
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 83
DDT AND ITS DERIVATIVES - ENVIRONMENTAL ASPECTS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1989
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154283 7
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DDT AND ITS DERIVATIVES - ENVIRONMENTAL
ASPECTS
1. SUMMARY AND CONCLUSIONS
1.1. Physical and chemical properties
1.2. Uptake, accumulation, and degradation
1.3. Toxicity to microorganisms
1.4. Toxicity to aquatic invertebrates
1.5. Toxicity to fish
1.6. Toxicity to amphibians
1.7. Toxicity to terrestrial invertebrates
1.8. Toxicity to birds
1.9. Toxicity to non-laboratory mammals
2. PHYSICAL AND CHEMICAL PROPERTIES OF DDT AND RELATED COMPOUNDS
3. KINETICS, METABOLISM, BIOTRANSFORMATION, AND BIOACCUMULATION
3.1. Retention in soils and sediments and plant uptake
3.2. Uptake and accumulation by organisms
3.2.1. Plants
3.2.2. Microorganisms
3.2.3. Aquatic invertebrates
3.2.4. Fish
3.2.5. Terrestrial invertebrates
3.2.6. Birds
3.2.7. Mammals
4. TOXICITY TO MICROORGANISMS
4.1. Bacteria and cyanobacteria (blue-green algae)
4.2. Freshwater microorganisms
4.3. Marine microorganisms
4.4. Soil microorganisms
4.5. Fungi
5. TOXICITY TO AQUATIC ORGANISMS
5.1. Aquatic invertebrates
5.1.1. Short-term and long-term toxicity
5.1.2. Physiological effects on aquatic invertebrates
5.2. Fish
5.2.1. Short-term and long-term direct toxicity to fish
5.2.2. Sublethal behavioural effects on fish
5.2.3. Physiological effects on fish
5.2.4. Development of tolerance
5.3. Toxicity to amphibians
6. TOXICITY TO TERRESTRIAL ORGANISMS
6.1. Terrestrial invertebrates
6.2. Birds
6.2.1. Short-term and long-term toxicity to birds
6.2.2. Toxicity to birds' eggs
6.2.3. Reproductive effects on birds
6.2.4. Reproductive hormones and behaviour
6.2.5. Reproductive effects on the male
6.2.6. Effects on the thyroid and adrenal glands in birds
6.2.7. Special studies in birds
6.2.8. Synergism with other compounds in birds
6.3. Non-laboratory mammals
7. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
8. EVALUATION
8.1. Aquatic organisms
8.2. Terrestrial organisms
REFERENCES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR
DDT AND ITS DERIVATIVES - ENVIRONMENTAL ASPECTS
Members
Dr L.A. Albert, Environmental Pollution Programme, National Institute
for Research on Biotic Resources, Xalapa, Mexico
Mr H. Craven, Ecological Effects Branch, Office of Pesticides
Programs, US Environmental Protection Agency, Washington DC, USA
Dr A.H. El-Sebae, Division of Pesticide Toxicology, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Dr J.W. Everts, Department of Toxicology, Agricultural University,
Wageningen, Netherlands
Dr W. Fabig, Fraunhofer Institute for Environmental Chemistry and
Ecotoxicology, Schmallenberg-Grafschaft, Federal Republic of
Germany
Dr R. Koch, Division of Toxicology, Research Institute for Hygiene and
Microbiology, Bad Elster, German Democratic Republic (Chairman)
Dr Y. Kurokawa, Division of Toxicology, Biological Safety Research
Centre, National Institute of Hygienic Sciences, Tokyo, Japan
Dr E.D. Magallona, Pesticide Toxicology and Chemistry Laboratory,
University of the Philippines at Los Baños, College of Agriculture,
Laguna, Philippines
Professor P.N. Viswanathan, Ecotoxicology Section, Industrial
Toxicology Research Centre, Lucknow, India
Observers
---------
Dr M.A.S. Burton, Monitoring and Assessment Research Centre, London,
United Kingdom
Dr I. Newton, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
Secretariat
-----------
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom ( Rapporteur )
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary )
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR DDT AND ITS DERIVATIVES -ENVIRONMENTAL
ASPECTS
A WHO Task Group on Environmental Health Criteria for DDT and its
Derivatives - Environmental Aspects met at the Institute of Terrestrial
Ecology, Monks Wood, United Kingdon, from 14 to 18 December 1987. Dr. I.
Newton welcomed the participants on behalf of the three co-sponsoring
organizations of the IPCS (ILO/UNEP/WHO). The Task Group reviewed and
revised the draft criteria document and made an evaluation of the risks
for the environment from exposure to DDT and its derivatives.
The first draft of this document was prepared by Dr. S. Dobson and
Mr. P.D. Howe, Institute of Terrestrial Ecology. Dr. M. Gilbert and Dr.
P.G. Jenkins, both members of the IPCS Central Unit, were responsible for
the overall scientific content and editing, respectively.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of Health
and Human Services, through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA - a WHO Collaborating Centre for Environmental Health Effects.
INTRODUCTION
There is a fundamental difference in approach between the
toxicologist and the ecotoxicologist concerning the appraisal of the
potential threat posed by chemicals. The toxicologist, because his
concern is with human health and welfare, is preoccupied with any
adverse effects on individuals, whether or not they have ultimate
effects on performance or survival. The ecotoxicologist, in contrast,
is concerned primarily with the maintenance of population levels of
organisms in the environment. In toxicity tests, he is interested in
effects on the performance of individuals - in their reproduction and
survival - only insofar as these might ultimately affect the population
size. To him, minor biochemical and physiological effects of toxicants
are irrelevant if they do not, in turn, affect reproduction, growth, or
survival.
It is the aim of this document to take the ecotoxicologist's point
of view and consider effects on populations of organisms in the
environment. The risk to human health of the use of DDT was evaluated
in Environmental Health Criteria 9: DDT and its Derivatives (WHO,
1979). This document did not consider effects on organisms in the
environment, but did consider environmental levels of DDT likely to
arise from recommended uses. No attempt has been made here to reassess
the human health risk; the interested reader should refer to the
original document, which contains the relevant literature in this
area.
This document, although based on a thorough survey of the
literature, is not intended to be exhaustive in the material included.
In order to keep the document concise, only those data which were
considered to be essential in the evaluation of the risk posed by DDT
to the environment have been included.
The term bioaccumulation indicates that organisms take up chemicals
to a greater concentration than that found in their environment or
their food. 'Bioconcentration factor' is a quantitative way of
expressing bioaccumulation: the ratio of the concentration of the
chemical in the organism to the concentration of the chemical in the
environment or food. Biomagnification refers, in this document, to the
progressive accumulation of chemicals along a food chain.
1. SUMMARY AND CONCLUSIONS
1.1 Physical and Chemical Properties
DDT is an organochlorine insecticide which is a white crystalline
solid, tasteless and almost odourless. Technical DDT, which is
principally the p,p' isomer, has been formulated in almost every
conceivable form.
1.2 Uptake, Accumulation, and Degradation
The physicochemical properties of DDT and its metabolites enable
these compounds to be taken up readily by organisms. High lipid
solubility and low water solubility lead to the retention of DDT and
its stable metabolites in fatty tissue. The rates of accumulation into
organisms vary with the species, with the duration and concentration of
exposure, and with environmental conditions. The high retention of DDT
metabolites means that toxic effects can occur in organisms remote in
time and geographical area from the point of exposure.
These compounds are resistant to breakdown and are readily adsorbed
to sediments and soils that can act both as sinks and as long-term
sources of exposure (e.g., for soil organisms).
Organisms can accumulate these chemicals from the surrounding
medium and from food. In aquatic organisms, uptake from the water is
generally more important, whereas, in terrestrial fauna, food provides
the major source.
In general, organisms at higher trophic levels tend to contain more
DDT-type compounds than those at lower trophic levels.
Such compounds can be transported around the world in the bodies of
migrant animals and in ocean and air currents.
1.3 Toxicity to Microorganisms
Aquatic microorganisms are more sensitive than terrestrial ones to
DDT.
An environmental exposure concentration of 0.1 µg/litre can cause
inhibition of growth and photosynthesis in green algae.
Repeated applications of DDT can lead to the development of
tolerance in some microorganisms.
There is no information concerning the effects on species
composition of microorganism communities. Therefore, it is difficult
to extrapolate the relevance of single-culture studies to aquatic or
terrestrial ecosystems. However, since microorganisms are basic in
food chains, adverse effects on their populations would influence
ecosystems. Thus, DDT and its metabolites should be regarded as a
major environmental hazard.
1.4 Toxicity to Aquatic Invertebrates
Both the acute and long-term toxicities of DDT vary between species
of aquatic invertebrates. Early developmental stages are more
sensitive than adults to DDT. Long-term effects occur after exposure
to concentrations ten to a hundred times lower than those causing
short-term effects.
DDT is highly toxic, in acute exposure, to aquatic invertebrates at
concentrations as low as 0.3 µg/litre. Toxic effects include impair-
ment of reproduction and development, cardiovascular modifications, and
neurological changes. Daphnia reproduction is adversely affected by
DDT at 0.5 µg/litre.
The influence of environmental variables (such as temperature,
water hardness, etc.) is documented but the mechanism is not fully
understood. In contrast to the data on DDT, there is little
information on the metabolites DDE or TDE. The reversibility of some
effects, once exposure ceases, and the development of resistance have
been reported.
1.5 Toxicity to Fish
DDT is highly toxic to fish; the 96-h LC50s reported (static
tests) range from 1.5 to 56 µg/litre (for largemouth bass and guppy,
respectively). Smaller fish are more susceptible than larger ones of
the same species. An increase in temperature decreases the toxicity of
DDT to fish.
The behaviour of fish is influenced by DDT. Goldfish exposed to
1 µg/litre exhibit hyperactivity. Changes in the feeding of young
fish are caused by DDT levels commonly found in nature, and effects on
temperature preference have been reported.
Residue levels of > 2.4 mg/kg in eggs of the winter flounder result
in abnormal embryos in the laboratory, and comparable residue levels
have been found to relate to the death of lake trout fry in the wild.
Cellular respiration may be the main toxic target of DDT since
there are reports of effects on ATPase.
The toxicity of TDE and DDE has been less studied than that of DDT.
However, the data available on rainbow trout and bluegill sunfish show
that TDE and DDE are both less toxic than DDT.
1.6 Toxicity to Amphibians
The toxicity of DDT and its metabolites to amphibians varies from
species to species; although only a few data are available, amphibian
larvae seem to be more sensitive than adults to DDT. TDE seems to be
more toxic than DDT to amphibians, but there are no data available for
DDE. All the studies reported have been static tests and, therefore,
results should be treated with caution.
1.7 Toxicity to Terrestrial Invertebrates
There have been few reports on the effects of DDT and its
metabolites on non-target terrestrial invertebrates.
Earthworms are insensitive to the acutely toxic effects of these
compounds at levels higher than those likely to be found in the
environment. The uptake of DDT by earthworms is related to the
concentrations in soil and to the activity of the worms; seasonally
greater activity increases uptake. Thus, although earthworms are
unlikely to be seriously affected by DDT, they pose a major hazard to
predators because of the residues they can tolerate.
Both DDT and DDE are classified as being relatively non-toxic to
honey bees, with a topical LD50 of 27 µg/bee.
There are no reports on laboratory studies using DDE or TDE, in
spite of the fact that these are major contaminants of soil.
1.8 Toxicity to Birds
DDT and its metabolites can lower the reproductive rate of birds by
causing eggshell thinning (which leads to egg breakage) and by causing
embryo deaths. However, different groups of birds vary greatly in
their sensitivity to these chemicals; predatory birds are extremely
sensitive and, in the wild, often show marked shell thinning, whilst
gallinaceous birds are relatively insensitive. Because of the
difficulties of breeding birds of prey in captivity, most of the
experimental work has been done with insensitive species, which have
often shown little or no shell thinning. The few studies on more
sensitive species have shown shell thinning at levels similar to those
found in the wild. The lowest dietary concentration of DDT reported to
cause shell thinning experimentally was 0.6 mg/kg for the black duck.
The mechanism of shell thinning is not fully understood.
1.9 Toxicity to non-laboratory Mammals
Experimental work suggests that some species, notably bats, may
have been affected by DDT and its metabolites. Species which show
marked seasonal cycles in fat content are most vulnerable, but few
experimental studies on such species have been made. In contrast to
the situation in birds, where the main effect of DDT is on
reproduction, the main known effect in mammals is to increase the
mortality of migrating adults. The lowest acute dose which kills
American big brown bats is 20 mg/kg. Bats collected from the wild (and
containing residues of DDE in fat) die after experimental starvation,
which simulates loss of fat during migration.
2. PHYSICAL AND CHEMICAL PROPERTIES OF DDT AND RELATED COMPOUNDS
The term DDT is generally understood throughout the world and
refers to p,p' -DDT (1,1 -[2,2,2-trichloroethylidine]-bis [4-chloro-
benzene]). The compound's structure permits several different isomeric
forms, such as o,p' -DDT (1-chloro-2-[2,2,2-trichloro-1-(4-chloro-
phenyl) ethyl] benzene). The term DDT is also applied to commercial
products consisting predominantly of p,p' -DDT with smaller amounts of
other compounds. A typical example of technical DDT had the following
constituents: p,p' -DDT, 77.1%; o,p' -DDT, 14.9%; p,p' -TDE, 0.3%;
o,p' -TDE, 0.1%; p,p' -DDE, 4%; o,p' -DDE, 0.1%; and unidentified
products, 3.5%.
All isomers of the compound DDT are white, crystalline,
tasteless, almost odourless solids, with the empirical formula
C14H9Cl5 and a relative molecular mass of 354.5. The melting
range of p,p' -DDT is 108.5 to 109 °C and its vapour pressure is
2.53 x 10-5 Pa (1.9 x 10-7 mmHg) at 20 °C. DDT is soluble in
organic solvents as follows (g/100 ml): benzene, 106; cyclohexanone,
100; chloroform, 96; petroleum solvents, 4-10; ethanol, 1.5. It is
highly insoluble in water (solubility approximately 1 µg/litre) but
very soluble in animal fats. The octanol-water partition coefficient
(log kow) is 7.48
The chemical structure of some of the analogues of DDT is shown in
Table 1. The structure of the o,p' - and m,p' -compounds can be
inferred from those of the p,p' -isomers presented in the table. The
table is confined to compounds that occur in commercial DDT,
metabolites formed from them, and analogues that have had some use as
insecticides. It must be emphasized that even the commercially-
available insecticidal analogues have strikingly different properties.
Especially remarkable is the slow metabolism and marked storage of DDT
and its metabolite DDE and the rapid metabolism and negligible storage
of methoxychlor.
Technical DDT has been formulated in almost every conceivable form
including solutions in xylene or petroleum distillates, emulsifiable
concentrates, water-wettable powders, granules, aerosols, smoke
candles, charges for vaporizers and lotions. Aerosols and other
household formulations are often combined with synergized pyrethroids.
This is a summary of part of the relevant section from
Environmental Health Criteria 9: DDT and its Derivatives (WHO, 1979).
Further details, including information on analysis, sources of
pollution, and environmental distribution can be found in this
document.
Table 1. Structure of p,p' -DDT and its analogues of the form:
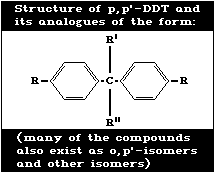 ------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chloroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethlyene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Some related insecticides
NO2
Bulan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-a-(4-chlorophenyl)- -Cl -OH -CH3
a-(methyl)benzenemethanol
dicocol 4-chloro-a-(4-chlorophenyl)-a- -Cl -OH -CCl3
(Kelthane(r)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-a-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
a-hydroxybenzeneacetate
chloropropopylatec 1-methylethyl 4-chloro-a- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-a-hydroxy-
benzeneacetate
Table 1. Structure of p,p' -DDT and its analogues of the form (continued)
------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Perthane(r) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
------------------------------------------------------------------------------------
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been
sold under the name Rothane(r); in metabloic studies the same compound has been
referred as DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
(r) Registered.
3. KINETICS, METABOLISM, BIOTRANSFORMATION, AND BIOACCUMULATION
Appraisal
The physicochemical properties of DDT and its metabolites enable
these compounds to be taken up readily by organisms. The rates of
accumulation vary with the species, with the duration and concentration
of exposure, and with environmental conditions.
These compounds are resistant to breakdown and are readily adsorbed
to sediments and soils, which can act both as sinks and as long-term
sources of exposure (e.g., for soil organisms).
Organisms can accumulate these chemicals from the surrounding
medium and from food. In aquatic organisms, uptake from the water is
generally more important, whereas, in terrestrial fauna, food provides
the major source.
In general, organisms at higher trophic levels tend to contain more
DDT-type compounds than those at lower trophic levels.
Such compounds can be transported around the world in the bodies of
migrant animals and in ocean and air currents.
Different organisms metabolise DDT via different pathways. Of the
two initial metabolites, DDE is the more persistent, though not all
organisms produce DDE from DDT. The alternative route of metabolism,
via TDE leads to more rapid elimination (WHO, 1979). Much of the
retained DDT and its metabolites are stored in lipid-rich tissues.
Because there is an annual cycle in lipid storage and utilization in
many organisms, there is also a related annual cyclic pattern in the
handling of DDT.
3.1 Retention in Soils and Sediments and Plant Uptake
Shin et al. (1970) investigated the adsorption of DDT by soils of
various different types and by isolated soil fractions. A sandy loam,
a clay soil, and a highly organic muck were either used intact or had
various components extracted before estimating their adsorptive
capacity for the insecticide. Adsorption was least in the sandy loam
and greatest in the muck (distribution coefficients [Kd] were in the
ratio 1:10:80 for sandy loam, clay soil, and organic muck,
respectively). All soils showed a strong adsorptive capacity for DDT.
The adsorption of DDT was closely related to the organic matter content
of the soils; progressive removal of lipids, resins, polysaccharides,
polyuronides, and humic matter identified the organic fractions which
bound the DDT. Humic material represents a major source of adsorptive
capacity for DDT; the degree of sorption, however, is strongly
connected with the degree of humification. Soil containing large
amounts of humic material may not adsorb DDT as greatly as other soils
where humification is more advanced. Wheatley (1965) estimated half-
times for the loss of DDT applied to soils. After surface application,
50% of DDT was lost within 16-20 days. The estimated time for the loss
of 90% of surface-applied DDT was 1.5 to 2 years. With DDT mixed into
the soil, 50% loss occurred in 5 to 8 years, and it was estimated that
90% of applied insecticide would be lost in 25-40 years.
Albone et al. (1972) investigated the capacity of river
sediments, from the Severn Estuary, United Kingdom, to degrade DDT.
p,p' -DDT (14C-labelled) was applied to sediments either in situ on
the mud flats or in the laboratory. Sediment movement in the area of
the in situ study was sufficiently small to neither bury nor expose
the incubation tubes set into the mud. Incubation in situ over 46
days led to very little metabolism of DDT in the sediments. Some
p,p' -TDE was produced, but the ratio of DDT to TDE was 13 : 1 and
48 : 1 in two replicate experiments. There was no production of
extractable polar products; metabolism beyond TDE did not occur.
Incubation of the same sediments in the laboratory, over 21 days, led
to much greater metabolism (ratios of 1 : 1.1 and 1 : 3.3, DDT to TDE,
in replicate incubations) and the production of some unidentified,
further breakdown products. Investigation of the microbial population
of the sediment showed that some of the organisms were capable of
degrading DDT; little metabolism appeared to take place in situ .
3.2 Uptake and Accumulation by Organisms
The uptake and accumulation of DDT and its metabolites into
organisms, as determined in controlled laboratory experiments, is
summarized in Table 2. Results are expressed as bioconcentration
factors (the ratio of the concentration of the compound in the organism
to the concentration in the medium).
Concentration factors can be misleading with compounds such as DDT
when exposure is high. The compound is readily taken up and retained
at very low concentrations. At high concentrations, no more material
can be taken up because a plateau has been reached. The only
meaningful way to assess the capacity of organisms to take up and
retain DDT is by looking over a wide range of exposure levels. The
low concentration factor quoted in Table 2 for earthworms, for example,
reflects the high exposure rather than a low capacity for uptake and
retention of DDT, because concentration factors are simple ratios
between "exposure" and final concentration in the organism.
Concentration factors for fish are generally higher than for their
invertebrate prey (Table 2). It is now generally agreed that most of
the DDT taken into aquatic organisms comes from the water rather than
from their food (Moriarty, 1975). Again, the concentration factors can
be misleading. Aquatic organisms take in a small proportion of
ingested DDT. However, they retain a large proportion of the DDT which
has been absorbed into the body from the food. There has been some
controversy in the past over explanations for higher accumulations of
DDT at higher trophic levels in aquatic systems. It now seems clear
that this is not due primarily to biomagnification up food chains but
rather to a tendency for organisms at higher trophic levels to
accumulate more DDT directly from the water.
Terrestrial organisms do not live in a uniform medium surrounded by
a relatively constant concentration of a chemical. Even soil organisms
live in a medium with very variable concentrations of DDT or its
metabolites at different levels of the soil profile or patchy distri-
bution of the chemical. Some terrestrial organisms could be directly
exposed to DDT during application of the insecticide, but most will be
exposed to what remains of the DDT after application. Therefore,
higher terrestrial organisms will accumulate DDT mostly from their
food. The data in Table 2 are taken from controlled laboratory
investigations. There is ample evidence from the field that DDT does
accumulate in many organisms in different media. There is similarly
evidence that the residues of DDT or its metabolites persist in
organisms for long periods after exposure has ceased. The following
should not be regarded as a comprehensive review of the literature on
this subject, which is too large to be included. Rather, these are
examples from different groups of organisms.
Table 2. Bioaccumulation of DDTa
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Bacteria
Aerobacter aerogenes 100 22 24 h 1.2 3736 Johnson &
Bacillus subtilis 130 22 24 h 0.676 4303 & Kennedy
Aerobacter aerogenes 25 22 4 h 0.64 10 639 (1973)
200 22 4 h 0.64 1784 Johnson &
Bacillus subtilis 43 22 4 h 0.64 13 880 Kennedy
348 22 4 h 0.64 1805 (1973)
Marine algae
Cyclotella nana 17 23 2 h 0.7 37 600 Rice & Sikka
8 23 2 h 0.7 58 100 (1973)
Isochrysis galbane 39 23 2 h 0.7 11 300 Rice & Sikka
19 23 2 h 0.7 28 800 (1973)
Olisthodiscus luteus 108 23 2 h 0.7 4600 Rice & Sikka
54 23 2 h 0.7 7000 (1973)
Amphidinium carteri 66 23 2 h 0.7 4300 Rice & Sikka
33 23 2 h 0.7 9600 (1973)
Tetraselmis chuii 106 23 2 h 0.7 5200 Rice & Sikka
53 23 2 h 0.7 6300 (1973)
Skeletonema costatum 29 23 2 h 0.7 31 900 Rice & Sikka
15 23 2 h 0.7 38 400 (1973)
Diatom
Cylindrotheca 21 days 100 300 Keil & Priester
closterium (1969)
Pond snail stat 6 days 3.0 6000 Reinbold et al.
(Physa 5 sp.) (1971)
Freshwater mussel flow 20 3 weeks 0.62 3990d Bedford & Zabik
(Anodonta grandis) (1973)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Earthworm 10 4 weeks 17 000 0.47d Davis (1971)
(Lumbricus terrestris)
Water flea stat 30 3 days 2.0 1330 Metcalf et al.
(1973)
(Daphnia magna) flow 21 3 days 0.08 114 100 Johnson et al.
(1971)
Scud flow 21 3 days 0.081 20 600 Johnson et al.
(Gammarus fasciatus) (1971)
Glass shrimp flow 21 3 days 0.1 5000 Johnson et al.
(Palaemonetes kadiakensis) (1974)
Pink shrimp flow 8-15 13 days 0.14 1500 Nimmo et al.
(Penaeus duorarum) (1970)
Crayfish flow 21 3 days 0.08 2900 Johnson et al.
(Orconectes nais) (1971)
Mayfly larva flow 21 3 days 0.052 32 600 Johnson et al.
(Hexagenia bilineata) (1971)
Mayfly larva flow 21 3 days 0.047 22 900 Johnson et al.
(Siphlonurus sp.) (1971)
Dragonfly nymph flow 21 2 days 0.101 3500 Johnson et al.
(Ischnura verticalis) (1971)
Dragonfly nymph flow 21 2 days 0.079 910 Johnson et al.
(Libellula sp.) (1971)
Midge larva flow 21 3 days 0.046 47 800 Johnson et al.
(Chironomus sp.) (1971)
Mosquito larva flow 21 2 days 0.105 133 600 Johnson et al.
(Culex pipiens) (1971)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Mosquito larva stat 30 3 days 2.0 110d Metcalf et al.
(Culex quinquifasciatus) stat 30 3 days 0.9 74d (1973)
Mosquito fish stat 30 3 days 2.0 344d Metcalf et al.
(Gambusia affinis) stat 30 3 days 0.9 217d (1973)
Rainbow trout flow 5 12 weeks 0.176 21 363d Reinert et al.
(Salmo gairdneri) flow 10 12 weeks 0.137 43 158d (1974)
flow 15 12 weeks 0.133 51 355d Reinert et al.
(1974)
Brook trout flow 14 120 days 3 mg 0.64d Macek & Korn
(Salvelinus fontinalis) /kg diet (1970)
flow 14 120 days 0.003 8533d Macek & Korn
(1970)
Pinfish flow 14 days 0.1 40 000d Hansen & Wilson
(Lagodon rhomboides) flow 14 days 1.0 11 020d (1970)
Atlantic croaker flow 14 days 0.1 12 500d Hansen & Wilson
(Micropogon undulatus) flow 14 days 1.0 12 170d (1970)
Fathead minnow flow 24-25.5 14 days 45.6 mg/kg 1.17d Jarvinen et al.
(Pimephales promelas) flow 24-25.5 14 days 0.5 85 400d (1977)
flow 24-25.5 14 days 2.0 69 100d Jarvinen et al.
flow 24-25.5 112 days 45.6 mg/kg 1.33d (1977)
flow 24-25.5 112 days 0.5 93 200d Jarvinen et al.
flow 24-25.5 112 days 2.0 154 100d (1977)
Tilapia stat 31 days 1.0 6800 Reinbold et al.
(Tilapia mossambica) 31 days 10 10 600 (1971)
Green sunfish stat 31 days 1.0 3900 Reinbold et al.
(Lepomis cyanellus) 31 days 10 4020 (1971)
stat 22 15 days 0.1-0.3 17 500d Sanborn et al.
(1975)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Chicken eggs 8 weeks 0.1 1.87d Foster et al.
fat 8 weeks 0.1 5.8d (1972)
Broiler hen fat 6 weeks 1.0 10.3d Kan et al.
(1978)
White pelican WB 10 weeks 72 11.9d Greichus et al.
(Pelecanus erythrorhynchos) (1975)
Double-crested cormorant WB 9 weeks 0.95 236.3d Greichus &
(Phalacrocorax a. auritus) Hannon (1973)
American kestrel WB 11-16 2.8 103.9 Porter &
(Falco sparverius) months Wiemeyer (1972)
Mule deere muscle 30 days 5 mg/day 122.8 ug Watson et al.
(Odocoileus heminonus) oral /kgd (1975)
---------------------------------------------------------------------------------------------------------
a Unless specified otherwise, bioconcentration factors are based on whole body (WB) measurements.
b Stat = static conditions (water unchanged for duration of experiment);
Flow = flow-through conditions (DDT concentration in water continuously maintained).
c Bioconcentration factor = concentration of DDT in organism/concentration of DDT in medium or food.
Concentrations of DDT in organisms represents total DDT, i.e., DDT plus its stable metabolites,
principally DDE. Bioconcentration factors calculated on a dry weight basis unless otherwise stated.
d Calculated on a wet weight basis.
e Oral dose (by capsule) given daily.
3.2.1 Plants
Fuhremann & Lichtenstein (1980) applied 14C-labelled p,p' -DDT to
loam or sandy soil (at 4 and 2 mg/kg, respectively) and grew oat plants
on the treated soils for 13 days. At harvest, residues of DDT and its
metabolites were analysed in soil and plant by scintillation counting,
thin layer chromatography, and GLC. Of the total applied DDT, 95.7%
was recovered from loam soil and 88.6% from sandy soil. Almost all of
the DDT present was extractable in organic solvent (only 2.8%, for
loam, and 0.7%, for sand, was present in a water-bound form),
indicating little or no metabolism of the compound except to persistent
organically extractable residues. DDE was detected in both soils,
accounting for 3.4% of the total extracted in loam soil and 2.2% in
sand. Other metabolites, including o,p' -DDT, TDE, and dicofol were
recovered in very small quantities. Very little DDT (and none of its
metabolites) was detected in oat roots grown on loam, amounting to 0.2%
of the total DDT applied. The uptake was greater (4.6%) in roots of
oats grown on sand, but the uptake of labelled carbon into plant tops,
from both soils, was so low that it could not be analysed.
DDT was not translocated into the foliage of alfalfa when applied
to the soil (Ware, 1968; Ware et al., 1970) or into soybeans (Eden &
Arthur, 1965). Harris & Sans (1967) found only trace amounts of DDT
or its metabolites in the storage roots of carrots, radishes, and
turnips after growing the plants in soils containing up to 14 mg
DDT/kg.
3.2.2 Microorganisms
The uptake and accumulation of DDT from the culture medium by
microorganisms has been reviewed by Lal & Saxena (1982). All of the
microorganisms studied showed some capacity to take up DDT from their
growth medium, but the relative amount taken up varied greatly from
species to species. Many species took up more than 90% of the DDT when
exposed to concentrations ranging from 1 to 1000 µg/litre, whereas a
few species took in only 0.5% of the available DDT. The concentration
factors (i.e., the concentration within the organism expressed as a
ratio against the concentration in the medium) for DDT were variable
but always high (Table 2).
3.2.3 Aquatic invertebrates
Concentration factors are also variable in aquatic invertebrates.
In all cases there is considerable uptake and retention of the DDT,
though often as DDE or other metabolites rather than as the parent
compound. The main point of interest is the ability of aquatic
organisms to take up large amounts of the compound, over time, from
water where DDT is present at very low concentrations, and to retain
it.
Risebrough et al. (1976) measured DDT in sea water and in mussels
( Mytilus sp.) from San Fransisco Bay and the French Mediterranean
coast. Concentration factors varied between 40 000 and 690 000 for DDT
and between 45 000 and 310 000 for DDE.
Eberhardt et al. (1971) applied radioactively labelled DDT, at a
rate of 220 g/ha, to a freshwater marsh and followed the distribution
of the compound and its metabolites. Concentration factors in ten
species of plants varied between 5500 and 84 000. Various invert-
ebrates showed high concentration factors: ramshorn snail
( Planorbidae ), 4700; backswimmer ( Notonectidae ), 10 000; crayfish
( Orconectes immunis ), 22 000; bloodworm ( Tendipes ), 25 000; and red
leech ( Erpobdella punctata ), 47 000. Reporting earlier on the same
study, over 15 months, Meeks (1968) showed that plants and invert-
ebrates accumulated DDT to a maximum mainly within the first week after
treatment, whereas vertebrates required longer to attain maximum
residues. Residues of DDT in the surface water and suspended particles
had fallen below detectable levels within 1 month. Residues in
sediments stabilized at about 0.3 mg/kg after 9 months.
3.2.4 Fish
The uptake of DDT from water is affected by the size of the fish;
smaller fish take up relatively more DDT from water than larger
specimens of the same species. A range in weight of mosquitofish
between 70 and 1000 mg led to a four-fold difference between the
smallest and largest fish in DDT uptake from water over 48 h (Murphy,
1971).
A rise in temperature results in increased uptake of DDT by fish
(Reinert et al., 1974). Rainbow trout were exposed to a single water
concentration of DDT (nominally 330 ng/litre) at temperatures of 5, 10,
or 15 °C; the actual concentrations of DDT in water varied with
temperature and were measured at 176, 137, and 133 ng/litre,
respectively, for 5, 10, and 15 °C. Whole body residues of DDT (total)
after 12 weeks exposure were 3.8, 5.9, and 6.8 mg/kg for the three
temperatures, respectively. Expressing the results as bioconcentration
factors to allow for the differences in dissolved DDT showed a similar,
clear increase in the relative amount of DDT taken up and retained
(Reinert et al., 1974).
Increasing salinity decreases DDT uptake significantly, but has
no effect on the uptake of DDE or TDE by fish (Murphy, 1970).
Increasing the salinity from 0.15o/oo to 10o/oo decreased DDT
uptake over 24 h from 22% of the dose to 18% (body residues decreased
from 658 to 329 ng). There was a further significant decrease in
uptake when the salinity was increased to 15o/oo (Murphy, 1970)
Fish accumulate DDT from food in a dose-dependent manner. When
Macek et al. (1970) fed rainbow trout on diets containing 0.2 or 1.0 mg
DDT/kg, the fish retained more than 90% of the dietary intake of DDT
(measured as total DDT) over the 90-day exposure period. The authors
estimated the time required for the elimination of 50% of accumulated
DDT to be 160 (± 18) days. When Warlen et al. (1977) fed Atlantic
menhaden on a diet containing 14C-labelled DDT at three dose levels,
the fish assimilated and retained between 17% and 27% of the cumulative
dose from food containing 0.58, 9.0, or 93 µg/kg. There was a
straight-line relationship between exposure time and body burden of
total DDT, with no tendency for residues to reach a plateau within the
45 days of feeding with DDT. At the end of the feeding period, the
fish had accumulated DDT or its metabolites, to levels of approximately
1.1, 11, and 110 µg/kg for the three doses respectively. The
biological half-time of DDT in the fish was estimated to be 428, 64,
and 137 days, for groups exposed to 0.58, 9.0, or 93 µg/kg diet,
respectively.
3.2.5 Terrestrial invertebrates
Relatively low concentration factors have been reported for
terrestrial molluscs by Dindal & Wurtzinger (1971), who also reviewed
the earlier literature. However, low concentration factors, derived
from short-term studies, can be misleading for these organisms because
of the high persistence of DDT in soil. Residues of DDT were as high
as 40 mg/kg and, therefore, molluscs represent a source of DDT which
will be concentrated by organisms which eat them. The same is true
for earthworms, which also show low concentration factors (Davis, 1971;
Edwards & Jeffs, 1974). Gish & Hughes (1982) investigated residues of
DDT and other pesticides in earthworms for 2 years following appli-
cation. They showed that body residue levels were cyclic, with higher
levels of DDT and its metabolites occuring between late spring and
early autumn and lower levels from late autumn to early spring. Peak
high levels occurred in May and low levels in January, coinciding with
the seasonal high and low activity periods of earthworms. These
changing residue levels presumably indicate that DDT is retained in
soil and that earthworms contain more of the residual metabolites when
they are processing more soil through the gut.
3.2.6 Birds
Laboratory studies on birds have shown them capable of accumulating
DDT from food, yielding high concentration factors (Table 2).
The accumulation of DDT and its metabolites in birds in the field
has been regularly and extensively reviewed (Moore, 1965; Moriarty,
1975; Newton, 1979). The results of an analysis of a long-term
sampling programme of birds in the United Kingdom (Cooke et al., 1982)
confirm many of the early theories. Birds with the highest residues of
DDT or its metabolites were either terrestrial predators feeding on
other birds or aquatic predators feeding on fish. Thus, residues of
DDE in the liver of the peregrine falcon, with birds as its principal
dietary component, averaged 7.56 mg/kg, whereas for the rough-legged
buzzard, with mammals as the principal dietary component, mean DDE
levels were 0.05 mg/kg over a period extending from the early 1960s to
the late 1970s.
There are marked geographical differences throughout the United
Kingdom, related to usage patterns of DDT (Cooke et al., 1982), and
also marked seasonal changes in residues. These seasonal changes
appear to relate more to physiological changes in body composition,
which occur with climatic and breeding seasons, than to the environ-
mental availability of pollutants. Some species, e.g., heron, barn
owl, and kingfisher, showed a decline in DDE residues with time, but
others, e.g., sparrowhawk, kestrel, and great-crested grebe, did not,
levels in 1977 being similar to those in 1963. Eventually residues of
DDT in wildlife decline with time after a ban is imposed on the use of
the pesticide. However, the highly persistent nature of DDE means that
significant residues will continue to be found for a considerable
period. The situation in the United Kingdom and the USA appears to be
broadly similar (O'Shea & Ludke, 1979).
3.2.7 Mammals
DDT is taken up by, and retained in, wild mammals. The degree of
uptake and retention varies with the species. In a study following a
single application of DDT to a forest to control spruce budworm at a
rate of 0.89 kg/ha, Dimond & Sherburne (1969) and Sherburne & Dimond
(1969) reported residues of DDT and its metabolites in mammals over 9
years. Herbivorous mice, voles, and hares contained less DDT than
carnivorous mink and insectivorous shrews. In herbivores, residues
approached pre-treatment levels after 6-7 years, whereas residues were
still significantly higher in shrews and mink than in the same species
taken from untreated areas 9 years after the single treatment with DDT.
In these species, the authors calculate that it would take at least 15
years for residues to reach background levels. They regard the high
residue levels in mammals at higher trophic levels as deriving
principally from DDT retained in the soil, since there is little long-
term retention on vegetation.
In a 3-year study, after treating a field ecosystem with 36Cl-
ring-labelled DDT at a dose rate of 0.92 kg/ha, Forsyth & Peterle
(1973) measured DDT residues in various tissues of two species of
shrew. The highest residue (135 mg/kg) occurred in fat, compared with
10, 10, and 4 mg/kg in liver, muscle, and brain, respectively. Shrews
of the species Blarina brevicauda released into treated areas accumu-
lated DDT to the same degree as resident shrews within 15-20 days of
exposure. Equilibrium between intake and excretion of DDT occurred
within approximately 30 days in muscle, liver, and brain and within
40 days in fat. The second species of shrew ( Sorex cinereus )
accumulated residue levels of DDT during the following 2 years which
were successively greater than levels present in the first year,
indicating that DDT was increasing in availability to this species
with the passage of time. The levels of DDT in muscle were not
influenced by sex but were influenced by breeding condition. Male
shrews with scrotal testes and lactating females developed lower
levels of DDT in muscle and viscera than did males with abdominal
testes or non-lactating females.
Benson & Smith (1972) measured levels of DDT and its metabolites in
deer exposed to DDT used for spruce budworm control, and found that, in
the year of spraying, there was up to 20 mg/kg in fat. Males had
considerably higher levels of DDT than females. Fawns also had higher
levels than their mothers, though this was from a small sample. The
majority of the residues consisted of p,p' -DDT, with almost insignifi-
cant levels of DDE. Five years later, the residue levels in males were
still higher than those in females, though these had fallen to about
1% of original levels. Most of the deer population was 3 years old or
less, and so the figures for 5 years after spraying represent DDT
ingested from the environment and not from direct exposure.
Some, though very little, DDT was detected in black bears by
Benson et al. (1974). There was no evidence that the area had been
directly sprayed with DDT. This study illustrates that there is a
general environmental contamination with DDT, which can be accumulated
by mammals, though to a small degree, without direct application of the
material to their habitat.
4. TOXICITY TO MICROORGANISMS
Appraisal
Aquatic microorganisms are more sensitive than terrestrial ones to
DDT.
An environmental exposure concentration of 0.1 µg/litre can cause
inhibition of growth and photosynthesis in green algae.
Repeated applications of DDT can lead to tolerance in some micro-
organisms.
There is no information on effects concerning the species compo-
sition of microorganism communities. Therefore, it is difficult to
extrapolate the relevance of single-culture studies to aquatic or
terrestrial ecosystems. However, since microorganisms are basic in
food chains, adverse effects on their populations would influence
ecosystems. Thus, DDT and its metabolites should be regarded as a
major environmental hazard.
Studies cited in this section will be restricted to those effects
produced by low concentrations of DDT. Some studies still use DDT at
concentrations above its water solubility. Reviews of other effects of
DDT and its analogues, at higher concentrations, on cell division and
several biochemical parameters have been produced by Luard (1973) and
Lal & Saxena (1979).
4.1 Bacteria and Cyanobacteria (Blue-green Algae)
Ledford & Chen (1969) cultured bacteria isolated from surface-
ripened cheese with 0.5 mg DDT/litre or 0.5 mg DDE/litre, but found no
effect on growth.
At a concentration of 10 µg/litre in the culture medium, DDT
stimulated the growth of the bacterium Escherichia coli (Keil et al.,
1972). Yields of cultures exposed to 100 µg/litre did not differ from
controls. There was no effect of DDT on denitrification (conversion of
nitrate to nitrite) at a concentration of 100 mg/kg in soil and,
similarly, no effect on this process when carried out by a bacterial
culture (Bollag & Henninger, 1976). DDT at up to 22 kg/ha did not
affect the numbers of soil bacteria in outdoor-treated plots (Bollen et
al., 1954), and five annual applications of DDT to a sandy loam soil
did not significantly affect the numbers of soil bacteria (Martin,
1966).
Concerning cyanobacteria (blue-green algae), Goulding & Ellis
(1981) found no effect on the growth of Anabaena variabilis at a DDT
concentration of 1 µg/litre. Batterton et al. (1972) suggested that
DDT reduced the tolerance of Anocystis nidulans to sodium chloride.
The organism is resistant to salt and to DDT, at concentrations up to
8000 mg/litre, but not to combinations of the two stressors.
4.2 Freshwater Microorganisms
Lee et al. (1976) showed that DDT inhibited photosynthesis in the
green alga Selenastrum capricornutum at concentrations between 3.6 and
36 µg/litre, inhibition increasing with time of exposure.
Two different species of green algae were shown to be resistant
to DDT and its metabolites, DDE and TDE, at concentrations up to
1000 mg/litre in culture. Scenedesmus and Dunaliella revealed rates of
photosynthetic uptake of 14C-labelled CO2 similar to those of
controls (Luard, 1973). Considerable variation exists between species
of microorganisms concerning the effect of DDT and its analogues;
resistance to DDT is not restricted to one taxonomic group, either
freshwater or marine (Luard, 1973). The source of the resistance is
unclear. The two species studied show very different characteristics;
Dunaliella has no cell wall, whereas Scenedesmus has a complex cell
wall. Since both show resistance to DDT, it is unlikely that the
chemical is excluded from the cell by the cell wall. Cell membranes
and chloroplast membranes are an alternative barrier to DDT uptake and
effect. It is not known how these structures might be involved in DDT
resistance; studies with isolated chloroplasts suggest that there is no
barrier to DDT uptake there.
Cole & Plapp (1974) found inhibition of growth and photosynthesis
of the green alga Chlorella pyrenoidosa with DDT at 1 µg/litre in the
medium. However, inhibition was inversely related to the number of
cells in the culture. With high cell counts, there was no
inhibition of either growth or photosynthesis with DDT present at up to
1 mg/litre. Inhibition only occurred at low cell densities in culture.
Goulding & Ellis (1981) found that the green alga Chlorella fusca
was affected by DDT at 0.1 µg/litre. The amount of inhibition of
growth varied with time and with the method of assessing the result.
Cell numbers were maximally affected (75% inhibition) after 72 hours,
and after 200 hours cell numbers had reached control levels. When
growth was assessed by chlorophyll content or biovolume, the initial
inhibition was more marked and cultures were only equivalent to
controls after 480 hours. The apparent anomaly is explained by
reductions in cell size in response to DDT.
Christie (1969) reported no effect of DDT on the growth of
Chlorella and attributed this to the ability of the organism to
metabolize the compound.
Lal & Saxena (1980) reported that DDT did not affect growth and DNA
synthesis in the ciliate Stylonychia notophora at concentrations of
1 mg/litre or less.
4.3 Marine Microorganisms
MacFarlane et al. (1972) showed that DDT, at concentrations
between 9.4 and 1000 µg/litre, reduced photosynthetic carbon
fixation and the cell content of chlorophyll a relative to controls
in a marine diatom Nitzschia delicatissima , over a 24-h period.
The diatom was cultured with DDT under four different light inten-
sities. The insecticide had the greatest effect at the highest light
intensity, where carbon fixation was reduced by 94% in water containing
100 µg DDT/litre. At higher DDT concentrations, there was no further
reduction in either carbon fixation or chlorophyll content.
The photosynthesis of several species of marine phytoplankton has
been found to be inhibited by DDT at concentrations of 100 µg/litre or
less (Wurster, 1968). Four different species showed increasing
inhibition up to DDT concentrations of 100 µg/litre, but no greater
effect at higher concentrations. A green alga, Pyramimonas , was
affected by DDT only at concentrations higher than 10 µg/litre. The
other three species, a diatom, a coccolithophore, and a dinoflagellate
were affected at DDT concentrations between 1 and 10 µg/litre. In
a similar study (Menzel et al., 1970) four different species of
marine phytoplankton were studied. Inhibition of photosynthesis,
where it occurred, followed a similar dose-response relationship.
For three species ( Skeletonema costatum , a diatom; Coccolithus
huxleyi , a coccolithophorid; and Cyclotella nana , a second diatom)
inhibition began between 1 and 10 µg DDT/litre and reached a maximum
at 100 µg/litre. The other organism, a green flagellate Dunaliella
tertiolecta , was unaffected by DDT at concentrations up to 1 mg/litre,
the highest exposure tested.
The marine dinoflagellate Exuviella baltica showed significant
inhibition of growth after exposure to DDT at concentrations as low as
0.1 µg/litre (Powers et al., 1979).
4.4 Soil Microorganisms
TDE had no significant effects on growth and reproduction of soil
amoebae except at concentrations higher than 1 mg/litre (Prescott &
Olson, 1972). Populations of protozoa in garden soil were reduced by
DDT at a concentration of 5 mg/kg (MacRae & Vinckx, 1973). Numbers
were still significantly reduced after 3 months.
4.5 Fungi
Two aquatic and one terrestrial fungi showed stimulated growth
in response to DDT present at concentrations of between 2 and
60 µg/litre of growth medium (Hodkinson & Dalton, 1973)
5. TOXICITY TO AQUATIC ORGANISMS
DDT and its derivatives are highly toxic to aquatic organisms;
water concentrations of a few micrograms per litre are sufficient to
kill a large proportion of populations of aquatic organisms in acute or
short-term exposure. In addition to its high short-term toxicity, DDT
also has long-term sublethal effects on aquatic organisms. Many
physiological and behavioural parameters have been reported to be
affected by the insecticide. This toxicity, coupled with its high
capacity for bioconcentration and biomagnification, means that DDT
presents a major hazard to aquatic organisms.
5.1 Aquatic Invertebrates
Appraisal
Both the acute and long-term toxicities of DDT vary between species
of aquatic invertebrates. Early developmental stages are more
sensitive than adults to DDT. Long-term effects occur after exposure
to concentrations ten to a hundred times lower than those causing
short-term effects.
DDT is highly toxic, in acute exposure, to aquatic invertebrate, at
concentrations as low as 0.3 µg/litre. Toxic effects include
impairment of reproduction and development, cardiovascular
modifications, and neurological changes. Daphnia reproduction is
adversely affected by DDT at 0.5 µg DDT/litre.
The influence of environmental variables (such as temperature,
water hardness, etc.) is documented but the mechanism is not fully
understood. In contrast to the data on DDT, there is less information
on the metabolites DDE or TDE. The reversibility of some effects once
exposure ceases has been reported, as well as the development of
resistance.
5.1.1 Short-term and long-term toxicity
The short-term toxicity to aquatic invertebrates is summarized in
Table 3.
Most aquatic invertebrates are killed by low water concentrations
of DDT and its metabolites, though the majority of the published data
is on DDT itself. Six invertebrate species studied by Macek & Sanders
(1970) showed 96-h LC50 values ranging from 1.8 to 54.0 µg/ litre.
Adult molluscs are relatively resistant to DDT and the compound has
been used to control crustacean pests on oyster beds (Loosanoff, 1959).
However, the larval stages of molluscs are affected by DDT; clam larvae
showed 90% mortality after exposure to DDT at 0.05 mg/litre (Calabrese,
1972). Molluscs exhibit effects on shell growth at low DDT concen-
trations. Tubifex worms are resistant to DDT; 3 mg/litre did not kill
any Tubifex tubifex (Naqvi & Ferguson, 1968). Many aquatic crustaceans
yield LC50 values less than 1 µg/litre. Muirhead-Thomson (1973)
showed that predator invertebrates, such as dragonfly nymphs, were
more tolerant of DDT than prey organisms. Since the prey organisms
are also food for fish, the balance of aquatic ecosystems could be
changed by very low levels of DDT. Lowe (1965) reported that juvenile
blue crabs ( Callinectes sapidus ), exposed to 0.25 µg DDT/litre for 9
months, grew and moulted normally; there were no apparent sublethal
effects. However, exposure to 5 µg DDT/litre killed all crabs.
The metabolite TDE has been studied in parallel tests with the
parent compound in some organisms. There is no consistent relationship
between the toxicity of the two compounds. TDE is considerably less
toxic to stonefly larvae than DDT, by a factor of about 100 (Sanders &
Cope, 1968). However, for other freshwater organisms TDE may have
similar, lower, or greater toxicity according to the organism and
duration of test (Table 3). For most marine invertebrates, DDT is most
toxic, followed by DDE and TDE (data from Mayer, 1987).
5.1.2 Physiological effects on aquatic invertebrates
Butler (1964) demonstrated a 50% reduction in shell growth in young
eastern oysters exposed for 96-h to DDT at 14 µg/litre. Roberts
(1975) showed that DDT at 50 µg/litre reduced the amplitude of
ventricular contractions in the isolated heart of the bivalve Mya
arenaria within 4 minutes. At higher concentrations, DDT stopped heart
contractions altogether. Recovery, even of the arrested heart, was
rapid after the immediate replacement of the DDT solution with clean
sea water.
Kouyoumjian & Uglow (1974) found that for the planarian worm
Polycelis felina , TDE was most toxic and DDT least toxic, with DDE
showing intermediate toxicity. Sublethal effects of DDT and TDE were
demonstrated. DDT reduced the rate of asexual fission. Both DDT and
TDE were shown to reduce the righting time of animals turned onto their
backs. This was presumed to be a nervous system effect.
Maki & Johnson (1975) report 50% reduction in three parameters of
reproduction in the water flea Daphnia magna at 0.5 µg/litre, for
total young produced, at 0.61 µg/litre for average brood size, and at
0.75 µg/litre for percentage of days reproducing.
In vitro effects on gill ATPases of two species of crab have been
reported (Jowett et al., 1978; Neufeld & Pritchard, 1979). There is a
transitory effect in vivo on gill ATPases and, thereby, an effect on
plasma osmolarity. However, this osmoregulatory effect soon disappears
(Pritchard & Neufeld, 1979). Leffler (1975) reported metabolic rate
elevation, decreased muscular coordination, inhibition of autotomy
reflex, and reduced carapace thickness/width ratio in juvenile crabs
exposed to DDT. Osmoregulation was not affected. The DDT was given in
the food of the crabs at a concentration of 0.8 mg/kg. DDT has been
found to accelerate limb regeneration and the onset of the next moult
in fiddler crabs (Weis & Mantel, 1976). The authors suggest that the
effect is on the central nervous system, with DDT causing changes in
neurosecretory activity.
Table 3. Toxicity of DDT and its derivatives to invertebrates
---------------------------------------------------------------------------------------------------------
Organismf Flow Temp Salinity Compound Parameter Water Reference
stata ( °C) o/oo concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine invertebrates
Eastern oyster (juv.) flow 30 23 DDTd 96-h EC50j 9 Mayer (1987)
(Crassostrea virginica) flow 12 25 DDEd 96-h EC50j 14 Mayer (1987)
flow 20 30 TDEd 96-h EC50j 25 Mayer (1987)
Shrimp stat 20 sea water DDTd 96-h LC50 0.4 McLeese &
(Crangon septemspinosa) stat 10 sea water DDTd 96-h LC50 31 Metcalfe (1980)
+ sediment
Mysid shrimp (adult) stat 25 23 DDTd 96-h LC50 0.45 Mayer (1987)
(Mysidopsis bahia) (0.39-0.52)
Pink shrimp (juv.) flow 24 28 DDTd 48-h LC50 0.6 Mayer (1987)
(Penaeus duorarum) flow 16 31 TDEd 48-h LC50 2.4 Mayer (1987)
White shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.7 Mayer (1987)
(Penaeus setiferus)
Grass shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.8 Mayer (1987)
(Palaemonetes pugio)
Brown shrimp (juv.) flow 28 17-27 DDEd 24-h LC50 52 Butler (1964)
(Penaeus aztecus) flow 28 17-27 DDEd 48-h LC50 28 Butler (1964)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata ( °C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Freshwater invertebrates
Water flea stat 20 192 138 8.2- DDTd 48-h LC50 1.1 (1.0-1.3) Randall et
8.5 al. (1979)
(Daphnia magna) stat 15 44 7.1 DDTd 48-h LC50 4.7 (2.8-5.6) Mayer &
Ellersieck
(1986)
stat 20 192 138 8.2- DDTe 48-h LC50 1.7 (1.5-1.8) Randall et
8.5 al. (1979)
statb 24 320- 7.6 DDT 14-day 0.67 (0.65-0.69) Maki &
340 (99%) LC50 Johnson
statb 24 320- 7.6 DDT 14-day 0.5 (0.48-0.52) (1975)
340 (99%) EC50g
statb 24 320- 7.6 DDT 14-day 0.61 (0.58-0.64) Maki &
340 (99%) EC50h Johnson
statb 24 320- 7.6 DDT 14-day 0.75 (0.71-0.79) (1975)
340 (99%) EC50i
stat 10 44 7.1 TDEd 48-h LC50 9.1 Mayer &
stat 21 44 7.1 TDEd 48-h LC50 8.9 Ellersieck
(1986)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 510 (230-1120) Berglind &
soft water stat 20.5 250 7.8-8.2 DDT 48-h LC50 1.1 (0.89-1.7) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 98 (75-127) Berglind &
50 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 1.3 (1.1-1.5) Dave (1984)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 71 (41-130) Berglind &
hard water stat 20.5 250 7.8-8.2 DDT 48-h LC50 0.68 (0.46-1.0) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 42 (32-56) Berglind &
300 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 0.5 (0.41-0.61) Dave
stat 20.5 50 7.8-8.2 DDT 24-h LC50 0.99 (0.66-1.49) (1984)
Water flea stat 15 44 7.1 DDTd 48-h LC50 0.36 (0.28-0.47) Mayer &
(Daphnia pulex) Ellersieck
(1986)
Water flea stat 15 44 7.1 DDTd 48-h LC50 2.5 (1.9-3.3) Mayer &
(Simocephalus stat 21 44 7.1 DDTd 48-h LC50 2.8 (2.3-3.5) Ellersieck
serrulatus) stat 15 44 7.1 TDEd 48-h LC50 3.2 (2.3-4.4) (1986)
stat 21 44 7.1 TDEd 48-h LC50 4.5 (3.1-6.6)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 21 35 44 7.1 TDEd 24-h LC50 4.6 (3.6-5.8) Sanders
(Gammarus fasciatus) stat 21 35 44 7.1 TDEd 96-h LC50 0.6 (0.05-1.2) (1972)
stat 21 35 44 7.1 DDTd 24-h LC50 15 (9.0-20) Sanders
stat 21 35 44 7.1 DDTd 96-h LC50 3.2 (1.8-5.6) (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 3.2 (2.1-4.3) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.86 (0.42-1.3) (1972)
stat 21 260 272 7.4 DDTd 24-h LC50 4.2 (1.8-5.6) Sanders
stat 21 260 272 7.4 DDTd 48-h LC50 3.1 (1972)
stat 21 260 272 7.4 DDTd 96-h LC50 1.8 (1.0-3.1) Sanders
(1972)
stat 21 260 272 7.4 DDTd 120-h LC50 0.32 Sanders
flow 18-21 260 272 7.4 DDTd 24-h LC50 1.1 (1972)
flow 18-21 260 272 7.4 DDTd 48-h LC50 1.0 Sanders
flow 18-21 260 272 7.4 DDTd 96-h LC50 0.8 (1972)
flow 18-21 260 272 7.4 DDTd 120-h LC50 0.6 Sanders
(1972)
Scud stat 21 44 7.1 DDTd 24-h LC50 4.7 (3.2-7.0) Mayer &
(Gammarus lacustris) stat 21 44 7.1 DDTd 96-h LC50 1.0 (0.68-1.5) Ellersieck
(1986)
stat 15 DDTe 96-h LC50 9.0 Gaufin et
al. (1965)
Glass shrimp stat 21 260 272 7.4 DDTd 24-h LC50 6.8 (6.2-7.5) Sanders
(Palaemonetes stat 21 260 272 7.4 DDTd 48-h LC50 4.7 (1972)
kadiakensis) stat 21 260 272 7.4 DDTd 96-h LC50 2.3 (1.3-4.9) Sanders
stat 21 260 272 7.4 DDTd 120-h LC50 1.0 (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 11 (8.4-16) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.68 (0.47-1.1) (1972)
flow 18-21 260 272 7.4 DDTd 24-h LC50 9.4 Sanders
flow 18-21 260 272 7.4 DDTd 48-h LC50 7.7 (1972)
flow 18-21 260 272 7.4 DDTd 96-h LC50 3.5 Sanders
flow 18-21 260 272 7.4 DDTd 120-h LC50 1.3 (1972)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Crayfish (Orconectes nais)
mature stat 21 260 7.4 DDTd 24-h LC50 1100 (1000-1400) Sanders
stat 21 260 7.4 DDTd 96-h LC50 100 (80-120) (1972)
1 day old - 15g stat 21 260 7.4 DDTd 24-h LC50 1.4 (1.1-4.2) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.3 (0.18-0.5) (1972)
1 week old - 20g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.18 (0.12-0.3) (1972)
2 weeks old - 23g stat 21 260 7.4 DDTd 24-h LC50 1.2 (0.9-5.5) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.2 (0.16-1.1) (1972)
3 weeks old - 30g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.24 (0.1-0.6) (1972)
5 weeks old - 50g stat 21 260 7.4 DDTd 24-h LC50 3.2 (1.8-8.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.9 (0.7-1.4) (1972)
8 weeks old - 500g stat 21 260 7.4 DDTd 24-h LC50 45 (40-52) Sanders
stat 21 260 7.4 DDTd 96-h LC50 28 (24-36) (1972)
10 weeks old - 1200g stat 21 260 7.4 DDTd 24-h LC50 50 (48-56) Sanders
stat 21 260 7.4 DDTd 96-h LC50 30 (26-42) (1972)
Sowbug (isopod) stat 21 35 7.1 DDTd 24-h LC50 8.7 (4.9-13.0) Sanders
(Asellus brevicaudus) stat 21 35 7.1 DDTd 96-h LC50 4.0 (1.2-6.5) (1972)
stat 21 35 7.1 TDEd 24-h LC50 18 (14-25) Sanders
stat 21 35 7.1 TDEd 96-h LC50 10 (7.0-14) (1972)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 48 Gaufin et
(Hydropsyche californica) 12 al. (1965)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 175 Gaufin et
(Arctopsyche grandis) 12 al. (1965)
May fly (nymph) stat 8.8- DDTe 96-h LC50 25 Gaufin et
(Ephemerella grandis) 10 al. (1965)
Stonefly (naiad) stat 11- DDTe 96-h LC50 320 Gaufin et
(Acroneuria pacifica) 12 al. (1965)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Stonefly (naiad) stat 11- DDTe 96-h LC50 1800 Gaufin et
(Pteronarcys 12 al. (1965)
californica) stat 15.5 35 DDT 24-h LC50 41 (27-62) Sanders &
stat 15.5 35 DDT 48-h LC50 19 (14-27) Cope (1968)
stat 15.5 35 DDT 96-h LC50 7 (4.9-9.9) Sanders &
stat 15.5 35 TDE 24-h LC50 3000 (2100-4300) Cope (1968)
stat 15.5 35 TDE 48-h LC50 1100 (800-1500) Sanders &
stat 15.5 35 TDE 96-h LC50 380 (280-520) Cope (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 12 (8.8-16) Sanders &
(Pteronarcella badia) stat 15.5 35 DDT 48-h LC50 9 (7-11) Cope
stat 15.5 35 DDT 96-h LC50 1.9 (1.3-2.7) (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 16 (12-20) Sanders &
(Claasenia sabulosa) stat 15.5 35 DDT 48-h LC50 6.4 (4.9-8.3) Cope
stat 15.5 35 DDT 96-h LC50 3.5 (2.9-4.2) (1968)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions (DDT
concentration in water continuously maintained).
b Static conditions but test solution renewed every 24 h.
c Alkalinity and hardness expressed as mg CaCO3/litre.
d Technical grade (99%).
e Emulsifiable concentrate (25% active ingredient).
f Juv. = juvenile.
g Value based on total number of young produced.
h Value based on average brood size.
i Value based on % days reproducing.
j Effect on shell growth.
Eggs of the Chironomid midge, contaminated with DDE by exposure of
the female during ovarian development, failed to hatch as many adults
as uncontaminated eggs. DDE in the water had less of an effect than
DDE contamination within the eggs obtained from the female. The
females had been maintained in water containing 30 µg DDE/litre; eggs
were kept in water containing 20 µg DDE/litre (Derr & Zabik, 1972).
Crayfish populations exposed over long periods to DDT develop some
tolerance to the insecticide (Albaugh, 1972). In 48-h tests, LC50
values for the crayfish Procambarus clarkii were 3.0 (2.5-3.6) µg/litre
for the unexposed population, and 7.2 (5.8-8.8) µg/litre for the
exposed population (95% confidence limits in parentheses). Naqvi &
Ferguson (1968) demonstrated the development of tolerance to DDT after
exposure to the insecticide, in a wide variety of aquatic
invertebrates, including cyclopoid copepods, tubifex worms, and pond
snails. These tolerant populations occurred in the Mississippi delta
in areas of cotton cultivation.
5.2 Fish
Appraisal
DDT is highly toxic to fish; the 96-h LC50s reported (static
tests) range from 1.5 to 56 µg/litre (for largemouth bass and guppy,
respectively). Smaller fish are more susceptible than larger ones of
the same species. An increase in temperature decreases the toxicity of
DDT to fish.
The behaviour of fish is influenced by DDT. Goldfish exposed to
1 µg/litre exhibit hyperactivity. Changes in the feeding of young
fish are caused by DDT levels commonly found in nature, and effects on
temperature preference have been reported.
Residue levels of > 2.4 mg/kg in eggs of the winter flounder result
in abnormal embryos in the laboratory, and comparable residue levels
have been found to relate to the death of lake trout fry in the wild.
Cellular respiration may be the main toxic target of DDT since
there are reports of effects on ATPase.
The toxicity of TDE and DDE has been less studied than that of DDT.
However, the data available show that TDE and DDE are both less toxic
than DDT.
The exact mode of action of DDT in fish remains unclear. There
have been many different suggestions to explain both lethal and
sublethal effects. Most of these are primarily the result of effects
on membranes. DDT is very soluble in lipid and, therefore, dissolves
in the lipid component of membranes. It may interfere both with
membrane function and with many enzyme systems that are located on
membranes. It has been shown experimentally to interfere with the
normal function of so many systems that a primary action of DDT is
difficult to determine.
5.2.1 Short-term and long-term direct toxicity to fish
The short-term toxicity of DDT to fish is summarized in Table 4.
The relatively few studies on TDE (Gardner, 1973; Korn & Earnest,
1974; Mayer & Ellersieck, 1986; Mayer, 1987) show it to be less toxic
than DDT, in the same test system, by factors of 5-10. The still fewer
studies on DDE indicate a similarly lowered toxicity relative to the
parent compound (Mayer & Ellersieck, 1986; Mayer, 1987). Whilst there
is some variation between species, DDT has proved highly toxic to all
fish tested; static 24-h LC50 values range from 2.1 µg/litre for the
largemouth bass (Mayer & Ellersieck, 1986) to 180 µg/litre for the
goldfish (Henderson et al., 1959). For 96-h tests, LC50 values range
from 1.5 µg/litre for largemouth bass (Mayer & Ellersieck, 1986) to
56 µg/litre for the guppy (Henderson et al., 1959). Several authors
have stated that DDT toxicity varies somewhat with temperature and
water hardness.
Buhler et al. (1969) studied the long-term effects, over 95 days,
of feeding DDT-contaminated diets to juvenile chinook and coho salmon.
The DDT was dissolved in corn-oil and then incorporated into a semi-
synthetic diet. Fish were fed until they stopped actively taking the
slowly sinking food. Pure p,p' -DDT was slightly more toxic to
juvenile salmon than the technical product, and chinook salmon were
2 to 3 times more sensitive to the same dose of DDT in the diet than
coho salmon. Size was an important factor in the toxicity of DDT,
smaller fish being more susceptible than larger ones. The authors
estimated, by extrapolation, a 90-day LD50 value of 27.5 µg/kg per
day for chinook and 64 µg/kg per day for coho salmon juveniles. In
fish exposed to higher doses of DDT, pre-death symptoms were marginal.
Some increased agitation and slight photophobia were reported. Fish
exposed to low doses of DDT took longer to die, and other symptoms were
noted. Many individuals developed ulceration of the nasal area. This
spread over the head and in some cases eyes were lost. Pathological
examination showed a specific and severe kidney lesion; this was
limited to one short section of the distal convoluted tubule, which
eventually degenerated almost completely. The authors suggested this
as the main lethal lesion in the fish.
In a later study (Buhler & Shanks, 1970), the same authors showed
that median survival time was directly proportional to body weight in
young coho salmon fed technical DDT. Fish were all given a diet
containing 200 mg DDT/kg and food consumption was monitored for each
group of fish. The main effect of body size on DDT lethality was
related to the intake of the chemical by the fish; smaller fish ate
more of the contaminated diet and consequently received the greatest
dose in mg/kg bodyweight terms. However, even after correcting for
dosage received, the smaller fish were more susceptible than larger
ones. The authors suggested that the lower lipid content of smaller
fish might have accounted for the remaining difference. Twelve groups
of 100 fish ranged in weight (average for each group) from 3 to 15 g.
Total DDT intake ranged from 0.4 to 3 mg/fish; daily intake was higher
in the smaller fish at 3 mg/kg per day, falling to 1.3 mg/kg per day
for the largest. The estimated LC50 ranged from 95 mg/kg for the
smallest to 135 mg/kg for the largest fish, and median survival time
increased from 30 days for the smallest fish to 106 days for the
largest.
Crawford & Guarino (1976) exposed killifish ( Fundulus heteroclitus )
to a twice-repeated schedule of 24 h in water containing DDT at a
concentration of 0.1 mg/litre and 24 h in clean water. At this
exposure level, there was a delay in the rate of development of ferti-
lized eggs but no apparent effect on the hatched fry. Fertilization of
killifish eggs was diminished when insemination was carried out in sea
water containing DDT at 0.1 mg/litre. Mortality at a late stage of
embryo development has been reported for a variety of salmonids and
related to egg residues of DDT (Allison et al., 1964, for cutthroat
trout; Burdick et al., 1964, for lake trout; Macek, 1968, for brook
trout; and Johnson & Pecor, 1969, for coho salmon).
Smith & Cole (1973) reported effects on embryos developing from
eggs laid by adult winter flounder ( Pseudopleuronectes americanus )
that were exposed to 2 µg DDT/litre for various times and, therefore,
accumulated different residue levels in the eggs. These residue levels
varied from 1.15 to 3.70 mg DDT/kg and from 0.07 to 0.4 mg DDE/kg.
Embryos showed abnormal gastrulation and a high incidence (mean 39%)
of vertebral deformities. Bone erosion and haemorrhaging at the
vertebral junctures were often associated with the vertebral deform-
ities.
Halter & Johnson (1974) report that DDT is toxic to the early
life-stages of coho salmon. Mean survival times were considerably
reduced by water concentrations of DDT greater than 0.5 µg/litre.
5.2.2 Sublethal behavioural effects on fish
Hansen (1969) and Hansen et al. (1972) investigated the avoidance
of DDT by sheepshead minnows and mosquitofish in a 'Y'-shaped avoidance
maze. Although there was some statistically significant avoidance of
DDT when fish were given the choice between DDT and clean water, this
only occurred at concentrations of the insecticide above the 24-h
LC50. Fish of both species, when given the choice between DDT at 0.1
and 0.01 mg/litre, chose the higher concentration of the chemical.
This suggests that the perception of DDT is poor and that fish could
not reliably avoid DDT in water at toxic concentrations.
Olofsson & Lindahl (1979) administered either 0.5 or 1.0 mg DDT/kg
body weight to cod by oral intubation. There was a significant effect,
at the higher dose but not the lower one, on the ability of the fish to
compensate its posture to cope with a rotating tube in which it was
swimming.
Hansen (1972) allowed mosquitofish to select a desired salinity in
a fluvarium with a salinity gradient. Fish selected a higher salinity
than controls when exposed to DDT, but only at exposure levels which
caused some mortality. The author suggested that DDT might have affec-
ted the osmoregulatory ability of the mosquitofish. Other possible
explanations include a change in sensitivity of nerves to stimuli or a
preference for the pre-exposure salinity, which was 15 g/litre.
Table 4. Toxicity of DDT and its derivatives to fish
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Salinity Compound Parameter Water Reference
(g)/ stata perat- o/oo concen-
agef ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine fish
Dwarf perch 1.2-11.0 Stat 13 28 DDTc 96-h LC50 4.6 Earnest &
(Micrometrus minimus) 1.2-11.0 flowb 14-18 26-28 DDTc 96-h LC50 0.26 Benville
(0.13-0.52) (1972)
Shiner perch 1.2-11.0 stat 13 26 DDTc 96-h LC50 7.6 Earnest &
(Cymatogaster aggregata) 1.2-11.0 flowb 14-18 13-23 DDTc 96-h LC50 0.45 Benville
(0.21-0.94) (1972)
Striped bass 2.7 flowb 17 28 DDT(77%) 96-h LC50 0.53 Korn &
(Morone saxatilis) (0.38-0.84) Earnest
0.6 flowb 17 30 TDEc 96-h LC50 2.5 (1974)
(1.6-4.0)
Sheepshead minnow juv. flow 15 30 DDTc 48-h LC50 2.0 Mayer
(Cyprinodon variegatus) (1987)
Longnose killifish juv. flow 15 30 DDTc 48-h LC50 2.8 Mayer
(Fundulus similis) juv. flow 16 28 TDEc 48-h LC50 42.0 (1987)
Pinfish juv. flow 22 29 DDTc 48-h LC50 0.3 Mayer
(Lagodon rhomboides) (1987)
Striped mullet juv. flow 15 30 DDTc 48-h LC50 0.4 Mayer
(Mugil cephalus) (1987)
Spot juv. flow 12 26 DDEc 48-h LC50 > 100 Mayer
(Leiostomus xanthurus) juv. flow 26 30 TDEc 48-h LC50 20.0 (1987)
Three-spined 0.4-0.8 stat 20 5 DDT 24-h LC50 22.0 Katz
stickleback 0.4-0.8 stat 20 5 DDT 48-h LC50 21.0 (1961)
(Gasterosteus 0.4-0.8 stat 20 5 DDT 72-h LC50 18.5 Katz
aculeatus) 0.4-0.8 stat 20 5 DDT 96-h LC50 18.0 (1961)
0.4-0.8 stat 20 25 DDT 24-h LC50 18.0 Katz
0.4-0.8 stat 20 25 DDT 48-h LC50 15.0 (1961)
0.4-0.8 stat 20 25 DDT 72-h LC50 14.5 Katz
0.4-0.8 stat 20 25 DDT 96-h LC50 11.5 (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Freshwater fish
Black bullhead 1.2 stat 18 44 7.1 DDTc 24-h LC50 36.8 Mayer &
(Ictalurus melas) (20.3-67.0)
1.2 stat 18 44 7.1 DDTc 96-h LC50 4.8
(3.4-6.8) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 26.2
(22.0-31.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 5.1
(3.9-6.7) Ellersieckg
Channel catfish 1.5 stat 18 44 7.1 DDTc 24-h LC50 22.0
(Ictalurus punctatus) (18.2-26.5) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 21.5
(17.7-26.1) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 18.4
(13.7-24.7) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 17.3
(13.0-23.1) Ellersieckg
0.7 stat 18 44 7.1 DDTc 24-h LC50 17.9
(12.7-25.3) Mayer &
0.7 stat 18 44 7.1 DDTc 96-h LC50 6.9
(5.7-8.5) Ellersieckg
1.6 stat 18 44 7.1 DDTc 24-h LC50 44.0
(37.0-52.0) Mayer &
1.6 stat 18 44 7.1 DDTc 96-h LC50 22.0
(19.0-26.0) Ellersieckg
1.4 stat 18 44 7.1 DDTc 24-h LC50 30.0
(22.0-41.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 16.0
(9.4-29.0) Ellersieckg
1.4 stat 18 272 7.7 DDTc 24-h LC50 29.0
(20.0-41.0) Mayer &
1.4 stat 18 272 7.7 DDTc 96-h LC50 7.0
(4.3-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Atlantic salmon 0.45 stat 12 40 7.5 DDTc 24-h LC50 6.2
(Salmo salar) (4.6-8.4) Mayer &
0.45 stat 12 40 7.5 DDTc 96-h LC50 1.8
(1.3-2.6) Ellersieckg
0.5 stat 12 44 7.5 DDEc 96-h LC50 96.0
(52.1-177) Mayer &
Ellersieckg
Coho salmon 2.7-4.1 stat 20 45-57 6.8-7.4 DDT 24-h LC50 66.0 Katz (1961)
(Oncorhynchus kisutch)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 48-h LC50 46.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 72-h LC50 44.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 96-h LC50 44.0 Katz (1961)
1.0 stat 13 44 7.1 DDTc 24-h LC50 10.0
(7.0-12.0) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 4.0
(3.0-6.0) Ellersieckg
6.0 stat 13 40 7.1 DDTc 24-h LC50 26.9
(18.1-40.0) Mayer &
6.0 stat 13 40 7.1 DDTc 96-h LC50 19.3
(9.6-38.8) Ellersieckg
Chinook salmon 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 24-h LC50 38.0 Katz (1961)
(Oncorhynchus
tshawytscha) 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 48-h LC50 17.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 72-h LC50 14.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 96-h LC50 11.5 Katz (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout 0.9 stat 7 44 7.1 DDTc 24-h LC50 7.5
(Salmo gairdneri) (6.7-8.3) Mayer &
0.9 stat 7 44 7.1 DDTc 96-h LC50 4.1
(3.6-4.6) Ellersieckg
0.9 stat 13 44 7.1 DDTc 24-h LC50 8.2
(7.2-9.2) Mayer &
0.9 stat 13 44 7.1 DDTc 96-h LC50 4.7
(4.2-5.3) Ellersieckg
0.9 stat 18 44 7.1 DDTc 24-h LC50 12.0
(1.0-13.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 5.8
(5.2-6.5) Ellersieckg
3.2 stat 20 45-57 6.8-7.4 DDT 24-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 48-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 72-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 96-h LC50 42.0 Katz (1961)
1.8 flow 17 272 7.4 DDTc 96-h LC50 > 3.0 Mayer &
0.8 stat 12 44 7.1 DDEc 96-h LC50 32.0
(26.0-40.0) Ellersieckg
1.0 stat 12 44 7.1 TDEc 96-h LC50 70.0
(57.0-87.0) Mayer &
1.0 stat 12 272 7.4 TDEc 96-h LC50 70.0
(58.0-85.0) Ellersieckg
Cutthroat trout 1.0 stat 13 44 7.1 DDTc 24-h LC50 8.4
(Salmo clarki) (7.6-9.2) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 5.5
(4.7-6.4) Ellersieckg
1.8 stat 9 162 7.4 DDTc 24-h LC50 11.3
(9.4-13.6) Mayer &
1.8 stat 9 162 7.4 DDTc 96-h LC50 7.9
(6.5-9.7) Ellersieckg
Brown trout 1.7 stat 13 44 7.1 DDTc 96-h LC50 1.8
(Salmo trutta) (1.3-2.5) Mayer &
Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Northern pike 0.7 stat 18 272 7.4 DDTc 24-h LC50 5.5 Mayer &
(Esox lucius) 0.7 stat 18 272 7.4 DDTc 96-h LC50 2.7 Ellersieckg
Guppy 0.1-0.2 stat 25 18 20 7.4 DDTc 24-h LC50 135 Henderson
(Lebistes 0.1-0.2 stat 25 18 20 7.4 DDTc 48-h LC50 72.0 et al.
reticulatus) 0.1-0.2 stat 25 18 20 7.4 DDTc 96-h LC50 56.0 (1959)
River shiner 0.3 stat 18 44 7.1 DDTc 24-h LC50 6.7
(Notropis blennius) (4.9-9.1) Mayer &
0.3 stat 18 44 7.1 DDTc 96-h LC50 5.8
(3.6-9.1) Ellersieckg
Fathead minnow 1.2 stat 18 44 7.1 DDTc 24-h LC50 14.2
(Pimephales (11.0-18.0) Mayer &
promelas) 1.2 stat 18 44 7.1 DDTc 96-h LC50 12.4
(10.0-15.4) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 13.8
(10.3-18.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 13.2
(10.1-17.3) Ellersieckg
0.9 flow 12 314 7.6 DDTc 96-h LC50 9.9
(6.5-15.0) Mayer &
Ellersieckg
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 56.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 42.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 24-h LC50 78.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDTc 48-h LC50 68.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 96-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 24-h LC50 32.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDT 48-h LC50 26.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 96-h LC50 26.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 24-h LC50 29.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDT 48-h LC50 27.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 96-h LC50 26.0 et al. (1959)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Mosquitofish 0.2 stat 25 DDTc 24-h LC50 22.7
(Gambusia affinis) (16.6-31.1) El-Sebae (1987)
0.2 stat 25 DDTc 96-h LC50 9.9
(7.3-13.4) El-Sebae (1987)
0.2 stat 25 DDTe 24-h LC50 58.6
(43.2-79.5) El-Sebae (1987)
0.2 stat 25 DDTe 96-h LC50 27.7
(21.3-36.0) El-Sebae (1987)
Bluegill sunfish 0.26 stat 19 138 192 8.2-8.5 DDTc 96-h LC50 3.4
(Lepomis macrochirus) (2.6-4.1) Randall
0.26 stat 19 138 192 8.2-8.5 DDT 96-h LC50 9.0
(7.4-10.6) et al. (1979)
(25%)
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 26.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 21.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 21.0 (1959)
1.5 stat 18 44 7.1 DDTc 24-h LC50 11.5
(8.4-16.0) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 8.6
(6.2-12.0) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 10.0
(8.5-12.9) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 6.3
(4.3-9.3) Ellersieckg
0.9 stat 17 44 7.1 DDEc 96-h LC50 240
(201-286) Mayer &
0.9 stat 24 44 7.4 TDEc 24-h LC50 56.0
(46.0-68.0) Ellersieckg
0.9 stat 24 44 7.4 TDEc 96-h LC50 42.0
(36.0-49.0) Mayer &
Ellersieckg
Redear sunfish 3.2 stat 24 44 7.1 DDTc 24-h LC50 19.0 Mayer &
(Lepomis microlophus) 3.2 stat 24 44 7.1 DDTc 96-h LC50 15.0 Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Green sunfish 1.1 stat 18 44 7.1 DDTc 24-h LC50 16.9
(Lepomis cyanellus) (12.7-22.3) Mayer &
1.1 stat 18 44 7.1 DDTc 96-h LC50 10.9
(7.3-15.6) Ellersieckg
0.8 stat 18 44 7.1 DDTc 24-h LC50 18.0
(13.0-24.0) Mayer &
0.8 stat 18 44 7.1 DDTc 96-h LC50 6.5
(4.1-10.4) Ellersieckg
1.1 stat 18 272 7.7 DDTc 24-h LC50 19.8
(15.0-25.6) Mayer &
1.1 stat 18 272 7.7 DDTc 96-h LC50 9.9
(6.4-15.0) Ellersieckg
Largemouth bass 0.8 stat 18 44 7.1 DDTc 24-h LC50 3.7
(Micropterus (3.1-4.5) Mayer &
Salmoides) 0.8 stat 18 44 7.1 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.8 stat 18 272 7.4 DDTc 24-h LC50 2.1
(1.6-2.9) Mayer &
0.8 stat 18 272 7.4 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.7 stat 18 44 7.1 TDEc 24-h LC50 50.0
(35.0-71.0) Mayer &
0.7 stat 18 44 7.1 TDEc 96-h LC50 42.0
(34.0-51.0) Ellersieckg
Black crappie 1.0 stat 18 44 7.1 DDTc 24-h LC50 6.5
(Pomoxis (5.4-7.8) Mayer &
nigromaculatus) 1.0 stat 18 44 7.1 DDTc 96-h LC50 5.6
(4.6-6.7) Ellersieckg
Yellow perch 1.4 stat 18 44 7.1 DDTc 24-h LC50 10.0
(Perca flavescens) (8.0-12.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 9.0
(7.0-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Walleye 1.4 stat 18 44 7.1 DDTc 24-h LC50 4.2
(Stizostedion v. (3.2-5.6) Mayer &
vitreum) 1.4 stat 18 44 7.1 DDTc 96-h LC50 2.9
(2.4-3.5) Ellersieckg
1.3 stat 18 272 7.4 DDTc 24-h LC50 4.6
(3.9-5.4) Mayer &
1.3 stat 18 272 7.4 DDTc 96-h LC50 4.6
(3.9-5.4) Ellersieckg
1.0 stat 18 44 7.1 TDEc 24-h LC50 20.0
(16.0-24.0) Mayer &
1.0 stat 18 44 7.1 TDEc 96-h LC50 14.0
(11.0-19.0) Ellersieckg
Tilapia 0.8 stat 24 44 7.1 DDTc 24-h LC50 19.0
(Tilapia mossambica) (16.0-23.0) Mayer &
0.8 stat 24 44 7.1 DDTc 96-h LC50 17.0
(14.0-21.0) Ellersieckg
0.8 stat 24 272 7.4 DDTc 24-h LC50 15.0
(13.0-17.0) Mayer &
0.8 stat 24 272 7.4 DDTc 96-h LC50 14.0
(12.0-16.0) Ellersieckg
0.8 flow 18 272 7.4 DDTc 24-h LC50 24.0
(17.0-32.0) Mayer &
0.8 flow 18 272 7.4 DDTc 96-h LC50 5.1
(3.2-8.1) Ellersieckg
Tilapia 0.8 stat 25 DDTc 24-h LC50 21.8
(Tilapia zilli) (17.0-28.0) El-Sebae (1987)
0.8 stat 25 DDTc 96-h LC50 15.5
(11.7-20.6) El-Sebae (1987)
0.8 stat 25 DDTe 24-h LC50 12.8
(9.6-17.1) El-Sebae (1987)
0.8 stat 25 DDTe 96-h LC50 9.5
(7.4-12.3) El-Sebae (1987)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Goldfish 1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 180 Henderson
(Carassius auratus) 1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 47.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 36.0 (1959)
0.9 stat 18 44 7.1 DDTc 24-h LC50 24.0
(17.0-33.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 15.5
(9.1-26.0) Ellersieckg
0.9 stat 18 272 7.4 DDTc 24-h LC50 22.2
(16.0-31.1) Mayer &
0.9 stat 18 272 7.4 DDTc 96-h LC50 14.7
(10.0-20.0) Ellersieckg
Common carp 0.6 stat 18 44 7.1 DDTc 24-h LC50 14.0
(Cyprinus carpio) (10.0-19.0) Mayer &
0.6 stat 18 44 7.1 DDTc 96-h LC50 9.7
(7.4-12.9) Ellersieckg
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions
(DDT concentration in water continuously maintained).
b Intermittent flow-through conditions.
c Technical grade (99%).
d Alkalinity and hardness expressed as mg CaCO3/litre.
e 25% emulsifiable concentrate.
f Juv. = juvenile.
g 1986.
Peterson (1973) monitored the selection of temperature by
juvenile Atlantic salmon ( Salmo salar ) previously exposed to DDT or
its metabolites. Low concentrations produced no effect on tem-
perature selection, but at higher levels of exposure the temperature
selected by the fish increased. Fish were most sensitive, in this
respect, to p,p' -DDE and showed decreasing sensitivity to
o,p' -DDT, p,p' -TDE, and p,p' -DDT. Increasing the exposure to
p,p' -DDE from 0 to 1.0 mg/litre increased the preferred temperature
from about 16 °C to 21 °C. There was no effect of p,p' -DDA on
temperature selection at concentrations as high as 8 mg/litre. In a
similar experiment, where brook trout (Salvelinus fontinalis) were
exposed to a vertical rather than horizontal temperature gradient, fish
previously exposed to p,p' -DDT and p,p' -TDE selected higher
temperatures than controls. Conversely, Gardner (1973) found that DDT
and its analogues induced selection of lower temperatures by the same
species of fish over a dose range between 0 and 50 µg/litre; DDE did
not produce any temperature preference. Ogilvie & Miller (1976)
reported that Atlantic salmon exposed to DDT at a concentration of
50 µg/litre selected higher temperatures, the effect persisting for at
least 4 weeks after exposure. The authors suggested that the tempera-
ture selection response to DDT exposure is "biphasic". At low
exposure levels, similar to those used by Gardner (1973), lower
temperatures are selected, whilst higher temperatures are preferred at
higher exposure levels.
Dill & Saunders (1974) exposed the eggs of Atlantic salmon
at gastrulation to DDT at water concentrations of 5, 10, 50, or
100 µg/litre, and observed behavioural development in hatched fry over
30 days following hatch. The two highest doses of DDT impaired balance
and retarded behavioural development of the fry (i.e., the appearance
of normal behaviour patterns was delayed). The authors considered that
the effects observed would affect predation rates and feeding, in
young fish, at "realistic" DDT exposure levels in the wild.
Davy et al. (1973) reported that exposure to DDT, at 10 µg/litre
for 4 days, affected the exploratory behaviour of goldfish experi-
encing a novel environment. They attributed the effect to a central
nervous system lesion caused by DDT. Weis & Weis (1974) showed an
increase in individual activity and an increase in school-size in
groups of goldfish exposed to DDT at 1 µg/litre for 7 days. After a
frightening stimulus, schools scattered further and did not regroup as
readily as control fish. The transfer of fish to clean water led to
a return to normal behaviour within one week. An effect on the
locomotor behaviour of goldfish after exposure to 10 µg DDT/litre per-
sisted for the remainder of the observation period of 130-139 days
(Davy et al., 1972).
5.2.3 Physiological effects on fish
Hanke et al. (1983) investigated the effects of DDT on a range of
physiological functions in carp ( Cyprinus carpio ). At water concen-
trations of 100 or 500 µg/litre, the insecticide induced changes in
plasma cortisol and glucose levels, liver glycogen level, and plasma
and brain acetylcholinesterase activity. The response was biphasic in
all cases. Initially, after 6 hours, there was a stimulation of these
parameters which, within 24 hours, changed to an inhibition.
Ramalingam & Ramalingam (1982) reported that the chronic effect of DDT
on glycogen utilization in fish led to the use of protein as an energy
source. The protein content of tissues declined after chronic exposure
to DDT.
Janicki & Kinter (1971) found that DDT impaired fluid absorption in
the intestinal sacs of eels adapted to sea water and exposed to the
insecticide at 50 µg/litre. DDT also inhibited Na+-, K+-, and
Mg2+-dependent ATPases in homogenates of the intestinal mucosa. In a
later study, Kinter et al. (1972) showed that plasma osmolarity was
also affected in sea-water-adapted eels exposed to DDT (1 mg/litre)
for 9 to 10 hours. Haux & Larsson (1979) reported effects of DDT on
plasma electrolytes in the flounder Platichthys flesus kept in
hypotonic, brackish water. The fish were force-fed with DDT in gelatin
capsules to give a total DDT dose of 1.5 or 15.0 mg/kg body weight.
Plasma sodium was reduced but not significantly; plasma chloride was
significantly reduced in a dose-related manner after 3 weeks but not
after 6 weeks. Waggoner & Zeeman (1975) reported similar effects on
plasma electrolytes in the black surfperch ( Embiotoca jacksoni ), but
only at high DDT exposure levels. They injected DDT doses of 1, 10,
100, or 200 mg/kg; the only effect occurred with the dose of 200 mg/kg,
but the fish did not survive to 72 h. The authors suggested that
osmoregulatory effects are not the major cause of DDT-induced mortality
in marine fish.
Desaiah et al. (1975) exposed fathead minnows ( Pimephales
promelas ) for long periods to DDT at water concentrations of 0.5 or
2.0 µg/litre and also via the food, and monitored the activity of
ATPases in brain and gill. This study followed up several previous
studies on in vitro effects on these enzymes. After 266 days of
exposure, there was an approximately 50% reduction in brain
oligomycin-sensitive (mitochondrial) Mg2+-ATPase activity. In
contrast, oligomycin-insensitive Mg2+-ATPase activity was increased
by almost 40%. Total Mg2+-ATPase activity was, therefore, almost
unaffected by DDT. There was a less obvious (about 18%) activation of
Na+-K+-ATPase activity in the brain. Gill tissue showed different
results; all the ATPases studied were inhibited by DDT. The authors
suggested that a major factor in the toxicity of DDT to fish (and other
organisms) could be the inhibition of oxidative phosphorylation.
Moffett & Yarbrough (1972) investigated the enzyme succinic
dehydrogenase in insecticide-resistant and insecticide-susceptible
mosquitofish ( Gambusia affinis ) in an attempt to discover if
resistance could be related to membrane effects of DDT. They found
that the effect on membrane-bound enzymes was, indeed, reduced in
resistant fish. This may not explain all the factors involved in
resistance, since DDT uptake from water may also be reduced.
5.2.4 Development of tolerance
The development of tolerance to DDT in fish has been reported.
Vinson et al. (1963) reported DDT tolerance in mosquitofish ( Gambusia
affinis ) exposed long-term to DDT in the wild, and Boyd & Ferguson
(1964) showed TDE tolerance in the same species. However, fish exposed
long-term to DDT do not always show tolerance. Ferguson et al. (1964)
recorded tolerance to a variety of organochlorine insecticides in three
species of freshwater fish from the Mississippi delta area of cotton
cultivation, but there was no tolerance to DDT. El-Sebae (1987)
determined the LC50 values for two populations of Tilapia zilli from
different areas of Egypt. Fish from the Behera Governate which had
been taken from agricultural drains showed exactly the same suscepti-
bility to DDT (25% EC) as fish taken from a less contaminated area in
the Alexandria Governate. Tolerance had developed to other insecti-
cides in these different strains.
5.3 Toxicity to Amphibians
Appraisal
The toxicity of DDT and its metabolites to amphibians varies from
species to species; although only a few data are available, amphibian
larvae seem to be more sensitive than adults to DDT. TDE seems to be
more toxic than DDT to amphibians, but there are no data available for
DDE. All the studies reported have been static tests and, therefore,
results should be treated with caution.
The toxicity of DDT and TDE to amphibians is summarized in
Table 5. Both compounds are toxic to amphibian larvae at low water
concentrations.
Two studies (Harri et al., 1979; Hudson et al., 1984) showed
that DDT is moderately toxic to adult frogs when given orally.
Repeated oral dosing of adult common frogs ( Rana temporaria ) with DDT
at 0.6 mg/kg body weight twice weekly for 8 weeks, led to no mortality
when the animals were fed (Harri et al., 1979). Frogs dosed in the
same way, but not fed, showed 50% mortality by the end of dosing. The
first animal died after the fifth dose and all others showed symptoms
of poisoning.
A study by Sanders (1970) indicated that the toxicity of DDT to
tadpoles of Fowler's toad increased with age of the tadpole. The 24-
and 96-h LC50 values of 5.3 and 0.75 mg/litre for one-week-old
tadpoles fell to 1.4 and 0.03 mg/litre, respectively, by the time the
tadpoles were 7 weeks old. TDE was only tested on one age range of
tadpoles for a maximum of 96 h, and was found to be 3-8 times more
toxic than DDT. The pattern of pesticide poisoning progressed through
irritability and loss of equilibrium to death. Tadpoles were affected
irreversibly by concentrations well below their calculated short-term
LC50 values and, therefore, would succumb to DDT over time. DDT was
re-tested several times during over the period of the study in an
attempt to identify any development of resistance in the population.
None was found; the 24-h LC50 values were stable throughout a 4-month
period.
Table 5. Toxicity of DDT and its derivatives to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Fowler's toad (tadpole)
(Bufo woodhousii)
1 week old - 15 mg stat 15.5 30 7.1 DDT 24-h LC50 5300 (2900-9900) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1800 (950-3300) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 750 (280-2000) Sanders
(1970)
2-3 weeks old - 56 mg stat 15.5 30 7.1 DDT 24-h LC50 5400 (2900-10 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1300 (320-5300) (1970)
4-5 weeks old - 74 mg stat 15.5 30 7.1 DDT 24-h LC50 2400 (730-8000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1000 (40-6500) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 1000 (20-3600) Sanders
stat 15.5 30 7.1 TDE 24-h LC50 700 (250-2000) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 320 (210-450) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 140 (100-210) (1970)
6 weeks old - 350 mg stat 15.5 30 7.1 DDT 24-h LC50 2200 (550-15 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 410 (280-600) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 100 (20-600) Sanders
(1970)
7 weeks old - 600 mg stat 15.5 30 7.1 DDT 24-h LC50 1400 (900-2000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 750 (610-1100) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 30 (6-400) Sanders
(1970)
Table 5. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Western chorus frog stat 15.5 30 7.1 DDT 24-h LC50 1400 (910-2800) Sanders
(Pseudacris stat 15.5 30 7.1 DDT 48-h LC50 900 (400-1500) (1970)
triseriata) stat 15.5 30 7.1 DDT 96-h LC50 800 (500-2300) Sanders
(1-week-old tadpole) stat 15.5 30 7.1 TDE 24-h LC50 610 (410-820) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 500 (210-750) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 400 (210-750) (1970)
Bullfrog DDT acute LD50c > 2000 ug/kg Hudson
(Rana catesbeiana) (77.2%) et al.
(1984)
Common frog 15 DDT acute LD50c 7600 ug/kg Harri
(Rana temporaria) et al.
(1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(DDT concentration in water continuously maintained).
b alkalinity expressed as mg CaCO3/litre.
c acute LD50 was calculated by administering a single oral dose.
Cooke (1970) exposed tadpoles of the common frog ( Rana temporaria )
to 0.1, 1.0, or 10 mg DDT/litre for only one hour and reported a
period of uncoordinated hyperactivity beginning less than one hour
after the end of the exposure period. Body weight decreased during
this hyperactive period and development was restricted in some of the
tadpoles. Smaller tadpoles were more vulnerable to the effects of DDT
than larger ones. In a later study (Cooke, 1979b), the same author
reared tadpoles of the common frog at two different densities. The
densities differed 5-fold and resulted in a 2-fold average difference
in body weight between the two groups. The larger tadpoles, reared at
the lower density, were completely tolerant of concentrations of DDT
that caused severe sublethal effects in smaller tadpoles. Field
populations of tadpoles included individuals with weights corresponding
to the two experimental groups, but these were at the two extremes of
the natural weight range.
Cooke (1972) exposed both spawn and tadpoles of the common frog
( Rana temporaria ), the common toad ( Bufo bufo ), and the smooth newt
( Triturus vulgaris ) for 24 and 48 hours to concentrations of DDT
between 0.8 µg/litre and 0.5 mg/litre. Results indicated that DDT did
not penetrate well-developed spawn and was only detectable in tadpoles
hatched from spawn that had been treated with DDT immediately after it
had been laid. Tadpoles hatching from spawn treated when newly laid
showed hyperactivity, symptomatic of DDT poisoning, only later in
their development at the point where external gills were lost. In the
experiments where tadpoles were exposed to DDT, they were most suscep-
tible either just before or just after the development of hindlimb
buds. At these two stages, the characteristic hyperactivity was
shown when DDT tissue concentrations reached between 2-3 mg/kg before
the tadpoles developed limb buds, and when they reached 3-4 mg/kg,
immediately after the tadpoles developed limb buds. During resorption
of the tail, small frogs, but not small toads, were susceptible to DDT
residues that had been acquired during larval development. At all
stages of development, toads were more resistant to DDT than were
frogs, and some toad tadpoles survived despite tissue residues in
excess of 300 mg/kg. The metabolite DDE was often detectable in newt
tadpoles and in frog and toad tadpoles with hindlimbs.
DDT has an anatomical effect on developing frog tadpoles (Cooke,
1970; Osborn et al., 1981). Exposure of tadpoles to 0.1 mg DDT/litre
for 2 days or to 0.1, 1.0, or 10 mg/litre for one hour produced some
individuals with abnormalities in the snout. A detailed histological
and behavioural study suggested that the effect was caused by two
separate factors. DDT had a direct effect on the development of skin
glands in the region above the upper mandible. The uncoordinated
hyperactivity that followed DDT treatment caused the lower mandible to
strike the upper, distorted mandible and resulted in further damage.
Some individuals recovered from this abnormality at various stages of
development. However, froglets that were affected at the tadpole stage
frequently have blunt snouts and deformed brains. The authors
suggested that DDT caused the disruption by preventing the organisation
of the epithelial cells into gland units, possibly by affecting cell
membranes and disrupting cell-to-cell communication. The mechanism of
recovery remained unclear and a full explanation of the very specific
nature of the abnormality was not possible.
This toxicity of DDT to amphibians is of significance in its use as
an insecticide. The use of DDT to control mosquito larvae has been a
major source of exposure of tadpoles and has led to toxic effects
(Mulla, 1963; Cooke, 1973a).
The widespread use of DDT has led to the development of some
resistance in two species of cricket frog ( Acris crepitans and Acris
gryllus ). Boyd et al. (1963) found that cricket frogs collected from
areas of high DDT usage for the control of cotton pests were more
tolerant to DDT than were frogs from other areas.
6. TOXICITY TO TERRESTRIAL ORGANISMS
There is evidence that DDT and its metabolites have affected
wildlife in terrestrial ecosystems. Laboratory studies covered in this
section give clear indication of a variety of lethal and sublethal
effects. The range of organisms studied is not comprehensive. No
review has been made here of the effects of DDT on insects, the target
organisms. The lethal effect of DDT on insects is thought to result
from changes in nerve transmission.
6.1 Terrestrial Invertebrates
Appraisal
There have been few reports on the effects of DDT and its
metabolites on non-target terrestrial invertebrates.
Earthworms are insensitive to the acutely toxic effects of these
compounds at levels higher than those likely to be found in the
environment. The uptake of DDT by earthworms is related to the concen-
trations in soil and to the activity of the worms; seasonally greater
activity increases uptake. Thus, although earthworms are unlikely to
be seriously affected by DDT, they pose a major hazard to predators
because of the residues they can tolerate.
Both DDT and DDE are classified as being relatively non-toxic to
honey bees, with a topical LD 50 at 27 µg/bee.
There are no reports on laboratory studies using DDE or TDE, in
spite of the fact that these are major contaminants of soil.
The toxicity of DDT to insects, the target organisms, is exten-
sively documented. Uptake of DDT and its metabolism by other
terrestrial invertebrates is also well covered in the literature.
However, there are few reports of effects of either DDT or its
metabolites on non-target invertebrates.
Johansen (1962) classified DDT as "moderately" toxic to honey
bees in both laboratory and field tests. Atkins et al. (1973) quoted
a topical LD50 for honey bees of 12.09 µg/bee and classified DDT as
"relatively non-toxic".
DDT has little or no effect on earthworms at dose levels likely to
be encountered in the field; worms were unaffected by 2000 mg/kg soil
(Goffart, 1949). The early literature has been examined by Davey
(1963), whose review includes reports on a variety of earthworm species
that live in surface soil or deeper layers. Thompson (1971) treated an
area of grassland with an emulsifiable concentrate of DDT at the rate
of 5.6 kg/ha. Although there was a reduction in earthworm numbers and
biomass of about 30%, the author considered this to be of little sig-
nificance. Results in tropical areas are similar to those of temperate
regions. Cook et al. (1980) examined the effects of cultivation and
DDT treatment on earthworm activity and populations in Nigeria
following the application of DDT (1 kg/ha) as a foliar spray on cowpea
plots. The number of casts on the surface was reduced by DDT
application, but there was no effect on the number of worms in the
soil.
Cooke & Pollard (1973) treated Roman snails ( Helix pomatia )
with p,p' -DDT applied to lettuce leaves. The snails were fed a
365 x 2.5 cm square of leaf that had been treated with 0.1 ml of an
acetone solution of DDT (either 0.025, 1.0, or 40 mg/ml). The dosing
started when the snails were 2 weeks old and continued for 17 weeks, at
which point the dose was doubled and continued for a further 12 weeks.
The snails were then transferred outside to stimulate hibernation. Low
doses of DDT reduced the weight of the shell and operculum whereas
higher doses did not. After re-emergence from hibernation, the
incidence of operculum eating was significantly higher among snails
hibernating late in the season, and as exposure to DDT increased so
operculum eating became more prevalent. The authors suggest that
shell-thinning is likely to have occurred in snails in heavily-treated
agricultural areas if the response of all snail species to DDT is
similar to that of Helix pomatia .
Critchley et al. (1980) investigated the effects of the use of
DDT for 4 years on a cultivated forest soil in Nigeria on the
numbers of epigeal (surface-living) and subterranean species of invert-
ebrates. DDT was applied as a foliar spray to crops of cowpeas at a
rate of 1 kg/ha annually. After the first application of DDT there was
no effect on ant or millipede numbers but the numbers of lycosid
spiders and crickets were reduced. At the end of the study, after four
applications of DDT, ants and millipedes were also reduced in number.
When Shires (1985) treated cereals on clay loam soil in experimen-
tal plots with DDT (1 kg/ha), the numbers of predatory beetles
(Carabidae) were reduced by 50% one week after application. However,
the numbers increased again after 4 to 6 weeks and remained at control
levels. The use of other insecticides led to a second decrease in
Carabidae numbers; this was attributed by the authors to a reduction
in the food supply of aphids. DDT failed to control the aphids, which
were tolerant to the compound.
6.2 Birds
Appraisal
DDT and its metabolites can lower the reproductive rate of birds by
causing eggshell thinning (which leads to egg breakage) and by causing
embryo deaths. However, different groups of birds vary greatly in
their sensitivity to these chemicals; predatory birds are extremely
sensitive and, in the wild, often show marked shell thinning, whilst
gallinaceous birds are relatively insensitive. Because of the
difficulties of breeding birds of prey in captivity, most of the
experimental work has been done with insensitive species, which have
often shown little or no shell thinning. The few studies on more
sensitive species have shown shell thinning at levels similar to those
found in the wild. The lowest dietary concentration of DDT reported to
cause shell thinning experimentally was 0.6 mg/kg for the black duck.
The mechanism of shell thinning is not fully understood.
6.2.1 Short-term and long-term toxicity to birds
DDT and its derivatives DDE and TDE have moderate to low toxicity
to birds when given as an acute oral dose or in the diet. The acute
oral and dietary toxicities of DDT, DDE, and TDE to birds are
summarized in Table 6.
These compounds have been studied in a wide variety of species in
tests ranging from a single acute dose to 100 days of dietary dosing.
All three compounds, DDT, DDE, and TDE, have low to moderate toxicity
to young and adult birds. There is no obvious pattern of relative
toxicity between the three compounds. In some species it is DDT that
is the most toxic, while in other species it is TDE. Most of these
laboratory tests have been conducted on species that are easy to
maintain and breed in captivity. These species are unusual in many
respects; they tend to be gallinaceous birds with young that are not
fed by the adults after hatching. They also tend to have long breeding
seasons untypical of most birds in the wild. In the wild, the most
severely affected species of birds are raptors at the top of food
chains. There is little direct laboratory data on toxicity to these
birds. Toxicity to small songbirds, which make up the majority of bird
species, has not been examined either in the laboratory or the field.
Porter & Wiemeyer (1972) fed American kestrels on a diet containing
p,p' -DDE at a concentration of 2.8 mg/kg. Two birds died after 14
and 16 months of treatment; they showed residues of DDE in brain
tissues of 212 and 301 mg/kg, respectively. This compared with mean
residues of 14.9 (range: 4.47-26.6) mg/kg in 11 adult males sacrificed
after 12-16 months on the diet. Van Velzen et al. (1972) investigated
the lethal effect of stored DDT mobilization by brown-headed
cowbirds. Cowbirds were fed for 13 days on a diet containing 100, 200,
or 300 mg p,p' -DDT/kg, and were then given reduced rations of
approximately 43% of normal daily intake for a 6-day period. Of 30
birds dosed, 21 died (6, 7, and 8 from the three dose levels,
respectively). After 4 months, the remaining birds were subjected
to a second period of 6 days on a reduced diet. Four more
birds, out of six, died. In a second experiment, cowbirds were fed
100 mg p,p' -DDT/kg diet for 13 days and then subjected to 4 days of
reduced food intake. Seven out of 20 birds died. There were no deaths
in any of the control groups (i.e., birds dosed but not starved,
undosed and starved, or undosed and unstarved).
6.2.2 Toxicity to birds' eggs
Dunachie & Fletcher (1969) injected chicken eggs with DDT or TDE to
give concentrations, in the egg, varying between 10 and 500 mg/kg.
Two different vehicles were used to dissolve the insecticides (corn oil
and acetone), controls being injected with vehicle alone. The authors
monitored egg hatchability and survival of chicks to 4 days of age.
Some chicks were fed and some were not. No dose of DDT, applied in
either vehicle, had any significant effect on egg hatchability when
compared to controls. However, there was a profound effect on the
chick survival rate. All chicks hatched from eggs treated with DDT at
100 mg/kg, and which were not fed, were dead within 4 days after
hatching. Feeding the chicks eliminated this effect; the survival rate
of fed young was similar to that of controls. Chicks hatched from
eggs treated with 50 mg DDT/kg survived as well as controls, whether
they were fed or not. TDE was found to affect hatchability, but only
when applied in corn oil; the acetone-dissolved material did not have
any significant effect. TDE dissolved in corn oil reduced
hatchability to 60% of control levels at 100 and 200 mg/kg, to 7% at
300 and 400 mg/kg, and to 0% at 500 mg/kg. The effects on chick
survivability were similar to those of DDT. All chicks hatched from
eggs treated with 100 mg TDE/kg were dead after 4 days if they were not
fed, whereas chicks from eggs treated with 50 mg/kg survived as well as
controls. Chicks from either 100 or 200 mg/kg treatments survived as
well as controls as long as they were fed. The significance of the
different vehicles was discussed by Cooke (1971) and Gilman et al.
(1978). Acetone causes coagulation of yolk protein whereas corn oil
allows the injected organochlorine to float through the yolk to a
position directly under the blastodisc.
Table 6. Toxicity of DDT and its derivatives to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
Bobwhite quail 23 days diet DDE 99.9 5-day LC50 825 (697-976) Hill
(Colinus virginianus) 23 days diet DDT 100 5-day LC50 611 (514-724) et al.
23 days diet TDE TG 5-day LC50 2178 (1835-2584) (1975)
young diet DDT 5-day LC50 881 (796-975) Stickel
& Heath (1964)
(wild) diet DDT TG 5-day LC50 1170 (830-1650) Hill et al.
(farm-reared) diet DDT TG 5-day LC50 1610 (1331-1948) (1971)
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 400 (1963)
adult diet DDT 10-day LC50 2500 DeWitt et al.
adult diet DDT 100-day LC50 1000 (1963)
Japanese quail 7 days diet DDE 99.9 5-day LC50 1355 (1111-1648) Hill
(Coturnix coturnix 7 days diet DDT 100 5-day LC50 568 (470-687) et al.
japonica) 7 days diet TDE TG 5-day LC50 3165 (2534-3978) (1975)
M 2 months oral DDT 77.2 acute LD50 841 (607-1170) Hudson et al.
(1984)
California quail M 6 months oral DDT TG acute LD50 595 (430-825) Hudson et al.
(Callipepla F 6 months oral TDE > 95 acute LD50 > 760 (1984)
californica)
Mallard duck 17 days diet DDE 99.9 5-day LC50 3572 (2811-4669) Hill
(Anas platyrhynchos) 17 days diet DDT 100 5-day LC50 1869 (1500-2372) et al.
17 days diet TDE TG 5-day LC50 4814 (3451-7054) (1975)
F 3 months oral DDT 77.2 acute LD50 > 2240 Hudson et al.
F 3 months oral TDE > 95 acute LD50 > 2000 (1984)
young diet DDT 5-day LC50 875 (650-1140) Stickel &
Heath (1964)
young diet DDT 10-day LC50 500 DeWitt
young diet DDT 100-day LC50 > 200 et al.
adult diet DDT 100-day LC50 1000 (1963)
Pheasant 10 days diet DDE 99.9 5-day LC50 829 (746-922) Hill
(Phasianus colchicus) 21 days diet DDT 100 5-day LC50 311 (256-374) et al.
10 days diet TDE TG 5-day LC50 445 (402-494) (1975)
F 3-4 months oral DDT > 99 acute LD50 1334 (894-1990) Hudson et al.
F 3-4 months oral TDE > 95 acute LD50 386 (270-551) (1984)
young diet DDT 5-day LC50 804 (686-942) Stickel &
Heath (1964)
Table 6. (Contd)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 100 (1963)
adult diet DDT 10-day LC50 1000 DeWitt et al.
adult diet DDT 100-day LC50 > 100 (1963)
Red-winged blackbird diet DDT 10-day LC50 1000 DeWitt et al.
(Agelaius phoeniceus) diet DDT 30-day LC50 500 (1963)
Cardinal diet DDT TG 5-day LC50 535 (420-700) Hill et al.
(Richmondena cardinalis) (1971)
House sparrow diet DDT TG 5-day LC50 415 (370-465) Hill et al.
(Passer domesticus) (1971)
Blue jay diet DDT TG 5-day LC50 415 (320-540) Hill et al.
(Cyanocitta cristata) (1971)
Rock dove M,F oral DDT 77.2 acute LD50 > 4000 Hudson et al.
(Columba livia) (1984)
Sandhill crane M,F adult oral DDT > 99 acute LD50 > 1200 Hudson et al.
(Grus canadensis) (1984)
Clapper rail M diet DDT 5-day LC50 1612 Van Velzen &
(1975) Kreitzer
(Rallus F diet DDT 5-day LC50 1896 (1975)
longirostris) (1975)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed
as mg/kg diet).
c TG = Technical grade.
6.2.3 Reproductive effects on birds
DDT, or more specifically its metabolite DDE, causes the shells of
birds' eggs to be thinner than normal. Results on eggshell thinning
are summarized in Table 7. There is considerable variation between
species for this effect. Galliform species are very resistant to shell
thinning whereas birds of prey are particularly susceptible.
Lincer (1975) dosed captive American kestrels and established a
clear relationship between dietary DDE and thinning of eggshells.
There was a similar close correlation between the residues of DDE in
individual eggs and the degree of shell thinning. The kestrels were
fed with day-old cockerels (which were injected with 0.2 ml of corn
oil, containing the DDE, into the breast muscle) and received either
0.3, 3, 6, or 10 mg DDE/kg diet. Residues of DDE in eggs laid by the
birds correlated closely with dietary DDE concentration; residues of
1.9 mg/kg wet weight were associated with the lowest dose and 245 mg/kg
with the highest dose given. There was no shell thinning associated
with the dose of 0.3 mg/kg. The other doses showed 15.1%, 22.8%, and
29.2% thinning (at 3, 6, and 10 mg/kg, respectively). There was a
straight-line relationship between the degree of shell thinning and
the logarithm of the DDE residue in the egg. Data obtained from the
field showed exactly the same trend (Fig. 1). This represents the best
evidence for the effect of DDE on shell thickness in a species actually
adversely affected in the field.
Table 7. Thinning effects of DDT and its derivatives on bird egg shells
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Ring dove diet DDE 10 - 9.2 0.01 Peakall et al. (1973)
(Streptopelia diet DDE 40 - 6.8 0.01 Haegele & Hudson (1973)
risoria)
Mallard diet TDE 10 - 2.6 NS Heath et al. (1969)
(Anas platyrhynchos) diet TDE 10 - 5.4 NS Heath et al. (1969)
diet TDE 40 - 2.6 NS Heath et al. (1969)
diet TDE 40 - 5.4 NS Heath et al. (1969)
diet DDT 2.5 - 5.3 NS Heath et al. (1969)
diet DDT 10 - 7.9 NS Heath et al. (1969)
diet DDT 40/25 -13.2 0.01 Heath et al. (1969)
White pekin duck diet DDE 40 -20.3 0.001 Peakall et al. (1973)
4 day diet DDE 40 - 3.3 0.01 Miller et al. (1976)
1-3 months diet DDE 40 -18.2 0.01 Miller et al. (1976)
Black duck diet DDE 10 -17.6 0.01 Longcore et al. (1971)
(Anas rubripes) diet DDE 30 -23.5 0.01 Longcore et al. (1971)
Screech owl diet DDE 2.8 -13.3 0.01 McLane & Hall (1972)
(Otus asio)
American kestrel diet DDE 3 -15.2 0.05 Peakall et al. (1973)
(Falco sparverius) diet DDE 6 -21.0 0.01 Peakall et al. (1973)
diet DDE 10 -26.3 0.001 Peakall et al. (1973)
diet DDE 2.8 - 8.7 0.001 Wiemeyer & Porter (1970)
diet DDE 0.3 + 2.1 NS Lincer (1975)
diet DDE 3 -15.1 0.05 Lincer (1975)
diet DDE 6 -22.8 0.01 Lincer (1975)
diet DDE 10 -29.2 0.001 Lincer (1975)
Table 7. (Contd).
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Japanese quail diet o,p'-DDT 100 - 4.0 0.001d Bitman et al. (1969)
(Coturnix coturnix diet DDT 100 - 5.6 0.001d Bitman et al. (1969)
japonica) diet DDT 100 0 NS Cecil et al. (1971)
diet DDE 100 - 2.5 NS Cecil et al. (1971)
diet DDE 2 + 1.9 NS Davison et al. (1976)
diet DDE 10 + 6.3 NS Davison et al. (1976)
diet DDE 40 + 5.0 NS Davison et al. (1976)
diet DDE 200 - 0.6 NS Davison et al. (1976)
strain 1a diet DDT 2.5 + 1.0 NS Davison et al. (1976)
diet DDT 10 - 1.5 NS Davison et al. (1976)
diet DDT 40 - 0.5 NS Davison et al. (1976)
strain 1b diet DDT 2.5 - 2.7 NS Davison et al. (1976)
diet DDT 10 - 1.6 NS Davison et al. (1976)
diet DDT 40 - 7.1 NS Davison et al. (1976)
strain 2a diet DDT 2.5 + 0.5 NS Davison et al. (1976)
diet DDT 10 + 1.6 NS Davison et al. (1976)
diet DDT 40 + 1.0 NS Davison et al. (1976)
strain 2b diet DDT 2.5 - 3.7 NS Davison et al. (1976)
diet DDT 10 - 2.6 NS Davison et al. (1976)
diet DDT 40 - 5.7 NS Davison et al. (1976)
---------------------------------------------------------------------------------------------------------
a Individually caged.
b Caged in pairs.
c DDT in the p,p' - form unless stated otherwise.
d Low calcium diet (0.56%).
e S = not significant.
------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chloroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethlyene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Some related insecticides
NO2
Bulan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-a-(4-chlorophenyl)- -Cl -OH -CH3
a-(methyl)benzenemethanol
dicocol 4-chloro-a-(4-chlorophenyl)-a- -Cl -OH -CCl3
(Kelthane(r)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-a-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
a-hydroxybenzeneacetate
chloropropopylatec 1-methylethyl 4-chloro-a- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-a-hydroxy-
benzeneacetate
Table 1. Structure of p,p' -DDT and its analogues of the form (continued)
------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Perthane(r) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
------------------------------------------------------------------------------------
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been
sold under the name Rothane(r); in metabloic studies the same compound has been
referred as DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
(r) Registered.
3. KINETICS, METABOLISM, BIOTRANSFORMATION, AND BIOACCUMULATION
Appraisal
The physicochemical properties of DDT and its metabolites enable
these compounds to be taken up readily by organisms. The rates of
accumulation vary with the species, with the duration and concentration
of exposure, and with environmental conditions.
These compounds are resistant to breakdown and are readily adsorbed
to sediments and soils, which can act both as sinks and as long-term
sources of exposure (e.g., for soil organisms).
Organisms can accumulate these chemicals from the surrounding
medium and from food. In aquatic organisms, uptake from the water is
generally more important, whereas, in terrestrial fauna, food provides
the major source.
In general, organisms at higher trophic levels tend to contain more
DDT-type compounds than those at lower trophic levels.
Such compounds can be transported around the world in the bodies of
migrant animals and in ocean and air currents.
Different organisms metabolise DDT via different pathways. Of the
two initial metabolites, DDE is the more persistent, though not all
organisms produce DDE from DDT. The alternative route of metabolism,
via TDE leads to more rapid elimination (WHO, 1979). Much of the
retained DDT and its metabolites are stored in lipid-rich tissues.
Because there is an annual cycle in lipid storage and utilization in
many organisms, there is also a related annual cyclic pattern in the
handling of DDT.
3.1 Retention in Soils and Sediments and Plant Uptake
Shin et al. (1970) investigated the adsorption of DDT by soils of
various different types and by isolated soil fractions. A sandy loam,
a clay soil, and a highly organic muck were either used intact or had
various components extracted before estimating their adsorptive
capacity for the insecticide. Adsorption was least in the sandy loam
and greatest in the muck (distribution coefficients [Kd] were in the
ratio 1:10:80 for sandy loam, clay soil, and organic muck,
respectively). All soils showed a strong adsorptive capacity for DDT.
The adsorption of DDT was closely related to the organic matter content
of the soils; progressive removal of lipids, resins, polysaccharides,
polyuronides, and humic matter identified the organic fractions which
bound the DDT. Humic material represents a major source of adsorptive
capacity for DDT; the degree of sorption, however, is strongly
connected with the degree of humification. Soil containing large
amounts of humic material may not adsorb DDT as greatly as other soils
where humification is more advanced. Wheatley (1965) estimated half-
times for the loss of DDT applied to soils. After surface application,
50% of DDT was lost within 16-20 days. The estimated time for the loss
of 90% of surface-applied DDT was 1.5 to 2 years. With DDT mixed into
the soil, 50% loss occurred in 5 to 8 years, and it was estimated that
90% of applied insecticide would be lost in 25-40 years.
Albone et al. (1972) investigated the capacity of river
sediments, from the Severn Estuary, United Kingdom, to degrade DDT.
p,p' -DDT (14C-labelled) was applied to sediments either in situ on
the mud flats or in the laboratory. Sediment movement in the area of
the in situ study was sufficiently small to neither bury nor expose
the incubation tubes set into the mud. Incubation in situ over 46
days led to very little metabolism of DDT in the sediments. Some
p,p' -TDE was produced, but the ratio of DDT to TDE was 13 : 1 and
48 : 1 in two replicate experiments. There was no production of
extractable polar products; metabolism beyond TDE did not occur.
Incubation of the same sediments in the laboratory, over 21 days, led
to much greater metabolism (ratios of 1 : 1.1 and 1 : 3.3, DDT to TDE,
in replicate incubations) and the production of some unidentified,
further breakdown products. Investigation of the microbial population
of the sediment showed that some of the organisms were capable of
degrading DDT; little metabolism appeared to take place in situ .
3.2 Uptake and Accumulation by Organisms
The uptake and accumulation of DDT and its metabolites into
organisms, as determined in controlled laboratory experiments, is
summarized in Table 2. Results are expressed as bioconcentration
factors (the ratio of the concentration of the compound in the organism
to the concentration in the medium).
Concentration factors can be misleading with compounds such as DDT
when exposure is high. The compound is readily taken up and retained
at very low concentrations. At high concentrations, no more material
can be taken up because a plateau has been reached. The only
meaningful way to assess the capacity of organisms to take up and
retain DDT is by looking over a wide range of exposure levels. The
low concentration factor quoted in Table 2 for earthworms, for example,
reflects the high exposure rather than a low capacity for uptake and
retention of DDT, because concentration factors are simple ratios
between "exposure" and final concentration in the organism.
Concentration factors for fish are generally higher than for their
invertebrate prey (Table 2). It is now generally agreed that most of
the DDT taken into aquatic organisms comes from the water rather than
from their food (Moriarty, 1975). Again, the concentration factors can
be misleading. Aquatic organisms take in a small proportion of
ingested DDT. However, they retain a large proportion of the DDT which
has been absorbed into the body from the food. There has been some
controversy in the past over explanations for higher accumulations of
DDT at higher trophic levels in aquatic systems. It now seems clear
that this is not due primarily to biomagnification up food chains but
rather to a tendency for organisms at higher trophic levels to
accumulate more DDT directly from the water.
Terrestrial organisms do not live in a uniform medium surrounded by
a relatively constant concentration of a chemical. Even soil organisms
live in a medium with very variable concentrations of DDT or its
metabolites at different levels of the soil profile or patchy distri-
bution of the chemical. Some terrestrial organisms could be directly
exposed to DDT during application of the insecticide, but most will be
exposed to what remains of the DDT after application. Therefore,
higher terrestrial organisms will accumulate DDT mostly from their
food. The data in Table 2 are taken from controlled laboratory
investigations. There is ample evidence from the field that DDT does
accumulate in many organisms in different media. There is similarly
evidence that the residues of DDT or its metabolites persist in
organisms for long periods after exposure has ceased. The following
should not be regarded as a comprehensive review of the literature on
this subject, which is too large to be included. Rather, these are
examples from different groups of organisms.
Table 2. Bioaccumulation of DDTa
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Bacteria
Aerobacter aerogenes 100 22 24 h 1.2 3736 Johnson &
Bacillus subtilis 130 22 24 h 0.676 4303 & Kennedy
Aerobacter aerogenes 25 22 4 h 0.64 10 639 (1973)
200 22 4 h 0.64 1784 Johnson &
Bacillus subtilis 43 22 4 h 0.64 13 880 Kennedy
348 22 4 h 0.64 1805 (1973)
Marine algae
Cyclotella nana 17 23 2 h 0.7 37 600 Rice & Sikka
8 23 2 h 0.7 58 100 (1973)
Isochrysis galbane 39 23 2 h 0.7 11 300 Rice & Sikka
19 23 2 h 0.7 28 800 (1973)
Olisthodiscus luteus 108 23 2 h 0.7 4600 Rice & Sikka
54 23 2 h 0.7 7000 (1973)
Amphidinium carteri 66 23 2 h 0.7 4300 Rice & Sikka
33 23 2 h 0.7 9600 (1973)
Tetraselmis chuii 106 23 2 h 0.7 5200 Rice & Sikka
53 23 2 h 0.7 6300 (1973)
Skeletonema costatum 29 23 2 h 0.7 31 900 Rice & Sikka
15 23 2 h 0.7 38 400 (1973)
Diatom
Cylindrotheca 21 days 100 300 Keil & Priester
closterium (1969)
Pond snail stat 6 days 3.0 6000 Reinbold et al.
(Physa 5 sp.) (1971)
Freshwater mussel flow 20 3 weeks 0.62 3990d Bedford & Zabik
(Anodonta grandis) (1973)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Earthworm 10 4 weeks 17 000 0.47d Davis (1971)
(Lumbricus terrestris)
Water flea stat 30 3 days 2.0 1330 Metcalf et al.
(1973)
(Daphnia magna) flow 21 3 days 0.08 114 100 Johnson et al.
(1971)
Scud flow 21 3 days 0.081 20 600 Johnson et al.
(Gammarus fasciatus) (1971)
Glass shrimp flow 21 3 days 0.1 5000 Johnson et al.
(Palaemonetes kadiakensis) (1974)
Pink shrimp flow 8-15 13 days 0.14 1500 Nimmo et al.
(Penaeus duorarum) (1970)
Crayfish flow 21 3 days 0.08 2900 Johnson et al.
(Orconectes nais) (1971)
Mayfly larva flow 21 3 days 0.052 32 600 Johnson et al.
(Hexagenia bilineata) (1971)
Mayfly larva flow 21 3 days 0.047 22 900 Johnson et al.
(Siphlonurus sp.) (1971)
Dragonfly nymph flow 21 2 days 0.101 3500 Johnson et al.
(Ischnura verticalis) (1971)
Dragonfly nymph flow 21 2 days 0.079 910 Johnson et al.
(Libellula sp.) (1971)
Midge larva flow 21 3 days 0.046 47 800 Johnson et al.
(Chironomus sp.) (1971)
Mosquito larva flow 21 2 days 0.105 133 600 Johnson et al.
(Culex pipiens) (1971)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Mosquito larva stat 30 3 days 2.0 110d Metcalf et al.
(Culex quinquifasciatus) stat 30 3 days 0.9 74d (1973)
Mosquito fish stat 30 3 days 2.0 344d Metcalf et al.
(Gambusia affinis) stat 30 3 days 0.9 217d (1973)
Rainbow trout flow 5 12 weeks 0.176 21 363d Reinert et al.
(Salmo gairdneri) flow 10 12 weeks 0.137 43 158d (1974)
flow 15 12 weeks 0.133 51 355d Reinert et al.
(1974)
Brook trout flow 14 120 days 3 mg 0.64d Macek & Korn
(Salvelinus fontinalis) /kg diet (1970)
flow 14 120 days 0.003 8533d Macek & Korn
(1970)
Pinfish flow 14 days 0.1 40 000d Hansen & Wilson
(Lagodon rhomboides) flow 14 days 1.0 11 020d (1970)
Atlantic croaker flow 14 days 0.1 12 500d Hansen & Wilson
(Micropogon undulatus) flow 14 days 1.0 12 170d (1970)
Fathead minnow flow 24-25.5 14 days 45.6 mg/kg 1.17d Jarvinen et al.
(Pimephales promelas) flow 24-25.5 14 days 0.5 85 400d (1977)
flow 24-25.5 14 days 2.0 69 100d Jarvinen et al.
flow 24-25.5 112 days 45.6 mg/kg 1.33d (1977)
flow 24-25.5 112 days 0.5 93 200d Jarvinen et al.
flow 24-25.5 112 days 2.0 154 100d (1977)
Tilapia stat 31 days 1.0 6800 Reinbold et al.
(Tilapia mossambica) 31 days 10 10 600 (1971)
Green sunfish stat 31 days 1.0 3900 Reinbold et al.
(Lepomis cyanellus) 31 days 10 4020 (1971)
stat 22 15 days 0.1-0.3 17 500d Sanborn et al.
(1975)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Chicken eggs 8 weeks 0.1 1.87d Foster et al.
fat 8 weeks 0.1 5.8d (1972)
Broiler hen fat 6 weeks 1.0 10.3d Kan et al.
(1978)
White pelican WB 10 weeks 72 11.9d Greichus et al.
(Pelecanus erythrorhynchos) (1975)
Double-crested cormorant WB 9 weeks 0.95 236.3d Greichus &
(Phalacrocorax a. auritus) Hannon (1973)
American kestrel WB 11-16 2.8 103.9 Porter &
(Falco sparverius) months Wiemeyer (1972)
Mule deere muscle 30 days 5 mg/day 122.8 ug Watson et al.
(Odocoileus heminonus) oral /kgd (1975)
---------------------------------------------------------------------------------------------------------
a Unless specified otherwise, bioconcentration factors are based on whole body (WB) measurements.
b Stat = static conditions (water unchanged for duration of experiment);
Flow = flow-through conditions (DDT concentration in water continuously maintained).
c Bioconcentration factor = concentration of DDT in organism/concentration of DDT in medium or food.
Concentrations of DDT in organisms represents total DDT, i.e., DDT plus its stable metabolites,
principally DDE. Bioconcentration factors calculated on a dry weight basis unless otherwise stated.
d Calculated on a wet weight basis.
e Oral dose (by capsule) given daily.
3.2.1 Plants
Fuhremann & Lichtenstein (1980) applied 14C-labelled p,p' -DDT to
loam or sandy soil (at 4 and 2 mg/kg, respectively) and grew oat plants
on the treated soils for 13 days. At harvest, residues of DDT and its
metabolites were analysed in soil and plant by scintillation counting,
thin layer chromatography, and GLC. Of the total applied DDT, 95.7%
was recovered from loam soil and 88.6% from sandy soil. Almost all of
the DDT present was extractable in organic solvent (only 2.8%, for
loam, and 0.7%, for sand, was present in a water-bound form),
indicating little or no metabolism of the compound except to persistent
organically extractable residues. DDE was detected in both soils,
accounting for 3.4% of the total extracted in loam soil and 2.2% in
sand. Other metabolites, including o,p' -DDT, TDE, and dicofol were
recovered in very small quantities. Very little DDT (and none of its
metabolites) was detected in oat roots grown on loam, amounting to 0.2%
of the total DDT applied. The uptake was greater (4.6%) in roots of
oats grown on sand, but the uptake of labelled carbon into plant tops,
from both soils, was so low that it could not be analysed.
DDT was not translocated into the foliage of alfalfa when applied
to the soil (Ware, 1968; Ware et al., 1970) or into soybeans (Eden &
Arthur, 1965). Harris & Sans (1967) found only trace amounts of DDT
or its metabolites in the storage roots of carrots, radishes, and
turnips after growing the plants in soils containing up to 14 mg
DDT/kg.
3.2.2 Microorganisms
The uptake and accumulation of DDT from the culture medium by
microorganisms has been reviewed by Lal & Saxena (1982). All of the
microorganisms studied showed some capacity to take up DDT from their
growth medium, but the relative amount taken up varied greatly from
species to species. Many species took up more than 90% of the DDT when
exposed to concentrations ranging from 1 to 1000 µg/litre, whereas a
few species took in only 0.5% of the available DDT. The concentration
factors (i.e., the concentration within the organism expressed as a
ratio against the concentration in the medium) for DDT were variable
but always high (Table 2).
3.2.3 Aquatic invertebrates
Concentration factors are also variable in aquatic invertebrates.
In all cases there is considerable uptake and retention of the DDT,
though often as DDE or other metabolites rather than as the parent
compound. The main point of interest is the ability of aquatic
organisms to take up large amounts of the compound, over time, from
water where DDT is present at very low concentrations, and to retain
it.
Risebrough et al. (1976) measured DDT in sea water and in mussels
( Mytilus sp.) from San Fransisco Bay and the French Mediterranean
coast. Concentration factors varied between 40 000 and 690 000 for DDT
and between 45 000 and 310 000 for DDE.
Eberhardt et al. (1971) applied radioactively labelled DDT, at a
rate of 220 g/ha, to a freshwater marsh and followed the distribution
of the compound and its metabolites. Concentration factors in ten
species of plants varied between 5500 and 84 000. Various invert-
ebrates showed high concentration factors: ramshorn snail
( Planorbidae ), 4700; backswimmer ( Notonectidae ), 10 000; crayfish
( Orconectes immunis ), 22 000; bloodworm ( Tendipes ), 25 000; and red
leech ( Erpobdella punctata ), 47 000. Reporting earlier on the same
study, over 15 months, Meeks (1968) showed that plants and invert-
ebrates accumulated DDT to a maximum mainly within the first week after
treatment, whereas vertebrates required longer to attain maximum
residues. Residues of DDT in the surface water and suspended particles
had fallen below detectable levels within 1 month. Residues in
sediments stabilized at about 0.3 mg/kg after 9 months.
3.2.4 Fish
The uptake of DDT from water is affected by the size of the fish;
smaller fish take up relatively more DDT from water than larger
specimens of the same species. A range in weight of mosquitofish
between 70 and 1000 mg led to a four-fold difference between the
smallest and largest fish in DDT uptake from water over 48 h (Murphy,
1971).
A rise in temperature results in increased uptake of DDT by fish
(Reinert et al., 1974). Rainbow trout were exposed to a single water
concentration of DDT (nominally 330 ng/litre) at temperatures of 5, 10,
or 15 °C; the actual concentrations of DDT in water varied with
temperature and were measured at 176, 137, and 133 ng/litre,
respectively, for 5, 10, and 15 °C. Whole body residues of DDT (total)
after 12 weeks exposure were 3.8, 5.9, and 6.8 mg/kg for the three
temperatures, respectively. Expressing the results as bioconcentration
factors to allow for the differences in dissolved DDT showed a similar,
clear increase in the relative amount of DDT taken up and retained
(Reinert et al., 1974).
Increasing salinity decreases DDT uptake significantly, but has
no effect on the uptake of DDE or TDE by fish (Murphy, 1970).
Increasing the salinity from 0.15o/oo to 10o/oo decreased DDT
uptake over 24 h from 22% of the dose to 18% (body residues decreased
from 658 to 329 ng). There was a further significant decrease in
uptake when the salinity was increased to 15o/oo (Murphy, 1970)
Fish accumulate DDT from food in a dose-dependent manner. When
Macek et al. (1970) fed rainbow trout on diets containing 0.2 or 1.0 mg
DDT/kg, the fish retained more than 90% of the dietary intake of DDT
(measured as total DDT) over the 90-day exposure period. The authors
estimated the time required for the elimination of 50% of accumulated
DDT to be 160 (± 18) days. When Warlen et al. (1977) fed Atlantic
menhaden on a diet containing 14C-labelled DDT at three dose levels,
the fish assimilated and retained between 17% and 27% of the cumulative
dose from food containing 0.58, 9.0, or 93 µg/kg. There was a
straight-line relationship between exposure time and body burden of
total DDT, with no tendency for residues to reach a plateau within the
45 days of feeding with DDT. At the end of the feeding period, the
fish had accumulated DDT or its metabolites, to levels of approximately
1.1, 11, and 110 µg/kg for the three doses respectively. The
biological half-time of DDT in the fish was estimated to be 428, 64,
and 137 days, for groups exposed to 0.58, 9.0, or 93 µg/kg diet,
respectively.
3.2.5 Terrestrial invertebrates
Relatively low concentration factors have been reported for
terrestrial molluscs by Dindal & Wurtzinger (1971), who also reviewed
the earlier literature. However, low concentration factors, derived
from short-term studies, can be misleading for these organisms because
of the high persistence of DDT in soil. Residues of DDT were as high
as 40 mg/kg and, therefore, molluscs represent a source of DDT which
will be concentrated by organisms which eat them. The same is true
for earthworms, which also show low concentration factors (Davis, 1971;
Edwards & Jeffs, 1974). Gish & Hughes (1982) investigated residues of
DDT and other pesticides in earthworms for 2 years following appli-
cation. They showed that body residue levels were cyclic, with higher
levels of DDT and its metabolites occuring between late spring and
early autumn and lower levels from late autumn to early spring. Peak
high levels occurred in May and low levels in January, coinciding with
the seasonal high and low activity periods of earthworms. These
changing residue levels presumably indicate that DDT is retained in
soil and that earthworms contain more of the residual metabolites when
they are processing more soil through the gut.
3.2.6 Birds
Laboratory studies on birds have shown them capable of accumulating
DDT from food, yielding high concentration factors (Table 2).
The accumulation of DDT and its metabolites in birds in the field
has been regularly and extensively reviewed (Moore, 1965; Moriarty,
1975; Newton, 1979). The results of an analysis of a long-term
sampling programme of birds in the United Kingdom (Cooke et al., 1982)
confirm many of the early theories. Birds with the highest residues of
DDT or its metabolites were either terrestrial predators feeding on
other birds or aquatic predators feeding on fish. Thus, residues of
DDE in the liver of the peregrine falcon, with birds as its principal
dietary component, averaged 7.56 mg/kg, whereas for the rough-legged
buzzard, with mammals as the principal dietary component, mean DDE
levels were 0.05 mg/kg over a period extending from the early 1960s to
the late 1970s.
There are marked geographical differences throughout the United
Kingdom, related to usage patterns of DDT (Cooke et al., 1982), and
also marked seasonal changes in residues. These seasonal changes
appear to relate more to physiological changes in body composition,
which occur with climatic and breeding seasons, than to the environ-
mental availability of pollutants. Some species, e.g., heron, barn
owl, and kingfisher, showed a decline in DDE residues with time, but
others, e.g., sparrowhawk, kestrel, and great-crested grebe, did not,
levels in 1977 being similar to those in 1963. Eventually residues of
DDT in wildlife decline with time after a ban is imposed on the use of
the pesticide. However, the highly persistent nature of DDE means that
significant residues will continue to be found for a considerable
period. The situation in the United Kingdom and the USA appears to be
broadly similar (O'Shea & Ludke, 1979).
3.2.7 Mammals
DDT is taken up by, and retained in, wild mammals. The degree of
uptake and retention varies with the species. In a study following a
single application of DDT to a forest to control spruce budworm at a
rate of 0.89 kg/ha, Dimond & Sherburne (1969) and Sherburne & Dimond
(1969) reported residues of DDT and its metabolites in mammals over 9
years. Herbivorous mice, voles, and hares contained less DDT than
carnivorous mink and insectivorous shrews. In herbivores, residues
approached pre-treatment levels after 6-7 years, whereas residues were
still significantly higher in shrews and mink than in the same species
taken from untreated areas 9 years after the single treatment with DDT.
In these species, the authors calculate that it would take at least 15
years for residues to reach background levels. They regard the high
residue levels in mammals at higher trophic levels as deriving
principally from DDT retained in the soil, since there is little long-
term retention on vegetation.
In a 3-year study, after treating a field ecosystem with 36Cl-
ring-labelled DDT at a dose rate of 0.92 kg/ha, Forsyth & Peterle
(1973) measured DDT residues in various tissues of two species of
shrew. The highest residue (135 mg/kg) occurred in fat, compared with
10, 10, and 4 mg/kg in liver, muscle, and brain, respectively. Shrews
of the species Blarina brevicauda released into treated areas accumu-
lated DDT to the same degree as resident shrews within 15-20 days of
exposure. Equilibrium between intake and excretion of DDT occurred
within approximately 30 days in muscle, liver, and brain and within
40 days in fat. The second species of shrew ( Sorex cinereus )
accumulated residue levels of DDT during the following 2 years which
were successively greater than levels present in the first year,
indicating that DDT was increasing in availability to this species
with the passage of time. The levels of DDT in muscle were not
influenced by sex but were influenced by breeding condition. Male
shrews with scrotal testes and lactating females developed lower
levels of DDT in muscle and viscera than did males with abdominal
testes or non-lactating females.
Benson & Smith (1972) measured levels of DDT and its metabolites in
deer exposed to DDT used for spruce budworm control, and found that, in
the year of spraying, there was up to 20 mg/kg in fat. Males had
considerably higher levels of DDT than females. Fawns also had higher
levels than their mothers, though this was from a small sample. The
majority of the residues consisted of p,p' -DDT, with almost insignifi-
cant levels of DDE. Five years later, the residue levels in males were
still higher than those in females, though these had fallen to about
1% of original levels. Most of the deer population was 3 years old or
less, and so the figures for 5 years after spraying represent DDT
ingested from the environment and not from direct exposure.
Some, though very little, DDT was detected in black bears by
Benson et al. (1974). There was no evidence that the area had been
directly sprayed with DDT. This study illustrates that there is a
general environmental contamination with DDT, which can be accumulated
by mammals, though to a small degree, without direct application of the
material to their habitat.
4. TOXICITY TO MICROORGANISMS
Appraisal
Aquatic microorganisms are more sensitive than terrestrial ones to
DDT.
An environmental exposure concentration of 0.1 µg/litre can cause
inhibition of growth and photosynthesis in green algae.
Repeated applications of DDT can lead to tolerance in some micro-
organisms.
There is no information on effects concerning the species compo-
sition of microorganism communities. Therefore, it is difficult to
extrapolate the relevance of single-culture studies to aquatic or
terrestrial ecosystems. However, since microorganisms are basic in
food chains, adverse effects on their populations would influence
ecosystems. Thus, DDT and its metabolites should be regarded as a
major environmental hazard.
Studies cited in this section will be restricted to those effects
produced by low concentrations of DDT. Some studies still use DDT at
concentrations above its water solubility. Reviews of other effects of
DDT and its analogues, at higher concentrations, on cell division and
several biochemical parameters have been produced by Luard (1973) and
Lal & Saxena (1979).
4.1 Bacteria and Cyanobacteria (Blue-green Algae)
Ledford & Chen (1969) cultured bacteria isolated from surface-
ripened cheese with 0.5 mg DDT/litre or 0.5 mg DDE/litre, but found no
effect on growth.
At a concentration of 10 µg/litre in the culture medium, DDT
stimulated the growth of the bacterium Escherichia coli (Keil et al.,
1972). Yields of cultures exposed to 100 µg/litre did not differ from
controls. There was no effect of DDT on denitrification (conversion of
nitrate to nitrite) at a concentration of 100 mg/kg in soil and,
similarly, no effect on this process when carried out by a bacterial
culture (Bollag & Henninger, 1976). DDT at up to 22 kg/ha did not
affect the numbers of soil bacteria in outdoor-treated plots (Bollen et
al., 1954), and five annual applications of DDT to a sandy loam soil
did not significantly affect the numbers of soil bacteria (Martin,
1966).
Concerning cyanobacteria (blue-green algae), Goulding & Ellis
(1981) found no effect on the growth of Anabaena variabilis at a DDT
concentration of 1 µg/litre. Batterton et al. (1972) suggested that
DDT reduced the tolerance of Anocystis nidulans to sodium chloride.
The organism is resistant to salt and to DDT, at concentrations up to
8000 mg/litre, but not to combinations of the two stressors.
4.2 Freshwater Microorganisms
Lee et al. (1976) showed that DDT inhibited photosynthesis in the
green alga Selenastrum capricornutum at concentrations between 3.6 and
36 µg/litre, inhibition increasing with time of exposure.
Two different species of green algae were shown to be resistant
to DDT and its metabolites, DDE and TDE, at concentrations up to
1000 mg/litre in culture. Scenedesmus and Dunaliella revealed rates of
photosynthetic uptake of 14C-labelled CO2 similar to those of
controls (Luard, 1973). Considerable variation exists between species
of microorganisms concerning the effect of DDT and its analogues;
resistance to DDT is not restricted to one taxonomic group, either
freshwater or marine (Luard, 1973). The source of the resistance is
unclear. The two species studied show very different characteristics;
Dunaliella has no cell wall, whereas Scenedesmus has a complex cell
wall. Since both show resistance to DDT, it is unlikely that the
chemical is excluded from the cell by the cell wall. Cell membranes
and chloroplast membranes are an alternative barrier to DDT uptake and
effect. It is not known how these structures might be involved in DDT
resistance; studies with isolated chloroplasts suggest that there is no
barrier to DDT uptake there.
Cole & Plapp (1974) found inhibition of growth and photosynthesis
of the green alga Chlorella pyrenoidosa with DDT at 1 µg/litre in the
medium. However, inhibition was inversely related to the number of
cells in the culture. With high cell counts, there was no
inhibition of either growth or photosynthesis with DDT present at up to
1 mg/litre. Inhibition only occurred at low cell densities in culture.
Goulding & Ellis (1981) found that the green alga Chlorella fusca
was affected by DDT at 0.1 µg/litre. The amount of inhibition of
growth varied with time and with the method of assessing the result.
Cell numbers were maximally affected (75% inhibition) after 72 hours,
and after 200 hours cell numbers had reached control levels. When
growth was assessed by chlorophyll content or biovolume, the initial
inhibition was more marked and cultures were only equivalent to
controls after 480 hours. The apparent anomaly is explained by
reductions in cell size in response to DDT.
Christie (1969) reported no effect of DDT on the growth of
Chlorella and attributed this to the ability of the organism to
metabolize the compound.
Lal & Saxena (1980) reported that DDT did not affect growth and DNA
synthesis in the ciliate Stylonychia notophora at concentrations of
1 mg/litre or less.
4.3 Marine Microorganisms
MacFarlane et al. (1972) showed that DDT, at concentrations
between 9.4 and 1000 µg/litre, reduced photosynthetic carbon
fixation and the cell content of chlorophyll a relative to controls
in a marine diatom Nitzschia delicatissima , over a 24-h period.
The diatom was cultured with DDT under four different light inten-
sities. The insecticide had the greatest effect at the highest light
intensity, where carbon fixation was reduced by 94% in water containing
100 µg DDT/litre. At higher DDT concentrations, there was no further
reduction in either carbon fixation or chlorophyll content.
The photosynthesis of several species of marine phytoplankton has
been found to be inhibited by DDT at concentrations of 100 µg/litre or
less (Wurster, 1968). Four different species showed increasing
inhibition up to DDT concentrations of 100 µg/litre, but no greater
effect at higher concentrations. A green alga, Pyramimonas , was
affected by DDT only at concentrations higher than 10 µg/litre. The
other three species, a diatom, a coccolithophore, and a dinoflagellate
were affected at DDT concentrations between 1 and 10 µg/litre. In
a similar study (Menzel et al., 1970) four different species of
marine phytoplankton were studied. Inhibition of photosynthesis,
where it occurred, followed a similar dose-response relationship.
For three species ( Skeletonema costatum , a diatom; Coccolithus
huxleyi , a coccolithophorid; and Cyclotella nana , a second diatom)
inhibition began between 1 and 10 µg DDT/litre and reached a maximum
at 100 µg/litre. The other organism, a green flagellate Dunaliella
tertiolecta , was unaffected by DDT at concentrations up to 1 mg/litre,
the highest exposure tested.
The marine dinoflagellate Exuviella baltica showed significant
inhibition of growth after exposure to DDT at concentrations as low as
0.1 µg/litre (Powers et al., 1979).
4.4 Soil Microorganisms
TDE had no significant effects on growth and reproduction of soil
amoebae except at concentrations higher than 1 mg/litre (Prescott &
Olson, 1972). Populations of protozoa in garden soil were reduced by
DDT at a concentration of 5 mg/kg (MacRae & Vinckx, 1973). Numbers
were still significantly reduced after 3 months.
4.5 Fungi
Two aquatic and one terrestrial fungi showed stimulated growth
in response to DDT present at concentrations of between 2 and
60 µg/litre of growth medium (Hodkinson & Dalton, 1973)
5. TOXICITY TO AQUATIC ORGANISMS
DDT and its derivatives are highly toxic to aquatic organisms;
water concentrations of a few micrograms per litre are sufficient to
kill a large proportion of populations of aquatic organisms in acute or
short-term exposure. In addition to its high short-term toxicity, DDT
also has long-term sublethal effects on aquatic organisms. Many
physiological and behavioural parameters have been reported to be
affected by the insecticide. This toxicity, coupled with its high
capacity for bioconcentration and biomagnification, means that DDT
presents a major hazard to aquatic organisms.
5.1 Aquatic Invertebrates
Appraisal
Both the acute and long-term toxicities of DDT vary between species
of aquatic invertebrates. Early developmental stages are more
sensitive than adults to DDT. Long-term effects occur after exposure
to concentrations ten to a hundred times lower than those causing
short-term effects.
DDT is highly toxic, in acute exposure, to aquatic invertebrate, at
concentrations as low as 0.3 µg/litre. Toxic effects include
impairment of reproduction and development, cardiovascular
modifications, and neurological changes. Daphnia reproduction is
adversely affected by DDT at 0.5 µg DDT/litre.
The influence of environmental variables (such as temperature,
water hardness, etc.) is documented but the mechanism is not fully
understood. In contrast to the data on DDT, there is less information
on the metabolites DDE or TDE. The reversibility of some effects once
exposure ceases has been reported, as well as the development of
resistance.
5.1.1 Short-term and long-term toxicity
The short-term toxicity to aquatic invertebrates is summarized in
Table 3.
Most aquatic invertebrates are killed by low water concentrations
of DDT and its metabolites, though the majority of the published data
is on DDT itself. Six invertebrate species studied by Macek & Sanders
(1970) showed 96-h LC50 values ranging from 1.8 to 54.0 µg/ litre.
Adult molluscs are relatively resistant to DDT and the compound has
been used to control crustacean pests on oyster beds (Loosanoff, 1959).
However, the larval stages of molluscs are affected by DDT; clam larvae
showed 90% mortality after exposure to DDT at 0.05 mg/litre (Calabrese,
1972). Molluscs exhibit effects on shell growth at low DDT concen-
trations. Tubifex worms are resistant to DDT; 3 mg/litre did not kill
any Tubifex tubifex (Naqvi & Ferguson, 1968). Many aquatic crustaceans
yield LC50 values less than 1 µg/litre. Muirhead-Thomson (1973)
showed that predator invertebrates, such as dragonfly nymphs, were
more tolerant of DDT than prey organisms. Since the prey organisms
are also food for fish, the balance of aquatic ecosystems could be
changed by very low levels of DDT. Lowe (1965) reported that juvenile
blue crabs ( Callinectes sapidus ), exposed to 0.25 µg DDT/litre for 9
months, grew and moulted normally; there were no apparent sublethal
effects. However, exposure to 5 µg DDT/litre killed all crabs.
The metabolite TDE has been studied in parallel tests with the
parent compound in some organisms. There is no consistent relationship
between the toxicity of the two compounds. TDE is considerably less
toxic to stonefly larvae than DDT, by a factor of about 100 (Sanders &
Cope, 1968). However, for other freshwater organisms TDE may have
similar, lower, or greater toxicity according to the organism and
duration of test (Table 3). For most marine invertebrates, DDT is most
toxic, followed by DDE and TDE (data from Mayer, 1987).
5.1.2 Physiological effects on aquatic invertebrates
Butler (1964) demonstrated a 50% reduction in shell growth in young
eastern oysters exposed for 96-h to DDT at 14 µg/litre. Roberts
(1975) showed that DDT at 50 µg/litre reduced the amplitude of
ventricular contractions in the isolated heart of the bivalve Mya
arenaria within 4 minutes. At higher concentrations, DDT stopped heart
contractions altogether. Recovery, even of the arrested heart, was
rapid after the immediate replacement of the DDT solution with clean
sea water.
Kouyoumjian & Uglow (1974) found that for the planarian worm
Polycelis felina , TDE was most toxic and DDT least toxic, with DDE
showing intermediate toxicity. Sublethal effects of DDT and TDE were
demonstrated. DDT reduced the rate of asexual fission. Both DDT and
TDE were shown to reduce the righting time of animals turned onto their
backs. This was presumed to be a nervous system effect.
Maki & Johnson (1975) report 50% reduction in three parameters of
reproduction in the water flea Daphnia magna at 0.5 µg/litre, for
total young produced, at 0.61 µg/litre for average brood size, and at
0.75 µg/litre for percentage of days reproducing.
In vitro effects on gill ATPases of two species of crab have been
reported (Jowett et al., 1978; Neufeld & Pritchard, 1979). There is a
transitory effect in vivo on gill ATPases and, thereby, an effect on
plasma osmolarity. However, this osmoregulatory effect soon disappears
(Pritchard & Neufeld, 1979). Leffler (1975) reported metabolic rate
elevation, decreased muscular coordination, inhibition of autotomy
reflex, and reduced carapace thickness/width ratio in juvenile crabs
exposed to DDT. Osmoregulation was not affected. The DDT was given in
the food of the crabs at a concentration of 0.8 mg/kg. DDT has been
found to accelerate limb regeneration and the onset of the next moult
in fiddler crabs (Weis & Mantel, 1976). The authors suggest that the
effect is on the central nervous system, with DDT causing changes in
neurosecretory activity.
Table 3. Toxicity of DDT and its derivatives to invertebrates
---------------------------------------------------------------------------------------------------------
Organismf Flow Temp Salinity Compound Parameter Water Reference
stata ( °C) o/oo concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine invertebrates
Eastern oyster (juv.) flow 30 23 DDTd 96-h EC50j 9 Mayer (1987)
(Crassostrea virginica) flow 12 25 DDEd 96-h EC50j 14 Mayer (1987)
flow 20 30 TDEd 96-h EC50j 25 Mayer (1987)
Shrimp stat 20 sea water DDTd 96-h LC50 0.4 McLeese &
(Crangon septemspinosa) stat 10 sea water DDTd 96-h LC50 31 Metcalfe (1980)
+ sediment
Mysid shrimp (adult) stat 25 23 DDTd 96-h LC50 0.45 Mayer (1987)
(Mysidopsis bahia) (0.39-0.52)
Pink shrimp (juv.) flow 24 28 DDTd 48-h LC50 0.6 Mayer (1987)
(Penaeus duorarum) flow 16 31 TDEd 48-h LC50 2.4 Mayer (1987)
White shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.7 Mayer (1987)
(Penaeus setiferus)
Grass shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.8 Mayer (1987)
(Palaemonetes pugio)
Brown shrimp (juv.) flow 28 17-27 DDEd 24-h LC50 52 Butler (1964)
(Penaeus aztecus) flow 28 17-27 DDEd 48-h LC50 28 Butler (1964)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata ( °C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Freshwater invertebrates
Water flea stat 20 192 138 8.2- DDTd 48-h LC50 1.1 (1.0-1.3) Randall et
8.5 al. (1979)
(Daphnia magna) stat 15 44 7.1 DDTd 48-h LC50 4.7 (2.8-5.6) Mayer &
Ellersieck
(1986)
stat 20 192 138 8.2- DDTe 48-h LC50 1.7 (1.5-1.8) Randall et
8.5 al. (1979)
statb 24 320- 7.6 DDT 14-day 0.67 (0.65-0.69) Maki &
340 (99%) LC50 Johnson
statb 24 320- 7.6 DDT 14-day 0.5 (0.48-0.52) (1975)
340 (99%) EC50g
statb 24 320- 7.6 DDT 14-day 0.61 (0.58-0.64) Maki &
340 (99%) EC50h Johnson
statb 24 320- 7.6 DDT 14-day 0.75 (0.71-0.79) (1975)
340 (99%) EC50i
stat 10 44 7.1 TDEd 48-h LC50 9.1 Mayer &
stat 21 44 7.1 TDEd 48-h LC50 8.9 Ellersieck
(1986)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 510 (230-1120) Berglind &
soft water stat 20.5 250 7.8-8.2 DDT 48-h LC50 1.1 (0.89-1.7) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 98 (75-127) Berglind &
50 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 1.3 (1.1-1.5) Dave (1984)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 71 (41-130) Berglind &
hard water stat 20.5 250 7.8-8.2 DDT 48-h LC50 0.68 (0.46-1.0) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 42 (32-56) Berglind &
300 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 0.5 (0.41-0.61) Dave
stat 20.5 50 7.8-8.2 DDT 24-h LC50 0.99 (0.66-1.49) (1984)
Water flea stat 15 44 7.1 DDTd 48-h LC50 0.36 (0.28-0.47) Mayer &
(Daphnia pulex) Ellersieck
(1986)
Water flea stat 15 44 7.1 DDTd 48-h LC50 2.5 (1.9-3.3) Mayer &
(Simocephalus stat 21 44 7.1 DDTd 48-h LC50 2.8 (2.3-3.5) Ellersieck
serrulatus) stat 15 44 7.1 TDEd 48-h LC50 3.2 (2.3-4.4) (1986)
stat 21 44 7.1 TDEd 48-h LC50 4.5 (3.1-6.6)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 21 35 44 7.1 TDEd 24-h LC50 4.6 (3.6-5.8) Sanders
(Gammarus fasciatus) stat 21 35 44 7.1 TDEd 96-h LC50 0.6 (0.05-1.2) (1972)
stat 21 35 44 7.1 DDTd 24-h LC50 15 (9.0-20) Sanders
stat 21 35 44 7.1 DDTd 96-h LC50 3.2 (1.8-5.6) (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 3.2 (2.1-4.3) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.86 (0.42-1.3) (1972)
stat 21 260 272 7.4 DDTd 24-h LC50 4.2 (1.8-5.6) Sanders
stat 21 260 272 7.4 DDTd 48-h LC50 3.1 (1972)
stat 21 260 272 7.4 DDTd 96-h LC50 1.8 (1.0-3.1) Sanders
(1972)
stat 21 260 272 7.4 DDTd 120-h LC50 0.32 Sanders
flow 18-21 260 272 7.4 DDTd 24-h LC50 1.1 (1972)
flow 18-21 260 272 7.4 DDTd 48-h LC50 1.0 Sanders
flow 18-21 260 272 7.4 DDTd 96-h LC50 0.8 (1972)
flow 18-21 260 272 7.4 DDTd 120-h LC50 0.6 Sanders
(1972)
Scud stat 21 44 7.1 DDTd 24-h LC50 4.7 (3.2-7.0) Mayer &
(Gammarus lacustris) stat 21 44 7.1 DDTd 96-h LC50 1.0 (0.68-1.5) Ellersieck
(1986)
stat 15 DDTe 96-h LC50 9.0 Gaufin et
al. (1965)
Glass shrimp stat 21 260 272 7.4 DDTd 24-h LC50 6.8 (6.2-7.5) Sanders
(Palaemonetes stat 21 260 272 7.4 DDTd 48-h LC50 4.7 (1972)
kadiakensis) stat 21 260 272 7.4 DDTd 96-h LC50 2.3 (1.3-4.9) Sanders
stat 21 260 272 7.4 DDTd 120-h LC50 1.0 (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 11 (8.4-16) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.68 (0.47-1.1) (1972)
flow 18-21 260 272 7.4 DDTd 24-h LC50 9.4 Sanders
flow 18-21 260 272 7.4 DDTd 48-h LC50 7.7 (1972)
flow 18-21 260 272 7.4 DDTd 96-h LC50 3.5 Sanders
flow 18-21 260 272 7.4 DDTd 120-h LC50 1.3 (1972)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Crayfish (Orconectes nais)
mature stat 21 260 7.4 DDTd 24-h LC50 1100 (1000-1400) Sanders
stat 21 260 7.4 DDTd 96-h LC50 100 (80-120) (1972)
1 day old - 15g stat 21 260 7.4 DDTd 24-h LC50 1.4 (1.1-4.2) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.3 (0.18-0.5) (1972)
1 week old - 20g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.18 (0.12-0.3) (1972)
2 weeks old - 23g stat 21 260 7.4 DDTd 24-h LC50 1.2 (0.9-5.5) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.2 (0.16-1.1) (1972)
3 weeks old - 30g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.24 (0.1-0.6) (1972)
5 weeks old - 50g stat 21 260 7.4 DDTd 24-h LC50 3.2 (1.8-8.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.9 (0.7-1.4) (1972)
8 weeks old - 500g stat 21 260 7.4 DDTd 24-h LC50 45 (40-52) Sanders
stat 21 260 7.4 DDTd 96-h LC50 28 (24-36) (1972)
10 weeks old - 1200g stat 21 260 7.4 DDTd 24-h LC50 50 (48-56) Sanders
stat 21 260 7.4 DDTd 96-h LC50 30 (26-42) (1972)
Sowbug (isopod) stat 21 35 7.1 DDTd 24-h LC50 8.7 (4.9-13.0) Sanders
(Asellus brevicaudus) stat 21 35 7.1 DDTd 96-h LC50 4.0 (1.2-6.5) (1972)
stat 21 35 7.1 TDEd 24-h LC50 18 (14-25) Sanders
stat 21 35 7.1 TDEd 96-h LC50 10 (7.0-14) (1972)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 48 Gaufin et
(Hydropsyche californica) 12 al. (1965)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 175 Gaufin et
(Arctopsyche grandis) 12 al. (1965)
May fly (nymph) stat 8.8- DDTe 96-h LC50 25 Gaufin et
(Ephemerella grandis) 10 al. (1965)
Stonefly (naiad) stat 11- DDTe 96-h LC50 320 Gaufin et
(Acroneuria pacifica) 12 al. (1965)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Stonefly (naiad) stat 11- DDTe 96-h LC50 1800 Gaufin et
(Pteronarcys 12 al. (1965)
californica) stat 15.5 35 DDT 24-h LC50 41 (27-62) Sanders &
stat 15.5 35 DDT 48-h LC50 19 (14-27) Cope (1968)
stat 15.5 35 DDT 96-h LC50 7 (4.9-9.9) Sanders &
stat 15.5 35 TDE 24-h LC50 3000 (2100-4300) Cope (1968)
stat 15.5 35 TDE 48-h LC50 1100 (800-1500) Sanders &
stat 15.5 35 TDE 96-h LC50 380 (280-520) Cope (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 12 (8.8-16) Sanders &
(Pteronarcella badia) stat 15.5 35 DDT 48-h LC50 9 (7-11) Cope
stat 15.5 35 DDT 96-h LC50 1.9 (1.3-2.7) (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 16 (12-20) Sanders &
(Claasenia sabulosa) stat 15.5 35 DDT 48-h LC50 6.4 (4.9-8.3) Cope
stat 15.5 35 DDT 96-h LC50 3.5 (2.9-4.2) (1968)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions (DDT
concentration in water continuously maintained).
b Static conditions but test solution renewed every 24 h.
c Alkalinity and hardness expressed as mg CaCO3/litre.
d Technical grade (99%).
e Emulsifiable concentrate (25% active ingredient).
f Juv. = juvenile.
g Value based on total number of young produced.
h Value based on average brood size.
i Value based on % days reproducing.
j Effect on shell growth.
Eggs of the Chironomid midge, contaminated with DDE by exposure of
the female during ovarian development, failed to hatch as many adults
as uncontaminated eggs. DDE in the water had less of an effect than
DDE contamination within the eggs obtained from the female. The
females had been maintained in water containing 30 µg DDE/litre; eggs
were kept in water containing 20 µg DDE/litre (Derr & Zabik, 1972).
Crayfish populations exposed over long periods to DDT develop some
tolerance to the insecticide (Albaugh, 1972). In 48-h tests, LC50
values for the crayfish Procambarus clarkii were 3.0 (2.5-3.6) µg/litre
for the unexposed population, and 7.2 (5.8-8.8) µg/litre for the
exposed population (95% confidence limits in parentheses). Naqvi &
Ferguson (1968) demonstrated the development of tolerance to DDT after
exposure to the insecticide, in a wide variety of aquatic
invertebrates, including cyclopoid copepods, tubifex worms, and pond
snails. These tolerant populations occurred in the Mississippi delta
in areas of cotton cultivation.
5.2 Fish
Appraisal
DDT is highly toxic to fish; the 96-h LC50s reported (static
tests) range from 1.5 to 56 µg/litre (for largemouth bass and guppy,
respectively). Smaller fish are more susceptible than larger ones of
the same species. An increase in temperature decreases the toxicity of
DDT to fish.
The behaviour of fish is influenced by DDT. Goldfish exposed to
1 µg/litre exhibit hyperactivity. Changes in the feeding of young
fish are caused by DDT levels commonly found in nature, and effects on
temperature preference have been reported.
Residue levels of > 2.4 mg/kg in eggs of the winter flounder result
in abnormal embryos in the laboratory, and comparable residue levels
have been found to relate to the death of lake trout fry in the wild.
Cellular respiration may be the main toxic target of DDT since
there are reports of effects on ATPase.
The toxicity of TDE and DDE has been less studied than that of DDT.
However, the data available show that TDE and DDE are both less toxic
than DDT.
The exact mode of action of DDT in fish remains unclear. There
have been many different suggestions to explain both lethal and
sublethal effects. Most of these are primarily the result of effects
on membranes. DDT is very soluble in lipid and, therefore, dissolves
in the lipid component of membranes. It may interfere both with
membrane function and with many enzyme systems that are located on
membranes. It has been shown experimentally to interfere with the
normal function of so many systems that a primary action of DDT is
difficult to determine.
5.2.1 Short-term and long-term direct toxicity to fish
The short-term toxicity of DDT to fish is summarized in Table 4.
The relatively few studies on TDE (Gardner, 1973; Korn & Earnest,
1974; Mayer & Ellersieck, 1986; Mayer, 1987) show it to be less toxic
than DDT, in the same test system, by factors of 5-10. The still fewer
studies on DDE indicate a similarly lowered toxicity relative to the
parent compound (Mayer & Ellersieck, 1986; Mayer, 1987). Whilst there
is some variation between species, DDT has proved highly toxic to all
fish tested; static 24-h LC50 values range from 2.1 µg/litre for the
largemouth bass (Mayer & Ellersieck, 1986) to 180 µg/litre for the
goldfish (Henderson et al., 1959). For 96-h tests, LC50 values range
from 1.5 µg/litre for largemouth bass (Mayer & Ellersieck, 1986) to
56 µg/litre for the guppy (Henderson et al., 1959). Several authors
have stated that DDT toxicity varies somewhat with temperature and
water hardness.
Buhler et al. (1969) studied the long-term effects, over 95 days,
of feeding DDT-contaminated diets to juvenile chinook and coho salmon.
The DDT was dissolved in corn-oil and then incorporated into a semi-
synthetic diet. Fish were fed until they stopped actively taking the
slowly sinking food. Pure p,p' -DDT was slightly more toxic to
juvenile salmon than the technical product, and chinook salmon were
2 to 3 times more sensitive to the same dose of DDT in the diet than
coho salmon. Size was an important factor in the toxicity of DDT,
smaller fish being more susceptible than larger ones. The authors
estimated, by extrapolation, a 90-day LD50 value of 27.5 µg/kg per
day for chinook and 64 µg/kg per day for coho salmon juveniles. In
fish exposed to higher doses of DDT, pre-death symptoms were marginal.
Some increased agitation and slight photophobia were reported. Fish
exposed to low doses of DDT took longer to die, and other symptoms were
noted. Many individuals developed ulceration of the nasal area. This
spread over the head and in some cases eyes were lost. Pathological
examination showed a specific and severe kidney lesion; this was
limited to one short section of the distal convoluted tubule, which
eventually degenerated almost completely. The authors suggested this
as the main lethal lesion in the fish.
In a later study (Buhler & Shanks, 1970), the same authors showed
that median survival time was directly proportional to body weight in
young coho salmon fed technical DDT. Fish were all given a diet
containing 200 mg DDT/kg and food consumption was monitored for each
group of fish. The main effect of body size on DDT lethality was
related to the intake of the chemical by the fish; smaller fish ate
more of the contaminated diet and consequently received the greatest
dose in mg/kg bodyweight terms. However, even after correcting for
dosage received, the smaller fish were more susceptible than larger
ones. The authors suggested that the lower lipid content of smaller
fish might have accounted for the remaining difference. Twelve groups
of 100 fish ranged in weight (average for each group) from 3 to 15 g.
Total DDT intake ranged from 0.4 to 3 mg/fish; daily intake was higher
in the smaller fish at 3 mg/kg per day, falling to 1.3 mg/kg per day
for the largest. The estimated LC50 ranged from 95 mg/kg for the
smallest to 135 mg/kg for the largest fish, and median survival time
increased from 30 days for the smallest fish to 106 days for the
largest.
Crawford & Guarino (1976) exposed killifish ( Fundulus heteroclitus )
to a twice-repeated schedule of 24 h in water containing DDT at a
concentration of 0.1 mg/litre and 24 h in clean water. At this
exposure level, there was a delay in the rate of development of ferti-
lized eggs but no apparent effect on the hatched fry. Fertilization of
killifish eggs was diminished when insemination was carried out in sea
water containing DDT at 0.1 mg/litre. Mortality at a late stage of
embryo development has been reported for a variety of salmonids and
related to egg residues of DDT (Allison et al., 1964, for cutthroat
trout; Burdick et al., 1964, for lake trout; Macek, 1968, for brook
trout; and Johnson & Pecor, 1969, for coho salmon).
Smith & Cole (1973) reported effects on embryos developing from
eggs laid by adult winter flounder ( Pseudopleuronectes americanus )
that were exposed to 2 µg DDT/litre for various times and, therefore,
accumulated different residue levels in the eggs. These residue levels
varied from 1.15 to 3.70 mg DDT/kg and from 0.07 to 0.4 mg DDE/kg.
Embryos showed abnormal gastrulation and a high incidence (mean 39%)
of vertebral deformities. Bone erosion and haemorrhaging at the
vertebral junctures were often associated with the vertebral deform-
ities.
Halter & Johnson (1974) report that DDT is toxic to the early
life-stages of coho salmon. Mean survival times were considerably
reduced by water concentrations of DDT greater than 0.5 µg/litre.
5.2.2 Sublethal behavioural effects on fish
Hansen (1969) and Hansen et al. (1972) investigated the avoidance
of DDT by sheepshead minnows and mosquitofish in a 'Y'-shaped avoidance
maze. Although there was some statistically significant avoidance of
DDT when fish were given the choice between DDT and clean water, this
only occurred at concentrations of the insecticide above the 24-h
LC50. Fish of both species, when given the choice between DDT at 0.1
and 0.01 mg/litre, chose the higher concentration of the chemical.
This suggests that the perception of DDT is poor and that fish could
not reliably avoid DDT in water at toxic concentrations.
Olofsson & Lindahl (1979) administered either 0.5 or 1.0 mg DDT/kg
body weight to cod by oral intubation. There was a significant effect,
at the higher dose but not the lower one, on the ability of the fish to
compensate its posture to cope with a rotating tube in which it was
swimming.
Hansen (1972) allowed mosquitofish to select a desired salinity in
a fluvarium with a salinity gradient. Fish selected a higher salinity
than controls when exposed to DDT, but only at exposure levels which
caused some mortality. The author suggested that DDT might have affec-
ted the osmoregulatory ability of the mosquitofish. Other possible
explanations include a change in sensitivity of nerves to stimuli or a
preference for the pre-exposure salinity, which was 15 g/litre.
Table 4. Toxicity of DDT and its derivatives to fish
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Salinity Compound Parameter Water Reference
(g)/ stata perat- o/oo concen-
agef ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine fish
Dwarf perch 1.2-11.0 Stat 13 28 DDTc 96-h LC50 4.6 Earnest &
(Micrometrus minimus) 1.2-11.0 flowb 14-18 26-28 DDTc 96-h LC50 0.26 Benville
(0.13-0.52) (1972)
Shiner perch 1.2-11.0 stat 13 26 DDTc 96-h LC50 7.6 Earnest &
(Cymatogaster aggregata) 1.2-11.0 flowb 14-18 13-23 DDTc 96-h LC50 0.45 Benville
(0.21-0.94) (1972)
Striped bass 2.7 flowb 17 28 DDT(77%) 96-h LC50 0.53 Korn &
(Morone saxatilis) (0.38-0.84) Earnest
0.6 flowb 17 30 TDEc 96-h LC50 2.5 (1974)
(1.6-4.0)
Sheepshead minnow juv. flow 15 30 DDTc 48-h LC50 2.0 Mayer
(Cyprinodon variegatus) (1987)
Longnose killifish juv. flow 15 30 DDTc 48-h LC50 2.8 Mayer
(Fundulus similis) juv. flow 16 28 TDEc 48-h LC50 42.0 (1987)
Pinfish juv. flow 22 29 DDTc 48-h LC50 0.3 Mayer
(Lagodon rhomboides) (1987)
Striped mullet juv. flow 15 30 DDTc 48-h LC50 0.4 Mayer
(Mugil cephalus) (1987)
Spot juv. flow 12 26 DDEc 48-h LC50 > 100 Mayer
(Leiostomus xanthurus) juv. flow 26 30 TDEc 48-h LC50 20.0 (1987)
Three-spined 0.4-0.8 stat 20 5 DDT 24-h LC50 22.0 Katz
stickleback 0.4-0.8 stat 20 5 DDT 48-h LC50 21.0 (1961)
(Gasterosteus 0.4-0.8 stat 20 5 DDT 72-h LC50 18.5 Katz
aculeatus) 0.4-0.8 stat 20 5 DDT 96-h LC50 18.0 (1961)
0.4-0.8 stat 20 25 DDT 24-h LC50 18.0 Katz
0.4-0.8 stat 20 25 DDT 48-h LC50 15.0 (1961)
0.4-0.8 stat 20 25 DDT 72-h LC50 14.5 Katz
0.4-0.8 stat 20 25 DDT 96-h LC50 11.5 (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Freshwater fish
Black bullhead 1.2 stat 18 44 7.1 DDTc 24-h LC50 36.8 Mayer &
(Ictalurus melas) (20.3-67.0)
1.2 stat 18 44 7.1 DDTc 96-h LC50 4.8
(3.4-6.8) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 26.2
(22.0-31.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 5.1
(3.9-6.7) Ellersieckg
Channel catfish 1.5 stat 18 44 7.1 DDTc 24-h LC50 22.0
(Ictalurus punctatus) (18.2-26.5) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 21.5
(17.7-26.1) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 18.4
(13.7-24.7) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 17.3
(13.0-23.1) Ellersieckg
0.7 stat 18 44 7.1 DDTc 24-h LC50 17.9
(12.7-25.3) Mayer &
0.7 stat 18 44 7.1 DDTc 96-h LC50 6.9
(5.7-8.5) Ellersieckg
1.6 stat 18 44 7.1 DDTc 24-h LC50 44.0
(37.0-52.0) Mayer &
1.6 stat 18 44 7.1 DDTc 96-h LC50 22.0
(19.0-26.0) Ellersieckg
1.4 stat 18 44 7.1 DDTc 24-h LC50 30.0
(22.0-41.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 16.0
(9.4-29.0) Ellersieckg
1.4 stat 18 272 7.7 DDTc 24-h LC50 29.0
(20.0-41.0) Mayer &
1.4 stat 18 272 7.7 DDTc 96-h LC50 7.0
(4.3-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Atlantic salmon 0.45 stat 12 40 7.5 DDTc 24-h LC50 6.2
(Salmo salar) (4.6-8.4) Mayer &
0.45 stat 12 40 7.5 DDTc 96-h LC50 1.8
(1.3-2.6) Ellersieckg
0.5 stat 12 44 7.5 DDEc 96-h LC50 96.0
(52.1-177) Mayer &
Ellersieckg
Coho salmon 2.7-4.1 stat 20 45-57 6.8-7.4 DDT 24-h LC50 66.0 Katz (1961)
(Oncorhynchus kisutch)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 48-h LC50 46.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 72-h LC50 44.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 96-h LC50 44.0 Katz (1961)
1.0 stat 13 44 7.1 DDTc 24-h LC50 10.0
(7.0-12.0) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 4.0
(3.0-6.0) Ellersieckg
6.0 stat 13 40 7.1 DDTc 24-h LC50 26.9
(18.1-40.0) Mayer &
6.0 stat 13 40 7.1 DDTc 96-h LC50 19.3
(9.6-38.8) Ellersieckg
Chinook salmon 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 24-h LC50 38.0 Katz (1961)
(Oncorhynchus
tshawytscha) 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 48-h LC50 17.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 72-h LC50 14.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 96-h LC50 11.5 Katz (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout 0.9 stat 7 44 7.1 DDTc 24-h LC50 7.5
(Salmo gairdneri) (6.7-8.3) Mayer &
0.9 stat 7 44 7.1 DDTc 96-h LC50 4.1
(3.6-4.6) Ellersieckg
0.9 stat 13 44 7.1 DDTc 24-h LC50 8.2
(7.2-9.2) Mayer &
0.9 stat 13 44 7.1 DDTc 96-h LC50 4.7
(4.2-5.3) Ellersieckg
0.9 stat 18 44 7.1 DDTc 24-h LC50 12.0
(1.0-13.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 5.8
(5.2-6.5) Ellersieckg
3.2 stat 20 45-57 6.8-7.4 DDT 24-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 48-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 72-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 96-h LC50 42.0 Katz (1961)
1.8 flow 17 272 7.4 DDTc 96-h LC50 > 3.0 Mayer &
0.8 stat 12 44 7.1 DDEc 96-h LC50 32.0
(26.0-40.0) Ellersieckg
1.0 stat 12 44 7.1 TDEc 96-h LC50 70.0
(57.0-87.0) Mayer &
1.0 stat 12 272 7.4 TDEc 96-h LC50 70.0
(58.0-85.0) Ellersieckg
Cutthroat trout 1.0 stat 13 44 7.1 DDTc 24-h LC50 8.4
(Salmo clarki) (7.6-9.2) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 5.5
(4.7-6.4) Ellersieckg
1.8 stat 9 162 7.4 DDTc 24-h LC50 11.3
(9.4-13.6) Mayer &
1.8 stat 9 162 7.4 DDTc 96-h LC50 7.9
(6.5-9.7) Ellersieckg
Brown trout 1.7 stat 13 44 7.1 DDTc 96-h LC50 1.8
(Salmo trutta) (1.3-2.5) Mayer &
Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Northern pike 0.7 stat 18 272 7.4 DDTc 24-h LC50 5.5 Mayer &
(Esox lucius) 0.7 stat 18 272 7.4 DDTc 96-h LC50 2.7 Ellersieckg
Guppy 0.1-0.2 stat 25 18 20 7.4 DDTc 24-h LC50 135 Henderson
(Lebistes 0.1-0.2 stat 25 18 20 7.4 DDTc 48-h LC50 72.0 et al.
reticulatus) 0.1-0.2 stat 25 18 20 7.4 DDTc 96-h LC50 56.0 (1959)
River shiner 0.3 stat 18 44 7.1 DDTc 24-h LC50 6.7
(Notropis blennius) (4.9-9.1) Mayer &
0.3 stat 18 44 7.1 DDTc 96-h LC50 5.8
(3.6-9.1) Ellersieckg
Fathead minnow 1.2 stat 18 44 7.1 DDTc 24-h LC50 14.2
(Pimephales (11.0-18.0) Mayer &
promelas) 1.2 stat 18 44 7.1 DDTc 96-h LC50 12.4
(10.0-15.4) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 13.8
(10.3-18.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 13.2
(10.1-17.3) Ellersieckg
0.9 flow 12 314 7.6 DDTc 96-h LC50 9.9
(6.5-15.0) Mayer &
Ellersieckg
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 56.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 42.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 24-h LC50 78.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDTc 48-h LC50 68.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 96-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 24-h LC50 32.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDT 48-h LC50 26.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 96-h LC50 26.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 24-h LC50 29.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDT 48-h LC50 27.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 96-h LC50 26.0 et al. (1959)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Mosquitofish 0.2 stat 25 DDTc 24-h LC50 22.7
(Gambusia affinis) (16.6-31.1) El-Sebae (1987)
0.2 stat 25 DDTc 96-h LC50 9.9
(7.3-13.4) El-Sebae (1987)
0.2 stat 25 DDTe 24-h LC50 58.6
(43.2-79.5) El-Sebae (1987)
0.2 stat 25 DDTe 96-h LC50 27.7
(21.3-36.0) El-Sebae (1987)
Bluegill sunfish 0.26 stat 19 138 192 8.2-8.5 DDTc 96-h LC50 3.4
(Lepomis macrochirus) (2.6-4.1) Randall
0.26 stat 19 138 192 8.2-8.5 DDT 96-h LC50 9.0
(7.4-10.6) et al. (1979)
(25%)
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 26.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 21.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 21.0 (1959)
1.5 stat 18 44 7.1 DDTc 24-h LC50 11.5
(8.4-16.0) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 8.6
(6.2-12.0) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 10.0
(8.5-12.9) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 6.3
(4.3-9.3) Ellersieckg
0.9 stat 17 44 7.1 DDEc 96-h LC50 240
(201-286) Mayer &
0.9 stat 24 44 7.4 TDEc 24-h LC50 56.0
(46.0-68.0) Ellersieckg
0.9 stat 24 44 7.4 TDEc 96-h LC50 42.0
(36.0-49.0) Mayer &
Ellersieckg
Redear sunfish 3.2 stat 24 44 7.1 DDTc 24-h LC50 19.0 Mayer &
(Lepomis microlophus) 3.2 stat 24 44 7.1 DDTc 96-h LC50 15.0 Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Green sunfish 1.1 stat 18 44 7.1 DDTc 24-h LC50 16.9
(Lepomis cyanellus) (12.7-22.3) Mayer &
1.1 stat 18 44 7.1 DDTc 96-h LC50 10.9
(7.3-15.6) Ellersieckg
0.8 stat 18 44 7.1 DDTc 24-h LC50 18.0
(13.0-24.0) Mayer &
0.8 stat 18 44 7.1 DDTc 96-h LC50 6.5
(4.1-10.4) Ellersieckg
1.1 stat 18 272 7.7 DDTc 24-h LC50 19.8
(15.0-25.6) Mayer &
1.1 stat 18 272 7.7 DDTc 96-h LC50 9.9
(6.4-15.0) Ellersieckg
Largemouth bass 0.8 stat 18 44 7.1 DDTc 24-h LC50 3.7
(Micropterus (3.1-4.5) Mayer &
Salmoides) 0.8 stat 18 44 7.1 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.8 stat 18 272 7.4 DDTc 24-h LC50 2.1
(1.6-2.9) Mayer &
0.8 stat 18 272 7.4 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.7 stat 18 44 7.1 TDEc 24-h LC50 50.0
(35.0-71.0) Mayer &
0.7 stat 18 44 7.1 TDEc 96-h LC50 42.0
(34.0-51.0) Ellersieckg
Black crappie 1.0 stat 18 44 7.1 DDTc 24-h LC50 6.5
(Pomoxis (5.4-7.8) Mayer &
nigromaculatus) 1.0 stat 18 44 7.1 DDTc 96-h LC50 5.6
(4.6-6.7) Ellersieckg
Yellow perch 1.4 stat 18 44 7.1 DDTc 24-h LC50 10.0
(Perca flavescens) (8.0-12.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 9.0
(7.0-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Walleye 1.4 stat 18 44 7.1 DDTc 24-h LC50 4.2
(Stizostedion v. (3.2-5.6) Mayer &
vitreum) 1.4 stat 18 44 7.1 DDTc 96-h LC50 2.9
(2.4-3.5) Ellersieckg
1.3 stat 18 272 7.4 DDTc 24-h LC50 4.6
(3.9-5.4) Mayer &
1.3 stat 18 272 7.4 DDTc 96-h LC50 4.6
(3.9-5.4) Ellersieckg
1.0 stat 18 44 7.1 TDEc 24-h LC50 20.0
(16.0-24.0) Mayer &
1.0 stat 18 44 7.1 TDEc 96-h LC50 14.0
(11.0-19.0) Ellersieckg
Tilapia 0.8 stat 24 44 7.1 DDTc 24-h LC50 19.0
(Tilapia mossambica) (16.0-23.0) Mayer &
0.8 stat 24 44 7.1 DDTc 96-h LC50 17.0
(14.0-21.0) Ellersieckg
0.8 stat 24 272 7.4 DDTc 24-h LC50 15.0
(13.0-17.0) Mayer &
0.8 stat 24 272 7.4 DDTc 96-h LC50 14.0
(12.0-16.0) Ellersieckg
0.8 flow 18 272 7.4 DDTc 24-h LC50 24.0
(17.0-32.0) Mayer &
0.8 flow 18 272 7.4 DDTc 96-h LC50 5.1
(3.2-8.1) Ellersieckg
Tilapia 0.8 stat 25 DDTc 24-h LC50 21.8
(Tilapia zilli) (17.0-28.0) El-Sebae (1987)
0.8 stat 25 DDTc 96-h LC50 15.5
(11.7-20.6) El-Sebae (1987)
0.8 stat 25 DDTe 24-h LC50 12.8
(9.6-17.1) El-Sebae (1987)
0.8 stat 25 DDTe 96-h LC50 9.5
(7.4-12.3) El-Sebae (1987)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Goldfish 1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 180 Henderson
(Carassius auratus) 1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 47.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 36.0 (1959)
0.9 stat 18 44 7.1 DDTc 24-h LC50 24.0
(17.0-33.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 15.5
(9.1-26.0) Ellersieckg
0.9 stat 18 272 7.4 DDTc 24-h LC50 22.2
(16.0-31.1) Mayer &
0.9 stat 18 272 7.4 DDTc 96-h LC50 14.7
(10.0-20.0) Ellersieckg
Common carp 0.6 stat 18 44 7.1 DDTc 24-h LC50 14.0
(Cyprinus carpio) (10.0-19.0) Mayer &
0.6 stat 18 44 7.1 DDTc 96-h LC50 9.7
(7.4-12.9) Ellersieckg
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions
(DDT concentration in water continuously maintained).
b Intermittent flow-through conditions.
c Technical grade (99%).
d Alkalinity and hardness expressed as mg CaCO3/litre.
e 25% emulsifiable concentrate.
f Juv. = juvenile.
g 1986.
Peterson (1973) monitored the selection of temperature by
juvenile Atlantic salmon ( Salmo salar ) previously exposed to DDT or
its metabolites. Low concentrations produced no effect on tem-
perature selection, but at higher levels of exposure the temperature
selected by the fish increased. Fish were most sensitive, in this
respect, to p,p' -DDE and showed decreasing sensitivity to
o,p' -DDT, p,p' -TDE, and p,p' -DDT. Increasing the exposure to
p,p' -DDE from 0 to 1.0 mg/litre increased the preferred temperature
from about 16 °C to 21 °C. There was no effect of p,p' -DDA on
temperature selection at concentrations as high as 8 mg/litre. In a
similar experiment, where brook trout (Salvelinus fontinalis) were
exposed to a vertical rather than horizontal temperature gradient, fish
previously exposed to p,p' -DDT and p,p' -TDE selected higher
temperatures than controls. Conversely, Gardner (1973) found that DDT
and its analogues induced selection of lower temperatures by the same
species of fish over a dose range between 0 and 50 µg/litre; DDE did
not produce any temperature preference. Ogilvie & Miller (1976)
reported that Atlantic salmon exposed to DDT at a concentration of
50 µg/litre selected higher temperatures, the effect persisting for at
least 4 weeks after exposure. The authors suggested that the tempera-
ture selection response to DDT exposure is "biphasic". At low
exposure levels, similar to those used by Gardner (1973), lower
temperatures are selected, whilst higher temperatures are preferred at
higher exposure levels.
Dill & Saunders (1974) exposed the eggs of Atlantic salmon
at gastrulation to DDT at water concentrations of 5, 10, 50, or
100 µg/litre, and observed behavioural development in hatched fry over
30 days following hatch. The two highest doses of DDT impaired balance
and retarded behavioural development of the fry (i.e., the appearance
of normal behaviour patterns was delayed). The authors considered that
the effects observed would affect predation rates and feeding, in
young fish, at "realistic" DDT exposure levels in the wild.
Davy et al. (1973) reported that exposure to DDT, at 10 µg/litre
for 4 days, affected the exploratory behaviour of goldfish experi-
encing a novel environment. They attributed the effect to a central
nervous system lesion caused by DDT. Weis & Weis (1974) showed an
increase in individual activity and an increase in school-size in
groups of goldfish exposed to DDT at 1 µg/litre for 7 days. After a
frightening stimulus, schools scattered further and did not regroup as
readily as control fish. The transfer of fish to clean water led to
a return to normal behaviour within one week. An effect on the
locomotor behaviour of goldfish after exposure to 10 µg DDT/litre per-
sisted for the remainder of the observation period of 130-139 days
(Davy et al., 1972).
5.2.3 Physiological effects on fish
Hanke et al. (1983) investigated the effects of DDT on a range of
physiological functions in carp ( Cyprinus carpio ). At water concen-
trations of 100 or 500 µg/litre, the insecticide induced changes in
plasma cortisol and glucose levels, liver glycogen level, and plasma
and brain acetylcholinesterase activity. The response was biphasic in
all cases. Initially, after 6 hours, there was a stimulation of these
parameters which, within 24 hours, changed to an inhibition.
Ramalingam & Ramalingam (1982) reported that the chronic effect of DDT
on glycogen utilization in fish led to the use of protein as an energy
source. The protein content of tissues declined after chronic exposure
to DDT.
Janicki & Kinter (1971) found that DDT impaired fluid absorption in
the intestinal sacs of eels adapted to sea water and exposed to the
insecticide at 50 µg/litre. DDT also inhibited Na+-, K+-, and
Mg2+-dependent ATPases in homogenates of the intestinal mucosa. In a
later study, Kinter et al. (1972) showed that plasma osmolarity was
also affected in sea-water-adapted eels exposed to DDT (1 mg/litre)
for 9 to 10 hours. Haux & Larsson (1979) reported effects of DDT on
plasma electrolytes in the flounder Platichthys flesus kept in
hypotonic, brackish water. The fish were force-fed with DDT in gelatin
capsules to give a total DDT dose of 1.5 or 15.0 mg/kg body weight.
Plasma sodium was reduced but not significantly; plasma chloride was
significantly reduced in a dose-related manner after 3 weeks but not
after 6 weeks. Waggoner & Zeeman (1975) reported similar effects on
plasma electrolytes in the black surfperch ( Embiotoca jacksoni ), but
only at high DDT exposure levels. They injected DDT doses of 1, 10,
100, or 200 mg/kg; the only effect occurred with the dose of 200 mg/kg,
but the fish did not survive to 72 h. The authors suggested that
osmoregulatory effects are not the major cause of DDT-induced mortality
in marine fish.
Desaiah et al. (1975) exposed fathead minnows ( Pimephales
promelas ) for long periods to DDT at water concentrations of 0.5 or
2.0 µg/litre and also via the food, and monitored the activity of
ATPases in brain and gill. This study followed up several previous
studies on in vitro effects on these enzymes. After 266 days of
exposure, there was an approximately 50% reduction in brain
oligomycin-sensitive (mitochondrial) Mg2+-ATPase activity. In
contrast, oligomycin-insensitive Mg2+-ATPase activity was increased
by almost 40%. Total Mg2+-ATPase activity was, therefore, almost
unaffected by DDT. There was a less obvious (about 18%) activation of
Na+-K+-ATPase activity in the brain. Gill tissue showed different
results; all the ATPases studied were inhibited by DDT. The authors
suggested that a major factor in the toxicity of DDT to fish (and other
organisms) could be the inhibition of oxidative phosphorylation.
Moffett & Yarbrough (1972) investigated the enzyme succinic
dehydrogenase in insecticide-resistant and insecticide-susceptible
mosquitofish ( Gambusia affinis ) in an attempt to discover if
resistance could be related to membrane effects of DDT. They found
that the effect on membrane-bound enzymes was, indeed, reduced in
resistant fish. This may not explain all the factors involved in
resistance, since DDT uptake from water may also be reduced.
5.2.4 Development of tolerance
The development of tolerance to DDT in fish has been reported.
Vinson et al. (1963) reported DDT tolerance in mosquitofish ( Gambusia
affinis ) exposed long-term to DDT in the wild, and Boyd & Ferguson
(1964) showed TDE tolerance in the same species. However, fish exposed
long-term to DDT do not always show tolerance. Ferguson et al. (1964)
recorded tolerance to a variety of organochlorine insecticides in three
species of freshwater fish from the Mississippi delta area of cotton
cultivation, but there was no tolerance to DDT. El-Sebae (1987)
determined the LC50 values for two populations of Tilapia zilli from
different areas of Egypt. Fish from the Behera Governate which had
been taken from agricultural drains showed exactly the same suscepti-
bility to DDT (25% EC) as fish taken from a less contaminated area in
the Alexandria Governate. Tolerance had developed to other insecti-
cides in these different strains.
5.3 Toxicity to Amphibians
Appraisal
The toxicity of DDT and its metabolites to amphibians varies from
species to species; although only a few data are available, amphibian
larvae seem to be more sensitive than adults to DDT. TDE seems to be
more toxic than DDT to amphibians, but there are no data available for
DDE. All the studies reported have been static tests and, therefore,
results should be treated with caution.
The toxicity of DDT and TDE to amphibians is summarized in
Table 5. Both compounds are toxic to amphibian larvae at low water
concentrations.
Two studies (Harri et al., 1979; Hudson et al., 1984) showed
that DDT is moderately toxic to adult frogs when given orally.
Repeated oral dosing of adult common frogs ( Rana temporaria ) with DDT
at 0.6 mg/kg body weight twice weekly for 8 weeks, led to no mortality
when the animals were fed (Harri et al., 1979). Frogs dosed in the
same way, but not fed, showed 50% mortality by the end of dosing. The
first animal died after the fifth dose and all others showed symptoms
of poisoning.
A study by Sanders (1970) indicated that the toxicity of DDT to
tadpoles of Fowler's toad increased with age of the tadpole. The 24-
and 96-h LC50 values of 5.3 and 0.75 mg/litre for one-week-old
tadpoles fell to 1.4 and 0.03 mg/litre, respectively, by the time the
tadpoles were 7 weeks old. TDE was only tested on one age range of
tadpoles for a maximum of 96 h, and was found to be 3-8 times more
toxic than DDT. The pattern of pesticide poisoning progressed through
irritability and loss of equilibrium to death. Tadpoles were affected
irreversibly by concentrations well below their calculated short-term
LC50 values and, therefore, would succumb to DDT over time. DDT was
re-tested several times during over the period of the study in an
attempt to identify any development of resistance in the population.
None was found; the 24-h LC50 values were stable throughout a 4-month
period.
Table 5. Toxicity of DDT and its derivatives to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Fowler's toad (tadpole)
(Bufo woodhousii)
1 week old - 15 mg stat 15.5 30 7.1 DDT 24-h LC50 5300 (2900-9900) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1800 (950-3300) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 750 (280-2000) Sanders
(1970)
2-3 weeks old - 56 mg stat 15.5 30 7.1 DDT 24-h LC50 5400 (2900-10 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1300 (320-5300) (1970)
4-5 weeks old - 74 mg stat 15.5 30 7.1 DDT 24-h LC50 2400 (730-8000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1000 (40-6500) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 1000 (20-3600) Sanders
stat 15.5 30 7.1 TDE 24-h LC50 700 (250-2000) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 320 (210-450) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 140 (100-210) (1970)
6 weeks old - 350 mg stat 15.5 30 7.1 DDT 24-h LC50 2200 (550-15 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 410 (280-600) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 100 (20-600) Sanders
(1970)
7 weeks old - 600 mg stat 15.5 30 7.1 DDT 24-h LC50 1400 (900-2000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 750 (610-1100) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 30 (6-400) Sanders
(1970)
Table 5. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Western chorus frog stat 15.5 30 7.1 DDT 24-h LC50 1400 (910-2800) Sanders
(Pseudacris stat 15.5 30 7.1 DDT 48-h LC50 900 (400-1500) (1970)
triseriata) stat 15.5 30 7.1 DDT 96-h LC50 800 (500-2300) Sanders
(1-week-old tadpole) stat 15.5 30 7.1 TDE 24-h LC50 610 (410-820) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 500 (210-750) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 400 (210-750) (1970)
Bullfrog DDT acute LD50c > 2000 ug/kg Hudson
(Rana catesbeiana) (77.2%) et al.
(1984)
Common frog 15 DDT acute LD50c 7600 ug/kg Harri
(Rana temporaria) et al.
(1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(DDT concentration in water continuously maintained).
b alkalinity expressed as mg CaCO3/litre.
c acute LD50 was calculated by administering a single oral dose.
Cooke (1970) exposed tadpoles of the common frog ( Rana temporaria )
to 0.1, 1.0, or 10 mg DDT/litre for only one hour and reported a
period of uncoordinated hyperactivity beginning less than one hour
after the end of the exposure period. Body weight decreased during
this hyperactive period and development was restricted in some of the
tadpoles. Smaller tadpoles were more vulnerable to the effects of DDT
than larger ones. In a later study (Cooke, 1979b), the same author
reared tadpoles of the common frog at two different densities. The
densities differed 5-fold and resulted in a 2-fold average difference
in body weight between the two groups. The larger tadpoles, reared at
the lower density, were completely tolerant of concentrations of DDT
that caused severe sublethal effects in smaller tadpoles. Field
populations of tadpoles included individuals with weights corresponding
to the two experimental groups, but these were at the two extremes of
the natural weight range.
Cooke (1972) exposed both spawn and tadpoles of the common frog
( Rana temporaria ), the common toad ( Bufo bufo ), and the smooth newt
( Triturus vulgaris ) for 24 and 48 hours to concentrations of DDT
between 0.8 µg/litre and 0.5 mg/litre. Results indicated that DDT did
not penetrate well-developed spawn and was only detectable in tadpoles
hatched from spawn that had been treated with DDT immediately after it
had been laid. Tadpoles hatching from spawn treated when newly laid
showed hyperactivity, symptomatic of DDT poisoning, only later in
their development at the point where external gills were lost. In the
experiments where tadpoles were exposed to DDT, they were most suscep-
tible either just before or just after the development of hindlimb
buds. At these two stages, the characteristic hyperactivity was
shown when DDT tissue concentrations reached between 2-3 mg/kg before
the tadpoles developed limb buds, and when they reached 3-4 mg/kg,
immediately after the tadpoles developed limb buds. During resorption
of the tail, small frogs, but not small toads, were susceptible to DDT
residues that had been acquired during larval development. At all
stages of development, toads were more resistant to DDT than were
frogs, and some toad tadpoles survived despite tissue residues in
excess of 300 mg/kg. The metabolite DDE was often detectable in newt
tadpoles and in frog and toad tadpoles with hindlimbs.
DDT has an anatomical effect on developing frog tadpoles (Cooke,
1970; Osborn et al., 1981). Exposure of tadpoles to 0.1 mg DDT/litre
for 2 days or to 0.1, 1.0, or 10 mg/litre for one hour produced some
individuals with abnormalities in the snout. A detailed histological
and behavioural study suggested that the effect was caused by two
separate factors. DDT had a direct effect on the development of skin
glands in the region above the upper mandible. The uncoordinated
hyperactivity that followed DDT treatment caused the lower mandible to
strike the upper, distorted mandible and resulted in further damage.
Some individuals recovered from this abnormality at various stages of
development. However, froglets that were affected at the tadpole stage
frequently have blunt snouts and deformed brains. The authors
suggested that DDT caused the disruption by preventing the organisation
of the epithelial cells into gland units, possibly by affecting cell
membranes and disrupting cell-to-cell communication. The mechanism of
recovery remained unclear and a full explanation of the very specific
nature of the abnormality was not possible.
This toxicity of DDT to amphibians is of significance in its use as
an insecticide. The use of DDT to control mosquito larvae has been a
major source of exposure of tadpoles and has led to toxic effects
(Mulla, 1963; Cooke, 1973a).
The widespread use of DDT has led to the development of some
resistance in two species of cricket frog ( Acris crepitans and Acris
gryllus ). Boyd et al. (1963) found that cricket frogs collected from
areas of high DDT usage for the control of cotton pests were more
tolerant to DDT than were frogs from other areas.
6. TOXICITY TO TERRESTRIAL ORGANISMS
There is evidence that DDT and its metabolites have affected
wildlife in terrestrial ecosystems. Laboratory studies covered in this
section give clear indication of a variety of lethal and sublethal
effects. The range of organisms studied is not comprehensive. No
review has been made here of the effects of DDT on insects, the target
organisms. The lethal effect of DDT on insects is thought to result
from changes in nerve transmission.
6.1 Terrestrial Invertebrates
Appraisal
There have been few reports on the effects of DDT and its
metabolites on non-target terrestrial invertebrates.
Earthworms are insensitive to the acutely toxic effects of these
compounds at levels higher than those likely to be found in the
environment. The uptake of DDT by earthworms is related to the concen-
trations in soil and to the activity of the worms; seasonally greater
activity increases uptake. Thus, although earthworms are unlikely to
be seriously affected by DDT, they pose a major hazard to predators
because of the residues they can tolerate.
Both DDT and DDE are classified as being relatively non-toxic to
honey bees, with a topical LD 50 at 27 µg/bee.
There are no reports on laboratory studies using DDE or TDE, in
spite of the fact that these are major contaminants of soil.
The toxicity of DDT to insects, the target organisms, is exten-
sively documented. Uptake of DDT and its metabolism by other
terrestrial invertebrates is also well covered in the literature.
However, there are few reports of effects of either DDT or its
metabolites on non-target invertebrates.
Johansen (1962) classified DDT as "moderately" toxic to honey
bees in both laboratory and field tests. Atkins et al. (1973) quoted
a topical LD50 for honey bees of 12.09 µg/bee and classified DDT as
"relatively non-toxic".
DDT has little or no effect on earthworms at dose levels likely to
be encountered in the field; worms were unaffected by 2000 mg/kg soil
(Goffart, 1949). The early literature has been examined by Davey
(1963), whose review includes reports on a variety of earthworm species
that live in surface soil or deeper layers. Thompson (1971) treated an
area of grassland with an emulsifiable concentrate of DDT at the rate
of 5.6 kg/ha. Although there was a reduction in earthworm numbers and
biomass of about 30%, the author considered this to be of little sig-
nificance. Results in tropical areas are similar to those of temperate
regions. Cook et al. (1980) examined the effects of cultivation and
DDT treatment on earthworm activity and populations in Nigeria
following the application of DDT (1 kg/ha) as a foliar spray on cowpea
plots. The number of casts on the surface was reduced by DDT
application, but there was no effect on the number of worms in the
soil.
Cooke & Pollard (1973) treated Roman snails ( Helix pomatia )
with p,p' -DDT applied to lettuce leaves. The snails were fed a
365 x 2.5 cm square of leaf that had been treated with 0.1 ml of an
acetone solution of DDT (either 0.025, 1.0, or 40 mg/ml). The dosing
started when the snails were 2 weeks old and continued for 17 weeks, at
which point the dose was doubled and continued for a further 12 weeks.
The snails were then transferred outside to stimulate hibernation. Low
doses of DDT reduced the weight of the shell and operculum whereas
higher doses did not. After re-emergence from hibernation, the
incidence of operculum eating was significantly higher among snails
hibernating late in the season, and as exposure to DDT increased so
operculum eating became more prevalent. The authors suggest that
shell-thinning is likely to have occurred in snails in heavily-treated
agricultural areas if the response of all snail species to DDT is
similar to that of Helix pomatia .
Critchley et al. (1980) investigated the effects of the use of
DDT for 4 years on a cultivated forest soil in Nigeria on the
numbers of epigeal (surface-living) and subterranean species of invert-
ebrates. DDT was applied as a foliar spray to crops of cowpeas at a
rate of 1 kg/ha annually. After the first application of DDT there was
no effect on ant or millipede numbers but the numbers of lycosid
spiders and crickets were reduced. At the end of the study, after four
applications of DDT, ants and millipedes were also reduced in number.
When Shires (1985) treated cereals on clay loam soil in experimen-
tal plots with DDT (1 kg/ha), the numbers of predatory beetles
(Carabidae) were reduced by 50% one week after application. However,
the numbers increased again after 4 to 6 weeks and remained at control
levels. The use of other insecticides led to a second decrease in
Carabidae numbers; this was attributed by the authors to a reduction
in the food supply of aphids. DDT failed to control the aphids, which
were tolerant to the compound.
6.2 Birds
Appraisal
DDT and its metabolites can lower the reproductive rate of birds by
causing eggshell thinning (which leads to egg breakage) and by causing
embryo deaths. However, different groups of birds vary greatly in
their sensitivity to these chemicals; predatory birds are extremely
sensitive and, in the wild, often show marked shell thinning, whilst
gallinaceous birds are relatively insensitive. Because of the
difficulties of breeding birds of prey in captivity, most of the
experimental work has been done with insensitive species, which have
often shown little or no shell thinning. The few studies on more
sensitive species have shown shell thinning at levels similar to those
found in the wild. The lowest dietary concentration of DDT reported to
cause shell thinning experimentally was 0.6 mg/kg for the black duck.
The mechanism of shell thinning is not fully understood.
6.2.1 Short-term and long-term toxicity to birds
DDT and its derivatives DDE and TDE have moderate to low toxicity
to birds when given as an acute oral dose or in the diet. The acute
oral and dietary toxicities of DDT, DDE, and TDE to birds are
summarized in Table 6.
These compounds have been studied in a wide variety of species in
tests ranging from a single acute dose to 100 days of dietary dosing.
All three compounds, DDT, DDE, and TDE, have low to moderate toxicity
to young and adult birds. There is no obvious pattern of relative
toxicity between the three compounds. In some species it is DDT that
is the most toxic, while in other species it is TDE. Most of these
laboratory tests have been conducted on species that are easy to
maintain and breed in captivity. These species are unusual in many
respects; they tend to be gallinaceous birds with young that are not
fed by the adults after hatching. They also tend to have long breeding
seasons untypical of most birds in the wild. In the wild, the most
severely affected species of birds are raptors at the top of food
chains. There is little direct laboratory data on toxicity to these
birds. Toxicity to small songbirds, which make up the majority of bird
species, has not been examined either in the laboratory or the field.
Porter & Wiemeyer (1972) fed American kestrels on a diet containing
p,p' -DDE at a concentration of 2.8 mg/kg. Two birds died after 14
and 16 months of treatment; they showed residues of DDE in brain
tissues of 212 and 301 mg/kg, respectively. This compared with mean
residues of 14.9 (range: 4.47-26.6) mg/kg in 11 adult males sacrificed
after 12-16 months on the diet. Van Velzen et al. (1972) investigated
the lethal effect of stored DDT mobilization by brown-headed
cowbirds. Cowbirds were fed for 13 days on a diet containing 100, 200,
or 300 mg p,p' -DDT/kg, and were then given reduced rations of
approximately 43% of normal daily intake for a 6-day period. Of 30
birds dosed, 21 died (6, 7, and 8 from the three dose levels,
respectively). After 4 months, the remaining birds were subjected
to a second period of 6 days on a reduced diet. Four more
birds, out of six, died. In a second experiment, cowbirds were fed
100 mg p,p' -DDT/kg diet for 13 days and then subjected to 4 days of
reduced food intake. Seven out of 20 birds died. There were no deaths
in any of the control groups (i.e., birds dosed but not starved,
undosed and starved, or undosed and unstarved).
6.2.2 Toxicity to birds' eggs
Dunachie & Fletcher (1969) injected chicken eggs with DDT or TDE to
give concentrations, in the egg, varying between 10 and 500 mg/kg.
Two different vehicles were used to dissolve the insecticides (corn oil
and acetone), controls being injected with vehicle alone. The authors
monitored egg hatchability and survival of chicks to 4 days of age.
Some chicks were fed and some were not. No dose of DDT, applied in
either vehicle, had any significant effect on egg hatchability when
compared to controls. However, there was a profound effect on the
chick survival rate. All chicks hatched from eggs treated with DDT at
100 mg/kg, and which were not fed, were dead within 4 days after
hatching. Feeding the chicks eliminated this effect; the survival rate
of fed young was similar to that of controls. Chicks hatched from
eggs treated with 50 mg DDT/kg survived as well as controls, whether
they were fed or not. TDE was found to affect hatchability, but only
when applied in corn oil; the acetone-dissolved material did not have
any significant effect. TDE dissolved in corn oil reduced
hatchability to 60% of control levels at 100 and 200 mg/kg, to 7% at
300 and 400 mg/kg, and to 0% at 500 mg/kg. The effects on chick
survivability were similar to those of DDT. All chicks hatched from
eggs treated with 100 mg TDE/kg were dead after 4 days if they were not
fed, whereas chicks from eggs treated with 50 mg/kg survived as well as
controls. Chicks from either 100 or 200 mg/kg treatments survived as
well as controls as long as they were fed. The significance of the
different vehicles was discussed by Cooke (1971) and Gilman et al.
(1978). Acetone causes coagulation of yolk protein whereas corn oil
allows the injected organochlorine to float through the yolk to a
position directly under the blastodisc.
Table 6. Toxicity of DDT and its derivatives to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
Bobwhite quail 23 days diet DDE 99.9 5-day LC50 825 (697-976) Hill
(Colinus virginianus) 23 days diet DDT 100 5-day LC50 611 (514-724) et al.
23 days diet TDE TG 5-day LC50 2178 (1835-2584) (1975)
young diet DDT 5-day LC50 881 (796-975) Stickel
& Heath (1964)
(wild) diet DDT TG 5-day LC50 1170 (830-1650) Hill et al.
(farm-reared) diet DDT TG 5-day LC50 1610 (1331-1948) (1971)
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 400 (1963)
adult diet DDT 10-day LC50 2500 DeWitt et al.
adult diet DDT 100-day LC50 1000 (1963)
Japanese quail 7 days diet DDE 99.9 5-day LC50 1355 (1111-1648) Hill
(Coturnix coturnix 7 days diet DDT 100 5-day LC50 568 (470-687) et al.
japonica) 7 days diet TDE TG 5-day LC50 3165 (2534-3978) (1975)
M 2 months oral DDT 77.2 acute LD50 841 (607-1170) Hudson et al.
(1984)
California quail M 6 months oral DDT TG acute LD50 595 (430-825) Hudson et al.
(Callipepla F 6 months oral TDE > 95 acute LD50 > 760 (1984)
californica)
Mallard duck 17 days diet DDE 99.9 5-day LC50 3572 (2811-4669) Hill
(Anas platyrhynchos) 17 days diet DDT 100 5-day LC50 1869 (1500-2372) et al.
17 days diet TDE TG 5-day LC50 4814 (3451-7054) (1975)
F 3 months oral DDT 77.2 acute LD50 > 2240 Hudson et al.
F 3 months oral TDE > 95 acute LD50 > 2000 (1984)
young diet DDT 5-day LC50 875 (650-1140) Stickel &
Heath (1964)
young diet DDT 10-day LC50 500 DeWitt
young diet DDT 100-day LC50 > 200 et al.
adult diet DDT 100-day LC50 1000 (1963)
Pheasant 10 days diet DDE 99.9 5-day LC50 829 (746-922) Hill
(Phasianus colchicus) 21 days diet DDT 100 5-day LC50 311 (256-374) et al.
10 days diet TDE TG 5-day LC50 445 (402-494) (1975)
F 3-4 months oral DDT > 99 acute LD50 1334 (894-1990) Hudson et al.
F 3-4 months oral TDE > 95 acute LD50 386 (270-551) (1984)
young diet DDT 5-day LC50 804 (686-942) Stickel &
Heath (1964)
Table 6. (Contd)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 100 (1963)
adult diet DDT 10-day LC50 1000 DeWitt et al.
adult diet DDT 100-day LC50 > 100 (1963)
Red-winged blackbird diet DDT 10-day LC50 1000 DeWitt et al.
(Agelaius phoeniceus) diet DDT 30-day LC50 500 (1963)
Cardinal diet DDT TG 5-day LC50 535 (420-700) Hill et al.
(Richmondena cardinalis) (1971)
House sparrow diet DDT TG 5-day LC50 415 (370-465) Hill et al.
(Passer domesticus) (1971)
Blue jay diet DDT TG 5-day LC50 415 (320-540) Hill et al.
(Cyanocitta cristata) (1971)
Rock dove M,F oral DDT 77.2 acute LD50 > 4000 Hudson et al.
(Columba livia) (1984)
Sandhill crane M,F adult oral DDT > 99 acute LD50 > 1200 Hudson et al.
(Grus canadensis) (1984)
Clapper rail M diet DDT 5-day LC50 1612 Van Velzen &
(1975) Kreitzer
(Rallus F diet DDT 5-day LC50 1896 (1975)
longirostris) (1975)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed
as mg/kg diet).
c TG = Technical grade.
6.2.3 Reproductive effects on birds
DDT, or more specifically its metabolite DDE, causes the shells of
birds' eggs to be thinner than normal. Results on eggshell thinning
are summarized in Table 7. There is considerable variation between
species for this effect. Galliform species are very resistant to shell
thinning whereas birds of prey are particularly susceptible.
Lincer (1975) dosed captive American kestrels and established a
clear relationship between dietary DDE and thinning of eggshells.
There was a similar close correlation between the residues of DDE in
individual eggs and the degree of shell thinning. The kestrels were
fed with day-old cockerels (which were injected with 0.2 ml of corn
oil, containing the DDE, into the breast muscle) and received either
0.3, 3, 6, or 10 mg DDE/kg diet. Residues of DDE in eggs laid by the
birds correlated closely with dietary DDE concentration; residues of
1.9 mg/kg wet weight were associated with the lowest dose and 245 mg/kg
with the highest dose given. There was no shell thinning associated
with the dose of 0.3 mg/kg. The other doses showed 15.1%, 22.8%, and
29.2% thinning (at 3, 6, and 10 mg/kg, respectively). There was a
straight-line relationship between the degree of shell thinning and
the logarithm of the DDE residue in the egg. Data obtained from the
field showed exactly the same trend (Fig. 1). This represents the best
evidence for the effect of DDE on shell thickness in a species actually
adversely affected in the field.
Table 7. Thinning effects of DDT and its derivatives on bird egg shells
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Ring dove diet DDE 10 - 9.2 0.01 Peakall et al. (1973)
(Streptopelia diet DDE 40 - 6.8 0.01 Haegele & Hudson (1973)
risoria)
Mallard diet TDE 10 - 2.6 NS Heath et al. (1969)
(Anas platyrhynchos) diet TDE 10 - 5.4 NS Heath et al. (1969)
diet TDE 40 - 2.6 NS Heath et al. (1969)
diet TDE 40 - 5.4 NS Heath et al. (1969)
diet DDT 2.5 - 5.3 NS Heath et al. (1969)
diet DDT 10 - 7.9 NS Heath et al. (1969)
diet DDT 40/25 -13.2 0.01 Heath et al. (1969)
White pekin duck diet DDE 40 -20.3 0.001 Peakall et al. (1973)
4 day diet DDE 40 - 3.3 0.01 Miller et al. (1976)
1-3 months diet DDE 40 -18.2 0.01 Miller et al. (1976)
Black duck diet DDE 10 -17.6 0.01 Longcore et al. (1971)
(Anas rubripes) diet DDE 30 -23.5 0.01 Longcore et al. (1971)
Screech owl diet DDE 2.8 -13.3 0.01 McLane & Hall (1972)
(Otus asio)
American kestrel diet DDE 3 -15.2 0.05 Peakall et al. (1973)
(Falco sparverius) diet DDE 6 -21.0 0.01 Peakall et al. (1973)
diet DDE 10 -26.3 0.001 Peakall et al. (1973)
diet DDE 2.8 - 8.7 0.001 Wiemeyer & Porter (1970)
diet DDE 0.3 + 2.1 NS Lincer (1975)
diet DDE 3 -15.1 0.05 Lincer (1975)
diet DDE 6 -22.8 0.01 Lincer (1975)
diet DDE 10 -29.2 0.001 Lincer (1975)
Table 7. (Contd).
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Japanese quail diet o,p'-DDT 100 - 4.0 0.001d Bitman et al. (1969)
(Coturnix coturnix diet DDT 100 - 5.6 0.001d Bitman et al. (1969)
japonica) diet DDT 100 0 NS Cecil et al. (1971)
diet DDE 100 - 2.5 NS Cecil et al. (1971)
diet DDE 2 + 1.9 NS Davison et al. (1976)
diet DDE 10 + 6.3 NS Davison et al. (1976)
diet DDE 40 + 5.0 NS Davison et al. (1976)
diet DDE 200 - 0.6 NS Davison et al. (1976)
strain 1a diet DDT 2.5 + 1.0 NS Davison et al. (1976)
diet DDT 10 - 1.5 NS Davison et al. (1976)
diet DDT 40 - 0.5 NS Davison et al. (1976)
strain 1b diet DDT 2.5 - 2.7 NS Davison et al. (1976)
diet DDT 10 - 1.6 NS Davison et al. (1976)
diet DDT 40 - 7.1 NS Davison et al. (1976)
strain 2a diet DDT 2.5 + 0.5 NS Davison et al. (1976)
diet DDT 10 + 1.6 NS Davison et al. (1976)
diet DDT 40 + 1.0 NS Davison et al. (1976)
strain 2b diet DDT 2.5 - 3.7 NS Davison et al. (1976)
diet DDT 10 - 2.6 NS Davison et al. (1976)
diet DDT 40 - 5.7 NS Davison et al. (1976)
---------------------------------------------------------------------------------------------------------
a Individually caged.
b Caged in pairs.
c DDT in the p,p' - form unless stated otherwise.
d Low calcium diet (0.56%).
e S = not significant.
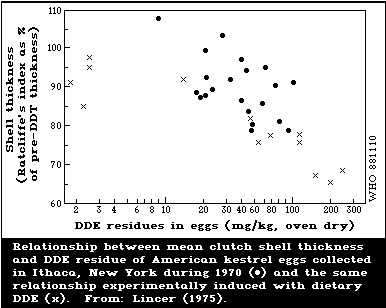 Haegele & Tucker (1974) dosed egg-laying Japanese quail with a
single oral dose of p,p' -DDE, o,p' -DDT, p,p' -DDT, or technical
DDT, all at 125 mg/kg body weight. None of the treatments caused
appreciable eggshell thinning. When Smith et al. (1969) fed Japanese
quail with DDT at 100, 200, or 400 mg/kg diet, the two lower doses had
no effect on hatchability or fertility of eggs laid. At 400 mg/kg,
there was 50% mortality amongst dosed birds; survivors showed a decline
in hatchability and fertility after 30 days. Bitman et al. (1969)
dosed Japanese quail with o,p' -DDT or p,p' -DDT at a dietary level
of 100 mg/kg. The quail were given a low calcium diet (0.56%) and
were, therefore, under calcium stress during egg laying. Both isomers
of DDT caused significant thinning of eggshells (P<0.001) and a
significant (P<0.01) reduction in shell calcium content. Eggs produced
by birds dosed with the p,p isomer were significantly lighter than
those laid by birds dosed with the o,p' isomer.
Cecil et al. (1971) investigated the effects of p,p' -DDT and
p,p' -DDE on the egg production and eggshell characteristics of
Japanese quail receiving an adequate calcium diet, and compared their
results with previous studies of the effects of these compounds on
quail receiving low calcium diets. They found a delay in the onset of
egg production in quail fed a concentration of 100 mg/kg of either DDT
or DDE for about 3 weeks. This result was similar to that of studies
with low calcium diets. In contrast to the earlier studies, there was
no effect of either DDT or DDE on shell thickness or egg weight when
dietary calcium was higher. There was an increased incidence of egg
breakage in birds fed DDT and DDE, but this was less pronounced than
with the low calcium diets.
Robson et al. (1976) studied the effects of DDE and DDT fed to
Japanese quail in two different diets containing adequate or low
calcium. DDT was fed at 100 mg/kg diet, whereas DDE was given at 0,
199, or 300 mg/kg diet, and the two calcium levels were 0.5% and 3%.
DDE at 300 mg/kg was detrimental to adult body weight, fertility, and
survivability. There was no effect of either DDT or of DDE at up to
100 mg/kg diet on adult body weight, food consumption, egg production,
egg weight, fertility, hatchability, cracking of eggs, or eggshell
thickness. Low dietary calcium had the effect of reducing the
thickness of eggshells, increasing the incidence of cracked shells and
decreasing egg production and hatchability.
Davison et al. (1976) fed DDE (0, 2, 10, 40, or 200 mg/kg diet)
to female Japanese quail that were individually caged and had 14 g of
food available each day. There was no effect on body weight, egg
laying, egg weight, eggshell thickness, or on shell calcium content.
Quail were then fed a diet containing DDT at 0, 2.5, 10, or 40 mg/kg.
There was no effect on eggshell thickness, number of eggs laid,
fertility, or hatchability. Quail fed 40 mg DDT/kg diet and caged in
pairs, broke more eggs than birds fed lower concentrations of DDT or
any concentration when the birds were caged individually. Paired quail
laid fewer eggs than single quail and in one experiment they laid eggs
with thinner shells.
When Davison & Sell (1972) dosed white leghorn hens with 100 or
200 mg DDT/kg diet for 12 weeks, the average egg production per bird,
egg weight, dry shell weight, shell thickness, and shell calcium were
all found to be unaffected by DDT at either dose level.
Egg-laying mallard ducks treated by Haegele & Tucker (1974) with a
single oral p,p' -DDE dose of 500, 1000, or 2000 mg/kg body weight
showed a clear effect on eggshell thickness at all dose levels.
Unfortunately, whilst the results are clear, no statistical analysis of
the results was presented. The effect on eggshells was dose related,
quick acting, and persistent. Heath et al. (1969) dosed mallard for
two seasons with DDE or TDE at 10 or 40 mg/kg diet and with DDT at 2.5,
10, or 40 mg/kg diet. The highest dose of DDT was reduced to 25 mg/kg
in the second season. DDE at both concentrations severely impaired
reproductive success, a more rapid initiation of the effect being seen
with the higher dose. DDE significantly affected eggshell thickness;
eggs from birds dosed with 40 mg/kg laid, in their second season, eggs
with shells 13% thinner than controls. There was a significant
increase in egg cracking and decrease in egg hatchability at both DDE
dose levels. TDE did not have a significant effect on shell thickness.
It impaired reproductive success, but not as severely as did DDE. DDT
induced eggshell thinning at a dose of 25 mg/kg, shells being 18%
thinner than controls, and reduced duckling survival during 14 days
post-hatch by 35%. DDT at 2.5 and 10 mg/kg had no effect.
Vangilder & Peterle (1980) fed mallard a diet containing 10 mg
DDE/kg, and brought the birds into breeding condition using long
daylength. Relative to controls, egg laying was delayed, eggshell
thickness was decreased, and hatchability was reduced in treated birds.
Ducklings, hatched from eggs laid by treated females, showed a signifi-
cantly reduced survival time, and a greater proportion of ducklings
were unable to initiate normal body temperature regulation.
When Longcore et al. (1971) dosed black ducks with 10 or 30 mg
DDE/kg diet, there was significant eggshell thinning and an increase in
shell cracking, compared to controls, at both dose levels. The
survival of ducklings to 21 days was also significantly reduced at both
dose levels. Longcore & Stendell (1977) fed DDE (10 mg/kg diet) to
black ducks over two breeding seasons and then untreated food for a
further 2 years. The eggshells of treated birds during dosing were 20%
thinner than controls. When dosing stopped, eggshell thickness
gradually increased but shells were still 10% thinner than controls 2
years after dosing had finished. Similarly, there was still a reduced
survival of ducklings, to 3 weeks of age, 2 years after dosing with DDE
had ceased.
Peakall et al. (1973) studied the effects of dietary DDE on
eggshell thinning in three species of bird (white pekin duck, American
kestrel, and ringdove). In addition to shell thinning, they reported a
reduced rate of water loss from eggs laid by DDE-treated birds; the
permeability constants of the eggs were significantly decreased.
Scanning electron micrographs revealed a decrease in the number of
pores per unit shell area and an increase in the number of globular
inclusions in eggshells from treated birds. Greenburg et al. (1979)
showed, also using scanning electron microscopy, that DDE affected both
organic and inorganic constituents of the eggshells of mallard dosed in
their diet. The literature concerning the effects of DDE on eggshell
structure has been reviewed in detail by Cooke (1973b).
In studies by Miller et al. (1976), laying white pekin ducks and
white leghorn hens were dosed with 40 mg DDE/kg diet. The ducks showed
significant eggshell thinning within 4 days, and again between 1 and 3
months of the start of dosing, but the hens did not show significant
eggshell effects within 2 weeks.
Peakall et al. (1975a) dosed white pekin ducks at a dietary level
of 250 mg DDE/kg for 10 days, and, approximately 2 months later,
started to collect eggs and measure shell thickness for a period of 27
weeks. At the beginning of the collection period, shells from treated
birds were found to be 20% thinner than controls. Recovery was slow
and shells were still 10% thinner at the end of the study. Haseltine
et al. (1974) dosed mallard and pheasant (10 mg DDE/kg diet) and ring
doves (40 mg DDE/kg diet) and found significant eggshell thinning and
depression of serum calcium levels in both mallard and ring dove.
However, neither parameter was significantly changed in pheasant.
Peakall et al. (1975b) maintained paired ring doves on a diet
containing 100 mg DDE/kg for 3 weeks and white pekin ducks on 250 mg
DDE/kg diet for 10 days. Although both species showed significant
eggshell thinning, there was no significant difference between the
levels of serum calcium of treated and control birds.
Miller et al. (1976) removed the shell glands from white pekin
ducks and white leghorn chickens, dosed with 40 mg DDE/kg diet, when a
calcifying egg was present within the gland, and assessed enzymatic
activity. There was a significant decrease in Ca2+-ATPase and
carbonic anhydrase activities in the shell glands removed from dosed
ducks, but no difference from controls in chicken shell glands. Kolaja
(1977) maintained mallard ducks on a diet containing either DDT, DDE,
DDT sulphonate, or DDE sulphonate at dose levels of 10 or 50 mg/kg.
Eggs were collected for 30 days and were weighed and measured. There
was no significant difference between egg weights at the different dose
levels. The thickness of eggshells of birds fed DDE was significantly
reduced. Ducks fed DDT laid eggs with significantly thinner shells
only after day 14. The two sulphonate-treated groups were not
significantly different from each other and were only significantly
different from controls on day 18; eggshell weights followed a similar
pattern.
Mendenhall et al. (1983) dosed breeding barn owls with 3 mg DDE/kg
diet during two breeding seasons, and found that treated birds laid
thin-shelled eggs and laid significantly more eggs per pair in both
seasons. In both years the percentage of eggs broken was increased,
relative to controls, and the mean number of eggs hatched and young
fledged per pair was reduced. There was a significant increase in
embryo deaths in one of the two years.
Eggshell thickness has been monitored in different ways by
different authors. Some direct measurement has been made with
membranes intact and some without. Other methods have been used to
compare recent eggs with museum specimens, which could not be broken to
measure thickness directly. The various methods were reviewed by Cooke
(1973b), who suggested standards. Generally a log-linear relationship
between DDE load and shell thinning is claimed. In a recent consider-
ation of the theoretical treatment of such data, Moriarty et al. (1986)
suggested that the main methods of assessing shell thickness do not
adequately take into consideration the effects of shell size and shape.
This does not detract from the conclusion that shell thinning occurs,
but suggests that the relationship may be more properly described as
curvilinear.
6.2.4 Reproductive hormones and behaviour
After feeding mallard a diet contaminated with 3 mg p,p' -DDE/kg
and artificially incubating eggs laid by the females, Heinz (1976)
found that the average egg residue of DDE was 5.8 mg/kg. Ducklings
from treated eggs were hyperresponsive to a tape-recorded maternal
call; treated ducklings were significantly more likely to approach the
recorder. In contrast, treated ducklings moved shorter distances away
from a frightening stimulus, compared to controls. Japanese quail
chicks fed a diet containing 50 mg DDE/kg for 8 days, starting at 7
days of age, and then a clean diet for a further 6 days showed no
significant effect on avoidance response to a moving silhouette.
Haegele & Hudson (1977) paired ring doves for 12.5 min each day,
for 5 days, prior to dosing their diet with 10 or 50 mg p,p' -DDE/kg.
The birds were also paired between days 31 and 35 and between days 59
and 63 after the start of dosing. Two measures of the courtship
behaviour of males were made: total courtship activity time and mean
bow-coo frequency. Bow-cooing behaviour is the initial behaviour
displayed by males to attract females. In control birds, the total
courtship activity time was 25% (days 31-35) and 23% (days 59-63)
longer than it was in the predose period. In birds dosed with 10 mg
DDE/kg, the courtship activity between days 31 and 35 was not different
from that in the predose period, whereas the final pairing produced a
decrease of 55% in activity. In birds dosed with 50 mg DDE/kg, the
courtship activity decreased by 30% and 67% for the two later pairing
periods compared to the predose period. After dosing at 10 mg/kg,
there was no change in bow-cooing between days 31 and 35, but a
reduction of 53% between days 59 and 63. Birds dosed at 50 mg/kg
showed decreases in bow-cooing behaviour of 43% and 84%, in the two
subsequent pairings respectively, when compared to the predose period.
When Richie & Peterle (1979) paired ring doves and fed them with
either 10 or 40 mg p,p' -DDE/kg diet, there was a significant delay in
the period between pairing and egg laying at both dose levels.
Leutinizing harmone levels in blood plasma, sampled throughout the
experiment, were not significantly altered by the DDE. Similarly,
Jefferies (1967) reported an increase in the time between
pairing and egg laying in Bengalese finches fed a range of doses
of p,p' -DDT between 75 and 1200 mg/kg diet. Treated birds were fed
for 2 h/day, immediately following a period of 1 h of starvation.
There was a significant correlation between DDT intake by the female
and the delay in egg laying. Dobson (1981) measured circulating
hormone levels and nest-building behaviour in pigeons dosed orally with
DDE and found a delay in egg laying. Hormone measurements showed that
ovulation was not delayed. Nest building was reduced in treated birds.
The delay in egg laying resulted from a lengthening of the period
between ovulation and oviposition. Since the laying of eggs is
dependent on the stimulus of adequate nest material, this lengthening
of the period between pairing and egg laying was considered to be
primarily an indirect effect on reproduction, triggered by a direct
effect on behaviour; the egg was retained longer in the oviduct.
Peakall (1970) maintained ring doves on a diet containing
10 mg p,p' -DDT/kg for 3 weeks. They were kept in isolation (with
short daylengths) and then paired (with long daylengths) to induce
breeding. The females were killed either 8 days after pairing or
after completion of their clutch of two eggs. In those killed 8 days
after pairing, circulating oestradiol levels were significantly
reduced and hepatic enzyme activity was significantly increased.
There was a significant delay in the laying of the first egg and a
decrease in egg weight. In the same study, the birds were given
oral 45Ca (7.4 x 104 Bq; 2 µCi) on the day before pairing. There
was a significant decrease in the radioactivity of eggs and in the
bones of females killed 8 days after pairing. In a separate
experiment, p,p' -DDT was injected intraperitoneally at a dose of
150 mg/kg body weight, into female ring doves within 1 day of their
first egg being laid. The birds were killed after completing their
clutch of two eggs. The shell weight of the second egg was
significantly reduced when compared to the first and there was a
significant decrease in carbonic anhydrase activity in the oviduct.
This enzyme is associated with deposition of calcium into the shell.
6.2.5 Reproductive effects on the male
Burlington & Lindeman (1950) administered a daily subcutaneous
injection of DDT to male white leghorn chicks, gradually increasing
the dose from 15 to 300 mg/kg body weight. The birds were treated for
60-89 days, and the cockerels were killed and their testes removed,
weighed, and sectioned. Treated birds were found to have smaller
testes, more intertubular tissue, and retarded tubular development.
These effects were accompanied by an inhibition of testosterone-
dependent secondary sexual characters; combs and wattles were reduced
in both size and colour development in treated birds. Locke et al.
(1966) dosed male bald eagles at dietary levels of 10 mg DDT/kg for 60
or 120 days, and found no effects on spermatogenic activity. There
were some degenerative effects on the testis, but only at doses which
had severe neurological effects and ultimately led to death.
6.2.6 Effects on the thyroid and adrenal glands in birds
When Jefferies & French (1972) fed pigeons on a diet containing
either 18, 36, or 72 mg p,p' -DDE/kg for a period of 56 days, paired
thyroid weights were found to be greater in treated birds than in
controls. There was no apparent dose relationship to this effect, but
bird numbers were small. Taking the dosed birds as a single group,
the results were significantly different from those of control birds.
Liver weights were similarly increased, and, at the two highest dose
levels, there was an increase in paired adrenal weights.
Biessman & von Faber (1981) dosed Japanese quail for 9 weeks
with technical DDT (either 50 or 250 mg/kg diet) or for 5 weeks with
p,p' -DDT or p,p' -DDE (250 and 300 mg/kg, respectively). Adrenal
weights increased with all treatments but the increase in size was only
significant for the 300 mg DDE/kg dose. The percentage of cortical
tissue, measured from areas of sections of the gland, showed a similar
trend, but results were not statistically significant. No changes were
detectable in nuclear size of either cortical or medullary cells.
Lehman et al. (1974) studied the effect of technical grade DDT on the
adrenal glands of bobwhite quail, which were maintained on a diet
containing 10, 50, or 150 mg DDT/kg for 242 days and then killed. No
effect was found on adrenal weight expressed as a percentage of body
weight, but there was a significant dose-related increase in the ratio
between areas of cortex and medulla.
6.2.7 Special studies in birds
Dieter (1974) fed Japanese quail on a diet containing 5, 25, or
100 mg DDE/kg and, after 12 weeks of dosing, assessed the activity of
five plasma enzymes (creatine kinase, aspartate aminotransferase,
lactate dehydrogenase, cholinesterase, and fructose-diphosphate
aldolase). There was an increase in the activity of all these enzymes,
which, in each case, was proportional to the logarithm of the DDE
dose.
Bend et al. (1977) dosed immature puffins, orally by intubation,
with DDE at 6 mg/day (equivalent to 50 mg/kg diet) for 16 to 21 days,
and, after killing the birds, determined the effect of DDE on hepatic
mixed-function oxidases. Both aniline hydroxylase and benzphetamine
demethylase activities were increased in treated birds; the yield of
microsomal protein remained unchanged. In contrast, Sell et al. (1972)
demonstrated a depression in aniline hydroxylase and N-demethylase
activities after feeding Japanese quail with DDT at 200 mg/kg diet.
Both DDT and DDE inhibited aniline hydroxylase in vitro activity, when
present at concentrations of 10-7 mol/litre or more.
Bunyan et al. (1970) measured the activities of glucose-6-
phosphate dehydrogenase (G-6-P) and 6-phosphogluconate dehydrogenase
(6-P-G) in the liver of Japanese quail fed diets containing low levels
of p,p' -DDT or a number of saturated and unsaturated analogues of
p,p' -DDT. Generally, saturated compounds lowered G-6-P levels and
increased 6-P-G levels. p,p' -DDMU was anomalous in elevating G-6-P.
The authors suggested that these effects might be due to interference
with protein metabolism primarily by the unsaturated analogues and
metabolites of DDT. Bunyan et al. (1972) fed either DDT or DDE to
Japanese quail and monitored hepatic microsomal protein, cytochrome
P450, aniline hydroxylase, aromatic nitroreductase, phenylbenzoate
esterase, and total vitamin C. Changes in these factors were more
readily explained in terms of residues of DDE in the liver than in
terms of dietary dose. DDE was found to be a more potent inducer of
microsomal protein, cytochrome P450, and aniline hydroxylase than was
DDT. The effects of DDT could be explained in terms of the effects of
the DDE produced by DDT metabolism. Aromatic nitroreductase was
unaffected by either compound. Vitamin C levels were raised by DDT
more than by DDE. Phenylbenzoate esterase showed a biphasic response
following the feeding of DDE. Bunyan & Page (1973) extended these
studies by examining the effects of DDE and DDMU on hepatic microsomal
enzyme systems. Most of the changes observed in quail were greater
with DDMU than with any other DDT metabolite. The authors suggested
that DDT metabolism in birds may be different to metabolism in mammals.
Metabolism probably gives rise, via the production of DDMU, to a
highly active liver inducer.
Heinz et al. (1980) fed ring doves on a diet containing 2, 20, or
200 mg DDE/kg for 8 weeks, and found at the end of the dosing period, a
significant decrease in dopamine concentration in brain tissue from
birds fed on the two higher doses. Brain noradrenalin concentration
was also affected but only at the highest dose. There was a signifi-
cant, negative correlation between concentration of both dopamine and
noradrenalin and the residue of DDE in the brain tissue.
Friend et al. (1973) fed a dietary dose of 10, 100, or
1000 mg DDE/kg. to male mallard that had been previously maintained on
either fresh water or 1% salt water. Birds were given a concentrated
salt solution either 1, 3, 6, or 9 days after the beginning of DDE
treatment, the salt being administered both intraperitoneally (12 ml of
a 10% solution) and intravenously (3ml of a 5% solution). The rate of
sodium chloride excretion was not reduced, relative to controls, in
DDE-treated birds maintained previously on salt water, but was reduced
significantly in DDE-treated birds not previously given salt.
When Mahoney (1975) fed caged white-throated sparrows on technical
DDT (either 5 or 25 mg/kg), the onset of spring nocturnal migratory
restlessness (Zugunruhe) and weight increase was delayed by at least 1
week. Although Zugunruhe onset was delayed, when migratory nocturnal
activity did commence it was more pronounced than in control birds.
The increase in Zugunruhe was related to body residues of DDT.
Haynes (1972) dosed male bobwhite quail with DDT (100 mg/kg diet)
for 10 weeks and, 1 week before the study was terminated, some birds
were transferred to clean food while others were starved for 4 days and
then given clean food for 3 days before being killed. There was no
significant effect on liver glycogen, either from dosing with DDT or
from starvation, but liver lipid levels were significantly increased by
both DDT and starvation. Body lipid levels were not significantly
affected by DDT but were reduced after starvation.
6.2.8 Synergism with other compounds in birds
Kreitzer & Spann (1973), in a study on combined effects of
pesticides, found that mixtures of DDT and dieldrin in Japanese
quail, and DDE and Ceresan M (organomercury fungicide) in pheasants,
were additive rather than synergistic in their action. The study
compared known LD50 values with expected ones. Mallard, maintained
on a diet containing a mixture of DDE (40 mg/kg) and Aroclor 1254 (40
mg/kg) for at least 30 days, laid eggs with significantly thinner
shells than did controls. This result was not significantly different
from that produced by DDE alone (Risebrough & Anderson, 1975). In a
similar study on American kestrels, Lincer (1972) dosed the birds with
Aroclor 1254 (10 mg/kg) and DDE (3 mg/kg) in the diet, both separately
and in combination. There was no eggshell thinning with Aroclor
alone, but Aroclor and DDE together had a significantly greater
effect on shell thickness than DDE alone, indicating synergism.
Japanese quail exposed to dietary doses of 5 or 50 mg DDE/kg for 12
weeks, and subsequently dosed orally with either parathion or paraoxon
at 2 µl/g body weight, showed synergism between the compounds with
respect to mortality and to inhibition of brain cholinesterase. The
synergistic action of DDE on cholinesterase inhibition was apparent 3
days after exposure to 50 mg/kg and one week after exposure to 5 mg/kg.
Mortality due to DDE was increased from 10% to 90% in the presence of
the organophosphorus compounds. Anticholinesterase effects were
increased by 50% in the presence of DDE (Ludke, 1977).
6.3 Non-laboratory Mammals
Appraisal
Experimental work suggests that some species, notably bats, may
have been affected by DDT and its metabolites. Species which show
marked seasonal cycles in fat content are most vulnerable, but few
experimental studies on such species have been made. In contrast to
the situation in birds, where the main effect of DDT is on repro-
duction, the main known effect in mammals is to increase the mortality
of migrating adults. The lowest acute dose which kills American big
brown bats is 20 mg/kg. Bats collected from the wild (and containing
residues of DDE in fat) die after experimental starvation which
simulates loss of fat during migration.
In studies into the effect of DDE on bats, Geluso et al. (1976)
captured young Mexican free-tailed bats ( Tadarida brasiliensis ) before
their first migratory flight and transferred them to the laboratory.
This species migrates north from Mexico to the USA in spring and
returns to winter in the south. Three groups of bats were used. A
reference set was killed on capture and, when the bats were analysed
for residues of organochlorines derived from environmental source, DDE
was the only chemical found in significant amounts. Brain residues of
DDE were low; the median being 3.7 (range: 1.5 to 17.0) mg/kg in eight
younger animals and 1.3 (range 1.1 to 11.0) mg/kg in older animals.
Two further groups were maintained in the laboratory where the bats
were given water but not fed. One group was regularly exercised, while
the other was given no exercise. All exercised bats died within 9
days; 4 bats in the unexercised group died and the other 4 were killed
after 9 days. Analysis of brain DDE residues showed considerably
elevated levels compared to the reference group. For the unexercised
bats, the median residue values were 47 (range 18 to 76) mg/kg in
younger animals and 70 (range 10 to 95) mg/kg in older animals. In
exercised bats, the values were 160 (range 66 to 330) mg/kg for younger
animals and 160 (range 37 to 260) mg/kg for older animals. Those
animals that died before the end of the study showed symptoms charac-
teristic of pesticide poisoning, including hyperactivity, intermittent
audiogenic seizures, and violent contractions of chest muscles. The
high brain residues of DDE were considered to be the cause of death of
the animals. It should be noted that these animals had not been arti-
ficially dosed with DDE. The effects resulted from residues of DDT in
body fat, taken up in the maternity roost. The authors considered that
their studies confirmed the suggestion that bats were being killed by
accumulated residues of DDE during the period of migration, when their
fat reserves were used up.
Clark & Kroll (1977) experimentally fed adult females of the same
species of bat ( Tadarida brasiliensis ) for 40 days with mealworms
containing 107 mg DDE/kg and then killed four of the bats. They had a
whole body burden of 2.345-2.929 mg DDE (and 78-90 mg DDE/kg in the
brain). Twelve of the dosed bats were then starved, and they died
within 8 days. The total body burden of DDE ranged from 1.952 to
3.711 mg DDE and brain residues from 379 to 564 mg/kg. These brain
residues were considered to be diagnostic of death from DDE poisoning.
Tremors characteristic of poisoning were seen in the bats before death
occurred.
The toxicity of single oral doses of DDT to bats has been
estimated in two studies. Jefferies (1972) derived an approximate
LD50 of 63 mg/kg body weight for the pipistrelle bat ( Pipistrellus
pipistrellus ), a small British species. There was no mortality at
doses below 45 mg/kg and 100% mortality at doses above 95 mg/kg.
Luckens & Davis (1964) found that the lowest dose which killed American
big brown bats ( Eptesicus fuscus ) was 20 mg/kg and that 40 mg/kg was
invariably lethal. The LD50 for this species lies somewhere between
25 and 40 mg/kg for a single oral dose.
Blus (1978) determined dietary LC50 values of DDT, given in food
either as a powder or dissolved in oil, for short-tailed shrews
( Blarina brevicauda ) of different ages and sex. In 2-week tests, the
range of LC50s for DDT dissolved in oil was 651 to 1160 mg/kg diet,
and for DDT added as powder it was 839 to >2552 mg/kg. The influence
of age and sex was sometimes more important in determining DDT toxicity
than was body weight, though heavier shrews tended to be more tolerant
of the chemical. Among older animals, males were more tolerant of DDT
than females. Braham & Neal (1974) found an effect of DDT on the
metabolic rate of the same species of shrew after feeding it with
earthworms contaminated with the insecticide. After one week of this
diet, the metabolic rate was significantly higher than that of undosed
shrews, but after 2-3 weeks of dosing there was a return to oxygen
consumption rates not different from controls. Two shrews were fasted
for 18 h, after being fed earthworms containing DDT for 3 weeks, and
compared to untreated shrews similarly fasted. The DDT-treated animals
showed 12.6% and 12.1% increases in metabolic rate after fasting,
whereas controls showed decreases of 8.7% and 8.0%. The DDT exposure
was environmentally realistic because earthworms used for feeding were
not artificially dosed with DDT but were collected from an area where
DDT had been used.
7. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
There have been kills of fish (Hunt & Linn, 1970) and aquatic
invertebrates (Ide, 1957) reported after normal usage of DDT as a
terrestrial insecticide and after its application to water for mosquito
control. Reproductive failure in commercial fisheries has also been
attributed to DDT (Hunt & Linn, 1970). In addition, it has been shown
to be toxic to amphibia after water application (section 5.3). The
setting of safe water levels of DDT and its metabolites is difficult
because its high bioaccumulation and high lipid solubility mean that it
can have effects remote in time from its application. The toxicity of
DDT to aquatic microorganisms and invertebrates is very variable
between species. Exposure to DDT or its stable metabolites would,
therefore, be expected to kill certain species selectively. Short-
term, there is close correspondence between the 96-h LC50 for a
moderately sensitive fish (16 µg/litre) and the expected water concen-
tration after application of DDT at the normal rate.
DDT and its metabolites, principally DDE, have been implicated in
reproductive effects on birds in the field. Large population declines
in some bird species, mainly birds of prey, have been blamed on DDT or
on combinations of DDT with other persistent organochlorines. The
evidence for this rests on correlations. There is a correlation in
time between the onset of effects on eggshells and the onset of major
DDT use in agriculture. There is also a correlation between geo-
graphical areas of high DDT use and effects on local populations of
birds (compared to populations living in areas of low use). There is a
clear correlation between DDE residues in eggs and the degree of
thinning of the shells of those eggs, collected from the wild. Storage
of DDT in body fat means that the effects of the compound can be remote
in time from the application of the chemical to an area. Only some
species of birds are affected by DDT or its metabolites. There are
considerable data on the variability between species in their suscepti-
bility to these compounds. Widespread monitoring programmes have
related the recovery of bird populations to reduced levels of DDE and
the residual material of aldrin/dieldrin use in the tissues of birds
sampled from the wild, following attempts to limit or ban the use of
the parent pesticide in agriculture. Because DDT is seldom the only
chemical residue found in bird tissues from the wild, there is some
disagreement on whether DDT alone can cause population declines in
birds.
Ratcliffe (1967, 1969, 1970), Hickey & Anderson (1968), and
Anderson & Hickey (1972, 1974) were the first, in Britain and North
America, respectively, to compare the thickness of eggshells sampled
from the wild with that of specimens measured from museums and private
collections which predated the use of DDT. These authors examined a
wide range of bird species but mainly those high in food chains. Later
studies, along the same lines, include those of Dilworth et al. (1972)
on the woodcock, Wiemeyer et al. (1975) on the osprey, Fox (1976) on
the common tern, Cooke et al. (1976) on the grey heron, and Koeman et
al. (1972) and Newton & Haas (1984) on the sparrowhawk. Ratcliffe
(1970) collected eggshell data on 17 species of British birds, 9 of
which showed significant decreases in shell thickness when comparing
the period before 1947 with the period after 1947. The birds affected
were predominantly raptors, exceptions being the carrion crow, rook,
and shag. Anderson & Hickey made eggshell comparisons between pre- and
post-DDT use on 25 different species of birds. The same species from
different geographical areas of North America were investigated, making
166 comparisons in all. Of these, 62% showed significant decreases, 37%
showed non-significant decreases or no change, and only 1% showed an
increase in shell thickness.
King et al. (1978) found significant decreases in eggshell thick-
ness in 15 out of 22 aquatic species of birds, in Texas, USA, when
comparing shells from 1970 with museum specimens from before 1943. All
of these studies, and many more, demonstrated that, in those species
that showed effects on eggshell thinning, the effect began suddenly and
markedly at the same time as the onset of DDT use. In Britain, the use
of DDT in large quantities began in 1947. Fig. 2 reproduces the data
(from Newton & Haas, 1984) on sparrowhawks from 1870 to 1980. The
persistence of DDT in bird tissues means that recovery is still not
complete, despite controls on the use of DDT. In Alaska, populations
of peregrine falcons did not show the effects of DDT until much later
than other regions of North America. These birds breed in Alaska,
where use of DDT was low, but winter in Central and South America.
Residues of DDT and its metabolites in Alaskan peregrines began to rise
in 1967, along with the use of DDT in its wintering grounds. Con-
comitant reductions in breeding success and populations of peregrines
occurred (White & Cade, 1977). These data are indicative of a bird
breeding and survival effect of DDT use, correlated both with time and
geographical area. The index of eggshell thickness has reflected the
pattern of use of the insecticide (Ratcliffe, 1970). Before 1947,
there was no significant geographical variation in the mean thickness
of peregrine falcon eggshells in Britain. Since 1947, eggshells from
non-agricultural areas, notably the central and eastern Scottish
highlands, have shown a smaller decrease in shell thickness than shells
from highly agricultural regions.
Anderson & Hickey (1974) showed that shells of the white-tailed
eagle in Greenland were thicker than shells of the same species
collected in the Baltic. Compared to early reference shells from
museums, the Greenland shells showed a slight increase in thickness of
3%, whereas Swedish shells showed a decrease of 16%.
Lincer (1975) established a dose relationship between dietary DDE
and eggshell thinning in captive American kestrels and, also, a
relationship between DDE residues in the eggs and the thickness of
their shells. He then compared shell thickness with egg DDE residue in
kestrels sampled from the wild. The relationship was identical. Many
other authors have shown a good correlation between egg DDE residues
and the degree of eggshell thinning. These studies cover the
following species: double-crested cormorant (Anderson et al., 1969);
great blue heron (Vermeer & Reynolds, 1970); prairie falcon (Enderson &
Berger, 1970); peregrine falcon (Peakall et al., 1975c); grey heron
(Cooke et al., 1976); sparrowhawk (Newton & Bogan, 1978); and gannet,
shag, and great black-backed gull (Cooke, 1979a). In many of these
studies, there is not only a correlation between eggshell thickness and
DDE but there are also correlations between DDE residues and residues
of other organochlorines. Therefore, it is often difficult to deter-
mine solely from the field data, exactly which chemical is responsible
for the effect. This problem has been addressed by Newton & Bogan
(1978). They conducted a statistical analysis of their data that
showed a correlation between DDE and shell thickness, egg breakage, egg
addling, and hatching failure, in addition to a correlation between
DDT, PCB, and dieldrin residues. After multivariate analysis, DDE
appeared only to be responsible for eggshell thinning and egg breakage.
Relating laboratory studies to field observations suggests that DDE is
the only organochlorine that causes eggshell thinning.
Haegele & Tucker (1974) dosed egg-laying Japanese quail with a
single oral dose of p,p' -DDE, o,p' -DDT, p,p' -DDT, or technical
DDT, all at 125 mg/kg body weight. None of the treatments caused
appreciable eggshell thinning. When Smith et al. (1969) fed Japanese
quail with DDT at 100, 200, or 400 mg/kg diet, the two lower doses had
no effect on hatchability or fertility of eggs laid. At 400 mg/kg,
there was 50% mortality amongst dosed birds; survivors showed a decline
in hatchability and fertility after 30 days. Bitman et al. (1969)
dosed Japanese quail with o,p' -DDT or p,p' -DDT at a dietary level
of 100 mg/kg. The quail were given a low calcium diet (0.56%) and
were, therefore, under calcium stress during egg laying. Both isomers
of DDT caused significant thinning of eggshells (P<0.001) and a
significant (P<0.01) reduction in shell calcium content. Eggs produced
by birds dosed with the p,p isomer were significantly lighter than
those laid by birds dosed with the o,p' isomer.
Cecil et al. (1971) investigated the effects of p,p' -DDT and
p,p' -DDE on the egg production and eggshell characteristics of
Japanese quail receiving an adequate calcium diet, and compared their
results with previous studies of the effects of these compounds on
quail receiving low calcium diets. They found a delay in the onset of
egg production in quail fed a concentration of 100 mg/kg of either DDT
or DDE for about 3 weeks. This result was similar to that of studies
with low calcium diets. In contrast to the earlier studies, there was
no effect of either DDT or DDE on shell thickness or egg weight when
dietary calcium was higher. There was an increased incidence of egg
breakage in birds fed DDT and DDE, but this was less pronounced than
with the low calcium diets.
Robson et al. (1976) studied the effects of DDE and DDT fed to
Japanese quail in two different diets containing adequate or low
calcium. DDT was fed at 100 mg/kg diet, whereas DDE was given at 0,
199, or 300 mg/kg diet, and the two calcium levels were 0.5% and 3%.
DDE at 300 mg/kg was detrimental to adult body weight, fertility, and
survivability. There was no effect of either DDT or of DDE at up to
100 mg/kg diet on adult body weight, food consumption, egg production,
egg weight, fertility, hatchability, cracking of eggs, or eggshell
thickness. Low dietary calcium had the effect of reducing the
thickness of eggshells, increasing the incidence of cracked shells and
decreasing egg production and hatchability.
Davison et al. (1976) fed DDE (0, 2, 10, 40, or 200 mg/kg diet)
to female Japanese quail that were individually caged and had 14 g of
food available each day. There was no effect on body weight, egg
laying, egg weight, eggshell thickness, or on shell calcium content.
Quail were then fed a diet containing DDT at 0, 2.5, 10, or 40 mg/kg.
There was no effect on eggshell thickness, number of eggs laid,
fertility, or hatchability. Quail fed 40 mg DDT/kg diet and caged in
pairs, broke more eggs than birds fed lower concentrations of DDT or
any concentration when the birds were caged individually. Paired quail
laid fewer eggs than single quail and in one experiment they laid eggs
with thinner shells.
When Davison & Sell (1972) dosed white leghorn hens with 100 or
200 mg DDT/kg diet for 12 weeks, the average egg production per bird,
egg weight, dry shell weight, shell thickness, and shell calcium were
all found to be unaffected by DDT at either dose level.
Egg-laying mallard ducks treated by Haegele & Tucker (1974) with a
single oral p,p' -DDE dose of 500, 1000, or 2000 mg/kg body weight
showed a clear effect on eggshell thickness at all dose levels.
Unfortunately, whilst the results are clear, no statistical analysis of
the results was presented. The effect on eggshells was dose related,
quick acting, and persistent. Heath et al. (1969) dosed mallard for
two seasons with DDE or TDE at 10 or 40 mg/kg diet and with DDT at 2.5,
10, or 40 mg/kg diet. The highest dose of DDT was reduced to 25 mg/kg
in the second season. DDE at both concentrations severely impaired
reproductive success, a more rapid initiation of the effect being seen
with the higher dose. DDE significantly affected eggshell thickness;
eggs from birds dosed with 40 mg/kg laid, in their second season, eggs
with shells 13% thinner than controls. There was a significant
increase in egg cracking and decrease in egg hatchability at both DDE
dose levels. TDE did not have a significant effect on shell thickness.
It impaired reproductive success, but not as severely as did DDE. DDT
induced eggshell thinning at a dose of 25 mg/kg, shells being 18%
thinner than controls, and reduced duckling survival during 14 days
post-hatch by 35%. DDT at 2.5 and 10 mg/kg had no effect.
Vangilder & Peterle (1980) fed mallard a diet containing 10 mg
DDE/kg, and brought the birds into breeding condition using long
daylength. Relative to controls, egg laying was delayed, eggshell
thickness was decreased, and hatchability was reduced in treated birds.
Ducklings, hatched from eggs laid by treated females, showed a signifi-
cantly reduced survival time, and a greater proportion of ducklings
were unable to initiate normal body temperature regulation.
When Longcore et al. (1971) dosed black ducks with 10 or 30 mg
DDE/kg diet, there was significant eggshell thinning and an increase in
shell cracking, compared to controls, at both dose levels. The
survival of ducklings to 21 days was also significantly reduced at both
dose levels. Longcore & Stendell (1977) fed DDE (10 mg/kg diet) to
black ducks over two breeding seasons and then untreated food for a
further 2 years. The eggshells of treated birds during dosing were 20%
thinner than controls. When dosing stopped, eggshell thickness
gradually increased but shells were still 10% thinner than controls 2
years after dosing had finished. Similarly, there was still a reduced
survival of ducklings, to 3 weeks of age, 2 years after dosing with DDE
had ceased.
Peakall et al. (1973) studied the effects of dietary DDE on
eggshell thinning in three species of bird (white pekin duck, American
kestrel, and ringdove). In addition to shell thinning, they reported a
reduced rate of water loss from eggs laid by DDE-treated birds; the
permeability constants of the eggs were significantly decreased.
Scanning electron micrographs revealed a decrease in the number of
pores per unit shell area and an increase in the number of globular
inclusions in eggshells from treated birds. Greenburg et al. (1979)
showed, also using scanning electron microscopy, that DDE affected both
organic and inorganic constituents of the eggshells of mallard dosed in
their diet. The literature concerning the effects of DDE on eggshell
structure has been reviewed in detail by Cooke (1973b).
In studies by Miller et al. (1976), laying white pekin ducks and
white leghorn hens were dosed with 40 mg DDE/kg diet. The ducks showed
significant eggshell thinning within 4 days, and again between 1 and 3
months of the start of dosing, but the hens did not show significant
eggshell effects within 2 weeks.
Peakall et al. (1975a) dosed white pekin ducks at a dietary level
of 250 mg DDE/kg for 10 days, and, approximately 2 months later,
started to collect eggs and measure shell thickness for a period of 27
weeks. At the beginning of the collection period, shells from treated
birds were found to be 20% thinner than controls. Recovery was slow
and shells were still 10% thinner at the end of the study. Haseltine
et al. (1974) dosed mallard and pheasant (10 mg DDE/kg diet) and ring
doves (40 mg DDE/kg diet) and found significant eggshell thinning and
depression of serum calcium levels in both mallard and ring dove.
However, neither parameter was significantly changed in pheasant.
Peakall et al. (1975b) maintained paired ring doves on a diet
containing 100 mg DDE/kg for 3 weeks and white pekin ducks on 250 mg
DDE/kg diet for 10 days. Although both species showed significant
eggshell thinning, there was no significant difference between the
levels of serum calcium of treated and control birds.
Miller et al. (1976) removed the shell glands from white pekin
ducks and white leghorn chickens, dosed with 40 mg DDE/kg diet, when a
calcifying egg was present within the gland, and assessed enzymatic
activity. There was a significant decrease in Ca2+-ATPase and
carbonic anhydrase activities in the shell glands removed from dosed
ducks, but no difference from controls in chicken shell glands. Kolaja
(1977) maintained mallard ducks on a diet containing either DDT, DDE,
DDT sulphonate, or DDE sulphonate at dose levels of 10 or 50 mg/kg.
Eggs were collected for 30 days and were weighed and measured. There
was no significant difference between egg weights at the different dose
levels. The thickness of eggshells of birds fed DDE was significantly
reduced. Ducks fed DDT laid eggs with significantly thinner shells
only after day 14. The two sulphonate-treated groups were not
significantly different from each other and were only significantly
different from controls on day 18; eggshell weights followed a similar
pattern.
Mendenhall et al. (1983) dosed breeding barn owls with 3 mg DDE/kg
diet during two breeding seasons, and found that treated birds laid
thin-shelled eggs and laid significantly more eggs per pair in both
seasons. In both years the percentage of eggs broken was increased,
relative to controls, and the mean number of eggs hatched and young
fledged per pair was reduced. There was a significant increase in
embryo deaths in one of the two years.
Eggshell thickness has been monitored in different ways by
different authors. Some direct measurement has been made with
membranes intact and some without. Other methods have been used to
compare recent eggs with museum specimens, which could not be broken to
measure thickness directly. The various methods were reviewed by Cooke
(1973b), who suggested standards. Generally a log-linear relationship
between DDE load and shell thinning is claimed. In a recent consider-
ation of the theoretical treatment of such data, Moriarty et al. (1986)
suggested that the main methods of assessing shell thickness do not
adequately take into consideration the effects of shell size and shape.
This does not detract from the conclusion that shell thinning occurs,
but suggests that the relationship may be more properly described as
curvilinear.
6.2.4 Reproductive hormones and behaviour
After feeding mallard a diet contaminated with 3 mg p,p' -DDE/kg
and artificially incubating eggs laid by the females, Heinz (1976)
found that the average egg residue of DDE was 5.8 mg/kg. Ducklings
from treated eggs were hyperresponsive to a tape-recorded maternal
call; treated ducklings were significantly more likely to approach the
recorder. In contrast, treated ducklings moved shorter distances away
from a frightening stimulus, compared to controls. Japanese quail
chicks fed a diet containing 50 mg DDE/kg for 8 days, starting at 7
days of age, and then a clean diet for a further 6 days showed no
significant effect on avoidance response to a moving silhouette.
Haegele & Hudson (1977) paired ring doves for 12.5 min each day,
for 5 days, prior to dosing their diet with 10 or 50 mg p,p' -DDE/kg.
The birds were also paired between days 31 and 35 and between days 59
and 63 after the start of dosing. Two measures of the courtship
behaviour of males were made: total courtship activity time and mean
bow-coo frequency. Bow-cooing behaviour is the initial behaviour
displayed by males to attract females. In control birds, the total
courtship activity time was 25% (days 31-35) and 23% (days 59-63)
longer than it was in the predose period. In birds dosed with 10 mg
DDE/kg, the courtship activity between days 31 and 35 was not different
from that in the predose period, whereas the final pairing produced a
decrease of 55% in activity. In birds dosed with 50 mg DDE/kg, the
courtship activity decreased by 30% and 67% for the two later pairing
periods compared to the predose period. After dosing at 10 mg/kg,
there was no change in bow-cooing between days 31 and 35, but a
reduction of 53% between days 59 and 63. Birds dosed at 50 mg/kg
showed decreases in bow-cooing behaviour of 43% and 84%, in the two
subsequent pairings respectively, when compared to the predose period.
When Richie & Peterle (1979) paired ring doves and fed them with
either 10 or 40 mg p,p' -DDE/kg diet, there was a significant delay in
the period between pairing and egg laying at both dose levels.
Leutinizing harmone levels in blood plasma, sampled throughout the
experiment, were not significantly altered by the DDE. Similarly,
Jefferies (1967) reported an increase in the time between
pairing and egg laying in Bengalese finches fed a range of doses
of p,p' -DDT between 75 and 1200 mg/kg diet. Treated birds were fed
for 2 h/day, immediately following a period of 1 h of starvation.
There was a significant correlation between DDT intake by the female
and the delay in egg laying. Dobson (1981) measured circulating
hormone levels and nest-building behaviour in pigeons dosed orally with
DDE and found a delay in egg laying. Hormone measurements showed that
ovulation was not delayed. Nest building was reduced in treated birds.
The delay in egg laying resulted from a lengthening of the period
between ovulation and oviposition. Since the laying of eggs is
dependent on the stimulus of adequate nest material, this lengthening
of the period between pairing and egg laying was considered to be
primarily an indirect effect on reproduction, triggered by a direct
effect on behaviour; the egg was retained longer in the oviduct.
Peakall (1970) maintained ring doves on a diet containing
10 mg p,p' -DDT/kg for 3 weeks. They were kept in isolation (with
short daylengths) and then paired (with long daylengths) to induce
breeding. The females were killed either 8 days after pairing or
after completion of their clutch of two eggs. In those killed 8 days
after pairing, circulating oestradiol levels were significantly
reduced and hepatic enzyme activity was significantly increased.
There was a significant delay in the laying of the first egg and a
decrease in egg weight. In the same study, the birds were given
oral 45Ca (7.4 x 104 Bq; 2 µCi) on the day before pairing. There
was a significant decrease in the radioactivity of eggs and in the
bones of females killed 8 days after pairing. In a separate
experiment, p,p' -DDT was injected intraperitoneally at a dose of
150 mg/kg body weight, into female ring doves within 1 day of their
first egg being laid. The birds were killed after completing their
clutch of two eggs. The shell weight of the second egg was
significantly reduced when compared to the first and there was a
significant decrease in carbonic anhydrase activity in the oviduct.
This enzyme is associated with deposition of calcium into the shell.
6.2.5 Reproductive effects on the male
Burlington & Lindeman (1950) administered a daily subcutaneous
injection of DDT to male white leghorn chicks, gradually increasing
the dose from 15 to 300 mg/kg body weight. The birds were treated for
60-89 days, and the cockerels were killed and their testes removed,
weighed, and sectioned. Treated birds were found to have smaller
testes, more intertubular tissue, and retarded tubular development.
These effects were accompanied by an inhibition of testosterone-
dependent secondary sexual characters; combs and wattles were reduced
in both size and colour development in treated birds. Locke et al.
(1966) dosed male bald eagles at dietary levels of 10 mg DDT/kg for 60
or 120 days, and found no effects on spermatogenic activity. There
were some degenerative effects on the testis, but only at doses which
had severe neurological effects and ultimately led to death.
6.2.6 Effects on the thyroid and adrenal glands in birds
When Jefferies & French (1972) fed pigeons on a diet containing
either 18, 36, or 72 mg p,p' -DDE/kg for a period of 56 days, paired
thyroid weights were found to be greater in treated birds than in
controls. There was no apparent dose relationship to this effect, but
bird numbers were small. Taking the dosed birds as a single group,
the results were significantly different from those of control birds.
Liver weights were similarly increased, and, at the two highest dose
levels, there was an increase in paired adrenal weights.
Biessman & von Faber (1981) dosed Japanese quail for 9 weeks
with technical DDT (either 50 or 250 mg/kg diet) or for 5 weeks with
p,p' -DDT or p,p' -DDE (250 and 300 mg/kg, respectively). Adrenal
weights increased with all treatments but the increase in size was only
significant for the 300 mg DDE/kg dose. The percentage of cortical
tissue, measured from areas of sections of the gland, showed a similar
trend, but results were not statistically significant. No changes were
detectable in nuclear size of either cortical or medullary cells.
Lehman et al. (1974) studied the effect of technical grade DDT on the
adrenal glands of bobwhite quail, which were maintained on a diet
containing 10, 50, or 150 mg DDT/kg for 242 days and then killed. No
effect was found on adrenal weight expressed as a percentage of body
weight, but there was a significant dose-related increase in the ratio
between areas of cortex and medulla.
6.2.7 Special studies in birds
Dieter (1974) fed Japanese quail on a diet containing 5, 25, or
100 mg DDE/kg and, after 12 weeks of dosing, assessed the activity of
five plasma enzymes (creatine kinase, aspartate aminotransferase,
lactate dehydrogenase, cholinesterase, and fructose-diphosphate
aldolase). There was an increase in the activity of all these enzymes,
which, in each case, was proportional to the logarithm of the DDE
dose.
Bend et al. (1977) dosed immature puffins, orally by intubation,
with DDE at 6 mg/day (equivalent to 50 mg/kg diet) for 16 to 21 days,
and, after killing the birds, determined the effect of DDE on hepatic
mixed-function oxidases. Both aniline hydroxylase and benzphetamine
demethylase activities were increased in treated birds; the yield of
microsomal protein remained unchanged. In contrast, Sell et al. (1972)
demonstrated a depression in aniline hydroxylase and N-demethylase
activities after feeding Japanese quail with DDT at 200 mg/kg diet.
Both DDT and DDE inhibited aniline hydroxylase in vitro activity, when
present at concentrations of 10-7 mol/litre or more.
Bunyan et al. (1970) measured the activities of glucose-6-
phosphate dehydrogenase (G-6-P) and 6-phosphogluconate dehydrogenase
(6-P-G) in the liver of Japanese quail fed diets containing low levels
of p,p' -DDT or a number of saturated and unsaturated analogues of
p,p' -DDT. Generally, saturated compounds lowered G-6-P levels and
increased 6-P-G levels. p,p' -DDMU was anomalous in elevating G-6-P.
The authors suggested that these effects might be due to interference
with protein metabolism primarily by the unsaturated analogues and
metabolites of DDT. Bunyan et al. (1972) fed either DDT or DDE to
Japanese quail and monitored hepatic microsomal protein, cytochrome
P450, aniline hydroxylase, aromatic nitroreductase, phenylbenzoate
esterase, and total vitamin C. Changes in these factors were more
readily explained in terms of residues of DDE in the liver than in
terms of dietary dose. DDE was found to be a more potent inducer of
microsomal protein, cytochrome P450, and aniline hydroxylase than was
DDT. The effects of DDT could be explained in terms of the effects of
the DDE produced by DDT metabolism. Aromatic nitroreductase was
unaffected by either compound. Vitamin C levels were raised by DDT
more than by DDE. Phenylbenzoate esterase showed a biphasic response
following the feeding of DDE. Bunyan & Page (1973) extended these
studies by examining the effects of DDE and DDMU on hepatic microsomal
enzyme systems. Most of the changes observed in quail were greater
with DDMU than with any other DDT metabolite. The authors suggested
that DDT metabolism in birds may be different to metabolism in mammals.
Metabolism probably gives rise, via the production of DDMU, to a
highly active liver inducer.
Heinz et al. (1980) fed ring doves on a diet containing 2, 20, or
200 mg DDE/kg for 8 weeks, and found at the end of the dosing period, a
significant decrease in dopamine concentration in brain tissue from
birds fed on the two higher doses. Brain noradrenalin concentration
was also affected but only at the highest dose. There was a signifi-
cant, negative correlation between concentration of both dopamine and
noradrenalin and the residue of DDE in the brain tissue.
Friend et al. (1973) fed a dietary dose of 10, 100, or
1000 mg DDE/kg. to male mallard that had been previously maintained on
either fresh water or 1% salt water. Birds were given a concentrated
salt solution either 1, 3, 6, or 9 days after the beginning of DDE
treatment, the salt being administered both intraperitoneally (12 ml of
a 10% solution) and intravenously (3ml of a 5% solution). The rate of
sodium chloride excretion was not reduced, relative to controls, in
DDE-treated birds maintained previously on salt water, but was reduced
significantly in DDE-treated birds not previously given salt.
When Mahoney (1975) fed caged white-throated sparrows on technical
DDT (either 5 or 25 mg/kg), the onset of spring nocturnal migratory
restlessness (Zugunruhe) and weight increase was delayed by at least 1
week. Although Zugunruhe onset was delayed, when migratory nocturnal
activity did commence it was more pronounced than in control birds.
The increase in Zugunruhe was related to body residues of DDT.
Haynes (1972) dosed male bobwhite quail with DDT (100 mg/kg diet)
for 10 weeks and, 1 week before the study was terminated, some birds
were transferred to clean food while others were starved for 4 days and
then given clean food for 3 days before being killed. There was no
significant effect on liver glycogen, either from dosing with DDT or
from starvation, but liver lipid levels were significantly increased by
both DDT and starvation. Body lipid levels were not significantly
affected by DDT but were reduced after starvation.
6.2.8 Synergism with other compounds in birds
Kreitzer & Spann (1973), in a study on combined effects of
pesticides, found that mixtures of DDT and dieldrin in Japanese
quail, and DDE and Ceresan M (organomercury fungicide) in pheasants,
were additive rather than synergistic in their action. The study
compared known LD50 values with expected ones. Mallard, maintained
on a diet containing a mixture of DDE (40 mg/kg) and Aroclor 1254 (40
mg/kg) for at least 30 days, laid eggs with significantly thinner
shells than did controls. This result was not significantly different
from that produced by DDE alone (Risebrough & Anderson, 1975). In a
similar study on American kestrels, Lincer (1972) dosed the birds with
Aroclor 1254 (10 mg/kg) and DDE (3 mg/kg) in the diet, both separately
and in combination. There was no eggshell thinning with Aroclor
alone, but Aroclor and DDE together had a significantly greater
effect on shell thickness than DDE alone, indicating synergism.
Japanese quail exposed to dietary doses of 5 or 50 mg DDE/kg for 12
weeks, and subsequently dosed orally with either parathion or paraoxon
at 2 µl/g body weight, showed synergism between the compounds with
respect to mortality and to inhibition of brain cholinesterase. The
synergistic action of DDE on cholinesterase inhibition was apparent 3
days after exposure to 50 mg/kg and one week after exposure to 5 mg/kg.
Mortality due to DDE was increased from 10% to 90% in the presence of
the organophosphorus compounds. Anticholinesterase effects were
increased by 50% in the presence of DDE (Ludke, 1977).
6.3 Non-laboratory Mammals
Appraisal
Experimental work suggests that some species, notably bats, may
have been affected by DDT and its metabolites. Species which show
marked seasonal cycles in fat content are most vulnerable, but few
experimental studies on such species have been made. In contrast to
the situation in birds, where the main effect of DDT is on repro-
duction, the main known effect in mammals is to increase the mortality
of migrating adults. The lowest acute dose which kills American big
brown bats is 20 mg/kg. Bats collected from the wild (and containing
residues of DDE in fat) die after experimental starvation which
simulates loss of fat during migration.
In studies into the effect of DDE on bats, Geluso et al. (1976)
captured young Mexican free-tailed bats ( Tadarida brasiliensis ) before
their first migratory flight and transferred them to the laboratory.
This species migrates north from Mexico to the USA in spring and
returns to winter in the south. Three groups of bats were used. A
reference set was killed on capture and, when the bats were analysed
for residues of organochlorines derived from environmental source, DDE
was the only chemical found in significant amounts. Brain residues of
DDE were low; the median being 3.7 (range: 1.5 to 17.0) mg/kg in eight
younger animals and 1.3 (range 1.1 to 11.0) mg/kg in older animals.
Two further groups were maintained in the laboratory where the bats
were given water but not fed. One group was regularly exercised, while
the other was given no exercise. All exercised bats died within 9
days; 4 bats in the unexercised group died and the other 4 were killed
after 9 days. Analysis of brain DDE residues showed considerably
elevated levels compared to the reference group. For the unexercised
bats, the median residue values were 47 (range 18 to 76) mg/kg in
younger animals and 70 (range 10 to 95) mg/kg in older animals. In
exercised bats, the values were 160 (range 66 to 330) mg/kg for younger
animals and 160 (range 37 to 260) mg/kg for older animals. Those
animals that died before the end of the study showed symptoms charac-
teristic of pesticide poisoning, including hyperactivity, intermittent
audiogenic seizures, and violent contractions of chest muscles. The
high brain residues of DDE were considered to be the cause of death of
the animals. It should be noted that these animals had not been arti-
ficially dosed with DDE. The effects resulted from residues of DDT in
body fat, taken up in the maternity roost. The authors considered that
their studies confirmed the suggestion that bats were being killed by
accumulated residues of DDE during the period of migration, when their
fat reserves were used up.
Clark & Kroll (1977) experimentally fed adult females of the same
species of bat ( Tadarida brasiliensis ) for 40 days with mealworms
containing 107 mg DDE/kg and then killed four of the bats. They had a
whole body burden of 2.345-2.929 mg DDE (and 78-90 mg DDE/kg in the
brain). Twelve of the dosed bats were then starved, and they died
within 8 days. The total body burden of DDE ranged from 1.952 to
3.711 mg DDE and brain residues from 379 to 564 mg/kg. These brain
residues were considered to be diagnostic of death from DDE poisoning.
Tremors characteristic of poisoning were seen in the bats before death
occurred.
The toxicity of single oral doses of DDT to bats has been
estimated in two studies. Jefferies (1972) derived an approximate
LD50 of 63 mg/kg body weight for the pipistrelle bat ( Pipistrellus
pipistrellus ), a small British species. There was no mortality at
doses below 45 mg/kg and 100% mortality at doses above 95 mg/kg.
Luckens & Davis (1964) found that the lowest dose which killed American
big brown bats ( Eptesicus fuscus ) was 20 mg/kg and that 40 mg/kg was
invariably lethal. The LD50 for this species lies somewhere between
25 and 40 mg/kg for a single oral dose.
Blus (1978) determined dietary LC50 values of DDT, given in food
either as a powder or dissolved in oil, for short-tailed shrews
( Blarina brevicauda ) of different ages and sex. In 2-week tests, the
range of LC50s for DDT dissolved in oil was 651 to 1160 mg/kg diet,
and for DDT added as powder it was 839 to >2552 mg/kg. The influence
of age and sex was sometimes more important in determining DDT toxicity
than was body weight, though heavier shrews tended to be more tolerant
of the chemical. Among older animals, males were more tolerant of DDT
than females. Braham & Neal (1974) found an effect of DDT on the
metabolic rate of the same species of shrew after feeding it with
earthworms contaminated with the insecticide. After one week of this
diet, the metabolic rate was significantly higher than that of undosed
shrews, but after 2-3 weeks of dosing there was a return to oxygen
consumption rates not different from controls. Two shrews were fasted
for 18 h, after being fed earthworms containing DDT for 3 weeks, and
compared to untreated shrews similarly fasted. The DDT-treated animals
showed 12.6% and 12.1% increases in metabolic rate after fasting,
whereas controls showed decreases of 8.7% and 8.0%. The DDT exposure
was environmentally realistic because earthworms used for feeding were
not artificially dosed with DDT but were collected from an area where
DDT had been used.
7. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
There have been kills of fish (Hunt & Linn, 1970) and aquatic
invertebrates (Ide, 1957) reported after normal usage of DDT as a
terrestrial insecticide and after its application to water for mosquito
control. Reproductive failure in commercial fisheries has also been
attributed to DDT (Hunt & Linn, 1970). In addition, it has been shown
to be toxic to amphibia after water application (section 5.3). The
setting of safe water levels of DDT and its metabolites is difficult
because its high bioaccumulation and high lipid solubility mean that it
can have effects remote in time from its application. The toxicity of
DDT to aquatic microorganisms and invertebrates is very variable
between species. Exposure to DDT or its stable metabolites would,
therefore, be expected to kill certain species selectively. Short-
term, there is close correspondence between the 96-h LC50 for a
moderately sensitive fish (16 µg/litre) and the expected water concen-
tration after application of DDT at the normal rate.
DDT and its metabolites, principally DDE, have been implicated in
reproductive effects on birds in the field. Large population declines
in some bird species, mainly birds of prey, have been blamed on DDT or
on combinations of DDT with other persistent organochlorines. The
evidence for this rests on correlations. There is a correlation in
time between the onset of effects on eggshells and the onset of major
DDT use in agriculture. There is also a correlation between geo-
graphical areas of high DDT use and effects on local populations of
birds (compared to populations living in areas of low use). There is a
clear correlation between DDE residues in eggs and the degree of
thinning of the shells of those eggs, collected from the wild. Storage
of DDT in body fat means that the effects of the compound can be remote
in time from the application of the chemical to an area. Only some
species of birds are affected by DDT or its metabolites. There are
considerable data on the variability between species in their suscepti-
bility to these compounds. Widespread monitoring programmes have
related the recovery of bird populations to reduced levels of DDE and
the residual material of aldrin/dieldrin use in the tissues of birds
sampled from the wild, following attempts to limit or ban the use of
the parent pesticide in agriculture. Because DDT is seldom the only
chemical residue found in bird tissues from the wild, there is some
disagreement on whether DDT alone can cause population declines in
birds.
Ratcliffe (1967, 1969, 1970), Hickey & Anderson (1968), and
Anderson & Hickey (1972, 1974) were the first, in Britain and North
America, respectively, to compare the thickness of eggshells sampled
from the wild with that of specimens measured from museums and private
collections which predated the use of DDT. These authors examined a
wide range of bird species but mainly those high in food chains. Later
studies, along the same lines, include those of Dilworth et al. (1972)
on the woodcock, Wiemeyer et al. (1975) on the osprey, Fox (1976) on
the common tern, Cooke et al. (1976) on the grey heron, and Koeman et
al. (1972) and Newton & Haas (1984) on the sparrowhawk. Ratcliffe
(1970) collected eggshell data on 17 species of British birds, 9 of
which showed significant decreases in shell thickness when comparing
the period before 1947 with the period after 1947. The birds affected
were predominantly raptors, exceptions being the carrion crow, rook,
and shag. Anderson & Hickey made eggshell comparisons between pre- and
post-DDT use on 25 different species of birds. The same species from
different geographical areas of North America were investigated, making
166 comparisons in all. Of these, 62% showed significant decreases, 37%
showed non-significant decreases or no change, and only 1% showed an
increase in shell thickness.
King et al. (1978) found significant decreases in eggshell thick-
ness in 15 out of 22 aquatic species of birds, in Texas, USA, when
comparing shells from 1970 with museum specimens from before 1943. All
of these studies, and many more, demonstrated that, in those species
that showed effects on eggshell thinning, the effect began suddenly and
markedly at the same time as the onset of DDT use. In Britain, the use
of DDT in large quantities began in 1947. Fig. 2 reproduces the data
(from Newton & Haas, 1984) on sparrowhawks from 1870 to 1980. The
persistence of DDT in bird tissues means that recovery is still not
complete, despite controls on the use of DDT. In Alaska, populations
of peregrine falcons did not show the effects of DDT until much later
than other regions of North America. These birds breed in Alaska,
where use of DDT was low, but winter in Central and South America.
Residues of DDT and its metabolites in Alaskan peregrines began to rise
in 1967, along with the use of DDT in its wintering grounds. Con-
comitant reductions in breeding success and populations of peregrines
occurred (White & Cade, 1977). These data are indicative of a bird
breeding and survival effect of DDT use, correlated both with time and
geographical area. The index of eggshell thickness has reflected the
pattern of use of the insecticide (Ratcliffe, 1970). Before 1947,
there was no significant geographical variation in the mean thickness
of peregrine falcon eggshells in Britain. Since 1947, eggshells from
non-agricultural areas, notably the central and eastern Scottish
highlands, have shown a smaller decrease in shell thickness than shells
from highly agricultural regions.
Anderson & Hickey (1974) showed that shells of the white-tailed
eagle in Greenland were thicker than shells of the same species
collected in the Baltic. Compared to early reference shells from
museums, the Greenland shells showed a slight increase in thickness of
3%, whereas Swedish shells showed a decrease of 16%.
Lincer (1975) established a dose relationship between dietary DDE
and eggshell thinning in captive American kestrels and, also, a
relationship between DDE residues in the eggs and the thickness of
their shells. He then compared shell thickness with egg DDE residue in
kestrels sampled from the wild. The relationship was identical. Many
other authors have shown a good correlation between egg DDE residues
and the degree of eggshell thinning. These studies cover the
following species: double-crested cormorant (Anderson et al., 1969);
great blue heron (Vermeer & Reynolds, 1970); prairie falcon (Enderson &
Berger, 1970); peregrine falcon (Peakall et al., 1975c); grey heron
(Cooke et al., 1976); sparrowhawk (Newton & Bogan, 1978); and gannet,
shag, and great black-backed gull (Cooke, 1979a). In many of these
studies, there is not only a correlation between eggshell thickness and
DDE but there are also correlations between DDE residues and residues
of other organochlorines. Therefore, it is often difficult to deter-
mine solely from the field data, exactly which chemical is responsible
for the effect. This problem has been addressed by Newton & Bogan
(1978). They conducted a statistical analysis of their data that
showed a correlation between DDE and shell thickness, egg breakage, egg
addling, and hatching failure, in addition to a correlation between
DDT, PCB, and dieldrin residues. After multivariate analysis, DDE
appeared only to be responsible for eggshell thinning and egg breakage.
Relating laboratory studies to field observations suggests that DDE is
the only organochlorine that causes eggshell thinning.
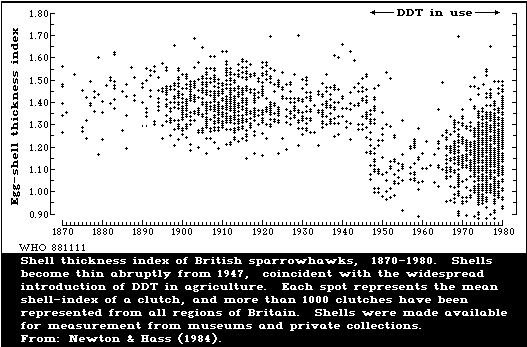 Population declines in birds of prey differed between much of North
America and eastern North America and western Europe. In North
America, apart from in the East, declines were gradual, whereas in
Europe and eastern North America declines were sudden and catastrophic.
The sudden declines in Europe have usually been attributed to the use
of the chlorinated cyclodienes, which kill adult birds, rather than to
DDT. A study of the recoveries of European birds of prey populations
provides evidence for this attribution. Populations began to rise at a
time when residues of DDE in tissues were stable but when use of the
cyclodienes and, therefore, residues of HEOD (dieldrin) were declining.
Some populations in North America did not show high contamination with
cyclodienes and may have declined due to DDT use alone. Henny (1972)
showed that in American populations of osprey, American kestrel, and
red-shouldered hawk there was a decrease in breeding performance, but
no increase in adult mortality, in response to DDT. The reproductive
effects of DDT may have prevented population recoveries after the
cessation of dieldrin use and the return of mortality rates to normal.
The question has been reviewed by Newton (1979) and Newton & Haas
(1984).
The populations of many species of birds of prey were monitored
throughout a period of high DDT use. This was done by large scale
surveys and studies of population dynamics (Ratcliffe, 1972; Henny,
1977; Lindberg, 1977; White & Cade, 1977), migration counts at obser-
vation points (Rosen, 1966; Hackman & Henny, 1971; Edelstam, 1972;
Ulfstrand et al., 1974; Nagy, 1977), and by sample counts (Ash, 1965;
Bezzel, 1969). Some species showed marked declines (in some areas this
led to local extinction), whilst others showed only temporary effects
or no effects at all. Declines were most marked in bird-eating
species, such as the sparrowhawk and peregrine falcon, and fish-eating
species, such as the white-tailed and bald eagles, and were less marked
in mammal-eating species, such as the kestrel, golden eagle, and
buzzard. These variations in decline correspond to the DDE levels
found in these particular species (Newton, 1979).
Perfect (1980) reported the results of a 4-year study on the
overall effects of the use of DDT as an insecticide on cowpeas crops in
a Nigerian forest soil. In addition to effects on soil invertebrates
(section 6.1), there were effects on the decomposition of plant
material. The remains of the plants after harvesting were ploughed
into the soil and this resulted in an increase in the residues of DDT
and its metabolites in lower levels of the soil. To confirm an effect
on decomposition, these plant remains were buried in mesh bags and the
loss of weight due to decomposition was recorded over time. There was
a significant reduction in the rate of decomposition of plant material
treated with DDT and also of untreated plant material buried in
contaminated soil. Shires (1985) reported no significant effect on the
decomposition of sweet chestnut leaf litter in a temperate area after
the application of DDT at 1 kg/ha.
Perfect et al. (1979) investigated the effects of repeated DDT
applications on cowpea crop yield in Nigeria. Yields varied
considerably from season to season and from year to year in untreated
plots because of differences in pest damage and climate. DDT was
applied to the treated plots weekly between planting and harvest at a
rate of 1 kg/ha, and the site was studied for 4 years. Over the 4-year
period there was a considerable benefit in yield from DDT application;
the yield was 1.45 tonnes/ha in the untreated and 3.42 tonnes/ha in the
treated plots. However, the benefit was most noticeable in the first
year of cultivation and declined over the four years to the point where
DDT use did not significantly increase yield. The authors attributed
the effect to the deleterious action of the insecticide on soil biota.
8. EVALUATION
In evaluating the environmental hazard of DDT and its metabolites
the following general points have to be kept in mind.
(a) The environmental distribution and effects of DDT are spread wider
than the area of use, because the parent compound or its
metabolites are carried worldwide by air and ocean currents and in
biota.
(b) Some of the breakdown products of DDT, principally DDE, are highly
persistent in soil, sediment, and biota. Thus, problems with
residues of these materials last long after the cessation of use.
(c) The bioaccumulation of DDT, or more usually of its metabolites, is
well established and occurs from very low environmental
concentrations of DDT. The use of "bioconcentration factors"
(the ratio of concentration in the organism with concentration in
the medium) to estimate the capacity of organisms to take up DDT
can be misleading if the exposure is high, since these values are
ratios.
(d) Residues and effects are often highly seasonal, corresponding to
changes in body fat, since DDT metabolites are very lipid-soluble.
Measurements of these metabolites in the tissues of organisms must
be conducted over a period of time if they are to give any
indication of the degree of contamination of the environment.
(e) There are insufficient data on the effects of DDT and its
metabolites on communities of organisms and ecosystem functioning.
Hazard assessment is, therefore, often made by extrapolation from
single species studies.
(f) Research and monitoring have concentrated on a few effects of DDT
observed in the wild. This could give the mistaken impression that
the effects of these compounds are restricted to a few species.
Other effects could be predicted but have received little or no
attention from the scientific community.
(g) The major remaining use of DDT is for malaria control operations
that are normally carried out in tropical countries. However, the
majority of environmental studies on DDT have been carried out in
conditions relevant to temperate regions. Care must be exercised
in extrapolating these results to tropical conditions.
8.1 Aquatic Organisms
The widespread use of DDT as an insecticide has resulted in
worldwide contamination of the environment. Due to the physicochemical
characteristics of DDT and its metabolites, concentrations have been
recorded in different environmental compartments, including soil,
sediments, and terrestrial and aquatic organisms. The bioconcentration
of DDT and its metabolites is a real hazard to non-target organisms.
DDT and its metabolites cause adverse effects at all trophic levels of
aquatic ecosystems, particularly on primary producers, which are the
most sensitive. Although no data are available for the effects of DDT
on ecosystem function, it should be regarded as a major environmental
hazard in this respect. DDT and its metabolites are highly toxic to
fish and, besides their lethal effect, they affect development,
behaviour, and biochemical processes. DDT and its metabolites, should
be regarded as hazardous to fish productivity and distribution and,
hence, to human food supplies. Accumulated DDT and its metabolites are
further transferred from aquatic organisms to consumers, including
birds, mammals, and, ultimately, human beings.
8.2 Terrestrial Organisms
DDT-type compounds are resistant to breakdown and are readily
adsorbed onto soils and sediments, from whence they can act as long-
term sources of exposure and contribute to terrestrial organisms.
Accumulation in terrestrial organisms is via the food chain.
These chemicals are hazardous to microorganisms, but repeated
application can lead to the development of tolerance in some species.
DDT causes fluctuations in some populations of microorganisms, and this
could eventually lead to changes in species composition, disruption of
nutrient cycles, and changes in soil fertility.
Earthworms are insensitive to the acute toxic effects of DDT
residues in soil. However, they are known to take up DDT from soil and
this uptake presents a major hazard to predators.
DDT is a non-selective insecticide and leads to mortality in
natural enemies of the insect pest. This results in impairment of the
balance between predators and prey and leads to outbreaks of secondary
pests and occurrence of the primary pest in larger numbers.
Laboratory studies confirm field findings that bat populations are
adversely affected by DDE, especially during migration. These studies
are indicative of the potential hazard to other mammals, exposed to DDT
in the environment, when fat containing DDT residues is mobilized,
e.g., during migration or temporary starvation.
One of the most widely studied effects of DDT is eggshell thinning
in birds, particularly in predatory species. The metabolite DDE, not
DDT, has been shown to be responsible for this effect. Other effects
on reproduction and survival of birds have been demonstrated. Large
population declines in birds of prey can be, at least partially,
attributed to DDT. It has been shown that DDE residues in birds and
their eggs reduced the rate of recovery of affected raptor
populations. A factor that has received less attention is the
secondary effect of the increasing numbers of pest rodents that were
controlled principally by birds of prey in some countries.
* * *
Because of their lack of degradation, their resulting widespread
persistence in the environment, their high acute toxicity to organisms
at the base of food chains, and their high potential for
bioaccumulation, DDT and its metabolites should be regarded as a major
hazard to the environment. DDT should not be used when an alternative
insecticide is available.
REFERENCES
ALBAUGH, D.W. (1972) Insecticide tolerances of two crayfish
populations ( Procambarus acutus ) in South-central Texas. Bull.
environ. Contam. Toxicol ., 8: 334-338.
ALBONE, E.S., EGLINTON, G., EVANS, N.C., HUNTER, J.M., & RHEAD,
M.M. (1972) Fate of DDT in severn estuary sediments. Environ. Sci.
Technol ., 6: 914-919.
ALLISON, D., KALLMAN, B.J., COPE, O.B., & VAN VALLIN, C.C. (1964)
Some chronic effects of DDT on cutthroat trout , Washington, DC, US
Department of the Interior, Bureau of Sport, Fisheries and Wildlife,
pp. 1-30 (Resource Report No. 64).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain north
American birds. Proc. Int. Ornithol. Congr ., 15: 514-540.
ANDERSON, D.W. & HICKEY, J.J. (1974) Eggshell changes in raptors from
the Baltic region. Oikos , 25: 395-401.
ANDERSON, D.W., HICKEY, J.J., RISEBROUGH, R.W., HUGHES, D.F., &
CHRISTENSEN, R.E. (1969) Significance of chlorinated hydrocarbon
residues to breeding pelicans and comorant. Can. field Nat ., 83: 91-
112.
ASH, J.S. (1965) A reduction in numbers of birds of prey in France.
Bird Study , 12: 17-26.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees , University
of California, 38 pp. (Laboratory Studies - Extension M-16).
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1972) DDT:
inhibition of sodium chloride tolerance by the blue-green
alga Anacystis nidulans . Science, 176: 1141-1143.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the aquatic
environment: uptake and loss of DDT and dieldrin by freshwater mussels.
Bull. environ. Contam. Toxicol ., 1: 97-111.
BEND, J.R., MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1977) DDE-
induced microsomal mixed-function oxidases in the puffin ( Fratercula
arctica). Biochem. Pharmacol ., 26: 1000-1001.
BENSON, W.W. & SMITH, P. (1972) Pesticide levels in deer. Bull.
environ. Contam. Toxicol ., 8: 1-9.
BENSON, W.W., GABICA, J., & BEECHAM, J. (1974) Pesticide and mercury
levels in bear. Bull. environ. Contam. Toxicol ., 11: 1-4.
BERGLIND, R. & DAVE, G. (1984) Acute toxicity of chromate, DDT, PCP,
TPBS and zinc to Daphnia magna cultured in hard and soft water. Bull.
environ. Contam. Toxicol ., 33: 63-68.
BEZZEL, E. (1969) [Results of quantitative observations of the
Accipitridae in Upper Bavaria.] Ornithol. Mitt ., 21: 29-36 (in
German).
BIESSMANN, A. & VON FABER, H. (1981) Effects of DDT and its
metabolites on the adrenal gland of the Japanese quail. Environ.
Pollut ., 25: 99-104.
BITMAN, J., CECIL, H.C., HARRIS, S.J., & FRIES, G.F. (1969) DDT
induces a decrease in eggshell calcium. Nature (Lond.) , 224: 44-46.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin and endrin. Arch. environ. Contam.
Toxicol ., 7: 83-98.
BOLLAG, J.-M. & HENNINGER, N.M. (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J. environ.
Qual ., 5: 15-18.
BOLLEN, W.B., MORRISON, H.E., & CROWELL, H.H. (1954) Effect of field
treatments of insecticides on numbers of bacteria, Streptomyces and
moulds in the soil. J. econ. Entomol ., 47: 302-306.
BOYD, C.E. & FERGUSON, D.E. (1964) Susceptibility and resistance of
mosquito fish to several insecticides. J. econ. Entomol ., 57: 430-431.
BOYD, C.E., VINSON, S.B., & FERGUSON, D.E. (1963) Possible DDT
resistance in two species of frogs. Copeia , 1963(2): 426-429.
BRAHAM, H.W. & NEAL, C.M. (1974) The effects of DDT on energetics of
the short-tailed shrew, Blarina brevicauda. Bull. environ. Contam.
Toxicol ., 12: 32-37.
BUHLER, D.R. & SHANKS, W.E. (1970) Influence of body weight on chronic
oral DDT toxicity in coho salmon. J. Fish Res. Board Can ., 27: 347-
358.
BUHLER, D.R., RASMUSSON, M.E., & SHANKS W.E. (1969) Chronic oral
DDT toxicity in juvenile coho and chinook salmon. Toxicol. appl.
Pharmacol ., 14: 535-555.
BUNYAN, P.J. & PAGE, J.M.J. (1973) Pesticide-induced changes in
hepatic microsomal enzyme systems. Some effects of 1,1-di(p-chloro-
phenyl)-2,2-dichloroethylene (DDE) and 1,1-di(p-chlorophenyl)-2-
chloroethylene (DDMU) in the rat and the Japanese quail. Chem.-biol.
Interact ., 6: 249-257.
BUNYAN, P.J., DAVIDSON, J., & SHORTHILL, M.J. (1970) Hepatic glucose-
6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase levels
in Japanese quail following the ingestion of p,p' -DDT and related
compounds. Chem.-biol. Interact ., 2: 175-182.
BUNYAN, P.J., TOWNSEND, M.G., & TAYLOR, A. (1972) Pesticide-induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-di(p-
chlorophenyl) -2,2,2-trichloroethane (DDT) and 1,1-di(p-chlorophenyl)-
2,2-dichloroethylene (DDE) in the rat and Japanese quail. Chem.-biol.
Interact ., 5: 13-26.
BURDICK, G.E., HARRIS, E.J., DEAN, H.J., WALKER, T.M., SKEA, J., &
COLBY, D. (1964) The accumulation of DDT in lake trout and the effect
on reproduction. Trans. Am. Fish. Soc ., 93: 127-136.
BURLINGTON, H. & LINDEMAN, V.F. (1950) Effect of DDT on testes and
secondary sex characters of white leghorn cockerels. Proc. Soc. Exp.
Biol ., 74: 48-51.
BUTLER, P.A. (1964) Commercial fishery investigations. In: The
effects of pesticides on fish and wildlife , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 65-77
(Circular No. 226).
CALABRESE, A. (1972) How some pollutants affect embryos & larvae of
American oyster & hard-shell clam. Mar. Fish. Rev ., 34: 66-77.
CECIL, H.C., BITMAN, J., & HARRIS, S.J. (1971) Effects of dietary
p,p' -DDT and p,p' -DDE on egg production and eggshell characteristics
of Japanese quail receiving an adequate calcium diet. Poult. Sci ., 50:
657-659.
CHRISTIE, A.E. (1969) Effect of insecticides on algae. Water Sewage
Works , 116: 172-176.
CLARK, D.R. & KROLL, J.C. (1977) Effects of DDE on experimentally
poisoned free-tailed bats ( Tadarida brasilensis ): lethal brain
concentrations. J. Toxicol. environ. Health, 3: 893-901.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyreniodosa by a polychlorinated biphenyl
and several insecticides. Environ. Entomol ., 3: 217-220.
COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U., PERFECT, T.J., &
YEADON, R. (1980) Effects of cultivation and DDT on earthworm
activity in a forest soil in the sub-humid tropics. J. appl. Ecol.,
17: 21-29.
COOKE, A.S. (1970) The effect of p,p -DDT on tadpoles of the common
frog (Rana temporaria). Environ. Pollut ., 1: 57-71.
COOKE, A.S. (1971) Uptake of DDT and DDE by the quail embryo and
chick. Pestic. Sci ., 2: 144-147.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on amphibian
spawn and tadpoles. Environ. Pollut ., 3: 51-68.
COOKE, A.S. (1973a) The effects of DDT, when used as a mosquito
larvicide, on tadpoles of the frog Rana temporaria. Environ. Pollut.,
5: 259-273.
COOKE, A.S. (1973b) Shell-thinning in avian eggs by environmental
pollutants. Environ. Pollut ., 4: 85-152.
COOKE, A.S. (1979a) Eggshell characteristics of gannets ( Sula
bassana ), shags ( Phalacrocorax aristotelis ) and great black-backed
gulls ( Larus marinus ) exposed to DDE and other environmental
pollutants. Environ. Pollut ., 19: 47-65.
COOKE, A.S. (1979b) The influence of rearing density on subsequent
response to DDT dosing for tadpoles of the frog Rana temporaria . Bull.
environ. Contam. Toxicol ., 21: 837-841.
COOKE, A.S. & POLLARD, E. (1973) Shell and operculum formation by
immature Roman snails, Helix pomatia L., when treated with p,p' -DDT.
Pestic. Biochem. Physiol ., 3: 230-236.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Eggshell characteristics
and incidence of shell breakage for grey herons Ardea cinerea exposed
to environmental pollutants. Environ. Pollut ., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution , Swindon, United Kingdom, Natural Environment
Research Council, 74 pp.
CRAWFORD, R.B. & GUARINO, A.M. (1976) Effects of DDT in Fundulus
studies on toxicity, fate and reproduction. Arch. environ. Contam.
Toxicol ., 4: 334-348.
CRITCHLEY, B.R., COOK, A.G., CRITCHLEY, U., PERFECT, T.J., &
RUSSELL-SMITH, A. (1980) The effects of crop protection with DDT on
some elements of the subterranean and surface active arthropod fauna of
a cultivated forest soil in the humid tropics. Pedobiologia , 20: 31-
38.
DAVEY, S.P. (1963) Effects of chemicals on earthworms: a review of the
literature , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 1-20 (Special Scientific Report, Wildlife No.
74).
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin and
DDT by earthworms. Soil Biol. Biochem ., 3: 221-233.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p' -DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol ., 7: 9-18.
DAVISON, K.L., ENGEBRETSON, K.A., & COX, J.H. (1976) p,p' -DDT and
p,p' -DDE effects on egg production, eggshell thickness and
reproduction of Japanese quail. Bull. environ. Contam. Toxicol ., 15:
265-270.
DAVY, F.B., KLEEREKOPER, H., & GENSLER, P. (1972) Effects of
exposure to sublethal DDT on the locomotor behavior of the goldfish
(Carassius auratus). J. Fish Res. Board Can ., 29: 1333-1336.
DAVY, F.B., KLEEREKOPER, H., & MATIS, J.H. (1973) Effects of exposure
to sublethal DDT on the exploratory behavior of goldfish (Carassius
auratus). Water Resour. Res ., 9: 900-905.
DERR, S.K. & ZABIK, M.J. (1972) Biologically active compounds in the
aquatic environment: the effect of DDT on the egg viability of
Chironomus tentans. Bull. environ. Contam. Toxicol ., 7: 366-368.
DESAIAH, D., CUTKOMP, L.K., KOCH, R.B., & JARVINEN, A. (1975)
DDT: effect of continuous exposure on ATPase activity in fish,
Pimephales promelas. Arch. environ. Contam. Toxicol ., 3: 132-141.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife studies,
Patuxent Wildlife Research Center 1961 - 1962 , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 74-96
(Circular No. 167).
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyl, malathion and mercuric
chloride. Toxicol. appl. Pharmacol ., 27: 86-98.
DILL, P.A. & SAUNDERS, R.C. (1974) Retarded behavioral development and
impaired balance in Atlantic salmon ( Salmo salar ) alevins hatched from
gastrulae exposed to DDT. J. Fish Res. Board Can ., 31: 1936-1938.
DILWORTH, T.G., KEITH, J.A., PEARCE, P.A., & REYNOLDS, L.M. (1972)
DDE and eggshell thickness in New Brunswick woodcock. J. wildl.
Manage ., 36: 1186-1193.
DIMOND, J.B. & SHERBURNE, J.A. (1969) Persistence of DDT in wild
populations of small mammals. Nature (Lond.) , 221: 486-487.
DINDAL, D.L. & WURZINGER, K.-H. (1971) Accumulation and excretion of
DDT by the terrestrial snail, Cepaea hortensis. Bull. environ. Contam.
Toxicol ., 4: 362-371.
DOBSON, S. (1981) [Physiological and behavioural effects of organo-
chlorines on pigeons.] Oekol. Vogel , 3(Suppl.): 39-43 (in German).
DUNACHIE, J.F. & FLETCHER, W.W. (1969) An investigation of the
toxicity of insecticides to birds' eggs using the egg-injection
technique. Ann. appl. Biol ., 64: 409-423.
EARNEST, R.D. & BENVILLE, P.E. (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game , 58: 127-132.
EBERHARDT, L., MEEKS, R.L., & PETERLE, T.J. (1971) Food chain model
for DDT kinetics in a fresh water marsh. Nature (Lond.) , 230: 60-62.
EDELSTAM, C. (1972) The visible migration of birds at Ottenby,
Sweden. Var Fagelvarld , 7(Suppl.): 1-360.
EDEN, W.G. & ARTHUR, B.W. (1965) Translocation of DDT and heptachlor
in soybeans. J. econ. Entomol ., 34: 161-162.
EDWARDS, C.A. & JEFFS, K. (1974) Rate of uptake of DDT from soil by
earthworms. Nature (Lond.) , 247: 157-158.
EL-SEBAE, A.H. (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region , Athens,
Mediterranean Action Plan, United Nations Environment Programme/Food
and Agriculture Organization of the United Nations, pp. 109-116. (MAP
Technical Report Series No. 10).
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning and
lowered production of young in prairie falcons. Bioscience , 20: 355-
356.
FERGUSON, D.E., CULLEY, D.D., COTTON, W.D., & DODDS, R.P. (1964)
Resistance to chlorinated hydrocarbon insecticides in three species of
freshwater fish. Bioscience , 14: 43-44.
FORSYTH, D.J. & PETERLE, T.J. (1973) Accumulation of chlorine-36 ring-
labeled DDT residues in various tissues of two species of shrew. Arch.
environ. Contam. Toxicol ., 1: 1-17.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R.,
& HUNT, J.R. (1972) Residues in eggs and tissues of hens fed a ration
containing low levels of pesticides with and without charcoal. J. econ.
Entomol ., 65: 982-988.
FOX, G.A. (1976) Eggshell quality: its ecological and physiological
significance in a DDE-contaminated common tern population. Wilson
Bull ., 88: 459-477.
FRIEND, M., HAEGELE, M.A., & WILSON, R. (1973) DDE: interference with
extra-renal salt excretion in the mallard. Bull. environ. Contam.
Toxicol ., 9: 49-53.
FUHREMANN, T.W. & LICHTENSTEIN, E.P. (1980) A comparative study
of the persistence, movement, and metabolism of six carbon-14
insecticides in soils and plants. J. agric. food Chem ., 28: 446-452.
GARDNER, D.R. (1973) The effect of some DDT and methoxychlor analogs on
temperature selection and lethality in brook trout fingerlings.
Pestic. Biochem. Physiol ., 2: 437-446.
GAUFIN, A.R., JENSEN, L.D., NEBEKER, A.V., NELSON, T., & TEEL,
R.W. (1965) The toxicity of ten organic insecticides to various
aquatic invertebrates. Water Sewage Works , 112: 276-279.
GELUSO, K.N., ALTENBACH, J.S., & WILSON, D.E. (1976) Bat mortality:
pesticide poisoning and migratory stress. Science , 194: 184-186.
GILMAN, A.P., HALLETT, D.J., FOX, G.A., ALLAN, L.J., LEARNING,
W.J., & PEAKALL, D.B. (1978) Effects of injected organochlorines on
naturally incubated herring gull eggs. J. wildl. Manage ., 42: 484-493.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 15 pp (Special Scientific Report No. 241).
GOFFART, H. (1949) [The effect of new kinds of insecticides on
earthworms.] Anz. Schadlingskd. (Berlin) , 22: 72-74 (in German).
GOULDING, K.H. & ELLIS, S.W. (1981) The interaction of DDT with two
species of freshwater algae. Environ. Pollut ., 25: 271-290.
GREENBURG, R.R., RISEBROUGH, R.W., & ANDERSON D.W. (1979)
p,p' -DDE-induced changes in the organic and inorganic structure of
eggshells of the mallard, Anas platyrhynchos. Toxicol. appl.
Pharmacol ., 48: 279-286.
GREICHUS, Y.A. & HANNON, M.R. (1973) Distribution and biochemical
effects of DDT, DDD and DDE in penned double-crested cormorants.
Toxicol. appl. Pharmacol ., 26: 483-494.
GREICHUS, Y.A., CALL, D.J., AMMANN, B.M., GREICHUS, A., &
SHAVE, H. (1975) Physiological effects of polychlorinated biphenyls or
a combination of DDT, DDD, and DDE in penned white pelicans. Arch.
environ. Contam. Toxicol ., 3: 330-343.
HACKMAN, C.D. & HENNY, C.J. (1971) Hawk migration over white marsh,
Maryland. Chesapeake Sci ., 12: 137-141.
HAEGELE, M.A. & HUDSON, R.H. (1973) DDE effects on reproduction of
ring doves. Environ. Pollut ., 4: 53-57.
HAEGELE, M.A. & HUDSON, R.H. (1977) Reduction of courtship behavior
induced by DDE in male ringed turtle doves. Wilson Bull ., 89: 593-601.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common pollutants
on eggshell thickness in mallards and coturnix. Bull. environ. Contam.
Toxicol ., 11: 98-102.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of coho salmon (Oncorhynchus kisutch). J. Fish Res.
Board Can ., 31: 1543-1547.
HANKE, W., GLUTH, G., BUBEL, H., & MULLER, R. (1983) Physiological
changes in carps induced by pollution. Ecotoxicol. environ. Saf ., 7:
229-241.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained sheepshead
minnows. Trans. Am. Fish. Soc ., 98: 426-429.
HANSEN, D.J. (1972) DDT and malathion: effect on salinity selection by
mosquitofish. Trans. Am. Fish. Soc ., 101: 346-350.
HANSEN, D.J. & WILSON, A.J. (1970) Significance of DDT residues from
the estuary near Pensacola, Fla. Pestic. monit . J., 4: 51-56.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquitofish, Gambusia affinis.
Bull. environ. Contam. Toxicol ., 8: 46-51.
HARRI, M.N.E., LAITINEN, J., & VALKAMA, E.-L. (1979) Toxicity and
retention of DDT in adult frogs, Rana temporaria L. Environ. Pollut.,
13: 45-55.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J. agric.
food Chem ., 15: 861-863.
HASELTINE, S., UEBELHART, K., PETERLE, T., & LUSTICK, S. (1974)
DDE, PTH and eggshell thinning in mallard, pheasant and ring dove.
Bull. environ. Contam. Toxicol ., 11: 139-145.
HAUX, C. & LARSSON, A. (1979) Effects of DDT on blood plasma
electrolytes in the flounder, Platichthys flesus L., in hypotonic
brackish water. Ambio , 8: 171-173.
HAYNES, R.J. (1972) Effects of DDT on glycogen and lipid levels in
bobwhites. J. wildl. Manage ., 36: 518-523.
HEATH, R.G., SPANN, J.W., & KREITZER, J.F. (1969) Marked DDE
impairment of mallard reproduction in controlled studies. Nature
( Lond .), 224: 47-48.
HEINZ, G.H. (1976) Behavior of mallard ducklings from parents fed 3
ppm DDE. Bull. environ. Contam. Toxicol ., 16: 640-645.
HEINZ, G.H., HILL, E.F., & CONTRERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin and Aroclor
1254. Toxicol. appl. Pharmacol ., 53: 75-82.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four species of
fish. Trans. Am. Fish. Soc ., 88: 23-32.
HENNY, C.J. (1972) An analysis of the population dynamics of selected
avian species , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 99 pp
(Research Report No. 1).
HENNY, C.J. (1977) Birds of prey, DDT, & tussock moths in Pacific
Northwest. In: Transactions of the Forty-second North American Wildlife
and Natural Resource Conference , Washington, DC, Wildlife Management
Institute, pp. 397-411.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish eating birds. Science , 162:
271-273.
HILL, E.F., DALE, W.E., & MILES, J.W. (1971) DDT intoxication in birds:
subchronic effects and brain residues. Toxicol. appl. Pharmacol ., 20:
502-514.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds , Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 61 pp
(Special Scientific Report No. 191).
HODKINSON, M. & DALTON, S.A. (1973) Interactions between DDT and
river fungi. II Influence of culture conditions on the compatibility of
fungi and p,p' -DDT. Bull. environ. Contam. Toxicol ., 10: 356-359.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife , Washington, DC, US Department of
the Interior, Fish and Wildlife Service, 90 pp (Resource Publication
No. 153).
HUNT, E.G. & LINN, J.D. (1970) Fish kills by pesticides. In: Gillett,
J.W., ed. The biological impact of pesticides in the environment
Corvallis, Oregon, Oregon State University, pp. 97-102.
IDE, F.P. (1957) Effect of forest spraying with DDT on aquatic insects
of salmon streams. Trans. Am. Fish. Soc ., 86: 208-219.
JANICKI, R.H. & KINTER, W.B. (1971) DDT disrupted osmoregulatory
events in the intestine of the eel Anguilla rostrata adapted to
seawater. Science , 173: 1146-1148.
JARVINEN, A.W., HOFFMAN, M.J., & THORSLUND, T.W. (1977) Long-
term toxic effects of DDT food and water exposure on fathead minnows.
J. Fish Res. Board Can ., 34: 2089-2103.
JEFFERIES, D.J. (1967) The delay in ovulation produced by p,p' -DDT and
its possible significance in the field. Ibis , 109: 266-272.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in British
bats and their significance. J. Zool ., 166: 245-263.
JEFFERIES, D.J. & FRENCH, M.C. (1972) Changes induced in the pigeon
thyroid by p,p' -DDE and dieldrin. J. wildl. Manage ., 36: 24-30.
JOHANSEN, C. (1961) Bee poisoning. A hazard of applying agricultural
chemicals , Washington, DC, Institute of Agricultural Sciences,
Washington State University (Circular No. 356).
JOHNSON, B.T. & KENNEDY, J.O. (1973) Biomagnification of p,p' -DDT and
methoxychlor by bacteria. Appl. Microbiol ., 26: 66-71.
JOHNSON, B.T., SAUNDERS, C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin by
freshwater invertebrates. J. Fish Res. Board Can ., 28: 705-709.
JOHNSON, H.E. & PECOR, C. (1969) Coho salmon mortality and DDT in Lake
Michigan. Trans. North Am. Wildl. Nat. Resour. Conf ., 34: 159-166.
JOWETT, P.E., RHEAD, M.M., & BAYNE, B.L. (1978) In vitro changes in the
activity of ATPases in the gills of Carcinus maenas exposed to various
concentrations of p,p' -DDT. Environ. Pollut ., 17: 1-6.
KAN, C.A., JONKER-DEN ROOYEN, J.C., TUINISTRA, L.G.M.T., ROOS,
A.H., & TRAAG, W. (1978) Possible influence of sex and embryonic
content on accumulation of some organochlorine pesticides in
broilers. J. agric. food Chem ., 26: 618-621.
KATZ, M. (1961) Acute toxicity of some organic insecticides to three
species of salmonids and to the threespine stickleback. Trans. Am.
Fish. Soc ., 90: 264-268.
KEIL, J.E. & PRIESTER, L.E. (1969) DDT uptake and metabolism by a
marine diatom. Bull. environ. Contam. Toxicol ., 4: 169-173.
KEIL, J.E., SANDIFER, S.H., GRABER, C.D., & PRIESTER, L.E. (1972) DDT
and polychlorinated biphenyl (Aroclor 1242). Effects of uptake on E.
coli growth. Water Res ., 6: 837-841.
KING, K.A., FLICKINGER, E.L., & HILDEBRAND, H.H. (1978) Shell
thinning and pesticide residues in Texas aquatic bird eggs,
1970. Pestic. monit. J ., 12: 16-21.
KINTER, W.B., MERKENS, L.S., JANICKI, R.H., & GUARINO, A.M. (1972)
Studies on the mechanism of toxicity of DDT and polychlorinated
biphenyls (PCBs): disruption of osmoregulation in marine fish.
Environ. Health Perspect ., 1: 169-173.
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972) Eggshell
and population changes in the sparrow-hawk (Accipiter nisus). TNO-
Nieuws , 27: 542-550.
KOLAJA, G.J. (1977) The effects of DDT, DDE and their sulfonated
derivatives on eggshell formation in the mallard duck. Bull. environ.
Contam. Toxicol ., 17: 697-701.
KORN, S. & EARNEST, R. (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game , 60: 128-131.
KOUYOUMJIAN, H.H. & UGLOW, R.F. (1974) Some aspects of the toxicity of
p,p' -DDT, p,p' -DDE and p,p' -DDD to the freshwater planarian
Polycelis felina (Tricladida). Environ. Pollut ., 7: 103-109.
KREITZER, J.F. & SPANN, J.W. (1973) Test of pesticidal synergism with
young pheasants and Japanese quail. Bull. environ. Contam. Toxicol.,
9: 250-256.
LAL, R. & SAXENA, D.M. (1979) Effect of DDT on cell population growth
of Tetrahymena pyriformis. Arch. Protistenkd ., 122: 382-386.
LAL, R. & SAXENA, D.M. (1980) Effect of DDT on cell population growth,
cell division, and DNA synthesis in Stylonychia notophora (Stokes).
Arch. environ. Contam. Toxicol ., 9: 163-170.
LAL, R. & SAXENA, D.M. (1982) Accumulation, metabolism, and effects of
organochlorine insecticides on microorganisms. Microbiol. Rev ., 46: 95-
127.
LEE, S.S., FANG, S.C., & FREED, V.H. (1976) Effect of DDT on
photosynthesis of Selenastrum capricornutum. Pestic. Biochem. Physiol.,
6: 46-51.
LEFFLER, C.W. (1975) Effects of ingested mirex and DDT on juvenile
Callinectes sapidus Rathbun. Environ. Pollut ., 8: 283-300.
LEDFORD, R.A. & CHEN, J.H. (1969) Degradation of DDT and DDE by
cheese microoganisms. J. food Sci ., 34: 386-388.
LEGMAN, J.W., PETERLE, T.J., & MILLS, C.M. (1974) Effects of DDT on
bobwhite quail ( Colinus virginianus ) adrenal gland. Bull. environ.
Contam. Toxicol ., 11: 407-414.
LINCER, J.L. (1972) The effects of organochlorines on the American
kestrel (Falco sparverius Linn.) , Ithaca, New York, Cornell
University (PhD Thesis).
LINCER, J.L. (1975) DDE-induced eggshell-thinning in the American
kestrel: a comparison of the field situation and laboratory results.
J. appl. Ecol ., 12: 781-793.
LINDBURG, P. (1977) The peregrine falcon in Sweden. In: Proceedings of
the International Council for Bird Preservation World Conference on
Birds of Prey, Vienna, 1975 , Cambridge, United Kingdom, International
Council for Bird Preservation, pp. 329-338.
LOCKE, L.N., CHURA, N.J., & STEWART, P.A. (1966) Spermatogenesis in
bald eagles experimentally fed a diet containing DDT. Condor , 68: 497-
502.
LONGCORE, J.R., SAMSON, F.B., & WHITTENDALE, T.W. (1971) DDE
thins eggshells and lowers reproductive success of captive black
ducks. Bull. environ. Contam. Toxicol ., 6: 485-490.
LONGCORE, J.R. & STENDELL, R.C. (1977) Shell thinning and reproductive
impairment in black ducks after cessation of DDE dosage. Arch. environ.
Contam. Toxicol ., 6: 293-304.
LOOSANOFF, V.L. (1960) Some effects of pesticides on marine arthropods
and mollusks. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Transactions of the Second Seminar, 1959 , Cincinnati, USA,
Robert, A. Taft Sanitation and Engineering Centre, pp. 89-93 (Technical
Report No. W60-3).
LOWE, J.I. (1965) Chronic exposure of blue crabs, Callinectes sapidus,
to sublethal concentrations of DDT. Ecology , 46: 899-900.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia , 12: 29-33.
LUCKENS, M.M. & DAVIS, W.H. (1964) Bats: sensitivity to DDT. Science,
146: 948.
LUDKE, J.L. (1977) DDE increases the toxicity of parathion to
Coturnix quail. Pestic. Biochem. Physiol ., 7: 28-33.
MACEK, K.J. (1968) Reproduction in brook trout ( Salvelinus fontinalis )
fed sublethal concentrations of DDT. J. Fish Res. Board Can ., 25:
1787-1795.
MACEK, K.J. & KORN, S. (1970) Significance of the food chain in DDT
accumulation by fish. J. Fish Res. Board Can ., 27: 1496-1498.
MACEK, K.J. & SANDERS, H.O. (1970) Biological variation in the
susceptibility of fish and aquatic invertebrates to DDT. Trans. Am.
Fish. Soc ., 99: 89-90.
MACEK, K.J., RODGERS, C.R., STALLING, D.L., & KORN, S. (1970) The
uptake, distribution and elimination of dietary 14C-DDT and
14C-dieldrin in rainbow trout. Trans. Am. Fish. Soc ., 99: 689-695.
MACFARLANE, R.B., GLOOSCHENKO, W.A., & HARRISS, R.C. (1972) The
interaction of light intensity and DDT concentration upon the marine
diatom, Nitzschia delicatissima Cleve. Hydrobiologia , 39: 373-382.
MCLANE, M.A.R. & HALL, L.C. (1972) DDE thins screech owl eggshells.
Bull. environ. Contam. Toxicol ., 8: 65-68.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol ., 25: 921-928.
MACRAE, I.C. & VINCKX, E. (1973) Effect of lindane and DDT on
populations of protozoa in a garden soil. Soil Biol. Biochem ., 5: 245-
247.
MAHONEY, J.J. (1975) DDT and DDE effects on migratory condition in
white-throated sparrows. J. wildl. Manage ., 39: 520-527.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p' -DDT on production and survival of Daphnia magna Strauss. Bull.
environ. Contam. Toxicol ., 13: 412-416.
MARTIN, J.P. (1966) Influence of pesticides on soil microbes and soil
properties. Am. Soc. Agron. Spec. Publ ., 8: 95-108.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms , Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity :
interpretation and data base for 410 chemicals and 66 species of
freshwater animals , Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp (Resource Publication No. 160).
MEEKS, R.L. (1968) The accumulation of 36Cl ring-labeled DDT in a
freshwater marsh. J. wildl. Manage ., 32: 376-398.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls ( Tyto alba ) fed low levels of DDE and dieldrin.
Arch. environ. Contam. Toxicol ., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine phyto-
plankton vary in their response to chlorinated hydrocarbons.
Science , 167: 1724-1726.
METCALF, R.L., KAPOOR, I.P., LU, P.-Y, SCHUTH, C.K., & SHERMAN,
P. (1973) Model ecosystem studies of the environmental fate of six
organochlorine pesticides. Environ. Health Perspect ., 4: 35-43.
MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1976a) Enzymatic basis
for DDE-induced eggshell thinning in a sensitive bird. Nature (Lond.),
259: 122-124.
MILLER, D.S., KINTER, W.B., PEAKALL, D.B., & RISEBROUGH, R.W.
(1976b) DDE feeding and plasma osmoregulation in ducks, guillemots,
and puffins. Am. J. Physiol ., 231: 370-376.
MOFFETT, G.B. & YARBROUGH, J.D. (1972) The effects of DDT, toxaphene
and dieldrin on succinic dehydrogenase activity in insecticide-
resistant and susceptible Gambusia affinis. J. agric. food Chem ., 20:
558-560.
MOORE, N.W. (1965) Pesticides in birds - a review of the situation in
Great Britain in 1965. Bird Study , 12: 222-252.
MORIARTY, F. (1975) Pollutants and animals: a factual perspective,
London, Allen & Unwin.
MORIARTY, F., BELL, A.A., & HANSON, H. (1986) Does p,p' -DDE thin
eggshells? Environ. Pollut ., 40: 257-286.
MUIRHEAD-THOMSON, R.C. (1973) Laboratory evaluation of pesticide
impact on stream invertebrates. Freshwater Biol ., 3: 479-498.
MULLA, M.S. (1963) Toxicity of organochlorine insecticides to the
mosquitofish Gambusia affinis and the bullfrog Rana catesbeiana.
Mosq. News , 23: 299-303.
MURPHY, P.G. (1970) Effects of salinity on uptake of DDT, DDE and DDD
by fish. Bull. environ. Contam. Toxicol ., 5: 404-407.
MURPHY, P.G. (1971) The effect of size on the uptake of DDT from water
by fish. Bull. environ. Contam. Toxicol ., 6: 20-23.
NAGY, A.C. (1977) Population trend indices based on 40 years of autumn
counts at hawk mountain. In: Proceedings of the International Council
for Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 243-253.
NAQVI, S.M. & FERGUSON, D.E. (1968) Pesticide tolerances of selected
freshwater invertebrates. J. Mississippi Acad. Sci ., 14: 121-127.
NEUFELD, G.J. & PRITCHARD, J.B. (1979) An assessment of DDT toxicity in
osmoregulation and gill Na,K-ATPase activity in the blue crab,
Philadelphia, American Society for Testing and Materials, pp. 23-34
(ASTM Special Technical Publication No. 667).
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted, United
Kingdom , T. & A.D. Poyser.
NEWTON, I. & BOGAN, J. (1978) The role of different organo-chlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol ., 15:
105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds , 77: 47-70.
NIMMO, D.R., WILSON, A.J., & BLACKMAN, R.R. (1970) Localization of
DDT in the body organs of pink and white shrimp. Bull. environ. Contam.
Toxicol ., 5: 333-341.
OGILVIE, D.M. & MILLER, D.L. (1976) Duration of a DDT-induced shift in
the selected temperature of Atlantic salmon (Salmo salar). Bull.
environ. Contam. Toxicol ., 16: 86-89.
OLOFSSON, S. & LINDAHL, P.E. (1979) Decreased fitness of cod ( Gadus
morrhua L.) from polluted waters. Mar. environ. Res ., 2: 33-45.
OSBORN, D., COOKE, A.S., & FREESTONE, S. (1981) Histology of a
teratogenic effect of DDT on Rana temporaria tadpoles. Environ.
Pollut ., 25: 305-319.
O'SHEA, T.J. & LUDKE, J.L. (1979) Monitoring fish and wildlife for
environmental pollutants , Fort Collins, Colorado, US Department of the
Interior, Fish and Wildlife Service.
PEAKALL, D.B. (1970) p,p' -DDT: effect on calcium metabolism and
concentration of estradiol in the blood. Science , 168: 592-594.
PEAKALL, D.B., LINCER, J.L., RISEBROUGH, R.W., PRITCHARD, J.B., &
KINTER, W.B. (1973) DDE-induced eggshell thinning: structural and
physiological effects in three species. Comp. gen. Pharmacol ., 4: 305-
313.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975a) Prolonged eggshell
thinning caused by DDE in the duck. Nature (Lond.) , 254: 421.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975b) Blood calcium
levels and the mechanism of DDE-induced eggshell thinning. Environ.
Pollut ., 9: 289-294.
PEAKALL, D.B., CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1975c)
Organochlorine residues in Alaskan peregrines. Pestic. monit. J. , 8:
255-260.
PERFECT, J. (1980) The environmental impact of DDT in a tropical agro-
ecosystem. Ambio , 9: 16-21.
PERFECT, T.J., COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U.,
DAVIES, A.L., SWIFT, M.J., RUSSELL-SMITH, A., & YEADON, R. (1979)
The effect of DDT contamination on the productivity of a cultivated
forest soil in the sub-humid tropics. J. appl. Ecol ., 16: 705-719.
PETERSON, R.H. (1973) Temperature selection of Atlantic salmon (Salmo
salar) and brook trout ( Salvalinus fontinalis ) as influenced by various
chlorinated hydrocarbons. J. Fish Res. Board Can ., 30: 1091-1097.
PORTER, R.D. & WIEMEYER, S.N. (1972) DDE at low dietary levels kills
captive American kestrels. Bull. environ. Contam. Toxicol ., 8: 193-
199.
POWERS, C.D., WURSTER, C.F., & ROWLAND, R.G. (1979) DDE inhalation
of marine algal cell division and photosynthesis per cell. Pestic.
Biochem. Physiol ., 10: 306-312.
PRESCOTT, L.M. & OLSON, D.L. (1972) The effect of pesticides on the
soil amoeba Acanthamoeba castellanii (Neff). Proc. South Dakota Acad.
Sci ., 51: 136-141.
RAMALINGAM, K. & RAMALINGAM, K. (1982) Effects of sublethal levels
of DDT, malathion and mercury on tissue proteins of Sarotherodon
mossambicus (Peters). Proc. Indian Acad. Sci. anim. Sci ., 91: 501-505.
RANDALL, W.F., DENNIS, W.H., & WARNER, M.C. (1979) Acute toxicity of
dechlorinated DDT, chlordane and lindane to bluegill ( Lepomis
machrochirus ) and Daphnia magna. Bull. environ. Contam. Toxicol ., 21:
849-854.
RATCLIFFE, D.A. (1967) Decrease in eggshell weight in certain birds of
prey. Nature (Lond.) , 215: 208-210.
RATCLIFFE, D.A. (1969) Population trends of the peregrine falcon in
Great Britain. In: Hinkley, J.J., ed. Peregrine falcon populations:
Their biology and decline , Madison, Milwaukee, London, University of
Wisconsin Press, pp. 239-269.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol ., 7: 67-107.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Study , 19: 117-156.
REINBOLD, K.A., KAPOOR, I.P., CHILDERS, W.F., BRUCE, W.N., &
METCALF, R.L. (1971) Comparative uptake and biodegradability of DDT
and methoxychlor by aquatic organisms. Illinois nat. Hist. Surv.
Bull ., 30: 405-415.
REINERT, R.E., STONE, L.J., & WILLFORD, W.A. (1974) Effect of
temperature on accumulation of methylmercuric chloride and p,p' -DDT by
rainbow trout (Salmo gairdneri). J. Fish Res. Board Can ., 31: 1649-
1652.
RICE, C.P. & SIKKA (1973) Uptake and metabolism of DDT by six species
of marine algae. J. agric. food Chem ., 21: 148-152.
RICHIE, P.J. & PETERLE, T.J. (1979) Effect of DDE on circulating
luteinizing hormone levels in ring doves during courtship and nesting.
Bull. environ. Contam. Toxicol ., 23: 220-226.
RISEBROUGH, R.W. & ANDERSON, D.W. (1975) Some effects of DDE and
PCB on mallards and their eggs. J. wildl. Manage ., 39: 508-513.
RISEBROUGH, R.W., DE LAPPE, B.W., & SCHMIDT, T.T. (1976)
Bioaccumulation factors of chlorinated hydrocarbons between mussels and
seawater. Mar. Pollut. Bull ., 7: 225-228.
ROBERTS, D. (1975) Sub-lethal effects of chlorinated hydrocarbons on
bivalves. Mar. Pollut. Bull ., 6: 20-24.
ROBSON, W.A., ARSCOTT, G.H., & TINSLEY, I.J. (1976) Effect of DDE,
DDT and calcium on the performance of adult Japanese quail (Coturnix
coturnix japonica). Poult. Sci ., 55: 2222-2227.
ROSEN, L. (1966) [The flight of birds of prey at Falsterbo.] Var
Fagelvarld , 25: 315-326 (in Swedish).
SANBORN, J.R., CHILDERS, W.F., & METCALF, R.L. (1975) Uptake of three
polychlorinated biphenyls, DDT, and DDE by the green sunfish, Lepomis
cyanellus Raf. Bull. environ. Contam. Toxicol ., 13: 209-217.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowlers toad Bufo woodhousii
fowleri. Copeia , 2: 246-251.
SANDERS, H.O. (1972) Toxicity of some insecticides to four species of
malacostracan crustaceans , Washington, DC, US Department of the
Interior, Bureau of Sport, Fisheries and Wildlife, pp. 3-19 (Technical
Paper No. 66).
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol.
Oceanogr ., 13: 112-117.
SELL, J.L., DAVISON, K.L., & POONACHA, K.B. (1972) Decreased aniline
hydroxylase activity in Japanese quail due to dietary DDT. J. agric.
food Chem ., 20: 553-557.
SHERBURNE, J.A. & DIMOND, J.B. (1969) DDT persistence in wild hares and
mink. J. wildl. Manage ., 33: 944-948.
SHIN, Y.-O., CHODAN, J.J., & WOLCOTT, A.R. (1970) Adsorption of DDT
by soils, soil fractions, and biological materials. J. agric. food
Chem ., 18: 1129-1133.
SHIRES, S.W. (1985) A comparison of the effects of cypermethrin,
parathion-methyl and DDT on cereal aphids, predatory beetles,
earthworms and litter decomposition in spring wheat. Crop Prot.,
4: 177-193.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of DDT
and dieldrin on development in winter flounder (Pseudopleuronectes
americanus). J. Fish Res. Board Can ., 30: 1894-1898.
SMITH, S.I., WEBER, C.W., & REID, B.L. (1969) The effect of high
levels of dietary DDT on egg production, mortality, fertility,
hatchability and pesticide content of yolks in Japanese quail. Poult.
Sci ., 48: 1000-1004.
STICKEL, L.F. & HEATH, R.G. (1964) Wildlife studies. Patuxent wildlife
research center. In: The effects of pesticides on fish and wildlife,
Washington, DC, Department of the Interior, Fish and Wildlife Service,
pp. 3-30 (Circular No. 226).
THOMPSON, A.R. (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull. environ. Contam. Toxicol ., 5:
577-586.
ULFSTRAND, S., ROOS, G., ALERSTAM, T., & OSTERDAHL, L. (1974)
Visible bird migration at Falsterbo, Sweden. Var Fagelvarld,
8(Suppl.): 1-244.
VANGILDER, L.D. & PETERLE, T.J. (1980) South Louisiana crude oil and
DDE in the diet of mallard hens: effects on reproduction and duckling
survival. Bull. environ. Contam. Toxicol ., 25: 23-28.
VAN VELZEN, A. & KREITZER, J.F. (1975) The toxicity of p,p' -DDT to
the clapper rail. J. wildl. Manage ., 39: 305-309.
VAN VELZEN, A.C., STILES, W.B., & STICKEL, L.F. (1972) Lethal
mobilization of DDT by cowbirds. J. wildl. Manage ., 36: 733-739.
VERMEER, K. & REYNOLDS, L.M. (1970) Organochlorine residues in aquatic
birds in the canadian prairie provinces. Can. field Nat ., 84: 117-130.
VINSON, S.B., BOYD, C.E., & FERGUSON, D.E. (1963) Resistance to DDT in
the mosquito fish, Gambusia affinis. Science , 139: 217-218.
WAGGONER, J.P. & ZEEMAN, M.G. (1975) DDT: short term effects on
osmoregulation in black surfperch (Embiotoca jacksoni). Bull. environ.
Contam. Toxicol ., 13: 297-300.
WARE, G.W. (1968) DDT-C14 translocation in alfalfa. J. econ.
Entomol ., 61: 1451-1452.
WARE, G.W., ESTESEN, B.J., & CAHILL, W.P. (1970) Uptake of C14-DDT
from soil by alfalfa. Bull. environ. Contam. Toxicol ., 5: 85-86.
WARLEN, S.M., WOLFE, D.A., LEWIS, C.W., & COLBY, D.R. (1977)
Accumulation and retention of dietary 14C-DDT by Atlantic menhaden.
Trans. Am. Fish. Soc ., 106: 95-104.
WATSON, M., PHARAOH, B., WYLLIE, J., & BENSON, W.W. (1975)
Metabolism of low oral doses of DDT and DDE by tame mule deer fawns.
Bull. environ. Contam. Toxicol ., 13: 316-323.
WEIS, J.S. & MANTEL, L.H. (1976) DDT as an accelerator of limb
regeneration and molting in fiddler crabs. Estuarine coastal mar. Sci.,
4: 461-466.
WEIS, P. & WEIS, J.S. (1974) DDT causes changes in activity and
schooling behavior in goldfish. Environ. Res ., 7: 68-74.
WHEATLEY, G.A. (1965) The assessment and persistence of residues of
organochlorine insecticides in soils and their uptake by crops. Ann.
appl. Biol ., 55: 325-329.
WHITE, C.M. & CADE, T.J. (1977) Long term trends of peregrine
populations in Alaska. In: Proceedings of the International Council for
Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 63-71.
WHO (1979) Environmental Health Criteria 9: DDT and its derivatives,
Geneva, World Health Organization, 194 pp.
WIEMEYER, S.N. & PORTER, R.D. (1970) DDE thins eggshells of captive
American kestrels. Nature (Lond.) , 227: 737-738.
WIEMEYER, S.N., SPITZER, P.R., KRANTZ, W.C., LAMONT, T.G., &
CROMARTIE, E. (1975) Effects of environmental pollutants on
Connecticut and Maryland ospreys. J. wildl. Manage ., 39: 124-139.
WURSTER, C.F. (1968) DDT reduces photosynthesis by marine
phytoplankton. Science , 159: 1474-1475.
Population declines in birds of prey differed between much of North
America and eastern North America and western Europe. In North
America, apart from in the East, declines were gradual, whereas in
Europe and eastern North America declines were sudden and catastrophic.
The sudden declines in Europe have usually been attributed to the use
of the chlorinated cyclodienes, which kill adult birds, rather than to
DDT. A study of the recoveries of European birds of prey populations
provides evidence for this attribution. Populations began to rise at a
time when residues of DDE in tissues were stable but when use of the
cyclodienes and, therefore, residues of HEOD (dieldrin) were declining.
Some populations in North America did not show high contamination with
cyclodienes and may have declined due to DDT use alone. Henny (1972)
showed that in American populations of osprey, American kestrel, and
red-shouldered hawk there was a decrease in breeding performance, but
no increase in adult mortality, in response to DDT. The reproductive
effects of DDT may have prevented population recoveries after the
cessation of dieldrin use and the return of mortality rates to normal.
The question has been reviewed by Newton (1979) and Newton & Haas
(1984).
The populations of many species of birds of prey were monitored
throughout a period of high DDT use. This was done by large scale
surveys and studies of population dynamics (Ratcliffe, 1972; Henny,
1977; Lindberg, 1977; White & Cade, 1977), migration counts at obser-
vation points (Rosen, 1966; Hackman & Henny, 1971; Edelstam, 1972;
Ulfstrand et al., 1974; Nagy, 1977), and by sample counts (Ash, 1965;
Bezzel, 1969). Some species showed marked declines (in some areas this
led to local extinction), whilst others showed only temporary effects
or no effects at all. Declines were most marked in bird-eating
species, such as the sparrowhawk and peregrine falcon, and fish-eating
species, such as the white-tailed and bald eagles, and were less marked
in mammal-eating species, such as the kestrel, golden eagle, and
buzzard. These variations in decline correspond to the DDE levels
found in these particular species (Newton, 1979).
Perfect (1980) reported the results of a 4-year study on the
overall effects of the use of DDT as an insecticide on cowpeas crops in
a Nigerian forest soil. In addition to effects on soil invertebrates
(section 6.1), there were effects on the decomposition of plant
material. The remains of the plants after harvesting were ploughed
into the soil and this resulted in an increase in the residues of DDT
and its metabolites in lower levels of the soil. To confirm an effect
on decomposition, these plant remains were buried in mesh bags and the
loss of weight due to decomposition was recorded over time. There was
a significant reduction in the rate of decomposition of plant material
treated with DDT and also of untreated plant material buried in
contaminated soil. Shires (1985) reported no significant effect on the
decomposition of sweet chestnut leaf litter in a temperate area after
the application of DDT at 1 kg/ha.
Perfect et al. (1979) investigated the effects of repeated DDT
applications on cowpea crop yield in Nigeria. Yields varied
considerably from season to season and from year to year in untreated
plots because of differences in pest damage and climate. DDT was
applied to the treated plots weekly between planting and harvest at a
rate of 1 kg/ha, and the site was studied for 4 years. Over the 4-year
period there was a considerable benefit in yield from DDT application;
the yield was 1.45 tonnes/ha in the untreated and 3.42 tonnes/ha in the
treated plots. However, the benefit was most noticeable in the first
year of cultivation and declined over the four years to the point where
DDT use did not significantly increase yield. The authors attributed
the effect to the deleterious action of the insecticide on soil biota.
8. EVALUATION
In evaluating the environmental hazard of DDT and its metabolites
the following general points have to be kept in mind.
(a) The environmental distribution and effects of DDT are spread wider
than the area of use, because the parent compound or its
metabolites are carried worldwide by air and ocean currents and in
biota.
(b) Some of the breakdown products of DDT, principally DDE, are highly
persistent in soil, sediment, and biota. Thus, problems with
residues of these materials last long after the cessation of use.
(c) The bioaccumulation of DDT, or more usually of its metabolites, is
well established and occurs from very low environmental
concentrations of DDT. The use of "bioconcentration factors"
(the ratio of concentration in the organism with concentration in
the medium) to estimate the capacity of organisms to take up DDT
can be misleading if the exposure is high, since these values are
ratios.
(d) Residues and effects are often highly seasonal, corresponding to
changes in body fat, since DDT metabolites are very lipid-soluble.
Measurements of these metabolites in the tissues of organisms must
be conducted over a period of time if they are to give any
indication of the degree of contamination of the environment.
(e) There are insufficient data on the effects of DDT and its
metabolites on communities of organisms and ecosystem functioning.
Hazard assessment is, therefore, often made by extrapolation from
single species studies.
(f) Research and monitoring have concentrated on a few effects of DDT
observed in the wild. This could give the mistaken impression that
the effects of these compounds are restricted to a few species.
Other effects could be predicted but have received little or no
attention from the scientific community.
(g) The major remaining use of DDT is for malaria control operations
that are normally carried out in tropical countries. However, the
majority of environmental studies on DDT have been carried out in
conditions relevant to temperate regions. Care must be exercised
in extrapolating these results to tropical conditions.
8.1 Aquatic Organisms
The widespread use of DDT as an insecticide has resulted in
worldwide contamination of the environment. Due to the physicochemical
characteristics of DDT and its metabolites, concentrations have been
recorded in different environmental compartments, including soil,
sediments, and terrestrial and aquatic organisms. The bioconcentration
of DDT and its metabolites is a real hazard to non-target organisms.
DDT and its metabolites cause adverse effects at all trophic levels of
aquatic ecosystems, particularly on primary producers, which are the
most sensitive. Although no data are available for the effects of DDT
on ecosystem function, it should be regarded as a major environmental
hazard in this respect. DDT and its metabolites are highly toxic to
fish and, besides their lethal effect, they affect development,
behaviour, and biochemical processes. DDT and its metabolites, should
be regarded as hazardous to fish productivity and distribution and,
hence, to human food supplies. Accumulated DDT and its metabolites are
further transferred from aquatic organisms to consumers, including
birds, mammals, and, ultimately, human beings.
8.2 Terrestrial Organisms
DDT-type compounds are resistant to breakdown and are readily
adsorbed onto soils and sediments, from whence they can act as long-
term sources of exposure and contribute to terrestrial organisms.
Accumulation in terrestrial organisms is via the food chain.
These chemicals are hazardous to microorganisms, but repeated
application can lead to the development of tolerance in some species.
DDT causes fluctuations in some populations of microorganisms, and this
could eventually lead to changes in species composition, disruption of
nutrient cycles, and changes in soil fertility.
Earthworms are insensitive to the acute toxic effects of DDT
residues in soil. However, they are known to take up DDT from soil and
this uptake presents a major hazard to predators.
DDT is a non-selective insecticide and leads to mortality in
natural enemies of the insect pest. This results in impairment of the
balance between predators and prey and leads to outbreaks of secondary
pests and occurrence of the primary pest in larger numbers.
Laboratory studies confirm field findings that bat populations are
adversely affected by DDE, especially during migration. These studies
are indicative of the potential hazard to other mammals, exposed to DDT
in the environment, when fat containing DDT residues is mobilized,
e.g., during migration or temporary starvation.
One of the most widely studied effects of DDT is eggshell thinning
in birds, particularly in predatory species. The metabolite DDE, not
DDT, has been shown to be responsible for this effect. Other effects
on reproduction and survival of birds have been demonstrated. Large
population declines in birds of prey can be, at least partially,
attributed to DDT. It has been shown that DDE residues in birds and
their eggs reduced the rate of recovery of affected raptor
populations. A factor that has received less attention is the
secondary effect of the increasing numbers of pest rodents that were
controlled principally by birds of prey in some countries.
* * *
Because of their lack of degradation, their resulting widespread
persistence in the environment, their high acute toxicity to organisms
at the base of food chains, and their high potential for
bioaccumulation, DDT and its metabolites should be regarded as a major
hazard to the environment. DDT should not be used when an alternative
insecticide is available.
REFERENCES
ALBAUGH, D.W. (1972) Insecticide tolerances of two crayfish
populations ( Procambarus acutus ) in South-central Texas. Bull.
environ. Contam. Toxicol ., 8: 334-338.
ALBONE, E.S., EGLINTON, G., EVANS, N.C., HUNTER, J.M., & RHEAD,
M.M. (1972) Fate of DDT in severn estuary sediments. Environ. Sci.
Technol ., 6: 914-919.
ALLISON, D., KALLMAN, B.J., COPE, O.B., & VAN VALLIN, C.C. (1964)
Some chronic effects of DDT on cutthroat trout , Washington, DC, US
Department of the Interior, Bureau of Sport, Fisheries and Wildlife,
pp. 1-30 (Resource Report No. 64).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain north
American birds. Proc. Int. Ornithol. Congr ., 15: 514-540.
ANDERSON, D.W. & HICKEY, J.J. (1974) Eggshell changes in raptors from
the Baltic region. Oikos , 25: 395-401.
ANDERSON, D.W., HICKEY, J.J., RISEBROUGH, R.W., HUGHES, D.F., &
CHRISTENSEN, R.E. (1969) Significance of chlorinated hydrocarbon
residues to breeding pelicans and comorant. Can. field Nat ., 83: 91-
112.
ASH, J.S. (1965) A reduction in numbers of birds of prey in France.
Bird Study , 12: 17-26.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees , University
of California, 38 pp. (Laboratory Studies - Extension M-16).
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1972) DDT:
inhibition of sodium chloride tolerance by the blue-green
alga Anacystis nidulans . Science, 176: 1141-1143.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the aquatic
environment: uptake and loss of DDT and dieldrin by freshwater mussels.
Bull. environ. Contam. Toxicol ., 1: 97-111.
BEND, J.R., MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1977) DDE-
induced microsomal mixed-function oxidases in the puffin ( Fratercula
arctica). Biochem. Pharmacol ., 26: 1000-1001.
BENSON, W.W. & SMITH, P. (1972) Pesticide levels in deer. Bull.
environ. Contam. Toxicol ., 8: 1-9.
BENSON, W.W., GABICA, J., & BEECHAM, J. (1974) Pesticide and mercury
levels in bear. Bull. environ. Contam. Toxicol ., 11: 1-4.
BERGLIND, R. & DAVE, G. (1984) Acute toxicity of chromate, DDT, PCP,
TPBS and zinc to Daphnia magna cultured in hard and soft water. Bull.
environ. Contam. Toxicol ., 33: 63-68.
BEZZEL, E. (1969) [Results of quantitative observations of the
Accipitridae in Upper Bavaria.] Ornithol. Mitt ., 21: 29-36 (in
German).
BIESSMANN, A. & VON FABER, H. (1981) Effects of DDT and its
metabolites on the adrenal gland of the Japanese quail. Environ.
Pollut ., 25: 99-104.
BITMAN, J., CECIL, H.C., HARRIS, S.J., & FRIES, G.F. (1969) DDT
induces a decrease in eggshell calcium. Nature (Lond.) , 224: 44-46.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin and endrin. Arch. environ. Contam.
Toxicol ., 7: 83-98.
BOLLAG, J.-M. & HENNINGER, N.M. (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J. environ.
Qual ., 5: 15-18.
BOLLEN, W.B., MORRISON, H.E., & CROWELL, H.H. (1954) Effect of field
treatments of insecticides on numbers of bacteria, Streptomyces and
moulds in the soil. J. econ. Entomol ., 47: 302-306.
BOYD, C.E. & FERGUSON, D.E. (1964) Susceptibility and resistance of
mosquito fish to several insecticides. J. econ. Entomol ., 57: 430-431.
BOYD, C.E., VINSON, S.B., & FERGUSON, D.E. (1963) Possible DDT
resistance in two species of frogs. Copeia , 1963(2): 426-429.
BRAHAM, H.W. & NEAL, C.M. (1974) The effects of DDT on energetics of
the short-tailed shrew, Blarina brevicauda. Bull. environ. Contam.
Toxicol ., 12: 32-37.
BUHLER, D.R. & SHANKS, W.E. (1970) Influence of body weight on chronic
oral DDT toxicity in coho salmon. J. Fish Res. Board Can ., 27: 347-
358.
BUHLER, D.R., RASMUSSON, M.E., & SHANKS W.E. (1969) Chronic oral
DDT toxicity in juvenile coho and chinook salmon. Toxicol. appl.
Pharmacol ., 14: 535-555.
BUNYAN, P.J. & PAGE, J.M.J. (1973) Pesticide-induced changes in
hepatic microsomal enzyme systems. Some effects of 1,1-di(p-chloro-
phenyl)-2,2-dichloroethylene (DDE) and 1,1-di(p-chlorophenyl)-2-
chloroethylene (DDMU) in the rat and the Japanese quail. Chem.-biol.
Interact ., 6: 249-257.
BUNYAN, P.J., DAVIDSON, J., & SHORTHILL, M.J. (1970) Hepatic glucose-
6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase levels
in Japanese quail following the ingestion of p,p' -DDT and related
compounds. Chem.-biol. Interact ., 2: 175-182.
BUNYAN, P.J., TOWNSEND, M.G., & TAYLOR, A. (1972) Pesticide-induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-di(p-
chlorophenyl) -2,2,2-trichloroethane (DDT) and 1,1-di(p-chlorophenyl)-
2,2-dichloroethylene (DDE) in the rat and Japanese quail. Chem.-biol.
Interact ., 5: 13-26.
BURDICK, G.E., HARRIS, E.J., DEAN, H.J., WALKER, T.M., SKEA, J., &
COLBY, D. (1964) The accumulation of DDT in lake trout and the effect
on reproduction. Trans. Am. Fish. Soc ., 93: 127-136.
BURLINGTON, H. & LINDEMAN, V.F. (1950) Effect of DDT on testes and
secondary sex characters of white leghorn cockerels. Proc. Soc. Exp.
Biol ., 74: 48-51.
BUTLER, P.A. (1964) Commercial fishery investigations. In: The
effects of pesticides on fish and wildlife , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 65-77
(Circular No. 226).
CALABRESE, A. (1972) How some pollutants affect embryos & larvae of
American oyster & hard-shell clam. Mar. Fish. Rev ., 34: 66-77.
CECIL, H.C., BITMAN, J., & HARRIS, S.J. (1971) Effects of dietary
p,p' -DDT and p,p' -DDE on egg production and eggshell characteristics
of Japanese quail receiving an adequate calcium diet. Poult. Sci ., 50:
657-659.
CHRISTIE, A.E. (1969) Effect of insecticides on algae. Water Sewage
Works , 116: 172-176.
CLARK, D.R. & KROLL, J.C. (1977) Effects of DDE on experimentally
poisoned free-tailed bats ( Tadarida brasilensis ): lethal brain
concentrations. J. Toxicol. environ. Health, 3: 893-901.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyreniodosa by a polychlorinated biphenyl
and several insecticides. Environ. Entomol ., 3: 217-220.
COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U., PERFECT, T.J., &
YEADON, R. (1980) Effects of cultivation and DDT on earthworm
activity in a forest soil in the sub-humid tropics. J. appl. Ecol.,
17: 21-29.
COOKE, A.S. (1970) The effect of p,p -DDT on tadpoles of the common
frog (Rana temporaria). Environ. Pollut ., 1: 57-71.
COOKE, A.S. (1971) Uptake of DDT and DDE by the quail embryo and
chick. Pestic. Sci ., 2: 144-147.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on amphibian
spawn and tadpoles. Environ. Pollut ., 3: 51-68.
COOKE, A.S. (1973a) The effects of DDT, when used as a mosquito
larvicide, on tadpoles of the frog Rana temporaria. Environ. Pollut.,
5: 259-273.
COOKE, A.S. (1973b) Shell-thinning in avian eggs by environmental
pollutants. Environ. Pollut ., 4: 85-152.
COOKE, A.S. (1979a) Eggshell characteristics of gannets ( Sula
bassana ), shags ( Phalacrocorax aristotelis ) and great black-backed
gulls ( Larus marinus ) exposed to DDE and other environmental
pollutants. Environ. Pollut ., 19: 47-65.
COOKE, A.S. (1979b) The influence of rearing density on subsequent
response to DDT dosing for tadpoles of the frog Rana temporaria . Bull.
environ. Contam. Toxicol ., 21: 837-841.
COOKE, A.S. & POLLARD, E. (1973) Shell and operculum formation by
immature Roman snails, Helix pomatia L., when treated with p,p' -DDT.
Pestic. Biochem. Physiol ., 3: 230-236.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Eggshell characteristics
and incidence of shell breakage for grey herons Ardea cinerea exposed
to environmental pollutants. Environ. Pollut ., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution , Swindon, United Kingdom, Natural Environment
Research Council, 74 pp.
CRAWFORD, R.B. & GUARINO, A.M. (1976) Effects of DDT in Fundulus
studies on toxicity, fate and reproduction. Arch. environ. Contam.
Toxicol ., 4: 334-348.
CRITCHLEY, B.R., COOK, A.G., CRITCHLEY, U., PERFECT, T.J., &
RUSSELL-SMITH, A. (1980) The effects of crop protection with DDT on
some elements of the subterranean and surface active arthropod fauna of
a cultivated forest soil in the humid tropics. Pedobiologia , 20: 31-
38.
DAVEY, S.P. (1963) Effects of chemicals on earthworms: a review of the
literature , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 1-20 (Special Scientific Report, Wildlife No.
74).
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin and
DDT by earthworms. Soil Biol. Biochem ., 3: 221-233.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p' -DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol ., 7: 9-18.
DAVISON, K.L., ENGEBRETSON, K.A., & COX, J.H. (1976) p,p' -DDT and
p,p' -DDE effects on egg production, eggshell thickness and
reproduction of Japanese quail. Bull. environ. Contam. Toxicol ., 15:
265-270.
DAVY, F.B., KLEEREKOPER, H., & GENSLER, P. (1972) Effects of
exposure to sublethal DDT on the locomotor behavior of the goldfish
(Carassius auratus). J. Fish Res. Board Can ., 29: 1333-1336.
DAVY, F.B., KLEEREKOPER, H., & MATIS, J.H. (1973) Effects of exposure
to sublethal DDT on the exploratory behavior of goldfish (Carassius
auratus). Water Resour. Res ., 9: 900-905.
DERR, S.K. & ZABIK, M.J. (1972) Biologically active compounds in the
aquatic environment: the effect of DDT on the egg viability of
Chironomus tentans. Bull. environ. Contam. Toxicol ., 7: 366-368.
DESAIAH, D., CUTKOMP, L.K., KOCH, R.B., & JARVINEN, A. (1975)
DDT: effect of continuous exposure on ATPase activity in fish,
Pimephales promelas. Arch. environ. Contam. Toxicol ., 3: 132-141.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife studies,
Patuxent Wildlife Research Center 1961 - 1962 , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 74-96
(Circular No. 167).
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyl, malathion and mercuric
chloride. Toxicol. appl. Pharmacol ., 27: 86-98.
DILL, P.A. & SAUNDERS, R.C. (1974) Retarded behavioral development and
impaired balance in Atlantic salmon ( Salmo salar ) alevins hatched from
gastrulae exposed to DDT. J. Fish Res. Board Can ., 31: 1936-1938.
DILWORTH, T.G., KEITH, J.A., PEARCE, P.A., & REYNOLDS, L.M. (1972)
DDE and eggshell thickness in New Brunswick woodcock. J. wildl.
Manage ., 36: 1186-1193.
DIMOND, J.B. & SHERBURNE, J.A. (1969) Persistence of DDT in wild
populations of small mammals. Nature (Lond.) , 221: 486-487.
DINDAL, D.L. & WURZINGER, K.-H. (1971) Accumulation and excretion of
DDT by the terrestrial snail, Cepaea hortensis. Bull. environ. Contam.
Toxicol ., 4: 362-371.
DOBSON, S. (1981) [Physiological and behavioural effects of organo-
chlorines on pigeons.] Oekol. Vogel , 3(Suppl.): 39-43 (in German).
DUNACHIE, J.F. & FLETCHER, W.W. (1969) An investigation of the
toxicity of insecticides to birds' eggs using the egg-injection
technique. Ann. appl. Biol ., 64: 409-423.
EARNEST, R.D. & BENVILLE, P.E. (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game , 58: 127-132.
EBERHARDT, L., MEEKS, R.L., & PETERLE, T.J. (1971) Food chain model
for DDT kinetics in a fresh water marsh. Nature (Lond.) , 230: 60-62.
EDELSTAM, C. (1972) The visible migration of birds at Ottenby,
Sweden. Var Fagelvarld , 7(Suppl.): 1-360.
EDEN, W.G. & ARTHUR, B.W. (1965) Translocation of DDT and heptachlor
in soybeans. J. econ. Entomol ., 34: 161-162.
EDWARDS, C.A. & JEFFS, K. (1974) Rate of uptake of DDT from soil by
earthworms. Nature (Lond.) , 247: 157-158.
EL-SEBAE, A.H. (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region , Athens,
Mediterranean Action Plan, United Nations Environment Programme/Food
and Agriculture Organization of the United Nations, pp. 109-116. (MAP
Technical Report Series No. 10).
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning and
lowered production of young in prairie falcons. Bioscience , 20: 355-
356.
FERGUSON, D.E., CULLEY, D.D., COTTON, W.D., & DODDS, R.P. (1964)
Resistance to chlorinated hydrocarbon insecticides in three species of
freshwater fish. Bioscience , 14: 43-44.
FORSYTH, D.J. & PETERLE, T.J. (1973) Accumulation of chlorine-36 ring-
labeled DDT residues in various tissues of two species of shrew. Arch.
environ. Contam. Toxicol ., 1: 1-17.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R.,
& HUNT, J.R. (1972) Residues in eggs and tissues of hens fed a ration
containing low levels of pesticides with and without charcoal. J. econ.
Entomol ., 65: 982-988.
FOX, G.A. (1976) Eggshell quality: its ecological and physiological
significance in a DDE-contaminated common tern population. Wilson
Bull ., 88: 459-477.
FRIEND, M., HAEGELE, M.A., & WILSON, R. (1973) DDE: interference with
extra-renal salt excretion in the mallard. Bull. environ. Contam.
Toxicol ., 9: 49-53.
FUHREMANN, T.W. & LICHTENSTEIN, E.P. (1980) A comparative study
of the persistence, movement, and metabolism of six carbon-14
insecticides in soils and plants. J. agric. food Chem ., 28: 446-452.
GARDNER, D.R. (1973) The effect of some DDT and methoxychlor analogs on
temperature selection and lethality in brook trout fingerlings.
Pestic. Biochem. Physiol ., 2: 437-446.
GAUFIN, A.R., JENSEN, L.D., NEBEKER, A.V., NELSON, T., & TEEL,
R.W. (1965) The toxicity of ten organic insecticides to various
aquatic invertebrates. Water Sewage Works , 112: 276-279.
GELUSO, K.N., ALTENBACH, J.S., & WILSON, D.E. (1976) Bat mortality:
pesticide poisoning and migratory stress. Science , 194: 184-186.
GILMAN, A.P., HALLETT, D.J., FOX, G.A., ALLAN, L.J., LEARNING,
W.J., & PEAKALL, D.B. (1978) Effects of injected organochlorines on
naturally incubated herring gull eggs. J. wildl. Manage ., 42: 484-493.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 15 pp (Special Scientific Report No. 241).
GOFFART, H. (1949) [The effect of new kinds of insecticides on
earthworms.] Anz. Schadlingskd. (Berlin) , 22: 72-74 (in German).
GOULDING, K.H. & ELLIS, S.W. (1981) The interaction of DDT with two
species of freshwater algae. Environ. Pollut ., 25: 271-290.
GREENBURG, R.R., RISEBROUGH, R.W., & ANDERSON D.W. (1979)
p,p' -DDE-induced changes in the organic and inorganic structure of
eggshells of the mallard, Anas platyrhynchos. Toxicol. appl.
Pharmacol ., 48: 279-286.
GREICHUS, Y.A. & HANNON, M.R. (1973) Distribution and biochemical
effects of DDT, DDD and DDE in penned double-crested cormorants.
Toxicol. appl. Pharmacol ., 26: 483-494.
GREICHUS, Y.A., CALL, D.J., AMMANN, B.M., GREICHUS, A., &
SHAVE, H. (1975) Physiological effects of polychlorinated biphenyls or
a combination of DDT, DDD, and DDE in penned white pelicans. Arch.
environ. Contam. Toxicol ., 3: 330-343.
HACKMAN, C.D. & HENNY, C.J. (1971) Hawk migration over white marsh,
Maryland. Chesapeake Sci ., 12: 137-141.
HAEGELE, M.A. & HUDSON, R.H. (1973) DDE effects on reproduction of
ring doves. Environ. Pollut ., 4: 53-57.
HAEGELE, M.A. & HUDSON, R.H. (1977) Reduction of courtship behavior
induced by DDE in male ringed turtle doves. Wilson Bull ., 89: 593-601.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common pollutants
on eggshell thickness in mallards and coturnix. Bull. environ. Contam.
Toxicol ., 11: 98-102.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of coho salmon (Oncorhynchus kisutch). J. Fish Res.
Board Can ., 31: 1543-1547.
HANKE, W., GLUTH, G., BUBEL, H., & MULLER, R. (1983) Physiological
changes in carps induced by pollution. Ecotoxicol. environ. Saf ., 7:
229-241.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained sheepshead
minnows. Trans. Am. Fish. Soc ., 98: 426-429.
HANSEN, D.J. (1972) DDT and malathion: effect on salinity selection by
mosquitofish. Trans. Am. Fish. Soc ., 101: 346-350.
HANSEN, D.J. & WILSON, A.J. (1970) Significance of DDT residues from
the estuary near Pensacola, Fla. Pestic. monit . J., 4: 51-56.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquitofish, Gambusia affinis.
Bull. environ. Contam. Toxicol ., 8: 46-51.
HARRI, M.N.E., LAITINEN, J., & VALKAMA, E.-L. (1979) Toxicity and
retention of DDT in adult frogs, Rana temporaria L. Environ. Pollut.,
13: 45-55.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J. agric.
food Chem ., 15: 861-863.
HASELTINE, S., UEBELHART, K., PETERLE, T., & LUSTICK, S. (1974)
DDE, PTH and eggshell thinning in mallard, pheasant and ring dove.
Bull. environ. Contam. Toxicol ., 11: 139-145.
HAUX, C. & LARSSON, A. (1979) Effects of DDT on blood plasma
electrolytes in the flounder, Platichthys flesus L., in hypotonic
brackish water. Ambio , 8: 171-173.
HAYNES, R.J. (1972) Effects of DDT on glycogen and lipid levels in
bobwhites. J. wildl. Manage ., 36: 518-523.
HEATH, R.G., SPANN, J.W., & KREITZER, J.F. (1969) Marked DDE
impairment of mallard reproduction in controlled studies. Nature
( Lond .), 224: 47-48.
HEINZ, G.H. (1976) Behavior of mallard ducklings from parents fed 3
ppm DDE. Bull. environ. Contam. Toxicol ., 16: 640-645.
HEINZ, G.H., HILL, E.F., & CONTRERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin and Aroclor
1254. Toxicol. appl. Pharmacol ., 53: 75-82.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four species of
fish. Trans. Am. Fish. Soc ., 88: 23-32.
HENNY, C.J. (1972) An analysis of the population dynamics of selected
avian species , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 99 pp
(Research Report No. 1).
HENNY, C.J. (1977) Birds of prey, DDT, & tussock moths in Pacific
Northwest. In: Transactions of the Forty-second North American Wildlife
and Natural Resource Conference , Washington, DC, Wildlife Management
Institute, pp. 397-411.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish eating birds. Science , 162:
271-273.
HILL, E.F., DALE, W.E., & MILES, J.W. (1971) DDT intoxication in birds:
subchronic effects and brain residues. Toxicol. appl. Pharmacol ., 20:
502-514.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds , Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 61 pp
(Special Scientific Report No. 191).
HODKINSON, M. & DALTON, S.A. (1973) Interactions between DDT and
river fungi. II Influence of culture conditions on the compatibility of
fungi and p,p' -DDT. Bull. environ. Contam. Toxicol ., 10: 356-359.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife , Washington, DC, US Department of
the Interior, Fish and Wildlife Service, 90 pp (Resource Publication
No. 153).
HUNT, E.G. & LINN, J.D. (1970) Fish kills by pesticides. In: Gillett,
J.W., ed. The biological impact of pesticides in the environment
Corvallis, Oregon, Oregon State University, pp. 97-102.
IDE, F.P. (1957) Effect of forest spraying with DDT on aquatic insects
of salmon streams. Trans. Am. Fish. Soc ., 86: 208-219.
JANICKI, R.H. & KINTER, W.B. (1971) DDT disrupted osmoregulatory
events in the intestine of the eel Anguilla rostrata adapted to
seawater. Science , 173: 1146-1148.
JARVINEN, A.W., HOFFMAN, M.J., & THORSLUND, T.W. (1977) Long-
term toxic effects of DDT food and water exposure on fathead minnows.
J. Fish Res. Board Can ., 34: 2089-2103.
JEFFERIES, D.J. (1967) The delay in ovulation produced by p,p' -DDT and
its possible significance in the field. Ibis , 109: 266-272.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in British
bats and their significance. J. Zool ., 166: 245-263.
JEFFERIES, D.J. & FRENCH, M.C. (1972) Changes induced in the pigeon
thyroid by p,p' -DDE and dieldrin. J. wildl. Manage ., 36: 24-30.
JOHANSEN, C. (1961) Bee poisoning. A hazard of applying agricultural
chemicals , Washington, DC, Institute of Agricultural Sciences,
Washington State University (Circular No. 356).
JOHNSON, B.T. & KENNEDY, J.O. (1973) Biomagnification of p,p' -DDT and
methoxychlor by bacteria. Appl. Microbiol ., 26: 66-71.
JOHNSON, B.T., SAUNDERS, C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin by
freshwater invertebrates. J. Fish Res. Board Can ., 28: 705-709.
JOHNSON, H.E. & PECOR, C. (1969) Coho salmon mortality and DDT in Lake
Michigan. Trans. North Am. Wildl. Nat. Resour. Conf ., 34: 159-166.
JOWETT, P.E., RHEAD, M.M., & BAYNE, B.L. (1978) In vitro changes in the
activity of ATPases in the gills of Carcinus maenas exposed to various
concentrations of p,p' -DDT. Environ. Pollut ., 17: 1-6.
KAN, C.A., JONKER-DEN ROOYEN, J.C., TUINISTRA, L.G.M.T., ROOS,
A.H., & TRAAG, W. (1978) Possible influence of sex and embryonic
content on accumulation of some organochlorine pesticides in
broilers. J. agric. food Chem ., 26: 618-621.
KATZ, M. (1961) Acute toxicity of some organic insecticides to three
species of salmonids and to the threespine stickleback. Trans. Am.
Fish. Soc ., 90: 264-268.
KEIL, J.E. & PRIESTER, L.E. (1969) DDT uptake and metabolism by a
marine diatom. Bull. environ. Contam. Toxicol ., 4: 169-173.
KEIL, J.E., SANDIFER, S.H., GRABER, C.D., & PRIESTER, L.E. (1972) DDT
and polychlorinated biphenyl (Aroclor 1242). Effects of uptake on E.
coli growth. Water Res ., 6: 837-841.
KING, K.A., FLICKINGER, E.L., & HILDEBRAND, H.H. (1978) Shell
thinning and pesticide residues in Texas aquatic bird eggs,
1970. Pestic. monit. J ., 12: 16-21.
KINTER, W.B., MERKENS, L.S., JANICKI, R.H., & GUARINO, A.M. (1972)
Studies on the mechanism of toxicity of DDT and polychlorinated
biphenyls (PCBs): disruption of osmoregulation in marine fish.
Environ. Health Perspect ., 1: 169-173.
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972) Eggshell
and population changes in the sparrow-hawk (Accipiter nisus). TNO-
Nieuws , 27: 542-550.
KOLAJA, G.J. (1977) The effects of DDT, DDE and their sulfonated
derivatives on eggshell formation in the mallard duck. Bull. environ.
Contam. Toxicol ., 17: 697-701.
KORN, S. & EARNEST, R. (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game , 60: 128-131.
KOUYOUMJIAN, H.H. & UGLOW, R.F. (1974) Some aspects of the toxicity of
p,p' -DDT, p,p' -DDE and p,p' -DDD to the freshwater planarian
Polycelis felina (Tricladida). Environ. Pollut ., 7: 103-109.
KREITZER, J.F. & SPANN, J.W. (1973) Test of pesticidal synergism with
young pheasants and Japanese quail. Bull. environ. Contam. Toxicol.,
9: 250-256.
LAL, R. & SAXENA, D.M. (1979) Effect of DDT on cell population growth
of Tetrahymena pyriformis. Arch. Protistenkd ., 122: 382-386.
LAL, R. & SAXENA, D.M. (1980) Effect of DDT on cell population growth,
cell division, and DNA synthesis in Stylonychia notophora (Stokes).
Arch. environ. Contam. Toxicol ., 9: 163-170.
LAL, R. & SAXENA, D.M. (1982) Accumulation, metabolism, and effects of
organochlorine insecticides on microorganisms. Microbiol. Rev ., 46: 95-
127.
LEE, S.S., FANG, S.C., & FREED, V.H. (1976) Effect of DDT on
photosynthesis of Selenastrum capricornutum. Pestic. Biochem. Physiol.,
6: 46-51.
LEFFLER, C.W. (1975) Effects of ingested mirex and DDT on juvenile
Callinectes sapidus Rathbun. Environ. Pollut ., 8: 283-300.
LEDFORD, R.A. & CHEN, J.H. (1969) Degradation of DDT and DDE by
cheese microoganisms. J. food Sci ., 34: 386-388.
LEGMAN, J.W., PETERLE, T.J., & MILLS, C.M. (1974) Effects of DDT on
bobwhite quail ( Colinus virginianus ) adrenal gland. Bull. environ.
Contam. Toxicol ., 11: 407-414.
LINCER, J.L. (1972) The effects of organochlorines on the American
kestrel (Falco sparverius Linn.) , Ithaca, New York, Cornell
University (PhD Thesis).
LINCER, J.L. (1975) DDE-induced eggshell-thinning in the American
kestrel: a comparison of the field situation and laboratory results.
J. appl. Ecol ., 12: 781-793.
LINDBURG, P. (1977) The peregrine falcon in Sweden. In: Proceedings of
the International Council for Bird Preservation World Conference on
Birds of Prey, Vienna, 1975 , Cambridge, United Kingdom, International
Council for Bird Preservation, pp. 329-338.
LOCKE, L.N., CHURA, N.J., & STEWART, P.A. (1966) Spermatogenesis in
bald eagles experimentally fed a diet containing DDT. Condor , 68: 497-
502.
LONGCORE, J.R., SAMSON, F.B., & WHITTENDALE, T.W. (1971) DDE
thins eggshells and lowers reproductive success of captive black
ducks. Bull. environ. Contam. Toxicol ., 6: 485-490.
LONGCORE, J.R. & STENDELL, R.C. (1977) Shell thinning and reproductive
impairment in black ducks after cessation of DDE dosage. Arch. environ.
Contam. Toxicol ., 6: 293-304.
LOOSANOFF, V.L. (1960) Some effects of pesticides on marine arthropods
and mollusks. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Transactions of the Second Seminar, 1959 , Cincinnati, USA,
Robert, A. Taft Sanitation and Engineering Centre, pp. 89-93 (Technical
Report No. W60-3).
LOWE, J.I. (1965) Chronic exposure of blue crabs, Callinectes sapidus,
to sublethal concentrations of DDT. Ecology , 46: 899-900.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia , 12: 29-33.
LUCKENS, M.M. & DAVIS, W.H. (1964) Bats: sensitivity to DDT. Science,
146: 948.
LUDKE, J.L. (1977) DDE increases the toxicity of parathion to
Coturnix quail. Pestic. Biochem. Physiol ., 7: 28-33.
MACEK, K.J. (1968) Reproduction in brook trout ( Salvelinus fontinalis )
fed sublethal concentrations of DDT. J. Fish Res. Board Can ., 25:
1787-1795.
MACEK, K.J. & KORN, S. (1970) Significance of the food chain in DDT
accumulation by fish. J. Fish Res. Board Can ., 27: 1496-1498.
MACEK, K.J. & SANDERS, H.O. (1970) Biological variation in the
susceptibility of fish and aquatic invertebrates to DDT. Trans. Am.
Fish. Soc ., 99: 89-90.
MACEK, K.J., RODGERS, C.R., STALLING, D.L., & KORN, S. (1970) The
uptake, distribution and elimination of dietary 14C-DDT and
14C-dieldrin in rainbow trout. Trans. Am. Fish. Soc ., 99: 689-695.
MACFARLANE, R.B., GLOOSCHENKO, W.A., & HARRISS, R.C. (1972) The
interaction of light intensity and DDT concentration upon the marine
diatom, Nitzschia delicatissima Cleve. Hydrobiologia , 39: 373-382.
MCLANE, M.A.R. & HALL, L.C. (1972) DDE thins screech owl eggshells.
Bull. environ. Contam. Toxicol ., 8: 65-68.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol ., 25: 921-928.
MACRAE, I.C. & VINCKX, E. (1973) Effect of lindane and DDT on
populations of protozoa in a garden soil. Soil Biol. Biochem ., 5: 245-
247.
MAHONEY, J.J. (1975) DDT and DDE effects on migratory condition in
white-throated sparrows. J. wildl. Manage ., 39: 520-527.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p' -DDT on production and survival of Daphnia magna Strauss. Bull.
environ. Contam. Toxicol ., 13: 412-416.
MARTIN, J.P. (1966) Influence of pesticides on soil microbes and soil
properties. Am. Soc. Agron. Spec. Publ ., 8: 95-108.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms , Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity :
interpretation and data base for 410 chemicals and 66 species of
freshwater animals , Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp (Resource Publication No. 160).
MEEKS, R.L. (1968) The accumulation of 36Cl ring-labeled DDT in a
freshwater marsh. J. wildl. Manage ., 32: 376-398.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls ( Tyto alba ) fed low levels of DDE and dieldrin.
Arch. environ. Contam. Toxicol ., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine phyto-
plankton vary in their response to chlorinated hydrocarbons.
Science , 167: 1724-1726.
METCALF, R.L., KAPOOR, I.P., LU, P.-Y, SCHUTH, C.K., & SHERMAN,
P. (1973) Model ecosystem studies of the environmental fate of six
organochlorine pesticides. Environ. Health Perspect ., 4: 35-43.
MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1976a) Enzymatic basis
for DDE-induced eggshell thinning in a sensitive bird. Nature (Lond.),
259: 122-124.
MILLER, D.S., KINTER, W.B., PEAKALL, D.B., & RISEBROUGH, R.W.
(1976b) DDE feeding and plasma osmoregulation in ducks, guillemots,
and puffins. Am. J. Physiol ., 231: 370-376.
MOFFETT, G.B. & YARBROUGH, J.D. (1972) The effects of DDT, toxaphene
and dieldrin on succinic dehydrogenase activity in insecticide-
resistant and susceptible Gambusia affinis. J. agric. food Chem ., 20:
558-560.
MOORE, N.W. (1965) Pesticides in birds - a review of the situation in
Great Britain in 1965. Bird Study , 12: 222-252.
MORIARTY, F. (1975) Pollutants and animals: a factual perspective,
London, Allen & Unwin.
MORIARTY, F., BELL, A.A., & HANSON, H. (1986) Does p,p' -DDE thin
eggshells? Environ. Pollut ., 40: 257-286.
MUIRHEAD-THOMSON, R.C. (1973) Laboratory evaluation of pesticide
impact on stream invertebrates. Freshwater Biol ., 3: 479-498.
MULLA, M.S. (1963) Toxicity of organochlorine insecticides to the
mosquitofish Gambusia affinis and the bullfrog Rana catesbeiana.
Mosq. News , 23: 299-303.
MURPHY, P.G. (1970) Effects of salinity on uptake of DDT, DDE and DDD
by fish. Bull. environ. Contam. Toxicol ., 5: 404-407.
MURPHY, P.G. (1971) The effect of size on the uptake of DDT from water
by fish. Bull. environ. Contam. Toxicol ., 6: 20-23.
NAGY, A.C. (1977) Population trend indices based on 40 years of autumn
counts at hawk mountain. In: Proceedings of the International Council
for Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 243-253.
NAQVI, S.M. & FERGUSON, D.E. (1968) Pesticide tolerances of selected
freshwater invertebrates. J. Mississippi Acad. Sci ., 14: 121-127.
NEUFELD, G.J. & PRITCHARD, J.B. (1979) An assessment of DDT toxicity in
osmoregulation and gill Na,K-ATPase activity in the blue crab,
Philadelphia, American Society for Testing and Materials, pp. 23-34
(ASTM Special Technical Publication No. 667).
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted, United
Kingdom , T. & A.D. Poyser.
NEWTON, I. & BOGAN, J. (1978) The role of different organo-chlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol ., 15:
105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds , 77: 47-70.
NIMMO, D.R., WILSON, A.J., & BLACKMAN, R.R. (1970) Localization of
DDT in the body organs of pink and white shrimp. Bull. environ. Contam.
Toxicol ., 5: 333-341.
OGILVIE, D.M. & MILLER, D.L. (1976) Duration of a DDT-induced shift in
the selected temperature of Atlantic salmon (Salmo salar). Bull.
environ. Contam. Toxicol ., 16: 86-89.
OLOFSSON, S. & LINDAHL, P.E. (1979) Decreased fitness of cod ( Gadus
morrhua L.) from polluted waters. Mar. environ. Res ., 2: 33-45.
OSBORN, D., COOKE, A.S., & FREESTONE, S. (1981) Histology of a
teratogenic effect of DDT on Rana temporaria tadpoles. Environ.
Pollut ., 25: 305-319.
O'SHEA, T.J. & LUDKE, J.L. (1979) Monitoring fish and wildlife for
environmental pollutants , Fort Collins, Colorado, US Department of the
Interior, Fish and Wildlife Service.
PEAKALL, D.B. (1970) p,p' -DDT: effect on calcium metabolism and
concentration of estradiol in the blood. Science , 168: 592-594.
PEAKALL, D.B., LINCER, J.L., RISEBROUGH, R.W., PRITCHARD, J.B., &
KINTER, W.B. (1973) DDE-induced eggshell thinning: structural and
physiological effects in three species. Comp. gen. Pharmacol ., 4: 305-
313.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975a) Prolonged eggshell
thinning caused by DDE in the duck. Nature (Lond.) , 254: 421.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975b) Blood calcium
levels and the mechanism of DDE-induced eggshell thinning. Environ.
Pollut ., 9: 289-294.
PEAKALL, D.B., CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1975c)
Organochlorine residues in Alaskan peregrines. Pestic. monit. J. , 8:
255-260.
PERFECT, J. (1980) The environmental impact of DDT in a tropical agro-
ecosystem. Ambio , 9: 16-21.
PERFECT, T.J., COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U.,
DAVIES, A.L., SWIFT, M.J., RUSSELL-SMITH, A., & YEADON, R. (1979)
The effect of DDT contamination on the productivity of a cultivated
forest soil in the sub-humid tropics. J. appl. Ecol ., 16: 705-719.
PETERSON, R.H. (1973) Temperature selection of Atlantic salmon (Salmo
salar) and brook trout ( Salvalinus fontinalis ) as influenced by various
chlorinated hydrocarbons. J. Fish Res. Board Can ., 30: 1091-1097.
PORTER, R.D. & WIEMEYER, S.N. (1972) DDE at low dietary levels kills
captive American kestrels. Bull. environ. Contam. Toxicol ., 8: 193-
199.
POWERS, C.D., WURSTER, C.F., & ROWLAND, R.G. (1979) DDE inhalation
of marine algal cell division and photosynthesis per cell. Pestic.
Biochem. Physiol ., 10: 306-312.
PRESCOTT, L.M. & OLSON, D.L. (1972) The effect of pesticides on the
soil amoeba Acanthamoeba castellanii (Neff). Proc. South Dakota Acad.
Sci ., 51: 136-141.
RAMALINGAM, K. & RAMALINGAM, K. (1982) Effects of sublethal levels
of DDT, malathion and mercury on tissue proteins of Sarotherodon
mossambicus (Peters). Proc. Indian Acad. Sci. anim. Sci ., 91: 501-505.
RANDALL, W.F., DENNIS, W.H., & WARNER, M.C. (1979) Acute toxicity of
dechlorinated DDT, chlordane and lindane to bluegill ( Lepomis
machrochirus ) and Daphnia magna. Bull. environ. Contam. Toxicol ., 21:
849-854.
RATCLIFFE, D.A. (1967) Decrease in eggshell weight in certain birds of
prey. Nature (Lond.) , 215: 208-210.
RATCLIFFE, D.A. (1969) Population trends of the peregrine falcon in
Great Britain. In: Hinkley, J.J., ed. Peregrine falcon populations:
Their biology and decline , Madison, Milwaukee, London, University of
Wisconsin Press, pp. 239-269.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol ., 7: 67-107.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Study , 19: 117-156.
REINBOLD, K.A., KAPOOR, I.P., CHILDERS, W.F., BRUCE, W.N., &
METCALF, R.L. (1971) Comparative uptake and biodegradability of DDT
and methoxychlor by aquatic organisms. Illinois nat. Hist. Surv.
Bull ., 30: 405-415.
REINERT, R.E., STONE, L.J., & WILLFORD, W.A. (1974) Effect of
temperature on accumulation of methylmercuric chloride and p,p' -DDT by
rainbow trout (Salmo gairdneri). J. Fish Res. Board Can ., 31: 1649-
1652.
RICE, C.P. & SIKKA (1973) Uptake and metabolism of DDT by six species
of marine algae. J. agric. food Chem ., 21: 148-152.
RICHIE, P.J. & PETERLE, T.J. (1979) Effect of DDE on circulating
luteinizing hormone levels in ring doves during courtship and nesting.
Bull. environ. Contam. Toxicol ., 23: 220-226.
RISEBROUGH, R.W. & ANDERSON, D.W. (1975) Some effects of DDE and
PCB on mallards and their eggs. J. wildl. Manage ., 39: 508-513.
RISEBROUGH, R.W., DE LAPPE, B.W., & SCHMIDT, T.T. (1976)
Bioaccumulation factors of chlorinated hydrocarbons between mussels and
seawater. Mar. Pollut. Bull ., 7: 225-228.
ROBERTS, D. (1975) Sub-lethal effects of chlorinated hydrocarbons on
bivalves. Mar. Pollut. Bull ., 6: 20-24.
ROBSON, W.A., ARSCOTT, G.H., & TINSLEY, I.J. (1976) Effect of DDE,
DDT and calcium on the performance of adult Japanese quail (Coturnix
coturnix japonica). Poult. Sci ., 55: 2222-2227.
ROSEN, L. (1966) [The flight of birds of prey at Falsterbo.] Var
Fagelvarld , 25: 315-326 (in Swedish).
SANBORN, J.R., CHILDERS, W.F., & METCALF, R.L. (1975) Uptake of three
polychlorinated biphenyls, DDT, and DDE by the green sunfish, Lepomis
cyanellus Raf. Bull. environ. Contam. Toxicol ., 13: 209-217.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowlers toad Bufo woodhousii
fowleri. Copeia , 2: 246-251.
SANDERS, H.O. (1972) Toxicity of some insecticides to four species of
malacostracan crustaceans , Washington, DC, US Department of the
Interior, Bureau of Sport, Fisheries and Wildlife, pp. 3-19 (Technical
Paper No. 66).
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol.
Oceanogr ., 13: 112-117.
SELL, J.L., DAVISON, K.L., & POONACHA, K.B. (1972) Decreased aniline
hydroxylase activity in Japanese quail due to dietary DDT. J. agric.
food Chem ., 20: 553-557.
SHERBURNE, J.A. & DIMOND, J.B. (1969) DDT persistence in wild hares and
mink. J. wildl. Manage ., 33: 944-948.
SHIN, Y.-O., CHODAN, J.J., & WOLCOTT, A.R. (1970) Adsorption of DDT
by soils, soil fractions, and biological materials. J. agric. food
Chem ., 18: 1129-1133.
SHIRES, S.W. (1985) A comparison of the effects of cypermethrin,
parathion-methyl and DDT on cereal aphids, predatory beetles,
earthworms and litter decomposition in spring wheat. Crop Prot.,
4: 177-193.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of DDT
and dieldrin on development in winter flounder (Pseudopleuronectes
americanus). J. Fish Res. Board Can ., 30: 1894-1898.
SMITH, S.I., WEBER, C.W., & REID, B.L. (1969) The effect of high
levels of dietary DDT on egg production, mortality, fertility,
hatchability and pesticide content of yolks in Japanese quail. Poult.
Sci ., 48: 1000-1004.
STICKEL, L.F. & HEATH, R.G. (1964) Wildlife studies. Patuxent wildlife
research center. In: The effects of pesticides on fish and wildlife,
Washington, DC, Department of the Interior, Fish and Wildlife Service,
pp. 3-30 (Circular No. 226).
THOMPSON, A.R. (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull. environ. Contam. Toxicol ., 5:
577-586.
ULFSTRAND, S., ROOS, G., ALERSTAM, T., & OSTERDAHL, L. (1974)
Visible bird migration at Falsterbo, Sweden. Var Fagelvarld,
8(Suppl.): 1-244.
VANGILDER, L.D. & PETERLE, T.J. (1980) South Louisiana crude oil and
DDE in the diet of mallard hens: effects on reproduction and duckling
survival. Bull. environ. Contam. Toxicol ., 25: 23-28.
VAN VELZEN, A. & KREITZER, J.F. (1975) The toxicity of p,p' -DDT to
the clapper rail. J. wildl. Manage ., 39: 305-309.
VAN VELZEN, A.C., STILES, W.B., & STICKEL, L.F. (1972) Lethal
mobilization of DDT by cowbirds. J. wildl. Manage ., 36: 733-739.
VERMEER, K. & REYNOLDS, L.M. (1970) Organochlorine residues in aquatic
birds in the canadian prairie provinces. Can. field Nat ., 84: 117-130.
VINSON, S.B., BOYD, C.E., & FERGUSON, D.E. (1963) Resistance to DDT in
the mosquito fish, Gambusia affinis. Science , 139: 217-218.
WAGGONER, J.P. & ZEEMAN, M.G. (1975) DDT: short term effects on
osmoregulation in black surfperch (Embiotoca jacksoni). Bull. environ.
Contam. Toxicol ., 13: 297-300.
WARE, G.W. (1968) DDT-C14 translocation in alfalfa. J. econ.
Entomol ., 61: 1451-1452.
WARE, G.W., ESTESEN, B.J., & CAHILL, W.P. (1970) Uptake of C14-DDT
from soil by alfalfa. Bull. environ. Contam. Toxicol ., 5: 85-86.
WARLEN, S.M., WOLFE, D.A., LEWIS, C.W., & COLBY, D.R. (1977)
Accumulation and retention of dietary 14C-DDT by Atlantic menhaden.
Trans. Am. Fish. Soc ., 106: 95-104.
WATSON, M., PHARAOH, B., WYLLIE, J., & BENSON, W.W. (1975)
Metabolism of low oral doses of DDT and DDE by tame mule deer fawns.
Bull. environ. Contam. Toxicol ., 13: 316-323.
WEIS, J.S. & MANTEL, L.H. (1976) DDT as an accelerator of limb
regeneration and molting in fiddler crabs. Estuarine coastal mar. Sci.,
4: 461-466.
WEIS, P. & WEIS, J.S. (1974) DDT causes changes in activity and
schooling behavior in goldfish. Environ. Res ., 7: 68-74.
WHEATLEY, G.A. (1965) The assessment and persistence of residues of
organochlorine insecticides in soils and their uptake by crops. Ann.
appl. Biol ., 55: 325-329.
WHITE, C.M. & CADE, T.J. (1977) Long term trends of peregrine
populations in Alaska. In: Proceedings of the International Council for
Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 63-71.
WHO (1979) Environmental Health Criteria 9: DDT and its derivatives,
Geneva, World Health Organization, 194 pp.
WIEMEYER, S.N. & PORTER, R.D. (1970) DDE thins eggshells of captive
American kestrels. Nature (Lond.) , 227: 737-738.
WIEMEYER, S.N., SPITZER, P.R., KRANTZ, W.C., LAMONT, T.G., &
CROMARTIE, E. (1975) Effects of environmental pollutants on
Connecticut and Maryland ospreys. J. wildl. Manage ., 39: 124-139.
WURSTER, C.F. (1968) DDT reduces photosynthesis by marine
phytoplankton. Science , 159: 1474-1475.
 ------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chloroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethlyene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Some related insecticides
NO2
Bulan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-a-(4-chlorophenyl)- -Cl -OH -CH3
a-(methyl)benzenemethanol
dicocol 4-chloro-a-(4-chlorophenyl)-a- -Cl -OH -CCl3
(Kelthane(r)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-a-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
a-hydroxybenzeneacetate
chloropropopylatec 1-methylethyl 4-chloro-a- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-a-hydroxy-
benzeneacetate
Table 1. Structure of p,p' -DDT and its analogues of the form (continued)
------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Perthane(r) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
------------------------------------------------------------------------------------
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been
sold under the name Rothane(r); in metabloic studies the same compound has been
referred as DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
(r) Registered.
3. KINETICS, METABOLISM, BIOTRANSFORMATION, AND BIOACCUMULATION
Appraisal
The physicochemical properties of DDT and its metabolites enable
these compounds to be taken up readily by organisms. The rates of
accumulation vary with the species, with the duration and concentration
of exposure, and with environmental conditions.
These compounds are resistant to breakdown and are readily adsorbed
to sediments and soils, which can act both as sinks and as long-term
sources of exposure (e.g., for soil organisms).
Organisms can accumulate these chemicals from the surrounding
medium and from food. In aquatic organisms, uptake from the water is
generally more important, whereas, in terrestrial fauna, food provides
the major source.
In general, organisms at higher trophic levels tend to contain more
DDT-type compounds than those at lower trophic levels.
Such compounds can be transported around the world in the bodies of
migrant animals and in ocean and air currents.
Different organisms metabolise DDT via different pathways. Of the
two initial metabolites, DDE is the more persistent, though not all
organisms produce DDE from DDT. The alternative route of metabolism,
via TDE leads to more rapid elimination (WHO, 1979). Much of the
retained DDT and its metabolites are stored in lipid-rich tissues.
Because there is an annual cycle in lipid storage and utilization in
many organisms, there is also a related annual cyclic pattern in the
handling of DDT.
3.1 Retention in Soils and Sediments and Plant Uptake
Shin et al. (1970) investigated the adsorption of DDT by soils of
various different types and by isolated soil fractions. A sandy loam,
a clay soil, and a highly organic muck were either used intact or had
various components extracted before estimating their adsorptive
capacity for the insecticide. Adsorption was least in the sandy loam
and greatest in the muck (distribution coefficients [Kd] were in the
ratio 1:10:80 for sandy loam, clay soil, and organic muck,
respectively). All soils showed a strong adsorptive capacity for DDT.
The adsorption of DDT was closely related to the organic matter content
of the soils; progressive removal of lipids, resins, polysaccharides,
polyuronides, and humic matter identified the organic fractions which
bound the DDT. Humic material represents a major source of adsorptive
capacity for DDT; the degree of sorption, however, is strongly
connected with the degree of humification. Soil containing large
amounts of humic material may not adsorb DDT as greatly as other soils
where humification is more advanced. Wheatley (1965) estimated half-
times for the loss of DDT applied to soils. After surface application,
50% of DDT was lost within 16-20 days. The estimated time for the loss
of 90% of surface-applied DDT was 1.5 to 2 years. With DDT mixed into
the soil, 50% loss occurred in 5 to 8 years, and it was estimated that
90% of applied insecticide would be lost in 25-40 years.
Albone et al. (1972) investigated the capacity of river
sediments, from the Severn Estuary, United Kingdom, to degrade DDT.
p,p' -DDT (14C-labelled) was applied to sediments either in situ on
the mud flats or in the laboratory. Sediment movement in the area of
the in situ study was sufficiently small to neither bury nor expose
the incubation tubes set into the mud. Incubation in situ over 46
days led to very little metabolism of DDT in the sediments. Some
p,p' -TDE was produced, but the ratio of DDT to TDE was 13 : 1 and
48 : 1 in two replicate experiments. There was no production of
extractable polar products; metabolism beyond TDE did not occur.
Incubation of the same sediments in the laboratory, over 21 days, led
to much greater metabolism (ratios of 1 : 1.1 and 1 : 3.3, DDT to TDE,
in replicate incubations) and the production of some unidentified,
further breakdown products. Investigation of the microbial population
of the sediment showed that some of the organisms were capable of
degrading DDT; little metabolism appeared to take place in situ .
3.2 Uptake and Accumulation by Organisms
The uptake and accumulation of DDT and its metabolites into
organisms, as determined in controlled laboratory experiments, is
summarized in Table 2. Results are expressed as bioconcentration
factors (the ratio of the concentration of the compound in the organism
to the concentration in the medium).
Concentration factors can be misleading with compounds such as DDT
when exposure is high. The compound is readily taken up and retained
at very low concentrations. At high concentrations, no more material
can be taken up because a plateau has been reached. The only
meaningful way to assess the capacity of organisms to take up and
retain DDT is by looking over a wide range of exposure levels. The
low concentration factor quoted in Table 2 for earthworms, for example,
reflects the high exposure rather than a low capacity for uptake and
retention of DDT, because concentration factors are simple ratios
between "exposure" and final concentration in the organism.
Concentration factors for fish are generally higher than for their
invertebrate prey (Table 2). It is now generally agreed that most of
the DDT taken into aquatic organisms comes from the water rather than
from their food (Moriarty, 1975). Again, the concentration factors can
be misleading. Aquatic organisms take in a small proportion of
ingested DDT. However, they retain a large proportion of the DDT which
has been absorbed into the body from the food. There has been some
controversy in the past over explanations for higher accumulations of
DDT at higher trophic levels in aquatic systems. It now seems clear
that this is not due primarily to biomagnification up food chains but
rather to a tendency for organisms at higher trophic levels to
accumulate more DDT directly from the water.
Terrestrial organisms do not live in a uniform medium surrounded by
a relatively constant concentration of a chemical. Even soil organisms
live in a medium with very variable concentrations of DDT or its
metabolites at different levels of the soil profile or patchy distri-
bution of the chemical. Some terrestrial organisms could be directly
exposed to DDT during application of the insecticide, but most will be
exposed to what remains of the DDT after application. Therefore,
higher terrestrial organisms will accumulate DDT mostly from their
food. The data in Table 2 are taken from controlled laboratory
investigations. There is ample evidence from the field that DDT does
accumulate in many organisms in different media. There is similarly
evidence that the residues of DDT or its metabolites persist in
organisms for long periods after exposure has ceased. The following
should not be regarded as a comprehensive review of the literature on
this subject, which is too large to be included. Rather, these are
examples from different groups of organisms.
Table 2. Bioaccumulation of DDTa
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Bacteria
Aerobacter aerogenes 100 22 24 h 1.2 3736 Johnson &
Bacillus subtilis 130 22 24 h 0.676 4303 & Kennedy
Aerobacter aerogenes 25 22 4 h 0.64 10 639 (1973)
200 22 4 h 0.64 1784 Johnson &
Bacillus subtilis 43 22 4 h 0.64 13 880 Kennedy
348 22 4 h 0.64 1805 (1973)
Marine algae
Cyclotella nana 17 23 2 h 0.7 37 600 Rice & Sikka
8 23 2 h 0.7 58 100 (1973)
Isochrysis galbane 39 23 2 h 0.7 11 300 Rice & Sikka
19 23 2 h 0.7 28 800 (1973)
Olisthodiscus luteus 108 23 2 h 0.7 4600 Rice & Sikka
54 23 2 h 0.7 7000 (1973)
Amphidinium carteri 66 23 2 h 0.7 4300 Rice & Sikka
33 23 2 h 0.7 9600 (1973)
Tetraselmis chuii 106 23 2 h 0.7 5200 Rice & Sikka
53 23 2 h 0.7 6300 (1973)
Skeletonema costatum 29 23 2 h 0.7 31 900 Rice & Sikka
15 23 2 h 0.7 38 400 (1973)
Diatom
Cylindrotheca 21 days 100 300 Keil & Priester
closterium (1969)
Pond snail stat 6 days 3.0 6000 Reinbold et al.
(Physa 5 sp.) (1971)
Freshwater mussel flow 20 3 weeks 0.62 3990d Bedford & Zabik
(Anodonta grandis) (1973)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Earthworm 10 4 weeks 17 000 0.47d Davis (1971)
(Lumbricus terrestris)
Water flea stat 30 3 days 2.0 1330 Metcalf et al.
(1973)
(Daphnia magna) flow 21 3 days 0.08 114 100 Johnson et al.
(1971)
Scud flow 21 3 days 0.081 20 600 Johnson et al.
(Gammarus fasciatus) (1971)
Glass shrimp flow 21 3 days 0.1 5000 Johnson et al.
(Palaemonetes kadiakensis) (1974)
Pink shrimp flow 8-15 13 days 0.14 1500 Nimmo et al.
(Penaeus duorarum) (1970)
Crayfish flow 21 3 days 0.08 2900 Johnson et al.
(Orconectes nais) (1971)
Mayfly larva flow 21 3 days 0.052 32 600 Johnson et al.
(Hexagenia bilineata) (1971)
Mayfly larva flow 21 3 days 0.047 22 900 Johnson et al.
(Siphlonurus sp.) (1971)
Dragonfly nymph flow 21 2 days 0.101 3500 Johnson et al.
(Ischnura verticalis) (1971)
Dragonfly nymph flow 21 2 days 0.079 910 Johnson et al.
(Libellula sp.) (1971)
Midge larva flow 21 3 days 0.046 47 800 Johnson et al.
(Chironomus sp.) (1971)
Mosquito larva flow 21 2 days 0.105 133 600 Johnson et al.
(Culex pipiens) (1971)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Mosquito larva stat 30 3 days 2.0 110d Metcalf et al.
(Culex quinquifasciatus) stat 30 3 days 0.9 74d (1973)
Mosquito fish stat 30 3 days 2.0 344d Metcalf et al.
(Gambusia affinis) stat 30 3 days 0.9 217d (1973)
Rainbow trout flow 5 12 weeks 0.176 21 363d Reinert et al.
(Salmo gairdneri) flow 10 12 weeks 0.137 43 158d (1974)
flow 15 12 weeks 0.133 51 355d Reinert et al.
(1974)
Brook trout flow 14 120 days 3 mg 0.64d Macek & Korn
(Salvelinus fontinalis) /kg diet (1970)
flow 14 120 days 0.003 8533d Macek & Korn
(1970)
Pinfish flow 14 days 0.1 40 000d Hansen & Wilson
(Lagodon rhomboides) flow 14 days 1.0 11 020d (1970)
Atlantic croaker flow 14 days 0.1 12 500d Hansen & Wilson
(Micropogon undulatus) flow 14 days 1.0 12 170d (1970)
Fathead minnow flow 24-25.5 14 days 45.6 mg/kg 1.17d Jarvinen et al.
(Pimephales promelas) flow 24-25.5 14 days 0.5 85 400d (1977)
flow 24-25.5 14 days 2.0 69 100d Jarvinen et al.
flow 24-25.5 112 days 45.6 mg/kg 1.33d (1977)
flow 24-25.5 112 days 0.5 93 200d Jarvinen et al.
flow 24-25.5 112 days 2.0 154 100d (1977)
Tilapia stat 31 days 1.0 6800 Reinbold et al.
(Tilapia mossambica) 31 days 10 10 600 (1971)
Green sunfish stat 31 days 1.0 3900 Reinbold et al.
(Lepomis cyanellus) 31 days 10 4020 (1971)
stat 22 15 days 0.1-0.3 17 500d Sanborn et al.
(1975)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Chicken eggs 8 weeks 0.1 1.87d Foster et al.
fat 8 weeks 0.1 5.8d (1972)
Broiler hen fat 6 weeks 1.0 10.3d Kan et al.
(1978)
White pelican WB 10 weeks 72 11.9d Greichus et al.
(Pelecanus erythrorhynchos) (1975)
Double-crested cormorant WB 9 weeks 0.95 236.3d Greichus &
(Phalacrocorax a. auritus) Hannon (1973)
American kestrel WB 11-16 2.8 103.9 Porter &
(Falco sparverius) months Wiemeyer (1972)
Mule deere muscle 30 days 5 mg/day 122.8 ug Watson et al.
(Odocoileus heminonus) oral /kgd (1975)
---------------------------------------------------------------------------------------------------------
a Unless specified otherwise, bioconcentration factors are based on whole body (WB) measurements.
b Stat = static conditions (water unchanged for duration of experiment);
Flow = flow-through conditions (DDT concentration in water continuously maintained).
c Bioconcentration factor = concentration of DDT in organism/concentration of DDT in medium or food.
Concentrations of DDT in organisms represents total DDT, i.e., DDT plus its stable metabolites,
principally DDE. Bioconcentration factors calculated on a dry weight basis unless otherwise stated.
d Calculated on a wet weight basis.
e Oral dose (by capsule) given daily.
3.2.1 Plants
Fuhremann & Lichtenstein (1980) applied 14C-labelled p,p' -DDT to
loam or sandy soil (at 4 and 2 mg/kg, respectively) and grew oat plants
on the treated soils for 13 days. At harvest, residues of DDT and its
metabolites were analysed in soil and plant by scintillation counting,
thin layer chromatography, and GLC. Of the total applied DDT, 95.7%
was recovered from loam soil and 88.6% from sandy soil. Almost all of
the DDT present was extractable in organic solvent (only 2.8%, for
loam, and 0.7%, for sand, was present in a water-bound form),
indicating little or no metabolism of the compound except to persistent
organically extractable residues. DDE was detected in both soils,
accounting for 3.4% of the total extracted in loam soil and 2.2% in
sand. Other metabolites, including o,p' -DDT, TDE, and dicofol were
recovered in very small quantities. Very little DDT (and none of its
metabolites) was detected in oat roots grown on loam, amounting to 0.2%
of the total DDT applied. The uptake was greater (4.6%) in roots of
oats grown on sand, but the uptake of labelled carbon into plant tops,
from both soils, was so low that it could not be analysed.
DDT was not translocated into the foliage of alfalfa when applied
to the soil (Ware, 1968; Ware et al., 1970) or into soybeans (Eden &
Arthur, 1965). Harris & Sans (1967) found only trace amounts of DDT
or its metabolites in the storage roots of carrots, radishes, and
turnips after growing the plants in soils containing up to 14 mg
DDT/kg.
3.2.2 Microorganisms
The uptake and accumulation of DDT from the culture medium by
microorganisms has been reviewed by Lal & Saxena (1982). All of the
microorganisms studied showed some capacity to take up DDT from their
growth medium, but the relative amount taken up varied greatly from
species to species. Many species took up more than 90% of the DDT when
exposed to concentrations ranging from 1 to 1000 µg/litre, whereas a
few species took in only 0.5% of the available DDT. The concentration
factors (i.e., the concentration within the organism expressed as a
ratio against the concentration in the medium) for DDT were variable
but always high (Table 2).
3.2.3 Aquatic invertebrates
Concentration factors are also variable in aquatic invertebrates.
In all cases there is considerable uptake and retention of the DDT,
though often as DDE or other metabolites rather than as the parent
compound. The main point of interest is the ability of aquatic
organisms to take up large amounts of the compound, over time, from
water where DDT is present at very low concentrations, and to retain
it.
Risebrough et al. (1976) measured DDT in sea water and in mussels
( Mytilus sp.) from San Fransisco Bay and the French Mediterranean
coast. Concentration factors varied between 40 000 and 690 000 for DDT
and between 45 000 and 310 000 for DDE.
Eberhardt et al. (1971) applied radioactively labelled DDT, at a
rate of 220 g/ha, to a freshwater marsh and followed the distribution
of the compound and its metabolites. Concentration factors in ten
species of plants varied between 5500 and 84 000. Various invert-
ebrates showed high concentration factors: ramshorn snail
( Planorbidae ), 4700; backswimmer ( Notonectidae ), 10 000; crayfish
( Orconectes immunis ), 22 000; bloodworm ( Tendipes ), 25 000; and red
leech ( Erpobdella punctata ), 47 000. Reporting earlier on the same
study, over 15 months, Meeks (1968) showed that plants and invert-
ebrates accumulated DDT to a maximum mainly within the first week after
treatment, whereas vertebrates required longer to attain maximum
residues. Residues of DDT in the surface water and suspended particles
had fallen below detectable levels within 1 month. Residues in
sediments stabilized at about 0.3 mg/kg after 9 months.
3.2.4 Fish
The uptake of DDT from water is affected by the size of the fish;
smaller fish take up relatively more DDT from water than larger
specimens of the same species. A range in weight of mosquitofish
between 70 and 1000 mg led to a four-fold difference between the
smallest and largest fish in DDT uptake from water over 48 h (Murphy,
1971).
A rise in temperature results in increased uptake of DDT by fish
(Reinert et al., 1974). Rainbow trout were exposed to a single water
concentration of DDT (nominally 330 ng/litre) at temperatures of 5, 10,
or 15 °C; the actual concentrations of DDT in water varied with
temperature and were measured at 176, 137, and 133 ng/litre,
respectively, for 5, 10, and 15 °C. Whole body residues of DDT (total)
after 12 weeks exposure were 3.8, 5.9, and 6.8 mg/kg for the three
temperatures, respectively. Expressing the results as bioconcentration
factors to allow for the differences in dissolved DDT showed a similar,
clear increase in the relative amount of DDT taken up and retained
(Reinert et al., 1974).
Increasing salinity decreases DDT uptake significantly, but has
no effect on the uptake of DDE or TDE by fish (Murphy, 1970).
Increasing the salinity from 0.15o/oo to 10o/oo decreased DDT
uptake over 24 h from 22% of the dose to 18% (body residues decreased
from 658 to 329 ng). There was a further significant decrease in
uptake when the salinity was increased to 15o/oo (Murphy, 1970)
Fish accumulate DDT from food in a dose-dependent manner. When
Macek et al. (1970) fed rainbow trout on diets containing 0.2 or 1.0 mg
DDT/kg, the fish retained more than 90% of the dietary intake of DDT
(measured as total DDT) over the 90-day exposure period. The authors
estimated the time required for the elimination of 50% of accumulated
DDT to be 160 (± 18) days. When Warlen et al. (1977) fed Atlantic
menhaden on a diet containing 14C-labelled DDT at three dose levels,
the fish assimilated and retained between 17% and 27% of the cumulative
dose from food containing 0.58, 9.0, or 93 µg/kg. There was a
straight-line relationship between exposure time and body burden of
total DDT, with no tendency for residues to reach a plateau within the
45 days of feeding with DDT. At the end of the feeding period, the
fish had accumulated DDT or its metabolites, to levels of approximately
1.1, 11, and 110 µg/kg for the three doses respectively. The
biological half-time of DDT in the fish was estimated to be 428, 64,
and 137 days, for groups exposed to 0.58, 9.0, or 93 µg/kg diet,
respectively.
3.2.5 Terrestrial invertebrates
Relatively low concentration factors have been reported for
terrestrial molluscs by Dindal & Wurtzinger (1971), who also reviewed
the earlier literature. However, low concentration factors, derived
from short-term studies, can be misleading for these organisms because
of the high persistence of DDT in soil. Residues of DDT were as high
as 40 mg/kg and, therefore, molluscs represent a source of DDT which
will be concentrated by organisms which eat them. The same is true
for earthworms, which also show low concentration factors (Davis, 1971;
Edwards & Jeffs, 1974). Gish & Hughes (1982) investigated residues of
DDT and other pesticides in earthworms for 2 years following appli-
cation. They showed that body residue levels were cyclic, with higher
levels of DDT and its metabolites occuring between late spring and
early autumn and lower levels from late autumn to early spring. Peak
high levels occurred in May and low levels in January, coinciding with
the seasonal high and low activity periods of earthworms. These
changing residue levels presumably indicate that DDT is retained in
soil and that earthworms contain more of the residual metabolites when
they are processing more soil through the gut.
3.2.6 Birds
Laboratory studies on birds have shown them capable of accumulating
DDT from food, yielding high concentration factors (Table 2).
The accumulation of DDT and its metabolites in birds in the field
has been regularly and extensively reviewed (Moore, 1965; Moriarty,
1975; Newton, 1979). The results of an analysis of a long-term
sampling programme of birds in the United Kingdom (Cooke et al., 1982)
confirm many of the early theories. Birds with the highest residues of
DDT or its metabolites were either terrestrial predators feeding on
other birds or aquatic predators feeding on fish. Thus, residues of
DDE in the liver of the peregrine falcon, with birds as its principal
dietary component, averaged 7.56 mg/kg, whereas for the rough-legged
buzzard, with mammals as the principal dietary component, mean DDE
levels were 0.05 mg/kg over a period extending from the early 1960s to
the late 1970s.
There are marked geographical differences throughout the United
Kingdom, related to usage patterns of DDT (Cooke et al., 1982), and
also marked seasonal changes in residues. These seasonal changes
appear to relate more to physiological changes in body composition,
which occur with climatic and breeding seasons, than to the environ-
mental availability of pollutants. Some species, e.g., heron, barn
owl, and kingfisher, showed a decline in DDE residues with time, but
others, e.g., sparrowhawk, kestrel, and great-crested grebe, did not,
levels in 1977 being similar to those in 1963. Eventually residues of
DDT in wildlife decline with time after a ban is imposed on the use of
the pesticide. However, the highly persistent nature of DDE means that
significant residues will continue to be found for a considerable
period. The situation in the United Kingdom and the USA appears to be
broadly similar (O'Shea & Ludke, 1979).
3.2.7 Mammals
DDT is taken up by, and retained in, wild mammals. The degree of
uptake and retention varies with the species. In a study following a
single application of DDT to a forest to control spruce budworm at a
rate of 0.89 kg/ha, Dimond & Sherburne (1969) and Sherburne & Dimond
(1969) reported residues of DDT and its metabolites in mammals over 9
years. Herbivorous mice, voles, and hares contained less DDT than
carnivorous mink and insectivorous shrews. In herbivores, residues
approached pre-treatment levels after 6-7 years, whereas residues were
still significantly higher in shrews and mink than in the same species
taken from untreated areas 9 years after the single treatment with DDT.
In these species, the authors calculate that it would take at least 15
years for residues to reach background levels. They regard the high
residue levels in mammals at higher trophic levels as deriving
principally from DDT retained in the soil, since there is little long-
term retention on vegetation.
In a 3-year study, after treating a field ecosystem with 36Cl-
ring-labelled DDT at a dose rate of 0.92 kg/ha, Forsyth & Peterle
(1973) measured DDT residues in various tissues of two species of
shrew. The highest residue (135 mg/kg) occurred in fat, compared with
10, 10, and 4 mg/kg in liver, muscle, and brain, respectively. Shrews
of the species Blarina brevicauda released into treated areas accumu-
lated DDT to the same degree as resident shrews within 15-20 days of
exposure. Equilibrium between intake and excretion of DDT occurred
within approximately 30 days in muscle, liver, and brain and within
40 days in fat. The second species of shrew ( Sorex cinereus )
accumulated residue levels of DDT during the following 2 years which
were successively greater than levels present in the first year,
indicating that DDT was increasing in availability to this species
with the passage of time. The levels of DDT in muscle were not
influenced by sex but were influenced by breeding condition. Male
shrews with scrotal testes and lactating females developed lower
levels of DDT in muscle and viscera than did males with abdominal
testes or non-lactating females.
Benson & Smith (1972) measured levels of DDT and its metabolites in
deer exposed to DDT used for spruce budworm control, and found that, in
the year of spraying, there was up to 20 mg/kg in fat. Males had
considerably higher levels of DDT than females. Fawns also had higher
levels than their mothers, though this was from a small sample. The
majority of the residues consisted of p,p' -DDT, with almost insignifi-
cant levels of DDE. Five years later, the residue levels in males were
still higher than those in females, though these had fallen to about
1% of original levels. Most of the deer population was 3 years old or
less, and so the figures for 5 years after spraying represent DDT
ingested from the environment and not from direct exposure.
Some, though very little, DDT was detected in black bears by
Benson et al. (1974). There was no evidence that the area had been
directly sprayed with DDT. This study illustrates that there is a
general environmental contamination with DDT, which can be accumulated
by mammals, though to a small degree, without direct application of the
material to their habitat.
4. TOXICITY TO MICROORGANISMS
Appraisal
Aquatic microorganisms are more sensitive than terrestrial ones to
DDT.
An environmental exposure concentration of 0.1 µg/litre can cause
inhibition of growth and photosynthesis in green algae.
Repeated applications of DDT can lead to tolerance in some micro-
organisms.
There is no information on effects concerning the species compo-
sition of microorganism communities. Therefore, it is difficult to
extrapolate the relevance of single-culture studies to aquatic or
terrestrial ecosystems. However, since microorganisms are basic in
food chains, adverse effects on their populations would influence
ecosystems. Thus, DDT and its metabolites should be regarded as a
major environmental hazard.
Studies cited in this section will be restricted to those effects
produced by low concentrations of DDT. Some studies still use DDT at
concentrations above its water solubility. Reviews of other effects of
DDT and its analogues, at higher concentrations, on cell division and
several biochemical parameters have been produced by Luard (1973) and
Lal & Saxena (1979).
4.1 Bacteria and Cyanobacteria (Blue-green Algae)
Ledford & Chen (1969) cultured bacteria isolated from surface-
ripened cheese with 0.5 mg DDT/litre or 0.5 mg DDE/litre, but found no
effect on growth.
At a concentration of 10 µg/litre in the culture medium, DDT
stimulated the growth of the bacterium Escherichia coli (Keil et al.,
1972). Yields of cultures exposed to 100 µg/litre did not differ from
controls. There was no effect of DDT on denitrification (conversion of
nitrate to nitrite) at a concentration of 100 mg/kg in soil and,
similarly, no effect on this process when carried out by a bacterial
culture (Bollag & Henninger, 1976). DDT at up to 22 kg/ha did not
affect the numbers of soil bacteria in outdoor-treated plots (Bollen et
al., 1954), and five annual applications of DDT to a sandy loam soil
did not significantly affect the numbers of soil bacteria (Martin,
1966).
Concerning cyanobacteria (blue-green algae), Goulding & Ellis
(1981) found no effect on the growth of Anabaena variabilis at a DDT
concentration of 1 µg/litre. Batterton et al. (1972) suggested that
DDT reduced the tolerance of Anocystis nidulans to sodium chloride.
The organism is resistant to salt and to DDT, at concentrations up to
8000 mg/litre, but not to combinations of the two stressors.
4.2 Freshwater Microorganisms
Lee et al. (1976) showed that DDT inhibited photosynthesis in the
green alga Selenastrum capricornutum at concentrations between 3.6 and
36 µg/litre, inhibition increasing with time of exposure.
Two different species of green algae were shown to be resistant
to DDT and its metabolites, DDE and TDE, at concentrations up to
1000 mg/litre in culture. Scenedesmus and Dunaliella revealed rates of
photosynthetic uptake of 14C-labelled CO2 similar to those of
controls (Luard, 1973). Considerable variation exists between species
of microorganisms concerning the effect of DDT and its analogues;
resistance to DDT is not restricted to one taxonomic group, either
freshwater or marine (Luard, 1973). The source of the resistance is
unclear. The two species studied show very different characteristics;
Dunaliella has no cell wall, whereas Scenedesmus has a complex cell
wall. Since both show resistance to DDT, it is unlikely that the
chemical is excluded from the cell by the cell wall. Cell membranes
and chloroplast membranes are an alternative barrier to DDT uptake and
effect. It is not known how these structures might be involved in DDT
resistance; studies with isolated chloroplasts suggest that there is no
barrier to DDT uptake there.
Cole & Plapp (1974) found inhibition of growth and photosynthesis
of the green alga Chlorella pyrenoidosa with DDT at 1 µg/litre in the
medium. However, inhibition was inversely related to the number of
cells in the culture. With high cell counts, there was no
inhibition of either growth or photosynthesis with DDT present at up to
1 mg/litre. Inhibition only occurred at low cell densities in culture.
Goulding & Ellis (1981) found that the green alga Chlorella fusca
was affected by DDT at 0.1 µg/litre. The amount of inhibition of
growth varied with time and with the method of assessing the result.
Cell numbers were maximally affected (75% inhibition) after 72 hours,
and after 200 hours cell numbers had reached control levels. When
growth was assessed by chlorophyll content or biovolume, the initial
inhibition was more marked and cultures were only equivalent to
controls after 480 hours. The apparent anomaly is explained by
reductions in cell size in response to DDT.
Christie (1969) reported no effect of DDT on the growth of
Chlorella and attributed this to the ability of the organism to
metabolize the compound.
Lal & Saxena (1980) reported that DDT did not affect growth and DNA
synthesis in the ciliate Stylonychia notophora at concentrations of
1 mg/litre or less.
4.3 Marine Microorganisms
MacFarlane et al. (1972) showed that DDT, at concentrations
between 9.4 and 1000 µg/litre, reduced photosynthetic carbon
fixation and the cell content of chlorophyll a relative to controls
in a marine diatom Nitzschia delicatissima , over a 24-h period.
The diatom was cultured with DDT under four different light inten-
sities. The insecticide had the greatest effect at the highest light
intensity, where carbon fixation was reduced by 94% in water containing
100 µg DDT/litre. At higher DDT concentrations, there was no further
reduction in either carbon fixation or chlorophyll content.
The photosynthesis of several species of marine phytoplankton has
been found to be inhibited by DDT at concentrations of 100 µg/litre or
less (Wurster, 1968). Four different species showed increasing
inhibition up to DDT concentrations of 100 µg/litre, but no greater
effect at higher concentrations. A green alga, Pyramimonas , was
affected by DDT only at concentrations higher than 10 µg/litre. The
other three species, a diatom, a coccolithophore, and a dinoflagellate
were affected at DDT concentrations between 1 and 10 µg/litre. In
a similar study (Menzel et al., 1970) four different species of
marine phytoplankton were studied. Inhibition of photosynthesis,
where it occurred, followed a similar dose-response relationship.
For three species ( Skeletonema costatum , a diatom; Coccolithus
huxleyi , a coccolithophorid; and Cyclotella nana , a second diatom)
inhibition began between 1 and 10 µg DDT/litre and reached a maximum
at 100 µg/litre. The other organism, a green flagellate Dunaliella
tertiolecta , was unaffected by DDT at concentrations up to 1 mg/litre,
the highest exposure tested.
The marine dinoflagellate Exuviella baltica showed significant
inhibition of growth after exposure to DDT at concentrations as low as
0.1 µg/litre (Powers et al., 1979).
4.4 Soil Microorganisms
TDE had no significant effects on growth and reproduction of soil
amoebae except at concentrations higher than 1 mg/litre (Prescott &
Olson, 1972). Populations of protozoa in garden soil were reduced by
DDT at a concentration of 5 mg/kg (MacRae & Vinckx, 1973). Numbers
were still significantly reduced after 3 months.
4.5 Fungi
Two aquatic and one terrestrial fungi showed stimulated growth
in response to DDT present at concentrations of between 2 and
60 µg/litre of growth medium (Hodkinson & Dalton, 1973)
5. TOXICITY TO AQUATIC ORGANISMS
DDT and its derivatives are highly toxic to aquatic organisms;
water concentrations of a few micrograms per litre are sufficient to
kill a large proportion of populations of aquatic organisms in acute or
short-term exposure. In addition to its high short-term toxicity, DDT
also has long-term sublethal effects on aquatic organisms. Many
physiological and behavioural parameters have been reported to be
affected by the insecticide. This toxicity, coupled with its high
capacity for bioconcentration and biomagnification, means that DDT
presents a major hazard to aquatic organisms.
5.1 Aquatic Invertebrates
Appraisal
Both the acute and long-term toxicities of DDT vary between species
of aquatic invertebrates. Early developmental stages are more
sensitive than adults to DDT. Long-term effects occur after exposure
to concentrations ten to a hundred times lower than those causing
short-term effects.
DDT is highly toxic, in acute exposure, to aquatic invertebrate, at
concentrations as low as 0.3 µg/litre. Toxic effects include
impairment of reproduction and development, cardiovascular
modifications, and neurological changes. Daphnia reproduction is
adversely affected by DDT at 0.5 µg DDT/litre.
The influence of environmental variables (such as temperature,
water hardness, etc.) is documented but the mechanism is not fully
understood. In contrast to the data on DDT, there is less information
on the metabolites DDE or TDE. The reversibility of some effects once
exposure ceases has been reported, as well as the development of
resistance.
5.1.1 Short-term and long-term toxicity
The short-term toxicity to aquatic invertebrates is summarized in
Table 3.
Most aquatic invertebrates are killed by low water concentrations
of DDT and its metabolites, though the majority of the published data
is on DDT itself. Six invertebrate species studied by Macek & Sanders
(1970) showed 96-h LC50 values ranging from 1.8 to 54.0 µg/ litre.
Adult molluscs are relatively resistant to DDT and the compound has
been used to control crustacean pests on oyster beds (Loosanoff, 1959).
However, the larval stages of molluscs are affected by DDT; clam larvae
showed 90% mortality after exposure to DDT at 0.05 mg/litre (Calabrese,
1972). Molluscs exhibit effects on shell growth at low DDT concen-
trations. Tubifex worms are resistant to DDT; 3 mg/litre did not kill
any Tubifex tubifex (Naqvi & Ferguson, 1968). Many aquatic crustaceans
yield LC50 values less than 1 µg/litre. Muirhead-Thomson (1973)
showed that predator invertebrates, such as dragonfly nymphs, were
more tolerant of DDT than prey organisms. Since the prey organisms
are also food for fish, the balance of aquatic ecosystems could be
changed by very low levels of DDT. Lowe (1965) reported that juvenile
blue crabs ( Callinectes sapidus ), exposed to 0.25 µg DDT/litre for 9
months, grew and moulted normally; there were no apparent sublethal
effects. However, exposure to 5 µg DDT/litre killed all crabs.
The metabolite TDE has been studied in parallel tests with the
parent compound in some organisms. There is no consistent relationship
between the toxicity of the two compounds. TDE is considerably less
toxic to stonefly larvae than DDT, by a factor of about 100 (Sanders &
Cope, 1968). However, for other freshwater organisms TDE may have
similar, lower, or greater toxicity according to the organism and
duration of test (Table 3). For most marine invertebrates, DDT is most
toxic, followed by DDE and TDE (data from Mayer, 1987).
5.1.2 Physiological effects on aquatic invertebrates
Butler (1964) demonstrated a 50% reduction in shell growth in young
eastern oysters exposed for 96-h to DDT at 14 µg/litre. Roberts
(1975) showed that DDT at 50 µg/litre reduced the amplitude of
ventricular contractions in the isolated heart of the bivalve Mya
arenaria within 4 minutes. At higher concentrations, DDT stopped heart
contractions altogether. Recovery, even of the arrested heart, was
rapid after the immediate replacement of the DDT solution with clean
sea water.
Kouyoumjian & Uglow (1974) found that for the planarian worm
Polycelis felina , TDE was most toxic and DDT least toxic, with DDE
showing intermediate toxicity. Sublethal effects of DDT and TDE were
demonstrated. DDT reduced the rate of asexual fission. Both DDT and
TDE were shown to reduce the righting time of animals turned onto their
backs. This was presumed to be a nervous system effect.
Maki & Johnson (1975) report 50% reduction in three parameters of
reproduction in the water flea Daphnia magna at 0.5 µg/litre, for
total young produced, at 0.61 µg/litre for average brood size, and at
0.75 µg/litre for percentage of days reproducing.
In vitro effects on gill ATPases of two species of crab have been
reported (Jowett et al., 1978; Neufeld & Pritchard, 1979). There is a
transitory effect in vivo on gill ATPases and, thereby, an effect on
plasma osmolarity. However, this osmoregulatory effect soon disappears
(Pritchard & Neufeld, 1979). Leffler (1975) reported metabolic rate
elevation, decreased muscular coordination, inhibition of autotomy
reflex, and reduced carapace thickness/width ratio in juvenile crabs
exposed to DDT. Osmoregulation was not affected. The DDT was given in
the food of the crabs at a concentration of 0.8 mg/kg. DDT has been
found to accelerate limb regeneration and the onset of the next moult
in fiddler crabs (Weis & Mantel, 1976). The authors suggest that the
effect is on the central nervous system, with DDT causing changes in
neurosecretory activity.
Table 3. Toxicity of DDT and its derivatives to invertebrates
---------------------------------------------------------------------------------------------------------
Organismf Flow Temp Salinity Compound Parameter Water Reference
stata ( °C) o/oo concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine invertebrates
Eastern oyster (juv.) flow 30 23 DDTd 96-h EC50j 9 Mayer (1987)
(Crassostrea virginica) flow 12 25 DDEd 96-h EC50j 14 Mayer (1987)
flow 20 30 TDEd 96-h EC50j 25 Mayer (1987)
Shrimp stat 20 sea water DDTd 96-h LC50 0.4 McLeese &
(Crangon septemspinosa) stat 10 sea water DDTd 96-h LC50 31 Metcalfe (1980)
+ sediment
Mysid shrimp (adult) stat 25 23 DDTd 96-h LC50 0.45 Mayer (1987)
(Mysidopsis bahia) (0.39-0.52)
Pink shrimp (juv.) flow 24 28 DDTd 48-h LC50 0.6 Mayer (1987)
(Penaeus duorarum) flow 16 31 TDEd 48-h LC50 2.4 Mayer (1987)
White shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.7 Mayer (1987)
(Penaeus setiferus)
Grass shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.8 Mayer (1987)
(Palaemonetes pugio)
Brown shrimp (juv.) flow 28 17-27 DDEd 24-h LC50 52 Butler (1964)
(Penaeus aztecus) flow 28 17-27 DDEd 48-h LC50 28 Butler (1964)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata ( °C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Freshwater invertebrates
Water flea stat 20 192 138 8.2- DDTd 48-h LC50 1.1 (1.0-1.3) Randall et
8.5 al. (1979)
(Daphnia magna) stat 15 44 7.1 DDTd 48-h LC50 4.7 (2.8-5.6) Mayer &
Ellersieck
(1986)
stat 20 192 138 8.2- DDTe 48-h LC50 1.7 (1.5-1.8) Randall et
8.5 al. (1979)
statb 24 320- 7.6 DDT 14-day 0.67 (0.65-0.69) Maki &
340 (99%) LC50 Johnson
statb 24 320- 7.6 DDT 14-day 0.5 (0.48-0.52) (1975)
340 (99%) EC50g
statb 24 320- 7.6 DDT 14-day 0.61 (0.58-0.64) Maki &
340 (99%) EC50h Johnson
statb 24 320- 7.6 DDT 14-day 0.75 (0.71-0.79) (1975)
340 (99%) EC50i
stat 10 44 7.1 TDEd 48-h LC50 9.1 Mayer &
stat 21 44 7.1 TDEd 48-h LC50 8.9 Ellersieck
(1986)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 510 (230-1120) Berglind &
soft water stat 20.5 250 7.8-8.2 DDT 48-h LC50 1.1 (0.89-1.7) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 98 (75-127) Berglind &
50 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 1.3 (1.1-1.5) Dave (1984)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 71 (41-130) Berglind &
hard water stat 20.5 250 7.8-8.2 DDT 48-h LC50 0.68 (0.46-1.0) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 42 (32-56) Berglind &
300 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 0.5 (0.41-0.61) Dave
stat 20.5 50 7.8-8.2 DDT 24-h LC50 0.99 (0.66-1.49) (1984)
Water flea stat 15 44 7.1 DDTd 48-h LC50 0.36 (0.28-0.47) Mayer &
(Daphnia pulex) Ellersieck
(1986)
Water flea stat 15 44 7.1 DDTd 48-h LC50 2.5 (1.9-3.3) Mayer &
(Simocephalus stat 21 44 7.1 DDTd 48-h LC50 2.8 (2.3-3.5) Ellersieck
serrulatus) stat 15 44 7.1 TDEd 48-h LC50 3.2 (2.3-4.4) (1986)
stat 21 44 7.1 TDEd 48-h LC50 4.5 (3.1-6.6)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 21 35 44 7.1 TDEd 24-h LC50 4.6 (3.6-5.8) Sanders
(Gammarus fasciatus) stat 21 35 44 7.1 TDEd 96-h LC50 0.6 (0.05-1.2) (1972)
stat 21 35 44 7.1 DDTd 24-h LC50 15 (9.0-20) Sanders
stat 21 35 44 7.1 DDTd 96-h LC50 3.2 (1.8-5.6) (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 3.2 (2.1-4.3) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.86 (0.42-1.3) (1972)
stat 21 260 272 7.4 DDTd 24-h LC50 4.2 (1.8-5.6) Sanders
stat 21 260 272 7.4 DDTd 48-h LC50 3.1 (1972)
stat 21 260 272 7.4 DDTd 96-h LC50 1.8 (1.0-3.1) Sanders
(1972)
stat 21 260 272 7.4 DDTd 120-h LC50 0.32 Sanders
flow 18-21 260 272 7.4 DDTd 24-h LC50 1.1 (1972)
flow 18-21 260 272 7.4 DDTd 48-h LC50 1.0 Sanders
flow 18-21 260 272 7.4 DDTd 96-h LC50 0.8 (1972)
flow 18-21 260 272 7.4 DDTd 120-h LC50 0.6 Sanders
(1972)
Scud stat 21 44 7.1 DDTd 24-h LC50 4.7 (3.2-7.0) Mayer &
(Gammarus lacustris) stat 21 44 7.1 DDTd 96-h LC50 1.0 (0.68-1.5) Ellersieck
(1986)
stat 15 DDTe 96-h LC50 9.0 Gaufin et
al. (1965)
Glass shrimp stat 21 260 272 7.4 DDTd 24-h LC50 6.8 (6.2-7.5) Sanders
(Palaemonetes stat 21 260 272 7.4 DDTd 48-h LC50 4.7 (1972)
kadiakensis) stat 21 260 272 7.4 DDTd 96-h LC50 2.3 (1.3-4.9) Sanders
stat 21 260 272 7.4 DDTd 120-h LC50 1.0 (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 11 (8.4-16) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.68 (0.47-1.1) (1972)
flow 18-21 260 272 7.4 DDTd 24-h LC50 9.4 Sanders
flow 18-21 260 272 7.4 DDTd 48-h LC50 7.7 (1972)
flow 18-21 260 272 7.4 DDTd 96-h LC50 3.5 Sanders
flow 18-21 260 272 7.4 DDTd 120-h LC50 1.3 (1972)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Crayfish (Orconectes nais)
mature stat 21 260 7.4 DDTd 24-h LC50 1100 (1000-1400) Sanders
stat 21 260 7.4 DDTd 96-h LC50 100 (80-120) (1972)
1 day old - 15g stat 21 260 7.4 DDTd 24-h LC50 1.4 (1.1-4.2) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.3 (0.18-0.5) (1972)
1 week old - 20g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.18 (0.12-0.3) (1972)
2 weeks old - 23g stat 21 260 7.4 DDTd 24-h LC50 1.2 (0.9-5.5) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.2 (0.16-1.1) (1972)
3 weeks old - 30g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.24 (0.1-0.6) (1972)
5 weeks old - 50g stat 21 260 7.4 DDTd 24-h LC50 3.2 (1.8-8.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.9 (0.7-1.4) (1972)
8 weeks old - 500g stat 21 260 7.4 DDTd 24-h LC50 45 (40-52) Sanders
stat 21 260 7.4 DDTd 96-h LC50 28 (24-36) (1972)
10 weeks old - 1200g stat 21 260 7.4 DDTd 24-h LC50 50 (48-56) Sanders
stat 21 260 7.4 DDTd 96-h LC50 30 (26-42) (1972)
Sowbug (isopod) stat 21 35 7.1 DDTd 24-h LC50 8.7 (4.9-13.0) Sanders
(Asellus brevicaudus) stat 21 35 7.1 DDTd 96-h LC50 4.0 (1.2-6.5) (1972)
stat 21 35 7.1 TDEd 24-h LC50 18 (14-25) Sanders
stat 21 35 7.1 TDEd 96-h LC50 10 (7.0-14) (1972)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 48 Gaufin et
(Hydropsyche californica) 12 al. (1965)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 175 Gaufin et
(Arctopsyche grandis) 12 al. (1965)
May fly (nymph) stat 8.8- DDTe 96-h LC50 25 Gaufin et
(Ephemerella grandis) 10 al. (1965)
Stonefly (naiad) stat 11- DDTe 96-h LC50 320 Gaufin et
(Acroneuria pacifica) 12 al. (1965)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Stonefly (naiad) stat 11- DDTe 96-h LC50 1800 Gaufin et
(Pteronarcys 12 al. (1965)
californica) stat 15.5 35 DDT 24-h LC50 41 (27-62) Sanders &
stat 15.5 35 DDT 48-h LC50 19 (14-27) Cope (1968)
stat 15.5 35 DDT 96-h LC50 7 (4.9-9.9) Sanders &
stat 15.5 35 TDE 24-h LC50 3000 (2100-4300) Cope (1968)
stat 15.5 35 TDE 48-h LC50 1100 (800-1500) Sanders &
stat 15.5 35 TDE 96-h LC50 380 (280-520) Cope (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 12 (8.8-16) Sanders &
(Pteronarcella badia) stat 15.5 35 DDT 48-h LC50 9 (7-11) Cope
stat 15.5 35 DDT 96-h LC50 1.9 (1.3-2.7) (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 16 (12-20) Sanders &
(Claasenia sabulosa) stat 15.5 35 DDT 48-h LC50 6.4 (4.9-8.3) Cope
stat 15.5 35 DDT 96-h LC50 3.5 (2.9-4.2) (1968)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions (DDT
concentration in water continuously maintained).
b Static conditions but test solution renewed every 24 h.
c Alkalinity and hardness expressed as mg CaCO3/litre.
d Technical grade (99%).
e Emulsifiable concentrate (25% active ingredient).
f Juv. = juvenile.
g Value based on total number of young produced.
h Value based on average brood size.
i Value based on % days reproducing.
j Effect on shell growth.
Eggs of the Chironomid midge, contaminated with DDE by exposure of
the female during ovarian development, failed to hatch as many adults
as uncontaminated eggs. DDE in the water had less of an effect than
DDE contamination within the eggs obtained from the female. The
females had been maintained in water containing 30 µg DDE/litre; eggs
were kept in water containing 20 µg DDE/litre (Derr & Zabik, 1972).
Crayfish populations exposed over long periods to DDT develop some
tolerance to the insecticide (Albaugh, 1972). In 48-h tests, LC50
values for the crayfish Procambarus clarkii were 3.0 (2.5-3.6) µg/litre
for the unexposed population, and 7.2 (5.8-8.8) µg/litre for the
exposed population (95% confidence limits in parentheses). Naqvi &
Ferguson (1968) demonstrated the development of tolerance to DDT after
exposure to the insecticide, in a wide variety of aquatic
invertebrates, including cyclopoid copepods, tubifex worms, and pond
snails. These tolerant populations occurred in the Mississippi delta
in areas of cotton cultivation.
5.2 Fish
Appraisal
DDT is highly toxic to fish; the 96-h LC50s reported (static
tests) range from 1.5 to 56 µg/litre (for largemouth bass and guppy,
respectively). Smaller fish are more susceptible than larger ones of
the same species. An increase in temperature decreases the toxicity of
DDT to fish.
The behaviour of fish is influenced by DDT. Goldfish exposed to
1 µg/litre exhibit hyperactivity. Changes in the feeding of young
fish are caused by DDT levels commonly found in nature, and effects on
temperature preference have been reported.
Residue levels of > 2.4 mg/kg in eggs of the winter flounder result
in abnormal embryos in the laboratory, and comparable residue levels
have been found to relate to the death of lake trout fry in the wild.
Cellular respiration may be the main toxic target of DDT since
there are reports of effects on ATPase.
The toxicity of TDE and DDE has been less studied than that of DDT.
However, the data available show that TDE and DDE are both less toxic
than DDT.
The exact mode of action of DDT in fish remains unclear. There
have been many different suggestions to explain both lethal and
sublethal effects. Most of these are primarily the result of effects
on membranes. DDT is very soluble in lipid and, therefore, dissolves
in the lipid component of membranes. It may interfere both with
membrane function and with many enzyme systems that are located on
membranes. It has been shown experimentally to interfere with the
normal function of so many systems that a primary action of DDT is
difficult to determine.
5.2.1 Short-term and long-term direct toxicity to fish
The short-term toxicity of DDT to fish is summarized in Table 4.
The relatively few studies on TDE (Gardner, 1973; Korn & Earnest,
1974; Mayer & Ellersieck, 1986; Mayer, 1987) show it to be less toxic
than DDT, in the same test system, by factors of 5-10. The still fewer
studies on DDE indicate a similarly lowered toxicity relative to the
parent compound (Mayer & Ellersieck, 1986; Mayer, 1987). Whilst there
is some variation between species, DDT has proved highly toxic to all
fish tested; static 24-h LC50 values range from 2.1 µg/litre for the
largemouth bass (Mayer & Ellersieck, 1986) to 180 µg/litre for the
goldfish (Henderson et al., 1959). For 96-h tests, LC50 values range
from 1.5 µg/litre for largemouth bass (Mayer & Ellersieck, 1986) to
56 µg/litre for the guppy (Henderson et al., 1959). Several authors
have stated that DDT toxicity varies somewhat with temperature and
water hardness.
Buhler et al. (1969) studied the long-term effects, over 95 days,
of feeding DDT-contaminated diets to juvenile chinook and coho salmon.
The DDT was dissolved in corn-oil and then incorporated into a semi-
synthetic diet. Fish were fed until they stopped actively taking the
slowly sinking food. Pure p,p' -DDT was slightly more toxic to
juvenile salmon than the technical product, and chinook salmon were
2 to 3 times more sensitive to the same dose of DDT in the diet than
coho salmon. Size was an important factor in the toxicity of DDT,
smaller fish being more susceptible than larger ones. The authors
estimated, by extrapolation, a 90-day LD50 value of 27.5 µg/kg per
day for chinook and 64 µg/kg per day for coho salmon juveniles. In
fish exposed to higher doses of DDT, pre-death symptoms were marginal.
Some increased agitation and slight photophobia were reported. Fish
exposed to low doses of DDT took longer to die, and other symptoms were
noted. Many individuals developed ulceration of the nasal area. This
spread over the head and in some cases eyes were lost. Pathological
examination showed a specific and severe kidney lesion; this was
limited to one short section of the distal convoluted tubule, which
eventually degenerated almost completely. The authors suggested this
as the main lethal lesion in the fish.
In a later study (Buhler & Shanks, 1970), the same authors showed
that median survival time was directly proportional to body weight in
young coho salmon fed technical DDT. Fish were all given a diet
containing 200 mg DDT/kg and food consumption was monitored for each
group of fish. The main effect of body size on DDT lethality was
related to the intake of the chemical by the fish; smaller fish ate
more of the contaminated diet and consequently received the greatest
dose in mg/kg bodyweight terms. However, even after correcting for
dosage received, the smaller fish were more susceptible than larger
ones. The authors suggested that the lower lipid content of smaller
fish might have accounted for the remaining difference. Twelve groups
of 100 fish ranged in weight (average for each group) from 3 to 15 g.
Total DDT intake ranged from 0.4 to 3 mg/fish; daily intake was higher
in the smaller fish at 3 mg/kg per day, falling to 1.3 mg/kg per day
for the largest. The estimated LC50 ranged from 95 mg/kg for the
smallest to 135 mg/kg for the largest fish, and median survival time
increased from 30 days for the smallest fish to 106 days for the
largest.
Crawford & Guarino (1976) exposed killifish ( Fundulus heteroclitus )
to a twice-repeated schedule of 24 h in water containing DDT at a
concentration of 0.1 mg/litre and 24 h in clean water. At this
exposure level, there was a delay in the rate of development of ferti-
lized eggs but no apparent effect on the hatched fry. Fertilization of
killifish eggs was diminished when insemination was carried out in sea
water containing DDT at 0.1 mg/litre. Mortality at a late stage of
embryo development has been reported for a variety of salmonids and
related to egg residues of DDT (Allison et al., 1964, for cutthroat
trout; Burdick et al., 1964, for lake trout; Macek, 1968, for brook
trout; and Johnson & Pecor, 1969, for coho salmon).
Smith & Cole (1973) reported effects on embryos developing from
eggs laid by adult winter flounder ( Pseudopleuronectes americanus )
that were exposed to 2 µg DDT/litre for various times and, therefore,
accumulated different residue levels in the eggs. These residue levels
varied from 1.15 to 3.70 mg DDT/kg and from 0.07 to 0.4 mg DDE/kg.
Embryos showed abnormal gastrulation and a high incidence (mean 39%)
of vertebral deformities. Bone erosion and haemorrhaging at the
vertebral junctures were often associated with the vertebral deform-
ities.
Halter & Johnson (1974) report that DDT is toxic to the early
life-stages of coho salmon. Mean survival times were considerably
reduced by water concentrations of DDT greater than 0.5 µg/litre.
5.2.2 Sublethal behavioural effects on fish
Hansen (1969) and Hansen et al. (1972) investigated the avoidance
of DDT by sheepshead minnows and mosquitofish in a 'Y'-shaped avoidance
maze. Although there was some statistically significant avoidance of
DDT when fish were given the choice between DDT and clean water, this
only occurred at concentrations of the insecticide above the 24-h
LC50. Fish of both species, when given the choice between DDT at 0.1
and 0.01 mg/litre, chose the higher concentration of the chemical.
This suggests that the perception of DDT is poor and that fish could
not reliably avoid DDT in water at toxic concentrations.
Olofsson & Lindahl (1979) administered either 0.5 or 1.0 mg DDT/kg
body weight to cod by oral intubation. There was a significant effect,
at the higher dose but not the lower one, on the ability of the fish to
compensate its posture to cope with a rotating tube in which it was
swimming.
Hansen (1972) allowed mosquitofish to select a desired salinity in
a fluvarium with a salinity gradient. Fish selected a higher salinity
than controls when exposed to DDT, but only at exposure levels which
caused some mortality. The author suggested that DDT might have affec-
ted the osmoregulatory ability of the mosquitofish. Other possible
explanations include a change in sensitivity of nerves to stimuli or a
preference for the pre-exposure salinity, which was 15 g/litre.
Table 4. Toxicity of DDT and its derivatives to fish
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Salinity Compound Parameter Water Reference
(g)/ stata perat- o/oo concen-
agef ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine fish
Dwarf perch 1.2-11.0 Stat 13 28 DDTc 96-h LC50 4.6 Earnest &
(Micrometrus minimus) 1.2-11.0 flowb 14-18 26-28 DDTc 96-h LC50 0.26 Benville
(0.13-0.52) (1972)
Shiner perch 1.2-11.0 stat 13 26 DDTc 96-h LC50 7.6 Earnest &
(Cymatogaster aggregata) 1.2-11.0 flowb 14-18 13-23 DDTc 96-h LC50 0.45 Benville
(0.21-0.94) (1972)
Striped bass 2.7 flowb 17 28 DDT(77%) 96-h LC50 0.53 Korn &
(Morone saxatilis) (0.38-0.84) Earnest
0.6 flowb 17 30 TDEc 96-h LC50 2.5 (1974)
(1.6-4.0)
Sheepshead minnow juv. flow 15 30 DDTc 48-h LC50 2.0 Mayer
(Cyprinodon variegatus) (1987)
Longnose killifish juv. flow 15 30 DDTc 48-h LC50 2.8 Mayer
(Fundulus similis) juv. flow 16 28 TDEc 48-h LC50 42.0 (1987)
Pinfish juv. flow 22 29 DDTc 48-h LC50 0.3 Mayer
(Lagodon rhomboides) (1987)
Striped mullet juv. flow 15 30 DDTc 48-h LC50 0.4 Mayer
(Mugil cephalus) (1987)
Spot juv. flow 12 26 DDEc 48-h LC50 > 100 Mayer
(Leiostomus xanthurus) juv. flow 26 30 TDEc 48-h LC50 20.0 (1987)
Three-spined 0.4-0.8 stat 20 5 DDT 24-h LC50 22.0 Katz
stickleback 0.4-0.8 stat 20 5 DDT 48-h LC50 21.0 (1961)
(Gasterosteus 0.4-0.8 stat 20 5 DDT 72-h LC50 18.5 Katz
aculeatus) 0.4-0.8 stat 20 5 DDT 96-h LC50 18.0 (1961)
0.4-0.8 stat 20 25 DDT 24-h LC50 18.0 Katz
0.4-0.8 stat 20 25 DDT 48-h LC50 15.0 (1961)
0.4-0.8 stat 20 25 DDT 72-h LC50 14.5 Katz
0.4-0.8 stat 20 25 DDT 96-h LC50 11.5 (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Freshwater fish
Black bullhead 1.2 stat 18 44 7.1 DDTc 24-h LC50 36.8 Mayer &
(Ictalurus melas) (20.3-67.0)
1.2 stat 18 44 7.1 DDTc 96-h LC50 4.8
(3.4-6.8) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 26.2
(22.0-31.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 5.1
(3.9-6.7) Ellersieckg
Channel catfish 1.5 stat 18 44 7.1 DDTc 24-h LC50 22.0
(Ictalurus punctatus) (18.2-26.5) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 21.5
(17.7-26.1) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 18.4
(13.7-24.7) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 17.3
(13.0-23.1) Ellersieckg
0.7 stat 18 44 7.1 DDTc 24-h LC50 17.9
(12.7-25.3) Mayer &
0.7 stat 18 44 7.1 DDTc 96-h LC50 6.9
(5.7-8.5) Ellersieckg
1.6 stat 18 44 7.1 DDTc 24-h LC50 44.0
(37.0-52.0) Mayer &
1.6 stat 18 44 7.1 DDTc 96-h LC50 22.0
(19.0-26.0) Ellersieckg
1.4 stat 18 44 7.1 DDTc 24-h LC50 30.0
(22.0-41.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 16.0
(9.4-29.0) Ellersieckg
1.4 stat 18 272 7.7 DDTc 24-h LC50 29.0
(20.0-41.0) Mayer &
1.4 stat 18 272 7.7 DDTc 96-h LC50 7.0
(4.3-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Atlantic salmon 0.45 stat 12 40 7.5 DDTc 24-h LC50 6.2
(Salmo salar) (4.6-8.4) Mayer &
0.45 stat 12 40 7.5 DDTc 96-h LC50 1.8
(1.3-2.6) Ellersieckg
0.5 stat 12 44 7.5 DDEc 96-h LC50 96.0
(52.1-177) Mayer &
Ellersieckg
Coho salmon 2.7-4.1 stat 20 45-57 6.8-7.4 DDT 24-h LC50 66.0 Katz (1961)
(Oncorhynchus kisutch)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 48-h LC50 46.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 72-h LC50 44.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 96-h LC50 44.0 Katz (1961)
1.0 stat 13 44 7.1 DDTc 24-h LC50 10.0
(7.0-12.0) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 4.0
(3.0-6.0) Ellersieckg
6.0 stat 13 40 7.1 DDTc 24-h LC50 26.9
(18.1-40.0) Mayer &
6.0 stat 13 40 7.1 DDTc 96-h LC50 19.3
(9.6-38.8) Ellersieckg
Chinook salmon 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 24-h LC50 38.0 Katz (1961)
(Oncorhynchus
tshawytscha) 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 48-h LC50 17.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 72-h LC50 14.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 96-h LC50 11.5 Katz (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout 0.9 stat 7 44 7.1 DDTc 24-h LC50 7.5
(Salmo gairdneri) (6.7-8.3) Mayer &
0.9 stat 7 44 7.1 DDTc 96-h LC50 4.1
(3.6-4.6) Ellersieckg
0.9 stat 13 44 7.1 DDTc 24-h LC50 8.2
(7.2-9.2) Mayer &
0.9 stat 13 44 7.1 DDTc 96-h LC50 4.7
(4.2-5.3) Ellersieckg
0.9 stat 18 44 7.1 DDTc 24-h LC50 12.0
(1.0-13.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 5.8
(5.2-6.5) Ellersieckg
3.2 stat 20 45-57 6.8-7.4 DDT 24-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 48-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 72-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 96-h LC50 42.0 Katz (1961)
1.8 flow 17 272 7.4 DDTc 96-h LC50 > 3.0 Mayer &
0.8 stat 12 44 7.1 DDEc 96-h LC50 32.0
(26.0-40.0) Ellersieckg
1.0 stat 12 44 7.1 TDEc 96-h LC50 70.0
(57.0-87.0) Mayer &
1.0 stat 12 272 7.4 TDEc 96-h LC50 70.0
(58.0-85.0) Ellersieckg
Cutthroat trout 1.0 stat 13 44 7.1 DDTc 24-h LC50 8.4
(Salmo clarki) (7.6-9.2) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 5.5
(4.7-6.4) Ellersieckg
1.8 stat 9 162 7.4 DDTc 24-h LC50 11.3
(9.4-13.6) Mayer &
1.8 stat 9 162 7.4 DDTc 96-h LC50 7.9
(6.5-9.7) Ellersieckg
Brown trout 1.7 stat 13 44 7.1 DDTc 96-h LC50 1.8
(Salmo trutta) (1.3-2.5) Mayer &
Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Northern pike 0.7 stat 18 272 7.4 DDTc 24-h LC50 5.5 Mayer &
(Esox lucius) 0.7 stat 18 272 7.4 DDTc 96-h LC50 2.7 Ellersieckg
Guppy 0.1-0.2 stat 25 18 20 7.4 DDTc 24-h LC50 135 Henderson
(Lebistes 0.1-0.2 stat 25 18 20 7.4 DDTc 48-h LC50 72.0 et al.
reticulatus) 0.1-0.2 stat 25 18 20 7.4 DDTc 96-h LC50 56.0 (1959)
River shiner 0.3 stat 18 44 7.1 DDTc 24-h LC50 6.7
(Notropis blennius) (4.9-9.1) Mayer &
0.3 stat 18 44 7.1 DDTc 96-h LC50 5.8
(3.6-9.1) Ellersieckg
Fathead minnow 1.2 stat 18 44 7.1 DDTc 24-h LC50 14.2
(Pimephales (11.0-18.0) Mayer &
promelas) 1.2 stat 18 44 7.1 DDTc 96-h LC50 12.4
(10.0-15.4) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 13.8
(10.3-18.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 13.2
(10.1-17.3) Ellersieckg
0.9 flow 12 314 7.6 DDTc 96-h LC50 9.9
(6.5-15.0) Mayer &
Ellersieckg
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 56.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 42.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 24-h LC50 78.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDTc 48-h LC50 68.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 96-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 24-h LC50 32.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDT 48-h LC50 26.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 96-h LC50 26.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 24-h LC50 29.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDT 48-h LC50 27.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 96-h LC50 26.0 et al. (1959)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Mosquitofish 0.2 stat 25 DDTc 24-h LC50 22.7
(Gambusia affinis) (16.6-31.1) El-Sebae (1987)
0.2 stat 25 DDTc 96-h LC50 9.9
(7.3-13.4) El-Sebae (1987)
0.2 stat 25 DDTe 24-h LC50 58.6
(43.2-79.5) El-Sebae (1987)
0.2 stat 25 DDTe 96-h LC50 27.7
(21.3-36.0) El-Sebae (1987)
Bluegill sunfish 0.26 stat 19 138 192 8.2-8.5 DDTc 96-h LC50 3.4
(Lepomis macrochirus) (2.6-4.1) Randall
0.26 stat 19 138 192 8.2-8.5 DDT 96-h LC50 9.0
(7.4-10.6) et al. (1979)
(25%)
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 26.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 21.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 21.0 (1959)
1.5 stat 18 44 7.1 DDTc 24-h LC50 11.5
(8.4-16.0) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 8.6
(6.2-12.0) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 10.0
(8.5-12.9) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 6.3
(4.3-9.3) Ellersieckg
0.9 stat 17 44 7.1 DDEc 96-h LC50 240
(201-286) Mayer &
0.9 stat 24 44 7.4 TDEc 24-h LC50 56.0
(46.0-68.0) Ellersieckg
0.9 stat 24 44 7.4 TDEc 96-h LC50 42.0
(36.0-49.0) Mayer &
Ellersieckg
Redear sunfish 3.2 stat 24 44 7.1 DDTc 24-h LC50 19.0 Mayer &
(Lepomis microlophus) 3.2 stat 24 44 7.1 DDTc 96-h LC50 15.0 Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Green sunfish 1.1 stat 18 44 7.1 DDTc 24-h LC50 16.9
(Lepomis cyanellus) (12.7-22.3) Mayer &
1.1 stat 18 44 7.1 DDTc 96-h LC50 10.9
(7.3-15.6) Ellersieckg
0.8 stat 18 44 7.1 DDTc 24-h LC50 18.0
(13.0-24.0) Mayer &
0.8 stat 18 44 7.1 DDTc 96-h LC50 6.5
(4.1-10.4) Ellersieckg
1.1 stat 18 272 7.7 DDTc 24-h LC50 19.8
(15.0-25.6) Mayer &
1.1 stat 18 272 7.7 DDTc 96-h LC50 9.9
(6.4-15.0) Ellersieckg
Largemouth bass 0.8 stat 18 44 7.1 DDTc 24-h LC50 3.7
(Micropterus (3.1-4.5) Mayer &
Salmoides) 0.8 stat 18 44 7.1 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.8 stat 18 272 7.4 DDTc 24-h LC50 2.1
(1.6-2.9) Mayer &
0.8 stat 18 272 7.4 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.7 stat 18 44 7.1 TDEc 24-h LC50 50.0
(35.0-71.0) Mayer &
0.7 stat 18 44 7.1 TDEc 96-h LC50 42.0
(34.0-51.0) Ellersieckg
Black crappie 1.0 stat 18 44 7.1 DDTc 24-h LC50 6.5
(Pomoxis (5.4-7.8) Mayer &
nigromaculatus) 1.0 stat 18 44 7.1 DDTc 96-h LC50 5.6
(4.6-6.7) Ellersieckg
Yellow perch 1.4 stat 18 44 7.1 DDTc 24-h LC50 10.0
(Perca flavescens) (8.0-12.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 9.0
(7.0-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Walleye 1.4 stat 18 44 7.1 DDTc 24-h LC50 4.2
(Stizostedion v. (3.2-5.6) Mayer &
vitreum) 1.4 stat 18 44 7.1 DDTc 96-h LC50 2.9
(2.4-3.5) Ellersieckg
1.3 stat 18 272 7.4 DDTc 24-h LC50 4.6
(3.9-5.4) Mayer &
1.3 stat 18 272 7.4 DDTc 96-h LC50 4.6
(3.9-5.4) Ellersieckg
1.0 stat 18 44 7.1 TDEc 24-h LC50 20.0
(16.0-24.0) Mayer &
1.0 stat 18 44 7.1 TDEc 96-h LC50 14.0
(11.0-19.0) Ellersieckg
Tilapia 0.8 stat 24 44 7.1 DDTc 24-h LC50 19.0
(Tilapia mossambica) (16.0-23.0) Mayer &
0.8 stat 24 44 7.1 DDTc 96-h LC50 17.0
(14.0-21.0) Ellersieckg
0.8 stat 24 272 7.4 DDTc 24-h LC50 15.0
(13.0-17.0) Mayer &
0.8 stat 24 272 7.4 DDTc 96-h LC50 14.0
(12.0-16.0) Ellersieckg
0.8 flow 18 272 7.4 DDTc 24-h LC50 24.0
(17.0-32.0) Mayer &
0.8 flow 18 272 7.4 DDTc 96-h LC50 5.1
(3.2-8.1) Ellersieckg
Tilapia 0.8 stat 25 DDTc 24-h LC50 21.8
(Tilapia zilli) (17.0-28.0) El-Sebae (1987)
0.8 stat 25 DDTc 96-h LC50 15.5
(11.7-20.6) El-Sebae (1987)
0.8 stat 25 DDTe 24-h LC50 12.8
(9.6-17.1) El-Sebae (1987)
0.8 stat 25 DDTe 96-h LC50 9.5
(7.4-12.3) El-Sebae (1987)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Goldfish 1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 180 Henderson
(Carassius auratus) 1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 47.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 36.0 (1959)
0.9 stat 18 44 7.1 DDTc 24-h LC50 24.0
(17.0-33.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 15.5
(9.1-26.0) Ellersieckg
0.9 stat 18 272 7.4 DDTc 24-h LC50 22.2
(16.0-31.1) Mayer &
0.9 stat 18 272 7.4 DDTc 96-h LC50 14.7
(10.0-20.0) Ellersieckg
Common carp 0.6 stat 18 44 7.1 DDTc 24-h LC50 14.0
(Cyprinus carpio) (10.0-19.0) Mayer &
0.6 stat 18 44 7.1 DDTc 96-h LC50 9.7
(7.4-12.9) Ellersieckg
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions
(DDT concentration in water continuously maintained).
b Intermittent flow-through conditions.
c Technical grade (99%).
d Alkalinity and hardness expressed as mg CaCO3/litre.
e 25% emulsifiable concentrate.
f Juv. = juvenile.
g 1986.
Peterson (1973) monitored the selection of temperature by
juvenile Atlantic salmon ( Salmo salar ) previously exposed to DDT or
its metabolites. Low concentrations produced no effect on tem-
perature selection, but at higher levels of exposure the temperature
selected by the fish increased. Fish were most sensitive, in this
respect, to p,p' -DDE and showed decreasing sensitivity to
o,p' -DDT, p,p' -TDE, and p,p' -DDT. Increasing the exposure to
p,p' -DDE from 0 to 1.0 mg/litre increased the preferred temperature
from about 16 °C to 21 °C. There was no effect of p,p' -DDA on
temperature selection at concentrations as high as 8 mg/litre. In a
similar experiment, where brook trout (Salvelinus fontinalis) were
exposed to a vertical rather than horizontal temperature gradient, fish
previously exposed to p,p' -DDT and p,p' -TDE selected higher
temperatures than controls. Conversely, Gardner (1973) found that DDT
and its analogues induced selection of lower temperatures by the same
species of fish over a dose range between 0 and 50 µg/litre; DDE did
not produce any temperature preference. Ogilvie & Miller (1976)
reported that Atlantic salmon exposed to DDT at a concentration of
50 µg/litre selected higher temperatures, the effect persisting for at
least 4 weeks after exposure. The authors suggested that the tempera-
ture selection response to DDT exposure is "biphasic". At low
exposure levels, similar to those used by Gardner (1973), lower
temperatures are selected, whilst higher temperatures are preferred at
higher exposure levels.
Dill & Saunders (1974) exposed the eggs of Atlantic salmon
at gastrulation to DDT at water concentrations of 5, 10, 50, or
100 µg/litre, and observed behavioural development in hatched fry over
30 days following hatch. The two highest doses of DDT impaired balance
and retarded behavioural development of the fry (i.e., the appearance
of normal behaviour patterns was delayed). The authors considered that
the effects observed would affect predation rates and feeding, in
young fish, at "realistic" DDT exposure levels in the wild.
Davy et al. (1973) reported that exposure to DDT, at 10 µg/litre
for 4 days, affected the exploratory behaviour of goldfish experi-
encing a novel environment. They attributed the effect to a central
nervous system lesion caused by DDT. Weis & Weis (1974) showed an
increase in individual activity and an increase in school-size in
groups of goldfish exposed to DDT at 1 µg/litre for 7 days. After a
frightening stimulus, schools scattered further and did not regroup as
readily as control fish. The transfer of fish to clean water led to
a return to normal behaviour within one week. An effect on the
locomotor behaviour of goldfish after exposure to 10 µg DDT/litre per-
sisted for the remainder of the observation period of 130-139 days
(Davy et al., 1972).
5.2.3 Physiological effects on fish
Hanke et al. (1983) investigated the effects of DDT on a range of
physiological functions in carp ( Cyprinus carpio ). At water concen-
trations of 100 or 500 µg/litre, the insecticide induced changes in
plasma cortisol and glucose levels, liver glycogen level, and plasma
and brain acetylcholinesterase activity. The response was biphasic in
all cases. Initially, after 6 hours, there was a stimulation of these
parameters which, within 24 hours, changed to an inhibition.
Ramalingam & Ramalingam (1982) reported that the chronic effect of DDT
on glycogen utilization in fish led to the use of protein as an energy
source. The protein content of tissues declined after chronic exposure
to DDT.
Janicki & Kinter (1971) found that DDT impaired fluid absorption in
the intestinal sacs of eels adapted to sea water and exposed to the
insecticide at 50 µg/litre. DDT also inhibited Na+-, K+-, and
Mg2+-dependent ATPases in homogenates of the intestinal mucosa. In a
later study, Kinter et al. (1972) showed that plasma osmolarity was
also affected in sea-water-adapted eels exposed to DDT (1 mg/litre)
for 9 to 10 hours. Haux & Larsson (1979) reported effects of DDT on
plasma electrolytes in the flounder Platichthys flesus kept in
hypotonic, brackish water. The fish were force-fed with DDT in gelatin
capsules to give a total DDT dose of 1.5 or 15.0 mg/kg body weight.
Plasma sodium was reduced but not significantly; plasma chloride was
significantly reduced in a dose-related manner after 3 weeks but not
after 6 weeks. Waggoner & Zeeman (1975) reported similar effects on
plasma electrolytes in the black surfperch ( Embiotoca jacksoni ), but
only at high DDT exposure levels. They injected DDT doses of 1, 10,
100, or 200 mg/kg; the only effect occurred with the dose of 200 mg/kg,
but the fish did not survive to 72 h. The authors suggested that
osmoregulatory effects are not the major cause of DDT-induced mortality
in marine fish.
Desaiah et al. (1975) exposed fathead minnows ( Pimephales
promelas ) for long periods to DDT at water concentrations of 0.5 or
2.0 µg/litre and also via the food, and monitored the activity of
ATPases in brain and gill. This study followed up several previous
studies on in vitro effects on these enzymes. After 266 days of
exposure, there was an approximately 50% reduction in brain
oligomycin-sensitive (mitochondrial) Mg2+-ATPase activity. In
contrast, oligomycin-insensitive Mg2+-ATPase activity was increased
by almost 40%. Total Mg2+-ATPase activity was, therefore, almost
unaffected by DDT. There was a less obvious (about 18%) activation of
Na+-K+-ATPase activity in the brain. Gill tissue showed different
results; all the ATPases studied were inhibited by DDT. The authors
suggested that a major factor in the toxicity of DDT to fish (and other
organisms) could be the inhibition of oxidative phosphorylation.
Moffett & Yarbrough (1972) investigated the enzyme succinic
dehydrogenase in insecticide-resistant and insecticide-susceptible
mosquitofish ( Gambusia affinis ) in an attempt to discover if
resistance could be related to membrane effects of DDT. They found
that the effect on membrane-bound enzymes was, indeed, reduced in
resistant fish. This may not explain all the factors involved in
resistance, since DDT uptake from water may also be reduced.
5.2.4 Development of tolerance
The development of tolerance to DDT in fish has been reported.
Vinson et al. (1963) reported DDT tolerance in mosquitofish ( Gambusia
affinis ) exposed long-term to DDT in the wild, and Boyd & Ferguson
(1964) showed TDE tolerance in the same species. However, fish exposed
long-term to DDT do not always show tolerance. Ferguson et al. (1964)
recorded tolerance to a variety of organochlorine insecticides in three
species of freshwater fish from the Mississippi delta area of cotton
cultivation, but there was no tolerance to DDT. El-Sebae (1987)
determined the LC50 values for two populations of Tilapia zilli from
different areas of Egypt. Fish from the Behera Governate which had
been taken from agricultural drains showed exactly the same suscepti-
bility to DDT (25% EC) as fish taken from a less contaminated area in
the Alexandria Governate. Tolerance had developed to other insecti-
cides in these different strains.
5.3 Toxicity to Amphibians
Appraisal
The toxicity of DDT and its metabolites to amphibians varies from
species to species; although only a few data are available, amphibian
larvae seem to be more sensitive than adults to DDT. TDE seems to be
more toxic than DDT to amphibians, but there are no data available for
DDE. All the studies reported have been static tests and, therefore,
results should be treated with caution.
The toxicity of DDT and TDE to amphibians is summarized in
Table 5. Both compounds are toxic to amphibian larvae at low water
concentrations.
Two studies (Harri et al., 1979; Hudson et al., 1984) showed
that DDT is moderately toxic to adult frogs when given orally.
Repeated oral dosing of adult common frogs ( Rana temporaria ) with DDT
at 0.6 mg/kg body weight twice weekly for 8 weeks, led to no mortality
when the animals were fed (Harri et al., 1979). Frogs dosed in the
same way, but not fed, showed 50% mortality by the end of dosing. The
first animal died after the fifth dose and all others showed symptoms
of poisoning.
A study by Sanders (1970) indicated that the toxicity of DDT to
tadpoles of Fowler's toad increased with age of the tadpole. The 24-
and 96-h LC50 values of 5.3 and 0.75 mg/litre for one-week-old
tadpoles fell to 1.4 and 0.03 mg/litre, respectively, by the time the
tadpoles were 7 weeks old. TDE was only tested on one age range of
tadpoles for a maximum of 96 h, and was found to be 3-8 times more
toxic than DDT. The pattern of pesticide poisoning progressed through
irritability and loss of equilibrium to death. Tadpoles were affected
irreversibly by concentrations well below their calculated short-term
LC50 values and, therefore, would succumb to DDT over time. DDT was
re-tested several times during over the period of the study in an
attempt to identify any development of resistance in the population.
None was found; the 24-h LC50 values were stable throughout a 4-month
period.
Table 5. Toxicity of DDT and its derivatives to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Fowler's toad (tadpole)
(Bufo woodhousii)
1 week old - 15 mg stat 15.5 30 7.1 DDT 24-h LC50 5300 (2900-9900) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1800 (950-3300) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 750 (280-2000) Sanders
(1970)
2-3 weeks old - 56 mg stat 15.5 30 7.1 DDT 24-h LC50 5400 (2900-10 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1300 (320-5300) (1970)
4-5 weeks old - 74 mg stat 15.5 30 7.1 DDT 24-h LC50 2400 (730-8000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1000 (40-6500) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 1000 (20-3600) Sanders
stat 15.5 30 7.1 TDE 24-h LC50 700 (250-2000) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 320 (210-450) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 140 (100-210) (1970)
6 weeks old - 350 mg stat 15.5 30 7.1 DDT 24-h LC50 2200 (550-15 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 410 (280-600) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 100 (20-600) Sanders
(1970)
7 weeks old - 600 mg stat 15.5 30 7.1 DDT 24-h LC50 1400 (900-2000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 750 (610-1100) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 30 (6-400) Sanders
(1970)
Table 5. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Western chorus frog stat 15.5 30 7.1 DDT 24-h LC50 1400 (910-2800) Sanders
(Pseudacris stat 15.5 30 7.1 DDT 48-h LC50 900 (400-1500) (1970)
triseriata) stat 15.5 30 7.1 DDT 96-h LC50 800 (500-2300) Sanders
(1-week-old tadpole) stat 15.5 30 7.1 TDE 24-h LC50 610 (410-820) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 500 (210-750) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 400 (210-750) (1970)
Bullfrog DDT acute LD50c > 2000 ug/kg Hudson
(Rana catesbeiana) (77.2%) et al.
(1984)
Common frog 15 DDT acute LD50c 7600 ug/kg Harri
(Rana temporaria) et al.
(1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(DDT concentration in water continuously maintained).
b alkalinity expressed as mg CaCO3/litre.
c acute LD50 was calculated by administering a single oral dose.
Cooke (1970) exposed tadpoles of the common frog ( Rana temporaria )
to 0.1, 1.0, or 10 mg DDT/litre for only one hour and reported a
period of uncoordinated hyperactivity beginning less than one hour
after the end of the exposure period. Body weight decreased during
this hyperactive period and development was restricted in some of the
tadpoles. Smaller tadpoles were more vulnerable to the effects of DDT
than larger ones. In a later study (Cooke, 1979b), the same author
reared tadpoles of the common frog at two different densities. The
densities differed 5-fold and resulted in a 2-fold average difference
in body weight between the two groups. The larger tadpoles, reared at
the lower density, were completely tolerant of concentrations of DDT
that caused severe sublethal effects in smaller tadpoles. Field
populations of tadpoles included individuals with weights corresponding
to the two experimental groups, but these were at the two extremes of
the natural weight range.
Cooke (1972) exposed both spawn and tadpoles of the common frog
( Rana temporaria ), the common toad ( Bufo bufo ), and the smooth newt
( Triturus vulgaris ) for 24 and 48 hours to concentrations of DDT
between 0.8 µg/litre and 0.5 mg/litre. Results indicated that DDT did
not penetrate well-developed spawn and was only detectable in tadpoles
hatched from spawn that had been treated with DDT immediately after it
had been laid. Tadpoles hatching from spawn treated when newly laid
showed hyperactivity, symptomatic of DDT poisoning, only later in
their development at the point where external gills were lost. In the
experiments where tadpoles were exposed to DDT, they were most suscep-
tible either just before or just after the development of hindlimb
buds. At these two stages, the characteristic hyperactivity was
shown when DDT tissue concentrations reached between 2-3 mg/kg before
the tadpoles developed limb buds, and when they reached 3-4 mg/kg,
immediately after the tadpoles developed limb buds. During resorption
of the tail, small frogs, but not small toads, were susceptible to DDT
residues that had been acquired during larval development. At all
stages of development, toads were more resistant to DDT than were
frogs, and some toad tadpoles survived despite tissue residues in
excess of 300 mg/kg. The metabolite DDE was often detectable in newt
tadpoles and in frog and toad tadpoles with hindlimbs.
DDT has an anatomical effect on developing frog tadpoles (Cooke,
1970; Osborn et al., 1981). Exposure of tadpoles to 0.1 mg DDT/litre
for 2 days or to 0.1, 1.0, or 10 mg/litre for one hour produced some
individuals with abnormalities in the snout. A detailed histological
and behavioural study suggested that the effect was caused by two
separate factors. DDT had a direct effect on the development of skin
glands in the region above the upper mandible. The uncoordinated
hyperactivity that followed DDT treatment caused the lower mandible to
strike the upper, distorted mandible and resulted in further damage.
Some individuals recovered from this abnormality at various stages of
development. However, froglets that were affected at the tadpole stage
frequently have blunt snouts and deformed brains. The authors
suggested that DDT caused the disruption by preventing the organisation
of the epithelial cells into gland units, possibly by affecting cell
membranes and disrupting cell-to-cell communication. The mechanism of
recovery remained unclear and a full explanation of the very specific
nature of the abnormality was not possible.
This toxicity of DDT to amphibians is of significance in its use as
an insecticide. The use of DDT to control mosquito larvae has been a
major source of exposure of tadpoles and has led to toxic effects
(Mulla, 1963; Cooke, 1973a).
The widespread use of DDT has led to the development of some
resistance in two species of cricket frog ( Acris crepitans and Acris
gryllus ). Boyd et al. (1963) found that cricket frogs collected from
areas of high DDT usage for the control of cotton pests were more
tolerant to DDT than were frogs from other areas.
6. TOXICITY TO TERRESTRIAL ORGANISMS
There is evidence that DDT and its metabolites have affected
wildlife in terrestrial ecosystems. Laboratory studies covered in this
section give clear indication of a variety of lethal and sublethal
effects. The range of organisms studied is not comprehensive. No
review has been made here of the effects of DDT on insects, the target
organisms. The lethal effect of DDT on insects is thought to result
from changes in nerve transmission.
6.1 Terrestrial Invertebrates
Appraisal
There have been few reports on the effects of DDT and its
metabolites on non-target terrestrial invertebrates.
Earthworms are insensitive to the acutely toxic effects of these
compounds at levels higher than those likely to be found in the
environment. The uptake of DDT by earthworms is related to the concen-
trations in soil and to the activity of the worms; seasonally greater
activity increases uptake. Thus, although earthworms are unlikely to
be seriously affected by DDT, they pose a major hazard to predators
because of the residues they can tolerate.
Both DDT and DDE are classified as being relatively non-toxic to
honey bees, with a topical LD 50 at 27 µg/bee.
There are no reports on laboratory studies using DDE or TDE, in
spite of the fact that these are major contaminants of soil.
The toxicity of DDT to insects, the target organisms, is exten-
sively documented. Uptake of DDT and its metabolism by other
terrestrial invertebrates is also well covered in the literature.
However, there are few reports of effects of either DDT or its
metabolites on non-target invertebrates.
Johansen (1962) classified DDT as "moderately" toxic to honey
bees in both laboratory and field tests. Atkins et al. (1973) quoted
a topical LD50 for honey bees of 12.09 µg/bee and classified DDT as
"relatively non-toxic".
DDT has little or no effect on earthworms at dose levels likely to
be encountered in the field; worms were unaffected by 2000 mg/kg soil
(Goffart, 1949). The early literature has been examined by Davey
(1963), whose review includes reports on a variety of earthworm species
that live in surface soil or deeper layers. Thompson (1971) treated an
area of grassland with an emulsifiable concentrate of DDT at the rate
of 5.6 kg/ha. Although there was a reduction in earthworm numbers and
biomass of about 30%, the author considered this to be of little sig-
nificance. Results in tropical areas are similar to those of temperate
regions. Cook et al. (1980) examined the effects of cultivation and
DDT treatment on earthworm activity and populations in Nigeria
following the application of DDT (1 kg/ha) as a foliar spray on cowpea
plots. The number of casts on the surface was reduced by DDT
application, but there was no effect on the number of worms in the
soil.
Cooke & Pollard (1973) treated Roman snails ( Helix pomatia )
with p,p' -DDT applied to lettuce leaves. The snails were fed a
365 x 2.5 cm square of leaf that had been treated with 0.1 ml of an
acetone solution of DDT (either 0.025, 1.0, or 40 mg/ml). The dosing
started when the snails were 2 weeks old and continued for 17 weeks, at
which point the dose was doubled and continued for a further 12 weeks.
The snails were then transferred outside to stimulate hibernation. Low
doses of DDT reduced the weight of the shell and operculum whereas
higher doses did not. After re-emergence from hibernation, the
incidence of operculum eating was significantly higher among snails
hibernating late in the season, and as exposure to DDT increased so
operculum eating became more prevalent. The authors suggest that
shell-thinning is likely to have occurred in snails in heavily-treated
agricultural areas if the response of all snail species to DDT is
similar to that of Helix pomatia .
Critchley et al. (1980) investigated the effects of the use of
DDT for 4 years on a cultivated forest soil in Nigeria on the
numbers of epigeal (surface-living) and subterranean species of invert-
ebrates. DDT was applied as a foliar spray to crops of cowpeas at a
rate of 1 kg/ha annually. After the first application of DDT there was
no effect on ant or millipede numbers but the numbers of lycosid
spiders and crickets were reduced. At the end of the study, after four
applications of DDT, ants and millipedes were also reduced in number.
When Shires (1985) treated cereals on clay loam soil in experimen-
tal plots with DDT (1 kg/ha), the numbers of predatory beetles
(Carabidae) were reduced by 50% one week after application. However,
the numbers increased again after 4 to 6 weeks and remained at control
levels. The use of other insecticides led to a second decrease in
Carabidae numbers; this was attributed by the authors to a reduction
in the food supply of aphids. DDT failed to control the aphids, which
were tolerant to the compound.
6.2 Birds
Appraisal
DDT and its metabolites can lower the reproductive rate of birds by
causing eggshell thinning (which leads to egg breakage) and by causing
embryo deaths. However, different groups of birds vary greatly in
their sensitivity to these chemicals; predatory birds are extremely
sensitive and, in the wild, often show marked shell thinning, whilst
gallinaceous birds are relatively insensitive. Because of the
difficulties of breeding birds of prey in captivity, most of the
experimental work has been done with insensitive species, which have
often shown little or no shell thinning. The few studies on more
sensitive species have shown shell thinning at levels similar to those
found in the wild. The lowest dietary concentration of DDT reported to
cause shell thinning experimentally was 0.6 mg/kg for the black duck.
The mechanism of shell thinning is not fully understood.
6.2.1 Short-term and long-term toxicity to birds
DDT and its derivatives DDE and TDE have moderate to low toxicity
to birds when given as an acute oral dose or in the diet. The acute
oral and dietary toxicities of DDT, DDE, and TDE to birds are
summarized in Table 6.
These compounds have been studied in a wide variety of species in
tests ranging from a single acute dose to 100 days of dietary dosing.
All three compounds, DDT, DDE, and TDE, have low to moderate toxicity
to young and adult birds. There is no obvious pattern of relative
toxicity between the three compounds. In some species it is DDT that
is the most toxic, while in other species it is TDE. Most of these
laboratory tests have been conducted on species that are easy to
maintain and breed in captivity. These species are unusual in many
respects; they tend to be gallinaceous birds with young that are not
fed by the adults after hatching. They also tend to have long breeding
seasons untypical of most birds in the wild. In the wild, the most
severely affected species of birds are raptors at the top of food
chains. There is little direct laboratory data on toxicity to these
birds. Toxicity to small songbirds, which make up the majority of bird
species, has not been examined either in the laboratory or the field.
Porter & Wiemeyer (1972) fed American kestrels on a diet containing
p,p' -DDE at a concentration of 2.8 mg/kg. Two birds died after 14
and 16 months of treatment; they showed residues of DDE in brain
tissues of 212 and 301 mg/kg, respectively. This compared with mean
residues of 14.9 (range: 4.47-26.6) mg/kg in 11 adult males sacrificed
after 12-16 months on the diet. Van Velzen et al. (1972) investigated
the lethal effect of stored DDT mobilization by brown-headed
cowbirds. Cowbirds were fed for 13 days on a diet containing 100, 200,
or 300 mg p,p' -DDT/kg, and were then given reduced rations of
approximately 43% of normal daily intake for a 6-day period. Of 30
birds dosed, 21 died (6, 7, and 8 from the three dose levels,
respectively). After 4 months, the remaining birds were subjected
to a second period of 6 days on a reduced diet. Four more
birds, out of six, died. In a second experiment, cowbirds were fed
100 mg p,p' -DDT/kg diet for 13 days and then subjected to 4 days of
reduced food intake. Seven out of 20 birds died. There were no deaths
in any of the control groups (i.e., birds dosed but not starved,
undosed and starved, or undosed and unstarved).
6.2.2 Toxicity to birds' eggs
Dunachie & Fletcher (1969) injected chicken eggs with DDT or TDE to
give concentrations, in the egg, varying between 10 and 500 mg/kg.
Two different vehicles were used to dissolve the insecticides (corn oil
and acetone), controls being injected with vehicle alone. The authors
monitored egg hatchability and survival of chicks to 4 days of age.
Some chicks were fed and some were not. No dose of DDT, applied in
either vehicle, had any significant effect on egg hatchability when
compared to controls. However, there was a profound effect on the
chick survival rate. All chicks hatched from eggs treated with DDT at
100 mg/kg, and which were not fed, were dead within 4 days after
hatching. Feeding the chicks eliminated this effect; the survival rate
of fed young was similar to that of controls. Chicks hatched from
eggs treated with 50 mg DDT/kg survived as well as controls, whether
they were fed or not. TDE was found to affect hatchability, but only
when applied in corn oil; the acetone-dissolved material did not have
any significant effect. TDE dissolved in corn oil reduced
hatchability to 60% of control levels at 100 and 200 mg/kg, to 7% at
300 and 400 mg/kg, and to 0% at 500 mg/kg. The effects on chick
survivability were similar to those of DDT. All chicks hatched from
eggs treated with 100 mg TDE/kg were dead after 4 days if they were not
fed, whereas chicks from eggs treated with 50 mg/kg survived as well as
controls. Chicks from either 100 or 200 mg/kg treatments survived as
well as controls as long as they were fed. The significance of the
different vehicles was discussed by Cooke (1971) and Gilman et al.
(1978). Acetone causes coagulation of yolk protein whereas corn oil
allows the injected organochlorine to float through the yolk to a
position directly under the blastodisc.
Table 6. Toxicity of DDT and its derivatives to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
Bobwhite quail 23 days diet DDE 99.9 5-day LC50 825 (697-976) Hill
(Colinus virginianus) 23 days diet DDT 100 5-day LC50 611 (514-724) et al.
23 days diet TDE TG 5-day LC50 2178 (1835-2584) (1975)
young diet DDT 5-day LC50 881 (796-975) Stickel
& Heath (1964)
(wild) diet DDT TG 5-day LC50 1170 (830-1650) Hill et al.
(farm-reared) diet DDT TG 5-day LC50 1610 (1331-1948) (1971)
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 400 (1963)
adult diet DDT 10-day LC50 2500 DeWitt et al.
adult diet DDT 100-day LC50 1000 (1963)
Japanese quail 7 days diet DDE 99.9 5-day LC50 1355 (1111-1648) Hill
(Coturnix coturnix 7 days diet DDT 100 5-day LC50 568 (470-687) et al.
japonica) 7 days diet TDE TG 5-day LC50 3165 (2534-3978) (1975)
M 2 months oral DDT 77.2 acute LD50 841 (607-1170) Hudson et al.
(1984)
California quail M 6 months oral DDT TG acute LD50 595 (430-825) Hudson et al.
(Callipepla F 6 months oral TDE > 95 acute LD50 > 760 (1984)
californica)
Mallard duck 17 days diet DDE 99.9 5-day LC50 3572 (2811-4669) Hill
(Anas platyrhynchos) 17 days diet DDT 100 5-day LC50 1869 (1500-2372) et al.
17 days diet TDE TG 5-day LC50 4814 (3451-7054) (1975)
F 3 months oral DDT 77.2 acute LD50 > 2240 Hudson et al.
F 3 months oral TDE > 95 acute LD50 > 2000 (1984)
young diet DDT 5-day LC50 875 (650-1140) Stickel &
Heath (1964)
young diet DDT 10-day LC50 500 DeWitt
young diet DDT 100-day LC50 > 200 et al.
adult diet DDT 100-day LC50 1000 (1963)
Pheasant 10 days diet DDE 99.9 5-day LC50 829 (746-922) Hill
(Phasianus colchicus) 21 days diet DDT 100 5-day LC50 311 (256-374) et al.
10 days diet TDE TG 5-day LC50 445 (402-494) (1975)
F 3-4 months oral DDT > 99 acute LD50 1334 (894-1990) Hudson et al.
F 3-4 months oral TDE > 95 acute LD50 386 (270-551) (1984)
young diet DDT 5-day LC50 804 (686-942) Stickel &
Heath (1964)
Table 6. (Contd)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 100 (1963)
adult diet DDT 10-day LC50 1000 DeWitt et al.
adult diet DDT 100-day LC50 > 100 (1963)
Red-winged blackbird diet DDT 10-day LC50 1000 DeWitt et al.
(Agelaius phoeniceus) diet DDT 30-day LC50 500 (1963)
Cardinal diet DDT TG 5-day LC50 535 (420-700) Hill et al.
(Richmondena cardinalis) (1971)
House sparrow diet DDT TG 5-day LC50 415 (370-465) Hill et al.
(Passer domesticus) (1971)
Blue jay diet DDT TG 5-day LC50 415 (320-540) Hill et al.
(Cyanocitta cristata) (1971)
Rock dove M,F oral DDT 77.2 acute LD50 > 4000 Hudson et al.
(Columba livia) (1984)
Sandhill crane M,F adult oral DDT > 99 acute LD50 > 1200 Hudson et al.
(Grus canadensis) (1984)
Clapper rail M diet DDT 5-day LC50 1612 Van Velzen &
(1975) Kreitzer
(Rallus F diet DDT 5-day LC50 1896 (1975)
longirostris) (1975)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed
as mg/kg diet).
c TG = Technical grade.
6.2.3 Reproductive effects on birds
DDT, or more specifically its metabolite DDE, causes the shells of
birds' eggs to be thinner than normal. Results on eggshell thinning
are summarized in Table 7. There is considerable variation between
species for this effect. Galliform species are very resistant to shell
thinning whereas birds of prey are particularly susceptible.
Lincer (1975) dosed captive American kestrels and established a
clear relationship between dietary DDE and thinning of eggshells.
There was a similar close correlation between the residues of DDE in
individual eggs and the degree of shell thinning. The kestrels were
fed with day-old cockerels (which were injected with 0.2 ml of corn
oil, containing the DDE, into the breast muscle) and received either
0.3, 3, 6, or 10 mg DDE/kg diet. Residues of DDE in eggs laid by the
birds correlated closely with dietary DDE concentration; residues of
1.9 mg/kg wet weight were associated with the lowest dose and 245 mg/kg
with the highest dose given. There was no shell thinning associated
with the dose of 0.3 mg/kg. The other doses showed 15.1%, 22.8%, and
29.2% thinning (at 3, 6, and 10 mg/kg, respectively). There was a
straight-line relationship between the degree of shell thinning and
the logarithm of the DDE residue in the egg. Data obtained from the
field showed exactly the same trend (Fig. 1). This represents the best
evidence for the effect of DDE on shell thickness in a species actually
adversely affected in the field.
Table 7. Thinning effects of DDT and its derivatives on bird egg shells
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Ring dove diet DDE 10 - 9.2 0.01 Peakall et al. (1973)
(Streptopelia diet DDE 40 - 6.8 0.01 Haegele & Hudson (1973)
risoria)
Mallard diet TDE 10 - 2.6 NS Heath et al. (1969)
(Anas platyrhynchos) diet TDE 10 - 5.4 NS Heath et al. (1969)
diet TDE 40 - 2.6 NS Heath et al. (1969)
diet TDE 40 - 5.4 NS Heath et al. (1969)
diet DDT 2.5 - 5.3 NS Heath et al. (1969)
diet DDT 10 - 7.9 NS Heath et al. (1969)
diet DDT 40/25 -13.2 0.01 Heath et al. (1969)
White pekin duck diet DDE 40 -20.3 0.001 Peakall et al. (1973)
4 day diet DDE 40 - 3.3 0.01 Miller et al. (1976)
1-3 months diet DDE 40 -18.2 0.01 Miller et al. (1976)
Black duck diet DDE 10 -17.6 0.01 Longcore et al. (1971)
(Anas rubripes) diet DDE 30 -23.5 0.01 Longcore et al. (1971)
Screech owl diet DDE 2.8 -13.3 0.01 McLane & Hall (1972)
(Otus asio)
American kestrel diet DDE 3 -15.2 0.05 Peakall et al. (1973)
(Falco sparverius) diet DDE 6 -21.0 0.01 Peakall et al. (1973)
diet DDE 10 -26.3 0.001 Peakall et al. (1973)
diet DDE 2.8 - 8.7 0.001 Wiemeyer & Porter (1970)
diet DDE 0.3 + 2.1 NS Lincer (1975)
diet DDE 3 -15.1 0.05 Lincer (1975)
diet DDE 6 -22.8 0.01 Lincer (1975)
diet DDE 10 -29.2 0.001 Lincer (1975)
Table 7. (Contd).
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Japanese quail diet o,p'-DDT 100 - 4.0 0.001d Bitman et al. (1969)
(Coturnix coturnix diet DDT 100 - 5.6 0.001d Bitman et al. (1969)
japonica) diet DDT 100 0 NS Cecil et al. (1971)
diet DDE 100 - 2.5 NS Cecil et al. (1971)
diet DDE 2 + 1.9 NS Davison et al. (1976)
diet DDE 10 + 6.3 NS Davison et al. (1976)
diet DDE 40 + 5.0 NS Davison et al. (1976)
diet DDE 200 - 0.6 NS Davison et al. (1976)
strain 1a diet DDT 2.5 + 1.0 NS Davison et al. (1976)
diet DDT 10 - 1.5 NS Davison et al. (1976)
diet DDT 40 - 0.5 NS Davison et al. (1976)
strain 1b diet DDT 2.5 - 2.7 NS Davison et al. (1976)
diet DDT 10 - 1.6 NS Davison et al. (1976)
diet DDT 40 - 7.1 NS Davison et al. (1976)
strain 2a diet DDT 2.5 + 0.5 NS Davison et al. (1976)
diet DDT 10 + 1.6 NS Davison et al. (1976)
diet DDT 40 + 1.0 NS Davison et al. (1976)
strain 2b diet DDT 2.5 - 3.7 NS Davison et al. (1976)
diet DDT 10 - 2.6 NS Davison et al. (1976)
diet DDT 40 - 5.7 NS Davison et al. (1976)
---------------------------------------------------------------------------------------------------------
a Individually caged.
b Caged in pairs.
c DDT in the p,p' - form unless stated otherwise.
d Low calcium diet (0.56%).
e S = not significant.
------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chloroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethlyene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Some related insecticides
NO2
Bulan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(r) 2-nitro-1,1-bis- -Cl -H |
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-a-(4-chlorophenyl)- -Cl -OH -CH3
a-(methyl)benzenemethanol
dicocol 4-chloro-a-(4-chlorophenyl)-a- -Cl -OH -CCl3
(Kelthane(r)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-a-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
a-hydroxybenzeneacetate
chloropropopylatec 1-methylethyl 4-chloro-a- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-a-hydroxy-
benzeneacetate
Table 1. Structure of p,p' -DDT and its analogues of the form (continued)
------------------------------------------------------------------------------------
Name Chemical name R R' R"
DDT and its major
metabolites
------------------------------------------------------------------------------------
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Perthane(r) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
------------------------------------------------------------------------------------
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been
sold under the name Rothane(r); in metabloic studies the same compound has been
referred as DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
(r) Registered.
3. KINETICS, METABOLISM, BIOTRANSFORMATION, AND BIOACCUMULATION
Appraisal
The physicochemical properties of DDT and its metabolites enable
these compounds to be taken up readily by organisms. The rates of
accumulation vary with the species, with the duration and concentration
of exposure, and with environmental conditions.
These compounds are resistant to breakdown and are readily adsorbed
to sediments and soils, which can act both as sinks and as long-term
sources of exposure (e.g., for soil organisms).
Organisms can accumulate these chemicals from the surrounding
medium and from food. In aquatic organisms, uptake from the water is
generally more important, whereas, in terrestrial fauna, food provides
the major source.
In general, organisms at higher trophic levels tend to contain more
DDT-type compounds than those at lower trophic levels.
Such compounds can be transported around the world in the bodies of
migrant animals and in ocean and air currents.
Different organisms metabolise DDT via different pathways. Of the
two initial metabolites, DDE is the more persistent, though not all
organisms produce DDE from DDT. The alternative route of metabolism,
via TDE leads to more rapid elimination (WHO, 1979). Much of the
retained DDT and its metabolites are stored in lipid-rich tissues.
Because there is an annual cycle in lipid storage and utilization in
many organisms, there is also a related annual cyclic pattern in the
handling of DDT.
3.1 Retention in Soils and Sediments and Plant Uptake
Shin et al. (1970) investigated the adsorption of DDT by soils of
various different types and by isolated soil fractions. A sandy loam,
a clay soil, and a highly organic muck were either used intact or had
various components extracted before estimating their adsorptive
capacity for the insecticide. Adsorption was least in the sandy loam
and greatest in the muck (distribution coefficients [Kd] were in the
ratio 1:10:80 for sandy loam, clay soil, and organic muck,
respectively). All soils showed a strong adsorptive capacity for DDT.
The adsorption of DDT was closely related to the organic matter content
of the soils; progressive removal of lipids, resins, polysaccharides,
polyuronides, and humic matter identified the organic fractions which
bound the DDT. Humic material represents a major source of adsorptive
capacity for DDT; the degree of sorption, however, is strongly
connected with the degree of humification. Soil containing large
amounts of humic material may not adsorb DDT as greatly as other soils
where humification is more advanced. Wheatley (1965) estimated half-
times for the loss of DDT applied to soils. After surface application,
50% of DDT was lost within 16-20 days. The estimated time for the loss
of 90% of surface-applied DDT was 1.5 to 2 years. With DDT mixed into
the soil, 50% loss occurred in 5 to 8 years, and it was estimated that
90% of applied insecticide would be lost in 25-40 years.
Albone et al. (1972) investigated the capacity of river
sediments, from the Severn Estuary, United Kingdom, to degrade DDT.
p,p' -DDT (14C-labelled) was applied to sediments either in situ on
the mud flats or in the laboratory. Sediment movement in the area of
the in situ study was sufficiently small to neither bury nor expose
the incubation tubes set into the mud. Incubation in situ over 46
days led to very little metabolism of DDT in the sediments. Some
p,p' -TDE was produced, but the ratio of DDT to TDE was 13 : 1 and
48 : 1 in two replicate experiments. There was no production of
extractable polar products; metabolism beyond TDE did not occur.
Incubation of the same sediments in the laboratory, over 21 days, led
to much greater metabolism (ratios of 1 : 1.1 and 1 : 3.3, DDT to TDE,
in replicate incubations) and the production of some unidentified,
further breakdown products. Investigation of the microbial population
of the sediment showed that some of the organisms were capable of
degrading DDT; little metabolism appeared to take place in situ .
3.2 Uptake and Accumulation by Organisms
The uptake and accumulation of DDT and its metabolites into
organisms, as determined in controlled laboratory experiments, is
summarized in Table 2. Results are expressed as bioconcentration
factors (the ratio of the concentration of the compound in the organism
to the concentration in the medium).
Concentration factors can be misleading with compounds such as DDT
when exposure is high. The compound is readily taken up and retained
at very low concentrations. At high concentrations, no more material
can be taken up because a plateau has been reached. The only
meaningful way to assess the capacity of organisms to take up and
retain DDT is by looking over a wide range of exposure levels. The
low concentration factor quoted in Table 2 for earthworms, for example,
reflects the high exposure rather than a low capacity for uptake and
retention of DDT, because concentration factors are simple ratios
between "exposure" and final concentration in the organism.
Concentration factors for fish are generally higher than for their
invertebrate prey (Table 2). It is now generally agreed that most of
the DDT taken into aquatic organisms comes from the water rather than
from their food (Moriarty, 1975). Again, the concentration factors can
be misleading. Aquatic organisms take in a small proportion of
ingested DDT. However, they retain a large proportion of the DDT which
has been absorbed into the body from the food. There has been some
controversy in the past over explanations for higher accumulations of
DDT at higher trophic levels in aquatic systems. It now seems clear
that this is not due primarily to biomagnification up food chains but
rather to a tendency for organisms at higher trophic levels to
accumulate more DDT directly from the water.
Terrestrial organisms do not live in a uniform medium surrounded by
a relatively constant concentration of a chemical. Even soil organisms
live in a medium with very variable concentrations of DDT or its
metabolites at different levels of the soil profile or patchy distri-
bution of the chemical. Some terrestrial organisms could be directly
exposed to DDT during application of the insecticide, but most will be
exposed to what remains of the DDT after application. Therefore,
higher terrestrial organisms will accumulate DDT mostly from their
food. The data in Table 2 are taken from controlled laboratory
investigations. There is ample evidence from the field that DDT does
accumulate in many organisms in different media. There is similarly
evidence that the residues of DDT or its metabolites persist in
organisms for long periods after exposure has ceased. The following
should not be regarded as a comprehensive review of the literature on
this subject, which is too large to be included. Rather, these are
examples from different groups of organisms.
Table 2. Bioaccumulation of DDTa
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Bacteria
Aerobacter aerogenes 100 22 24 h 1.2 3736 Johnson &
Bacillus subtilis 130 22 24 h 0.676 4303 & Kennedy
Aerobacter aerogenes 25 22 4 h 0.64 10 639 (1973)
200 22 4 h 0.64 1784 Johnson &
Bacillus subtilis 43 22 4 h 0.64 13 880 Kennedy
348 22 4 h 0.64 1805 (1973)
Marine algae
Cyclotella nana 17 23 2 h 0.7 37 600 Rice & Sikka
8 23 2 h 0.7 58 100 (1973)
Isochrysis galbane 39 23 2 h 0.7 11 300 Rice & Sikka
19 23 2 h 0.7 28 800 (1973)
Olisthodiscus luteus 108 23 2 h 0.7 4600 Rice & Sikka
54 23 2 h 0.7 7000 (1973)
Amphidinium carteri 66 23 2 h 0.7 4300 Rice & Sikka
33 23 2 h 0.7 9600 (1973)
Tetraselmis chuii 106 23 2 h 0.7 5200 Rice & Sikka
53 23 2 h 0.7 6300 (1973)
Skeletonema costatum 29 23 2 h 0.7 31 900 Rice & Sikka
15 23 2 h 0.7 38 400 (1973)
Diatom
Cylindrotheca 21 days 100 300 Keil & Priester
closterium (1969)
Pond snail stat 6 days 3.0 6000 Reinbold et al.
(Physa 5 sp.) (1971)
Freshwater mussel flow 20 3 weeks 0.62 3990d Bedford & Zabik
(Anodonta grandis) (1973)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Earthworm 10 4 weeks 17 000 0.47d Davis (1971)
(Lumbricus terrestris)
Water flea stat 30 3 days 2.0 1330 Metcalf et al.
(1973)
(Daphnia magna) flow 21 3 days 0.08 114 100 Johnson et al.
(1971)
Scud flow 21 3 days 0.081 20 600 Johnson et al.
(Gammarus fasciatus) (1971)
Glass shrimp flow 21 3 days 0.1 5000 Johnson et al.
(Palaemonetes kadiakensis) (1974)
Pink shrimp flow 8-15 13 days 0.14 1500 Nimmo et al.
(Penaeus duorarum) (1970)
Crayfish flow 21 3 days 0.08 2900 Johnson et al.
(Orconectes nais) (1971)
Mayfly larva flow 21 3 days 0.052 32 600 Johnson et al.
(Hexagenia bilineata) (1971)
Mayfly larva flow 21 3 days 0.047 22 900 Johnson et al.
(Siphlonurus sp.) (1971)
Dragonfly nymph flow 21 2 days 0.101 3500 Johnson et al.
(Ischnura verticalis) (1971)
Dragonfly nymph flow 21 2 days 0.079 910 Johnson et al.
(Libellula sp.) (1971)
Midge larva flow 21 3 days 0.046 47 800 Johnson et al.
(Chironomus sp.) (1971)
Mosquito larva flow 21 2 days 0.105 133 600 Johnson et al.
(Culex pipiens) (1971)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Mosquito larva stat 30 3 days 2.0 110d Metcalf et al.
(Culex quinquifasciatus) stat 30 3 days 0.9 74d (1973)
Mosquito fish stat 30 3 days 2.0 344d Metcalf et al.
(Gambusia affinis) stat 30 3 days 0.9 217d (1973)
Rainbow trout flow 5 12 weeks 0.176 21 363d Reinert et al.
(Salmo gairdneri) flow 10 12 weeks 0.137 43 158d (1974)
flow 15 12 weeks 0.133 51 355d Reinert et al.
(1974)
Brook trout flow 14 120 days 3 mg 0.64d Macek & Korn
(Salvelinus fontinalis) /kg diet (1970)
flow 14 120 days 0.003 8533d Macek & Korn
(1970)
Pinfish flow 14 days 0.1 40 000d Hansen & Wilson
(Lagodon rhomboides) flow 14 days 1.0 11 020d (1970)
Atlantic croaker flow 14 days 0.1 12 500d Hansen & Wilson
(Micropogon undulatus) flow 14 days 1.0 12 170d (1970)
Fathead minnow flow 24-25.5 14 days 45.6 mg/kg 1.17d Jarvinen et al.
(Pimephales promelas) flow 24-25.5 14 days 0.5 85 400d (1977)
flow 24-25.5 14 days 2.0 69 100d Jarvinen et al.
flow 24-25.5 112 days 45.6 mg/kg 1.33d (1977)
flow 24-25.5 112 days 0.5 93 200d Jarvinen et al.
flow 24-25.5 112 days 2.0 154 100d (1977)
Tilapia stat 31 days 1.0 6800 Reinbold et al.
(Tilapia mossambica) 31 days 10 10 600 (1971)
Green sunfish stat 31 days 1.0 3900 Reinbold et al.
(Lepomis cyanellus) 31 days 10 4020 (1971)
stat 22 15 days 0.1-0.3 17 500d Sanborn et al.
(1975)
Table 2. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Biomass Flow Organ Tem- Duration Exposure Bioconcen- Reference
(µg/ml) statb perature (µg/litre) tration
( °C) factorc
---------------------------------------------------------------------------------------------------------
Chicken eggs 8 weeks 0.1 1.87d Foster et al.
fat 8 weeks 0.1 5.8d (1972)
Broiler hen fat 6 weeks 1.0 10.3d Kan et al.
(1978)
White pelican WB 10 weeks 72 11.9d Greichus et al.
(Pelecanus erythrorhynchos) (1975)
Double-crested cormorant WB 9 weeks 0.95 236.3d Greichus &
(Phalacrocorax a. auritus) Hannon (1973)
American kestrel WB 11-16 2.8 103.9 Porter &
(Falco sparverius) months Wiemeyer (1972)
Mule deere muscle 30 days 5 mg/day 122.8 ug Watson et al.
(Odocoileus heminonus) oral /kgd (1975)
---------------------------------------------------------------------------------------------------------
a Unless specified otherwise, bioconcentration factors are based on whole body (WB) measurements.
b Stat = static conditions (water unchanged for duration of experiment);
Flow = flow-through conditions (DDT concentration in water continuously maintained).
c Bioconcentration factor = concentration of DDT in organism/concentration of DDT in medium or food.
Concentrations of DDT in organisms represents total DDT, i.e., DDT plus its stable metabolites,
principally DDE. Bioconcentration factors calculated on a dry weight basis unless otherwise stated.
d Calculated on a wet weight basis.
e Oral dose (by capsule) given daily.
3.2.1 Plants
Fuhremann & Lichtenstein (1980) applied 14C-labelled p,p' -DDT to
loam or sandy soil (at 4 and 2 mg/kg, respectively) and grew oat plants
on the treated soils for 13 days. At harvest, residues of DDT and its
metabolites were analysed in soil and plant by scintillation counting,
thin layer chromatography, and GLC. Of the total applied DDT, 95.7%
was recovered from loam soil and 88.6% from sandy soil. Almost all of
the DDT present was extractable in organic solvent (only 2.8%, for
loam, and 0.7%, for sand, was present in a water-bound form),
indicating little or no metabolism of the compound except to persistent
organically extractable residues. DDE was detected in both soils,
accounting for 3.4% of the total extracted in loam soil and 2.2% in
sand. Other metabolites, including o,p' -DDT, TDE, and dicofol were
recovered in very small quantities. Very little DDT (and none of its
metabolites) was detected in oat roots grown on loam, amounting to 0.2%
of the total DDT applied. The uptake was greater (4.6%) in roots of
oats grown on sand, but the uptake of labelled carbon into plant tops,
from both soils, was so low that it could not be analysed.
DDT was not translocated into the foliage of alfalfa when applied
to the soil (Ware, 1968; Ware et al., 1970) or into soybeans (Eden &
Arthur, 1965). Harris & Sans (1967) found only trace amounts of DDT
or its metabolites in the storage roots of carrots, radishes, and
turnips after growing the plants in soils containing up to 14 mg
DDT/kg.
3.2.2 Microorganisms
The uptake and accumulation of DDT from the culture medium by
microorganisms has been reviewed by Lal & Saxena (1982). All of the
microorganisms studied showed some capacity to take up DDT from their
growth medium, but the relative amount taken up varied greatly from
species to species. Many species took up more than 90% of the DDT when
exposed to concentrations ranging from 1 to 1000 µg/litre, whereas a
few species took in only 0.5% of the available DDT. The concentration
factors (i.e., the concentration within the organism expressed as a
ratio against the concentration in the medium) for DDT were variable
but always high (Table 2).
3.2.3 Aquatic invertebrates
Concentration factors are also variable in aquatic invertebrates.
In all cases there is considerable uptake and retention of the DDT,
though often as DDE or other metabolites rather than as the parent
compound. The main point of interest is the ability of aquatic
organisms to take up large amounts of the compound, over time, from
water where DDT is present at very low concentrations, and to retain
it.
Risebrough et al. (1976) measured DDT in sea water and in mussels
( Mytilus sp.) from San Fransisco Bay and the French Mediterranean
coast. Concentration factors varied between 40 000 and 690 000 for DDT
and between 45 000 and 310 000 for DDE.
Eberhardt et al. (1971) applied radioactively labelled DDT, at a
rate of 220 g/ha, to a freshwater marsh and followed the distribution
of the compound and its metabolites. Concentration factors in ten
species of plants varied between 5500 and 84 000. Various invert-
ebrates showed high concentration factors: ramshorn snail
( Planorbidae ), 4700; backswimmer ( Notonectidae ), 10 000; crayfish
( Orconectes immunis ), 22 000; bloodworm ( Tendipes ), 25 000; and red
leech ( Erpobdella punctata ), 47 000. Reporting earlier on the same
study, over 15 months, Meeks (1968) showed that plants and invert-
ebrates accumulated DDT to a maximum mainly within the first week after
treatment, whereas vertebrates required longer to attain maximum
residues. Residues of DDT in the surface water and suspended particles
had fallen below detectable levels within 1 month. Residues in
sediments stabilized at about 0.3 mg/kg after 9 months.
3.2.4 Fish
The uptake of DDT from water is affected by the size of the fish;
smaller fish take up relatively more DDT from water than larger
specimens of the same species. A range in weight of mosquitofish
between 70 and 1000 mg led to a four-fold difference between the
smallest and largest fish in DDT uptake from water over 48 h (Murphy,
1971).
A rise in temperature results in increased uptake of DDT by fish
(Reinert et al., 1974). Rainbow trout were exposed to a single water
concentration of DDT (nominally 330 ng/litre) at temperatures of 5, 10,
or 15 °C; the actual concentrations of DDT in water varied with
temperature and were measured at 176, 137, and 133 ng/litre,
respectively, for 5, 10, and 15 °C. Whole body residues of DDT (total)
after 12 weeks exposure were 3.8, 5.9, and 6.8 mg/kg for the three
temperatures, respectively. Expressing the results as bioconcentration
factors to allow for the differences in dissolved DDT showed a similar,
clear increase in the relative amount of DDT taken up and retained
(Reinert et al., 1974).
Increasing salinity decreases DDT uptake significantly, but has
no effect on the uptake of DDE or TDE by fish (Murphy, 1970).
Increasing the salinity from 0.15o/oo to 10o/oo decreased DDT
uptake over 24 h from 22% of the dose to 18% (body residues decreased
from 658 to 329 ng). There was a further significant decrease in
uptake when the salinity was increased to 15o/oo (Murphy, 1970)
Fish accumulate DDT from food in a dose-dependent manner. When
Macek et al. (1970) fed rainbow trout on diets containing 0.2 or 1.0 mg
DDT/kg, the fish retained more than 90% of the dietary intake of DDT
(measured as total DDT) over the 90-day exposure period. The authors
estimated the time required for the elimination of 50% of accumulated
DDT to be 160 (± 18) days. When Warlen et al. (1977) fed Atlantic
menhaden on a diet containing 14C-labelled DDT at three dose levels,
the fish assimilated and retained between 17% and 27% of the cumulative
dose from food containing 0.58, 9.0, or 93 µg/kg. There was a
straight-line relationship between exposure time and body burden of
total DDT, with no tendency for residues to reach a plateau within the
45 days of feeding with DDT. At the end of the feeding period, the
fish had accumulated DDT or its metabolites, to levels of approximately
1.1, 11, and 110 µg/kg for the three doses respectively. The
biological half-time of DDT in the fish was estimated to be 428, 64,
and 137 days, for groups exposed to 0.58, 9.0, or 93 µg/kg diet,
respectively.
3.2.5 Terrestrial invertebrates
Relatively low concentration factors have been reported for
terrestrial molluscs by Dindal & Wurtzinger (1971), who also reviewed
the earlier literature. However, low concentration factors, derived
from short-term studies, can be misleading for these organisms because
of the high persistence of DDT in soil. Residues of DDT were as high
as 40 mg/kg and, therefore, molluscs represent a source of DDT which
will be concentrated by organisms which eat them. The same is true
for earthworms, which also show low concentration factors (Davis, 1971;
Edwards & Jeffs, 1974). Gish & Hughes (1982) investigated residues of
DDT and other pesticides in earthworms for 2 years following appli-
cation. They showed that body residue levels were cyclic, with higher
levels of DDT and its metabolites occuring between late spring and
early autumn and lower levels from late autumn to early spring. Peak
high levels occurred in May and low levels in January, coinciding with
the seasonal high and low activity periods of earthworms. These
changing residue levels presumably indicate that DDT is retained in
soil and that earthworms contain more of the residual metabolites when
they are processing more soil through the gut.
3.2.6 Birds
Laboratory studies on birds have shown them capable of accumulating
DDT from food, yielding high concentration factors (Table 2).
The accumulation of DDT and its metabolites in birds in the field
has been regularly and extensively reviewed (Moore, 1965; Moriarty,
1975; Newton, 1979). The results of an analysis of a long-term
sampling programme of birds in the United Kingdom (Cooke et al., 1982)
confirm many of the early theories. Birds with the highest residues of
DDT or its metabolites were either terrestrial predators feeding on
other birds or aquatic predators feeding on fish. Thus, residues of
DDE in the liver of the peregrine falcon, with birds as its principal
dietary component, averaged 7.56 mg/kg, whereas for the rough-legged
buzzard, with mammals as the principal dietary component, mean DDE
levels were 0.05 mg/kg over a period extending from the early 1960s to
the late 1970s.
There are marked geographical differences throughout the United
Kingdom, related to usage patterns of DDT (Cooke et al., 1982), and
also marked seasonal changes in residues. These seasonal changes
appear to relate more to physiological changes in body composition,
which occur with climatic and breeding seasons, than to the environ-
mental availability of pollutants. Some species, e.g., heron, barn
owl, and kingfisher, showed a decline in DDE residues with time, but
others, e.g., sparrowhawk, kestrel, and great-crested grebe, did not,
levels in 1977 being similar to those in 1963. Eventually residues of
DDT in wildlife decline with time after a ban is imposed on the use of
the pesticide. However, the highly persistent nature of DDE means that
significant residues will continue to be found for a considerable
period. The situation in the United Kingdom and the USA appears to be
broadly similar (O'Shea & Ludke, 1979).
3.2.7 Mammals
DDT is taken up by, and retained in, wild mammals. The degree of
uptake and retention varies with the species. In a study following a
single application of DDT to a forest to control spruce budworm at a
rate of 0.89 kg/ha, Dimond & Sherburne (1969) and Sherburne & Dimond
(1969) reported residues of DDT and its metabolites in mammals over 9
years. Herbivorous mice, voles, and hares contained less DDT than
carnivorous mink and insectivorous shrews. In herbivores, residues
approached pre-treatment levels after 6-7 years, whereas residues were
still significantly higher in shrews and mink than in the same species
taken from untreated areas 9 years after the single treatment with DDT.
In these species, the authors calculate that it would take at least 15
years for residues to reach background levels. They regard the high
residue levels in mammals at higher trophic levels as deriving
principally from DDT retained in the soil, since there is little long-
term retention on vegetation.
In a 3-year study, after treating a field ecosystem with 36Cl-
ring-labelled DDT at a dose rate of 0.92 kg/ha, Forsyth & Peterle
(1973) measured DDT residues in various tissues of two species of
shrew. The highest residue (135 mg/kg) occurred in fat, compared with
10, 10, and 4 mg/kg in liver, muscle, and brain, respectively. Shrews
of the species Blarina brevicauda released into treated areas accumu-
lated DDT to the same degree as resident shrews within 15-20 days of
exposure. Equilibrium between intake and excretion of DDT occurred
within approximately 30 days in muscle, liver, and brain and within
40 days in fat. The second species of shrew ( Sorex cinereus )
accumulated residue levels of DDT during the following 2 years which
were successively greater than levels present in the first year,
indicating that DDT was increasing in availability to this species
with the passage of time. The levels of DDT in muscle were not
influenced by sex but were influenced by breeding condition. Male
shrews with scrotal testes and lactating females developed lower
levels of DDT in muscle and viscera than did males with abdominal
testes or non-lactating females.
Benson & Smith (1972) measured levels of DDT and its metabolites in
deer exposed to DDT used for spruce budworm control, and found that, in
the year of spraying, there was up to 20 mg/kg in fat. Males had
considerably higher levels of DDT than females. Fawns also had higher
levels than their mothers, though this was from a small sample. The
majority of the residues consisted of p,p' -DDT, with almost insignifi-
cant levels of DDE. Five years later, the residue levels in males were
still higher than those in females, though these had fallen to about
1% of original levels. Most of the deer population was 3 years old or
less, and so the figures for 5 years after spraying represent DDT
ingested from the environment and not from direct exposure.
Some, though very little, DDT was detected in black bears by
Benson et al. (1974). There was no evidence that the area had been
directly sprayed with DDT. This study illustrates that there is a
general environmental contamination with DDT, which can be accumulated
by mammals, though to a small degree, without direct application of the
material to their habitat.
4. TOXICITY TO MICROORGANISMS
Appraisal
Aquatic microorganisms are more sensitive than terrestrial ones to
DDT.
An environmental exposure concentration of 0.1 µg/litre can cause
inhibition of growth and photosynthesis in green algae.
Repeated applications of DDT can lead to tolerance in some micro-
organisms.
There is no information on effects concerning the species compo-
sition of microorganism communities. Therefore, it is difficult to
extrapolate the relevance of single-culture studies to aquatic or
terrestrial ecosystems. However, since microorganisms are basic in
food chains, adverse effects on their populations would influence
ecosystems. Thus, DDT and its metabolites should be regarded as a
major environmental hazard.
Studies cited in this section will be restricted to those effects
produced by low concentrations of DDT. Some studies still use DDT at
concentrations above its water solubility. Reviews of other effects of
DDT and its analogues, at higher concentrations, on cell division and
several biochemical parameters have been produced by Luard (1973) and
Lal & Saxena (1979).
4.1 Bacteria and Cyanobacteria (Blue-green Algae)
Ledford & Chen (1969) cultured bacteria isolated from surface-
ripened cheese with 0.5 mg DDT/litre or 0.5 mg DDE/litre, but found no
effect on growth.
At a concentration of 10 µg/litre in the culture medium, DDT
stimulated the growth of the bacterium Escherichia coli (Keil et al.,
1972). Yields of cultures exposed to 100 µg/litre did not differ from
controls. There was no effect of DDT on denitrification (conversion of
nitrate to nitrite) at a concentration of 100 mg/kg in soil and,
similarly, no effect on this process when carried out by a bacterial
culture (Bollag & Henninger, 1976). DDT at up to 22 kg/ha did not
affect the numbers of soil bacteria in outdoor-treated plots (Bollen et
al., 1954), and five annual applications of DDT to a sandy loam soil
did not significantly affect the numbers of soil bacteria (Martin,
1966).
Concerning cyanobacteria (blue-green algae), Goulding & Ellis
(1981) found no effect on the growth of Anabaena variabilis at a DDT
concentration of 1 µg/litre. Batterton et al. (1972) suggested that
DDT reduced the tolerance of Anocystis nidulans to sodium chloride.
The organism is resistant to salt and to DDT, at concentrations up to
8000 mg/litre, but not to combinations of the two stressors.
4.2 Freshwater Microorganisms
Lee et al. (1976) showed that DDT inhibited photosynthesis in the
green alga Selenastrum capricornutum at concentrations between 3.6 and
36 µg/litre, inhibition increasing with time of exposure.
Two different species of green algae were shown to be resistant
to DDT and its metabolites, DDE and TDE, at concentrations up to
1000 mg/litre in culture. Scenedesmus and Dunaliella revealed rates of
photosynthetic uptake of 14C-labelled CO2 similar to those of
controls (Luard, 1973). Considerable variation exists between species
of microorganisms concerning the effect of DDT and its analogues;
resistance to DDT is not restricted to one taxonomic group, either
freshwater or marine (Luard, 1973). The source of the resistance is
unclear. The two species studied show very different characteristics;
Dunaliella has no cell wall, whereas Scenedesmus has a complex cell
wall. Since both show resistance to DDT, it is unlikely that the
chemical is excluded from the cell by the cell wall. Cell membranes
and chloroplast membranes are an alternative barrier to DDT uptake and
effect. It is not known how these structures might be involved in DDT
resistance; studies with isolated chloroplasts suggest that there is no
barrier to DDT uptake there.
Cole & Plapp (1974) found inhibition of growth and photosynthesis
of the green alga Chlorella pyrenoidosa with DDT at 1 µg/litre in the
medium. However, inhibition was inversely related to the number of
cells in the culture. With high cell counts, there was no
inhibition of either growth or photosynthesis with DDT present at up to
1 mg/litre. Inhibition only occurred at low cell densities in culture.
Goulding & Ellis (1981) found that the green alga Chlorella fusca
was affected by DDT at 0.1 µg/litre. The amount of inhibition of
growth varied with time and with the method of assessing the result.
Cell numbers were maximally affected (75% inhibition) after 72 hours,
and after 200 hours cell numbers had reached control levels. When
growth was assessed by chlorophyll content or biovolume, the initial
inhibition was more marked and cultures were only equivalent to
controls after 480 hours. The apparent anomaly is explained by
reductions in cell size in response to DDT.
Christie (1969) reported no effect of DDT on the growth of
Chlorella and attributed this to the ability of the organism to
metabolize the compound.
Lal & Saxena (1980) reported that DDT did not affect growth and DNA
synthesis in the ciliate Stylonychia notophora at concentrations of
1 mg/litre or less.
4.3 Marine Microorganisms
MacFarlane et al. (1972) showed that DDT, at concentrations
between 9.4 and 1000 µg/litre, reduced photosynthetic carbon
fixation and the cell content of chlorophyll a relative to controls
in a marine diatom Nitzschia delicatissima , over a 24-h period.
The diatom was cultured with DDT under four different light inten-
sities. The insecticide had the greatest effect at the highest light
intensity, where carbon fixation was reduced by 94% in water containing
100 µg DDT/litre. At higher DDT concentrations, there was no further
reduction in either carbon fixation or chlorophyll content.
The photosynthesis of several species of marine phytoplankton has
been found to be inhibited by DDT at concentrations of 100 µg/litre or
less (Wurster, 1968). Four different species showed increasing
inhibition up to DDT concentrations of 100 µg/litre, but no greater
effect at higher concentrations. A green alga, Pyramimonas , was
affected by DDT only at concentrations higher than 10 µg/litre. The
other three species, a diatom, a coccolithophore, and a dinoflagellate
were affected at DDT concentrations between 1 and 10 µg/litre. In
a similar study (Menzel et al., 1970) four different species of
marine phytoplankton were studied. Inhibition of photosynthesis,
where it occurred, followed a similar dose-response relationship.
For three species ( Skeletonema costatum , a diatom; Coccolithus
huxleyi , a coccolithophorid; and Cyclotella nana , a second diatom)
inhibition began between 1 and 10 µg DDT/litre and reached a maximum
at 100 µg/litre. The other organism, a green flagellate Dunaliella
tertiolecta , was unaffected by DDT at concentrations up to 1 mg/litre,
the highest exposure tested.
The marine dinoflagellate Exuviella baltica showed significant
inhibition of growth after exposure to DDT at concentrations as low as
0.1 µg/litre (Powers et al., 1979).
4.4 Soil Microorganisms
TDE had no significant effects on growth and reproduction of soil
amoebae except at concentrations higher than 1 mg/litre (Prescott &
Olson, 1972). Populations of protozoa in garden soil were reduced by
DDT at a concentration of 5 mg/kg (MacRae & Vinckx, 1973). Numbers
were still significantly reduced after 3 months.
4.5 Fungi
Two aquatic and one terrestrial fungi showed stimulated growth
in response to DDT present at concentrations of between 2 and
60 µg/litre of growth medium (Hodkinson & Dalton, 1973)
5. TOXICITY TO AQUATIC ORGANISMS
DDT and its derivatives are highly toxic to aquatic organisms;
water concentrations of a few micrograms per litre are sufficient to
kill a large proportion of populations of aquatic organisms in acute or
short-term exposure. In addition to its high short-term toxicity, DDT
also has long-term sublethal effects on aquatic organisms. Many
physiological and behavioural parameters have been reported to be
affected by the insecticide. This toxicity, coupled with its high
capacity for bioconcentration and biomagnification, means that DDT
presents a major hazard to aquatic organisms.
5.1 Aquatic Invertebrates
Appraisal
Both the acute and long-term toxicities of DDT vary between species
of aquatic invertebrates. Early developmental stages are more
sensitive than adults to DDT. Long-term effects occur after exposure
to concentrations ten to a hundred times lower than those causing
short-term effects.
DDT is highly toxic, in acute exposure, to aquatic invertebrate, at
concentrations as low as 0.3 µg/litre. Toxic effects include
impairment of reproduction and development, cardiovascular
modifications, and neurological changes. Daphnia reproduction is
adversely affected by DDT at 0.5 µg DDT/litre.
The influence of environmental variables (such as temperature,
water hardness, etc.) is documented but the mechanism is not fully
understood. In contrast to the data on DDT, there is less information
on the metabolites DDE or TDE. The reversibility of some effects once
exposure ceases has been reported, as well as the development of
resistance.
5.1.1 Short-term and long-term toxicity
The short-term toxicity to aquatic invertebrates is summarized in
Table 3.
Most aquatic invertebrates are killed by low water concentrations
of DDT and its metabolites, though the majority of the published data
is on DDT itself. Six invertebrate species studied by Macek & Sanders
(1970) showed 96-h LC50 values ranging from 1.8 to 54.0 µg/ litre.
Adult molluscs are relatively resistant to DDT and the compound has
been used to control crustacean pests on oyster beds (Loosanoff, 1959).
However, the larval stages of molluscs are affected by DDT; clam larvae
showed 90% mortality after exposure to DDT at 0.05 mg/litre (Calabrese,
1972). Molluscs exhibit effects on shell growth at low DDT concen-
trations. Tubifex worms are resistant to DDT; 3 mg/litre did not kill
any Tubifex tubifex (Naqvi & Ferguson, 1968). Many aquatic crustaceans
yield LC50 values less than 1 µg/litre. Muirhead-Thomson (1973)
showed that predator invertebrates, such as dragonfly nymphs, were
more tolerant of DDT than prey organisms. Since the prey organisms
are also food for fish, the balance of aquatic ecosystems could be
changed by very low levels of DDT. Lowe (1965) reported that juvenile
blue crabs ( Callinectes sapidus ), exposed to 0.25 µg DDT/litre for 9
months, grew and moulted normally; there were no apparent sublethal
effects. However, exposure to 5 µg DDT/litre killed all crabs.
The metabolite TDE has been studied in parallel tests with the
parent compound in some organisms. There is no consistent relationship
between the toxicity of the two compounds. TDE is considerably less
toxic to stonefly larvae than DDT, by a factor of about 100 (Sanders &
Cope, 1968). However, for other freshwater organisms TDE may have
similar, lower, or greater toxicity according to the organism and
duration of test (Table 3). For most marine invertebrates, DDT is most
toxic, followed by DDE and TDE (data from Mayer, 1987).
5.1.2 Physiological effects on aquatic invertebrates
Butler (1964) demonstrated a 50% reduction in shell growth in young
eastern oysters exposed for 96-h to DDT at 14 µg/litre. Roberts
(1975) showed that DDT at 50 µg/litre reduced the amplitude of
ventricular contractions in the isolated heart of the bivalve Mya
arenaria within 4 minutes. At higher concentrations, DDT stopped heart
contractions altogether. Recovery, even of the arrested heart, was
rapid after the immediate replacement of the DDT solution with clean
sea water.
Kouyoumjian & Uglow (1974) found that for the planarian worm
Polycelis felina , TDE was most toxic and DDT least toxic, with DDE
showing intermediate toxicity. Sublethal effects of DDT and TDE were
demonstrated. DDT reduced the rate of asexual fission. Both DDT and
TDE were shown to reduce the righting time of animals turned onto their
backs. This was presumed to be a nervous system effect.
Maki & Johnson (1975) report 50% reduction in three parameters of
reproduction in the water flea Daphnia magna at 0.5 µg/litre, for
total young produced, at 0.61 µg/litre for average brood size, and at
0.75 µg/litre for percentage of days reproducing.
In vitro effects on gill ATPases of two species of crab have been
reported (Jowett et al., 1978; Neufeld & Pritchard, 1979). There is a
transitory effect in vivo on gill ATPases and, thereby, an effect on
plasma osmolarity. However, this osmoregulatory effect soon disappears
(Pritchard & Neufeld, 1979). Leffler (1975) reported metabolic rate
elevation, decreased muscular coordination, inhibition of autotomy
reflex, and reduced carapace thickness/width ratio in juvenile crabs
exposed to DDT. Osmoregulation was not affected. The DDT was given in
the food of the crabs at a concentration of 0.8 mg/kg. DDT has been
found to accelerate limb regeneration and the onset of the next moult
in fiddler crabs (Weis & Mantel, 1976). The authors suggest that the
effect is on the central nervous system, with DDT causing changes in
neurosecretory activity.
Table 3. Toxicity of DDT and its derivatives to invertebrates
---------------------------------------------------------------------------------------------------------
Organismf Flow Temp Salinity Compound Parameter Water Reference
stata ( °C) o/oo concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine invertebrates
Eastern oyster (juv.) flow 30 23 DDTd 96-h EC50j 9 Mayer (1987)
(Crassostrea virginica) flow 12 25 DDEd 96-h EC50j 14 Mayer (1987)
flow 20 30 TDEd 96-h EC50j 25 Mayer (1987)
Shrimp stat 20 sea water DDTd 96-h LC50 0.4 McLeese &
(Crangon septemspinosa) stat 10 sea water DDTd 96-h LC50 31 Metcalfe (1980)
+ sediment
Mysid shrimp (adult) stat 25 23 DDTd 96-h LC50 0.45 Mayer (1987)
(Mysidopsis bahia) (0.39-0.52)
Pink shrimp (juv.) flow 24 28 DDTd 48-h LC50 0.6 Mayer (1987)
(Penaeus duorarum) flow 16 31 TDEd 48-h LC50 2.4 Mayer (1987)
White shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.7 Mayer (1987)
(Penaeus setiferus)
Grass shrimp (juv.) flow 27 28 DDTd 24-h LC50 0.8 Mayer (1987)
(Palaemonetes pugio)
Brown shrimp (juv.) flow 28 17-27 DDEd 24-h LC50 52 Butler (1964)
(Penaeus aztecus) flow 28 17-27 DDEd 48-h LC50 28 Butler (1964)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata ( °C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Freshwater invertebrates
Water flea stat 20 192 138 8.2- DDTd 48-h LC50 1.1 (1.0-1.3) Randall et
8.5 al. (1979)
(Daphnia magna) stat 15 44 7.1 DDTd 48-h LC50 4.7 (2.8-5.6) Mayer &
Ellersieck
(1986)
stat 20 192 138 8.2- DDTe 48-h LC50 1.7 (1.5-1.8) Randall et
8.5 al. (1979)
statb 24 320- 7.6 DDT 14-day 0.67 (0.65-0.69) Maki &
340 (99%) LC50 Johnson
statb 24 320- 7.6 DDT 14-day 0.5 (0.48-0.52) (1975)
340 (99%) EC50g
statb 24 320- 7.6 DDT 14-day 0.61 (0.58-0.64) Maki &
340 (99%) EC50h Johnson
statb 24 320- 7.6 DDT 14-day 0.75 (0.71-0.79) (1975)
340 (99%) EC50i
stat 10 44 7.1 TDEd 48-h LC50 9.1 Mayer &
stat 21 44 7.1 TDEd 48-h LC50 8.9 Ellersieck
(1986)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 510 (230-1120) Berglind &
soft water stat 20.5 250 7.8-8.2 DDT 48-h LC50 1.1 (0.89-1.7) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 98 (75-127) Berglind &
50 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 1.3 (1.1-1.5) Dave (1984)
reared in stat 20.5 250 7.8-8.2 DDT 24-h LC50 71 (41-130) Berglind &
hard water stat 20.5 250 7.8-8.2 DDT 48-h LC50 0.68 (0.46-1.0) Dave (1984)
(CaCO3: stat 20.5 250 8.4-8.5 DDT 24-h LC50 42 (32-56) Berglind &
300 mg/litre) stat 20.5 250 8.4-8.5 DDT 48-h LC50 0.5 (0.41-0.61) Dave
stat 20.5 50 7.8-8.2 DDT 24-h LC50 0.99 (0.66-1.49) (1984)
Water flea stat 15 44 7.1 DDTd 48-h LC50 0.36 (0.28-0.47) Mayer &
(Daphnia pulex) Ellersieck
(1986)
Water flea stat 15 44 7.1 DDTd 48-h LC50 2.5 (1.9-3.3) Mayer &
(Simocephalus stat 21 44 7.1 DDTd 48-h LC50 2.8 (2.3-3.5) Ellersieck
serrulatus) stat 15 44 7.1 TDEd 48-h LC50 3.2 (2.3-4.4) (1986)
stat 21 44 7.1 TDEd 48-h LC50 4.5 (3.1-6.6)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Scud stat 21 35 44 7.1 TDEd 24-h LC50 4.6 (3.6-5.8) Sanders
(Gammarus fasciatus) stat 21 35 44 7.1 TDEd 96-h LC50 0.6 (0.05-1.2) (1972)
stat 21 35 44 7.1 DDTd 24-h LC50 15 (9.0-20) Sanders
stat 21 35 44 7.1 DDTd 96-h LC50 3.2 (1.8-5.6) (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 3.2 (2.1-4.3) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.86 (0.42-1.3) (1972)
stat 21 260 272 7.4 DDTd 24-h LC50 4.2 (1.8-5.6) Sanders
stat 21 260 272 7.4 DDTd 48-h LC50 3.1 (1972)
stat 21 260 272 7.4 DDTd 96-h LC50 1.8 (1.0-3.1) Sanders
(1972)
stat 21 260 272 7.4 DDTd 120-h LC50 0.32 Sanders
flow 18-21 260 272 7.4 DDTd 24-h LC50 1.1 (1972)
flow 18-21 260 272 7.4 DDTd 48-h LC50 1.0 Sanders
flow 18-21 260 272 7.4 DDTd 96-h LC50 0.8 (1972)
flow 18-21 260 272 7.4 DDTd 120-h LC50 0.6 Sanders
(1972)
Scud stat 21 44 7.1 DDTd 24-h LC50 4.7 (3.2-7.0) Mayer &
(Gammarus lacustris) stat 21 44 7.1 DDTd 96-h LC50 1.0 (0.68-1.5) Ellersieck
(1986)
stat 15 DDTe 96-h LC50 9.0 Gaufin et
al. (1965)
Glass shrimp stat 21 260 272 7.4 DDTd 24-h LC50 6.8 (6.2-7.5) Sanders
(Palaemonetes stat 21 260 272 7.4 DDTd 48-h LC50 4.7 (1972)
kadiakensis) stat 21 260 272 7.4 DDTd 96-h LC50 2.3 (1.3-4.9) Sanders
stat 21 260 272 7.4 DDTd 120-h LC50 1.0 (1972)
stat 21 260 272 7.4 TDEd 24-h LC50 11 (8.4-16) Sanders
stat 21 260 272 7.4 TDEd 96-h LC50 0.68 (0.47-1.1) (1972)
flow 18-21 260 272 7.4 DDTd 24-h LC50 9.4 Sanders
flow 18-21 260 272 7.4 DDTd 48-h LC50 7.7 (1972)
flow 18-21 260 272 7.4 DDTd 96-h LC50 3.5 Sanders
flow 18-21 260 272 7.4 DDTd 120-h LC50 1.3 (1972)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Crayfish (Orconectes nais)
mature stat 21 260 7.4 DDTd 24-h LC50 1100 (1000-1400) Sanders
stat 21 260 7.4 DDTd 96-h LC50 100 (80-120) (1972)
1 day old - 15g stat 21 260 7.4 DDTd 24-h LC50 1.4 (1.1-4.2) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.3 (0.18-0.5) (1972)
1 week old - 20g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.18 (0.12-0.3) (1972)
2 weeks old - 23g stat 21 260 7.4 DDTd 24-h LC50 1.2 (0.9-5.5) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.2 (0.16-1.1) (1972)
3 weeks old - 30g stat 21 260 7.4 DDTd 24-h LC50 1.0 (0.6-5.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.24 (0.1-0.6) (1972)
5 weeks old - 50g stat 21 260 7.4 DDTd 24-h LC50 3.2 (1.8-8.0) Sanders
stat 21 260 7.4 DDTd 96-h LC50 0.9 (0.7-1.4) (1972)
8 weeks old - 500g stat 21 260 7.4 DDTd 24-h LC50 45 (40-52) Sanders
stat 21 260 7.4 DDTd 96-h LC50 28 (24-36) (1972)
10 weeks old - 1200g stat 21 260 7.4 DDTd 24-h LC50 50 (48-56) Sanders
stat 21 260 7.4 DDTd 96-h LC50 30 (26-42) (1972)
Sowbug (isopod) stat 21 35 7.1 DDTd 24-h LC50 8.7 (4.9-13.0) Sanders
(Asellus brevicaudus) stat 21 35 7.1 DDTd 96-h LC50 4.0 (1.2-6.5) (1972)
stat 21 35 7.1 TDEd 24-h LC50 18 (14-25) Sanders
stat 21 35 7.1 TDEd 96-h LC50 10 (7.0-14) (1972)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 48 Gaufin et
(Hydropsyche californica) 12 al. (1965)
Caddis fly (nymph) stat 10.5- DDTe 96-h LC50 175 Gaufin et
(Arctopsyche grandis) 12 al. (1965)
May fly (nymph) stat 8.8- DDTe 96-h LC50 25 Gaufin et
(Ephemerella grandis) 10 al. (1965)
Stonefly (naiad) stat 11- DDTe 96-h LC50 320 Gaufin et
(Acroneuria pacifica) 12 al. (1965)
Table 3. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow Temp Alkali- Hard- pH Comp- Parameter Water Reference
Stata (°C) nityc nessc ound concentration
(µg/litre)
---------------------------------------------------------------------------------------------------------
Stonefly (naiad) stat 11- DDTe 96-h LC50 1800 Gaufin et
(Pteronarcys 12 al. (1965)
californica) stat 15.5 35 DDT 24-h LC50 41 (27-62) Sanders &
stat 15.5 35 DDT 48-h LC50 19 (14-27) Cope (1968)
stat 15.5 35 DDT 96-h LC50 7 (4.9-9.9) Sanders &
stat 15.5 35 TDE 24-h LC50 3000 (2100-4300) Cope (1968)
stat 15.5 35 TDE 48-h LC50 1100 (800-1500) Sanders &
stat 15.5 35 TDE 96-h LC50 380 (280-520) Cope (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 12 (8.8-16) Sanders &
(Pteronarcella badia) stat 15.5 35 DDT 48-h LC50 9 (7-11) Cope
stat 15.5 35 DDT 96-h LC50 1.9 (1.3-2.7) (1968)
Stonefly (naiad) stat 15.5 35 DDT 24-h LC50 16 (12-20) Sanders &
(Claasenia sabulosa) stat 15.5 35 DDT 48-h LC50 6.4 (4.9-8.3) Cope
stat 15.5 35 DDT 96-h LC50 3.5 (2.9-4.2) (1968)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions (DDT
concentration in water continuously maintained).
b Static conditions but test solution renewed every 24 h.
c Alkalinity and hardness expressed as mg CaCO3/litre.
d Technical grade (99%).
e Emulsifiable concentrate (25% active ingredient).
f Juv. = juvenile.
g Value based on total number of young produced.
h Value based on average brood size.
i Value based on % days reproducing.
j Effect on shell growth.
Eggs of the Chironomid midge, contaminated with DDE by exposure of
the female during ovarian development, failed to hatch as many adults
as uncontaminated eggs. DDE in the water had less of an effect than
DDE contamination within the eggs obtained from the female. The
females had been maintained in water containing 30 µg DDE/litre; eggs
were kept in water containing 20 µg DDE/litre (Derr & Zabik, 1972).
Crayfish populations exposed over long periods to DDT develop some
tolerance to the insecticide (Albaugh, 1972). In 48-h tests, LC50
values for the crayfish Procambarus clarkii were 3.0 (2.5-3.6) µg/litre
for the unexposed population, and 7.2 (5.8-8.8) µg/litre for the
exposed population (95% confidence limits in parentheses). Naqvi &
Ferguson (1968) demonstrated the development of tolerance to DDT after
exposure to the insecticide, in a wide variety of aquatic
invertebrates, including cyclopoid copepods, tubifex worms, and pond
snails. These tolerant populations occurred in the Mississippi delta
in areas of cotton cultivation.
5.2 Fish
Appraisal
DDT is highly toxic to fish; the 96-h LC50s reported (static
tests) range from 1.5 to 56 µg/litre (for largemouth bass and guppy,
respectively). Smaller fish are more susceptible than larger ones of
the same species. An increase in temperature decreases the toxicity of
DDT to fish.
The behaviour of fish is influenced by DDT. Goldfish exposed to
1 µg/litre exhibit hyperactivity. Changes in the feeding of young
fish are caused by DDT levels commonly found in nature, and effects on
temperature preference have been reported.
Residue levels of > 2.4 mg/kg in eggs of the winter flounder result
in abnormal embryos in the laboratory, and comparable residue levels
have been found to relate to the death of lake trout fry in the wild.
Cellular respiration may be the main toxic target of DDT since
there are reports of effects on ATPase.
The toxicity of TDE and DDE has been less studied than that of DDT.
However, the data available show that TDE and DDE are both less toxic
than DDT.
The exact mode of action of DDT in fish remains unclear. There
have been many different suggestions to explain both lethal and
sublethal effects. Most of these are primarily the result of effects
on membranes. DDT is very soluble in lipid and, therefore, dissolves
in the lipid component of membranes. It may interfere both with
membrane function and with many enzyme systems that are located on
membranes. It has been shown experimentally to interfere with the
normal function of so many systems that a primary action of DDT is
difficult to determine.
5.2.1 Short-term and long-term direct toxicity to fish
The short-term toxicity of DDT to fish is summarized in Table 4.
The relatively few studies on TDE (Gardner, 1973; Korn & Earnest,
1974; Mayer & Ellersieck, 1986; Mayer, 1987) show it to be less toxic
than DDT, in the same test system, by factors of 5-10. The still fewer
studies on DDE indicate a similarly lowered toxicity relative to the
parent compound (Mayer & Ellersieck, 1986; Mayer, 1987). Whilst there
is some variation between species, DDT has proved highly toxic to all
fish tested; static 24-h LC50 values range from 2.1 µg/litre for the
largemouth bass (Mayer & Ellersieck, 1986) to 180 µg/litre for the
goldfish (Henderson et al., 1959). For 96-h tests, LC50 values range
from 1.5 µg/litre for largemouth bass (Mayer & Ellersieck, 1986) to
56 µg/litre for the guppy (Henderson et al., 1959). Several authors
have stated that DDT toxicity varies somewhat with temperature and
water hardness.
Buhler et al. (1969) studied the long-term effects, over 95 days,
of feeding DDT-contaminated diets to juvenile chinook and coho salmon.
The DDT was dissolved in corn-oil and then incorporated into a semi-
synthetic diet. Fish were fed until they stopped actively taking the
slowly sinking food. Pure p,p' -DDT was slightly more toxic to
juvenile salmon than the technical product, and chinook salmon were
2 to 3 times more sensitive to the same dose of DDT in the diet than
coho salmon. Size was an important factor in the toxicity of DDT,
smaller fish being more susceptible than larger ones. The authors
estimated, by extrapolation, a 90-day LD50 value of 27.5 µg/kg per
day for chinook and 64 µg/kg per day for coho salmon juveniles. In
fish exposed to higher doses of DDT, pre-death symptoms were marginal.
Some increased agitation and slight photophobia were reported. Fish
exposed to low doses of DDT took longer to die, and other symptoms were
noted. Many individuals developed ulceration of the nasal area. This
spread over the head and in some cases eyes were lost. Pathological
examination showed a specific and severe kidney lesion; this was
limited to one short section of the distal convoluted tubule, which
eventually degenerated almost completely. The authors suggested this
as the main lethal lesion in the fish.
In a later study (Buhler & Shanks, 1970), the same authors showed
that median survival time was directly proportional to body weight in
young coho salmon fed technical DDT. Fish were all given a diet
containing 200 mg DDT/kg and food consumption was monitored for each
group of fish. The main effect of body size on DDT lethality was
related to the intake of the chemical by the fish; smaller fish ate
more of the contaminated diet and consequently received the greatest
dose in mg/kg bodyweight terms. However, even after correcting for
dosage received, the smaller fish were more susceptible than larger
ones. The authors suggested that the lower lipid content of smaller
fish might have accounted for the remaining difference. Twelve groups
of 100 fish ranged in weight (average for each group) from 3 to 15 g.
Total DDT intake ranged from 0.4 to 3 mg/fish; daily intake was higher
in the smaller fish at 3 mg/kg per day, falling to 1.3 mg/kg per day
for the largest. The estimated LC50 ranged from 95 mg/kg for the
smallest to 135 mg/kg for the largest fish, and median survival time
increased from 30 days for the smallest fish to 106 days for the
largest.
Crawford & Guarino (1976) exposed killifish ( Fundulus heteroclitus )
to a twice-repeated schedule of 24 h in water containing DDT at a
concentration of 0.1 mg/litre and 24 h in clean water. At this
exposure level, there was a delay in the rate of development of ferti-
lized eggs but no apparent effect on the hatched fry. Fertilization of
killifish eggs was diminished when insemination was carried out in sea
water containing DDT at 0.1 mg/litre. Mortality at a late stage of
embryo development has been reported for a variety of salmonids and
related to egg residues of DDT (Allison et al., 1964, for cutthroat
trout; Burdick et al., 1964, for lake trout; Macek, 1968, for brook
trout; and Johnson & Pecor, 1969, for coho salmon).
Smith & Cole (1973) reported effects on embryos developing from
eggs laid by adult winter flounder ( Pseudopleuronectes americanus )
that were exposed to 2 µg DDT/litre for various times and, therefore,
accumulated different residue levels in the eggs. These residue levels
varied from 1.15 to 3.70 mg DDT/kg and from 0.07 to 0.4 mg DDE/kg.
Embryos showed abnormal gastrulation and a high incidence (mean 39%)
of vertebral deformities. Bone erosion and haemorrhaging at the
vertebral junctures were often associated with the vertebral deform-
ities.
Halter & Johnson (1974) report that DDT is toxic to the early
life-stages of coho salmon. Mean survival times were considerably
reduced by water concentrations of DDT greater than 0.5 µg/litre.
5.2.2 Sublethal behavioural effects on fish
Hansen (1969) and Hansen et al. (1972) investigated the avoidance
of DDT by sheepshead minnows and mosquitofish in a 'Y'-shaped avoidance
maze. Although there was some statistically significant avoidance of
DDT when fish were given the choice between DDT and clean water, this
only occurred at concentrations of the insecticide above the 24-h
LC50. Fish of both species, when given the choice between DDT at 0.1
and 0.01 mg/litre, chose the higher concentration of the chemical.
This suggests that the perception of DDT is poor and that fish could
not reliably avoid DDT in water at toxic concentrations.
Olofsson & Lindahl (1979) administered either 0.5 or 1.0 mg DDT/kg
body weight to cod by oral intubation. There was a significant effect,
at the higher dose but not the lower one, on the ability of the fish to
compensate its posture to cope with a rotating tube in which it was
swimming.
Hansen (1972) allowed mosquitofish to select a desired salinity in
a fluvarium with a salinity gradient. Fish selected a higher salinity
than controls when exposed to DDT, but only at exposure levels which
caused some mortality. The author suggested that DDT might have affec-
ted the osmoregulatory ability of the mosquitofish. Other possible
explanations include a change in sensitivity of nerves to stimuli or a
preference for the pre-exposure salinity, which was 15 g/litre.
Table 4. Toxicity of DDT and its derivatives to fish
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Salinity Compound Parameter Water Reference
(g)/ stata perat- o/oo concen-
agef ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Estuarine and marine fish
Dwarf perch 1.2-11.0 Stat 13 28 DDTc 96-h LC50 4.6 Earnest &
(Micrometrus minimus) 1.2-11.0 flowb 14-18 26-28 DDTc 96-h LC50 0.26 Benville
(0.13-0.52) (1972)
Shiner perch 1.2-11.0 stat 13 26 DDTc 96-h LC50 7.6 Earnest &
(Cymatogaster aggregata) 1.2-11.0 flowb 14-18 13-23 DDTc 96-h LC50 0.45 Benville
(0.21-0.94) (1972)
Striped bass 2.7 flowb 17 28 DDT(77%) 96-h LC50 0.53 Korn &
(Morone saxatilis) (0.38-0.84) Earnest
0.6 flowb 17 30 TDEc 96-h LC50 2.5 (1974)
(1.6-4.0)
Sheepshead minnow juv. flow 15 30 DDTc 48-h LC50 2.0 Mayer
(Cyprinodon variegatus) (1987)
Longnose killifish juv. flow 15 30 DDTc 48-h LC50 2.8 Mayer
(Fundulus similis) juv. flow 16 28 TDEc 48-h LC50 42.0 (1987)
Pinfish juv. flow 22 29 DDTc 48-h LC50 0.3 Mayer
(Lagodon rhomboides) (1987)
Striped mullet juv. flow 15 30 DDTc 48-h LC50 0.4 Mayer
(Mugil cephalus) (1987)
Spot juv. flow 12 26 DDEc 48-h LC50 > 100 Mayer
(Leiostomus xanthurus) juv. flow 26 30 TDEc 48-h LC50 20.0 (1987)
Three-spined 0.4-0.8 stat 20 5 DDT 24-h LC50 22.0 Katz
stickleback 0.4-0.8 stat 20 5 DDT 48-h LC50 21.0 (1961)
(Gasterosteus 0.4-0.8 stat 20 5 DDT 72-h LC50 18.5 Katz
aculeatus) 0.4-0.8 stat 20 5 DDT 96-h LC50 18.0 (1961)
0.4-0.8 stat 20 25 DDT 24-h LC50 18.0 Katz
0.4-0.8 stat 20 25 DDT 48-h LC50 15.0 (1961)
0.4-0.8 stat 20 25 DDT 72-h LC50 14.5 Katz
0.4-0.8 stat 20 25 DDT 96-h LC50 11.5 (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Freshwater fish
Black bullhead 1.2 stat 18 44 7.1 DDTc 24-h LC50 36.8 Mayer &
(Ictalurus melas) (20.3-67.0)
1.2 stat 18 44 7.1 DDTc 96-h LC50 4.8
(3.4-6.8) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 26.2
(22.0-31.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 5.1
(3.9-6.7) Ellersieckg
Channel catfish 1.5 stat 18 44 7.1 DDTc 24-h LC50 22.0
(Ictalurus punctatus) (18.2-26.5) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 21.5
(17.7-26.1) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 18.4
(13.7-24.7) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 17.3
(13.0-23.1) Ellersieckg
0.7 stat 18 44 7.1 DDTc 24-h LC50 17.9
(12.7-25.3) Mayer &
0.7 stat 18 44 7.1 DDTc 96-h LC50 6.9
(5.7-8.5) Ellersieckg
1.6 stat 18 44 7.1 DDTc 24-h LC50 44.0
(37.0-52.0) Mayer &
1.6 stat 18 44 7.1 DDTc 96-h LC50 22.0
(19.0-26.0) Ellersieckg
1.4 stat 18 44 7.1 DDTc 24-h LC50 30.0
(22.0-41.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 16.0
(9.4-29.0) Ellersieckg
1.4 stat 18 272 7.7 DDTc 24-h LC50 29.0
(20.0-41.0) Mayer &
1.4 stat 18 272 7.7 DDTc 96-h LC50 7.0
(4.3-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Atlantic salmon 0.45 stat 12 40 7.5 DDTc 24-h LC50 6.2
(Salmo salar) (4.6-8.4) Mayer &
0.45 stat 12 40 7.5 DDTc 96-h LC50 1.8
(1.3-2.6) Ellersieckg
0.5 stat 12 44 7.5 DDEc 96-h LC50 96.0
(52.1-177) Mayer &
Ellersieckg
Coho salmon 2.7-4.1 stat 20 45-57 6.8-7.4 DDT 24-h LC50 66.0 Katz (1961)
(Oncorhynchus kisutch)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 48-h LC50 46.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 72-h LC50 44.0 Katz (1961)
2.7-4.1 stat 20 45-57 6.8-7.4 DDT 96-h LC50 44.0 Katz (1961)
1.0 stat 13 44 7.1 DDTc 24-h LC50 10.0
(7.0-12.0) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 4.0
(3.0-6.0) Ellersieckg
6.0 stat 13 40 7.1 DDTc 24-h LC50 26.9
(18.1-40.0) Mayer &
6.0 stat 13 40 7.1 DDTc 96-h LC50 19.3
(9.6-38.8) Ellersieckg
Chinook salmon 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 24-h LC50 38.0 Katz (1961)
(Oncorhynchus
tshawytscha) 1.5-5.0 stat 20 45-57 6.8-7.4 DDT 48-h LC50 17.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 72-h LC50 14.0 Katz (1961)
1.5-5.0 stat 20 45-57 6.8-7.4 DDT 96-h LC50 11.5 Katz (1961)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Rainbow trout 0.9 stat 7 44 7.1 DDTc 24-h LC50 7.5
(Salmo gairdneri) (6.7-8.3) Mayer &
0.9 stat 7 44 7.1 DDTc 96-h LC50 4.1
(3.6-4.6) Ellersieckg
0.9 stat 13 44 7.1 DDTc 24-h LC50 8.2
(7.2-9.2) Mayer &
0.9 stat 13 44 7.1 DDTc 96-h LC50 4.7
(4.2-5.3) Ellersieckg
0.9 stat 18 44 7.1 DDTc 24-h LC50 12.0
(1.0-13.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 5.8
(5.2-6.5) Ellersieckg
3.2 stat 20 45-57 6.8-7.4 DDT 24-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 48-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 72-h LC50 42.0 Katz (1961)
3.2 stat 20 45-57 6.8-7.4 DDT 96-h LC50 42.0 Katz (1961)
1.8 flow 17 272 7.4 DDTc 96-h LC50 > 3.0 Mayer &
0.8 stat 12 44 7.1 DDEc 96-h LC50 32.0
(26.0-40.0) Ellersieckg
1.0 stat 12 44 7.1 TDEc 96-h LC50 70.0
(57.0-87.0) Mayer &
1.0 stat 12 272 7.4 TDEc 96-h LC50 70.0
(58.0-85.0) Ellersieckg
Cutthroat trout 1.0 stat 13 44 7.1 DDTc 24-h LC50 8.4
(Salmo clarki) (7.6-9.2) Mayer &
1.0 stat 13 44 7.1 DDTc 96-h LC50 5.5
(4.7-6.4) Ellersieckg
1.8 stat 9 162 7.4 DDTc 24-h LC50 11.3
(9.4-13.6) Mayer &
1.8 stat 9 162 7.4 DDTc 96-h LC50 7.9
(6.5-9.7) Ellersieckg
Brown trout 1.7 stat 13 44 7.1 DDTc 96-h LC50 1.8
(Salmo trutta) (1.3-2.5) Mayer &
Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Northern pike 0.7 stat 18 272 7.4 DDTc 24-h LC50 5.5 Mayer &
(Esox lucius) 0.7 stat 18 272 7.4 DDTc 96-h LC50 2.7 Ellersieckg
Guppy 0.1-0.2 stat 25 18 20 7.4 DDTc 24-h LC50 135 Henderson
(Lebistes 0.1-0.2 stat 25 18 20 7.4 DDTc 48-h LC50 72.0 et al.
reticulatus) 0.1-0.2 stat 25 18 20 7.4 DDTc 96-h LC50 56.0 (1959)
River shiner 0.3 stat 18 44 7.1 DDTc 24-h LC50 6.7
(Notropis blennius) (4.9-9.1) Mayer &
0.3 stat 18 44 7.1 DDTc 96-h LC50 5.8
(3.6-9.1) Ellersieckg
Fathead minnow 1.2 stat 18 44 7.1 DDTc 24-h LC50 14.2
(Pimephales (11.0-18.0) Mayer &
promelas) 1.2 stat 18 44 7.1 DDTc 96-h LC50 12.4
(10.0-15.4) Ellersieckg
1.2 stat 18 272 7.4 DDTc 24-h LC50 13.8
(10.3-18.3) Mayer &
1.2 stat 18 272 7.4 DDTc 96-h LC50 13.2
(10.1-17.3) Ellersieckg
0.9 flow 12 314 7.6 DDTc 96-h LC50 9.9
(6.5-15.0) Mayer &
Ellersieckg
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 56.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 42.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 24-h LC50 78.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDTc 48-h LC50 68.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDTc 96-h LC50 45.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 24-h LC50 32.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDT 48-h LC50 26.0 et al. (1959)
1.0-2.0 stat 25 18 20 7.4 DDT 96-h LC50 26.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 24-h LC50 29.0 et al. (1959)
1.0-2.0 stat 25 360 400 8.2 DDT 48-h LC50 27.0 Henderson
1.0-2.0 stat 25 360 400 8.2 DDT 96-h LC50 26.0 et al. (1959)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Mosquitofish 0.2 stat 25 DDTc 24-h LC50 22.7
(Gambusia affinis) (16.6-31.1) El-Sebae (1987)
0.2 stat 25 DDTc 96-h LC50 9.9
(7.3-13.4) El-Sebae (1987)
0.2 stat 25 DDTe 24-h LC50 58.6
(43.2-79.5) El-Sebae (1987)
0.2 stat 25 DDTe 96-h LC50 27.7
(21.3-36.0) El-Sebae (1987)
Bluegill sunfish 0.26 stat 19 138 192 8.2-8.5 DDTc 96-h LC50 3.4
(Lepomis macrochirus) (2.6-4.1) Randall
0.26 stat 19 138 192 8.2-8.5 DDT 96-h LC50 9.0
(7.4-10.6) et al. (1979)
(25%)
1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 26.0 Henderson
1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 21.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 21.0 (1959)
1.5 stat 18 44 7.1 DDTc 24-h LC50 11.5
(8.4-16.0) Mayer &
1.5 stat 18 44 7.1 DDTc 96-h LC50 8.6
(6.2-12.0) Ellersieckg
1.5 stat 18 272 7.4 DDTc 24-h LC50 10.0
(8.5-12.9) Mayer &
1.5 stat 18 272 7.4 DDTc 96-h LC50 6.3
(4.3-9.3) Ellersieckg
0.9 stat 17 44 7.1 DDEc 96-h LC50 240
(201-286) Mayer &
0.9 stat 24 44 7.4 TDEc 24-h LC50 56.0
(46.0-68.0) Ellersieckg
0.9 stat 24 44 7.4 TDEc 96-h LC50 42.0
(36.0-49.0) Mayer &
Ellersieckg
Redear sunfish 3.2 stat 24 44 7.1 DDTc 24-h LC50 19.0 Mayer &
(Lepomis microlophus) 3.2 stat 24 44 7.1 DDTc 96-h LC50 15.0 Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Green sunfish 1.1 stat 18 44 7.1 DDTc 24-h LC50 16.9
(Lepomis cyanellus) (12.7-22.3) Mayer &
1.1 stat 18 44 7.1 DDTc 96-h LC50 10.9
(7.3-15.6) Ellersieckg
0.8 stat 18 44 7.1 DDTc 24-h LC50 18.0
(13.0-24.0) Mayer &
0.8 stat 18 44 7.1 DDTc 96-h LC50 6.5
(4.1-10.4) Ellersieckg
1.1 stat 18 272 7.7 DDTc 24-h LC50 19.8
(15.0-25.6) Mayer &
1.1 stat 18 272 7.7 DDTc 96-h LC50 9.9
(6.4-15.0) Ellersieckg
Largemouth bass 0.8 stat 18 44 7.1 DDTc 24-h LC50 3.7
(Micropterus (3.1-4.5) Mayer &
Salmoides) 0.8 stat 18 44 7.1 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.8 stat 18 272 7.4 DDTc 24-h LC50 2.1
(1.6-2.9) Mayer &
0.8 stat 18 272 7.4 DDTc 96-h LC50 1.5
(0.9-2.4) Ellersieckg
0.7 stat 18 44 7.1 TDEc 24-h LC50 50.0
(35.0-71.0) Mayer &
0.7 stat 18 44 7.1 TDEc 96-h LC50 42.0
(34.0-51.0) Ellersieckg
Black crappie 1.0 stat 18 44 7.1 DDTc 24-h LC50 6.5
(Pomoxis (5.4-7.8) Mayer &
nigromaculatus) 1.0 stat 18 44 7.1 DDTc 96-h LC50 5.6
(4.6-6.7) Ellersieckg
Yellow perch 1.4 stat 18 44 7.1 DDTc 24-h LC50 10.0
(Perca flavescens) (8.0-12.0) Mayer &
1.4 stat 18 44 7.1 DDTc 96-h LC50 9.0
(7.0-11.0) Ellersieckg
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Walleye 1.4 stat 18 44 7.1 DDTc 24-h LC50 4.2
(Stizostedion v. (3.2-5.6) Mayer &
vitreum) 1.4 stat 18 44 7.1 DDTc 96-h LC50 2.9
(2.4-3.5) Ellersieckg
1.3 stat 18 272 7.4 DDTc 24-h LC50 4.6
(3.9-5.4) Mayer &
1.3 stat 18 272 7.4 DDTc 96-h LC50 4.6
(3.9-5.4) Ellersieckg
1.0 stat 18 44 7.1 TDEc 24-h LC50 20.0
(16.0-24.0) Mayer &
1.0 stat 18 44 7.1 TDEc 96-h LC50 14.0
(11.0-19.0) Ellersieckg
Tilapia 0.8 stat 24 44 7.1 DDTc 24-h LC50 19.0
(Tilapia mossambica) (16.0-23.0) Mayer &
0.8 stat 24 44 7.1 DDTc 96-h LC50 17.0
(14.0-21.0) Ellersieckg
0.8 stat 24 272 7.4 DDTc 24-h LC50 15.0
(13.0-17.0) Mayer &
0.8 stat 24 272 7.4 DDTc 96-h LC50 14.0
(12.0-16.0) Ellersieckg
0.8 flow 18 272 7.4 DDTc 24-h LC50 24.0
(17.0-32.0) Mayer &
0.8 flow 18 272 7.4 DDTc 96-h LC50 5.1
(3.2-8.1) Ellersieckg
Tilapia 0.8 stat 25 DDTc 24-h LC50 21.8
(Tilapia zilli) (17.0-28.0) El-Sebae (1987)
0.8 stat 25 DDTc 96-h LC50 15.5
(11.7-20.6) El-Sebae (1987)
0.8 stat 25 DDTe 24-h LC50 12.8
(9.6-17.1) El-Sebae (1987)
0.8 stat 25 DDTe 96-h LC50 9.5
(7.4-12.3) El-Sebae (1987)
Table 4. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Size Flow/ Tem- Alkali- Hard- pH Com- Parameter Water Reference
(g) stata perat- nityd nessd pound concen-
ure tration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Goldfish 1.0-2.0 stat 25 18 20 7.4 DDTc 24-h LC50 180 Henderson
(Carassius auratus) 1.0-2.0 stat 25 18 20 7.4 DDTc 48-h LC50 47.0 et al.
1.0-2.0 stat 25 18 20 7.4 DDTc 96-h LC50 36.0 (1959)
0.9 stat 18 44 7.1 DDTc 24-h LC50 24.0
(17.0-33.0) Mayer &
0.9 stat 18 44 7.1 DDTc 96-h LC50 15.5
(9.1-26.0) Ellersieckg
0.9 stat 18 272 7.4 DDTc 24-h LC50 22.2
(16.0-31.1) Mayer &
0.9 stat 18 272 7.4 DDTc 96-h LC50 14.7
(10.0-20.0) Ellersieckg
Common carp 0.6 stat 18 44 7.1 DDTc 24-h LC50 14.0
(Cyprinus carpio) (10.0-19.0) Mayer &
0.6 stat 18 44 7.1 DDTc 96-h LC50 9.7
(7.4-12.9) Ellersieckg
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); Flow = flow-through conditions
(DDT concentration in water continuously maintained).
b Intermittent flow-through conditions.
c Technical grade (99%).
d Alkalinity and hardness expressed as mg CaCO3/litre.
e 25% emulsifiable concentrate.
f Juv. = juvenile.
g 1986.
Peterson (1973) monitored the selection of temperature by
juvenile Atlantic salmon ( Salmo salar ) previously exposed to DDT or
its metabolites. Low concentrations produced no effect on tem-
perature selection, but at higher levels of exposure the temperature
selected by the fish increased. Fish were most sensitive, in this
respect, to p,p' -DDE and showed decreasing sensitivity to
o,p' -DDT, p,p' -TDE, and p,p' -DDT. Increasing the exposure to
p,p' -DDE from 0 to 1.0 mg/litre increased the preferred temperature
from about 16 °C to 21 °C. There was no effect of p,p' -DDA on
temperature selection at concentrations as high as 8 mg/litre. In a
similar experiment, where brook trout (Salvelinus fontinalis) were
exposed to a vertical rather than horizontal temperature gradient, fish
previously exposed to p,p' -DDT and p,p' -TDE selected higher
temperatures than controls. Conversely, Gardner (1973) found that DDT
and its analogues induced selection of lower temperatures by the same
species of fish over a dose range between 0 and 50 µg/litre; DDE did
not produce any temperature preference. Ogilvie & Miller (1976)
reported that Atlantic salmon exposed to DDT at a concentration of
50 µg/litre selected higher temperatures, the effect persisting for at
least 4 weeks after exposure. The authors suggested that the tempera-
ture selection response to DDT exposure is "biphasic". At low
exposure levels, similar to those used by Gardner (1973), lower
temperatures are selected, whilst higher temperatures are preferred at
higher exposure levels.
Dill & Saunders (1974) exposed the eggs of Atlantic salmon
at gastrulation to DDT at water concentrations of 5, 10, 50, or
100 µg/litre, and observed behavioural development in hatched fry over
30 days following hatch. The two highest doses of DDT impaired balance
and retarded behavioural development of the fry (i.e., the appearance
of normal behaviour patterns was delayed). The authors considered that
the effects observed would affect predation rates and feeding, in
young fish, at "realistic" DDT exposure levels in the wild.
Davy et al. (1973) reported that exposure to DDT, at 10 µg/litre
for 4 days, affected the exploratory behaviour of goldfish experi-
encing a novel environment. They attributed the effect to a central
nervous system lesion caused by DDT. Weis & Weis (1974) showed an
increase in individual activity and an increase in school-size in
groups of goldfish exposed to DDT at 1 µg/litre for 7 days. After a
frightening stimulus, schools scattered further and did not regroup as
readily as control fish. The transfer of fish to clean water led to
a return to normal behaviour within one week. An effect on the
locomotor behaviour of goldfish after exposure to 10 µg DDT/litre per-
sisted for the remainder of the observation period of 130-139 days
(Davy et al., 1972).
5.2.3 Physiological effects on fish
Hanke et al. (1983) investigated the effects of DDT on a range of
physiological functions in carp ( Cyprinus carpio ). At water concen-
trations of 100 or 500 µg/litre, the insecticide induced changes in
plasma cortisol and glucose levels, liver glycogen level, and plasma
and brain acetylcholinesterase activity. The response was biphasic in
all cases. Initially, after 6 hours, there was a stimulation of these
parameters which, within 24 hours, changed to an inhibition.
Ramalingam & Ramalingam (1982) reported that the chronic effect of DDT
on glycogen utilization in fish led to the use of protein as an energy
source. The protein content of tissues declined after chronic exposure
to DDT.
Janicki & Kinter (1971) found that DDT impaired fluid absorption in
the intestinal sacs of eels adapted to sea water and exposed to the
insecticide at 50 µg/litre. DDT also inhibited Na+-, K+-, and
Mg2+-dependent ATPases in homogenates of the intestinal mucosa. In a
later study, Kinter et al. (1972) showed that plasma osmolarity was
also affected in sea-water-adapted eels exposed to DDT (1 mg/litre)
for 9 to 10 hours. Haux & Larsson (1979) reported effects of DDT on
plasma electrolytes in the flounder Platichthys flesus kept in
hypotonic, brackish water. The fish were force-fed with DDT in gelatin
capsules to give a total DDT dose of 1.5 or 15.0 mg/kg body weight.
Plasma sodium was reduced but not significantly; plasma chloride was
significantly reduced in a dose-related manner after 3 weeks but not
after 6 weeks. Waggoner & Zeeman (1975) reported similar effects on
plasma electrolytes in the black surfperch ( Embiotoca jacksoni ), but
only at high DDT exposure levels. They injected DDT doses of 1, 10,
100, or 200 mg/kg; the only effect occurred with the dose of 200 mg/kg,
but the fish did not survive to 72 h. The authors suggested that
osmoregulatory effects are not the major cause of DDT-induced mortality
in marine fish.
Desaiah et al. (1975) exposed fathead minnows ( Pimephales
promelas ) for long periods to DDT at water concentrations of 0.5 or
2.0 µg/litre and also via the food, and monitored the activity of
ATPases in brain and gill. This study followed up several previous
studies on in vitro effects on these enzymes. After 266 days of
exposure, there was an approximately 50% reduction in brain
oligomycin-sensitive (mitochondrial) Mg2+-ATPase activity. In
contrast, oligomycin-insensitive Mg2+-ATPase activity was increased
by almost 40%. Total Mg2+-ATPase activity was, therefore, almost
unaffected by DDT. There was a less obvious (about 18%) activation of
Na+-K+-ATPase activity in the brain. Gill tissue showed different
results; all the ATPases studied were inhibited by DDT. The authors
suggested that a major factor in the toxicity of DDT to fish (and other
organisms) could be the inhibition of oxidative phosphorylation.
Moffett & Yarbrough (1972) investigated the enzyme succinic
dehydrogenase in insecticide-resistant and insecticide-susceptible
mosquitofish ( Gambusia affinis ) in an attempt to discover if
resistance could be related to membrane effects of DDT. They found
that the effect on membrane-bound enzymes was, indeed, reduced in
resistant fish. This may not explain all the factors involved in
resistance, since DDT uptake from water may also be reduced.
5.2.4 Development of tolerance
The development of tolerance to DDT in fish has been reported.
Vinson et al. (1963) reported DDT tolerance in mosquitofish ( Gambusia
affinis ) exposed long-term to DDT in the wild, and Boyd & Ferguson
(1964) showed TDE tolerance in the same species. However, fish exposed
long-term to DDT do not always show tolerance. Ferguson et al. (1964)
recorded tolerance to a variety of organochlorine insecticides in three
species of freshwater fish from the Mississippi delta area of cotton
cultivation, but there was no tolerance to DDT. El-Sebae (1987)
determined the LC50 values for two populations of Tilapia zilli from
different areas of Egypt. Fish from the Behera Governate which had
been taken from agricultural drains showed exactly the same suscepti-
bility to DDT (25% EC) as fish taken from a less contaminated area in
the Alexandria Governate. Tolerance had developed to other insecti-
cides in these different strains.
5.3 Toxicity to Amphibians
Appraisal
The toxicity of DDT and its metabolites to amphibians varies from
species to species; although only a few data are available, amphibian
larvae seem to be more sensitive than adults to DDT. TDE seems to be
more toxic than DDT to amphibians, but there are no data available for
DDE. All the studies reported have been static tests and, therefore,
results should be treated with caution.
The toxicity of DDT and TDE to amphibians is summarized in
Table 5. Both compounds are toxic to amphibian larvae at low water
concentrations.
Two studies (Harri et al., 1979; Hudson et al., 1984) showed
that DDT is moderately toxic to adult frogs when given orally.
Repeated oral dosing of adult common frogs ( Rana temporaria ) with DDT
at 0.6 mg/kg body weight twice weekly for 8 weeks, led to no mortality
when the animals were fed (Harri et al., 1979). Frogs dosed in the
same way, but not fed, showed 50% mortality by the end of dosing. The
first animal died after the fifth dose and all others showed symptoms
of poisoning.
A study by Sanders (1970) indicated that the toxicity of DDT to
tadpoles of Fowler's toad increased with age of the tadpole. The 24-
and 96-h LC50 values of 5.3 and 0.75 mg/litre for one-week-old
tadpoles fell to 1.4 and 0.03 mg/litre, respectively, by the time the
tadpoles were 7 weeks old. TDE was only tested on one age range of
tadpoles for a maximum of 96 h, and was found to be 3-8 times more
toxic than DDT. The pattern of pesticide poisoning progressed through
irritability and loss of equilibrium to death. Tadpoles were affected
irreversibly by concentrations well below their calculated short-term
LC50 values and, therefore, would succumb to DDT over time. DDT was
re-tested several times during over the period of the study in an
attempt to identify any development of resistance in the population.
None was found; the 24-h LC50 values were stable throughout a 4-month
period.
Table 5. Toxicity of DDT and its derivatives to amphibians
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Fowler's toad (tadpole)
(Bufo woodhousii)
1 week old - 15 mg stat 15.5 30 7.1 DDT 24-h LC50 5300 (2900-9900) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1800 (950-3300) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 750 (280-2000) Sanders
(1970)
2-3 weeks old - 56 mg stat 15.5 30 7.1 DDT 24-h LC50 5400 (2900-10 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1300 (320-5300) (1970)
4-5 weeks old - 74 mg stat 15.5 30 7.1 DDT 24-h LC50 2400 (730-8000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 1000 (40-6500) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 1000 (20-3600) Sanders
stat 15.5 30 7.1 TDE 24-h LC50 700 (250-2000) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 320 (210-450) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 140 (100-210) (1970)
6 weeks old - 350 mg stat 15.5 30 7.1 DDT 24-h LC50 2200 (550-15 000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 410 (280-600) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 100 (20-600) Sanders
(1970)
7 weeks old - 600 mg stat 15.5 30 7.1 DDT 24-h LC50 1400 (900-2000) Sanders
stat 15.5 30 7.1 DDT 48-h LC50 750 (610-1100) (1970)
stat 15.5 30 7.1 DDT 96-h LC50 30 (6-400) Sanders
(1970)
Table 5. (Contd).
---------------------------------------------------------------------------------------------------------
Organism Flow/ Tem- Alkali- pH Compound Parameter Water Reference
Stata perature nityb concentration
(°C) (ug/litre)
---------------------------------------------------------------------------------------------------------
Western chorus frog stat 15.5 30 7.1 DDT 24-h LC50 1400 (910-2800) Sanders
(Pseudacris stat 15.5 30 7.1 DDT 48-h LC50 900 (400-1500) (1970)
triseriata) stat 15.5 30 7.1 DDT 96-h LC50 800 (500-2300) Sanders
(1-week-old tadpole) stat 15.5 30 7.1 TDE 24-h LC50 610 (410-820) (1970)
stat 15.5 30 7.1 TDE 48-h LC50 500 (210-750) Sanders
stat 15.5 30 7.1 TDE 96-h LC50 400 (210-750) (1970)
Bullfrog DDT acute LD50c > 2000 ug/kg Hudson
(Rana catesbeiana) (77.2%) et al.
(1984)
Common frog 15 DDT acute LD50c 7600 ug/kg Harri
(Rana temporaria) et al.
(1979)
---------------------------------------------------------------------------------------------------------
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions
(DDT concentration in water continuously maintained).
b alkalinity expressed as mg CaCO3/litre.
c acute LD50 was calculated by administering a single oral dose.
Cooke (1970) exposed tadpoles of the common frog ( Rana temporaria )
to 0.1, 1.0, or 10 mg DDT/litre for only one hour and reported a
period of uncoordinated hyperactivity beginning less than one hour
after the end of the exposure period. Body weight decreased during
this hyperactive period and development was restricted in some of the
tadpoles. Smaller tadpoles were more vulnerable to the effects of DDT
than larger ones. In a later study (Cooke, 1979b), the same author
reared tadpoles of the common frog at two different densities. The
densities differed 5-fold and resulted in a 2-fold average difference
in body weight between the two groups. The larger tadpoles, reared at
the lower density, were completely tolerant of concentrations of DDT
that caused severe sublethal effects in smaller tadpoles. Field
populations of tadpoles included individuals with weights corresponding
to the two experimental groups, but these were at the two extremes of
the natural weight range.
Cooke (1972) exposed both spawn and tadpoles of the common frog
( Rana temporaria ), the common toad ( Bufo bufo ), and the smooth newt
( Triturus vulgaris ) for 24 and 48 hours to concentrations of DDT
between 0.8 µg/litre and 0.5 mg/litre. Results indicated that DDT did
not penetrate well-developed spawn and was only detectable in tadpoles
hatched from spawn that had been treated with DDT immediately after it
had been laid. Tadpoles hatching from spawn treated when newly laid
showed hyperactivity, symptomatic of DDT poisoning, only later in
their development at the point where external gills were lost. In the
experiments where tadpoles were exposed to DDT, they were most suscep-
tible either just before or just after the development of hindlimb
buds. At these two stages, the characteristic hyperactivity was
shown when DDT tissue concentrations reached between 2-3 mg/kg before
the tadpoles developed limb buds, and when they reached 3-4 mg/kg,
immediately after the tadpoles developed limb buds. During resorption
of the tail, small frogs, but not small toads, were susceptible to DDT
residues that had been acquired during larval development. At all
stages of development, toads were more resistant to DDT than were
frogs, and some toad tadpoles survived despite tissue residues in
excess of 300 mg/kg. The metabolite DDE was often detectable in newt
tadpoles and in frog and toad tadpoles with hindlimbs.
DDT has an anatomical effect on developing frog tadpoles (Cooke,
1970; Osborn et al., 1981). Exposure of tadpoles to 0.1 mg DDT/litre
for 2 days or to 0.1, 1.0, or 10 mg/litre for one hour produced some
individuals with abnormalities in the snout. A detailed histological
and behavioural study suggested that the effect was caused by two
separate factors. DDT had a direct effect on the development of skin
glands in the region above the upper mandible. The uncoordinated
hyperactivity that followed DDT treatment caused the lower mandible to
strike the upper, distorted mandible and resulted in further damage.
Some individuals recovered from this abnormality at various stages of
development. However, froglets that were affected at the tadpole stage
frequently have blunt snouts and deformed brains. The authors
suggested that DDT caused the disruption by preventing the organisation
of the epithelial cells into gland units, possibly by affecting cell
membranes and disrupting cell-to-cell communication. The mechanism of
recovery remained unclear and a full explanation of the very specific
nature of the abnormality was not possible.
This toxicity of DDT to amphibians is of significance in its use as
an insecticide. The use of DDT to control mosquito larvae has been a
major source of exposure of tadpoles and has led to toxic effects
(Mulla, 1963; Cooke, 1973a).
The widespread use of DDT has led to the development of some
resistance in two species of cricket frog ( Acris crepitans and Acris
gryllus ). Boyd et al. (1963) found that cricket frogs collected from
areas of high DDT usage for the control of cotton pests were more
tolerant to DDT than were frogs from other areas.
6. TOXICITY TO TERRESTRIAL ORGANISMS
There is evidence that DDT and its metabolites have affected
wildlife in terrestrial ecosystems. Laboratory studies covered in this
section give clear indication of a variety of lethal and sublethal
effects. The range of organisms studied is not comprehensive. No
review has been made here of the effects of DDT on insects, the target
organisms. The lethal effect of DDT on insects is thought to result
from changes in nerve transmission.
6.1 Terrestrial Invertebrates
Appraisal
There have been few reports on the effects of DDT and its
metabolites on non-target terrestrial invertebrates.
Earthworms are insensitive to the acutely toxic effects of these
compounds at levels higher than those likely to be found in the
environment. The uptake of DDT by earthworms is related to the concen-
trations in soil and to the activity of the worms; seasonally greater
activity increases uptake. Thus, although earthworms are unlikely to
be seriously affected by DDT, they pose a major hazard to predators
because of the residues they can tolerate.
Both DDT and DDE are classified as being relatively non-toxic to
honey bees, with a topical LD 50 at 27 µg/bee.
There are no reports on laboratory studies using DDE or TDE, in
spite of the fact that these are major contaminants of soil.
The toxicity of DDT to insects, the target organisms, is exten-
sively documented. Uptake of DDT and its metabolism by other
terrestrial invertebrates is also well covered in the literature.
However, there are few reports of effects of either DDT or its
metabolites on non-target invertebrates.
Johansen (1962) classified DDT as "moderately" toxic to honey
bees in both laboratory and field tests. Atkins et al. (1973) quoted
a topical LD50 for honey bees of 12.09 µg/bee and classified DDT as
"relatively non-toxic".
DDT has little or no effect on earthworms at dose levels likely to
be encountered in the field; worms were unaffected by 2000 mg/kg soil
(Goffart, 1949). The early literature has been examined by Davey
(1963), whose review includes reports on a variety of earthworm species
that live in surface soil or deeper layers. Thompson (1971) treated an
area of grassland with an emulsifiable concentrate of DDT at the rate
of 5.6 kg/ha. Although there was a reduction in earthworm numbers and
biomass of about 30%, the author considered this to be of little sig-
nificance. Results in tropical areas are similar to those of temperate
regions. Cook et al. (1980) examined the effects of cultivation and
DDT treatment on earthworm activity and populations in Nigeria
following the application of DDT (1 kg/ha) as a foliar spray on cowpea
plots. The number of casts on the surface was reduced by DDT
application, but there was no effect on the number of worms in the
soil.
Cooke & Pollard (1973) treated Roman snails ( Helix pomatia )
with p,p' -DDT applied to lettuce leaves. The snails were fed a
365 x 2.5 cm square of leaf that had been treated with 0.1 ml of an
acetone solution of DDT (either 0.025, 1.0, or 40 mg/ml). The dosing
started when the snails were 2 weeks old and continued for 17 weeks, at
which point the dose was doubled and continued for a further 12 weeks.
The snails were then transferred outside to stimulate hibernation. Low
doses of DDT reduced the weight of the shell and operculum whereas
higher doses did not. After re-emergence from hibernation, the
incidence of operculum eating was significantly higher among snails
hibernating late in the season, and as exposure to DDT increased so
operculum eating became more prevalent. The authors suggest that
shell-thinning is likely to have occurred in snails in heavily-treated
agricultural areas if the response of all snail species to DDT is
similar to that of Helix pomatia .
Critchley et al. (1980) investigated the effects of the use of
DDT for 4 years on a cultivated forest soil in Nigeria on the
numbers of epigeal (surface-living) and subterranean species of invert-
ebrates. DDT was applied as a foliar spray to crops of cowpeas at a
rate of 1 kg/ha annually. After the first application of DDT there was
no effect on ant or millipede numbers but the numbers of lycosid
spiders and crickets were reduced. At the end of the study, after four
applications of DDT, ants and millipedes were also reduced in number.
When Shires (1985) treated cereals on clay loam soil in experimen-
tal plots with DDT (1 kg/ha), the numbers of predatory beetles
(Carabidae) were reduced by 50% one week after application. However,
the numbers increased again after 4 to 6 weeks and remained at control
levels. The use of other insecticides led to a second decrease in
Carabidae numbers; this was attributed by the authors to a reduction
in the food supply of aphids. DDT failed to control the aphids, which
were tolerant to the compound.
6.2 Birds
Appraisal
DDT and its metabolites can lower the reproductive rate of birds by
causing eggshell thinning (which leads to egg breakage) and by causing
embryo deaths. However, different groups of birds vary greatly in
their sensitivity to these chemicals; predatory birds are extremely
sensitive and, in the wild, often show marked shell thinning, whilst
gallinaceous birds are relatively insensitive. Because of the
difficulties of breeding birds of prey in captivity, most of the
experimental work has been done with insensitive species, which have
often shown little or no shell thinning. The few studies on more
sensitive species have shown shell thinning at levels similar to those
found in the wild. The lowest dietary concentration of DDT reported to
cause shell thinning experimentally was 0.6 mg/kg for the black duck.
The mechanism of shell thinning is not fully understood.
6.2.1 Short-term and long-term toxicity to birds
DDT and its derivatives DDE and TDE have moderate to low toxicity
to birds when given as an acute oral dose or in the diet. The acute
oral and dietary toxicities of DDT, DDE, and TDE to birds are
summarized in Table 6.
These compounds have been studied in a wide variety of species in
tests ranging from a single acute dose to 100 days of dietary dosing.
All three compounds, DDT, DDE, and TDE, have low to moderate toxicity
to young and adult birds. There is no obvious pattern of relative
toxicity between the three compounds. In some species it is DDT that
is the most toxic, while in other species it is TDE. Most of these
laboratory tests have been conducted on species that are easy to
maintain and breed in captivity. These species are unusual in many
respects; they tend to be gallinaceous birds with young that are not
fed by the adults after hatching. They also tend to have long breeding
seasons untypical of most birds in the wild. In the wild, the most
severely affected species of birds are raptors at the top of food
chains. There is little direct laboratory data on toxicity to these
birds. Toxicity to small songbirds, which make up the majority of bird
species, has not been examined either in the laboratory or the field.
Porter & Wiemeyer (1972) fed American kestrels on a diet containing
p,p' -DDE at a concentration of 2.8 mg/kg. Two birds died after 14
and 16 months of treatment; they showed residues of DDE in brain
tissues of 212 and 301 mg/kg, respectively. This compared with mean
residues of 14.9 (range: 4.47-26.6) mg/kg in 11 adult males sacrificed
after 12-16 months on the diet. Van Velzen et al. (1972) investigated
the lethal effect of stored DDT mobilization by brown-headed
cowbirds. Cowbirds were fed for 13 days on a diet containing 100, 200,
or 300 mg p,p' -DDT/kg, and were then given reduced rations of
approximately 43% of normal daily intake for a 6-day period. Of 30
birds dosed, 21 died (6, 7, and 8 from the three dose levels,
respectively). After 4 months, the remaining birds were subjected
to a second period of 6 days on a reduced diet. Four more
birds, out of six, died. In a second experiment, cowbirds were fed
100 mg p,p' -DDT/kg diet for 13 days and then subjected to 4 days of
reduced food intake. Seven out of 20 birds died. There were no deaths
in any of the control groups (i.e., birds dosed but not starved,
undosed and starved, or undosed and unstarved).
6.2.2 Toxicity to birds' eggs
Dunachie & Fletcher (1969) injected chicken eggs with DDT or TDE to
give concentrations, in the egg, varying between 10 and 500 mg/kg.
Two different vehicles were used to dissolve the insecticides (corn oil
and acetone), controls being injected with vehicle alone. The authors
monitored egg hatchability and survival of chicks to 4 days of age.
Some chicks were fed and some were not. No dose of DDT, applied in
either vehicle, had any significant effect on egg hatchability when
compared to controls. However, there was a profound effect on the
chick survival rate. All chicks hatched from eggs treated with DDT at
100 mg/kg, and which were not fed, were dead within 4 days after
hatching. Feeding the chicks eliminated this effect; the survival rate
of fed young was similar to that of controls. Chicks hatched from
eggs treated with 50 mg DDT/kg survived as well as controls, whether
they were fed or not. TDE was found to affect hatchability, but only
when applied in corn oil; the acetone-dissolved material did not have
any significant effect. TDE dissolved in corn oil reduced
hatchability to 60% of control levels at 100 and 200 mg/kg, to 7% at
300 and 400 mg/kg, and to 0% at 500 mg/kg. The effects on chick
survivability were similar to those of DDT. All chicks hatched from
eggs treated with 100 mg TDE/kg were dead after 4 days if they were not
fed, whereas chicks from eggs treated with 50 mg/kg survived as well as
controls. Chicks from either 100 or 200 mg/kg treatments survived as
well as controls as long as they were fed. The significance of the
different vehicles was discussed by Cooke (1971) and Gilman et al.
(1978). Acetone causes coagulation of yolk protein whereas corn oil
allows the injected organochlorine to float through the yolk to a
position directly under the blastodisc.
Table 6. Toxicity of DDT and its derivatives to birds
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
Bobwhite quail 23 days diet DDE 99.9 5-day LC50 825 (697-976) Hill
(Colinus virginianus) 23 days diet DDT 100 5-day LC50 611 (514-724) et al.
23 days diet TDE TG 5-day LC50 2178 (1835-2584) (1975)
young diet DDT 5-day LC50 881 (796-975) Stickel
& Heath (1964)
(wild) diet DDT TG 5-day LC50 1170 (830-1650) Hill et al.
(farm-reared) diet DDT TG 5-day LC50 1610 (1331-1948) (1971)
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 400 (1963)
adult diet DDT 10-day LC50 2500 DeWitt et al.
adult diet DDT 100-day LC50 1000 (1963)
Japanese quail 7 days diet DDE 99.9 5-day LC50 1355 (1111-1648) Hill
(Coturnix coturnix 7 days diet DDT 100 5-day LC50 568 (470-687) et al.
japonica) 7 days diet TDE TG 5-day LC50 3165 (2534-3978) (1975)
M 2 months oral DDT 77.2 acute LD50 841 (607-1170) Hudson et al.
(1984)
California quail M 6 months oral DDT TG acute LD50 595 (430-825) Hudson et al.
(Callipepla F 6 months oral TDE > 95 acute LD50 > 760 (1984)
californica)
Mallard duck 17 days diet DDE 99.9 5-day LC50 3572 (2811-4669) Hill
(Anas platyrhynchos) 17 days diet DDT 100 5-day LC50 1869 (1500-2372) et al.
17 days diet TDE TG 5-day LC50 4814 (3451-7054) (1975)
F 3 months oral DDT 77.2 acute LD50 > 2240 Hudson et al.
F 3 months oral TDE > 95 acute LD50 > 2000 (1984)
young diet DDT 5-day LC50 875 (650-1140) Stickel &
Heath (1964)
young diet DDT 10-day LC50 500 DeWitt
young diet DDT 100-day LC50 > 200 et al.
adult diet DDT 100-day LC50 1000 (1963)
Pheasant 10 days diet DDE 99.9 5-day LC50 829 (746-922) Hill
(Phasianus colchicus) 21 days diet DDT 100 5-day LC50 311 (256-374) et al.
10 days diet TDE TG 5-day LC50 445 (402-494) (1975)
F 3-4 months oral DDT > 99 acute LD50 1334 (894-1990) Hudson et al.
F 3-4 months oral TDE > 95 acute LD50 386 (270-551) (1984)
young diet DDT 5-day LC50 804 (686-942) Stickel &
Heath (1964)
Table 6. (Contd)
---------------------------------------------------------------------------------------------------------
Species Sexa Age Routeb Comp- Purityc Parameter Concentration Reference
ound (%) (mg/kg)
---------------------------------------------------------------------------------------------------------
young diet DDT 10-day LC50 1000 DeWitt et al.
young diet DDT 100-day LC50 100 (1963)
adult diet DDT 10-day LC50 1000 DeWitt et al.
adult diet DDT 100-day LC50 > 100 (1963)
Red-winged blackbird diet DDT 10-day LC50 1000 DeWitt et al.
(Agelaius phoeniceus) diet DDT 30-day LC50 500 (1963)
Cardinal diet DDT TG 5-day LC50 535 (420-700) Hill et al.
(Richmondena cardinalis) (1971)
House sparrow diet DDT TG 5-day LC50 415 (370-465) Hill et al.
(Passer domesticus) (1971)
Blue jay diet DDT TG 5-day LC50 415 (320-540) Hill et al.
(Cyanocitta cristata) (1971)
Rock dove M,F oral DDT 77.2 acute LD50 > 4000 Hudson et al.
(Columba livia) (1984)
Sandhill crane M,F adult oral DDT > 99 acute LD50 > 1200 Hudson et al.
(Grus canadensis) (1984)
Clapper rail M diet DDT 5-day LC50 1612 Van Velzen &
(1975) Kreitzer
(Rallus F diet DDT 5-day LC50 1896 (1975)
longirostris) (1975)
---------------------------------------------------------------------------------------------------------
a M = male; F = female.
b oral = acute oral test (result expressed as mg/kg body weight); diet = dietary test (result expressed
as mg/kg diet).
c TG = Technical grade.
6.2.3 Reproductive effects on birds
DDT, or more specifically its metabolite DDE, causes the shells of
birds' eggs to be thinner than normal. Results on eggshell thinning
are summarized in Table 7. There is considerable variation between
species for this effect. Galliform species are very resistant to shell
thinning whereas birds of prey are particularly susceptible.
Lincer (1975) dosed captive American kestrels and established a
clear relationship between dietary DDE and thinning of eggshells.
There was a similar close correlation between the residues of DDE in
individual eggs and the degree of shell thinning. The kestrels were
fed with day-old cockerels (which were injected with 0.2 ml of corn
oil, containing the DDE, into the breast muscle) and received either
0.3, 3, 6, or 10 mg DDE/kg diet. Residues of DDE in eggs laid by the
birds correlated closely with dietary DDE concentration; residues of
1.9 mg/kg wet weight were associated with the lowest dose and 245 mg/kg
with the highest dose given. There was no shell thinning associated
with the dose of 0.3 mg/kg. The other doses showed 15.1%, 22.8%, and
29.2% thinning (at 3, 6, and 10 mg/kg, respectively). There was a
straight-line relationship between the degree of shell thinning and
the logarithm of the DDE residue in the egg. Data obtained from the
field showed exactly the same trend (Fig. 1). This represents the best
evidence for the effect of DDE on shell thickness in a species actually
adversely affected in the field.
Table 7. Thinning effects of DDT and its derivatives on bird egg shells
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Ring dove diet DDE 10 - 9.2 0.01 Peakall et al. (1973)
(Streptopelia diet DDE 40 - 6.8 0.01 Haegele & Hudson (1973)
risoria)
Mallard diet TDE 10 - 2.6 NS Heath et al. (1969)
(Anas platyrhynchos) diet TDE 10 - 5.4 NS Heath et al. (1969)
diet TDE 40 - 2.6 NS Heath et al. (1969)
diet TDE 40 - 5.4 NS Heath et al. (1969)
diet DDT 2.5 - 5.3 NS Heath et al. (1969)
diet DDT 10 - 7.9 NS Heath et al. (1969)
diet DDT 40/25 -13.2 0.01 Heath et al. (1969)
White pekin duck diet DDE 40 -20.3 0.001 Peakall et al. (1973)
4 day diet DDE 40 - 3.3 0.01 Miller et al. (1976)
1-3 months diet DDE 40 -18.2 0.01 Miller et al. (1976)
Black duck diet DDE 10 -17.6 0.01 Longcore et al. (1971)
(Anas rubripes) diet DDE 30 -23.5 0.01 Longcore et al. (1971)
Screech owl diet DDE 2.8 -13.3 0.01 McLane & Hall (1972)
(Otus asio)
American kestrel diet DDE 3 -15.2 0.05 Peakall et al. (1973)
(Falco sparverius) diet DDE 6 -21.0 0.01 Peakall et al. (1973)
diet DDE 10 -26.3 0.001 Peakall et al. (1973)
diet DDE 2.8 - 8.7 0.001 Wiemeyer & Porter (1970)
diet DDE 0.3 + 2.1 NS Lincer (1975)
diet DDE 3 -15.1 0.05 Lincer (1975)
diet DDE 6 -22.8 0.01 Lincer (1975)
diet DDE 10 -29.2 0.001 Lincer (1975)
Table 7. (Contd).
---------------------------------------------------------------------------------------------------------
Species Route Compoundc Dose Percentage Significancee Reference
(mg/kg) change (p)
---------------------------------------------------------------------------------------------------------
Japanese quail diet o,p'-DDT 100 - 4.0 0.001d Bitman et al. (1969)
(Coturnix coturnix diet DDT 100 - 5.6 0.001d Bitman et al. (1969)
japonica) diet DDT 100 0 NS Cecil et al. (1971)
diet DDE 100 - 2.5 NS Cecil et al. (1971)
diet DDE 2 + 1.9 NS Davison et al. (1976)
diet DDE 10 + 6.3 NS Davison et al. (1976)
diet DDE 40 + 5.0 NS Davison et al. (1976)
diet DDE 200 - 0.6 NS Davison et al. (1976)
strain 1a diet DDT 2.5 + 1.0 NS Davison et al. (1976)
diet DDT 10 - 1.5 NS Davison et al. (1976)
diet DDT 40 - 0.5 NS Davison et al. (1976)
strain 1b diet DDT 2.5 - 2.7 NS Davison et al. (1976)
diet DDT 10 - 1.6 NS Davison et al. (1976)
diet DDT 40 - 7.1 NS Davison et al. (1976)
strain 2a diet DDT 2.5 + 0.5 NS Davison et al. (1976)
diet DDT 10 + 1.6 NS Davison et al. (1976)
diet DDT 40 + 1.0 NS Davison et al. (1976)
strain 2b diet DDT 2.5 - 3.7 NS Davison et al. (1976)
diet DDT 10 - 2.6 NS Davison et al. (1976)
diet DDT 40 - 5.7 NS Davison et al. (1976)
---------------------------------------------------------------------------------------------------------
a Individually caged.
b Caged in pairs.
c DDT in the p,p' - form unless stated otherwise.
d Low calcium diet (0.56%).
e S = not significant.
 Haegele & Tucker (1974) dosed egg-laying Japanese quail with a
single oral dose of p,p' -DDE, o,p' -DDT, p,p' -DDT, or technical
DDT, all at 125 mg/kg body weight. None of the treatments caused
appreciable eggshell thinning. When Smith et al. (1969) fed Japanese
quail with DDT at 100, 200, or 400 mg/kg diet, the two lower doses had
no effect on hatchability or fertility of eggs laid. At 400 mg/kg,
there was 50% mortality amongst dosed birds; survivors showed a decline
in hatchability and fertility after 30 days. Bitman et al. (1969)
dosed Japanese quail with o,p' -DDT or p,p' -DDT at a dietary level
of 100 mg/kg. The quail were given a low calcium diet (0.56%) and
were, therefore, under calcium stress during egg laying. Both isomers
of DDT caused significant thinning of eggshells (P<0.001) and a
significant (P<0.01) reduction in shell calcium content. Eggs produced
by birds dosed with the p,p isomer were significantly lighter than
those laid by birds dosed with the o,p' isomer.
Cecil et al. (1971) investigated the effects of p,p' -DDT and
p,p' -DDE on the egg production and eggshell characteristics of
Japanese quail receiving an adequate calcium diet, and compared their
results with previous studies of the effects of these compounds on
quail receiving low calcium diets. They found a delay in the onset of
egg production in quail fed a concentration of 100 mg/kg of either DDT
or DDE for about 3 weeks. This result was similar to that of studies
with low calcium diets. In contrast to the earlier studies, there was
no effect of either DDT or DDE on shell thickness or egg weight when
dietary calcium was higher. There was an increased incidence of egg
breakage in birds fed DDT and DDE, but this was less pronounced than
with the low calcium diets.
Robson et al. (1976) studied the effects of DDE and DDT fed to
Japanese quail in two different diets containing adequate or low
calcium. DDT was fed at 100 mg/kg diet, whereas DDE was given at 0,
199, or 300 mg/kg diet, and the two calcium levels were 0.5% and 3%.
DDE at 300 mg/kg was detrimental to adult body weight, fertility, and
survivability. There was no effect of either DDT or of DDE at up to
100 mg/kg diet on adult body weight, food consumption, egg production,
egg weight, fertility, hatchability, cracking of eggs, or eggshell
thickness. Low dietary calcium had the effect of reducing the
thickness of eggshells, increasing the incidence of cracked shells and
decreasing egg production and hatchability.
Davison et al. (1976) fed DDE (0, 2, 10, 40, or 200 mg/kg diet)
to female Japanese quail that were individually caged and had 14 g of
food available each day. There was no effect on body weight, egg
laying, egg weight, eggshell thickness, or on shell calcium content.
Quail were then fed a diet containing DDT at 0, 2.5, 10, or 40 mg/kg.
There was no effect on eggshell thickness, number of eggs laid,
fertility, or hatchability. Quail fed 40 mg DDT/kg diet and caged in
pairs, broke more eggs than birds fed lower concentrations of DDT or
any concentration when the birds were caged individually. Paired quail
laid fewer eggs than single quail and in one experiment they laid eggs
with thinner shells.
When Davison & Sell (1972) dosed white leghorn hens with 100 or
200 mg DDT/kg diet for 12 weeks, the average egg production per bird,
egg weight, dry shell weight, shell thickness, and shell calcium were
all found to be unaffected by DDT at either dose level.
Egg-laying mallard ducks treated by Haegele & Tucker (1974) with a
single oral p,p' -DDE dose of 500, 1000, or 2000 mg/kg body weight
showed a clear effect on eggshell thickness at all dose levels.
Unfortunately, whilst the results are clear, no statistical analysis of
the results was presented. The effect on eggshells was dose related,
quick acting, and persistent. Heath et al. (1969) dosed mallard for
two seasons with DDE or TDE at 10 or 40 mg/kg diet and with DDT at 2.5,
10, or 40 mg/kg diet. The highest dose of DDT was reduced to 25 mg/kg
in the second season. DDE at both concentrations severely impaired
reproductive success, a more rapid initiation of the effect being seen
with the higher dose. DDE significantly affected eggshell thickness;
eggs from birds dosed with 40 mg/kg laid, in their second season, eggs
with shells 13% thinner than controls. There was a significant
increase in egg cracking and decrease in egg hatchability at both DDE
dose levels. TDE did not have a significant effect on shell thickness.
It impaired reproductive success, but not as severely as did DDE. DDT
induced eggshell thinning at a dose of 25 mg/kg, shells being 18%
thinner than controls, and reduced duckling survival during 14 days
post-hatch by 35%. DDT at 2.5 and 10 mg/kg had no effect.
Vangilder & Peterle (1980) fed mallard a diet containing 10 mg
DDE/kg, and brought the birds into breeding condition using long
daylength. Relative to controls, egg laying was delayed, eggshell
thickness was decreased, and hatchability was reduced in treated birds.
Ducklings, hatched from eggs laid by treated females, showed a signifi-
cantly reduced survival time, and a greater proportion of ducklings
were unable to initiate normal body temperature regulation.
When Longcore et al. (1971) dosed black ducks with 10 or 30 mg
DDE/kg diet, there was significant eggshell thinning and an increase in
shell cracking, compared to controls, at both dose levels. The
survival of ducklings to 21 days was also significantly reduced at both
dose levels. Longcore & Stendell (1977) fed DDE (10 mg/kg diet) to
black ducks over two breeding seasons and then untreated food for a
further 2 years. The eggshells of treated birds during dosing were 20%
thinner than controls. When dosing stopped, eggshell thickness
gradually increased but shells were still 10% thinner than controls 2
years after dosing had finished. Similarly, there was still a reduced
survival of ducklings, to 3 weeks of age, 2 years after dosing with DDE
had ceased.
Peakall et al. (1973) studied the effects of dietary DDE on
eggshell thinning in three species of bird (white pekin duck, American
kestrel, and ringdove). In addition to shell thinning, they reported a
reduced rate of water loss from eggs laid by DDE-treated birds; the
permeability constants of the eggs were significantly decreased.
Scanning electron micrographs revealed a decrease in the number of
pores per unit shell area and an increase in the number of globular
inclusions in eggshells from treated birds. Greenburg et al. (1979)
showed, also using scanning electron microscopy, that DDE affected both
organic and inorganic constituents of the eggshells of mallard dosed in
their diet. The literature concerning the effects of DDE on eggshell
structure has been reviewed in detail by Cooke (1973b).
In studies by Miller et al. (1976), laying white pekin ducks and
white leghorn hens were dosed with 40 mg DDE/kg diet. The ducks showed
significant eggshell thinning within 4 days, and again between 1 and 3
months of the start of dosing, but the hens did not show significant
eggshell effects within 2 weeks.
Peakall et al. (1975a) dosed white pekin ducks at a dietary level
of 250 mg DDE/kg for 10 days, and, approximately 2 months later,
started to collect eggs and measure shell thickness for a period of 27
weeks. At the beginning of the collection period, shells from treated
birds were found to be 20% thinner than controls. Recovery was slow
and shells were still 10% thinner at the end of the study. Haseltine
et al. (1974) dosed mallard and pheasant (10 mg DDE/kg diet) and ring
doves (40 mg DDE/kg diet) and found significant eggshell thinning and
depression of serum calcium levels in both mallard and ring dove.
However, neither parameter was significantly changed in pheasant.
Peakall et al. (1975b) maintained paired ring doves on a diet
containing 100 mg DDE/kg for 3 weeks and white pekin ducks on 250 mg
DDE/kg diet for 10 days. Although both species showed significant
eggshell thinning, there was no significant difference between the
levels of serum calcium of treated and control birds.
Miller et al. (1976) removed the shell glands from white pekin
ducks and white leghorn chickens, dosed with 40 mg DDE/kg diet, when a
calcifying egg was present within the gland, and assessed enzymatic
activity. There was a significant decrease in Ca2+-ATPase and
carbonic anhydrase activities in the shell glands removed from dosed
ducks, but no difference from controls in chicken shell glands. Kolaja
(1977) maintained mallard ducks on a diet containing either DDT, DDE,
DDT sulphonate, or DDE sulphonate at dose levels of 10 or 50 mg/kg.
Eggs were collected for 30 days and were weighed and measured. There
was no significant difference between egg weights at the different dose
levels. The thickness of eggshells of birds fed DDE was significantly
reduced. Ducks fed DDT laid eggs with significantly thinner shells
only after day 14. The two sulphonate-treated groups were not
significantly different from each other and were only significantly
different from controls on day 18; eggshell weights followed a similar
pattern.
Mendenhall et al. (1983) dosed breeding barn owls with 3 mg DDE/kg
diet during two breeding seasons, and found that treated birds laid
thin-shelled eggs and laid significantly more eggs per pair in both
seasons. In both years the percentage of eggs broken was increased,
relative to controls, and the mean number of eggs hatched and young
fledged per pair was reduced. There was a significant increase in
embryo deaths in one of the two years.
Eggshell thickness has been monitored in different ways by
different authors. Some direct measurement has been made with
membranes intact and some without. Other methods have been used to
compare recent eggs with museum specimens, which could not be broken to
measure thickness directly. The various methods were reviewed by Cooke
(1973b), who suggested standards. Generally a log-linear relationship
between DDE load and shell thinning is claimed. In a recent consider-
ation of the theoretical treatment of such data, Moriarty et al. (1986)
suggested that the main methods of assessing shell thickness do not
adequately take into consideration the effects of shell size and shape.
This does not detract from the conclusion that shell thinning occurs,
but suggests that the relationship may be more properly described as
curvilinear.
6.2.4 Reproductive hormones and behaviour
After feeding mallard a diet contaminated with 3 mg p,p' -DDE/kg
and artificially incubating eggs laid by the females, Heinz (1976)
found that the average egg residue of DDE was 5.8 mg/kg. Ducklings
from treated eggs were hyperresponsive to a tape-recorded maternal
call; treated ducklings were significantly more likely to approach the
recorder. In contrast, treated ducklings moved shorter distances away
from a frightening stimulus, compared to controls. Japanese quail
chicks fed a diet containing 50 mg DDE/kg for 8 days, starting at 7
days of age, and then a clean diet for a further 6 days showed no
significant effect on avoidance response to a moving silhouette.
Haegele & Hudson (1977) paired ring doves for 12.5 min each day,
for 5 days, prior to dosing their diet with 10 or 50 mg p,p' -DDE/kg.
The birds were also paired between days 31 and 35 and between days 59
and 63 after the start of dosing. Two measures of the courtship
behaviour of males were made: total courtship activity time and mean
bow-coo frequency. Bow-cooing behaviour is the initial behaviour
displayed by males to attract females. In control birds, the total
courtship activity time was 25% (days 31-35) and 23% (days 59-63)
longer than it was in the predose period. In birds dosed with 10 mg
DDE/kg, the courtship activity between days 31 and 35 was not different
from that in the predose period, whereas the final pairing produced a
decrease of 55% in activity. In birds dosed with 50 mg DDE/kg, the
courtship activity decreased by 30% and 67% for the two later pairing
periods compared to the predose period. After dosing at 10 mg/kg,
there was no change in bow-cooing between days 31 and 35, but a
reduction of 53% between days 59 and 63. Birds dosed at 50 mg/kg
showed decreases in bow-cooing behaviour of 43% and 84%, in the two
subsequent pairings respectively, when compared to the predose period.
When Richie & Peterle (1979) paired ring doves and fed them with
either 10 or 40 mg p,p' -DDE/kg diet, there was a significant delay in
the period between pairing and egg laying at both dose levels.
Leutinizing harmone levels in blood plasma, sampled throughout the
experiment, were not significantly altered by the DDE. Similarly,
Jefferies (1967) reported an increase in the time between
pairing and egg laying in Bengalese finches fed a range of doses
of p,p' -DDT between 75 and 1200 mg/kg diet. Treated birds were fed
for 2 h/day, immediately following a period of 1 h of starvation.
There was a significant correlation between DDT intake by the female
and the delay in egg laying. Dobson (1981) measured circulating
hormone levels and nest-building behaviour in pigeons dosed orally with
DDE and found a delay in egg laying. Hormone measurements showed that
ovulation was not delayed. Nest building was reduced in treated birds.
The delay in egg laying resulted from a lengthening of the period
between ovulation and oviposition. Since the laying of eggs is
dependent on the stimulus of adequate nest material, this lengthening
of the period between pairing and egg laying was considered to be
primarily an indirect effect on reproduction, triggered by a direct
effect on behaviour; the egg was retained longer in the oviduct.
Peakall (1970) maintained ring doves on a diet containing
10 mg p,p' -DDT/kg for 3 weeks. They were kept in isolation (with
short daylengths) and then paired (with long daylengths) to induce
breeding. The females were killed either 8 days after pairing or
after completion of their clutch of two eggs. In those killed 8 days
after pairing, circulating oestradiol levels were significantly
reduced and hepatic enzyme activity was significantly increased.
There was a significant delay in the laying of the first egg and a
decrease in egg weight. In the same study, the birds were given
oral 45Ca (7.4 x 104 Bq; 2 µCi) on the day before pairing. There
was a significant decrease in the radioactivity of eggs and in the
bones of females killed 8 days after pairing. In a separate
experiment, p,p' -DDT was injected intraperitoneally at a dose of
150 mg/kg body weight, into female ring doves within 1 day of their
first egg being laid. The birds were killed after completing their
clutch of two eggs. The shell weight of the second egg was
significantly reduced when compared to the first and there was a
significant decrease in carbonic anhydrase activity in the oviduct.
This enzyme is associated with deposition of calcium into the shell.
6.2.5 Reproductive effects on the male
Burlington & Lindeman (1950) administered a daily subcutaneous
injection of DDT to male white leghorn chicks, gradually increasing
the dose from 15 to 300 mg/kg body weight. The birds were treated for
60-89 days, and the cockerels were killed and their testes removed,
weighed, and sectioned. Treated birds were found to have smaller
testes, more intertubular tissue, and retarded tubular development.
These effects were accompanied by an inhibition of testosterone-
dependent secondary sexual characters; combs and wattles were reduced
in both size and colour development in treated birds. Locke et al.
(1966) dosed male bald eagles at dietary levels of 10 mg DDT/kg for 60
or 120 days, and found no effects on spermatogenic activity. There
were some degenerative effects on the testis, but only at doses which
had severe neurological effects and ultimately led to death.
6.2.6 Effects on the thyroid and adrenal glands in birds
When Jefferies & French (1972) fed pigeons on a diet containing
either 18, 36, or 72 mg p,p' -DDE/kg for a period of 56 days, paired
thyroid weights were found to be greater in treated birds than in
controls. There was no apparent dose relationship to this effect, but
bird numbers were small. Taking the dosed birds as a single group,
the results were significantly different from those of control birds.
Liver weights were similarly increased, and, at the two highest dose
levels, there was an increase in paired adrenal weights.
Biessman & von Faber (1981) dosed Japanese quail for 9 weeks
with technical DDT (either 50 or 250 mg/kg diet) or for 5 weeks with
p,p' -DDT or p,p' -DDE (250 and 300 mg/kg, respectively). Adrenal
weights increased with all treatments but the increase in size was only
significant for the 300 mg DDE/kg dose. The percentage of cortical
tissue, measured from areas of sections of the gland, showed a similar
trend, but results were not statistically significant. No changes were
detectable in nuclear size of either cortical or medullary cells.
Lehman et al. (1974) studied the effect of technical grade DDT on the
adrenal glands of bobwhite quail, which were maintained on a diet
containing 10, 50, or 150 mg DDT/kg for 242 days and then killed. No
effect was found on adrenal weight expressed as a percentage of body
weight, but there was a significant dose-related increase in the ratio
between areas of cortex and medulla.
6.2.7 Special studies in birds
Dieter (1974) fed Japanese quail on a diet containing 5, 25, or
100 mg DDE/kg and, after 12 weeks of dosing, assessed the activity of
five plasma enzymes (creatine kinase, aspartate aminotransferase,
lactate dehydrogenase, cholinesterase, and fructose-diphosphate
aldolase). There was an increase in the activity of all these enzymes,
which, in each case, was proportional to the logarithm of the DDE
dose.
Bend et al. (1977) dosed immature puffins, orally by intubation,
with DDE at 6 mg/day (equivalent to 50 mg/kg diet) for 16 to 21 days,
and, after killing the birds, determined the effect of DDE on hepatic
mixed-function oxidases. Both aniline hydroxylase and benzphetamine
demethylase activities were increased in treated birds; the yield of
microsomal protein remained unchanged. In contrast, Sell et al. (1972)
demonstrated a depression in aniline hydroxylase and N-demethylase
activities after feeding Japanese quail with DDT at 200 mg/kg diet.
Both DDT and DDE inhibited aniline hydroxylase in vitro activity, when
present at concentrations of 10-7 mol/litre or more.
Bunyan et al. (1970) measured the activities of glucose-6-
phosphate dehydrogenase (G-6-P) and 6-phosphogluconate dehydrogenase
(6-P-G) in the liver of Japanese quail fed diets containing low levels
of p,p' -DDT or a number of saturated and unsaturated analogues of
p,p' -DDT. Generally, saturated compounds lowered G-6-P levels and
increased 6-P-G levels. p,p' -DDMU was anomalous in elevating G-6-P.
The authors suggested that these effects might be due to interference
with protein metabolism primarily by the unsaturated analogues and
metabolites of DDT. Bunyan et al. (1972) fed either DDT or DDE to
Japanese quail and monitored hepatic microsomal protein, cytochrome
P450, aniline hydroxylase, aromatic nitroreductase, phenylbenzoate
esterase, and total vitamin C. Changes in these factors were more
readily explained in terms of residues of DDE in the liver than in
terms of dietary dose. DDE was found to be a more potent inducer of
microsomal protein, cytochrome P450, and aniline hydroxylase than was
DDT. The effects of DDT could be explained in terms of the effects of
the DDE produced by DDT metabolism. Aromatic nitroreductase was
unaffected by either compound. Vitamin C levels were raised by DDT
more than by DDE. Phenylbenzoate esterase showed a biphasic response
following the feeding of DDE. Bunyan & Page (1973) extended these
studies by examining the effects of DDE and DDMU on hepatic microsomal
enzyme systems. Most of the changes observed in quail were greater
with DDMU than with any other DDT metabolite. The authors suggested
that DDT metabolism in birds may be different to metabolism in mammals.
Metabolism probably gives rise, via the production of DDMU, to a
highly active liver inducer.
Heinz et al. (1980) fed ring doves on a diet containing 2, 20, or
200 mg DDE/kg for 8 weeks, and found at the end of the dosing period, a
significant decrease in dopamine concentration in brain tissue from
birds fed on the two higher doses. Brain noradrenalin concentration
was also affected but only at the highest dose. There was a signifi-
cant, negative correlation between concentration of both dopamine and
noradrenalin and the residue of DDE in the brain tissue.
Friend et al. (1973) fed a dietary dose of 10, 100, or
1000 mg DDE/kg. to male mallard that had been previously maintained on
either fresh water or 1% salt water. Birds were given a concentrated
salt solution either 1, 3, 6, or 9 days after the beginning of DDE
treatment, the salt being administered both intraperitoneally (12 ml of
a 10% solution) and intravenously (3ml of a 5% solution). The rate of
sodium chloride excretion was not reduced, relative to controls, in
DDE-treated birds maintained previously on salt water, but was reduced
significantly in DDE-treated birds not previously given salt.
When Mahoney (1975) fed caged white-throated sparrows on technical
DDT (either 5 or 25 mg/kg), the onset of spring nocturnal migratory
restlessness (Zugunruhe) and weight increase was delayed by at least 1
week. Although Zugunruhe onset was delayed, when migratory nocturnal
activity did commence it was more pronounced than in control birds.
The increase in Zugunruhe was related to body residues of DDT.
Haynes (1972) dosed male bobwhite quail with DDT (100 mg/kg diet)
for 10 weeks and, 1 week before the study was terminated, some birds
were transferred to clean food while others were starved for 4 days and
then given clean food for 3 days before being killed. There was no
significant effect on liver glycogen, either from dosing with DDT or
from starvation, but liver lipid levels were significantly increased by
both DDT and starvation. Body lipid levels were not significantly
affected by DDT but were reduced after starvation.
6.2.8 Synergism with other compounds in birds
Kreitzer & Spann (1973), in a study on combined effects of
pesticides, found that mixtures of DDT and dieldrin in Japanese
quail, and DDE and Ceresan M (organomercury fungicide) in pheasants,
were additive rather than synergistic in their action. The study
compared known LD50 values with expected ones. Mallard, maintained
on a diet containing a mixture of DDE (40 mg/kg) and Aroclor 1254 (40
mg/kg) for at least 30 days, laid eggs with significantly thinner
shells than did controls. This result was not significantly different
from that produced by DDE alone (Risebrough & Anderson, 1975). In a
similar study on American kestrels, Lincer (1972) dosed the birds with
Aroclor 1254 (10 mg/kg) and DDE (3 mg/kg) in the diet, both separately
and in combination. There was no eggshell thinning with Aroclor
alone, but Aroclor and DDE together had a significantly greater
effect on shell thickness than DDE alone, indicating synergism.
Japanese quail exposed to dietary doses of 5 or 50 mg DDE/kg for 12
weeks, and subsequently dosed orally with either parathion or paraoxon
at 2 µl/g body weight, showed synergism between the compounds with
respect to mortality and to inhibition of brain cholinesterase. The
synergistic action of DDE on cholinesterase inhibition was apparent 3
days after exposure to 50 mg/kg and one week after exposure to 5 mg/kg.
Mortality due to DDE was increased from 10% to 90% in the presence of
the organophosphorus compounds. Anticholinesterase effects were
increased by 50% in the presence of DDE (Ludke, 1977).
6.3 Non-laboratory Mammals
Appraisal
Experimental work suggests that some species, notably bats, may
have been affected by DDT and its metabolites. Species which show
marked seasonal cycles in fat content are most vulnerable, but few
experimental studies on such species have been made. In contrast to
the situation in birds, where the main effect of DDT is on repro-
duction, the main known effect in mammals is to increase the mortality
of migrating adults. The lowest acute dose which kills American big
brown bats is 20 mg/kg. Bats collected from the wild (and containing
residues of DDE in fat) die after experimental starvation which
simulates loss of fat during migration.
In studies into the effect of DDE on bats, Geluso et al. (1976)
captured young Mexican free-tailed bats ( Tadarida brasiliensis ) before
their first migratory flight and transferred them to the laboratory.
This species migrates north from Mexico to the USA in spring and
returns to winter in the south. Three groups of bats were used. A
reference set was killed on capture and, when the bats were analysed
for residues of organochlorines derived from environmental source, DDE
was the only chemical found in significant amounts. Brain residues of
DDE were low; the median being 3.7 (range: 1.5 to 17.0) mg/kg in eight
younger animals and 1.3 (range 1.1 to 11.0) mg/kg in older animals.
Two further groups were maintained in the laboratory where the bats
were given water but not fed. One group was regularly exercised, while
the other was given no exercise. All exercised bats died within 9
days; 4 bats in the unexercised group died and the other 4 were killed
after 9 days. Analysis of brain DDE residues showed considerably
elevated levels compared to the reference group. For the unexercised
bats, the median residue values were 47 (range 18 to 76) mg/kg in
younger animals and 70 (range 10 to 95) mg/kg in older animals. In
exercised bats, the values were 160 (range 66 to 330) mg/kg for younger
animals and 160 (range 37 to 260) mg/kg for older animals. Those
animals that died before the end of the study showed symptoms charac-
teristic of pesticide poisoning, including hyperactivity, intermittent
audiogenic seizures, and violent contractions of chest muscles. The
high brain residues of DDE were considered to be the cause of death of
the animals. It should be noted that these animals had not been arti-
ficially dosed with DDE. The effects resulted from residues of DDT in
body fat, taken up in the maternity roost. The authors considered that
their studies confirmed the suggestion that bats were being killed by
accumulated residues of DDE during the period of migration, when their
fat reserves were used up.
Clark & Kroll (1977) experimentally fed adult females of the same
species of bat ( Tadarida brasiliensis ) for 40 days with mealworms
containing 107 mg DDE/kg and then killed four of the bats. They had a
whole body burden of 2.345-2.929 mg DDE (and 78-90 mg DDE/kg in the
brain). Twelve of the dosed bats were then starved, and they died
within 8 days. The total body burden of DDE ranged from 1.952 to
3.711 mg DDE and brain residues from 379 to 564 mg/kg. These brain
residues were considered to be diagnostic of death from DDE poisoning.
Tremors characteristic of poisoning were seen in the bats before death
occurred.
The toxicity of single oral doses of DDT to bats has been
estimated in two studies. Jefferies (1972) derived an approximate
LD50 of 63 mg/kg body weight for the pipistrelle bat ( Pipistrellus
pipistrellus ), a small British species. There was no mortality at
doses below 45 mg/kg and 100% mortality at doses above 95 mg/kg.
Luckens & Davis (1964) found that the lowest dose which killed American
big brown bats ( Eptesicus fuscus ) was 20 mg/kg and that 40 mg/kg was
invariably lethal. The LD50 for this species lies somewhere between
25 and 40 mg/kg for a single oral dose.
Blus (1978) determined dietary LC50 values of DDT, given in food
either as a powder or dissolved in oil, for short-tailed shrews
( Blarina brevicauda ) of different ages and sex. In 2-week tests, the
range of LC50s for DDT dissolved in oil was 651 to 1160 mg/kg diet,
and for DDT added as powder it was 839 to >2552 mg/kg. The influence
of age and sex was sometimes more important in determining DDT toxicity
than was body weight, though heavier shrews tended to be more tolerant
of the chemical. Among older animals, males were more tolerant of DDT
than females. Braham & Neal (1974) found an effect of DDT on the
metabolic rate of the same species of shrew after feeding it with
earthworms contaminated with the insecticide. After one week of this
diet, the metabolic rate was significantly higher than that of undosed
shrews, but after 2-3 weeks of dosing there was a return to oxygen
consumption rates not different from controls. Two shrews were fasted
for 18 h, after being fed earthworms containing DDT for 3 weeks, and
compared to untreated shrews similarly fasted. The DDT-treated animals
showed 12.6% and 12.1% increases in metabolic rate after fasting,
whereas controls showed decreases of 8.7% and 8.0%. The DDT exposure
was environmentally realistic because earthworms used for feeding were
not artificially dosed with DDT but were collected from an area where
DDT had been used.
7. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
There have been kills of fish (Hunt & Linn, 1970) and aquatic
invertebrates (Ide, 1957) reported after normal usage of DDT as a
terrestrial insecticide and after its application to water for mosquito
control. Reproductive failure in commercial fisheries has also been
attributed to DDT (Hunt & Linn, 1970). In addition, it has been shown
to be toxic to amphibia after water application (section 5.3). The
setting of safe water levels of DDT and its metabolites is difficult
because its high bioaccumulation and high lipid solubility mean that it
can have effects remote in time from its application. The toxicity of
DDT to aquatic microorganisms and invertebrates is very variable
between species. Exposure to DDT or its stable metabolites would,
therefore, be expected to kill certain species selectively. Short-
term, there is close correspondence between the 96-h LC50 for a
moderately sensitive fish (16 µg/litre) and the expected water concen-
tration after application of DDT at the normal rate.
DDT and its metabolites, principally DDE, have been implicated in
reproductive effects on birds in the field. Large population declines
in some bird species, mainly birds of prey, have been blamed on DDT or
on combinations of DDT with other persistent organochlorines. The
evidence for this rests on correlations. There is a correlation in
time between the onset of effects on eggshells and the onset of major
DDT use in agriculture. There is also a correlation between geo-
graphical areas of high DDT use and effects on local populations of
birds (compared to populations living in areas of low use). There is a
clear correlation between DDE residues in eggs and the degree of
thinning of the shells of those eggs, collected from the wild. Storage
of DDT in body fat means that the effects of the compound can be remote
in time from the application of the chemical to an area. Only some
species of birds are affected by DDT or its metabolites. There are
considerable data on the variability between species in their suscepti-
bility to these compounds. Widespread monitoring programmes have
related the recovery of bird populations to reduced levels of DDE and
the residual material of aldrin/dieldrin use in the tissues of birds
sampled from the wild, following attempts to limit or ban the use of
the parent pesticide in agriculture. Because DDT is seldom the only
chemical residue found in bird tissues from the wild, there is some
disagreement on whether DDT alone can cause population declines in
birds.
Ratcliffe (1967, 1969, 1970), Hickey & Anderson (1968), and
Anderson & Hickey (1972, 1974) were the first, in Britain and North
America, respectively, to compare the thickness of eggshells sampled
from the wild with that of specimens measured from museums and private
collections which predated the use of DDT. These authors examined a
wide range of bird species but mainly those high in food chains. Later
studies, along the same lines, include those of Dilworth et al. (1972)
on the woodcock, Wiemeyer et al. (1975) on the osprey, Fox (1976) on
the common tern, Cooke et al. (1976) on the grey heron, and Koeman et
al. (1972) and Newton & Haas (1984) on the sparrowhawk. Ratcliffe
(1970) collected eggshell data on 17 species of British birds, 9 of
which showed significant decreases in shell thickness when comparing
the period before 1947 with the period after 1947. The birds affected
were predominantly raptors, exceptions being the carrion crow, rook,
and shag. Anderson & Hickey made eggshell comparisons between pre- and
post-DDT use on 25 different species of birds. The same species from
different geographical areas of North America were investigated, making
166 comparisons in all. Of these, 62% showed significant decreases, 37%
showed non-significant decreases or no change, and only 1% showed an
increase in shell thickness.
King et al. (1978) found significant decreases in eggshell thick-
ness in 15 out of 22 aquatic species of birds, in Texas, USA, when
comparing shells from 1970 with museum specimens from before 1943. All
of these studies, and many more, demonstrated that, in those species
that showed effects on eggshell thinning, the effect began suddenly and
markedly at the same time as the onset of DDT use. In Britain, the use
of DDT in large quantities began in 1947. Fig. 2 reproduces the data
(from Newton & Haas, 1984) on sparrowhawks from 1870 to 1980. The
persistence of DDT in bird tissues means that recovery is still not
complete, despite controls on the use of DDT. In Alaska, populations
of peregrine falcons did not show the effects of DDT until much later
than other regions of North America. These birds breed in Alaska,
where use of DDT was low, but winter in Central and South America.
Residues of DDT and its metabolites in Alaskan peregrines began to rise
in 1967, along with the use of DDT in its wintering grounds. Con-
comitant reductions in breeding success and populations of peregrines
occurred (White & Cade, 1977). These data are indicative of a bird
breeding and survival effect of DDT use, correlated both with time and
geographical area. The index of eggshell thickness has reflected the
pattern of use of the insecticide (Ratcliffe, 1970). Before 1947,
there was no significant geographical variation in the mean thickness
of peregrine falcon eggshells in Britain. Since 1947, eggshells from
non-agricultural areas, notably the central and eastern Scottish
highlands, have shown a smaller decrease in shell thickness than shells
from highly agricultural regions.
Anderson & Hickey (1974) showed that shells of the white-tailed
eagle in Greenland were thicker than shells of the same species
collected in the Baltic. Compared to early reference shells from
museums, the Greenland shells showed a slight increase in thickness of
3%, whereas Swedish shells showed a decrease of 16%.
Lincer (1975) established a dose relationship between dietary DDE
and eggshell thinning in captive American kestrels and, also, a
relationship between DDE residues in the eggs and the thickness of
their shells. He then compared shell thickness with egg DDE residue in
kestrels sampled from the wild. The relationship was identical. Many
other authors have shown a good correlation between egg DDE residues
and the degree of eggshell thinning. These studies cover the
following species: double-crested cormorant (Anderson et al., 1969);
great blue heron (Vermeer & Reynolds, 1970); prairie falcon (Enderson &
Berger, 1970); peregrine falcon (Peakall et al., 1975c); grey heron
(Cooke et al., 1976); sparrowhawk (Newton & Bogan, 1978); and gannet,
shag, and great black-backed gull (Cooke, 1979a). In many of these
studies, there is not only a correlation between eggshell thickness and
DDE but there are also correlations between DDE residues and residues
of other organochlorines. Therefore, it is often difficult to deter-
mine solely from the field data, exactly which chemical is responsible
for the effect. This problem has been addressed by Newton & Bogan
(1978). They conducted a statistical analysis of their data that
showed a correlation between DDE and shell thickness, egg breakage, egg
addling, and hatching failure, in addition to a correlation between
DDT, PCB, and dieldrin residues. After multivariate analysis, DDE
appeared only to be responsible for eggshell thinning and egg breakage.
Relating laboratory studies to field observations suggests that DDE is
the only organochlorine that causes eggshell thinning.
Haegele & Tucker (1974) dosed egg-laying Japanese quail with a
single oral dose of p,p' -DDE, o,p' -DDT, p,p' -DDT, or technical
DDT, all at 125 mg/kg body weight. None of the treatments caused
appreciable eggshell thinning. When Smith et al. (1969) fed Japanese
quail with DDT at 100, 200, or 400 mg/kg diet, the two lower doses had
no effect on hatchability or fertility of eggs laid. At 400 mg/kg,
there was 50% mortality amongst dosed birds; survivors showed a decline
in hatchability and fertility after 30 days. Bitman et al. (1969)
dosed Japanese quail with o,p' -DDT or p,p' -DDT at a dietary level
of 100 mg/kg. The quail were given a low calcium diet (0.56%) and
were, therefore, under calcium stress during egg laying. Both isomers
of DDT caused significant thinning of eggshells (P<0.001) and a
significant (P<0.01) reduction in shell calcium content. Eggs produced
by birds dosed with the p,p isomer were significantly lighter than
those laid by birds dosed with the o,p' isomer.
Cecil et al. (1971) investigated the effects of p,p' -DDT and
p,p' -DDE on the egg production and eggshell characteristics of
Japanese quail receiving an adequate calcium diet, and compared their
results with previous studies of the effects of these compounds on
quail receiving low calcium diets. They found a delay in the onset of
egg production in quail fed a concentration of 100 mg/kg of either DDT
or DDE for about 3 weeks. This result was similar to that of studies
with low calcium diets. In contrast to the earlier studies, there was
no effect of either DDT or DDE on shell thickness or egg weight when
dietary calcium was higher. There was an increased incidence of egg
breakage in birds fed DDT and DDE, but this was less pronounced than
with the low calcium diets.
Robson et al. (1976) studied the effects of DDE and DDT fed to
Japanese quail in two different diets containing adequate or low
calcium. DDT was fed at 100 mg/kg diet, whereas DDE was given at 0,
199, or 300 mg/kg diet, and the two calcium levels were 0.5% and 3%.
DDE at 300 mg/kg was detrimental to adult body weight, fertility, and
survivability. There was no effect of either DDT or of DDE at up to
100 mg/kg diet on adult body weight, food consumption, egg production,
egg weight, fertility, hatchability, cracking of eggs, or eggshell
thickness. Low dietary calcium had the effect of reducing the
thickness of eggshells, increasing the incidence of cracked shells and
decreasing egg production and hatchability.
Davison et al. (1976) fed DDE (0, 2, 10, 40, or 200 mg/kg diet)
to female Japanese quail that were individually caged and had 14 g of
food available each day. There was no effect on body weight, egg
laying, egg weight, eggshell thickness, or on shell calcium content.
Quail were then fed a diet containing DDT at 0, 2.5, 10, or 40 mg/kg.
There was no effect on eggshell thickness, number of eggs laid,
fertility, or hatchability. Quail fed 40 mg DDT/kg diet and caged in
pairs, broke more eggs than birds fed lower concentrations of DDT or
any concentration when the birds were caged individually. Paired quail
laid fewer eggs than single quail and in one experiment they laid eggs
with thinner shells.
When Davison & Sell (1972) dosed white leghorn hens with 100 or
200 mg DDT/kg diet for 12 weeks, the average egg production per bird,
egg weight, dry shell weight, shell thickness, and shell calcium were
all found to be unaffected by DDT at either dose level.
Egg-laying mallard ducks treated by Haegele & Tucker (1974) with a
single oral p,p' -DDE dose of 500, 1000, or 2000 mg/kg body weight
showed a clear effect on eggshell thickness at all dose levels.
Unfortunately, whilst the results are clear, no statistical analysis of
the results was presented. The effect on eggshells was dose related,
quick acting, and persistent. Heath et al. (1969) dosed mallard for
two seasons with DDE or TDE at 10 or 40 mg/kg diet and with DDT at 2.5,
10, or 40 mg/kg diet. The highest dose of DDT was reduced to 25 mg/kg
in the second season. DDE at both concentrations severely impaired
reproductive success, a more rapid initiation of the effect being seen
with the higher dose. DDE significantly affected eggshell thickness;
eggs from birds dosed with 40 mg/kg laid, in their second season, eggs
with shells 13% thinner than controls. There was a significant
increase in egg cracking and decrease in egg hatchability at both DDE
dose levels. TDE did not have a significant effect on shell thickness.
It impaired reproductive success, but not as severely as did DDE. DDT
induced eggshell thinning at a dose of 25 mg/kg, shells being 18%
thinner than controls, and reduced duckling survival during 14 days
post-hatch by 35%. DDT at 2.5 and 10 mg/kg had no effect.
Vangilder & Peterle (1980) fed mallard a diet containing 10 mg
DDE/kg, and brought the birds into breeding condition using long
daylength. Relative to controls, egg laying was delayed, eggshell
thickness was decreased, and hatchability was reduced in treated birds.
Ducklings, hatched from eggs laid by treated females, showed a signifi-
cantly reduced survival time, and a greater proportion of ducklings
were unable to initiate normal body temperature regulation.
When Longcore et al. (1971) dosed black ducks with 10 or 30 mg
DDE/kg diet, there was significant eggshell thinning and an increase in
shell cracking, compared to controls, at both dose levels. The
survival of ducklings to 21 days was also significantly reduced at both
dose levels. Longcore & Stendell (1977) fed DDE (10 mg/kg diet) to
black ducks over two breeding seasons and then untreated food for a
further 2 years. The eggshells of treated birds during dosing were 20%
thinner than controls. When dosing stopped, eggshell thickness
gradually increased but shells were still 10% thinner than controls 2
years after dosing had finished. Similarly, there was still a reduced
survival of ducklings, to 3 weeks of age, 2 years after dosing with DDE
had ceased.
Peakall et al. (1973) studied the effects of dietary DDE on
eggshell thinning in three species of bird (white pekin duck, American
kestrel, and ringdove). In addition to shell thinning, they reported a
reduced rate of water loss from eggs laid by DDE-treated birds; the
permeability constants of the eggs were significantly decreased.
Scanning electron micrographs revealed a decrease in the number of
pores per unit shell area and an increase in the number of globular
inclusions in eggshells from treated birds. Greenburg et al. (1979)
showed, also using scanning electron microscopy, that DDE affected both
organic and inorganic constituents of the eggshells of mallard dosed in
their diet. The literature concerning the effects of DDE on eggshell
structure has been reviewed in detail by Cooke (1973b).
In studies by Miller et al. (1976), laying white pekin ducks and
white leghorn hens were dosed with 40 mg DDE/kg diet. The ducks showed
significant eggshell thinning within 4 days, and again between 1 and 3
months of the start of dosing, but the hens did not show significant
eggshell effects within 2 weeks.
Peakall et al. (1975a) dosed white pekin ducks at a dietary level
of 250 mg DDE/kg for 10 days, and, approximately 2 months later,
started to collect eggs and measure shell thickness for a period of 27
weeks. At the beginning of the collection period, shells from treated
birds were found to be 20% thinner than controls. Recovery was slow
and shells were still 10% thinner at the end of the study. Haseltine
et al. (1974) dosed mallard and pheasant (10 mg DDE/kg diet) and ring
doves (40 mg DDE/kg diet) and found significant eggshell thinning and
depression of serum calcium levels in both mallard and ring dove.
However, neither parameter was significantly changed in pheasant.
Peakall et al. (1975b) maintained paired ring doves on a diet
containing 100 mg DDE/kg for 3 weeks and white pekin ducks on 250 mg
DDE/kg diet for 10 days. Although both species showed significant
eggshell thinning, there was no significant difference between the
levels of serum calcium of treated and control birds.
Miller et al. (1976) removed the shell glands from white pekin
ducks and white leghorn chickens, dosed with 40 mg DDE/kg diet, when a
calcifying egg was present within the gland, and assessed enzymatic
activity. There was a significant decrease in Ca2+-ATPase and
carbonic anhydrase activities in the shell glands removed from dosed
ducks, but no difference from controls in chicken shell glands. Kolaja
(1977) maintained mallard ducks on a diet containing either DDT, DDE,
DDT sulphonate, or DDE sulphonate at dose levels of 10 or 50 mg/kg.
Eggs were collected for 30 days and were weighed and measured. There
was no significant difference between egg weights at the different dose
levels. The thickness of eggshells of birds fed DDE was significantly
reduced. Ducks fed DDT laid eggs with significantly thinner shells
only after day 14. The two sulphonate-treated groups were not
significantly different from each other and were only significantly
different from controls on day 18; eggshell weights followed a similar
pattern.
Mendenhall et al. (1983) dosed breeding barn owls with 3 mg DDE/kg
diet during two breeding seasons, and found that treated birds laid
thin-shelled eggs and laid significantly more eggs per pair in both
seasons. In both years the percentage of eggs broken was increased,
relative to controls, and the mean number of eggs hatched and young
fledged per pair was reduced. There was a significant increase in
embryo deaths in one of the two years.
Eggshell thickness has been monitored in different ways by
different authors. Some direct measurement has been made with
membranes intact and some without. Other methods have been used to
compare recent eggs with museum specimens, which could not be broken to
measure thickness directly. The various methods were reviewed by Cooke
(1973b), who suggested standards. Generally a log-linear relationship
between DDE load and shell thinning is claimed. In a recent consider-
ation of the theoretical treatment of such data, Moriarty et al. (1986)
suggested that the main methods of assessing shell thickness do not
adequately take into consideration the effects of shell size and shape.
This does not detract from the conclusion that shell thinning occurs,
but suggests that the relationship may be more properly described as
curvilinear.
6.2.4 Reproductive hormones and behaviour
After feeding mallard a diet contaminated with 3 mg p,p' -DDE/kg
and artificially incubating eggs laid by the females, Heinz (1976)
found that the average egg residue of DDE was 5.8 mg/kg. Ducklings
from treated eggs were hyperresponsive to a tape-recorded maternal
call; treated ducklings were significantly more likely to approach the
recorder. In contrast, treated ducklings moved shorter distances away
from a frightening stimulus, compared to controls. Japanese quail
chicks fed a diet containing 50 mg DDE/kg for 8 days, starting at 7
days of age, and then a clean diet for a further 6 days showed no
significant effect on avoidance response to a moving silhouette.
Haegele & Hudson (1977) paired ring doves for 12.5 min each day,
for 5 days, prior to dosing their diet with 10 or 50 mg p,p' -DDE/kg.
The birds were also paired between days 31 and 35 and between days 59
and 63 after the start of dosing. Two measures of the courtship
behaviour of males were made: total courtship activity time and mean
bow-coo frequency. Bow-cooing behaviour is the initial behaviour
displayed by males to attract females. In control birds, the total
courtship activity time was 25% (days 31-35) and 23% (days 59-63)
longer than it was in the predose period. In birds dosed with 10 mg
DDE/kg, the courtship activity between days 31 and 35 was not different
from that in the predose period, whereas the final pairing produced a
decrease of 55% in activity. In birds dosed with 50 mg DDE/kg, the
courtship activity decreased by 30% and 67% for the two later pairing
periods compared to the predose period. After dosing at 10 mg/kg,
there was no change in bow-cooing between days 31 and 35, but a
reduction of 53% between days 59 and 63. Birds dosed at 50 mg/kg
showed decreases in bow-cooing behaviour of 43% and 84%, in the two
subsequent pairings respectively, when compared to the predose period.
When Richie & Peterle (1979) paired ring doves and fed them with
either 10 or 40 mg p,p' -DDE/kg diet, there was a significant delay in
the period between pairing and egg laying at both dose levels.
Leutinizing harmone levels in blood plasma, sampled throughout the
experiment, were not significantly altered by the DDE. Similarly,
Jefferies (1967) reported an increase in the time between
pairing and egg laying in Bengalese finches fed a range of doses
of p,p' -DDT between 75 and 1200 mg/kg diet. Treated birds were fed
for 2 h/day, immediately following a period of 1 h of starvation.
There was a significant correlation between DDT intake by the female
and the delay in egg laying. Dobson (1981) measured circulating
hormone levels and nest-building behaviour in pigeons dosed orally with
DDE and found a delay in egg laying. Hormone measurements showed that
ovulation was not delayed. Nest building was reduced in treated birds.
The delay in egg laying resulted from a lengthening of the period
between ovulation and oviposition. Since the laying of eggs is
dependent on the stimulus of adequate nest material, this lengthening
of the period between pairing and egg laying was considered to be
primarily an indirect effect on reproduction, triggered by a direct
effect on behaviour; the egg was retained longer in the oviduct.
Peakall (1970) maintained ring doves on a diet containing
10 mg p,p' -DDT/kg for 3 weeks. They were kept in isolation (with
short daylengths) and then paired (with long daylengths) to induce
breeding. The females were killed either 8 days after pairing or
after completion of their clutch of two eggs. In those killed 8 days
after pairing, circulating oestradiol levels were significantly
reduced and hepatic enzyme activity was significantly increased.
There was a significant delay in the laying of the first egg and a
decrease in egg weight. In the same study, the birds were given
oral 45Ca (7.4 x 104 Bq; 2 µCi) on the day before pairing. There
was a significant decrease in the radioactivity of eggs and in the
bones of females killed 8 days after pairing. In a separate
experiment, p,p' -DDT was injected intraperitoneally at a dose of
150 mg/kg body weight, into female ring doves within 1 day of their
first egg being laid. The birds were killed after completing their
clutch of two eggs. The shell weight of the second egg was
significantly reduced when compared to the first and there was a
significant decrease in carbonic anhydrase activity in the oviduct.
This enzyme is associated with deposition of calcium into the shell.
6.2.5 Reproductive effects on the male
Burlington & Lindeman (1950) administered a daily subcutaneous
injection of DDT to male white leghorn chicks, gradually increasing
the dose from 15 to 300 mg/kg body weight. The birds were treated for
60-89 days, and the cockerels were killed and their testes removed,
weighed, and sectioned. Treated birds were found to have smaller
testes, more intertubular tissue, and retarded tubular development.
These effects were accompanied by an inhibition of testosterone-
dependent secondary sexual characters; combs and wattles were reduced
in both size and colour development in treated birds. Locke et al.
(1966) dosed male bald eagles at dietary levels of 10 mg DDT/kg for 60
or 120 days, and found no effects on spermatogenic activity. There
were some degenerative effects on the testis, but only at doses which
had severe neurological effects and ultimately led to death.
6.2.6 Effects on the thyroid and adrenal glands in birds
When Jefferies & French (1972) fed pigeons on a diet containing
either 18, 36, or 72 mg p,p' -DDE/kg for a period of 56 days, paired
thyroid weights were found to be greater in treated birds than in
controls. There was no apparent dose relationship to this effect, but
bird numbers were small. Taking the dosed birds as a single group,
the results were significantly different from those of control birds.
Liver weights were similarly increased, and, at the two highest dose
levels, there was an increase in paired adrenal weights.
Biessman & von Faber (1981) dosed Japanese quail for 9 weeks
with technical DDT (either 50 or 250 mg/kg diet) or for 5 weeks with
p,p' -DDT or p,p' -DDE (250 and 300 mg/kg, respectively). Adrenal
weights increased with all treatments but the increase in size was only
significant for the 300 mg DDE/kg dose. The percentage of cortical
tissue, measured from areas of sections of the gland, showed a similar
trend, but results were not statistically significant. No changes were
detectable in nuclear size of either cortical or medullary cells.
Lehman et al. (1974) studied the effect of technical grade DDT on the
adrenal glands of bobwhite quail, which were maintained on a diet
containing 10, 50, or 150 mg DDT/kg for 242 days and then killed. No
effect was found on adrenal weight expressed as a percentage of body
weight, but there was a significant dose-related increase in the ratio
between areas of cortex and medulla.
6.2.7 Special studies in birds
Dieter (1974) fed Japanese quail on a diet containing 5, 25, or
100 mg DDE/kg and, after 12 weeks of dosing, assessed the activity of
five plasma enzymes (creatine kinase, aspartate aminotransferase,
lactate dehydrogenase, cholinesterase, and fructose-diphosphate
aldolase). There was an increase in the activity of all these enzymes,
which, in each case, was proportional to the logarithm of the DDE
dose.
Bend et al. (1977) dosed immature puffins, orally by intubation,
with DDE at 6 mg/day (equivalent to 50 mg/kg diet) for 16 to 21 days,
and, after killing the birds, determined the effect of DDE on hepatic
mixed-function oxidases. Both aniline hydroxylase and benzphetamine
demethylase activities were increased in treated birds; the yield of
microsomal protein remained unchanged. In contrast, Sell et al. (1972)
demonstrated a depression in aniline hydroxylase and N-demethylase
activities after feeding Japanese quail with DDT at 200 mg/kg diet.
Both DDT and DDE inhibited aniline hydroxylase in vitro activity, when
present at concentrations of 10-7 mol/litre or more.
Bunyan et al. (1970) measured the activities of glucose-6-
phosphate dehydrogenase (G-6-P) and 6-phosphogluconate dehydrogenase
(6-P-G) in the liver of Japanese quail fed diets containing low levels
of p,p' -DDT or a number of saturated and unsaturated analogues of
p,p' -DDT. Generally, saturated compounds lowered G-6-P levels and
increased 6-P-G levels. p,p' -DDMU was anomalous in elevating G-6-P.
The authors suggested that these effects might be due to interference
with protein metabolism primarily by the unsaturated analogues and
metabolites of DDT. Bunyan et al. (1972) fed either DDT or DDE to
Japanese quail and monitored hepatic microsomal protein, cytochrome
P450, aniline hydroxylase, aromatic nitroreductase, phenylbenzoate
esterase, and total vitamin C. Changes in these factors were more
readily explained in terms of residues of DDE in the liver than in
terms of dietary dose. DDE was found to be a more potent inducer of
microsomal protein, cytochrome P450, and aniline hydroxylase than was
DDT. The effects of DDT could be explained in terms of the effects of
the DDE produced by DDT metabolism. Aromatic nitroreductase was
unaffected by either compound. Vitamin C levels were raised by DDT
more than by DDE. Phenylbenzoate esterase showed a biphasic response
following the feeding of DDE. Bunyan & Page (1973) extended these
studies by examining the effects of DDE and DDMU on hepatic microsomal
enzyme systems. Most of the changes observed in quail were greater
with DDMU than with any other DDT metabolite. The authors suggested
that DDT metabolism in birds may be different to metabolism in mammals.
Metabolism probably gives rise, via the production of DDMU, to a
highly active liver inducer.
Heinz et al. (1980) fed ring doves on a diet containing 2, 20, or
200 mg DDE/kg for 8 weeks, and found at the end of the dosing period, a
significant decrease in dopamine concentration in brain tissue from
birds fed on the two higher doses. Brain noradrenalin concentration
was also affected but only at the highest dose. There was a signifi-
cant, negative correlation between concentration of both dopamine and
noradrenalin and the residue of DDE in the brain tissue.
Friend et al. (1973) fed a dietary dose of 10, 100, or
1000 mg DDE/kg. to male mallard that had been previously maintained on
either fresh water or 1% salt water. Birds were given a concentrated
salt solution either 1, 3, 6, or 9 days after the beginning of DDE
treatment, the salt being administered both intraperitoneally (12 ml of
a 10% solution) and intravenously (3ml of a 5% solution). The rate of
sodium chloride excretion was not reduced, relative to controls, in
DDE-treated birds maintained previously on salt water, but was reduced
significantly in DDE-treated birds not previously given salt.
When Mahoney (1975) fed caged white-throated sparrows on technical
DDT (either 5 or 25 mg/kg), the onset of spring nocturnal migratory
restlessness (Zugunruhe) and weight increase was delayed by at least 1
week. Although Zugunruhe onset was delayed, when migratory nocturnal
activity did commence it was more pronounced than in control birds.
The increase in Zugunruhe was related to body residues of DDT.
Haynes (1972) dosed male bobwhite quail with DDT (100 mg/kg diet)
for 10 weeks and, 1 week before the study was terminated, some birds
were transferred to clean food while others were starved for 4 days and
then given clean food for 3 days before being killed. There was no
significant effect on liver glycogen, either from dosing with DDT or
from starvation, but liver lipid levels were significantly increased by
both DDT and starvation. Body lipid levels were not significantly
affected by DDT but were reduced after starvation.
6.2.8 Synergism with other compounds in birds
Kreitzer & Spann (1973), in a study on combined effects of
pesticides, found that mixtures of DDT and dieldrin in Japanese
quail, and DDE and Ceresan M (organomercury fungicide) in pheasants,
were additive rather than synergistic in their action. The study
compared known LD50 values with expected ones. Mallard, maintained
on a diet containing a mixture of DDE (40 mg/kg) and Aroclor 1254 (40
mg/kg) for at least 30 days, laid eggs with significantly thinner
shells than did controls. This result was not significantly different
from that produced by DDE alone (Risebrough & Anderson, 1975). In a
similar study on American kestrels, Lincer (1972) dosed the birds with
Aroclor 1254 (10 mg/kg) and DDE (3 mg/kg) in the diet, both separately
and in combination. There was no eggshell thinning with Aroclor
alone, but Aroclor and DDE together had a significantly greater
effect on shell thickness than DDE alone, indicating synergism.
Japanese quail exposed to dietary doses of 5 or 50 mg DDE/kg for 12
weeks, and subsequently dosed orally with either parathion or paraoxon
at 2 µl/g body weight, showed synergism between the compounds with
respect to mortality and to inhibition of brain cholinesterase. The
synergistic action of DDE on cholinesterase inhibition was apparent 3
days after exposure to 50 mg/kg and one week after exposure to 5 mg/kg.
Mortality due to DDE was increased from 10% to 90% in the presence of
the organophosphorus compounds. Anticholinesterase effects were
increased by 50% in the presence of DDE (Ludke, 1977).
6.3 Non-laboratory Mammals
Appraisal
Experimental work suggests that some species, notably bats, may
have been affected by DDT and its metabolites. Species which show
marked seasonal cycles in fat content are most vulnerable, but few
experimental studies on such species have been made. In contrast to
the situation in birds, where the main effect of DDT is on repro-
duction, the main known effect in mammals is to increase the mortality
of migrating adults. The lowest acute dose which kills American big
brown bats is 20 mg/kg. Bats collected from the wild (and containing
residues of DDE in fat) die after experimental starvation which
simulates loss of fat during migration.
In studies into the effect of DDE on bats, Geluso et al. (1976)
captured young Mexican free-tailed bats ( Tadarida brasiliensis ) before
their first migratory flight and transferred them to the laboratory.
This species migrates north from Mexico to the USA in spring and
returns to winter in the south. Three groups of bats were used. A
reference set was killed on capture and, when the bats were analysed
for residues of organochlorines derived from environmental source, DDE
was the only chemical found in significant amounts. Brain residues of
DDE were low; the median being 3.7 (range: 1.5 to 17.0) mg/kg in eight
younger animals and 1.3 (range 1.1 to 11.0) mg/kg in older animals.
Two further groups were maintained in the laboratory where the bats
were given water but not fed. One group was regularly exercised, while
the other was given no exercise. All exercised bats died within 9
days; 4 bats in the unexercised group died and the other 4 were killed
after 9 days. Analysis of brain DDE residues showed considerably
elevated levels compared to the reference group. For the unexercised
bats, the median residue values were 47 (range 18 to 76) mg/kg in
younger animals and 70 (range 10 to 95) mg/kg in older animals. In
exercised bats, the values were 160 (range 66 to 330) mg/kg for younger
animals and 160 (range 37 to 260) mg/kg for older animals. Those
animals that died before the end of the study showed symptoms charac-
teristic of pesticide poisoning, including hyperactivity, intermittent
audiogenic seizures, and violent contractions of chest muscles. The
high brain residues of DDE were considered to be the cause of death of
the animals. It should be noted that these animals had not been arti-
ficially dosed with DDE. The effects resulted from residues of DDT in
body fat, taken up in the maternity roost. The authors considered that
their studies confirmed the suggestion that bats were being killed by
accumulated residues of DDE during the period of migration, when their
fat reserves were used up.
Clark & Kroll (1977) experimentally fed adult females of the same
species of bat ( Tadarida brasiliensis ) for 40 days with mealworms
containing 107 mg DDE/kg and then killed four of the bats. They had a
whole body burden of 2.345-2.929 mg DDE (and 78-90 mg DDE/kg in the
brain). Twelve of the dosed bats were then starved, and they died
within 8 days. The total body burden of DDE ranged from 1.952 to
3.711 mg DDE and brain residues from 379 to 564 mg/kg. These brain
residues were considered to be diagnostic of death from DDE poisoning.
Tremors characteristic of poisoning were seen in the bats before death
occurred.
The toxicity of single oral doses of DDT to bats has been
estimated in two studies. Jefferies (1972) derived an approximate
LD50 of 63 mg/kg body weight for the pipistrelle bat ( Pipistrellus
pipistrellus ), a small British species. There was no mortality at
doses below 45 mg/kg and 100% mortality at doses above 95 mg/kg.
Luckens & Davis (1964) found that the lowest dose which killed American
big brown bats ( Eptesicus fuscus ) was 20 mg/kg and that 40 mg/kg was
invariably lethal. The LD50 for this species lies somewhere between
25 and 40 mg/kg for a single oral dose.
Blus (1978) determined dietary LC50 values of DDT, given in food
either as a powder or dissolved in oil, for short-tailed shrews
( Blarina brevicauda ) of different ages and sex. In 2-week tests, the
range of LC50s for DDT dissolved in oil was 651 to 1160 mg/kg diet,
and for DDT added as powder it was 839 to >2552 mg/kg. The influence
of age and sex was sometimes more important in determining DDT toxicity
than was body weight, though heavier shrews tended to be more tolerant
of the chemical. Among older animals, males were more tolerant of DDT
than females. Braham & Neal (1974) found an effect of DDT on the
metabolic rate of the same species of shrew after feeding it with
earthworms contaminated with the insecticide. After one week of this
diet, the metabolic rate was significantly higher than that of undosed
shrews, but after 2-3 weeks of dosing there was a return to oxygen
consumption rates not different from controls. Two shrews were fasted
for 18 h, after being fed earthworms containing DDT for 3 weeks, and
compared to untreated shrews similarly fasted. The DDT-treated animals
showed 12.6% and 12.1% increases in metabolic rate after fasting,
whereas controls showed decreases of 8.7% and 8.0%. The DDT exposure
was environmentally realistic because earthworms used for feeding were
not artificially dosed with DDT but were collected from an area where
DDT had been used.
7. ECOLOGICAL EFFECTS FROM FIELD APPLICATION
There have been kills of fish (Hunt & Linn, 1970) and aquatic
invertebrates (Ide, 1957) reported after normal usage of DDT as a
terrestrial insecticide and after its application to water for mosquito
control. Reproductive failure in commercial fisheries has also been
attributed to DDT (Hunt & Linn, 1970). In addition, it has been shown
to be toxic to amphibia after water application (section 5.3). The
setting of safe water levels of DDT and its metabolites is difficult
because its high bioaccumulation and high lipid solubility mean that it
can have effects remote in time from its application. The toxicity of
DDT to aquatic microorganisms and invertebrates is very variable
between species. Exposure to DDT or its stable metabolites would,
therefore, be expected to kill certain species selectively. Short-
term, there is close correspondence between the 96-h LC50 for a
moderately sensitive fish (16 µg/litre) and the expected water concen-
tration after application of DDT at the normal rate.
DDT and its metabolites, principally DDE, have been implicated in
reproductive effects on birds in the field. Large population declines
in some bird species, mainly birds of prey, have been blamed on DDT or
on combinations of DDT with other persistent organochlorines. The
evidence for this rests on correlations. There is a correlation in
time between the onset of effects on eggshells and the onset of major
DDT use in agriculture. There is also a correlation between geo-
graphical areas of high DDT use and effects on local populations of
birds (compared to populations living in areas of low use). There is a
clear correlation between DDE residues in eggs and the degree of
thinning of the shells of those eggs, collected from the wild. Storage
of DDT in body fat means that the effects of the compound can be remote
in time from the application of the chemical to an area. Only some
species of birds are affected by DDT or its metabolites. There are
considerable data on the variability between species in their suscepti-
bility to these compounds. Widespread monitoring programmes have
related the recovery of bird populations to reduced levels of DDE and
the residual material of aldrin/dieldrin use in the tissues of birds
sampled from the wild, following attempts to limit or ban the use of
the parent pesticide in agriculture. Because DDT is seldom the only
chemical residue found in bird tissues from the wild, there is some
disagreement on whether DDT alone can cause population declines in
birds.
Ratcliffe (1967, 1969, 1970), Hickey & Anderson (1968), and
Anderson & Hickey (1972, 1974) were the first, in Britain and North
America, respectively, to compare the thickness of eggshells sampled
from the wild with that of specimens measured from museums and private
collections which predated the use of DDT. These authors examined a
wide range of bird species but mainly those high in food chains. Later
studies, along the same lines, include those of Dilworth et al. (1972)
on the woodcock, Wiemeyer et al. (1975) on the osprey, Fox (1976) on
the common tern, Cooke et al. (1976) on the grey heron, and Koeman et
al. (1972) and Newton & Haas (1984) on the sparrowhawk. Ratcliffe
(1970) collected eggshell data on 17 species of British birds, 9 of
which showed significant decreases in shell thickness when comparing
the period before 1947 with the period after 1947. The birds affected
were predominantly raptors, exceptions being the carrion crow, rook,
and shag. Anderson & Hickey made eggshell comparisons between pre- and
post-DDT use on 25 different species of birds. The same species from
different geographical areas of North America were investigated, making
166 comparisons in all. Of these, 62% showed significant decreases, 37%
showed non-significant decreases or no change, and only 1% showed an
increase in shell thickness.
King et al. (1978) found significant decreases in eggshell thick-
ness in 15 out of 22 aquatic species of birds, in Texas, USA, when
comparing shells from 1970 with museum specimens from before 1943. All
of these studies, and many more, demonstrated that, in those species
that showed effects on eggshell thinning, the effect began suddenly and
markedly at the same time as the onset of DDT use. In Britain, the use
of DDT in large quantities began in 1947. Fig. 2 reproduces the data
(from Newton & Haas, 1984) on sparrowhawks from 1870 to 1980. The
persistence of DDT in bird tissues means that recovery is still not
complete, despite controls on the use of DDT. In Alaska, populations
of peregrine falcons did not show the effects of DDT until much later
than other regions of North America. These birds breed in Alaska,
where use of DDT was low, but winter in Central and South America.
Residues of DDT and its metabolites in Alaskan peregrines began to rise
in 1967, along with the use of DDT in its wintering grounds. Con-
comitant reductions in breeding success and populations of peregrines
occurred (White & Cade, 1977). These data are indicative of a bird
breeding and survival effect of DDT use, correlated both with time and
geographical area. The index of eggshell thickness has reflected the
pattern of use of the insecticide (Ratcliffe, 1970). Before 1947,
there was no significant geographical variation in the mean thickness
of peregrine falcon eggshells in Britain. Since 1947, eggshells from
non-agricultural areas, notably the central and eastern Scottish
highlands, have shown a smaller decrease in shell thickness than shells
from highly agricultural regions.
Anderson & Hickey (1974) showed that shells of the white-tailed
eagle in Greenland were thicker than shells of the same species
collected in the Baltic. Compared to early reference shells from
museums, the Greenland shells showed a slight increase in thickness of
3%, whereas Swedish shells showed a decrease of 16%.
Lincer (1975) established a dose relationship between dietary DDE
and eggshell thinning in captive American kestrels and, also, a
relationship between DDE residues in the eggs and the thickness of
their shells. He then compared shell thickness with egg DDE residue in
kestrels sampled from the wild. The relationship was identical. Many
other authors have shown a good correlation between egg DDE residues
and the degree of eggshell thinning. These studies cover the
following species: double-crested cormorant (Anderson et al., 1969);
great blue heron (Vermeer & Reynolds, 1970); prairie falcon (Enderson &
Berger, 1970); peregrine falcon (Peakall et al., 1975c); grey heron
(Cooke et al., 1976); sparrowhawk (Newton & Bogan, 1978); and gannet,
shag, and great black-backed gull (Cooke, 1979a). In many of these
studies, there is not only a correlation between eggshell thickness and
DDE but there are also correlations between DDE residues and residues
of other organochlorines. Therefore, it is often difficult to deter-
mine solely from the field data, exactly which chemical is responsible
for the effect. This problem has been addressed by Newton & Bogan
(1978). They conducted a statistical analysis of their data that
showed a correlation between DDE and shell thickness, egg breakage, egg
addling, and hatching failure, in addition to a correlation between
DDT, PCB, and dieldrin residues. After multivariate analysis, DDE
appeared only to be responsible for eggshell thinning and egg breakage.
Relating laboratory studies to field observations suggests that DDE is
the only organochlorine that causes eggshell thinning.
 Population declines in birds of prey differed between much of North
America and eastern North America and western Europe. In North
America, apart from in the East, declines were gradual, whereas in
Europe and eastern North America declines were sudden and catastrophic.
The sudden declines in Europe have usually been attributed to the use
of the chlorinated cyclodienes, which kill adult birds, rather than to
DDT. A study of the recoveries of European birds of prey populations
provides evidence for this attribution. Populations began to rise at a
time when residues of DDE in tissues were stable but when use of the
cyclodienes and, therefore, residues of HEOD (dieldrin) were declining.
Some populations in North America did not show high contamination with
cyclodienes and may have declined due to DDT use alone. Henny (1972)
showed that in American populations of osprey, American kestrel, and
red-shouldered hawk there was a decrease in breeding performance, but
no increase in adult mortality, in response to DDT. The reproductive
effects of DDT may have prevented population recoveries after the
cessation of dieldrin use and the return of mortality rates to normal.
The question has been reviewed by Newton (1979) and Newton & Haas
(1984).
The populations of many species of birds of prey were monitored
throughout a period of high DDT use. This was done by large scale
surveys and studies of population dynamics (Ratcliffe, 1972; Henny,
1977; Lindberg, 1977; White & Cade, 1977), migration counts at obser-
vation points (Rosen, 1966; Hackman & Henny, 1971; Edelstam, 1972;
Ulfstrand et al., 1974; Nagy, 1977), and by sample counts (Ash, 1965;
Bezzel, 1969). Some species showed marked declines (in some areas this
led to local extinction), whilst others showed only temporary effects
or no effects at all. Declines were most marked in bird-eating
species, such as the sparrowhawk and peregrine falcon, and fish-eating
species, such as the white-tailed and bald eagles, and were less marked
in mammal-eating species, such as the kestrel, golden eagle, and
buzzard. These variations in decline correspond to the DDE levels
found in these particular species (Newton, 1979).
Perfect (1980) reported the results of a 4-year study on the
overall effects of the use of DDT as an insecticide on cowpeas crops in
a Nigerian forest soil. In addition to effects on soil invertebrates
(section 6.1), there were effects on the decomposition of plant
material. The remains of the plants after harvesting were ploughed
into the soil and this resulted in an increase in the residues of DDT
and its metabolites in lower levels of the soil. To confirm an effect
on decomposition, these plant remains were buried in mesh bags and the
loss of weight due to decomposition was recorded over time. There was
a significant reduction in the rate of decomposition of plant material
treated with DDT and also of untreated plant material buried in
contaminated soil. Shires (1985) reported no significant effect on the
decomposition of sweet chestnut leaf litter in a temperate area after
the application of DDT at 1 kg/ha.
Perfect et al. (1979) investigated the effects of repeated DDT
applications on cowpea crop yield in Nigeria. Yields varied
considerably from season to season and from year to year in untreated
plots because of differences in pest damage and climate. DDT was
applied to the treated plots weekly between planting and harvest at a
rate of 1 kg/ha, and the site was studied for 4 years. Over the 4-year
period there was a considerable benefit in yield from DDT application;
the yield was 1.45 tonnes/ha in the untreated and 3.42 tonnes/ha in the
treated plots. However, the benefit was most noticeable in the first
year of cultivation and declined over the four years to the point where
DDT use did not significantly increase yield. The authors attributed
the effect to the deleterious action of the insecticide on soil biota.
8. EVALUATION
In evaluating the environmental hazard of DDT and its metabolites
the following general points have to be kept in mind.
(a) The environmental distribution and effects of DDT are spread wider
than the area of use, because the parent compound or its
metabolites are carried worldwide by air and ocean currents and in
biota.
(b) Some of the breakdown products of DDT, principally DDE, are highly
persistent in soil, sediment, and biota. Thus, problems with
residues of these materials last long after the cessation of use.
(c) The bioaccumulation of DDT, or more usually of its metabolites, is
well established and occurs from very low environmental
concentrations of DDT. The use of "bioconcentration factors"
(the ratio of concentration in the organism with concentration in
the medium) to estimate the capacity of organisms to take up DDT
can be misleading if the exposure is high, since these values are
ratios.
(d) Residues and effects are often highly seasonal, corresponding to
changes in body fat, since DDT metabolites are very lipid-soluble.
Measurements of these metabolites in the tissues of organisms must
be conducted over a period of time if they are to give any
indication of the degree of contamination of the environment.
(e) There are insufficient data on the effects of DDT and its
metabolites on communities of organisms and ecosystem functioning.
Hazard assessment is, therefore, often made by extrapolation from
single species studies.
(f) Research and monitoring have concentrated on a few effects of DDT
observed in the wild. This could give the mistaken impression that
the effects of these compounds are restricted to a few species.
Other effects could be predicted but have received little or no
attention from the scientific community.
(g) The major remaining use of DDT is for malaria control operations
that are normally carried out in tropical countries. However, the
majority of environmental studies on DDT have been carried out in
conditions relevant to temperate regions. Care must be exercised
in extrapolating these results to tropical conditions.
8.1 Aquatic Organisms
The widespread use of DDT as an insecticide has resulted in
worldwide contamination of the environment. Due to the physicochemical
characteristics of DDT and its metabolites, concentrations have been
recorded in different environmental compartments, including soil,
sediments, and terrestrial and aquatic organisms. The bioconcentration
of DDT and its metabolites is a real hazard to non-target organisms.
DDT and its metabolites cause adverse effects at all trophic levels of
aquatic ecosystems, particularly on primary producers, which are the
most sensitive. Although no data are available for the effects of DDT
on ecosystem function, it should be regarded as a major environmental
hazard in this respect. DDT and its metabolites are highly toxic to
fish and, besides their lethal effect, they affect development,
behaviour, and biochemical processes. DDT and its metabolites, should
be regarded as hazardous to fish productivity and distribution and,
hence, to human food supplies. Accumulated DDT and its metabolites are
further transferred from aquatic organisms to consumers, including
birds, mammals, and, ultimately, human beings.
8.2 Terrestrial Organisms
DDT-type compounds are resistant to breakdown and are readily
adsorbed onto soils and sediments, from whence they can act as long-
term sources of exposure and contribute to terrestrial organisms.
Accumulation in terrestrial organisms is via the food chain.
These chemicals are hazardous to microorganisms, but repeated
application can lead to the development of tolerance in some species.
DDT causes fluctuations in some populations of microorganisms, and this
could eventually lead to changes in species composition, disruption of
nutrient cycles, and changes in soil fertility.
Earthworms are insensitive to the acute toxic effects of DDT
residues in soil. However, they are known to take up DDT from soil and
this uptake presents a major hazard to predators.
DDT is a non-selective insecticide and leads to mortality in
natural enemies of the insect pest. This results in impairment of the
balance between predators and prey and leads to outbreaks of secondary
pests and occurrence of the primary pest in larger numbers.
Laboratory studies confirm field findings that bat populations are
adversely affected by DDE, especially during migration. These studies
are indicative of the potential hazard to other mammals, exposed to DDT
in the environment, when fat containing DDT residues is mobilized,
e.g., during migration or temporary starvation.
One of the most widely studied effects of DDT is eggshell thinning
in birds, particularly in predatory species. The metabolite DDE, not
DDT, has been shown to be responsible for this effect. Other effects
on reproduction and survival of birds have been demonstrated. Large
population declines in birds of prey can be, at least partially,
attributed to DDT. It has been shown that DDE residues in birds and
their eggs reduced the rate of recovery of affected raptor
populations. A factor that has received less attention is the
secondary effect of the increasing numbers of pest rodents that were
controlled principally by birds of prey in some countries.
* * *
Because of their lack of degradation, their resulting widespread
persistence in the environment, their high acute toxicity to organisms
at the base of food chains, and their high potential for
bioaccumulation, DDT and its metabolites should be regarded as a major
hazard to the environment. DDT should not be used when an alternative
insecticide is available.
REFERENCES
ALBAUGH, D.W. (1972) Insecticide tolerances of two crayfish
populations ( Procambarus acutus ) in South-central Texas. Bull.
environ. Contam. Toxicol ., 8: 334-338.
ALBONE, E.S., EGLINTON, G., EVANS, N.C., HUNTER, J.M., & RHEAD,
M.M. (1972) Fate of DDT in severn estuary sediments. Environ. Sci.
Technol ., 6: 914-919.
ALLISON, D., KALLMAN, B.J., COPE, O.B., & VAN VALLIN, C.C. (1964)
Some chronic effects of DDT on cutthroat trout , Washington, DC, US
Department of the Interior, Bureau of Sport, Fisheries and Wildlife,
pp. 1-30 (Resource Report No. 64).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain north
American birds. Proc. Int. Ornithol. Congr ., 15: 514-540.
ANDERSON, D.W. & HICKEY, J.J. (1974) Eggshell changes in raptors from
the Baltic region. Oikos , 25: 395-401.
ANDERSON, D.W., HICKEY, J.J., RISEBROUGH, R.W., HUGHES, D.F., &
CHRISTENSEN, R.E. (1969) Significance of chlorinated hydrocarbon
residues to breeding pelicans and comorant. Can. field Nat ., 83: 91-
112.
ASH, J.S. (1965) A reduction in numbers of birds of prey in France.
Bird Study , 12: 17-26.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees , University
of California, 38 pp. (Laboratory Studies - Extension M-16).
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1972) DDT:
inhibition of sodium chloride tolerance by the blue-green
alga Anacystis nidulans . Science, 176: 1141-1143.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the aquatic
environment: uptake and loss of DDT and dieldrin by freshwater mussels.
Bull. environ. Contam. Toxicol ., 1: 97-111.
BEND, J.R., MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1977) DDE-
induced microsomal mixed-function oxidases in the puffin ( Fratercula
arctica). Biochem. Pharmacol ., 26: 1000-1001.
BENSON, W.W. & SMITH, P. (1972) Pesticide levels in deer. Bull.
environ. Contam. Toxicol ., 8: 1-9.
BENSON, W.W., GABICA, J., & BEECHAM, J. (1974) Pesticide and mercury
levels in bear. Bull. environ. Contam. Toxicol ., 11: 1-4.
BERGLIND, R. & DAVE, G. (1984) Acute toxicity of chromate, DDT, PCP,
TPBS and zinc to Daphnia magna cultured in hard and soft water. Bull.
environ. Contam. Toxicol ., 33: 63-68.
BEZZEL, E. (1969) [Results of quantitative observations of the
Accipitridae in Upper Bavaria.] Ornithol. Mitt ., 21: 29-36 (in
German).
BIESSMANN, A. & VON FABER, H. (1981) Effects of DDT and its
metabolites on the adrenal gland of the Japanese quail. Environ.
Pollut ., 25: 99-104.
BITMAN, J., CECIL, H.C., HARRIS, S.J., & FRIES, G.F. (1969) DDT
induces a decrease in eggshell calcium. Nature (Lond.) , 224: 44-46.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin and endrin. Arch. environ. Contam.
Toxicol ., 7: 83-98.
BOLLAG, J.-M. & HENNINGER, N.M. (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J. environ.
Qual ., 5: 15-18.
BOLLEN, W.B., MORRISON, H.E., & CROWELL, H.H. (1954) Effect of field
treatments of insecticides on numbers of bacteria, Streptomyces and
moulds in the soil. J. econ. Entomol ., 47: 302-306.
BOYD, C.E. & FERGUSON, D.E. (1964) Susceptibility and resistance of
mosquito fish to several insecticides. J. econ. Entomol ., 57: 430-431.
BOYD, C.E., VINSON, S.B., & FERGUSON, D.E. (1963) Possible DDT
resistance in two species of frogs. Copeia , 1963(2): 426-429.
BRAHAM, H.W. & NEAL, C.M. (1974) The effects of DDT on energetics of
the short-tailed shrew, Blarina brevicauda. Bull. environ. Contam.
Toxicol ., 12: 32-37.
BUHLER, D.R. & SHANKS, W.E. (1970) Influence of body weight on chronic
oral DDT toxicity in coho salmon. J. Fish Res. Board Can ., 27: 347-
358.
BUHLER, D.R., RASMUSSON, M.E., & SHANKS W.E. (1969) Chronic oral
DDT toxicity in juvenile coho and chinook salmon. Toxicol. appl.
Pharmacol ., 14: 535-555.
BUNYAN, P.J. & PAGE, J.M.J. (1973) Pesticide-induced changes in
hepatic microsomal enzyme systems. Some effects of 1,1-di(p-chloro-
phenyl)-2,2-dichloroethylene (DDE) and 1,1-di(p-chlorophenyl)-2-
chloroethylene (DDMU) in the rat and the Japanese quail. Chem.-biol.
Interact ., 6: 249-257.
BUNYAN, P.J., DAVIDSON, J., & SHORTHILL, M.J. (1970) Hepatic glucose-
6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase levels
in Japanese quail following the ingestion of p,p' -DDT and related
compounds. Chem.-biol. Interact ., 2: 175-182.
BUNYAN, P.J., TOWNSEND, M.G., & TAYLOR, A. (1972) Pesticide-induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-di(p-
chlorophenyl) -2,2,2-trichloroethane (DDT) and 1,1-di(p-chlorophenyl)-
2,2-dichloroethylene (DDE) in the rat and Japanese quail. Chem.-biol.
Interact ., 5: 13-26.
BURDICK, G.E., HARRIS, E.J., DEAN, H.J., WALKER, T.M., SKEA, J., &
COLBY, D. (1964) The accumulation of DDT in lake trout and the effect
on reproduction. Trans. Am. Fish. Soc ., 93: 127-136.
BURLINGTON, H. & LINDEMAN, V.F. (1950) Effect of DDT on testes and
secondary sex characters of white leghorn cockerels. Proc. Soc. Exp.
Biol ., 74: 48-51.
BUTLER, P.A. (1964) Commercial fishery investigations. In: The
effects of pesticides on fish and wildlife , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 65-77
(Circular No. 226).
CALABRESE, A. (1972) How some pollutants affect embryos & larvae of
American oyster & hard-shell clam. Mar. Fish. Rev ., 34: 66-77.
CECIL, H.C., BITMAN, J., & HARRIS, S.J. (1971) Effects of dietary
p,p' -DDT and p,p' -DDE on egg production and eggshell characteristics
of Japanese quail receiving an adequate calcium diet. Poult. Sci ., 50:
657-659.
CHRISTIE, A.E. (1969) Effect of insecticides on algae. Water Sewage
Works , 116: 172-176.
CLARK, D.R. & KROLL, J.C. (1977) Effects of DDE on experimentally
poisoned free-tailed bats ( Tadarida brasilensis ): lethal brain
concentrations. J. Toxicol. environ. Health, 3: 893-901.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyreniodosa by a polychlorinated biphenyl
and several insecticides. Environ. Entomol ., 3: 217-220.
COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U., PERFECT, T.J., &
YEADON, R. (1980) Effects of cultivation and DDT on earthworm
activity in a forest soil in the sub-humid tropics. J. appl. Ecol.,
17: 21-29.
COOKE, A.S. (1970) The effect of p,p -DDT on tadpoles of the common
frog (Rana temporaria). Environ. Pollut ., 1: 57-71.
COOKE, A.S. (1971) Uptake of DDT and DDE by the quail embryo and
chick. Pestic. Sci ., 2: 144-147.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on amphibian
spawn and tadpoles. Environ. Pollut ., 3: 51-68.
COOKE, A.S. (1973a) The effects of DDT, when used as a mosquito
larvicide, on tadpoles of the frog Rana temporaria. Environ. Pollut.,
5: 259-273.
COOKE, A.S. (1973b) Shell-thinning in avian eggs by environmental
pollutants. Environ. Pollut ., 4: 85-152.
COOKE, A.S. (1979a) Eggshell characteristics of gannets ( Sula
bassana ), shags ( Phalacrocorax aristotelis ) and great black-backed
gulls ( Larus marinus ) exposed to DDE and other environmental
pollutants. Environ. Pollut ., 19: 47-65.
COOKE, A.S. (1979b) The influence of rearing density on subsequent
response to DDT dosing for tadpoles of the frog Rana temporaria . Bull.
environ. Contam. Toxicol ., 21: 837-841.
COOKE, A.S. & POLLARD, E. (1973) Shell and operculum formation by
immature Roman snails, Helix pomatia L., when treated with p,p' -DDT.
Pestic. Biochem. Physiol ., 3: 230-236.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Eggshell characteristics
and incidence of shell breakage for grey herons Ardea cinerea exposed
to environmental pollutants. Environ. Pollut ., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution , Swindon, United Kingdom, Natural Environment
Research Council, 74 pp.
CRAWFORD, R.B. & GUARINO, A.M. (1976) Effects of DDT in Fundulus
studies on toxicity, fate and reproduction. Arch. environ. Contam.
Toxicol ., 4: 334-348.
CRITCHLEY, B.R., COOK, A.G., CRITCHLEY, U., PERFECT, T.J., &
RUSSELL-SMITH, A. (1980) The effects of crop protection with DDT on
some elements of the subterranean and surface active arthropod fauna of
a cultivated forest soil in the humid tropics. Pedobiologia , 20: 31-
38.
DAVEY, S.P. (1963) Effects of chemicals on earthworms: a review of the
literature , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 1-20 (Special Scientific Report, Wildlife No.
74).
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin and
DDT by earthworms. Soil Biol. Biochem ., 3: 221-233.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p' -DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol ., 7: 9-18.
DAVISON, K.L., ENGEBRETSON, K.A., & COX, J.H. (1976) p,p' -DDT and
p,p' -DDE effects on egg production, eggshell thickness and
reproduction of Japanese quail. Bull. environ. Contam. Toxicol ., 15:
265-270.
DAVY, F.B., KLEEREKOPER, H., & GENSLER, P. (1972) Effects of
exposure to sublethal DDT on the locomotor behavior of the goldfish
(Carassius auratus). J. Fish Res. Board Can ., 29: 1333-1336.
DAVY, F.B., KLEEREKOPER, H., & MATIS, J.H. (1973) Effects of exposure
to sublethal DDT on the exploratory behavior of goldfish (Carassius
auratus). Water Resour. Res ., 9: 900-905.
DERR, S.K. & ZABIK, M.J. (1972) Biologically active compounds in the
aquatic environment: the effect of DDT on the egg viability of
Chironomus tentans. Bull. environ. Contam. Toxicol ., 7: 366-368.
DESAIAH, D., CUTKOMP, L.K., KOCH, R.B., & JARVINEN, A. (1975)
DDT: effect of continuous exposure on ATPase activity in fish,
Pimephales promelas. Arch. environ. Contam. Toxicol ., 3: 132-141.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife studies,
Patuxent Wildlife Research Center 1961 - 1962 , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 74-96
(Circular No. 167).
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyl, malathion and mercuric
chloride. Toxicol. appl. Pharmacol ., 27: 86-98.
DILL, P.A. & SAUNDERS, R.C. (1974) Retarded behavioral development and
impaired balance in Atlantic salmon ( Salmo salar ) alevins hatched from
gastrulae exposed to DDT. J. Fish Res. Board Can ., 31: 1936-1938.
DILWORTH, T.G., KEITH, J.A., PEARCE, P.A., & REYNOLDS, L.M. (1972)
DDE and eggshell thickness in New Brunswick woodcock. J. wildl.
Manage ., 36: 1186-1193.
DIMOND, J.B. & SHERBURNE, J.A. (1969) Persistence of DDT in wild
populations of small mammals. Nature (Lond.) , 221: 486-487.
DINDAL, D.L. & WURZINGER, K.-H. (1971) Accumulation and excretion of
DDT by the terrestrial snail, Cepaea hortensis. Bull. environ. Contam.
Toxicol ., 4: 362-371.
DOBSON, S. (1981) [Physiological and behavioural effects of organo-
chlorines on pigeons.] Oekol. Vogel , 3(Suppl.): 39-43 (in German).
DUNACHIE, J.F. & FLETCHER, W.W. (1969) An investigation of the
toxicity of insecticides to birds' eggs using the egg-injection
technique. Ann. appl. Biol ., 64: 409-423.
EARNEST, R.D. & BENVILLE, P.E. (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game , 58: 127-132.
EBERHARDT, L., MEEKS, R.L., & PETERLE, T.J. (1971) Food chain model
for DDT kinetics in a fresh water marsh. Nature (Lond.) , 230: 60-62.
EDELSTAM, C. (1972) The visible migration of birds at Ottenby,
Sweden. Var Fagelvarld , 7(Suppl.): 1-360.
EDEN, W.G. & ARTHUR, B.W. (1965) Translocation of DDT and heptachlor
in soybeans. J. econ. Entomol ., 34: 161-162.
EDWARDS, C.A. & JEFFS, K. (1974) Rate of uptake of DDT from soil by
earthworms. Nature (Lond.) , 247: 157-158.
EL-SEBAE, A.H. (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region , Athens,
Mediterranean Action Plan, United Nations Environment Programme/Food
and Agriculture Organization of the United Nations, pp. 109-116. (MAP
Technical Report Series No. 10).
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning and
lowered production of young in prairie falcons. Bioscience , 20: 355-
356.
FERGUSON, D.E., CULLEY, D.D., COTTON, W.D., & DODDS, R.P. (1964)
Resistance to chlorinated hydrocarbon insecticides in three species of
freshwater fish. Bioscience , 14: 43-44.
FORSYTH, D.J. & PETERLE, T.J. (1973) Accumulation of chlorine-36 ring-
labeled DDT residues in various tissues of two species of shrew. Arch.
environ. Contam. Toxicol ., 1: 1-17.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R.,
& HUNT, J.R. (1972) Residues in eggs and tissues of hens fed a ration
containing low levels of pesticides with and without charcoal. J. econ.
Entomol ., 65: 982-988.
FOX, G.A. (1976) Eggshell quality: its ecological and physiological
significance in a DDE-contaminated common tern population. Wilson
Bull ., 88: 459-477.
FRIEND, M., HAEGELE, M.A., & WILSON, R. (1973) DDE: interference with
extra-renal salt excretion in the mallard. Bull. environ. Contam.
Toxicol ., 9: 49-53.
FUHREMANN, T.W. & LICHTENSTEIN, E.P. (1980) A comparative study
of the persistence, movement, and metabolism of six carbon-14
insecticides in soils and plants. J. agric. food Chem ., 28: 446-452.
GARDNER, D.R. (1973) The effect of some DDT and methoxychlor analogs on
temperature selection and lethality in brook trout fingerlings.
Pestic. Biochem. Physiol ., 2: 437-446.
GAUFIN, A.R., JENSEN, L.D., NEBEKER, A.V., NELSON, T., & TEEL,
R.W. (1965) The toxicity of ten organic insecticides to various
aquatic invertebrates. Water Sewage Works , 112: 276-279.
GELUSO, K.N., ALTENBACH, J.S., & WILSON, D.E. (1976) Bat mortality:
pesticide poisoning and migratory stress. Science , 194: 184-186.
GILMAN, A.P., HALLETT, D.J., FOX, G.A., ALLAN, L.J., LEARNING,
W.J., & PEAKALL, D.B. (1978) Effects of injected organochlorines on
naturally incubated herring gull eggs. J. wildl. Manage ., 42: 484-493.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 15 pp (Special Scientific Report No. 241).
GOFFART, H. (1949) [The effect of new kinds of insecticides on
earthworms.] Anz. Schadlingskd. (Berlin) , 22: 72-74 (in German).
GOULDING, K.H. & ELLIS, S.W. (1981) The interaction of DDT with two
species of freshwater algae. Environ. Pollut ., 25: 271-290.
GREENBURG, R.R., RISEBROUGH, R.W., & ANDERSON D.W. (1979)
p,p' -DDE-induced changes in the organic and inorganic structure of
eggshells of the mallard, Anas platyrhynchos. Toxicol. appl.
Pharmacol ., 48: 279-286.
GREICHUS, Y.A. & HANNON, M.R. (1973) Distribution and biochemical
effects of DDT, DDD and DDE in penned double-crested cormorants.
Toxicol. appl. Pharmacol ., 26: 483-494.
GREICHUS, Y.A., CALL, D.J., AMMANN, B.M., GREICHUS, A., &
SHAVE, H. (1975) Physiological effects of polychlorinated biphenyls or
a combination of DDT, DDD, and DDE in penned white pelicans. Arch.
environ. Contam. Toxicol ., 3: 330-343.
HACKMAN, C.D. & HENNY, C.J. (1971) Hawk migration over white marsh,
Maryland. Chesapeake Sci ., 12: 137-141.
HAEGELE, M.A. & HUDSON, R.H. (1973) DDE effects on reproduction of
ring doves. Environ. Pollut ., 4: 53-57.
HAEGELE, M.A. & HUDSON, R.H. (1977) Reduction of courtship behavior
induced by DDE in male ringed turtle doves. Wilson Bull ., 89: 593-601.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common pollutants
on eggshell thickness in mallards and coturnix. Bull. environ. Contam.
Toxicol ., 11: 98-102.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of coho salmon (Oncorhynchus kisutch). J. Fish Res.
Board Can ., 31: 1543-1547.
HANKE, W., GLUTH, G., BUBEL, H., & MULLER, R. (1983) Physiological
changes in carps induced by pollution. Ecotoxicol. environ. Saf ., 7:
229-241.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained sheepshead
minnows. Trans. Am. Fish. Soc ., 98: 426-429.
HANSEN, D.J. (1972) DDT and malathion: effect on salinity selection by
mosquitofish. Trans. Am. Fish. Soc ., 101: 346-350.
HANSEN, D.J. & WILSON, A.J. (1970) Significance of DDT residues from
the estuary near Pensacola, Fla. Pestic. monit . J., 4: 51-56.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquitofish, Gambusia affinis.
Bull. environ. Contam. Toxicol ., 8: 46-51.
HARRI, M.N.E., LAITINEN, J., & VALKAMA, E.-L. (1979) Toxicity and
retention of DDT in adult frogs, Rana temporaria L. Environ. Pollut.,
13: 45-55.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J. agric.
food Chem ., 15: 861-863.
HASELTINE, S., UEBELHART, K., PETERLE, T., & LUSTICK, S. (1974)
DDE, PTH and eggshell thinning in mallard, pheasant and ring dove.
Bull. environ. Contam. Toxicol ., 11: 139-145.
HAUX, C. & LARSSON, A. (1979) Effects of DDT on blood plasma
electrolytes in the flounder, Platichthys flesus L., in hypotonic
brackish water. Ambio , 8: 171-173.
HAYNES, R.J. (1972) Effects of DDT on glycogen and lipid levels in
bobwhites. J. wildl. Manage ., 36: 518-523.
HEATH, R.G., SPANN, J.W., & KREITZER, J.F. (1969) Marked DDE
impairment of mallard reproduction in controlled studies. Nature
( Lond .), 224: 47-48.
HEINZ, G.H. (1976) Behavior of mallard ducklings from parents fed 3
ppm DDE. Bull. environ. Contam. Toxicol ., 16: 640-645.
HEINZ, G.H., HILL, E.F., & CONTRERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin and Aroclor
1254. Toxicol. appl. Pharmacol ., 53: 75-82.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four species of
fish. Trans. Am. Fish. Soc ., 88: 23-32.
HENNY, C.J. (1972) An analysis of the population dynamics of selected
avian species , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 99 pp
(Research Report No. 1).
HENNY, C.J. (1977) Birds of prey, DDT, & tussock moths in Pacific
Northwest. In: Transactions of the Forty-second North American Wildlife
and Natural Resource Conference , Washington, DC, Wildlife Management
Institute, pp. 397-411.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish eating birds. Science , 162:
271-273.
HILL, E.F., DALE, W.E., & MILES, J.W. (1971) DDT intoxication in birds:
subchronic effects and brain residues. Toxicol. appl. Pharmacol ., 20:
502-514.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds , Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 61 pp
(Special Scientific Report No. 191).
HODKINSON, M. & DALTON, S.A. (1973) Interactions between DDT and
river fungi. II Influence of culture conditions on the compatibility of
fungi and p,p' -DDT. Bull. environ. Contam. Toxicol ., 10: 356-359.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife , Washington, DC, US Department of
the Interior, Fish and Wildlife Service, 90 pp (Resource Publication
No. 153).
HUNT, E.G. & LINN, J.D. (1970) Fish kills by pesticides. In: Gillett,
J.W., ed. The biological impact of pesticides in the environment
Corvallis, Oregon, Oregon State University, pp. 97-102.
IDE, F.P. (1957) Effect of forest spraying with DDT on aquatic insects
of salmon streams. Trans. Am. Fish. Soc ., 86: 208-219.
JANICKI, R.H. & KINTER, W.B. (1971) DDT disrupted osmoregulatory
events in the intestine of the eel Anguilla rostrata adapted to
seawater. Science , 173: 1146-1148.
JARVINEN, A.W., HOFFMAN, M.J., & THORSLUND, T.W. (1977) Long-
term toxic effects of DDT food and water exposure on fathead minnows.
J. Fish Res. Board Can ., 34: 2089-2103.
JEFFERIES, D.J. (1967) The delay in ovulation produced by p,p' -DDT and
its possible significance in the field. Ibis , 109: 266-272.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in British
bats and their significance. J. Zool ., 166: 245-263.
JEFFERIES, D.J. & FRENCH, M.C. (1972) Changes induced in the pigeon
thyroid by p,p' -DDE and dieldrin. J. wildl. Manage ., 36: 24-30.
JOHANSEN, C. (1961) Bee poisoning. A hazard of applying agricultural
chemicals , Washington, DC, Institute of Agricultural Sciences,
Washington State University (Circular No. 356).
JOHNSON, B.T. & KENNEDY, J.O. (1973) Biomagnification of p,p' -DDT and
methoxychlor by bacteria. Appl. Microbiol ., 26: 66-71.
JOHNSON, B.T., SAUNDERS, C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin by
freshwater invertebrates. J. Fish Res. Board Can ., 28: 705-709.
JOHNSON, H.E. & PECOR, C. (1969) Coho salmon mortality and DDT in Lake
Michigan. Trans. North Am. Wildl. Nat. Resour. Conf ., 34: 159-166.
JOWETT, P.E., RHEAD, M.M., & BAYNE, B.L. (1978) In vitro changes in the
activity of ATPases in the gills of Carcinus maenas exposed to various
concentrations of p,p' -DDT. Environ. Pollut ., 17: 1-6.
KAN, C.A., JONKER-DEN ROOYEN, J.C., TUINISTRA, L.G.M.T., ROOS,
A.H., & TRAAG, W. (1978) Possible influence of sex and embryonic
content on accumulation of some organochlorine pesticides in
broilers. J. agric. food Chem ., 26: 618-621.
KATZ, M. (1961) Acute toxicity of some organic insecticides to three
species of salmonids and to the threespine stickleback. Trans. Am.
Fish. Soc ., 90: 264-268.
KEIL, J.E. & PRIESTER, L.E. (1969) DDT uptake and metabolism by a
marine diatom. Bull. environ. Contam. Toxicol ., 4: 169-173.
KEIL, J.E., SANDIFER, S.H., GRABER, C.D., & PRIESTER, L.E. (1972) DDT
and polychlorinated biphenyl (Aroclor 1242). Effects of uptake on E.
coli growth. Water Res ., 6: 837-841.
KING, K.A., FLICKINGER, E.L., & HILDEBRAND, H.H. (1978) Shell
thinning and pesticide residues in Texas aquatic bird eggs,
1970. Pestic. monit. J ., 12: 16-21.
KINTER, W.B., MERKENS, L.S., JANICKI, R.H., & GUARINO, A.M. (1972)
Studies on the mechanism of toxicity of DDT and polychlorinated
biphenyls (PCBs): disruption of osmoregulation in marine fish.
Environ. Health Perspect ., 1: 169-173.
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972) Eggshell
and population changes in the sparrow-hawk (Accipiter nisus). TNO-
Nieuws , 27: 542-550.
KOLAJA, G.J. (1977) The effects of DDT, DDE and their sulfonated
derivatives on eggshell formation in the mallard duck. Bull. environ.
Contam. Toxicol ., 17: 697-701.
KORN, S. & EARNEST, R. (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game , 60: 128-131.
KOUYOUMJIAN, H.H. & UGLOW, R.F. (1974) Some aspects of the toxicity of
p,p' -DDT, p,p' -DDE and p,p' -DDD to the freshwater planarian
Polycelis felina (Tricladida). Environ. Pollut ., 7: 103-109.
KREITZER, J.F. & SPANN, J.W. (1973) Test of pesticidal synergism with
young pheasants and Japanese quail. Bull. environ. Contam. Toxicol.,
9: 250-256.
LAL, R. & SAXENA, D.M. (1979) Effect of DDT on cell population growth
of Tetrahymena pyriformis. Arch. Protistenkd ., 122: 382-386.
LAL, R. & SAXENA, D.M. (1980) Effect of DDT on cell population growth,
cell division, and DNA synthesis in Stylonychia notophora (Stokes).
Arch. environ. Contam. Toxicol ., 9: 163-170.
LAL, R. & SAXENA, D.M. (1982) Accumulation, metabolism, and effects of
organochlorine insecticides on microorganisms. Microbiol. Rev ., 46: 95-
127.
LEE, S.S., FANG, S.C., & FREED, V.H. (1976) Effect of DDT on
photosynthesis of Selenastrum capricornutum. Pestic. Biochem. Physiol.,
6: 46-51.
LEFFLER, C.W. (1975) Effects of ingested mirex and DDT on juvenile
Callinectes sapidus Rathbun. Environ. Pollut ., 8: 283-300.
LEDFORD, R.A. & CHEN, J.H. (1969) Degradation of DDT and DDE by
cheese microoganisms. J. food Sci ., 34: 386-388.
LEGMAN, J.W., PETERLE, T.J., & MILLS, C.M. (1974) Effects of DDT on
bobwhite quail ( Colinus virginianus ) adrenal gland. Bull. environ.
Contam. Toxicol ., 11: 407-414.
LINCER, J.L. (1972) The effects of organochlorines on the American
kestrel (Falco sparverius Linn.) , Ithaca, New York, Cornell
University (PhD Thesis).
LINCER, J.L. (1975) DDE-induced eggshell-thinning in the American
kestrel: a comparison of the field situation and laboratory results.
J. appl. Ecol ., 12: 781-793.
LINDBURG, P. (1977) The peregrine falcon in Sweden. In: Proceedings of
the International Council for Bird Preservation World Conference on
Birds of Prey, Vienna, 1975 , Cambridge, United Kingdom, International
Council for Bird Preservation, pp. 329-338.
LOCKE, L.N., CHURA, N.J., & STEWART, P.A. (1966) Spermatogenesis in
bald eagles experimentally fed a diet containing DDT. Condor , 68: 497-
502.
LONGCORE, J.R., SAMSON, F.B., & WHITTENDALE, T.W. (1971) DDE
thins eggshells and lowers reproductive success of captive black
ducks. Bull. environ. Contam. Toxicol ., 6: 485-490.
LONGCORE, J.R. & STENDELL, R.C. (1977) Shell thinning and reproductive
impairment in black ducks after cessation of DDE dosage. Arch. environ.
Contam. Toxicol ., 6: 293-304.
LOOSANOFF, V.L. (1960) Some effects of pesticides on marine arthropods
and mollusks. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Transactions of the Second Seminar, 1959 , Cincinnati, USA,
Robert, A. Taft Sanitation and Engineering Centre, pp. 89-93 (Technical
Report No. W60-3).
LOWE, J.I. (1965) Chronic exposure of blue crabs, Callinectes sapidus,
to sublethal concentrations of DDT. Ecology , 46: 899-900.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia , 12: 29-33.
LUCKENS, M.M. & DAVIS, W.H. (1964) Bats: sensitivity to DDT. Science,
146: 948.
LUDKE, J.L. (1977) DDE increases the toxicity of parathion to
Coturnix quail. Pestic. Biochem. Physiol ., 7: 28-33.
MACEK, K.J. (1968) Reproduction in brook trout ( Salvelinus fontinalis )
fed sublethal concentrations of DDT. J. Fish Res. Board Can ., 25:
1787-1795.
MACEK, K.J. & KORN, S. (1970) Significance of the food chain in DDT
accumulation by fish. J. Fish Res. Board Can ., 27: 1496-1498.
MACEK, K.J. & SANDERS, H.O. (1970) Biological variation in the
susceptibility of fish and aquatic invertebrates to DDT. Trans. Am.
Fish. Soc ., 99: 89-90.
MACEK, K.J., RODGERS, C.R., STALLING, D.L., & KORN, S. (1970) The
uptake, distribution and elimination of dietary 14C-DDT and
14C-dieldrin in rainbow trout. Trans. Am. Fish. Soc ., 99: 689-695.
MACFARLANE, R.B., GLOOSCHENKO, W.A., & HARRISS, R.C. (1972) The
interaction of light intensity and DDT concentration upon the marine
diatom, Nitzschia delicatissima Cleve. Hydrobiologia , 39: 373-382.
MCLANE, M.A.R. & HALL, L.C. (1972) DDE thins screech owl eggshells.
Bull. environ. Contam. Toxicol ., 8: 65-68.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol ., 25: 921-928.
MACRAE, I.C. & VINCKX, E. (1973) Effect of lindane and DDT on
populations of protozoa in a garden soil. Soil Biol. Biochem ., 5: 245-
247.
MAHONEY, J.J. (1975) DDT and DDE effects on migratory condition in
white-throated sparrows. J. wildl. Manage ., 39: 520-527.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p' -DDT on production and survival of Daphnia magna Strauss. Bull.
environ. Contam. Toxicol ., 13: 412-416.
MARTIN, J.P. (1966) Influence of pesticides on soil microbes and soil
properties. Am. Soc. Agron. Spec. Publ ., 8: 95-108.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms , Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity :
interpretation and data base for 410 chemicals and 66 species of
freshwater animals , Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp (Resource Publication No. 160).
MEEKS, R.L. (1968) The accumulation of 36Cl ring-labeled DDT in a
freshwater marsh. J. wildl. Manage ., 32: 376-398.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls ( Tyto alba ) fed low levels of DDE and dieldrin.
Arch. environ. Contam. Toxicol ., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine phyto-
plankton vary in their response to chlorinated hydrocarbons.
Science , 167: 1724-1726.
METCALF, R.L., KAPOOR, I.P., LU, P.-Y, SCHUTH, C.K., & SHERMAN,
P. (1973) Model ecosystem studies of the environmental fate of six
organochlorine pesticides. Environ. Health Perspect ., 4: 35-43.
MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1976a) Enzymatic basis
for DDE-induced eggshell thinning in a sensitive bird. Nature (Lond.),
259: 122-124.
MILLER, D.S., KINTER, W.B., PEAKALL, D.B., & RISEBROUGH, R.W.
(1976b) DDE feeding and plasma osmoregulation in ducks, guillemots,
and puffins. Am. J. Physiol ., 231: 370-376.
MOFFETT, G.B. & YARBROUGH, J.D. (1972) The effects of DDT, toxaphene
and dieldrin on succinic dehydrogenase activity in insecticide-
resistant and susceptible Gambusia affinis. J. agric. food Chem ., 20:
558-560.
MOORE, N.W. (1965) Pesticides in birds - a review of the situation in
Great Britain in 1965. Bird Study , 12: 222-252.
MORIARTY, F. (1975) Pollutants and animals: a factual perspective,
London, Allen & Unwin.
MORIARTY, F., BELL, A.A., & HANSON, H. (1986) Does p,p' -DDE thin
eggshells? Environ. Pollut ., 40: 257-286.
MUIRHEAD-THOMSON, R.C. (1973) Laboratory evaluation of pesticide
impact on stream invertebrates. Freshwater Biol ., 3: 479-498.
MULLA, M.S. (1963) Toxicity of organochlorine insecticides to the
mosquitofish Gambusia affinis and the bullfrog Rana catesbeiana.
Mosq. News , 23: 299-303.
MURPHY, P.G. (1970) Effects of salinity on uptake of DDT, DDE and DDD
by fish. Bull. environ. Contam. Toxicol ., 5: 404-407.
MURPHY, P.G. (1971) The effect of size on the uptake of DDT from water
by fish. Bull. environ. Contam. Toxicol ., 6: 20-23.
NAGY, A.C. (1977) Population trend indices based on 40 years of autumn
counts at hawk mountain. In: Proceedings of the International Council
for Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 243-253.
NAQVI, S.M. & FERGUSON, D.E. (1968) Pesticide tolerances of selected
freshwater invertebrates. J. Mississippi Acad. Sci ., 14: 121-127.
NEUFELD, G.J. & PRITCHARD, J.B. (1979) An assessment of DDT toxicity in
osmoregulation and gill Na,K-ATPase activity in the blue crab,
Philadelphia, American Society for Testing and Materials, pp. 23-34
(ASTM Special Technical Publication No. 667).
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted, United
Kingdom , T. & A.D. Poyser.
NEWTON, I. & BOGAN, J. (1978) The role of different organo-chlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol ., 15:
105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds , 77: 47-70.
NIMMO, D.R., WILSON, A.J., & BLACKMAN, R.R. (1970) Localization of
DDT in the body organs of pink and white shrimp. Bull. environ. Contam.
Toxicol ., 5: 333-341.
OGILVIE, D.M. & MILLER, D.L. (1976) Duration of a DDT-induced shift in
the selected temperature of Atlantic salmon (Salmo salar). Bull.
environ. Contam. Toxicol ., 16: 86-89.
OLOFSSON, S. & LINDAHL, P.E. (1979) Decreased fitness of cod ( Gadus
morrhua L.) from polluted waters. Mar. environ. Res ., 2: 33-45.
OSBORN, D., COOKE, A.S., & FREESTONE, S. (1981) Histology of a
teratogenic effect of DDT on Rana temporaria tadpoles. Environ.
Pollut ., 25: 305-319.
O'SHEA, T.J. & LUDKE, J.L. (1979) Monitoring fish and wildlife for
environmental pollutants , Fort Collins, Colorado, US Department of the
Interior, Fish and Wildlife Service.
PEAKALL, D.B. (1970) p,p' -DDT: effect on calcium metabolism and
concentration of estradiol in the blood. Science , 168: 592-594.
PEAKALL, D.B., LINCER, J.L., RISEBROUGH, R.W., PRITCHARD, J.B., &
KINTER, W.B. (1973) DDE-induced eggshell thinning: structural and
physiological effects in three species. Comp. gen. Pharmacol ., 4: 305-
313.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975a) Prolonged eggshell
thinning caused by DDE in the duck. Nature (Lond.) , 254: 421.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975b) Blood calcium
levels and the mechanism of DDE-induced eggshell thinning. Environ.
Pollut ., 9: 289-294.
PEAKALL, D.B., CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1975c)
Organochlorine residues in Alaskan peregrines. Pestic. monit. J. , 8:
255-260.
PERFECT, J. (1980) The environmental impact of DDT in a tropical agro-
ecosystem. Ambio , 9: 16-21.
PERFECT, T.J., COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U.,
DAVIES, A.L., SWIFT, M.J., RUSSELL-SMITH, A., & YEADON, R. (1979)
The effect of DDT contamination on the productivity of a cultivated
forest soil in the sub-humid tropics. J. appl. Ecol ., 16: 705-719.
PETERSON, R.H. (1973) Temperature selection of Atlantic salmon (Salmo
salar) and brook trout ( Salvalinus fontinalis ) as influenced by various
chlorinated hydrocarbons. J. Fish Res. Board Can ., 30: 1091-1097.
PORTER, R.D. & WIEMEYER, S.N. (1972) DDE at low dietary levels kills
captive American kestrels. Bull. environ. Contam. Toxicol ., 8: 193-
199.
POWERS, C.D., WURSTER, C.F., & ROWLAND, R.G. (1979) DDE inhalation
of marine algal cell division and photosynthesis per cell. Pestic.
Biochem. Physiol ., 10: 306-312.
PRESCOTT, L.M. & OLSON, D.L. (1972) The effect of pesticides on the
soil amoeba Acanthamoeba castellanii (Neff). Proc. South Dakota Acad.
Sci ., 51: 136-141.
RAMALINGAM, K. & RAMALINGAM, K. (1982) Effects of sublethal levels
of DDT, malathion and mercury on tissue proteins of Sarotherodon
mossambicus (Peters). Proc. Indian Acad. Sci. anim. Sci ., 91: 501-505.
RANDALL, W.F., DENNIS, W.H., & WARNER, M.C. (1979) Acute toxicity of
dechlorinated DDT, chlordane and lindane to bluegill ( Lepomis
machrochirus ) and Daphnia magna. Bull. environ. Contam. Toxicol ., 21:
849-854.
RATCLIFFE, D.A. (1967) Decrease in eggshell weight in certain birds of
prey. Nature (Lond.) , 215: 208-210.
RATCLIFFE, D.A. (1969) Population trends of the peregrine falcon in
Great Britain. In: Hinkley, J.J., ed. Peregrine falcon populations:
Their biology and decline , Madison, Milwaukee, London, University of
Wisconsin Press, pp. 239-269.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol ., 7: 67-107.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Study , 19: 117-156.
REINBOLD, K.A., KAPOOR, I.P., CHILDERS, W.F., BRUCE, W.N., &
METCALF, R.L. (1971) Comparative uptake and biodegradability of DDT
and methoxychlor by aquatic organisms. Illinois nat. Hist. Surv.
Bull ., 30: 405-415.
REINERT, R.E., STONE, L.J., & WILLFORD, W.A. (1974) Effect of
temperature on accumulation of methylmercuric chloride and p,p' -DDT by
rainbow trout (Salmo gairdneri). J. Fish Res. Board Can ., 31: 1649-
1652.
RICE, C.P. & SIKKA (1973) Uptake and metabolism of DDT by six species
of marine algae. J. agric. food Chem ., 21: 148-152.
RICHIE, P.J. & PETERLE, T.J. (1979) Effect of DDE on circulating
luteinizing hormone levels in ring doves during courtship and nesting.
Bull. environ. Contam. Toxicol ., 23: 220-226.
RISEBROUGH, R.W. & ANDERSON, D.W. (1975) Some effects of DDE and
PCB on mallards and their eggs. J. wildl. Manage ., 39: 508-513.
RISEBROUGH, R.W., DE LAPPE, B.W., & SCHMIDT, T.T. (1976)
Bioaccumulation factors of chlorinated hydrocarbons between mussels and
seawater. Mar. Pollut. Bull ., 7: 225-228.
ROBERTS, D. (1975) Sub-lethal effects of chlorinated hydrocarbons on
bivalves. Mar. Pollut. Bull ., 6: 20-24.
ROBSON, W.A., ARSCOTT, G.H., & TINSLEY, I.J. (1976) Effect of DDE,
DDT and calcium on the performance of adult Japanese quail (Coturnix
coturnix japonica). Poult. Sci ., 55: 2222-2227.
ROSEN, L. (1966) [The flight of birds of prey at Falsterbo.] Var
Fagelvarld , 25: 315-326 (in Swedish).
SANBORN, J.R., CHILDERS, W.F., & METCALF, R.L. (1975) Uptake of three
polychlorinated biphenyls, DDT, and DDE by the green sunfish, Lepomis
cyanellus Raf. Bull. environ. Contam. Toxicol ., 13: 209-217.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowlers toad Bufo woodhousii
fowleri. Copeia , 2: 246-251.
SANDERS, H.O. (1972) Toxicity of some insecticides to four species of
malacostracan crustaceans , Washington, DC, US Department of the
Interior, Bureau of Sport, Fisheries and Wildlife, pp. 3-19 (Technical
Paper No. 66).
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol.
Oceanogr ., 13: 112-117.
SELL, J.L., DAVISON, K.L., & POONACHA, K.B. (1972) Decreased aniline
hydroxylase activity in Japanese quail due to dietary DDT. J. agric.
food Chem ., 20: 553-557.
SHERBURNE, J.A. & DIMOND, J.B. (1969) DDT persistence in wild hares and
mink. J. wildl. Manage ., 33: 944-948.
SHIN, Y.-O., CHODAN, J.J., & WOLCOTT, A.R. (1970) Adsorption of DDT
by soils, soil fractions, and biological materials. J. agric. food
Chem ., 18: 1129-1133.
SHIRES, S.W. (1985) A comparison of the effects of cypermethrin,
parathion-methyl and DDT on cereal aphids, predatory beetles,
earthworms and litter decomposition in spring wheat. Crop Prot.,
4: 177-193.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of DDT
and dieldrin on development in winter flounder (Pseudopleuronectes
americanus). J. Fish Res. Board Can ., 30: 1894-1898.
SMITH, S.I., WEBER, C.W., & REID, B.L. (1969) The effect of high
levels of dietary DDT on egg production, mortality, fertility,
hatchability and pesticide content of yolks in Japanese quail. Poult.
Sci ., 48: 1000-1004.
STICKEL, L.F. & HEATH, R.G. (1964) Wildlife studies. Patuxent wildlife
research center. In: The effects of pesticides on fish and wildlife,
Washington, DC, Department of the Interior, Fish and Wildlife Service,
pp. 3-30 (Circular No. 226).
THOMPSON, A.R. (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull. environ. Contam. Toxicol ., 5:
577-586.
ULFSTRAND, S., ROOS, G., ALERSTAM, T., & OSTERDAHL, L. (1974)
Visible bird migration at Falsterbo, Sweden. Var Fagelvarld,
8(Suppl.): 1-244.
VANGILDER, L.D. & PETERLE, T.J. (1980) South Louisiana crude oil and
DDE in the diet of mallard hens: effects on reproduction and duckling
survival. Bull. environ. Contam. Toxicol ., 25: 23-28.
VAN VELZEN, A. & KREITZER, J.F. (1975) The toxicity of p,p' -DDT to
the clapper rail. J. wildl. Manage ., 39: 305-309.
VAN VELZEN, A.C., STILES, W.B., & STICKEL, L.F. (1972) Lethal
mobilization of DDT by cowbirds. J. wildl. Manage ., 36: 733-739.
VERMEER, K. & REYNOLDS, L.M. (1970) Organochlorine residues in aquatic
birds in the canadian prairie provinces. Can. field Nat ., 84: 117-130.
VINSON, S.B., BOYD, C.E., & FERGUSON, D.E. (1963) Resistance to DDT in
the mosquito fish, Gambusia affinis. Science , 139: 217-218.
WAGGONER, J.P. & ZEEMAN, M.G. (1975) DDT: short term effects on
osmoregulation in black surfperch (Embiotoca jacksoni). Bull. environ.
Contam. Toxicol ., 13: 297-300.
WARE, G.W. (1968) DDT-C14 translocation in alfalfa. J. econ.
Entomol ., 61: 1451-1452.
WARE, G.W., ESTESEN, B.J., & CAHILL, W.P. (1970) Uptake of C14-DDT
from soil by alfalfa. Bull. environ. Contam. Toxicol ., 5: 85-86.
WARLEN, S.M., WOLFE, D.A., LEWIS, C.W., & COLBY, D.R. (1977)
Accumulation and retention of dietary 14C-DDT by Atlantic menhaden.
Trans. Am. Fish. Soc ., 106: 95-104.
WATSON, M., PHARAOH, B., WYLLIE, J., & BENSON, W.W. (1975)
Metabolism of low oral doses of DDT and DDE by tame mule deer fawns.
Bull. environ. Contam. Toxicol ., 13: 316-323.
WEIS, J.S. & MANTEL, L.H. (1976) DDT as an accelerator of limb
regeneration and molting in fiddler crabs. Estuarine coastal mar. Sci.,
4: 461-466.
WEIS, P. & WEIS, J.S. (1974) DDT causes changes in activity and
schooling behavior in goldfish. Environ. Res ., 7: 68-74.
WHEATLEY, G.A. (1965) The assessment and persistence of residues of
organochlorine insecticides in soils and their uptake by crops. Ann.
appl. Biol ., 55: 325-329.
WHITE, C.M. & CADE, T.J. (1977) Long term trends of peregrine
populations in Alaska. In: Proceedings of the International Council for
Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 63-71.
WHO (1979) Environmental Health Criteria 9: DDT and its derivatives,
Geneva, World Health Organization, 194 pp.
WIEMEYER, S.N. & PORTER, R.D. (1970) DDE thins eggshells of captive
American kestrels. Nature (Lond.) , 227: 737-738.
WIEMEYER, S.N., SPITZER, P.R., KRANTZ, W.C., LAMONT, T.G., &
CROMARTIE, E. (1975) Effects of environmental pollutants on
Connecticut and Maryland ospreys. J. wildl. Manage ., 39: 124-139.
WURSTER, C.F. (1968) DDT reduces photosynthesis by marine
phytoplankton. Science , 159: 1474-1475.
Population declines in birds of prey differed between much of North
America and eastern North America and western Europe. In North
America, apart from in the East, declines were gradual, whereas in
Europe and eastern North America declines were sudden and catastrophic.
The sudden declines in Europe have usually been attributed to the use
of the chlorinated cyclodienes, which kill adult birds, rather than to
DDT. A study of the recoveries of European birds of prey populations
provides evidence for this attribution. Populations began to rise at a
time when residues of DDE in tissues were stable but when use of the
cyclodienes and, therefore, residues of HEOD (dieldrin) were declining.
Some populations in North America did not show high contamination with
cyclodienes and may have declined due to DDT use alone. Henny (1972)
showed that in American populations of osprey, American kestrel, and
red-shouldered hawk there was a decrease in breeding performance, but
no increase in adult mortality, in response to DDT. The reproductive
effects of DDT may have prevented population recoveries after the
cessation of dieldrin use and the return of mortality rates to normal.
The question has been reviewed by Newton (1979) and Newton & Haas
(1984).
The populations of many species of birds of prey were monitored
throughout a period of high DDT use. This was done by large scale
surveys and studies of population dynamics (Ratcliffe, 1972; Henny,
1977; Lindberg, 1977; White & Cade, 1977), migration counts at obser-
vation points (Rosen, 1966; Hackman & Henny, 1971; Edelstam, 1972;
Ulfstrand et al., 1974; Nagy, 1977), and by sample counts (Ash, 1965;
Bezzel, 1969). Some species showed marked declines (in some areas this
led to local extinction), whilst others showed only temporary effects
or no effects at all. Declines were most marked in bird-eating
species, such as the sparrowhawk and peregrine falcon, and fish-eating
species, such as the white-tailed and bald eagles, and were less marked
in mammal-eating species, such as the kestrel, golden eagle, and
buzzard. These variations in decline correspond to the DDE levels
found in these particular species (Newton, 1979).
Perfect (1980) reported the results of a 4-year study on the
overall effects of the use of DDT as an insecticide on cowpeas crops in
a Nigerian forest soil. In addition to effects on soil invertebrates
(section 6.1), there were effects on the decomposition of plant
material. The remains of the plants after harvesting were ploughed
into the soil and this resulted in an increase in the residues of DDT
and its metabolites in lower levels of the soil. To confirm an effect
on decomposition, these plant remains were buried in mesh bags and the
loss of weight due to decomposition was recorded over time. There was
a significant reduction in the rate of decomposition of plant material
treated with DDT and also of untreated plant material buried in
contaminated soil. Shires (1985) reported no significant effect on the
decomposition of sweet chestnut leaf litter in a temperate area after
the application of DDT at 1 kg/ha.
Perfect et al. (1979) investigated the effects of repeated DDT
applications on cowpea crop yield in Nigeria. Yields varied
considerably from season to season and from year to year in untreated
plots because of differences in pest damage and climate. DDT was
applied to the treated plots weekly between planting and harvest at a
rate of 1 kg/ha, and the site was studied for 4 years. Over the 4-year
period there was a considerable benefit in yield from DDT application;
the yield was 1.45 tonnes/ha in the untreated and 3.42 tonnes/ha in the
treated plots. However, the benefit was most noticeable in the first
year of cultivation and declined over the four years to the point where
DDT use did not significantly increase yield. The authors attributed
the effect to the deleterious action of the insecticide on soil biota.
8. EVALUATION
In evaluating the environmental hazard of DDT and its metabolites
the following general points have to be kept in mind.
(a) The environmental distribution and effects of DDT are spread wider
than the area of use, because the parent compound or its
metabolites are carried worldwide by air and ocean currents and in
biota.
(b) Some of the breakdown products of DDT, principally DDE, are highly
persistent in soil, sediment, and biota. Thus, problems with
residues of these materials last long after the cessation of use.
(c) The bioaccumulation of DDT, or more usually of its metabolites, is
well established and occurs from very low environmental
concentrations of DDT. The use of "bioconcentration factors"
(the ratio of concentration in the organism with concentration in
the medium) to estimate the capacity of organisms to take up DDT
can be misleading if the exposure is high, since these values are
ratios.
(d) Residues and effects are often highly seasonal, corresponding to
changes in body fat, since DDT metabolites are very lipid-soluble.
Measurements of these metabolites in the tissues of organisms must
be conducted over a period of time if they are to give any
indication of the degree of contamination of the environment.
(e) There are insufficient data on the effects of DDT and its
metabolites on communities of organisms and ecosystem functioning.
Hazard assessment is, therefore, often made by extrapolation from
single species studies.
(f) Research and monitoring have concentrated on a few effects of DDT
observed in the wild. This could give the mistaken impression that
the effects of these compounds are restricted to a few species.
Other effects could be predicted but have received little or no
attention from the scientific community.
(g) The major remaining use of DDT is for malaria control operations
that are normally carried out in tropical countries. However, the
majority of environmental studies on DDT have been carried out in
conditions relevant to temperate regions. Care must be exercised
in extrapolating these results to tropical conditions.
8.1 Aquatic Organisms
The widespread use of DDT as an insecticide has resulted in
worldwide contamination of the environment. Due to the physicochemical
characteristics of DDT and its metabolites, concentrations have been
recorded in different environmental compartments, including soil,
sediments, and terrestrial and aquatic organisms. The bioconcentration
of DDT and its metabolites is a real hazard to non-target organisms.
DDT and its metabolites cause adverse effects at all trophic levels of
aquatic ecosystems, particularly on primary producers, which are the
most sensitive. Although no data are available for the effects of DDT
on ecosystem function, it should be regarded as a major environmental
hazard in this respect. DDT and its metabolites are highly toxic to
fish and, besides their lethal effect, they affect development,
behaviour, and biochemical processes. DDT and its metabolites, should
be regarded as hazardous to fish productivity and distribution and,
hence, to human food supplies. Accumulated DDT and its metabolites are
further transferred from aquatic organisms to consumers, including
birds, mammals, and, ultimately, human beings.
8.2 Terrestrial Organisms
DDT-type compounds are resistant to breakdown and are readily
adsorbed onto soils and sediments, from whence they can act as long-
term sources of exposure and contribute to terrestrial organisms.
Accumulation in terrestrial organisms is via the food chain.
These chemicals are hazardous to microorganisms, but repeated
application can lead to the development of tolerance in some species.
DDT causes fluctuations in some populations of microorganisms, and this
could eventually lead to changes in species composition, disruption of
nutrient cycles, and changes in soil fertility.
Earthworms are insensitive to the acute toxic effects of DDT
residues in soil. However, they are known to take up DDT from soil and
this uptake presents a major hazard to predators.
DDT is a non-selective insecticide and leads to mortality in
natural enemies of the insect pest. This results in impairment of the
balance between predators and prey and leads to outbreaks of secondary
pests and occurrence of the primary pest in larger numbers.
Laboratory studies confirm field findings that bat populations are
adversely affected by DDE, especially during migration. These studies
are indicative of the potential hazard to other mammals, exposed to DDT
in the environment, when fat containing DDT residues is mobilized,
e.g., during migration or temporary starvation.
One of the most widely studied effects of DDT is eggshell thinning
in birds, particularly in predatory species. The metabolite DDE, not
DDT, has been shown to be responsible for this effect. Other effects
on reproduction and survival of birds have been demonstrated. Large
population declines in birds of prey can be, at least partially,
attributed to DDT. It has been shown that DDE residues in birds and
their eggs reduced the rate of recovery of affected raptor
populations. A factor that has received less attention is the
secondary effect of the increasing numbers of pest rodents that were
controlled principally by birds of prey in some countries.
* * *
Because of their lack of degradation, their resulting widespread
persistence in the environment, their high acute toxicity to organisms
at the base of food chains, and their high potential for
bioaccumulation, DDT and its metabolites should be regarded as a major
hazard to the environment. DDT should not be used when an alternative
insecticide is available.
REFERENCES
ALBAUGH, D.W. (1972) Insecticide tolerances of two crayfish
populations ( Procambarus acutus ) in South-central Texas. Bull.
environ. Contam. Toxicol ., 8: 334-338.
ALBONE, E.S., EGLINTON, G., EVANS, N.C., HUNTER, J.M., & RHEAD,
M.M. (1972) Fate of DDT in severn estuary sediments. Environ. Sci.
Technol ., 6: 914-919.
ALLISON, D., KALLMAN, B.J., COPE, O.B., & VAN VALLIN, C.C. (1964)
Some chronic effects of DDT on cutthroat trout , Washington, DC, US
Department of the Interior, Bureau of Sport, Fisheries and Wildlife,
pp. 1-30 (Resource Report No. 64).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain north
American birds. Proc. Int. Ornithol. Congr ., 15: 514-540.
ANDERSON, D.W. & HICKEY, J.J. (1974) Eggshell changes in raptors from
the Baltic region. Oikos , 25: 395-401.
ANDERSON, D.W., HICKEY, J.J., RISEBROUGH, R.W., HUGHES, D.F., &
CHRISTENSEN, R.E. (1969) Significance of chlorinated hydrocarbon
residues to breeding pelicans and comorant. Can. field Nat ., 83: 91-
112.
ASH, J.S. (1965) A reduction in numbers of birds of prey in France.
Bird Study , 12: 17-26.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees , University
of California, 38 pp. (Laboratory Studies - Extension M-16).
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1972) DDT:
inhibition of sodium chloride tolerance by the blue-green
alga Anacystis nidulans . Science, 176: 1141-1143.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the aquatic
environment: uptake and loss of DDT and dieldrin by freshwater mussels.
Bull. environ. Contam. Toxicol ., 1: 97-111.
BEND, J.R., MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1977) DDE-
induced microsomal mixed-function oxidases in the puffin ( Fratercula
arctica). Biochem. Pharmacol ., 26: 1000-1001.
BENSON, W.W. & SMITH, P. (1972) Pesticide levels in deer. Bull.
environ. Contam. Toxicol ., 8: 1-9.
BENSON, W.W., GABICA, J., & BEECHAM, J. (1974) Pesticide and mercury
levels in bear. Bull. environ. Contam. Toxicol ., 11: 1-4.
BERGLIND, R. & DAVE, G. (1984) Acute toxicity of chromate, DDT, PCP,
TPBS and zinc to Daphnia magna cultured in hard and soft water. Bull.
environ. Contam. Toxicol ., 33: 63-68.
BEZZEL, E. (1969) [Results of quantitative observations of the
Accipitridae in Upper Bavaria.] Ornithol. Mitt ., 21: 29-36 (in
German).
BIESSMANN, A. & VON FABER, H. (1981) Effects of DDT and its
metabolites on the adrenal gland of the Japanese quail. Environ.
Pollut ., 25: 99-104.
BITMAN, J., CECIL, H.C., HARRIS, S.J., & FRIES, G.F. (1969) DDT
induces a decrease in eggshell calcium. Nature (Lond.) , 224: 44-46.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin and endrin. Arch. environ. Contam.
Toxicol ., 7: 83-98.
BOLLAG, J.-M. & HENNINGER, N.M. (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J. environ.
Qual ., 5: 15-18.
BOLLEN, W.B., MORRISON, H.E., & CROWELL, H.H. (1954) Effect of field
treatments of insecticides on numbers of bacteria, Streptomyces and
moulds in the soil. J. econ. Entomol ., 47: 302-306.
BOYD, C.E. & FERGUSON, D.E. (1964) Susceptibility and resistance of
mosquito fish to several insecticides. J. econ. Entomol ., 57: 430-431.
BOYD, C.E., VINSON, S.B., & FERGUSON, D.E. (1963) Possible DDT
resistance in two species of frogs. Copeia , 1963(2): 426-429.
BRAHAM, H.W. & NEAL, C.M. (1974) The effects of DDT on energetics of
the short-tailed shrew, Blarina brevicauda. Bull. environ. Contam.
Toxicol ., 12: 32-37.
BUHLER, D.R. & SHANKS, W.E. (1970) Influence of body weight on chronic
oral DDT toxicity in coho salmon. J. Fish Res. Board Can ., 27: 347-
358.
BUHLER, D.R., RASMUSSON, M.E., & SHANKS W.E. (1969) Chronic oral
DDT toxicity in juvenile coho and chinook salmon. Toxicol. appl.
Pharmacol ., 14: 535-555.
BUNYAN, P.J. & PAGE, J.M.J. (1973) Pesticide-induced changes in
hepatic microsomal enzyme systems. Some effects of 1,1-di(p-chloro-
phenyl)-2,2-dichloroethylene (DDE) and 1,1-di(p-chlorophenyl)-2-
chloroethylene (DDMU) in the rat and the Japanese quail. Chem.-biol.
Interact ., 6: 249-257.
BUNYAN, P.J., DAVIDSON, J., & SHORTHILL, M.J. (1970) Hepatic glucose-
6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase levels
in Japanese quail following the ingestion of p,p' -DDT and related
compounds. Chem.-biol. Interact ., 2: 175-182.
BUNYAN, P.J., TOWNSEND, M.G., & TAYLOR, A. (1972) Pesticide-induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-di(p-
chlorophenyl) -2,2,2-trichloroethane (DDT) and 1,1-di(p-chlorophenyl)-
2,2-dichloroethylene (DDE) in the rat and Japanese quail. Chem.-biol.
Interact ., 5: 13-26.
BURDICK, G.E., HARRIS, E.J., DEAN, H.J., WALKER, T.M., SKEA, J., &
COLBY, D. (1964) The accumulation of DDT in lake trout and the effect
on reproduction. Trans. Am. Fish. Soc ., 93: 127-136.
BURLINGTON, H. & LINDEMAN, V.F. (1950) Effect of DDT on testes and
secondary sex characters of white leghorn cockerels. Proc. Soc. Exp.
Biol ., 74: 48-51.
BUTLER, P.A. (1964) Commercial fishery investigations. In: The
effects of pesticides on fish and wildlife , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 65-77
(Circular No. 226).
CALABRESE, A. (1972) How some pollutants affect embryos & larvae of
American oyster & hard-shell clam. Mar. Fish. Rev ., 34: 66-77.
CECIL, H.C., BITMAN, J., & HARRIS, S.J. (1971) Effects of dietary
p,p' -DDT and p,p' -DDE on egg production and eggshell characteristics
of Japanese quail receiving an adequate calcium diet. Poult. Sci ., 50:
657-659.
CHRISTIE, A.E. (1969) Effect of insecticides on algae. Water Sewage
Works , 116: 172-176.
CLARK, D.R. & KROLL, J.C. (1977) Effects of DDE on experimentally
poisoned free-tailed bats ( Tadarida brasilensis ): lethal brain
concentrations. J. Toxicol. environ. Health, 3: 893-901.
COLE, D.R. & PLAPP, F.W. (1974) Inhibition of growth and
photosynthesis in Chlorella pyreniodosa by a polychlorinated biphenyl
and several insecticides. Environ. Entomol ., 3: 217-220.
COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U., PERFECT, T.J., &
YEADON, R. (1980) Effects of cultivation and DDT on earthworm
activity in a forest soil in the sub-humid tropics. J. appl. Ecol.,
17: 21-29.
COOKE, A.S. (1970) The effect of p,p -DDT on tadpoles of the common
frog (Rana temporaria). Environ. Pollut ., 1: 57-71.
COOKE, A.S. (1971) Uptake of DDT and DDE by the quail embryo and
chick. Pestic. Sci ., 2: 144-147.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on amphibian
spawn and tadpoles. Environ. Pollut ., 3: 51-68.
COOKE, A.S. (1973a) The effects of DDT, when used as a mosquito
larvicide, on tadpoles of the frog Rana temporaria. Environ. Pollut.,
5: 259-273.
COOKE, A.S. (1973b) Shell-thinning in avian eggs by environmental
pollutants. Environ. Pollut ., 4: 85-152.
COOKE, A.S. (1979a) Eggshell characteristics of gannets ( Sula
bassana ), shags ( Phalacrocorax aristotelis ) and great black-backed
gulls ( Larus marinus ) exposed to DDE and other environmental
pollutants. Environ. Pollut ., 19: 47-65.
COOKE, A.S. (1979b) The influence of rearing density on subsequent
response to DDT dosing for tadpoles of the frog Rana temporaria . Bull.
environ. Contam. Toxicol ., 21: 837-841.
COOKE, A.S. & POLLARD, E. (1973) Shell and operculum formation by
immature Roman snails, Helix pomatia L., when treated with p,p' -DDT.
Pestic. Biochem. Physiol ., 3: 230-236.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Eggshell characteristics
and incidence of shell breakage for grey herons Ardea cinerea exposed
to environmental pollutants. Environ. Pollut ., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides and pollution , Swindon, United Kingdom, Natural Environment
Research Council, 74 pp.
CRAWFORD, R.B. & GUARINO, A.M. (1976) Effects of DDT in Fundulus
studies on toxicity, fate and reproduction. Arch. environ. Contam.
Toxicol ., 4: 334-348.
CRITCHLEY, B.R., COOK, A.G., CRITCHLEY, U., PERFECT, T.J., &
RUSSELL-SMITH, A. (1980) The effects of crop protection with DDT on
some elements of the subterranean and surface active arthropod fauna of
a cultivated forest soil in the humid tropics. Pedobiologia , 20: 31-
38.
DAVEY, S.P. (1963) Effects of chemicals on earthworms: a review of the
literature , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 1-20 (Special Scientific Report, Wildlife No.
74).
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin and
DDT by earthworms. Soil Biol. Biochem ., 3: 221-233.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p' -DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol ., 7: 9-18.
DAVISON, K.L., ENGEBRETSON, K.A., & COX, J.H. (1976) p,p' -DDT and
p,p' -DDE effects on egg production, eggshell thickness and
reproduction of Japanese quail. Bull. environ. Contam. Toxicol ., 15:
265-270.
DAVY, F.B., KLEEREKOPER, H., & GENSLER, P. (1972) Effects of
exposure to sublethal DDT on the locomotor behavior of the goldfish
(Carassius auratus). J. Fish Res. Board Can ., 29: 1333-1336.
DAVY, F.B., KLEEREKOPER, H., & MATIS, J.H. (1973) Effects of exposure
to sublethal DDT on the exploratory behavior of goldfish (Carassius
auratus). Water Resour. Res ., 9: 900-905.
DERR, S.K. & ZABIK, M.J. (1972) Biologically active compounds in the
aquatic environment: the effect of DDT on the egg viability of
Chironomus tentans. Bull. environ. Contam. Toxicol ., 7: 366-368.
DESAIAH, D., CUTKOMP, L.K., KOCH, R.B., & JARVINEN, A. (1975)
DDT: effect of continuous exposure on ATPase activity in fish,
Pimephales promelas. Arch. environ. Contam. Toxicol ., 3: 132-141.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963) Wildlife studies,
Patuxent Wildlife Research Center 1961 - 1962 , Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 74-96
(Circular No. 167).
DIETER, M.P. (1974) Plasma enzyme activities in Coturnix quail fed
graded doses of DDE, polychlorinated biphenyl, malathion and mercuric
chloride. Toxicol. appl. Pharmacol ., 27: 86-98.
DILL, P.A. & SAUNDERS, R.C. (1974) Retarded behavioral development and
impaired balance in Atlantic salmon ( Salmo salar ) alevins hatched from
gastrulae exposed to DDT. J. Fish Res. Board Can ., 31: 1936-1938.
DILWORTH, T.G., KEITH, J.A., PEARCE, P.A., & REYNOLDS, L.M. (1972)
DDE and eggshell thickness in New Brunswick woodcock. J. wildl.
Manage ., 36: 1186-1193.
DIMOND, J.B. & SHERBURNE, J.A. (1969) Persistence of DDT in wild
populations of small mammals. Nature (Lond.) , 221: 486-487.
DINDAL, D.L. & WURZINGER, K.-H. (1971) Accumulation and excretion of
DDT by the terrestrial snail, Cepaea hortensis. Bull. environ. Contam.
Toxicol ., 4: 362-371.
DOBSON, S. (1981) [Physiological and behavioural effects of organo-
chlorines on pigeons.] Oekol. Vogel , 3(Suppl.): 39-43 (in German).
DUNACHIE, J.F. & FLETCHER, W.W. (1969) An investigation of the
toxicity of insecticides to birds' eggs using the egg-injection
technique. Ann. appl. Biol ., 64: 409-423.
EARNEST, R.D. & BENVILLE, P.E. (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game , 58: 127-132.
EBERHARDT, L., MEEKS, R.L., & PETERLE, T.J. (1971) Food chain model
for DDT kinetics in a fresh water marsh. Nature (Lond.) , 230: 60-62.
EDELSTAM, C. (1972) The visible migration of birds at Ottenby,
Sweden. Var Fagelvarld , 7(Suppl.): 1-360.
EDEN, W.G. & ARTHUR, B.W. (1965) Translocation of DDT and heptachlor
in soybeans. J. econ. Entomol ., 34: 161-162.
EDWARDS, C.A. & JEFFS, K. (1974) Rate of uptake of DDT from soil by
earthworms. Nature (Lond.) , 247: 157-158.
EL-SEBAE, A.H. (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region , Athens,
Mediterranean Action Plan, United Nations Environment Programme/Food
and Agriculture Organization of the United Nations, pp. 109-116. (MAP
Technical Report Series No. 10).
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning and
lowered production of young in prairie falcons. Bioscience , 20: 355-
356.
FERGUSON, D.E., CULLEY, D.D., COTTON, W.D., & DODDS, R.P. (1964)
Resistance to chlorinated hydrocarbon insecticides in three species of
freshwater fish. Bioscience , 14: 43-44.
FORSYTH, D.J. & PETERLE, T.J. (1973) Accumulation of chlorine-36 ring-
labeled DDT residues in various tissues of two species of shrew. Arch.
environ. Contam. Toxicol ., 1: 1-17.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R.,
& HUNT, J.R. (1972) Residues in eggs and tissues of hens fed a ration
containing low levels of pesticides with and without charcoal. J. econ.
Entomol ., 65: 982-988.
FOX, G.A. (1976) Eggshell quality: its ecological and physiological
significance in a DDE-contaminated common tern population. Wilson
Bull ., 88: 459-477.
FRIEND, M., HAEGELE, M.A., & WILSON, R. (1973) DDE: interference with
extra-renal salt excretion in the mallard. Bull. environ. Contam.
Toxicol ., 9: 49-53.
FUHREMANN, T.W. & LICHTENSTEIN, E.P. (1980) A comparative study
of the persistence, movement, and metabolism of six carbon-14
insecticides in soils and plants. J. agric. food Chem ., 28: 446-452.
GARDNER, D.R. (1973) The effect of some DDT and methoxychlor analogs on
temperature selection and lethality in brook trout fingerlings.
Pestic. Biochem. Physiol ., 2: 437-446.
GAUFIN, A.R., JENSEN, L.D., NEBEKER, A.V., NELSON, T., & TEEL,
R.W. (1965) The toxicity of ten organic insecticides to various
aquatic invertebrates. Water Sewage Works , 112: 276-279.
GELUSO, K.N., ALTENBACH, J.S., & WILSON, D.E. (1976) Bat mortality:
pesticide poisoning and migratory stress. Science , 194: 184-186.
GILMAN, A.P., HALLETT, D.J., FOX, G.A., ALLAN, L.J., LEARNING,
W.J., & PEAKALL, D.B. (1978) Effects of injected organochlorines on
naturally incubated herring gull eggs. J. wildl. Manage ., 42: 484-493.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 15 pp (Special Scientific Report No. 241).
GOFFART, H. (1949) [The effect of new kinds of insecticides on
earthworms.] Anz. Schadlingskd. (Berlin) , 22: 72-74 (in German).
GOULDING, K.H. & ELLIS, S.W. (1981) The interaction of DDT with two
species of freshwater algae. Environ. Pollut ., 25: 271-290.
GREENBURG, R.R., RISEBROUGH, R.W., & ANDERSON D.W. (1979)
p,p' -DDE-induced changes in the organic and inorganic structure of
eggshells of the mallard, Anas platyrhynchos. Toxicol. appl.
Pharmacol ., 48: 279-286.
GREICHUS, Y.A. & HANNON, M.R. (1973) Distribution and biochemical
effects of DDT, DDD and DDE in penned double-crested cormorants.
Toxicol. appl. Pharmacol ., 26: 483-494.
GREICHUS, Y.A., CALL, D.J., AMMANN, B.M., GREICHUS, A., &
SHAVE, H. (1975) Physiological effects of polychlorinated biphenyls or
a combination of DDT, DDD, and DDE in penned white pelicans. Arch.
environ. Contam. Toxicol ., 3: 330-343.
HACKMAN, C.D. & HENNY, C.J. (1971) Hawk migration over white marsh,
Maryland. Chesapeake Sci ., 12: 137-141.
HAEGELE, M.A. & HUDSON, R.H. (1973) DDE effects on reproduction of
ring doves. Environ. Pollut ., 4: 53-57.
HAEGELE, M.A. & HUDSON, R.H. (1977) Reduction of courtship behavior
induced by DDE in male ringed turtle doves. Wilson Bull ., 89: 593-601.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common pollutants
on eggshell thickness in mallards and coturnix. Bull. environ. Contam.
Toxicol ., 11: 98-102.
HALTER, M.T. & JOHNSON, H.E. (1974) Acute toxicities of a
polychlorinated biphenyl (PCB) and DDT alone and in combination to
early life stages of coho salmon (Oncorhynchus kisutch). J. Fish Res.
Board Can ., 31: 1543-1547.
HANKE, W., GLUTH, G., BUBEL, H., & MULLER, R. (1983) Physiological
changes in carps induced by pollution. Ecotoxicol. environ. Saf ., 7:
229-241.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained sheepshead
minnows. Trans. Am. Fish. Soc ., 98: 426-429.
HANSEN, D.J. (1972) DDT and malathion: effect on salinity selection by
mosquitofish. Trans. Am. Fish. Soc ., 101: 346-350.
HANSEN, D.J. & WILSON, A.J. (1970) Significance of DDT residues from
the estuary near Pensacola, Fla. Pestic. monit . J., 4: 51-56.
HANSEN, D.J., MATTHEWS, E., NALL, S.L., & DUMAS, D.P. (1972)
Avoidance of pesticides by untrained mosquitofish, Gambusia affinis.
Bull. environ. Contam. Toxicol ., 8: 46-51.
HARRI, M.N.E., LAITINEN, J., & VALKAMA, E.-L. (1979) Toxicity and
retention of DDT in adult frogs, Rana temporaria L. Environ. Pollut.,
13: 45-55.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J. agric.
food Chem ., 15: 861-863.
HASELTINE, S., UEBELHART, K., PETERLE, T., & LUSTICK, S. (1974)
DDE, PTH and eggshell thinning in mallard, pheasant and ring dove.
Bull. environ. Contam. Toxicol ., 11: 139-145.
HAUX, C. & LARSSON, A. (1979) Effects of DDT on blood plasma
electrolytes in the flounder, Platichthys flesus L., in hypotonic
brackish water. Ambio , 8: 171-173.
HAYNES, R.J. (1972) Effects of DDT on glycogen and lipid levels in
bobwhites. J. wildl. Manage ., 36: 518-523.
HEATH, R.G., SPANN, J.W., & KREITZER, J.F. (1969) Marked DDE
impairment of mallard reproduction in controlled studies. Nature
( Lond .), 224: 47-48.
HEINZ, G.H. (1976) Behavior of mallard ducklings from parents fed 3
ppm DDE. Bull. environ. Contam. Toxicol ., 16: 640-645.
HEINZ, G.H., HILL, E.F., & CONTRERA, J.F. (1980) Dopamine and
norepinephrine depletion in ring doves fed DDE, dieldrin and Aroclor
1254. Toxicol. appl. Pharmacol ., 53: 75-82.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four species of
fish. Trans. Am. Fish. Soc ., 88: 23-32.
HENNY, C.J. (1972) An analysis of the population dynamics of selected
avian species , Washington, DC, US Department of the Interior, Fish and
Wildlife Service, Bureau of Sport, Fisheries and Wildlife, 99 pp
(Research Report No. 1).
HENNY, C.J. (1977) Birds of prey, DDT, & tussock moths in Pacific
Northwest. In: Transactions of the Forty-second North American Wildlife
and Natural Resource Conference , Washington, DC, Wildlife Management
Institute, pp. 397-411.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish eating birds. Science , 162:
271-273.
HILL, E.F., DALE, W.E., & MILES, J.W. (1971) DDT intoxication in birds:
subchronic effects and brain residues. Toxicol. appl. Pharmacol ., 20:
502-514.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds , Washington,
DC, US Department of the Interior, Fish and Wildlife Service, 61 pp
(Special Scientific Report No. 191).
HODKINSON, M. & DALTON, S.A. (1973) Interactions between DDT and
river fungi. II Influence of culture conditions on the compatibility of
fungi and p,p' -DDT. Bull. environ. Contam. Toxicol ., 10: 356-359.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife , Washington, DC, US Department of
the Interior, Fish and Wildlife Service, 90 pp (Resource Publication
No. 153).
HUNT, E.G. & LINN, J.D. (1970) Fish kills by pesticides. In: Gillett,
J.W., ed. The biological impact of pesticides in the environment
Corvallis, Oregon, Oregon State University, pp. 97-102.
IDE, F.P. (1957) Effect of forest spraying with DDT on aquatic insects
of salmon streams. Trans. Am. Fish. Soc ., 86: 208-219.
JANICKI, R.H. & KINTER, W.B. (1971) DDT disrupted osmoregulatory
events in the intestine of the eel Anguilla rostrata adapted to
seawater. Science , 173: 1146-1148.
JARVINEN, A.W., HOFFMAN, M.J., & THORSLUND, T.W. (1977) Long-
term toxic effects of DDT food and water exposure on fathead minnows.
J. Fish Res. Board Can ., 34: 2089-2103.
JEFFERIES, D.J. (1967) The delay in ovulation produced by p,p' -DDT and
its possible significance in the field. Ibis , 109: 266-272.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in British
bats and their significance. J. Zool ., 166: 245-263.
JEFFERIES, D.J. & FRENCH, M.C. (1972) Changes induced in the pigeon
thyroid by p,p' -DDE and dieldrin. J. wildl. Manage ., 36: 24-30.
JOHANSEN, C. (1961) Bee poisoning. A hazard of applying agricultural
chemicals , Washington, DC, Institute of Agricultural Sciences,
Washington State University (Circular No. 356).
JOHNSON, B.T. & KENNEDY, J.O. (1973) Biomagnification of p,p' -DDT and
methoxychlor by bacteria. Appl. Microbiol ., 26: 66-71.
JOHNSON, B.T., SAUNDERS, C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin by
freshwater invertebrates. J. Fish Res. Board Can ., 28: 705-709.
JOHNSON, H.E. & PECOR, C. (1969) Coho salmon mortality and DDT in Lake
Michigan. Trans. North Am. Wildl. Nat. Resour. Conf ., 34: 159-166.
JOWETT, P.E., RHEAD, M.M., & BAYNE, B.L. (1978) In vitro changes in the
activity of ATPases in the gills of Carcinus maenas exposed to various
concentrations of p,p' -DDT. Environ. Pollut ., 17: 1-6.
KAN, C.A., JONKER-DEN ROOYEN, J.C., TUINISTRA, L.G.M.T., ROOS,
A.H., & TRAAG, W. (1978) Possible influence of sex and embryonic
content on accumulation of some organochlorine pesticides in
broilers. J. agric. food Chem ., 26: 618-621.
KATZ, M. (1961) Acute toxicity of some organic insecticides to three
species of salmonids and to the threespine stickleback. Trans. Am.
Fish. Soc ., 90: 264-268.
KEIL, J.E. & PRIESTER, L.E. (1969) DDT uptake and metabolism by a
marine diatom. Bull. environ. Contam. Toxicol ., 4: 169-173.
KEIL, J.E., SANDIFER, S.H., GRABER, C.D., & PRIESTER, L.E. (1972) DDT
and polychlorinated biphenyl (Aroclor 1242). Effects of uptake on E.
coli growth. Water Res ., 6: 837-841.
KING, K.A., FLICKINGER, E.L., & HILDEBRAND, H.H. (1978) Shell
thinning and pesticide residues in Texas aquatic bird eggs,
1970. Pestic. monit. J ., 12: 16-21.
KINTER, W.B., MERKENS, L.S., JANICKI, R.H., & GUARINO, A.M. (1972)
Studies on the mechanism of toxicity of DDT and polychlorinated
biphenyls (PCBs): disruption of osmoregulation in marine fish.
Environ. Health Perspect ., 1: 169-173.
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972) Eggshell
and population changes in the sparrow-hawk (Accipiter nisus). TNO-
Nieuws , 27: 542-550.
KOLAJA, G.J. (1977) The effects of DDT, DDE and their sulfonated
derivatives on eggshell formation in the mallard duck. Bull. environ.
Contam. Toxicol ., 17: 697-701.
KORN, S. & EARNEST, R. (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game , 60: 128-131.
KOUYOUMJIAN, H.H. & UGLOW, R.F. (1974) Some aspects of the toxicity of
p,p' -DDT, p,p' -DDE and p,p' -DDD to the freshwater planarian
Polycelis felina (Tricladida). Environ. Pollut ., 7: 103-109.
KREITZER, J.F. & SPANN, J.W. (1973) Test of pesticidal synergism with
young pheasants and Japanese quail. Bull. environ. Contam. Toxicol.,
9: 250-256.
LAL, R. & SAXENA, D.M. (1979) Effect of DDT on cell population growth
of Tetrahymena pyriformis. Arch. Protistenkd ., 122: 382-386.
LAL, R. & SAXENA, D.M. (1980) Effect of DDT on cell population growth,
cell division, and DNA synthesis in Stylonychia notophora (Stokes).
Arch. environ. Contam. Toxicol ., 9: 163-170.
LAL, R. & SAXENA, D.M. (1982) Accumulation, metabolism, and effects of
organochlorine insecticides on microorganisms. Microbiol. Rev ., 46: 95-
127.
LEE, S.S., FANG, S.C., & FREED, V.H. (1976) Effect of DDT on
photosynthesis of Selenastrum capricornutum. Pestic. Biochem. Physiol.,
6: 46-51.
LEFFLER, C.W. (1975) Effects of ingested mirex and DDT on juvenile
Callinectes sapidus Rathbun. Environ. Pollut ., 8: 283-300.
LEDFORD, R.A. & CHEN, J.H. (1969) Degradation of DDT and DDE by
cheese microoganisms. J. food Sci ., 34: 386-388.
LEGMAN, J.W., PETERLE, T.J., & MILLS, C.M. (1974) Effects of DDT on
bobwhite quail ( Colinus virginianus ) adrenal gland. Bull. environ.
Contam. Toxicol ., 11: 407-414.
LINCER, J.L. (1972) The effects of organochlorines on the American
kestrel (Falco sparverius Linn.) , Ithaca, New York, Cornell
University (PhD Thesis).
LINCER, J.L. (1975) DDE-induced eggshell-thinning in the American
kestrel: a comparison of the field situation and laboratory results.
J. appl. Ecol ., 12: 781-793.
LINDBURG, P. (1977) The peregrine falcon in Sweden. In: Proceedings of
the International Council for Bird Preservation World Conference on
Birds of Prey, Vienna, 1975 , Cambridge, United Kingdom, International
Council for Bird Preservation, pp. 329-338.
LOCKE, L.N., CHURA, N.J., & STEWART, P.A. (1966) Spermatogenesis in
bald eagles experimentally fed a diet containing DDT. Condor , 68: 497-
502.
LONGCORE, J.R., SAMSON, F.B., & WHITTENDALE, T.W. (1971) DDE
thins eggshells and lowers reproductive success of captive black
ducks. Bull. environ. Contam. Toxicol ., 6: 485-490.
LONGCORE, J.R. & STENDELL, R.C. (1977) Shell thinning and reproductive
impairment in black ducks after cessation of DDE dosage. Arch. environ.
Contam. Toxicol ., 6: 293-304.
LOOSANOFF, V.L. (1960) Some effects of pesticides on marine arthropods
and mollusks. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Transactions of the Second Seminar, 1959 , Cincinnati, USA,
Robert, A. Taft Sanitation and Engineering Centre, pp. 89-93 (Technical
Report No. W60-3).
LOWE, J.I. (1965) Chronic exposure of blue crabs, Callinectes sapidus,
to sublethal concentrations of DDT. Ecology , 46: 899-900.
LUARD, E.J. (1973) Sensitivity of Dunaliella and Scenedesmus
(Chlorophyceae) to chlorinated hydrocarbons. Phycologia , 12: 29-33.
LUCKENS, M.M. & DAVIS, W.H. (1964) Bats: sensitivity to DDT. Science,
146: 948.
LUDKE, J.L. (1977) DDE increases the toxicity of parathion to
Coturnix quail. Pestic. Biochem. Physiol ., 7: 28-33.
MACEK, K.J. (1968) Reproduction in brook trout ( Salvelinus fontinalis )
fed sublethal concentrations of DDT. J. Fish Res. Board Can ., 25:
1787-1795.
MACEK, K.J. & KORN, S. (1970) Significance of the food chain in DDT
accumulation by fish. J. Fish Res. Board Can ., 27: 1496-1498.
MACEK, K.J. & SANDERS, H.O. (1970) Biological variation in the
susceptibility of fish and aquatic invertebrates to DDT. Trans. Am.
Fish. Soc ., 99: 89-90.
MACEK, K.J., RODGERS, C.R., STALLING, D.L., & KORN, S. (1970) The
uptake, distribution and elimination of dietary 14C-DDT and
14C-dieldrin in rainbow trout. Trans. Am. Fish. Soc ., 99: 689-695.
MACFARLANE, R.B., GLOOSCHENKO, W.A., & HARRISS, R.C. (1972) The
interaction of light intensity and DDT concentration upon the marine
diatom, Nitzschia delicatissima Cleve. Hydrobiologia , 39: 373-382.
MCLANE, M.A.R. & HALL, L.C. (1972) DDE thins screech owl eggshells.
Bull. environ. Contam. Toxicol ., 8: 65-68.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol ., 25: 921-928.
MACRAE, I.C. & VINCKX, E. (1973) Effect of lindane and DDT on
populations of protozoa in a garden soil. Soil Biol. Biochem ., 5: 245-
247.
MAHONEY, J.J. (1975) DDT and DDE effects on migratory condition in
white-throated sparrows. J. wildl. Manage ., 39: 520-527.
MAKI, A.W. & JOHNSON, H.E. (1975) Effects of PCB (Aroclor 1254) and
p,p' -DDT on production and survival of Daphnia magna Strauss. Bull.
environ. Contam. Toxicol ., 13: 412-416.
MARTIN, J.P. (1966) Influence of pesticides on soil microbes and soil
properties. Am. Soc. Agron. Spec. Publ ., 8: 95-108.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms , Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity :
interpretation and data base for 410 chemicals and 66 species of
freshwater animals , Washington, DC, US Department of the Interior, Fish
and Wildlife Service, 506 pp (Resource Publication No. 160).
MEEKS, R.L. (1968) The accumulation of 36Cl ring-labeled DDT in a
freshwater marsh. J. wildl. Manage ., 32: 376-398.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls ( Tyto alba ) fed low levels of DDE and dieldrin.
Arch. environ. Contam. Toxicol ., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine phyto-
plankton vary in their response to chlorinated hydrocarbons.
Science , 167: 1724-1726.
METCALF, R.L., KAPOOR, I.P., LU, P.-Y, SCHUTH, C.K., & SHERMAN,
P. (1973) Model ecosystem studies of the environmental fate of six
organochlorine pesticides. Environ. Health Perspect ., 4: 35-43.
MILLER, D.S., KINTER, W.B., & PEAKALL, D.B. (1976a) Enzymatic basis
for DDE-induced eggshell thinning in a sensitive bird. Nature (Lond.),
259: 122-124.
MILLER, D.S., KINTER, W.B., PEAKALL, D.B., & RISEBROUGH, R.W.
(1976b) DDE feeding and plasma osmoregulation in ducks, guillemots,
and puffins. Am. J. Physiol ., 231: 370-376.
MOFFETT, G.B. & YARBROUGH, J.D. (1972) The effects of DDT, toxaphene
and dieldrin on succinic dehydrogenase activity in insecticide-
resistant and susceptible Gambusia affinis. J. agric. food Chem ., 20:
558-560.
MOORE, N.W. (1965) Pesticides in birds - a review of the situation in
Great Britain in 1965. Bird Study , 12: 222-252.
MORIARTY, F. (1975) Pollutants and animals: a factual perspective,
London, Allen & Unwin.
MORIARTY, F., BELL, A.A., & HANSON, H. (1986) Does p,p' -DDE thin
eggshells? Environ. Pollut ., 40: 257-286.
MUIRHEAD-THOMSON, R.C. (1973) Laboratory evaluation of pesticide
impact on stream invertebrates. Freshwater Biol ., 3: 479-498.
MULLA, M.S. (1963) Toxicity of organochlorine insecticides to the
mosquitofish Gambusia affinis and the bullfrog Rana catesbeiana.
Mosq. News , 23: 299-303.
MURPHY, P.G. (1970) Effects of salinity on uptake of DDT, DDE and DDD
by fish. Bull. environ. Contam. Toxicol ., 5: 404-407.
MURPHY, P.G. (1971) The effect of size on the uptake of DDT from water
by fish. Bull. environ. Contam. Toxicol ., 6: 20-23.
NAGY, A.C. (1977) Population trend indices based on 40 years of autumn
counts at hawk mountain. In: Proceedings of the International Council
for Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 243-253.
NAQVI, S.M. & FERGUSON, D.E. (1968) Pesticide tolerances of selected
freshwater invertebrates. J. Mississippi Acad. Sci ., 14: 121-127.
NEUFELD, G.J. & PRITCHARD, J.B. (1979) An assessment of DDT toxicity in
osmoregulation and gill Na,K-ATPase activity in the blue crab,
Philadelphia, American Society for Testing and Materials, pp. 23-34
(ASTM Special Technical Publication No. 667).
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted, United
Kingdom , T. & A.D. Poyser.
NEWTON, I. & BOGAN, J. (1978) The role of different organo-chlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol ., 15:
105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds , 77: 47-70.
NIMMO, D.R., WILSON, A.J., & BLACKMAN, R.R. (1970) Localization of
DDT in the body organs of pink and white shrimp. Bull. environ. Contam.
Toxicol ., 5: 333-341.
OGILVIE, D.M. & MILLER, D.L. (1976) Duration of a DDT-induced shift in
the selected temperature of Atlantic salmon (Salmo salar). Bull.
environ. Contam. Toxicol ., 16: 86-89.
OLOFSSON, S. & LINDAHL, P.E. (1979) Decreased fitness of cod ( Gadus
morrhua L.) from polluted waters. Mar. environ. Res ., 2: 33-45.
OSBORN, D., COOKE, A.S., & FREESTONE, S. (1981) Histology of a
teratogenic effect of DDT on Rana temporaria tadpoles. Environ.
Pollut ., 25: 305-319.
O'SHEA, T.J. & LUDKE, J.L. (1979) Monitoring fish and wildlife for
environmental pollutants , Fort Collins, Colorado, US Department of the
Interior, Fish and Wildlife Service.
PEAKALL, D.B. (1970) p,p' -DDT: effect on calcium metabolism and
concentration of estradiol in the blood. Science , 168: 592-594.
PEAKALL, D.B., LINCER, J.L., RISEBROUGH, R.W., PRITCHARD, J.B., &
KINTER, W.B. (1973) DDE-induced eggshell thinning: structural and
physiological effects in three species. Comp. gen. Pharmacol ., 4: 305-
313.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975a) Prolonged eggshell
thinning caused by DDE in the duck. Nature (Lond.) , 254: 421.
PEAKALL, D.B., MILLER, D.S., & KINTER, W.B. (1975b) Blood calcium
levels and the mechanism of DDE-induced eggshell thinning. Environ.
Pollut ., 9: 289-294.
PEAKALL, D.B., CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1975c)
Organochlorine residues in Alaskan peregrines. Pestic. monit. J. , 8:
255-260.
PERFECT, J. (1980) The environmental impact of DDT in a tropical agro-
ecosystem. Ambio , 9: 16-21.
PERFECT, T.J., COOK, A.G., CRITCHLEY, B.R., CRITCHLEY, U.,
DAVIES, A.L., SWIFT, M.J., RUSSELL-SMITH, A., & YEADON, R. (1979)
The effect of DDT contamination on the productivity of a cultivated
forest soil in the sub-humid tropics. J. appl. Ecol ., 16: 705-719.
PETERSON, R.H. (1973) Temperature selection of Atlantic salmon (Salmo
salar) and brook trout ( Salvalinus fontinalis ) as influenced by various
chlorinated hydrocarbons. J. Fish Res. Board Can ., 30: 1091-1097.
PORTER, R.D. & WIEMEYER, S.N. (1972) DDE at low dietary levels kills
captive American kestrels. Bull. environ. Contam. Toxicol ., 8: 193-
199.
POWERS, C.D., WURSTER, C.F., & ROWLAND, R.G. (1979) DDE inhalation
of marine algal cell division and photosynthesis per cell. Pestic.
Biochem. Physiol ., 10: 306-312.
PRESCOTT, L.M. & OLSON, D.L. (1972) The effect of pesticides on the
soil amoeba Acanthamoeba castellanii (Neff). Proc. South Dakota Acad.
Sci ., 51: 136-141.
RAMALINGAM, K. & RAMALINGAM, K. (1982) Effects of sublethal levels
of DDT, malathion and mercury on tissue proteins of Sarotherodon
mossambicus (Peters). Proc. Indian Acad. Sci. anim. Sci ., 91: 501-505.
RANDALL, W.F., DENNIS, W.H., & WARNER, M.C. (1979) Acute toxicity of
dechlorinated DDT, chlordane and lindane to bluegill ( Lepomis
machrochirus ) and Daphnia magna. Bull. environ. Contam. Toxicol ., 21:
849-854.
RATCLIFFE, D.A. (1967) Decrease in eggshell weight in certain birds of
prey. Nature (Lond.) , 215: 208-210.
RATCLIFFE, D.A. (1969) Population trends of the peregrine falcon in
Great Britain. In: Hinkley, J.J., ed. Peregrine falcon populations:
Their biology and decline , Madison, Milwaukee, London, University of
Wisconsin Press, pp. 239-269.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol ., 7: 67-107.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Study , 19: 117-156.
REINBOLD, K.A., KAPOOR, I.P., CHILDERS, W.F., BRUCE, W.N., &
METCALF, R.L. (1971) Comparative uptake and biodegradability of DDT
and methoxychlor by aquatic organisms. Illinois nat. Hist. Surv.
Bull ., 30: 405-415.
REINERT, R.E., STONE, L.J., & WILLFORD, W.A. (1974) Effect of
temperature on accumulation of methylmercuric chloride and p,p' -DDT by
rainbow trout (Salmo gairdneri). J. Fish Res. Board Can ., 31: 1649-
1652.
RICE, C.P. & SIKKA (1973) Uptake and metabolism of DDT by six species
of marine algae. J. agric. food Chem ., 21: 148-152.
RICHIE, P.J. & PETERLE, T.J. (1979) Effect of DDE on circulating
luteinizing hormone levels in ring doves during courtship and nesting.
Bull. environ. Contam. Toxicol ., 23: 220-226.
RISEBROUGH, R.W. & ANDERSON, D.W. (1975) Some effects of DDE and
PCB on mallards and their eggs. J. wildl. Manage ., 39: 508-513.
RISEBROUGH, R.W., DE LAPPE, B.W., & SCHMIDT, T.T. (1976)
Bioaccumulation factors of chlorinated hydrocarbons between mussels and
seawater. Mar. Pollut. Bull ., 7: 225-228.
ROBERTS, D. (1975) Sub-lethal effects of chlorinated hydrocarbons on
bivalves. Mar. Pollut. Bull ., 6: 20-24.
ROBSON, W.A., ARSCOTT, G.H., & TINSLEY, I.J. (1976) Effect of DDE,
DDT and calcium on the performance of adult Japanese quail (Coturnix
coturnix japonica). Poult. Sci ., 55: 2222-2227.
ROSEN, L. (1966) [The flight of birds of prey at Falsterbo.] Var
Fagelvarld , 25: 315-326 (in Swedish).
SANBORN, J.R., CHILDERS, W.F., & METCALF, R.L. (1975) Uptake of three
polychlorinated biphenyls, DDT, and DDE by the green sunfish, Lepomis
cyanellus Raf. Bull. environ. Contam. Toxicol ., 13: 209-217.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the western
chorus frog Pseudacris triseriata and Fowlers toad Bufo woodhousii
fowleri. Copeia , 2: 246-251.
SANDERS, H.O. (1972) Toxicity of some insecticides to four species of
malacostracan crustaceans , Washington, DC, US Department of the
Interior, Bureau of Sport, Fisheries and Wildlife, pp. 3-19 (Technical
Paper No. 66).
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of several
pesticides to naiads of three species of stoneflies. Limnol.
Oceanogr ., 13: 112-117.
SELL, J.L., DAVISON, K.L., & POONACHA, K.B. (1972) Decreased aniline
hydroxylase activity in Japanese quail due to dietary DDT. J. agric.
food Chem ., 20: 553-557.
SHERBURNE, J.A. & DIMOND, J.B. (1969) DDT persistence in wild hares and
mink. J. wildl. Manage ., 33: 944-948.
SHIN, Y.-O., CHODAN, J.J., & WOLCOTT, A.R. (1970) Adsorption of DDT
by soils, soil fractions, and biological materials. J. agric. food
Chem ., 18: 1129-1133.
SHIRES, S.W. (1985) A comparison of the effects of cypermethrin,
parathion-methyl and DDT on cereal aphids, predatory beetles,
earthworms and litter decomposition in spring wheat. Crop Prot.,
4: 177-193.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of DDT
and dieldrin on development in winter flounder (Pseudopleuronectes
americanus). J. Fish Res. Board Can ., 30: 1894-1898.
SMITH, S.I., WEBER, C.W., & REID, B.L. (1969) The effect of high
levels of dietary DDT on egg production, mortality, fertility,
hatchability and pesticide content of yolks in Japanese quail. Poult.
Sci ., 48: 1000-1004.
STICKEL, L.F. & HEATH, R.G. (1964) Wildlife studies. Patuxent wildlife
research center. In: The effects of pesticides on fish and wildlife,
Washington, DC, Department of the Interior, Fish and Wildlife Service,
pp. 3-30 (Circular No. 226).
THOMPSON, A.R. (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull. environ. Contam. Toxicol ., 5:
577-586.
ULFSTRAND, S., ROOS, G., ALERSTAM, T., & OSTERDAHL, L. (1974)
Visible bird migration at Falsterbo, Sweden. Var Fagelvarld,
8(Suppl.): 1-244.
VANGILDER, L.D. & PETERLE, T.J. (1980) South Louisiana crude oil and
DDE in the diet of mallard hens: effects on reproduction and duckling
survival. Bull. environ. Contam. Toxicol ., 25: 23-28.
VAN VELZEN, A. & KREITZER, J.F. (1975) The toxicity of p,p' -DDT to
the clapper rail. J. wildl. Manage ., 39: 305-309.
VAN VELZEN, A.C., STILES, W.B., & STICKEL, L.F. (1972) Lethal
mobilization of DDT by cowbirds. J. wildl. Manage ., 36: 733-739.
VERMEER, K. & REYNOLDS, L.M. (1970) Organochlorine residues in aquatic
birds in the canadian prairie provinces. Can. field Nat ., 84: 117-130.
VINSON, S.B., BOYD, C.E., & FERGUSON, D.E. (1963) Resistance to DDT in
the mosquito fish, Gambusia affinis. Science , 139: 217-218.
WAGGONER, J.P. & ZEEMAN, M.G. (1975) DDT: short term effects on
osmoregulation in black surfperch (Embiotoca jacksoni). Bull. environ.
Contam. Toxicol ., 13: 297-300.
WARE, G.W. (1968) DDT-C14 translocation in alfalfa. J. econ.
Entomol ., 61: 1451-1452.
WARE, G.W., ESTESEN, B.J., & CAHILL, W.P. (1970) Uptake of C14-DDT
from soil by alfalfa. Bull. environ. Contam. Toxicol ., 5: 85-86.
WARLEN, S.M., WOLFE, D.A., LEWIS, C.W., & COLBY, D.R. (1977)
Accumulation and retention of dietary 14C-DDT by Atlantic menhaden.
Trans. Am. Fish. Soc ., 106: 95-104.
WATSON, M., PHARAOH, B., WYLLIE, J., & BENSON, W.W. (1975)
Metabolism of low oral doses of DDT and DDE by tame mule deer fawns.
Bull. environ. Contam. Toxicol ., 13: 316-323.
WEIS, J.S. & MANTEL, L.H. (1976) DDT as an accelerator of limb
regeneration and molting in fiddler crabs. Estuarine coastal mar. Sci.,
4: 461-466.
WEIS, P. & WEIS, J.S. (1974) DDT causes changes in activity and
schooling behavior in goldfish. Environ. Res ., 7: 68-74.
WHEATLEY, G.A. (1965) The assessment and persistence of residues of
organochlorine insecticides in soils and their uptake by crops. Ann.
appl. Biol ., 55: 325-329.
WHITE, C.M. & CADE, T.J. (1977) Long term trends of peregrine
populations in Alaska. In: Proceedings of the International Council for
Bird Preservation World Conference on Birds of Prey, Vienna, 1975,
Cambridge, United Kingdom, International Council for Bird
Preservation, pp. 63-71.
WHO (1979) Environmental Health Criteria 9: DDT and its derivatives,
Geneva, World Health Organization, 194 pp.
WIEMEYER, S.N. & PORTER, R.D. (1970) DDE thins eggshells of captive
American kestrels. Nature (Lond.) , 227: 737-738.
WIEMEYER, S.N., SPITZER, P.R., KRANTZ, W.C., LAMONT, T.G., &
CROMARTIE, E. (1975) Effects of environmental pollutants on
Connecticut and Maryland ospreys. J. wildl. Manage ., 39: 124-139.
WURSTER, C.F. (1968) DDT reduces photosynthesis by marine
phytoplankton. Science , 159: 1474-1475.
