
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 95
FENVALERATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Fenvalerate.
(Environmental health criteria ; 95)
1.Pyrethrins I.Series
ISBN 92 4 154295 0 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR FENVALERATE
INTRODUCTION
1. SUMMARY, EVALUATIONS, CONCLUSIONS, AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties,
analytical methods
1.1.2. Production and use
1.1.3. Human exposure
1.1.4. Environmental fate
1.1.5. Kinetics and metabolism
1.1.6. Effects on organisms in the environment
1.1.7. Effects on experimental animals and in vitro test systems
1.1.8. Effects on human beings
1.2. Conclusions
1.2.1. General population
1.2.2. Occupational exposure
1.2.3. Environment
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF ENVIRONMENTAL POLLUTION AND ENVIRONMENTAL LEVELS
3.1. Industrial production
3.2. Use patterns
3.3. Residues in food
3.4. Residues in the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Photodecomposition
4.3. Decomposition in plants
4.4. Decomposition in soils
4.5. Decomposition in water
5. KINETICS AND METABOLISM
5.1. Metabolism in mammals
5.1.1. Rat
5.1.2. Mouse
5.1.3. Domestic animals
5.2. Enzymatic systems for biotransformation
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Toxicity to aquatic invertebrates
6.1.2. Toxicity to fish
6.1.3. Field studies and community effects
6.2. Terrestrial organisms
6.2.1. Toxicity to soil microorganisms
6.2.2. Toxicity to beneficial insects
6.2.3. Toxicity to birds
6.3. Uptake, loss, and bioaccumulation
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.2. Short-term exposures
7.2.1. Oral administration
7.2.2. Inhalation
7.2.3. Dermal application
7.3. Skin and eye irritation; sensitization
7.3.1. Skin and eye irritation
7.3.2. Skin sensitization
7.4. Long-term exposures and carcinogenicity
7.4.1. Mouse
7.4.2. Rat
7.5. Mutagenicity
7.5.1. Microorganisms and insects
7.5.2. Rat
7.5.3. Mouse
7.5.4. Hamster
7.6. Teratogenicity and reproduction studies
7.6.1. Teratogenicity
7.6.2. Reproduction studies
7.7. Neurotoxicity
7.8. Behavioural studies
7.9. Miscellaneous studies
7.10. Mechanism of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. Occupational exposure
8.2. Clinical studies
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX I
FRENCH TRANSLATION OF SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FENVALERATE
Members
Dr V. Benes, Toxicology and Reference Laboratory,
Institute of Hygiene and Epidemiology, Prague,
Czechoslovakia
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
Dr Y. Hayashi, Division of Pathology, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr S. Johnson, Hazard Evaluation Division, Office of
Pesticide Programme, US Environmental Protection
Agency, Washington DC, USA (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health
(I.C.M.R.) Ahmedabad, India (Vice Chairman)
Dr Yu. I. Kundiev, Research Institute of Labour, Hygiene,
and Occupational Diseases, Kiev, USSR
Dr J.P. Leahey, ICI Agrochemicals, Jealotts Hill Research
Station, Bracknell, United Kingdom (Rapporteur)
Dr J. Miyamoto, Takarazuka Research Centre, Sumitomo
Chemical Company, Takarazuka, Hyogo, Japan
Dr J. Sekizawa, Section of Information and Investigation,
Division of Information on Chemical Safety, National
Institute of Hygienic Sciences, Tokyo, Japan
(Rapporteur)
Dr Y. Takenaka, Division of Information on Chemical
Safety, Tokyo, Japan
Representatives
Dr M. Ikeda, International Commission on Occupational
Health, Department of Environmental Health, Tohoku
University School of Medicine, Sendai, Japan
Dr H. Naito, World Federation of Poison Control Centres
and Clinical Toxicology, Institute of Clinical Medi-
cine, University of Tsukuba, Tsukuba-Shi, Ibaraki,
Japan
Dr N. Punja, Groupement International des Associations
Nationales de Fabricants de Produits Agrochimiques
(GIFAP), ICI Plant Protection Division, Fenhurst,
Haslemere, United Kingdom
Observers
Dr M. Matsuo, Sumitomo Chemical Company, Biochemistry &
Toxicology Laboratory, Osaka, Japan
Dr Y. Okuno, Sumitomo Chemical Company, Biochemistry &
Toxicology Laboratory, Osaka, Japan
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
(Secretary)
Dr R. Plestina, Division of Vector Control, Delivery and
Management of Vector Control, World Health Organiz-
ation Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria documents,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be
obtained from the International Register of Potentially
Toxic Chemicals, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 7988400 - 7985850).
* * *
The proprietary information contained in this document
cannot replace documentation for registration purposes,
because the latter has to be closely linked to the source,
the manufacturing route, and the purity/impurities of the
substance to be registered. The data should be used in
accordance with paragraph 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation
[39].
ENVIRONMENTAL HEALTH CRITERIA FOR FENVALERATE
A WHO Task Group on Environmental Health Criteria for
Fenvalerate, Permethrin, and d-Phenothrin met in Tokyo
from 4 to 8 July 1988. This meeting was convened with the
financial assistance of the Ministry of Health and
Welfare, Tokyo, Japan, and was hosted by the National
Institute of Hygienic Sciences (NIHS) in Tokyo.
Dr T. Furukawa and Dr K. Shirota opened the meeting on
behalf of the Ministry of Health and Welfare, and Dr A.
Tanimura, Director-General of the NIHS welcomed the par-
ticipants to the institute. Dr M. Mercier, Manager of the
IPCS, welcomed the participants on behalf of the three
IPCS cooperating organizations (UNEP/ILO/WHO). The group
reviewed and revised the draft monograph and made an
evaluation of the risks for human health and the environ-
ment from exposure to fenvalerate.
The first draft of this document was prepared by
Dr J. MIYAMOTO and Dr M. MATSUO of the Sumitomo
Chemical Company, Japan, with the assistance of the staff
of the National Institute of Hygienic Sciences, Tokyo,
Japan. Dr I. Yamamoto of the Tokyo University of Agricul-
ture and Dr M. Eto of Kyushu University, Japan, assisted
with the finalization of the draft.
The second draft was prepared by Dr J. SEKIZAWA, NIHS,
Tokyo, incorporating comments received following circu-
lation of the first draft to the IPCS contact points for
Environmental Health Criteria documents. Dr K.W. Jager and
Dr P.G. Jenkins, both members of the IPCS Central Unit,
were responsible for the technical development and
editing, respectively, of this monograph.
The assistance of the Sumitomo Chemical Company in
making available to the IPCS and the Task Group its toxi-
cological proprietary information on fenvalerate is grate-
fully acknowledged. This allowed the Task Group to make
its evaluation on the basis of more complete data.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
ABBREVIATIONS
ai active ingredient
Cl-Vacid (= CPIA) 2-(4-chlorophenyl)isovaleric acid
ECD-GC gas chromatography with electron capture detector
FID-GC gas chromatography with flame ionization detector
GLC gas-liquid chromatography
HPLC high-performance liquid chromatography
NOEL no-observed-effect level
PBacid 3-phenoxybenzoic acid
PBalc 3-phenoxybenzyl alcohol
PBald 3-phenoxybenzaldehyde
PCB polychlorinated biphenyl
TOCP tri- ortho- cresyl phosphate
INTRODUCTION
SYNTHETIC PYRETHROIDS - A PROFILE
1. During investigations to modify the chemical struc-
tures of natural pyrethrins, a certain number of syn-
thetic pyrethroids were produced with improved physi-
cal and chemical properties and greater biological
activity. Several of the earlier synthetic pyrethroids
were successfully commercialized, mainly for the con-
trol of household insects. Other more recent
pyrethroids have been introduced as agricultural in-
secticides because of their excellent activity against
a wide range of insect pests and their non-persistence
in the environment.
2. The pyrethroids constitute another group of insecti-
cides in addition to organochlorine, organophosphorus,
carbamate, and other compounds. Pyrethroids commer-
cially available, to date include allethrin, res-
methrin, d-phenothrin, and tetramethrin (for insects
of public health importance), and cypermethrin, delta-
methrin, fenvalerate, and permethrin (mainly for agri-
cultural insects). Other pyrethroids are also avail-
able, including furamethrin, kadethrin, and tellalle-
thrin (usually for household insects), fenpropathrin,
tralomethrin, cyhalothrin, lambda-cyhalothrin, teflu-
thrin, cufluthrin, flucythrinate, fluvalinate, and
biphenate (for agricultural insects).
3. Toxicological evaluations of several synthetic
pyrethroids have been performed by the FAO/WHO Joint
Meeting on Pesticide Residues (JMPR). The acceptable
daily intake (ADI) has been estimated by the JMPR for
cypermethrin, deltamethrin, fenvalerate, permethrin,
d-phenothrin, cyfluthrin, cyhalothrin, and flucythri-
nate.
4. Chemically, synthetic pyrethroids are esters of spe-
cific acids (e.g., chrysanthemic acid, halo-substi-
tuted chrysanthemic acid, 2-(4-chlorophenyl)-3-methyl-
butyric acid) and alcohols (e.g., allethrolone, 3-
phenoxybenzyl alcohol). For certain pyrethroids, the
asymmetric centre(s) exist in the acid and/or alcohol
moiety, and the commercial products sometimes consist
of a mixture of both optical (IR/1S or d/1) and geo-
metric ( cis/trans ) isomers. However, most of the
insecticidal activity of such products may reside in
only one or two isomers. Some of the products (e.g.,
d-phenothrin, deltamethrin) consist only of such
active isomer(s).
5. Synthetic pyrethroids are neuropoisons acting on the
axons in the peripheral and central nervous systems by
interacting with sodium channels in mammals and/or
insects. A single dose produces toxic signs in mam-
mals, such as tremors, hyperexcitability, salivation,
choreoathetosis, and paralysis. The signs disappear
fairly rapidly, and the animals recover, generally
within a week. At near-lethal dose levels, synthetic
pyrethroids cause transient changes in the nervous
system, such as axonal swelling and/or breaks and
myelin degeneration in sciatic nerves. They are not
considered to cause delayed neurotoxicity of the kind
induced by some organophosphorus compounds. The mech-
anism of toxicity of synthetic pyrethroids and their
classification into two types are discussed in the
Appendix.
6. Some pyrethroids (e.g., deltamethrin, fenvalerate,
flucythrinate, and cypermethrin) may cause a transient
itching and/or burning sensation in exposed human
skin.
7. Synthetic pyrethroids are generally metabolized in
mammals through ester hydrolysis, oxidation, and con-
jugation, and there is no tendency to accumulate in
tissues. In the environment, synthetic pyrethroids are
fairly rapidly degraded in soil and in plants. Ester
hydrolysis and oxidation at various sites on the mol-
ecule are the major degradation processes. The
pyrethroids are strongly adsorbed on soil and sedi-
ments, and hardly eluted with water. There is little
tendency for bioaccumulation in organisms.
8. Because of low application rates and rapid degradation
in the environment, residues in food are generally
low.
9. Synthetic pyrethroids have been shown to be toxic for
fish, aquatic arthropods, and honey bees in laboratory
tests. But, in practical usage, no serious adverse
effects have been noticed because of the low rates of
application and lack of persistence in the environ-
ment. The toxicity of synthetic pyrethroids in birds
and domestic animals is low.
10. In addition to the evaluation documents of FAO/WHO,
there are several good reviews and books on the chem-
istry, metabolism, mammalian toxicity, environmental
effects, etc., of synthetic pyrethroids, including
those by Elliott [36], Miyamoto [126], Miyamoto &
Kearney [127], and Leahey [101].
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and Evaluation
1.1.1 Identity, physical and chemical properties,
analytical methods
Fenvalerate is a potent insecticide that has been in
use since 1976. It is an ester of 2-(4-chlorophenyl)-3-
methylbutyric acid and alpha-cyano-3-phenoxybenzyl alcohol,
but lacks a cyclopropane ring. However, in terms of its
insecticidal behaviour, it belongs to the pyrethroid
insecticides. It is a racemic mixture of four optical
isomers with the configurations [2S, alphaS], [2S, alphaR],
[2R, alphaS], and [2R, alphaR]. The [2S, alphaS] isomer is
the most biologically active, followed by the [2S, alphaR]
isomer.
Technical grade fenvalerate is a yellow or brown vis-
cous liquid having a specific gravity of 1.175 at 25 °C.
The vapour pressure is 0.037 mPa at 25 °C and it is rela-
tively non-volatile. It is practically insoluble in water
(approximately 2 µg/litre), but soluble in organic sol-
vents such as acetone, xylene, and kerosene. It is stable
to light, heat, and moisture, but unstable in alkaline
media due to hydrolysis of the ester linkage.
Residue and environmental analysis can be carried out
using a gas chromatograph equipped with an electron cap-
ture detector, the minimum detectable concentration being
0.005 mg/kg. A gas chromatograph with a flame ionization
detector is used for product analysis.
1.1.2 Production and use
Approximately 1000 tonnes per year of fenvalerate are
used worldwide (1979-1983 figures). It is mostly employed
in agriculture but also for insect control in homes and
gardens and on cattle, alone or in combination with other
insecticides. It is formulated as emulsifiable concen-
trate, ultra-low-volume concentrate, dust, and wettable
powder.
1.1.3 Human exposure
Exposure of the general population to fenvalerate is
mainly via dietary residues. Residue levels in crops grown
by good agricultural practice are generally low. The
resulting exposure of the general population is expected
to be very low, but data from total-diet studies are
lacking.
Analysis of residues in stored grain showed that over
70% of the applied dose remained on wheat after 10 months
at 25 °C. Following milling and baking, white bread has
about the same residue level as white flour (approximately
0.06-0.1 mg/kg).
Information on occupational exposure to fenvalerate is
very limited.
1.1.4 Environmental fate
In soil, degradation occurs via ester cleavage,
diphenyl ether cleavage, ring hydroxylation, hydration of
the cyano group to amide, and further oxidation of the
fragments formed to yield carbon dioxide as a major final
product. Studies to investigate the leaching potential of
fenvalerate and its degradation products showed that very
little downward movement will occur in soils.
In water and on soil surfaces, fenvalerate is photo-
degraded by sunlight. Ester cleavage, hydrolysis of the
cyano group, decarboxylation to yield 2-(3-phenoxyphenyl)-
3-(4-chlorophenyl)-4-methylpentane-nitrile (decarboxy-
fenvalerate), and other radical-initiated reactions have
been shown to occur.
On plants, fenvalerate has a half-life of approximate-
ly 14 days. Ester cleavage is a major reaction, followed
by oxidation and/or conjugation of the fragments formed.
Decarboxylation to yield decarboxy-fenvalerate also oc-
curs.
In general, the degradative processes which occur in
the environment lead to less toxic products.
The degradation of fenvalerate in the environment is
rather rapid. Half-lives are 4-15 days in river water,
8-14 days on plants, 1-18 days by photodegradation on soil
and 15 days-3 months in soil.
There is virtually no leaching of fenvalerate in soil.
Thus, it is unlikely that the compound will attain signif-
icant levels in the aquatic environment.
1.1.5 Kinetics and metabolism
The fate of fenvalerate in rats and mice has been
studied using fenvalerate radiolabelled in the acid moiety
or benzyl or cyano groups. The administered radioac-
tivity, except that of the cyano-labelled compounds, is
readily excreted (up to 99% in 6 days). The major meta-
bolic reactions are ester cleavage and hydroxylation at
the 4'position. Various oxidative and conjugation
reactions that lead to a complex mixture of products have
been shown to occur. When studies were carried out with
fenvalerate radiolabelled in the cyano group, elimination
of the radioactive dose was less rapid (up to 81% in 6
days). The remaining radioactivity was retained mainly in
the skin, hair, and stomach as thiocyanate. A minor, but
very important, metabolic pathway is the formation of a
lipophilic conjugate of [2R]-2-(4-chlorophenyl)isovalerate.
This conjugate, which is implicated in the formation of
granuloma, has been detected in the adrenals, liver and
mesenteric lymph nodes of rats, mice, and some other
species.
1.1.6 Effects on organisms in the environment
In laboratory tests, fenvalerate is highly toxic
for aquatic organisms. The LC50 values range from
0.008 µg/litre for newly hatched mysid shrimps to
2 µg/litre for a stonefly. The no-observed-effect level
in life-cycle tests using Daphnia galeata mendotae is less
than 0.005 µg/litre. Fenvalerate is also highly toxic
for fish. The 96-h LC50 values range from 0.3 µg/litre
for larval grunion to 200 µg/litre for adult Tilapia. The
no-observed-effect level, over 28 days, for early-life
stages of the sheepshead minnow is 0.56 µg/litre. Fenval-
erate is less toxic for aquatic algae and molluscs, with
96-h LC50 values > 1000 µg/litre.
In field tests and in the use of the compound under
practical conditions, the potentially high toxicity to
aquatic organisms is not manifested. Some aquatic
invertebrates are killed when water is oversprayed, but
the effect on populations is temporary. There have been
no reports of fish kills. This reduced toxicity in field
use is related to the strong adsorption of the compound to
sediments.
Fenvalerate is highly toxic to honey bees. The topical
LD50 is 0.41 µg/bee, but there is a strong repellent
effect of fenvalerate to bees, which reduces the effect in
practice. There is no evidence of significant kills of
honey bees under normal use. Fenvalerate is more toxic to
predator mites than to the target pest species.
Fenvalerate has very low toxicity to birds when given
orally or applied to the diet. LD50 values are > 1500
mg/kg body weight for acute oral dosage and an LD50 value
for dietary exposure of Bobwhite quail has been reported
at > 15 000 mg/kg diet.
Fenvalerate is readily taken up by aquatic organisms.
Bioconcentration factors ranged from 120 to 4700 for vari-
ous organisms (algae, snail, Daphnia and fish) in model
ecosystem studies. The fenvalerate taken up by aquatic
organisms is rapidly lost on transfer to clean water. The
compound can, therefore, be regarded as having no tendency
to bioaccumulate in practice.
1.1.7 Effects on experimental animals and in vitro test systems
Fenvalerate has moderate to low acute oral toxicity.
However, LD50 values differ considerably (82 to > 3200
mg/kg) according to animal species and vehicle of adminis-
tration. The acute clinical signs of poisoning appear
rapidly but survivors become asymptomatic within 3-4 days.
The toxic signs of the racemic mixture, as well as of its
[2S, alphaS] isomer, include restlessness, tremors, pilo-
erection, diarrhoea, abnormal gait, choreo-athetosis, and
salivation (CS-syndrome); it is classified as a Type II
pyrethroid. Electrophysiologically it produces bursts of
spikes in the cercal motor nerve of the cockroach. There
is, however, no clear-cut link between electro-physiologi-
cal findings in insects and toxicity to mammals.
Rats fed fenvalerate at 2000 mg/kg diet for 8-10 days
showed typical signs of acute intoxication. Reversible
morphological changes in the sciatic nerve were observed
in rats administered fenvalerate at 3000 mg/kg diet.
Histopathological changes in sciatic nerves were also
observed in rats and mice treated with a single oral does
of fenvalerate at lethal or sublethal levels.
Hens administered fenvalerate orally at 1000 mg/kg per
day for 5 days did not show any clinical or morphological
signs of delayed neurotoxicity.
The acute intraperitoneal toxicity of fenvalerate
metabolites in mice was no greater than that of
fenvalerate itself.
In subacute and subchronic toxicity studies, mice,
rats, dogs, and rabbits were treated with fenvalerate by
oral, dermal, and inhalational routes for 3 weeks to 6
months. In 4-week mouse and rat inhalation studies, a no-
observed-effect level (NOEL) of 7 mg/m3 was established
in both species. The NOEL in a 90-day rat study was
125 mg/kg diet, in a 2-year feeding study it was 250 mg/kg
diet (12.5 mg/kg body weight), and in a 24-28 month study
it was 150 mg/kg diet, (7.5 mg/kg body weight). The NOEL
in a 2-year mouse study was 50 mg/kg diet, corresponding
to 6.0 mg/kg body weight, and 30 mg/kg diet, corresponding
to 3.5 mg/kg body weight, in a 20-month feeding study.
For dogs the NOEL was 12.5 mg/kg body weight in a 90-day
feeding study. Some fenvalerate formulations have caused
skin and eye irritation. However, technical fenvalerate
is non-irritant and has no sensitizing effects.
In long-term toxicity studies, microgranulomatous
changes were observed in mice, specifically when treated
with the [2R, alphaS] isomer of fenvalerate (125 mg/kg
diet) for 1 to 3 months. These changes were reversed when
fenvalerate was eliminated from the diet. The causative
agent for this change was the cholesterol ester of 2-(4-
chlorophenyl)isovaleric acid, a lipophilic metabolite of
fenvalerate from the [2R, alphaS] isomer. The NOEL for
these microgranulomatous changes in mice was 30 mg fenval-
erate per kg diet.
In a long-term toxicity study, microgranulomatous
changes were also observed in rats at a dose level of
500 mg/kg diet, the NOEL for these changes being 150 mg/kg
diet.
Fenvalerate was not carcinogenic to mice, when fed at
dietary levels up to 3000 mg/kg for 78 weeks or 1250 mg/kg
for 2 years. It was also not carcinogenic to rats when
fed at dietary levels up to 1000 mg/kg for 2 years.
Fenvalerate did not show any mutagenic or chromosome-
damaging activity in several in vitro and in vivo assays.
Fenvalerate is not teratogenic to mice or rabbits at
dose levels of up to 50 mg/kg body weight per day, nor did
it show any toxic effects (at up to 250 mg/kg diet) on
reproductive parameters in a 3-generation rat reproduction
study.
1.1.8 Effects on human beings
Fenvalerate can induce numbness, itching, tingling,
and burning sensations in exposed workers, which develop
after a latent period of approximately 30 min, peak by
8 h, and disappear within 24 hours. Some poisoning cases
have resulted from occupational exposure, owing to over-
exposure due to neglect of safety precautions.
There are no indications that fenvalerate will have an
adverse effect on human beings, provided it is used as
recommended.
1.2 Conclusions
1.2.1 General population
The exposure of the general population to fenvalerate
is expected to be very low. It is not likely to present a
hazard provided it is used as recommended.
1.2.2 Occupational exposure
With reasonable work practices, hygiene measures, and
safety precautions, fenvalerate is unlikely to present a
hazard to those occupationally exposed to it.
1.2.3 Environment
It is unlikely that fenvalerate or its degradation
products will attain levels of environmental significance
provided that recommended application rates are used.
Under laboratory conditions fenvalerate is highly toxic to
fish, aquatic arthropods, and honey bees. However, lasting
adverse effects are not likely to occur under field con-
ditions provided it is used as recommended.
1.3 Recommendations
Although dietary levels arising from recommended usage
are considered to be very low, confirmation of this
through inclusion of fenvalerate in monitoring studies
should be considered.
Fenvalerate has been used for many years and only a
few cases of temporary effects from occupational exposure
have been reported. Nevertheless, it would be wise to
maintain observations of human exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
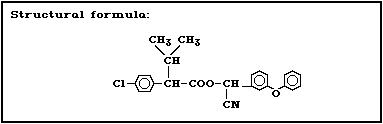
2.1 Identity
Fenvalerate is a synthetic pyrethroid having no cyclo-
propane ring in the molecule. It is prepared by the es-
terification of (2RS)-2-(4-chlorophenyl)-3-methylbutyric
acid (also known as (2RS)-2-(4-chlorophenyl)isovaleric
acid, CPIA, or Cl-Vacid) with (alphaRS)-alpha-cyano-3-
phenoxybenzyl alcohol [137]. It has four stereoisomers as
a result of the two chiral centres in the acid and alcohol
moieties (Fig. 1).
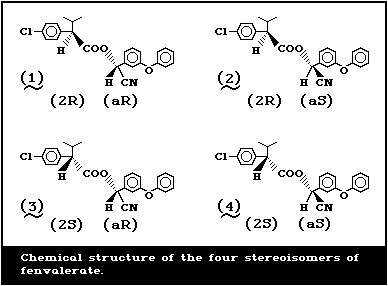 The composition of the product is a racemic mixture of
the four isomers in equal proportions (Table 1). Technical
grade fenvalerate contains 90-94% of fenvalerate [41]. The
molecular formula is C25H22ClNO3.
Table 1. Chemical identity of fenvalerate and its various stereoisomers
-----------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry no./ compositionc trade names
NIOSH Accession no.a Stereospecific nameb
-----------------------------------------------------------------------------------------------------
Fenvalerate Benzeneacetic acid, (1):(2):(3):(4) Sumicidin, Belmark,
51630-58-1 4-chloro- alpha-(1-methylethyl)-, = 1:1:1:1
CY1576350 cyano(3-phenoxyphenyl)methyl ester Pydrin, S-5602
SD43775, WL43775
(RS)- alpha-cyano-3-phenoxybenzyl
(RS)-2-(4-chlorophenyl)-3-
methylbutyrate
alpha-Fenvalerate Same as fenvalerate
66230-04-4
Benzeneacetic acid, 4-chloro- alpha-
(1-methylethyl)-, cyano-3-phenylbenzyl
ester, [S-(R*,R*)]-
beta-Fenvalerate Same as fenvalerate
66267-77-4
Benzeneacetic acid, 4-chloro- alpha-
(1-methylethyl)-, cyano-3-phenoxybenzyl
ester, [R-R*, S*)]-
(S,S)-Fenvalerate
CY1576350
-----------------------------------------------------------------------------------------------------
a Registry of Toxic Effects of Chemical Substances (1981-1982 edition).
b (2S), d, (+) or (2R), 1, (-) in the acid part of fenvalerate signify the same stereospecific
conformation, respectively.
c Numbers in parantheses identify the structures shown in Fig. 1.
The composition of the product is a racemic mixture of
the four isomers in equal proportions (Table 1). Technical
grade fenvalerate contains 90-94% of fenvalerate [41]. The
molecular formula is C25H22ClNO3.
Table 1. Chemical identity of fenvalerate and its various stereoisomers
-----------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry no./ compositionc trade names
NIOSH Accession no.a Stereospecific nameb
-----------------------------------------------------------------------------------------------------
Fenvalerate Benzeneacetic acid, (1):(2):(3):(4) Sumicidin, Belmark,
51630-58-1 4-chloro- alpha-(1-methylethyl)-, = 1:1:1:1
CY1576350 cyano(3-phenoxyphenyl)methyl ester Pydrin, S-5602
SD43775, WL43775
(RS)- alpha-cyano-3-phenoxybenzyl
(RS)-2-(4-chlorophenyl)-3-
methylbutyrate
alpha-Fenvalerate Same as fenvalerate
66230-04-4
Benzeneacetic acid, 4-chloro- alpha-
(1-methylethyl)-, cyano-3-phenylbenzyl
ester, [S-(R*,R*)]-
beta-Fenvalerate Same as fenvalerate
66267-77-4
Benzeneacetic acid, 4-chloro- alpha-
(1-methylethyl)-, cyano-3-phenoxybenzyl
ester, [R-R*, S*)]-
(S,S)-Fenvalerate
CY1576350
-----------------------------------------------------------------------------------------------------
a Registry of Toxic Effects of Chemical Substances (1981-1982 edition).
b (2S), d, (+) or (2R), 1, (-) in the acid part of fenvalerate signify the same stereospecific
conformation, respectively.
c Numbers in parantheses identify the structures shown in Fig. 1.
 2.2 Physical and Chemical Properties
Some physical and chemical properties of fenvalerate
are given in Table 2. It is stable to heat and moisture
and is relatively stable (compared with natural py-
rethrins) when exposed to light. It is more stable in
acidic than in alkaline media, optimum stability being at
pH 4 [41, 117, 207].
Table 2. Some physical and chemical properties of fenvalerate
--------------------------------------------------------------
Physical state viscous liquid
Colour yellow or brown
Odour mild "chemical" odour
Relative molecular mass 419.9
Boiling point 300 °C at 4.93 kPa (37 mmHg)
Water solubility 2 µg/Litre
Solubility in organic solvents solublea
Relative density (25 °C) 1.175
Vapour pressure (25 °C) 0.037 mPa
Log octanol-water partition
coefficient (log Pow) 6.2
--------------------------------------------------------------
a Acetone (>1 kg/kg), hexane (155 g/kg), xylene (>1 kg/kg),
ethanol, cyclohexanone, ether, kerosene, chloroform.
2.3 Analytical Methods
Methods for the analysis of fenvalerate are summarized
in Table 3. This table includes the procedures for (a) ex-
traction with solvent, (b) liquid-liquid partition, (c)
chromatographic separation (clean up), and (d) quantitat-
ive and qualitative determination by suitable analytical
instruments, and also includes minimum detectable concen-
tration (MDC) and percentage recovery data.
The separation of the cis and trans isomers of
fenvalerate has been carried out using a commercially
available Pirkle type 1-A chiralphase HPLC with, as sol-
vent system, 0.025% propen-2-ol in hexane (1 ml/min)
[24].
Fenvalerate can be determined by gas-liquid chromato-
graphy with a flame ionization detector (FID-GC) (3% OV-17
glass column with temperature programming) [11].
A laminar flow, microwave-induced plasma torch has
been evaluated for its use in gas chromatography [19]. The
detection limit of fenvalerate on the carbon channel was
0.054 µg/ml.
To analyse technical grade fenvalerate, the product is
dissolved in chloroform together with 2-(4-biphenyl)-5-
phenyl-1,3,4-oxadiazole (an internal standard), and the
solution is injected into an FID-GC system [79].
The Joint FAO/WHO Codex Alimentarius Commission has
published recommendations for methods for the analysis of
fenvalerate residues [48].
Table 3. Analytical methods for fenvalerate
--------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCb % Recovery Reference
GLC or HPLC; detector, (fortification
Extraction Partition Clean-up carrier flow, column, level)
solvent temperature, retention (mg/kg)c
Column Elution time
--------------------------------------------------------------------------------------------------------------------------------------
Residue analysis
apple n-hexane ext.sol.a silica gel CH2Cl2 ECD-GC, N2, 50 ml/min, 0.01 89-108 6
pear acetone /H2O 1 m, 3% OV-7, 235 °C (0.1-1.0)
cabbage (1/1)
potato
grape acetone saturated Florisil acetone/ ECD-GC, N2, 30 ml/min, 0.005 94-99 67
pepper NaCl/ petroleum 1.1 m, 2% XE-60, (0.005-1.0)
petroleum ether (1/99) 215 °C, 7 min
ether
cabbage CH3CN 1% NaCl/ Florisil benzene/ n- ECD-GC, argon/methane 0.005 88-104 103
lettuce petroleum hexane (1/1) (95/5), 45 ml/min, 1.8 m, (0.012-1.2)
ether silica gel benzene/ 4% SE-30/6% QF-1 or 15%
acetone (3/1) OV-101, 225 °C, 25-30 min
beef CH3CN/ n-hexane/ Florisil CH3CN/ ECD-GC, N2, 100 ml/min, 0.005 82-94 16
muscle H20 2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01-1.0)
egg yolk (85/15) solution n-hexane 220 °C, 11.5, 14.2 min
milk or CH3CN (0.35/50/50)
Environmental analysis
soil acetone, 2% NaCl/ alumina ether/ ECD-GC, argon/methane 78-105 74
n-hexane/ ext.sol.a n-hexane (95/5), 60 ml/min, (0.005-1.0)
acetone (1/9) 0.97 m, 6% OV-210,
(1/1), 230 °C, 10.6, 11.8 min
hexane
Product analysis
Technical CHCl3 FID-GC, He, 60 ml/min, 79
grade 1.0 m, 2% Apiezon L,
245 °C
--------------------------------------------------------------------------------------------------------------------------------------
a extraction solvent.
b minimum detectable concentration (mg/kg).
c fortification level indicates the concentration of fenvalerate added to control samples for the measurement of recovery.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE:
ENVIRONMENTAL LEVELS
3.1 Industrial Production
Fenvalerate was first marketed in 1976 and the esti-
mated production was 1000 tonnes in 1979 and 889 tonnes in
1982 [203]. Recent world-wide production figures are
listed in Table 4.
Table 4. World-wide production of
fenvalerate
-------------------------------------
Year Production Reference
(tonnes)
-------------------------------------
1979 1016 200
1980 1067 201
1981 914 202, 203
1982 903 203
1983 1280 204
1984 919 9
-------------------------------------
3.2 Use Patterns
Of the total world-wide consumption of 473 tonnes of
fenvalerate in 1980 [8], 271 tonnes were used in the USA,
103 tonnes in Latin America, 43 tonnes in Africa, 28
tonnes in Western Europe, and 26 tonnes each in Australia
and Turkey. It was mostly used on cotton (90.3% of the
consumption) but some was used on other crops such as
vines, tomatoes, potatoes, pomes, and other fruit.
Fenvalerate has also been used for homes and gardens
and for the control of cattle insect infestation [8]. It
is formulated in emulsifiable concentrates (25-300
g/litre), ultra-low volume concentrates (25-75 g/litre),
dusts, and wettable powder, and is also used in combi-
nation with other pesticides (e.g., fenitrothion).
3.3 Residues in Food
Supervised trials have been carried out on a wide
variety of crops and comprehensive summaries of the re-
sults of residue analysis in these trials are contained in
the evaluation reports of the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) [41, 43, 45, 47, 50]. A compre-
hensive list of Maximum Residue Limits (MRLs) for a large
number of commodities resulted from these evaluations
[51].
In one study, apples in the USA were treated four
times with 30% emulsifiable concentrate at a rate of 0.67
kg active ingredient/ha. The residue levels were 2.2 mg/kg
in whole apples, 7.3 mg/kg in peel, and 0.03 mg/kg in
peeled fruits 6 weeks after the last application [41].
When wheat grain treated with fenvalerate at a rate of
1.01 mg/kg was stored at 25 °C, the residue levels were
0.86 mg/kg after 6.5 months of storage and 0.74 mg/kg
after 10 monthsa.
Three lactating cows were fed 14C-(acid-labelled)-
fenvalerate at a dose level of 0.11 mg/kg diet daily for
21 days and sacrificed 12 h after receiving the last dose.
The recovery of 14C in the milk was less than 1% and the
levels ranged from < 0.0006 to 0.0019 µg/litre, with a
plateau occurring after 1 week of feeding. No 14C was
detectable in fat (< 0.02 mg/kg) or muscle (< 0.01 mg/kg).
In another study, fenvalerate was sprayed on cows at a
rate of 0.2, 0.4, or 2 g/animal. The residue level did not
exceed 0.01 mg/kg muscle. Maximum residues were 0.22 mg/kg
in fat and 0.02 mg/kg in milk at the dose rate of 2 g/cow
[132, 154].
When wheat containing 0.6 mg fenvalerate/kg was sub-
jected to milling and baking, white bread was found to
have about the same residue level as white flour, i.e.,
about 0.06-0.1 and 0.08-0.09 mg/kg, respectivelyb.
3.4 Residues in the Environment
Data on actual levels of fenvalerate residues in air,
water, or soil are not available. Residues in air would
not be expected for a compound with a vapour pressure of
0.037 mPa at 25 °C.
----------------------------------------------------------
a M. Bengston (1979), personal communication from final
report on silo-scale experiments 1977-1978 to the
Australian Wheat Board Working Party on grain protect-
ants. Queensland Department of Primary Industries
(unpublished report cited from FAO/WHO [41].
b B.W. Simpson (1979), draft report to be published by
Queensland Department of Primary Industries Analyti-
cal Chemical Branch, Brisbane, Australia (unpublished
report cited in FAO/WHO [41].
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Appraisal
The major photodegradation routes for fenvalerate are de-
carboxylation to yield 3-(4-chlorophenyl)-4-methyl-2-(3-phenoxy-
phenyl)-valeronitrile, ester and ether cleavage, hydrolysis of
the cyanide group, and other radical-initiated reactions. Ester
cleavage and some photo-initiated reactions are the major
routes of decomposition on plants. In soils, the formation of
bound material and the evolution of carbon dioxide are the
major processes observed under both aerobic and anaerobic con-
ditions.
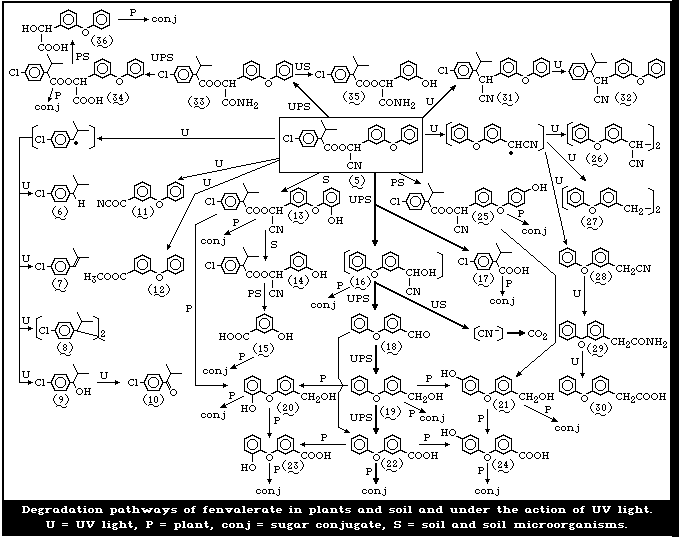
The degradation pathways of fenvalerate are summarized
in Fig. 2.
2.2 Physical and Chemical Properties
Some physical and chemical properties of fenvalerate
are given in Table 2. It is stable to heat and moisture
and is relatively stable (compared with natural py-
rethrins) when exposed to light. It is more stable in
acidic than in alkaline media, optimum stability being at
pH 4 [41, 117, 207].
Table 2. Some physical and chemical properties of fenvalerate
--------------------------------------------------------------
Physical state viscous liquid
Colour yellow or brown
Odour mild "chemical" odour
Relative molecular mass 419.9
Boiling point 300 °C at 4.93 kPa (37 mmHg)
Water solubility 2 µg/Litre
Solubility in organic solvents solublea
Relative density (25 °C) 1.175
Vapour pressure (25 °C) 0.037 mPa
Log octanol-water partition
coefficient (log Pow) 6.2
--------------------------------------------------------------
a Acetone (>1 kg/kg), hexane (155 g/kg), xylene (>1 kg/kg),
ethanol, cyclohexanone, ether, kerosene, chloroform.
2.3 Analytical Methods
Methods for the analysis of fenvalerate are summarized
in Table 3. This table includes the procedures for (a) ex-
traction with solvent, (b) liquid-liquid partition, (c)
chromatographic separation (clean up), and (d) quantitat-
ive and qualitative determination by suitable analytical
instruments, and also includes minimum detectable concen-
tration (MDC) and percentage recovery data.
The separation of the cis and trans isomers of
fenvalerate has been carried out using a commercially
available Pirkle type 1-A chiralphase HPLC with, as sol-
vent system, 0.025% propen-2-ol in hexane (1 ml/min)
[24].
Fenvalerate can be determined by gas-liquid chromato-
graphy with a flame ionization detector (FID-GC) (3% OV-17
glass column with temperature programming) [11].
A laminar flow, microwave-induced plasma torch has
been evaluated for its use in gas chromatography [19]. The
detection limit of fenvalerate on the carbon channel was
0.054 µg/ml.
To analyse technical grade fenvalerate, the product is
dissolved in chloroform together with 2-(4-biphenyl)-5-
phenyl-1,3,4-oxadiazole (an internal standard), and the
solution is injected into an FID-GC system [79].
The Joint FAO/WHO Codex Alimentarius Commission has
published recommendations for methods for the analysis of
fenvalerate residues [48].
Table 3. Analytical methods for fenvalerate
--------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCb % Recovery Reference
GLC or HPLC; detector, (fortification
Extraction Partition Clean-up carrier flow, column, level)
solvent temperature, retention (mg/kg)c
Column Elution time
--------------------------------------------------------------------------------------------------------------------------------------
Residue analysis
apple n-hexane ext.sol.a silica gel CH2Cl2 ECD-GC, N2, 50 ml/min, 0.01 89-108 6
pear acetone /H2O 1 m, 3% OV-7, 235 °C (0.1-1.0)
cabbage (1/1)
potato
grape acetone saturated Florisil acetone/ ECD-GC, N2, 30 ml/min, 0.005 94-99 67
pepper NaCl/ petroleum 1.1 m, 2% XE-60, (0.005-1.0)
petroleum ether (1/99) 215 °C, 7 min
ether
cabbage CH3CN 1% NaCl/ Florisil benzene/ n- ECD-GC, argon/methane 0.005 88-104 103
lettuce petroleum hexane (1/1) (95/5), 45 ml/min, 1.8 m, (0.012-1.2)
ether silica gel benzene/ 4% SE-30/6% QF-1 or 15%
acetone (3/1) OV-101, 225 °C, 25-30 min
beef CH3CN/ n-hexane/ Florisil CH3CN/ ECD-GC, N2, 100 ml/min, 0.005 82-94 16
muscle H20 2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01-1.0)
egg yolk (85/15) solution n-hexane 220 °C, 11.5, 14.2 min
milk or CH3CN (0.35/50/50)
Environmental analysis
soil acetone, 2% NaCl/ alumina ether/ ECD-GC, argon/methane 78-105 74
n-hexane/ ext.sol.a n-hexane (95/5), 60 ml/min, (0.005-1.0)
acetone (1/9) 0.97 m, 6% OV-210,
(1/1), 230 °C, 10.6, 11.8 min
hexane
Product analysis
Technical CHCl3 FID-GC, He, 60 ml/min, 79
grade 1.0 m, 2% Apiezon L,
245 °C
--------------------------------------------------------------------------------------------------------------------------------------
a extraction solvent.
b minimum detectable concentration (mg/kg).
c fortification level indicates the concentration of fenvalerate added to control samples for the measurement of recovery.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE:
ENVIRONMENTAL LEVELS
3.1 Industrial Production
Fenvalerate was first marketed in 1976 and the esti-
mated production was 1000 tonnes in 1979 and 889 tonnes in
1982 [203]. Recent world-wide production figures are
listed in Table 4.
Table 4. World-wide production of
fenvalerate
-------------------------------------
Year Production Reference
(tonnes)
-------------------------------------
1979 1016 200
1980 1067 201
1981 914 202, 203
1982 903 203
1983 1280 204
1984 919 9
-------------------------------------
3.2 Use Patterns
Of the total world-wide consumption of 473 tonnes of
fenvalerate in 1980 [8], 271 tonnes were used in the USA,
103 tonnes in Latin America, 43 tonnes in Africa, 28
tonnes in Western Europe, and 26 tonnes each in Australia
and Turkey. It was mostly used on cotton (90.3% of the
consumption) but some was used on other crops such as
vines, tomatoes, potatoes, pomes, and other fruit.
Fenvalerate has also been used for homes and gardens
and for the control of cattle insect infestation [8]. It
is formulated in emulsifiable concentrates (25-300
g/litre), ultra-low volume concentrates (25-75 g/litre),
dusts, and wettable powder, and is also used in combi-
nation with other pesticides (e.g., fenitrothion).
3.3 Residues in Food
Supervised trials have been carried out on a wide
variety of crops and comprehensive summaries of the re-
sults of residue analysis in these trials are contained in
the evaluation reports of the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) [41, 43, 45, 47, 50]. A compre-
hensive list of Maximum Residue Limits (MRLs) for a large
number of commodities resulted from these evaluations
[51].
In one study, apples in the USA were treated four
times with 30% emulsifiable concentrate at a rate of 0.67
kg active ingredient/ha. The residue levels were 2.2 mg/kg
in whole apples, 7.3 mg/kg in peel, and 0.03 mg/kg in
peeled fruits 6 weeks after the last application [41].
When wheat grain treated with fenvalerate at a rate of
1.01 mg/kg was stored at 25 °C, the residue levels were
0.86 mg/kg after 6.5 months of storage and 0.74 mg/kg
after 10 monthsa.
Three lactating cows were fed 14C-(acid-labelled)-
fenvalerate at a dose level of 0.11 mg/kg diet daily for
21 days and sacrificed 12 h after receiving the last dose.
The recovery of 14C in the milk was less than 1% and the
levels ranged from < 0.0006 to 0.0019 µg/litre, with a
plateau occurring after 1 week of feeding. No 14C was
detectable in fat (< 0.02 mg/kg) or muscle (< 0.01 mg/kg).
In another study, fenvalerate was sprayed on cows at a
rate of 0.2, 0.4, or 2 g/animal. The residue level did not
exceed 0.01 mg/kg muscle. Maximum residues were 0.22 mg/kg
in fat and 0.02 mg/kg in milk at the dose rate of 2 g/cow
[132, 154].
When wheat containing 0.6 mg fenvalerate/kg was sub-
jected to milling and baking, white bread was found to
have about the same residue level as white flour, i.e.,
about 0.06-0.1 and 0.08-0.09 mg/kg, respectivelyb.
3.4 Residues in the Environment
Data on actual levels of fenvalerate residues in air,
water, or soil are not available. Residues in air would
not be expected for a compound with a vapour pressure of
0.037 mPa at 25 °C.
----------------------------------------------------------
a M. Bengston (1979), personal communication from final
report on silo-scale experiments 1977-1978 to the
Australian Wheat Board Working Party on grain protect-
ants. Queensland Department of Primary Industries
(unpublished report cited from FAO/WHO [41].
b B.W. Simpson (1979), draft report to be published by
Queensland Department of Primary Industries Analyti-
cal Chemical Branch, Brisbane, Australia (unpublished
report cited in FAO/WHO [41].
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Appraisal
The major photodegradation routes for fenvalerate are de-
carboxylation to yield 3-(4-chlorophenyl)-4-methyl-2-(3-phenoxy-
phenyl)-valeronitrile, ester and ether cleavage, hydrolysis of
the cyanide group, and other radical-initiated reactions. Ester
cleavage and some photo-initiated reactions are the major
routes of decomposition on plants. In soils, the formation of
bound material and the evolution of carbon dioxide are the
major processes observed under both aerobic and anaerobic con-
ditions.
The degradation pathways of fenvalerate are summarized
in Fig. 2.
 4.1 Transportation and Distribution Between Media
Hill [74] investigated the distribution of fenvalerate
residues in soil under field conditions using a microplot
technique. The microplots (20 x 20 cm) were treated with
fenvalerate at a rate of 150 g/ha. After 45 weeks, 11% of
the applied fenvalerate was located in the 0-2.5 cm soil
layer and less than 0.5% in the 2.5-5 cm soil samples.
Less than 0.1% of the applied fenvalerate was detected in
any of the soil samples taken after 3 or 4 weeks, despite
a rainfall of 95.4 mm during the first 4 weeks (including
a 25.9 mm downpour 15 days after application). These
results indicate that fenvalerate does not readily leach
downward and that lateral surface movement is very
limited.
A similar conclusion was obtained from laboratory
soil-leaching studies. More than 95% of the applied
fenvalerate remained in the treated portion of soil
columns when leaching was started immediately or 30 days
after treatment of the soil [133]. The possibility of
fenvalerate accumulating in orchard soils was assessed by
monitoring soil and leaf litter in an orchard in the
Okanagan valley, British Columbia, Canada, following
multiple annual application of the pesticide. Belmark 300
(30% fenvalerate EC formulation) had been sprayed at a
rate of 188-500 ml/ha (one to three times per year) for
more than three years. To obtain initial concentration,
organic litter samples were sprayed at a rate of 450 ml/ha
and samples were collected 2 h later. While the initial
concentration thus obtained in the litter was 0.214 mg/kg
(average value), orchard litter samples contained
0.30-0.63 mg/kg while samples from a non-treated block
contained < 0.002 mg/kg. Orchard soil samples (0-15 cm
depth) in two orchards contained fenvalerate residues of
< 0.0035-0.006 mg/kg and 0.0063-0.024 mg/kg [188].
4.2 Photodecompositiona
In studies by Holmstead et al. [78], fenvalerate (5),
at a concentration of 0.01 mol/litre in methanol, hexane,
or acetonitrilewater (60:40), underwent rapid photodegra-
dation under the action of UV light (290-320 nm) with a
half-life of 16-18 min (Fig. 2). With 90-95% conversion
after 60 min, 2-(3-phenoxyphenyl)-3-(4-chlorophenyl)-4-
methyl-pentanenitrile (31) (decarboxy-fenvalerate) was the
major photoproduct, amounting to 54-57% of the total reac-
tion mixture. There were smaller amounts of the dechlori-
nated analogue (32) of decarboxy-fenvalerate and the dimer
(8) of 2,2-dimethyl-4-chlorostyrene. 3-Phenoxybenzoyl cy-
anide (4), 3-phenoxybenzaldehyde (18) (PBald), 4-chloro-
isobutylbenzene (6), and 2,2-dimethyl-4-chlorostyrene (7)
were detected in small amounts in hexane or methanol. 3-
Phenoxybenzyl cyanide (28), its dimer (26), and 1,2-bis-
(phenoxyphenyl)ethane (27) were found only in hexane, and
methyl 3-phenoxybenzoate (12) was detected only in meth-
anol. Products found uniquely in acetonitrile-water were
2-(4-chlorophenyl)-3-methylbutyric acid (17) (CPIA), 3-
phenoxybenzoic acid (22) (PBacid) and 1-(4-chlorophenyl)-
2-methylpropanol (9). Several unknown compounds were ob-
served in the remaining 5-10%. Fenvalerate, in a thin film
(1 mg/cm2) on glass, decomposed in sunlight with a half-
life of approximately 4 days. About 10% of the applied
material remained after 43 days. In addition to the photo-
products formed in solution, small amounts of 3-phenoxy-
benzyl alcohol (19) (PBalc) and isopropyl 4-chlorophenyl-
ketone (10) were detected.
On exposure to autumn sunlight in Japan, the
[2S, alphaS] isomer of fenvalerate in distilled water
decomposed with a half-life of approximate 10 days. This
isomer photodecomposed via pathways that included decar-
boxylation, hydration of the CN group to a carbamoyl
(CONH2) group, hydrolysis of the CONH2 group to a car-
boxyl (COOH) group, and cleavage of the ester or diphenyl
ether linkage. Cleavage of the ester linkage was a major
photochemical reaction and led to the formation of 2-(4-
chlorophenyl)-3-methylbutyric acid (17) (17.3% of the
applied 14C, 10 days after exposure). There was no sig-
nificant difference between fenvalerate and the [2S, alphaS]
isomer in the rates and routes of photodegradation [179].
The photodegradation of fenvalerate (0.3-0.4 ng/cm2)
on two kinds of soil in natural sunlight was compared with
that of the [2S, alphaS] isomer. Fenvalerate and its isomer
photodecomposed with half-lives of 1.4-2.4 days and 1.1-
2.5 days, respectively. The pathways included hydration
of the cyano group to the carbamoyl group (19.2-48.4% at
10 days) with subsequent hydrolysis to the carboxyl group
(0.9-2.0% at 10 days), ester-bond cleavage (3.4-4.5% at 10
days), and decarboxylation (0.3-0.9% at 10 days). Little
2S/2R and alphaS/alphaR isomerization (as determined by
HPLC) occurred on the soils. There was no significant
difference between the two compounds in the rates and
pathways of photodegradation [89].
Holmstead & Fullmer [77] investigated the photodecar-
boxylation of several cyanohydrin esters in methanol and
hexane under artificial light as models for pyrethroid
photodecomposition. The cyanohydrin esters gave rise to
decarboxylated products, to a greater or lesser extent,
whereas the analogous compounds without the cyano group
did not produce the photodecarboxylated compounds.
alpha-Cyanobenzyl phenylacetate, which yielded the stable
benzyl radical, gave substantially larger amounts of the
decarboxylated product than alpha-cyanobenzyl benzoate,
which produced the unstable phenyl radical.
The photodegradation of fenvalerate in water and on
soil was investigated using compounds labelled with 14C
at the following positions: carbonyl group (CO-fenval-
----------------------------------------------------------------
a The numbers in brackets following chemical names refer to the
numbers given in Fig. 2.
erate), alpha-carbon in the benzyl group (Calpha-fenvale-
rate), and cyano group (CN-fenvalerate) [118]. On exposure
to sunlight, fenvalerate in very dilute solution in distil-
led water, in 2% aqueous acetone, in filter-sterilized
river water, or in sea water underwent rapid photolysis
with half-lives of approximately 4 days in summer and 13-
15 days in winter. The quantum yield was calculated at
6.6 x 10-3 (at 313 nm in water) and the half-life of dis-
appearance at latitude 40°N was calculated at 4.1 days in
summer and 12.4 days in winter, values which were close to
the experimental ones. Photodegradation of 14CN-fenval-
erate resulted in the formation of greater amounts of
14CO2 than 14CN-. After 6 weeks irradiation, ap-
proximately 30% (in aqueous acetone or river water) or
approximately 55-60% (in distilled water or sea water) of
the 14C was recovered as 14CO2, while the correspond-
ing figures for 14CN- were 5% and 30%. One of the major
photodegradation products was decarboxy-fenvalerate (31),
which increased to approximately 20% (in distilled water)
in summer after 1 week and decreased thereafter. In win-
ter, the amount was approximately 20% after 6 weeks. Other
major products were PBacid (22) and CPIA (17), derived
from the ester bond cleavage, amounting to 43% and 58%,
respectively, of the applied radioactivity after 6 weeks
in winter. In addition, small amounts of alpha-carbamoyl-
3-phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (33)
(CONH2-fenvalerate), alpha-carboxy-3-phenoxybenzyl-2-(4-
chlorophenyl)-3-methylbutyrate (34) (COOH-fenvalerate), 3-
phenoxybenzyl cyanide (28), 3-phenoxyphenylacetamide (29),
3-phenoxyphenylacetic acid (30), PBalc, and PBald were
detected.
Fenvalerate, as a deposit (5.5-5.9 µg/100 cm2) on
Kodaira light clay, Azuchi sandy clay loam, and Katano
sandy loam soil from Japan, was decomposed by autumn sun-
light with the respective half-lives of 2, 6, and 18 days
[118]. The major product was CONH2-fenvalerate (33),
which amounted to 7.9-25.7% of the applied radioactivity
after 10 days; it was formed in greatest amounts in sun-
light but also formed in the dark. Smaller amounts of
decarboxy-fenvalerate (31), the desphenyl analogue of
CONH2-fenvalerate (35), COOH-fenvalerate (34), PBacid,
and PBalc were also detected. Of the applied radiocarbon,
3-10% remained unidentified.
4.3 Decomposition in Plants
Fenvalerate (2.4% emulsifiable concentrate (EC)),
permethrin (2% EC), and deltamethrin (25 g/litre) were
sprayed onto cotton fields in Arizona, USA, at respective
rates of 0.11, 0.11, and 0.23 kg/ha, and dislodgeable
residues of the insecticides on cotton foliage were exam-
ined. Of the original deposits of fenvalerate, 65%
remained at the end of 96 h (there were two rains between
24 and 48 h), compared with 47% and 32% for permethrin and
deltamethrin, respectively [57].
Fenvalerate deposits on cotton plants (0.8 mg/plant)
disappeared rapidly, with only half the material remaining
after 8 days of exposure. After 23 days, decarboxy-fenval-
erate and ester-cleavage products such as PBacid, PBald,
PBalc, and CPIA were detectable, but not quantifiable.
Decarboxy-fenvalerate was considerably more stable to UV
light than fenvalerate, but it decomposed at a somewhat
faster rate than p,p'-DDT, yielding mainly the dechlori-
nated analogue [78].
The metabolism of fenvalerate in kidney bean plants
has been studied under laboratory conditions by Ohkawa et
al. [136]. Fenvalerate labelled with 14C at the cyano
group and the [2S, alphaRS] isomer labelled separately at
the cyano, carboxy, and benzylic carbon atoms were used to
treat individual bean leaves of 14-day-old seedlings at a
rate of 10 µg per leaf. After 60 days, 85-86% of the
applied 14C was recovered from plants treated with the
carboxy and benzyl labels, whereas 67% was recovered from
plants treated with the cyano label. Only limited translo-
cation was observed and only very low levels of radioac-
tive residues (2-9 µg/kg) were detected in seeds.
Fenvalerate and the [2S, alphaRS] isomer disappeared at a
similar rate from the treated leaves with an initial half-
life of 14 days.
The metabolism of racemic fenvalerate and of its
[2S, alphaS] isomer was examined in cabbage plants grown
under laboratory conditions and treated (20 µg per leaf)
with [14C]-chlorophenyl- and [phenyl-14C]-benzyl-labelled
preparations of the two compounds. Both compounds disap-
peared from the treated leaves with similar half-lives of
approximately 12-14 days. They underwent ester cleavage
to a significant extent, together with some hydroxylation
at the 2- or 4-position of the phenoxy ring and hydrolysis
of the nitrile group to amide and carboxyl groups. Most of
the carboxylic acids and phenols thus produced occurred as
glycoside conjugates. In a separate experiment, the uptake
and metabolism of CPIA (17) was examined in the laboratory
using abscised leaves of kidney bean, cabbage, cotton, cu-
cumber, and tomato plants. The acid (17) was found to be
readily converted, mainly into glucose or 6-O-malonylglu-
cose esters in kidney bean, cabbage, and cucumber, into
glucosylxylose, sophorose, and gentiobiose esters in
cotton, and into two types of triglucose esters with dif-
fering isomerism in tomato. One of the acetyl-derived
glucoside conjugates was identical with the authentic
deca-acetyl derivative of the [1-6]-triglucose ester
[121].
In studies by Ohkawa et al. [136], fenvalerate was
metabolized or degraded in bean plants via several routes.
A minor route was hydrolysis of the cyano group leading to
the formation of the amide (33) and carboxylic acid (34)
derivatives of fenvalerate. The 3-phen-oxybenzyl moiety
underwent metabolism to yield PBacid, 3-(2'-hydroxy-
phenoxy)benzoic acid (23) and PBalc, which occurred mainly
as sugar conjugates. In addition, glucoside conjugates
of alpha-carboxy-3-phenoxybenzyl alcohol (36) were detected
to a lesser extent. The presence of alpha-cyano-3-phenoxyben-
zyl alcohol (16) conjugates was inferred since PBald was
released upon treatment with beta-glucosidase. A major
metabolite of the acid moiety was CPIA (17), which also
occurred mainly as glucoside conjugates. The decarboxy
derivative (31) of fenvalerate, presumably formed by
photochemical reaction on plant foliage as discussed pre-
viously, was detected in leaf extracts. When bean plant
seedlings were planted and left for 30 days in light clay
and sandy loam soils treated with 14C-fenvalerate at
1 mg/kg, the roots retained fairly large amounts of radio-
carbon. However, only limited radiocarbon was found in
the shoots (0.02 mg/kg), pods and seeds (0.01 mg/kg), and
there was no parent compound in the shoots.
Additional studies were carried out to investigate the
fate of 3-phenoxybenzoic acid (an important metabolite of
fenvalerate and most other pyrethroids) in plants. Using
abscised leaves of cabbage, cotton, cucumber, kidney bean
and tomato plants, 14C-3-phenoxybenzoic acid was shown to
conjugate with a complex range of sugars [120].
4.4 Decomposition in Soils
The degradation of fenvalerate in soils has been
studied under various conditions (aerobic or anaerobic
conditions, laboratory or field conditions, using radioac-
tive or non-radioactive material).
Samples of 14C-fenvalerate labelled separately in the
carboxy and cyano groups were used for soil studies by
Ohkawa et al. [133]. When several types of soil were
treated at a rate of 1 mg/kg and stored at 25°C under
aerobic conditions, the initial half-life of fenvalerate
ranged from 15 days to 3 months. As with other
pyrethroids, hydrolysis at the ester linkage was a major
degradation route, and ring hydroxylation in the 4'-pos-
ition (25), together with hydrolysis of the cyano group to
the amide and carboxyl groups, occurred to smaller ex-
tents. The degradation route unique to fenvalerate was
ether-bond cleavage yielding alpha-cyano-3-hydroxybenzyl-
1-2-(4-chlorophenyl)-3-methylbutyrate (14), which could be
produced through hydroxylation at the 2'7-position (13) in
the alcohol moiety. No H14CN released during ester-bond
cleavage was detected owing to its rapid conversion to
14CO2. The amount of 14CO2 was greater with the
cyano label than the carboxy label. For example, after
30 days in Katano sandy loam soil, 47.5% and 38.2% of the
applied radiolabel was evolved as 14CO2 from the cyano
and carboxyl groups, respectively. In a laboratory soil-
leaching study, less than 1% of the applied radiocarbon
appeared in the effluent when leaching was started
immediately after treatment of the soil. Even after a
30-day incubation, only a trace amount of CPIA (17) was
detected in the effluent from soil columns treated with
14C-carbonyl-fenvalerate. In a separate experiment, the
degradation of 14C-fenvalerate was studied in a soil-
nutrient liquid suspension system. Separate cultures of
bacteria and fungi were used for the system. After 2 weeks
of incubation, larger amounts of 14CO2 (35-42% of the
applied radiolabel) were formed from both culture media
when the 14CN-labelled compound was used than when the
14CO-labelled compound was used (1.1-2.3%). In the
latter case, the main degradation product was CPIA, which
amounted to 34-69% [133].
Studies using 14C-fenvalerate, labelled separately in
the chlorophenyl and benzyl groups, confirmed the degra-
dation pathways mentioned above. These studies also showed
that the labelled aromatic rings were also readily de-
graded to 14CO2 (up to 66%). In addition, it was found
that any "bound residues" formed could be further de-
graded to 14CO2 by admixture with fresh soils [119].
The rate of degradation of the individual isomers of
fenvalerate has been investigated. In one soil, the half-
lives of the RR, RS, SR, and SS isomers were shown to be
178, 89, 155, and 108 days, respectively [105]. Different
rates for the various isomers were similarly obtained in
loam and sandy loam soils [160].
Under flooded conditions fenvalerate degrades more
slowly than under aerobic conditions. In sterile soil,
degradation is minimal, indicating that microbial activity
is the major cause of this degradation [102]. Ohkawa et
al. [133] reported similar findings.
Studies in which crops were sown in soils containing
aged residues of 14C-fenvalerate (aging periods of 30,
120, and 345 days) showed that residues from fenvalerate
should not carry over into rotated crops [102].
The persistence of fenvalerate in Lethbridge (Canada)
soil has been studied under field and laboratory con-
ditions [74]. Formulated fenvalerate (30% emulsifiable
concentrate) was applied once to soil microplots in the
field at a dose rate of 600 µg/plot (150 g/ha) or to
soil in pots at a dose of 88.7 µg/pot (10 g/ha). The
treated pots were maintained at a daily temperature regime
of 20°C for 16 h and 10°C for 8 h in the environmental
chamber. Fenvalerate was found mainly in the top 2.5 cm
of the field soil, and 16 weeks later, 15% of the applied
fenvalerate remained. The initial half-lives were 5.9
weeks for the [2S, alphaR] [2R, alphaS] enantiomeric pair
and 6 weeks for the [2R, alphaR] [2S, alphaS] pair. The
spring soil samples, taken 45 weeks after application, con-
tained 11% of the total fenvalerate. Limited degradation
occurred during the winter. The degradation of fenvalerate
in soil incubated in the environmental chamber was similar
to the field results. The [2S, alphaR] [2R, alphaS] enant-
iomeric pair had a half-life of 5 weeks while the [2R,
alphaR] [2S, alphaS] pair had a half-life of 5.3 weeks.
These results were comparable to the average half-life of
7 weeks for fenvalerate incubated in British Columbia soils
[198].
In studies by Harris et al. [69], the persistence of
fenvalerate in subtropical field soil (average soil tem-
perature, 20-30°C) was investigated after applying 20%
emulsifiable concentrate at a rate of 1 kg active ingredi-
ent (ai)/ha twice a year (spring and autumn) over a 2.5-
year period. Residues in the top 15 cm of soil were moni-
tored for up to one year after the final application.
Fenvalerate levels declined rapidly after the spring
application and relatively slowly after the autumn appli-
cation. There was no carry over of the insecticide from
year to year, and after 2.5 years of application only 2%
of the total fenvalerate remained. The rate of disappear-
ance became slightly slower when fenvalerate application
ceased [181]. The degradation of fenvalerate (14.9 mg/kg)
in plainfield sand (5% moisture) at 25°C was relatively
slow, with an initial half-life of 2 months, as compared
with initial half-lives of 0.5, 1, and 2 months for
fenpropathrin (7.1 mg/kg), permethrin (8.8 mg/kg), and
cypermethrin (7.3 mg/kg), respectively, under laboratory
conditions.
A 2-year field study on the relative persistence of
permethrin, cypermethrin, fenpropathrin, and fenvalerate
in soils was carried out by Chapman & Harris [25]. The
pyrethroids were applied as emulsifiable concentrates at a
rate of 280 g ai/ha or 140 g ai/ha to duplicate plots in
Ontario, Canada, containing either sand or organic soil.
For plots treated at the higher rate, the insecticide was
immediately raked into the soil, while the plots receiving
the lower rate were left undisturbed and the upper 4-5 cm
of soil was subjected to gas-liquid chromatography (GLC)
analyses. The concentrations of the four pyrethroids
incorporated in both soils or remaining on the upper soil
layer decreased to less than 50% of the initial values
within one month. Again, fenvalerate was slightly more
persistent, with 7% of the initial application remaining
in organic soil 28 months after treatment.
Reed et al. [157] demonstrated that when fenvalerate
was applied to soil, adsorption prevented significant
leaching of the pesticide. Soil metabolites produced
either by photolytic or microbial degradation did not
accumulate to a significant level or present a problem in
subsequent rotation crops (lettuce, beets, and wheat)
planted at 30 days, 60 days, 120 days, or 1 year after
soil treatment. Although fenvalerate has intrinsically
high toxicity to a variety of aquatic organisms, these
field studies demonstrated that the toxicant was unavail-
able to non-target organisms. Therefore, it had little or
no impact in this test system following its use at the
maximum allowed rate of 2.24 kg/ha per year.
4.5 Decomposition in Water
The hydrolysis of racemic fenvalerate in buffered
aqueous solutions at pH 5.0, 7.0, and 9.0 was compared by
Katagi et al. [90] with that of the [2S, alphaS] isomer.
Both compounds were fairly stable at pH 5.0 and 7.0 (half-
lives of 130-220 days), while at pH 9.0 they underwent
hydrolysis (half-lives of 64.6-67.2 days) mainly via ester
bond cleavage. The main product was 2-(4-chlorophenyl)-3-
methylbutyric acid (17), which amounted to 14.9% of the
applied 14C after 28 days. As the [2S, alphaS] isomer
underwent alphaS/alphaR epimerization in the alcohol moiety
at pH 7.0 and 9.0, its rate of hydrolysis appeared to be
rather faster than that of fenvalerate. However, the half-
life estimated from the total amounts of [2S, alphaS] and
[2S, alphaR] epimer was close to that of fenvalerate, which
indicates no significant difference in hydrolysis rate.
The persistence of fenvalerate has been evaluated
in water and sediment contained in open trenches
(3 m x 1 m x 30 cm) lined with alkathene sheet [1]. Insec-
ticide emulsion was sprayed on the surface of the water at
the normal rate and at twice the recommended dosage. The
dissipation of the insecticide from water was rapid. About
74-80% of the pesticide was lost within 24 h at both ap-
plication rates. However, residues were found to be ad-
sorbed onto sediment, and these persisted beyond 30 days.
In soil, persistence was moderate, lasting around
30 days.
5. KINETICS AND METABOLISM
Appraisal
The metabolic fate of fenvalerate in rats, mice, and cows
has been studied using variously labelled racemic fenvalerate
(acid moiety or benzyl or cyano groups labelled).
From oral administration studies, fenvalerate appears to be
absorbed rapidly through the gastrointestinal wall.
Following a single oral administration of labelled fenval-
erate to rats, the excretion of radiocarbon from the acid or
benzyl moieties was fairly rapid. However, the excretion of
radiocarbon originating from the cyano group was relatively
slow, the rest of the radioactivity being retained in various
tissues, particularly in hair and stomach as thiocyanate. The
major routes of metabolism were ester cleavage, hydroxylation
at the 4'position of the alcohol moiety, and thiocyanate
formation from the cyano group. Major metabolites were 2-(4-
chlorophenyl)isovaleric acid (Cl-Vacid) and 3-OH-Cl-Vacid (Cl-
Vacid hydroxylated at the 3 position) from the acid moiety, and
the sulfate conjugate of 3-(4'-hydroxyphenyl)benzoic acid and
thiocyanate from the alcohol moiety. A lipophilic metabolite,
cholesteryl-[2R]-2-(4-chlorophenyl)isovalerate, which was related
to granuloma formation, was detected in the adrenals, liver,
and the mesenteric lymph nodes of rats, mice, and some other
species. The excretion of fenvalerate in the milk from orally
dosed cows was very low (0.44-0.64% of the total dose).
The metabolic fate of fenvalerate in mammals is sum-
marized in Fig. 3.
5.1 Metabolism in Mammals
5.1.1 Rat
Following the single oral administration of fenval-
erate, labelled with 14C in the carbonyl of the acid
moiety (14CO) and the benzylic carbon (14Calpha), to
male rats (7-30 mg/kg body weight), the radiocarbon from
the acid and alcohol moieties was rapidly and completely
excreted [86, 134]. The tissue residues were generally
very low, except for those in the fat. The total recovery
of 14C in urine, faeces, and expired air was 93-99% in
6 days. However, on dosing with 14CN-labelled fenval-
erate, the radiocarbon derived from the CN group was
excreted relatively slowly into the urine and faeces, and
a considerable amount (10%) of the radiocarbon was also
excreted as CO2. Total recovery of 14C in urine,
faeces, and expired air was 75-81% in 6 days in this case.
The tissue residue levels were generally higher than those
from the acid and alcohol moieties. Hair, skin, and
stomach contents showed high residue levels, due to reten-
tion as 14C-thiocyanate. These excretion and tissue resi-
due patterns for the radiocarbon from the CN group were
similar to those with 14C dosed as KCN and KSCN in male
rats [134].
4.1 Transportation and Distribution Between Media
Hill [74] investigated the distribution of fenvalerate
residues in soil under field conditions using a microplot
technique. The microplots (20 x 20 cm) were treated with
fenvalerate at a rate of 150 g/ha. After 45 weeks, 11% of
the applied fenvalerate was located in the 0-2.5 cm soil
layer and less than 0.5% in the 2.5-5 cm soil samples.
Less than 0.1% of the applied fenvalerate was detected in
any of the soil samples taken after 3 or 4 weeks, despite
a rainfall of 95.4 mm during the first 4 weeks (including
a 25.9 mm downpour 15 days after application). These
results indicate that fenvalerate does not readily leach
downward and that lateral surface movement is very
limited.
A similar conclusion was obtained from laboratory
soil-leaching studies. More than 95% of the applied
fenvalerate remained in the treated portion of soil
columns when leaching was started immediately or 30 days
after treatment of the soil [133]. The possibility of
fenvalerate accumulating in orchard soils was assessed by
monitoring soil and leaf litter in an orchard in the
Okanagan valley, British Columbia, Canada, following
multiple annual application of the pesticide. Belmark 300
(30% fenvalerate EC formulation) had been sprayed at a
rate of 188-500 ml/ha (one to three times per year) for
more than three years. To obtain initial concentration,
organic litter samples were sprayed at a rate of 450 ml/ha
and samples were collected 2 h later. While the initial
concentration thus obtained in the litter was 0.214 mg/kg
(average value), orchard litter samples contained
0.30-0.63 mg/kg while samples from a non-treated block
contained < 0.002 mg/kg. Orchard soil samples (0-15 cm
depth) in two orchards contained fenvalerate residues of
< 0.0035-0.006 mg/kg and 0.0063-0.024 mg/kg [188].
4.2 Photodecompositiona
In studies by Holmstead et al. [78], fenvalerate (5),
at a concentration of 0.01 mol/litre in methanol, hexane,
or acetonitrilewater (60:40), underwent rapid photodegra-
dation under the action of UV light (290-320 nm) with a
half-life of 16-18 min (Fig. 2). With 90-95% conversion
after 60 min, 2-(3-phenoxyphenyl)-3-(4-chlorophenyl)-4-
methyl-pentanenitrile (31) (decarboxy-fenvalerate) was the
major photoproduct, amounting to 54-57% of the total reac-
tion mixture. There were smaller amounts of the dechlori-
nated analogue (32) of decarboxy-fenvalerate and the dimer
(8) of 2,2-dimethyl-4-chlorostyrene. 3-Phenoxybenzoyl cy-
anide (4), 3-phenoxybenzaldehyde (18) (PBald), 4-chloro-
isobutylbenzene (6), and 2,2-dimethyl-4-chlorostyrene (7)
were detected in small amounts in hexane or methanol. 3-
Phenoxybenzyl cyanide (28), its dimer (26), and 1,2-bis-
(phenoxyphenyl)ethane (27) were found only in hexane, and
methyl 3-phenoxybenzoate (12) was detected only in meth-
anol. Products found uniquely in acetonitrile-water were
2-(4-chlorophenyl)-3-methylbutyric acid (17) (CPIA), 3-
phenoxybenzoic acid (22) (PBacid) and 1-(4-chlorophenyl)-
2-methylpropanol (9). Several unknown compounds were ob-
served in the remaining 5-10%. Fenvalerate, in a thin film
(1 mg/cm2) on glass, decomposed in sunlight with a half-
life of approximately 4 days. About 10% of the applied
material remained after 43 days. In addition to the photo-
products formed in solution, small amounts of 3-phenoxy-
benzyl alcohol (19) (PBalc) and isopropyl 4-chlorophenyl-
ketone (10) were detected.
On exposure to autumn sunlight in Japan, the
[2S, alphaS] isomer of fenvalerate in distilled water
decomposed with a half-life of approximate 10 days. This
isomer photodecomposed via pathways that included decar-
boxylation, hydration of the CN group to a carbamoyl
(CONH2) group, hydrolysis of the CONH2 group to a car-
boxyl (COOH) group, and cleavage of the ester or diphenyl
ether linkage. Cleavage of the ester linkage was a major
photochemical reaction and led to the formation of 2-(4-
chlorophenyl)-3-methylbutyric acid (17) (17.3% of the
applied 14C, 10 days after exposure). There was no sig-
nificant difference between fenvalerate and the [2S, alphaS]
isomer in the rates and routes of photodegradation [179].
The photodegradation of fenvalerate (0.3-0.4 ng/cm2)
on two kinds of soil in natural sunlight was compared with
that of the [2S, alphaS] isomer. Fenvalerate and its isomer
photodecomposed with half-lives of 1.4-2.4 days and 1.1-
2.5 days, respectively. The pathways included hydration
of the cyano group to the carbamoyl group (19.2-48.4% at
10 days) with subsequent hydrolysis to the carboxyl group
(0.9-2.0% at 10 days), ester-bond cleavage (3.4-4.5% at 10
days), and decarboxylation (0.3-0.9% at 10 days). Little
2S/2R and alphaS/alphaR isomerization (as determined by
HPLC) occurred on the soils. There was no significant
difference between the two compounds in the rates and
pathways of photodegradation [89].
Holmstead & Fullmer [77] investigated the photodecar-
boxylation of several cyanohydrin esters in methanol and
hexane under artificial light as models for pyrethroid
photodecomposition. The cyanohydrin esters gave rise to
decarboxylated products, to a greater or lesser extent,
whereas the analogous compounds without the cyano group
did not produce the photodecarboxylated compounds.
alpha-Cyanobenzyl phenylacetate, which yielded the stable
benzyl radical, gave substantially larger amounts of the
decarboxylated product than alpha-cyanobenzyl benzoate,
which produced the unstable phenyl radical.
The photodegradation of fenvalerate in water and on
soil was investigated using compounds labelled with 14C
at the following positions: carbonyl group (CO-fenval-
----------------------------------------------------------------
a The numbers in brackets following chemical names refer to the
numbers given in Fig. 2.
erate), alpha-carbon in the benzyl group (Calpha-fenvale-
rate), and cyano group (CN-fenvalerate) [118]. On exposure
to sunlight, fenvalerate in very dilute solution in distil-
led water, in 2% aqueous acetone, in filter-sterilized
river water, or in sea water underwent rapid photolysis
with half-lives of approximately 4 days in summer and 13-
15 days in winter. The quantum yield was calculated at
6.6 x 10-3 (at 313 nm in water) and the half-life of dis-
appearance at latitude 40°N was calculated at 4.1 days in
summer and 12.4 days in winter, values which were close to
the experimental ones. Photodegradation of 14CN-fenval-
erate resulted in the formation of greater amounts of
14CO2 than 14CN-. After 6 weeks irradiation, ap-
proximately 30% (in aqueous acetone or river water) or
approximately 55-60% (in distilled water or sea water) of
the 14C was recovered as 14CO2, while the correspond-
ing figures for 14CN- were 5% and 30%. One of the major
photodegradation products was decarboxy-fenvalerate (31),
which increased to approximately 20% (in distilled water)
in summer after 1 week and decreased thereafter. In win-
ter, the amount was approximately 20% after 6 weeks. Other
major products were PBacid (22) and CPIA (17), derived
from the ester bond cleavage, amounting to 43% and 58%,
respectively, of the applied radioactivity after 6 weeks
in winter. In addition, small amounts of alpha-carbamoyl-
3-phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (33)
(CONH2-fenvalerate), alpha-carboxy-3-phenoxybenzyl-2-(4-
chlorophenyl)-3-methylbutyrate (34) (COOH-fenvalerate), 3-
phenoxybenzyl cyanide (28), 3-phenoxyphenylacetamide (29),
3-phenoxyphenylacetic acid (30), PBalc, and PBald were
detected.
Fenvalerate, as a deposit (5.5-5.9 µg/100 cm2) on
Kodaira light clay, Azuchi sandy clay loam, and Katano
sandy loam soil from Japan, was decomposed by autumn sun-
light with the respective half-lives of 2, 6, and 18 days
[118]. The major product was CONH2-fenvalerate (33),
which amounted to 7.9-25.7% of the applied radioactivity
after 10 days; it was formed in greatest amounts in sun-
light but also formed in the dark. Smaller amounts of
decarboxy-fenvalerate (31), the desphenyl analogue of
CONH2-fenvalerate (35), COOH-fenvalerate (34), PBacid,
and PBalc were also detected. Of the applied radiocarbon,
3-10% remained unidentified.
4.3 Decomposition in Plants
Fenvalerate (2.4% emulsifiable concentrate (EC)),
permethrin (2% EC), and deltamethrin (25 g/litre) were
sprayed onto cotton fields in Arizona, USA, at respective
rates of 0.11, 0.11, and 0.23 kg/ha, and dislodgeable
residues of the insecticides on cotton foliage were exam-
ined. Of the original deposits of fenvalerate, 65%
remained at the end of 96 h (there were two rains between
24 and 48 h), compared with 47% and 32% for permethrin and
deltamethrin, respectively [57].
Fenvalerate deposits on cotton plants (0.8 mg/plant)
disappeared rapidly, with only half the material remaining
after 8 days of exposure. After 23 days, decarboxy-fenval-
erate and ester-cleavage products such as PBacid, PBald,
PBalc, and CPIA were detectable, but not quantifiable.
Decarboxy-fenvalerate was considerably more stable to UV
light than fenvalerate, but it decomposed at a somewhat
faster rate than p,p'-DDT, yielding mainly the dechlori-
nated analogue [78].
The metabolism of fenvalerate in kidney bean plants
has been studied under laboratory conditions by Ohkawa et
al. [136]. Fenvalerate labelled with 14C at the cyano
group and the [2S, alphaRS] isomer labelled separately at
the cyano, carboxy, and benzylic carbon atoms were used to
treat individual bean leaves of 14-day-old seedlings at a
rate of 10 µg per leaf. After 60 days, 85-86% of the
applied 14C was recovered from plants treated with the
carboxy and benzyl labels, whereas 67% was recovered from
plants treated with the cyano label. Only limited translo-
cation was observed and only very low levels of radioac-
tive residues (2-9 µg/kg) were detected in seeds.
Fenvalerate and the [2S, alphaRS] isomer disappeared at a
similar rate from the treated leaves with an initial half-
life of 14 days.
The metabolism of racemic fenvalerate and of its
[2S, alphaS] isomer was examined in cabbage plants grown
under laboratory conditions and treated (20 µg per leaf)
with [14C]-chlorophenyl- and [phenyl-14C]-benzyl-labelled
preparations of the two compounds. Both compounds disap-
peared from the treated leaves with similar half-lives of
approximately 12-14 days. They underwent ester cleavage
to a significant extent, together with some hydroxylation
at the 2- or 4-position of the phenoxy ring and hydrolysis
of the nitrile group to amide and carboxyl groups. Most of
the carboxylic acids and phenols thus produced occurred as
glycoside conjugates. In a separate experiment, the uptake
and metabolism of CPIA (17) was examined in the laboratory
using abscised leaves of kidney bean, cabbage, cotton, cu-
cumber, and tomato plants. The acid (17) was found to be
readily converted, mainly into glucose or 6-O-malonylglu-
cose esters in kidney bean, cabbage, and cucumber, into
glucosylxylose, sophorose, and gentiobiose esters in
cotton, and into two types of triglucose esters with dif-
fering isomerism in tomato. One of the acetyl-derived
glucoside conjugates was identical with the authentic
deca-acetyl derivative of the [1-6]-triglucose ester
[121].
In studies by Ohkawa et al. [136], fenvalerate was
metabolized or degraded in bean plants via several routes.
A minor route was hydrolysis of the cyano group leading to
the formation of the amide (33) and carboxylic acid (34)
derivatives of fenvalerate. The 3-phen-oxybenzyl moiety
underwent metabolism to yield PBacid, 3-(2'-hydroxy-
phenoxy)benzoic acid (23) and PBalc, which occurred mainly
as sugar conjugates. In addition, glucoside conjugates
of alpha-carboxy-3-phenoxybenzyl alcohol (36) were detected
to a lesser extent. The presence of alpha-cyano-3-phenoxyben-
zyl alcohol (16) conjugates was inferred since PBald was
released upon treatment with beta-glucosidase. A major
metabolite of the acid moiety was CPIA (17), which also
occurred mainly as glucoside conjugates. The decarboxy
derivative (31) of fenvalerate, presumably formed by
photochemical reaction on plant foliage as discussed pre-
viously, was detected in leaf extracts. When bean plant
seedlings were planted and left for 30 days in light clay
and sandy loam soils treated with 14C-fenvalerate at
1 mg/kg, the roots retained fairly large amounts of radio-
carbon. However, only limited radiocarbon was found in
the shoots (0.02 mg/kg), pods and seeds (0.01 mg/kg), and
there was no parent compound in the shoots.
Additional studies were carried out to investigate the
fate of 3-phenoxybenzoic acid (an important metabolite of
fenvalerate and most other pyrethroids) in plants. Using
abscised leaves of cabbage, cotton, cucumber, kidney bean
and tomato plants, 14C-3-phenoxybenzoic acid was shown to
conjugate with a complex range of sugars [120].
4.4 Decomposition in Soils
The degradation of fenvalerate in soils has been
studied under various conditions (aerobic or anaerobic
conditions, laboratory or field conditions, using radioac-
tive or non-radioactive material).
Samples of 14C-fenvalerate labelled separately in the
carboxy and cyano groups were used for soil studies by
Ohkawa et al. [133]. When several types of soil were
treated at a rate of 1 mg/kg and stored at 25°C under
aerobic conditions, the initial half-life of fenvalerate
ranged from 15 days to 3 months. As with other
pyrethroids, hydrolysis at the ester linkage was a major
degradation route, and ring hydroxylation in the 4'-pos-
ition (25), together with hydrolysis of the cyano group to
the amide and carboxyl groups, occurred to smaller ex-
tents. The degradation route unique to fenvalerate was
ether-bond cleavage yielding alpha-cyano-3-hydroxybenzyl-
1-2-(4-chlorophenyl)-3-methylbutyrate (14), which could be
produced through hydroxylation at the 2'7-position (13) in
the alcohol moiety. No H14CN released during ester-bond
cleavage was detected owing to its rapid conversion to
14CO2. The amount of 14CO2 was greater with the
cyano label than the carboxy label. For example, after
30 days in Katano sandy loam soil, 47.5% and 38.2% of the
applied radiolabel was evolved as 14CO2 from the cyano
and carboxyl groups, respectively. In a laboratory soil-
leaching study, less than 1% of the applied radiocarbon
appeared in the effluent when leaching was started
immediately after treatment of the soil. Even after a
30-day incubation, only a trace amount of CPIA (17) was
detected in the effluent from soil columns treated with
14C-carbonyl-fenvalerate. In a separate experiment, the
degradation of 14C-fenvalerate was studied in a soil-
nutrient liquid suspension system. Separate cultures of
bacteria and fungi were used for the system. After 2 weeks
of incubation, larger amounts of 14CO2 (35-42% of the
applied radiolabel) were formed from both culture media
when the 14CN-labelled compound was used than when the
14CO-labelled compound was used (1.1-2.3%). In the
latter case, the main degradation product was CPIA, which
amounted to 34-69% [133].
Studies using 14C-fenvalerate, labelled separately in
the chlorophenyl and benzyl groups, confirmed the degra-
dation pathways mentioned above. These studies also showed
that the labelled aromatic rings were also readily de-
graded to 14CO2 (up to 66%). In addition, it was found
that any "bound residues" formed could be further de-
graded to 14CO2 by admixture with fresh soils [119].
The rate of degradation of the individual isomers of
fenvalerate has been investigated. In one soil, the half-
lives of the RR, RS, SR, and SS isomers were shown to be
178, 89, 155, and 108 days, respectively [105]. Different
rates for the various isomers were similarly obtained in
loam and sandy loam soils [160].
Under flooded conditions fenvalerate degrades more
slowly than under aerobic conditions. In sterile soil,
degradation is minimal, indicating that microbial activity
is the major cause of this degradation [102]. Ohkawa et
al. [133] reported similar findings.
Studies in which crops were sown in soils containing
aged residues of 14C-fenvalerate (aging periods of 30,
120, and 345 days) showed that residues from fenvalerate
should not carry over into rotated crops [102].
The persistence of fenvalerate in Lethbridge (Canada)
soil has been studied under field and laboratory con-
ditions [74]. Formulated fenvalerate (30% emulsifiable
concentrate) was applied once to soil microplots in the
field at a dose rate of 600 µg/plot (150 g/ha) or to
soil in pots at a dose of 88.7 µg/pot (10 g/ha). The
treated pots were maintained at a daily temperature regime
of 20°C for 16 h and 10°C for 8 h in the environmental
chamber. Fenvalerate was found mainly in the top 2.5 cm
of the field soil, and 16 weeks later, 15% of the applied
fenvalerate remained. The initial half-lives were 5.9
weeks for the [2S, alphaR] [2R, alphaS] enantiomeric pair
and 6 weeks for the [2R, alphaR] [2S, alphaS] pair. The
spring soil samples, taken 45 weeks after application, con-
tained 11% of the total fenvalerate. Limited degradation
occurred during the winter. The degradation of fenvalerate
in soil incubated in the environmental chamber was similar
to the field results. The [2S, alphaR] [2R, alphaS] enant-
iomeric pair had a half-life of 5 weeks while the [2R,
alphaR] [2S, alphaS] pair had a half-life of 5.3 weeks.
These results were comparable to the average half-life of
7 weeks for fenvalerate incubated in British Columbia soils
[198].
In studies by Harris et al. [69], the persistence of
fenvalerate in subtropical field soil (average soil tem-
perature, 20-30°C) was investigated after applying 20%
emulsifiable concentrate at a rate of 1 kg active ingredi-
ent (ai)/ha twice a year (spring and autumn) over a 2.5-
year period. Residues in the top 15 cm of soil were moni-
tored for up to one year after the final application.
Fenvalerate levels declined rapidly after the spring
application and relatively slowly after the autumn appli-
cation. There was no carry over of the insecticide from
year to year, and after 2.5 years of application only 2%
of the total fenvalerate remained. The rate of disappear-
ance became slightly slower when fenvalerate application
ceased [181]. The degradation of fenvalerate (14.9 mg/kg)
in plainfield sand (5% moisture) at 25°C was relatively
slow, with an initial half-life of 2 months, as compared
with initial half-lives of 0.5, 1, and 2 months for
fenpropathrin (7.1 mg/kg), permethrin (8.8 mg/kg), and
cypermethrin (7.3 mg/kg), respectively, under laboratory
conditions.
A 2-year field study on the relative persistence of
permethrin, cypermethrin, fenpropathrin, and fenvalerate
in soils was carried out by Chapman & Harris [25]. The
pyrethroids were applied as emulsifiable concentrates at a
rate of 280 g ai/ha or 140 g ai/ha to duplicate plots in
Ontario, Canada, containing either sand or organic soil.
For plots treated at the higher rate, the insecticide was
immediately raked into the soil, while the plots receiving
the lower rate were left undisturbed and the upper 4-5 cm
of soil was subjected to gas-liquid chromatography (GLC)
analyses. The concentrations of the four pyrethroids
incorporated in both soils or remaining on the upper soil
layer decreased to less than 50% of the initial values
within one month. Again, fenvalerate was slightly more
persistent, with 7% of the initial application remaining
in organic soil 28 months after treatment.
Reed et al. [157] demonstrated that when fenvalerate
was applied to soil, adsorption prevented significant
leaching of the pesticide. Soil metabolites produced
either by photolytic or microbial degradation did not
accumulate to a significant level or present a problem in
subsequent rotation crops (lettuce, beets, and wheat)
planted at 30 days, 60 days, 120 days, or 1 year after
soil treatment. Although fenvalerate has intrinsically
high toxicity to a variety of aquatic organisms, these
field studies demonstrated that the toxicant was unavail-
able to non-target organisms. Therefore, it had little or
no impact in this test system following its use at the
maximum allowed rate of 2.24 kg/ha per year.
4.5 Decomposition in Water
The hydrolysis of racemic fenvalerate in buffered
aqueous solutions at pH 5.0, 7.0, and 9.0 was compared by
Katagi et al. [90] with that of the [2S, alphaS] isomer.
Both compounds were fairly stable at pH 5.0 and 7.0 (half-
lives of 130-220 days), while at pH 9.0 they underwent
hydrolysis (half-lives of 64.6-67.2 days) mainly via ester
bond cleavage. The main product was 2-(4-chlorophenyl)-3-
methylbutyric acid (17), which amounted to 14.9% of the
applied 14C after 28 days. As the [2S, alphaS] isomer
underwent alphaS/alphaR epimerization in the alcohol moiety
at pH 7.0 and 9.0, its rate of hydrolysis appeared to be
rather faster than that of fenvalerate. However, the half-
life estimated from the total amounts of [2S, alphaS] and
[2S, alphaR] epimer was close to that of fenvalerate, which
indicates no significant difference in hydrolysis rate.
The persistence of fenvalerate has been evaluated
in water and sediment contained in open trenches
(3 m x 1 m x 30 cm) lined with alkathene sheet [1]. Insec-
ticide emulsion was sprayed on the surface of the water at
the normal rate and at twice the recommended dosage. The
dissipation of the insecticide from water was rapid. About
74-80% of the pesticide was lost within 24 h at both ap-
plication rates. However, residues were found to be ad-
sorbed onto sediment, and these persisted beyond 30 days.
In soil, persistence was moderate, lasting around
30 days.
5. KINETICS AND METABOLISM
Appraisal
The metabolic fate of fenvalerate in rats, mice, and cows
has been studied using variously labelled racemic fenvalerate
(acid moiety or benzyl or cyano groups labelled).
From oral administration studies, fenvalerate appears to be
absorbed rapidly through the gastrointestinal wall.
Following a single oral administration of labelled fenval-
erate to rats, the excretion of radiocarbon from the acid or
benzyl moieties was fairly rapid. However, the excretion of
radiocarbon originating from the cyano group was relatively
slow, the rest of the radioactivity being retained in various
tissues, particularly in hair and stomach as thiocyanate. The
major routes of metabolism were ester cleavage, hydroxylation
at the 4'position of the alcohol moiety, and thiocyanate
formation from the cyano group. Major metabolites were 2-(4-
chlorophenyl)isovaleric acid (Cl-Vacid) and 3-OH-Cl-Vacid (Cl-
Vacid hydroxylated at the 3 position) from the acid moiety, and
the sulfate conjugate of 3-(4'-hydroxyphenyl)benzoic acid and
thiocyanate from the alcohol moiety. A lipophilic metabolite,
cholesteryl-[2R]-2-(4-chlorophenyl)isovalerate, which was related
to granuloma formation, was detected in the adrenals, liver,
and the mesenteric lymph nodes of rats, mice, and some other
species. The excretion of fenvalerate in the milk from orally
dosed cows was very low (0.44-0.64% of the total dose).
The metabolic fate of fenvalerate in mammals is sum-
marized in Fig. 3.
5.1 Metabolism in Mammals
5.1.1 Rat
Following the single oral administration of fenval-
erate, labelled with 14C in the carbonyl of the acid
moiety (14CO) and the benzylic carbon (14Calpha), to
male rats (7-30 mg/kg body weight), the radiocarbon from
the acid and alcohol moieties was rapidly and completely
excreted [86, 134]. The tissue residues were generally
very low, except for those in the fat. The total recovery
of 14C in urine, faeces, and expired air was 93-99% in
6 days. However, on dosing with 14CN-labelled fenval-
erate, the radiocarbon derived from the CN group was
excreted relatively slowly into the urine and faeces, and
a considerable amount (10%) of the radiocarbon was also
excreted as CO2. Total recovery of 14C in urine,
faeces, and expired air was 75-81% in 6 days in this case.
The tissue residue levels were generally higher than those
from the acid and alcohol moieties. Hair, skin, and
stomach contents showed high residue levels, due to reten-
tion as 14C-thiocyanate. These excretion and tissue resi-
due patterns for the radiocarbon from the CN group were
similar to those with 14C dosed as KCN and KSCN in male
rats [134].
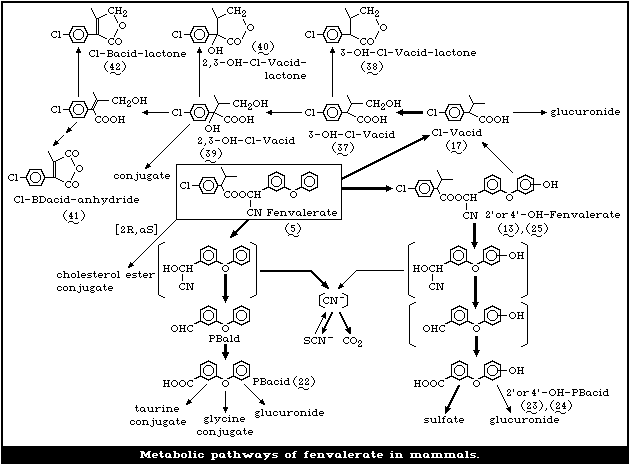 It was shown in the same study that fenvalerate under-
went oxidation at the 2'and 4'positions of the alcohol
moiety, as well as at the 2 and 3 positions of the acid
moiety, ester cleavage, and the conjugation of resultant
phenols and carboxylic acids with glucuronic acid, sul-
furic acid, and glycine. Cleavage of fenvalerate and its
ester metabolites appeared to release cyanohydrins, which
were, however, unstable under physiological conditions and
decomposed easily to cyanide and aldehydes (Fig. 3). The
cyanide ion was converted mainly to thiocyanate and
CO2, and 2-iminothiazolidine-4-carboxylic acid, a metab-
olite detected with other pyrethroids containing a cyanide
group, was not positively identified [134]. The major fae-
cal metabolites from 14CO-, 14Calpha-, and 14CN-fenval-
erate were unchanged fenvalerate (5) and two ester metab-
olites of 2'-hydroxy-(13) and 4'-hydroxy-fenvalerate (25).
The major metabolites in 0- to 2-day pooled urine (50-55%
of the dosed radioactivity) from the acid-labelling were
2-(4-chlorophenyl)isovaleric acid (17) (Cl-Vacid), 2-(4-
chlorophenyl)-3-hydroxymethylbutyric acid (37) (3-OH-Cl-
Vacid), and its lactone (38) (3-OH-Cl-Vacid-lactone).
Other minor metabolites were 2-(4-chlorophenyl)-2-hydroxy-
3-hydroxymethylbutyric acid (39) (2,3-OH-Cl-Vacid) in the
free, the lactone (40) (2,3-OH-Cl-Vacid-lactone), and the
conjugated forms, 2-(4-chlorophenyl)- cis-2-butenedioic
acid anhydride (41) (Cl-BDacid anhydride), and 2-(4-
chlorophenyl)-3-methyl-2-butene-4-olide (42) (Cl-B-acid-
lactone). On the other hand, the predominant urinary
metabolite from the alcohol moiety was the sulfate conju-
gate of 3-(4'-hydroxyphenoxy)benzoic acid (23) (4'-OH-
PBacid), accounting for approximately 40% of the dose.
Other major metabolites were 3-phenoxybenzoic acid (22)
(PBacid) in the free (6%), the glucuronide (2%), and the
glycine (2%) conjugated forms, 4'-OH-PBacid in the free
(5%) and the glucuronide (2%) forms, and the sulfate of 3-
(2'-hydroxyphenoxy)benzoic acid (24) (2'-OH-PBacid) (3%).
With 14CN-fenvalerate, the major urinary metabolite was
thiocyanate (43) [134].
Pydrin insecticide (Y-rich) is an isomerically
enriched form of fenvalerate containing an excess ratio of
the active diastereomers SS and RR (designated Y) over the
less active diastereomers RS and SR (designated X) at a
ratio of approximately 85:15. Fenvalerate contains Y:X in
a ratio of 45:55. Following a single oral dose of the Y-
rich insecticide (8.4 mg/kg) to male and female Sprague-
Dawley rats, more than 90% of the administered radioac-
tivity from the acid moiety (chlorophenyl-14C) and the
alcohol moiety (phenoxyphenyl-14C) was eliminated within
the first 24 h. There was no major difference between the
two different fenvalerate preparations in either the elim-
ination rate or the metabolites distribution profile.
Cleavage of the ester linkage was the primary metabolic
pathway. The acid and alcohol portions of the parent mole-
cule underwent hydroxylation, oxidation, and conjugation.
These metabolic reactions were not dependent on the iso-
meric composition of the test material. Tissue residue
data showed that 14C residues were not retained in the
various organs [104].
The fate of sugar conjugates, which may be formed as
plant metabolites, has been investigated by Mikami et al.
[122]. Upon single oral administration to male Sprague-
Dawley rats at a concentration of 3.8 mg/kg, the mono-,
di-, and tri-glucose conjugates of [14C]-3-phenoxybenzyl
alcohol (19) and the mono-glucose conjugate of [14C]-3-
phenoxybenzoic acid (22) were rapidly hydrolysed and
extensively eliminated in the urine, mostly as the sulfate
conjugate of 3-(4-hydroxyphenoxy)benzoic acid (24).
Faecal elimination was a minor route, whereas biliary
excretion was responsible for about 42% of the dose, and
the glucuronide conjugates of (19), (22), and (24) were
common major metabolites. The biliary glucuronides were
metabolized in the small intestine to the respective
aglycones, which were reabsorbed, metabolized further, and
excreted in the urine as the sulfate conjugate of (24).
Although small amounts of the mono-, di-, and tri-gluco-
sides were found in the 30-min blood and liver samples
following oral administration of the tri-glucoside of
(19), they were not detected in the urine, bile, or fae-
ces. Similarly, the sulfate conjugate was one of the major
urinary metabolites in germ-free male rats, when dosed
with the 14C-glucosides at a rate of 9 µmol/kg via the
oral or intraperitoneal route, although certain amounts
were excreted unchanged in the urine and faeces. The glu-
cose conjugates were metabolized in vitro by intestinal
microflora and in various rat tissues including blood,
liver, small intestine, and small intestinal mucosa. The
tissue enzymes showed a different substrate specificity in
hydrolysing the glucosides. However, they were not metab-
olized in gastric juice, bile, pancreatic juice, or
urine.
5.1.2 Mouse
In mice, fenvalerate is metabolized in a similar way
to that in rats, but the following significant species
differences were found by Kaneko et al. [86]: (a) the
taurine conjugate of PBacid was found in mice but not in
rats; (b) 4'-OH-PBacid sulfate occurred to a greater
extent in rats than in mice; and (c) a greater amount of
thiocyanate was excreted in mice than in rats. No sig-
nificant sex differences were observed in rats and mice.
The metabolism of the stereoisomers of fenvalerate,
([2S, alphaRS] and [2S, alphaS]) was apparently similar to
that of racemic fenvalerate.
Following a single oral administration of the four
chiral isomers of [14C-chlorophenyl]-fenvalerate to
Sprague-Dawley rats and ddY mice (2.5 mg/kg body weight),
the [2R, alphaS] isomer showed, in both rats and mice, rel-
atively greater residues in the analyzed tissues (except
fat), particularly in adrenal glands, compared with the
other three isomers. Similarly, this isomer showed
higher tissue concentrations than the other isomers when
mice were fed a diet containing 500 mg/kg of the
[2S, alphaS], [2R, alphaS], or [2R, alphaR] isomers for
two weeks. The greater amount of radioactive residues from
the administration of [2R, alphaS] isomer, as compared
with those of other isomers, was explained by the
preferential formation of a lipophilic metabolite from the
[2R, alphaS] isomer found in all examined tissues, which
was not easily excreted. The amounts of the lipophilic
metabolite differed among tissues, being higher in adrenal,
liver, and mesenteric lymph nodes. This metabolite was
identified as cholesteryl [2R]-2-(4-chlorophenyl)isoval-
erate. The presence of the same metabolite was also indi-
cated in rat tissues [87].
5.1.3 Domestic animals
Two 3-month-old lambs were fed a diet containing
45 mg/kg fenvalerate for 10 days and then killed to deter-
mine the concentrations of fenvalerate in the kidney,
liver, leg muscle, and renal fat [210]. Among the analyzed
tissues, fat showed the highest fenvalerate level (3.6-4.4
mg/kg dry weight) while other tissues contained less than
0.3 mg/kg. Fenvalerate gave two gas chromatographic peaks
and each peak contained a pair of its enantiomers. In all
cases, the ratio of the areas of these peaks (peak 1
(RS,SR)/peak 2 (SS,RR)) was 1.08 both for fenvalerate in
the diet and for fenvalerate recovered from the fortified
control fat. In contrast, the fenvalerate isolated from
lamb fat had a peak area ratio of 0.76-0.78. Thus, one or
both of the first eluting enantiomers appeared to be
metabolized more rapidly than the other enantiomers.
In a study by Wszolek et al. [209], two Holstein cows
were fed fenvalerate at 5 and 15 mg/kg diet for 4 days and
were then given a clean diet for 6 days. Total excretion
of fenvalerate in milk amounted to 0.44 and 0.64% of the
total dose for the 5 and 15 mg/kg levels, respectively,
whereas about 25% of the dose was eliminated in the
faeces.
A lactating Holstein cow was fed grain fortified with
227 mg fenvalerate daily for 4 days and the urine was ana-
lysed. Intact fenvalerate was not detected in any samples
of the urine excreted by the cow during the 10-day feeding
study, nor was the acid metabolite (Cl-Vacid) (17) ident-
ified. The in vitro study on fenvalerate degradation in
rumen fluid indicated that no significant degradation of
fenvalerate was observed during the 6-h incubation [211].
Saleh et al. [161] gave a single oral dose of fenval-
erate (10 mg/kg body weight) to chickens and monitored the
persistence and distribution of the insecticide over 15
days. A concentration of 4.7 mg/litre in blood after 24 h
fell to 0.05 mg/litre after 7 days. Levels in other tis-
sues reached maxima of less than 1.0 mg/kg and fell rap-
idly. However, brain residues rose to a level of 4.0 mg/kg
over 7 days and persisted for the 15 days of the exper-
iment. Concentrations in eggs reached a maximum of
0.3 mg/kg yolk after 4 to 5 days, and a maximum of
0.24 mg/kg egg white. By day 6, levels had returned to the
pre-dosing level.
5.2 Enzymatic Systems for Biotransformation
The [2R, alphaRS] isomer of fenvalerate has been found
to be more rapidly hydrolysed by mouse liver esterase than
the [2S, alphaRS] isomer, but less rapidly metabolised than
the [2R, alphaRS] isomer with an oxidase system. A similar
correlation was observed with the [2S] and [2R] isomers of
S-5439 (3-phenoxybenzyl-2-(4-chlorophenyl)isovalerate) [165].
In an in vitro study on the metabolism of the four
chiral isomers of fenvalerate using homogenates from vari-
ous tissues of mice, rats, dogs, and monkeys, only the
[2R, alphaS] isomer yielded cholesteryl-[2R]-2-(4-chloro-
phenyl)isovalerate (CPIA-cholesterol ester) as a major
metabolite. Mouse tissues exhibited a higher rate of CPIA-
cholesterol ester formation than those of other animals.
Of the mouse tissues tested, the kidney, brain, and spleen
showed the greatest ability to form this ester, the rel-
evant enzyme activity being mainly localized in the
microsomal fractions. Carboxyesterases for mouse kidney
microsomes hydrolyzed the [2R, alphaS] isomer only of
fenvalerate to give CPIA and yielded the corresponding
cholesterol ester in the presence of artificial liposomes
containing cholesterol. It appears that the CPIA-
cholesterol ester resulted from the stereoselective
([2R, alphaS] only) formation of the CPIA-carboxyesterase
complex, which subsequently reacted with cholesterol to
yield the CPIA-cholesterol ester [128].
Hydrolysis of the four chiral isomers of fenvalerate
by microsomes of various mouse tissues has been investi-
gated by Takamatsu et al. [180]. The kidney, spleen and
brain hydrolyzed only the [2R, alphaS] isomer. Liver hydro-
lyzed the [2R, alphaS] and [2R, alphaR] isomers to a great-
er extent than the [2S, alphaR] and [2S, alphaS] isomers,
while plasma hydrolysed the [2S, alphaR] and [2R, alphaR]
isomers more rapidly than the [2S, alphaS] and [2R, alphaS]
isomers. The stereoselectivity of hydrolysis of the four
isomers by mouse liver microsomes was found to be same as
that in vivo. Of the four isomers, the [2R, alphaS] isomer
alone was transformed to cholesteryl-[2R]-2-(4-chlorophenyl)
isovalerate (CPIA-cholesterol ester) by microsomes of the
brain, kidney, spleen, or liver but not by plasma. The
rate of CPIA-cholesterol ester formation was lower in the
liver than in other tissues. The optimum pH (7.4-9.0) for
the formation of this ester was nearly the same as that
for hydrolysis of the [2R, alphaS] isomer to form CPIA in
mouse kidney microsomes.
The substrate specificity of microsomal carboxyester-
ase(s) responsible for the formation of cholesteryl-[2R]-
2-(4-chlorophenyl)isovalerate from fenvalerate was inves-
tigated by incubating mouse kidney microsomes with 14C-
cholesterol and fenvalerate or its analogues. Of the four
isomers of fenvalerate, only the [2R, alphaS] isomer yielded
a cholesterol ester. This specificity of cholesterol ester
formation was the same as that in the in vivo study. Some
of the fenvalerate analogues also produced similar choles-
terol esters. Steroids other than cholesterol were also
investigated as acceptors of the acid moiety of the
[2R, alphaS] isomer by incubating egg lecithin and several
steroids with the [2R, alphaS] isomer in the presence of
solubilized carboxyesterase(s). Dehydroisoandrosterone and
pregnenolone reacted with the [2R, alphaS] isomer to give the
corresponding ester conjugates [88].
One or more carboxyesterases located in the soluble
fraction of mouse brain homogenates hydrolyzed several
pyrethroid esters with a substrate specificity different
from that of the hepatic esterases. In particular,
fenvalerate and fluvalinate were hydrolyzed by brain
esterases at rates equal to or greater than that measured
for trans-permethrin. The results suggest that hydrolysis
in the brain may contribute to the detoxication of some
pyrethroids in mammals [64].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The acute toxicity data of fenvalerate to aquatic and
terrestrial non-target organisms are summarized in Tables
5, 6, and 7.
6.1 Aquatic Organisms
6.1.1 Toxicity to aquatic invertebrates
Non-target invertebrates, except molluscs, are more
susceptible to the insecticide than fish, the LC50
ranging from 0.08 to 2 µg/litre.
Fenvalerate is relatively non-toxic to oysters and
algae (LC50 >1000 µg/litre) over short exposure periods.
Snails (Heliosoma trivolvis) exposed for 28 days to 0.79
µg/litre, the highest concentration tested, showed no
change in behaviour or survival [4].
Day & Kaushik [32] conducted short-term toxicity
tests on three species of cladoceran and one species of
calanoid (Diaptomus oregonensis). The 48-h LC50 values
for daphnids were: 2.52 µg/litre for adult Daphnia magna,
0.83 µg/litre for D. magna aged 48 h (or less);
0.29 µg/litre for adult Daphnia galeata mendotae;
0.21 µg/litre for adult Ceriodaphnia lacustris; 0.16
µg/litre for D. galeata mendotae aged 48 h (or less).
Diaptomus oregonensis was the most sensitive species with
a 48-h LC50 of 0.12 µg/litre. No toxicity was found with
the emulsifiable concentrate from which fenvalerate was
omitted (EC control). Rates of filtration of algae were
reduced at sub-lethal concentrations of fenvalerate.
Ceriodaphnia lacustris was the most sensitive species,
with rates of filtration significantly decreased at
fenvalerate concentrations of 0.01 µg/litre. Rates of
assimilation of algae were decreased at fenvalerate con-
centrations of 0.05 µg/litre or more.
Day & Kaushik [33] conducted life-cycle studies on the
toxicity of fenvalerate to Daphnia galeata mendotae.
Lifetable methods were used to generate statistical
comparisons between treatments. At a concentration of
0.005 µg/litre, fenvalerate increased the longevity of
the daphnids significantly from 37.6 to 51.6 days. How-
ever, at the same concentration, production of young was
decreased. Higher concentrations of fenvalerate caused
reduced survival of the adults. The intrinsic rate of
natural increase in the population was reduced at a con-
centration of 0.5 µg/litre. At 0.01 µg/litre, the net
reproductive rate decreased from 126 to 73 offspring per
female and the generation time from 20.3 to 17.3 days.
Table 5. Acute toxicity of fenvalerate to non-target freshwater organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formu- Systemc Temperature pH Hardnessd Reference
(µg/litre) lationb (° C)
--------------------------------------------------------------------------------------------------------------------------------------------
Arthropods
Gammarus pseudolimnaeus adult-juv 96-h LC50 0.03 T F 15 7.6-7.8 46-48 4
Gammarus pseudolimnaeus 1-3 mm, juv 96-h LC50 0.05 T R 17 7.6-7.8 46-48 4
Waterflea 1st instar 96-h LC50 0.032 T S 17 7.4 44 115
(Daphnia magna)
Midge 3rd instar 48-h LC50 0.43 T S 22 7.4 44 115
(Chironomus pulmosus)
Mayfly larva 9-day LC50 0.08 T F 15 7.6 46-48 4
(Ephemerella sp.)
Rhagionid fly larva 28-day LC50 0.03 T F 15 7.6-7.8 46-48 4
(Atherix)
Stonefly naiad 72-h EC50 0.13 T F 15 7.6-7.8 46-48 4
(Pteronarcys dorsata)
Stonefly 3-6 weeks old 96-h LC50 1.9 EC S 20-22 7.8 7% 107
(Nitocra spinipes)
Fish
Atlantic salmon 6.2 cm, 5.3 g 96-h LC50 1.2 T R 10 110
(Salmo salar)
Rainbow trout 5-6 cm 48-h LC50 3.0 EC S 12-25.5 129
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 76 T S 10 7.5 110 28
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 21 EC S 10 7.5 110 28
(Salmo gairdneri)
Mosquitofish 4-5 cm 48-h LC50 15.0 EC S 8.8-16 129
(Gambusia affinis)
Mosquitofish 3-days old 72-h LC50 2.6 T S 24-27 124
(Gambusia affinis)
Desert pupfish 4-5 cm 48-h LC50 25.0 EC S 11-16.6 129
(Cyprinodon macularis)
Tilapia mossambica 5-6 cm 48-h LC50 200.0 EC S 15-21.4 129
Bluegill sunfish adult 96-h LC50 0.76 T S 22 7.4 40 115
(Lepomis macrochirus)
Fathead minnow adult 96-h LC50 2.35 T S 22 7.1 49 115
(Pimephales promelas)
--------------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
d expressed as mg CaCO3 per litre.
Table 6. Acute toxicity of fenvalerate to non-target estuarine & marine organisms
---------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formul- Systemc Temper- pH Salinity Reference
(µg/litre) ationb ature o/oo
(° C)
---------------------------------------------------------------------------------------------------------------------------------------
Algae
Skeletonema costatum 96-h EC50 > 1000 T 20 30 212
Isochrysis galbana 96-h EC50 > 1000 T 20 30 212
Thalassiosira pseudonana 96-h EC50 > 1000 T 20 30 212
Nitzschia angularum 96-h EC50 > 1000 T 20 30 212
Molluscs
Eastern oyster 2-h larva 48-h EC50 >
1000 T S 25 20 212
(Crassostrea virginica)
Arthropods
Lobster (Homarus americanus) 450 g 96-h LC50 0.14 T R 10 30 110
Shrimp (Crangon septemspinosa) 1.3 g 96-h LC50 0.04 T R 10 110
Shrimp (Mysidopsis bahia) 1-day juv 96-h LC50 0.021 T S 25 20 212
Shrimp (Mysidopsis bahia) newly hatched 96-h LC50 0.008 T F 25.4 25.3 163
Shrimp (Penalus duorarum) adult 96-h LC50 0.84 T F 24.8 24.9 163
California grunion 3-day larva 96-h LC50 0.29 T F 26 25 114
(Leuresthes tenuis)
California grunion juv 96-h LC50 0.60 T F 25 22 114
(Leuresthes tenuis)
Inland silverside 26-day larva 96-h LC50 1.00 T F 24 20 114
(Nenidia beryllina)
Tidewater silverside juv 96-h LC50 1.00 T F 25 20 114
(Menidia peninsulae)
Fish
Sheepshead minnow 28-day fry 96-h LC50 121 T S 25 20 212
(Cyprinodon variegatus)
Sheepshead minnow adult 96-h LC50 5 T F 30 26.5 163
(Cyprinodon variegatus)
Bleak (Alburnus alburnus) 8 cm 96-h LC50 2-3 EC S 10 7.8 7 107
Atlantic silverside adult 96-h LC50 0.31 T F 24.1 25 163
(Menidia menidia)
Striped mullet adult 96-h LC50 0.58 T F 25.9 25.8 163
(Mugil cephalus)
Gulf toadfish adult 96-h LC50 5.4 T F 30 24.8 163
(Opsanus beta)
---------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
Table 7. Acute toxicity of fenvalerate to non-target terrestrial organisms
-------------------------------------------------------------------------------------------------------------------------
Species Size Application Parameter Toxicity Temper- Reference
ature
(°C)
-------------------------------------------------------------------------------------------------------------------------
Bird
Broiler chicks 8-12 weeks old, oral LD50 12 590 (mg/kg) 31-32 155
0.99-2.2 kg
Hen oral LD50 > 1500 (mg/kg) 123
Arthropods
Insect parasite
Ichneumoid adult male film 24-h LC50 1760 (ng/vial) 152
(Campoletis sonorensis)
Insect predators
Lacewing adult 2.74 mg topical LD50 4.3 (mg/kg) 15 176
(Austromicromus tasmaniae) larva 2.52 mg topical LD50 67 (mg/kg) 20 176
Lacewing (Chrysopa carnea) 3rd instar, topical 72-h ED50 > 25 (mg/g) 28 164
larva 9.9-10 mg topical ED50 ~ 1 (mg/g) 28 164
one generation
Lacewing (Chrysopa carnea) larva film LC50 0.073 (mg/vial) 25 151
5-6 days old
Beetle 11.2 mg topical LD50 0.38 (mg/kg) 15 164
(Coccinella undecimpunctata)
Earwig (Labidura riparia) mature soil 0.11 kg ai/ha mortality 6% 205
0.22 kg ai/ha mortality 25% 205
0.44 kg ai/ha mortality 50% 205
Honey bee (Apis mellifera) adult topical LD50 410 ng/bee - 5
Predaceous mite species
Amblyseius fallacis adult female slide dip method LC50 2.6 (mg ai/litre) 27 158
Amblyseius fallacis adult female slide dip method LC50 7.0 (mg ai/litre) 26 199
Typhlodromus pyri adult female slide dip method LC50 8.1 (mg ai/litre) 26 199
Typhlodromus occidentalis adult female slide dip method LC50 2.1 (mg ai/litre) 26 199
-------------------------------------------------------------------------------------------------------------------------
McKenney & Hamaker [109] exposed the estuarine grass
shrimp Palaemonetes pugio to fenvalerate, in a flow-
through system to maintain constant exposure, throughout
20 days of larval development. The study was conducted
under optimal salinity conditions (20 o/oo). A nominal
concentration of 3.2 ng/litre significantly reduced the
percentage of larvae successfully completing metamor-
phosis. Exposure to 1.6 ng/litre prolonged larval develop-
ment. Larvae were also found to be less capable of
responding successfully to osmotic stress after exposure
to fenvalerate at 0.1 or 0.2 ng/litre.
6.1.2 Toxicity to fish
Fenvalerate is toxic to fish, LC50 values being
0.29-200 µg/litre (Tables 5 and 6). The LC50 value for
rainbow trout obtained with an emulsifiable concentrate
was 3.6 times lower than that for the technical product
[28]. The toxicity of fenvalerate to adult bluegill sun-
fish (Lepomis macrochirus) was unaffected by changes in
water hardness and pH [115].
The acute toxicities (96-h LC50) of fenvalerate to
juvenile steelhead trout were 172 ng/litre and 88
ng/litre, respectively, under continuous and intermittent
exposure (approximate peak concentration: 460 ± 40
ng/litre for 4.5 h). Prolonged intermittent exposure
(70 days) of the early life-stage resulted in marked
lethality (32%) and reduced terminal weight (50% of con-
trol) (mean concentration: 80 ng/litre, peak concen-
tration: 461 ng/litre). However, continuous exposure to
80 ng/litre for 70 days did not effect these parameters
[31].
Fenvalerate has narrow safety margins for fish
(LC50 of fish : LC50 of mosquito larvae is in the ratio
of 1:24) when the insecticide is used against mosquitoes
[129].
Four rainbow trout (Salmo gairdneri) died within 11
hours when exposed to 412 µg fenvalerate/litre. Visible
signs of poisoning included elevated cough rate, tremors,
and seizures. Ventilatory and cardiac activity stopped
during the seizures. Histopathological examination of
gill tissue showed damage consistent with irritation, and
Na+ and K+ excretion rates were elevated. Fenvalerate
concentrations in brain, liver, and carcass at death were
0.16, 3.62, and 0.25 mg/kg, respectively. The study
suggested that, apart from effects on the nervous system,
effects on respiratory surfaces and renal ion regulation
may be associated with fenvalerate toxicity in fish [15].
When sheepshead minnows (Cyprinodon variegatus) were
studied during 28 days for early-life-stage toxicity,
3.9 µg fenvalerate/litre significantly reduced the sur-
vival of hatched fish and 2.2 µg/litre reduced both
length and weight, but no effects were detected at
0.56 µg/litre [68].
6.1.3 Field studies and community effects
Caplan et al. [23] applied fenvalerate at concen-
trations of 0.2 and 1.0 mg/kg to sediment in a tidal marsh
sediment model ecosystem. No adverse effects were seen on
the heterotrophic microorganisms in the sediment after a
7-day exposure to either concentration. Plate counts to
assess numbers of organisms and measurements of substrate
degradation were not different from those of controls. The
half-life of fenvalerate was 6.3 days for the treatment at
0.2 mg/kg and 8.9 days at 1.0 mg/kg.
In the field, fenvalerate was applied to ponds at
rates of 28-112 g ai/ha as a mosquito larvicide [124].
Populations of plankton, crustaceans, and mayfly nymphs
decreased but recovered quickly. Corixids, notonectids,
and aquatic beetle populations decreased slightly and the
effects remained throughout the study. Chironomid larval
populations were suppressed and emergence was inhibited.
However, no deleterious effects were observed on rotifer
populations.
When fenvalerate was applied to ponds at rates of
11.2-56 g ai/ha for mosquito control, the insecticide
produced complete mortality of mayfly naiads [130]. A
single treatment by fenvalerate at 28 g/ha controlled
mosquito larvae for more than 7 days, and it also affected
populations of mayfly naiads, dragonfly naiads, and diving
beetle larva, but not ostracods or damselfly naiads
[131].
Studies into the effects of fenvalerate on estuarine
benthic communities were conducted in a flow-through
system for 8 weeks and 1 week for laboratory- and field-
colonized communities, respectively. Technical grade
fenvalerate (100%), dissolved in a stock solution con-
sisting of 15% acetone and 85% triethylene glycol, was
metered by syringe pump into, and mixed with, the sea
water entering the centre of the constant-head box of each
apparatus receiving fenvalerate. The same amount of
carrier solvent (10 ml/day, 5 mg/litre) was metered into
the control apparatus. Nominal concentrations of fenval-
erate in sea water were 0.01, 0.1, and 1.0 µg/litre.
Samples of water were taken from the constant-head boxes
once a week for chemical analyses for fenvalerate concen-
tration. Community structure was altered significantly in
both cases by fenvalerate at 0.1 or 1 µg/litre, but not
by 0.01 µg/litre. The groups most sensitive to the
insecticide were chordates (Branchiostoma caribaeum) and
amphipods, while annelids and molluscs tolerated concen-
trations up to 10 µg/litre [177].
Tagatz et al. [178] placed boxes containing sand,
either uncontaminated or contaminated (nominal concen-
tration of fenvalerate of 0.1, 1.0, or 10 mg/kg), in an
estuary for 8 weeks, and the community structure of
benthic organisms colonising the boxes was assessed. The
average number of species colonising the sand at the
highest treatment level was significantly less than for
the controls (35.6 compared to 47.8); lower concentrations
had no effect on species diversity. Colonisation by
annelids, molluscs, and arthropods was unaffected even at
the highest dose. The only organisms deterred by the
fenvalerate were chordates (primarily lanceolets).
6.2 Terrestrial Organisms
6.2.1 Toxicity to soil microorganisms
In laboratory trials for effects on soil algae,
Megharaj et al. [116] applied fenvalerate to a black
cotton soil, taken from a fallow cotton field. Fenvalerate
applied once at a dose equivalent to 0.5 or 1.0 kg/ha had
no inhibitory effect on soil algae, but two applications
of fenvalerate, at concentrations of 0.75 or 5.0 kg/ha,
resulted in increased algal populations.
6.2.2 Toxicity to beneficial insects
Fenvalerate is highly toxic to honey bees (Apis
mellifera) with a topical LD50 of 0.41 µg/bee. However,
in field tests at a normal application rate of 0.22 kg/ha,
the hazard is low because the residue repels bees for
about 10 h following application and decreases to non-
toxic levels within one day. During the first 5 days after
application, fenvalerate caused only light bee mortality.
At higher application rates (0.44 kg/ha), however, mor-
tality remained high 8 hours after application [5, 63, 84,
85].
Fenvalerate is toxic to the tobacco budworm (Heliothis
virescens) and to its predator green lacewing (Chrysopa
carnea) as well as to the parasite (Campoletis sonorensis)
of the tobacco budworm. But, it is more toxic to the pest
than to either the predator or the parasite. Comparison of
the LC50 value for the parasite (C. sonorensis) with that
for the host (H. virescens) indicated similar toxicity,
the value for the host being 1.5 times that for the para-
site [152]. However, in the case of the predator (C.
carnea), the insecticide was much less toxic to the pred-
ator than to the pest, the selectivity ratio being 0.037
[151].
When third instar larvae of C. carnea were topically
dosed with 250 µg/insect, they exhibited marked toler-
ance during a 72-h period. The ED50 value (paralysis,
failure to pupate, knockdown, and mortality) for fenval-
erate through one generation (larva to larva) was approxi-
mately 1000 µg/g [164].
Syrett & Penman [176] compared LC50 values for
fenvalerate when applied topically to lucerne-infesting
aphids (Acyrthosipho kondoi and A. pisum) and to their
predators, namely the brown lacewing ( Austromicromus
tasmaniae, adult and larva) and the ladybird (Coccinella
undecimpunctata). The values were 0.071, 0.033, 4.3, 67,
and 0.38 mg/kg, respectively. From these data, the lady-
bird was slightly (5-10 times) more tolerant than the
aphid species, but lacewing adults were 60-120 times as
tolerant as the aphids. Furthermore, the larvae were 15
times more tolerant than the adults. There was a negative
temperature coefficient for A. tasmaniae, with greater
toxicity (approximately 3 times) at 10 °C than at 25 °C
[176].
When fenvalerate was applied to loamy sand and then
striped earwigs (Labidura riparia), a predator of the
cabbage looper (Trichoplusia ni), were added to the soil,
fenvalerate was of low toxicity at rates giving good
looper control [205].
Laboratory studies of the activity of fenvalerate on
spider mites and their predators showed that the spider
mite (Tetranychus urticae) was considerably more (67-548
times) resistant to fenvalerate than were its predators
(Amblyseius fallacis, Typhlodromus pyri, and Typhlodromus
occidentalis) [199]. The LC50 value for T. urticae was
approximately 25 times greater than that for the predator
(A. fallacis) [158].
In the field, the predatory mite (T. pyri) disappeared
during the first 4-6 weeks after fenvalerate was sprayed
at 25 mg/litre to drip-off, and then small numbers were
found 7 weeks after spraying. The insecticide had no
appreciable toxicity for spider mites (Panonychus ulmi).
The virtual elimination of the predatory mite led to a
marked population increase of P. ulmi later in the same
season [3].
In apple and pear orchards, dramatic increases in the
populations of spider mites (T. urticae, Tetranychus
mcdanieli, or P. ulmi) were seen after the application of
fenvalerate at rates of 7.5 and 15 mg ai/litre. This was
due to a reduction in the numbers of the predatory mite
(Mataseiulus occidentalis) to zero or near zero [80].
From these results, it was suggested that the rec-
ommended application rates for fenvalerate would sometimes
be detrimental to integrated mite control programs in or-
chards, and these would require careful reconsideration.
6.2.3 Toxicity to birds
The toxicity of fenvalerate to birds is very low. The
acute LD50 for the chicken is more than 12 g/kg
(Table 7). The toxicity to the bobwhite quail (Colinus
virginianus) and American kestrel is similarly low.
Bradbury & Coats [13] measured the toxicity of
fenvalerate for the bobwhite quail. Acute oral dosing
yielded an LC50 in excess of 4 g/kg body weight for adult
birds and 1.785 g/kg body weight for 5-week-old juveniles.
Dietary dosing of 2-week-old chicks for 5 days (with a
further 3 days of observation) indicated an LC50 of
> 15 g/kg diet.
Rattner & Franson [156] dosed American kestrels with
fenvalerate (1-4 g/kg body weight) and examined the birds
for toxic effects over 10 h after dosing. Some birds were
kept at temperatures of 22 °C and others under cold stress
at -5 °C. Fenvalerate, at exposures far greater than could
be expected in the environment, caused mild intoxication
and elevated plasma alanine aminotransferase activity.
Cold did not increase the toxicity of the pyrethroid.
6.3 Uptake, Loss, and Bioaccumulation
Fenvalerate is taken up readily by aquatic organisms
and rapidly reaches, within the organism, a plateau level
related to the water concentration of the pyrethroid. Loss
of fenvalerate from organisms is rapid when they are
transferred to uncontaminated water. There is no sugges-
tion of biomagnification in food chains.
Under laboratory conditions, the half-life of fenval-
erate in sea water containing 100 g sediment per litre sea
water was 34 (27-42) days in foil-covered samples and 8
8 days in sunlight-exposed ones. Eastern oysters
(Crassostrea virginica) kept for 28 days in sea water
containing 24 µg fenvalerate/litre gave a steady state
bioconcentration factor of 4700. After treatment ceased,
fenvalerate was depurated by the oysters to non-detectable
concentrations within a week [163].
Snails exposed for 28 days to fenvalerate (0.79 µg
per litre) did not show any changes in behaviour or
survival. The bioaccumulation ratios ranged from 356 to
1167 [4].
In a study by Spehar et al. [166], embryonic, larval,
and early juvenile stages of fathead minnows (Pimephales
promelas) were exposed to fenvalerate in a continuous-flow
system for 30 days. At 0.33 µg/litre the only effect was
a temporary initial impairment of swimming in some larvae.
This was more marked at 0.43 µg/litre at which level
survival of the larvae was also reduced. The 30-day bio-
concentration factor was 3000 ± 1500, but 25 days after
transfer to clean water the fenvalerate had again been
eliminated.
Rainbow trout (Salmo gairdneri) were used to evaluate
the gill uptake and toxicokinetics of [3H]-fenvalerate.
Fish (weight between 0.64 and 0.97 kg) were exposed in a
respirometer-metabolism chamber to technical grade
fenvalerate (0.28 or 23 ng/litre) or an emulsifiable-
concentrate formulation (16 ng/litre) at 11.0-11.5 °C for
36 to 48 h. No significant effects of emulsifiers or
fenvalerate concentration on uptake were observed. The
overall mean gill uptake efficiency was 28.6 ± 4.4%. After
8- to 48-h depuration periods, carcass and bile contained
80-90% and 10-20% of the gill-absorbed material, respect-
ively. Urine, faeces, and blood each contained less than
2% of the dose. Significant excretion and blood transport
of fenvalerate equivalents were completed within 8-12 h
after exposure ceased. Specific tissues from trout exposed
to 0.28 ng/litre were analyzed for fenvalerate equival-
ents. After a 48-h depuration period, bile contained the
highest concentration of fenvalerate equivalents (7 ng/g),
followed by fat (0.2 ng/g). Remaining tissues contained
0.015-0.045 ng/g. Analysis of biliary metabolites indi-
cated that the glucuronide of 4 -OH-fenvalerate was the
only significant degradation product. Results from the
present study suggest that efficient gill uptake does not
explain the sensitivity of fish to fenvalerate. Instead,
a low rate of biotransformation and excretion may play a
significant role in the susceptibility of rainbow trout
[14].
When juvenile Atlantic salmon were exposed to static
water containing 0.8-9.3 µg fenvalerate/litre for 16-96 h,
the concentration of fenvalerate in dead fish ranged from
0.16 to 0.43 mg/kg. The insecticide was not detected (de-
tection limit: 5 µg/kg) either in dead lobster hepatopan-
creas or in dead shrimps [110].
When carp (Cyprinus carpio) was exposed to [14C-CN]-
[2S, alphaRS]-fenvalerate (0.8 µg/litre) under semi-static
conditions for 7 days, the radioactivity in fish increased
to a level of 922 µg/kg. Once the fish were transferred
to fresh water, the levels of radioactivity in the fish
decreased with an initial half-life of 5 days [135].
In studies by Ohkawa et al. [135], carp, snails,
Daphnia, and algae were exposed to fenvalerate in an
aquatic model ecosystem where 14C-[2S, alphaRS] fenval-
erate (0.3 mg/kg) was applied to the bottom sandy loam
soil. During a 30-day run, concentrations of fenval-
erate in the water were 0.35-0.63 µg/litre and 0.14-
0.21 µg/litre on days 7 and 30, respectively. The bio-
concentration factors for fenvalerate were 122, 617, 683,
and 477 on day 7 (162-300, 993-1110, 629-829, and 714-1180
on day 30) in carp, snails, Daphnia, and algae, respect-
ively. In carp, large amounts of CP-Vacid (17) and
3-phenoxybenzoic acid (22) were detected, together with
small amounts of alpha-cyano-3-(4'-hydroxyphenoxy)benzyl-2-
(4-chlorophenyl)-3-methylbutyrate (4'-OH-Fen) (25). Small
amounts of alpha-carbamoyl-3-phenoxybenzyl-2-(4-chlorophe-
nyl)-3-methylbutyrate (CONH2-Fen) (33), alpha-carboxy-3-
phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (COOH-
Fen) (34), and 4 -OH-Fen (25) were detected in snails.
CPIA was specifically present in both Daphnia (prey) and
carp (predator). CONH2-Fen (33) and alpha-carboxy-3-phenoxy-
benzyl alcohol (36) were common to algae (prey) and carp
(predator). Based on the products identified, degradation
pathways were proposed for this aquatic model ecosystem
(Fig. 4).
In a 28-day early-life stage study (see section
6.1.2), the mean bioconcentration factor in whole fish was
570 [68].
It was shown in the same study that fenvalerate under-
went oxidation at the 2'and 4'positions of the alcohol
moiety, as well as at the 2 and 3 positions of the acid
moiety, ester cleavage, and the conjugation of resultant
phenols and carboxylic acids with glucuronic acid, sul-
furic acid, and glycine. Cleavage of fenvalerate and its
ester metabolites appeared to release cyanohydrins, which
were, however, unstable under physiological conditions and
decomposed easily to cyanide and aldehydes (Fig. 3). The
cyanide ion was converted mainly to thiocyanate and
CO2, and 2-iminothiazolidine-4-carboxylic acid, a metab-
olite detected with other pyrethroids containing a cyanide
group, was not positively identified [134]. The major fae-
cal metabolites from 14CO-, 14Calpha-, and 14CN-fenval-
erate were unchanged fenvalerate (5) and two ester metab-
olites of 2'-hydroxy-(13) and 4'-hydroxy-fenvalerate (25).
The major metabolites in 0- to 2-day pooled urine (50-55%
of the dosed radioactivity) from the acid-labelling were
2-(4-chlorophenyl)isovaleric acid (17) (Cl-Vacid), 2-(4-
chlorophenyl)-3-hydroxymethylbutyric acid (37) (3-OH-Cl-
Vacid), and its lactone (38) (3-OH-Cl-Vacid-lactone).
Other minor metabolites were 2-(4-chlorophenyl)-2-hydroxy-
3-hydroxymethylbutyric acid (39) (2,3-OH-Cl-Vacid) in the
free, the lactone (40) (2,3-OH-Cl-Vacid-lactone), and the
conjugated forms, 2-(4-chlorophenyl)- cis-2-butenedioic
acid anhydride (41) (Cl-BDacid anhydride), and 2-(4-
chlorophenyl)-3-methyl-2-butene-4-olide (42) (Cl-B-acid-
lactone). On the other hand, the predominant urinary
metabolite from the alcohol moiety was the sulfate conju-
gate of 3-(4'-hydroxyphenoxy)benzoic acid (23) (4'-OH-
PBacid), accounting for approximately 40% of the dose.
Other major metabolites were 3-phenoxybenzoic acid (22)
(PBacid) in the free (6%), the glucuronide (2%), and the
glycine (2%) conjugated forms, 4'-OH-PBacid in the free
(5%) and the glucuronide (2%) forms, and the sulfate of 3-
(2'-hydroxyphenoxy)benzoic acid (24) (2'-OH-PBacid) (3%).
With 14CN-fenvalerate, the major urinary metabolite was
thiocyanate (43) [134].
Pydrin insecticide (Y-rich) is an isomerically
enriched form of fenvalerate containing an excess ratio of
the active diastereomers SS and RR (designated Y) over the
less active diastereomers RS and SR (designated X) at a
ratio of approximately 85:15. Fenvalerate contains Y:X in
a ratio of 45:55. Following a single oral dose of the Y-
rich insecticide (8.4 mg/kg) to male and female Sprague-
Dawley rats, more than 90% of the administered radioac-
tivity from the acid moiety (chlorophenyl-14C) and the
alcohol moiety (phenoxyphenyl-14C) was eliminated within
the first 24 h. There was no major difference between the
two different fenvalerate preparations in either the elim-
ination rate or the metabolites distribution profile.
Cleavage of the ester linkage was the primary metabolic
pathway. The acid and alcohol portions of the parent mole-
cule underwent hydroxylation, oxidation, and conjugation.
These metabolic reactions were not dependent on the iso-
meric composition of the test material. Tissue residue
data showed that 14C residues were not retained in the
various organs [104].
The fate of sugar conjugates, which may be formed as
plant metabolites, has been investigated by Mikami et al.
[122]. Upon single oral administration to male Sprague-
Dawley rats at a concentration of 3.8 mg/kg, the mono-,
di-, and tri-glucose conjugates of [14C]-3-phenoxybenzyl
alcohol (19) and the mono-glucose conjugate of [14C]-3-
phenoxybenzoic acid (22) were rapidly hydrolysed and
extensively eliminated in the urine, mostly as the sulfate
conjugate of 3-(4-hydroxyphenoxy)benzoic acid (24).
Faecal elimination was a minor route, whereas biliary
excretion was responsible for about 42% of the dose, and
the glucuronide conjugates of (19), (22), and (24) were
common major metabolites. The biliary glucuronides were
metabolized in the small intestine to the respective
aglycones, which were reabsorbed, metabolized further, and
excreted in the urine as the sulfate conjugate of (24).
Although small amounts of the mono-, di-, and tri-gluco-
sides were found in the 30-min blood and liver samples
following oral administration of the tri-glucoside of
(19), they were not detected in the urine, bile, or fae-
ces. Similarly, the sulfate conjugate was one of the major
urinary metabolites in germ-free male rats, when dosed
with the 14C-glucosides at a rate of 9 µmol/kg via the
oral or intraperitoneal route, although certain amounts
were excreted unchanged in the urine and faeces. The glu-
cose conjugates were metabolized in vitro by intestinal
microflora and in various rat tissues including blood,
liver, small intestine, and small intestinal mucosa. The
tissue enzymes showed a different substrate specificity in
hydrolysing the glucosides. However, they were not metab-
olized in gastric juice, bile, pancreatic juice, or
urine.
5.1.2 Mouse
In mice, fenvalerate is metabolized in a similar way
to that in rats, but the following significant species
differences were found by Kaneko et al. [86]: (a) the
taurine conjugate of PBacid was found in mice but not in
rats; (b) 4'-OH-PBacid sulfate occurred to a greater
extent in rats than in mice; and (c) a greater amount of
thiocyanate was excreted in mice than in rats. No sig-
nificant sex differences were observed in rats and mice.
The metabolism of the stereoisomers of fenvalerate,
([2S, alphaRS] and [2S, alphaS]) was apparently similar to
that of racemic fenvalerate.
Following a single oral administration of the four
chiral isomers of [14C-chlorophenyl]-fenvalerate to
Sprague-Dawley rats and ddY mice (2.5 mg/kg body weight),
the [2R, alphaS] isomer showed, in both rats and mice, rel-
atively greater residues in the analyzed tissues (except
fat), particularly in adrenal glands, compared with the
other three isomers. Similarly, this isomer showed
higher tissue concentrations than the other isomers when
mice were fed a diet containing 500 mg/kg of the
[2S, alphaS], [2R, alphaS], or [2R, alphaR] isomers for
two weeks. The greater amount of radioactive residues from
the administration of [2R, alphaS] isomer, as compared
with those of other isomers, was explained by the
preferential formation of a lipophilic metabolite from the
[2R, alphaS] isomer found in all examined tissues, which
was not easily excreted. The amounts of the lipophilic
metabolite differed among tissues, being higher in adrenal,
liver, and mesenteric lymph nodes. This metabolite was
identified as cholesteryl [2R]-2-(4-chlorophenyl)isoval-
erate. The presence of the same metabolite was also indi-
cated in rat tissues [87].
5.1.3 Domestic animals
Two 3-month-old lambs were fed a diet containing
45 mg/kg fenvalerate for 10 days and then killed to deter-
mine the concentrations of fenvalerate in the kidney,
liver, leg muscle, and renal fat [210]. Among the analyzed
tissues, fat showed the highest fenvalerate level (3.6-4.4
mg/kg dry weight) while other tissues contained less than
0.3 mg/kg. Fenvalerate gave two gas chromatographic peaks
and each peak contained a pair of its enantiomers. In all
cases, the ratio of the areas of these peaks (peak 1
(RS,SR)/peak 2 (SS,RR)) was 1.08 both for fenvalerate in
the diet and for fenvalerate recovered from the fortified
control fat. In contrast, the fenvalerate isolated from
lamb fat had a peak area ratio of 0.76-0.78. Thus, one or
both of the first eluting enantiomers appeared to be
metabolized more rapidly than the other enantiomers.
In a study by Wszolek et al. [209], two Holstein cows
were fed fenvalerate at 5 and 15 mg/kg diet for 4 days and
were then given a clean diet for 6 days. Total excretion
of fenvalerate in milk amounted to 0.44 and 0.64% of the
total dose for the 5 and 15 mg/kg levels, respectively,
whereas about 25% of the dose was eliminated in the
faeces.
A lactating Holstein cow was fed grain fortified with
227 mg fenvalerate daily for 4 days and the urine was ana-
lysed. Intact fenvalerate was not detected in any samples
of the urine excreted by the cow during the 10-day feeding
study, nor was the acid metabolite (Cl-Vacid) (17) ident-
ified. The in vitro study on fenvalerate degradation in
rumen fluid indicated that no significant degradation of
fenvalerate was observed during the 6-h incubation [211].
Saleh et al. [161] gave a single oral dose of fenval-
erate (10 mg/kg body weight) to chickens and monitored the
persistence and distribution of the insecticide over 15
days. A concentration of 4.7 mg/litre in blood after 24 h
fell to 0.05 mg/litre after 7 days. Levels in other tis-
sues reached maxima of less than 1.0 mg/kg and fell rap-
idly. However, brain residues rose to a level of 4.0 mg/kg
over 7 days and persisted for the 15 days of the exper-
iment. Concentrations in eggs reached a maximum of
0.3 mg/kg yolk after 4 to 5 days, and a maximum of
0.24 mg/kg egg white. By day 6, levels had returned to the
pre-dosing level.
5.2 Enzymatic Systems for Biotransformation
The [2R, alphaRS] isomer of fenvalerate has been found
to be more rapidly hydrolysed by mouse liver esterase than
the [2S, alphaRS] isomer, but less rapidly metabolised than
the [2R, alphaRS] isomer with an oxidase system. A similar
correlation was observed with the [2S] and [2R] isomers of
S-5439 (3-phenoxybenzyl-2-(4-chlorophenyl)isovalerate) [165].
In an in vitro study on the metabolism of the four
chiral isomers of fenvalerate using homogenates from vari-
ous tissues of mice, rats, dogs, and monkeys, only the
[2R, alphaS] isomer yielded cholesteryl-[2R]-2-(4-chloro-
phenyl)isovalerate (CPIA-cholesterol ester) as a major
metabolite. Mouse tissues exhibited a higher rate of CPIA-
cholesterol ester formation than those of other animals.
Of the mouse tissues tested, the kidney, brain, and spleen
showed the greatest ability to form this ester, the rel-
evant enzyme activity being mainly localized in the
microsomal fractions. Carboxyesterases for mouse kidney
microsomes hydrolyzed the [2R, alphaS] isomer only of
fenvalerate to give CPIA and yielded the corresponding
cholesterol ester in the presence of artificial liposomes
containing cholesterol. It appears that the CPIA-
cholesterol ester resulted from the stereoselective
([2R, alphaS] only) formation of the CPIA-carboxyesterase
complex, which subsequently reacted with cholesterol to
yield the CPIA-cholesterol ester [128].
Hydrolysis of the four chiral isomers of fenvalerate
by microsomes of various mouse tissues has been investi-
gated by Takamatsu et al. [180]. The kidney, spleen and
brain hydrolyzed only the [2R, alphaS] isomer. Liver hydro-
lyzed the [2R, alphaS] and [2R, alphaR] isomers to a great-
er extent than the [2S, alphaR] and [2S, alphaS] isomers,
while plasma hydrolysed the [2S, alphaR] and [2R, alphaR]
isomers more rapidly than the [2S, alphaS] and [2R, alphaS]
isomers. The stereoselectivity of hydrolysis of the four
isomers by mouse liver microsomes was found to be same as
that in vivo. Of the four isomers, the [2R, alphaS] isomer
alone was transformed to cholesteryl-[2R]-2-(4-chlorophenyl)
isovalerate (CPIA-cholesterol ester) by microsomes of the
brain, kidney, spleen, or liver but not by plasma. The
rate of CPIA-cholesterol ester formation was lower in the
liver than in other tissues. The optimum pH (7.4-9.0) for
the formation of this ester was nearly the same as that
for hydrolysis of the [2R, alphaS] isomer to form CPIA in
mouse kidney microsomes.
The substrate specificity of microsomal carboxyester-
ase(s) responsible for the formation of cholesteryl-[2R]-
2-(4-chlorophenyl)isovalerate from fenvalerate was inves-
tigated by incubating mouse kidney microsomes with 14C-
cholesterol and fenvalerate or its analogues. Of the four
isomers of fenvalerate, only the [2R, alphaS] isomer yielded
a cholesterol ester. This specificity of cholesterol ester
formation was the same as that in the in vivo study. Some
of the fenvalerate analogues also produced similar choles-
terol esters. Steroids other than cholesterol were also
investigated as acceptors of the acid moiety of the
[2R, alphaS] isomer by incubating egg lecithin and several
steroids with the [2R, alphaS] isomer in the presence of
solubilized carboxyesterase(s). Dehydroisoandrosterone and
pregnenolone reacted with the [2R, alphaS] isomer to give the
corresponding ester conjugates [88].
One or more carboxyesterases located in the soluble
fraction of mouse brain homogenates hydrolyzed several
pyrethroid esters with a substrate specificity different
from that of the hepatic esterases. In particular,
fenvalerate and fluvalinate were hydrolyzed by brain
esterases at rates equal to or greater than that measured
for trans-permethrin. The results suggest that hydrolysis
in the brain may contribute to the detoxication of some
pyrethroids in mammals [64].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The acute toxicity data of fenvalerate to aquatic and
terrestrial non-target organisms are summarized in Tables
5, 6, and 7.
6.1 Aquatic Organisms
6.1.1 Toxicity to aquatic invertebrates
Non-target invertebrates, except molluscs, are more
susceptible to the insecticide than fish, the LC50
ranging from 0.08 to 2 µg/litre.
Fenvalerate is relatively non-toxic to oysters and
algae (LC50 >1000 µg/litre) over short exposure periods.
Snails (Heliosoma trivolvis) exposed for 28 days to 0.79
µg/litre, the highest concentration tested, showed no
change in behaviour or survival [4].
Day & Kaushik [32] conducted short-term toxicity
tests on three species of cladoceran and one species of
calanoid (Diaptomus oregonensis). The 48-h LC50 values
for daphnids were: 2.52 µg/litre for adult Daphnia magna,
0.83 µg/litre for D. magna aged 48 h (or less);
0.29 µg/litre for adult Daphnia galeata mendotae;
0.21 µg/litre for adult Ceriodaphnia lacustris; 0.16
µg/litre for D. galeata mendotae aged 48 h (or less).
Diaptomus oregonensis was the most sensitive species with
a 48-h LC50 of 0.12 µg/litre. No toxicity was found with
the emulsifiable concentrate from which fenvalerate was
omitted (EC control). Rates of filtration of algae were
reduced at sub-lethal concentrations of fenvalerate.
Ceriodaphnia lacustris was the most sensitive species,
with rates of filtration significantly decreased at
fenvalerate concentrations of 0.01 µg/litre. Rates of
assimilation of algae were decreased at fenvalerate con-
centrations of 0.05 µg/litre or more.
Day & Kaushik [33] conducted life-cycle studies on the
toxicity of fenvalerate to Daphnia galeata mendotae.
Lifetable methods were used to generate statistical
comparisons between treatments. At a concentration of
0.005 µg/litre, fenvalerate increased the longevity of
the daphnids significantly from 37.6 to 51.6 days. How-
ever, at the same concentration, production of young was
decreased. Higher concentrations of fenvalerate caused
reduced survival of the adults. The intrinsic rate of
natural increase in the population was reduced at a con-
centration of 0.5 µg/litre. At 0.01 µg/litre, the net
reproductive rate decreased from 126 to 73 offspring per
female and the generation time from 20.3 to 17.3 days.
Table 5. Acute toxicity of fenvalerate to non-target freshwater organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formu- Systemc Temperature pH Hardnessd Reference
(µg/litre) lationb (° C)
--------------------------------------------------------------------------------------------------------------------------------------------
Arthropods
Gammarus pseudolimnaeus adult-juv 96-h LC50 0.03 T F 15 7.6-7.8 46-48 4
Gammarus pseudolimnaeus 1-3 mm, juv 96-h LC50 0.05 T R 17 7.6-7.8 46-48 4
Waterflea 1st instar 96-h LC50 0.032 T S 17 7.4 44 115
(Daphnia magna)
Midge 3rd instar 48-h LC50 0.43 T S 22 7.4 44 115
(Chironomus pulmosus)
Mayfly larva 9-day LC50 0.08 T F 15 7.6 46-48 4
(Ephemerella sp.)
Rhagionid fly larva 28-day LC50 0.03 T F 15 7.6-7.8 46-48 4
(Atherix)
Stonefly naiad 72-h EC50 0.13 T F 15 7.6-7.8 46-48 4
(Pteronarcys dorsata)
Stonefly 3-6 weeks old 96-h LC50 1.9 EC S 20-22 7.8 7% 107
(Nitocra spinipes)
Fish
Atlantic salmon 6.2 cm, 5.3 g 96-h LC50 1.2 T R 10 110
(Salmo salar)
Rainbow trout 5-6 cm 48-h LC50 3.0 EC S 12-25.5 129
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 76 T S 10 7.5 110 28
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 21 EC S 10 7.5 110 28
(Salmo gairdneri)
Mosquitofish 4-5 cm 48-h LC50 15.0 EC S 8.8-16 129
(Gambusia affinis)
Mosquitofish 3-days old 72-h LC50 2.6 T S 24-27 124
(Gambusia affinis)
Desert pupfish 4-5 cm 48-h LC50 25.0 EC S 11-16.6 129
(Cyprinodon macularis)
Tilapia mossambica 5-6 cm 48-h LC50 200.0 EC S 15-21.4 129
Bluegill sunfish adult 96-h LC50 0.76 T S 22 7.4 40 115
(Lepomis macrochirus)
Fathead minnow adult 96-h LC50 2.35 T S 22 7.1 49 115
(Pimephales promelas)
--------------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
d expressed as mg CaCO3 per litre.
Table 6. Acute toxicity of fenvalerate to non-target estuarine & marine organisms
---------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formul- Systemc Temper- pH Salinity Reference
(µg/litre) ationb ature o/oo
(° C)
---------------------------------------------------------------------------------------------------------------------------------------
Algae
Skeletonema costatum 96-h EC50 > 1000 T 20 30 212
Isochrysis galbana 96-h EC50 > 1000 T 20 30 212
Thalassiosira pseudonana 96-h EC50 > 1000 T 20 30 212
Nitzschia angularum 96-h EC50 > 1000 T 20 30 212
Molluscs
Eastern oyster 2-h larva 48-h EC50 >
1000 T S 25 20 212
(Crassostrea virginica)
Arthropods
Lobster (Homarus americanus) 450 g 96-h LC50 0.14 T R 10 30 110
Shrimp (Crangon septemspinosa) 1.3 g 96-h LC50 0.04 T R 10 110
Shrimp (Mysidopsis bahia) 1-day juv 96-h LC50 0.021 T S 25 20 212
Shrimp (Mysidopsis bahia) newly hatched 96-h LC50 0.008 T F 25.4 25.3 163
Shrimp (Penalus duorarum) adult 96-h LC50 0.84 T F 24.8 24.9 163
California grunion 3-day larva 96-h LC50 0.29 T F 26 25 114
(Leuresthes tenuis)
California grunion juv 96-h LC50 0.60 T F 25 22 114
(Leuresthes tenuis)
Inland silverside 26-day larva 96-h LC50 1.00 T F 24 20 114
(Nenidia beryllina)
Tidewater silverside juv 96-h LC50 1.00 T F 25 20 114
(Menidia peninsulae)
Fish
Sheepshead minnow 28-day fry 96-h LC50 121 T S 25 20 212
(Cyprinodon variegatus)
Sheepshead minnow adult 96-h LC50 5 T F 30 26.5 163
(Cyprinodon variegatus)
Bleak (Alburnus alburnus) 8 cm 96-h LC50 2-3 EC S 10 7.8 7 107
Atlantic silverside adult 96-h LC50 0.31 T F 24.1 25 163
(Menidia menidia)
Striped mullet adult 96-h LC50 0.58 T F 25.9 25.8 163
(Mugil cephalus)
Gulf toadfish adult 96-h LC50 5.4 T F 30 24.8 163
(Opsanus beta)
---------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
Table 7. Acute toxicity of fenvalerate to non-target terrestrial organisms
-------------------------------------------------------------------------------------------------------------------------
Species Size Application Parameter Toxicity Temper- Reference
ature
(°C)
-------------------------------------------------------------------------------------------------------------------------
Bird
Broiler chicks 8-12 weeks old, oral LD50 12 590 (mg/kg) 31-32 155
0.99-2.2 kg
Hen oral LD50 > 1500 (mg/kg) 123
Arthropods
Insect parasite
Ichneumoid adult male film 24-h LC50 1760 (ng/vial) 152
(Campoletis sonorensis)
Insect predators
Lacewing adult 2.74 mg topical LD50 4.3 (mg/kg) 15 176
(Austromicromus tasmaniae) larva 2.52 mg topical LD50 67 (mg/kg) 20 176
Lacewing (Chrysopa carnea) 3rd instar, topical 72-h ED50 > 25 (mg/g) 28 164
larva 9.9-10 mg topical ED50 ~ 1 (mg/g) 28 164
one generation
Lacewing (Chrysopa carnea) larva film LC50 0.073 (mg/vial) 25 151
5-6 days old
Beetle 11.2 mg topical LD50 0.38 (mg/kg) 15 164
(Coccinella undecimpunctata)
Earwig (Labidura riparia) mature soil 0.11 kg ai/ha mortality 6% 205
0.22 kg ai/ha mortality 25% 205
0.44 kg ai/ha mortality 50% 205
Honey bee (Apis mellifera) adult topical LD50 410 ng/bee - 5
Predaceous mite species
Amblyseius fallacis adult female slide dip method LC50 2.6 (mg ai/litre) 27 158
Amblyseius fallacis adult female slide dip method LC50 7.0 (mg ai/litre) 26 199
Typhlodromus pyri adult female slide dip method LC50 8.1 (mg ai/litre) 26 199
Typhlodromus occidentalis adult female slide dip method LC50 2.1 (mg ai/litre) 26 199
-------------------------------------------------------------------------------------------------------------------------
McKenney & Hamaker [109] exposed the estuarine grass
shrimp Palaemonetes pugio to fenvalerate, in a flow-
through system to maintain constant exposure, throughout
20 days of larval development. The study was conducted
under optimal salinity conditions (20 o/oo). A nominal
concentration of 3.2 ng/litre significantly reduced the
percentage of larvae successfully completing metamor-
phosis. Exposure to 1.6 ng/litre prolonged larval develop-
ment. Larvae were also found to be less capable of
responding successfully to osmotic stress after exposure
to fenvalerate at 0.1 or 0.2 ng/litre.
6.1.2 Toxicity to fish
Fenvalerate is toxic to fish, LC50 values being
0.29-200 µg/litre (Tables 5 and 6). The LC50 value for
rainbow trout obtained with an emulsifiable concentrate
was 3.6 times lower than that for the technical product
[28]. The toxicity of fenvalerate to adult bluegill sun-
fish (Lepomis macrochirus) was unaffected by changes in
water hardness and pH [115].
The acute toxicities (96-h LC50) of fenvalerate to
juvenile steelhead trout were 172 ng/litre and 88
ng/litre, respectively, under continuous and intermittent
exposure (approximate peak concentration: 460 ± 40
ng/litre for 4.5 h). Prolonged intermittent exposure
(70 days) of the early life-stage resulted in marked
lethality (32%) and reduced terminal weight (50% of con-
trol) (mean concentration: 80 ng/litre, peak concen-
tration: 461 ng/litre). However, continuous exposure to
80 ng/litre for 70 days did not effect these parameters
[31].
Fenvalerate has narrow safety margins for fish
(LC50 of fish : LC50 of mosquito larvae is in the ratio
of 1:24) when the insecticide is used against mosquitoes
[129].
Four rainbow trout (Salmo gairdneri) died within 11
hours when exposed to 412 µg fenvalerate/litre. Visible
signs of poisoning included elevated cough rate, tremors,
and seizures. Ventilatory and cardiac activity stopped
during the seizures. Histopathological examination of
gill tissue showed damage consistent with irritation, and
Na+ and K+ excretion rates were elevated. Fenvalerate
concentrations in brain, liver, and carcass at death were
0.16, 3.62, and 0.25 mg/kg, respectively. The study
suggested that, apart from effects on the nervous system,
effects on respiratory surfaces and renal ion regulation
may be associated with fenvalerate toxicity in fish [15].
When sheepshead minnows (Cyprinodon variegatus) were
studied during 28 days for early-life-stage toxicity,
3.9 µg fenvalerate/litre significantly reduced the sur-
vival of hatched fish and 2.2 µg/litre reduced both
length and weight, but no effects were detected at
0.56 µg/litre [68].
6.1.3 Field studies and community effects
Caplan et al. [23] applied fenvalerate at concen-
trations of 0.2 and 1.0 mg/kg to sediment in a tidal marsh
sediment model ecosystem. No adverse effects were seen on
the heterotrophic microorganisms in the sediment after a
7-day exposure to either concentration. Plate counts to
assess numbers of organisms and measurements of substrate
degradation were not different from those of controls. The
half-life of fenvalerate was 6.3 days for the treatment at
0.2 mg/kg and 8.9 days at 1.0 mg/kg.
In the field, fenvalerate was applied to ponds at
rates of 28-112 g ai/ha as a mosquito larvicide [124].
Populations of plankton, crustaceans, and mayfly nymphs
decreased but recovered quickly. Corixids, notonectids,
and aquatic beetle populations decreased slightly and the
effects remained throughout the study. Chironomid larval
populations were suppressed and emergence was inhibited.
However, no deleterious effects were observed on rotifer
populations.
When fenvalerate was applied to ponds at rates of
11.2-56 g ai/ha for mosquito control, the insecticide
produced complete mortality of mayfly naiads [130]. A
single treatment by fenvalerate at 28 g/ha controlled
mosquito larvae for more than 7 days, and it also affected
populations of mayfly naiads, dragonfly naiads, and diving
beetle larva, but not ostracods or damselfly naiads
[131].
Studies into the effects of fenvalerate on estuarine
benthic communities were conducted in a flow-through
system for 8 weeks and 1 week for laboratory- and field-
colonized communities, respectively. Technical grade
fenvalerate (100%), dissolved in a stock solution con-
sisting of 15% acetone and 85% triethylene glycol, was
metered by syringe pump into, and mixed with, the sea
water entering the centre of the constant-head box of each
apparatus receiving fenvalerate. The same amount of
carrier solvent (10 ml/day, 5 mg/litre) was metered into
the control apparatus. Nominal concentrations of fenval-
erate in sea water were 0.01, 0.1, and 1.0 µg/litre.
Samples of water were taken from the constant-head boxes
once a week for chemical analyses for fenvalerate concen-
tration. Community structure was altered significantly in
both cases by fenvalerate at 0.1 or 1 µg/litre, but not
by 0.01 µg/litre. The groups most sensitive to the
insecticide were chordates (Branchiostoma caribaeum) and
amphipods, while annelids and molluscs tolerated concen-
trations up to 10 µg/litre [177].
Tagatz et al. [178] placed boxes containing sand,
either uncontaminated or contaminated (nominal concen-
tration of fenvalerate of 0.1, 1.0, or 10 mg/kg), in an
estuary for 8 weeks, and the community structure of
benthic organisms colonising the boxes was assessed. The
average number of species colonising the sand at the
highest treatment level was significantly less than for
the controls (35.6 compared to 47.8); lower concentrations
had no effect on species diversity. Colonisation by
annelids, molluscs, and arthropods was unaffected even at
the highest dose. The only organisms deterred by the
fenvalerate were chordates (primarily lanceolets).
6.2 Terrestrial Organisms
6.2.1 Toxicity to soil microorganisms
In laboratory trials for effects on soil algae,
Megharaj et al. [116] applied fenvalerate to a black
cotton soil, taken from a fallow cotton field. Fenvalerate
applied once at a dose equivalent to 0.5 or 1.0 kg/ha had
no inhibitory effect on soil algae, but two applications
of fenvalerate, at concentrations of 0.75 or 5.0 kg/ha,
resulted in increased algal populations.
6.2.2 Toxicity to beneficial insects
Fenvalerate is highly toxic to honey bees (Apis
mellifera) with a topical LD50 of 0.41 µg/bee. However,
in field tests at a normal application rate of 0.22 kg/ha,
the hazard is low because the residue repels bees for
about 10 h following application and decreases to non-
toxic levels within one day. During the first 5 days after
application, fenvalerate caused only light bee mortality.
At higher application rates (0.44 kg/ha), however, mor-
tality remained high 8 hours after application [5, 63, 84,
85].
Fenvalerate is toxic to the tobacco budworm (Heliothis
virescens) and to its predator green lacewing (Chrysopa
carnea) as well as to the parasite (Campoletis sonorensis)
of the tobacco budworm. But, it is more toxic to the pest
than to either the predator or the parasite. Comparison of
the LC50 value for the parasite (C. sonorensis) with that
for the host (H. virescens) indicated similar toxicity,
the value for the host being 1.5 times that for the para-
site [152]. However, in the case of the predator (C.
carnea), the insecticide was much less toxic to the pred-
ator than to the pest, the selectivity ratio being 0.037
[151].
When third instar larvae of C. carnea were topically
dosed with 250 µg/insect, they exhibited marked toler-
ance during a 72-h period. The ED50 value (paralysis,
failure to pupate, knockdown, and mortality) for fenval-
erate through one generation (larva to larva) was approxi-
mately 1000 µg/g [164].
Syrett & Penman [176] compared LC50 values for
fenvalerate when applied topically to lucerne-infesting
aphids (Acyrthosipho kondoi and A. pisum) and to their
predators, namely the brown lacewing ( Austromicromus
tasmaniae, adult and larva) and the ladybird (Coccinella
undecimpunctata). The values were 0.071, 0.033, 4.3, 67,
and 0.38 mg/kg, respectively. From these data, the lady-
bird was slightly (5-10 times) more tolerant than the
aphid species, but lacewing adults were 60-120 times as
tolerant as the aphids. Furthermore, the larvae were 15
times more tolerant than the adults. There was a negative
temperature coefficient for A. tasmaniae, with greater
toxicity (approximately 3 times) at 10 °C than at 25 °C
[176].
When fenvalerate was applied to loamy sand and then
striped earwigs (Labidura riparia), a predator of the
cabbage looper (Trichoplusia ni), were added to the soil,
fenvalerate was of low toxicity at rates giving good
looper control [205].
Laboratory studies of the activity of fenvalerate on
spider mites and their predators showed that the spider
mite (Tetranychus urticae) was considerably more (67-548
times) resistant to fenvalerate than were its predators
(Amblyseius fallacis, Typhlodromus pyri, and Typhlodromus
occidentalis) [199]. The LC50 value for T. urticae was
approximately 25 times greater than that for the predator
(A. fallacis) [158].
In the field, the predatory mite (T. pyri) disappeared
during the first 4-6 weeks after fenvalerate was sprayed
at 25 mg/litre to drip-off, and then small numbers were
found 7 weeks after spraying. The insecticide had no
appreciable toxicity for spider mites (Panonychus ulmi).
The virtual elimination of the predatory mite led to a
marked population increase of P. ulmi later in the same
season [3].
In apple and pear orchards, dramatic increases in the
populations of spider mites (T. urticae, Tetranychus
mcdanieli, or P. ulmi) were seen after the application of
fenvalerate at rates of 7.5 and 15 mg ai/litre. This was
due to a reduction in the numbers of the predatory mite
(Mataseiulus occidentalis) to zero or near zero [80].
From these results, it was suggested that the rec-
ommended application rates for fenvalerate would sometimes
be detrimental to integrated mite control programs in or-
chards, and these would require careful reconsideration.
6.2.3 Toxicity to birds
The toxicity of fenvalerate to birds is very low. The
acute LD50 for the chicken is more than 12 g/kg
(Table 7). The toxicity to the bobwhite quail (Colinus
virginianus) and American kestrel is similarly low.
Bradbury & Coats [13] measured the toxicity of
fenvalerate for the bobwhite quail. Acute oral dosing
yielded an LC50 in excess of 4 g/kg body weight for adult
birds and 1.785 g/kg body weight for 5-week-old juveniles.
Dietary dosing of 2-week-old chicks for 5 days (with a
further 3 days of observation) indicated an LC50 of
> 15 g/kg diet.
Rattner & Franson [156] dosed American kestrels with
fenvalerate (1-4 g/kg body weight) and examined the birds
for toxic effects over 10 h after dosing. Some birds were
kept at temperatures of 22 °C and others under cold stress
at -5 °C. Fenvalerate, at exposures far greater than could
be expected in the environment, caused mild intoxication
and elevated plasma alanine aminotransferase activity.
Cold did not increase the toxicity of the pyrethroid.
6.3 Uptake, Loss, and Bioaccumulation
Fenvalerate is taken up readily by aquatic organisms
and rapidly reaches, within the organism, a plateau level
related to the water concentration of the pyrethroid. Loss
of fenvalerate from organisms is rapid when they are
transferred to uncontaminated water. There is no sugges-
tion of biomagnification in food chains.
Under laboratory conditions, the half-life of fenval-
erate in sea water containing 100 g sediment per litre sea
water was 34 (27-42) days in foil-covered samples and 8
8 days in sunlight-exposed ones. Eastern oysters
(Crassostrea virginica) kept for 28 days in sea water
containing 24 µg fenvalerate/litre gave a steady state
bioconcentration factor of 4700. After treatment ceased,
fenvalerate was depurated by the oysters to non-detectable
concentrations within a week [163].
Snails exposed for 28 days to fenvalerate (0.79 µg
per litre) did not show any changes in behaviour or
survival. The bioaccumulation ratios ranged from 356 to
1167 [4].
In a study by Spehar et al. [166], embryonic, larval,
and early juvenile stages of fathead minnows (Pimephales
promelas) were exposed to fenvalerate in a continuous-flow
system for 30 days. At 0.33 µg/litre the only effect was
a temporary initial impairment of swimming in some larvae.
This was more marked at 0.43 µg/litre at which level
survival of the larvae was also reduced. The 30-day bio-
concentration factor was 3000 ± 1500, but 25 days after
transfer to clean water the fenvalerate had again been
eliminated.
Rainbow trout (Salmo gairdneri) were used to evaluate
the gill uptake and toxicokinetics of [3H]-fenvalerate.
Fish (weight between 0.64 and 0.97 kg) were exposed in a
respirometer-metabolism chamber to technical grade
fenvalerate (0.28 or 23 ng/litre) or an emulsifiable-
concentrate formulation (16 ng/litre) at 11.0-11.5 °C for
36 to 48 h. No significant effects of emulsifiers or
fenvalerate concentration on uptake were observed. The
overall mean gill uptake efficiency was 28.6 ± 4.4%. After
8- to 48-h depuration periods, carcass and bile contained
80-90% and 10-20% of the gill-absorbed material, respect-
ively. Urine, faeces, and blood each contained less than
2% of the dose. Significant excretion and blood transport
of fenvalerate equivalents were completed within 8-12 h
after exposure ceased. Specific tissues from trout exposed
to 0.28 ng/litre were analyzed for fenvalerate equival-
ents. After a 48-h depuration period, bile contained the
highest concentration of fenvalerate equivalents (7 ng/g),
followed by fat (0.2 ng/g). Remaining tissues contained
0.015-0.045 ng/g. Analysis of biliary metabolites indi-
cated that the glucuronide of 4 -OH-fenvalerate was the
only significant degradation product. Results from the
present study suggest that efficient gill uptake does not
explain the sensitivity of fish to fenvalerate. Instead,
a low rate of biotransformation and excretion may play a
significant role in the susceptibility of rainbow trout
[14].
When juvenile Atlantic salmon were exposed to static
water containing 0.8-9.3 µg fenvalerate/litre for 16-96 h,
the concentration of fenvalerate in dead fish ranged from
0.16 to 0.43 mg/kg. The insecticide was not detected (de-
tection limit: 5 µg/kg) either in dead lobster hepatopan-
creas or in dead shrimps [110].
When carp (Cyprinus carpio) was exposed to [14C-CN]-
[2S, alphaRS]-fenvalerate (0.8 µg/litre) under semi-static
conditions for 7 days, the radioactivity in fish increased
to a level of 922 µg/kg. Once the fish were transferred
to fresh water, the levels of radioactivity in the fish
decreased with an initial half-life of 5 days [135].
In studies by Ohkawa et al. [135], carp, snails,
Daphnia, and algae were exposed to fenvalerate in an
aquatic model ecosystem where 14C-[2S, alphaRS] fenval-
erate (0.3 mg/kg) was applied to the bottom sandy loam
soil. During a 30-day run, concentrations of fenval-
erate in the water were 0.35-0.63 µg/litre and 0.14-
0.21 µg/litre on days 7 and 30, respectively. The bio-
concentration factors for fenvalerate were 122, 617, 683,
and 477 on day 7 (162-300, 993-1110, 629-829, and 714-1180
on day 30) in carp, snails, Daphnia, and algae, respect-
ively. In carp, large amounts of CP-Vacid (17) and
3-phenoxybenzoic acid (22) were detected, together with
small amounts of alpha-cyano-3-(4'-hydroxyphenoxy)benzyl-2-
(4-chlorophenyl)-3-methylbutyrate (4'-OH-Fen) (25). Small
amounts of alpha-carbamoyl-3-phenoxybenzyl-2-(4-chlorophe-
nyl)-3-methylbutyrate (CONH2-Fen) (33), alpha-carboxy-3-
phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (COOH-
Fen) (34), and 4 -OH-Fen (25) were detected in snails.
CPIA was specifically present in both Daphnia (prey) and
carp (predator). CONH2-Fen (33) and alpha-carboxy-3-phenoxy-
benzyl alcohol (36) were common to algae (prey) and carp
(predator). Based on the products identified, degradation
pathways were proposed for this aquatic model ecosystem
(Fig. 4).
In a 28-day early-life stage study (see section
6.1.2), the mean bioconcentration factor in whole fish was
570 [68].
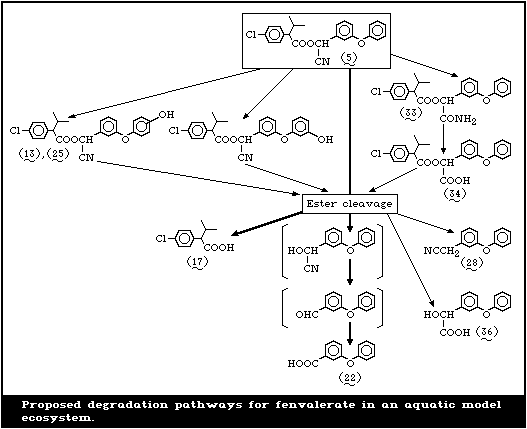 7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
Table 8 shows the results of acute toxicity tests with
technical grade fenvalerate in various animal species.
The acute toxic signs in rats were restlessness,
tremors, piloerection, occasional diarrhoea, and an abnor-
mal gait. Following oral administration, surviving rats
recovered rapidly from acute clinical signs of poisoning
and were asymptomatic within 3-4 days [20].
Table 9 shows the results of an acute intraperitoneal
toxicity study of fenvalerate metabolites in mice [96].
All the compounds were dissolved in corn oil except
3-phenoxybenzoic acid, which was dissolved in DMSO. The
acute intraperitoneal toxicity in mice of the proposed
decarboxylated photo-products was found to be similar to,
or greater than, that of fenvalerate [78].
In a study by Blair and Roderick [12], groups of four
male and four female rats were exposed by inhalation (head
only) to an aerosol formulation (77-µm particle size)
generated from an aqueous suspension containing 3 g/litre.
Following a single administration (4 h) of this non-
inhalable particulate, acute signs of poisoning were noted
for a short period, presumably from oral ingestion of the
large particles. There was no mortality and all animals
appeared normal within 3 days following exposure.
7.2 Short-Term Exposures
7.2.1 Oral administration
Groups of Carworth Farm E rats (12 of each sex per
group) were fed fenvalerate in the diet at dose levels of
0, 125, 500, 1000, and 2000 mg/kg for 90 days [72]. Mor-
tality (11/12 male, 9/12 female) was observed at the
highest concentration. Body weight gain and food consump-
tion were decreased and blood urea nitrogen concentrations
were increased at 1000 and 2000 mg/kg. There were no
treatment-related changes in any groups of rats in the
haematological parameters examined. Increases in liver to
body weight ratios and kidney to body weight ratios were
observed at 500 mg/kg or more. Gross and microscopic
examinations revealed no compound-related changes in any
groups. The NOEL was 125 mg/kg diet.
Table 8. Acute toxicity of fenvalerate (technical grade) administered to various species
-------------------------------------------------------------------------------------------------
Species Route Sex Vehiclea LD50 (mg/kg) Reference
-------------------------------------------------------------------------------------------------
Rat oral DMSO 451 195
oral PEG:water > 3200 168
dermal 5000 (24 h) 140
inhalation M, F water > 101 mg/m3 (3 h) 94
intraperitoneal 340 162
Mouse oral M DMSO 200-300 195
F 100-200
oral PEG:water 1202 169
intraperitoneal M, F corn oil 85-89 96
intraperitoneal 132 162
intravenous glycerolformol 65 2
inhalation M, F water > 101 mg/m3 (3 h) 94
Chinese oral M DMSO 98 195
hamster F 82
Rabbit percutaneous undiluted 1000-3200 75
Hen oral > 1500 123
-------------------------------------------------------------------------------------------------
a PEG = polyethylene glycol; DMSO = dimethylsulfoxide.
Table 9. Acute intraperitoneal toxicity of fenvalerate metabolites in mice
---------------------------------------------------------------------------
Chemical No.a LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------------
Fenvalerate (5) 88.5 85
2-(4-Chlorophenyl)isovaleric acid (17) 351 350
3-Phenoxybenzyl alcohol (19) 371 424
3-(4'-Hydroxyphenoxyl) benzyl alcohol (21) 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol (20) 876 778
3-Phenoxybenzoic acid (22) 154 169
3-(4'-Hydroxyphenoxy) benzoic acid (24) 783 745
3-(2'-Hydroxyphenoxy) benzoic acid (23) 859 912
3-Phenoxybenzaldehyde (18) 415 416
NaSCN 604 578
---------------------------------------------------------------------------
a Refers to chemical identification no. in Fig. 3 and in text.
In a study by Parker et al. [150], Fischer 344 rats
(30 of each sex per group) were fed decarboxyfenvalerate
(one of the major photodegradation products) at concen-
trations of 0, 30, 100, 300, 3000 or 10 000 mg/kg diet for
up to 13 weeks. Body weight was decreased in male rats fed
10 000 mg/kg, but no treatment-related mortality or clini-
cal signs were observed. Absolute and relative liver
weight of male and female rats fed 300, 3000, or 10 000
mg/kg were all higher than those of the controls. Signifi-
cant increases in absolute or relative kidney weights were
observed in male and female rats fed 3000 or 10 000 mg/kg.
Significant treatment-related microscopic effects were
limited to glomerulonephrosis in male and female rats fed
10 000 mg/kg and hepatocellular hypertrophy with pale
eosinophilic cytoplasm and hepatocellular focal necrosis
in male and female rats fed 3000 or 10 000 mg/kg. A NOEL
of 300 mg/kg diet was established in this study.
Groups of young adult beagle dogs (four of each sex
per group) were fed fenvalerate in the diet at dose levels
of 0, 0.25, 0.5, 1.25, or 12.5 mg/kg body weight for 90
days [70]. There were no treatment-related changes in body
weight, food consumption, clinical signs, and clinical
laboratory data. Gross and microscopic examinations
revealed no effects of the fenvalerate. Thus, daily
administration at a level of 12.5 mg/kg body weight for a
period of 90 days produced no detectable evidence of toxi-
cological effect.
In a study by Parker et al. [148], male and female
beagle dogs (six of each sex per group) were fed diets
containing 0, 250, 500, or 1000 mg fenvalerate/kg diet for
a period of 6 months. Prominent clinical signs related to
treatment were emesis, head shaking, biting of the ex-
tremities, and tremors. The mean body weights of female
dogs fed fenvalerate at 1000 mg/kg were significantly
lower than those of controls. Red blood cell counts and
haematocrit and haemoglobin values in both male and female
dogs fed the highest dose were significantly lower. Serum
cholesterol and alkaline phosphatase levels were also
increased, mostly in the group fed 1000 mg/kg. Hepatic
multifocal microgranulomas observed during microscopic
examination increased in incidence and severity in a dose-
dependent way and were considered to be related to treat-
ment. Histiocytic cell infiltrates in the mesenteric lymph
nodes of some female dogs fed 500 or 1000 mg/kg and of
male dogs fed 1000 mg/kg were the only other treatment-
related effects observed microscopically.
7.2.2 Inhalation
Groups of Sprague-Dawley rats and ICR mice (10 of each
sex per group) were exposed to fenvalerate by inhalation
for 3 h daily for 4 weeks at concentration levels of 0, 2,
7, or 20 mg/m3 (fully respirable particle size). Although
animals showed acute signs of poisoning at the highest
dose level, no mortality was observed in any group. There
were no treatment-related effects in body weight, haema-
tology, or clinical biochemistry parameters, nor were
there any gross or microscopic abnormal findings [82,
95].
7.2.3 Dermal application
In a study by Hine [75], groups of rabbits (7-8 male
rabbits per group) were administered fenvalerate dermally
at dose levels of 0, 100, or 400 mg/kg daily for 6 h
(14 exposures were performed over a 22-day period).
Severe weight loss, clinical signs of poisoning, and gross
dermal effects were observed at 400 mg/kg, where mortality
was also observed.
7.3 Skin and Eye Irritation; Sensitization
7.3.1 Skin and eye irritation
Two formulated products (an emulsifiable concentrate
and an ultra-low-volume formulation) were found to be
severe eye and skin irritants in rabbits. Dermal irri-
tation was evident for 7 days after a 24-h exposure, and
severe conjunctivitis, corneal opacity, and iritis were
observed within 30 min of an application of 0.2 ml of the
formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation [29, 30]. However,
when experiments were carried out using pure (non-formu-
lated) fenvalerate, there was no irritation [138].
7.3.2 Skin sensitization
Skin sensitization by pure fenvalerate (95%) has been
evaluated using the Landsteiner-Draize method on guinea-
pigs. No sensitization was detected by Okuno et al.
[139].
7.4 Long-Term Exposures and Carcinogenicity
7.4.1 Mouse
When groups of ddY mice (35-47 of each sex per group)
were administered fenvalerate in the diet for 78 weeks at
levels of 0, 100, 300, 1000, or 3000 mg/kg, mortality
occurred at the highest dose level. Hyperexcitability was
observed at 1000 mg/kg or more, and body weight was
depressed at 3000 mg/kg over the 18-month period and at
1000 and 3000 mg/kg over the first 3 months. A variety of
haematological parameters were affected at 3 months, pre-
dominantly at the highest dose level, but no haematol-
ogical changes were observed at the end of the study.
Several biochemical changes suggestive of hepatotoxicity
were observed at 3 months and at termination of the study
in the 300, 1000, and 3000 mg/kg groups. There were gross
changes in several organ weights and in organ-to-body
weight ratios, predominantly in the liver. Microscopic
examination revealed changes in the liver, mesenteric
lymph nodes, and kidney. Dose-dependent granulomatous
changes were observed in the liver and/or mesenteric lymph
nodes in all treatment groups. At the 3-month interim
sacrifice, multiple small necrotic foci in the liver and
changes in the epithelial cells of the proximal convoluted
tubules were noted at the two highest dose levels. There
were no indications in this study of tumorigenicity or
carcinogenicity as a result of fenvalerate administration
[81, 83, 173, 175].
In studies by Okuno et al. [144], male ddY mice were
fed diets containing the [2S, alphaS], [2S, alphaRS], [2R,
alphaS], and [2R, alphaR] isomers of fenvalerate at diet-
ary dose levels of 0, 500, or 1000 mg/kg, 500, 1000, or
2000 mg/kg, 0, 125, or 1000 mg/kg, and 125, or 1000 mg/kg
for 52, 52, 13, and 13 weeks, respectively. Microgranulo-
matous changes were observed in the mice treated with the
[2R, alphaS] isomer after 1, 2, or 3 months. In contrast,
the changes did not occur in mice treated with the [2R,
alphaR] isomer under the same conditions. Neither [2S,
alphaS] nor [2S, alphaRS] isomers caused microgranulomatous
changes at 500 or 1000 mg/kg after 1 year. To clarify the
causative agent of granuloma formation, the cholesterol
ester of 2-(4-chlorophenyl)isovaleric acid (CPIA), a
lipophilic conjugate from the [2R, alphaS] isomer of fenva-
lerate, was injected intravenously into ddY mice. Micro-
granulomatous changes were observed in the liver of mice
treated with the [2R]-, [2S]-, or [2RS]-CPIA-cholesterol
esters 1 week after a single treatment of 1, 10, or 100
mg/kg body weight, as well as in the liver of mice treated
with a single dose of 10 or 30 mg/kg body weight of the
[2R]-CPIA-cholesterol ester and kept up to 26 weeks after-
wards. Histochemical examination and microscopic autoradio-
graphy of the liver demonstrated the presence of tritium,
derived from 3H-labelled [2R]-CPIA and cholesterol in giant
cells and Kuppfer cells. Another histochemical examination
showed the presence of cholesterol ester in the liver of
mice treated with the [2R, alphaS] isomer. These results
support the hypothesis that the CPIA-cholesterol ester is
the causative agent of the microgranulomatous changes ind-
uced by fenvalerate.
In further studies by Okuno et al. [145], male and
female ddY mice were fed diets containing technical
fenvalerate (either 0, 10, 30, 100, or 300 mg/kg diet for
20 months or 0, 100, 300, 1000, or 3000 for 17-18 months).
Microgranulomatous changes were observed in the lymph
nodes, liver, and spleen, the NOEL for such changes being
30 mg/kg. To examine the reversibility of these changes,
ddY mice (male and female) were fed a diet containing
technical fenvalerate at dose levels of 1000 and 3000
mg/kg for 6 weeks, followed by a control diet for up to
12 months. The size and number of the microgranulomatous
changes were reduced with time. These changes were typical
of foreign body granulomas and did not have the appearance
of granulomas formed in response to an immunological
stimulus.
When B6C3F1 mice (50 of each sex per group) were fed
fenvalerate at dietary concentrations of 0, 10, 50, 250,
or 1250 mg/kg for 2 years, mortality was increased and
body weight significantly decreased in male and female
mice fed 1250 mg/kg. The mean body weight of female mice
fed 250 mg/kg was also generally lower than that of con-
trols after the 60th week of feeding. The only treatment-
related non-neoplastic pathological effect observed in the
study was multifocal microgranulomata in the lymph nodes,
liver, and spleen of male mice fed 1250 mg/kg and of
female mice fed 250 or 1250 mg/kg. No statistically sig-
nificant differences were observed in either the number or
type of neoplasms in mice fed fenvalerate (compared to
concurrent controls). Thus, fenvalerate was found not to
be carcinogenic in B6C3F1 mice under the conditions of
the test [146].
7.4.2 Rat
When groups of Wistar rats (15 of each sex per group)
were fed fenvalerate at concentrations of 0, 50, 150, 500,
or 1500 mg/kg diet for 15 months, there was no mortality
attributable to fenvalerate. The hyperexcitability ob-
served during the early stages of the study disappeared
within 3 months. Body weight was significantly depressed
in both sexes at the highest dose level. No compound-
related changes were detected in the urine or in the eyes,
but the haemoglobin concentration was depressed in males
at the highest dose level and the females at 150 mg/kg or
more. Several blood biochemistry parameters were signifi-
cantly altered at the highest dose level (blood urea
nitrogen was increased in both sexes; protein content and
plasma cholinesterase were decreased in females). Gross
and microscopic examination revealed no dose-related
effects [174].
In a study by Gordon & Weir [66], groups of Sprague-
Dawley rats (93 males and 93 females per treated group;
183 of each sex used as the control group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25,
250, or 500 mg/kg. There was no compound-related mor-
tality, although body weight was reduced at the highest
dose level. The group fed 500 mg/kg and a separate control
group were sacrificed at 26 weeks while the other animals
were maintained for 2 years. There were no significant ef-
fects on food consumption, growth, behavior, haematology,
blood biochemical composition or urine consumption. At the
conclusion of the study, organ weight and organ-to-body
weight ratios were normal. Gross and microscopic findings
in the treated groups did not differ significantly from
those of the controls. A specific pathology examination
of the sciatic nerve of animals fed 250 mg/kg revealed no
treatment-related changes. Thus, the no-observed-effect
level in this study was 250 mg/kg diet.
Parker et al. [147] fed Sprague-Dawley rats (93 of
each sex per group) diets containing 0.1, 5, 25, or 250 mg
fenvalerate/kg for up to 2 years. The control group con-
sisted of 183 males and 183 females. Ten treated and 20
control rats of each sex from each group were killed at
intervals of 3, 6, 12, and 18 months. When body weight,
organ weight, food consumption, haematology, and clinical
chemical analysis measurements did not reveal any effect
resulting from the treatment, two additional groups of
rats (50 of each sex per group) were fed 0 or 1000 mg
fenvalerate/kg diet for 2 years. Body weight was decreased
and organ-to-body weight ratios were increased in brain,
liver, spleen, testes, kidneys (females only), and heart
(females only), in the treated animals. Mammary and pitu-
itary tumours were commonly observed, along with a variety
of other tumours that occurred randomly among all control
and treatment groups. No statistically significant differ-
ences in the number and type of neoplasms were observed,
except for mammary tumours in females in the main study.
These effects were judged not to be toxicologically sig-
nificant, since mammary tumour incidences did not exceed
expected incidences in aged female Sprague-Dawley rats.
In addition, the time taken for tumours to appear was un-
changed, and no change in the ratio of benign to malignant
tumours occurred. Sarcomas identified in the subcutis and
dermis in 5 out of 51 males fed 1000 mg/kg were also
identified in 2% (1/50), 2% (2/102), and 0-6% of concur-
rent, original, and historical controls, respectively.
The no-observed-effect level was 250 mg/kg.
When male and female Wistar rates were fed a diet
containing technical fenvalerate at 0, 50, 150, 500, or
1500 mg/diet for 24-28 months, microgranulomatous changes
were observed in lymph nodes, liver, spleen, and adrenal
glands. The no-observed-effect level for these microgranu-
lomatous changes was 150 mg/kg [145].
7.5 Mutagenicity
7.5.1 Microorganism and insects
The DNA-damaging capacity of fenvalerate has been
examined in a Rec-assay with Bacillus subtilis M45 rec-
and H17 wild type strains at concentrations up to 10
mg/disk per plate. Fenvalerate had no inhibitory effect on
the growth of indicator strains, and was judged to be non-
mutagenic [170].
Fenvalerate has also been examined for its mutagenic
potency with the Ames test in Salmonella typhimurium
(TA 1535, TA 1538, TA 98, and TA 100), using dose levels
of up to 1 mg/plate both with and without a metabolic
enzyme system. Fenvalerate was non-mutagenic in these
tests [170]. It was also tested using hepatic metabolic
enzyme systems prepared from various PCB-treated animals
(three strains of rats, six strains of mice and the Syrian
golden hamster). At dose levels of up to 1 mg/plate,
Fenvalerate was non-mutagenic [171, 172].
In further studies by Suzuki & Miyamoto [170], fenval-
erate was given orally at doses of 60 and 125 mg/kg to
groups of mice, and indicator cells (S. typhimurium G46)
were injected intraperitoneally. Fenvalerate did not
induce any significant level of mutation among the indi-
cator cells recovered from the abdominal cavity. On the
other hand, the positive control, dimethylnitrosamine,
significantly increased the mutation frequency of the
indicator organism.
Another host-mediated assay of fenvalerate in mice was
conducted using Saccharomyces cerevisiae as indicator
microorganism. Groups of mice were administered fenval-
erate orally at doses of 25 and 50 mg/kg, and were in-
jected with a suspension of indicator cells intraperito-
neally. No mutagenic effect on the indicator cells was
detected [17].
Fenvalerate was not found to be mutagenic in S.
typhimurium strains TA 100 or TA 98 in the presence or
absence of a rat liver activation system by fluctuation
tests at a concentration of up to 10 µg/ml or in V79
Chinese hamster cells in the presence or absence of
hepatocytes at a concentration of up to 40 µg/ml [153].
Fenvalerate did not induce sex-linked recessive
lethals, sex-chromosome losses, or non-disjunction in
Drosophila melanogaster when it was given to adults (up
to 20 mg/litre in the diet) or larvae (up to 50 mg/litre
in the diet), or was injected into adults (at 20 µg/ml)
[7].
7.5.2 Rat
In a study by Chatterjee et al. [26], groups of rats
(21 per group) were administered fenvalerate orally at
doses of 50, 75, or 100 mg/kg per day for 3 weeks. The
rats were killed 24 h after the last treatment and bone
marrow cells were examined for chromosomal aberrations.
Although an increase in the frequency of chromosomal aber-
rations was observed in fenvalerate-treated animals, it
was not possible to draw any definite conclusion because
it was not dose related and may have been non-specific.
Fenvalerate has also been studied for the enhancement
of gamma-glutamyl transpeptidase-positive enzyme-altered
focus incidence in partially hepatectomized, nitrosodi-
ethylamine-initiated male Sprague-Dawley rats. Fenvalerate
administered peritoneally (75 mg/kg body weight per day,
5 days a week for 10 weeks) induced significantly more
foci per cm3 and a larger percentage of liver tissue
occupied by focus tissue, compared with a vehicle-control
group. Analysis of the size distribution of foci in
fenvalerate- and vehicle-treated rats showed elevated
focus incidences in fenvalerate-treated rats at all focus
sizes. Fenvalerate induced no hepatotoxic effects, as
judged by serum transaminase activities and histopatho-
logical analysis [58].
7.5.3 Mouse
In a dominant lethal assay, groups of male mice (10-11
per group) were administered fenvalerate orally at doses
of 25, 50, or 100 mg/kg body weight. Each male was mated
with three virgin females for 7 days. The procedure was
repeated weekly as a standard dominant lethal test. The
females were sacrificed and examined for dominant
lethality at the 13th day of gestation. Fetal implants in
females, mated to males that had been treated with 100
mg/kg for 2 weeks, showed a significant reduction in
viability. A significant increase in early fetal death
was observed in females mated with males that had been
treated for 4 weeks with the highest dose [34].
The significance of the above data was further studied
as follows:
(1) By using a two-way analysis of variance, it was
judged that the reduction in fetal implants in females
mated to males the second week after dosing at 100 mg/kg
and the increase in early fetal deaths in the 4th week
were statistically significant. But these increases or
decreases appeared to be random and were not considered to
be biologically significant.
(2) Using a t-test and the Mann-Whitney U-test, no
significance was shown in any mean proportions for the
above parameters.
From these findings, it was concluded that fenvalerate
did not cause dominant lethal effects in micea.
----------------------------------------------------------
a Personal communication, J. Miyamoto, 1981, Comments on
"Further work information" required by 1979 JMPR on
fenvalerate, Laboratory of Biochemistry and Toxicology
(Unpublished report submitted to WHO by Sumitomo
Chemical Co. Ltd).
7.5.4 Hamster
In a study by Dean & Senner [35], fenvalerate was
administered orally to groups of hamsters (six males and
six females per group) at two successive daily doses of
12.5 and 25 mg/kg. The chromosomal preparations were made
8 or 24 h after administration. Fenvalerate did not induce
any chromosomal damage in the bone marrow cells from
treated animals, whereas the positive control, methyl
methanesulfonate (50 mg/kg), had induced a substantial
number of chromatid gaps within 8 h of dosing.
Fenvalerate, and the fenvalerate metabolite 2-(4-
chlorophenyl)isovaleric acid, were investigated for the
inhibition of gap-junctional intercellular communication
in vitro in the Chinese hamster lung fibroblast (V79)
metabolic cooperation assay [58]. This study showed that
both fenvalerate and 2-(4-chlorophenyl)isovaleric acid
were inhibitors of intercellular communication at non-
cytotoxic concentrations.
7.6 Teratogenicity and Reproduction Studies
7.6.1 Teratogenicity
In studies by Kohda et al. [93], groups of pregnant
ICR mice (32-33 per group) were orally administered
fenvalerate at dose levels of 0, 5, 15, or 50 mg/kg per
day on days 6 to 15 of gestation. Groups of 20 mice were
sacrificed on day 18, and the fetuses were removed and
examined for visceral and skeletal abnormalities. The
remaining dams were allowed to deliver naturally and the
young were maintained until weaning to evaluate postnatal
deficits. Additionally two male and two female weanlings
from each dam were maintained for 8 weeks and mated to
investigate their reproductive potential. Although toxic
signs were noted in the dams at the highest dose level,
there was no significant mortality. Examination of the
fetuses revealed no external, visceral, or skeletal abnor-
malities. Treatment of the dams with fenvalerate did not
affect the reproductive performance of the offspring.
Van Der Pauw et al. [187] dosed groups of pregnant
Dutch rabbits (20 to 31 per group) orally with fenvalerate
(0, 12.5, 25, or 50 mg/kg body weight per day) from day 6
to day 18 of gestation. The dams were sacrificed on day
28 and standard teratogenic assessments made. The body
weights of the dams given the highest dose were reduced.
There were no significant differences from controls in any
of the other parameters examined. Fenvalerate was not
found to be teratogenic in this study.
7.6.2 Reproduction studies
In studies by Stein [167] and Beliles et al. [10],
groups of Sprague-Dawley rats (11 males and 22 females per
group) were fed fenvalerate in the diet at levels of 0, 1,
5, 25, or 250 mg/kg. The animals were dosed for 9 weeks
prior to mating and the initiation of a standard three-
generation (two litters per generation) reproduction
study. Fertility, viability, gestation, and lactation in-
dices were calculated for each group of rats and were com-
pared to control values. Ten of the female weanlings and
all of the males from the F3b litters were examined his-
tologically at the conclusion of the study. The mean body
weight of the F2b adults was decreased at 250 mg/kg, but
no pathological changes were noted to account for this
weight loss. No effects on reproductive parameters in any
of the three generations were observed. Histological
examination revealed no treatment-related changes in any
group.
Groups of pregnant ICR mice (32-33 mice per group)
were orally administered fenvalerate at dose levels of 0,
5, 15, or 50 mg/kg body weight per day on days 6 to 15 of
gestation in a standard teratogenicity bioassay. Two male
and two female weanlings from each dam were maintained for
8 weeks and mated to investigate their reproductive poten-
tial. Toxic signs were noted in maternal mice at the
highest dose level. There was no significant mortality
over the course of the study, and no effects were noted on
any of the other animals as a result of continuous admin-
istration of fenvalerate. The animals maintained in the
abbreviated reproduction study showed no differences from
the control value in their ability to reproduce. There
were no changes in the reproduction indices with any
animals examined [93].
7.7 Neurotoxicity
In a study by Butterworth & Carter [20], histopatho-
logical examination was performed on the sciatic nerve and
posterior tibial nerve of rats that had been exposed to
acutely toxic levels of fenvalerate. After poisoning, and
for 9 days during the course of recovery, axonal breaks,
swelling, and vacuolisation, accompanied by phagocytosis
of myelin, were seen. The degree to which myelin was dis-
rupted was dose dependent and was closely associated with
the acute signs of toxicity.
Acute oral administration of fenvalerate, cyper-
methrin, resmethrin, permethrin, and natural pyrethrum to
rats at very high dose levels resulted in severe clinical
signs of poisoning and mortality within 24 h. Histopatho-
logical lesions were observed in the sciatic nerve with
all compounds tested. Fenvalerate did not cause the clini-
cal signs or histopathological lesions at a lower dose
level (200 mg/kg), nor did the other compounds [141,
142].
When groups of six male and six female rats were fed
fenvalerate in the diet at a concentration of 2000 mg/kg
for 8 to 10 days, all the animals showed typical signs of
acute intoxication, such as ataxia, tremors, and hyperex-
citability. Histopathological examinations did not reveal
any adverse effects of fenvalerate on the sciatic nerve
[73].
In order to evaluate the reversibility of the lesions
induced in the sciatic nerve, rats were administered
fenvalerate in the diet at dose levels of 0 or 3000 mg/kg
diet for 10 days. This was followed by a control diet for
12 weeks. During the treatment period, mortality was 60%
in the animals treated with fenvalerate. Rats on the
recovery control diets, sacrificed at 3 weeks, continued
to show swelling and disintegration of axons of the
sciatic nerves. However, there were no histopathological
lesions after 6, 9, or 12 weeks of the recovery period.
These results showed the reversibility of the sciatic
nerve lesions caused by fenvalerate [143].
In studies by Butterworth & Hend [21], fenvalerate was
administered orally to Wistar or Carworth Farm E (CFE)
rats either as single doses or in the diet. When given in
large quantities by a single dose of 250, 500, 800, or
1000 mg/kg body weight, which were sufficient to kill some
of the treated animals, fenvalerate produced sporadic
Wallerian degeneration in the sciatic nerve. The neuro-
pathy was never severe and was not seen in animals given
the compound in sub-lethal doses. In feeding studies (for
5 weeks at 1000 mg/kg diet and for 3 months at 2000 mg/kg
diet), no lesions were seen in the peripheral nerve,
brain, or spinal cord, and there was no evidence of cumu-
lative neurotoxicity.
B6C3F1 mice and Sprague-Dawley rats showed the
characteristic signs of intoxication following single oral
doses of fenvalerate ranging from 56 to 320 and 133 to
1000 mg/kg body weight, respectively. Neurological signs,
such as splayed gait, tremors, ataxia, and hind limb
incoordination, were observed at doses of 100 mg/kg or
more (mice) and 133 mg/kg or more (rats) within 1-8 h
after dosing. These signs had disappeared in most animals
within 72 h. Slight peripheral nerve fibre damage was
detected in surviving mice and rats sacrificed 10 days
after dosing. The incidence and severity were dose related
at doses > 56 and > 180 mg/kg; however, even at lethal
doses, there was no evidence of nerve lesions in some
animals. Thus, two distinct neurological effects were
observed, i.e., (a) a reversible ataxia and (b) incoordi-
nation plus a neuropathological effect manifested as
sparse axonal damage in peripheral nerves [149].
In a study by Milner & Butterworth [123], groups of
six hens were administered fenvalerate orally at dose
levels of 0 or 1000 mg/kg per day for 5 days. A positive
control of tri- ortho-cresyl phosphate (0.5 ml/kg) (TOCP)
was also included in the study. The fenvalerate-treated
birds were retreated, using the same dose regimen, after
3 weeks. The TOCP-treated hens showed signs of delayed
neurotoxicity and histopathological lesions in their
sciatic nerve and spinal cord. As would be expected for a
non-organophosphorus insecticide, there were no typical
clinical signs and histopathological lesions related to
fenvalerate.
7.8 Behavioural Studies
Guinea-pigs responded to dermal applications of
fenvalerate by scratching the treated sites of the skin.
This characteristic response was essentially over within
3-4 h. When the powerful skin irritant oil of mustard was
applied to fenvalerate-treated sites of skin 4-72 h after
the fenvalerate treatment, the behavioural skin sensory
response was re-stimulated. Oil of mustard alone did not
produce skin sensory stimulation. These results indicate
that pyrethroid treatment causes a transient sensitivity
to stimulation produced by chemical irritants [111].
To develop an animal model for studying skin sensory
stimulation, Duncan-Hartley guinea-pigs were treated with
pyrethroid solutions on one side and control substances on
the other side of their shaved back. The animals responded
by licking, scratching, or biting the test sites, and
activity was quantified by counting the number of times
the animals responded. This behavioural activity reached
a maximum 1-4 h after treatment. A chemical irritant (oil
of mustard) was able to restimulate the behavioural ac-
tivity when applied within 24 h after pyrethroid appli-
cation. Skin sensory stimulation produced by cyano-
containing pyrethroids, including fenvalerate, was
significantly greater than that produced by non-cyano-
containing pyrethroids. This behavioural model provides a
quantitative means of evaluating pyrethroid non-erythema-
tous skin sensory stimulation [22].
7.9 Miscellaneous Studies
In an antidotal study, phenobarbital, pentobarbital,
and diphenylhydantoin were found to be effective in
relieving the acute signs of intoxication in the rat.
Intraperitoneal injection of phenobarbital (50 mg/kg)
prevented tremor, diphenylhydantoin (100 mg/kg) by the
same route reduced the toxic reaction, and pentobarbital
(35 mg/kg intraperitoneally) removed the tremor reaction
completely within 30 min. The combination of diphenyl-
hydantoin with either of the barbiturates was effective in
reducing the onset and severity of tremors whereas various
other agents d-tubocurarine, atropine, meprobamate,
diazepam, biperiden, and trimethadione) were ineffective
[113].
The therapeutic potency of intraperitoneally admin-
istered methocarbamol was examined as an antidote against
the acute oral intoxication of rats by a lethal dose of
fenvalerate. Methocarbamol was initially administered at
a dose of 400 mg/kg body weight, followed by repeated
doses of 200 mg/kg body weight when tremors or hyperexcit-
ability to sound were observed. Methocarbamol markedly
decreased the mortality from 80%, which would be caused by
an administration of 850 mg fenvalerate/kg, to 0%, and was
effective in alleviating motor symptoms such as fibril-
lation, tremors, hyperexcitability, clonic seizures, and
choreoathetotic movements. A subcutaneous administration
of atropine sulfate (25 mg/kg body weight) was also effec-
tive in reducing the salivation produced by fenvalerate
[76].
Effective treatments against fenvalerate-mediated ef-
fects have been investigated by quantifying behavioural
skin sensory responses such as licking, scratching, or
biting of the treated sites by fenvalerate-treated guinea-
pigs. Preparations containing vitamin E, corn oil, or the
local anesthetic benzocaine were most effective [111].
Intraperitoneal administration of O-ethyl- O-(4-nitro-
phenyl)phenylphosphonothioate (EPN) or S,S,S-tributylphos-
phorotrithioate (DEF) to mice at 25 mg/kg increased the
intraperitoneal toxicity of fenvalerate (administered 1 h
later) by more than 25-fold; the LD50 decreased from
> 1000 mg/kg to 37 or 42 mg/kg. This suggests that mam-
malian esterases highly sensitive to inhibition by certain
organophosphorus compounds may play a critical role in
fenvalerate detoxication. This kind of synergism among
pesticides would be detrimental in increasing the toxicity
of certain pyrethroids to mammals [62].
Fenvalerate, administered to dogs at a dose sufficient
to induce toxic signs, showed no consistent cardiovascular
effects. Respiratory stimulation was noted at high levels,
and this was not reduced by anaesthetic supplements
(urethane, chloralose, and pentobarbital) [91].
7.10 Mechanism of Toxicity - Mode of Action
The intravenous toxicity of fenvalerate (50-100 mg/kg)
to rats was examined by Verschoyle & Aldridge [189].
[2S, alphaS]-Fenvalerate induced choreoathetosis with sali-
vation (CS-syndrome), and was classified as a Type II
pyrethroid. For the mode of action of pyrethroids in
general see Appendix 1.
The intracerebral injection of [2S, alphaS]-fenvaler-
ate (0.01 mg/kg) to mice produced the Type II syndrome,
consisting of choreoathetosis, convulsion, and saliv-
ation [98]. The Type II syndrome is produced character-
istically by pyrethroids with an alpha-cyano group and
the site of action in mammals is considered to be the
central nervous system.
In intact locusts and neuromuscular preparations of
locusts, fenvalerate caused (a) prolonged firing in the
crural nerve without associated muscle contractions; (b)
sustained muscle contractions; and (c) a block of neurally
evoked muscle contractions at low concentration (10-8 to
10-5 mol/litre). However, fenvalerate did not cause
repetitive firing and after-discharges with associated
muscle contractions [27]. The fenvalerate stereoisomers
with an (S) configuration in the alcohol moiety are more
active pharmacologically and toxicologically than those
with the (R) configuration or the racemate (R,S). It is
also apparent that stereoisomers with the (S) configur-
ation in the acid moiety are more active than those with
the (R) configuration or the racemate (R,S) [27].
[S,S]-Fenvalerate does not induce repetitive firing in
the cockroach cercal sensory nerves either in vivo or in
vitro. It does, however, cause different signs, including
bursts of spikes in the cercal motor nerve [60].
There are no clear-cut links between electrophysio-
logical findings in insects and toxicity to mammals.
8. EFFECTS ON HUMANS
8.1 Occupational Exposure
Appraisal
Fenvalerate has been found to induce skin sensations in
some of the workers who handle this insecticide. Clinical
studies showed that the skin sensations develop with a latent
period of approximately 30 min, peak by 8 h and deteriorate
after 24 h. Numbness, itching, tingling, and burning are symp-
toms frequently reported. Alpha-tocopheryl acetate has been
found to inhibit the occurrence of these skin sensations.
In a study by Kolmodin-Hedman et al. [97], personnel
(52 people) at various plant nurseries who had handled
conifer seedlings treated with fenvalerate were examined.
The symptoms were mainly irritative, such as itching,
paraesthesia and burning of the skin, and itching and
irritation of the eyes. The frequency (% of people who
reported these signs) was about 10%. Increased nasal
secretion was reported by 19% of the personnel.
No clinical case of pyrethroid poisoning had been
reported until outbreaks of acute deltamethrin and fenval-
erate poisoning occurred among cotton growers in China in
1982. Having been told (in error) that pyrethroids were
non-toxic, the farmers handled the pyrethroid insecticides
without taking any precautions. After repeated spraying
in the cotton fields, the mild cases presented severe
headaches, dizziness, fatigue, nausea, and anorexia, with
transient changes in the electroencephalogram (EEG), while
a severe case developed muscular fasciculation, repetitive
discharges in the electromyogram (EMG), and frequent con-
vulsions. However, all were found by follow-up studies to
have completely recovered and the prognosis of acute
pyrethroid poisoning proved to be correct [71]a.
----------------------------------------------------------
a More recently, the same author reviewed 573 cases of
acute pyrethroid poisoning reported in the Chinese
medical literature during 1983-1988 [213]. Among these
there were 196 cases of acute fenvalerate poisoning,
63 of which were occupational, due to inappropriate
handling and 133 accidental, mostly due to ingestion.
Two died of convulsions. All others recovered with
symptomatic and supportive treatment within 1-6 days.
A comprehensive review of clinical manifestations is
included.
Among 23 workers exposed to synthetic pyrethroids,
including fenvalerate, 19 experienced one or more episodes
of abnormal facial sensation, developing between 30 min
and 3 h after exposure and persisting for 30 min to 8 h
[106]. However, there were no abnormal neurological signs,
and electrophysiological studies showed normal responses
in the arms and legs. The symptoms were most likely due
to transient lowering of the threshold of sensory nerve
fibres or sensory nerve endings following exposure of the
facial skin to pyrethroids.
In a study by Tucker & Flannigan [182], selected
individuals who had worked extensively with fenvalerate in
the delta region of the Mississippi and Alabama, USA, were
interviewed and examined. They had, on some occasions,
noted paraesthesia associated with exposure to this insec-
ticide. The cutaneous sensation was described as a sting-
ing or burning, which progressed to numbness in approxi-
mately one-third of the exposed workers. The sensation
typically began a number of hours after contact, peaked in
the evening, and rarely was present the following morning.
The intensity of the sensation varied according to the
type and extent of exposure. Clinical signs of inflam-
mation such as oedema or vesiculation were not apparent.
Erythema was present in a few individuals but this was
difficult to distinguish from sunburn. Several environ-
mental factors were found to affect the cutaneous sen-
sation associated with fenvalerate exposure.
8.2 Clinical Studies
A double-blind study with 29 male volunteers was
performed to test the skin reaction to formulated fenval-
erate. The emulsifiable concentrate formulation was
diluted with water and applied to one side of the face, on
the cheek, with a control formulation on the opposite
cheek. There were no signs of dermatitis 24 h after appli-
cation, nor did the fenvalerate formulation produce any
abnormal skin sensations. There were no indications that
any of the symptoms such as tingling, itching, or burning
were associated with fenvalerate [18].
A double-blind study was performed to compare human
discrimination of technical fenvalerate, the heavy-ends
fraction of distilled fenvalerate, and ethyl alcohol
(vehicle) applied to the lower edge of each earlobe of 36
adult (both male and female) volunteers on three separate
occasions. Both forms of fenvalerate caused a statisti-
cally significant increase in paraesthesia, compared with
the vehicle alone. The onset of the cutaneous sensations
occurred 1 h after application, peaked at 3-6 h, and
lasted approximately 24 h. Numbness, itching, burning,
tingling, and warmth were the most frequently reported
sensations. The difference between the effects of the two
fractions of fenvalerate was not statistically significant
[92].
Flannigan & Tucker [55], Flannigan et al. [56, 57],
and Malley et al. [112] studied the difference in the
degree of paraesthesia induced by a number of pyrethroids.
Applications of 0.05 ml fenvalerate formulated to field
strength (0.13 mg/cm2) were made to a 4 cm2 area of
earlobe on five occasions, the opposite earlobe receiving
distilled water. Participant evaluation after each appli-
cation continued for 48 h and involved description of the
cutaneous sensations. Each participant was treated after
each application with one of the remaining compounds.
Fenvalerate (like the other pyrethroids) induced skin sen-
sations. The paraesthesia developed with a latent period
of approximately 30 min, peaked by 8 h, and deteriorated
as early as 24 h. The local application of dl-alpha
tocopheryl acetate markedly inhibited the occurrence of
skin sensations.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
has discussed and evaluated fenvalerate at its meetings in
1979, 1981, 1982, 1984, 1986, and 1987 [40-47, 49, 50, 52-
4].
Since 1986, an acceptable daily intake (ADI) of 0-0.02
mg/kg body weight has been established.
In the WHO Recommended Classification of Pesticides by
Hazard, technical fenvalerate is classified as "moder-
ately hazardous" (Class II) [197].
REFERENCES
1. AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10(2): 147-151.
2. ALBERT, J.R. & SUMMITT, L.M. (1976) Intravenous toxicity of WL 43775
(6-1-0-0) in the mouse (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
3. ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri and its prey,
Panonychus ulmi on apples in southeast England. Environ. Entomol., 9:
436-439.
4. ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several non-target aquatic invertebrates. Environ. Entomol., 11:
1251-1257.
5. ATKINS, E.L., KELLUM, D., & ATKINS, K.W. (1981) Reducing pesticide
hazards to honey bees: mortality prediction techniques and integrated
management strategies, Berkeley, California, University of
California, Division of Agricultural Sciences (Leaflet No. 2883).
6. BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
7. BATISTE-ALENTORN, M., XAMENA, N., VELAZQUEZ, A., CREUS, A., & MARCOS,
R. (1987) Non-mutagenicity of fenvalerate in Drosophilia.
Mutagenesis, 2: 7-10.
8. BATTELLE (1982) Pesticide programme of research and market planning.
Part I. Insecticides, Geneva, Battelle Research Centre.
9. BATTELLE (1986) World pesticide programme: Insecticide II, Geneva,
Battelle Research Centre.
10. BELILES R.P., MARIS, S.L., & WEIR, R.J. (1978) Three-generation
reproduction study in rats, Kensington, Maryland, Litton Bionetics
Inc. (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
11. BIRDIE, N.S., BANERJI, R.K., & CHAUHAN, A.K. (1986) Gas liquid
chromatographic separation of pyrethrins from some synthetic
pyrethroids in formulations. Pyrethrum Post, 16(3): 77-80.
12. BLAIR, D. & RODERICK, H. (1975) Toxicity studies on the pyrethroid
insecticide WL 43775, emulsifiable concentrate FX 3368: Acute
inhalation exposure to an aqueous spray (Unpublished report submitted
to WHO by Shell Development Co., Ltd).
13. BRADBURY, S.P. & COATS, J.R. (1982) Toxicity of fenvalerate to
bobwhite quail (Colinus virginianus) including brain and liver
residues associated with mortality. J. Toxicol. environ. Health, 10:
307-319.
14. BRADBURY, S.P., COATS, J.R., & MCKIM, J.M. (1986) Toxicokinetics of
fenvalerate in rainbow trout (Salmo gairdneri). Environ. Toxicol.
Chem., 5: 567-576.
15. BRADBURY, S.P., MCKIM, J.M., & COATS, J.R. (1987) Physiological
response of rainbow trout (Salmo gairdneri) to acute fenvalerate
intoxication. Pestic. Biochem. Physiol., 27: 275-288.
16. BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-
residue method to determination of synthetic pyrethroid residues in
celery and animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
17. BROOKS, T.M. (1976) Toxicity studies with WL 43775: Mutagenicity
studies with WL43775 in the host-mediated assay (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
18. BROWN, L.J. & SLOMKA, M.B. (1979) Skin reaction potential of use
dilution (1.33%) PYDRIN EC (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
19. BRUCE, M.L. & CARUSO, J.A. (1985) The laminar flow torch for gas
chromatographic He microwave plasma detection of pyrethroids and
dioxins. Appl. Spectrosc., 39(6): 942-949.
20. BUTTERWORTH, S.T.G. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL 43775: Acute oral toxicity and neuropathological
effects in rats (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
21. BUTTERWORTH, S.T.G. & HEND, R.W. (in press) Peripheral nerve lesions
induced by large doses of pyrethroid. Neuropathol. appl. Neurobiol.
22. CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin sensory stimulation
characterized by a new behavioural paradigm. Toxicol. appl.
Pharmacol., 76: 270-279.
23. CAPLAN, J.R., ISENSEE, A.R., & NELSON, J.O. (1984) Fate and effect of
[14C]fenvalerate in a tidal marsh sediment ecosystem model. J. agric.
food Chem., 32: 166-171.
24. CAYLEY, G.R. & SIMPSON, B.W. (1986) Separation of pyrethroid enan-
tiomers by chiral high-performance liquid chromatography. J.
Chromatogr., 356: 123-134.
25. CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four pyrethroid
insecticide in a mineral and organic soil. J. environ. Sci. Health,
B16: 605-615.
26. CHATTERJEE, K.K., TALUKDER, G., & SHARMA, A. (1982) Effects of
synthetic pyrethroids on mammalian chromosomes I. Sumicidin. Mutat.
Res., 105: 101-106.
27. CLEMENTS, A.N. & MAY, T.E. (1977) The actions of pyrethroids upon the
peripheral nervous system and associated organs in the locust.
Pestic. Sci., 8: 661-680.
28. COATS, J.R. & O'DONNELL-JEFFREY, N.L. (1979) Toxicity of four syn-
thetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
29. COOMBS, A.D. & CARTER, B.I. (1975) Toxicity studies on insecticide WL
43775: Acute toxicity, skin irritancy potential of the emulsifiable
concentrate FX 3368 (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
30. COOMBS, A.D. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL43775: Toxicity and skin and eye irritancy potential of
the ULV formulation FX4353 (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
31. CURTIS, L.R., SEIM, W.K., & CHAPMAN, G.A. (1985) Toxicity of
fenvalerate to developing steelhead trout following continuous or
intermittent exposure. J. Toxicol. environ. Health, 15: 445-457.
32. DAY, K. & KAUSHIK, N.K. (1987a) Short-term exposure of zooplankton to
the synthetic pyrethroid, fenvalerate, and its effects on rates of
filtration and assimilation of the alga Chlamydomonas reinhardii.
Arch. environ. Contam. Toxicol., 16: 423-432.
33. DAY, K. & KAUSHIK, N.K. (1987b) An assessment of the chronic toxicity
of the synthetic pyrethroid, fenvalerate, to Daphnia galeata
mendotae, using life tables. Environ. Pollut., 44: 13-26.
34. DEAN, B.J. (1975) Toxicity studies with WL 43775: Dominant lethal
assays in male mice after single oral doses of WL 43775 (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
35. DEAN, B.J. & SENNER, R.K. (1975) Toxicity studies with WL 43775:
Chromosome studies on bone marrow cells of Chinese hamster after two
daily oral doses of WL 43775 (Unpublished report to WHO submitted by
Shell Development Co., Ltd).
36. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series No. 42).
37. ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1979) Dislodgable
insecticide residue on cotton foliage: permethrin, curacron,
fenvalerate, sulprofos, decis, and endosulfan. Bull. environ. Contam.
Toxicol., 22: 245-248.
38. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
39. FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
40. FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49
(FAO Plant Production and Protection Paper 20).
41. FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
299-353 (FAO Plant Production and Protection Paper 20 Sup.).
42. FAO/WHO (1982a) Pesticide residues in food. Report of the 1981 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 26-28
(FAO Plant Production and Protection Paper 37).
43. FAO/WHO (1982b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
209-256 (FAO Plant Production and Protection Paper 42).
44. FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 28 (FAO
Plant Production and Protection Paper 46).
45. FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, p. 420
(FAO Plant Production and Protection Paper 49).
46. FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49,
83, 90-93 (FAO Plant Production and Protection Paper 62).
47. FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
343-353 (FAO Plant Production and Protection Paper 67).
48. FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations.
49. FAO/WHO (1986a) Pesticide residues in food. Report of the 1986 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 28-29,
61 (FAO Plant Production and Protection Paper 77).
50. FAO/WHO (1986b) 1986 Evaluations of some pesticide residues in food.
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 189-193, 355 (FAO Plant Production and Protection
Paper 78/1).
51. FAO/WHO (1986c) Codex maximum limits for pesticide residues, 2nd ed.,
Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC XIII).
52. FAO/WHO (1987a) 1986 Evaluations of some pesticide residues in food.
Part II - Toxicology, Rome, Food and Agriculture Organization of the
United Nations, p. 219 (FAO Plant Production and Protection Paper
78/2).
53. FAO/WHO (1987b) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 27 (FAO
Plant Production and Protection Paper 84).
54. FAO/WHO (1988) 1987 Evaluations of some pesticide residues in food.
Part 1 - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 57-59 (FAO Plant Production and Protection Paper
86/1).
55. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
56. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary irritant contact
dermatitis from synthetic pyrethroid insecticide exposure. Arch.
Toxicol., 56: 288-294.
57. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Brit. J. ind. Med., 42:
363-372.
58. FLODSTROM, S., WARNGARD, L., LJUNGQUIST, S., & AHLBORG, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch. Toxicol., 61: 218-223.
59. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
60. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
61. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
62. GAUGHAN, L.C., ENGEL, J.I., & CASIDA, J.E. (1980) Pesticide
interactions: effects on organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pestic. Biochem. Physiol., 14: 81-85.
63. GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid
insecticides to bees. Pestic. Sci., 16: 206-207.
64. GHIASUDDIN, S.M. & SODERLUND, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. appl.
Pharmacol., 74: 390-396.
65. GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav. Toxicol.
Teratol., 4:(6) 793-799.
66. GORDON, E.B. & WEIR, R.J. (1978) Lifetime feeding study in rats, SD-
43775 Technical, Kensington, Maryland, Litton Bionetics, Inc.
(Project No. 2054, Report AT-81-0181) (Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd).
67. GREENBERG, R.S. (1981) Determination of fenvalerate, a synthetic
pyrethroid, in grapes, peppers, apples, and cotton seeds by gas-
liquid chromatography. J. agric. food Chem., 29: 856-860.
68. HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage toxicity tests.
Environ. Toxicol. Chem., 2(2): 251-8
69. HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory studies
on the persistence and behavior in soil of four pyrethroid
insecticides. Can. Entomol., 113: 685-694.
70. HART, E.R. (1975) 90-day subacute toxicity study in dogs, SD 43775
Technical (sumicidin), Kensington, Maryland, Litton Bionetics, Inc.
(Technical Report No. AT-51-0013) (Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd).
71. HE, FENSHENG (1987) Occupational neurotoxicology: Current problems
and trends. Presented at the XXII International Congress on
Occupational Health, Sydney, Australia, 27 September-2 October, 1987.
72. HEND, R.W. & BUTTERWORTH, S.T.G. (1975) Toxicity studies of the
insecticide WL 43775: A three-month feeding study in rats
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
73. HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies of the
insecticide WL 43775: A short-term feeding study in rats (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
74. HILL, B.D. (1981) Persistence and distribution of fenvalerate
residues in soil under field and laboratory conditions. J. agric.
food Chem., 29: 107-110.
75. HINE, C. (1975) SD-43775 Toxicity: Acute and repeated (14-day) dermal
toxicity in the rabbit, San Francisco, Hine Inc. (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
76. HIROMORI, T., NAKANISHI, T., KAWAGUCHI, S., SAKO, H., SUZUKI, T., &
MIYAMOTO, J. (1986) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci., 11: 9-14.
77. HOLMSTEAD, R.L. & FULLMER, D.G. (1977) Photodecarboxylation of
cyanohydrine esters. Models for pyrethroid photodecomposition. J.
agric. food Chem., 25: 56-58.
78. HOLMSTEAD, R.L., FULLMER, D.G., & RUZO, L.O. (1978) Pyrethroid
photodecomposition: Pydrin. J. agric. food Chem., 26: 954-959.
79. HORIBA, M., KITAHARA, H., TAKAHASHI, K., YAMAMOTO, S., & MURANO, A.
(1980) Gas chromatographic determination of fenvalerate (S-5602) in
technical preparations. Agric. biol. Chem., 44: 1197-1199.
80. HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
81. ITO, N. (1976a) Histopathological findings of mice treated with S5602
for three months (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
82. ITO, N. (1976b) Histopathological findings of mice and rats exposed
to mist of S5602 for four weeks (Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd).
83. ITO, N. (1978) Histopathological findings in mice treated with S5602,
Nagoya, Nagoya City University Medical School (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
84. JOHANSEN, C., MAYER, D., MADSEN, R., & ROBINSON, W. (1975) Bee
research investigations, 1975, Pullman, Washington, Washington State
University, Department of Entomology (Unpublished report).
85. JOHANSEN, C., KIOUS, C., SCHULTZ, G., GUPTA, R., & STANFORD, A.
(1978) Bee research investigations, 1978, Pullman, Washington,
Washington State University, Department of Entomology (Unpublished
report).
86. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Comparative metabolism
of fenvalerate and the [2S, S]-isomer in rats and mice. J. Pestic.
Sci., 6: 317-326.
87. KANEKO, H., MATSUO, M., & MIYAMOTO, J. (1986) Differential metabolism
of fenvalerate and granuloma formation. I. Identification of
cholesterol ester derived from a specific chiral isomer of
fenvalerate. Toxicol. appl. Pharmacol., 83: 148-156.
88. KANEKO, H., TAKAMATSU, Y., OKUNO, Y., ABIKO, J., YOSHITAKE, A., &
MIYAMOTO, J. (1988) Substrate specificity for formation of
cholesterol ester conjugates from fenvalerate analogues and for
granuloma formation. Xenobiotica, 18(1): 11-19.
89. KATAGI, T., MIKAMI, N., MATSUDA., T., & MIYAMOTO, J. (1985a)
Photodegradation of fenvalerate and esfenvalerate on soils
(Unpublished Report No. LLM-50-0005 submitted to WHO by Sumitomo
Chemical Co., Ltd).
90. KATAGI, T., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985b)
Hydrolysis of fenvalerate and esfenvalerate in buffered aqueous
solutions (Unpublished Report No. LLM-50-0006 submitted to WHO by
Sumitomo Chemical Co., Ltd).
91. KIRKLAND, V.L. & ALBERT, J.R. (1977) Preliminary pharmacodynamic
investigation of SD 43775 (6-10-0) administered intravenously to the
anesthetized dog. I. Effects on the cardiovascular system and
respiration. II. Electrocardiographic (ECG) findings (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
92. KNOX, J.M., II, TUCKER, S.B., & FLANNIGAN, S.A. (1984) Paraesthesia
from cutaneous exposure to a synthetic pyrethroid insecticide. Arch.
Dermatol., 120: 744-746.
93. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic study on
S5602 in mice (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
94. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Acute inhalation
toxicity of S3206 and S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
95. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976c) Subacute inhalation
toxicity study of S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
96. KOHDA, H., KANEKO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J. (1979)
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
97. KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and fenvalerate).
Arch. Toxicol., 50: 27-33.
98. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
99. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
100. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
101. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
102. LEE, P.W. (1985) Fate of fenvalerate (Pydrin insecticide) in the soil
environment. J. agric. food Chem., 33: 993-998.
103. LEE, P.W., WESTCOTT, N.D., & REICHLE, R.A. (1978) Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871.
104. LEE, P.W., STEARNS, S.M., & POWELL, W.R. (1985) Rat metabolism of
fenvalerate (Pydrin insecticide). J. agric. food Chem., 33: 988-993.
105. LEE, P.W., POWELL, W.R., STEARNS, S.M. & MCCONNEL, O.J. (1987)
Comparative aerobic soil metabolism of fenvalerate isomers. J. agric.
food Chem., 35: 384-387.
106. LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
107. LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 11/12: 843-851.
108. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol.,
20: 203-216.
109. MCKENNEY, C.L., Jr & HAMAKER, D.B. (1984) Effects of fenvalerate on
larval development of Palaemonetes pugio (Holthuis) and on larval
metabolism during osmotic stress. Aquat. Toxicol., 5: 343-355.
110. MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and
shrimp. Bull. environ. Contam. Toxicol., 25: 950-955.
111. MALLEY, L.A., CAGEN, S.Z., GARDINER, T.H., & VAN GELDER, G.A. (1984)
Characterization of fenvalerate mediated skin sensory stimulation.
Neurotoxicology, 5: 71-72.
112. MALLEY, L.A., CAGEN, S.Z., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & ROSE, G.P. (1985) Effect of vitamin E and other amelioratory
agents on the fenvalerate-mediated skin sensation. Toxicol. Lett.,
29: 51-58.
113. MATSUBARA, R.A., SUZUKI, T., KADOTA, T., & MIYAMOTO, J. (1977)
Antidotes against poisoning by S5602 in rats (Unpublished report
submitted by Sumitomo Chemical Co., Ltd).
114. MAYER, F.L., Jr (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Environmental Protection
Agency, p. 115 (EPA-600/8-87/017).
115. MAYER, F.L., Jr & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp. 235-236.
116. MEGHARAJ, M., VENKATESWARLU, K., & RAO, A.S. (1986) Influence of
cypermethrin and fenvalerate on natural soil algal populations.
Ecotoxicol. environ. Saf., 12: 141-145.
117. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Willoughby,
Ohio, Meister Publishing Co., pp. C104-C105.
118. MIKAMI, N., TAKAHASHI, N., HAYASHI, K., & MIYAMOTO, J. (1980)
Photodegradation of fenvalerate (Sumicidin) in water and on soil
surface. J. Pestic. Sci., 5: 225-236.
119. MIKAMI, N., SAKATA, S., YAMADA, H., & MIYAMOTO, J. (1984a) Further
studies on degradation of the pyrethroid insecticide fenvalerate in
soils. J. Pestic. Sci., 9: 697-702.
120. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1984b) New
conjugated metabolites of 3-phenoxybenzoic acid in plants. Pestic.
Sci., 15: 531-542.
121. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1985a) The
metabolism of fenvalerate in plants: The conjugation of the acid
moiety. Pestic Sci., 16: 46-58.
122. MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO, J.
(1985b) Metabolism in rats of 3-phenoxybenzyl alcohol and 3-
phenoxybenzoic acid glucoside conjugates formed in plants. Pestic.
Sci., 16: 33-45.
123. MILNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticide. Investigation of neurotoxic potential of WL 43775
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
124. MIURA, T. & TAKAHASHI, R.M. (1976) Effects of a synthetic pyrethroid,
SD43775, on nontarget organisms when utilized as a mosquito
larvicide. Mosq. News, 36: 322-326.
125. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
126. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
127. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress
of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982,
Oxford, Pergamon Press, Vol. 1-4.
128. MIYAMOTO, J., KANEKO, H., & TAKAMATSU, Y. (1986) Stereoselective
formation of a cholesterol ester conjugate from fenvalerate by mouse
microsomal carboxyesterase(s). J. Biochem. Toxicol., 1(2): 79-94.
129. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a) Toxicity
of mosquito larvicidal pyrethroids to four species of freshwater
fishes. Environ. Entomol., 7: 428-430.
130. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978b)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
131. MULLA, M.S., DARWAZEH, H.A., & DHILLON, M.S. (1980) New pyrethroids
as mosquito larvicides and their effects on nontarget organisms.
Mosq. News, 40: 6-12.
132. NOBLE, P.J. (1976) AF1117 residue data in animal tissues, milk and
cream (Unpublished data submitted to WHO by Shell Chemical
(Australia), Ltd).
133. OHKAWA, H., NAMBU, K., INUI, H., & MIYAMOTO, J. (1978) Meta-
bolic fate of fenvalerate (Sumicidin) in soil and by microorganisms.
J. Pestic. Sci., 3: 129-141.
134. OHKAWA, H., KANEKO, H., TSUJI, H., & MIYAMOTO, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
135. OHKAWA, H., KIKUCHI, R., & MIYAMOTO, J. (1980a) Bioaccumulation and
biodegradation of the [S]-acid isomer of fenvalerate (Sumicidin) in
an aquatic model ecosystem. J. Pestic. Sci., 5: 11-12.
136. OHKAWA, H., NAMBU, K., & MIYAMOTO, J. (1980b) Metabolic fate of
fenvalerate (Sumicidin) in bean plants. J. Pestic. Sci., 5: 215-223.
137. OHNO, N., FUJIMOTO, K., OKUNO, Y., MIZUTANI, T., HIRANO, M., ITAYA,
N., HONDA, T., & YOSHIOKA, H. (1976) 2-Arylalkanoates, a new group of
synthetic pyrethroid esters not containing cyclopropane-carboxylates.
Pestic. Sci., 7: 241-246.
138. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975a) Eye and skin irritation
of S-3206 and S-5602 in rabbits (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
139. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975b) Skin sensitization
study of S-5602 in guinea pigs (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
140. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by dermal
applications in rats (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
141. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977a) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by oral
administration in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
142. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977b) Neurotoxic effects of
S5602 and natural pyrethrins by oral administration in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
143. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977c) Recovery of
histopathological lesions in rats caused by short-term feeding of
S5602 (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
144. OKUNO, Y., SEKI, T., ITO, S., KANEKO, H., WATANABE, T., YAMADA, T., &
MIYAMOTO, J. (1986a) Differential metabolism of fenvalerate and
granuloma formation. II. Toxicological significance of lipophilic
conjugate from fenvalerate. Toxicol. appl. Pharmacol., 83: 157-169.
145. OKUNO, Y., ITO, S., SEKI, T., HIROMORI, T., MURAKAMI, M., KADOTA, T.,
& MIYAMOTO, J. (1986b) Fenvalerate-induced granulomatous changes in
rats and mice. J. toxicol. Sci., 11: 53-66.
146. PARKER, C.M., MCCULLOUGH, C.B., GELLATLY, J.B.M., & JOHNSTON, C.D.
(1983) Toxicologic and carcinogenic evaluation of fenvalerate in the
B6C3F1 mouse. Fundam. appl. Toxicol., 3: 114-120.
147. PARKER, C.M., PATTERSON, D.R., VAN GELDER, G.A., GORDON, E.B.,
VALERIO, M.G., & HALL, W.C. (1984a) Chronic toxicity and
carcinogenicity evaluation of fenvalerate in rats. J. Toxicol.
environ. Health, 13: 83-97.
148. PARKER, C.M., PICCIRILLO, V.J., KURTZ, S.L., GARNER, F.M., GARDINER,
T.H., & VAN GELDER, G.A. (1984b) Six-month feeding study of
fenvalerate in dogs. Fundam. appl. Toxicol., 4: 577-586.
149. PARKER, C.M., ALBERT, J.R., VAN GELDER, G.A., PATTERSON, D.R., &
TAYLOR, J.L. (1985) Neuropharmacologic and neuropathologic effect of
fenvalerate in mice and rats. Fundam. appl. Toxicol., 5: 278-286.
150. PARKER, C.M., WIMBERLY, H.C., LAM, A.S., GARDINER, T.H., & VAN
GELDER, G.A. (1986) Subchronic feeding study of decarboxyfenvalerate
in rats. J. Toxicol environ. Health, 18: 77-90.
151. PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
152. PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
153. PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137:7-15.
154. POTTER, J.C. (1976) (a) Tissues of rats fed SD43775-C-14; (b) and (c)
milk and tissues of cows; (d) eggs and tissues from laying hens; (e)
cream from the milk of cows fed SD 43775 (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
155. RANGACHAR, T.R.S., SURESH, T.P., & THINMMAIAH, K. (1981) Acute oral
toxicity of Sumicidin 20E in poultry. Indian vet. J., 58: 941-942.
156. RATTNER, B.A. & FRANSON, J.C. (1983) Methyl parathion and fenvalerate
toxicity in American kestrels: acute physiological responses and
effects of cold. Can. J. Physiol. Pharmacol., 62: 787-792.
157. REED, W.T., EHMAN, A., LEE, P., BARBER, G., & BISHOP, J. (1983)
Pydrin insecticide (fenvalerate) on non-target systems following
field applications. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: Human welfare and the environment, Oxford, Pergamon Press,
Vol. 2, pp. 213-221.
158. ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J.
econ. Entomol., 72: 293-294.
159. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve - muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
160. SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Degradation of esfenvalerate and its isomers in soils (Unpublished
Report No. LLM-30-0003 submitted to WHO by Sumitomo Chemical Co.,
Ltd).
161. SALEH, M.A., IBRAHIM, N.A., SOLIMAN, N.Z., & EL SHEIMY, M.K. (1986)
Persistence and distribution of cypermethrin, deltamethrin and
fenvalerate in laying chickens. J. agric. food Chem., 34: 895-898.
162. SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygiene
rules for synthetic pyrethroid content in the work zone air.] Gig.
tr. prof. Zabol., 8: 48-50 (in Russian).
163. SCHIMMEL, S.C., GARNAS, R.L., PATRICK, J.M., & MOORE, J.C. (1983)
Acute toxicity, bioconcentration, and persistence of AC 222,705,
benthiocarb, chlorpyrifos, fenvalerate, methyl parathion, and
permethrin in the estuarine environment, J. agric. food Chem., 31:
104-113.
164. SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
165. SODERLUND, D.M. & CASIDA, J.E. (1977) Effect of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pestic. Biochem. Physiol., 7: 391-401.
166. SPEHAR, R.L., TANNER, D.K., & GIBSON, J.H. (1982) Effects of Kelthane
and Pydrin on early life stages of Fathead Minnows (Pimephales
promelas) and Amphipods (Hyalella azteca), Philadelphia, American
Society for Testing and Materials (Special Technical Publication No.
766).
167. STEIN, A.A. (1977) Histopathology evaluation of animals from 3-
generation reproduction study. Albany, New York, Biological Research,
Ltd (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
168. SUMMIT, L.M. & ALBERT, J.R. (1977a) Oral lethality of WL 43775 (6-1-
0-0) in the rat (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
169. SUMMIT, L.M. & ALBERT, J.R. (1977b) Determination of the acute oral
lethality of WL 43775 (6-1-0-0) in the male and female mouse
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
170. SUZUKI, H. & MIYAMOTO, J. (1976) Studies on mutagenicity of S 5602
with bacteria systems (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
171. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of some
pyrethroids on salmonella strains in the presence of mouse hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
172. SUZUKI, H., KISHIDA, F., & MIYAMOTO, J. (1979) Studies on
mutagenicity of S5602 in Ames test in the presence of hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
173. SUZUKI, T., KADATA, T., & MIYAMOTO, J. (1976) One-year chronic
toxicity study of S5602 in mice (three month interim report)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
174. SUZUKI, T., OKUNO, Y., HIROMORI, T., ITO, S., KADOTA, T., & MIYAMOTO,
J. (1977a) Fifteen-month chronic toxicity study of S5602 in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
175. SUZUKI, T., OKUNO, Y., HIROMORI, T., KADOTA, T., & MIYAMOTO, J.
(1977b) Eighteen-month chronic toxicity study of S5602 in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
176. SYRETT, P. & PENMAN, D.R. (1980) Studies of insecticide toxicity to
lucerne aphids and their predators. N.Z. J. agric. Res., 23: 575-580.
177. TAGATZ, M.E. & IVEY, J.M. (1981) Effects of fenvalerate on field- and
laboratory-developed estuarine benthic communities. Bull. environ.
Contam. Toxicol., 27: 256-267.
178. TAGATZ, M.E., STANLEY, R.S., PLAIA, G.R., & DEANS, C.H. (1987)
Responses of estuarine macrofauna colonizing sediments contaminated
with fenvalerate. Environ. Toxicol. Chem., 6: 21-25.
179. TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Photodegradation of the [2S, alphaS] isomer of fenvalerate in distilled
water (Unpublished Report No. LLM-50-0001 submitted to WHO by
Sumitomo Chemical Co., Ltd).
180. TAKAMATSU, Y., KANEKO, H., ABIKO, J., YOSHITAKE, A., & MIYAMOTO, J.
(1987) In vivo and in vitro stereoselective hydrolysis of four chiral
isomers of fenvalerate. J. Pestic. Sci., 12: 397-404.
181. TALEKER, N.S., CHEN, J.S., & KAO, H.T. (1983) Persistence of
fenvalerate in subtropical soil. J. econ. Entomol., 76: 223-226.
182. TUCKER, S.B. & FLANNIGAN, S.A. (1983) Cutaneous effects from
occupational exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
183. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
184. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect: Neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
185. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M., (1973)
DDT-like action of allethrin in the sensory nervous system of Xenopus
laevis. Eur. J. Phamacol., 21: 95-106.
186. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979)
Effect of insecticides on the sensory nervous system. In: Narahashi,
T., ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 183-210.
187. VAN DER PAUW, C.L., DIX, K.M., BLANCHARD, K., & MCCARTHY, W.V. (1975)
Teratological studies in rabbits given WL 43775 orally (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
188. VAKENTI, J.M. (1986) Persistence of fenvalerate in orchard soils,
Okanagan Valley, British Columbia (Unpublished report of cooperative
study of Agriculture Canada and Ciba-Geigy Canada, Ltd).
189. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relation-
ship of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
190. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog myelinated
nerve fibres. Eur. J. Phamacol., 58: 501-504.
191. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
192. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
193. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in mylinated nerves. Nature (Lond.), 295: 601-603.
194. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN
DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
195. WALKER, B.J., HEND, R.W., & LINNETT, S. (1975) Toxicity studies on
the insecticide WL 43775: Summary of results of preliminary
experiments (Unpublished report submitted to WHO by Shell Development
Co., Ltd).
196. WHO (1979) WHO Technical Report Series No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp.18-23.
197. WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
198. WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
199. WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic pyrethroid
insecticides to predaceous phytoseiid mites and their prey. Aust. J.
agric. Res., 30: 497-501.
200. WOOD MACKENZIE (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
201. WOOD MACKENZIE (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
202. WOOD MACKENZIE (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
203. WOOD MACKENZIE (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
204. WOOD MACKENZIE (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
205. WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida. State Hortic. Soc., 90: 401-402.
206. WORTHING, C.R. (1979) The pesticide manual, 6th ed., Croydon, British
Crop Protection Council, p. 270.
207. WORTHING, C.R. & WALKER, S.B. (1987) Fenvalerate In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 395-
396.
208. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
209. WSZOLEK, P.C., LEIN, D.H., & LISK, D.J. (1980) Excretion of fenval-
erate insecticide in the milk of dairy cows. Bull. environ. Contam.
Toxicol., 24: 296-298.
210. WSZOLEK, P.C., HOGUE, D.E., & LISK, D.J. (1981a) Accumulation of
fenvalerate insecticide in lamb tissues. Bull. environ. Contam.
Toxicol., 27: 869-871.
211. WSZOLEK, P.C., LAFAUNCE, N.A., WACHS, T., & LISK, D.J. (1981b)
Studies of possible bovine urinary excretion and rumen decomposition
of fenvalerate insecticide and a metabolite. Bull. environ. Contam.
Toxicol., 26: 262-266.
212. BORTHWICK, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
static acute toxicity tests with selected estuarine algae,
invertebrates, and fish. Gulf Breeze, Florida, US Environmental
Protection Agency (EPA-600/4-81-076).
213. HE, F., WANG, S., LIU, L., CHEN, S., ZHANG, Z., & SUN, J. (1989)
Clinical manifestations and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63: 54-58.
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs (Xenopus laevis; Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [65, 98, 186,
189, 196]. The same distinction was found in studies on
cockroaches [60].
Based on the binding assay on the gamma-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types:
the alpha-cyano-3-phenoxybenzyl pyrethroids and the non-
cyano pyrethroids [59, 61, 99, 100].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin,
cismethrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [185]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [183]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [194].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [55].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [184]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [194].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans
[38], are almost identical to those of allethrin, i.e.,
presynaptic repetitive firing, and no significant effects
on transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [185, 186, 193].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [108].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [159].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cypermethrin, fenval-
erate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [192]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [190,
194].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during
excitation, presumably by slowing down the closing of the
activation gate of the sodium channel [190, 194]. The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than
100 milliseconds. Apparently, the amplitude of the pro-
longed sodium current after cypermethrin is too small to
induce repetitive activity in nerve fibres, but is suf-
ficient to cause the long-lasting repetitive firing in the
lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [108].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [61].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the tox-
icity of Type II pyrethroids and their potency to inhibit
the [35S]-TBPS binding was established using a radio-
ligand, [35S]- t-butyl-bicyclophosphoro-thionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute corre-
lation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all
toxic cyano compounds including deltamethrin, 1R, cis-
cypermethrin, 1R, trans-cypermethrin, and [2S, alphaS]-
fenvalerate were inhibitors, but their non-toxic stereo-
isomers were not; non-cyano pyrethroids were much less
potent or were inactive [99].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-di-
hydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [100].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S, alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex
[59].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [159].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-
lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation.
This results in long-lasting trains of repetitive impulses
in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [196, 208].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [191].
RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes
d'analyse
La fenvalérate est un insecticide puissant utilisé
depuis 1976. C'est un ester de l'acide (chloro-4 phényle)-
2 méthyl-3 butyrique et de l'alcool alpha-cyano-phénoxy-
benzylique. Malgré l'absence du cycle cyclopropane, ses
propriétés insecticides le rattachent au groupe des
pyréthroïdes. Il s'agit d'un mélange racémique de quatre
isomères optiques dont les configurations sont [2S, alphaS],
[2S, alphaR], [2R, alphaS] et [2R, alphaR]. L'isomère [2S,
alphaS] est le plus actif biologiquement; vient ensuite
l'isomère [2S, alphaR].
Le fenvalérate de qualité technique se présente sous
la forme d'un liquide visqueux jaune ou brun dont la
densité est de 1,175 à 25 °C. Il est relativement non
volatil, sa tension de vapeur étant de 0,037 mPa à 25 °C.
Pratiquement insoluble dans l'eau (environ 2 µg/litre),
il est soluble dans les solvants organiques comme
l'acétone, le xylène et le kérosène. Il est stable à la
lumière, à la chaleur et à l'humidité, mais instable en
milieu alcalin par suite de l'hydrolyse du groupement
ester.
Le dosage des résidus et les analyses écotoxico-
logiques peuvent s'effectuer par chromatographie en phase
gazeuse avec détection par capture d'électrons, la
concentration minimale décelable étant de 0,005 mg/kg.
Pour l'analyse des produits techniques on utilise la même
méthode mais avec un détecteur à ionisation de flamme.
1.2 Production et usage
On utilise dans le monde environ 1000 tonnes de
fenvalérate par an (chiffres de 1979-1983), essentielle-
ment en agriculture mais également pour la désinsecti-
sation des habitations et des jardins et le déparasitage
des bestiaux, soit seul, soit en association avec d'autres
insecticides. Il est présenté sous forme de concentré
émulsionnable, de concentré pour épandage à très bas
volume, de poudre pour poudrage et de poudre mouillable.
1.3 Exposition humaine
C'est essentiellement du fait de la présence de
résidus dans les aliments que la population dans son
ensemble est exposée à cet insecticide. Le respect des
règles de bonne pratique permet en général de maintenir
les résidus dans les récoltes à un faible niveau. L'expo-
sition qui en découle pour la population générale devrait
a priori être très faible mais on ne dispose pas de
données tirées d'études de la ration totale.
L'analyse des résidus présents dans les céréales
ensilées a montré que plus de 70% de la dose appliquée
subsistent sur le blé au bout de dix mois à 25 °C. Après
mouture et panification, la teneur en résidus du pain
blanc et de la farine de froment est à peu près la même
(environ 0,06-0,1 mg/kg).
Les informations relatives à l'exposition profession-
nelle au fenvalérate sont très fragmentaires.
1.4 Destinée dans l'environnement
Dans le sol, il y a dégradation par coupure de la
liaison ester et du groupement diphényl-éther, hydroxyl-
ation du cycle benzénique, hydratation du nitrile en
amide, l'oxydation des fragments se poursuivant jusqu'à
l'obtention d'anhydride carbonique qui constitue le
principal produit final. L'étude du potentiel de lixivi-
ation du fenvalérate et de ses produits de dégradation a
montré qu'il n'y avait guère de pénétration au niveau du
sol.
Dans l'eau et à la surface du sol, la fenvalérate
subit une photodégradation par la lumière solaire. On a
montré qu'il se produisait une coupure du groupement
ester, une hydrolyse du groupement cyano, une décarboxy-
lation conduisant au (phénoxy-3)-2 (chloro-4 phényle)-3
méthyl-4 pentane-nitrile (décarboxy-fenvalérate), ainsi que
d'autres réactions à initiation radicalaire.
Sur les végétaux, la fenvalérate a une demi-vie
d'environ 14 jours. La principale réaction consiste dans
la rupture de la liaison ester suivie d'une oxydation
et/ou d'une conjugaison des fragments. Il se produit
également une décarboxylation en décarboxy-fenvalérate.
En général, la dégradation dans l'environnement
conduit à des produits moins toxiques.
Dans l'environnement, le fenvalérate est assez
rapidement dégradé. La demi-vie est de 4 à 15 jours dans
les rivières, 8 à 14 jours sur les végétaux, 1 à 18 jours
par photodégradation à la surface du sol et 15 jours à
3 mois dans le sol.
Il n'y a pratiquement aucune lixiviation du fenva-
lérate présent dans le sol. Il est donc improbable que ce
composé puisse s'accumuler de façon importante dans le
milieu aquatique.
1.5 Cinétique et métabolisme
On a étudié la destinée du fenvalérate chez le rat et
la souris au moyen de fenvalérate radio-marqué au niveau
du groupement carboxylate, ou des groupements benzyle ou
cyano. Sauf dans le cas des composés marqués au niveau du
groupement cyano, la radioactivité administrée est
rapidement excrétée (jusqu'à 99% en six jours). Les
principales réactions métaboliques consistent en une
rupture du groupement ester et une hydroxylation en
position 4. On a également observé diverses réactions
d'oxydation et de conjugaison conduisant à un mélange
complexe. Lorsque le fenvalérate est radio-marqué au
niveau du groupement cyano, la dose radioactive s'élimine
moins rapidement (jusqu'à 81% en six jours). La
radioactivité restante est principalement confinée dans la
peau, les poils et l'estomac sous forme de thiocyanate.
Il existe aussi une voie métabolique secondaire, quoique
très importante, qui consiste dans la formation d'un
conjugué lipophile de (chloro-4 phényle)-2-[2R] iso-
valérate. Ce conjugué qui intervient dans la formation de
granulomes a été décelé dans les surrénales, le foie et
les ganglions mésentériques des rats, des souris et de
certaines autres espèces.
1.6 Effets sur les êtres vivant dans leur milieu naturel
Au laboratoire, le fenvalérate se révèle extrêment
toxique pour les organismes aquatiques. La CL50 varie de
0,008 µg/litre pour des mysidacées nouvellement écloses
à 2 µg/litre pour une espèce d'éphéméroptère. Les
épreuves portant sur le cycle évolutif de Daphnia galeata
mendotae ont révélé que la dose sans effet observable
était de 0,005 µg/litre. Le fenvalérate est également
extrêment toxique pour les poissons. Les valeurs de la
CL50 à 96 heures vont de 0,03 µg/litre pour la larve de
Leuresthes tenuis à 200 µg/litre pour le Tilapia
adulte. La dose sans effet observable sur 28 jours
s'établit à 0,56 µg/litre pour les premiers stades de
certains vairons. Le fenvalérate est moins toxique pour
les algues et les mollusques aquatiques, la CL50 à 96
heures étant supérieure à 1000 µg/litre.
La forte toxicité potentielle du fenvalérate pour les
organismes aquatiques ne se manifeste pas dans les essais
sur le terrain ni dans les conditions d'utilisation
pratique. Certains invertébrés aquatiques sont détruits
par un épandage à la surface des eaux mais l'effet sur les
populations est temporaire. On n'a pas signalé de
mortalité chez les poissons. La toxicité moindre observée
lors des épandages de plein champ s'explique par une forte
adsorption du composé par les sédiments.
Le fenvalérate est très toxique pour l'abeille. En
applications topiques la DL50 est de 0,41 µg/abeille,
toutefois l'effet répulsif intense qu'exerce le fenva-
lérate sur ces insectes en réduit l'action dans la
pratique. Rien n'indique qu'il y ait eu des destructions
importantes d'abeilles dans les conditions normales
d'utilisation. Le fenvalérate est plus toxique pour les
acariens prédateurs que pour les espèces cibles de
ravageurs.
Administré par voie orale ou mêlé à la nourriture, le
fenvalérate est très peu toxique pour les oiseaux. La
DL50 est supérieure à 1500 mg/kg de poids corporel en
administration orale directe et dépasse 15 000 mg/kg de
nourriture en administration dans la ration alimentaire
pour le colin de Virginie.
Les organismes aquatiques fixent rapidement le fenva-
lérate. Le facteur de bioconcentration varie de 120 à
4700 selon l'organisme en cause (algues, mollusques,
daphnies et poissons) selon les études effectuées sur des
modèles d'écosystèmes. Toutefois, ce fenvalérate s'élimine
rapidement lorsque les organismes sont replacés en eau
propre. On peut donc considérer qu'en pratique, ce composé
ne présente aucune tendance à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
La toxicité aiguë par voie orale du fenvalérate est
modérée à faible. Toutefois, les valeurs de la DL50
peuvent varier considérablement (de 82 à plus de 3200
mg/kg) selon l'espèce animale en cause et le véhicule
d'administration. Les signes cliniques d'intoxication
aiguë apparaissent rapidement mais les survivants
redeviennent asymptomatiques au bout de trois à quatre
jours. Parmi les signes d'intoxication produits par le
mélange racémique, ainsi que par l'isomère [2S, alphaS], on
note de l'agitation, des tremblements, une horripilation,
de la diarrhée, une démarche anormale, une choréo-athétose
et une salivation (syndrome CS); il est classé comme
pyréthroïde du type II. Du point de vue électrophysio-
logique, il produit des bouffées de pointes au niveau des
nerfs moteurs des cerques de la blatte. Toutefois, il n'y
a pas de relation bien définie entre les effets électro-
physiologiques chez l'insecte et la toxicité pour les
mammifères.
Des rats ayant reçu du fenvalérate pendant 8 à 10
jours à raison de 2000 mg/kg de nourriture ont présenté
des signes typiques d'intoxication aiguë. A la dose de
3000 mg/kg de nourriture, on observait des modifications
morphologiques réversibles au niveau du nerf sciatique.
On a également observé des modifications histopatho-
logiques au niveau du même nerf chez des rats et des
souris ayant reçu en une seule fois du fenvalérate par
voie orale à des doses létales ou sublétales.
Des poulets ayant reçu par voie orale du fenvalérate
pendant cinq jours à raison de 1000 mg/kg par jour n'ont
pas présenté de signes cliniques ou morphologiques de
neurotoxicité retardée.
Chez la souris, la toxicité aiguë par voie intra-
péritonéale des métabolites du fenvalérate n'est pas
supérieure à celle du fenvalérate lui-même.
Lors d'études de toxicité subaiguë et subchronique,
des souris, des rats, des chiens et des lapins ont reçu
pendant trois semaines à six mois, du fenvalérate par voie
orale, percutanée et respiratoire. Chez le rat et la
souris, des études d'inhalation de quatre semaines ont
permis de fixer la dose sans effet observable à 7 mg/m3.
Chez le rat, lors d'une étude de 90 jours, elle
s'établissait à 125 mg/kg de nourriture, et sur deux ans à
250 mg/kg de nourriture (soit 12,5 mg/kg de poids
corporel). Dans une étude de 24 à 28 mois, elle s'est
établie à 150 mg/kg de nourriture, soit 7,5 mg/kg de poids
corporel. Une étude de deux ans sur la souris a permis de
fixer la dose à 50 mg/kg de nourriture, soit 6 mg/kg de
poids corporel et une étude de 20 mois, à 30 mg/kg de
nourriture, soit 3,5 mg/kg de poids corporel. Chez le
chien cette dose a été établie à 12,5 mg/kg de poids
corporel, lors d'une étude de 90 jours. Certaines formu-
lations de fenvalérate ont provoqué une irritation cutanée
et oculaire. Toutefois, le fenvalérate technique n'est
pas irritant et n'a pas d'effet sensibilisateur.
Lors d'études de toxicité à long terme, on a noté
l'apparition de microgranulomes chez des souris qui
avaient été traitées avec l'isomère [2R, alphaS] (125 mg/kg
de nourriture) sur une période de un à trois mois. Ces
anomalies disparaissaient lorsqu'on supprimait le fenval-
érate. L'agent causal en était l'ester cholestérique de
l'acide (chloro-4 phényl)-2 isovalérique, un métabolite
lipophile de l'isomère [2R, alphaS] du fenvalérate. La
dose sans effet observable relative à la formation de
microgranulomes chez la souris s'établit à 30 mg de
fenvalérate par kg de nourriture.
Lors d'une autre étude de toxicité à long terme, on a
également observé ces anomalies microgranulomateuses chez
des rats à la dose de 500 mg/kg de nourriture, la dose
sans effet observable étant dans ce cas de 150 mg par kg
de nourriture.
Administré à des souris dans leur nourriture pendant
78 semaines en doses allant jusqu'à 3000 mg/kg ou pendant
deux ans à raison de 1250 mg/kg, le fenvalérate ne s'est
pas révélé cancérogène. Il ne l'a pas été non plus chez
des rats qui avaient reçu pendant deux ans une alimen-
tation contenant jusqu'à 1000 mg d'insecticide par kg.
Le fenvalérate n'est ni mutagène, ni délétère pour les
chromosomes ainsi qu'il ressort d'un certain nombre
d'épreuves in vitro et in vivo.
Il n'est pas non plus tératogène pour la souris et le
lapin à des doses quotidiennes allant jusqu'à 50 mg par kg
de poids corporel et, lors d'une étude de reproduction
portant sur trois générations de rats où les animaux
recevaient des doses allant jusqu'à 250 mg/kg de
nourriture, il n'a affecté aucun des paramètres de la
fonction de reproduction.
1.8 Effets sur l'être humain
Le fenvalérate peut provoquer des sensations
d'engourdissement, de démangeaison, de picotement et de
brûlure chez les travailleurs exposés; les symptômes
apparaissent après une période de latence d'environ
30 minutes, atteignent leur acmé au bout de 8 heures et
disparaissent dans les 24 heures suivantes. Certains cas
d'intoxication se sont produits à la suite d'une expo-
sition professionnelle due au non respect des mesures de
sécurité.
Rien n'indique que le fenvalérate soit nocif pour
l'être humain dans la mesure où il est utilisé conformé-
ment aux recommandations.
2. Conclusions
2.1 Population générale
L'exposition de la population générale au fenvalérate
est probablement très faible. Il n'y a sans doute aucun
risque si on l'emploie conformément aux recommandations.
2.2 Exposition professionnelle
Utilisé de manière raisonnable, et moyennant certaines
mesures d'hygiène et de sécurité, le fenvalérate ne
devrait pas être dangereux pour les personnes qui lui sont
exposées de par leur profession.
2.3 Environnement
Il est improbable que le fenvalérate ou ses produits
de dégradation puissent s'accumuler dans l'environnement
en quantité suffisante pour créer des problèmes, dans la
mesure où l'on respecte les doses d'emploi recommandées.
Au laboratoire, le fenvalérate se révèle extrêmement
toxique pour les poissons, les arthropodes aquatiques et
les abeilles. Toutefois, il ne semble pas que des effets
nocifs durables puissent se produire sur le terrain si
l'insecticide est utilisé conformément aux recomman-
dations.
3. Recommandations
Les concentrations alimentaires qui résultent d'une
utilisation conforme aux recommandations sont considérées
comme très faibles; toutefois il conviendrait de confirmer
ce point de vue en étendant les études de surveillance au
fenvalérate.
Le fenvalérate est utilisé depuis de nombreuses années
et seuls quelques effets temporaires ont été observés ça
et là à la suite d'expositions professionnelles. Néan-
moins, il serait bon de poursuivre les observations sur
l'exposition humaine.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
Table 8 shows the results of acute toxicity tests with
technical grade fenvalerate in various animal species.
The acute toxic signs in rats were restlessness,
tremors, piloerection, occasional diarrhoea, and an abnor-
mal gait. Following oral administration, surviving rats
recovered rapidly from acute clinical signs of poisoning
and were asymptomatic within 3-4 days [20].
Table 9 shows the results of an acute intraperitoneal
toxicity study of fenvalerate metabolites in mice [96].
All the compounds were dissolved in corn oil except
3-phenoxybenzoic acid, which was dissolved in DMSO. The
acute intraperitoneal toxicity in mice of the proposed
decarboxylated photo-products was found to be similar to,
or greater than, that of fenvalerate [78].
In a study by Blair and Roderick [12], groups of four
male and four female rats were exposed by inhalation (head
only) to an aerosol formulation (77-µm particle size)
generated from an aqueous suspension containing 3 g/litre.
Following a single administration (4 h) of this non-
inhalable particulate, acute signs of poisoning were noted
for a short period, presumably from oral ingestion of the
large particles. There was no mortality and all animals
appeared normal within 3 days following exposure.
7.2 Short-Term Exposures
7.2.1 Oral administration
Groups of Carworth Farm E rats (12 of each sex per
group) were fed fenvalerate in the diet at dose levels of
0, 125, 500, 1000, and 2000 mg/kg for 90 days [72]. Mor-
tality (11/12 male, 9/12 female) was observed at the
highest concentration. Body weight gain and food consump-
tion were decreased and blood urea nitrogen concentrations
were increased at 1000 and 2000 mg/kg. There were no
treatment-related changes in any groups of rats in the
haematological parameters examined. Increases in liver to
body weight ratios and kidney to body weight ratios were
observed at 500 mg/kg or more. Gross and microscopic
examinations revealed no compound-related changes in any
groups. The NOEL was 125 mg/kg diet.
Table 8. Acute toxicity of fenvalerate (technical grade) administered to various species
-------------------------------------------------------------------------------------------------
Species Route Sex Vehiclea LD50 (mg/kg) Reference
-------------------------------------------------------------------------------------------------
Rat oral DMSO 451 195
oral PEG:water > 3200 168
dermal 5000 (24 h) 140
inhalation M, F water > 101 mg/m3 (3 h) 94
intraperitoneal 340 162
Mouse oral M DMSO 200-300 195
F 100-200
oral PEG:water 1202 169
intraperitoneal M, F corn oil 85-89 96
intraperitoneal 132 162
intravenous glycerolformol 65 2
inhalation M, F water > 101 mg/m3 (3 h) 94
Chinese oral M DMSO 98 195
hamster F 82
Rabbit percutaneous undiluted 1000-3200 75
Hen oral > 1500 123
-------------------------------------------------------------------------------------------------
a PEG = polyethylene glycol; DMSO = dimethylsulfoxide.
Table 9. Acute intraperitoneal toxicity of fenvalerate metabolites in mice
---------------------------------------------------------------------------
Chemical No.a LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------------
Fenvalerate (5) 88.5 85
2-(4-Chlorophenyl)isovaleric acid (17) 351 350
3-Phenoxybenzyl alcohol (19) 371 424
3-(4'-Hydroxyphenoxyl) benzyl alcohol (21) 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol (20) 876 778
3-Phenoxybenzoic acid (22) 154 169
3-(4'-Hydroxyphenoxy) benzoic acid (24) 783 745
3-(2'-Hydroxyphenoxy) benzoic acid (23) 859 912
3-Phenoxybenzaldehyde (18) 415 416
NaSCN 604 578
---------------------------------------------------------------------------
a Refers to chemical identification no. in Fig. 3 and in text.
In a study by Parker et al. [150], Fischer 344 rats
(30 of each sex per group) were fed decarboxyfenvalerate
(one of the major photodegradation products) at concen-
trations of 0, 30, 100, 300, 3000 or 10 000 mg/kg diet for
up to 13 weeks. Body weight was decreased in male rats fed
10 000 mg/kg, but no treatment-related mortality or clini-
cal signs were observed. Absolute and relative liver
weight of male and female rats fed 300, 3000, or 10 000
mg/kg were all higher than those of the controls. Signifi-
cant increases in absolute or relative kidney weights were
observed in male and female rats fed 3000 or 10 000 mg/kg.
Significant treatment-related microscopic effects were
limited to glomerulonephrosis in male and female rats fed
10 000 mg/kg and hepatocellular hypertrophy with pale
eosinophilic cytoplasm and hepatocellular focal necrosis
in male and female rats fed 3000 or 10 000 mg/kg. A NOEL
of 300 mg/kg diet was established in this study.
Groups of young adult beagle dogs (four of each sex
per group) were fed fenvalerate in the diet at dose levels
of 0, 0.25, 0.5, 1.25, or 12.5 mg/kg body weight for 90
days [70]. There were no treatment-related changes in body
weight, food consumption, clinical signs, and clinical
laboratory data. Gross and microscopic examinations
revealed no effects of the fenvalerate. Thus, daily
administration at a level of 12.5 mg/kg body weight for a
period of 90 days produced no detectable evidence of toxi-
cological effect.
In a study by Parker et al. [148], male and female
beagle dogs (six of each sex per group) were fed diets
containing 0, 250, 500, or 1000 mg fenvalerate/kg diet for
a period of 6 months. Prominent clinical signs related to
treatment were emesis, head shaking, biting of the ex-
tremities, and tremors. The mean body weights of female
dogs fed fenvalerate at 1000 mg/kg were significantly
lower than those of controls. Red blood cell counts and
haematocrit and haemoglobin values in both male and female
dogs fed the highest dose were significantly lower. Serum
cholesterol and alkaline phosphatase levels were also
increased, mostly in the group fed 1000 mg/kg. Hepatic
multifocal microgranulomas observed during microscopic
examination increased in incidence and severity in a dose-
dependent way and were considered to be related to treat-
ment. Histiocytic cell infiltrates in the mesenteric lymph
nodes of some female dogs fed 500 or 1000 mg/kg and of
male dogs fed 1000 mg/kg were the only other treatment-
related effects observed microscopically.
7.2.2 Inhalation
Groups of Sprague-Dawley rats and ICR mice (10 of each
sex per group) were exposed to fenvalerate by inhalation
for 3 h daily for 4 weeks at concentration levels of 0, 2,
7, or 20 mg/m3 (fully respirable particle size). Although
animals showed acute signs of poisoning at the highest
dose level, no mortality was observed in any group. There
were no treatment-related effects in body weight, haema-
tology, or clinical biochemistry parameters, nor were
there any gross or microscopic abnormal findings [82,
95].
7.2.3 Dermal application
In a study by Hine [75], groups of rabbits (7-8 male
rabbits per group) were administered fenvalerate dermally
at dose levels of 0, 100, or 400 mg/kg daily for 6 h
(14 exposures were performed over a 22-day period).
Severe weight loss, clinical signs of poisoning, and gross
dermal effects were observed at 400 mg/kg, where mortality
was also observed.
7.3 Skin and Eye Irritation; Sensitization
7.3.1 Skin and eye irritation
Two formulated products (an emulsifiable concentrate
and an ultra-low-volume formulation) were found to be
severe eye and skin irritants in rabbits. Dermal irri-
tation was evident for 7 days after a 24-h exposure, and
severe conjunctivitis, corneal opacity, and iritis were
observed within 30 min of an application of 0.2 ml of the
formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation [29, 30]. However,
when experiments were carried out using pure (non-formu-
lated) fenvalerate, there was no irritation [138].
7.3.2 Skin sensitization
Skin sensitization by pure fenvalerate (95%) has been
evaluated using the Landsteiner-Draize method on guinea-
pigs. No sensitization was detected by Okuno et al.
[139].
7.4 Long-Term Exposures and Carcinogenicity
7.4.1 Mouse
When groups of ddY mice (35-47 of each sex per group)
were administered fenvalerate in the diet for 78 weeks at
levels of 0, 100, 300, 1000, or 3000 mg/kg, mortality
occurred at the highest dose level. Hyperexcitability was
observed at 1000 mg/kg or more, and body weight was
depressed at 3000 mg/kg over the 18-month period and at
1000 and 3000 mg/kg over the first 3 months. A variety of
haematological parameters were affected at 3 months, pre-
dominantly at the highest dose level, but no haematol-
ogical changes were observed at the end of the study.
Several biochemical changes suggestive of hepatotoxicity
were observed at 3 months and at termination of the study
in the 300, 1000, and 3000 mg/kg groups. There were gross
changes in several organ weights and in organ-to-body
weight ratios, predominantly in the liver. Microscopic
examination revealed changes in the liver, mesenteric
lymph nodes, and kidney. Dose-dependent granulomatous
changes were observed in the liver and/or mesenteric lymph
nodes in all treatment groups. At the 3-month interim
sacrifice, multiple small necrotic foci in the liver and
changes in the epithelial cells of the proximal convoluted
tubules were noted at the two highest dose levels. There
were no indications in this study of tumorigenicity or
carcinogenicity as a result of fenvalerate administration
[81, 83, 173, 175].
In studies by Okuno et al. [144], male ddY mice were
fed diets containing the [2S, alphaS], [2S, alphaRS], [2R,
alphaS], and [2R, alphaR] isomers of fenvalerate at diet-
ary dose levels of 0, 500, or 1000 mg/kg, 500, 1000, or
2000 mg/kg, 0, 125, or 1000 mg/kg, and 125, or 1000 mg/kg
for 52, 52, 13, and 13 weeks, respectively. Microgranulo-
matous changes were observed in the mice treated with the
[2R, alphaS] isomer after 1, 2, or 3 months. In contrast,
the changes did not occur in mice treated with the [2R,
alphaR] isomer under the same conditions. Neither [2S,
alphaS] nor [2S, alphaRS] isomers caused microgranulomatous
changes at 500 or 1000 mg/kg after 1 year. To clarify the
causative agent of granuloma formation, the cholesterol
ester of 2-(4-chlorophenyl)isovaleric acid (CPIA), a
lipophilic conjugate from the [2R, alphaS] isomer of fenva-
lerate, was injected intravenously into ddY mice. Micro-
granulomatous changes were observed in the liver of mice
treated with the [2R]-, [2S]-, or [2RS]-CPIA-cholesterol
esters 1 week after a single treatment of 1, 10, or 100
mg/kg body weight, as well as in the liver of mice treated
with a single dose of 10 or 30 mg/kg body weight of the
[2R]-CPIA-cholesterol ester and kept up to 26 weeks after-
wards. Histochemical examination and microscopic autoradio-
graphy of the liver demonstrated the presence of tritium,
derived from 3H-labelled [2R]-CPIA and cholesterol in giant
cells and Kuppfer cells. Another histochemical examination
showed the presence of cholesterol ester in the liver of
mice treated with the [2R, alphaS] isomer. These results
support the hypothesis that the CPIA-cholesterol ester is
the causative agent of the microgranulomatous changes ind-
uced by fenvalerate.
In further studies by Okuno et al. [145], male and
female ddY mice were fed diets containing technical
fenvalerate (either 0, 10, 30, 100, or 300 mg/kg diet for
20 months or 0, 100, 300, 1000, or 3000 for 17-18 months).
Microgranulomatous changes were observed in the lymph
nodes, liver, and spleen, the NOEL for such changes being
30 mg/kg. To examine the reversibility of these changes,
ddY mice (male and female) were fed a diet containing
technical fenvalerate at dose levels of 1000 and 3000
mg/kg for 6 weeks, followed by a control diet for up to
12 months. The size and number of the microgranulomatous
changes were reduced with time. These changes were typical
of foreign body granulomas and did not have the appearance
of granulomas formed in response to an immunological
stimulus.
When B6C3F1 mice (50 of each sex per group) were fed
fenvalerate at dietary concentrations of 0, 10, 50, 250,
or 1250 mg/kg for 2 years, mortality was increased and
body weight significantly decreased in male and female
mice fed 1250 mg/kg. The mean body weight of female mice
fed 250 mg/kg was also generally lower than that of con-
trols after the 60th week of feeding. The only treatment-
related non-neoplastic pathological effect observed in the
study was multifocal microgranulomata in the lymph nodes,
liver, and spleen of male mice fed 1250 mg/kg and of
female mice fed 250 or 1250 mg/kg. No statistically sig-
nificant differences were observed in either the number or
type of neoplasms in mice fed fenvalerate (compared to
concurrent controls). Thus, fenvalerate was found not to
be carcinogenic in B6C3F1 mice under the conditions of
the test [146].
7.4.2 Rat
When groups of Wistar rats (15 of each sex per group)
were fed fenvalerate at concentrations of 0, 50, 150, 500,
or 1500 mg/kg diet for 15 months, there was no mortality
attributable to fenvalerate. The hyperexcitability ob-
served during the early stages of the study disappeared
within 3 months. Body weight was significantly depressed
in both sexes at the highest dose level. No compound-
related changes were detected in the urine or in the eyes,
but the haemoglobin concentration was depressed in males
at the highest dose level and the females at 150 mg/kg or
more. Several blood biochemistry parameters were signifi-
cantly altered at the highest dose level (blood urea
nitrogen was increased in both sexes; protein content and
plasma cholinesterase were decreased in females). Gross
and microscopic examination revealed no dose-related
effects [174].
In a study by Gordon & Weir [66], groups of Sprague-
Dawley rats (93 males and 93 females per treated group;
183 of each sex used as the control group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25,
250, or 500 mg/kg. There was no compound-related mor-
tality, although body weight was reduced at the highest
dose level. The group fed 500 mg/kg and a separate control
group were sacrificed at 26 weeks while the other animals
were maintained for 2 years. There were no significant ef-
fects on food consumption, growth, behavior, haematology,
blood biochemical composition or urine consumption. At the
conclusion of the study, organ weight and organ-to-body
weight ratios were normal. Gross and microscopic findings
in the treated groups did not differ significantly from
those of the controls. A specific pathology examination
of the sciatic nerve of animals fed 250 mg/kg revealed no
treatment-related changes. Thus, the no-observed-effect
level in this study was 250 mg/kg diet.
Parker et al. [147] fed Sprague-Dawley rats (93 of
each sex per group) diets containing 0.1, 5, 25, or 250 mg
fenvalerate/kg for up to 2 years. The control group con-
sisted of 183 males and 183 females. Ten treated and 20
control rats of each sex from each group were killed at
intervals of 3, 6, 12, and 18 months. When body weight,
organ weight, food consumption, haematology, and clinical
chemical analysis measurements did not reveal any effect
resulting from the treatment, two additional groups of
rats (50 of each sex per group) were fed 0 or 1000 mg
fenvalerate/kg diet for 2 years. Body weight was decreased
and organ-to-body weight ratios were increased in brain,
liver, spleen, testes, kidneys (females only), and heart
(females only), in the treated animals. Mammary and pitu-
itary tumours were commonly observed, along with a variety
of other tumours that occurred randomly among all control
and treatment groups. No statistically significant differ-
ences in the number and type of neoplasms were observed,
except for mammary tumours in females in the main study.
These effects were judged not to be toxicologically sig-
nificant, since mammary tumour incidences did not exceed
expected incidences in aged female Sprague-Dawley rats.
In addition, the time taken for tumours to appear was un-
changed, and no change in the ratio of benign to malignant
tumours occurred. Sarcomas identified in the subcutis and
dermis in 5 out of 51 males fed 1000 mg/kg were also
identified in 2% (1/50), 2% (2/102), and 0-6% of concur-
rent, original, and historical controls, respectively.
The no-observed-effect level was 250 mg/kg.
When male and female Wistar rates were fed a diet
containing technical fenvalerate at 0, 50, 150, 500, or
1500 mg/diet for 24-28 months, microgranulomatous changes
were observed in lymph nodes, liver, spleen, and adrenal
glands. The no-observed-effect level for these microgranu-
lomatous changes was 150 mg/kg [145].
7.5 Mutagenicity
7.5.1 Microorganism and insects
The DNA-damaging capacity of fenvalerate has been
examined in a Rec-assay with Bacillus subtilis M45 rec-
and H17 wild type strains at concentrations up to 10
mg/disk per plate. Fenvalerate had no inhibitory effect on
the growth of indicator strains, and was judged to be non-
mutagenic [170].
Fenvalerate has also been examined for its mutagenic
potency with the Ames test in Salmonella typhimurium
(TA 1535, TA 1538, TA 98, and TA 100), using dose levels
of up to 1 mg/plate both with and without a metabolic
enzyme system. Fenvalerate was non-mutagenic in these
tests [170]. It was also tested using hepatic metabolic
enzyme systems prepared from various PCB-treated animals
(three strains of rats, six strains of mice and the Syrian
golden hamster). At dose levels of up to 1 mg/plate,
Fenvalerate was non-mutagenic [171, 172].
In further studies by Suzuki & Miyamoto [170], fenval-
erate was given orally at doses of 60 and 125 mg/kg to
groups of mice, and indicator cells (S. typhimurium G46)
were injected intraperitoneally. Fenvalerate did not
induce any significant level of mutation among the indi-
cator cells recovered from the abdominal cavity. On the
other hand, the positive control, dimethylnitrosamine,
significantly increased the mutation frequency of the
indicator organism.
Another host-mediated assay of fenvalerate in mice was
conducted using Saccharomyces cerevisiae as indicator
microorganism. Groups of mice were administered fenval-
erate orally at doses of 25 and 50 mg/kg, and were in-
jected with a suspension of indicator cells intraperito-
neally. No mutagenic effect on the indicator cells was
detected [17].
Fenvalerate was not found to be mutagenic in S.
typhimurium strains TA 100 or TA 98 in the presence or
absence of a rat liver activation system by fluctuation
tests at a concentration of up to 10 µg/ml or in V79
Chinese hamster cells in the presence or absence of
hepatocytes at a concentration of up to 40 µg/ml [153].
Fenvalerate did not induce sex-linked recessive
lethals, sex-chromosome losses, or non-disjunction in
Drosophila melanogaster when it was given to adults (up
to 20 mg/litre in the diet) or larvae (up to 50 mg/litre
in the diet), or was injected into adults (at 20 µg/ml)
[7].
7.5.2 Rat
In a study by Chatterjee et al. [26], groups of rats
(21 per group) were administered fenvalerate orally at
doses of 50, 75, or 100 mg/kg per day for 3 weeks. The
rats were killed 24 h after the last treatment and bone
marrow cells were examined for chromosomal aberrations.
Although an increase in the frequency of chromosomal aber-
rations was observed in fenvalerate-treated animals, it
was not possible to draw any definite conclusion because
it was not dose related and may have been non-specific.
Fenvalerate has also been studied for the enhancement
of gamma-glutamyl transpeptidase-positive enzyme-altered
focus incidence in partially hepatectomized, nitrosodi-
ethylamine-initiated male Sprague-Dawley rats. Fenvalerate
administered peritoneally (75 mg/kg body weight per day,
5 days a week for 10 weeks) induced significantly more
foci per cm3 and a larger percentage of liver tissue
occupied by focus tissue, compared with a vehicle-control
group. Analysis of the size distribution of foci in
fenvalerate- and vehicle-treated rats showed elevated
focus incidences in fenvalerate-treated rats at all focus
sizes. Fenvalerate induced no hepatotoxic effects, as
judged by serum transaminase activities and histopatho-
logical analysis [58].
7.5.3 Mouse
In a dominant lethal assay, groups of male mice (10-11
per group) were administered fenvalerate orally at doses
of 25, 50, or 100 mg/kg body weight. Each male was mated
with three virgin females for 7 days. The procedure was
repeated weekly as a standard dominant lethal test. The
females were sacrificed and examined for dominant
lethality at the 13th day of gestation. Fetal implants in
females, mated to males that had been treated with 100
mg/kg for 2 weeks, showed a significant reduction in
viability. A significant increase in early fetal death
was observed in females mated with males that had been
treated for 4 weeks with the highest dose [34].
The significance of the above data was further studied
as follows:
(1) By using a two-way analysis of variance, it was
judged that the reduction in fetal implants in females
mated to males the second week after dosing at 100 mg/kg
and the increase in early fetal deaths in the 4th week
were statistically significant. But these increases or
decreases appeared to be random and were not considered to
be biologically significant.
(2) Using a t-test and the Mann-Whitney U-test, no
significance was shown in any mean proportions for the
above parameters.
From these findings, it was concluded that fenvalerate
did not cause dominant lethal effects in micea.
----------------------------------------------------------
a Personal communication, J. Miyamoto, 1981, Comments on
"Further work information" required by 1979 JMPR on
fenvalerate, Laboratory of Biochemistry and Toxicology
(Unpublished report submitted to WHO by Sumitomo
Chemical Co. Ltd).
7.5.4 Hamster
In a study by Dean & Senner [35], fenvalerate was
administered orally to groups of hamsters (six males and
six females per group) at two successive daily doses of
12.5 and 25 mg/kg. The chromosomal preparations were made
8 or 24 h after administration. Fenvalerate did not induce
any chromosomal damage in the bone marrow cells from
treated animals, whereas the positive control, methyl
methanesulfonate (50 mg/kg), had induced a substantial
number of chromatid gaps within 8 h of dosing.
Fenvalerate, and the fenvalerate metabolite 2-(4-
chlorophenyl)isovaleric acid, were investigated for the
inhibition of gap-junctional intercellular communication
in vitro in the Chinese hamster lung fibroblast (V79)
metabolic cooperation assay [58]. This study showed that
both fenvalerate and 2-(4-chlorophenyl)isovaleric acid
were inhibitors of intercellular communication at non-
cytotoxic concentrations.
7.6 Teratogenicity and Reproduction Studies
7.6.1 Teratogenicity
In studies by Kohda et al. [93], groups of pregnant
ICR mice (32-33 per group) were orally administered
fenvalerate at dose levels of 0, 5, 15, or 50 mg/kg per
day on days 6 to 15 of gestation. Groups of 20 mice were
sacrificed on day 18, and the fetuses were removed and
examined for visceral and skeletal abnormalities. The
remaining dams were allowed to deliver naturally and the
young were maintained until weaning to evaluate postnatal
deficits. Additionally two male and two female weanlings
from each dam were maintained for 8 weeks and mated to
investigate their reproductive potential. Although toxic
signs were noted in the dams at the highest dose level,
there was no significant mortality. Examination of the
fetuses revealed no external, visceral, or skeletal abnor-
malities. Treatment of the dams with fenvalerate did not
affect the reproductive performance of the offspring.
Van Der Pauw et al. [187] dosed groups of pregnant
Dutch rabbits (20 to 31 per group) orally with fenvalerate
(0, 12.5, 25, or 50 mg/kg body weight per day) from day 6
to day 18 of gestation. The dams were sacrificed on day
28 and standard teratogenic assessments made. The body
weights of the dams given the highest dose were reduced.
There were no significant differences from controls in any
of the other parameters examined. Fenvalerate was not
found to be teratogenic in this study.
7.6.2 Reproduction studies
In studies by Stein [167] and Beliles et al. [10],
groups of Sprague-Dawley rats (11 males and 22 females per
group) were fed fenvalerate in the diet at levels of 0, 1,
5, 25, or 250 mg/kg. The animals were dosed for 9 weeks
prior to mating and the initiation of a standard three-
generation (two litters per generation) reproduction
study. Fertility, viability, gestation, and lactation in-
dices were calculated for each group of rats and were com-
pared to control values. Ten of the female weanlings and
all of the males from the F3b litters were examined his-
tologically at the conclusion of the study. The mean body
weight of the F2b adults was decreased at 250 mg/kg, but
no pathological changes were noted to account for this
weight loss. No effects on reproductive parameters in any
of the three generations were observed. Histological
examination revealed no treatment-related changes in any
group.
Groups of pregnant ICR mice (32-33 mice per group)
were orally administered fenvalerate at dose levels of 0,
5, 15, or 50 mg/kg body weight per day on days 6 to 15 of
gestation in a standard teratogenicity bioassay. Two male
and two female weanlings from each dam were maintained for
8 weeks and mated to investigate their reproductive poten-
tial. Toxic signs were noted in maternal mice at the
highest dose level. There was no significant mortality
over the course of the study, and no effects were noted on
any of the other animals as a result of continuous admin-
istration of fenvalerate. The animals maintained in the
abbreviated reproduction study showed no differences from
the control value in their ability to reproduce. There
were no changes in the reproduction indices with any
animals examined [93].
7.7 Neurotoxicity
In a study by Butterworth & Carter [20], histopatho-
logical examination was performed on the sciatic nerve and
posterior tibial nerve of rats that had been exposed to
acutely toxic levels of fenvalerate. After poisoning, and
for 9 days during the course of recovery, axonal breaks,
swelling, and vacuolisation, accompanied by phagocytosis
of myelin, were seen. The degree to which myelin was dis-
rupted was dose dependent and was closely associated with
the acute signs of toxicity.
Acute oral administration of fenvalerate, cyper-
methrin, resmethrin, permethrin, and natural pyrethrum to
rats at very high dose levels resulted in severe clinical
signs of poisoning and mortality within 24 h. Histopatho-
logical lesions were observed in the sciatic nerve with
all compounds tested. Fenvalerate did not cause the clini-
cal signs or histopathological lesions at a lower dose
level (200 mg/kg), nor did the other compounds [141,
142].
When groups of six male and six female rats were fed
fenvalerate in the diet at a concentration of 2000 mg/kg
for 8 to 10 days, all the animals showed typical signs of
acute intoxication, such as ataxia, tremors, and hyperex-
citability. Histopathological examinations did not reveal
any adverse effects of fenvalerate on the sciatic nerve
[73].
In order to evaluate the reversibility of the lesions
induced in the sciatic nerve, rats were administered
fenvalerate in the diet at dose levels of 0 or 3000 mg/kg
diet for 10 days. This was followed by a control diet for
12 weeks. During the treatment period, mortality was 60%
in the animals treated with fenvalerate. Rats on the
recovery control diets, sacrificed at 3 weeks, continued
to show swelling and disintegration of axons of the
sciatic nerves. However, there were no histopathological
lesions after 6, 9, or 12 weeks of the recovery period.
These results showed the reversibility of the sciatic
nerve lesions caused by fenvalerate [143].
In studies by Butterworth & Hend [21], fenvalerate was
administered orally to Wistar or Carworth Farm E (CFE)
rats either as single doses or in the diet. When given in
large quantities by a single dose of 250, 500, 800, or
1000 mg/kg body weight, which were sufficient to kill some
of the treated animals, fenvalerate produced sporadic
Wallerian degeneration in the sciatic nerve. The neuro-
pathy was never severe and was not seen in animals given
the compound in sub-lethal doses. In feeding studies (for
5 weeks at 1000 mg/kg diet and for 3 months at 2000 mg/kg
diet), no lesions were seen in the peripheral nerve,
brain, or spinal cord, and there was no evidence of cumu-
lative neurotoxicity.
B6C3F1 mice and Sprague-Dawley rats showed the
characteristic signs of intoxication following single oral
doses of fenvalerate ranging from 56 to 320 and 133 to
1000 mg/kg body weight, respectively. Neurological signs,
such as splayed gait, tremors, ataxia, and hind limb
incoordination, were observed at doses of 100 mg/kg or
more (mice) and 133 mg/kg or more (rats) within 1-8 h
after dosing. These signs had disappeared in most animals
within 72 h. Slight peripheral nerve fibre damage was
detected in surviving mice and rats sacrificed 10 days
after dosing. The incidence and severity were dose related
at doses > 56 and > 180 mg/kg; however, even at lethal
doses, there was no evidence of nerve lesions in some
animals. Thus, two distinct neurological effects were
observed, i.e., (a) a reversible ataxia and (b) incoordi-
nation plus a neuropathological effect manifested as
sparse axonal damage in peripheral nerves [149].
In a study by Milner & Butterworth [123], groups of
six hens were administered fenvalerate orally at dose
levels of 0 or 1000 mg/kg per day for 5 days. A positive
control of tri- ortho-cresyl phosphate (0.5 ml/kg) (TOCP)
was also included in the study. The fenvalerate-treated
birds were retreated, using the same dose regimen, after
3 weeks. The TOCP-treated hens showed signs of delayed
neurotoxicity and histopathological lesions in their
sciatic nerve and spinal cord. As would be expected for a
non-organophosphorus insecticide, there were no typical
clinical signs and histopathological lesions related to
fenvalerate.
7.8 Behavioural Studies
Guinea-pigs responded to dermal applications of
fenvalerate by scratching the treated sites of the skin.
This characteristic response was essentially over within
3-4 h. When the powerful skin irritant oil of mustard was
applied to fenvalerate-treated sites of skin 4-72 h after
the fenvalerate treatment, the behavioural skin sensory
response was re-stimulated. Oil of mustard alone did not
produce skin sensory stimulation. These results indicate
that pyrethroid treatment causes a transient sensitivity
to stimulation produced by chemical irritants [111].
To develop an animal model for studying skin sensory
stimulation, Duncan-Hartley guinea-pigs were treated with
pyrethroid solutions on one side and control substances on
the other side of their shaved back. The animals responded
by licking, scratching, or biting the test sites, and
activity was quantified by counting the number of times
the animals responded. This behavioural activity reached
a maximum 1-4 h after treatment. A chemical irritant (oil
of mustard) was able to restimulate the behavioural ac-
tivity when applied within 24 h after pyrethroid appli-
cation. Skin sensory stimulation produced by cyano-
containing pyrethroids, including fenvalerate, was
significantly greater than that produced by non-cyano-
containing pyrethroids. This behavioural model provides a
quantitative means of evaluating pyrethroid non-erythema-
tous skin sensory stimulation [22].
7.9 Miscellaneous Studies
In an antidotal study, phenobarbital, pentobarbital,
and diphenylhydantoin were found to be effective in
relieving the acute signs of intoxication in the rat.
Intraperitoneal injection of phenobarbital (50 mg/kg)
prevented tremor, diphenylhydantoin (100 mg/kg) by the
same route reduced the toxic reaction, and pentobarbital
(35 mg/kg intraperitoneally) removed the tremor reaction
completely within 30 min. The combination of diphenyl-
hydantoin with either of the barbiturates was effective in
reducing the onset and severity of tremors whereas various
other agents d-tubocurarine, atropine, meprobamate,
diazepam, biperiden, and trimethadione) were ineffective
[113].
The therapeutic potency of intraperitoneally admin-
istered methocarbamol was examined as an antidote against
the acute oral intoxication of rats by a lethal dose of
fenvalerate. Methocarbamol was initially administered at
a dose of 400 mg/kg body weight, followed by repeated
doses of 200 mg/kg body weight when tremors or hyperexcit-
ability to sound were observed. Methocarbamol markedly
decreased the mortality from 80%, which would be caused by
an administration of 850 mg fenvalerate/kg, to 0%, and was
effective in alleviating motor symptoms such as fibril-
lation, tremors, hyperexcitability, clonic seizures, and
choreoathetotic movements. A subcutaneous administration
of atropine sulfate (25 mg/kg body weight) was also effec-
tive in reducing the salivation produced by fenvalerate
[76].
Effective treatments against fenvalerate-mediated ef-
fects have been investigated by quantifying behavioural
skin sensory responses such as licking, scratching, or
biting of the treated sites by fenvalerate-treated guinea-
pigs. Preparations containing vitamin E, corn oil, or the
local anesthetic benzocaine were most effective [111].
Intraperitoneal administration of O-ethyl- O-(4-nitro-
phenyl)phenylphosphonothioate (EPN) or S,S,S-tributylphos-
phorotrithioate (DEF) to mice at 25 mg/kg increased the
intraperitoneal toxicity of fenvalerate (administered 1 h
later) by more than 25-fold; the LD50 decreased from
> 1000 mg/kg to 37 or 42 mg/kg. This suggests that mam-
malian esterases highly sensitive to inhibition by certain
organophosphorus compounds may play a critical role in
fenvalerate detoxication. This kind of synergism among
pesticides would be detrimental in increasing the toxicity
of certain pyrethroids to mammals [62].
Fenvalerate, administered to dogs at a dose sufficient
to induce toxic signs, showed no consistent cardiovascular
effects. Respiratory stimulation was noted at high levels,
and this was not reduced by anaesthetic supplements
(urethane, chloralose, and pentobarbital) [91].
7.10 Mechanism of Toxicity - Mode of Action
The intravenous toxicity of fenvalerate (50-100 mg/kg)
to rats was examined by Verschoyle & Aldridge [189].
[2S, alphaS]-Fenvalerate induced choreoathetosis with sali-
vation (CS-syndrome), and was classified as a Type II
pyrethroid. For the mode of action of pyrethroids in
general see Appendix 1.
The intracerebral injection of [2S, alphaS]-fenvaler-
ate (0.01 mg/kg) to mice produced the Type II syndrome,
consisting of choreoathetosis, convulsion, and saliv-
ation [98]. The Type II syndrome is produced character-
istically by pyrethroids with an alpha-cyano group and
the site of action in mammals is considered to be the
central nervous system.
In intact locusts and neuromuscular preparations of
locusts, fenvalerate caused (a) prolonged firing in the
crural nerve without associated muscle contractions; (b)
sustained muscle contractions; and (c) a block of neurally
evoked muscle contractions at low concentration (10-8 to
10-5 mol/litre). However, fenvalerate did not cause
repetitive firing and after-discharges with associated
muscle contractions [27]. The fenvalerate stereoisomers
with an (S) configuration in the alcohol moiety are more
active pharmacologically and toxicologically than those
with the (R) configuration or the racemate (R,S). It is
also apparent that stereoisomers with the (S) configur-
ation in the acid moiety are more active than those with
the (R) configuration or the racemate (R,S) [27].
[S,S]-Fenvalerate does not induce repetitive firing in
the cockroach cercal sensory nerves either in vivo or in
vitro. It does, however, cause different signs, including
bursts of spikes in the cercal motor nerve [60].
There are no clear-cut links between electrophysio-
logical findings in insects and toxicity to mammals.
8. EFFECTS ON HUMANS
8.1 Occupational Exposure
Appraisal
Fenvalerate has been found to induce skin sensations in
some of the workers who handle this insecticide. Clinical
studies showed that the skin sensations develop with a latent
period of approximately 30 min, peak by 8 h and deteriorate
after 24 h. Numbness, itching, tingling, and burning are symp-
toms frequently reported. Alpha-tocopheryl acetate has been
found to inhibit the occurrence of these skin sensations.
In a study by Kolmodin-Hedman et al. [97], personnel
(52 people) at various plant nurseries who had handled
conifer seedlings treated with fenvalerate were examined.
The symptoms were mainly irritative, such as itching,
paraesthesia and burning of the skin, and itching and
irritation of the eyes. The frequency (% of people who
reported these signs) was about 10%. Increased nasal
secretion was reported by 19% of the personnel.
No clinical case of pyrethroid poisoning had been
reported until outbreaks of acute deltamethrin and fenval-
erate poisoning occurred among cotton growers in China in
1982. Having been told (in error) that pyrethroids were
non-toxic, the farmers handled the pyrethroid insecticides
without taking any precautions. After repeated spraying
in the cotton fields, the mild cases presented severe
headaches, dizziness, fatigue, nausea, and anorexia, with
transient changes in the electroencephalogram (EEG), while
a severe case developed muscular fasciculation, repetitive
discharges in the electromyogram (EMG), and frequent con-
vulsions. However, all were found by follow-up studies to
have completely recovered and the prognosis of acute
pyrethroid poisoning proved to be correct [71]a.
----------------------------------------------------------
a More recently, the same author reviewed 573 cases of
acute pyrethroid poisoning reported in the Chinese
medical literature during 1983-1988 [213]. Among these
there were 196 cases of acute fenvalerate poisoning,
63 of which were occupational, due to inappropriate
handling and 133 accidental, mostly due to ingestion.
Two died of convulsions. All others recovered with
symptomatic and supportive treatment within 1-6 days.
A comprehensive review of clinical manifestations is
included.
Among 23 workers exposed to synthetic pyrethroids,
including fenvalerate, 19 experienced one or more episodes
of abnormal facial sensation, developing between 30 min
and 3 h after exposure and persisting for 30 min to 8 h
[106]. However, there were no abnormal neurological signs,
and electrophysiological studies showed normal responses
in the arms and legs. The symptoms were most likely due
to transient lowering of the threshold of sensory nerve
fibres or sensory nerve endings following exposure of the
facial skin to pyrethroids.
In a study by Tucker & Flannigan [182], selected
individuals who had worked extensively with fenvalerate in
the delta region of the Mississippi and Alabama, USA, were
interviewed and examined. They had, on some occasions,
noted paraesthesia associated with exposure to this insec-
ticide. The cutaneous sensation was described as a sting-
ing or burning, which progressed to numbness in approxi-
mately one-third of the exposed workers. The sensation
typically began a number of hours after contact, peaked in
the evening, and rarely was present the following morning.
The intensity of the sensation varied according to the
type and extent of exposure. Clinical signs of inflam-
mation such as oedema or vesiculation were not apparent.
Erythema was present in a few individuals but this was
difficult to distinguish from sunburn. Several environ-
mental factors were found to affect the cutaneous sen-
sation associated with fenvalerate exposure.
8.2 Clinical Studies
A double-blind study with 29 male volunteers was
performed to test the skin reaction to formulated fenval-
erate. The emulsifiable concentrate formulation was
diluted with water and applied to one side of the face, on
the cheek, with a control formulation on the opposite
cheek. There were no signs of dermatitis 24 h after appli-
cation, nor did the fenvalerate formulation produce any
abnormal skin sensations. There were no indications that
any of the symptoms such as tingling, itching, or burning
were associated with fenvalerate [18].
A double-blind study was performed to compare human
discrimination of technical fenvalerate, the heavy-ends
fraction of distilled fenvalerate, and ethyl alcohol
(vehicle) applied to the lower edge of each earlobe of 36
adult (both male and female) volunteers on three separate
occasions. Both forms of fenvalerate caused a statisti-
cally significant increase in paraesthesia, compared with
the vehicle alone. The onset of the cutaneous sensations
occurred 1 h after application, peaked at 3-6 h, and
lasted approximately 24 h. Numbness, itching, burning,
tingling, and warmth were the most frequently reported
sensations. The difference between the effects of the two
fractions of fenvalerate was not statistically significant
[92].
Flannigan & Tucker [55], Flannigan et al. [56, 57],
and Malley et al. [112] studied the difference in the
degree of paraesthesia induced by a number of pyrethroids.
Applications of 0.05 ml fenvalerate formulated to field
strength (0.13 mg/cm2) were made to a 4 cm2 area of
earlobe on five occasions, the opposite earlobe receiving
distilled water. Participant evaluation after each appli-
cation continued for 48 h and involved description of the
cutaneous sensations. Each participant was treated after
each application with one of the remaining compounds.
Fenvalerate (like the other pyrethroids) induced skin sen-
sations. The paraesthesia developed with a latent period
of approximately 30 min, peaked by 8 h, and deteriorated
as early as 24 h. The local application of dl-alpha
tocopheryl acetate markedly inhibited the occurrence of
skin sensations.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
has discussed and evaluated fenvalerate at its meetings in
1979, 1981, 1982, 1984, 1986, and 1987 [40-47, 49, 50, 52-
4].
Since 1986, an acceptable daily intake (ADI) of 0-0.02
mg/kg body weight has been established.
In the WHO Recommended Classification of Pesticides by
Hazard, technical fenvalerate is classified as "moder-
ately hazardous" (Class II) [197].
REFERENCES
1. AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10(2): 147-151.
2. ALBERT, J.R. & SUMMITT, L.M. (1976) Intravenous toxicity of WL 43775
(6-1-0-0) in the mouse (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
3. ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri and its prey,
Panonychus ulmi on apples in southeast England. Environ. Entomol., 9:
436-439.
4. ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several non-target aquatic invertebrates. Environ. Entomol., 11:
1251-1257.
5. ATKINS, E.L., KELLUM, D., & ATKINS, K.W. (1981) Reducing pesticide
hazards to honey bees: mortality prediction techniques and integrated
management strategies, Berkeley, California, University of
California, Division of Agricultural Sciences (Leaflet No. 2883).
6. BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
7. BATISTE-ALENTORN, M., XAMENA, N., VELAZQUEZ, A., CREUS, A., & MARCOS,
R. (1987) Non-mutagenicity of fenvalerate in Drosophilia.
Mutagenesis, 2: 7-10.
8. BATTELLE (1982) Pesticide programme of research and market planning.
Part I. Insecticides, Geneva, Battelle Research Centre.
9. BATTELLE (1986) World pesticide programme: Insecticide II, Geneva,
Battelle Research Centre.
10. BELILES R.P., MARIS, S.L., & WEIR, R.J. (1978) Three-generation
reproduction study in rats, Kensington, Maryland, Litton Bionetics
Inc. (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
11. BIRDIE, N.S., BANERJI, R.K., & CHAUHAN, A.K. (1986) Gas liquid
chromatographic separation of pyrethrins from some synthetic
pyrethroids in formulations. Pyrethrum Post, 16(3): 77-80.
12. BLAIR, D. & RODERICK, H. (1975) Toxicity studies on the pyrethroid
insecticide WL 43775, emulsifiable concentrate FX 3368: Acute
inhalation exposure to an aqueous spray (Unpublished report submitted
to WHO by Shell Development Co., Ltd).
13. BRADBURY, S.P. & COATS, J.R. (1982) Toxicity of fenvalerate to
bobwhite quail (Colinus virginianus) including brain and liver
residues associated with mortality. J. Toxicol. environ. Health, 10:
307-319.
14. BRADBURY, S.P., COATS, J.R., & MCKIM, J.M. (1986) Toxicokinetics of
fenvalerate in rainbow trout (Salmo gairdneri). Environ. Toxicol.
Chem., 5: 567-576.
15. BRADBURY, S.P., MCKIM, J.M., & COATS, J.R. (1987) Physiological
response of rainbow trout (Salmo gairdneri) to acute fenvalerate
intoxication. Pestic. Biochem. Physiol., 27: 275-288.
16. BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-
residue method to determination of synthetic pyrethroid residues in
celery and animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
17. BROOKS, T.M. (1976) Toxicity studies with WL 43775: Mutagenicity
studies with WL43775 in the host-mediated assay (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
18. BROWN, L.J. & SLOMKA, M.B. (1979) Skin reaction potential of use
dilution (1.33%) PYDRIN EC (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
19. BRUCE, M.L. & CARUSO, J.A. (1985) The laminar flow torch for gas
chromatographic He microwave plasma detection of pyrethroids and
dioxins. Appl. Spectrosc., 39(6): 942-949.
20. BUTTERWORTH, S.T.G. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL 43775: Acute oral toxicity and neuropathological
effects in rats (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
21. BUTTERWORTH, S.T.G. & HEND, R.W. (in press) Peripheral nerve lesions
induced by large doses of pyrethroid. Neuropathol. appl. Neurobiol.
22. CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin sensory stimulation
characterized by a new behavioural paradigm. Toxicol. appl.
Pharmacol., 76: 270-279.
23. CAPLAN, J.R., ISENSEE, A.R., & NELSON, J.O. (1984) Fate and effect of
[14C]fenvalerate in a tidal marsh sediment ecosystem model. J. agric.
food Chem., 32: 166-171.
24. CAYLEY, G.R. & SIMPSON, B.W. (1986) Separation of pyrethroid enan-
tiomers by chiral high-performance liquid chromatography. J.
Chromatogr., 356: 123-134.
25. CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four pyrethroid
insecticide in a mineral and organic soil. J. environ. Sci. Health,
B16: 605-615.
26. CHATTERJEE, K.K., TALUKDER, G., & SHARMA, A. (1982) Effects of
synthetic pyrethroids on mammalian chromosomes I. Sumicidin. Mutat.
Res., 105: 101-106.
27. CLEMENTS, A.N. & MAY, T.E. (1977) The actions of pyrethroids upon the
peripheral nervous system and associated organs in the locust.
Pestic. Sci., 8: 661-680.
28. COATS, J.R. & O'DONNELL-JEFFREY, N.L. (1979) Toxicity of four syn-
thetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
29. COOMBS, A.D. & CARTER, B.I. (1975) Toxicity studies on insecticide WL
43775: Acute toxicity, skin irritancy potential of the emulsifiable
concentrate FX 3368 (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
30. COOMBS, A.D. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL43775: Toxicity and skin and eye irritancy potential of
the ULV formulation FX4353 (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
31. CURTIS, L.R., SEIM, W.K., & CHAPMAN, G.A. (1985) Toxicity of
fenvalerate to developing steelhead trout following continuous or
intermittent exposure. J. Toxicol. environ. Health, 15: 445-457.
32. DAY, K. & KAUSHIK, N.K. (1987a) Short-term exposure of zooplankton to
the synthetic pyrethroid, fenvalerate, and its effects on rates of
filtration and assimilation of the alga Chlamydomonas reinhardii.
Arch. environ. Contam. Toxicol., 16: 423-432.
33. DAY, K. & KAUSHIK, N.K. (1987b) An assessment of the chronic toxicity
of the synthetic pyrethroid, fenvalerate, to Daphnia galeata
mendotae, using life tables. Environ. Pollut., 44: 13-26.
34. DEAN, B.J. (1975) Toxicity studies with WL 43775: Dominant lethal
assays in male mice after single oral doses of WL 43775 (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
35. DEAN, B.J. & SENNER, R.K. (1975) Toxicity studies with WL 43775:
Chromosome studies on bone marrow cells of Chinese hamster after two
daily oral doses of WL 43775 (Unpublished report to WHO submitted by
Shell Development Co., Ltd).
36. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series No. 42).
37. ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1979) Dislodgable
insecticide residue on cotton foliage: permethrin, curacron,
fenvalerate, sulprofos, decis, and endosulfan. Bull. environ. Contam.
Toxicol., 22: 245-248.
38. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
39. FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
40. FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49
(FAO Plant Production and Protection Paper 20).
41. FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
299-353 (FAO Plant Production and Protection Paper 20 Sup.).
42. FAO/WHO (1982a) Pesticide residues in food. Report of the 1981 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 26-28
(FAO Plant Production and Protection Paper 37).
43. FAO/WHO (1982b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
209-256 (FAO Plant Production and Protection Paper 42).
44. FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 28 (FAO
Plant Production and Protection Paper 46).
45. FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, p. 420
(FAO Plant Production and Protection Paper 49).
46. FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49,
83, 90-93 (FAO Plant Production and Protection Paper 62).
47. FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
343-353 (FAO Plant Production and Protection Paper 67).
48. FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations.
49. FAO/WHO (1986a) Pesticide residues in food. Report of the 1986 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 28-29,
61 (FAO Plant Production and Protection Paper 77).
50. FAO/WHO (1986b) 1986 Evaluations of some pesticide residues in food.
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 189-193, 355 (FAO Plant Production and Protection
Paper 78/1).
51. FAO/WHO (1986c) Codex maximum limits for pesticide residues, 2nd ed.,
Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC XIII).
52. FAO/WHO (1987a) 1986 Evaluations of some pesticide residues in food.
Part II - Toxicology, Rome, Food and Agriculture Organization of the
United Nations, p. 219 (FAO Plant Production and Protection Paper
78/2).
53. FAO/WHO (1987b) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 27 (FAO
Plant Production and Protection Paper 84).
54. FAO/WHO (1988) 1987 Evaluations of some pesticide residues in food.
Part 1 - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 57-59 (FAO Plant Production and Protection Paper
86/1).
55. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
56. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary irritant contact
dermatitis from synthetic pyrethroid insecticide exposure. Arch.
Toxicol., 56: 288-294.
57. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Brit. J. ind. Med., 42:
363-372.
58. FLODSTROM, S., WARNGARD, L., LJUNGQUIST, S., & AHLBORG, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch. Toxicol., 61: 218-223.
59. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
60. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
61. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
62. GAUGHAN, L.C., ENGEL, J.I., & CASIDA, J.E. (1980) Pesticide
interactions: effects on organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pestic. Biochem. Physiol., 14: 81-85.
63. GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid
insecticides to bees. Pestic. Sci., 16: 206-207.
64. GHIASUDDIN, S.M. & SODERLUND, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. appl.
Pharmacol., 74: 390-396.
65. GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav. Toxicol.
Teratol., 4:(6) 793-799.
66. GORDON, E.B. & WEIR, R.J. (1978) Lifetime feeding study in rats, SD-
43775 Technical, Kensington, Maryland, Litton Bionetics, Inc.
(Project No. 2054, Report AT-81-0181) (Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd).
67. GREENBERG, R.S. (1981) Determination of fenvalerate, a synthetic
pyrethroid, in grapes, peppers, apples, and cotton seeds by gas-
liquid chromatography. J. agric. food Chem., 29: 856-860.
68. HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage toxicity tests.
Environ. Toxicol. Chem., 2(2): 251-8
69. HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory studies
on the persistence and behavior in soil of four pyrethroid
insecticides. Can. Entomol., 113: 685-694.
70. HART, E.R. (1975) 90-day subacute toxicity study in dogs, SD 43775
Technical (sumicidin), Kensington, Maryland, Litton Bionetics, Inc.
(Technical Report No. AT-51-0013) (Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd).
71. HE, FENSHENG (1987) Occupational neurotoxicology: Current problems
and trends. Presented at the XXII International Congress on
Occupational Health, Sydney, Australia, 27 September-2 October, 1987.
72. HEND, R.W. & BUTTERWORTH, S.T.G. (1975) Toxicity studies of the
insecticide WL 43775: A three-month feeding study in rats
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
73. HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies of the
insecticide WL 43775: A short-term feeding study in rats (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
74. HILL, B.D. (1981) Persistence and distribution of fenvalerate
residues in soil under field and laboratory conditions. J. agric.
food Chem., 29: 107-110.
75. HINE, C. (1975) SD-43775 Toxicity: Acute and repeated (14-day) dermal
toxicity in the rabbit, San Francisco, Hine Inc. (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
76. HIROMORI, T., NAKANISHI, T., KAWAGUCHI, S., SAKO, H., SUZUKI, T., &
MIYAMOTO, J. (1986) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci., 11: 9-14.
77. HOLMSTEAD, R.L. & FULLMER, D.G. (1977) Photodecarboxylation of
cyanohydrine esters. Models for pyrethroid photodecomposition. J.
agric. food Chem., 25: 56-58.
78. HOLMSTEAD, R.L., FULLMER, D.G., & RUZO, L.O. (1978) Pyrethroid
photodecomposition: Pydrin. J. agric. food Chem., 26: 954-959.
79. HORIBA, M., KITAHARA, H., TAKAHASHI, K., YAMAMOTO, S., & MURANO, A.
(1980) Gas chromatographic determination of fenvalerate (S-5602) in
technical preparations. Agric. biol. Chem., 44: 1197-1199.
80. HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
81. ITO, N. (1976a) Histopathological findings of mice treated with S5602
for three months (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
82. ITO, N. (1976b) Histopathological findings of mice and rats exposed
to mist of S5602 for four weeks (Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd).
83. ITO, N. (1978) Histopathological findings in mice treated with S5602,
Nagoya, Nagoya City University Medical School (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
84. JOHANSEN, C., MAYER, D., MADSEN, R., & ROBINSON, W. (1975) Bee
research investigations, 1975, Pullman, Washington, Washington State
University, Department of Entomology (Unpublished report).
85. JOHANSEN, C., KIOUS, C., SCHULTZ, G., GUPTA, R., & STANFORD, A.
(1978) Bee research investigations, 1978, Pullman, Washington,
Washington State University, Department of Entomology (Unpublished
report).
86. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Comparative metabolism
of fenvalerate and the [2S, S]-isomer in rats and mice. J. Pestic.
Sci., 6: 317-326.
87. KANEKO, H., MATSUO, M., & MIYAMOTO, J. (1986) Differential metabolism
of fenvalerate and granuloma formation. I. Identification of
cholesterol ester derived from a specific chiral isomer of
fenvalerate. Toxicol. appl. Pharmacol., 83: 148-156.
88. KANEKO, H., TAKAMATSU, Y., OKUNO, Y., ABIKO, J., YOSHITAKE, A., &
MIYAMOTO, J. (1988) Substrate specificity for formation of
cholesterol ester conjugates from fenvalerate analogues and for
granuloma formation. Xenobiotica, 18(1): 11-19.
89. KATAGI, T., MIKAMI, N., MATSUDA., T., & MIYAMOTO, J. (1985a)
Photodegradation of fenvalerate and esfenvalerate on soils
(Unpublished Report No. LLM-50-0005 submitted to WHO by Sumitomo
Chemical Co., Ltd).
90. KATAGI, T., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985b)
Hydrolysis of fenvalerate and esfenvalerate in buffered aqueous
solutions (Unpublished Report No. LLM-50-0006 submitted to WHO by
Sumitomo Chemical Co., Ltd).
91. KIRKLAND, V.L. & ALBERT, J.R. (1977) Preliminary pharmacodynamic
investigation of SD 43775 (6-10-0) administered intravenously to the
anesthetized dog. I. Effects on the cardiovascular system and
respiration. II. Electrocardiographic (ECG) findings (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
92. KNOX, J.M., II, TUCKER, S.B., & FLANNIGAN, S.A. (1984) Paraesthesia
from cutaneous exposure to a synthetic pyrethroid insecticide. Arch.
Dermatol., 120: 744-746.
93. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic study on
S5602 in mice (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
94. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Acute inhalation
toxicity of S3206 and S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
95. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976c) Subacute inhalation
toxicity study of S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
96. KOHDA, H., KANEKO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J. (1979)
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
97. KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and fenvalerate).
Arch. Toxicol., 50: 27-33.
98. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
99. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
100. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
101. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
102. LEE, P.W. (1985) Fate of fenvalerate (Pydrin insecticide) in the soil
environment. J. agric. food Chem., 33: 993-998.
103. LEE, P.W., WESTCOTT, N.D., & REICHLE, R.A. (1978) Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871.
104. LEE, P.W., STEARNS, S.M., & POWELL, W.R. (1985) Rat metabolism of
fenvalerate (Pydrin insecticide). J. agric. food Chem., 33: 988-993.
105. LEE, P.W., POWELL, W.R., STEARNS, S.M. & MCCONNEL, O.J. (1987)
Comparative aerobic soil metabolism of fenvalerate isomers. J. agric.
food Chem., 35: 384-387.
106. LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
107. LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 11/12: 843-851.
108. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol.,
20: 203-216.
109. MCKENNEY, C.L., Jr & HAMAKER, D.B. (1984) Effects of fenvalerate on
larval development of Palaemonetes pugio (Holthuis) and on larval
metabolism during osmotic stress. Aquat. Toxicol., 5: 343-355.
110. MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and
shrimp. Bull. environ. Contam. Toxicol., 25: 950-955.
111. MALLEY, L.A., CAGEN, S.Z., GARDINER, T.H., & VAN GELDER, G.A. (1984)
Characterization of fenvalerate mediated skin sensory stimulation.
Neurotoxicology, 5: 71-72.
112. MALLEY, L.A., CAGEN, S.Z., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & ROSE, G.P. (1985) Effect of vitamin E and other amelioratory
agents on the fenvalerate-mediated skin sensation. Toxicol. Lett.,
29: 51-58.
113. MATSUBARA, R.A., SUZUKI, T., KADOTA, T., & MIYAMOTO, J. (1977)
Antidotes against poisoning by S5602 in rats (Unpublished report
submitted by Sumitomo Chemical Co., Ltd).
114. MAYER, F.L., Jr (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Environmental Protection
Agency, p. 115 (EPA-600/8-87/017).
115. MAYER, F.L., Jr & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp. 235-236.
116. MEGHARAJ, M., VENKATESWARLU, K., & RAO, A.S. (1986) Influence of
cypermethrin and fenvalerate on natural soil algal populations.
Ecotoxicol. environ. Saf., 12: 141-145.
117. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Willoughby,
Ohio, Meister Publishing Co., pp. C104-C105.
118. MIKAMI, N., TAKAHASHI, N., HAYASHI, K., & MIYAMOTO, J. (1980)
Photodegradation of fenvalerate (Sumicidin) in water and on soil
surface. J. Pestic. Sci., 5: 225-236.
119. MIKAMI, N., SAKATA, S., YAMADA, H., & MIYAMOTO, J. (1984a) Further
studies on degradation of the pyrethroid insecticide fenvalerate in
soils. J. Pestic. Sci., 9: 697-702.
120. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1984b) New
conjugated metabolites of 3-phenoxybenzoic acid in plants. Pestic.
Sci., 15: 531-542.
121. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1985a) The
metabolism of fenvalerate in plants: The conjugation of the acid
moiety. Pestic Sci., 16: 46-58.
122. MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO, J.
(1985b) Metabolism in rats of 3-phenoxybenzyl alcohol and 3-
phenoxybenzoic acid glucoside conjugates formed in plants. Pestic.
Sci., 16: 33-45.
123. MILNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticide. Investigation of neurotoxic potential of WL 43775
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
124. MIURA, T. & TAKAHASHI, R.M. (1976) Effects of a synthetic pyrethroid,
SD43775, on nontarget organisms when utilized as a mosquito
larvicide. Mosq. News, 36: 322-326.
125. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
126. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
127. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress
of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982,
Oxford, Pergamon Press, Vol. 1-4.
128. MIYAMOTO, J., KANEKO, H., & TAKAMATSU, Y. (1986) Stereoselective
formation of a cholesterol ester conjugate from fenvalerate by mouse
microsomal carboxyesterase(s). J. Biochem. Toxicol., 1(2): 79-94.
129. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a) Toxicity
of mosquito larvicidal pyrethroids to four species of freshwater
fishes. Environ. Entomol., 7: 428-430.
130. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978b)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
131. MULLA, M.S., DARWAZEH, H.A., & DHILLON, M.S. (1980) New pyrethroids
as mosquito larvicides and their effects on nontarget organisms.
Mosq. News, 40: 6-12.
132. NOBLE, P.J. (1976) AF1117 residue data in animal tissues, milk and
cream (Unpublished data submitted to WHO by Shell Chemical
(Australia), Ltd).
133. OHKAWA, H., NAMBU, K., INUI, H., & MIYAMOTO, J. (1978) Meta-
bolic fate of fenvalerate (Sumicidin) in soil and by microorganisms.
J. Pestic. Sci., 3: 129-141.
134. OHKAWA, H., KANEKO, H., TSUJI, H., & MIYAMOTO, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
135. OHKAWA, H., KIKUCHI, R., & MIYAMOTO, J. (1980a) Bioaccumulation and
biodegradation of the [S]-acid isomer of fenvalerate (Sumicidin) in
an aquatic model ecosystem. J. Pestic. Sci., 5: 11-12.
136. OHKAWA, H., NAMBU, K., & MIYAMOTO, J. (1980b) Metabolic fate of
fenvalerate (Sumicidin) in bean plants. J. Pestic. Sci., 5: 215-223.
137. OHNO, N., FUJIMOTO, K., OKUNO, Y., MIZUTANI, T., HIRANO, M., ITAYA,
N., HONDA, T., & YOSHIOKA, H. (1976) 2-Arylalkanoates, a new group of
synthetic pyrethroid esters not containing cyclopropane-carboxylates.
Pestic. Sci., 7: 241-246.
138. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975a) Eye and skin irritation
of S-3206 and S-5602 in rabbits (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
139. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975b) Skin sensitization
study of S-5602 in guinea pigs (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
140. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by dermal
applications in rats (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
141. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977a) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by oral
administration in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
142. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977b) Neurotoxic effects of
S5602 and natural pyrethrins by oral administration in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
143. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977c) Recovery of
histopathological lesions in rats caused by short-term feeding of
S5602 (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
144. OKUNO, Y., SEKI, T., ITO, S., KANEKO, H., WATANABE, T., YAMADA, T., &
MIYAMOTO, J. (1986a) Differential metabolism of fenvalerate and
granuloma formation. II. Toxicological significance of lipophilic
conjugate from fenvalerate. Toxicol. appl. Pharmacol., 83: 157-169.
145. OKUNO, Y., ITO, S., SEKI, T., HIROMORI, T., MURAKAMI, M., KADOTA, T.,
& MIYAMOTO, J. (1986b) Fenvalerate-induced granulomatous changes in
rats and mice. J. toxicol. Sci., 11: 53-66.
146. PARKER, C.M., MCCULLOUGH, C.B., GELLATLY, J.B.M., & JOHNSTON, C.D.
(1983) Toxicologic and carcinogenic evaluation of fenvalerate in the
B6C3F1 mouse. Fundam. appl. Toxicol., 3: 114-120.
147. PARKER, C.M., PATTERSON, D.R., VAN GELDER, G.A., GORDON, E.B.,
VALERIO, M.G., & HALL, W.C. (1984a) Chronic toxicity and
carcinogenicity evaluation of fenvalerate in rats. J. Toxicol.
environ. Health, 13: 83-97.
148. PARKER, C.M., PICCIRILLO, V.J., KURTZ, S.L., GARNER, F.M., GARDINER,
T.H., & VAN GELDER, G.A. (1984b) Six-month feeding study of
fenvalerate in dogs. Fundam. appl. Toxicol., 4: 577-586.
149. PARKER, C.M., ALBERT, J.R., VAN GELDER, G.A., PATTERSON, D.R., &
TAYLOR, J.L. (1985) Neuropharmacologic and neuropathologic effect of
fenvalerate in mice and rats. Fundam. appl. Toxicol., 5: 278-286.
150. PARKER, C.M., WIMBERLY, H.C., LAM, A.S., GARDINER, T.H., & VAN
GELDER, G.A. (1986) Subchronic feeding study of decarboxyfenvalerate
in rats. J. Toxicol environ. Health, 18: 77-90.
151. PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
152. PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
153. PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137:7-15.
154. POTTER, J.C. (1976) (a) Tissues of rats fed SD43775-C-14; (b) and (c)
milk and tissues of cows; (d) eggs and tissues from laying hens; (e)
cream from the milk of cows fed SD 43775 (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
155. RANGACHAR, T.R.S., SURESH, T.P., & THINMMAIAH, K. (1981) Acute oral
toxicity of Sumicidin 20E in poultry. Indian vet. J., 58: 941-942.
156. RATTNER, B.A. & FRANSON, J.C. (1983) Methyl parathion and fenvalerate
toxicity in American kestrels: acute physiological responses and
effects of cold. Can. J. Physiol. Pharmacol., 62: 787-792.
157. REED, W.T., EHMAN, A., LEE, P., BARBER, G., & BISHOP, J. (1983)
Pydrin insecticide (fenvalerate) on non-target systems following
field applications. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: Human welfare and the environment, Oxford, Pergamon Press,
Vol. 2, pp. 213-221.
158. ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J.
econ. Entomol., 72: 293-294.
159. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve - muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
160. SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Degradation of esfenvalerate and its isomers in soils (Unpublished
Report No. LLM-30-0003 submitted to WHO by Sumitomo Chemical Co.,
Ltd).
161. SALEH, M.A., IBRAHIM, N.A., SOLIMAN, N.Z., & EL SHEIMY, M.K. (1986)
Persistence and distribution of cypermethrin, deltamethrin and
fenvalerate in laying chickens. J. agric. food Chem., 34: 895-898.
162. SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygiene
rules for synthetic pyrethroid content in the work zone air.] Gig.
tr. prof. Zabol., 8: 48-50 (in Russian).
163. SCHIMMEL, S.C., GARNAS, R.L., PATRICK, J.M., & MOORE, J.C. (1983)
Acute toxicity, bioconcentration, and persistence of AC 222,705,
benthiocarb, chlorpyrifos, fenvalerate, methyl parathion, and
permethrin in the estuarine environment, J. agric. food Chem., 31:
104-113.
164. SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
165. SODERLUND, D.M. & CASIDA, J.E. (1977) Effect of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pestic. Biochem. Physiol., 7: 391-401.
166. SPEHAR, R.L., TANNER, D.K., & GIBSON, J.H. (1982) Effects of Kelthane
and Pydrin on early life stages of Fathead Minnows (Pimephales
promelas) and Amphipods (Hyalella azteca), Philadelphia, American
Society for Testing and Materials (Special Technical Publication No.
766).
167. STEIN, A.A. (1977) Histopathology evaluation of animals from 3-
generation reproduction study. Albany, New York, Biological Research,
Ltd (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
168. SUMMIT, L.M. & ALBERT, J.R. (1977a) Oral lethality of WL 43775 (6-1-
0-0) in the rat (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
169. SUMMIT, L.M. & ALBERT, J.R. (1977b) Determination of the acute oral
lethality of WL 43775 (6-1-0-0) in the male and female mouse
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
170. SUZUKI, H. & MIYAMOTO, J. (1976) Studies on mutagenicity of S 5602
with bacteria systems (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
171. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of some
pyrethroids on salmonella strains in the presence of mouse hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
172. SUZUKI, H., KISHIDA, F., & MIYAMOTO, J. (1979) Studies on
mutagenicity of S5602 in Ames test in the presence of hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
173. SUZUKI, T., KADATA, T., & MIYAMOTO, J. (1976) One-year chronic
toxicity study of S5602 in mice (three month interim report)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
174. SUZUKI, T., OKUNO, Y., HIROMORI, T., ITO, S., KADOTA, T., & MIYAMOTO,
J. (1977a) Fifteen-month chronic toxicity study of S5602 in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
175. SUZUKI, T., OKUNO, Y., HIROMORI, T., KADOTA, T., & MIYAMOTO, J.
(1977b) Eighteen-month chronic toxicity study of S5602 in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
176. SYRETT, P. & PENMAN, D.R. (1980) Studies of insecticide toxicity to
lucerne aphids and their predators. N.Z. J. agric. Res., 23: 575-580.
177. TAGATZ, M.E. & IVEY, J.M. (1981) Effects of fenvalerate on field- and
laboratory-developed estuarine benthic communities. Bull. environ.
Contam. Toxicol., 27: 256-267.
178. TAGATZ, M.E., STANLEY, R.S., PLAIA, G.R., & DEANS, C.H. (1987)
Responses of estuarine macrofauna colonizing sediments contaminated
with fenvalerate. Environ. Toxicol. Chem., 6: 21-25.
179. TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Photodegradation of the [2S, alphaS] isomer of fenvalerate in distilled
water (Unpublished Report No. LLM-50-0001 submitted to WHO by
Sumitomo Chemical Co., Ltd).
180. TAKAMATSU, Y., KANEKO, H., ABIKO, J., YOSHITAKE, A., & MIYAMOTO, J.
(1987) In vivo and in vitro stereoselective hydrolysis of four chiral
isomers of fenvalerate. J. Pestic. Sci., 12: 397-404.
181. TALEKER, N.S., CHEN, J.S., & KAO, H.T. (1983) Persistence of
fenvalerate in subtropical soil. J. econ. Entomol., 76: 223-226.
182. TUCKER, S.B. & FLANNIGAN, S.A. (1983) Cutaneous effects from
occupational exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
183. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
184. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect: Neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
185. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M., (1973)
DDT-like action of allethrin in the sensory nervous system of Xenopus
laevis. Eur. J. Phamacol., 21: 95-106.
186. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979)
Effect of insecticides on the sensory nervous system. In: Narahashi,
T., ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 183-210.
187. VAN DER PAUW, C.L., DIX, K.M., BLANCHARD, K., & MCCARTHY, W.V. (1975)
Teratological studies in rabbits given WL 43775 orally (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
188. VAKENTI, J.M. (1986) Persistence of fenvalerate in orchard soils,
Okanagan Valley, British Columbia (Unpublished report of cooperative
study of Agriculture Canada and Ciba-Geigy Canada, Ltd).
189. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relation-
ship of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
190. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog myelinated
nerve fibres. Eur. J. Phamacol., 58: 501-504.
191. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
192. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
193. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in mylinated nerves. Nature (Lond.), 295: 601-603.
194. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN
DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
195. WALKER, B.J., HEND, R.W., & LINNETT, S. (1975) Toxicity studies on
the insecticide WL 43775: Summary of results of preliminary
experiments (Unpublished report submitted to WHO by Shell Development
Co., Ltd).
196. WHO (1979) WHO Technical Report Series No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp.18-23.
197. WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
198. WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
199. WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic pyrethroid
insecticides to predaceous phytoseiid mites and their prey. Aust. J.
agric. Res., 30: 497-501.
200. WOOD MACKENZIE (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
201. WOOD MACKENZIE (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
202. WOOD MACKENZIE (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
203. WOOD MACKENZIE (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
204. WOOD MACKENZIE (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
205. WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida. State Hortic. Soc., 90: 401-402.
206. WORTHING, C.R. (1979) The pesticide manual, 6th ed., Croydon, British
Crop Protection Council, p. 270.
207. WORTHING, C.R. & WALKER, S.B. (1987) Fenvalerate In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 395-
396.
208. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
209. WSZOLEK, P.C., LEIN, D.H., & LISK, D.J. (1980) Excretion of fenval-
erate insecticide in the milk of dairy cows. Bull. environ. Contam.
Toxicol., 24: 296-298.
210. WSZOLEK, P.C., HOGUE, D.E., & LISK, D.J. (1981a) Accumulation of
fenvalerate insecticide in lamb tissues. Bull. environ. Contam.
Toxicol., 27: 869-871.
211. WSZOLEK, P.C., LAFAUNCE, N.A., WACHS, T., & LISK, D.J. (1981b)
Studies of possible bovine urinary excretion and rumen decomposition
of fenvalerate insecticide and a metabolite. Bull. environ. Contam.
Toxicol., 26: 262-266.
212. BORTHWICK, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
static acute toxicity tests with selected estuarine algae,
invertebrates, and fish. Gulf Breeze, Florida, US Environmental
Protection Agency (EPA-600/4-81-076).
213. HE, F., WANG, S., LIU, L., CHEN, S., ZHANG, Z., & SUN, J. (1989)
Clinical manifestations and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63: 54-58.
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs (Xenopus laevis; Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [65, 98, 186,
189, 196]. The same distinction was found in studies on
cockroaches [60].
Based on the binding assay on the gamma-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types:
the alpha-cyano-3-phenoxybenzyl pyrethroids and the non-
cyano pyrethroids [59, 61, 99, 100].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin,
cismethrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [185]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [183]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [194].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [55].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [184]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [194].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans
[38], are almost identical to those of allethrin, i.e.,
presynaptic repetitive firing, and no significant effects
on transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [185, 186, 193].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [108].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [159].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cypermethrin, fenval-
erate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [192]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [190,
194].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during
excitation, presumably by slowing down the closing of the
activation gate of the sodium channel [190, 194]. The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than
100 milliseconds. Apparently, the amplitude of the pro-
longed sodium current after cypermethrin is too small to
induce repetitive activity in nerve fibres, but is suf-
ficient to cause the long-lasting repetitive firing in the
lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [108].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [61].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the tox-
icity of Type II pyrethroids and their potency to inhibit
the [35S]-TBPS binding was established using a radio-
ligand, [35S]- t-butyl-bicyclophosphoro-thionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute corre-
lation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all
toxic cyano compounds including deltamethrin, 1R, cis-
cypermethrin, 1R, trans-cypermethrin, and [2S, alphaS]-
fenvalerate were inhibitors, but their non-toxic stereo-
isomers were not; non-cyano pyrethroids were much less
potent or were inactive [99].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-di-
hydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [100].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S, alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex
[59].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [159].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-
lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation.
This results in long-lasting trains of repetitive impulses
in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [196, 208].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [191].
RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes
d'analyse
La fenvalérate est un insecticide puissant utilisé
depuis 1976. C'est un ester de l'acide (chloro-4 phényle)-
2 méthyl-3 butyrique et de l'alcool alpha-cyano-phénoxy-
benzylique. Malgré l'absence du cycle cyclopropane, ses
propriétés insecticides le rattachent au groupe des
pyréthroïdes. Il s'agit d'un mélange racémique de quatre
isomères optiques dont les configurations sont [2S, alphaS],
[2S, alphaR], [2R, alphaS] et [2R, alphaR]. L'isomère [2S,
alphaS] est le plus actif biologiquement; vient ensuite
l'isomère [2S, alphaR].
Le fenvalérate de qualité technique se présente sous
la forme d'un liquide visqueux jaune ou brun dont la
densité est de 1,175 à 25 °C. Il est relativement non
volatil, sa tension de vapeur étant de 0,037 mPa à 25 °C.
Pratiquement insoluble dans l'eau (environ 2 µg/litre),
il est soluble dans les solvants organiques comme
l'acétone, le xylène et le kérosène. Il est stable à la
lumière, à la chaleur et à l'humidité, mais instable en
milieu alcalin par suite de l'hydrolyse du groupement
ester.
Le dosage des résidus et les analyses écotoxico-
logiques peuvent s'effectuer par chromatographie en phase
gazeuse avec détection par capture d'électrons, la
concentration minimale décelable étant de 0,005 mg/kg.
Pour l'analyse des produits techniques on utilise la même
méthode mais avec un détecteur à ionisation de flamme.
1.2 Production et usage
On utilise dans le monde environ 1000 tonnes de
fenvalérate par an (chiffres de 1979-1983), essentielle-
ment en agriculture mais également pour la désinsecti-
sation des habitations et des jardins et le déparasitage
des bestiaux, soit seul, soit en association avec d'autres
insecticides. Il est présenté sous forme de concentré
émulsionnable, de concentré pour épandage à très bas
volume, de poudre pour poudrage et de poudre mouillable.
1.3 Exposition humaine
C'est essentiellement du fait de la présence de
résidus dans les aliments que la population dans son
ensemble est exposée à cet insecticide. Le respect des
règles de bonne pratique permet en général de maintenir
les résidus dans les récoltes à un faible niveau. L'expo-
sition qui en découle pour la population générale devrait
a priori être très faible mais on ne dispose pas de
données tirées d'études de la ration totale.
L'analyse des résidus présents dans les céréales
ensilées a montré que plus de 70% de la dose appliquée
subsistent sur le blé au bout de dix mois à 25 °C. Après
mouture et panification, la teneur en résidus du pain
blanc et de la farine de froment est à peu près la même
(environ 0,06-0,1 mg/kg).
Les informations relatives à l'exposition profession-
nelle au fenvalérate sont très fragmentaires.
1.4 Destinée dans l'environnement
Dans le sol, il y a dégradation par coupure de la
liaison ester et du groupement diphényl-éther, hydroxyl-
ation du cycle benzénique, hydratation du nitrile en
amide, l'oxydation des fragments se poursuivant jusqu'à
l'obtention d'anhydride carbonique qui constitue le
principal produit final. L'étude du potentiel de lixivi-
ation du fenvalérate et de ses produits de dégradation a
montré qu'il n'y avait guère de pénétration au niveau du
sol.
Dans l'eau et à la surface du sol, la fenvalérate
subit une photodégradation par la lumière solaire. On a
montré qu'il se produisait une coupure du groupement
ester, une hydrolyse du groupement cyano, une décarboxy-
lation conduisant au (phénoxy-3)-2 (chloro-4 phényle)-3
méthyl-4 pentane-nitrile (décarboxy-fenvalérate), ainsi que
d'autres réactions à initiation radicalaire.
Sur les végétaux, la fenvalérate a une demi-vie
d'environ 14 jours. La principale réaction consiste dans
la rupture de la liaison ester suivie d'une oxydation
et/ou d'une conjugaison des fragments. Il se produit
également une décarboxylation en décarboxy-fenvalérate.
En général, la dégradation dans l'environnement
conduit à des produits moins toxiques.
Dans l'environnement, le fenvalérate est assez
rapidement dégradé. La demi-vie est de 4 à 15 jours dans
les rivières, 8 à 14 jours sur les végétaux, 1 à 18 jours
par photodégradation à la surface du sol et 15 jours à
3 mois dans le sol.
Il n'y a pratiquement aucune lixiviation du fenva-
lérate présent dans le sol. Il est donc improbable que ce
composé puisse s'accumuler de façon importante dans le
milieu aquatique.
1.5 Cinétique et métabolisme
On a étudié la destinée du fenvalérate chez le rat et
la souris au moyen de fenvalérate radio-marqué au niveau
du groupement carboxylate, ou des groupements benzyle ou
cyano. Sauf dans le cas des composés marqués au niveau du
groupement cyano, la radioactivité administrée est
rapidement excrétée (jusqu'à 99% en six jours). Les
principales réactions métaboliques consistent en une
rupture du groupement ester et une hydroxylation en
position 4. On a également observé diverses réactions
d'oxydation et de conjugaison conduisant à un mélange
complexe. Lorsque le fenvalérate est radio-marqué au
niveau du groupement cyano, la dose radioactive s'élimine
moins rapidement (jusqu'à 81% en six jours). La
radioactivité restante est principalement confinée dans la
peau, les poils et l'estomac sous forme de thiocyanate.
Il existe aussi une voie métabolique secondaire, quoique
très importante, qui consiste dans la formation d'un
conjugué lipophile de (chloro-4 phényle)-2-[2R] iso-
valérate. Ce conjugué qui intervient dans la formation de
granulomes a été décelé dans les surrénales, le foie et
les ganglions mésentériques des rats, des souris et de
certaines autres espèces.
1.6 Effets sur les êtres vivant dans leur milieu naturel
Au laboratoire, le fenvalérate se révèle extrêment
toxique pour les organismes aquatiques. La CL50 varie de
0,008 µg/litre pour des mysidacées nouvellement écloses
à 2 µg/litre pour une espèce d'éphéméroptère. Les
épreuves portant sur le cycle évolutif de Daphnia galeata
mendotae ont révélé que la dose sans effet observable
était de 0,005 µg/litre. Le fenvalérate est également
extrêment toxique pour les poissons. Les valeurs de la
CL50 à 96 heures vont de 0,03 µg/litre pour la larve de
Leuresthes tenuis à 200 µg/litre pour le Tilapia
adulte. La dose sans effet observable sur 28 jours
s'établit à 0,56 µg/litre pour les premiers stades de
certains vairons. Le fenvalérate est moins toxique pour
les algues et les mollusques aquatiques, la CL50 à 96
heures étant supérieure à 1000 µg/litre.
La forte toxicité potentielle du fenvalérate pour les
organismes aquatiques ne se manifeste pas dans les essais
sur le terrain ni dans les conditions d'utilisation
pratique. Certains invertébrés aquatiques sont détruits
par un épandage à la surface des eaux mais l'effet sur les
populations est temporaire. On n'a pas signalé de
mortalité chez les poissons. La toxicité moindre observée
lors des épandages de plein champ s'explique par une forte
adsorption du composé par les sédiments.
Le fenvalérate est très toxique pour l'abeille. En
applications topiques la DL50 est de 0,41 µg/abeille,
toutefois l'effet répulsif intense qu'exerce le fenva-
lérate sur ces insectes en réduit l'action dans la
pratique. Rien n'indique qu'il y ait eu des destructions
importantes d'abeilles dans les conditions normales
d'utilisation. Le fenvalérate est plus toxique pour les
acariens prédateurs que pour les espèces cibles de
ravageurs.
Administré par voie orale ou mêlé à la nourriture, le
fenvalérate est très peu toxique pour les oiseaux. La
DL50 est supérieure à 1500 mg/kg de poids corporel en
administration orale directe et dépasse 15 000 mg/kg de
nourriture en administration dans la ration alimentaire
pour le colin de Virginie.
Les organismes aquatiques fixent rapidement le fenva-
lérate. Le facteur de bioconcentration varie de 120 à
4700 selon l'organisme en cause (algues, mollusques,
daphnies et poissons) selon les études effectuées sur des
modèles d'écosystèmes. Toutefois, ce fenvalérate s'élimine
rapidement lorsque les organismes sont replacés en eau
propre. On peut donc considérer qu'en pratique, ce composé
ne présente aucune tendance à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
La toxicité aiguë par voie orale du fenvalérate est
modérée à faible. Toutefois, les valeurs de la DL50
peuvent varier considérablement (de 82 à plus de 3200
mg/kg) selon l'espèce animale en cause et le véhicule
d'administration. Les signes cliniques d'intoxication
aiguë apparaissent rapidement mais les survivants
redeviennent asymptomatiques au bout de trois à quatre
jours. Parmi les signes d'intoxication produits par le
mélange racémique, ainsi que par l'isomère [2S, alphaS], on
note de l'agitation, des tremblements, une horripilation,
de la diarrhée, une démarche anormale, une choréo-athétose
et une salivation (syndrome CS); il est classé comme
pyréthroïde du type II. Du point de vue électrophysio-
logique, il produit des bouffées de pointes au niveau des
nerfs moteurs des cerques de la blatte. Toutefois, il n'y
a pas de relation bien définie entre les effets électro-
physiologiques chez l'insecte et la toxicité pour les
mammifères.
Des rats ayant reçu du fenvalérate pendant 8 à 10
jours à raison de 2000 mg/kg de nourriture ont présenté
des signes typiques d'intoxication aiguë. A la dose de
3000 mg/kg de nourriture, on observait des modifications
morphologiques réversibles au niveau du nerf sciatique.
On a également observé des modifications histopatho-
logiques au niveau du même nerf chez des rats et des
souris ayant reçu en une seule fois du fenvalérate par
voie orale à des doses létales ou sublétales.
Des poulets ayant reçu par voie orale du fenvalérate
pendant cinq jours à raison de 1000 mg/kg par jour n'ont
pas présenté de signes cliniques ou morphologiques de
neurotoxicité retardée.
Chez la souris, la toxicité aiguë par voie intra-
péritonéale des métabolites du fenvalérate n'est pas
supérieure à celle du fenvalérate lui-même.
Lors d'études de toxicité subaiguë et subchronique,
des souris, des rats, des chiens et des lapins ont reçu
pendant trois semaines à six mois, du fenvalérate par voie
orale, percutanée et respiratoire. Chez le rat et la
souris, des études d'inhalation de quatre semaines ont
permis de fixer la dose sans effet observable à 7 mg/m3.
Chez le rat, lors d'une étude de 90 jours, elle
s'établissait à 125 mg/kg de nourriture, et sur deux ans à
250 mg/kg de nourriture (soit 12,5 mg/kg de poids
corporel). Dans une étude de 24 à 28 mois, elle s'est
établie à 150 mg/kg de nourriture, soit 7,5 mg/kg de poids
corporel. Une étude de deux ans sur la souris a permis de
fixer la dose à 50 mg/kg de nourriture, soit 6 mg/kg de
poids corporel et une étude de 20 mois, à 30 mg/kg de
nourriture, soit 3,5 mg/kg de poids corporel. Chez le
chien cette dose a été établie à 12,5 mg/kg de poids
corporel, lors d'une étude de 90 jours. Certaines formu-
lations de fenvalérate ont provoqué une irritation cutanée
et oculaire. Toutefois, le fenvalérate technique n'est
pas irritant et n'a pas d'effet sensibilisateur.
Lors d'études de toxicité à long terme, on a noté
l'apparition de microgranulomes chez des souris qui
avaient été traitées avec l'isomère [2R, alphaS] (125 mg/kg
de nourriture) sur une période de un à trois mois. Ces
anomalies disparaissaient lorsqu'on supprimait le fenval-
érate. L'agent causal en était l'ester cholestérique de
l'acide (chloro-4 phényl)-2 isovalérique, un métabolite
lipophile de l'isomère [2R, alphaS] du fenvalérate. La
dose sans effet observable relative à la formation de
microgranulomes chez la souris s'établit à 30 mg de
fenvalérate par kg de nourriture.
Lors d'une autre étude de toxicité à long terme, on a
également observé ces anomalies microgranulomateuses chez
des rats à la dose de 500 mg/kg de nourriture, la dose
sans effet observable étant dans ce cas de 150 mg par kg
de nourriture.
Administré à des souris dans leur nourriture pendant
78 semaines en doses allant jusqu'à 3000 mg/kg ou pendant
deux ans à raison de 1250 mg/kg, le fenvalérate ne s'est
pas révélé cancérogène. Il ne l'a pas été non plus chez
des rats qui avaient reçu pendant deux ans une alimen-
tation contenant jusqu'à 1000 mg d'insecticide par kg.
Le fenvalérate n'est ni mutagène, ni délétère pour les
chromosomes ainsi qu'il ressort d'un certain nombre
d'épreuves in vitro et in vivo.
Il n'est pas non plus tératogène pour la souris et le
lapin à des doses quotidiennes allant jusqu'à 50 mg par kg
de poids corporel et, lors d'une étude de reproduction
portant sur trois générations de rats où les animaux
recevaient des doses allant jusqu'à 250 mg/kg de
nourriture, il n'a affecté aucun des paramètres de la
fonction de reproduction.
1.8 Effets sur l'être humain
Le fenvalérate peut provoquer des sensations
d'engourdissement, de démangeaison, de picotement et de
brûlure chez les travailleurs exposés; les symptômes
apparaissent après une période de latence d'environ
30 minutes, atteignent leur acmé au bout de 8 heures et
disparaissent dans les 24 heures suivantes. Certains cas
d'intoxication se sont produits à la suite d'une expo-
sition professionnelle due au non respect des mesures de
sécurité.
Rien n'indique que le fenvalérate soit nocif pour
l'être humain dans la mesure où il est utilisé conformé-
ment aux recommandations.
2. Conclusions
2.1 Population générale
L'exposition de la population générale au fenvalérate
est probablement très faible. Il n'y a sans doute aucun
risque si on l'emploie conformément aux recommandations.
2.2 Exposition professionnelle
Utilisé de manière raisonnable, et moyennant certaines
mesures d'hygiène et de sécurité, le fenvalérate ne
devrait pas être dangereux pour les personnes qui lui sont
exposées de par leur profession.
2.3 Environnement
Il est improbable que le fenvalérate ou ses produits
de dégradation puissent s'accumuler dans l'environnement
en quantité suffisante pour créer des problèmes, dans la
mesure où l'on respecte les doses d'emploi recommandées.
Au laboratoire, le fenvalérate se révèle extrêmement
toxique pour les poissons, les arthropodes aquatiques et
les abeilles. Toutefois, il ne semble pas que des effets
nocifs durables puissent se produire sur le terrain si
l'insecticide est utilisé conformément aux recomman-
dations.
3. Recommandations
Les concentrations alimentaires qui résultent d'une
utilisation conforme aux recommandations sont considérées
comme très faibles; toutefois il conviendrait de confirmer
ce point de vue en étendant les études de surveillance au
fenvalérate.
Le fenvalérate est utilisé depuis de nombreuses années
et seuls quelques effets temporaires ont été observés ça
et là à la suite d'expositions professionnelles. Néan-
moins, il serait bon de poursuivre les observations sur
l'exposition humaine.
 The composition of the product is a racemic mixture of
the four isomers in equal proportions (Table 1). Technical
grade fenvalerate contains 90-94% of fenvalerate [41]. The
molecular formula is C25H22ClNO3.
Table 1. Chemical identity of fenvalerate and its various stereoisomers
-----------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry no./ compositionc trade names
NIOSH Accession no.a Stereospecific nameb
-----------------------------------------------------------------------------------------------------
Fenvalerate Benzeneacetic acid, (1):(2):(3):(4) Sumicidin, Belmark,
The composition of the product is a racemic mixture of
the four isomers in equal proportions (Table 1). Technical
grade fenvalerate contains 90-94% of fenvalerate [41]. The
molecular formula is C25H22ClNO3.
Table 1. Chemical identity of fenvalerate and its various stereoisomers
-----------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry no./ compositionc trade names
NIOSH Accession no.a Stereospecific nameb
-----------------------------------------------------------------------------------------------------
Fenvalerate Benzeneacetic acid, (1):(2):(3):(4) Sumicidin, Belmark,
 2.2 Physical and Chemical Properties
Some physical and chemical properties of fenvalerate
are given in Table 2. It is stable to heat and moisture
and is relatively stable (compared with natural py-
rethrins) when exposed to light. It is more stable in
acidic than in alkaline media, optimum stability being at
pH 4 [41, 117, 207].
Table 2. Some physical and chemical properties of fenvalerate
--------------------------------------------------------------
Physical state viscous liquid
Colour yellow or brown
Odour mild "chemical" odour
Relative molecular mass 419.9
Boiling point 300 °C at 4.93 kPa (37 mmHg)
Water solubility 2 µg/Litre
Solubility in organic solvents solublea
Relative density (25 °C) 1.175
Vapour pressure (25 °C) 0.037 mPa
Log octanol-water partition
coefficient (log Pow) 6.2
--------------------------------------------------------------
a Acetone (>1 kg/kg), hexane (155 g/kg), xylene (>1 kg/kg),
ethanol, cyclohexanone, ether, kerosene, chloroform.
2.3 Analytical Methods
Methods for the analysis of fenvalerate are summarized
in Table 3. This table includes the procedures for (a) ex-
traction with solvent, (b) liquid-liquid partition, (c)
chromatographic separation (clean up), and (d) quantitat-
ive and qualitative determination by suitable analytical
instruments, and also includes minimum detectable concen-
tration (MDC) and percentage recovery data.
The separation of the cis and trans isomers of
fenvalerate has been carried out using a commercially
available Pirkle type 1-A chiralphase HPLC with, as sol-
vent system, 0.025% propen-2-ol in hexane (1 ml/min)
[24].
Fenvalerate can be determined by gas-liquid chromato-
graphy with a flame ionization detector (FID-GC) (3% OV-17
glass column with temperature programming) [11].
A laminar flow, microwave-induced plasma torch has
been evaluated for its use in gas chromatography [19]. The
detection limit of fenvalerate on the carbon channel was
0.054 µg/ml.
To analyse technical grade fenvalerate, the product is
dissolved in chloroform together with 2-(4-biphenyl)-5-
phenyl-1,3,4-oxadiazole (an internal standard), and the
solution is injected into an FID-GC system [79].
The Joint FAO/WHO Codex Alimentarius Commission has
published recommendations for methods for the analysis of
fenvalerate residues [48].
Table 3. Analytical methods for fenvalerate
--------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCb % Recovery Reference
GLC or HPLC; detector, (fortification
Extraction Partition Clean-up carrier flow, column, level)
solvent temperature, retention (mg/kg)c
Column Elution time
--------------------------------------------------------------------------------------------------------------------------------------
Residue analysis
apple n-hexane ext.sol.a silica gel CH2Cl2 ECD-GC, N2, 50 ml/min, 0.01 89-108 6
pear acetone /H2O 1 m, 3% OV-7, 235 °C (0.1-1.0)
cabbage (1/1)
potato
grape acetone saturated Florisil acetone/ ECD-GC, N2, 30 ml/min, 0.005 94-99 67
pepper NaCl/ petroleum 1.1 m, 2% XE-60, (0.005-1.0)
petroleum ether (1/99) 215 °C, 7 min
ether
cabbage CH3CN 1% NaCl/ Florisil benzene/ n- ECD-GC, argon/methane 0.005 88-104 103
lettuce petroleum hexane (1/1) (95/5), 45 ml/min, 1.8 m, (0.012-1.2)
ether silica gel benzene/ 4% SE-30/6% QF-1 or 15%
acetone (3/1) OV-101, 225 °C, 25-30 min
beef CH3CN/ n-hexane/ Florisil CH3CN/ ECD-GC, N2, 100 ml/min, 0.005 82-94 16
muscle H20 2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01-1.0)
egg yolk (85/15) solution n-hexane 220 °C, 11.5, 14.2 min
milk or CH3CN (0.35/50/50)
Environmental analysis
soil acetone, 2% NaCl/ alumina ether/ ECD-GC, argon/methane 78-105 74
n-hexane/ ext.sol.a n-hexane (95/5), 60 ml/min, (0.005-1.0)
acetone (1/9) 0.97 m, 6% OV-210,
(1/1), 230 °C, 10.6, 11.8 min
hexane
Product analysis
Technical CHCl3 FID-GC, He, 60 ml/min, 79
grade 1.0 m, 2% Apiezon L,
245 °C
--------------------------------------------------------------------------------------------------------------------------------------
a extraction solvent.
b minimum detectable concentration (mg/kg).
c fortification level indicates the concentration of fenvalerate added to control samples for the measurement of recovery.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE:
ENVIRONMENTAL LEVELS
3.1 Industrial Production
Fenvalerate was first marketed in 1976 and the esti-
mated production was 1000 tonnes in 1979 and 889 tonnes in
1982 [203]. Recent world-wide production figures are
listed in Table 4.
Table 4. World-wide production of
fenvalerate
-------------------------------------
Year Production Reference
(tonnes)
-------------------------------------
1979 1016 200
1980 1067 201
1981 914 202, 203
1982 903 203
1983 1280 204
1984 919 9
-------------------------------------
3.2 Use Patterns
Of the total world-wide consumption of 473 tonnes of
fenvalerate in 1980 [8], 271 tonnes were used in the USA,
103 tonnes in Latin America, 43 tonnes in Africa, 28
tonnes in Western Europe, and 26 tonnes each in Australia
and Turkey. It was mostly used on cotton (90.3% of the
consumption) but some was used on other crops such as
vines, tomatoes, potatoes, pomes, and other fruit.
Fenvalerate has also been used for homes and gardens
and for the control of cattle insect infestation [8]. It
is formulated in emulsifiable concentrates (25-300
g/litre), ultra-low volume concentrates (25-75 g/litre),
dusts, and wettable powder, and is also used in combi-
nation with other pesticides (e.g., fenitrothion).
3.3 Residues in Food
Supervised trials have been carried out on a wide
variety of crops and comprehensive summaries of the re-
sults of residue analysis in these trials are contained in
the evaluation reports of the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) [41, 43, 45, 47, 50]. A compre-
hensive list of Maximum Residue Limits (MRLs) for a large
number of commodities resulted from these evaluations
[51].
In one study, apples in the USA were treated four
times with 30% emulsifiable concentrate at a rate of 0.67
kg active ingredient/ha. The residue levels were 2.2 mg/kg
in whole apples, 7.3 mg/kg in peel, and 0.03 mg/kg in
peeled fruits 6 weeks after the last application [41].
When wheat grain treated with fenvalerate at a rate of
1.01 mg/kg was stored at 25 °C, the residue levels were
0.86 mg/kg after 6.5 months of storage and 0.74 mg/kg
after 10 monthsa.
Three lactating cows were fed 14C-(acid-labelled)-
fenvalerate at a dose level of 0.11 mg/kg diet daily for
21 days and sacrificed 12 h after receiving the last dose.
The recovery of 14C in the milk was less than 1% and the
levels ranged from < 0.0006 to 0.0019 µg/litre, with a
plateau occurring after 1 week of feeding. No 14C was
detectable in fat (< 0.02 mg/kg) or muscle (< 0.01 mg/kg).
In another study, fenvalerate was sprayed on cows at a
rate of 0.2, 0.4, or 2 g/animal. The residue level did not
exceed 0.01 mg/kg muscle. Maximum residues were 0.22 mg/kg
in fat and 0.02 mg/kg in milk at the dose rate of 2 g/cow
[132, 154].
When wheat containing 0.6 mg fenvalerate/kg was sub-
jected to milling and baking, white bread was found to
have about the same residue level as white flour, i.e.,
about 0.06-0.1 and 0.08-0.09 mg/kg, respectivelyb.
3.4 Residues in the Environment
Data on actual levels of fenvalerate residues in air,
water, or soil are not available. Residues in air would
not be expected for a compound with a vapour pressure of
0.037 mPa at 25 °C.
----------------------------------------------------------
a M. Bengston (1979), personal communication from final
report on silo-scale experiments 1977-1978 to the
Australian Wheat Board Working Party on grain protect-
ants. Queensland Department of Primary Industries
(unpublished report cited from FAO/WHO [41].
b B.W. Simpson (1979), draft report to be published by
Queensland Department of Primary Industries Analyti-
cal Chemical Branch, Brisbane, Australia (unpublished
report cited in FAO/WHO [41].
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Appraisal
The major photodegradation routes for fenvalerate are de-
carboxylation to yield 3-(4-chlorophenyl)-4-methyl-2-(3-phenoxy-
phenyl)-valeronitrile, ester and ether cleavage, hydrolysis of
the cyanide group, and other radical-initiated reactions. Ester
cleavage and some photo-initiated reactions are the major
routes of decomposition on plants. In soils, the formation of
bound material and the evolution of carbon dioxide are the
major processes observed under both aerobic and anaerobic con-
ditions.
The degradation pathways of fenvalerate are summarized
in Fig. 2.
2.2 Physical and Chemical Properties
Some physical and chemical properties of fenvalerate
are given in Table 2. It is stable to heat and moisture
and is relatively stable (compared with natural py-
rethrins) when exposed to light. It is more stable in
acidic than in alkaline media, optimum stability being at
pH 4 [41, 117, 207].
Table 2. Some physical and chemical properties of fenvalerate
--------------------------------------------------------------
Physical state viscous liquid
Colour yellow or brown
Odour mild "chemical" odour
Relative molecular mass 419.9
Boiling point 300 °C at 4.93 kPa (37 mmHg)
Water solubility 2 µg/Litre
Solubility in organic solvents solublea
Relative density (25 °C) 1.175
Vapour pressure (25 °C) 0.037 mPa
Log octanol-water partition
coefficient (log Pow) 6.2
--------------------------------------------------------------
a Acetone (>1 kg/kg), hexane (155 g/kg), xylene (>1 kg/kg),
ethanol, cyclohexanone, ether, kerosene, chloroform.
2.3 Analytical Methods
Methods for the analysis of fenvalerate are summarized
in Table 3. This table includes the procedures for (a) ex-
traction with solvent, (b) liquid-liquid partition, (c)
chromatographic separation (clean up), and (d) quantitat-
ive and qualitative determination by suitable analytical
instruments, and also includes minimum detectable concen-
tration (MDC) and percentage recovery data.
The separation of the cis and trans isomers of
fenvalerate has been carried out using a commercially
available Pirkle type 1-A chiralphase HPLC with, as sol-
vent system, 0.025% propen-2-ol in hexane (1 ml/min)
[24].
Fenvalerate can be determined by gas-liquid chromato-
graphy with a flame ionization detector (FID-GC) (3% OV-17
glass column with temperature programming) [11].
A laminar flow, microwave-induced plasma torch has
been evaluated for its use in gas chromatography [19]. The
detection limit of fenvalerate on the carbon channel was
0.054 µg/ml.
To analyse technical grade fenvalerate, the product is
dissolved in chloroform together with 2-(4-biphenyl)-5-
phenyl-1,3,4-oxadiazole (an internal standard), and the
solution is injected into an FID-GC system [79].
The Joint FAO/WHO Codex Alimentarius Commission has
published recommendations for methods for the analysis of
fenvalerate residues [48].
Table 3. Analytical methods for fenvalerate
--------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCb % Recovery Reference
GLC or HPLC; detector, (fortification
Extraction Partition Clean-up carrier flow, column, level)
solvent temperature, retention (mg/kg)c
Column Elution time
--------------------------------------------------------------------------------------------------------------------------------------
Residue analysis
apple n-hexane ext.sol.a silica gel CH2Cl2 ECD-GC, N2, 50 ml/min, 0.01 89-108 6
pear acetone /H2O 1 m, 3% OV-7, 235 °C (0.1-1.0)
cabbage (1/1)
potato
grape acetone saturated Florisil acetone/ ECD-GC, N2, 30 ml/min, 0.005 94-99 67
pepper NaCl/ petroleum 1.1 m, 2% XE-60, (0.005-1.0)
petroleum ether (1/99) 215 °C, 7 min
ether
cabbage CH3CN 1% NaCl/ Florisil benzene/ n- ECD-GC, argon/methane 0.005 88-104 103
lettuce petroleum hexane (1/1) (95/5), 45 ml/min, 1.8 m, (0.012-1.2)
ether silica gel benzene/ 4% SE-30/6% QF-1 or 15%
acetone (3/1) OV-101, 225 °C, 25-30 min
beef CH3CN/ n-hexane/ Florisil CH3CN/ ECD-GC, N2, 100 ml/min, 0.005 82-94 16
muscle H20 2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01-1.0)
egg yolk (85/15) solution n-hexane 220 °C, 11.5, 14.2 min
milk or CH3CN (0.35/50/50)
Environmental analysis
soil acetone, 2% NaCl/ alumina ether/ ECD-GC, argon/methane 78-105 74
n-hexane/ ext.sol.a n-hexane (95/5), 60 ml/min, (0.005-1.0)
acetone (1/9) 0.97 m, 6% OV-210,
(1/1), 230 °C, 10.6, 11.8 min
hexane
Product analysis
Technical CHCl3 FID-GC, He, 60 ml/min, 79
grade 1.0 m, 2% Apiezon L,
245 °C
--------------------------------------------------------------------------------------------------------------------------------------
a extraction solvent.
b minimum detectable concentration (mg/kg).
c fortification level indicates the concentration of fenvalerate added to control samples for the measurement of recovery.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE:
ENVIRONMENTAL LEVELS
3.1 Industrial Production
Fenvalerate was first marketed in 1976 and the esti-
mated production was 1000 tonnes in 1979 and 889 tonnes in
1982 [203]. Recent world-wide production figures are
listed in Table 4.
Table 4. World-wide production of
fenvalerate
-------------------------------------
Year Production Reference
(tonnes)
-------------------------------------
1979 1016 200
1980 1067 201
1981 914 202, 203
1982 903 203
1983 1280 204
1984 919 9
-------------------------------------
3.2 Use Patterns
Of the total world-wide consumption of 473 tonnes of
fenvalerate in 1980 [8], 271 tonnes were used in the USA,
103 tonnes in Latin America, 43 tonnes in Africa, 28
tonnes in Western Europe, and 26 tonnes each in Australia
and Turkey. It was mostly used on cotton (90.3% of the
consumption) but some was used on other crops such as
vines, tomatoes, potatoes, pomes, and other fruit.
Fenvalerate has also been used for homes and gardens
and for the control of cattle insect infestation [8]. It
is formulated in emulsifiable concentrates (25-300
g/litre), ultra-low volume concentrates (25-75 g/litre),
dusts, and wettable powder, and is also used in combi-
nation with other pesticides (e.g., fenitrothion).
3.3 Residues in Food
Supervised trials have been carried out on a wide
variety of crops and comprehensive summaries of the re-
sults of residue analysis in these trials are contained in
the evaluation reports of the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) [41, 43, 45, 47, 50]. A compre-
hensive list of Maximum Residue Limits (MRLs) for a large
number of commodities resulted from these evaluations
[51].
In one study, apples in the USA were treated four
times with 30% emulsifiable concentrate at a rate of 0.67
kg active ingredient/ha. The residue levels were 2.2 mg/kg
in whole apples, 7.3 mg/kg in peel, and 0.03 mg/kg in
peeled fruits 6 weeks after the last application [41].
When wheat grain treated with fenvalerate at a rate of
1.01 mg/kg was stored at 25 °C, the residue levels were
0.86 mg/kg after 6.5 months of storage and 0.74 mg/kg
after 10 monthsa.
Three lactating cows were fed 14C-(acid-labelled)-
fenvalerate at a dose level of 0.11 mg/kg diet daily for
21 days and sacrificed 12 h after receiving the last dose.
The recovery of 14C in the milk was less than 1% and the
levels ranged from < 0.0006 to 0.0019 µg/litre, with a
plateau occurring after 1 week of feeding. No 14C was
detectable in fat (< 0.02 mg/kg) or muscle (< 0.01 mg/kg).
In another study, fenvalerate was sprayed on cows at a
rate of 0.2, 0.4, or 2 g/animal. The residue level did not
exceed 0.01 mg/kg muscle. Maximum residues were 0.22 mg/kg
in fat and 0.02 mg/kg in milk at the dose rate of 2 g/cow
[132, 154].
When wheat containing 0.6 mg fenvalerate/kg was sub-
jected to milling and baking, white bread was found to
have about the same residue level as white flour, i.e.,
about 0.06-0.1 and 0.08-0.09 mg/kg, respectivelyb.
3.4 Residues in the Environment
Data on actual levels of fenvalerate residues in air,
water, or soil are not available. Residues in air would
not be expected for a compound with a vapour pressure of
0.037 mPa at 25 °C.
----------------------------------------------------------
a M. Bengston (1979), personal communication from final
report on silo-scale experiments 1977-1978 to the
Australian Wheat Board Working Party on grain protect-
ants. Queensland Department of Primary Industries
(unpublished report cited from FAO/WHO [41].
b B.W. Simpson (1979), draft report to be published by
Queensland Department of Primary Industries Analyti-
cal Chemical Branch, Brisbane, Australia (unpublished
report cited in FAO/WHO [41].
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Appraisal
The major photodegradation routes for fenvalerate are de-
carboxylation to yield 3-(4-chlorophenyl)-4-methyl-2-(3-phenoxy-
phenyl)-valeronitrile, ester and ether cleavage, hydrolysis of
the cyanide group, and other radical-initiated reactions. Ester
cleavage and some photo-initiated reactions are the major
routes of decomposition on plants. In soils, the formation of
bound material and the evolution of carbon dioxide are the
major processes observed under both aerobic and anaerobic con-
ditions.
The degradation pathways of fenvalerate are summarized
in Fig. 2.
 4.1 Transportation and Distribution Between Media
Hill [74] investigated the distribution of fenvalerate
residues in soil under field conditions using a microplot
technique. The microplots (20 x 20 cm) were treated with
fenvalerate at a rate of 150 g/ha. After 45 weeks, 11% of
the applied fenvalerate was located in the 0-2.5 cm soil
layer and less than 0.5% in the 2.5-5 cm soil samples.
Less than 0.1% of the applied fenvalerate was detected in
any of the soil samples taken after 3 or 4 weeks, despite
a rainfall of 95.4 mm during the first 4 weeks (including
a 25.9 mm downpour 15 days after application). These
results indicate that fenvalerate does not readily leach
downward and that lateral surface movement is very
limited.
A similar conclusion was obtained from laboratory
soil-leaching studies. More than 95% of the applied
fenvalerate remained in the treated portion of soil
columns when leaching was started immediately or 30 days
after treatment of the soil [133]. The possibility of
fenvalerate accumulating in orchard soils was assessed by
monitoring soil and leaf litter in an orchard in the
Okanagan valley, British Columbia, Canada, following
multiple annual application of the pesticide. Belmark 300
(30% fenvalerate EC formulation) had been sprayed at a
rate of 188-500 ml/ha (one to three times per year) for
more than three years. To obtain initial concentration,
organic litter samples were sprayed at a rate of 450 ml/ha
and samples were collected 2 h later. While the initial
concentration thus obtained in the litter was 0.214 mg/kg
(average value), orchard litter samples contained
0.30-0.63 mg/kg while samples from a non-treated block
contained < 0.002 mg/kg. Orchard soil samples (0-15 cm
depth) in two orchards contained fenvalerate residues of
< 0.0035-0.006 mg/kg and 0.0063-0.024 mg/kg [188].
4.2 Photodecompositiona
In studies by Holmstead et al. [78], fenvalerate (5),
at a concentration of 0.01 mol/litre in methanol, hexane,
or acetonitrilewater (60:40), underwent rapid photodegra-
dation under the action of UV light (290-320 nm) with a
half-life of 16-18 min (Fig. 2). With 90-95% conversion
after 60 min, 2-(3-phenoxyphenyl)-3-(4-chlorophenyl)-4-
methyl-pentanenitrile (31) (decarboxy-fenvalerate) was the
major photoproduct, amounting to 54-57% of the total reac-
tion mixture. There were smaller amounts of the dechlori-
nated analogue (32) of decarboxy-fenvalerate and the dimer
(8) of 2,2-dimethyl-4-chlorostyrene. 3-Phenoxybenzoyl cy-
anide (4), 3-phenoxybenzaldehyde (18) (PBald), 4-chloro-
isobutylbenzene (6), and 2,2-dimethyl-4-chlorostyrene (7)
were detected in small amounts in hexane or methanol. 3-
Phenoxybenzyl cyanide (28), its dimer (26), and 1,2-bis-
(phenoxyphenyl)ethane (27) were found only in hexane, and
methyl 3-phenoxybenzoate (12) was detected only in meth-
anol. Products found uniquely in acetonitrile-water were
2-(4-chlorophenyl)-3-methylbutyric acid (17) (CPIA), 3-
phenoxybenzoic acid (22) (PBacid) and 1-(4-chlorophenyl)-
2-methylpropanol (9). Several unknown compounds were ob-
served in the remaining 5-10%. Fenvalerate, in a thin film
(1 mg/cm2) on glass, decomposed in sunlight with a half-
life of approximately 4 days. About 10% of the applied
material remained after 43 days. In addition to the photo-
products formed in solution, small amounts of 3-phenoxy-
benzyl alcohol (19) (PBalc) and isopropyl 4-chlorophenyl-
ketone (10) were detected.
On exposure to autumn sunlight in Japan, the
[2S, alphaS] isomer of fenvalerate in distilled water
decomposed with a half-life of approximate 10 days. This
isomer photodecomposed via pathways that included decar-
boxylation, hydration of the CN group to a carbamoyl
(CONH2) group, hydrolysis of the CONH2 group to a car-
boxyl (COOH) group, and cleavage of the ester or diphenyl
ether linkage. Cleavage of the ester linkage was a major
photochemical reaction and led to the formation of 2-(4-
chlorophenyl)-3-methylbutyric acid (17) (17.3% of the
applied 14C, 10 days after exposure). There was no sig-
nificant difference between fenvalerate and the [2S, alphaS]
isomer in the rates and routes of photodegradation [179].
The photodegradation of fenvalerate (0.3-0.4 ng/cm2)
on two kinds of soil in natural sunlight was compared with
that of the [2S, alphaS] isomer. Fenvalerate and its isomer
photodecomposed with half-lives of 1.4-2.4 days and 1.1-
2.5 days, respectively. The pathways included hydration
of the cyano group to the carbamoyl group (19.2-48.4% at
10 days) with subsequent hydrolysis to the carboxyl group
(0.9-2.0% at 10 days), ester-bond cleavage (3.4-4.5% at 10
days), and decarboxylation (0.3-0.9% at 10 days). Little
2S/2R and alphaS/alphaR isomerization (as determined by
HPLC) occurred on the soils. There was no significant
difference between the two compounds in the rates and
pathways of photodegradation [89].
Holmstead & Fullmer [77] investigated the photodecar-
boxylation of several cyanohydrin esters in methanol and
hexane under artificial light as models for pyrethroid
photodecomposition. The cyanohydrin esters gave rise to
decarboxylated products, to a greater or lesser extent,
whereas the analogous compounds without the cyano group
did not produce the photodecarboxylated compounds.
alpha-Cyanobenzyl phenylacetate, which yielded the stable
benzyl radical, gave substantially larger amounts of the
decarboxylated product than alpha-cyanobenzyl benzoate,
which produced the unstable phenyl radical.
The photodegradation of fenvalerate in water and on
soil was investigated using compounds labelled with 14C
at the following positions: carbonyl group (CO-fenval-
----------------------------------------------------------------
a The numbers in brackets following chemical names refer to the
numbers given in Fig. 2.
erate), alpha-carbon in the benzyl group (Calpha-fenvale-
rate), and cyano group (CN-fenvalerate) [118]. On exposure
to sunlight, fenvalerate in very dilute solution in distil-
led water, in 2% aqueous acetone, in filter-sterilized
river water, or in sea water underwent rapid photolysis
with half-lives of approximately 4 days in summer and 13-
15 days in winter. The quantum yield was calculated at
6.6 x 10-3 (at 313 nm in water) and the half-life of dis-
appearance at latitude 40°N was calculated at 4.1 days in
summer and 12.4 days in winter, values which were close to
the experimental ones. Photodegradation of 14CN-fenval-
erate resulted in the formation of greater amounts of
14CO2 than 14CN-. After 6 weeks irradiation, ap-
proximately 30% (in aqueous acetone or river water) or
approximately 55-60% (in distilled water or sea water) of
the 14C was recovered as 14CO2, while the correspond-
ing figures for 14CN- were 5% and 30%. One of the major
photodegradation products was decarboxy-fenvalerate (31),
which increased to approximately 20% (in distilled water)
in summer after 1 week and decreased thereafter. In win-
ter, the amount was approximately 20% after 6 weeks. Other
major products were PBacid (22) and CPIA (17), derived
from the ester bond cleavage, amounting to 43% and 58%,
respectively, of the applied radioactivity after 6 weeks
in winter. In addition, small amounts of alpha-carbamoyl-
3-phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (33)
(CONH2-fenvalerate), alpha-carboxy-3-phenoxybenzyl-2-(4-
chlorophenyl)-3-methylbutyrate (34) (COOH-fenvalerate), 3-
phenoxybenzyl cyanide (28), 3-phenoxyphenylacetamide (29),
3-phenoxyphenylacetic acid (30), PBalc, and PBald were
detected.
Fenvalerate, as a deposit (5.5-5.9 µg/100 cm2) on
Kodaira light clay, Azuchi sandy clay loam, and Katano
sandy loam soil from Japan, was decomposed by autumn sun-
light with the respective half-lives of 2, 6, and 18 days
[118]. The major product was CONH2-fenvalerate (33),
which amounted to 7.9-25.7% of the applied radioactivity
after 10 days; it was formed in greatest amounts in sun-
light but also formed in the dark. Smaller amounts of
decarboxy-fenvalerate (31), the desphenyl analogue of
CONH2-fenvalerate (35), COOH-fenvalerate (34), PBacid,
and PBalc were also detected. Of the applied radiocarbon,
3-10% remained unidentified.
4.3 Decomposition in Plants
Fenvalerate (2.4% emulsifiable concentrate (EC)),
permethrin (2% EC), and deltamethrin (25 g/litre) were
sprayed onto cotton fields in Arizona, USA, at respective
rates of 0.11, 0.11, and 0.23 kg/ha, and dislodgeable
residues of the insecticides on cotton foliage were exam-
ined. Of the original deposits of fenvalerate, 65%
remained at the end of 96 h (there were two rains between
24 and 48 h), compared with 47% and 32% for permethrin and
deltamethrin, respectively [57].
Fenvalerate deposits on cotton plants (0.8 mg/plant)
disappeared rapidly, with only half the material remaining
after 8 days of exposure. After 23 days, decarboxy-fenval-
erate and ester-cleavage products such as PBacid, PBald,
PBalc, and CPIA were detectable, but not quantifiable.
Decarboxy-fenvalerate was considerably more stable to UV
light than fenvalerate, but it decomposed at a somewhat
faster rate than p,p'-DDT, yielding mainly the dechlori-
nated analogue [78].
The metabolism of fenvalerate in kidney bean plants
has been studied under laboratory conditions by Ohkawa et
al. [136]. Fenvalerate labelled with 14C at the cyano
group and the [2S, alphaRS] isomer labelled separately at
the cyano, carboxy, and benzylic carbon atoms were used to
treat individual bean leaves of 14-day-old seedlings at a
rate of 10 µg per leaf. After 60 days, 85-86% of the
applied 14C was recovered from plants treated with the
carboxy and benzyl labels, whereas 67% was recovered from
plants treated with the cyano label. Only limited translo-
cation was observed and only very low levels of radioac-
tive residues (2-9 µg/kg) were detected in seeds.
Fenvalerate and the [2S, alphaRS] isomer disappeared at a
similar rate from the treated leaves with an initial half-
life of 14 days.
The metabolism of racemic fenvalerate and of its
[2S, alphaS] isomer was examined in cabbage plants grown
under laboratory conditions and treated (20 µg per leaf)
with [14C]-chlorophenyl- and [phenyl-14C]-benzyl-labelled
preparations of the two compounds. Both compounds disap-
peared from the treated leaves with similar half-lives of
approximately 12-14 days. They underwent ester cleavage
to a significant extent, together with some hydroxylation
at the 2- or 4-position of the phenoxy ring and hydrolysis
of the nitrile group to amide and carboxyl groups. Most of
the carboxylic acids and phenols thus produced occurred as
glycoside conjugates. In a separate experiment, the uptake
and metabolism of CPIA (17) was examined in the laboratory
using abscised leaves of kidney bean, cabbage, cotton, cu-
cumber, and tomato plants. The acid (17) was found to be
readily converted, mainly into glucose or 6-O-malonylglu-
cose esters in kidney bean, cabbage, and cucumber, into
glucosylxylose, sophorose, and gentiobiose esters in
cotton, and into two types of triglucose esters with dif-
fering isomerism in tomato. One of the acetyl-derived
glucoside conjugates was identical with the authentic
deca-acetyl derivative of the [1-6]-triglucose ester
[121].
In studies by Ohkawa et al. [136], fenvalerate was
metabolized or degraded in bean plants via several routes.
A minor route was hydrolysis of the cyano group leading to
the formation of the amide (33) and carboxylic acid (34)
derivatives of fenvalerate. The 3-phen-oxybenzyl moiety
underwent metabolism to yield PBacid, 3-(2'-hydroxy-
phenoxy)benzoic acid (23) and PBalc, which occurred mainly
as sugar conjugates. In addition, glucoside conjugates
of alpha-carboxy-3-phenoxybenzyl alcohol (36) were detected
to a lesser extent. The presence of alpha-cyano-3-phenoxyben-
zyl alcohol (16) conjugates was inferred since PBald was
released upon treatment with beta-glucosidase. A major
metabolite of the acid moiety was CPIA (17), which also
occurred mainly as glucoside conjugates. The decarboxy
derivative (31) of fenvalerate, presumably formed by
photochemical reaction on plant foliage as discussed pre-
viously, was detected in leaf extracts. When bean plant
seedlings were planted and left for 30 days in light clay
and sandy loam soils treated with 14C-fenvalerate at
1 mg/kg, the roots retained fairly large amounts of radio-
carbon. However, only limited radiocarbon was found in
the shoots (0.02 mg/kg), pods and seeds (0.01 mg/kg), and
there was no parent compound in the shoots.
Additional studies were carried out to investigate the
fate of 3-phenoxybenzoic acid (an important metabolite of
fenvalerate and most other pyrethroids) in plants. Using
abscised leaves of cabbage, cotton, cucumber, kidney bean
and tomato plants, 14C-3-phenoxybenzoic acid was shown to
conjugate with a complex range of sugars [120].
4.4 Decomposition in Soils
The degradation of fenvalerate in soils has been
studied under various conditions (aerobic or anaerobic
conditions, laboratory or field conditions, using radioac-
tive or non-radioactive material).
Samples of 14C-fenvalerate labelled separately in the
carboxy and cyano groups were used for soil studies by
Ohkawa et al. [133]. When several types of soil were
treated at a rate of 1 mg/kg and stored at 25°C under
aerobic conditions, the initial half-life of fenvalerate
ranged from 15 days to 3 months. As with other
pyrethroids, hydrolysis at the ester linkage was a major
degradation route, and ring hydroxylation in the 4'-pos-
ition (25), together with hydrolysis of the cyano group to
the amide and carboxyl groups, occurred to smaller ex-
tents. The degradation route unique to fenvalerate was
ether-bond cleavage yielding alpha-cyano-3-hydroxybenzyl-
1-2-(4-chlorophenyl)-3-methylbutyrate (14), which could be
produced through hydroxylation at the 2'7-position (13) in
the alcohol moiety. No H14CN released during ester-bond
cleavage was detected owing to its rapid conversion to
14CO2. The amount of 14CO2 was greater with the
cyano label than the carboxy label. For example, after
30 days in Katano sandy loam soil, 47.5% and 38.2% of the
applied radiolabel was evolved as 14CO2 from the cyano
and carboxyl groups, respectively. In a laboratory soil-
leaching study, less than 1% of the applied radiocarbon
appeared in the effluent when leaching was started
immediately after treatment of the soil. Even after a
30-day incubation, only a trace amount of CPIA (17) was
detected in the effluent from soil columns treated with
14C-carbonyl-fenvalerate. In a separate experiment, the
degradation of 14C-fenvalerate was studied in a soil-
nutrient liquid suspension system. Separate cultures of
bacteria and fungi were used for the system. After 2 weeks
of incubation, larger amounts of 14CO2 (35-42% of the
applied radiolabel) were formed from both culture media
when the 14CN-labelled compound was used than when the
14CO-labelled compound was used (1.1-2.3%). In the
latter case, the main degradation product was CPIA, which
amounted to 34-69% [133].
Studies using 14C-fenvalerate, labelled separately in
the chlorophenyl and benzyl groups, confirmed the degra-
dation pathways mentioned above. These studies also showed
that the labelled aromatic rings were also readily de-
graded to 14CO2 (up to 66%). In addition, it was found
that any "bound residues" formed could be further de-
graded to 14CO2 by admixture with fresh soils [119].
The rate of degradation of the individual isomers of
fenvalerate has been investigated. In one soil, the half-
lives of the RR, RS, SR, and SS isomers were shown to be
178, 89, 155, and 108 days, respectively [105]. Different
rates for the various isomers were similarly obtained in
loam and sandy loam soils [160].
Under flooded conditions fenvalerate degrades more
slowly than under aerobic conditions. In sterile soil,
degradation is minimal, indicating that microbial activity
is the major cause of this degradation [102]. Ohkawa et
al. [133] reported similar findings.
Studies in which crops were sown in soils containing
aged residues of 14C-fenvalerate (aging periods of 30,
120, and 345 days) showed that residues from fenvalerate
should not carry over into rotated crops [102].
The persistence of fenvalerate in Lethbridge (Canada)
soil has been studied under field and laboratory con-
ditions [74]. Formulated fenvalerate (30% emulsifiable
concentrate) was applied once to soil microplots in the
field at a dose rate of 600 µg/plot (150 g/ha) or to
soil in pots at a dose of 88.7 µg/pot (10 g/ha). The
treated pots were maintained at a daily temperature regime
of 20°C for 16 h and 10°C for 8 h in the environmental
chamber. Fenvalerate was found mainly in the top 2.5 cm
of the field soil, and 16 weeks later, 15% of the applied
fenvalerate remained. The initial half-lives were 5.9
weeks for the [2S, alphaR] [2R, alphaS] enantiomeric pair
and 6 weeks for the [2R, alphaR] [2S, alphaS] pair. The
spring soil samples, taken 45 weeks after application, con-
tained 11% of the total fenvalerate. Limited degradation
occurred during the winter. The degradation of fenvalerate
in soil incubated in the environmental chamber was similar
to the field results. The [2S, alphaR] [2R, alphaS] enant-
iomeric pair had a half-life of 5 weeks while the [2R,
alphaR] [2S, alphaS] pair had a half-life of 5.3 weeks.
These results were comparable to the average half-life of
7 weeks for fenvalerate incubated in British Columbia soils
[198].
In studies by Harris et al. [69], the persistence of
fenvalerate in subtropical field soil (average soil tem-
perature, 20-30°C) was investigated after applying 20%
emulsifiable concentrate at a rate of 1 kg active ingredi-
ent (ai)/ha twice a year (spring and autumn) over a 2.5-
year period. Residues in the top 15 cm of soil were moni-
tored for up to one year after the final application.
Fenvalerate levels declined rapidly after the spring
application and relatively slowly after the autumn appli-
cation. There was no carry over of the insecticide from
year to year, and after 2.5 years of application only 2%
of the total fenvalerate remained. The rate of disappear-
ance became slightly slower when fenvalerate application
ceased [181]. The degradation of fenvalerate (14.9 mg/kg)
in plainfield sand (5% moisture) at 25°C was relatively
slow, with an initial half-life of 2 months, as compared
with initial half-lives of 0.5, 1, and 2 months for
fenpropathrin (7.1 mg/kg), permethrin (8.8 mg/kg), and
cypermethrin (7.3 mg/kg), respectively, under laboratory
conditions.
A 2-year field study on the relative persistence of
permethrin, cypermethrin, fenpropathrin, and fenvalerate
in soils was carried out by Chapman & Harris [25]. The
pyrethroids were applied as emulsifiable concentrates at a
rate of 280 g ai/ha or 140 g ai/ha to duplicate plots in
Ontario, Canada, containing either sand or organic soil.
For plots treated at the higher rate, the insecticide was
immediately raked into the soil, while the plots receiving
the lower rate were left undisturbed and the upper 4-5 cm
of soil was subjected to gas-liquid chromatography (GLC)
analyses. The concentrations of the four pyrethroids
incorporated in both soils or remaining on the upper soil
layer decreased to less than 50% of the initial values
within one month. Again, fenvalerate was slightly more
persistent, with 7% of the initial application remaining
in organic soil 28 months after treatment.
Reed et al. [157] demonstrated that when fenvalerate
was applied to soil, adsorption prevented significant
leaching of the pesticide. Soil metabolites produced
either by photolytic or microbial degradation did not
accumulate to a significant level or present a problem in
subsequent rotation crops (lettuce, beets, and wheat)
planted at 30 days, 60 days, 120 days, or 1 year after
soil treatment. Although fenvalerate has intrinsically
high toxicity to a variety of aquatic organisms, these
field studies demonstrated that the toxicant was unavail-
able to non-target organisms. Therefore, it had little or
no impact in this test system following its use at the
maximum allowed rate of 2.24 kg/ha per year.
4.5 Decomposition in Water
The hydrolysis of racemic fenvalerate in buffered
aqueous solutions at pH 5.0, 7.0, and 9.0 was compared by
Katagi et al. [90] with that of the [2S, alphaS] isomer.
Both compounds were fairly stable at pH 5.0 and 7.0 (half-
lives of 130-220 days), while at pH 9.0 they underwent
hydrolysis (half-lives of 64.6-67.2 days) mainly via ester
bond cleavage. The main product was 2-(4-chlorophenyl)-3-
methylbutyric acid (17), which amounted to 14.9% of the
applied 14C after 28 days. As the [2S, alphaS] isomer
underwent alphaS/alphaR epimerization in the alcohol moiety
at pH 7.0 and 9.0, its rate of hydrolysis appeared to be
rather faster than that of fenvalerate. However, the half-
life estimated from the total amounts of [2S, alphaS] and
[2S, alphaR] epimer was close to that of fenvalerate, which
indicates no significant difference in hydrolysis rate.
The persistence of fenvalerate has been evaluated
in water and sediment contained in open trenches
(3 m x 1 m x 30 cm) lined with alkathene sheet [1]. Insec-
ticide emulsion was sprayed on the surface of the water at
the normal rate and at twice the recommended dosage. The
dissipation of the insecticide from water was rapid. About
74-80% of the pesticide was lost within 24 h at both ap-
plication rates. However, residues were found to be ad-
sorbed onto sediment, and these persisted beyond 30 days.
In soil, persistence was moderate, lasting around
30 days.
5. KINETICS AND METABOLISM
Appraisal
The metabolic fate of fenvalerate in rats, mice, and cows
has been studied using variously labelled racemic fenvalerate
(acid moiety or benzyl or cyano groups labelled).
From oral administration studies, fenvalerate appears to be
absorbed rapidly through the gastrointestinal wall.
Following a single oral administration of labelled fenval-
erate to rats, the excretion of radiocarbon from the acid or
benzyl moieties was fairly rapid. However, the excretion of
radiocarbon originating from the cyano group was relatively
slow, the rest of the radioactivity being retained in various
tissues, particularly in hair and stomach as thiocyanate. The
major routes of metabolism were ester cleavage, hydroxylation
at the 4'position of the alcohol moiety, and thiocyanate
formation from the cyano group. Major metabolites were 2-(4-
chlorophenyl)isovaleric acid (Cl-Vacid) and 3-OH-Cl-Vacid (Cl-
Vacid hydroxylated at the 3 position) from the acid moiety, and
the sulfate conjugate of 3-(4'-hydroxyphenyl)benzoic acid and
thiocyanate from the alcohol moiety. A lipophilic metabolite,
cholesteryl-[2R]-2-(4-chlorophenyl)isovalerate, which was related
to granuloma formation, was detected in the adrenals, liver,
and the mesenteric lymph nodes of rats, mice, and some other
species. The excretion of fenvalerate in the milk from orally
dosed cows was very low (0.44-0.64% of the total dose).
The metabolic fate of fenvalerate in mammals is sum-
marized in Fig. 3.
5.1 Metabolism in Mammals
5.1.1 Rat
Following the single oral administration of fenval-
erate, labelled with 14C in the carbonyl of the acid
moiety (14CO) and the benzylic carbon (14Calpha), to
male rats (7-30 mg/kg body weight), the radiocarbon from
the acid and alcohol moieties was rapidly and completely
excreted [86, 134]. The tissue residues were generally
very low, except for those in the fat. The total recovery
of 14C in urine, faeces, and expired air was 93-99% in
6 days. However, on dosing with 14CN-labelled fenval-
erate, the radiocarbon derived from the CN group was
excreted relatively slowly into the urine and faeces, and
a considerable amount (10%) of the radiocarbon was also
excreted as CO2. Total recovery of 14C in urine,
faeces, and expired air was 75-81% in 6 days in this case.
The tissue residue levels were generally higher than those
from the acid and alcohol moieties. Hair, skin, and
stomach contents showed high residue levels, due to reten-
tion as 14C-thiocyanate. These excretion and tissue resi-
due patterns for the radiocarbon from the CN group were
similar to those with 14C dosed as KCN and KSCN in male
rats [134].
4.1 Transportation and Distribution Between Media
Hill [74] investigated the distribution of fenvalerate
residues in soil under field conditions using a microplot
technique. The microplots (20 x 20 cm) were treated with
fenvalerate at a rate of 150 g/ha. After 45 weeks, 11% of
the applied fenvalerate was located in the 0-2.5 cm soil
layer and less than 0.5% in the 2.5-5 cm soil samples.
Less than 0.1% of the applied fenvalerate was detected in
any of the soil samples taken after 3 or 4 weeks, despite
a rainfall of 95.4 mm during the first 4 weeks (including
a 25.9 mm downpour 15 days after application). These
results indicate that fenvalerate does not readily leach
downward and that lateral surface movement is very
limited.
A similar conclusion was obtained from laboratory
soil-leaching studies. More than 95% of the applied
fenvalerate remained in the treated portion of soil
columns when leaching was started immediately or 30 days
after treatment of the soil [133]. The possibility of
fenvalerate accumulating in orchard soils was assessed by
monitoring soil and leaf litter in an orchard in the
Okanagan valley, British Columbia, Canada, following
multiple annual application of the pesticide. Belmark 300
(30% fenvalerate EC formulation) had been sprayed at a
rate of 188-500 ml/ha (one to three times per year) for
more than three years. To obtain initial concentration,
organic litter samples were sprayed at a rate of 450 ml/ha
and samples were collected 2 h later. While the initial
concentration thus obtained in the litter was 0.214 mg/kg
(average value), orchard litter samples contained
0.30-0.63 mg/kg while samples from a non-treated block
contained < 0.002 mg/kg. Orchard soil samples (0-15 cm
depth) in two orchards contained fenvalerate residues of
< 0.0035-0.006 mg/kg and 0.0063-0.024 mg/kg [188].
4.2 Photodecompositiona
In studies by Holmstead et al. [78], fenvalerate (5),
at a concentration of 0.01 mol/litre in methanol, hexane,
or acetonitrilewater (60:40), underwent rapid photodegra-
dation under the action of UV light (290-320 nm) with a
half-life of 16-18 min (Fig. 2). With 90-95% conversion
after 60 min, 2-(3-phenoxyphenyl)-3-(4-chlorophenyl)-4-
methyl-pentanenitrile (31) (decarboxy-fenvalerate) was the
major photoproduct, amounting to 54-57% of the total reac-
tion mixture. There were smaller amounts of the dechlori-
nated analogue (32) of decarboxy-fenvalerate and the dimer
(8) of 2,2-dimethyl-4-chlorostyrene. 3-Phenoxybenzoyl cy-
anide (4), 3-phenoxybenzaldehyde (18) (PBald), 4-chloro-
isobutylbenzene (6), and 2,2-dimethyl-4-chlorostyrene (7)
were detected in small amounts in hexane or methanol. 3-
Phenoxybenzyl cyanide (28), its dimer (26), and 1,2-bis-
(phenoxyphenyl)ethane (27) were found only in hexane, and
methyl 3-phenoxybenzoate (12) was detected only in meth-
anol. Products found uniquely in acetonitrile-water were
2-(4-chlorophenyl)-3-methylbutyric acid (17) (CPIA), 3-
phenoxybenzoic acid (22) (PBacid) and 1-(4-chlorophenyl)-
2-methylpropanol (9). Several unknown compounds were ob-
served in the remaining 5-10%. Fenvalerate, in a thin film
(1 mg/cm2) on glass, decomposed in sunlight with a half-
life of approximately 4 days. About 10% of the applied
material remained after 43 days. In addition to the photo-
products formed in solution, small amounts of 3-phenoxy-
benzyl alcohol (19) (PBalc) and isopropyl 4-chlorophenyl-
ketone (10) were detected.
On exposure to autumn sunlight in Japan, the
[2S, alphaS] isomer of fenvalerate in distilled water
decomposed with a half-life of approximate 10 days. This
isomer photodecomposed via pathways that included decar-
boxylation, hydration of the CN group to a carbamoyl
(CONH2) group, hydrolysis of the CONH2 group to a car-
boxyl (COOH) group, and cleavage of the ester or diphenyl
ether linkage. Cleavage of the ester linkage was a major
photochemical reaction and led to the formation of 2-(4-
chlorophenyl)-3-methylbutyric acid (17) (17.3% of the
applied 14C, 10 days after exposure). There was no sig-
nificant difference between fenvalerate and the [2S, alphaS]
isomer in the rates and routes of photodegradation [179].
The photodegradation of fenvalerate (0.3-0.4 ng/cm2)
on two kinds of soil in natural sunlight was compared with
that of the [2S, alphaS] isomer. Fenvalerate and its isomer
photodecomposed with half-lives of 1.4-2.4 days and 1.1-
2.5 days, respectively. The pathways included hydration
of the cyano group to the carbamoyl group (19.2-48.4% at
10 days) with subsequent hydrolysis to the carboxyl group
(0.9-2.0% at 10 days), ester-bond cleavage (3.4-4.5% at 10
days), and decarboxylation (0.3-0.9% at 10 days). Little
2S/2R and alphaS/alphaR isomerization (as determined by
HPLC) occurred on the soils. There was no significant
difference between the two compounds in the rates and
pathways of photodegradation [89].
Holmstead & Fullmer [77] investigated the photodecar-
boxylation of several cyanohydrin esters in methanol and
hexane under artificial light as models for pyrethroid
photodecomposition. The cyanohydrin esters gave rise to
decarboxylated products, to a greater or lesser extent,
whereas the analogous compounds without the cyano group
did not produce the photodecarboxylated compounds.
alpha-Cyanobenzyl phenylacetate, which yielded the stable
benzyl radical, gave substantially larger amounts of the
decarboxylated product than alpha-cyanobenzyl benzoate,
which produced the unstable phenyl radical.
The photodegradation of fenvalerate in water and on
soil was investigated using compounds labelled with 14C
at the following positions: carbonyl group (CO-fenval-
----------------------------------------------------------------
a The numbers in brackets following chemical names refer to the
numbers given in Fig. 2.
erate), alpha-carbon in the benzyl group (Calpha-fenvale-
rate), and cyano group (CN-fenvalerate) [118]. On exposure
to sunlight, fenvalerate in very dilute solution in distil-
led water, in 2% aqueous acetone, in filter-sterilized
river water, or in sea water underwent rapid photolysis
with half-lives of approximately 4 days in summer and 13-
15 days in winter. The quantum yield was calculated at
6.6 x 10-3 (at 313 nm in water) and the half-life of dis-
appearance at latitude 40°N was calculated at 4.1 days in
summer and 12.4 days in winter, values which were close to
the experimental ones. Photodegradation of 14CN-fenval-
erate resulted in the formation of greater amounts of
14CO2 than 14CN-. After 6 weeks irradiation, ap-
proximately 30% (in aqueous acetone or river water) or
approximately 55-60% (in distilled water or sea water) of
the 14C was recovered as 14CO2, while the correspond-
ing figures for 14CN- were 5% and 30%. One of the major
photodegradation products was decarboxy-fenvalerate (31),
which increased to approximately 20% (in distilled water)
in summer after 1 week and decreased thereafter. In win-
ter, the amount was approximately 20% after 6 weeks. Other
major products were PBacid (22) and CPIA (17), derived
from the ester bond cleavage, amounting to 43% and 58%,
respectively, of the applied radioactivity after 6 weeks
in winter. In addition, small amounts of alpha-carbamoyl-
3-phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (33)
(CONH2-fenvalerate), alpha-carboxy-3-phenoxybenzyl-2-(4-
chlorophenyl)-3-methylbutyrate (34) (COOH-fenvalerate), 3-
phenoxybenzyl cyanide (28), 3-phenoxyphenylacetamide (29),
3-phenoxyphenylacetic acid (30), PBalc, and PBald were
detected.
Fenvalerate, as a deposit (5.5-5.9 µg/100 cm2) on
Kodaira light clay, Azuchi sandy clay loam, and Katano
sandy loam soil from Japan, was decomposed by autumn sun-
light with the respective half-lives of 2, 6, and 18 days
[118]. The major product was CONH2-fenvalerate (33),
which amounted to 7.9-25.7% of the applied radioactivity
after 10 days; it was formed in greatest amounts in sun-
light but also formed in the dark. Smaller amounts of
decarboxy-fenvalerate (31), the desphenyl analogue of
CONH2-fenvalerate (35), COOH-fenvalerate (34), PBacid,
and PBalc were also detected. Of the applied radiocarbon,
3-10% remained unidentified.
4.3 Decomposition in Plants
Fenvalerate (2.4% emulsifiable concentrate (EC)),
permethrin (2% EC), and deltamethrin (25 g/litre) were
sprayed onto cotton fields in Arizona, USA, at respective
rates of 0.11, 0.11, and 0.23 kg/ha, and dislodgeable
residues of the insecticides on cotton foliage were exam-
ined. Of the original deposits of fenvalerate, 65%
remained at the end of 96 h (there were two rains between
24 and 48 h), compared with 47% and 32% for permethrin and
deltamethrin, respectively [57].
Fenvalerate deposits on cotton plants (0.8 mg/plant)
disappeared rapidly, with only half the material remaining
after 8 days of exposure. After 23 days, decarboxy-fenval-
erate and ester-cleavage products such as PBacid, PBald,
PBalc, and CPIA were detectable, but not quantifiable.
Decarboxy-fenvalerate was considerably more stable to UV
light than fenvalerate, but it decomposed at a somewhat
faster rate than p,p'-DDT, yielding mainly the dechlori-
nated analogue [78].
The metabolism of fenvalerate in kidney bean plants
has been studied under laboratory conditions by Ohkawa et
al. [136]. Fenvalerate labelled with 14C at the cyano
group and the [2S, alphaRS] isomer labelled separately at
the cyano, carboxy, and benzylic carbon atoms were used to
treat individual bean leaves of 14-day-old seedlings at a
rate of 10 µg per leaf. After 60 days, 85-86% of the
applied 14C was recovered from plants treated with the
carboxy and benzyl labels, whereas 67% was recovered from
plants treated with the cyano label. Only limited translo-
cation was observed and only very low levels of radioac-
tive residues (2-9 µg/kg) were detected in seeds.
Fenvalerate and the [2S, alphaRS] isomer disappeared at a
similar rate from the treated leaves with an initial half-
life of 14 days.
The metabolism of racemic fenvalerate and of its
[2S, alphaS] isomer was examined in cabbage plants grown
under laboratory conditions and treated (20 µg per leaf)
with [14C]-chlorophenyl- and [phenyl-14C]-benzyl-labelled
preparations of the two compounds. Both compounds disap-
peared from the treated leaves with similar half-lives of
approximately 12-14 days. They underwent ester cleavage
to a significant extent, together with some hydroxylation
at the 2- or 4-position of the phenoxy ring and hydrolysis
of the nitrile group to amide and carboxyl groups. Most of
the carboxylic acids and phenols thus produced occurred as
glycoside conjugates. In a separate experiment, the uptake
and metabolism of CPIA (17) was examined in the laboratory
using abscised leaves of kidney bean, cabbage, cotton, cu-
cumber, and tomato plants. The acid (17) was found to be
readily converted, mainly into glucose or 6-O-malonylglu-
cose esters in kidney bean, cabbage, and cucumber, into
glucosylxylose, sophorose, and gentiobiose esters in
cotton, and into two types of triglucose esters with dif-
fering isomerism in tomato. One of the acetyl-derived
glucoside conjugates was identical with the authentic
deca-acetyl derivative of the [1-6]-triglucose ester
[121].
In studies by Ohkawa et al. [136], fenvalerate was
metabolized or degraded in bean plants via several routes.
A minor route was hydrolysis of the cyano group leading to
the formation of the amide (33) and carboxylic acid (34)
derivatives of fenvalerate. The 3-phen-oxybenzyl moiety
underwent metabolism to yield PBacid, 3-(2'-hydroxy-
phenoxy)benzoic acid (23) and PBalc, which occurred mainly
as sugar conjugates. In addition, glucoside conjugates
of alpha-carboxy-3-phenoxybenzyl alcohol (36) were detected
to a lesser extent. The presence of alpha-cyano-3-phenoxyben-
zyl alcohol (16) conjugates was inferred since PBald was
released upon treatment with beta-glucosidase. A major
metabolite of the acid moiety was CPIA (17), which also
occurred mainly as glucoside conjugates. The decarboxy
derivative (31) of fenvalerate, presumably formed by
photochemical reaction on plant foliage as discussed pre-
viously, was detected in leaf extracts. When bean plant
seedlings were planted and left for 30 days in light clay
and sandy loam soils treated with 14C-fenvalerate at
1 mg/kg, the roots retained fairly large amounts of radio-
carbon. However, only limited radiocarbon was found in
the shoots (0.02 mg/kg), pods and seeds (0.01 mg/kg), and
there was no parent compound in the shoots.
Additional studies were carried out to investigate the
fate of 3-phenoxybenzoic acid (an important metabolite of
fenvalerate and most other pyrethroids) in plants. Using
abscised leaves of cabbage, cotton, cucumber, kidney bean
and tomato plants, 14C-3-phenoxybenzoic acid was shown to
conjugate with a complex range of sugars [120].
4.4 Decomposition in Soils
The degradation of fenvalerate in soils has been
studied under various conditions (aerobic or anaerobic
conditions, laboratory or field conditions, using radioac-
tive or non-radioactive material).
Samples of 14C-fenvalerate labelled separately in the
carboxy and cyano groups were used for soil studies by
Ohkawa et al. [133]. When several types of soil were
treated at a rate of 1 mg/kg and stored at 25°C under
aerobic conditions, the initial half-life of fenvalerate
ranged from 15 days to 3 months. As with other
pyrethroids, hydrolysis at the ester linkage was a major
degradation route, and ring hydroxylation in the 4'-pos-
ition (25), together with hydrolysis of the cyano group to
the amide and carboxyl groups, occurred to smaller ex-
tents. The degradation route unique to fenvalerate was
ether-bond cleavage yielding alpha-cyano-3-hydroxybenzyl-
1-2-(4-chlorophenyl)-3-methylbutyrate (14), which could be
produced through hydroxylation at the 2'7-position (13) in
the alcohol moiety. No H14CN released during ester-bond
cleavage was detected owing to its rapid conversion to
14CO2. The amount of 14CO2 was greater with the
cyano label than the carboxy label. For example, after
30 days in Katano sandy loam soil, 47.5% and 38.2% of the
applied radiolabel was evolved as 14CO2 from the cyano
and carboxyl groups, respectively. In a laboratory soil-
leaching study, less than 1% of the applied radiocarbon
appeared in the effluent when leaching was started
immediately after treatment of the soil. Even after a
30-day incubation, only a trace amount of CPIA (17) was
detected in the effluent from soil columns treated with
14C-carbonyl-fenvalerate. In a separate experiment, the
degradation of 14C-fenvalerate was studied in a soil-
nutrient liquid suspension system. Separate cultures of
bacteria and fungi were used for the system. After 2 weeks
of incubation, larger amounts of 14CO2 (35-42% of the
applied radiolabel) were formed from both culture media
when the 14CN-labelled compound was used than when the
14CO-labelled compound was used (1.1-2.3%). In the
latter case, the main degradation product was CPIA, which
amounted to 34-69% [133].
Studies using 14C-fenvalerate, labelled separately in
the chlorophenyl and benzyl groups, confirmed the degra-
dation pathways mentioned above. These studies also showed
that the labelled aromatic rings were also readily de-
graded to 14CO2 (up to 66%). In addition, it was found
that any "bound residues" formed could be further de-
graded to 14CO2 by admixture with fresh soils [119].
The rate of degradation of the individual isomers of
fenvalerate has been investigated. In one soil, the half-
lives of the RR, RS, SR, and SS isomers were shown to be
178, 89, 155, and 108 days, respectively [105]. Different
rates for the various isomers were similarly obtained in
loam and sandy loam soils [160].
Under flooded conditions fenvalerate degrades more
slowly than under aerobic conditions. In sterile soil,
degradation is minimal, indicating that microbial activity
is the major cause of this degradation [102]. Ohkawa et
al. [133] reported similar findings.
Studies in which crops were sown in soils containing
aged residues of 14C-fenvalerate (aging periods of 30,
120, and 345 days) showed that residues from fenvalerate
should not carry over into rotated crops [102].
The persistence of fenvalerate in Lethbridge (Canada)
soil has been studied under field and laboratory con-
ditions [74]. Formulated fenvalerate (30% emulsifiable
concentrate) was applied once to soil microplots in the
field at a dose rate of 600 µg/plot (150 g/ha) or to
soil in pots at a dose of 88.7 µg/pot (10 g/ha). The
treated pots were maintained at a daily temperature regime
of 20°C for 16 h and 10°C for 8 h in the environmental
chamber. Fenvalerate was found mainly in the top 2.5 cm
of the field soil, and 16 weeks later, 15% of the applied
fenvalerate remained. The initial half-lives were 5.9
weeks for the [2S, alphaR] [2R, alphaS] enantiomeric pair
and 6 weeks for the [2R, alphaR] [2S, alphaS] pair. The
spring soil samples, taken 45 weeks after application, con-
tained 11% of the total fenvalerate. Limited degradation
occurred during the winter. The degradation of fenvalerate
in soil incubated in the environmental chamber was similar
to the field results. The [2S, alphaR] [2R, alphaS] enant-
iomeric pair had a half-life of 5 weeks while the [2R,
alphaR] [2S, alphaS] pair had a half-life of 5.3 weeks.
These results were comparable to the average half-life of
7 weeks for fenvalerate incubated in British Columbia soils
[198].
In studies by Harris et al. [69], the persistence of
fenvalerate in subtropical field soil (average soil tem-
perature, 20-30°C) was investigated after applying 20%
emulsifiable concentrate at a rate of 1 kg active ingredi-
ent (ai)/ha twice a year (spring and autumn) over a 2.5-
year period. Residues in the top 15 cm of soil were moni-
tored for up to one year after the final application.
Fenvalerate levels declined rapidly after the spring
application and relatively slowly after the autumn appli-
cation. There was no carry over of the insecticide from
year to year, and after 2.5 years of application only 2%
of the total fenvalerate remained. The rate of disappear-
ance became slightly slower when fenvalerate application
ceased [181]. The degradation of fenvalerate (14.9 mg/kg)
in plainfield sand (5% moisture) at 25°C was relatively
slow, with an initial half-life of 2 months, as compared
with initial half-lives of 0.5, 1, and 2 months for
fenpropathrin (7.1 mg/kg), permethrin (8.8 mg/kg), and
cypermethrin (7.3 mg/kg), respectively, under laboratory
conditions.
A 2-year field study on the relative persistence of
permethrin, cypermethrin, fenpropathrin, and fenvalerate
in soils was carried out by Chapman & Harris [25]. The
pyrethroids were applied as emulsifiable concentrates at a
rate of 280 g ai/ha or 140 g ai/ha to duplicate plots in
Ontario, Canada, containing either sand or organic soil.
For plots treated at the higher rate, the insecticide was
immediately raked into the soil, while the plots receiving
the lower rate were left undisturbed and the upper 4-5 cm
of soil was subjected to gas-liquid chromatography (GLC)
analyses. The concentrations of the four pyrethroids
incorporated in both soils or remaining on the upper soil
layer decreased to less than 50% of the initial values
within one month. Again, fenvalerate was slightly more
persistent, with 7% of the initial application remaining
in organic soil 28 months after treatment.
Reed et al. [157] demonstrated that when fenvalerate
was applied to soil, adsorption prevented significant
leaching of the pesticide. Soil metabolites produced
either by photolytic or microbial degradation did not
accumulate to a significant level or present a problem in
subsequent rotation crops (lettuce, beets, and wheat)
planted at 30 days, 60 days, 120 days, or 1 year after
soil treatment. Although fenvalerate has intrinsically
high toxicity to a variety of aquatic organisms, these
field studies demonstrated that the toxicant was unavail-
able to non-target organisms. Therefore, it had little or
no impact in this test system following its use at the
maximum allowed rate of 2.24 kg/ha per year.
4.5 Decomposition in Water
The hydrolysis of racemic fenvalerate in buffered
aqueous solutions at pH 5.0, 7.0, and 9.0 was compared by
Katagi et al. [90] with that of the [2S, alphaS] isomer.
Both compounds were fairly stable at pH 5.0 and 7.0 (half-
lives of 130-220 days), while at pH 9.0 they underwent
hydrolysis (half-lives of 64.6-67.2 days) mainly via ester
bond cleavage. The main product was 2-(4-chlorophenyl)-3-
methylbutyric acid (17), which amounted to 14.9% of the
applied 14C after 28 days. As the [2S, alphaS] isomer
underwent alphaS/alphaR epimerization in the alcohol moiety
at pH 7.0 and 9.0, its rate of hydrolysis appeared to be
rather faster than that of fenvalerate. However, the half-
life estimated from the total amounts of [2S, alphaS] and
[2S, alphaR] epimer was close to that of fenvalerate, which
indicates no significant difference in hydrolysis rate.
The persistence of fenvalerate has been evaluated
in water and sediment contained in open trenches
(3 m x 1 m x 30 cm) lined with alkathene sheet [1]. Insec-
ticide emulsion was sprayed on the surface of the water at
the normal rate and at twice the recommended dosage. The
dissipation of the insecticide from water was rapid. About
74-80% of the pesticide was lost within 24 h at both ap-
plication rates. However, residues were found to be ad-
sorbed onto sediment, and these persisted beyond 30 days.
In soil, persistence was moderate, lasting around
30 days.
5. KINETICS AND METABOLISM
Appraisal
The metabolic fate of fenvalerate in rats, mice, and cows
has been studied using variously labelled racemic fenvalerate
(acid moiety or benzyl or cyano groups labelled).
From oral administration studies, fenvalerate appears to be
absorbed rapidly through the gastrointestinal wall.
Following a single oral administration of labelled fenval-
erate to rats, the excretion of radiocarbon from the acid or
benzyl moieties was fairly rapid. However, the excretion of
radiocarbon originating from the cyano group was relatively
slow, the rest of the radioactivity being retained in various
tissues, particularly in hair and stomach as thiocyanate. The
major routes of metabolism were ester cleavage, hydroxylation
at the 4'position of the alcohol moiety, and thiocyanate
formation from the cyano group. Major metabolites were 2-(4-
chlorophenyl)isovaleric acid (Cl-Vacid) and 3-OH-Cl-Vacid (Cl-
Vacid hydroxylated at the 3 position) from the acid moiety, and
the sulfate conjugate of 3-(4'-hydroxyphenyl)benzoic acid and
thiocyanate from the alcohol moiety. A lipophilic metabolite,
cholesteryl-[2R]-2-(4-chlorophenyl)isovalerate, which was related
to granuloma formation, was detected in the adrenals, liver,
and the mesenteric lymph nodes of rats, mice, and some other
species. The excretion of fenvalerate in the milk from orally
dosed cows was very low (0.44-0.64% of the total dose).
The metabolic fate of fenvalerate in mammals is sum-
marized in Fig. 3.
5.1 Metabolism in Mammals
5.1.1 Rat
Following the single oral administration of fenval-
erate, labelled with 14C in the carbonyl of the acid
moiety (14CO) and the benzylic carbon (14Calpha), to
male rats (7-30 mg/kg body weight), the radiocarbon from
the acid and alcohol moieties was rapidly and completely
excreted [86, 134]. The tissue residues were generally
very low, except for those in the fat. The total recovery
of 14C in urine, faeces, and expired air was 93-99% in
6 days. However, on dosing with 14CN-labelled fenval-
erate, the radiocarbon derived from the CN group was
excreted relatively slowly into the urine and faeces, and
a considerable amount (10%) of the radiocarbon was also
excreted as CO2. Total recovery of 14C in urine,
faeces, and expired air was 75-81% in 6 days in this case.
The tissue residue levels were generally higher than those
from the acid and alcohol moieties. Hair, skin, and
stomach contents showed high residue levels, due to reten-
tion as 14C-thiocyanate. These excretion and tissue resi-
due patterns for the radiocarbon from the CN group were
similar to those with 14C dosed as KCN and KSCN in male
rats [134].
 It was shown in the same study that fenvalerate under-
went oxidation at the 2'and 4'positions of the alcohol
moiety, as well as at the 2 and 3 positions of the acid
moiety, ester cleavage, and the conjugation of resultant
phenols and carboxylic acids with glucuronic acid, sul-
furic acid, and glycine. Cleavage of fenvalerate and its
ester metabolites appeared to release cyanohydrins, which
were, however, unstable under physiological conditions and
decomposed easily to cyanide and aldehydes (Fig. 3). The
cyanide ion was converted mainly to thiocyanate and
CO2, and 2-iminothiazolidine-4-carboxylic acid, a metab-
olite detected with other pyrethroids containing a cyanide
group, was not positively identified [134]. The major fae-
cal metabolites from 14CO-, 14Calpha-, and 14CN-fenval-
erate were unchanged fenvalerate (5) and two ester metab-
olites of 2'-hydroxy-(13) and 4'-hydroxy-fenvalerate (25).
The major metabolites in 0- to 2-day pooled urine (50-55%
of the dosed radioactivity) from the acid-labelling were
2-(4-chlorophenyl)isovaleric acid (17) (Cl-Vacid), 2-(4-
chlorophenyl)-3-hydroxymethylbutyric acid (37) (3-OH-Cl-
Vacid), and its lactone (38) (3-OH-Cl-Vacid-lactone).
Other minor metabolites were 2-(4-chlorophenyl)-2-hydroxy-
3-hydroxymethylbutyric acid (39) (2,3-OH-Cl-Vacid) in the
free, the lactone (40) (2,3-OH-Cl-Vacid-lactone), and the
conjugated forms, 2-(4-chlorophenyl)- cis-2-butenedioic
acid anhydride (41) (Cl-BDacid anhydride), and 2-(4-
chlorophenyl)-3-methyl-2-butene-4-olide (42) (Cl-B-acid-
lactone). On the other hand, the predominant urinary
metabolite from the alcohol moiety was the sulfate conju-
gate of 3-(4'-hydroxyphenoxy)benzoic acid (23) (4'-OH-
PBacid), accounting for approximately 40% of the dose.
Other major metabolites were 3-phenoxybenzoic acid (22)
(PBacid) in the free (6%), the glucuronide (2%), and the
glycine (2%) conjugated forms, 4'-OH-PBacid in the free
(5%) and the glucuronide (2%) forms, and the sulfate of 3-
(2'-hydroxyphenoxy)benzoic acid (24) (2'-OH-PBacid) (3%).
With 14CN-fenvalerate, the major urinary metabolite was
thiocyanate (43) [134].
Pydrin insecticide (Y-rich) is an isomerically
enriched form of fenvalerate containing an excess ratio of
the active diastereomers SS and RR (designated Y) over the
less active diastereomers RS and SR (designated X) at a
ratio of approximately 85:15. Fenvalerate contains Y:X in
a ratio of 45:55. Following a single oral dose of the Y-
rich insecticide (8.4 mg/kg) to male and female Sprague-
Dawley rats, more than 90% of the administered radioac-
tivity from the acid moiety (chlorophenyl-14C) and the
alcohol moiety (phenoxyphenyl-14C) was eliminated within
the first 24 h. There was no major difference between the
two different fenvalerate preparations in either the elim-
ination rate or the metabolites distribution profile.
Cleavage of the ester linkage was the primary metabolic
pathway. The acid and alcohol portions of the parent mole-
cule underwent hydroxylation, oxidation, and conjugation.
These metabolic reactions were not dependent on the iso-
meric composition of the test material. Tissue residue
data showed that 14C residues were not retained in the
various organs [104].
The fate of sugar conjugates, which may be formed as
plant metabolites, has been investigated by Mikami et al.
[122]. Upon single oral administration to male Sprague-
Dawley rats at a concentration of 3.8 mg/kg, the mono-,
di-, and tri-glucose conjugates of [14C]-3-phenoxybenzyl
alcohol (19) and the mono-glucose conjugate of [14C]-3-
phenoxybenzoic acid (22) were rapidly hydrolysed and
extensively eliminated in the urine, mostly as the sulfate
conjugate of 3-(4-hydroxyphenoxy)benzoic acid (24).
Faecal elimination was a minor route, whereas biliary
excretion was responsible for about 42% of the dose, and
the glucuronide conjugates of (19), (22), and (24) were
common major metabolites. The biliary glucuronides were
metabolized in the small intestine to the respective
aglycones, which were reabsorbed, metabolized further, and
excreted in the urine as the sulfate conjugate of (24).
Although small amounts of the mono-, di-, and tri-gluco-
sides were found in the 30-min blood and liver samples
following oral administration of the tri-glucoside of
(19), they were not detected in the urine, bile, or fae-
ces. Similarly, the sulfate conjugate was one of the major
urinary metabolites in germ-free male rats, when dosed
with the 14C-glucosides at a rate of 9 µmol/kg via the
oral or intraperitoneal route, although certain amounts
were excreted unchanged in the urine and faeces. The glu-
cose conjugates were metabolized in vitro by intestinal
microflora and in various rat tissues including blood,
liver, small intestine, and small intestinal mucosa. The
tissue enzymes showed a different substrate specificity in
hydrolysing the glucosides. However, they were not metab-
olized in gastric juice, bile, pancreatic juice, or
urine.
5.1.2 Mouse
In mice, fenvalerate is metabolized in a similar way
to that in rats, but the following significant species
differences were found by Kaneko et al. [86]: (a) the
taurine conjugate of PBacid was found in mice but not in
rats; (b) 4'-OH-PBacid sulfate occurred to a greater
extent in rats than in mice; and (c) a greater amount of
thiocyanate was excreted in mice than in rats. No sig-
nificant sex differences were observed in rats and mice.
The metabolism of the stereoisomers of fenvalerate,
([2S, alphaRS] and [2S, alphaS]) was apparently similar to
that of racemic fenvalerate.
Following a single oral administration of the four
chiral isomers of [14C-chlorophenyl]-fenvalerate to
Sprague-Dawley rats and ddY mice (2.5 mg/kg body weight),
the [2R, alphaS] isomer showed, in both rats and mice, rel-
atively greater residues in the analyzed tissues (except
fat), particularly in adrenal glands, compared with the
other three isomers. Similarly, this isomer showed
higher tissue concentrations than the other isomers when
mice were fed a diet containing 500 mg/kg of the
[2S, alphaS], [2R, alphaS], or [2R, alphaR] isomers for
two weeks. The greater amount of radioactive residues from
the administration of [2R, alphaS] isomer, as compared
with those of other isomers, was explained by the
preferential formation of a lipophilic metabolite from the
[2R, alphaS] isomer found in all examined tissues, which
was not easily excreted. The amounts of the lipophilic
metabolite differed among tissues, being higher in adrenal,
liver, and mesenteric lymph nodes. This metabolite was
identified as cholesteryl [2R]-2-(4-chlorophenyl)isoval-
erate. The presence of the same metabolite was also indi-
cated in rat tissues [87].
5.1.3 Domestic animals
Two 3-month-old lambs were fed a diet containing
45 mg/kg fenvalerate for 10 days and then killed to deter-
mine the concentrations of fenvalerate in the kidney,
liver, leg muscle, and renal fat [210]. Among the analyzed
tissues, fat showed the highest fenvalerate level (3.6-4.4
mg/kg dry weight) while other tissues contained less than
0.3 mg/kg. Fenvalerate gave two gas chromatographic peaks
and each peak contained a pair of its enantiomers. In all
cases, the ratio of the areas of these peaks (peak 1
(RS,SR)/peak 2 (SS,RR)) was 1.08 both for fenvalerate in
the diet and for fenvalerate recovered from the fortified
control fat. In contrast, the fenvalerate isolated from
lamb fat had a peak area ratio of 0.76-0.78. Thus, one or
both of the first eluting enantiomers appeared to be
metabolized more rapidly than the other enantiomers.
In a study by Wszolek et al. [209], two Holstein cows
were fed fenvalerate at 5 and 15 mg/kg diet for 4 days and
were then given a clean diet for 6 days. Total excretion
of fenvalerate in milk amounted to 0.44 and 0.64% of the
total dose for the 5 and 15 mg/kg levels, respectively,
whereas about 25% of the dose was eliminated in the
faeces.
A lactating Holstein cow was fed grain fortified with
227 mg fenvalerate daily for 4 days and the urine was ana-
lysed. Intact fenvalerate was not detected in any samples
of the urine excreted by the cow during the 10-day feeding
study, nor was the acid metabolite (Cl-Vacid) (17) ident-
ified. The in vitro study on fenvalerate degradation in
rumen fluid indicated that no significant degradation of
fenvalerate was observed during the 6-h incubation [211].
Saleh et al. [161] gave a single oral dose of fenval-
erate (10 mg/kg body weight) to chickens and monitored the
persistence and distribution of the insecticide over 15
days. A concentration of 4.7 mg/litre in blood after 24 h
fell to 0.05 mg/litre after 7 days. Levels in other tis-
sues reached maxima of less than 1.0 mg/kg and fell rap-
idly. However, brain residues rose to a level of 4.0 mg/kg
over 7 days and persisted for the 15 days of the exper-
iment. Concentrations in eggs reached a maximum of
0.3 mg/kg yolk after 4 to 5 days, and a maximum of
0.24 mg/kg egg white. By day 6, levels had returned to the
pre-dosing level.
5.2 Enzymatic Systems for Biotransformation
The [2R, alphaRS] isomer of fenvalerate has been found
to be more rapidly hydrolysed by mouse liver esterase than
the [2S, alphaRS] isomer, but less rapidly metabolised than
the [2R, alphaRS] isomer with an oxidase system. A similar
correlation was observed with the [2S] and [2R] isomers of
S-5439 (3-phenoxybenzyl-2-(4-chlorophenyl)isovalerate) [165].
In an in vitro study on the metabolism of the four
chiral isomers of fenvalerate using homogenates from vari-
ous tissues of mice, rats, dogs, and monkeys, only the
[2R, alphaS] isomer yielded cholesteryl-[2R]-2-(4-chloro-
phenyl)isovalerate (CPIA-cholesterol ester) as a major
metabolite. Mouse tissues exhibited a higher rate of CPIA-
cholesterol ester formation than those of other animals.
Of the mouse tissues tested, the kidney, brain, and spleen
showed the greatest ability to form this ester, the rel-
evant enzyme activity being mainly localized in the
microsomal fractions. Carboxyesterases for mouse kidney
microsomes hydrolyzed the [2R, alphaS] isomer only of
fenvalerate to give CPIA and yielded the corresponding
cholesterol ester in the presence of artificial liposomes
containing cholesterol. It appears that the CPIA-
cholesterol ester resulted from the stereoselective
([2R, alphaS] only) formation of the CPIA-carboxyesterase
complex, which subsequently reacted with cholesterol to
yield the CPIA-cholesterol ester [128].
Hydrolysis of the four chiral isomers of fenvalerate
by microsomes of various mouse tissues has been investi-
gated by Takamatsu et al. [180]. The kidney, spleen and
brain hydrolyzed only the [2R, alphaS] isomer. Liver hydro-
lyzed the [2R, alphaS] and [2R, alphaR] isomers to a great-
er extent than the [2S, alphaR] and [2S, alphaS] isomers,
while plasma hydrolysed the [2S, alphaR] and [2R, alphaR]
isomers more rapidly than the [2S, alphaS] and [2R, alphaS]
isomers. The stereoselectivity of hydrolysis of the four
isomers by mouse liver microsomes was found to be same as
that in vivo. Of the four isomers, the [2R, alphaS] isomer
alone was transformed to cholesteryl-[2R]-2-(4-chlorophenyl)
isovalerate (CPIA-cholesterol ester) by microsomes of the
brain, kidney, spleen, or liver but not by plasma. The
rate of CPIA-cholesterol ester formation was lower in the
liver than in other tissues. The optimum pH (7.4-9.0) for
the formation of this ester was nearly the same as that
for hydrolysis of the [2R, alphaS] isomer to form CPIA in
mouse kidney microsomes.
The substrate specificity of microsomal carboxyester-
ase(s) responsible for the formation of cholesteryl-[2R]-
2-(4-chlorophenyl)isovalerate from fenvalerate was inves-
tigated by incubating mouse kidney microsomes with 14C-
cholesterol and fenvalerate or its analogues. Of the four
isomers of fenvalerate, only the [2R, alphaS] isomer yielded
a cholesterol ester. This specificity of cholesterol ester
formation was the same as that in the in vivo study. Some
of the fenvalerate analogues also produced similar choles-
terol esters. Steroids other than cholesterol were also
investigated as acceptors of the acid moiety of the
[2R, alphaS] isomer by incubating egg lecithin and several
steroids with the [2R, alphaS] isomer in the presence of
solubilized carboxyesterase(s). Dehydroisoandrosterone and
pregnenolone reacted with the [2R, alphaS] isomer to give the
corresponding ester conjugates [88].
One or more carboxyesterases located in the soluble
fraction of mouse brain homogenates hydrolyzed several
pyrethroid esters with a substrate specificity different
from that of the hepatic esterases. In particular,
fenvalerate and fluvalinate were hydrolyzed by brain
esterases at rates equal to or greater than that measured
for trans-permethrin. The results suggest that hydrolysis
in the brain may contribute to the detoxication of some
pyrethroids in mammals [64].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The acute toxicity data of fenvalerate to aquatic and
terrestrial non-target organisms are summarized in Tables
5, 6, and 7.
6.1 Aquatic Organisms
6.1.1 Toxicity to aquatic invertebrates
Non-target invertebrates, except molluscs, are more
susceptible to the insecticide than fish, the LC50
ranging from 0.08 to 2 µg/litre.
Fenvalerate is relatively non-toxic to oysters and
algae (LC50 >1000 µg/litre) over short exposure periods.
Snails (Heliosoma trivolvis) exposed for 28 days to 0.79
µg/litre, the highest concentration tested, showed no
change in behaviour or survival [4].
Day & Kaushik [32] conducted short-term toxicity
tests on three species of cladoceran and one species of
calanoid (Diaptomus oregonensis). The 48-h LC50 values
for daphnids were: 2.52 µg/litre for adult Daphnia magna,
0.83 µg/litre for D. magna aged 48 h (or less);
0.29 µg/litre for adult Daphnia galeata mendotae;
0.21 µg/litre for adult Ceriodaphnia lacustris; 0.16
µg/litre for D. galeata mendotae aged 48 h (or less).
Diaptomus oregonensis was the most sensitive species with
a 48-h LC50 of 0.12 µg/litre. No toxicity was found with
the emulsifiable concentrate from which fenvalerate was
omitted (EC control). Rates of filtration of algae were
reduced at sub-lethal concentrations of fenvalerate.
Ceriodaphnia lacustris was the most sensitive species,
with rates of filtration significantly decreased at
fenvalerate concentrations of 0.01 µg/litre. Rates of
assimilation of algae were decreased at fenvalerate con-
centrations of 0.05 µg/litre or more.
Day & Kaushik [33] conducted life-cycle studies on the
toxicity of fenvalerate to Daphnia galeata mendotae.
Lifetable methods were used to generate statistical
comparisons between treatments. At a concentration of
0.005 µg/litre, fenvalerate increased the longevity of
the daphnids significantly from 37.6 to 51.6 days. How-
ever, at the same concentration, production of young was
decreased. Higher concentrations of fenvalerate caused
reduced survival of the adults. The intrinsic rate of
natural increase in the population was reduced at a con-
centration of 0.5 µg/litre. At 0.01 µg/litre, the net
reproductive rate decreased from 126 to 73 offspring per
female and the generation time from 20.3 to 17.3 days.
Table 5. Acute toxicity of fenvalerate to non-target freshwater organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formu- Systemc Temperature pH Hardnessd Reference
(µg/litre) lationb (° C)
--------------------------------------------------------------------------------------------------------------------------------------------
Arthropods
Gammarus pseudolimnaeus adult-juv 96-h LC50 0.03 T F 15 7.6-7.8 46-48 4
Gammarus pseudolimnaeus 1-3 mm, juv 96-h LC50 0.05 T R 17 7.6-7.8 46-48 4
Waterflea 1st instar 96-h LC50 0.032 T S 17 7.4 44 115
(Daphnia magna)
Midge 3rd instar 48-h LC50 0.43 T S 22 7.4 44 115
(Chironomus pulmosus)
Mayfly larva 9-day LC50 0.08 T F 15 7.6 46-48 4
(Ephemerella sp.)
Rhagionid fly larva 28-day LC50 0.03 T F 15 7.6-7.8 46-48 4
(Atherix)
Stonefly naiad 72-h EC50 0.13 T F 15 7.6-7.8 46-48 4
(Pteronarcys dorsata)
Stonefly 3-6 weeks old 96-h LC50 1.9 EC S 20-22 7.8 7% 107
(Nitocra spinipes)
Fish
Atlantic salmon 6.2 cm, 5.3 g 96-h LC50 1.2 T R 10 110
(Salmo salar)
Rainbow trout 5-6 cm 48-h LC50 3.0 EC S 12-25.5 129
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 76 T S 10 7.5 110 28
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 21 EC S 10 7.5 110 28
(Salmo gairdneri)
Mosquitofish 4-5 cm 48-h LC50 15.0 EC S 8.8-16 129
(Gambusia affinis)
Mosquitofish 3-days old 72-h LC50 2.6 T S 24-27 124
(Gambusia affinis)
Desert pupfish 4-5 cm 48-h LC50 25.0 EC S 11-16.6 129
(Cyprinodon macularis)
Tilapia mossambica 5-6 cm 48-h LC50 200.0 EC S 15-21.4 129
Bluegill sunfish adult 96-h LC50 0.76 T S 22 7.4 40 115
(Lepomis macrochirus)
Fathead minnow adult 96-h LC50 2.35 T S 22 7.1 49 115
(Pimephales promelas)
--------------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
d expressed as mg CaCO3 per litre.
Table 6. Acute toxicity of fenvalerate to non-target estuarine & marine organisms
---------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formul- Systemc Temper- pH Salinity Reference
(µg/litre) ationb ature o/oo
(° C)
---------------------------------------------------------------------------------------------------------------------------------------
Algae
Skeletonema costatum 96-h EC50 > 1000 T 20 30 212
Isochrysis galbana 96-h EC50 > 1000 T 20 30 212
Thalassiosira pseudonana 96-h EC50 > 1000 T 20 30 212
Nitzschia angularum 96-h EC50 > 1000 T 20 30 212
Molluscs
Eastern oyster 2-h larva 48-h EC50 >
1000 T S 25 20 212
(Crassostrea virginica)
Arthropods
Lobster (Homarus americanus) 450 g 96-h LC50 0.14 T R 10 30 110
Shrimp (Crangon septemspinosa) 1.3 g 96-h LC50 0.04 T R 10 110
Shrimp (Mysidopsis bahia) 1-day juv 96-h LC50 0.021 T S 25 20 212
Shrimp (Mysidopsis bahia) newly hatched 96-h LC50 0.008 T F 25.4 25.3 163
Shrimp (Penalus duorarum) adult 96-h LC50 0.84 T F 24.8 24.9 163
California grunion 3-day larva 96-h LC50 0.29 T F 26 25 114
(Leuresthes tenuis)
California grunion juv 96-h LC50 0.60 T F 25 22 114
(Leuresthes tenuis)
Inland silverside 26-day larva 96-h LC50 1.00 T F 24 20 114
(Nenidia beryllina)
Tidewater silverside juv 96-h LC50 1.00 T F 25 20 114
(Menidia peninsulae)
Fish
Sheepshead minnow 28-day fry 96-h LC50 121 T S 25 20 212
(Cyprinodon variegatus)
Sheepshead minnow adult 96-h LC50 5 T F 30 26.5 163
(Cyprinodon variegatus)
Bleak (Alburnus alburnus) 8 cm 96-h LC50 2-3 EC S 10 7.8 7 107
Atlantic silverside adult 96-h LC50 0.31 T F 24.1 25 163
(Menidia menidia)
Striped mullet adult 96-h LC50 0.58 T F 25.9 25.8 163
(Mugil cephalus)
Gulf toadfish adult 96-h LC50 5.4 T F 30 24.8 163
(Opsanus beta)
---------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
Table 7. Acute toxicity of fenvalerate to non-target terrestrial organisms
-------------------------------------------------------------------------------------------------------------------------
Species Size Application Parameter Toxicity Temper- Reference
ature
(°C)
-------------------------------------------------------------------------------------------------------------------------
Bird
Broiler chicks 8-12 weeks old, oral LD50 12 590 (mg/kg) 31-32 155
0.99-2.2 kg
Hen oral LD50 > 1500 (mg/kg) 123
Arthropods
Insect parasite
Ichneumoid adult male film 24-h LC50 1760 (ng/vial) 152
(Campoletis sonorensis)
Insect predators
Lacewing adult 2.74 mg topical LD50 4.3 (mg/kg) 15 176
(Austromicromus tasmaniae) larva 2.52 mg topical LD50 67 (mg/kg) 20 176
Lacewing (Chrysopa carnea) 3rd instar, topical 72-h ED50 > 25 (mg/g) 28 164
larva 9.9-10 mg topical ED50 ~ 1 (mg/g) 28 164
one generation
Lacewing (Chrysopa carnea) larva film LC50 0.073 (mg/vial) 25 151
5-6 days old
Beetle 11.2 mg topical LD50 0.38 (mg/kg) 15 164
(Coccinella undecimpunctata)
Earwig (Labidura riparia) mature soil 0.11 kg ai/ha mortality 6% 205
0.22 kg ai/ha mortality 25% 205
0.44 kg ai/ha mortality 50% 205
Honey bee (Apis mellifera) adult topical LD50 410 ng/bee - 5
Predaceous mite species
Amblyseius fallacis adult female slide dip method LC50 2.6 (mg ai/litre) 27 158
Amblyseius fallacis adult female slide dip method LC50 7.0 (mg ai/litre) 26 199
Typhlodromus pyri adult female slide dip method LC50 8.1 (mg ai/litre) 26 199
Typhlodromus occidentalis adult female slide dip method LC50 2.1 (mg ai/litre) 26 199
-------------------------------------------------------------------------------------------------------------------------
McKenney & Hamaker [109] exposed the estuarine grass
shrimp Palaemonetes pugio to fenvalerate, in a flow-
through system to maintain constant exposure, throughout
20 days of larval development. The study was conducted
under optimal salinity conditions (20 o/oo). A nominal
concentration of 3.2 ng/litre significantly reduced the
percentage of larvae successfully completing metamor-
phosis. Exposure to 1.6 ng/litre prolonged larval develop-
ment. Larvae were also found to be less capable of
responding successfully to osmotic stress after exposure
to fenvalerate at 0.1 or 0.2 ng/litre.
6.1.2 Toxicity to fish
Fenvalerate is toxic to fish, LC50 values being
0.29-200 µg/litre (Tables 5 and 6). The LC50 value for
rainbow trout obtained with an emulsifiable concentrate
was 3.6 times lower than that for the technical product
[28]. The toxicity of fenvalerate to adult bluegill sun-
fish (Lepomis macrochirus) was unaffected by changes in
water hardness and pH [115].
The acute toxicities (96-h LC50) of fenvalerate to
juvenile steelhead trout were 172 ng/litre and 88
ng/litre, respectively, under continuous and intermittent
exposure (approximate peak concentration: 460 ± 40
ng/litre for 4.5 h). Prolonged intermittent exposure
(70 days) of the early life-stage resulted in marked
lethality (32%) and reduced terminal weight (50% of con-
trol) (mean concentration: 80 ng/litre, peak concen-
tration: 461 ng/litre). However, continuous exposure to
80 ng/litre for 70 days did not effect these parameters
[31].
Fenvalerate has narrow safety margins for fish
(LC50 of fish : LC50 of mosquito larvae is in the ratio
of 1:24) when the insecticide is used against mosquitoes
[129].
Four rainbow trout (Salmo gairdneri) died within 11
hours when exposed to 412 µg fenvalerate/litre. Visible
signs of poisoning included elevated cough rate, tremors,
and seizures. Ventilatory and cardiac activity stopped
during the seizures. Histopathological examination of
gill tissue showed damage consistent with irritation, and
Na+ and K+ excretion rates were elevated. Fenvalerate
concentrations in brain, liver, and carcass at death were
0.16, 3.62, and 0.25 mg/kg, respectively. The study
suggested that, apart from effects on the nervous system,
effects on respiratory surfaces and renal ion regulation
may be associated with fenvalerate toxicity in fish [15].
When sheepshead minnows (Cyprinodon variegatus) were
studied during 28 days for early-life-stage toxicity,
3.9 µg fenvalerate/litre significantly reduced the sur-
vival of hatched fish and 2.2 µg/litre reduced both
length and weight, but no effects were detected at
0.56 µg/litre [68].
6.1.3 Field studies and community effects
Caplan et al. [23] applied fenvalerate at concen-
trations of 0.2 and 1.0 mg/kg to sediment in a tidal marsh
sediment model ecosystem. No adverse effects were seen on
the heterotrophic microorganisms in the sediment after a
7-day exposure to either concentration. Plate counts to
assess numbers of organisms and measurements of substrate
degradation were not different from those of controls. The
half-life of fenvalerate was 6.3 days for the treatment at
0.2 mg/kg and 8.9 days at 1.0 mg/kg.
In the field, fenvalerate was applied to ponds at
rates of 28-112 g ai/ha as a mosquito larvicide [124].
Populations of plankton, crustaceans, and mayfly nymphs
decreased but recovered quickly. Corixids, notonectids,
and aquatic beetle populations decreased slightly and the
effects remained throughout the study. Chironomid larval
populations were suppressed and emergence was inhibited.
However, no deleterious effects were observed on rotifer
populations.
When fenvalerate was applied to ponds at rates of
11.2-56 g ai/ha for mosquito control, the insecticide
produced complete mortality of mayfly naiads [130]. A
single treatment by fenvalerate at 28 g/ha controlled
mosquito larvae for more than 7 days, and it also affected
populations of mayfly naiads, dragonfly naiads, and diving
beetle larva, but not ostracods or damselfly naiads
[131].
Studies into the effects of fenvalerate on estuarine
benthic communities were conducted in a flow-through
system for 8 weeks and 1 week for laboratory- and field-
colonized communities, respectively. Technical grade
fenvalerate (100%), dissolved in a stock solution con-
sisting of 15% acetone and 85% triethylene glycol, was
metered by syringe pump into, and mixed with, the sea
water entering the centre of the constant-head box of each
apparatus receiving fenvalerate. The same amount of
carrier solvent (10 ml/day, 5 mg/litre) was metered into
the control apparatus. Nominal concentrations of fenval-
erate in sea water were 0.01, 0.1, and 1.0 µg/litre.
Samples of water were taken from the constant-head boxes
once a week for chemical analyses for fenvalerate concen-
tration. Community structure was altered significantly in
both cases by fenvalerate at 0.1 or 1 µg/litre, but not
by 0.01 µg/litre. The groups most sensitive to the
insecticide were chordates (Branchiostoma caribaeum) and
amphipods, while annelids and molluscs tolerated concen-
trations up to 10 µg/litre [177].
Tagatz et al. [178] placed boxes containing sand,
either uncontaminated or contaminated (nominal concen-
tration of fenvalerate of 0.1, 1.0, or 10 mg/kg), in an
estuary for 8 weeks, and the community structure of
benthic organisms colonising the boxes was assessed. The
average number of species colonising the sand at the
highest treatment level was significantly less than for
the controls (35.6 compared to 47.8); lower concentrations
had no effect on species diversity. Colonisation by
annelids, molluscs, and arthropods was unaffected even at
the highest dose. The only organisms deterred by the
fenvalerate were chordates (primarily lanceolets).
6.2 Terrestrial Organisms
6.2.1 Toxicity to soil microorganisms
In laboratory trials for effects on soil algae,
Megharaj et al. [116] applied fenvalerate to a black
cotton soil, taken from a fallow cotton field. Fenvalerate
applied once at a dose equivalent to 0.5 or 1.0 kg/ha had
no inhibitory effect on soil algae, but two applications
of fenvalerate, at concentrations of 0.75 or 5.0 kg/ha,
resulted in increased algal populations.
6.2.2 Toxicity to beneficial insects
Fenvalerate is highly toxic to honey bees (Apis
mellifera) with a topical LD50 of 0.41 µg/bee. However,
in field tests at a normal application rate of 0.22 kg/ha,
the hazard is low because the residue repels bees for
about 10 h following application and decreases to non-
toxic levels within one day. During the first 5 days after
application, fenvalerate caused only light bee mortality.
At higher application rates (0.44 kg/ha), however, mor-
tality remained high 8 hours after application [5, 63, 84,
85].
Fenvalerate is toxic to the tobacco budworm (Heliothis
virescens) and to its predator green lacewing (Chrysopa
carnea) as well as to the parasite (Campoletis sonorensis)
of the tobacco budworm. But, it is more toxic to the pest
than to either the predator or the parasite. Comparison of
the LC50 value for the parasite (C. sonorensis) with that
for the host (H. virescens) indicated similar toxicity,
the value for the host being 1.5 times that for the para-
site [152]. However, in the case of the predator (C.
carnea), the insecticide was much less toxic to the pred-
ator than to the pest, the selectivity ratio being 0.037
[151].
When third instar larvae of C. carnea were topically
dosed with 250 µg/insect, they exhibited marked toler-
ance during a 72-h period. The ED50 value (paralysis,
failure to pupate, knockdown, and mortality) for fenval-
erate through one generation (larva to larva) was approxi-
mately 1000 µg/g [164].
Syrett & Penman [176] compared LC50 values for
fenvalerate when applied topically to lucerne-infesting
aphids (Acyrthosipho kondoi and A. pisum) and to their
predators, namely the brown lacewing ( Austromicromus
tasmaniae, adult and larva) and the ladybird (Coccinella
undecimpunctata). The values were 0.071, 0.033, 4.3, 67,
and 0.38 mg/kg, respectively. From these data, the lady-
bird was slightly (5-10 times) more tolerant than the
aphid species, but lacewing adults were 60-120 times as
tolerant as the aphids. Furthermore, the larvae were 15
times more tolerant than the adults. There was a negative
temperature coefficient for A. tasmaniae, with greater
toxicity (approximately 3 times) at 10 °C than at 25 °C
[176].
When fenvalerate was applied to loamy sand and then
striped earwigs (Labidura riparia), a predator of the
cabbage looper (Trichoplusia ni), were added to the soil,
fenvalerate was of low toxicity at rates giving good
looper control [205].
Laboratory studies of the activity of fenvalerate on
spider mites and their predators showed that the spider
mite (Tetranychus urticae) was considerably more (67-548
times) resistant to fenvalerate than were its predators
(Amblyseius fallacis, Typhlodromus pyri, and Typhlodromus
occidentalis) [199]. The LC50 value for T. urticae was
approximately 25 times greater than that for the predator
(A. fallacis) [158].
In the field, the predatory mite (T. pyri) disappeared
during the first 4-6 weeks after fenvalerate was sprayed
at 25 mg/litre to drip-off, and then small numbers were
found 7 weeks after spraying. The insecticide had no
appreciable toxicity for spider mites (Panonychus ulmi).
The virtual elimination of the predatory mite led to a
marked population increase of P. ulmi later in the same
season [3].
In apple and pear orchards, dramatic increases in the
populations of spider mites (T. urticae, Tetranychus
mcdanieli, or P. ulmi) were seen after the application of
fenvalerate at rates of 7.5 and 15 mg ai/litre. This was
due to a reduction in the numbers of the predatory mite
(Mataseiulus occidentalis) to zero or near zero [80].
From these results, it was suggested that the rec-
ommended application rates for fenvalerate would sometimes
be detrimental to integrated mite control programs in or-
chards, and these would require careful reconsideration.
6.2.3 Toxicity to birds
The toxicity of fenvalerate to birds is very low. The
acute LD50 for the chicken is more than 12 g/kg
(Table 7). The toxicity to the bobwhite quail (Colinus
virginianus) and American kestrel is similarly low.
Bradbury & Coats [13] measured the toxicity of
fenvalerate for the bobwhite quail. Acute oral dosing
yielded an LC50 in excess of 4 g/kg body weight for adult
birds and 1.785 g/kg body weight for 5-week-old juveniles.
Dietary dosing of 2-week-old chicks for 5 days (with a
further 3 days of observation) indicated an LC50 of
> 15 g/kg diet.
Rattner & Franson [156] dosed American kestrels with
fenvalerate (1-4 g/kg body weight) and examined the birds
for toxic effects over 10 h after dosing. Some birds were
kept at temperatures of 22 °C and others under cold stress
at -5 °C. Fenvalerate, at exposures far greater than could
be expected in the environment, caused mild intoxication
and elevated plasma alanine aminotransferase activity.
Cold did not increase the toxicity of the pyrethroid.
6.3 Uptake, Loss, and Bioaccumulation
Fenvalerate is taken up readily by aquatic organisms
and rapidly reaches, within the organism, a plateau level
related to the water concentration of the pyrethroid. Loss
of fenvalerate from organisms is rapid when they are
transferred to uncontaminated water. There is no sugges-
tion of biomagnification in food chains.
Under laboratory conditions, the half-life of fenval-
erate in sea water containing 100 g sediment per litre sea
water was 34 (27-42) days in foil-covered samples and 8
8 days in sunlight-exposed ones. Eastern oysters
(Crassostrea virginica) kept for 28 days in sea water
containing 24 µg fenvalerate/litre gave a steady state
bioconcentration factor of 4700. After treatment ceased,
fenvalerate was depurated by the oysters to non-detectable
concentrations within a week [163].
Snails exposed for 28 days to fenvalerate (0.79 µg
per litre) did not show any changes in behaviour or
survival. The bioaccumulation ratios ranged from 356 to
1167 [4].
In a study by Spehar et al. [166], embryonic, larval,
and early juvenile stages of fathead minnows (Pimephales
promelas) were exposed to fenvalerate in a continuous-flow
system for 30 days. At 0.33 µg/litre the only effect was
a temporary initial impairment of swimming in some larvae.
This was more marked at 0.43 µg/litre at which level
survival of the larvae was also reduced. The 30-day bio-
concentration factor was 3000 ± 1500, but 25 days after
transfer to clean water the fenvalerate had again been
eliminated.
Rainbow trout (Salmo gairdneri) were used to evaluate
the gill uptake and toxicokinetics of [3H]-fenvalerate.
Fish (weight between 0.64 and 0.97 kg) were exposed in a
respirometer-metabolism chamber to technical grade
fenvalerate (0.28 or 23 ng/litre) or an emulsifiable-
concentrate formulation (16 ng/litre) at 11.0-11.5 °C for
36 to 48 h. No significant effects of emulsifiers or
fenvalerate concentration on uptake were observed. The
overall mean gill uptake efficiency was 28.6 ± 4.4%. After
8- to 48-h depuration periods, carcass and bile contained
80-90% and 10-20% of the gill-absorbed material, respect-
ively. Urine, faeces, and blood each contained less than
2% of the dose. Significant excretion and blood transport
of fenvalerate equivalents were completed within 8-12 h
after exposure ceased. Specific tissues from trout exposed
to 0.28 ng/litre were analyzed for fenvalerate equival-
ents. After a 48-h depuration period, bile contained the
highest concentration of fenvalerate equivalents (7 ng/g),
followed by fat (0.2 ng/g). Remaining tissues contained
0.015-0.045 ng/g. Analysis of biliary metabolites indi-
cated that the glucuronide of 4 -OH-fenvalerate was the
only significant degradation product. Results from the
present study suggest that efficient gill uptake does not
explain the sensitivity of fish to fenvalerate. Instead,
a low rate of biotransformation and excretion may play a
significant role in the susceptibility of rainbow trout
[14].
When juvenile Atlantic salmon were exposed to static
water containing 0.8-9.3 µg fenvalerate/litre for 16-96 h,
the concentration of fenvalerate in dead fish ranged from
0.16 to 0.43 mg/kg. The insecticide was not detected (de-
tection limit: 5 µg/kg) either in dead lobster hepatopan-
creas or in dead shrimps [110].
When carp (Cyprinus carpio) was exposed to [14C-CN]-
[2S, alphaRS]-fenvalerate (0.8 µg/litre) under semi-static
conditions for 7 days, the radioactivity in fish increased
to a level of 922 µg/kg. Once the fish were transferred
to fresh water, the levels of radioactivity in the fish
decreased with an initial half-life of 5 days [135].
In studies by Ohkawa et al. [135], carp, snails,
Daphnia, and algae were exposed to fenvalerate in an
aquatic model ecosystem where 14C-[2S, alphaRS] fenval-
erate (0.3 mg/kg) was applied to the bottom sandy loam
soil. During a 30-day run, concentrations of fenval-
erate in the water were 0.35-0.63 µg/litre and 0.14-
0.21 µg/litre on days 7 and 30, respectively. The bio-
concentration factors for fenvalerate were 122, 617, 683,
and 477 on day 7 (162-300, 993-1110, 629-829, and 714-1180
on day 30) in carp, snails, Daphnia, and algae, respect-
ively. In carp, large amounts of CP-Vacid (17) and
3-phenoxybenzoic acid (22) were detected, together with
small amounts of alpha-cyano-3-(4'-hydroxyphenoxy)benzyl-2-
(4-chlorophenyl)-3-methylbutyrate (4'-OH-Fen) (25). Small
amounts of alpha-carbamoyl-3-phenoxybenzyl-2-(4-chlorophe-
nyl)-3-methylbutyrate (CONH2-Fen) (33), alpha-carboxy-3-
phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (COOH-
Fen) (34), and 4 -OH-Fen (25) were detected in snails.
CPIA was specifically present in both Daphnia (prey) and
carp (predator). CONH2-Fen (33) and alpha-carboxy-3-phenoxy-
benzyl alcohol (36) were common to algae (prey) and carp
(predator). Based on the products identified, degradation
pathways were proposed for this aquatic model ecosystem
(Fig. 4).
In a 28-day early-life stage study (see section
6.1.2), the mean bioconcentration factor in whole fish was
570 [68].
It was shown in the same study that fenvalerate under-
went oxidation at the 2'and 4'positions of the alcohol
moiety, as well as at the 2 and 3 positions of the acid
moiety, ester cleavage, and the conjugation of resultant
phenols and carboxylic acids with glucuronic acid, sul-
furic acid, and glycine. Cleavage of fenvalerate and its
ester metabolites appeared to release cyanohydrins, which
were, however, unstable under physiological conditions and
decomposed easily to cyanide and aldehydes (Fig. 3). The
cyanide ion was converted mainly to thiocyanate and
CO2, and 2-iminothiazolidine-4-carboxylic acid, a metab-
olite detected with other pyrethroids containing a cyanide
group, was not positively identified [134]. The major fae-
cal metabolites from 14CO-, 14Calpha-, and 14CN-fenval-
erate were unchanged fenvalerate (5) and two ester metab-
olites of 2'-hydroxy-(13) and 4'-hydroxy-fenvalerate (25).
The major metabolites in 0- to 2-day pooled urine (50-55%
of the dosed radioactivity) from the acid-labelling were
2-(4-chlorophenyl)isovaleric acid (17) (Cl-Vacid), 2-(4-
chlorophenyl)-3-hydroxymethylbutyric acid (37) (3-OH-Cl-
Vacid), and its lactone (38) (3-OH-Cl-Vacid-lactone).
Other minor metabolites were 2-(4-chlorophenyl)-2-hydroxy-
3-hydroxymethylbutyric acid (39) (2,3-OH-Cl-Vacid) in the
free, the lactone (40) (2,3-OH-Cl-Vacid-lactone), and the
conjugated forms, 2-(4-chlorophenyl)- cis-2-butenedioic
acid anhydride (41) (Cl-BDacid anhydride), and 2-(4-
chlorophenyl)-3-methyl-2-butene-4-olide (42) (Cl-B-acid-
lactone). On the other hand, the predominant urinary
metabolite from the alcohol moiety was the sulfate conju-
gate of 3-(4'-hydroxyphenoxy)benzoic acid (23) (4'-OH-
PBacid), accounting for approximately 40% of the dose.
Other major metabolites were 3-phenoxybenzoic acid (22)
(PBacid) in the free (6%), the glucuronide (2%), and the
glycine (2%) conjugated forms, 4'-OH-PBacid in the free
(5%) and the glucuronide (2%) forms, and the sulfate of 3-
(2'-hydroxyphenoxy)benzoic acid (24) (2'-OH-PBacid) (3%).
With 14CN-fenvalerate, the major urinary metabolite was
thiocyanate (43) [134].
Pydrin insecticide (Y-rich) is an isomerically
enriched form of fenvalerate containing an excess ratio of
the active diastereomers SS and RR (designated Y) over the
less active diastereomers RS and SR (designated X) at a
ratio of approximately 85:15. Fenvalerate contains Y:X in
a ratio of 45:55. Following a single oral dose of the Y-
rich insecticide (8.4 mg/kg) to male and female Sprague-
Dawley rats, more than 90% of the administered radioac-
tivity from the acid moiety (chlorophenyl-14C) and the
alcohol moiety (phenoxyphenyl-14C) was eliminated within
the first 24 h. There was no major difference between the
two different fenvalerate preparations in either the elim-
ination rate or the metabolites distribution profile.
Cleavage of the ester linkage was the primary metabolic
pathway. The acid and alcohol portions of the parent mole-
cule underwent hydroxylation, oxidation, and conjugation.
These metabolic reactions were not dependent on the iso-
meric composition of the test material. Tissue residue
data showed that 14C residues were not retained in the
various organs [104].
The fate of sugar conjugates, which may be formed as
plant metabolites, has been investigated by Mikami et al.
[122]. Upon single oral administration to male Sprague-
Dawley rats at a concentration of 3.8 mg/kg, the mono-,
di-, and tri-glucose conjugates of [14C]-3-phenoxybenzyl
alcohol (19) and the mono-glucose conjugate of [14C]-3-
phenoxybenzoic acid (22) were rapidly hydrolysed and
extensively eliminated in the urine, mostly as the sulfate
conjugate of 3-(4-hydroxyphenoxy)benzoic acid (24).
Faecal elimination was a minor route, whereas biliary
excretion was responsible for about 42% of the dose, and
the glucuronide conjugates of (19), (22), and (24) were
common major metabolites. The biliary glucuronides were
metabolized in the small intestine to the respective
aglycones, which were reabsorbed, metabolized further, and
excreted in the urine as the sulfate conjugate of (24).
Although small amounts of the mono-, di-, and tri-gluco-
sides were found in the 30-min blood and liver samples
following oral administration of the tri-glucoside of
(19), they were not detected in the urine, bile, or fae-
ces. Similarly, the sulfate conjugate was one of the major
urinary metabolites in germ-free male rats, when dosed
with the 14C-glucosides at a rate of 9 µmol/kg via the
oral or intraperitoneal route, although certain amounts
were excreted unchanged in the urine and faeces. The glu-
cose conjugates were metabolized in vitro by intestinal
microflora and in various rat tissues including blood,
liver, small intestine, and small intestinal mucosa. The
tissue enzymes showed a different substrate specificity in
hydrolysing the glucosides. However, they were not metab-
olized in gastric juice, bile, pancreatic juice, or
urine.
5.1.2 Mouse
In mice, fenvalerate is metabolized in a similar way
to that in rats, but the following significant species
differences were found by Kaneko et al. [86]: (a) the
taurine conjugate of PBacid was found in mice but not in
rats; (b) 4'-OH-PBacid sulfate occurred to a greater
extent in rats than in mice; and (c) a greater amount of
thiocyanate was excreted in mice than in rats. No sig-
nificant sex differences were observed in rats and mice.
The metabolism of the stereoisomers of fenvalerate,
([2S, alphaRS] and [2S, alphaS]) was apparently similar to
that of racemic fenvalerate.
Following a single oral administration of the four
chiral isomers of [14C-chlorophenyl]-fenvalerate to
Sprague-Dawley rats and ddY mice (2.5 mg/kg body weight),
the [2R, alphaS] isomer showed, in both rats and mice, rel-
atively greater residues in the analyzed tissues (except
fat), particularly in adrenal glands, compared with the
other three isomers. Similarly, this isomer showed
higher tissue concentrations than the other isomers when
mice were fed a diet containing 500 mg/kg of the
[2S, alphaS], [2R, alphaS], or [2R, alphaR] isomers for
two weeks. The greater amount of radioactive residues from
the administration of [2R, alphaS] isomer, as compared
with those of other isomers, was explained by the
preferential formation of a lipophilic metabolite from the
[2R, alphaS] isomer found in all examined tissues, which
was not easily excreted. The amounts of the lipophilic
metabolite differed among tissues, being higher in adrenal,
liver, and mesenteric lymph nodes. This metabolite was
identified as cholesteryl [2R]-2-(4-chlorophenyl)isoval-
erate. The presence of the same metabolite was also indi-
cated in rat tissues [87].
5.1.3 Domestic animals
Two 3-month-old lambs were fed a diet containing
45 mg/kg fenvalerate for 10 days and then killed to deter-
mine the concentrations of fenvalerate in the kidney,
liver, leg muscle, and renal fat [210]. Among the analyzed
tissues, fat showed the highest fenvalerate level (3.6-4.4
mg/kg dry weight) while other tissues contained less than
0.3 mg/kg. Fenvalerate gave two gas chromatographic peaks
and each peak contained a pair of its enantiomers. In all
cases, the ratio of the areas of these peaks (peak 1
(RS,SR)/peak 2 (SS,RR)) was 1.08 both for fenvalerate in
the diet and for fenvalerate recovered from the fortified
control fat. In contrast, the fenvalerate isolated from
lamb fat had a peak area ratio of 0.76-0.78. Thus, one or
both of the first eluting enantiomers appeared to be
metabolized more rapidly than the other enantiomers.
In a study by Wszolek et al. [209], two Holstein cows
were fed fenvalerate at 5 and 15 mg/kg diet for 4 days and
were then given a clean diet for 6 days. Total excretion
of fenvalerate in milk amounted to 0.44 and 0.64% of the
total dose for the 5 and 15 mg/kg levels, respectively,
whereas about 25% of the dose was eliminated in the
faeces.
A lactating Holstein cow was fed grain fortified with
227 mg fenvalerate daily for 4 days and the urine was ana-
lysed. Intact fenvalerate was not detected in any samples
of the urine excreted by the cow during the 10-day feeding
study, nor was the acid metabolite (Cl-Vacid) (17) ident-
ified. The in vitro study on fenvalerate degradation in
rumen fluid indicated that no significant degradation of
fenvalerate was observed during the 6-h incubation [211].
Saleh et al. [161] gave a single oral dose of fenval-
erate (10 mg/kg body weight) to chickens and monitored the
persistence and distribution of the insecticide over 15
days. A concentration of 4.7 mg/litre in blood after 24 h
fell to 0.05 mg/litre after 7 days. Levels in other tis-
sues reached maxima of less than 1.0 mg/kg and fell rap-
idly. However, brain residues rose to a level of 4.0 mg/kg
over 7 days and persisted for the 15 days of the exper-
iment. Concentrations in eggs reached a maximum of
0.3 mg/kg yolk after 4 to 5 days, and a maximum of
0.24 mg/kg egg white. By day 6, levels had returned to the
pre-dosing level.
5.2 Enzymatic Systems for Biotransformation
The [2R, alphaRS] isomer of fenvalerate has been found
to be more rapidly hydrolysed by mouse liver esterase than
the [2S, alphaRS] isomer, but less rapidly metabolised than
the [2R, alphaRS] isomer with an oxidase system. A similar
correlation was observed with the [2S] and [2R] isomers of
S-5439 (3-phenoxybenzyl-2-(4-chlorophenyl)isovalerate) [165].
In an in vitro study on the metabolism of the four
chiral isomers of fenvalerate using homogenates from vari-
ous tissues of mice, rats, dogs, and monkeys, only the
[2R, alphaS] isomer yielded cholesteryl-[2R]-2-(4-chloro-
phenyl)isovalerate (CPIA-cholesterol ester) as a major
metabolite. Mouse tissues exhibited a higher rate of CPIA-
cholesterol ester formation than those of other animals.
Of the mouse tissues tested, the kidney, brain, and spleen
showed the greatest ability to form this ester, the rel-
evant enzyme activity being mainly localized in the
microsomal fractions. Carboxyesterases for mouse kidney
microsomes hydrolyzed the [2R, alphaS] isomer only of
fenvalerate to give CPIA and yielded the corresponding
cholesterol ester in the presence of artificial liposomes
containing cholesterol. It appears that the CPIA-
cholesterol ester resulted from the stereoselective
([2R, alphaS] only) formation of the CPIA-carboxyesterase
complex, which subsequently reacted with cholesterol to
yield the CPIA-cholesterol ester [128].
Hydrolysis of the four chiral isomers of fenvalerate
by microsomes of various mouse tissues has been investi-
gated by Takamatsu et al. [180]. The kidney, spleen and
brain hydrolyzed only the [2R, alphaS] isomer. Liver hydro-
lyzed the [2R, alphaS] and [2R, alphaR] isomers to a great-
er extent than the [2S, alphaR] and [2S, alphaS] isomers,
while plasma hydrolysed the [2S, alphaR] and [2R, alphaR]
isomers more rapidly than the [2S, alphaS] and [2R, alphaS]
isomers. The stereoselectivity of hydrolysis of the four
isomers by mouse liver microsomes was found to be same as
that in vivo. Of the four isomers, the [2R, alphaS] isomer
alone was transformed to cholesteryl-[2R]-2-(4-chlorophenyl)
isovalerate (CPIA-cholesterol ester) by microsomes of the
brain, kidney, spleen, or liver but not by plasma. The
rate of CPIA-cholesterol ester formation was lower in the
liver than in other tissues. The optimum pH (7.4-9.0) for
the formation of this ester was nearly the same as that
for hydrolysis of the [2R, alphaS] isomer to form CPIA in
mouse kidney microsomes.
The substrate specificity of microsomal carboxyester-
ase(s) responsible for the formation of cholesteryl-[2R]-
2-(4-chlorophenyl)isovalerate from fenvalerate was inves-
tigated by incubating mouse kidney microsomes with 14C-
cholesterol and fenvalerate or its analogues. Of the four
isomers of fenvalerate, only the [2R, alphaS] isomer yielded
a cholesterol ester. This specificity of cholesterol ester
formation was the same as that in the in vivo study. Some
of the fenvalerate analogues also produced similar choles-
terol esters. Steroids other than cholesterol were also
investigated as acceptors of the acid moiety of the
[2R, alphaS] isomer by incubating egg lecithin and several
steroids with the [2R, alphaS] isomer in the presence of
solubilized carboxyesterase(s). Dehydroisoandrosterone and
pregnenolone reacted with the [2R, alphaS] isomer to give the
corresponding ester conjugates [88].
One or more carboxyesterases located in the soluble
fraction of mouse brain homogenates hydrolyzed several
pyrethroid esters with a substrate specificity different
from that of the hepatic esterases. In particular,
fenvalerate and fluvalinate were hydrolyzed by brain
esterases at rates equal to or greater than that measured
for trans-permethrin. The results suggest that hydrolysis
in the brain may contribute to the detoxication of some
pyrethroids in mammals [64].
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The acute toxicity data of fenvalerate to aquatic and
terrestrial non-target organisms are summarized in Tables
5, 6, and 7.
6.1 Aquatic Organisms
6.1.1 Toxicity to aquatic invertebrates
Non-target invertebrates, except molluscs, are more
susceptible to the insecticide than fish, the LC50
ranging from 0.08 to 2 µg/litre.
Fenvalerate is relatively non-toxic to oysters and
algae (LC50 >1000 µg/litre) over short exposure periods.
Snails (Heliosoma trivolvis) exposed for 28 days to 0.79
µg/litre, the highest concentration tested, showed no
change in behaviour or survival [4].
Day & Kaushik [32] conducted short-term toxicity
tests on three species of cladoceran and one species of
calanoid (Diaptomus oregonensis). The 48-h LC50 values
for daphnids were: 2.52 µg/litre for adult Daphnia magna,
0.83 µg/litre for D. magna aged 48 h (or less);
0.29 µg/litre for adult Daphnia galeata mendotae;
0.21 µg/litre for adult Ceriodaphnia lacustris; 0.16
µg/litre for D. galeata mendotae aged 48 h (or less).
Diaptomus oregonensis was the most sensitive species with
a 48-h LC50 of 0.12 µg/litre. No toxicity was found with
the emulsifiable concentrate from which fenvalerate was
omitted (EC control). Rates of filtration of algae were
reduced at sub-lethal concentrations of fenvalerate.
Ceriodaphnia lacustris was the most sensitive species,
with rates of filtration significantly decreased at
fenvalerate concentrations of 0.01 µg/litre. Rates of
assimilation of algae were decreased at fenvalerate con-
centrations of 0.05 µg/litre or more.
Day & Kaushik [33] conducted life-cycle studies on the
toxicity of fenvalerate to Daphnia galeata mendotae.
Lifetable methods were used to generate statistical
comparisons between treatments. At a concentration of
0.005 µg/litre, fenvalerate increased the longevity of
the daphnids significantly from 37.6 to 51.6 days. How-
ever, at the same concentration, production of young was
decreased. Higher concentrations of fenvalerate caused
reduced survival of the adults. The intrinsic rate of
natural increase in the population was reduced at a con-
centration of 0.5 µg/litre. At 0.01 µg/litre, the net
reproductive rate decreased from 126 to 73 offspring per
female and the generation time from 20.3 to 17.3 days.
Table 5. Acute toxicity of fenvalerate to non-target freshwater organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formu- Systemc Temperature pH Hardnessd Reference
(µg/litre) lationb (° C)
--------------------------------------------------------------------------------------------------------------------------------------------
Arthropods
Gammarus pseudolimnaeus adult-juv 96-h LC50 0.03 T F 15 7.6-7.8 46-48 4
Gammarus pseudolimnaeus 1-3 mm, juv 96-h LC50 0.05 T R 17 7.6-7.8 46-48 4
Waterflea 1st instar 96-h LC50 0.032 T S 17 7.4 44 115
(Daphnia magna)
Midge 3rd instar 48-h LC50 0.43 T S 22 7.4 44 115
(Chironomus pulmosus)
Mayfly larva 9-day LC50 0.08 T F 15 7.6 46-48 4
(Ephemerella sp.)
Rhagionid fly larva 28-day LC50 0.03 T F 15 7.6-7.8 46-48 4
(Atherix)
Stonefly naiad 72-h EC50 0.13 T F 15 7.6-7.8 46-48 4
(Pteronarcys dorsata)
Stonefly 3-6 weeks old 96-h LC50 1.9 EC S 20-22 7.8 7% 107
(Nitocra spinipes)
Fish
Atlantic salmon 6.2 cm, 5.3 g 96-h LC50 1.2 T R 10 110
(Salmo salar)
Rainbow trout 5-6 cm 48-h LC50 3.0 EC S 12-25.5 129
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 76 T S 10 7.5 110 28
(Salmo gairdneri)
Rainbow trout 6 cm, 3 g 24-h LC50 21 EC S 10 7.5 110 28
(Salmo gairdneri)
Mosquitofish 4-5 cm 48-h LC50 15.0 EC S 8.8-16 129
(Gambusia affinis)
Mosquitofish 3-days old 72-h LC50 2.6 T S 24-27 124
(Gambusia affinis)
Desert pupfish 4-5 cm 48-h LC50 25.0 EC S 11-16.6 129
(Cyprinodon macularis)
Tilapia mossambica 5-6 cm 48-h LC50 200.0 EC S 15-21.4 129
Bluegill sunfish adult 96-h LC50 0.76 T S 22 7.4 40 115
(Lepomis macrochirus)
Fathead minnow adult 96-h LC50 2.35 T S 22 7.1 49 115
(Pimephales promelas)
--------------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
d expressed as mg CaCO3 per litre.
Table 6. Acute toxicity of fenvalerate to non-target estuarine & marine organisms
---------------------------------------------------------------------------------------------------------------------------------------
Species Sizea Parameter Toxicity Formul- Systemc Temper- pH Salinity Reference
(µg/litre) ationb ature o/oo
(° C)
---------------------------------------------------------------------------------------------------------------------------------------
Algae
Skeletonema costatum 96-h EC50 > 1000 T 20 30 212
Isochrysis galbana 96-h EC50 > 1000 T 20 30 212
Thalassiosira pseudonana 96-h EC50 > 1000 T 20 30 212
Nitzschia angularum 96-h EC50 > 1000 T 20 30 212
Molluscs
Eastern oyster 2-h larva 48-h EC50 >
1000 T S 25 20 212
(Crassostrea virginica)
Arthropods
Lobster (Homarus americanus) 450 g 96-h LC50 0.14 T R 10 30 110
Shrimp (Crangon septemspinosa) 1.3 g 96-h LC50 0.04 T R 10 110
Shrimp (Mysidopsis bahia) 1-day juv 96-h LC50 0.021 T S 25 20 212
Shrimp (Mysidopsis bahia) newly hatched 96-h LC50 0.008 T F 25.4 25.3 163
Shrimp (Penalus duorarum) adult 96-h LC50 0.84 T F 24.8 24.9 163
California grunion 3-day larva 96-h LC50 0.29 T F 26 25 114
(Leuresthes tenuis)
California grunion juv 96-h LC50 0.60 T F 25 22 114
(Leuresthes tenuis)
Inland silverside 26-day larva 96-h LC50 1.00 T F 24 20 114
(Nenidia beryllina)
Tidewater silverside juv 96-h LC50 1.00 T F 25 20 114
(Menidia peninsulae)
Fish
Sheepshead minnow 28-day fry 96-h LC50 121 T S 25 20 212
(Cyprinodon variegatus)
Sheepshead minnow adult 96-h LC50 5 T F 30 26.5 163
(Cyprinodon variegatus)
Bleak (Alburnus alburnus) 8 cm 96-h LC50 2-3 EC S 10 7.8 7 107
Atlantic silverside adult 96-h LC50 0.31 T F 24.1 25 163
(Menidia menidia)
Striped mullet adult 96-h LC50 0.58 T F 25.9 25.8 163
(Mugil cephalus)
Gulf toadfish adult 96-h LC50 5.4 T F 30 24.8 163
(Opsanus beta)
---------------------------------------------------------------------------------------------------------------------------------------
a juv = juvenile.
b T = Technical, EC = Emulsifiable concentrate.
c R = Renewal, S = Static, F = Flow-through.
Table 7. Acute toxicity of fenvalerate to non-target terrestrial organisms
-------------------------------------------------------------------------------------------------------------------------
Species Size Application Parameter Toxicity Temper- Reference
ature
(°C)
-------------------------------------------------------------------------------------------------------------------------
Bird
Broiler chicks 8-12 weeks old, oral LD50 12 590 (mg/kg) 31-32 155
0.99-2.2 kg
Hen oral LD50 > 1500 (mg/kg) 123
Arthropods
Insect parasite
Ichneumoid adult male film 24-h LC50 1760 (ng/vial) 152
(Campoletis sonorensis)
Insect predators
Lacewing adult 2.74 mg topical LD50 4.3 (mg/kg) 15 176
(Austromicromus tasmaniae) larva 2.52 mg topical LD50 67 (mg/kg) 20 176
Lacewing (Chrysopa carnea) 3rd instar, topical 72-h ED50 > 25 (mg/g) 28 164
larva 9.9-10 mg topical ED50 ~ 1 (mg/g) 28 164
one generation
Lacewing (Chrysopa carnea) larva film LC50 0.073 (mg/vial) 25 151
5-6 days old
Beetle 11.2 mg topical LD50 0.38 (mg/kg) 15 164
(Coccinella undecimpunctata)
Earwig (Labidura riparia) mature soil 0.11 kg ai/ha mortality 6% 205
0.22 kg ai/ha mortality 25% 205
0.44 kg ai/ha mortality 50% 205
Honey bee (Apis mellifera) adult topical LD50 410 ng/bee - 5
Predaceous mite species
Amblyseius fallacis adult female slide dip method LC50 2.6 (mg ai/litre) 27 158
Amblyseius fallacis adult female slide dip method LC50 7.0 (mg ai/litre) 26 199
Typhlodromus pyri adult female slide dip method LC50 8.1 (mg ai/litre) 26 199
Typhlodromus occidentalis adult female slide dip method LC50 2.1 (mg ai/litre) 26 199
-------------------------------------------------------------------------------------------------------------------------
McKenney & Hamaker [109] exposed the estuarine grass
shrimp Palaemonetes pugio to fenvalerate, in a flow-
through system to maintain constant exposure, throughout
20 days of larval development. The study was conducted
under optimal salinity conditions (20 o/oo). A nominal
concentration of 3.2 ng/litre significantly reduced the
percentage of larvae successfully completing metamor-
phosis. Exposure to 1.6 ng/litre prolonged larval develop-
ment. Larvae were also found to be less capable of
responding successfully to osmotic stress after exposure
to fenvalerate at 0.1 or 0.2 ng/litre.
6.1.2 Toxicity to fish
Fenvalerate is toxic to fish, LC50 values being
0.29-200 µg/litre (Tables 5 and 6). The LC50 value for
rainbow trout obtained with an emulsifiable concentrate
was 3.6 times lower than that for the technical product
[28]. The toxicity of fenvalerate to adult bluegill sun-
fish (Lepomis macrochirus) was unaffected by changes in
water hardness and pH [115].
The acute toxicities (96-h LC50) of fenvalerate to
juvenile steelhead trout were 172 ng/litre and 88
ng/litre, respectively, under continuous and intermittent
exposure (approximate peak concentration: 460 ± 40
ng/litre for 4.5 h). Prolonged intermittent exposure
(70 days) of the early life-stage resulted in marked
lethality (32%) and reduced terminal weight (50% of con-
trol) (mean concentration: 80 ng/litre, peak concen-
tration: 461 ng/litre). However, continuous exposure to
80 ng/litre for 70 days did not effect these parameters
[31].
Fenvalerate has narrow safety margins for fish
(LC50 of fish : LC50 of mosquito larvae is in the ratio
of 1:24) when the insecticide is used against mosquitoes
[129].
Four rainbow trout (Salmo gairdneri) died within 11
hours when exposed to 412 µg fenvalerate/litre. Visible
signs of poisoning included elevated cough rate, tremors,
and seizures. Ventilatory and cardiac activity stopped
during the seizures. Histopathological examination of
gill tissue showed damage consistent with irritation, and
Na+ and K+ excretion rates were elevated. Fenvalerate
concentrations in brain, liver, and carcass at death were
0.16, 3.62, and 0.25 mg/kg, respectively. The study
suggested that, apart from effects on the nervous system,
effects on respiratory surfaces and renal ion regulation
may be associated with fenvalerate toxicity in fish [15].
When sheepshead minnows (Cyprinodon variegatus) were
studied during 28 days for early-life-stage toxicity,
3.9 µg fenvalerate/litre significantly reduced the sur-
vival of hatched fish and 2.2 µg/litre reduced both
length and weight, but no effects were detected at
0.56 µg/litre [68].
6.1.3 Field studies and community effects
Caplan et al. [23] applied fenvalerate at concen-
trations of 0.2 and 1.0 mg/kg to sediment in a tidal marsh
sediment model ecosystem. No adverse effects were seen on
the heterotrophic microorganisms in the sediment after a
7-day exposure to either concentration. Plate counts to
assess numbers of organisms and measurements of substrate
degradation were not different from those of controls. The
half-life of fenvalerate was 6.3 days for the treatment at
0.2 mg/kg and 8.9 days at 1.0 mg/kg.
In the field, fenvalerate was applied to ponds at
rates of 28-112 g ai/ha as a mosquito larvicide [124].
Populations of plankton, crustaceans, and mayfly nymphs
decreased but recovered quickly. Corixids, notonectids,
and aquatic beetle populations decreased slightly and the
effects remained throughout the study. Chironomid larval
populations were suppressed and emergence was inhibited.
However, no deleterious effects were observed on rotifer
populations.
When fenvalerate was applied to ponds at rates of
11.2-56 g ai/ha for mosquito control, the insecticide
produced complete mortality of mayfly naiads [130]. A
single treatment by fenvalerate at 28 g/ha controlled
mosquito larvae for more than 7 days, and it also affected
populations of mayfly naiads, dragonfly naiads, and diving
beetle larva, but not ostracods or damselfly naiads
[131].
Studies into the effects of fenvalerate on estuarine
benthic communities were conducted in a flow-through
system for 8 weeks and 1 week for laboratory- and field-
colonized communities, respectively. Technical grade
fenvalerate (100%), dissolved in a stock solution con-
sisting of 15% acetone and 85% triethylene glycol, was
metered by syringe pump into, and mixed with, the sea
water entering the centre of the constant-head box of each
apparatus receiving fenvalerate. The same amount of
carrier solvent (10 ml/day, 5 mg/litre) was metered into
the control apparatus. Nominal concentrations of fenval-
erate in sea water were 0.01, 0.1, and 1.0 µg/litre.
Samples of water were taken from the constant-head boxes
once a week for chemical analyses for fenvalerate concen-
tration. Community structure was altered significantly in
both cases by fenvalerate at 0.1 or 1 µg/litre, but not
by 0.01 µg/litre. The groups most sensitive to the
insecticide were chordates (Branchiostoma caribaeum) and
amphipods, while annelids and molluscs tolerated concen-
trations up to 10 µg/litre [177].
Tagatz et al. [178] placed boxes containing sand,
either uncontaminated or contaminated (nominal concen-
tration of fenvalerate of 0.1, 1.0, or 10 mg/kg), in an
estuary for 8 weeks, and the community structure of
benthic organisms colonising the boxes was assessed. The
average number of species colonising the sand at the
highest treatment level was significantly less than for
the controls (35.6 compared to 47.8); lower concentrations
had no effect on species diversity. Colonisation by
annelids, molluscs, and arthropods was unaffected even at
the highest dose. The only organisms deterred by the
fenvalerate were chordates (primarily lanceolets).
6.2 Terrestrial Organisms
6.2.1 Toxicity to soil microorganisms
In laboratory trials for effects on soil algae,
Megharaj et al. [116] applied fenvalerate to a black
cotton soil, taken from a fallow cotton field. Fenvalerate
applied once at a dose equivalent to 0.5 or 1.0 kg/ha had
no inhibitory effect on soil algae, but two applications
of fenvalerate, at concentrations of 0.75 or 5.0 kg/ha,
resulted in increased algal populations.
6.2.2 Toxicity to beneficial insects
Fenvalerate is highly toxic to honey bees (Apis
mellifera) with a topical LD50 of 0.41 µg/bee. However,
in field tests at a normal application rate of 0.22 kg/ha,
the hazard is low because the residue repels bees for
about 10 h following application and decreases to non-
toxic levels within one day. During the first 5 days after
application, fenvalerate caused only light bee mortality.
At higher application rates (0.44 kg/ha), however, mor-
tality remained high 8 hours after application [5, 63, 84,
85].
Fenvalerate is toxic to the tobacco budworm (Heliothis
virescens) and to its predator green lacewing (Chrysopa
carnea) as well as to the parasite (Campoletis sonorensis)
of the tobacco budworm. But, it is more toxic to the pest
than to either the predator or the parasite. Comparison of
the LC50 value for the parasite (C. sonorensis) with that
for the host (H. virescens) indicated similar toxicity,
the value for the host being 1.5 times that for the para-
site [152]. However, in the case of the predator (C.
carnea), the insecticide was much less toxic to the pred-
ator than to the pest, the selectivity ratio being 0.037
[151].
When third instar larvae of C. carnea were topically
dosed with 250 µg/insect, they exhibited marked toler-
ance during a 72-h period. The ED50 value (paralysis,
failure to pupate, knockdown, and mortality) for fenval-
erate through one generation (larva to larva) was approxi-
mately 1000 µg/g [164].
Syrett & Penman [176] compared LC50 values for
fenvalerate when applied topically to lucerne-infesting
aphids (Acyrthosipho kondoi and A. pisum) and to their
predators, namely the brown lacewing ( Austromicromus
tasmaniae, adult and larva) and the ladybird (Coccinella
undecimpunctata). The values were 0.071, 0.033, 4.3, 67,
and 0.38 mg/kg, respectively. From these data, the lady-
bird was slightly (5-10 times) more tolerant than the
aphid species, but lacewing adults were 60-120 times as
tolerant as the aphids. Furthermore, the larvae were 15
times more tolerant than the adults. There was a negative
temperature coefficient for A. tasmaniae, with greater
toxicity (approximately 3 times) at 10 °C than at 25 °C
[176].
When fenvalerate was applied to loamy sand and then
striped earwigs (Labidura riparia), a predator of the
cabbage looper (Trichoplusia ni), were added to the soil,
fenvalerate was of low toxicity at rates giving good
looper control [205].
Laboratory studies of the activity of fenvalerate on
spider mites and their predators showed that the spider
mite (Tetranychus urticae) was considerably more (67-548
times) resistant to fenvalerate than were its predators
(Amblyseius fallacis, Typhlodromus pyri, and Typhlodromus
occidentalis) [199]. The LC50 value for T. urticae was
approximately 25 times greater than that for the predator
(A. fallacis) [158].
In the field, the predatory mite (T. pyri) disappeared
during the first 4-6 weeks after fenvalerate was sprayed
at 25 mg/litre to drip-off, and then small numbers were
found 7 weeks after spraying. The insecticide had no
appreciable toxicity for spider mites (Panonychus ulmi).
The virtual elimination of the predatory mite led to a
marked population increase of P. ulmi later in the same
season [3].
In apple and pear orchards, dramatic increases in the
populations of spider mites (T. urticae, Tetranychus
mcdanieli, or P. ulmi) were seen after the application of
fenvalerate at rates of 7.5 and 15 mg ai/litre. This was
due to a reduction in the numbers of the predatory mite
(Mataseiulus occidentalis) to zero or near zero [80].
From these results, it was suggested that the rec-
ommended application rates for fenvalerate would sometimes
be detrimental to integrated mite control programs in or-
chards, and these would require careful reconsideration.
6.2.3 Toxicity to birds
The toxicity of fenvalerate to birds is very low. The
acute LD50 for the chicken is more than 12 g/kg
(Table 7). The toxicity to the bobwhite quail (Colinus
virginianus) and American kestrel is similarly low.
Bradbury & Coats [13] measured the toxicity of
fenvalerate for the bobwhite quail. Acute oral dosing
yielded an LC50 in excess of 4 g/kg body weight for adult
birds and 1.785 g/kg body weight for 5-week-old juveniles.
Dietary dosing of 2-week-old chicks for 5 days (with a
further 3 days of observation) indicated an LC50 of
> 15 g/kg diet.
Rattner & Franson [156] dosed American kestrels with
fenvalerate (1-4 g/kg body weight) and examined the birds
for toxic effects over 10 h after dosing. Some birds were
kept at temperatures of 22 °C and others under cold stress
at -5 °C. Fenvalerate, at exposures far greater than could
be expected in the environment, caused mild intoxication
and elevated plasma alanine aminotransferase activity.
Cold did not increase the toxicity of the pyrethroid.
6.3 Uptake, Loss, and Bioaccumulation
Fenvalerate is taken up readily by aquatic organisms
and rapidly reaches, within the organism, a plateau level
related to the water concentration of the pyrethroid. Loss
of fenvalerate from organisms is rapid when they are
transferred to uncontaminated water. There is no sugges-
tion of biomagnification in food chains.
Under laboratory conditions, the half-life of fenval-
erate in sea water containing 100 g sediment per litre sea
water was 34 (27-42) days in foil-covered samples and 8
8 days in sunlight-exposed ones. Eastern oysters
(Crassostrea virginica) kept for 28 days in sea water
containing 24 µg fenvalerate/litre gave a steady state
bioconcentration factor of 4700. After treatment ceased,
fenvalerate was depurated by the oysters to non-detectable
concentrations within a week [163].
Snails exposed for 28 days to fenvalerate (0.79 µg
per litre) did not show any changes in behaviour or
survival. The bioaccumulation ratios ranged from 356 to
1167 [4].
In a study by Spehar et al. [166], embryonic, larval,
and early juvenile stages of fathead minnows (Pimephales
promelas) were exposed to fenvalerate in a continuous-flow
system for 30 days. At 0.33 µg/litre the only effect was
a temporary initial impairment of swimming in some larvae.
This was more marked at 0.43 µg/litre at which level
survival of the larvae was also reduced. The 30-day bio-
concentration factor was 3000 ± 1500, but 25 days after
transfer to clean water the fenvalerate had again been
eliminated.
Rainbow trout (Salmo gairdneri) were used to evaluate
the gill uptake and toxicokinetics of [3H]-fenvalerate.
Fish (weight between 0.64 and 0.97 kg) were exposed in a
respirometer-metabolism chamber to technical grade
fenvalerate (0.28 or 23 ng/litre) or an emulsifiable-
concentrate formulation (16 ng/litre) at 11.0-11.5 °C for
36 to 48 h. No significant effects of emulsifiers or
fenvalerate concentration on uptake were observed. The
overall mean gill uptake efficiency was 28.6 ± 4.4%. After
8- to 48-h depuration periods, carcass and bile contained
80-90% and 10-20% of the gill-absorbed material, respect-
ively. Urine, faeces, and blood each contained less than
2% of the dose. Significant excretion and blood transport
of fenvalerate equivalents were completed within 8-12 h
after exposure ceased. Specific tissues from trout exposed
to 0.28 ng/litre were analyzed for fenvalerate equival-
ents. After a 48-h depuration period, bile contained the
highest concentration of fenvalerate equivalents (7 ng/g),
followed by fat (0.2 ng/g). Remaining tissues contained
0.015-0.045 ng/g. Analysis of biliary metabolites indi-
cated that the glucuronide of 4 -OH-fenvalerate was the
only significant degradation product. Results from the
present study suggest that efficient gill uptake does not
explain the sensitivity of fish to fenvalerate. Instead,
a low rate of biotransformation and excretion may play a
significant role in the susceptibility of rainbow trout
[14].
When juvenile Atlantic salmon were exposed to static
water containing 0.8-9.3 µg fenvalerate/litre for 16-96 h,
the concentration of fenvalerate in dead fish ranged from
0.16 to 0.43 mg/kg. The insecticide was not detected (de-
tection limit: 5 µg/kg) either in dead lobster hepatopan-
creas or in dead shrimps [110].
When carp (Cyprinus carpio) was exposed to [14C-CN]-
[2S, alphaRS]-fenvalerate (0.8 µg/litre) under semi-static
conditions for 7 days, the radioactivity in fish increased
to a level of 922 µg/kg. Once the fish were transferred
to fresh water, the levels of radioactivity in the fish
decreased with an initial half-life of 5 days [135].
In studies by Ohkawa et al. [135], carp, snails,
Daphnia, and algae were exposed to fenvalerate in an
aquatic model ecosystem where 14C-[2S, alphaRS] fenval-
erate (0.3 mg/kg) was applied to the bottom sandy loam
soil. During a 30-day run, concentrations of fenval-
erate in the water were 0.35-0.63 µg/litre and 0.14-
0.21 µg/litre on days 7 and 30, respectively. The bio-
concentration factors for fenvalerate were 122, 617, 683,
and 477 on day 7 (162-300, 993-1110, 629-829, and 714-1180
on day 30) in carp, snails, Daphnia, and algae, respect-
ively. In carp, large amounts of CP-Vacid (17) and
3-phenoxybenzoic acid (22) were detected, together with
small amounts of alpha-cyano-3-(4'-hydroxyphenoxy)benzyl-2-
(4-chlorophenyl)-3-methylbutyrate (4'-OH-Fen) (25). Small
amounts of alpha-carbamoyl-3-phenoxybenzyl-2-(4-chlorophe-
nyl)-3-methylbutyrate (CONH2-Fen) (33), alpha-carboxy-3-
phenoxybenzyl-2-(4-chlorophenyl)-3-methylbutyrate (COOH-
Fen) (34), and 4 -OH-Fen (25) were detected in snails.
CPIA was specifically present in both Daphnia (prey) and
carp (predator). CONH2-Fen (33) and alpha-carboxy-3-phenoxy-
benzyl alcohol (36) were common to algae (prey) and carp
(predator). Based on the products identified, degradation
pathways were proposed for this aquatic model ecosystem
(Fig. 4).
In a 28-day early-life stage study (see section
6.1.2), the mean bioconcentration factor in whole fish was
570 [68].
 7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
Table 8 shows the results of acute toxicity tests with
technical grade fenvalerate in various animal species.
The acute toxic signs in rats were restlessness,
tremors, piloerection, occasional diarrhoea, and an abnor-
mal gait. Following oral administration, surviving rats
recovered rapidly from acute clinical signs of poisoning
and were asymptomatic within 3-4 days [20].
Table 9 shows the results of an acute intraperitoneal
toxicity study of fenvalerate metabolites in mice [96].
All the compounds were dissolved in corn oil except
3-phenoxybenzoic acid, which was dissolved in DMSO. The
acute intraperitoneal toxicity in mice of the proposed
decarboxylated photo-products was found to be similar to,
or greater than, that of fenvalerate [78].
In a study by Blair and Roderick [12], groups of four
male and four female rats were exposed by inhalation (head
only) to an aerosol formulation (77-µm particle size)
generated from an aqueous suspension containing 3 g/litre.
Following a single administration (4 h) of this non-
inhalable particulate, acute signs of poisoning were noted
for a short period, presumably from oral ingestion of the
large particles. There was no mortality and all animals
appeared normal within 3 days following exposure.
7.2 Short-Term Exposures
7.2.1 Oral administration
Groups of Carworth Farm E rats (12 of each sex per
group) were fed fenvalerate in the diet at dose levels of
0, 125, 500, 1000, and 2000 mg/kg for 90 days [72]. Mor-
tality (11/12 male, 9/12 female) was observed at the
highest concentration. Body weight gain and food consump-
tion were decreased and blood urea nitrogen concentrations
were increased at 1000 and 2000 mg/kg. There were no
treatment-related changes in any groups of rats in the
haematological parameters examined. Increases in liver to
body weight ratios and kidney to body weight ratios were
observed at 500 mg/kg or more. Gross and microscopic
examinations revealed no compound-related changes in any
groups. The NOEL was 125 mg/kg diet.
Table 8. Acute toxicity of fenvalerate (technical grade) administered to various species
-------------------------------------------------------------------------------------------------
Species Route Sex Vehiclea LD50 (mg/kg) Reference
-------------------------------------------------------------------------------------------------
Rat oral DMSO 451 195
oral PEG:water > 3200 168
dermal 5000 (24 h) 140
inhalation M, F water > 101 mg/m3 (3 h) 94
intraperitoneal 340 162
Mouse oral M DMSO 200-300 195
F 100-200
oral PEG:water 1202 169
intraperitoneal M, F corn oil 85-89 96
intraperitoneal 132 162
intravenous glycerolformol 65 2
inhalation M, F water > 101 mg/m3 (3 h) 94
Chinese oral M DMSO 98 195
hamster F 82
Rabbit percutaneous undiluted 1000-3200 75
Hen oral > 1500 123
-------------------------------------------------------------------------------------------------
a PEG = polyethylene glycol; DMSO = dimethylsulfoxide.
Table 9. Acute intraperitoneal toxicity of fenvalerate metabolites in mice
---------------------------------------------------------------------------
Chemical No.a LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------------
Fenvalerate (5) 88.5 85
2-(4-Chlorophenyl)isovaleric acid (17) 351 350
3-Phenoxybenzyl alcohol (19) 371 424
3-(4'-Hydroxyphenoxyl) benzyl alcohol (21) 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol (20) 876 778
3-Phenoxybenzoic acid (22) 154 169
3-(4'-Hydroxyphenoxy) benzoic acid (24) 783 745
3-(2'-Hydroxyphenoxy) benzoic acid (23) 859 912
3-Phenoxybenzaldehyde (18) 415 416
NaSCN 604 578
---------------------------------------------------------------------------
a Refers to chemical identification no. in Fig. 3 and in text.
In a study by Parker et al. [150], Fischer 344 rats
(30 of each sex per group) were fed decarboxyfenvalerate
(one of the major photodegradation products) at concen-
trations of 0, 30, 100, 300, 3000 or 10 000 mg/kg diet for
up to 13 weeks. Body weight was decreased in male rats fed
10 000 mg/kg, but no treatment-related mortality or clini-
cal signs were observed. Absolute and relative liver
weight of male and female rats fed 300, 3000, or 10 000
mg/kg were all higher than those of the controls. Signifi-
cant increases in absolute or relative kidney weights were
observed in male and female rats fed 3000 or 10 000 mg/kg.
Significant treatment-related microscopic effects were
limited to glomerulonephrosis in male and female rats fed
10 000 mg/kg and hepatocellular hypertrophy with pale
eosinophilic cytoplasm and hepatocellular focal necrosis
in male and female rats fed 3000 or 10 000 mg/kg. A NOEL
of 300 mg/kg diet was established in this study.
Groups of young adult beagle dogs (four of each sex
per group) were fed fenvalerate in the diet at dose levels
of 0, 0.25, 0.5, 1.25, or 12.5 mg/kg body weight for 90
days [70]. There were no treatment-related changes in body
weight, food consumption, clinical signs, and clinical
laboratory data. Gross and microscopic examinations
revealed no effects of the fenvalerate. Thus, daily
administration at a level of 12.5 mg/kg body weight for a
period of 90 days produced no detectable evidence of toxi-
cological effect.
In a study by Parker et al. [148], male and female
beagle dogs (six of each sex per group) were fed diets
containing 0, 250, 500, or 1000 mg fenvalerate/kg diet for
a period of 6 months. Prominent clinical signs related to
treatment were emesis, head shaking, biting of the ex-
tremities, and tremors. The mean body weights of female
dogs fed fenvalerate at 1000 mg/kg were significantly
lower than those of controls. Red blood cell counts and
haematocrit and haemoglobin values in both male and female
dogs fed the highest dose were significantly lower. Serum
cholesterol and alkaline phosphatase levels were also
increased, mostly in the group fed 1000 mg/kg. Hepatic
multifocal microgranulomas observed during microscopic
examination increased in incidence and severity in a dose-
dependent way and were considered to be related to treat-
ment. Histiocytic cell infiltrates in the mesenteric lymph
nodes of some female dogs fed 500 or 1000 mg/kg and of
male dogs fed 1000 mg/kg were the only other treatment-
related effects observed microscopically.
7.2.2 Inhalation
Groups of Sprague-Dawley rats and ICR mice (10 of each
sex per group) were exposed to fenvalerate by inhalation
for 3 h daily for 4 weeks at concentration levels of 0, 2,
7, or 20 mg/m3 (fully respirable particle size). Although
animals showed acute signs of poisoning at the highest
dose level, no mortality was observed in any group. There
were no treatment-related effects in body weight, haema-
tology, or clinical biochemistry parameters, nor were
there any gross or microscopic abnormal findings [82,
95].
7.2.3 Dermal application
In a study by Hine [75], groups of rabbits (7-8 male
rabbits per group) were administered fenvalerate dermally
at dose levels of 0, 100, or 400 mg/kg daily for 6 h
(14 exposures were performed over a 22-day period).
Severe weight loss, clinical signs of poisoning, and gross
dermal effects were observed at 400 mg/kg, where mortality
was also observed.
7.3 Skin and Eye Irritation; Sensitization
7.3.1 Skin and eye irritation
Two formulated products (an emulsifiable concentrate
and an ultra-low-volume formulation) were found to be
severe eye and skin irritants in rabbits. Dermal irri-
tation was evident for 7 days after a 24-h exposure, and
severe conjunctivitis, corneal opacity, and iritis were
observed within 30 min of an application of 0.2 ml of the
formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation [29, 30]. However,
when experiments were carried out using pure (non-formu-
lated) fenvalerate, there was no irritation [138].
7.3.2 Skin sensitization
Skin sensitization by pure fenvalerate (95%) has been
evaluated using the Landsteiner-Draize method on guinea-
pigs. No sensitization was detected by Okuno et al.
[139].
7.4 Long-Term Exposures and Carcinogenicity
7.4.1 Mouse
When groups of ddY mice (35-47 of each sex per group)
were administered fenvalerate in the diet for 78 weeks at
levels of 0, 100, 300, 1000, or 3000 mg/kg, mortality
occurred at the highest dose level. Hyperexcitability was
observed at 1000 mg/kg or more, and body weight was
depressed at 3000 mg/kg over the 18-month period and at
1000 and 3000 mg/kg over the first 3 months. A variety of
haematological parameters were affected at 3 months, pre-
dominantly at the highest dose level, but no haematol-
ogical changes were observed at the end of the study.
Several biochemical changes suggestive of hepatotoxicity
were observed at 3 months and at termination of the study
in the 300, 1000, and 3000 mg/kg groups. There were gross
changes in several organ weights and in organ-to-body
weight ratios, predominantly in the liver. Microscopic
examination revealed changes in the liver, mesenteric
lymph nodes, and kidney. Dose-dependent granulomatous
changes were observed in the liver and/or mesenteric lymph
nodes in all treatment groups. At the 3-month interim
sacrifice, multiple small necrotic foci in the liver and
changes in the epithelial cells of the proximal convoluted
tubules were noted at the two highest dose levels. There
were no indications in this study of tumorigenicity or
carcinogenicity as a result of fenvalerate administration
[81, 83, 173, 175].
In studies by Okuno et al. [144], male ddY mice were
fed diets containing the [2S, alphaS], [2S, alphaRS], [2R,
alphaS], and [2R, alphaR] isomers of fenvalerate at diet-
ary dose levels of 0, 500, or 1000 mg/kg, 500, 1000, or
2000 mg/kg, 0, 125, or 1000 mg/kg, and 125, or 1000 mg/kg
for 52, 52, 13, and 13 weeks, respectively. Microgranulo-
matous changes were observed in the mice treated with the
[2R, alphaS] isomer after 1, 2, or 3 months. In contrast,
the changes did not occur in mice treated with the [2R,
alphaR] isomer under the same conditions. Neither [2S,
alphaS] nor [2S, alphaRS] isomers caused microgranulomatous
changes at 500 or 1000 mg/kg after 1 year. To clarify the
causative agent of granuloma formation, the cholesterol
ester of 2-(4-chlorophenyl)isovaleric acid (CPIA), a
lipophilic conjugate from the [2R, alphaS] isomer of fenva-
lerate, was injected intravenously into ddY mice. Micro-
granulomatous changes were observed in the liver of mice
treated with the [2R]-, [2S]-, or [2RS]-CPIA-cholesterol
esters 1 week after a single treatment of 1, 10, or 100
mg/kg body weight, as well as in the liver of mice treated
with a single dose of 10 or 30 mg/kg body weight of the
[2R]-CPIA-cholesterol ester and kept up to 26 weeks after-
wards. Histochemical examination and microscopic autoradio-
graphy of the liver demonstrated the presence of tritium,
derived from 3H-labelled [2R]-CPIA and cholesterol in giant
cells and Kuppfer cells. Another histochemical examination
showed the presence of cholesterol ester in the liver of
mice treated with the [2R, alphaS] isomer. These results
support the hypothesis that the CPIA-cholesterol ester is
the causative agent of the microgranulomatous changes ind-
uced by fenvalerate.
In further studies by Okuno et al. [145], male and
female ddY mice were fed diets containing technical
fenvalerate (either 0, 10, 30, 100, or 300 mg/kg diet for
20 months or 0, 100, 300, 1000, or 3000 for 17-18 months).
Microgranulomatous changes were observed in the lymph
nodes, liver, and spleen, the NOEL for such changes being
30 mg/kg. To examine the reversibility of these changes,
ddY mice (male and female) were fed a diet containing
technical fenvalerate at dose levels of 1000 and 3000
mg/kg for 6 weeks, followed by a control diet for up to
12 months. The size and number of the microgranulomatous
changes were reduced with time. These changes were typical
of foreign body granulomas and did not have the appearance
of granulomas formed in response to an immunological
stimulus.
When B6C3F1 mice (50 of each sex per group) were fed
fenvalerate at dietary concentrations of 0, 10, 50, 250,
or 1250 mg/kg for 2 years, mortality was increased and
body weight significantly decreased in male and female
mice fed 1250 mg/kg. The mean body weight of female mice
fed 250 mg/kg was also generally lower than that of con-
trols after the 60th week of feeding. The only treatment-
related non-neoplastic pathological effect observed in the
study was multifocal microgranulomata in the lymph nodes,
liver, and spleen of male mice fed 1250 mg/kg and of
female mice fed 250 or 1250 mg/kg. No statistically sig-
nificant differences were observed in either the number or
type of neoplasms in mice fed fenvalerate (compared to
concurrent controls). Thus, fenvalerate was found not to
be carcinogenic in B6C3F1 mice under the conditions of
the test [146].
7.4.2 Rat
When groups of Wistar rats (15 of each sex per group)
were fed fenvalerate at concentrations of 0, 50, 150, 500,
or 1500 mg/kg diet for 15 months, there was no mortality
attributable to fenvalerate. The hyperexcitability ob-
served during the early stages of the study disappeared
within 3 months. Body weight was significantly depressed
in both sexes at the highest dose level. No compound-
related changes were detected in the urine or in the eyes,
but the haemoglobin concentration was depressed in males
at the highest dose level and the females at 150 mg/kg or
more. Several blood biochemistry parameters were signifi-
cantly altered at the highest dose level (blood urea
nitrogen was increased in both sexes; protein content and
plasma cholinesterase were decreased in females). Gross
and microscopic examination revealed no dose-related
effects [174].
In a study by Gordon & Weir [66], groups of Sprague-
Dawley rats (93 males and 93 females per treated group;
183 of each sex used as the control group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25,
250, or 500 mg/kg. There was no compound-related mor-
tality, although body weight was reduced at the highest
dose level. The group fed 500 mg/kg and a separate control
group were sacrificed at 26 weeks while the other animals
were maintained for 2 years. There were no significant ef-
fects on food consumption, growth, behavior, haematology,
blood biochemical composition or urine consumption. At the
conclusion of the study, organ weight and organ-to-body
weight ratios were normal. Gross and microscopic findings
in the treated groups did not differ significantly from
those of the controls. A specific pathology examination
of the sciatic nerve of animals fed 250 mg/kg revealed no
treatment-related changes. Thus, the no-observed-effect
level in this study was 250 mg/kg diet.
Parker et al. [147] fed Sprague-Dawley rats (93 of
each sex per group) diets containing 0.1, 5, 25, or 250 mg
fenvalerate/kg for up to 2 years. The control group con-
sisted of 183 males and 183 females. Ten treated and 20
control rats of each sex from each group were killed at
intervals of 3, 6, 12, and 18 months. When body weight,
organ weight, food consumption, haematology, and clinical
chemical analysis measurements did not reveal any effect
resulting from the treatment, two additional groups of
rats (50 of each sex per group) were fed 0 or 1000 mg
fenvalerate/kg diet for 2 years. Body weight was decreased
and organ-to-body weight ratios were increased in brain,
liver, spleen, testes, kidneys (females only), and heart
(females only), in the treated animals. Mammary and pitu-
itary tumours were commonly observed, along with a variety
of other tumours that occurred randomly among all control
and treatment groups. No statistically significant differ-
ences in the number and type of neoplasms were observed,
except for mammary tumours in females in the main study.
These effects were judged not to be toxicologically sig-
nificant, since mammary tumour incidences did not exceed
expected incidences in aged female Sprague-Dawley rats.
In addition, the time taken for tumours to appear was un-
changed, and no change in the ratio of benign to malignant
tumours occurred. Sarcomas identified in the subcutis and
dermis in 5 out of 51 males fed 1000 mg/kg were also
identified in 2% (1/50), 2% (2/102), and 0-6% of concur-
rent, original, and historical controls, respectively.
The no-observed-effect level was 250 mg/kg.
When male and female Wistar rates were fed a diet
containing technical fenvalerate at 0, 50, 150, 500, or
1500 mg/diet for 24-28 months, microgranulomatous changes
were observed in lymph nodes, liver, spleen, and adrenal
glands. The no-observed-effect level for these microgranu-
lomatous changes was 150 mg/kg [145].
7.5 Mutagenicity
7.5.1 Microorganism and insects
The DNA-damaging capacity of fenvalerate has been
examined in a Rec-assay with Bacillus subtilis M45 rec-
and H17 wild type strains at concentrations up to 10
mg/disk per plate. Fenvalerate had no inhibitory effect on
the growth of indicator strains, and was judged to be non-
mutagenic [170].
Fenvalerate has also been examined for its mutagenic
potency with the Ames test in Salmonella typhimurium
(TA 1535, TA 1538, TA 98, and TA 100), using dose levels
of up to 1 mg/plate both with and without a metabolic
enzyme system. Fenvalerate was non-mutagenic in these
tests [170]. It was also tested using hepatic metabolic
enzyme systems prepared from various PCB-treated animals
(three strains of rats, six strains of mice and the Syrian
golden hamster). At dose levels of up to 1 mg/plate,
Fenvalerate was non-mutagenic [171, 172].
In further studies by Suzuki & Miyamoto [170], fenval-
erate was given orally at doses of 60 and 125 mg/kg to
groups of mice, and indicator cells (S. typhimurium G46)
were injected intraperitoneally. Fenvalerate did not
induce any significant level of mutation among the indi-
cator cells recovered from the abdominal cavity. On the
other hand, the positive control, dimethylnitrosamine,
significantly increased the mutation frequency of the
indicator organism.
Another host-mediated assay of fenvalerate in mice was
conducted using Saccharomyces cerevisiae as indicator
microorganism. Groups of mice were administered fenval-
erate orally at doses of 25 and 50 mg/kg, and were in-
jected with a suspension of indicator cells intraperito-
neally. No mutagenic effect on the indicator cells was
detected [17].
Fenvalerate was not found to be mutagenic in S.
typhimurium strains TA 100 or TA 98 in the presence or
absence of a rat liver activation system by fluctuation
tests at a concentration of up to 10 µg/ml or in V79
Chinese hamster cells in the presence or absence of
hepatocytes at a concentration of up to 40 µg/ml [153].
Fenvalerate did not induce sex-linked recessive
lethals, sex-chromosome losses, or non-disjunction in
Drosophila melanogaster when it was given to adults (up
to 20 mg/litre in the diet) or larvae (up to 50 mg/litre
in the diet), or was injected into adults (at 20 µg/ml)
[7].
7.5.2 Rat
In a study by Chatterjee et al. [26], groups of rats
(21 per group) were administered fenvalerate orally at
doses of 50, 75, or 100 mg/kg per day for 3 weeks. The
rats were killed 24 h after the last treatment and bone
marrow cells were examined for chromosomal aberrations.
Although an increase in the frequency of chromosomal aber-
rations was observed in fenvalerate-treated animals, it
was not possible to draw any definite conclusion because
it was not dose related and may have been non-specific.
Fenvalerate has also been studied for the enhancement
of gamma-glutamyl transpeptidase-positive enzyme-altered
focus incidence in partially hepatectomized, nitrosodi-
ethylamine-initiated male Sprague-Dawley rats. Fenvalerate
administered peritoneally (75 mg/kg body weight per day,
5 days a week for 10 weeks) induced significantly more
foci per cm3 and a larger percentage of liver tissue
occupied by focus tissue, compared with a vehicle-control
group. Analysis of the size distribution of foci in
fenvalerate- and vehicle-treated rats showed elevated
focus incidences in fenvalerate-treated rats at all focus
sizes. Fenvalerate induced no hepatotoxic effects, as
judged by serum transaminase activities and histopatho-
logical analysis [58].
7.5.3 Mouse
In a dominant lethal assay, groups of male mice (10-11
per group) were administered fenvalerate orally at doses
of 25, 50, or 100 mg/kg body weight. Each male was mated
with three virgin females for 7 days. The procedure was
repeated weekly as a standard dominant lethal test. The
females were sacrificed and examined for dominant
lethality at the 13th day of gestation. Fetal implants in
females, mated to males that had been treated with 100
mg/kg for 2 weeks, showed a significant reduction in
viability. A significant increase in early fetal death
was observed in females mated with males that had been
treated for 4 weeks with the highest dose [34].
The significance of the above data was further studied
as follows:
(1) By using a two-way analysis of variance, it was
judged that the reduction in fetal implants in females
mated to males the second week after dosing at 100 mg/kg
and the increase in early fetal deaths in the 4th week
were statistically significant. But these increases or
decreases appeared to be random and were not considered to
be biologically significant.
(2) Using a t-test and the Mann-Whitney U-test, no
significance was shown in any mean proportions for the
above parameters.
From these findings, it was concluded that fenvalerate
did not cause dominant lethal effects in micea.
----------------------------------------------------------
a Personal communication, J. Miyamoto, 1981, Comments on
"Further work information" required by 1979 JMPR on
fenvalerate, Laboratory of Biochemistry and Toxicology
(Unpublished report submitted to WHO by Sumitomo
Chemical Co. Ltd).
7.5.4 Hamster
In a study by Dean & Senner [35], fenvalerate was
administered orally to groups of hamsters (six males and
six females per group) at two successive daily doses of
12.5 and 25 mg/kg. The chromosomal preparations were made
8 or 24 h after administration. Fenvalerate did not induce
any chromosomal damage in the bone marrow cells from
treated animals, whereas the positive control, methyl
methanesulfonate (50 mg/kg), had induced a substantial
number of chromatid gaps within 8 h of dosing.
Fenvalerate, and the fenvalerate metabolite 2-(4-
chlorophenyl)isovaleric acid, were investigated for the
inhibition of gap-junctional intercellular communication
in vitro in the Chinese hamster lung fibroblast (V79)
metabolic cooperation assay [58]. This study showed that
both fenvalerate and 2-(4-chlorophenyl)isovaleric acid
were inhibitors of intercellular communication at non-
cytotoxic concentrations.
7.6 Teratogenicity and Reproduction Studies
7.6.1 Teratogenicity
In studies by Kohda et al. [93], groups of pregnant
ICR mice (32-33 per group) were orally administered
fenvalerate at dose levels of 0, 5, 15, or 50 mg/kg per
day on days 6 to 15 of gestation. Groups of 20 mice were
sacrificed on day 18, and the fetuses were removed and
examined for visceral and skeletal abnormalities. The
remaining dams were allowed to deliver naturally and the
young were maintained until weaning to evaluate postnatal
deficits. Additionally two male and two female weanlings
from each dam were maintained for 8 weeks and mated to
investigate their reproductive potential. Although toxic
signs were noted in the dams at the highest dose level,
there was no significant mortality. Examination of the
fetuses revealed no external, visceral, or skeletal abnor-
malities. Treatment of the dams with fenvalerate did not
affect the reproductive performance of the offspring.
Van Der Pauw et al. [187] dosed groups of pregnant
Dutch rabbits (20 to 31 per group) orally with fenvalerate
(0, 12.5, 25, or 50 mg/kg body weight per day) from day 6
to day 18 of gestation. The dams were sacrificed on day
28 and standard teratogenic assessments made. The body
weights of the dams given the highest dose were reduced.
There were no significant differences from controls in any
of the other parameters examined. Fenvalerate was not
found to be teratogenic in this study.
7.6.2 Reproduction studies
In studies by Stein [167] and Beliles et al. [10],
groups of Sprague-Dawley rats (11 males and 22 females per
group) were fed fenvalerate in the diet at levels of 0, 1,
5, 25, or 250 mg/kg. The animals were dosed for 9 weeks
prior to mating and the initiation of a standard three-
generation (two litters per generation) reproduction
study. Fertility, viability, gestation, and lactation in-
dices were calculated for each group of rats and were com-
pared to control values. Ten of the female weanlings and
all of the males from the F3b litters were examined his-
tologically at the conclusion of the study. The mean body
weight of the F2b adults was decreased at 250 mg/kg, but
no pathological changes were noted to account for this
weight loss. No effects on reproductive parameters in any
of the three generations were observed. Histological
examination revealed no treatment-related changes in any
group.
Groups of pregnant ICR mice (32-33 mice per group)
were orally administered fenvalerate at dose levels of 0,
5, 15, or 50 mg/kg body weight per day on days 6 to 15 of
gestation in a standard teratogenicity bioassay. Two male
and two female weanlings from each dam were maintained for
8 weeks and mated to investigate their reproductive poten-
tial. Toxic signs were noted in maternal mice at the
highest dose level. There was no significant mortality
over the course of the study, and no effects were noted on
any of the other animals as a result of continuous admin-
istration of fenvalerate. The animals maintained in the
abbreviated reproduction study showed no differences from
the control value in their ability to reproduce. There
were no changes in the reproduction indices with any
animals examined [93].
7.7 Neurotoxicity
In a study by Butterworth & Carter [20], histopatho-
logical examination was performed on the sciatic nerve and
posterior tibial nerve of rats that had been exposed to
acutely toxic levels of fenvalerate. After poisoning, and
for 9 days during the course of recovery, axonal breaks,
swelling, and vacuolisation, accompanied by phagocytosis
of myelin, were seen. The degree to which myelin was dis-
rupted was dose dependent and was closely associated with
the acute signs of toxicity.
Acute oral administration of fenvalerate, cyper-
methrin, resmethrin, permethrin, and natural pyrethrum to
rats at very high dose levels resulted in severe clinical
signs of poisoning and mortality within 24 h. Histopatho-
logical lesions were observed in the sciatic nerve with
all compounds tested. Fenvalerate did not cause the clini-
cal signs or histopathological lesions at a lower dose
level (200 mg/kg), nor did the other compounds [141,
142].
When groups of six male and six female rats were fed
fenvalerate in the diet at a concentration of 2000 mg/kg
for 8 to 10 days, all the animals showed typical signs of
acute intoxication, such as ataxia, tremors, and hyperex-
citability. Histopathological examinations did not reveal
any adverse effects of fenvalerate on the sciatic nerve
[73].
In order to evaluate the reversibility of the lesions
induced in the sciatic nerve, rats were administered
fenvalerate in the diet at dose levels of 0 or 3000 mg/kg
diet for 10 days. This was followed by a control diet for
12 weeks. During the treatment period, mortality was 60%
in the animals treated with fenvalerate. Rats on the
recovery control diets, sacrificed at 3 weeks, continued
to show swelling and disintegration of axons of the
sciatic nerves. However, there were no histopathological
lesions after 6, 9, or 12 weeks of the recovery period.
These results showed the reversibility of the sciatic
nerve lesions caused by fenvalerate [143].
In studies by Butterworth & Hend [21], fenvalerate was
administered orally to Wistar or Carworth Farm E (CFE)
rats either as single doses or in the diet. When given in
large quantities by a single dose of 250, 500, 800, or
1000 mg/kg body weight, which were sufficient to kill some
of the treated animals, fenvalerate produced sporadic
Wallerian degeneration in the sciatic nerve. The neuro-
pathy was never severe and was not seen in animals given
the compound in sub-lethal doses. In feeding studies (for
5 weeks at 1000 mg/kg diet and for 3 months at 2000 mg/kg
diet), no lesions were seen in the peripheral nerve,
brain, or spinal cord, and there was no evidence of cumu-
lative neurotoxicity.
B6C3F1 mice and Sprague-Dawley rats showed the
characteristic signs of intoxication following single oral
doses of fenvalerate ranging from 56 to 320 and 133 to
1000 mg/kg body weight, respectively. Neurological signs,
such as splayed gait, tremors, ataxia, and hind limb
incoordination, were observed at doses of 100 mg/kg or
more (mice) and 133 mg/kg or more (rats) within 1-8 h
after dosing. These signs had disappeared in most animals
within 72 h. Slight peripheral nerve fibre damage was
detected in surviving mice and rats sacrificed 10 days
after dosing. The incidence and severity were dose related
at doses > 56 and > 180 mg/kg; however, even at lethal
doses, there was no evidence of nerve lesions in some
animals. Thus, two distinct neurological effects were
observed, i.e., (a) a reversible ataxia and (b) incoordi-
nation plus a neuropathological effect manifested as
sparse axonal damage in peripheral nerves [149].
In a study by Milner & Butterworth [123], groups of
six hens were administered fenvalerate orally at dose
levels of 0 or 1000 mg/kg per day for 5 days. A positive
control of tri- ortho-cresyl phosphate (0.5 ml/kg) (TOCP)
was also included in the study. The fenvalerate-treated
birds were retreated, using the same dose regimen, after
3 weeks. The TOCP-treated hens showed signs of delayed
neurotoxicity and histopathological lesions in their
sciatic nerve and spinal cord. As would be expected for a
non-organophosphorus insecticide, there were no typical
clinical signs and histopathological lesions related to
fenvalerate.
7.8 Behavioural Studies
Guinea-pigs responded to dermal applications of
fenvalerate by scratching the treated sites of the skin.
This characteristic response was essentially over within
3-4 h. When the powerful skin irritant oil of mustard was
applied to fenvalerate-treated sites of skin 4-72 h after
the fenvalerate treatment, the behavioural skin sensory
response was re-stimulated. Oil of mustard alone did not
produce skin sensory stimulation. These results indicate
that pyrethroid treatment causes a transient sensitivity
to stimulation produced by chemical irritants [111].
To develop an animal model for studying skin sensory
stimulation, Duncan-Hartley guinea-pigs were treated with
pyrethroid solutions on one side and control substances on
the other side of their shaved back. The animals responded
by licking, scratching, or biting the test sites, and
activity was quantified by counting the number of times
the animals responded. This behavioural activity reached
a maximum 1-4 h after treatment. A chemical irritant (oil
of mustard) was able to restimulate the behavioural ac-
tivity when applied within 24 h after pyrethroid appli-
cation. Skin sensory stimulation produced by cyano-
containing pyrethroids, including fenvalerate, was
significantly greater than that produced by non-cyano-
containing pyrethroids. This behavioural model provides a
quantitative means of evaluating pyrethroid non-erythema-
tous skin sensory stimulation [22].
7.9 Miscellaneous Studies
In an antidotal study, phenobarbital, pentobarbital,
and diphenylhydantoin were found to be effective in
relieving the acute signs of intoxication in the rat.
Intraperitoneal injection of phenobarbital (50 mg/kg)
prevented tremor, diphenylhydantoin (100 mg/kg) by the
same route reduced the toxic reaction, and pentobarbital
(35 mg/kg intraperitoneally) removed the tremor reaction
completely within 30 min. The combination of diphenyl-
hydantoin with either of the barbiturates was effective in
reducing the onset and severity of tremors whereas various
other agents d-tubocurarine, atropine, meprobamate,
diazepam, biperiden, and trimethadione) were ineffective
[113].
The therapeutic potency of intraperitoneally admin-
istered methocarbamol was examined as an antidote against
the acute oral intoxication of rats by a lethal dose of
fenvalerate. Methocarbamol was initially administered at
a dose of 400 mg/kg body weight, followed by repeated
doses of 200 mg/kg body weight when tremors or hyperexcit-
ability to sound were observed. Methocarbamol markedly
decreased the mortality from 80%, which would be caused by
an administration of 850 mg fenvalerate/kg, to 0%, and was
effective in alleviating motor symptoms such as fibril-
lation, tremors, hyperexcitability, clonic seizures, and
choreoathetotic movements. A subcutaneous administration
of atropine sulfate (25 mg/kg body weight) was also effec-
tive in reducing the salivation produced by fenvalerate
[76].
Effective treatments against fenvalerate-mediated ef-
fects have been investigated by quantifying behavioural
skin sensory responses such as licking, scratching, or
biting of the treated sites by fenvalerate-treated guinea-
pigs. Preparations containing vitamin E, corn oil, or the
local anesthetic benzocaine were most effective [111].
Intraperitoneal administration of O-ethyl- O-(4-nitro-
phenyl)phenylphosphonothioate (EPN) or S,S,S-tributylphos-
phorotrithioate (DEF) to mice at 25 mg/kg increased the
intraperitoneal toxicity of fenvalerate (administered 1 h
later) by more than 25-fold; the LD50 decreased from
> 1000 mg/kg to 37 or 42 mg/kg. This suggests that mam-
malian esterases highly sensitive to inhibition by certain
organophosphorus compounds may play a critical role in
fenvalerate detoxication. This kind of synergism among
pesticides would be detrimental in increasing the toxicity
of certain pyrethroids to mammals [62].
Fenvalerate, administered to dogs at a dose sufficient
to induce toxic signs, showed no consistent cardiovascular
effects. Respiratory stimulation was noted at high levels,
and this was not reduced by anaesthetic supplements
(urethane, chloralose, and pentobarbital) [91].
7.10 Mechanism of Toxicity - Mode of Action
The intravenous toxicity of fenvalerate (50-100 mg/kg)
to rats was examined by Verschoyle & Aldridge [189].
[2S, alphaS]-Fenvalerate induced choreoathetosis with sali-
vation (CS-syndrome), and was classified as a Type II
pyrethroid. For the mode of action of pyrethroids in
general see Appendix 1.
The intracerebral injection of [2S, alphaS]-fenvaler-
ate (0.01 mg/kg) to mice produced the Type II syndrome,
consisting of choreoathetosis, convulsion, and saliv-
ation [98]. The Type II syndrome is produced character-
istically by pyrethroids with an alpha-cyano group and
the site of action in mammals is considered to be the
central nervous system.
In intact locusts and neuromuscular preparations of
locusts, fenvalerate caused (a) prolonged firing in the
crural nerve without associated muscle contractions; (b)
sustained muscle contractions; and (c) a block of neurally
evoked muscle contractions at low concentration (10-8 to
10-5 mol/litre). However, fenvalerate did not cause
repetitive firing and after-discharges with associated
muscle contractions [27]. The fenvalerate stereoisomers
with an (S) configuration in the alcohol moiety are more
active pharmacologically and toxicologically than those
with the (R) configuration or the racemate (R,S). It is
also apparent that stereoisomers with the (S) configur-
ation in the acid moiety are more active than those with
the (R) configuration or the racemate (R,S) [27].
[S,S]-Fenvalerate does not induce repetitive firing in
the cockroach cercal sensory nerves either in vivo or in
vitro. It does, however, cause different signs, including
bursts of spikes in the cercal motor nerve [60].
There are no clear-cut links between electrophysio-
logical findings in insects and toxicity to mammals.
8. EFFECTS ON HUMANS
8.1 Occupational Exposure
Appraisal
Fenvalerate has been found to induce skin sensations in
some of the workers who handle this insecticide. Clinical
studies showed that the skin sensations develop with a latent
period of approximately 30 min, peak by 8 h and deteriorate
after 24 h. Numbness, itching, tingling, and burning are symp-
toms frequently reported. Alpha-tocopheryl acetate has been
found to inhibit the occurrence of these skin sensations.
In a study by Kolmodin-Hedman et al. [97], personnel
(52 people) at various plant nurseries who had handled
conifer seedlings treated with fenvalerate were examined.
The symptoms were mainly irritative, such as itching,
paraesthesia and burning of the skin, and itching and
irritation of the eyes. The frequency (% of people who
reported these signs) was about 10%. Increased nasal
secretion was reported by 19% of the personnel.
No clinical case of pyrethroid poisoning had been
reported until outbreaks of acute deltamethrin and fenval-
erate poisoning occurred among cotton growers in China in
1982. Having been told (in error) that pyrethroids were
non-toxic, the farmers handled the pyrethroid insecticides
without taking any precautions. After repeated spraying
in the cotton fields, the mild cases presented severe
headaches, dizziness, fatigue, nausea, and anorexia, with
transient changes in the electroencephalogram (EEG), while
a severe case developed muscular fasciculation, repetitive
discharges in the electromyogram (EMG), and frequent con-
vulsions. However, all were found by follow-up studies to
have completely recovered and the prognosis of acute
pyrethroid poisoning proved to be correct [71]a.
----------------------------------------------------------
a More recently, the same author reviewed 573 cases of
acute pyrethroid poisoning reported in the Chinese
medical literature during 1983-1988 [213]. Among these
there were 196 cases of acute fenvalerate poisoning,
63 of which were occupational, due to inappropriate
handling and 133 accidental, mostly due to ingestion.
Two died of convulsions. All others recovered with
symptomatic and supportive treatment within 1-6 days.
A comprehensive review of clinical manifestations is
included.
Among 23 workers exposed to synthetic pyrethroids,
including fenvalerate, 19 experienced one or more episodes
of abnormal facial sensation, developing between 30 min
and 3 h after exposure and persisting for 30 min to 8 h
[106]. However, there were no abnormal neurological signs,
and electrophysiological studies showed normal responses
in the arms and legs. The symptoms were most likely due
to transient lowering of the threshold of sensory nerve
fibres or sensory nerve endings following exposure of the
facial skin to pyrethroids.
In a study by Tucker & Flannigan [182], selected
individuals who had worked extensively with fenvalerate in
the delta region of the Mississippi and Alabama, USA, were
interviewed and examined. They had, on some occasions,
noted paraesthesia associated with exposure to this insec-
ticide. The cutaneous sensation was described as a sting-
ing or burning, which progressed to numbness in approxi-
mately one-third of the exposed workers. The sensation
typically began a number of hours after contact, peaked in
the evening, and rarely was present the following morning.
The intensity of the sensation varied according to the
type and extent of exposure. Clinical signs of inflam-
mation such as oedema or vesiculation were not apparent.
Erythema was present in a few individuals but this was
difficult to distinguish from sunburn. Several environ-
mental factors were found to affect the cutaneous sen-
sation associated with fenvalerate exposure.
8.2 Clinical Studies
A double-blind study with 29 male volunteers was
performed to test the skin reaction to formulated fenval-
erate. The emulsifiable concentrate formulation was
diluted with water and applied to one side of the face, on
the cheek, with a control formulation on the opposite
cheek. There were no signs of dermatitis 24 h after appli-
cation, nor did the fenvalerate formulation produce any
abnormal skin sensations. There were no indications that
any of the symptoms such as tingling, itching, or burning
were associated with fenvalerate [18].
A double-blind study was performed to compare human
discrimination of technical fenvalerate, the heavy-ends
fraction of distilled fenvalerate, and ethyl alcohol
(vehicle) applied to the lower edge of each earlobe of 36
adult (both male and female) volunteers on three separate
occasions. Both forms of fenvalerate caused a statisti-
cally significant increase in paraesthesia, compared with
the vehicle alone. The onset of the cutaneous sensations
occurred 1 h after application, peaked at 3-6 h, and
lasted approximately 24 h. Numbness, itching, burning,
tingling, and warmth were the most frequently reported
sensations. The difference between the effects of the two
fractions of fenvalerate was not statistically significant
[92].
Flannigan & Tucker [55], Flannigan et al. [56, 57],
and Malley et al. [112] studied the difference in the
degree of paraesthesia induced by a number of pyrethroids.
Applications of 0.05 ml fenvalerate formulated to field
strength (0.13 mg/cm2) were made to a 4 cm2 area of
earlobe on five occasions, the opposite earlobe receiving
distilled water. Participant evaluation after each appli-
cation continued for 48 h and involved description of the
cutaneous sensations. Each participant was treated after
each application with one of the remaining compounds.
Fenvalerate (like the other pyrethroids) induced skin sen-
sations. The paraesthesia developed with a latent period
of approximately 30 min, peaked by 8 h, and deteriorated
as early as 24 h. The local application of dl-alpha
tocopheryl acetate markedly inhibited the occurrence of
skin sensations.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
has discussed and evaluated fenvalerate at its meetings in
1979, 1981, 1982, 1984, 1986, and 1987 [40-47, 49, 50, 52-
4].
Since 1986, an acceptable daily intake (ADI) of 0-0.02
mg/kg body weight has been established.
In the WHO Recommended Classification of Pesticides by
Hazard, technical fenvalerate is classified as "moder-
ately hazardous" (Class II) [197].
REFERENCES
1. AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10(2): 147-151.
2. ALBERT, J.R. & SUMMITT, L.M. (1976) Intravenous toxicity of WL 43775
(6-1-0-0) in the mouse (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
3. ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri and its prey,
Panonychus ulmi on apples in southeast England. Environ. Entomol., 9:
436-439.
4. ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several non-target aquatic invertebrates. Environ. Entomol., 11:
1251-1257.
5. ATKINS, E.L., KELLUM, D., & ATKINS, K.W. (1981) Reducing pesticide
hazards to honey bees: mortality prediction techniques and integrated
management strategies, Berkeley, California, University of
California, Division of Agricultural Sciences (Leaflet No. 2883).
6. BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
7. BATISTE-ALENTORN, M., XAMENA, N., VELAZQUEZ, A., CREUS, A., & MARCOS,
R. (1987) Non-mutagenicity of fenvalerate in Drosophilia.
Mutagenesis, 2: 7-10.
8. BATTELLE (1982) Pesticide programme of research and market planning.
Part I. Insecticides, Geneva, Battelle Research Centre.
9. BATTELLE (1986) World pesticide programme: Insecticide II, Geneva,
Battelle Research Centre.
10. BELILES R.P., MARIS, S.L., & WEIR, R.J. (1978) Three-generation
reproduction study in rats, Kensington, Maryland, Litton Bionetics
Inc. (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
11. BIRDIE, N.S., BANERJI, R.K., & CHAUHAN, A.K. (1986) Gas liquid
chromatographic separation of pyrethrins from some synthetic
pyrethroids in formulations. Pyrethrum Post, 16(3): 77-80.
12. BLAIR, D. & RODERICK, H. (1975) Toxicity studies on the pyrethroid
insecticide WL 43775, emulsifiable concentrate FX 3368: Acute
inhalation exposure to an aqueous spray (Unpublished report submitted
to WHO by Shell Development Co., Ltd).
13. BRADBURY, S.P. & COATS, J.R. (1982) Toxicity of fenvalerate to
bobwhite quail (Colinus virginianus) including brain and liver
residues associated with mortality. J. Toxicol. environ. Health, 10:
307-319.
14. BRADBURY, S.P., COATS, J.R., & MCKIM, J.M. (1986) Toxicokinetics of
fenvalerate in rainbow trout (Salmo gairdneri). Environ. Toxicol.
Chem., 5: 567-576.
15. BRADBURY, S.P., MCKIM, J.M., & COATS, J.R. (1987) Physiological
response of rainbow trout (Salmo gairdneri) to acute fenvalerate
intoxication. Pestic. Biochem. Physiol., 27: 275-288.
16. BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-
residue method to determination of synthetic pyrethroid residues in
celery and animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
17. BROOKS, T.M. (1976) Toxicity studies with WL 43775: Mutagenicity
studies with WL43775 in the host-mediated assay (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
18. BROWN, L.J. & SLOMKA, M.B. (1979) Skin reaction potential of use
dilution (1.33%) PYDRIN EC (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
19. BRUCE, M.L. & CARUSO, J.A. (1985) The laminar flow torch for gas
chromatographic He microwave plasma detection of pyrethroids and
dioxins. Appl. Spectrosc., 39(6): 942-949.
20. BUTTERWORTH, S.T.G. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL 43775: Acute oral toxicity and neuropathological
effects in rats (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
21. BUTTERWORTH, S.T.G. & HEND, R.W. (in press) Peripheral nerve lesions
induced by large doses of pyrethroid. Neuropathol. appl. Neurobiol.
22. CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin sensory stimulation
characterized by a new behavioural paradigm. Toxicol. appl.
Pharmacol., 76: 270-279.
23. CAPLAN, J.R., ISENSEE, A.R., & NELSON, J.O. (1984) Fate and effect of
[14C]fenvalerate in a tidal marsh sediment ecosystem model. J. agric.
food Chem., 32: 166-171.
24. CAYLEY, G.R. & SIMPSON, B.W. (1986) Separation of pyrethroid enan-
tiomers by chiral high-performance liquid chromatography. J.
Chromatogr., 356: 123-134.
25. CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four pyrethroid
insecticide in a mineral and organic soil. J. environ. Sci. Health,
B16: 605-615.
26. CHATTERJEE, K.K., TALUKDER, G., & SHARMA, A. (1982) Effects of
synthetic pyrethroids on mammalian chromosomes I. Sumicidin. Mutat.
Res., 105: 101-106.
27. CLEMENTS, A.N. & MAY, T.E. (1977) The actions of pyrethroids upon the
peripheral nervous system and associated organs in the locust.
Pestic. Sci., 8: 661-680.
28. COATS, J.R. & O'DONNELL-JEFFREY, N.L. (1979) Toxicity of four syn-
thetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
29. COOMBS, A.D. & CARTER, B.I. (1975) Toxicity studies on insecticide WL
43775: Acute toxicity, skin irritancy potential of the emulsifiable
concentrate FX 3368 (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
30. COOMBS, A.D. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL43775: Toxicity and skin and eye irritancy potential of
the ULV formulation FX4353 (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
31. CURTIS, L.R., SEIM, W.K., & CHAPMAN, G.A. (1985) Toxicity of
fenvalerate to developing steelhead trout following continuous or
intermittent exposure. J. Toxicol. environ. Health, 15: 445-457.
32. DAY, K. & KAUSHIK, N.K. (1987a) Short-term exposure of zooplankton to
the synthetic pyrethroid, fenvalerate, and its effects on rates of
filtration and assimilation of the alga Chlamydomonas reinhardii.
Arch. environ. Contam. Toxicol., 16: 423-432.
33. DAY, K. & KAUSHIK, N.K. (1987b) An assessment of the chronic toxicity
of the synthetic pyrethroid, fenvalerate, to Daphnia galeata
mendotae, using life tables. Environ. Pollut., 44: 13-26.
34. DEAN, B.J. (1975) Toxicity studies with WL 43775: Dominant lethal
assays in male mice after single oral doses of WL 43775 (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
35. DEAN, B.J. & SENNER, R.K. (1975) Toxicity studies with WL 43775:
Chromosome studies on bone marrow cells of Chinese hamster after two
daily oral doses of WL 43775 (Unpublished report to WHO submitted by
Shell Development Co., Ltd).
36. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series No. 42).
37. ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1979) Dislodgable
insecticide residue on cotton foliage: permethrin, curacron,
fenvalerate, sulprofos, decis, and endosulfan. Bull. environ. Contam.
Toxicol., 22: 245-248.
38. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
39. FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
40. FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49
(FAO Plant Production and Protection Paper 20).
41. FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
299-353 (FAO Plant Production and Protection Paper 20 Sup.).
42. FAO/WHO (1982a) Pesticide residues in food. Report of the 1981 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 26-28
(FAO Plant Production and Protection Paper 37).
43. FAO/WHO (1982b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
209-256 (FAO Plant Production and Protection Paper 42).
44. FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 28 (FAO
Plant Production and Protection Paper 46).
45. FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, p. 420
(FAO Plant Production and Protection Paper 49).
46. FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49,
83, 90-93 (FAO Plant Production and Protection Paper 62).
47. FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
343-353 (FAO Plant Production and Protection Paper 67).
48. FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations.
49. FAO/WHO (1986a) Pesticide residues in food. Report of the 1986 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 28-29,
61 (FAO Plant Production and Protection Paper 77).
50. FAO/WHO (1986b) 1986 Evaluations of some pesticide residues in food.
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 189-193, 355 (FAO Plant Production and Protection
Paper 78/1).
51. FAO/WHO (1986c) Codex maximum limits for pesticide residues, 2nd ed.,
Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC XIII).
52. FAO/WHO (1987a) 1986 Evaluations of some pesticide residues in food.
Part II - Toxicology, Rome, Food and Agriculture Organization of the
United Nations, p. 219 (FAO Plant Production and Protection Paper
78/2).
53. FAO/WHO (1987b) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 27 (FAO
Plant Production and Protection Paper 84).
54. FAO/WHO (1988) 1987 Evaluations of some pesticide residues in food.
Part 1 - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 57-59 (FAO Plant Production and Protection Paper
86/1).
55. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
56. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary irritant contact
dermatitis from synthetic pyrethroid insecticide exposure. Arch.
Toxicol., 56: 288-294.
57. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Brit. J. ind. Med., 42:
363-372.
58. FLODSTROM, S., WARNGARD, L., LJUNGQUIST, S., & AHLBORG, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch. Toxicol., 61: 218-223.
59. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
60. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
61. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
62. GAUGHAN, L.C., ENGEL, J.I., & CASIDA, J.E. (1980) Pesticide
interactions: effects on organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pestic. Biochem. Physiol., 14: 81-85.
63. GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid
insecticides to bees. Pestic. Sci., 16: 206-207.
64. GHIASUDDIN, S.M. & SODERLUND, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. appl.
Pharmacol., 74: 390-396.
65. GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav. Toxicol.
Teratol., 4:(6) 793-799.
66. GORDON, E.B. & WEIR, R.J. (1978) Lifetime feeding study in rats, SD-
43775 Technical, Kensington, Maryland, Litton Bionetics, Inc.
(Project No. 2054, Report AT-81-0181) (Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd).
67. GREENBERG, R.S. (1981) Determination of fenvalerate, a synthetic
pyrethroid, in grapes, peppers, apples, and cotton seeds by gas-
liquid chromatography. J. agric. food Chem., 29: 856-860.
68. HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage toxicity tests.
Environ. Toxicol. Chem., 2(2): 251-8
69. HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory studies
on the persistence and behavior in soil of four pyrethroid
insecticides. Can. Entomol., 113: 685-694.
70. HART, E.R. (1975) 90-day subacute toxicity study in dogs, SD 43775
Technical (sumicidin), Kensington, Maryland, Litton Bionetics, Inc.
(Technical Report No. AT-51-0013) (Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd).
71. HE, FENSHENG (1987) Occupational neurotoxicology: Current problems
and trends. Presented at the XXII International Congress on
Occupational Health, Sydney, Australia, 27 September-2 October, 1987.
72. HEND, R.W. & BUTTERWORTH, S.T.G. (1975) Toxicity studies of the
insecticide WL 43775: A three-month feeding study in rats
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
73. HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies of the
insecticide WL 43775: A short-term feeding study in rats (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
74. HILL, B.D. (1981) Persistence and distribution of fenvalerate
residues in soil under field and laboratory conditions. J. agric.
food Chem., 29: 107-110.
75. HINE, C. (1975) SD-43775 Toxicity: Acute and repeated (14-day) dermal
toxicity in the rabbit, San Francisco, Hine Inc. (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
76. HIROMORI, T., NAKANISHI, T., KAWAGUCHI, S., SAKO, H., SUZUKI, T., &
MIYAMOTO, J. (1986) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci., 11: 9-14.
77. HOLMSTEAD, R.L. & FULLMER, D.G. (1977) Photodecarboxylation of
cyanohydrine esters. Models for pyrethroid photodecomposition. J.
agric. food Chem., 25: 56-58.
78. HOLMSTEAD, R.L., FULLMER, D.G., & RUZO, L.O. (1978) Pyrethroid
photodecomposition: Pydrin. J. agric. food Chem., 26: 954-959.
79. HORIBA, M., KITAHARA, H., TAKAHASHI, K., YAMAMOTO, S., & MURANO, A.
(1980) Gas chromatographic determination of fenvalerate (S-5602) in
technical preparations. Agric. biol. Chem., 44: 1197-1199.
80. HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
81. ITO, N. (1976a) Histopathological findings of mice treated with S5602
for three months (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
82. ITO, N. (1976b) Histopathological findings of mice and rats exposed
to mist of S5602 for four weeks (Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd).
83. ITO, N. (1978) Histopathological findings in mice treated with S5602,
Nagoya, Nagoya City University Medical School (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
84. JOHANSEN, C., MAYER, D., MADSEN, R., & ROBINSON, W. (1975) Bee
research investigations, 1975, Pullman, Washington, Washington State
University, Department of Entomology (Unpublished report).
85. JOHANSEN, C., KIOUS, C., SCHULTZ, G., GUPTA, R., & STANFORD, A.
(1978) Bee research investigations, 1978, Pullman, Washington,
Washington State University, Department of Entomology (Unpublished
report).
86. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Comparative metabolism
of fenvalerate and the [2S, S]-isomer in rats and mice. J. Pestic.
Sci., 6: 317-326.
87. KANEKO, H., MATSUO, M., & MIYAMOTO, J. (1986) Differential metabolism
of fenvalerate and granuloma formation. I. Identification of
cholesterol ester derived from a specific chiral isomer of
fenvalerate. Toxicol. appl. Pharmacol., 83: 148-156.
88. KANEKO, H., TAKAMATSU, Y., OKUNO, Y., ABIKO, J., YOSHITAKE, A., &
MIYAMOTO, J. (1988) Substrate specificity for formation of
cholesterol ester conjugates from fenvalerate analogues and for
granuloma formation. Xenobiotica, 18(1): 11-19.
89. KATAGI, T., MIKAMI, N., MATSUDA., T., & MIYAMOTO, J. (1985a)
Photodegradation of fenvalerate and esfenvalerate on soils
(Unpublished Report No. LLM-50-0005 submitted to WHO by Sumitomo
Chemical Co., Ltd).
90. KATAGI, T., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985b)
Hydrolysis of fenvalerate and esfenvalerate in buffered aqueous
solutions (Unpublished Report No. LLM-50-0006 submitted to WHO by
Sumitomo Chemical Co., Ltd).
91. KIRKLAND, V.L. & ALBERT, J.R. (1977) Preliminary pharmacodynamic
investigation of SD 43775 (6-10-0) administered intravenously to the
anesthetized dog. I. Effects on the cardiovascular system and
respiration. II. Electrocardiographic (ECG) findings (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
92. KNOX, J.M., II, TUCKER, S.B., & FLANNIGAN, S.A. (1984) Paraesthesia
from cutaneous exposure to a synthetic pyrethroid insecticide. Arch.
Dermatol., 120: 744-746.
93. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic study on
S5602 in mice (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
94. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Acute inhalation
toxicity of S3206 and S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
95. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976c) Subacute inhalation
toxicity study of S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
96. KOHDA, H., KANEKO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J. (1979)
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
97. KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and fenvalerate).
Arch. Toxicol., 50: 27-33.
98. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
99. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
100. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
101. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
102. LEE, P.W. (1985) Fate of fenvalerate (Pydrin insecticide) in the soil
environment. J. agric. food Chem., 33: 993-998.
103. LEE, P.W., WESTCOTT, N.D., & REICHLE, R.A. (1978) Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871.
104. LEE, P.W., STEARNS, S.M., & POWELL, W.R. (1985) Rat metabolism of
fenvalerate (Pydrin insecticide). J. agric. food Chem., 33: 988-993.
105. LEE, P.W., POWELL, W.R., STEARNS, S.M. & MCCONNEL, O.J. (1987)
Comparative aerobic soil metabolism of fenvalerate isomers. J. agric.
food Chem., 35: 384-387.
106. LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
107. LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 11/12: 843-851.
108. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol.,
20: 203-216.
109. MCKENNEY, C.L., Jr & HAMAKER, D.B. (1984) Effects of fenvalerate on
larval development of Palaemonetes pugio (Holthuis) and on larval
metabolism during osmotic stress. Aquat. Toxicol., 5: 343-355.
110. MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and
shrimp. Bull. environ. Contam. Toxicol., 25: 950-955.
111. MALLEY, L.A., CAGEN, S.Z., GARDINER, T.H., & VAN GELDER, G.A. (1984)
Characterization of fenvalerate mediated skin sensory stimulation.
Neurotoxicology, 5: 71-72.
112. MALLEY, L.A., CAGEN, S.Z., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & ROSE, G.P. (1985) Effect of vitamin E and other amelioratory
agents on the fenvalerate-mediated skin sensation. Toxicol. Lett.,
29: 51-58.
113. MATSUBARA, R.A., SUZUKI, T., KADOTA, T., & MIYAMOTO, J. (1977)
Antidotes against poisoning by S5602 in rats (Unpublished report
submitted by Sumitomo Chemical Co., Ltd).
114. MAYER, F.L., Jr (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Environmental Protection
Agency, p. 115 (EPA-600/8-87/017).
115. MAYER, F.L., Jr & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp. 235-236.
116. MEGHARAJ, M., VENKATESWARLU, K., & RAO, A.S. (1986) Influence of
cypermethrin and fenvalerate on natural soil algal populations.
Ecotoxicol. environ. Saf., 12: 141-145.
117. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Willoughby,
Ohio, Meister Publishing Co., pp. C104-C105.
118. MIKAMI, N., TAKAHASHI, N., HAYASHI, K., & MIYAMOTO, J. (1980)
Photodegradation of fenvalerate (Sumicidin) in water and on soil
surface. J. Pestic. Sci., 5: 225-236.
119. MIKAMI, N., SAKATA, S., YAMADA, H., & MIYAMOTO, J. (1984a) Further
studies on degradation of the pyrethroid insecticide fenvalerate in
soils. J. Pestic. Sci., 9: 697-702.
120. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1984b) New
conjugated metabolites of 3-phenoxybenzoic acid in plants. Pestic.
Sci., 15: 531-542.
121. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1985a) The
metabolism of fenvalerate in plants: The conjugation of the acid
moiety. Pestic Sci., 16: 46-58.
122. MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO, J.
(1985b) Metabolism in rats of 3-phenoxybenzyl alcohol and 3-
phenoxybenzoic acid glucoside conjugates formed in plants. Pestic.
Sci., 16: 33-45.
123. MILNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticide. Investigation of neurotoxic potential of WL 43775
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
124. MIURA, T. & TAKAHASHI, R.M. (1976) Effects of a synthetic pyrethroid,
SD43775, on nontarget organisms when utilized as a mosquito
larvicide. Mosq. News, 36: 322-326.
125. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
126. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
127. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress
of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982,
Oxford, Pergamon Press, Vol. 1-4.
128. MIYAMOTO, J., KANEKO, H., & TAKAMATSU, Y. (1986) Stereoselective
formation of a cholesterol ester conjugate from fenvalerate by mouse
microsomal carboxyesterase(s). J. Biochem. Toxicol., 1(2): 79-94.
129. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a) Toxicity
of mosquito larvicidal pyrethroids to four species of freshwater
fishes. Environ. Entomol., 7: 428-430.
130. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978b)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
131. MULLA, M.S., DARWAZEH, H.A., & DHILLON, M.S. (1980) New pyrethroids
as mosquito larvicides and their effects on nontarget organisms.
Mosq. News, 40: 6-12.
132. NOBLE, P.J. (1976) AF1117 residue data in animal tissues, milk and
cream (Unpublished data submitted to WHO by Shell Chemical
(Australia), Ltd).
133. OHKAWA, H., NAMBU, K., INUI, H., & MIYAMOTO, J. (1978) Meta-
bolic fate of fenvalerate (Sumicidin) in soil and by microorganisms.
J. Pestic. Sci., 3: 129-141.
134. OHKAWA, H., KANEKO, H., TSUJI, H., & MIYAMOTO, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
135. OHKAWA, H., KIKUCHI, R., & MIYAMOTO, J. (1980a) Bioaccumulation and
biodegradation of the [S]-acid isomer of fenvalerate (Sumicidin) in
an aquatic model ecosystem. J. Pestic. Sci., 5: 11-12.
136. OHKAWA, H., NAMBU, K., & MIYAMOTO, J. (1980b) Metabolic fate of
fenvalerate (Sumicidin) in bean plants. J. Pestic. Sci., 5: 215-223.
137. OHNO, N., FUJIMOTO, K., OKUNO, Y., MIZUTANI, T., HIRANO, M., ITAYA,
N., HONDA, T., & YOSHIOKA, H. (1976) 2-Arylalkanoates, a new group of
synthetic pyrethroid esters not containing cyclopropane-carboxylates.
Pestic. Sci., 7: 241-246.
138. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975a) Eye and skin irritation
of S-3206 and S-5602 in rabbits (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
139. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975b) Skin sensitization
study of S-5602 in guinea pigs (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
140. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by dermal
applications in rats (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
141. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977a) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by oral
administration in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
142. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977b) Neurotoxic effects of
S5602 and natural pyrethrins by oral administration in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
143. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977c) Recovery of
histopathological lesions in rats caused by short-term feeding of
S5602 (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
144. OKUNO, Y., SEKI, T., ITO, S., KANEKO, H., WATANABE, T., YAMADA, T., &
MIYAMOTO, J. (1986a) Differential metabolism of fenvalerate and
granuloma formation. II. Toxicological significance of lipophilic
conjugate from fenvalerate. Toxicol. appl. Pharmacol., 83: 157-169.
145. OKUNO, Y., ITO, S., SEKI, T., HIROMORI, T., MURAKAMI, M., KADOTA, T.,
& MIYAMOTO, J. (1986b) Fenvalerate-induced granulomatous changes in
rats and mice. J. toxicol. Sci., 11: 53-66.
146. PARKER, C.M., MCCULLOUGH, C.B., GELLATLY, J.B.M., & JOHNSTON, C.D.
(1983) Toxicologic and carcinogenic evaluation of fenvalerate in the
B6C3F1 mouse. Fundam. appl. Toxicol., 3: 114-120.
147. PARKER, C.M., PATTERSON, D.R., VAN GELDER, G.A., GORDON, E.B.,
VALERIO, M.G., & HALL, W.C. (1984a) Chronic toxicity and
carcinogenicity evaluation of fenvalerate in rats. J. Toxicol.
environ. Health, 13: 83-97.
148. PARKER, C.M., PICCIRILLO, V.J., KURTZ, S.L., GARNER, F.M., GARDINER,
T.H., & VAN GELDER, G.A. (1984b) Six-month feeding study of
fenvalerate in dogs. Fundam. appl. Toxicol., 4: 577-586.
149. PARKER, C.M., ALBERT, J.R., VAN GELDER, G.A., PATTERSON, D.R., &
TAYLOR, J.L. (1985) Neuropharmacologic and neuropathologic effect of
fenvalerate in mice and rats. Fundam. appl. Toxicol., 5: 278-286.
150. PARKER, C.M., WIMBERLY, H.C., LAM, A.S., GARDINER, T.H., & VAN
GELDER, G.A. (1986) Subchronic feeding study of decarboxyfenvalerate
in rats. J. Toxicol environ. Health, 18: 77-90.
151. PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
152. PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
153. PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137:7-15.
154. POTTER, J.C. (1976) (a) Tissues of rats fed SD43775-C-14; (b) and (c)
milk and tissues of cows; (d) eggs and tissues from laying hens; (e)
cream from the milk of cows fed SD 43775 (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
155. RANGACHAR, T.R.S., SURESH, T.P., & THINMMAIAH, K. (1981) Acute oral
toxicity of Sumicidin 20E in poultry. Indian vet. J., 58: 941-942.
156. RATTNER, B.A. & FRANSON, J.C. (1983) Methyl parathion and fenvalerate
toxicity in American kestrels: acute physiological responses and
effects of cold. Can. J. Physiol. Pharmacol., 62: 787-792.
157. REED, W.T., EHMAN, A., LEE, P., BARBER, G., & BISHOP, J. (1983)
Pydrin insecticide (fenvalerate) on non-target systems following
field applications. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: Human welfare and the environment, Oxford, Pergamon Press,
Vol. 2, pp. 213-221.
158. ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J.
econ. Entomol., 72: 293-294.
159. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve - muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
160. SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Degradation of esfenvalerate and its isomers in soils (Unpublished
Report No. LLM-30-0003 submitted to WHO by Sumitomo Chemical Co.,
Ltd).
161. SALEH, M.A., IBRAHIM, N.A., SOLIMAN, N.Z., & EL SHEIMY, M.K. (1986)
Persistence and distribution of cypermethrin, deltamethrin and
fenvalerate in laying chickens. J. agric. food Chem., 34: 895-898.
162. SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygiene
rules for synthetic pyrethroid content in the work zone air.] Gig.
tr. prof. Zabol., 8: 48-50 (in Russian).
163. SCHIMMEL, S.C., GARNAS, R.L., PATRICK, J.M., & MOORE, J.C. (1983)
Acute toxicity, bioconcentration, and persistence of AC 222,705,
benthiocarb, chlorpyrifos, fenvalerate, methyl parathion, and
permethrin in the estuarine environment, J. agric. food Chem., 31:
104-113.
164. SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
165. SODERLUND, D.M. & CASIDA, J.E. (1977) Effect of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pestic. Biochem. Physiol., 7: 391-401.
166. SPEHAR, R.L., TANNER, D.K., & GIBSON, J.H. (1982) Effects of Kelthane
and Pydrin on early life stages of Fathead Minnows (Pimephales
promelas) and Amphipods (Hyalella azteca), Philadelphia, American
Society for Testing and Materials (Special Technical Publication No.
766).
167. STEIN, A.A. (1977) Histopathology evaluation of animals from 3-
generation reproduction study. Albany, New York, Biological Research,
Ltd (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
168. SUMMIT, L.M. & ALBERT, J.R. (1977a) Oral lethality of WL 43775 (6-1-
0-0) in the rat (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
169. SUMMIT, L.M. & ALBERT, J.R. (1977b) Determination of the acute oral
lethality of WL 43775 (6-1-0-0) in the male and female mouse
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
170. SUZUKI, H. & MIYAMOTO, J. (1976) Studies on mutagenicity of S 5602
with bacteria systems (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
171. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of some
pyrethroids on salmonella strains in the presence of mouse hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
172. SUZUKI, H., KISHIDA, F., & MIYAMOTO, J. (1979) Studies on
mutagenicity of S5602 in Ames test in the presence of hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
173. SUZUKI, T., KADATA, T., & MIYAMOTO, J. (1976) One-year chronic
toxicity study of S5602 in mice (three month interim report)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
174. SUZUKI, T., OKUNO, Y., HIROMORI, T., ITO, S., KADOTA, T., & MIYAMOTO,
J. (1977a) Fifteen-month chronic toxicity study of S5602 in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
175. SUZUKI, T., OKUNO, Y., HIROMORI, T., KADOTA, T., & MIYAMOTO, J.
(1977b) Eighteen-month chronic toxicity study of S5602 in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
176. SYRETT, P. & PENMAN, D.R. (1980) Studies of insecticide toxicity to
lucerne aphids and their predators. N.Z. J. agric. Res., 23: 575-580.
177. TAGATZ, M.E. & IVEY, J.M. (1981) Effects of fenvalerate on field- and
laboratory-developed estuarine benthic communities. Bull. environ.
Contam. Toxicol., 27: 256-267.
178. TAGATZ, M.E., STANLEY, R.S., PLAIA, G.R., & DEANS, C.H. (1987)
Responses of estuarine macrofauna colonizing sediments contaminated
with fenvalerate. Environ. Toxicol. Chem., 6: 21-25.
179. TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Photodegradation of the [2S, alphaS] isomer of fenvalerate in distilled
water (Unpublished Report No. LLM-50-0001 submitted to WHO by
Sumitomo Chemical Co., Ltd).
180. TAKAMATSU, Y., KANEKO, H., ABIKO, J., YOSHITAKE, A., & MIYAMOTO, J.
(1987) In vivo and in vitro stereoselective hydrolysis of four chiral
isomers of fenvalerate. J. Pestic. Sci., 12: 397-404.
181. TALEKER, N.S., CHEN, J.S., & KAO, H.T. (1983) Persistence of
fenvalerate in subtropical soil. J. econ. Entomol., 76: 223-226.
182. TUCKER, S.B. & FLANNIGAN, S.A. (1983) Cutaneous effects from
occupational exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
183. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
184. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect: Neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
185. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M., (1973)
DDT-like action of allethrin in the sensory nervous system of Xenopus
laevis. Eur. J. Phamacol., 21: 95-106.
186. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979)
Effect of insecticides on the sensory nervous system. In: Narahashi,
T., ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 183-210.
187. VAN DER PAUW, C.L., DIX, K.M., BLANCHARD, K., & MCCARTHY, W.V. (1975)
Teratological studies in rabbits given WL 43775 orally (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
188. VAKENTI, J.M. (1986) Persistence of fenvalerate in orchard soils,
Okanagan Valley, British Columbia (Unpublished report of cooperative
study of Agriculture Canada and Ciba-Geigy Canada, Ltd).
189. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relation-
ship of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
190. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog myelinated
nerve fibres. Eur. J. Phamacol., 58: 501-504.
191. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
192. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
193. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in mylinated nerves. Nature (Lond.), 295: 601-603.
194. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN
DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
195. WALKER, B.J., HEND, R.W., & LINNETT, S. (1975) Toxicity studies on
the insecticide WL 43775: Summary of results of preliminary
experiments (Unpublished report submitted to WHO by Shell Development
Co., Ltd).
196. WHO (1979) WHO Technical Report Series No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp.18-23.
197. WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
198. WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
199. WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic pyrethroid
insecticides to predaceous phytoseiid mites and their prey. Aust. J.
agric. Res., 30: 497-501.
200. WOOD MACKENZIE (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
201. WOOD MACKENZIE (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
202. WOOD MACKENZIE (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
203. WOOD MACKENZIE (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
204. WOOD MACKENZIE (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
205. WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida. State Hortic. Soc., 90: 401-402.
206. WORTHING, C.R. (1979) The pesticide manual, 6th ed., Croydon, British
Crop Protection Council, p. 270.
207. WORTHING, C.R. & WALKER, S.B. (1987) Fenvalerate In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 395-
396.
208. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
209. WSZOLEK, P.C., LEIN, D.H., & LISK, D.J. (1980) Excretion of fenval-
erate insecticide in the milk of dairy cows. Bull. environ. Contam.
Toxicol., 24: 296-298.
210. WSZOLEK, P.C., HOGUE, D.E., & LISK, D.J. (1981a) Accumulation of
fenvalerate insecticide in lamb tissues. Bull. environ. Contam.
Toxicol., 27: 869-871.
211. WSZOLEK, P.C., LAFAUNCE, N.A., WACHS, T., & LISK, D.J. (1981b)
Studies of possible bovine urinary excretion and rumen decomposition
of fenvalerate insecticide and a metabolite. Bull. environ. Contam.
Toxicol., 26: 262-266.
212. BORTHWICK, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
static acute toxicity tests with selected estuarine algae,
invertebrates, and fish. Gulf Breeze, Florida, US Environmental
Protection Agency (EPA-600/4-81-076).
213. HE, F., WANG, S., LIU, L., CHEN, S., ZHANG, Z., & SUN, J. (1989)
Clinical manifestations and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63: 54-58.
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs (Xenopus laevis; Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [65, 98, 186,
189, 196]. The same distinction was found in studies on
cockroaches [60].
Based on the binding assay on the gamma-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types:
the alpha-cyano-3-phenoxybenzyl pyrethroids and the non-
cyano pyrethroids [59, 61, 99, 100].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin,
cismethrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [185]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [183]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [194].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [55].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [184]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [194].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans
[38], are almost identical to those of allethrin, i.e.,
presynaptic repetitive firing, and no significant effects
on transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [185, 186, 193].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [108].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [159].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cypermethrin, fenval-
erate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [192]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [190,
194].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during
excitation, presumably by slowing down the closing of the
activation gate of the sodium channel [190, 194]. The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than
100 milliseconds. Apparently, the amplitude of the pro-
longed sodium current after cypermethrin is too small to
induce repetitive activity in nerve fibres, but is suf-
ficient to cause the long-lasting repetitive firing in the
lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [108].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [61].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the tox-
icity of Type II pyrethroids and their potency to inhibit
the [35S]-TBPS binding was established using a radio-
ligand, [35S]- t-butyl-bicyclophosphoro-thionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute corre-
lation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all
toxic cyano compounds including deltamethrin, 1R, cis-
cypermethrin, 1R, trans-cypermethrin, and [2S, alphaS]-
fenvalerate were inhibitors, but their non-toxic stereo-
isomers were not; non-cyano pyrethroids were much less
potent or were inactive [99].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-di-
hydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [100].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S, alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex
[59].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [159].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-
lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation.
This results in long-lasting trains of repetitive impulses
in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [196, 208].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [191].
RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes
d'analyse
La fenvalérate est un insecticide puissant utilisé
depuis 1976. C'est un ester de l'acide (chloro-4 phényle)-
2 méthyl-3 butyrique et de l'alcool alpha-cyano-phénoxy-
benzylique. Malgré l'absence du cycle cyclopropane, ses
propriétés insecticides le rattachent au groupe des
pyréthroïdes. Il s'agit d'un mélange racémique de quatre
isomères optiques dont les configurations sont [2S, alphaS],
[2S, alphaR], [2R, alphaS] et [2R, alphaR]. L'isomère [2S,
alphaS] est le plus actif biologiquement; vient ensuite
l'isomère [2S, alphaR].
Le fenvalérate de qualité technique se présente sous
la forme d'un liquide visqueux jaune ou brun dont la
densité est de 1,175 à 25 °C. Il est relativement non
volatil, sa tension de vapeur étant de 0,037 mPa à 25 °C.
Pratiquement insoluble dans l'eau (environ 2 µg/litre),
il est soluble dans les solvants organiques comme
l'acétone, le xylène et le kérosène. Il est stable à la
lumière, à la chaleur et à l'humidité, mais instable en
milieu alcalin par suite de l'hydrolyse du groupement
ester.
Le dosage des résidus et les analyses écotoxico-
logiques peuvent s'effectuer par chromatographie en phase
gazeuse avec détection par capture d'électrons, la
concentration minimale décelable étant de 0,005 mg/kg.
Pour l'analyse des produits techniques on utilise la même
méthode mais avec un détecteur à ionisation de flamme.
1.2 Production et usage
On utilise dans le monde environ 1000 tonnes de
fenvalérate par an (chiffres de 1979-1983), essentielle-
ment en agriculture mais également pour la désinsecti-
sation des habitations et des jardins et le déparasitage
des bestiaux, soit seul, soit en association avec d'autres
insecticides. Il est présenté sous forme de concentré
émulsionnable, de concentré pour épandage à très bas
volume, de poudre pour poudrage et de poudre mouillable.
1.3 Exposition humaine
C'est essentiellement du fait de la présence de
résidus dans les aliments que la population dans son
ensemble est exposée à cet insecticide. Le respect des
règles de bonne pratique permet en général de maintenir
les résidus dans les récoltes à un faible niveau. L'expo-
sition qui en découle pour la population générale devrait
a priori être très faible mais on ne dispose pas de
données tirées d'études de la ration totale.
L'analyse des résidus présents dans les céréales
ensilées a montré que plus de 70% de la dose appliquée
subsistent sur le blé au bout de dix mois à 25 °C. Après
mouture et panification, la teneur en résidus du pain
blanc et de la farine de froment est à peu près la même
(environ 0,06-0,1 mg/kg).
Les informations relatives à l'exposition profession-
nelle au fenvalérate sont très fragmentaires.
1.4 Destinée dans l'environnement
Dans le sol, il y a dégradation par coupure de la
liaison ester et du groupement diphényl-éther, hydroxyl-
ation du cycle benzénique, hydratation du nitrile en
amide, l'oxydation des fragments se poursuivant jusqu'à
l'obtention d'anhydride carbonique qui constitue le
principal produit final. L'étude du potentiel de lixivi-
ation du fenvalérate et de ses produits de dégradation a
montré qu'il n'y avait guère de pénétration au niveau du
sol.
Dans l'eau et à la surface du sol, la fenvalérate
subit une photodégradation par la lumière solaire. On a
montré qu'il se produisait une coupure du groupement
ester, une hydrolyse du groupement cyano, une décarboxy-
lation conduisant au (phénoxy-3)-2 (chloro-4 phényle)-3
méthyl-4 pentane-nitrile (décarboxy-fenvalérate), ainsi que
d'autres réactions à initiation radicalaire.
Sur les végétaux, la fenvalérate a une demi-vie
d'environ 14 jours. La principale réaction consiste dans
la rupture de la liaison ester suivie d'une oxydation
et/ou d'une conjugaison des fragments. Il se produit
également une décarboxylation en décarboxy-fenvalérate.
En général, la dégradation dans l'environnement
conduit à des produits moins toxiques.
Dans l'environnement, le fenvalérate est assez
rapidement dégradé. La demi-vie est de 4 à 15 jours dans
les rivières, 8 à 14 jours sur les végétaux, 1 à 18 jours
par photodégradation à la surface du sol et 15 jours à
3 mois dans le sol.
Il n'y a pratiquement aucune lixiviation du fenva-
lérate présent dans le sol. Il est donc improbable que ce
composé puisse s'accumuler de façon importante dans le
milieu aquatique.
1.5 Cinétique et métabolisme
On a étudié la destinée du fenvalérate chez le rat et
la souris au moyen de fenvalérate radio-marqué au niveau
du groupement carboxylate, ou des groupements benzyle ou
cyano. Sauf dans le cas des composés marqués au niveau du
groupement cyano, la radioactivité administrée est
rapidement excrétée (jusqu'à 99% en six jours). Les
principales réactions métaboliques consistent en une
rupture du groupement ester et une hydroxylation en
position 4. On a également observé diverses réactions
d'oxydation et de conjugaison conduisant à un mélange
complexe. Lorsque le fenvalérate est radio-marqué au
niveau du groupement cyano, la dose radioactive s'élimine
moins rapidement (jusqu'à 81% en six jours). La
radioactivité restante est principalement confinée dans la
peau, les poils et l'estomac sous forme de thiocyanate.
Il existe aussi une voie métabolique secondaire, quoique
très importante, qui consiste dans la formation d'un
conjugué lipophile de (chloro-4 phényle)-2-[2R] iso-
valérate. Ce conjugué qui intervient dans la formation de
granulomes a été décelé dans les surrénales, le foie et
les ganglions mésentériques des rats, des souris et de
certaines autres espèces.
1.6 Effets sur les êtres vivant dans leur milieu naturel
Au laboratoire, le fenvalérate se révèle extrêment
toxique pour les organismes aquatiques. La CL50 varie de
0,008 µg/litre pour des mysidacées nouvellement écloses
à 2 µg/litre pour une espèce d'éphéméroptère. Les
épreuves portant sur le cycle évolutif de Daphnia galeata
mendotae ont révélé que la dose sans effet observable
était de 0,005 µg/litre. Le fenvalérate est également
extrêment toxique pour les poissons. Les valeurs de la
CL50 à 96 heures vont de 0,03 µg/litre pour la larve de
Leuresthes tenuis à 200 µg/litre pour le Tilapia
adulte. La dose sans effet observable sur 28 jours
s'établit à 0,56 µg/litre pour les premiers stades de
certains vairons. Le fenvalérate est moins toxique pour
les algues et les mollusques aquatiques, la CL50 à 96
heures étant supérieure à 1000 µg/litre.
La forte toxicité potentielle du fenvalérate pour les
organismes aquatiques ne se manifeste pas dans les essais
sur le terrain ni dans les conditions d'utilisation
pratique. Certains invertébrés aquatiques sont détruits
par un épandage à la surface des eaux mais l'effet sur les
populations est temporaire. On n'a pas signalé de
mortalité chez les poissons. La toxicité moindre observée
lors des épandages de plein champ s'explique par une forte
adsorption du composé par les sédiments.
Le fenvalérate est très toxique pour l'abeille. En
applications topiques la DL50 est de 0,41 µg/abeille,
toutefois l'effet répulsif intense qu'exerce le fenva-
lérate sur ces insectes en réduit l'action dans la
pratique. Rien n'indique qu'il y ait eu des destructions
importantes d'abeilles dans les conditions normales
d'utilisation. Le fenvalérate est plus toxique pour les
acariens prédateurs que pour les espèces cibles de
ravageurs.
Administré par voie orale ou mêlé à la nourriture, le
fenvalérate est très peu toxique pour les oiseaux. La
DL50 est supérieure à 1500 mg/kg de poids corporel en
administration orale directe et dépasse 15 000 mg/kg de
nourriture en administration dans la ration alimentaire
pour le colin de Virginie.
Les organismes aquatiques fixent rapidement le fenva-
lérate. Le facteur de bioconcentration varie de 120 à
4700 selon l'organisme en cause (algues, mollusques,
daphnies et poissons) selon les études effectuées sur des
modèles d'écosystèmes. Toutefois, ce fenvalérate s'élimine
rapidement lorsque les organismes sont replacés en eau
propre. On peut donc considérer qu'en pratique, ce composé
ne présente aucune tendance à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
La toxicité aiguë par voie orale du fenvalérate est
modérée à faible. Toutefois, les valeurs de la DL50
peuvent varier considérablement (de 82 à plus de 3200
mg/kg) selon l'espèce animale en cause et le véhicule
d'administration. Les signes cliniques d'intoxication
aiguë apparaissent rapidement mais les survivants
redeviennent asymptomatiques au bout de trois à quatre
jours. Parmi les signes d'intoxication produits par le
mélange racémique, ainsi que par l'isomère [2S, alphaS], on
note de l'agitation, des tremblements, une horripilation,
de la diarrhée, une démarche anormale, une choréo-athétose
et une salivation (syndrome CS); il est classé comme
pyréthroïde du type II. Du point de vue électrophysio-
logique, il produit des bouffées de pointes au niveau des
nerfs moteurs des cerques de la blatte. Toutefois, il n'y
a pas de relation bien définie entre les effets électro-
physiologiques chez l'insecte et la toxicité pour les
mammifères.
Des rats ayant reçu du fenvalérate pendant 8 à 10
jours à raison de 2000 mg/kg de nourriture ont présenté
des signes typiques d'intoxication aiguë. A la dose de
3000 mg/kg de nourriture, on observait des modifications
morphologiques réversibles au niveau du nerf sciatique.
On a également observé des modifications histopatho-
logiques au niveau du même nerf chez des rats et des
souris ayant reçu en une seule fois du fenvalérate par
voie orale à des doses létales ou sublétales.
Des poulets ayant reçu par voie orale du fenvalérate
pendant cinq jours à raison de 1000 mg/kg par jour n'ont
pas présenté de signes cliniques ou morphologiques de
neurotoxicité retardée.
Chez la souris, la toxicité aiguë par voie intra-
péritonéale des métabolites du fenvalérate n'est pas
supérieure à celle du fenvalérate lui-même.
Lors d'études de toxicité subaiguë et subchronique,
des souris, des rats, des chiens et des lapins ont reçu
pendant trois semaines à six mois, du fenvalérate par voie
orale, percutanée et respiratoire. Chez le rat et la
souris, des études d'inhalation de quatre semaines ont
permis de fixer la dose sans effet observable à 7 mg/m3.
Chez le rat, lors d'une étude de 90 jours, elle
s'établissait à 125 mg/kg de nourriture, et sur deux ans à
250 mg/kg de nourriture (soit 12,5 mg/kg de poids
corporel). Dans une étude de 24 à 28 mois, elle s'est
établie à 150 mg/kg de nourriture, soit 7,5 mg/kg de poids
corporel. Une étude de deux ans sur la souris a permis de
fixer la dose à 50 mg/kg de nourriture, soit 6 mg/kg de
poids corporel et une étude de 20 mois, à 30 mg/kg de
nourriture, soit 3,5 mg/kg de poids corporel. Chez le
chien cette dose a été établie à 12,5 mg/kg de poids
corporel, lors d'une étude de 90 jours. Certaines formu-
lations de fenvalérate ont provoqué une irritation cutanée
et oculaire. Toutefois, le fenvalérate technique n'est
pas irritant et n'a pas d'effet sensibilisateur.
Lors d'études de toxicité à long terme, on a noté
l'apparition de microgranulomes chez des souris qui
avaient été traitées avec l'isomère [2R, alphaS] (125 mg/kg
de nourriture) sur une période de un à trois mois. Ces
anomalies disparaissaient lorsqu'on supprimait le fenval-
érate. L'agent causal en était l'ester cholestérique de
l'acide (chloro-4 phényl)-2 isovalérique, un métabolite
lipophile de l'isomère [2R, alphaS] du fenvalérate. La
dose sans effet observable relative à la formation de
microgranulomes chez la souris s'établit à 30 mg de
fenvalérate par kg de nourriture.
Lors d'une autre étude de toxicité à long terme, on a
également observé ces anomalies microgranulomateuses chez
des rats à la dose de 500 mg/kg de nourriture, la dose
sans effet observable étant dans ce cas de 150 mg par kg
de nourriture.
Administré à des souris dans leur nourriture pendant
78 semaines en doses allant jusqu'à 3000 mg/kg ou pendant
deux ans à raison de 1250 mg/kg, le fenvalérate ne s'est
pas révélé cancérogène. Il ne l'a pas été non plus chez
des rats qui avaient reçu pendant deux ans une alimen-
tation contenant jusqu'à 1000 mg d'insecticide par kg.
Le fenvalérate n'est ni mutagène, ni délétère pour les
chromosomes ainsi qu'il ressort d'un certain nombre
d'épreuves in vitro et in vivo.
Il n'est pas non plus tératogène pour la souris et le
lapin à des doses quotidiennes allant jusqu'à 50 mg par kg
de poids corporel et, lors d'une étude de reproduction
portant sur trois générations de rats où les animaux
recevaient des doses allant jusqu'à 250 mg/kg de
nourriture, il n'a affecté aucun des paramètres de la
fonction de reproduction.
1.8 Effets sur l'être humain
Le fenvalérate peut provoquer des sensations
d'engourdissement, de démangeaison, de picotement et de
brûlure chez les travailleurs exposés; les symptômes
apparaissent après une période de latence d'environ
30 minutes, atteignent leur acmé au bout de 8 heures et
disparaissent dans les 24 heures suivantes. Certains cas
d'intoxication se sont produits à la suite d'une expo-
sition professionnelle due au non respect des mesures de
sécurité.
Rien n'indique que le fenvalérate soit nocif pour
l'être humain dans la mesure où il est utilisé conformé-
ment aux recommandations.
2. Conclusions
2.1 Population générale
L'exposition de la population générale au fenvalérate
est probablement très faible. Il n'y a sans doute aucun
risque si on l'emploie conformément aux recommandations.
2.2 Exposition professionnelle
Utilisé de manière raisonnable, et moyennant certaines
mesures d'hygiène et de sécurité, le fenvalérate ne
devrait pas être dangereux pour les personnes qui lui sont
exposées de par leur profession.
2.3 Environnement
Il est improbable que le fenvalérate ou ses produits
de dégradation puissent s'accumuler dans l'environnement
en quantité suffisante pour créer des problèmes, dans la
mesure où l'on respecte les doses d'emploi recommandées.
Au laboratoire, le fenvalérate se révèle extrêmement
toxique pour les poissons, les arthropodes aquatiques et
les abeilles. Toutefois, il ne semble pas que des effets
nocifs durables puissent se produire sur le terrain si
l'insecticide est utilisé conformément aux recomman-
dations.
3. Recommandations
Les concentrations alimentaires qui résultent d'une
utilisation conforme aux recommandations sont considérées
comme très faibles; toutefois il conviendrait de confirmer
ce point de vue en étendant les études de surveillance au
fenvalérate.
Le fenvalérate est utilisé depuis de nombreuses années
et seuls quelques effets temporaires ont été observés ça
et là à la suite d'expositions professionnelles. Néan-
moins, il serait bon de poursuivre les observations sur
l'exposition humaine.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single Exposures
Table 8 shows the results of acute toxicity tests with
technical grade fenvalerate in various animal species.
The acute toxic signs in rats were restlessness,
tremors, piloerection, occasional diarrhoea, and an abnor-
mal gait. Following oral administration, surviving rats
recovered rapidly from acute clinical signs of poisoning
and were asymptomatic within 3-4 days [20].
Table 9 shows the results of an acute intraperitoneal
toxicity study of fenvalerate metabolites in mice [96].
All the compounds were dissolved in corn oil except
3-phenoxybenzoic acid, which was dissolved in DMSO. The
acute intraperitoneal toxicity in mice of the proposed
decarboxylated photo-products was found to be similar to,
or greater than, that of fenvalerate [78].
In a study by Blair and Roderick [12], groups of four
male and four female rats were exposed by inhalation (head
only) to an aerosol formulation (77-µm particle size)
generated from an aqueous suspension containing 3 g/litre.
Following a single administration (4 h) of this non-
inhalable particulate, acute signs of poisoning were noted
for a short period, presumably from oral ingestion of the
large particles. There was no mortality and all animals
appeared normal within 3 days following exposure.
7.2 Short-Term Exposures
7.2.1 Oral administration
Groups of Carworth Farm E rats (12 of each sex per
group) were fed fenvalerate in the diet at dose levels of
0, 125, 500, 1000, and 2000 mg/kg for 90 days [72]. Mor-
tality (11/12 male, 9/12 female) was observed at the
highest concentration. Body weight gain and food consump-
tion were decreased and blood urea nitrogen concentrations
were increased at 1000 and 2000 mg/kg. There were no
treatment-related changes in any groups of rats in the
haematological parameters examined. Increases in liver to
body weight ratios and kidney to body weight ratios were
observed at 500 mg/kg or more. Gross and microscopic
examinations revealed no compound-related changes in any
groups. The NOEL was 125 mg/kg diet.
Table 8. Acute toxicity of fenvalerate (technical grade) administered to various species
-------------------------------------------------------------------------------------------------
Species Route Sex Vehiclea LD50 (mg/kg) Reference
-------------------------------------------------------------------------------------------------
Rat oral DMSO 451 195
oral PEG:water > 3200 168
dermal 5000 (24 h) 140
inhalation M, F water > 101 mg/m3 (3 h) 94
intraperitoneal 340 162
Mouse oral M DMSO 200-300 195
F 100-200
oral PEG:water 1202 169
intraperitoneal M, F corn oil 85-89 96
intraperitoneal 132 162
intravenous glycerolformol 65 2
inhalation M, F water > 101 mg/m3 (3 h) 94
Chinese oral M DMSO 98 195
hamster F 82
Rabbit percutaneous undiluted 1000-3200 75
Hen oral > 1500 123
-------------------------------------------------------------------------------------------------
a PEG = polyethylene glycol; DMSO = dimethylsulfoxide.
Table 9. Acute intraperitoneal toxicity of fenvalerate metabolites in mice
---------------------------------------------------------------------------
Chemical No.a LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------------
Fenvalerate (5) 88.5 85
2-(4-Chlorophenyl)isovaleric acid (17) 351 350
3-Phenoxybenzyl alcohol (19) 371 424
3-(4'-Hydroxyphenoxyl) benzyl alcohol (21) 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol (20) 876 778
3-Phenoxybenzoic acid (22) 154 169
3-(4'-Hydroxyphenoxy) benzoic acid (24) 783 745
3-(2'-Hydroxyphenoxy) benzoic acid (23) 859 912
3-Phenoxybenzaldehyde (18) 415 416
NaSCN 604 578
---------------------------------------------------------------------------
a Refers to chemical identification no. in Fig. 3 and in text.
In a study by Parker et al. [150], Fischer 344 rats
(30 of each sex per group) were fed decarboxyfenvalerate
(one of the major photodegradation products) at concen-
trations of 0, 30, 100, 300, 3000 or 10 000 mg/kg diet for
up to 13 weeks. Body weight was decreased in male rats fed
10 000 mg/kg, but no treatment-related mortality or clini-
cal signs were observed. Absolute and relative liver
weight of male and female rats fed 300, 3000, or 10 000
mg/kg were all higher than those of the controls. Signifi-
cant increases in absolute or relative kidney weights were
observed in male and female rats fed 3000 or 10 000 mg/kg.
Significant treatment-related microscopic effects were
limited to glomerulonephrosis in male and female rats fed
10 000 mg/kg and hepatocellular hypertrophy with pale
eosinophilic cytoplasm and hepatocellular focal necrosis
in male and female rats fed 3000 or 10 000 mg/kg. A NOEL
of 300 mg/kg diet was established in this study.
Groups of young adult beagle dogs (four of each sex
per group) were fed fenvalerate in the diet at dose levels
of 0, 0.25, 0.5, 1.25, or 12.5 mg/kg body weight for 90
days [70]. There were no treatment-related changes in body
weight, food consumption, clinical signs, and clinical
laboratory data. Gross and microscopic examinations
revealed no effects of the fenvalerate. Thus, daily
administration at a level of 12.5 mg/kg body weight for a
period of 90 days produced no detectable evidence of toxi-
cological effect.
In a study by Parker et al. [148], male and female
beagle dogs (six of each sex per group) were fed diets
containing 0, 250, 500, or 1000 mg fenvalerate/kg diet for
a period of 6 months. Prominent clinical signs related to
treatment were emesis, head shaking, biting of the ex-
tremities, and tremors. The mean body weights of female
dogs fed fenvalerate at 1000 mg/kg were significantly
lower than those of controls. Red blood cell counts and
haematocrit and haemoglobin values in both male and female
dogs fed the highest dose were significantly lower. Serum
cholesterol and alkaline phosphatase levels were also
increased, mostly in the group fed 1000 mg/kg. Hepatic
multifocal microgranulomas observed during microscopic
examination increased in incidence and severity in a dose-
dependent way and were considered to be related to treat-
ment. Histiocytic cell infiltrates in the mesenteric lymph
nodes of some female dogs fed 500 or 1000 mg/kg and of
male dogs fed 1000 mg/kg were the only other treatment-
related effects observed microscopically.
7.2.2 Inhalation
Groups of Sprague-Dawley rats and ICR mice (10 of each
sex per group) were exposed to fenvalerate by inhalation
for 3 h daily for 4 weeks at concentration levels of 0, 2,
7, or 20 mg/m3 (fully respirable particle size). Although
animals showed acute signs of poisoning at the highest
dose level, no mortality was observed in any group. There
were no treatment-related effects in body weight, haema-
tology, or clinical biochemistry parameters, nor were
there any gross or microscopic abnormal findings [82,
95].
7.2.3 Dermal application
In a study by Hine [75], groups of rabbits (7-8 male
rabbits per group) were administered fenvalerate dermally
at dose levels of 0, 100, or 400 mg/kg daily for 6 h
(14 exposures were performed over a 22-day period).
Severe weight loss, clinical signs of poisoning, and gross
dermal effects were observed at 400 mg/kg, where mortality
was also observed.
7.3 Skin and Eye Irritation; Sensitization
7.3.1 Skin and eye irritation
Two formulated products (an emulsifiable concentrate
and an ultra-low-volume formulation) were found to be
severe eye and skin irritants in rabbits. Dermal irri-
tation was evident for 7 days after a 24-h exposure, and
severe conjunctivitis, corneal opacity, and iritis were
observed within 30 min of an application of 0.2 ml of the
formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation [29, 30]. However,
when experiments were carried out using pure (non-formu-
lated) fenvalerate, there was no irritation [138].
7.3.2 Skin sensitization
Skin sensitization by pure fenvalerate (95%) has been
evaluated using the Landsteiner-Draize method on guinea-
pigs. No sensitization was detected by Okuno et al.
[139].
7.4 Long-Term Exposures and Carcinogenicity
7.4.1 Mouse
When groups of ddY mice (35-47 of each sex per group)
were administered fenvalerate in the diet for 78 weeks at
levels of 0, 100, 300, 1000, or 3000 mg/kg, mortality
occurred at the highest dose level. Hyperexcitability was
observed at 1000 mg/kg or more, and body weight was
depressed at 3000 mg/kg over the 18-month period and at
1000 and 3000 mg/kg over the first 3 months. A variety of
haematological parameters were affected at 3 months, pre-
dominantly at the highest dose level, but no haematol-
ogical changes were observed at the end of the study.
Several biochemical changes suggestive of hepatotoxicity
were observed at 3 months and at termination of the study
in the 300, 1000, and 3000 mg/kg groups. There were gross
changes in several organ weights and in organ-to-body
weight ratios, predominantly in the liver. Microscopic
examination revealed changes in the liver, mesenteric
lymph nodes, and kidney. Dose-dependent granulomatous
changes were observed in the liver and/or mesenteric lymph
nodes in all treatment groups. At the 3-month interim
sacrifice, multiple small necrotic foci in the liver and
changes in the epithelial cells of the proximal convoluted
tubules were noted at the two highest dose levels. There
were no indications in this study of tumorigenicity or
carcinogenicity as a result of fenvalerate administration
[81, 83, 173, 175].
In studies by Okuno et al. [144], male ddY mice were
fed diets containing the [2S, alphaS], [2S, alphaRS], [2R,
alphaS], and [2R, alphaR] isomers of fenvalerate at diet-
ary dose levels of 0, 500, or 1000 mg/kg, 500, 1000, or
2000 mg/kg, 0, 125, or 1000 mg/kg, and 125, or 1000 mg/kg
for 52, 52, 13, and 13 weeks, respectively. Microgranulo-
matous changes were observed in the mice treated with the
[2R, alphaS] isomer after 1, 2, or 3 months. In contrast,
the changes did not occur in mice treated with the [2R,
alphaR] isomer under the same conditions. Neither [2S,
alphaS] nor [2S, alphaRS] isomers caused microgranulomatous
changes at 500 or 1000 mg/kg after 1 year. To clarify the
causative agent of granuloma formation, the cholesterol
ester of 2-(4-chlorophenyl)isovaleric acid (CPIA), a
lipophilic conjugate from the [2R, alphaS] isomer of fenva-
lerate, was injected intravenously into ddY mice. Micro-
granulomatous changes were observed in the liver of mice
treated with the [2R]-, [2S]-, or [2RS]-CPIA-cholesterol
esters 1 week after a single treatment of 1, 10, or 100
mg/kg body weight, as well as in the liver of mice treated
with a single dose of 10 or 30 mg/kg body weight of the
[2R]-CPIA-cholesterol ester and kept up to 26 weeks after-
wards. Histochemical examination and microscopic autoradio-
graphy of the liver demonstrated the presence of tritium,
derived from 3H-labelled [2R]-CPIA and cholesterol in giant
cells and Kuppfer cells. Another histochemical examination
showed the presence of cholesterol ester in the liver of
mice treated with the [2R, alphaS] isomer. These results
support the hypothesis that the CPIA-cholesterol ester is
the causative agent of the microgranulomatous changes ind-
uced by fenvalerate.
In further studies by Okuno et al. [145], male and
female ddY mice were fed diets containing technical
fenvalerate (either 0, 10, 30, 100, or 300 mg/kg diet for
20 months or 0, 100, 300, 1000, or 3000 for 17-18 months).
Microgranulomatous changes were observed in the lymph
nodes, liver, and spleen, the NOEL for such changes being
30 mg/kg. To examine the reversibility of these changes,
ddY mice (male and female) were fed a diet containing
technical fenvalerate at dose levels of 1000 and 3000
mg/kg for 6 weeks, followed by a control diet for up to
12 months. The size and number of the microgranulomatous
changes were reduced with time. These changes were typical
of foreign body granulomas and did not have the appearance
of granulomas formed in response to an immunological
stimulus.
When B6C3F1 mice (50 of each sex per group) were fed
fenvalerate at dietary concentrations of 0, 10, 50, 250,
or 1250 mg/kg for 2 years, mortality was increased and
body weight significantly decreased in male and female
mice fed 1250 mg/kg. The mean body weight of female mice
fed 250 mg/kg was also generally lower than that of con-
trols after the 60th week of feeding. The only treatment-
related non-neoplastic pathological effect observed in the
study was multifocal microgranulomata in the lymph nodes,
liver, and spleen of male mice fed 1250 mg/kg and of
female mice fed 250 or 1250 mg/kg. No statistically sig-
nificant differences were observed in either the number or
type of neoplasms in mice fed fenvalerate (compared to
concurrent controls). Thus, fenvalerate was found not to
be carcinogenic in B6C3F1 mice under the conditions of
the test [146].
7.4.2 Rat
When groups of Wistar rats (15 of each sex per group)
were fed fenvalerate at concentrations of 0, 50, 150, 500,
or 1500 mg/kg diet for 15 months, there was no mortality
attributable to fenvalerate. The hyperexcitability ob-
served during the early stages of the study disappeared
within 3 months. Body weight was significantly depressed
in both sexes at the highest dose level. No compound-
related changes were detected in the urine or in the eyes,
but the haemoglobin concentration was depressed in males
at the highest dose level and the females at 150 mg/kg or
more. Several blood biochemistry parameters were signifi-
cantly altered at the highest dose level (blood urea
nitrogen was increased in both sexes; protein content and
plasma cholinesterase were decreased in females). Gross
and microscopic examination revealed no dose-related
effects [174].
In a study by Gordon & Weir [66], groups of Sprague-
Dawley rats (93 males and 93 females per treated group;
183 of each sex used as the control group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25,
250, or 500 mg/kg. There was no compound-related mor-
tality, although body weight was reduced at the highest
dose level. The group fed 500 mg/kg and a separate control
group were sacrificed at 26 weeks while the other animals
were maintained for 2 years. There were no significant ef-
fects on food consumption, growth, behavior, haematology,
blood biochemical composition or urine consumption. At the
conclusion of the study, organ weight and organ-to-body
weight ratios were normal. Gross and microscopic findings
in the treated groups did not differ significantly from
those of the controls. A specific pathology examination
of the sciatic nerve of animals fed 250 mg/kg revealed no
treatment-related changes. Thus, the no-observed-effect
level in this study was 250 mg/kg diet.
Parker et al. [147] fed Sprague-Dawley rats (93 of
each sex per group) diets containing 0.1, 5, 25, or 250 mg
fenvalerate/kg for up to 2 years. The control group con-
sisted of 183 males and 183 females. Ten treated and 20
control rats of each sex from each group were killed at
intervals of 3, 6, 12, and 18 months. When body weight,
organ weight, food consumption, haematology, and clinical
chemical analysis measurements did not reveal any effect
resulting from the treatment, two additional groups of
rats (50 of each sex per group) were fed 0 or 1000 mg
fenvalerate/kg diet for 2 years. Body weight was decreased
and organ-to-body weight ratios were increased in brain,
liver, spleen, testes, kidneys (females only), and heart
(females only), in the treated animals. Mammary and pitu-
itary tumours were commonly observed, along with a variety
of other tumours that occurred randomly among all control
and treatment groups. No statistically significant differ-
ences in the number and type of neoplasms were observed,
except for mammary tumours in females in the main study.
These effects were judged not to be toxicologically sig-
nificant, since mammary tumour incidences did not exceed
expected incidences in aged female Sprague-Dawley rats.
In addition, the time taken for tumours to appear was un-
changed, and no change in the ratio of benign to malignant
tumours occurred. Sarcomas identified in the subcutis and
dermis in 5 out of 51 males fed 1000 mg/kg were also
identified in 2% (1/50), 2% (2/102), and 0-6% of concur-
rent, original, and historical controls, respectively.
The no-observed-effect level was 250 mg/kg.
When male and female Wistar rates were fed a diet
containing technical fenvalerate at 0, 50, 150, 500, or
1500 mg/diet for 24-28 months, microgranulomatous changes
were observed in lymph nodes, liver, spleen, and adrenal
glands. The no-observed-effect level for these microgranu-
lomatous changes was 150 mg/kg [145].
7.5 Mutagenicity
7.5.1 Microorganism and insects
The DNA-damaging capacity of fenvalerate has been
examined in a Rec-assay with Bacillus subtilis M45 rec-
and H17 wild type strains at concentrations up to 10
mg/disk per plate. Fenvalerate had no inhibitory effect on
the growth of indicator strains, and was judged to be non-
mutagenic [170].
Fenvalerate has also been examined for its mutagenic
potency with the Ames test in Salmonella typhimurium
(TA 1535, TA 1538, TA 98, and TA 100), using dose levels
of up to 1 mg/plate both with and without a metabolic
enzyme system. Fenvalerate was non-mutagenic in these
tests [170]. It was also tested using hepatic metabolic
enzyme systems prepared from various PCB-treated animals
(three strains of rats, six strains of mice and the Syrian
golden hamster). At dose levels of up to 1 mg/plate,
Fenvalerate was non-mutagenic [171, 172].
In further studies by Suzuki & Miyamoto [170], fenval-
erate was given orally at doses of 60 and 125 mg/kg to
groups of mice, and indicator cells (S. typhimurium G46)
were injected intraperitoneally. Fenvalerate did not
induce any significant level of mutation among the indi-
cator cells recovered from the abdominal cavity. On the
other hand, the positive control, dimethylnitrosamine,
significantly increased the mutation frequency of the
indicator organism.
Another host-mediated assay of fenvalerate in mice was
conducted using Saccharomyces cerevisiae as indicator
microorganism. Groups of mice were administered fenval-
erate orally at doses of 25 and 50 mg/kg, and were in-
jected with a suspension of indicator cells intraperito-
neally. No mutagenic effect on the indicator cells was
detected [17].
Fenvalerate was not found to be mutagenic in S.
typhimurium strains TA 100 or TA 98 in the presence or
absence of a rat liver activation system by fluctuation
tests at a concentration of up to 10 µg/ml or in V79
Chinese hamster cells in the presence or absence of
hepatocytes at a concentration of up to 40 µg/ml [153].
Fenvalerate did not induce sex-linked recessive
lethals, sex-chromosome losses, or non-disjunction in
Drosophila melanogaster when it was given to adults (up
to 20 mg/litre in the diet) or larvae (up to 50 mg/litre
in the diet), or was injected into adults (at 20 µg/ml)
[7].
7.5.2 Rat
In a study by Chatterjee et al. [26], groups of rats
(21 per group) were administered fenvalerate orally at
doses of 50, 75, or 100 mg/kg per day for 3 weeks. The
rats were killed 24 h after the last treatment and bone
marrow cells were examined for chromosomal aberrations.
Although an increase in the frequency of chromosomal aber-
rations was observed in fenvalerate-treated animals, it
was not possible to draw any definite conclusion because
it was not dose related and may have been non-specific.
Fenvalerate has also been studied for the enhancement
of gamma-glutamyl transpeptidase-positive enzyme-altered
focus incidence in partially hepatectomized, nitrosodi-
ethylamine-initiated male Sprague-Dawley rats. Fenvalerate
administered peritoneally (75 mg/kg body weight per day,
5 days a week for 10 weeks) induced significantly more
foci per cm3 and a larger percentage of liver tissue
occupied by focus tissue, compared with a vehicle-control
group. Analysis of the size distribution of foci in
fenvalerate- and vehicle-treated rats showed elevated
focus incidences in fenvalerate-treated rats at all focus
sizes. Fenvalerate induced no hepatotoxic effects, as
judged by serum transaminase activities and histopatho-
logical analysis [58].
7.5.3 Mouse
In a dominant lethal assay, groups of male mice (10-11
per group) were administered fenvalerate orally at doses
of 25, 50, or 100 mg/kg body weight. Each male was mated
with three virgin females for 7 days. The procedure was
repeated weekly as a standard dominant lethal test. The
females were sacrificed and examined for dominant
lethality at the 13th day of gestation. Fetal implants in
females, mated to males that had been treated with 100
mg/kg for 2 weeks, showed a significant reduction in
viability. A significant increase in early fetal death
was observed in females mated with males that had been
treated for 4 weeks with the highest dose [34].
The significance of the above data was further studied
as follows:
(1) By using a two-way analysis of variance, it was
judged that the reduction in fetal implants in females
mated to males the second week after dosing at 100 mg/kg
and the increase in early fetal deaths in the 4th week
were statistically significant. But these increases or
decreases appeared to be random and were not considered to
be biologically significant.
(2) Using a t-test and the Mann-Whitney U-test, no
significance was shown in any mean proportions for the
above parameters.
From these findings, it was concluded that fenvalerate
did not cause dominant lethal effects in micea.
----------------------------------------------------------
a Personal communication, J. Miyamoto, 1981, Comments on
"Further work information" required by 1979 JMPR on
fenvalerate, Laboratory of Biochemistry and Toxicology
(Unpublished report submitted to WHO by Sumitomo
Chemical Co. Ltd).
7.5.4 Hamster
In a study by Dean & Senner [35], fenvalerate was
administered orally to groups of hamsters (six males and
six females per group) at two successive daily doses of
12.5 and 25 mg/kg. The chromosomal preparations were made
8 or 24 h after administration. Fenvalerate did not induce
any chromosomal damage in the bone marrow cells from
treated animals, whereas the positive control, methyl
methanesulfonate (50 mg/kg), had induced a substantial
number of chromatid gaps within 8 h of dosing.
Fenvalerate, and the fenvalerate metabolite 2-(4-
chlorophenyl)isovaleric acid, were investigated for the
inhibition of gap-junctional intercellular communication
in vitro in the Chinese hamster lung fibroblast (V79)
metabolic cooperation assay [58]. This study showed that
both fenvalerate and 2-(4-chlorophenyl)isovaleric acid
were inhibitors of intercellular communication at non-
cytotoxic concentrations.
7.6 Teratogenicity and Reproduction Studies
7.6.1 Teratogenicity
In studies by Kohda et al. [93], groups of pregnant
ICR mice (32-33 per group) were orally administered
fenvalerate at dose levels of 0, 5, 15, or 50 mg/kg per
day on days 6 to 15 of gestation. Groups of 20 mice were
sacrificed on day 18, and the fetuses were removed and
examined for visceral and skeletal abnormalities. The
remaining dams were allowed to deliver naturally and the
young were maintained until weaning to evaluate postnatal
deficits. Additionally two male and two female weanlings
from each dam were maintained for 8 weeks and mated to
investigate their reproductive potential. Although toxic
signs were noted in the dams at the highest dose level,
there was no significant mortality. Examination of the
fetuses revealed no external, visceral, or skeletal abnor-
malities. Treatment of the dams with fenvalerate did not
affect the reproductive performance of the offspring.
Van Der Pauw et al. [187] dosed groups of pregnant
Dutch rabbits (20 to 31 per group) orally with fenvalerate
(0, 12.5, 25, or 50 mg/kg body weight per day) from day 6
to day 18 of gestation. The dams were sacrificed on day
28 and standard teratogenic assessments made. The body
weights of the dams given the highest dose were reduced.
There were no significant differences from controls in any
of the other parameters examined. Fenvalerate was not
found to be teratogenic in this study.
7.6.2 Reproduction studies
In studies by Stein [167] and Beliles et al. [10],
groups of Sprague-Dawley rats (11 males and 22 females per
group) were fed fenvalerate in the diet at levels of 0, 1,
5, 25, or 250 mg/kg. The animals were dosed for 9 weeks
prior to mating and the initiation of a standard three-
generation (two litters per generation) reproduction
study. Fertility, viability, gestation, and lactation in-
dices were calculated for each group of rats and were com-
pared to control values. Ten of the female weanlings and
all of the males from the F3b litters were examined his-
tologically at the conclusion of the study. The mean body
weight of the F2b adults was decreased at 250 mg/kg, but
no pathological changes were noted to account for this
weight loss. No effects on reproductive parameters in any
of the three generations were observed. Histological
examination revealed no treatment-related changes in any
group.
Groups of pregnant ICR mice (32-33 mice per group)
were orally administered fenvalerate at dose levels of 0,
5, 15, or 50 mg/kg body weight per day on days 6 to 15 of
gestation in a standard teratogenicity bioassay. Two male
and two female weanlings from each dam were maintained for
8 weeks and mated to investigate their reproductive poten-
tial. Toxic signs were noted in maternal mice at the
highest dose level. There was no significant mortality
over the course of the study, and no effects were noted on
any of the other animals as a result of continuous admin-
istration of fenvalerate. The animals maintained in the
abbreviated reproduction study showed no differences from
the control value in their ability to reproduce. There
were no changes in the reproduction indices with any
animals examined [93].
7.7 Neurotoxicity
In a study by Butterworth & Carter [20], histopatho-
logical examination was performed on the sciatic nerve and
posterior tibial nerve of rats that had been exposed to
acutely toxic levels of fenvalerate. After poisoning, and
for 9 days during the course of recovery, axonal breaks,
swelling, and vacuolisation, accompanied by phagocytosis
of myelin, were seen. The degree to which myelin was dis-
rupted was dose dependent and was closely associated with
the acute signs of toxicity.
Acute oral administration of fenvalerate, cyper-
methrin, resmethrin, permethrin, and natural pyrethrum to
rats at very high dose levels resulted in severe clinical
signs of poisoning and mortality within 24 h. Histopatho-
logical lesions were observed in the sciatic nerve with
all compounds tested. Fenvalerate did not cause the clini-
cal signs or histopathological lesions at a lower dose
level (200 mg/kg), nor did the other compounds [141,
142].
When groups of six male and six female rats were fed
fenvalerate in the diet at a concentration of 2000 mg/kg
for 8 to 10 days, all the animals showed typical signs of
acute intoxication, such as ataxia, tremors, and hyperex-
citability. Histopathological examinations did not reveal
any adverse effects of fenvalerate on the sciatic nerve
[73].
In order to evaluate the reversibility of the lesions
induced in the sciatic nerve, rats were administered
fenvalerate in the diet at dose levels of 0 or 3000 mg/kg
diet for 10 days. This was followed by a control diet for
12 weeks. During the treatment period, mortality was 60%
in the animals treated with fenvalerate. Rats on the
recovery control diets, sacrificed at 3 weeks, continued
to show swelling and disintegration of axons of the
sciatic nerves. However, there were no histopathological
lesions after 6, 9, or 12 weeks of the recovery period.
These results showed the reversibility of the sciatic
nerve lesions caused by fenvalerate [143].
In studies by Butterworth & Hend [21], fenvalerate was
administered orally to Wistar or Carworth Farm E (CFE)
rats either as single doses or in the diet. When given in
large quantities by a single dose of 250, 500, 800, or
1000 mg/kg body weight, which were sufficient to kill some
of the treated animals, fenvalerate produced sporadic
Wallerian degeneration in the sciatic nerve. The neuro-
pathy was never severe and was not seen in animals given
the compound in sub-lethal doses. In feeding studies (for
5 weeks at 1000 mg/kg diet and for 3 months at 2000 mg/kg
diet), no lesions were seen in the peripheral nerve,
brain, or spinal cord, and there was no evidence of cumu-
lative neurotoxicity.
B6C3F1 mice and Sprague-Dawley rats showed the
characteristic signs of intoxication following single oral
doses of fenvalerate ranging from 56 to 320 and 133 to
1000 mg/kg body weight, respectively. Neurological signs,
such as splayed gait, tremors, ataxia, and hind limb
incoordination, were observed at doses of 100 mg/kg or
more (mice) and 133 mg/kg or more (rats) within 1-8 h
after dosing. These signs had disappeared in most animals
within 72 h. Slight peripheral nerve fibre damage was
detected in surviving mice and rats sacrificed 10 days
after dosing. The incidence and severity were dose related
at doses > 56 and > 180 mg/kg; however, even at lethal
doses, there was no evidence of nerve lesions in some
animals. Thus, two distinct neurological effects were
observed, i.e., (a) a reversible ataxia and (b) incoordi-
nation plus a neuropathological effect manifested as
sparse axonal damage in peripheral nerves [149].
In a study by Milner & Butterworth [123], groups of
six hens were administered fenvalerate orally at dose
levels of 0 or 1000 mg/kg per day for 5 days. A positive
control of tri- ortho-cresyl phosphate (0.5 ml/kg) (TOCP)
was also included in the study. The fenvalerate-treated
birds were retreated, using the same dose regimen, after
3 weeks. The TOCP-treated hens showed signs of delayed
neurotoxicity and histopathological lesions in their
sciatic nerve and spinal cord. As would be expected for a
non-organophosphorus insecticide, there were no typical
clinical signs and histopathological lesions related to
fenvalerate.
7.8 Behavioural Studies
Guinea-pigs responded to dermal applications of
fenvalerate by scratching the treated sites of the skin.
This characteristic response was essentially over within
3-4 h. When the powerful skin irritant oil of mustard was
applied to fenvalerate-treated sites of skin 4-72 h after
the fenvalerate treatment, the behavioural skin sensory
response was re-stimulated. Oil of mustard alone did not
produce skin sensory stimulation. These results indicate
that pyrethroid treatment causes a transient sensitivity
to stimulation produced by chemical irritants [111].
To develop an animal model for studying skin sensory
stimulation, Duncan-Hartley guinea-pigs were treated with
pyrethroid solutions on one side and control substances on
the other side of their shaved back. The animals responded
by licking, scratching, or biting the test sites, and
activity was quantified by counting the number of times
the animals responded. This behavioural activity reached
a maximum 1-4 h after treatment. A chemical irritant (oil
of mustard) was able to restimulate the behavioural ac-
tivity when applied within 24 h after pyrethroid appli-
cation. Skin sensory stimulation produced by cyano-
containing pyrethroids, including fenvalerate, was
significantly greater than that produced by non-cyano-
containing pyrethroids. This behavioural model provides a
quantitative means of evaluating pyrethroid non-erythema-
tous skin sensory stimulation [22].
7.9 Miscellaneous Studies
In an antidotal study, phenobarbital, pentobarbital,
and diphenylhydantoin were found to be effective in
relieving the acute signs of intoxication in the rat.
Intraperitoneal injection of phenobarbital (50 mg/kg)
prevented tremor, diphenylhydantoin (100 mg/kg) by the
same route reduced the toxic reaction, and pentobarbital
(35 mg/kg intraperitoneally) removed the tremor reaction
completely within 30 min. The combination of diphenyl-
hydantoin with either of the barbiturates was effective in
reducing the onset and severity of tremors whereas various
other agents d-tubocurarine, atropine, meprobamate,
diazepam, biperiden, and trimethadione) were ineffective
[113].
The therapeutic potency of intraperitoneally admin-
istered methocarbamol was examined as an antidote against
the acute oral intoxication of rats by a lethal dose of
fenvalerate. Methocarbamol was initially administered at
a dose of 400 mg/kg body weight, followed by repeated
doses of 200 mg/kg body weight when tremors or hyperexcit-
ability to sound were observed. Methocarbamol markedly
decreased the mortality from 80%, which would be caused by
an administration of 850 mg fenvalerate/kg, to 0%, and was
effective in alleviating motor symptoms such as fibril-
lation, tremors, hyperexcitability, clonic seizures, and
choreoathetotic movements. A subcutaneous administration
of atropine sulfate (25 mg/kg body weight) was also effec-
tive in reducing the salivation produced by fenvalerate
[76].
Effective treatments against fenvalerate-mediated ef-
fects have been investigated by quantifying behavioural
skin sensory responses such as licking, scratching, or
biting of the treated sites by fenvalerate-treated guinea-
pigs. Preparations containing vitamin E, corn oil, or the
local anesthetic benzocaine were most effective [111].
Intraperitoneal administration of O-ethyl- O-(4-nitro-
phenyl)phenylphosphonothioate (EPN) or S,S,S-tributylphos-
phorotrithioate (DEF) to mice at 25 mg/kg increased the
intraperitoneal toxicity of fenvalerate (administered 1 h
later) by more than 25-fold; the LD50 decreased from
> 1000 mg/kg to 37 or 42 mg/kg. This suggests that mam-
malian esterases highly sensitive to inhibition by certain
organophosphorus compounds may play a critical role in
fenvalerate detoxication. This kind of synergism among
pesticides would be detrimental in increasing the toxicity
of certain pyrethroids to mammals [62].
Fenvalerate, administered to dogs at a dose sufficient
to induce toxic signs, showed no consistent cardiovascular
effects. Respiratory stimulation was noted at high levels,
and this was not reduced by anaesthetic supplements
(urethane, chloralose, and pentobarbital) [91].
7.10 Mechanism of Toxicity - Mode of Action
The intravenous toxicity of fenvalerate (50-100 mg/kg)
to rats was examined by Verschoyle & Aldridge [189].
[2S, alphaS]-Fenvalerate induced choreoathetosis with sali-
vation (CS-syndrome), and was classified as a Type II
pyrethroid. For the mode of action of pyrethroids in
general see Appendix 1.
The intracerebral injection of [2S, alphaS]-fenvaler-
ate (0.01 mg/kg) to mice produced the Type II syndrome,
consisting of choreoathetosis, convulsion, and saliv-
ation [98]. The Type II syndrome is produced character-
istically by pyrethroids with an alpha-cyano group and
the site of action in mammals is considered to be the
central nervous system.
In intact locusts and neuromuscular preparations of
locusts, fenvalerate caused (a) prolonged firing in the
crural nerve without associated muscle contractions; (b)
sustained muscle contractions; and (c) a block of neurally
evoked muscle contractions at low concentration (10-8 to
10-5 mol/litre). However, fenvalerate did not cause
repetitive firing and after-discharges with associated
muscle contractions [27]. The fenvalerate stereoisomers
with an (S) configuration in the alcohol moiety are more
active pharmacologically and toxicologically than those
with the (R) configuration or the racemate (R,S). It is
also apparent that stereoisomers with the (S) configur-
ation in the acid moiety are more active than those with
the (R) configuration or the racemate (R,S) [27].
[S,S]-Fenvalerate does not induce repetitive firing in
the cockroach cercal sensory nerves either in vivo or in
vitro. It does, however, cause different signs, including
bursts of spikes in the cercal motor nerve [60].
There are no clear-cut links between electrophysio-
logical findings in insects and toxicity to mammals.
8. EFFECTS ON HUMANS
8.1 Occupational Exposure
Appraisal
Fenvalerate has been found to induce skin sensations in
some of the workers who handle this insecticide. Clinical
studies showed that the skin sensations develop with a latent
period of approximately 30 min, peak by 8 h and deteriorate
after 24 h. Numbness, itching, tingling, and burning are symp-
toms frequently reported. Alpha-tocopheryl acetate has been
found to inhibit the occurrence of these skin sensations.
In a study by Kolmodin-Hedman et al. [97], personnel
(52 people) at various plant nurseries who had handled
conifer seedlings treated with fenvalerate were examined.
The symptoms were mainly irritative, such as itching,
paraesthesia and burning of the skin, and itching and
irritation of the eyes. The frequency (% of people who
reported these signs) was about 10%. Increased nasal
secretion was reported by 19% of the personnel.
No clinical case of pyrethroid poisoning had been
reported until outbreaks of acute deltamethrin and fenval-
erate poisoning occurred among cotton growers in China in
1982. Having been told (in error) that pyrethroids were
non-toxic, the farmers handled the pyrethroid insecticides
without taking any precautions. After repeated spraying
in the cotton fields, the mild cases presented severe
headaches, dizziness, fatigue, nausea, and anorexia, with
transient changes in the electroencephalogram (EEG), while
a severe case developed muscular fasciculation, repetitive
discharges in the electromyogram (EMG), and frequent con-
vulsions. However, all were found by follow-up studies to
have completely recovered and the prognosis of acute
pyrethroid poisoning proved to be correct [71]a.
----------------------------------------------------------
a More recently, the same author reviewed 573 cases of
acute pyrethroid poisoning reported in the Chinese
medical literature during 1983-1988 [213]. Among these
there were 196 cases of acute fenvalerate poisoning,
63 of which were occupational, due to inappropriate
handling and 133 accidental, mostly due to ingestion.
Two died of convulsions. All others recovered with
symptomatic and supportive treatment within 1-6 days.
A comprehensive review of clinical manifestations is
included.
Among 23 workers exposed to synthetic pyrethroids,
including fenvalerate, 19 experienced one or more episodes
of abnormal facial sensation, developing between 30 min
and 3 h after exposure and persisting for 30 min to 8 h
[106]. However, there were no abnormal neurological signs,
and electrophysiological studies showed normal responses
in the arms and legs. The symptoms were most likely due
to transient lowering of the threshold of sensory nerve
fibres or sensory nerve endings following exposure of the
facial skin to pyrethroids.
In a study by Tucker & Flannigan [182], selected
individuals who had worked extensively with fenvalerate in
the delta region of the Mississippi and Alabama, USA, were
interviewed and examined. They had, on some occasions,
noted paraesthesia associated with exposure to this insec-
ticide. The cutaneous sensation was described as a sting-
ing or burning, which progressed to numbness in approxi-
mately one-third of the exposed workers. The sensation
typically began a number of hours after contact, peaked in
the evening, and rarely was present the following morning.
The intensity of the sensation varied according to the
type and extent of exposure. Clinical signs of inflam-
mation such as oedema or vesiculation were not apparent.
Erythema was present in a few individuals but this was
difficult to distinguish from sunburn. Several environ-
mental factors were found to affect the cutaneous sen-
sation associated with fenvalerate exposure.
8.2 Clinical Studies
A double-blind study with 29 male volunteers was
performed to test the skin reaction to formulated fenval-
erate. The emulsifiable concentrate formulation was
diluted with water and applied to one side of the face, on
the cheek, with a control formulation on the opposite
cheek. There were no signs of dermatitis 24 h after appli-
cation, nor did the fenvalerate formulation produce any
abnormal skin sensations. There were no indications that
any of the symptoms such as tingling, itching, or burning
were associated with fenvalerate [18].
A double-blind study was performed to compare human
discrimination of technical fenvalerate, the heavy-ends
fraction of distilled fenvalerate, and ethyl alcohol
(vehicle) applied to the lower edge of each earlobe of 36
adult (both male and female) volunteers on three separate
occasions. Both forms of fenvalerate caused a statisti-
cally significant increase in paraesthesia, compared with
the vehicle alone. The onset of the cutaneous sensations
occurred 1 h after application, peaked at 3-6 h, and
lasted approximately 24 h. Numbness, itching, burning,
tingling, and warmth were the most frequently reported
sensations. The difference between the effects of the two
fractions of fenvalerate was not statistically significant
[92].
Flannigan & Tucker [55], Flannigan et al. [56, 57],
and Malley et al. [112] studied the difference in the
degree of paraesthesia induced by a number of pyrethroids.
Applications of 0.05 ml fenvalerate formulated to field
strength (0.13 mg/cm2) were made to a 4 cm2 area of
earlobe on five occasions, the opposite earlobe receiving
distilled water. Participant evaluation after each appli-
cation continued for 48 h and involved description of the
cutaneous sensations. Each participant was treated after
each application with one of the remaining compounds.
Fenvalerate (like the other pyrethroids) induced skin sen-
sations. The paraesthesia developed with a latent period
of approximately 30 min, peaked by 8 h, and deteriorated
as early as 24 h. The local application of dl-alpha
tocopheryl acetate markedly inhibited the occurrence of
skin sensations.
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
has discussed and evaluated fenvalerate at its meetings in
1979, 1981, 1982, 1984, 1986, and 1987 [40-47, 49, 50, 52-
4].
Since 1986, an acceptable daily intake (ADI) of 0-0.02
mg/kg body weight has been established.
In the WHO Recommended Classification of Pesticides by
Hazard, technical fenvalerate is classified as "moder-
ately hazardous" (Class II) [197].
REFERENCES
1. AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10(2): 147-151.
2. ALBERT, J.R. & SUMMITT, L.M. (1976) Intravenous toxicity of WL 43775
(6-1-0-0) in the mouse (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
3. ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri and its prey,
Panonychus ulmi on apples in southeast England. Environ. Entomol., 9:
436-439.
4. ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several non-target aquatic invertebrates. Environ. Entomol., 11:
1251-1257.
5. ATKINS, E.L., KELLUM, D., & ATKINS, K.W. (1981) Reducing pesticide
hazards to honey bees: mortality prediction techniques and integrated
management strategies, Berkeley, California, University of
California, Division of Agricultural Sciences (Leaflet No. 2883).
6. BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
7. BATISTE-ALENTORN, M., XAMENA, N., VELAZQUEZ, A., CREUS, A., & MARCOS,
R. (1987) Non-mutagenicity of fenvalerate in Drosophilia.
Mutagenesis, 2: 7-10.
8. BATTELLE (1982) Pesticide programme of research and market planning.
Part I. Insecticides, Geneva, Battelle Research Centre.
9. BATTELLE (1986) World pesticide programme: Insecticide II, Geneva,
Battelle Research Centre.
10. BELILES R.P., MARIS, S.L., & WEIR, R.J. (1978) Three-generation
reproduction study in rats, Kensington, Maryland, Litton Bionetics
Inc. (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
11. BIRDIE, N.S., BANERJI, R.K., & CHAUHAN, A.K. (1986) Gas liquid
chromatographic separation of pyrethrins from some synthetic
pyrethroids in formulations. Pyrethrum Post, 16(3): 77-80.
12. BLAIR, D. & RODERICK, H. (1975) Toxicity studies on the pyrethroid
insecticide WL 43775, emulsifiable concentrate FX 3368: Acute
inhalation exposure to an aqueous spray (Unpublished report submitted
to WHO by Shell Development Co., Ltd).
13. BRADBURY, S.P. & COATS, J.R. (1982) Toxicity of fenvalerate to
bobwhite quail (Colinus virginianus) including brain and liver
residues associated with mortality. J. Toxicol. environ. Health, 10:
307-319.
14. BRADBURY, S.P., COATS, J.R., & MCKIM, J.M. (1986) Toxicokinetics of
fenvalerate in rainbow trout (Salmo gairdneri). Environ. Toxicol.
Chem., 5: 567-576.
15. BRADBURY, S.P., MCKIM, J.M., & COATS, J.R. (1987) Physiological
response of rainbow trout (Salmo gairdneri) to acute fenvalerate
intoxication. Pestic. Biochem. Physiol., 27: 275-288.
16. BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-
residue method to determination of synthetic pyrethroid residues in
celery and animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
17. BROOKS, T.M. (1976) Toxicity studies with WL 43775: Mutagenicity
studies with WL43775 in the host-mediated assay (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
18. BROWN, L.J. & SLOMKA, M.B. (1979) Skin reaction potential of use
dilution (1.33%) PYDRIN EC (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
19. BRUCE, M.L. & CARUSO, J.A. (1985) The laminar flow torch for gas
chromatographic He microwave plasma detection of pyrethroids and
dioxins. Appl. Spectrosc., 39(6): 942-949.
20. BUTTERWORTH, S.T.G. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL 43775: Acute oral toxicity and neuropathological
effects in rats (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
21. BUTTERWORTH, S.T.G. & HEND, R.W. (in press) Peripheral nerve lesions
induced by large doses of pyrethroid. Neuropathol. appl. Neurobiol.
22. CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin sensory stimulation
characterized by a new behavioural paradigm. Toxicol. appl.
Pharmacol., 76: 270-279.
23. CAPLAN, J.R., ISENSEE, A.R., & NELSON, J.O. (1984) Fate and effect of
[14C]fenvalerate in a tidal marsh sediment ecosystem model. J. agric.
food Chem., 32: 166-171.
24. CAYLEY, G.R. & SIMPSON, B.W. (1986) Separation of pyrethroid enan-
tiomers by chiral high-performance liquid chromatography. J.
Chromatogr., 356: 123-134.
25. CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four pyrethroid
insecticide in a mineral and organic soil. J. environ. Sci. Health,
B16: 605-615.
26. CHATTERJEE, K.K., TALUKDER, G., & SHARMA, A. (1982) Effects of
synthetic pyrethroids on mammalian chromosomes I. Sumicidin. Mutat.
Res., 105: 101-106.
27. CLEMENTS, A.N. & MAY, T.E. (1977) The actions of pyrethroids upon the
peripheral nervous system and associated organs in the locust.
Pestic. Sci., 8: 661-680.
28. COATS, J.R. & O'DONNELL-JEFFREY, N.L. (1979) Toxicity of four syn-
thetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
29. COOMBS, A.D. & CARTER, B.I. (1975) Toxicity studies on insecticide WL
43775: Acute toxicity, skin irritancy potential of the emulsifiable
concentrate FX 3368 (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
30. COOMBS, A.D. & CARTER, B.I. (1976) Toxicity studies on the
insecticide WL43775: Toxicity and skin and eye irritancy potential of
the ULV formulation FX4353 (Unpublished report submitted to WHO by
Shell Development Co., Ltd).
31. CURTIS, L.R., SEIM, W.K., & CHAPMAN, G.A. (1985) Toxicity of
fenvalerate to developing steelhead trout following continuous or
intermittent exposure. J. Toxicol. environ. Health, 15: 445-457.
32. DAY, K. & KAUSHIK, N.K. (1987a) Short-term exposure of zooplankton to
the synthetic pyrethroid, fenvalerate, and its effects on rates of
filtration and assimilation of the alga Chlamydomonas reinhardii.
Arch. environ. Contam. Toxicol., 16: 423-432.
33. DAY, K. & KAUSHIK, N.K. (1987b) An assessment of the chronic toxicity
of the synthetic pyrethroid, fenvalerate, to Daphnia galeata
mendotae, using life tables. Environ. Pollut., 44: 13-26.
34. DEAN, B.J. (1975) Toxicity studies with WL 43775: Dominant lethal
assays in male mice after single oral doses of WL 43775 (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
35. DEAN, B.J. & SENNER, R.K. (1975) Toxicity studies with WL 43775:
Chromosome studies on bone marrow cells of Chinese hamster after two
daily oral doses of WL 43775 (Unpublished report to WHO submitted by
Shell Development Co., Ltd).
36. ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series No. 42).
37. ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1979) Dislodgable
insecticide residue on cotton foliage: permethrin, curacron,
fenvalerate, sulprofos, decis, and endosulfan. Bull. environ. Contam.
Toxicol., 22: 245-248.
38. EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
39. FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
40. FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49
(FAO Plant Production and Protection Paper 20).
41. FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
299-353 (FAO Plant Production and Protection Paper 20 Sup.).
42. FAO/WHO (1982a) Pesticide residues in food. Report of the 1981 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 26-28
(FAO Plant Production and Protection Paper 37).
43. FAO/WHO (1982b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
209-256 (FAO Plant Production and Protection Paper 42).
44. FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 28 (FAO
Plant Production and Protection Paper 46).
45. FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, p. 420
(FAO Plant Production and Protection Paper 49).
46. FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 46-49,
83, 90-93 (FAO Plant Production and Protection Paper 62).
47. FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations, pp.
343-353 (FAO Plant Production and Protection Paper 67).
48. FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations.
49. FAO/WHO (1986a) Pesticide residues in food. Report of the 1986 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 28-29,
61 (FAO Plant Production and Protection Paper 77).
50. FAO/WHO (1986b) 1986 Evaluations of some pesticide residues in food.
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 189-193, 355 (FAO Plant Production and Protection
Paper 78/1).
51. FAO/WHO (1986c) Codex maximum limits for pesticide residues, 2nd ed.,
Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC XIII).
52. FAO/WHO (1987a) 1986 Evaluations of some pesticide residues in food.
Part II - Toxicology, Rome, Food and Agriculture Organization of the
United Nations, p. 219 (FAO Plant Production and Protection Paper
78/2).
53. FAO/WHO (1987b) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 27 (FAO
Plant Production and Protection Paper 84).
54. FAO/WHO (1988) 1987 Evaluations of some pesticide residues in food.
Part 1 - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 57-59 (FAO Plant Production and Protection Paper
86/1).
55. FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
56. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary irritant contact
dermatitis from synthetic pyrethroid insecticide exposure. Arch.
Toxicol., 56: 288-294.
57. FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Brit. J. ind. Med., 42:
363-372.
58. FLODSTROM, S., WARNGARD, L., LJUNGQUIST, S., & AHLBORG, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch. Toxicol., 61: 218-223.
59. GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
60. GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
61. GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
62. GAUGHAN, L.C., ENGEL, J.I., & CASIDA, J.E. (1980) Pesticide
interactions: effects on organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pestic. Biochem. Physiol., 14: 81-85.
63. GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid
insecticides to bees. Pestic. Sci., 16: 206-207.
64. GHIASUDDIN, S.M. & SODERLUND, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. appl.
Pharmacol., 74: 390-396.
65. GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav. Toxicol.
Teratol., 4:(6) 793-799.
66. GORDON, E.B. & WEIR, R.J. (1978) Lifetime feeding study in rats, SD-
43775 Technical, Kensington, Maryland, Litton Bionetics, Inc.
(Project No. 2054, Report AT-81-0181) (Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd).
67. GREENBERG, R.S. (1981) Determination of fenvalerate, a synthetic
pyrethroid, in grapes, peppers, apples, and cotton seeds by gas-
liquid chromatography. J. agric. food Chem., 29: 856-860.
68. HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage toxicity tests.
Environ. Toxicol. Chem., 2(2): 251-8
69. HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory studies
on the persistence and behavior in soil of four pyrethroid
insecticides. Can. Entomol., 113: 685-694.
70. HART, E.R. (1975) 90-day subacute toxicity study in dogs, SD 43775
Technical (sumicidin), Kensington, Maryland, Litton Bionetics, Inc.
(Technical Report No. AT-51-0013) (Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd).
71. HE, FENSHENG (1987) Occupational neurotoxicology: Current problems
and trends. Presented at the XXII International Congress on
Occupational Health, Sydney, Australia, 27 September-2 October, 1987.
72. HEND, R.W. & BUTTERWORTH, S.T.G. (1975) Toxicity studies of the
insecticide WL 43775: A three-month feeding study in rats
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
73. HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies of the
insecticide WL 43775: A short-term feeding study in rats (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
74. HILL, B.D. (1981) Persistence and distribution of fenvalerate
residues in soil under field and laboratory conditions. J. agric.
food Chem., 29: 107-110.
75. HINE, C. (1975) SD-43775 Toxicity: Acute and repeated (14-day) dermal
toxicity in the rabbit, San Francisco, Hine Inc. (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
76. HIROMORI, T., NAKANISHI, T., KAWAGUCHI, S., SAKO, H., SUZUKI, T., &
MIYAMOTO, J. (1986) Therapeutic effects of methocarbamol on acute
intoxication by pyrethroids in rats. J. Pestic. Sci., 11: 9-14.
77. HOLMSTEAD, R.L. & FULLMER, D.G. (1977) Photodecarboxylation of
cyanohydrine esters. Models for pyrethroid photodecomposition. J.
agric. food Chem., 25: 56-58.
78. HOLMSTEAD, R.L., FULLMER, D.G., & RUZO, L.O. (1978) Pyrethroid
photodecomposition: Pydrin. J. agric. food Chem., 26: 954-959.
79. HORIBA, M., KITAHARA, H., TAKAHASHI, K., YAMAMOTO, S., & MURANO, A.
(1980) Gas chromatographic determination of fenvalerate (S-5602) in
technical preparations. Agric. biol. Chem., 44: 1197-1199.
80. HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
81. ITO, N. (1976a) Histopathological findings of mice treated with S5602
for three months (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
82. ITO, N. (1976b) Histopathological findings of mice and rats exposed
to mist of S5602 for four weeks (Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd).
83. ITO, N. (1978) Histopathological findings in mice treated with S5602,
Nagoya, Nagoya City University Medical School (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
84. JOHANSEN, C., MAYER, D., MADSEN, R., & ROBINSON, W. (1975) Bee
research investigations, 1975, Pullman, Washington, Washington State
University, Department of Entomology (Unpublished report).
85. JOHANSEN, C., KIOUS, C., SCHULTZ, G., GUPTA, R., & STANFORD, A.
(1978) Bee research investigations, 1978, Pullman, Washington,
Washington State University, Department of Entomology (Unpublished
report).
86. KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1981) Comparative metabolism
of fenvalerate and the [2S, S]-isomer in rats and mice. J. Pestic.
Sci., 6: 317-326.
87. KANEKO, H., MATSUO, M., & MIYAMOTO, J. (1986) Differential metabolism
of fenvalerate and granuloma formation. I. Identification of
cholesterol ester derived from a specific chiral isomer of
fenvalerate. Toxicol. appl. Pharmacol., 83: 148-156.
88. KANEKO, H., TAKAMATSU, Y., OKUNO, Y., ABIKO, J., YOSHITAKE, A., &
MIYAMOTO, J. (1988) Substrate specificity for formation of
cholesterol ester conjugates from fenvalerate analogues and for
granuloma formation. Xenobiotica, 18(1): 11-19.
89. KATAGI, T., MIKAMI, N., MATSUDA., T., & MIYAMOTO, J. (1985a)
Photodegradation of fenvalerate and esfenvalerate on soils
(Unpublished Report No. LLM-50-0005 submitted to WHO by Sumitomo
Chemical Co., Ltd).
90. KATAGI, T., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985b)
Hydrolysis of fenvalerate and esfenvalerate in buffered aqueous
solutions (Unpublished Report No. LLM-50-0006 submitted to WHO by
Sumitomo Chemical Co., Ltd).
91. KIRKLAND, V.L. & ALBERT, J.R. (1977) Preliminary pharmacodynamic
investigation of SD 43775 (6-10-0) administered intravenously to the
anesthetized dog. I. Effects on the cardiovascular system and
respiration. II. Electrocardiographic (ECG) findings (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
92. KNOX, J.M., II, TUCKER, S.B., & FLANNIGAN, S.A. (1984) Paraesthesia
from cutaneous exposure to a synthetic pyrethroid insecticide. Arch.
Dermatol., 120: 744-746.
93. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic study on
S5602 in mice (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
94. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Acute inhalation
toxicity of S3206 and S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
95. KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976c) Subacute inhalation
toxicity study of S5602 in mice and rats (Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd).
96. KOHDA, H., KANEKO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J. (1979)
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
97. KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and fenvalerate).
Arch. Toxicol., 50: 27-33.
98. LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
99. LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
100. LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
101. LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
102. LEE, P.W. (1985) Fate of fenvalerate (Pydrin insecticide) in the soil
environment. J. agric. food Chem., 33: 993-998.
103. LEE, P.W., WESTCOTT, N.D., & REICHLE, R.A. (1978) Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871.
104. LEE, P.W., STEARNS, S.M., & POWELL, W.R. (1985) Rat metabolism of
fenvalerate (Pydrin insecticide). J. agric. food Chem., 33: 988-993.
105. LEE, P.W., POWELL, W.R., STEARNS, S.M. & MCCONNEL, O.J. (1987)
Comparative aerobic soil metabolism of fenvalerate isomers. J. agric.
food Chem., 35: 384-387.
106. LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurotoxicology, 2: 1-11.
107. LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 11/12: 843-851.
108. LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol.,
20: 203-216.
109. MCKENNEY, C.L., Jr & HAMAKER, D.B. (1984) Effects of fenvalerate on
larval development of Palaemonetes pugio (Holthuis) and on larval
metabolism during osmotic stress. Aquat. Toxicol., 5: 343-355.
110. MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and
shrimp. Bull. environ. Contam. Toxicol., 25: 950-955.
111. MALLEY, L.A., CAGEN, S.Z., GARDINER, T.H., & VAN GELDER, G.A. (1984)
Characterization of fenvalerate mediated skin sensory stimulation.
Neurotoxicology, 5: 71-72.
112. MALLEY, L.A., CAGEN, S.Z., PARKER, C.M., GARDINER, T.H., VAN GELDER,
G.A., & ROSE, G.P. (1985) Effect of vitamin E and other amelioratory
agents on the fenvalerate-mediated skin sensation. Toxicol. Lett.,
29: 51-58.
113. MATSUBARA, R.A., SUZUKI, T., KADOTA, T., & MIYAMOTO, J. (1977)
Antidotes against poisoning by S5602 in rats (Unpublished report
submitted by Sumitomo Chemical Co., Ltd).
114. MAYER, F.L., Jr (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Washington, DC, US Environmental Protection
Agency, p. 115 (EPA-600/8-87/017).
115. MAYER, F.L., Jr & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp. 235-236.
116. MEGHARAJ, M., VENKATESWARLU, K., & RAO, A.S. (1986) Influence of
cypermethrin and fenvalerate on natural soil algal populations.
Ecotoxicol. environ. Saf., 12: 141-145.
117. MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Willoughby,
Ohio, Meister Publishing Co., pp. C104-C105.
118. MIKAMI, N., TAKAHASHI, N., HAYASHI, K., & MIYAMOTO, J. (1980)
Photodegradation of fenvalerate (Sumicidin) in water and on soil
surface. J. Pestic. Sci., 5: 225-236.
119. MIKAMI, N., SAKATA, S., YAMADA, H., & MIYAMOTO, J. (1984a) Further
studies on degradation of the pyrethroid insecticide fenvalerate in
soils. J. Pestic. Sci., 9: 697-702.
120. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1984b) New
conjugated metabolites of 3-phenoxybenzoic acid in plants. Pestic.
Sci., 15: 531-542.
121. MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J. (1985a) The
metabolism of fenvalerate in plants: The conjugation of the acid
moiety. Pestic Sci., 16: 46-58.
122. MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO, J.
(1985b) Metabolism in rats of 3-phenoxybenzyl alcohol and 3-
phenoxybenzoic acid glucoside conjugates formed in plants. Pestic.
Sci., 16: 33-45.
123. MILNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticide. Investigation of neurotoxic potential of WL 43775
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
124. MIURA, T. & TAKAHASHI, R.M. (1976) Effects of a synthetic pyrethroid,
SD43775, on nontarget organisms when utilized as a mosquito
larvicide. Mosq. News, 36: 322-326.
125. MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
126. MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
127. MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress
of Pesticide Chemistry, Kyoto, Japan, 29 August-4 September, 1982,
Oxford, Pergamon Press, Vol. 1-4.
128. MIYAMOTO, J., KANEKO, H., & TAKAMATSU, Y. (1986) Stereoselective
formation of a cholesterol ester conjugate from fenvalerate by mouse
microsomal carboxyesterase(s). J. Biochem. Toxicol., 1(2): 79-94.
129. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a) Toxicity
of mosquito larvicidal pyrethroids to four species of freshwater
fishes. Environ. Entomol., 7: 428-430.
130. MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978b)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
131. MULLA, M.S., DARWAZEH, H.A., & DHILLON, M.S. (1980) New pyrethroids
as mosquito larvicides and their effects on nontarget organisms.
Mosq. News, 40: 6-12.
132. NOBLE, P.J. (1976) AF1117 residue data in animal tissues, milk and
cream (Unpublished data submitted to WHO by Shell Chemical
(Australia), Ltd).
133. OHKAWA, H., NAMBU, K., INUI, H., & MIYAMOTO, J. (1978) Meta-
bolic fate of fenvalerate (Sumicidin) in soil and by microorganisms.
J. Pestic. Sci., 3: 129-141.
134. OHKAWA, H., KANEKO, H., TSUJI, H., & MIYAMOTO, J. (1979) Metabolism
of fenvalerate (Sumicidin) in rats. J. Pestic. Sci., 4: 143-155.
135. OHKAWA, H., KIKUCHI, R., & MIYAMOTO, J. (1980a) Bioaccumulation and
biodegradation of the [S]-acid isomer of fenvalerate (Sumicidin) in
an aquatic model ecosystem. J. Pestic. Sci., 5: 11-12.
136. OHKAWA, H., NAMBU, K., & MIYAMOTO, J. (1980b) Metabolic fate of
fenvalerate (Sumicidin) in bean plants. J. Pestic. Sci., 5: 215-223.
137. OHNO, N., FUJIMOTO, K., OKUNO, Y., MIZUTANI, T., HIRANO, M., ITAYA,
N., HONDA, T., & YOSHIOKA, H. (1976) 2-Arylalkanoates, a new group of
synthetic pyrethroid esters not containing cyclopropane-carboxylates.
Pestic. Sci., 7: 241-246.
138. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975a) Eye and skin irritation
of S-3206 and S-5602 in rabbits (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
139. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1975b) Skin sensitization
study of S-5602 in guinea pigs (Technical Report No. AT-50-0214)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
140. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1976) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by dermal
applications in rats (Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd).
141. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977a) Neurotoxic effects of
some synthetic pyrethroids and natural pyrethrins by oral
administration in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
142. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977b) Neurotoxic effects of
S5602 and natural pyrethrins by oral administration in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
143. OKUNO, Y., KADOTA, T., & MIYAMOTO, J. (1977c) Recovery of
histopathological lesions in rats caused by short-term feeding of
S5602 (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
144. OKUNO, Y., SEKI, T., ITO, S., KANEKO, H., WATANABE, T., YAMADA, T., &
MIYAMOTO, J. (1986a) Differential metabolism of fenvalerate and
granuloma formation. II. Toxicological significance of lipophilic
conjugate from fenvalerate. Toxicol. appl. Pharmacol., 83: 157-169.
145. OKUNO, Y., ITO, S., SEKI, T., HIROMORI, T., MURAKAMI, M., KADOTA, T.,
& MIYAMOTO, J. (1986b) Fenvalerate-induced granulomatous changes in
rats and mice. J. toxicol. Sci., 11: 53-66.
146. PARKER, C.M., MCCULLOUGH, C.B., GELLATLY, J.B.M., & JOHNSTON, C.D.
(1983) Toxicologic and carcinogenic evaluation of fenvalerate in the
B6C3F1 mouse. Fundam. appl. Toxicol., 3: 114-120.
147. PARKER, C.M., PATTERSON, D.R., VAN GELDER, G.A., GORDON, E.B.,
VALERIO, M.G., & HALL, W.C. (1984a) Chronic toxicity and
carcinogenicity evaluation of fenvalerate in rats. J. Toxicol.
environ. Health, 13: 83-97.
148. PARKER, C.M., PICCIRILLO, V.J., KURTZ, S.L., GARNER, F.M., GARDINER,
T.H., & VAN GELDER, G.A. (1984b) Six-month feeding study of
fenvalerate in dogs. Fundam. appl. Toxicol., 4: 577-586.
149. PARKER, C.M., ALBERT, J.R., VAN GELDER, G.A., PATTERSON, D.R., &
TAYLOR, J.L. (1985) Neuropharmacologic and neuropathologic effect of
fenvalerate in mice and rats. Fundam. appl. Toxicol., 5: 278-286.
150. PARKER, C.M., WIMBERLY, H.C., LAM, A.S., GARDINER, T.H., & VAN
GELDER, G.A. (1986) Subchronic feeding study of decarboxyfenvalerate
in rats. J. Toxicol environ. Health, 18: 77-90.
151. PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
152. PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
153. PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137:7-15.
154. POTTER, J.C. (1976) (a) Tissues of rats fed SD43775-C-14; (b) and (c)
milk and tissues of cows; (d) eggs and tissues from laying hens; (e)
cream from the milk of cows fed SD 43775 (Unpublished report
submitted to WHO by Shell Development Co., Ltd).
155. RANGACHAR, T.R.S., SURESH, T.P., & THINMMAIAH, K. (1981) Acute oral
toxicity of Sumicidin 20E in poultry. Indian vet. J., 58: 941-942.
156. RATTNER, B.A. & FRANSON, J.C. (1983) Methyl parathion and fenvalerate
toxicity in American kestrels: acute physiological responses and
effects of cold. Can. J. Physiol. Pharmacol., 62: 787-792.
157. REED, W.T., EHMAN, A., LEE, P., BARBER, G., & BISHOP, J. (1983)
Pydrin insecticide (fenvalerate) on non-target systems following
field applications. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: Human welfare and the environment, Oxford, Pergamon Press,
Vol. 2, pp. 213-221.
158. ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J.
econ. Entomol., 72: 293-294.
159. RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve - muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
160. SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Degradation of esfenvalerate and its isomers in soils (Unpublished
Report No. LLM-30-0003 submitted to WHO by Sumitomo Chemical Co.,
Ltd).
161. SALEH, M.A., IBRAHIM, N.A., SOLIMAN, N.Z., & EL SHEIMY, M.K. (1986)
Persistence and distribution of cypermethrin, deltamethrin and
fenvalerate in laying chickens. J. agric. food Chem., 34: 895-898.
162. SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygiene
rules for synthetic pyrethroid content in the work zone air.] Gig.
tr. prof. Zabol., 8: 48-50 (in Russian).
163. SCHIMMEL, S.C., GARNAS, R.L., PATRICK, J.M., & MOORE, J.C. (1983)
Acute toxicity, bioconcentration, and persistence of AC 222,705,
benthiocarb, chlorpyrifos, fenvalerate, methyl parathion, and
permethrin in the estuarine environment, J. agric. food Chem., 31:
104-113.
164. SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
165. SODERLUND, D.M. & CASIDA, J.E. (1977) Effect of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pestic. Biochem. Physiol., 7: 391-401.
166. SPEHAR, R.L., TANNER, D.K., & GIBSON, J.H. (1982) Effects of Kelthane
and Pydrin on early life stages of Fathead Minnows (Pimephales
promelas) and Amphipods (Hyalella azteca), Philadelphia, American
Society for Testing and Materials (Special Technical Publication No.
766).
167. STEIN, A.A. (1977) Histopathology evaluation of animals from 3-
generation reproduction study. Albany, New York, Biological Research,
Ltd (Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd).
168. SUMMIT, L.M. & ALBERT, J.R. (1977a) Oral lethality of WL 43775 (6-1-
0-0) in the rat (Unpublished report submitted to WHO by Shell
Development Co., Ltd).
169. SUMMIT, L.M. & ALBERT, J.R. (1977b) Determination of the acute oral
lethality of WL 43775 (6-1-0-0) in the male and female mouse
(Unpublished report submitted to WHO by Shell Development Co., Ltd).
170. SUZUKI, H. & MIYAMOTO, J. (1976) Studies on mutagenicity of S 5602
with bacteria systems (Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd).
171. SUZUKI, H. & MIYAMOTO, J. (1977) Studies on mutagenicity of some
pyrethroids on salmonella strains in the presence of mouse hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
172. SUZUKI, H., KISHIDA, F., & MIYAMOTO, J. (1979) Studies on
mutagenicity of S5602 in Ames test in the presence of hepatic S9
fractions (Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd).
173. SUZUKI, T., KADATA, T., & MIYAMOTO, J. (1976) One-year chronic
toxicity study of S5602 in mice (three month interim report)
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
174. SUZUKI, T., OKUNO, Y., HIROMORI, T., ITO, S., KADOTA, T., & MIYAMOTO,
J. (1977a) Fifteen-month chronic toxicity study of S5602 in rats
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
175. SUZUKI, T., OKUNO, Y., HIROMORI, T., KADOTA, T., & MIYAMOTO, J.
(1977b) Eighteen-month chronic toxicity study of S5602 in mice
(Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd).
176. SYRETT, P. & PENMAN, D.R. (1980) Studies of insecticide toxicity to
lucerne aphids and their predators. N.Z. J. agric. Res., 23: 575-580.
177. TAGATZ, M.E. & IVEY, J.M. (1981) Effects of fenvalerate on field- and
laboratory-developed estuarine benthic communities. Bull. environ.
Contam. Toxicol., 27: 256-267.
178. TAGATZ, M.E., STANLEY, R.S., PLAIA, G.R., & DEANS, C.H. (1987)
Responses of estuarine macrofauna colonizing sediments contaminated
with fenvalerate. Environ. Toxicol. Chem., 6: 21-25.
179. TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1985)
Photodegradation of the [2S, alphaS] isomer of fenvalerate in distilled
water (Unpublished Report No. LLM-50-0001 submitted to WHO by
Sumitomo Chemical Co., Ltd).
180. TAKAMATSU, Y., KANEKO, H., ABIKO, J., YOSHITAKE, A., & MIYAMOTO, J.
(1987) In vivo and in vitro stereoselective hydrolysis of four chiral
isomers of fenvalerate. J. Pestic. Sci., 12: 397-404.
181. TALEKER, N.S., CHEN, J.S., & KAO, H.T. (1983) Persistence of
fenvalerate in subtropical soil. J. econ. Entomol., 76: 223-226.
182. TUCKER, S.B. & FLANNIGAN, S.A. (1983) Cutaneous effects from
occupational exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
183. VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
184. VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect: Neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
185. VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M., (1973)
DDT-like action of allethrin in the sensory nervous system of Xenopus
laevis. Eur. J. Phamacol., 21: 95-106.
186. VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A. (1979)
Effect of insecticides on the sensory nervous system. In: Narahashi,
T., ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 183-210.
187. VAN DER PAUW, C.L., DIX, K.M., BLANCHARD, K., & MCCARTHY, W.V. (1975)
Teratological studies in rabbits given WL 43775 orally (Unpublished
report submitted to WHO by Shell Development Co., Ltd).
188. VAKENTI, J.M. (1986) Persistence of fenvalerate in orchard soils,
Okanagan Valley, British Columbia (Unpublished report of cooperative
study of Agriculture Canada and Ciba-Geigy Canada, Ltd).
189. VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity relation-
ship of some pyrethroids in rats. Arch. Toxicol., 45: 325-329.
190. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog myelinated
nerve fibres. Eur. J. Phamacol., 58: 501-504.
191. VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
192. VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
193. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in mylinated nerves. Nature (Lond.), 295: 601-603.
194. VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN
DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
195. WALKER, B.J., HEND, R.W., & LINNETT, S. (1975) Toxicity studies on
the insecticide WL 43775: Summary of results of preliminary
experiments (Unpublished report submitted to WHO by Shell Development
Co., Ltd).
196. WHO (1979) WHO Technical Report Series No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp.18-23.
197. WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
198. WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
199. WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic pyrethroid
insecticides to predaceous phytoseiid mites and their prey. Aust. J.
agric. Res., 30: 497-501.
200. WOOD MACKENZIE (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
201. WOOD MACKENZIE (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
202. WOOD MACKENZIE (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
203. WOOD MACKENZIE (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
204. WOOD MACKENZIE (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
205. WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida. State Hortic. Soc., 90: 401-402.
206. WORTHING, C.R. (1979) The pesticide manual, 6th ed., Croydon, British
Crop Protection Council, p. 270.
207. WORTHING, C.R. & WALKER, S.B. (1987) Fenvalerate In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 395-
396.
208. WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
209. WSZOLEK, P.C., LEIN, D.H., & LISK, D.J. (1980) Excretion of fenval-
erate insecticide in the milk of dairy cows. Bull. environ. Contam.
Toxicol., 24: 296-298.
210. WSZOLEK, P.C., HOGUE, D.E., & LISK, D.J. (1981a) Accumulation of
fenvalerate insecticide in lamb tissues. Bull. environ. Contam.
Toxicol., 27: 869-871.
211. WSZOLEK, P.C., LAFAUNCE, N.A., WACHS, T., & LISK, D.J. (1981b)
Studies of possible bovine urinary excretion and rumen decomposition
of fenvalerate insecticide and a metabolite. Bull. environ. Contam.
Toxicol., 26: 262-266.
212. BORTHWICK, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
static acute toxicity tests with selected estuarine algae,
invertebrates, and fish. Gulf Breeze, Florida, US Environmental
Protection Agency (EPA-600/4-81-076).
213. HE, F., WANG, S., LIU, L., CHEN, S., ZHANG, Z., & SUN, J. (1989)
Clinical manifestations and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63: 54-58.
APPENDIX I
On the basis of electrophysiological studies with per-
ipheral nerve preparations of frogs (Xenopus laevis; Rana
temporaria, and Rana esculenta), it is possible to dis-
tinguish between 2 classes of pyrethroid insecticides:
(Type I and Type II). A similar distinction between these
2 classes of pyrethroids has been made on the basis of the
symptoms of toxicity in mammals and insects [65, 98, 186,
189, 196]. The same distinction was found in studies on
cockroaches [60].
Based on the binding assay on the gamma-aminobutyric
acid (GABA) receptor-ionophore complex, synthetic
pyrethroids can also be classified into two types:
the alpha-cyano-3-phenoxybenzyl pyrethroids and the non-
cyano pyrethroids [59, 61, 99, 100].
Pyrethroids that do not contain an alpha-cyano group
(allethrin, d-phenothrin, permethrin, tetramethrin,
cismethrin, and bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group
give rise to pronounced repetitive activity in sense
organs and in sensory nerve fibres [185]. At room tempera-
ture, this repetitive activity usually consists of trains
of 3-10 impulses and occasionally up to 25 impulses. Train
duration is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive
firing of the presynaptic motor nerve terminal in the
neuromuscular junction [183]. There was no significant
effect of the insecticide on neurotransmitter release or
on the sensitivity of the subsynaptic membrane, nor on the
muscle fibre membrane. Presynaptic repetitive firing was
also observed in the sympathetic ganglion treated with
these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in
sensory nerve fibres, the pyrethroid-induced repetitive
activity increases dramatically as the temperature is
lowered, and a decrease of 5 °C in temperature may cause a
more than 3-fold increase in the number of repetitive im-
pulses per train. This effect is easily reversed by rais-
ing the temperature. The origin of this "negative tem-
perature coefficient" is not clear [194].
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve im-
pulse. The transitional state of the sodium channel is
controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation
gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the
activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is
open, the activation gate is restrained in the open pos-
ition by the pyrethroid molecule. While all pyrethroids
have essentially the same basic mechanism of action, how-
ever, the rate of relaxation differs substantially for the
various pyrethroids [55].
In the isolated node of Ranvier, allethrin causes pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation [184]. Evidence so
far available indicates that allethrin selectively slows
down the closing of the activation gate of a fraction of
the sodium channels that open during depolarization of the
membrane. The time constant of closing of the activation
gate in the allethrin-affected channels is about 100
milliseconds compared with less than 100 microseconds in
the normal sodium channel, i.e., it is slowed down by a
factor of more than 100. This results in a marked pro-
longation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is
directly responsible for the repetitive activity induced
by allethrin [194].
The effects of cismethrin on synaptic transmission in
the frog neuromuscular junction, as reported by Evans
[38], are almost identical to those of allethrin, i.e.,
presynaptic repetitive firing, and no significant effects
on transmitter release or on the subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral
nervous system of the frog. DDT also causes pronounced
repetitive activity in sense organs, in sensory nerve
fibres, and in motor nerve terminals, due to a pro-
longation of the transient increase in sodium permeability
of the nerve membrane during excitation. Recently, it was
demonstrated that allethrin and DDT have essentially the
same effect on sodium channels in frog myelinated nerve
membrane. Both compounds slow down the rate of closing of
a fraction of the sodium channels that open on depolariz-
ation of the membrane [185, 186, 193].
In the electrophysiological experiments using giant
axons of crayfish, the type I pyrethroids and DDT ana-
logues retain sodium channels in a modified open state
only intermittently, cause large depolarizing after-poten-
tials, and evoke repetitive firing with minimal effect on
the resting potential [108].
These results strongly suggest that permethrin and
cismethrin, like allethrin, primarily affect the sodium
channels in the nerve membrane and cause a prolongation of
the transient increase in sodium permeability of the mem-
brane during excitation.
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type I pyrethroids (allethrin, cismethrin, bioresmethrin,
and 1R, cis-phenothrin) caused moderate presynaptic re-
petitive activity, resulting in the occurrence of multiple
end-plate potentials [159].
Pyrethroids with an alpha-cyano group on the 3-phenoxy-
benzyl alcohol (deltamethrin, cypermethrin, fenval-
erate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an in-
tense repetitive activity in the lateral line organ in the
form of long-lasting trains of impulses [192]. Such a
train may last for up to 1 min and contains thousands of
impulses. The duration of the trains and the number of im-
pulses per train increase markedly on lowering the tem-
perature. Cypermethrin does not cause repetitive activity
in myelinated nerve fibres. Instead, this pyrethroid
causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of de-
polarizing after-potentials during train stimulation [190,
194].
In the isolated node of Ranvier, cypermethrin, like
allethrin, specifically affects the sodium channels of the
nerve membrane and causes a long-lasting prolongation of
the transient increase in sodium permeability during
excitation, presumably by slowing down the closing of the
activation gate of the sodium channel [190, 194]. The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than
100 milliseconds. Apparently, the amplitude of the pro-
longed sodium current after cypermethrin is too small to
induce repetitive activity in nerve fibres, but is suf-
ficient to cause the long-lasting repetitive firing in the
lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids pri-
marily affect the sodium channels in the nerve membrane
and cause a long-lasting prolongation of the transient
increase in sodium permeability of the membrane during
excitation.
In the electrophysiological experiments using giant
axons of crayfish, the Type II pyrethroids retain sodium
channels in a modified continuous open state persistently,
depolarize the membrane, and block the action potential
without causing repetitive firing [108].
Diazepam, which facilitates GABA reaction, delayed the
onset of action of deltamethrin and fenvalerate, but not
permethrin and allethrin, in both the mouse and cockroach.
Possible mechanisms of the Type II pyrethroid syndrome
include action at the GABA receptor complex or a closely
linked class of neuroreceptor [61].
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant
picrotoxin (PTX). Deltamethrin inhibits the binding of
[3H]-dihydropicrotoxin to rat brain synaptic membranes,
whereas the non-toxic R epimer of deltamethrin is inac-
tive. These findings suggest a possible relation between
the Type II pyrethroid action and the GABA receptor com-
plex. The stereospecific correlation between the tox-
icity of Type II pyrethroids and their potency to inhibit
the [35S]-TBPS binding was established using a radio-
ligand, [35S]- t-butyl-bicyclophosphoro-thionate [35S]-TBPS.
Studies with 37 pyrethroids revealed an absolute corre-
lation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all
toxic cyano compounds including deltamethrin, 1R, cis-
cypermethrin, 1R, trans-cypermethrin, and [2S, alphaS]-
fenvalerate were inhibitors, but their non-toxic stereo-
isomers were not; non-cyano pyrethroids were much less
potent or were inactive [99].
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory
potencies of pyrethroids were closely related to their
mammalian toxicities. The most toxic pyrethroids of Type
II were the most potent inhibitors of [3H]-Ro 5-4864
specific binding to rat brain membranes. The [3H]-di-
hydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an in-
teraction with a GABA-regulated chloride ionophore-associ-
ated binding site. Moreover, studies with [3H]-Ro 5-4864
support this hypothesis and, in addition, indicate that
the pyrethroid-binding site may be very closely related to
the convulsant benzodiazepine site of action [100].
The Type II pyrethroids (deltamethrin, 1R, cis-cyper-
methrin and [2S, alphaS]-fenvalerate) increased the input
resistance of crayfish claw opener muscle fibres bathed in
GABA. In contrast, two non-insecticidal stereoisomers and
Type I pyrethroids (permethrin, resmethrin, allethrin)
were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex
[59].
The effects of pyrethroids on end-plate and muscle
action potentials were studied in the pectoralis nerve-
muscle preparation of the clawed frog (Xenopus laevis).
Type II pyrethroids (cypermethrin and deltamethrin) in-
duced trains of repetitive muscle action potentials with-
out presynaptic repetitive activity. However, an inter-
mediate group of pyrethroids (1R-permethrin, cyphenothrin,
and fenvalerate) caused both types of effect. Thus, in
muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current.
But no clear distinction was observed between non-cyano
and alpha-cyano pyrethroids [159].
Appraisal
In summary, the results strongly suggest that the
primary target site of pyrethroid insecticides in the
vertebrate nervous system is the sodium channel in the
nerve membrane. Pyrethroids without an alpha-cyano group
(allethrin, d-phenothrin, permethrin, and cismethrin)
cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during exci-
tation. This results in relatively short trains of repeti-
tive nerve impulses in sense organs, sensory (afferent)
nerve fibres, and, in effect, nerve terminals. On the
other hand, the alpha-cyano pyrethroids cause a long-
lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation.
This results in long-lasting trains of repetitive impulses
in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects
between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-
cyano group on the phenoxybenzyl alcohol, indicates that
it is this alpha-cyano group that is responsible for the
long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse
generation and conduction are basically the same through-
out the entire nervous system, pyrethroids may also
induce repetitive activity in various parts of the brain.
The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of
action. It may be related to the long-lasting repetitive
activity in sense organs and possibly in other parts of
the nervous system, which, in a more advance state of
poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity
and a prolongation of the transient increase in sodium
permeability of the nerve membrane in insects and other
invertebrates. Available information indicates that the
sodium channel in the nerve membrane is also the most
important target site of pyrethroids in the invertebrate
nervous system [196, 208].
Because of the universal character of the processes
underlying nerve excitability, the action of pyrethroids
should not be considered restricted to particular animal
species, or to a certain region of the nervous system.
Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of
pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions,
and on the chemical structure and physical characteristics
of the pyrethroid molecule [191].
RESUME, EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes
d'analyse
La fenvalérate est un insecticide puissant utilisé
depuis 1976. C'est un ester de l'acide (chloro-4 phényle)-
2 méthyl-3 butyrique et de l'alcool alpha-cyano-phénoxy-
benzylique. Malgré l'absence du cycle cyclopropane, ses
propriétés insecticides le rattachent au groupe des
pyréthroïdes. Il s'agit d'un mélange racémique de quatre
isomères optiques dont les configurations sont [2S, alphaS],
[2S, alphaR], [2R, alphaS] et [2R, alphaR]. L'isomère [2S,
alphaS] est le plus actif biologiquement; vient ensuite
l'isomère [2S, alphaR].
Le fenvalérate de qualité technique se présente sous
la forme d'un liquide visqueux jaune ou brun dont la
densité est de 1,175 à 25 °C. Il est relativement non
volatil, sa tension de vapeur étant de 0,037 mPa à 25 °C.
Pratiquement insoluble dans l'eau (environ 2 µg/litre),
il est soluble dans les solvants organiques comme
l'acétone, le xylène et le kérosène. Il est stable à la
lumière, à la chaleur et à l'humidité, mais instable en
milieu alcalin par suite de l'hydrolyse du groupement
ester.
Le dosage des résidus et les analyses écotoxico-
logiques peuvent s'effectuer par chromatographie en phase
gazeuse avec détection par capture d'électrons, la
concentration minimale décelable étant de 0,005 mg/kg.
Pour l'analyse des produits techniques on utilise la même
méthode mais avec un détecteur à ionisation de flamme.
1.2 Production et usage
On utilise dans le monde environ 1000 tonnes de
fenvalérate par an (chiffres de 1979-1983), essentielle-
ment en agriculture mais également pour la désinsecti-
sation des habitations et des jardins et le déparasitage
des bestiaux, soit seul, soit en association avec d'autres
insecticides. Il est présenté sous forme de concentré
émulsionnable, de concentré pour épandage à très bas
volume, de poudre pour poudrage et de poudre mouillable.
1.3 Exposition humaine
C'est essentiellement du fait de la présence de
résidus dans les aliments que la population dans son
ensemble est exposée à cet insecticide. Le respect des
règles de bonne pratique permet en général de maintenir
les résidus dans les récoltes à un faible niveau. L'expo-
sition qui en découle pour la population générale devrait
a priori être très faible mais on ne dispose pas de
données tirées d'études de la ration totale.
L'analyse des résidus présents dans les céréales
ensilées a montré que plus de 70% de la dose appliquée
subsistent sur le blé au bout de dix mois à 25 °C. Après
mouture et panification, la teneur en résidus du pain
blanc et de la farine de froment est à peu près la même
(environ 0,06-0,1 mg/kg).
Les informations relatives à l'exposition profession-
nelle au fenvalérate sont très fragmentaires.
1.4 Destinée dans l'environnement
Dans le sol, il y a dégradation par coupure de la
liaison ester et du groupement diphényl-éther, hydroxyl-
ation du cycle benzénique, hydratation du nitrile en
amide, l'oxydation des fragments se poursuivant jusqu'à
l'obtention d'anhydride carbonique qui constitue le
principal produit final. L'étude du potentiel de lixivi-
ation du fenvalérate et de ses produits de dégradation a
montré qu'il n'y avait guère de pénétration au niveau du
sol.
Dans l'eau et à la surface du sol, la fenvalérate
subit une photodégradation par la lumière solaire. On a
montré qu'il se produisait une coupure du groupement
ester, une hydrolyse du groupement cyano, une décarboxy-
lation conduisant au (phénoxy-3)-2 (chloro-4 phényle)-3
méthyl-4 pentane-nitrile (décarboxy-fenvalérate), ainsi que
d'autres réactions à initiation radicalaire.
Sur les végétaux, la fenvalérate a une demi-vie
d'environ 14 jours. La principale réaction consiste dans
la rupture de la liaison ester suivie d'une oxydation
et/ou d'une conjugaison des fragments. Il se produit
également une décarboxylation en décarboxy-fenvalérate.
En général, la dégradation dans l'environnement
conduit à des produits moins toxiques.
Dans l'environnement, le fenvalérate est assez
rapidement dégradé. La demi-vie est de 4 à 15 jours dans
les rivières, 8 à 14 jours sur les végétaux, 1 à 18 jours
par photodégradation à la surface du sol et 15 jours à
3 mois dans le sol.
Il n'y a pratiquement aucune lixiviation du fenva-
lérate présent dans le sol. Il est donc improbable que ce
composé puisse s'accumuler de façon importante dans le
milieu aquatique.
1.5 Cinétique et métabolisme
On a étudié la destinée du fenvalérate chez le rat et
la souris au moyen de fenvalérate radio-marqué au niveau
du groupement carboxylate, ou des groupements benzyle ou
cyano. Sauf dans le cas des composés marqués au niveau du
groupement cyano, la radioactivité administrée est
rapidement excrétée (jusqu'à 99% en six jours). Les
principales réactions métaboliques consistent en une
rupture du groupement ester et une hydroxylation en
position 4. On a également observé diverses réactions
d'oxydation et de conjugaison conduisant à un mélange
complexe. Lorsque le fenvalérate est radio-marqué au
niveau du groupement cyano, la dose radioactive s'élimine
moins rapidement (jusqu'à 81% en six jours). La
radioactivité restante est principalement confinée dans la
peau, les poils et l'estomac sous forme de thiocyanate.
Il existe aussi une voie métabolique secondaire, quoique
très importante, qui consiste dans la formation d'un
conjugué lipophile de (chloro-4 phényle)-2-[2R] iso-
valérate. Ce conjugué qui intervient dans la formation de
granulomes a été décelé dans les surrénales, le foie et
les ganglions mésentériques des rats, des souris et de
certaines autres espèces.
1.6 Effets sur les êtres vivant dans leur milieu naturel
Au laboratoire, le fenvalérate se révèle extrêment
toxique pour les organismes aquatiques. La CL50 varie de
0,008 µg/litre pour des mysidacées nouvellement écloses
à 2 µg/litre pour une espèce d'éphéméroptère. Les
épreuves portant sur le cycle évolutif de Daphnia galeata
mendotae ont révélé que la dose sans effet observable
était de 0,005 µg/litre. Le fenvalérate est également
extrêment toxique pour les poissons. Les valeurs de la
CL50 à 96 heures vont de 0,03 µg/litre pour la larve de
Leuresthes tenuis à 200 µg/litre pour le Tilapia
adulte. La dose sans effet observable sur 28 jours
s'établit à 0,56 µg/litre pour les premiers stades de
certains vairons. Le fenvalérate est moins toxique pour
les algues et les mollusques aquatiques, la CL50 à 96
heures étant supérieure à 1000 µg/litre.
La forte toxicité potentielle du fenvalérate pour les
organismes aquatiques ne se manifeste pas dans les essais
sur le terrain ni dans les conditions d'utilisation
pratique. Certains invertébrés aquatiques sont détruits
par un épandage à la surface des eaux mais l'effet sur les
populations est temporaire. On n'a pas signalé de
mortalité chez les poissons. La toxicité moindre observée
lors des épandages de plein champ s'explique par une forte
adsorption du composé par les sédiments.
Le fenvalérate est très toxique pour l'abeille. En
applications topiques la DL50 est de 0,41 µg/abeille,
toutefois l'effet répulsif intense qu'exerce le fenva-
lérate sur ces insectes en réduit l'action dans la
pratique. Rien n'indique qu'il y ait eu des destructions
importantes d'abeilles dans les conditions normales
d'utilisation. Le fenvalérate est plus toxique pour les
acariens prédateurs que pour les espèces cibles de
ravageurs.
Administré par voie orale ou mêlé à la nourriture, le
fenvalérate est très peu toxique pour les oiseaux. La
DL50 est supérieure à 1500 mg/kg de poids corporel en
administration orale directe et dépasse 15 000 mg/kg de
nourriture en administration dans la ration alimentaire
pour le colin de Virginie.
Les organismes aquatiques fixent rapidement le fenva-
lérate. Le facteur de bioconcentration varie de 120 à
4700 selon l'organisme en cause (algues, mollusques,
daphnies et poissons) selon les études effectuées sur des
modèles d'écosystèmes. Toutefois, ce fenvalérate s'élimine
rapidement lorsque les organismes sont replacés en eau
propre. On peut donc considérer qu'en pratique, ce composé
ne présente aucune tendance à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et les systèmes
d'épreuve in vitro
La toxicité aiguë par voie orale du fenvalérate est
modérée à faible. Toutefois, les valeurs de la DL50
peuvent varier considérablement (de 82 à plus de 3200
mg/kg) selon l'espèce animale en cause et le véhicule
d'administration. Les signes cliniques d'intoxication
aiguë apparaissent rapidement mais les survivants
redeviennent asymptomatiques au bout de trois à quatre
jours. Parmi les signes d'intoxication produits par le
mélange racémique, ainsi que par l'isomère [2S, alphaS], on
note de l'agitation, des tremblements, une horripilation,
de la diarrhée, une démarche anormale, une choréo-athétose
et une salivation (syndrome CS); il est classé comme
pyréthroïde du type II. Du point de vue électrophysio-
logique, il produit des bouffées de pointes au niveau des
nerfs moteurs des cerques de la blatte. Toutefois, il n'y
a pas de relation bien définie entre les effets électro-
physiologiques chez l'insecte et la toxicité pour les
mammifères.
Des rats ayant reçu du fenvalérate pendant 8 à 10
jours à raison de 2000 mg/kg de nourriture ont présenté
des signes typiques d'intoxication aiguë. A la dose de
3000 mg/kg de nourriture, on observait des modifications
morphologiques réversibles au niveau du nerf sciatique.
On a également observé des modifications histopatho-
logiques au niveau du même nerf chez des rats et des
souris ayant reçu en une seule fois du fenvalérate par
voie orale à des doses létales ou sublétales.
Des poulets ayant reçu par voie orale du fenvalérate
pendant cinq jours à raison de 1000 mg/kg par jour n'ont
pas présenté de signes cliniques ou morphologiques de
neurotoxicité retardée.
Chez la souris, la toxicité aiguë par voie intra-
péritonéale des métabolites du fenvalérate n'est pas
supérieure à celle du fenvalérate lui-même.
Lors d'études de toxicité subaiguë et subchronique,
des souris, des rats, des chiens et des lapins ont reçu
pendant trois semaines à six mois, du fenvalérate par voie
orale, percutanée et respiratoire. Chez le rat et la
souris, des études d'inhalation de quatre semaines ont
permis de fixer la dose sans effet observable à 7 mg/m3.
Chez le rat, lors d'une étude de 90 jours, elle
s'établissait à 125 mg/kg de nourriture, et sur deux ans à
250 mg/kg de nourriture (soit 12,5 mg/kg de poids
corporel). Dans une étude de 24 à 28 mois, elle s'est
établie à 150 mg/kg de nourriture, soit 7,5 mg/kg de poids
corporel. Une étude de deux ans sur la souris a permis de
fixer la dose à 50 mg/kg de nourriture, soit 6 mg/kg de
poids corporel et une étude de 20 mois, à 30 mg/kg de
nourriture, soit 3,5 mg/kg de poids corporel. Chez le
chien cette dose a été établie à 12,5 mg/kg de poids
corporel, lors d'une étude de 90 jours. Certaines formu-
lations de fenvalérate ont provoqué une irritation cutanée
et oculaire. Toutefois, le fenvalérate technique n'est
pas irritant et n'a pas d'effet sensibilisateur.
Lors d'études de toxicité à long terme, on a noté
l'apparition de microgranulomes chez des souris qui
avaient été traitées avec l'isomère [2R, alphaS] (125 mg/kg
de nourriture) sur une période de un à trois mois. Ces
anomalies disparaissaient lorsqu'on supprimait le fenval-
érate. L'agent causal en était l'ester cholestérique de
l'acide (chloro-4 phényl)-2 isovalérique, un métabolite
lipophile de l'isomère [2R, alphaS] du fenvalérate. La
dose sans effet observable relative à la formation de
microgranulomes chez la souris s'établit à 30 mg de
fenvalérate par kg de nourriture.
Lors d'une autre étude de toxicité à long terme, on a
également observé ces anomalies microgranulomateuses chez
des rats à la dose de 500 mg/kg de nourriture, la dose
sans effet observable étant dans ce cas de 150 mg par kg
de nourriture.
Administré à des souris dans leur nourriture pendant
78 semaines en doses allant jusqu'à 3000 mg/kg ou pendant
deux ans à raison de 1250 mg/kg, le fenvalérate ne s'est
pas révélé cancérogène. Il ne l'a pas été non plus chez
des rats qui avaient reçu pendant deux ans une alimen-
tation contenant jusqu'à 1000 mg d'insecticide par kg.
Le fenvalérate n'est ni mutagène, ni délétère pour les
chromosomes ainsi qu'il ressort d'un certain nombre
d'épreuves in vitro et in vivo.
Il n'est pas non plus tératogène pour la souris et le
lapin à des doses quotidiennes allant jusqu'à 50 mg par kg
de poids corporel et, lors d'une étude de reproduction
portant sur trois générations de rats où les animaux
recevaient des doses allant jusqu'à 250 mg/kg de
nourriture, il n'a affecté aucun des paramètres de la
fonction de reproduction.
1.8 Effets sur l'être humain
Le fenvalérate peut provoquer des sensations
d'engourdissement, de démangeaison, de picotement et de
brûlure chez les travailleurs exposés; les symptômes
apparaissent après une période de latence d'environ
30 minutes, atteignent leur acmé au bout de 8 heures et
disparaissent dans les 24 heures suivantes. Certains cas
d'intoxication se sont produits à la suite d'une expo-
sition professionnelle due au non respect des mesures de
sécurité.
Rien n'indique que le fenvalérate soit nocif pour
l'être humain dans la mesure où il est utilisé conformé-
ment aux recommandations.
2. Conclusions
2.1 Population générale
L'exposition de la population générale au fenvalérate
est probablement très faible. Il n'y a sans doute aucun
risque si on l'emploie conformément aux recommandations.
2.2 Exposition professionnelle
Utilisé de manière raisonnable, et moyennant certaines
mesures d'hygiène et de sécurité, le fenvalérate ne
devrait pas être dangereux pour les personnes qui lui sont
exposées de par leur profession.
2.3 Environnement
Il est improbable que le fenvalérate ou ses produits
de dégradation puissent s'accumuler dans l'environnement
en quantité suffisante pour créer des problèmes, dans la
mesure où l'on respecte les doses d'emploi recommandées.
Au laboratoire, le fenvalérate se révèle extrêmement
toxique pour les poissons, les arthropodes aquatiques et
les abeilles. Toutefois, il ne semble pas que des effets
nocifs durables puissent se produire sur le terrain si
l'insecticide est utilisé conformément aux recomman-
dations.
3. Recommandations
Les concentrations alimentaires qui résultent d'une
utilisation conforme aux recommandations sont considérées
comme très faibles; toutefois il conviendrait de confirmer
ce point de vue en étendant les études de surveillance au
fenvalérate.
Le fenvalérate est utilisé depuis de nombreuses années
et seuls quelques effets temporaires ont été observés ça
et là à la suite d'expositions professionnelles. Néan-
moins, il serait bon de poursuivre les observations sur
l'exposition humaine.
