
ne l'utilise, conformément aux recommandations, que dans
les habitations, il est peu probable qu'il puisse excercer
des effets nocifs sur l'environnement.
1.1.7 Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in-vitro
La tétraméthrine a une faible toxicité aiguë par voie
orale. La DL50 pour le rat est >5000 mg/kg, qu'il
s'agisse du racémique ou de l'isomère (1R,cis/trans),
tandis que pour la souris elle est d'environ 2000 mg/kg
(racémique) et de 1060 mg/kg (1R,cis/trans). Chez le rat,
la souris et le lapin la toxicité aiguë par voie percu-
tanée e st également faible; la DL50 chez le rat et la
souris étant <5000 mg/kg et <2000 mg/kg chez le lapin
(toutes les études portaient sur le racémique). Les études
de toxicité aiguë par inhalation ont donné une CL50 chez
le rat et la souris de 2500 mg/m3 pour le racémique et
>1180 mg/m3 pour l'isomère (1R,cis/trans). Parmi les
signes d'intoxication on a noté une hyperexcitabilité, des
tremblements, de l'ataxie et une dépression (signes
généraux observés dans l'ensemble des études de toxicité
aiguë). Les souris se sont révélées un peu plus sensibles
que les rats mais il n'y avait pas de différences de
sensibilité entre mâles et femelles. Qu'ils s'agisse du
racémique ou de l'isomère (1R,cis/trans), la tétraméthrine
ne provoque pratiquement aucune irritation oculaire ou
cutanée chez le lapin. En outre, ni l'un ni l'autre de
ces produits n'exercent d'effet sensibilisateur chez le
cobaye.
La tétraméthrine est un pyréthroïde du type I. Chez
les mammifères ce sont les tremblements (syndrome-T) qui
constituent le symptôme d'intoxication caractéristique.
Chez des rats ayant reçu de la tétraméthrine mêlée à
leur nourriture à des concentrations allant jusqu'à 5000
mg/kg de nourriture pendant 91 jours, on a noté une réduc-
tion du gain de poids à la dose la plus forte. D'après les
résultats d'études de 3 et 6 mois, au cours desquelles des
rats ont reçu l'isomère 1R(cis/trans) dans leur nourriture
à des doses allant de 25 mg/kg à 3000 mg/kg d'aliment, la
dose sans effet observable était de 200 mg/kg de nourri-
ture pour les mâles et de 300 mg/kg pour les femelles
(parmi les anomalies observées, on notait une réduction du
gain de poids et du poids final du corps ainsi que
certains effets sur les reins et le foie). Les effets sur
le foie résultent, semble-t-il, d'une réaction d'adapt-
ation à la présence dans l'alimentation du véhicule
utilisé, à savoir l'huile de maïs.
Une étude de 26 semaines sur des chiens a fait
ressortir une dose sans effet observable de 1250 mg/kg de
nourriture.
Des souris et des rats à qui l'on avait fait inhaler
de la tétraméthrine en aérosol à une concentration de 200
mg/m3, 3 à 4 heures par jour pendant des périodes allant
jusqu'à quatre semaines, n'ont présenté aucune anomalie
imputable à ce produit. Lors d'une autre étude de ce
type, au cours de laquelle des rats ont été exposés à une
brumisation (gouttelettes de 1,2-1,5 µm de diamètre)
d'isomère (1R,cis/trans) dans du kérosène désodorisé à des
concentrations allant jusqu'à 87 mg/m3, trois heures par
jour et sept jours par semaine pendant 28 jours, on a
obtenu, pour la dose sans effet observable, une valeur de
49 mg/m3. Les signes d'intoxication n'ont été observés
qu'au cours de l'exposition.
Ni la tétraméthrine ni ses isomères (1R,cis/trans) ne
se sont révélés mutagènes dans divers systèmes d'épreuve
in vivo et in vitro utilisés pour étudier les mutations
génétiques, les lésions et les réparations de l'ADN ainsi
que les effets sur les chromosomes.
Trois études, dont deux chez le rat et une chez la
souris ont été menées pendant 104 semaines afin d'étudier
la cancérogénicité à long terme de la tétraméthrine. Les
souris ont reçu de la tétraméthrine dans leur nourriture à
des doses allant jusqu'à 1500 mg/kg de nourriture. Aucun
effet oncogène n'a été observé. A partir de 60 mg/kg de
nourriture on observait une réduction du poids de
l'hypophyse, de la thyroïde et de la parathyroïde. Chez
la souris, la dose sans effet général observable se
situait à 12 mg/kg de nourriture. Quant aux rats, ils ont
été exposés à de la tétraméthrine à des doses allant
jusqu'à 5000 mg/kg de nourriture soit in utero soit au
cours d'une période prolongée. Les deux études ont fait
ressortir un gain de poids sensiblement moindre chez les
animaux recevant 3000 mg de tétraméthrine par kg de
nourriture ou davantage. En outre, à ces concentrations,
on a observé une augmentation du poids du foie. Pour ce
qui est des effets généraux, la dose sans effet observ-
able se situait dans les deux études, à 1000 mg/kg de
nourriture. A la dose de 3000 mg/kg de nourriture,
l'incidence des tumeurs testiculaires à cellules de Leydig
était supérieure à la valeur notée dans le groupe témoin
et ce, pour les deux études. Les tumeurs à cellules de
Leydig se produisent spontanément chez les rats âgés et
leur incidence peut varier énormément dans les groupes
témoins. On pense que cette tumeur est d'origine hormon-
ale. Aucun signe de malignité et aucune tumeur de ce type
n'ont été relevés chez les souris. On peut en conclure
que cet effet oncogène, s'il existe réellement, ne peut
être pris en considération pour ce qui concerne l'homme.
La tétraméthrine ne s'est révélée ni tératogène ni
embryotoxique à des doses allant jusqu'à 1000 mg par kg de
poids corporel chez les rats et jusqu'à 500 mg/kg chez des
lapins (il s'agit des concentrations les plus fortes
étudiées). Lors d'une étude de fécondité au cours de
laquelle des rats ont reçu de la tétraméthrine à des doses
allant jusqu'à 1000 mg/kg de poids corporel par jour, la
dose sans effet observable sur la reproduction des parents
et la croissance des foetus, se situait à 300 mg/kg de
poids corporel par jour. Une étude de reproduction chez
le rat, portant sur la période périnatale et sur la
période post-natale, a permis de fixer à 100 mg/kg de
poids corporel la dose quotidienne sans effet observable
(la dose la plus forte administrée au cours de cette
étude).
Lors d'une étude de reproduction portant sur une
génération de rats, on a administré aux animaux 1000-6000
mg de tétraméthrine par kg de nourriture et constaté que
la dose sans effet observable était de 1000 mg/kg. Selon
une autre étude portant cette fois sur deux générations,
au cours de laquelle les rats ont reçu de l'isomère
(1R,cis/trans) à des doses allant de 100 à 3000 mg/kg, la
dose sans effet observable était de 500 mg/kg de
nourriture.
1.1.8 Effets sur l'homme
Bien que la tétraméthrine et son isomère 1R soient
utilisées depuis des années, on ne signale aucun cas
d'intoxication ou d'effets indésirables chez l'homme.
Rien n'indique que la tétraméthrine ou son isomère 1R
puissent avoir des effets nocifs sur l'homme si on
continue de les utiliser à faibles concentrations et
seulement pour la destruction des nuisibles à l'intérieur
des habitations.
1.2 Conclusions
a) Population générale: l'exposition de la population
générale à la tétraméthrine, dans son utilisation actu-
elle, est vraisemblablement faible. Si ce produit est
utilisé conformément aux recommandations, il ne présente
probablement aucun risque.
b) L'exposition professionnelle: moyennant de bonnes
méthodes de travail, l'application de mesures d'hygiène et
avec quelques précautions, la tétraméthrine ne devrait pas
présenter de danger pour les personnes qui y sont exposées
de par leur profession.
c) Environnement: il est tout à fait improbable que la
tétraméthrine ou ses produits de décomposition s'accum-
ulent au point d'avoir des effets nocifs sur l'environne-
ment.
1.3 Recommandations
Bien que la tétraméthrine et son isomère 1R soient
utilisés depuis des années sans qu'on ait à déplorer
d'effets nocifs chez l'homme, il est souhaitable que
l'exposition humaine continue d'être surveillée.
 Tetramethrin was first synthesized in 1964 by Kato
et al. [19] and is prepared by the esterificaton of
(1RS ,cis,trans)-2,2-dimethyl-3- (2,2-dimethylvinyl)-cyclo-
propanecarboxylic acid (chrysanthemic acid) with 3,4,5,6-
tetrahydrophthalimidomethyl alcohol. It is a mixture of
four stereoisomers (Fig. 1). The cis:trans ratio is re-
ported to be 1:4 and the optical ratio of 1R:1S is 1:1
(racemic). Thus its composition is roughly 4:1:4:1 for
the [IR,trans], [IR,cis],[IS,trans], and [IS,cis] isomers.
The [1R,trans] isomer is the most active biologically of
the isomers, followed by the [1R,cis] isomer. Neo-Pynamin
Forte is a mixture of the [1R,cis,] and [IR,trans] isomers
in the ratio of 1:4 (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of tetramethrin
are given in Table 2.
No data are available for boiling point and n-
octanol/water partition coefficient. Technical grade
tetramethrin is stable to heat (50 °C for 6 months) but
unstable to light and air and to alkaline condition [30,
31, 76].
2.3 Analytical Methods
Dislodgeable residues of tetramethrin can be analysed
by dual-wavelength densitometry after clean-up of the hex-
ane washings by high-performance silica gel thin-layer
chromatography (Table 3). To analyse technical grade
tetramethrin, the product and tributoxyethyl phosphate (an
internal standard) were dissolved in acetone, and the sol-
ution was injected into a gas chromatograph equipped with
flame ionization detector (FID) [37]). Analysis of tetra-
methrin formulations can also be carried out using high
performance liquid chromatography (HPLC) with an infra-red
selective detector [42].
Table 1. Chemical identity of tetramethrins of various stereoisomeric
compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry no./ Stereospecific nameb,c compositiond trade names
NIOSH Accession no.a
Tetramethrine Cyclopropanecarboxylic acid, (1):(2):(3):(4) Tetramethrine,
(racemic mixture) 2,2-dimethyl-3-(2-methyl-1-propenyl)-, = 4:1:4:1 Phthalthrin,
Tetramethrin was first synthesized in 1964 by Kato
et al. [19] and is prepared by the esterificaton of
(1RS ,cis,trans)-2,2-dimethyl-3- (2,2-dimethylvinyl)-cyclo-
propanecarboxylic acid (chrysanthemic acid) with 3,4,5,6-
tetrahydrophthalimidomethyl alcohol. It is a mixture of
four stereoisomers (Fig. 1). The cis:trans ratio is re-
ported to be 1:4 and the optical ratio of 1R:1S is 1:1
(racemic). Thus its composition is roughly 4:1:4:1 for
the [IR,trans], [IR,cis],[IS,trans], and [IS,cis] isomers.
The [1R,trans] isomer is the most active biologically of
the isomers, followed by the [1R,cis] isomer. Neo-Pynamin
Forte is a mixture of the [1R,cis,] and [IR,trans] isomers
in the ratio of 1:4 (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of tetramethrin
are given in Table 2.
No data are available for boiling point and n-
octanol/water partition coefficient. Technical grade
tetramethrin is stable to heat (50 °C for 6 months) but
unstable to light and air and to alkaline condition [30,
31, 76].
2.3 Analytical Methods
Dislodgeable residues of tetramethrin can be analysed
by dual-wavelength densitometry after clean-up of the hex-
ane washings by high-performance silica gel thin-layer
chromatography (Table 3). To analyse technical grade
tetramethrin, the product and tributoxyethyl phosphate (an
internal standard) were dissolved in acetone, and the sol-
ution was injected into a gas chromatograph equipped with
flame ionization detector (FID) [37]). Analysis of tetra-
methrin formulations can also be carried out using high
performance liquid chromatography (HPLC) with an infra-red
selective detector [42].
Table 1. Chemical identity of tetramethrins of various stereoisomeric
compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry no./ Stereospecific nameb,c compositiond trade names
NIOSH Accession no.a
Tetramethrine Cyclopropanecarboxylic acid, (1):(2):(3):(4) Tetramethrine,
(racemic mixture) 2,2-dimethyl-3-(2-methyl-1-propenyl)-, = 4:1:4:1 Phthalthrin,
 a Numbers in parentheses refer to numbered chemical structures in
Figures 2 and 3.
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
The metabolic pathways of tetramethrin in mammals are
summarized in Fig. 3.
Tetramethrin is readily absorbed and excreted by rats.
Following a single oral administration of [1RS, trans]-
tetramethrin (17], labelled with 14C at the carbonyl
group of the alcohol moiety, to male Wistar rats at a con-
centration of 500 mg/kg, 47% and 42% of the radiolabel
were excreted into the urine and faeces, respectively,
during the subsequent 2 days and 95% was recovered during
the 5-day period that followed dosing. The tissue levels
during the first 2 days after administration were very low
and the tetramethrin content in tissues was less than
0.01% of the dosed radioactivity. Unmetabolized trans-
tetramethrin (17) was not excreted into the urine, and the
major urinary metabolite was 3-hydroxy-cyclohexane-1,2-di-
carboximide (19) (3-OH-HPI) in free and glucuronide forms.
N-(Hydroxymethyl)-3,4,5,6-tetrahydrophthalimide (20) (MTI),
3,4,5,6-tetrahydrophthalimide (21) (TPI), and cyclohexane-
1,2-dicarboximide (22) (HPI) were identified as minor uri-
nary and faecal metabolites [36].
Following a single oral or subcutaneous administration
to Sprague-Dawley rats of [1R, trans]- or [1R, cis]-tetra-
methrin (17,18), labelled with 14C in the acid or alcohol
moieties at concentrations of 3.2-5.3 mg/kg, the radiocar-
bon was rapidly and almost completely eliminated from the
rat body. The total recoveries 7 days after administration
were 93-97% for the trans isomer and 90-101% for the cis
isomer (approximately equal amounts being eliminated in
urine and faeces). In the case of the oral dose of acid-
labelled tetramethrin, 1-3% of the radiolabel was ex-
creted as 14CO2, whereas in other cases the amount of
14CO2 accounted for less than 1% of the dose. The tissue
residue 7 days after administration was very low. The
transisomer yielded somewhat more complete radiolabel re-
covery and lower tissue residues than the cis isomer. In
addition, labelling resulted in slightly lower tissue
residues than did alcohol labelling. However, there were
no significant differences, according to sex or adminis-
tration route, in the total radiocarbon recoveries and
tissue residue levels [18]. The major metabolic reactions
of both [1R, trans]- and [1R, cis]-tetramethrin were
ester cleavage, loss of the hydroxymethyl group from the
alcohol moiety, reduction of the 1-2 bond of the alcohol
moiety, and oxidation at the isobutenyl group of the acid
moiety and at the 2-, 3-, and 4-positions of the alcohol
moiety. The metabolites produced via these reactions were
in part conjugated with glucuronic acid. None of the trans
isomer remained unmetabolized, whereas 0.3-1.2% of the cis
isomer was found unchanged in the faeces. The major metab-
olites from the acid moiety of both isomers were chrysan-
themic acid (23, 24) (CA) and its derivatives oxidized at
the trans-methyl of the isobutenyl group. 3-(2'-E-Carboxy-
1'-propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid (25,
26) ( w t-acid- t,c-CA) accounted for 17-27% and 7-9% of
the dose of the trans and cis isomers, respectively. Other
significant metabolites were 3-(2'-E-hydroxymethyl-1'-
propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid(27,28)
( w t-alc-t,c-CA), 3-(2'-Z-carboxy-1'-propenyl)-2,2-dimethyl-
1-cyclopropanecarboxylic acid (29, 30) ( w c-acid- t,c-CA),and
3-(2'-Z-hydroxymethyl-1'-propenyl)-2,2-dimethyl-1-cyclopro-
panecarboxylic acid (31, 32) ( w c-alc- t,c-CA).
a Numbers in parentheses refer to numbered chemical structures in
Figures 2 and 3.
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
The metabolic pathways of tetramethrin in mammals are
summarized in Fig. 3.
Tetramethrin is readily absorbed and excreted by rats.
Following a single oral administration of [1RS, trans]-
tetramethrin (17], labelled with 14C at the carbonyl
group of the alcohol moiety, to male Wistar rats at a con-
centration of 500 mg/kg, 47% and 42% of the radiolabel
were excreted into the urine and faeces, respectively,
during the subsequent 2 days and 95% was recovered during
the 5-day period that followed dosing. The tissue levels
during the first 2 days after administration were very low
and the tetramethrin content in tissues was less than
0.01% of the dosed radioactivity. Unmetabolized trans-
tetramethrin (17) was not excreted into the urine, and the
major urinary metabolite was 3-hydroxy-cyclohexane-1,2-di-
carboximide (19) (3-OH-HPI) in free and glucuronide forms.
N-(Hydroxymethyl)-3,4,5,6-tetrahydrophthalimide (20) (MTI),
3,4,5,6-tetrahydrophthalimide (21) (TPI), and cyclohexane-
1,2-dicarboximide (22) (HPI) were identified as minor uri-
nary and faecal metabolites [36].
Following a single oral or subcutaneous administration
to Sprague-Dawley rats of [1R, trans]- or [1R, cis]-tetra-
methrin (17,18), labelled with 14C in the acid or alcohol
moieties at concentrations of 3.2-5.3 mg/kg, the radiocar-
bon was rapidly and almost completely eliminated from the
rat body. The total recoveries 7 days after administration
were 93-97% for the trans isomer and 90-101% for the cis
isomer (approximately equal amounts being eliminated in
urine and faeces). In the case of the oral dose of acid-
labelled tetramethrin, 1-3% of the radiolabel was ex-
creted as 14CO2, whereas in other cases the amount of
14CO2 accounted for less than 1% of the dose. The tissue
residue 7 days after administration was very low. The
transisomer yielded somewhat more complete radiolabel re-
covery and lower tissue residues than the cis isomer. In
addition, labelling resulted in slightly lower tissue
residues than did alcohol labelling. However, there were
no significant differences, according to sex or adminis-
tration route, in the total radiocarbon recoveries and
tissue residue levels [18]. The major metabolic reactions
of both [1R, trans]- and [1R, cis]-tetramethrin were
ester cleavage, loss of the hydroxymethyl group from the
alcohol moiety, reduction of the 1-2 bond of the alcohol
moiety, and oxidation at the isobutenyl group of the acid
moiety and at the 2-, 3-, and 4-positions of the alcohol
moiety. The metabolites produced via these reactions were
in part conjugated with glucuronic acid. None of the trans
isomer remained unmetabolized, whereas 0.3-1.2% of the cis
isomer was found unchanged in the faeces. The major metab-
olites from the acid moiety of both isomers were chrysan-
themic acid (23, 24) (CA) and its derivatives oxidized at
the trans-methyl of the isobutenyl group. 3-(2'-E-Carboxy-
1'-propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid (25,
26) ( w t-acid- t,c-CA) accounted for 17-27% and 7-9% of
the dose of the trans and cis isomers, respectively. Other
significant metabolites were 3-(2'-E-hydroxymethyl-1'-
propenyl)-2,2-dimethyl-1-cyclopropanecarboxylic acid(27,28)
( w t-alc-t,c-CA), 3-(2'-Z-carboxy-1'-propenyl)-2,2-dimethyl-
1-cyclopropanecarboxylic acid (29, 30) ( w c-acid- t,c-CA),and
3-(2'-Z-hydroxymethyl-1'-propenyl)-2,2-dimethyl-1-cyclopro-
panecarboxylic acid (31, 32) ( w c-alc- t,c-CA).
 Judging from the metabolites derived from the acid
moiety, cis to trans isomerization of the oxidized deriva-
tives of CA occurs, as happens in resmethrin metabolism
[60].
Although both cis to trans and trans to cis isomeriza-
tions of tetramethrin were observed by Kaneko et al. [18],
cis to trans conversion seemed to be predominant. On the
other hand, the detected metabolites from the alcohol
moiety were TPI, HPI, 3-OH-TPI (33), 3,4,5,6-tetrahydro-
phthalic acid amide (34) (TPIA), 2-OH-HPI (35), 3-OH-HPI
(19), and 4-OH-HPI (36). Of these metabolites, 2-OH-HPI
was found in relatively large amounts.
Smith et al. [55] found that tetramethrin and TPI
readily underwent the Michael addition with thiols. The
tetramethrin-gluthathione (GSH) conjugate was formed under
physiological conditions in the presence of mouse liver
homogenate fractions, probably by a non-enzymatic
reaction. The soluble thiol level of mouse liver was de-
creased by intraperitoneal administration of TPI. However,
mercapturic acid and GSH conjugates of tetramethrin were
not detected in the bile or urine of rats or mice treated
intraperitoneally with tetramethrin.
5.2 Enzymatic Systems for Biotransformation
When alcohol- or acid-labelled [1RS, trans]-tetra-
methrin (1 mmol/litre) was incubated for 1 h at 37 °C with
30 mg protein of a rat liver subcellular fraction (i.e.
nuclei plus mitochondria, microsomes, and soluble frac-
tion), the microsomes and nuclei plus mitochondria frac-
tions were active in degrading tetramethrin. Rat micro-
somal fraction degraded [1RS, trans]-tetramethrin to CA,
MTI, and TPI in the absence of NADPH. In the presence of
NADPH, tetramethrin was more rapidly degraded to yield
oxidized tetramethrin ( wt-alc-, wt-ald-, and wt-acid-tetra-
methrin), oxidized CA ( wt-alc-, wt-ald-, and wt-acid-CA),
TPI, and unidentified metabolites in larger amounts. The
major metabolite TPI was shown to be produced non-enzy-
matically from MTI. The degradation rate of tetramethrin
was greatly reduced by the inhibition of ester hydrolysis
with paraoxon [57].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
As the use of tetramethrin is limited to indoor pest
control, there is a paucity of data concerning its effect
on the environment.
6.1 Aquatic Organisms
Data on the toxicity of tetramethrin to non-target
aquatic organisms are given in Table 4.
Tetramethrin is highly acutely toxic to fish in lab-
oratory tests, the 96-h LC50s for two species being ap-
proximately 20 µg/litrea. The 48-h LC50 for killifish
is about 200 µg/litre, with a no-observed-effect level
(NOEL) of 50 µg/litre [33]. The NOEL for Daphnia pulex
was reported by Miyamoto [33] to be 50 µg/litre for
racemic tetramethrin and 10 µg/litre for [1R, trans]- or
[1R, cis]-tetramethrin.
6.2 Terrestrial Organisms
Tetramethrin has low toxicity to birds. The acute oral
LD50 for Bobwhite quail is >2510 mg/kg body weight, and
the 8-day dietary LC50 to Mallard duck and Bobwhite quail
is >5620 mg/kga.
Tetramethrin is toxic to bees [14].
a written comment from US EPA to IPCS, 1987.
Table 4. Acute toxicity of tetramethrin to non-target aquatic organisms
Species Size Parameter Toxicity Formulation System Temperature Refer-
(mg/litre) (°C) ence
Fish
Killifish adult 48-h LC50 0.2 Technical Static 25 33
( Oryzias latipes) adult 48-h LC50 0.2 (+)-trans Static 25 33
adult 48-h LC50 0.15 (+)-cis Static 25 33
Bluegill sunfish 96-h LC50 0.019 a
( Lepomis macrochirur)
Rainbow trout 96-h LC50 0.021 a
( Salmo gairdneri)
Arthropods
Daphnia pulex 3-h LC50 >50 Technical Static 25 33
3-h LC50 >50 (+)-trans Static 25 33
3-h LC50 >50 (+)-cis Static 25 33
a Written comment from US EPA to IPCS, 1987.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
The acute toxicity of tetramethrin to rats and mice is
low (Table 5).
Table 5. Acute toxicity of tetramethrin to rats and mice
Compound Species Sex Route LD50 Reference
(mg/kg body
weight)
Racemic rat M,F oral 4600 41
rata M,F oralb >5000 17
rat M,F dermalb >10 000 41
mousea M oralb 1920 17
mousea F oralb 2000 17
(1R, cis/trans)- rata M,F oralb >5000 16
rat M,F subcutaneous >5000 16
rat M intraperitoneal 770 16
rat F intraperitoneal 548 16
rat M,F dermal(>24 h)b >5000 16
mousea M oral 1060 16
mousea F oral 1040 16
mouse M subcutaneous 2020 16
mouse F subcutaneous 1950 16
mouse M intraperitoneal 631 16
mouse F intraperitoneal 527 16
mouse M,F dermal (>24 h)b >5000 16
rabbit dermal (>24 h)b >2000 20
a Animals were not fasted.
b Corn oil was used as vehicle.
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1.2-
1.5 µm) of [1R, cis/trans]-tetramethrin (technical grade,
95.6% purity) in deodorized kerosene (0, 26, 131, 243,
595, and 1180 mg active ingredient per m3 air) for a
duration of 3 h. At 131 mg/m3 or more, salivation, hyper-
excitability, irregular respiration, urinary incontinence,
muscular fibrillation, limb paralysis, decrease in spon-
taneous activity, and other toxic signs were observed in
males and females. At 1180 mg/m3, 10% of female animals
died, but the body weight gain was similar to that of the
control rats. The no-observed-effect level (NOEL) for
inhalation of the compound in rats was 26 mg/m3, and the
LC50 value was >1180 mg/m3 in both sexes [58].
The toxic symptoms observed following [1R, cis/trans]-
tetramethrin administration were hyperexcitability, muscle
twitching, tremor, ataxia, irregular respiration, and
depression. Mice were invariably more susceptible than
rats. No differences in susceptibility were observed
between male and female animals [16, 17, 33].
7.2 Irritation and Sensitization
7.2.1 Eye irritation
In a study by Okuno et al. [39], 50 mg of the techni-
cal product (91.3% purity) was instilled in one eye of
Japanese albino male rabbits. The treated eye was washed
with distilled water 5 min (group I) or 24 h (group II)
thereafter. The conjunctiva, cornea, and pupil were exam-
ined, 1, 24, 72 h and 7, 14, and 21 days after appli-
cation. No particular changes were noted except that a
very slight erythema and oedema of the conjunctiva was
transiently observed in the rabbits in group II.
In a separate study, 0.1 ml [1R, cis/trans]-tetra-
methrin (technical grade, 95.6% purity) was applied to one
eye of Japanese albino rabbits. The treated eye was sub-
sequently washed in five rabbits but not in three other
rabbits. The material did not produce any lesions in the
cornea or iris of the treated eyes that were not washed,
but slight hyperemia and/or chemosis of the conjunctiva
was observed 1 h after application. In the washed eyes,
slight hyperemia of the conjunctiva was observed in all
animals 1 h after treatment. These changes, however, had
disappeared by 48 h after application in the unwashed eyes
and 24 h in the washed eyes. The irritating potency of the
material was judged to be minimal in the unwashed eyes and
negative in the washed eyes [11].
7.2.2 Skin irritation
In a study by Okuno et al. [39], 0.5 g of the techni-
cal product (91.3% purity) was applied on a lint patch
(3.8 x 3.8 cm) to the abraded or intact skin of six rab-
bits. The skin was assessed for severity of erythema and
oedema 4, 24, 48, 72 h and 7, 14, and 21 days after appli-
cation, but no particular changes were noted.
When 0.5 ml [1R, cis/trans]-tetramethrin (technical
grade, 95.6% purity) was applied on a lint patch
(2.5 x 2.5 cm) to abraded or intact skin on the back of
rabbits, again no irritating reactions such as erythema
and oedema were observed [11].
7.2.3 Sensitization
In a skin-sensitization study of tetramethrin in
guinea-pigs, Hartley male guinea-pigs (seven per group)
were sensitized ten times at intervals of one or two days
by intracutaneous injections (first injection: 0.05 ml,
subsequent ones: 0.1 ml) of a 1% solution of the technical
product (91.3% purity) in corn oil. The sensitized animals
were then challenged against the same concentration in the
same manner (0.5 ml injection) 14 days later, but no skin-
sensitization reaction was noted [40].
In another skin-sensitization test on Hartley male
guinea-pigs, 0.5 ml [1R, cis/trans]-tetramethrin (techni-
cal product, 95.6% purity) in 0.5 ml acetone was applied
topically by lint patch to the back of animals ten times
(three times per week). The animals were challenged in the
same manner 2 weeks after the last sensitizing treatment,
but no allergic reactions were observed 24 h later [12].
7.3 Short-Term Exposure Studies
7.3.1 Oral
When groups of 10 male Wistar rats were maintained for
91 days on a diet containing 0, 500, 1000, 3000, or 5000
mg tetramethrin/kg diet, there was a reduced rate of body
weight gain at 5000 mg/kg but not at 3000 mg/kg or less.
The liver glycogen level was reduced at 3000 mg/kg and
5000 mg/kg. The kidney, spleen, heart, small intestine,
and brain showed no abnormal signs, either macroscopically
or microscopically, and there were no significant changes
in blood parameters. There was no increase in the protein
or glucose levels in the urine of test animals [59].
When technical (1R, cis/trans)-tetramethrin in corn
oil was administered to Sprague-Dawley rats for 3 or 6
months at 0, 100, 300, 1000, or 3000 mg/kg diet, no treat-
ment-related changes were observed in clinical signs, or
food and water consumption, or in an ophthalmological
examination. However, the body weight gain and final body
weight of males and females in the 3000-mg/kg group were
significantly lower than those of the controls. There were
slight increases in urine protein level in the rats fed
more than 1000 mg/kg and in serum calcium level in the
male rats fed more than 300 mg/kg. During a histopatho-
logical examination, eosinophilic bodies in tubular epi-
thelial cells and hyaline droplets in kidney tubular epi-
thelium cytoplasm were observed in males fed 3000 mg/kg
diet, along with an increase in relative organ weight.
There were dose-dependent increases in absolute and rela-
tive liver weight in all treated male rats and in female
rats fed more than 1000 mg/kg diet. There were also
slightly higher serum cholesterol concentrations in rats
of both sexes fed more than 1000 mg/kg and a significant
reduction in liver lipid content among males fed 1000
mg/kg or more. However, these liver effects were not
accompanied by damage to hepatocytes and were therefore
considered to be an adaptation to the corn oil without
toxicological significance. Furthermore, there were no
marked effects, even at 200 mg/kg, when an additional sub-
chronic study was conducted at tetramethrin levels of 25,
50, 100, and 200 mg/kg diet without corn oil in order to
confirm the NOEL in male rats. The NOEL for tetramethrin
in rats in the 6-month study was concluded to be 200 mg/kg
diet for males and 300 mg/kg diet for females [16].
When technical grade tetramethrin (94.6% purity) was
administered for 26 weeks to beagle dogs (six of each sex
per group) at levels of 0, 1250, 2500, and 5000 mg/kg
diet, nervousness and tremors were observed in both males
and females at 2500 and 5000 mg/kg diet. A lack of oestrus
activity in females was also noted clinically and a lack
of corpora lutea was confirmed histologically at 5000
mg/kg diet. Absolute liver weight was increased in males
at 2500 and 5000 mg/kg and relative liver weight was sig-
nificantly increased in males and females at 5000 mg/kg.
Decreased absolute/relative ovary weights were noted for
females at 5000 mg/kg. No other treatment-related changes
were observed with respect to survival, body weight gain,
food consumption, haematology, urinalysis, ophthalmology,
gross pathology or histopathology. The NOEL was 1250 mg/kg
diet [43].
In a study by Weir & Crus (1966), groups of three male
and three female beagle dogs were fed tetramethrin dis-
solved in corn oil for 13 weeks at levels of 0, 1250,
2500, and 5000 mg/kg diet. There were no effects on haema-
tological, clinical chemistry, or urinary parameters.
Organ weights were not affected by the treatment, and
there were no significant histopathological findings.
Clinical signs were not recorded. The NOEL was >5000 mg/kg
diet.
7.3.2 Inhalation
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1-2 µm)
of tetramethrin at concentrations of 0, 26, 49, and 87 mg
active ingredient per m3 air, 3 h a day, 7 days a week,
for a period of 28 days. At 87 mg/m3, irregular respir-
ation, slight salivation, and hyperexcitability were
observed as toxic signs every day during the exposure
period, but no cumulative toxicity was noted. There were
no compound-related effects on body weight gain, food and
water consumption, urinalysis, haematology, biochemistry,
organ weight, and histopathology. The NOEL in subacute
inhalation was considered to be 49 mg/m3 [58]. This NOEL
is approximately 100 times higher than the aerial concen-
tration attained during normal use of tetramethrin [33].
7.4 Long-Term Exposures and Carcinogenicity
Appraisal
Testicular interstitial cell tumours occur spontaneously in
aged rats, and the incidence can vary greatly in control
groups. This tumour is believed to be hormonally mediated.
There was no evidence of malignancy in three rat studies and no
evidence of this type of tumour in mice. It can be concluded
that the tumorigenic effect, if real, is most unlikely to be
relevant to human exposure.
When tetramethrin (technical grade) was administered
to Sprague-Dawley CRCDR rats (50 of each sex per group,
F1A weanlings from parental animals pre-treated with the
compound at dose levels of 1000, 3000, and 6000 mg/kg
diet) at dose levels of 0, 1000, 3000, or 5000 mg/kg diet
for 104 weeks, no compound-related effects were detected
in investigations of appearance, behaviour, survival,
haematology, blood chemistry, urinalysis, eye examination,
and organ weight at up to 5000 mg/kg diet. However, the
body weight gain of male and female rats fed 3000 mg/kg or
more was significantly lower than that of controls. The
incidence for testicular interstitial cell tumours was
increased at dose levels of 3000 mg/kg or more [49].
Tetramethrin (technical grade, 90.0/93.6% purity) was
tested for long-term toxic effects and tumorigenic poten-
tial in Sprague-Dawley CRCDR and Long-Evans hooded rats
by in utero exposure and 104-week chronic exposure at dose
levels of 0, 200, 1000, and 5000 mg/kg diet. No distinct
compound-related effects were observed in either strain
with regard to fertility rate, mortality, clinical signs,
and clinical laboratory data. However, body weight gains
were significantly lower in both strains at 5000 mg/kg
diet, and absolute and relative liver weights were in-
creased in both strains at 5000 mg/kg diet. The incidence
of interstitial cell tumours in both strains at 5000 mg/kg
diet was above the level in the concurrent control groups
[44].
When tetramethrin (technical product, 93.3% purity)
was fed daily to B6C3F1 mice (dose levels of 0, 12, 60,
300, or 1500 mg/kg diet) for 104 weeks, there were no sig-
nificant dose-related changes in survival, clinical signs,
mean body weight, or food consumption. However, the mor-
tality of male mice at 300 mg/kg was significantly lower
than that of control males. The absolute and relative
weight of pituitary and thyroid/parathyroid glands was
decreased for males fed 60 mg/kg diet or more. Absolute
spleen weights were also decreased for males fed 300 mg/kg
diet or more. However, gross and microscopic examination
of these tissues did not reveal any treatment-related
histomorphological changes. There were no other histo-
pathological findings attributable to tetramethrin admin-
istration. The NOEL was considered to be 12 mg/kg diet
[45].
7.5 Mutagenicity
The results of mutagenicity tests on tetramethrin are
summarized in Table 6.
Ding et al. [2] reported the induction of unscheduled
DNA synthesis in human amnion FL cells by tetramethrin
(72% industrial grade of unknown origin). The same product
gave weakly positive results in an Ames test with Salmon-
ella typhimurium TA 97. It is not clear if the effect was
caused by tetramethrin itself or by the unidentified (28%)
portion of the industrial grade material.
7.6 Reproduction, Embryotoxicity, and Teratogenicity
In a study by Miyamoto [33], groups of 10-15 pregnant
New Zealand white rabbits received tetramethrin orally on
days 6-18 of gestation at doses of 0, 30, or 90 mg/kg per
day. Fetuses were obtained by caesarean section prior to
parturition and were examined for external and skeletal
abnormalities. Seven extra pregnant animals were allowed
to give birth naturally and the pups were examined for
several weeks to check their growth and development. No
significant adverse effects were observed.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to 6-week-old male Slc: SD rats (SPF, 20
per group) for not less than 9 weeks and to 9-week-old
females (20 per group) for 2 weeks of the non-pregnant
period and up to day 7 of pregnancy. The effects of the
material on the mating ability of male and female animals
and on the fetuses were investigated. In males, the liver
weight increased at all dose levels and a kidney weight
increase was noted at 1000 mg/kg. Salivation and a slower
body weight increase were observed during the latter half
of the administration period at 300 and 1000 mg/kg. How-
ever, no effects on the reproductive ability of males were
noted. In females, no changes were observed in the rate of
pregnancy, but there were effects on the sexual cycle and
an ovulation-inhibiting effect at 1000 mg/kg. In fetuses,
growth inhibition was suspected at 1000 mg/kg. However,
all these changes were slight. The NOEL was considered to
be 300 mg/kg body weight per day for reproductive ability
of parents and growth of fetuses [51].
Table 6. Mutagenicity studies on tetramethrin
Test System Test object Concentration Purity/ Results Reference
Compound
Ames test S. typhimurium up to 10 mg/plate 93 - 100% Negative 33
TA 1535 without activation
TA 1538
Ames test Escherichia coli up to 10 mg/plate 93 - 100% Negative 33
W 3623 without activation
W 3012
Ames test S. typhimurium 1 - 10 000 ug/plate technical Negative 56
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1538
Ames test S. typhimurium 100 - 5000 ug/plate 94.0% Negative 81
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1537
TA 97
Ames test E. coli 100 - 5000 ug/plate 94.0% Negative 81
WP2 uvr A with and without racemic
activation
Ames test S. typhimurium 10 - 5000 ug/plate 95.6%, Negative 22
TA 100 with and without 1R, cis/trans
TA 98 activation
TA 1535
TA 1537
TA 1538
Ames test E. coli 10 - 5000 ug/plate 95.6%, Negative 22
WP2 uvr A 1R, cis/trans
Ames test S. typhimurium 5 - 500 ug/plate 72% Positivea 2
TA 97 without activation industrial
grade
Rec-assay Bacillus 1 - 10 000 ug/disk technical Negative 56
subtilis racemic
M45 rec- and H17
(wild type)
Rec-assay Bacillus 50 - 5000 ug/disk 95.6%, Negative 22
subtilis 1R, cis/trans
M45 rec- and H17
(wild type)
Table 6 (contd).
Test System Test object Concentration Purity/ Results Reference
Compound
Host-mediated ICR male mice 200 - 1000 mg/kg technical Negative 56
assay S. typhimurium body weight (oral) racemic
G46
In vivo
chromosomal ICR male mice 1200, 2400, 5000 93.4% Negative 80
aberration bone marrow mg/kg body racemic
weight (ip)
In vivo
chromosomal ICR male mice 150, 300, 600 mg/kg 95.6%, Negative 13
aberration bone marrow body weight (ip) 1R, cis/trans
Unscheduled
DNA Human amnion Not recorded 72%, Positivea 2
synthesis FL cells industrial
grade
Unscheduled
DNA Rat hepatocyte 0, 0.2, 1, 94.0% Negative 21
synthesis primary cultures 5, 25, 50, racemic
and 100 ug/ml
a The test material was of unknown origin and it was unclear whether or not
positive results were due to the 28% impurities.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 30 per group) on
days 7-17 of pregnancy, and its effects on the dams,
fetuses, and growth of pups were investigated. In dams, an
inhibition of body weight increase and a decrease of food
consumption were observed at 1000 mg/kg, in addition to an
increase in liver and kidney weights at the time of
caesarean section. In fetuses, no abnormalities such as
embryo lethality, growth inhibition, or teratogenic
effects were detected. In addition, the tetramethrin had
no effect on the growth of the young after birth or on the
reproductive ability of the offspring. The NOEL was con-
sidered to be 300 mg/kg body weight per day for the dams
and >1000 mg/kg body weight per day for teratogenicity
[52].
When tetramethrin (technical product) was orally
administered at dose levels of 0, 50, 150, or 500 mg/kg
body weight per day to pregnant Japanese white rabbits,
(10 per group) on days 8-18 of pregnancy, a slight transi-
ent decline in the body weight of the dams was noted in
the middle of the treatment period at 500 mg/kg. No
adverse effects such as embryo lethality, inhibition of
fetal growth, or teratogenic action were observed at any
dose level. The NOEL for teratogenicity in rabbits was
considered to be >500 mg/kg body weight per day [53].
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 20 per group) from
day 17 of gestation to day 21 of lactation (perinatal and
postnatal period). In dams, a liver weight increase was
noted at 300 and 1000 mg/kg but there were no abnormali-
ties at delivery or during lactation. Tetramethrin had no
detectable effects on the survival rate of pups, growth
and development, sensory function, motor function, learn-
ing ability, or reproductive ability. The NOEL was
considered to be 100 mg/kg body weight per day for dams
and >1000 mg/kg body weight per day for pups [54].
In studies by Rutter [48], tetramethrin (technical
grade) was administered to Sprague-Dawley rats at dose
levels of 0, 1000, 3000, and 6000 mg/kg diet for 9 weeks
through weaning of the F1A generation. Body weight
reduction occurred at 6000 mg/kg diet in the parent rats.
The lactation index was depressed for the F1A generation
at 6000 mg/kg diet, and the weaning body weights for both
sexes of the F1A generation were reduced at doses of 3000
mg/kg diet or more. There were no other compound-related
adverse effects. The NOEL was considered to be 1000 mg/kg
diet.
(1R, cis/trans)-tetramethrin (technical product, 93.4%
purity) was administered at dose levels of 0, 100, 500,
and 3000 mg/kg diet to two successive generations of
Sprague-Dawley CDR albino rats to determine the effects on
the growth and reproductive performance. The body weights
of parental females were significantly lower during the
pre-mating growth, gestation, lactation, and post-weaning
periods, and the body weight of offsprings of both gener-
ations decreased during lactation at 3000 mg/kg diet.
Slight bile duct hyperplasia was noted in F1 females
sacrificed after a 30-day feeding period following weaning
of the F2 offspring at 3000 mg/kg diet. This was, how-
ever, a commonly observed change in old rats. Thus, tetra-
methrin did not affect the reproductive performance of
male and female rats in two successive generations at up
to 500 mg/kg diet [46].
7.7 Neurotoxicity - Mode of Action
Tetramethrin is classified as a Type I pyrethroid. The
mode of action of pyrethroids in general is described in
Appendix I.
In electrophysiological studies, tetramethrin produced
repetitive discharges in housefly muscle and uncoupling
in motor units [1, 32] and caused repetitive firing in
cockroach cercal sensory nerves at a concentration of
3 x 10-13 mol/litre [8].
The effects of tetramethrin on the sodium channel
gating mechanism were studied using the squid giant axons
under voltage clamp conditions [27, 28]. Tetramethrin pro-
longed the falling phase of sodium current during depolar-
ization and increased and prolonged the tail current
associated with repolarization. The prolongation of the
sodium current was due to the channel remaining open. The
channel returned slowly to the resting state upon repolar-
ization.
Analysis of the dose dependence of the two kinetic
phases of tail current development suggests that the ap-
parent dissociation constant for 1R, trans-tetramethrin
depends on the conformational state of the channel. Thus,
it can be concluded that tetramethrin binds to sodium
channels and modifies the state of the channel in the
resting, open, or inactivated state [28].
1R, trans-Tetramethrin markedly prolongs the open
time of single sodium channels recorded by the gigaohm-
seal voltage clamp technique in a membrane patch excised
from the N1E-115 neuroblastoma cell. Single channel con-
ductance is not altered by tetramethrin. The modification
by tetramethrin occurs in an all-or-nothing manner in a
population of sodium channels. The observed tetramethrin-
induced modification of single sodium channels is compat-
ible with previous sodium current data from axons [78].
Tetramethrin greatly prolongs the sodium current
during step depolarization and the sodium tail current
associated with step repolarization of the squid axon mem-
brane. Non-linear current-voltage relationships for the
sodium tail current were analyzed to assess the open
sodium channel properties, which included the permeation
of various cations, calcium block, and cation selectivity.
Tetramethrin had no effect on any of these properties. It
was concluded that tetramethrin modifies the sodium chan-
nel gating mechanism without affecting the pore properties
[79].
8. EFFECTS ON HUMANS
Although tetramethrin has been used for many years, no
adverse effects and no cases of human poisoning have been
reported in the published literature.
In a semi-closed patch test, an aqueous emulsion con-
taining 1.0% tetramethrin was applied to the skin of 200
human volunteers (aged 15-80, both male and female), using
cotton gauze, for 4 days. After 2 weeks, an additional ap-
plication was made in a same manner. Dermatological exam-
ination showed that tetramethrin is neither a primary
irritant nor a human skin sensitizer [73].
9. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by
Hazard, technical tetramethrin is classified as unlikely
to present an acute hazard in normal use [75].
REFERENCES
1. ADAMS, M.E. & MILLER, T.A. (1980) Neurophysiological correlation of
pyrethroid and DDT-type insecticide poisoning in the housefly Musca
domestica L. In: Insect neurobiology and pesticide action (Neutox 79),
London, Society of Chemical Industry, pp. 431-439.
2. DING, C., YU, Y., ZHANG, J., CAI, Z., & CHAN, X. (1985) Genotoxic
effects of tetramethrin on cultured mammalian cells and Salmonella
typhimurium. Zhejiang Yike Daxue Xuebao, 14(1): 1-4.
3. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
4. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
5. FAO (1982) Second Government Consultation on International Harmonization
of Pesticide Registration Requirements, Rome, 11-15 October 1982, Rome,
Food and Agriculture Organization of the United Nations.
6. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
7. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
8. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15: 181-
191.
9. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in the mouse
and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
10 GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:(6)
793-799.
11. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980a) Primary eye and skin
irritation tests of Neopynamin Forte, technical in rabbits (Technical
Report No. IT-00-0073) (Submitted to WHO by Sumitomo Chemical Co.).
12. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980b) Skin sensitization
test of Neopynamin Forte, technical in guinea-pigs (Technical Report No.
IT-00-0082) (Submitted to WHO by Sumitomo Chemical Co.).
13. HARA, M., SUZUKI, H., & MIYAMOTO, J. (1981) In vivo chromosomal
aberration test of Neopynamin Forte, on bone marrow cells of mice (Tech-
nical Report No. 81042) (Submitted to WHO by Sumitomo Chemical Co.).
14. HARTLEY, D., KIDD, H., & KENNEDY, J.M. (1987) Agrochemicals
Handbook, 2nd ed., Nottingham, United Kingdom, Royal Society of
Chemistry.
15. HAYASHI, A. (1977) [How to use insecticides safely.] Bo-satsu-kyo
News, 1: 6-11 (in Japanese).
16. HIROMORI, T., HOSOKAWA, S., OKUNO, Y., SEKI, T., SUZUKI, T.,
& MIYAMOTO, J. (1982) [Mammalian toxicity of d-isomer of phthalthrin
(Neopynamin Forte).] Pharmacometrics, 24: 179-201 (in Japanese).
17. KADOTA, T., KOHDA, H., & MIYAMOTO, J. (1977) Acute oral and
dermal toxicities of Neopynamin in mice and rats (Technical Report No.
IT-70-0003) (Submitted to WHO by Sumitomo Chemical Co.).
18. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Metabolism of
tetramethrin isomers in rats. J. Pestic. Sci., 6: 425-435.
19. KATO, T., UEDA, K., & FUJIMOTO, K. (1964) New insecticidally active
chrysanthemates. Agric. biol. Chem., 28: 914-915.
20. KATO, T., SUZUKI, T., SAKO, H., OKUNO, Y., & MIYAMOTO, J.
(1987) Acute dermal toxicity of Neopynamin in rabbits (Technical
Report No. IT-70-0207) Submitted to WHO by Sumitomo Chemical Co.).
21. KAWAMOTO, M., HARA, M., KOGISO, S., YOSHITAKE, A., &
YAMADA, H. (1988) In vitro unscheduled DNA synthesis (UDS) assay of
Neopynamin in rat hepatocytes (Technical Report No. IT-80-0213)
(Submitted to WHO by Sumitomo Chemical Co.).
22. KISHIDA, F., SUZUKI, H., & MIYAMOTO, J. (1981) Mutagenicity test of
Neopynamin Forte, in bacterial system (Technical Report No. IT-00-0101)
(Submitted to WHO by Sumitomo Chemical Co.).
23. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
24. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore
complex. Science, 221: 1399-1401.
25. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions
of pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
26. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd., p. 440.
27. LUND, A.E. & NARAHASHI, T. (1981) Kinetics of sodium channel
modification by the insecticide tetramethrin in squid axon membranes.
J. Pharmacol. exp. Ther., 219: 464-473.
28. LUND, A.E. & NARAHASHI, T. (1982) Dose-dependent interaction of the
pyrethroid isomers with sodium channels of squid axon membranes.
Neurotoxicology, 3: 11-24.
29. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane effects
of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
30. MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, p. 502.
31. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J.
(1983) Farm chemicals handbook. Section C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, p. C232.
32. MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington DC, American Chemical Society,
pp. 98-115 (ACS Symposium Series No. 42).
33. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
34. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
35. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry, human
welfare and the environment, Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September
1982, Oxford, Pergamon Press, Vol. 1-4.
36. MIYAMOTO, J., SATO, Y., & YAMAMOTO, K. (1968) Biochemical
studies on the mode of action of pyrethroidal insecticides. Part I.
Metabolic fate of phthalthrin in mammals. Agric. biol. Chem., 32:
628-640.
37. MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem., 36:
2203-2211.
38. OKUNO, Y. (1982) Exposure study with spraying of Neopynamin
aerosol (Technical Report No. IF-20-002) (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
39. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976a) Eye and skin
irritation of Neopynamin in rabbits (Technical Report No. IT-60-0014)
(Submitted to WHO by Sumitomo Chemical Co.).
40. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976b) Skin sensitization
study of Neopynamin with guinea-pigs (Technical Report No. IT-60-0013)
(Submitted to WHO by Sumitomo Chemical Co.).
41. PANSHINA, T.N. & SASINOVICH, L.M. (1983) Toxicology of synthetic
pyrethroids. Khim. Selsk. Khoz. 12: 51-53.
42. PAPADOPOULOU-MOURKIDOU, E., IWATA, Y., & GUNTHER, F.A.
(1981) Utilization of an infrared detector for selective liquid
chromatographic analysis. 2. Formulation analysis of the pyrethroid
insecticides allethrin, decamethrin, cypermethrin, phenothrin, and tetra-
methrin. J. agric. food. Chem., 29(6): 1105-1111.
43. PENCE, D.H., HAGEN, W.H., ALSAKER, R.D., DAWKINS, B.G., MARSHALL,
P.M., & TACEY, R.L. (1981a) Subchronic toxicity study in dogs.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories Inc.
(Technical Report No. IT-11-0098) (Submitted to WHO by Sumitomo Chemical
Co.).
44. PENCE, D.H., SEROTA, D.G., ALSAKER, R.D., BANAS, D.A., &
KUNDZINS, W. (1981b) Chronic toxicity study in rats. Neopynamin
Technical Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-11-0097) (Submitted to WHO by Sumitomo
Chemical Co.).
45. PENCE, D.H., COX, R.H., DUDECK, L.E., ALSAKER, R.D., JONES, S.R.,
PARKER, G.A., HEPNER, K.E., & ZOETIS, T. (1986a) Combined chronic
toxicity and oncogenicity study in mice. Neopynamin Final Report, Vienna,
Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-61-0193)
(Submitted to WHO by Sumitomo Chemical Co.).
46. PENCE, D.H., WOLFE, G.W., KULWICH, B.A., HEPNER, K.E., &
SNYDER, F.G. (1986b) Two-generation reproduction study in rats.
Neopynamin Forte, Final Report, Hazleton Laboratories, Inc. (Technical
Report No. IT-61-0201) (Submitted to WHO by Sumitomo Chemical Co.).
47. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids
on a nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
48. RUTTER, H.A., Jr (1974) One-generation reproduction study in rats.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-01-0081) (Submitted to WHO by Sumitomo
Chemical Co.).
49. RUTTER, H.A., Jr, NELSON, L.W., KUNDZINS, W., & MCHUGH (1974)
Two-year dietary administration in the rat. Neopynamin Final Report,
Vienna, Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-
41-0024) (Submitted to WHO by Sumitomo Chemical Co.).
50. RUZO, L.O., SMITH, I.H., & CASIDA, J.E. (1982) Pyrethroid
photochemistry: photooxidation reactions of the chrysanthemates,
phenothrin and tetramethrin. J. agric. food Chem., 30: 110-115.
51. SATO, T. & NARAMA, K. (1980a) Reproduction test of Neopynamin. Part 1:
Fertility study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0075) (Submitted to WHO by Sumitomo Chemical Co.).
52. SATO, T. & NARAMA, K. (1980b) Reproduction test of Neopynamin. Part 2:
Teratology study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0076) (Submitted to WHO by Sumitomo Chemical Co.).
53. SATO, T. & NARAMA, K. (1980c) Reproduction test of Neopynamin. Part 3:
Teratology study in rabbits, Shizuoka, Hamamatsu Seigiken Research (Tech-
nical Report No. IT-01-0077) (Submitted to WHO by Sumitomo Chemical Co.).
54. SATO, T., TAGAWA, G., & NARAMA, K. (1980) Reproduction test of
Neopynamin. Part 4: Perinatal and postnatal study in rats, Shizuoka,
Hamamatsu Seigiken Research (Technical Report No. IT-01-0078) (Submitted
to WHO by Sumitomo Chemical Co.).
55. SMITH, I.H., WOOD, E.J., & CASIDA, J.E. (1982) Glutathione conjugate
of the pyrethroid tetramethrin. J. agric. food Chem., 30: 598-600.
56. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of
Neopynamin with bacterial systems (Technical Report No. IT-70-0023)
(Submitted to WHO by Sumitomo Chemical Co.).
57. SUZUKI, T. & MIYAMOTO, J. (1974) Metabolism of tetramethrin in
houseflies and rats in vitro. Pestic. Biochem. Physiol., 4: 86-97.
58. SUZUKI, T., KOHDA, H., MISAKI, Y., OKUNO, Y., KOYAMA, Y.,
& MIYAMOTO, J. (1980) Acute and subacute inhalation toxicity studies of
Neopynamin Forte in rats (Technical Report No. IT-10-0144) (Submitted to
WHO by Sumitomo Chemical Co.).
59. TANAKA, H., TAKADA, S., ISEKI, M., SANO, R., AIHARA, H.,
KURODA, H., MAEBO, Y., MOTOYOSHI, I., & WAKAI, Y. (1967)
Toxicological studies of various insecticides to mammalia. 2. On the
acute and chronic toxicity of pyrethroid insecticide phthalthrin to
albino rat or guinea-pig. Med. J. Osaka City Univ., 16: 509-517.
60. UEDA, K., GAUGHAN, L.C., & CASIDA, J E. (1975) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food Chem., 23:
106-115.
61. UNO, M., OKADA, T., OHMAE, T., TERADA, I., & TANIGAWA, K.
(1982) [Determination of pyrethroid insecticides by dual-wavelength
densitometry.] Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
62. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
63. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels
in frog myelinated nerve membrane. In: Insect neurobiology and
pesticide action, London, Society of Chemical Industry, pp. 79-85.
64. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M. (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
65. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and phermones,
New York, London, Plenum Press, pp. 183-210.
66. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
67. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
68. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
69. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
70. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN,
J. (1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in myelinated nerves. Nature (Lond.), 295: 601-603.
71. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M.,
& VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
72. WEIR, R.J. & CREWS, L.M. (1966) Three month dietary administration
to dogs of Neopynamin - final report (Technical Report No. IT-61-0011)
(Submitted to WHO by Sumitomo Chemical Co.).
73. WEIR, R.J. & OSBOURN, R.A. (1966) Report of human patch test on
coded substance : 2301 D-61-1, Vienna, Virginia, Hazleton
Laboratories, Inc. (Technical Report No. IT-61-0008) (Submitted to
WHO by Sumitomo Chemical Co.).
74. WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
75. WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988/89, Geneva, World
Health Organization (Unpublished report VBC/88.953).
76. WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 520.
77. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids.
Gen. Pharmacol., 9: 387-398.
78. YAMAMOTO, D., QUANDT, F.N., & NARAHASHI, T. (1983)
Modification of single sodium channels by the insecticide
tetramethrin. Brain Res. 274: 344-349.
79. YAMAMOTO, D., YEH, J.Z., & NARAHASHI, T. (1986) Ion permeation
and selectivity of squid axon sodium channels modified by
tetramethrin. Brain Res., 372: 193-197.
80. YOSHITAKE, A., KOGISO, S., HARA, M., & MIYAMOTO, J. (1986)
In vivo chromosomal aberration test of Neopynamin in mouse bone
marrow cells (Technical Report No. IT-60-0197) (Submitted to WHO
by Sumitomo Chemical Co.).
81. YOSHITAKE, A., KOGISO, S., YAMADA, F., HARA, M., & MIYAMOTO,
J. (1987) Reverse mutation test of Neopynamin in Salmonella
typhimurium and Escherichia coli (Technical Report No. IT-70-0205)
(Submitted to WHO by Sumitomo Chemical Co.).
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs ( Xenopus laevis, Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [10, 23, 65,
66, 74]. The same distinction was found in studies on
cockroaches [8].
Based on the binding assay on the alpha-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types: the
alpha-cyano-3-phenoxybenzyl pyrethroids and the non-cyano
pyrethroids [7, 9, 24, 25].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin, cisme-
thrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [64]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [62]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [71].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [6].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [63]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [71].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans [4],
are almost identical to those of allethrin, i.e., presyn-
aptic repetitive firing, and no significant effects on
transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [64, 65, 70].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [29].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [47].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cyhalothrin, lambda-cyhalo-
thrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [69]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [67,
71].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during exci-
tation, presumably by slowing down the closing of the
activation gate of the sodium channel [67, 71]. The time
constant of closing of the activation gate in the cyper-
methrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged
sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to
cause the long-lasting repetitive firing in the lateral-
line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [29].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [9].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the toxicity
of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand,
[35S]- t-butylbicyclophosphorothionate [35S]-TBPS. Studies
with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic
cyano compounds including deltamethrin, 1R, cis-cyper-
methrin, 1R, trans-cypermethrin, and [2S, alphaS]-fenvalerate
were inhibitors, but their non-toxic stereoisomers were
not; non-cyano pyrethroids were much less potent or were
inactive [24].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [25].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S,alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex [7].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [47].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-lasting
prolongation of the transient increase in sodium per-
meability of the nerve membrane during excitation. This
results in long-lasting trains of repetitive impulses in
sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [74, 77].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [68].
1. RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1.1 Résumé et évaluation
1.1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La tétraméthrine a été synthétisée pour la première
fois en 1964 et commercialisée et en 1965. Sur la plan
chimique, c'est un ester de l'acide chrysanthémique (acide
diméthyl-2,2(diméthyl-2,2 vinyl)-3 cyclopropanecarboxylique
et de l'alcool tétrahydro-3,4,5,6 phatlimidométhylique.
Elle est constituée d'un mélange de quatre stéréoisomères
[1R,trans], [1R,cis], [1S,trans] et [1S,cis]. Les stéréo-
isomères qui entrent dans la composition des produits
techniques sont à peu près dans la proportion de 4:1:4:1.
De tous les isomères, c'est l'isomère [1R,trans] qui est
le plus actif biologiquement; vient ensuite l'isomère
1R,cis]. On commercialise sous le nom de "Neo-pynamine
Forte" (désignée dans la présente monographie sous le nom
de 1R, cis/trans-tetraméthrine), un mélange des isomères
[1R,cis] et [1R,trans] dans le proportion de 1:4.
La tétraméthrine de qualité technique est un solide
incolore dont le point de fusion est de 60-80 °C. Sa
densité est de 1,11 à 20 °C et sa tension de vapeur de
0,946 mPa (7,1 x 10-6 mm Hg) à 30 °C. Peu soluble dans
l'eau (4,6 mg/litre à 30 °C), elle est en revanche soluble
dans certains solvants organiques tels que l'hexane, le
méthanol et le xylène. Elle est stable à la chaleur mais
instable à la lumière et à l'air. L'isomère [1R,cis/trans]
est un liquide visqueux de couleur jaune dont les autres
propriétés physiques et les propriétés chimiques sont les
mêmes que celles de la tétraméthrine.
Le dosage des résidus s'effectue par densitométrie à
deux longueurs d'onde (370-230 nm). Pour l'analyse des
produits techniques, on utilise la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
Une analyse des différentes formulations peut s'effectuer
au moyen d'un chromatographe en phase liquide à haute
performance muni d'un détecteur infrarouge.
1.1.2 Production et usage
La production mondiale annuelle de tétraméthrine est
évaluée à quelques centaines de tonnes. Elle est princi-
palement utilisée pour la lutte contre les nuisibles à
l'intérieur des habitations, sous forme d'aérosols, de
concentrés émulsionnables ou de serpentins anti-moust-
iques. La tétraméthrine entre également dans la compos-
ition d'autres formulations insecticides additionnées ou
non de synergisants.
1.1.3 Exposition humaine
L'exposition de la population dans son ensemble peut
résulter de l'utilisation de ce produit pour la destruc-
tion des nuisibles dans les habitations. Lorsqu'on utilise
la tétraméthrine conformément aux recommandations, sa con-
centration atmosphérique ainsi que celle de l'isomère 1R
ne devraient pas dépasser 0,5 mg/m3; par ailleurs le
composé se dégrade rapidement. L'exposition de la popu-
lation générale est donc vraisemblablement très faible. On
n'utilise pas de tétraméthrine pour traiter les cultures
vivrières.
1.1.4 Exposition et destinée dans l'environnement
Une fine pellicule de tétraméthrine exposée à la
lumière solaire se dégrade rapidement. On a observé que
les principales réactions photochimiques qui se produis-
aient au cours d'une exposition de 2 heures (conversion de
30%) étaient: une époxydation au niveau de la double
liaison du radical isobutényle, une oxydation en hydroxy-
méthyle, en aldéhyde et en acide carboxylique du groupe
méthyle en position trans du groupement isobutényle;
enfin, une hydroperoxydation en hydroperoxyde allylique.
On ne connaît pas avec exactitude les concentrations
exactes de tétraméthrine dans l'environnement, mais compte
tenu de l'utilisation qui en est faite actuellement pour
traiter les habitations et pourvu que le produit soit
utilisé conformément aux recommandations, il est vraisem-
blable que l'exposition dans l'environnement devrait être
très faible. La tétraméthrine se décompose rapidement en
produits moins toxiques.
1.1.5 Absorption, métabolisme, et excrétion
Des rats à qui l'on avait administré par voie orale ou
sous-cutanée de la tétraméthrine radio-marquée au niveau
du reste acide ou du reste alcool ont rapidement absorbé,
métabolisé et excrété le produit. L'excrétion s'effectue
en 5 à 7 jours dans la proportion d'environ 95%, à peu
près autant par la voie urinaire que par la voie fécale.
Par ces deux voies, les résidus présents dans les tissus
sont très faibles. La métabolisation s'effectue par les
réactions suivantes: coupure de l'ester; élimination du
groupe hydroxyméthyl du reste alcool; réduction de la
double liaison 1-2 du reste alcool; oxydation du groupe-
ment méthyle de l'isobutényle au niveau du reste acide et
en 2, 3 et 4 au niveau du reste alcool; conjugaison des
acides et des alcools résultant avec l'acide glucuronique
et enfin isomérisation cis/trans.
1.1.6 Effets sur les êtres vivant dans leur milieu naturel
On ne dispose que de très peu d'informations à ce
sujet. La tétramethrine est extrêmement toxique pour les
poissons, la valeur de la CL50 à 96-h pour deux espèces
se situant respectivement à 19 et 21 µg/litre. Pour une
troisième espèce, on a obtenu une CL50 à 48 heures de
200 µg/litre et la dose sans effet observable était de
50 µg/litre. Pour la daphnie, la dose sans effet
observable est également de 50 µg/litre. La tétraméthrine
est en revanche très peu toxique pour les oiseaux mais
elle est toxique pour les abeilles. Cependant du fait que
le produit est rapidement dégradé et dans la mesure où on
ne l'utilise, conformément aux recommandations, que dans
les habitations, il est peu probable qu'il puisse excercer
des effets nocifs sur l'environnement.
1.1.7 Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in-vitro
La tétraméthrine a une faible toxicité aiguë par voie
orale. La DL50 pour le rat est >5000 mg/kg, qu'il
s'agisse du racémique ou de l'isomère (1R,cis/trans),
tandis que pour la souris elle est d'environ 2000 mg/kg
(racémique) et de 1060 mg/kg (1R,cis/trans). Chez le rat,
la souris et le lapin la toxicité aiguë par voie percu-
tanée e st également faible; la DL50 chez le rat et la
souris étant <5000 mg/kg et <2000 mg/kg chez le lapin
(toutes les études portaient sur le racémique). Les études
de toxicité aiguë par inhalation ont donné une CL50 chez
le rat et la souris de 2500 mg/m3 pour le racémique et
>1180 mg/m3 pour l'isomère (1R,cis/trans). Parmi les
signes d'intoxication on a noté une hyperexcitabilité, des
tremblements, de l'ataxie et une dépression (signes
généraux observés dans l'ensemble des études de toxicité
aiguë). Les souris se sont révélées un peu plus sensibles
que les rats mais il n'y avait pas de différences de
sensibilité entre mâles et femelles. Qu'ils s'agisse du
racémique ou de l'isomère (1R,cis/trans), la tétraméthrine
ne provoque pratiquement aucune irritation oculaire ou
cutanée chez le lapin. En outre, ni l'un ni l'autre de
ces produits n'exercent d'effet sensibilisateur chez le
cobaye.
La tétraméthrine est un pyréthroïde du type I. Chez
les mammifères ce sont les tremblements (syndrome-T) qui
constituent le symptôme d'intoxication caractéristique.
Chez des rats ayant reçu de la tétraméthrine mêlée à
leur nourriture à des concentrations allant jusqu'à 5000
mg/kg de nourriture pendant 91 jours, on a noté une réduc-
tion du gain de poids à la dose la plus forte. D'après les
résultats d'études de 3 et 6 mois, au cours desquelles des
rats ont reçu l'isomère 1R(cis/trans) dans leur nourriture
à des doses allant de 25 mg/kg à 3000 mg/kg d'aliment, la
dose sans effet observable était de 200 mg/kg de nourri-
ture pour les mâles et de 300 mg/kg pour les femelles
(parmi les anomalies observées, on notait une réduction du
gain de poids et du poids final du corps ainsi que
certains effets sur les reins et le foie). Les effets sur
le foie résultent, semble-t-il, d'une réaction d'adapt-
ation à la présence dans l'alimentation du véhicule
utilisé, à savoir l'huile de maïs.
Une étude de 26 semaines sur des chiens a fait
ressortir une dose sans effet observable de 1250 mg/kg de
nourriture.
Des souris et des rats à qui l'on avait fait inhaler
de la tétraméthrine en aérosol à une concentration de 200
mg/m3, 3 à 4 heures par jour pendant des périodes allant
jusqu'à quatre semaines, n'ont présenté aucune anomalie
imputable à ce produit. Lors d'une autre étude de ce
type, au cours de laquelle des rats ont été exposés à une
brumisation (gouttelettes de 1,2-1,5 µm de diamètre)
d'isomère (1R,cis/trans) dans du kérosène désodorisé à des
concentrations allant jusqu'à 87 mg/m3, trois heures par
jour et sept jours par semaine pendant 28 jours, on a
obtenu, pour la dose sans effet observable, une valeur de
49 mg/m3. Les signes d'intoxication n'ont été observés
qu'au cours de l'exposition.
Ni la tétraméthrine ni ses isomères (1R,cis/trans) ne
se sont révélés mutagènes dans divers systèmes d'épreuve
in vivo et in vitro utilisés pour étudier les mutations
génétiques, les lésions et les réparations de l'ADN ainsi
que les effets sur les chromosomes.
Trois études, dont deux chez le rat et une chez la
souris ont été menées pendant 104 semaines afin d'étudier
la cancérogénicité à long terme de la tétraméthrine. Les
souris ont reçu de la tétraméthrine dans leur nourriture à
des doses allant jusqu'à 1500 mg/kg de nourriture. Aucun
effet oncogène n'a été observé. A partir de 60 mg/kg de
nourriture on observait une réduction du poids de
l'hypophyse, de la thyroïde et de la parathyroïde. Chez
la souris, la dose sans effet général observable se
situait à 12 mg/kg de nourriture. Quant aux rats, ils ont
été exposés à de la tétraméthrine à des doses allant
jusqu'à 5000 mg/kg de nourriture soit in utero soit au
cours d'une période prolongée. Les deux études ont fait
ressortir un gain de poids sensiblement moindre chez les
animaux recevant 3000 mg de tétraméthrine par kg de
nourriture ou davantage. En outre, à ces concentrations,
on a observé une augmentation du poids du foie. Pour ce
qui est des effets généraux, la dose sans effet observ-
able se situait dans les deux études, à 1000 mg/kg de
nourriture. A la dose de 3000 mg/kg de nourriture,
l'incidence des tumeurs testiculaires à cellules de Leydig
était supérieure à la valeur notée dans le groupe témoin
et ce, pour les deux études. Les tumeurs à cellules de
Leydig se produisent spontanément chez les rats âgés et
leur incidence peut varier énormément dans les groupes
témoins. On pense que cette tumeur est d'origine hormon-
ale. Aucun signe de malignité et aucune tumeur de ce type
n'ont été relevés chez les souris. On peut en conclure
que cet effet oncogène, s'il existe réellement, ne peut
être pris en considération pour ce qui concerne l'homme.
La tétraméthrine ne s'est révélée ni tératogène ni
embryotoxique à des doses allant jusqu'à 1000 mg par kg de
poids corporel chez les rats et jusqu'à 500 mg/kg chez des
lapins (il s'agit des concentrations les plus fortes
étudiées). Lors d'une étude de fécondité au cours de
laquelle des rats ont reçu de la tétraméthrine à des doses
allant jusqu'à 1000 mg/kg de poids corporel par jour, la
dose sans effet observable sur la reproduction des parents
et la croissance des foetus, se situait à 300 mg/kg de
poids corporel par jour. Une étude de reproduction chez
le rat, portant sur la période périnatale et sur la
période post-natale, a permis de fixer à 100 mg/kg de
poids corporel la dose quotidienne sans effet observable
(la dose la plus forte administrée au cours de cette
étude).
Lors d'une étude de reproduction portant sur une
génération de rats, on a administré aux animaux 1000-6000
mg de tétraméthrine par kg de nourriture et constaté que
la dose sans effet observable était de 1000 mg/kg. Selon
une autre étude portant cette fois sur deux générations,
au cours de laquelle les rats ont reçu de l'isomère
(1R,cis/trans) à des doses allant de 100 à 3000 mg/kg, la
dose sans effet observable était de 500 mg/kg de
nourriture.
1.1.8 Effets sur l'homme
Bien que la tétraméthrine et son isomère 1R soient
utilisées depuis des années, on ne signale aucun cas
d'intoxication ou d'effets indésirables chez l'homme.
Rien n'indique que la tétraméthrine ou son isomère 1R
puissent avoir des effets nocifs sur l'homme si on
continue de les utiliser à faibles concentrations et
seulement pour la destruction des nuisibles à l'intérieur
des habitations.
1.2 Conclusions
a) Population générale: l'exposition de la population
générale à la tétraméthrine, dans son utilisation actu-
elle, est vraisemblablement faible. Si ce produit est
utilisé conformément aux recommandations, il ne présente
probablement aucun risque.
b) L'exposition professionnelle: moyennant de bonnes
méthodes de travail, l'application de mesures d'hygiène et
avec quelques précautions, la tétraméthrine ne devrait pas
présenter de danger pour les personnes qui y sont exposées
de par leur profession.
c) Environnement: il est tout à fait improbable que la
tétraméthrine ou ses produits de décomposition s'accum-
ulent au point d'avoir des effets nocifs sur l'environne-
ment.
1.3 Recommandations
Bien que la tétraméthrine et son isomère 1R soient
utilisés depuis des années sans qu'on ait à déplorer
d'effets nocifs chez l'homme, il est souhaitable que
l'exposition humaine continue d'être surveillée.
Judging from the metabolites derived from the acid
moiety, cis to trans isomerization of the oxidized deriva-
tives of CA occurs, as happens in resmethrin metabolism
[60].
Although both cis to trans and trans to cis isomeriza-
tions of tetramethrin were observed by Kaneko et al. [18],
cis to trans conversion seemed to be predominant. On the
other hand, the detected metabolites from the alcohol
moiety were TPI, HPI, 3-OH-TPI (33), 3,4,5,6-tetrahydro-
phthalic acid amide (34) (TPIA), 2-OH-HPI (35), 3-OH-HPI
(19), and 4-OH-HPI (36). Of these metabolites, 2-OH-HPI
was found in relatively large amounts.
Smith et al. [55] found that tetramethrin and TPI
readily underwent the Michael addition with thiols. The
tetramethrin-gluthathione (GSH) conjugate was formed under
physiological conditions in the presence of mouse liver
homogenate fractions, probably by a non-enzymatic
reaction. The soluble thiol level of mouse liver was de-
creased by intraperitoneal administration of TPI. However,
mercapturic acid and GSH conjugates of tetramethrin were
not detected in the bile or urine of rats or mice treated
intraperitoneally with tetramethrin.
5.2 Enzymatic Systems for Biotransformation
When alcohol- or acid-labelled [1RS, trans]-tetra-
methrin (1 mmol/litre) was incubated for 1 h at 37 °C with
30 mg protein of a rat liver subcellular fraction (i.e.
nuclei plus mitochondria, microsomes, and soluble frac-
tion), the microsomes and nuclei plus mitochondria frac-
tions were active in degrading tetramethrin. Rat micro-
somal fraction degraded [1RS, trans]-tetramethrin to CA,
MTI, and TPI in the absence of NADPH. In the presence of
NADPH, tetramethrin was more rapidly degraded to yield
oxidized tetramethrin ( wt-alc-, wt-ald-, and wt-acid-tetra-
methrin), oxidized CA ( wt-alc-, wt-ald-, and wt-acid-CA),
TPI, and unidentified metabolites in larger amounts. The
major metabolite TPI was shown to be produced non-enzy-
matically from MTI. The degradation rate of tetramethrin
was greatly reduced by the inhibition of ester hydrolysis
with paraoxon [57].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
As the use of tetramethrin is limited to indoor pest
control, there is a paucity of data concerning its effect
on the environment.
6.1 Aquatic Organisms
Data on the toxicity of tetramethrin to non-target
aquatic organisms are given in Table 4.
Tetramethrin is highly acutely toxic to fish in lab-
oratory tests, the 96-h LC50s for two species being ap-
proximately 20 µg/litrea. The 48-h LC50 for killifish
is about 200 µg/litre, with a no-observed-effect level
(NOEL) of 50 µg/litre [33]. The NOEL for Daphnia pulex
was reported by Miyamoto [33] to be 50 µg/litre for
racemic tetramethrin and 10 µg/litre for [1R, trans]- or
[1R, cis]-tetramethrin.
6.2 Terrestrial Organisms
Tetramethrin has low toxicity to birds. The acute oral
LD50 for Bobwhite quail is >2510 mg/kg body weight, and
the 8-day dietary LC50 to Mallard duck and Bobwhite quail
is >5620 mg/kga.
Tetramethrin is toxic to bees [14].
a written comment from US EPA to IPCS, 1987.
Table 4. Acute toxicity of tetramethrin to non-target aquatic organisms
Species Size Parameter Toxicity Formulation System Temperature Refer-
(mg/litre) (°C) ence
Fish
Killifish adult 48-h LC50 0.2 Technical Static 25 33
( Oryzias latipes) adult 48-h LC50 0.2 (+)-trans Static 25 33
adult 48-h LC50 0.15 (+)-cis Static 25 33
Bluegill sunfish 96-h LC50 0.019 a
( Lepomis macrochirur)
Rainbow trout 96-h LC50 0.021 a
( Salmo gairdneri)
Arthropods
Daphnia pulex 3-h LC50 >50 Technical Static 25 33
3-h LC50 >50 (+)-trans Static 25 33
3-h LC50 >50 (+)-cis Static 25 33
a Written comment from US EPA to IPCS, 1987.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
The acute toxicity of tetramethrin to rats and mice is
low (Table 5).
Table 5. Acute toxicity of tetramethrin to rats and mice
Compound Species Sex Route LD50 Reference
(mg/kg body
weight)
Racemic rat M,F oral 4600 41
rata M,F oralb >5000 17
rat M,F dermalb >10 000 41
mousea M oralb 1920 17
mousea F oralb 2000 17
(1R, cis/trans)- rata M,F oralb >5000 16
rat M,F subcutaneous >5000 16
rat M intraperitoneal 770 16
rat F intraperitoneal 548 16
rat M,F dermal(>24 h)b >5000 16
mousea M oral 1060 16
mousea F oral 1040 16
mouse M subcutaneous 2020 16
mouse F subcutaneous 1950 16
mouse M intraperitoneal 631 16
mouse F intraperitoneal 527 16
mouse M,F dermal (>24 h)b >5000 16
rabbit dermal (>24 h)b >2000 20
a Animals were not fasted.
b Corn oil was used as vehicle.
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1.2-
1.5 µm) of [1R, cis/trans]-tetramethrin (technical grade,
95.6% purity) in deodorized kerosene (0, 26, 131, 243,
595, and 1180 mg active ingredient per m3 air) for a
duration of 3 h. At 131 mg/m3 or more, salivation, hyper-
excitability, irregular respiration, urinary incontinence,
muscular fibrillation, limb paralysis, decrease in spon-
taneous activity, and other toxic signs were observed in
males and females. At 1180 mg/m3, 10% of female animals
died, but the body weight gain was similar to that of the
control rats. The no-observed-effect level (NOEL) for
inhalation of the compound in rats was 26 mg/m3, and the
LC50 value was >1180 mg/m3 in both sexes [58].
The toxic symptoms observed following [1R, cis/trans]-
tetramethrin administration were hyperexcitability, muscle
twitching, tremor, ataxia, irregular respiration, and
depression. Mice were invariably more susceptible than
rats. No differences in susceptibility were observed
between male and female animals [16, 17, 33].
7.2 Irritation and Sensitization
7.2.1 Eye irritation
In a study by Okuno et al. [39], 50 mg of the techni-
cal product (91.3% purity) was instilled in one eye of
Japanese albino male rabbits. The treated eye was washed
with distilled water 5 min (group I) or 24 h (group II)
thereafter. The conjunctiva, cornea, and pupil were exam-
ined, 1, 24, 72 h and 7, 14, and 21 days after appli-
cation. No particular changes were noted except that a
very slight erythema and oedema of the conjunctiva was
transiently observed in the rabbits in group II.
In a separate study, 0.1 ml [1R, cis/trans]-tetra-
methrin (technical grade, 95.6% purity) was applied to one
eye of Japanese albino rabbits. The treated eye was sub-
sequently washed in five rabbits but not in three other
rabbits. The material did not produce any lesions in the
cornea or iris of the treated eyes that were not washed,
but slight hyperemia and/or chemosis of the conjunctiva
was observed 1 h after application. In the washed eyes,
slight hyperemia of the conjunctiva was observed in all
animals 1 h after treatment. These changes, however, had
disappeared by 48 h after application in the unwashed eyes
and 24 h in the washed eyes. The irritating potency of the
material was judged to be minimal in the unwashed eyes and
negative in the washed eyes [11].
7.2.2 Skin irritation
In a study by Okuno et al. [39], 0.5 g of the techni-
cal product (91.3% purity) was applied on a lint patch
(3.8 x 3.8 cm) to the abraded or intact skin of six rab-
bits. The skin was assessed for severity of erythema and
oedema 4, 24, 48, 72 h and 7, 14, and 21 days after appli-
cation, but no particular changes were noted.
When 0.5 ml [1R, cis/trans]-tetramethrin (technical
grade, 95.6% purity) was applied on a lint patch
(2.5 x 2.5 cm) to abraded or intact skin on the back of
rabbits, again no irritating reactions such as erythema
and oedema were observed [11].
7.2.3 Sensitization
In a skin-sensitization study of tetramethrin in
guinea-pigs, Hartley male guinea-pigs (seven per group)
were sensitized ten times at intervals of one or two days
by intracutaneous injections (first injection: 0.05 ml,
subsequent ones: 0.1 ml) of a 1% solution of the technical
product (91.3% purity) in corn oil. The sensitized animals
were then challenged against the same concentration in the
same manner (0.5 ml injection) 14 days later, but no skin-
sensitization reaction was noted [40].
In another skin-sensitization test on Hartley male
guinea-pigs, 0.5 ml [1R, cis/trans]-tetramethrin (techni-
cal product, 95.6% purity) in 0.5 ml acetone was applied
topically by lint patch to the back of animals ten times
(three times per week). The animals were challenged in the
same manner 2 weeks after the last sensitizing treatment,
but no allergic reactions were observed 24 h later [12].
7.3 Short-Term Exposure Studies
7.3.1 Oral
When groups of 10 male Wistar rats were maintained for
91 days on a diet containing 0, 500, 1000, 3000, or 5000
mg tetramethrin/kg diet, there was a reduced rate of body
weight gain at 5000 mg/kg but not at 3000 mg/kg or less.
The liver glycogen level was reduced at 3000 mg/kg and
5000 mg/kg. The kidney, spleen, heart, small intestine,
and brain showed no abnormal signs, either macroscopically
or microscopically, and there were no significant changes
in blood parameters. There was no increase in the protein
or glucose levels in the urine of test animals [59].
When technical (1R, cis/trans)-tetramethrin in corn
oil was administered to Sprague-Dawley rats for 3 or 6
months at 0, 100, 300, 1000, or 3000 mg/kg diet, no treat-
ment-related changes were observed in clinical signs, or
food and water consumption, or in an ophthalmological
examination. However, the body weight gain and final body
weight of males and females in the 3000-mg/kg group were
significantly lower than those of the controls. There were
slight increases in urine protein level in the rats fed
more than 1000 mg/kg and in serum calcium level in the
male rats fed more than 300 mg/kg. During a histopatho-
logical examination, eosinophilic bodies in tubular epi-
thelial cells and hyaline droplets in kidney tubular epi-
thelium cytoplasm were observed in males fed 3000 mg/kg
diet, along with an increase in relative organ weight.
There were dose-dependent increases in absolute and rela-
tive liver weight in all treated male rats and in female
rats fed more than 1000 mg/kg diet. There were also
slightly higher serum cholesterol concentrations in rats
of both sexes fed more than 1000 mg/kg and a significant
reduction in liver lipid content among males fed 1000
mg/kg or more. However, these liver effects were not
accompanied by damage to hepatocytes and were therefore
considered to be an adaptation to the corn oil without
toxicological significance. Furthermore, there were no
marked effects, even at 200 mg/kg, when an additional sub-
chronic study was conducted at tetramethrin levels of 25,
50, 100, and 200 mg/kg diet without corn oil in order to
confirm the NOEL in male rats. The NOEL for tetramethrin
in rats in the 6-month study was concluded to be 200 mg/kg
diet for males and 300 mg/kg diet for females [16].
When technical grade tetramethrin (94.6% purity) was
administered for 26 weeks to beagle dogs (six of each sex
per group) at levels of 0, 1250, 2500, and 5000 mg/kg
diet, nervousness and tremors were observed in both males
and females at 2500 and 5000 mg/kg diet. A lack of oestrus
activity in females was also noted clinically and a lack
of corpora lutea was confirmed histologically at 5000
mg/kg diet. Absolute liver weight was increased in males
at 2500 and 5000 mg/kg and relative liver weight was sig-
nificantly increased in males and females at 5000 mg/kg.
Decreased absolute/relative ovary weights were noted for
females at 5000 mg/kg. No other treatment-related changes
were observed with respect to survival, body weight gain,
food consumption, haematology, urinalysis, ophthalmology,
gross pathology or histopathology. The NOEL was 1250 mg/kg
diet [43].
In a study by Weir & Crus (1966), groups of three male
and three female beagle dogs were fed tetramethrin dis-
solved in corn oil for 13 weeks at levels of 0, 1250,
2500, and 5000 mg/kg diet. There were no effects on haema-
tological, clinical chemistry, or urinary parameters.
Organ weights were not affected by the treatment, and
there were no significant histopathological findings.
Clinical signs were not recorded. The NOEL was >5000 mg/kg
diet.
7.3.2 Inhalation
Sprague-Dawley rats (10 of each sex per group) were
exposed to a respirable mist (droplet diameter of 1-2 µm)
of tetramethrin at concentrations of 0, 26, 49, and 87 mg
active ingredient per m3 air, 3 h a day, 7 days a week,
for a period of 28 days. At 87 mg/m3, irregular respir-
ation, slight salivation, and hyperexcitability were
observed as toxic signs every day during the exposure
period, but no cumulative toxicity was noted. There were
no compound-related effects on body weight gain, food and
water consumption, urinalysis, haematology, biochemistry,
organ weight, and histopathology. The NOEL in subacute
inhalation was considered to be 49 mg/m3 [58]. This NOEL
is approximately 100 times higher than the aerial concen-
tration attained during normal use of tetramethrin [33].
7.4 Long-Term Exposures and Carcinogenicity
Appraisal
Testicular interstitial cell tumours occur spontaneously in
aged rats, and the incidence can vary greatly in control
groups. This tumour is believed to be hormonally mediated.
There was no evidence of malignancy in three rat studies and no
evidence of this type of tumour in mice. It can be concluded
that the tumorigenic effect, if real, is most unlikely to be
relevant to human exposure.
When tetramethrin (technical grade) was administered
to Sprague-Dawley CRCDR rats (50 of each sex per group,
F1A weanlings from parental animals pre-treated with the
compound at dose levels of 1000, 3000, and 6000 mg/kg
diet) at dose levels of 0, 1000, 3000, or 5000 mg/kg diet
for 104 weeks, no compound-related effects were detected
in investigations of appearance, behaviour, survival,
haematology, blood chemistry, urinalysis, eye examination,
and organ weight at up to 5000 mg/kg diet. However, the
body weight gain of male and female rats fed 3000 mg/kg or
more was significantly lower than that of controls. The
incidence for testicular interstitial cell tumours was
increased at dose levels of 3000 mg/kg or more [49].
Tetramethrin (technical grade, 90.0/93.6% purity) was
tested for long-term toxic effects and tumorigenic poten-
tial in Sprague-Dawley CRCDR and Long-Evans hooded rats
by in utero exposure and 104-week chronic exposure at dose
levels of 0, 200, 1000, and 5000 mg/kg diet. No distinct
compound-related effects were observed in either strain
with regard to fertility rate, mortality, clinical signs,
and clinical laboratory data. However, body weight gains
were significantly lower in both strains at 5000 mg/kg
diet, and absolute and relative liver weights were in-
creased in both strains at 5000 mg/kg diet. The incidence
of interstitial cell tumours in both strains at 5000 mg/kg
diet was above the level in the concurrent control groups
[44].
When tetramethrin (technical product, 93.3% purity)
was fed daily to B6C3F1 mice (dose levels of 0, 12, 60,
300, or 1500 mg/kg diet) for 104 weeks, there were no sig-
nificant dose-related changes in survival, clinical signs,
mean body weight, or food consumption. However, the mor-
tality of male mice at 300 mg/kg was significantly lower
than that of control males. The absolute and relative
weight of pituitary and thyroid/parathyroid glands was
decreased for males fed 60 mg/kg diet or more. Absolute
spleen weights were also decreased for males fed 300 mg/kg
diet or more. However, gross and microscopic examination
of these tissues did not reveal any treatment-related
histomorphological changes. There were no other histo-
pathological findings attributable to tetramethrin admin-
istration. The NOEL was considered to be 12 mg/kg diet
[45].
7.5 Mutagenicity
The results of mutagenicity tests on tetramethrin are
summarized in Table 6.
Ding et al. [2] reported the induction of unscheduled
DNA synthesis in human amnion FL cells by tetramethrin
(72% industrial grade of unknown origin). The same product
gave weakly positive results in an Ames test with Salmon-
ella typhimurium TA 97. It is not clear if the effect was
caused by tetramethrin itself or by the unidentified (28%)
portion of the industrial grade material.
7.6 Reproduction, Embryotoxicity, and Teratogenicity
In a study by Miyamoto [33], groups of 10-15 pregnant
New Zealand white rabbits received tetramethrin orally on
days 6-18 of gestation at doses of 0, 30, or 90 mg/kg per
day. Fetuses were obtained by caesarean section prior to
parturition and were examined for external and skeletal
abnormalities. Seven extra pregnant animals were allowed
to give birth naturally and the pups were examined for
several weeks to check their growth and development. No
significant adverse effects were observed.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to 6-week-old male Slc: SD rats (SPF, 20
per group) for not less than 9 weeks and to 9-week-old
females (20 per group) for 2 weeks of the non-pregnant
period and up to day 7 of pregnancy. The effects of the
material on the mating ability of male and female animals
and on the fetuses were investigated. In males, the liver
weight increased at all dose levels and a kidney weight
increase was noted at 1000 mg/kg. Salivation and a slower
body weight increase were observed during the latter half
of the administration period at 300 and 1000 mg/kg. How-
ever, no effects on the reproductive ability of males were
noted. In females, no changes were observed in the rate of
pregnancy, but there were effects on the sexual cycle and
an ovulation-inhibiting effect at 1000 mg/kg. In fetuses,
growth inhibition was suspected at 1000 mg/kg. However,
all these changes were slight. The NOEL was considered to
be 300 mg/kg body weight per day for reproductive ability
of parents and growth of fetuses [51].
Table 6. Mutagenicity studies on tetramethrin
Test System Test object Concentration Purity/ Results Reference
Compound
Ames test S. typhimurium up to 10 mg/plate 93 - 100% Negative 33
TA 1535 without activation
TA 1538
Ames test Escherichia coli up to 10 mg/plate 93 - 100% Negative 33
W 3623 without activation
W 3012
Ames test S. typhimurium 1 - 10 000 ug/plate technical Negative 56
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1538
Ames test S. typhimurium 100 - 5000 ug/plate 94.0% Negative 81
TA 100 with and without racemic
TA 98 activation
TA 1535
TA 1537
TA 97
Ames test E. coli 100 - 5000 ug/plate 94.0% Negative 81
WP2 uvr A with and without racemic
activation
Ames test S. typhimurium 10 - 5000 ug/plate 95.6%, Negative 22
TA 100 with and without 1R, cis/trans
TA 98 activation
TA 1535
TA 1537
TA 1538
Ames test E. coli 10 - 5000 ug/plate 95.6%, Negative 22
WP2 uvr A 1R, cis/trans
Ames test S. typhimurium 5 - 500 ug/plate 72% Positivea 2
TA 97 without activation industrial
grade
Rec-assay Bacillus 1 - 10 000 ug/disk technical Negative 56
subtilis racemic
M45 rec- and H17
(wild type)
Rec-assay Bacillus 50 - 5000 ug/disk 95.6%, Negative 22
subtilis 1R, cis/trans
M45 rec- and H17
(wild type)
Table 6 (contd).
Test System Test object Concentration Purity/ Results Reference
Compound
Host-mediated ICR male mice 200 - 1000 mg/kg technical Negative 56
assay S. typhimurium body weight (oral) racemic
G46
In vivo
chromosomal ICR male mice 1200, 2400, 5000 93.4% Negative 80
aberration bone marrow mg/kg body racemic
weight (ip)
In vivo
chromosomal ICR male mice 150, 300, 600 mg/kg 95.6%, Negative 13
aberration bone marrow body weight (ip) 1R, cis/trans
Unscheduled
DNA Human amnion Not recorded 72%, Positivea 2
synthesis FL cells industrial
grade
Unscheduled
DNA Rat hepatocyte 0, 0.2, 1, 94.0% Negative 21
synthesis primary cultures 5, 25, 50, racemic
and 100 ug/ml
a The test material was of unknown origin and it was unclear whether or not
positive results were due to the 28% impurities.
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 30 per group) on
days 7-17 of pregnancy, and its effects on the dams,
fetuses, and growth of pups were investigated. In dams, an
inhibition of body weight increase and a decrease of food
consumption were observed at 1000 mg/kg, in addition to an
increase in liver and kidney weights at the time of
caesarean section. In fetuses, no abnormalities such as
embryo lethality, growth inhibition, or teratogenic
effects were detected. In addition, the tetramethrin had
no effect on the growth of the young after birth or on the
reproductive ability of the offspring. The NOEL was con-
sidered to be 300 mg/kg body weight per day for the dams
and >1000 mg/kg body weight per day for teratogenicity
[52].
When tetramethrin (technical product) was orally
administered at dose levels of 0, 50, 150, or 500 mg/kg
body weight per day to pregnant Japanese white rabbits,
(10 per group) on days 8-18 of pregnancy, a slight transi-
ent decline in the body weight of the dams was noted in
the middle of the treatment period at 500 mg/kg. No
adverse effects such as embryo lethality, inhibition of
fetal growth, or teratogenic action were observed at any
dose level. The NOEL for teratogenicity in rabbits was
considered to be >500 mg/kg body weight per day [53].
Tetramethrin (technical product) was orally adminis-
tered (dose levels of 0, 100, 300, and 1000 mg/kg body
weight per day) to Slc: SD rats (SPF, 20 per group) from
day 17 of gestation to day 21 of lactation (perinatal and
postnatal period). In dams, a liver weight increase was
noted at 300 and 1000 mg/kg but there were no abnormali-
ties at delivery or during lactation. Tetramethrin had no
detectable effects on the survival rate of pups, growth
and development, sensory function, motor function, learn-
ing ability, or reproductive ability. The NOEL was
considered to be 100 mg/kg body weight per day for dams
and >1000 mg/kg body weight per day for pups [54].
In studies by Rutter [48], tetramethrin (technical
grade) was administered to Sprague-Dawley rats at dose
levels of 0, 1000, 3000, and 6000 mg/kg diet for 9 weeks
through weaning of the F1A generation. Body weight
reduction occurred at 6000 mg/kg diet in the parent rats.
The lactation index was depressed for the F1A generation
at 6000 mg/kg diet, and the weaning body weights for both
sexes of the F1A generation were reduced at doses of 3000
mg/kg diet or more. There were no other compound-related
adverse effects. The NOEL was considered to be 1000 mg/kg
diet.
(1R, cis/trans)-tetramethrin (technical product, 93.4%
purity) was administered at dose levels of 0, 100, 500,
and 3000 mg/kg diet to two successive generations of
Sprague-Dawley CDR albino rats to determine the effects on
the growth and reproductive performance. The body weights
of parental females were significantly lower during the
pre-mating growth, gestation, lactation, and post-weaning
periods, and the body weight of offsprings of both gener-
ations decreased during lactation at 3000 mg/kg diet.
Slight bile duct hyperplasia was noted in F1 females
sacrificed after a 30-day feeding period following weaning
of the F2 offspring at 3000 mg/kg diet. This was, how-
ever, a commonly observed change in old rats. Thus, tetra-
methrin did not affect the reproductive performance of
male and female rats in two successive generations at up
to 500 mg/kg diet [46].
7.7 Neurotoxicity - Mode of Action
Tetramethrin is classified as a Type I pyrethroid. The
mode of action of pyrethroids in general is described in
Appendix I.
In electrophysiological studies, tetramethrin produced
repetitive discharges in housefly muscle and uncoupling
in motor units [1, 32] and caused repetitive firing in
cockroach cercal sensory nerves at a concentration of
3 x 10-13 mol/litre [8].
The effects of tetramethrin on the sodium channel
gating mechanism were studied using the squid giant axons
under voltage clamp conditions [27, 28]. Tetramethrin pro-
longed the falling phase of sodium current during depolar-
ization and increased and prolonged the tail current
associated with repolarization. The prolongation of the
sodium current was due to the channel remaining open. The
channel returned slowly to the resting state upon repolar-
ization.
Analysis of the dose dependence of the two kinetic
phases of tail current development suggests that the ap-
parent dissociation constant for 1R, trans-tetramethrin
depends on the conformational state of the channel. Thus,
it can be concluded that tetramethrin binds to sodium
channels and modifies the state of the channel in the
resting, open, or inactivated state [28].
1R, trans-Tetramethrin markedly prolongs the open
time of single sodium channels recorded by the gigaohm-
seal voltage clamp technique in a membrane patch excised
from the N1E-115 neuroblastoma cell. Single channel con-
ductance is not altered by tetramethrin. The modification
by tetramethrin occurs in an all-or-nothing manner in a
population of sodium channels. The observed tetramethrin-
induced modification of single sodium channels is compat-
ible with previous sodium current data from axons [78].
Tetramethrin greatly prolongs the sodium current
during step depolarization and the sodium tail current
associated with step repolarization of the squid axon mem-
brane. Non-linear current-voltage relationships for the
sodium tail current were analyzed to assess the open
sodium channel properties, which included the permeation
of various cations, calcium block, and cation selectivity.
Tetramethrin had no effect on any of these properties. It
was concluded that tetramethrin modifies the sodium chan-
nel gating mechanism without affecting the pore properties
[79].
8. EFFECTS ON HUMANS
Although tetramethrin has been used for many years, no
adverse effects and no cases of human poisoning have been
reported in the published literature.
In a semi-closed patch test, an aqueous emulsion con-
taining 1.0% tetramethrin was applied to the skin of 200
human volunteers (aged 15-80, both male and female), using
cotton gauze, for 4 days. After 2 weeks, an additional ap-
plication was made in a same manner. Dermatological exam-
ination showed that tetramethrin is neither a primary
irritant nor a human skin sensitizer [73].
9. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
In the WHO Recommended Classification of Pesticides by
Hazard, technical tetramethrin is classified as unlikely
to present an acute hazard in normal use [75].
REFERENCES
1. ADAMS, M.E. & MILLER, T.A. (1980) Neurophysiological correlation of
pyrethroid and DDT-type insecticide poisoning in the housefly Musca
domestica L. In: Insect neurobiology and pesticide action (Neutox 79),
London, Society of Chemical Industry, pp. 431-439.
2. DING, C., YU, Y., ZHANG, J., CAI, Z., & CHAN, X. (1985) Genotoxic
effects of tetramethrin on cultured mammalian cells and Salmonella
typhimurium. Zhejiang Yike Daxue Xuebao, 14(1): 1-4.
3. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
4. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
5. FAO (1982) Second Government Consultation on International Harmonization
of Pesticide Registration Requirements, Rome, 11-15 October 1982, Rome,
Food and Agriculture Organization of the United Nations.
6. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
7. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
8. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15: 181-
191.
9. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in the mouse
and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
10 GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:(6)
793-799.
11. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980a) Primary eye and skin
irritation tests of Neopynamin Forte, technical in rabbits (Technical
Report No. IT-00-0073) (Submitted to WHO by Sumitomo Chemical Co.).
12. HARA, S., SUZUKI, T., & MIYAMOTO, J. (1980b) Skin sensitization
test of Neopynamin Forte, technical in guinea-pigs (Technical Report No.
IT-00-0082) (Submitted to WHO by Sumitomo Chemical Co.).
13. HARA, M., SUZUKI, H., & MIYAMOTO, J. (1981) In vivo chromosomal
aberration test of Neopynamin Forte, on bone marrow cells of mice (Tech-
nical Report No. 81042) (Submitted to WHO by Sumitomo Chemical Co.).
14. HARTLEY, D., KIDD, H., & KENNEDY, J.M. (1987) Agrochemicals
Handbook, 2nd ed., Nottingham, United Kingdom, Royal Society of
Chemistry.
15. HAYASHI, A. (1977) [How to use insecticides safely.] Bo-satsu-kyo
News, 1: 6-11 (in Japanese).
16. HIROMORI, T., HOSOKAWA, S., OKUNO, Y., SEKI, T., SUZUKI, T.,
& MIYAMOTO, J. (1982) [Mammalian toxicity of d-isomer of phthalthrin
(Neopynamin Forte).] Pharmacometrics, 24: 179-201 (in Japanese).
17. KADOTA, T., KOHDA, H., & MIYAMOTO, J. (1977) Acute oral and
dermal toxicities of Neopynamin in mice and rats (Technical Report No.
IT-70-0003) (Submitted to WHO by Sumitomo Chemical Co.).
18. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Metabolism of
tetramethrin isomers in rats. J. Pestic. Sci., 6: 425-435.
19. KATO, T., UEDA, K., & FUJIMOTO, K. (1964) New insecticidally active
chrysanthemates. Agric. biol. Chem., 28: 914-915.
20. KATO, T., SUZUKI, T., SAKO, H., OKUNO, Y., & MIYAMOTO, J.
(1987) Acute dermal toxicity of Neopynamin in rabbits (Technical
Report No. IT-70-0207) Submitted to WHO by Sumitomo Chemical Co.).
21. KAWAMOTO, M., HARA, M., KOGISO, S., YOSHITAKE, A., &
YAMADA, H. (1988) In vitro unscheduled DNA synthesis (UDS) assay of
Neopynamin in rat hepatocytes (Technical Report No. IT-80-0213)
(Submitted to WHO by Sumitomo Chemical Co.).
22. KISHIDA, F., SUZUKI, H., & MIYAMOTO, J. (1981) Mutagenicity test of
Neopynamin Forte, in bacterial system (Technical Report No. IT-00-0101)
(Submitted to WHO by Sumitomo Chemical Co.).
23. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
24. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore
complex. Science, 221: 1399-1401.
25. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions
of pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
26. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd., p. 440.
27. LUND, A.E. & NARAHASHI, T. (1981) Kinetics of sodium channel
modification by the insecticide tetramethrin in squid axon membranes.
J. Pharmacol. exp. Ther., 219: 464-473.
28. LUND, A.E. & NARAHASHI, T. (1982) Dose-dependent interaction of the
pyrethroid isomers with sodium channels of squid axon membranes.
Neurotoxicology, 3: 11-24.
29. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane effects
of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20: 203-216.
30. MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, p. 502.
31. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J.
(1983) Farm chemicals handbook. Section C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, p. C232.
32. MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington DC, American Chemical Society,
pp. 98-115 (ACS Symposium Series No. 42).
33. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
34. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
35. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry, human
welfare and the environment, Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September
1982, Oxford, Pergamon Press, Vol. 1-4.
36. MIYAMOTO, J., SATO, Y., & YAMAMOTO, K. (1968) Biochemical
studies on the mode of action of pyrethroidal insecticides. Part I.
Metabolic fate of phthalthrin in mammals. Agric. biol. Chem., 32:
628-640.
37. MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem., 36:
2203-2211.
38. OKUNO, Y. (1982) Exposure study with spraying of Neopynamin
aerosol (Technical Report No. IF-20-002) (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
39. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976a) Eye and skin
irritation of Neopynamin in rabbits (Technical Report No. IT-60-0014)
(Submitted to WHO by Sumitomo Chemical Co.).
40. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976b) Skin sensitization
study of Neopynamin with guinea-pigs (Technical Report No. IT-60-0013)
(Submitted to WHO by Sumitomo Chemical Co.).
41. PANSHINA, T.N. & SASINOVICH, L.M. (1983) Toxicology of synthetic
pyrethroids. Khim. Selsk. Khoz. 12: 51-53.
42. PAPADOPOULOU-MOURKIDOU, E., IWATA, Y., & GUNTHER, F.A.
(1981) Utilization of an infrared detector for selective liquid
chromatographic analysis. 2. Formulation analysis of the pyrethroid
insecticides allethrin, decamethrin, cypermethrin, phenothrin, and tetra-
methrin. J. agric. food. Chem., 29(6): 1105-1111.
43. PENCE, D.H., HAGEN, W.H., ALSAKER, R.D., DAWKINS, B.G., MARSHALL,
P.M., & TACEY, R.L. (1981a) Subchronic toxicity study in dogs.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories Inc.
(Technical Report No. IT-11-0098) (Submitted to WHO by Sumitomo Chemical
Co.).
44. PENCE, D.H., SEROTA, D.G., ALSAKER, R.D., BANAS, D.A., &
KUNDZINS, W. (1981b) Chronic toxicity study in rats. Neopynamin
Technical Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-11-0097) (Submitted to WHO by Sumitomo
Chemical Co.).
45. PENCE, D.H., COX, R.H., DUDECK, L.E., ALSAKER, R.D., JONES, S.R.,
PARKER, G.A., HEPNER, K.E., & ZOETIS, T. (1986a) Combined chronic
toxicity and oncogenicity study in mice. Neopynamin Final Report, Vienna,
Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-61-0193)
(Submitted to WHO by Sumitomo Chemical Co.).
46. PENCE, D.H., WOLFE, G.W., KULWICH, B.A., HEPNER, K.E., &
SNYDER, F.G. (1986b) Two-generation reproduction study in rats.
Neopynamin Forte, Final Report, Hazleton Laboratories, Inc. (Technical
Report No. IT-61-0201) (Submitted to WHO by Sumitomo Chemical Co.).
47. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids
on a nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
48. RUTTER, H.A., Jr (1974) One-generation reproduction study in rats.
Neopynamin Final Report, Vienna, Virginia, Hazleton Laboratories, Inc.
(Technical Report No. IT-01-0081) (Submitted to WHO by Sumitomo
Chemical Co.).
49. RUTTER, H.A., Jr, NELSON, L.W., KUNDZINS, W., & MCHUGH (1974)
Two-year dietary administration in the rat. Neopynamin Final Report,
Vienna, Virginia, Hazleton Laboratories, Inc. (Technical Report No. IT-
41-0024) (Submitted to WHO by Sumitomo Chemical Co.).
50. RUZO, L.O., SMITH, I.H., & CASIDA, J.E. (1982) Pyrethroid
photochemistry: photooxidation reactions of the chrysanthemates,
phenothrin and tetramethrin. J. agric. food Chem., 30: 110-115.
51. SATO, T. & NARAMA, K. (1980a) Reproduction test of Neopynamin. Part 1:
Fertility study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0075) (Submitted to WHO by Sumitomo Chemical Co.).
52. SATO, T. & NARAMA, K. (1980b) Reproduction test of Neopynamin. Part 2:
Teratology study in rats, Shizuoka, Hamamatsu Seigiken Research (Technical
Report No. IT-01-0076) (Submitted to WHO by Sumitomo Chemical Co.).
53. SATO, T. & NARAMA, K. (1980c) Reproduction test of Neopynamin. Part 3:
Teratology study in rabbits, Shizuoka, Hamamatsu Seigiken Research (Tech-
nical Report No. IT-01-0077) (Submitted to WHO by Sumitomo Chemical Co.).
54. SATO, T., TAGAWA, G., & NARAMA, K. (1980) Reproduction test of
Neopynamin. Part 4: Perinatal and postnatal study in rats, Shizuoka,
Hamamatsu Seigiken Research (Technical Report No. IT-01-0078) (Submitted
to WHO by Sumitomo Chemical Co.).
55. SMITH, I.H., WOOD, E.J., & CASIDA, J.E. (1982) Glutathione conjugate
of the pyrethroid tetramethrin. J. agric. food Chem., 30: 598-600.
56. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of
Neopynamin with bacterial systems (Technical Report No. IT-70-0023)
(Submitted to WHO by Sumitomo Chemical Co.).
57. SUZUKI, T. & MIYAMOTO, J. (1974) Metabolism of tetramethrin in
houseflies and rats in vitro. Pestic. Biochem. Physiol., 4: 86-97.
58. SUZUKI, T., KOHDA, H., MISAKI, Y., OKUNO, Y., KOYAMA, Y.,
& MIYAMOTO, J. (1980) Acute and subacute inhalation toxicity studies of
Neopynamin Forte in rats (Technical Report No. IT-10-0144) (Submitted to
WHO by Sumitomo Chemical Co.).
59. TANAKA, H., TAKADA, S., ISEKI, M., SANO, R., AIHARA, H.,
KURODA, H., MAEBO, Y., MOTOYOSHI, I., & WAKAI, Y. (1967)
Toxicological studies of various insecticides to mammalia. 2. On the
acute and chronic toxicity of pyrethroid insecticide phthalthrin to
albino rat or guinea-pig. Med. J. Osaka City Univ., 16: 509-517.
60. UEDA, K., GAUGHAN, L.C., & CASIDA, J E. (1975) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food Chem., 23:
106-115.
61. UNO, M., OKADA, T., OHMAE, T., TERADA, I., & TANIGAWA, K.
(1982) [Determination of pyrethroid insecticides by dual-wavelength
densitometry.] Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
62. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
63. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels
in frog myelinated nerve membrane. In: Insect neurobiology and
pesticide action, London, Society of Chemical Industry, pp. 79-85.
64. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M. (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
65. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and phermones,
New York, London, Plenum Press, pp. 183-210.
66. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
67. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
68. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
69. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
70. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN,
J. (1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in myelinated nerves. Nature (Lond.), 295: 601-603.
71. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M.,
& VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
72. WEIR, R.J. & CREWS, L.M. (1966) Three month dietary administration
to dogs of Neopynamin - final report (Technical Report No. IT-61-0011)
(Submitted to WHO by Sumitomo Chemical Co.).
73. WEIR, R.J. & OSBOURN, R.A. (1966) Report of human patch test on
coded substance : 2301 D-61-1, Vienna, Virginia, Hazleton
Laboratories, Inc. (Technical Report No. IT-61-0008) (Submitted to
WHO by Sumitomo Chemical Co.).
74. WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
75. WHO (1988) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1988/89, Geneva, World
Health Organization (Unpublished report VBC/88.953).
76. WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 520.
77. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids.
Gen. Pharmacol., 9: 387-398.
78. YAMAMOTO, D., QUANDT, F.N., & NARAHASHI, T. (1983)
Modification of single sodium channels by the insecticide
tetramethrin. Brain Res. 274: 344-349.
79. YAMAMOTO, D., YEH, J.Z., & NARAHASHI, T. (1986) Ion permeation
and selectivity of squid axon sodium channels modified by
tetramethrin. Brain Res., 372: 193-197.
80. YOSHITAKE, A., KOGISO, S., HARA, M., & MIYAMOTO, J. (1986)
In vivo chromosomal aberration test of Neopynamin in mouse bone
marrow cells (Technical Report No. IT-60-0197) (Submitted to WHO
by Sumitomo Chemical Co.).
81. YOSHITAKE, A., KOGISO, S., YAMADA, F., HARA, M., & MIYAMOTO,
J. (1987) Reverse mutation test of Neopynamin in Salmonella
typhimurium and Escherichia coli (Technical Report No. IT-70-0205)
(Submitted to WHO by Sumitomo Chemical Co.).
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs ( Xenopus laevis, Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [10, 23, 65,
66, 74]. The same distinction was found in studies on
cockroaches [8].
Based on the binding assay on the alpha-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types: the
alpha-cyano-3-phenoxybenzyl pyrethroids and the non-cyano
pyrethroids [7, 9, 24, 25].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin, cisme-
thrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [64]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [62]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [71].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [6].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [63]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [71].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans [4],
are almost identical to those of allethrin, i.e., presyn-
aptic repetitive firing, and no significant effects on
transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [64, 65, 70].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [29].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [47].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cyhalothrin, lambda-cyhalo-
thrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [69]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [67,
71].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during exci-
tation, presumably by slowing down the closing of the
activation gate of the sodium channel [67, 71]. The time
constant of closing of the activation gate in the cyper-
methrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged
sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to
cause the long-lasting repetitive firing in the lateral-
line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [29].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [9].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the toxicity
of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand,
[35S]- t-butylbicyclophosphorothionate [35S]-TBPS. Studies
with 37 pyrethroids revealed an absolute correlation,
without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic
cyano compounds including deltamethrin, 1R, cis-cyper-
methrin, 1R, trans-cypermethrin, and [2S, alphaS]-fenvalerate
were inhibitors, but their non-toxic stereoisomers were
not; non-cyano pyrethroids were much less potent or were
inactive [24].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [25].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S,alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex [7].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog ( Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [47].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-lasting
prolongation of the transient increase in sodium per-
meability of the nerve membrane during excitation. This
results in long-lasting trains of repetitive impulses in
sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [74, 77].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [68].
1. RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1.1 Résumé et évaluation
1.1.1 Identité, propriétés physiques et chimiques, méthodes d'analyse
La tétraméthrine a été synthétisée pour la première
fois en 1964 et commercialisée et en 1965. Sur la plan
chimique, c'est un ester de l'acide chrysanthémique (acide
diméthyl-2,2(diméthyl-2,2 vinyl)-3 cyclopropanecarboxylique
et de l'alcool tétrahydro-3,4,5,6 phatlimidométhylique.
Elle est constituée d'un mélange de quatre stéréoisomères
[1R,trans], [1R,cis], [1S,trans] et [1S,cis]. Les stéréo-
isomères qui entrent dans la composition des produits
techniques sont à peu près dans la proportion de 4:1:4:1.
De tous les isomères, c'est l'isomère [1R,trans] qui est
le plus actif biologiquement; vient ensuite l'isomère
1R,cis]. On commercialise sous le nom de "Neo-pynamine
Forte" (désignée dans la présente monographie sous le nom
de 1R, cis/trans-tetraméthrine), un mélange des isomères
[1R,cis] et [1R,trans] dans le proportion de 1:4.
La tétraméthrine de qualité technique est un solide
incolore dont le point de fusion est de 60-80 °C. Sa
densité est de 1,11 à 20 °C et sa tension de vapeur de
0,946 mPa (7,1 x 10-6 mm Hg) à 30 °C. Peu soluble dans
l'eau (4,6 mg/litre à 30 °C), elle est en revanche soluble
dans certains solvants organiques tels que l'hexane, le
méthanol et le xylène. Elle est stable à la chaleur mais
instable à la lumière et à l'air. L'isomère [1R,cis/trans]
est un liquide visqueux de couleur jaune dont les autres
propriétés physiques et les propriétés chimiques sont les
mêmes que celles de la tétraméthrine.
Le dosage des résidus s'effectue par densitométrie à
deux longueurs d'onde (370-230 nm). Pour l'analyse des
produits techniques, on utilise la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
Une analyse des différentes formulations peut s'effectuer
au moyen d'un chromatographe en phase liquide à haute
performance muni d'un détecteur infrarouge.
1.1.2 Production et usage
La production mondiale annuelle de tétraméthrine est
évaluée à quelques centaines de tonnes. Elle est princi-
palement utilisée pour la lutte contre les nuisibles à
l'intérieur des habitations, sous forme d'aérosols, de
concentrés émulsionnables ou de serpentins anti-moust-
iques. La tétraméthrine entre également dans la compos-
ition d'autres formulations insecticides additionnées ou
non de synergisants.
1.1.3 Exposition humaine
L'exposition de la population dans son ensemble peut
résulter de l'utilisation de ce produit pour la destruc-
tion des nuisibles dans les habitations. Lorsqu'on utilise
la tétraméthrine conformément aux recommandations, sa con-
centration atmosphérique ainsi que celle de l'isomère 1R
ne devraient pas dépasser 0,5 mg/m3; par ailleurs le
composé se dégrade rapidement. L'exposition de la popu-
lation générale est donc vraisemblablement très faible. On
n'utilise pas de tétraméthrine pour traiter les cultures
vivrières.
1.1.4 Exposition et destinée dans l'environnement
Une fine pellicule de tétraméthrine exposée à la
lumière solaire se dégrade rapidement. On a observé que
les principales réactions photochimiques qui se produis-
aient au cours d'une exposition de 2 heures (conversion de
30%) étaient: une époxydation au niveau de la double
liaison du radical isobutényle, une oxydation en hydroxy-
méthyle, en aldéhyde et en acide carboxylique du groupe
méthyle en position trans du groupement isobutényle;
enfin, une hydroperoxydation en hydroperoxyde allylique.
On ne connaît pas avec exactitude les concentrations
exactes de tétraméthrine dans l'environnement, mais compte
tenu de l'utilisation qui en est faite actuellement pour
traiter les habitations et pourvu que le produit soit
utilisé conformément aux recommandations, il est vraisem-
blable que l'exposition dans l'environnement devrait être
très faible. La tétraméthrine se décompose rapidement en
produits moins toxiques.
1.1.5 Absorption, métabolisme, et excrétion
Des rats à qui l'on avait administré par voie orale ou
sous-cutanée de la tétraméthrine radio-marquée au niveau
du reste acide ou du reste alcool ont rapidement absorbé,
métabolisé et excrété le produit. L'excrétion s'effectue
en 5 à 7 jours dans la proportion d'environ 95%, à peu
près autant par la voie urinaire que par la voie fécale.
Par ces deux voies, les résidus présents dans les tissus
sont très faibles. La métabolisation s'effectue par les
réactions suivantes: coupure de l'ester; élimination du
groupe hydroxyméthyl du reste alcool; réduction de la
double liaison 1-2 du reste alcool; oxydation du groupe-
ment méthyle de l'isobutényle au niveau du reste acide et
en 2, 3 et 4 au niveau du reste alcool; conjugaison des
acides et des alcools résultant avec l'acide glucuronique
et enfin isomérisation cis/trans.
1.1.6 Effets sur les êtres vivant dans leur milieu naturel
On ne dispose que de très peu d'informations à ce
sujet. La tétramethrine est extrêmement toxique pour les
poissons, la valeur de la CL50 à 96-h pour deux espèces
se situant respectivement à 19 et 21 µg/litre. Pour une
troisième espèce, on a obtenu une CL50 à 48 heures de
200 µg/litre et la dose sans effet observable était de
50 µg/litre. Pour la daphnie, la dose sans effet
observable est également de 50 µg/litre. La tétraméthrine
est en revanche très peu toxique pour les oiseaux mais
elle est toxique pour les abeilles. Cependant du fait que
le produit est rapidement dégradé et dans la mesure où on
ne l'utilise, conformément aux recommandations, que dans
les habitations, il est peu probable qu'il puisse excercer
des effets nocifs sur l'environnement.
1.1.7 Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in-vitro
La tétraméthrine a une faible toxicité aiguë par voie
orale. La DL50 pour le rat est >5000 mg/kg, qu'il
s'agisse du racémique ou de l'isomère (1R,cis/trans),
tandis que pour la souris elle est d'environ 2000 mg/kg
(racémique) et de 1060 mg/kg (1R,cis/trans). Chez le rat,
la souris et le lapin la toxicité aiguë par voie percu-
tanée e st également faible; la DL50 chez le rat et la
souris étant <5000 mg/kg et <2000 mg/kg chez le lapin
(toutes les études portaient sur le racémique). Les études
de toxicité aiguë par inhalation ont donné une CL50 chez
le rat et la souris de 2500 mg/m3 pour le racémique et
>1180 mg/m3 pour l'isomère (1R,cis/trans). Parmi les
signes d'intoxication on a noté une hyperexcitabilité, des
tremblements, de l'ataxie et une dépression (signes
généraux observés dans l'ensemble des études de toxicité
aiguë). Les souris se sont révélées un peu plus sensibles
que les rats mais il n'y avait pas de différences de
sensibilité entre mâles et femelles. Qu'ils s'agisse du
racémique ou de l'isomère (1R,cis/trans), la tétraméthrine
ne provoque pratiquement aucune irritation oculaire ou
cutanée chez le lapin. En outre, ni l'un ni l'autre de
ces produits n'exercent d'effet sensibilisateur chez le
cobaye.
La tétraméthrine est un pyréthroïde du type I. Chez
les mammifères ce sont les tremblements (syndrome-T) qui
constituent le symptôme d'intoxication caractéristique.
Chez des rats ayant reçu de la tétraméthrine mêlée à
leur nourriture à des concentrations allant jusqu'à 5000
mg/kg de nourriture pendant 91 jours, on a noté une réduc-
tion du gain de poids à la dose la plus forte. D'après les
résultats d'études de 3 et 6 mois, au cours desquelles des
rats ont reçu l'isomère 1R(cis/trans) dans leur nourriture
à des doses allant de 25 mg/kg à 3000 mg/kg d'aliment, la
dose sans effet observable était de 200 mg/kg de nourri-
ture pour les mâles et de 300 mg/kg pour les femelles
(parmi les anomalies observées, on notait une réduction du
gain de poids et du poids final du corps ainsi que
certains effets sur les reins et le foie). Les effets sur
le foie résultent, semble-t-il, d'une réaction d'adapt-
ation à la présence dans l'alimentation du véhicule
utilisé, à savoir l'huile de maïs.
Une étude de 26 semaines sur des chiens a fait
ressortir une dose sans effet observable de 1250 mg/kg de
nourriture.
Des souris et des rats à qui l'on avait fait inhaler
de la tétraméthrine en aérosol à une concentration de 200
mg/m3, 3 à 4 heures par jour pendant des périodes allant
jusqu'à quatre semaines, n'ont présenté aucune anomalie
imputable à ce produit. Lors d'une autre étude de ce
type, au cours de laquelle des rats ont été exposés à une
brumisation (gouttelettes de 1,2-1,5 µm de diamètre)
d'isomère (1R,cis/trans) dans du kérosène désodorisé à des
concentrations allant jusqu'à 87 mg/m3, trois heures par
jour et sept jours par semaine pendant 28 jours, on a
obtenu, pour la dose sans effet observable, une valeur de
49 mg/m3. Les signes d'intoxication n'ont été observés
qu'au cours de l'exposition.
Ni la tétraméthrine ni ses isomères (1R,cis/trans) ne
se sont révélés mutagènes dans divers systèmes d'épreuve
in vivo et in vitro utilisés pour étudier les mutations
génétiques, les lésions et les réparations de l'ADN ainsi
que les effets sur les chromosomes.
Trois études, dont deux chez le rat et une chez la
souris ont été menées pendant 104 semaines afin d'étudier
la cancérogénicité à long terme de la tétraméthrine. Les
souris ont reçu de la tétraméthrine dans leur nourriture à
des doses allant jusqu'à 1500 mg/kg de nourriture. Aucun
effet oncogène n'a été observé. A partir de 60 mg/kg de
nourriture on observait une réduction du poids de
l'hypophyse, de la thyroïde et de la parathyroïde. Chez
la souris, la dose sans effet général observable se
situait à 12 mg/kg de nourriture. Quant aux rats, ils ont
été exposés à de la tétraméthrine à des doses allant
jusqu'à 5000 mg/kg de nourriture soit in utero soit au
cours d'une période prolongée. Les deux études ont fait
ressortir un gain de poids sensiblement moindre chez les
animaux recevant 3000 mg de tétraméthrine par kg de
nourriture ou davantage. En outre, à ces concentrations,
on a observé une augmentation du poids du foie. Pour ce
qui est des effets généraux, la dose sans effet observ-
able se situait dans les deux études, à 1000 mg/kg de
nourriture. A la dose de 3000 mg/kg de nourriture,
l'incidence des tumeurs testiculaires à cellules de Leydig
était supérieure à la valeur notée dans le groupe témoin
et ce, pour les deux études. Les tumeurs à cellules de
Leydig se produisent spontanément chez les rats âgés et
leur incidence peut varier énormément dans les groupes
témoins. On pense que cette tumeur est d'origine hormon-
ale. Aucun signe de malignité et aucune tumeur de ce type
n'ont été relevés chez les souris. On peut en conclure
que cet effet oncogène, s'il existe réellement, ne peut
être pris en considération pour ce qui concerne l'homme.
La tétraméthrine ne s'est révélée ni tératogène ni
embryotoxique à des doses allant jusqu'à 1000 mg par kg de
poids corporel chez les rats et jusqu'à 500 mg/kg chez des
lapins (il s'agit des concentrations les plus fortes
étudiées). Lors d'une étude de fécondité au cours de
laquelle des rats ont reçu de la tétraméthrine à des doses
allant jusqu'à 1000 mg/kg de poids corporel par jour, la
dose sans effet observable sur la reproduction des parents
et la croissance des foetus, se situait à 300 mg/kg de
poids corporel par jour. Une étude de reproduction chez
le rat, portant sur la période périnatale et sur la
période post-natale, a permis de fixer à 100 mg/kg de
poids corporel la dose quotidienne sans effet observable
(la dose la plus forte administrée au cours de cette
étude).
Lors d'une étude de reproduction portant sur une
génération de rats, on a administré aux animaux 1000-6000
mg de tétraméthrine par kg de nourriture et constaté que
la dose sans effet observable était de 1000 mg/kg. Selon
une autre étude portant cette fois sur deux générations,
au cours de laquelle les rats ont reçu de l'isomère
(1R,cis/trans) à des doses allant de 100 à 3000 mg/kg, la
dose sans effet observable était de 500 mg/kg de
nourriture.
1.1.8 Effets sur l'homme
Bien que la tétraméthrine et son isomère 1R soient
utilisées depuis des années, on ne signale aucun cas
d'intoxication ou d'effets indésirables chez l'homme.
Rien n'indique que la tétraméthrine ou son isomère 1R
puissent avoir des effets nocifs sur l'homme si on
continue de les utiliser à faibles concentrations et
seulement pour la destruction des nuisibles à l'intérieur
des habitations.
1.2 Conclusions
a) Population générale: l'exposition de la population
générale à la tétraméthrine, dans son utilisation actu-
elle, est vraisemblablement faible. Si ce produit est
utilisé conformément aux recommandations, il ne présente
probablement aucun risque.
b) L'exposition professionnelle: moyennant de bonnes
méthodes de travail, l'application de mesures d'hygiène et
avec quelques précautions, la tétraméthrine ne devrait pas
présenter de danger pour les personnes qui y sont exposées
de par leur profession.
c) Environnement: il est tout à fait improbable que la
tétraméthrine ou ses produits de décomposition s'accum-
ulent au point d'avoir des effets nocifs sur l'environne-
ment.
1.3 Recommandations
Bien que la tétraméthrine et son isomère 1R soient
utilisés depuis des années sans qu'on ait à déplorer
d'effets nocifs chez l'homme, il est souhaitable que
l'exposition humaine continue d'être surveillée.
