
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 84
2,4-DICHLOROPHENOXYACETIC ACID (2,4-D) - ENVIRONMENTAL ASPECTS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1989
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154284 5
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 2,4-DICHLOROPHENOXYACETIC ACID
(2,4-D) - ENVIRONMENTAL ASPECTS
1. SUMMARY AND CONCLUSIONS
1.1. Uptake, accumulation, elimination, and biodegradation
1.2. Toxicity to microorganisms
1.3. Toxicity to aquatic organisms
1.4. Toxicity to terrestrial organisms
1.5. Effects of 2,4-D in the field
2. PHYSICAL AND CHEMICAL PROPERTIES
2.1. Synthesis of 2,4-D
2.2. Important chemical reactions of 2,4-D
2.3. Volatility of 2,4-D derivatives
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Production of 2,4-D herbicides
3.2. Uses
3.3. Disposal of wastes
4. UPTAKE, ACCUMULATION, ELIMINATION, AND BIODEGRADATION
4.1. Biodegradation
4.2. Uptake and accumulation by organisms
4.2.1. Laboratory studies
4.2.2. Field studies
4.3. Elimination
5. TOXICITY TO MICROORGANISMS
5.1. Aquatic microorganisms
5.2. Soil microorganisms
6. TOXICITY TO AQUATIC ORGANISMS
6.1. Toxicity to aquatic invertebrates
6.1.1. Short-term toxicity
6.1.2. Behavioural effects
6.2. Toxicity to fish
6.2.1. Effect of formulation on short-term toxicity to fish
6.2.1.1 Tolerance and potentiation
6.2.2. No-observed-effect-levels in short-term tests with fish
6.2.3. Species differences in short-term toxicity to fish
6.2.4. Toxicity to early life-stages of fish
6.2.5. Long-term toxicity to fish
6.2.6. Behavioural effects on fish
6.2.7. Effects of environmental variables on toxicity to fish
6.2.8. Special studies on fish
6.3. Toxicity to amphibians
7. TOXICITY TO TERRESTRIAL ORGANISMS
7.1. Toxicity to terrestrial invertebrates
7.2. Toxicity to birds
7.2.1. Toxicity to birds' eggs
7.2.2. Toxicity to birds after short-term and long-term dosing
7.2.3. Special studies on birds
7.3. Toxicity to non-laboratory mammals
8. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
9. EVALUATION
9.1. Aquatic organisms
9.2. Terrestrial organisms
10. RECOMMENDATIONS FOR FURTHER RESEARCH
REFERENCES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR
2,4-DICHLOROPHENOXYACETIC ACID (2,4-D) - ENVIRONMENTAL ASPECTS
Members
Dr L.A. Albert, Director, Environmental Pollution Programme, National
Institute for Research on Biotic Resources, Veracruz, Mexico
Mr H. Craven, Ecological Effects Branch, Office of Pesticides
Programs, US Environmental Protection Agency, Washington DC, USA
Dr A.H. El-Sebae, Division of Pesticide Toxicology, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Dr J.W. Everts, Department of Toxicology, Agricultural University,
Wageningen, Netherlands
Dr W. Fabig, Fraunhofer Institute for Environmental Chemistry and
Ecotoxicology, Schmallenberg-Grafschaft, Federal Republic of
Germany
Dr R. Koch, Division of Toxicology, Research Institute for Hygiene and
Microbiology, Bad Elster, German Democratic Republic (Chairman)
Dr Y. Kurokawa, Division of Toxicology, Biological Safety Research
Centre, National Institute of Hygienic Sciences, Tokyo, Japan
Dr E.D. Magallona, Pesticide Toxicology and Chemistry Laboratory,
University of the Philippines at Los Banos, College of Agriculture,
Laguna, Philippines
Professor P.N. Viswanathan, Ecotoxicology Section, Industrial Toxi-
cology Research Centre, Lucknow, India
Observers
Dr M.A.S. Burton, The Monitoring and Assessment Research Centre,
London, United Kingdom
Dr I. Newton, The Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
Secretariat
Dr S. Dobson, The Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom (Rapporteur)
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Mr P.D. Howe, The Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR 2,4-DICHLOROPHENOXYACETIC ACID
(2,4-D) - ENVIRONMENTAL ASPECTS
A WHO Task Group on Environmental Health Criteria for
2,4-Dichlorophenoxyacetic acid (2,4-D) - Environmental Aspects met at
the Institute of Terrestrial Ecology, Monks Wood, United Kingdom, from
14 to 18 December 1987. Dr I. Newton welcomed the participants on
behalf of the host institution, and Dr M. Gilbert opened the meeting on
behalf of the three co-sponsoring organizations of the IPCS
(ILO/UNEP/WHO). The Task Group reviewed and revised the draft criteria
document and made an evaluation of the risks for the environment from
exposure to 2,4-D.
The first draft of this document was prepared by Dr S. Dobson and
Mr P.D. Howe, Institute of Terrestrial Ecology. Dr M. Gilbert and
Dr P.G. Jenkins, both members of the IPCS Central Unit, were respon-
sible for the overall scientific content and editing, respectively.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of Health
and Human Services, through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA - a WHO Collaborating Centre for Environmental Health Effects.
INTRODUCTION
There is a fundamental difference in approach between the
toxicologist and the ecotoxicologist concerning the appraisal of the
potential threat posed by chemicals. The toxicologist, because his
concern is with human health and welfare, is preoccupied with any
adverse effects on individuals, whether or not they have ultimate
effects on performance or survival. The ecotoxicologist, in contrast,
is concerned primarily with the maintenance of population levels of
organisms in the environment. In toxicity tests, he is interested in
effects on the performance of individuals - in their reproduction and
survival - only insofar as these might ultimately affect the population
size. To him, minor biochemical and physiological effects of toxicants
are irrelevant if they do not, in turn, affect reproduction, growth, or
survival.
It is the aim of this document to take the ecotoxicologist's point
of view and consider effects on populations of organisms in the
environment. The risk to human health of the use of 2,4-D was
evaluated in Environmental Health Criteria 29: 2,4-Dichlorophenoxy-
acetic acid (WHO, 1984). This document did not consider effects on
organisms in the environment, but did consider environmental levels of
2,4-D likely to arise from recommended uses. No attempt has been made
here to reassess the human health risk; the interested reader should
refer to the original document, which contains the relevant literature
in this area.
This document, although based on a thorough survey of the
literature, is not intended to be exhaustive in the material included.
In order to keep the document concise, only those data which were
considered to be essential in the evaluation of the risk posed by 2,4-D
to the environment have been included.
The term bioaccumulation indicates that organisms take up chemicals
to a greater concentration than that found in their environment or
their food. `Bioconcentration factor' is a quantitative way of
expressing bioaccumulation: the ratio of the concentration of the
chemical in the organism to the concentration of the chemical in the
environment or food. Biomagnification refers, in this document, to the
progressive accumulation of chemicals along a food chain.
1. SUMMARY AND CONCLUSIONS
2,4-D is a selective herbicide which kills broad-leaved plants but
not grasses or conifers. Its chemical structure is a modification of a
naturally occuring plant hormone. 2,4-D is available as the free acid
but is used, in agriculture and forestry, in formulations as a salt or
ester.
1.1. Uptake, Accumulation, Elimination, and Biodegradation
2,4-D does not persist in soil because of its rapid degradation.
The physico-chemical properties of 2,4-D acid and its formulations
have an important effect on its behaviour in environmental
compartments.
The bioavailability to, and uptake by, aquatic and terrestrial
organisms is strongly influenced by the organic matter content of
soils, microbiological activity, and by environmental conditions such
as temperature and pH. Although highly inconsistent, the data on
dissipation and bioavailability in various soils demonstrate a marked
influence of differences in the texture and mineral composition of the
soil. In aerobic soils, with a high content of organic material, and
at high pH values and temperatures, toxic effects are limited because
of rapid degradation of 2,4-D.
Uptake is followed by rapid excretion in most organisms. With the
exception of some algae, the retention of 2,4-D by organisms in the
environment cannot be expected, because of its rapid degradation.
Some microorganisms are capable of utilizing 2,4-D as their sole
carbon source. Repeated application to soil stimulates the number of
organisms capable of degrading the compound.
1.2. Toxicity to Microorganisms
In general 2,4-D is relatively non-toxic to water and soil
microorganisms at recommended field application rates. No effect of
2,4-D was recorded on 17 genera of freshwater and two genera of marine
algae at concentrations up to 222 mg/litre. No effect of 2,4-D on
respiration of either sandy loam or clay loam soils was observed at
concentrations up to 200 mg/kg.
N-fixation by aquatic algae is affected at high concentrations of
2,4-D acid (400 mg/litre). An effect of 2,4-D esters on N-fixation
occurs from a concentration of 36 mg/litre upwards. N-fixing algae in
topsoils appear to be more vulnerable to 2,4-D acid than other algal
species. The Cyanobacteria (blue-green algae) are important as the
major N2 source in tropical ponds and soils.
In the range of 25.2 to 50.4 mg/litre, 2,4-D was inhibitory to all
types of soil fungi.
Cell division was reduced in a green alga by 2,4-D at 20 mg/litre
and stopped at 50 mg/litre. No effect was observed on a natural
phytoplankton community after exposure to 2,4-D at 1 mg/litre.
However, exposure to esters of 2,4-D reduced productivity in these
organisms.
1.3. Toxicity to Aquatic Organisms
At recommended application rates, the concentration of 2,4-D in
water has been estimated to be a maximum of 50 mg/litre. Most
applications would lead to water concentrations much lower than this
(between 0.1 and 1.0 mg/litre).
The short-term toxicity data on the effects of 2,4-D free acid,
its salts, and esters on aquatic invertebrates is extensive. Ester
formulations are more toxic than the free acids or salts. Sensitivity
variations exist among species in response to the same formulation.
Organisms become more sensitive to 2,4-D when the water temperature
increases. Reproductive impairment occurred at concentrations below
0.1 of the short-term toxic levels determined for these formulations.
LC50 values for fish vary considerably. This variation is partly
due to differences in species sensitivity, chemical structure (esters,
salts, or free acid), and formulation of the herbicide.
Although the free acid is the physiologically toxic entity, the
ester formulations represent a major hazard to fish when used directly
as aquatic herbicides (because they are more readily taken up by fish).
Amine salt formulations used to control aquatic weeds do not affect
adult fish.
The no-observed-effect-level (NOEL) varies with the species and the
formulation: less than 1 mg/litre (coho salmon) to 50 mg/litre (rainbow
trout).
Fish larvae are the most sensitive life stage but are unlikely to
be affected under normal usage of the herbicide.
Long-term adverse effects on fish are observed only at
concentrations higher than those produced after 2,4-D has been applied
at recommended rates.
Few studies are related to the effects of environmental variables,
such as temperature and water hardness, on 2,4-D toxicity to fish.
Higher temperature possibly increases the toxicity. This might be
considered when assessing the safety of 2,4-D to fish during control of
aquatic weeds.
Fish detect and avoid 2,4-D only at higher concentrations than
those obtained under normal conditions of use.
Amphibian larvae are generally tolerant to amine salts of 2,4-D;
the 96-h LC50 values exceed 100 mg/litre. Of the species tested, only
one was sensitive. No information is available on reproductive
development and differentiation or on tissue levels.
1.4. Toxicity to Terrestrial Organisms
Based on the widespread use of 2,4-D and its formulations, insects
of many kinds could be exposed to the material. Although the compounds
are generally classified as non-toxic for beneficial insects, such as
honey bees and natural enemies of pests, some adverse effects have been
reported on the early life-stages and adults of some insects.
Esters are less toxic to insects than are salts or the free acid.
Birds, and particularly the eggs of ground-nesting species, would
be exposed to 2,4-D after spraying. Food items could also be expected
to be contaminated by the herbicide. However, most studies on birds
and their eggs have been conducted at exposures far higher than could
be expected in the field.
LD50 values from acute oral and from short-term dietary dosing
indicate low toxicity of 2,4-D to birds. In longer-term studies,
effects have only been reported at extremely high exposures (for
example, kidney effects after dosing in drinking water with
concentrations in excess of the solubility of the material). There
have been no reported effects on reproductive parameters, even at
excessive exposure levels.
A single study reported adverse effects on the embryos of birds'
eggs sprayed with 2,4-D. Many studies since have shown no effect on
hatchability of eggs and no increased incidence of abnormalities in
chicks even after very high exposure to 2,4-D. Other work indicates a
very poor penetration of the eggshell by the herbicide. It can only be
concluded that after normal, or even after excessive, 2,4-D use, there
would be no effect on birds' eggs.
Based on the available data, no generalization can be made about
the hazard of 2,4-D to mammals in the field. Data on voles indicate
that the herbicide poses no hazard.
1.5. Effects of 2,4-D in the Field
No direct toxic effects, acute or long-term, of 2,4-D applications
under field conditions on any animals species have been observed thus
far.
There are, inevitably, indirect effects resulting from the intended
selective herbicidal properties of the compound. These effects would
result from the use of any herbicide or from other methods of land
management. There will, therefore, be effects for mammals, birds, and
insects because of food deprivation, modification of habitat,
requirements for nesting, shelter, etc.
The application of 2,4-D appears to be less hazardous to the
beneficial epigeal arthropod community than physical cultivation.
2. PHYSICAL AND CHEMICAL PROPERTIES
Details of the physical and chemical properties of 2,4-
dichloropheoxyacetic acid (2,4-D) are given in Environmental Health
Criteria 29: 2,4-D (WHO, 1984). The relevant chapter is summarized
here.
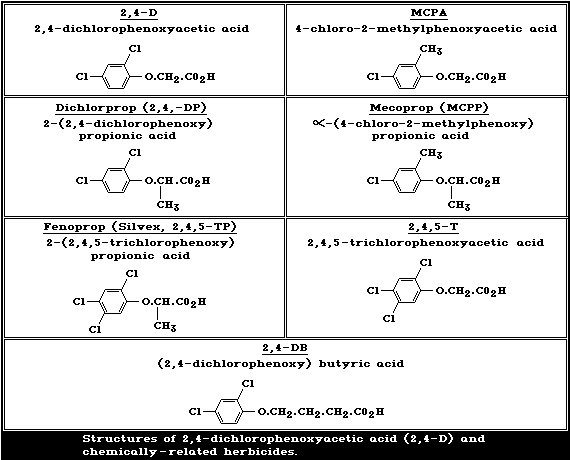
The structures of 2,4-D and of chemically-related phenoxy
herbicides in common use are given in Fig. 1. 2,4-D is a chlorinated
form of a natural plant hormone (auxin).
Some physical properties of 2,4-D and of the 2,4-D derivatives that
are used in agriculture are summarized in Tables 1 & 2.
2,4-D has growth-regulating and herbicidal properties in broad-
leaved plants. Because of its solubility, 2,4-D is rarely used in the
form of the acid; commercial 2,4-D herbicide formulations consist of
the more soluble forms such as alkali salts, amine salts, or esters.
These are combined with solvents, carriers, or surfactants and are
marketed in the form of dusts, granules, emulsions, or oil and water
solutions in a wide range of concentrations.
Table 1. Physical properties of 2,4-D
-------------------------------------------------------
Molecular formula C8H6Cl2O3
Relative molecular mass 221.0
Melting point 140 - 141 °C
Solubility in water slightly soluble
Solubility in organic solvents soluble
Vapour pressure 52.3 Pa at 160 °C
pKa at 25 °C 2.64 - 3.31
-------------------------------------------------------
2.1. Synthesis of 2,4-D
2,4-D is commonly prepared by the condensation of 2,4-dichloro-
phenol with monochloroacetic acid in a strongly alkaline medium at
moderate temperatures or by the chlorination of phenoxyacetic acid, but
this method leads to a product with a high content of 2,4-dichloro-
phenol and other impurities. Higher reaction temperatures and
alkaline conditions during the manufacture of 2,4-D increase the
formation of polychlorinated dibenzo- p -dioxin (CDD) by-products. One
formulation of 2,4-D was found to contain 6.8 µg/kg of 2,3,7,8-
tetrachlorinated dibenzo- para -dioxin (Hagenmaier, 1986). In other
amine and ester formulations, levels of this dioxin were non-
detectable, i.e., < 1 µg/kg (WHO, 1984). The alkali metal salts of
2,4-D are produced by the reaction of 2,4-D with the appropriate metal
base. Amine salts are obtained by reacting stoichiometric quantities
of amine and 2,4-D in a compatible solvent. Esters are formed by
acid-catalysed esterification with azeotropic distillation of water or
by direct synthesis in which the appropriate ester of monochloroacetic
acid is reacted with dichlorophenol to form the 2,4-D ester.
 2.2. Important Chemical Reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides. Pyrolysis of 2,4-D and its derivatives is likely
to produce certain CDD isomers. 2,4-D is readily photodegraded.
2.3. Volatility of 2,4-D Derivatives
2,4-D esters with short-chain alcohols are highly volatile. This
influences the effectiveness of their application to target crops,
their effects on neighbouring crops, and the degree of contamination of
the atmosphere. 2,4-D alkali salts or amine salts are much less
volatile than esters, and these products are to be preferred when the
use of 2,4-D esters might lead to evaporative 2,4-D losses and to crop
damage or damage to the surrounding environment.
Details of technical compositions, impurities, and analytical
methods can be found in Environmental Health Criteria 29: 2,4-
Dichlorophenoxyacetic acid (WHO, 1984).
Table 2. Vapour pressure and solubility of 2,4-D salts and esters
--------------------------------------------------------------------------------
Compound Vapour pressurea Solubility
--------------------------------------------------------------------------------
2,4-D free acid 0.4 mmHg (160 °C) 0.09% in water (25 °C),
85% in acetone (25 °C)
dimethylamine salt 300% in water (20 °C),
soluble in acetone
isopropyl ester 1.4 x 10-3 mmHgb insoluble in water, soluble
4.6 x 10-5 mmHgb in most organic solvents
butoxyethanol ester 4.5 x 10-6 mmHgb insoluble in water, soluble
(butylethyl ester) in most organic solvents
ethylhexyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
isooctyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
propyleneglycol butyl 3.0 x 10-6 mmHgb insoluble in water, soluble
ether ester in organic solvents
methyl ester 2.3 x 10-3 mmHgb
ethyl ester 1.1 x 10-3 mmHgb
butyl ester 3.97 x 10-4 mmHgb
--------------------------------------------------------------------------------
a 1 mmHg = 0.133 kPa.
b Vapour pressures of esters were determined at high temperatures by gas-
liquid chromatography, and these values are the result of extrapolation
to 25 °C. Values vary considerably between authors as a result of this
extrapolation; original values at high temperatures agree. Results are
presented here as an indication of relative vapour pressure at working
temperature. Values from Flint et al. (1968) and Jensen & Schall
(1966).
3. SOURCES OF ENVIRONMENTAL POLLUTION
The following is a summary of the chapter from Environmental Health
Criteria 29: 2,4-Dichlorophenoxyacetic acid (WHO, 1984).
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use were
not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United Kingdom.
World-wide use of herbicides and annual production, which probably
exceeds 5 x 107 kg/year, are increasing.
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaved weeds in cereal crops, as well as on
pastures and lawns, in parks, and on golf courses, at rates of about
0.2 to 2.0 kg active ingredient (acid equivalent) per hectare. Esters
are also used at rates of up to 6.0 kg (acid equivalent) per hectare to
suppress weeds, brush, and deciduous trees along rights-of-way and in
conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides in or
along irrigation and other canals, in ponds, and lakes at rates ranging
from 1 to 122 kg/ha.
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays containing 20
to 40 mg 2,4-D/litre on apple trees to reduce premature fruit-drop, on
potato plants to increase the proportion of medium-size tubers or to
intensify the tuber skin colour of the red varieties, and in citrus
culture to reduce pre-harvest fruit-drop and to increase fruit storage
life.
The highly volatile ethyl, isopropyl, and butyl esters are being
replaced by low-volatile esters or by amine salts to reduce crop damage
resulting from 2,4-D vapour drift, and to decrease atmospheric
pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public, has
been reduced in some countries because of increasing concern about
possible toxic effects, especially in relation to CDDs.
3.3. Disposal of Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localized to
the production site and to areas of waste dumping, and it is likely to
be more dispersed if disposal or leaching has occurred into water
courses. Disposal of unused 2,4-D in agriculture and washing of
equipment may result in localized land pollution and also pollution of
water supplies through direct contamination or leaching from soil.
4. UPTAKE, ACCUMULATION, ELIMINATION, AND BIODEGRADATION
Appraisal
2,4-D does not persist in soil because of its rapid degradation.
The physico-chemical properties of 2,4-D acid and its formulations
have an important effect on its behaviour in environmental
compartments.
The bioavailability to, and uptake by, aquatic and terrestrial
organisms is strongly influenced by the organic matter content of
soils, microbiological activity, and by environmental conditions such
as temperature and pH. Although highly inconsistent, the data on
dissipation and bioavailability in various soils demonstrate a marked
influence of differences in the texture and mineral composition of the
soil (Graham-Bryce, 1972). In aerobic soils, with a high content of
organic material, and at high pH values and temperatures, toxic effects
are limited because of rapid degradation of 2,4-D.
Uptake is followed by rapid excretion in most organisms. With the
exception of some algae, the retention of 2,4-D by organisms in the
environment cannot be expected, because of its rapid degradation.
Some microorganisms are capable of utilizing 2,4-D as their sole
carbon source. Repeated application to soil stimulates the number of
organisms capable of degrading the compound.
4.1. Biodegradation
2,4-D is readily and rapidly degraded in soil. Warm, moist
conditions and addition of organic matter stimulate degradation.
Autoclaving the soil and inhibiting bacterial metabolism reduce
degradation. The kinetics of 2,4-D disappearance suggest that
microorganisms are responsible. Particular species of microorganisms,
of various types, have been isolated and shown to degrade phenoxyacetic
acid herbicides in pure culture. Degradation of the phenoxyacetic
acids proceeds by two main pathways. These are via a hydroxyphenoxy
acetic acid intermediate or via the corresponding phenol. The
literature has been reviewed by the two workers principally responsible
for this evidence (Audus, 1960, 1964; Loos, 1969). Some microorganisms
are capable of using 2,4-D as their sole carbon source. More often,
2,4-D is co-metabolized with another carbon source. Regular treatment
of soil with 2,4-D stimulates the numbers of organisms which are
capable of degrading the compound. Treatment with other phenoxy
herbicides can also lead to an increase in organisms capable of
degrading 2,4-D.
Butler et al. (1975a) exposed 21 species of freshwater algae
isolated from natural lake water to 2,4-D butoxyethanol ester, at a
concentration of 0.01 mg/litre, and looked for degrading ability. Most
of the cultures fully degraded 2,4-D within 2 weeks. A single culture
retained 64% of the added 2,4-D, while seven isolates reduced 2,4-D to
less than 20% of the amount added. The remaining isolates showed 2,4-D
recoveries ranging from 22% to 53%.
Le Van To (1984) isolated six species of microorganisms
from soil previously treated with herbicides. These were
Flavobacterium peregrinum, Pseudomonas fluorescens, Arthrobacter
globiformis, Brevibacterium sp., Streptomyces viridochromogenes, and
an unidentified Streptomyces species. Flavobacterium was the most
active organism in degrading 2,4-D; degradation of 20 mg/kg of
2,4-D was complete after 20 to 30 days. In a liquid medium,
Flavobacterium degraded 93.5% of added 2,4-D within 80 h. The time
required to degrade half of the 2,4-D added to a sterilized soil along
with nutrient was estimated at 3 days. Li-Tse Ou (1984) investigated
the breakdown of 2,4-D in two types of soil under dry and moist
conditions and at two different temperatures. Numbers of
microorganisms degrading 2,4-D were also estimated. Generally, 2,4-D
disappeared more rapidly from moist soil; after 14 days of a slow rate
of disappearance, however, the removal rate from dry, sandy soil
increased. Numbers of organisms degrading 2,4-D were initially much
lower in sandy than in clay loams. However, numbers increased rapidly
in sandy soils after the addition of the herbicide and, as a result,
2,4-D was eventually degraded more rapidly in sandy than in clay loams.
In moist conditions, at 25 °C, the half-life of 2,4-D was 7 days or
less, whereas in dry conditions, at 35 °C, it could be as long as 250
days. These latter conditions are unlikely to apply in most natural
conditions where 2,4-D is likely to be used.
Rosenberg & Alexander (1980) incubated sewage-sludge bacteria with
2,4-D and found that nearly all of the herbicide had disappeared after
7 days. Subsequent additions of 2,4-D led to destruction of the
compound without a lag period; this suggests selection for organisms
capable of degrading the compound. Similar results were obtained using
bacteria from soil. The time needed for the disappearance of 90% of
the added 2,4-D was 14 days with soil inocula. 2,4-D added
subsequently was reduced by 70% within 3 to 4 days. Various tropical
soils were used in the experiment and all showed a high capacity for
degrading 2,4-D. Thompson et al. (1984) determined the persistence of
2,4-D applied at recommended rates in agricultural soils in Canada. In
all but one soil, a sandy loam, the concentration had declined by 50%
within 7 days. Sattar & Paasivirta (1980) showed slower degradation
of 2,4-D in acid soils. It took 6 weeks for 50% of the 2,4-D to
disappear from the soil and 7% was still left after 24 weeks. In
water-logged soil, there was reduced degradation of the herbicide.
Lewis et al. (1984) studied bacterial breakdown of 2,4-D
butoxyethyl ester and the effects of adding various extra components to
the medium. The addition of unfiltered, spent fungal medium from which
the majority of the fungus had settled out could be either stimulatory
or inhibitory to degradation rates of the herbicide; this depended on
the particular fungus species cultured in the medium. Further
investigation showed that effects were primarily due to differences in
pH. Reduction of the pH below 6 inhibited bacterial transformation of
the compound. Fungi commonly release large amount of organic acids.
The addition of spent fungal medium inhibited the breakdown of 2,4-D
ester. Buffering the added fungal medium reduced this inhibitory
effect; indeed, some stimulation of breakdown occurred after the
addition of buffered, spent medium. The addition of nutrients, or
other bacteria which did not transform 2,4-D, stimulated the
transformation of the herbicide. The authors consider that the most
likely explanation for this phenomenon is induction of other
transforming enzymes. With increasing substrate concentration, further
enzyme systems are induced in bacteria. The presence of other
organisms may stimulate the induction of these other enzymes at lower
substrate concentrations than would normally induce them. Increased
biomass of transforming bacteria in the presence of competing organisms
contributes to increased transformation rates. The nature of the
microbial community can, therefore, greatly change the ability of
degrading bacteria to transform 2,4-D and other xenobiotics.
O'Connor et al. (1981) found that 2,4-D applied at about 1.5 mg/kg
was readily degraded in soil. Adding extra carbon in the form of
dried, digested sewage sludge had a short-term effect in enhancing
degradation of the compound. Torstensson (1975) measured the half-life
of 2,4-D degradation in cultures of soil microorganisms at different
pH. In the pH range of 8.5 to 5.0, the half-life changed little,
ranging from 5 to 8 days. At pH 4.5, the half-life increased to 21
days and, at pH 4.0, increased further to 41 days.
Lieberman & Alexander (1981) added 2,4-D to inocula of municipal
sewage and monitored the biological oxygen depletion (BOD) as a measure
of degradation. The herbicide was added to carbon-depleted inocula
such that the 2,4-D represented the sole carbon source. Less than 5%
of the available oxygen was depleted, indicating poor biodegradation of
2,4-D because of low numbers of organisms capable of degrading the
herbicide as their sole carbon source. A separate study showed that
2,4-D was not toxic to microorganisms in sewage.
Fournier (1980) showed that, while 2,4-D treatment increased the
numbers of soil microorganisms capable of metabolizing 2,4-D as the
sole carbon source and those capable of co-metabolizing the herbicide,
this increase was dependent on the concentration of 2,4-D used. At
concentrations of 2,4-D between 5 and 50 mg/litre, there was a
significant increase in the numbers of organisms metabolizing 2,4-D,
and at 5 mg/litre there was a very pronounced increase in organisms co-
metabolizing the compound. At much higher (500 mg/litre) or much lower
(1.2 µg/litre) 2,4-D concentrations, there was no increase in the
numbers of either metabolizing or co-metabolizing organisms.
Sandmann & Loos (1984) estimated the numbers of microorganisms
capable of degrading 2,4-D in soils with and without the `rhizosphere
effect' of two plants, African clover (Trifolium africanum) and sugar
cane (Saccharum officinarum). The `rhizosphere effect' is a
phenomenon which occurs in close association with the roots of plants,
where material from the root or the metabolic activity of the root
tissue affects the surrounding soil. Particularly high, stimulated
populations were associated with sugar cane. A similar effect, but to
a lesser degree, was found with clover. In the three sugar cane soils
examined, and their corresponding controls, the numbers of organisms
were 46 400, 156 000, and 40 700 per g of soil, with rhizospheres, and
178, 1480, and 6170 per g of soil, without rhizospheres, respectively.
Seibert et al. (1982) failed to demonstrate a rhizosphere effect on
2,4-D degradation in glasshouse studies using soils with and without
maize roots.
Norris & Greiner (1967) investigated the degradation of 2,4-D in
forest leaf litter. Litter from either alder, ceanothus, vine maple,
bigleaf maple or Douglas fir showed comparable ability to degrade
2,4-D, the recovery of 2,4-D being between 60% and 70% after 15 days of
incubation. In a second series of experiments, different formulations
of 2,4-D were added to alder litter. About 50% of the free acid of
2,4-D was degraded within 15 days. Triethanolamine salt and two
commercial formulations (`solubilized acid' and isooctyl ester) were
degraded less than the pure acid. There was between 30% and 40%
degradation of these preparations over 15 days.
Nesbitt & Watson (1980) related the degradation rate of 2,4-D in
river water to the nutrient levels, sediment load, and dissolved
organic carbon content of the water. The addition of sediment or
inorganic nutrients increased the rate of 2,4-D degradation, whereas
the addition of organisms capable of degrading 2,4-D did not increase
the rate of breakdown of the herbicide. This finding indicated that
the limiting factor in breakdown of 2,4-D in river water was not
numbers of organisms but the nutrient status of the river. The authors
noted that in winter, when the river was in peak flow and the water
temperature below that for optimum microbial activity, appreciable
amounts of the herbicide would be washed into the estuary. An earlier
pilot study of seasonal changes in the capacity of river water in
Western Australia to degrade 2,4-D (Watson, 1977) indicated clear
seasonal differences in both river water concentrations of the
herbicide and the degrading capacity of river water. Several rivers
were studied and differences were related to the amount of
agricultural run-off, the sediment content of the water, river flow,
and temperature. Rivers receiving agricultural run-off degraded 2,4-D
better than those receiving run-off principally from forests. This was
presumed to be the result of the preconditioning of organisms to the
herbicide; the investigation corrected for nutrient content of the
water which had been previously shown to affect degradation.
Spain & Van Veld (1983) looked at the degrading ability of
microbial communities taken from sediment cores from freshwater,
estuarine, and marine sites. Some cores were pre-exposed to 2,4-D.
Cores from freshwater sites showed increased degradation of 2,4-D after
pre-exposure to the compound, whereas estuarine and marine cores did
not show this effect. The adaptation of freshwater cores was maximal
after 2 weeks and no longer detectable 6 weeks after pre-exposure.
4.2. Uptake and Accumulation by Organisms
Appraisal
Many studies on the accumulation of 2,4-D have used radioactively
labelled herbicide and have monitored uptake by simple counting of the
label. This fails to take into account that the label could have been
removed from the parent molecule by metabolic breakdown. Values for
uptake should, therefore, be treated as a maximum possible uptake
value for 2,4-D. Such data would not normally be considered
acceptable. However, the accumulation of 2,4-D is so low that these
data serve to illustrate that little of the herbicide is accumulated.
4.2.1. Laboratory studies
Eliasson (1973) sprayed leaves of 3-year-old aspen (Populus
tremens) with the butoxyethanol ester of 2,4-D at 0.5 kg acid
equivalent/litre. The plants were then kept in an open-sided
glasshouse and residues of 2,4-D were monitored. Most of the
herbicide remained in, or on, the sprayed leaves. The average residue
level was 2300 mg/kg fresh weight 1 day after spraying. This level had
fallen to 1300 mg/kg after 37 days and, by day 365, the average residue
level was 870 mg/kg. This was a very high application rate and
indicates that there is no foliar uptake of 2,4-D by plants.
Glynn et al. (1984) exposed coral Pocillopora damicornis to three
concentrations of 2,4-D sodium or amine salts at 0.1, 1.0, or
10.0 mg/litre. The maximum concentration of 2,4-D found in coral
tissue was 0.137 mg/kg after exposure to the amine salt at 10 mg/litre,
but residues were not related to the 2,4-D exposure concentration. The
highest bioconcentration factor (BCF) of 1.33 was found after exposure
to 0.1 mg/litre of the amine salt of 2,4-D, i.e., the coral contained
1.33 times the concentration of 2,4-D in water.
Metcalf & Sanborn (1975) introduced 14C-labelled 2,4-D into
model ecosystems consisting of an alga Oedogonium, an aquatic plant
Elodea, a snail Physa, and the mosquito fish Gambusia. Total
14C in the water was equivalent to 0.205 mg 2,4-D/litre. The highest
BCF was in the alga (26.8, based on measurement of radioactivity).
Analysis of all components of the ecosystem for 2,4-D, rather than the
radiolabel, revealed none of the parent compound. The BCF, therefore,
refers to breakdown products rather than 2,4-D itself. Gile (1983)
introduced 14C-labelled 2,4-D, as the butyl ester, into a simulated
ryegrass ecosystem. The system consisted of a sandy loam soil, annual
ryegrass, several invertebrates, and grey-tailed voles. Voles were
introduced 10 days after spraying 2,4-D as a foliar spray at the
equivalent of 1 kg/ha. The experiment was terminated after 1 month.
Plant material contained an average of 8.9 mg/kg; this was identified
as being mostly 2,5-dichloro-4-hydroxyphenoxyacetic acid. Residue
levels in animals (based on unidentified 14C residues) ranged from
0.31 mg/kg in snails to 5.28 mg/kg in pillbugs (isopods).
Freitag et al.(1982) measured the bioaccumulation of 14C-2,4-D in
an alga Chlorella fusca and a fish, the golden orfe. They measured a
24-h static BCF of 6 for the alga, and a 3-day static BCF of <10 for
the fish. This measurement was based on radioactivity and, therefore,
did not distinguish between the parent compound and its breakdown
products.
Schultz (1973) examined uptake and loss of 14C-2,4-D dimethyl-
amine salt by organs of three species of fish (channel catfish,
bluegill sunfish, and largemouth bass), exposed to 0.5, 1.0, or
2.0 mg/litre of 2,4-D acid equivalent. After exposure to the highest
concentration of 2,4-D dimethylamine salt, there was detectable
radioactivity in all organs examined. Bile showed the highest residues
of 14C in all three species after 1 week. For the remainder of the
exposure period of 12 weeks, there was an increase of radioactivity in
other organs and a decrease in the bile. At the end of the exposure
period, there was no clear pattern to residue levels of 14C in
different organs. These levels ranged from 5.04 mg/kg in bile to
35.5 mg/kg in posterior kidney for the channel catfish. For largemouth
bass, the range was from 1.32 mg/kg in muscle to 7.29 mg/kg in liver.
For the sunfish, the lowest residue was 24.75 mg/kg in bile and the
highest 322.7 mg/kg in the pyloric caeca of the gut. After 84 days
exposure to the dimethylamine salt at 2 mg/litre, levels of 14C in
the muscle of catfish, bass, and sunfish were equivalent to 0.953,
0.035, and 1.065 mg 2,4-D/kg, respectively. No analysis for 2,4-D
itself was carried out. A second study exposed the three fish species
for 2 weeks to 14C-2,4-D dimethylamine salt at 1 mg/litre and then
for a further 4 weeks to clean water. The disappearance of 14C was
measured. Loss of 14C was slow at first but by 4 weeks most tissues
had shown a decline in residues. Samples were analysed for 2,4-D but
none was detectable, suggesting that the 14C measured was in
breakdown products. The values for 2,4-D residues in this and other
studies using 14C-labelled material should, therefore, be regarded as
overestimates of retained 2,4-D. Uptake of 14C-2,4-D was examined at
two different temperatures, 17 °C and 25 °C. The highest residues of
14C detected in fish were equivalent to 0.122 mg 2,4-D/kg, but no
2,4-D could be found after analysis, except in bluegill sunfish after
14 days. Loss of 2,4-D did not, therefore, seem to change with
differing temperature over this range. A similar study, at two
different water pH values, showed significantly more 14C uptake in
all three species at the more acidic pH. Analysis of fish tissues for
2,4-D by gas-liquid chromatography showed non-detectable, or trace,
levels in most samples. Only in bluegill sunfish after 7 and 14 days
were residues measurable. These 2,4-D residues showed the opposite
trend to the 14C results; there was more 2,4-D in fish exposed at the
more alkaline pH. The authors suggest that metabolism of the herbicide
in the fish is suppressed at alkaline pH.
Sigmon (1979) exposed bluegill sunfish to 2,4-D butyl ethyl ester
(3 mg/litre) at three different temperatures, 20, 25, and 30 °C, and
measured the tissue content of 2,4-D after 8 days. None of the groups
differed from the controls, residues being <0.05 mg/kg.
Bluegill sunfish and channel catfish took up <0.5% of the
available 14C when exposed to 14C-2,4-D dimethylamine salt at 2
mg/litre (with 1 litre of water per fish) for 7 days (Sikka et al.,
1977). A maximum total 14C concentration in the fish was
reached after 24 h and did not change significantly over 14 days.
Bluegill sunfish attained a total body concentration of 0.9 mg/kg and
catfish 0.2 mg/kg at 24 h. These values were 2,4-D equivalents of
14C measured; the compound was not analyzed directly. When bluegill
sunfish were injected intraperitoneally with 14C-2,4-D dimethylamine
salt, at dose levels of 1 or 2.5 mg/kg body weight, they
excreted 90% of the dose within 6 h of treatment. In a similar
experiment, Stalling & Huckins (1978) exposed bluegill sunfish to
14C-2,4-D dimethylamine salt at 2 mg/litre and measured both
14C and 2,4-D in fish and water samples over the following 12 weeks.
Radioactivity was detected in tissues and increased over the
experimental period, but there was no measurable 2,4-D; the detection
limit of the method was 0.1 mg/kg. An in vivo intraperitoneal
injection of 110 µg of 14C-2,4-D was followed by rapid elimination.
Rodgers & Stalling (1972) measured uptake of 14C-2,4-D butoxy-
ethanol ester by three species of fish, which were exposed to either
0.3 or 1.0 mg/litre and sampled over the next 168 h. Some fish were
fed and some fasted. Radioactivity in a variety of tissues was
determined; the maximum levels were found within 3 h of exposure in fed
fish. After this, levels declined over the remaining sampling period,
and by the end of the experiment, residues were negligible. The one
exception was the gall bladder, which consistently contained more 2,4-D
than other tissues. Results were different for fasted fish. In almost
all organs of fasted fish, uptake of 2,4-D was slower than for fed
fish, although the levels reached were eventually two to five times
higher than in fed fish. Analysis of the residues showed that only the
liver ever contained the herbicide in the ester form. In all other
tissues, only the acid was present.
Shcherbakov & Poluboyarinova (1973) monitored the accumulation of
2,4-D in carp and Daphnia. The 2,4-D was added as the butyl ester
at concentrations ranging from 0.006 to 5 mg/litre; the recommended
usage rate for this ester leads to water concentrations of about
0.5 mg/litre. Analyses of fish tissues were made for both the ester
and the acid. The highest BCF for the ester, at 395, was found with
fish after a 7-day exposure to 0.5 mg/litre. Acid accumulation was
lower than that of the ester. The experiment lasted for 70 days. At
day 10 and after, only trace amounts of ester were found in fish.
Small amounts of 2,4-D acid were found at day 10, but only trace
amounts after day 70. Residues of 2,4-D ester in Daphnia varied from
23.9 to 518 mg/kg, according to the exposure concentration.
Two experiments have been carried out on the grey slug Derocerus
reticulatum by Haque & Ebing (1983) using 14C-labelled 2,4-D acid.
The first study, a contact experiment, exposed the slugs to 2,4-D in
contaminated soil at 1.1 mg/kg. The body content of 2,4-D in slugs
reached equilibrium (0.014 mg/kg) after 15 days; this represented a
BCF of 0.013 based on radioactivity. In the second experiment,
slugs were exposed via the food using carrot discs containing 1.1 mg/kg
slug body weight per day over 5 days. Residues of 14C in the slugs
increased during the feeding period, peaking at 5.5 mg/kg. During the
following 7 days, residues were monitored to investigate loss of
radioactive material. At the end of the experiment, on day 12,
residues were comparable to those at the end of the feeding period.
During the course of feeding 2,4-D-contaminated carrots, more than 80%
of the ingested dose of radioactivity was excreted rapidly; only 20%
was retained. There was no attempt to characterize the 14C residues;
these may, therefore, represent either 2,4-D or its breakdown
products.
Chickens given a single oral dose of 100, 200, or 300 mg/kg
body weight reached maximum plasma levels of 2,4-D of 90, 130, and
250 µg/ml, respectively. Plasma levels in all groups had fallen to
15 µg/ml or less after 24 h. Continuous dosing of chickens at
300 mg/kg per day led to a faster rate of elimination of the daily dose
of 2,4-D with time (Bjorklund & Erne, 1966).
4.2.2. Field studies
Cope et al. (1970) treated experimental ponds with 2,4-D propylene
glycol butyl ether ester to give water concentrations up to and
including 10 mg/litre. No detectable 2,4-D was found in fish exposed
to 1 mg/litre or less of the herbicide, but residues were found in
bluegill sunfish exposed to 5 or 10 mg/litre. The highest residue
(2 mg/kg) was found 1 day after application. Residues were still
detectable after 3 days but not subsequently. Vegetation
(Potamogeton nodosus) and bottom sediment contained residues of
50.0 and 3.0 mg/kg, respectively, 2 days after treatment with the 2,4-D
ester at 10 mg/litre. The herbicide was still detectable at 0.1 mg/kg
in sediment after 44 days but not thereafter. At 44 days after
treatment, there were residues in the plant of 1.2 mg/kg; this amount
declined to 0.1 mg/kg after 94 days.
Following the field application of 2,4-D butoxyethanol ester at
22.5 kg/ha, Whitney et al. (1973) measured residues of the herbicide in
fish, crustacea, and insect larvae over a 3-week period. The herbicide
had been applied to control eurasian water milfoil. Some 2,4-D was
taken up by these various species; the highest residue concentration
was 0.24 mg/kg in largemouth bass after 8 days. All residues in
organisms were below 0.1 mg/kg after 3 weeks. No 2,4-D could be
detected in water in 33 samples taken after treatment, the detection
limit being 0.10 mg/litre. The highest reported concentration of 2,4-D
in mud was 0.65 mg/kg, 10 days after treatment, but in most samples the
herbicide level in mud was much lower and in several it was
undetectable.
Hoeppel & Westerdahl (1983) treated four areas (10 ha each) of
dense water milfoil beds in Lake Seminole, Georgia, with either 2,4-D
dimethylamine salt or 2,4-D butoxyethanol ester, at each of two
application rates (22.5 or 45 kg/ha). Both formulations were converted
to 2,4-D free acid within 24 h. Maximum water concentrations achieved
in the high rate (45 kg/ha) areas were 3.6 and 0.68 mg/litre for the
dimethylamine salt and butoxyethanol ester, respectively. There was no
detectable uptake of 2,4-D into fish in those areas treated with the
dimethylamine salt. In the ester-treated areas, 4 out of 24 game fish
sampled contained low levels of 2,4-D in muscle (the highest residue
being 0.29 mg/kg) and 18 out of 20 gizzard shad contained detectable
2,4-D in muscle (the highest residue being 6.9 mg/kg). No fish sampled
more than 13 days after treatment contained detectable 2,4-D.
Schultz & Harman (1974) treated nine experimental ponds with 2,4-D
dimethylamine salt at three concentrations: 2.24, 4.48, and 8.96 kg/ha.
Samples of water, bottom sediment, and fish were taken over 147 days.
Maximum water and sediment concentrations of 2,4-D were 0.692 mg/litre
and 0.17 mg/kg, respectively. Of 307 fish sampled, 45 contained
detectable residues of 2,4-D. The highest residue measured was in a
channel catfish at 1.075 mg/kg 1 day after treatment. All residues in
fish after 28 days were less than 0.005 mg/kg; most were undetectable.
Smith & Isom (1967) measured uptake and retention of 2,4-D after
treatment of two field sites for control of watermilfoil with the
butoxyethanol ester. The first site was treated with a granular
formulation at a rate of 112 kg/ha. One bluegill sunfish (Lepomis
macrochirus) contained 0.15 mg 2,4-D/kg on day 50 after treatment. All
other fish, sampled between 72 h and 50 days after treatment,
contained less than 0.14 mg/kg, which was the limit of detection. Two
samples of several species of mussel, held in cages for 96 h following
spraying, showed residues of 0.38 and 0.7 mg/kg. Water levels of 2,4-D
reached a peak of 37 mg/litre within 1 h of application and had fallen
to less than 1 µg/litre within 8 h. Mud samples contained very
variable levels of 2,4-D residues, ranging between 0.14 and 58.8 mg/kg.
The highest residue was found 10 months after application. The second
site was treated at the lower rate of 45 kg/ha. All fish sampled
between 15 days and 9 months after 2,4-D application showed residues
of less than 0.14 mg/kg. Mussels sampled between 1 and 42 days after
application contained residues ranging between <0.14 and 1.12 mg/kg.
Water levels peaked at 157 µg/litre, 1 h after spraying, and mud
residues ranged from <0.14 to 33.6 mg/kg.
Coakley et al. (1964) measured residues in organisms at the center
of a 0.4-ha field plot sprayed with 2,4-D butoxyethanol ester at a rate
of 33.7 kg/ha for watermilfoil control. Two days after application,
oysters (Crassostrea virginica), clams (Mya arenaria), fish (Lepomis
gibbus), and blue crabs (Callinectes sapidus) contained 3.5, 3.7, 0.3,
and <0.8 mg/kg, respectively.
In 1971, over 2800 ha in Loxahatchee National Wildlife Refuge
were sprayed with the dodecyl-tetradecyl amine salts of 2,4-D at a
rate of 4.48 kg/ha. The initial application of 2,4-D was followed by
spot treatments of the same formulation and/or the dimethylamine salt
of 2,4-D. The highest water concentration (0.037 mg/litre of 2,4-D)
was measured 1 day after the initial application. Of 60 fish sampled
in the area, 19 had measurable residues of 2,4-D but only three of
these were greater than 0.1 mg/kg; the highest recorded residue was
0.162 mg/kg. Breast muscle and liver of a bird, the common Florida
gallinule Gallinula chloropus, had residues of 0.3 and 0.675 mg/kg,
respectively, 1 day after spraying. No residues were found in the bird
4 days after spraying (Schultz & Whitney, 1974).
Plumb et al. (1977) treated sprouting chamise (Adenostoma
fasciculatum) with the polyethylene glycol butyl ether ester of 2,4-D
at a rate of 3.4 kg acid equivalent/ha. A maximum concentration of
herbicide (95.2 mg/kg) was found in the plant within 15 min of
application. A residue of 3.8 mg 2,4-D/kg remained in, or on, the
plants (shoots which had been originally sprayed) 1 year after
treatment. When Radosevich & Winterlin (1977) applied the butoxypropyl
ester of 2,4-D to a chaparral area at a rate of 4.5 kg/ha, the
residues measured in chamise were 221 mg/kg and in grass and forbs
269 mg/kg within 2 h of application. After 30 days, these levels had
dropped to 60 mg/kg for chamise and 21 mg/kg for grass and forbs, and,
after 360 days, 0.1 mg/kg was present in chamise. Siltanen et al.
(1981) monitored residues of 2,4-D in the fruit of bilberries 1 year
after the application of 0.25, 0.75, or 2.25 kg/ha acid equivalent. No
residues were detected, the limit of detection being 0.05 mg/kg.
Raatikainen et al. (1979), in a controlled field experiment,
sprayed cowberry and bilberry with an ester formulation of 2,4-D.
Three application rates were used, 0.25, 0.75, and 2.25 kg acid
equivalent/ha, and residues of 2,4-D were measured approximately
1 month after application. Thirty-four days after the application of
0.25 kg/ha, residues in cowberry were 0.3 mg/kg. Cowberries exposed to
0.75 or 2.25 kg/ha were analysed after 35 days and contained residues
of 1.0 and 3.7 mg/kg, respectively. Bilberries treated with 0.25,
0.75, or 2.25 kg/ha were analysed 29 days later; residues were 0.1,
1.3, and 4.8 mg/kg, respectively.
4.3 Elimination
James (1979) studied tissue distribution of 14C-labelled 2,4-D in
the spiny lobster (Panulirus argus). Labelled herbicide was injected
into the pericardial sinus and animals were sacrificed at regular
intervals. 2,4-D was taken up from the haemolymph, by the green gland,
and excreted unchanged, with an overall half-time of about 8 h. Tuey &
James (1980), in a similar study, found that the clearance of 2,4-D
from haemolymph, via the green gland, was three to five times greater
than the rate of metabolism in the hepatopancreas.
Pritchard & James (1979) studied the renal handling of intra-
venously injected 2,4-D by the winter flounder (Pseudopleuronectes
americanus). 2,4-D, at a concentration of 1 µmol/litre of plasma,
was actively secreted into the glomerular filtrate of the kidney
with clearances of nearly 500 times the glomerular filtration rate.
At higher plasma concentrations of between 10 and 60 µmol/litre, a
transport maximum of 0.85 µmol/g of kidney per h was observed.
Koschier & Pritchard (1980) reported a similar study using
an elasmobranch fish Squalus acanthias. They administered
2.5 µmol 14C-2,4-D/kg to the fish intramuscularly and monitored
blood and urine 14C levels. Clearance of total 2,4-D was more than
25 times greater than the glomerular filtration rate, indicating that
2,4-D was being actively secreted by the kidney. 2,4-D was eliminated
in the urine as a taurine conjugate, this representing about 95% of the
excretory products. The plasma contained, primarily, unconjugated
2,4-D (>90%). It seemed, therefore, that 2,4-D was conjugated with
taurine before being excreted in the urine. Guarino et al. (1977), in
a similar study on the dogfish Squalus, also found that 2,4-D was
extensively conjugated to taurine (>90%) and was eliminated
predominantly via the urine; 70% of the administered dose appeared in
the urine within 4 to 6 days. The highest tissue concentration of
2,4-D (14.5 mg/kg) was found in the kidney after 4 h. Plasma
elimination was rapid, with a half-time of 44 min; similarly rapid
clearance was seen from the kidney. Half-time estimates for muscle and
liver were 2 to 3 days and 5 days, respectively.
5. TOXICITY TO MICROORGANISMS
Appraisal
In general 2,4-D is relatively non-toxic to water and soil
microorganisms at recommended field application rates.
No effect of 2,4-D was recorded on 17 genera of freshwater and two
genera of marine algae at concentrations up to 222 mg/litre.
No effect of 2,4-D was observed on respiration of either sandy loam
or clay loam soils at concentrations up to 200 mg/kg.
N-fixation by aquatic algae is affected at high concentrations of
2,4-D acid (400 mg/litre). An effect of 2,4-D esters on N-fixation
occurs from a concentration of 36 mg/litre upwards. N-fixing algae in
topsoils appear to be more vulnerable to 2,4-D acid than other algal
species. The Cyanobacteria (blue-green algae) are important as the
major N2 source in tropical ponds and soils.
In the range of 25.2 to 50.4 mg/litre, 2,4-D was inhibitory to all
types of soil fungi.
Cell division was reduced in a green alga by 2,4-D at 20 mg/litre
and stopped at 50 mg/litre. No effect was observed on a natural
phytoplankton community after exposure to 2,4-D at 1 mg/litre.
However, exposure to esters of 2,4-D reduced productivity in these
organisms.
5.1. Aquatic Microorganisms
Hawxby et al. (1977) exposed cultures of three algae
(Chlorella pyrenoidosa, Chlorococcum sp., and Lyngbya sp.,) and
one cyanobacterium (blue-green alga) (Anabaena variabilis) to
concentrations of 2,4-D in the medium of up to 10 µmol /litre
(= 2.21 mg/litre). There was no effect on growth, respiration, or
photosynthetic rate.
Gangawane et al. (1980) studied the effects of 2,4-D on growth and
heterocyst formation in the nitrogen-fixing cyanobacterium (blue-green
alga) Nostoc. The organism was cultured for 30 days in 0, 10, 100,
1000, or 1500 mg 2,4-D/litre. Growth was measured by optical density
and cells forming heterocysts were counted. Growth was inhibited at
both 10 and 100 mg 2,4-D/litre and was eliminated at higher
concentrations. There was also reduced heterocyst formation.
Lembi & Coleridge (1975) demonstrated a marked effect of 2,4-D,
at concentrations of 110 or 220 mg/litre, on cultures of the
green algae Scenedesmus, Ankistrodesmus, and Pediastrum. After 14
days of culture, the three species under control conditions produced
456 x 102, 634 x 104, and 227 cells or colonies per ml of medium,
respectively. Corresponding figures after exposure to 110 mg/litre
were 54 x 102, 41 x 104, and 74 cells or colonies per ml,
respectively. For both Scenedesmus and Ankistrodesmus, these values
were less than the pre-treatment cell concentrations.
Butler et al. (1975b) exposed unialgal cultures of green algae
isolated from Warrior River water to 2,4-D butoxyethanol ester at
0.001, 0.01, 0.1, 1.0, or 4.0 mg/litre. Thirty separate isolates were
used. Concentrations less than or equal to 1 mg/litre of the 2,4-D
ester did not change the growth pattern of the isolates. However, with
a concentration of 4 mg/litre, there was some inhibition of growth, as
indicated by a 10% increase in the number of incubates which showed
poor growth, or no growth, when compared to controls. Some isolates
were unaffected even at this concentration and it can therefore be
assumed that 2,4-D butoxyethanol ester might change the species
composition of green algae populations.
Bednarz (1981) used 12 pure cultures of green algae and
cyanobacteria (blue-green algae) separately and in combination to
investigate the effects of 2,4-D acid. Cultures were exposed to
concentrations of 2,4-D ranging from 0.001 to 10 mg/litre. Low
concentrations of 2,4-D stimulated the growth of most species of algae,
whereas high concentrations inhibited growth. Chlorococcal green algae
were more sensitive to 2,4-D than were filamentous green algae or
cyanobacteria. In further experiments, the authors cultured
combinations of sensitive and tolerant species in the same range of
2,4-D concentrations. Tolerant species used in combinations were
Chlorella pyrenoidosa, Dictyosphaerium pulchellum, and Scenedesmus
quadricaudata. The first two of these tolerant species reduced the
toxicity of 2,4-D to sensitive species in mixed culture. This
protective effect was not seen with Scenedesmus.
Singh (1974) cultured a filamentous, nitrogen-fixing,
cyanobacterium Cylindrospermum sp. in concentrations of 2,4-D acid of
0, 100, 300, 400, 500, 600, 800, 1000, or 1200 mg/litre and examined
growth and heterocyst formation after 8 days. Both parameters were
affected at concentrations higher than 300 mg/litre and cultures were
killed at a concentration of 1000 mg/litre. Kapoor & Sharma (1980)
exposed cultures of the nitrogen-fixing, filamentous cyanobacterium
Anabaena doliolum to 2,4-D ethyl ester (as `Weedone 48' concentrate)
at concentrations of 36, 108, 180, 252, or 324 mg/litre. There was a
dose-related decrease in cell nitrogen over the whole range of 2,4-D
ester exposures. Cell growth was stimulated by lower concentrations of
2,4-D and only inhibited by the highest dose. Tiwari et al. (1984)
exposed cultures of a similar nitrogen-fixing, filamentous
cyanobacterium (Anabaena cylindrica) to 2,4-D acid at concentrations of
0, 100, 500, 700, 1000, or 1500 mg/litre, and examined growth,
heterocyst formation, and nitrogen fixation. For all these parameters,
there was a stimulatory effect of 2,4-D at 100 mg/litre and a
progressive inhibition with higher concentrations. These and similar
algae are considered to be a major source of nitrogen in tropical
ponds and soils. Das & Singh (1977) cultured the nitrogen-fixing
cyanobacterium Anaebaenopsis raciborskii in concentrations of 2,4-D
acid (sodium salt) of 10, 100, 400, 600, 800, and 1000 mg/litre and
measured nitrogen fixation. Control cultures and those exposed at 10
and 100 mg 2,4-D/litre showed no significant differences.
Nitrogen-fixation was inhibited at 400 mg/litre or more and eliminated
at 600 mg/litre.
Butler (1963) reported no effect on a natural phytoplankton
community of exposure to a 1 mg/litre concentration of 2,4-D (as the
acid or dimethylamine salt), or of the dimethylamine salt on pure
cultures of Dunaliella euchlora or Platymonas over 4 h. In a later
study (Butler, 1965), natural phytoplankton communities were exposed to
esters of 2,4-D. Butoxyethanol ester, propylene glycol butyl ether
ester, and ethylhexyl ester reduced productivity (as measured by
carbon fixation) by 16%, 44%, and 49%, respectively, at a concentration
of 1 mg/litre.
Sarma & Tripathi (1980) monitored cell division in the filamentous
green alga Oedogonium acmandrium exposed to 2,4-D acid at 1, 5, 10,
20, or 50 mg/litre of culture medium. At up to 10 mg/litre, 2,4-D
was found to stimulate cell division; a 168 h exposure to 5 mg/litre
increased the incidence of dividing cells by 15% over controls.
However, cell division was reduced at 20 mg/litre and stopped at
50 mg/litre. Abnormalities in chromosomes during cell division
increased with increasing 2,4-D exposure.
Chai & Chung (1975) examined the effects on growth,
photosynthesis, respiration, and chemical composition of exposing
cultures of the green alga Chlorella ellipsoidea to 2,4-D acid at 22 or
88 mg/litre. At 22 mg/litre, 2,4-D increased growth, photosynthesis,
and the cell content of protein and nucleic acids. Carbohydrate
content was unchanged. However, at 88 mg/litre, growth was inhibited,
photosynthesis was no different from controls, and the cell content of
carbohydrate, protein, and nucleic acids was decreased.
Elder et al. (1970) examined the effect of 2,4-D acid on 17 genera
of freshwater and two genera of marine algae exposed at 22, 111, or
222 mg/litre. There was no effect on the growth of any of the
cultures, even at the highest dose of 2,4-D.
Cultures of the flagellate Euglena gracilis were exposed for 24 h
to concentrations of 1, 5, 10, 50, or 100 mg/litre or for 7 days to 10,
50, or 100 mg/litre of 2,4-D acid by Poorman (1973). Cultures in 50
and 100 mg 2,4-D/litre yielded 84% and 74%, respectively, relative to
controls, over 24 h. Lower concentrations of 2,4-D had a slightly
stimulatory effect. After 7 days, there was significant stimulation of
yield with 10 mg/litre; the culture yielded 161% compared to a control.
There was slight stimulation of growth by 50 mg/litre and a reduction
to 78% of control levels with 100 mg/litre.
George et al. (1982) exposed the rotifer Brachionus
calyciflorus to 2,4-D at 5 mg/litre. Median lethal time (LT50) was
24 h and LT100 was 31 h.
5.2. Soil Microorganisms
Pachpande & David (1980) isolated the soil alga Chlorococcum
infusionum from paddy fields and cultured the organism in the presence
of 2,4-D acid at concentrations of 0, 1, 2, 3, 4, and 5 mg/litre.
Growth was estimated as dry weight of algal cells filtered out of the
medium. All concentrations of 2,4-D were inhibitory to growth.
At the highest 2,4-D concentration of 5 mg/litre, the culture yield
was reduced from a control level of 720 mg dry wt/litre of medium to
520 mg/litre.
Cullimore & McCann (1977) applied 2,4-D acid to isolated cores
taken from a prairie, loam soil to give approximate concentrations of
1 or 100 mg/kg in the top 2 cm of soil. Soil algal populations were
estimated from subsamples of cores taken before treatment and 1, 5, or
20 days after treatment with herbicide. Thirty-one genera of algae
were identified, of which five were very sensitive to 2,4-D and were
rarely found after treatment. These were Chlamydomonas, Chlorococcum,
Hormidium, Palmella, and Ulothrix. The most resistant genera were
Chlorella, Lyngbya, Nostoc, and Hantzschia; the `percent sensitivity'
of these genera (% of the total number of treatments in which the
genus was absent) was 28%, 6%, 22%, and 44%, respectively. The
reduction in cell numbers of algae in the top layer of the soil after
herbicide treatment was soon offset by an increase in the population of
Chlorella, Stichococcus, Oscillatoria, and Spongiochloris, all of
which recovered very rapidly from the herbicide effects. There was,
however, an overall reduction in cell numbers of nitrogen-fixing algae.
Mukhopadhyay (1980) measured the bacterial, fungal, and
actinomycete populations of soils supporting rice or maize plants which
had been treated with various herbicides for weed control. There was
no effect of 2,4-D, applied at the recommended rate, either on soil
microorganism numbers or on the evolution of carbon dioxide by soil
cultures.
Huber et al. (1980) examined the effect of 2,4-D at 0.3, 0.2, or
0.1 mmol/litre (= 66, 44, and 22 mg/litre, respectively) on seven
cultures of soil microorganisms. There was no effect on the growth of
five of the cultures; these were Nocardia sp., Pseudomonas fluorescens
in both aerobic and anaerobic culture, Bacillus subtilis, and
Ustilago maydis. There was a small reduction in growth at the
highest 2,4-D dose in cultures of Rhizopus japonicus and Aspergillus
niger. 2,4-D had no effect on mycelium growth of three out of four
plant pathogenic fungi in culture; Phytophthora cryptogea showed
reduced mycelial growth at 0.1, 0.2, and 0.3 mmol 2,4-D/litre,
but Fusarium oxysporum, Alternia radicina, and Rhizoctonia solani
were unaffected.
Moubasher et al. (1981) added 2,4-D at three doses (1.9, 7.6, and
15.2 mg/kg) either to soil or to agar medium inoculated with soil
fungi, and the effects on fungal populations were monitored. In soil,
at all three doses, 2,4-D stimulated the fungi. When incorporated in
the agar medium, 2,4-D was stimulatory to overall fungal growth and to
four individual species of fungus at the lowest dose of 6.3 mg/litre,
but inhibitory to two other species. At higher doses of 25.2 or
50.4 mg/litre, the herbicide was inhibitory to all fungi.
2,4-D had a significant inhibitory effect on culture yields of the
bacterium Escherichia coli only at 10-3mol/litre (= 220 mg/litre).
There was no effect at 10-4mol/litre (= 22 mg/litre) (Toure & Stenz,
1977).
Prescot & Olson (1972) added 2,4-D, at doses of 0, 0.1, 1.0, 10, or
100 mg/litre, to cultures of the soil amoeba Acanthamoeba castellanii
and monitored growth and reproduction. There was a stimulatory effect
of 2,4-D at all dose levels; this effect was most marked at the lowest
dose and declined with increasing exposure to 2,4-D. The authors
suggest that the amoeba may degrade the 2,4-D and utilize it as a
carbon source. However, Pons & Pussard (1980) found no effect of 2,4-D
(at 28, 54, or 84 mg/litre) on the reproduction of 23 different strains
of free-living soil amoebae.
2,4-D, at 10-3mol/litre in cultures of the ascomycete Neurospora
crassa, stimulated DNA synthesis but had no effect at lower
concentrations of 10-4 to 10-6mol/litre. These concentrations had
no significant effect on either RNA or protein (Schroder et al.,
1970).
Naguib et al. (1980) measured growth, respiration, and absorption
and utilization of sugar and nitrogen in pre-formed fungal mats of
Aspergillus terreus over 72 h in the presence of 200 mg/litre of
2,4-D. The herbicide inhibited sugar inversion and consequently sugar
absorption. It also reduced the incorporation of nitrogen into protein.
Respiration was depressed. Growth of the fungus was suppressed and, on
a dry weight basis, culture mass was reduced to below the initial
level.
Trevors & Starodub (1983) added 2,4-D to sandy loam and clay
loam soils and measured both respiration and electron transport
system (ETS) activity. ETS was assessed by measuring the capacity
of the soil to reduce 2-( p -iodophenyl)-3-( p -nitrophenyl)-5-phenyl
tetrazolium chloride (INT) to iodonitrotetrazolium formazan (INT
formazan). The effects of 2,4-D were tested at concentrations of the
herbicide in soil of 0, 10, 25, 50, 75, 100, or 200 mg/kg. There was
no effect on soil respiration, monitored either as oxygen consumption
or carbon dioxide evolution, at any of the concentrations of 2,4-D in
either soil. There was similarly no effect on ETS in the sandy loam.
However, in the clay loam, there was a progressive inhibition of ETS
over the whole range of concentrations of the herbicide. The control
soil had an ETS activity of 37.3 µg INT formazan production/g soil,
whereas the ETS activity of soil treated with 10 mg 2,4-D/kg was
25.1 µg INT formazan/g, significantly lower than that of the
control. The activity was reduced further with increasing
concentrations of 2,4-D, until an activity of 16.3 µg INT formazan/g
was found at 200 mg 2,4-D/kg.
Deshmukh & Shrikhande (1975) added 2,4-D, at recommended field
rates, and at five times the recommended field rates, to two
types of soil from India. Both doses of 2,4-D inhibited numbers
of Azobacter in both soil types, and the high, but not the low, dose of
2,4-D reduced nitrogen fixation in both soils. The same authors
(Deshmukh & Shrikhande, 1974) monitored the populations of various
microorganisms under the same dosing conditions. 2,4-D stimulated
the numbers of actinomycetes throughout the 6-week incubation period at
both dose levels. Fungal populations were reduced in the first week of
incubation at both dose levels in sandy loam, but only at the higher
dose level in clay loam. This reduction in fungal populations
persisted until the second week with the high dose in the sandy soil
and throughout the incubation period with the high dose in clay soil.
There was a temporary (1 week) reduction in total bacterial numbers
with both 2,4-D dose levels in the sandy soil and with the higher level
in clay soil. Schroder & Pilz (1983) reported that 2,4-D at
approximately 10-4mol/litre (= 22 mg/kg) had no long-term effect on
soil nitrification.
Welp & Brummer (1985) measured the influence of 2,4-D on the
reducing capacity of soil microorganisms, reduction being monitored as
the capacity to reduce Fe(III) oxides to soluble Fe(II) ions. They
determined no-observed-effect levels (NOEL) of 115 and 95 mg 2,4-D/kg
for two different soil types and corresponding EC50 values on
reduction capacity of 200 and 530 mg 2,4-D/kg soil.
Ruggiero & Radogna (1985) extracted and partially purified soil
diphenolase (laccase) from forest soil. This enzyme, which exists free
in the soil, plays an important role in the metabolism of humic
materials in soil. Oxygen consumption was monitored during the
enzymatic reaction, using either catechol or p -phenylenediamine as
substrate, and the effect of 2,4-D was investigated. The herbicide
inhibited diphenolase activity, and Lineweaver-Burk plots of the
data suggested that 2,4-D acts as a non-competitive inhibitor.
Apparent K values of 28.7 and 6.0 mol/litre were obtained for catechol
and p -phenylenediamine, respectively.
6. TOXICITY TO AQUATIC ORGANISMS
6.1. Toxicity to Aquatic Invertebrates
Appraisal
The short-term toxicity data on the effects of 2,4-D free acid,
its salts, and esters on aquatic invertebrates is extensive. Ester
formulations are more toxic than the free acids or salts. Sensitivity
variations exist among species in response to the same formulation.
Organisms become more sensitive to 2,4-D when the water temperature
increases. Reproductive impairment occurred at concentrations below
0.1 of the short-term toxic levels determined for these formulations.
6.1.1. Short-term toxicity
The short-term toxicity of 2,4-D to aquatic invertebrates is
summarized in tables 3 - 5.
Unfortunately, there are few studies where both the free acid (or
its salts) and ester preparations have been tested on the same organism
under the same conditions. The only organisms for which this applies
are the oyster (Butler, 1963; Butler, 1965), the stonefly (Sanders &
Cope, 1968), and daphnids and shrimp (Sanders, 1970a). These studies
all show that the free acid and its salts are less toxic than ester
formulations; for example the free acid is at least 20 times less toxic
to the water flea Daphnia magna than the least toxic of the esters
tested (Sanders, 1970a). Comparing studies carried out by different
authors and in different systems also suggests a much greater toxicity
of the ester preparations.
Liu & Lee (1975) found that 2,4-D could adversely affect the bay
mussel (Mytilus edulis) at all stages of its life cycle. The attachment
of young mussels to test chamber walls was reduced (data in Table 3).
The authors evaluated, in two duplicate experiments, the effects of
2,4-D acid, at concentrations in sea water of 22.8, 45.7, 91.4, and
182.8 mg/litre, on the growth of larval mussels. After 10 days
exposure, there was a significant reduction in the growth of larvae
exposed to 91.4 mg 2,4-D/litre; larvae were 11.6% smaller than
controls. This reduction was found in only one experimental replicate.
In both experiments, there was reduced growth after 10 days exposure
to 182.8 mg/litre; larvae were 31.9% and 34.9% smaller than controls
in the two experiments. Exposure for 20 days at 91.4 mg/litre
led to reduced growth in both experiments. All larvae exposed to
182.8 mg/litre died within 12 days, but only in one experimental
replicate. Extension of the growth study in the second experiment led
to all larvae dying within 22 days of exposure to 182.8 mg/litre and,
therefore, failing to undergo metamorphosis. The metamorphosis of
larvae exposed from age 30 to 70 days was not affected by 2,4-D at
concentrations up to 176 mg/litre.
Presing (1981) monitored reproduction over four broods in the water
flea Daphnia magna exposed to 0, 5, 10, 25, or 50 mg/litre of
`Dikonirt' (sodium salt of 2,4-D). For the first brood, the only
significant effect was at 50 mg/litre, whereas the fourth brood was
delayed even at 5 or 10 mg/litre. Significant reductions in the
average number of young produced for each female were found with
the two highest concentrations. Young kept until maturity from
each of the tests were themselves exposed to 2,4-D in a repeat
experiment. Again there was a significant effect on young produced at
25 and 50 mg/litre.
Table 3. Toxicity of 2,4-D to estuarine or marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Salinity pH Formulationc Parameter Water Reference
stata (°C) (o/oo) concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bay mussel 17.2- 22.9- 6.4- free acid 96-h LC50 259 Liu &
(Mytilus edulis) 18.6 24.5 7.8 (232-289) Lee (1975)
17.2- 22.9- 6.4- free acid 96-h EC50 262 Liu &
18.6 24.5 7.8 attachment Lee (1975)
(trocophore larva) 17.2- 22.9- 6.4- free acid 48-h EC50 211.7 Liu &
18.6 24.5 7.8 normal Lee (1975)
development
Eastern oyster flow 18 29 butoxyethanol 96-h EC50 3.75 Butler
(Crassostrea virginica) shell growth (1963)
flow 29 25 isooctyl 96-h EC50 1.0 Mayer
shell growth (1987)
flow 28 25 PGBEE 96-h EC50 0.055 Mayer
shell growth (1987)
Copepod 21 7 7.8 butoxyethanol 96-h LC50 3.1 Linden
(Nitocra spinipes) (2.4-4.1) et al.
(1979)
Brown shrimp (adult) flow 30 PGBEE 24-h EC50 0.55 Butler
(Penaeus aztecus) loss of (1963)
equilibrium
(adult) flow 30 PGBEE 48-h EC50 0.55 Butler
loss of (1963)
equilibrium
(juv.)b stat 26 30 butoxyethanol 48-h LC50 5.6 Mayer
(1987)
(adult) flow 29 26 isooctyl 48-h LC50 0.48 Mayer
(1987)
Dungeness crab (1st zoel) stat 13 25 acid (tech) 96-h LC50 > 10 Caldwell
(Cancer magister) (1977)
(1st instar juv.)b stat 13 25 acid (tech) 96-h LC50 > 100 Caldwell
(1977)
Blue crab (juv.)b stat 24 29 PGBEE 48-h LC50 2.8 Mayer
(Callinectes sapidus) (1987)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration
in water continuously maintained.
b juv. = juvenile.
c PGBEE = propylene glycol butyl ethyl ester.
Table 4. Toxicity of 2,4-D to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Oligochaete worm flow 20 30 30 7.8 free acid 48-h LC50 122.2 Bailey &
(Lumbriculus flow 20 30 30 7.8 free acid 96-h LC50 122.2 Liu (1980)
variegatus)
Water flea stat 21 260 272 7.4 PGBEE 48-h LC50 0.1 Sanders (1970a)
(Daphnia magna) stat 21 260 272 7.4 dimethylamine 48-h LC50 4.0 Sanders (1970a)
stat 17 39 7.2 dimethylamine 48-h LC50 > 100.0 Mayer &
Ellersieck(1986)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 5.6 Sanders (1970a)
stat 21 260 272 7.4 free acid 48-h LC50 > 100.0 Sanders (1970a)
20 8.4- free acid 96-h LC50 417.8 Presing (1981)
8.6
20 8.4- sodium salt 96-h LC50 932.1 Presing (1981)
8.6
Water flea 15.6 44 7.4 PGBEE 48-h LC50 4.9 Sanders &
(Simocephalus (4.0-6.7) Cope (1966)
serrulatus)
Water flea 15.6 PGBEE 48-h LC50 3.2 Sanders &
(Daphnia pulex) (2.4-4.3) Cope (1966)
Copepod (nauplius larva)
(Cyclops vernalis) stat 20 31.6 70 6.7 free acid 96-h LC50 8.72 Robertson (1975)
(5.34-11.57)
stat 20 31.6 70 6.7 alkanolamine 96-h LC50 54.8 Robertson (1975)
(46.45-64.6)
Scud stat 21.1 30 7.1 butoxyethanol 24-h LC50 1.4 (1.1-1.8) Sanders (1969)
(Gammarus stat 21.1 30 7.1 butoxyethanol 48-h LC50 0.76 (0.51
lacustris) -1.1) Sanders (1969)
stat 21.1 30 7.1 butoxyethanol 96-h LC50 0.44 (0.31
-0.62) Sanders (1969)
stat 21.1 30 7.1 PGBEE 24-h LC50 2.1 (1.7-2.5) Sanders (1969)
stat 21.1 30 7.1 PGBEE 48-h LC50 1.8 (1.4-2.3) Sanders (1969)
stat 21.1 30 7.1 PGBEE 96-h LC50 1.6 (1.2-2.1) Sanders (1969)
stat 21.1 30 7.1 isooctyl 24-h LC50 6.8 (4.8-9.7) Sanders (1969)
stat 21.1 30 7.1 isooctyl 48-h LC50 4.6 (2.9-7.3) Sanders (1969)
stat 21.1 30 7.1 isooctyl 96-h LC50 2.4 (1.9-4.8) Sanders (1969)
stat 15.5 260 272 7.4 PGBEE 24-h LC50 4.1 (2.8-5.8) Sanders (1970a)
stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.6 (1.7-3.9) Sanders (1970a)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 15.5 260 272 7.4 PGBEE 96-h LC50 2.5 (1.7-3.7) Sanders (1970a)
(Gammarus stat 15.5 260 272 7.4 butoxyethanol 24-h LC50 6.5 (1.0-8.6) Sanders (1970a)
lacustris) (contd.) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 5.9 (3.1-11) Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 96-h LC50 5.9 (3.1-11) Sanders (1970a)
Scud stat 15 272 7.4 dimethylamine 24-h LC50 > 100 Mayer &
(Gammarus fasciatus) stat 15 272 7.4 dimethylamine 96-h LC50 > 100 Ellersieck (1986)
Glass shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 2.7 Sanders (1970a)
(Palaemonetes stat 21 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
kadiakensis stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.4 Sanders (1970a)
Seed shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 0.32 Sanders (1970a)
(Cypridopsis vidua) stat 21 260 272 7.4 dimethylamine 48-h LC50 8.0 Sanders (1970a)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.8 Sanders (1970a)
Freshwater prawn stat 27 113.9 7.5 sodium salt 24-h LC50 2342 Shukla &
(Macrobranchium stat 27 113.9 7.5 sodium salt 48-h LC50 2309 Omkar (1983)
lamarrei) stat 27 113.9 7.5 sodium salt 72-h LC50 2267 Shukla &
stat 27 113.9 7.5 sodium salt 96-h LC50 2224 Omkar (1983)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2644 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2536 Shukla (1984)
naso) stat 28 112.7 7.5 sodium salt 72-h LC50 2435 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2397 Shukla (1984)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2474 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2381 Shukla (1984)
dayanum) stat 28 112.7 7.5 sodium salt 72-h LC50 2333 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2275 Shukla (1984)
Crayfish stat 15.5 260 272 7.4 PGBEE 48-h LC50 > 100 Sanders (1970a)
(Orconectes nais) stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 > 100 Sanders (1970a)
Red swamp stat 20 100 8.4 alkanolamine 96-h LC50 1389 Cheah et al.
crayfish (imm.)c (1174-1681) (1980)
(Procambarus clarki)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Sowbug stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.2 Sanders (1970a)
(Asellus stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
brevicaudus) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 3.2 Sanders (1970a)
Stone fly (naiad) 15.5 35 7.1 butoxyethanol 24-h LC50 8.5 (5.7-13) Sanders &
(Pteronarcys 15.5 35 7.1 butoxyethanol 48-h LC50 1.8 (1.5-2.7) Cope (1968)
californica) 15.5 35 7.1 butoxyethanol 96-h LC50 1.6 (1.3-1.9) Sanders &
15.5 35 7.1 acid (tech) 24-h LC50 56 (50-63) Cope (1968)
15.5 35 7.1 acid (tech) 48-h LC50 44 (32-59) Sanders &
15.5 35 7.1 acid (tech) 96-h LC50 15 (10-22) Cope (1968)
Midge (larva) 15 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1490 Bunting &
(Chaoborus 15 78-95 55 7.3-7.8 dimethylamine 96-h LC50 890 (421-1211)Robertson
punctipennis) 20 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1124 (1975)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained.
b Alkalinity and hardness expressed as mg CaCO3/litre.
c imm. = immature.
d PGBEE = propylene glycol butyl ether ester.
Table 5. Toxicity of 2,4-D to aquatic invertebrates: no observed effect levels
---------------------------------------------------------------------------------------------------------
Flow/ Temp Sali- Alkali- Hard- Water con- Refer-
Organism stata (°C) nity nityb nessb pH Formulationc Parameterd centration ence
(o/oo) (mg/litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster flow 9 19 free acid 96-h EC0 2.0 Butler
(Crassostrea shell growth (1963)
virginica) flow 30 23 free acid 96-h EC0 2.0 Butler
shell growth (1963)
flow 25 28 dimethylamine 96-h EC0 2.0 Butler
shell growth (1963)
Freshwater oligochaete flow 20 30 30 7.8 free acid 96-h LC0 86.7 Bailey
(Lumbriculus & Liu
variegatus) (1980)
Scud stat 21.1 30 7.1 dimethylamine 96-h LC0 100 Sanders
(Gammarus lacustris) (1969)
Grass shrimp stat 20 20 butoxyethanol 24-h LC0 10 Hansen
(Palaemonetes pugio) et al.
(1973)
Pink shrimp butoxyethanol 48-h LC0 1.0 Butler
(Penaeus duorarum) (1965)
PGBEE 48-h LC0 1.0 Butler
(1965)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester.
d LC0 and EC0 represent the highest dose used which cause no death or no effect, respectively;
they are not mathematically determined no-effect levels.
George et al. (1982) measured lethal times (LT) after exposure of
the water flea Daphnia lumholtzi to 10 or 20 mg 2,4-D/litre. They
reported, for 10 mg/litre, an LT50 of 38 h and an LT100 of 71 h. For
20 mg/litre, the LT50 was 21 h and the LT100 was 31 h. Doses of
2,4-D ranging from 0.1 to 50 mg/litre did not affect the behaviour of,
or kill, the copepod Mesocyclops leuckarti within a 30-day exposure
period and so lethal times could not be calculated.
Caldwell (1977) and Caldwell et al. (1979) found the zoeal larva to
be the most sensitive life-cycle stage of the Dungeness crab (Cancer
magister) to the free acid of 2,4-D. Based on the herbicide's toxicity
to this stage, the authors suggest a maximum acceptable toxicant level
(MATC) of <1 mg/litre. At this concentration, there was no mortality,
but there was an effect on moulting.
6.1.2. Behavioural effects
Folmar (1978) tested mayfly nymphs (Ephemerella walkeri) in a `Y'-
shaped avoidance maze. A 2,4-D dimethylamine salt solution was run
into one arm of the maze and clean water was run into a second arm,
both at 400 ml/min. Numbers of nymphs in each arm of the maze were
counted after 1 h. No avoidance of 2,4-D was found at concentrations
of 10 mg/litre and there was no mortality. At 100 mg/litre there was
70% mortality in the test nymphs but still no avoidance of the
herbicide. In a similar experiment using the grass shrimp
(Palaemonetes pugio) exposed to the butoxyethanol ester of 2,4-D,
there was significant avoidance of the herbicide at 1 mg/litre (Hansen
et al., 1973).
6.2. Toxicity to Fish
Appraisal
At recommended application rates, the concentration of 2,4-D in
water has been estimated to be a maximum of 50 mg/litre. Most
applications would lead to water concentrations much lower than this
(between 0.1 and 1.0 mg/litre).
LC50 values for fish vary considerably. This variation is due to
differences in species sensitivity, chemical structure (esters, salts,
or free acid), and formulation of the herbicide.
Although the free acid is the physiologically toxic entity, the
ester formulations represent a major hazard to fish when used directly
as aquatic herbicides (because they are more readily taken up by fish).
Amine salt formulations used to control aquatic weeds do not affect
adult fish.
The NOEL varies with the species and the formulation: <1 mg/litre
(coho salmon) to 50 mg/litre (rainbow trout).
Fish larvae are the most sensitive life stage but are unlikely to
be affected under normal usage of the herbicide.
Long-term adverse effects on fish are observed only at
concentrations higher than those produced after 2,4-D has been applied
at recommended rates.
Few studies are related to the effects of environmental variables,
such as temperature and water hardness, on 2,4-D toxicity to fish.
Higher temperature possibly increases the toxicity. This might be
considered when assessing the safety of 2,4-D to fish during control of
aquatic weeds.
Fish detect and avoid 2,4-D only at higher concentrations than
those obtained under normal conditions of use.
6.2.1. Effect of formulation on short-term toxicity to fish
The toxicity of different formulations of 2,4-D to fish is
summarized in Table 6.
The most comprehensive study on the effects of different
formulations of 2,4-D using the same test fish, fingerling bluegill
sunfish (Lepomis macrochirus), was performed by Hughes & Davis (1963)
in static 24-h and 48-h tests. Ester formulations were invariably more
toxic than amine salt formulations. Dimethylamine and alkanolamine
preparations ranged in toxicity from 166 to 900 mg/litre (LC50 in 24-h
tests), depending on the commercial preparation used. Although esters
were always more toxic than amine salts, there was some variation
between different ester formulations (range: 0.9 to 66.3 mg/litre; 24-h
LC50). Most of this variation was between different preparations of
the least toxic of the esters, the isooctyl ester, which ranged in
toxicity from 8.8 to 66.3 mg/litre. All other esters tested produced
LC50 values of 8 mg/litre or less, the most toxic being the isopropyl
with a 24-h LC50 of 0.9 mg/litre. The addition of emulsifiers to acid
preparations increased 2,4-D toxicity; a formulation with emulsifiers
gave an LC50 of 8 mg/litre over 24 h, making it comparable to the
esters in toxicity. All ester formulations were considered by the
authors to present a major hazard to fish when used directly as an
aquatic herbicide, whereas the amine salt formulations could be safely
used to control aquatic weeds without adversely affecting adult fish
(Hughes & Davis, 1963).
A study on a range of ester formulations, using salmonids as test
fish, conducted by Finlayson & Verrue (1985), showed that the toxicity
for salmonids was similar to that for bluegill sunfish. These authors
argue that static tests underestimate the toxicity of 2,4-D esters
because some of the ester is hydrolysed to the less-toxic free acid
during the course of even short-term tests. The presence of test fish
increases the rate of hydrolysis of 2,4-D esters. In a static test,
with two different stocking rates of fish, the apparent toxicity of
2,4-D ester decreased with a greater density of test fish (rainbow
trout) because of this enhanced hydrolysis. Results are given in
Table 6. In their flow-through tests, results were adjusted to take
account of the hydrolysis of ester to 2,4-D acid during the course of
the experiment. Two values are given in Table 6 for each test. The
first is the calculated effect of the non-hydrolysed ester and the
second, entered as `total 2,4-D', is the observed effect of the mixture
of ester and free acid produced by hydrolysis during the course of the
experiment. There is as much as a five-fold difference between the two
values. Alabaster (1969) examined several formulations of 2,4-D in two
species of fish, and found that pelleted herbicide, either as clay-
based or resin-based pellets, was the least toxic to fish of any of the
formulations tested.
Table 6. Toxicity of 2,4-D to fish: effects of different formulations
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
--------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 40 29 6.9 alkanolamine 24-h LC50 450-900 Hughes &
(Lepomis macrochirus) stat 25 40 29 6.9 alkanolamine 48-h LC50 435-840 Davis (1963)
stat 25 40 29 6.9 dimethylamine 24-h LC50 166-542 Hughes &
stat 25 40 29 6.9 dimethylamine 48-h LC50 166-458 Davis (1963)
stat 25 40 29 6.9 di-N,N 24-h LC50 1.5 Hughes &
stat 25 40 29 6.9 di-N,N 48-h LC50 1.5 Davis
stat 25 40 29 6.9 2,4-D acid + 24-h LC50 8.0 (1963)
emulsifiers
stat 25 40 29 6.9 2,4-D acid + 48-h LC50 8.0 Hughes &
emulsifiers Davis (1963)
stat 25 40 29 6.9 isooctyl ester 24-h LC50 8.8-66.3 Hughes &
stat 25 40 29 6.9 isooctyl ester 48-h LC50 8.8-59.7 Davis (1963)
stat 25 40 29 6.9 PGBEE 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 PGBEE 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butoxyethanol 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 butoxyethanol 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butyl ester 24-h LC50 1.3 Hughes &
stat 25 40 29 6.9 butyl ester 48-h LC50 1.3 Davis (1963)
stat 25 40 29 6.9 mixed butyl + 24-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 mixed butyl + 48-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 isopropylester 24-h LC50 0.9 Hughes &
stat 25 40 29 6.9 isopropylester 48-h LC50 0.8 Davis (1963)
stat 25 40 29 6.9 ethyl ester 24-h LC50 1.4 Hughes &
stat 25 40 29 6.9 ethyl ester 48-h LC50 1.4 Davis (1963)
Cutthroat trout butyl ester 96-h LC50 0.78 Woodward (1982)
(juvenile) (Salmo clarki) (0.66-0.92)
PGBEE 96-h LC50 0.77 Woodward (1982)
(0.62-0.96)
isooctyl ester 96-h LC50 > 50 Woodward (1982)
Chinook salmon (fry) flow 9 18 17 7.1 butoxyethanol 96-h LC50 0.315 Finlayson &
(Oncorhynchus flow 9 18 17 7.1 total 2,4-D 96-h LC50 0.373 Verrue (1985)
tshawytscha)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.375 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.250 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.246 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.117 Verrue (1985)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout (fry) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.518 Finlayson &
(Salmo gairdneri) flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.642 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.329 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.514 Verrue (1985)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.468 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.338 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.342 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.555 Verrue (1985)
loading factor stat 14 18 17 7.1 butoxyethanol 96-h LC50 1.206 Finlayson &
4.2 g fish/litre Verrue (1985)
stat 14 18 17 7.1 total 2,4-D 96-h LC50 1.422 Finlayson &
loading factor stat 15 18 17 7.1 butoxyethanol 96-h LC50 3.689 Verrue (1985)
8.8 g fish/litre Finlayson &
stat 15 18 17 7.1 total 2,4-D 96-h LC50 4.487 Verrue (1985)
Harlequin fish flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Rasbora heteromorpha) pellets
flow 20 250 7.2 resin-based 24-h LC50 3950 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 3100 Alabaster (1969)
pellets
flow 20 20 7.2 sodium salt 24-h LC50 1160 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 24-h LC50 1.0 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 48-h LC50 1.0 Alabaster (1969)
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Salmo gairdneri) pellets
flow 20 250 7.2 clay-based 48-h LC50 4800 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 24-h LC50 3400 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 2400 Alabaster (1969)
pellets
flow 20 250 7.2 amine salt 24-h LC50 250 Alabaster (1969)
flow 20 250 7.2 amine salt 48-h LC50 210 Alabaster (1969)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test);
flow = flow-through conditions (2,4-D concentration in water
continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c di-N,N = di-N,N-dimethylcocoamine; PGBEE = propylene glycol
butyl ether ester; total 2,4-D = the effect actually observed in the
flow-through test; the value which preceeds each "total 2,4-D" value is
the calculated effect of the ester alone. The authors determined the
degree of hydrolysis of the ester during the course of the test and
subtracted the effect due to the free acid produced by this hydrolysis.
6.2.1.1 Tolerance and potentiation
Chambers et al. (1977) used insecticide-tolerant and insecticide-
susceptible populations of mosquito fish and an esterase inhibitor to
investigate hydrolytic activation and detoxification of 2,4-D esters.
Mosquito fish taken from a wild population which had developed some
tolerance to insecticides also showed some slight tolerance to 2,4-D
ethyl and butyl esters. This tolerance was most pronounced with the
butyl ester, where the 48-h LC50 was raised from 0.98 mg/litre in the
susceptible fish, to 1.70 mg/litre in the tolerant fish. Further
experiments were carried out to find the basis for this tolerance and
for the higher toxicity of 2,4-D esters over that of the free acid.
The addition of DEF (S,S,S-tributyl phosphorotrithioate), a carboxyl
esterase inhibitor, to the toxicity test medium slightly reduced the
toxicity of both 2,4-D esters. This finding suggested that the toxic
effect of the esters required initial hydrolysis to the acid. The
increased toxicity of the esters would result from esters being more
readily absorbed into the fish through the gills. The resistance of
the insecticide-tolerant population was, at least partially, explained
by measuring esterase activity in homogenates of gill and liver from
the two fish populations. The tolerant fish hydrolyzed less of both
2,4-D esters than susceptible fish. This effect was most marked with
liver homogenates; results from gill homogenates were equivocal. The
overall conclusion, put forward by the authors, is that liver
hydrolysis `activates' 2,4-D esters by converting them to the toxic
free acid. Hydrolysis in the gill is a `detoxification' reaction
because it reduces the uptake of toxic material. The slight increase
in tolerance in the insecticide-resistant mosquito fish is largely the
result of decreased activation of the 2,4-D esters by the liver.
Antagonism to 2,4-D ester toxicity by DEF is largely the result of
inhibition of activation in the liver rather than increased
detoxification in either liver or gill (Chambers et al., 1977).
Carbaryl, a cholinesterase inhibitor, potentiates the toxicity of 2,4-D
butyl ester to brown trout (Salmo trutta) (Statham & Lech, 1975).
The 4.5-h LC50 for 2,4-D butyl ester in static tests was shifted from
30 mg/litre to 11 mg/litre by the addition of carbaryl, at a
concentration of 1 mg/litre, to the test water. Carbaryl has no
toxicity to the fish at this concentration; the 24-h LC50 for carbaryl
alone is 6.8 mg/litre. The potentiating effect of carbaryl was itself
blocked by atropine, a muscarinic blocker, at a water concentration of
10 mg/litre; the atropine itself was not toxic to the fish at this
concentration. In a similar way, carbaryl potentiated the toxicity of
several other compounds. The authors suggested a non-specific action,
possibly by increasing the uptake of 2,4-D ester from the water. The
same potentiation was demonstrated for trout in flow-through tests
(Statham & Lech, 1975). Combinations of 2,4-D esters (butyl or
propylene glycol butyl) with the herbicide picloram increased the
toxicity to fish above the combined toxicity of the individual
compounds (Woodward, 1982).
6.2.2. No-observed-effect levels in short-term tests with fish
The NOELs of 2,4-D on fish in short-term tests are summarized in
Table 7. Values quoted from Meehan et al. (1974) and from Butler
(1965) are based on the lowest dose used in their studies. The values
from Birge et al. (1979) are calculated 1% mortality values derived
mathematically from a full toxicity curve, and based on young fish
exposed to 2,4-D from shortly after fertilization of the eggs until
4 days after hatching. The differing exposure times for the three
species tested is due to differences in time to hatching. The
variation in these data is, therefore, partly due to species
differences in sensitivity to the compound and partly due to exposure
times. Newly hatched young fish are more sensitive to 2,4-D than
unhatched embryos (see section 6.2.4).
6.2.3. Species differences in short-term toxicity to fish
Variation in the toxicity of 2,4-D to fish with species is
summarized in Table 8. Of a range of fish species, examined in the
same test conditions, using 2,4-D as the free acid, by Rehwoldt et al.
(1977), the most sensitive was the white perch (Roccus americanus)
with a 24 h LC50 of 55 mg/litre, and the least sensitive was the
eel Anguilla rostrata with an LC50 of 427 mg/litre. The grass carp,
often used together with herbicides to control aquatic vegetation, was
the least sensitive of all species examined, with a 24 h LC50 for an
amine salt formulation of 3080 mg/litre (Tooby et al., 1980).
6.2.4. Toxicity to early life-stages of fish
Short-term studies on the toxicity of 2,4-D to early life-stages of
fish are summarized in Table 9.
Studies on fish eggs and larvae immediately after hatching have
been conducted on few species and mainly with simple salts of 2,4-D.
There is little information on the effects of the more toxic esters.
2,4-D is clearly toxic for fish early life-stages, within the likely
range of water concentrations which would be found after use of the
herbicide to control aquatic weeds.
At the 16 - 32 cell blastomere stage, eggs of the bleak Alburnus
alburnus, developed more slowly than control eggs when exposed to 2,4-D
solutions. After 48-h exposure, mortality reached 68% and 79% in
those eggs exposed to 2,4-D at 25 and 50 mg/litre, respectively.
Control mortality after 48-h exposure was 37%. After 23 h of
development, eggs exposed to 25 mg 2,4-D/litre showed normal
development while those exposed to 100 mg/litre showed slower
embryogenesis or development halted at the morula-gastrula stage.
Free-swimming larvae were more sensitive to 2,4-D than eggs; the rate
of survival of embryos in tests lasting for between 12 and 48 h was
higher than for larvae. In tests lasting for between 24 and 48 h, at
concentrations of 2,4-D above 400 mg/litre, no larvae survived.
Embryos showed malformations and reduced mobility at concentrations
above 100 mg/litre, and at concentrations of 800 mg/litre or more,
embryos were immobile (Biro, 1979).
Birge et al. (1979) examined the effects of 2,4-D, as the potassium
salt, on the eggs and larvae of three species of fish. Rainbow trout
eggs were the most sensitive, largemouth bass eggs less sensitive, and
goldfish eggs extremely tolerant to 2,4-D. In all species tested,
the larval stages were more sensitive to the herbicide than were the
eggs.
Table 7. Toxicity of 2,4-D to fish: no-observed-effect levels
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Pink salmon (fry) stat 10 10-34 free acid 96-h LC0 < 1 Meehan
(Oncorhynchus stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
gorbuscha stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Chum salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus keto) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Coho salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus kisutch) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Sockeye salmon (smolts) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus nerka) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Alaska coho salmon stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
stat 10 10-34 PGBEE 96-h LC0 < 1
Oregon coho salmon stat 10 10-34 free acid 96-h LC0 10 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Dolly Varden stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salvelinus malma) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Rainbow trout stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salmo gairdneri) (1974)
Spot stat free acid 48-h LC0 50 Butler
(Leistomus xanthurus) (1965)
Longnose killifish stat dimethylamine 48-h LC0 15 Butler
(Fundulus similis) (1965)
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mullet (Mugil cephalus) stat ethylhexyl 48-h LC0 10 Butler
(1965)
White mullet (juvenile) flow sea free acid 48-h LC0 50.0 Butler
(Mugil curema) water (1963)
Goldfish flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC1 8.2 Birge et al.
(Carassius auratus) 25.8 (2.7-15.0) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC1 8.9 Birge et al.
25.8 (3.8-14.6) (1979)d
Largemouth bass flow 18.2- 66.7 53.5 7.84 pot. salt 7.5-day LC1 13.1 Birge et al.
(Micropterus salmoides) 25.8 (4.4-21-9) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC1 3.2 Birge et al.
25.8 (1.2-6.0) (1979)d
Rainbow trout flow 12.5- 66.7 53.5 7.84 pot. salt 27-day LC1 0.032 Birge et al.
(Salmo gairdneri) 14.5 (0.008-0.084) (1979)d
flow 12.5- 65.3 197.5 7.78 pot. salt 27-day LC1 0.022 Birge et al.
14.5 (0.006-0.055) (1979)d
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c pot. salt = potassium salt; PGBEE = propylene glycol butyl ether ester.
LC0 obtained by extrapolation and LC1 mathematically calculated.
d Birge et al. (1979) exposed fish from four days after hatching.
Table 8. Toxicity of 2,4-D to fish: species variation
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Striped bass stat 20 50 7.2 free acid 24-h LC50 85.6 Rehwoldt
(Morone saxatilis) stat 20 50 7.2 free acid 96-h LC50 70.1 et al.
(1977)
Banded killifish stat 20 50 7.2 free acid 24-h LC50 306.2 Rehwoldt
(Fundulus diaphanus) stat 20 50 7.2 free acid 96-h LC50 26.7 et al.
(1977)
Pumpkinseed sunfish stat 20 50 7.2 free acid 24-h LC50 120 Rehwoldt
(Lepomis gibbosus) stat 20 50 7.2 free acid 96-h LC50 94.6 et al.
(1977)
White perch stat 20 50 7.2 free acid 24-h LC50 55.5 Rehwoldt
(Roccus americanus) stat 20 50 7.2 free acid 96-h LC50 40 et al.
(1977)
American eel stat 20 50 7.2 free acid 24-h LC50 427.2 Rehwoldt
(Anguilla rostrata) stat 20 50 7.2 free acid 96-h LC50 300.6 et al.
(1977)
Carp stat 20 50 7.2 free acid 24-h LC50 175.2 Rehwoldt
(Cyprinus carpio) stat 20 50 7.2 free acid 96-h LC50 96.5 et al.
(1977)
Guppy stat 20 50 7.2 free acid 24-h LC50 76.7 Rehwoldt
(Lebistes reticulata) stat 20 50 7.2 free acid 96-h LC50 70.7 et al.
(1977)
Grass carp flow 13 270 8.1 amine salt 24-h LC50 3080 Tooby et
(Ctenopharyngodon (2622-3618) al. (1980)
idella) flow 13 270 8.1 amine salt 48-h LC50 2540 Tooby et
(2184-2952) al. (1980)
flow 13 270 8.1 amine salt 96-h LC50 1313 Tooby et
(1116-1544) al. (1980)
Bleak stat 10 15 7.8 butoxyethanol 96-h LC50 3.2-3.7 Linden et
(Alburnus alburnus) al. (1979)
Mosquito fish stat 21-22 amine salt 24-h LC50 500 Johnson
(Gambusia affinis) stat 21-22 amine salt 48-h LC50 445 (1978)
stat 21-22 amine salt 96-h LC50 405
Mullet stat sodium salt 24-h LC50 68.0 Tag El-Din
(Mugil cephalus) stat sodium salt 96-h LC50 32.0 et al.
(1981)
Longnosed killifish stat butoxyethanol 48-h LC50 5.0 Butler
(Fundulus similis) stat PGBEE 48-h LC50 4.5 (1965)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 19 7.0 dimethylamine 24-h LC50 390 Davis &
(Lepomis macrochirus) stat 25 19 7.0 dimethylamine 48-h LC50 375 Hardcastle
(1959)
Largemouth bass stat 25 19 7.0 dimethylamine 24-h LC50 375 Davis &
(Micropterus salmoides) stat 25 19 7.0 dimethylamine 48-h LC50 350 Hardcastle
(1959)
Punti (Puntius ticto) stat 23.5 ethyl ester 24-h LC50 1.6 Verma et
al. (1984)
Medaka (Oryzias latipes) sodium salt 48-h LC50 > 40 Hashimoto &
Nishiuchi
(1978)
Longnose killifish (juv.) flow sea water PGBEE 24-h LC50 5.0 Butler
(Fundulus similis) flow sea water PGBEE 48-h LC50 4.5 (1963)
flow sea water butoxyethanol 24-h LC50 5.0 Butler
flow sea water butoxyethanol 48-h LC50 5.0 (1963)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester
Table 9. Toxicity of 2,4-D to fish early life-stages
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bleak (embryo) sodium salt 12-h LC50 159.4 Biro (1979)
(Alburnus alburnus) sodium salt 24-h LC50 129.0 Biro (1979)
sodium salt 36-h LC50 63.9 Biro (1979)
sodium salt 48-h LC50 12.9 Biro (1979)
(larvae) sodium salt 12-h LC50 111.2 Biro (1979)
sodium salt 24-h LC50 70.6 Biro (1979)
sodium salt 36-h LC50 62.1 Biro (1979)
sodium salt 48-h LC50 51.6 Biro (1979)
Goldfish (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 4-day LC50 > 187 Birge et
(Carassius auratus) 25.8 al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 4-day LC50 > 201 Birge et
25.8 al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC50 133.1 Birge et
post-hatch) 25.8 (108.6-174.8) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC50 119.1 Birge et
25.8 (98.5-150.6) al. (1979)
Largemouth bass (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 3.5-day LC50 165.4 Birge et
(Micropterus salmoides) 25.8 (130.6-274.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 3.5-day LC50 160.7 Birge et
25.8 (122.9-230.6) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 7.5-day LC50 108.6 Birge et
post-hatch) 25.8 (92.5-138.4) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC50 81.6 Birge et
25.8 (64.8-103.5) al. (1979)
Rainbow trout (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 23-day LC50 11.0 Birge et
(Salmo gairdneri) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 23-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 27-day LC50 11.0 Birge et
post-hatch) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 27-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (2,4-D concentration
in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
Pot. salt = potassium salt.
Only one study has examined the effects of 2,4-D esters on newly-
hatched fish fry and on fertilized eggs. Unfortunately, this study, by
Hiltibran (1967), does not record the full experimental details. Four
species of fish were used in the study but complete results were given
only for the bluegill sunfish. The most toxic preparations were the
propylene glycol butyl ether (PGBE) ester and mixed isopropyl and butyl
esters with no-observed-effect levels of 2 and 3 mg/litre,
respectively. The dimethylamine salt, ethylhexyl ester, and sodium
salt formulations were less toxic with no-observed-effect levels at 40,
50, and 100 mg/litre, respectively.
6.2.5. Long-term toxicity to fish
Chronic exposure of sub-adult fish of a variety of species to 2,4-D
for 10 months, at a concentration of 0.1 mg/litre, led to no mortality
and to no change in the acute response to 2,4-D. The 24-h LC50 for
the compound was unchanged after 10 months exposure to sub-lethal
doses; i.e., no tolerance developed. One species of fish, the guppy
Lebistes reticulatus, reproduced in captivity. Reproductive success
was compared between control fish and fish breeding in water
containing 2,4-D at 0.1 mg/litre over 10 months. The ratio of numbers
of offspring of treated and control fish was 1.2 (Rehwoldt et al.,
1977). Mount & Stephan (1967) conducted a 10-month study on fathead
minnows (Pimephales promelas) exposed to the butoxyethanol ester of
2,4-D at 0, 0.01, 0.04, 0.2, or 0.8 mg/litre water in a flow-through
test system. Concentrations of 2,4-D of 0.2 mg/litre or less had no
observable effect on growth, survival, or reproductive success of the
fish, but the highest concentration tested was toxic to eggs. The
highest tested no-observed-effect concentration was approximately
1/45 of the 96-h LC50 for this species. Finlayson & Verrue (1985)
conducted a chronic egg-to-fry test over 86 days using chinook
salmon in 2,4-D butoxyethanol ester solutions ranging up to
118 µg/litre. The mortality of salmon during the alevin to fry period
was 4.7% and 47.6% for exposures to 60 and 118 µg/litre, respectively.
When compared to controls, the length of salmon at 36 days
post hatch was significantly reduced after exposure to 60 and
118 µg/litre. Neither survival nor growth of fry were affected at
2,4-D concentrations of 40 µg/litre or less. This maximum acceptable
toxicant concentration (MATC) represents 0.13 and 0.11 of the 96-h
LC50 values for fry and smolts of this species, respectively.
6.2.6. Behavioural effects on fish
Folmar (1976) used rainbow trout fry in an investigation to
determine whether fish avoided water contaminated by herbicides. Trout
fry were initially placed in a `Y'-shaped maze for 15 min. The
dimethylamine salt of 2,4-D, then was added to one arm of the maze and
clean water in a second arm, both at flow rates of 400 ml/min. The
number of fish in each arm was counted after 1 hour and results tested
statistically using a Chi-squared test. At concentrations of 2,4-D of
0.1 mg/litre water (approximately equal to water levels after the use
of this preparation as an aquatic herbicide), there was no avoidance
of the compound; the numbers of fish in each arm of the maze were
equal. However, at concentrations of 1.0 or 10.0 mg/litre of 2,4-D,
there was significant avoidance of the chemical.
Hidaka et al. (1984) conducted a similar study using medakas
Oryzias latipes, and tested a wide range of doses for a variety of
pesticides and herbicides. In all cases, the fish avoided the
chemical in a dose-related manner, but only over a limited range of
concentrations. Above this range, presumably because of the onset of
toxic effects, there was a dose-related decrease in avoidance response.
The authors calculated two values from these chevron-shaped graphs,
the avoidance response EC65 taken from the increasing curve
(AR65) and the DAR60 taken from the decreasing curve. For 2,4-D, the
value for AR65 was 177 (171-182; 95% confidence limits) µg/litre, and
for DAR60 was 288 (245-338) µg/litre. Compared to other chemicals
commonly used or found in water, 2,4-D has a high threshold of
detection by fish as indicated by the high avoidance threshold.
Rand & Barthalmus (1980) exposed goldfish to 2,4-D at 20 mg/litre
(10% of the 96-h LC50 for this species) at different stages during the
training period for conditioning the fish to avoid electric shocks.
They found that the herbicide had no effect when given for 24 h on the
9th day of conditioning but was effective in changing the magnitude of
the avoidance response when given for the first 24 h of conditioning.
Fish exposed for 2 weeks showed significant differences in the pattern,
rate of acquisition, and maintenance of the avoidance baseline.
Behavioural differences persisted into the post-exposure period in fish
exposed to 2,4-D for 2 weeks. The authors point out that short-term
toxicity tests do not examine subtle behavioural effects which could be
of considerable importance in the wild.
Dodson & Mayfield (1979) assessed the effect of 2,4-D on the
``reotaxic response'' of rainbow trout, that is their tendency to swim
upstream to compensate for flowing water. There was a dose-related
effect of 2,4-D at concentrations between 0 and 7 mg/litre. Above this
concentration range, there was a fall of more than 50% in the frequency
of positive reotaxic response to a revolving drum marked in alternate
light and dark stripes and an increase in the frequency of `no-
response'. The authors state that, at ``realistic concentrations'' of
2,4-D in water, there would be a tendency for fish to be moved
downstream because of a reduced reotaxic response.
6.2.7. Effects of environmental variables on toxicity to fish
The toxicity of 2,4-D to fish is related to season. Vardia &
Durve (1981) obtained different values for 96-h LC50 for the carp
Cyprinus carpio at different times of the year. Water
characteristics did not differ, except for temperature which varied
from 39 °C in May, its highest value, to 17 °C in February, its lowest.
There was a positive correlation between temperature and toxicity. At
39 °C, the 96-h LC50 was 5.6 mg/litre, and, at 17 °C, the LC50 was
40.83 mg/litre. As the authors point out, the temperature of the water
must be borne in mind when assessing the safety of this compound to
fish during control of aquatic weeds. The effect may be one of season
rather than temperature; the physiology of the fish also changes
throughout the year.
There is some effect of water hardness and pH on 2,4-D toxicity to
fish but this is very dependent on the species of test fish (Birge et
al., 1979). This effect has not been studied systematically.
6.2.8. Special studies on fish
Chronic exposure of carp to sub-lethal concentrations of 2,4-D
(5 mg/litre) led to ultrastructural changes in the liver of the fish
(Benedeczky et al., 1984). After 2 months, there was detectable
swelling of mitochondria and loss of cristae. There were also large
numbers of inclusions in the cytoplasm, interpreted by the authors as
bile pigments. Their presence in the bile canaliculi indicated the
onset of cholestasis (reduction in bile flow). After 3, 4, or 5 months
of exposure, the cholestasis was pronounced with cholesterin crystals
appearing as cytoplasmic inclusions. Later, in the 6th month of
exposure, there were endoplasmic reticulum changes indicative of
changed protein synthesis.
Oxygen consumption by bluegill sunfish was not affected by 2,4-D at
a concentration of 3 mg/litre water (Sigmon, 1979).
2,4-D at 10-4 mol/litre of medium did not affect the activity of
Na/K-ATPase in microsomes from trout gill (Davis et al., 1972).
Verma et al. (1984) detected effects on pituitary and pineal
histology in punti Puntius ticto exposed for 96 h to 1 mg/litre of
Weedone (ethyl ester of 2,4-D). There was a significant effect on cell
size of acidophilic pituitary cells but a much more marked effect on
cyanophils. Pineal epithelium cell height was significantly greater in
exposed fish.
6.3. Toxicity to Amphibians
Appraisal
Amphibian larvae are generally tolerant to amine salts of 2,4-D;
the 96-h LC50 values exceed 100 mg/litre. Of the species tested, only
one was sensitive.
No information is available on reproductive development and
differentiation or on tissue levels.
The toxicity of 2,4-D to amphibians is summarized in Table 10.
Tadpoles of the Indian toad are particularly susceptible to the
compound (Vardia et al., 1984). Lhoste & Roth (1946) showed that
2,4-D, at 5 g/litre, prevented development of the eggs of the common
frog (Rana temporaria). At doses between 500 mg/litre and 4 g/litre,
there was some development which decreased with increasing dose. These
levels are far higher than those likely to be encountered in the
environment.
Table 10. Toxicity of 2,4-D to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Refer-
stata (°C) nityb nessb concentration ence
(mg/litre)
-------------------------------------------------------------------------------------------------------------------------------------------------
Chorus frog (tadpole) stat 15.5 30 7.1 dimethylamine 24-h LC50 > 100 Sanders
(Pseudacris triseriata) stat 15.5 30 7.1 dimethylamine 96-h LC50 > 100 (1970b)
Indian toad (tadpole) 25 210 220 8.3 free acid 24-h LC50 13.77 Vardia et
(11.81-16.05) al. (1984)
(Bufo melanostictus) 25 210 220 8.3 free acid 48-h LC50 9.03 Vardia et
(8.23-9.91) al. (1984)
25 210 220 8.3 free acid 96-h LC50 8.05 Vardia et
(7.29-8.81) al. (1984)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 255 Johnson
(Adelotus brevis) stat 21-22 amine salt 48-h LC50 228 (1976)
stat 21-22 amine salt 96-h LC50 200 Johnson
(1976)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 321 Johnson
(Limnodynastes peroni) stat 21-22 amine salt 48-h LC50 300 (1976)
stat 21-22 amine salt 96-h LC50 287 Johnson
(1976)
Toad (tadpole) stat 21-22 amine salt 24-h LC50 346 Johnson
(Bufo marinus) stat 21-22 amine salt 48-h LC50 333 (1976)
stat 21-22 amine salt 96-h LC50 288 Johnson
(1976)
Common frog (tadpole) 17-29 free acid 48-h LC0 50 Cooke
(Rana temporaria) (1972)
-------------------------------------------------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
For terrestrial application, 2,4-D is usually used in the form of
the less volatile, longer-chain esters to reduce drift damage of sprays
to broad-leaved crop plants. The herbicide is used on cereal crops and
on rangeland against broad-leaved weeds and also in forestry. Thus,
insects of all kinds will be exposed to 2,4-D. Birds' eggs in the nest
are more likely to be exposed than the adult birds, though adult
exposure remains possible, particularly for sitting birds. Ground-
nesting species will be exposed from the use of 2,4-D on rangeland and
cereals; tree-nesting species will be exposed in forests.
The insecticidal action of 2,4-D is low enough that the compound
does not represent a hazard to beneficial insects; there is an adequate
safety margin with usage at recommended levels.
Although there is some disagreement in the literature about the
toxicity of 2,4-D to birds' eggs, the low uptake of the material
through the egg shell suggests that exposure would not affect hatching
in normal use of the compound. Adult birds are not affected by short-
term exposure to 2,4-D. The likelihood of prolonged exposure of
either adult birds or eggs to high levels of 2,4-D is small.
7.1. Toxicity to Terrestrial Invertebrates
Appraisal
Based on the widespread use of 2,4-D and its formulations, insects
of many kinds could be exposed to the material. Although the compounds
are generally classified as non-toxic for beneficial insects, such as
honey bees and natural enemies of pests, some adverse effects have been
reported on the early life-stages and adults of some insects.
Esters are less toxic to insects than are salts or the free acid.
Feeding studies dosing worker honey bees (Apis mellifera) with
2,4-D salts in sucrose syrup have generated two estimates of 24-h
LC50: 104 and 115 µg/bee (Jones & Connell, 1954; Beran & Neururer,
1955). Morton et al. (1972) fed 2,4-D acid to honey bees in 60%
sucrose syrup at 10, 100, or 1000 mg/litre and monitored half-time,
i.e., the time taken for 50% of the bees in a cage to die. The half-
time was significantly longer than that of controls for the two lowest
doses (37.2 days at 10 mg/litre and 40.4 days at 100 mg/litre,
compared to a control value of 33.4 days), but was significantly
reduced at 1000 mg/litre (18.6 days). The butoxyethanol and isooctyl
(commercial formulation) esters of 2,4-D had no effect on survival
times at 10, 100, or 1000 mg/litre of syrup when fed under the same
conditions as the acid. The dimethylamine salt of 2,4-D (commercial
formulation) had no effect at 10 or 100 mg/litre, but did shorten the
half-time at 1000 mg/litre.
The effects of 2,4-D on beneficial coccinellid larvae were studied
by Adams (1960). Larvae were sprayed with a preparation of mixed amine
salts of 2,4-D, at a rate equivalent to 0.56 kg acid equivalent/ha, at
different stages of their development 1, 3, 6, 9, or 12 days after
hatching. There was a lengthening of the development period when the
larvae were treated on days 3, 6, 9, or 12 but no effect when they were
sprayed on the first day after hatching. Mortality before pupation was
more than doubled in all treated groups, but mortality during pupation
was not different from that of controls.
Trumble & Kok (1980) dosed adult thistle-rosette weevils
(Ceuthorhynchidius horridus), which are used for the biological
control of musk thistle, with 2,4-D amine salt at five dose levels
between 0.17 and 147.8 kg/ha. No significant mortality was
observed, up to 175 days after treatment, at doses up to and including
1.68 kg/ha. At 16.8 and 84 kg/ha, there was significantly increased
mortality after day 3 post-treatment. At the highest dose level of
147.8 kg/ha, mortality was increased both on day 3 and subsequently.
Five-day LC50 values for males and females were calculated at 70.2 and
61.4 kg/ha, respectively. This is 41.8 times the recommended
application rate of 2,4-D for males and 36.6 for females. Riviere
(1976) reared the European cockroach (Blatella germanica) on food
containing 1000 mg/kg and reported negligible effects on reproduction.
Gall & Dogger (1967) wetted wheat plants with a 0.3% solution of
mixed isopropyl and butyl esters of 2,4-D and exposed the plants to
females of the wheat stem sawfly (Cephus cinctus). Spraying of the
wheat plants was performed at different times relative to oviposition;
times were 7 days prior to oviposition, at the time of oviposition, or
7, 14, or 21 days after oviposition. Eggs took about 7 days to hatch.
The highest larval mortality (96.4%) occurred after spraying at the
time of egg laying. The effectiveness of the 2,4-D in killing larvae
decreased with later exposure times. For plants sprayed 7, 14, and 21
days after oviposition, larval mortalities were 68.1%, 60.8%, and 37%,
respectively, compared to a control mortality of 30%. When plants were
sprayed 7 days before egg laying, larval mortality was 46.9%. Adult
flies were not affected by 2,4-D spray.
Muller (1971) exposed beetles (Carabidae) to sand dose d with
2,4-D at 0.2, 1.0, or 2.0 g/m2. Two species, Bembidion
femoratum and B. ustulatum, both showed more than 50% mortality within
4 days of exposure to 1.0 g 2,4-D/m2. B. ustulatum showed 100%
mortality within 10 days when exposed to 1.0 g/m2 and similar
mortality within 4 days when exposed to 2.0 g/m2. About 20% of the
individuals of B. femoratum survived the 14-day exposure to both 1.0
and 2.0 g/m2.
Roberts & Dorough (1984) exposed earthworms to 2,4-D acid sprayed
on to filter paper. The papers were wetted and the earthworms exposed
to the wetted paper in glass vials. The calculated 48-h LC50 value
was 61.6 (41 - 92.4; 95% confidence limits) µg/cm2.
Rapoport & Cangioli (1963) treated turf with a mixture of
(4-chloro-2-methylphenoxy)acetic acid (MCPA), at recommended rates, and
the butyl ester of 2,4-D, at 10 times the recommended rate. They
reported no effect on soil microarthropods.
7.2. Toxicity to Birds
Appraisal
Birds, and particularly the eggs of ground-nesting species, would
be exposed to 2,4-D after spraying. Food items could also be expected
to be contaminated by the herbicide. However, most studies on birds
and their eggs have been conducted at exposures far higher than could
be expected in the field.
LD50 values from acute oral and from short-term dietary dosing
indicate low toxicity of 2,4-D to birds. In longer-term studies,
effects have only been reported at extremely high exposures (for
example, kidney effects after dosing in drinking water with
concentrations in excess of the solubility of the material). There
have been no reported effects on reproductive parameters, even at
excessive exposure levels.
A single study reported adverse effects on the embryos of birds'
eggs sprayed with 2,4-D. Many studies since have shown no effect on
hatchability of eggs and no increased incidence of abnormalities in
chicks even after very high exposure to 2,4-D. Other work indicates a
very poor penetration of the eggshell by the herbicide. It can only be
concluded that after normal, or even after excessive, 2,4-D use, there
would be no effect on birds' eggs.
7.2.1. Toxicity to birds' eggs
There have been several studies on the toxicity of various 2,4-D
formulations to birds' eggs dosed by different routes.
Spraying eggs of pheasant, red-legged partridge, and grey partridge
with the equivalent of 0.55 to 1.1 kg 2,4-D/ha, either before
incubation or after 3 days of incubation, was found by Lutz-Ostertag &
Lutz (1970) and Lutz & Lutz-Ostertag (1972) to cause embryonic
abnormalities. They reported 77% mortality in pheasant eggs, 77% in
grey partridge eggs, and 43% in red-legged partridge eggs within the
first 19 days of incubation. The eggs were broken open, between days
20 and 22 of incubation for histopathological examination of the
embryos; hatching takes place at about 24 days in all species used. A
majority of surviving embryos were either wholly or partially
paralyzed. Histopathological effects were mainly gonadal. In both
male and female embryos, there were abnormalities of the gonad, often
severe enough to lead to sterility, and, in the male, abnormal
regression of the Mullerian ducts. No control embryos were examined.
In a second study by Lutz & Lutz-Ostertag (1973), quail, pheasant, and
partridge eggs were sprayed with 2,4-D from two different commercial
sources either before incubation or on days 3 or 7 after the start of
incubation. The authors reported increased mortality in embryos,
reduced hatchability, and increased abnormality in chicks. The only
control eggs reported were those in a single incubation of quail.
Later work has failed to repeat these results.
Kopischke (1972) found no adverse effects on hatchability and no
increase in deformities or later mortality of hatched chicks after
spraying eggs of pheasants or chickens with an isooctyl ester
formulation of 2,4-D on day 13 of incubation at a dose equivalent to
0.28 kg/ha.
Somers et al. (1974) sprayed chicken eggs, prior to incubation,
with concentrations of an amine salt of 2,4-D at up to 15 times the
recommended field application rate of approximately 3 kg/ha. There was
no effect on hatching success or on the survival of chicks in the
period 3 to 4 weeks post hatch. Spraying chicken eggs on days 0, 4, or
18 of incubation with 2,4-D (as a PGBE ester formulation) at up to 10
times the field application rate, had no effect on hatchability or on
survival and growth of chicks after hatching (Somers et al., 1978a).
Birds hatched from eggs, similarly treated by spraying, showed no
significant adverse effects on later reproductive performance (egg
laying performance of the females; testis weight or sperm count of
males) (Somers et al., 1978b). Hilbig et al. (1976a) found no effect
on egg hatch rate or on body weight or malformation rate in chicks,
after spraying (at 20 kg/ha) the eggs of Japanese quail, pheasants, and
chickens prior to incubation, or 3 days after the start of incubation.
In a follow-up study on the reproductive performance of birds hatched
from these dosed eggs, Hilbig et al. (1976b) reported no effects on
laying capacity, fertility, or hatchability of their eggs.
The effects of 2,4-D dimethylamine salt on the eggs of Japanese
quail, grey partridge, and red-legged partridge were studied by
Grolleau et al. (1974). Eggs were sprayed with 2,4-D at dose levels
equivalent to the recommended application rate (1.2 kg/ha) and at two
higher dose levels equivalent to 2.4 and 6 kg/ha. There were no
effects on hatching rate, embryonic mortality, or chick mortality in
the first month after hatching or on embryonic or chick malformations.
In addition, the histopathological examination of partridge thyroids
revealed no effects. Residues of 2,4-D were measured in those
partridge eggs receiving the highest dose. Very little 2,4-D
penetrated the egg shell and the highest residue measured was a total
egg content of 19.3 µg (in an 11-g egg, 15 days after treatment). The
lack of effect of 2,4-D on sprayed eggs was attributed to the poor
penetration of the herbicide. Spittler (1976) found no adverse
effects of 2,4-D on hatchability and no increase in chick abnormalities
in pheasant or quail eggs sprayed 24 h before hatching with a dose 12
times higher than the recommended application rate. Only at a dose 30
times higher than the recommended rate did hatchability fall by 10% to
15%, relative to controls. No increased incidence of abnormalities was
reported at this dose rate.
Hoffman & Albers (1984) immersed mallard eggs for 30 seconds in
aqueous emulsions of 2,4-D and calculated an LC50 equivalent to a
field application rate of 216 (155 - 300) kg/ha. This is 32 times the
recommended field application rate. Dunachie & Fletcher (1967)
injected chicken eggs with 10, 100, or 200 mg 2,4-D/kg, equivalent to
0.5, 5, or 10 mg/egg, and found reduced hatchability relative to
control eggs injected with solvent only. Treated eggs showed 80-90%,
70%, and 50% of the control hatch rate for the three dose rates,
respectively. In a similar study, Gyrd-Hansen & Dalgaard-Mikkelsen
(1974) found that injecting 1 mg/egg or less of the dimethylamine salt
of 2,4-D had no effect. Injections of 2 mg/egg reduced both
hatchability of the eggs and survival of hatched chicks. An injected
dose of 5 mg/egg reduced the hatching rate to 15% of control levels and
there were no surviving chicks after 1 week. There was no successful
hatching after an injection of 10 mg/egg. The same authors also dosed
eggs by immersion in solutions of 2,4-D for 10 seconds. There was no
effect after immersion in a solution of 10 g/litre and only a slight
effect after immersion in 50 g/litre. The hatching success and
survival of the chicks up to 4 weeks post hatch, after immersion in
50 g/litre, was more than 80% of control values.
7.2.2. Toxicity to birds after short-term and long-term dosing
The toxicity of 2,4-D (given either orally by capsule or in the
diet) to birds is summarized in Table 11. The studies reported in this
Table include single oral dosing, repeated oral dosing, and dietary
tests over 5 to 100 days. Studies lasting 10 days or less show that
high dosage (in excess of 1000 mg/kg food) is required to kill birds.
2,4-D is, therefore, of low toxicity to birds.
Haegele & Tucker (1974) dosed egg-laying Japanese quail and mallard
a single oral dose of 250 or 1500 mg 2,4-D acid/kg body weight and
monitored egg shell thickness. There was a short-term effect; thin-
shelled eggs were produced during the first 3 days after dosing. This
was considered to be an indirect effect, i.e., the result of reduced
food consumption. When Bjorklund & Erne (1966) gave single oral doses
of 100, 200, or 300 mg 2,4-D amine/kg body weight to chickens, all
clinical and gross pathological findings were negative, with the
exception of a single bird showing gastritis after the highest dose.
2,4-D amine was given orally at 300 mg/kg body weight to a second group
of chickens each day. One bird died after 5 days and was shown on
autopsy to have developed renal and visceral gout. The other birds
were killed on days 12 or 24 of dosing. Slight kidney enlargement was
seen, and there was an enhancement of the rate of 2,4-D elimination
with time.
Bjorn & Northen (1948) orally dosed white-rock chicks on alternate
days for a period of 4 weeks (12 doses in total) with an alkanolamine
salt formulation of 2,4-D. All chicks weighed approximately 50 g at
the beginning of dosing and doses were adjusted for weight gain of the
chicks through the dosing period. No effect on weight gain was noted
at doses of 2,4-D up to 280 mg acid equivalent/kg body weight. In a
further study, single oral doses of up to 380 mg/kg body weight were
without effect, but a single oral dose of 765 mg/kg body weight killed
all the birds.
Whitehead & Pettigrew (1972a) dosed 28-week-old laying hens daily,
by gelatin capsule, with the butoxyethyl ester of 2,4-D at either 6.2
or 18.7 mg acid equivalent/bird for 20 weeks. There were no adverse
effects on egg production, egg or yolk weight, egg shell thickness,
hatchability, or growth rate of the progeny.
Chickens were given oral doses of 2,4-D amine salt at 100, 250, or
500 mg/kg body weight or PGBE ester at 50, 100, or 250 mg/kg body
weight for 10 days in a study by Palmer & Radeleff (1969). Birds given
2,4-D amine salt did not differ from controls in weight gain, even at
the highest dose. There was similarly no effect from the lowest dose
of the ester. However, a growth rate reduction was seen with the
medium dose of the ester (only 19% weight gain relative to a control
weight gain of 41%), and at the highest dose, there was complete
mortality within 4 days, associated with a weight loss of 13%. In a
comparable study, Palmer (1972) dosed chickens with 2,4-D
dimethylamine salt at 25 to 500 mg/kg body weight for 10 consecutive
days. There were effects on weight gain at doses of 100 mg/kg or more.
At 100 mg/kg, the weight gain was 38%, compared to a control value of
57%. At 175, 250, and 375 mg/kg, the weight gain was 30%, similar to
the control value. At the highest dose, three out of five treated
birds died, and the survivors showed a weight gain of 26%. There was
no effect of 2,4-D ethylhexyl ester at 100 mg/kg on weight gain, but at
250 and 500 mg/kg, weight gain was 42% and 36%, respectively, compared
to a control value of 59%.
Solomon et al. (1973) studied the effects of 2,4-D acid and two
unspecified amine salt formulations of 2,4-D. Pheasants were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing one of
the preparations at either 75 or 150 mg/bird. No effects were observed
on fertility and there was no increase in the number of abnormal
embryos.
Table 11. Toxicity of 2,4-D to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mallard duck M 4 months oral acid (technical) acute LD50 > 2000 Hudson et
(Anas platyrhynchos) M 3-5 months oral sodium salt acute LD50 > 2050 al. (1984)
M 7 months oral amine salt acute LD50 < 2000 Hudson et
F 3-5 months oral acid (technical) acute LD50 > 1000 al. (1984)
23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et al.
17 days diet dimethylamine 5-day LC50 > 5000 c (1975)
young diet acetamide 100-day LC50 > 500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 2500 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 > 5000 DeWitt et
al. (1963)
Japanese quail M 2 months oral acid (technical) acute LD50 668 Hudson et
(530-842) al. (1984)
(Coturnix coturnix 14 days diet acetamide 5-day LC50 > 5000 c Hill et
(japonica) 12 days diet butoxyethanol 5-day LC50 > 5000 c al. (1975)
20 days diet dimethylamine 5-day LC50 > 5000 c Hill et
al. (1975)
Bobwhite quail 23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et
(Colinus virginianus) 23 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 2500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 10-day LC50 5000 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
Pheasant F 3-4 months oral acid (technical) acute LD50 472 Hudson et
(340-654) al. (1984)
(Phasianus colchicus) 10 days diet butoxyethanol 5-day LC50 > 5000 Hill et
10 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 1000 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 5000 DeWitt et
adult diet dimethylamine 100-day LC50 > 5000 al. (1963)
young diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
10 days diet butoxyethanol 5-day LC17 5000 Hill et al.
(1975)
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Chukar partridge M,F 4 months oral acid (technical) acute LD50 200-400 Hudson et
(Alectoris chukar) al. (1984)
Rock dove M,F oral acid (technical) acute LD50 668 Hudson et
(Columba livia) (530-842) al. (1984)
Chicken M,F 21 days oral free acid 14-day LD50 541 Rowe & Hymas
(358-817) (1954)
M,F 21 days oral isopropyl 14-day LD50 1420 Rowe & Hymas
(1127-1789) (1954)
M,F 21 days oral mixed butyl 14-day LD50 2000 Rowe & Hymas
esters (1350-2960) (1954)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b Acute oral doses are given as mg/kg body weight; all other doses are as mg/kg diet.
c Dose level of 5000 mg/kg diet produced no mortality.
Whitehead & Pettigrew (1972b) fed day-old chicken chicks with the
butoxyethanol ester of 2,4-D at concentrations up to 7500 mg/kg diet
for 3 weeks. Dietary levels up to 1000 mg/kg had no adverse effect,
but at 2000 mg/kg diet 2,4-D ester reduced the food consumption and
growth rate of the chicks. Although there was no mortality at the
higher doses, necropsy of birds sacrificed at the end of the experiment
showed swollen kidneys in all birds and some mottling of the spleen.
Bjorklund & Erne (1966) fed chickens with the amine salt of 2,4-D at
500 mg/kg diet. One bird died of renal gout after 5 months dosing;
autopsy showed hypoplasia (possibly congenital) of the right kidney and
hyperplasia of the left kidney. Other birds were killed at 1, 2, 9,
or 18 months after dosing began, but there was no consistent pattern to
autopsy findings.
Erne & Bjorklund (1970) examined the long-term effects of
phenoxyherbicides on chickens. Groups of day-old broiler chicks were
given 2,4-D in the drinking water at 1000 mg/litre for up to 7 months.
During the dosing period, chickens were sacrificed at regular intervals
for autopsy and samples were prepared for electron microscopy. There
was decreased food and water intake in dosed birds. The most
pronounced effect was on the kidney; there was noticeable kidney
enlargement after 14 days of dosing and this increased with time.
2,4-D concentrations in body tissues reached a plateau after 7 days,
with the highest residue in kidney tissue. Histologically, the kidney
enlargement was shown to be due to hypertrophy of proximal tubule
epithelium. These hypertrophied cells, under the electron microscope,
were shown to display an increased mitochondrial content and
pronounced mitochondrial pleomorphy. The number of microbodies was
also increased and nuclear bodies were observed. These findings were
stated to reflect alterations in intermediate metabolism in the
tubular cells. In an earlier study, using chicks dosed similarly with
1000 mg/litre of drinking water (Bjorklund & Erne, 1966), the birds
were followed through to sexual maturity. No significant effects were
observed on weight gain, age at sexual maturity, or onset of egg
production, but the number of eggs laid was reduced during the first 2
months of egg laying. The number of birds dying during the course of
the study did not differ from the control value. Surviving birds were
killed and autopsied at intervals of between 2 and 18 months after the
onset of egg laying. The primary effect was consistent enlargement of
the kidneys.
7.2.3. Special studies on birds
Lundholm & Mathson (1983) studied the effect of 2,4-D on the ATP-
dependent Ca2+ binding of the particulate fraction of egg shell
gland mucosa cells from egg-laying hens. This parameter had been
found to be a sensitive indicator of potential shell-thinning effects
of chemicals. They calculated a 5-min IC50, for Ca binding
inhibition, of 30.7 x 10-8 mmol 2,4-D/litre incubation medium.
This makes 2,4-D 13.5 times less effective in this respect than
1,1'-(2,2-dichlorethenylidine)-bis[4-chlorobenzene] ( p-p' -DDE), the
major agent causing eggshell-thinning in birds.
Percutaneous absorption of 2,4-D through the feet of red-winged
blackbirds was measured by Rogers et al. (1974). A 24-h exposure to
14C-labelled 2,4-D at 0.01 mmol/litre resulted in a blood
concentration of 1.24 x 10-3 mmol 2,4-D/litre.
7.3. Toxicity to Non-laboratory Mammals
Appraisal
Based on the available data, no generalization can be made about
the hazard of 2,4-D to mammals in the field. Data on voles indicate
that the herbicide poses no hazard.
Cholakis et al. (1982) obtained acute oral LD50 estimates for
two species of voles by determining mortality 14 days after the
administration of a single dose of 2,4-D acid. Values were 2110
(1800 - 2570) and 2100 (1900 - 2390) mg/kg body weight for males
and females, respectively, of the prairie vole (Microtus
orchrogaster). Values for the grey-tailed vole (Microtus canicaudus)
were 1200 (955 - 1150) for males and 1310 (1010 - 1790) mg/kg body
weight for females.
Skokova (1975) orally dosed 24 male bank voles with 400 to
405 mg/kg body weight (10% of the LD50) daily for 10 or 20 days and
examined reproductive parameters. Testis weight, an index of
spermatogenesis, and divisions in spermatogonia were all significantly
reduced relative to control values. Gile (1983) applied a foliar spray
of butyl ester of 2,4-D to a simulated ryegrass ecosystem at 1 kg/ha.
The system included voles, which showed a weight loss after exposure to
2,4-D when compared to similar animals in an untreated system. This
loss was considered to be the result of protein deficiency.
8. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
Appraisal
No direct toxic effects, acute or long-term, of 2,4-D applications
under field conditions on any animals species have been observed thus
far.
There are, inevitably, indirect effects resulting from the intended
selective herbicidal properties of the compound. These effects would
result from the use of any herbicide or from other methods of land
management. There will, therefore, be effects for mammals, birds, and
insects because of food deprivation, modification of habitat,
requirements for nesting, shelter, etc.
The application of 2,4-D appears to present no hazard to the
beneficial epigeal arthropod community. Physical cultivation present a
greater hazard to sensitive soil arthropods than the use of 2,4-D
herbicides.
Oka & Pimental (1976) observed increased numbers of insect pests
and increased occurrence of blight infection in maize (Zea mays) crops
treated with 2,4-D as the triethanolamine salt. The crops had been
treated with 2,4-D at 0.14, 0.55, or 4.4 kg/ha; 0.55 kg/ha is the
normal rate of application for this crop. The number of aphids
increased from 1420 on control untreated plants to 2449 on plants
treated at 0.14 kg/ha, 3116 on plants treated at 0.55 kg/ha, and 2023
on plants treated at 4.4 kg/ha. The percentage of plants attacked by
the European corn borer (Ostrinia nubialis) increased from 63% on
controls to 83% and 70% for treatments at 0.14 and 0.55 kg/ha,
respectively. Controlled studies also showed an increase in infection
with fungal blight. Laboratory investigation of these effects
confirmed that the treated maize had higher protein levels than the
untreated. This was thought to be the reason for the increased success
of the pests.
Everts et al. (1986) investigated the effects of various pesticides
on soil arthropods. Spiders were found to be a sensitive indicator of
effect. No side-effects of the use of 2,4-D amine were observed on
these organisms. Lahr et al. (1987) showed that spider numbers were
reduced by ploughing but not by the use of 2,4-D herbicides.
Matida et al. (1975) examined aquatic organisms in a stream running
through a mountainous area of 9.4 ha, in Shizuoka prefecture in Japan,
which had been aerially sprayed with a mixture of 2,4-D and 2,4,5-T at
a rate of 150 kg/ha. There was no effect on the number or species
diversity of aquatic invertebrates. Caged cherry salmon and dace
fingerlings showed no mortality, abnormal behaviour, or pathological
change after spraying. An extensive ecological survey of an area in
Florida treated with military mixtures of herbicides, including 2,4-D,
revealed no major change in species diversity or population size for
aquatic invertebrates, fish, a lizard, or the beach mouse (Young et
al., 1975).
The weevil Rhinocyllus conicus is used in the biological control of
musk thistle, an invasive weed. In an investigation of the
practicality of combining biological with chemical control, Lee & Evans
(1980) investigated the toxicity to the weevil of 2,4-D. They sprayed
musk thistle with 2,4-D at a rate of 4.48 kg/ha. One week later, the
terminal seed heads of the thistle were covered with cloth bags to
contain the weevils. The number of dead larvae, pupae, and adults
were counted and compared to the number on unsprayed, control thistle
heads. No significant differences were observed.
Dwernychuk & Boag (1973) studied the effect of herbicide spraying
on several species of nesting ducks (lesser scaup, gadwall, white-
winged scoter, mallard, pintail, and American wigeon) in Canada. An
ester of 2,4-D was applied to two islands. This application
significantly reduced the areas dominated by broad-leaved plants and
permitted invasion of these areas by grasses. Ducks preferred to nest
amongst broad-leaved vegetation and avoided grass. As the areas of
broad-leaved plants disappeared, there was an increase in nest density
in those broad-leaved areas still present. Total numbers of nesting
ducks declined over the 3-year study period. This decline was
attributed, by the authors, entirely to the effect of the herbicide on
vegetation type.
Keith et al. (1959) studied the effects on populations of the
pocket gopher (Thomomys talpoides) of spraying weedy rangeland in
Colorado with 2,4-D, as the butyl ester, at 3.4 kg/ha. Numbers of
gophers were estimated using two different methods, either by trapping
or by counting numbers of newly excavated mounds. Both methods showed
a highly significant difference between sprayed and non-sprayed areas
1 year after spraying. The total numbers of gophers trapped in the two
areas before 2,4-D application were 101 and 110, respectively. In the
same two areas, 1 year after spraying, numbers were 117 (untreated
area) and 15 (treated area), respectively. This represents a fall in
gopher numbers of 87% in the treated area and a slight increase in
numbers in the unsprayed area. Newly excavated mounds in the treated
area were only 28% as numerous as in control areas. Spraying had
reduced production of forbs (broad-leaved plants) from 445 kg/ha
before spraying to 75 kg/ha afterwards, a reduction of 83%. Grass
production had increased by 37%. The overall reduction in vegetation
was 232 kg/ha or 35% on sprayed plots.
In a similar study by Tietjen et al. (1967), 2,4-D butyl ester
applied at 3.4 kg/ha to identical high-altitude rangeland initially
reduced forb density and gopher populations. Pocket gopher numbers
were reduced by between 80% and 90%. Both forbs and gopher populations
remained low in one treated area but not in a second one. The decline
in gopher numbers was considered by the authors to be a result of food
deficiency; the grasses available represented a marginal diet for the
animals. This decline was not due either to movement of animals out of
the area or to the direct toxic effects of the herbicide. Reduction in
numbers was, therefore, primarily a result of reduced breeding success.
Johnson & Hansen (1969) studied the after-effects on wild mammals
of treating perennial forb and shrub/grass ranges with 2,4-D either
aerially or from a ground rig at rates of 2.2 or 3.4 kg/ha using a
diesel-oil carrier. The density and litter size of the deer mouse
(Peromyscus maniculatus) was little affected by the treatment, but the
densities of northern pocket gophers (Thomomys talpoides) and least
chipmunks (Eutamias minimus) were reduced. Montane voles (Microtus
montanus) increased their abundance in treated perennial forb range.
Gopher and vole populations returned to normal with the re-
establishment of forb dominance. Density changes were considered by
the authors to be primarily due to changes in availability of food for
the gophers, availability of both food and cover for chipmunks, and
availability of a close-canopied grass cover for the voles. There were
no direct toxic effects of the herbicide.
Fagerstone et al. (1977) observed two colonies of black-tailed
prairie dogs (Cynomys ludovicianus) in North America and the effect of
spraying their feeding areas with 2,4-D. The herbicide was applied
initially as the dimethylamine salt, followed by two further sprays of
the butyl ester after 1 month and 1 year. All applications of 2,4-D
were at 2.2 kg/ha. One colony lived in a sprayed area, initially rich
in broad-leaved plants. The second colony lived in an unsprayed area
poor in dicotyledonous plants and rich in grass. The effect of
spraying was, therefore, to make the treated area similar to the
control area with less broad-leaved herbage and less cover. Prior to
treatment, the first colony preferentially ate the forbs; diet was 73%
forbs and 5% grass. After spraying, they ate 9% forbs and 82% grass.
The control colony ate a similar diet to the first colony post-
treatment, i.e., mostly grass. Prairie dogs remained in the same area
after treatment with herbicide and there was no evidence of starvation.
Body weight was maintained, activity was comparable to the pre-
treatment level, and reproduction was unaffected.
Spencer & Barrett (1980) monitored the population response of
meadow voles (Microtus pennsylvanicus) to application of 2,4-D as the
N-oleyl 1,3 propylenediamine salt at 567.5 g/ha. Two 0.4-ha plots
were compared, one sprayed and the other untreated. The population
fluctuation of the voles was monitored for 6 months, from June to
December, a period spanning the breeding season. The control area
reached a population peak of 116 animals on 6 November; a peak of 68
voles was reached on 9 October in the treated area. There was a skewed
sex ratio in the treated area, mainly because of a reduced survival
rate in females. Voles in the treated plot were protein-deficient,
compared to controls
9. EVALUATION
In evaluating the environmental hazards of 2,4-D, the following
general points should be borne in mind:
(a) the chlorinated dibenzo- p -dioxins (CDDs) are present in
2,4-D only in trace amounts which are difficult to separate and
identify;
(b) 2,4-D is rapidly degraded in the environment;
(c) the environmental effects are indirect, and are the
consequence of vegetation diversity being modified; care should be
taken to avoid unintentional vegetation damage; the mode of
application and the formulation should be carefully selected;
esters should be avoided in aquatic applications (because of
toxicity to aquatic organisms);
(d) there are limited data on the effects of 2,4-D and its
formulations on communities of organisms; hazard assessment is,
therefore, often by extrapolation from single species studies;
(e) minor adverse effects shown in laboratory studies on
terrestrial organisms have resulted from exposures far in excess of
likely exposures in the field.
9.1. Aquatic Organisms
Sources of exposure of aquatic ecosystems to 2,4-D include direct
application, run-off, and spray drift. Because of low adsorption rate
and rapid degradation, the herbicide is not accumulated in compartments
of the aquatic system.
2,4-D acid and its salts are less toxic to aquatic organisms than
are the esters.
2,4-D acid and its salts are of low to moderate toxicity to aquatic
organisms. However, the growth and nitrogen fixation of some
cyanobacteria (blue-green algae) are inhibited, but only at
concentrations above levels expected from direct application of the
herbicide to water. These microorganisms are the source of most
nitrogen in wet tropical soils. This inhibitory effect could be of
concern when these compounds are applied to rice fields at a high
dosage.
Because of the toxicity of esters, particularly the propylene
glycol butyl ether ester, for early life-stages of several fish
species, they should be regarded as hazardous to aquatic ecosystems.
9.2. Terrestrial Organisms
2,4-D does not persist in soil and other compartments of the
terrestrial environment.
Nitrogen-fixing microorganisms appear to be particularly sensitive
to 2,4-D; this might be especially important in tropical soils.
Some terrestrial invertebrates have shown adverse effects, but only
at high exposure levels. Therefore, 2,4-D does not constitute a hazard
to this group of organisms.
2,4-D has low acute toxicity to birds, as indicated by the
LD50. Most studies on birds and their eggs have been conducted at
exposures exceeding those that could be expected in the field; even
under these conditions, no significant adverse effects have been
observed.
Under field conditions, 2,4-D does not cause direct toxic effects
on animals. However, the change of species composition and structure
of the vegetation, resulting from the use of this herbicide, leads to
indirect effects on terrestrial ecosystems. This indirect effect would
also result from the use of any herbicide or from other methods of land
management in either temperate or tropical regions.
* * *
There is evidence only for minor effects on the environment arising
from the use of 2,4-D, as long as the following simple recommendations
are followed:
(a) amine formulations, rather than esters, should be used to
control aquatic weeds;
(b) accidental spread of the herbicide to other vegetation should
be avoided;
(c) the margins of agricultural land should be left untreated with
herbicide to avoid even the indirect effects of the material on
wildlife.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
There are indications that 2,4-D affects nitrogen fixation by
algae. Since this is the major source of nitrogen in tropical soils,
it is recommended that this should be further investigated,
particularly with reference to varying soil conditions. This should be
extended to a study on the functioning of a rice-paddy at the ecosystem
level.
REFERENCES
ADAMS, B.J. (1960) Effects of spraying 2,4-D amine on Coccinellid
larvae. Can. J. Zool., 38: 285-288.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous substances.
Int. Pest Control, 11: 29-35.
AUDUS, L.J. (1960) Microbiological breakdown of herbicides in soils.
In: Woodford, E.K. & Sagar, G.R., ed. Herbicides and soil, Oxford
Blackwell Scientific Publications, pp. 1-19.
AUDUS, L.J. (1964) Herbicide behaviour in the soil. In: Audus, L.J.,
ed. The physiology and biochemistry of herbicides, London, New York,
Academic Press, pp. 163-206.
BAILEY, H.C. & LIU, D.H.W. (1980) Lumbriculus variegatus, a benthic
oligochaete, as a bioassay organism. In: Eaton, J.C., Parish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
for Testing and Materials, pp. 205-215 (Special Technical Publication
707).
BEDNARZ, T. (1981) The effect of 2,4-D acid on green and blue-
green algae in unialgal and mixed cultures. Acta hydrobiol., 23: 173-
182.
BENEDECZKY, I., BIRO, P., & SCHAFF, Z. (1984) The effect of
2,4-D-containing herbicide (Dikonirt) on the ultrastructure of carp
(Cyprinus carpio) liver cells. Acta biol. (Szeged), 30: 107-125.
BERAN, F. & NEURURER, J. (1955) [Effect of plant protection
materials on the honeybee. I. Toxicity to bees.]
Pflanzenschutzberichte, 15: 97-147 (in German).
BIRGE, W.J., BLACK, J.A., & BRUSER, D.M. (1979) Toxicity of organic
chemicals to embryo-larval stages of fish. Washington, DC, US
Environmental Protection Agency (EPA 560-1179-007).
BIRO, P. (1979) Acute effects of the sodium salt of 2,4-D on the
early developmental stages of bleak, Alburnus alburnus. J. Fish Biol.,
14: 101-109.
BJORKLUND, N-E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. Scand., 7: 364-390.
BJORN, M.K. & NORTHEN, H.T. (1948) Effects of 2,4-
dichlorophenoxyacetic acid on chicks. Science, 108: 479-480.
BUNTING, D.L. & ROBERTSON, E.B. (1975) Lethal and sublethal effects
of herbicides on zooplankton species, University of Tennessee, Water
Resources Research Center, 34 pp. (Res. Report No. 43, NTIS Report No.
PB-241 337).
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975a) Loss of
five pesticides from cultures of twenty-one planktonic algae. Bull.
environ. Contam. Toxicol., 13: 149-152.
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975b) The effect
of atrazine, 2,4-D, methoxychlor, carbaryl and diazinon on the growth
of planktonic algae. Br. phycol. J., 10: 371-376.
BUTLER, P.A. (1963) A review of Fish and Wildlife Service
investigations during 1961 and 1962, Washington, DC, US Department of
the Interior, Fish and Wildlife Service, pp. 11-25 (Circular No. 167).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna. Proc.
South. Weed Conf., 18: 576-580.
CALDWELL, R.S. (1977) Biological effects of pesticides on the
Dungeness crab, Gulf Breeze, Florida, US Environmental Protection
Agency (EPA-600/3-77-131).
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON,
M.H., & MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D,
DEF, propanil and trifluarin to the Dungeness crab, Cancer magister.
Arch. environ. Contam. Toxicol., 8: 383-386.
CHAI, I.K. & CHUNG, Y.S. (1975) [Physiological effects of 2,4-
dichlorophenoxyacetic acid (2,4-D) on Chlorella ellipsoidea.]
Misaengmul Hakhoe Chi., 13: 101-108 (in Korean).
CHAMBERS, H., DZIUK, L.J., & WATKINS, J. (1977) Hydrolytic
activation and detoxification of 2,4-dichlorophenoxyacetic acid esters
in mosquito fish. Pestic. Biochem. Physiol., 7: 297-300.
CHEAH, M.L., AVAULT, J.W., & GRAVES, J.B. (1980) Acute toxicity
of selected rice pesticides to crayfish Procambarus clarkii. Prog. Fish
Cult., 42: 169-172.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1982)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avaian and Mammalian Wildlife
Toxicology: 2nd Conference, Philadelphia, American Society for Testing
and Materials, pp. 143-154 (Special Technical Publication 757).
COAKLEY, J.E., CAMPBELL, J.E., & MCFARREN, E.F. (1964)
Determination of butoxyethanol ester of 2,4-dichlorophenoxyacetic acid
in shellfish and fish. J. agric. food Chem., 12: 262-265.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99: 1-12.
CULLIMORE, D.R. & MCCANN, A.E. (1977) Influence of four
herbicides on the algal flora of a prairie soil. Plant Soil, 46: 499-
510.
DAS, B. & SINGH, P.K. (1977) The effect of 2,4-dichlorophenoxyacetic
acid on growth and nitrogen-fixation of blue-green alga Anabaenopsis
raciborskii. Arch. environ. Contam. Toxicol., 5: 437-445.
DAVIS, J.T. & HARDCASTLE, W.S. (1959) Biological assay of
herbicides for fish toxicity. Weeds, 7: 397-404.
DAVIS, P.W., FRIEDHOFF, J.M., & WEDERMEYER, G.A. (1972)
Organochlorine insecticide, herbicide and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8: 69-72.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1974) Effect of some
herbicides on soil microflora. J. Indian Soc. Soil Sci., 22: 300-303.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1975) Effect of some
herbicides on Azotobacter and non-symbiotic nitrogen fixation in the
soil. Indian J. Agric. Chem., 8: 95-98.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife
studies, Patuxent Wildlife Research Center. Pesticide-wildlife
studies. A review of Fish and Wildlife Service investigations during
1961 and 1962. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular No. 167).
DODSON, J.J. & MAYFIELD, C.I. (1979) The dynamics and
behavioural toxicology of Aqua-Kleen (2,4-D butoxyethanol ester) as
revealed by the modification of rheotropism in rainbow trout. Trans.
Am. Fish. Soc., 108: 632-640.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some
herbicides on the hatching rate of hen's eggs. Nature (Lond.), 215:
1406-1407.
DWERNYCHUK, L.W. & BOAG, D.A. (1973) Effect of
herbicide-induced changes in vegetation on nesting ducks. Can. field
Nat., 87: 155-165.
ELDER, J.H., LEMBI, C.A., & MORRE, D.J. (1970) Toxicity of 2,4-D
and picloram to fresh and saltwater algae. Proc. North-cent. Weed
Control Conf., 25: 96-98.
ELIASSON, L. (1973) Translocation and persistence of 2,4-D in
Populus tremula L. Weed Res., 13: 140-147.
ERNE, K. & BJORKLUND, N-E. (1970) Nephrotoxic effects of
phenoxy acetic herbicides. In: Proceedings of the 7th International
Congress on Plant Protection, Paris, France, pp. 768-769.
EVERTS, J.W., BEURSKENS, J.E.M., BOUWHUIS, J.S., BUYSE, A.D.,
HENGEVELD, R., WOUTERS, L., & KOEMAN, J.H. (1986) Terrestrial
arthropods as indicators for side-effects caused by insecticides in
arable farm systems in the Netherlands. In: Proceedings of the 1st
International TNO Conference on Contaminated Soil, Utrecht, The
Netherlands, November 1985, Dordrecht, Boston, Martinus Nijhoff, pp.
423-425.
FAGERSTONE, K.A., TIETJEN, H.P., & LAVOIE, G.K. (1977) Effects
of range treatment with 2,4-D on prairie dog diet. J. Range Manage.,
30: 57-60.
FINLAYSON, B.J. & VERRUE, K.M. (1985) Toxicities of
butoxyethanol ester and propylene glycol butyl ether ester formulations
of 2,4-dichlorophenoxy acetic acid (2,4-D) to juvenile salmonids.
Arch. environ. Contam. Toxicol., 14: 153-160.
FLINT, G.W., ALEXANDER, J.J., & FUNDERBURK, O.P. (1968)
Vapor pressures of low-volatile esters of 2,4-D. Weed Sci., 16: 541-
544.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry to
nine herbicides. Bull. environ. Contam. Toxicol., 15: 509-514.
FOLMAR, L.C. (1978) Avoidance chamber responses of mayfly nymphs
exposed to eight herbicides. Bull. environ. Contam. Toxicol., 19: 312-
318.
FOURNIER, J.C. (1980) Enumeration of the soil micro-organisms able
to degrade 2,4-D by metabolism or co-metabolism. Chemosphere, 9: 169-
174.
FREITAG, D., GEYER, H., KRAUS, A., VISWANATHAN, R., KOTZIAS,
D., ATTAR, A., KLEIN, W., & KORTE, F. (1982) Ecotoxicological profile
analysis. VII, Screening chemicals for their environmental behaviour
by comparative evaluation. Ecotoxicol. environ. Saf., 6: 60-81.
GALL, A. & DOGGER, J.R. (1967) Effect of 2,4-D on the wheat stem
sawfly. J. econ. Entomol., 60: 75-77.
GANGAWANE, L.V., SALER, R.S., & KULKARNI, L. (1980) Effect
of pesticides on growth and heterocyst formation in Nostoc sp.
Marathwada Univ. J. Sci., 19B: 3-6.
GEORGE, J.P., HINGORANI, H.G., & RAO, K.S. (1982) Herbicide
toxicity to fish-food organisms. Environ. Pollut., 28: 183-188.
GILE, J.D. (1983) 2,4-D - its distribution and effects in a ryegrass
ecosystem. J. environ. Qual., 12: 406-412.
GLYNN, P.W., HOWARD, L.S., CORCORAN, E., & FREAY, A.D.
(1984) The occurrence and toxicity of herbicides in reef-building
corals. Mar. Pollut. Bull., 15: 370-374.
GRAHAM-BRYCE, I.J. (1972) Herbicide movement and availability in
soils. Proc. Br. Weed Control Conf., 11: 1193-1202.
GROLLEAU, G., DE LAVAUR, E., & SIOU, G. (1974) Effets du
2,4-D sur la reproduction des cailles et des perdrix, aprčs application
du produit par pulvérisation sur les oeufs. Ann. Zool. Ecol. anim.,
6: 313-331.
GUARINO, A.M., JAMES, M.O., & BEND, J.R. (1977) Fate and
distribution of the herbicides 2,4-dichlorophenoxyacetic acid (2,4-D)
and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in the dogfish shark.
Xenobiotica, 7: 623-631.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The
effect of phenoxy-herbicides on the hatchability of eggs and the
viability of the chicks. Acta pharmacol. toxicol., 35: 300-308.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
coturnix. Bull. environ. Contam. Toxicol., 11: 98-102.
HAGENMAIER, H. (1986) Determination of 2,3,7,8-tetrachlorodibenzo-p-
dioxin in commercial chlorophenols and related products. Fresenius Z.
anal. Chem., 325: 603-606.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973)
Avoidance of pesticides by grass shrimp (Palaemonetes pugio). Bull.
environ. Contam. Toxicol., 9: 129-133.
HAQUE, A. & EBING, W. (1983) Uptake, accumulation, and
elimination of HCB and 2,4-D by the terrestrial slug, Deroceras
reticulatum (Muller). Bull. environ. Contam. Toxicol., 31: 727-733.
HASHIMOTO & NISHIUCHI (1978) quoted by Hidaka et al. (1984).
HAWXBY, K., TUBEA, B., OWNBY, J., & BASLER, E. (1977) Effects
of various classes of herbicides on four species of algae. Pestic.
Biochem. Physiol., 7: 203-209.
HIDAKA, H., HATTANDA, M., & TATSUKAWA, R. (1984)
[Avoidance of pesticides with medakas (Oryzias latipes).] Nippon
Nogeikagaku Kaishi, 58: 145-151 (in Japanese).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (First report).] Anz. schädlingskd. Pflanzenschutz Umweltschutz,
49: 21-25 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976b) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (Second report).] Anz. schädlingskd. Pflanzenschutz
Umweltschutz, 49: 65-68 (in German).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish Wildlife Service,
61 pp. (Special Science Report, Wildlife No. 191).
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized fish
eggs and fry. Trans. Am. Fish. Soc., 96: 414-416.
HILTIBRAN, R.C. (1975) Oxygen and phosphate metabolism of bluegill
liver mitochondria in the presence of some pesticides. Environ. Qual.
Saf. (Suppl), 3: 549-553.
HOEPPEL, R.E. & WESTERDAHL, H.E. (1983) Dissipation of 2,4-D
DMA and BEE from water, mud and fish at Lake Seminole, Georgia.
Water Resour. Bull., 19: 197-204.
HOFFMAN, D.J. & ALBERS, P.H. (1984) Evaluation of potential
embryotoxicity and teratogenicity of 42 herbicides, insecticides and
petroleum contaminants to mallard eggs. Arch. environ. Contam.
Toxicol., 13: 15-27.
HUBER, S.J., POSCHENREIDER, G., & WALLNOFER, P.R. (1980)
[Effect of pesticides and the corresponding metabolites on growth and
respiration of some soil microorganisms.] Z. Pflanzenkr.
Pflanzenschutz, 87: 533-545 (in German).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook
of toxicity of pesticides to wildlife, 2nd edition, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 90 (Resource
Publication 153).
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to bluegill
sunfish of phenoxy herbicides. Weeds, 11: 50-53.
JAMES, M.O. (1979) Disposition of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Panulirus argus).
Pharmacologist, 21: 251.
JANA, B.B. & DE, U.K. (1982) Stimulatory effect of herbicide 2,4-D
on the heterotrophic microbial community in the water of three fish
ponds. Acta microbiol. Acad. Sci. Hung., 29: 77-82.
JENSEN, D.A. & SCHALL, E.D. (1966) Determination of vapor
pressures of some phenoxyacetic herbicides by gas-liquid
chromatography. J. agric. food Chem., 14: 123-125.
JOHNSON, C.R. (1976) Herbicide toxicities in some Australian anurans
and the effect of subacute dosages on temperature tolerance. Zool. J.
Linnean Soc., 59: 79-83.
JOHNSON, C.R. (1978) Herbicide toxicities in the mosquito fish
Gambusia affinis. Proc. R. Soc. Queensland., 89: 25-27.
JOHNSON, D.R. & HANSEN, R.M. (1969) Effects of range treatment
with 2,4-D on rodent populations. J. wildl. Manage., 33: 125-132.
JONES, G.D.E. & CONNELL, J.U. (1954) Studies of the toxicity to
worker honey-bees (Apis mellifera L.) of certain chemicals used in
plant protection. Ann. appl. Biol., 41: 271-279.
KAPOOR, K. & SHARMA V.K. (1980) Effect of certain herbicides on
survival, growth and nitrogen fixation of blue-green alga Anabaena
doliolum Bharadwaja. Z. allg. Mikrobiol., 20: 465-469.
KEITH, J.O., HANSEN, R.M., & WARD, A.L. (1959) Effect of 2,4-D
on abundance and foods of pocket gophers. J. wildl. Manage., 23: 137-
145.
KOPISCHKE, E.D. (1972) Effect of 2,4-D and diesel fuel on egg
hatchability. J. wildl. Manage., 36: 1353-1356.
KOSCHIER, F.J. & PRITCHARD, J.B. (1980) Renal handling of 2,4-D
by dogfish shark (Squalus acanthias). Xenobiotica, 10: 1-6.
LAHR, J., GOEDEJOHAN, N., & EVERTS, J.W. (1987) Side effects of
pesticides on terrestrial arthropods. I. Long-term effects,
geographical variability and effects of cultural practices. In:
Proceedings of the Symposium ``25 Years After Silent Spring'',
November 1987, Amsterdam, Toxicological Society.
LE VAN TO (1984) [Degradation of defoliants 2,4-D and 2,4,5-T by
selected soil microorganisms.] Acta agrar. Silvestria, Ser. agrar.,
23: 225-234 (in Polish).
LEE, R.D. & EVANS, J.O. (1980) The influence of selected herbicides
on the development of Rhinocyllus conicus, an insect used in biocontrol
of musk thistle. Proc. West. Soc. Weed Sci., 33: 104-110.
LEMBI, C.A. & COLERIDGE, S.E. (1975) Selective toxicity of detergents
and herbicides to phytoplankton, West Lafayette, Indiana, Purdue
University, Water Resources Research Center, 71 pp. (Technical Report
No. 71).
LEWIS, D.L., HODSON, R.E., & FREEMAN, L.F. (1984) Effects of
microbial community interactions on transformation rates of xenobiotic
chemicals. Appl. environ. Microbiol., 48: 561-565.
LHOSTE, J. & ROTH, P. (1946) Sur l'action des solutions aqueuses de
2,4-dichlorophénoxyacétate de sodium sur l'évolution des oeufs de Rana
temporaria L. Soc. Biol., 140: 272-273.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides
on decomposition of organic matter and nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LINDEN, E., BENGTSSON, B-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LI-TSE OU (1984) 2,4-D degradation and 2,4-D degrading
microorganisms in soils. Soil Sci., 137: 100-107.
LIU, H.W. & LEE, M. (1975) Toxicity of selected pesticides to the bay
mussel Mytilus edulis, Washington, DC, US Department of Commerce,
National Technical Information Service (NTIS Report No. PB-243 221).
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel Dekker,
Inc., pp. 1-49.
LUNDHOLM, C.E. & MATHSON, K. (1983) The inhibitory effect of
some chlorinated hydrocarbon pesticides on the ATP-dependent Ca2+
binding of the particulate fraction of the eggshell gland mucosa
cells. Acta pharmacol. toxicol., 52: 390-394.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1972) The action of different
pesticides on the development of bird embryos. Adv. exp. Med. Biol.,
27: 127-150.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1973) Pesticides, tératogénčse et
survie chez les oiseaux. Arch. Anat. Histol. Embryol., 56: 65-78.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de
l'herbicide 2,4-D sur le développement embryonnaire et la fécondité du
gibier ŕ plumes. C. R. Acad. Sci. Paris, Ser. D, 271: 2418-2421.
MATIDA, Y., FURUTA, Y., KUMADA, H., TANAKA, H., YOKOTE,
M., & KIMURA, S. (1975) Effects of some herbicides applied in the
forest to the freshwater fishes and other aquatic organisms - I. Survey
on the effects of aerially applied sodium chlorate and a mixture of
2,4-D and 2,4,5-T on the stream community. Bull. freshwater Fish Res.
Lab. (Tokyo), 25: 41-53.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp. (NTIS Report No. PB-87 188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp. (Resource Publication 160).
MEEHAN, W.R., NORRIS, L.A., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in southeast Alaska. J. Fish
Res. Board Can., 31: 480-485.
METCALF, R.L. & SANBORN, J.R. (1975) Pesticides and
environmental quality in Illinois. Illinois nat. Hist. Surv. Bull.,
31: 381-435.
MORTON, H.L., MOFFETT, J.O., & MACDONALD, R.H. (1972)
Toxicity of herbicides to newly emerged honey bees. Environ.
Entomol., 1: 102-104.
MOUBASHER, A.H., EL-HISSY, F.T., & ABDEL-KADER, M.I.A.
(1981) Selective effects of three herbicides on the fungus flora of
Egyptian soil. Folia microbiol., 26: 37-44.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxyethanol
ester of 2,4-D. Trans. Am. Fish. Soc., 96: 185-193.
MUKHOPADHYAY, S.K. (1980) Effects of herbicides and insecticides
alone and their combinations on soil microflora. Indian J. Weed Sci.,
12: 53-60.
MULLER, G. (1971) [Laboratory studies on effects of herbicides on
Carabidae.] Arch. Pflanzenschutz, 7: 351-364 (in German).
NAGUIB, K., YOUNIS, A.E., & EL-ESSAWY, M.A. (1980) The effects
of 2,4-dichlorophenoxyacetic acid on respiration, nitrogen and
carbohydrate metabolism of Aspergillus terreus Thom. Zbl. Bakteriol.
II. Abt., 135: 252-259.
NESBITT, H.J. & WATSON, J.R. (1980) Degradation of the herbicide
2,4-D in river water. II. The role of suspended sediment, nutrients and
water temperature. Water Res., 14: 1689-1694.
NORRIS, L.A. & GREINER, D. (1967) The degradation of 2,4-D in
forest litter. Bull. environ. Contam. Toxicol., 2: 65-74.
O'CONNOR, G.A., FAIRBANKS, B.C., & DOYLE, E.A. (1981) Effects
of sewage sludge on phenoxy herbicide adsorption and degradation in
soils. J. environ. Qual., 10: 510-515.
OKA, I.N. & PIMENTAL, D. (1976) Herbicide (2,4-D) increases insect
and pathogen pests on corn. Science, 193: 239-240.
OMKAR & SHUKLA, G.S. (1984) Toxicity of the herbicide 2,4-D-Na
to two species of freshwater prawn of the genus Macrobranchium. Acta
hydrochim. hydrobiol., 12: 285-289.
PACHPANDE, R.R. & DAVID, S.B. (1980) Effect of selected herbicides
on the growth of soil alga Chlorococcum infusionum meneghini
(Schrank). Phykos, 19: 138-141.
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens, Washington, DC, US Department of Agriculture,
Agricultural Research Service, 41 pp. (Production Research Report No.
137).
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some organic
herbicides to cattle, sheep, and chickens. Washington, DC, US
Department of Agriculture, Agricultural Research Service, 26 pp.
(Production Research Report No. 106).
PLUMB, T.R., NORRIS, L.A., & MONTGOMERY, M.L. (1977)
Persistence of 2,4-D and 2,4,5-T in chaparral soil and
vegetation. Bull. environ. Contam. Toxicol., 17: 1-8.
PONS, R. & PUSSARD, M. (1980) Action des herbicides sur les amibes
libres (Rhizopoda, Protozoa). Etude préliminaire. Acta oecol. oegol.
appl., 1: 15-20.
POORMAN, A.E. (1973) Effects of pesticides on Euglena gracilis. I.
Growth studies. Bull. environ. Contam. Toxicol., 10: 25-28.
PRESCOT, L.M. & OLSON, D.L. (1972) Effects of pesticides on the
soil amoeba, Acanthamoeba castellanii. Proc. South Dakota Acad. Sci.,
51: 136-141.
PRESING, M. (1981) On the effects of Dikonirt (sodium salt of 2,4-
dichlorophenoxyacetic acid) on the mortality and reproduction of
Daphnia magna. Hydrobiologia, 83: 511-516.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder. J.
Pharmacol. exp. Ther., 208: 280-286.
RAATIKAINEN, M., SILTANEN, H., ROSENBERG, C., RAATIKAINEN,
T., & MUKULA, J. (1979) Herbicide residues in cowberries, bilberries
and lichens in controlled ground spraying experiments on woodland.
Ann. agric. Fenniae, 18: 112-116.
RADOSEVICH, S.R. & WINTERLIN, W.L. (1977) Persistence of 2,4-D
and 2,4,5-T in chaparral vegetation and soil. Weed Sci., 25: 423-425.
RAND, G.M. & BARTHALMUS, G.T. (1980) Use of an unsignalled
avoidance technique to evaluate the effects of the herbicide 2,4-
dichlorophenoxyacetic acid on goldfish, Philadelphia, American Society
for Testing and Materials, pp. 341-353 (Special Technical Publication
707).
RAPOPORT, E.H. & CANGIOLI, G. (1963) Herbicides and the soil
fauna. Pedobiologia, 2: 235-238.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977)
Investigations into the acute toxicity and some chronic effects of
selected herbicides and pesticides on several fresh water fish
species. Bull. environ. Contam. Toxicol., 18: 361-365.
RIVIERE, J-L. (1976) Les effets secondaires des pesticides agricoles.
II. Action de quelques herbicides sur la croissance et la fécondité de
Blattella germanica (L.). Ann. Zool. Ecol. anim., 8: 543-550.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3: 67-78.
ROBERTSON, E.B. (1975) The acute toxicity of four herbicides to 0 -
4 hour nauplii of the copepod Cyclops vernalis Fisher, Washington, DC,
US Department of Commerce, National Technical Information Service, 70
pp. (M.S. Thesis, University of Tennessee) (NTIS Report No. PB-269
495).
RODGERS, C.A. & STALLING, D.L. (1972) Dynamics of an ester of
2,4-D in organs of three fish species. Weed Sci., 20: 101-105.
ROGERS, J.G., CAGAN, R.H., & KARE, M.R. (1974) Percutaneous
absorption of several chemicals, some pesticides included, in the red-
winged blackbird. Environ. Physiol. Biochem., 4: 104-111.
ROSENBERG, A. & ALEXANDER, M. (1980) 2,4,5-trichlorophenoxy-
acetic acid (2,4,5-T) decomposition in tropical soil and its co-
metabolism by bacteria in vitro. J. agric. food Chem., 28: 705-709.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation of
the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUGGIERO, P. & RADOGNA, V.M. (1985) Inhibition of soil humus-
laccase complexes by some phenoxyacetic and s- triazine
herbicides. Soil Biol. Biochem., 17: 309-312.
SANDERS, H.O. (1969) Toxicity of pesticides to the crustacean,
Gammers lacustris. Washington, DC, US Department of the Interior, Fish
and Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 18 pp.
(Technical Paper No. 25).
SANDERS, H.O. (1970a) Toxicities of some herbicides to six species of
freshwater crustaceans. J. Water Pollut. Control Fed., 42: 1544-1550.
SANDERS, H.O. (1970b) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowler's toad Bufo woodhousii
fowleri. Copeia, 1970(2): 246-251.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several pesticides to
two species of cladocerans. Trans. Am. Fish. Soc., 95: 165-169.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol. Oceanogr.,
13: 112-117.
SANDMANN, E.R.I.C. & LOOS, M.A. (1984) Enumeration of
2,4-D-degrading microorganisms in soils and crop plant rhizospheres
using indicator media; high populations associated with sugarcane
(Saccharum officinarum). Chemosphere, 13: 1073-1084.
SARMA, Y.S.R.K. & TRIPATHI, S.N. (1980) Effects of some chemicals
(2,4-dichlorophenoxyacetic acid, coumarin and acenaphthene) on the
green alga, Oedogonium acmandrium Elfving. Phykos, 19: 142-152.
SATTAR, M.A. & PAASIVIRTA, J. (1980) Fate of chlorophenoxyacetic
acids in acid soil. Chemosphere, 9 745-752.
SCHRODER, H. & PILZ, E. (1983) [Effect of herbicides on nitrification
in different soils.] Zbl. Mikrobiol., :138: 223-234 (in German).
SCHRODER, I., MEYER, M., & MUCKE, D. (1970) [Effects of the
herbicides 2,4-D, aminotriazole, atrazine, chlorpropham and
chlorfurenol on nucleic-acid biosynthesis in the ascomycete Neurospora
crassa.] Weed Res., 10: 172-177 (in German).
SCHULTZ, D.P. (1973) Dynamics of a salt of (2,4-dichlorophenoxy)-
acetic acid in fish, water, and hydrosol. J. agric. food Chem., 21:
186-192.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8: 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues
at Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7: 146-
152.
SEIBERT, K., FUHR, F., & MITTELSTAEDT, W. (1982) Experiments
on the influence of roots and soils on 2,4-D degradation. Landwirtsch.
Forsch., 35: 5-13.
SHCHERBAKOV, Y.A. & POLUBOYARINOVA, I.V. (1973) The
persistence of 2,4-D butyl ester in water and its accumulation in
aquatic life. In: Andrushaitis, G.P., ed. Experimental water
toxicology, Charlotte, North Carolina, Army Foreign Service and
Technology Center, pp. 19-21 (NTIS Report No. AD-760 400).
SHUKLA, G.S. & OMKAR (1983) Toxicity of 2,4-D-Na salt to fresh
water prawn, Macrobrachium lamarrei (M. Edwards). Comp. Physiol.
Ecol., 8: 282-284.
SIGMON, C. (1979) Oxygen consumption in Lepomis macrochirus
exposed to 2,4-D or 2,4,5-T. Bull. environ. Contam. Toxicol., 21: 826-
830.
SIKKA, M.C., APPLETON, H.T., & GANGSTAD, E.O. (1977) Uptake
and metabolism of dimethylamine salt of 2,4-D acid by fish. J. agric.
food Chem., 25: 1030-1033.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., &
RAATIKAINEN, T. (1981) Triclopyr, glycophosphate, and
phenoxyherbicide residues in cowberries, bilberries and lichen. Bull.
environ. Contam. Toxicol., 27: 731-737.
SINGH, P.K. (1974) Algicidal effect of 2,4-dichlorophenoxy acetic
acid on blue-green alga Cylindrospermum sp. Arch. Microbiol., 97: 69-
72.
SKOKOVA, N.N. (1975) [Effect of the butyl ester of 2,4-D on the
reproductive function of male bank voles.] Nauchn. Osn. Okhr. Prir.,
3: 172-178 (in Russian).
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of large-
scale applications of 2,4-D on aquatic fauna and water quality.
Pestic. monit. J., 1: 16-21.
SOLOMON, K.E., DAHLGREN, R.B., & LINDER, R.L. (1973)
Abnormal embryos in eggs of pheasants given 2,4-D or PCB. Proc. South
Dakota Acad. Sci., 52: 95-99.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11: 511-516.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978a) Hatching
success and early performance of chicks from eggs sprayed with 2,4-D,
2,4,5-T and picloram at various stages of embryonic development.
Bull. environ. Contam. Toxicol., 20: 289-293.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978b)
Reproductive success of hens and cockerels originating from eggs
sprayed with 2,4-D, 2,4,5-T and picloram followed by early performance
of their progeny after a comparable in ovo exposure. Bull. environ.
Contam. Toxicol., 20: 111-119.
SPAIN, J.C. & VAN VELD, P.A. (1983) Adaptation of natural microbial
communities to degradation of xenobiotic compounds: Effects of
concentration, exposure time, inoculum and chemical structure. Appl.
environ. Microbiol., 45: 428-435.
SPENCER, S.R. & BARRETT, G.W. (1980) Meadow vole population
responses to vegetational changes resulting from 2,4-D application.
Am. Midl. Nat., 103: 32-46.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on the
embryo development of Phasianus colchicus var. and Coturnix coturnix
japonica.] Z. Jagdwiss., 22: 197-201 (in German).
STALLING, D.L. & HUCKINS, J.N. (1978) Metabolism of 2,4-dichloro-
phenoxyacetic acid (2,4-D) in bluegills and water. J. agric. food
Chem., 26: 447-452.
STATHAM, C.N. & LECH, J.J. (1975) Potentiation of the acute toxicity
of several pesticides and herbicides in trout by carbaryl. Toxicol.
appl. Pharmacol., 34: 83-87.
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., &
ASKAR, A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46: 387-391.
THOMPSON, D.G., STEPHENSON, G.R., SOLOMON, K.R., &
SKEPASTS, A.V. (1984) Persistence of (2,4-dichlorophenoxy) acetic
acid and 2-(2,4-dichlorophenoxy) propionic acid in agricultural and
forest soils of northern and southern Ontario. J. agric. food
Chem., 32: 578-581.
TIETJEN, H.P., HALVORSON, C.H., HEGDAL, P.L., & JOHNSON, A.M.
(1967) 2,4-D herbicide, vegetation, and pocket gopher relationships
Black Mesa, Colorado. Ecology, 48: 634-643.
TIWARI, D.N., PANDEY, A.K., & MISRA, A.K. (1984) Toxicity of
2,4-dichlorophenoxy acetic acid on growth and nitrogen fixation of
blue-green alga Anabaena cylindrica. Pesticides (Bombay), 18: 16-18.
TOOBY, T.E., LUCEY, J., & STOTT, B. (1980) The tolerance of grass
carp Ctenopharyngodon idella Val., to aquatic herbicides. J. Fish
Biol., 16: 591-597.
TORSTENSSON, N.T.L. (1975) Degradation of 2,4-D and MCPA in
soils of low pH. Environ. Qual. Saf. (Suppl.), 3: 262-265.
TOURE, I.M. & STENZ, E. (1977) [The action of selected herbicides on
bacteriophages and Escherichia coli.] Zbl. Bakteriol. Parasitol.
Infektionskr. 132: 163-177 (in German).
TREVORS, J.T. & STARODUB, M.E. (1983) Effect of 2,4-D on
electron transport system (ETS) activity and respiration in soil.
Bull. environ. Contam. Toxicol., 31: 595-598.
TRUMBLE, J.T. & KOK, L.T. (1980) Impact of 2,4-D on
Ceuthorhychidius horridus (Coleoptera: Curculionidae) and their
compatibility for integrated control of Carduus thistles.
Weed Res., 20: 73-75.
TUEY, D.B. & JAMES, M.O. (1980) Compartmental analysis of 2,4-
dichlorophenoxyacetic acid pharmacokinetics in spiny lobster. Fed.
Proc., 39: 525.
VARDIA, H.K. & DURVE, V.S. (1981) The toxicity of 2,4-D to
Cyprinus carpio var. communis in relation to the seasonal variation in
the temperature. Hydrobiologia, 77: 155-159.
VARDIA, H.K., RAO, P.S., & DURVE, V.S. (1984) Sensitivity of toad
larvae to 2,4-D and endosulfan pesticides. Arch. Hydrobiol., 100: 395-
400.
VERMA, D., CHOUHAN, M.S., & PANDEY, A.K. (1984) Effect of
Weedone (a selective 2,4-D herbicide) on neuroendocrine complex
of Puntius ticto (Ham.). J. environ. Biol., 5: 249-254.
WATSON, J.R. (1977) Seasonal variation in the biodegradation of 2,4-D
in river water. Water Res., 11: 153-157.
WELP, G. & BRUMMER, G. (1985) [The Fe(III)-reduction test - a
simple procedure to determine the effects of environmental chemicals on
the microbial activity in soils.] Z. Pflanzenernaehr. Bodenkd.,
148: 10-23 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972a) The subacute toxicity
of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid
to chicks. Toxicol. appl. Pharmacol., 21: 348-354.
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972b) The effect of 2,4-
dichlorophenoxyacetic acid on laying hens. Br. poult. Sci., 13: 191-
195.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., &
GANGSTAD, E.O. (1973) The effects of a 2,4-D application on the
biota and water quality in Currituck Sound, North Carolina. Hyacinth
Control J., 11: 13-17.
WHO (1984) Environmental Health Criteria 29: 2,4-dichlorophenoxy-
acetic acid (2,4-D), Geneva, World Health Organization, 151 pp.
WOODWARD, D.F. (1982) Acute toxicity of mixtures of range
management herbicides to cutthroat trout. J. Range Manage., 35: 539-
540.
YOUNG, A.L., THALKEN, C.E., & WARD, W.E. (1975) Studies of the
ecological impact of repetitive aerial applications of herbicides on
the ecosystem of Test Area C-52A, Elgin AFB, Florida. Florida Air Force
Armament Laboratory, Elgin Air Force Base, 126 pp. (Report No. AFATL-
TR-75-142).
2.2. Important Chemical Reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides. Pyrolysis of 2,4-D and its derivatives is likely
to produce certain CDD isomers. 2,4-D is readily photodegraded.
2.3. Volatility of 2,4-D Derivatives
2,4-D esters with short-chain alcohols are highly volatile. This
influences the effectiveness of their application to target crops,
their effects on neighbouring crops, and the degree of contamination of
the atmosphere. 2,4-D alkali salts or amine salts are much less
volatile than esters, and these products are to be preferred when the
use of 2,4-D esters might lead to evaporative 2,4-D losses and to crop
damage or damage to the surrounding environment.
Details of technical compositions, impurities, and analytical
methods can be found in Environmental Health Criteria 29: 2,4-
Dichlorophenoxyacetic acid (WHO, 1984).
Table 2. Vapour pressure and solubility of 2,4-D salts and esters
--------------------------------------------------------------------------------
Compound Vapour pressurea Solubility
--------------------------------------------------------------------------------
2,4-D free acid 0.4 mmHg (160 °C) 0.09% in water (25 °C),
85% in acetone (25 °C)
dimethylamine salt 300% in water (20 °C),
soluble in acetone
isopropyl ester 1.4 x 10-3 mmHgb insoluble in water, soluble
4.6 x 10-5 mmHgb in most organic solvents
butoxyethanol ester 4.5 x 10-6 mmHgb insoluble in water, soluble
(butylethyl ester) in most organic solvents
ethylhexyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
isooctyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
propyleneglycol butyl 3.0 x 10-6 mmHgb insoluble in water, soluble
ether ester in organic solvents
methyl ester 2.3 x 10-3 mmHgb
ethyl ester 1.1 x 10-3 mmHgb
butyl ester 3.97 x 10-4 mmHgb
--------------------------------------------------------------------------------
a 1 mmHg = 0.133 kPa.
b Vapour pressures of esters were determined at high temperatures by gas-
liquid chromatography, and these values are the result of extrapolation
to 25 °C. Values vary considerably between authors as a result of this
extrapolation; original values at high temperatures agree. Results are
presented here as an indication of relative vapour pressure at working
temperature. Values from Flint et al. (1968) and Jensen & Schall
(1966).
3. SOURCES OF ENVIRONMENTAL POLLUTION
The following is a summary of the chapter from Environmental Health
Criteria 29: 2,4-Dichlorophenoxyacetic acid (WHO, 1984).
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use were
not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United Kingdom.
World-wide use of herbicides and annual production, which probably
exceeds 5 x 107 kg/year, are increasing.
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaved weeds in cereal crops, as well as on
pastures and lawns, in parks, and on golf courses, at rates of about
0.2 to 2.0 kg active ingredient (acid equivalent) per hectare. Esters
are also used at rates of up to 6.0 kg (acid equivalent) per hectare to
suppress weeds, brush, and deciduous trees along rights-of-way and in
conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides in or
along irrigation and other canals, in ponds, and lakes at rates ranging
from 1 to 122 kg/ha.
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays containing 20
to 40 mg 2,4-D/litre on apple trees to reduce premature fruit-drop, on
potato plants to increase the proportion of medium-size tubers or to
intensify the tuber skin colour of the red varieties, and in citrus
culture to reduce pre-harvest fruit-drop and to increase fruit storage
life.
The highly volatile ethyl, isopropyl, and butyl esters are being
replaced by low-volatile esters or by amine salts to reduce crop damage
resulting from 2,4-D vapour drift, and to decrease atmospheric
pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public, has
been reduced in some countries because of increasing concern about
possible toxic effects, especially in relation to CDDs.
3.3. Disposal of Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localized to
the production site and to areas of waste dumping, and it is likely to
be more dispersed if disposal or leaching has occurred into water
courses. Disposal of unused 2,4-D in agriculture and washing of
equipment may result in localized land pollution and also pollution of
water supplies through direct contamination or leaching from soil.
4. UPTAKE, ACCUMULATION, ELIMINATION, AND BIODEGRADATION
Appraisal
2,4-D does not persist in soil because of its rapid degradation.
The physico-chemical properties of 2,4-D acid and its formulations
have an important effect on its behaviour in environmental
compartments.
The bioavailability to, and uptake by, aquatic and terrestrial
organisms is strongly influenced by the organic matter content of
soils, microbiological activity, and by environmental conditions such
as temperature and pH. Although highly inconsistent, the data on
dissipation and bioavailability in various soils demonstrate a marked
influence of differences in the texture and mineral composition of the
soil (Graham-Bryce, 1972). In aerobic soils, with a high content of
organic material, and at high pH values and temperatures, toxic effects
are limited because of rapid degradation of 2,4-D.
Uptake is followed by rapid excretion in most organisms. With the
exception of some algae, the retention of 2,4-D by organisms in the
environment cannot be expected, because of its rapid degradation.
Some microorganisms are capable of utilizing 2,4-D as their sole
carbon source. Repeated application to soil stimulates the number of
organisms capable of degrading the compound.
4.1. Biodegradation
2,4-D is readily and rapidly degraded in soil. Warm, moist
conditions and addition of organic matter stimulate degradation.
Autoclaving the soil and inhibiting bacterial metabolism reduce
degradation. The kinetics of 2,4-D disappearance suggest that
microorganisms are responsible. Particular species of microorganisms,
of various types, have been isolated and shown to degrade phenoxyacetic
acid herbicides in pure culture. Degradation of the phenoxyacetic
acids proceeds by two main pathways. These are via a hydroxyphenoxy
acetic acid intermediate or via the corresponding phenol. The
literature has been reviewed by the two workers principally responsible
for this evidence (Audus, 1960, 1964; Loos, 1969). Some microorganisms
are capable of using 2,4-D as their sole carbon source. More often,
2,4-D is co-metabolized with another carbon source. Regular treatment
of soil with 2,4-D stimulates the numbers of organisms which are
capable of degrading the compound. Treatment with other phenoxy
herbicides can also lead to an increase in organisms capable of
degrading 2,4-D.
Butler et al. (1975a) exposed 21 species of freshwater algae
isolated from natural lake water to 2,4-D butoxyethanol ester, at a
concentration of 0.01 mg/litre, and looked for degrading ability. Most
of the cultures fully degraded 2,4-D within 2 weeks. A single culture
retained 64% of the added 2,4-D, while seven isolates reduced 2,4-D to
less than 20% of the amount added. The remaining isolates showed 2,4-D
recoveries ranging from 22% to 53%.
Le Van To (1984) isolated six species of microorganisms
from soil previously treated with herbicides. These were
Flavobacterium peregrinum, Pseudomonas fluorescens, Arthrobacter
globiformis, Brevibacterium sp., Streptomyces viridochromogenes, and
an unidentified Streptomyces species. Flavobacterium was the most
active organism in degrading 2,4-D; degradation of 20 mg/kg of
2,4-D was complete after 20 to 30 days. In a liquid medium,
Flavobacterium degraded 93.5% of added 2,4-D within 80 h. The time
required to degrade half of the 2,4-D added to a sterilized soil along
with nutrient was estimated at 3 days. Li-Tse Ou (1984) investigated
the breakdown of 2,4-D in two types of soil under dry and moist
conditions and at two different temperatures. Numbers of
microorganisms degrading 2,4-D were also estimated. Generally, 2,4-D
disappeared more rapidly from moist soil; after 14 days of a slow rate
of disappearance, however, the removal rate from dry, sandy soil
increased. Numbers of organisms degrading 2,4-D were initially much
lower in sandy than in clay loams. However, numbers increased rapidly
in sandy soils after the addition of the herbicide and, as a result,
2,4-D was eventually degraded more rapidly in sandy than in clay loams.
In moist conditions, at 25 °C, the half-life of 2,4-D was 7 days or
less, whereas in dry conditions, at 35 °C, it could be as long as 250
days. These latter conditions are unlikely to apply in most natural
conditions where 2,4-D is likely to be used.
Rosenberg & Alexander (1980) incubated sewage-sludge bacteria with
2,4-D and found that nearly all of the herbicide had disappeared after
7 days. Subsequent additions of 2,4-D led to destruction of the
compound without a lag period; this suggests selection for organisms
capable of degrading the compound. Similar results were obtained using
bacteria from soil. The time needed for the disappearance of 90% of
the added 2,4-D was 14 days with soil inocula. 2,4-D added
subsequently was reduced by 70% within 3 to 4 days. Various tropical
soils were used in the experiment and all showed a high capacity for
degrading 2,4-D. Thompson et al. (1984) determined the persistence of
2,4-D applied at recommended rates in agricultural soils in Canada. In
all but one soil, a sandy loam, the concentration had declined by 50%
within 7 days. Sattar & Paasivirta (1980) showed slower degradation
of 2,4-D in acid soils. It took 6 weeks for 50% of the 2,4-D to
disappear from the soil and 7% was still left after 24 weeks. In
water-logged soil, there was reduced degradation of the herbicide.
Lewis et al. (1984) studied bacterial breakdown of 2,4-D
butoxyethyl ester and the effects of adding various extra components to
the medium. The addition of unfiltered, spent fungal medium from which
the majority of the fungus had settled out could be either stimulatory
or inhibitory to degradation rates of the herbicide; this depended on
the particular fungus species cultured in the medium. Further
investigation showed that effects were primarily due to differences in
pH. Reduction of the pH below 6 inhibited bacterial transformation of
the compound. Fungi commonly release large amount of organic acids.
The addition of spent fungal medium inhibited the breakdown of 2,4-D
ester. Buffering the added fungal medium reduced this inhibitory
effect; indeed, some stimulation of breakdown occurred after the
addition of buffered, spent medium. The addition of nutrients, or
other bacteria which did not transform 2,4-D, stimulated the
transformation of the herbicide. The authors consider that the most
likely explanation for this phenomenon is induction of other
transforming enzymes. With increasing substrate concentration, further
enzyme systems are induced in bacteria. The presence of other
organisms may stimulate the induction of these other enzymes at lower
substrate concentrations than would normally induce them. Increased
biomass of transforming bacteria in the presence of competing organisms
contributes to increased transformation rates. The nature of the
microbial community can, therefore, greatly change the ability of
degrading bacteria to transform 2,4-D and other xenobiotics.
O'Connor et al. (1981) found that 2,4-D applied at about 1.5 mg/kg
was readily degraded in soil. Adding extra carbon in the form of
dried, digested sewage sludge had a short-term effect in enhancing
degradation of the compound. Torstensson (1975) measured the half-life
of 2,4-D degradation in cultures of soil microorganisms at different
pH. In the pH range of 8.5 to 5.0, the half-life changed little,
ranging from 5 to 8 days. At pH 4.5, the half-life increased to 21
days and, at pH 4.0, increased further to 41 days.
Lieberman & Alexander (1981) added 2,4-D to inocula of municipal
sewage and monitored the biological oxygen depletion (BOD) as a measure
of degradation. The herbicide was added to carbon-depleted inocula
such that the 2,4-D represented the sole carbon source. Less than 5%
of the available oxygen was depleted, indicating poor biodegradation of
2,4-D because of low numbers of organisms capable of degrading the
herbicide as their sole carbon source. A separate study showed that
2,4-D was not toxic to microorganisms in sewage.
Fournier (1980) showed that, while 2,4-D treatment increased the
numbers of soil microorganisms capable of metabolizing 2,4-D as the
sole carbon source and those capable of co-metabolizing the herbicide,
this increase was dependent on the concentration of 2,4-D used. At
concentrations of 2,4-D between 5 and 50 mg/litre, there was a
significant increase in the numbers of organisms metabolizing 2,4-D,
and at 5 mg/litre there was a very pronounced increase in organisms co-
metabolizing the compound. At much higher (500 mg/litre) or much lower
(1.2 µg/litre) 2,4-D concentrations, there was no increase in the
numbers of either metabolizing or co-metabolizing organisms.
Sandmann & Loos (1984) estimated the numbers of microorganisms
capable of degrading 2,4-D in soils with and without the `rhizosphere
effect' of two plants, African clover (Trifolium africanum) and sugar
cane (Saccharum officinarum). The `rhizosphere effect' is a
phenomenon which occurs in close association with the roots of plants,
where material from the root or the metabolic activity of the root
tissue affects the surrounding soil. Particularly high, stimulated
populations were associated with sugar cane. A similar effect, but to
a lesser degree, was found with clover. In the three sugar cane soils
examined, and their corresponding controls, the numbers of organisms
were 46 400, 156 000, and 40 700 per g of soil, with rhizospheres, and
178, 1480, and 6170 per g of soil, without rhizospheres, respectively.
Seibert et al. (1982) failed to demonstrate a rhizosphere effect on
2,4-D degradation in glasshouse studies using soils with and without
maize roots.
Norris & Greiner (1967) investigated the degradation of 2,4-D in
forest leaf litter. Litter from either alder, ceanothus, vine maple,
bigleaf maple or Douglas fir showed comparable ability to degrade
2,4-D, the recovery of 2,4-D being between 60% and 70% after 15 days of
incubation. In a second series of experiments, different formulations
of 2,4-D were added to alder litter. About 50% of the free acid of
2,4-D was degraded within 15 days. Triethanolamine salt and two
commercial formulations (`solubilized acid' and isooctyl ester) were
degraded less than the pure acid. There was between 30% and 40%
degradation of these preparations over 15 days.
Nesbitt & Watson (1980) related the degradation rate of 2,4-D in
river water to the nutrient levels, sediment load, and dissolved
organic carbon content of the water. The addition of sediment or
inorganic nutrients increased the rate of 2,4-D degradation, whereas
the addition of organisms capable of degrading 2,4-D did not increase
the rate of breakdown of the herbicide. This finding indicated that
the limiting factor in breakdown of 2,4-D in river water was not
numbers of organisms but the nutrient status of the river. The authors
noted that in winter, when the river was in peak flow and the water
temperature below that for optimum microbial activity, appreciable
amounts of the herbicide would be washed into the estuary. An earlier
pilot study of seasonal changes in the capacity of river water in
Western Australia to degrade 2,4-D (Watson, 1977) indicated clear
seasonal differences in both river water concentrations of the
herbicide and the degrading capacity of river water. Several rivers
were studied and differences were related to the amount of
agricultural run-off, the sediment content of the water, river flow,
and temperature. Rivers receiving agricultural run-off degraded 2,4-D
better than those receiving run-off principally from forests. This was
presumed to be the result of the preconditioning of organisms to the
herbicide; the investigation corrected for nutrient content of the
water which had been previously shown to affect degradation.
Spain & Van Veld (1983) looked at the degrading ability of
microbial communities taken from sediment cores from freshwater,
estuarine, and marine sites. Some cores were pre-exposed to 2,4-D.
Cores from freshwater sites showed increased degradation of 2,4-D after
pre-exposure to the compound, whereas estuarine and marine cores did
not show this effect. The adaptation of freshwater cores was maximal
after 2 weeks and no longer detectable 6 weeks after pre-exposure.
4.2. Uptake and Accumulation by Organisms
Appraisal
Many studies on the accumulation of 2,4-D have used radioactively
labelled herbicide and have monitored uptake by simple counting of the
label. This fails to take into account that the label could have been
removed from the parent molecule by metabolic breakdown. Values for
uptake should, therefore, be treated as a maximum possible uptake
value for 2,4-D. Such data would not normally be considered
acceptable. However, the accumulation of 2,4-D is so low that these
data serve to illustrate that little of the herbicide is accumulated.
4.2.1. Laboratory studies
Eliasson (1973) sprayed leaves of 3-year-old aspen (Populus
tremens) with the butoxyethanol ester of 2,4-D at 0.5 kg acid
equivalent/litre. The plants were then kept in an open-sided
glasshouse and residues of 2,4-D were monitored. Most of the
herbicide remained in, or on, the sprayed leaves. The average residue
level was 2300 mg/kg fresh weight 1 day after spraying. This level had
fallen to 1300 mg/kg after 37 days and, by day 365, the average residue
level was 870 mg/kg. This was a very high application rate and
indicates that there is no foliar uptake of 2,4-D by plants.
Glynn et al. (1984) exposed coral Pocillopora damicornis to three
concentrations of 2,4-D sodium or amine salts at 0.1, 1.0, or
10.0 mg/litre. The maximum concentration of 2,4-D found in coral
tissue was 0.137 mg/kg after exposure to the amine salt at 10 mg/litre,
but residues were not related to the 2,4-D exposure concentration. The
highest bioconcentration factor (BCF) of 1.33 was found after exposure
to 0.1 mg/litre of the amine salt of 2,4-D, i.e., the coral contained
1.33 times the concentration of 2,4-D in water.
Metcalf & Sanborn (1975) introduced 14C-labelled 2,4-D into
model ecosystems consisting of an alga Oedogonium, an aquatic plant
Elodea, a snail Physa, and the mosquito fish Gambusia. Total
14C in the water was equivalent to 0.205 mg 2,4-D/litre. The highest
BCF was in the alga (26.8, based on measurement of radioactivity).
Analysis of all components of the ecosystem for 2,4-D, rather than the
radiolabel, revealed none of the parent compound. The BCF, therefore,
refers to breakdown products rather than 2,4-D itself. Gile (1983)
introduced 14C-labelled 2,4-D, as the butyl ester, into a simulated
ryegrass ecosystem. The system consisted of a sandy loam soil, annual
ryegrass, several invertebrates, and grey-tailed voles. Voles were
introduced 10 days after spraying 2,4-D as a foliar spray at the
equivalent of 1 kg/ha. The experiment was terminated after 1 month.
Plant material contained an average of 8.9 mg/kg; this was identified
as being mostly 2,5-dichloro-4-hydroxyphenoxyacetic acid. Residue
levels in animals (based on unidentified 14C residues) ranged from
0.31 mg/kg in snails to 5.28 mg/kg in pillbugs (isopods).
Freitag et al.(1982) measured the bioaccumulation of 14C-2,4-D in
an alga Chlorella fusca and a fish, the golden orfe. They measured a
24-h static BCF of 6 for the alga, and a 3-day static BCF of <10 for
the fish. This measurement was based on radioactivity and, therefore,
did not distinguish between the parent compound and its breakdown
products.
Schultz (1973) examined uptake and loss of 14C-2,4-D dimethyl-
amine salt by organs of three species of fish (channel catfish,
bluegill sunfish, and largemouth bass), exposed to 0.5, 1.0, or
2.0 mg/litre of 2,4-D acid equivalent. After exposure to the highest
concentration of 2,4-D dimethylamine salt, there was detectable
radioactivity in all organs examined. Bile showed the highest residues
of 14C in all three species after 1 week. For the remainder of the
exposure period of 12 weeks, there was an increase of radioactivity in
other organs and a decrease in the bile. At the end of the exposure
period, there was no clear pattern to residue levels of 14C in
different organs. These levels ranged from 5.04 mg/kg in bile to
35.5 mg/kg in posterior kidney for the channel catfish. For largemouth
bass, the range was from 1.32 mg/kg in muscle to 7.29 mg/kg in liver.
For the sunfish, the lowest residue was 24.75 mg/kg in bile and the
highest 322.7 mg/kg in the pyloric caeca of the gut. After 84 days
exposure to the dimethylamine salt at 2 mg/litre, levels of 14C in
the muscle of catfish, bass, and sunfish were equivalent to 0.953,
0.035, and 1.065 mg 2,4-D/kg, respectively. No analysis for 2,4-D
itself was carried out. A second study exposed the three fish species
for 2 weeks to 14C-2,4-D dimethylamine salt at 1 mg/litre and then
for a further 4 weeks to clean water. The disappearance of 14C was
measured. Loss of 14C was slow at first but by 4 weeks most tissues
had shown a decline in residues. Samples were analysed for 2,4-D but
none was detectable, suggesting that the 14C measured was in
breakdown products. The values for 2,4-D residues in this and other
studies using 14C-labelled material should, therefore, be regarded as
overestimates of retained 2,4-D. Uptake of 14C-2,4-D was examined at
two different temperatures, 17 °C and 25 °C. The highest residues of
14C detected in fish were equivalent to 0.122 mg 2,4-D/kg, but no
2,4-D could be found after analysis, except in bluegill sunfish after
14 days. Loss of 2,4-D did not, therefore, seem to change with
differing temperature over this range. A similar study, at two
different water pH values, showed significantly more 14C uptake in
all three species at the more acidic pH. Analysis of fish tissues for
2,4-D by gas-liquid chromatography showed non-detectable, or trace,
levels in most samples. Only in bluegill sunfish after 7 and 14 days
were residues measurable. These 2,4-D residues showed the opposite
trend to the 14C results; there was more 2,4-D in fish exposed at the
more alkaline pH. The authors suggest that metabolism of the herbicide
in the fish is suppressed at alkaline pH.
Sigmon (1979) exposed bluegill sunfish to 2,4-D butyl ethyl ester
(3 mg/litre) at three different temperatures, 20, 25, and 30 °C, and
measured the tissue content of 2,4-D after 8 days. None of the groups
differed from the controls, residues being <0.05 mg/kg.
Bluegill sunfish and channel catfish took up <0.5% of the
available 14C when exposed to 14C-2,4-D dimethylamine salt at 2
mg/litre (with 1 litre of water per fish) for 7 days (Sikka et al.,
1977). A maximum total 14C concentration in the fish was
reached after 24 h and did not change significantly over 14 days.
Bluegill sunfish attained a total body concentration of 0.9 mg/kg and
catfish 0.2 mg/kg at 24 h. These values were 2,4-D equivalents of
14C measured; the compound was not analyzed directly. When bluegill
sunfish were injected intraperitoneally with 14C-2,4-D dimethylamine
salt, at dose levels of 1 or 2.5 mg/kg body weight, they
excreted 90% of the dose within 6 h of treatment. In a similar
experiment, Stalling & Huckins (1978) exposed bluegill sunfish to
14C-2,4-D dimethylamine salt at 2 mg/litre and measured both
14C and 2,4-D in fish and water samples over the following 12 weeks.
Radioactivity was detected in tissues and increased over the
experimental period, but there was no measurable 2,4-D; the detection
limit of the method was 0.1 mg/kg. An in vivo intraperitoneal
injection of 110 µg of 14C-2,4-D was followed by rapid elimination.
Rodgers & Stalling (1972) measured uptake of 14C-2,4-D butoxy-
ethanol ester by three species of fish, which were exposed to either
0.3 or 1.0 mg/litre and sampled over the next 168 h. Some fish were
fed and some fasted. Radioactivity in a variety of tissues was
determined; the maximum levels were found within 3 h of exposure in fed
fish. After this, levels declined over the remaining sampling period,
and by the end of the experiment, residues were negligible. The one
exception was the gall bladder, which consistently contained more 2,4-D
than other tissues. Results were different for fasted fish. In almost
all organs of fasted fish, uptake of 2,4-D was slower than for fed
fish, although the levels reached were eventually two to five times
higher than in fed fish. Analysis of the residues showed that only the
liver ever contained the herbicide in the ester form. In all other
tissues, only the acid was present.
Shcherbakov & Poluboyarinova (1973) monitored the accumulation of
2,4-D in carp and Daphnia. The 2,4-D was added as the butyl ester
at concentrations ranging from 0.006 to 5 mg/litre; the recommended
usage rate for this ester leads to water concentrations of about
0.5 mg/litre. Analyses of fish tissues were made for both the ester
and the acid. The highest BCF for the ester, at 395, was found with
fish after a 7-day exposure to 0.5 mg/litre. Acid accumulation was
lower than that of the ester. The experiment lasted for 70 days. At
day 10 and after, only trace amounts of ester were found in fish.
Small amounts of 2,4-D acid were found at day 10, but only trace
amounts after day 70. Residues of 2,4-D ester in Daphnia varied from
23.9 to 518 mg/kg, according to the exposure concentration.
Two experiments have been carried out on the grey slug Derocerus
reticulatum by Haque & Ebing (1983) using 14C-labelled 2,4-D acid.
The first study, a contact experiment, exposed the slugs to 2,4-D in
contaminated soil at 1.1 mg/kg. The body content of 2,4-D in slugs
reached equilibrium (0.014 mg/kg) after 15 days; this represented a
BCF of 0.013 based on radioactivity. In the second experiment,
slugs were exposed via the food using carrot discs containing 1.1 mg/kg
slug body weight per day over 5 days. Residues of 14C in the slugs
increased during the feeding period, peaking at 5.5 mg/kg. During the
following 7 days, residues were monitored to investigate loss of
radioactive material. At the end of the experiment, on day 12,
residues were comparable to those at the end of the feeding period.
During the course of feeding 2,4-D-contaminated carrots, more than 80%
of the ingested dose of radioactivity was excreted rapidly; only 20%
was retained. There was no attempt to characterize the 14C residues;
these may, therefore, represent either 2,4-D or its breakdown
products.
Chickens given a single oral dose of 100, 200, or 300 mg/kg
body weight reached maximum plasma levels of 2,4-D of 90, 130, and
250 µg/ml, respectively. Plasma levels in all groups had fallen to
15 µg/ml or less after 24 h. Continuous dosing of chickens at
300 mg/kg per day led to a faster rate of elimination of the daily dose
of 2,4-D with time (Bjorklund & Erne, 1966).
4.2.2. Field studies
Cope et al. (1970) treated experimental ponds with 2,4-D propylene
glycol butyl ether ester to give water concentrations up to and
including 10 mg/litre. No detectable 2,4-D was found in fish exposed
to 1 mg/litre or less of the herbicide, but residues were found in
bluegill sunfish exposed to 5 or 10 mg/litre. The highest residue
(2 mg/kg) was found 1 day after application. Residues were still
detectable after 3 days but not subsequently. Vegetation
(Potamogeton nodosus) and bottom sediment contained residues of
50.0 and 3.0 mg/kg, respectively, 2 days after treatment with the 2,4-D
ester at 10 mg/litre. The herbicide was still detectable at 0.1 mg/kg
in sediment after 44 days but not thereafter. At 44 days after
treatment, there were residues in the plant of 1.2 mg/kg; this amount
declined to 0.1 mg/kg after 94 days.
Following the field application of 2,4-D butoxyethanol ester at
22.5 kg/ha, Whitney et al. (1973) measured residues of the herbicide in
fish, crustacea, and insect larvae over a 3-week period. The herbicide
had been applied to control eurasian water milfoil. Some 2,4-D was
taken up by these various species; the highest residue concentration
was 0.24 mg/kg in largemouth bass after 8 days. All residues in
organisms were below 0.1 mg/kg after 3 weeks. No 2,4-D could be
detected in water in 33 samples taken after treatment, the detection
limit being 0.10 mg/litre. The highest reported concentration of 2,4-D
in mud was 0.65 mg/kg, 10 days after treatment, but in most samples the
herbicide level in mud was much lower and in several it was
undetectable.
Hoeppel & Westerdahl (1983) treated four areas (10 ha each) of
dense water milfoil beds in Lake Seminole, Georgia, with either 2,4-D
dimethylamine salt or 2,4-D butoxyethanol ester, at each of two
application rates (22.5 or 45 kg/ha). Both formulations were converted
to 2,4-D free acid within 24 h. Maximum water concentrations achieved
in the high rate (45 kg/ha) areas were 3.6 and 0.68 mg/litre for the
dimethylamine salt and butoxyethanol ester, respectively. There was no
detectable uptake of 2,4-D into fish in those areas treated with the
dimethylamine salt. In the ester-treated areas, 4 out of 24 game fish
sampled contained low levels of 2,4-D in muscle (the highest residue
being 0.29 mg/kg) and 18 out of 20 gizzard shad contained detectable
2,4-D in muscle (the highest residue being 6.9 mg/kg). No fish sampled
more than 13 days after treatment contained detectable 2,4-D.
Schultz & Harman (1974) treated nine experimental ponds with 2,4-D
dimethylamine salt at three concentrations: 2.24, 4.48, and 8.96 kg/ha.
Samples of water, bottom sediment, and fish were taken over 147 days.
Maximum water and sediment concentrations of 2,4-D were 0.692 mg/litre
and 0.17 mg/kg, respectively. Of 307 fish sampled, 45 contained
detectable residues of 2,4-D. The highest residue measured was in a
channel catfish at 1.075 mg/kg 1 day after treatment. All residues in
fish after 28 days were less than 0.005 mg/kg; most were undetectable.
Smith & Isom (1967) measured uptake and retention of 2,4-D after
treatment of two field sites for control of watermilfoil with the
butoxyethanol ester. The first site was treated with a granular
formulation at a rate of 112 kg/ha. One bluegill sunfish (Lepomis
macrochirus) contained 0.15 mg 2,4-D/kg on day 50 after treatment. All
other fish, sampled between 72 h and 50 days after treatment,
contained less than 0.14 mg/kg, which was the limit of detection. Two
samples of several species of mussel, held in cages for 96 h following
spraying, showed residues of 0.38 and 0.7 mg/kg. Water levels of 2,4-D
reached a peak of 37 mg/litre within 1 h of application and had fallen
to less than 1 µg/litre within 8 h. Mud samples contained very
variable levels of 2,4-D residues, ranging between 0.14 and 58.8 mg/kg.
The highest residue was found 10 months after application. The second
site was treated at the lower rate of 45 kg/ha. All fish sampled
between 15 days and 9 months after 2,4-D application showed residues
of less than 0.14 mg/kg. Mussels sampled between 1 and 42 days after
application contained residues ranging between <0.14 and 1.12 mg/kg.
Water levels peaked at 157 µg/litre, 1 h after spraying, and mud
residues ranged from <0.14 to 33.6 mg/kg.
Coakley et al. (1964) measured residues in organisms at the center
of a 0.4-ha field plot sprayed with 2,4-D butoxyethanol ester at a rate
of 33.7 kg/ha for watermilfoil control. Two days after application,
oysters (Crassostrea virginica), clams (Mya arenaria), fish (Lepomis
gibbus), and blue crabs (Callinectes sapidus) contained 3.5, 3.7, 0.3,
and <0.8 mg/kg, respectively.
In 1971, over 2800 ha in Loxahatchee National Wildlife Refuge
were sprayed with the dodecyl-tetradecyl amine salts of 2,4-D at a
rate of 4.48 kg/ha. The initial application of 2,4-D was followed by
spot treatments of the same formulation and/or the dimethylamine salt
of 2,4-D. The highest water concentration (0.037 mg/litre of 2,4-D)
was measured 1 day after the initial application. Of 60 fish sampled
in the area, 19 had measurable residues of 2,4-D but only three of
these were greater than 0.1 mg/kg; the highest recorded residue was
0.162 mg/kg. Breast muscle and liver of a bird, the common Florida
gallinule Gallinula chloropus, had residues of 0.3 and 0.675 mg/kg,
respectively, 1 day after spraying. No residues were found in the bird
4 days after spraying (Schultz & Whitney, 1974).
Plumb et al. (1977) treated sprouting chamise (Adenostoma
fasciculatum) with the polyethylene glycol butyl ether ester of 2,4-D
at a rate of 3.4 kg acid equivalent/ha. A maximum concentration of
herbicide (95.2 mg/kg) was found in the plant within 15 min of
application. A residue of 3.8 mg 2,4-D/kg remained in, or on, the
plants (shoots which had been originally sprayed) 1 year after
treatment. When Radosevich & Winterlin (1977) applied the butoxypropyl
ester of 2,4-D to a chaparral area at a rate of 4.5 kg/ha, the
residues measured in chamise were 221 mg/kg and in grass and forbs
269 mg/kg within 2 h of application. After 30 days, these levels had
dropped to 60 mg/kg for chamise and 21 mg/kg for grass and forbs, and,
after 360 days, 0.1 mg/kg was present in chamise. Siltanen et al.
(1981) monitored residues of 2,4-D in the fruit of bilberries 1 year
after the application of 0.25, 0.75, or 2.25 kg/ha acid equivalent. No
residues were detected, the limit of detection being 0.05 mg/kg.
Raatikainen et al. (1979), in a controlled field experiment,
sprayed cowberry and bilberry with an ester formulation of 2,4-D.
Three application rates were used, 0.25, 0.75, and 2.25 kg acid
equivalent/ha, and residues of 2,4-D were measured approximately
1 month after application. Thirty-four days after the application of
0.25 kg/ha, residues in cowberry were 0.3 mg/kg. Cowberries exposed to
0.75 or 2.25 kg/ha were analysed after 35 days and contained residues
of 1.0 and 3.7 mg/kg, respectively. Bilberries treated with 0.25,
0.75, or 2.25 kg/ha were analysed 29 days later; residues were 0.1,
1.3, and 4.8 mg/kg, respectively.
4.3 Elimination
James (1979) studied tissue distribution of 14C-labelled 2,4-D in
the spiny lobster (Panulirus argus). Labelled herbicide was injected
into the pericardial sinus and animals were sacrificed at regular
intervals. 2,4-D was taken up from the haemolymph, by the green gland,
and excreted unchanged, with an overall half-time of about 8 h. Tuey &
James (1980), in a similar study, found that the clearance of 2,4-D
from haemolymph, via the green gland, was three to five times greater
than the rate of metabolism in the hepatopancreas.
Pritchard & James (1979) studied the renal handling of intra-
venously injected 2,4-D by the winter flounder (Pseudopleuronectes
americanus). 2,4-D, at a concentration of 1 µmol/litre of plasma,
was actively secreted into the glomerular filtrate of the kidney
with clearances of nearly 500 times the glomerular filtration rate.
At higher plasma concentrations of between 10 and 60 µmol/litre, a
transport maximum of 0.85 µmol/g of kidney per h was observed.
Koschier & Pritchard (1980) reported a similar study using
an elasmobranch fish Squalus acanthias. They administered
2.5 µmol 14C-2,4-D/kg to the fish intramuscularly and monitored
blood and urine 14C levels. Clearance of total 2,4-D was more than
25 times greater than the glomerular filtration rate, indicating that
2,4-D was being actively secreted by the kidney. 2,4-D was eliminated
in the urine as a taurine conjugate, this representing about 95% of the
excretory products. The plasma contained, primarily, unconjugated
2,4-D (>90%). It seemed, therefore, that 2,4-D was conjugated with
taurine before being excreted in the urine. Guarino et al. (1977), in
a similar study on the dogfish Squalus, also found that 2,4-D was
extensively conjugated to taurine (>90%) and was eliminated
predominantly via the urine; 70% of the administered dose appeared in
the urine within 4 to 6 days. The highest tissue concentration of
2,4-D (14.5 mg/kg) was found in the kidney after 4 h. Plasma
elimination was rapid, with a half-time of 44 min; similarly rapid
clearance was seen from the kidney. Half-time estimates for muscle and
liver were 2 to 3 days and 5 days, respectively.
5. TOXICITY TO MICROORGANISMS
Appraisal
In general 2,4-D is relatively non-toxic to water and soil
microorganisms at recommended field application rates.
No effect of 2,4-D was recorded on 17 genera of freshwater and two
genera of marine algae at concentrations up to 222 mg/litre.
No effect of 2,4-D was observed on respiration of either sandy loam
or clay loam soils at concentrations up to 200 mg/kg.
N-fixation by aquatic algae is affected at high concentrations of
2,4-D acid (400 mg/litre). An effect of 2,4-D esters on N-fixation
occurs from a concentration of 36 mg/litre upwards. N-fixing algae in
topsoils appear to be more vulnerable to 2,4-D acid than other algal
species. The Cyanobacteria (blue-green algae) are important as the
major N2 source in tropical ponds and soils.
In the range of 25.2 to 50.4 mg/litre, 2,4-D was inhibitory to all
types of soil fungi.
Cell division was reduced in a green alga by 2,4-D at 20 mg/litre
and stopped at 50 mg/litre. No effect was observed on a natural
phytoplankton community after exposure to 2,4-D at 1 mg/litre.
However, exposure to esters of 2,4-D reduced productivity in these
organisms.
5.1. Aquatic Microorganisms
Hawxby et al. (1977) exposed cultures of three algae
(Chlorella pyrenoidosa, Chlorococcum sp., and Lyngbya sp.,) and
one cyanobacterium (blue-green alga) (Anabaena variabilis) to
concentrations of 2,4-D in the medium of up to 10 µmol /litre
(= 2.21 mg/litre). There was no effect on growth, respiration, or
photosynthetic rate.
Gangawane et al. (1980) studied the effects of 2,4-D on growth and
heterocyst formation in the nitrogen-fixing cyanobacterium (blue-green
alga) Nostoc. The organism was cultured for 30 days in 0, 10, 100,
1000, or 1500 mg 2,4-D/litre. Growth was measured by optical density
and cells forming heterocysts were counted. Growth was inhibited at
both 10 and 100 mg 2,4-D/litre and was eliminated at higher
concentrations. There was also reduced heterocyst formation.
Lembi & Coleridge (1975) demonstrated a marked effect of 2,4-D,
at concentrations of 110 or 220 mg/litre, on cultures of the
green algae Scenedesmus, Ankistrodesmus, and Pediastrum. After 14
days of culture, the three species under control conditions produced
456 x 102, 634 x 104, and 227 cells or colonies per ml of medium,
respectively. Corresponding figures after exposure to 110 mg/litre
were 54 x 102, 41 x 104, and 74 cells or colonies per ml,
respectively. For both Scenedesmus and Ankistrodesmus, these values
were less than the pre-treatment cell concentrations.
Butler et al. (1975b) exposed unialgal cultures of green algae
isolated from Warrior River water to 2,4-D butoxyethanol ester at
0.001, 0.01, 0.1, 1.0, or 4.0 mg/litre. Thirty separate isolates were
used. Concentrations less than or equal to 1 mg/litre of the 2,4-D
ester did not change the growth pattern of the isolates. However, with
a concentration of 4 mg/litre, there was some inhibition of growth, as
indicated by a 10% increase in the number of incubates which showed
poor growth, or no growth, when compared to controls. Some isolates
were unaffected even at this concentration and it can therefore be
assumed that 2,4-D butoxyethanol ester might change the species
composition of green algae populations.
Bednarz (1981) used 12 pure cultures of green algae and
cyanobacteria (blue-green algae) separately and in combination to
investigate the effects of 2,4-D acid. Cultures were exposed to
concentrations of 2,4-D ranging from 0.001 to 10 mg/litre. Low
concentrations of 2,4-D stimulated the growth of most species of algae,
whereas high concentrations inhibited growth. Chlorococcal green algae
were more sensitive to 2,4-D than were filamentous green algae or
cyanobacteria. In further experiments, the authors cultured
combinations of sensitive and tolerant species in the same range of
2,4-D concentrations. Tolerant species used in combinations were
Chlorella pyrenoidosa, Dictyosphaerium pulchellum, and Scenedesmus
quadricaudata. The first two of these tolerant species reduced the
toxicity of 2,4-D to sensitive species in mixed culture. This
protective effect was not seen with Scenedesmus.
Singh (1974) cultured a filamentous, nitrogen-fixing,
cyanobacterium Cylindrospermum sp. in concentrations of 2,4-D acid of
0, 100, 300, 400, 500, 600, 800, 1000, or 1200 mg/litre and examined
growth and heterocyst formation after 8 days. Both parameters were
affected at concentrations higher than 300 mg/litre and cultures were
killed at a concentration of 1000 mg/litre. Kapoor & Sharma (1980)
exposed cultures of the nitrogen-fixing, filamentous cyanobacterium
Anabaena doliolum to 2,4-D ethyl ester (as `Weedone 48' concentrate)
at concentrations of 36, 108, 180, 252, or 324 mg/litre. There was a
dose-related decrease in cell nitrogen over the whole range of 2,4-D
ester exposures. Cell growth was stimulated by lower concentrations of
2,4-D and only inhibited by the highest dose. Tiwari et al. (1984)
exposed cultures of a similar nitrogen-fixing, filamentous
cyanobacterium (Anabaena cylindrica) to 2,4-D acid at concentrations of
0, 100, 500, 700, 1000, or 1500 mg/litre, and examined growth,
heterocyst formation, and nitrogen fixation. For all these parameters,
there was a stimulatory effect of 2,4-D at 100 mg/litre and a
progressive inhibition with higher concentrations. These and similar
algae are considered to be a major source of nitrogen in tropical
ponds and soils. Das & Singh (1977) cultured the nitrogen-fixing
cyanobacterium Anaebaenopsis raciborskii in concentrations of 2,4-D
acid (sodium salt) of 10, 100, 400, 600, 800, and 1000 mg/litre and
measured nitrogen fixation. Control cultures and those exposed at 10
and 100 mg 2,4-D/litre showed no significant differences.
Nitrogen-fixation was inhibited at 400 mg/litre or more and eliminated
at 600 mg/litre.
Butler (1963) reported no effect on a natural phytoplankton
community of exposure to a 1 mg/litre concentration of 2,4-D (as the
acid or dimethylamine salt), or of the dimethylamine salt on pure
cultures of Dunaliella euchlora or Platymonas over 4 h. In a later
study (Butler, 1965), natural phytoplankton communities were exposed to
esters of 2,4-D. Butoxyethanol ester, propylene glycol butyl ether
ester, and ethylhexyl ester reduced productivity (as measured by
carbon fixation) by 16%, 44%, and 49%, respectively, at a concentration
of 1 mg/litre.
Sarma & Tripathi (1980) monitored cell division in the filamentous
green alga Oedogonium acmandrium exposed to 2,4-D acid at 1, 5, 10,
20, or 50 mg/litre of culture medium. At up to 10 mg/litre, 2,4-D
was found to stimulate cell division; a 168 h exposure to 5 mg/litre
increased the incidence of dividing cells by 15% over controls.
However, cell division was reduced at 20 mg/litre and stopped at
50 mg/litre. Abnormalities in chromosomes during cell division
increased with increasing 2,4-D exposure.
Chai & Chung (1975) examined the effects on growth,
photosynthesis, respiration, and chemical composition of exposing
cultures of the green alga Chlorella ellipsoidea to 2,4-D acid at 22 or
88 mg/litre. At 22 mg/litre, 2,4-D increased growth, photosynthesis,
and the cell content of protein and nucleic acids. Carbohydrate
content was unchanged. However, at 88 mg/litre, growth was inhibited,
photosynthesis was no different from controls, and the cell content of
carbohydrate, protein, and nucleic acids was decreased.
Elder et al. (1970) examined the effect of 2,4-D acid on 17 genera
of freshwater and two genera of marine algae exposed at 22, 111, or
222 mg/litre. There was no effect on the growth of any of the
cultures, even at the highest dose of 2,4-D.
Cultures of the flagellate Euglena gracilis were exposed for 24 h
to concentrations of 1, 5, 10, 50, or 100 mg/litre or for 7 days to 10,
50, or 100 mg/litre of 2,4-D acid by Poorman (1973). Cultures in 50
and 100 mg 2,4-D/litre yielded 84% and 74%, respectively, relative to
controls, over 24 h. Lower concentrations of 2,4-D had a slightly
stimulatory effect. After 7 days, there was significant stimulation of
yield with 10 mg/litre; the culture yielded 161% compared to a control.
There was slight stimulation of growth by 50 mg/litre and a reduction
to 78% of control levels with 100 mg/litre.
George et al. (1982) exposed the rotifer Brachionus
calyciflorus to 2,4-D at 5 mg/litre. Median lethal time (LT50) was
24 h and LT100 was 31 h.
5.2. Soil Microorganisms
Pachpande & David (1980) isolated the soil alga Chlorococcum
infusionum from paddy fields and cultured the organism in the presence
of 2,4-D acid at concentrations of 0, 1, 2, 3, 4, and 5 mg/litre.
Growth was estimated as dry weight of algal cells filtered out of the
medium. All concentrations of 2,4-D were inhibitory to growth.
At the highest 2,4-D concentration of 5 mg/litre, the culture yield
was reduced from a control level of 720 mg dry wt/litre of medium to
520 mg/litre.
Cullimore & McCann (1977) applied 2,4-D acid to isolated cores
taken from a prairie, loam soil to give approximate concentrations of
1 or 100 mg/kg in the top 2 cm of soil. Soil algal populations were
estimated from subsamples of cores taken before treatment and 1, 5, or
20 days after treatment with herbicide. Thirty-one genera of algae
were identified, of which five were very sensitive to 2,4-D and were
rarely found after treatment. These were Chlamydomonas, Chlorococcum,
Hormidium, Palmella, and Ulothrix. The most resistant genera were
Chlorella, Lyngbya, Nostoc, and Hantzschia; the `percent sensitivity'
of these genera (% of the total number of treatments in which the
genus was absent) was 28%, 6%, 22%, and 44%, respectively. The
reduction in cell numbers of algae in the top layer of the soil after
herbicide treatment was soon offset by an increase in the population of
Chlorella, Stichococcus, Oscillatoria, and Spongiochloris, all of
which recovered very rapidly from the herbicide effects. There was,
however, an overall reduction in cell numbers of nitrogen-fixing algae.
Mukhopadhyay (1980) measured the bacterial, fungal, and
actinomycete populations of soils supporting rice or maize plants which
had been treated with various herbicides for weed control. There was
no effect of 2,4-D, applied at the recommended rate, either on soil
microorganism numbers or on the evolution of carbon dioxide by soil
cultures.
Huber et al. (1980) examined the effect of 2,4-D at 0.3, 0.2, or
0.1 mmol/litre (= 66, 44, and 22 mg/litre, respectively) on seven
cultures of soil microorganisms. There was no effect on the growth of
five of the cultures; these were Nocardia sp., Pseudomonas fluorescens
in both aerobic and anaerobic culture, Bacillus subtilis, and
Ustilago maydis. There was a small reduction in growth at the
highest 2,4-D dose in cultures of Rhizopus japonicus and Aspergillus
niger. 2,4-D had no effect on mycelium growth of three out of four
plant pathogenic fungi in culture; Phytophthora cryptogea showed
reduced mycelial growth at 0.1, 0.2, and 0.3 mmol 2,4-D/litre,
but Fusarium oxysporum, Alternia radicina, and Rhizoctonia solani
were unaffected.
Moubasher et al. (1981) added 2,4-D at three doses (1.9, 7.6, and
15.2 mg/kg) either to soil or to agar medium inoculated with soil
fungi, and the effects on fungal populations were monitored. In soil,
at all three doses, 2,4-D stimulated the fungi. When incorporated in
the agar medium, 2,4-D was stimulatory to overall fungal growth and to
four individual species of fungus at the lowest dose of 6.3 mg/litre,
but inhibitory to two other species. At higher doses of 25.2 or
50.4 mg/litre, the herbicide was inhibitory to all fungi.
2,4-D had a significant inhibitory effect on culture yields of the
bacterium Escherichia coli only at 10-3mol/litre (= 220 mg/litre).
There was no effect at 10-4mol/litre (= 22 mg/litre) (Toure & Stenz,
1977).
Prescot & Olson (1972) added 2,4-D, at doses of 0, 0.1, 1.0, 10, or
100 mg/litre, to cultures of the soil amoeba Acanthamoeba castellanii
and monitored growth and reproduction. There was a stimulatory effect
of 2,4-D at all dose levels; this effect was most marked at the lowest
dose and declined with increasing exposure to 2,4-D. The authors
suggest that the amoeba may degrade the 2,4-D and utilize it as a
carbon source. However, Pons & Pussard (1980) found no effect of 2,4-D
(at 28, 54, or 84 mg/litre) on the reproduction of 23 different strains
of free-living soil amoebae.
2,4-D, at 10-3mol/litre in cultures of the ascomycete Neurospora
crassa, stimulated DNA synthesis but had no effect at lower
concentrations of 10-4 to 10-6mol/litre. These concentrations had
no significant effect on either RNA or protein (Schroder et al.,
1970).
Naguib et al. (1980) measured growth, respiration, and absorption
and utilization of sugar and nitrogen in pre-formed fungal mats of
Aspergillus terreus over 72 h in the presence of 200 mg/litre of
2,4-D. The herbicide inhibited sugar inversion and consequently sugar
absorption. It also reduced the incorporation of nitrogen into protein.
Respiration was depressed. Growth of the fungus was suppressed and, on
a dry weight basis, culture mass was reduced to below the initial
level.
Trevors & Starodub (1983) added 2,4-D to sandy loam and clay
loam soils and measured both respiration and electron transport
system (ETS) activity. ETS was assessed by measuring the capacity
of the soil to reduce 2-( p -iodophenyl)-3-( p -nitrophenyl)-5-phenyl
tetrazolium chloride (INT) to iodonitrotetrazolium formazan (INT
formazan). The effects of 2,4-D were tested at concentrations of the
herbicide in soil of 0, 10, 25, 50, 75, 100, or 200 mg/kg. There was
no effect on soil respiration, monitored either as oxygen consumption
or carbon dioxide evolution, at any of the concentrations of 2,4-D in
either soil. There was similarly no effect on ETS in the sandy loam.
However, in the clay loam, there was a progressive inhibition of ETS
over the whole range of concentrations of the herbicide. The control
soil had an ETS activity of 37.3 µg INT formazan production/g soil,
whereas the ETS activity of soil treated with 10 mg 2,4-D/kg was
25.1 µg INT formazan/g, significantly lower than that of the
control. The activity was reduced further with increasing
concentrations of 2,4-D, until an activity of 16.3 µg INT formazan/g
was found at 200 mg 2,4-D/kg.
Deshmukh & Shrikhande (1975) added 2,4-D, at recommended field
rates, and at five times the recommended field rates, to two
types of soil from India. Both doses of 2,4-D inhibited numbers
of Azobacter in both soil types, and the high, but not the low, dose of
2,4-D reduced nitrogen fixation in both soils. The same authors
(Deshmukh & Shrikhande, 1974) monitored the populations of various
microorganisms under the same dosing conditions. 2,4-D stimulated
the numbers of actinomycetes throughout the 6-week incubation period at
both dose levels. Fungal populations were reduced in the first week of
incubation at both dose levels in sandy loam, but only at the higher
dose level in clay loam. This reduction in fungal populations
persisted until the second week with the high dose in the sandy soil
and throughout the incubation period with the high dose in clay soil.
There was a temporary (1 week) reduction in total bacterial numbers
with both 2,4-D dose levels in the sandy soil and with the higher level
in clay soil. Schroder & Pilz (1983) reported that 2,4-D at
approximately 10-4mol/litre (= 22 mg/kg) had no long-term effect on
soil nitrification.
Welp & Brummer (1985) measured the influence of 2,4-D on the
reducing capacity of soil microorganisms, reduction being monitored as
the capacity to reduce Fe(III) oxides to soluble Fe(II) ions. They
determined no-observed-effect levels (NOEL) of 115 and 95 mg 2,4-D/kg
for two different soil types and corresponding EC50 values on
reduction capacity of 200 and 530 mg 2,4-D/kg soil.
Ruggiero & Radogna (1985) extracted and partially purified soil
diphenolase (laccase) from forest soil. This enzyme, which exists free
in the soil, plays an important role in the metabolism of humic
materials in soil. Oxygen consumption was monitored during the
enzymatic reaction, using either catechol or p -phenylenediamine as
substrate, and the effect of 2,4-D was investigated. The herbicide
inhibited diphenolase activity, and Lineweaver-Burk plots of the
data suggested that 2,4-D acts as a non-competitive inhibitor.
Apparent K values of 28.7 and 6.0 mol/litre were obtained for catechol
and p -phenylenediamine, respectively.
6. TOXICITY TO AQUATIC ORGANISMS
6.1. Toxicity to Aquatic Invertebrates
Appraisal
The short-term toxicity data on the effects of 2,4-D free acid,
its salts, and esters on aquatic invertebrates is extensive. Ester
formulations are more toxic than the free acids or salts. Sensitivity
variations exist among species in response to the same formulation.
Organisms become more sensitive to 2,4-D when the water temperature
increases. Reproductive impairment occurred at concentrations below
0.1 of the short-term toxic levels determined for these formulations.
6.1.1. Short-term toxicity
The short-term toxicity of 2,4-D to aquatic invertebrates is
summarized in tables 3 - 5.
Unfortunately, there are few studies where both the free acid (or
its salts) and ester preparations have been tested on the same organism
under the same conditions. The only organisms for which this applies
are the oyster (Butler, 1963; Butler, 1965), the stonefly (Sanders &
Cope, 1968), and daphnids and shrimp (Sanders, 1970a). These studies
all show that the free acid and its salts are less toxic than ester
formulations; for example the free acid is at least 20 times less toxic
to the water flea Daphnia magna than the least toxic of the esters
tested (Sanders, 1970a). Comparing studies carried out by different
authors and in different systems also suggests a much greater toxicity
of the ester preparations.
Liu & Lee (1975) found that 2,4-D could adversely affect the bay
mussel (Mytilus edulis) at all stages of its life cycle. The attachment
of young mussels to test chamber walls was reduced (data in Table 3).
The authors evaluated, in two duplicate experiments, the effects of
2,4-D acid, at concentrations in sea water of 22.8, 45.7, 91.4, and
182.8 mg/litre, on the growth of larval mussels. After 10 days
exposure, there was a significant reduction in the growth of larvae
exposed to 91.4 mg 2,4-D/litre; larvae were 11.6% smaller than
controls. This reduction was found in only one experimental replicate.
In both experiments, there was reduced growth after 10 days exposure
to 182.8 mg/litre; larvae were 31.9% and 34.9% smaller than controls
in the two experiments. Exposure for 20 days at 91.4 mg/litre
led to reduced growth in both experiments. All larvae exposed to
182.8 mg/litre died within 12 days, but only in one experimental
replicate. Extension of the growth study in the second experiment led
to all larvae dying within 22 days of exposure to 182.8 mg/litre and,
therefore, failing to undergo metamorphosis. The metamorphosis of
larvae exposed from age 30 to 70 days was not affected by 2,4-D at
concentrations up to 176 mg/litre.
Presing (1981) monitored reproduction over four broods in the water
flea Daphnia magna exposed to 0, 5, 10, 25, or 50 mg/litre of
`Dikonirt' (sodium salt of 2,4-D). For the first brood, the only
significant effect was at 50 mg/litre, whereas the fourth brood was
delayed even at 5 or 10 mg/litre. Significant reductions in the
average number of young produced for each female were found with
the two highest concentrations. Young kept until maturity from
each of the tests were themselves exposed to 2,4-D in a repeat
experiment. Again there was a significant effect on young produced at
25 and 50 mg/litre.
Table 3. Toxicity of 2,4-D to estuarine or marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Salinity pH Formulationc Parameter Water Reference
stata (°C) (o/oo) concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bay mussel 17.2- 22.9- 6.4- free acid 96-h LC50 259 Liu &
(Mytilus edulis) 18.6 24.5 7.8 (232-289) Lee (1975)
17.2- 22.9- 6.4- free acid 96-h EC50 262 Liu &
18.6 24.5 7.8 attachment Lee (1975)
(trocophore larva) 17.2- 22.9- 6.4- free acid 48-h EC50 211.7 Liu &
18.6 24.5 7.8 normal Lee (1975)
development
Eastern oyster flow 18 29 butoxyethanol 96-h EC50 3.75 Butler
(Crassostrea virginica) shell growth (1963)
flow 29 25 isooctyl 96-h EC50 1.0 Mayer
shell growth (1987)
flow 28 25 PGBEE 96-h EC50 0.055 Mayer
shell growth (1987)
Copepod 21 7 7.8 butoxyethanol 96-h LC50 3.1 Linden
(Nitocra spinipes) (2.4-4.1) et al.
(1979)
Brown shrimp (adult) flow 30 PGBEE 24-h EC50 0.55 Butler
(Penaeus aztecus) loss of (1963)
equilibrium
(adult) flow 30 PGBEE 48-h EC50 0.55 Butler
loss of (1963)
equilibrium
(juv.)b stat 26 30 butoxyethanol 48-h LC50 5.6 Mayer
(1987)
(adult) flow 29 26 isooctyl 48-h LC50 0.48 Mayer
(1987)
Dungeness crab (1st zoel) stat 13 25 acid (tech) 96-h LC50 > 10 Caldwell
(Cancer magister) (1977)
(1st instar juv.)b stat 13 25 acid (tech) 96-h LC50 > 100 Caldwell
(1977)
Blue crab (juv.)b stat 24 29 PGBEE 48-h LC50 2.8 Mayer
(Callinectes sapidus) (1987)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration
in water continuously maintained.
b juv. = juvenile.
c PGBEE = propylene glycol butyl ethyl ester.
Table 4. Toxicity of 2,4-D to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Oligochaete worm flow 20 30 30 7.8 free acid 48-h LC50 122.2 Bailey &
(Lumbriculus flow 20 30 30 7.8 free acid 96-h LC50 122.2 Liu (1980)
variegatus)
Water flea stat 21 260 272 7.4 PGBEE 48-h LC50 0.1 Sanders (1970a)
(Daphnia magna) stat 21 260 272 7.4 dimethylamine 48-h LC50 4.0 Sanders (1970a)
stat 17 39 7.2 dimethylamine 48-h LC50 > 100.0 Mayer &
Ellersieck(1986)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 5.6 Sanders (1970a)
stat 21 260 272 7.4 free acid 48-h LC50 > 100.0 Sanders (1970a)
20 8.4- free acid 96-h LC50 417.8 Presing (1981)
8.6
20 8.4- sodium salt 96-h LC50 932.1 Presing (1981)
8.6
Water flea 15.6 44 7.4 PGBEE 48-h LC50 4.9 Sanders &
(Simocephalus (4.0-6.7) Cope (1966)
serrulatus)
Water flea 15.6 PGBEE 48-h LC50 3.2 Sanders &
(Daphnia pulex) (2.4-4.3) Cope (1966)
Copepod (nauplius larva)
(Cyclops vernalis) stat 20 31.6 70 6.7 free acid 96-h LC50 8.72 Robertson (1975)
(5.34-11.57)
stat 20 31.6 70 6.7 alkanolamine 96-h LC50 54.8 Robertson (1975)
(46.45-64.6)
Scud stat 21.1 30 7.1 butoxyethanol 24-h LC50 1.4 (1.1-1.8) Sanders (1969)
(Gammarus stat 21.1 30 7.1 butoxyethanol 48-h LC50 0.76 (0.51
lacustris) -1.1) Sanders (1969)
stat 21.1 30 7.1 butoxyethanol 96-h LC50 0.44 (0.31
-0.62) Sanders (1969)
stat 21.1 30 7.1 PGBEE 24-h LC50 2.1 (1.7-2.5) Sanders (1969)
stat 21.1 30 7.1 PGBEE 48-h LC50 1.8 (1.4-2.3) Sanders (1969)
stat 21.1 30 7.1 PGBEE 96-h LC50 1.6 (1.2-2.1) Sanders (1969)
stat 21.1 30 7.1 isooctyl 24-h LC50 6.8 (4.8-9.7) Sanders (1969)
stat 21.1 30 7.1 isooctyl 48-h LC50 4.6 (2.9-7.3) Sanders (1969)
stat 21.1 30 7.1 isooctyl 96-h LC50 2.4 (1.9-4.8) Sanders (1969)
stat 15.5 260 272 7.4 PGBEE 24-h LC50 4.1 (2.8-5.8) Sanders (1970a)
stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.6 (1.7-3.9) Sanders (1970a)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 15.5 260 272 7.4 PGBEE 96-h LC50 2.5 (1.7-3.7) Sanders (1970a)
(Gammarus stat 15.5 260 272 7.4 butoxyethanol 24-h LC50 6.5 (1.0-8.6) Sanders (1970a)
lacustris) (contd.) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 5.9 (3.1-11) Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 96-h LC50 5.9 (3.1-11) Sanders (1970a)
Scud stat 15 272 7.4 dimethylamine 24-h LC50 > 100 Mayer &
(Gammarus fasciatus) stat 15 272 7.4 dimethylamine 96-h LC50 > 100 Ellersieck (1986)
Glass shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 2.7 Sanders (1970a)
(Palaemonetes stat 21 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
kadiakensis stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.4 Sanders (1970a)
Seed shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 0.32 Sanders (1970a)
(Cypridopsis vidua) stat 21 260 272 7.4 dimethylamine 48-h LC50 8.0 Sanders (1970a)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.8 Sanders (1970a)
Freshwater prawn stat 27 113.9 7.5 sodium salt 24-h LC50 2342 Shukla &
(Macrobranchium stat 27 113.9 7.5 sodium salt 48-h LC50 2309 Omkar (1983)
lamarrei) stat 27 113.9 7.5 sodium salt 72-h LC50 2267 Shukla &
stat 27 113.9 7.5 sodium salt 96-h LC50 2224 Omkar (1983)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2644 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2536 Shukla (1984)
naso) stat 28 112.7 7.5 sodium salt 72-h LC50 2435 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2397 Shukla (1984)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2474 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2381 Shukla (1984)
dayanum) stat 28 112.7 7.5 sodium salt 72-h LC50 2333 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2275 Shukla (1984)
Crayfish stat 15.5 260 272 7.4 PGBEE 48-h LC50 > 100 Sanders (1970a)
(Orconectes nais) stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 > 100 Sanders (1970a)
Red swamp stat 20 100 8.4 alkanolamine 96-h LC50 1389 Cheah et al.
crayfish (imm.)c (1174-1681) (1980)
(Procambarus clarki)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Sowbug stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.2 Sanders (1970a)
(Asellus stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
brevicaudus) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 3.2 Sanders (1970a)
Stone fly (naiad) 15.5 35 7.1 butoxyethanol 24-h LC50 8.5 (5.7-13) Sanders &
(Pteronarcys 15.5 35 7.1 butoxyethanol 48-h LC50 1.8 (1.5-2.7) Cope (1968)
californica) 15.5 35 7.1 butoxyethanol 96-h LC50 1.6 (1.3-1.9) Sanders &
15.5 35 7.1 acid (tech) 24-h LC50 56 (50-63) Cope (1968)
15.5 35 7.1 acid (tech) 48-h LC50 44 (32-59) Sanders &
15.5 35 7.1 acid (tech) 96-h LC50 15 (10-22) Cope (1968)
Midge (larva) 15 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1490 Bunting &
(Chaoborus 15 78-95 55 7.3-7.8 dimethylamine 96-h LC50 890 (421-1211)Robertson
punctipennis) 20 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1124 (1975)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained.
b Alkalinity and hardness expressed as mg CaCO3/litre.
c imm. = immature.
d PGBEE = propylene glycol butyl ether ester.
Table 5. Toxicity of 2,4-D to aquatic invertebrates: no observed effect levels
---------------------------------------------------------------------------------------------------------
Flow/ Temp Sali- Alkali- Hard- Water con- Refer-
Organism stata (°C) nity nityb nessb pH Formulationc Parameterd centration ence
(o/oo) (mg/litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster flow 9 19 free acid 96-h EC0 2.0 Butler
(Crassostrea shell growth (1963)
virginica) flow 30 23 free acid 96-h EC0 2.0 Butler
shell growth (1963)
flow 25 28 dimethylamine 96-h EC0 2.0 Butler
shell growth (1963)
Freshwater oligochaete flow 20 30 30 7.8 free acid 96-h LC0 86.7 Bailey
(Lumbriculus & Liu
variegatus) (1980)
Scud stat 21.1 30 7.1 dimethylamine 96-h LC0 100 Sanders
(Gammarus lacustris) (1969)
Grass shrimp stat 20 20 butoxyethanol 24-h LC0 10 Hansen
(Palaemonetes pugio) et al.
(1973)
Pink shrimp butoxyethanol 48-h LC0 1.0 Butler
(Penaeus duorarum) (1965)
PGBEE 48-h LC0 1.0 Butler
(1965)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester.
d LC0 and EC0 represent the highest dose used which cause no death or no effect, respectively;
they are not mathematically determined no-effect levels.
George et al. (1982) measured lethal times (LT) after exposure of
the water flea Daphnia lumholtzi to 10 or 20 mg 2,4-D/litre. They
reported, for 10 mg/litre, an LT50 of 38 h and an LT100 of 71 h. For
20 mg/litre, the LT50 was 21 h and the LT100 was 31 h. Doses of
2,4-D ranging from 0.1 to 50 mg/litre did not affect the behaviour of,
or kill, the copepod Mesocyclops leuckarti within a 30-day exposure
period and so lethal times could not be calculated.
Caldwell (1977) and Caldwell et al. (1979) found the zoeal larva to
be the most sensitive life-cycle stage of the Dungeness crab (Cancer
magister) to the free acid of 2,4-D. Based on the herbicide's toxicity
to this stage, the authors suggest a maximum acceptable toxicant level
(MATC) of <1 mg/litre. At this concentration, there was no mortality,
but there was an effect on moulting.
6.1.2. Behavioural effects
Folmar (1978) tested mayfly nymphs (Ephemerella walkeri) in a `Y'-
shaped avoidance maze. A 2,4-D dimethylamine salt solution was run
into one arm of the maze and clean water was run into a second arm,
both at 400 ml/min. Numbers of nymphs in each arm of the maze were
counted after 1 h. No avoidance of 2,4-D was found at concentrations
of 10 mg/litre and there was no mortality. At 100 mg/litre there was
70% mortality in the test nymphs but still no avoidance of the
herbicide. In a similar experiment using the grass shrimp
(Palaemonetes pugio) exposed to the butoxyethanol ester of 2,4-D,
there was significant avoidance of the herbicide at 1 mg/litre (Hansen
et al., 1973).
6.2. Toxicity to Fish
Appraisal
At recommended application rates, the concentration of 2,4-D in
water has been estimated to be a maximum of 50 mg/litre. Most
applications would lead to water concentrations much lower than this
(between 0.1 and 1.0 mg/litre).
LC50 values for fish vary considerably. This variation is due to
differences in species sensitivity, chemical structure (esters, salts,
or free acid), and formulation of the herbicide.
Although the free acid is the physiologically toxic entity, the
ester formulations represent a major hazard to fish when used directly
as aquatic herbicides (because they are more readily taken up by fish).
Amine salt formulations used to control aquatic weeds do not affect
adult fish.
The NOEL varies with the species and the formulation: <1 mg/litre
(coho salmon) to 50 mg/litre (rainbow trout).
Fish larvae are the most sensitive life stage but are unlikely to
be affected under normal usage of the herbicide.
Long-term adverse effects on fish are observed only at
concentrations higher than those produced after 2,4-D has been applied
at recommended rates.
Few studies are related to the effects of environmental variables,
such as temperature and water hardness, on 2,4-D toxicity to fish.
Higher temperature possibly increases the toxicity. This might be
considered when assessing the safety of 2,4-D to fish during control of
aquatic weeds.
Fish detect and avoid 2,4-D only at higher concentrations than
those obtained under normal conditions of use.
6.2.1. Effect of formulation on short-term toxicity to fish
The toxicity of different formulations of 2,4-D to fish is
summarized in Table 6.
The most comprehensive study on the effects of different
formulations of 2,4-D using the same test fish, fingerling bluegill
sunfish (Lepomis macrochirus), was performed by Hughes & Davis (1963)
in static 24-h and 48-h tests. Ester formulations were invariably more
toxic than amine salt formulations. Dimethylamine and alkanolamine
preparations ranged in toxicity from 166 to 900 mg/litre (LC50 in 24-h
tests), depending on the commercial preparation used. Although esters
were always more toxic than amine salts, there was some variation
between different ester formulations (range: 0.9 to 66.3 mg/litre; 24-h
LC50). Most of this variation was between different preparations of
the least toxic of the esters, the isooctyl ester, which ranged in
toxicity from 8.8 to 66.3 mg/litre. All other esters tested produced
LC50 values of 8 mg/litre or less, the most toxic being the isopropyl
with a 24-h LC50 of 0.9 mg/litre. The addition of emulsifiers to acid
preparations increased 2,4-D toxicity; a formulation with emulsifiers
gave an LC50 of 8 mg/litre over 24 h, making it comparable to the
esters in toxicity. All ester formulations were considered by the
authors to present a major hazard to fish when used directly as an
aquatic herbicide, whereas the amine salt formulations could be safely
used to control aquatic weeds without adversely affecting adult fish
(Hughes & Davis, 1963).
A study on a range of ester formulations, using salmonids as test
fish, conducted by Finlayson & Verrue (1985), showed that the toxicity
for salmonids was similar to that for bluegill sunfish. These authors
argue that static tests underestimate the toxicity of 2,4-D esters
because some of the ester is hydrolysed to the less-toxic free acid
during the course of even short-term tests. The presence of test fish
increases the rate of hydrolysis of 2,4-D esters. In a static test,
with two different stocking rates of fish, the apparent toxicity of
2,4-D ester decreased with a greater density of test fish (rainbow
trout) because of this enhanced hydrolysis. Results are given in
Table 6. In their flow-through tests, results were adjusted to take
account of the hydrolysis of ester to 2,4-D acid during the course of
the experiment. Two values are given in Table 6 for each test. The
first is the calculated effect of the non-hydrolysed ester and the
second, entered as `total 2,4-D', is the observed effect of the mixture
of ester and free acid produced by hydrolysis during the course of the
experiment. There is as much as a five-fold difference between the two
values. Alabaster (1969) examined several formulations of 2,4-D in two
species of fish, and found that pelleted herbicide, either as clay-
based or resin-based pellets, was the least toxic to fish of any of the
formulations tested.
Table 6. Toxicity of 2,4-D to fish: effects of different formulations
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
--------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 40 29 6.9 alkanolamine 24-h LC50 450-900 Hughes &
(Lepomis macrochirus) stat 25 40 29 6.9 alkanolamine 48-h LC50 435-840 Davis (1963)
stat 25 40 29 6.9 dimethylamine 24-h LC50 166-542 Hughes &
stat 25 40 29 6.9 dimethylamine 48-h LC50 166-458 Davis (1963)
stat 25 40 29 6.9 di-N,N 24-h LC50 1.5 Hughes &
stat 25 40 29 6.9 di-N,N 48-h LC50 1.5 Davis
stat 25 40 29 6.9 2,4-D acid + 24-h LC50 8.0 (1963)
emulsifiers
stat 25 40 29 6.9 2,4-D acid + 48-h LC50 8.0 Hughes &
emulsifiers Davis (1963)
stat 25 40 29 6.9 isooctyl ester 24-h LC50 8.8-66.3 Hughes &
stat 25 40 29 6.9 isooctyl ester 48-h LC50 8.8-59.7 Davis (1963)
stat 25 40 29 6.9 PGBEE 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 PGBEE 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butoxyethanol 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 butoxyethanol 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butyl ester 24-h LC50 1.3 Hughes &
stat 25 40 29 6.9 butyl ester 48-h LC50 1.3 Davis (1963)
stat 25 40 29 6.9 mixed butyl + 24-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 mixed butyl + 48-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 isopropylester 24-h LC50 0.9 Hughes &
stat 25 40 29 6.9 isopropylester 48-h LC50 0.8 Davis (1963)
stat 25 40 29 6.9 ethyl ester 24-h LC50 1.4 Hughes &
stat 25 40 29 6.9 ethyl ester 48-h LC50 1.4 Davis (1963)
Cutthroat trout butyl ester 96-h LC50 0.78 Woodward (1982)
(juvenile) (Salmo clarki) (0.66-0.92)
PGBEE 96-h LC50 0.77 Woodward (1982)
(0.62-0.96)
isooctyl ester 96-h LC50 > 50 Woodward (1982)
Chinook salmon (fry) flow 9 18 17 7.1 butoxyethanol 96-h LC50 0.315 Finlayson &
(Oncorhynchus flow 9 18 17 7.1 total 2,4-D 96-h LC50 0.373 Verrue (1985)
tshawytscha)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.375 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.250 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.246 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.117 Verrue (1985)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout (fry) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.518 Finlayson &
(Salmo gairdneri) flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.642 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.329 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.514 Verrue (1985)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.468 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.338 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.342 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.555 Verrue (1985)
loading factor stat 14 18 17 7.1 butoxyethanol 96-h LC50 1.206 Finlayson &
4.2 g fish/litre Verrue (1985)
stat 14 18 17 7.1 total 2,4-D 96-h LC50 1.422 Finlayson &
loading factor stat 15 18 17 7.1 butoxyethanol 96-h LC50 3.689 Verrue (1985)
8.8 g fish/litre Finlayson &
stat 15 18 17 7.1 total 2,4-D 96-h LC50 4.487 Verrue (1985)
Harlequin fish flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Rasbora heteromorpha) pellets
flow 20 250 7.2 resin-based 24-h LC50 3950 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 3100 Alabaster (1969)
pellets
flow 20 20 7.2 sodium salt 24-h LC50 1160 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 24-h LC50 1.0 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 48-h LC50 1.0 Alabaster (1969)
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Salmo gairdneri) pellets
flow 20 250 7.2 clay-based 48-h LC50 4800 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 24-h LC50 3400 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 2400 Alabaster (1969)
pellets
flow 20 250 7.2 amine salt 24-h LC50 250 Alabaster (1969)
flow 20 250 7.2 amine salt 48-h LC50 210 Alabaster (1969)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test);
flow = flow-through conditions (2,4-D concentration in water
continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c di-N,N = di-N,N-dimethylcocoamine; PGBEE = propylene glycol
butyl ether ester; total 2,4-D = the effect actually observed in the
flow-through test; the value which preceeds each "total 2,4-D" value is
the calculated effect of the ester alone. The authors determined the
degree of hydrolysis of the ester during the course of the test and
subtracted the effect due to the free acid produced by this hydrolysis.
6.2.1.1 Tolerance and potentiation
Chambers et al. (1977) used insecticide-tolerant and insecticide-
susceptible populations of mosquito fish and an esterase inhibitor to
investigate hydrolytic activation and detoxification of 2,4-D esters.
Mosquito fish taken from a wild population which had developed some
tolerance to insecticides also showed some slight tolerance to 2,4-D
ethyl and butyl esters. This tolerance was most pronounced with the
butyl ester, where the 48-h LC50 was raised from 0.98 mg/litre in the
susceptible fish, to 1.70 mg/litre in the tolerant fish. Further
experiments were carried out to find the basis for this tolerance and
for the higher toxicity of 2,4-D esters over that of the free acid.
The addition of DEF (S,S,S-tributyl phosphorotrithioate), a carboxyl
esterase inhibitor, to the toxicity test medium slightly reduced the
toxicity of both 2,4-D esters. This finding suggested that the toxic
effect of the esters required initial hydrolysis to the acid. The
increased toxicity of the esters would result from esters being more
readily absorbed into the fish through the gills. The resistance of
the insecticide-tolerant population was, at least partially, explained
by measuring esterase activity in homogenates of gill and liver from
the two fish populations. The tolerant fish hydrolyzed less of both
2,4-D esters than susceptible fish. This effect was most marked with
liver homogenates; results from gill homogenates were equivocal. The
overall conclusion, put forward by the authors, is that liver
hydrolysis `activates' 2,4-D esters by converting them to the toxic
free acid. Hydrolysis in the gill is a `detoxification' reaction
because it reduces the uptake of toxic material. The slight increase
in tolerance in the insecticide-resistant mosquito fish is largely the
result of decreased activation of the 2,4-D esters by the liver.
Antagonism to 2,4-D ester toxicity by DEF is largely the result of
inhibition of activation in the liver rather than increased
detoxification in either liver or gill (Chambers et al., 1977).
Carbaryl, a cholinesterase inhibitor, potentiates the toxicity of 2,4-D
butyl ester to brown trout (Salmo trutta) (Statham & Lech, 1975).
The 4.5-h LC50 for 2,4-D butyl ester in static tests was shifted from
30 mg/litre to 11 mg/litre by the addition of carbaryl, at a
concentration of 1 mg/litre, to the test water. Carbaryl has no
toxicity to the fish at this concentration; the 24-h LC50 for carbaryl
alone is 6.8 mg/litre. The potentiating effect of carbaryl was itself
blocked by atropine, a muscarinic blocker, at a water concentration of
10 mg/litre; the atropine itself was not toxic to the fish at this
concentration. In a similar way, carbaryl potentiated the toxicity of
several other compounds. The authors suggested a non-specific action,
possibly by increasing the uptake of 2,4-D ester from the water. The
same potentiation was demonstrated for trout in flow-through tests
(Statham & Lech, 1975). Combinations of 2,4-D esters (butyl or
propylene glycol butyl) with the herbicide picloram increased the
toxicity to fish above the combined toxicity of the individual
compounds (Woodward, 1982).
6.2.2. No-observed-effect levels in short-term tests with fish
The NOELs of 2,4-D on fish in short-term tests are summarized in
Table 7. Values quoted from Meehan et al. (1974) and from Butler
(1965) are based on the lowest dose used in their studies. The values
from Birge et al. (1979) are calculated 1% mortality values derived
mathematically from a full toxicity curve, and based on young fish
exposed to 2,4-D from shortly after fertilization of the eggs until
4 days after hatching. The differing exposure times for the three
species tested is due to differences in time to hatching. The
variation in these data is, therefore, partly due to species
differences in sensitivity to the compound and partly due to exposure
times. Newly hatched young fish are more sensitive to 2,4-D than
unhatched embryos (see section 6.2.4).
6.2.3. Species differences in short-term toxicity to fish
Variation in the toxicity of 2,4-D to fish with species is
summarized in Table 8. Of a range of fish species, examined in the
same test conditions, using 2,4-D as the free acid, by Rehwoldt et al.
(1977), the most sensitive was the white perch (Roccus americanus)
with a 24 h LC50 of 55 mg/litre, and the least sensitive was the
eel Anguilla rostrata with an LC50 of 427 mg/litre. The grass carp,
often used together with herbicides to control aquatic vegetation, was
the least sensitive of all species examined, with a 24 h LC50 for an
amine salt formulation of 3080 mg/litre (Tooby et al., 1980).
6.2.4. Toxicity to early life-stages of fish
Short-term studies on the toxicity of 2,4-D to early life-stages of
fish are summarized in Table 9.
Studies on fish eggs and larvae immediately after hatching have
been conducted on few species and mainly with simple salts of 2,4-D.
There is little information on the effects of the more toxic esters.
2,4-D is clearly toxic for fish early life-stages, within the likely
range of water concentrations which would be found after use of the
herbicide to control aquatic weeds.
At the 16 - 32 cell blastomere stage, eggs of the bleak Alburnus
alburnus, developed more slowly than control eggs when exposed to 2,4-D
solutions. After 48-h exposure, mortality reached 68% and 79% in
those eggs exposed to 2,4-D at 25 and 50 mg/litre, respectively.
Control mortality after 48-h exposure was 37%. After 23 h of
development, eggs exposed to 25 mg 2,4-D/litre showed normal
development while those exposed to 100 mg/litre showed slower
embryogenesis or development halted at the morula-gastrula stage.
Free-swimming larvae were more sensitive to 2,4-D than eggs; the rate
of survival of embryos in tests lasting for between 12 and 48 h was
higher than for larvae. In tests lasting for between 24 and 48 h, at
concentrations of 2,4-D above 400 mg/litre, no larvae survived.
Embryos showed malformations and reduced mobility at concentrations
above 100 mg/litre, and at concentrations of 800 mg/litre or more,
embryos were immobile (Biro, 1979).
Birge et al. (1979) examined the effects of 2,4-D, as the potassium
salt, on the eggs and larvae of three species of fish. Rainbow trout
eggs were the most sensitive, largemouth bass eggs less sensitive, and
goldfish eggs extremely tolerant to 2,4-D. In all species tested,
the larval stages were more sensitive to the herbicide than were the
eggs.
Table 7. Toxicity of 2,4-D to fish: no-observed-effect levels
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Pink salmon (fry) stat 10 10-34 free acid 96-h LC0 < 1 Meehan
(Oncorhynchus stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
gorbuscha stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Chum salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus keto) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Coho salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus kisutch) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Sockeye salmon (smolts) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus nerka) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Alaska coho salmon stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
stat 10 10-34 PGBEE 96-h LC0 < 1
Oregon coho salmon stat 10 10-34 free acid 96-h LC0 10 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Dolly Varden stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salvelinus malma) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Rainbow trout stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salmo gairdneri) (1974)
Spot stat free acid 48-h LC0 50 Butler
(Leistomus xanthurus) (1965)
Longnose killifish stat dimethylamine 48-h LC0 15 Butler
(Fundulus similis) (1965)
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mullet (Mugil cephalus) stat ethylhexyl 48-h LC0 10 Butler
(1965)
White mullet (juvenile) flow sea free acid 48-h LC0 50.0 Butler
(Mugil curema) water (1963)
Goldfish flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC1 8.2 Birge et al.
(Carassius auratus) 25.8 (2.7-15.0) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC1 8.9 Birge et al.
25.8 (3.8-14.6) (1979)d
Largemouth bass flow 18.2- 66.7 53.5 7.84 pot. salt 7.5-day LC1 13.1 Birge et al.
(Micropterus salmoides) 25.8 (4.4-21-9) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC1 3.2 Birge et al.
25.8 (1.2-6.0) (1979)d
Rainbow trout flow 12.5- 66.7 53.5 7.84 pot. salt 27-day LC1 0.032 Birge et al.
(Salmo gairdneri) 14.5 (0.008-0.084) (1979)d
flow 12.5- 65.3 197.5 7.78 pot. salt 27-day LC1 0.022 Birge et al.
14.5 (0.006-0.055) (1979)d
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c pot. salt = potassium salt; PGBEE = propylene glycol butyl ether ester.
LC0 obtained by extrapolation and LC1 mathematically calculated.
d Birge et al. (1979) exposed fish from four days after hatching.
Table 8. Toxicity of 2,4-D to fish: species variation
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Striped bass stat 20 50 7.2 free acid 24-h LC50 85.6 Rehwoldt
(Morone saxatilis) stat 20 50 7.2 free acid 96-h LC50 70.1 et al.
(1977)
Banded killifish stat 20 50 7.2 free acid 24-h LC50 306.2 Rehwoldt
(Fundulus diaphanus) stat 20 50 7.2 free acid 96-h LC50 26.7 et al.
(1977)
Pumpkinseed sunfish stat 20 50 7.2 free acid 24-h LC50 120 Rehwoldt
(Lepomis gibbosus) stat 20 50 7.2 free acid 96-h LC50 94.6 et al.
(1977)
White perch stat 20 50 7.2 free acid 24-h LC50 55.5 Rehwoldt
(Roccus americanus) stat 20 50 7.2 free acid 96-h LC50 40 et al.
(1977)
American eel stat 20 50 7.2 free acid 24-h LC50 427.2 Rehwoldt
(Anguilla rostrata) stat 20 50 7.2 free acid 96-h LC50 300.6 et al.
(1977)
Carp stat 20 50 7.2 free acid 24-h LC50 175.2 Rehwoldt
(Cyprinus carpio) stat 20 50 7.2 free acid 96-h LC50 96.5 et al.
(1977)
Guppy stat 20 50 7.2 free acid 24-h LC50 76.7 Rehwoldt
(Lebistes reticulata) stat 20 50 7.2 free acid 96-h LC50 70.7 et al.
(1977)
Grass carp flow 13 270 8.1 amine salt 24-h LC50 3080 Tooby et
(Ctenopharyngodon (2622-3618) al. (1980)
idella) flow 13 270 8.1 amine salt 48-h LC50 2540 Tooby et
(2184-2952) al. (1980)
flow 13 270 8.1 amine salt 96-h LC50 1313 Tooby et
(1116-1544) al. (1980)
Bleak stat 10 15 7.8 butoxyethanol 96-h LC50 3.2-3.7 Linden et
(Alburnus alburnus) al. (1979)
Mosquito fish stat 21-22 amine salt 24-h LC50 500 Johnson
(Gambusia affinis) stat 21-22 amine salt 48-h LC50 445 (1978)
stat 21-22 amine salt 96-h LC50 405
Mullet stat sodium salt 24-h LC50 68.0 Tag El-Din
(Mugil cephalus) stat sodium salt 96-h LC50 32.0 et al.
(1981)
Longnosed killifish stat butoxyethanol 48-h LC50 5.0 Butler
(Fundulus similis) stat PGBEE 48-h LC50 4.5 (1965)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 19 7.0 dimethylamine 24-h LC50 390 Davis &
(Lepomis macrochirus) stat 25 19 7.0 dimethylamine 48-h LC50 375 Hardcastle
(1959)
Largemouth bass stat 25 19 7.0 dimethylamine 24-h LC50 375 Davis &
(Micropterus salmoides) stat 25 19 7.0 dimethylamine 48-h LC50 350 Hardcastle
(1959)
Punti (Puntius ticto) stat 23.5 ethyl ester 24-h LC50 1.6 Verma et
al. (1984)
Medaka (Oryzias latipes) sodium salt 48-h LC50 > 40 Hashimoto &
Nishiuchi
(1978)
Longnose killifish (juv.) flow sea water PGBEE 24-h LC50 5.0 Butler
(Fundulus similis) flow sea water PGBEE 48-h LC50 4.5 (1963)
flow sea water butoxyethanol 24-h LC50 5.0 Butler
flow sea water butoxyethanol 48-h LC50 5.0 (1963)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester
Table 9. Toxicity of 2,4-D to fish early life-stages
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bleak (embryo) sodium salt 12-h LC50 159.4 Biro (1979)
(Alburnus alburnus) sodium salt 24-h LC50 129.0 Biro (1979)
sodium salt 36-h LC50 63.9 Biro (1979)
sodium salt 48-h LC50 12.9 Biro (1979)
(larvae) sodium salt 12-h LC50 111.2 Biro (1979)
sodium salt 24-h LC50 70.6 Biro (1979)
sodium salt 36-h LC50 62.1 Biro (1979)
sodium salt 48-h LC50 51.6 Biro (1979)
Goldfish (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 4-day LC50 > 187 Birge et
(Carassius auratus) 25.8 al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 4-day LC50 > 201 Birge et
25.8 al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC50 133.1 Birge et
post-hatch) 25.8 (108.6-174.8) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC50 119.1 Birge et
25.8 (98.5-150.6) al. (1979)
Largemouth bass (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 3.5-day LC50 165.4 Birge et
(Micropterus salmoides) 25.8 (130.6-274.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 3.5-day LC50 160.7 Birge et
25.8 (122.9-230.6) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 7.5-day LC50 108.6 Birge et
post-hatch) 25.8 (92.5-138.4) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC50 81.6 Birge et
25.8 (64.8-103.5) al. (1979)
Rainbow trout (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 23-day LC50 11.0 Birge et
(Salmo gairdneri) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 23-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 27-day LC50 11.0 Birge et
post-hatch) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 27-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (2,4-D concentration
in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
Pot. salt = potassium salt.
Only one study has examined the effects of 2,4-D esters on newly-
hatched fish fry and on fertilized eggs. Unfortunately, this study, by
Hiltibran (1967), does not record the full experimental details. Four
species of fish were used in the study but complete results were given
only for the bluegill sunfish. The most toxic preparations were the
propylene glycol butyl ether (PGBE) ester and mixed isopropyl and butyl
esters with no-observed-effect levels of 2 and 3 mg/litre,
respectively. The dimethylamine salt, ethylhexyl ester, and sodium
salt formulations were less toxic with no-observed-effect levels at 40,
50, and 100 mg/litre, respectively.
6.2.5. Long-term toxicity to fish
Chronic exposure of sub-adult fish of a variety of species to 2,4-D
for 10 months, at a concentration of 0.1 mg/litre, led to no mortality
and to no change in the acute response to 2,4-D. The 24-h LC50 for
the compound was unchanged after 10 months exposure to sub-lethal
doses; i.e., no tolerance developed. One species of fish, the guppy
Lebistes reticulatus, reproduced in captivity. Reproductive success
was compared between control fish and fish breeding in water
containing 2,4-D at 0.1 mg/litre over 10 months. The ratio of numbers
of offspring of treated and control fish was 1.2 (Rehwoldt et al.,
1977). Mount & Stephan (1967) conducted a 10-month study on fathead
minnows (Pimephales promelas) exposed to the butoxyethanol ester of
2,4-D at 0, 0.01, 0.04, 0.2, or 0.8 mg/litre water in a flow-through
test system. Concentrations of 2,4-D of 0.2 mg/litre or less had no
observable effect on growth, survival, or reproductive success of the
fish, but the highest concentration tested was toxic to eggs. The
highest tested no-observed-effect concentration was approximately
1/45 of the 96-h LC50 for this species. Finlayson & Verrue (1985)
conducted a chronic egg-to-fry test over 86 days using chinook
salmon in 2,4-D butoxyethanol ester solutions ranging up to
118 µg/litre. The mortality of salmon during the alevin to fry period
was 4.7% and 47.6% for exposures to 60 and 118 µg/litre, respectively.
When compared to controls, the length of salmon at 36 days
post hatch was significantly reduced after exposure to 60 and
118 µg/litre. Neither survival nor growth of fry were affected at
2,4-D concentrations of 40 µg/litre or less. This maximum acceptable
toxicant concentration (MATC) represents 0.13 and 0.11 of the 96-h
LC50 values for fry and smolts of this species, respectively.
6.2.6. Behavioural effects on fish
Folmar (1976) used rainbow trout fry in an investigation to
determine whether fish avoided water contaminated by herbicides. Trout
fry were initially placed in a `Y'-shaped maze for 15 min. The
dimethylamine salt of 2,4-D, then was added to one arm of the maze and
clean water in a second arm, both at flow rates of 400 ml/min. The
number of fish in each arm was counted after 1 hour and results tested
statistically using a Chi-squared test. At concentrations of 2,4-D of
0.1 mg/litre water (approximately equal to water levels after the use
of this preparation as an aquatic herbicide), there was no avoidance
of the compound; the numbers of fish in each arm of the maze were
equal. However, at concentrations of 1.0 or 10.0 mg/litre of 2,4-D,
there was significant avoidance of the chemical.
Hidaka et al. (1984) conducted a similar study using medakas
Oryzias latipes, and tested a wide range of doses for a variety of
pesticides and herbicides. In all cases, the fish avoided the
chemical in a dose-related manner, but only over a limited range of
concentrations. Above this range, presumably because of the onset of
toxic effects, there was a dose-related decrease in avoidance response.
The authors calculated two values from these chevron-shaped graphs,
the avoidance response EC65 taken from the increasing curve
(AR65) and the DAR60 taken from the decreasing curve. For 2,4-D, the
value for AR65 was 177 (171-182; 95% confidence limits) µg/litre, and
for DAR60 was 288 (245-338) µg/litre. Compared to other chemicals
commonly used or found in water, 2,4-D has a high threshold of
detection by fish as indicated by the high avoidance threshold.
Rand & Barthalmus (1980) exposed goldfish to 2,4-D at 20 mg/litre
(10% of the 96-h LC50 for this species) at different stages during the
training period for conditioning the fish to avoid electric shocks.
They found that the herbicide had no effect when given for 24 h on the
9th day of conditioning but was effective in changing the magnitude of
the avoidance response when given for the first 24 h of conditioning.
Fish exposed for 2 weeks showed significant differences in the pattern,
rate of acquisition, and maintenance of the avoidance baseline.
Behavioural differences persisted into the post-exposure period in fish
exposed to 2,4-D for 2 weeks. The authors point out that short-term
toxicity tests do not examine subtle behavioural effects which could be
of considerable importance in the wild.
Dodson & Mayfield (1979) assessed the effect of 2,4-D on the
``reotaxic response'' of rainbow trout, that is their tendency to swim
upstream to compensate for flowing water. There was a dose-related
effect of 2,4-D at concentrations between 0 and 7 mg/litre. Above this
concentration range, there was a fall of more than 50% in the frequency
of positive reotaxic response to a revolving drum marked in alternate
light and dark stripes and an increase in the frequency of `no-
response'. The authors state that, at ``realistic concentrations'' of
2,4-D in water, there would be a tendency for fish to be moved
downstream because of a reduced reotaxic response.
6.2.7. Effects of environmental variables on toxicity to fish
The toxicity of 2,4-D to fish is related to season. Vardia &
Durve (1981) obtained different values for 96-h LC50 for the carp
Cyprinus carpio at different times of the year. Water
characteristics did not differ, except for temperature which varied
from 39 °C in May, its highest value, to 17 °C in February, its lowest.
There was a positive correlation between temperature and toxicity. At
39 °C, the 96-h LC50 was 5.6 mg/litre, and, at 17 °C, the LC50 was
40.83 mg/litre. As the authors point out, the temperature of the water
must be borne in mind when assessing the safety of this compound to
fish during control of aquatic weeds. The effect may be one of season
rather than temperature; the physiology of the fish also changes
throughout the year.
There is some effect of water hardness and pH on 2,4-D toxicity to
fish but this is very dependent on the species of test fish (Birge et
al., 1979). This effect has not been studied systematically.
6.2.8. Special studies on fish
Chronic exposure of carp to sub-lethal concentrations of 2,4-D
(5 mg/litre) led to ultrastructural changes in the liver of the fish
(Benedeczky et al., 1984). After 2 months, there was detectable
swelling of mitochondria and loss of cristae. There were also large
numbers of inclusions in the cytoplasm, interpreted by the authors as
bile pigments. Their presence in the bile canaliculi indicated the
onset of cholestasis (reduction in bile flow). After 3, 4, or 5 months
of exposure, the cholestasis was pronounced with cholesterin crystals
appearing as cytoplasmic inclusions. Later, in the 6th month of
exposure, there were endoplasmic reticulum changes indicative of
changed protein synthesis.
Oxygen consumption by bluegill sunfish was not affected by 2,4-D at
a concentration of 3 mg/litre water (Sigmon, 1979).
2,4-D at 10-4 mol/litre of medium did not affect the activity of
Na/K-ATPase in microsomes from trout gill (Davis et al., 1972).
Verma et al. (1984) detected effects on pituitary and pineal
histology in punti Puntius ticto exposed for 96 h to 1 mg/litre of
Weedone (ethyl ester of 2,4-D). There was a significant effect on cell
size of acidophilic pituitary cells but a much more marked effect on
cyanophils. Pineal epithelium cell height was significantly greater in
exposed fish.
6.3. Toxicity to Amphibians
Appraisal
Amphibian larvae are generally tolerant to amine salts of 2,4-D;
the 96-h LC50 values exceed 100 mg/litre. Of the species tested, only
one was sensitive.
No information is available on reproductive development and
differentiation or on tissue levels.
The toxicity of 2,4-D to amphibians is summarized in Table 10.
Tadpoles of the Indian toad are particularly susceptible to the
compound (Vardia et al., 1984). Lhoste & Roth (1946) showed that
2,4-D, at 5 g/litre, prevented development of the eggs of the common
frog (Rana temporaria). At doses between 500 mg/litre and 4 g/litre,
there was some development which decreased with increasing dose. These
levels are far higher than those likely to be encountered in the
environment.
Table 10. Toxicity of 2,4-D to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Refer-
stata (°C) nityb nessb concentration ence
(mg/litre)
-------------------------------------------------------------------------------------------------------------------------------------------------
Chorus frog (tadpole) stat 15.5 30 7.1 dimethylamine 24-h LC50 > 100 Sanders
(Pseudacris triseriata) stat 15.5 30 7.1 dimethylamine 96-h LC50 > 100 (1970b)
Indian toad (tadpole) 25 210 220 8.3 free acid 24-h LC50 13.77 Vardia et
(11.81-16.05) al. (1984)
(Bufo melanostictus) 25 210 220 8.3 free acid 48-h LC50 9.03 Vardia et
(8.23-9.91) al. (1984)
25 210 220 8.3 free acid 96-h LC50 8.05 Vardia et
(7.29-8.81) al. (1984)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 255 Johnson
(Adelotus brevis) stat 21-22 amine salt 48-h LC50 228 (1976)
stat 21-22 amine salt 96-h LC50 200 Johnson
(1976)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 321 Johnson
(Limnodynastes peroni) stat 21-22 amine salt 48-h LC50 300 (1976)
stat 21-22 amine salt 96-h LC50 287 Johnson
(1976)
Toad (tadpole) stat 21-22 amine salt 24-h LC50 346 Johnson
(Bufo marinus) stat 21-22 amine salt 48-h LC50 333 (1976)
stat 21-22 amine salt 96-h LC50 288 Johnson
(1976)
Common frog (tadpole) 17-29 free acid 48-h LC0 50 Cooke
(Rana temporaria) (1972)
-------------------------------------------------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
For terrestrial application, 2,4-D is usually used in the form of
the less volatile, longer-chain esters to reduce drift damage of sprays
to broad-leaved crop plants. The herbicide is used on cereal crops and
on rangeland against broad-leaved weeds and also in forestry. Thus,
insects of all kinds will be exposed to 2,4-D. Birds' eggs in the nest
are more likely to be exposed than the adult birds, though adult
exposure remains possible, particularly for sitting birds. Ground-
nesting species will be exposed from the use of 2,4-D on rangeland and
cereals; tree-nesting species will be exposed in forests.
The insecticidal action of 2,4-D is low enough that the compound
does not represent a hazard to beneficial insects; there is an adequate
safety margin with usage at recommended levels.
Although there is some disagreement in the literature about the
toxicity of 2,4-D to birds' eggs, the low uptake of the material
through the egg shell suggests that exposure would not affect hatching
in normal use of the compound. Adult birds are not affected by short-
term exposure to 2,4-D. The likelihood of prolonged exposure of
either adult birds or eggs to high levels of 2,4-D is small.
7.1. Toxicity to Terrestrial Invertebrates
Appraisal
Based on the widespread use of 2,4-D and its formulations, insects
of many kinds could be exposed to the material. Although the compounds
are generally classified as non-toxic for beneficial insects, such as
honey bees and natural enemies of pests, some adverse effects have been
reported on the early life-stages and adults of some insects.
Esters are less toxic to insects than are salts or the free acid.
Feeding studies dosing worker honey bees (Apis mellifera) with
2,4-D salts in sucrose syrup have generated two estimates of 24-h
LC50: 104 and 115 µg/bee (Jones & Connell, 1954; Beran & Neururer,
1955). Morton et al. (1972) fed 2,4-D acid to honey bees in 60%
sucrose syrup at 10, 100, or 1000 mg/litre and monitored half-time,
i.e., the time taken for 50% of the bees in a cage to die. The half-
time was significantly longer than that of controls for the two lowest
doses (37.2 days at 10 mg/litre and 40.4 days at 100 mg/litre,
compared to a control value of 33.4 days), but was significantly
reduced at 1000 mg/litre (18.6 days). The butoxyethanol and isooctyl
(commercial formulation) esters of 2,4-D had no effect on survival
times at 10, 100, or 1000 mg/litre of syrup when fed under the same
conditions as the acid. The dimethylamine salt of 2,4-D (commercial
formulation) had no effect at 10 or 100 mg/litre, but did shorten the
half-time at 1000 mg/litre.
The effects of 2,4-D on beneficial coccinellid larvae were studied
by Adams (1960). Larvae were sprayed with a preparation of mixed amine
salts of 2,4-D, at a rate equivalent to 0.56 kg acid equivalent/ha, at
different stages of their development 1, 3, 6, 9, or 12 days after
hatching. There was a lengthening of the development period when the
larvae were treated on days 3, 6, 9, or 12 but no effect when they were
sprayed on the first day after hatching. Mortality before pupation was
more than doubled in all treated groups, but mortality during pupation
was not different from that of controls.
Trumble & Kok (1980) dosed adult thistle-rosette weevils
(Ceuthorhynchidius horridus), which are used for the biological
control of musk thistle, with 2,4-D amine salt at five dose levels
between 0.17 and 147.8 kg/ha. No significant mortality was
observed, up to 175 days after treatment, at doses up to and including
1.68 kg/ha. At 16.8 and 84 kg/ha, there was significantly increased
mortality after day 3 post-treatment. At the highest dose level of
147.8 kg/ha, mortality was increased both on day 3 and subsequently.
Five-day LC50 values for males and females were calculated at 70.2 and
61.4 kg/ha, respectively. This is 41.8 times the recommended
application rate of 2,4-D for males and 36.6 for females. Riviere
(1976) reared the European cockroach (Blatella germanica) on food
containing 1000 mg/kg and reported negligible effects on reproduction.
Gall & Dogger (1967) wetted wheat plants with a 0.3% solution of
mixed isopropyl and butyl esters of 2,4-D and exposed the plants to
females of the wheat stem sawfly (Cephus cinctus). Spraying of the
wheat plants was performed at different times relative to oviposition;
times were 7 days prior to oviposition, at the time of oviposition, or
7, 14, or 21 days after oviposition. Eggs took about 7 days to hatch.
The highest larval mortality (96.4%) occurred after spraying at the
time of egg laying. The effectiveness of the 2,4-D in killing larvae
decreased with later exposure times. For plants sprayed 7, 14, and 21
days after oviposition, larval mortalities were 68.1%, 60.8%, and 37%,
respectively, compared to a control mortality of 30%. When plants were
sprayed 7 days before egg laying, larval mortality was 46.9%. Adult
flies were not affected by 2,4-D spray.
Muller (1971) exposed beetles (Carabidae) to sand dose d with
2,4-D at 0.2, 1.0, or 2.0 g/m2. Two species, Bembidion
femoratum and B. ustulatum, both showed more than 50% mortality within
4 days of exposure to 1.0 g 2,4-D/m2. B. ustulatum showed 100%
mortality within 10 days when exposed to 1.0 g/m2 and similar
mortality within 4 days when exposed to 2.0 g/m2. About 20% of the
individuals of B. femoratum survived the 14-day exposure to both 1.0
and 2.0 g/m2.
Roberts & Dorough (1984) exposed earthworms to 2,4-D acid sprayed
on to filter paper. The papers were wetted and the earthworms exposed
to the wetted paper in glass vials. The calculated 48-h LC50 value
was 61.6 (41 - 92.4; 95% confidence limits) µg/cm2.
Rapoport & Cangioli (1963) treated turf with a mixture of
(4-chloro-2-methylphenoxy)acetic acid (MCPA), at recommended rates, and
the butyl ester of 2,4-D, at 10 times the recommended rate. They
reported no effect on soil microarthropods.
7.2. Toxicity to Birds
Appraisal
Birds, and particularly the eggs of ground-nesting species, would
be exposed to 2,4-D after spraying. Food items could also be expected
to be contaminated by the herbicide. However, most studies on birds
and their eggs have been conducted at exposures far higher than could
be expected in the field.
LD50 values from acute oral and from short-term dietary dosing
indicate low toxicity of 2,4-D to birds. In longer-term studies,
effects have only been reported at extremely high exposures (for
example, kidney effects after dosing in drinking water with
concentrations in excess of the solubility of the material). There
have been no reported effects on reproductive parameters, even at
excessive exposure levels.
A single study reported adverse effects on the embryos of birds'
eggs sprayed with 2,4-D. Many studies since have shown no effect on
hatchability of eggs and no increased incidence of abnormalities in
chicks even after very high exposure to 2,4-D. Other work indicates a
very poor penetration of the eggshell by the herbicide. It can only be
concluded that after normal, or even after excessive, 2,4-D use, there
would be no effect on birds' eggs.
7.2.1. Toxicity to birds' eggs
There have been several studies on the toxicity of various 2,4-D
formulations to birds' eggs dosed by different routes.
Spraying eggs of pheasant, red-legged partridge, and grey partridge
with the equivalent of 0.55 to 1.1 kg 2,4-D/ha, either before
incubation or after 3 days of incubation, was found by Lutz-Ostertag &
Lutz (1970) and Lutz & Lutz-Ostertag (1972) to cause embryonic
abnormalities. They reported 77% mortality in pheasant eggs, 77% in
grey partridge eggs, and 43% in red-legged partridge eggs within the
first 19 days of incubation. The eggs were broken open, between days
20 and 22 of incubation for histopathological examination of the
embryos; hatching takes place at about 24 days in all species used. A
majority of surviving embryos were either wholly or partially
paralyzed. Histopathological effects were mainly gonadal. In both
male and female embryos, there were abnormalities of the gonad, often
severe enough to lead to sterility, and, in the male, abnormal
regression of the Mullerian ducts. No control embryos were examined.
In a second study by Lutz & Lutz-Ostertag (1973), quail, pheasant, and
partridge eggs were sprayed with 2,4-D from two different commercial
sources either before incubation or on days 3 or 7 after the start of
incubation. The authors reported increased mortality in embryos,
reduced hatchability, and increased abnormality in chicks. The only
control eggs reported were those in a single incubation of quail.
Later work has failed to repeat these results.
Kopischke (1972) found no adverse effects on hatchability and no
increase in deformities or later mortality of hatched chicks after
spraying eggs of pheasants or chickens with an isooctyl ester
formulation of 2,4-D on day 13 of incubation at a dose equivalent to
0.28 kg/ha.
Somers et al. (1974) sprayed chicken eggs, prior to incubation,
with concentrations of an amine salt of 2,4-D at up to 15 times the
recommended field application rate of approximately 3 kg/ha. There was
no effect on hatching success or on the survival of chicks in the
period 3 to 4 weeks post hatch. Spraying chicken eggs on days 0, 4, or
18 of incubation with 2,4-D (as a PGBE ester formulation) at up to 10
times the field application rate, had no effect on hatchability or on
survival and growth of chicks after hatching (Somers et al., 1978a).
Birds hatched from eggs, similarly treated by spraying, showed no
significant adverse effects on later reproductive performance (egg
laying performance of the females; testis weight or sperm count of
males) (Somers et al., 1978b). Hilbig et al. (1976a) found no effect
on egg hatch rate or on body weight or malformation rate in chicks,
after spraying (at 20 kg/ha) the eggs of Japanese quail, pheasants, and
chickens prior to incubation, or 3 days after the start of incubation.
In a follow-up study on the reproductive performance of birds hatched
from these dosed eggs, Hilbig et al. (1976b) reported no effects on
laying capacity, fertility, or hatchability of their eggs.
The effects of 2,4-D dimethylamine salt on the eggs of Japanese
quail, grey partridge, and red-legged partridge were studied by
Grolleau et al. (1974). Eggs were sprayed with 2,4-D at dose levels
equivalent to the recommended application rate (1.2 kg/ha) and at two
higher dose levels equivalent to 2.4 and 6 kg/ha. There were no
effects on hatching rate, embryonic mortality, or chick mortality in
the first month after hatching or on embryonic or chick malformations.
In addition, the histopathological examination of partridge thyroids
revealed no effects. Residues of 2,4-D were measured in those
partridge eggs receiving the highest dose. Very little 2,4-D
penetrated the egg shell and the highest residue measured was a total
egg content of 19.3 µg (in an 11-g egg, 15 days after treatment). The
lack of effect of 2,4-D on sprayed eggs was attributed to the poor
penetration of the herbicide. Spittler (1976) found no adverse
effects of 2,4-D on hatchability and no increase in chick abnormalities
in pheasant or quail eggs sprayed 24 h before hatching with a dose 12
times higher than the recommended application rate. Only at a dose 30
times higher than the recommended rate did hatchability fall by 10% to
15%, relative to controls. No increased incidence of abnormalities was
reported at this dose rate.
Hoffman & Albers (1984) immersed mallard eggs for 30 seconds in
aqueous emulsions of 2,4-D and calculated an LC50 equivalent to a
field application rate of 216 (155 - 300) kg/ha. This is 32 times the
recommended field application rate. Dunachie & Fletcher (1967)
injected chicken eggs with 10, 100, or 200 mg 2,4-D/kg, equivalent to
0.5, 5, or 10 mg/egg, and found reduced hatchability relative to
control eggs injected with solvent only. Treated eggs showed 80-90%,
70%, and 50% of the control hatch rate for the three dose rates,
respectively. In a similar study, Gyrd-Hansen & Dalgaard-Mikkelsen
(1974) found that injecting 1 mg/egg or less of the dimethylamine salt
of 2,4-D had no effect. Injections of 2 mg/egg reduced both
hatchability of the eggs and survival of hatched chicks. An injected
dose of 5 mg/egg reduced the hatching rate to 15% of control levels and
there were no surviving chicks after 1 week. There was no successful
hatching after an injection of 10 mg/egg. The same authors also dosed
eggs by immersion in solutions of 2,4-D for 10 seconds. There was no
effect after immersion in a solution of 10 g/litre and only a slight
effect after immersion in 50 g/litre. The hatching success and
survival of the chicks up to 4 weeks post hatch, after immersion in
50 g/litre, was more than 80% of control values.
7.2.2. Toxicity to birds after short-term and long-term dosing
The toxicity of 2,4-D (given either orally by capsule or in the
diet) to birds is summarized in Table 11. The studies reported in this
Table include single oral dosing, repeated oral dosing, and dietary
tests over 5 to 100 days. Studies lasting 10 days or less show that
high dosage (in excess of 1000 mg/kg food) is required to kill birds.
2,4-D is, therefore, of low toxicity to birds.
Haegele & Tucker (1974) dosed egg-laying Japanese quail and mallard
a single oral dose of 250 or 1500 mg 2,4-D acid/kg body weight and
monitored egg shell thickness. There was a short-term effect; thin-
shelled eggs were produced during the first 3 days after dosing. This
was considered to be an indirect effect, i.e., the result of reduced
food consumption. When Bjorklund & Erne (1966) gave single oral doses
of 100, 200, or 300 mg 2,4-D amine/kg body weight to chickens, all
clinical and gross pathological findings were negative, with the
exception of a single bird showing gastritis after the highest dose.
2,4-D amine was given orally at 300 mg/kg body weight to a second group
of chickens each day. One bird died after 5 days and was shown on
autopsy to have developed renal and visceral gout. The other birds
were killed on days 12 or 24 of dosing. Slight kidney enlargement was
seen, and there was an enhancement of the rate of 2,4-D elimination
with time.
Bjorn & Northen (1948) orally dosed white-rock chicks on alternate
days for a period of 4 weeks (12 doses in total) with an alkanolamine
salt formulation of 2,4-D. All chicks weighed approximately 50 g at
the beginning of dosing and doses were adjusted for weight gain of the
chicks through the dosing period. No effect on weight gain was noted
at doses of 2,4-D up to 280 mg acid equivalent/kg body weight. In a
further study, single oral doses of up to 380 mg/kg body weight were
without effect, but a single oral dose of 765 mg/kg body weight killed
all the birds.
Whitehead & Pettigrew (1972a) dosed 28-week-old laying hens daily,
by gelatin capsule, with the butoxyethyl ester of 2,4-D at either 6.2
or 18.7 mg acid equivalent/bird for 20 weeks. There were no adverse
effects on egg production, egg or yolk weight, egg shell thickness,
hatchability, or growth rate of the progeny.
Chickens were given oral doses of 2,4-D amine salt at 100, 250, or
500 mg/kg body weight or PGBE ester at 50, 100, or 250 mg/kg body
weight for 10 days in a study by Palmer & Radeleff (1969). Birds given
2,4-D amine salt did not differ from controls in weight gain, even at
the highest dose. There was similarly no effect from the lowest dose
of the ester. However, a growth rate reduction was seen with the
medium dose of the ester (only 19% weight gain relative to a control
weight gain of 41%), and at the highest dose, there was complete
mortality within 4 days, associated with a weight loss of 13%. In a
comparable study, Palmer (1972) dosed chickens with 2,4-D
dimethylamine salt at 25 to 500 mg/kg body weight for 10 consecutive
days. There were effects on weight gain at doses of 100 mg/kg or more.
At 100 mg/kg, the weight gain was 38%, compared to a control value of
57%. At 175, 250, and 375 mg/kg, the weight gain was 30%, similar to
the control value. At the highest dose, three out of five treated
birds died, and the survivors showed a weight gain of 26%. There was
no effect of 2,4-D ethylhexyl ester at 100 mg/kg on weight gain, but at
250 and 500 mg/kg, weight gain was 42% and 36%, respectively, compared
to a control value of 59%.
Solomon et al. (1973) studied the effects of 2,4-D acid and two
unspecified amine salt formulations of 2,4-D. Pheasants were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing one of
the preparations at either 75 or 150 mg/bird. No effects were observed
on fertility and there was no increase in the number of abnormal
embryos.
Table 11. Toxicity of 2,4-D to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mallard duck M 4 months oral acid (technical) acute LD50 > 2000 Hudson et
(Anas platyrhynchos) M 3-5 months oral sodium salt acute LD50 > 2050 al. (1984)
M 7 months oral amine salt acute LD50 < 2000 Hudson et
F 3-5 months oral acid (technical) acute LD50 > 1000 al. (1984)
23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et al.
17 days diet dimethylamine 5-day LC50 > 5000 c (1975)
young diet acetamide 100-day LC50 > 500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 2500 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 > 5000 DeWitt et
al. (1963)
Japanese quail M 2 months oral acid (technical) acute LD50 668 Hudson et
(530-842) al. (1984)
(Coturnix coturnix 14 days diet acetamide 5-day LC50 > 5000 c Hill et
(japonica) 12 days diet butoxyethanol 5-day LC50 > 5000 c al. (1975)
20 days diet dimethylamine 5-day LC50 > 5000 c Hill et
al. (1975)
Bobwhite quail 23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et
(Colinus virginianus) 23 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 2500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 10-day LC50 5000 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
Pheasant F 3-4 months oral acid (technical) acute LD50 472 Hudson et
(340-654) al. (1984)
(Phasianus colchicus) 10 days diet butoxyethanol 5-day LC50 > 5000 Hill et
10 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 1000 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 5000 DeWitt et
adult diet dimethylamine 100-day LC50 > 5000 al. (1963)
young diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
10 days diet butoxyethanol 5-day LC17 5000 Hill et al.
(1975)
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Chukar partridge M,F 4 months oral acid (technical) acute LD50 200-400 Hudson et
(Alectoris chukar) al. (1984)
Rock dove M,F oral acid (technical) acute LD50 668 Hudson et
(Columba livia) (530-842) al. (1984)
Chicken M,F 21 days oral free acid 14-day LD50 541 Rowe & Hymas
(358-817) (1954)
M,F 21 days oral isopropyl 14-day LD50 1420 Rowe & Hymas
(1127-1789) (1954)
M,F 21 days oral mixed butyl 14-day LD50 2000 Rowe & Hymas
esters (1350-2960) (1954)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b Acute oral doses are given as mg/kg body weight; all other doses are as mg/kg diet.
c Dose level of 5000 mg/kg diet produced no mortality.
Whitehead & Pettigrew (1972b) fed day-old chicken chicks with the
butoxyethanol ester of 2,4-D at concentrations up to 7500 mg/kg diet
for 3 weeks. Dietary levels up to 1000 mg/kg had no adverse effect,
but at 2000 mg/kg diet 2,4-D ester reduced the food consumption and
growth rate of the chicks. Although there was no mortality at the
higher doses, necropsy of birds sacrificed at the end of the experiment
showed swollen kidneys in all birds and some mottling of the spleen.
Bjorklund & Erne (1966) fed chickens with the amine salt of 2,4-D at
500 mg/kg diet. One bird died of renal gout after 5 months dosing;
autopsy showed hypoplasia (possibly congenital) of the right kidney and
hyperplasia of the left kidney. Other birds were killed at 1, 2, 9,
or 18 months after dosing began, but there was no consistent pattern to
autopsy findings.
Erne & Bjorklund (1970) examined the long-term effects of
phenoxyherbicides on chickens. Groups of day-old broiler chicks were
given 2,4-D in the drinking water at 1000 mg/litre for up to 7 months.
During the dosing period, chickens were sacrificed at regular intervals
for autopsy and samples were prepared for electron microscopy. There
was decreased food and water intake in dosed birds. The most
pronounced effect was on the kidney; there was noticeable kidney
enlargement after 14 days of dosing and this increased with time.
2,4-D concentrations in body tissues reached a plateau after 7 days,
with the highest residue in kidney tissue. Histologically, the kidney
enlargement was shown to be due to hypertrophy of proximal tubule
epithelium. These hypertrophied cells, under the electron microscope,
were shown to display an increased mitochondrial content and
pronounced mitochondrial pleomorphy. The number of microbodies was
also increased and nuclear bodies were observed. These findings were
stated to reflect alterations in intermediate metabolism in the
tubular cells. In an earlier study, using chicks dosed similarly with
1000 mg/litre of drinking water (Bjorklund & Erne, 1966), the birds
were followed through to sexual maturity. No significant effects were
observed on weight gain, age at sexual maturity, or onset of egg
production, but the number of eggs laid was reduced during the first 2
months of egg laying. The number of birds dying during the course of
the study did not differ from the control value. Surviving birds were
killed and autopsied at intervals of between 2 and 18 months after the
onset of egg laying. The primary effect was consistent enlargement of
the kidneys.
7.2.3. Special studies on birds
Lundholm & Mathson (1983) studied the effect of 2,4-D on the ATP-
dependent Ca2+ binding of the particulate fraction of egg shell
gland mucosa cells from egg-laying hens. This parameter had been
found to be a sensitive indicator of potential shell-thinning effects
of chemicals. They calculated a 5-min IC50, for Ca binding
inhibition, of 30.7 x 10-8 mmol 2,4-D/litre incubation medium.
This makes 2,4-D 13.5 times less effective in this respect than
1,1'-(2,2-dichlorethenylidine)-bis[4-chlorobenzene] ( p-p' -DDE), the
major agent causing eggshell-thinning in birds.
Percutaneous absorption of 2,4-D through the feet of red-winged
blackbirds was measured by Rogers et al. (1974). A 24-h exposure to
14C-labelled 2,4-D at 0.01 mmol/litre resulted in a blood
concentration of 1.24 x 10-3 mmol 2,4-D/litre.
7.3. Toxicity to Non-laboratory Mammals
Appraisal
Based on the available data, no generalization can be made about
the hazard of 2,4-D to mammals in the field. Data on voles indicate
that the herbicide poses no hazard.
Cholakis et al. (1982) obtained acute oral LD50 estimates for
two species of voles by determining mortality 14 days after the
administration of a single dose of 2,4-D acid. Values were 2110
(1800 - 2570) and 2100 (1900 - 2390) mg/kg body weight for males
and females, respectively, of the prairie vole (Microtus
orchrogaster). Values for the grey-tailed vole (Microtus canicaudus)
were 1200 (955 - 1150) for males and 1310 (1010 - 1790) mg/kg body
weight for females.
Skokova (1975) orally dosed 24 male bank voles with 400 to
405 mg/kg body weight (10% of the LD50) daily for 10 or 20 days and
examined reproductive parameters. Testis weight, an index of
spermatogenesis, and divisions in spermatogonia were all significantly
reduced relative to control values. Gile (1983) applied a foliar spray
of butyl ester of 2,4-D to a simulated ryegrass ecosystem at 1 kg/ha.
The system included voles, which showed a weight loss after exposure to
2,4-D when compared to similar animals in an untreated system. This
loss was considered to be the result of protein deficiency.
8. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
Appraisal
No direct toxic effects, acute or long-term, of 2,4-D applications
under field conditions on any animals species have been observed thus
far.
There are, inevitably, indirect effects resulting from the intended
selective herbicidal properties of the compound. These effects would
result from the use of any herbicide or from other methods of land
management. There will, therefore, be effects for mammals, birds, and
insects because of food deprivation, modification of habitat,
requirements for nesting, shelter, etc.
The application of 2,4-D appears to present no hazard to the
beneficial epigeal arthropod community. Physical cultivation present a
greater hazard to sensitive soil arthropods than the use of 2,4-D
herbicides.
Oka & Pimental (1976) observed increased numbers of insect pests
and increased occurrence of blight infection in maize (Zea mays) crops
treated with 2,4-D as the triethanolamine salt. The crops had been
treated with 2,4-D at 0.14, 0.55, or 4.4 kg/ha; 0.55 kg/ha is the
normal rate of application for this crop. The number of aphids
increased from 1420 on control untreated plants to 2449 on plants
treated at 0.14 kg/ha, 3116 on plants treated at 0.55 kg/ha, and 2023
on plants treated at 4.4 kg/ha. The percentage of plants attacked by
the European corn borer (Ostrinia nubialis) increased from 63% on
controls to 83% and 70% for treatments at 0.14 and 0.55 kg/ha,
respectively. Controlled studies also showed an increase in infection
with fungal blight. Laboratory investigation of these effects
confirmed that the treated maize had higher protein levels than the
untreated. This was thought to be the reason for the increased success
of the pests.
Everts et al. (1986) investigated the effects of various pesticides
on soil arthropods. Spiders were found to be a sensitive indicator of
effect. No side-effects of the use of 2,4-D amine were observed on
these organisms. Lahr et al. (1987) showed that spider numbers were
reduced by ploughing but not by the use of 2,4-D herbicides.
Matida et al. (1975) examined aquatic organisms in a stream running
through a mountainous area of 9.4 ha, in Shizuoka prefecture in Japan,
which had been aerially sprayed with a mixture of 2,4-D and 2,4,5-T at
a rate of 150 kg/ha. There was no effect on the number or species
diversity of aquatic invertebrates. Caged cherry salmon and dace
fingerlings showed no mortality, abnormal behaviour, or pathological
change after spraying. An extensive ecological survey of an area in
Florida treated with military mixtures of herbicides, including 2,4-D,
revealed no major change in species diversity or population size for
aquatic invertebrates, fish, a lizard, or the beach mouse (Young et
al., 1975).
The weevil Rhinocyllus conicus is used in the biological control of
musk thistle, an invasive weed. In an investigation of the
practicality of combining biological with chemical control, Lee & Evans
(1980) investigated the toxicity to the weevil of 2,4-D. They sprayed
musk thistle with 2,4-D at a rate of 4.48 kg/ha. One week later, the
terminal seed heads of the thistle were covered with cloth bags to
contain the weevils. The number of dead larvae, pupae, and adults
were counted and compared to the number on unsprayed, control thistle
heads. No significant differences were observed.
Dwernychuk & Boag (1973) studied the effect of herbicide spraying
on several species of nesting ducks (lesser scaup, gadwall, white-
winged scoter, mallard, pintail, and American wigeon) in Canada. An
ester of 2,4-D was applied to two islands. This application
significantly reduced the areas dominated by broad-leaved plants and
permitted invasion of these areas by grasses. Ducks preferred to nest
amongst broad-leaved vegetation and avoided grass. As the areas of
broad-leaved plants disappeared, there was an increase in nest density
in those broad-leaved areas still present. Total numbers of nesting
ducks declined over the 3-year study period. This decline was
attributed, by the authors, entirely to the effect of the herbicide on
vegetation type.
Keith et al. (1959) studied the effects on populations of the
pocket gopher (Thomomys talpoides) of spraying weedy rangeland in
Colorado with 2,4-D, as the butyl ester, at 3.4 kg/ha. Numbers of
gophers were estimated using two different methods, either by trapping
or by counting numbers of newly excavated mounds. Both methods showed
a highly significant difference between sprayed and non-sprayed areas
1 year after spraying. The total numbers of gophers trapped in the two
areas before 2,4-D application were 101 and 110, respectively. In the
same two areas, 1 year after spraying, numbers were 117 (untreated
area) and 15 (treated area), respectively. This represents a fall in
gopher numbers of 87% in the treated area and a slight increase in
numbers in the unsprayed area. Newly excavated mounds in the treated
area were only 28% as numerous as in control areas. Spraying had
reduced production of forbs (broad-leaved plants) from 445 kg/ha
before spraying to 75 kg/ha afterwards, a reduction of 83%. Grass
production had increased by 37%. The overall reduction in vegetation
was 232 kg/ha or 35% on sprayed plots.
In a similar study by Tietjen et al. (1967), 2,4-D butyl ester
applied at 3.4 kg/ha to identical high-altitude rangeland initially
reduced forb density and gopher populations. Pocket gopher numbers
were reduced by between 80% and 90%. Both forbs and gopher populations
remained low in one treated area but not in a second one. The decline
in gopher numbers was considered by the authors to be a result of food
deficiency; the grasses available represented a marginal diet for the
animals. This decline was not due either to movement of animals out of
the area or to the direct toxic effects of the herbicide. Reduction in
numbers was, therefore, primarily a result of reduced breeding success.
Johnson & Hansen (1969) studied the after-effects on wild mammals
of treating perennial forb and shrub/grass ranges with 2,4-D either
aerially or from a ground rig at rates of 2.2 or 3.4 kg/ha using a
diesel-oil carrier. The density and litter size of the deer mouse
(Peromyscus maniculatus) was little affected by the treatment, but the
densities of northern pocket gophers (Thomomys talpoides) and least
chipmunks (Eutamias minimus) were reduced. Montane voles (Microtus
montanus) increased their abundance in treated perennial forb range.
Gopher and vole populations returned to normal with the re-
establishment of forb dominance. Density changes were considered by
the authors to be primarily due to changes in availability of food for
the gophers, availability of both food and cover for chipmunks, and
availability of a close-canopied grass cover for the voles. There were
no direct toxic effects of the herbicide.
Fagerstone et al. (1977) observed two colonies of black-tailed
prairie dogs (Cynomys ludovicianus) in North America and the effect of
spraying their feeding areas with 2,4-D. The herbicide was applied
initially as the dimethylamine salt, followed by two further sprays of
the butyl ester after 1 month and 1 year. All applications of 2,4-D
were at 2.2 kg/ha. One colony lived in a sprayed area, initially rich
in broad-leaved plants. The second colony lived in an unsprayed area
poor in dicotyledonous plants and rich in grass. The effect of
spraying was, therefore, to make the treated area similar to the
control area with less broad-leaved herbage and less cover. Prior to
treatment, the first colony preferentially ate the forbs; diet was 73%
forbs and 5% grass. After spraying, they ate 9% forbs and 82% grass.
The control colony ate a similar diet to the first colony post-
treatment, i.e., mostly grass. Prairie dogs remained in the same area
after treatment with herbicide and there was no evidence of starvation.
Body weight was maintained, activity was comparable to the pre-
treatment level, and reproduction was unaffected.
Spencer & Barrett (1980) monitored the population response of
meadow voles (Microtus pennsylvanicus) to application of 2,4-D as the
N-oleyl 1,3 propylenediamine salt at 567.5 g/ha. Two 0.4-ha plots
were compared, one sprayed and the other untreated. The population
fluctuation of the voles was monitored for 6 months, from June to
December, a period spanning the breeding season. The control area
reached a population peak of 116 animals on 6 November; a peak of 68
voles was reached on 9 October in the treated area. There was a skewed
sex ratio in the treated area, mainly because of a reduced survival
rate in females. Voles in the treated plot were protein-deficient,
compared to controls
9. EVALUATION
In evaluating the environmental hazards of 2,4-D, the following
general points should be borne in mind:
(a) the chlorinated dibenzo- p -dioxins (CDDs) are present in
2,4-D only in trace amounts which are difficult to separate and
identify;
(b) 2,4-D is rapidly degraded in the environment;
(c) the environmental effects are indirect, and are the
consequence of vegetation diversity being modified; care should be
taken to avoid unintentional vegetation damage; the mode of
application and the formulation should be carefully selected;
esters should be avoided in aquatic applications (because of
toxicity to aquatic organisms);
(d) there are limited data on the effects of 2,4-D and its
formulations on communities of organisms; hazard assessment is,
therefore, often by extrapolation from single species studies;
(e) minor adverse effects shown in laboratory studies on
terrestrial organisms have resulted from exposures far in excess of
likely exposures in the field.
9.1. Aquatic Organisms
Sources of exposure of aquatic ecosystems to 2,4-D include direct
application, run-off, and spray drift. Because of low adsorption rate
and rapid degradation, the herbicide is not accumulated in compartments
of the aquatic system.
2,4-D acid and its salts are less toxic to aquatic organisms than
are the esters.
2,4-D acid and its salts are of low to moderate toxicity to aquatic
organisms. However, the growth and nitrogen fixation of some
cyanobacteria (blue-green algae) are inhibited, but only at
concentrations above levels expected from direct application of the
herbicide to water. These microorganisms are the source of most
nitrogen in wet tropical soils. This inhibitory effect could be of
concern when these compounds are applied to rice fields at a high
dosage.
Because of the toxicity of esters, particularly the propylene
glycol butyl ether ester, for early life-stages of several fish
species, they should be regarded as hazardous to aquatic ecosystems.
9.2. Terrestrial Organisms
2,4-D does not persist in soil and other compartments of the
terrestrial environment.
Nitrogen-fixing microorganisms appear to be particularly sensitive
to 2,4-D; this might be especially important in tropical soils.
Some terrestrial invertebrates have shown adverse effects, but only
at high exposure levels. Therefore, 2,4-D does not constitute a hazard
to this group of organisms.
2,4-D has low acute toxicity to birds, as indicated by the
LD50. Most studies on birds and their eggs have been conducted at
exposures exceeding those that could be expected in the field; even
under these conditions, no significant adverse effects have been
observed.
Under field conditions, 2,4-D does not cause direct toxic effects
on animals. However, the change of species composition and structure
of the vegetation, resulting from the use of this herbicide, leads to
indirect effects on terrestrial ecosystems. This indirect effect would
also result from the use of any herbicide or from other methods of land
management in either temperate or tropical regions.
* * *
There is evidence only for minor effects on the environment arising
from the use of 2,4-D, as long as the following simple recommendations
are followed:
(a) amine formulations, rather than esters, should be used to
control aquatic weeds;
(b) accidental spread of the herbicide to other vegetation should
be avoided;
(c) the margins of agricultural land should be left untreated with
herbicide to avoid even the indirect effects of the material on
wildlife.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
There are indications that 2,4-D affects nitrogen fixation by
algae. Since this is the major source of nitrogen in tropical soils,
it is recommended that this should be further investigated,
particularly with reference to varying soil conditions. This should be
extended to a study on the functioning of a rice-paddy at the ecosystem
level.
REFERENCES
ADAMS, B.J. (1960) Effects of spraying 2,4-D amine on Coccinellid
larvae. Can. J. Zool., 38: 285-288.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous substances.
Int. Pest Control, 11: 29-35.
AUDUS, L.J. (1960) Microbiological breakdown of herbicides in soils.
In: Woodford, E.K. & Sagar, G.R., ed. Herbicides and soil, Oxford
Blackwell Scientific Publications, pp. 1-19.
AUDUS, L.J. (1964) Herbicide behaviour in the soil. In: Audus, L.J.,
ed. The physiology and biochemistry of herbicides, London, New York,
Academic Press, pp. 163-206.
BAILEY, H.C. & LIU, D.H.W. (1980) Lumbriculus variegatus, a benthic
oligochaete, as a bioassay organism. In: Eaton, J.C., Parish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
for Testing and Materials, pp. 205-215 (Special Technical Publication
707).
BEDNARZ, T. (1981) The effect of 2,4-D acid on green and blue-
green algae in unialgal and mixed cultures. Acta hydrobiol., 23: 173-
182.
BENEDECZKY, I., BIRO, P., & SCHAFF, Z. (1984) The effect of
2,4-D-containing herbicide (Dikonirt) on the ultrastructure of carp
(Cyprinus carpio) liver cells. Acta biol. (Szeged), 30: 107-125.
BERAN, F. & NEURURER, J. (1955) [Effect of plant protection
materials on the honeybee. I. Toxicity to bees.]
Pflanzenschutzberichte, 15: 97-147 (in German).
BIRGE, W.J., BLACK, J.A., & BRUSER, D.M. (1979) Toxicity of organic
chemicals to embryo-larval stages of fish. Washington, DC, US
Environmental Protection Agency (EPA 560-1179-007).
BIRO, P. (1979) Acute effects of the sodium salt of 2,4-D on the
early developmental stages of bleak, Alburnus alburnus. J. Fish Biol.,
14: 101-109.
BJORKLUND, N-E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. Scand., 7: 364-390.
BJORN, M.K. & NORTHEN, H.T. (1948) Effects of 2,4-
dichlorophenoxyacetic acid on chicks. Science, 108: 479-480.
BUNTING, D.L. & ROBERTSON, E.B. (1975) Lethal and sublethal effects
of herbicides on zooplankton species, University of Tennessee, Water
Resources Research Center, 34 pp. (Res. Report No. 43, NTIS Report No.
PB-241 337).
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975a) Loss of
five pesticides from cultures of twenty-one planktonic algae. Bull.
environ. Contam. Toxicol., 13: 149-152.
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975b) The effect
of atrazine, 2,4-D, methoxychlor, carbaryl and diazinon on the growth
of planktonic algae. Br. phycol. J., 10: 371-376.
BUTLER, P.A. (1963) A review of Fish and Wildlife Service
investigations during 1961 and 1962, Washington, DC, US Department of
the Interior, Fish and Wildlife Service, pp. 11-25 (Circular No. 167).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna. Proc.
South. Weed Conf., 18: 576-580.
CALDWELL, R.S. (1977) Biological effects of pesticides on the
Dungeness crab, Gulf Breeze, Florida, US Environmental Protection
Agency (EPA-600/3-77-131).
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON,
M.H., & MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D,
DEF, propanil and trifluarin to the Dungeness crab, Cancer magister.
Arch. environ. Contam. Toxicol., 8: 383-386.
CHAI, I.K. & CHUNG, Y.S. (1975) [Physiological effects of 2,4-
dichlorophenoxyacetic acid (2,4-D) on Chlorella ellipsoidea.]
Misaengmul Hakhoe Chi., 13: 101-108 (in Korean).
CHAMBERS, H., DZIUK, L.J., & WATKINS, J. (1977) Hydrolytic
activation and detoxification of 2,4-dichlorophenoxyacetic acid esters
in mosquito fish. Pestic. Biochem. Physiol., 7: 297-300.
CHEAH, M.L., AVAULT, J.W., & GRAVES, J.B. (1980) Acute toxicity
of selected rice pesticides to crayfish Procambarus clarkii. Prog. Fish
Cult., 42: 169-172.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1982)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avaian and Mammalian Wildlife
Toxicology: 2nd Conference, Philadelphia, American Society for Testing
and Materials, pp. 143-154 (Special Technical Publication 757).
COAKLEY, J.E., CAMPBELL, J.E., & MCFARREN, E.F. (1964)
Determination of butoxyethanol ester of 2,4-dichlorophenoxyacetic acid
in shellfish and fish. J. agric. food Chem., 12: 262-265.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99: 1-12.
CULLIMORE, D.R. & MCCANN, A.E. (1977) Influence of four
herbicides on the algal flora of a prairie soil. Plant Soil, 46: 499-
510.
DAS, B. & SINGH, P.K. (1977) The effect of 2,4-dichlorophenoxyacetic
acid on growth and nitrogen-fixation of blue-green alga Anabaenopsis
raciborskii. Arch. environ. Contam. Toxicol., 5: 437-445.
DAVIS, J.T. & HARDCASTLE, W.S. (1959) Biological assay of
herbicides for fish toxicity. Weeds, 7: 397-404.
DAVIS, P.W., FRIEDHOFF, J.M., & WEDERMEYER, G.A. (1972)
Organochlorine insecticide, herbicide and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8: 69-72.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1974) Effect of some
herbicides on soil microflora. J. Indian Soc. Soil Sci., 22: 300-303.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1975) Effect of some
herbicides on Azotobacter and non-symbiotic nitrogen fixation in the
soil. Indian J. Agric. Chem., 8: 95-98.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife
studies, Patuxent Wildlife Research Center. Pesticide-wildlife
studies. A review of Fish and Wildlife Service investigations during
1961 and 1962. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular No. 167).
DODSON, J.J. & MAYFIELD, C.I. (1979) The dynamics and
behavioural toxicology of Aqua-Kleen (2,4-D butoxyethanol ester) as
revealed by the modification of rheotropism in rainbow trout. Trans.
Am. Fish. Soc., 108: 632-640.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some
herbicides on the hatching rate of hen's eggs. Nature (Lond.), 215:
1406-1407.
DWERNYCHUK, L.W. & BOAG, D.A. (1973) Effect of
herbicide-induced changes in vegetation on nesting ducks. Can. field
Nat., 87: 155-165.
ELDER, J.H., LEMBI, C.A., & MORRE, D.J. (1970) Toxicity of 2,4-D
and picloram to fresh and saltwater algae. Proc. North-cent. Weed
Control Conf., 25: 96-98.
ELIASSON, L. (1973) Translocation and persistence of 2,4-D in
Populus tremula L. Weed Res., 13: 140-147.
ERNE, K. & BJORKLUND, N-E. (1970) Nephrotoxic effects of
phenoxy acetic herbicides. In: Proceedings of the 7th International
Congress on Plant Protection, Paris, France, pp. 768-769.
EVERTS, J.W., BEURSKENS, J.E.M., BOUWHUIS, J.S., BUYSE, A.D.,
HENGEVELD, R., WOUTERS, L., & KOEMAN, J.H. (1986) Terrestrial
arthropods as indicators for side-effects caused by insecticides in
arable farm systems in the Netherlands. In: Proceedings of the 1st
International TNO Conference on Contaminated Soil, Utrecht, The
Netherlands, November 1985, Dordrecht, Boston, Martinus Nijhoff, pp.
423-425.
FAGERSTONE, K.A., TIETJEN, H.P., & LAVOIE, G.K. (1977) Effects
of range treatment with 2,4-D on prairie dog diet. J. Range Manage.,
30: 57-60.
FINLAYSON, B.J. & VERRUE, K.M. (1985) Toxicities of
butoxyethanol ester and propylene glycol butyl ether ester formulations
of 2,4-dichlorophenoxy acetic acid (2,4-D) to juvenile salmonids.
Arch. environ. Contam. Toxicol., 14: 153-160.
FLINT, G.W., ALEXANDER, J.J., & FUNDERBURK, O.P. (1968)
Vapor pressures of low-volatile esters of 2,4-D. Weed Sci., 16: 541-
544.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry to
nine herbicides. Bull. environ. Contam. Toxicol., 15: 509-514.
FOLMAR, L.C. (1978) Avoidance chamber responses of mayfly nymphs
exposed to eight herbicides. Bull. environ. Contam. Toxicol., 19: 312-
318.
FOURNIER, J.C. (1980) Enumeration of the soil micro-organisms able
to degrade 2,4-D by metabolism or co-metabolism. Chemosphere, 9: 169-
174.
FREITAG, D., GEYER, H., KRAUS, A., VISWANATHAN, R., KOTZIAS,
D., ATTAR, A., KLEIN, W., & KORTE, F. (1982) Ecotoxicological profile
analysis. VII, Screening chemicals for their environmental behaviour
by comparative evaluation. Ecotoxicol. environ. Saf., 6: 60-81.
GALL, A. & DOGGER, J.R. (1967) Effect of 2,4-D on the wheat stem
sawfly. J. econ. Entomol., 60: 75-77.
GANGAWANE, L.V., SALER, R.S., & KULKARNI, L. (1980) Effect
of pesticides on growth and heterocyst formation in Nostoc sp.
Marathwada Univ. J. Sci., 19B: 3-6.
GEORGE, J.P., HINGORANI, H.G., & RAO, K.S. (1982) Herbicide
toxicity to fish-food organisms. Environ. Pollut., 28: 183-188.
GILE, J.D. (1983) 2,4-D - its distribution and effects in a ryegrass
ecosystem. J. environ. Qual., 12: 406-412.
GLYNN, P.W., HOWARD, L.S., CORCORAN, E., & FREAY, A.D.
(1984) The occurrence and toxicity of herbicides in reef-building
corals. Mar. Pollut. Bull., 15: 370-374.
GRAHAM-BRYCE, I.J. (1972) Herbicide movement and availability in
soils. Proc. Br. Weed Control Conf., 11: 1193-1202.
GROLLEAU, G., DE LAVAUR, E., & SIOU, G. (1974) Effets du
2,4-D sur la reproduction des cailles et des perdrix, aprčs application
du produit par pulvérisation sur les oeufs. Ann. Zool. Ecol. anim.,
6: 313-331.
GUARINO, A.M., JAMES, M.O., & BEND, J.R. (1977) Fate and
distribution of the herbicides 2,4-dichlorophenoxyacetic acid (2,4-D)
and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in the dogfish shark.
Xenobiotica, 7: 623-631.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The
effect of phenoxy-herbicides on the hatchability of eggs and the
viability of the chicks. Acta pharmacol. toxicol., 35: 300-308.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
coturnix. Bull. environ. Contam. Toxicol., 11: 98-102.
HAGENMAIER, H. (1986) Determination of 2,3,7,8-tetrachlorodibenzo-p-
dioxin in commercial chlorophenols and related products. Fresenius Z.
anal. Chem., 325: 603-606.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973)
Avoidance of pesticides by grass shrimp (Palaemonetes pugio). Bull.
environ. Contam. Toxicol., 9: 129-133.
HAQUE, A. & EBING, W. (1983) Uptake, accumulation, and
elimination of HCB and 2,4-D by the terrestrial slug, Deroceras
reticulatum (Muller). Bull. environ. Contam. Toxicol., 31: 727-733.
HASHIMOTO & NISHIUCHI (1978) quoted by Hidaka et al. (1984).
HAWXBY, K., TUBEA, B., OWNBY, J., & BASLER, E. (1977) Effects
of various classes of herbicides on four species of algae. Pestic.
Biochem. Physiol., 7: 203-209.
HIDAKA, H., HATTANDA, M., & TATSUKAWA, R. (1984)
[Avoidance of pesticides with medakas (Oryzias latipes).] Nippon
Nogeikagaku Kaishi, 58: 145-151 (in Japanese).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (First report).] Anz. schädlingskd. Pflanzenschutz Umweltschutz,
49: 21-25 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976b) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (Second report).] Anz. schädlingskd. Pflanzenschutz
Umweltschutz, 49: 65-68 (in German).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish Wildlife Service,
61 pp. (Special Science Report, Wildlife No. 191).
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized fish
eggs and fry. Trans. Am. Fish. Soc., 96: 414-416.
HILTIBRAN, R.C. (1975) Oxygen and phosphate metabolism of bluegill
liver mitochondria in the presence of some pesticides. Environ. Qual.
Saf. (Suppl), 3: 549-553.
HOEPPEL, R.E. & WESTERDAHL, H.E. (1983) Dissipation of 2,4-D
DMA and BEE from water, mud and fish at Lake Seminole, Georgia.
Water Resour. Bull., 19: 197-204.
HOFFMAN, D.J. & ALBERS, P.H. (1984) Evaluation of potential
embryotoxicity and teratogenicity of 42 herbicides, insecticides and
petroleum contaminants to mallard eggs. Arch. environ. Contam.
Toxicol., 13: 15-27.
HUBER, S.J., POSCHENREIDER, G., & WALLNOFER, P.R. (1980)
[Effect of pesticides and the corresponding metabolites on growth and
respiration of some soil microorganisms.] Z. Pflanzenkr.
Pflanzenschutz, 87: 533-545 (in German).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook
of toxicity of pesticides to wildlife, 2nd edition, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 90 (Resource
Publication 153).
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to bluegill
sunfish of phenoxy herbicides. Weeds, 11: 50-53.
JAMES, M.O. (1979) Disposition of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Panulirus argus).
Pharmacologist, 21: 251.
JANA, B.B. & DE, U.K. (1982) Stimulatory effect of herbicide 2,4-D
on the heterotrophic microbial community in the water of three fish
ponds. Acta microbiol. Acad. Sci. Hung., 29: 77-82.
JENSEN, D.A. & SCHALL, E.D. (1966) Determination of vapor
pressures of some phenoxyacetic herbicides by gas-liquid
chromatography. J. agric. food Chem., 14: 123-125.
JOHNSON, C.R. (1976) Herbicide toxicities in some Australian anurans
and the effect of subacute dosages on temperature tolerance. Zool. J.
Linnean Soc., 59: 79-83.
JOHNSON, C.R. (1978) Herbicide toxicities in the mosquito fish
Gambusia affinis. Proc. R. Soc. Queensland., 89: 25-27.
JOHNSON, D.R. & HANSEN, R.M. (1969) Effects of range treatment
with 2,4-D on rodent populations. J. wildl. Manage., 33: 125-132.
JONES, G.D.E. & CONNELL, J.U. (1954) Studies of the toxicity to
worker honey-bees (Apis mellifera L.) of certain chemicals used in
plant protection. Ann. appl. Biol., 41: 271-279.
KAPOOR, K. & SHARMA V.K. (1980) Effect of certain herbicides on
survival, growth and nitrogen fixation of blue-green alga Anabaena
doliolum Bharadwaja. Z. allg. Mikrobiol., 20: 465-469.
KEITH, J.O., HANSEN, R.M., & WARD, A.L. (1959) Effect of 2,4-D
on abundance and foods of pocket gophers. J. wildl. Manage., 23: 137-
145.
KOPISCHKE, E.D. (1972) Effect of 2,4-D and diesel fuel on egg
hatchability. J. wildl. Manage., 36: 1353-1356.
KOSCHIER, F.J. & PRITCHARD, J.B. (1980) Renal handling of 2,4-D
by dogfish shark (Squalus acanthias). Xenobiotica, 10: 1-6.
LAHR, J., GOEDEJOHAN, N., & EVERTS, J.W. (1987) Side effects of
pesticides on terrestrial arthropods. I. Long-term effects,
geographical variability and effects of cultural practices. In:
Proceedings of the Symposium ``25 Years After Silent Spring'',
November 1987, Amsterdam, Toxicological Society.
LE VAN TO (1984) [Degradation of defoliants 2,4-D and 2,4,5-T by
selected soil microorganisms.] Acta agrar. Silvestria, Ser. agrar.,
23: 225-234 (in Polish).
LEE, R.D. & EVANS, J.O. (1980) The influence of selected herbicides
on the development of Rhinocyllus conicus, an insect used in biocontrol
of musk thistle. Proc. West. Soc. Weed Sci., 33: 104-110.
LEMBI, C.A. & COLERIDGE, S.E. (1975) Selective toxicity of detergents
and herbicides to phytoplankton, West Lafayette, Indiana, Purdue
University, Water Resources Research Center, 71 pp. (Technical Report
No. 71).
LEWIS, D.L., HODSON, R.E., & FREEMAN, L.F. (1984) Effects of
microbial community interactions on transformation rates of xenobiotic
chemicals. Appl. environ. Microbiol., 48: 561-565.
LHOSTE, J. & ROTH, P. (1946) Sur l'action des solutions aqueuses de
2,4-dichlorophénoxyacétate de sodium sur l'évolution des oeufs de Rana
temporaria L. Soc. Biol., 140: 272-273.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides
on decomposition of organic matter and nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LINDEN, E., BENGTSSON, B-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LI-TSE OU (1984) 2,4-D degradation and 2,4-D degrading
microorganisms in soils. Soil Sci., 137: 100-107.
LIU, H.W. & LEE, M. (1975) Toxicity of selected pesticides to the bay
mussel Mytilus edulis, Washington, DC, US Department of Commerce,
National Technical Information Service (NTIS Report No. PB-243 221).
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel Dekker,
Inc., pp. 1-49.
LUNDHOLM, C.E. & MATHSON, K. (1983) The inhibitory effect of
some chlorinated hydrocarbon pesticides on the ATP-dependent Ca2+
binding of the particulate fraction of the eggshell gland mucosa
cells. Acta pharmacol. toxicol., 52: 390-394.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1972) The action of different
pesticides on the development of bird embryos. Adv. exp. Med. Biol.,
27: 127-150.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1973) Pesticides, tératogénčse et
survie chez les oiseaux. Arch. Anat. Histol. Embryol., 56: 65-78.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de
l'herbicide 2,4-D sur le développement embryonnaire et la fécondité du
gibier ŕ plumes. C. R. Acad. Sci. Paris, Ser. D, 271: 2418-2421.
MATIDA, Y., FURUTA, Y., KUMADA, H., TANAKA, H., YOKOTE,
M., & KIMURA, S. (1975) Effects of some herbicides applied in the
forest to the freshwater fishes and other aquatic organisms - I. Survey
on the effects of aerially applied sodium chlorate and a mixture of
2,4-D and 2,4,5-T on the stream community. Bull. freshwater Fish Res.
Lab. (Tokyo), 25: 41-53.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp. (NTIS Report No. PB-87 188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp. (Resource Publication 160).
MEEHAN, W.R., NORRIS, L.A., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in southeast Alaska. J. Fish
Res. Board Can., 31: 480-485.
METCALF, R.L. & SANBORN, J.R. (1975) Pesticides and
environmental quality in Illinois. Illinois nat. Hist. Surv. Bull.,
31: 381-435.
MORTON, H.L., MOFFETT, J.O., & MACDONALD, R.H. (1972)
Toxicity of herbicides to newly emerged honey bees. Environ.
Entomol., 1: 102-104.
MOUBASHER, A.H., EL-HISSY, F.T., & ABDEL-KADER, M.I.A.
(1981) Selective effects of three herbicides on the fungus flora of
Egyptian soil. Folia microbiol., 26: 37-44.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxyethanol
ester of 2,4-D. Trans. Am. Fish. Soc., 96: 185-193.
MUKHOPADHYAY, S.K. (1980) Effects of herbicides and insecticides
alone and their combinations on soil microflora. Indian J. Weed Sci.,
12: 53-60.
MULLER, G. (1971) [Laboratory studies on effects of herbicides on
Carabidae.] Arch. Pflanzenschutz, 7: 351-364 (in German).
NAGUIB, K., YOUNIS, A.E., & EL-ESSAWY, M.A. (1980) The effects
of 2,4-dichlorophenoxyacetic acid on respiration, nitrogen and
carbohydrate metabolism of Aspergillus terreus Thom. Zbl. Bakteriol.
II. Abt., 135: 252-259.
NESBITT, H.J. & WATSON, J.R. (1980) Degradation of the herbicide
2,4-D in river water. II. The role of suspended sediment, nutrients and
water temperature. Water Res., 14: 1689-1694.
NORRIS, L.A. & GREINER, D. (1967) The degradation of 2,4-D in
forest litter. Bull. environ. Contam. Toxicol., 2: 65-74.
O'CONNOR, G.A., FAIRBANKS, B.C., & DOYLE, E.A. (1981) Effects
of sewage sludge on phenoxy herbicide adsorption and degradation in
soils. J. environ. Qual., 10: 510-515.
OKA, I.N. & PIMENTAL, D. (1976) Herbicide (2,4-D) increases insect
and pathogen pests on corn. Science, 193: 239-240.
OMKAR & SHUKLA, G.S. (1984) Toxicity of the herbicide 2,4-D-Na
to two species of freshwater prawn of the genus Macrobranchium. Acta
hydrochim. hydrobiol., 12: 285-289.
PACHPANDE, R.R. & DAVID, S.B. (1980) Effect of selected herbicides
on the growth of soil alga Chlorococcum infusionum meneghini
(Schrank). Phykos, 19: 138-141.
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens, Washington, DC, US Department of Agriculture,
Agricultural Research Service, 41 pp. (Production Research Report No.
137).
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some organic
herbicides to cattle, sheep, and chickens. Washington, DC, US
Department of Agriculture, Agricultural Research Service, 26 pp.
(Production Research Report No. 106).
PLUMB, T.R., NORRIS, L.A., & MONTGOMERY, M.L. (1977)
Persistence of 2,4-D and 2,4,5-T in chaparral soil and
vegetation. Bull. environ. Contam. Toxicol., 17: 1-8.
PONS, R. & PUSSARD, M. (1980) Action des herbicides sur les amibes
libres (Rhizopoda, Protozoa). Etude préliminaire. Acta oecol. oegol.
appl., 1: 15-20.
POORMAN, A.E. (1973) Effects of pesticides on Euglena gracilis. I.
Growth studies. Bull. environ. Contam. Toxicol., 10: 25-28.
PRESCOT, L.M. & OLSON, D.L. (1972) Effects of pesticides on the
soil amoeba, Acanthamoeba castellanii. Proc. South Dakota Acad. Sci.,
51: 136-141.
PRESING, M. (1981) On the effects of Dikonirt (sodium salt of 2,4-
dichlorophenoxyacetic acid) on the mortality and reproduction of
Daphnia magna. Hydrobiologia, 83: 511-516.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder. J.
Pharmacol. exp. Ther., 208: 280-286.
RAATIKAINEN, M., SILTANEN, H., ROSENBERG, C., RAATIKAINEN,
T., & MUKULA, J. (1979) Herbicide residues in cowberries, bilberries
and lichens in controlled ground spraying experiments on woodland.
Ann. agric. Fenniae, 18: 112-116.
RADOSEVICH, S.R. & WINTERLIN, W.L. (1977) Persistence of 2,4-D
and 2,4,5-T in chaparral vegetation and soil. Weed Sci., 25: 423-425.
RAND, G.M. & BARTHALMUS, G.T. (1980) Use of an unsignalled
avoidance technique to evaluate the effects of the herbicide 2,4-
dichlorophenoxyacetic acid on goldfish, Philadelphia, American Society
for Testing and Materials, pp. 341-353 (Special Technical Publication
707).
RAPOPORT, E.H. & CANGIOLI, G. (1963) Herbicides and the soil
fauna. Pedobiologia, 2: 235-238.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977)
Investigations into the acute toxicity and some chronic effects of
selected herbicides and pesticides on several fresh water fish
species. Bull. environ. Contam. Toxicol., 18: 361-365.
RIVIERE, J-L. (1976) Les effets secondaires des pesticides agricoles.
II. Action de quelques herbicides sur la croissance et la fécondité de
Blattella germanica (L.). Ann. Zool. Ecol. anim., 8: 543-550.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3: 67-78.
ROBERTSON, E.B. (1975) The acute toxicity of four herbicides to 0 -
4 hour nauplii of the copepod Cyclops vernalis Fisher, Washington, DC,
US Department of Commerce, National Technical Information Service, 70
pp. (M.S. Thesis, University of Tennessee) (NTIS Report No. PB-269
495).
RODGERS, C.A. & STALLING, D.L. (1972) Dynamics of an ester of
2,4-D in organs of three fish species. Weed Sci., 20: 101-105.
ROGERS, J.G., CAGAN, R.H., & KARE, M.R. (1974) Percutaneous
absorption of several chemicals, some pesticides included, in the red-
winged blackbird. Environ. Physiol. Biochem., 4: 104-111.
ROSENBERG, A. & ALEXANDER, M. (1980) 2,4,5-trichlorophenoxy-
acetic acid (2,4,5-T) decomposition in tropical soil and its co-
metabolism by bacteria in vitro. J. agric. food Chem., 28: 705-709.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation of
the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUGGIERO, P. & RADOGNA, V.M. (1985) Inhibition of soil humus-
laccase complexes by some phenoxyacetic and s- triazine
herbicides. Soil Biol. Biochem., 17: 309-312.
SANDERS, H.O. (1969) Toxicity of pesticides to the crustacean,
Gammers lacustris. Washington, DC, US Department of the Interior, Fish
and Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 18 pp.
(Technical Paper No. 25).
SANDERS, H.O. (1970a) Toxicities of some herbicides to six species of
freshwater crustaceans. J. Water Pollut. Control Fed., 42: 1544-1550.
SANDERS, H.O. (1970b) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowler's toad Bufo woodhousii
fowleri. Copeia, 1970(2): 246-251.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several pesticides to
two species of cladocerans. Trans. Am. Fish. Soc., 95: 165-169.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol. Oceanogr.,
13: 112-117.
SANDMANN, E.R.I.C. & LOOS, M.A. (1984) Enumeration of
2,4-D-degrading microorganisms in soils and crop plant rhizospheres
using indicator media; high populations associated with sugarcane
(Saccharum officinarum). Chemosphere, 13: 1073-1084.
SARMA, Y.S.R.K. & TRIPATHI, S.N. (1980) Effects of some chemicals
(2,4-dichlorophenoxyacetic acid, coumarin and acenaphthene) on the
green alga, Oedogonium acmandrium Elfving. Phykos, 19: 142-152.
SATTAR, M.A. & PAASIVIRTA, J. (1980) Fate of chlorophenoxyacetic
acids in acid soil. Chemosphere, 9 745-752.
SCHRODER, H. & PILZ, E. (1983) [Effect of herbicides on nitrification
in different soils.] Zbl. Mikrobiol., :138: 223-234 (in German).
SCHRODER, I., MEYER, M., & MUCKE, D. (1970) [Effects of the
herbicides 2,4-D, aminotriazole, atrazine, chlorpropham and
chlorfurenol on nucleic-acid biosynthesis in the ascomycete Neurospora
crassa.] Weed Res., 10: 172-177 (in German).
SCHULTZ, D.P. (1973) Dynamics of a salt of (2,4-dichlorophenoxy)-
acetic acid in fish, water, and hydrosol. J. agric. food Chem., 21:
186-192.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8: 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues
at Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7: 146-
152.
SEIBERT, K., FUHR, F., & MITTELSTAEDT, W. (1982) Experiments
on the influence of roots and soils on 2,4-D degradation. Landwirtsch.
Forsch., 35: 5-13.
SHCHERBAKOV, Y.A. & POLUBOYARINOVA, I.V. (1973) The
persistence of 2,4-D butyl ester in water and its accumulation in
aquatic life. In: Andrushaitis, G.P., ed. Experimental water
toxicology, Charlotte, North Carolina, Army Foreign Service and
Technology Center, pp. 19-21 (NTIS Report No. AD-760 400).
SHUKLA, G.S. & OMKAR (1983) Toxicity of 2,4-D-Na salt to fresh
water prawn, Macrobrachium lamarrei (M. Edwards). Comp. Physiol.
Ecol., 8: 282-284.
SIGMON, C. (1979) Oxygen consumption in Lepomis macrochirus
exposed to 2,4-D or 2,4,5-T. Bull. environ. Contam. Toxicol., 21: 826-
830.
SIKKA, M.C., APPLETON, H.T., & GANGSTAD, E.O. (1977) Uptake
and metabolism of dimethylamine salt of 2,4-D acid by fish. J. agric.
food Chem., 25: 1030-1033.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., &
RAATIKAINEN, T. (1981) Triclopyr, glycophosphate, and
phenoxyherbicide residues in cowberries, bilberries and lichen. Bull.
environ. Contam. Toxicol., 27: 731-737.
SINGH, P.K. (1974) Algicidal effect of 2,4-dichlorophenoxy acetic
acid on blue-green alga Cylindrospermum sp. Arch. Microbiol., 97: 69-
72.
SKOKOVA, N.N. (1975) [Effect of the butyl ester of 2,4-D on the
reproductive function of male bank voles.] Nauchn. Osn. Okhr. Prir.,
3: 172-178 (in Russian).
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of large-
scale applications of 2,4-D on aquatic fauna and water quality.
Pestic. monit. J., 1: 16-21.
SOLOMON, K.E., DAHLGREN, R.B., & LINDER, R.L. (1973)
Abnormal embryos in eggs of pheasants given 2,4-D or PCB. Proc. South
Dakota Acad. Sci., 52: 95-99.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11: 511-516.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978a) Hatching
success and early performance of chicks from eggs sprayed with 2,4-D,
2,4,5-T and picloram at various stages of embryonic development.
Bull. environ. Contam. Toxicol., 20: 289-293.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978b)
Reproductive success of hens and cockerels originating from eggs
sprayed with 2,4-D, 2,4,5-T and picloram followed by early performance
of their progeny after a comparable in ovo exposure. Bull. environ.
Contam. Toxicol., 20: 111-119.
SPAIN, J.C. & VAN VELD, P.A. (1983) Adaptation of natural microbial
communities to degradation of xenobiotic compounds: Effects of
concentration, exposure time, inoculum and chemical structure. Appl.
environ. Microbiol., 45: 428-435.
SPENCER, S.R. & BARRETT, G.W. (1980) Meadow vole population
responses to vegetational changes resulting from 2,4-D application.
Am. Midl. Nat., 103: 32-46.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on the
embryo development of Phasianus colchicus var. and Coturnix coturnix
japonica.] Z. Jagdwiss., 22: 197-201 (in German).
STALLING, D.L. & HUCKINS, J.N. (1978) Metabolism of 2,4-dichloro-
phenoxyacetic acid (2,4-D) in bluegills and water. J. agric. food
Chem., 26: 447-452.
STATHAM, C.N. & LECH, J.J. (1975) Potentiation of the acute toxicity
of several pesticides and herbicides in trout by carbaryl. Toxicol.
appl. Pharmacol., 34: 83-87.
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., &
ASKAR, A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46: 387-391.
THOMPSON, D.G., STEPHENSON, G.R., SOLOMON, K.R., &
SKEPASTS, A.V. (1984) Persistence of (2,4-dichlorophenoxy) acetic
acid and 2-(2,4-dichlorophenoxy) propionic acid in agricultural and
forest soils of northern and southern Ontario. J. agric. food
Chem., 32: 578-581.
TIETJEN, H.P., HALVORSON, C.H., HEGDAL, P.L., & JOHNSON, A.M.
(1967) 2,4-D herbicide, vegetation, and pocket gopher relationships
Black Mesa, Colorado. Ecology, 48: 634-643.
TIWARI, D.N., PANDEY, A.K., & MISRA, A.K. (1984) Toxicity of
2,4-dichlorophenoxy acetic acid on growth and nitrogen fixation of
blue-green alga Anabaena cylindrica. Pesticides (Bombay), 18: 16-18.
TOOBY, T.E., LUCEY, J., & STOTT, B. (1980) The tolerance of grass
carp Ctenopharyngodon idella Val., to aquatic herbicides. J. Fish
Biol., 16: 591-597.
TORSTENSSON, N.T.L. (1975) Degradation of 2,4-D and MCPA in
soils of low pH. Environ. Qual. Saf. (Suppl.), 3: 262-265.
TOURE, I.M. & STENZ, E. (1977) [The action of selected herbicides on
bacteriophages and Escherichia coli.] Zbl. Bakteriol. Parasitol.
Infektionskr. 132: 163-177 (in German).
TREVORS, J.T. & STARODUB, M.E. (1983) Effect of 2,4-D on
electron transport system (ETS) activity and respiration in soil.
Bull. environ. Contam. Toxicol., 31: 595-598.
TRUMBLE, J.T. & KOK, L.T. (1980) Impact of 2,4-D on
Ceuthorhychidius horridus (Coleoptera: Curculionidae) and their
compatibility for integrated control of Carduus thistles.
Weed Res., 20: 73-75.
TUEY, D.B. & JAMES, M.O. (1980) Compartmental analysis of 2,4-
dichlorophenoxyacetic acid pharmacokinetics in spiny lobster. Fed.
Proc., 39: 525.
VARDIA, H.K. & DURVE, V.S. (1981) The toxicity of 2,4-D to
Cyprinus carpio var. communis in relation to the seasonal variation in
the temperature. Hydrobiologia, 77: 155-159.
VARDIA, H.K., RAO, P.S., & DURVE, V.S. (1984) Sensitivity of toad
larvae to 2,4-D and endosulfan pesticides. Arch. Hydrobiol., 100: 395-
400.
VERMA, D., CHOUHAN, M.S., & PANDEY, A.K. (1984) Effect of
Weedone (a selective 2,4-D herbicide) on neuroendocrine complex
of Puntius ticto (Ham.). J. environ. Biol., 5: 249-254.
WATSON, J.R. (1977) Seasonal variation in the biodegradation of 2,4-D
in river water. Water Res., 11: 153-157.
WELP, G. & BRUMMER, G. (1985) [The Fe(III)-reduction test - a
simple procedure to determine the effects of environmental chemicals on
the microbial activity in soils.] Z. Pflanzenernaehr. Bodenkd.,
148: 10-23 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972a) The subacute toxicity
of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid
to chicks. Toxicol. appl. Pharmacol., 21: 348-354.
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972b) The effect of 2,4-
dichlorophenoxyacetic acid on laying hens. Br. poult. Sci., 13: 191-
195.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., &
GANGSTAD, E.O. (1973) The effects of a 2,4-D application on the
biota and water quality in Currituck Sound, North Carolina. Hyacinth
Control J., 11: 13-17.
WHO (1984) Environmental Health Criteria 29: 2,4-dichlorophenoxy-
acetic acid (2,4-D), Geneva, World Health Organization, 151 pp.
WOODWARD, D.F. (1982) Acute toxicity of mixtures of range
management herbicides to cutthroat trout. J. Range Manage., 35: 539-
540.
YOUNG, A.L., THALKEN, C.E., & WARD, W.E. (1975) Studies of the
ecological impact of repetitive aerial applications of herbicides on
the ecosystem of Test Area C-52A, Elgin AFB, Florida. Florida Air Force
Armament Laboratory, Elgin Air Force Base, 126 pp. (Report No. AFATL-
TR-75-142).
 2.2. Important Chemical Reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides. Pyrolysis of 2,4-D and its derivatives is likely
to produce certain CDD isomers. 2,4-D is readily photodegraded.
2.3. Volatility of 2,4-D Derivatives
2,4-D esters with short-chain alcohols are highly volatile. This
influences the effectiveness of their application to target crops,
their effects on neighbouring crops, and the degree of contamination of
the atmosphere. 2,4-D alkali salts or amine salts are much less
volatile than esters, and these products are to be preferred when the
use of 2,4-D esters might lead to evaporative 2,4-D losses and to crop
damage or damage to the surrounding environment.
Details of technical compositions, impurities, and analytical
methods can be found in Environmental Health Criteria 29: 2,4-
Dichlorophenoxyacetic acid (WHO, 1984).
Table 2. Vapour pressure and solubility of 2,4-D salts and esters
--------------------------------------------------------------------------------
Compound Vapour pressurea Solubility
--------------------------------------------------------------------------------
2,4-D free acid 0.4 mmHg (160 °C) 0.09% in water (25 °C),
85% in acetone (25 °C)
dimethylamine salt 300% in water (20 °C),
soluble in acetone
isopropyl ester 1.4 x 10-3 mmHgb insoluble in water, soluble
4.6 x 10-5 mmHgb in most organic solvents
butoxyethanol ester 4.5 x 10-6 mmHgb insoluble in water, soluble
(butylethyl ester) in most organic solvents
ethylhexyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
isooctyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
propyleneglycol butyl 3.0 x 10-6 mmHgb insoluble in water, soluble
ether ester in organic solvents
methyl ester 2.3 x 10-3 mmHgb
ethyl ester 1.1 x 10-3 mmHgb
butyl ester 3.97 x 10-4 mmHgb
--------------------------------------------------------------------------------
a 1 mmHg = 0.133 kPa.
b Vapour pressures of esters were determined at high temperatures by gas-
liquid chromatography, and these values are the result of extrapolation
to 25 °C. Values vary considerably between authors as a result of this
extrapolation; original values at high temperatures agree. Results are
presented here as an indication of relative vapour pressure at working
temperature. Values from Flint et al. (1968) and Jensen & Schall
(1966).
3. SOURCES OF ENVIRONMENTAL POLLUTION
The following is a summary of the chapter from Environmental Health
Criteria 29: 2,4-Dichlorophenoxyacetic acid (WHO, 1984).
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use were
not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United Kingdom.
World-wide use of herbicides and annual production, which probably
exceeds 5 x 107 kg/year, are increasing.
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaved weeds in cereal crops, as well as on
pastures and lawns, in parks, and on golf courses, at rates of about
0.2 to 2.0 kg active ingredient (acid equivalent) per hectare. Esters
are also used at rates of up to 6.0 kg (acid equivalent) per hectare to
suppress weeds, brush, and deciduous trees along rights-of-way and in
conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides in or
along irrigation and other canals, in ponds, and lakes at rates ranging
from 1 to 122 kg/ha.
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays containing 20
to 40 mg 2,4-D/litre on apple trees to reduce premature fruit-drop, on
potato plants to increase the proportion of medium-size tubers or to
intensify the tuber skin colour of the red varieties, and in citrus
culture to reduce pre-harvest fruit-drop and to increase fruit storage
life.
The highly volatile ethyl, isopropyl, and butyl esters are being
replaced by low-volatile esters or by amine salts to reduce crop damage
resulting from 2,4-D vapour drift, and to decrease atmospheric
pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public, has
been reduced in some countries because of increasing concern about
possible toxic effects, especially in relation to CDDs.
3.3. Disposal of Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localized to
the production site and to areas of waste dumping, and it is likely to
be more dispersed if disposal or leaching has occurred into water
courses. Disposal of unused 2,4-D in agriculture and washing of
equipment may result in localized land pollution and also pollution of
water supplies through direct contamination or leaching from soil.
4. UPTAKE, ACCUMULATION, ELIMINATION, AND BIODEGRADATION
Appraisal
2,4-D does not persist in soil because of its rapid degradation.
The physico-chemical properties of 2,4-D acid and its formulations
have an important effect on its behaviour in environmental
compartments.
The bioavailability to, and uptake by, aquatic and terrestrial
organisms is strongly influenced by the organic matter content of
soils, microbiological activity, and by environmental conditions such
as temperature and pH. Although highly inconsistent, the data on
dissipation and bioavailability in various soils demonstrate a marked
influence of differences in the texture and mineral composition of the
soil (Graham-Bryce, 1972). In aerobic soils, with a high content of
organic material, and at high pH values and temperatures, toxic effects
are limited because of rapid degradation of 2,4-D.
Uptake is followed by rapid excretion in most organisms. With the
exception of some algae, the retention of 2,4-D by organisms in the
environment cannot be expected, because of its rapid degradation.
Some microorganisms are capable of utilizing 2,4-D as their sole
carbon source. Repeated application to soil stimulates the number of
organisms capable of degrading the compound.
4.1. Biodegradation
2,4-D is readily and rapidly degraded in soil. Warm, moist
conditions and addition of organic matter stimulate degradation.
Autoclaving the soil and inhibiting bacterial metabolism reduce
degradation. The kinetics of 2,4-D disappearance suggest that
microorganisms are responsible. Particular species of microorganisms,
of various types, have been isolated and shown to degrade phenoxyacetic
acid herbicides in pure culture. Degradation of the phenoxyacetic
acids proceeds by two main pathways. These are via a hydroxyphenoxy
acetic acid intermediate or via the corresponding phenol. The
literature has been reviewed by the two workers principally responsible
for this evidence (Audus, 1960, 1964; Loos, 1969). Some microorganisms
are capable of using 2,4-D as their sole carbon source. More often,
2,4-D is co-metabolized with another carbon source. Regular treatment
of soil with 2,4-D stimulates the numbers of organisms which are
capable of degrading the compound. Treatment with other phenoxy
herbicides can also lead to an increase in organisms capable of
degrading 2,4-D.
Butler et al. (1975a) exposed 21 species of freshwater algae
isolated from natural lake water to 2,4-D butoxyethanol ester, at a
concentration of 0.01 mg/litre, and looked for degrading ability. Most
of the cultures fully degraded 2,4-D within 2 weeks. A single culture
retained 64% of the added 2,4-D, while seven isolates reduced 2,4-D to
less than 20% of the amount added. The remaining isolates showed 2,4-D
recoveries ranging from 22% to 53%.
Le Van To (1984) isolated six species of microorganisms
from soil previously treated with herbicides. These were
Flavobacterium peregrinum, Pseudomonas fluorescens, Arthrobacter
globiformis, Brevibacterium sp., Streptomyces viridochromogenes, and
an unidentified Streptomyces species. Flavobacterium was the most
active organism in degrading 2,4-D; degradation of 20 mg/kg of
2,4-D was complete after 20 to 30 days. In a liquid medium,
Flavobacterium degraded 93.5% of added 2,4-D within 80 h. The time
required to degrade half of the 2,4-D added to a sterilized soil along
with nutrient was estimated at 3 days. Li-Tse Ou (1984) investigated
the breakdown of 2,4-D in two types of soil under dry and moist
conditions and at two different temperatures. Numbers of
microorganisms degrading 2,4-D were also estimated. Generally, 2,4-D
disappeared more rapidly from moist soil; after 14 days of a slow rate
of disappearance, however, the removal rate from dry, sandy soil
increased. Numbers of organisms degrading 2,4-D were initially much
lower in sandy than in clay loams. However, numbers increased rapidly
in sandy soils after the addition of the herbicide and, as a result,
2,4-D was eventually degraded more rapidly in sandy than in clay loams.
In moist conditions, at 25 °C, the half-life of 2,4-D was 7 days or
less, whereas in dry conditions, at 35 °C, it could be as long as 250
days. These latter conditions are unlikely to apply in most natural
conditions where 2,4-D is likely to be used.
Rosenberg & Alexander (1980) incubated sewage-sludge bacteria with
2,4-D and found that nearly all of the herbicide had disappeared after
7 days. Subsequent additions of 2,4-D led to destruction of the
compound without a lag period; this suggests selection for organisms
capable of degrading the compound. Similar results were obtained using
bacteria from soil. The time needed for the disappearance of 90% of
the added 2,4-D was 14 days with soil inocula. 2,4-D added
subsequently was reduced by 70% within 3 to 4 days. Various tropical
soils were used in the experiment and all showed a high capacity for
degrading 2,4-D. Thompson et al. (1984) determined the persistence of
2,4-D applied at recommended rates in agricultural soils in Canada. In
all but one soil, a sandy loam, the concentration had declined by 50%
within 7 days. Sattar & Paasivirta (1980) showed slower degradation
of 2,4-D in acid soils. It took 6 weeks for 50% of the 2,4-D to
disappear from the soil and 7% was still left after 24 weeks. In
water-logged soil, there was reduced degradation of the herbicide.
Lewis et al. (1984) studied bacterial breakdown of 2,4-D
butoxyethyl ester and the effects of adding various extra components to
the medium. The addition of unfiltered, spent fungal medium from which
the majority of the fungus had settled out could be either stimulatory
or inhibitory to degradation rates of the herbicide; this depended on
the particular fungus species cultured in the medium. Further
investigation showed that effects were primarily due to differences in
pH. Reduction of the pH below 6 inhibited bacterial transformation of
the compound. Fungi commonly release large amount of organic acids.
The addition of spent fungal medium inhibited the breakdown of 2,4-D
ester. Buffering the added fungal medium reduced this inhibitory
effect; indeed, some stimulation of breakdown occurred after the
addition of buffered, spent medium. The addition of nutrients, or
other bacteria which did not transform 2,4-D, stimulated the
transformation of the herbicide. The authors consider that the most
likely explanation for this phenomenon is induction of other
transforming enzymes. With increasing substrate concentration, further
enzyme systems are induced in bacteria. The presence of other
organisms may stimulate the induction of these other enzymes at lower
substrate concentrations than would normally induce them. Increased
biomass of transforming bacteria in the presence of competing organisms
contributes to increased transformation rates. The nature of the
microbial community can, therefore, greatly change the ability of
degrading bacteria to transform 2,4-D and other xenobiotics.
O'Connor et al. (1981) found that 2,4-D applied at about 1.5 mg/kg
was readily degraded in soil. Adding extra carbon in the form of
dried, digested sewage sludge had a short-term effect in enhancing
degradation of the compound. Torstensson (1975) measured the half-life
of 2,4-D degradation in cultures of soil microorganisms at different
pH. In the pH range of 8.5 to 5.0, the half-life changed little,
ranging from 5 to 8 days. At pH 4.5, the half-life increased to 21
days and, at pH 4.0, increased further to 41 days.
Lieberman & Alexander (1981) added 2,4-D to inocula of municipal
sewage and monitored the biological oxygen depletion (BOD) as a measure
of degradation. The herbicide was added to carbon-depleted inocula
such that the 2,4-D represented the sole carbon source. Less than 5%
of the available oxygen was depleted, indicating poor biodegradation of
2,4-D because of low numbers of organisms capable of degrading the
herbicide as their sole carbon source. A separate study showed that
2,4-D was not toxic to microorganisms in sewage.
Fournier (1980) showed that, while 2,4-D treatment increased the
numbers of soil microorganisms capable of metabolizing 2,4-D as the
sole carbon source and those capable of co-metabolizing the herbicide,
this increase was dependent on the concentration of 2,4-D used. At
concentrations of 2,4-D between 5 and 50 mg/litre, there was a
significant increase in the numbers of organisms metabolizing 2,4-D,
and at 5 mg/litre there was a very pronounced increase in organisms co-
metabolizing the compound. At much higher (500 mg/litre) or much lower
(1.2 µg/litre) 2,4-D concentrations, there was no increase in the
numbers of either metabolizing or co-metabolizing organisms.
Sandmann & Loos (1984) estimated the numbers of microorganisms
capable of degrading 2,4-D in soils with and without the `rhizosphere
effect' of two plants, African clover (Trifolium africanum) and sugar
cane (Saccharum officinarum). The `rhizosphere effect' is a
phenomenon which occurs in close association with the roots of plants,
where material from the root or the metabolic activity of the root
tissue affects the surrounding soil. Particularly high, stimulated
populations were associated with sugar cane. A similar effect, but to
a lesser degree, was found with clover. In the three sugar cane soils
examined, and their corresponding controls, the numbers of organisms
were 46 400, 156 000, and 40 700 per g of soil, with rhizospheres, and
178, 1480, and 6170 per g of soil, without rhizospheres, respectively.
Seibert et al. (1982) failed to demonstrate a rhizosphere effect on
2,4-D degradation in glasshouse studies using soils with and without
maize roots.
Norris & Greiner (1967) investigated the degradation of 2,4-D in
forest leaf litter. Litter from either alder, ceanothus, vine maple,
bigleaf maple or Douglas fir showed comparable ability to degrade
2,4-D, the recovery of 2,4-D being between 60% and 70% after 15 days of
incubation. In a second series of experiments, different formulations
of 2,4-D were added to alder litter. About 50% of the free acid of
2,4-D was degraded within 15 days. Triethanolamine salt and two
commercial formulations (`solubilized acid' and isooctyl ester) were
degraded less than the pure acid. There was between 30% and 40%
degradation of these preparations over 15 days.
Nesbitt & Watson (1980) related the degradation rate of 2,4-D in
river water to the nutrient levels, sediment load, and dissolved
organic carbon content of the water. The addition of sediment or
inorganic nutrients increased the rate of 2,4-D degradation, whereas
the addition of organisms capable of degrading 2,4-D did not increase
the rate of breakdown of the herbicide. This finding indicated that
the limiting factor in breakdown of 2,4-D in river water was not
numbers of organisms but the nutrient status of the river. The authors
noted that in winter, when the river was in peak flow and the water
temperature below that for optimum microbial activity, appreciable
amounts of the herbicide would be washed into the estuary. An earlier
pilot study of seasonal changes in the capacity of river water in
Western Australia to degrade 2,4-D (Watson, 1977) indicated clear
seasonal differences in both river water concentrations of the
herbicide and the degrading capacity of river water. Several rivers
were studied and differences were related to the amount of
agricultural run-off, the sediment content of the water, river flow,
and temperature. Rivers receiving agricultural run-off degraded 2,4-D
better than those receiving run-off principally from forests. This was
presumed to be the result of the preconditioning of organisms to the
herbicide; the investigation corrected for nutrient content of the
water which had been previously shown to affect degradation.
Spain & Van Veld (1983) looked at the degrading ability of
microbial communities taken from sediment cores from freshwater,
estuarine, and marine sites. Some cores were pre-exposed to 2,4-D.
Cores from freshwater sites showed increased degradation of 2,4-D after
pre-exposure to the compound, whereas estuarine and marine cores did
not show this effect. The adaptation of freshwater cores was maximal
after 2 weeks and no longer detectable 6 weeks after pre-exposure.
4.2. Uptake and Accumulation by Organisms
Appraisal
Many studies on the accumulation of 2,4-D have used radioactively
labelled herbicide and have monitored uptake by simple counting of the
label. This fails to take into account that the label could have been
removed from the parent molecule by metabolic breakdown. Values for
uptake should, therefore, be treated as a maximum possible uptake
value for 2,4-D. Such data would not normally be considered
acceptable. However, the accumulation of 2,4-D is so low that these
data serve to illustrate that little of the herbicide is accumulated.
4.2.1. Laboratory studies
Eliasson (1973) sprayed leaves of 3-year-old aspen (Populus
tremens) with the butoxyethanol ester of 2,4-D at 0.5 kg acid
equivalent/litre. The plants were then kept in an open-sided
glasshouse and residues of 2,4-D were monitored. Most of the
herbicide remained in, or on, the sprayed leaves. The average residue
level was 2300 mg/kg fresh weight 1 day after spraying. This level had
fallen to 1300 mg/kg after 37 days and, by day 365, the average residue
level was 870 mg/kg. This was a very high application rate and
indicates that there is no foliar uptake of 2,4-D by plants.
Glynn et al. (1984) exposed coral Pocillopora damicornis to three
concentrations of 2,4-D sodium or amine salts at 0.1, 1.0, or
10.0 mg/litre. The maximum concentration of 2,4-D found in coral
tissue was 0.137 mg/kg after exposure to the amine salt at 10 mg/litre,
but residues were not related to the 2,4-D exposure concentration. The
highest bioconcentration factor (BCF) of 1.33 was found after exposure
to 0.1 mg/litre of the amine salt of 2,4-D, i.e., the coral contained
1.33 times the concentration of 2,4-D in water.
Metcalf & Sanborn (1975) introduced 14C-labelled 2,4-D into
model ecosystems consisting of an alga Oedogonium, an aquatic plant
Elodea, a snail Physa, and the mosquito fish Gambusia. Total
14C in the water was equivalent to 0.205 mg 2,4-D/litre. The highest
BCF was in the alga (26.8, based on measurement of radioactivity).
Analysis of all components of the ecosystem for 2,4-D, rather than the
radiolabel, revealed none of the parent compound. The BCF, therefore,
refers to breakdown products rather than 2,4-D itself. Gile (1983)
introduced 14C-labelled 2,4-D, as the butyl ester, into a simulated
ryegrass ecosystem. The system consisted of a sandy loam soil, annual
ryegrass, several invertebrates, and grey-tailed voles. Voles were
introduced 10 days after spraying 2,4-D as a foliar spray at the
equivalent of 1 kg/ha. The experiment was terminated after 1 month.
Plant material contained an average of 8.9 mg/kg; this was identified
as being mostly 2,5-dichloro-4-hydroxyphenoxyacetic acid. Residue
levels in animals (based on unidentified 14C residues) ranged from
0.31 mg/kg in snails to 5.28 mg/kg in pillbugs (isopods).
Freitag et al.(1982) measured the bioaccumulation of 14C-2,4-D in
an alga Chlorella fusca and a fish, the golden orfe. They measured a
24-h static BCF of 6 for the alga, and a 3-day static BCF of <10 for
the fish. This measurement was based on radioactivity and, therefore,
did not distinguish between the parent compound and its breakdown
products.
Schultz (1973) examined uptake and loss of 14C-2,4-D dimethyl-
amine salt by organs of three species of fish (channel catfish,
bluegill sunfish, and largemouth bass), exposed to 0.5, 1.0, or
2.0 mg/litre of 2,4-D acid equivalent. After exposure to the highest
concentration of 2,4-D dimethylamine salt, there was detectable
radioactivity in all organs examined. Bile showed the highest residues
of 14C in all three species after 1 week. For the remainder of the
exposure period of 12 weeks, there was an increase of radioactivity in
other organs and a decrease in the bile. At the end of the exposure
period, there was no clear pattern to residue levels of 14C in
different organs. These levels ranged from 5.04 mg/kg in bile to
35.5 mg/kg in posterior kidney for the channel catfish. For largemouth
bass, the range was from 1.32 mg/kg in muscle to 7.29 mg/kg in liver.
For the sunfish, the lowest residue was 24.75 mg/kg in bile and the
highest 322.7 mg/kg in the pyloric caeca of the gut. After 84 days
exposure to the dimethylamine salt at 2 mg/litre, levels of 14C in
the muscle of catfish, bass, and sunfish were equivalent to 0.953,
0.035, and 1.065 mg 2,4-D/kg, respectively. No analysis for 2,4-D
itself was carried out. A second study exposed the three fish species
for 2 weeks to 14C-2,4-D dimethylamine salt at 1 mg/litre and then
for a further 4 weeks to clean water. The disappearance of 14C was
measured. Loss of 14C was slow at first but by 4 weeks most tissues
had shown a decline in residues. Samples were analysed for 2,4-D but
none was detectable, suggesting that the 14C measured was in
breakdown products. The values for 2,4-D residues in this and other
studies using 14C-labelled material should, therefore, be regarded as
overestimates of retained 2,4-D. Uptake of 14C-2,4-D was examined at
two different temperatures, 17 °C and 25 °C. The highest residues of
14C detected in fish were equivalent to 0.122 mg 2,4-D/kg, but no
2,4-D could be found after analysis, except in bluegill sunfish after
14 days. Loss of 2,4-D did not, therefore, seem to change with
differing temperature over this range. A similar study, at two
different water pH values, showed significantly more 14C uptake in
all three species at the more acidic pH. Analysis of fish tissues for
2,4-D by gas-liquid chromatography showed non-detectable, or trace,
levels in most samples. Only in bluegill sunfish after 7 and 14 days
were residues measurable. These 2,4-D residues showed the opposite
trend to the 14C results; there was more 2,4-D in fish exposed at the
more alkaline pH. The authors suggest that metabolism of the herbicide
in the fish is suppressed at alkaline pH.
Sigmon (1979) exposed bluegill sunfish to 2,4-D butyl ethyl ester
(3 mg/litre) at three different temperatures, 20, 25, and 30 °C, and
measured the tissue content of 2,4-D after 8 days. None of the groups
differed from the controls, residues being <0.05 mg/kg.
Bluegill sunfish and channel catfish took up <0.5% of the
available 14C when exposed to 14C-2,4-D dimethylamine salt at 2
mg/litre (with 1 litre of water per fish) for 7 days (Sikka et al.,
1977). A maximum total 14C concentration in the fish was
reached after 24 h and did not change significantly over 14 days.
Bluegill sunfish attained a total body concentration of 0.9 mg/kg and
catfish 0.2 mg/kg at 24 h. These values were 2,4-D equivalents of
14C measured; the compound was not analyzed directly. When bluegill
sunfish were injected intraperitoneally with 14C-2,4-D dimethylamine
salt, at dose levels of 1 or 2.5 mg/kg body weight, they
excreted 90% of the dose within 6 h of treatment. In a similar
experiment, Stalling & Huckins (1978) exposed bluegill sunfish to
14C-2,4-D dimethylamine salt at 2 mg/litre and measured both
14C and 2,4-D in fish and water samples over the following 12 weeks.
Radioactivity was detected in tissues and increased over the
experimental period, but there was no measurable 2,4-D; the detection
limit of the method was 0.1 mg/kg. An in vivo intraperitoneal
injection of 110 µg of 14C-2,4-D was followed by rapid elimination.
Rodgers & Stalling (1972) measured uptake of 14C-2,4-D butoxy-
ethanol ester by three species of fish, which were exposed to either
0.3 or 1.0 mg/litre and sampled over the next 168 h. Some fish were
fed and some fasted. Radioactivity in a variety of tissues was
determined; the maximum levels were found within 3 h of exposure in fed
fish. After this, levels declined over the remaining sampling period,
and by the end of the experiment, residues were negligible. The one
exception was the gall bladder, which consistently contained more 2,4-D
than other tissues. Results were different for fasted fish. In almost
all organs of fasted fish, uptake of 2,4-D was slower than for fed
fish, although the levels reached were eventually two to five times
higher than in fed fish. Analysis of the residues showed that only the
liver ever contained the herbicide in the ester form. In all other
tissues, only the acid was present.
Shcherbakov & Poluboyarinova (1973) monitored the accumulation of
2,4-D in carp and Daphnia. The 2,4-D was added as the butyl ester
at concentrations ranging from 0.006 to 5 mg/litre; the recommended
usage rate for this ester leads to water concentrations of about
0.5 mg/litre. Analyses of fish tissues were made for both the ester
and the acid. The highest BCF for the ester, at 395, was found with
fish after a 7-day exposure to 0.5 mg/litre. Acid accumulation was
lower than that of the ester. The experiment lasted for 70 days. At
day 10 and after, only trace amounts of ester were found in fish.
Small amounts of 2,4-D acid were found at day 10, but only trace
amounts after day 70. Residues of 2,4-D ester in Daphnia varied from
23.9 to 518 mg/kg, according to the exposure concentration.
Two experiments have been carried out on the grey slug Derocerus
reticulatum by Haque & Ebing (1983) using 14C-labelled 2,4-D acid.
The first study, a contact experiment, exposed the slugs to 2,4-D in
contaminated soil at 1.1 mg/kg. The body content of 2,4-D in slugs
reached equilibrium (0.014 mg/kg) after 15 days; this represented a
BCF of 0.013 based on radioactivity. In the second experiment,
slugs were exposed via the food using carrot discs containing 1.1 mg/kg
slug body weight per day over 5 days. Residues of 14C in the slugs
increased during the feeding period, peaking at 5.5 mg/kg. During the
following 7 days, residues were monitored to investigate loss of
radioactive material. At the end of the experiment, on day 12,
residues were comparable to those at the end of the feeding period.
During the course of feeding 2,4-D-contaminated carrots, more than 80%
of the ingested dose of radioactivity was excreted rapidly; only 20%
was retained. There was no attempt to characterize the 14C residues;
these may, therefore, represent either 2,4-D or its breakdown
products.
Chickens given a single oral dose of 100, 200, or 300 mg/kg
body weight reached maximum plasma levels of 2,4-D of 90, 130, and
250 µg/ml, respectively. Plasma levels in all groups had fallen to
15 µg/ml or less after 24 h. Continuous dosing of chickens at
300 mg/kg per day led to a faster rate of elimination of the daily dose
of 2,4-D with time (Bjorklund & Erne, 1966).
4.2.2. Field studies
Cope et al. (1970) treated experimental ponds with 2,4-D propylene
glycol butyl ether ester to give water concentrations up to and
including 10 mg/litre. No detectable 2,4-D was found in fish exposed
to 1 mg/litre or less of the herbicide, but residues were found in
bluegill sunfish exposed to 5 or 10 mg/litre. The highest residue
(2 mg/kg) was found 1 day after application. Residues were still
detectable after 3 days but not subsequently. Vegetation
(Potamogeton nodosus) and bottom sediment contained residues of
50.0 and 3.0 mg/kg, respectively, 2 days after treatment with the 2,4-D
ester at 10 mg/litre. The herbicide was still detectable at 0.1 mg/kg
in sediment after 44 days but not thereafter. At 44 days after
treatment, there were residues in the plant of 1.2 mg/kg; this amount
declined to 0.1 mg/kg after 94 days.
Following the field application of 2,4-D butoxyethanol ester at
22.5 kg/ha, Whitney et al. (1973) measured residues of the herbicide in
fish, crustacea, and insect larvae over a 3-week period. The herbicide
had been applied to control eurasian water milfoil. Some 2,4-D was
taken up by these various species; the highest residue concentration
was 0.24 mg/kg in largemouth bass after 8 days. All residues in
organisms were below 0.1 mg/kg after 3 weeks. No 2,4-D could be
detected in water in 33 samples taken after treatment, the detection
limit being 0.10 mg/litre. The highest reported concentration of 2,4-D
in mud was 0.65 mg/kg, 10 days after treatment, but in most samples the
herbicide level in mud was much lower and in several it was
undetectable.
Hoeppel & Westerdahl (1983) treated four areas (10 ha each) of
dense water milfoil beds in Lake Seminole, Georgia, with either 2,4-D
dimethylamine salt or 2,4-D butoxyethanol ester, at each of two
application rates (22.5 or 45 kg/ha). Both formulations were converted
to 2,4-D free acid within 24 h. Maximum water concentrations achieved
in the high rate (45 kg/ha) areas were 3.6 and 0.68 mg/litre for the
dimethylamine salt and butoxyethanol ester, respectively. There was no
detectable uptake of 2,4-D into fish in those areas treated with the
dimethylamine salt. In the ester-treated areas, 4 out of 24 game fish
sampled contained low levels of 2,4-D in muscle (the highest residue
being 0.29 mg/kg) and 18 out of 20 gizzard shad contained detectable
2,4-D in muscle (the highest residue being 6.9 mg/kg). No fish sampled
more than 13 days after treatment contained detectable 2,4-D.
Schultz & Harman (1974) treated nine experimental ponds with 2,4-D
dimethylamine salt at three concentrations: 2.24, 4.48, and 8.96 kg/ha.
Samples of water, bottom sediment, and fish were taken over 147 days.
Maximum water and sediment concentrations of 2,4-D were 0.692 mg/litre
and 0.17 mg/kg, respectively. Of 307 fish sampled, 45 contained
detectable residues of 2,4-D. The highest residue measured was in a
channel catfish at 1.075 mg/kg 1 day after treatment. All residues in
fish after 28 days were less than 0.005 mg/kg; most were undetectable.
Smith & Isom (1967) measured uptake and retention of 2,4-D after
treatment of two field sites for control of watermilfoil with the
butoxyethanol ester. The first site was treated with a granular
formulation at a rate of 112 kg/ha. One bluegill sunfish (Lepomis
macrochirus) contained 0.15 mg 2,4-D/kg on day 50 after treatment. All
other fish, sampled between 72 h and 50 days after treatment,
contained less than 0.14 mg/kg, which was the limit of detection. Two
samples of several species of mussel, held in cages for 96 h following
spraying, showed residues of 0.38 and 0.7 mg/kg. Water levels of 2,4-D
reached a peak of 37 mg/litre within 1 h of application and had fallen
to less than 1 µg/litre within 8 h. Mud samples contained very
variable levels of 2,4-D residues, ranging between 0.14 and 58.8 mg/kg.
The highest residue was found 10 months after application. The second
site was treated at the lower rate of 45 kg/ha. All fish sampled
between 15 days and 9 months after 2,4-D application showed residues
of less than 0.14 mg/kg. Mussels sampled between 1 and 42 days after
application contained residues ranging between <0.14 and 1.12 mg/kg.
Water levels peaked at 157 µg/litre, 1 h after spraying, and mud
residues ranged from <0.14 to 33.6 mg/kg.
Coakley et al. (1964) measured residues in organisms at the center
of a 0.4-ha field plot sprayed with 2,4-D butoxyethanol ester at a rate
of 33.7 kg/ha for watermilfoil control. Two days after application,
oysters (Crassostrea virginica), clams (Mya arenaria), fish (Lepomis
gibbus), and blue crabs (Callinectes sapidus) contained 3.5, 3.7, 0.3,
and <0.8 mg/kg, respectively.
In 1971, over 2800 ha in Loxahatchee National Wildlife Refuge
were sprayed with the dodecyl-tetradecyl amine salts of 2,4-D at a
rate of 4.48 kg/ha. The initial application of 2,4-D was followed by
spot treatments of the same formulation and/or the dimethylamine salt
of 2,4-D. The highest water concentration (0.037 mg/litre of 2,4-D)
was measured 1 day after the initial application. Of 60 fish sampled
in the area, 19 had measurable residues of 2,4-D but only three of
these were greater than 0.1 mg/kg; the highest recorded residue was
0.162 mg/kg. Breast muscle and liver of a bird, the common Florida
gallinule Gallinula chloropus, had residues of 0.3 and 0.675 mg/kg,
respectively, 1 day after spraying. No residues were found in the bird
4 days after spraying (Schultz & Whitney, 1974).
Plumb et al. (1977) treated sprouting chamise (Adenostoma
fasciculatum) with the polyethylene glycol butyl ether ester of 2,4-D
at a rate of 3.4 kg acid equivalent/ha. A maximum concentration of
herbicide (95.2 mg/kg) was found in the plant within 15 min of
application. A residue of 3.8 mg 2,4-D/kg remained in, or on, the
plants (shoots which had been originally sprayed) 1 year after
treatment. When Radosevich & Winterlin (1977) applied the butoxypropyl
ester of 2,4-D to a chaparral area at a rate of 4.5 kg/ha, the
residues measured in chamise were 221 mg/kg and in grass and forbs
269 mg/kg within 2 h of application. After 30 days, these levels had
dropped to 60 mg/kg for chamise and 21 mg/kg for grass and forbs, and,
after 360 days, 0.1 mg/kg was present in chamise. Siltanen et al.
(1981) monitored residues of 2,4-D in the fruit of bilberries 1 year
after the application of 0.25, 0.75, or 2.25 kg/ha acid equivalent. No
residues were detected, the limit of detection being 0.05 mg/kg.
Raatikainen et al. (1979), in a controlled field experiment,
sprayed cowberry and bilberry with an ester formulation of 2,4-D.
Three application rates were used, 0.25, 0.75, and 2.25 kg acid
equivalent/ha, and residues of 2,4-D were measured approximately
1 month after application. Thirty-four days after the application of
0.25 kg/ha, residues in cowberry were 0.3 mg/kg. Cowberries exposed to
0.75 or 2.25 kg/ha were analysed after 35 days and contained residues
of 1.0 and 3.7 mg/kg, respectively. Bilberries treated with 0.25,
0.75, or 2.25 kg/ha were analysed 29 days later; residues were 0.1,
1.3, and 4.8 mg/kg, respectively.
4.3 Elimination
James (1979) studied tissue distribution of 14C-labelled 2,4-D in
the spiny lobster (Panulirus argus). Labelled herbicide was injected
into the pericardial sinus and animals were sacrificed at regular
intervals. 2,4-D was taken up from the haemolymph, by the green gland,
and excreted unchanged, with an overall half-time of about 8 h. Tuey &
James (1980), in a similar study, found that the clearance of 2,4-D
from haemolymph, via the green gland, was three to five times greater
than the rate of metabolism in the hepatopancreas.
Pritchard & James (1979) studied the renal handling of intra-
venously injected 2,4-D by the winter flounder (Pseudopleuronectes
americanus). 2,4-D, at a concentration of 1 µmol/litre of plasma,
was actively secreted into the glomerular filtrate of the kidney
with clearances of nearly 500 times the glomerular filtration rate.
At higher plasma concentrations of between 10 and 60 µmol/litre, a
transport maximum of 0.85 µmol/g of kidney per h was observed.
Koschier & Pritchard (1980) reported a similar study using
an elasmobranch fish Squalus acanthias. They administered
2.5 µmol 14C-2,4-D/kg to the fish intramuscularly and monitored
blood and urine 14C levels. Clearance of total 2,4-D was more than
25 times greater than the glomerular filtration rate, indicating that
2,4-D was being actively secreted by the kidney. 2,4-D was eliminated
in the urine as a taurine conjugate, this representing about 95% of the
excretory products. The plasma contained, primarily, unconjugated
2,4-D (>90%). It seemed, therefore, that 2,4-D was conjugated with
taurine before being excreted in the urine. Guarino et al. (1977), in
a similar study on the dogfish Squalus, also found that 2,4-D was
extensively conjugated to taurine (>90%) and was eliminated
predominantly via the urine; 70% of the administered dose appeared in
the urine within 4 to 6 days. The highest tissue concentration of
2,4-D (14.5 mg/kg) was found in the kidney after 4 h. Plasma
elimination was rapid, with a half-time of 44 min; similarly rapid
clearance was seen from the kidney. Half-time estimates for muscle and
liver were 2 to 3 days and 5 days, respectively.
5. TOXICITY TO MICROORGANISMS
Appraisal
In general 2,4-D is relatively non-toxic to water and soil
microorganisms at recommended field application rates.
No effect of 2,4-D was recorded on 17 genera of freshwater and two
genera of marine algae at concentrations up to 222 mg/litre.
No effect of 2,4-D was observed on respiration of either sandy loam
or clay loam soils at concentrations up to 200 mg/kg.
N-fixation by aquatic algae is affected at high concentrations of
2,4-D acid (400 mg/litre). An effect of 2,4-D esters on N-fixation
occurs from a concentration of 36 mg/litre upwards. N-fixing algae in
topsoils appear to be more vulnerable to 2,4-D acid than other algal
species. The Cyanobacteria (blue-green algae) are important as the
major N2 source in tropical ponds and soils.
In the range of 25.2 to 50.4 mg/litre, 2,4-D was inhibitory to all
types of soil fungi.
Cell division was reduced in a green alga by 2,4-D at 20 mg/litre
and stopped at 50 mg/litre. No effect was observed on a natural
phytoplankton community after exposure to 2,4-D at 1 mg/litre.
However, exposure to esters of 2,4-D reduced productivity in these
organisms.
5.1. Aquatic Microorganisms
Hawxby et al. (1977) exposed cultures of three algae
(Chlorella pyrenoidosa, Chlorococcum sp., and Lyngbya sp.,) and
one cyanobacterium (blue-green alga) (Anabaena variabilis) to
concentrations of 2,4-D in the medium of up to 10 µmol /litre
(= 2.21 mg/litre). There was no effect on growth, respiration, or
photosynthetic rate.
Gangawane et al. (1980) studied the effects of 2,4-D on growth and
heterocyst formation in the nitrogen-fixing cyanobacterium (blue-green
alga) Nostoc. The organism was cultured for 30 days in 0, 10, 100,
1000, or 1500 mg 2,4-D/litre. Growth was measured by optical density
and cells forming heterocysts were counted. Growth was inhibited at
both 10 and 100 mg 2,4-D/litre and was eliminated at higher
concentrations. There was also reduced heterocyst formation.
Lembi & Coleridge (1975) demonstrated a marked effect of 2,4-D,
at concentrations of 110 or 220 mg/litre, on cultures of the
green algae Scenedesmus, Ankistrodesmus, and Pediastrum. After 14
days of culture, the three species under control conditions produced
456 x 102, 634 x 104, and 227 cells or colonies per ml of medium,
respectively. Corresponding figures after exposure to 110 mg/litre
were 54 x 102, 41 x 104, and 74 cells or colonies per ml,
respectively. For both Scenedesmus and Ankistrodesmus, these values
were less than the pre-treatment cell concentrations.
Butler et al. (1975b) exposed unialgal cultures of green algae
isolated from Warrior River water to 2,4-D butoxyethanol ester at
0.001, 0.01, 0.1, 1.0, or 4.0 mg/litre. Thirty separate isolates were
used. Concentrations less than or equal to 1 mg/litre of the 2,4-D
ester did not change the growth pattern of the isolates. However, with
a concentration of 4 mg/litre, there was some inhibition of growth, as
indicated by a 10% increase in the number of incubates which showed
poor growth, or no growth, when compared to controls. Some isolates
were unaffected even at this concentration and it can therefore be
assumed that 2,4-D butoxyethanol ester might change the species
composition of green algae populations.
Bednarz (1981) used 12 pure cultures of green algae and
cyanobacteria (blue-green algae) separately and in combination to
investigate the effects of 2,4-D acid. Cultures were exposed to
concentrations of 2,4-D ranging from 0.001 to 10 mg/litre. Low
concentrations of 2,4-D stimulated the growth of most species of algae,
whereas high concentrations inhibited growth. Chlorococcal green algae
were more sensitive to 2,4-D than were filamentous green algae or
cyanobacteria. In further experiments, the authors cultured
combinations of sensitive and tolerant species in the same range of
2,4-D concentrations. Tolerant species used in combinations were
Chlorella pyrenoidosa, Dictyosphaerium pulchellum, and Scenedesmus
quadricaudata. The first two of these tolerant species reduced the
toxicity of 2,4-D to sensitive species in mixed culture. This
protective effect was not seen with Scenedesmus.
Singh (1974) cultured a filamentous, nitrogen-fixing,
cyanobacterium Cylindrospermum sp. in concentrations of 2,4-D acid of
0, 100, 300, 400, 500, 600, 800, 1000, or 1200 mg/litre and examined
growth and heterocyst formation after 8 days. Both parameters were
affected at concentrations higher than 300 mg/litre and cultures were
killed at a concentration of 1000 mg/litre. Kapoor & Sharma (1980)
exposed cultures of the nitrogen-fixing, filamentous cyanobacterium
Anabaena doliolum to 2,4-D ethyl ester (as `Weedone 48' concentrate)
at concentrations of 36, 108, 180, 252, or 324 mg/litre. There was a
dose-related decrease in cell nitrogen over the whole range of 2,4-D
ester exposures. Cell growth was stimulated by lower concentrations of
2,4-D and only inhibited by the highest dose. Tiwari et al. (1984)
exposed cultures of a similar nitrogen-fixing, filamentous
cyanobacterium (Anabaena cylindrica) to 2,4-D acid at concentrations of
0, 100, 500, 700, 1000, or 1500 mg/litre, and examined growth,
heterocyst formation, and nitrogen fixation. For all these parameters,
there was a stimulatory effect of 2,4-D at 100 mg/litre and a
progressive inhibition with higher concentrations. These and similar
algae are considered to be a major source of nitrogen in tropical
ponds and soils. Das & Singh (1977) cultured the nitrogen-fixing
cyanobacterium Anaebaenopsis raciborskii in concentrations of 2,4-D
acid (sodium salt) of 10, 100, 400, 600, 800, and 1000 mg/litre and
measured nitrogen fixation. Control cultures and those exposed at 10
and 100 mg 2,4-D/litre showed no significant differences.
Nitrogen-fixation was inhibited at 400 mg/litre or more and eliminated
at 600 mg/litre.
Butler (1963) reported no effect on a natural phytoplankton
community of exposure to a 1 mg/litre concentration of 2,4-D (as the
acid or dimethylamine salt), or of the dimethylamine salt on pure
cultures of Dunaliella euchlora or Platymonas over 4 h. In a later
study (Butler, 1965), natural phytoplankton communities were exposed to
esters of 2,4-D. Butoxyethanol ester, propylene glycol butyl ether
ester, and ethylhexyl ester reduced productivity (as measured by
carbon fixation) by 16%, 44%, and 49%, respectively, at a concentration
of 1 mg/litre.
Sarma & Tripathi (1980) monitored cell division in the filamentous
green alga Oedogonium acmandrium exposed to 2,4-D acid at 1, 5, 10,
20, or 50 mg/litre of culture medium. At up to 10 mg/litre, 2,4-D
was found to stimulate cell division; a 168 h exposure to 5 mg/litre
increased the incidence of dividing cells by 15% over controls.
However, cell division was reduced at 20 mg/litre and stopped at
50 mg/litre. Abnormalities in chromosomes during cell division
increased with increasing 2,4-D exposure.
Chai & Chung (1975) examined the effects on growth,
photosynthesis, respiration, and chemical composition of exposing
cultures of the green alga Chlorella ellipsoidea to 2,4-D acid at 22 or
88 mg/litre. At 22 mg/litre, 2,4-D increased growth, photosynthesis,
and the cell content of protein and nucleic acids. Carbohydrate
content was unchanged. However, at 88 mg/litre, growth was inhibited,
photosynthesis was no different from controls, and the cell content of
carbohydrate, protein, and nucleic acids was decreased.
Elder et al. (1970) examined the effect of 2,4-D acid on 17 genera
of freshwater and two genera of marine algae exposed at 22, 111, or
222 mg/litre. There was no effect on the growth of any of the
cultures, even at the highest dose of 2,4-D.
Cultures of the flagellate Euglena gracilis were exposed for 24 h
to concentrations of 1, 5, 10, 50, or 100 mg/litre or for 7 days to 10,
50, or 100 mg/litre of 2,4-D acid by Poorman (1973). Cultures in 50
and 100 mg 2,4-D/litre yielded 84% and 74%, respectively, relative to
controls, over 24 h. Lower concentrations of 2,4-D had a slightly
stimulatory effect. After 7 days, there was significant stimulation of
yield with 10 mg/litre; the culture yielded 161% compared to a control.
There was slight stimulation of growth by 50 mg/litre and a reduction
to 78% of control levels with 100 mg/litre.
George et al. (1982) exposed the rotifer Brachionus
calyciflorus to 2,4-D at 5 mg/litre. Median lethal time (LT50) was
24 h and LT100 was 31 h.
5.2. Soil Microorganisms
Pachpande & David (1980) isolated the soil alga Chlorococcum
infusionum from paddy fields and cultured the organism in the presence
of 2,4-D acid at concentrations of 0, 1, 2, 3, 4, and 5 mg/litre.
Growth was estimated as dry weight of algal cells filtered out of the
medium. All concentrations of 2,4-D were inhibitory to growth.
At the highest 2,4-D concentration of 5 mg/litre, the culture yield
was reduced from a control level of 720 mg dry wt/litre of medium to
520 mg/litre.
Cullimore & McCann (1977) applied 2,4-D acid to isolated cores
taken from a prairie, loam soil to give approximate concentrations of
1 or 100 mg/kg in the top 2 cm of soil. Soil algal populations were
estimated from subsamples of cores taken before treatment and 1, 5, or
20 days after treatment with herbicide. Thirty-one genera of algae
were identified, of which five were very sensitive to 2,4-D and were
rarely found after treatment. These were Chlamydomonas, Chlorococcum,
Hormidium, Palmella, and Ulothrix. The most resistant genera were
Chlorella, Lyngbya, Nostoc, and Hantzschia; the `percent sensitivity'
of these genera (% of the total number of treatments in which the
genus was absent) was 28%, 6%, 22%, and 44%, respectively. The
reduction in cell numbers of algae in the top layer of the soil after
herbicide treatment was soon offset by an increase in the population of
Chlorella, Stichococcus, Oscillatoria, and Spongiochloris, all of
which recovered very rapidly from the herbicide effects. There was,
however, an overall reduction in cell numbers of nitrogen-fixing algae.
Mukhopadhyay (1980) measured the bacterial, fungal, and
actinomycete populations of soils supporting rice or maize plants which
had been treated with various herbicides for weed control. There was
no effect of 2,4-D, applied at the recommended rate, either on soil
microorganism numbers or on the evolution of carbon dioxide by soil
cultures.
Huber et al. (1980) examined the effect of 2,4-D at 0.3, 0.2, or
0.1 mmol/litre (= 66, 44, and 22 mg/litre, respectively) on seven
cultures of soil microorganisms. There was no effect on the growth of
five of the cultures; these were Nocardia sp., Pseudomonas fluorescens
in both aerobic and anaerobic culture, Bacillus subtilis, and
Ustilago maydis. There was a small reduction in growth at the
highest 2,4-D dose in cultures of Rhizopus japonicus and Aspergillus
niger. 2,4-D had no effect on mycelium growth of three out of four
plant pathogenic fungi in culture; Phytophthora cryptogea showed
reduced mycelial growth at 0.1, 0.2, and 0.3 mmol 2,4-D/litre,
but Fusarium oxysporum, Alternia radicina, and Rhizoctonia solani
were unaffected.
Moubasher et al. (1981) added 2,4-D at three doses (1.9, 7.6, and
15.2 mg/kg) either to soil or to agar medium inoculated with soil
fungi, and the effects on fungal populations were monitored. In soil,
at all three doses, 2,4-D stimulated the fungi. When incorporated in
the agar medium, 2,4-D was stimulatory to overall fungal growth and to
four individual species of fungus at the lowest dose of 6.3 mg/litre,
but inhibitory to two other species. At higher doses of 25.2 or
50.4 mg/litre, the herbicide was inhibitory to all fungi.
2,4-D had a significant inhibitory effect on culture yields of the
bacterium Escherichia coli only at 10-3mol/litre (= 220 mg/litre).
There was no effect at 10-4mol/litre (= 22 mg/litre) (Toure & Stenz,
1977).
Prescot & Olson (1972) added 2,4-D, at doses of 0, 0.1, 1.0, 10, or
100 mg/litre, to cultures of the soil amoeba Acanthamoeba castellanii
and monitored growth and reproduction. There was a stimulatory effect
of 2,4-D at all dose levels; this effect was most marked at the lowest
dose and declined with increasing exposure to 2,4-D. The authors
suggest that the amoeba may degrade the 2,4-D and utilize it as a
carbon source. However, Pons & Pussard (1980) found no effect of 2,4-D
(at 28, 54, or 84 mg/litre) on the reproduction of 23 different strains
of free-living soil amoebae.
2,4-D, at 10-3mol/litre in cultures of the ascomycete Neurospora
crassa, stimulated DNA synthesis but had no effect at lower
concentrations of 10-4 to 10-6mol/litre. These concentrations had
no significant effect on either RNA or protein (Schroder et al.,
1970).
Naguib et al. (1980) measured growth, respiration, and absorption
and utilization of sugar and nitrogen in pre-formed fungal mats of
Aspergillus terreus over 72 h in the presence of 200 mg/litre of
2,4-D. The herbicide inhibited sugar inversion and consequently sugar
absorption. It also reduced the incorporation of nitrogen into protein.
Respiration was depressed. Growth of the fungus was suppressed and, on
a dry weight basis, culture mass was reduced to below the initial
level.
Trevors & Starodub (1983) added 2,4-D to sandy loam and clay
loam soils and measured both respiration and electron transport
system (ETS) activity. ETS was assessed by measuring the capacity
of the soil to reduce 2-( p -iodophenyl)-3-( p -nitrophenyl)-5-phenyl
tetrazolium chloride (INT) to iodonitrotetrazolium formazan (INT
formazan). The effects of 2,4-D were tested at concentrations of the
herbicide in soil of 0, 10, 25, 50, 75, 100, or 200 mg/kg. There was
no effect on soil respiration, monitored either as oxygen consumption
or carbon dioxide evolution, at any of the concentrations of 2,4-D in
either soil. There was similarly no effect on ETS in the sandy loam.
However, in the clay loam, there was a progressive inhibition of ETS
over the whole range of concentrations of the herbicide. The control
soil had an ETS activity of 37.3 µg INT formazan production/g soil,
whereas the ETS activity of soil treated with 10 mg 2,4-D/kg was
25.1 µg INT formazan/g, significantly lower than that of the
control. The activity was reduced further with increasing
concentrations of 2,4-D, until an activity of 16.3 µg INT formazan/g
was found at 200 mg 2,4-D/kg.
Deshmukh & Shrikhande (1975) added 2,4-D, at recommended field
rates, and at five times the recommended field rates, to two
types of soil from India. Both doses of 2,4-D inhibited numbers
of Azobacter in both soil types, and the high, but not the low, dose of
2,4-D reduced nitrogen fixation in both soils. The same authors
(Deshmukh & Shrikhande, 1974) monitored the populations of various
microorganisms under the same dosing conditions. 2,4-D stimulated
the numbers of actinomycetes throughout the 6-week incubation period at
both dose levels. Fungal populations were reduced in the first week of
incubation at both dose levels in sandy loam, but only at the higher
dose level in clay loam. This reduction in fungal populations
persisted until the second week with the high dose in the sandy soil
and throughout the incubation period with the high dose in clay soil.
There was a temporary (1 week) reduction in total bacterial numbers
with both 2,4-D dose levels in the sandy soil and with the higher level
in clay soil. Schroder & Pilz (1983) reported that 2,4-D at
approximately 10-4mol/litre (= 22 mg/kg) had no long-term effect on
soil nitrification.
Welp & Brummer (1985) measured the influence of 2,4-D on the
reducing capacity of soil microorganisms, reduction being monitored as
the capacity to reduce Fe(III) oxides to soluble Fe(II) ions. They
determined no-observed-effect levels (NOEL) of 115 and 95 mg 2,4-D/kg
for two different soil types and corresponding EC50 values on
reduction capacity of 200 and 530 mg 2,4-D/kg soil.
Ruggiero & Radogna (1985) extracted and partially purified soil
diphenolase (laccase) from forest soil. This enzyme, which exists free
in the soil, plays an important role in the metabolism of humic
materials in soil. Oxygen consumption was monitored during the
enzymatic reaction, using either catechol or p -phenylenediamine as
substrate, and the effect of 2,4-D was investigated. The herbicide
inhibited diphenolase activity, and Lineweaver-Burk plots of the
data suggested that 2,4-D acts as a non-competitive inhibitor.
Apparent K values of 28.7 and 6.0 mol/litre were obtained for catechol
and p -phenylenediamine, respectively.
6. TOXICITY TO AQUATIC ORGANISMS
6.1. Toxicity to Aquatic Invertebrates
Appraisal
The short-term toxicity data on the effects of 2,4-D free acid,
its salts, and esters on aquatic invertebrates is extensive. Ester
formulations are more toxic than the free acids or salts. Sensitivity
variations exist among species in response to the same formulation.
Organisms become more sensitive to 2,4-D when the water temperature
increases. Reproductive impairment occurred at concentrations below
0.1 of the short-term toxic levels determined for these formulations.
6.1.1. Short-term toxicity
The short-term toxicity of 2,4-D to aquatic invertebrates is
summarized in tables 3 - 5.
Unfortunately, there are few studies where both the free acid (or
its salts) and ester preparations have been tested on the same organism
under the same conditions. The only organisms for which this applies
are the oyster (Butler, 1963; Butler, 1965), the stonefly (Sanders &
Cope, 1968), and daphnids and shrimp (Sanders, 1970a). These studies
all show that the free acid and its salts are less toxic than ester
formulations; for example the free acid is at least 20 times less toxic
to the water flea Daphnia magna than the least toxic of the esters
tested (Sanders, 1970a). Comparing studies carried out by different
authors and in different systems also suggests a much greater toxicity
of the ester preparations.
Liu & Lee (1975) found that 2,4-D could adversely affect the bay
mussel (Mytilus edulis) at all stages of its life cycle. The attachment
of young mussels to test chamber walls was reduced (data in Table 3).
The authors evaluated, in two duplicate experiments, the effects of
2,4-D acid, at concentrations in sea water of 22.8, 45.7, 91.4, and
182.8 mg/litre, on the growth of larval mussels. After 10 days
exposure, there was a significant reduction in the growth of larvae
exposed to 91.4 mg 2,4-D/litre; larvae were 11.6% smaller than
controls. This reduction was found in only one experimental replicate.
In both experiments, there was reduced growth after 10 days exposure
to 182.8 mg/litre; larvae were 31.9% and 34.9% smaller than controls
in the two experiments. Exposure for 20 days at 91.4 mg/litre
led to reduced growth in both experiments. All larvae exposed to
182.8 mg/litre died within 12 days, but only in one experimental
replicate. Extension of the growth study in the second experiment led
to all larvae dying within 22 days of exposure to 182.8 mg/litre and,
therefore, failing to undergo metamorphosis. The metamorphosis of
larvae exposed from age 30 to 70 days was not affected by 2,4-D at
concentrations up to 176 mg/litre.
Presing (1981) monitored reproduction over four broods in the water
flea Daphnia magna exposed to 0, 5, 10, 25, or 50 mg/litre of
`Dikonirt' (sodium salt of 2,4-D). For the first brood, the only
significant effect was at 50 mg/litre, whereas the fourth brood was
delayed even at 5 or 10 mg/litre. Significant reductions in the
average number of young produced for each female were found with
the two highest concentrations. Young kept until maturity from
each of the tests were themselves exposed to 2,4-D in a repeat
experiment. Again there was a significant effect on young produced at
25 and 50 mg/litre.
Table 3. Toxicity of 2,4-D to estuarine or marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Salinity pH Formulationc Parameter Water Reference
stata (°C) (o/oo) concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bay mussel 17.2- 22.9- 6.4- free acid 96-h LC50 259 Liu &
(Mytilus edulis) 18.6 24.5 7.8 (232-289) Lee (1975)
17.2- 22.9- 6.4- free acid 96-h EC50 262 Liu &
18.6 24.5 7.8 attachment Lee (1975)
(trocophore larva) 17.2- 22.9- 6.4- free acid 48-h EC50 211.7 Liu &
18.6 24.5 7.8 normal Lee (1975)
development
Eastern oyster flow 18 29 butoxyethanol 96-h EC50 3.75 Butler
(Crassostrea virginica) shell growth (1963)
flow 29 25 isooctyl 96-h EC50 1.0 Mayer
shell growth (1987)
flow 28 25 PGBEE 96-h EC50 0.055 Mayer
shell growth (1987)
Copepod 21 7 7.8 butoxyethanol 96-h LC50 3.1 Linden
(Nitocra spinipes) (2.4-4.1) et al.
(1979)
Brown shrimp (adult) flow 30 PGBEE 24-h EC50 0.55 Butler
(Penaeus aztecus) loss of (1963)
equilibrium
(adult) flow 30 PGBEE 48-h EC50 0.55 Butler
loss of (1963)
equilibrium
(juv.)b stat 26 30 butoxyethanol 48-h LC50 5.6 Mayer
(1987)
(adult) flow 29 26 isooctyl 48-h LC50 0.48 Mayer
(1987)
Dungeness crab (1st zoel) stat 13 25 acid (tech) 96-h LC50 > 10 Caldwell
(Cancer magister) (1977)
(1st instar juv.)b stat 13 25 acid (tech) 96-h LC50 > 100 Caldwell
(1977)
Blue crab (juv.)b stat 24 29 PGBEE 48-h LC50 2.8 Mayer
(Callinectes sapidus) (1987)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration
in water continuously maintained.
b juv. = juvenile.
c PGBEE = propylene glycol butyl ethyl ester.
Table 4. Toxicity of 2,4-D to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Oligochaete worm flow 20 30 30 7.8 free acid 48-h LC50 122.2 Bailey &
(Lumbriculus flow 20 30 30 7.8 free acid 96-h LC50 122.2 Liu (1980)
variegatus)
Water flea stat 21 260 272 7.4 PGBEE 48-h LC50 0.1 Sanders (1970a)
(Daphnia magna) stat 21 260 272 7.4 dimethylamine 48-h LC50 4.0 Sanders (1970a)
stat 17 39 7.2 dimethylamine 48-h LC50 > 100.0 Mayer &
Ellersieck(1986)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 5.6 Sanders (1970a)
stat 21 260 272 7.4 free acid 48-h LC50 > 100.0 Sanders (1970a)
20 8.4- free acid 96-h LC50 417.8 Presing (1981)
8.6
20 8.4- sodium salt 96-h LC50 932.1 Presing (1981)
8.6
Water flea 15.6 44 7.4 PGBEE 48-h LC50 4.9 Sanders &
(Simocephalus (4.0-6.7) Cope (1966)
serrulatus)
Water flea 15.6 PGBEE 48-h LC50 3.2 Sanders &
(Daphnia pulex) (2.4-4.3) Cope (1966)
Copepod (nauplius larva)
(Cyclops vernalis) stat 20 31.6 70 6.7 free acid 96-h LC50 8.72 Robertson (1975)
(5.34-11.57)
stat 20 31.6 70 6.7 alkanolamine 96-h LC50 54.8 Robertson (1975)
(46.45-64.6)
Scud stat 21.1 30 7.1 butoxyethanol 24-h LC50 1.4 (1.1-1.8) Sanders (1969)
(Gammarus stat 21.1 30 7.1 butoxyethanol 48-h LC50 0.76 (0.51
lacustris) -1.1) Sanders (1969)
stat 21.1 30 7.1 butoxyethanol 96-h LC50 0.44 (0.31
-0.62) Sanders (1969)
stat 21.1 30 7.1 PGBEE 24-h LC50 2.1 (1.7-2.5) Sanders (1969)
stat 21.1 30 7.1 PGBEE 48-h LC50 1.8 (1.4-2.3) Sanders (1969)
stat 21.1 30 7.1 PGBEE 96-h LC50 1.6 (1.2-2.1) Sanders (1969)
stat 21.1 30 7.1 isooctyl 24-h LC50 6.8 (4.8-9.7) Sanders (1969)
stat 21.1 30 7.1 isooctyl 48-h LC50 4.6 (2.9-7.3) Sanders (1969)
stat 21.1 30 7.1 isooctyl 96-h LC50 2.4 (1.9-4.8) Sanders (1969)
stat 15.5 260 272 7.4 PGBEE 24-h LC50 4.1 (2.8-5.8) Sanders (1970a)
stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.6 (1.7-3.9) Sanders (1970a)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 15.5 260 272 7.4 PGBEE 96-h LC50 2.5 (1.7-3.7) Sanders (1970a)
(Gammarus stat 15.5 260 272 7.4 butoxyethanol 24-h LC50 6.5 (1.0-8.6) Sanders (1970a)
lacustris) (contd.) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 5.9 (3.1-11) Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 96-h LC50 5.9 (3.1-11) Sanders (1970a)
Scud stat 15 272 7.4 dimethylamine 24-h LC50 > 100 Mayer &
(Gammarus fasciatus) stat 15 272 7.4 dimethylamine 96-h LC50 > 100 Ellersieck (1986)
Glass shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 2.7 Sanders (1970a)
(Palaemonetes stat 21 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
kadiakensis stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.4 Sanders (1970a)
Seed shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 0.32 Sanders (1970a)
(Cypridopsis vidua) stat 21 260 272 7.4 dimethylamine 48-h LC50 8.0 Sanders (1970a)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.8 Sanders (1970a)
Freshwater prawn stat 27 113.9 7.5 sodium salt 24-h LC50 2342 Shukla &
(Macrobranchium stat 27 113.9 7.5 sodium salt 48-h LC50 2309 Omkar (1983)
lamarrei) stat 27 113.9 7.5 sodium salt 72-h LC50 2267 Shukla &
stat 27 113.9 7.5 sodium salt 96-h LC50 2224 Omkar (1983)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2644 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2536 Shukla (1984)
naso) stat 28 112.7 7.5 sodium salt 72-h LC50 2435 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2397 Shukla (1984)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2474 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2381 Shukla (1984)
dayanum) stat 28 112.7 7.5 sodium salt 72-h LC50 2333 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2275 Shukla (1984)
Crayfish stat 15.5 260 272 7.4 PGBEE 48-h LC50 > 100 Sanders (1970a)
(Orconectes nais) stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 > 100 Sanders (1970a)
Red swamp stat 20 100 8.4 alkanolamine 96-h LC50 1389 Cheah et al.
crayfish (imm.)c (1174-1681) (1980)
(Procambarus clarki)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Sowbug stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.2 Sanders (1970a)
(Asellus stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
brevicaudus) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 3.2 Sanders (1970a)
Stone fly (naiad) 15.5 35 7.1 butoxyethanol 24-h LC50 8.5 (5.7-13) Sanders &
(Pteronarcys 15.5 35 7.1 butoxyethanol 48-h LC50 1.8 (1.5-2.7) Cope (1968)
californica) 15.5 35 7.1 butoxyethanol 96-h LC50 1.6 (1.3-1.9) Sanders &
15.5 35 7.1 acid (tech) 24-h LC50 56 (50-63) Cope (1968)
15.5 35 7.1 acid (tech) 48-h LC50 44 (32-59) Sanders &
15.5 35 7.1 acid (tech) 96-h LC50 15 (10-22) Cope (1968)
Midge (larva) 15 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1490 Bunting &
(Chaoborus 15 78-95 55 7.3-7.8 dimethylamine 96-h LC50 890 (421-1211)Robertson
punctipennis) 20 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1124 (1975)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained.
b Alkalinity and hardness expressed as mg CaCO3/litre.
c imm. = immature.
d PGBEE = propylene glycol butyl ether ester.
Table 5. Toxicity of 2,4-D to aquatic invertebrates: no observed effect levels
---------------------------------------------------------------------------------------------------------
Flow/ Temp Sali- Alkali- Hard- Water con- Refer-
Organism stata (°C) nity nityb nessb pH Formulationc Parameterd centration ence
(o/oo) (mg/litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster flow 9 19 free acid 96-h EC0 2.0 Butler
(Crassostrea shell growth (1963)
virginica) flow 30 23 free acid 96-h EC0 2.0 Butler
shell growth (1963)
flow 25 28 dimethylamine 96-h EC0 2.0 Butler
shell growth (1963)
Freshwater oligochaete flow 20 30 30 7.8 free acid 96-h LC0 86.7 Bailey
(Lumbriculus & Liu
variegatus) (1980)
Scud stat 21.1 30 7.1 dimethylamine 96-h LC0 100 Sanders
(Gammarus lacustris) (1969)
Grass shrimp stat 20 20 butoxyethanol 24-h LC0 10 Hansen
(Palaemonetes pugio) et al.
(1973)
Pink shrimp butoxyethanol 48-h LC0 1.0 Butler
(Penaeus duorarum) (1965)
PGBEE 48-h LC0 1.0 Butler
(1965)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester.
d LC0 and EC0 represent the highest dose used which cause no death or no effect, respectively;
they are not mathematically determined no-effect levels.
George et al. (1982) measured lethal times (LT) after exposure of
the water flea Daphnia lumholtzi to 10 or 20 mg 2,4-D/litre. They
reported, for 10 mg/litre, an LT50 of 38 h and an LT100 of 71 h. For
20 mg/litre, the LT50 was 21 h and the LT100 was 31 h. Doses of
2,4-D ranging from 0.1 to 50 mg/litre did not affect the behaviour of,
or kill, the copepod Mesocyclops leuckarti within a 30-day exposure
period and so lethal times could not be calculated.
Caldwell (1977) and Caldwell et al. (1979) found the zoeal larva to
be the most sensitive life-cycle stage of the Dungeness crab (Cancer
magister) to the free acid of 2,4-D. Based on the herbicide's toxicity
to this stage, the authors suggest a maximum acceptable toxicant level
(MATC) of <1 mg/litre. At this concentration, there was no mortality,
but there was an effect on moulting.
6.1.2. Behavioural effects
Folmar (1978) tested mayfly nymphs (Ephemerella walkeri) in a `Y'-
shaped avoidance maze. A 2,4-D dimethylamine salt solution was run
into one arm of the maze and clean water was run into a second arm,
both at 400 ml/min. Numbers of nymphs in each arm of the maze were
counted after 1 h. No avoidance of 2,4-D was found at concentrations
of 10 mg/litre and there was no mortality. At 100 mg/litre there was
70% mortality in the test nymphs but still no avoidance of the
herbicide. In a similar experiment using the grass shrimp
(Palaemonetes pugio) exposed to the butoxyethanol ester of 2,4-D,
there was significant avoidance of the herbicide at 1 mg/litre (Hansen
et al., 1973).
6.2. Toxicity to Fish
Appraisal
At recommended application rates, the concentration of 2,4-D in
water has been estimated to be a maximum of 50 mg/litre. Most
applications would lead to water concentrations much lower than this
(between 0.1 and 1.0 mg/litre).
LC50 values for fish vary considerably. This variation is due to
differences in species sensitivity, chemical structure (esters, salts,
or free acid), and formulation of the herbicide.
Although the free acid is the physiologically toxic entity, the
ester formulations represent a major hazard to fish when used directly
as aquatic herbicides (because they are more readily taken up by fish).
Amine salt formulations used to control aquatic weeds do not affect
adult fish.
The NOEL varies with the species and the formulation: <1 mg/litre
(coho salmon) to 50 mg/litre (rainbow trout).
Fish larvae are the most sensitive life stage but are unlikely to
be affected under normal usage of the herbicide.
Long-term adverse effects on fish are observed only at
concentrations higher than those produced after 2,4-D has been applied
at recommended rates.
Few studies are related to the effects of environmental variables,
such as temperature and water hardness, on 2,4-D toxicity to fish.
Higher temperature possibly increases the toxicity. This might be
considered when assessing the safety of 2,4-D to fish during control of
aquatic weeds.
Fish detect and avoid 2,4-D only at higher concentrations than
those obtained under normal conditions of use.
6.2.1. Effect of formulation on short-term toxicity to fish
The toxicity of different formulations of 2,4-D to fish is
summarized in Table 6.
The most comprehensive study on the effects of different
formulations of 2,4-D using the same test fish, fingerling bluegill
sunfish (Lepomis macrochirus), was performed by Hughes & Davis (1963)
in static 24-h and 48-h tests. Ester formulations were invariably more
toxic than amine salt formulations. Dimethylamine and alkanolamine
preparations ranged in toxicity from 166 to 900 mg/litre (LC50 in 24-h
tests), depending on the commercial preparation used. Although esters
were always more toxic than amine salts, there was some variation
between different ester formulations (range: 0.9 to 66.3 mg/litre; 24-h
LC50). Most of this variation was between different preparations of
the least toxic of the esters, the isooctyl ester, which ranged in
toxicity from 8.8 to 66.3 mg/litre. All other esters tested produced
LC50 values of 8 mg/litre or less, the most toxic being the isopropyl
with a 24-h LC50 of 0.9 mg/litre. The addition of emulsifiers to acid
preparations increased 2,4-D toxicity; a formulation with emulsifiers
gave an LC50 of 8 mg/litre over 24 h, making it comparable to the
esters in toxicity. All ester formulations were considered by the
authors to present a major hazard to fish when used directly as an
aquatic herbicide, whereas the amine salt formulations could be safely
used to control aquatic weeds without adversely affecting adult fish
(Hughes & Davis, 1963).
A study on a range of ester formulations, using salmonids as test
fish, conducted by Finlayson & Verrue (1985), showed that the toxicity
for salmonids was similar to that for bluegill sunfish. These authors
argue that static tests underestimate the toxicity of 2,4-D esters
because some of the ester is hydrolysed to the less-toxic free acid
during the course of even short-term tests. The presence of test fish
increases the rate of hydrolysis of 2,4-D esters. In a static test,
with two different stocking rates of fish, the apparent toxicity of
2,4-D ester decreased with a greater density of test fish (rainbow
trout) because of this enhanced hydrolysis. Results are given in
Table 6. In their flow-through tests, results were adjusted to take
account of the hydrolysis of ester to 2,4-D acid during the course of
the experiment. Two values are given in Table 6 for each test. The
first is the calculated effect of the non-hydrolysed ester and the
second, entered as `total 2,4-D', is the observed effect of the mixture
of ester and free acid produced by hydrolysis during the course of the
experiment. There is as much as a five-fold difference between the two
values. Alabaster (1969) examined several formulations of 2,4-D in two
species of fish, and found that pelleted herbicide, either as clay-
based or resin-based pellets, was the least toxic to fish of any of the
formulations tested.
Table 6. Toxicity of 2,4-D to fish: effects of different formulations
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
--------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 40 29 6.9 alkanolamine 24-h LC50 450-900 Hughes &
(Lepomis macrochirus) stat 25 40 29 6.9 alkanolamine 48-h LC50 435-840 Davis (1963)
stat 25 40 29 6.9 dimethylamine 24-h LC50 166-542 Hughes &
stat 25 40 29 6.9 dimethylamine 48-h LC50 166-458 Davis (1963)
stat 25 40 29 6.9 di-N,N 24-h LC50 1.5 Hughes &
stat 25 40 29 6.9 di-N,N 48-h LC50 1.5 Davis
stat 25 40 29 6.9 2,4-D acid + 24-h LC50 8.0 (1963)
emulsifiers
stat 25 40 29 6.9 2,4-D acid + 48-h LC50 8.0 Hughes &
emulsifiers Davis (1963)
stat 25 40 29 6.9 isooctyl ester 24-h LC50 8.8-66.3 Hughes &
stat 25 40 29 6.9 isooctyl ester 48-h LC50 8.8-59.7 Davis (1963)
stat 25 40 29 6.9 PGBEE 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 PGBEE 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butoxyethanol 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 butoxyethanol 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butyl ester 24-h LC50 1.3 Hughes &
stat 25 40 29 6.9 butyl ester 48-h LC50 1.3 Davis (1963)
stat 25 40 29 6.9 mixed butyl + 24-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 mixed butyl + 48-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 isopropylester 24-h LC50 0.9 Hughes &
stat 25 40 29 6.9 isopropylester 48-h LC50 0.8 Davis (1963)
stat 25 40 29 6.9 ethyl ester 24-h LC50 1.4 Hughes &
stat 25 40 29 6.9 ethyl ester 48-h LC50 1.4 Davis (1963)
Cutthroat trout butyl ester 96-h LC50 0.78 Woodward (1982)
(juvenile) (Salmo clarki) (0.66-0.92)
PGBEE 96-h LC50 0.77 Woodward (1982)
(0.62-0.96)
isooctyl ester 96-h LC50 > 50 Woodward (1982)
Chinook salmon (fry) flow 9 18 17 7.1 butoxyethanol 96-h LC50 0.315 Finlayson &
(Oncorhynchus flow 9 18 17 7.1 total 2,4-D 96-h LC50 0.373 Verrue (1985)
tshawytscha)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.375 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.250 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.246 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.117 Verrue (1985)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout (fry) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.518 Finlayson &
(Salmo gairdneri) flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.642 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.329 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.514 Verrue (1985)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.468 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.338 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.342 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.555 Verrue (1985)
loading factor stat 14 18 17 7.1 butoxyethanol 96-h LC50 1.206 Finlayson &
4.2 g fish/litre Verrue (1985)
stat 14 18 17 7.1 total 2,4-D 96-h LC50 1.422 Finlayson &
loading factor stat 15 18 17 7.1 butoxyethanol 96-h LC50 3.689 Verrue (1985)
8.8 g fish/litre Finlayson &
stat 15 18 17 7.1 total 2,4-D 96-h LC50 4.487 Verrue (1985)
Harlequin fish flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Rasbora heteromorpha) pellets
flow 20 250 7.2 resin-based 24-h LC50 3950 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 3100 Alabaster (1969)
pellets
flow 20 20 7.2 sodium salt 24-h LC50 1160 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 24-h LC50 1.0 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 48-h LC50 1.0 Alabaster (1969)
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Salmo gairdneri) pellets
flow 20 250 7.2 clay-based 48-h LC50 4800 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 24-h LC50 3400 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 2400 Alabaster (1969)
pellets
flow 20 250 7.2 amine salt 24-h LC50 250 Alabaster (1969)
flow 20 250 7.2 amine salt 48-h LC50 210 Alabaster (1969)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test);
flow = flow-through conditions (2,4-D concentration in water
continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c di-N,N = di-N,N-dimethylcocoamine; PGBEE = propylene glycol
butyl ether ester; total 2,4-D = the effect actually observed in the
flow-through test; the value which preceeds each "total 2,4-D" value is
the calculated effect of the ester alone. The authors determined the
degree of hydrolysis of the ester during the course of the test and
subtracted the effect due to the free acid produced by this hydrolysis.
6.2.1.1 Tolerance and potentiation
Chambers et al. (1977) used insecticide-tolerant and insecticide-
susceptible populations of mosquito fish and an esterase inhibitor to
investigate hydrolytic activation and detoxification of 2,4-D esters.
Mosquito fish taken from a wild population which had developed some
tolerance to insecticides also showed some slight tolerance to 2,4-D
ethyl and butyl esters. This tolerance was most pronounced with the
butyl ester, where the 48-h LC50 was raised from 0.98 mg/litre in the
susceptible fish, to 1.70 mg/litre in the tolerant fish. Further
experiments were carried out to find the basis for this tolerance and
for the higher toxicity of 2,4-D esters over that of the free acid.
The addition of DEF (S,S,S-tributyl phosphorotrithioate), a carboxyl
esterase inhibitor, to the toxicity test medium slightly reduced the
toxicity of both 2,4-D esters. This finding suggested that the toxic
effect of the esters required initial hydrolysis to the acid. The
increased toxicity of the esters would result from esters being more
readily absorbed into the fish through the gills. The resistance of
the insecticide-tolerant population was, at least partially, explained
by measuring esterase activity in homogenates of gill and liver from
the two fish populations. The tolerant fish hydrolyzed less of both
2,4-D esters than susceptible fish. This effect was most marked with
liver homogenates; results from gill homogenates were equivocal. The
overall conclusion, put forward by the authors, is that liver
hydrolysis `activates' 2,4-D esters by converting them to the toxic
free acid. Hydrolysis in the gill is a `detoxification' reaction
because it reduces the uptake of toxic material. The slight increase
in tolerance in the insecticide-resistant mosquito fish is largely the
result of decreased activation of the 2,4-D esters by the liver.
Antagonism to 2,4-D ester toxicity by DEF is largely the result of
inhibition of activation in the liver rather than increased
detoxification in either liver or gill (Chambers et al., 1977).
Carbaryl, a cholinesterase inhibitor, potentiates the toxicity of 2,4-D
butyl ester to brown trout (Salmo trutta) (Statham & Lech, 1975).
The 4.5-h LC50 for 2,4-D butyl ester in static tests was shifted from
30 mg/litre to 11 mg/litre by the addition of carbaryl, at a
concentration of 1 mg/litre, to the test water. Carbaryl has no
toxicity to the fish at this concentration; the 24-h LC50 for carbaryl
alone is 6.8 mg/litre. The potentiating effect of carbaryl was itself
blocked by atropine, a muscarinic blocker, at a water concentration of
10 mg/litre; the atropine itself was not toxic to the fish at this
concentration. In a similar way, carbaryl potentiated the toxicity of
several other compounds. The authors suggested a non-specific action,
possibly by increasing the uptake of 2,4-D ester from the water. The
same potentiation was demonstrated for trout in flow-through tests
(Statham & Lech, 1975). Combinations of 2,4-D esters (butyl or
propylene glycol butyl) with the herbicide picloram increased the
toxicity to fish above the combined toxicity of the individual
compounds (Woodward, 1982).
6.2.2. No-observed-effect levels in short-term tests with fish
The NOELs of 2,4-D on fish in short-term tests are summarized in
Table 7. Values quoted from Meehan et al. (1974) and from Butler
(1965) are based on the lowest dose used in their studies. The values
from Birge et al. (1979) are calculated 1% mortality values derived
mathematically from a full toxicity curve, and based on young fish
exposed to 2,4-D from shortly after fertilization of the eggs until
4 days after hatching. The differing exposure times for the three
species tested is due to differences in time to hatching. The
variation in these data is, therefore, partly due to species
differences in sensitivity to the compound and partly due to exposure
times. Newly hatched young fish are more sensitive to 2,4-D than
unhatched embryos (see section 6.2.4).
6.2.3. Species differences in short-term toxicity to fish
Variation in the toxicity of 2,4-D to fish with species is
summarized in Table 8. Of a range of fish species, examined in the
same test conditions, using 2,4-D as the free acid, by Rehwoldt et al.
(1977), the most sensitive was the white perch (Roccus americanus)
with a 24 h LC50 of 55 mg/litre, and the least sensitive was the
eel Anguilla rostrata with an LC50 of 427 mg/litre. The grass carp,
often used together with herbicides to control aquatic vegetation, was
the least sensitive of all species examined, with a 24 h LC50 for an
amine salt formulation of 3080 mg/litre (Tooby et al., 1980).
6.2.4. Toxicity to early life-stages of fish
Short-term studies on the toxicity of 2,4-D to early life-stages of
fish are summarized in Table 9.
Studies on fish eggs and larvae immediately after hatching have
been conducted on few species and mainly with simple salts of 2,4-D.
There is little information on the effects of the more toxic esters.
2,4-D is clearly toxic for fish early life-stages, within the likely
range of water concentrations which would be found after use of the
herbicide to control aquatic weeds.
At the 16 - 32 cell blastomere stage, eggs of the bleak Alburnus
alburnus, developed more slowly than control eggs when exposed to 2,4-D
solutions. After 48-h exposure, mortality reached 68% and 79% in
those eggs exposed to 2,4-D at 25 and 50 mg/litre, respectively.
Control mortality after 48-h exposure was 37%. After 23 h of
development, eggs exposed to 25 mg 2,4-D/litre showed normal
development while those exposed to 100 mg/litre showed slower
embryogenesis or development halted at the morula-gastrula stage.
Free-swimming larvae were more sensitive to 2,4-D than eggs; the rate
of survival of embryos in tests lasting for between 12 and 48 h was
higher than for larvae. In tests lasting for between 24 and 48 h, at
concentrations of 2,4-D above 400 mg/litre, no larvae survived.
Embryos showed malformations and reduced mobility at concentrations
above 100 mg/litre, and at concentrations of 800 mg/litre or more,
embryos were immobile (Biro, 1979).
Birge et al. (1979) examined the effects of 2,4-D, as the potassium
salt, on the eggs and larvae of three species of fish. Rainbow trout
eggs were the most sensitive, largemouth bass eggs less sensitive, and
goldfish eggs extremely tolerant to 2,4-D. In all species tested,
the larval stages were more sensitive to the herbicide than were the
eggs.
Table 7. Toxicity of 2,4-D to fish: no-observed-effect levels
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Pink salmon (fry) stat 10 10-34 free acid 96-h LC0 < 1 Meehan
(Oncorhynchus stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
gorbuscha stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Chum salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus keto) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Coho salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus kisutch) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Sockeye salmon (smolts) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus nerka) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Alaska coho salmon stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
stat 10 10-34 PGBEE 96-h LC0 < 1
Oregon coho salmon stat 10 10-34 free acid 96-h LC0 10 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Dolly Varden stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salvelinus malma) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Rainbow trout stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salmo gairdneri) (1974)
Spot stat free acid 48-h LC0 50 Butler
(Leistomus xanthurus) (1965)
Longnose killifish stat dimethylamine 48-h LC0 15 Butler
(Fundulus similis) (1965)
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mullet (Mugil cephalus) stat ethylhexyl 48-h LC0 10 Butler
(1965)
White mullet (juvenile) flow sea free acid 48-h LC0 50.0 Butler
(Mugil curema) water (1963)
Goldfish flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC1 8.2 Birge et al.
(Carassius auratus) 25.8 (2.7-15.0) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC1 8.9 Birge et al.
25.8 (3.8-14.6) (1979)d
Largemouth bass flow 18.2- 66.7 53.5 7.84 pot. salt 7.5-day LC1 13.1 Birge et al.
(Micropterus salmoides) 25.8 (4.4-21-9) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC1 3.2 Birge et al.
25.8 (1.2-6.0) (1979)d
Rainbow trout flow 12.5- 66.7 53.5 7.84 pot. salt 27-day LC1 0.032 Birge et al.
(Salmo gairdneri) 14.5 (0.008-0.084) (1979)d
flow 12.5- 65.3 197.5 7.78 pot. salt 27-day LC1 0.022 Birge et al.
14.5 (0.006-0.055) (1979)d
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c pot. salt = potassium salt; PGBEE = propylene glycol butyl ether ester.
LC0 obtained by extrapolation and LC1 mathematically calculated.
d Birge et al. (1979) exposed fish from four days after hatching.
Table 8. Toxicity of 2,4-D to fish: species variation
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Striped bass stat 20 50 7.2 free acid 24-h LC50 85.6 Rehwoldt
(Morone saxatilis) stat 20 50 7.2 free acid 96-h LC50 70.1 et al.
(1977)
Banded killifish stat 20 50 7.2 free acid 24-h LC50 306.2 Rehwoldt
(Fundulus diaphanus) stat 20 50 7.2 free acid 96-h LC50 26.7 et al.
(1977)
Pumpkinseed sunfish stat 20 50 7.2 free acid 24-h LC50 120 Rehwoldt
(Lepomis gibbosus) stat 20 50 7.2 free acid 96-h LC50 94.6 et al.
(1977)
White perch stat 20 50 7.2 free acid 24-h LC50 55.5 Rehwoldt
(Roccus americanus) stat 20 50 7.2 free acid 96-h LC50 40 et al.
(1977)
American eel stat 20 50 7.2 free acid 24-h LC50 427.2 Rehwoldt
(Anguilla rostrata) stat 20 50 7.2 free acid 96-h LC50 300.6 et al.
(1977)
Carp stat 20 50 7.2 free acid 24-h LC50 175.2 Rehwoldt
(Cyprinus carpio) stat 20 50 7.2 free acid 96-h LC50 96.5 et al.
(1977)
Guppy stat 20 50 7.2 free acid 24-h LC50 76.7 Rehwoldt
(Lebistes reticulata) stat 20 50 7.2 free acid 96-h LC50 70.7 et al.
(1977)
Grass carp flow 13 270 8.1 amine salt 24-h LC50 3080 Tooby et
(Ctenopharyngodon (2622-3618) al. (1980)
idella) flow 13 270 8.1 amine salt 48-h LC50 2540 Tooby et
(2184-2952) al. (1980)
flow 13 270 8.1 amine salt 96-h LC50 1313 Tooby et
(1116-1544) al. (1980)
Bleak stat 10 15 7.8 butoxyethanol 96-h LC50 3.2-3.7 Linden et
(Alburnus alburnus) al. (1979)
Mosquito fish stat 21-22 amine salt 24-h LC50 500 Johnson
(Gambusia affinis) stat 21-22 amine salt 48-h LC50 445 (1978)
stat 21-22 amine salt 96-h LC50 405
Mullet stat sodium salt 24-h LC50 68.0 Tag El-Din
(Mugil cephalus) stat sodium salt 96-h LC50 32.0 et al.
(1981)
Longnosed killifish stat butoxyethanol 48-h LC50 5.0 Butler
(Fundulus similis) stat PGBEE 48-h LC50 4.5 (1965)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 19 7.0 dimethylamine 24-h LC50 390 Davis &
(Lepomis macrochirus) stat 25 19 7.0 dimethylamine 48-h LC50 375 Hardcastle
(1959)
Largemouth bass stat 25 19 7.0 dimethylamine 24-h LC50 375 Davis &
(Micropterus salmoides) stat 25 19 7.0 dimethylamine 48-h LC50 350 Hardcastle
(1959)
Punti (Puntius ticto) stat 23.5 ethyl ester 24-h LC50 1.6 Verma et
al. (1984)
Medaka (Oryzias latipes) sodium salt 48-h LC50 > 40 Hashimoto &
Nishiuchi
(1978)
Longnose killifish (juv.) flow sea water PGBEE 24-h LC50 5.0 Butler
(Fundulus similis) flow sea water PGBEE 48-h LC50 4.5 (1963)
flow sea water butoxyethanol 24-h LC50 5.0 Butler
flow sea water butoxyethanol 48-h LC50 5.0 (1963)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester
Table 9. Toxicity of 2,4-D to fish early life-stages
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bleak (embryo) sodium salt 12-h LC50 159.4 Biro (1979)
(Alburnus alburnus) sodium salt 24-h LC50 129.0 Biro (1979)
sodium salt 36-h LC50 63.9 Biro (1979)
sodium salt 48-h LC50 12.9 Biro (1979)
(larvae) sodium salt 12-h LC50 111.2 Biro (1979)
sodium salt 24-h LC50 70.6 Biro (1979)
sodium salt 36-h LC50 62.1 Biro (1979)
sodium salt 48-h LC50 51.6 Biro (1979)
Goldfish (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 4-day LC50 > 187 Birge et
(Carassius auratus) 25.8 al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 4-day LC50 > 201 Birge et
25.8 al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC50 133.1 Birge et
post-hatch) 25.8 (108.6-174.8) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC50 119.1 Birge et
25.8 (98.5-150.6) al. (1979)
Largemouth bass (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 3.5-day LC50 165.4 Birge et
(Micropterus salmoides) 25.8 (130.6-274.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 3.5-day LC50 160.7 Birge et
25.8 (122.9-230.6) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 7.5-day LC50 108.6 Birge et
post-hatch) 25.8 (92.5-138.4) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC50 81.6 Birge et
25.8 (64.8-103.5) al. (1979)
Rainbow trout (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 23-day LC50 11.0 Birge et
(Salmo gairdneri) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 23-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 27-day LC50 11.0 Birge et
post-hatch) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 27-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (2,4-D concentration
in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
Pot. salt = potassium salt.
Only one study has examined the effects of 2,4-D esters on newly-
hatched fish fry and on fertilized eggs. Unfortunately, this study, by
Hiltibran (1967), does not record the full experimental details. Four
species of fish were used in the study but complete results were given
only for the bluegill sunfish. The most toxic preparations were the
propylene glycol butyl ether (PGBE) ester and mixed isopropyl and butyl
esters with no-observed-effect levels of 2 and 3 mg/litre,
respectively. The dimethylamine salt, ethylhexyl ester, and sodium
salt formulations were less toxic with no-observed-effect levels at 40,
50, and 100 mg/litre, respectively.
6.2.5. Long-term toxicity to fish
Chronic exposure of sub-adult fish of a variety of species to 2,4-D
for 10 months, at a concentration of 0.1 mg/litre, led to no mortality
and to no change in the acute response to 2,4-D. The 24-h LC50 for
the compound was unchanged after 10 months exposure to sub-lethal
doses; i.e., no tolerance developed. One species of fish, the guppy
Lebistes reticulatus, reproduced in captivity. Reproductive success
was compared between control fish and fish breeding in water
containing 2,4-D at 0.1 mg/litre over 10 months. The ratio of numbers
of offspring of treated and control fish was 1.2 (Rehwoldt et al.,
1977). Mount & Stephan (1967) conducted a 10-month study on fathead
minnows (Pimephales promelas) exposed to the butoxyethanol ester of
2,4-D at 0, 0.01, 0.04, 0.2, or 0.8 mg/litre water in a flow-through
test system. Concentrations of 2,4-D of 0.2 mg/litre or less had no
observable effect on growth, survival, or reproductive success of the
fish, but the highest concentration tested was toxic to eggs. The
highest tested no-observed-effect concentration was approximately
1/45 of the 96-h LC50 for this species. Finlayson & Verrue (1985)
conducted a chronic egg-to-fry test over 86 days using chinook
salmon in 2,4-D butoxyethanol ester solutions ranging up to
118 µg/litre. The mortality of salmon during the alevin to fry period
was 4.7% and 47.6% for exposures to 60 and 118 µg/litre, respectively.
When compared to controls, the length of salmon at 36 days
post hatch was significantly reduced after exposure to 60 and
118 µg/litre. Neither survival nor growth of fry were affected at
2,4-D concentrations of 40 µg/litre or less. This maximum acceptable
toxicant concentration (MATC) represents 0.13 and 0.11 of the 96-h
LC50 values for fry and smolts of this species, respectively.
6.2.6. Behavioural effects on fish
Folmar (1976) used rainbow trout fry in an investigation to
determine whether fish avoided water contaminated by herbicides. Trout
fry were initially placed in a `Y'-shaped maze for 15 min. The
dimethylamine salt of 2,4-D, then was added to one arm of the maze and
clean water in a second arm, both at flow rates of 400 ml/min. The
number of fish in each arm was counted after 1 hour and results tested
statistically using a Chi-squared test. At concentrations of 2,4-D of
0.1 mg/litre water (approximately equal to water levels after the use
of this preparation as an aquatic herbicide), there was no avoidance
of the compound; the numbers of fish in each arm of the maze were
equal. However, at concentrations of 1.0 or 10.0 mg/litre of 2,4-D,
there was significant avoidance of the chemical.
Hidaka et al. (1984) conducted a similar study using medakas
Oryzias latipes, and tested a wide range of doses for a variety of
pesticides and herbicides. In all cases, the fish avoided the
chemical in a dose-related manner, but only over a limited range of
concentrations. Above this range, presumably because of the onset of
toxic effects, there was a dose-related decrease in avoidance response.
The authors calculated two values from these chevron-shaped graphs,
the avoidance response EC65 taken from the increasing curve
(AR65) and the DAR60 taken from the decreasing curve. For 2,4-D, the
value for AR65 was 177 (171-182; 95% confidence limits) µg/litre, and
for DAR60 was 288 (245-338) µg/litre. Compared to other chemicals
commonly used or found in water, 2,4-D has a high threshold of
detection by fish as indicated by the high avoidance threshold.
Rand & Barthalmus (1980) exposed goldfish to 2,4-D at 20 mg/litre
(10% of the 96-h LC50 for this species) at different stages during the
training period for conditioning the fish to avoid electric shocks.
They found that the herbicide had no effect when given for 24 h on the
9th day of conditioning but was effective in changing the magnitude of
the avoidance response when given for the first 24 h of conditioning.
Fish exposed for 2 weeks showed significant differences in the pattern,
rate of acquisition, and maintenance of the avoidance baseline.
Behavioural differences persisted into the post-exposure period in fish
exposed to 2,4-D for 2 weeks. The authors point out that short-term
toxicity tests do not examine subtle behavioural effects which could be
of considerable importance in the wild.
Dodson & Mayfield (1979) assessed the effect of 2,4-D on the
``reotaxic response'' of rainbow trout, that is their tendency to swim
upstream to compensate for flowing water. There was a dose-related
effect of 2,4-D at concentrations between 0 and 7 mg/litre. Above this
concentration range, there was a fall of more than 50% in the frequency
of positive reotaxic response to a revolving drum marked in alternate
light and dark stripes and an increase in the frequency of `no-
response'. The authors state that, at ``realistic concentrations'' of
2,4-D in water, there would be a tendency for fish to be moved
downstream because of a reduced reotaxic response.
6.2.7. Effects of environmental variables on toxicity to fish
The toxicity of 2,4-D to fish is related to season. Vardia &
Durve (1981) obtained different values for 96-h LC50 for the carp
Cyprinus carpio at different times of the year. Water
characteristics did not differ, except for temperature which varied
from 39 °C in May, its highest value, to 17 °C in February, its lowest.
There was a positive correlation between temperature and toxicity. At
39 °C, the 96-h LC50 was 5.6 mg/litre, and, at 17 °C, the LC50 was
40.83 mg/litre. As the authors point out, the temperature of the water
must be borne in mind when assessing the safety of this compound to
fish during control of aquatic weeds. The effect may be one of season
rather than temperature; the physiology of the fish also changes
throughout the year.
There is some effect of water hardness and pH on 2,4-D toxicity to
fish but this is very dependent on the species of test fish (Birge et
al., 1979). This effect has not been studied systematically.
6.2.8. Special studies on fish
Chronic exposure of carp to sub-lethal concentrations of 2,4-D
(5 mg/litre) led to ultrastructural changes in the liver of the fish
(Benedeczky et al., 1984). After 2 months, there was detectable
swelling of mitochondria and loss of cristae. There were also large
numbers of inclusions in the cytoplasm, interpreted by the authors as
bile pigments. Their presence in the bile canaliculi indicated the
onset of cholestasis (reduction in bile flow). After 3, 4, or 5 months
of exposure, the cholestasis was pronounced with cholesterin crystals
appearing as cytoplasmic inclusions. Later, in the 6th month of
exposure, there were endoplasmic reticulum changes indicative of
changed protein synthesis.
Oxygen consumption by bluegill sunfish was not affected by 2,4-D at
a concentration of 3 mg/litre water (Sigmon, 1979).
2,4-D at 10-4 mol/litre of medium did not affect the activity of
Na/K-ATPase in microsomes from trout gill (Davis et al., 1972).
Verma et al. (1984) detected effects on pituitary and pineal
histology in punti Puntius ticto exposed for 96 h to 1 mg/litre of
Weedone (ethyl ester of 2,4-D). There was a significant effect on cell
size of acidophilic pituitary cells but a much more marked effect on
cyanophils. Pineal epithelium cell height was significantly greater in
exposed fish.
6.3. Toxicity to Amphibians
Appraisal
Amphibian larvae are generally tolerant to amine salts of 2,4-D;
the 96-h LC50 values exceed 100 mg/litre. Of the species tested, only
one was sensitive.
No information is available on reproductive development and
differentiation or on tissue levels.
The toxicity of 2,4-D to amphibians is summarized in Table 10.
Tadpoles of the Indian toad are particularly susceptible to the
compound (Vardia et al., 1984). Lhoste & Roth (1946) showed that
2,4-D, at 5 g/litre, prevented development of the eggs of the common
frog (Rana temporaria). At doses between 500 mg/litre and 4 g/litre,
there was some development which decreased with increasing dose. These
levels are far higher than those likely to be encountered in the
environment.
Table 10. Toxicity of 2,4-D to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Refer-
stata (°C) nityb nessb concentration ence
(mg/litre)
-------------------------------------------------------------------------------------------------------------------------------------------------
Chorus frog (tadpole) stat 15.5 30 7.1 dimethylamine 24-h LC50 > 100 Sanders
(Pseudacris triseriata) stat 15.5 30 7.1 dimethylamine 96-h LC50 > 100 (1970b)
Indian toad (tadpole) 25 210 220 8.3 free acid 24-h LC50 13.77 Vardia et
(11.81-16.05) al. (1984)
(Bufo melanostictus) 25 210 220 8.3 free acid 48-h LC50 9.03 Vardia et
(8.23-9.91) al. (1984)
25 210 220 8.3 free acid 96-h LC50 8.05 Vardia et
(7.29-8.81) al. (1984)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 255 Johnson
(Adelotus brevis) stat 21-22 amine salt 48-h LC50 228 (1976)
stat 21-22 amine salt 96-h LC50 200 Johnson
(1976)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 321 Johnson
(Limnodynastes peroni) stat 21-22 amine salt 48-h LC50 300 (1976)
stat 21-22 amine salt 96-h LC50 287 Johnson
(1976)
Toad (tadpole) stat 21-22 amine salt 24-h LC50 346 Johnson
(Bufo marinus) stat 21-22 amine salt 48-h LC50 333 (1976)
stat 21-22 amine salt 96-h LC50 288 Johnson
(1976)
Common frog (tadpole) 17-29 free acid 48-h LC0 50 Cooke
(Rana temporaria) (1972)
-------------------------------------------------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
For terrestrial application, 2,4-D is usually used in the form of
the less volatile, longer-chain esters to reduce drift damage of sprays
to broad-leaved crop plants. The herbicide is used on cereal crops and
on rangeland against broad-leaved weeds and also in forestry. Thus,
insects of all kinds will be exposed to 2,4-D. Birds' eggs in the nest
are more likely to be exposed than the adult birds, though adult
exposure remains possible, particularly for sitting birds. Ground-
nesting species will be exposed from the use of 2,4-D on rangeland and
cereals; tree-nesting species will be exposed in forests.
The insecticidal action of 2,4-D is low enough that the compound
does not represent a hazard to beneficial insects; there is an adequate
safety margin with usage at recommended levels.
Although there is some disagreement in the literature about the
toxicity of 2,4-D to birds' eggs, the low uptake of the material
through the egg shell suggests that exposure would not affect hatching
in normal use of the compound. Adult birds are not affected by short-
term exposure to 2,4-D. The likelihood of prolonged exposure of
either adult birds or eggs to high levels of 2,4-D is small.
7.1. Toxicity to Terrestrial Invertebrates
Appraisal
Based on the widespread use of 2,4-D and its formulations, insects
of many kinds could be exposed to the material. Although the compounds
are generally classified as non-toxic for beneficial insects, such as
honey bees and natural enemies of pests, some adverse effects have been
reported on the early life-stages and adults of some insects.
Esters are less toxic to insects than are salts or the free acid.
Feeding studies dosing worker honey bees (Apis mellifera) with
2,4-D salts in sucrose syrup have generated two estimates of 24-h
LC50: 104 and 115 µg/bee (Jones & Connell, 1954; Beran & Neururer,
1955). Morton et al. (1972) fed 2,4-D acid to honey bees in 60%
sucrose syrup at 10, 100, or 1000 mg/litre and monitored half-time,
i.e., the time taken for 50% of the bees in a cage to die. The half-
time was significantly longer than that of controls for the two lowest
doses (37.2 days at 10 mg/litre and 40.4 days at 100 mg/litre,
compared to a control value of 33.4 days), but was significantly
reduced at 1000 mg/litre (18.6 days). The butoxyethanol and isooctyl
(commercial formulation) esters of 2,4-D had no effect on survival
times at 10, 100, or 1000 mg/litre of syrup when fed under the same
conditions as the acid. The dimethylamine salt of 2,4-D (commercial
formulation) had no effect at 10 or 100 mg/litre, but did shorten the
half-time at 1000 mg/litre.
The effects of 2,4-D on beneficial coccinellid larvae were studied
by Adams (1960). Larvae were sprayed with a preparation of mixed amine
salts of 2,4-D, at a rate equivalent to 0.56 kg acid equivalent/ha, at
different stages of their development 1, 3, 6, 9, or 12 days after
hatching. There was a lengthening of the development period when the
larvae were treated on days 3, 6, 9, or 12 but no effect when they were
sprayed on the first day after hatching. Mortality before pupation was
more than doubled in all treated groups, but mortality during pupation
was not different from that of controls.
Trumble & Kok (1980) dosed adult thistle-rosette weevils
(Ceuthorhynchidius horridus), which are used for the biological
control of musk thistle, with 2,4-D amine salt at five dose levels
between 0.17 and 147.8 kg/ha. No significant mortality was
observed, up to 175 days after treatment, at doses up to and including
1.68 kg/ha. At 16.8 and 84 kg/ha, there was significantly increased
mortality after day 3 post-treatment. At the highest dose level of
147.8 kg/ha, mortality was increased both on day 3 and subsequently.
Five-day LC50 values for males and females were calculated at 70.2 and
61.4 kg/ha, respectively. This is 41.8 times the recommended
application rate of 2,4-D for males and 36.6 for females. Riviere
(1976) reared the European cockroach (Blatella germanica) on food
containing 1000 mg/kg and reported negligible effects on reproduction.
Gall & Dogger (1967) wetted wheat plants with a 0.3% solution of
mixed isopropyl and butyl esters of 2,4-D and exposed the plants to
females of the wheat stem sawfly (Cephus cinctus). Spraying of the
wheat plants was performed at different times relative to oviposition;
times were 7 days prior to oviposition, at the time of oviposition, or
7, 14, or 21 days after oviposition. Eggs took about 7 days to hatch.
The highest larval mortality (96.4%) occurred after spraying at the
time of egg laying. The effectiveness of the 2,4-D in killing larvae
decreased with later exposure times. For plants sprayed 7, 14, and 21
days after oviposition, larval mortalities were 68.1%, 60.8%, and 37%,
respectively, compared to a control mortality of 30%. When plants were
sprayed 7 days before egg laying, larval mortality was 46.9%. Adult
flies were not affected by 2,4-D spray.
Muller (1971) exposed beetles (Carabidae) to sand dose d with
2,4-D at 0.2, 1.0, or 2.0 g/m2. Two species, Bembidion
femoratum and B. ustulatum, both showed more than 50% mortality within
4 days of exposure to 1.0 g 2,4-D/m2. B. ustulatum showed 100%
mortality within 10 days when exposed to 1.0 g/m2 and similar
mortality within 4 days when exposed to 2.0 g/m2. About 20% of the
individuals of B. femoratum survived the 14-day exposure to both 1.0
and 2.0 g/m2.
Roberts & Dorough (1984) exposed earthworms to 2,4-D acid sprayed
on to filter paper. The papers were wetted and the earthworms exposed
to the wetted paper in glass vials. The calculated 48-h LC50 value
was 61.6 (41 - 92.4; 95% confidence limits) µg/cm2.
Rapoport & Cangioli (1963) treated turf with a mixture of
(4-chloro-2-methylphenoxy)acetic acid (MCPA), at recommended rates, and
the butyl ester of 2,4-D, at 10 times the recommended rate. They
reported no effect on soil microarthropods.
7.2. Toxicity to Birds
Appraisal
Birds, and particularly the eggs of ground-nesting species, would
be exposed to 2,4-D after spraying. Food items could also be expected
to be contaminated by the herbicide. However, most studies on birds
and their eggs have been conducted at exposures far higher than could
be expected in the field.
LD50 values from acute oral and from short-term dietary dosing
indicate low toxicity of 2,4-D to birds. In longer-term studies,
effects have only been reported at extremely high exposures (for
example, kidney effects after dosing in drinking water with
concentrations in excess of the solubility of the material). There
have been no reported effects on reproductive parameters, even at
excessive exposure levels.
A single study reported adverse effects on the embryos of birds'
eggs sprayed with 2,4-D. Many studies since have shown no effect on
hatchability of eggs and no increased incidence of abnormalities in
chicks even after very high exposure to 2,4-D. Other work indicates a
very poor penetration of the eggshell by the herbicide. It can only be
concluded that after normal, or even after excessive, 2,4-D use, there
would be no effect on birds' eggs.
7.2.1. Toxicity to birds' eggs
There have been several studies on the toxicity of various 2,4-D
formulations to birds' eggs dosed by different routes.
Spraying eggs of pheasant, red-legged partridge, and grey partridge
with the equivalent of 0.55 to 1.1 kg 2,4-D/ha, either before
incubation or after 3 days of incubation, was found by Lutz-Ostertag &
Lutz (1970) and Lutz & Lutz-Ostertag (1972) to cause embryonic
abnormalities. They reported 77% mortality in pheasant eggs, 77% in
grey partridge eggs, and 43% in red-legged partridge eggs within the
first 19 days of incubation. The eggs were broken open, between days
20 and 22 of incubation for histopathological examination of the
embryos; hatching takes place at about 24 days in all species used. A
majority of surviving embryos were either wholly or partially
paralyzed. Histopathological effects were mainly gonadal. In both
male and female embryos, there were abnormalities of the gonad, often
severe enough to lead to sterility, and, in the male, abnormal
regression of the Mullerian ducts. No control embryos were examined.
In a second study by Lutz & Lutz-Ostertag (1973), quail, pheasant, and
partridge eggs were sprayed with 2,4-D from two different commercial
sources either before incubation or on days 3 or 7 after the start of
incubation. The authors reported increased mortality in embryos,
reduced hatchability, and increased abnormality in chicks. The only
control eggs reported were those in a single incubation of quail.
Later work has failed to repeat these results.
Kopischke (1972) found no adverse effects on hatchability and no
increase in deformities or later mortality of hatched chicks after
spraying eggs of pheasants or chickens with an isooctyl ester
formulation of 2,4-D on day 13 of incubation at a dose equivalent to
0.28 kg/ha.
Somers et al. (1974) sprayed chicken eggs, prior to incubation,
with concentrations of an amine salt of 2,4-D at up to 15 times the
recommended field application rate of approximately 3 kg/ha. There was
no effect on hatching success or on the survival of chicks in the
period 3 to 4 weeks post hatch. Spraying chicken eggs on days 0, 4, or
18 of incubation with 2,4-D (as a PGBE ester formulation) at up to 10
times the field application rate, had no effect on hatchability or on
survival and growth of chicks after hatching (Somers et al., 1978a).
Birds hatched from eggs, similarly treated by spraying, showed no
significant adverse effects on later reproductive performance (egg
laying performance of the females; testis weight or sperm count of
males) (Somers et al., 1978b). Hilbig et al. (1976a) found no effect
on egg hatch rate or on body weight or malformation rate in chicks,
after spraying (at 20 kg/ha) the eggs of Japanese quail, pheasants, and
chickens prior to incubation, or 3 days after the start of incubation.
In a follow-up study on the reproductive performance of birds hatched
from these dosed eggs, Hilbig et al. (1976b) reported no effects on
laying capacity, fertility, or hatchability of their eggs.
The effects of 2,4-D dimethylamine salt on the eggs of Japanese
quail, grey partridge, and red-legged partridge were studied by
Grolleau et al. (1974). Eggs were sprayed with 2,4-D at dose levels
equivalent to the recommended application rate (1.2 kg/ha) and at two
higher dose levels equivalent to 2.4 and 6 kg/ha. There were no
effects on hatching rate, embryonic mortality, or chick mortality in
the first month after hatching or on embryonic or chick malformations.
In addition, the histopathological examination of partridge thyroids
revealed no effects. Residues of 2,4-D were measured in those
partridge eggs receiving the highest dose. Very little 2,4-D
penetrated the egg shell and the highest residue measured was a total
egg content of 19.3 µg (in an 11-g egg, 15 days after treatment). The
lack of effect of 2,4-D on sprayed eggs was attributed to the poor
penetration of the herbicide. Spittler (1976) found no adverse
effects of 2,4-D on hatchability and no increase in chick abnormalities
in pheasant or quail eggs sprayed 24 h before hatching with a dose 12
times higher than the recommended application rate. Only at a dose 30
times higher than the recommended rate did hatchability fall by 10% to
15%, relative to controls. No increased incidence of abnormalities was
reported at this dose rate.
Hoffman & Albers (1984) immersed mallard eggs for 30 seconds in
aqueous emulsions of 2,4-D and calculated an LC50 equivalent to a
field application rate of 216 (155 - 300) kg/ha. This is 32 times the
recommended field application rate. Dunachie & Fletcher (1967)
injected chicken eggs with 10, 100, or 200 mg 2,4-D/kg, equivalent to
0.5, 5, or 10 mg/egg, and found reduced hatchability relative to
control eggs injected with solvent only. Treated eggs showed 80-90%,
70%, and 50% of the control hatch rate for the three dose rates,
respectively. In a similar study, Gyrd-Hansen & Dalgaard-Mikkelsen
(1974) found that injecting 1 mg/egg or less of the dimethylamine salt
of 2,4-D had no effect. Injections of 2 mg/egg reduced both
hatchability of the eggs and survival of hatched chicks. An injected
dose of 5 mg/egg reduced the hatching rate to 15% of control levels and
there were no surviving chicks after 1 week. There was no successful
hatching after an injection of 10 mg/egg. The same authors also dosed
eggs by immersion in solutions of 2,4-D for 10 seconds. There was no
effect after immersion in a solution of 10 g/litre and only a slight
effect after immersion in 50 g/litre. The hatching success and
survival of the chicks up to 4 weeks post hatch, after immersion in
50 g/litre, was more than 80% of control values.
7.2.2. Toxicity to birds after short-term and long-term dosing
The toxicity of 2,4-D (given either orally by capsule or in the
diet) to birds is summarized in Table 11. The studies reported in this
Table include single oral dosing, repeated oral dosing, and dietary
tests over 5 to 100 days. Studies lasting 10 days or less show that
high dosage (in excess of 1000 mg/kg food) is required to kill birds.
2,4-D is, therefore, of low toxicity to birds.
Haegele & Tucker (1974) dosed egg-laying Japanese quail and mallard
a single oral dose of 250 or 1500 mg 2,4-D acid/kg body weight and
monitored egg shell thickness. There was a short-term effect; thin-
shelled eggs were produced during the first 3 days after dosing. This
was considered to be an indirect effect, i.e., the result of reduced
food consumption. When Bjorklund & Erne (1966) gave single oral doses
of 100, 200, or 300 mg 2,4-D amine/kg body weight to chickens, all
clinical and gross pathological findings were negative, with the
exception of a single bird showing gastritis after the highest dose.
2,4-D amine was given orally at 300 mg/kg body weight to a second group
of chickens each day. One bird died after 5 days and was shown on
autopsy to have developed renal and visceral gout. The other birds
were killed on days 12 or 24 of dosing. Slight kidney enlargement was
seen, and there was an enhancement of the rate of 2,4-D elimination
with time.
Bjorn & Northen (1948) orally dosed white-rock chicks on alternate
days for a period of 4 weeks (12 doses in total) with an alkanolamine
salt formulation of 2,4-D. All chicks weighed approximately 50 g at
the beginning of dosing and doses were adjusted for weight gain of the
chicks through the dosing period. No effect on weight gain was noted
at doses of 2,4-D up to 280 mg acid equivalent/kg body weight. In a
further study, single oral doses of up to 380 mg/kg body weight were
without effect, but a single oral dose of 765 mg/kg body weight killed
all the birds.
Whitehead & Pettigrew (1972a) dosed 28-week-old laying hens daily,
by gelatin capsule, with the butoxyethyl ester of 2,4-D at either 6.2
or 18.7 mg acid equivalent/bird for 20 weeks. There were no adverse
effects on egg production, egg or yolk weight, egg shell thickness,
hatchability, or growth rate of the progeny.
Chickens were given oral doses of 2,4-D amine salt at 100, 250, or
500 mg/kg body weight or PGBE ester at 50, 100, or 250 mg/kg body
weight for 10 days in a study by Palmer & Radeleff (1969). Birds given
2,4-D amine salt did not differ from controls in weight gain, even at
the highest dose. There was similarly no effect from the lowest dose
of the ester. However, a growth rate reduction was seen with the
medium dose of the ester (only 19% weight gain relative to a control
weight gain of 41%), and at the highest dose, there was complete
mortality within 4 days, associated with a weight loss of 13%. In a
comparable study, Palmer (1972) dosed chickens with 2,4-D
dimethylamine salt at 25 to 500 mg/kg body weight for 10 consecutive
days. There were effects on weight gain at doses of 100 mg/kg or more.
At 100 mg/kg, the weight gain was 38%, compared to a control value of
57%. At 175, 250, and 375 mg/kg, the weight gain was 30%, similar to
the control value. At the highest dose, three out of five treated
birds died, and the survivors showed a weight gain of 26%. There was
no effect of 2,4-D ethylhexyl ester at 100 mg/kg on weight gain, but at
250 and 500 mg/kg, weight gain was 42% and 36%, respectively, compared
to a control value of 59%.
Solomon et al. (1973) studied the effects of 2,4-D acid and two
unspecified amine salt formulations of 2,4-D. Pheasants were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing one of
the preparations at either 75 or 150 mg/bird. No effects were observed
on fertility and there was no increase in the number of abnormal
embryos.
Table 11. Toxicity of 2,4-D to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mallard duck M 4 months oral acid (technical) acute LD50 > 2000 Hudson et
(Anas platyrhynchos) M 3-5 months oral sodium salt acute LD50 > 2050 al. (1984)
M 7 months oral amine salt acute LD50 < 2000 Hudson et
F 3-5 months oral acid (technical) acute LD50 > 1000 al. (1984)
23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et al.
17 days diet dimethylamine 5-day LC50 > 5000 c (1975)
young diet acetamide 100-day LC50 > 500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 2500 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 > 5000 DeWitt et
al. (1963)
Japanese quail M 2 months oral acid (technical) acute LD50 668 Hudson et
(530-842) al. (1984)
(Coturnix coturnix 14 days diet acetamide 5-day LC50 > 5000 c Hill et
(japonica) 12 days diet butoxyethanol 5-day LC50 > 5000 c al. (1975)
20 days diet dimethylamine 5-day LC50 > 5000 c Hill et
al. (1975)
Bobwhite quail 23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et
(Colinus virginianus) 23 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 2500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 10-day LC50 5000 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
Pheasant F 3-4 months oral acid (technical) acute LD50 472 Hudson et
(340-654) al. (1984)
(Phasianus colchicus) 10 days diet butoxyethanol 5-day LC50 > 5000 Hill et
10 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 1000 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 5000 DeWitt et
adult diet dimethylamine 100-day LC50 > 5000 al. (1963)
young diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
10 days diet butoxyethanol 5-day LC17 5000 Hill et al.
(1975)
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Chukar partridge M,F 4 months oral acid (technical) acute LD50 200-400 Hudson et
(Alectoris chukar) al. (1984)
Rock dove M,F oral acid (technical) acute LD50 668 Hudson et
(Columba livia) (530-842) al. (1984)
Chicken M,F 21 days oral free acid 14-day LD50 541 Rowe & Hymas
(358-817) (1954)
M,F 21 days oral isopropyl 14-day LD50 1420 Rowe & Hymas
(1127-1789) (1954)
M,F 21 days oral mixed butyl 14-day LD50 2000 Rowe & Hymas
esters (1350-2960) (1954)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b Acute oral doses are given as mg/kg body weight; all other doses are as mg/kg diet.
c Dose level of 5000 mg/kg diet produced no mortality.
Whitehead & Pettigrew (1972b) fed day-old chicken chicks with the
butoxyethanol ester of 2,4-D at concentrations up to 7500 mg/kg diet
for 3 weeks. Dietary levels up to 1000 mg/kg had no adverse effect,
but at 2000 mg/kg diet 2,4-D ester reduced the food consumption and
growth rate of the chicks. Although there was no mortality at the
higher doses, necropsy of birds sacrificed at the end of the experiment
showed swollen kidneys in all birds and some mottling of the spleen.
Bjorklund & Erne (1966) fed chickens with the amine salt of 2,4-D at
500 mg/kg diet. One bird died of renal gout after 5 months dosing;
autopsy showed hypoplasia (possibly congenital) of the right kidney and
hyperplasia of the left kidney. Other birds were killed at 1, 2, 9,
or 18 months after dosing began, but there was no consistent pattern to
autopsy findings.
Erne & Bjorklund (1970) examined the long-term effects of
phenoxyherbicides on chickens. Groups of day-old broiler chicks were
given 2,4-D in the drinking water at 1000 mg/litre for up to 7 months.
During the dosing period, chickens were sacrificed at regular intervals
for autopsy and samples were prepared for electron microscopy. There
was decreased food and water intake in dosed birds. The most
pronounced effect was on the kidney; there was noticeable kidney
enlargement after 14 days of dosing and this increased with time.
2,4-D concentrations in body tissues reached a plateau after 7 days,
with the highest residue in kidney tissue. Histologically, the kidney
enlargement was shown to be due to hypertrophy of proximal tubule
epithelium. These hypertrophied cells, under the electron microscope,
were shown to display an increased mitochondrial content and
pronounced mitochondrial pleomorphy. The number of microbodies was
also increased and nuclear bodies were observed. These findings were
stated to reflect alterations in intermediate metabolism in the
tubular cells. In an earlier study, using chicks dosed similarly with
1000 mg/litre of drinking water (Bjorklund & Erne, 1966), the birds
were followed through to sexual maturity. No significant effects were
observed on weight gain, age at sexual maturity, or onset of egg
production, but the number of eggs laid was reduced during the first 2
months of egg laying. The number of birds dying during the course of
the study did not differ from the control value. Surviving birds were
killed and autopsied at intervals of between 2 and 18 months after the
onset of egg laying. The primary effect was consistent enlargement of
the kidneys.
7.2.3. Special studies on birds
Lundholm & Mathson (1983) studied the effect of 2,4-D on the ATP-
dependent Ca2+ binding of the particulate fraction of egg shell
gland mucosa cells from egg-laying hens. This parameter had been
found to be a sensitive indicator of potential shell-thinning effects
of chemicals. They calculated a 5-min IC50, for Ca binding
inhibition, of 30.7 x 10-8 mmol 2,4-D/litre incubation medium.
This makes 2,4-D 13.5 times less effective in this respect than
1,1'-(2,2-dichlorethenylidine)-bis[4-chlorobenzene] ( p-p' -DDE), the
major agent causing eggshell-thinning in birds.
Percutaneous absorption of 2,4-D through the feet of red-winged
blackbirds was measured by Rogers et al. (1974). A 24-h exposure to
14C-labelled 2,4-D at 0.01 mmol/litre resulted in a blood
concentration of 1.24 x 10-3 mmol 2,4-D/litre.
7.3. Toxicity to Non-laboratory Mammals
Appraisal
Based on the available data, no generalization can be made about
the hazard of 2,4-D to mammals in the field. Data on voles indicate
that the herbicide poses no hazard.
Cholakis et al. (1982) obtained acute oral LD50 estimates for
two species of voles by determining mortality 14 days after the
administration of a single dose of 2,4-D acid. Values were 2110
(1800 - 2570) and 2100 (1900 - 2390) mg/kg body weight for males
and females, respectively, of the prairie vole (Microtus
orchrogaster). Values for the grey-tailed vole (Microtus canicaudus)
were 1200 (955 - 1150) for males and 1310 (1010 - 1790) mg/kg body
weight for females.
Skokova (1975) orally dosed 24 male bank voles with 400 to
405 mg/kg body weight (10% of the LD50) daily for 10 or 20 days and
examined reproductive parameters. Testis weight, an index of
spermatogenesis, and divisions in spermatogonia were all significantly
reduced relative to control values. Gile (1983) applied a foliar spray
of butyl ester of 2,4-D to a simulated ryegrass ecosystem at 1 kg/ha.
The system included voles, which showed a weight loss after exposure to
2,4-D when compared to similar animals in an untreated system. This
loss was considered to be the result of protein deficiency.
8. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
Appraisal
No direct toxic effects, acute or long-term, of 2,4-D applications
under field conditions on any animals species have been observed thus
far.
There are, inevitably, indirect effects resulting from the intended
selective herbicidal properties of the compound. These effects would
result from the use of any herbicide or from other methods of land
management. There will, therefore, be effects for mammals, birds, and
insects because of food deprivation, modification of habitat,
requirements for nesting, shelter, etc.
The application of 2,4-D appears to present no hazard to the
beneficial epigeal arthropod community. Physical cultivation present a
greater hazard to sensitive soil arthropods than the use of 2,4-D
herbicides.
Oka & Pimental (1976) observed increased numbers of insect pests
and increased occurrence of blight infection in maize (Zea mays) crops
treated with 2,4-D as the triethanolamine salt. The crops had been
treated with 2,4-D at 0.14, 0.55, or 4.4 kg/ha; 0.55 kg/ha is the
normal rate of application for this crop. The number of aphids
increased from 1420 on control untreated plants to 2449 on plants
treated at 0.14 kg/ha, 3116 on plants treated at 0.55 kg/ha, and 2023
on plants treated at 4.4 kg/ha. The percentage of plants attacked by
the European corn borer (Ostrinia nubialis) increased from 63% on
controls to 83% and 70% for treatments at 0.14 and 0.55 kg/ha,
respectively. Controlled studies also showed an increase in infection
with fungal blight. Laboratory investigation of these effects
confirmed that the treated maize had higher protein levels than the
untreated. This was thought to be the reason for the increased success
of the pests.
Everts et al. (1986) investigated the effects of various pesticides
on soil arthropods. Spiders were found to be a sensitive indicator of
effect. No side-effects of the use of 2,4-D amine were observed on
these organisms. Lahr et al. (1987) showed that spider numbers were
reduced by ploughing but not by the use of 2,4-D herbicides.
Matida et al. (1975) examined aquatic organisms in a stream running
through a mountainous area of 9.4 ha, in Shizuoka prefecture in Japan,
which had been aerially sprayed with a mixture of 2,4-D and 2,4,5-T at
a rate of 150 kg/ha. There was no effect on the number or species
diversity of aquatic invertebrates. Caged cherry salmon and dace
fingerlings showed no mortality, abnormal behaviour, or pathological
change after spraying. An extensive ecological survey of an area in
Florida treated with military mixtures of herbicides, including 2,4-D,
revealed no major change in species diversity or population size for
aquatic invertebrates, fish, a lizard, or the beach mouse (Young et
al., 1975).
The weevil Rhinocyllus conicus is used in the biological control of
musk thistle, an invasive weed. In an investigation of the
practicality of combining biological with chemical control, Lee & Evans
(1980) investigated the toxicity to the weevil of 2,4-D. They sprayed
musk thistle with 2,4-D at a rate of 4.48 kg/ha. One week later, the
terminal seed heads of the thistle were covered with cloth bags to
contain the weevils. The number of dead larvae, pupae, and adults
were counted and compared to the number on unsprayed, control thistle
heads. No significant differences were observed.
Dwernychuk & Boag (1973) studied the effect of herbicide spraying
on several species of nesting ducks (lesser scaup, gadwall, white-
winged scoter, mallard, pintail, and American wigeon) in Canada. An
ester of 2,4-D was applied to two islands. This application
significantly reduced the areas dominated by broad-leaved plants and
permitted invasion of these areas by grasses. Ducks preferred to nest
amongst broad-leaved vegetation and avoided grass. As the areas of
broad-leaved plants disappeared, there was an increase in nest density
in those broad-leaved areas still present. Total numbers of nesting
ducks declined over the 3-year study period. This decline was
attributed, by the authors, entirely to the effect of the herbicide on
vegetation type.
Keith et al. (1959) studied the effects on populations of the
pocket gopher (Thomomys talpoides) of spraying weedy rangeland in
Colorado with 2,4-D, as the butyl ester, at 3.4 kg/ha. Numbers of
gophers were estimated using two different methods, either by trapping
or by counting numbers of newly excavated mounds. Both methods showed
a highly significant difference between sprayed and non-sprayed areas
1 year after spraying. The total numbers of gophers trapped in the two
areas before 2,4-D application were 101 and 110, respectively. In the
same two areas, 1 year after spraying, numbers were 117 (untreated
area) and 15 (treated area), respectively. This represents a fall in
gopher numbers of 87% in the treated area and a slight increase in
numbers in the unsprayed area. Newly excavated mounds in the treated
area were only 28% as numerous as in control areas. Spraying had
reduced production of forbs (broad-leaved plants) from 445 kg/ha
before spraying to 75 kg/ha afterwards, a reduction of 83%. Grass
production had increased by 37%. The overall reduction in vegetation
was 232 kg/ha or 35% on sprayed plots.
In a similar study by Tietjen et al. (1967), 2,4-D butyl ester
applied at 3.4 kg/ha to identical high-altitude rangeland initially
reduced forb density and gopher populations. Pocket gopher numbers
were reduced by between 80% and 90%. Both forbs and gopher populations
remained low in one treated area but not in a second one. The decline
in gopher numbers was considered by the authors to be a result of food
deficiency; the grasses available represented a marginal diet for the
animals. This decline was not due either to movement of animals out of
the area or to the direct toxic effects of the herbicide. Reduction in
numbers was, therefore, primarily a result of reduced breeding success.
Johnson & Hansen (1969) studied the after-effects on wild mammals
of treating perennial forb and shrub/grass ranges with 2,4-D either
aerially or from a ground rig at rates of 2.2 or 3.4 kg/ha using a
diesel-oil carrier. The density and litter size of the deer mouse
(Peromyscus maniculatus) was little affected by the treatment, but the
densities of northern pocket gophers (Thomomys talpoides) and least
chipmunks (Eutamias minimus) were reduced. Montane voles (Microtus
montanus) increased their abundance in treated perennial forb range.
Gopher and vole populations returned to normal with the re-
establishment of forb dominance. Density changes were considered by
the authors to be primarily due to changes in availability of food for
the gophers, availability of both food and cover for chipmunks, and
availability of a close-canopied grass cover for the voles. There were
no direct toxic effects of the herbicide.
Fagerstone et al. (1977) observed two colonies of black-tailed
prairie dogs (Cynomys ludovicianus) in North America and the effect of
spraying their feeding areas with 2,4-D. The herbicide was applied
initially as the dimethylamine salt, followed by two further sprays of
the butyl ester after 1 month and 1 year. All applications of 2,4-D
were at 2.2 kg/ha. One colony lived in a sprayed area, initially rich
in broad-leaved plants. The second colony lived in an unsprayed area
poor in dicotyledonous plants and rich in grass. The effect of
spraying was, therefore, to make the treated area similar to the
control area with less broad-leaved herbage and less cover. Prior to
treatment, the first colony preferentially ate the forbs; diet was 73%
forbs and 5% grass. After spraying, they ate 9% forbs and 82% grass.
The control colony ate a similar diet to the first colony post-
treatment, i.e., mostly grass. Prairie dogs remained in the same area
after treatment with herbicide and there was no evidence of starvation.
Body weight was maintained, activity was comparable to the pre-
treatment level, and reproduction was unaffected.
Spencer & Barrett (1980) monitored the population response of
meadow voles (Microtus pennsylvanicus) to application of 2,4-D as the
N-oleyl 1,3 propylenediamine salt at 567.5 g/ha. Two 0.4-ha plots
were compared, one sprayed and the other untreated. The population
fluctuation of the voles was monitored for 6 months, from June to
December, a period spanning the breeding season. The control area
reached a population peak of 116 animals on 6 November; a peak of 68
voles was reached on 9 October in the treated area. There was a skewed
sex ratio in the treated area, mainly because of a reduced survival
rate in females. Voles in the treated plot were protein-deficient,
compared to controls
9. EVALUATION
In evaluating the environmental hazards of 2,4-D, the following
general points should be borne in mind:
(a) the chlorinated dibenzo- p -dioxins (CDDs) are present in
2,4-D only in trace amounts which are difficult to separate and
identify;
(b) 2,4-D is rapidly degraded in the environment;
(c) the environmental effects are indirect, and are the
consequence of vegetation diversity being modified; care should be
taken to avoid unintentional vegetation damage; the mode of
application and the formulation should be carefully selected;
esters should be avoided in aquatic applications (because of
toxicity to aquatic organisms);
(d) there are limited data on the effects of 2,4-D and its
formulations on communities of organisms; hazard assessment is,
therefore, often by extrapolation from single species studies;
(e) minor adverse effects shown in laboratory studies on
terrestrial organisms have resulted from exposures far in excess of
likely exposures in the field.
9.1. Aquatic Organisms
Sources of exposure of aquatic ecosystems to 2,4-D include direct
application, run-off, and spray drift. Because of low adsorption rate
and rapid degradation, the herbicide is not accumulated in compartments
of the aquatic system.
2,4-D acid and its salts are less toxic to aquatic organisms than
are the esters.
2,4-D acid and its salts are of low to moderate toxicity to aquatic
organisms. However, the growth and nitrogen fixation of some
cyanobacteria (blue-green algae) are inhibited, but only at
concentrations above levels expected from direct application of the
herbicide to water. These microorganisms are the source of most
nitrogen in wet tropical soils. This inhibitory effect could be of
concern when these compounds are applied to rice fields at a high
dosage.
Because of the toxicity of esters, particularly the propylene
glycol butyl ether ester, for early life-stages of several fish
species, they should be regarded as hazardous to aquatic ecosystems.
9.2. Terrestrial Organisms
2,4-D does not persist in soil and other compartments of the
terrestrial environment.
Nitrogen-fixing microorganisms appear to be particularly sensitive
to 2,4-D; this might be especially important in tropical soils.
Some terrestrial invertebrates have shown adverse effects, but only
at high exposure levels. Therefore, 2,4-D does not constitute a hazard
to this group of organisms.
2,4-D has low acute toxicity to birds, as indicated by the
LD50. Most studies on birds and their eggs have been conducted at
exposures exceeding those that could be expected in the field; even
under these conditions, no significant adverse effects have been
observed.
Under field conditions, 2,4-D does not cause direct toxic effects
on animals. However, the change of species composition and structure
of the vegetation, resulting from the use of this herbicide, leads to
indirect effects on terrestrial ecosystems. This indirect effect would
also result from the use of any herbicide or from other methods of land
management in either temperate or tropical regions.
* * *
There is evidence only for minor effects on the environment arising
from the use of 2,4-D, as long as the following simple recommendations
are followed:
(a) amine formulations, rather than esters, should be used to
control aquatic weeds;
(b) accidental spread of the herbicide to other vegetation should
be avoided;
(c) the margins of agricultural land should be left untreated with
herbicide to avoid even the indirect effects of the material on
wildlife.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
There are indications that 2,4-D affects nitrogen fixation by
algae. Since this is the major source of nitrogen in tropical soils,
it is recommended that this should be further investigated,
particularly with reference to varying soil conditions. This should be
extended to a study on the functioning of a rice-paddy at the ecosystem
level.
REFERENCES
ADAMS, B.J. (1960) Effects of spraying 2,4-D amine on Coccinellid
larvae. Can. J. Zool., 38: 285-288.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous substances.
Int. Pest Control, 11: 29-35.
AUDUS, L.J. (1960) Microbiological breakdown of herbicides in soils.
In: Woodford, E.K. & Sagar, G.R., ed. Herbicides and soil, Oxford
Blackwell Scientific Publications, pp. 1-19.
AUDUS, L.J. (1964) Herbicide behaviour in the soil. In: Audus, L.J.,
ed. The physiology and biochemistry of herbicides, London, New York,
Academic Press, pp. 163-206.
BAILEY, H.C. & LIU, D.H.W. (1980) Lumbriculus variegatus, a benthic
oligochaete, as a bioassay organism. In: Eaton, J.C., Parish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
for Testing and Materials, pp. 205-215 (Special Technical Publication
707).
BEDNARZ, T. (1981) The effect of 2,4-D acid on green and blue-
green algae in unialgal and mixed cultures. Acta hydrobiol., 23: 173-
182.
BENEDECZKY, I., BIRO, P., & SCHAFF, Z. (1984) The effect of
2,4-D-containing herbicide (Dikonirt) on the ultrastructure of carp
(Cyprinus carpio) liver cells. Acta biol. (Szeged), 30: 107-125.
BERAN, F. & NEURURER, J. (1955) [Effect of plant protection
materials on the honeybee. I. Toxicity to bees.]
Pflanzenschutzberichte, 15: 97-147 (in German).
BIRGE, W.J., BLACK, J.A., & BRUSER, D.M. (1979) Toxicity of organic
chemicals to embryo-larval stages of fish. Washington, DC, US
Environmental Protection Agency (EPA 560-1179-007).
BIRO, P. (1979) Acute effects of the sodium salt of 2,4-D on the
early developmental stages of bleak, Alburnus alburnus. J. Fish Biol.,
14: 101-109.
BJORKLUND, N-E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. Scand., 7: 364-390.
BJORN, M.K. & NORTHEN, H.T. (1948) Effects of 2,4-
dichlorophenoxyacetic acid on chicks. Science, 108: 479-480.
BUNTING, D.L. & ROBERTSON, E.B. (1975) Lethal and sublethal effects
of herbicides on zooplankton species, University of Tennessee, Water
Resources Research Center, 34 pp. (Res. Report No. 43, NTIS Report No.
PB-241 337).
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975a) Loss of
five pesticides from cultures of twenty-one planktonic algae. Bull.
environ. Contam. Toxicol., 13: 149-152.
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975b) The effect
of atrazine, 2,4-D, methoxychlor, carbaryl and diazinon on the growth
of planktonic algae. Br. phycol. J., 10: 371-376.
BUTLER, P.A. (1963) A review of Fish and Wildlife Service
investigations during 1961 and 1962, Washington, DC, US Department of
the Interior, Fish and Wildlife Service, pp. 11-25 (Circular No. 167).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna. Proc.
South. Weed Conf., 18: 576-580.
CALDWELL, R.S. (1977) Biological effects of pesticides on the
Dungeness crab, Gulf Breeze, Florida, US Environmental Protection
Agency (EPA-600/3-77-131).
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON,
M.H., & MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D,
DEF, propanil and trifluarin to the Dungeness crab, Cancer magister.
Arch. environ. Contam. Toxicol., 8: 383-386.
CHAI, I.K. & CHUNG, Y.S. (1975) [Physiological effects of 2,4-
dichlorophenoxyacetic acid (2,4-D) on Chlorella ellipsoidea.]
Misaengmul Hakhoe Chi., 13: 101-108 (in Korean).
CHAMBERS, H., DZIUK, L.J., & WATKINS, J. (1977) Hydrolytic
activation and detoxification of 2,4-dichlorophenoxyacetic acid esters
in mosquito fish. Pestic. Biochem. Physiol., 7: 297-300.
CHEAH, M.L., AVAULT, J.W., & GRAVES, J.B. (1980) Acute toxicity
of selected rice pesticides to crayfish Procambarus clarkii. Prog. Fish
Cult., 42: 169-172.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1982)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avaian and Mammalian Wildlife
Toxicology: 2nd Conference, Philadelphia, American Society for Testing
and Materials, pp. 143-154 (Special Technical Publication 757).
COAKLEY, J.E., CAMPBELL, J.E., & MCFARREN, E.F. (1964)
Determination of butoxyethanol ester of 2,4-dichlorophenoxyacetic acid
in shellfish and fish. J. agric. food Chem., 12: 262-265.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99: 1-12.
CULLIMORE, D.R. & MCCANN, A.E. (1977) Influence of four
herbicides on the algal flora of a prairie soil. Plant Soil, 46: 499-
510.
DAS, B. & SINGH, P.K. (1977) The effect of 2,4-dichlorophenoxyacetic
acid on growth and nitrogen-fixation of blue-green alga Anabaenopsis
raciborskii. Arch. environ. Contam. Toxicol., 5: 437-445.
DAVIS, J.T. & HARDCASTLE, W.S. (1959) Biological assay of
herbicides for fish toxicity. Weeds, 7: 397-404.
DAVIS, P.W., FRIEDHOFF, J.M., & WEDERMEYER, G.A. (1972)
Organochlorine insecticide, herbicide and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8: 69-72.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1974) Effect of some
herbicides on soil microflora. J. Indian Soc. Soil Sci., 22: 300-303.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1975) Effect of some
herbicides on Azotobacter and non-symbiotic nitrogen fixation in the
soil. Indian J. Agric. Chem., 8: 95-98.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife
studies, Patuxent Wildlife Research Center. Pesticide-wildlife
studies. A review of Fish and Wildlife Service investigations during
1961 and 1962. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular No. 167).
DODSON, J.J. & MAYFIELD, C.I. (1979) The dynamics and
behavioural toxicology of Aqua-Kleen (2,4-D butoxyethanol ester) as
revealed by the modification of rheotropism in rainbow trout. Trans.
Am. Fish. Soc., 108: 632-640.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some
herbicides on the hatching rate of hen's eggs. Nature (Lond.), 215:
1406-1407.
DWERNYCHUK, L.W. & BOAG, D.A. (1973) Effect of
herbicide-induced changes in vegetation on nesting ducks. Can. field
Nat., 87: 155-165.
ELDER, J.H., LEMBI, C.A., & MORRE, D.J. (1970) Toxicity of 2,4-D
and picloram to fresh and saltwater algae. Proc. North-cent. Weed
Control Conf., 25: 96-98.
ELIASSON, L. (1973) Translocation and persistence of 2,4-D in
Populus tremula L. Weed Res., 13: 140-147.
ERNE, K. & BJORKLUND, N-E. (1970) Nephrotoxic effects of
phenoxy acetic herbicides. In: Proceedings of the 7th International
Congress on Plant Protection, Paris, France, pp. 768-769.
EVERTS, J.W., BEURSKENS, J.E.M., BOUWHUIS, J.S., BUYSE, A.D.,
HENGEVELD, R., WOUTERS, L., & KOEMAN, J.H. (1986) Terrestrial
arthropods as indicators for side-effects caused by insecticides in
arable farm systems in the Netherlands. In: Proceedings of the 1st
International TNO Conference on Contaminated Soil, Utrecht, The
Netherlands, November 1985, Dordrecht, Boston, Martinus Nijhoff, pp.
423-425.
FAGERSTONE, K.A., TIETJEN, H.P., & LAVOIE, G.K. (1977) Effects
of range treatment with 2,4-D on prairie dog diet. J. Range Manage.,
30: 57-60.
FINLAYSON, B.J. & VERRUE, K.M. (1985) Toxicities of
butoxyethanol ester and propylene glycol butyl ether ester formulations
of 2,4-dichlorophenoxy acetic acid (2,4-D) to juvenile salmonids.
Arch. environ. Contam. Toxicol., 14: 153-160.
FLINT, G.W., ALEXANDER, J.J., & FUNDERBURK, O.P. (1968)
Vapor pressures of low-volatile esters of 2,4-D. Weed Sci., 16: 541-
544.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry to
nine herbicides. Bull. environ. Contam. Toxicol., 15: 509-514.
FOLMAR, L.C. (1978) Avoidance chamber responses of mayfly nymphs
exposed to eight herbicides. Bull. environ. Contam. Toxicol., 19: 312-
318.
FOURNIER, J.C. (1980) Enumeration of the soil micro-organisms able
to degrade 2,4-D by metabolism or co-metabolism. Chemosphere, 9: 169-
174.
FREITAG, D., GEYER, H., KRAUS, A., VISWANATHAN, R., KOTZIAS,
D., ATTAR, A., KLEIN, W., & KORTE, F. (1982) Ecotoxicological profile
analysis. VII, Screening chemicals for their environmental behaviour
by comparative evaluation. Ecotoxicol. environ. Saf., 6: 60-81.
GALL, A. & DOGGER, J.R. (1967) Effect of 2,4-D on the wheat stem
sawfly. J. econ. Entomol., 60: 75-77.
GANGAWANE, L.V., SALER, R.S., & KULKARNI, L. (1980) Effect
of pesticides on growth and heterocyst formation in Nostoc sp.
Marathwada Univ. J. Sci., 19B: 3-6.
GEORGE, J.P., HINGORANI, H.G., & RAO, K.S. (1982) Herbicide
toxicity to fish-food organisms. Environ. Pollut., 28: 183-188.
GILE, J.D. (1983) 2,4-D - its distribution and effects in a ryegrass
ecosystem. J. environ. Qual., 12: 406-412.
GLYNN, P.W., HOWARD, L.S., CORCORAN, E., & FREAY, A.D.
(1984) The occurrence and toxicity of herbicides in reef-building
corals. Mar. Pollut. Bull., 15: 370-374.
GRAHAM-BRYCE, I.J. (1972) Herbicide movement and availability in
soils. Proc. Br. Weed Control Conf., 11: 1193-1202.
GROLLEAU, G., DE LAVAUR, E., & SIOU, G. (1974) Effets du
2,4-D sur la reproduction des cailles et des perdrix, aprčs application
du produit par pulvérisation sur les oeufs. Ann. Zool. Ecol. anim.,
6: 313-331.
GUARINO, A.M., JAMES, M.O., & BEND, J.R. (1977) Fate and
distribution of the herbicides 2,4-dichlorophenoxyacetic acid (2,4-D)
and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in the dogfish shark.
Xenobiotica, 7: 623-631.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The
effect of phenoxy-herbicides on the hatchability of eggs and the
viability of the chicks. Acta pharmacol. toxicol., 35: 300-308.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
coturnix. Bull. environ. Contam. Toxicol., 11: 98-102.
HAGENMAIER, H. (1986) Determination of 2,3,7,8-tetrachlorodibenzo-p-
dioxin in commercial chlorophenols and related products. Fresenius Z.
anal. Chem., 325: 603-606.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973)
Avoidance of pesticides by grass shrimp (Palaemonetes pugio). Bull.
environ. Contam. Toxicol., 9: 129-133.
HAQUE, A. & EBING, W. (1983) Uptake, accumulation, and
elimination of HCB and 2,4-D by the terrestrial slug, Deroceras
reticulatum (Muller). Bull. environ. Contam. Toxicol., 31: 727-733.
HASHIMOTO & NISHIUCHI (1978) quoted by Hidaka et al. (1984).
HAWXBY, K., TUBEA, B., OWNBY, J., & BASLER, E. (1977) Effects
of various classes of herbicides on four species of algae. Pestic.
Biochem. Physiol., 7: 203-209.
HIDAKA, H., HATTANDA, M., & TATSUKAWA, R. (1984)
[Avoidance of pesticides with medakas (Oryzias latipes).] Nippon
Nogeikagaku Kaishi, 58: 145-151 (in Japanese).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (First report).] Anz. schädlingskd. Pflanzenschutz Umweltschutz,
49: 21-25 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976b) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (Second report).] Anz. schädlingskd. Pflanzenschutz
Umweltschutz, 49: 65-68 (in German).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish Wildlife Service,
61 pp. (Special Science Report, Wildlife No. 191).
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized fish
eggs and fry. Trans. Am. Fish. Soc., 96: 414-416.
HILTIBRAN, R.C. (1975) Oxygen and phosphate metabolism of bluegill
liver mitochondria in the presence of some pesticides. Environ. Qual.
Saf. (Suppl), 3: 549-553.
HOEPPEL, R.E. & WESTERDAHL, H.E. (1983) Dissipation of 2,4-D
DMA and BEE from water, mud and fish at Lake Seminole, Georgia.
Water Resour. Bull., 19: 197-204.
HOFFMAN, D.J. & ALBERS, P.H. (1984) Evaluation of potential
embryotoxicity and teratogenicity of 42 herbicides, insecticides and
petroleum contaminants to mallard eggs. Arch. environ. Contam.
Toxicol., 13: 15-27.
HUBER, S.J., POSCHENREIDER, G., & WALLNOFER, P.R. (1980)
[Effect of pesticides and the corresponding metabolites on growth and
respiration of some soil microorganisms.] Z. Pflanzenkr.
Pflanzenschutz, 87: 533-545 (in German).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook
of toxicity of pesticides to wildlife, 2nd edition, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 90 (Resource
Publication 153).
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to bluegill
sunfish of phenoxy herbicides. Weeds, 11: 50-53.
JAMES, M.O. (1979) Disposition of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Panulirus argus).
Pharmacologist, 21: 251.
JANA, B.B. & DE, U.K. (1982) Stimulatory effect of herbicide 2,4-D
on the heterotrophic microbial community in the water of three fish
ponds. Acta microbiol. Acad. Sci. Hung., 29: 77-82.
JENSEN, D.A. & SCHALL, E.D. (1966) Determination of vapor
pressures of some phenoxyacetic herbicides by gas-liquid
chromatography. J. agric. food Chem., 14: 123-125.
JOHNSON, C.R. (1976) Herbicide toxicities in some Australian anurans
and the effect of subacute dosages on temperature tolerance. Zool. J.
Linnean Soc., 59: 79-83.
JOHNSON, C.R. (1978) Herbicide toxicities in the mosquito fish
Gambusia affinis. Proc. R. Soc. Queensland., 89: 25-27.
JOHNSON, D.R. & HANSEN, R.M. (1969) Effects of range treatment
with 2,4-D on rodent populations. J. wildl. Manage., 33: 125-132.
JONES, G.D.E. & CONNELL, J.U. (1954) Studies of the toxicity to
worker honey-bees (Apis mellifera L.) of certain chemicals used in
plant protection. Ann. appl. Biol., 41: 271-279.
KAPOOR, K. & SHARMA V.K. (1980) Effect of certain herbicides on
survival, growth and nitrogen fixation of blue-green alga Anabaena
doliolum Bharadwaja. Z. allg. Mikrobiol., 20: 465-469.
KEITH, J.O., HANSEN, R.M., & WARD, A.L. (1959) Effect of 2,4-D
on abundance and foods of pocket gophers. J. wildl. Manage., 23: 137-
145.
KOPISCHKE, E.D. (1972) Effect of 2,4-D and diesel fuel on egg
hatchability. J. wildl. Manage., 36: 1353-1356.
KOSCHIER, F.J. & PRITCHARD, J.B. (1980) Renal handling of 2,4-D
by dogfish shark (Squalus acanthias). Xenobiotica, 10: 1-6.
LAHR, J., GOEDEJOHAN, N., & EVERTS, J.W. (1987) Side effects of
pesticides on terrestrial arthropods. I. Long-term effects,
geographical variability and effects of cultural practices. In:
Proceedings of the Symposium ``25 Years After Silent Spring'',
November 1987, Amsterdam, Toxicological Society.
LE VAN TO (1984) [Degradation of defoliants 2,4-D and 2,4,5-T by
selected soil microorganisms.] Acta agrar. Silvestria, Ser. agrar.,
23: 225-234 (in Polish).
LEE, R.D. & EVANS, J.O. (1980) The influence of selected herbicides
on the development of Rhinocyllus conicus, an insect used in biocontrol
of musk thistle. Proc. West. Soc. Weed Sci., 33: 104-110.
LEMBI, C.A. & COLERIDGE, S.E. (1975) Selective toxicity of detergents
and herbicides to phytoplankton, West Lafayette, Indiana, Purdue
University, Water Resources Research Center, 71 pp. (Technical Report
No. 71).
LEWIS, D.L., HODSON, R.E., & FREEMAN, L.F. (1984) Effects of
microbial community interactions on transformation rates of xenobiotic
chemicals. Appl. environ. Microbiol., 48: 561-565.
LHOSTE, J. & ROTH, P. (1946) Sur l'action des solutions aqueuses de
2,4-dichlorophénoxyacétate de sodium sur l'évolution des oeufs de Rana
temporaria L. Soc. Biol., 140: 272-273.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides
on decomposition of organic matter and nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LINDEN, E., BENGTSSON, B-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LI-TSE OU (1984) 2,4-D degradation and 2,4-D degrading
microorganisms in soils. Soil Sci., 137: 100-107.
LIU, H.W. & LEE, M. (1975) Toxicity of selected pesticides to the bay
mussel Mytilus edulis, Washington, DC, US Department of Commerce,
National Technical Information Service (NTIS Report No. PB-243 221).
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel Dekker,
Inc., pp. 1-49.
LUNDHOLM, C.E. & MATHSON, K. (1983) The inhibitory effect of
some chlorinated hydrocarbon pesticides on the ATP-dependent Ca2+
binding of the particulate fraction of the eggshell gland mucosa
cells. Acta pharmacol. toxicol., 52: 390-394.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1972) The action of different
pesticides on the development of bird embryos. Adv. exp. Med. Biol.,
27: 127-150.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1973) Pesticides, tératogénčse et
survie chez les oiseaux. Arch. Anat. Histol. Embryol., 56: 65-78.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de
l'herbicide 2,4-D sur le développement embryonnaire et la fécondité du
gibier ŕ plumes. C. R. Acad. Sci. Paris, Ser. D, 271: 2418-2421.
MATIDA, Y., FURUTA, Y., KUMADA, H., TANAKA, H., YOKOTE,
M., & KIMURA, S. (1975) Effects of some herbicides applied in the
forest to the freshwater fishes and other aquatic organisms - I. Survey
on the effects of aerially applied sodium chlorate and a mixture of
2,4-D and 2,4,5-T on the stream community. Bull. freshwater Fish Res.
Lab. (Tokyo), 25: 41-53.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp. (NTIS Report No. PB-87 188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp. (Resource Publication 160).
MEEHAN, W.R., NORRIS, L.A., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in southeast Alaska. J. Fish
Res. Board Can., 31: 480-485.
METCALF, R.L. & SANBORN, J.R. (1975) Pesticides and
environmental quality in Illinois. Illinois nat. Hist. Surv. Bull.,
31: 381-435.
MORTON, H.L., MOFFETT, J.O., & MACDONALD, R.H. (1972)
Toxicity of herbicides to newly emerged honey bees. Environ.
Entomol., 1: 102-104.
MOUBASHER, A.H., EL-HISSY, F.T., & ABDEL-KADER, M.I.A.
(1981) Selective effects of three herbicides on the fungus flora of
Egyptian soil. Folia microbiol., 26: 37-44.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxyethanol
ester of 2,4-D. Trans. Am. Fish. Soc., 96: 185-193.
MUKHOPADHYAY, S.K. (1980) Effects of herbicides and insecticides
alone and their combinations on soil microflora. Indian J. Weed Sci.,
12: 53-60.
MULLER, G. (1971) [Laboratory studies on effects of herbicides on
Carabidae.] Arch. Pflanzenschutz, 7: 351-364 (in German).
NAGUIB, K., YOUNIS, A.E., & EL-ESSAWY, M.A. (1980) The effects
of 2,4-dichlorophenoxyacetic acid on respiration, nitrogen and
carbohydrate metabolism of Aspergillus terreus Thom. Zbl. Bakteriol.
II. Abt., 135: 252-259.
NESBITT, H.J. & WATSON, J.R. (1980) Degradation of the herbicide
2,4-D in river water. II. The role of suspended sediment, nutrients and
water temperature. Water Res., 14: 1689-1694.
NORRIS, L.A. & GREINER, D. (1967) The degradation of 2,4-D in
forest litter. Bull. environ. Contam. Toxicol., 2: 65-74.
O'CONNOR, G.A., FAIRBANKS, B.C., & DOYLE, E.A. (1981) Effects
of sewage sludge on phenoxy herbicide adsorption and degradation in
soils. J. environ. Qual., 10: 510-515.
OKA, I.N. & PIMENTAL, D. (1976) Herbicide (2,4-D) increases insect
and pathogen pests on corn. Science, 193: 239-240.
OMKAR & SHUKLA, G.S. (1984) Toxicity of the herbicide 2,4-D-Na
to two species of freshwater prawn of the genus Macrobranchium. Acta
hydrochim. hydrobiol., 12: 285-289.
PACHPANDE, R.R. & DAVID, S.B. (1980) Effect of selected herbicides
on the growth of soil alga Chlorococcum infusionum meneghini
(Schrank). Phykos, 19: 138-141.
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens, Washington, DC, US Department of Agriculture,
Agricultural Research Service, 41 pp. (Production Research Report No.
137).
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some organic
herbicides to cattle, sheep, and chickens. Washington, DC, US
Department of Agriculture, Agricultural Research Service, 26 pp.
(Production Research Report No. 106).
PLUMB, T.R., NORRIS, L.A., & MONTGOMERY, M.L. (1977)
Persistence of 2,4-D and 2,4,5-T in chaparral soil and
vegetation. Bull. environ. Contam. Toxicol., 17: 1-8.
PONS, R. & PUSSARD, M. (1980) Action des herbicides sur les amibes
libres (Rhizopoda, Protozoa). Etude préliminaire. Acta oecol. oegol.
appl., 1: 15-20.
POORMAN, A.E. (1973) Effects of pesticides on Euglena gracilis. I.
Growth studies. Bull. environ. Contam. Toxicol., 10: 25-28.
PRESCOT, L.M. & OLSON, D.L. (1972) Effects of pesticides on the
soil amoeba, Acanthamoeba castellanii. Proc. South Dakota Acad. Sci.,
51: 136-141.
PRESING, M. (1981) On the effects of Dikonirt (sodium salt of 2,4-
dichlorophenoxyacetic acid) on the mortality and reproduction of
Daphnia magna. Hydrobiologia, 83: 511-516.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder. J.
Pharmacol. exp. Ther., 208: 280-286.
RAATIKAINEN, M., SILTANEN, H., ROSENBERG, C., RAATIKAINEN,
T., & MUKULA, J. (1979) Herbicide residues in cowberries, bilberries
and lichens in controlled ground spraying experiments on woodland.
Ann. agric. Fenniae, 18: 112-116.
RADOSEVICH, S.R. & WINTERLIN, W.L. (1977) Persistence of 2,4-D
and 2,4,5-T in chaparral vegetation and soil. Weed Sci., 25: 423-425.
RAND, G.M. & BARTHALMUS, G.T. (1980) Use of an unsignalled
avoidance technique to evaluate the effects of the herbicide 2,4-
dichlorophenoxyacetic acid on goldfish, Philadelphia, American Society
for Testing and Materials, pp. 341-353 (Special Technical Publication
707).
RAPOPORT, E.H. & CANGIOLI, G. (1963) Herbicides and the soil
fauna. Pedobiologia, 2: 235-238.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977)
Investigations into the acute toxicity and some chronic effects of
selected herbicides and pesticides on several fresh water fish
species. Bull. environ. Contam. Toxicol., 18: 361-365.
RIVIERE, J-L. (1976) Les effets secondaires des pesticides agricoles.
II. Action de quelques herbicides sur la croissance et la fécondité de
Blattella germanica (L.). Ann. Zool. Ecol. anim., 8: 543-550.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3: 67-78.
ROBERTSON, E.B. (1975) The acute toxicity of four herbicides to 0 -
4 hour nauplii of the copepod Cyclops vernalis Fisher, Washington, DC,
US Department of Commerce, National Technical Information Service, 70
pp. (M.S. Thesis, University of Tennessee) (NTIS Report No. PB-269
495).
RODGERS, C.A. & STALLING, D.L. (1972) Dynamics of an ester of
2,4-D in organs of three fish species. Weed Sci., 20: 101-105.
ROGERS, J.G., CAGAN, R.H., & KARE, M.R. (1974) Percutaneous
absorption of several chemicals, some pesticides included, in the red-
winged blackbird. Environ. Physiol. Biochem., 4: 104-111.
ROSENBERG, A. & ALEXANDER, M. (1980) 2,4,5-trichlorophenoxy-
acetic acid (2,4,5-T) decomposition in tropical soil and its co-
metabolism by bacteria in vitro. J. agric. food Chem., 28: 705-709.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation of
the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUGGIERO, P. & RADOGNA, V.M. (1985) Inhibition of soil humus-
laccase complexes by some phenoxyacetic and s- triazine
herbicides. Soil Biol. Biochem., 17: 309-312.
SANDERS, H.O. (1969) Toxicity of pesticides to the crustacean,
Gammers lacustris. Washington, DC, US Department of the Interior, Fish
and Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 18 pp.
(Technical Paper No. 25).
SANDERS, H.O. (1970a) Toxicities of some herbicides to six species of
freshwater crustaceans. J. Water Pollut. Control Fed., 42: 1544-1550.
SANDERS, H.O. (1970b) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowler's toad Bufo woodhousii
fowleri. Copeia, 1970(2): 246-251.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several pesticides to
two species of cladocerans. Trans. Am. Fish. Soc., 95: 165-169.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol. Oceanogr.,
13: 112-117.
SANDMANN, E.R.I.C. & LOOS, M.A. (1984) Enumeration of
2,4-D-degrading microorganisms in soils and crop plant rhizospheres
using indicator media; high populations associated with sugarcane
(Saccharum officinarum). Chemosphere, 13: 1073-1084.
SARMA, Y.S.R.K. & TRIPATHI, S.N. (1980) Effects of some chemicals
(2,4-dichlorophenoxyacetic acid, coumarin and acenaphthene) on the
green alga, Oedogonium acmandrium Elfving. Phykos, 19: 142-152.
SATTAR, M.A. & PAASIVIRTA, J. (1980) Fate of chlorophenoxyacetic
acids in acid soil. Chemosphere, 9 745-752.
SCHRODER, H. & PILZ, E. (1983) [Effect of herbicides on nitrification
in different soils.] Zbl. Mikrobiol., :138: 223-234 (in German).
SCHRODER, I., MEYER, M., & MUCKE, D. (1970) [Effects of the
herbicides 2,4-D, aminotriazole, atrazine, chlorpropham and
chlorfurenol on nucleic-acid biosynthesis in the ascomycete Neurospora
crassa.] Weed Res., 10: 172-177 (in German).
SCHULTZ, D.P. (1973) Dynamics of a salt of (2,4-dichlorophenoxy)-
acetic acid in fish, water, and hydrosol. J. agric. food Chem., 21:
186-192.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8: 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues
at Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7: 146-
152.
SEIBERT, K., FUHR, F., & MITTELSTAEDT, W. (1982) Experiments
on the influence of roots and soils on 2,4-D degradation. Landwirtsch.
Forsch., 35: 5-13.
SHCHERBAKOV, Y.A. & POLUBOYARINOVA, I.V. (1973) The
persistence of 2,4-D butyl ester in water and its accumulation in
aquatic life. In: Andrushaitis, G.P., ed. Experimental water
toxicology, Charlotte, North Carolina, Army Foreign Service and
Technology Center, pp. 19-21 (NTIS Report No. AD-760 400).
SHUKLA, G.S. & OMKAR (1983) Toxicity of 2,4-D-Na salt to fresh
water prawn, Macrobrachium lamarrei (M. Edwards). Comp. Physiol.
Ecol., 8: 282-284.
SIGMON, C. (1979) Oxygen consumption in Lepomis macrochirus
exposed to 2,4-D or 2,4,5-T. Bull. environ. Contam. Toxicol., 21: 826-
830.
SIKKA, M.C., APPLETON, H.T., & GANGSTAD, E.O. (1977) Uptake
and metabolism of dimethylamine salt of 2,4-D acid by fish. J. agric.
food Chem., 25: 1030-1033.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., &
RAATIKAINEN, T. (1981) Triclopyr, glycophosphate, and
phenoxyherbicide residues in cowberries, bilberries and lichen. Bull.
environ. Contam. Toxicol., 27: 731-737.
SINGH, P.K. (1974) Algicidal effect of 2,4-dichlorophenoxy acetic
acid on blue-green alga Cylindrospermum sp. Arch. Microbiol., 97: 69-
72.
SKOKOVA, N.N. (1975) [Effect of the butyl ester of 2,4-D on the
reproductive function of male bank voles.] Nauchn. Osn. Okhr. Prir.,
3: 172-178 (in Russian).
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of large-
scale applications of 2,4-D on aquatic fauna and water quality.
Pestic. monit. J., 1: 16-21.
SOLOMON, K.E., DAHLGREN, R.B., & LINDER, R.L. (1973)
Abnormal embryos in eggs of pheasants given 2,4-D or PCB. Proc. South
Dakota Acad. Sci., 52: 95-99.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11: 511-516.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978a) Hatching
success and early performance of chicks from eggs sprayed with 2,4-D,
2,4,5-T and picloram at various stages of embryonic development.
Bull. environ. Contam. Toxicol., 20: 289-293.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978b)
Reproductive success of hens and cockerels originating from eggs
sprayed with 2,4-D, 2,4,5-T and picloram followed by early performance
of their progeny after a comparable in ovo exposure. Bull. environ.
Contam. Toxicol., 20: 111-119.
SPAIN, J.C. & VAN VELD, P.A. (1983) Adaptation of natural microbial
communities to degradation of xenobiotic compounds: Effects of
concentration, exposure time, inoculum and chemical structure. Appl.
environ. Microbiol., 45: 428-435.
SPENCER, S.R. & BARRETT, G.W. (1980) Meadow vole population
responses to vegetational changes resulting from 2,4-D application.
Am. Midl. Nat., 103: 32-46.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on the
embryo development of Phasianus colchicus var. and Coturnix coturnix
japonica.] Z. Jagdwiss., 22: 197-201 (in German).
STALLING, D.L. & HUCKINS, J.N. (1978) Metabolism of 2,4-dichloro-
phenoxyacetic acid (2,4-D) in bluegills and water. J. agric. food
Chem., 26: 447-452.
STATHAM, C.N. & LECH, J.J. (1975) Potentiation of the acute toxicity
of several pesticides and herbicides in trout by carbaryl. Toxicol.
appl. Pharmacol., 34: 83-87.
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., &
ASKAR, A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46: 387-391.
THOMPSON, D.G., STEPHENSON, G.R., SOLOMON, K.R., &
SKEPASTS, A.V. (1984) Persistence of (2,4-dichlorophenoxy) acetic
acid and 2-(2,4-dichlorophenoxy) propionic acid in agricultural and
forest soils of northern and southern Ontario. J. agric. food
Chem., 32: 578-581.
TIETJEN, H.P., HALVORSON, C.H., HEGDAL, P.L., & JOHNSON, A.M.
(1967) 2,4-D herbicide, vegetation, and pocket gopher relationships
Black Mesa, Colorado. Ecology, 48: 634-643.
TIWARI, D.N., PANDEY, A.K., & MISRA, A.K. (1984) Toxicity of
2,4-dichlorophenoxy acetic acid on growth and nitrogen fixation of
blue-green alga Anabaena cylindrica. Pesticides (Bombay), 18: 16-18.
TOOBY, T.E., LUCEY, J., & STOTT, B. (1980) The tolerance of grass
carp Ctenopharyngodon idella Val., to aquatic herbicides. J. Fish
Biol., 16: 591-597.
TORSTENSSON, N.T.L. (1975) Degradation of 2,4-D and MCPA in
soils of low pH. Environ. Qual. Saf. (Suppl.), 3: 262-265.
TOURE, I.M. & STENZ, E. (1977) [The action of selected herbicides on
bacteriophages and Escherichia coli.] Zbl. Bakteriol. Parasitol.
Infektionskr. 132: 163-177 (in German).
TREVORS, J.T. & STARODUB, M.E. (1983) Effect of 2,4-D on
electron transport system (ETS) activity and respiration in soil.
Bull. environ. Contam. Toxicol., 31: 595-598.
TRUMBLE, J.T. & KOK, L.T. (1980) Impact of 2,4-D on
Ceuthorhychidius horridus (Coleoptera: Curculionidae) and their
compatibility for integrated control of Carduus thistles.
Weed Res., 20: 73-75.
TUEY, D.B. & JAMES, M.O. (1980) Compartmental analysis of 2,4-
dichlorophenoxyacetic acid pharmacokinetics in spiny lobster. Fed.
Proc., 39: 525.
VARDIA, H.K. & DURVE, V.S. (1981) The toxicity of 2,4-D to
Cyprinus carpio var. communis in relation to the seasonal variation in
the temperature. Hydrobiologia, 77: 155-159.
VARDIA, H.K., RAO, P.S., & DURVE, V.S. (1984) Sensitivity of toad
larvae to 2,4-D and endosulfan pesticides. Arch. Hydrobiol., 100: 395-
400.
VERMA, D., CHOUHAN, M.S., & PANDEY, A.K. (1984) Effect of
Weedone (a selective 2,4-D herbicide) on neuroendocrine complex
of Puntius ticto (Ham.). J. environ. Biol., 5: 249-254.
WATSON, J.R. (1977) Seasonal variation in the biodegradation of 2,4-D
in river water. Water Res., 11: 153-157.
WELP, G. & BRUMMER, G. (1985) [The Fe(III)-reduction test - a
simple procedure to determine the effects of environmental chemicals on
the microbial activity in soils.] Z. Pflanzenernaehr. Bodenkd.,
148: 10-23 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972a) The subacute toxicity
of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid
to chicks. Toxicol. appl. Pharmacol., 21: 348-354.
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972b) The effect of 2,4-
dichlorophenoxyacetic acid on laying hens. Br. poult. Sci., 13: 191-
195.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., &
GANGSTAD, E.O. (1973) The effects of a 2,4-D application on the
biota and water quality in Currituck Sound, North Carolina. Hyacinth
Control J., 11: 13-17.
WHO (1984) Environmental Health Criteria 29: 2,4-dichlorophenoxy-
acetic acid (2,4-D), Geneva, World Health Organization, 151 pp.
WOODWARD, D.F. (1982) Acute toxicity of mixtures of range
management herbicides to cutthroat trout. J. Range Manage., 35: 539-
540.
YOUNG, A.L., THALKEN, C.E., & WARD, W.E. (1975) Studies of the
ecological impact of repetitive aerial applications of herbicides on
the ecosystem of Test Area C-52A, Elgin AFB, Florida. Florida Air Force
Armament Laboratory, Elgin Air Force Base, 126 pp. (Report No. AFATL-
TR-75-142).
2.2. Important Chemical Reactions of 2,4-D
Pyrolysis converts various amine salts of 2,4-D to the
corresponding amides. Pyrolysis of 2,4-D and its derivatives is likely
to produce certain CDD isomers. 2,4-D is readily photodegraded.
2.3. Volatility of 2,4-D Derivatives
2,4-D esters with short-chain alcohols are highly volatile. This
influences the effectiveness of their application to target crops,
their effects on neighbouring crops, and the degree of contamination of
the atmosphere. 2,4-D alkali salts or amine salts are much less
volatile than esters, and these products are to be preferred when the
use of 2,4-D esters might lead to evaporative 2,4-D losses and to crop
damage or damage to the surrounding environment.
Details of technical compositions, impurities, and analytical
methods can be found in Environmental Health Criteria 29: 2,4-
Dichlorophenoxyacetic acid (WHO, 1984).
Table 2. Vapour pressure and solubility of 2,4-D salts and esters
--------------------------------------------------------------------------------
Compound Vapour pressurea Solubility
--------------------------------------------------------------------------------
2,4-D free acid 0.4 mmHg (160 °C) 0.09% in water (25 °C),
85% in acetone (25 °C)
dimethylamine salt 300% in water (20 °C),
soluble in acetone
isopropyl ester 1.4 x 10-3 mmHgb insoluble in water, soluble
4.6 x 10-5 mmHgb in most organic solvents
butoxyethanol ester 4.5 x 10-6 mmHgb insoluble in water, soluble
(butylethyl ester) in most organic solvents
ethylhexyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
isooctyl ester 2.0 x 10-6 mmHgb insoluble in water, soluble
in organic solvents
propyleneglycol butyl 3.0 x 10-6 mmHgb insoluble in water, soluble
ether ester in organic solvents
methyl ester 2.3 x 10-3 mmHgb
ethyl ester 1.1 x 10-3 mmHgb
butyl ester 3.97 x 10-4 mmHgb
--------------------------------------------------------------------------------
a 1 mmHg = 0.133 kPa.
b Vapour pressures of esters were determined at high temperatures by gas-
liquid chromatography, and these values are the result of extrapolation
to 25 °C. Values vary considerably between authors as a result of this
extrapolation; original values at high temperatures agree. Results are
presented here as an indication of relative vapour pressure at working
temperature. Values from Flint et al. (1968) and Jensen & Schall
(1966).
3. SOURCES OF ENVIRONMENTAL POLLUTION
The following is a summary of the chapter from Environmental Health
Criteria 29: 2,4-Dichlorophenoxyacetic acid (WHO, 1984).
3.1. Production of 2,4-D Herbicides
Comprehensive statistics on 2,4-D herbicide production or use were
not available for review. According to the US Department of
Agriculture, 3 x 108 kg of total herbicides were used in the USA
alone, in 1981. In the past, 10% of the herbicide used was 2,4-D,
which would account for a total use in the USA of about 3 x 107 kg.
In 1975, an estimated 5 x 106 kg were produced in the United Kingdom.
World-wide use of herbicides and annual production, which probably
exceeds 5 x 107 kg/year, are increasing.
3.2. Uses
2,4-D alkali or amine salts or esters are used as agricultural
herbicides against broad-leaved weeds in cereal crops, as well as on
pastures and lawns, in parks, and on golf courses, at rates of about
0.2 to 2.0 kg active ingredient (acid equivalent) per hectare. Esters
are also used at rates of up to 6.0 kg (acid equivalent) per hectare to
suppress weeds, brush, and deciduous trees along rights-of-way and in
conifer plantations and conifer reafforestation areas.
Granular formulations of 2,4-D are used as aquatic herbicides in or
along irrigation and other canals, in ponds, and lakes at rates ranging
from 1 to 122 kg/ha.
2,4-D products can be used at very low application rates as
growth regulators by application of aqueous foliar sprays containing 20
to 40 mg 2,4-D/litre on apple trees to reduce premature fruit-drop, on
potato plants to increase the proportion of medium-size tubers or to
intensify the tuber skin colour of the red varieties, and in citrus
culture to reduce pre-harvest fruit-drop and to increase fruit storage
life.
The highly volatile ethyl, isopropyl, and butyl esters are being
replaced by low-volatile esters or by amine salts to reduce crop damage
resulting from 2,4-D vapour drift, and to decrease atmospheric
pollution.
During recent years, the use of 2,4-D and 2,4,5-T in parks,
forested recreation, and other areas frequently used by the public, has
been reduced in some countries because of increasing concern about
possible toxic effects, especially in relation to CDDs.
3.3. Disposal of Wastes
Environmental pollution with 2,4-D may occur as a result of the
production and disposal of 2,4-D, or of its by-products, and of
industrial effluents. Such pollution will be generally localized to
the production site and to areas of waste dumping, and it is likely to
be more dispersed if disposal or leaching has occurred into water
courses. Disposal of unused 2,4-D in agriculture and washing of
equipment may result in localized land pollution and also pollution of
water supplies through direct contamination or leaching from soil.
4. UPTAKE, ACCUMULATION, ELIMINATION, AND BIODEGRADATION
Appraisal
2,4-D does not persist in soil because of its rapid degradation.
The physico-chemical properties of 2,4-D acid and its formulations
have an important effect on its behaviour in environmental
compartments.
The bioavailability to, and uptake by, aquatic and terrestrial
organisms is strongly influenced by the organic matter content of
soils, microbiological activity, and by environmental conditions such
as temperature and pH. Although highly inconsistent, the data on
dissipation and bioavailability in various soils demonstrate a marked
influence of differences in the texture and mineral composition of the
soil (Graham-Bryce, 1972). In aerobic soils, with a high content of
organic material, and at high pH values and temperatures, toxic effects
are limited because of rapid degradation of 2,4-D.
Uptake is followed by rapid excretion in most organisms. With the
exception of some algae, the retention of 2,4-D by organisms in the
environment cannot be expected, because of its rapid degradation.
Some microorganisms are capable of utilizing 2,4-D as their sole
carbon source. Repeated application to soil stimulates the number of
organisms capable of degrading the compound.
4.1. Biodegradation
2,4-D is readily and rapidly degraded in soil. Warm, moist
conditions and addition of organic matter stimulate degradation.
Autoclaving the soil and inhibiting bacterial metabolism reduce
degradation. The kinetics of 2,4-D disappearance suggest that
microorganisms are responsible. Particular species of microorganisms,
of various types, have been isolated and shown to degrade phenoxyacetic
acid herbicides in pure culture. Degradation of the phenoxyacetic
acids proceeds by two main pathways. These are via a hydroxyphenoxy
acetic acid intermediate or via the corresponding phenol. The
literature has been reviewed by the two workers principally responsible
for this evidence (Audus, 1960, 1964; Loos, 1969). Some microorganisms
are capable of using 2,4-D as their sole carbon source. More often,
2,4-D is co-metabolized with another carbon source. Regular treatment
of soil with 2,4-D stimulates the numbers of organisms which are
capable of degrading the compound. Treatment with other phenoxy
herbicides can also lead to an increase in organisms capable of
degrading 2,4-D.
Butler et al. (1975a) exposed 21 species of freshwater algae
isolated from natural lake water to 2,4-D butoxyethanol ester, at a
concentration of 0.01 mg/litre, and looked for degrading ability. Most
of the cultures fully degraded 2,4-D within 2 weeks. A single culture
retained 64% of the added 2,4-D, while seven isolates reduced 2,4-D to
less than 20% of the amount added. The remaining isolates showed 2,4-D
recoveries ranging from 22% to 53%.
Le Van To (1984) isolated six species of microorganisms
from soil previously treated with herbicides. These were
Flavobacterium peregrinum, Pseudomonas fluorescens, Arthrobacter
globiformis, Brevibacterium sp., Streptomyces viridochromogenes, and
an unidentified Streptomyces species. Flavobacterium was the most
active organism in degrading 2,4-D; degradation of 20 mg/kg of
2,4-D was complete after 20 to 30 days. In a liquid medium,
Flavobacterium degraded 93.5% of added 2,4-D within 80 h. The time
required to degrade half of the 2,4-D added to a sterilized soil along
with nutrient was estimated at 3 days. Li-Tse Ou (1984) investigated
the breakdown of 2,4-D in two types of soil under dry and moist
conditions and at two different temperatures. Numbers of
microorganisms degrading 2,4-D were also estimated. Generally, 2,4-D
disappeared more rapidly from moist soil; after 14 days of a slow rate
of disappearance, however, the removal rate from dry, sandy soil
increased. Numbers of organisms degrading 2,4-D were initially much
lower in sandy than in clay loams. However, numbers increased rapidly
in sandy soils after the addition of the herbicide and, as a result,
2,4-D was eventually degraded more rapidly in sandy than in clay loams.
In moist conditions, at 25 °C, the half-life of 2,4-D was 7 days or
less, whereas in dry conditions, at 35 °C, it could be as long as 250
days. These latter conditions are unlikely to apply in most natural
conditions where 2,4-D is likely to be used.
Rosenberg & Alexander (1980) incubated sewage-sludge bacteria with
2,4-D and found that nearly all of the herbicide had disappeared after
7 days. Subsequent additions of 2,4-D led to destruction of the
compound without a lag period; this suggests selection for organisms
capable of degrading the compound. Similar results were obtained using
bacteria from soil. The time needed for the disappearance of 90% of
the added 2,4-D was 14 days with soil inocula. 2,4-D added
subsequently was reduced by 70% within 3 to 4 days. Various tropical
soils were used in the experiment and all showed a high capacity for
degrading 2,4-D. Thompson et al. (1984) determined the persistence of
2,4-D applied at recommended rates in agricultural soils in Canada. In
all but one soil, a sandy loam, the concentration had declined by 50%
within 7 days. Sattar & Paasivirta (1980) showed slower degradation
of 2,4-D in acid soils. It took 6 weeks for 50% of the 2,4-D to
disappear from the soil and 7% was still left after 24 weeks. In
water-logged soil, there was reduced degradation of the herbicide.
Lewis et al. (1984) studied bacterial breakdown of 2,4-D
butoxyethyl ester and the effects of adding various extra components to
the medium. The addition of unfiltered, spent fungal medium from which
the majority of the fungus had settled out could be either stimulatory
or inhibitory to degradation rates of the herbicide; this depended on
the particular fungus species cultured in the medium. Further
investigation showed that effects were primarily due to differences in
pH. Reduction of the pH below 6 inhibited bacterial transformation of
the compound. Fungi commonly release large amount of organic acids.
The addition of spent fungal medium inhibited the breakdown of 2,4-D
ester. Buffering the added fungal medium reduced this inhibitory
effect; indeed, some stimulation of breakdown occurred after the
addition of buffered, spent medium. The addition of nutrients, or
other bacteria which did not transform 2,4-D, stimulated the
transformation of the herbicide. The authors consider that the most
likely explanation for this phenomenon is induction of other
transforming enzymes. With increasing substrate concentration, further
enzyme systems are induced in bacteria. The presence of other
organisms may stimulate the induction of these other enzymes at lower
substrate concentrations than would normally induce them. Increased
biomass of transforming bacteria in the presence of competing organisms
contributes to increased transformation rates. The nature of the
microbial community can, therefore, greatly change the ability of
degrading bacteria to transform 2,4-D and other xenobiotics.
O'Connor et al. (1981) found that 2,4-D applied at about 1.5 mg/kg
was readily degraded in soil. Adding extra carbon in the form of
dried, digested sewage sludge had a short-term effect in enhancing
degradation of the compound. Torstensson (1975) measured the half-life
of 2,4-D degradation in cultures of soil microorganisms at different
pH. In the pH range of 8.5 to 5.0, the half-life changed little,
ranging from 5 to 8 days. At pH 4.5, the half-life increased to 21
days and, at pH 4.0, increased further to 41 days.
Lieberman & Alexander (1981) added 2,4-D to inocula of municipal
sewage and monitored the biological oxygen depletion (BOD) as a measure
of degradation. The herbicide was added to carbon-depleted inocula
such that the 2,4-D represented the sole carbon source. Less than 5%
of the available oxygen was depleted, indicating poor biodegradation of
2,4-D because of low numbers of organisms capable of degrading the
herbicide as their sole carbon source. A separate study showed that
2,4-D was not toxic to microorganisms in sewage.
Fournier (1980) showed that, while 2,4-D treatment increased the
numbers of soil microorganisms capable of metabolizing 2,4-D as the
sole carbon source and those capable of co-metabolizing the herbicide,
this increase was dependent on the concentration of 2,4-D used. At
concentrations of 2,4-D between 5 and 50 mg/litre, there was a
significant increase in the numbers of organisms metabolizing 2,4-D,
and at 5 mg/litre there was a very pronounced increase in organisms co-
metabolizing the compound. At much higher (500 mg/litre) or much lower
(1.2 µg/litre) 2,4-D concentrations, there was no increase in the
numbers of either metabolizing or co-metabolizing organisms.
Sandmann & Loos (1984) estimated the numbers of microorganisms
capable of degrading 2,4-D in soils with and without the `rhizosphere
effect' of two plants, African clover (Trifolium africanum) and sugar
cane (Saccharum officinarum). The `rhizosphere effect' is a
phenomenon which occurs in close association with the roots of plants,
where material from the root or the metabolic activity of the root
tissue affects the surrounding soil. Particularly high, stimulated
populations were associated with sugar cane. A similar effect, but to
a lesser degree, was found with clover. In the three sugar cane soils
examined, and their corresponding controls, the numbers of organisms
were 46 400, 156 000, and 40 700 per g of soil, with rhizospheres, and
178, 1480, and 6170 per g of soil, without rhizospheres, respectively.
Seibert et al. (1982) failed to demonstrate a rhizosphere effect on
2,4-D degradation in glasshouse studies using soils with and without
maize roots.
Norris & Greiner (1967) investigated the degradation of 2,4-D in
forest leaf litter. Litter from either alder, ceanothus, vine maple,
bigleaf maple or Douglas fir showed comparable ability to degrade
2,4-D, the recovery of 2,4-D being between 60% and 70% after 15 days of
incubation. In a second series of experiments, different formulations
of 2,4-D were added to alder litter. About 50% of the free acid of
2,4-D was degraded within 15 days. Triethanolamine salt and two
commercial formulations (`solubilized acid' and isooctyl ester) were
degraded less than the pure acid. There was between 30% and 40%
degradation of these preparations over 15 days.
Nesbitt & Watson (1980) related the degradation rate of 2,4-D in
river water to the nutrient levels, sediment load, and dissolved
organic carbon content of the water. The addition of sediment or
inorganic nutrients increased the rate of 2,4-D degradation, whereas
the addition of organisms capable of degrading 2,4-D did not increase
the rate of breakdown of the herbicide. This finding indicated that
the limiting factor in breakdown of 2,4-D in river water was not
numbers of organisms but the nutrient status of the river. The authors
noted that in winter, when the river was in peak flow and the water
temperature below that for optimum microbial activity, appreciable
amounts of the herbicide would be washed into the estuary. An earlier
pilot study of seasonal changes in the capacity of river water in
Western Australia to degrade 2,4-D (Watson, 1977) indicated clear
seasonal differences in both river water concentrations of the
herbicide and the degrading capacity of river water. Several rivers
were studied and differences were related to the amount of
agricultural run-off, the sediment content of the water, river flow,
and temperature. Rivers receiving agricultural run-off degraded 2,4-D
better than those receiving run-off principally from forests. This was
presumed to be the result of the preconditioning of organisms to the
herbicide; the investigation corrected for nutrient content of the
water which had been previously shown to affect degradation.
Spain & Van Veld (1983) looked at the degrading ability of
microbial communities taken from sediment cores from freshwater,
estuarine, and marine sites. Some cores were pre-exposed to 2,4-D.
Cores from freshwater sites showed increased degradation of 2,4-D after
pre-exposure to the compound, whereas estuarine and marine cores did
not show this effect. The adaptation of freshwater cores was maximal
after 2 weeks and no longer detectable 6 weeks after pre-exposure.
4.2. Uptake and Accumulation by Organisms
Appraisal
Many studies on the accumulation of 2,4-D have used radioactively
labelled herbicide and have monitored uptake by simple counting of the
label. This fails to take into account that the label could have been
removed from the parent molecule by metabolic breakdown. Values for
uptake should, therefore, be treated as a maximum possible uptake
value for 2,4-D. Such data would not normally be considered
acceptable. However, the accumulation of 2,4-D is so low that these
data serve to illustrate that little of the herbicide is accumulated.
4.2.1. Laboratory studies
Eliasson (1973) sprayed leaves of 3-year-old aspen (Populus
tremens) with the butoxyethanol ester of 2,4-D at 0.5 kg acid
equivalent/litre. The plants were then kept in an open-sided
glasshouse and residues of 2,4-D were monitored. Most of the
herbicide remained in, or on, the sprayed leaves. The average residue
level was 2300 mg/kg fresh weight 1 day after spraying. This level had
fallen to 1300 mg/kg after 37 days and, by day 365, the average residue
level was 870 mg/kg. This was a very high application rate and
indicates that there is no foliar uptake of 2,4-D by plants.
Glynn et al. (1984) exposed coral Pocillopora damicornis to three
concentrations of 2,4-D sodium or amine salts at 0.1, 1.0, or
10.0 mg/litre. The maximum concentration of 2,4-D found in coral
tissue was 0.137 mg/kg after exposure to the amine salt at 10 mg/litre,
but residues were not related to the 2,4-D exposure concentration. The
highest bioconcentration factor (BCF) of 1.33 was found after exposure
to 0.1 mg/litre of the amine salt of 2,4-D, i.e., the coral contained
1.33 times the concentration of 2,4-D in water.
Metcalf & Sanborn (1975) introduced 14C-labelled 2,4-D into
model ecosystems consisting of an alga Oedogonium, an aquatic plant
Elodea, a snail Physa, and the mosquito fish Gambusia. Total
14C in the water was equivalent to 0.205 mg 2,4-D/litre. The highest
BCF was in the alga (26.8, based on measurement of radioactivity).
Analysis of all components of the ecosystem for 2,4-D, rather than the
radiolabel, revealed none of the parent compound. The BCF, therefore,
refers to breakdown products rather than 2,4-D itself. Gile (1983)
introduced 14C-labelled 2,4-D, as the butyl ester, into a simulated
ryegrass ecosystem. The system consisted of a sandy loam soil, annual
ryegrass, several invertebrates, and grey-tailed voles. Voles were
introduced 10 days after spraying 2,4-D as a foliar spray at the
equivalent of 1 kg/ha. The experiment was terminated after 1 month.
Plant material contained an average of 8.9 mg/kg; this was identified
as being mostly 2,5-dichloro-4-hydroxyphenoxyacetic acid. Residue
levels in animals (based on unidentified 14C residues) ranged from
0.31 mg/kg in snails to 5.28 mg/kg in pillbugs (isopods).
Freitag et al.(1982) measured the bioaccumulation of 14C-2,4-D in
an alga Chlorella fusca and a fish, the golden orfe. They measured a
24-h static BCF of 6 for the alga, and a 3-day static BCF of <10 for
the fish. This measurement was based on radioactivity and, therefore,
did not distinguish between the parent compound and its breakdown
products.
Schultz (1973) examined uptake and loss of 14C-2,4-D dimethyl-
amine salt by organs of three species of fish (channel catfish,
bluegill sunfish, and largemouth bass), exposed to 0.5, 1.0, or
2.0 mg/litre of 2,4-D acid equivalent. After exposure to the highest
concentration of 2,4-D dimethylamine salt, there was detectable
radioactivity in all organs examined. Bile showed the highest residues
of 14C in all three species after 1 week. For the remainder of the
exposure period of 12 weeks, there was an increase of radioactivity in
other organs and a decrease in the bile. At the end of the exposure
period, there was no clear pattern to residue levels of 14C in
different organs. These levels ranged from 5.04 mg/kg in bile to
35.5 mg/kg in posterior kidney for the channel catfish. For largemouth
bass, the range was from 1.32 mg/kg in muscle to 7.29 mg/kg in liver.
For the sunfish, the lowest residue was 24.75 mg/kg in bile and the
highest 322.7 mg/kg in the pyloric caeca of the gut. After 84 days
exposure to the dimethylamine salt at 2 mg/litre, levels of 14C in
the muscle of catfish, bass, and sunfish were equivalent to 0.953,
0.035, and 1.065 mg 2,4-D/kg, respectively. No analysis for 2,4-D
itself was carried out. A second study exposed the three fish species
for 2 weeks to 14C-2,4-D dimethylamine salt at 1 mg/litre and then
for a further 4 weeks to clean water. The disappearance of 14C was
measured. Loss of 14C was slow at first but by 4 weeks most tissues
had shown a decline in residues. Samples were analysed for 2,4-D but
none was detectable, suggesting that the 14C measured was in
breakdown products. The values for 2,4-D residues in this and other
studies using 14C-labelled material should, therefore, be regarded as
overestimates of retained 2,4-D. Uptake of 14C-2,4-D was examined at
two different temperatures, 17 °C and 25 °C. The highest residues of
14C detected in fish were equivalent to 0.122 mg 2,4-D/kg, but no
2,4-D could be found after analysis, except in bluegill sunfish after
14 days. Loss of 2,4-D did not, therefore, seem to change with
differing temperature over this range. A similar study, at two
different water pH values, showed significantly more 14C uptake in
all three species at the more acidic pH. Analysis of fish tissues for
2,4-D by gas-liquid chromatography showed non-detectable, or trace,
levels in most samples. Only in bluegill sunfish after 7 and 14 days
were residues measurable. These 2,4-D residues showed the opposite
trend to the 14C results; there was more 2,4-D in fish exposed at the
more alkaline pH. The authors suggest that metabolism of the herbicide
in the fish is suppressed at alkaline pH.
Sigmon (1979) exposed bluegill sunfish to 2,4-D butyl ethyl ester
(3 mg/litre) at three different temperatures, 20, 25, and 30 °C, and
measured the tissue content of 2,4-D after 8 days. None of the groups
differed from the controls, residues being <0.05 mg/kg.
Bluegill sunfish and channel catfish took up <0.5% of the
available 14C when exposed to 14C-2,4-D dimethylamine salt at 2
mg/litre (with 1 litre of water per fish) for 7 days (Sikka et al.,
1977). A maximum total 14C concentration in the fish was
reached after 24 h and did not change significantly over 14 days.
Bluegill sunfish attained a total body concentration of 0.9 mg/kg and
catfish 0.2 mg/kg at 24 h. These values were 2,4-D equivalents of
14C measured; the compound was not analyzed directly. When bluegill
sunfish were injected intraperitoneally with 14C-2,4-D dimethylamine
salt, at dose levels of 1 or 2.5 mg/kg body weight, they
excreted 90% of the dose within 6 h of treatment. In a similar
experiment, Stalling & Huckins (1978) exposed bluegill sunfish to
14C-2,4-D dimethylamine salt at 2 mg/litre and measured both
14C and 2,4-D in fish and water samples over the following 12 weeks.
Radioactivity was detected in tissues and increased over the
experimental period, but there was no measurable 2,4-D; the detection
limit of the method was 0.1 mg/kg. An in vivo intraperitoneal
injection of 110 µg of 14C-2,4-D was followed by rapid elimination.
Rodgers & Stalling (1972) measured uptake of 14C-2,4-D butoxy-
ethanol ester by three species of fish, which were exposed to either
0.3 or 1.0 mg/litre and sampled over the next 168 h. Some fish were
fed and some fasted. Radioactivity in a variety of tissues was
determined; the maximum levels were found within 3 h of exposure in fed
fish. After this, levels declined over the remaining sampling period,
and by the end of the experiment, residues were negligible. The one
exception was the gall bladder, which consistently contained more 2,4-D
than other tissues. Results were different for fasted fish. In almost
all organs of fasted fish, uptake of 2,4-D was slower than for fed
fish, although the levels reached were eventually two to five times
higher than in fed fish. Analysis of the residues showed that only the
liver ever contained the herbicide in the ester form. In all other
tissues, only the acid was present.
Shcherbakov & Poluboyarinova (1973) monitored the accumulation of
2,4-D in carp and Daphnia. The 2,4-D was added as the butyl ester
at concentrations ranging from 0.006 to 5 mg/litre; the recommended
usage rate for this ester leads to water concentrations of about
0.5 mg/litre. Analyses of fish tissues were made for both the ester
and the acid. The highest BCF for the ester, at 395, was found with
fish after a 7-day exposure to 0.5 mg/litre. Acid accumulation was
lower than that of the ester. The experiment lasted for 70 days. At
day 10 and after, only trace amounts of ester were found in fish.
Small amounts of 2,4-D acid were found at day 10, but only trace
amounts after day 70. Residues of 2,4-D ester in Daphnia varied from
23.9 to 518 mg/kg, according to the exposure concentration.
Two experiments have been carried out on the grey slug Derocerus
reticulatum by Haque & Ebing (1983) using 14C-labelled 2,4-D acid.
The first study, a contact experiment, exposed the slugs to 2,4-D in
contaminated soil at 1.1 mg/kg. The body content of 2,4-D in slugs
reached equilibrium (0.014 mg/kg) after 15 days; this represented a
BCF of 0.013 based on radioactivity. In the second experiment,
slugs were exposed via the food using carrot discs containing 1.1 mg/kg
slug body weight per day over 5 days. Residues of 14C in the slugs
increased during the feeding period, peaking at 5.5 mg/kg. During the
following 7 days, residues were monitored to investigate loss of
radioactive material. At the end of the experiment, on day 12,
residues were comparable to those at the end of the feeding period.
During the course of feeding 2,4-D-contaminated carrots, more than 80%
of the ingested dose of radioactivity was excreted rapidly; only 20%
was retained. There was no attempt to characterize the 14C residues;
these may, therefore, represent either 2,4-D or its breakdown
products.
Chickens given a single oral dose of 100, 200, or 300 mg/kg
body weight reached maximum plasma levels of 2,4-D of 90, 130, and
250 µg/ml, respectively. Plasma levels in all groups had fallen to
15 µg/ml or less after 24 h. Continuous dosing of chickens at
300 mg/kg per day led to a faster rate of elimination of the daily dose
of 2,4-D with time (Bjorklund & Erne, 1966).
4.2.2. Field studies
Cope et al. (1970) treated experimental ponds with 2,4-D propylene
glycol butyl ether ester to give water concentrations up to and
including 10 mg/litre. No detectable 2,4-D was found in fish exposed
to 1 mg/litre or less of the herbicide, but residues were found in
bluegill sunfish exposed to 5 or 10 mg/litre. The highest residue
(2 mg/kg) was found 1 day after application. Residues were still
detectable after 3 days but not subsequently. Vegetation
(Potamogeton nodosus) and bottom sediment contained residues of
50.0 and 3.0 mg/kg, respectively, 2 days after treatment with the 2,4-D
ester at 10 mg/litre. The herbicide was still detectable at 0.1 mg/kg
in sediment after 44 days but not thereafter. At 44 days after
treatment, there were residues in the plant of 1.2 mg/kg; this amount
declined to 0.1 mg/kg after 94 days.
Following the field application of 2,4-D butoxyethanol ester at
22.5 kg/ha, Whitney et al. (1973) measured residues of the herbicide in
fish, crustacea, and insect larvae over a 3-week period. The herbicide
had been applied to control eurasian water milfoil. Some 2,4-D was
taken up by these various species; the highest residue concentration
was 0.24 mg/kg in largemouth bass after 8 days. All residues in
organisms were below 0.1 mg/kg after 3 weeks. No 2,4-D could be
detected in water in 33 samples taken after treatment, the detection
limit being 0.10 mg/litre. The highest reported concentration of 2,4-D
in mud was 0.65 mg/kg, 10 days after treatment, but in most samples the
herbicide level in mud was much lower and in several it was
undetectable.
Hoeppel & Westerdahl (1983) treated four areas (10 ha each) of
dense water milfoil beds in Lake Seminole, Georgia, with either 2,4-D
dimethylamine salt or 2,4-D butoxyethanol ester, at each of two
application rates (22.5 or 45 kg/ha). Both formulations were converted
to 2,4-D free acid within 24 h. Maximum water concentrations achieved
in the high rate (45 kg/ha) areas were 3.6 and 0.68 mg/litre for the
dimethylamine salt and butoxyethanol ester, respectively. There was no
detectable uptake of 2,4-D into fish in those areas treated with the
dimethylamine salt. In the ester-treated areas, 4 out of 24 game fish
sampled contained low levels of 2,4-D in muscle (the highest residue
being 0.29 mg/kg) and 18 out of 20 gizzard shad contained detectable
2,4-D in muscle (the highest residue being 6.9 mg/kg). No fish sampled
more than 13 days after treatment contained detectable 2,4-D.
Schultz & Harman (1974) treated nine experimental ponds with 2,4-D
dimethylamine salt at three concentrations: 2.24, 4.48, and 8.96 kg/ha.
Samples of water, bottom sediment, and fish were taken over 147 days.
Maximum water and sediment concentrations of 2,4-D were 0.692 mg/litre
and 0.17 mg/kg, respectively. Of 307 fish sampled, 45 contained
detectable residues of 2,4-D. The highest residue measured was in a
channel catfish at 1.075 mg/kg 1 day after treatment. All residues in
fish after 28 days were less than 0.005 mg/kg; most were undetectable.
Smith & Isom (1967) measured uptake and retention of 2,4-D after
treatment of two field sites for control of watermilfoil with the
butoxyethanol ester. The first site was treated with a granular
formulation at a rate of 112 kg/ha. One bluegill sunfish (Lepomis
macrochirus) contained 0.15 mg 2,4-D/kg on day 50 after treatment. All
other fish, sampled between 72 h and 50 days after treatment,
contained less than 0.14 mg/kg, which was the limit of detection. Two
samples of several species of mussel, held in cages for 96 h following
spraying, showed residues of 0.38 and 0.7 mg/kg. Water levels of 2,4-D
reached a peak of 37 mg/litre within 1 h of application and had fallen
to less than 1 µg/litre within 8 h. Mud samples contained very
variable levels of 2,4-D residues, ranging between 0.14 and 58.8 mg/kg.
The highest residue was found 10 months after application. The second
site was treated at the lower rate of 45 kg/ha. All fish sampled
between 15 days and 9 months after 2,4-D application showed residues
of less than 0.14 mg/kg. Mussels sampled between 1 and 42 days after
application contained residues ranging between <0.14 and 1.12 mg/kg.
Water levels peaked at 157 µg/litre, 1 h after spraying, and mud
residues ranged from <0.14 to 33.6 mg/kg.
Coakley et al. (1964) measured residues in organisms at the center
of a 0.4-ha field plot sprayed with 2,4-D butoxyethanol ester at a rate
of 33.7 kg/ha for watermilfoil control. Two days after application,
oysters (Crassostrea virginica), clams (Mya arenaria), fish (Lepomis
gibbus), and blue crabs (Callinectes sapidus) contained 3.5, 3.7, 0.3,
and <0.8 mg/kg, respectively.
In 1971, over 2800 ha in Loxahatchee National Wildlife Refuge
were sprayed with the dodecyl-tetradecyl amine salts of 2,4-D at a
rate of 4.48 kg/ha. The initial application of 2,4-D was followed by
spot treatments of the same formulation and/or the dimethylamine salt
of 2,4-D. The highest water concentration (0.037 mg/litre of 2,4-D)
was measured 1 day after the initial application. Of 60 fish sampled
in the area, 19 had measurable residues of 2,4-D but only three of
these were greater than 0.1 mg/kg; the highest recorded residue was
0.162 mg/kg. Breast muscle and liver of a bird, the common Florida
gallinule Gallinula chloropus, had residues of 0.3 and 0.675 mg/kg,
respectively, 1 day after spraying. No residues were found in the bird
4 days after spraying (Schultz & Whitney, 1974).
Plumb et al. (1977) treated sprouting chamise (Adenostoma
fasciculatum) with the polyethylene glycol butyl ether ester of 2,4-D
at a rate of 3.4 kg acid equivalent/ha. A maximum concentration of
herbicide (95.2 mg/kg) was found in the plant within 15 min of
application. A residue of 3.8 mg 2,4-D/kg remained in, or on, the
plants (shoots which had been originally sprayed) 1 year after
treatment. When Radosevich & Winterlin (1977) applied the butoxypropyl
ester of 2,4-D to a chaparral area at a rate of 4.5 kg/ha, the
residues measured in chamise were 221 mg/kg and in grass and forbs
269 mg/kg within 2 h of application. After 30 days, these levels had
dropped to 60 mg/kg for chamise and 21 mg/kg for grass and forbs, and,
after 360 days, 0.1 mg/kg was present in chamise. Siltanen et al.
(1981) monitored residues of 2,4-D in the fruit of bilberries 1 year
after the application of 0.25, 0.75, or 2.25 kg/ha acid equivalent. No
residues were detected, the limit of detection being 0.05 mg/kg.
Raatikainen et al. (1979), in a controlled field experiment,
sprayed cowberry and bilberry with an ester formulation of 2,4-D.
Three application rates were used, 0.25, 0.75, and 2.25 kg acid
equivalent/ha, and residues of 2,4-D were measured approximately
1 month after application. Thirty-four days after the application of
0.25 kg/ha, residues in cowberry were 0.3 mg/kg. Cowberries exposed to
0.75 or 2.25 kg/ha were analysed after 35 days and contained residues
of 1.0 and 3.7 mg/kg, respectively. Bilberries treated with 0.25,
0.75, or 2.25 kg/ha were analysed 29 days later; residues were 0.1,
1.3, and 4.8 mg/kg, respectively.
4.3 Elimination
James (1979) studied tissue distribution of 14C-labelled 2,4-D in
the spiny lobster (Panulirus argus). Labelled herbicide was injected
into the pericardial sinus and animals were sacrificed at regular
intervals. 2,4-D was taken up from the haemolymph, by the green gland,
and excreted unchanged, with an overall half-time of about 8 h. Tuey &
James (1980), in a similar study, found that the clearance of 2,4-D
from haemolymph, via the green gland, was three to five times greater
than the rate of metabolism in the hepatopancreas.
Pritchard & James (1979) studied the renal handling of intra-
venously injected 2,4-D by the winter flounder (Pseudopleuronectes
americanus). 2,4-D, at a concentration of 1 µmol/litre of plasma,
was actively secreted into the glomerular filtrate of the kidney
with clearances of nearly 500 times the glomerular filtration rate.
At higher plasma concentrations of between 10 and 60 µmol/litre, a
transport maximum of 0.85 µmol/g of kidney per h was observed.
Koschier & Pritchard (1980) reported a similar study using
an elasmobranch fish Squalus acanthias. They administered
2.5 µmol 14C-2,4-D/kg to the fish intramuscularly and monitored
blood and urine 14C levels. Clearance of total 2,4-D was more than
25 times greater than the glomerular filtration rate, indicating that
2,4-D was being actively secreted by the kidney. 2,4-D was eliminated
in the urine as a taurine conjugate, this representing about 95% of the
excretory products. The plasma contained, primarily, unconjugated
2,4-D (>90%). It seemed, therefore, that 2,4-D was conjugated with
taurine before being excreted in the urine. Guarino et al. (1977), in
a similar study on the dogfish Squalus, also found that 2,4-D was
extensively conjugated to taurine (>90%) and was eliminated
predominantly via the urine; 70% of the administered dose appeared in
the urine within 4 to 6 days. The highest tissue concentration of
2,4-D (14.5 mg/kg) was found in the kidney after 4 h. Plasma
elimination was rapid, with a half-time of 44 min; similarly rapid
clearance was seen from the kidney. Half-time estimates for muscle and
liver were 2 to 3 days and 5 days, respectively.
5. TOXICITY TO MICROORGANISMS
Appraisal
In general 2,4-D is relatively non-toxic to water and soil
microorganisms at recommended field application rates.
No effect of 2,4-D was recorded on 17 genera of freshwater and two
genera of marine algae at concentrations up to 222 mg/litre.
No effect of 2,4-D was observed on respiration of either sandy loam
or clay loam soils at concentrations up to 200 mg/kg.
N-fixation by aquatic algae is affected at high concentrations of
2,4-D acid (400 mg/litre). An effect of 2,4-D esters on N-fixation
occurs from a concentration of 36 mg/litre upwards. N-fixing algae in
topsoils appear to be more vulnerable to 2,4-D acid than other algal
species. The Cyanobacteria (blue-green algae) are important as the
major N2 source in tropical ponds and soils.
In the range of 25.2 to 50.4 mg/litre, 2,4-D was inhibitory to all
types of soil fungi.
Cell division was reduced in a green alga by 2,4-D at 20 mg/litre
and stopped at 50 mg/litre. No effect was observed on a natural
phytoplankton community after exposure to 2,4-D at 1 mg/litre.
However, exposure to esters of 2,4-D reduced productivity in these
organisms.
5.1. Aquatic Microorganisms
Hawxby et al. (1977) exposed cultures of three algae
(Chlorella pyrenoidosa, Chlorococcum sp., and Lyngbya sp.,) and
one cyanobacterium (blue-green alga) (Anabaena variabilis) to
concentrations of 2,4-D in the medium of up to 10 µmol /litre
(= 2.21 mg/litre). There was no effect on growth, respiration, or
photosynthetic rate.
Gangawane et al. (1980) studied the effects of 2,4-D on growth and
heterocyst formation in the nitrogen-fixing cyanobacterium (blue-green
alga) Nostoc. The organism was cultured for 30 days in 0, 10, 100,
1000, or 1500 mg 2,4-D/litre. Growth was measured by optical density
and cells forming heterocysts were counted. Growth was inhibited at
both 10 and 100 mg 2,4-D/litre and was eliminated at higher
concentrations. There was also reduced heterocyst formation.
Lembi & Coleridge (1975) demonstrated a marked effect of 2,4-D,
at concentrations of 110 or 220 mg/litre, on cultures of the
green algae Scenedesmus, Ankistrodesmus, and Pediastrum. After 14
days of culture, the three species under control conditions produced
456 x 102, 634 x 104, and 227 cells or colonies per ml of medium,
respectively. Corresponding figures after exposure to 110 mg/litre
were 54 x 102, 41 x 104, and 74 cells or colonies per ml,
respectively. For both Scenedesmus and Ankistrodesmus, these values
were less than the pre-treatment cell concentrations.
Butler et al. (1975b) exposed unialgal cultures of green algae
isolated from Warrior River water to 2,4-D butoxyethanol ester at
0.001, 0.01, 0.1, 1.0, or 4.0 mg/litre. Thirty separate isolates were
used. Concentrations less than or equal to 1 mg/litre of the 2,4-D
ester did not change the growth pattern of the isolates. However, with
a concentration of 4 mg/litre, there was some inhibition of growth, as
indicated by a 10% increase in the number of incubates which showed
poor growth, or no growth, when compared to controls. Some isolates
were unaffected even at this concentration and it can therefore be
assumed that 2,4-D butoxyethanol ester might change the species
composition of green algae populations.
Bednarz (1981) used 12 pure cultures of green algae and
cyanobacteria (blue-green algae) separately and in combination to
investigate the effects of 2,4-D acid. Cultures were exposed to
concentrations of 2,4-D ranging from 0.001 to 10 mg/litre. Low
concentrations of 2,4-D stimulated the growth of most species of algae,
whereas high concentrations inhibited growth. Chlorococcal green algae
were more sensitive to 2,4-D than were filamentous green algae or
cyanobacteria. In further experiments, the authors cultured
combinations of sensitive and tolerant species in the same range of
2,4-D concentrations. Tolerant species used in combinations were
Chlorella pyrenoidosa, Dictyosphaerium pulchellum, and Scenedesmus
quadricaudata. The first two of these tolerant species reduced the
toxicity of 2,4-D to sensitive species in mixed culture. This
protective effect was not seen with Scenedesmus.
Singh (1974) cultured a filamentous, nitrogen-fixing,
cyanobacterium Cylindrospermum sp. in concentrations of 2,4-D acid of
0, 100, 300, 400, 500, 600, 800, 1000, or 1200 mg/litre and examined
growth and heterocyst formation after 8 days. Both parameters were
affected at concentrations higher than 300 mg/litre and cultures were
killed at a concentration of 1000 mg/litre. Kapoor & Sharma (1980)
exposed cultures of the nitrogen-fixing, filamentous cyanobacterium
Anabaena doliolum to 2,4-D ethyl ester (as `Weedone 48' concentrate)
at concentrations of 36, 108, 180, 252, or 324 mg/litre. There was a
dose-related decrease in cell nitrogen over the whole range of 2,4-D
ester exposures. Cell growth was stimulated by lower concentrations of
2,4-D and only inhibited by the highest dose. Tiwari et al. (1984)
exposed cultures of a similar nitrogen-fixing, filamentous
cyanobacterium (Anabaena cylindrica) to 2,4-D acid at concentrations of
0, 100, 500, 700, 1000, or 1500 mg/litre, and examined growth,
heterocyst formation, and nitrogen fixation. For all these parameters,
there was a stimulatory effect of 2,4-D at 100 mg/litre and a
progressive inhibition with higher concentrations. These and similar
algae are considered to be a major source of nitrogen in tropical
ponds and soils. Das & Singh (1977) cultured the nitrogen-fixing
cyanobacterium Anaebaenopsis raciborskii in concentrations of 2,4-D
acid (sodium salt) of 10, 100, 400, 600, 800, and 1000 mg/litre and
measured nitrogen fixation. Control cultures and those exposed at 10
and 100 mg 2,4-D/litre showed no significant differences.
Nitrogen-fixation was inhibited at 400 mg/litre or more and eliminated
at 600 mg/litre.
Butler (1963) reported no effect on a natural phytoplankton
community of exposure to a 1 mg/litre concentration of 2,4-D (as the
acid or dimethylamine salt), or of the dimethylamine salt on pure
cultures of Dunaliella euchlora or Platymonas over 4 h. In a later
study (Butler, 1965), natural phytoplankton communities were exposed to
esters of 2,4-D. Butoxyethanol ester, propylene glycol butyl ether
ester, and ethylhexyl ester reduced productivity (as measured by
carbon fixation) by 16%, 44%, and 49%, respectively, at a concentration
of 1 mg/litre.
Sarma & Tripathi (1980) monitored cell division in the filamentous
green alga Oedogonium acmandrium exposed to 2,4-D acid at 1, 5, 10,
20, or 50 mg/litre of culture medium. At up to 10 mg/litre, 2,4-D
was found to stimulate cell division; a 168 h exposure to 5 mg/litre
increased the incidence of dividing cells by 15% over controls.
However, cell division was reduced at 20 mg/litre and stopped at
50 mg/litre. Abnormalities in chromosomes during cell division
increased with increasing 2,4-D exposure.
Chai & Chung (1975) examined the effects on growth,
photosynthesis, respiration, and chemical composition of exposing
cultures of the green alga Chlorella ellipsoidea to 2,4-D acid at 22 or
88 mg/litre. At 22 mg/litre, 2,4-D increased growth, photosynthesis,
and the cell content of protein and nucleic acids. Carbohydrate
content was unchanged. However, at 88 mg/litre, growth was inhibited,
photosynthesis was no different from controls, and the cell content of
carbohydrate, protein, and nucleic acids was decreased.
Elder et al. (1970) examined the effect of 2,4-D acid on 17 genera
of freshwater and two genera of marine algae exposed at 22, 111, or
222 mg/litre. There was no effect on the growth of any of the
cultures, even at the highest dose of 2,4-D.
Cultures of the flagellate Euglena gracilis were exposed for 24 h
to concentrations of 1, 5, 10, 50, or 100 mg/litre or for 7 days to 10,
50, or 100 mg/litre of 2,4-D acid by Poorman (1973). Cultures in 50
and 100 mg 2,4-D/litre yielded 84% and 74%, respectively, relative to
controls, over 24 h. Lower concentrations of 2,4-D had a slightly
stimulatory effect. After 7 days, there was significant stimulation of
yield with 10 mg/litre; the culture yielded 161% compared to a control.
There was slight stimulation of growth by 50 mg/litre and a reduction
to 78% of control levels with 100 mg/litre.
George et al. (1982) exposed the rotifer Brachionus
calyciflorus to 2,4-D at 5 mg/litre. Median lethal time (LT50) was
24 h and LT100 was 31 h.
5.2. Soil Microorganisms
Pachpande & David (1980) isolated the soil alga Chlorococcum
infusionum from paddy fields and cultured the organism in the presence
of 2,4-D acid at concentrations of 0, 1, 2, 3, 4, and 5 mg/litre.
Growth was estimated as dry weight of algal cells filtered out of the
medium. All concentrations of 2,4-D were inhibitory to growth.
At the highest 2,4-D concentration of 5 mg/litre, the culture yield
was reduced from a control level of 720 mg dry wt/litre of medium to
520 mg/litre.
Cullimore & McCann (1977) applied 2,4-D acid to isolated cores
taken from a prairie, loam soil to give approximate concentrations of
1 or 100 mg/kg in the top 2 cm of soil. Soil algal populations were
estimated from subsamples of cores taken before treatment and 1, 5, or
20 days after treatment with herbicide. Thirty-one genera of algae
were identified, of which five were very sensitive to 2,4-D and were
rarely found after treatment. These were Chlamydomonas, Chlorococcum,
Hormidium, Palmella, and Ulothrix. The most resistant genera were
Chlorella, Lyngbya, Nostoc, and Hantzschia; the `percent sensitivity'
of these genera (% of the total number of treatments in which the
genus was absent) was 28%, 6%, 22%, and 44%, respectively. The
reduction in cell numbers of algae in the top layer of the soil after
herbicide treatment was soon offset by an increase in the population of
Chlorella, Stichococcus, Oscillatoria, and Spongiochloris, all of
which recovered very rapidly from the herbicide effects. There was,
however, an overall reduction in cell numbers of nitrogen-fixing algae.
Mukhopadhyay (1980) measured the bacterial, fungal, and
actinomycete populations of soils supporting rice or maize plants which
had been treated with various herbicides for weed control. There was
no effect of 2,4-D, applied at the recommended rate, either on soil
microorganism numbers or on the evolution of carbon dioxide by soil
cultures.
Huber et al. (1980) examined the effect of 2,4-D at 0.3, 0.2, or
0.1 mmol/litre (= 66, 44, and 22 mg/litre, respectively) on seven
cultures of soil microorganisms. There was no effect on the growth of
five of the cultures; these were Nocardia sp., Pseudomonas fluorescens
in both aerobic and anaerobic culture, Bacillus subtilis, and
Ustilago maydis. There was a small reduction in growth at the
highest 2,4-D dose in cultures of Rhizopus japonicus and Aspergillus
niger. 2,4-D had no effect on mycelium growth of three out of four
plant pathogenic fungi in culture; Phytophthora cryptogea showed
reduced mycelial growth at 0.1, 0.2, and 0.3 mmol 2,4-D/litre,
but Fusarium oxysporum, Alternia radicina, and Rhizoctonia solani
were unaffected.
Moubasher et al. (1981) added 2,4-D at three doses (1.9, 7.6, and
15.2 mg/kg) either to soil or to agar medium inoculated with soil
fungi, and the effects on fungal populations were monitored. In soil,
at all three doses, 2,4-D stimulated the fungi. When incorporated in
the agar medium, 2,4-D was stimulatory to overall fungal growth and to
four individual species of fungus at the lowest dose of 6.3 mg/litre,
but inhibitory to two other species. At higher doses of 25.2 or
50.4 mg/litre, the herbicide was inhibitory to all fungi.
2,4-D had a significant inhibitory effect on culture yields of the
bacterium Escherichia coli only at 10-3mol/litre (= 220 mg/litre).
There was no effect at 10-4mol/litre (= 22 mg/litre) (Toure & Stenz,
1977).
Prescot & Olson (1972) added 2,4-D, at doses of 0, 0.1, 1.0, 10, or
100 mg/litre, to cultures of the soil amoeba Acanthamoeba castellanii
and monitored growth and reproduction. There was a stimulatory effect
of 2,4-D at all dose levels; this effect was most marked at the lowest
dose and declined with increasing exposure to 2,4-D. The authors
suggest that the amoeba may degrade the 2,4-D and utilize it as a
carbon source. However, Pons & Pussard (1980) found no effect of 2,4-D
(at 28, 54, or 84 mg/litre) on the reproduction of 23 different strains
of free-living soil amoebae.
2,4-D, at 10-3mol/litre in cultures of the ascomycete Neurospora
crassa, stimulated DNA synthesis but had no effect at lower
concentrations of 10-4 to 10-6mol/litre. These concentrations had
no significant effect on either RNA or protein (Schroder et al.,
1970).
Naguib et al. (1980) measured growth, respiration, and absorption
and utilization of sugar and nitrogen in pre-formed fungal mats of
Aspergillus terreus over 72 h in the presence of 200 mg/litre of
2,4-D. The herbicide inhibited sugar inversion and consequently sugar
absorption. It also reduced the incorporation of nitrogen into protein.
Respiration was depressed. Growth of the fungus was suppressed and, on
a dry weight basis, culture mass was reduced to below the initial
level.
Trevors & Starodub (1983) added 2,4-D to sandy loam and clay
loam soils and measured both respiration and electron transport
system (ETS) activity. ETS was assessed by measuring the capacity
of the soil to reduce 2-( p -iodophenyl)-3-( p -nitrophenyl)-5-phenyl
tetrazolium chloride (INT) to iodonitrotetrazolium formazan (INT
formazan). The effects of 2,4-D were tested at concentrations of the
herbicide in soil of 0, 10, 25, 50, 75, 100, or 200 mg/kg. There was
no effect on soil respiration, monitored either as oxygen consumption
or carbon dioxide evolution, at any of the concentrations of 2,4-D in
either soil. There was similarly no effect on ETS in the sandy loam.
However, in the clay loam, there was a progressive inhibition of ETS
over the whole range of concentrations of the herbicide. The control
soil had an ETS activity of 37.3 µg INT formazan production/g soil,
whereas the ETS activity of soil treated with 10 mg 2,4-D/kg was
25.1 µg INT formazan/g, significantly lower than that of the
control. The activity was reduced further with increasing
concentrations of 2,4-D, until an activity of 16.3 µg INT formazan/g
was found at 200 mg 2,4-D/kg.
Deshmukh & Shrikhande (1975) added 2,4-D, at recommended field
rates, and at five times the recommended field rates, to two
types of soil from India. Both doses of 2,4-D inhibited numbers
of Azobacter in both soil types, and the high, but not the low, dose of
2,4-D reduced nitrogen fixation in both soils. The same authors
(Deshmukh & Shrikhande, 1974) monitored the populations of various
microorganisms under the same dosing conditions. 2,4-D stimulated
the numbers of actinomycetes throughout the 6-week incubation period at
both dose levels. Fungal populations were reduced in the first week of
incubation at both dose levels in sandy loam, but only at the higher
dose level in clay loam. This reduction in fungal populations
persisted until the second week with the high dose in the sandy soil
and throughout the incubation period with the high dose in clay soil.
There was a temporary (1 week) reduction in total bacterial numbers
with both 2,4-D dose levels in the sandy soil and with the higher level
in clay soil. Schroder & Pilz (1983) reported that 2,4-D at
approximately 10-4mol/litre (= 22 mg/kg) had no long-term effect on
soil nitrification.
Welp & Brummer (1985) measured the influence of 2,4-D on the
reducing capacity of soil microorganisms, reduction being monitored as
the capacity to reduce Fe(III) oxides to soluble Fe(II) ions. They
determined no-observed-effect levels (NOEL) of 115 and 95 mg 2,4-D/kg
for two different soil types and corresponding EC50 values on
reduction capacity of 200 and 530 mg 2,4-D/kg soil.
Ruggiero & Radogna (1985) extracted and partially purified soil
diphenolase (laccase) from forest soil. This enzyme, which exists free
in the soil, plays an important role in the metabolism of humic
materials in soil. Oxygen consumption was monitored during the
enzymatic reaction, using either catechol or p -phenylenediamine as
substrate, and the effect of 2,4-D was investigated. The herbicide
inhibited diphenolase activity, and Lineweaver-Burk plots of the
data suggested that 2,4-D acts as a non-competitive inhibitor.
Apparent K values of 28.7 and 6.0 mol/litre were obtained for catechol
and p -phenylenediamine, respectively.
6. TOXICITY TO AQUATIC ORGANISMS
6.1. Toxicity to Aquatic Invertebrates
Appraisal
The short-term toxicity data on the effects of 2,4-D free acid,
its salts, and esters on aquatic invertebrates is extensive. Ester
formulations are more toxic than the free acids or salts. Sensitivity
variations exist among species in response to the same formulation.
Organisms become more sensitive to 2,4-D when the water temperature
increases. Reproductive impairment occurred at concentrations below
0.1 of the short-term toxic levels determined for these formulations.
6.1.1. Short-term toxicity
The short-term toxicity of 2,4-D to aquatic invertebrates is
summarized in tables 3 - 5.
Unfortunately, there are few studies where both the free acid (or
its salts) and ester preparations have been tested on the same organism
under the same conditions. The only organisms for which this applies
are the oyster (Butler, 1963; Butler, 1965), the stonefly (Sanders &
Cope, 1968), and daphnids and shrimp (Sanders, 1970a). These studies
all show that the free acid and its salts are less toxic than ester
formulations; for example the free acid is at least 20 times less toxic
to the water flea Daphnia magna than the least toxic of the esters
tested (Sanders, 1970a). Comparing studies carried out by different
authors and in different systems also suggests a much greater toxicity
of the ester preparations.
Liu & Lee (1975) found that 2,4-D could adversely affect the bay
mussel (Mytilus edulis) at all stages of its life cycle. The attachment
of young mussels to test chamber walls was reduced (data in Table 3).
The authors evaluated, in two duplicate experiments, the effects of
2,4-D acid, at concentrations in sea water of 22.8, 45.7, 91.4, and
182.8 mg/litre, on the growth of larval mussels. After 10 days
exposure, there was a significant reduction in the growth of larvae
exposed to 91.4 mg 2,4-D/litre; larvae were 11.6% smaller than
controls. This reduction was found in only one experimental replicate.
In both experiments, there was reduced growth after 10 days exposure
to 182.8 mg/litre; larvae were 31.9% and 34.9% smaller than controls
in the two experiments. Exposure for 20 days at 91.4 mg/litre
led to reduced growth in both experiments. All larvae exposed to
182.8 mg/litre died within 12 days, but only in one experimental
replicate. Extension of the growth study in the second experiment led
to all larvae dying within 22 days of exposure to 182.8 mg/litre and,
therefore, failing to undergo metamorphosis. The metamorphosis of
larvae exposed from age 30 to 70 days was not affected by 2,4-D at
concentrations up to 176 mg/litre.
Presing (1981) monitored reproduction over four broods in the water
flea Daphnia magna exposed to 0, 5, 10, 25, or 50 mg/litre of
`Dikonirt' (sodium salt of 2,4-D). For the first brood, the only
significant effect was at 50 mg/litre, whereas the fourth brood was
delayed even at 5 or 10 mg/litre. Significant reductions in the
average number of young produced for each female were found with
the two highest concentrations. Young kept until maturity from
each of the tests were themselves exposed to 2,4-D in a repeat
experiment. Again there was a significant effect on young produced at
25 and 50 mg/litre.
Table 3. Toxicity of 2,4-D to estuarine or marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Salinity pH Formulationc Parameter Water Reference
stata (°C) (o/oo) concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bay mussel 17.2- 22.9- 6.4- free acid 96-h LC50 259 Liu &
(Mytilus edulis) 18.6 24.5 7.8 (232-289) Lee (1975)
17.2- 22.9- 6.4- free acid 96-h EC50 262 Liu &
18.6 24.5 7.8 attachment Lee (1975)
(trocophore larva) 17.2- 22.9- 6.4- free acid 48-h EC50 211.7 Liu &
18.6 24.5 7.8 normal Lee (1975)
development
Eastern oyster flow 18 29 butoxyethanol 96-h EC50 3.75 Butler
(Crassostrea virginica) shell growth (1963)
flow 29 25 isooctyl 96-h EC50 1.0 Mayer
shell growth (1987)
flow 28 25 PGBEE 96-h EC50 0.055 Mayer
shell growth (1987)
Copepod 21 7 7.8 butoxyethanol 96-h LC50 3.1 Linden
(Nitocra spinipes) (2.4-4.1) et al.
(1979)
Brown shrimp (adult) flow 30 PGBEE 24-h EC50 0.55 Butler
(Penaeus aztecus) loss of (1963)
equilibrium
(adult) flow 30 PGBEE 48-h EC50 0.55 Butler
loss of (1963)
equilibrium
(juv.)b stat 26 30 butoxyethanol 48-h LC50 5.6 Mayer
(1987)
(adult) flow 29 26 isooctyl 48-h LC50 0.48 Mayer
(1987)
Dungeness crab (1st zoel) stat 13 25 acid (tech) 96-h LC50 > 10 Caldwell
(Cancer magister) (1977)
(1st instar juv.)b stat 13 25 acid (tech) 96-h LC50 > 100 Caldwell
(1977)
Blue crab (juv.)b stat 24 29 PGBEE 48-h LC50 2.8 Mayer
(Callinectes sapidus) (1987)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration
in water continuously maintained.
b juv. = juvenile.
c PGBEE = propylene glycol butyl ethyl ester.
Table 4. Toxicity of 2,4-D to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Oligochaete worm flow 20 30 30 7.8 free acid 48-h LC50 122.2 Bailey &
(Lumbriculus flow 20 30 30 7.8 free acid 96-h LC50 122.2 Liu (1980)
variegatus)
Water flea stat 21 260 272 7.4 PGBEE 48-h LC50 0.1 Sanders (1970a)
(Daphnia magna) stat 21 260 272 7.4 dimethylamine 48-h LC50 4.0 Sanders (1970a)
stat 17 39 7.2 dimethylamine 48-h LC50 > 100.0 Mayer &
Ellersieck(1986)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 5.6 Sanders (1970a)
stat 21 260 272 7.4 free acid 48-h LC50 > 100.0 Sanders (1970a)
20 8.4- free acid 96-h LC50 417.8 Presing (1981)
8.6
20 8.4- sodium salt 96-h LC50 932.1 Presing (1981)
8.6
Water flea 15.6 44 7.4 PGBEE 48-h LC50 4.9 Sanders &
(Simocephalus (4.0-6.7) Cope (1966)
serrulatus)
Water flea 15.6 PGBEE 48-h LC50 3.2 Sanders &
(Daphnia pulex) (2.4-4.3) Cope (1966)
Copepod (nauplius larva)
(Cyclops vernalis) stat 20 31.6 70 6.7 free acid 96-h LC50 8.72 Robertson (1975)
(5.34-11.57)
stat 20 31.6 70 6.7 alkanolamine 96-h LC50 54.8 Robertson (1975)
(46.45-64.6)
Scud stat 21.1 30 7.1 butoxyethanol 24-h LC50 1.4 (1.1-1.8) Sanders (1969)
(Gammarus stat 21.1 30 7.1 butoxyethanol 48-h LC50 0.76 (0.51
lacustris) -1.1) Sanders (1969)
stat 21.1 30 7.1 butoxyethanol 96-h LC50 0.44 (0.31
-0.62) Sanders (1969)
stat 21.1 30 7.1 PGBEE 24-h LC50 2.1 (1.7-2.5) Sanders (1969)
stat 21.1 30 7.1 PGBEE 48-h LC50 1.8 (1.4-2.3) Sanders (1969)
stat 21.1 30 7.1 PGBEE 96-h LC50 1.6 (1.2-2.1) Sanders (1969)
stat 21.1 30 7.1 isooctyl 24-h LC50 6.8 (4.8-9.7) Sanders (1969)
stat 21.1 30 7.1 isooctyl 48-h LC50 4.6 (2.9-7.3) Sanders (1969)
stat 21.1 30 7.1 isooctyl 96-h LC50 2.4 (1.9-4.8) Sanders (1969)
stat 15.5 260 272 7.4 PGBEE 24-h LC50 4.1 (2.8-5.8) Sanders (1970a)
stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.6 (1.7-3.9) Sanders (1970a)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 15.5 260 272 7.4 PGBEE 96-h LC50 2.5 (1.7-3.7) Sanders (1970a)
(Gammarus stat 15.5 260 272 7.4 butoxyethanol 24-h LC50 6.5 (1.0-8.6) Sanders (1970a)
lacustris) (contd.) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 5.9 (3.1-11) Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 96-h LC50 5.9 (3.1-11) Sanders (1970a)
Scud stat 15 272 7.4 dimethylamine 24-h LC50 > 100 Mayer &
(Gammarus fasciatus) stat 15 272 7.4 dimethylamine 96-h LC50 > 100 Ellersieck (1986)
Glass shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 2.7 Sanders (1970a)
(Palaemonetes stat 21 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
kadiakensis stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.4 Sanders (1970a)
Seed shrimp stat 21 260 272 7.4 PGBEE 48-h LC50 0.32 Sanders (1970a)
(Cypridopsis vidua) stat 21 260 272 7.4 dimethylamine 48-h LC50 8.0 Sanders (1970a)
stat 21 260 272 7.4 butoxyethanol 48-h LC50 1.8 Sanders (1970a)
Freshwater prawn stat 27 113.9 7.5 sodium salt 24-h LC50 2342 Shukla &
(Macrobranchium stat 27 113.9 7.5 sodium salt 48-h LC50 2309 Omkar (1983)
lamarrei) stat 27 113.9 7.5 sodium salt 72-h LC50 2267 Shukla &
stat 27 113.9 7.5 sodium salt 96-h LC50 2224 Omkar (1983)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2644 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2536 Shukla (1984)
naso) stat 28 112.7 7.5 sodium salt 72-h LC50 2435 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2397 Shukla (1984)
Freshwater prawn stat 28 112.7 7.5 sodium salt 24-h LC50 2474 Omkar &
(Macrobranchium stat 28 112.7 7.5 sodium salt 48-h LC50 2381 Shukla (1984)
dayanum) stat 28 112.7 7.5 sodium salt 72-h LC50 2333 Omkar &
stat 28 112.7 7.5 sodium salt 96-h LC50 2275 Shukla (1984)
Crayfish stat 15.5 260 272 7.4 PGBEE 48-h LC50 > 100 Sanders (1970a)
(Orconectes nais) stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 > 100 Sanders (1970a)
Red swamp stat 20 100 8.4 alkanolamine 96-h LC50 1389 Cheah et al.
crayfish (imm.)c (1174-1681) (1980)
(Procambarus clarki)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationd Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Sowbug stat 15.5 260 272 7.4 PGBEE 48-h LC50 2.2 Sanders (1970a)
(Asellus stat 15.5 260 272 7.4 dimethylamine 48-h LC50 > 100 Sanders (1970a)
brevicaudus) stat 15.5 260 272 7.4 butoxyethanol 48-h LC50 3.2 Sanders (1970a)
Stone fly (naiad) 15.5 35 7.1 butoxyethanol 24-h LC50 8.5 (5.7-13) Sanders &
(Pteronarcys 15.5 35 7.1 butoxyethanol 48-h LC50 1.8 (1.5-2.7) Cope (1968)
californica) 15.5 35 7.1 butoxyethanol 96-h LC50 1.6 (1.3-1.9) Sanders &
15.5 35 7.1 acid (tech) 24-h LC50 56 (50-63) Cope (1968)
15.5 35 7.1 acid (tech) 48-h LC50 44 (32-59) Sanders &
15.5 35 7.1 acid (tech) 96-h LC50 15 (10-22) Cope (1968)
Midge (larva) 15 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1490 Bunting &
(Chaoborus 15 78-95 55 7.3-7.8 dimethylamine 96-h LC50 890 (421-1211)Robertson
punctipennis) 20 78-95 55 7.3-7.8 dimethylamine 24-h LC50 1124 (1975)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained.
b Alkalinity and hardness expressed as mg CaCO3/litre.
c imm. = immature.
d PGBEE = propylene glycol butyl ether ester.
Table 5. Toxicity of 2,4-D to aquatic invertebrates: no observed effect levels
---------------------------------------------------------------------------------------------------------
Flow/ Temp Sali- Alkali- Hard- Water con- Refer-
Organism stata (°C) nity nityb nessb pH Formulationc Parameterd centration ence
(o/oo) (mg/litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster flow 9 19 free acid 96-h EC0 2.0 Butler
(Crassostrea shell growth (1963)
virginica) flow 30 23 free acid 96-h EC0 2.0 Butler
shell growth (1963)
flow 25 28 dimethylamine 96-h EC0 2.0 Butler
shell growth (1963)
Freshwater oligochaete flow 20 30 30 7.8 free acid 96-h LC0 86.7 Bailey
(Lumbriculus & Liu
variegatus) (1980)
Scud stat 21.1 30 7.1 dimethylamine 96-h LC0 100 Sanders
(Gammarus lacustris) (1969)
Grass shrimp stat 20 20 butoxyethanol 24-h LC0 10 Hansen
(Palaemonetes pugio) et al.
(1973)
Pink shrimp butoxyethanol 48-h LC0 1.0 Butler
(Penaeus duorarum) (1965)
PGBEE 48-h LC0 1.0 Butler
(1965)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester.
d LC0 and EC0 represent the highest dose used which cause no death or no effect, respectively;
they are not mathematically determined no-effect levels.
George et al. (1982) measured lethal times (LT) after exposure of
the water flea Daphnia lumholtzi to 10 or 20 mg 2,4-D/litre. They
reported, for 10 mg/litre, an LT50 of 38 h and an LT100 of 71 h. For
20 mg/litre, the LT50 was 21 h and the LT100 was 31 h. Doses of
2,4-D ranging from 0.1 to 50 mg/litre did not affect the behaviour of,
or kill, the copepod Mesocyclops leuckarti within a 30-day exposure
period and so lethal times could not be calculated.
Caldwell (1977) and Caldwell et al. (1979) found the zoeal larva to
be the most sensitive life-cycle stage of the Dungeness crab (Cancer
magister) to the free acid of 2,4-D. Based on the herbicide's toxicity
to this stage, the authors suggest a maximum acceptable toxicant level
(MATC) of <1 mg/litre. At this concentration, there was no mortality,
but there was an effect on moulting.
6.1.2. Behavioural effects
Folmar (1978) tested mayfly nymphs (Ephemerella walkeri) in a `Y'-
shaped avoidance maze. A 2,4-D dimethylamine salt solution was run
into one arm of the maze and clean water was run into a second arm,
both at 400 ml/min. Numbers of nymphs in each arm of the maze were
counted after 1 h. No avoidance of 2,4-D was found at concentrations
of 10 mg/litre and there was no mortality. At 100 mg/litre there was
70% mortality in the test nymphs but still no avoidance of the
herbicide. In a similar experiment using the grass shrimp
(Palaemonetes pugio) exposed to the butoxyethanol ester of 2,4-D,
there was significant avoidance of the herbicide at 1 mg/litre (Hansen
et al., 1973).
6.2. Toxicity to Fish
Appraisal
At recommended application rates, the concentration of 2,4-D in
water has been estimated to be a maximum of 50 mg/litre. Most
applications would lead to water concentrations much lower than this
(between 0.1 and 1.0 mg/litre).
LC50 values for fish vary considerably. This variation is due to
differences in species sensitivity, chemical structure (esters, salts,
or free acid), and formulation of the herbicide.
Although the free acid is the physiologically toxic entity, the
ester formulations represent a major hazard to fish when used directly
as aquatic herbicides (because they are more readily taken up by fish).
Amine salt formulations used to control aquatic weeds do not affect
adult fish.
The NOEL varies with the species and the formulation: <1 mg/litre
(coho salmon) to 50 mg/litre (rainbow trout).
Fish larvae are the most sensitive life stage but are unlikely to
be affected under normal usage of the herbicide.
Long-term adverse effects on fish are observed only at
concentrations higher than those produced after 2,4-D has been applied
at recommended rates.
Few studies are related to the effects of environmental variables,
such as temperature and water hardness, on 2,4-D toxicity to fish.
Higher temperature possibly increases the toxicity. This might be
considered when assessing the safety of 2,4-D to fish during control of
aquatic weeds.
Fish detect and avoid 2,4-D only at higher concentrations than
those obtained under normal conditions of use.
6.2.1. Effect of formulation on short-term toxicity to fish
The toxicity of different formulations of 2,4-D to fish is
summarized in Table 6.
The most comprehensive study on the effects of different
formulations of 2,4-D using the same test fish, fingerling bluegill
sunfish (Lepomis macrochirus), was performed by Hughes & Davis (1963)
in static 24-h and 48-h tests. Ester formulations were invariably more
toxic than amine salt formulations. Dimethylamine and alkanolamine
preparations ranged in toxicity from 166 to 900 mg/litre (LC50 in 24-h
tests), depending on the commercial preparation used. Although esters
were always more toxic than amine salts, there was some variation
between different ester formulations (range: 0.9 to 66.3 mg/litre; 24-h
LC50). Most of this variation was between different preparations of
the least toxic of the esters, the isooctyl ester, which ranged in
toxicity from 8.8 to 66.3 mg/litre. All other esters tested produced
LC50 values of 8 mg/litre or less, the most toxic being the isopropyl
with a 24-h LC50 of 0.9 mg/litre. The addition of emulsifiers to acid
preparations increased 2,4-D toxicity; a formulation with emulsifiers
gave an LC50 of 8 mg/litre over 24 h, making it comparable to the
esters in toxicity. All ester formulations were considered by the
authors to present a major hazard to fish when used directly as an
aquatic herbicide, whereas the amine salt formulations could be safely
used to control aquatic weeds without adversely affecting adult fish
(Hughes & Davis, 1963).
A study on a range of ester formulations, using salmonids as test
fish, conducted by Finlayson & Verrue (1985), showed that the toxicity
for salmonids was similar to that for bluegill sunfish. These authors
argue that static tests underestimate the toxicity of 2,4-D esters
because some of the ester is hydrolysed to the less-toxic free acid
during the course of even short-term tests. The presence of test fish
increases the rate of hydrolysis of 2,4-D esters. In a static test,
with two different stocking rates of fish, the apparent toxicity of
2,4-D ester decreased with a greater density of test fish (rainbow
trout) because of this enhanced hydrolysis. Results are given in
Table 6. In their flow-through tests, results were adjusted to take
account of the hydrolysis of ester to 2,4-D acid during the course of
the experiment. Two values are given in Table 6 for each test. The
first is the calculated effect of the non-hydrolysed ester and the
second, entered as `total 2,4-D', is the observed effect of the mixture
of ester and free acid produced by hydrolysis during the course of the
experiment. There is as much as a five-fold difference between the two
values. Alabaster (1969) examined several formulations of 2,4-D in two
species of fish, and found that pelleted herbicide, either as clay-
based or resin-based pellets, was the least toxic to fish of any of the
formulations tested.
Table 6. Toxicity of 2,4-D to fish: effects of different formulations
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
--------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 40 29 6.9 alkanolamine 24-h LC50 450-900 Hughes &
(Lepomis macrochirus) stat 25 40 29 6.9 alkanolamine 48-h LC50 435-840 Davis (1963)
stat 25 40 29 6.9 dimethylamine 24-h LC50 166-542 Hughes &
stat 25 40 29 6.9 dimethylamine 48-h LC50 166-458 Davis (1963)
stat 25 40 29 6.9 di-N,N 24-h LC50 1.5 Hughes &
stat 25 40 29 6.9 di-N,N 48-h LC50 1.5 Davis
stat 25 40 29 6.9 2,4-D acid + 24-h LC50 8.0 (1963)
emulsifiers
stat 25 40 29 6.9 2,4-D acid + 48-h LC50 8.0 Hughes &
emulsifiers Davis (1963)
stat 25 40 29 6.9 isooctyl ester 24-h LC50 8.8-66.3 Hughes &
stat 25 40 29 6.9 isooctyl ester 48-h LC50 8.8-59.7 Davis (1963)
stat 25 40 29 6.9 PGBEE 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 PGBEE 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butoxyethanol 24-h LC50 2.1 Hughes &
stat 25 40 29 6.9 butoxyethanol 48-h LC50 2.1 Davis (1963)
stat 25 40 29 6.9 butyl ester 24-h LC50 1.3 Hughes &
stat 25 40 29 6.9 butyl ester 48-h LC50 1.3 Davis (1963)
stat 25 40 29 6.9 mixed butyl + 24-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 mixed butyl + 48-h LC50 1.7 Hughes &
isopropyl esters Davis (1963)
stat 25 40 29 6.9 isopropylester 24-h LC50 0.9 Hughes &
stat 25 40 29 6.9 isopropylester 48-h LC50 0.8 Davis (1963)
stat 25 40 29 6.9 ethyl ester 24-h LC50 1.4 Hughes &
stat 25 40 29 6.9 ethyl ester 48-h LC50 1.4 Davis (1963)
Cutthroat trout butyl ester 96-h LC50 0.78 Woodward (1982)
(juvenile) (Salmo clarki) (0.66-0.92)
PGBEE 96-h LC50 0.77 Woodward (1982)
(0.62-0.96)
isooctyl ester 96-h LC50 > 50 Woodward (1982)
Chinook salmon (fry) flow 9 18 17 7.1 butoxyethanol 96-h LC50 0.315 Finlayson &
(Oncorhynchus flow 9 18 17 7.1 total 2,4-D 96-h LC50 0.373 Verrue (1985)
tshawytscha)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.375 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.250 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.246 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.117 Verrue (1985)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout (fry) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.518 Finlayson &
(Salmo gairdneri) flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.642 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.329 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 0.514 Verrue (1985)
(smolts) flow 15 18 17 7.1 butoxyethanol 96-h LC50 0.468 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.338 Verrue (1985)
flow 15 18 17 7.1 PGBEE 96-h LC50 0.342 Finlayson &
flow 15 18 17 7.1 total 2,4-D 96-h LC50 1.555 Verrue (1985)
loading factor stat 14 18 17 7.1 butoxyethanol 96-h LC50 1.206 Finlayson &
4.2 g fish/litre Verrue (1985)
stat 14 18 17 7.1 total 2,4-D 96-h LC50 1.422 Finlayson &
loading factor stat 15 18 17 7.1 butoxyethanol 96-h LC50 3.689 Verrue (1985)
8.8 g fish/litre Finlayson &
stat 15 18 17 7.1 total 2,4-D 96-h LC50 4.487 Verrue (1985)
Harlequin fish flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Rasbora heteromorpha) pellets
flow 20 250 7.2 resin-based 24-h LC50 3950 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 3100 Alabaster (1969)
pellets
flow 20 20 7.2 sodium salt 24-h LC50 1160 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 24-h LC50 1.0 Alabaster (1969)
flow 20 20 7.2 butoxyethyl 48-h LC50 1.0 Alabaster (1969)
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout flow 20 250 7.2 clay-based 24-h LC50 7000 Alabaster (1969)
(Salmo gairdneri) pellets
flow 20 250 7.2 clay-based 48-h LC50 4800 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 24-h LC50 3400 Alabaster (1969)
pellets
flow 20 250 7.2 resin-based 48-h LC50 2400 Alabaster (1969)
pellets
flow 20 250 7.2 amine salt 24-h LC50 250 Alabaster (1969)
flow 20 250 7.2 amine salt 48-h LC50 210 Alabaster (1969)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test);
flow = flow-through conditions (2,4-D concentration in water
continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c di-N,N = di-N,N-dimethylcocoamine; PGBEE = propylene glycol
butyl ether ester; total 2,4-D = the effect actually observed in the
flow-through test; the value which preceeds each "total 2,4-D" value is
the calculated effect of the ester alone. The authors determined the
degree of hydrolysis of the ester during the course of the test and
subtracted the effect due to the free acid produced by this hydrolysis.
6.2.1.1 Tolerance and potentiation
Chambers et al. (1977) used insecticide-tolerant and insecticide-
susceptible populations of mosquito fish and an esterase inhibitor to
investigate hydrolytic activation and detoxification of 2,4-D esters.
Mosquito fish taken from a wild population which had developed some
tolerance to insecticides also showed some slight tolerance to 2,4-D
ethyl and butyl esters. This tolerance was most pronounced with the
butyl ester, where the 48-h LC50 was raised from 0.98 mg/litre in the
susceptible fish, to 1.70 mg/litre in the tolerant fish. Further
experiments were carried out to find the basis for this tolerance and
for the higher toxicity of 2,4-D esters over that of the free acid.
The addition of DEF (S,S,S-tributyl phosphorotrithioate), a carboxyl
esterase inhibitor, to the toxicity test medium slightly reduced the
toxicity of both 2,4-D esters. This finding suggested that the toxic
effect of the esters required initial hydrolysis to the acid. The
increased toxicity of the esters would result from esters being more
readily absorbed into the fish through the gills. The resistance of
the insecticide-tolerant population was, at least partially, explained
by measuring esterase activity in homogenates of gill and liver from
the two fish populations. The tolerant fish hydrolyzed less of both
2,4-D esters than susceptible fish. This effect was most marked with
liver homogenates; results from gill homogenates were equivocal. The
overall conclusion, put forward by the authors, is that liver
hydrolysis `activates' 2,4-D esters by converting them to the toxic
free acid. Hydrolysis in the gill is a `detoxification' reaction
because it reduces the uptake of toxic material. The slight increase
in tolerance in the insecticide-resistant mosquito fish is largely the
result of decreased activation of the 2,4-D esters by the liver.
Antagonism to 2,4-D ester toxicity by DEF is largely the result of
inhibition of activation in the liver rather than increased
detoxification in either liver or gill (Chambers et al., 1977).
Carbaryl, a cholinesterase inhibitor, potentiates the toxicity of 2,4-D
butyl ester to brown trout (Salmo trutta) (Statham & Lech, 1975).
The 4.5-h LC50 for 2,4-D butyl ester in static tests was shifted from
30 mg/litre to 11 mg/litre by the addition of carbaryl, at a
concentration of 1 mg/litre, to the test water. Carbaryl has no
toxicity to the fish at this concentration; the 24-h LC50 for carbaryl
alone is 6.8 mg/litre. The potentiating effect of carbaryl was itself
blocked by atropine, a muscarinic blocker, at a water concentration of
10 mg/litre; the atropine itself was not toxic to the fish at this
concentration. In a similar way, carbaryl potentiated the toxicity of
several other compounds. The authors suggested a non-specific action,
possibly by increasing the uptake of 2,4-D ester from the water. The
same potentiation was demonstrated for trout in flow-through tests
(Statham & Lech, 1975). Combinations of 2,4-D esters (butyl or
propylene glycol butyl) with the herbicide picloram increased the
toxicity to fish above the combined toxicity of the individual
compounds (Woodward, 1982).
6.2.2. No-observed-effect levels in short-term tests with fish
The NOELs of 2,4-D on fish in short-term tests are summarized in
Table 7. Values quoted from Meehan et al. (1974) and from Butler
(1965) are based on the lowest dose used in their studies. The values
from Birge et al. (1979) are calculated 1% mortality values derived
mathematically from a full toxicity curve, and based on young fish
exposed to 2,4-D from shortly after fertilization of the eggs until
4 days after hatching. The differing exposure times for the three
species tested is due to differences in time to hatching. The
variation in these data is, therefore, partly due to species
differences in sensitivity to the compound and partly due to exposure
times. Newly hatched young fish are more sensitive to 2,4-D than
unhatched embryos (see section 6.2.4).
6.2.3. Species differences in short-term toxicity to fish
Variation in the toxicity of 2,4-D to fish with species is
summarized in Table 8. Of a range of fish species, examined in the
same test conditions, using 2,4-D as the free acid, by Rehwoldt et al.
(1977), the most sensitive was the white perch (Roccus americanus)
with a 24 h LC50 of 55 mg/litre, and the least sensitive was the
eel Anguilla rostrata with an LC50 of 427 mg/litre. The grass carp,
often used together with herbicides to control aquatic vegetation, was
the least sensitive of all species examined, with a 24 h LC50 for an
amine salt formulation of 3080 mg/litre (Tooby et al., 1980).
6.2.4. Toxicity to early life-stages of fish
Short-term studies on the toxicity of 2,4-D to early life-stages of
fish are summarized in Table 9.
Studies on fish eggs and larvae immediately after hatching have
been conducted on few species and mainly with simple salts of 2,4-D.
There is little information on the effects of the more toxic esters.
2,4-D is clearly toxic for fish early life-stages, within the likely
range of water concentrations which would be found after use of the
herbicide to control aquatic weeds.
At the 16 - 32 cell blastomere stage, eggs of the bleak Alburnus
alburnus, developed more slowly than control eggs when exposed to 2,4-D
solutions. After 48-h exposure, mortality reached 68% and 79% in
those eggs exposed to 2,4-D at 25 and 50 mg/litre, respectively.
Control mortality after 48-h exposure was 37%. After 23 h of
development, eggs exposed to 25 mg 2,4-D/litre showed normal
development while those exposed to 100 mg/litre showed slower
embryogenesis or development halted at the morula-gastrula stage.
Free-swimming larvae were more sensitive to 2,4-D than eggs; the rate
of survival of embryos in tests lasting for between 12 and 48 h was
higher than for larvae. In tests lasting for between 24 and 48 h, at
concentrations of 2,4-D above 400 mg/litre, no larvae survived.
Embryos showed malformations and reduced mobility at concentrations
above 100 mg/litre, and at concentrations of 800 mg/litre or more,
embryos were immobile (Biro, 1979).
Birge et al. (1979) examined the effects of 2,4-D, as the potassium
salt, on the eggs and larvae of three species of fish. Rainbow trout
eggs were the most sensitive, largemouth bass eggs less sensitive, and
goldfish eggs extremely tolerant to 2,4-D. In all species tested,
the larval stages were more sensitive to the herbicide than were the
eggs.
Table 7. Toxicity of 2,4-D to fish: no-observed-effect levels
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Pink salmon (fry) stat 10 10-34 free acid 96-h LC0 < 1 Meehan
(Oncorhynchus stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
gorbuscha stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Chum salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus keto) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Coho salmon (fry) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus kisutch) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 1 (1974)
Sockeye salmon (smolts) stat 10 10-34 free acid 96-h LC0 10 Meehan
(Oncorhynchus nerka) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
Alaska coho salmon stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 < 1 (1974)
stat 10 10-34 PGBEE 96-h LC0 < 1
Oregon coho salmon stat 10 10-34 free acid 96-h LC0 10 Meehan
(fingerlings) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Oncorhynchus kisutch) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Dolly Varden stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salvelinus malma) stat 10 10-34 isooctyl 96-h LC0 10 (1974)
Rainbow trout stat 10 10-34 free acid 96-h LC0 50 Meehan
(fingerling) stat 10 10-34 butyl ester 96-h LC0 < 1 et al.
(Salmo gairdneri) (1974)
Spot stat free acid 48-h LC0 50 Butler
(Leistomus xanthurus) (1965)
Longnose killifish stat dimethylamine 48-h LC0 15 Butler
(Fundulus similis) (1965)
---------------------------------------------------------------------------------------------------------
Table 7. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mullet (Mugil cephalus) stat ethylhexyl 48-h LC0 10 Butler
(1965)
White mullet (juvenile) flow sea free acid 48-h LC0 50.0 Butler
(Mugil curema) water (1963)
Goldfish flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC1 8.2 Birge et al.
(Carassius auratus) 25.8 (2.7-15.0) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC1 8.9 Birge et al.
25.8 (3.8-14.6) (1979)d
Largemouth bass flow 18.2- 66.7 53.5 7.84 pot. salt 7.5-day LC1 13.1 Birge et al.
(Micropterus salmoides) 25.8 (4.4-21-9) (1979)d
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC1 3.2 Birge et al.
25.8 (1.2-6.0) (1979)d
Rainbow trout flow 12.5- 66.7 53.5 7.84 pot. salt 27-day LC1 0.032 Birge et al.
(Salmo gairdneri) 14.5 (0.008-0.084) (1979)d
flow 12.5- 65.3 197.5 7.78 pot. salt 27-day LC1 0.022 Birge et al.
14.5 (0.006-0.055) (1979)d
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c pot. salt = potassium salt; PGBEE = propylene glycol butyl ether ester.
LC0 obtained by extrapolation and LC1 mathematically calculated.
d Birge et al. (1979) exposed fish from four days after hatching.
Table 8. Toxicity of 2,4-D to fish: species variation
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Striped bass stat 20 50 7.2 free acid 24-h LC50 85.6 Rehwoldt
(Morone saxatilis) stat 20 50 7.2 free acid 96-h LC50 70.1 et al.
(1977)
Banded killifish stat 20 50 7.2 free acid 24-h LC50 306.2 Rehwoldt
(Fundulus diaphanus) stat 20 50 7.2 free acid 96-h LC50 26.7 et al.
(1977)
Pumpkinseed sunfish stat 20 50 7.2 free acid 24-h LC50 120 Rehwoldt
(Lepomis gibbosus) stat 20 50 7.2 free acid 96-h LC50 94.6 et al.
(1977)
White perch stat 20 50 7.2 free acid 24-h LC50 55.5 Rehwoldt
(Roccus americanus) stat 20 50 7.2 free acid 96-h LC50 40 et al.
(1977)
American eel stat 20 50 7.2 free acid 24-h LC50 427.2 Rehwoldt
(Anguilla rostrata) stat 20 50 7.2 free acid 96-h LC50 300.6 et al.
(1977)
Carp stat 20 50 7.2 free acid 24-h LC50 175.2 Rehwoldt
(Cyprinus carpio) stat 20 50 7.2 free acid 96-h LC50 96.5 et al.
(1977)
Guppy stat 20 50 7.2 free acid 24-h LC50 76.7 Rehwoldt
(Lebistes reticulata) stat 20 50 7.2 free acid 96-h LC50 70.7 et al.
(1977)
Grass carp flow 13 270 8.1 amine salt 24-h LC50 3080 Tooby et
(Ctenopharyngodon (2622-3618) al. (1980)
idella) flow 13 270 8.1 amine salt 48-h LC50 2540 Tooby et
(2184-2952) al. (1980)
flow 13 270 8.1 amine salt 96-h LC50 1313 Tooby et
(1116-1544) al. (1980)
Bleak stat 10 15 7.8 butoxyethanol 96-h LC50 3.2-3.7 Linden et
(Alburnus alburnus) al. (1979)
Mosquito fish stat 21-22 amine salt 24-h LC50 500 Johnson
(Gambusia affinis) stat 21-22 amine salt 48-h LC50 445 (1978)
stat 21-22 amine salt 96-h LC50 405
Mullet stat sodium salt 24-h LC50 68.0 Tag El-Din
(Mugil cephalus) stat sodium salt 96-h LC50 32.0 et al.
(1981)
Longnosed killifish stat butoxyethanol 48-h LC50 5.0 Butler
(Fundulus similis) stat PGBEE 48-h LC50 4.5 (1965)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulationc Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bluegill sunfish stat 25 19 7.0 dimethylamine 24-h LC50 390 Davis &
(Lepomis macrochirus) stat 25 19 7.0 dimethylamine 48-h LC50 375 Hardcastle
(1959)
Largemouth bass stat 25 19 7.0 dimethylamine 24-h LC50 375 Davis &
(Micropterus salmoides) stat 25 19 7.0 dimethylamine 48-h LC50 350 Hardcastle
(1959)
Punti (Puntius ticto) stat 23.5 ethyl ester 24-h LC50 1.6 Verma et
al. (1984)
Medaka (Oryzias latipes) sodium salt 48-h LC50 > 40 Hashimoto &
Nishiuchi
(1978)
Longnose killifish (juv.) flow sea water PGBEE 24-h LC50 5.0 Butler
(Fundulus similis) flow sea water PGBEE 48-h LC50 4.5 (1963)
flow sea water butoxyethanol 24-h LC50 5.0 Butler
flow sea water butoxyethanol 48-h LC50 5.0 (1963)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
c PGBEE = propylene glycol butyl ether ester
Table 9. Toxicity of 2,4-D to fish early life-stages
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Reference
stata (°C) nityb nessb concentration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Bleak (embryo) sodium salt 12-h LC50 159.4 Biro (1979)
(Alburnus alburnus) sodium salt 24-h LC50 129.0 Biro (1979)
sodium salt 36-h LC50 63.9 Biro (1979)
sodium salt 48-h LC50 12.9 Biro (1979)
(larvae) sodium salt 12-h LC50 111.2 Biro (1979)
sodium salt 24-h LC50 70.6 Biro (1979)
sodium salt 36-h LC50 62.1 Biro (1979)
sodium salt 48-h LC50 51.6 Biro (1979)
Goldfish (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 4-day LC50 > 187 Birge et
(Carassius auratus) 25.8 al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 4-day LC50 > 201 Birge et
25.8 al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 8-day LC50 133.1 Birge et
post-hatch) 25.8 (108.6-174.8) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 8-day LC50 119.1 Birge et
25.8 (98.5-150.6) al. (1979)
Largemouth bass (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 3.5-day LC50 165.4 Birge et
(Micropterus salmoides) 25.8 (130.6-274.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 3.5-day LC50 160.7 Birge et
25.8 (122.9-230.6) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 7.5-day LC50 108.6 Birge et
post-hatch) 25.8 (92.5-138.4) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 7.5-day LC50 81.6 Birge et
25.8 (64.8-103.5) al. (1979)
Rainbow trout (embryo) flow 18.2- 66.7 53.3 7.84 pot. salt 23-day LC50 11.0 Birge et
(Salmo gairdneri) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 23-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
(4-day flow 18.2- 66.7 53.3 7.84 pot. salt 27-day LC50 11.0 Birge et
post-hatch) 25.8 (7.8-15.1) al. (1979)
flow 18.2- 65.3 197.5 7.78 pot. salt 27-day LC50 4.2 Birge et
25.8 (2.8-5.9) al. (1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (2,4-D concentration
in water continuously maintained).
b Alkalinity & hardness expressed as mg CaCO3/litre.
Pot. salt = potassium salt.
Only one study has examined the effects of 2,4-D esters on newly-
hatched fish fry and on fertilized eggs. Unfortunately, this study, by
Hiltibran (1967), does not record the full experimental details. Four
species of fish were used in the study but complete results were given
only for the bluegill sunfish. The most toxic preparations were the
propylene glycol butyl ether (PGBE) ester and mixed isopropyl and butyl
esters with no-observed-effect levels of 2 and 3 mg/litre,
respectively. The dimethylamine salt, ethylhexyl ester, and sodium
salt formulations were less toxic with no-observed-effect levels at 40,
50, and 100 mg/litre, respectively.
6.2.5. Long-term toxicity to fish
Chronic exposure of sub-adult fish of a variety of species to 2,4-D
for 10 months, at a concentration of 0.1 mg/litre, led to no mortality
and to no change in the acute response to 2,4-D. The 24-h LC50 for
the compound was unchanged after 10 months exposure to sub-lethal
doses; i.e., no tolerance developed. One species of fish, the guppy
Lebistes reticulatus, reproduced in captivity. Reproductive success
was compared between control fish and fish breeding in water
containing 2,4-D at 0.1 mg/litre over 10 months. The ratio of numbers
of offspring of treated and control fish was 1.2 (Rehwoldt et al.,
1977). Mount & Stephan (1967) conducted a 10-month study on fathead
minnows (Pimephales promelas) exposed to the butoxyethanol ester of
2,4-D at 0, 0.01, 0.04, 0.2, or 0.8 mg/litre water in a flow-through
test system. Concentrations of 2,4-D of 0.2 mg/litre or less had no
observable effect on growth, survival, or reproductive success of the
fish, but the highest concentration tested was toxic to eggs. The
highest tested no-observed-effect concentration was approximately
1/45 of the 96-h LC50 for this species. Finlayson & Verrue (1985)
conducted a chronic egg-to-fry test over 86 days using chinook
salmon in 2,4-D butoxyethanol ester solutions ranging up to
118 µg/litre. The mortality of salmon during the alevin to fry period
was 4.7% and 47.6% for exposures to 60 and 118 µg/litre, respectively.
When compared to controls, the length of salmon at 36 days
post hatch was significantly reduced after exposure to 60 and
118 µg/litre. Neither survival nor growth of fry were affected at
2,4-D concentrations of 40 µg/litre or less. This maximum acceptable
toxicant concentration (MATC) represents 0.13 and 0.11 of the 96-h
LC50 values for fry and smolts of this species, respectively.
6.2.6. Behavioural effects on fish
Folmar (1976) used rainbow trout fry in an investigation to
determine whether fish avoided water contaminated by herbicides. Trout
fry were initially placed in a `Y'-shaped maze for 15 min. The
dimethylamine salt of 2,4-D, then was added to one arm of the maze and
clean water in a second arm, both at flow rates of 400 ml/min. The
number of fish in each arm was counted after 1 hour and results tested
statistically using a Chi-squared test. At concentrations of 2,4-D of
0.1 mg/litre water (approximately equal to water levels after the use
of this preparation as an aquatic herbicide), there was no avoidance
of the compound; the numbers of fish in each arm of the maze were
equal. However, at concentrations of 1.0 or 10.0 mg/litre of 2,4-D,
there was significant avoidance of the chemical.
Hidaka et al. (1984) conducted a similar study using medakas
Oryzias latipes, and tested a wide range of doses for a variety of
pesticides and herbicides. In all cases, the fish avoided the
chemical in a dose-related manner, but only over a limited range of
concentrations. Above this range, presumably because of the onset of
toxic effects, there was a dose-related decrease in avoidance response.
The authors calculated two values from these chevron-shaped graphs,
the avoidance response EC65 taken from the increasing curve
(AR65) and the DAR60 taken from the decreasing curve. For 2,4-D, the
value for AR65 was 177 (171-182; 95% confidence limits) µg/litre, and
for DAR60 was 288 (245-338) µg/litre. Compared to other chemicals
commonly used or found in water, 2,4-D has a high threshold of
detection by fish as indicated by the high avoidance threshold.
Rand & Barthalmus (1980) exposed goldfish to 2,4-D at 20 mg/litre
(10% of the 96-h LC50 for this species) at different stages during the
training period for conditioning the fish to avoid electric shocks.
They found that the herbicide had no effect when given for 24 h on the
9th day of conditioning but was effective in changing the magnitude of
the avoidance response when given for the first 24 h of conditioning.
Fish exposed for 2 weeks showed significant differences in the pattern,
rate of acquisition, and maintenance of the avoidance baseline.
Behavioural differences persisted into the post-exposure period in fish
exposed to 2,4-D for 2 weeks. The authors point out that short-term
toxicity tests do not examine subtle behavioural effects which could be
of considerable importance in the wild.
Dodson & Mayfield (1979) assessed the effect of 2,4-D on the
``reotaxic response'' of rainbow trout, that is their tendency to swim
upstream to compensate for flowing water. There was a dose-related
effect of 2,4-D at concentrations between 0 and 7 mg/litre. Above this
concentration range, there was a fall of more than 50% in the frequency
of positive reotaxic response to a revolving drum marked in alternate
light and dark stripes and an increase in the frequency of `no-
response'. The authors state that, at ``realistic concentrations'' of
2,4-D in water, there would be a tendency for fish to be moved
downstream because of a reduced reotaxic response.
6.2.7. Effects of environmental variables on toxicity to fish
The toxicity of 2,4-D to fish is related to season. Vardia &
Durve (1981) obtained different values for 96-h LC50 for the carp
Cyprinus carpio at different times of the year. Water
characteristics did not differ, except for temperature which varied
from 39 °C in May, its highest value, to 17 °C in February, its lowest.
There was a positive correlation between temperature and toxicity. At
39 °C, the 96-h LC50 was 5.6 mg/litre, and, at 17 °C, the LC50 was
40.83 mg/litre. As the authors point out, the temperature of the water
must be borne in mind when assessing the safety of this compound to
fish during control of aquatic weeds. The effect may be one of season
rather than temperature; the physiology of the fish also changes
throughout the year.
There is some effect of water hardness and pH on 2,4-D toxicity to
fish but this is very dependent on the species of test fish (Birge et
al., 1979). This effect has not been studied systematically.
6.2.8. Special studies on fish
Chronic exposure of carp to sub-lethal concentrations of 2,4-D
(5 mg/litre) led to ultrastructural changes in the liver of the fish
(Benedeczky et al., 1984). After 2 months, there was detectable
swelling of mitochondria and loss of cristae. There were also large
numbers of inclusions in the cytoplasm, interpreted by the authors as
bile pigments. Their presence in the bile canaliculi indicated the
onset of cholestasis (reduction in bile flow). After 3, 4, or 5 months
of exposure, the cholestasis was pronounced with cholesterin crystals
appearing as cytoplasmic inclusions. Later, in the 6th month of
exposure, there were endoplasmic reticulum changes indicative of
changed protein synthesis.
Oxygen consumption by bluegill sunfish was not affected by 2,4-D at
a concentration of 3 mg/litre water (Sigmon, 1979).
2,4-D at 10-4 mol/litre of medium did not affect the activity of
Na/K-ATPase in microsomes from trout gill (Davis et al., 1972).
Verma et al. (1984) detected effects on pituitary and pineal
histology in punti Puntius ticto exposed for 96 h to 1 mg/litre of
Weedone (ethyl ester of 2,4-D). There was a significant effect on cell
size of acidophilic pituitary cells but a much more marked effect on
cyanophils. Pineal epithelium cell height was significantly greater in
exposed fish.
6.3. Toxicity to Amphibians
Appraisal
Amphibian larvae are generally tolerant to amine salts of 2,4-D;
the 96-h LC50 values exceed 100 mg/litre. Of the species tested, only
one was sensitive.
No information is available on reproductive development and
differentiation or on tissue levels.
The toxicity of 2,4-D to amphibians is summarized in Table 10.
Tadpoles of the Indian toad are particularly susceptible to the
compound (Vardia et al., 1984). Lhoste & Roth (1946) showed that
2,4-D, at 5 g/litre, prevented development of the eggs of the common
frog (Rana temporaria). At doses between 500 mg/litre and 4 g/litre,
there was some development which decreased with increasing dose. These
levels are far higher than those likely to be encountered in the
environment.
Table 10. Toxicity of 2,4-D to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Alkali- Hard- pH Formulation Parameter Water Refer-
stata (°C) nityb nessb concentration ence
(mg/litre)
-------------------------------------------------------------------------------------------------------------------------------------------------
Chorus frog (tadpole) stat 15.5 30 7.1 dimethylamine 24-h LC50 > 100 Sanders
(Pseudacris triseriata) stat 15.5 30 7.1 dimethylamine 96-h LC50 > 100 (1970b)
Indian toad (tadpole) 25 210 220 8.3 free acid 24-h LC50 13.77 Vardia et
(11.81-16.05) al. (1984)
(Bufo melanostictus) 25 210 220 8.3 free acid 48-h LC50 9.03 Vardia et
(8.23-9.91) al. (1984)
25 210 220 8.3 free acid 96-h LC50 8.05 Vardia et
(7.29-8.81) al. (1984)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 255 Johnson
(Adelotus brevis) stat 21-22 amine salt 48-h LC50 228 (1976)
stat 21-22 amine salt 96-h LC50 200 Johnson
(1976)
Frog (tadpole) stat 21-22 amine salt 24-h LC50 321 Johnson
(Limnodynastes peroni) stat 21-22 amine salt 48-h LC50 300 (1976)
stat 21-22 amine salt 96-h LC50 287 Johnson
(1976)
Toad (tadpole) stat 21-22 amine salt 24-h LC50 346 Johnson
(Bufo marinus) stat 21-22 amine salt 48-h LC50 333 (1976)
stat 21-22 amine salt 96-h LC50 288 Johnson
(1976)
Common frog (tadpole) 17-29 free acid 48-h LC0 50 Cooke
(Rana temporaria) (1972)
-------------------------------------------------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(2,4-D concentration in water continuously maintained).
b Alkalinity and hardness expressed as mg CaCO3/litre.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
For terrestrial application, 2,4-D is usually used in the form of
the less volatile, longer-chain esters to reduce drift damage of sprays
to broad-leaved crop plants. The herbicide is used on cereal crops and
on rangeland against broad-leaved weeds and also in forestry. Thus,
insects of all kinds will be exposed to 2,4-D. Birds' eggs in the nest
are more likely to be exposed than the adult birds, though adult
exposure remains possible, particularly for sitting birds. Ground-
nesting species will be exposed from the use of 2,4-D on rangeland and
cereals; tree-nesting species will be exposed in forests.
The insecticidal action of 2,4-D is low enough that the compound
does not represent a hazard to beneficial insects; there is an adequate
safety margin with usage at recommended levels.
Although there is some disagreement in the literature about the
toxicity of 2,4-D to birds' eggs, the low uptake of the material
through the egg shell suggests that exposure would not affect hatching
in normal use of the compound. Adult birds are not affected by short-
term exposure to 2,4-D. The likelihood of prolonged exposure of
either adult birds or eggs to high levels of 2,4-D is small.
7.1. Toxicity to Terrestrial Invertebrates
Appraisal
Based on the widespread use of 2,4-D and its formulations, insects
of many kinds could be exposed to the material. Although the compounds
are generally classified as non-toxic for beneficial insects, such as
honey bees and natural enemies of pests, some adverse effects have been
reported on the early life-stages and adults of some insects.
Esters are less toxic to insects than are salts or the free acid.
Feeding studies dosing worker honey bees (Apis mellifera) with
2,4-D salts in sucrose syrup have generated two estimates of 24-h
LC50: 104 and 115 µg/bee (Jones & Connell, 1954; Beran & Neururer,
1955). Morton et al. (1972) fed 2,4-D acid to honey bees in 60%
sucrose syrup at 10, 100, or 1000 mg/litre and monitored half-time,
i.e., the time taken for 50% of the bees in a cage to die. The half-
time was significantly longer than that of controls for the two lowest
doses (37.2 days at 10 mg/litre and 40.4 days at 100 mg/litre,
compared to a control value of 33.4 days), but was significantly
reduced at 1000 mg/litre (18.6 days). The butoxyethanol and isooctyl
(commercial formulation) esters of 2,4-D had no effect on survival
times at 10, 100, or 1000 mg/litre of syrup when fed under the same
conditions as the acid. The dimethylamine salt of 2,4-D (commercial
formulation) had no effect at 10 or 100 mg/litre, but did shorten the
half-time at 1000 mg/litre.
The effects of 2,4-D on beneficial coccinellid larvae were studied
by Adams (1960). Larvae were sprayed with a preparation of mixed amine
salts of 2,4-D, at a rate equivalent to 0.56 kg acid equivalent/ha, at
different stages of their development 1, 3, 6, 9, or 12 days after
hatching. There was a lengthening of the development period when the
larvae were treated on days 3, 6, 9, or 12 but no effect when they were
sprayed on the first day after hatching. Mortality before pupation was
more than doubled in all treated groups, but mortality during pupation
was not different from that of controls.
Trumble & Kok (1980) dosed adult thistle-rosette weevils
(Ceuthorhynchidius horridus), which are used for the biological
control of musk thistle, with 2,4-D amine salt at five dose levels
between 0.17 and 147.8 kg/ha. No significant mortality was
observed, up to 175 days after treatment, at doses up to and including
1.68 kg/ha. At 16.8 and 84 kg/ha, there was significantly increased
mortality after day 3 post-treatment. At the highest dose level of
147.8 kg/ha, mortality was increased both on day 3 and subsequently.
Five-day LC50 values for males and females were calculated at 70.2 and
61.4 kg/ha, respectively. This is 41.8 times the recommended
application rate of 2,4-D for males and 36.6 for females. Riviere
(1976) reared the European cockroach (Blatella germanica) on food
containing 1000 mg/kg and reported negligible effects on reproduction.
Gall & Dogger (1967) wetted wheat plants with a 0.3% solution of
mixed isopropyl and butyl esters of 2,4-D and exposed the plants to
females of the wheat stem sawfly (Cephus cinctus). Spraying of the
wheat plants was performed at different times relative to oviposition;
times were 7 days prior to oviposition, at the time of oviposition, or
7, 14, or 21 days after oviposition. Eggs took about 7 days to hatch.
The highest larval mortality (96.4%) occurred after spraying at the
time of egg laying. The effectiveness of the 2,4-D in killing larvae
decreased with later exposure times. For plants sprayed 7, 14, and 21
days after oviposition, larval mortalities were 68.1%, 60.8%, and 37%,
respectively, compared to a control mortality of 30%. When plants were
sprayed 7 days before egg laying, larval mortality was 46.9%. Adult
flies were not affected by 2,4-D spray.
Muller (1971) exposed beetles (Carabidae) to sand dose d with
2,4-D at 0.2, 1.0, or 2.0 g/m2. Two species, Bembidion
femoratum and B. ustulatum, both showed more than 50% mortality within
4 days of exposure to 1.0 g 2,4-D/m2. B. ustulatum showed 100%
mortality within 10 days when exposed to 1.0 g/m2 and similar
mortality within 4 days when exposed to 2.0 g/m2. About 20% of the
individuals of B. femoratum survived the 14-day exposure to both 1.0
and 2.0 g/m2.
Roberts & Dorough (1984) exposed earthworms to 2,4-D acid sprayed
on to filter paper. The papers were wetted and the earthworms exposed
to the wetted paper in glass vials. The calculated 48-h LC50 value
was 61.6 (41 - 92.4; 95% confidence limits) µg/cm2.
Rapoport & Cangioli (1963) treated turf with a mixture of
(4-chloro-2-methylphenoxy)acetic acid (MCPA), at recommended rates, and
the butyl ester of 2,4-D, at 10 times the recommended rate. They
reported no effect on soil microarthropods.
7.2. Toxicity to Birds
Appraisal
Birds, and particularly the eggs of ground-nesting species, would
be exposed to 2,4-D after spraying. Food items could also be expected
to be contaminated by the herbicide. However, most studies on birds
and their eggs have been conducted at exposures far higher than could
be expected in the field.
LD50 values from acute oral and from short-term dietary dosing
indicate low toxicity of 2,4-D to birds. In longer-term studies,
effects have only been reported at extremely high exposures (for
example, kidney effects after dosing in drinking water with
concentrations in excess of the solubility of the material). There
have been no reported effects on reproductive parameters, even at
excessive exposure levels.
A single study reported adverse effects on the embryos of birds'
eggs sprayed with 2,4-D. Many studies since have shown no effect on
hatchability of eggs and no increased incidence of abnormalities in
chicks even after very high exposure to 2,4-D. Other work indicates a
very poor penetration of the eggshell by the herbicide. It can only be
concluded that after normal, or even after excessive, 2,4-D use, there
would be no effect on birds' eggs.
7.2.1. Toxicity to birds' eggs
There have been several studies on the toxicity of various 2,4-D
formulations to birds' eggs dosed by different routes.
Spraying eggs of pheasant, red-legged partridge, and grey partridge
with the equivalent of 0.55 to 1.1 kg 2,4-D/ha, either before
incubation or after 3 days of incubation, was found by Lutz-Ostertag &
Lutz (1970) and Lutz & Lutz-Ostertag (1972) to cause embryonic
abnormalities. They reported 77% mortality in pheasant eggs, 77% in
grey partridge eggs, and 43% in red-legged partridge eggs within the
first 19 days of incubation. The eggs were broken open, between days
20 and 22 of incubation for histopathological examination of the
embryos; hatching takes place at about 24 days in all species used. A
majority of surviving embryos were either wholly or partially
paralyzed. Histopathological effects were mainly gonadal. In both
male and female embryos, there were abnormalities of the gonad, often
severe enough to lead to sterility, and, in the male, abnormal
regression of the Mullerian ducts. No control embryos were examined.
In a second study by Lutz & Lutz-Ostertag (1973), quail, pheasant, and
partridge eggs were sprayed with 2,4-D from two different commercial
sources either before incubation or on days 3 or 7 after the start of
incubation. The authors reported increased mortality in embryos,
reduced hatchability, and increased abnormality in chicks. The only
control eggs reported were those in a single incubation of quail.
Later work has failed to repeat these results.
Kopischke (1972) found no adverse effects on hatchability and no
increase in deformities or later mortality of hatched chicks after
spraying eggs of pheasants or chickens with an isooctyl ester
formulation of 2,4-D on day 13 of incubation at a dose equivalent to
0.28 kg/ha.
Somers et al. (1974) sprayed chicken eggs, prior to incubation,
with concentrations of an amine salt of 2,4-D at up to 15 times the
recommended field application rate of approximately 3 kg/ha. There was
no effect on hatching success or on the survival of chicks in the
period 3 to 4 weeks post hatch. Spraying chicken eggs on days 0, 4, or
18 of incubation with 2,4-D (as a PGBE ester formulation) at up to 10
times the field application rate, had no effect on hatchability or on
survival and growth of chicks after hatching (Somers et al., 1978a).
Birds hatched from eggs, similarly treated by spraying, showed no
significant adverse effects on later reproductive performance (egg
laying performance of the females; testis weight or sperm count of
males) (Somers et al., 1978b). Hilbig et al. (1976a) found no effect
on egg hatch rate or on body weight or malformation rate in chicks,
after spraying (at 20 kg/ha) the eggs of Japanese quail, pheasants, and
chickens prior to incubation, or 3 days after the start of incubation.
In a follow-up study on the reproductive performance of birds hatched
from these dosed eggs, Hilbig et al. (1976b) reported no effects on
laying capacity, fertility, or hatchability of their eggs.
The effects of 2,4-D dimethylamine salt on the eggs of Japanese
quail, grey partridge, and red-legged partridge were studied by
Grolleau et al. (1974). Eggs were sprayed with 2,4-D at dose levels
equivalent to the recommended application rate (1.2 kg/ha) and at two
higher dose levels equivalent to 2.4 and 6 kg/ha. There were no
effects on hatching rate, embryonic mortality, or chick mortality in
the first month after hatching or on embryonic or chick malformations.
In addition, the histopathological examination of partridge thyroids
revealed no effects. Residues of 2,4-D were measured in those
partridge eggs receiving the highest dose. Very little 2,4-D
penetrated the egg shell and the highest residue measured was a total
egg content of 19.3 µg (in an 11-g egg, 15 days after treatment). The
lack of effect of 2,4-D on sprayed eggs was attributed to the poor
penetration of the herbicide. Spittler (1976) found no adverse
effects of 2,4-D on hatchability and no increase in chick abnormalities
in pheasant or quail eggs sprayed 24 h before hatching with a dose 12
times higher than the recommended application rate. Only at a dose 30
times higher than the recommended rate did hatchability fall by 10% to
15%, relative to controls. No increased incidence of abnormalities was
reported at this dose rate.
Hoffman & Albers (1984) immersed mallard eggs for 30 seconds in
aqueous emulsions of 2,4-D and calculated an LC50 equivalent to a
field application rate of 216 (155 - 300) kg/ha. This is 32 times the
recommended field application rate. Dunachie & Fletcher (1967)
injected chicken eggs with 10, 100, or 200 mg 2,4-D/kg, equivalent to
0.5, 5, or 10 mg/egg, and found reduced hatchability relative to
control eggs injected with solvent only. Treated eggs showed 80-90%,
70%, and 50% of the control hatch rate for the three dose rates,
respectively. In a similar study, Gyrd-Hansen & Dalgaard-Mikkelsen
(1974) found that injecting 1 mg/egg or less of the dimethylamine salt
of 2,4-D had no effect. Injections of 2 mg/egg reduced both
hatchability of the eggs and survival of hatched chicks. An injected
dose of 5 mg/egg reduced the hatching rate to 15% of control levels and
there were no surviving chicks after 1 week. There was no successful
hatching after an injection of 10 mg/egg. The same authors also dosed
eggs by immersion in solutions of 2,4-D for 10 seconds. There was no
effect after immersion in a solution of 10 g/litre and only a slight
effect after immersion in 50 g/litre. The hatching success and
survival of the chicks up to 4 weeks post hatch, after immersion in
50 g/litre, was more than 80% of control values.
7.2.2. Toxicity to birds after short-term and long-term dosing
The toxicity of 2,4-D (given either orally by capsule or in the
diet) to birds is summarized in Table 11. The studies reported in this
Table include single oral dosing, repeated oral dosing, and dietary
tests over 5 to 100 days. Studies lasting 10 days or less show that
high dosage (in excess of 1000 mg/kg food) is required to kill birds.
2,4-D is, therefore, of low toxicity to birds.
Haegele & Tucker (1974) dosed egg-laying Japanese quail and mallard
a single oral dose of 250 or 1500 mg 2,4-D acid/kg body weight and
monitored egg shell thickness. There was a short-term effect; thin-
shelled eggs were produced during the first 3 days after dosing. This
was considered to be an indirect effect, i.e., the result of reduced
food consumption. When Bjorklund & Erne (1966) gave single oral doses
of 100, 200, or 300 mg 2,4-D amine/kg body weight to chickens, all
clinical and gross pathological findings were negative, with the
exception of a single bird showing gastritis after the highest dose.
2,4-D amine was given orally at 300 mg/kg body weight to a second group
of chickens each day. One bird died after 5 days and was shown on
autopsy to have developed renal and visceral gout. The other birds
were killed on days 12 or 24 of dosing. Slight kidney enlargement was
seen, and there was an enhancement of the rate of 2,4-D elimination
with time.
Bjorn & Northen (1948) orally dosed white-rock chicks on alternate
days for a period of 4 weeks (12 doses in total) with an alkanolamine
salt formulation of 2,4-D. All chicks weighed approximately 50 g at
the beginning of dosing and doses were adjusted for weight gain of the
chicks through the dosing period. No effect on weight gain was noted
at doses of 2,4-D up to 280 mg acid equivalent/kg body weight. In a
further study, single oral doses of up to 380 mg/kg body weight were
without effect, but a single oral dose of 765 mg/kg body weight killed
all the birds.
Whitehead & Pettigrew (1972a) dosed 28-week-old laying hens daily,
by gelatin capsule, with the butoxyethyl ester of 2,4-D at either 6.2
or 18.7 mg acid equivalent/bird for 20 weeks. There were no adverse
effects on egg production, egg or yolk weight, egg shell thickness,
hatchability, or growth rate of the progeny.
Chickens were given oral doses of 2,4-D amine salt at 100, 250, or
500 mg/kg body weight or PGBE ester at 50, 100, or 250 mg/kg body
weight for 10 days in a study by Palmer & Radeleff (1969). Birds given
2,4-D amine salt did not differ from controls in weight gain, even at
the highest dose. There was similarly no effect from the lowest dose
of the ester. However, a growth rate reduction was seen with the
medium dose of the ester (only 19% weight gain relative to a control
weight gain of 41%), and at the highest dose, there was complete
mortality within 4 days, associated with a weight loss of 13%. In a
comparable study, Palmer (1972) dosed chickens with 2,4-D
dimethylamine salt at 25 to 500 mg/kg body weight for 10 consecutive
days. There were effects on weight gain at doses of 100 mg/kg or more.
At 100 mg/kg, the weight gain was 38%, compared to a control value of
57%. At 175, 250, and 375 mg/kg, the weight gain was 30%, similar to
the control value. At the highest dose, three out of five treated
birds died, and the survivors showed a weight gain of 26%. There was
no effect of 2,4-D ethylhexyl ester at 100 mg/kg on weight gain, but at
250 and 500 mg/kg, weight gain was 42% and 36%, respectively, compared
to a control value of 59%.
Solomon et al. (1973) studied the effects of 2,4-D acid and two
unspecified amine salt formulations of 2,4-D. Pheasants were dosed at
weekly intervals, for 17 weeks, with gelatin capsules containing one of
the preparations at either 75 or 150 mg/bird. No effects were observed
on fertility and there was no increase in the number of abnormal
embryos.
Table 11. Toxicity of 2,4-D to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Mallard duck M 4 months oral acid (technical) acute LD50 > 2000 Hudson et
(Anas platyrhynchos) M 3-5 months oral sodium salt acute LD50 > 2050 al. (1984)
M 7 months oral amine salt acute LD50 < 2000 Hudson et
F 3-5 months oral acid (technical) acute LD50 > 1000 al. (1984)
23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et al.
17 days diet dimethylamine 5-day LC50 > 5000 c (1975)
young diet acetamide 100-day LC50 > 500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 2500 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 > 5000 DeWitt et
al. (1963)
Japanese quail M 2 months oral acid (technical) acute LD50 668 Hudson et
(530-842) al. (1984)
(Coturnix coturnix 14 days diet acetamide 5-day LC50 > 5000 c Hill et
(japonica) 12 days diet butoxyethanol 5-day LC50 > 5000 c al. (1975)
20 days diet dimethylamine 5-day LC50 > 5000 c Hill et
al. (1975)
Bobwhite quail 23 days diet butoxyethanol 5-day LC50 > 5000 c Hill et
(Colinus virginianus) 23 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 2500 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 10-day LC50 5000 DeWitt et
young diet butoxyethanol 100-day LC50 5000 al. (1963)
adult diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
Pheasant F 3-4 months oral acid (technical) acute LD50 472 Hudson et
(340-654) al. (1984)
(Phasianus colchicus) 10 days diet butoxyethanol 5-day LC50 > 5000 Hill et
10 days diet dimethylamine 5-day LC50 > 5000 c al. (1975)
young diet acetamide 10-day LC50 1000 DeWitt et
adult diet acetamide 100-day LC50 > 2500 al. (1963)
young diet dimethylamine 100-day LC50 5000 DeWitt et
adult diet dimethylamine 100-day LC50 > 5000 al. (1963)
young diet butoxyethanol 100-day LC50 5000 DeWitt et
al. (1963)
10 days diet butoxyethanol 5-day LC17 5000 Hill et al.
(1975)
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Route Formulation Parameter Concentrationb Reference
(mg/litre)
---------------------------------------------------------------------------------------------------------
Chukar partridge M,F 4 months oral acid (technical) acute LD50 200-400 Hudson et
(Alectoris chukar) al. (1984)
Rock dove M,F oral acid (technical) acute LD50 668 Hudson et
(Columba livia) (530-842) al. (1984)
Chicken M,F 21 days oral free acid 14-day LD50 541 Rowe & Hymas
(358-817) (1954)
M,F 21 days oral isopropyl 14-day LD50 1420 Rowe & Hymas
(1127-1789) (1954)
M,F 21 days oral mixed butyl 14-day LD50 2000 Rowe & Hymas
esters (1350-2960) (1954)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b Acute oral doses are given as mg/kg body weight; all other doses are as mg/kg diet.
c Dose level of 5000 mg/kg diet produced no mortality.
Whitehead & Pettigrew (1972b) fed day-old chicken chicks with the
butoxyethanol ester of 2,4-D at concentrations up to 7500 mg/kg diet
for 3 weeks. Dietary levels up to 1000 mg/kg had no adverse effect,
but at 2000 mg/kg diet 2,4-D ester reduced the food consumption and
growth rate of the chicks. Although there was no mortality at the
higher doses, necropsy of birds sacrificed at the end of the experiment
showed swollen kidneys in all birds and some mottling of the spleen.
Bjorklund & Erne (1966) fed chickens with the amine salt of 2,4-D at
500 mg/kg diet. One bird died of renal gout after 5 months dosing;
autopsy showed hypoplasia (possibly congenital) of the right kidney and
hyperplasia of the left kidney. Other birds were killed at 1, 2, 9,
or 18 months after dosing began, but there was no consistent pattern to
autopsy findings.
Erne & Bjorklund (1970) examined the long-term effects of
phenoxyherbicides on chickens. Groups of day-old broiler chicks were
given 2,4-D in the drinking water at 1000 mg/litre for up to 7 months.
During the dosing period, chickens were sacrificed at regular intervals
for autopsy and samples were prepared for electron microscopy. There
was decreased food and water intake in dosed birds. The most
pronounced effect was on the kidney; there was noticeable kidney
enlargement after 14 days of dosing and this increased with time.
2,4-D concentrations in body tissues reached a plateau after 7 days,
with the highest residue in kidney tissue. Histologically, the kidney
enlargement was shown to be due to hypertrophy of proximal tubule
epithelium. These hypertrophied cells, under the electron microscope,
were shown to display an increased mitochondrial content and
pronounced mitochondrial pleomorphy. The number of microbodies was
also increased and nuclear bodies were observed. These findings were
stated to reflect alterations in intermediate metabolism in the
tubular cells. In an earlier study, using chicks dosed similarly with
1000 mg/litre of drinking water (Bjorklund & Erne, 1966), the birds
were followed through to sexual maturity. No significant effects were
observed on weight gain, age at sexual maturity, or onset of egg
production, but the number of eggs laid was reduced during the first 2
months of egg laying. The number of birds dying during the course of
the study did not differ from the control value. Surviving birds were
killed and autopsied at intervals of between 2 and 18 months after the
onset of egg laying. The primary effect was consistent enlargement of
the kidneys.
7.2.3. Special studies on birds
Lundholm & Mathson (1983) studied the effect of 2,4-D on the ATP-
dependent Ca2+ binding of the particulate fraction of egg shell
gland mucosa cells from egg-laying hens. This parameter had been
found to be a sensitive indicator of potential shell-thinning effects
of chemicals. They calculated a 5-min IC50, for Ca binding
inhibition, of 30.7 x 10-8 mmol 2,4-D/litre incubation medium.
This makes 2,4-D 13.5 times less effective in this respect than
1,1'-(2,2-dichlorethenylidine)-bis[4-chlorobenzene] ( p-p' -DDE), the
major agent causing eggshell-thinning in birds.
Percutaneous absorption of 2,4-D through the feet of red-winged
blackbirds was measured by Rogers et al. (1974). A 24-h exposure to
14C-labelled 2,4-D at 0.01 mmol/litre resulted in a blood
concentration of 1.24 x 10-3 mmol 2,4-D/litre.
7.3. Toxicity to Non-laboratory Mammals
Appraisal
Based on the available data, no generalization can be made about
the hazard of 2,4-D to mammals in the field. Data on voles indicate
that the herbicide poses no hazard.
Cholakis et al. (1982) obtained acute oral LD50 estimates for
two species of voles by determining mortality 14 days after the
administration of a single dose of 2,4-D acid. Values were 2110
(1800 - 2570) and 2100 (1900 - 2390) mg/kg body weight for males
and females, respectively, of the prairie vole (Microtus
orchrogaster). Values for the grey-tailed vole (Microtus canicaudus)
were 1200 (955 - 1150) for males and 1310 (1010 - 1790) mg/kg body
weight for females.
Skokova (1975) orally dosed 24 male bank voles with 400 to
405 mg/kg body weight (10% of the LD50) daily for 10 or 20 days and
examined reproductive parameters. Testis weight, an index of
spermatogenesis, and divisions in spermatogonia were all significantly
reduced relative to control values. Gile (1983) applied a foliar spray
of butyl ester of 2,4-D to a simulated ryegrass ecosystem at 1 kg/ha.
The system included voles, which showed a weight loss after exposure to
2,4-D when compared to similar animals in an untreated system. This
loss was considered to be the result of protein deficiency.
8. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
Appraisal
No direct toxic effects, acute or long-term, of 2,4-D applications
under field conditions on any animals species have been observed thus
far.
There are, inevitably, indirect effects resulting from the intended
selective herbicidal properties of the compound. These effects would
result from the use of any herbicide or from other methods of land
management. There will, therefore, be effects for mammals, birds, and
insects because of food deprivation, modification of habitat,
requirements for nesting, shelter, etc.
The application of 2,4-D appears to present no hazard to the
beneficial epigeal arthropod community. Physical cultivation present a
greater hazard to sensitive soil arthropods than the use of 2,4-D
herbicides.
Oka & Pimental (1976) observed increased numbers of insect pests
and increased occurrence of blight infection in maize (Zea mays) crops
treated with 2,4-D as the triethanolamine salt. The crops had been
treated with 2,4-D at 0.14, 0.55, or 4.4 kg/ha; 0.55 kg/ha is the
normal rate of application for this crop. The number of aphids
increased from 1420 on control untreated plants to 2449 on plants
treated at 0.14 kg/ha, 3116 on plants treated at 0.55 kg/ha, and 2023
on plants treated at 4.4 kg/ha. The percentage of plants attacked by
the European corn borer (Ostrinia nubialis) increased from 63% on
controls to 83% and 70% for treatments at 0.14 and 0.55 kg/ha,
respectively. Controlled studies also showed an increase in infection
with fungal blight. Laboratory investigation of these effects
confirmed that the treated maize had higher protein levels than the
untreated. This was thought to be the reason for the increased success
of the pests.
Everts et al. (1986) investigated the effects of various pesticides
on soil arthropods. Spiders were found to be a sensitive indicator of
effect. No side-effects of the use of 2,4-D amine were observed on
these organisms. Lahr et al. (1987) showed that spider numbers were
reduced by ploughing but not by the use of 2,4-D herbicides.
Matida et al. (1975) examined aquatic organisms in a stream running
through a mountainous area of 9.4 ha, in Shizuoka prefecture in Japan,
which had been aerially sprayed with a mixture of 2,4-D and 2,4,5-T at
a rate of 150 kg/ha. There was no effect on the number or species
diversity of aquatic invertebrates. Caged cherry salmon and dace
fingerlings showed no mortality, abnormal behaviour, or pathological
change after spraying. An extensive ecological survey of an area in
Florida treated with military mixtures of herbicides, including 2,4-D,
revealed no major change in species diversity or population size for
aquatic invertebrates, fish, a lizard, or the beach mouse (Young et
al., 1975).
The weevil Rhinocyllus conicus is used in the biological control of
musk thistle, an invasive weed. In an investigation of the
practicality of combining biological with chemical control, Lee & Evans
(1980) investigated the toxicity to the weevil of 2,4-D. They sprayed
musk thistle with 2,4-D at a rate of 4.48 kg/ha. One week later, the
terminal seed heads of the thistle were covered with cloth bags to
contain the weevils. The number of dead larvae, pupae, and adults
were counted and compared to the number on unsprayed, control thistle
heads. No significant differences were observed.
Dwernychuk & Boag (1973) studied the effect of herbicide spraying
on several species of nesting ducks (lesser scaup, gadwall, white-
winged scoter, mallard, pintail, and American wigeon) in Canada. An
ester of 2,4-D was applied to two islands. This application
significantly reduced the areas dominated by broad-leaved plants and
permitted invasion of these areas by grasses. Ducks preferred to nest
amongst broad-leaved vegetation and avoided grass. As the areas of
broad-leaved plants disappeared, there was an increase in nest density
in those broad-leaved areas still present. Total numbers of nesting
ducks declined over the 3-year study period. This decline was
attributed, by the authors, entirely to the effect of the herbicide on
vegetation type.
Keith et al. (1959) studied the effects on populations of the
pocket gopher (Thomomys talpoides) of spraying weedy rangeland in
Colorado with 2,4-D, as the butyl ester, at 3.4 kg/ha. Numbers of
gophers were estimated using two different methods, either by trapping
or by counting numbers of newly excavated mounds. Both methods showed
a highly significant difference between sprayed and non-sprayed areas
1 year after spraying. The total numbers of gophers trapped in the two
areas before 2,4-D application were 101 and 110, respectively. In the
same two areas, 1 year after spraying, numbers were 117 (untreated
area) and 15 (treated area), respectively. This represents a fall in
gopher numbers of 87% in the treated area and a slight increase in
numbers in the unsprayed area. Newly excavated mounds in the treated
area were only 28% as numerous as in control areas. Spraying had
reduced production of forbs (broad-leaved plants) from 445 kg/ha
before spraying to 75 kg/ha afterwards, a reduction of 83%. Grass
production had increased by 37%. The overall reduction in vegetation
was 232 kg/ha or 35% on sprayed plots.
In a similar study by Tietjen et al. (1967), 2,4-D butyl ester
applied at 3.4 kg/ha to identical high-altitude rangeland initially
reduced forb density and gopher populations. Pocket gopher numbers
were reduced by between 80% and 90%. Both forbs and gopher populations
remained low in one treated area but not in a second one. The decline
in gopher numbers was considered by the authors to be a result of food
deficiency; the grasses available represented a marginal diet for the
animals. This decline was not due either to movement of animals out of
the area or to the direct toxic effects of the herbicide. Reduction in
numbers was, therefore, primarily a result of reduced breeding success.
Johnson & Hansen (1969) studied the after-effects on wild mammals
of treating perennial forb and shrub/grass ranges with 2,4-D either
aerially or from a ground rig at rates of 2.2 or 3.4 kg/ha using a
diesel-oil carrier. The density and litter size of the deer mouse
(Peromyscus maniculatus) was little affected by the treatment, but the
densities of northern pocket gophers (Thomomys talpoides) and least
chipmunks (Eutamias minimus) were reduced. Montane voles (Microtus
montanus) increased their abundance in treated perennial forb range.
Gopher and vole populations returned to normal with the re-
establishment of forb dominance. Density changes were considered by
the authors to be primarily due to changes in availability of food for
the gophers, availability of both food and cover for chipmunks, and
availability of a close-canopied grass cover for the voles. There were
no direct toxic effects of the herbicide.
Fagerstone et al. (1977) observed two colonies of black-tailed
prairie dogs (Cynomys ludovicianus) in North America and the effect of
spraying their feeding areas with 2,4-D. The herbicide was applied
initially as the dimethylamine salt, followed by two further sprays of
the butyl ester after 1 month and 1 year. All applications of 2,4-D
were at 2.2 kg/ha. One colony lived in a sprayed area, initially rich
in broad-leaved plants. The second colony lived in an unsprayed area
poor in dicotyledonous plants and rich in grass. The effect of
spraying was, therefore, to make the treated area similar to the
control area with less broad-leaved herbage and less cover. Prior to
treatment, the first colony preferentially ate the forbs; diet was 73%
forbs and 5% grass. After spraying, they ate 9% forbs and 82% grass.
The control colony ate a similar diet to the first colony post-
treatment, i.e., mostly grass. Prairie dogs remained in the same area
after treatment with herbicide and there was no evidence of starvation.
Body weight was maintained, activity was comparable to the pre-
treatment level, and reproduction was unaffected.
Spencer & Barrett (1980) monitored the population response of
meadow voles (Microtus pennsylvanicus) to application of 2,4-D as the
N-oleyl 1,3 propylenediamine salt at 567.5 g/ha. Two 0.4-ha plots
were compared, one sprayed and the other untreated. The population
fluctuation of the voles was monitored for 6 months, from June to
December, a period spanning the breeding season. The control area
reached a population peak of 116 animals on 6 November; a peak of 68
voles was reached on 9 October in the treated area. There was a skewed
sex ratio in the treated area, mainly because of a reduced survival
rate in females. Voles in the treated plot were protein-deficient,
compared to controls
9. EVALUATION
In evaluating the environmental hazards of 2,4-D, the following
general points should be borne in mind:
(a) the chlorinated dibenzo- p -dioxins (CDDs) are present in
2,4-D only in trace amounts which are difficult to separate and
identify;
(b) 2,4-D is rapidly degraded in the environment;
(c) the environmental effects are indirect, and are the
consequence of vegetation diversity being modified; care should be
taken to avoid unintentional vegetation damage; the mode of
application and the formulation should be carefully selected;
esters should be avoided in aquatic applications (because of
toxicity to aquatic organisms);
(d) there are limited data on the effects of 2,4-D and its
formulations on communities of organisms; hazard assessment is,
therefore, often by extrapolation from single species studies;
(e) minor adverse effects shown in laboratory studies on
terrestrial organisms have resulted from exposures far in excess of
likely exposures in the field.
9.1. Aquatic Organisms
Sources of exposure of aquatic ecosystems to 2,4-D include direct
application, run-off, and spray drift. Because of low adsorption rate
and rapid degradation, the herbicide is not accumulated in compartments
of the aquatic system.
2,4-D acid and its salts are less toxic to aquatic organisms than
are the esters.
2,4-D acid and its salts are of low to moderate toxicity to aquatic
organisms. However, the growth and nitrogen fixation of some
cyanobacteria (blue-green algae) are inhibited, but only at
concentrations above levels expected from direct application of the
herbicide to water. These microorganisms are the source of most
nitrogen in wet tropical soils. This inhibitory effect could be of
concern when these compounds are applied to rice fields at a high
dosage.
Because of the toxicity of esters, particularly the propylene
glycol butyl ether ester, for early life-stages of several fish
species, they should be regarded as hazardous to aquatic ecosystems.
9.2. Terrestrial Organisms
2,4-D does not persist in soil and other compartments of the
terrestrial environment.
Nitrogen-fixing microorganisms appear to be particularly sensitive
to 2,4-D; this might be especially important in tropical soils.
Some terrestrial invertebrates have shown adverse effects, but only
at high exposure levels. Therefore, 2,4-D does not constitute a hazard
to this group of organisms.
2,4-D has low acute toxicity to birds, as indicated by the
LD50. Most studies on birds and their eggs have been conducted at
exposures exceeding those that could be expected in the field; even
under these conditions, no significant adverse effects have been
observed.
Under field conditions, 2,4-D does not cause direct toxic effects
on animals. However, the change of species composition and structure
of the vegetation, resulting from the use of this herbicide, leads to
indirect effects on terrestrial ecosystems. This indirect effect would
also result from the use of any herbicide or from other methods of land
management in either temperate or tropical regions.
* * *
There is evidence only for minor effects on the environment arising
from the use of 2,4-D, as long as the following simple recommendations
are followed:
(a) amine formulations, rather than esters, should be used to
control aquatic weeds;
(b) accidental spread of the herbicide to other vegetation should
be avoided;
(c) the margins of agricultural land should be left untreated with
herbicide to avoid even the indirect effects of the material on
wildlife.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
There are indications that 2,4-D affects nitrogen fixation by
algae. Since this is the major source of nitrogen in tropical soils,
it is recommended that this should be further investigated,
particularly with reference to varying soil conditions. This should be
extended to a study on the functioning of a rice-paddy at the ecosystem
level.
REFERENCES
ADAMS, B.J. (1960) Effects of spraying 2,4-D amine on Coccinellid
larvae. Can. J. Zool., 38: 285-288.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents and miscellaneous substances.
Int. Pest Control, 11: 29-35.
AUDUS, L.J. (1960) Microbiological breakdown of herbicides in soils.
In: Woodford, E.K. & Sagar, G.R., ed. Herbicides and soil, Oxford
Blackwell Scientific Publications, pp. 1-19.
AUDUS, L.J. (1964) Herbicide behaviour in the soil. In: Audus, L.J.,
ed. The physiology and biochemistry of herbicides, London, New York,
Academic Press, pp. 163-206.
BAILEY, H.C. & LIU, D.H.W. (1980) Lumbriculus variegatus, a benthic
oligochaete, as a bioassay organism. In: Eaton, J.C., Parish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
for Testing and Materials, pp. 205-215 (Special Technical Publication
707).
BEDNARZ, T. (1981) The effect of 2,4-D acid on green and blue-
green algae in unialgal and mixed cultures. Acta hydrobiol., 23: 173-
182.
BENEDECZKY, I., BIRO, P., & SCHAFF, Z. (1984) The effect of
2,4-D-containing herbicide (Dikonirt) on the ultrastructure of carp
(Cyprinus carpio) liver cells. Acta biol. (Szeged), 30: 107-125.
BERAN, F. & NEURURER, J. (1955) [Effect of plant protection
materials on the honeybee. I. Toxicity to bees.]
Pflanzenschutzberichte, 15: 97-147 (in German).
BIRGE, W.J., BLACK, J.A., & BRUSER, D.M. (1979) Toxicity of organic
chemicals to embryo-larval stages of fish. Washington, DC, US
Environmental Protection Agency (EPA 560-1179-007).
BIRO, P. (1979) Acute effects of the sodium salt of 2,4-D on the
early developmental stages of bleak, Alburnus alburnus. J. Fish Biol.,
14: 101-109.
BJORKLUND, N-E. & ERNE, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta vet. Scand., 7: 364-390.
BJORN, M.K. & NORTHEN, H.T. (1948) Effects of 2,4-
dichlorophenoxyacetic acid on chicks. Science, 108: 479-480.
BUNTING, D.L. & ROBERTSON, E.B. (1975) Lethal and sublethal effects
of herbicides on zooplankton species, University of Tennessee, Water
Resources Research Center, 34 pp. (Res. Report No. 43, NTIS Report No.
PB-241 337).
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975a) Loss of
five pesticides from cultures of twenty-one planktonic algae. Bull.
environ. Contam. Toxicol., 13: 149-152.
BUTLER, G.L., DEASON, T.R., & O'KELLEY, J.C. (1975b) The effect
of atrazine, 2,4-D, methoxychlor, carbaryl and diazinon on the growth
of planktonic algae. Br. phycol. J., 10: 371-376.
BUTLER, P.A. (1963) A review of Fish and Wildlife Service
investigations during 1961 and 1962, Washington, DC, US Department of
the Interior, Fish and Wildlife Service, pp. 11-25 (Circular No. 167).
BUTLER, P.A. (1965) Effects of herbicides on estuarine fauna. Proc.
South. Weed Conf., 18: 576-580.
CALDWELL, R.S. (1977) Biological effects of pesticides on the
Dungeness crab, Gulf Breeze, Florida, US Environmental Protection
Agency (EPA-600/3-77-131).
CALDWELL, R.S., BUCHANAN, D.V., ARMSTRONG, D.A., MALLON,
M.H., & MILLEMANN, R.E. (1979) Toxicity of the herbicides 2,4-D,
DEF, propanil and trifluarin to the Dungeness crab, Cancer magister.
Arch. environ. Contam. Toxicol., 8: 383-386.
CHAI, I.K. & CHUNG, Y.S. (1975) [Physiological effects of 2,4-
dichlorophenoxyacetic acid (2,4-D) on Chlorella ellipsoidea.]
Misaengmul Hakhoe Chi., 13: 101-108 (in Korean).
CHAMBERS, H., DZIUK, L.J., & WATKINS, J. (1977) Hydrolytic
activation and detoxification of 2,4-dichlorophenoxyacetic acid esters
in mosquito fish. Pestic. Biochem. Physiol., 7: 297-300.
CHEAH, M.L., AVAULT, J.W., & GRAVES, J.B. (1980) Acute toxicity
of selected rice pesticides to crayfish Procambarus clarkii. Prog. Fish
Cult., 42: 169-172.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1982)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avaian and Mammalian Wildlife
Toxicology: 2nd Conference, Philadelphia, American Society for Testing
and Materials, pp. 143-154 (Special Technical Publication 757).
COAKLEY, J.E., CAMPBELL, J.E., & MCFARREN, E.F. (1964)
Determination of butoxyethanol ester of 2,4-dichlorophenoxyacetic acid
in shellfish and fish. J. agric. food Chem., 12: 262-265.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COPE, O.B., WOOD, E.M., & WALLEN, G.H. (1970) Some chronic
effects of 2,4-D on the bluegill (Lepomis macrochirus). Trans. Am.
Fish. Soc., 99: 1-12.
CULLIMORE, D.R. & MCCANN, A.E. (1977) Influence of four
herbicides on the algal flora of a prairie soil. Plant Soil, 46: 499-
510.
DAS, B. & SINGH, P.K. (1977) The effect of 2,4-dichlorophenoxyacetic
acid on growth and nitrogen-fixation of blue-green alga Anabaenopsis
raciborskii. Arch. environ. Contam. Toxicol., 5: 437-445.
DAVIS, J.T. & HARDCASTLE, W.S. (1959) Biological assay of
herbicides for fish toxicity. Weeds, 7: 397-404.
DAVIS, P.W., FRIEDHOFF, J.M., & WEDERMEYER, G.A. (1972)
Organochlorine insecticide, herbicide and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8: 69-72.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1974) Effect of some
herbicides on soil microflora. J. Indian Soc. Soil Sci., 22: 300-303.
DESHMUKH, V.A. & SHRIKHANDE, J.G. (1975) Effect of some
herbicides on Azotobacter and non-symbiotic nitrogen fixation in the
soil. Indian J. Agric. Chem., 8: 95-98.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife
studies, Patuxent Wildlife Research Center. Pesticide-wildlife
studies. A review of Fish and Wildlife Service investigations during
1961 and 1962. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular No. 167).
DODSON, J.J. & MAYFIELD, C.I. (1979) The dynamics and
behavioural toxicology of Aqua-Kleen (2,4-D butoxyethanol ester) as
revealed by the modification of rheotropism in rainbow trout. Trans.
Am. Fish. Soc., 108: 632-640.
DUNACHIE, J.F. & FLETCHER, W.W. (1967) Effect of some
herbicides on the hatching rate of hen's eggs. Nature (Lond.), 215:
1406-1407.
DWERNYCHUK, L.W. & BOAG, D.A. (1973) Effect of
herbicide-induced changes in vegetation on nesting ducks. Can. field
Nat., 87: 155-165.
ELDER, J.H., LEMBI, C.A., & MORRE, D.J. (1970) Toxicity of 2,4-D
and picloram to fresh and saltwater algae. Proc. North-cent. Weed
Control Conf., 25: 96-98.
ELIASSON, L. (1973) Translocation and persistence of 2,4-D in
Populus tremula L. Weed Res., 13: 140-147.
ERNE, K. & BJORKLUND, N-E. (1970) Nephrotoxic effects of
phenoxy acetic herbicides. In: Proceedings of the 7th International
Congress on Plant Protection, Paris, France, pp. 768-769.
EVERTS, J.W., BEURSKENS, J.E.M., BOUWHUIS, J.S., BUYSE, A.D.,
HENGEVELD, R., WOUTERS, L., & KOEMAN, J.H. (1986) Terrestrial
arthropods as indicators for side-effects caused by insecticides in
arable farm systems in the Netherlands. In: Proceedings of the 1st
International TNO Conference on Contaminated Soil, Utrecht, The
Netherlands, November 1985, Dordrecht, Boston, Martinus Nijhoff, pp.
423-425.
FAGERSTONE, K.A., TIETJEN, H.P., & LAVOIE, G.K. (1977) Effects
of range treatment with 2,4-D on prairie dog diet. J. Range Manage.,
30: 57-60.
FINLAYSON, B.J. & VERRUE, K.M. (1985) Toxicities of
butoxyethanol ester and propylene glycol butyl ether ester formulations
of 2,4-dichlorophenoxy acetic acid (2,4-D) to juvenile salmonids.
Arch. environ. Contam. Toxicol., 14: 153-160.
FLINT, G.W., ALEXANDER, J.J., & FUNDERBURK, O.P. (1968)
Vapor pressures of low-volatile esters of 2,4-D. Weed Sci., 16: 541-
544.
FOLMAR, L.C. (1976) Overt avoidance reaction of rainbow trout fry to
nine herbicides. Bull. environ. Contam. Toxicol., 15: 509-514.
FOLMAR, L.C. (1978) Avoidance chamber responses of mayfly nymphs
exposed to eight herbicides. Bull. environ. Contam. Toxicol., 19: 312-
318.
FOURNIER, J.C. (1980) Enumeration of the soil micro-organisms able
to degrade 2,4-D by metabolism or co-metabolism. Chemosphere, 9: 169-
174.
FREITAG, D., GEYER, H., KRAUS, A., VISWANATHAN, R., KOTZIAS,
D., ATTAR, A., KLEIN, W., & KORTE, F. (1982) Ecotoxicological profile
analysis. VII, Screening chemicals for their environmental behaviour
by comparative evaluation. Ecotoxicol. environ. Saf., 6: 60-81.
GALL, A. & DOGGER, J.R. (1967) Effect of 2,4-D on the wheat stem
sawfly. J. econ. Entomol., 60: 75-77.
GANGAWANE, L.V., SALER, R.S., & KULKARNI, L. (1980) Effect
of pesticides on growth and heterocyst formation in Nostoc sp.
Marathwada Univ. J. Sci., 19B: 3-6.
GEORGE, J.P., HINGORANI, H.G., & RAO, K.S. (1982) Herbicide
toxicity to fish-food organisms. Environ. Pollut., 28: 183-188.
GILE, J.D. (1983) 2,4-D - its distribution and effects in a ryegrass
ecosystem. J. environ. Qual., 12: 406-412.
GLYNN, P.W., HOWARD, L.S., CORCORAN, E., & FREAY, A.D.
(1984) The occurrence and toxicity of herbicides in reef-building
corals. Mar. Pollut. Bull., 15: 370-374.
GRAHAM-BRYCE, I.J. (1972) Herbicide movement and availability in
soils. Proc. Br. Weed Control Conf., 11: 1193-1202.
GROLLEAU, G., DE LAVAUR, E., & SIOU, G. (1974) Effets du
2,4-D sur la reproduction des cailles et des perdrix, aprčs application
du produit par pulvérisation sur les oeufs. Ann. Zool. Ecol. anim.,
6: 313-331.
GUARINO, A.M., JAMES, M.O., & BEND, J.R. (1977) Fate and
distribution of the herbicides 2,4-dichlorophenoxyacetic acid (2,4-D)
and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in the dogfish shark.
Xenobiotica, 7: 623-631.
GYRD-HANSEN, N. & DALGAARD-MIKKELSEN, S. (1974) The
effect of phenoxy-herbicides on the hatchability of eggs and the
viability of the chicks. Acta pharmacol. toxicol., 35: 300-308.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
coturnix. Bull. environ. Contam. Toxicol., 11: 98-102.
HAGENMAIER, H. (1986) Determination of 2,3,7,8-tetrachlorodibenzo-p-
dioxin in commercial chlorophenols and related products. Fresenius Z.
anal. Chem., 325: 603-606.
HANSEN, D.J., SCHIMMEL, S.C., & KELTNER, J.M. (1973)
Avoidance of pesticides by grass shrimp (Palaemonetes pugio). Bull.
environ. Contam. Toxicol., 9: 129-133.
HAQUE, A. & EBING, W. (1983) Uptake, accumulation, and
elimination of HCB and 2,4-D by the terrestrial slug, Deroceras
reticulatum (Muller). Bull. environ. Contam. Toxicol., 31: 727-733.
HASHIMOTO & NISHIUCHI (1978) quoted by Hidaka et al. (1984).
HAWXBY, K., TUBEA, B., OWNBY, J., & BASLER, E. (1977) Effects
of various classes of herbicides on four species of algae. Pestic.
Biochem. Physiol., 7: 203-209.
HIDAKA, H., HATTANDA, M., & TATSUKAWA, R. (1984)
[Avoidance of pesticides with medakas (Oryzias latipes).] Nippon
Nogeikagaku Kaishi, 58: 145-151 (in Japanese).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976a) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (First report).] Anz. schädlingskd. Pflanzenschutz Umweltschutz,
49: 21-25 (in German).
HILBIG, V., LUCAS, K., & SEBEK, V. (1976b) [Studies on the
susceptibility of eggs of gallinaceous birds to 2,4,5-T and
2,4-D (Second report).] Anz. schädlingskd. Pflanzenschutz
Umweltschutz, 49: 65-68 (in German).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish Wildlife Service,
61 pp. (Special Science Report, Wildlife No. 191).
HILTIBRAN, R.C. (1967) Effects of some herbicides on fertilized fish
eggs and fry. Trans. Am. Fish. Soc., 96: 414-416.
HILTIBRAN, R.C. (1975) Oxygen and phosphate metabolism of bluegill
liver mitochondria in the presence of some pesticides. Environ. Qual.
Saf. (Suppl), 3: 549-553.
HOEPPEL, R.E. & WESTERDAHL, H.E. (1983) Dissipation of 2,4-D
DMA and BEE from water, mud and fish at Lake Seminole, Georgia.
Water Resour. Bull., 19: 197-204.
HOFFMAN, D.J. & ALBERS, P.H. (1984) Evaluation of potential
embryotoxicity and teratogenicity of 42 herbicides, insecticides and
petroleum contaminants to mallard eggs. Arch. environ. Contam.
Toxicol., 13: 15-27.
HUBER, S.J., POSCHENREIDER, G., & WALLNOFER, P.R. (1980)
[Effect of pesticides and the corresponding metabolites on growth and
respiration of some soil microorganisms.] Z. Pflanzenkr.
Pflanzenschutz, 87: 533-545 (in German).
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook
of toxicity of pesticides to wildlife, 2nd edition, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 90 (Resource
Publication 153).
HUGHES, J.S. & DAVIS, J.T. (1963) Variations in toxicity to bluegill
sunfish of phenoxy herbicides. Weeds, 11: 50-53.
JAMES, M.O. (1979) Disposition of 2,4-D, 2,4,5-T, DDA and
phenylacetic acid in the spiny lobster (Panulirus argus).
Pharmacologist, 21: 251.
JANA, B.B. & DE, U.K. (1982) Stimulatory effect of herbicide 2,4-D
on the heterotrophic microbial community in the water of three fish
ponds. Acta microbiol. Acad. Sci. Hung., 29: 77-82.
JENSEN, D.A. & SCHALL, E.D. (1966) Determination of vapor
pressures of some phenoxyacetic herbicides by gas-liquid
chromatography. J. agric. food Chem., 14: 123-125.
JOHNSON, C.R. (1976) Herbicide toxicities in some Australian anurans
and the effect of subacute dosages on temperature tolerance. Zool. J.
Linnean Soc., 59: 79-83.
JOHNSON, C.R. (1978) Herbicide toxicities in the mosquito fish
Gambusia affinis. Proc. R. Soc. Queensland., 89: 25-27.
JOHNSON, D.R. & HANSEN, R.M. (1969) Effects of range treatment
with 2,4-D on rodent populations. J. wildl. Manage., 33: 125-132.
JONES, G.D.E. & CONNELL, J.U. (1954) Studies of the toxicity to
worker honey-bees (Apis mellifera L.) of certain chemicals used in
plant protection. Ann. appl. Biol., 41: 271-279.
KAPOOR, K. & SHARMA V.K. (1980) Effect of certain herbicides on
survival, growth and nitrogen fixation of blue-green alga Anabaena
doliolum Bharadwaja. Z. allg. Mikrobiol., 20: 465-469.
KEITH, J.O., HANSEN, R.M., & WARD, A.L. (1959) Effect of 2,4-D
on abundance and foods of pocket gophers. J. wildl. Manage., 23: 137-
145.
KOPISCHKE, E.D. (1972) Effect of 2,4-D and diesel fuel on egg
hatchability. J. wildl. Manage., 36: 1353-1356.
KOSCHIER, F.J. & PRITCHARD, J.B. (1980) Renal handling of 2,4-D
by dogfish shark (Squalus acanthias). Xenobiotica, 10: 1-6.
LAHR, J., GOEDEJOHAN, N., & EVERTS, J.W. (1987) Side effects of
pesticides on terrestrial arthropods. I. Long-term effects,
geographical variability and effects of cultural practices. In:
Proceedings of the Symposium ``25 Years After Silent Spring'',
November 1987, Amsterdam, Toxicological Society.
LE VAN TO (1984) [Degradation of defoliants 2,4-D and 2,4,5-T by
selected soil microorganisms.] Acta agrar. Silvestria, Ser. agrar.,
23: 225-234 (in Polish).
LEE, R.D. & EVANS, J.O. (1980) The influence of selected herbicides
on the development of Rhinocyllus conicus, an insect used in biocontrol
of musk thistle. Proc. West. Soc. Weed Sci., 33: 104-110.
LEMBI, C.A. & COLERIDGE, S.E. (1975) Selective toxicity of detergents
and herbicides to phytoplankton, West Lafayette, Indiana, Purdue
University, Water Resources Research Center, 71 pp. (Technical Report
No. 71).
LEWIS, D.L., HODSON, R.E., & FREEMAN, L.F. (1984) Effects of
microbial community interactions on transformation rates of xenobiotic
chemicals. Appl. environ. Microbiol., 48: 561-565.
LHOSTE, J. & ROTH, P. (1946) Sur l'action des solutions aqueuses de
2,4-dichlorophénoxyacétate de sodium sur l'évolution des oeufs de Rana
temporaria L. Soc. Biol., 140: 272-273.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides
on decomposition of organic matter and nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LINDEN, E., BENGTSSON, B-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the bleak (Alburnus alburnus)
and the harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LI-TSE OU (1984) 2,4-D degradation and 2,4-D degrading
microorganisms in soils. Soil Sci., 137: 100-107.
LIU, H.W. & LEE, M. (1975) Toxicity of selected pesticides to the bay
mussel Mytilus edulis, Washington, DC, US Department of Commerce,
National Technical Information Service (NTIS Report No. PB-243 221).
LOOS, M.A. (1969) Phenoxyalkanoic acids. In: Kearney, P.C. &
Kaufman, D.D., ed. Degradation of herbicides, New York, Marcel Dekker,
Inc., pp. 1-49.
LUNDHOLM, C.E. & MATHSON, K. (1983) The inhibitory effect of
some chlorinated hydrocarbon pesticides on the ATP-dependent Ca2+
binding of the particulate fraction of the eggshell gland mucosa
cells. Acta pharmacol. toxicol., 52: 390-394.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1972) The action of different
pesticides on the development of bird embryos. Adv. exp. Med. Biol.,
27: 127-150.
LUTZ, H. & LUTZ-OSTERTAG, Y. (1973) Pesticides, tératogénčse et
survie chez les oiseaux. Arch. Anat. Histol. Embryol., 56: 65-78.
LUTZ-OSTERTAG, Y. & LUTZ, H. (1970) Action néfaste de
l'herbicide 2,4-D sur le développement embryonnaire et la fécondité du
gibier ŕ plumes. C. R. Acad. Sci. Paris, Ser. D, 271: 2418-2421.
MATIDA, Y., FURUTA, Y., KUMADA, H., TANAKA, H., YOKOTE,
M., & KIMURA, S. (1975) Effects of some herbicides applied in the
forest to the freshwater fishes and other aquatic organisms - I. Survey
on the effects of aerially applied sodium chlorate and a mixture of
2,4-D and 2,4,5-T on the stream community. Bull. freshwater Fish Res.
Lab. (Tokyo), 25: 41-53.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp. (NTIS Report No. PB-87 188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp. (Resource Publication 160).
MEEHAN, W.R., NORRIS, L.A., & SEARS, H.S. (1974) Toxicity of
various formulations of 2,4-D to salmonids in southeast Alaska. J. Fish
Res. Board Can., 31: 480-485.
METCALF, R.L. & SANBORN, J.R. (1975) Pesticides and
environmental quality in Illinois. Illinois nat. Hist. Surv. Bull.,
31: 381-435.
MORTON, H.L., MOFFETT, J.O., & MACDONALD, R.H. (1972)
Toxicity of herbicides to newly emerged honey bees. Environ.
Entomol., 1: 102-104.
MOUBASHER, A.H., EL-HISSY, F.T., & ABDEL-KADER, M.I.A.
(1981) Selective effects of three herbicides on the fungus flora of
Egyptian soil. Folia microbiol., 26: 37-44.
MOUNT, D.I. & STEPHAN, C.E. (1967) A method for establishing
acceptable toxicant limits for fish - malathion and the butoxyethanol
ester of 2,4-D. Trans. Am. Fish. Soc., 96: 185-193.
MUKHOPADHYAY, S.K. (1980) Effects of herbicides and insecticides
alone and their combinations on soil microflora. Indian J. Weed Sci.,
12: 53-60.
MULLER, G. (1971) [Laboratory studies on effects of herbicides on
Carabidae.] Arch. Pflanzenschutz, 7: 351-364 (in German).
NAGUIB, K., YOUNIS, A.E., & EL-ESSAWY, M.A. (1980) The effects
of 2,4-dichlorophenoxyacetic acid on respiration, nitrogen and
carbohydrate metabolism of Aspergillus terreus Thom. Zbl. Bakteriol.
II. Abt., 135: 252-259.
NESBITT, H.J. & WATSON, J.R. (1980) Degradation of the herbicide
2,4-D in river water. II. The role of suspended sediment, nutrients and
water temperature. Water Res., 14: 1689-1694.
NORRIS, L.A. & GREINER, D. (1967) The degradation of 2,4-D in
forest litter. Bull. environ. Contam. Toxicol., 2: 65-74.
O'CONNOR, G.A., FAIRBANKS, B.C., & DOYLE, E.A. (1981) Effects
of sewage sludge on phenoxy herbicide adsorption and degradation in
soils. J. environ. Qual., 10: 510-515.
OKA, I.N. & PIMENTAL, D. (1976) Herbicide (2,4-D) increases insect
and pathogen pests on corn. Science, 193: 239-240.
OMKAR & SHUKLA, G.S. (1984) Toxicity of the herbicide 2,4-D-Na
to two species of freshwater prawn of the genus Macrobranchium. Acta
hydrochim. hydrobiol., 12: 285-289.
PACHPANDE, R.R. & DAVID, S.B. (1980) Effect of selected herbicides
on the growth of soil alga Chlorococcum infusionum meneghini
(Schrank). Phykos, 19: 138-141.
PALMER, J.S. (1972) Toxicity of 45 organic herbicides to cattle,
sheep, and chickens, Washington, DC, US Department of Agriculture,
Agricultural Research Service, 41 pp. (Production Research Report No.
137).
PALMER, J.S. & RADELEFF, R.D. (1969) The toxicity of some organic
herbicides to cattle, sheep, and chickens. Washington, DC, US
Department of Agriculture, Agricultural Research Service, 26 pp.
(Production Research Report No. 106).
PLUMB, T.R., NORRIS, L.A., & MONTGOMERY, M.L. (1977)
Persistence of 2,4-D and 2,4,5-T in chaparral soil and
vegetation. Bull. environ. Contam. Toxicol., 17: 1-8.
PONS, R. & PUSSARD, M. (1980) Action des herbicides sur les amibes
libres (Rhizopoda, Protozoa). Etude préliminaire. Acta oecol. oegol.
appl., 1: 15-20.
POORMAN, A.E. (1973) Effects of pesticides on Euglena gracilis. I.
Growth studies. Bull. environ. Contam. Toxicol., 10: 25-28.
PRESCOT, L.M. & OLSON, D.L. (1972) Effects of pesticides on the
soil amoeba, Acanthamoeba castellanii. Proc. South Dakota Acad. Sci.,
51: 136-141.
PRESING, M. (1981) On the effects of Dikonirt (sodium salt of 2,4-
dichlorophenoxyacetic acid) on the mortality and reproduction of
Daphnia magna. Hydrobiologia, 83: 511-516.
PRITCHARD, J.B. & JAMES, M.O. (1979) Determinants of the renal
handling of 2,4-dichlorophenoxyacetic acid by winter flounder. J.
Pharmacol. exp. Ther., 208: 280-286.
RAATIKAINEN, M., SILTANEN, H., ROSENBERG, C., RAATIKAINEN,
T., & MUKULA, J. (1979) Herbicide residues in cowberries, bilberries
and lichens in controlled ground spraying experiments on woodland.
Ann. agric. Fenniae, 18: 112-116.
RADOSEVICH, S.R. & WINTERLIN, W.L. (1977) Persistence of 2,4-D
and 2,4,5-T in chaparral vegetation and soil. Weed Sci., 25: 423-425.
RAND, G.M. & BARTHALMUS, G.T. (1980) Use of an unsignalled
avoidance technique to evaluate the effects of the herbicide 2,4-
dichlorophenoxyacetic acid on goldfish, Philadelphia, American Society
for Testing and Materials, pp. 341-353 (Special Technical Publication
707).
RAPOPORT, E.H. & CANGIOLI, G. (1963) Herbicides and the soil
fauna. Pedobiologia, 2: 235-238.
REHWOLDT, R.E., KELLEY, E., & MAHONEY, M. (1977)
Investigations into the acute toxicity and some chronic effects of
selected herbicides and pesticides on several fresh water fish
species. Bull. environ. Contam. Toxicol., 18: 361-365.
RIVIERE, J-L. (1976) Les effets secondaires des pesticides agricoles.
II. Action de quelques herbicides sur la croissance et la fécondité de
Blattella germanica (L.). Ann. Zool. Ecol. anim., 8: 543-550.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3: 67-78.
ROBERTSON, E.B. (1975) The acute toxicity of four herbicides to 0 -
4 hour nauplii of the copepod Cyclops vernalis Fisher, Washington, DC,
US Department of Commerce, National Technical Information Service, 70
pp. (M.S. Thesis, University of Tennessee) (NTIS Report No. PB-269
495).
RODGERS, C.A. & STALLING, D.L. (1972) Dynamics of an ester of
2,4-D in organs of three fish species. Weed Sci., 20: 101-105.
ROGERS, J.G., CAGAN, R.H., & KARE, M.R. (1974) Percutaneous
absorption of several chemicals, some pesticides included, in the red-
winged blackbird. Environ. Physiol. Biochem., 4: 104-111.
ROSENBERG, A. & ALEXANDER, M. (1980) 2,4,5-trichlorophenoxy-
acetic acid (2,4,5-T) decomposition in tropical soil and its co-
metabolism by bacteria in vitro. J. agric. food Chem., 28: 705-709.
ROWE, V.K. & HYMAS, T.A. (1954) Summary of toxicological
information on 2,4-D and 2,4,5-T type herbicides and an evaluation of
the hazards to livestock associated with their use. Am. J. vet.
Res., 15: 622-629.
RUGGIERO, P. & RADOGNA, V.M. (1985) Inhibition of soil humus-
laccase complexes by some phenoxyacetic and s- triazine
herbicides. Soil Biol. Biochem., 17: 309-312.
SANDERS, H.O. (1969) Toxicity of pesticides to the crustacean,
Gammers lacustris. Washington, DC, US Department of the Interior, Fish
and Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 18 pp.
(Technical Paper No. 25).
SANDERS, H.O. (1970a) Toxicities of some herbicides to six species of
freshwater crustaceans. J. Water Pollut. Control Fed., 42: 1544-1550.
SANDERS, H.O. (1970b) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowler's toad Bufo woodhousii
fowleri. Copeia, 1970(2): 246-251.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several pesticides to
two species of cladocerans. Trans. Am. Fish. Soc., 95: 165-169.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol. Oceanogr.,
13: 112-117.
SANDMANN, E.R.I.C. & LOOS, M.A. (1984) Enumeration of
2,4-D-degrading microorganisms in soils and crop plant rhizospheres
using indicator media; high populations associated with sugarcane
(Saccharum officinarum). Chemosphere, 13: 1073-1084.
SARMA, Y.S.R.K. & TRIPATHI, S.N. (1980) Effects of some chemicals
(2,4-dichlorophenoxyacetic acid, coumarin and acenaphthene) on the
green alga, Oedogonium acmandrium Elfving. Phykos, 19: 142-152.
SATTAR, M.A. & PAASIVIRTA, J. (1980) Fate of chlorophenoxyacetic
acids in acid soil. Chemosphere, 9 745-752.
SCHRODER, H. & PILZ, E. (1983) [Effect of herbicides on nitrification
in different soils.] Zbl. Mikrobiol., :138: 223-234 (in German).
SCHRODER, I., MEYER, M., & MUCKE, D. (1970) [Effects of the
herbicides 2,4-D, aminotriazole, atrazine, chlorpropham and
chlorfurenol on nucleic-acid biosynthesis in the ascomycete Neurospora
crassa.] Weed Res., 10: 172-177 (in German).
SCHULTZ, D.P. (1973) Dynamics of a salt of (2,4-dichlorophenoxy)-
acetic acid in fish, water, and hydrosol. J. agric. food Chem., 21:
186-192.
SCHULTZ, D.P. & HARMAN, P.D. (1974) Residues of 2,4-D in pond
waters, mud, and fish, 1971. Pestic. monit. J., 8: 173-179.
SCHULTZ, D.P. & WHITNEY, E.W. (1974) Monitoring 2,4-D residues
at Loxahatchee National Wildlife Refuge. Pestic. monit. J., 7: 146-
152.
SEIBERT, K., FUHR, F., & MITTELSTAEDT, W. (1982) Experiments
on the influence of roots and soils on 2,4-D degradation. Landwirtsch.
Forsch., 35: 5-13.
SHCHERBAKOV, Y.A. & POLUBOYARINOVA, I.V. (1973) The
persistence of 2,4-D butyl ester in water and its accumulation in
aquatic life. In: Andrushaitis, G.P., ed. Experimental water
toxicology, Charlotte, North Carolina, Army Foreign Service and
Technology Center, pp. 19-21 (NTIS Report No. AD-760 400).
SHUKLA, G.S. & OMKAR (1983) Toxicity of 2,4-D-Na salt to fresh
water prawn, Macrobrachium lamarrei (M. Edwards). Comp. Physiol.
Ecol., 8: 282-284.
SIGMON, C. (1979) Oxygen consumption in Lepomis macrochirus
exposed to 2,4-D or 2,4,5-T. Bull. environ. Contam. Toxicol., 21: 826-
830.
SIKKA, M.C., APPLETON, H.T., & GANGSTAD, E.O. (1977) Uptake
and metabolism of dimethylamine salt of 2,4-D acid by fish. J. agric.
food Chem., 25: 1030-1033.
SILTANEN, H., ROSENBERG, C., RAATIKAINEN, M., &
RAATIKAINEN, T. (1981) Triclopyr, glycophosphate, and
phenoxyherbicide residues in cowberries, bilberries and lichen. Bull.
environ. Contam. Toxicol., 27: 731-737.
SINGH, P.K. (1974) Algicidal effect of 2,4-dichlorophenoxy acetic
acid on blue-green alga Cylindrospermum sp. Arch. Microbiol., 97: 69-
72.
SKOKOVA, N.N. (1975) [Effect of the butyl ester of 2,4-D on the
reproductive function of male bank voles.] Nauchn. Osn. Okhr. Prir.,
3: 172-178 (in Russian).
SMITH, G.E. & ISOM, B.G. (1967) Investigation of effects of large-
scale applications of 2,4-D on aquatic fauna and water quality.
Pestic. monit. J., 1: 16-21.
SOLOMON, K.E., DAHLGREN, R.B., & LINDER, R.L. (1973)
Abnormal embryos in eggs of pheasants given 2,4-D or PCB. Proc. South
Dakota Acad. Sci., 52: 95-99.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1974) Effect of
external application of pesticides to the fertile egg on hatching
success and early chick performance. 3. Consequences of combining
2,4-D with picloram and extremes in contamination. Bull. environ.
Contam. Toxicol., 11: 511-516.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978a) Hatching
success and early performance of chicks from eggs sprayed with 2,4-D,
2,4,5-T and picloram at various stages of embryonic development.
Bull. environ. Contam. Toxicol., 20: 289-293.
SOMERS, J.D., MORAN, E.T., & REINHART, B.S. (1978b)
Reproductive success of hens and cockerels originating from eggs
sprayed with 2,4-D, 2,4,5-T and picloram followed by early performance
of their progeny after a comparable in ovo exposure. Bull. environ.
Contam. Toxicol., 20: 111-119.
SPAIN, J.C. & VAN VELD, P.A. (1983) Adaptation of natural microbial
communities to degradation of xenobiotic compounds: Effects of
concentration, exposure time, inoculum and chemical structure. Appl.
environ. Microbiol., 45: 428-435.
SPENCER, S.R. & BARRETT, G.W. (1980) Meadow vole population
responses to vegetational changes resulting from 2,4-D application.
Am. Midl. Nat., 103: 32-46.
SPITTLER, H. (1976) [Experiments on the influence of herbicides on the
embryo development of Phasianus colchicus var. and Coturnix coturnix
japonica.] Z. Jagdwiss., 22: 197-201 (in German).
STALLING, D.L. & HUCKINS, J.N. (1978) Metabolism of 2,4-dichloro-
phenoxyacetic acid (2,4-D) in bluegills and water. J. agric. food
Chem., 26: 447-452.
STATHAM, C.N. & LECH, J.J. (1975) Potentiation of the acute toxicity
of several pesticides and herbicides in trout by carbaryl. Toxicol.
appl. Pharmacol., 34: 83-87.
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., &
ASKAR, A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46: 387-391.
THOMPSON, D.G., STEPHENSON, G.R., SOLOMON, K.R., &
SKEPASTS, A.V. (1984) Persistence of (2,4-dichlorophenoxy) acetic
acid and 2-(2,4-dichlorophenoxy) propionic acid in agricultural and
forest soils of northern and southern Ontario. J. agric. food
Chem., 32: 578-581.
TIETJEN, H.P., HALVORSON, C.H., HEGDAL, P.L., & JOHNSON, A.M.
(1967) 2,4-D herbicide, vegetation, and pocket gopher relationships
Black Mesa, Colorado. Ecology, 48: 634-643.
TIWARI, D.N., PANDEY, A.K., & MISRA, A.K. (1984) Toxicity of
2,4-dichlorophenoxy acetic acid on growth and nitrogen fixation of
blue-green alga Anabaena cylindrica. Pesticides (Bombay), 18: 16-18.
TOOBY, T.E., LUCEY, J., & STOTT, B. (1980) The tolerance of grass
carp Ctenopharyngodon idella Val., to aquatic herbicides. J. Fish
Biol., 16: 591-597.
TORSTENSSON, N.T.L. (1975) Degradation of 2,4-D and MCPA in
soils of low pH. Environ. Qual. Saf. (Suppl.), 3: 262-265.
TOURE, I.M. & STENZ, E. (1977) [The action of selected herbicides on
bacteriophages and Escherichia coli.] Zbl. Bakteriol. Parasitol.
Infektionskr. 132: 163-177 (in German).
TREVORS, J.T. & STARODUB, M.E. (1983) Effect of 2,4-D on
electron transport system (ETS) activity and respiration in soil.
Bull. environ. Contam. Toxicol., 31: 595-598.
TRUMBLE, J.T. & KOK, L.T. (1980) Impact of 2,4-D on
Ceuthorhychidius horridus (Coleoptera: Curculionidae) and their
compatibility for integrated control of Carduus thistles.
Weed Res., 20: 73-75.
TUEY, D.B. & JAMES, M.O. (1980) Compartmental analysis of 2,4-
dichlorophenoxyacetic acid pharmacokinetics in spiny lobster. Fed.
Proc., 39: 525.
VARDIA, H.K. & DURVE, V.S. (1981) The toxicity of 2,4-D to
Cyprinus carpio var. communis in relation to the seasonal variation in
the temperature. Hydrobiologia, 77: 155-159.
VARDIA, H.K., RAO, P.S., & DURVE, V.S. (1984) Sensitivity of toad
larvae to 2,4-D and endosulfan pesticides. Arch. Hydrobiol., 100: 395-
400.
VERMA, D., CHOUHAN, M.S., & PANDEY, A.K. (1984) Effect of
Weedone (a selective 2,4-D herbicide) on neuroendocrine complex
of Puntius ticto (Ham.). J. environ. Biol., 5: 249-254.
WATSON, J.R. (1977) Seasonal variation in the biodegradation of 2,4-D
in river water. Water Res., 11: 153-157.
WELP, G. & BRUMMER, G. (1985) [The Fe(III)-reduction test - a
simple procedure to determine the effects of environmental chemicals on
the microbial activity in soils.] Z. Pflanzenernaehr. Bodenkd.,
148: 10-23 (in German).
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972a) The subacute toxicity
of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid
to chicks. Toxicol. appl. Pharmacol., 21: 348-354.
WHITEHEAD, C.C. & PETTIGREW, R.J. (1972b) The effect of 2,4-
dichlorophenoxyacetic acid on laying hens. Br. poult. Sci., 13: 191-
195.
WHITNEY, E.W., MONTGOMERY, A.B., MARTIN, E.C., &
GANGSTAD, E.O. (1973) The effects of a 2,4-D application on the
biota and water quality in Currituck Sound, North Carolina. Hyacinth
Control J., 11: 13-17.
WHO (1984) Environmental Health Criteria 29: 2,4-dichlorophenoxy-
acetic acid (2,4-D), Geneva, World Health Organization, 151 pp.
WOODWARD, D.F. (1982) Acute toxicity of mixtures of range
management herbicides to cutthroat trout. J. Range Manage., 35: 539-
540.
YOUNG, A.L., THALKEN, C.E., & WARD, W.E. (1975) Studies of the
ecological impact of repetitive aerial applications of herbicides on
the ecosystem of Test Area C-52A, Elgin AFB, Florida. Florida Air Force
Armament Laboratory, Elgin Air Force Base, 126 pp. (Report No. AFATL-
TR-75-142).
