
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 75
TOLUENE DIISOCYANATES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TOLUENE DIISOCYANATES
1. SUMMARY AND CONCLUSIONS
1.1. Summary
1.1.1. Identity, properties, analytical methods
1.1.2. Production, uses, and sources of exposure
1.1.3. Kinetics, biotransformation, and elimination
1.1.4. Effects on experimental animals
1.1.5. Effects on human beings
1.1.6. Effects on organisms in the environment
1.2. Evaluation of hazards from long-term exposure to toluene
diisocyanates
1.2.1. Conclusions and recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 World production figures
3.2.1.2 Manufacturing processes: release into the
environment
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Air
4.2. Water
4.3. Soil
4.4. Biotransformation
4.5. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. General population exposure
5.2. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation and elimination
6.4. Reaction with body components
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.2. Short-term exposures
8.2.1. Inhalation
8.2.1.1 Guinea-pig
8.2.1.2 Mouse
8.2.1.3 Rat
8.2.1.4 Dog
8.2.2. Dermal
8.2.2.1 Guinea-pig
8.2.2.2 Mouse
8.2.3. Oral
8.3. Long-term exposure
8.3.1. Inhalation
8.3.1.1 Mouse
8.3.1.2 Dog
8.4. Reproduction, embryotoxicity, and teratogenicity
8.5. Mutagenicity and related end-points
8.5.1. Bacterial mutagenicity
8.5.2. Mammalian cell transformation
8.5.3. Mammalian in vivo study
8.6. Carcinogenicity
8.6.1. Oral
8.6.2. Inhalation
8.7. Special studies and mechanisms of toxicity
9. EFFECTS ON MAN
9.1. General population exposure - controlled human studies
9.1.1. Single exposures
9.2. Occupational exposure
9.2.1. Acute toxicity
9.2.2. Effects of short- and long-term occupational
exposure - epidemiological studies
9.2.2.1 Ocular
9.2.2.2 Dermal
9.2.2.3 Respiratory tract
9.2.2.4 Cancer epidemiology
9.2.2.5 Immunotoxicity
9.2.3. Potential mechanisms of action
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Exposure to toluene diisocyanates
10.1.1. Acute and short-term effects
10.1.2. Health risks of long-term exposure to toluene
diisocyanates
10.2. Evaluation of effects on the environment
10.3. Conclusions and recommendations
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON TOLUENE DIISOCYANATES (TDIs)
Members
Dr X. Baur, Pulmonary Section, Klinikum Grosshaden, University of
Munich, Munich, Federal Republic of Germany
Dr L. Belin, Department of Medicine, Sahlgren's Hospital, Goteborg,
Sweden
Ms Andrea Blaska, Office of Toxic Substances, US Environmental
Protection Agency, Washington DC, USA (Co-Rapporteur)
Dr M. Dieter, US National Institute for Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
(Co-Rapporteur)
Dr M. Greenberg, Department of Health and Social Security, London,
United Kingdom.
Dr I. Gut, Institute of Hygiene and Epidemiology, Prague,
Czechoslovakia (Chairman)
Dr M. Mann, Bayer AG, Leverkusen, Bayerwerk, Federal Republic of
Germany
Dr C. Rosenburg, Institute of Occupational Health, Department of
Industrial Hygiene and Toxicology, Helsinki, Finland
Professor H. Sakurai, School of Medicine, Keio University, Tokyo,
Japan
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA (Secretary)
Mr A.C. Fletcher, International Agency for Research on Cancer,
Lyons, France
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR TOLUENE DIISOCYANATES
A WHO Task Group on Environmental Health Criteria for
Diaminotoluenes met at the Monitoring and Assessment Research
Centre, London, United Kingdom, from 20 to 25 October 1986.
Professor P.J. Petersen welcomed the participants on behalf of
the host Institution, and Dr G.C. Becking opened the meeting on
behalf of the three co-sponsoring organizations of the IPCS
(ILO/UNEP/WHO). The Task Group reviewed and revised the draft
criteria document and made an evaluation of the health risks of
exposure to diaminotoluenes.
The efforts of DR M. DIETER, US NATIONAL INSTITUTE OF
ENVIRONMENTAL HEALTH SCIENCES, Research Triangle Park, North
Carolina, USA, in the preparation of the draft, and of all others
who helped in the preparation and finalization of the document are
gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
1. SUMMARY AND CONCLUSIONS
1.1. Summary
1.1.1. Identity, properties, analytical methods
Toluene diisocyanates (TDIs) are synthetic organic chemicals of
low relative molecular mass (174.17). They are colourless to pale
yellow liquids, at room temperature, with a distinct pungent odour
detectable around 0.7 mg/m3, which is well above current exposure
limits. The different commercial grades solidify at between 4.7 °C
and 22 °C and boil at 251 °C at 760 mmHg. Toluene diisocyanates
react with water to form polyureas, carbon dioxide gas, and small
amounts of diaminotoluenes, depending on the amount of water
present. They also react with basic chemicals, including proteins.
Adequate analytical methods have been developed to measure air
levels of toluene diisocyanates in the work-place, for both area
and personal monitoring. The methods include: (a) high-pressure
liquid chromatography; (b) photometric methods; and (c) the use of
direct-reading instruments in which a chemically impregnated paper
tape changes colour on exposure to toluene diisocyanates. The
detection limits, ranging from 0.0001 to 0.07 mg/m3, depend on the
sampling and analytical procedure.
Analytical methods have also been developed to measure toluene
diisocyanates levels in environmental media and consumer products.
1.1.2. Production, uses, and sources of exposure
Toluene diisocyanates are commercially available as > 99.5%
pure 2,4-toluene diisocyanate (2,4-TDI), and 80:20 or 65:35
mixtures of the 2,4- and 2,6-isomers. "Crude"-TDI, with an
unidentified isomer ratio, is also commercially available, but not
widely used. By far the most widely used compound is the 80:20
isomer mixture.
Toluene diisocyanates are important industrial intermediates
used in conjunction with polyether and polyester polyols as co-
reactants in the manufacture of polyurethane foams, paints,
varnishes, elastomers, and coatings. TDI-based polyurethane foams
are widely used in the automotive and furniture industries, and in
packaging and insulation.
Toluene diisocyanates released into the environment, will tend
to partition into water and undergo rapid hydrolysis (half-life of
0.5 seconds - 3 days in water, depending on pH and water turbidity)
leading predominantly to the formation of relatively inert
polymeric ureas. Toluene diisocyanates would also be expected
to undergo photolysis and hydroxy radical oxidation. Therefore,
transport and occurrence would be limited to the immediate vicinity
of effluents or spills, and the resulting polyureas would probably
be resistant to further biodegradation.
Consumer products, including single-pack paints and lacquers,
may include traces of free toluene diisocyanates.
Exposure may occur in a wide variety of occupations, including
the manufacture and use of chemicals, and work with polyurethane-
coating products. It may also occur during transport, as a result
of spills or leaks. Excursions above safety limits are of
particular concern for sprayers and their co-workers.
1.1.3. Kinetics, biotransformation, and elimination
Toluene diisocyanates are highly reactive in body fluids with a
reported half-life of less than 30 seconds in serum and < 20 min
in stomach contents. However, in oral administration studies using
high doses, TDI forms insoluble polyurea-coated globules and
persists much longer. It is believed that TDIs react with the
tissues they contact rather than being absorbed and distributed
in the body in free form. Isocyanates react with hydroxyl, amino,
carboxyl, and sulfhydryl groups and can inactivate proteins by
covalent bonding. Animals, treated orally with 2,6-TDI,
excreted mono- and di-acetylated diaminotoluene, indicating that
diaminotoluene may be formed as a TDI metabolite. Its immunogenic
action may derive from relations with proteins or polyaccharides to
form a hapten complex and new antigenic determinants.
1.1.4. Effects on experimental animals
When inhaled, toluene diisocyanates are very toxic for animals.
The 4-h LC50 ranges from 70 to 356 mg/m3. Animals die of pulmonary
oedema and haemorrhage. TDIs, ingested orally or in contact with
the skin, are relatively less toxic in terms of lethal dose. The
oral LD50 ranges from 3.06 to 4.13 g/kg body weight, and the dermal
LD50 in rabbits is 10 g/kg body weight. Liver, kidney,
gastrointestinal, and skin damage occur via these routes.
Toluene diisocyanates are irritants for the mucous membranes of
the respiratory tract, eyes, and skin and are sensitizers of the
respiratory tract and skin.
Dermal application of toluene diisocyanates in one animal model
resulted in sensitization, and subsequent bronchial challenge
produced a hypersensitive response.
The mechanism of the sensitization reaction has been the
subject of extensive research and is still under debate. It has
been suggested that sensitization, which may develop gradually or
suddenly after exposure to toluene diisocyanates, may be due both
to immunological factors, as evidenced by the production of
TDI-specific antibodies, and to non-immunological factors, as
evidenced by increased carbachol-induced contractibility.
TDI was positive in two bacterial mutagenicity tests.
Toluene diisocyanates were negative for cell transformation
in two mammalian in vitro systems and one in vivo system.
The results of 2-year, inhalation studies on mice and rats,
using commercial grade 80:20 TDI at doses of 0.356 and 1.068 mg/m3,
administered for 6 h/day, 5 days per week, for periods ranging from
104 to 108 weeks, were negative for carcinogenicity. In 2-year
oral gavage studies with an 80:20 commercial grade mixture of
TDI in corn oil (30 - 240 mg/kg), the incidences of a variety of
tumours increased in both male and female rats and in female mice.
The tumours consisted of subcutaneous fibromas/fibrosarcomas and
pancreatic acinar cell adenomas in male rats, subcutaneous
fibromas/fibrosarcomas, pancreatic islet cell adenomas, neoplastic
nodules of the liver, and mammary gland fibroadenomas in female
rats, and haemangiomas/haemangiosarcomas and hepatocullular
adenomas in female, but not male, mice.
1.1.5. Effects on human beings
Exposure to toluene diisocyanates can lead to adverse effects
on the respiratory tract, skin, eyes, and gastrointestinal tract.
A variety of respiratory illnesses have been induced in workers
exposed occupationally to toluene diisocyanates, including
irritation of the upper and lower respiratory tract, an asthma-
like sensitization response, and individual and group mean
decreases in lung function. These decreases have been noted,
in some cases, after exposure to an estimated average TDI
concentration of > 0.014 mg/m3 (for short-term as well as
long-term occupational exposure).
Irritation of the eye, nose, and respiratory tract has been
reported at levels of > 0.35 mg/m3. The respiratory tract
sensitization response, producing bronchial asthma in up to 10%
of previously exposed individuals, may occur at a level of 0.036
mg/m3.
1.1.6. Effects on organisms in the environment
TDIs have been lethal for certain aquatic organisms at
concentrations of between 10.5 and 508.3 mg/litre; the LD50 for two
avian species was about 100 mg/kg body weight.
1.2. Evaluation of Hazards from Long-Term Exposure to Toluene
Diisocyanates
The risk of respiratory toxicity from repeated exposure can be
summarized as follows:
(a) chronic loss of ventilatory capacity, as measured by
forced expiratory volume and forced vital capacity; and
(b) immediate and/or delayed asthmatic responses.
Estimates of past mean exposures to TDI have been made in
many epidemiological studies in attempts to quantify dose-
response relationships for respiratory ill-health. Because of
inconsistencies in the hygiene sampling and measurements used in
the past, it is difficult to be confident about the exact levels at
which TDI causes the above-mentioned health effects. It should be
remembered that fluctuations in true individual exposure occur and,
as the size and extent of the intermittent peaks is not known,
their biological significance cannot be evaluated.
Once individuals are sensitized to toluene diisocyanates, low
concentrations, much below current occupational exposure limits,
can induce asthma. Studies on experimental animals have shown that
skin application of TDI can lead to pulmonary sensitization; thus,
it is prudent to avoid repeated skin contact.
No data were available on the carcinogenic effects of toluene
diisocyanates in human beings.
No carcinogenic effects of TDI were noted in an inhalation
study on rats and mice. However, gavage of the 80:20 mixture in
corn oil produced dose-related carcinogenic effects in male and
female rats and female mice. It is considered that there is
sufficient evidence for the carcinogenicity of TDI for experimental
animals.
There is evidence of mutagenicity in two bacterial tests.
It is not possible, on the basis of available data, to evaluate
the hazards for non-human targets from environmental levels of TDI.
1.2.1. Conclusions and recommendations
1. There is sufficient knowledge about TDI to classify it as a
very toxic compound, when inhaled, and it should be treated
as a potential human carcinogen and as a known animal
carcinogen. Consequently, the greatest priority should be
given to safe methods of use, and the education, training,
and supervision of operatives, together with state
enforcement of legislation by an effective inspectorate.
Special attention should be paid to the prevention and
adequate treatment of unscheduled releases and spills.
2. Additional animal carcinogenicity testing using the
inhalation route should be carried out.
3. Morbidity and mortality studies are required on
occupational groups, for whom reliable exposure levels are
available, to address the question of cancer, and to
evaluate potential long-term human hazards under current
standards of good working practice.
4. Because it is not possible to reach confident conclusions
from data on the neurotoxicity of TDI, neurophysiological
and behavioural studies should be carried out on
asymptomatic workers exposed at current hygiene standards.
5. For the foreseeable future, exposed workers require health
monitoring by systematic symptom enquiry and by
standardized measurement of ventilatory function, with
subsequent analysis of trends in individual, and group
mean, values.
6. Appropriate sampling strategies, together with existing
analytical methods, have to be developed and used to obtain
better information about exposure. Special attention
should be given to the detection and characterization of
peak values. The results of these analyses should be
evaluated in parallel with careful health studies.
7. Further metabolic studies of a qualitative and quantitative
nature are required with a view to developing methods of
measuring TDI uptake and monitoring exposure.
8. Whether TDI produces sensitization in human beings by
pharmacological or immune mechanisms needs to be elucidated
with a view to determining whether restrictions placed on
the employment of atopic subjects, in areas where TDI is
produced or used, are justified.
9. Studies are required to determine whether TDI has embryo-
toxic and teratogenic properties or induces adverse
reproductive effects at current exposure levels.
10. Further environmental studies are required to monitor
general environmental levels of TDI in the neighborhood of
sources and to collect ecotoxicity data.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Toluene diisocyanates (TDIs) are synthetic organic chemicals
with a molecular formula of C9H6N2O2; a relative molecular mass of
174.17; and the following chemical structure (R = -N=C=O):
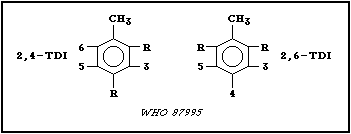 Toluene diisocyanates are produced as 2 isomers (2,4-toluene
diisocyanate (2,4-TDI) and 2,6-toluene diisocyanate (2,6-TDI))
and are commercially available in 3 isomer ratios: (a) > 99.5%
2,4-TDI; (b) 80% 2,4-TDI/20% 2,6-TDI, which is the most common
and referred to in this document as 80:20 mixture; and (c) 65%
2,4-TDI/35% 2,6-TDI. "Crude" toluene diisocyanate (Crude-TDI),
with an unidentified isomer ratio, is also commercially available,
but not widely used. Various identification codes for the most
commonly marketed toluene diisocyanates are listed in Table 1.
Table 1. Identification codes of commercial toluene diisocyanatesa
-------------------------------------------------------------------
Numeric index 2,4-TDI 2,6-TDI Commercial
(80:20)
mixture
-------------------------------------------------------------------
CAS register number 584-84-9 91-08-7 26471-62-5
RTECS access number CZ6300000 CZ6310000 CZ6200000
Wiswesser line notation OCNR Bl ENCO OCNR Bl CNCO -
Shipping ID number UN 2078 - UN 2078
OHM TADS number - 1- 7217313
Hazard substances data
bank number 0874 5272 6003
-------------------------------------------------------------------
a "Crude" toluene diisocyanate (unidentified isomers). CAS No. 1321-
38-6 and chemical abstracts name benzene, diisocyanatomethyl-.
The chemical, common, and trade names for toluene diisocyanates
are listed in Table 2.
Table 2. Toluene diisocyanates: Synonymous and Trade Names
--------------------------------------------------------------------
I. Commercial mixtures: 2,4-, 2,6-isomers
Chemical abstracts name benzene, 1,3-diisocyanatomethyl-
Other chemical names diisocyanatotoluene; isocyanic acid;
methyl- m-phenylene ester; methyl- m-
phenylene isocyanate; methylphenylene
isocyanate; toluene diisocyanate; tolylene
diisocyanate
Common name TDI
Trade names Desmodur T65 (also-T80); Hylene T (also,
-TCPA, -TLC, -TM, -TM65, -TRF); Isocyanic
acid; Lupranat T80; Mondur TD (also,
-TD80, -TDS); Nacconate 100; NCI-C50533;
Niax TDI; Niax TDI-P; Rubinate TDI 80/20;
TDI 80
II. 2,4-TDI
Chemical abstracts name benzene, 2,4-diisocyanato-1-methyl-
Other chemical names di-iso-cyanatoluene; di-isocyanate de
toluylene; diisocyanat-toluol; isocyanic
acid; 4-methyl- m-phenylene ester; tolueen-
diisocyanaat; toluen-disocianato; toluene
diisocyanate; toluene-2,4-diisocyanate;
toluene, 2,4-diisocyanato-;
toluilenodwuizocyjanian; toluylene-2,4-
diisocyanate; tolylene-2,4-diisocyanate;
tolylene diisocyanate;
tuluylendiisocyanat; 2,4-Dicyanato-1-
methyl-phenylene; 2,4-diisocyanato-1-
methylbenzene; 2,4-diisocyanatotoluene;
2,4-toluene diisocyanate; 2,4-toluene
diisocyanate; 4-methyl- m-phenylene
diisocyanate; 4-methyl- m-phenylene
isocyanate; 4-methyl-phenylene
diisocyanate; 4-methyl-phenylene
isocyanate
Common names TDI, 2,4-TDI
Trade names Desmodur T65 (also-T80); Hylene T (also,
-TCPA, -TLC, -TM, -TM65, -TRF); Isocyanic
acid; Lupranat T80; Mondur TD (also,
-TD80, -TDS); Nacconate 100; NCI-C50533;
Niax TDI; Niax TDI-P; Rubinate TDI 80/20;
TDI 80
--------------------------------------------------------------------
Table 2. (contd.)
--------------------------------------------------------------------
III. 2,6-TDI
Chemical abstracts name benzene, 2,6-diisocyanato-1-methy-
Other chemical names benzene, 1,3-diisocyanato-2-methyl-;
isocyanic acid; meta-tolylene diisocyanate;
2-methyl- m-phenylene isocyanate; 2,6-
toluene diisocyanates
Common name 2,6-TDI
Trade names not commercially available
--------------------------------------------------------------------
The isomer or mixture studied is often not reported in the
literature. For the purposes of this document, in such cases,
the chemical will be referred to as toluene diisocyanates. When
identified, the name of the particular isomer or mixture will be
used.
2.2. Physical and Chemical Properties
Toluene diisocyanates are colourless liquids or crystals,
turning pale yellow on standing, and having a characteristic sharp
pungent, sweet, fruity odour. Some of the physical and chemical
properties of toluene diisocyanates are listed in Table 3.
No properties of 2,6-TDI were found in the published
literature, except for a boiling point of 129 - 133 °C at 18 mmHg,
and a specific gravity similar to that of 2,4-TDI (Pollock &
Stevens, 1974).
Toluene diisocyanates are soluble in acetone, ethyl acetate,
ether, benzene, carbon tetrachloride, chlorobenzene, kerosene,
and various oils, e.g., corn oil. They may react violently with
compounds containing active hydrogen, such as alcohols, with the
generation of enough heat to lead to self-ignition and subsequent
release of toxic combustion products. Other such solvents that
must not be mixed with toluene diisocyanates include water, acids,
bases, and strong alkaline materials, such as sodium hydroxide and
tertiary amines, etc.
Toluene diisocyanates react with water and most acids to
produce unstable carbanic acids, which subsequently decarboxylate
(raising the pressure in closed containers) to yield relatively
chemically inert and insoluble polymeric urea (Hardy & Purnell,
1978). According to Holdren et al. (1984), reaction of TDI-vapour
with water vapour does not take place in the gaseous phase. They
concluded that loss due to surface adsorption takes place first,
since no diaminotoluenes or TDI-ureas could be detected in an
environmental chamber. Toluene diisocyanates also react with
(-NH-)-containing compounds to form ureides or ureas. Each
reaction pathway is important in terms of the health hazard
potential associated with toluene diisocyanates, since both
pathways are biologically, as well as commercially, significant,
and occur at room temperature (Chadwick & Cleveland, 1981).
Table 3. Physical and chemical properties of toluene diisocyanates
------------------------------------------------------------------------
Properties 2,4-TDI Commercial mixture
(2,4-, 2,6-isomers)
------------------------------------------------------------------------
Freezing point ( °C) 14 - 20a 11.5 - 13.5 (80:20 mix)
15c 11 - 14 (80:20 mix)
3 - 5 (65:35 mix)b
T80 = 12.5 - 13.5°
T65 = 4.7 - 6
Melting point ( °C) 22b 12.5 - 13.5 (80:20 mix)
4.7 - 6 (65:35 mix)
Boiling Point ( °C)
at 10 mmHg 120b 121c
at 760 mmHg 251c 25l (both mixes)
Flash point ( °C)
open cup 135a 132 (both mixes)
closed cup 127b -
Explosive limits:
Concentration (% v/v)
lower 0.9 0.9
upper 9.5 9.5
Temperature ( °C)
lower - 118
upper - 150
Fire temperature ( °C) - 142
Autoignition temperature 620 620
( °C)
Volatility; vapour pressure 1 mmHg (80 °C)d 1.9 mmHg (94 °C)a
0.01 mmHg (20 °C)
Vapour density (air = 1) 6d 6e
Density (g/cm3)b,c 1.22 25/15 1.22 25/15 (both mixes)
1.2244 20/4c -
Odour threshold 0.36 - 0.92 mg/m3
------------------------------------------------------------------------
a From: Woolrich & Rye (1969).
b From: Chadwick & Cleveland (1981).
c From: Windholz (1983).
d From: Hartung (1982).
e From: NIOSH (1978).
f From: Olin product literature.
Toluene diisocyanates dimerize slowly at ambient temperatures
and more rapidly at elevated temperatures. Trimerization occurs at
100 - 200 °C and, above 175 °C, carbodiimides form with the release
of carbon dioxide (CO2) (Chadwick & Cleveland, 1981; Ulrich, 1983).
2.3. Conversion Factors
At 25 °C and 760 mmHg:
1 mg/m3 = 0.14 ppm in air
1 mg/litre = 140.5 ppm.
2.4. Analytical Methods
The sampling and determination of toluene diisocyanates in air
has been the subject of several studies. The method originally
published by Marcali (1957) has been modified by several
investigators (Grim & Linch, 1964; Meddle & Wood, 1970).
Photometric methods are non-specific and most of them pool all the
isocyanates. Also, most procedures are severely hampered because
other agents, particularly aromatic amines, interfere in a way that
may result in falsely high readings. In contrast, chromatographic
techniques are specific and measure individual isocyanate species
(Table 4).
Most recent analytical methods involve high-performance
liquid chromatography (HPLC) using ultraviolet, fluorescence, or
electrochemical detection (Dunlap et al., 1976; Sango & Zimerson,
1980; Warwick et al., 1981). Improved sampling techniques include
the use of solid adsorbents (Tucker & Arnold, 1982). The Marcali
method has been evaluated for its response for the two isomers of
toluene diisocyanate. The simple modification involving changes in
diazotization time and temperature eliminates the isomeric effect
(Rando & Hammad, 1985). An extension of the spectrophotometric
method is the development of a tape method involving a chemically
impregnated paper tape that changes colour on exposure to toluene
diisocyanates (Reilly, 1968). However, the presence of
diaminotoluenes at concentrations similar to that of the toluene
diisocyanates leads to significant negative interference with the
colour-forming reaction (Walker & Pinches, 1981). Also, at low
humidity, the tape monitor tends to give falsely low readings
(Mazur et al., 1986).
Levels of toluene diisocyanates in consumer products (lacquers)
are usually determined, after appropriate extraction techniques, by
gas chromatography and high-performance liquid chromatographic
methods (McFadyen, 1976; Conte & Cossi, 1981).
In general, it should be understood that, with all analytical
methods, reliable figures for isocyanate concentrations in air
can be obtained only in the range of at least 5 - 10 times the
detection limit.
Table 4. Analytical methods for the determination of toluene diisocyanates
---------------------------------------------------------------------------------------------------------
Purpose/method Detection limit Reference
---------------------------------------------------------------------------------------------------------
I. Detection of TDIs in work-place air
1. Spectrophotometry
TDIs hydrolysed to the corresponding diamines, 0.07 mg 2,4-TDI/m3; Marcali (1957)
diazotized, coupled to N-1-naphthyethylene- field kit, 0.14 mg/m3
diamine, and final colour measured at 550 nm;
Note: as the concentration of 2,6-TDI increases
in the mixture, the total recovery of TDIs is
reduced
a modification of the Marcali method, in an Meddle & Wood (1970)
attempt to circumvent interference by primary
aromatic amines
a further modification to eliminate the Rando & Hammad (1985)
difference in response for the two isomers
2. Gas chromatography
2,4-TDI hydrolysed in dilute hydrochloric acid 0.06 - 0.23 mg/m3 De Pascale et al. (1983)
and subsequent determination by gas-liquid
chromatography/mass fragmentography 2,4-DAT;
instrument detection limit is 500 pg
gas liquid chromatography: TDIs hydrolysed to the 0.004 mg/m3 Audunsson & Mathiasson
corresponding amines in dilute sulfuric acid; (1983)
2,4- and 2,6-TDI detection limit depends on
sample volume
3. High-performance liquid chromatography
TDI sampled in derivatizing absorber ( N-(4-nitro 14 µg/m3 Dunlap et al. (1976)
benzyl)propylamine) and subsequent determination
of the urea derivative formed by UV detection;
sample volume 20 litre at 1 - 2 litre/min
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Purpose/method Detection limit Reference
---------------------------------------------------------------------------------------------------------
3. High-performance liquid chromatography (contd.)
TDI derivatized during sampling with gamma- 0.0001 mg/m3 Sangö & Zimerson (1980)
( N-methylaminomethyl)anthracene and analysis by
fluorescence or UV detection; sample volume
15 litre at 1 litre/min
TDI sampled in absorbed solution containing 0.0002 mg/m3 Warwick et al. (1981)
1-(2-methoxyphenyl) piperazine; the derivative
formed is detected by electrochemical or UV
detection; instrument detection limit 200 pg;
sampling time 10 min at 1 litre/min
TDI detected with electrochemical detection of 5 µg/m3 Meyer & Tallman (1983)
the derivative formed after treatment with
p-aminophenol, instrument detection limit 100 pg;
flow rate of 1 litre/min, for 10 min
Note: formation of multiple derivatives might
lead to somewhat complex chromatograms
TDIs determined as N-(4-nitrobenzyl)propylamine 1 µg/m3 Rosenberg (1984)
derivatives with UV detection using a 10-litre
sample
4. Direct read-out
Tape-monitor using impregnated paper that yields 0.07 mg/m3 Reilly (1968)
colour stain on exposure to isocyanate vapour
TDIs absorbed on a piezoelectric quartz crystal 0.14 mg/m3 Alder & Isaac (1981a,b)
coated with polyethylene glycol (PEG 400); (portable kit);
resulting change in weight of crystal is 0.043 mg/m3
monitored by the associated change in the
oscillation frequency; PEG 400 minimizes the
effect of water vapour
detection of TDIs with a coated quartz piezo- 0.07 mg/m3 Fielden et al. (1984)
electric crystal 76 Hz cps SI
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Purpose/method Detection limit Reference
---------------------------------------------------------------------------------------------------------
II. Determination of free TDIs in flexible foam
1. Infrared spectroscopy
based on the N=C=O stretching vibration; detects Conte & Cossi (1981)
the presence of N=C=O groups independently of
the molecular structure; extraction of unreacted
TDIs in the foam with o-dichlorobenzene and
gas chromatographic determination using a flame
ionization detector; free TDIs present at levels
of about w/w foam in fresh foam (1 h after
production) disappear after 24 h, under all
storage conditions (both ambient and dry air)
---------------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Toluene diisocyanates are not known to occur as natural
products.
3.2. Man-Made Sources
3.2.1. Production levels and processes
Toluene diisocyanates are manufactured by the reaction of
diaminotoluenes with phosgene. The reaction temperature increases
from ambient in the first reactor to about 200 °C in the last
reactor. The isomer mixture is stripped of solvent and separated
by distillation (NIOSH, 1978; IARC, 1979; Chadwick & Cleveland,
1981).
The most widely marketed grade of toluene diisocyanate is the
80:20 mixture of the 2,4- and 2,6-isomers. A mixture of 65:35
of 2,4-; 2,6-isomers is also available. "Pure" 2,4-TDI is
manufactured in small quantities and used for special applications.
The residue product (i.e., "crude"-TDI) is sold as a speciality
isocyanate (Chadwick & Cleveland, 1981; Ulrich, 1983).
3.2.1.1. World production figures
The production of 80:20 mixture accounts for > 90% of
the total toluene diisocyanates produced in USA. In 1983,
approximately 300 x 106 kg were produced in the USA; 29% of the
1983 production was exported to Belgium, Canada, the Federal
Republic of Germany, Japan, Korea, the Netherlands, and other
countries (US ITC, 1985). In the USA, it has been projected that
the domestic demand for toluene diisocyanates will increase at the
rate of 1 - 3% annually, until the year 1990 (Anon., 1983).
Production of toluene diisocyanates in Canada in 1975 amounted
to 9 x 106 kg. In 1982, the annual production capacity for toluene
diisocyanates was 20 x 106 kg for Brazil and 12 x 106 kg for
Mexico. Western European nations (mainly Belgium, France, the
Federal Republic of Germany, Italy, and Spain) reported a combined
annual capacity for the production of toluene diisocyanates in 1982
of > 326 x 106 kg. Production capacity during 1982 within the
Netherlands, Portugal, and the United Kingdom was not reported;
however, production in 1976 in the United Kingdom was 25 x 106 kg.
Seventy percent of the western European production was consumed
nationally and 30% was exported, primarily to eastern Europe, the
Middle East, and North Africa. In eastern Europe, the German
Democratic Republic and Yugoslavia had a combined production
capacity of 47 x 106 kg toluene diisocyanates in 1982. No figures
were available for production within the USSR. Japan reported an
effective annual production capacity of 78 x 106 kg in 1982, and
actual production reached 67 x 106 kg. The global capacity for
the production of toluene diisocyanates in 1982 was reported to
be > 817 x 106 kg (Ulrich, 1983).
3.2.1.2. Manufacturing processes: release into the environment
Toluene diisocyanates are manufactured in a closed system, and
air emission is minimal. However, toluene diisocyanates may be
emitted into the atmosphere during the removal of phosgene and
hydrogen chloride from the first fractionating column. It is the
belief of the Task Group that few of these products are emitted,
because of improved manufacturing facilities. After gas scrubbing,
they may be discharged into the waste effluent (Dyson & Hermann,
1971; Bagon & Hardy, 1978; Dharmarajan et al., 1978).
Levels of toluene diisocyanates ranging from 0.1 to 17.7 mg/m3
have been monitored in stack gases from 3 plants manufacturing
polyurethane foams (Grieveson & Reeve, 1983). It was estimated
that approximately 50 ± 5 g toluene diisocyanates/tonne of TDI
processed within the plant was emitted during the manufacture of
soft-block foams (Grieveson & Reeve, 1983).
3.2.2. Uses
Toluene diisocyanates are reactive intermediates that are used
in combination with polyether and polyester polyols to produce
polyurethane products. The production of flexible polyurethane
foams represents the primary use of toluene diisocyanates (~ 90% of
the total supply). The 80:20 mixture is used in their production
at an average of 30% by weight. Domestic consumption of flexible
polyurethane foam in the USA in 1981, estimated at 499 x 106 kg,
can be broken down into the following uses (in million kg):
furniture (208.7); transportation (99.8); bedding (63.5); carpet
underlay (72.6); and other uses (11.3). An estimated 27 x 106 kg
of rigid polyurethane foams, used in refrigeration equipment, was
produced with "crude"-TDI in the USA in 1982 (US EPA, 1984).
Polyurethane coatings represent the second largest market for
toluene diisocyanates. Toluene diisocyanates are also used in the
production of polyurethane elastomeric casting systems, adhesives,
sealants, and other limited uses (Brandt, 1972; Granatek et al.,
1975; Aragon et al., 1980).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
There are very few studies on the overall environmental fate
of toluene diisocyanates in the published literature. Available
studies have been summarized by Duff (1983). On the basis of this
review and available information on the physical and chemical
properties, the following statements can be supported.
4.1. Air
It has been demonstrated in environmental chambers that, in the
gaseous phase, TDI vapour and water vapour do not react to form
diaminotoluenes, since not even trace amounts of these compounds
were detected (Holdren et al., 1984). A rate of loss of about 20%
of TDI-vapour per hour could be explained by surface adsorption.
This rate of loss was much higher and more rapid when comparable
concentrations of an aliphatic amine were simultaneously present in
the chamber. Again, no hydrolysis products of TDI could be
detected.
4.2. Water
In most industrial situations, toluene diisocyanates are
hydrolysed by water to give the corresponding polymeric ureas and
carbon dioxide (Chadwick & Cleveland, 1981). However, when toluene
diisocyanates come into contact with water without agitation, as in
spills, a hard crystalline crust of polymeric ureas forms slowing
down further degradation of the toluene diisocyanates, unless the
crust is mechanically broken. The solid reaction products are
insoluble and biologically inert (Brochhagen & Grieveson, 1984).
4.3. Soil
A computerized partitioning model proposed by Mackay (1979)
indicated that toluene diisocyanates released into the environment
will tend to partition into water. However, in making this
prediction, the reactivity of the compounds was not taken into
consideration.
4.4. Biotransformation
Studies were conducted under laboratory or environmental
conditions to evaluate the potential degradation of soft
polyurethane foams, with either a polyester or a polyether base,
both prepared with an isomeric mixture of 2,4 and 2,6-diisocyanates
(Martens & Domsch, 1981). Polyurethane-ether foams were highly
resistant to chemical and microbial degradation. Polyurethane-
ester foams were quite susceptible to degradation, especially at
elevated temperatures (50 °C), yielding 0.25% 2,4-toluenediamine
and 0.38% 2,6-toluenediamine in acidic (pH 1) water extracts of
leachate after 3 months incubation in the laboratory. The
incubation mixture contained 1 g of finely chopped soft
polyurethane foam of a polyester base prepared with the isomeric
mixture of 2,4- and 2,6-diisocyanates, in 100 ml of leachate (pH
7.5) from a refuse tip near Braunschweig, Federal Republic of
Germany. An experimental study conducted near this site
corroborated the laboratory results. Soft polyurethane foam cubes
were checked for weight loss after 13 months incubation in the
refuse tip, where they were found in the refuse layer of a 25:10:1
(weight) mixture of municipal refuse, sewage sludge, and caustic
lime. The polyurethane-ester foam cubes lost 17 - 31% of their
initial weight in the stratified filling, and, if the layers of
fill were mixed, weight loss ranged between 35 and 86%. The
polyurethane-ether foam cubes did not degrade under these
conditions. It was concluded that soft polyurethane foams prepared
with toluene diisocyanate isomers are susceptible to chemical
hydrolysis under extreme environmental conditions, and that under
these circumstances, an accumulation of aromatic amines can occur,
if their microbial degradation is impeded.
4.5. Bioaccumulation
There are no data on the bioaccumulation of TDIs.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
No data were found on levels of toluene diisocyanates in the
general environment.
5.1. General Population Exposure
Human beings and animals would be exposed to toluene
diisocyanates in environmental media only in the immediate vicinity
of effluents, factories, or areas of spillage (sections 3.2.1.2 and
4). Consumers may also be exposed to toluene diisocyanates through
the indiscriminate use of several commercially available household
products, such as polyurethane foam kits (US EPA, 1984). For
example, Peters & Murphy (1971) tested 5 "instant" polyurethane
foam products and found that concentrations of toluene
diisocyanates in the air ranged from 0 to 0.15 mg/m3 during
applications. They also noted that labels on the cans were
inadequate regarding contents, precautions, and toxicity warnings.
Consumers may also be exposed to toluene diisocyanates during the
application of polyurethane varnishes (US EPA, 1984).
Beall & Ulsamer (1981) suggested that toluene diisocyanates
might be an indoor air pollutant. During pyrolysis of
polyurethanes, the general public could be exposed to the pyrolytic
products of toluene diisocyanates. No monitoring levels were
given.
5.2. Occupational Exposure
Because of the volatility of toluene diisocyanates, exposure
can occur in all phases of their manufacture and use (Sittig,
1979). Monitoring data for toluene diisocyanates in the work-
place is extensive with levels found between 0.014 and 1.050 mg/m3
(NIOSH, 1978; Hosein & Farkas, 1981; Belin et al., 1983). During
the production of polyurethane-coated wire, toluene diisocyanates
may be found in the work-place, during the different stages of the
coating process, at concentrations ranging between < 0.001 and
0.11 mg/m3 (Rosenberg, 1984). The highest levels have occurred
during spraying with polyurethane foam, a procedure that is usually
conducted in confined spaces (Hosein & Farkas, 1981). Isocyanate
lacquers contain 0.2 - 1% monomeric toluene diisocyanates (Tu &
Fetsch, 1980), and short-term excursions above safe limits are of
a particular concern for spray workers and their assistants.
Sittig (1979) estimated that approximately 40 000 workers in
the USA were potentially exposed to toluene diisocyanates in such
jobs as adhesive production, insulation, application and production
of toluene diisocyanate resins and lacquers; organic chemical
synthesis, paint spraying, polyurethane foam production, working
with rubber, shipbuilding, textile processing, and wire-coating.
Consumer use of products containing TDI could result in many more
cases of exposure.
6. KINETICS AND METABOLISM
6.1. Absorption
Absorption of toluene diisocyanates through the respiratory
tract is suggested by: (a) their high acute toxicity for animals
via inhalation (section 8.1); and (b) reports on systemic effects
and antibody formation in individuals exposed to toluene
diisocyanates primarily via inhalation (Sharonova & Kryzhanovskya,
1976; Steinmetz et al., 1976; White et al., 1980; Sharonova et al.,
1982).
6.2. Distribution
No information was found regarding the distribution of
toluene diisocyanates in mammalian systems. Because of the wide
distribution of water and other nucleophiles in tissues, it is
likely that toluene diisocyanates will react with the tissues they
initially contact and be transformed into various products, rather
than that they will be absorbed and distributed throughout the body
as toluene diisocyanates.
6.3. Metabolic Transformation and Elimination
No published studies on the biotransformation of toluene
diisocyanates were found. However, one report (NTP, 1985) on the
disposition of 2,6-TDI in Fischer 344 rats was available to the
Task Group. The apparent half-life of 2,6-TDI was dependent on
the vehicle in which it was administered, its concentration in the
solvent, and the rate of mixing as the compound was added. In an
aqueous suspension of stomach contents, the half-life of 2,6-TDI
was < 2 min, whereas in serum, the half-life was < 30 seconds.
When [C14]-2,6-TDI was given orally to rats in corn oil, most
of the compound formed polymers in the gastrointestinal tract. At
doses of 900 mg/kg body weight, the insoluble polyureas usually
lined the stomach, slowing down or preventing the migration of
stomach contents into the intestine. At a 60 mg/kg dose level,
these results were not observed (NTP, 1985). Most 2,6-TDI-derived
materials were eliminated in the faeces or were found in the
gastrointestinal tract 72 h after dosing. Approximately 12% of
the low dose (60 mg/kg body weight) and only 5% of the high dose
(900 mg/kg) were excreted in the urine (24-h after treatment),
mainly in the form of 2,6-bis(acetylamino) toluene (54%). Increased
urinary excretion of 2,6-TDI metabolites with decreasing dosage was
consistent with the lower concentration of the compound in the
stomach permitting increasing amounts of the 2,6-TDI to be
hydrolysed completely to monomeric 2,6-diaminotoluene rather than
forming polymers. The 2,6-diaminotoluene could then be absorbed,
acetylated, and excreted in the urine. Materials derived from
2,6-TDI were not concentrated in any tissue (NTP, 1985). In rats
exposed dermally to 2,4-TDI, no unreacted isocyanate was detected
in the urine, but 2,4-TDA was detected after hydrolysis of the
urine (Rosenberg & Savolainen, 1985). The same authors studied
workers occupationally exposed to the 80:20 TDI isomer mixture, and
reported that concentrations of TDA in the urine after hydrolytic
treatment were linearly related to the estimated TDI dose
(Rosenberg & Savolainen, 1986). A possible biochemical pathway
would involve the formation of TDA, its conjugation and excretion,
but it was not known if this had taken place.
6.4. Reaction with Body Components
Toluene diisocyanates are highly reactive towards a large
number of active hydrogen and basic nitrogen compounds (Ozawa,
1967; Alarie, 1973; Brown & Wold, 1973; Brown et al., 1982). Thus,
more than one reaction may occur in a system at a given time. The
results of an in vitro study reported by the NTP (1985) showed that
2,6-TDI would react with both rat serum and stomach contents at
37 °C. The 2,6-TDI appeared to form a polymeric film, which
encapsulated globules of 2,6-TDI, thus limiting the availability of
the compound in the interior of the globules for further reaction.
Mixtures of TDI isomers, such as 80:20, may behave in a
different manner to single 2,6- or 2,4-TDI isomers.
Isocyanates react with carboxyl groups and form amines, acid
anhydrides, and ureas (Fry, 1953). Ozawa (1967) and Brown & Wold
(1973) demonstrated that diisocyanates were active-site-specific
reagents towards the hydroxyl groups of serine in proteases. Such
reactions can result in the irreversible inactivation of enzymes,
such as adenylate cyclase, serine proteases, alcohol dehydrogenase,
and cholinestrase (Brown & Wold, 1973; Twe & Wold, 1973; Butcher et
al., 1979; Dewair et al., 1983). Isocyanates react with amino
groups to form ureas, which are also highly stable and unlikely to
dissociate under biological conditions.
Isocyanates form thiolic acid and esters when reacting with
sulfhydryl groups in proteins. However, this reaction takes place
at low pH only, whereas the products are unstable at pH 7 or higher
(Twe & Wold, 1973).
Toluene diisocyanates may react with naturally occurring
proteins or polysaccharides and form immuno hapten complexes. The
results of an in vitro study by Ted Tse & Pesce (1979) showed that
toluene diisocyanates will react with human serum-albumin via one,
or both, of the isocyanate groups to form mono- or bisureido
protein derivatives. Such derivatives may be immunogenic and may
possibly lead to allergenic responses, as well as new antigenic
determinants (Baur, 1983).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Lethality data for some avian and aquatic species are listed in
Table 5.
Although practically insoluble in water, dispersed TDI can form
droplets and cause toxicity in aquatic systems.
Curtis et al. (1979) reported that 2,4-toluene diisocyanates
appeared to be toxic for fathead minnows only in the unreacted
form, and most lethality occurred during the first 12 h of
the test. The LC50 for aquatic species ranged from 10.5 to
508.3 mg/litre in static tests. The oral LD50 for avian species
was > 100 mg/kg body weight (Schafer et al., 1983).
Table 5. Lethality of toluene diisocyanates for aquatic and avian species
---------------------------------------------------------------------------------------------------------
Species Dose/concen- Condition Lethality Reference
tration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Freshwater
Fathead Minnow 194a static 24-h LC50 Curtis et al. (1979)
(Pimephales promelas) 172.1 static 48-h LC50
164.5 static 96-h LC50
reconstituted
softwater (20 °C)
Saltwater
Grass shrimp 508.3a salinity 25 parts/ mortality less than Curtis et al.
(Palaemonetes pugio) thousand; 22 °C; 65% below this level (1979)
static within 96 h
Harpacticoid copepod 11.8b salinity 7 parts/ 96-h LC50 Bengtsson & Tarkpea
( Nitocra spinipes (10.5 - 13.2) thousand; static (1983)
Boeck) Crustacea
Avian
Redwinged blackbird 100 mg/kgc oral LD50 Schafer et al. (1983)
(Agelaius phoeniceus)
Starling > 100 mg/kgc oral LD50 Schafer et al. (1983)
(Sturnus vulgaris)
---------------------------------------------------------------------------------------------------------
a 2,4-isomer.
b Authors did not state the units; mg/litre were assumed on the basis of previous publication of Lindén
et al. (1979).
c 2,6-isomer.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Application of drops of toluene diisocyanates in the eyes of
rabbits caused immediate reaction suggestive of pain, lachrymation,
swelling of the eyelids, a conjunctival reaction, and mild damage
to the cornea (Zapp, 1957; Grant, 1974; Duprat et al., 1976;
Woolrich, 1982).
Intratracheal administration of 0.3 ml toluene diisocyanates in
guinea-pigs resulted in coagulation of proteins in the respiratory
tract and rapid death from respiratory distress (Friebel &
Lüchtralh, 1955). The acute toxicity of toluene diisocyanates via
various routes of exposure is summarized in Table 6.
Table 6. Lethality of toluene diisocyanates
------------------------------------------------------------------------
Route/species Concentration Duration Lethality Reference
(h)
------------------------------------------------------------------------
Inhalation
Rat 56.96 mg/m3 1 LC50 Harton & Rawl (1976)
Rat 98.96 ± 8.6 4 LC50 Duncan et al. (1962)
mg/m3
Rat (male) 348.88 mg/m3 4 LC50 Bunge et al. (1977)
(female) 356 mg/m3 4 LC50
Mouse 69.1 ± 9.96 4 LC50 Duncan et al. (1962)
mg/m3
Rabbit 78.32 mg/m3 4 LC50 Duncan et al. (1962)
Guinea-pig 90.4 ± 19.2 4 LC50 Duncan et al. (1962)
mg/m3
Oral
Rat 3060 mg/kg - LD50 Harton & Rawl (1976)
body weight
Mouse (male) 4130 mg/kg - LD50 Woolrich (1982)
body weight
Dermal
Rabbit 10 000 mg/kg - LD50 Harton & Rawl (1976)
------------------------------------------------------------------------
The toxicity of toluene diisocyanates, administered orally, is
low, but they are very toxic after inhalation exposure. Animals
reportedly died of acute pulmonary congestion, oedema, and
haemorrhage. Duncan et al. (1962) reported that during inhalation
exposures, all animals (Table 6) exhibited irritation. Tracheitis
and bronchitis, with sloughing of superficial epithelium, occurred
after exposure to 14.2 mg/m3 for 4 h. Rapid coagulation and
necrosis of the epithelium was evident following exposure to
35.8 mg/m3, suggesting direct chemical injury.
8.2. Short-Term Exposures
8.2.1. Inhalation
8.2.1.1. Guinea-pig
Exposure of guinea-pigs to 1200 mg toluene diisocyanates/m3,
as an aerosol, or 250 - 550 mg/m3, as a vapour, for 10 - 20 min at
irregular intervals, for one month, caused asthmatic reactions
after the first few inhalations, changing into continuous dyspnoea
in the course of the study (Friebel & Lüchtralh, 1955). Attainment
of these concentrations could only be achieved with both aerosol
and vapours. Bronchiolitis obliterans, pneumonia, and emphysema
occurred with little healing. Guinea-pigs sensitized to chicken
albumin responded in the same manner as untreated animals,
indicating the predominance of a primary toxic effect. However,
a possible role of allergic type reactions could not be excluded
completely.
Immunological sensitization and pulmonary hypersensitivity to
an 80:20 mixture were evaluated in the guinea-pig. Five days after
exposure to 1.78 mg/m3 (3 h/day for 5 days), 3 out of 16 exposed
guinea-pigs had developed antibody against TDI-guinea-pig serum-
albumin antigen, as demonstrated by immuno-diffusion, compared with
0/16 before exposure (Karol et al., 1980). Three additional
animals showed antibody responses with the more sensitive passive
cutaneous anaphylaxis assay (PCA). That is, a total of 6/16
exposed animals were antibody positive. No consistent increases
indicative of a pulmonary hypersensitive reaction were observed.
Concentration-dependent immunological responses to toluene
diisocyanates were measured following exposures that mimicked
industrial exposures and might lead to allergic reactions in
exposed workers (Karol, 1983). Guinea-pigs were exposed to 0.85 -
71.2 mg 80:20 mixture/m3, 3 h/day, for 5 days. On day 22, assays
for TDI-specific antibody, skin sensitivity, and pulmonary
sensitivity to toluene diisocyanates were performed. No antibody
to toluene diisocyanates was detected in animals exposed to a
concentration of 0.85 mg/m3, whereas 55% of the animals exposed
to > 2.56 mg 80:20 mixture/m3 displayed TDI-specific antibody in a
dose-related fashion. Pulmonary sensitivity to TDI-protein antigen
was observed at concentrations exceeding 2.6 mg/m3. Doses higher
than 14.2 mg/m3 resulted in pneumotoxicity and fewer pulmonary
hypersensitivity reactions. Exposure of a group of 24 guinea-pigs
to 0.14 mg 80:20 mixture/m3 (6 h/day, 5 days/week, for 70 days) in
whole-body exposure chambers, did not elicit these reactions
(Karol, 1983).
8.2.1.2. Mouse
The effects of single and repeated exposures to 2,4-TDI (99.7%
pure) vapour at concentrations ranging from 0.05 to 14.2 mg/m3 were
investigated in male Swiss-Webster mice, to detect the level of
sensory irritation caused by this chemical (Sangha & Alarie, 1979).
The results obtained demonstrated that the level of response not
only depended on the concentration, but also on the duration of
exposure; recovery rates also depended on the duration of exposure.
The RD50 (respiratory rate decrease of 50%) values decreased
significantly between 10 and 180 min and the levels of response
were exactly the same at 180 and 240 min of exposure. The RD50
found at 180 or 240 min of exposure was 1.42 mg/m3. Repeated
exposures to 2,4-TDI concentrations of or above 0.14 mg/m3 resulted
in cumulative effects, because of incomplete recovery prior to a
repeat exposure.
Weyel et al. (1982) measured the sensory irritation of 2,6-TDI
vapour at 0.37 - 7.6 mg/m3 in male Swiss-Webster mice. After a 3-h
exposure, a decrease in respiratory rate occurred with a pattern
indicative of sensory irritation of the upper respiratory tract,
which was similar to that noted by Sangha & Alarie (1979) with
exposure to 2,4-TDI at levels of > 0.14 mg/m3. An inverse linear
relationship (respiratory rate decrease versus log concentration of
2,6-TDI) was obtained that was identical to the slope and
relationship obtained in the earlier study on 2,4-TDI. From the
concentration-response (sensory irritation) relationship for
2,6-TDI, the RD50 was determined to be 1.85 mg/m3.
Lesions in the nasal cavity with a distinct anterior-posterior
severity gradient developed in mice after exposure to toluene
diisocyanates at 2.84 mg/m3, for 6 h/day, over 5 days (Buckley et
al., 1984). The lesions ranged from slight epithelial hypertrophy
or hyperplasia to epithelial erosion, ulceration, and necrosis with
variable inflamation of the subepithelial tissues. There was also
an associated loss of the olfactory nerves in the lamina prioria in
exposed animals.
8.2.1.3. Rat
Studies by Henschler et al. (1962) on the inhalation toxicity
of toluene diisocyanates are summarized in Table 7.
At exposure levels of 35.6 and 71.2 mg toluene
diisocyanates/m3, death was due to mechanical blocking of the
respiratory passages by mucosal tissue detached from bronchi and
trachea. At lower exposure levels, the fatal sequelae also
included heavy peribronchitis and spreading bronchopneumonia.
After cessation of exposure, partial reversal of pulmonary changes
occurred after several months in the 7.12 mg/m3 exposure group, and
complete remission occurred in the 3.56 mg/m3 exposure group.
After 40 exposures at 0.712 mg/m3, there were no definitive changes
in the respiratory tract, the only toxicological response being a
depression in body weight (Henschler et al., 1962).
Table 7. Inhalation toxicity of toluene diisocyanatesa
-------------------------------------------------------------------
Number of exposures Concentration Lethality
(schedule) (mg/m3)
-------------------------------------------------------------------
24 3.56 45% fatality of animals
(6 h/day, 6 days/week, with initial body weight
twice, miss 4 weeks, of 91 - 124 g; 0% fatality
repeat second 12 of animals with initial
exposures) body weight of 140 - 180 g
10 7.12 75% fatality
4 35.6 65% fatality
2 71.2 lethal for most animals;
2,4-isomer more toxic than
2,6-isomer
3 or 5 71.2 lethal for all
exposed animals
-------------------------------------------------------------------
a The 71.2 level mg/m3 was for 2,4- or 2,6-isomer or a 65:35
mixture of isomers. The 35.6, 7.12, and 3.56 mg/m3 levels were
for the 80:20 mixture. From: Henschler et al. (1962).
8.2.1.4. Dog
Four male dogs were exposed to concentrations of toluene
diisocyanates averaging 10.68 mg/m3, 35 - 37 times, for various
lengths of exposure (30 - 120 min), over a period of 4 months.
The dogs showed lachrymation, coughing, restlessness, and
expectoration of white frothy material. When killed after the last
exposure, all dogs showed mild congestion and inflammation of the
trachea and large bronchi. A conspicuous feature was the presence
of thick mucous plugs in some of the bronchial branches (Zapp,
1957).
8.2.2. Dermal
Toluene diisocyanates were described as a "medium" irritant for
rabbits and guinea-pigs, capable of producing cutaneous sensitivity
similar to contact allergy. The allergenic capacity was long-
lasting and depended on the allergen concentration (Zapp, 1957;
Duprat et al., 1976).
8.2.2.1. Guinea-pig
Dermal contact with toluene diisocyanates in the animal model
resulted in "rare" cases of sensitization (Peschel, 1970) and in
subsequent respiratory tract hypersensitivity (Karol et al., 1981).
Dermal contact sensitivity developed by the 7th day, following
applications of 1 - 100% solutions of 80:20 mixture (diluted with
olive oil) to the dorsal skin of the guinea-pig. After 14 days,
animals were evaluated for toluene diisocyanates sensitivity by
serological analysis and by bronchial provocation challenge.
Bronchial challenge with 0.03 mg toluene diisocyanates/m3, or
aerosols of TDI-protein conjugates, or p-tolyl isocyanate resulted
in respiratory hypersensitivity (Karol et al., 1981). These
responses were immediate; the respiratory rate increases were 3
times those in non-sensitive guinea-pigs. In challenges using
isocyanate conjugates, TDI-specific pulmonary reactions were
elicited more effectively when the hapten-protein conjugates rather
than the toluene diisocyanates vapour served as the challenging
agent.
Koschier et al. (1983) evaluated the dose-dependent eliciting
of dermal sensitization in young adult guinea-pigs treated with
2,4-TDI (2,6-isomer was < 2.5%). Induction was by cutaneous
application (25 µl) of 8 - 40% 2,4-TDI in n-butyl ether on 2
separate uncovered dorsal sites. Five days later, animals were
challenged with 0 - 0.4% 2,4-TDI (25 µl per site). All challenge
applications from 0.025% (6.25 µg) elicited a positive response in
75 - 100% of the animals. The results demonstrated that 2,4-TDI
induced sensitization and that the severity of the dermal response
was correlated with the concentration used at induction and
challenge. In a second study, in the group induced with 4% 2,4-
TDI, no effects were elicited after a dermal challenge application
of 3 µg, and a minimum effect was seen with 6.25 µg 2,4-TDI
(Koschier et al., 1983).
8.2.2.2. Mouse
In a dose-response study (Tanaka, 1979), a 100% increase in
ear-swelling in C3H/He mice, 24 h after challenge with 0.5 ml of 5%
TDI, was reduced to 50% after 72 h. In a second trial, 81% ear-
swelling resulted from a 5% challenge with TDI; there was a 6.5%
ear-swelling response to a 1% TDI challenge, compared with 2.4% in
vehicle controls. There was a cross-reactivity between TDI and
monodiisocyanate (MDI), so that sensitization with either
diisocyanate resulted in equal ear-swelling responses to challenge
with the opposite diisocyanate. The degree of response in cross-
reactivity was 4-times that in controls and about 35% of a
challenge response by the same diisocyanate used for sensitization.
Thymectomy did not change the ear-swelling responses to TDI. In a
subsequent study, Tanaka et al. (1984) reported that TDI induced a
delayed-type hypersensitivity reaction in the ear skin of male ICR
mice, and that 7-week-old mice sensitized with 1 - 5% (100 µl/dose)
TDI solutions showed ear-swelling with a challenge of 1% (2 µl) TDI
solution. The responses in 5-, 7-, and 13-week-old mice were the
same but were very slight in 16-week-old mice. Seven-week-old
BalB/C mice showed similar responses to ICR mice, but reactions in
ddY mice were much weaker.
Allergic dermatitis developed in mice by sensitization to
toluene diisocyanates, followed by inhalation exposure to the test
compound to determine if a delayed type allergy plays a role in
lung disorders caused by toluene diisocyanates. At a concentration
of 4.27 mg/m3, inhaled for 2 h, allergic dermatitis did develop,
but without noticeable pathological changes in the respiratory
organs (Ohsawa, 1983).
8.2.3. Oral
Oral gavage of 1500 mg/kg per day resulted in the death of 50%
of rats within a total of 10 treatments. Pathological examination
revealed injury to the gastrointestinal tract and liver (Zapp,
1957). No other toxic effects were reported from these studies.
8.3. Long-Term Exposure
8.3.1. Inhalation
8.3.1.1. Mouse
A dose-related increase in the incidence and severity of either
chronic or necrotic rhinitis occurred in mice exposed through
inhalation to the 80:20 mixture of toluene diisocyanates at 0.36 or
1.07 mg/m3, for 6 h/day, 5 days/week, over 2 years. In addition,
lesions of variable incidence and severity were seen in the lower
respiratory tract (interstitial pneumonitis, catarrhal bronchitis)
and eyes (keratitis) of some mice, with a higher incidence in the
1.07 mg/m3 group. Morbidity and mortality due to rhinitis occurred
in both treated groups (Loeser, 1983).
8.3.1.2. Dog
Patterson et al. (1983) immunized 3 dogs (by endotracheal tube)
with an aerosol of toluene diisocyanates at 1 mg/kg body weight,
every 2 weeks, for 4 months (2 - 3 times the maximal TLV for
occupational exposure). Thereafter, 2 dogs were dosed with 1 mg/kg
body weight every 4 weeks for 6 months (stated to be the cumulative
dose analagous to long-term exposure to 0.14 mg/m3). Systemic
immune responses to TDI-dog serum-albumin, including elevated
specific antibody titers of IgG, IgA, IgM, and development of
specific lymphocyte reactivity, were seen in all animals. Elevated
IgG and IgA titers were persistent. Although increased, the lower
titer of IgM antibody was of short duration. The antibody IgE was
detected, but levels fluctuated and became negative, even with
continued exposure to toluene diisocyanates. Airway responses
that occurred immediately after exposure to the aerosol included
abnormalities of selected pulmonary function parameters. They were
clearly not immunologically mediated, because they occurred with
initial exposure. However, other immediate airway responses
occurred that qualitatively simulated IgE-mediated, antigen-induced
airway responses in dogs. There was a statistically-significant
correlation between the latter airway responses and immediate skin
reactions (Patterson et al., 1983).
8.4. Reproduction, Embryotoxicity and Teratogenicity
No published data were found on the effects of toluene
diisocyanates on reproduction, or on the embryotoxicity or
teratogenicity of these compounds.
8.5. Mutagenicity and Related End-Points
8.5.1. Bacterial mutagenicity
There are conflicting reports about the mutagenicity of toluene
diisocyanates. Anderson & Styles (1978) reported that toluene
diisocyanate of unknown purity was non-mutagenic in a study of 120
chemicals tested by Purchase et al. (1978), but the fact that
several known mutagens failed to give positive results means that
the original report was suspect. Andersen et al. (1980) later
optimized the procedures to test the reactive isocyanates and
showed that a mixture of 2,4- and 2,6-toluene diisocyanates caused
a dose-dependent mutagenic response, using S-9 activation, in
S. typhimurium strains TA 98, TA 100, and TA 1538. The positive
control for these mutagen tests was the hydrolysis product of 2,4-
TDI, 2,4-diaminotoluene, reported by Ames et al. (1975) to be
mutagenic. The NTP has also tested toluene diisocyanates using the
Salmonella test system and found that both 2,6-TDI and a mixture
of 2,4- and 2,6-TDI (80:20) were mutagenic in S. typhimurium
strains TA 98 and TA 100 in the presence (but not the absence) of
Aroclor 1254-induced male Sprague Dawley or Syrian hamster liver
S9. Neither sample was mutagenic in S. typhimurium strains TA
1535 or TA 1537, with or without metabolic activation.
8.5.2. Mammalian cell transformation
Toluene diisocyanates were negative in two in vitro cell
transformation assays using human lung and hamster kidney cells
(Styles, 1978).
8.5.3. Mammalian in vivo study
Studies by Loeser (1983) failed to show a dose- or treatment-
related percentage increase in micronucleated erythrocytes from the
bone marrow of rats and mice exposed through inhalation to 80:20
mixture at 0.35 or 1.06 mg/m3, for 6 h/day, 5 days/week, over 4
weeks.
8.6. Carcinogenicity
8.6.1. Oral
Long-term oral (gavage) administration of the 80:20 mixture
of 2,4-, 2,6-TDI in corn oil resulted in increased incidences of
various types of tumours in Fischer 344/N rats and B6C3F1 mice
(NTP, 1986). Female rats and mice were dosed with 60 or 120 mg/kg
body weight; male rats received 30 or 60 mg/kg body weight; and
male mice were dosed with 120 or 240 mg/kg body weight, for 5 days
per week, over 2 years. However, it is worth noting that the
reaction of toluene diisocyanates with the moisture in the corn oil
resulted in unknown reaction products and in doses qualitatively
and quantitatively different from those reported, possibly as much
as 23% below the target dose. Long-term treatment, by gavage, with
the TDI-corn oil mixture caused dose-related reductions in body
weight gain. A dose-dependent pattern of cumulative toxicity began
at weeks 70 - 75, culminating at 103 weeks in the following
percentage mortality in control, low-, and high-dose groups,
respectively: male rats: 28%, 72%, and 84%; female rats: 28%, 62%,
and 88%; male mice: 8%, 20%, and 48%; and female mice: 32%, 14%,
and 34%.
Significant increases were noted in the incidence of
subcutaneous fibromas and fibrosarcomas (combined) in male and
female rats; pancreatic acinar-cell adenomas in male rats;
pancreatic islet-cell adenomas, neoplastic nodules of the liver,
and mammary gland fibroadenomas in female rats; haemangiomas and
haemangiosarcomas (combined), and hepatocellular adenomas in
female mice (NTP, 1986). It was concluded that the 80:20 mixture
of 2,4-, 2,6-TDI in corn oil was carcinogenic for male and female
rats and female mice, when administered orally by gavage. The 1986
NTP report was reviewed during its preparation by Rampy et al.
(1983).
8.6.2. Inhalation
In a long-term inhalation study, Sprague Dawley CD rats and
CD-1 mice were exposed to the 80:20 isomer mixture at nominal
levels of 0.356 mg/m3 (0.05 ppm) or 1.068 mg/m3 (0.15 ppm), for
6 h/day, 5 days per week, for 108 weeks (female rats), 110 weeks
(male rats), or 104 weeks (male and female mice) (Loeser, 1983).
The type and incidence of tumours and the number of tumour-bearing
animals of either species did not indicate any carcinogenic effect.
The main pathological changes in mice occurred in the nasal cavity
and included dose-related incidences of epithelial atrophy, mucuous
and squamous metaplasia, inflammation, and focal destructive
rhinitis with debris.
In this study, there was an unexplained high mortality in both
the control and treated rats and mice. In the high-exposure groups
(1.068 mg/m3), significantly lower weight gains were noted
throughout the study in mice and during the first 12 weeks in rats.
Haematological indices, clinical chemistry, and urinalyses were not
affected by the doses of 80:20 mixture used, nor were there dose-
related changes in organ weights. It was tentatively concluded
that, under these experimental conditions, the 80:20 mixture at
0.356 or 1.068 mg/m3 did not lead to a carcinogenic response or to
other adverse clinical responses. Although some reductions in
weight gain were noted, the doses used were probably below maximal
tolerated doses. In addition, the high mortality rate reported
reduced the sensitivity of this bioassay.
8.7. Special Studies and Mechanisms of Toxicity
On the basis of the possible reactions to toluene diisocyanates
(section 7.5), it is most likely that covalent binding and slow
recovery could follow reaction of toluene diisocyanates with
hydroxyl and amino groups of receptor proteins in the nasal
mucosa. Under the conditions proposed by Brown & Wold (1971), it
is possible that only the first "reversible noncovalent" complex
may have been formed after short durations of exposure to toluene
diisocyanates, since recovery was rapid (Sangha & Alarie, 1979)
(section 8.2.1.2). With longer exposure, the slow recovery would
be due to the formation of the covalent irreversible complex (Brown
& Wold, 1971).
In a subsequent study, McKay & Brooks (1984) reported a
significant difference in carbachol-stimulated tracheal smooth
muscle strips from guinea-pigs exposed to toluene diisocyanates
(0.02 mg/m3, 5 h/day, for 20 days) compared with controls. The
observed increase in maximal tension and the shift of the dose-
effect curve for exposed animals suggested a direct effect of
toluene diisocyanates on tracheal smooth muscle. The toluene
diisocyanate tested had an isomer content of 97.8% 2,4-TDI and 2.2%
2,6-TDI.
Kido et al. (1983a) measured histamine release from the
leukocytes of guinea-pigs exposed to a toluene diisocyanate level
of less than 7 mg/m3 (1 ppm), to study the mechanism of induction
of asthma by toluene diisocyanates. In guinea-pigs with IgE
antibody, histamine release was > 20% with an average peak value
(apv) of 40% compared with < 20% and an apv of 8.8% in controls,
and a histamine release of approximately 20% with an apv of 24.1%
in guinea-pigs without elevated IgE antibody levels.
The authors hypothesized that, after exposure to toluene
diisocyanates, the IgE antibody, which is homocytotropic to
basophils, resulted in histamine release in vitro by the TDI-human
serum-albumin (TDI-HSA) conjugated antigen, and that it is possible
that an immediate-type allergic reaction by the IgE antibody is
involved in the mechanism of induction of TDI-asthma.
Studies by Chen & Bernstein (1982) have shown the presence of
hapten-specific IgE antibodies in the sera of guinea-pigs immunized
with either toluene-diisocyanate-human serum-albumin or
hexamethylene diisocyanate-human serum-albumin or hexamethylene
diisocyanate-human serum-albumin and subsequently challenged with
conjugates of the respective ligands coupled to transferrin. In
these studies, both homocytotropic (IgG and IgE), and precipitating
antibodies were produced under appropriate conditions of parenteral
immunization. The authors postulated that the complex nature of
the immune response generated by diisocyanate compounds in guinea-
pigs might also serve as an appropriate model for isocyanate-
induced human sensitivity reactions, which are known to involve
diverse immunological and nonimmunological mechanisms.
Tanaka et al. (1984) induced nasal allergy in guinea-pigs by
painting a 10% solution of toluene diisocyanates in ethyl acetate
on the bilateral nasal vestibules, once a day, for 5 days. After
waiting 3 weeks, the animals were challenged in a similar manner
with a 5% toluene diisocyanates solution; the process was repeated
2 times per week, for 3 months. Sneezing and rhinorrhea occurred
in guinea-pigs, either with or without dyspnoea, and many
eosinophils were found in nasal smears. Histopathology indicated
enhanced secretory function, eosinophil infiltration, and probable
degranulation of mast cells in the nasal mucosa. A significant
release of histamine from the nasal mucosa in TDI-sensitized
guinea-pigs was noted, when stimulated in vitro by TDI-guinea-pig
serum-albumin.
9. EFFECTS ON MAN
9.1. General Population Exposure - Controlled Human Studies
9.1.1. Single exposures
In human volunteers, eye and nose irritation began at acute
concentrations of 0.35 - 0.92 mg/m3, while skin irritation
generally occurred at higher levels (Brugsch & Elkins, 1963;
Bruckner et al., 1968; Sittig, 1981; Woolrich, 1982). The odour
threshold for aerosols of toluene diisocyanates was tested in human
volunteers by several investigators (Munn, 1960; Henschler et al.,
1962; Brugsch & Elkins, 1963). The responses were not uniform,
probably because of differences in chemical purity, protocol, etc.
Ehrlicher's group reported the following: slight odour = 0.92 mg/m3
(0.13 ppm); odour without irritation = 4.28 mg/m3 (0.60 ppm);
burning eyes and nose = 13.57 mg/m3 (1.9 ppm); and severe
irritation of eyes and respiratory tract = 27.8 mg/m3 (3.9 ppm).
Henschler et al. (1962) reported values that were about 10 times
lower for the irritation effects of TDI in human exposure. In a
30-min exposure of 6 persons, levels of 0.07 and 0.14 mg/m3 (0.01
and 0.02 ppm) were not perceived, a level of 0.35 mg/m3 (0.05 ppm)
was recognized by everyone, slight irritation of the eye, nose, and
throat occurred at concentrations of between 0.35 and 0.7 mg/m3
(0.05 and 0.1 ppm), secretions in the eye and nose occurred in most
persons at 0.7 mg/m3 (0.1 ppm) and always at 3.5 mg/m3 (0.5 ppm),
and, overall, the irritative effect was greater in response to 2,6-
than to 2,4-TDI. The difference in threshold response can perhaps
be attributed to more accurate analytical procedures used by
Henschler et al. (1962).
Brugsch & Elkins (1963) reported that the minimum concentration
of toluene diisocyanates for irritation was 0.35 - 0.7 mg/m3 (0.05 -
0.1 ppm) and that all subjects were irritated at 3.5 mg/m3 (0.5
ppm).
Odour threshold values varied from 0.35 to 0.92 mg/m3 (0.05 to
0.13 ppm) in these studies.
9.2. Occupational Exposure
9.2.1. Acute toxicity
The signs and symptoms of acute exposure are non-specific and
include: complaints of irritation of the nose and throat,
shortness of breath, choking, coughing, retrosternal discomfort or
pain, and gastrointestinal stress (e.g., nausea, vomiting, and
abdominal pain). The onset of signs and symptoms may be delayed
following exposure, and may persist for several days, months, or
years following removal from the contaminated environment
(Ehrlicher & Pilz, 1956; Walworth & Virchow, 1959; Munn, 1960,
1968; NIOSH, 1978).
Eye contact with toluene diisocyanates (vapour, aerosols, or
liquids) causes mild irritation, characterized by itching and
lachrymation, which may progress to conjunctivitis and
keratoconjunctivitis (Brugsch & Elkins, 1963; Luckenbach & Kieler,
1980). Oculorhinitis may also occur and be delayed by a few hours
(Paggiaro et al., 1985).
Systemic symptoms, which developed after acute occupational
exposure to toluene diisocyanates, have been reported by Axford et
al. (1976) and Le Quesne et al. (1976). These two reports describe
the findings from one accident in which the victims were firemen
involved in both fire-fighting and clean-up operations at a
polyurethane foam factory, where a large quantity of toluene
diisocyanates (4500 litres) had leaked.
Exposed firemen experienced symptoms during and/or after the
fire. Symptomatology included 15 cases of gastrointestinal
distress, 4 of which, though asymptomatic during the fire,
developed gastrointestinal symptoms the following day, 3
experiencing abdominal pain with diarrhoea, while the other
complained of nausea and vomiting. All symptoms eased within 2
days without any apparent long-term effects (following 4 years of
monitoring).
In addition to gastrointestinal symptoms, 23 firemen complained
of neurological symptoms. Five firemen experienced symptoms (i.e.,
euphoria, ataxia, intermittent shaking of the limbs, dizziness, and
loss of consciousness) immediately on exposure. Symptoms such as
headaches, difficulty in concentrating, poor memory, and confusion
persisted for 3 weeks in 14 of the firemen. After 4 years, poor
memory was the most common complaint, followed by personality
change, irritability, or depression, in a total of 13 firemen
(Le Quesne et al., 1976). Interpretation of these findings is
complicated by simultaneous exposure to other toxic components
released during these types of fires.
In an earlier investigation, Hama (1957) reported cold-like
symptoms and nocturnal sweating, without fever, in addition to the
gastrointestinal and neurological symptoms described above.
9.2.2. Effects of short- and long-term occupational exposure -
epidemiological studies
9.2.2.1. Ocular
Luckenbach & Kieler (1980) reported evidence of microcystic
corneal oedema and conjuctival infection in both eyes in a
polyurethane foam worker (40-year-old female). Clouded vision,
decreased visual acuity, and loss of light perception developed
within one week of employment. Both corneas and conjunctivae
returned to normal after 3 days without exposure to the
occupational chemicals. Similar visual effects have been
attributed to some amine catalysts (Belin et al., 1983).
9.2.2.2. Dermal
Skin sensitization on repeated exposure to toluene
diisocyanates may occur. Urticaria, dermatitis, and allergic
contact dermatitis have been reported in workers exposed to toluene
diisocyanates-based photopolymerized resins (Brugsch & Elkins,
1963; Calas et al., 1977). The dermatological symptoms included
skin lesions of an eczematous, and also, of an irritant,
pruriginous and erythaematous nature.
A 21-year-old female developed a rash following direct skin
contact with toluene diisocyanates. The urticaria or maculopapular
lesions occurred primarily over exposed areas, but occasionally
spread to covered areas and lasted for up to 10 days after
exposure. Titers of specific IgE antibodies gradually declined
over the period of observation from a high level of 1050 net cpm
[by radioallergosorbent test (RAST)] to 270 net cpm after
occupational exposure ceased. The lower level corresponds to those
found in non-sensitized toluene diisocyanates workers (Karol et
al., 1978).
9.2.2.3. Respiratory tract
Occupational exposure to toluene diisocyanates has produced a
variety of respiratory effects in workers including irritation of
the upper and lower respiratory tract, an asthma-like sensitization
response, dyspnoea, cyanosis, and pulmonitis and decreases in lung
function (Swensson et al., 1955; Brugsch & Elkins, 1963; Gandevia,
1963; Peters et al., 1968, 1969; Peters, 1970; Gaffuri & Brugnone,
1971; Charles et al., 1976; Wegmen et al., 1977; Burge et al.,
1979; Burge, 1982). Short-term, as well as long-term exposures to
toluene diisocyanates, in some instances at levels < 0.007 mg/m3,
have been reported to result in significant decreases in lung
function (NIOSH, 1978). However, the results of more recent
studies by Musk et al. (1982) failed to support such effects at
levels of isocyanates of the order of 0.007 mg/m3 (0.001 ppm).
Sensitization after a single exposure was not demonstrated by Pepys
(1980). Irritation of the respiratory tract can occur at levels
ranging between 0.712 and 3.560 mg/m3 (Henschler et al., 1962).
The asthmatic response, evident in up to 10% of previously exposed
individuals, may occur at levels of toluene diisocyanates > 0.0356
mg/m3 (Bernstein, 1982). The basis for this response is still
uncertain, but there is evidence supporting either immunological or
pharmacological mechanisms, or a combination of both (Scheel et
al., 1964; Weill et al., 1975; Butcher et al., 1977, 1980;
Cockcroft & Mink, 1979; Chadwick & Cleveland, 1981; NIOSH, 1981;
Bernstein, 1982; Karol, 1983).
Toluene diisocyanate-induced asthma may not be evident until
after many years of exposure (Salvaggio, 1979). However, TDI may
cause immediate, delayed, or biphasic asthmatic reactions (Baur et
al., 1983; NIOSH, 1981). The immediate reaction reaches a peak
within minutes. Late reactions occur from 2 to 8 h after exposure
and may show a recurring pattern. Most affected people have non-
specific bronchial hyperreactivity, as measured by mechanical
intratracheal challenge tests. This reaction may continue for
several years after cessation of exposure implying persisting
asthmatic symptoms. Immunologically sensitized workers can be
identified by means of RAST and skin testing.
A 43-year-old non-smoking molder (female) first exhibited
throat irritation and a non-productive cough after 4 months of
exposure to toluene diisocyanates. Dyspnoea was noted, which
worsened during work-days, resulting finally in an episode that
required emergency treatment. One month later, after cessation of
exposure, the patient was without symptoms, and pulmonary function
had returned to normal. Subsequent symptomatic episodes were
successfully treated with isoproterenol (Smith et al., 1980).
Other symptoms, such as faintness, nausea, vomiting of "foamy"
materials, anxiety, rapid pulse rate, elevated blood pressure,
fever, and cyanosis, were reported by Brugsch & Elkins (1963).
The patient, who was a 62-year-old spray painter, was coating the
inside of a large tank for 7 days without respiratory protection.
An ECG showed hypertrophy of the left ventrical, and a chest
roentgenogram showed prominent bronchovesicular markings and an
enlarged cardiac silhouette. The patient improved and was
discharged after 4 days.
Epidemiological studies of health effects from occupational
exposure to toluene diisocyanates are summarized in Table 8.
9.2.2.4. Cancer epidemiology
No epidemiological studies of mortality or cancer incidence
among workers exposed to toluene diisocyanates were available to
the Task Group.
One case report of adenocarcinoma in a 47-year-old non-smoking
spray-painter has been published. The subject had been exposed to
toluene diisocyanate and 4,4-methylene diisocyanate for 15 years.
The levels of exposure to isocyanates were not reported and neither
were other chemicals to which the subject may have been exposed
(Mortillaro & Schiavon, 1982).
9.2.2.5. Immunotoxicity
Several investigators have detected toluene diisocyanates
sensitization with the lymphocyte transformation test and other
immunoassays, but have been unable to consistently demonstrate
elevated antibody titers (Bruckner et al., 1968; Avery et al.,
1969; Danks et al., 1981; Game, 1982). Studies on the immune
response following exposure to toluene diisocyanates are
summarized in Table 9.
Table 8. Epidemiological studies of health effects from occupational exposure to toluene diisocyanates
---------------------------------------------------------------------------------------------------------
Concentration (mg/m3) Effects Reference
---------------------------------------------------------------------------------------------------------
< 0.213 (0.03 ppm); 38 workers at a polyurethane foam factory; after 1 Peters et al. (1968)
mostly < 0.142 day exposure (Monday), statistically significant
(0.02 ppm) decreases in FVC, FEV1 peak-flow rate, and FEF25-50%;
after 5 days' exposure (Friday), in 34 workers, the
FVC returned to baseline, the FEV1 was still
depressed, and the respiratory flow rates were more
depressed; diurnal variation could not account for
these changes; workers with respiratory symptoms
showed greater decreases in FEV1
0.014 - 0.093 111 workers at a polyurethane foam factory; changes Wegman et al. (1974);
(0.002 - 0.013 ppm) measured in FEV1 between Monday AM and PM of work Peters & Wegman (1975)
day; 51 workers (0.014 mg/m3 exposure): 78 ml
decrease; 43 workers (0.028 mg/m3 exposure): 106 to
112 ml decrease; 17 workers (0.064 - 0.093 mg/m3
exposure): 180 ml decrease
0.072 (0.001 ppm) 107 workers from 2 polyurethane manufacturing plants; Musk et al. (1982)
(time-weighted average) 5-year change in FEV did not exceed that expected for
aging; no significant change in FEV was noted between
Monday AM and PM of work day
0.356 - 0.712 287 workers in 2 TDI plants; lung function (FEV1 and Adams (1975)
FVC) measured annually for 8 years; 180 workers with
no respiratory symptoms: FEV1 and FVC normal; 46
workers with respiratory symptoms and still employed:
only questioned and reported more respiratory effects
than controls; 61 workers with respiratory symptoms
no longer at plant: FEV1 averaged 271 ml and FVC
averaged 269 ml lower than predicted from 608 controls
< 0.01 - > 0.025 57 workers at toluene diisocyanates plant; a dose- Wegman et al. (1977)
(< 0.0015 - > 0.0035 response relationship observed; exposure to < 0.01
ppm) mg/m3 did not affect FEV1; exposure to > 0.025 mg/m3
resulted in decrease in FEV1 of 103 ml/year, which
exceeded expected value by 3 - 4 times; workers
exposed to < 0.01 mg/m3 showed normal 2-year decline
(-12 ml in 2 years); differences in FEV1 not explained
by age, time employed, smoking habits, or lung size
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Concentration Effects Reference
(mg/m3)
---------------------------------------------------------------------------------------------------------
0.0007 - 0.178 223 workers at a new TDI plant; measured for Diem et al. (1982)
(0.0001 - 0.025 ppm) pulmonary function, with 3 or more data points to
(TWA) calculate slope of annual response (during 5-year
exposure); low and high cumulative exposure groups
exposed for 2% and 15% of time, respectively, toluene
diisocyanates exceeding 0.036 mg/m3; significant
effects of smoking on spirometric tests and lung
volumes; after adjustment for pack-years smoking,
the FEV1, %FEV, and FEF25-75% declined more in
"high" exposure group (74 workers) than in "low"
exposure group (149 workers); in non-smokers,
average FEV1 decline was 38 ml/year greater in
high- compared with low-exposure groups; a 24 ml/year
excess average decline attributed to longer exposure
to levels above 0.014 mg/m3
0.007 - > 0.014 145 workers at TDI plant surveyed for lung function Omae (1984)
(0.007 - > 0.002 ppm) 1980 and workers re-examined in 1982; short-term
exposure to > 0.014 mg/m3 occurred in 9.3% of samples
in 1980, and 1.9% in 1982; no dose response in loss of
pulmonary function; 0.007 mg/m3 was not associated
with acute or chronic effects on pulmonary function
---------------------------------------------------------------------------------------------------------
Note: Diem et al. (1982) clarified that the toluene diisocyanates workers were compared according to
cumulative exposure, not concentration categories, and that the low-exposure group's 8-h TWA was
spent at < 0.036 mg TDI/m3 and the high-exposure at > 0.036 mg TDI/m3. There was a mean increase
in FEV1 of 1 ml/year for the low-exposure category and a mean decrease in FEV1 of 1 ml/year for
the high-exposure category. They indicated that, because of variations in daily exposure to
toluene diisocyanates, an average 8-h TLV < 0.036 mg/m3 might be necessary to achieve compliance.
FVC = forced vital capacity.
FEV1 = forced expiratory volume in 1 second.
FEV1/FVC% = ratio of FEV1/FVC x 100.
FEF25-50% and FEF25-75% = forced expiratory flow between 25% and 50% or 25% and 75% of FVE, respectively.
Table 9. Immune response in workers exposed to toluene diisocyanates
---------------------------------------------------------------------------------------------------------
Level (mg/m3) Sample number Immune response References
---------------------------------------------------------------------------------------------------------
0.028 - 0.142 32 workers hypersensitive responses Porter et al. (1975)
correlated with TDI levels
> 0.35 mg/m3; IgG antibodies
present and broncho-constriction
occurred
frequently > 0.14 166 workers; increased pulmonary function normal; Butcher et al. (1977)
incidence of TDI-specific positive skin test and broncho-
IgE antibodies constriction noted
0.142 challenge 23 workers (4 sensitive specific IgE antibodies in 3 out Karol et al. (1978)
to TDI) of 4 sensitive workers;
19 non-sensitized workers had
antibody titers comparable with
controls; high levels to specific
IgE antibodies not correlated
with serum-IgE levels
< 0.175 87 workers respiratory symptoms with Kido et al. (1983b)
reduction in FEV1; tolyl-specific
IgE detected in 2 workers only;
RAST indicated generally low
levels in all sensitized workers
0.119 - 0.147 39 workers Significant elevation of serum- Hobara et al. (1984)
IgE levels in only 4 out of 10
workers exposed for less than 1
year, all having obstructive
impairment of liver function
---------------------------------------------------------------------------------------------------------
The results of these studies indicated that exposure to
low concentrations of toluene diisocyanates (> 0.14 mg/m3)
induced hypersensitivity in a variable and unknown percentage
of the individuals at risk. The duration of exposure to
toluene diisocyanates necessary to induce hypersensitivity was
also highly variable, sometimes occurring immediately or
requiring months or even years of exposure. There has been
some success in evaluating the hypersensitivity to toluene
diisocyanates with RAST assays using the TDI-HSA antigen, but
neither the antibody responses to this specific antigen, nor
the level of IgE antibody were consistently elevated in
individuals with hypersensitivity and asthmatic responses
(Baur, 1983; Belin et al., 1983; Barkman et al., 1984).
Present immunoassay techniques will not detect all susceptible
individuals.
9.2.3. Potential mechanisms of action
Irritation and toxic effects are presumed to stem from the
reactivity of the isocyanate groups (i.e., -NCO) on toluene
diisocyanates and their quasipolymers (Brugsch & Elkins, 1963).
Wegman et al. (1974) demonstrated a dose-response relationship for
these symptoms. Irritation will stimulate mucous-secreting goblet
cells, with a proportional decrease in ciliated epithelial cells.
Impaired clearance and mucal stagnation may result, followed by
epithelial desquamation, submucosal glandular hypertrophy, and
basement membrane thickening as in chronic tracheo-bronchitis
(Braman & Teplitz, 1978).
The mechanisms concerned (immunological or pharmacological) in
the production of asthma and hypersensitivity pneumonitis through
occupational exposure to toluene diisocyanates are still the
subject of debate. The controversy stems from the paradoxical
nature of the sensitivity reactions. Karol et al. (1978) reported
specific IgE antibodies in sera from workers sensitive to toluene
diisocyanates, suggesting an IgE-mediated mechanism for sensitivity
to the compound. It was reported by Thurman et al (1978) that
toluene diisocyanates induced lymphocytes to undergo blastogenesis,
suggesting antigenic stimulation. In many instances, no specific
IgE antibodies against TDI-HSA conjugates were demonstrated
(Gaffuri & Brugnone, 1971; Butcher et al., 1977, 1980). Positive
results were observed in 14 - 19% of toluene diisocyanates
reactors, depending on the method of evaluation (Butcher et al.,
1980; Baur et al., 1983). Smith et al. (1980) also found a worker
sensitized to toluene diisocyanates, but with no IgE antibodies,
leukocyte inhibition factor for isocyanate antigen, or even
bronchial hyperreactivity to methacholine, an important component
of bronchial asthma. Asthma resulting from toluene diisocyanates
exposure appears to be a complex syndrome with several possible
mechanisms of causation, including an IgE mechanism in some
individuals.
Salvaggio (1979) suggests that preexisting asthmatic
conditions, together with partial andrenergic blockage or abnormal
cholinergic receptor activity, may increase bronchial airway
hyperreactivity to irritants, such as toluene diisocyanates. A
significant decrease in erythrocyte-cholinestrase activity was
found in in vitro studies in 70% of a group of 30 workers exposed
to toluene diisocyanates (Brown et al. 1982; Dewair et al., 1983;
Manno & Lotti, 1976).
The results of several in vitro studies showed evidence of
inhibition of elevated levels of intracellular cyclic AMP
production by TDI at doses as low as 6.7 x 10-7 mol (Butcher et
al., 1977; Davies et al., 1977). The explanation for these
responses is unclear.
The controversy over immune-controlled versus pharmaco-
logically mediated response to toluene diisocyanates in sensitized
workers has yet to be resolved.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Exposure to Toluene Diisocyanates
In the USA, it has been estimated that approximately 40 000
workers are involved in the manufacture or processing of toluene
diisocyanates. Occupational exposure levels have been reported to
range from 0.001 to 1 mg/m3.
Figures are not available on the total discharge of unreacted
TDI into the environment. However, releases into the air ranging
from 0.1 to 17.7 mg/m3 have been measured in stack gases emitted
from a plant manufacturing polyurethane foams. It has been
reported that approximately 50 g of TDI are released per tonne
of processed TDI in the manufacture of polyurethane foams, the
manufacture of which consumes about 90% of TDI produced.
As far as the general population is concerned, intake of
toluene diisocyanates, apart from their use in the form of
polyurethane lacquers and paints, is of a very low order, because
of the short persistence of TDI.
10.1.1. Acute and short-term effects
The odour threshold for toluene diisocyanates in human beings
is estimated to range between 0.35 and 0.92 mg/m3 (0.05 and 0.13
ppm).
The lowest levels of TDI associated with acute effects were
reported to be: 0.035 - 0.70 mg/m3, eye and nose irritation,
burning nose and throat, and a choking sensation; 0.70 - 3.5 mg/m3,
a respiratory response of irritation, cough, and chest discomfort.
At higher levels, chemical pneumonitis may be expected.
10.1.2. Health risks of long-term exposure to toluene diisocyanates
Risk of respiratory toxicity from repeated exposure can be
summarized as follows: (a) chronic loss of ventilatory capacity as
measured by forced expiratory volume and forced vital capacity; (b)
immediate and/or delayed asthmatic responses.
In many epidemiological studies, past mean exposures to TDI
have been estimated in an attempt to quantify dose-response
relationships for respiratory ill health. Because of the
uncertainties in the sampling procedures and analytical
measurements used in past industrial health surveys, it is
difficult to be confident about the exact levels at which TDI
causes the above-mentioned health effects. It should be remembered
that fluctuations in the true individual exposure occur and, as
both the size and extent of the intermittent peaks are unknown,
their biological significance cannot be evaluated.
Once individuals are sensitized to toluene diisocyanates, low
concentrations, much below current occupational exposure limits,
can induce asthma. Studies on experimental animal have shown that
skin application of TDI can lead to pulmonary sensitization; thus,
it is prudent to avoid repeated skin contact.
No data were available on the carcinogenic effects of toluene
diisocyanates in human beings.
No carcinogenic effects of TDI were noted in an inhalation
study on rats and mice. However, the 80:20 mixture in corn oil,
administered by gavage, was carcinogenic for male and female rats
and female mice in a dose-related manner. It is considered that
there is sufficient evidence for the carcinogenicity of TDI for
experimental animals.
There is evidence of mutagenicity in two bacterial tests.
10.2. Evaluation of Effects on the Environment
An evaluation of the hazards for non-human targets from
environmental levels of TDI is not possible on the basis of
available data.
10.3. Conclusions and Recommendations
1. There is sufficient knowledge about TDI to classify it as a
very toxic compound by inhalation, and TDI should be treated
as a potential human carcinogen and as a known animal
carcinogen. Consequently, the greatest priority should be
given to safe methods of use, the education, training, and
supervision of operatives, and state enforcement of
legislation by an effective inspectorate. Special attention
should be paid to the prevention and adequate treatment of
unscheduled releases and spills.
2. Additional animal carcinogenicity testing by the inhalation
route should be carried out.
3. Morbidity and mortality studies are required on occupational
groups for whom reliable exposure data are available, to
address the question of cancer and to evaluate the potential
of toluene diisocyanates to cause long-term human health
hazards under current standards of good working practice.
4. Because it is not possible to reach confident conclusions from
the data on the neurotoxicity of TDI, neurophysiological and
behavioural studies should be carried out on asymptomatic
workers, exposed at current hygiene standards.
5. For the foreseeable future, exposed workers require health
monitoring by systematic symptom enquiry and by standardized
measurement of ventilatory function, with subsequent analysis
of trends for individuals and for group mean values.
6. Appropriate sampling strategies together with existing
analytical methods have to be developed and used to obtain
better information about exposure, with special reference to
the detection and characterization of peak values. The
results of these analyses need to be evaluated in special
studies in parallel with careful health studies.
7. Further metabolic studies of a qualitative and quantitative
nature should be carried out with a view to developing methods
of measuring TDI uptake and monitoring exposure.
8. Whether TDI produces sensitization in human beings by
pharmacological or immune mechanisms needs to be elucidated
with a view to determining whether restrictions placed on the
employment of atopic subjects, in areas where TDI is produced
or used, are justified.
9. Studies are required to determine whether TDI has embryo-toxic
and teratogenic properties or has adverse reproductive effects
at current exposure levels.
10. Further environmental studies are required to monitor general
environmental levels of TDI in the neighbourhood of sources
and to collect ecotoxicity data.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1979) evaluated the data on the carcinogenicity of
toluene diisocyanates and found insufficient experimental animal
or human data on which to base an evaluation. An evaluation of
additional data by IARC (1986) led to the conclusion that there is
sufficient evidence for the carcinogenicity of toluene
diisocyanates for experimental animals.
In the absence of adequate case reports or epidemiological
studies, there is insufficient data to assess the carcinogenicity
of toluene diisocyanates for human beings (IARC 1986).
REFERENCES
ADAMS, W.G.F. (1975) Long-term effects on the health of men
engaged in the manufacture of tolylene di-isocyanate. Br. J. ind.
Med., 32: 72-78.
ALARIE, Y. (1973) Sensory irritation of the upper airways by
airborne chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
ALDER, J.F. & ISAAC, C.A. (1981a) Detection of toluene
diisocyanate in air with a coated piezoelectric crystal. Part
1. A study of coating materials. Anal. Chim. Acta, 129:
163-174.
ALDER, J.F. & ISAAC, C.A. (1981b) Detection of toluene
diisocyanate in air with a coated piezoelectric crystal. Part
2. Development of an instrumental method for personal
monitoring. Anal. Chim. Acta, 129: 175-188.
AMES, B.N., KAMMEN, H.O., & YAMASAKI, E. (1975) Hair dyes are
mutagenic: identification of a variety of mutagenic ingredients.
Proc. Natl Acad. Sci. (USA), 72: 2423-2327.
ANDERSEN, M., BINDRUP, M., KIEL, P., LARSEN, H., & MAXILD, J.
(1980) Mutagenic action of isocyanates used in the production
of polyurethanes. Scand. J. Work environ. Health, 6: 221-226.
ANDERSON, D. & STYLES, J.A. (1978). Appendix II. The bacterial
mutation test. Br. J. Cancer, 37: 924-930.
ANON. (1983) Polyurethane markets may be maturing. Chem. Eng.
News, 61(46): 16.
ARAGON, J.J., FELIU, J.E., FRENKEL, R.A., & SOLS, A. (1980)
Permeabilization of animal cells for kinetic studies of
intracellular enzymes: in situ behavior of the glycolytic
enzymes of erythrocytes. Proc. Natl Acad. Sci. (USA), 77(11):
6324-6328.
AUDUNSSON, G. & MATHIASSON, L. (1983) Simultaneous determination
of amines and isocyanates in working atmospheres by gas-liquid
chromatography. J. Chromatogr., 261: 253-264.
AVERY, S.B., STETSON, D.M., PAN, P.M., & MATHEWS, K.P. (1969)
Immunological investigation of individuals with toluene
diisocyanate asthma. Clin. exp. Immunol., 4: 585-596.
AXFORD, A.T., MCKERROW, C.B., JONES, P.A., & LE QUESNE, P.M. (1976)
Accidental exposure to isocyanate fumes in a group of firemen. Br.
J. ind. Med., 33: 65-71.
BAGON, D.A. & HARDY, H.L. (1978) Determination of free monomeric
toluene diisocyanate (TDI) and 4,4'-diisocyanate-diphenylmethant
(MDI) prepolymers, respectively, by high performance liquid
chromatography. J. Chromatogr., 152(2): 560-564.
BARKMAN, H.W., BANKS, D.E., HENDRICK, D.J., JONES, R.N.,
ABDELKADER, H.M., HAMMAD, Y.Y., GLINDMEYER, H.W., & WEILL, H.
(1984) Inhalation challenge testing with 2,4 and 2,6 toluene
diisocyanate isomers. Chest, 86(2): 340.
BAUR, X. (1983) Immunologic cross-reactivity between different
albumin-bound isocyanates. J. Allergy clin. Immunol., 71(2): 197-205.
BAUR, X. & FRUHMANN, G. (1981) Specific IgE antibodies in
patients with isocyanate asthma. Chest, 80: 73-76.
BAUR, X., DEWAIR, M., & FRUHMANN, G. (1983) Detection of
immunologically sensitized isocyanate workers by RAST and
intracutaneous skin tests. J. clin. Immunol., 73: 610.
BEALL, J.R. & ULSAMER, A.G. (1981) Toxicity of volatile organic
compounds present indoors. Bull N.Y. Acad. Med., 57(1): 978-996.
BELIN, L., WASS, U., AUDUNSSON, G., & MATHIASSON, L. (1983)
Amines: possible causative agents in the development of bronchial
hyperreactivity in workers manufacturing poly-urethanes from
isocyanates. Br. J. ind. Med., 40: 251-257.
BENGTSSON, B.-E. & TARKPEA, M. (1983) The acute aquatic toxicity
of some substances carried by ships. Mar. Pollut. Bull., 14(6): 213-214.
BERNSTEIN, I. (1982) Isocyanate-induced pulmonary diseases: a
current perspective. J. Allergy clin. Immunol., 70(1): 25-31.
BRAMAN, S.S. & TEPLITZ, C. (1978) Occupational lung disease.
Primary Care, 5(3): 425-445.
BRANDT, G.H. (1972) Soil stabilizers. In: Goring, C.A.I. &
Hamaker, J.W., ed. Organic chemicals in the soil environment, New
York, Marcel Dekker, pp. 692-693, 719-720.
BROCHHAGEN, F.K. & GRIEVESON, B.M. (1983) Environmental aspects
of isocyanates in water and soil. Cell. Polym., 3: 11-17.
BROWN, W.E. & WOLD, F. (1971) Alkyl isocyanates as active-site
specific inhibitors of chymotrypsim and elastase. Science, 174:
608-610.
BROWN, W.E. & WOLD, F. (1973) Alkyl isocyanates as active-site-
specific reagents for serine proteases. Biochemistry, 12: 828-834.
BROWN, W.E., GREEN, A.H., KAROL, M.H., & ALARIE, Y.C.E (1982)
Inhibition of cholinesterase activity by isocyanates. Toxicol.
appl. Pharmacol., 63: 45-52.
BRUCKNER, H.C., AVERY, S.B., STETSON, D.M., & DODSON, V.N. (1968)
Clinical and immunologic appraisal of workers exposed to
diisocyanates. Arch. environ. Health, 16: 619-625.
BRUGSCH, H.G. & ELKINS, H.B. (1963) Toluene diisocyanate (TDI)
toxicity. New Engl. J. Med., 268(7): 353-357.
BUCKLEY, L.A., JIANG, X.Z., JAMES, K.T., MORGAN, K.T., & BARROW,
C.S. (1984) Respiratory tract leisons induced by sensory
irritants at the RD50 concentration. Toxicol. appl. Pharmacol., 74:
417-429.
BUNGE, W., EHRLICHER, H., & KIMMERLE, G. (1977) Medical aspects of
work with surface coating systems using the spraying technique. Z.
Arbeitsmed. Arbeitsschutz Prophyl., 4(special ed.): 1-46
BURGE, P.S. (1982) Non-specific bronchial hyper-reactivity in
workers exposed to toluene di-isocyanate, diphenyl methane
diisocyanate and colophony. Eur. J. respir. Dis., 63(123): 91-96.
BURGE, P.S., O'BRIEN, I.M., & HARRIES, M.G. (1979) Peak flow rate
records in the diagnosis of occupational asthma due to isocyanates.
Thorax, 34: 317-323.
BUTCHER, B.T., JONES, R.N., O'NEIL, C.E., GLINDMEYER, H.W., DIEM,
J.E., DHARMARAJAN, V., WEILL, H., & SALVAGGIO, J.E. (1977)
Longitudinal study of workers employed in the manufacture of
toluene-diisocyanate. Am. Rev. respir. Dis., 116: 411-421.
BUTCHER, B.T., KARR, R.M., O'NEIL, C.E., WILSON, M.R., DHARMARAJAN,
V., SALVAGGIO, J.E., & WEILL, H. (1979) Inhalation challenge and
pharmacologic studies of toluene diisocyanate (TDI)-sensitive
workers. J. Allergy clin. Immunol., 64(2): 146-152.
BUTCHER, B.T., O'NEIL, C.E., REED, M.A., & SALVAGGIO, J.E. (1980)
Radioallergosorbent testing of toluene diisocyanate-reactive
individuals using p-tolyl isocyanate antigen. J. Allergy clin.
Immunol., 66(3): 213-216.
CALAS, E., CASTELAIN, P.Y., LAPOINTE, H.R., DUCOS, P., CAVELIER,
C., DUPRAT, P., & POITOU, P. (1977) Allergic contact dermatitis to
a photopolymerizable resin used in printing. Contact Dermat., 3(4):
186-194.
CHADWICK, D.H. & CLEVELAND, T.H. (1981) Isocyanates, organic. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed., New York,
John Wiley and Sons, Vol. 13, pp. 789-818.
CHARLES, J., BERNSTEIN, A., JONES, B., JONES, D.J., EDWARDS, J.H.,
SEAL, R.M.E., & SEATON, A. (1976) Hypersensitivity pneumonitis
after exposure to isocyanates. Thorax, 31: 127-136.
CHEN, S.E. & BERNSTEIN, I.L. (1982) The guinea-pig model of
diisocyanate sensitization immunologic studies. J. Allergy clin.
Immunol., 70(5): 383-392.
COCKCROFT, D.W. & MINK, J.T. (1979) Isocyanate-induced asthma in
an automobile spray painter. Can. Med. Assoc. J., 121(5): 602-604.
CONTE, A. & COSSI, G. (1981) Gas chromatographic determination of
free toluene diisocyanate in flexible urethane foams. J.
Chromatogr., 213: 162-165.
CURTIS, M.W., COPELAND, T.L., & WARD, C.H. (1979) Acute toxicity
of 12 industrial chemicals to freshwater and saltwater organisms.
Water Res., 13: 137-141.
DANKS, J.M., CROMWELL, O., BUCKINGHAM, J., NEWMAN-TAYLOR, A., &
DAVIES, R. (1981) Toluene-diisocyanate induced asthma: evaluation
of antibodies in the serum of affected workers against a tolyl
mono-isocyanate protein conjugate. Clin. Allergy, 11: 161-168.
DAVEY, J.E. & EDWARDS, A.D. (1983) Determination of toluene-2,4-
diisocyanate in rubber fumes. Analyst, 108: 407-411.
DAVIES, R.J., BUTCHER, B.T., O'NEIL, C.E., & SALVAGGIO, J.E.
(1977) The in vitro effects of toluene diisocyanate on lymphocyte
cyclic adenosine monophosphate production by isoproterenol,
prostaglandin, and histamine. J. Allergy clin. Immunol., 60(4): 223-229.
DE PASCALE, A., COBELLI, L., PALADINO, R., PASTORELLO, L., &
FRIGERIO, A. (1983) Quantitative determination of airborne 2,4-
toluene diisocyanate. J. Chromatogr., 256: 352-358.
DEWAIR, M., BAUR, X., & MAUERMAYER, R. (1983) Inhibition of
acetylcholinesterase by diisocyanates and its spontaneous
reactivation. Int. Arch. occup. environ. Health, 52: 257-261.
DHARMARAJAN, V., WEIL, H., & SELF, C.W. (1978) Environmental
characterization of toluene diisocyanate (TDI) in a manufacturing
plant. Am. Ind. Hyg. Assoc. J., 39(5): 414-418.
DIEM, J.E., JONES, R.N., HENDRICK, D.J., GLINDMEYER, H.W.,
DHARMARAIAN, V., BUTCHER, B.T., SALVAGGIO, J.E., & WEILL, H.
(1982) Five-year longitudinal study of workers employed in a new
toluene diisocyanate manufacturing plant. Am. Rev. respir.
Dis., 126(3): 426-428.
DUFF, P.B. (1983) The fate of TDI in the environment. In:
Proceedings of the Society of the Plastic Industry's 6th
International Technical Conference, San Diego, California, New
York, Society of Plastics Industry, pp. 408-412.
DUNCAN, B., SCHEEL, L.D., FAIRCHILD, E.J., RILLENS, R., & GRAHAM,
S. (1962) Toluene diisocyanate inhalation toxicity: Pathology and
mortality. Am. Ind. Hyg. Assoc. J., 23: 447-456.
DUNLAP, K.L., SANDRIDGE, R.L., & KELLER, J. (1976) Determination of
isocyanates in working atmospheres by high speed liquid
chromatography. Anal. Chem., 48: 497-499.
DUPRAT, P., GRADISKI, B., & MARIGNAC, B. (1976) Pouvoir irritant
et allergisant de deux isocyanates: toluène diisocyanate (TDI)
and diphénylméthane diisocyanate (MDI). Eur. J. Toxicol., 9(1): 41-53.
DYSON, W.L. & HERMANN, E.R. (1971) Reduction of atmospheric
toluene diisocyanate by water vapor. Am. Ind. Hyg. Assoc. J., 32(1):
741-744.
FIELDEN, P.R., MCCALLUM, J.J., STANIOS, T., & ALDER, J.F. (1984)
Detection of toluene diisocyanate with a coated quartz
piezoelectric crystal. Anal. Chim. Acta, 162: 85-96.
FRIEBEL, H. & LUCHTRALH H. (1955) [The effect of toluene
diisocyanate (Desmodur T) on the respiratory airways.] Arch. exp.
Pathol. Pharmakol., 277: 93-110 (in German).
FRY, A. (1953) A tracer study of the reaction of isocyanates with
carboxylic acids. J. Am. Chem. Soc., 75: 2686-2688.
GAFFURI, E. & BRUGNONE, F. (1971) [Respiratory troubles from
isocyanates in varnishers.] Med. Lav., 62(2-3): 151-157 (in Italian).
GAME, C.J.A. (1982) Australian TDI workers' sera assayed for IgE
against a p-tolyl-isocyanate-human serum albumin conjugate. Am.
Ind. Hyg. Assoc. J., 43(10): 759-763.
GANDEVIA, B. (1963) Studies of ventilatory capacity and histamine
response during exposure to isocyanate vapour in polyurethane foam
manufacture. Br. J. ind. Med., 20: 204-209.
GRANATEK, C.H., CORRY, P.M., & GOLKIN, E.M. (1975) Use of
fluorescent x-ray spectroscopy to quantitate binding of anti-
embryonic antibody to human tumor cells. AACR Abstr., 16: 47.
GRANT, W.A. (1974) Toxicology of the eye, 2nd ed., Springfield,
Illinois, Charles C. Thomas, p. 1028.
GRIEVESON, B.M. & REEVE, B. (1983) Isocyanate emissions: a review
of work on environmental aspects of handling toluene diisocyanate.
Cell. Polym., 2: 165-175.
GRIM, K.E. & LINCH, A.L. (1964) Recent isocyanate-in-air analysis
studies. Ind. Hyg. J., May-June: 285-290.
HAMA, G.M. (1957) Symptoms in workers exposed to isocyanates:
suggested exposure concentrations. Am. Med. Assoc. Arch. Ind.
Health, 16: 232-233.
HARDY, H.L. & PURNELL, C.J. (1978) Use of foam for the emergency
suppression of vapour emissions from organic isocyanate liquid
surfaces. Ann. occup. Hyg., 21: 95-98.
HARTON, E.E., Jr & RAWL, R.R. (1976) Toxicological and skin
corrosion testing of selected hazardous materials, Springfield,
Virginia, US Department of Commerce (Final report NTIS Publication
264 975).
HARTUNG, R. (1982) Cyanides and nitriles. In: Clayton, G.D. &
Clayton, I.I., ed. Patty's industrial hygiene and toxicology, 3rd
revised ed., New York, John Wiley and Sons, pp. 4845-4850,
4892-4893, 4898-4900.
HENSCHLER, D., ASSMANN, W., & MEYER, K.-O. (1962) [Toxicology of
toluene diisocyanate.] Arch. Toxikol., 19: 364-387 (in German).
HOBARA, T., KOBAYASHI, H., HIGASHIHARA, E., KAWAMOTO, T., IWAMOTO,
S., SHIMAZU, W., & SAKAI, T. (1984) Health hazard by exposure to
toluene-diisocyanate in the shipyard. Bull. environ. Contam.
Toxicol., 32: 134-139.
HOLDREN, M.W., SPICER, C.W., & RIGGIN, R.M. (1984) Gas phase
reaction of toluene diisocyanate with water vapor. Am. Ind. Hyg.
Assoc. J., 45(9): 626-633.
HOSEIN, H.R. & FARKAS, S. (1981) Risk associated with the spray
application of polyurethane foam. Am. Ind. Hyg. Assoc. J., 42(9):
663-665.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein, Lyons, International Agency for Research on Cancer,
p. 303 (Monographs on The Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 19).
IARC (1986) Toluene diisocyanate. In: Some chemicals used in
plastics and elastomers, Lyons, International Agency for Research
on Cancer, pp. 287-323 (Monographs on The Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 39).
III (1980) Technical information: recommendations for the
handling of toluene diisocyanate (TDI), New York, International
Isocyanate Institute Inc.
KAROL, M.H. (1983) Concentration-dependent immunologic response
to toluene diisocyanate (TDI) following inhalation exposure.
Toxicol. appl. Pharmacol., 68: 229-241.
KAROL, M.H., IOSET, H.H., & ALARIE, Y.C. (1978) Tolyl-specific
IgE antibodies in workers with hypersensitivity to toluene
diisocyanate. Am. Ind. Hyg. Assoc. J., 39: 454-458.
KAROL, M.H., DIXON, C., BRADY, M., & ALARIE, Y.C. (1980)
Immunologic sensitization and pulmonary hypersensitivity by
repeated inhalation of aromatic isocyanates. Toxicol. appl.
Pharmacol., 53: 260-270.
KAROL, M.H., HAUTH, B.A., RILEY, E.J., & MAGRENI, C.M. (1981)
Dermal contact with toluene diisocyanate (TDI) produces respiratory
tract hypersensitivity in guinea-pigs. Toxicol. appl. Pharmacol.,
58: 221-230.
KIDO, T., YAMADA, Y., & TERANISHI, H. (1983a) [Studies on toluene
diisocyanate asthma. Part 2. Histamine release from guinea pig
leukocytes by toluene diisocyanate.] Jpn. J. Allergol., 32: 131-137 (in
Japanese).
KIDO, T., YAMADA, Y., TERANISHI, H., DAIMON, N., & SHIMIZU, T.
(1983b) [Studies on toluene diisocyanate asthma. I. Clinico-
allergological Tests.] Jpn. J. Allergol., 32: 111-120 (in Japanese).
KOSCHIER, F.J., BURDEN, E.J., BRUNKHORST, C.S., & FRIEDMAN, M.A.
(1983) Concentration-dependent elicitation of dermal sensitization
in guinea pigs treated with 2,4-toluene diisocyanate. Toxicol.
appl. Pharmacol., 67: 401-407.
LE QUESNE, P.M., AXFORD, A.T., MCKERROW, C.B., & PARRY JONES, A.
(1976) Neurological complications after a single severe exposure
to toluene diisocyanates. Br. J. ind. Med., 33: 72-78.
LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROM, G. (1979)
The acute toxicity of 78 chemicals and pesticide formulations
against 2 brackish water organisms the Bleak (Alburnus alburnus)
and the Harpacticoid (Nitocra spinipes). Chemosphere, 8: 843-851.
LOESER, E. (1983) Long-term toxicity and carcinogenicity studies
with 2,4/2,6-toluene-diisocyanate (80/20) in rats and mice.
Toxicol. Lett., 15: 71-81.
LUCKENBACH, M. & KIELER, R. (1980) Toxic corneal epithelial edema
from exposure to high atmospheric concentration of toluene
diisocyanates. Am. J. Ophthalmol., 90: 682-686.
MCFADYEN, P. (1976) Determination of free toluene diisocyanate in
polyurethane prepolymers by high-performance liquid chromatography.
J. Chromatogr., 123: 468-473.
MACKAY, D. (1979) Finding fungacity feasible. Environ. Sci.
Technol., 13(10): 1218-1223.
MCKAY, R.T. & BROOKS, S.M. (1983) Induction of tracheal smooth-
muscle hyperresponsiveness to carbachol following exposure to
toluene diisocyanate (TDI) vapors. Am. Rev. respir. Dis., 127(4): 174.
MANNO, M. & LOTTI, M. (1976) Cholinesterases in human toluene
diisocyanate exposure. Int. Arch. occup. environ. Health, 38: 55-60.
MARCALI, K. (1957) Microdetermination of toluene diisocyanates in
atmosphere. Anal. Chem., 29: 552-558.
MARTENS, R. & DOMSCH, K.H. (1981) Microbial degradation of
polyurethane foams and isocyanate based polyureas in different
media. Water Air Soil Pollut., 15: 503-509.
MAZUR, G., BAUR, X., PFALLER, A., & ROMMELT, H. (1986)
Determination of toluene diisocyanate in air by HPLC on band-tape
monitors. Int. Arch. occup. environ. Health, 58: 269-276.
MEDDLE, D.W. & WOOD, R. (1970) A method for the determination of
aromatic isocyanates in air in the presence of primary aromatic
amines. Analyst, 95: 402-407.
MEYER, S.D. & TALLMAN, D.E. (1983) The determination of toluene
diisocyanate in air by high-performance liquid chromatography with
electrochemical detection. Anal. Chim. Acta, 146: 227-236.
MORTILLARO, P.T. & SCHIAVON, M. (1982) [One case of lung cancer
that developed in the course of a bronchopulmonary disease due to
isocyanates.] Med. Lav., 3: 207-209 (in Italian).
MUNN, A. (1960) Experiences with diisocyanates. Trans. Assoc.
Ind. Med. Off., 9: 134-138.
MUNN, A. (1968) Health hazards from isocyanates. Adv.
Polyurethane Technol., 16: 299-306.
MUSK, A.W., PETERS, J.M., DEBERARDINIS, L., & MURPHY, R.L.H.
(1982) Absence of respiratory effects in subjects exposed to low
concentrations of TDI and MDI. J. occup. Med., 24(10): 746-749.
NIOSH (1978) Criteria for a recommended standard occupational
exposure to diisocyanates, Rockville, Maryland, US National
Institute for Occupational Safety and Health (NIOSH 78-215;
PB 81-226615).
NIOSH (1981) Respiratory and immunologic evaluation of isocyanate
exposure in a new manufacturing plant, Washington DC, US National
Institute of Occupational Safety and Health, Department of Health
and Human Services, (DHHS (NIOSH) Publication No. 81-125).
NTP (1985) Disposition of 2,6-toluene diisocyanate in fischer 344
rats, Research Triangle Park, North Carolina, US National
Toxicology Program (Project Report No. 7; Contract No. N01-ES-1-
5007, RTI/2227/00-06P).
NTP (1986) Toxicology and carcinogenesis studies of commercial
grade 2,4(80%)- and 2,6(20%)-toluene diisocyanate (CAS No. 26471-
62-5) in F344/N rats and B6C3F1 mice (gavage studies), Research
Triangle Park, North Carolina, US National Toxicology Program
(Technical Report No. 251; NIH Publication No. 86-2507).
OHSAWA, T. (1983) [Experimental studies of the biological effects
on TDI: an inhalation study in skin-sensitized mice.] J. Tokyo
Women Med. Coll., 53(3): 237-246 (in Japanese).
OMAE, K. (1984) Two-year observation of pulmonary function in
workers exposed to low concentrations of toluene diisocyanate. Int.
Arch. occup. environ. Health, 55: 1-12.
OZAWA, H. (1967) Bridging reagent for protein. I. The reaction of
diisocyanates with lysine and enzyme protein. J. Biochem., 62(4):
419-423.
PAGGIARO, P.L., ROSSI, O., LASTRUCCI, L., PARDI, F., PEZZINI, A., &
BUSCHIERI, L. (1985) TDI-induced oculorhinitis and bronchial
asthma. J. occup. Med., 27: 51-52.
PATTERSON, R., ZEISS, C.R., & HARRIS, K.E. (1983) Immunologic and
respiratory responses to airway challenges of dogs with toluene
diisocyanate. J. Allergy clin. Immunol., 71: 604-611.
PEPYS, J. (1980) Occupational asthm: a review of present clinical
and immunologic status. J. Allergy clin. Immunol., 66: 179-185.
PESCHEL, H. (1970) [Skin alterations by isocyanate (Desmodur).]
Derm. Mschr., 156: 691-697 (in German).
PETERS, J.M. (1970) Cumulative pulmonary effects in workers
exposed to tolylene (sic) diisocyanate. Proc. R. Soc. Med., 63:
372-375.
PETERS, J.M. & MURPHY, R.L.H. (1971) Hazards to health: do it
yourself polyurethane foam. Am. Rev. respir. Dis., 104: 432-433.
PETERS, J.M. & WEGMAN, D.H. (1975) Epidemiology of toluene
diisocyanate (TDI)-induced respiratory disease. Environ. Health
Perspect., 11: 97-100.
PETERS, J.M., MURPHY, R.L.H., PAGNOTTO, L.D., & VAN GANSE, W.F.
(1968) Acute respiratory effects in workers exposed to low levels
of toluene diisocyanate (TDI). Arch. environ. Health, 16: 642-647.
PETERS, J.M., MURPHY, R.L.H., & FERRIS, B.G. (1969) Ventilatory
function in workers exposed to low levels of toluene diisocyanate
(TDI). Br. J. ind. Med., 26: 115-120.
POLLOCK, J.R.A. & STEVENS, R. (1974) Dictionary of organic
compounds, 4th ed., New York, Oxford University Press, Suppl. 10,
p. 337.
PORTER, C., HIGGINS, R.L., & SCHEEL, L.D. (1975) A retrospective
study of clinical, physiologic and immunologic changes in workers
exposed to toluene diisocyanate. Am. Ind. Hyg. Assoc. J., 36: 159-163.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J., ANDERSON,
D., LEFEVRE, P., & WESTWOOD, F. (1978) An evaluation of six
short-term tests for detecting organic chemical carcinogens. Br. J.
Cancer, 37(6): 873-959.
RAMPY, L.W., LOESER, E., LYON, J.P., & CARNEY, I. (1983) Two
carcinogenicity studies of toluene diisocyanate. In: Proceedings of
the Society of the Plastic Industry's 6th International Technical
Conference, San Diego, California, New York, Society of Plastics
Industry.
RANDO, R.J. & HAMMAD, Y.Y. (1985) Modified Marcali method for the
determination of total toluene diisocyanate in air. Am. Ind. Hyg.
Assoc. J., 46(4): 206-210.
REILLY, D.A. (1968) A test-paper method for the determination of
tolylene diisocyanate vapour in air. Analyst, 93: 178-185.
ROSENBERG, C. (1984) Direct determination of isocyanates and
amines as degradation products in the industrial production of
polyurethane-coated wire. Analyst, 109: 859-866.
ROSENBERG, C. & SAVOLAINEN, H. (1985) Detection of urinary amine
metabolites in toluene diisocyanate exposed rats. J. Chromatogr.,
323: 429-433.
ROSENBERG, C. & SAVOLAINEN, H. (1986) Determination of
occupational exposure to toluene diisocyanate by biological
monitoring. J. Chromatogr., 367: 385-392.
SALVAGGIO, J.E. (1979) Occupational asthma: overview of
mechanisms. J. Allergy clin. Immunol., 64: 646-649.
SANGHA, G.K. & ALARIE, Y. (1979) Sensory irritation by toluene
diisocyanate in single and repeated exposures. Toxicol. appl.
Pharmacol., 50: 533-547.
SANGO, C. & ZIMERSON, E. (1980) A new reagent for determination
of isocyanates in working atmosphere by HPLC using UV or
fluorescence detection. J. liq. Chromatogr., 3(7): 971-990.
SCHAFER, E.W., Jr, BOWLES, W.A., Jr, & HURLBUT, J. (1983) The
acute oral toxicity, repellency, and hazard potential of 998
chemicals to one or more species of wild & domestic birds. Arch.
environ. Contam. Toxicol., 12: 355-382.
SCHEEL, L.D., KILLENS, R., & JOSEPHSON, A. (1964) Immuno-
chemical aspects of toluene diisocyanate (TDI) toxicity. Am. Ind.
Hyg. Assoc. J., 25: 179-184.
SHARONOVA, Z.V. & KRYZHANOVSKAYA, N.A. (1976) [Tolylene
diisocyanate induced occupational liver pathology.] Gig. Tr. prof.
Zabol., 11: 27-31 (in Russian).
SHARONOVA, Z.V., PENKNOVICH, A.A., DOROFEEVA, E.D., KRYZHANOV-
SKAYA, N.A., MELNIKOVA, N.D., VOLKOVA, I.D., GOLOVA, I.A.,
ARZYAEVA, E.YA., KLIMOVA, E.I., & MAZE, E.N. (1982) [Changes in
the cardiovascular system of workers engaged in the manufacture of
toluyene diisocyanate.] Gig. Tr. prof. Zabol., 1: 16-19 (in Russian).
SITTIG, M. (1979) Isocyanates. In: Hazardous and toxic effects of
industrial chemicals, Park Ridge, New Jersey, Noyes Data
Corporation, pp. 266-277.
SITTIG, M. (1981) Handbook of toxic and hazardous chemicals, Park
Ridge, New Jersey, Noyes Publications, pp. 664-665.
SMITH, A.B., BROOKS, S.M., BLANCHARD, J., BERNSTEIN, I.L.L., &
GALLAGHER, J. (1980) Absense of airway hyperreactivity to
methacholine in a worker sensitized to toluene diisocyanate (TDI).
J. occup. Med., 22: 327-331.
STEINMETZ, P.R., AL-AWQATI, Q., & LAWTON, W.J. (1976) Specialty
rounds. Nephrology rounds. University of Iowa Hospitals: renal
tubular acidosis. Am. J. med. Sci., 271(1): 40-54.
STYLES, J.A. (1978) Appendix III. Mammalian cell transformation
in vitro. Br. J. Cancer, 37: 931-936.
SWENSSON, A., HOLMQUIST, C.-E., & LUNDGREN, K.-D. (1955) Injury
to the respiratory tract by isocyanates used in making lacquers.
Br. J. ind. Med., 12: 50-53.
TANAKA, K.-I. (1979) [On the induction of contact sensitivity by
tolylene diisocyanate (TDI) in mice.] Jpn. J. ind. Health, 21: 456-457
(in Japanese).
TANAKA, K.-I., OKAMOTO, Y., TAKEOKA, A., & INO, T. (1984) [Nasal
allergy observed in an asthma model by toluene diisocyanate.] Jpn.
J. Allergol., 33(4): 199-206 (in Japanese).
TED TSE, C.S. & PESCE, A.J. (1979) Chemical characterization of
isocyanate-protein conjugates. Toxicol. appl. Pharmacol., 51: 39-46.
THURMAN, G.B., SIMMS, B.G., & GOLDSTEIN, A.L. (1978) The effects
of organic compounds used in the manufacture of plastics on the
responsivity of murine and human lymphocytes. Toxicol. appl.
Pharmacol., 44: 617-641.
TUCKER, S.P. & ARNOLD, J.E. (1982) Sampling and determination
of 2,4-Bis (carbonylamino)toluene and 4,4'-bis(carbonylamino)
diphenylmethane in air. Anal. Chem., 54: 1137-1141.
TWE, J. & WOLD, F. (1973) Butyl isocyanate, an active-site-
specific reagent for yeast alcohol dehydrogenase. Biochemistry,
12(3): 381-386.
ULRICH, H. (1983) Urethane polymers. In: Kirk-Othmer encyclopedia
of chemical technology, 3rd ed., New York, John Wiley and Sons,
Vol. 23, pp. 576-608.
US EPA (1984) Toluene diisocyanates (TDI) 584-84-9; 91-08-7;
26471-62-5; 1321-38-6, Washington DC, US Environmental Protection
Agency (Chemical Hazard Information Profile Report).
US ITC (1985) Preliminary report on US production of selected
synthetic organic chemicals. Imports, exports and sales,
Washington DC, US International Trade Commission.
WALKER, R.F. & PINCHES, M.A. (1981) Chemical interference effects
in the measurement of atmospheric toluene diisocyanate
concentrations when sampling with an impregnated paper tape. Am.
Ind. Hyg. Assoc. J., 42: 392-397.
WALWORTH, H.T. & VIRCHOW, W.E. (1959) Industrial hygiene
experiences with toluene diisocyanate. Am. Ind. Hyg. Assoc. J., 20:
205-210.
WARWICK, C.J., BAGON, D.A., & PURNELL, C.J. (1981) Application of
electrochemical detection to the measurement of free monomeric
aromatic and aliphatic isocyanates in air by high-performance
liquid chromatography. Analyst, 106: 676-685.
WEGMAN, D.H., PAGNOTTO, L.D., FINE, L.J., & PETERS, J.M. (1974) A
dose-response relationship in TDI workers. J. occup. Med., 16: 258-260.
WEGMAN, D.H., PETERS, J.M., PAGNOTTO, L.D., & FINE, L.J. (1977)
Chronic pulmonary function loss from exposure to toluene
diisocyanate. Br. J. ind. Med., 34: 195-200.
WEILL, H., SALVAGGIO, J., NEILSON, A., BUTCHER, B., & ZISKIND, M.
(1975) Respiratory effects in toluene diisocyanate manufacture: a
multidisciplinary approach. Environ. Health Perspect., 11: 101-108.
WEILL, H., BUTCHER, B., DIEM, J.E., ET AL. (1979) Respiratory and
immunologic evaluation of isocyanate exposure in a new
manufacturing plant, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (Final Report, NIOSH Contract No.
210-75-006).
WEYEL, D.A., RODNEY, B.S., & ALARIE, Y. (1982) Sensory
irritation, pulmonary irritation, and acute lethality of a
polymeric isocyanate and sensory irritation of 2,6-toluene
diisocyanate. Toxicol. appl. Pharmacol., 64: 423-430.
WHITE, W.G., MORRIS, M.J., SUGDEN, E., & ZAPATA E. (1980)
Isocyanate-induced asthma in a car factory. Lancet, 1(8171): 756-760.
WINDHOLZ, M., ed. (1983) The Merck Index: an encyclopedia of
chemicals and drugs, 10th ed., Rahway, New Jersey, Merck and
Company, p. 1363.
WOOLRICH, P.F. (1982) Toxicology, industrial hygiene and medical
control of TDI, MDI and PMPPI. Am. Ind. Hyg. Assoc. J., 43(1): 89-97.
WOOLRICH, P.F. & RYE, W.A. (1969) Urethanes. Engineering, medical
control and toxicologic consideration. J. occup. Med.,11(4): 184-190.
ZAPP, J.A., Jr (1957) Hazards of isocyanates in polyurethane foam
plastic production. Arch. ind. Health, 15(4): 324-330.
Toluene diisocyanates are produced as 2 isomers (2,4-toluene
diisocyanate (2,4-TDI) and 2,6-toluene diisocyanate (2,6-TDI))
and are commercially available in 3 isomer ratios: (a) > 99.5%
2,4-TDI; (b) 80% 2,4-TDI/20% 2,6-TDI, which is the most common
and referred to in this document as 80:20 mixture; and (c) 65%
2,4-TDI/35% 2,6-TDI. "Crude" toluene diisocyanate (Crude-TDI),
with an unidentified isomer ratio, is also commercially available,
but not widely used. Various identification codes for the most
commonly marketed toluene diisocyanates are listed in Table 1.
Table 1. Identification codes of commercial toluene diisocyanatesa
-------------------------------------------------------------------
Numeric index 2,4-TDI 2,6-TDI Commercial
(80:20)
mixture
-------------------------------------------------------------------
CAS register number 584-84-9 91-08-7 26471-62-5
RTECS access number CZ6300000 CZ6310000 CZ6200000
Wiswesser line notation OCNR Bl ENCO OCNR Bl CNCO -
Shipping ID number UN 2078 - UN 2078
OHM TADS number - 1- 7217313
Hazard substances data
bank number 0874 5272 6003
-------------------------------------------------------------------
a "Crude" toluene diisocyanate (unidentified isomers). CAS No. 1321-
38-6 and chemical abstracts name benzene, diisocyanatomethyl-.
The chemical, common, and trade names for toluene diisocyanates
are listed in Table 2.
Table 2. Toluene diisocyanates: Synonymous and Trade Names
--------------------------------------------------------------------
I. Commercial mixtures: 2,4-, 2,6-isomers
Chemical abstracts name benzene, 1,3-diisocyanatomethyl-
Other chemical names diisocyanatotoluene; isocyanic acid;
methyl- m-phenylene ester; methyl- m-
phenylene isocyanate; methylphenylene
isocyanate; toluene diisocyanate; tolylene
diisocyanate
Common name TDI
Trade names Desmodur T65 (also-T80); Hylene T (also,
-TCPA, -TLC, -TM, -TM65, -TRF); Isocyanic
acid; Lupranat T80; Mondur TD (also,
-TD80, -TDS); Nacconate 100; NCI-C50533;
Niax TDI; Niax TDI-P; Rubinate TDI 80/20;
TDI 80
II. 2,4-TDI
Chemical abstracts name benzene, 2,4-diisocyanato-1-methyl-
Other chemical names di-iso-cyanatoluene; di-isocyanate de
toluylene; diisocyanat-toluol; isocyanic
acid; 4-methyl- m-phenylene ester; tolueen-
diisocyanaat; toluen-disocianato; toluene
diisocyanate; toluene-2,4-diisocyanate;
toluene, 2,4-diisocyanato-;
toluilenodwuizocyjanian; toluylene-2,4-
diisocyanate; tolylene-2,4-diisocyanate;
tolylene diisocyanate;
tuluylendiisocyanat; 2,4-Dicyanato-1-
methyl-phenylene; 2,4-diisocyanato-1-
methylbenzene; 2,4-diisocyanatotoluene;
2,4-toluene diisocyanate; 2,4-toluene
diisocyanate; 4-methyl- m-phenylene
diisocyanate; 4-methyl- m-phenylene
isocyanate; 4-methyl-phenylene
diisocyanate; 4-methyl-phenylene
isocyanate
Common names TDI, 2,4-TDI
Trade names Desmodur T65 (also-T80); Hylene T (also,
-TCPA, -TLC, -TM, -TM65, -TRF); Isocyanic
acid; Lupranat T80; Mondur TD (also,
-TD80, -TDS); Nacconate 100; NCI-C50533;
Niax TDI; Niax TDI-P; Rubinate TDI 80/20;
TDI 80
--------------------------------------------------------------------
Table 2. (contd.)
--------------------------------------------------------------------
III. 2,6-TDI
Chemical abstracts name benzene, 2,6-diisocyanato-1-methy-
Other chemical names benzene, 1,3-diisocyanato-2-methyl-;
isocyanic acid; meta-tolylene diisocyanate;
2-methyl- m-phenylene isocyanate; 2,6-
toluene diisocyanates
Common name 2,6-TDI
Trade names not commercially available
--------------------------------------------------------------------
The isomer or mixture studied is often not reported in the
literature. For the purposes of this document, in such cases,
the chemical will be referred to as toluene diisocyanates. When
identified, the name of the particular isomer or mixture will be
used.
2.2. Physical and Chemical Properties
Toluene diisocyanates are colourless liquids or crystals,
turning pale yellow on standing, and having a characteristic sharp
pungent, sweet, fruity odour. Some of the physical and chemical
properties of toluene diisocyanates are listed in Table 3.
No properties of 2,6-TDI were found in the published
literature, except for a boiling point of 129 - 133 °C at 18 mmHg,
and a specific gravity similar to that of 2,4-TDI (Pollock &
Stevens, 1974).
Toluene diisocyanates are soluble in acetone, ethyl acetate,
ether, benzene, carbon tetrachloride, chlorobenzene, kerosene,
and various oils, e.g., corn oil. They may react violently with
compounds containing active hydrogen, such as alcohols, with the
generation of enough heat to lead to self-ignition and subsequent
release of toxic combustion products. Other such solvents that
must not be mixed with toluene diisocyanates include water, acids,
bases, and strong alkaline materials, such as sodium hydroxide and
tertiary amines, etc.
Toluene diisocyanates react with water and most acids to
produce unstable carbanic acids, which subsequently decarboxylate
(raising the pressure in closed containers) to yield relatively
chemically inert and insoluble polymeric urea (Hardy & Purnell,
1978). According to Holdren et al. (1984), reaction of TDI-vapour
with water vapour does not take place in the gaseous phase. They
concluded that loss due to surface adsorption takes place first,
since no diaminotoluenes or TDI-ureas could be detected in an
environmental chamber. Toluene diisocyanates also react with
(-NH-)-containing compounds to form ureides or ureas. Each
reaction pathway is important in terms of the health hazard
potential associated with toluene diisocyanates, since both
pathways are biologically, as well as commercially, significant,
and occur at room temperature (Chadwick & Cleveland, 1981).
Table 3. Physical and chemical properties of toluene diisocyanates
------------------------------------------------------------------------
Properties 2,4-TDI Commercial mixture
(2,4-, 2,6-isomers)
------------------------------------------------------------------------
Freezing point ( °C) 14 - 20a 11.5 - 13.5 (80:20 mix)
15c 11 - 14 (80:20 mix)
3 - 5 (65:35 mix)b
T80 = 12.5 - 13.5°
T65 = 4.7 - 6
Melting point ( °C) 22b 12.5 - 13.5 (80:20 mix)
4.7 - 6 (65:35 mix)
Boiling Point ( °C)
at 10 mmHg 120b 121c
at 760 mmHg 251c 25l (both mixes)
Flash point ( °C)
open cup 135a 132 (both mixes)
closed cup 127b -
Explosive limits:
Concentration (% v/v)
lower 0.9 0.9
upper 9.5 9.5
Temperature ( °C)
lower - 118
upper - 150
Fire temperature ( °C) - 142
Autoignition temperature 620 620
( °C)
Volatility; vapour pressure 1 mmHg (80 °C)d 1.9 mmHg (94 °C)a
0.01 mmHg (20 °C)
Vapour density (air = 1) 6d 6e
Density (g/cm3)b,c 1.22 25/15 1.22 25/15 (both mixes)
1.2244 20/4c -
Odour threshold 0.36 - 0.92 mg/m3
------------------------------------------------------------------------
a From: Woolrich & Rye (1969).
b From: Chadwick & Cleveland (1981).
c From: Windholz (1983).
d From: Hartung (1982).
e From: NIOSH (1978).
f From: Olin product literature.
Toluene diisocyanates dimerize slowly at ambient temperatures
and more rapidly at elevated temperatures. Trimerization occurs at
100 - 200 °C and, above 175 °C, carbodiimides form with the release
of carbon dioxide (CO2) (Chadwick & Cleveland, 1981; Ulrich, 1983).
2.3. Conversion Factors
At 25 °C and 760 mmHg:
1 mg/m3 = 0.14 ppm in air
1 mg/litre = 140.5 ppm.
2.4. Analytical Methods
The sampling and determination of toluene diisocyanates in air
has been the subject of several studies. The method originally
published by Marcali (1957) has been modified by several
investigators (Grim & Linch, 1964; Meddle & Wood, 1970).
Photometric methods are non-specific and most of them pool all the
isocyanates. Also, most procedures are severely hampered because
other agents, particularly aromatic amines, interfere in a way that
may result in falsely high readings. In contrast, chromatographic
techniques are specific and measure individual isocyanate species
(Table 4).
Most recent analytical methods involve high-performance
liquid chromatography (HPLC) using ultraviolet, fluorescence, or
electrochemical detection (Dunlap et al., 1976; Sango & Zimerson,
1980; Warwick et al., 1981). Improved sampling techniques include
the use of solid adsorbents (Tucker & Arnold, 1982). The Marcali
method has been evaluated for its response for the two isomers of
toluene diisocyanate. The simple modification involving changes in
diazotization time and temperature eliminates the isomeric effect
(Rando & Hammad, 1985). An extension of the spectrophotometric
method is the development of a tape method involving a chemically
impregnated paper tape that changes colour on exposure to toluene
diisocyanates (Reilly, 1968). However, the presence of
diaminotoluenes at concentrations similar to that of the toluene
diisocyanates leads to significant negative interference with the
colour-forming reaction (Walker & Pinches, 1981). Also, at low
humidity, the tape monitor tends to give falsely low readings
(Mazur et al., 1986).
Levels of toluene diisocyanates in consumer products (lacquers)
are usually determined, after appropriate extraction techniques, by
gas chromatography and high-performance liquid chromatographic
methods (McFadyen, 1976; Conte & Cossi, 1981).
In general, it should be understood that, with all analytical
methods, reliable figures for isocyanate concentrations in air
can be obtained only in the range of at least 5 - 10 times the
detection limit.
Table 4. Analytical methods for the determination of toluene diisocyanates
---------------------------------------------------------------------------------------------------------
Purpose/method Detection limit Reference
---------------------------------------------------------------------------------------------------------
I. Detection of TDIs in work-place air
1. Spectrophotometry
TDIs hydrolysed to the corresponding diamines, 0.07 mg 2,4-TDI/m3; Marcali (1957)
diazotized, coupled to N-1-naphthyethylene- field kit, 0.14 mg/m3
diamine, and final colour measured at 550 nm;
Note: as the concentration of 2,6-TDI increases
in the mixture, the total recovery of TDIs is
reduced
a modification of the Marcali method, in an Meddle & Wood (1970)
attempt to circumvent interference by primary
aromatic amines
a further modification to eliminate the Rando & Hammad (1985)
difference in response for the two isomers
2. Gas chromatography
2,4-TDI hydrolysed in dilute hydrochloric acid 0.06 - 0.23 mg/m3 De Pascale et al. (1983)
and subsequent determination by gas-liquid
chromatography/mass fragmentography 2,4-DAT;
instrument detection limit is 500 pg
gas liquid chromatography: TDIs hydrolysed to the 0.004 mg/m3 Audunsson & Mathiasson
corresponding amines in dilute sulfuric acid; (1983)
2,4- and 2,6-TDI detection limit depends on
sample volume
3. High-performance liquid chromatography
TDI sampled in derivatizing absorber ( N-(4-nitro 14 µg/m3 Dunlap et al. (1976)
benzyl)propylamine) and subsequent determination
of the urea derivative formed by UV detection;
sample volume 20 litre at 1 - 2 litre/min
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Purpose/method Detection limit Reference
---------------------------------------------------------------------------------------------------------
3. High-performance liquid chromatography (contd.)
TDI derivatized during sampling with gamma- 0.0001 mg/m3 Sangö & Zimerson (1980)
( N-methylaminomethyl)anthracene and analysis by
fluorescence or UV detection; sample volume
15 litre at 1 litre/min
TDI sampled in absorbed solution containing 0.0002 mg/m3 Warwick et al. (1981)
1-(2-methoxyphenyl) piperazine; the derivative
formed is detected by electrochemical or UV
detection; instrument detection limit 200 pg;
sampling time 10 min at 1 litre/min
TDI detected with electrochemical detection of 5 µg/m3 Meyer & Tallman (1983)
the derivative formed after treatment with
p-aminophenol, instrument detection limit 100 pg;
flow rate of 1 litre/min, for 10 min
Note: formation of multiple derivatives might
lead to somewhat complex chromatograms
TDIs determined as N-(4-nitrobenzyl)propylamine 1 µg/m3 Rosenberg (1984)
derivatives with UV detection using a 10-litre
sample
4. Direct read-out
Tape-monitor using impregnated paper that yields 0.07 mg/m3 Reilly (1968)
colour stain on exposure to isocyanate vapour
TDIs absorbed on a piezoelectric quartz crystal 0.14 mg/m3 Alder & Isaac (1981a,b)
coated with polyethylene glycol (PEG 400); (portable kit);
resulting change in weight of crystal is 0.043 mg/m3
monitored by the associated change in the
oscillation frequency; PEG 400 minimizes the
effect of water vapour
detection of TDIs with a coated quartz piezo- 0.07 mg/m3 Fielden et al. (1984)
electric crystal 76 Hz cps SI
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Purpose/method Detection limit Reference
---------------------------------------------------------------------------------------------------------
II. Determination of free TDIs in flexible foam
1. Infrared spectroscopy
based on the N=C=O stretching vibration; detects Conte & Cossi (1981)
the presence of N=C=O groups independently of
the molecular structure; extraction of unreacted
TDIs in the foam with o-dichlorobenzene and
gas chromatographic determination using a flame
ionization detector; free TDIs present at levels
of about w/w foam in fresh foam (1 h after
production) disappear after 24 h, under all
storage conditions (both ambient and dry air)
---------------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Toluene diisocyanates are not known to occur as natural
products.
3.2. Man-Made Sources
3.2.1. Production levels and processes
Toluene diisocyanates are manufactured by the reaction of
diaminotoluenes with phosgene. The reaction temperature increases
from ambient in the first reactor to about 200 °C in the last
reactor. The isomer mixture is stripped of solvent and separated
by distillation (NIOSH, 1978; IARC, 1979; Chadwick & Cleveland,
1981).
The most widely marketed grade of toluene diisocyanate is the
80:20 mixture of the 2,4- and 2,6-isomers. A mixture of 65:35
of 2,4-; 2,6-isomers is also available. "Pure" 2,4-TDI is
manufactured in small quantities and used for special applications.
The residue product (i.e., "crude"-TDI) is sold as a speciality
isocyanate (Chadwick & Cleveland, 1981; Ulrich, 1983).
3.2.1.1. World production figures
The production of 80:20 mixture accounts for > 90% of
the total toluene diisocyanates produced in USA. In 1983,
approximately 300 x 106 kg were produced in the USA; 29% of the
1983 production was exported to Belgium, Canada, the Federal
Republic of Germany, Japan, Korea, the Netherlands, and other
countries (US ITC, 1985). In the USA, it has been projected that
the domestic demand for toluene diisocyanates will increase at the
rate of 1 - 3% annually, until the year 1990 (Anon., 1983).
Production of toluene diisocyanates in Canada in 1975 amounted
to 9 x 106 kg. In 1982, the annual production capacity for toluene
diisocyanates was 20 x 106 kg for Brazil and 12 x 106 kg for
Mexico. Western European nations (mainly Belgium, France, the
Federal Republic of Germany, Italy, and Spain) reported a combined
annual capacity for the production of toluene diisocyanates in 1982
of > 326 x 106 kg. Production capacity during 1982 within the
Netherlands, Portugal, and the United Kingdom was not reported;
however, production in 1976 in the United Kingdom was 25 x 106 kg.
Seventy percent of the western European production was consumed
nationally and 30% was exported, primarily to eastern Europe, the
Middle East, and North Africa. In eastern Europe, the German
Democratic Republic and Yugoslavia had a combined production
capacity of 47 x 106 kg toluene diisocyanates in 1982. No figures
were available for production within the USSR. Japan reported an
effective annual production capacity of 78 x 106 kg in 1982, and
actual production reached 67 x 106 kg. The global capacity for
the production of toluene diisocyanates in 1982 was reported to
be > 817 x 106 kg (Ulrich, 1983).
3.2.1.2. Manufacturing processes: release into the environment
Toluene diisocyanates are manufactured in a closed system, and
air emission is minimal. However, toluene diisocyanates may be
emitted into the atmosphere during the removal of phosgene and
hydrogen chloride from the first fractionating column. It is the
belief of the Task Group that few of these products are emitted,
because of improved manufacturing facilities. After gas scrubbing,
they may be discharged into the waste effluent (Dyson & Hermann,
1971; Bagon & Hardy, 1978; Dharmarajan et al., 1978).
Levels of toluene diisocyanates ranging from 0.1 to 17.7 mg/m3
have been monitored in stack gases from 3 plants manufacturing
polyurethane foams (Grieveson & Reeve, 1983). It was estimated
that approximately 50 ± 5 g toluene diisocyanates/tonne of TDI
processed within the plant was emitted during the manufacture of
soft-block foams (Grieveson & Reeve, 1983).
3.2.2. Uses
Toluene diisocyanates are reactive intermediates that are used
in combination with polyether and polyester polyols to produce
polyurethane products. The production of flexible polyurethane
foams represents the primary use of toluene diisocyanates (~ 90% of
the total supply). The 80:20 mixture is used in their production
at an average of 30% by weight. Domestic consumption of flexible
polyurethane foam in the USA in 1981, estimated at 499 x 106 kg,
can be broken down into the following uses (in million kg):
furniture (208.7); transportation (99.8); bedding (63.5); carpet
underlay (72.6); and other uses (11.3). An estimated 27 x 106 kg
of rigid polyurethane foams, used in refrigeration equipment, was
produced with "crude"-TDI in the USA in 1982 (US EPA, 1984).
Polyurethane coatings represent the second largest market for
toluene diisocyanates. Toluene diisocyanates are also used in the
production of polyurethane elastomeric casting systems, adhesives,
sealants, and other limited uses (Brandt, 1972; Granatek et al.,
1975; Aragon et al., 1980).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
There are very few studies on the overall environmental fate
of toluene diisocyanates in the published literature. Available
studies have been summarized by Duff (1983). On the basis of this
review and available information on the physical and chemical
properties, the following statements can be supported.
4.1. Air
It has been demonstrated in environmental chambers that, in the
gaseous phase, TDI vapour and water vapour do not react to form
diaminotoluenes, since not even trace amounts of these compounds
were detected (Holdren et al., 1984). A rate of loss of about 20%
of TDI-vapour per hour could be explained by surface adsorption.
This rate of loss was much higher and more rapid when comparable
concentrations of an aliphatic amine were simultaneously present in
the chamber. Again, no hydrolysis products of TDI could be
detected.
4.2. Water
In most industrial situations, toluene diisocyanates are
hydrolysed by water to give the corresponding polymeric ureas and
carbon dioxide (Chadwick & Cleveland, 1981). However, when toluene
diisocyanates come into contact with water without agitation, as in
spills, a hard crystalline crust of polymeric ureas forms slowing
down further degradation of the toluene diisocyanates, unless the
crust is mechanically broken. The solid reaction products are
insoluble and biologically inert (Brochhagen & Grieveson, 1984).
4.3. Soil
A computerized partitioning model proposed by Mackay (1979)
indicated that toluene diisocyanates released into the environment
will tend to partition into water. However, in making this
prediction, the reactivity of the compounds was not taken into
consideration.
4.4. Biotransformation
Studies were conducted under laboratory or environmental
conditions to evaluate the potential degradation of soft
polyurethane foams, with either a polyester or a polyether base,
both prepared with an isomeric mixture of 2,4 and 2,6-diisocyanates
(Martens & Domsch, 1981). Polyurethane-ether foams were highly
resistant to chemical and microbial degradation. Polyurethane-
ester foams were quite susceptible to degradation, especially at
elevated temperatures (50 °C), yielding 0.25% 2,4-toluenediamine
and 0.38% 2,6-toluenediamine in acidic (pH 1) water extracts of
leachate after 3 months incubation in the laboratory. The
incubation mixture contained 1 g of finely chopped soft
polyurethane foam of a polyester base prepared with the isomeric
mixture of 2,4- and 2,6-diisocyanates, in 100 ml of leachate (pH
7.5) from a refuse tip near Braunschweig, Federal Republic of
Germany. An experimental study conducted near this site
corroborated the laboratory results. Soft polyurethane foam cubes
were checked for weight loss after 13 months incubation in the
refuse tip, where they were found in the refuse layer of a 25:10:1
(weight) mixture of municipal refuse, sewage sludge, and caustic
lime. The polyurethane-ester foam cubes lost 17 - 31% of their
initial weight in the stratified filling, and, if the layers of
fill were mixed, weight loss ranged between 35 and 86%. The
polyurethane-ether foam cubes did not degrade under these
conditions. It was concluded that soft polyurethane foams prepared
with toluene diisocyanate isomers are susceptible to chemical
hydrolysis under extreme environmental conditions, and that under
these circumstances, an accumulation of aromatic amines can occur,
if their microbial degradation is impeded.
4.5. Bioaccumulation
There are no data on the bioaccumulation of TDIs.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
No data were found on levels of toluene diisocyanates in the
general environment.
5.1. General Population Exposure
Human beings and animals would be exposed to toluene
diisocyanates in environmental media only in the immediate vicinity
of effluents, factories, or areas of spillage (sections 3.2.1.2 and
4). Consumers may also be exposed to toluene diisocyanates through
the indiscriminate use of several commercially available household
products, such as polyurethane foam kits (US EPA, 1984). For
example, Peters & Murphy (1971) tested 5 "instant" polyurethane
foam products and found that concentrations of toluene
diisocyanates in the air ranged from 0 to 0.15 mg/m3 during
applications. They also noted that labels on the cans were
inadequate regarding contents, precautions, and toxicity warnings.
Consumers may also be exposed to toluene diisocyanates during the
application of polyurethane varnishes (US EPA, 1984).
Beall & Ulsamer (1981) suggested that toluene diisocyanates
might be an indoor air pollutant. During pyrolysis of
polyurethanes, the general public could be exposed to the pyrolytic
products of toluene diisocyanates. No monitoring levels were
given.
5.2. Occupational Exposure
Because of the volatility of toluene diisocyanates, exposure
can occur in all phases of their manufacture and use (Sittig,
1979). Monitoring data for toluene diisocyanates in the work-
place is extensive with levels found between 0.014 and 1.050 mg/m3
(NIOSH, 1978; Hosein & Farkas, 1981; Belin et al., 1983). During
the production of polyurethane-coated wire, toluene diisocyanates
may be found in the work-place, during the different stages of the
coating process, at concentrations ranging between < 0.001 and
0.11 mg/m3 (Rosenberg, 1984). The highest levels have occurred
during spraying with polyurethane foam, a procedure that is usually
conducted in confined spaces (Hosein & Farkas, 1981). Isocyanate
lacquers contain 0.2 - 1% monomeric toluene diisocyanates (Tu &
Fetsch, 1980), and short-term excursions above safe limits are of
a particular concern for spray workers and their assistants.
Sittig (1979) estimated that approximately 40 000 workers in
the USA were potentially exposed to toluene diisocyanates in such
jobs as adhesive production, insulation, application and production
of toluene diisocyanate resins and lacquers; organic chemical
synthesis, paint spraying, polyurethane foam production, working
with rubber, shipbuilding, textile processing, and wire-coating.
Consumer use of products containing TDI could result in many more
cases of exposure.
6. KINETICS AND METABOLISM
6.1. Absorption
Absorption of toluene diisocyanates through the respiratory
tract is suggested by: (a) their high acute toxicity for animals
via inhalation (section 8.1); and (b) reports on systemic effects
and antibody formation in individuals exposed to toluene
diisocyanates primarily via inhalation (Sharonova & Kryzhanovskya,
1976; Steinmetz et al., 1976; White et al., 1980; Sharonova et al.,
1982).
6.2. Distribution
No information was found regarding the distribution of
toluene diisocyanates in mammalian systems. Because of the wide
distribution of water and other nucleophiles in tissues, it is
likely that toluene diisocyanates will react with the tissues they
initially contact and be transformed into various products, rather
than that they will be absorbed and distributed throughout the body
as toluene diisocyanates.
6.3. Metabolic Transformation and Elimination
No published studies on the biotransformation of toluene
diisocyanates were found. However, one report (NTP, 1985) on the
disposition of 2,6-TDI in Fischer 344 rats was available to the
Task Group. The apparent half-life of 2,6-TDI was dependent on
the vehicle in which it was administered, its concentration in the
solvent, and the rate of mixing as the compound was added. In an
aqueous suspension of stomach contents, the half-life of 2,6-TDI
was < 2 min, whereas in serum, the half-life was < 30 seconds.
When [C14]-2,6-TDI was given orally to rats in corn oil, most
of the compound formed polymers in the gastrointestinal tract. At
doses of 900 mg/kg body weight, the insoluble polyureas usually
lined the stomach, slowing down or preventing the migration of
stomach contents into the intestine. At a 60 mg/kg dose level,
these results were not observed (NTP, 1985). Most 2,6-TDI-derived
materials were eliminated in the faeces or were found in the
gastrointestinal tract 72 h after dosing. Approximately 12% of
the low dose (60 mg/kg body weight) and only 5% of the high dose
(900 mg/kg) were excreted in the urine (24-h after treatment),
mainly in the form of 2,6-bis(acetylamino) toluene (54%). Increased
urinary excretion of 2,6-TDI metabolites with decreasing dosage was
consistent with the lower concentration of the compound in the
stomach permitting increasing amounts of the 2,6-TDI to be
hydrolysed completely to monomeric 2,6-diaminotoluene rather than
forming polymers. The 2,6-diaminotoluene could then be absorbed,
acetylated, and excreted in the urine. Materials derived from
2,6-TDI were not concentrated in any tissue (NTP, 1985). In rats
exposed dermally to 2,4-TDI, no unreacted isocyanate was detected
in the urine, but 2,4-TDA was detected after hydrolysis of the
urine (Rosenberg & Savolainen, 1985). The same authors studied
workers occupationally exposed to the 80:20 TDI isomer mixture, and
reported that concentrations of TDA in the urine after hydrolytic
treatment were linearly related to the estimated TDI dose
(Rosenberg & Savolainen, 1986). A possible biochemical pathway
would involve the formation of TDA, its conjugation and excretion,
but it was not known if this had taken place.
6.4. Reaction with Body Components
Toluene diisocyanates are highly reactive towards a large
number of active hydrogen and basic nitrogen compounds (Ozawa,
1967; Alarie, 1973; Brown & Wold, 1973; Brown et al., 1982). Thus,
more than one reaction may occur in a system at a given time. The
results of an in vitro study reported by the NTP (1985) showed that
2,6-TDI would react with both rat serum and stomach contents at
37 °C. The 2,6-TDI appeared to form a polymeric film, which
encapsulated globules of 2,6-TDI, thus limiting the availability of
the compound in the interior of the globules for further reaction.
Mixtures of TDI isomers, such as 80:20, may behave in a
different manner to single 2,6- or 2,4-TDI isomers.
Isocyanates react with carboxyl groups and form amines, acid
anhydrides, and ureas (Fry, 1953). Ozawa (1967) and Brown & Wold
(1973) demonstrated that diisocyanates were active-site-specific
reagents towards the hydroxyl groups of serine in proteases. Such
reactions can result in the irreversible inactivation of enzymes,
such as adenylate cyclase, serine proteases, alcohol dehydrogenase,
and cholinestrase (Brown & Wold, 1973; Twe & Wold, 1973; Butcher et
al., 1979; Dewair et al., 1983). Isocyanates react with amino
groups to form ureas, which are also highly stable and unlikely to
dissociate under biological conditions.
Isocyanates form thiolic acid and esters when reacting with
sulfhydryl groups in proteins. However, this reaction takes place
at low pH only, whereas the products are unstable at pH 7 or higher
(Twe & Wold, 1973).
Toluene diisocyanates may react with naturally occurring
proteins or polysaccharides and form immuno hapten complexes. The
results of an in vitro study by Ted Tse & Pesce (1979) showed that
toluene diisocyanates will react with human serum-albumin via one,
or both, of the isocyanate groups to form mono- or bisureido
protein derivatives. Such derivatives may be immunogenic and may
possibly lead to allergenic responses, as well as new antigenic
determinants (Baur, 1983).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Lethality data for some avian and aquatic species are listed in
Table 5.
Although practically insoluble in water, dispersed TDI can form
droplets and cause toxicity in aquatic systems.
Curtis et al. (1979) reported that 2,4-toluene diisocyanates
appeared to be toxic for fathead minnows only in the unreacted
form, and most lethality occurred during the first 12 h of
the test. The LC50 for aquatic species ranged from 10.5 to
508.3 mg/litre in static tests. The oral LD50 for avian species
was > 100 mg/kg body weight (Schafer et al., 1983).
Table 5. Lethality of toluene diisocyanates for aquatic and avian species
---------------------------------------------------------------------------------------------------------
Species Dose/concen- Condition Lethality Reference
tration
(mg/litre)
---------------------------------------------------------------------------------------------------------
Freshwater
Fathead Minnow 194a static 24-h LC50 Curtis et al. (1979)
(Pimephales promelas) 172.1 static 48-h LC50
164.5 static 96-h LC50
reconstituted
softwater (20 °C)
Saltwater
Grass shrimp 508.3a salinity 25 parts/ mortality less than Curtis et al.
(Palaemonetes pugio) thousand; 22 °C; 65% below this level (1979)
static within 96 h
Harpacticoid copepod 11.8b salinity 7 parts/ 96-h LC50 Bengtsson & Tarkpea
( Nitocra spinipes (10.5 - 13.2) thousand; static (1983)
Boeck) Crustacea
Avian
Redwinged blackbird 100 mg/kgc oral LD50 Schafer et al. (1983)
(Agelaius phoeniceus)
Starling > 100 mg/kgc oral LD50 Schafer et al. (1983)
(Sturnus vulgaris)
---------------------------------------------------------------------------------------------------------
a 2,4-isomer.
b Authors did not state the units; mg/litre were assumed on the basis of previous publication of Lindén
et al. (1979).
c 2,6-isomer.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Application of drops of toluene diisocyanates in the eyes of
rabbits caused immediate reaction suggestive of pain, lachrymation,
swelling of the eyelids, a conjunctival reaction, and mild damage
to the cornea (Zapp, 1957; Grant, 1974; Duprat et al., 1976;
Woolrich, 1982).
Intratracheal administration of 0.3 ml toluene diisocyanates in
guinea-pigs resulted in coagulation of proteins in the respiratory
tract and rapid death from respiratory distress (Friebel &
Lüchtralh, 1955). The acute toxicity of toluene diisocyanates via
various routes of exposure is summarized in Table 6.
Table 6. Lethality of toluene diisocyanates
------------------------------------------------------------------------
Route/species Concentration Duration Lethality Reference
(h)
------------------------------------------------------------------------
Inhalation
Rat 56.96 mg/m3 1 LC50 Harton & Rawl (1976)
Rat 98.96 ± 8.6 4 LC50 Duncan et al. (1962)
mg/m3
Rat (male) 348.88 mg/m3 4 LC50 Bunge et al. (1977)
(female) 356 mg/m3 4 LC50
Mouse 69.1 ± 9.96 4 LC50 Duncan et al. (1962)
mg/m3
Rabbit 78.32 mg/m3 4 LC50 Duncan et al. (1962)
Guinea-pig 90.4 ± 19.2 4 LC50 Duncan et al. (1962)
mg/m3
Oral
Rat 3060 mg/kg - LD50 Harton & Rawl (1976)
body weight
Mouse (male) 4130 mg/kg - LD50 Woolrich (1982)
body weight
Dermal
Rabbit 10 000 mg/kg - LD50 Harton & Rawl (1976)
------------------------------------------------------------------------
The toxicity of toluene diisocyanates, administered orally, is
low, but they are very toxic after inhalation exposure. Animals
reportedly died of acute pulmonary congestion, oedema, and
haemorrhage. Duncan et al. (1962) reported that during inhalation
exposures, all animals (Table 6) exhibited irritation. Tracheitis
and bronchitis, with sloughing of superficial epithelium, occurred
after exposure to 14.2 mg/m3 for 4 h. Rapid coagulation and
necrosis of the epithelium was evident following exposure to
35.8 mg/m3, suggesting direct chemical injury.
8.2. Short-Term Exposures
8.2.1. Inhalation
8.2.1.1. Guinea-pig
Exposure of guinea-pigs to 1200 mg toluene diisocyanates/m3,
as an aerosol, or 250 - 550 mg/m3, as a vapour, for 10 - 20 min at
irregular intervals, for one month, caused asthmatic reactions
after the first few inhalations, changing into continuous dyspnoea
in the course of the study (Friebel & Lüchtralh, 1955). Attainment
of these concentrations could only be achieved with both aerosol
and vapours. Bronchiolitis obliterans, pneumonia, and emphysema
occurred with little healing. Guinea-pigs sensitized to chicken
albumin responded in the same manner as untreated animals,
indicating the predominance of a primary toxic effect. However,
a possible role of allergic type reactions could not be excluded
completely.
Immunological sensitization and pulmonary hypersensitivity to
an 80:20 mixture were evaluated in the guinea-pig. Five days after
exposure to 1.78 mg/m3 (3 h/day for 5 days), 3 out of 16 exposed
guinea-pigs had developed antibody against TDI-guinea-pig serum-
albumin antigen, as demonstrated by immuno-diffusion, compared with
0/16 before exposure (Karol et al., 1980). Three additional
animals showed antibody responses with the more sensitive passive
cutaneous anaphylaxis assay (PCA). That is, a total of 6/16
exposed animals were antibody positive. No consistent increases
indicative of a pulmonary hypersensitive reaction were observed.
Concentration-dependent immunological responses to toluene
diisocyanates were measured following exposures that mimicked
industrial exposures and might lead to allergic reactions in
exposed workers (Karol, 1983). Guinea-pigs were exposed to 0.85 -
71.2 mg 80:20 mixture/m3, 3 h/day, for 5 days. On day 22, assays
for TDI-specific antibody, skin sensitivity, and pulmonary
sensitivity to toluene diisocyanates were performed. No antibody
to toluene diisocyanates was detected in animals exposed to a
concentration of 0.85 mg/m3, whereas 55% of the animals exposed
to > 2.56 mg 80:20 mixture/m3 displayed TDI-specific antibody in a
dose-related fashion. Pulmonary sensitivity to TDI-protein antigen
was observed at concentrations exceeding 2.6 mg/m3. Doses higher
than 14.2 mg/m3 resulted in pneumotoxicity and fewer pulmonary
hypersensitivity reactions. Exposure of a group of 24 guinea-pigs
to 0.14 mg 80:20 mixture/m3 (6 h/day, 5 days/week, for 70 days) in
whole-body exposure chambers, did not elicit these reactions
(Karol, 1983).
8.2.1.2. Mouse
The effects of single and repeated exposures to 2,4-TDI (99.7%
pure) vapour at concentrations ranging from 0.05 to 14.2 mg/m3 were
investigated in male Swiss-Webster mice, to detect the level of
sensory irritation caused by this chemical (Sangha & Alarie, 1979).
The results obtained demonstrated that the level of response not
only depended on the concentration, but also on the duration of
exposure; recovery rates also depended on the duration of exposure.
The RD50 (respiratory rate decrease of 50%) values decreased
significantly between 10 and 180 min and the levels of response
were exactly the same at 180 and 240 min of exposure. The RD50
found at 180 or 240 min of exposure was 1.42 mg/m3. Repeated
exposures to 2,4-TDI concentrations of or above 0.14 mg/m3 resulted
in cumulative effects, because of incomplete recovery prior to a
repeat exposure.
Weyel et al. (1982) measured the sensory irritation of 2,6-TDI
vapour at 0.37 - 7.6 mg/m3 in male Swiss-Webster mice. After a 3-h
exposure, a decrease in respiratory rate occurred with a pattern
indicative of sensory irritation of the upper respiratory tract,
which was similar to that noted by Sangha & Alarie (1979) with
exposure to 2,4-TDI at levels of > 0.14 mg/m3. An inverse linear
relationship (respiratory rate decrease versus log concentration of
2,6-TDI) was obtained that was identical to the slope and
relationship obtained in the earlier study on 2,4-TDI. From the
concentration-response (sensory irritation) relationship for
2,6-TDI, the RD50 was determined to be 1.85 mg/m3.
Lesions in the nasal cavity with a distinct anterior-posterior
severity gradient developed in mice after exposure to toluene
diisocyanates at 2.84 mg/m3, for 6 h/day, over 5 days (Buckley et
al., 1984). The lesions ranged from slight epithelial hypertrophy
or hyperplasia to epithelial erosion, ulceration, and necrosis with
variable inflamation of the subepithelial tissues. There was also
an associated loss of the olfactory nerves in the lamina prioria in
exposed animals.
8.2.1.3. Rat
Studies by Henschler et al. (1962) on the inhalation toxicity
of toluene diisocyanates are summarized in Table 7.
At exposure levels of 35.6 and 71.2 mg toluene
diisocyanates/m3, death was due to mechanical blocking of the
respiratory passages by mucosal tissue detached from bronchi and
trachea. At lower exposure levels, the fatal sequelae also
included heavy peribronchitis and spreading bronchopneumonia.
After cessation of exposure, partial reversal of pulmonary changes
occurred after several months in the 7.12 mg/m3 exposure group, and
complete remission occurred in the 3.56 mg/m3 exposure group.
After 40 exposures at 0.712 mg/m3, there were no definitive changes
in the respiratory tract, the only toxicological response being a
depression in body weight (Henschler et al., 1962).
Table 7. Inhalation toxicity of toluene diisocyanatesa
-------------------------------------------------------------------
Number of exposures Concentration Lethality
(schedule) (mg/m3)
-------------------------------------------------------------------
24 3.56 45% fatality of animals
(6 h/day, 6 days/week, with initial body weight
twice, miss 4 weeks, of 91 - 124 g; 0% fatality
repeat second 12 of animals with initial
exposures) body weight of 140 - 180 g
10 7.12 75% fatality
4 35.6 65% fatality
2 71.2 lethal for most animals;
2,4-isomer more toxic than
2,6-isomer
3 or 5 71.2 lethal for all
exposed animals
-------------------------------------------------------------------
a The 71.2 level mg/m3 was for 2,4- or 2,6-isomer or a 65:35
mixture of isomers. The 35.6, 7.12, and 3.56 mg/m3 levels were
for the 80:20 mixture. From: Henschler et al. (1962).
8.2.1.4. Dog
Four male dogs were exposed to concentrations of toluene
diisocyanates averaging 10.68 mg/m3, 35 - 37 times, for various
lengths of exposure (30 - 120 min), over a period of 4 months.
The dogs showed lachrymation, coughing, restlessness, and
expectoration of white frothy material. When killed after the last
exposure, all dogs showed mild congestion and inflammation of the
trachea and large bronchi. A conspicuous feature was the presence
of thick mucous plugs in some of the bronchial branches (Zapp,
1957).
8.2.2. Dermal
Toluene diisocyanates were described as a "medium" irritant for
rabbits and guinea-pigs, capable of producing cutaneous sensitivity
similar to contact allergy. The allergenic capacity was long-
lasting and depended on the allergen concentration (Zapp, 1957;
Duprat et al., 1976).
8.2.2.1. Guinea-pig
Dermal contact with toluene diisocyanates in the animal model
resulted in "rare" cases of sensitization (Peschel, 1970) and in
subsequent respiratory tract hypersensitivity (Karol et al., 1981).
Dermal contact sensitivity developed by the 7th day, following
applications of 1 - 100% solutions of 80:20 mixture (diluted with
olive oil) to the dorsal skin of the guinea-pig. After 14 days,
animals were evaluated for toluene diisocyanates sensitivity by
serological analysis and by bronchial provocation challenge.
Bronchial challenge with 0.03 mg toluene diisocyanates/m3, or
aerosols of TDI-protein conjugates, or p-tolyl isocyanate resulted
in respiratory hypersensitivity (Karol et al., 1981). These
responses were immediate; the respiratory rate increases were 3
times those in non-sensitive guinea-pigs. In challenges using
isocyanate conjugates, TDI-specific pulmonary reactions were
elicited more effectively when the hapten-protein conjugates rather
than the toluene diisocyanates vapour served as the challenging
agent.
Koschier et al. (1983) evaluated the dose-dependent eliciting
of dermal sensitization in young adult guinea-pigs treated with
2,4-TDI (2,6-isomer was < 2.5%). Induction was by cutaneous
application (25 µl) of 8 - 40% 2,4-TDI in n-butyl ether on 2
separate uncovered dorsal sites. Five days later, animals were
challenged with 0 - 0.4% 2,4-TDI (25 µl per site). All challenge
applications from 0.025% (6.25 µg) elicited a positive response in
75 - 100% of the animals. The results demonstrated that 2,4-TDI
induced sensitization and that the severity of the dermal response
was correlated with the concentration used at induction and
challenge. In a second study, in the group induced with 4% 2,4-
TDI, no effects were elicited after a dermal challenge application
of 3 µg, and a minimum effect was seen with 6.25 µg 2,4-TDI
(Koschier et al., 1983).
8.2.2.2. Mouse
In a dose-response study (Tanaka, 1979), a 100% increase in
ear-swelling in C3H/He mice, 24 h after challenge with 0.5 ml of 5%
TDI, was reduced to 50% after 72 h. In a second trial, 81% ear-
swelling resulted from a 5% challenge with TDI; there was a 6.5%
ear-swelling response to a 1% TDI challenge, compared with 2.4% in
vehicle controls. There was a cross-reactivity between TDI and
monodiisocyanate (MDI), so that sensitization with either
diisocyanate resulted in equal ear-swelling responses to challenge
with the opposite diisocyanate. The degree of response in cross-
reactivity was 4-times that in controls and about 35% of a
challenge response by the same diisocyanate used for sensitization.
Thymectomy did not change the ear-swelling responses to TDI. In a
subsequent study, Tanaka et al. (1984) reported that TDI induced a
delayed-type hypersensitivity reaction in the ear skin of male ICR
mice, and that 7-week-old mice sensitized with 1 - 5% (100 µl/dose)
TDI solutions showed ear-swelling with a challenge of 1% (2 µl) TDI
solution. The responses in 5-, 7-, and 13-week-old mice were the
same but were very slight in 16-week-old mice. Seven-week-old
BalB/C mice showed similar responses to ICR mice, but reactions in
ddY mice were much weaker.
Allergic dermatitis developed in mice by sensitization to
toluene diisocyanates, followed by inhalation exposure to the test
compound to determine if a delayed type allergy plays a role in
lung disorders caused by toluene diisocyanates. At a concentration
of 4.27 mg/m3, inhaled for 2 h, allergic dermatitis did develop,
but without noticeable pathological changes in the respiratory
organs (Ohsawa, 1983).
8.2.3. Oral
Oral gavage of 1500 mg/kg per day resulted in the death of 50%
of rats within a total of 10 treatments. Pathological examination
revealed injury to the gastrointestinal tract and liver (Zapp,
1957). No other toxic effects were reported from these studies.
8.3. Long-Term Exposure
8.3.1. Inhalation
8.3.1.1. Mouse
A dose-related increase in the incidence and severity of either
chronic or necrotic rhinitis occurred in mice exposed through
inhalation to the 80:20 mixture of toluene diisocyanates at 0.36 or
1.07 mg/m3, for 6 h/day, 5 days/week, over 2 years. In addition,
lesions of variable incidence and severity were seen in the lower
respiratory tract (interstitial pneumonitis, catarrhal bronchitis)
and eyes (keratitis) of some mice, with a higher incidence in the
1.07 mg/m3 group. Morbidity and mortality due to rhinitis occurred
in both treated groups (Loeser, 1983).
8.3.1.2. Dog
Patterson et al. (1983) immunized 3 dogs (by endotracheal tube)
with an aerosol of toluene diisocyanates at 1 mg/kg body weight,
every 2 weeks, for 4 months (2 - 3 times the maximal TLV for
occupational exposure). Thereafter, 2 dogs were dosed with 1 mg/kg
body weight every 4 weeks for 6 months (stated to be the cumulative
dose analagous to long-term exposure to 0.14 mg/m3). Systemic
immune responses to TDI-dog serum-albumin, including elevated
specific antibody titers of IgG, IgA, IgM, and development of
specific lymphocyte reactivity, were seen in all animals. Elevated
IgG and IgA titers were persistent. Although increased, the lower
titer of IgM antibody was of short duration. The antibody IgE was
detected, but levels fluctuated and became negative, even with
continued exposure to toluene diisocyanates. Airway responses
that occurred immediately after exposure to the aerosol included
abnormalities of selected pulmonary function parameters. They were
clearly not immunologically mediated, because they occurred with
initial exposure. However, other immediate airway responses
occurred that qualitatively simulated IgE-mediated, antigen-induced
airway responses in dogs. There was a statistically-significant
correlation between the latter airway responses and immediate skin
reactions (Patterson et al., 1983).
8.4. Reproduction, Embryotoxicity and Teratogenicity
No published data were found on the effects of toluene
diisocyanates on reproduction, or on the embryotoxicity or
teratogenicity of these compounds.
8.5. Mutagenicity and Related End-Points
8.5.1. Bacterial mutagenicity
There are conflicting reports about the mutagenicity of toluene
diisocyanates. Anderson & Styles (1978) reported that toluene
diisocyanate of unknown purity was non-mutagenic in a study of 120
chemicals tested by Purchase et al. (1978), but the fact that
several known mutagens failed to give positive results means that
the original report was suspect. Andersen et al. (1980) later
optimized the procedures to test the reactive isocyanates and
showed that a mixture of 2,4- and 2,6-toluene diisocyanates caused
a dose-dependent mutagenic response, using S-9 activation, in
S. typhimurium strains TA 98, TA 100, and TA 1538. The positive
control for these mutagen tests was the hydrolysis product of 2,4-
TDI, 2,4-diaminotoluene, reported by Ames et al. (1975) to be
mutagenic. The NTP has also tested toluene diisocyanates using the
Salmonella test system and found that both 2,6-TDI and a mixture
of 2,4- and 2,6-TDI (80:20) were mutagenic in S. typhimurium
strains TA 98 and TA 100 in the presence (but not the absence) of
Aroclor 1254-induced male Sprague Dawley or Syrian hamster liver
S9. Neither sample was mutagenic in S. typhimurium strains TA
1535 or TA 1537, with or without metabolic activation.
8.5.2. Mammalian cell transformation
Toluene diisocyanates were negative in two in vitro cell
transformation assays using human lung and hamster kidney cells
(Styles, 1978).
8.5.3. Mammalian in vivo study
Studies by Loeser (1983) failed to show a dose- or treatment-
related percentage increase in micronucleated erythrocytes from the
bone marrow of rats and mice exposed through inhalation to 80:20
mixture at 0.35 or 1.06 mg/m3, for 6 h/day, 5 days/week, over 4
weeks.
8.6. Carcinogenicity
8.6.1. Oral
Long-term oral (gavage) administration of the 80:20 mixture
of 2,4-, 2,6-TDI in corn oil resulted in increased incidences of
various types of tumours in Fischer 344/N rats and B6C3F1 mice
(NTP, 1986). Female rats and mice were dosed with 60 or 120 mg/kg
body weight; male rats received 30 or 60 mg/kg body weight; and
male mice were dosed with 120 or 240 mg/kg body weight, for 5 days
per week, over 2 years. However, it is worth noting that the
reaction of toluene diisocyanates with the moisture in the corn oil
resulted in unknown reaction products and in doses qualitatively
and quantitatively different from those reported, possibly as much
as 23% below the target dose. Long-term treatment, by gavage, with
the TDI-corn oil mixture caused dose-related reductions in body
weight gain. A dose-dependent pattern of cumulative toxicity began
at weeks 70 - 75, culminating at 103 weeks in the following
percentage mortality in control, low-, and high-dose groups,
respectively: male rats: 28%, 72%, and 84%; female rats: 28%, 62%,
and 88%; male mice: 8%, 20%, and 48%; and female mice: 32%, 14%,
and 34%.
Significant increases were noted in the incidence of
subcutaneous fibromas and fibrosarcomas (combined) in male and
female rats; pancreatic acinar-cell adenomas in male rats;
pancreatic islet-cell adenomas, neoplastic nodules of the liver,
and mammary gland fibroadenomas in female rats; haemangiomas and
haemangiosarcomas (combined), and hepatocellular adenomas in
female mice (NTP, 1986). It was concluded that the 80:20 mixture
of 2,4-, 2,6-TDI in corn oil was carcinogenic for male and female
rats and female mice, when administered orally by gavage. The 1986
NTP report was reviewed during its preparation by Rampy et al.
(1983).
8.6.2. Inhalation
In a long-term inhalation study, Sprague Dawley CD rats and
CD-1 mice were exposed to the 80:20 isomer mixture at nominal
levels of 0.356 mg/m3 (0.05 ppm) or 1.068 mg/m3 (0.15 ppm), for
6 h/day, 5 days per week, for 108 weeks (female rats), 110 weeks
(male rats), or 104 weeks (male and female mice) (Loeser, 1983).
The type and incidence of tumours and the number of tumour-bearing
animals of either species did not indicate any carcinogenic effect.
The main pathological changes in mice occurred in the nasal cavity
and included dose-related incidences of epithelial atrophy, mucuous
and squamous metaplasia, inflammation, and focal destructive
rhinitis with debris.
In this study, there was an unexplained high mortality in both
the control and treated rats and mice. In the high-exposure groups
(1.068 mg/m3), significantly lower weight gains were noted
throughout the study in mice and during the first 12 weeks in rats.
Haematological indices, clinical chemistry, and urinalyses were not
affected by the doses of 80:20 mixture used, nor were there dose-
related changes in organ weights. It was tentatively concluded
that, under these experimental conditions, the 80:20 mixture at
0.356 or 1.068 mg/m3 did not lead to a carcinogenic response or to
other adverse clinical responses. Although some reductions in
weight gain were noted, the doses used were probably below maximal
tolerated doses. In addition, the high mortality rate reported
reduced the sensitivity of this bioassay.
8.7. Special Studies and Mechanisms of Toxicity
On the basis of the possible reactions to toluene diisocyanates
(section 7.5), it is most likely that covalent binding and slow
recovery could follow reaction of toluene diisocyanates with
hydroxyl and amino groups of receptor proteins in the nasal
mucosa. Under the conditions proposed by Brown & Wold (1971), it
is possible that only the first "reversible noncovalent" complex
may have been formed after short durations of exposure to toluene
diisocyanates, since recovery was rapid (Sangha & Alarie, 1979)
(section 8.2.1.2). With longer exposure, the slow recovery would
be due to the formation of the covalent irreversible complex (Brown
& Wold, 1971).
In a subsequent study, McKay & Brooks (1984) reported a
significant difference in carbachol-stimulated tracheal smooth
muscle strips from guinea-pigs exposed to toluene diisocyanates
(0.02 mg/m3, 5 h/day, for 20 days) compared with controls. The
observed increase in maximal tension and the shift of the dose-
effect curve for exposed animals suggested a direct effect of
toluene diisocyanates on tracheal smooth muscle. The toluene
diisocyanate tested had an isomer content of 97.8% 2,4-TDI and 2.2%
2,6-TDI.
Kido et al. (1983a) measured histamine release from the
leukocytes of guinea-pigs exposed to a toluene diisocyanate level
of less than 7 mg/m3 (1 ppm), to study the mechanism of induction
of asthma by toluene diisocyanates. In guinea-pigs with IgE
antibody, histamine release was > 20% with an average peak value
(apv) of 40% compared with < 20% and an apv of 8.8% in controls,
and a histamine release of approximately 20% with an apv of 24.1%
in guinea-pigs without elevated IgE antibody levels.
The authors hypothesized that, after exposure to toluene
diisocyanates, the IgE antibody, which is homocytotropic to
basophils, resulted in histamine release in vitro by the TDI-human
serum-albumin (TDI-HSA) conjugated antigen, and that it is possible
that an immediate-type allergic reaction by the IgE antibody is
involved in the mechanism of induction of TDI-asthma.
Studies by Chen & Bernstein (1982) have shown the presence of
hapten-specific IgE antibodies in the sera of guinea-pigs immunized
with either toluene-diisocyanate-human serum-albumin or
hexamethylene diisocyanate-human serum-albumin or hexamethylene
diisocyanate-human serum-albumin and subsequently challenged with
conjugates of the respective ligands coupled to transferrin. In
these studies, both homocytotropic (IgG and IgE), and precipitating
antibodies were produced under appropriate conditions of parenteral
immunization. The authors postulated that the complex nature of
the immune response generated by diisocyanate compounds in guinea-
pigs might also serve as an appropriate model for isocyanate-
induced human sensitivity reactions, which are known to involve
diverse immunological and nonimmunological mechanisms.
Tanaka et al. (1984) induced nasal allergy in guinea-pigs by
painting a 10% solution of toluene diisocyanates in ethyl acetate
on the bilateral nasal vestibules, once a day, for 5 days. After
waiting 3 weeks, the animals were challenged in a similar manner
with a 5% toluene diisocyanates solution; the process was repeated
2 times per week, for 3 months. Sneezing and rhinorrhea occurred
in guinea-pigs, either with or without dyspnoea, and many
eosinophils were found in nasal smears. Histopathology indicated
enhanced secretory function, eosinophil infiltration, and probable
degranulation of mast cells in the nasal mucosa. A significant
release of histamine from the nasal mucosa in TDI-sensitized
guinea-pigs was noted, when stimulated in vitro by TDI-guinea-pig
serum-albumin.
9. EFFECTS ON MAN
9.1. General Population Exposure - Controlled Human Studies
9.1.1. Single exposures
In human volunteers, eye and nose irritation began at acute
concentrations of 0.35 - 0.92 mg/m3, while skin irritation
generally occurred at higher levels (Brugsch & Elkins, 1963;
Bruckner et al., 1968; Sittig, 1981; Woolrich, 1982). The odour
threshold for aerosols of toluene diisocyanates was tested in human
volunteers by several investigators (Munn, 1960; Henschler et al.,
1962; Brugsch & Elkins, 1963). The responses were not uniform,
probably because of differences in chemical purity, protocol, etc.
Ehrlicher's group reported the following: slight odour = 0.92 mg/m3
(0.13 ppm); odour without irritation = 4.28 mg/m3 (0.60 ppm);
burning eyes and nose = 13.57 mg/m3 (1.9 ppm); and severe
irritation of eyes and respiratory tract = 27.8 mg/m3 (3.9 ppm).
Henschler et al. (1962) reported values that were about 10 times
lower for the irritation effects of TDI in human exposure. In a
30-min exposure of 6 persons, levels of 0.07 and 0.14 mg/m3 (0.01
and 0.02 ppm) were not perceived, a level of 0.35 mg/m3 (0.05 ppm)
was recognized by everyone, slight irritation of the eye, nose, and
throat occurred at concentrations of between 0.35 and 0.7 mg/m3
(0.05 and 0.1 ppm), secretions in the eye and nose occurred in most
persons at 0.7 mg/m3 (0.1 ppm) and always at 3.5 mg/m3 (0.5 ppm),
and, overall, the irritative effect was greater in response to 2,6-
than to 2,4-TDI. The difference in threshold response can perhaps
be attributed to more accurate analytical procedures used by
Henschler et al. (1962).
Brugsch & Elkins (1963) reported that the minimum concentration
of toluene diisocyanates for irritation was 0.35 - 0.7 mg/m3 (0.05 -
0.1 ppm) and that all subjects were irritated at 3.5 mg/m3 (0.5
ppm).
Odour threshold values varied from 0.35 to 0.92 mg/m3 (0.05 to
0.13 ppm) in these studies.
9.2. Occupational Exposure
9.2.1. Acute toxicity
The signs and symptoms of acute exposure are non-specific and
include: complaints of irritation of the nose and throat,
shortness of breath, choking, coughing, retrosternal discomfort or
pain, and gastrointestinal stress (e.g., nausea, vomiting, and
abdominal pain). The onset of signs and symptoms may be delayed
following exposure, and may persist for several days, months, or
years following removal from the contaminated environment
(Ehrlicher & Pilz, 1956; Walworth & Virchow, 1959; Munn, 1960,
1968; NIOSH, 1978).
Eye contact with toluene diisocyanates (vapour, aerosols, or
liquids) causes mild irritation, characterized by itching and
lachrymation, which may progress to conjunctivitis and
keratoconjunctivitis (Brugsch & Elkins, 1963; Luckenbach & Kieler,
1980). Oculorhinitis may also occur and be delayed by a few hours
(Paggiaro et al., 1985).
Systemic symptoms, which developed after acute occupational
exposure to toluene diisocyanates, have been reported by Axford et
al. (1976) and Le Quesne et al. (1976). These two reports describe
the findings from one accident in which the victims were firemen
involved in both fire-fighting and clean-up operations at a
polyurethane foam factory, where a large quantity of toluene
diisocyanates (4500 litres) had leaked.
Exposed firemen experienced symptoms during and/or after the
fire. Symptomatology included 15 cases of gastrointestinal
distress, 4 of which, though asymptomatic during the fire,
developed gastrointestinal symptoms the following day, 3
experiencing abdominal pain with diarrhoea, while the other
complained of nausea and vomiting. All symptoms eased within 2
days without any apparent long-term effects (following 4 years of
monitoring).
In addition to gastrointestinal symptoms, 23 firemen complained
of neurological symptoms. Five firemen experienced symptoms (i.e.,
euphoria, ataxia, intermittent shaking of the limbs, dizziness, and
loss of consciousness) immediately on exposure. Symptoms such as
headaches, difficulty in concentrating, poor memory, and confusion
persisted for 3 weeks in 14 of the firemen. After 4 years, poor
memory was the most common complaint, followed by personality
change, irritability, or depression, in a total of 13 firemen
(Le Quesne et al., 1976). Interpretation of these findings is
complicated by simultaneous exposure to other toxic components
released during these types of fires.
In an earlier investigation, Hama (1957) reported cold-like
symptoms and nocturnal sweating, without fever, in addition to the
gastrointestinal and neurological symptoms described above.
9.2.2. Effects of short- and long-term occupational exposure -
epidemiological studies
9.2.2.1. Ocular
Luckenbach & Kieler (1980) reported evidence of microcystic
corneal oedema and conjuctival infection in both eyes in a
polyurethane foam worker (40-year-old female). Clouded vision,
decreased visual acuity, and loss of light perception developed
within one week of employment. Both corneas and conjunctivae
returned to normal after 3 days without exposure to the
occupational chemicals. Similar visual effects have been
attributed to some amine catalysts (Belin et al., 1983).
9.2.2.2. Dermal
Skin sensitization on repeated exposure to toluene
diisocyanates may occur. Urticaria, dermatitis, and allergic
contact dermatitis have been reported in workers exposed to toluene
diisocyanates-based photopolymerized resins (Brugsch & Elkins,
1963; Calas et al., 1977). The dermatological symptoms included
skin lesions of an eczematous, and also, of an irritant,
pruriginous and erythaematous nature.
A 21-year-old female developed a rash following direct skin
contact with toluene diisocyanates. The urticaria or maculopapular
lesions occurred primarily over exposed areas, but occasionally
spread to covered areas and lasted for up to 10 days after
exposure. Titers of specific IgE antibodies gradually declined
over the period of observation from a high level of 1050 net cpm
[by radioallergosorbent test (RAST)] to 270 net cpm after
occupational exposure ceased. The lower level corresponds to those
found in non-sensitized toluene diisocyanates workers (Karol et
al., 1978).
9.2.2.3. Respiratory tract
Occupational exposure to toluene diisocyanates has produced a
variety of respiratory effects in workers including irritation of
the upper and lower respiratory tract, an asthma-like sensitization
response, dyspnoea, cyanosis, and pulmonitis and decreases in lung
function (Swensson et al., 1955; Brugsch & Elkins, 1963; Gandevia,
1963; Peters et al., 1968, 1969; Peters, 1970; Gaffuri & Brugnone,
1971; Charles et al., 1976; Wegmen et al., 1977; Burge et al.,
1979; Burge, 1982). Short-term, as well as long-term exposures to
toluene diisocyanates, in some instances at levels < 0.007 mg/m3,
have been reported to result in significant decreases in lung
function (NIOSH, 1978). However, the results of more recent
studies by Musk et al. (1982) failed to support such effects at
levels of isocyanates of the order of 0.007 mg/m3 (0.001 ppm).
Sensitization after a single exposure was not demonstrated by Pepys
(1980). Irritation of the respiratory tract can occur at levels
ranging between 0.712 and 3.560 mg/m3 (Henschler et al., 1962).
The asthmatic response, evident in up to 10% of previously exposed
individuals, may occur at levels of toluene diisocyanates > 0.0356
mg/m3 (Bernstein, 1982). The basis for this response is still
uncertain, but there is evidence supporting either immunological or
pharmacological mechanisms, or a combination of both (Scheel et
al., 1964; Weill et al., 1975; Butcher et al., 1977, 1980;
Cockcroft & Mink, 1979; Chadwick & Cleveland, 1981; NIOSH, 1981;
Bernstein, 1982; Karol, 1983).
Toluene diisocyanate-induced asthma may not be evident until
after many years of exposure (Salvaggio, 1979). However, TDI may
cause immediate, delayed, or biphasic asthmatic reactions (Baur et
al., 1983; NIOSH, 1981). The immediate reaction reaches a peak
within minutes. Late reactions occur from 2 to 8 h after exposure
and may show a recurring pattern. Most affected people have non-
specific bronchial hyperreactivity, as measured by mechanical
intratracheal challenge tests. This reaction may continue for
several years after cessation of exposure implying persisting
asthmatic symptoms. Immunologically sensitized workers can be
identified by means of RAST and skin testing.
A 43-year-old non-smoking molder (female) first exhibited
throat irritation and a non-productive cough after 4 months of
exposure to toluene diisocyanates. Dyspnoea was noted, which
worsened during work-days, resulting finally in an episode that
required emergency treatment. One month later, after cessation of
exposure, the patient was without symptoms, and pulmonary function
had returned to normal. Subsequent symptomatic episodes were
successfully treated with isoproterenol (Smith et al., 1980).
Other symptoms, such as faintness, nausea, vomiting of "foamy"
materials, anxiety, rapid pulse rate, elevated blood pressure,
fever, and cyanosis, were reported by Brugsch & Elkins (1963).
The patient, who was a 62-year-old spray painter, was coating the
inside of a large tank for 7 days without respiratory protection.
An ECG showed hypertrophy of the left ventrical, and a chest
roentgenogram showed prominent bronchovesicular markings and an
enlarged cardiac silhouette. The patient improved and was
discharged after 4 days.
Epidemiological studies of health effects from occupational
exposure to toluene diisocyanates are summarized in Table 8.
9.2.2.4. Cancer epidemiology
No epidemiological studies of mortality or cancer incidence
among workers exposed to toluene diisocyanates were available to
the Task Group.
One case report of adenocarcinoma in a 47-year-old non-smoking
spray-painter has been published. The subject had been exposed to
toluene diisocyanate and 4,4-methylene diisocyanate for 15 years.
The levels of exposure to isocyanates were not reported and neither
were other chemicals to which the subject may have been exposed
(Mortillaro & Schiavon, 1982).
9.2.2.5. Immunotoxicity
Several investigators have detected toluene diisocyanates
sensitization with the lymphocyte transformation test and other
immunoassays, but have been unable to consistently demonstrate
elevated antibody titers (Bruckner et al., 1968; Avery et al.,
1969; Danks et al., 1981; Game, 1982). Studies on the immune
response following exposure to toluene diisocyanates are
summarized in Table 9.
Table 8. Epidemiological studies of health effects from occupational exposure to toluene diisocyanates
---------------------------------------------------------------------------------------------------------
Concentration (mg/m3) Effects Reference
---------------------------------------------------------------------------------------------------------
< 0.213 (0.03 ppm); 38 workers at a polyurethane foam factory; after 1 Peters et al. (1968)
mostly < 0.142 day exposure (Monday), statistically significant
(0.02 ppm) decreases in FVC, FEV1 peak-flow rate, and FEF25-50%;
after 5 days' exposure (Friday), in 34 workers, the
FVC returned to baseline, the FEV1 was still
depressed, and the respiratory flow rates were more
depressed; diurnal variation could not account for
these changes; workers with respiratory symptoms
showed greater decreases in FEV1
0.014 - 0.093 111 workers at a polyurethane foam factory; changes Wegman et al. (1974);
(0.002 - 0.013 ppm) measured in FEV1 between Monday AM and PM of work Peters & Wegman (1975)
day; 51 workers (0.014 mg/m3 exposure): 78 ml
decrease; 43 workers (0.028 mg/m3 exposure): 106 to
112 ml decrease; 17 workers (0.064 - 0.093 mg/m3
exposure): 180 ml decrease
0.072 (0.001 ppm) 107 workers from 2 polyurethane manufacturing plants; Musk et al. (1982)
(time-weighted average) 5-year change in FEV did not exceed that expected for
aging; no significant change in FEV was noted between
Monday AM and PM of work day
0.356 - 0.712 287 workers in 2 TDI plants; lung function (FEV1 and Adams (1975)
FVC) measured annually for 8 years; 180 workers with
no respiratory symptoms: FEV1 and FVC normal; 46
workers with respiratory symptoms and still employed:
only questioned and reported more respiratory effects
than controls; 61 workers with respiratory symptoms
no longer at plant: FEV1 averaged 271 ml and FVC
averaged 269 ml lower than predicted from 608 controls
< 0.01 - > 0.025 57 workers at toluene diisocyanates plant; a dose- Wegman et al. (1977)
(< 0.0015 - > 0.0035 response relationship observed; exposure to < 0.01
ppm) mg/m3 did not affect FEV1; exposure to > 0.025 mg/m3
resulted in decrease in FEV1 of 103 ml/year, which
exceeded expected value by 3 - 4 times; workers
exposed to < 0.01 mg/m3 showed normal 2-year decline
(-12 ml in 2 years); differences in FEV1 not explained
by age, time employed, smoking habits, or lung size
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Concentration Effects Reference
(mg/m3)
---------------------------------------------------------------------------------------------------------
0.0007 - 0.178 223 workers at a new TDI plant; measured for Diem et al. (1982)
(0.0001 - 0.025 ppm) pulmonary function, with 3 or more data points to
(TWA) calculate slope of annual response (during 5-year
exposure); low and high cumulative exposure groups
exposed for 2% and 15% of time, respectively, toluene
diisocyanates exceeding 0.036 mg/m3; significant
effects of smoking on spirometric tests and lung
volumes; after adjustment for pack-years smoking,
the FEV1, %FEV, and FEF25-75% declined more in
"high" exposure group (74 workers) than in "low"
exposure group (149 workers); in non-smokers,
average FEV1 decline was 38 ml/year greater in
high- compared with low-exposure groups; a 24 ml/year
excess average decline attributed to longer exposure
to levels above 0.014 mg/m3
0.007 - > 0.014 145 workers at TDI plant surveyed for lung function Omae (1984)
(0.007 - > 0.002 ppm) 1980 and workers re-examined in 1982; short-term
exposure to > 0.014 mg/m3 occurred in 9.3% of samples
in 1980, and 1.9% in 1982; no dose response in loss of
pulmonary function; 0.007 mg/m3 was not associated
with acute or chronic effects on pulmonary function
---------------------------------------------------------------------------------------------------------
Note: Diem et al. (1982) clarified that the toluene diisocyanates workers were compared according to
cumulative exposure, not concentration categories, and that the low-exposure group's 8-h TWA was
spent at < 0.036 mg TDI/m3 and the high-exposure at > 0.036 mg TDI/m3. There was a mean increase
in FEV1 of 1 ml/year for the low-exposure category and a mean decrease in FEV1 of 1 ml/year for
the high-exposure category. They indicated that, because of variations in daily exposure to
toluene diisocyanates, an average 8-h TLV < 0.036 mg/m3 might be necessary to achieve compliance.
FVC = forced vital capacity.
FEV1 = forced expiratory volume in 1 second.
FEV1/FVC% = ratio of FEV1/FVC x 100.
FEF25-50% and FEF25-75% = forced expiratory flow between 25% and 50% or 25% and 75% of FVE, respectively.
Table 9. Immune response in workers exposed to toluene diisocyanates
---------------------------------------------------------------------------------------------------------
Level (mg/m3) Sample number Immune response References
---------------------------------------------------------------------------------------------------------
0.028 - 0.142 32 workers hypersensitive responses Porter et al. (1975)
correlated with TDI levels
> 0.35 mg/m3; IgG antibodies
present and broncho-constriction
occurred
frequently > 0.14 166 workers; increased pulmonary function normal; Butcher et al. (1977)
incidence of TDI-specific positive skin test and broncho-
IgE antibodies constriction noted
0.142 challenge 23 workers (4 sensitive specific IgE antibodies in 3 out Karol et al. (1978)
to TDI) of 4 sensitive workers;
19 non-sensitized workers had
antibody titers comparable with
controls; high levels to specific
IgE antibodies not correlated
with serum-IgE levels
< 0.175 87 workers respiratory symptoms with Kido et al. (1983b)
reduction in FEV1; tolyl-specific
IgE detected in 2 workers only;
RAST indicated generally low
levels in all sensitized workers
0.119 - 0.147 39 workers Significant elevation of serum- Hobara et al. (1984)
IgE levels in only 4 out of 10
workers exposed for less than 1
year, all having obstructive
impairment of liver function
---------------------------------------------------------------------------------------------------------
The results of these studies indicated that exposure to
low concentrations of toluene diisocyanates (> 0.14 mg/m3)
induced hypersensitivity in a variable and unknown percentage
of the individuals at risk. The duration of exposure to
toluene diisocyanates necessary to induce hypersensitivity was
also highly variable, sometimes occurring immediately or
requiring months or even years of exposure. There has been
some success in evaluating the hypersensitivity to toluene
diisocyanates with RAST assays using the TDI-HSA antigen, but
neither the antibody responses to this specific antigen, nor
the level of IgE antibody were consistently elevated in
individuals with hypersensitivity and asthmatic responses
(Baur, 1983; Belin et al., 1983; Barkman et al., 1984).
Present immunoassay techniques will not detect all susceptible
individuals.
9.2.3. Potential mechanisms of action
Irritation and toxic effects are presumed to stem from the
reactivity of the isocyanate groups (i.e., -NCO) on toluene
diisocyanates and their quasipolymers (Brugsch & Elkins, 1963).
Wegman et al. (1974) demonstrated a dose-response relationship for
these symptoms. Irritation will stimulate mucous-secreting goblet
cells, with a proportional decrease in ciliated epithelial cells.
Impaired clearance and mucal stagnation may result, followed by
epithelial desquamation, submucosal glandular hypertrophy, and
basement membrane thickening as in chronic tracheo-bronchitis
(Braman & Teplitz, 1978).
The mechanisms concerned (immunological or pharmacological) in
the production of asthma and hypersensitivity pneumonitis through
occupational exposure to toluene diisocyanates are still the
subject of debate. The controversy stems from the paradoxical
nature of the sensitivity reactions. Karol et al. (1978) reported
specific IgE antibodies in sera from workers sensitive to toluene
diisocyanates, suggesting an IgE-mediated mechanism for sensitivity
to the compound. It was reported by Thurman et al (1978) that
toluene diisocyanates induced lymphocytes to undergo blastogenesis,
suggesting antigenic stimulation. In many instances, no specific
IgE antibodies against TDI-HSA conjugates were demonstrated
(Gaffuri & Brugnone, 1971; Butcher et al., 1977, 1980). Positive
results were observed in 14 - 19% of toluene diisocyanates
reactors, depending on the method of evaluation (Butcher et al.,
1980; Baur et al., 1983). Smith et al. (1980) also found a worker
sensitized to toluene diisocyanates, but with no IgE antibodies,
leukocyte inhibition factor for isocyanate antigen, or even
bronchial hyperreactivity to methacholine, an important component
of bronchial asthma. Asthma resulting from toluene diisocyanates
exposure appears to be a complex syndrome with several possible
mechanisms of causation, including an IgE mechanism in some
individuals.
Salvaggio (1979) suggests that preexisting asthmatic
conditions, together with partial andrenergic blockage or abnormal
cholinergic receptor activity, may increase bronchial airway
hyperreactivity to irritants, such as toluene diisocyanates. A
significant decrease in erythrocyte-cholinestrase activity was
found in in vitro studies in 70% of a group of 30 workers exposed
to toluene diisocyanates (Brown et al. 1982; Dewair et al., 1983;
Manno & Lotti, 1976).
The results of several in vitro studies showed evidence of
inhibition of elevated levels of intracellular cyclic AMP
production by TDI at doses as low as 6.7 x 10-7 mol (Butcher et
al., 1977; Davies et al., 1977). The explanation for these
responses is unclear.
The controversy over immune-controlled versus pharmaco-
logically mediated response to toluene diisocyanates in sensitized
workers has yet to be resolved.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Exposure to Toluene Diisocyanates
In the USA, it has been estimated that approximately 40 000
workers are involved in the manufacture or processing of toluene
diisocyanates. Occupational exposure levels have been reported to
range from 0.001 to 1 mg/m3.
Figures are not available on the total discharge of unreacted
TDI into the environment. However, releases into the air ranging
from 0.1 to 17.7 mg/m3 have been measured in stack gases emitted
from a plant manufacturing polyurethane foams. It has been
reported that approximately 50 g of TDI are released per tonne
of processed TDI in the manufacture of polyurethane foams, the
manufacture of which consumes about 90% of TDI produced.
As far as the general population is concerned, intake of
toluene diisocyanates, apart from their use in the form of
polyurethane lacquers and paints, is of a very low order, because
of the short persistence of TDI.
10.1.1. Acute and short-term effects
The odour threshold for toluene diisocyanates in human beings
is estimated to range between 0.35 and 0.92 mg/m3 (0.05 and 0.13
ppm).
The lowest levels of TDI associated with acute effects were
reported to be: 0.035 - 0.70 mg/m3, eye and nose irritation,
burning nose and throat, and a choking sensation; 0.70 - 3.5 mg/m3,
a respiratory response of irritation, cough, and chest discomfort.
At higher levels, chemical pneumonitis may be expected.
10.1.2. Health risks of long-term exposure to toluene diisocyanates
Risk of respiratory toxicity from repeated exposure can be
summarized as follows: (a) chronic loss of ventilatory capacity as
measured by forced expiratory volume and forced vital capacity; (b)
immediate and/or delayed asthmatic responses.
In many epidemiological studies, past mean exposures to TDI
have been estimated in an attempt to quantify dose-response
relationships for respiratory ill health. Because of the
uncertainties in the sampling procedures and analytical
measurements used in past industrial health surveys, it is
difficult to be confident about the exact levels at which TDI
causes the above-mentioned health effects. It should be remembered
that fluctuations in the true individual exposure occur and, as
both the size and extent of the intermittent peaks are unknown,
their biological significance cannot be evaluated.
Once individuals are sensitized to toluene diisocyanates, low
concentrations, much below current occupational exposure limits,
can induce asthma. Studies on experimental animal have shown that
skin application of TDI can lead to pulmonary sensitization; thus,
it is prudent to avoid repeated skin contact.
No data were available on the carcinogenic effects of toluene
diisocyanates in human beings.
No carcinogenic effects of TDI were noted in an inhalation
study on rats and mice. However, the 80:20 mixture in corn oil,
administered by gavage, was carcinogenic for male and female rats
and female mice in a dose-related manner. It is considered that
there is sufficient evidence for the carcinogenicity of TDI for
experimental animals.
There is evidence of mutagenicity in two bacterial tests.
10.2. Evaluation of Effects on the Environment
An evaluation of the hazards for non-human targets from
environmental levels of TDI is not possible on the basis of
available data.
10.3. Conclusions and Recommendations
1. There is sufficient knowledge about TDI to classify it as a
very toxic compound by inhalation, and TDI should be treated
as a potential human carcinogen and as a known animal
carcinogen. Consequently, the greatest priority should be
given to safe methods of use, the education, training, and
supervision of operatives, and state enforcement of
legislation by an effective inspectorate. Special attention
should be paid to the prevention and adequate treatment of
unscheduled releases and spills.
2. Additional animal carcinogenicity testing by the inhalation
route should be carried out.
3. Morbidity and mortality studies are required on occupational
groups for whom reliable exposure data are available, to
address the question of cancer and to evaluate the potential
of toluene diisocyanates to cause long-term human health
hazards under current standards of good working practice.
4. Because it is not possible to reach confident conclusions from
the data on the neurotoxicity of TDI, neurophysiological and
behavioural studies should be carried out on asymptomatic
workers, exposed at current hygiene standards.
5. For the foreseeable future, exposed workers require health
monitoring by systematic symptom enquiry and by standardized
measurement of ventilatory function, with subsequent analysis
of trends for individuals and for group mean values.
6. Appropriate sampling strategies together with existing
analytical methods have to be developed and used to obtain
better information about exposure, with special reference to
the detection and characterization of peak values. The
results of these analyses need to be evaluated in special
studies in parallel with careful health studies.
7. Further metabolic studies of a qualitative and quantitative
nature should be carried out with a view to developing methods
of measuring TDI uptake and monitoring exposure.
8. Whether TDI produces sensitization in human beings by
pharmacological or immune mechanisms needs to be elucidated
with a view to determining whether restrictions placed on the
employment of atopic subjects, in areas where TDI is produced
or used, are justified.
9. Studies are required to determine whether TDI has embryo-toxic
and teratogenic properties or has adverse reproductive effects
at current exposure levels.
10. Further environmental studies are required to monitor general
environmental levels of TDI in the neighbourhood of sources
and to collect ecotoxicity data.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1979) evaluated the data on the carcinogenicity of
toluene diisocyanates and found insufficient experimental animal
or human data on which to base an evaluation. An evaluation of
additional data by IARC (1986) led to the conclusion that there is
sufficient evidence for the carcinogenicity of toluene
diisocyanates for experimental animals.
In the absence of adequate case reports or epidemiological
studies, there is insufficient data to assess the carcinogenicity
of toluene diisocyanates for human beings (IARC 1986).
REFERENCES
ADAMS, W.G.F. (1975) Long-term effects on the health of men
engaged in the manufacture of tolylene di-isocyanate. Br. J. ind.
Med., 32: 72-78.
ALARIE, Y. (1973) Sensory irritation of the upper airways by
airborne chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
ALDER, J.F. & ISAAC, C.A. (1981a) Detection of toluene
diisocyanate in air with a coated piezoelectric crystal. Part
1. A study of coating materials. Anal. Chim. Acta, 129:
163-174.
ALDER, J.F. & ISAAC, C.A. (1981b) Detection of toluene
diisocyanate in air with a coated piezoelectric crystal. Part
2. Development of an instrumental method for personal
monitoring. Anal. Chim. Acta, 129: 175-188.
AMES, B.N., KAMMEN, H.O., & YAMASAKI, E. (1975) Hair dyes are
mutagenic: identification of a variety of mutagenic ingredients.
Proc. Natl Acad. Sci. (USA), 72: 2423-2327.
ANDERSEN, M., BINDRUP, M., KIEL, P., LARSEN, H., & MAXILD, J.
(1980) Mutagenic action of isocyanates used in the production
of polyurethanes. Scand. J. Work environ. Health, 6: 221-226.
ANDERSON, D. & STYLES, J.A. (1978). Appendix II. The bacterial
mutation test. Br. J. Cancer, 37: 924-930.
ANON. (1983) Polyurethane markets may be maturing. Chem. Eng.
News, 61(46): 16.
ARAGON, J.J., FELIU, J.E., FRENKEL, R.A., & SOLS, A. (1980)
Permeabilization of animal cells for kinetic studies of
intracellular enzymes: in situ behavior of the glycolytic
enzymes of erythrocytes. Proc. Natl Acad. Sci. (USA), 77(11):
6324-6328.
AUDUNSSON, G. & MATHIASSON, L. (1983) Simultaneous determination
of amines and isocyanates in working atmospheres by gas-liquid
chromatography. J. Chromatogr., 261: 253-264.
AVERY, S.B., STETSON, D.M., PAN, P.M., & MATHEWS, K.P. (1969)
Immunological investigation of individuals with toluene
diisocyanate asthma. Clin. exp. Immunol., 4: 585-596.
AXFORD, A.T., MCKERROW, C.B., JONES, P.A., & LE QUESNE, P.M. (1976)
Accidental exposure to isocyanate fumes in a group of firemen. Br.
J. ind. Med., 33: 65-71.
BAGON, D.A. & HARDY, H.L. (1978) Determination of free monomeric
toluene diisocyanate (TDI) and 4,4'-diisocyanate-diphenylmethant
(MDI) prepolymers, respectively, by high performance liquid
chromatography. J. Chromatogr., 152(2): 560-564.
BARKMAN, H.W., BANKS, D.E., HENDRICK, D.J., JONES, R.N.,
ABDELKADER, H.M., HAMMAD, Y.Y., GLINDMEYER, H.W., & WEILL, H.
(1984) Inhalation challenge testing with 2,4 and 2,6 toluene
diisocyanate isomers. Chest, 86(2): 340.
BAUR, X. (1983) Immunologic cross-reactivity between different
albumin-bound isocyanates. J. Allergy clin. Immunol., 71(2): 197-205.
BAUR, X. & FRUHMANN, G. (1981) Specific IgE antibodies in
patients with isocyanate asthma. Chest, 80: 73-76.
BAUR, X., DEWAIR, M., & FRUHMANN, G. (1983) Detection of
immunologically sensitized isocyanate workers by RAST and
intracutaneous skin tests. J. clin. Immunol., 73: 610.
BEALL, J.R. & ULSAMER, A.G. (1981) Toxicity of volatile organic
compounds present indoors. Bull N.Y. Acad. Med., 57(1): 978-996.
BELIN, L., WASS, U., AUDUNSSON, G., & MATHIASSON, L. (1983)
Amines: possible causative agents in the development of bronchial
hyperreactivity in workers manufacturing poly-urethanes from
isocyanates. Br. J. ind. Med., 40: 251-257.
BENGTSSON, B.-E. & TARKPEA, M. (1983) The acute aquatic toxicity
of some substances carried by ships. Mar. Pollut. Bull., 14(6): 213-214.
BERNSTEIN, I. (1982) Isocyanate-induced pulmonary diseases: a
current perspective. J. Allergy clin. Immunol., 70(1): 25-31.
BRAMAN, S.S. & TEPLITZ, C. (1978) Occupational lung disease.
Primary Care, 5(3): 425-445.
BRANDT, G.H. (1972) Soil stabilizers. In: Goring, C.A.I. &
Hamaker, J.W., ed. Organic chemicals in the soil environment, New
York, Marcel Dekker, pp. 692-693, 719-720.
BROCHHAGEN, F.K. & GRIEVESON, B.M. (1983) Environmental aspects
of isocyanates in water and soil. Cell. Polym., 3: 11-17.
BROWN, W.E. & WOLD, F. (1971) Alkyl isocyanates as active-site
specific inhibitors of chymotrypsim and elastase. Science, 174:
608-610.
BROWN, W.E. & WOLD, F. (1973) Alkyl isocyanates as active-site-
specific reagents for serine proteases. Biochemistry, 12: 828-834.
BROWN, W.E., GREEN, A.H., KAROL, M.H., & ALARIE, Y.C.E (1982)
Inhibition of cholinesterase activity by isocyanates. Toxicol.
appl. Pharmacol., 63: 45-52.
BRUCKNER, H.C., AVERY, S.B., STETSON, D.M., & DODSON, V.N. (1968)
Clinical and immunologic appraisal of workers exposed to
diisocyanates. Arch. environ. Health, 16: 619-625.
BRUGSCH, H.G. & ELKINS, H.B. (1963) Toluene diisocyanate (TDI)
toxicity. New Engl. J. Med., 268(7): 353-357.
BUCKLEY, L.A., JIANG, X.Z., JAMES, K.T., MORGAN, K.T., & BARROW,
C.S. (1984) Respiratory tract leisons induced by sensory
irritants at the RD50 concentration. Toxicol. appl. Pharmacol., 74:
417-429.
BUNGE, W., EHRLICHER, H., & KIMMERLE, G. (1977) Medical aspects of
work with surface coating systems using the spraying technique. Z.
Arbeitsmed. Arbeitsschutz Prophyl., 4(special ed.): 1-46
BURGE, P.S. (1982) Non-specific bronchial hyper-reactivity in
workers exposed to toluene di-isocyanate, diphenyl methane
diisocyanate and colophony. Eur. J. respir. Dis., 63(123): 91-96.
BURGE, P.S., O'BRIEN, I.M., & HARRIES, M.G. (1979) Peak flow rate
records in the diagnosis of occupational asthma due to isocyanates.
Thorax, 34: 317-323.
BUTCHER, B.T., JONES, R.N., O'NEIL, C.E., GLINDMEYER, H.W., DIEM,
J.E., DHARMARAJAN, V., WEILL, H., & SALVAGGIO, J.E. (1977)
Longitudinal study of workers employed in the manufacture of
toluene-diisocyanate. Am. Rev. respir. Dis., 116: 411-421.
BUTCHER, B.T., KARR, R.M., O'NEIL, C.E., WILSON, M.R., DHARMARAJAN,
V., SALVAGGIO, J.E., & WEILL, H. (1979) Inhalation challenge and
pharmacologic studies of toluene diisocyanate (TDI)-sensitive
workers. J. Allergy clin. Immunol., 64(2): 146-152.
BUTCHER, B.T., O'NEIL, C.E., REED, M.A., & SALVAGGIO, J.E. (1980)
Radioallergosorbent testing of toluene diisocyanate-reactive
individuals using p-tolyl isocyanate antigen. J. Allergy clin.
Immunol., 66(3): 213-216.
CALAS, E., CASTELAIN, P.Y., LAPOINTE, H.R., DUCOS, P., CAVELIER,
C., DUPRAT, P., & POITOU, P. (1977) Allergic contact dermatitis to
a photopolymerizable resin used in printing. Contact Dermat., 3(4):
186-194.
CHADWICK, D.H. & CLEVELAND, T.H. (1981) Isocyanates, organic. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed., New York,
John Wiley and Sons, Vol. 13, pp. 789-818.
CHARLES, J., BERNSTEIN, A., JONES, B., JONES, D.J., EDWARDS, J.H.,
SEAL, R.M.E., & SEATON, A. (1976) Hypersensitivity pneumonitis
after exposure to isocyanates. Thorax, 31: 127-136.
CHEN, S.E. & BERNSTEIN, I.L. (1982) The guinea-pig model of
diisocyanate sensitization immunologic studies. J. Allergy clin.
Immunol., 70(5): 383-392.
COCKCROFT, D.W. & MINK, J.T. (1979) Isocyanate-induced asthma in
an automobile spray painter. Can. Med. Assoc. J., 121(5): 602-604.
CONTE, A. & COSSI, G. (1981) Gas chromatographic determination of
free toluene diisocyanate in flexible urethane foams. J.
Chromatogr., 213: 162-165.
CURTIS, M.W., COPELAND, T.L., & WARD, C.H. (1979) Acute toxicity
of 12 industrial chemicals to freshwater and saltwater organisms.
Water Res., 13: 137-141.
DANKS, J.M., CROMWELL, O., BUCKINGHAM, J., NEWMAN-TAYLOR, A., &
DAVIES, R. (1981) Toluene-diisocyanate induced asthma: evaluation
of antibodies in the serum of affected workers against a tolyl
mono-isocyanate protein conjugate. Clin. Allergy, 11: 161-168.
DAVEY, J.E. & EDWARDS, A.D. (1983) Determination of toluene-2,4-
diisocyanate in rubber fumes. Analyst, 108: 407-411.
DAVIES, R.J., BUTCHER, B.T., O'NEIL, C.E., & SALVAGGIO, J.E.
(1977) The in vitro effects of toluene diisocyanate on lymphocyte
cyclic adenosine monophosphate production by isoproterenol,
prostaglandin, and histamine. J. Allergy clin. Immunol., 60(4): 223-229.
DE PASCALE, A., COBELLI, L., PALADINO, R., PASTORELLO, L., &
FRIGERIO, A. (1983) Quantitative determination of airborne 2,4-
toluene diisocyanate. J. Chromatogr., 256: 352-358.
DEWAIR, M., BAUR, X., & MAUERMAYER, R. (1983) Inhibition of
acetylcholinesterase by diisocyanates and its spontaneous
reactivation. Int. Arch. occup. environ. Health, 52: 257-261.
DHARMARAJAN, V., WEIL, H., & SELF, C.W. (1978) Environmental
characterization of toluene diisocyanate (TDI) in a manufacturing
plant. Am. Ind. Hyg. Assoc. J., 39(5): 414-418.
DIEM, J.E., JONES, R.N., HENDRICK, D.J., GLINDMEYER, H.W.,
DHARMARAIAN, V., BUTCHER, B.T., SALVAGGIO, J.E., & WEILL, H.
(1982) Five-year longitudinal study of workers employed in a new
toluene diisocyanate manufacturing plant. Am. Rev. respir.
Dis., 126(3): 426-428.
DUFF, P.B. (1983) The fate of TDI in the environment. In:
Proceedings of the Society of the Plastic Industry's 6th
International Technical Conference, San Diego, California, New
York, Society of Plastics Industry, pp. 408-412.
DUNCAN, B., SCHEEL, L.D., FAIRCHILD, E.J., RILLENS, R., & GRAHAM,
S. (1962) Toluene diisocyanate inhalation toxicity: Pathology and
mortality. Am. Ind. Hyg. Assoc. J., 23: 447-456.
DUNLAP, K.L., SANDRIDGE, R.L., & KELLER, J. (1976) Determination of
isocyanates in working atmospheres by high speed liquid
chromatography. Anal. Chem., 48: 497-499.
DUPRAT, P., GRADISKI, B., & MARIGNAC, B. (1976) Pouvoir irritant
et allergisant de deux isocyanates: toluène diisocyanate (TDI)
and diphénylméthane diisocyanate (MDI). Eur. J. Toxicol., 9(1): 41-53.
DYSON, W.L. & HERMANN, E.R. (1971) Reduction of atmospheric
toluene diisocyanate by water vapor. Am. Ind. Hyg. Assoc. J., 32(1):
741-744.
FIELDEN, P.R., MCCALLUM, J.J., STANIOS, T., & ALDER, J.F. (1984)
Detection of toluene diisocyanate with a coated quartz
piezoelectric crystal. Anal. Chim. Acta, 162: 85-96.
FRIEBEL, H. & LUCHTRALH H. (1955) [The effect of toluene
diisocyanate (Desmodur T) on the respiratory airways.] Arch. exp.
Pathol. Pharmakol., 277: 93-110 (in German).
FRY, A. (1953) A tracer study of the reaction of isocyanates with
carboxylic acids. J. Am. Chem. Soc., 75: 2686-2688.
GAFFURI, E. & BRUGNONE, F. (1971) [Respiratory troubles from
isocyanates in varnishers.] Med. Lav., 62(2-3): 151-157 (in Italian).
GAME, C.J.A. (1982) Australian TDI workers' sera assayed for IgE
against a p-tolyl-isocyanate-human serum albumin conjugate. Am.
Ind. Hyg. Assoc. J., 43(10): 759-763.
GANDEVIA, B. (1963) Studies of ventilatory capacity and histamine
response during exposure to isocyanate vapour in polyurethane foam
manufacture. Br. J. ind. Med., 20: 204-209.
GRANATEK, C.H., CORRY, P.M., & GOLKIN, E.M. (1975) Use of
fluorescent x-ray spectroscopy to quantitate binding of anti-
embryonic antibody to human tumor cells. AACR Abstr., 16: 47.
GRANT, W.A. (1974) Toxicology of the eye, 2nd ed., Springfield,
Illinois, Charles C. Thomas, p. 1028.
GRIEVESON, B.M. & REEVE, B. (1983) Isocyanate emissions: a review
of work on environmental aspects of handling toluene diisocyanate.
Cell. Polym., 2: 165-175.
GRIM, K.E. & LINCH, A.L. (1964) Recent isocyanate-in-air analysis
studies. Ind. Hyg. J., May-June: 285-290.
HAMA, G.M. (1957) Symptoms in workers exposed to isocyanates:
suggested exposure concentrations. Am. Med. Assoc. Arch. Ind.
Health, 16: 232-233.
HARDY, H.L. & PURNELL, C.J. (1978) Use of foam for the emergency
suppression of vapour emissions from organic isocyanate liquid
surfaces. Ann. occup. Hyg., 21: 95-98.
HARTON, E.E., Jr & RAWL, R.R. (1976) Toxicological and skin
corrosion testing of selected hazardous materials, Springfield,
Virginia, US Department of Commerce (Final report NTIS Publication
264 975).
HARTUNG, R. (1982) Cyanides and nitriles. In: Clayton, G.D. &
Clayton, I.I., ed. Patty's industrial hygiene and toxicology, 3rd
revised ed., New York, John Wiley and Sons, pp. 4845-4850,
4892-4893, 4898-4900.
HENSCHLER, D., ASSMANN, W., & MEYER, K.-O. (1962) [Toxicology of
toluene diisocyanate.] Arch. Toxikol., 19: 364-387 (in German).
HOBARA, T., KOBAYASHI, H., HIGASHIHARA, E., KAWAMOTO, T., IWAMOTO,
S., SHIMAZU, W., & SAKAI, T. (1984) Health hazard by exposure to
toluene-diisocyanate in the shipyard. Bull. environ. Contam.
Toxicol., 32: 134-139.
HOLDREN, M.W., SPICER, C.W., & RIGGIN, R.M. (1984) Gas phase
reaction of toluene diisocyanate with water vapor. Am. Ind. Hyg.
Assoc. J., 45(9): 626-633.
HOSEIN, H.R. & FARKAS, S. (1981) Risk associated with the spray
application of polyurethane foam. Am. Ind. Hyg. Assoc. J., 42(9):
663-665.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein, Lyons, International Agency for Research on Cancer,
p. 303 (Monographs on The Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 19).
IARC (1986) Toluene diisocyanate. In: Some chemicals used in
plastics and elastomers, Lyons, International Agency for Research
on Cancer, pp. 287-323 (Monographs on The Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 39).
III (1980) Technical information: recommendations for the
handling of toluene diisocyanate (TDI), New York, International
Isocyanate Institute Inc.
KAROL, M.H. (1983) Concentration-dependent immunologic response
to toluene diisocyanate (TDI) following inhalation exposure.
Toxicol. appl. Pharmacol., 68: 229-241.
KAROL, M.H., IOSET, H.H., & ALARIE, Y.C. (1978) Tolyl-specific
IgE antibodies in workers with hypersensitivity to toluene
diisocyanate. Am. Ind. Hyg. Assoc. J., 39: 454-458.
KAROL, M.H., DIXON, C., BRADY, M., & ALARIE, Y.C. (1980)
Immunologic sensitization and pulmonary hypersensitivity by
repeated inhalation of aromatic isocyanates. Toxicol. appl.
Pharmacol., 53: 260-270.
KAROL, M.H., HAUTH, B.A., RILEY, E.J., & MAGRENI, C.M. (1981)
Dermal contact with toluene diisocyanate (TDI) produces respiratory
tract hypersensitivity in guinea-pigs. Toxicol. appl. Pharmacol.,
58: 221-230.
KIDO, T., YAMADA, Y., & TERANISHI, H. (1983a) [Studies on toluene
diisocyanate asthma. Part 2. Histamine release from guinea pig
leukocytes by toluene diisocyanate.] Jpn. J. Allergol., 32: 131-137 (in
Japanese).
KIDO, T., YAMADA, Y., TERANISHI, H., DAIMON, N., & SHIMIZU, T.
(1983b) [Studies on toluene diisocyanate asthma. I. Clinico-
allergological Tests.] Jpn. J. Allergol., 32: 111-120 (in Japanese).
KOSCHIER, F.J., BURDEN, E.J., BRUNKHORST, C.S., & FRIEDMAN, M.A.
(1983) Concentration-dependent elicitation of dermal sensitization
in guinea pigs treated with 2,4-toluene diisocyanate. Toxicol.
appl. Pharmacol., 67: 401-407.
LE QUESNE, P.M., AXFORD, A.T., MCKERROW, C.B., & PARRY JONES, A.
(1976) Neurological complications after a single severe exposure
to toluene diisocyanates. Br. J. ind. Med., 33: 72-78.
LINDEN, E., BENGTSSON, B.E., SVANBERG, O., & SUNDSTROM, G. (1979)
The acute toxicity of 78 chemicals and pesticide formulations
against 2 brackish water organisms the Bleak (Alburnus alburnus)
and the Harpacticoid (Nitocra spinipes). Chemosphere, 8: 843-851.
LOESER, E. (1983) Long-term toxicity and carcinogenicity studies
with 2,4/2,6-toluene-diisocyanate (80/20) in rats and mice.
Toxicol. Lett., 15: 71-81.
LUCKENBACH, M. & KIELER, R. (1980) Toxic corneal epithelial edema
from exposure to high atmospheric concentration of toluene
diisocyanates. Am. J. Ophthalmol., 90: 682-686.
MCFADYEN, P. (1976) Determination of free toluene diisocyanate in
polyurethane prepolymers by high-performance liquid chromatography.
J. Chromatogr., 123: 468-473.
MACKAY, D. (1979) Finding fungacity feasible. Environ. Sci.
Technol., 13(10): 1218-1223.
MCKAY, R.T. & BROOKS, S.M. (1983) Induction of tracheal smooth-
muscle hyperresponsiveness to carbachol following exposure to
toluene diisocyanate (TDI) vapors. Am. Rev. respir. Dis., 127(4): 174.
MANNO, M. & LOTTI, M. (1976) Cholinesterases in human toluene
diisocyanate exposure. Int. Arch. occup. environ. Health, 38: 55-60.
MARCALI, K. (1957) Microdetermination of toluene diisocyanates in
atmosphere. Anal. Chem., 29: 552-558.
MARTENS, R. & DOMSCH, K.H. (1981) Microbial degradation of
polyurethane foams and isocyanate based polyureas in different
media. Water Air Soil Pollut., 15: 503-509.
MAZUR, G., BAUR, X., PFALLER, A., & ROMMELT, H. (1986)
Determination of toluene diisocyanate in air by HPLC on band-tape
monitors. Int. Arch. occup. environ. Health, 58: 269-276.
MEDDLE, D.W. & WOOD, R. (1970) A method for the determination of
aromatic isocyanates in air in the presence of primary aromatic
amines. Analyst, 95: 402-407.
MEYER, S.D. & TALLMAN, D.E. (1983) The determination of toluene
diisocyanate in air by high-performance liquid chromatography with
electrochemical detection. Anal. Chim. Acta, 146: 227-236.
MORTILLARO, P.T. & SCHIAVON, M. (1982) [One case of lung cancer
that developed in the course of a bronchopulmonary disease due to
isocyanates.] Med. Lav., 3: 207-209 (in Italian).
MUNN, A. (1960) Experiences with diisocyanates. Trans. Assoc.
Ind. Med. Off., 9: 134-138.
MUNN, A. (1968) Health hazards from isocyanates. Adv.
Polyurethane Technol., 16: 299-306.
MUSK, A.W., PETERS, J.M., DEBERARDINIS, L., & MURPHY, R.L.H.
(1982) Absence of respiratory effects in subjects exposed to low
concentrations of TDI and MDI. J. occup. Med., 24(10): 746-749.
NIOSH (1978) Criteria for a recommended standard occupational
exposure to diisocyanates, Rockville, Maryland, US National
Institute for Occupational Safety and Health (NIOSH 78-215;
PB 81-226615).
NIOSH (1981) Respiratory and immunologic evaluation of isocyanate
exposure in a new manufacturing plant, Washington DC, US National
Institute of Occupational Safety and Health, Department of Health
and Human Services, (DHHS (NIOSH) Publication No. 81-125).
NTP (1985) Disposition of 2,6-toluene diisocyanate in fischer 344
rats, Research Triangle Park, North Carolina, US National
Toxicology Program (Project Report No. 7; Contract No. N01-ES-1-
5007, RTI/2227/00-06P).
NTP (1986) Toxicology and carcinogenesis studies of commercial
grade 2,4(80%)- and 2,6(20%)-toluene diisocyanate (CAS No. 26471-
62-5) in F344/N rats and B6C3F1 mice (gavage studies), Research
Triangle Park, North Carolina, US National Toxicology Program
(Technical Report No. 251; NIH Publication No. 86-2507).
OHSAWA, T. (1983) [Experimental studies of the biological effects
on TDI: an inhalation study in skin-sensitized mice.] J. Tokyo
Women Med. Coll., 53(3): 237-246 (in Japanese).
OMAE, K. (1984) Two-year observation of pulmonary function in
workers exposed to low concentrations of toluene diisocyanate. Int.
Arch. occup. environ. Health, 55: 1-12.
OZAWA, H. (1967) Bridging reagent for protein. I. The reaction of
diisocyanates with lysine and enzyme protein. J. Biochem., 62(4):
419-423.
PAGGIARO, P.L., ROSSI, O., LASTRUCCI, L., PARDI, F., PEZZINI, A., &
BUSCHIERI, L. (1985) TDI-induced oculorhinitis and bronchial
asthma. J. occup. Med., 27: 51-52.
PATTERSON, R., ZEISS, C.R., & HARRIS, K.E. (1983) Immunologic and
respiratory responses to airway challenges of dogs with toluene
diisocyanate. J. Allergy clin. Immunol., 71: 604-611.
PEPYS, J. (1980) Occupational asthm: a review of present clinical
and immunologic status. J. Allergy clin. Immunol., 66: 179-185.
PESCHEL, H. (1970) [Skin alterations by isocyanate (Desmodur).]
Derm. Mschr., 156: 691-697 (in German).
PETERS, J.M. (1970) Cumulative pulmonary effects in workers
exposed to tolylene (sic) diisocyanate. Proc. R. Soc. Med., 63:
372-375.
PETERS, J.M. & MURPHY, R.L.H. (1971) Hazards to health: do it
yourself polyurethane foam. Am. Rev. respir. Dis., 104: 432-433.
PETERS, J.M. & WEGMAN, D.H. (1975) Epidemiology of toluene
diisocyanate (TDI)-induced respiratory disease. Environ. Health
Perspect., 11: 97-100.
PETERS, J.M., MURPHY, R.L.H., PAGNOTTO, L.D., & VAN GANSE, W.F.
(1968) Acute respiratory effects in workers exposed to low levels
of toluene diisocyanate (TDI). Arch. environ. Health, 16: 642-647.
PETERS, J.M., MURPHY, R.L.H., & FERRIS, B.G. (1969) Ventilatory
function in workers exposed to low levels of toluene diisocyanate
(TDI). Br. J. ind. Med., 26: 115-120.
POLLOCK, J.R.A. & STEVENS, R. (1974) Dictionary of organic
compounds, 4th ed., New York, Oxford University Press, Suppl. 10,
p. 337.
PORTER, C., HIGGINS, R.L., & SCHEEL, L.D. (1975) A retrospective
study of clinical, physiologic and immunologic changes in workers
exposed to toluene diisocyanate. Am. Ind. Hyg. Assoc. J., 36: 159-163.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J., ANDERSON,
D., LEFEVRE, P., & WESTWOOD, F. (1978) An evaluation of six
short-term tests for detecting organic chemical carcinogens. Br. J.
Cancer, 37(6): 873-959.
RAMPY, L.W., LOESER, E., LYON, J.P., & CARNEY, I. (1983) Two
carcinogenicity studies of toluene diisocyanate. In: Proceedings of
the Society of the Plastic Industry's 6th International Technical
Conference, San Diego, California, New York, Society of Plastics
Industry.
RANDO, R.J. & HAMMAD, Y.Y. (1985) Modified Marcali method for the
determination of total toluene diisocyanate in air. Am. Ind. Hyg.
Assoc. J., 46(4): 206-210.
REILLY, D.A. (1968) A test-paper method for the determination of
tolylene diisocyanate vapour in air. Analyst, 93: 178-185.
ROSENBERG, C. (1984) Direct determination of isocyanates and
amines as degradation products in the industrial production of
polyurethane-coated wire. Analyst, 109: 859-866.
ROSENBERG, C. & SAVOLAINEN, H. (1985) Detection of urinary amine
metabolites in toluene diisocyanate exposed rats. J. Chromatogr.,
323: 429-433.
ROSENBERG, C. & SAVOLAINEN, H. (1986) Determination of
occupational exposure to toluene diisocyanate by biological
monitoring. J. Chromatogr., 367: 385-392.
SALVAGGIO, J.E. (1979) Occupational asthma: overview of
mechanisms. J. Allergy clin. Immunol., 64: 646-649.
SANGHA, G.K. & ALARIE, Y. (1979) Sensory irritation by toluene
diisocyanate in single and repeated exposures. Toxicol. appl.
Pharmacol., 50: 533-547.
SANGO, C. & ZIMERSON, E. (1980) A new reagent for determination
of isocyanates in working atmosphere by HPLC using UV or
fluorescence detection. J. liq. Chromatogr., 3(7): 971-990.
SCHAFER, E.W., Jr, BOWLES, W.A., Jr, & HURLBUT, J. (1983) The
acute oral toxicity, repellency, and hazard potential of 998
chemicals to one or more species of wild & domestic birds. Arch.
environ. Contam. Toxicol., 12: 355-382.
SCHEEL, L.D., KILLENS, R., & JOSEPHSON, A. (1964) Immuno-
chemical aspects of toluene diisocyanate (TDI) toxicity. Am. Ind.
Hyg. Assoc. J., 25: 179-184.
SHARONOVA, Z.V. & KRYZHANOVSKAYA, N.A. (1976) [Tolylene
diisocyanate induced occupational liver pathology.] Gig. Tr. prof.
Zabol., 11: 27-31 (in Russian).
SHARONOVA, Z.V., PENKNOVICH, A.A., DOROFEEVA, E.D., KRYZHANOV-
SKAYA, N.A., MELNIKOVA, N.D., VOLKOVA, I.D., GOLOVA, I.A.,
ARZYAEVA, E.YA., KLIMOVA, E.I., & MAZE, E.N. (1982) [Changes in
the cardiovascular system of workers engaged in the manufacture of
toluyene diisocyanate.] Gig. Tr. prof. Zabol., 1: 16-19 (in Russian).
SITTIG, M. (1979) Isocyanates. In: Hazardous and toxic effects of
industrial chemicals, Park Ridge, New Jersey, Noyes Data
Corporation, pp. 266-277.
SITTIG, M. (1981) Handbook of toxic and hazardous chemicals, Park
Ridge, New Jersey, Noyes Publications, pp. 664-665.
SMITH, A.B., BROOKS, S.M., BLANCHARD, J., BERNSTEIN, I.L.L., &
GALLAGHER, J. (1980) Absense of airway hyperreactivity to
methacholine in a worker sensitized to toluene diisocyanate (TDI).
J. occup. Med., 22: 327-331.
STEINMETZ, P.R., AL-AWQATI, Q., & LAWTON, W.J. (1976) Specialty
rounds. Nephrology rounds. University of Iowa Hospitals: renal
tubular acidosis. Am. J. med. Sci., 271(1): 40-54.
STYLES, J.A. (1978) Appendix III. Mammalian cell transformation
in vitro. Br. J. Cancer, 37: 931-936.
SWENSSON, A., HOLMQUIST, C.-E., & LUNDGREN, K.-D. (1955) Injury
to the respiratory tract by isocyanates used in making lacquers.
Br. J. ind. Med., 12: 50-53.
TANAKA, K.-I. (1979) [On the induction of contact sensitivity by
tolylene diisocyanate (TDI) in mice.] Jpn. J. ind. Health, 21: 456-457
(in Japanese).
TANAKA, K.-I., OKAMOTO, Y., TAKEOKA, A., & INO, T. (1984) [Nasal
allergy observed in an asthma model by toluene diisocyanate.] Jpn.
J. Allergol., 33(4): 199-206 (in Japanese).
TED TSE, C.S. & PESCE, A.J. (1979) Chemical characterization of
isocyanate-protein conjugates. Toxicol. appl. Pharmacol., 51: 39-46.
THURMAN, G.B., SIMMS, B.G., & GOLDSTEIN, A.L. (1978) The effects
of organic compounds used in the manufacture of plastics on the
responsivity of murine and human lymphocytes. Toxicol. appl.
Pharmacol., 44: 617-641.
TUCKER, S.P. & ARNOLD, J.E. (1982) Sampling and determination
of 2,4-Bis (carbonylamino)toluene and 4,4'-bis(carbonylamino)
diphenylmethane in air. Anal. Chem., 54: 1137-1141.
TWE, J. & WOLD, F. (1973) Butyl isocyanate, an active-site-
specific reagent for yeast alcohol dehydrogenase. Biochemistry,
12(3): 381-386.
ULRICH, H. (1983) Urethane polymers. In: Kirk-Othmer encyclopedia
of chemical technology, 3rd ed., New York, John Wiley and Sons,
Vol. 23, pp. 576-608.
US EPA (1984) Toluene diisocyanates (TDI) 584-84-9; 91-08-7;
26471-62-5; 1321-38-6, Washington DC, US Environmental Protection
Agency (Chemical Hazard Information Profile Report).
US ITC (1985) Preliminary report on US production of selected
synthetic organic chemicals. Imports, exports and sales,
Washington DC, US International Trade Commission.
WALKER, R.F. & PINCHES, M.A. (1981) Chemical interference effects
in the measurement of atmospheric toluene diisocyanate
concentrations when sampling with an impregnated paper tape. Am.
Ind. Hyg. Assoc. J., 42: 392-397.
WALWORTH, H.T. & VIRCHOW, W.E. (1959) Industrial hygiene
experiences with toluene diisocyanate. Am. Ind. Hyg. Assoc. J., 20:
205-210.
WARWICK, C.J., BAGON, D.A., & PURNELL, C.J. (1981) Application of
electrochemical detection to the measurement of free monomeric
aromatic and aliphatic isocyanates in air by high-performance
liquid chromatography. Analyst, 106: 676-685.
WEGMAN, D.H., PAGNOTTO, L.D., FINE, L.J., & PETERS, J.M. (1974) A
dose-response relationship in TDI workers. J. occup. Med., 16: 258-260.
WEGMAN, D.H., PETERS, J.M., PAGNOTTO, L.D., & FINE, L.J. (1977)
Chronic pulmonary function loss from exposure to toluene
diisocyanate. Br. J. ind. Med., 34: 195-200.
WEILL, H., SALVAGGIO, J., NEILSON, A., BUTCHER, B., & ZISKIND, M.
(1975) Respiratory effects in toluene diisocyanate manufacture: a
multidisciplinary approach. Environ. Health Perspect., 11: 101-108.
WEILL, H., BUTCHER, B., DIEM, J.E., ET AL. (1979) Respiratory and
immunologic evaluation of isocyanate exposure in a new
manufacturing plant, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health (Final Report, NIOSH Contract No.
210-75-006).
WEYEL, D.A., RODNEY, B.S., & ALARIE, Y. (1982) Sensory
irritation, pulmonary irritation, and acute lethality of a
polymeric isocyanate and sensory irritation of 2,6-toluene
diisocyanate. Toxicol. appl. Pharmacol., 64: 423-430.
WHITE, W.G., MORRIS, M.J., SUGDEN, E., & ZAPATA E. (1980)
Isocyanate-induced asthma in a car factory. Lancet, 1(8171): 756-760.
WINDHOLZ, M., ed. (1983) The Merck Index: an encyclopedia of
chemicals and drugs, 10th ed., Rahway, New Jersey, Merck and
Company, p. 1363.
WOOLRICH, P.F. (1982) Toxicology, industrial hygiene and medical
control of TDI, MDI and PMPPI. Am. Ind. Hyg. Assoc. J., 43(1): 89-97.
WOOLRICH, P.F. & RYE, W.A. (1969) Urethanes. Engineering, medical
control and toxicologic consideration. J. occup. Med.,11(4): 184-190.
ZAPP, J.A., Jr (1957) Hazards of isocyanates in polyurethane foam
plastic production. Arch. ind. Health, 15(4): 324-330.
 Toluene diisocyanates are produced as 2 isomers (2,4-toluene
diisocyanate (2,4-TDI) and 2,6-toluene diisocyanate (2,6-TDI))
and are commercially available in 3 isomer ratios: (a) > 99.5%
2,4-TDI; (b) 80% 2,4-TDI/20% 2,6-TDI, which is the most common
and referred to in this document as 80:20 mixture; and (c) 65%
2,4-TDI/35% 2,6-TDI. "Crude" toluene diisocyanate (Crude-TDI),
with an unidentified isomer ratio, is also commercially available,
but not widely used. Various identification codes for the most
commonly marketed toluene diisocyanates are listed in Table 1.
Table 1. Identification codes of commercial toluene diisocyanatesa
-------------------------------------------------------------------
Numeric index 2,4-TDI 2,6-TDI Commercial
(80:20)
mixture
-------------------------------------------------------------------
CAS register number
Toluene diisocyanates are produced as 2 isomers (2,4-toluene
diisocyanate (2,4-TDI) and 2,6-toluene diisocyanate (2,6-TDI))
and are commercially available in 3 isomer ratios: (a) > 99.5%
2,4-TDI; (b) 80% 2,4-TDI/20% 2,6-TDI, which is the most common
and referred to in this document as 80:20 mixture; and (c) 65%
2,4-TDI/35% 2,6-TDI. "Crude" toluene diisocyanate (Crude-TDI),
with an unidentified isomer ratio, is also commercially available,
but not widely used. Various identification codes for the most
commonly marketed toluene diisocyanates are listed in Table 1.
Table 1. Identification codes of commercial toluene diisocyanatesa
-------------------------------------------------------------------
Numeric index 2,4-TDI 2,6-TDI Commercial
(80:20)
mixture
-------------------------------------------------------------------
CAS register number 