
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 74
DIAMINOTOLUENES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1987
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154274 8
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1987
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DIAMINOTOLUENES
1. SUMMARY AND CONCLUSIONS
1.1. Summary
1.1.1. Identity and analytical methods
1.1.2. Production, uses, and sources of exposure
1.1.3. Kinetics
1.1.3.1 Animal studies
1.1.3.2 Human studies
1.1.4. Effects on organisms in the environment
1.1.5. Effects on experimental animals
1.1.6. Effects on human beings
1.2. Conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Natural occurrence
3.2. Production
3.3. Uses
3.4. Release into the environment, distribution, and
transformation
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental levels
4.2. General population exposure
4.3. Occupational exposure
5. KINETICS AND METABOLISM
5.1. Studies on experimental animals
5.1.1. Absorption and retention
5.1.2. Distribution and reaction with body components
5.1.3. Metabolism
5.1.4. Excretion
5.2. Human studies
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.2. Short-term exposures
7.3. Long-term exposure
7.4. Reproduction and teratogenicity
7.4.1. Reproduction
7.4.2. Teratogenicity
7.5. Mutagenicity and related end-points
7.5.1. DNA damage
7.5.2. Mutation
7.5.3. Cell transformation
7.5.4. Chromosomal effects
7.6. Carcinogenicity
8. EFFECTS ON MAN
8.1. Single and short-term exposures
8.2. Long-term occupational exposure - epidemiological studies
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
9.1. Evaluation of human health risks
9.1.1. General considerations
9.1.2. Assessment of exposure
9.1.3. Single and short-term exposures
9.1.4. Long-term exposure
9.1.4.1 Carcinogenicity and mutagenicity
9.1.4.2 Reproduction and teratogenicity
9.2. Evaluation of effects on the environment
9.3. Conclusions
10. RECOMMENDATIONS
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON DIAMINOTOLUENES
Members
Dr X. Baur, Pulmonary Section, Klinikum Grosshaden, University of
Munich, Munich, Federal Republic of Germany
Dr L. Belin, Department of Medicine, Sahlgren's Hospital, Goteborg,
Sweden
Ms Andrea Blaschka, Office of Toxic Substances, US Environmental
Protection Agency, Washington DC, USA (Co-Rapporteur)
Dr M. Dieter, US National Institute for Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
(Co-Rapporteur)
Dr M. Greenberg, Department of Health and Social Security, London,
United Kingdom
Dr I. Gut, Institute of Hygiene and Epidemiology, Prague,
Czechoslovakia (Chairman)
Dr M. Mann, Bayer AG, Leverkusen, Bayerwerk, Federal Republic of
Germany
Dr C. Rosenburg, Institute of Occupational Health, Department of
Industrial Hygiene and Toxicology, Helsinki, Finland
Professor H. Sakurai, School of Medicine, Keio University, Tokyo,
Japan
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization,
Research Triangle Park, North Carolina, USA (Secretary)
Mr A.C. Fletcher, International Agency for Research on Cancer,
Lyons, France
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR DIAMINOTOLUENES
A WHO Task Group on Environmental Health Criteria for
Diaminotoluenes met at the Monitoring and Assessment Research
Centre, London, United Kingdom, from 20 to 25 October 1986.
Professor P.J. Petersen welcomed the participants on behalf of the
host Institution, and Dr G.C. Becking opened the meeting on behalf
of the three co-sponsoring organizations of the IPCS
(ILO/UNEP/WHO). The Task Group reviewed and revised the draft
criteria document and made an evaluation of the health risks of
exposure to diaminotoluenes.
The efforts of MS ANDREA BLASCHKA, US ENVIRONMENTAL PROTECTION
AGENCY, Washington DC, USA, in the preparation of the draft, and of
all others who helped in the preparation and finalization of the
document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
1. SUMMARY AND CONCLUSIONS
1.1. Summary
1.1.1. Identity and analytical methods
Diaminotoluenes are synthetic aromatic amines (total of 6
isomers). The isolated, purified isomers are colourless crystals,
while the commercial isomeric mixtures are light yellow to tan
(Meta-diaminotoluene), or light grey to purple (Ortho-diamino-
toluene) solids. Diaminotoluenes are soluble in hot water,
alcohol, ether, and hot benzene. When heated, they emit toxic
fumes of nitrogen oxides.
Several qualitative and quantitative procedures for the
determination of diaminotoluenes have been developed using thin-
layer, high-performance-liquid, or gas chromatography, methods.
Detection limits in air samples range from 0.1 to 10 µg/m3. The
isomeric ratios in technical grade mixtures have been determined by
nuclear magnetic resonance and infra-red spectrometry.
1.1.2. Production, uses, and sources of exposure
Diaminotoluenes are produced from dinitrotoluenes through a
catalytic hydrogenation procedure, or by the reaction of iron and
hydrochloric acid with dinitrotoluenes. Diaminotoluenes are large-
volume intermediates used in the production of a wide variety of
industrial and consumer products. The mixture of 2,4- and 2,6-
isomers is used predominantly as an intermediate in the manufacture
of toluene diisocyanate. Commercial mixtures of 2,3- and 3,4-
isomers, as well as the 2,4- and 2,6-isomers, are used as co-
reactants or as raw materials in the manufacture of urethane
products, dyes, corrosion inhibitors, and rubber antioxidants.
Diaminotoluene isomers have relatively limited use as epoxy curing
agents and as photographic developers. The most commonly marketed
isomers and isomer mixtures are 2,4-diaminotoluene (2,4-DAT), 3,4-
DAT, Meta-DAT (an 80:20 or 65:35 mixture of the 2,4- and 2,6-
isomers), and Ortho-DAT (3,4-, 2,3-isomers, as 60:40 mixture); 2,5-
diaminotoluene is also marketed in small quantities. These isomers
and their mixtures are reviewed together, because any single
commercial product will contain various levels of the other
isomers.
The major sources of environmental pollution are the
manufacture of diaminotoluenes and their products. Over 50% of the
losses into the environment are through industrial wastes deposited
in landfills. Diaminotoluenes are soluble in water; therefore,
leakage from landfills or storage sites, and spillage during
shipping and handling may also represent sources of surface and
groundwater contamination.
Despite the wide use and the water solubility of diamino-
toluenes, there is a lack of information concerning their levels in
the environment, as well as data on their transport and their fate
in the ecosystem.
Data are not available on the exposure of the general
population to diaminotoluenes and there is a paucity of data on the
exposure of workers to diaminotoluenes, though work-place air
levels ranging up to 0.44 mg/m3, with occasional excursions up to
11 mg/m3, have been reported.
1.1.3. Kinetics
1.1.3.1. Animal studies
Diaminotoluenes have been absorbed via all exposure routes
tested. Skin penetration by diaminotoluenes is affected by the
type of vehicle and site of application. The greatest absorption
of 2,4-diaminotoluene (approximately 50%) resulted when the test
material was dissolved in acetone and applied to the abdominal skin
of monkeys. Following intraperitoneal injection of [14C]-2,4-
diaminotoluene, absorption was rapid and peak concentrations in rat
and mouse blood and plasma occurred within 1 h and decreased
rapidly for 7 h.
Distribution varies with different species. However, data
indicate that, in most species, the organs with the highest
concentrations are the liver, kidneys, and adrenal glands. High
concentrations are also observed in the gastrointestinal tract,
while the lowest levels are found in the heart, gonads, brain, and
blood. A dose-dependent binding of the 2,4-isomer to hepatic and
renal proteins has been demonstrated.
The acetylation of amino groups, oxidation of methyl groups,
and ring hydroxylation appear to be the major metabolic steps.
Phenolic metabolites and trace amounts of unchanged diaminotoluenes
are excreted in the urine of experimental animals. Elimination of
diaminotoluene metabolites takes place via both urine and faeces.
However, the primary route and rate of elimination varies with
different species, e.g., urinary elimination is faster and more
complete in mice (2 days) than in rats (6 days).
1.1.3.2. Human studies
Data are not available on the kinetics and metabolism of
diaminotoluenes after oral or inhalation exposure. The results of
skin penetration studies correspond with those from experimental
animal studies. After 40 min of dermal contact, the highest rate
of urinary excretion occurred 4 - 8 h after exposure. During 24 h
of dermal contact, the highest absorption of 2,4-diaminotoluene
resulted when test material was dissolved in acetone and applied to
the skin of the forearm (23.7%). Data from studies on human
volunteers showed that, after subcutaneous injection of 5.5 mg 2,5-
diaminotoluene, 47.6% of the dose was excreted in the urine as
N,N'-diacetyl-2,5-diaminotoluene.
1.1.4. Effects on organisms in the environment
Diaminotoluenes are toxic for aquatic species. Daphnia, the
most sensitive species of those tested, was adversely affected at
concentrations of 2 - 5 mg/litre. At higher concentrations,
diaminotoluenes were toxic for ostracods, fish, and algae, the
algal species tested being the most tolerant. No data are
available on other non-mammalian species in the environment.
1.1.5. Effects on experimental animals
2,4- and 2,5-Diaminotoluenes are ocular and dermal irritants.
Instillation of 100 µg 2,4-diaminotoluene in the rabbit eye caused
severe eye irritation within 24 h. In rabbits, irritation and
blisters developed after 24-h dermal contact with 500 mg 2,4-
diaminotoluene or 12.5 mg 2,5-diaminotoluene.
Dermal contact with 1 - 10% solutions of 2,5-diaminotoluene
resulted in the development of severe irritation and leukocyte
infiltration in 25 - 50% of exposed guinea-pigs. In addition, 35%
of the exposed animals were sensitized to the test compound.
Dermal contact was for 24 h/day for 2 periods of 5 days, separated
by 2 days free of exposure.
Diaminotoluenes are mild cumulative poisons, and their toxicity
in different species varies considerably. The acute oral LD50 of
Meta-diaminotoluene for the mouse was 350 mg/kg body weight; for
the rat, it ranged from 270 to 300 mg/kg body weight. The acute
oral LD50 of Ortho-diaminotoluene for the rat was 810 mg/kg (range,
590 - 1120 mg/kg body weight). The dermal LD50 of Meta-
diaminotoluene for the rat was 1200 mg/kg body weight, while the
dermal LD50 of Ortho-diaminotoluene for the rabbit was 1120 mg/kg
(range, 650 - 2040 mg/kg body weight).
At extremely high exposure levels, diaminotoluenes are toxic
for the central nervous system, produce jaundice, and induce
anaemia by destruction of the red blood cells after methaemoglobin
formation.
In short-term studies, the toxic effects of 2,4-diaminotoluene
are characterized by a decrease in body weight and an increase in
the liver:body weight ratio. Following a 5-day oral treatment of
male F-344 rats with 70 mg 2,4-diaminotoluene/kg body weight, per
day, the activities of microsomal cytochrome P-450-dependent
enzymes were depressed, while that of epoxide hydrolase was
markedly elevated (3 - 8 times that in controls). 2,4-
Diaminotoluene or one of its metabolites has been shown to bind
irreversibly to hepatic and renal proteins and to liver ribosomal
RNA. Oral ingestion of 2,4-diaminotoluene at 50 or 100 mg/kg for 2
years accelerated the development of chronic renal disease in F-344
rats, an effect that contributed to a marked decrease in survival.
The reproductive and teratogenic effects of diaminotoluenes
depend on the route of administration, the isomer studied, and the
species of the experimental animal. Results of recent studies have
shown that 2,4-diaminotoluene (98% pure) is a potent reproductive
toxin in the male rat. At a level of 0.3 g/kg diet for 10 weeks
(~ 15 mg/kg body weight per day), this agent produced marked toxic
effects on spermatogenesis (66% reduction) associated with a
significant reduction in the weights of the seminal vesicles and
epididymides, as well as a diminished level of circulating
testosterone, and an elevation of serum-luteinizing hormone.
The 2,6-isomer, but not the 2,4-isomer, is embryotoxic in the
rat and rabbit and has been reported to cause malformation in the
rat. The no-observed-adverse-effect level for 2,6-diaminotoluene
was 10 mg/kg body weight in the rat, and 30 mg/kg body weight in
the rabbit. Ortho-diaminotoluene (2,3-, 3,4-isomer mixture) is
toxic for the treated dams, their embryos, and fetuses. The no-
observed-adverse-effect level is 30 mg/kg body weight in both the
rat and rabbit.
Diaminotoluenes have been shown to be mutagenic in several in
vitro assays and in Drosophila, but the results in several in vivo
mammalian assays were negative.
2,4-Diaminotoluene is the only isomer that has been reported to
produce an increased incidence of tumours in rodents. This isomer
produces hepatocellular, subcutaneous, and mammary gland tumours in
rats and hepatocellular and vascular tumours in mice, when present
in the diet at levels > 79 mg/kg. On the other hand, it was
reported that the 2,6-isomer was not carcinogenic for rodents.
Tumours in the same organs as those affected by 2,4-diaminotoluene
were found after administration of 2,6-diaminotoluene at > 250
mg/kg for 103 weeks, but they were considered not significant after
extensive statistical evaluation.
1.1.6. Effects on human beings
Diaminotoluenes are irritant to the eyes and the skin. Local
actions include severe dermatitis, blistering, and urticaria, and,
in the eye, lachrymation, corneal opacities, and permanent
blindness, if untreated. In the case of inhalation of fumes,
coughing, dyspnoea, and respiratory distress may result.
The epidemiological assessment of the reproductive hazards for
males exposed to DAT (in most cases, together with dinitrotoluene)
revealed inconclusive findings suggesting adverse effects on sperm
production and on the viability of pregnancies in women whose
husbands have been exposed. Sperm samples from workers in 3 DAT
production plants showed a reduced sperm count in one plant (with
the smallest study group and an unusually high sperm count in the
control group), but also a reduced proportion of large
morphological sperm. Studies of the reproductive history of the
wives of workers in 3 plants (in 2 of which semen analysis was also
carried out) revealed excess miscarriage rates, which are related
to DAT exposure in two populations, though both suffered from
limited size and the risk of some self selection of volunteers who
participated in the study. Given the animal evidence of adverse
effects on spermatogenesis, these findings are of concern.
1.2. Conclusions
Diaminotoluenes are highly irritating to the skin and eyes, and
the fumes are irritating to the respiratory tract. Diaminotoluenes
are readily absorbed through the skin, and exposure may result in
methaemoglobinaemia. Renal toxicity after oral administration of
2,4-diaminotoluene has been reported in experimental animals. 2,4-
Diaminotoluene has been shown to be carcinogenic for animals, but
there is inadequate evidence to evaluate the carcinogenic potential
of 2,5- and 2,6-diaminotoluene. All three of these isomers have
been shown to be mutagenic. They are reproductive toxins in
experimental animals, but human reproduction data are limited.
Diaminotoluenes should be handled as hazardous chemicals.
Preventive measures should be taken to avoid exposure of workers
and to prevent environmental pollution.
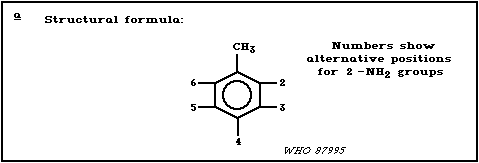
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Diaminotoluenes are synthetic aromatic amines (total of 6
isomers) with two amino groups and a methyl group attached to a
benzene ring (Table 1). The molecular formula is C7H10N2 and the
relative molecular mass, 122.17.
Table 1. Identity of diaminotoluene isomersa
-----------------------------------------------
Diaminotoluenes CAS RTECS
registry accession number
number index
-----------------------------------------------
Isomers
2,3-DAT 2687-25-4 ---
2,4-DAT 95-80-7 XS9625000
2,5-DAT 95-70-5 XS9700000
2,6-DAT 823-40-5 XS9750000
3,4-DAT 496-72-0 XS9820000
3,5-DAT 108-71-4 ---
Commercial mixture
Meta-DAT
(2,4-, 2,6- 95-80-7 ---
isomers mix) 823-40-5
(80:20)
Ortho-DAT 26787-25-4 ---
(2,3-, 3,4- 496-72-0
isomer mix)
(40:60)
-----------------------------------------------
a STRUCTURAL FORMULA:
 Commercial grades of diaminotoluenes are available; however,
the most commonly marketed diaminotoluenes are: (a) "crude"
diaminotoluenes-mixture, containing all 6 isomers (Table 1); (b)
Meta-diaminotoluene (Meta-DAT), containing approximately 80% 2,4-
and 20% 2,6-isomers (also produced in smaller amounts as 65:35
mixture); and (c) Ortho-diaminotoluene (Ortho-DAT), consisting of
approximately 40% 2,3- and 60% 3,4-isomers. All commercial grades
contain traces of the other isomers; therefore, diaminotoluenes and
their mixtures are reviewed together in this document.
Most of the common and trade names for commercial
diaminotoluenes are listed in Table 2.
Table 2. Diaminotoluenes synonyms and trade names
----------------------------------------------------------------------------
A. Commercial mixtures
I. Meta-Diaminotoluenes
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names benzenediamine,ar-methyl (RTECS: TDB);
diaminotoluene (RTECS: TDB);
phenylenediamine,ar-methyl- (TDB);
Meta-diaminotoluene; Meta-toluene-
diamine (MTD);
toluene-ar,ar-diamine (8CI) (CAS: TDB);
toluenediamine (RTECS: TDB, DOT);
tolylenediamine (RTECS, TDB)
Common name diaminotoluene; toluenediamine; TDA
II. Ortho-Diaminotoluene
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names o-TDA
Common name Ortho-toluenediamine; OTD
B. Diaminotoluene isomers
I. 2,3-isomer
Chemical abstract name 1,2-benzenediamine,3-methyl- (9CI)
Other chemical names toluene-2,3-diamine (8CI) (CAS: TDB);
1-methyl-1,2,3-phenylenediamine (TDB);
1,2-diamino-3-methylbenzene (TDB);
2,3-diaminotoluene (TDB*);
2,3-toluylenediamine (TDB);
2,3-tolylenediamine (TDB);
3-methyl-o-phenylenediamine (TDB);
3-methyl-1,2-phenylenediamine (TDB)
Common names 2,3-TDA
II. 2,4-isomer
Chemical abstract name 1,3-benzenediamine,4-methyl- (9CI)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
II. 2,4-isomer (contd.)
Other chemical names m-toluenediamine (RTECS);
m-toluylendiamin (Czech, RTECS: TDB);
m-toluylenediamine (RTECS: TDB);
m-tolyenediamine (RTECS: TDB);
m-tolylenediamine (RTECS);
Meta-toluylene diamine (RTECS: TDB);
toluene-2,4-diamine (8CI) (CAS, RTECS:
TDB*);
tolylene-2,4-diamine (RTECS: TDB);
1,3-diamino-4-methylbenzene (RTECS: TDB);
2,4-diamino-1-methylbenzene (RTECS: TDB);
2,4-diamino-1-toluene (RTECS: TDB);
2,4-diaminotoluen (Czech, RTECS: TDB);
2,4-diaminotoluene (MESH, RTECS: TDB);
2,4-diaminotoluol (RTECS: TDB);
2,4-tolamine (RTECS: TDB);
2,4-toluenediamine (MESH, RTECS: TDB);
2,4-toluylenediamine (DOT, RTECS: TDB);
2,4-tolylenediamine (RTECS: TDB);
3-amino- p-toluidine (RTECS: TDB);
4- m-tolylenediamine (RTECS: TDB);
4-methyl- m-phenylenediamine (RTECS: TDB);
4-methyl-1,3-benzenediamine (RTECS: TDB);
5-amino- o-toluidine (RTECS: TDB)
Common names TDA; MTD; 2,4-TDA (CAS, RTECS: TDB)
Trade names Azogen Developer H; Benzofur MT;
C.I. Oxidation Base (RTECS);
C.I. Oxidation Base 20;
C.I. Oxidation Base 35 (RTECS);
C.I. Oxidation Base 200;
Developer B (RTECS: TDB); Developer DB
(RTECS: TDB);
Developer DBJ (RTECS: TDB); Developer H;
Developer MC (RTECS: TDB); Developer MT
(RTECS: TDB);
Developer MT-CF (RTECS: TDB);
Developer MTD (RTECS; TDB);
Developer T (RTECS: TDB); Developer 14;
Eucanine GB (RTECS: TDB); Fouramine;
Fouramine J (RTECS: TDB); Fourrine M
(RTECS: TDB);
Fourrine 94 (RTECS: TDB);
Lekutherm-Haerter VP-KU 6546;
Nako TMT (RTECS: TDB); NCI-C02302 (RTECS:
TDB);
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
Trade names (contd.) Pelagol J (RTECS: TDB); Pelagol Grey J
(RTECS: TDB);
Pontamine Developer TN (RTECS: TDB);
Renel MD (RTECS: TDB); Tertral G;
Zoba GKE (RTECS: TDB);
Zogen Developer H (RTECS: TDB).
Colour index number 76035
III. 2,5-isomer
Chemical abstract name 1,4-benzenediamine,2-methyl-(9CI)
Other chemical names p-toluenediamine (MESH, RTECS: TDB);
p-toluylendiamine (RTECS: TDB);
P,m-tolylenediamine (RTECS: TDB);
para-meta-tolylenediamine;
para-toluenediamine;
para-toluylenediamine;
para-tolylenediamine;
toluene-2,5-diamine (8CI) (CAS, RTECS:
TDB);
toluylene-2,5-diamine (RTECS: TDB);
2-methyl- p-phenylenediamine (RTECS: TDB);
2-methyl-1,4-benzenediamine (RTECS: TDB*);
2,5-diaminotoluene (MESH, RTECS: TDB);
2,5-toluenediamine;
4-amino-2-methylaniline (RTECS: TDB)
Common name 2,5-TDA
Trade names Oxidation Base 4 (as sulfate)
Colour index number C.I. 76042 (RTECS); 76043 (as sulfate)
IV. 2,6-isomer
Chemical abstract name 1,3-benzenediamine,2-methyl- (9CI)
Other chemical names m-phenylenediamine-2-methyl;
toluene-2,6-diamine (8CI) (CAS, RTECS:
TDB);
tolylene-2,6-diamine;
1,3-benzenediamine;
2-methyl- m-phenylenediamine (TDB);
2-methyl-1,3-benzenediamine (TDB);
2-methyl-1,3-phenylenediamine (TDB);
2,6-diamino-1-methylbenzene (TDB);
2,6-diaminotoluene (MESH, RTECS: TDB*);
2,6-toluenediamine;
2,6-toluylenediamine (RTECS: TDB);
2,6-tolylenediamine (RTECS: TDB)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
IV. 2,6-isomer (contd.)
Common names 2,6-TDA
V. 3,4-isomer
Chemical abstract names 1,2-benzenediamine,4-methyl- (9CI)
Other chemical names o-toluenediamine;
toluene-3,4-diamine (8CI) (CAS, RTECS:
TDB);
1,2-benzenediamine,4-methyl- (RTECS: TDB);
1,2-diamino-4-methylbenzene (TDB);
3,4-diamino-1-methylbenzene (TDB);
3,4-diaminotoluene (RTECS: TDB);
3,4-toluenediamine;
3,4-toluylenediamine (RTECS);
3,4-tolylenediamine (RTECS: TDB);
4-methyl- o-phenylenediamine (TDB);
4-methyl-1,2-benzenediamine (TDB);
4-methyl-1,2-diaminobenzene (TDB);
4-methyl-1,2-phenylenediamine (TDB)
Common name 3,4-TDA
VI. 3,5-isomer
Chemical abstract name 1,3-benzenediamine,5-methyl- (9CI)
Other chemical names 3,5-diaminotoluene;
3,5-toluenediamine
Common name 3,5-TDA
----------------------------------------------------------------------------
2.2. Physical and Chemical Properties
Diaminotoluenes are colourless crystals that are freely soluble
in hot water, alcohol, ether, and hot benzene. Some of the
physical properties of the 6 isomers are listed in Table 3 (Buist,
1970; CRC, 1975). Diaminotoluenes are oxidized readily in neutral
or alkaline solution to form dark-coloured products and tars. The
oxidation products have not been fully characterized. When heated,
diaminotoluenes emit toxic fumes of nitrogen oxides.
The composition and physical properties of the commercial
mixtures vary considerably. Some of the physical properties of the
2 most widely-used commercial mixtures are summarized in Table 4.
Meta- and Ortho-diaminotoluenes are weakly basic and react with
mineral acids to form water-soluble amine salts. These salts are
more resistant to oxidation than the parent amine.
Table 3. Physical properties of the diaminotoluene isomers
-------------------------------------------------------------------
Property Diaminotoluene isomers
2,3- 2,4- 2,5- 2,6- 3,4- 3,5-
-------------------------------------------------------------------
Melting point (°C) 63-64 99 64 105 88.5 -
Boiling point (°C) 255 280 273-274 289b 265 283-285
(subl)
Vapour pressurea (kPa)
at 150 °C 1.20 1.47 - 2.13 - -
at 160 °C 1.87 2.27 - 3.33 - -
at 180 °C 2.67 4.80 - 7.60 - -
-------------------------------------------------------------------
a To convert kPa to mmHg, divide by 0.133.
b Obtained by extrapolation from vapour pressure-temperature data
and Antoine constants. From: Willeboordse et al. (1968).
Table 4. Physical and chemical properties of commercial grades of
diaminotoluene
----------------------------------------------------------------------
Meta-DAT Ortho-DAT
(80:20, 2,4-/2,6-isomers) (60:40, 3,4-/2,3-isomers)
----------------------------------------------------------------------
Appearance solid, light yellow to tan; light grey to purple
darkens on storage and solid
exposure to air
Odour slight ammonia-like slight ammonia-like
Melting range 80 - 90 °C (176 - 194 °F) 40 - 50 °C (104 - 122 °F)
Boiling point 283 °C (541 °F) at 760 mmHg > 250 °C (> 480 °F)
Flash point 140 °C (284 °F) > 110 °C (> 230 °F)
Autoignition 450 °C (842 °F) 540 °C (1005 °F)
temperature
Vapour 0.34 x 10-3 mmHg at 37.8 °C 2.23 mmHg at 100 °C
pressure 1 mmHg at 106.5 °C 27.8 mmHg at 140 °C
100 mmHg at 212 °C 43.5 mmHg at 160 °C
Specific - 1.045 at 100 °C
gravity
Density 0.086 kg/litre at 105 °C -
Solubility in hot water, alcohol, in hot water, alcohol,
ether and many polar ether and many polar
organic solvents organic solvents
----------------------------------------------------------------------
2.3. Conversion Factors
1 ppm in air = 5 mg/m3 at 25 °C and 760 mmHg.
2.4. Analytical Methods
Analytical methods for the determination of diaminotoluenes in
water, air, different consumer products, and biological fluids are
listed in Table 5.
Diaminotoluenes may be analysed as free bases by reversed phase
high-performance liquid chromatography using both ultraviolet (UV)
and electrochemical detection (Purnell & Warwick, 1981; Purnell et
al., 1982; Nieminen et al., 1983). Gas chromatographic methods
usually involve derivatization to facilitate separation and
increase sensitivity (Olufsen, 1979; Skarping et al., 1983a,b).
Detection limits in air samples range from 0.1 to 10 µg/m3.
Diaminotoluenes and their derivatives have been studied in
blood, urine, and liver cytosol preparations using thin-layer
chromatography and gas chromatography/mass spectrometry (GC/MS)
(Kiese & Rauscher, 1968; Kiese et al., 1968; Glinsukon et al.,
1975; Waring & Pheasant, 1976). A high-performance liquid
chromatographic method for the determination of diaminotoluenes in
urine and plasma has been described by Unger & Friedman (1979).
Table 5. Analytical methods for the determination of diaminotoluenes
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Water high-performance liquid chrom- 2,4-, 2,5-, 2,6-, 0.2 - 0.7 ng Riggin & Howard
atography with ultraviolet and 3,4-isomers (1983)
electrochemical detection
Air gas-liquid chromatography/ 2,4-isomer 3 µg/m3 Becher (1981)
nitrogen-phosphorus detector
on glass capillary columns
high-performance liquid chrom- 0.1 - Purnell et al.
atography with ultraviolet 10 µg/m3 (1982)
and electrochemical detection
high-performance liquid chrom- 2,4-isomer - Nieminen et al.
atography with ultraviolet (1983)
detection
gas-liquid chromatography with 2,4- and 2,6-isomers ~ 0.1 - Skarping et al.
electron capture detection on 0.4 pg (1983a)
glass capillary column
gas-liquid chromatography/ 2,4- and 2,6-isomers 10 - 20 pg Skarping et al.
nitrogen-phosphorus detector amine (1983b)
on glass capillary columns
Hair dyes gas-liquid chromatography/ 2,5-isomer 5 mg/litre Choudhary (1980)
flame ionization detector
thin-layer chromatography 2,4-, 2,5-, and 0.2 mg/litre Kottemann (1966)
3,4-isomer
high-performance liquid chrom- 2,4-, 2,5-, 0.5 mg/litre Johansson et al.
atography/ultraviolet detection and 2,6-isomers (1981)
high-performance liquid chrom- 2,4-, 2,5-, - Liem & Rooselaar
atography/ultraviolet detection and 3,4-isomers (1981)
------------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Hair dyes high-performance liquid chrom- 2,4- and 2,6-isomers 0.1 µg/litre Snyder et al.
(contd.) atography/ultraviolet detection (1982)
gas-liquid chromatography/mass
spectrometry
Polyurethane thin-layer chromatography/ 2,4- and 2,6-isomers 1 µg/g Guthrie &
foams fluorimetry McKinney (1977)
Biological high-performance liquid chrom- 2,4- and 2,6-isomers 2 mg/litre Unger & Friedman
tissues and atography/ultraviolet detection (1979)
fluids
Isomeric nuclear magnetic resonance all isomers - Mathias (1966)
mixtures spectrometry
thin-layer chromatography all isomers - Macke (1968)
gas-liquid chromatography/ all isomers - Boufford (1968)
flame ionization detector
gas-liquid chromatography/ all isomers - Willeboordse et
thermal detector al. (1968)
infra-red spectroscopy 2,4- and 2,6-isomers - Biernacka et al.
(1974)
------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Natural Occurrence
Diaminotoluenes are not known to occur as natural products.
3.2. Production
Currently, diaminotoluenes are produced commercially through
the catalytic hydrogenation of dinitrotoluenes. This procedure,
economic only for large-scale production, is used in the
manufacture of toluene diisocyanates. At dye plants, diamino-
toluenes are produced by the reaction of hydrochloric acid on
dinitrotoluenes, in the presence of an iron catalyst (Austin,
1974).
Most diaminotoluenes produced are used on site by the
manufacturer; therefore, published production figures do not
adequately reflect the true world production of diaminotoluenes.
Between 1972 and 1976, the average annual production of
diaminotoluenes in the USA was 89 x 106 kg, ranging from 76 x 106
kg in 1972 to 105 x 106 kg in 1976 (US ITC, 1977). Thereafter, the
production was estimated from the known production of toluene
diisocyanates ranging between 305 x 106 kg annually in the period
1977 - 81, and 360 x 106 kg in 1984 (US ITC, 1982, 1985).
Up to 1978, an estimated 180 - 200 x 106 kg of 2,4-DAT was
produced annually in western Europe (IARC, 1978). During
1971 - 75, the the annual production of 2,4-DAT in Japan was
approximately 210 x 103 kg; the compound was neither imported nor
exported (IARC, l978). However, in 1981, the production of 2,4-DAT
in Japan was estimated to have declined to 50 x 103 kg (CIC Japan,
1983).
3.3. Uses
Diaminotoluenes are used extensively within the chemical
industry as intermediates in the manufacture of widely different
commercial products (Table 6). Minor applications of diamino-
toluene isomers include their use as raw materials, co-reactants,
and curing agents. Toluene diisocyanates represent the largest
end-use accounting for more than 90% of the total annual production
of diaminotoluenes, largely a mixture of 2,4- and 2,6-isomers
(Backus, 1974; Milligan & Gilbert, 1978).
Diaminotoluenes are intermediates in the synthesis of dyes used
for textiles, furs, leathers, biological stains and indicators,
spirit varnishes and wood stains, and pigments. Previously used in
hair-dyes, 2,4-DAT was removed from use by many countries after it
was found to be a hepatocarcinogen in rats (Ito et al., 1969).
Meta-DAT is used to produce diethyltoluenediamine (DETDA) for the
manufacture of certain urethane elastomers (Milligan & Gilbert,
1978). Ortho-DAT is used to produce mercaptotoluimidazole (MTI)
and its zinc salt, both of which are used primarily as specialty
antioxidants in nitrile rubber elastomers (Gan et al., 1975).
Table 6. End-use application(s) of individual
diaminotoluene isomers
------------------------------------------------------
Application 2,3- 2,6- 2,3- 3,4- 2,5-
------------------------------------------------------
Toluene diisocyanate X X
(> 90% of total use of
diaminotoluenes)
Urethane co-reactants X X X X
(DAT-initiated polyols)
DETDAa X X
DYESb Xd X d
Tolyltriazole X X
Epoxy curing X X X
Mercaptotoluimidazole X X
Photographic developer X
------------------------------------------------------
a DETDA = Diethyltoluenediamine.
b DYES = Fur, leather, biological stains, indicators,
textiles, hair, spirit varnishes, wood stains, and
pigments.
c Use in hair dyes and cosmetics prohibited in USA
since 1971.
d Forbidden in Italy - 1978.
3.4. Release into the Environment, Distribution, and Transformation
Data are lacking on the extent of the global release of
diaminotoluenes, as well as their transport, distribution, and
degradation within the environment.
Releases of 2,4-DAT into the environment have been estimated in
the USA, the largest contribution being over 6 x 106 kg dumped in
authorized landfills. Releases of 1.4 x 106 kg were estimated to
occur from the production of diaminotoluenes, and 0.3 x 106 kg
during dye production and usage; unknown quantities of DAT may
derive from the hydrolysis of TDI released into the environment.
Information on the transport, distribution, and degradation of
DAT isomers under conditions approaching those found in natural
bodies of water have not been reported in the literature. However,
a bench-scale treatability study for 2,4-DAT using acclimated
sludge from a treatment plant showed that the isomers are
degradable. The observed total organic carbon removal was 45% in
4 h (Matsui et al., 1975).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
No information was available in the literature reviewed from
which environmental levels could be calculated. Two properties of
the diaminotoluenes are relevant to this problem. Since the vapour
pressure is low (Tables 4 and 5), the risk of contaminating the
environment through evaporation is minimal. However, air emissions
from inappropriately operated plants may pose a hazard. Since the
chemical is soluble in water, the potential for exposure through
water contamination is of concern. No data are available on levels
of diaminotoluenes in surface and groundwater, in soil, and/or air.
4.2. General Population Exposure
No information is available on the exposure of the general
population to diaminotoluenes.
4.3. Occupational Exposure
Filatova et al. (1970) reported concentrations of diamino-
toluenes in manufacturing plants of up to 0.2 mg/m3, with
occasional excursions up to 11 mg/m3.
The results of studies conducted in 3 plants manufacturing
diaminotoluenes in the USA showed that the work-place ambient air
levels ranged from 0.005 to 0.44 mg/m3 (NIOSH, 1980, 1981, 1982).
The highest level of diaminotoluenes (0.44 mg/m3) was found in the
filter room at one plant (NIOSH, 1980). A level of 0.39 mg
diaminotoluenes/m3 was measured in a sample taken at the breathing
zone of an operator in a second plant (NIOSH, 1981). All values
were calculated as time-weighted averages.
5. KINETICS AND METABOLISM
5.1. Studies on Experimental Animals
5.1.1. Absorption and retention
Skin penetration by test materials varied amoung species
(monkeys, swine), and was affected by vehicle and site of
application. In one study, [14C]-2,4-DAT (4 µg/cm2), dissolved in
acetone, methanol, or a skin lotion, was applied to 3 - 15 cm2 of
the ventral forearm, abdomen, or back of 3 - 6 monkeys/group (9
groups). The material was removed after 24 h by washing with soap
and water. The greatest absorption (53.8 ± 15.4%) resulted when
[14C]-2,4-DAT in acetone was applied to the abdominal skin of
monkeys (Marzulli et al., 1981). The permeability of diamino-
toluenes across the epidermis was highly dependent on the
formulation used. When 1.4 g of 2,5-DAT was applied in a gel
to the abdominal skin of dogs for a contact period of 3 h, 2.9%
(40 mg) was absorbed. Addition of hydrogen peroxide, similar to
the formulation used in hair dye, reduced the amount absorbed
to < 0.21% (Kiese et al., 1968) or < 0.13% (Hruby, 1977). The
hair in the exposed area retained 4% of the 14C activity, 5 days
after application (Hruby, 1977).
Hruby (1977) studied the absorption of [14C]-2,5-DAT following
oral and subcutaneous single-dose administrations to rats. Five
days following subcutaneous injection of 3 - 5 mg [14C]-2,5-DAT (in
water), 6.9% of the dose was found in the total-body homogenate and
1.7% remained at the injection site. Five days after oral (gavage)
administration of 10 mg [14C]-2,5-DAT (in water), the rat gastro-
intestinal tract retained 1.4% of the applied radioactivity and
1.2% was found in the total body homogenate (Hruby, 1977).
No studies on uptake after inhalation were found.
5.1.2. Distribution and reaction with body components
The distribution of diaminotoluenes and their reaction with
body components have been investigated, mainly after
intraperitoneal injection of radioactive labelled compounds. No
data on the distribution of diaminotoluenes and reaction with
tissues after inhalation or oral ingestion were found in published
reports.
Distribution of [14C]-2,4-DAT after intraperitoneal injection
was rapid, and the peak concentration in rat and mouse blood and
plasma occurred in 1 h, then decreased rapidly for 7 h. On a
comparative basis, all tissue concentrations of bound 14C were
considerably lower in the male NIH-Swiss mice than in the male
Fischer rats (Grantham et al., 1980).
Tissue distribution of [Me-14C]-2,4-DAT hydrochloride was
studied in male B6C3F1 mice given a single intraperitoneal
injection (1 µCi, 0.667 mg/kg body weight) (Unger et al., 1980).
The highest concentrations, 1/2 h after dosing, were found in the
kidneys, gonads, epididymis, lungs, muscle, and blood. One hour
after dosing, the liver contained the greatest amount, accounting
for nearly 12% of the dose. The concentration in the adrenal
glands exceeded that in the kidney, 1 and 2 h after dosing. High
concentrations of radioactivity were also observed in the
gastrointestinal tract.
Four hours after an intraperitoneal injection of 100 mg (0.8
mmol/kg, ring-labelled [3H]-2,4-DAT) in male Wistar rats, 0.3 nmol
was found covalently bound per mg liver protein. A similar degree
of binding was seen in the kidneys. Subcellular fractionation of
the liver showed that most of the bound material was in the
microsomal fraction (Dybing et al., 1978). No significant binding
to DNA in vitro or in vivo could be demonstrated using [3H]-2,4-
DAT, whereas it was found to bind covalently to hepatic RNA in
vivo. These findings were confirmed by Aune et al. (1979).
5.1.3. Metabolism
Glinsukon et al. (1975, 1976) found that the 2,4-isomer was
selectively N-acetylated at the p-amino group by liver cytosol
prepared from hamsters, guinea-pigs, rabbits, mice, and rats. The
cytosol from liver, kidney, intestinal mucosa, and lung of hamsters
and rabbits was studied for N-acetyl transferase activity using
2,4-DAT and 4-acetylamino-2-amino-toluene as substrates. All
tissues showed marked species differences in enzyme activity.
Tissues with high N-acetyl transferase levels, such as liver,
could produce both 4-acetylamino-2-aminotoluene and 2,4-
diacetylaminotoluene (Glinsukon et al., 1975). There were also sex
differences in the N-acetylation capacity of the liver cytosol.
After a single ip injection of 2,4-DAT (77 mg/kg body weight)
in male rats, 69.4% of the dose was eliminated in the urine and
faeces after 24 h as a complex mixture of metabolites, indicating
both free and conjugated derivatives. The major urinary
metabolites identified were 4-acetylamino-2-aminotoluene, 2,4-
diacetylaminotoluene, and 4-acetylamino-2-aminobenzoic acid. In
mice, oxidation of the methyl group to a benzoic acid was the major
reaction and the major urinary metabolites in mice were 4-
acetylamino-2-aminobenzoic, 4-acetylamino-2-aminotoluene, and 2,4-
diacetylaminobenzoic acid (Grantham et al., 1980). Waring &
Pheasant (1976) investigated the metabolism of 2,4-DAT in female
rabbits, rats, and guinea-pigs to determine whether the isomer gave
rise to hydroxylamines or aminophenols, which might account for the
observed toxic and carcinogenic effects. After oral administration
(gavage) of 2,4-DAT (50 mg/kg body weight), phenolic metabolites
were excreted in the urine. When free and conjugated metabolites
were combined, 5-hydroxy-2,4-DAT was the major metabolite in all 3
species (Table 7).
Table 7. Excretion of metabolites after dosing with 2,4-diamino-
toluene
-------------------------------------------------------------------
Metabolite Percentage dose excreteda
Rabbit Rat Guinea-pig
-------------------------------------------------------------------
2,4-DAT trace 1.3 trace
3-hydroxy-2,4-DAT 10 8 trace
5-hydroxy-2,4-DAT 22 12 9
6-hydroxy-2,4-DAT ( m-aminophenol) trace 5 trace
3-hydroxy-4-acetylamino-2-aminotoluene 10 18 trace
5-hydroxy-4-acetylamino-2-aminotoluene 6 14 17
glucuronide I, 3-hydroxy-DAT 10 16 15
glucuronide II, 5 hydroxy-DAT 32 12 46
glucuronide III, 6-hydroxy-DAT 2 6 4
unidentified phenolic compounds 0 trace trace
-------------------------------------------------------------------
a Results are given as percentage dose, average of 10 studies,
standard deviation 6.4% for metabolites 1 - 5, and 12.8% for
metabolites 6 - 8. Animals were dosed orally at 50 mg/kg; urine
was collected for 48 h.
From: Waring & Pheasant (1976).
The levels of methaemoglobin found in the rabbit, rat, and
guinea-pig correlated well with the total urinary excretion of
aminophenol. The methaemoglobin levels reached a peak 6 - 12 h
after the administration of 2,4-DAT and then slowly declined. The
highest levels of aminophenols and of methaemoglobin were found in
the rabbit (Waring & Pheasant, 1976).
5.1.4. Excretion
During a 24-h period of dermal contact with [14C]-2,4-DAT, 14C
urinary excretion in monkeys reached a peak at 8 - 12 h (Marzulli
et al., 1981).
Data from studies on the rat, rabbit, mouse, guinea-pig, and
dog exposed to diaminotoluenes (cutaneous, subcutaneous,
intravenous, intraperitoneal, or oral by gavage) showed fast
elimination rates (Kiese et al., 1968; Waring & Pheasant, 1976;
Hruby, 1977; Grantham et al., 1980; Unger et al., 1980). The
elimination of radioactivity from various tissues in rodents
followed a well-defined biphasic pattern. Rapid elimination over
7 h was followed by a rather slow decline in the isotopic contents
of tissues (Grantham et al., 1980; Unger et al., 1980). The half-
lives of tissue elimination during the fast phase were 0.89, 0.43,
and 1.51 h for male mouse liver, kidneys, and blood, respectively.
During the slow phase of elimination, the half-lives for liver,
kidneys, and blood in male mice were 11.7, 9.1, and 12.6 h,
respectively. The half-lives of elimination of radioactivity,
during the slow phase, were greater for muscle (23.9 h) and skin
(29.2 h) than for any other tissue (Wagner, 1975; Unger et al.,
1980).
The primary route of elimination in rodents was via the kidneys
during the first hour after exposure. However, after 2 h, the
predominant route shifted from urinary to faecal, probably a
reflection of biliary excretion. Only 1.25% of the administered
radioactivity had been exhaled after 24 h (Unger et al., 1980). On
a comparative basis, faecal elimination was greater in rats than in
mice, but the rate of urinary excretion was more rapid in mice than
in rats. Approximately 90% of a dose was eliminated in the urine
of mice in 24 h compared with 74% in the urine of rats (Grantham et
al., 1980). Complete elimination was accomplished in 2 days in
mice, while rats required 6 days.
Male and female rats, injected subcutaneously with 3 - 5 mg
[14C]-2,5-DAT hydrochloride, eliminated 65% of the dose in the
urine and 5% in faeces after 24 h. The same pattern of elimination
was found after oral administration of 10 mg of the labelled
compound (Hruby, 1977).
When beagle dogs were intravenously injected with a dose of
224 mg, infused over 3 h, the total amounts of radioactivity
eliminated in the urine and faeces were 60% and 19%, respectively.
After 4 days, elimination mainly occurred within the first 24 h.
After a skin application of 1.4 g [14C]-2,4-DAT for 3 h (in 50 ml
of a dye formulation), only 0.092 and 0.84% of the dose were
eliminated, respectively, in the urine and faeces of beagle dogs
over 4 days, reflecting the inhibitory effect of the dye
formulation on the absorption of the 2,4-DAT (Hruby, 1977). In
another study on dogs, about 40 mg 2,5-diaminotoluene was absorbed
through the skin from a gel containing 1.4 g of the material. The
addition of hydrogen peroxide to the gel reduced the amount
absorbed to less than 3 mg. The amount excreted unchanged in the
urine was 60 - 70 µg (Kiese et al., 1968).
5.2. Human Studies
As only limited information is available on the absorption,
distribution, metabolism, and excretion of diaminotoluenes in human
beings, these aspects are discussed together, rather than in
separate sections.
Although the high boiling point of diaminotoluenes makes
absorption through the lungs unlikely under normal working
conditions, inhalation may occur when hot vapours escape from
stills. Possible inhalation and dermal exposure to dusts may occur
if diaminotoluenes are handled in a less than optimal manner.
Since diaminotoluenes are soluble in water, absorption from the
gastrointestinal tract could occur following ingestion. However,
no data were found on the kinetics and metabolism of diamino-
toluenes after oral or inhalation exposures.
Skin penetration by [14C]-2,4-DAT was measured in human beings
(Marzulli et al., 1981). When 4 µg [14C]-2,4-DAT in acetone/cm2
was applied to the skin of the forearm, the highest absorption of
the chemical (23.7 % ± 16.1% of the applied dose) resulted after
24 h of dermal contact. Urinary excretion reached a peak after
4 - 8 h of skin contact. In a study by Kiese & Rauscher (1968),
the hair of 5 human subjects was dyed (40 min) with a formula
containing 2.5 g 2,5-DAT; absorption of approximately 0.2% of the
applied material occurred. No data were given on the retention and
distribution of diaminotoluenes after this dermal contact.
Data from studies on 6 volunteers (3 males and 3 females)
showed that, after subcutaneous injection of 5.54 mg 2,5-DAT, 47.6%
of the dose was excreted in the urine as N,N'-diacetyl-2,5-DAT.
The rate of excretion was highest during the first 24 h, and only a
trace appeared in the urine excreted on the third day, in one
study. When the compound was applied as a hair dye (40 min), the
highest rate of excretion was observed during a period of 5 - 8 h
after application. On average, a total amount of 3.7 mg N,N'-
diacetyl-2,5-DAT (i.e., 0.09% of the applied dose) was calculated
to have been excreted in urine taken over 2 days from 5 subjects
(Kiese & Rauscher, 1968).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Little information is available on the effects of diamino-
toluenes on animal populations found in the environment. The
effects of 2,4-DAT at concentrations ranging from 1 to 1000
mg/litre were observed for Daphnia ( Daphnia magna Straus),
ostracoda, guppies ( Lebistes reticulatus Peters), and channel
seaweed (Scenedesmus obliquus). Daphnia was the most sensitive
species; 5 mg/litre was lethal in 5 - 10 days, and prolonged
exposure to 2 mg/litre caused a reduction in the number of
offspring produced. A concentration of 20 mg/litre was not lethal
for ostracods after 10 days, but 50 mg/litre was lethal in 5 - 8
days. Fish survived for 10 days at 200 mg/litre, but a
concentration of 500 mg/litre was lethal in 2 - 3 days. The algae
tested were the most resistant, surviving for 10 days at a
concentration of 1000 mg/litre (Smirnova et al., 1967).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The early literature (1881 - 1939) contains several reports on
toxicological manifestations associated with the administration of
diaminotoluenes in experimental animals. Most of the papers are
difficult to interpret and to use in the assessment of chemical
hazards, because massive doses of chemicals of unknown purity and
isomeric composition were used, and because of the experimental
designs chosen. The toxic effects were characterized by icterus,
haemoglobinuria, disposition of haemosiderin in the spleen, bone
marrow, and liver, respiratory and generalized central nervous
system (CNS) depression, pulmonary and cerebral oedema, and
increased bile acids in the liver, blood, and/or urine of exposed
animals (Von Oettingen, 1941).
7.1. Single Exposures
Diaminotoluenes are considered to be dermal and eye irritants.
In studies on rabbits, 12.5 mg 2,5-DAT or 500 mg 2,4-DAT caused
skin irritation, defined as erythema and oedema, after 24 h of
dermal contact. Instillation of 100 µg of the 2,4-isomer into the
rabbit eye caused severe eye irritation within 24 h. Data showing
the extent of the acute toxicity of diaminotoluenes in various
laboratory animals are summarized in Tables 8 and 9.
The acute toxic effects of diaminotoluenes were characterized
by marked central nervous system depression during exposure (e.g.,
decreased locomotor activity, piloerection, ptosis, ataxia,
tremors) and production of methaemoglobin, 6 - 8 h after exposure.
Duodenal and glandular mucosal damage in the stomach were
observed in fed, unrestrained rats, 24 h following a single
subcutaneous dose of 3,4-DAT. The optimal ulcerogenic dose of 3,4-
DAT (i.e., the dose causing a low mortality and a maximal incidence
of duodenal damage within 24 h), was 350 mg/kg body weight (Perkins
& Green, 1975).
7.2. Short-Term Exposures
When guinea-pigs were treated with 1 - 10% 2,5-DAT (24 h/day
for 5 days, 2 days without treatment, followed by exposure for
another 5 days), sensitization was obtained in 35% of treated
animals (Schäfer et al., 1978).
Both the 2,4- and 3,4-isomers caused severe icterus in rats.
In male and female rats, 3,4-DAT (unlike the 2,4-isomer), given
orally or parenterally, produced a high incidence of perforating
duodenal ulcers within a few days (Selye, 1973). These effects
were obtained in animals allowed to move freely with access to food
and water during the period of observation. A dose of 500 mg
diaminotoluenes/kg body weight was administered in 2 ml water,
twice daily.
Table 8. Lethality of diaminotoluenes
---------------------------------------------------------------------------
Species Exposure LD50 Reference
route (mg/kg
body
weight)
---------------------------------------------------------------------------
Ortho-DAT (2,3-, 3,4- mix)
Rat oral 810 Carpenter et al. (1974)
Rabbit dermal 1120 Carpenter et al. (1974)
Meta-DAT (2,4-, 2,6- mix)
Rat oral 300 Izmerov et al. (1982)
Rat (male) oral 270 Weisbrod & Stephan (1983)
Mouse (male) oral 350 Weisbrod & Stephan (1983)
Rat (male) ip 230a Weisbrod & Stephan (1983)
Mouse (male) ip 240b Weisbrod & Stephan (1983)
Rat (male) iv 350 Weisbrod & Stephan (1983)
Mouse (male) iv 90-105 Weisbrod & Stephan (1983)
Rat dermal 1200 Izmerov et al. (1982)
2,4-DAT (technical grade)
Fischer rat (male) ip 325 Grantham et al. (1980)
NIH-Swiss mouse (male) ip 480 Grantham et al. (1980)
HaM/ICR mouse (male) ip 80 Weisburger et al. (1978)
HaM/ICR mouse (female) ip 90 Weisburger et al. (1978)
---------------------------------------------------------------------------
a A methaemoglobin level of 8.4% was observed, 6 h after ip administration.
b A methaemoglobin level of 7.8% was observed, 6 h after ip treatment.
Note: No published data on the acute toxicity of the 2,3-isomer were
available.
The effects of 2,4-DAT on the liver microsomal mixed-function
oxidase system, DT-diaphorase, and epoxide hydrolase were reported
by Dent & Graichen (1982). Following oral treatment with 2,4-DAT
at 70 mg/kg body weight per day for 5 days, the activities of
microsomal cytochrome P-450-dependent enzymes were depressed, while
epoxide hydrolase activity was markedly elevated (3 - 8 times
control) in male F-344 rats. Under these experimental conditions,
an increase in the liver to body weight ratio (3.2 - 4%), and in
the liver microsomal protein concentration (19.3 - 27.4 g/kg) were
induced by 2,4-DAT.
7.3. Long-Term Exposure
Oral administration of 2,4-DAT (see Table 11, section 7.6, for
doses) for 79 - 103 weeks accelerated the appearance of renal
toxicity in male F-344 rats, associated with a high incidence of
secondary hyperparathyroidism (NCI, 1979). The chronic renal
disease reported was believed to have decreased the longevity of
the treated rats, either directly or through inhibition of the
clearance of toxic metabolites (Cardy, 1979).
Table 9. Summary of some single-dose studies
--------------------------------------------------------------------------
Species Route of Dose Effects Reference
exposure (mg/kg
body
weight)
--------------------------------------------------------------------------
2,4-DAT (technical grade)
Wistar rat oral 50 produced metHba; highest Waring &
(male) amounts (5 - 6%) were Pheasant (1976)
found 6 - 8 h after
exposure
> 50 toxic
Sprague oral 500 developed icterus and Selye (1973)
Dawley rat death
(male and
female)
NZW rabbit oral 50 MetHb reached 18 - 20% Waring &
level 6 - 8 h after Pheasant (1976)
application
> 50 toxic
Dunkin- oral 50 MetHb reached 3 - 4% level Waring &
Harvey 6 - 8 h after application Pheasant (1976)
guinea-pig
> 50 toxic
3,4-DAT (97% pure)
Sprague subcut- 125-500 discrete, non-perforated Perkins &
Dawley rat aneous duodenal lesions were Green (1975)
(female) observed immediately
distal to the gastroduo-
denal junction 24 h
following the administra-
tion of a single dose
--------------------------------------------------------------------------
a metHb = methaemoglobin.
No studies were found on the effects of diaminotoluenes on the
nervous system or the immune system after long-term exposure.
7.4. Reproduction and Teratogenicity
7.4.1. Reproduction
There are 2 studies on experimental animals that evaluate the
reproductive toxicity of diaminotoluenes. Soares & Lock (1980)
administered 2,4-DAT orally or ip at 40 mg/kg body weight for
2 days to DBA/2J male mice. Forty-eight hours after treatment,
mating trials were conducted for 8 weeks. There were no treatment-
related effects on sperm morphology or fertility, as measured by
this dominant lethal assay. However, in male Sprague Dawley rats,
long-term exposure to 2,4-DAT in the feed impaired reproductive
performance and capacity (Thysen et al., 1985a,b). Dietary levels
of 0.03% 2,4-DAT for 10 weeks (~ 15 mg/kg body weight per day)
decreased fertility and exerted an inhibitory effect on sperm
production in male rats. Eleven weeks after treatment, the sperm
count remained significantly depressed ( P < 0.001), suggesting
irreversible damage to the germinal components in the testes. Data
from hormone analyses at the end of the 10 weeks of exposure, and
at the end of 11 weeks after treatment, showed a significant
decrease in serum-testosterone and an elevation of serum-luteinizing
hormone concentrations, which were associated with a reduction in
seminal vesicle weight. Histological changes found in the
reproductive organs from treated males were correlated with these
physiological changes. At a lower dose (0.01% or ~ 5 mg/kg body
weight), 2,4-DAT did not cause any of these toxic responses.
7.4.2. Teratogenicity
Studies on the teratogenic potential of diaminotoluenes are
summarized in Table 10. Skin application of 2,4-DAT induced a low
incidence of skeletal changes in rats (Burnett et al., 1976). Oral
or intraperitoneal administration of this isomer did not produce
any effects on the fertility or reproductive performance of male
mice (Soares & Lock, 1980). Subcutaneous or intraperitoneal
injection of 2,5-DAT in mice on day 8 of gestation, at levels of 50
or 75 mg/kg body weight, caused crainiofacial malformation and
fused or distorted thoracic vertebrae associated with the absence
of, or fused, ribs (Inouye & Murakami, 1976, 1977). However, 2,5-
DAT sulfate, at levels of 16 - 64 mg/kg body weight per day
administered subcutaneously on days 6 - 15 of gestation, did not
cause any malformations in mice or rats (Marks et al., 1981).
The results of oral administration of 2,6-DAT to rats and
rabbits, at doses of between 10 and 300 mg/kg body weight
(Knickerbocker et al., 1980), showed that, in rats, doses of
between 100 and 300 mg/kg body weight increased the occurrence of
incomplete vertebrae and that the highest dose resulted in missing
sternebrae and incomplete closure of the skull. A no-observed-
adverse-effect level of 10 mg/kg body weight was reported in rats.
No skeletal or soft-tissue abnormalities were observed in the
offspring of rabbits, but, using fetal toxicity indices, a no-
observed-adverse-effect level of 30 mg/kg body weight was reported.
Becci et al. (1983) administered Ortho-DAT (2,3-, 3,4-isomer
mixture) by gavage to rats and rabbits (Table 10). Reduced body
weight during gestation was noted at 300 mg/kg body weight in rats
and 100 mg/kg body weight in rabbits. An increased incidence of
several skeletal variations in the fetuses was noted, probably due,
in part, to the maternal toxicity. The no-observed-adverse-effect
level in both rats and rabbits was 30 mg/kg body weight.
Table 10. Teratogenicity studies with diaminotoluenes
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,4- Charles skin 2 ml/kg body weight skeletal changes seen in 6/169 Burnett et al.
(3% in River/CD on days 1, 4, 7, 10, live fetuses ( P > 0.05) (1976)
hair-dye rat 13, 16, and 19 of
formula) (female) gestation
2,5- Charles skin 2 ml/kg body weight no increase in abnormalities in Burnett et al.
(sulfate) River/CD on days 1, 4, 7, 10, treated groups (1976)
(3% in rat 13, 16, and 19 of
hair-dye gestation
formula)
2,5- JCL:ddn subcutaneous 50 mg/kg body weight In groups treated sc or ip on day Inouye & Murakami
dihydro- mice or intraper- on one day of days 8 of gestation, there was evidence (1976, 1977)
chloride (female) itoneal 7 - 14 of gestation of craniofacial malformation:
single dose or 75 mg/kg on day exencephaly, prosoposchisis, and
8 of gestation or hair lip with cleft palate, and
50 mg/kg on day 8 of high incidence of skeletal
gestation malformation: fused or distorted
thoracic vertebrae associated with
absence of, or fused, ribs; no
such malformed fetuses were found
in groups treated on days 10 - 14
of gestation; only a very low
incidence of vertebral and rib
anomalies followed treatment on
day 7 or 9; maternal toxicity was
reported at 75 mg/kg but not at
50 mg/kg
2,5- CD-1 mice subcutaneous 16, 32, 48, or 64 no teratogenic effects were noted; Marks et al.
(sulfate) mg/kg body weight maternal toxicity was evident at (1981)
per day on days 48 and 64 mg/kg; reduced fetal
6 - 15 of gestation weight was noted at > 32 mg/kg
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,5- rat oral (gavage) 10, 50, or 80 mg/kg maternal toxicity and embryo- Spengler et al.
(sulfate) body weight per toxicity evident at 80 mg/kg; no (1986)
day on day 15 of effects observed at lower doses
gestation
rabbit oral (gavage) 10, 25, or 50 mg/kg no effects observed
body weight per day
on days 6 - 18 of
gestation
2,6- Sprague oral (gavage) 10, 30, 100, or 300 no effects on pregnancy, number Knickerbocker et
Dawley mg/kg body weight of live fetuses, and resorption al. (1980)
rat per day on days sites/dam; 300 mg/kg produced
6 - 15 of gestation smaller body weight gain in the
dams; 30 - 300 mg/kg produced
increased haemorrhagic abdomens
in the fetuses; 100 and 300 mg/kg
increased the occurrence of
incomplete vertebrae, and 300
mg/kg showed missing sternebrae
and incomplete skull closure in
the fetuses; the no-observed-
adverse-effect dose was 10 mg/kg
per day
2,6 Dutch oral (gavage) 3, 10, 30, or 100 100 mg/kg per day reduced dam Knickerbocker et
belted mg/kg body weight weights, increased resorptions, al. (1980)
rabbit per day on days decreased fetal weights, and
(female) 6 - 18 of gestation neonatal survival;
oral (gavage) 3, 10, 30, or 100 there were no differences in Knickerbocker et
mg/kg body weight skeletal or soft-tissue al. (1980)
per day on days abnormalities between treated
6 - 18 of gestation animals and controls; the no-
observed-adverse-effect dose was
30 mg/kg per day
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
o-DAT Sprague oral 10, 30, 100, or 300 maternal toxicity was indicated Becci et al.
(2,3-, Dawley mg/kg body weight at 300 mg/kg per day by reduced (1983)
3,4- rat per day on days body weight gain during gestation;
isomer 6 - 15 of gestation No significant differences in
mix) numbers of live fetuses,
implantation or resorption sites;
fetal body weight was reduced at
the highest dose ( P < 0.05); no
evidence of teratogenic effects
or effects on dams at doses < 30
mg/kg; no skeletal or soft-tissue
malformations that could be
related to treatment; however,
increased incidence of missing
sternebrae at 300 mg/kg per day
and incomplete ossified vertebrae
at 100 and 300 mg/kg per day were
noted compared with controls
o-DAT Dutch oral 3, 10, 30, or 100 maternal toxicity at 100 mg/kg per Becci et al.
(2,3-, belted mg/kg body weight day elicited by reduced body (1983)
3,4- rabbit per day on days weight gain during pregnancy; no
isomer 6 - 18 of gestation significant difference in the
mix) number of implantations; at 100
mg/kg per day, fetal body weight
was reduced and the number of
resorption sites was increased;
no skeletal or soft-tissue
malformations that could be
related to treatment were noted
-----------------------------------------------------------------------------------------------------------------
7.5. Mutagenicity and Related End-Points
7.5.1. DNA damage
At concentrations of 1 x 10-4 mol/litre and below, 2,4-DAT, but
not 2,6-DAT, induced unscheduled DNA synthesis in primary cultures
of rat hepatocytes (Bermudez et al., 1979). 2,4-DAT produced a
significant elevation in unscheduled DNA synthesis at 2 and 12 h in
the in vivo/in vitro hepatocyte DNA repair assay (Mirsalis &
Butterworth, 1982; Mirsalis et al., 1982). 2,5-DAT produced a
positive response in a DNA-repair assay in rat hepatocytes and a
weak positive response in hamster hepatocytes at 10-5 mol, the
highest concentration that was not toxic to the cells that were
tested (Kornbrust & Barfknecht, 1984).
Shooter & Venitt (1979) used continuous administration of 2,4-
DAT in the drinking-water to determine whether phosphotriesters
could be detected in the DNA of the liver of treated rats.
Positive results were obtained at 10 mg/litre. A low, but
significant, level of these lesions was produced. Shooter &
Venitt's studies on rodents indicated that methyl- and
ethylphosphotriesters persist for many weeks in the DNA of certain
organs (notably liver, kidney, and lung) and that such lesions are
not eliminated by DNA repair.
The results of studies by Greene et al. (1981) showed that
2,4-, 2,5-, 2,6-, and 3,4-isomers significantly inhibited the
incorporation of [125I]-iododeoxyuridine into mouse testicular DNA
and demonstrated dose-response characteristics. The 2,4-, 2,5-,
and 3,4-isomers were capable of reaching the testes and of passing
target cell membranes at this site. They concluded that the 3
isomers may present a genetic health hazard for an intact animal.
The inhibition induced by 2,6-DAT might have been caused by a
chemically-induced decrease in body temperature (Greene et al.,
1981).
DNA damage was not found in human cultured fibroblasts after
exposure to 100 µmol 2,4-DAT alone (3.5 ± 1.5 increase in percent
single-strand DNA). When the cells were incubated in the presence
of 1 mg ram seminal vesicle microsomes/ml and 100 µmol arachidonic
acid, a significant increase in the fraction of single-strand DNA
(21.3 ± 3.7, P < 0.001) was found in cells exposed to 2,4-DAT.
DNA strand breaks were not induced when prostaglandin synthase
(PGS) was inhibited by adding indomethacin (100 µmol) or
acetylsalicylic acid (1 mmol) (Nordenskjöld et al., 1984). These
results, which suggest that 2,4-DAT may be activated by PGS to form
products that cause DNA damage in cultured human fibroblasts, are
in agreement with the findings of Rahimtula et al. (1982).
7.5.2. Mutation
Several studies have shown that 2,4-, 2,6-, and 2,5-isomers can
induce reverse mutations in Salmonella typhimurium strains TA 1538
and TA 98, in the presence of various metabolic activation systems
(McCann et al., 1975; Dybing & Thorgeirsson, 1977; Dybing et al.,
1977; Pienta et al., 1977b; Cinkotai et al., 1978; Aune et al.,
1979; Shahin et al., 1980). However, Mori et al. (1982) showed
that 2,4-DAT was inactive for strains TA 98 and TA 100 at doses
ranging from 5 to 1000 µg/plate. While 2,3-DAT is inactive in S.
typhimurium (Florin et al., 1980), its homologue 3,4-DAT showed a
marginal response in strains TA 98 and TA 1538 (Greene et al.,
1979).
2,4-DAT was shown to be a weak mutagen in Drosophila
melanogaster, inducing sex-linked recessive lethals when fed to
adult males at a concentration of 15.2 mmol (Blijleven, 1977;
Venitt, 1978). In a study reported by Fahmy & Fahmy (1977), 2,4-
DAT was injected around the testes of adult male Drosophila at
doses ranging from 5 to 20 mmol. Mutagenicity was measured at the
various stages of spermatogenesis, both on the X-chromosome and RNA
genes. The overall induced frequency of X-recessives was extremely
low. It was also observed that mutation yield was not dose-related
and that it was maximal in the earliest progeny fraction,
suggesting a greater toxicity for mature sperm.
2,4-DAT was mutagenic in L5178Y mouse lymphoma cells and CHO-
AT3-2 cells (Matheson & Creasy, 1976; Coppinger et al., 1984).
Mutagenic activity was observed in L5178Y cells, only in the
absence of exogenous metabolic activation, but was observed in CHO-
AT3-2 cells both with and without activation.
The in vivo mutagenic activity of 2,4-DAT was studied in
DBA/2J male mice by the dominant lethal assay, sperm abnormality
assay, and the recessive spot test. Mice were administered the
compound by intraperitoneal injection and orally by gavage (2 daily
doses of 40 mg/kg body weight), just before mating (Soares & Lock,
1980). No induction of dominant lethals was noted, nor was any
increase in abnormal sperm or recessive spots reported.
No dominant lethals were induced in Charles River rats injected
intraperitoneally, 3 times weekly for 8 weeks, with 20 mg 2,5-
DAT/kg body weight, before mating (Burnett et al., 1977).
7.5.3. Cell transformation
Several studies have shown that the 2,4-, 2,5-, 2,6-, and 3,4-
isomers can induce morphological transformations in Syrian golden
hamster embryo cells (Pienta et al., 1977a; Greene & Friedman,
1980). Each isomer chemically transformed secondary hamster embryo
cells, but none were active in more than 50% of the 5 or 6 separate
tests performed on each isomer (Greene & Freidman, 1980).
7.5.4. Chromosomal effects
Cytogenetic preparations were made from the bone marrow of male
mice, 30 and 48 h after intraperitoneal injection of 2 daily doses
of 2,4-DAT at 40 mg/kg body weight. The treatment did not induce
any obvious chromosome breaks (Soares & Lock, 1980).
2,5-DAT did not induce micronucleated cells in bone marrow
after oral administration of 120 mg/kg body weight to male and
female rats, in 2 doses separated by an interval of 24 h (Hossack &
Richardson, 1977).
7.6. Carcinogenicity
Several long-term studies on the carcinogenic potential of the
2,4-, 2,5-, and 2,6-DAT isomers have been published. The
experimental designs used in these studies are summarized in Table
11. The experimental designs used in 2 studies on the carcinogenic
effects of hair dye formulations are also given in Table 11.
Although the early studies of Umeda (1955) and Ito et al.
(1969) used protocols that generated data of minimal use for a
hazard evaluation, they did produce qualitative information showing
that 2,4-DAT was carcinogenic for rats. These studies have been
extended to the 2,5-, 2,6-, as well as 2,4-DATs using more animals
per study and well-defined protocols (NCI, 1978, 1979, 1980;
Weisburger et al., 1978).
Groups of 25 male Charles River/CD rats were administered 2,4-
DAT in the diet at time-weighted levels of 300 and 625 mg/kg for 18
months. Similarly, groups of 25 male and female CD-1 mice were
given diets containing 500 and 1000 mg 2,4-DAT/kg for 18 months
(Weisburger et al., 1978). The rats and mice used in these studies
had a high incidence of spontaneous tumours. However, there was a
statistically significant increase in subcutaneous fibromas in male
rats and hepatocellular carcinomas and vascular tumours in male and
female mice compared with controls.
Studies by the US National Cancer Institute on the
carcinogenicity of 2,4-DAT in rats and mice (NCI, 1979) confirmed
the report by Weisburger et al. (1978). Administration of time-
weighted average doses of 79 and 176 mg 2,4-DAT/kg diet to groups
of 50 male and 50 female Fisher 344 rats, for 103 weeks, led to a
severe depression in body weight gain, high mortality, and a dose-
related development of hepatocellular carcinomas or neoplastic
nodules in treated rats of both sexes. In addition, NCI reported
that carcinomas and adenomas of the mammary gland occurred in
female rats at incidences that were dose related and significantly
greater than those in the controls in both the high- and low-dose
groups. Groups of 50 male and 50 female B6C3F1 mice were similarly
administered 2,4-DAT at 100 or 200 mg/kg diet, for 101 weeks. In
male mice, tumour incidence was not significantly increased
compared with that in the control animals. However, the incidence
of hepatocellular carcinomas in female mice in both treated groups
was dose related and significantly higher than that in the
controls. Numbers of lymphomas were also higher in low-dose female
mice. On the basis of these results, it was concluded that 2,4-DAT
was carcinogenic for Fisher 344 rats of both sexes and for female
B6C3F1 mice (NCI, 1979).
Table 11. Experimental designs of carcinogenicity studies on diaminotoluenes
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,4- rat (male and female) 20 subcutaneous; 2 mg in 0.5 ml Umeda (1955)
propylene glycol weekly;
total of 28 injections; 452
days
2,4- Wistar rat (male) 12/12 (6) oral (diet); 0.6 and 1 g/kg; Ito et al.
36 weeks (1969)
2,4- Charles River/CD rat 25/25 (25) oral (diet); 500 and 1000 Weisburger et
(male) mg/kg for 4 months and 250 al. (1978)
Charles River mouse 25/25 (25) and 500 mg/kg for 14 months;
(male) oral (diet); 500 and 1000
Charles River mouse 25/25 (25) mg/kg for 18 months
(female)
2,5- Fisher 344 rat (male) 50/50 (50/25) oral (diet); 600 and 2000 NCI (1978)
Fisher 344 rat (female) 50/50 (50/25) mg/kg for 78 weeks + 31
weeks observation
B6C3F1 mouse (male) 50/50 (50/50) oral (diet); 600 and 2000
B6C3F1 mouse (female) 50/50 (50/50) mg/kg for 78 weeks + 19
weeks observation
2,4- Fisher 344 rat (male) 50/50 (20) oral (diet); 125 and 250 NCI (1979)
Fisher 344 rat (female) 50/50 (20) mg/kg for 40 weeks reduced
to 50 and 100 mg/kg for 63
weeks (time-weighted-average
79 and 176 mg/kg); high-dose
males killed after 79 weeks
and high-dose females after
84 weeks
2,4 B6C3F1 mouse (male) 50/50 (20) oral (diet); 100 and 200 NCI (1979)
B6C3F1 mouse (female) 50/50 (20) mg/kg for 101 weeks
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,6- Fisher 344 rat (male) 50/50 (50) oral (diet) 250 and 500 NCI (1980)
Fisher 344 rat (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
B6C3F1 mouse (male) 50/50 (50) oral (diet); 250 and 500
B6C3F1 mouse (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
2,5- Sprague Dawley rat (male) 50 (50) dermal application twice Kinkel &
(formulations Sprague Dawley rat 50 (50) weekly of 0.5 g of synthetic Holzmann (1973)
with 6% (female) formulation containing 4%
hydrogen 2,5-DAT mixed with equal
peroxide volume of 6% H202; treated 2
added) years
2,5- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(equal mixture male and ml weekly of formulation to (1975)
female) which equal volume of 6%
H202 had been added;
treatment period, 18 months
2,5- + 2,4- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(formulations (equal mixture male and ml weekly of formulation to (1975)
with 6% female) which equal volume of 6%
hydrogen H202 had been added;
peroxide treatment period, 18 months
added) (same control group as
above study)
---------------------------------------------------------------------------------------------------------
The carcinogenic potential of 2,5-DAT and 2,6-DAT for rats and
mice was determined by the US National Cancer Institute. After
administration of 2,5-DAT at 600 and 2000 mg/kg feed to groups of
50 male and 50 female Fisher 344 rats and 50 male and 50 female
B6C3F1 mice, for 78 weeks, there was not sufficient evidence to
demonstrate the carcinogenicity of 2,5-DAT (NCI, 1978). However,
the study had been curtailed and this reduced the potential of the
test for detecting carcinogenicity.
Using a similar protocol, 2,6-DAT was incorporated at levels of
250 and 500 mg/kg into the diet of groups of 50 male and 50 female
Fisher 344 rats, and B6C3F1 mice for 103 weeks (NCI, 1980). There
was some question of whether mice of either sex received a maximum
tolerated dose, but the dose given to rats appeared to be at the
maximum tolerated level. As reported by NCI (1980), islet-cell
adenomas of the pancreas and neoplastic nodules or carcinomas of
the liver occurred in male rats in dose-related trends that were
significant using the Cochran-Armitage test, but not using the
Fisher exact test. In the low-dose male mice, NCI reported that
the incidence of lymphomas was greater than that in the controls.
However, the incidence was not significant when the Bonferroni
criterion of multiple comparison was used. Similarly, the
occurrence of hepatocellular carcinomas in female mice was dose
related, but not significant by the Fisher exact test, when the
incidence in the high-dose groups was compared with that in the
controls. Under the conditions of the bioassay, it was concluded
that 2,6-DAT was not carcinogenic for male and female F344 rats or
for male and female B6C3F1 mice (NCI, l980). Summaries of the 3
NCI bioassays have been published by Cardy (1979), Reuber (1979),
and Sontag (1981).
Using the protocols outlined in Table 11, no evidence of
carcinogenicity was obtained when hair-dye formulations containing
2,5- and 2,5- plus 2,4-DAT were painted on the skin of rats and
mice (Kinkel & Holzmann, 1973; Burnett et al., 1975). Given the
duration of exposure, the amounts of diaminotoluenes placed on the
skin, and the use of hydrogen peroxide prior to administration,
such negative results would be expected in studies on the formula
containing 2,4-DAT (Burnett et al., 1975). These studies have
shown a low order of dermal toxicity for these hair dyes, even
after long-term exposure. However, no definitive statement can be
made on the carcinogenic potential of 2,4- and 2,5-DAT after dermal
administration.
8. EFFECTS ON MAN
8.1. Single and Short-Term Exposures
In human beings, as in animals, diaminotoluenes are considered
to be irritants for the mucous membranes and skin, and to lead to
conjunctivitis and corneal opacities. When solutions come into
contact with skin, they can cause irritation, severe dermatitis,
and blistering (Von Oettingen, 1941). In case of the inhalation of
fumes, coughing, dyspnoea, and respiratory distress can result. No
data are available for evaluating the sensitizing potential of
diaminotoluenes. In the case of ingestion of massive amounts,
nausea, vomiting, and diarrhoea would occur, with the possible
production of methaemoglobinaemia. No cases of human poisoning
from short-term exposures to 2,4-DAT have been documented in the
published literature.
8.2. Long-Term Occupational Exposure - Epidemiological Studies
Filatova et al. (1970) investigated the physiological and
biochemical status of workers in a plant manufacturing
diaminotoluenes. Fifty-two of the 59 workers (58 males and 1
female) had worked in the plant for > 2 1/2 years. Seventeen
workers were between 30 and 45 years of age and 42 workers were
under 30 years old. In general, all workers had equal exposure to
the toxic chemicals used at the plant, namely, dinitrotoluene,
diaminotoluenes, methanol, and o-dichlorobenzene. There were a
few complaints of headache (2 cases), excess coughing (2 cases),
stomach pains (2 cases), and chest pain (4 cases); however, the
majority of the workers did not exhibit any exposure-related
adverse effects. Nevertheless, the investigators concluded that
the 10 workers exhibiting the symptoms listed were indeed affected
by their exposure to the complex of chemicals at this factory. It
is impossible to delineate the role played by the diaminotoluenes
in the production of these adverse effects.
The US National Institute for Occupational Safety and Health
(NIOSH) evaluated the reproductive health of workers in 3 plants
manufacturing diaminotoluenes (NIOSH, 1980, 1981, 1982). Exposure
usually involved both DAT and dinitrotoluene (DNT). All 3 surveys
were conducted in response to requests from employees or their
unions. The reason for the first request was the workers' belief
that their wives were suffering increased rates of spontaneous
abortion. The other 2 studies were prompted by the publicity given
to the first study.
In 2 of the studies, environmental hygiene sampling took place
(section 4.2) and workers were invited to volunteer for a medical
examination, to complete a reproductive history questionnaire, and
to provide semen and blood samples. Semen samples were analysed
for volume, sperm count, and sperm morphology. Blood samples were
analysed for various markers of renal and hepatic function, neither
of which showed any significant inter-group differences in any of
the studies. Wives of workers were given a more detailed
reproductive history questionnaire.
In the first study (NIOSH, 1980), there were 44 volunteers, 30
of whom provided usable semen specimens. The total potential study
population was not given. Of the 30, 9 were exposed, 9 were
controls, and 12 were in an intermediate category. The rate of
spontaneous abortions was higher among the wives of exposed workers
(6/18 pregnancies while the husband was exposed) compared with the
controls (4/23 pregnancies), with 6/28 for the intermediate group.
The small number of congenital malformations was not exposure
related. The sperm count for the exposed (median = 49 million) was
significantly ( P < 0.03) lower than that for the control group
(median = 121 million); however, the latter figure was unusually
high. The exposed group showed a significant reduction in the
proportion of the large morphological type. The second study
involved only a reproductive history questionnaire and the
reporting of hygiene data by the company. Thirty-five out of 41
workers in DAT- and DNT-production areas were interviewed. The
rates of congenital abnormalities or spontaneous abortion did not
significantly differ between exposure groups. Where the husband
was employed in DNT production, 1 out of 9 pregnancies ended in a
spontaneous abortion; the equivalent data for DAT was 1 miscarriage
out of 14 pregnancies.
In the third study, 50 volunteers were examined, 41 of whom
provided semen specimens. The total eligible population was not
given, though it was reported that 25 workers were regularly
exposed, 15 of whom participated in the study. There were no
significant differences in sperm count morphology between exposure
groups, but the miscarriage rate was reported to be significantly
( P < 0.05) higher for the wives of workers in the DAT-exposed area
of the plant, where 6 out of 15 pregnancies ended in miscarriage
compared with 1 out of 7 for the wives of DNT-exposed workers, and
3 out of 38 for the wives of unexposed workers.
The ranges of levels of reported exposures overlapped between
the 3 studies and were all within the OSHA recommended standard of
1.5 mg/m3 for dinitrotoluenes (NIOSH, 1982). The first study might
have been expected to show an excess, because it was provoked by a
cluster of miscarriages. All the studies were of limited size and
subject to some risk of selection bias, because the population was
restricted to those who volunteered. Also, in all 3 studies, crude
figures, with no adjustment for age, were presented. However, in
the 2 follow-up studies, where apparently neither of the
populations held a prior belief that there was an excess
miscarriage rate, it is significant that an apparent excess of
miscarriages was found in the wives of DAT-exposed workers.
An epidemiological assessment of the reproduction hazards for
males after occupational exposure to diaminotoluenes and
dinitrotoluenes was carried out by Hamill et al. (1982). Reported
occupational exposures were similar to those reported by NIOSH
(1980, 1981, 1982) and were generally well within the OSHA
recommended level of 1.5 mg/m3 for dinitrotoluenes. Examination of
84 workers and 119 unexposed subjects consisted of semen analysis,
blood testing, medical examination, and an interview. Seventy-two
percent of non-vasectomized exposed workers provided semen samples.
These groups of workers were defined by exposure intensity,
frequency, and recency, and compared with controls. Although no
significant differences in miscarriage rates were reported between
exposure categories, the categories were as defined at the time of
the study, not at the time of pregnancy. Fertility rates were
reported to be unaffected by exposure, but no figures were given.
There were no statistically significant differences in semen
analysis, sperm count and morphology, and FSH levels, between the 3
exposure groups and unexposed workers. The authors concluded that
the results of their study suggested that no detectable
reproductive effects existed among male workers exposed to
dinitrotoluenes and diaminotoluenes.
Levine et al. (1985) reported an analysis of the fertility of
workers exposed to DNT and DAT. The approach taken consisted of
workers completing reproductive history questionnaires and of
observed births being compared with expected births for the married
workers. Expected births were derived from US birth rates by age,
calendar year, and parity, and the ratio of observed to expected
was expressed as a standardized fertility ratio (SFR). Populations
of 137, 207, and 235 persons, respectively, were studied and were
largely, but not exclusively, male. It is not clear whether any of
the plants were the same as those described above. Comparisons of
SFR between different exposure categories, both for the whole
population and also among individuals who spent at least part of
their reproductive life exposed, did not reveal any significant
effects on SFR between exposure groups. The authors estimated that
the power of this study to detect a 50% reduction in fertility
would have been 90%. In the third NIOSH study, the miscarriage
rate for wives of DAT-exposed workers was 6.8 times higher than
that for wives of unexposed workers (rates given as miscarriages
per 100 person-years), but the fertility rate was only 0.8 times
lower (ratio of rates of live births per 100 person-years). Thus,
overall fertility may not be a sensitive index of adverse
reproductive outcome.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1. Evaluation of Human Health Risks
9.1.1. General considerations
There are insufficient data on the effects of diaminotoluenes
on human beings to carry out a detailed hazard assessment or risk
evaluation. However, absorption and metabolic studies in human
beings have indicated that diaminotoluenes are rapidly absorbed,
metabolized, and excreted in the urine in a manner similar to that
found in experimental animals. Therefore, the risk evaluation that
follows is based largely on data from animals, supported by data
from human studies, where available.
9.1.2. Assessment of exposure
Diaminotoluenes can be absorbed through the skin and
gastrointestinal tract, and by inhalation. Given the properties of
this class of chemicals, the major route of human exposure is
dermal, in the work-place, with a possibility of the inhalation of
fumes during heating. Exposure through ingestion is minimal,
except in case of accidents.
No data exist on general ambient levels of diaminotoluenes in
air, water, and food. Bioaccumulation of diaminotoluenes in the
food-chain should not occur. Levels in the work-place air of up to
0.44 mg/m3, with occasional excursions up to 11 mg/m3, have been
reported.
9.1.3. Single and short-term exposures
Diaminotoluenes are classed as toxic, highly irritant
chemicals. The oral LD50 for animals is between 270 and 350 mg/kg
body weight. Dermal contact has been shown to cause irritation,
severe dermatitis, blistering, and possible skin sensitization.
Single oral doses of diaminotoluenes of 50 mg/kg body weight have
led to methaemoglobinaemia in rats, rabbits, and guinea-pigs. Eye
contact with diaminotoluenes has led to conjunctivitis and corneal
opacities. In case of inhalation of fumes, coughing, dyspnoea, and
respiratory distress can result.
9.1.4. Long-term exposure
9.1.4.1. Carcinogenicity and mutagenicity
No epidemiological data are available on the incidence of
cancer in human beings after exposure to diaminotoluenes.
Several studies using 2,4-DAT have been carried out on
experimental animals and, in each, the isomer was shown to be
carcinogenic for rats and mice. In the most recent study, doses
of, or greater than, 79 mg/kg diet led to an increase in
hepatocellular carcinomas or neoplastic nodules in rats; there was
an increase in hepatocellular carcinomas and lymphomas in female
mice at doses exceeding 100 mg/kg diet.
Using a similar protocol, the US NCI concluded that 2,6-DAT was
not carcinogenic for rats and mice after administration of up to
500 mg/kg diet for 103 weeks. It should be noted that
hepatocellular carcinomas, neoplastic nodules, and lymphomas were
detected, as in the bioassay for 2,4-DAT, however, they were
considered not significant after detailed statistical analyses.
There was no evidence of carcinogenicity in mice and rats after
administration of 2,5-DAT at levels of up to 2000 mg/kg diet, for
78 weeks. However, the short duration of the study reduced the
potential of the test for detecting carcinogenicity. No evidence
of carcinogenicity was noted after the dermal application of hair-
dye formulations containing 2,5-DAT (following application of
hydrogen peroxide) or a mixture of 2,5-DAT and 2,4-DAT with
hydrogen peroxide.
Positive mutagenic activity was noted in S. typhimurium when
2,4-, 2,5-, 2,6-, and 3,4-DAT were tested. In addition, DAT
isomers were mutagenic in mammalian cells in vitro. Significant
DNA damage was produced by 2,4-DAT in human cultured fibroblasts,
only after activation by prostoglandin synthase. The isomer 2,4-
DAT was weakly mutagenic in Drosophila melanogaster and induced
unscheduled DNA synthesis in primary rat hepatocytes in vitro.
The 2,4- and 2,5-isomers were inactive in in vivo mammalian
mutagenicity assays. Micronuclei and dominant lethals were not
produced by 2,5-DAT, and 2,4-DAT did not produce chromosomal
breaks, dominant lethals, abnormal sperm morphology, or recessive
spots.
It has been shown that 2,4-, 2,5-, and 2,6-DAT can inhibit DNA
synthesis in the testes after ip injection of high doses. On the
basis of this study, 2,4-DAT may pose a genetic hazard in addition
to its potential to cause adverse effects on reproduction.
9.1.4.2. Reproduction and teratogenicity
The results of limited studies on the reproduction hazards for
male workers exposed to diaminotoluenes are equivocal. In surveys
of reproductive outcome in 3 plants, an excess of spontaneous
abortions among the wives of male workers exposed to DAT and
dinitrotoluene was reported in 2 surveys, though these excesses
were based on small numbers, and not all workers in the plants
participated in the studies. In 1 out of the 3 plants studied,
some adverse effects on spermatogenesis were suggested. Analysis
of the overall fertility of workers in 3 other production plants
did not reveal any adverse effects from exposure to DAT.
In a study on animals fed 2,4-DAT, there was a significant and
persistent decrease in the sperm count.
Embryotoxicity was observed in animal studies after oral and
dermal doses exceeding 30 mg/kg body weight for the 2,3-and 3,4-
isomers and 10 mg/kg body weight for 2,6-DAT.
Skeletal changes were noted after dermal application of a hair-
dye formula containing 3% 2,4-DAT at 2 ml/kg body weight.
9.2. Evaluation of Effects on the Environment
Information is lacking concerning levels of diaminotoluenes in
the environment, and their transport, bioconcentration,
biotransformation, and biodegradation.
A few data indicate that diaminotoluenes may be hazardous for
aquatic organisms. No data on the effects of diaminotoluenes on
other non-mammalian targets in the environment could be found.
9.3. Conclusions
Diaminotoluenes are highly irritating to the skin and eyes and
the fumes are irritating to the respiratory tract. They are
readily absorbed through the skin. Methaemoglobinaemia may occur
in exposed individuals. Renal toxicity after oral administration
of 2,4-DAT has been reported in experimental animals. 2,4-DAT has
been shown to be carcinogenic for animals, but there is inadequate
evidence to evaluate the carcinogenic potential of 2,5- and 2,6-
diaminotoluene. All 3 of these isomers have been shown to be
mutagenic. Limited data are available concerning a reproductive
hazard for male workers handling DATs. DATs have been shown to
impair spermatogenesis in experimental animals and to be both
embryotoxic and teratogenic.
10. RECOMMENDATIONS
1. Monitoring should be undertaken to determine the sources,
levels, and fate of diaminotoluenes in the environment.
Ecotoxicity data should be collected.
2. For a better evaluation of occupational exposure and effects,
studies on the uptake, kinetics, and metabolism of DAT and the
relevant routes of exposure are important to provide a sound
basis for biological monitoring.
3. To assist in the development of appropriate health
surveillance systems, a systematic evaluation of the toxicity
of diaminotoluenes should be carried out to compliment
available data on carcinogenicity and reproductive effects.
4. Additional data should be obtained on human morbidity and
mortality related to exposure to diaminotoluenes, with
particular emphasis on carcinogenic, teratogenic, and
reproductive end-points.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1978) evaluated the data on the carcinogenicity of
diaminotoluenes and concluded that there was sufficient evidence of
the carcinogenicity of 2,4-diaminotoluene in experimental animals.
An evaluation of additional data by IARC (1986) further supported
this conclusion.
In the absence of case reports or epidemiological studies,
there was inadequate data to assess the carcinogenicity of
diaminotoluenes for human beings (IARC, 1978).
REFERENCES
AUNE, T., NELSON, S.D., & DYBING, E. (1979) Mutagenicity and
irreversible binding of the hepatocarcinogen 2,4-diaminotoluene.
Chem.-biol. Interact., 25(1): 23-24.
AUSTIN, G.T. (1974) Industrially significant organic chemicals.
Part 9. Chem. Eng., 11: 96-100.
BACKUS, J.K. (1974) Urethanes. In: Considine, D.M., ed.
Chemical and process technology encyclopedia, New York, McGraw
Hill Book Co., pp. 1121-1125.
BECCI, P.J., REAGAN, E.L., KNICKERBOCKER, M.J., BARBEE, S.J.,
& WEDIG, J.H. (1983) Teratogenesis study of o-toluenediamine
in rats and rabbits. Toxicol. appl. Pharmacol., 71: 323-329.
BECHER, G. (198l) Glass capillary columns in the gas
chromatographic separation of aromatic amines. 2. Application
of samples from workplace atmospheres using nitrogen-selective
detection. J. Chromatogr., 211(1): 103-111.
BERMUDEZ, E. & BUTTERWORTH, B.E. (1979) Analysis of the
activities of 2,4-diaminotoluene and 2,4- and 2,6-dinitrotoluene
in the primary hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 1: 168-169.
BERMUDEZ, E., TILLERY, D., & BUTTERWORTH, B.E. (1979) Effect
of 2,4-diaminotoluene and isomers of dinitrotoluene on
unscheduled DNA synthesis in primary rat hepatocytes. Environ.
Mutagen., 1: 39l-398.
BIERNACKA, T., SEKOWSKA, B., & MICHONSKA, J. (1974) [Determination
of 2,4- and 2,6-diaminotoluene in technical products by infra-red
spectroscopy.] Chem. Anal. (Warsaw), 19: 6l9-632 (in Polish).
BLIJLEVEN, W.G.H. (1977) Mutagenicity of four hair dyes in
Drosophila melanogaster. Mutat. Res., 48: 181-186.
BOUFFORD, C.E. (l968) Determination of isomeric diaminotoluenes
by direct gas liquid chromatography. J. Gas Chromatogr., 6:
438-440.
BUIST, J.M. (1970) Isocyanates in industry. Proc. R. Soc. Med.,
63: 365-366.
BURNETT, C., LANMAN, B., GIOVACCHINI, R., WOLCOTT, G., & SCALA, R.
(1975) Long-term toxicity studies on oxidation hair dyes.
Food Cosmet. Toxicol., 13(3): 353-357.
BURNETT, C., GOLDENTHAL, E.I., HARRIS, S.B., WAZETER, F.X.,
STRAUSBURG, J., KAPP, R., & VOELKER, R. (1976) Teratology
and percutaneous toxicity studies on hair dyes. J. Toxicol.
environ. Health, 1: 1027-1040.
BURNETT, C., LOEHR, R., & CORBETT, J. (1977) Dominant lethal
mutagenicity study on hair dyes. J. Toxicol. environ. Health,
2: 657-662.
CARDY, R.H. (1979) Carcinogenicity and chronic toxicity of
2,4-toluenediamine in F344 rats. J. Natl Cancer Inst., 62:
1107-1116.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F., Jr (1974)
Range-finding toxicity data. Toxicol. appl. Pharmacol., 28:
313-319.
CHOUDHARY, G. (1980) Gas-liquid chromatographic determination
of toxic diamines in permanent hair dyes. J. Chromatogr., 193:
277-284.
CIC JAPAN (1983) Commercial industrial chemicals, Tokyo,
Chemical Daily Co., Ltd.
CINKOTAI, K.I., JONES, C.C., TOPHAM, J.C., & WATKINS, P.A. (1978)
Experience with mutagenic tests as indicators of carcinogenic
activity. Mutat. Res., 53: 167-168.
COPPINGER, W.J., BRENNAN, S.A., CARVER, J.H., & THOMPSON, E.D.
(1984) Locus specificity of mutagenicity of 2,4-diaminotoluene in
both L5l78Y mouse lymphoma and AT3-2 chinese hamster ovary cells.
Mutat. Res., 135: 115-123.
CRC (1975) CRC handbook of chemistry and physics, 56th ed.,
Cleveland, Ohio, CRC Press.
DENT, J.G. & GRAICHEN, M.E. (1982) Effect of hepatocarcinogens
on epoxide hydrolase and other xenobiotic metabolizing enzymes.
Carcinogenesis, 3(7): 733-738.
DYBING, E. & THORGEIRSSON, S.S. (1977) Metabolic activation
of 2,4-diaminoanisole, a hair dye component. 1. Role of
cytochrome P-450 metabolism in mutagenicity in vitro. Biochem.
Pharmacol., 26: 729-734.
DYBING, E., AUNE, T., & SODERLUND, E.J. (1977) Use of the
Salmonella mutagenicity test in drug metabolism studies. Acta
pharmacol. toxicol., 41: 31.
DYBING, E., AUNE, T., & NELSON, S. (1978) Covalent binding
of 2,4-diaminoanisole and 2,4-diaminotoluene in vivo. Toxicol.
Aspects Food Saf. Arch. Toxicol., Suppl. 1: 213-217.
FAHMY, M.J. & FAHMY, O.G. (1977) Mutagenicity of hair dye
components relative to the carcinogens benzidine in Drosophila
melanogaster. Mutat. Res., 56: 31-38.
FILATOVA, V.S., TUBINA, A.Ya., SHARONOVA, Z.V., GOLOVA, I.A.,
FILINA, V.I., & DOROFEEVA, E.D. (1970) [Hygienic aspects of
work and health of workers in the production of toluylene-
diamine.] Gig. i Sanit., 35(2): 16-30 (in Russian with English
summary).
GAN, L.H., BLAIS, P., CARLSSON, D.J., SUPRUNCHUCK, T., & WILES, D.M.
(1975) Physicochemical characterization of some fully aromatic
polyamides. J. appl. polym. Sci., 19: 69-82.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., WEISBURGER, E.K., &
ROLLER, P.P. (1975) Enzymic N-acetylation of 2,4-toluenediamine
by liver cytosols from various species. Xenobiotica, 5(8): 475-483.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., LEWIS, N.L., &
WEISBURGER, E.K. (1976) N-acetylation as a route of 2,4-
toluenediamine metabolism by hamster liver cytosol. Biochem.
Pharmacol., 25: 95-97.
GRANTHAM, P.H., MOHAN, L., BENJAMIN, T., ROLLER, P.P., MILLER, J.R.,
& WEISBURGER, E.K. (1980) Comparison of the metabolism of 2,4-
toluenediamine in rats and mice. J. environ. Pathol. Toxicol.,
3(1-2): 149-l66.
GREENE, E.J. & FRIEDMAN, M.A. (1980) In vitro cell transformation
screening of 4 toluene diamine isomers. Mutat. Res., 79: 363-375.
GREENE, E.J., FRIEDMAN, M.A., & SHERROD, J.A. (1979) In vitro
mutagenicity and cell transformation testing of four toluene
diamine isomers. Environ. Mutagen., 1: 194.
GREENE, E.J., SALERNO, A.J., & FRIEDMAN, M.A. (1981) Effect
of 4 toluene diamine isomers on murine testicular DNA synthesis.
Mutat. Res., 91: 75-79.
GUTHRIE, J.L. & MCKINNEY, R.W. (1977) Determination of 2,4-
and 2,6-diaminotoluene in flexible urethane foams. Anal.
Chem., 49: 1676-1680.
HAMILL, V.V., STEINBERGER, E., LEVINE, R.J., RODRIGUEZ-RIGAU,
L.J., LEMESHOW, S., & AVRUNIN, J.S. (1982) The epidemiologic
assessment of male reproductive hazard from occupational
exposure to TDA and DNT. J. occup. Med., 24: 982-993.
HOSSACK, D.J.N. & RICHARDSON, J.C. (1977) Examination of the
potential mutagenicity of hair dye constituents using the
micronucleus test. Experientia (Basel), 33: 377-378.
HRUBY, R. (1977) The absorption of p-toluenediamine by the
skin of rats and dogs. Food Cosmet. Toxicol., 15: 595-599.
IARC (1978) 2,4-Diaminotoluene. In: Some aromatic amines and
related nitro compounds: hair dyes, colouring agents, and
miscellaneous industrial compounds, Lyons, International
Agency for Research on Cancer, pp. 83-95 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 16).
IARC (1986) Some chemicals used in plastics and elastomers,
Lyons, International Agency for Research on Cancer, p. 304
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 39).
INOUYE, M. & MURAKAMI, U. (1976) Teratogenicity of 2,5-
diaminotoluene, a hair dye component in mice. Teratology, 14:
241-242.
INOUYE, M. & MURAKAMI, U. (1977) Teratogenicity of 2,5-
diaminotoluene, a hair dye constituent in mice. Food Cosmet.
Toxicol., 15: 447-451.
ITO, N., HIASA, Y., KONISHI, Y., & MARUGAMI, M. (1969) The
development of carcinoma in liver of rats treated with
m-toluylenediamine and the synergistic and antagonistic
effects with other chemicals. Cancer Res., 29: 1137-1145.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982)
Toxicometric paramaters of industrial toxic chemicals under
single exposure, Moscow, USSR/SCST/USSR Commission for UNEP,
Centre of International Projects.
JOHANSSON, K., RAPPE, C., LINDBERG, W., & NYGREN, M. (1981)
Determination of aromatic diamines in hair dyes using liquid
chromatography. In: Egan, H., Fishbein, L., O'Neil, I.K.,
Castegnaro, M., & Bartsch, H., ed. Environmental carcinogens
selected methods of analysis. Volume 4: Some aromatic amines
and azo dyes in the general and industrial environment. Lyons,
International Agency for Research on Cancer (IARC Publications
No. 40).
KIESE, M. & RAUSCHER, E. (1968) The absorbtion of p-toluene-
diamine through human skin in hair dyeing. Toxicol. appl.
Pharmacol., 13: 325-331.
KIESE, M., RACHOR, M., & RAUSCHER, E. (1968) The absorption
of some phenylenediamines through the skin of dogs. Toxicol.
appl. Pharmacol., 12: 495-507.
KINKEL, H.J. & HOLZMANN, S. (1973) Study of long-term
percutaneous toxicity and carcinogenicity of hair dyes
(oxidizing dyes) in rats. Food Cosmet. Toxicol., 11: 641-648.
KNICKERBOCKER, M., RE, T.A., PARENT, R.A., & WEDING, J.H.
(1980) Teratogenic evaluation of ortho-toluenediamine
(o-TDA) in Sprague Dawley rats and Dutch Belted rabbits.
Toxicologist, 19: A.89.
KORNBRUST, D.J. & BARFKNECHT, T.R. (1984) Comparison of 7
azo dyes and their reduction products in the rat and hamster
hepatocyte primary culture/DNA-repair assays. Mutat. Res.,
136: 255-266.
KOTTEMANN, C.M. (1966) Two dimensional thin-layer
chromatographic procedure for the identification of dye
intermediates in arylamine oxidation hair dyes. J. Assoc. Off.
Anal. Chem., 49(5): 954-959.
LEVINE, R.J., CORSO, D.D., & BLUNDEN, P.B. (1985) Fertility
of workers exposed to dinitrotoluene and toluenediamine at
three chemical plants. In: Rickert, D.E., ed. Toxicity of
nitroaromatic compounds, Washington DC, Hemisphere.
LIEM, D.H. & ROOSELAAR, J. (1981) HPLC of oxidation hair
colours. Mitt. Geb. Lebensm. Hyg., 72: 164-176.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975)
Detection of carcinogens as mutagens in the Salmonella microsome
test: Assay of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72:
5135-5139.
MACKE, G.F. (1968) Use of tetracyanoethylene as a thin-layer
chromatographic spray reagent. J. Chromatogr., 36: 537-539.
MARKS, T.A., GUPTA, B.N., LEDOUX, T.A., & STAPLES, R.E.
(1981) Teratogenic evaluation of 2-nitro-p-phenyl-enediamine,
4-nitro-o-phenylendiamine, and 2,5-toluenediamine sulfate in
the mouse. Teratology, 24: 253-265.
MARZULLI, F.N., ANJO, D.M., & MAIBACH, H.I. (1981) In vivo
skin penetration studies of 2,4-toluenediamine, 2,4-diamino-
anisole, 2-nitro-p-phenylenediamine, p-dioxane and n-nitro-
sodiethanolamine in cosmetics. Food Cosmet. Toxicol., 19: 743.
MATHESON, D. & CREASY, B. (1976) Use of the L5178Y (TK+/-)
mouse lymphoma cell line coupled with an in vitro microsomal
enzyme activation system to study chemical promutagens. Mutat.
Res., 38: 400-401.
MATHIAS, A. (1966) Analysis of diaminotoluene isomer
mixtures by nuclear magnetic resonance spectrometry. Anal.
Chem., 38: 1931-1932.
MATSUI, S., MURAKAMI, T., SASAKI, T., HIROSE, Y., & IGUMA, Y.
(1975) Activated sludge degradability of organic substances
in the wastewater of the Kashima petroleum and petrochemical
industrial complex in Japan. Prog. Water Technol., 7:645-659.
MILLIGAN, B. & GILBERT, K.E. (1978) Diaminotoluenes. In:
Kirk-Othmer encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 1, pp. 321-329.
MIRSALIS, J.C. & BUTTERWORTH, B.E. (1982) Induction of
unscheduled DNA synthesis in rat hepatocytes following in vivo
treatment with dinitrotoluene. Carcinogenesis, 3: 241-245.
MIRSALIS, J.C., TYSON, C.M., & BUTTERWORTH, B.E. (1982)
Detection of genotoxic carcinogens in the in vivo/in vitro
hepatocyte DNA repair assay. Environ. Mutagen., 4: 553-562.
MORI, M.A., MIYAHARA, T., TANIGUCHI, K., HASEGAWA, K., KOZUKA,
H., MIYAGOSHI, M., & NAGAYAMA, T. (1982) Mutagenicity of
2,4-dinitrotoluene and its metabolites in Salmonella typhimurium.
Toxicol. Lett., 13: 1-5.
NCI (1978) Bioassay of 2,5-toluenediamine sulfate for
possible carcinogenicity, Bethesda, Maryland, National Cancer
Institute (NCI Carcinogenesis Technical Report Series No. 126;
DHEW Publication No. (NIH) 78-1381).
NCI (1979) Bioassay of 2,4-diaminotoluene for Possible
Carcinogenicity, Bethesda, Maryland, National Cancer Institute
(NCI Carcinogenesis Technical Report Series No. 162).
NCI (1980) Bioassay of 2,6-toluenediamine dihydrochloride
for possible carcinogenicity, Bethesda, Maryland, National
Cancer Institute (NCI Carcinogenesis Technical Report Series
No. 200; NTP No. 80-20; NIH Publication No. 80-1756).
NIEMINEN, E.H., SAARINAN, L.H., & LAAKSO, J.T. (1983)
Simultaneous determination of aromatic isocyanates and some
carcinogenic amines in the work atmosphere by reversed-phase
high-performance liquid chromatography. J. liq. Chromatogr.,
6: 453-469.
NIOSH (1978) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Service, Center for Disease Control,
Vol. 4 (Publication No. 78-175).
NIOSH (1980) Health hazard evaluation determination report
HE 79-113-728, Brandenburg, Kentucky, Olin Chemical Company,
and Cincinnati, Ohio, National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1981) Interim Report No. 1. HETA 81-118,
New Martinsville, West Virginia, Mobay Chemical Corporation,
and Cincinatti, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1982) Interim report No. 1. HETA 81-295-1155,
Moundsville, West Virginia, Allied Chemical Company, and
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NORDENSKJOLD, M., ANDERSSON, B., RAHIMTULA, A., & MOLDEUS, P.
(1984) Prostaglandin synthesis-catalyzed metabolic activation
of some aromatic amines to genotoxic products. Mutat. Res.,
127: 107-112.
OLUFSEN, B. (1979) Glass capillary columns in the gas
chromatographic separation of aromatic amines. I. J. Chromatogr.,
179: 97-103.
OSHA (1983) Code of federal regulations, Washington DC, US
Occupational Safety and Health Administration, pp. 660-664
(29CFR, 19101000, Table Z-1).
PERKINS, W.E. & GREEN, T.J. (1975) Effect of 3,4-toluenediamine
on output from in situ rat brunner's glands pouches (38941).
Proc. Soc. Exp. Biol. Med., 149: 991-994.
PIENTA, R.J., POILEY, J.A., & LEBHERZ, W.B., III (1977a)
Morphological transformation of early passage golden Syrian
hamster embryo cells derived from cryopreserved primary
cultures as a reliable in vitro bioassay for identifying
diverse carcinogens. Int. J. Cancer, 19: 642-655.
PIENTA, R.J., SHAH, M.J., LEBHERZ, W.B., & ANDREWS, A.W.
(1977b) Correlation of bacterial mutagenicity and hamster
cell transformation with mutagenicity induced by 2,4-
toluenediamine. Cancer Lett., 3: 45-52.
PURNELL, C.J. & WARWICK, C.J. (1981) Application of electro-
chemical detection in high-performance liquid chromatography
to the measurement of toxic substances in air. Anal. Proc.,
18: 151-154.
PURNELL, C.J., BAGON, D.A., & WARWICK, C.J. (1982) The
determination of organic contaminant concentrations in
workplace atmospheres by high-performance liquid chromatography.
Pergamon Ser. environ. Sci., 7: 203-219.
RAHIMTULA, A., MOLDEUS, P., ANDERSSON, B., & NORDENSKJOLD, M.
(1982) Prostaglandin synthetase catalyzed DNA strand breaks
by aromatic amines. In: Prostaglandins and related lipids,
New York, Alan R. Liss, Vol. 2, pp. 159-162.
REUBER, M.D. (1979) Carcinomas of the liver in female mice
fed toluene-2,4-diamine. Gann, 70: 453-457.
RIGGIN, R.M. & HOWARD, C.C. (1983) High performance liquid
chromatographic determination of phenylenediamines in aqueous
environmental samples. J. liq. Chromatogr., 6: 1897-1905.
SCHAFER, U. METZ, J., PEVNY, I., & ROCKL, H. (1978)
[Attempts of sensitizing guinea pigs with five different
derivates of para-substituted benzene.] Arch dermatol. Res.,
216: 153-161 (in German).
SELYE, H. (1973) Production of perforating duodenal ulcers
by 3,4-toluenediamine in the rat. Proc. Soc. Exp. Biol. Med.,
142: 1192-1194.
SHAHIN, M.M., BUGAUT, A., & KALOPISSIS, G. (1980) Structure-
activity relationship within a series of m-diaminobenzene
derivatives. Mutat. Res., 78: 25-31.
SHOOTER, K.V. & VENITT, S. (1979) Phosphotriesters in DNA:
non-repairable lesions as markers for the early detection of
chemical carcinogens in intact animals. Mutat. Res., 64: 106.
SKARPING, G., RENMAN, L., & SMITH, B.E.F. (1983a) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. I. Determination of
aromatic amines as perfluoro-fatty acid amines using electron-
capture detection. J. Chromatogr., 267: 315-327.
SKARPING, G., RENMAN, L., & DALENE, M. (1983b) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. II Determination of
aromatic amines as perfluoro-fatty acid amines using nitrogen-
selective detection. J. Chromatogr., 270: 207-218.
SMIRNOVA, A.N., TOROPOVA, L.A., & DOBROVOL'SKAYA, V.V.
(1967) [Effect of toluylenediamine and ortho-toluidine on
aquatic organisms.] Vodosnarzh Kanaltz Giurotekh, 5: 17-23 (in
Russian) (EPA translation).
SNYDER, R.C. & BREDER, C.V. (1982) High performance liquid
chromatographic determination of 2,4- and 2,6-toluenediamine
in aqueous extracts. J. Chromatogr., 236: 429-440.
SNYDER, R.C., BRUMLEY, W.C., BREDER, C.V., & FAZIO, T.
(1982) Gas chromatographic and gas chromatographic-mass
spectrometric confirmation of 2,4- and 2,6-toluenediamine
determined by liquid chromatography in aqueous extracts. J.
Assoc. Off. Anal. Chem., 65(6): 1388-1394.
SOARES, E.R. & LOCK, L.F. (1980) Lack of an indication of
mutagenic effects of dinitrotoluenes and diaminotoluenes in
mice. Environ. Mutagen., 2: 111-124.
SONTAG, J.M. (1981) Carcinogenicity of substituted-benzene-
diamines (phenylenediamines) in rats and mice. J. Natl Cancer
Inst., 66: 591-602.
SPENGLER, J., OSTERBURG, I., & KORTE, R. (1986) Abstract:
Teratogenic evaluation of p-toluenediamine sulphate.
Resorcinal and p-aminophenol in rats and rabbits. Teratology,
33(2): 31.
THYSEN, B., BLOCH, E., & VARMA, S.K. (1985a) Reproductive
toxicity of 2,4-toluenediamine in the rat. 2. Spermatogenic
and hormonal effects. J. Toxicol. environ. Health, 16: 763-769.
THYSEN, B., VARMA, S.K., & BLOCH, E (1985b) Reproductive
toxicity of 2,4-toluenediamine in the rat. 1. Effect on male
fertility. J. Toxicol. environ. Health, 16: 753-761.
UMEDA, M. (1955) Production of rat sarcoma by injections of
propylene glycol solution of m-toluylenediamine. Gann, 46:
597-606.
UNGER, P.D. & FRIEDMAN, M.A. (1979) High-performance liquid
chromatography of 2,6- and 2,4-diaminotoluene, and its
application to the determination of 2,4-diaminotoluene in
urine and plasma. J. Chromatogr., 174: 379-384.
UNGER, P.D., SALERNO, A.J., NESS, W.C., & FRIEDMAN, M.A.
(1980) Tissue distribution and excretion of
2,4[143]-toluenediamine in the mouse. J. Toxicol. environ.
Health, 6: 107-114.
US EPA (1980) Materials balance for 2,4-diaminotoluene.
Level 1. Preliminary, Washington DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/13:
79-016).
US ITC (1977) Synthetic organic chemicals: US production and
sales, 1975, Washington DC, US International Trade Commission,
pp. 22, 36, 42, 49, 60, 62, 65, 74 (US ITC Publication 804).
US ITC (1982) Preliminary report on US production of
selected synthetic organic chemicals. Preliminary totals 1981,
Washington DC, US International Trade Commission (SOC Series
C/P-82-1).
US ITC (1985) Preliminary report on US production of
selected synthetic organic chemicals. November, December, and
cummulative totals, 1984, Washington DC, US International
Trade Commission (SOC Series C/P-85.1).
VENITT, S. (1978) Mutagenicity of hair dyes: some more
evidence and the problems of its interpretation. Mutat. Res.,
53: 278-279.
VON OETTINGEN, W.F. (1941) The aromatic amino and nitro
compounds: their toxicity and potential dangers. A review of
the literature, Washington DC, Division of Industrial Hygiene,
National Institute of Health, pp. 55-63.
WAGNER, J.C. (1975) Linear compartment models. In:
Fundamentals of clinical pharmacokinetics, Hamilton, Illinois,
Drug Intelligence Publications, pp. 57-63.
WARING, R.H. & PHEASANT, A.E. (1976) Some phenolic metabolites
of 2,4-diaminotoluene in the rabbit, rat and guinea-pig.
Xenobiotica, 6: 257-262.
WEISBROD, D. & STEPHAN, U. (1983) [Studies of the toxic,
methaemoglobin-producing and erythrocyte-damaging effects of
diaminotoluene after a single administration.] Z. gesamte
Hyg., 29: 395-397 (in German).
WEISBURGER, E.K., RUSSFIELD, A.B., HOMBURGER, F., WEISBURGER,
J.H., BOGER, E., VAN DONGEN, C.G., & CHU, K.C. (1978)
Testing of twenty-one environmental aromatic amines or
derivatives for long-term toxicity or carcinogenicity.
J. environ. Pathol. Toxicol., 2(2): 325-356.
WILLEBOORDSE, F., QUICK, Q., & BISHOP, E. (1968) Direct gas
chromatographic analysis of isomeric diaminotoluenes. Anal.
Chem., 40: 1455-1458.
WILLIAM, R.T. (1971) The metabolism of certain drugs and
food chemicals in man. Ann. N.Y. Acad. Sci., 179: 141-154.
WILLIAMS, G.M. (1977) Detection of chemical carcinogens by
unscheduled DNA synthesis in rat liver primary cell cultures.
Cancer Res., 37: 1845-1851.
WILLIAMS, G.M. (1978) Further improvements in the hepatocyte
primary culture DNA repair test for carcinogens: detection of
carcinogenic biphenyl derivatives. Cancer Lett., 4: 69-75.
WILLIAMS, G.M. & LASPIA, M.F. (1979) The detection of
various nitrosamines in the hepatocyte primary culture/DNA
repair test. Cancer Lett., 66: 199-206.
Commercial grades of diaminotoluenes are available; however,
the most commonly marketed diaminotoluenes are: (a) "crude"
diaminotoluenes-mixture, containing all 6 isomers (Table 1); (b)
Meta-diaminotoluene (Meta-DAT), containing approximately 80% 2,4-
and 20% 2,6-isomers (also produced in smaller amounts as 65:35
mixture); and (c) Ortho-diaminotoluene (Ortho-DAT), consisting of
approximately 40% 2,3- and 60% 3,4-isomers. All commercial grades
contain traces of the other isomers; therefore, diaminotoluenes and
their mixtures are reviewed together in this document.
Most of the common and trade names for commercial
diaminotoluenes are listed in Table 2.
Table 2. Diaminotoluenes synonyms and trade names
----------------------------------------------------------------------------
A. Commercial mixtures
I. Meta-Diaminotoluenes
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names benzenediamine,ar-methyl (RTECS: TDB);
diaminotoluene (RTECS: TDB);
phenylenediamine,ar-methyl- (TDB);
Meta-diaminotoluene; Meta-toluene-
diamine (MTD);
toluene-ar,ar-diamine (8CI) (CAS: TDB);
toluenediamine (RTECS: TDB, DOT);
tolylenediamine (RTECS, TDB)
Common name diaminotoluene; toluenediamine; TDA
II. Ortho-Diaminotoluene
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names o-TDA
Common name Ortho-toluenediamine; OTD
B. Diaminotoluene isomers
I. 2,3-isomer
Chemical abstract name 1,2-benzenediamine,3-methyl- (9CI)
Other chemical names toluene-2,3-diamine (8CI) (CAS: TDB);
1-methyl-1,2,3-phenylenediamine (TDB);
1,2-diamino-3-methylbenzene (TDB);
2,3-diaminotoluene (TDB*);
2,3-toluylenediamine (TDB);
2,3-tolylenediamine (TDB);
3-methyl-o-phenylenediamine (TDB);
3-methyl-1,2-phenylenediamine (TDB)
Common names 2,3-TDA
II. 2,4-isomer
Chemical abstract name 1,3-benzenediamine,4-methyl- (9CI)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
II. 2,4-isomer (contd.)
Other chemical names m-toluenediamine (RTECS);
m-toluylendiamin (Czech, RTECS: TDB);
m-toluylenediamine (RTECS: TDB);
m-tolyenediamine (RTECS: TDB);
m-tolylenediamine (RTECS);
Meta-toluylene diamine (RTECS: TDB);
toluene-2,4-diamine (8CI) (CAS, RTECS:
TDB*);
tolylene-2,4-diamine (RTECS: TDB);
1,3-diamino-4-methylbenzene (RTECS: TDB);
2,4-diamino-1-methylbenzene (RTECS: TDB);
2,4-diamino-1-toluene (RTECS: TDB);
2,4-diaminotoluen (Czech, RTECS: TDB);
2,4-diaminotoluene (MESH, RTECS: TDB);
2,4-diaminotoluol (RTECS: TDB);
2,4-tolamine (RTECS: TDB);
2,4-toluenediamine (MESH, RTECS: TDB);
2,4-toluylenediamine (DOT, RTECS: TDB);
2,4-tolylenediamine (RTECS: TDB);
3-amino- p-toluidine (RTECS: TDB);
4- m-tolylenediamine (RTECS: TDB);
4-methyl- m-phenylenediamine (RTECS: TDB);
4-methyl-1,3-benzenediamine (RTECS: TDB);
5-amino- o-toluidine (RTECS: TDB)
Common names TDA; MTD; 2,4-TDA (CAS, RTECS: TDB)
Trade names Azogen Developer H; Benzofur MT;
C.I. Oxidation Base (RTECS);
C.I. Oxidation Base 20;
C.I. Oxidation Base 35 (RTECS);
C.I. Oxidation Base 200;
Developer B (RTECS: TDB); Developer DB
(RTECS: TDB);
Developer DBJ (RTECS: TDB); Developer H;
Developer MC (RTECS: TDB); Developer MT
(RTECS: TDB);
Developer MT-CF (RTECS: TDB);
Developer MTD (RTECS; TDB);
Developer T (RTECS: TDB); Developer 14;
Eucanine GB (RTECS: TDB); Fouramine;
Fouramine J (RTECS: TDB); Fourrine M
(RTECS: TDB);
Fourrine 94 (RTECS: TDB);
Lekutherm-Haerter VP-KU 6546;
Nako TMT (RTECS: TDB); NCI-C02302 (RTECS:
TDB);
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
Trade names (contd.) Pelagol J (RTECS: TDB); Pelagol Grey J
(RTECS: TDB);
Pontamine Developer TN (RTECS: TDB);
Renel MD (RTECS: TDB); Tertral G;
Zoba GKE (RTECS: TDB);
Zogen Developer H (RTECS: TDB).
Colour index number 76035
III. 2,5-isomer
Chemical abstract name 1,4-benzenediamine,2-methyl-(9CI)
Other chemical names p-toluenediamine (MESH, RTECS: TDB);
p-toluylendiamine (RTECS: TDB);
P,m-tolylenediamine (RTECS: TDB);
para-meta-tolylenediamine;
para-toluenediamine;
para-toluylenediamine;
para-tolylenediamine;
toluene-2,5-diamine (8CI) (CAS, RTECS:
TDB);
toluylene-2,5-diamine (RTECS: TDB);
2-methyl- p-phenylenediamine (RTECS: TDB);
2-methyl-1,4-benzenediamine (RTECS: TDB*);
2,5-diaminotoluene (MESH, RTECS: TDB);
2,5-toluenediamine;
4-amino-2-methylaniline (RTECS: TDB)
Common name 2,5-TDA
Trade names Oxidation Base 4 (as sulfate)
Colour index number C.I. 76042 (RTECS); 76043 (as sulfate)
IV. 2,6-isomer
Chemical abstract name 1,3-benzenediamine,2-methyl- (9CI)
Other chemical names m-phenylenediamine-2-methyl;
toluene-2,6-diamine (8CI) (CAS, RTECS:
TDB);
tolylene-2,6-diamine;
1,3-benzenediamine;
2-methyl- m-phenylenediamine (TDB);
2-methyl-1,3-benzenediamine (TDB);
2-methyl-1,3-phenylenediamine (TDB);
2,6-diamino-1-methylbenzene (TDB);
2,6-diaminotoluene (MESH, RTECS: TDB*);
2,6-toluenediamine;
2,6-toluylenediamine (RTECS: TDB);
2,6-tolylenediamine (RTECS: TDB)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
IV. 2,6-isomer (contd.)
Common names 2,6-TDA
V. 3,4-isomer
Chemical abstract names 1,2-benzenediamine,4-methyl- (9CI)
Other chemical names o-toluenediamine;
toluene-3,4-diamine (8CI) (CAS, RTECS:
TDB);
1,2-benzenediamine,4-methyl- (RTECS: TDB);
1,2-diamino-4-methylbenzene (TDB);
3,4-diamino-1-methylbenzene (TDB);
3,4-diaminotoluene (RTECS: TDB);
3,4-toluenediamine;
3,4-toluylenediamine (RTECS);
3,4-tolylenediamine (RTECS: TDB);
4-methyl- o-phenylenediamine (TDB);
4-methyl-1,2-benzenediamine (TDB);
4-methyl-1,2-diaminobenzene (TDB);
4-methyl-1,2-phenylenediamine (TDB)
Common name 3,4-TDA
VI. 3,5-isomer
Chemical abstract name 1,3-benzenediamine,5-methyl- (9CI)
Other chemical names 3,5-diaminotoluene;
3,5-toluenediamine
Common name 3,5-TDA
----------------------------------------------------------------------------
2.2. Physical and Chemical Properties
Diaminotoluenes are colourless crystals that are freely soluble
in hot water, alcohol, ether, and hot benzene. Some of the
physical properties of the 6 isomers are listed in Table 3 (Buist,
1970; CRC, 1975). Diaminotoluenes are oxidized readily in neutral
or alkaline solution to form dark-coloured products and tars. The
oxidation products have not been fully characterized. When heated,
diaminotoluenes emit toxic fumes of nitrogen oxides.
The composition and physical properties of the commercial
mixtures vary considerably. Some of the physical properties of the
2 most widely-used commercial mixtures are summarized in Table 4.
Meta- and Ortho-diaminotoluenes are weakly basic and react with
mineral acids to form water-soluble amine salts. These salts are
more resistant to oxidation than the parent amine.
Table 3. Physical properties of the diaminotoluene isomers
-------------------------------------------------------------------
Property Diaminotoluene isomers
2,3- 2,4- 2,5- 2,6- 3,4- 3,5-
-------------------------------------------------------------------
Melting point (°C) 63-64 99 64 105 88.5 -
Boiling point (°C) 255 280 273-274 289b 265 283-285
(subl)
Vapour pressurea (kPa)
at 150 °C 1.20 1.47 - 2.13 - -
at 160 °C 1.87 2.27 - 3.33 - -
at 180 °C 2.67 4.80 - 7.60 - -
-------------------------------------------------------------------
a To convert kPa to mmHg, divide by 0.133.
b Obtained by extrapolation from vapour pressure-temperature data
and Antoine constants. From: Willeboordse et al. (1968).
Table 4. Physical and chemical properties of commercial grades of
diaminotoluene
----------------------------------------------------------------------
Meta-DAT Ortho-DAT
(80:20, 2,4-/2,6-isomers) (60:40, 3,4-/2,3-isomers)
----------------------------------------------------------------------
Appearance solid, light yellow to tan; light grey to purple
darkens on storage and solid
exposure to air
Odour slight ammonia-like slight ammonia-like
Melting range 80 - 90 °C (176 - 194 °F) 40 - 50 °C (104 - 122 °F)
Boiling point 283 °C (541 °F) at 760 mmHg > 250 °C (> 480 °F)
Flash point 140 °C (284 °F) > 110 °C (> 230 °F)
Autoignition 450 °C (842 °F) 540 °C (1005 °F)
temperature
Vapour 0.34 x 10-3 mmHg at 37.8 °C 2.23 mmHg at 100 °C
pressure 1 mmHg at 106.5 °C 27.8 mmHg at 140 °C
100 mmHg at 212 °C 43.5 mmHg at 160 °C
Specific - 1.045 at 100 °C
gravity
Density 0.086 kg/litre at 105 °C -
Solubility in hot water, alcohol, in hot water, alcohol,
ether and many polar ether and many polar
organic solvents organic solvents
----------------------------------------------------------------------
2.3. Conversion Factors
1 ppm in air = 5 mg/m3 at 25 °C and 760 mmHg.
2.4. Analytical Methods
Analytical methods for the determination of diaminotoluenes in
water, air, different consumer products, and biological fluids are
listed in Table 5.
Diaminotoluenes may be analysed as free bases by reversed phase
high-performance liquid chromatography using both ultraviolet (UV)
and electrochemical detection (Purnell & Warwick, 1981; Purnell et
al., 1982; Nieminen et al., 1983). Gas chromatographic methods
usually involve derivatization to facilitate separation and
increase sensitivity (Olufsen, 1979; Skarping et al., 1983a,b).
Detection limits in air samples range from 0.1 to 10 µg/m3.
Diaminotoluenes and their derivatives have been studied in
blood, urine, and liver cytosol preparations using thin-layer
chromatography and gas chromatography/mass spectrometry (GC/MS)
(Kiese & Rauscher, 1968; Kiese et al., 1968; Glinsukon et al.,
1975; Waring & Pheasant, 1976). A high-performance liquid
chromatographic method for the determination of diaminotoluenes in
urine and plasma has been described by Unger & Friedman (1979).
Table 5. Analytical methods for the determination of diaminotoluenes
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Water high-performance liquid chrom- 2,4-, 2,5-, 2,6-, 0.2 - 0.7 ng Riggin & Howard
atography with ultraviolet and 3,4-isomers (1983)
electrochemical detection
Air gas-liquid chromatography/ 2,4-isomer 3 µg/m3 Becher (1981)
nitrogen-phosphorus detector
on glass capillary columns
high-performance liquid chrom- 0.1 - Purnell et al.
atography with ultraviolet 10 µg/m3 (1982)
and electrochemical detection
high-performance liquid chrom- 2,4-isomer - Nieminen et al.
atography with ultraviolet (1983)
detection
gas-liquid chromatography with 2,4- and 2,6-isomers ~ 0.1 - Skarping et al.
electron capture detection on 0.4 pg (1983a)
glass capillary column
gas-liquid chromatography/ 2,4- and 2,6-isomers 10 - 20 pg Skarping et al.
nitrogen-phosphorus detector amine (1983b)
on glass capillary columns
Hair dyes gas-liquid chromatography/ 2,5-isomer 5 mg/litre Choudhary (1980)
flame ionization detector
thin-layer chromatography 2,4-, 2,5-, and 0.2 mg/litre Kottemann (1966)
3,4-isomer
high-performance liquid chrom- 2,4-, 2,5-, 0.5 mg/litre Johansson et al.
atography/ultraviolet detection and 2,6-isomers (1981)
high-performance liquid chrom- 2,4-, 2,5-, - Liem & Rooselaar
atography/ultraviolet detection and 3,4-isomers (1981)
------------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Hair dyes high-performance liquid chrom- 2,4- and 2,6-isomers 0.1 µg/litre Snyder et al.
(contd.) atography/ultraviolet detection (1982)
gas-liquid chromatography/mass
spectrometry
Polyurethane thin-layer chromatography/ 2,4- and 2,6-isomers 1 µg/g Guthrie &
foams fluorimetry McKinney (1977)
Biological high-performance liquid chrom- 2,4- and 2,6-isomers 2 mg/litre Unger & Friedman
tissues and atography/ultraviolet detection (1979)
fluids
Isomeric nuclear magnetic resonance all isomers - Mathias (1966)
mixtures spectrometry
thin-layer chromatography all isomers - Macke (1968)
gas-liquid chromatography/ all isomers - Boufford (1968)
flame ionization detector
gas-liquid chromatography/ all isomers - Willeboordse et
thermal detector al. (1968)
infra-red spectroscopy 2,4- and 2,6-isomers - Biernacka et al.
(1974)
------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Natural Occurrence
Diaminotoluenes are not known to occur as natural products.
3.2. Production
Currently, diaminotoluenes are produced commercially through
the catalytic hydrogenation of dinitrotoluenes. This procedure,
economic only for large-scale production, is used in the
manufacture of toluene diisocyanates. At dye plants, diamino-
toluenes are produced by the reaction of hydrochloric acid on
dinitrotoluenes, in the presence of an iron catalyst (Austin,
1974).
Most diaminotoluenes produced are used on site by the
manufacturer; therefore, published production figures do not
adequately reflect the true world production of diaminotoluenes.
Between 1972 and 1976, the average annual production of
diaminotoluenes in the USA was 89 x 106 kg, ranging from 76 x 106
kg in 1972 to 105 x 106 kg in 1976 (US ITC, 1977). Thereafter, the
production was estimated from the known production of toluene
diisocyanates ranging between 305 x 106 kg annually in the period
1977 - 81, and 360 x 106 kg in 1984 (US ITC, 1982, 1985).
Up to 1978, an estimated 180 - 200 x 106 kg of 2,4-DAT was
produced annually in western Europe (IARC, 1978). During
1971 - 75, the the annual production of 2,4-DAT in Japan was
approximately 210 x 103 kg; the compound was neither imported nor
exported (IARC, l978). However, in 1981, the production of 2,4-DAT
in Japan was estimated to have declined to 50 x 103 kg (CIC Japan,
1983).
3.3. Uses
Diaminotoluenes are used extensively within the chemical
industry as intermediates in the manufacture of widely different
commercial products (Table 6). Minor applications of diamino-
toluene isomers include their use as raw materials, co-reactants,
and curing agents. Toluene diisocyanates represent the largest
end-use accounting for more than 90% of the total annual production
of diaminotoluenes, largely a mixture of 2,4- and 2,6-isomers
(Backus, 1974; Milligan & Gilbert, 1978).
Diaminotoluenes are intermediates in the synthesis of dyes used
for textiles, furs, leathers, biological stains and indicators,
spirit varnishes and wood stains, and pigments. Previously used in
hair-dyes, 2,4-DAT was removed from use by many countries after it
was found to be a hepatocarcinogen in rats (Ito et al., 1969).
Meta-DAT is used to produce diethyltoluenediamine (DETDA) for the
manufacture of certain urethane elastomers (Milligan & Gilbert,
1978). Ortho-DAT is used to produce mercaptotoluimidazole (MTI)
and its zinc salt, both of which are used primarily as specialty
antioxidants in nitrile rubber elastomers (Gan et al., 1975).
Table 6. End-use application(s) of individual
diaminotoluene isomers
------------------------------------------------------
Application 2,3- 2,6- 2,3- 3,4- 2,5-
------------------------------------------------------
Toluene diisocyanate X X
(> 90% of total use of
diaminotoluenes)
Urethane co-reactants X X X X
(DAT-initiated polyols)
DETDAa X X
DYESb Xd X d
Tolyltriazole X X
Epoxy curing X X X
Mercaptotoluimidazole X X
Photographic developer X
------------------------------------------------------
a DETDA = Diethyltoluenediamine.
b DYES = Fur, leather, biological stains, indicators,
textiles, hair, spirit varnishes, wood stains, and
pigments.
c Use in hair dyes and cosmetics prohibited in USA
since 1971.
d Forbidden in Italy - 1978.
3.4. Release into the Environment, Distribution, and Transformation
Data are lacking on the extent of the global release of
diaminotoluenes, as well as their transport, distribution, and
degradation within the environment.
Releases of 2,4-DAT into the environment have been estimated in
the USA, the largest contribution being over 6 x 106 kg dumped in
authorized landfills. Releases of 1.4 x 106 kg were estimated to
occur from the production of diaminotoluenes, and 0.3 x 106 kg
during dye production and usage; unknown quantities of DAT may
derive from the hydrolysis of TDI released into the environment.
Information on the transport, distribution, and degradation of
DAT isomers under conditions approaching those found in natural
bodies of water have not been reported in the literature. However,
a bench-scale treatability study for 2,4-DAT using acclimated
sludge from a treatment plant showed that the isomers are
degradable. The observed total organic carbon removal was 45% in
4 h (Matsui et al., 1975).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
No information was available in the literature reviewed from
which environmental levels could be calculated. Two properties of
the diaminotoluenes are relevant to this problem. Since the vapour
pressure is low (Tables 4 and 5), the risk of contaminating the
environment through evaporation is minimal. However, air emissions
from inappropriately operated plants may pose a hazard. Since the
chemical is soluble in water, the potential for exposure through
water contamination is of concern. No data are available on levels
of diaminotoluenes in surface and groundwater, in soil, and/or air.
4.2. General Population Exposure
No information is available on the exposure of the general
population to diaminotoluenes.
4.3. Occupational Exposure
Filatova et al. (1970) reported concentrations of diamino-
toluenes in manufacturing plants of up to 0.2 mg/m3, with
occasional excursions up to 11 mg/m3.
The results of studies conducted in 3 plants manufacturing
diaminotoluenes in the USA showed that the work-place ambient air
levels ranged from 0.005 to 0.44 mg/m3 (NIOSH, 1980, 1981, 1982).
The highest level of diaminotoluenes (0.44 mg/m3) was found in the
filter room at one plant (NIOSH, 1980). A level of 0.39 mg
diaminotoluenes/m3 was measured in a sample taken at the breathing
zone of an operator in a second plant (NIOSH, 1981). All values
were calculated as time-weighted averages.
5. KINETICS AND METABOLISM
5.1. Studies on Experimental Animals
5.1.1. Absorption and retention
Skin penetration by test materials varied amoung species
(monkeys, swine), and was affected by vehicle and site of
application. In one study, [14C]-2,4-DAT (4 µg/cm2), dissolved in
acetone, methanol, or a skin lotion, was applied to 3 - 15 cm2 of
the ventral forearm, abdomen, or back of 3 - 6 monkeys/group (9
groups). The material was removed after 24 h by washing with soap
and water. The greatest absorption (53.8 ± 15.4%) resulted when
[14C]-2,4-DAT in acetone was applied to the abdominal skin of
monkeys (Marzulli et al., 1981). The permeability of diamino-
toluenes across the epidermis was highly dependent on the
formulation used. When 1.4 g of 2,5-DAT was applied in a gel
to the abdominal skin of dogs for a contact period of 3 h, 2.9%
(40 mg) was absorbed. Addition of hydrogen peroxide, similar to
the formulation used in hair dye, reduced the amount absorbed
to < 0.21% (Kiese et al., 1968) or < 0.13% (Hruby, 1977). The
hair in the exposed area retained 4% of the 14C activity, 5 days
after application (Hruby, 1977).
Hruby (1977) studied the absorption of [14C]-2,5-DAT following
oral and subcutaneous single-dose administrations to rats. Five
days following subcutaneous injection of 3 - 5 mg [14C]-2,5-DAT (in
water), 6.9% of the dose was found in the total-body homogenate and
1.7% remained at the injection site. Five days after oral (gavage)
administration of 10 mg [14C]-2,5-DAT (in water), the rat gastro-
intestinal tract retained 1.4% of the applied radioactivity and
1.2% was found in the total body homogenate (Hruby, 1977).
No studies on uptake after inhalation were found.
5.1.2. Distribution and reaction with body components
The distribution of diaminotoluenes and their reaction with
body components have been investigated, mainly after
intraperitoneal injection of radioactive labelled compounds. No
data on the distribution of diaminotoluenes and reaction with
tissues after inhalation or oral ingestion were found in published
reports.
Distribution of [14C]-2,4-DAT after intraperitoneal injection
was rapid, and the peak concentration in rat and mouse blood and
plasma occurred in 1 h, then decreased rapidly for 7 h. On a
comparative basis, all tissue concentrations of bound 14C were
considerably lower in the male NIH-Swiss mice than in the male
Fischer rats (Grantham et al., 1980).
Tissue distribution of [Me-14C]-2,4-DAT hydrochloride was
studied in male B6C3F1 mice given a single intraperitoneal
injection (1 µCi, 0.667 mg/kg body weight) (Unger et al., 1980).
The highest concentrations, 1/2 h after dosing, were found in the
kidneys, gonads, epididymis, lungs, muscle, and blood. One hour
after dosing, the liver contained the greatest amount, accounting
for nearly 12% of the dose. The concentration in the adrenal
glands exceeded that in the kidney, 1 and 2 h after dosing. High
concentrations of radioactivity were also observed in the
gastrointestinal tract.
Four hours after an intraperitoneal injection of 100 mg (0.8
mmol/kg, ring-labelled [3H]-2,4-DAT) in male Wistar rats, 0.3 nmol
was found covalently bound per mg liver protein. A similar degree
of binding was seen in the kidneys. Subcellular fractionation of
the liver showed that most of the bound material was in the
microsomal fraction (Dybing et al., 1978). No significant binding
to DNA in vitro or in vivo could be demonstrated using [3H]-2,4-
DAT, whereas it was found to bind covalently to hepatic RNA in
vivo. These findings were confirmed by Aune et al. (1979).
5.1.3. Metabolism
Glinsukon et al. (1975, 1976) found that the 2,4-isomer was
selectively N-acetylated at the p-amino group by liver cytosol
prepared from hamsters, guinea-pigs, rabbits, mice, and rats. The
cytosol from liver, kidney, intestinal mucosa, and lung of hamsters
and rabbits was studied for N-acetyl transferase activity using
2,4-DAT and 4-acetylamino-2-amino-toluene as substrates. All
tissues showed marked species differences in enzyme activity.
Tissues with high N-acetyl transferase levels, such as liver,
could produce both 4-acetylamino-2-aminotoluene and 2,4-
diacetylaminotoluene (Glinsukon et al., 1975). There were also sex
differences in the N-acetylation capacity of the liver cytosol.
After a single ip injection of 2,4-DAT (77 mg/kg body weight)
in male rats, 69.4% of the dose was eliminated in the urine and
faeces after 24 h as a complex mixture of metabolites, indicating
both free and conjugated derivatives. The major urinary
metabolites identified were 4-acetylamino-2-aminotoluene, 2,4-
diacetylaminotoluene, and 4-acetylamino-2-aminobenzoic acid. In
mice, oxidation of the methyl group to a benzoic acid was the major
reaction and the major urinary metabolites in mice were 4-
acetylamino-2-aminobenzoic, 4-acetylamino-2-aminotoluene, and 2,4-
diacetylaminobenzoic acid (Grantham et al., 1980). Waring &
Pheasant (1976) investigated the metabolism of 2,4-DAT in female
rabbits, rats, and guinea-pigs to determine whether the isomer gave
rise to hydroxylamines or aminophenols, which might account for the
observed toxic and carcinogenic effects. After oral administration
(gavage) of 2,4-DAT (50 mg/kg body weight), phenolic metabolites
were excreted in the urine. When free and conjugated metabolites
were combined, 5-hydroxy-2,4-DAT was the major metabolite in all 3
species (Table 7).
Table 7. Excretion of metabolites after dosing with 2,4-diamino-
toluene
-------------------------------------------------------------------
Metabolite Percentage dose excreteda
Rabbit Rat Guinea-pig
-------------------------------------------------------------------
2,4-DAT trace 1.3 trace
3-hydroxy-2,4-DAT 10 8 trace
5-hydroxy-2,4-DAT 22 12 9
6-hydroxy-2,4-DAT ( m-aminophenol) trace 5 trace
3-hydroxy-4-acetylamino-2-aminotoluene 10 18 trace
5-hydroxy-4-acetylamino-2-aminotoluene 6 14 17
glucuronide I, 3-hydroxy-DAT 10 16 15
glucuronide II, 5 hydroxy-DAT 32 12 46
glucuronide III, 6-hydroxy-DAT 2 6 4
unidentified phenolic compounds 0 trace trace
-------------------------------------------------------------------
a Results are given as percentage dose, average of 10 studies,
standard deviation 6.4% for metabolites 1 - 5, and 12.8% for
metabolites 6 - 8. Animals were dosed orally at 50 mg/kg; urine
was collected for 48 h.
From: Waring & Pheasant (1976).
The levels of methaemoglobin found in the rabbit, rat, and
guinea-pig correlated well with the total urinary excretion of
aminophenol. The methaemoglobin levels reached a peak 6 - 12 h
after the administration of 2,4-DAT and then slowly declined. The
highest levels of aminophenols and of methaemoglobin were found in
the rabbit (Waring & Pheasant, 1976).
5.1.4. Excretion
During a 24-h period of dermal contact with [14C]-2,4-DAT, 14C
urinary excretion in monkeys reached a peak at 8 - 12 h (Marzulli
et al., 1981).
Data from studies on the rat, rabbit, mouse, guinea-pig, and
dog exposed to diaminotoluenes (cutaneous, subcutaneous,
intravenous, intraperitoneal, or oral by gavage) showed fast
elimination rates (Kiese et al., 1968; Waring & Pheasant, 1976;
Hruby, 1977; Grantham et al., 1980; Unger et al., 1980). The
elimination of radioactivity from various tissues in rodents
followed a well-defined biphasic pattern. Rapid elimination over
7 h was followed by a rather slow decline in the isotopic contents
of tissues (Grantham et al., 1980; Unger et al., 1980). The half-
lives of tissue elimination during the fast phase were 0.89, 0.43,
and 1.51 h for male mouse liver, kidneys, and blood, respectively.
During the slow phase of elimination, the half-lives for liver,
kidneys, and blood in male mice were 11.7, 9.1, and 12.6 h,
respectively. The half-lives of elimination of radioactivity,
during the slow phase, were greater for muscle (23.9 h) and skin
(29.2 h) than for any other tissue (Wagner, 1975; Unger et al.,
1980).
The primary route of elimination in rodents was via the kidneys
during the first hour after exposure. However, after 2 h, the
predominant route shifted from urinary to faecal, probably a
reflection of biliary excretion. Only 1.25% of the administered
radioactivity had been exhaled after 24 h (Unger et al., 1980). On
a comparative basis, faecal elimination was greater in rats than in
mice, but the rate of urinary excretion was more rapid in mice than
in rats. Approximately 90% of a dose was eliminated in the urine
of mice in 24 h compared with 74% in the urine of rats (Grantham et
al., 1980). Complete elimination was accomplished in 2 days in
mice, while rats required 6 days.
Male and female rats, injected subcutaneously with 3 - 5 mg
[14C]-2,5-DAT hydrochloride, eliminated 65% of the dose in the
urine and 5% in faeces after 24 h. The same pattern of elimination
was found after oral administration of 10 mg of the labelled
compound (Hruby, 1977).
When beagle dogs were intravenously injected with a dose of
224 mg, infused over 3 h, the total amounts of radioactivity
eliminated in the urine and faeces were 60% and 19%, respectively.
After 4 days, elimination mainly occurred within the first 24 h.
After a skin application of 1.4 g [14C]-2,4-DAT for 3 h (in 50 ml
of a dye formulation), only 0.092 and 0.84% of the dose were
eliminated, respectively, in the urine and faeces of beagle dogs
over 4 days, reflecting the inhibitory effect of the dye
formulation on the absorption of the 2,4-DAT (Hruby, 1977). In
another study on dogs, about 40 mg 2,5-diaminotoluene was absorbed
through the skin from a gel containing 1.4 g of the material. The
addition of hydrogen peroxide to the gel reduced the amount
absorbed to less than 3 mg. The amount excreted unchanged in the
urine was 60 - 70 µg (Kiese et al., 1968).
5.2. Human Studies
As only limited information is available on the absorption,
distribution, metabolism, and excretion of diaminotoluenes in human
beings, these aspects are discussed together, rather than in
separate sections.
Although the high boiling point of diaminotoluenes makes
absorption through the lungs unlikely under normal working
conditions, inhalation may occur when hot vapours escape from
stills. Possible inhalation and dermal exposure to dusts may occur
if diaminotoluenes are handled in a less than optimal manner.
Since diaminotoluenes are soluble in water, absorption from the
gastrointestinal tract could occur following ingestion. However,
no data were found on the kinetics and metabolism of diamino-
toluenes after oral or inhalation exposures.
Skin penetration by [14C]-2,4-DAT was measured in human beings
(Marzulli et al., 1981). When 4 µg [14C]-2,4-DAT in acetone/cm2
was applied to the skin of the forearm, the highest absorption of
the chemical (23.7 % ± 16.1% of the applied dose) resulted after
24 h of dermal contact. Urinary excretion reached a peak after
4 - 8 h of skin contact. In a study by Kiese & Rauscher (1968),
the hair of 5 human subjects was dyed (40 min) with a formula
containing 2.5 g 2,5-DAT; absorption of approximately 0.2% of the
applied material occurred. No data were given on the retention and
distribution of diaminotoluenes after this dermal contact.
Data from studies on 6 volunteers (3 males and 3 females)
showed that, after subcutaneous injection of 5.54 mg 2,5-DAT, 47.6%
of the dose was excreted in the urine as N,N'-diacetyl-2,5-DAT.
The rate of excretion was highest during the first 24 h, and only a
trace appeared in the urine excreted on the third day, in one
study. When the compound was applied as a hair dye (40 min), the
highest rate of excretion was observed during a period of 5 - 8 h
after application. On average, a total amount of 3.7 mg N,N'-
diacetyl-2,5-DAT (i.e., 0.09% of the applied dose) was calculated
to have been excreted in urine taken over 2 days from 5 subjects
(Kiese & Rauscher, 1968).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Little information is available on the effects of diamino-
toluenes on animal populations found in the environment. The
effects of 2,4-DAT at concentrations ranging from 1 to 1000
mg/litre were observed for Daphnia ( Daphnia magna Straus),
ostracoda, guppies ( Lebistes reticulatus Peters), and channel
seaweed (Scenedesmus obliquus). Daphnia was the most sensitive
species; 5 mg/litre was lethal in 5 - 10 days, and prolonged
exposure to 2 mg/litre caused a reduction in the number of
offspring produced. A concentration of 20 mg/litre was not lethal
for ostracods after 10 days, but 50 mg/litre was lethal in 5 - 8
days. Fish survived for 10 days at 200 mg/litre, but a
concentration of 500 mg/litre was lethal in 2 - 3 days. The algae
tested were the most resistant, surviving for 10 days at a
concentration of 1000 mg/litre (Smirnova et al., 1967).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The early literature (1881 - 1939) contains several reports on
toxicological manifestations associated with the administration of
diaminotoluenes in experimental animals. Most of the papers are
difficult to interpret and to use in the assessment of chemical
hazards, because massive doses of chemicals of unknown purity and
isomeric composition were used, and because of the experimental
designs chosen. The toxic effects were characterized by icterus,
haemoglobinuria, disposition of haemosiderin in the spleen, bone
marrow, and liver, respiratory and generalized central nervous
system (CNS) depression, pulmonary and cerebral oedema, and
increased bile acids in the liver, blood, and/or urine of exposed
animals (Von Oettingen, 1941).
7.1. Single Exposures
Diaminotoluenes are considered to be dermal and eye irritants.
In studies on rabbits, 12.5 mg 2,5-DAT or 500 mg 2,4-DAT caused
skin irritation, defined as erythema and oedema, after 24 h of
dermal contact. Instillation of 100 µg of the 2,4-isomer into the
rabbit eye caused severe eye irritation within 24 h. Data showing
the extent of the acute toxicity of diaminotoluenes in various
laboratory animals are summarized in Tables 8 and 9.
The acute toxic effects of diaminotoluenes were characterized
by marked central nervous system depression during exposure (e.g.,
decreased locomotor activity, piloerection, ptosis, ataxia,
tremors) and production of methaemoglobin, 6 - 8 h after exposure.
Duodenal and glandular mucosal damage in the stomach were
observed in fed, unrestrained rats, 24 h following a single
subcutaneous dose of 3,4-DAT. The optimal ulcerogenic dose of 3,4-
DAT (i.e., the dose causing a low mortality and a maximal incidence
of duodenal damage within 24 h), was 350 mg/kg body weight (Perkins
& Green, 1975).
7.2. Short-Term Exposures
When guinea-pigs were treated with 1 - 10% 2,5-DAT (24 h/day
for 5 days, 2 days without treatment, followed by exposure for
another 5 days), sensitization was obtained in 35% of treated
animals (Schäfer et al., 1978).
Both the 2,4- and 3,4-isomers caused severe icterus in rats.
In male and female rats, 3,4-DAT (unlike the 2,4-isomer), given
orally or parenterally, produced a high incidence of perforating
duodenal ulcers within a few days (Selye, 1973). These effects
were obtained in animals allowed to move freely with access to food
and water during the period of observation. A dose of 500 mg
diaminotoluenes/kg body weight was administered in 2 ml water,
twice daily.
Table 8. Lethality of diaminotoluenes
---------------------------------------------------------------------------
Species Exposure LD50 Reference
route (mg/kg
body
weight)
---------------------------------------------------------------------------
Ortho-DAT (2,3-, 3,4- mix)
Rat oral 810 Carpenter et al. (1974)
Rabbit dermal 1120 Carpenter et al. (1974)
Meta-DAT (2,4-, 2,6- mix)
Rat oral 300 Izmerov et al. (1982)
Rat (male) oral 270 Weisbrod & Stephan (1983)
Mouse (male) oral 350 Weisbrod & Stephan (1983)
Rat (male) ip 230a Weisbrod & Stephan (1983)
Mouse (male) ip 240b Weisbrod & Stephan (1983)
Rat (male) iv 350 Weisbrod & Stephan (1983)
Mouse (male) iv 90-105 Weisbrod & Stephan (1983)
Rat dermal 1200 Izmerov et al. (1982)
2,4-DAT (technical grade)
Fischer rat (male) ip 325 Grantham et al. (1980)
NIH-Swiss mouse (male) ip 480 Grantham et al. (1980)
HaM/ICR mouse (male) ip 80 Weisburger et al. (1978)
HaM/ICR mouse (female) ip 90 Weisburger et al. (1978)
---------------------------------------------------------------------------
a A methaemoglobin level of 8.4% was observed, 6 h after ip administration.
b A methaemoglobin level of 7.8% was observed, 6 h after ip treatment.
Note: No published data on the acute toxicity of the 2,3-isomer were
available.
The effects of 2,4-DAT on the liver microsomal mixed-function
oxidase system, DT-diaphorase, and epoxide hydrolase were reported
by Dent & Graichen (1982). Following oral treatment with 2,4-DAT
at 70 mg/kg body weight per day for 5 days, the activities of
microsomal cytochrome P-450-dependent enzymes were depressed, while
epoxide hydrolase activity was markedly elevated (3 - 8 times
control) in male F-344 rats. Under these experimental conditions,
an increase in the liver to body weight ratio (3.2 - 4%), and in
the liver microsomal protein concentration (19.3 - 27.4 g/kg) were
induced by 2,4-DAT.
7.3. Long-Term Exposure
Oral administration of 2,4-DAT (see Table 11, section 7.6, for
doses) for 79 - 103 weeks accelerated the appearance of renal
toxicity in male F-344 rats, associated with a high incidence of
secondary hyperparathyroidism (NCI, 1979). The chronic renal
disease reported was believed to have decreased the longevity of
the treated rats, either directly or through inhibition of the
clearance of toxic metabolites (Cardy, 1979).
Table 9. Summary of some single-dose studies
--------------------------------------------------------------------------
Species Route of Dose Effects Reference
exposure (mg/kg
body
weight)
--------------------------------------------------------------------------
2,4-DAT (technical grade)
Wistar rat oral 50 produced metHba; highest Waring &
(male) amounts (5 - 6%) were Pheasant (1976)
found 6 - 8 h after
exposure
> 50 toxic
Sprague oral 500 developed icterus and Selye (1973)
Dawley rat death
(male and
female)
NZW rabbit oral 50 MetHb reached 18 - 20% Waring &
level 6 - 8 h after Pheasant (1976)
application
> 50 toxic
Dunkin- oral 50 MetHb reached 3 - 4% level Waring &
Harvey 6 - 8 h after application Pheasant (1976)
guinea-pig
> 50 toxic
3,4-DAT (97% pure)
Sprague subcut- 125-500 discrete, non-perforated Perkins &
Dawley rat aneous duodenal lesions were Green (1975)
(female) observed immediately
distal to the gastroduo-
denal junction 24 h
following the administra-
tion of a single dose
--------------------------------------------------------------------------
a metHb = methaemoglobin.
No studies were found on the effects of diaminotoluenes on the
nervous system or the immune system after long-term exposure.
7.4. Reproduction and Teratogenicity
7.4.1. Reproduction
There are 2 studies on experimental animals that evaluate the
reproductive toxicity of diaminotoluenes. Soares & Lock (1980)
administered 2,4-DAT orally or ip at 40 mg/kg body weight for
2 days to DBA/2J male mice. Forty-eight hours after treatment,
mating trials were conducted for 8 weeks. There were no treatment-
related effects on sperm morphology or fertility, as measured by
this dominant lethal assay. However, in male Sprague Dawley rats,
long-term exposure to 2,4-DAT in the feed impaired reproductive
performance and capacity (Thysen et al., 1985a,b). Dietary levels
of 0.03% 2,4-DAT for 10 weeks (~ 15 mg/kg body weight per day)
decreased fertility and exerted an inhibitory effect on sperm
production in male rats. Eleven weeks after treatment, the sperm
count remained significantly depressed ( P < 0.001), suggesting
irreversible damage to the germinal components in the testes. Data
from hormone analyses at the end of the 10 weeks of exposure, and
at the end of 11 weeks after treatment, showed a significant
decrease in serum-testosterone and an elevation of serum-luteinizing
hormone concentrations, which were associated with a reduction in
seminal vesicle weight. Histological changes found in the
reproductive organs from treated males were correlated with these
physiological changes. At a lower dose (0.01% or ~ 5 mg/kg body
weight), 2,4-DAT did not cause any of these toxic responses.
7.4.2. Teratogenicity
Studies on the teratogenic potential of diaminotoluenes are
summarized in Table 10. Skin application of 2,4-DAT induced a low
incidence of skeletal changes in rats (Burnett et al., 1976). Oral
or intraperitoneal administration of this isomer did not produce
any effects on the fertility or reproductive performance of male
mice (Soares & Lock, 1980). Subcutaneous or intraperitoneal
injection of 2,5-DAT in mice on day 8 of gestation, at levels of 50
or 75 mg/kg body weight, caused crainiofacial malformation and
fused or distorted thoracic vertebrae associated with the absence
of, or fused, ribs (Inouye & Murakami, 1976, 1977). However, 2,5-
DAT sulfate, at levels of 16 - 64 mg/kg body weight per day
administered subcutaneously on days 6 - 15 of gestation, did not
cause any malformations in mice or rats (Marks et al., 1981).
The results of oral administration of 2,6-DAT to rats and
rabbits, at doses of between 10 and 300 mg/kg body weight
(Knickerbocker et al., 1980), showed that, in rats, doses of
between 100 and 300 mg/kg body weight increased the occurrence of
incomplete vertebrae and that the highest dose resulted in missing
sternebrae and incomplete closure of the skull. A no-observed-
adverse-effect level of 10 mg/kg body weight was reported in rats.
No skeletal or soft-tissue abnormalities were observed in the
offspring of rabbits, but, using fetal toxicity indices, a no-
observed-adverse-effect level of 30 mg/kg body weight was reported.
Becci et al. (1983) administered Ortho-DAT (2,3-, 3,4-isomer
mixture) by gavage to rats and rabbits (Table 10). Reduced body
weight during gestation was noted at 300 mg/kg body weight in rats
and 100 mg/kg body weight in rabbits. An increased incidence of
several skeletal variations in the fetuses was noted, probably due,
in part, to the maternal toxicity. The no-observed-adverse-effect
level in both rats and rabbits was 30 mg/kg body weight.
Table 10. Teratogenicity studies with diaminotoluenes
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,4- Charles skin 2 ml/kg body weight skeletal changes seen in 6/169 Burnett et al.
(3% in River/CD on days 1, 4, 7, 10, live fetuses ( P > 0.05) (1976)
hair-dye rat 13, 16, and 19 of
formula) (female) gestation
2,5- Charles skin 2 ml/kg body weight no increase in abnormalities in Burnett et al.
(sulfate) River/CD on days 1, 4, 7, 10, treated groups (1976)
(3% in rat 13, 16, and 19 of
hair-dye gestation
formula)
2,5- JCL:ddn subcutaneous 50 mg/kg body weight In groups treated sc or ip on day Inouye & Murakami
dihydro- mice or intraper- on one day of days 8 of gestation, there was evidence (1976, 1977)
chloride (female) itoneal 7 - 14 of gestation of craniofacial malformation:
single dose or 75 mg/kg on day exencephaly, prosoposchisis, and
8 of gestation or hair lip with cleft palate, and
50 mg/kg on day 8 of high incidence of skeletal
gestation malformation: fused or distorted
thoracic vertebrae associated with
absence of, or fused, ribs; no
such malformed fetuses were found
in groups treated on days 10 - 14
of gestation; only a very low
incidence of vertebral and rib
anomalies followed treatment on
day 7 or 9; maternal toxicity was
reported at 75 mg/kg but not at
50 mg/kg
2,5- CD-1 mice subcutaneous 16, 32, 48, or 64 no teratogenic effects were noted; Marks et al.
(sulfate) mg/kg body weight maternal toxicity was evident at (1981)
per day on days 48 and 64 mg/kg; reduced fetal
6 - 15 of gestation weight was noted at > 32 mg/kg
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,5- rat oral (gavage) 10, 50, or 80 mg/kg maternal toxicity and embryo- Spengler et al.
(sulfate) body weight per toxicity evident at 80 mg/kg; no (1986)
day on day 15 of effects observed at lower doses
gestation
rabbit oral (gavage) 10, 25, or 50 mg/kg no effects observed
body weight per day
on days 6 - 18 of
gestation
2,6- Sprague oral (gavage) 10, 30, 100, or 300 no effects on pregnancy, number Knickerbocker et
Dawley mg/kg body weight of live fetuses, and resorption al. (1980)
rat per day on days sites/dam; 300 mg/kg produced
6 - 15 of gestation smaller body weight gain in the
dams; 30 - 300 mg/kg produced
increased haemorrhagic abdomens
in the fetuses; 100 and 300 mg/kg
increased the occurrence of
incomplete vertebrae, and 300
mg/kg showed missing sternebrae
and incomplete skull closure in
the fetuses; the no-observed-
adverse-effect dose was 10 mg/kg
per day
2,6 Dutch oral (gavage) 3, 10, 30, or 100 100 mg/kg per day reduced dam Knickerbocker et
belted mg/kg body weight weights, increased resorptions, al. (1980)
rabbit per day on days decreased fetal weights, and
(female) 6 - 18 of gestation neonatal survival;
oral (gavage) 3, 10, 30, or 100 there were no differences in Knickerbocker et
mg/kg body weight skeletal or soft-tissue al. (1980)
per day on days abnormalities between treated
6 - 18 of gestation animals and controls; the no-
observed-adverse-effect dose was
30 mg/kg per day
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
o-DAT Sprague oral 10, 30, 100, or 300 maternal toxicity was indicated Becci et al.
(2,3-, Dawley mg/kg body weight at 300 mg/kg per day by reduced (1983)
3,4- rat per day on days body weight gain during gestation;
isomer 6 - 15 of gestation No significant differences in
mix) numbers of live fetuses,
implantation or resorption sites;
fetal body weight was reduced at
the highest dose ( P < 0.05); no
evidence of teratogenic effects
or effects on dams at doses < 30
mg/kg; no skeletal or soft-tissue
malformations that could be
related to treatment; however,
increased incidence of missing
sternebrae at 300 mg/kg per day
and incomplete ossified vertebrae
at 100 and 300 mg/kg per day were
noted compared with controls
o-DAT Dutch oral 3, 10, 30, or 100 maternal toxicity at 100 mg/kg per Becci et al.
(2,3-, belted mg/kg body weight day elicited by reduced body (1983)
3,4- rabbit per day on days weight gain during pregnancy; no
isomer 6 - 18 of gestation significant difference in the
mix) number of implantations; at 100
mg/kg per day, fetal body weight
was reduced and the number of
resorption sites was increased;
no skeletal or soft-tissue
malformations that could be
related to treatment were noted
-----------------------------------------------------------------------------------------------------------------
7.5. Mutagenicity and Related End-Points
7.5.1. DNA damage
At concentrations of 1 x 10-4 mol/litre and below, 2,4-DAT, but
not 2,6-DAT, induced unscheduled DNA synthesis in primary cultures
of rat hepatocytes (Bermudez et al., 1979). 2,4-DAT produced a
significant elevation in unscheduled DNA synthesis at 2 and 12 h in
the in vivo/in vitro hepatocyte DNA repair assay (Mirsalis &
Butterworth, 1982; Mirsalis et al., 1982). 2,5-DAT produced a
positive response in a DNA-repair assay in rat hepatocytes and a
weak positive response in hamster hepatocytes at 10-5 mol, the
highest concentration that was not toxic to the cells that were
tested (Kornbrust & Barfknecht, 1984).
Shooter & Venitt (1979) used continuous administration of 2,4-
DAT in the drinking-water to determine whether phosphotriesters
could be detected in the DNA of the liver of treated rats.
Positive results were obtained at 10 mg/litre. A low, but
significant, level of these lesions was produced. Shooter &
Venitt's studies on rodents indicated that methyl- and
ethylphosphotriesters persist for many weeks in the DNA of certain
organs (notably liver, kidney, and lung) and that such lesions are
not eliminated by DNA repair.
The results of studies by Greene et al. (1981) showed that
2,4-, 2,5-, 2,6-, and 3,4-isomers significantly inhibited the
incorporation of [125I]-iododeoxyuridine into mouse testicular DNA
and demonstrated dose-response characteristics. The 2,4-, 2,5-,
and 3,4-isomers were capable of reaching the testes and of passing
target cell membranes at this site. They concluded that the 3
isomers may present a genetic health hazard for an intact animal.
The inhibition induced by 2,6-DAT might have been caused by a
chemically-induced decrease in body temperature (Greene et al.,
1981).
DNA damage was not found in human cultured fibroblasts after
exposure to 100 µmol 2,4-DAT alone (3.5 ± 1.5 increase in percent
single-strand DNA). When the cells were incubated in the presence
of 1 mg ram seminal vesicle microsomes/ml and 100 µmol arachidonic
acid, a significant increase in the fraction of single-strand DNA
(21.3 ± 3.7, P < 0.001) was found in cells exposed to 2,4-DAT.
DNA strand breaks were not induced when prostaglandin synthase
(PGS) was inhibited by adding indomethacin (100 µmol) or
acetylsalicylic acid (1 mmol) (Nordenskjöld et al., 1984). These
results, which suggest that 2,4-DAT may be activated by PGS to form
products that cause DNA damage in cultured human fibroblasts, are
in agreement with the findings of Rahimtula et al. (1982).
7.5.2. Mutation
Several studies have shown that 2,4-, 2,6-, and 2,5-isomers can
induce reverse mutations in Salmonella typhimurium strains TA 1538
and TA 98, in the presence of various metabolic activation systems
(McCann et al., 1975; Dybing & Thorgeirsson, 1977; Dybing et al.,
1977; Pienta et al., 1977b; Cinkotai et al., 1978; Aune et al.,
1979; Shahin et al., 1980). However, Mori et al. (1982) showed
that 2,4-DAT was inactive for strains TA 98 and TA 100 at doses
ranging from 5 to 1000 µg/plate. While 2,3-DAT is inactive in S.
typhimurium (Florin et al., 1980), its homologue 3,4-DAT showed a
marginal response in strains TA 98 and TA 1538 (Greene et al.,
1979).
2,4-DAT was shown to be a weak mutagen in Drosophila
melanogaster, inducing sex-linked recessive lethals when fed to
adult males at a concentration of 15.2 mmol (Blijleven, 1977;
Venitt, 1978). In a study reported by Fahmy & Fahmy (1977), 2,4-
DAT was injected around the testes of adult male Drosophila at
doses ranging from 5 to 20 mmol. Mutagenicity was measured at the
various stages of spermatogenesis, both on the X-chromosome and RNA
genes. The overall induced frequency of X-recessives was extremely
low. It was also observed that mutation yield was not dose-related
and that it was maximal in the earliest progeny fraction,
suggesting a greater toxicity for mature sperm.
2,4-DAT was mutagenic in L5178Y mouse lymphoma cells and CHO-
AT3-2 cells (Matheson & Creasy, 1976; Coppinger et al., 1984).
Mutagenic activity was observed in L5178Y cells, only in the
absence of exogenous metabolic activation, but was observed in CHO-
AT3-2 cells both with and without activation.
The in vivo mutagenic activity of 2,4-DAT was studied in
DBA/2J male mice by the dominant lethal assay, sperm abnormality
assay, and the recessive spot test. Mice were administered the
compound by intraperitoneal injection and orally by gavage (2 daily
doses of 40 mg/kg body weight), just before mating (Soares & Lock,
1980). No induction of dominant lethals was noted, nor was any
increase in abnormal sperm or recessive spots reported.
No dominant lethals were induced in Charles River rats injected
intraperitoneally, 3 times weekly for 8 weeks, with 20 mg 2,5-
DAT/kg body weight, before mating (Burnett et al., 1977).
7.5.3. Cell transformation
Several studies have shown that the 2,4-, 2,5-, 2,6-, and 3,4-
isomers can induce morphological transformations in Syrian golden
hamster embryo cells (Pienta et al., 1977a; Greene & Friedman,
1980). Each isomer chemically transformed secondary hamster embryo
cells, but none were active in more than 50% of the 5 or 6 separate
tests performed on each isomer (Greene & Freidman, 1980).
7.5.4. Chromosomal effects
Cytogenetic preparations were made from the bone marrow of male
mice, 30 and 48 h after intraperitoneal injection of 2 daily doses
of 2,4-DAT at 40 mg/kg body weight. The treatment did not induce
any obvious chromosome breaks (Soares & Lock, 1980).
2,5-DAT did not induce micronucleated cells in bone marrow
after oral administration of 120 mg/kg body weight to male and
female rats, in 2 doses separated by an interval of 24 h (Hossack &
Richardson, 1977).
7.6. Carcinogenicity
Several long-term studies on the carcinogenic potential of the
2,4-, 2,5-, and 2,6-DAT isomers have been published. The
experimental designs used in these studies are summarized in Table
11. The experimental designs used in 2 studies on the carcinogenic
effects of hair dye formulations are also given in Table 11.
Although the early studies of Umeda (1955) and Ito et al.
(1969) used protocols that generated data of minimal use for a
hazard evaluation, they did produce qualitative information showing
that 2,4-DAT was carcinogenic for rats. These studies have been
extended to the 2,5-, 2,6-, as well as 2,4-DATs using more animals
per study and well-defined protocols (NCI, 1978, 1979, 1980;
Weisburger et al., 1978).
Groups of 25 male Charles River/CD rats were administered 2,4-
DAT in the diet at time-weighted levels of 300 and 625 mg/kg for 18
months. Similarly, groups of 25 male and female CD-1 mice were
given diets containing 500 and 1000 mg 2,4-DAT/kg for 18 months
(Weisburger et al., 1978). The rats and mice used in these studies
had a high incidence of spontaneous tumours. However, there was a
statistically significant increase in subcutaneous fibromas in male
rats and hepatocellular carcinomas and vascular tumours in male and
female mice compared with controls.
Studies by the US National Cancer Institute on the
carcinogenicity of 2,4-DAT in rats and mice (NCI, 1979) confirmed
the report by Weisburger et al. (1978). Administration of time-
weighted average doses of 79 and 176 mg 2,4-DAT/kg diet to groups
of 50 male and 50 female Fisher 344 rats, for 103 weeks, led to a
severe depression in body weight gain, high mortality, and a dose-
related development of hepatocellular carcinomas or neoplastic
nodules in treated rats of both sexes. In addition, NCI reported
that carcinomas and adenomas of the mammary gland occurred in
female rats at incidences that were dose related and significantly
greater than those in the controls in both the high- and low-dose
groups. Groups of 50 male and 50 female B6C3F1 mice were similarly
administered 2,4-DAT at 100 or 200 mg/kg diet, for 101 weeks. In
male mice, tumour incidence was not significantly increased
compared with that in the control animals. However, the incidence
of hepatocellular carcinomas in female mice in both treated groups
was dose related and significantly higher than that in the
controls. Numbers of lymphomas were also higher in low-dose female
mice. On the basis of these results, it was concluded that 2,4-DAT
was carcinogenic for Fisher 344 rats of both sexes and for female
B6C3F1 mice (NCI, 1979).
Table 11. Experimental designs of carcinogenicity studies on diaminotoluenes
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,4- rat (male and female) 20 subcutaneous; 2 mg in 0.5 ml Umeda (1955)
propylene glycol weekly;
total of 28 injections; 452
days
2,4- Wistar rat (male) 12/12 (6) oral (diet); 0.6 and 1 g/kg; Ito et al.
36 weeks (1969)
2,4- Charles River/CD rat 25/25 (25) oral (diet); 500 and 1000 Weisburger et
(male) mg/kg for 4 months and 250 al. (1978)
Charles River mouse 25/25 (25) and 500 mg/kg for 14 months;
(male) oral (diet); 500 and 1000
Charles River mouse 25/25 (25) mg/kg for 18 months
(female)
2,5- Fisher 344 rat (male) 50/50 (50/25) oral (diet); 600 and 2000 NCI (1978)
Fisher 344 rat (female) 50/50 (50/25) mg/kg for 78 weeks + 31
weeks observation
B6C3F1 mouse (male) 50/50 (50/50) oral (diet); 600 and 2000
B6C3F1 mouse (female) 50/50 (50/50) mg/kg for 78 weeks + 19
weeks observation
2,4- Fisher 344 rat (male) 50/50 (20) oral (diet); 125 and 250 NCI (1979)
Fisher 344 rat (female) 50/50 (20) mg/kg for 40 weeks reduced
to 50 and 100 mg/kg for 63
weeks (time-weighted-average
79 and 176 mg/kg); high-dose
males killed after 79 weeks
and high-dose females after
84 weeks
2,4 B6C3F1 mouse (male) 50/50 (20) oral (diet); 100 and 200 NCI (1979)
B6C3F1 mouse (female) 50/50 (20) mg/kg for 101 weeks
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,6- Fisher 344 rat (male) 50/50 (50) oral (diet) 250 and 500 NCI (1980)
Fisher 344 rat (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
B6C3F1 mouse (male) 50/50 (50) oral (diet); 250 and 500
B6C3F1 mouse (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
2,5- Sprague Dawley rat (male) 50 (50) dermal application twice Kinkel &
(formulations Sprague Dawley rat 50 (50) weekly of 0.5 g of synthetic Holzmann (1973)
with 6% (female) formulation containing 4%
hydrogen 2,5-DAT mixed with equal
peroxide volume of 6% H202; treated 2
added) years
2,5- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(equal mixture male and ml weekly of formulation to (1975)
female) which equal volume of 6%
H202 had been added;
treatment period, 18 months
2,5- + 2,4- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(formulations (equal mixture male and ml weekly of formulation to (1975)
with 6% female) which equal volume of 6%
hydrogen H202 had been added;
peroxide treatment period, 18 months
added) (same control group as
above study)
---------------------------------------------------------------------------------------------------------
The carcinogenic potential of 2,5-DAT and 2,6-DAT for rats and
mice was determined by the US National Cancer Institute. After
administration of 2,5-DAT at 600 and 2000 mg/kg feed to groups of
50 male and 50 female Fisher 344 rats and 50 male and 50 female
B6C3F1 mice, for 78 weeks, there was not sufficient evidence to
demonstrate the carcinogenicity of 2,5-DAT (NCI, 1978). However,
the study had been curtailed and this reduced the potential of the
test for detecting carcinogenicity.
Using a similar protocol, 2,6-DAT was incorporated at levels of
250 and 500 mg/kg into the diet of groups of 50 male and 50 female
Fisher 344 rats, and B6C3F1 mice for 103 weeks (NCI, 1980). There
was some question of whether mice of either sex received a maximum
tolerated dose, but the dose given to rats appeared to be at the
maximum tolerated level. As reported by NCI (1980), islet-cell
adenomas of the pancreas and neoplastic nodules or carcinomas of
the liver occurred in male rats in dose-related trends that were
significant using the Cochran-Armitage test, but not using the
Fisher exact test. In the low-dose male mice, NCI reported that
the incidence of lymphomas was greater than that in the controls.
However, the incidence was not significant when the Bonferroni
criterion of multiple comparison was used. Similarly, the
occurrence of hepatocellular carcinomas in female mice was dose
related, but not significant by the Fisher exact test, when the
incidence in the high-dose groups was compared with that in the
controls. Under the conditions of the bioassay, it was concluded
that 2,6-DAT was not carcinogenic for male and female F344 rats or
for male and female B6C3F1 mice (NCI, l980). Summaries of the 3
NCI bioassays have been published by Cardy (1979), Reuber (1979),
and Sontag (1981).
Using the protocols outlined in Table 11, no evidence of
carcinogenicity was obtained when hair-dye formulations containing
2,5- and 2,5- plus 2,4-DAT were painted on the skin of rats and
mice (Kinkel & Holzmann, 1973; Burnett et al., 1975). Given the
duration of exposure, the amounts of diaminotoluenes placed on the
skin, and the use of hydrogen peroxide prior to administration,
such negative results would be expected in studies on the formula
containing 2,4-DAT (Burnett et al., 1975). These studies have
shown a low order of dermal toxicity for these hair dyes, even
after long-term exposure. However, no definitive statement can be
made on the carcinogenic potential of 2,4- and 2,5-DAT after dermal
administration.
8. EFFECTS ON MAN
8.1. Single and Short-Term Exposures
In human beings, as in animals, diaminotoluenes are considered
to be irritants for the mucous membranes and skin, and to lead to
conjunctivitis and corneal opacities. When solutions come into
contact with skin, they can cause irritation, severe dermatitis,
and blistering (Von Oettingen, 1941). In case of the inhalation of
fumes, coughing, dyspnoea, and respiratory distress can result. No
data are available for evaluating the sensitizing potential of
diaminotoluenes. In the case of ingestion of massive amounts,
nausea, vomiting, and diarrhoea would occur, with the possible
production of methaemoglobinaemia. No cases of human poisoning
from short-term exposures to 2,4-DAT have been documented in the
published literature.
8.2. Long-Term Occupational Exposure - Epidemiological Studies
Filatova et al. (1970) investigated the physiological and
biochemical status of workers in a plant manufacturing
diaminotoluenes. Fifty-two of the 59 workers (58 males and 1
female) had worked in the plant for > 2 1/2 years. Seventeen
workers were between 30 and 45 years of age and 42 workers were
under 30 years old. In general, all workers had equal exposure to
the toxic chemicals used at the plant, namely, dinitrotoluene,
diaminotoluenes, methanol, and o-dichlorobenzene. There were a
few complaints of headache (2 cases), excess coughing (2 cases),
stomach pains (2 cases), and chest pain (4 cases); however, the
majority of the workers did not exhibit any exposure-related
adverse effects. Nevertheless, the investigators concluded that
the 10 workers exhibiting the symptoms listed were indeed affected
by their exposure to the complex of chemicals at this factory. It
is impossible to delineate the role played by the diaminotoluenes
in the production of these adverse effects.
The US National Institute for Occupational Safety and Health
(NIOSH) evaluated the reproductive health of workers in 3 plants
manufacturing diaminotoluenes (NIOSH, 1980, 1981, 1982). Exposure
usually involved both DAT and dinitrotoluene (DNT). All 3 surveys
were conducted in response to requests from employees or their
unions. The reason for the first request was the workers' belief
that their wives were suffering increased rates of spontaneous
abortion. The other 2 studies were prompted by the publicity given
to the first study.
In 2 of the studies, environmental hygiene sampling took place
(section 4.2) and workers were invited to volunteer for a medical
examination, to complete a reproductive history questionnaire, and
to provide semen and blood samples. Semen samples were analysed
for volume, sperm count, and sperm morphology. Blood samples were
analysed for various markers of renal and hepatic function, neither
of which showed any significant inter-group differences in any of
the studies. Wives of workers were given a more detailed
reproductive history questionnaire.
In the first study (NIOSH, 1980), there were 44 volunteers, 30
of whom provided usable semen specimens. The total potential study
population was not given. Of the 30, 9 were exposed, 9 were
controls, and 12 were in an intermediate category. The rate of
spontaneous abortions was higher among the wives of exposed workers
(6/18 pregnancies while the husband was exposed) compared with the
controls (4/23 pregnancies), with 6/28 for the intermediate group.
The small number of congenital malformations was not exposure
related. The sperm count for the exposed (median = 49 million) was
significantly ( P < 0.03) lower than that for the control group
(median = 121 million); however, the latter figure was unusually
high. The exposed group showed a significant reduction in the
proportion of the large morphological type. The second study
involved only a reproductive history questionnaire and the
reporting of hygiene data by the company. Thirty-five out of 41
workers in DAT- and DNT-production areas were interviewed. The
rates of congenital abnormalities or spontaneous abortion did not
significantly differ between exposure groups. Where the husband
was employed in DNT production, 1 out of 9 pregnancies ended in a
spontaneous abortion; the equivalent data for DAT was 1 miscarriage
out of 14 pregnancies.
In the third study, 50 volunteers were examined, 41 of whom
provided semen specimens. The total eligible population was not
given, though it was reported that 25 workers were regularly
exposed, 15 of whom participated in the study. There were no
significant differences in sperm count morphology between exposure
groups, but the miscarriage rate was reported to be significantly
( P < 0.05) higher for the wives of workers in the DAT-exposed area
of the plant, where 6 out of 15 pregnancies ended in miscarriage
compared with 1 out of 7 for the wives of DNT-exposed workers, and
3 out of 38 for the wives of unexposed workers.
The ranges of levels of reported exposures overlapped between
the 3 studies and were all within the OSHA recommended standard of
1.5 mg/m3 for dinitrotoluenes (NIOSH, 1982). The first study might
have been expected to show an excess, because it was provoked by a
cluster of miscarriages. All the studies were of limited size and
subject to some risk of selection bias, because the population was
restricted to those who volunteered. Also, in all 3 studies, crude
figures, with no adjustment for age, were presented. However, in
the 2 follow-up studies, where apparently neither of the
populations held a prior belief that there was an excess
miscarriage rate, it is significant that an apparent excess of
miscarriages was found in the wives of DAT-exposed workers.
An epidemiological assessment of the reproduction hazards for
males after occupational exposure to diaminotoluenes and
dinitrotoluenes was carried out by Hamill et al. (1982). Reported
occupational exposures were similar to those reported by NIOSH
(1980, 1981, 1982) and were generally well within the OSHA
recommended level of 1.5 mg/m3 for dinitrotoluenes. Examination of
84 workers and 119 unexposed subjects consisted of semen analysis,
blood testing, medical examination, and an interview. Seventy-two
percent of non-vasectomized exposed workers provided semen samples.
These groups of workers were defined by exposure intensity,
frequency, and recency, and compared with controls. Although no
significant differences in miscarriage rates were reported between
exposure categories, the categories were as defined at the time of
the study, not at the time of pregnancy. Fertility rates were
reported to be unaffected by exposure, but no figures were given.
There were no statistically significant differences in semen
analysis, sperm count and morphology, and FSH levels, between the 3
exposure groups and unexposed workers. The authors concluded that
the results of their study suggested that no detectable
reproductive effects existed among male workers exposed to
dinitrotoluenes and diaminotoluenes.
Levine et al. (1985) reported an analysis of the fertility of
workers exposed to DNT and DAT. The approach taken consisted of
workers completing reproductive history questionnaires and of
observed births being compared with expected births for the married
workers. Expected births were derived from US birth rates by age,
calendar year, and parity, and the ratio of observed to expected
was expressed as a standardized fertility ratio (SFR). Populations
of 137, 207, and 235 persons, respectively, were studied and were
largely, but not exclusively, male. It is not clear whether any of
the plants were the same as those described above. Comparisons of
SFR between different exposure categories, both for the whole
population and also among individuals who spent at least part of
their reproductive life exposed, did not reveal any significant
effects on SFR between exposure groups. The authors estimated that
the power of this study to detect a 50% reduction in fertility
would have been 90%. In the third NIOSH study, the miscarriage
rate for wives of DAT-exposed workers was 6.8 times higher than
that for wives of unexposed workers (rates given as miscarriages
per 100 person-years), but the fertility rate was only 0.8 times
lower (ratio of rates of live births per 100 person-years). Thus,
overall fertility may not be a sensitive index of adverse
reproductive outcome.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1. Evaluation of Human Health Risks
9.1.1. General considerations
There are insufficient data on the effects of diaminotoluenes
on human beings to carry out a detailed hazard assessment or risk
evaluation. However, absorption and metabolic studies in human
beings have indicated that diaminotoluenes are rapidly absorbed,
metabolized, and excreted in the urine in a manner similar to that
found in experimental animals. Therefore, the risk evaluation that
follows is based largely on data from animals, supported by data
from human studies, where available.
9.1.2. Assessment of exposure
Diaminotoluenes can be absorbed through the skin and
gastrointestinal tract, and by inhalation. Given the properties of
this class of chemicals, the major route of human exposure is
dermal, in the work-place, with a possibility of the inhalation of
fumes during heating. Exposure through ingestion is minimal,
except in case of accidents.
No data exist on general ambient levels of diaminotoluenes in
air, water, and food. Bioaccumulation of diaminotoluenes in the
food-chain should not occur. Levels in the work-place air of up to
0.44 mg/m3, with occasional excursions up to 11 mg/m3, have been
reported.
9.1.3. Single and short-term exposures
Diaminotoluenes are classed as toxic, highly irritant
chemicals. The oral LD50 for animals is between 270 and 350 mg/kg
body weight. Dermal contact has been shown to cause irritation,
severe dermatitis, blistering, and possible skin sensitization.
Single oral doses of diaminotoluenes of 50 mg/kg body weight have
led to methaemoglobinaemia in rats, rabbits, and guinea-pigs. Eye
contact with diaminotoluenes has led to conjunctivitis and corneal
opacities. In case of inhalation of fumes, coughing, dyspnoea, and
respiratory distress can result.
9.1.4. Long-term exposure
9.1.4.1. Carcinogenicity and mutagenicity
No epidemiological data are available on the incidence of
cancer in human beings after exposure to diaminotoluenes.
Several studies using 2,4-DAT have been carried out on
experimental animals and, in each, the isomer was shown to be
carcinogenic for rats and mice. In the most recent study, doses
of, or greater than, 79 mg/kg diet led to an increase in
hepatocellular carcinomas or neoplastic nodules in rats; there was
an increase in hepatocellular carcinomas and lymphomas in female
mice at doses exceeding 100 mg/kg diet.
Using a similar protocol, the US NCI concluded that 2,6-DAT was
not carcinogenic for rats and mice after administration of up to
500 mg/kg diet for 103 weeks. It should be noted that
hepatocellular carcinomas, neoplastic nodules, and lymphomas were
detected, as in the bioassay for 2,4-DAT, however, they were
considered not significant after detailed statistical analyses.
There was no evidence of carcinogenicity in mice and rats after
administration of 2,5-DAT at levels of up to 2000 mg/kg diet, for
78 weeks. However, the short duration of the study reduced the
potential of the test for detecting carcinogenicity. No evidence
of carcinogenicity was noted after the dermal application of hair-
dye formulations containing 2,5-DAT (following application of
hydrogen peroxide) or a mixture of 2,5-DAT and 2,4-DAT with
hydrogen peroxide.
Positive mutagenic activity was noted in S. typhimurium when
2,4-, 2,5-, 2,6-, and 3,4-DAT were tested. In addition, DAT
isomers were mutagenic in mammalian cells in vitro. Significant
DNA damage was produced by 2,4-DAT in human cultured fibroblasts,
only after activation by prostoglandin synthase. The isomer 2,4-
DAT was weakly mutagenic in Drosophila melanogaster and induced
unscheduled DNA synthesis in primary rat hepatocytes in vitro.
The 2,4- and 2,5-isomers were inactive in in vivo mammalian
mutagenicity assays. Micronuclei and dominant lethals were not
produced by 2,5-DAT, and 2,4-DAT did not produce chromosomal
breaks, dominant lethals, abnormal sperm morphology, or recessive
spots.
It has been shown that 2,4-, 2,5-, and 2,6-DAT can inhibit DNA
synthesis in the testes after ip injection of high doses. On the
basis of this study, 2,4-DAT may pose a genetic hazard in addition
to its potential to cause adverse effects on reproduction.
9.1.4.2. Reproduction and teratogenicity
The results of limited studies on the reproduction hazards for
male workers exposed to diaminotoluenes are equivocal. In surveys
of reproductive outcome in 3 plants, an excess of spontaneous
abortions among the wives of male workers exposed to DAT and
dinitrotoluene was reported in 2 surveys, though these excesses
were based on small numbers, and not all workers in the plants
participated in the studies. In 1 out of the 3 plants studied,
some adverse effects on spermatogenesis were suggested. Analysis
of the overall fertility of workers in 3 other production plants
did not reveal any adverse effects from exposure to DAT.
In a study on animals fed 2,4-DAT, there was a significant and
persistent decrease in the sperm count.
Embryotoxicity was observed in animal studies after oral and
dermal doses exceeding 30 mg/kg body weight for the 2,3-and 3,4-
isomers and 10 mg/kg body weight for 2,6-DAT.
Skeletal changes were noted after dermal application of a hair-
dye formula containing 3% 2,4-DAT at 2 ml/kg body weight.
9.2. Evaluation of Effects on the Environment
Information is lacking concerning levels of diaminotoluenes in
the environment, and their transport, bioconcentration,
biotransformation, and biodegradation.
A few data indicate that diaminotoluenes may be hazardous for
aquatic organisms. No data on the effects of diaminotoluenes on
other non-mammalian targets in the environment could be found.
9.3. Conclusions
Diaminotoluenes are highly irritating to the skin and eyes and
the fumes are irritating to the respiratory tract. They are
readily absorbed through the skin. Methaemoglobinaemia may occur
in exposed individuals. Renal toxicity after oral administration
of 2,4-DAT has been reported in experimental animals. 2,4-DAT has
been shown to be carcinogenic for animals, but there is inadequate
evidence to evaluate the carcinogenic potential of 2,5- and 2,6-
diaminotoluene. All 3 of these isomers have been shown to be
mutagenic. Limited data are available concerning a reproductive
hazard for male workers handling DATs. DATs have been shown to
impair spermatogenesis in experimental animals and to be both
embryotoxic and teratogenic.
10. RECOMMENDATIONS
1. Monitoring should be undertaken to determine the sources,
levels, and fate of diaminotoluenes in the environment.
Ecotoxicity data should be collected.
2. For a better evaluation of occupational exposure and effects,
studies on the uptake, kinetics, and metabolism of DAT and the
relevant routes of exposure are important to provide a sound
basis for biological monitoring.
3. To assist in the development of appropriate health
surveillance systems, a systematic evaluation of the toxicity
of diaminotoluenes should be carried out to compliment
available data on carcinogenicity and reproductive effects.
4. Additional data should be obtained on human morbidity and
mortality related to exposure to diaminotoluenes, with
particular emphasis on carcinogenic, teratogenic, and
reproductive end-points.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1978) evaluated the data on the carcinogenicity of
diaminotoluenes and concluded that there was sufficient evidence of
the carcinogenicity of 2,4-diaminotoluene in experimental animals.
An evaluation of additional data by IARC (1986) further supported
this conclusion.
In the absence of case reports or epidemiological studies,
there was inadequate data to assess the carcinogenicity of
diaminotoluenes for human beings (IARC, 1978).
REFERENCES
AUNE, T., NELSON, S.D., & DYBING, E. (1979) Mutagenicity and
irreversible binding of the hepatocarcinogen 2,4-diaminotoluene.
Chem.-biol. Interact., 25(1): 23-24.
AUSTIN, G.T. (1974) Industrially significant organic chemicals.
Part 9. Chem. Eng., 11: 96-100.
BACKUS, J.K. (1974) Urethanes. In: Considine, D.M., ed.
Chemical and process technology encyclopedia, New York, McGraw
Hill Book Co., pp. 1121-1125.
BECCI, P.J., REAGAN, E.L., KNICKERBOCKER, M.J., BARBEE, S.J.,
& WEDIG, J.H. (1983) Teratogenesis study of o-toluenediamine
in rats and rabbits. Toxicol. appl. Pharmacol., 71: 323-329.
BECHER, G. (198l) Glass capillary columns in the gas
chromatographic separation of aromatic amines. 2. Application
of samples from workplace atmospheres using nitrogen-selective
detection. J. Chromatogr., 211(1): 103-111.
BERMUDEZ, E. & BUTTERWORTH, B.E. (1979) Analysis of the
activities of 2,4-diaminotoluene and 2,4- and 2,6-dinitrotoluene
in the primary hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 1: 168-169.
BERMUDEZ, E., TILLERY, D., & BUTTERWORTH, B.E. (1979) Effect
of 2,4-diaminotoluene and isomers of dinitrotoluene on
unscheduled DNA synthesis in primary rat hepatocytes. Environ.
Mutagen., 1: 39l-398.
BIERNACKA, T., SEKOWSKA, B., & MICHONSKA, J. (1974) [Determination
of 2,4- and 2,6-diaminotoluene in technical products by infra-red
spectroscopy.] Chem. Anal. (Warsaw), 19: 6l9-632 (in Polish).
BLIJLEVEN, W.G.H. (1977) Mutagenicity of four hair dyes in
Drosophila melanogaster. Mutat. Res., 48: 181-186.
BOUFFORD, C.E. (l968) Determination of isomeric diaminotoluenes
by direct gas liquid chromatography. J. Gas Chromatogr., 6:
438-440.
BUIST, J.M. (1970) Isocyanates in industry. Proc. R. Soc. Med.,
63: 365-366.
BURNETT, C., LANMAN, B., GIOVACCHINI, R., WOLCOTT, G., & SCALA, R.
(1975) Long-term toxicity studies on oxidation hair dyes.
Food Cosmet. Toxicol., 13(3): 353-357.
BURNETT, C., GOLDENTHAL, E.I., HARRIS, S.B., WAZETER, F.X.,
STRAUSBURG, J., KAPP, R., & VOELKER, R. (1976) Teratology
and percutaneous toxicity studies on hair dyes. J. Toxicol.
environ. Health, 1: 1027-1040.
BURNETT, C., LOEHR, R., & CORBETT, J. (1977) Dominant lethal
mutagenicity study on hair dyes. J. Toxicol. environ. Health,
2: 657-662.
CARDY, R.H. (1979) Carcinogenicity and chronic toxicity of
2,4-toluenediamine in F344 rats. J. Natl Cancer Inst., 62:
1107-1116.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F., Jr (1974)
Range-finding toxicity data. Toxicol. appl. Pharmacol., 28:
313-319.
CHOUDHARY, G. (1980) Gas-liquid chromatographic determination
of toxic diamines in permanent hair dyes. J. Chromatogr., 193:
277-284.
CIC JAPAN (1983) Commercial industrial chemicals, Tokyo,
Chemical Daily Co., Ltd.
CINKOTAI, K.I., JONES, C.C., TOPHAM, J.C., & WATKINS, P.A. (1978)
Experience with mutagenic tests as indicators of carcinogenic
activity. Mutat. Res., 53: 167-168.
COPPINGER, W.J., BRENNAN, S.A., CARVER, J.H., & THOMPSON, E.D.
(1984) Locus specificity of mutagenicity of 2,4-diaminotoluene in
both L5l78Y mouse lymphoma and AT3-2 chinese hamster ovary cells.
Mutat. Res., 135: 115-123.
CRC (1975) CRC handbook of chemistry and physics, 56th ed.,
Cleveland, Ohio, CRC Press.
DENT, J.G. & GRAICHEN, M.E. (1982) Effect of hepatocarcinogens
on epoxide hydrolase and other xenobiotic metabolizing enzymes.
Carcinogenesis, 3(7): 733-738.
DYBING, E. & THORGEIRSSON, S.S. (1977) Metabolic activation
of 2,4-diaminoanisole, a hair dye component. 1. Role of
cytochrome P-450 metabolism in mutagenicity in vitro. Biochem.
Pharmacol., 26: 729-734.
DYBING, E., AUNE, T., & SODERLUND, E.J. (1977) Use of the
Salmonella mutagenicity test in drug metabolism studies. Acta
pharmacol. toxicol., 41: 31.
DYBING, E., AUNE, T., & NELSON, S. (1978) Covalent binding
of 2,4-diaminoanisole and 2,4-diaminotoluene in vivo. Toxicol.
Aspects Food Saf. Arch. Toxicol., Suppl. 1: 213-217.
FAHMY, M.J. & FAHMY, O.G. (1977) Mutagenicity of hair dye
components relative to the carcinogens benzidine in Drosophila
melanogaster. Mutat. Res., 56: 31-38.
FILATOVA, V.S., TUBINA, A.Ya., SHARONOVA, Z.V., GOLOVA, I.A.,
FILINA, V.I., & DOROFEEVA, E.D. (1970) [Hygienic aspects of
work and health of workers in the production of toluylene-
diamine.] Gig. i Sanit., 35(2): 16-30 (in Russian with English
summary).
GAN, L.H., BLAIS, P., CARLSSON, D.J., SUPRUNCHUCK, T., & WILES, D.M.
(1975) Physicochemical characterization of some fully aromatic
polyamides. J. appl. polym. Sci., 19: 69-82.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., WEISBURGER, E.K., &
ROLLER, P.P. (1975) Enzymic N-acetylation of 2,4-toluenediamine
by liver cytosols from various species. Xenobiotica, 5(8): 475-483.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., LEWIS, N.L., &
WEISBURGER, E.K. (1976) N-acetylation as a route of 2,4-
toluenediamine metabolism by hamster liver cytosol. Biochem.
Pharmacol., 25: 95-97.
GRANTHAM, P.H., MOHAN, L., BENJAMIN, T., ROLLER, P.P., MILLER, J.R.,
& WEISBURGER, E.K. (1980) Comparison of the metabolism of 2,4-
toluenediamine in rats and mice. J. environ. Pathol. Toxicol.,
3(1-2): 149-l66.
GREENE, E.J. & FRIEDMAN, M.A. (1980) In vitro cell transformation
screening of 4 toluene diamine isomers. Mutat. Res., 79: 363-375.
GREENE, E.J., FRIEDMAN, M.A., & SHERROD, J.A. (1979) In vitro
mutagenicity and cell transformation testing of four toluene
diamine isomers. Environ. Mutagen., 1: 194.
GREENE, E.J., SALERNO, A.J., & FRIEDMAN, M.A. (1981) Effect
of 4 toluene diamine isomers on murine testicular DNA synthesis.
Mutat. Res., 91: 75-79.
GUTHRIE, J.L. & MCKINNEY, R.W. (1977) Determination of 2,4-
and 2,6-diaminotoluene in flexible urethane foams. Anal.
Chem., 49: 1676-1680.
HAMILL, V.V., STEINBERGER, E., LEVINE, R.J., RODRIGUEZ-RIGAU,
L.J., LEMESHOW, S., & AVRUNIN, J.S. (1982) The epidemiologic
assessment of male reproductive hazard from occupational
exposure to TDA and DNT. J. occup. Med., 24: 982-993.
HOSSACK, D.J.N. & RICHARDSON, J.C. (1977) Examination of the
potential mutagenicity of hair dye constituents using the
micronucleus test. Experientia (Basel), 33: 377-378.
HRUBY, R. (1977) The absorption of p-toluenediamine by the
skin of rats and dogs. Food Cosmet. Toxicol., 15: 595-599.
IARC (1978) 2,4-Diaminotoluene. In: Some aromatic amines and
related nitro compounds: hair dyes, colouring agents, and
miscellaneous industrial compounds, Lyons, International
Agency for Research on Cancer, pp. 83-95 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 16).
IARC (1986) Some chemicals used in plastics and elastomers,
Lyons, International Agency for Research on Cancer, p. 304
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 39).
INOUYE, M. & MURAKAMI, U. (1976) Teratogenicity of 2,5-
diaminotoluene, a hair dye component in mice. Teratology, 14:
241-242.
INOUYE, M. & MURAKAMI, U. (1977) Teratogenicity of 2,5-
diaminotoluene, a hair dye constituent in mice. Food Cosmet.
Toxicol., 15: 447-451.
ITO, N., HIASA, Y., KONISHI, Y., & MARUGAMI, M. (1969) The
development of carcinoma in liver of rats treated with
m-toluylenediamine and the synergistic and antagonistic
effects with other chemicals. Cancer Res., 29: 1137-1145.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982)
Toxicometric paramaters of industrial toxic chemicals under
single exposure, Moscow, USSR/SCST/USSR Commission for UNEP,
Centre of International Projects.
JOHANSSON, K., RAPPE, C., LINDBERG, W., & NYGREN, M. (1981)
Determination of aromatic diamines in hair dyes using liquid
chromatography. In: Egan, H., Fishbein, L., O'Neil, I.K.,
Castegnaro, M., & Bartsch, H., ed. Environmental carcinogens
selected methods of analysis. Volume 4: Some aromatic amines
and azo dyes in the general and industrial environment. Lyons,
International Agency for Research on Cancer (IARC Publications
No. 40).
KIESE, M. & RAUSCHER, E. (1968) The absorbtion of p-toluene-
diamine through human skin in hair dyeing. Toxicol. appl.
Pharmacol., 13: 325-331.
KIESE, M., RACHOR, M., & RAUSCHER, E. (1968) The absorption
of some phenylenediamines through the skin of dogs. Toxicol.
appl. Pharmacol., 12: 495-507.
KINKEL, H.J. & HOLZMANN, S. (1973) Study of long-term
percutaneous toxicity and carcinogenicity of hair dyes
(oxidizing dyes) in rats. Food Cosmet. Toxicol., 11: 641-648.
KNICKERBOCKER, M., RE, T.A., PARENT, R.A., & WEDING, J.H.
(1980) Teratogenic evaluation of ortho-toluenediamine
(o-TDA) in Sprague Dawley rats and Dutch Belted rabbits.
Toxicologist, 19: A.89.
KORNBRUST, D.J. & BARFKNECHT, T.R. (1984) Comparison of 7
azo dyes and their reduction products in the rat and hamster
hepatocyte primary culture/DNA-repair assays. Mutat. Res.,
136: 255-266.
KOTTEMANN, C.M. (1966) Two dimensional thin-layer
chromatographic procedure for the identification of dye
intermediates in arylamine oxidation hair dyes. J. Assoc. Off.
Anal. Chem., 49(5): 954-959.
LEVINE, R.J., CORSO, D.D., & BLUNDEN, P.B. (1985) Fertility
of workers exposed to dinitrotoluene and toluenediamine at
three chemical plants. In: Rickert, D.E., ed. Toxicity of
nitroaromatic compounds, Washington DC, Hemisphere.
LIEM, D.H. & ROOSELAAR, J. (1981) HPLC of oxidation hair
colours. Mitt. Geb. Lebensm. Hyg., 72: 164-176.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975)
Detection of carcinogens as mutagens in the Salmonella microsome
test: Assay of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72:
5135-5139.
MACKE, G.F. (1968) Use of tetracyanoethylene as a thin-layer
chromatographic spray reagent. J. Chromatogr., 36: 537-539.
MARKS, T.A., GUPTA, B.N., LEDOUX, T.A., & STAPLES, R.E.
(1981) Teratogenic evaluation of 2-nitro-p-phenyl-enediamine,
4-nitro-o-phenylendiamine, and 2,5-toluenediamine sulfate in
the mouse. Teratology, 24: 253-265.
MARZULLI, F.N., ANJO, D.M., & MAIBACH, H.I. (1981) In vivo
skin penetration studies of 2,4-toluenediamine, 2,4-diamino-
anisole, 2-nitro-p-phenylenediamine, p-dioxane and n-nitro-
sodiethanolamine in cosmetics. Food Cosmet. Toxicol., 19: 743.
MATHESON, D. & CREASY, B. (1976) Use of the L5178Y (TK+/-)
mouse lymphoma cell line coupled with an in vitro microsomal
enzyme activation system to study chemical promutagens. Mutat.
Res., 38: 400-401.
MATHIAS, A. (1966) Analysis of diaminotoluene isomer
mixtures by nuclear magnetic resonance spectrometry. Anal.
Chem., 38: 1931-1932.
MATSUI, S., MURAKAMI, T., SASAKI, T., HIROSE, Y., & IGUMA, Y.
(1975) Activated sludge degradability of organic substances
in the wastewater of the Kashima petroleum and petrochemical
industrial complex in Japan. Prog. Water Technol., 7:645-659.
MILLIGAN, B. & GILBERT, K.E. (1978) Diaminotoluenes. In:
Kirk-Othmer encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 1, pp. 321-329.
MIRSALIS, J.C. & BUTTERWORTH, B.E. (1982) Induction of
unscheduled DNA synthesis in rat hepatocytes following in vivo
treatment with dinitrotoluene. Carcinogenesis, 3: 241-245.
MIRSALIS, J.C., TYSON, C.M., & BUTTERWORTH, B.E. (1982)
Detection of genotoxic carcinogens in the in vivo/in vitro
hepatocyte DNA repair assay. Environ. Mutagen., 4: 553-562.
MORI, M.A., MIYAHARA, T., TANIGUCHI, K., HASEGAWA, K., KOZUKA,
H., MIYAGOSHI, M., & NAGAYAMA, T. (1982) Mutagenicity of
2,4-dinitrotoluene and its metabolites in Salmonella typhimurium.
Toxicol. Lett., 13: 1-5.
NCI (1978) Bioassay of 2,5-toluenediamine sulfate for
possible carcinogenicity, Bethesda, Maryland, National Cancer
Institute (NCI Carcinogenesis Technical Report Series No. 126;
DHEW Publication No. (NIH) 78-1381).
NCI (1979) Bioassay of 2,4-diaminotoluene for Possible
Carcinogenicity, Bethesda, Maryland, National Cancer Institute
(NCI Carcinogenesis Technical Report Series No. 162).
NCI (1980) Bioassay of 2,6-toluenediamine dihydrochloride
for possible carcinogenicity, Bethesda, Maryland, National
Cancer Institute (NCI Carcinogenesis Technical Report Series
No. 200; NTP No. 80-20; NIH Publication No. 80-1756).
NIEMINEN, E.H., SAARINAN, L.H., & LAAKSO, J.T. (1983)
Simultaneous determination of aromatic isocyanates and some
carcinogenic amines in the work atmosphere by reversed-phase
high-performance liquid chromatography. J. liq. Chromatogr.,
6: 453-469.
NIOSH (1978) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Service, Center for Disease Control,
Vol. 4 (Publication No. 78-175).
NIOSH (1980) Health hazard evaluation determination report
HE 79-113-728, Brandenburg, Kentucky, Olin Chemical Company,
and Cincinnati, Ohio, National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1981) Interim Report No. 1. HETA 81-118,
New Martinsville, West Virginia, Mobay Chemical Corporation,
and Cincinatti, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1982) Interim report No. 1. HETA 81-295-1155,
Moundsville, West Virginia, Allied Chemical Company, and
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NORDENSKJOLD, M., ANDERSSON, B., RAHIMTULA, A., & MOLDEUS, P.
(1984) Prostaglandin synthesis-catalyzed metabolic activation
of some aromatic amines to genotoxic products. Mutat. Res.,
127: 107-112.
OLUFSEN, B. (1979) Glass capillary columns in the gas
chromatographic separation of aromatic amines. I. J. Chromatogr.,
179: 97-103.
OSHA (1983) Code of federal regulations, Washington DC, US
Occupational Safety and Health Administration, pp. 660-664
(29CFR, 19101000, Table Z-1).
PERKINS, W.E. & GREEN, T.J. (1975) Effect of 3,4-toluenediamine
on output from in situ rat brunner's glands pouches (38941).
Proc. Soc. Exp. Biol. Med., 149: 991-994.
PIENTA, R.J., POILEY, J.A., & LEBHERZ, W.B., III (1977a)
Morphological transformation of early passage golden Syrian
hamster embryo cells derived from cryopreserved primary
cultures as a reliable in vitro bioassay for identifying
diverse carcinogens. Int. J. Cancer, 19: 642-655.
PIENTA, R.J., SHAH, M.J., LEBHERZ, W.B., & ANDREWS, A.W.
(1977b) Correlation of bacterial mutagenicity and hamster
cell transformation with mutagenicity induced by 2,4-
toluenediamine. Cancer Lett., 3: 45-52.
PURNELL, C.J. & WARWICK, C.J. (1981) Application of electro-
chemical detection in high-performance liquid chromatography
to the measurement of toxic substances in air. Anal. Proc.,
18: 151-154.
PURNELL, C.J., BAGON, D.A., & WARWICK, C.J. (1982) The
determination of organic contaminant concentrations in
workplace atmospheres by high-performance liquid chromatography.
Pergamon Ser. environ. Sci., 7: 203-219.
RAHIMTULA, A., MOLDEUS, P., ANDERSSON, B., & NORDENSKJOLD, M.
(1982) Prostaglandin synthetase catalyzed DNA strand breaks
by aromatic amines. In: Prostaglandins and related lipids,
New York, Alan R. Liss, Vol. 2, pp. 159-162.
REUBER, M.D. (1979) Carcinomas of the liver in female mice
fed toluene-2,4-diamine. Gann, 70: 453-457.
RIGGIN, R.M. & HOWARD, C.C. (1983) High performance liquid
chromatographic determination of phenylenediamines in aqueous
environmental samples. J. liq. Chromatogr., 6: 1897-1905.
SCHAFER, U. METZ, J., PEVNY, I., & ROCKL, H. (1978)
[Attempts of sensitizing guinea pigs with five different
derivates of para-substituted benzene.] Arch dermatol. Res.,
216: 153-161 (in German).
SELYE, H. (1973) Production of perforating duodenal ulcers
by 3,4-toluenediamine in the rat. Proc. Soc. Exp. Biol. Med.,
142: 1192-1194.
SHAHIN, M.M., BUGAUT, A., & KALOPISSIS, G. (1980) Structure-
activity relationship within a series of m-diaminobenzene
derivatives. Mutat. Res., 78: 25-31.
SHOOTER, K.V. & VENITT, S. (1979) Phosphotriesters in DNA:
non-repairable lesions as markers for the early detection of
chemical carcinogens in intact animals. Mutat. Res., 64: 106.
SKARPING, G., RENMAN, L., & SMITH, B.E.F. (1983a) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. I. Determination of
aromatic amines as perfluoro-fatty acid amines using electron-
capture detection. J. Chromatogr., 267: 315-327.
SKARPING, G., RENMAN, L., & DALENE, M. (1983b) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. II Determination of
aromatic amines as perfluoro-fatty acid amines using nitrogen-
selective detection. J. Chromatogr., 270: 207-218.
SMIRNOVA, A.N., TOROPOVA, L.A., & DOBROVOL'SKAYA, V.V.
(1967) [Effect of toluylenediamine and ortho-toluidine on
aquatic organisms.] Vodosnarzh Kanaltz Giurotekh, 5: 17-23 (in
Russian) (EPA translation).
SNYDER, R.C. & BREDER, C.V. (1982) High performance liquid
chromatographic determination of 2,4- and 2,6-toluenediamine
in aqueous extracts. J. Chromatogr., 236: 429-440.
SNYDER, R.C., BRUMLEY, W.C., BREDER, C.V., & FAZIO, T.
(1982) Gas chromatographic and gas chromatographic-mass
spectrometric confirmation of 2,4- and 2,6-toluenediamine
determined by liquid chromatography in aqueous extracts. J.
Assoc. Off. Anal. Chem., 65(6): 1388-1394.
SOARES, E.R. & LOCK, L.F. (1980) Lack of an indication of
mutagenic effects of dinitrotoluenes and diaminotoluenes in
mice. Environ. Mutagen., 2: 111-124.
SONTAG, J.M. (1981) Carcinogenicity of substituted-benzene-
diamines (phenylenediamines) in rats and mice. J. Natl Cancer
Inst., 66: 591-602.
SPENGLER, J., OSTERBURG, I., & KORTE, R. (1986) Abstract:
Teratogenic evaluation of p-toluenediamine sulphate.
Resorcinal and p-aminophenol in rats and rabbits. Teratology,
33(2): 31.
THYSEN, B., BLOCH, E., & VARMA, S.K. (1985a) Reproductive
toxicity of 2,4-toluenediamine in the rat. 2. Spermatogenic
and hormonal effects. J. Toxicol. environ. Health, 16: 763-769.
THYSEN, B., VARMA, S.K., & BLOCH, E (1985b) Reproductive
toxicity of 2,4-toluenediamine in the rat. 1. Effect on male
fertility. J. Toxicol. environ. Health, 16: 753-761.
UMEDA, M. (1955) Production of rat sarcoma by injections of
propylene glycol solution of m-toluylenediamine. Gann, 46:
597-606.
UNGER, P.D. & FRIEDMAN, M.A. (1979) High-performance liquid
chromatography of 2,6- and 2,4-diaminotoluene, and its
application to the determination of 2,4-diaminotoluene in
urine and plasma. J. Chromatogr., 174: 379-384.
UNGER, P.D., SALERNO, A.J., NESS, W.C., & FRIEDMAN, M.A.
(1980) Tissue distribution and excretion of
2,4[143]-toluenediamine in the mouse. J. Toxicol. environ.
Health, 6: 107-114.
US EPA (1980) Materials balance for 2,4-diaminotoluene.
Level 1. Preliminary, Washington DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/13:
79-016).
US ITC (1977) Synthetic organic chemicals: US production and
sales, 1975, Washington DC, US International Trade Commission,
pp. 22, 36, 42, 49, 60, 62, 65, 74 (US ITC Publication 804).
US ITC (1982) Preliminary report on US production of
selected synthetic organic chemicals. Preliminary totals 1981,
Washington DC, US International Trade Commission (SOC Series
C/P-82-1).
US ITC (1985) Preliminary report on US production of
selected synthetic organic chemicals. November, December, and
cummulative totals, 1984, Washington DC, US International
Trade Commission (SOC Series C/P-85.1).
VENITT, S. (1978) Mutagenicity of hair dyes: some more
evidence and the problems of its interpretation. Mutat. Res.,
53: 278-279.
VON OETTINGEN, W.F. (1941) The aromatic amino and nitro
compounds: their toxicity and potential dangers. A review of
the literature, Washington DC, Division of Industrial Hygiene,
National Institute of Health, pp. 55-63.
WAGNER, J.C. (1975) Linear compartment models. In:
Fundamentals of clinical pharmacokinetics, Hamilton, Illinois,
Drug Intelligence Publications, pp. 57-63.
WARING, R.H. & PHEASANT, A.E. (1976) Some phenolic metabolites
of 2,4-diaminotoluene in the rabbit, rat and guinea-pig.
Xenobiotica, 6: 257-262.
WEISBROD, D. & STEPHAN, U. (1983) [Studies of the toxic,
methaemoglobin-producing and erythrocyte-damaging effects of
diaminotoluene after a single administration.] Z. gesamte
Hyg., 29: 395-397 (in German).
WEISBURGER, E.K., RUSSFIELD, A.B., HOMBURGER, F., WEISBURGER,
J.H., BOGER, E., VAN DONGEN, C.G., & CHU, K.C. (1978)
Testing of twenty-one environmental aromatic amines or
derivatives for long-term toxicity or carcinogenicity.
J. environ. Pathol. Toxicol., 2(2): 325-356.
WILLEBOORDSE, F., QUICK, Q., & BISHOP, E. (1968) Direct gas
chromatographic analysis of isomeric diaminotoluenes. Anal.
Chem., 40: 1455-1458.
WILLIAM, R.T. (1971) The metabolism of certain drugs and
food chemicals in man. Ann. N.Y. Acad. Sci., 179: 141-154.
WILLIAMS, G.M. (1977) Detection of chemical carcinogens by
unscheduled DNA synthesis in rat liver primary cell cultures.
Cancer Res., 37: 1845-1851.
WILLIAMS, G.M. (1978) Further improvements in the hepatocyte
primary culture DNA repair test for carcinogens: detection of
carcinogenic biphenyl derivatives. Cancer Lett., 4: 69-75.
WILLIAMS, G.M. & LASPIA, M.F. (1979) The detection of
various nitrosamines in the hepatocyte primary culture/DNA
repair test. Cancer Lett., 66: 199-206.
 Commercial grades of diaminotoluenes are available; however,
the most commonly marketed diaminotoluenes are: (a) "crude"
diaminotoluenes-mixture, containing all 6 isomers (Table 1); (b)
Meta-diaminotoluene (Meta-DAT), containing approximately 80% 2,4-
and 20% 2,6-isomers (also produced in smaller amounts as 65:35
mixture); and (c) Ortho-diaminotoluene (Ortho-DAT), consisting of
approximately 40% 2,3- and 60% 3,4-isomers. All commercial grades
contain traces of the other isomers; therefore, diaminotoluenes and
their mixtures are reviewed together in this document.
Most of the common and trade names for commercial
diaminotoluenes are listed in Table 2.
Table 2. Diaminotoluenes synonyms and trade names
----------------------------------------------------------------------------
A. Commercial mixtures
I. Meta-Diaminotoluenes
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names benzenediamine,ar-methyl (RTECS: TDB);
diaminotoluene (RTECS: TDB);
phenylenediamine,ar-methyl- (TDB);
Meta-diaminotoluene; Meta-toluene-
diamine (MTD);
toluene-ar,ar-diamine (8CI) (CAS: TDB);
toluenediamine (RTECS: TDB, DOT);
tolylenediamine (RTECS, TDB)
Common name diaminotoluene; toluenediamine; TDA
II. Ortho-Diaminotoluene
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names o-TDA
Common name Ortho-toluenediamine; OTD
B. Diaminotoluene isomers
I. 2,3-isomer
Chemical abstract name 1,2-benzenediamine,3-methyl- (9CI)
Other chemical names toluene-2,3-diamine (8CI) (CAS: TDB);
1-methyl-1,2,3-phenylenediamine (TDB);
1,2-diamino-3-methylbenzene (TDB);
2,3-diaminotoluene (TDB*);
2,3-toluylenediamine (TDB);
2,3-tolylenediamine (TDB);
3-methyl-o-phenylenediamine (TDB);
3-methyl-1,2-phenylenediamine (TDB)
Common names 2,3-TDA
II. 2,4-isomer
Chemical abstract name 1,3-benzenediamine,4-methyl- (9CI)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
II. 2,4-isomer (contd.)
Other chemical names m-toluenediamine (RTECS);
m-toluylendiamin (Czech, RTECS: TDB);
m-toluylenediamine (RTECS: TDB);
m-tolyenediamine (RTECS: TDB);
m-tolylenediamine (RTECS);
Meta-toluylene diamine (RTECS: TDB);
toluene-2,4-diamine (8CI) (CAS, RTECS:
TDB*);
tolylene-2,4-diamine (RTECS: TDB);
1,3-diamino-4-methylbenzene (RTECS: TDB);
2,4-diamino-1-methylbenzene (RTECS: TDB);
2,4-diamino-1-toluene (RTECS: TDB);
2,4-diaminotoluen (Czech, RTECS: TDB);
2,4-diaminotoluene (MESH, RTECS: TDB);
2,4-diaminotoluol (RTECS: TDB);
2,4-tolamine (RTECS: TDB);
2,4-toluenediamine (MESH, RTECS: TDB);
2,4-toluylenediamine (DOT, RTECS: TDB);
2,4-tolylenediamine (RTECS: TDB);
3-amino- p-toluidine (RTECS: TDB);
4- m-tolylenediamine (RTECS: TDB);
4-methyl- m-phenylenediamine (RTECS: TDB);
4-methyl-1,3-benzenediamine (RTECS: TDB);
5-amino- o-toluidine (RTECS: TDB)
Common names TDA; MTD; 2,4-TDA (CAS, RTECS: TDB)
Trade names Azogen Developer H; Benzofur MT;
C.I. Oxidation Base (RTECS);
C.I. Oxidation Base 20;
C.I. Oxidation Base 35 (RTECS);
C.I. Oxidation Base 200;
Developer B (RTECS: TDB); Developer DB
(RTECS: TDB);
Developer DBJ (RTECS: TDB); Developer H;
Developer MC (RTECS: TDB); Developer MT
(RTECS: TDB);
Developer MT-CF (RTECS: TDB);
Developer MTD (RTECS; TDB);
Developer T (RTECS: TDB); Developer 14;
Eucanine GB (RTECS: TDB); Fouramine;
Fouramine J (RTECS: TDB); Fourrine M
(RTECS: TDB);
Fourrine 94 (RTECS: TDB);
Lekutherm-Haerter VP-KU 6546;
Nako TMT (RTECS: TDB); NCI-C02302 (RTECS:
TDB);
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
Trade names (contd.) Pelagol J (RTECS: TDB); Pelagol Grey J
(RTECS: TDB);
Pontamine Developer TN (RTECS: TDB);
Renel MD (RTECS: TDB); Tertral G;
Zoba GKE (RTECS: TDB);
Zogen Developer H (RTECS: TDB).
Colour index number 76035
III. 2,5-isomer
Chemical abstract name 1,4-benzenediamine,2-methyl-(9CI)
Other chemical names p-toluenediamine (MESH, RTECS: TDB);
p-toluylendiamine (RTECS: TDB);
P,m-tolylenediamine (RTECS: TDB);
para-meta-tolylenediamine;
para-toluenediamine;
para-toluylenediamine;
para-tolylenediamine;
toluene-2,5-diamine (8CI) (CAS, RTECS:
TDB);
toluylene-2,5-diamine (RTECS: TDB);
2-methyl- p-phenylenediamine (RTECS: TDB);
2-methyl-1,4-benzenediamine (RTECS: TDB*);
2,5-diaminotoluene (MESH, RTECS: TDB);
2,5-toluenediamine;
4-amino-2-methylaniline (RTECS: TDB)
Common name 2,5-TDA
Trade names Oxidation Base 4 (as sulfate)
Colour index number C.I. 76042 (RTECS); 76043 (as sulfate)
IV. 2,6-isomer
Chemical abstract name 1,3-benzenediamine,2-methyl- (9CI)
Other chemical names m-phenylenediamine-2-methyl;
toluene-2,6-diamine (8CI) (CAS, RTECS:
TDB);
tolylene-2,6-diamine;
1,3-benzenediamine;
2-methyl- m-phenylenediamine (TDB);
2-methyl-1,3-benzenediamine (TDB);
2-methyl-1,3-phenylenediamine (TDB);
2,6-diamino-1-methylbenzene (TDB);
2,6-diaminotoluene (MESH, RTECS: TDB*);
2,6-toluenediamine;
2,6-toluylenediamine (RTECS: TDB);
2,6-tolylenediamine (RTECS: TDB)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
IV. 2,6-isomer (contd.)
Common names 2,6-TDA
V. 3,4-isomer
Chemical abstract names 1,2-benzenediamine,4-methyl- (9CI)
Other chemical names o-toluenediamine;
toluene-3,4-diamine (8CI) (CAS, RTECS:
TDB);
1,2-benzenediamine,4-methyl- (RTECS: TDB);
1,2-diamino-4-methylbenzene (TDB);
3,4-diamino-1-methylbenzene (TDB);
3,4-diaminotoluene (RTECS: TDB);
3,4-toluenediamine;
3,4-toluylenediamine (RTECS);
3,4-tolylenediamine (RTECS: TDB);
4-methyl- o-phenylenediamine (TDB);
4-methyl-1,2-benzenediamine (TDB);
4-methyl-1,2-diaminobenzene (TDB);
4-methyl-1,2-phenylenediamine (TDB)
Common name 3,4-TDA
VI. 3,5-isomer
Chemical abstract name 1,3-benzenediamine,5-methyl- (9CI)
Other chemical names 3,5-diaminotoluene;
3,5-toluenediamine
Common name 3,5-TDA
----------------------------------------------------------------------------
2.2. Physical and Chemical Properties
Diaminotoluenes are colourless crystals that are freely soluble
in hot water, alcohol, ether, and hot benzene. Some of the
physical properties of the 6 isomers are listed in Table 3 (Buist,
1970; CRC, 1975). Diaminotoluenes are oxidized readily in neutral
or alkaline solution to form dark-coloured products and tars. The
oxidation products have not been fully characterized. When heated,
diaminotoluenes emit toxic fumes of nitrogen oxides.
The composition and physical properties of the commercial
mixtures vary considerably. Some of the physical properties of the
2 most widely-used commercial mixtures are summarized in Table 4.
Meta- and Ortho-diaminotoluenes are weakly basic and react with
mineral acids to form water-soluble amine salts. These salts are
more resistant to oxidation than the parent amine.
Table 3. Physical properties of the diaminotoluene isomers
-------------------------------------------------------------------
Property Diaminotoluene isomers
2,3- 2,4- 2,5- 2,6- 3,4- 3,5-
-------------------------------------------------------------------
Melting point (°C) 63-64 99 64 105 88.5 -
Boiling point (°C) 255 280 273-274 289b 265 283-285
(subl)
Vapour pressurea (kPa)
at 150 °C 1.20 1.47 - 2.13 - -
at 160 °C 1.87 2.27 - 3.33 - -
at 180 °C 2.67 4.80 - 7.60 - -
-------------------------------------------------------------------
a To convert kPa to mmHg, divide by 0.133.
b Obtained by extrapolation from vapour pressure-temperature data
and Antoine constants. From: Willeboordse et al. (1968).
Table 4. Physical and chemical properties of commercial grades of
diaminotoluene
----------------------------------------------------------------------
Meta-DAT Ortho-DAT
(80:20, 2,4-/2,6-isomers) (60:40, 3,4-/2,3-isomers)
----------------------------------------------------------------------
Appearance solid, light yellow to tan; light grey to purple
darkens on storage and solid
exposure to air
Odour slight ammonia-like slight ammonia-like
Melting range 80 - 90 °C (176 - 194 °F) 40 - 50 °C (104 - 122 °F)
Boiling point 283 °C (541 °F) at 760 mmHg > 250 °C (> 480 °F)
Flash point 140 °C (284 °F) > 110 °C (> 230 °F)
Autoignition 450 °C (842 °F) 540 °C (1005 °F)
temperature
Vapour 0.34 x 10-3 mmHg at 37.8 °C 2.23 mmHg at 100 °C
pressure 1 mmHg at 106.5 °C 27.8 mmHg at 140 °C
100 mmHg at 212 °C 43.5 mmHg at 160 °C
Specific - 1.045 at 100 °C
gravity
Density 0.086 kg/litre at 105 °C -
Solubility in hot water, alcohol, in hot water, alcohol,
ether and many polar ether and many polar
organic solvents organic solvents
----------------------------------------------------------------------
2.3. Conversion Factors
1 ppm in air = 5 mg/m3 at 25 °C and 760 mmHg.
2.4. Analytical Methods
Analytical methods for the determination of diaminotoluenes in
water, air, different consumer products, and biological fluids are
listed in Table 5.
Diaminotoluenes may be analysed as free bases by reversed phase
high-performance liquid chromatography using both ultraviolet (UV)
and electrochemical detection (Purnell & Warwick, 1981; Purnell et
al., 1982; Nieminen et al., 1983). Gas chromatographic methods
usually involve derivatization to facilitate separation and
increase sensitivity (Olufsen, 1979; Skarping et al., 1983a,b).
Detection limits in air samples range from 0.1 to 10 µg/m3.
Diaminotoluenes and their derivatives have been studied in
blood, urine, and liver cytosol preparations using thin-layer
chromatography and gas chromatography/mass spectrometry (GC/MS)
(Kiese & Rauscher, 1968; Kiese et al., 1968; Glinsukon et al.,
1975; Waring & Pheasant, 1976). A high-performance liquid
chromatographic method for the determination of diaminotoluenes in
urine and plasma has been described by Unger & Friedman (1979).
Table 5. Analytical methods for the determination of diaminotoluenes
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Water high-performance liquid chrom- 2,4-, 2,5-, 2,6-, 0.2 - 0.7 ng Riggin & Howard
atography with ultraviolet and 3,4-isomers (1983)
electrochemical detection
Air gas-liquid chromatography/ 2,4-isomer 3 µg/m3 Becher (1981)
nitrogen-phosphorus detector
on glass capillary columns
high-performance liquid chrom- 0.1 - Purnell et al.
atography with ultraviolet 10 µg/m3 (1982)
and electrochemical detection
high-performance liquid chrom- 2,4-isomer - Nieminen et al.
atography with ultraviolet (1983)
detection
gas-liquid chromatography with 2,4- and 2,6-isomers ~ 0.1 - Skarping et al.
electron capture detection on 0.4 pg (1983a)
glass capillary column
gas-liquid chromatography/ 2,4- and 2,6-isomers 10 - 20 pg Skarping et al.
nitrogen-phosphorus detector amine (1983b)
on glass capillary columns
Hair dyes gas-liquid chromatography/ 2,5-isomer 5 mg/litre Choudhary (1980)
flame ionization detector
thin-layer chromatography 2,4-, 2,5-, and 0.2 mg/litre Kottemann (1966)
3,4-isomer
high-performance liquid chrom- 2,4-, 2,5-, 0.5 mg/litre Johansson et al.
atography/ultraviolet detection and 2,6-isomers (1981)
high-performance liquid chrom- 2,4-, 2,5-, - Liem & Rooselaar
atography/ultraviolet detection and 3,4-isomers (1981)
------------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Hair dyes high-performance liquid chrom- 2,4- and 2,6-isomers 0.1 µg/litre Snyder et al.
(contd.) atography/ultraviolet detection (1982)
gas-liquid chromatography/mass
spectrometry
Polyurethane thin-layer chromatography/ 2,4- and 2,6-isomers 1 µg/g Guthrie &
foams fluorimetry McKinney (1977)
Biological high-performance liquid chrom- 2,4- and 2,6-isomers 2 mg/litre Unger & Friedman
tissues and atography/ultraviolet detection (1979)
fluids
Isomeric nuclear magnetic resonance all isomers - Mathias (1966)
mixtures spectrometry
thin-layer chromatography all isomers - Macke (1968)
gas-liquid chromatography/ all isomers - Boufford (1968)
flame ionization detector
gas-liquid chromatography/ all isomers - Willeboordse et
thermal detector al. (1968)
infra-red spectroscopy 2,4- and 2,6-isomers - Biernacka et al.
(1974)
------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Natural Occurrence
Diaminotoluenes are not known to occur as natural products.
3.2. Production
Currently, diaminotoluenes are produced commercially through
the catalytic hydrogenation of dinitrotoluenes. This procedure,
economic only for large-scale production, is used in the
manufacture of toluene diisocyanates. At dye plants, diamino-
toluenes are produced by the reaction of hydrochloric acid on
dinitrotoluenes, in the presence of an iron catalyst (Austin,
1974).
Most diaminotoluenes produced are used on site by the
manufacturer; therefore, published production figures do not
adequately reflect the true world production of diaminotoluenes.
Between 1972 and 1976, the average annual production of
diaminotoluenes in the USA was 89 x 106 kg, ranging from 76 x 106
kg in 1972 to 105 x 106 kg in 1976 (US ITC, 1977). Thereafter, the
production was estimated from the known production of toluene
diisocyanates ranging between 305 x 106 kg annually in the period
1977 - 81, and 360 x 106 kg in 1984 (US ITC, 1982, 1985).
Up to 1978, an estimated 180 - 200 x 106 kg of 2,4-DAT was
produced annually in western Europe (IARC, 1978). During
1971 - 75, the the annual production of 2,4-DAT in Japan was
approximately 210 x 103 kg; the compound was neither imported nor
exported (IARC, l978). However, in 1981, the production of 2,4-DAT
in Japan was estimated to have declined to 50 x 103 kg (CIC Japan,
1983).
3.3. Uses
Diaminotoluenes are used extensively within the chemical
industry as intermediates in the manufacture of widely different
commercial products (Table 6). Minor applications of diamino-
toluene isomers include their use as raw materials, co-reactants,
and curing agents. Toluene diisocyanates represent the largest
end-use accounting for more than 90% of the total annual production
of diaminotoluenes, largely a mixture of 2,4- and 2,6-isomers
(Backus, 1974; Milligan & Gilbert, 1978).
Diaminotoluenes are intermediates in the synthesis of dyes used
for textiles, furs, leathers, biological stains and indicators,
spirit varnishes and wood stains, and pigments. Previously used in
hair-dyes, 2,4-DAT was removed from use by many countries after it
was found to be a hepatocarcinogen in rats (Ito et al., 1969).
Meta-DAT is used to produce diethyltoluenediamine (DETDA) for the
manufacture of certain urethane elastomers (Milligan & Gilbert,
1978). Ortho-DAT is used to produce mercaptotoluimidazole (MTI)
and its zinc salt, both of which are used primarily as specialty
antioxidants in nitrile rubber elastomers (Gan et al., 1975).
Table 6. End-use application(s) of individual
diaminotoluene isomers
------------------------------------------------------
Application 2,3- 2,6- 2,3- 3,4- 2,5-
------------------------------------------------------
Toluene diisocyanate X X
(> 90% of total use of
diaminotoluenes)
Urethane co-reactants X X X X
(DAT-initiated polyols)
DETDAa X X
DYESb Xd X d
Tolyltriazole X X
Epoxy curing X X X
Mercaptotoluimidazole X X
Photographic developer X
------------------------------------------------------
a DETDA = Diethyltoluenediamine.
b DYES = Fur, leather, biological stains, indicators,
textiles, hair, spirit varnishes, wood stains, and
pigments.
c Use in hair dyes and cosmetics prohibited in USA
since 1971.
d Forbidden in Italy - 1978.
3.4. Release into the Environment, Distribution, and Transformation
Data are lacking on the extent of the global release of
diaminotoluenes, as well as their transport, distribution, and
degradation within the environment.
Releases of 2,4-DAT into the environment have been estimated in
the USA, the largest contribution being over 6 x 106 kg dumped in
authorized landfills. Releases of 1.4 x 106 kg were estimated to
occur from the production of diaminotoluenes, and 0.3 x 106 kg
during dye production and usage; unknown quantities of DAT may
derive from the hydrolysis of TDI released into the environment.
Information on the transport, distribution, and degradation of
DAT isomers under conditions approaching those found in natural
bodies of water have not been reported in the literature. However,
a bench-scale treatability study for 2,4-DAT using acclimated
sludge from a treatment plant showed that the isomers are
degradable. The observed total organic carbon removal was 45% in
4 h (Matsui et al., 1975).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
No information was available in the literature reviewed from
which environmental levels could be calculated. Two properties of
the diaminotoluenes are relevant to this problem. Since the vapour
pressure is low (Tables 4 and 5), the risk of contaminating the
environment through evaporation is minimal. However, air emissions
from inappropriately operated plants may pose a hazard. Since the
chemical is soluble in water, the potential for exposure through
water contamination is of concern. No data are available on levels
of diaminotoluenes in surface and groundwater, in soil, and/or air.
4.2. General Population Exposure
No information is available on the exposure of the general
population to diaminotoluenes.
4.3. Occupational Exposure
Filatova et al. (1970) reported concentrations of diamino-
toluenes in manufacturing plants of up to 0.2 mg/m3, with
occasional excursions up to 11 mg/m3.
The results of studies conducted in 3 plants manufacturing
diaminotoluenes in the USA showed that the work-place ambient air
levels ranged from 0.005 to 0.44 mg/m3 (NIOSH, 1980, 1981, 1982).
The highest level of diaminotoluenes (0.44 mg/m3) was found in the
filter room at one plant (NIOSH, 1980). A level of 0.39 mg
diaminotoluenes/m3 was measured in a sample taken at the breathing
zone of an operator in a second plant (NIOSH, 1981). All values
were calculated as time-weighted averages.
5. KINETICS AND METABOLISM
5.1. Studies on Experimental Animals
5.1.1. Absorption and retention
Skin penetration by test materials varied amoung species
(monkeys, swine), and was affected by vehicle and site of
application. In one study, [14C]-2,4-DAT (4 µg/cm2), dissolved in
acetone, methanol, or a skin lotion, was applied to 3 - 15 cm2 of
the ventral forearm, abdomen, or back of 3 - 6 monkeys/group (9
groups). The material was removed after 24 h by washing with soap
and water. The greatest absorption (53.8 ± 15.4%) resulted when
[14C]-2,4-DAT in acetone was applied to the abdominal skin of
monkeys (Marzulli et al., 1981). The permeability of diamino-
toluenes across the epidermis was highly dependent on the
formulation used. When 1.4 g of 2,5-DAT was applied in a gel
to the abdominal skin of dogs for a contact period of 3 h, 2.9%
(40 mg) was absorbed. Addition of hydrogen peroxide, similar to
the formulation used in hair dye, reduced the amount absorbed
to < 0.21% (Kiese et al., 1968) or < 0.13% (Hruby, 1977). The
hair in the exposed area retained 4% of the 14C activity, 5 days
after application (Hruby, 1977).
Hruby (1977) studied the absorption of [14C]-2,5-DAT following
oral and subcutaneous single-dose administrations to rats. Five
days following subcutaneous injection of 3 - 5 mg [14C]-2,5-DAT (in
water), 6.9% of the dose was found in the total-body homogenate and
1.7% remained at the injection site. Five days after oral (gavage)
administration of 10 mg [14C]-2,5-DAT (in water), the rat gastro-
intestinal tract retained 1.4% of the applied radioactivity and
1.2% was found in the total body homogenate (Hruby, 1977).
No studies on uptake after inhalation were found.
5.1.2. Distribution and reaction with body components
The distribution of diaminotoluenes and their reaction with
body components have been investigated, mainly after
intraperitoneal injection of radioactive labelled compounds. No
data on the distribution of diaminotoluenes and reaction with
tissues after inhalation or oral ingestion were found in published
reports.
Distribution of [14C]-2,4-DAT after intraperitoneal injection
was rapid, and the peak concentration in rat and mouse blood and
plasma occurred in 1 h, then decreased rapidly for 7 h. On a
comparative basis, all tissue concentrations of bound 14C were
considerably lower in the male NIH-Swiss mice than in the male
Fischer rats (Grantham et al., 1980).
Tissue distribution of [Me-14C]-2,4-DAT hydrochloride was
studied in male B6C3F1 mice given a single intraperitoneal
injection (1 µCi, 0.667 mg/kg body weight) (Unger et al., 1980).
The highest concentrations, 1/2 h after dosing, were found in the
kidneys, gonads, epididymis, lungs, muscle, and blood. One hour
after dosing, the liver contained the greatest amount, accounting
for nearly 12% of the dose. The concentration in the adrenal
glands exceeded that in the kidney, 1 and 2 h after dosing. High
concentrations of radioactivity were also observed in the
gastrointestinal tract.
Four hours after an intraperitoneal injection of 100 mg (0.8
mmol/kg, ring-labelled [3H]-2,4-DAT) in male Wistar rats, 0.3 nmol
was found covalently bound per mg liver protein. A similar degree
of binding was seen in the kidneys. Subcellular fractionation of
the liver showed that most of the bound material was in the
microsomal fraction (Dybing et al., 1978). No significant binding
to DNA in vitro or in vivo could be demonstrated using [3H]-2,4-
DAT, whereas it was found to bind covalently to hepatic RNA in
vivo. These findings were confirmed by Aune et al. (1979).
5.1.3. Metabolism
Glinsukon et al. (1975, 1976) found that the 2,4-isomer was
selectively N-acetylated at the p-amino group by liver cytosol
prepared from hamsters, guinea-pigs, rabbits, mice, and rats. The
cytosol from liver, kidney, intestinal mucosa, and lung of hamsters
and rabbits was studied for N-acetyl transferase activity using
2,4-DAT and 4-acetylamino-2-amino-toluene as substrates. All
tissues showed marked species differences in enzyme activity.
Tissues with high N-acetyl transferase levels, such as liver,
could produce both 4-acetylamino-2-aminotoluene and 2,4-
diacetylaminotoluene (Glinsukon et al., 1975). There were also sex
differences in the N-acetylation capacity of the liver cytosol.
After a single ip injection of 2,4-DAT (77 mg/kg body weight)
in male rats, 69.4% of the dose was eliminated in the urine and
faeces after 24 h as a complex mixture of metabolites, indicating
both free and conjugated derivatives. The major urinary
metabolites identified were 4-acetylamino-2-aminotoluene, 2,4-
diacetylaminotoluene, and 4-acetylamino-2-aminobenzoic acid. In
mice, oxidation of the methyl group to a benzoic acid was the major
reaction and the major urinary metabolites in mice were 4-
acetylamino-2-aminobenzoic, 4-acetylamino-2-aminotoluene, and 2,4-
diacetylaminobenzoic acid (Grantham et al., 1980). Waring &
Pheasant (1976) investigated the metabolism of 2,4-DAT in female
rabbits, rats, and guinea-pigs to determine whether the isomer gave
rise to hydroxylamines or aminophenols, which might account for the
observed toxic and carcinogenic effects. After oral administration
(gavage) of 2,4-DAT (50 mg/kg body weight), phenolic metabolites
were excreted in the urine. When free and conjugated metabolites
were combined, 5-hydroxy-2,4-DAT was the major metabolite in all 3
species (Table 7).
Table 7. Excretion of metabolites after dosing with 2,4-diamino-
toluene
-------------------------------------------------------------------
Metabolite Percentage dose excreteda
Rabbit Rat Guinea-pig
-------------------------------------------------------------------
2,4-DAT trace 1.3 trace
3-hydroxy-2,4-DAT 10 8 trace
5-hydroxy-2,4-DAT 22 12 9
6-hydroxy-2,4-DAT ( m-aminophenol) trace 5 trace
3-hydroxy-4-acetylamino-2-aminotoluene 10 18 trace
5-hydroxy-4-acetylamino-2-aminotoluene 6 14 17
glucuronide I, 3-hydroxy-DAT 10 16 15
glucuronide II, 5 hydroxy-DAT 32 12 46
glucuronide III, 6-hydroxy-DAT 2 6 4
unidentified phenolic compounds 0 trace trace
-------------------------------------------------------------------
a Results are given as percentage dose, average of 10 studies,
standard deviation 6.4% for metabolites 1 - 5, and 12.8% for
metabolites 6 - 8. Animals were dosed orally at 50 mg/kg; urine
was collected for 48 h.
From: Waring & Pheasant (1976).
The levels of methaemoglobin found in the rabbit, rat, and
guinea-pig correlated well with the total urinary excretion of
aminophenol. The methaemoglobin levels reached a peak 6 - 12 h
after the administration of 2,4-DAT and then slowly declined. The
highest levels of aminophenols and of methaemoglobin were found in
the rabbit (Waring & Pheasant, 1976).
5.1.4. Excretion
During a 24-h period of dermal contact with [14C]-2,4-DAT, 14C
urinary excretion in monkeys reached a peak at 8 - 12 h (Marzulli
et al., 1981).
Data from studies on the rat, rabbit, mouse, guinea-pig, and
dog exposed to diaminotoluenes (cutaneous, subcutaneous,
intravenous, intraperitoneal, or oral by gavage) showed fast
elimination rates (Kiese et al., 1968; Waring & Pheasant, 1976;
Hruby, 1977; Grantham et al., 1980; Unger et al., 1980). The
elimination of radioactivity from various tissues in rodents
followed a well-defined biphasic pattern. Rapid elimination over
7 h was followed by a rather slow decline in the isotopic contents
of tissues (Grantham et al., 1980; Unger et al., 1980). The half-
lives of tissue elimination during the fast phase were 0.89, 0.43,
and 1.51 h for male mouse liver, kidneys, and blood, respectively.
During the slow phase of elimination, the half-lives for liver,
kidneys, and blood in male mice were 11.7, 9.1, and 12.6 h,
respectively. The half-lives of elimination of radioactivity,
during the slow phase, were greater for muscle (23.9 h) and skin
(29.2 h) than for any other tissue (Wagner, 1975; Unger et al.,
1980).
The primary route of elimination in rodents was via the kidneys
during the first hour after exposure. However, after 2 h, the
predominant route shifted from urinary to faecal, probably a
reflection of biliary excretion. Only 1.25% of the administered
radioactivity had been exhaled after 24 h (Unger et al., 1980). On
a comparative basis, faecal elimination was greater in rats than in
mice, but the rate of urinary excretion was more rapid in mice than
in rats. Approximately 90% of a dose was eliminated in the urine
of mice in 24 h compared with 74% in the urine of rats (Grantham et
al., 1980). Complete elimination was accomplished in 2 days in
mice, while rats required 6 days.
Male and female rats, injected subcutaneously with 3 - 5 mg
[14C]-2,5-DAT hydrochloride, eliminated 65% of the dose in the
urine and 5% in faeces after 24 h. The same pattern of elimination
was found after oral administration of 10 mg of the labelled
compound (Hruby, 1977).
When beagle dogs were intravenously injected with a dose of
224 mg, infused over 3 h, the total amounts of radioactivity
eliminated in the urine and faeces were 60% and 19%, respectively.
After 4 days, elimination mainly occurred within the first 24 h.
After a skin application of 1.4 g [14C]-2,4-DAT for 3 h (in 50 ml
of a dye formulation), only 0.092 and 0.84% of the dose were
eliminated, respectively, in the urine and faeces of beagle dogs
over 4 days, reflecting the inhibitory effect of the dye
formulation on the absorption of the 2,4-DAT (Hruby, 1977). In
another study on dogs, about 40 mg 2,5-diaminotoluene was absorbed
through the skin from a gel containing 1.4 g of the material. The
addition of hydrogen peroxide to the gel reduced the amount
absorbed to less than 3 mg. The amount excreted unchanged in the
urine was 60 - 70 µg (Kiese et al., 1968).
5.2. Human Studies
As only limited information is available on the absorption,
distribution, metabolism, and excretion of diaminotoluenes in human
beings, these aspects are discussed together, rather than in
separate sections.
Although the high boiling point of diaminotoluenes makes
absorption through the lungs unlikely under normal working
conditions, inhalation may occur when hot vapours escape from
stills. Possible inhalation and dermal exposure to dusts may occur
if diaminotoluenes are handled in a less than optimal manner.
Since diaminotoluenes are soluble in water, absorption from the
gastrointestinal tract could occur following ingestion. However,
no data were found on the kinetics and metabolism of diamino-
toluenes after oral or inhalation exposures.
Skin penetration by [14C]-2,4-DAT was measured in human beings
(Marzulli et al., 1981). When 4 µg [14C]-2,4-DAT in acetone/cm2
was applied to the skin of the forearm, the highest absorption of
the chemical (23.7 % ± 16.1% of the applied dose) resulted after
24 h of dermal contact. Urinary excretion reached a peak after
4 - 8 h of skin contact. In a study by Kiese & Rauscher (1968),
the hair of 5 human subjects was dyed (40 min) with a formula
containing 2.5 g 2,5-DAT; absorption of approximately 0.2% of the
applied material occurred. No data were given on the retention and
distribution of diaminotoluenes after this dermal contact.
Data from studies on 6 volunteers (3 males and 3 females)
showed that, after subcutaneous injection of 5.54 mg 2,5-DAT, 47.6%
of the dose was excreted in the urine as N,N'-diacetyl-2,5-DAT.
The rate of excretion was highest during the first 24 h, and only a
trace appeared in the urine excreted on the third day, in one
study. When the compound was applied as a hair dye (40 min), the
highest rate of excretion was observed during a period of 5 - 8 h
after application. On average, a total amount of 3.7 mg N,N'-
diacetyl-2,5-DAT (i.e., 0.09% of the applied dose) was calculated
to have been excreted in urine taken over 2 days from 5 subjects
(Kiese & Rauscher, 1968).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Little information is available on the effects of diamino-
toluenes on animal populations found in the environment. The
effects of 2,4-DAT at concentrations ranging from 1 to 1000
mg/litre were observed for Daphnia ( Daphnia magna Straus),
ostracoda, guppies ( Lebistes reticulatus Peters), and channel
seaweed (Scenedesmus obliquus). Daphnia was the most sensitive
species; 5 mg/litre was lethal in 5 - 10 days, and prolonged
exposure to 2 mg/litre caused a reduction in the number of
offspring produced. A concentration of 20 mg/litre was not lethal
for ostracods after 10 days, but 50 mg/litre was lethal in 5 - 8
days. Fish survived for 10 days at 200 mg/litre, but a
concentration of 500 mg/litre was lethal in 2 - 3 days. The algae
tested were the most resistant, surviving for 10 days at a
concentration of 1000 mg/litre (Smirnova et al., 1967).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The early literature (1881 - 1939) contains several reports on
toxicological manifestations associated with the administration of
diaminotoluenes in experimental animals. Most of the papers are
difficult to interpret and to use in the assessment of chemical
hazards, because massive doses of chemicals of unknown purity and
isomeric composition were used, and because of the experimental
designs chosen. The toxic effects were characterized by icterus,
haemoglobinuria, disposition of haemosiderin in the spleen, bone
marrow, and liver, respiratory and generalized central nervous
system (CNS) depression, pulmonary and cerebral oedema, and
increased bile acids in the liver, blood, and/or urine of exposed
animals (Von Oettingen, 1941).
7.1. Single Exposures
Diaminotoluenes are considered to be dermal and eye irritants.
In studies on rabbits, 12.5 mg 2,5-DAT or 500 mg 2,4-DAT caused
skin irritation, defined as erythema and oedema, after 24 h of
dermal contact. Instillation of 100 µg of the 2,4-isomer into the
rabbit eye caused severe eye irritation within 24 h. Data showing
the extent of the acute toxicity of diaminotoluenes in various
laboratory animals are summarized in Tables 8 and 9.
The acute toxic effects of diaminotoluenes were characterized
by marked central nervous system depression during exposure (e.g.,
decreased locomotor activity, piloerection, ptosis, ataxia,
tremors) and production of methaemoglobin, 6 - 8 h after exposure.
Duodenal and glandular mucosal damage in the stomach were
observed in fed, unrestrained rats, 24 h following a single
subcutaneous dose of 3,4-DAT. The optimal ulcerogenic dose of 3,4-
DAT (i.e., the dose causing a low mortality and a maximal incidence
of duodenal damage within 24 h), was 350 mg/kg body weight (Perkins
& Green, 1975).
7.2. Short-Term Exposures
When guinea-pigs were treated with 1 - 10% 2,5-DAT (24 h/day
for 5 days, 2 days without treatment, followed by exposure for
another 5 days), sensitization was obtained in 35% of treated
animals (Schäfer et al., 1978).
Both the 2,4- and 3,4-isomers caused severe icterus in rats.
In male and female rats, 3,4-DAT (unlike the 2,4-isomer), given
orally or parenterally, produced a high incidence of perforating
duodenal ulcers within a few days (Selye, 1973). These effects
were obtained in animals allowed to move freely with access to food
and water during the period of observation. A dose of 500 mg
diaminotoluenes/kg body weight was administered in 2 ml water,
twice daily.
Table 8. Lethality of diaminotoluenes
---------------------------------------------------------------------------
Species Exposure LD50 Reference
route (mg/kg
body
weight)
---------------------------------------------------------------------------
Ortho-DAT (2,3-, 3,4- mix)
Rat oral 810 Carpenter et al. (1974)
Rabbit dermal 1120 Carpenter et al. (1974)
Meta-DAT (2,4-, 2,6- mix)
Rat oral 300 Izmerov et al. (1982)
Rat (male) oral 270 Weisbrod & Stephan (1983)
Mouse (male) oral 350 Weisbrod & Stephan (1983)
Rat (male) ip 230a Weisbrod & Stephan (1983)
Mouse (male) ip 240b Weisbrod & Stephan (1983)
Rat (male) iv 350 Weisbrod & Stephan (1983)
Mouse (male) iv 90-105 Weisbrod & Stephan (1983)
Rat dermal 1200 Izmerov et al. (1982)
2,4-DAT (technical grade)
Fischer rat (male) ip 325 Grantham et al. (1980)
NIH-Swiss mouse (male) ip 480 Grantham et al. (1980)
HaM/ICR mouse (male) ip 80 Weisburger et al. (1978)
HaM/ICR mouse (female) ip 90 Weisburger et al. (1978)
---------------------------------------------------------------------------
a A methaemoglobin level of 8.4% was observed, 6 h after ip administration.
b A methaemoglobin level of 7.8% was observed, 6 h after ip treatment.
Note: No published data on the acute toxicity of the 2,3-isomer were
available.
The effects of 2,4-DAT on the liver microsomal mixed-function
oxidase system, DT-diaphorase, and epoxide hydrolase were reported
by Dent & Graichen (1982). Following oral treatment with 2,4-DAT
at 70 mg/kg body weight per day for 5 days, the activities of
microsomal cytochrome P-450-dependent enzymes were depressed, while
epoxide hydrolase activity was markedly elevated (3 - 8 times
control) in male F-344 rats. Under these experimental conditions,
an increase in the liver to body weight ratio (3.2 - 4%), and in
the liver microsomal protein concentration (19.3 - 27.4 g/kg) were
induced by 2,4-DAT.
7.3. Long-Term Exposure
Oral administration of 2,4-DAT (see Table 11, section 7.6, for
doses) for 79 - 103 weeks accelerated the appearance of renal
toxicity in male F-344 rats, associated with a high incidence of
secondary hyperparathyroidism (NCI, 1979). The chronic renal
disease reported was believed to have decreased the longevity of
the treated rats, either directly or through inhibition of the
clearance of toxic metabolites (Cardy, 1979).
Table 9. Summary of some single-dose studies
--------------------------------------------------------------------------
Species Route of Dose Effects Reference
exposure (mg/kg
body
weight)
--------------------------------------------------------------------------
2,4-DAT (technical grade)
Wistar rat oral 50 produced metHba; highest Waring &
(male) amounts (5 - 6%) were Pheasant (1976)
found 6 - 8 h after
exposure
> 50 toxic
Sprague oral 500 developed icterus and Selye (1973)
Dawley rat death
(male and
female)
NZW rabbit oral 50 MetHb reached 18 - 20% Waring &
level 6 - 8 h after Pheasant (1976)
application
> 50 toxic
Dunkin- oral 50 MetHb reached 3 - 4% level Waring &
Harvey 6 - 8 h after application Pheasant (1976)
guinea-pig
> 50 toxic
3,4-DAT (97% pure)
Sprague subcut- 125-500 discrete, non-perforated Perkins &
Dawley rat aneous duodenal lesions were Green (1975)
(female) observed immediately
distal to the gastroduo-
denal junction 24 h
following the administra-
tion of a single dose
--------------------------------------------------------------------------
a metHb = methaemoglobin.
No studies were found on the effects of diaminotoluenes on the
nervous system or the immune system after long-term exposure.
7.4. Reproduction and Teratogenicity
7.4.1. Reproduction
There are 2 studies on experimental animals that evaluate the
reproductive toxicity of diaminotoluenes. Soares & Lock (1980)
administered 2,4-DAT orally or ip at 40 mg/kg body weight for
2 days to DBA/2J male mice. Forty-eight hours after treatment,
mating trials were conducted for 8 weeks. There were no treatment-
related effects on sperm morphology or fertility, as measured by
this dominant lethal assay. However, in male Sprague Dawley rats,
long-term exposure to 2,4-DAT in the feed impaired reproductive
performance and capacity (Thysen et al., 1985a,b). Dietary levels
of 0.03% 2,4-DAT for 10 weeks (~ 15 mg/kg body weight per day)
decreased fertility and exerted an inhibitory effect on sperm
production in male rats. Eleven weeks after treatment, the sperm
count remained significantly depressed ( P < 0.001), suggesting
irreversible damage to the germinal components in the testes. Data
from hormone analyses at the end of the 10 weeks of exposure, and
at the end of 11 weeks after treatment, showed a significant
decrease in serum-testosterone and an elevation of serum-luteinizing
hormone concentrations, which were associated with a reduction in
seminal vesicle weight. Histological changes found in the
reproductive organs from treated males were correlated with these
physiological changes. At a lower dose (0.01% or ~ 5 mg/kg body
weight), 2,4-DAT did not cause any of these toxic responses.
7.4.2. Teratogenicity
Studies on the teratogenic potential of diaminotoluenes are
summarized in Table 10. Skin application of 2,4-DAT induced a low
incidence of skeletal changes in rats (Burnett et al., 1976). Oral
or intraperitoneal administration of this isomer did not produce
any effects on the fertility or reproductive performance of male
mice (Soares & Lock, 1980). Subcutaneous or intraperitoneal
injection of 2,5-DAT in mice on day 8 of gestation, at levels of 50
or 75 mg/kg body weight, caused crainiofacial malformation and
fused or distorted thoracic vertebrae associated with the absence
of, or fused, ribs (Inouye & Murakami, 1976, 1977). However, 2,5-
DAT sulfate, at levels of 16 - 64 mg/kg body weight per day
administered subcutaneously on days 6 - 15 of gestation, did not
cause any malformations in mice or rats (Marks et al., 1981).
The results of oral administration of 2,6-DAT to rats and
rabbits, at doses of between 10 and 300 mg/kg body weight
(Knickerbocker et al., 1980), showed that, in rats, doses of
between 100 and 300 mg/kg body weight increased the occurrence of
incomplete vertebrae and that the highest dose resulted in missing
sternebrae and incomplete closure of the skull. A no-observed-
adverse-effect level of 10 mg/kg body weight was reported in rats.
No skeletal or soft-tissue abnormalities were observed in the
offspring of rabbits, but, using fetal toxicity indices, a no-
observed-adverse-effect level of 30 mg/kg body weight was reported.
Becci et al. (1983) administered Ortho-DAT (2,3-, 3,4-isomer
mixture) by gavage to rats and rabbits (Table 10). Reduced body
weight during gestation was noted at 300 mg/kg body weight in rats
and 100 mg/kg body weight in rabbits. An increased incidence of
several skeletal variations in the fetuses was noted, probably due,
in part, to the maternal toxicity. The no-observed-adverse-effect
level in both rats and rabbits was 30 mg/kg body weight.
Table 10. Teratogenicity studies with diaminotoluenes
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,4- Charles skin 2 ml/kg body weight skeletal changes seen in 6/169 Burnett et al.
(3% in River/CD on days 1, 4, 7, 10, live fetuses ( P > 0.05) (1976)
hair-dye rat 13, 16, and 19 of
formula) (female) gestation
2,5- Charles skin 2 ml/kg body weight no increase in abnormalities in Burnett et al.
(sulfate) River/CD on days 1, 4, 7, 10, treated groups (1976)
(3% in rat 13, 16, and 19 of
hair-dye gestation
formula)
2,5- JCL:ddn subcutaneous 50 mg/kg body weight In groups treated sc or ip on day Inouye & Murakami
dihydro- mice or intraper- on one day of days 8 of gestation, there was evidence (1976, 1977)
chloride (female) itoneal 7 - 14 of gestation of craniofacial malformation:
single dose or 75 mg/kg on day exencephaly, prosoposchisis, and
8 of gestation or hair lip with cleft palate, and
50 mg/kg on day 8 of high incidence of skeletal
gestation malformation: fused or distorted
thoracic vertebrae associated with
absence of, or fused, ribs; no
such malformed fetuses were found
in groups treated on days 10 - 14
of gestation; only a very low
incidence of vertebral and rib
anomalies followed treatment on
day 7 or 9; maternal toxicity was
reported at 75 mg/kg but not at
50 mg/kg
2,5- CD-1 mice subcutaneous 16, 32, 48, or 64 no teratogenic effects were noted; Marks et al.
(sulfate) mg/kg body weight maternal toxicity was evident at (1981)
per day on days 48 and 64 mg/kg; reduced fetal
6 - 15 of gestation weight was noted at > 32 mg/kg
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,5- rat oral (gavage) 10, 50, or 80 mg/kg maternal toxicity and embryo- Spengler et al.
(sulfate) body weight per toxicity evident at 80 mg/kg; no (1986)
day on day 15 of effects observed at lower doses
gestation
rabbit oral (gavage) 10, 25, or 50 mg/kg no effects observed
body weight per day
on days 6 - 18 of
gestation
2,6- Sprague oral (gavage) 10, 30, 100, or 300 no effects on pregnancy, number Knickerbocker et
Dawley mg/kg body weight of live fetuses, and resorption al. (1980)
rat per day on days sites/dam; 300 mg/kg produced
6 - 15 of gestation smaller body weight gain in the
dams; 30 - 300 mg/kg produced
increased haemorrhagic abdomens
in the fetuses; 100 and 300 mg/kg
increased the occurrence of
incomplete vertebrae, and 300
mg/kg showed missing sternebrae
and incomplete skull closure in
the fetuses; the no-observed-
adverse-effect dose was 10 mg/kg
per day
2,6 Dutch oral (gavage) 3, 10, 30, or 100 100 mg/kg per day reduced dam Knickerbocker et
belted mg/kg body weight weights, increased resorptions, al. (1980)
rabbit per day on days decreased fetal weights, and
(female) 6 - 18 of gestation neonatal survival;
oral (gavage) 3, 10, 30, or 100 there were no differences in Knickerbocker et
mg/kg body weight skeletal or soft-tissue al. (1980)
per day on days abnormalities between treated
6 - 18 of gestation animals and controls; the no-
observed-adverse-effect dose was
30 mg/kg per day
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
o-DAT Sprague oral 10, 30, 100, or 300 maternal toxicity was indicated Becci et al.
(2,3-, Dawley mg/kg body weight at 300 mg/kg per day by reduced (1983)
3,4- rat per day on days body weight gain during gestation;
isomer 6 - 15 of gestation No significant differences in
mix) numbers of live fetuses,
implantation or resorption sites;
fetal body weight was reduced at
the highest dose ( P < 0.05); no
evidence of teratogenic effects
or effects on dams at doses < 30
mg/kg; no skeletal or soft-tissue
malformations that could be
related to treatment; however,
increased incidence of missing
sternebrae at 300 mg/kg per day
and incomplete ossified vertebrae
at 100 and 300 mg/kg per day were
noted compared with controls
o-DAT Dutch oral 3, 10, 30, or 100 maternal toxicity at 100 mg/kg per Becci et al.
(2,3-, belted mg/kg body weight day elicited by reduced body (1983)
3,4- rabbit per day on days weight gain during pregnancy; no
isomer 6 - 18 of gestation significant difference in the
mix) number of implantations; at 100
mg/kg per day, fetal body weight
was reduced and the number of
resorption sites was increased;
no skeletal or soft-tissue
malformations that could be
related to treatment were noted
-----------------------------------------------------------------------------------------------------------------
7.5. Mutagenicity and Related End-Points
7.5.1. DNA damage
At concentrations of 1 x 10-4 mol/litre and below, 2,4-DAT, but
not 2,6-DAT, induced unscheduled DNA synthesis in primary cultures
of rat hepatocytes (Bermudez et al., 1979). 2,4-DAT produced a
significant elevation in unscheduled DNA synthesis at 2 and 12 h in
the in vivo/in vitro hepatocyte DNA repair assay (Mirsalis &
Butterworth, 1982; Mirsalis et al., 1982). 2,5-DAT produced a
positive response in a DNA-repair assay in rat hepatocytes and a
weak positive response in hamster hepatocytes at 10-5 mol, the
highest concentration that was not toxic to the cells that were
tested (Kornbrust & Barfknecht, 1984).
Shooter & Venitt (1979) used continuous administration of 2,4-
DAT in the drinking-water to determine whether phosphotriesters
could be detected in the DNA of the liver of treated rats.
Positive results were obtained at 10 mg/litre. A low, but
significant, level of these lesions was produced. Shooter &
Venitt's studies on rodents indicated that methyl- and
ethylphosphotriesters persist for many weeks in the DNA of certain
organs (notably liver, kidney, and lung) and that such lesions are
not eliminated by DNA repair.
The results of studies by Greene et al. (1981) showed that
2,4-, 2,5-, 2,6-, and 3,4-isomers significantly inhibited the
incorporation of [125I]-iododeoxyuridine into mouse testicular DNA
and demonstrated dose-response characteristics. The 2,4-, 2,5-,
and 3,4-isomers were capable of reaching the testes and of passing
target cell membranes at this site. They concluded that the 3
isomers may present a genetic health hazard for an intact animal.
The inhibition induced by 2,6-DAT might have been caused by a
chemically-induced decrease in body temperature (Greene et al.,
1981).
DNA damage was not found in human cultured fibroblasts after
exposure to 100 µmol 2,4-DAT alone (3.5 ± 1.5 increase in percent
single-strand DNA). When the cells were incubated in the presence
of 1 mg ram seminal vesicle microsomes/ml and 100 µmol arachidonic
acid, a significant increase in the fraction of single-strand DNA
(21.3 ± 3.7, P < 0.001) was found in cells exposed to 2,4-DAT.
DNA strand breaks were not induced when prostaglandin synthase
(PGS) was inhibited by adding indomethacin (100 µmol) or
acetylsalicylic acid (1 mmol) (Nordenskjöld et al., 1984). These
results, which suggest that 2,4-DAT may be activated by PGS to form
products that cause DNA damage in cultured human fibroblasts, are
in agreement with the findings of Rahimtula et al. (1982).
7.5.2. Mutation
Several studies have shown that 2,4-, 2,6-, and 2,5-isomers can
induce reverse mutations in Salmonella typhimurium strains TA 1538
and TA 98, in the presence of various metabolic activation systems
(McCann et al., 1975; Dybing & Thorgeirsson, 1977; Dybing et al.,
1977; Pienta et al., 1977b; Cinkotai et al., 1978; Aune et al.,
1979; Shahin et al., 1980). However, Mori et al. (1982) showed
that 2,4-DAT was inactive for strains TA 98 and TA 100 at doses
ranging from 5 to 1000 µg/plate. While 2,3-DAT is inactive in S.
typhimurium (Florin et al., 1980), its homologue 3,4-DAT showed a
marginal response in strains TA 98 and TA 1538 (Greene et al.,
1979).
2,4-DAT was shown to be a weak mutagen in Drosophila
melanogaster, inducing sex-linked recessive lethals when fed to
adult males at a concentration of 15.2 mmol (Blijleven, 1977;
Venitt, 1978). In a study reported by Fahmy & Fahmy (1977), 2,4-
DAT was injected around the testes of adult male Drosophila at
doses ranging from 5 to 20 mmol. Mutagenicity was measured at the
various stages of spermatogenesis, both on the X-chromosome and RNA
genes. The overall induced frequency of X-recessives was extremely
low. It was also observed that mutation yield was not dose-related
and that it was maximal in the earliest progeny fraction,
suggesting a greater toxicity for mature sperm.
2,4-DAT was mutagenic in L5178Y mouse lymphoma cells and CHO-
AT3-2 cells (Matheson & Creasy, 1976; Coppinger et al., 1984).
Mutagenic activity was observed in L5178Y cells, only in the
absence of exogenous metabolic activation, but was observed in CHO-
AT3-2 cells both with and without activation.
The in vivo mutagenic activity of 2,4-DAT was studied in
DBA/2J male mice by the dominant lethal assay, sperm abnormality
assay, and the recessive spot test. Mice were administered the
compound by intraperitoneal injection and orally by gavage (2 daily
doses of 40 mg/kg body weight), just before mating (Soares & Lock,
1980). No induction of dominant lethals was noted, nor was any
increase in abnormal sperm or recessive spots reported.
No dominant lethals were induced in Charles River rats injected
intraperitoneally, 3 times weekly for 8 weeks, with 20 mg 2,5-
DAT/kg body weight, before mating (Burnett et al., 1977).
7.5.3. Cell transformation
Several studies have shown that the 2,4-, 2,5-, 2,6-, and 3,4-
isomers can induce morphological transformations in Syrian golden
hamster embryo cells (Pienta et al., 1977a; Greene & Friedman,
1980). Each isomer chemically transformed secondary hamster embryo
cells, but none were active in more than 50% of the 5 or 6 separate
tests performed on each isomer (Greene & Freidman, 1980).
7.5.4. Chromosomal effects
Cytogenetic preparations were made from the bone marrow of male
mice, 30 and 48 h after intraperitoneal injection of 2 daily doses
of 2,4-DAT at 40 mg/kg body weight. The treatment did not induce
any obvious chromosome breaks (Soares & Lock, 1980).
2,5-DAT did not induce micronucleated cells in bone marrow
after oral administration of 120 mg/kg body weight to male and
female rats, in 2 doses separated by an interval of 24 h (Hossack &
Richardson, 1977).
7.6. Carcinogenicity
Several long-term studies on the carcinogenic potential of the
2,4-, 2,5-, and 2,6-DAT isomers have been published. The
experimental designs used in these studies are summarized in Table
11. The experimental designs used in 2 studies on the carcinogenic
effects of hair dye formulations are also given in Table 11.
Although the early studies of Umeda (1955) and Ito et al.
(1969) used protocols that generated data of minimal use for a
hazard evaluation, they did produce qualitative information showing
that 2,4-DAT was carcinogenic for rats. These studies have been
extended to the 2,5-, 2,6-, as well as 2,4-DATs using more animals
per study and well-defined protocols (NCI, 1978, 1979, 1980;
Weisburger et al., 1978).
Groups of 25 male Charles River/CD rats were administered 2,4-
DAT in the diet at time-weighted levels of 300 and 625 mg/kg for 18
months. Similarly, groups of 25 male and female CD-1 mice were
given diets containing 500 and 1000 mg 2,4-DAT/kg for 18 months
(Weisburger et al., 1978). The rats and mice used in these studies
had a high incidence of spontaneous tumours. However, there was a
statistically significant increase in subcutaneous fibromas in male
rats and hepatocellular carcinomas and vascular tumours in male and
female mice compared with controls.
Studies by the US National Cancer Institute on the
carcinogenicity of 2,4-DAT in rats and mice (NCI, 1979) confirmed
the report by Weisburger et al. (1978). Administration of time-
weighted average doses of 79 and 176 mg 2,4-DAT/kg diet to groups
of 50 male and 50 female Fisher 344 rats, for 103 weeks, led to a
severe depression in body weight gain, high mortality, and a dose-
related development of hepatocellular carcinomas or neoplastic
nodules in treated rats of both sexes. In addition, NCI reported
that carcinomas and adenomas of the mammary gland occurred in
female rats at incidences that were dose related and significantly
greater than those in the controls in both the high- and low-dose
groups. Groups of 50 male and 50 female B6C3F1 mice were similarly
administered 2,4-DAT at 100 or 200 mg/kg diet, for 101 weeks. In
male mice, tumour incidence was not significantly increased
compared with that in the control animals. However, the incidence
of hepatocellular carcinomas in female mice in both treated groups
was dose related and significantly higher than that in the
controls. Numbers of lymphomas were also higher in low-dose female
mice. On the basis of these results, it was concluded that 2,4-DAT
was carcinogenic for Fisher 344 rats of both sexes and for female
B6C3F1 mice (NCI, 1979).
Table 11. Experimental designs of carcinogenicity studies on diaminotoluenes
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,4- rat (male and female) 20 subcutaneous; 2 mg in 0.5 ml Umeda (1955)
propylene glycol weekly;
total of 28 injections; 452
days
2,4- Wistar rat (male) 12/12 (6) oral (diet); 0.6 and 1 g/kg; Ito et al.
36 weeks (1969)
2,4- Charles River/CD rat 25/25 (25) oral (diet); 500 and 1000 Weisburger et
(male) mg/kg for 4 months and 250 al. (1978)
Charles River mouse 25/25 (25) and 500 mg/kg for 14 months;
(male) oral (diet); 500 and 1000
Charles River mouse 25/25 (25) mg/kg for 18 months
(female)
2,5- Fisher 344 rat (male) 50/50 (50/25) oral (diet); 600 and 2000 NCI (1978)
Fisher 344 rat (female) 50/50 (50/25) mg/kg for 78 weeks + 31
weeks observation
B6C3F1 mouse (male) 50/50 (50/50) oral (diet); 600 and 2000
B6C3F1 mouse (female) 50/50 (50/50) mg/kg for 78 weeks + 19
weeks observation
2,4- Fisher 344 rat (male) 50/50 (20) oral (diet); 125 and 250 NCI (1979)
Fisher 344 rat (female) 50/50 (20) mg/kg for 40 weeks reduced
to 50 and 100 mg/kg for 63
weeks (time-weighted-average
79 and 176 mg/kg); high-dose
males killed after 79 weeks
and high-dose females after
84 weeks
2,4 B6C3F1 mouse (male) 50/50 (20) oral (diet); 100 and 200 NCI (1979)
B6C3F1 mouse (female) 50/50 (20) mg/kg for 101 weeks
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,6- Fisher 344 rat (male) 50/50 (50) oral (diet) 250 and 500 NCI (1980)
Fisher 344 rat (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
B6C3F1 mouse (male) 50/50 (50) oral (diet); 250 and 500
B6C3F1 mouse (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
2,5- Sprague Dawley rat (male) 50 (50) dermal application twice Kinkel &
(formulations Sprague Dawley rat 50 (50) weekly of 0.5 g of synthetic Holzmann (1973)
with 6% (female) formulation containing 4%
hydrogen 2,5-DAT mixed with equal
peroxide volume of 6% H202; treated 2
added) years
2,5- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(equal mixture male and ml weekly of formulation to (1975)
female) which equal volume of 6%
H202 had been added;
treatment period, 18 months
2,5- + 2,4- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(formulations (equal mixture male and ml weekly of formulation to (1975)
with 6% female) which equal volume of 6%
hydrogen H202 had been added;
peroxide treatment period, 18 months
added) (same control group as
above study)
---------------------------------------------------------------------------------------------------------
The carcinogenic potential of 2,5-DAT and 2,6-DAT for rats and
mice was determined by the US National Cancer Institute. After
administration of 2,5-DAT at 600 and 2000 mg/kg feed to groups of
50 male and 50 female Fisher 344 rats and 50 male and 50 female
B6C3F1 mice, for 78 weeks, there was not sufficient evidence to
demonstrate the carcinogenicity of 2,5-DAT (NCI, 1978). However,
the study had been curtailed and this reduced the potential of the
test for detecting carcinogenicity.
Using a similar protocol, 2,6-DAT was incorporated at levels of
250 and 500 mg/kg into the diet of groups of 50 male and 50 female
Fisher 344 rats, and B6C3F1 mice for 103 weeks (NCI, 1980). There
was some question of whether mice of either sex received a maximum
tolerated dose, but the dose given to rats appeared to be at the
maximum tolerated level. As reported by NCI (1980), islet-cell
adenomas of the pancreas and neoplastic nodules or carcinomas of
the liver occurred in male rats in dose-related trends that were
significant using the Cochran-Armitage test, but not using the
Fisher exact test. In the low-dose male mice, NCI reported that
the incidence of lymphomas was greater than that in the controls.
However, the incidence was not significant when the Bonferroni
criterion of multiple comparison was used. Similarly, the
occurrence of hepatocellular carcinomas in female mice was dose
related, but not significant by the Fisher exact test, when the
incidence in the high-dose groups was compared with that in the
controls. Under the conditions of the bioassay, it was concluded
that 2,6-DAT was not carcinogenic for male and female F344 rats or
for male and female B6C3F1 mice (NCI, l980). Summaries of the 3
NCI bioassays have been published by Cardy (1979), Reuber (1979),
and Sontag (1981).
Using the protocols outlined in Table 11, no evidence of
carcinogenicity was obtained when hair-dye formulations containing
2,5- and 2,5- plus 2,4-DAT were painted on the skin of rats and
mice (Kinkel & Holzmann, 1973; Burnett et al., 1975). Given the
duration of exposure, the amounts of diaminotoluenes placed on the
skin, and the use of hydrogen peroxide prior to administration,
such negative results would be expected in studies on the formula
containing 2,4-DAT (Burnett et al., 1975). These studies have
shown a low order of dermal toxicity for these hair dyes, even
after long-term exposure. However, no definitive statement can be
made on the carcinogenic potential of 2,4- and 2,5-DAT after dermal
administration.
8. EFFECTS ON MAN
8.1. Single and Short-Term Exposures
In human beings, as in animals, diaminotoluenes are considered
to be irritants for the mucous membranes and skin, and to lead to
conjunctivitis and corneal opacities. When solutions come into
contact with skin, they can cause irritation, severe dermatitis,
and blistering (Von Oettingen, 1941). In case of the inhalation of
fumes, coughing, dyspnoea, and respiratory distress can result. No
data are available for evaluating the sensitizing potential of
diaminotoluenes. In the case of ingestion of massive amounts,
nausea, vomiting, and diarrhoea would occur, with the possible
production of methaemoglobinaemia. No cases of human poisoning
from short-term exposures to 2,4-DAT have been documented in the
published literature.
8.2. Long-Term Occupational Exposure - Epidemiological Studies
Filatova et al. (1970) investigated the physiological and
biochemical status of workers in a plant manufacturing
diaminotoluenes. Fifty-two of the 59 workers (58 males and 1
female) had worked in the plant for > 2 1/2 years. Seventeen
workers were between 30 and 45 years of age and 42 workers were
under 30 years old. In general, all workers had equal exposure to
the toxic chemicals used at the plant, namely, dinitrotoluene,
diaminotoluenes, methanol, and o-dichlorobenzene. There were a
few complaints of headache (2 cases), excess coughing (2 cases),
stomach pains (2 cases), and chest pain (4 cases); however, the
majority of the workers did not exhibit any exposure-related
adverse effects. Nevertheless, the investigators concluded that
the 10 workers exhibiting the symptoms listed were indeed affected
by their exposure to the complex of chemicals at this factory. It
is impossible to delineate the role played by the diaminotoluenes
in the production of these adverse effects.
The US National Institute for Occupational Safety and Health
(NIOSH) evaluated the reproductive health of workers in 3 plants
manufacturing diaminotoluenes (NIOSH, 1980, 1981, 1982). Exposure
usually involved both DAT and dinitrotoluene (DNT). All 3 surveys
were conducted in response to requests from employees or their
unions. The reason for the first request was the workers' belief
that their wives were suffering increased rates of spontaneous
abortion. The other 2 studies were prompted by the publicity given
to the first study.
In 2 of the studies, environmental hygiene sampling took place
(section 4.2) and workers were invited to volunteer for a medical
examination, to complete a reproductive history questionnaire, and
to provide semen and blood samples. Semen samples were analysed
for volume, sperm count, and sperm morphology. Blood samples were
analysed for various markers of renal and hepatic function, neither
of which showed any significant inter-group differences in any of
the studies. Wives of workers were given a more detailed
reproductive history questionnaire.
In the first study (NIOSH, 1980), there were 44 volunteers, 30
of whom provided usable semen specimens. The total potential study
population was not given. Of the 30, 9 were exposed, 9 were
controls, and 12 were in an intermediate category. The rate of
spontaneous abortions was higher among the wives of exposed workers
(6/18 pregnancies while the husband was exposed) compared with the
controls (4/23 pregnancies), with 6/28 for the intermediate group.
The small number of congenital malformations was not exposure
related. The sperm count for the exposed (median = 49 million) was
significantly ( P < 0.03) lower than that for the control group
(median = 121 million); however, the latter figure was unusually
high. The exposed group showed a significant reduction in the
proportion of the large morphological type. The second study
involved only a reproductive history questionnaire and the
reporting of hygiene data by the company. Thirty-five out of 41
workers in DAT- and DNT-production areas were interviewed. The
rates of congenital abnormalities or spontaneous abortion did not
significantly differ between exposure groups. Where the husband
was employed in DNT production, 1 out of 9 pregnancies ended in a
spontaneous abortion; the equivalent data for DAT was 1 miscarriage
out of 14 pregnancies.
In the third study, 50 volunteers were examined, 41 of whom
provided semen specimens. The total eligible population was not
given, though it was reported that 25 workers were regularly
exposed, 15 of whom participated in the study. There were no
significant differences in sperm count morphology between exposure
groups, but the miscarriage rate was reported to be significantly
( P < 0.05) higher for the wives of workers in the DAT-exposed area
of the plant, where 6 out of 15 pregnancies ended in miscarriage
compared with 1 out of 7 for the wives of DNT-exposed workers, and
3 out of 38 for the wives of unexposed workers.
The ranges of levels of reported exposures overlapped between
the 3 studies and were all within the OSHA recommended standard of
1.5 mg/m3 for dinitrotoluenes (NIOSH, 1982). The first study might
have been expected to show an excess, because it was provoked by a
cluster of miscarriages. All the studies were of limited size and
subject to some risk of selection bias, because the population was
restricted to those who volunteered. Also, in all 3 studies, crude
figures, with no adjustment for age, were presented. However, in
the 2 follow-up studies, where apparently neither of the
populations held a prior belief that there was an excess
miscarriage rate, it is significant that an apparent excess of
miscarriages was found in the wives of DAT-exposed workers.
An epidemiological assessment of the reproduction hazards for
males after occupational exposure to diaminotoluenes and
dinitrotoluenes was carried out by Hamill et al. (1982). Reported
occupational exposures were similar to those reported by NIOSH
(1980, 1981, 1982) and were generally well within the OSHA
recommended level of 1.5 mg/m3 for dinitrotoluenes. Examination of
84 workers and 119 unexposed subjects consisted of semen analysis,
blood testing, medical examination, and an interview. Seventy-two
percent of non-vasectomized exposed workers provided semen samples.
These groups of workers were defined by exposure intensity,
frequency, and recency, and compared with controls. Although no
significant differences in miscarriage rates were reported between
exposure categories, the categories were as defined at the time of
the study, not at the time of pregnancy. Fertility rates were
reported to be unaffected by exposure, but no figures were given.
There were no statistically significant differences in semen
analysis, sperm count and morphology, and FSH levels, between the 3
exposure groups and unexposed workers. The authors concluded that
the results of their study suggested that no detectable
reproductive effects existed among male workers exposed to
dinitrotoluenes and diaminotoluenes.
Levine et al. (1985) reported an analysis of the fertility of
workers exposed to DNT and DAT. The approach taken consisted of
workers completing reproductive history questionnaires and of
observed births being compared with expected births for the married
workers. Expected births were derived from US birth rates by age,
calendar year, and parity, and the ratio of observed to expected
was expressed as a standardized fertility ratio (SFR). Populations
of 137, 207, and 235 persons, respectively, were studied and were
largely, but not exclusively, male. It is not clear whether any of
the plants were the same as those described above. Comparisons of
SFR between different exposure categories, both for the whole
population and also among individuals who spent at least part of
their reproductive life exposed, did not reveal any significant
effects on SFR between exposure groups. The authors estimated that
the power of this study to detect a 50% reduction in fertility
would have been 90%. In the third NIOSH study, the miscarriage
rate for wives of DAT-exposed workers was 6.8 times higher than
that for wives of unexposed workers (rates given as miscarriages
per 100 person-years), but the fertility rate was only 0.8 times
lower (ratio of rates of live births per 100 person-years). Thus,
overall fertility may not be a sensitive index of adverse
reproductive outcome.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1. Evaluation of Human Health Risks
9.1.1. General considerations
There are insufficient data on the effects of diaminotoluenes
on human beings to carry out a detailed hazard assessment or risk
evaluation. However, absorption and metabolic studies in human
beings have indicated that diaminotoluenes are rapidly absorbed,
metabolized, and excreted in the urine in a manner similar to that
found in experimental animals. Therefore, the risk evaluation that
follows is based largely on data from animals, supported by data
from human studies, where available.
9.1.2. Assessment of exposure
Diaminotoluenes can be absorbed through the skin and
gastrointestinal tract, and by inhalation. Given the properties of
this class of chemicals, the major route of human exposure is
dermal, in the work-place, with a possibility of the inhalation of
fumes during heating. Exposure through ingestion is minimal,
except in case of accidents.
No data exist on general ambient levels of diaminotoluenes in
air, water, and food. Bioaccumulation of diaminotoluenes in the
food-chain should not occur. Levels in the work-place air of up to
0.44 mg/m3, with occasional excursions up to 11 mg/m3, have been
reported.
9.1.3. Single and short-term exposures
Diaminotoluenes are classed as toxic, highly irritant
chemicals. The oral LD50 for animals is between 270 and 350 mg/kg
body weight. Dermal contact has been shown to cause irritation,
severe dermatitis, blistering, and possible skin sensitization.
Single oral doses of diaminotoluenes of 50 mg/kg body weight have
led to methaemoglobinaemia in rats, rabbits, and guinea-pigs. Eye
contact with diaminotoluenes has led to conjunctivitis and corneal
opacities. In case of inhalation of fumes, coughing, dyspnoea, and
respiratory distress can result.
9.1.4. Long-term exposure
9.1.4.1. Carcinogenicity and mutagenicity
No epidemiological data are available on the incidence of
cancer in human beings after exposure to diaminotoluenes.
Several studies using 2,4-DAT have been carried out on
experimental animals and, in each, the isomer was shown to be
carcinogenic for rats and mice. In the most recent study, doses
of, or greater than, 79 mg/kg diet led to an increase in
hepatocellular carcinomas or neoplastic nodules in rats; there was
an increase in hepatocellular carcinomas and lymphomas in female
mice at doses exceeding 100 mg/kg diet.
Using a similar protocol, the US NCI concluded that 2,6-DAT was
not carcinogenic for rats and mice after administration of up to
500 mg/kg diet for 103 weeks. It should be noted that
hepatocellular carcinomas, neoplastic nodules, and lymphomas were
detected, as in the bioassay for 2,4-DAT, however, they were
considered not significant after detailed statistical analyses.
There was no evidence of carcinogenicity in mice and rats after
administration of 2,5-DAT at levels of up to 2000 mg/kg diet, for
78 weeks. However, the short duration of the study reduced the
potential of the test for detecting carcinogenicity. No evidence
of carcinogenicity was noted after the dermal application of hair-
dye formulations containing 2,5-DAT (following application of
hydrogen peroxide) or a mixture of 2,5-DAT and 2,4-DAT with
hydrogen peroxide.
Positive mutagenic activity was noted in S. typhimurium when
2,4-, 2,5-, 2,6-, and 3,4-DAT were tested. In addition, DAT
isomers were mutagenic in mammalian cells in vitro. Significant
DNA damage was produced by 2,4-DAT in human cultured fibroblasts,
only after activation by prostoglandin synthase. The isomer 2,4-
DAT was weakly mutagenic in Drosophila melanogaster and induced
unscheduled DNA synthesis in primary rat hepatocytes in vitro.
The 2,4- and 2,5-isomers were inactive in in vivo mammalian
mutagenicity assays. Micronuclei and dominant lethals were not
produced by 2,5-DAT, and 2,4-DAT did not produce chromosomal
breaks, dominant lethals, abnormal sperm morphology, or recessive
spots.
It has been shown that 2,4-, 2,5-, and 2,6-DAT can inhibit DNA
synthesis in the testes after ip injection of high doses. On the
basis of this study, 2,4-DAT may pose a genetic hazard in addition
to its potential to cause adverse effects on reproduction.
9.1.4.2. Reproduction and teratogenicity
The results of limited studies on the reproduction hazards for
male workers exposed to diaminotoluenes are equivocal. In surveys
of reproductive outcome in 3 plants, an excess of spontaneous
abortions among the wives of male workers exposed to DAT and
dinitrotoluene was reported in 2 surveys, though these excesses
were based on small numbers, and not all workers in the plants
participated in the studies. In 1 out of the 3 plants studied,
some adverse effects on spermatogenesis were suggested. Analysis
of the overall fertility of workers in 3 other production plants
did not reveal any adverse effects from exposure to DAT.
In a study on animals fed 2,4-DAT, there was a significant and
persistent decrease in the sperm count.
Embryotoxicity was observed in animal studies after oral and
dermal doses exceeding 30 mg/kg body weight for the 2,3-and 3,4-
isomers and 10 mg/kg body weight for 2,6-DAT.
Skeletal changes were noted after dermal application of a hair-
dye formula containing 3% 2,4-DAT at 2 ml/kg body weight.
9.2. Evaluation of Effects on the Environment
Information is lacking concerning levels of diaminotoluenes in
the environment, and their transport, bioconcentration,
biotransformation, and biodegradation.
A few data indicate that diaminotoluenes may be hazardous for
aquatic organisms. No data on the effects of diaminotoluenes on
other non-mammalian targets in the environment could be found.
9.3. Conclusions
Diaminotoluenes are highly irritating to the skin and eyes and
the fumes are irritating to the respiratory tract. They are
readily absorbed through the skin. Methaemoglobinaemia may occur
in exposed individuals. Renal toxicity after oral administration
of 2,4-DAT has been reported in experimental animals. 2,4-DAT has
been shown to be carcinogenic for animals, but there is inadequate
evidence to evaluate the carcinogenic potential of 2,5- and 2,6-
diaminotoluene. All 3 of these isomers have been shown to be
mutagenic. Limited data are available concerning a reproductive
hazard for male workers handling DATs. DATs have been shown to
impair spermatogenesis in experimental animals and to be both
embryotoxic and teratogenic.
10. RECOMMENDATIONS
1. Monitoring should be undertaken to determine the sources,
levels, and fate of diaminotoluenes in the environment.
Ecotoxicity data should be collected.
2. For a better evaluation of occupational exposure and effects,
studies on the uptake, kinetics, and metabolism of DAT and the
relevant routes of exposure are important to provide a sound
basis for biological monitoring.
3. To assist in the development of appropriate health
surveillance systems, a systematic evaluation of the toxicity
of diaminotoluenes should be carried out to compliment
available data on carcinogenicity and reproductive effects.
4. Additional data should be obtained on human morbidity and
mortality related to exposure to diaminotoluenes, with
particular emphasis on carcinogenic, teratogenic, and
reproductive end-points.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1978) evaluated the data on the carcinogenicity of
diaminotoluenes and concluded that there was sufficient evidence of
the carcinogenicity of 2,4-diaminotoluene in experimental animals.
An evaluation of additional data by IARC (1986) further supported
this conclusion.
In the absence of case reports or epidemiological studies,
there was inadequate data to assess the carcinogenicity of
diaminotoluenes for human beings (IARC, 1978).
REFERENCES
AUNE, T., NELSON, S.D., & DYBING, E. (1979) Mutagenicity and
irreversible binding of the hepatocarcinogen 2,4-diaminotoluene.
Chem.-biol. Interact., 25(1): 23-24.
AUSTIN, G.T. (1974) Industrially significant organic chemicals.
Part 9. Chem. Eng., 11: 96-100.
BACKUS, J.K. (1974) Urethanes. In: Considine, D.M., ed.
Chemical and process technology encyclopedia, New York, McGraw
Hill Book Co., pp. 1121-1125.
BECCI, P.J., REAGAN, E.L., KNICKERBOCKER, M.J., BARBEE, S.J.,
& WEDIG, J.H. (1983) Teratogenesis study of o-toluenediamine
in rats and rabbits. Toxicol. appl. Pharmacol., 71: 323-329.
BECHER, G. (198l) Glass capillary columns in the gas
chromatographic separation of aromatic amines. 2. Application
of samples from workplace atmospheres using nitrogen-selective
detection. J. Chromatogr., 211(1): 103-111.
BERMUDEZ, E. & BUTTERWORTH, B.E. (1979) Analysis of the
activities of 2,4-diaminotoluene and 2,4- and 2,6-dinitrotoluene
in the primary hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 1: 168-169.
BERMUDEZ, E., TILLERY, D., & BUTTERWORTH, B.E. (1979) Effect
of 2,4-diaminotoluene and isomers of dinitrotoluene on
unscheduled DNA synthesis in primary rat hepatocytes. Environ.
Mutagen., 1: 39l-398.
BIERNACKA, T., SEKOWSKA, B., & MICHONSKA, J. (1974) [Determination
of 2,4- and 2,6-diaminotoluene in technical products by infra-red
spectroscopy.] Chem. Anal. (Warsaw), 19: 6l9-632 (in Polish).
BLIJLEVEN, W.G.H. (1977) Mutagenicity of four hair dyes in
Drosophila melanogaster. Mutat. Res., 48: 181-186.
BOUFFORD, C.E. (l968) Determination of isomeric diaminotoluenes
by direct gas liquid chromatography. J. Gas Chromatogr., 6:
438-440.
BUIST, J.M. (1970) Isocyanates in industry. Proc. R. Soc. Med.,
63: 365-366.
BURNETT, C., LANMAN, B., GIOVACCHINI, R., WOLCOTT, G., & SCALA, R.
(1975) Long-term toxicity studies on oxidation hair dyes.
Food Cosmet. Toxicol., 13(3): 353-357.
BURNETT, C., GOLDENTHAL, E.I., HARRIS, S.B., WAZETER, F.X.,
STRAUSBURG, J., KAPP, R., & VOELKER, R. (1976) Teratology
and percutaneous toxicity studies on hair dyes. J. Toxicol.
environ. Health, 1: 1027-1040.
BURNETT, C., LOEHR, R., & CORBETT, J. (1977) Dominant lethal
mutagenicity study on hair dyes. J. Toxicol. environ. Health,
2: 657-662.
CARDY, R.H. (1979) Carcinogenicity and chronic toxicity of
2,4-toluenediamine in F344 rats. J. Natl Cancer Inst., 62:
1107-1116.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F., Jr (1974)
Range-finding toxicity data. Toxicol. appl. Pharmacol., 28:
313-319.
CHOUDHARY, G. (1980) Gas-liquid chromatographic determination
of toxic diamines in permanent hair dyes. J. Chromatogr., 193:
277-284.
CIC JAPAN (1983) Commercial industrial chemicals, Tokyo,
Chemical Daily Co., Ltd.
CINKOTAI, K.I., JONES, C.C., TOPHAM, J.C., & WATKINS, P.A. (1978)
Experience with mutagenic tests as indicators of carcinogenic
activity. Mutat. Res., 53: 167-168.
COPPINGER, W.J., BRENNAN, S.A., CARVER, J.H., & THOMPSON, E.D.
(1984) Locus specificity of mutagenicity of 2,4-diaminotoluene in
both L5l78Y mouse lymphoma and AT3-2 chinese hamster ovary cells.
Mutat. Res., 135: 115-123.
CRC (1975) CRC handbook of chemistry and physics, 56th ed.,
Cleveland, Ohio, CRC Press.
DENT, J.G. & GRAICHEN, M.E. (1982) Effect of hepatocarcinogens
on epoxide hydrolase and other xenobiotic metabolizing enzymes.
Carcinogenesis, 3(7): 733-738.
DYBING, E. & THORGEIRSSON, S.S. (1977) Metabolic activation
of 2,4-diaminoanisole, a hair dye component. 1. Role of
cytochrome P-450 metabolism in mutagenicity in vitro. Biochem.
Pharmacol., 26: 729-734.
DYBING, E., AUNE, T., & SODERLUND, E.J. (1977) Use of the
Salmonella mutagenicity test in drug metabolism studies. Acta
pharmacol. toxicol., 41: 31.
DYBING, E., AUNE, T., & NELSON, S. (1978) Covalent binding
of 2,4-diaminoanisole and 2,4-diaminotoluene in vivo. Toxicol.
Aspects Food Saf. Arch. Toxicol., Suppl. 1: 213-217.
FAHMY, M.J. & FAHMY, O.G. (1977) Mutagenicity of hair dye
components relative to the carcinogens benzidine in Drosophila
melanogaster. Mutat. Res., 56: 31-38.
FILATOVA, V.S., TUBINA, A.Ya., SHARONOVA, Z.V., GOLOVA, I.A.,
FILINA, V.I., & DOROFEEVA, E.D. (1970) [Hygienic aspects of
work and health of workers in the production of toluylene-
diamine.] Gig. i Sanit., 35(2): 16-30 (in Russian with English
summary).
GAN, L.H., BLAIS, P., CARLSSON, D.J., SUPRUNCHUCK, T., & WILES, D.M.
(1975) Physicochemical characterization of some fully aromatic
polyamides. J. appl. polym. Sci., 19: 69-82.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., WEISBURGER, E.K., &
ROLLER, P.P. (1975) Enzymic N-acetylation of 2,4-toluenediamine
by liver cytosols from various species. Xenobiotica, 5(8): 475-483.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., LEWIS, N.L., &
WEISBURGER, E.K. (1976) N-acetylation as a route of 2,4-
toluenediamine metabolism by hamster liver cytosol. Biochem.
Pharmacol., 25: 95-97.
GRANTHAM, P.H., MOHAN, L., BENJAMIN, T., ROLLER, P.P., MILLER, J.R.,
& WEISBURGER, E.K. (1980) Comparison of the metabolism of 2,4-
toluenediamine in rats and mice. J. environ. Pathol. Toxicol.,
3(1-2): 149-l66.
GREENE, E.J. & FRIEDMAN, M.A. (1980) In vitro cell transformation
screening of 4 toluene diamine isomers. Mutat. Res., 79: 363-375.
GREENE, E.J., FRIEDMAN, M.A., & SHERROD, J.A. (1979) In vitro
mutagenicity and cell transformation testing of four toluene
diamine isomers. Environ. Mutagen., 1: 194.
GREENE, E.J., SALERNO, A.J., & FRIEDMAN, M.A. (1981) Effect
of 4 toluene diamine isomers on murine testicular DNA synthesis.
Mutat. Res., 91: 75-79.
GUTHRIE, J.L. & MCKINNEY, R.W. (1977) Determination of 2,4-
and 2,6-diaminotoluene in flexible urethane foams. Anal.
Chem., 49: 1676-1680.
HAMILL, V.V., STEINBERGER, E., LEVINE, R.J., RODRIGUEZ-RIGAU,
L.J., LEMESHOW, S., & AVRUNIN, J.S. (1982) The epidemiologic
assessment of male reproductive hazard from occupational
exposure to TDA and DNT. J. occup. Med., 24: 982-993.
HOSSACK, D.J.N. & RICHARDSON, J.C. (1977) Examination of the
potential mutagenicity of hair dye constituents using the
micronucleus test. Experientia (Basel), 33: 377-378.
HRUBY, R. (1977) The absorption of p-toluenediamine by the
skin of rats and dogs. Food Cosmet. Toxicol., 15: 595-599.
IARC (1978) 2,4-Diaminotoluene. In: Some aromatic amines and
related nitro compounds: hair dyes, colouring agents, and
miscellaneous industrial compounds, Lyons, International
Agency for Research on Cancer, pp. 83-95 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 16).
IARC (1986) Some chemicals used in plastics and elastomers,
Lyons, International Agency for Research on Cancer, p. 304
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 39).
INOUYE, M. & MURAKAMI, U. (1976) Teratogenicity of 2,5-
diaminotoluene, a hair dye component in mice. Teratology, 14:
241-242.
INOUYE, M. & MURAKAMI, U. (1977) Teratogenicity of 2,5-
diaminotoluene, a hair dye constituent in mice. Food Cosmet.
Toxicol., 15: 447-451.
ITO, N., HIASA, Y., KONISHI, Y., & MARUGAMI, M. (1969) The
development of carcinoma in liver of rats treated with
m-toluylenediamine and the synergistic and antagonistic
effects with other chemicals. Cancer Res., 29: 1137-1145.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982)
Toxicometric paramaters of industrial toxic chemicals under
single exposure, Moscow, USSR/SCST/USSR Commission for UNEP,
Centre of International Projects.
JOHANSSON, K., RAPPE, C., LINDBERG, W., & NYGREN, M. (1981)
Determination of aromatic diamines in hair dyes using liquid
chromatography. In: Egan, H., Fishbein, L., O'Neil, I.K.,
Castegnaro, M., & Bartsch, H., ed. Environmental carcinogens
selected methods of analysis. Volume 4: Some aromatic amines
and azo dyes in the general and industrial environment. Lyons,
International Agency for Research on Cancer (IARC Publications
No. 40).
KIESE, M. & RAUSCHER, E. (1968) The absorbtion of p-toluene-
diamine through human skin in hair dyeing. Toxicol. appl.
Pharmacol., 13: 325-331.
KIESE, M., RACHOR, M., & RAUSCHER, E. (1968) The absorption
of some phenylenediamines through the skin of dogs. Toxicol.
appl. Pharmacol., 12: 495-507.
KINKEL, H.J. & HOLZMANN, S. (1973) Study of long-term
percutaneous toxicity and carcinogenicity of hair dyes
(oxidizing dyes) in rats. Food Cosmet. Toxicol., 11: 641-648.
KNICKERBOCKER, M., RE, T.A., PARENT, R.A., & WEDING, J.H.
(1980) Teratogenic evaluation of ortho-toluenediamine
(o-TDA) in Sprague Dawley rats and Dutch Belted rabbits.
Toxicologist, 19: A.89.
KORNBRUST, D.J. & BARFKNECHT, T.R. (1984) Comparison of 7
azo dyes and their reduction products in the rat and hamster
hepatocyte primary culture/DNA-repair assays. Mutat. Res.,
136: 255-266.
KOTTEMANN, C.M. (1966) Two dimensional thin-layer
chromatographic procedure for the identification of dye
intermediates in arylamine oxidation hair dyes. J. Assoc. Off.
Anal. Chem., 49(5): 954-959.
LEVINE, R.J., CORSO, D.D., & BLUNDEN, P.B. (1985) Fertility
of workers exposed to dinitrotoluene and toluenediamine at
three chemical plants. In: Rickert, D.E., ed. Toxicity of
nitroaromatic compounds, Washington DC, Hemisphere.
LIEM, D.H. & ROOSELAAR, J. (1981) HPLC of oxidation hair
colours. Mitt. Geb. Lebensm. Hyg., 72: 164-176.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975)
Detection of carcinogens as mutagens in the Salmonella microsome
test: Assay of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72:
5135-5139.
MACKE, G.F. (1968) Use of tetracyanoethylene as a thin-layer
chromatographic spray reagent. J. Chromatogr., 36: 537-539.
MARKS, T.A., GUPTA, B.N., LEDOUX, T.A., & STAPLES, R.E.
(1981) Teratogenic evaluation of 2-nitro-p-phenyl-enediamine,
4-nitro-o-phenylendiamine, and 2,5-toluenediamine sulfate in
the mouse. Teratology, 24: 253-265.
MARZULLI, F.N., ANJO, D.M., & MAIBACH, H.I. (1981) In vivo
skin penetration studies of 2,4-toluenediamine, 2,4-diamino-
anisole, 2-nitro-p-phenylenediamine, p-dioxane and n-nitro-
sodiethanolamine in cosmetics. Food Cosmet. Toxicol., 19: 743.
MATHESON, D. & CREASY, B. (1976) Use of the L5178Y (TK+/-)
mouse lymphoma cell line coupled with an in vitro microsomal
enzyme activation system to study chemical promutagens. Mutat.
Res., 38: 400-401.
MATHIAS, A. (1966) Analysis of diaminotoluene isomer
mixtures by nuclear magnetic resonance spectrometry. Anal.
Chem., 38: 1931-1932.
MATSUI, S., MURAKAMI, T., SASAKI, T., HIROSE, Y., & IGUMA, Y.
(1975) Activated sludge degradability of organic substances
in the wastewater of the Kashima petroleum and petrochemical
industrial complex in Japan. Prog. Water Technol., 7:645-659.
MILLIGAN, B. & GILBERT, K.E. (1978) Diaminotoluenes. In:
Kirk-Othmer encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 1, pp. 321-329.
MIRSALIS, J.C. & BUTTERWORTH, B.E. (1982) Induction of
unscheduled DNA synthesis in rat hepatocytes following in vivo
treatment with dinitrotoluene. Carcinogenesis, 3: 241-245.
MIRSALIS, J.C., TYSON, C.M., & BUTTERWORTH, B.E. (1982)
Detection of genotoxic carcinogens in the in vivo/in vitro
hepatocyte DNA repair assay. Environ. Mutagen., 4: 553-562.
MORI, M.A., MIYAHARA, T., TANIGUCHI, K., HASEGAWA, K., KOZUKA,
H., MIYAGOSHI, M., & NAGAYAMA, T. (1982) Mutagenicity of
2,4-dinitrotoluene and its metabolites in Salmonella typhimurium.
Toxicol. Lett., 13: 1-5.
NCI (1978) Bioassay of 2,5-toluenediamine sulfate for
possible carcinogenicity, Bethesda, Maryland, National Cancer
Institute (NCI Carcinogenesis Technical Report Series No. 126;
DHEW Publication No. (NIH) 78-1381).
NCI (1979) Bioassay of 2,4-diaminotoluene for Possible
Carcinogenicity, Bethesda, Maryland, National Cancer Institute
(NCI Carcinogenesis Technical Report Series No. 162).
NCI (1980) Bioassay of 2,6-toluenediamine dihydrochloride
for possible carcinogenicity, Bethesda, Maryland, National
Cancer Institute (NCI Carcinogenesis Technical Report Series
No. 200; NTP No. 80-20; NIH Publication No. 80-1756).
NIEMINEN, E.H., SAARINAN, L.H., & LAAKSO, J.T. (1983)
Simultaneous determination of aromatic isocyanates and some
carcinogenic amines in the work atmosphere by reversed-phase
high-performance liquid chromatography. J. liq. Chromatogr.,
6: 453-469.
NIOSH (1978) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Service, Center for Disease Control,
Vol. 4 (Publication No. 78-175).
NIOSH (1980) Health hazard evaluation determination report
HE 79-113-728, Brandenburg, Kentucky, Olin Chemical Company,
and Cincinnati, Ohio, National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1981) Interim Report No. 1. HETA 81-118,
New Martinsville, West Virginia, Mobay Chemical Corporation,
and Cincinatti, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1982) Interim report No. 1. HETA 81-295-1155,
Moundsville, West Virginia, Allied Chemical Company, and
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NORDENSKJOLD, M., ANDERSSON, B., RAHIMTULA, A., & MOLDEUS, P.
(1984) Prostaglandin synthesis-catalyzed metabolic activation
of some aromatic amines to genotoxic products. Mutat. Res.,
127: 107-112.
OLUFSEN, B. (1979) Glass capillary columns in the gas
chromatographic separation of aromatic amines. I. J. Chromatogr.,
179: 97-103.
OSHA (1983) Code of federal regulations, Washington DC, US
Occupational Safety and Health Administration, pp. 660-664
(29CFR, 19101000, Table Z-1).
PERKINS, W.E. & GREEN, T.J. (1975) Effect of 3,4-toluenediamine
on output from in situ rat brunner's glands pouches (38941).
Proc. Soc. Exp. Biol. Med., 149: 991-994.
PIENTA, R.J., POILEY, J.A., & LEBHERZ, W.B., III (1977a)
Morphological transformation of early passage golden Syrian
hamster embryo cells derived from cryopreserved primary
cultures as a reliable in vitro bioassay for identifying
diverse carcinogens. Int. J. Cancer, 19: 642-655.
PIENTA, R.J., SHAH, M.J., LEBHERZ, W.B., & ANDREWS, A.W.
(1977b) Correlation of bacterial mutagenicity and hamster
cell transformation with mutagenicity induced by 2,4-
toluenediamine. Cancer Lett., 3: 45-52.
PURNELL, C.J. & WARWICK, C.J. (1981) Application of electro-
chemical detection in high-performance liquid chromatography
to the measurement of toxic substances in air. Anal. Proc.,
18: 151-154.
PURNELL, C.J., BAGON, D.A., & WARWICK, C.J. (1982) The
determination of organic contaminant concentrations in
workplace atmospheres by high-performance liquid chromatography.
Pergamon Ser. environ. Sci., 7: 203-219.
RAHIMTULA, A., MOLDEUS, P., ANDERSSON, B., & NORDENSKJOLD, M.
(1982) Prostaglandin synthetase catalyzed DNA strand breaks
by aromatic amines. In: Prostaglandins and related lipids,
New York, Alan R. Liss, Vol. 2, pp. 159-162.
REUBER, M.D. (1979) Carcinomas of the liver in female mice
fed toluene-2,4-diamine. Gann, 70: 453-457.
RIGGIN, R.M. & HOWARD, C.C. (1983) High performance liquid
chromatographic determination of phenylenediamines in aqueous
environmental samples. J. liq. Chromatogr., 6: 1897-1905.
SCHAFER, U. METZ, J., PEVNY, I., & ROCKL, H. (1978)
[Attempts of sensitizing guinea pigs with five different
derivates of para-substituted benzene.] Arch dermatol. Res.,
216: 153-161 (in German).
SELYE, H. (1973) Production of perforating duodenal ulcers
by 3,4-toluenediamine in the rat. Proc. Soc. Exp. Biol. Med.,
142: 1192-1194.
SHAHIN, M.M., BUGAUT, A., & KALOPISSIS, G. (1980) Structure-
activity relationship within a series of m-diaminobenzene
derivatives. Mutat. Res., 78: 25-31.
SHOOTER, K.V. & VENITT, S. (1979) Phosphotriesters in DNA:
non-repairable lesions as markers for the early detection of
chemical carcinogens in intact animals. Mutat. Res., 64: 106.
SKARPING, G., RENMAN, L., & SMITH, B.E.F. (1983a) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. I. Determination of
aromatic amines as perfluoro-fatty acid amines using electron-
capture detection. J. Chromatogr., 267: 315-327.
SKARPING, G., RENMAN, L., & DALENE, M. (1983b) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. II Determination of
aromatic amines as perfluoro-fatty acid amines using nitrogen-
selective detection. J. Chromatogr., 270: 207-218.
SMIRNOVA, A.N., TOROPOVA, L.A., & DOBROVOL'SKAYA, V.V.
(1967) [Effect of toluylenediamine and ortho-toluidine on
aquatic organisms.] Vodosnarzh Kanaltz Giurotekh, 5: 17-23 (in
Russian) (EPA translation).
SNYDER, R.C. & BREDER, C.V. (1982) High performance liquid
chromatographic determination of 2,4- and 2,6-toluenediamine
in aqueous extracts. J. Chromatogr., 236: 429-440.
SNYDER, R.C., BRUMLEY, W.C., BREDER, C.V., & FAZIO, T.
(1982) Gas chromatographic and gas chromatographic-mass
spectrometric confirmation of 2,4- and 2,6-toluenediamine
determined by liquid chromatography in aqueous extracts. J.
Assoc. Off. Anal. Chem., 65(6): 1388-1394.
SOARES, E.R. & LOCK, L.F. (1980) Lack of an indication of
mutagenic effects of dinitrotoluenes and diaminotoluenes in
mice. Environ. Mutagen., 2: 111-124.
SONTAG, J.M. (1981) Carcinogenicity of substituted-benzene-
diamines (phenylenediamines) in rats and mice. J. Natl Cancer
Inst., 66: 591-602.
SPENGLER, J., OSTERBURG, I., & KORTE, R. (1986) Abstract:
Teratogenic evaluation of p-toluenediamine sulphate.
Resorcinal and p-aminophenol in rats and rabbits. Teratology,
33(2): 31.
THYSEN, B., BLOCH, E., & VARMA, S.K. (1985a) Reproductive
toxicity of 2,4-toluenediamine in the rat. 2. Spermatogenic
and hormonal effects. J. Toxicol. environ. Health, 16: 763-769.
THYSEN, B., VARMA, S.K., & BLOCH, E (1985b) Reproductive
toxicity of 2,4-toluenediamine in the rat. 1. Effect on male
fertility. J. Toxicol. environ. Health, 16: 753-761.
UMEDA, M. (1955) Production of rat sarcoma by injections of
propylene glycol solution of m-toluylenediamine. Gann, 46:
597-606.
UNGER, P.D. & FRIEDMAN, M.A. (1979) High-performance liquid
chromatography of 2,6- and 2,4-diaminotoluene, and its
application to the determination of 2,4-diaminotoluene in
urine and plasma. J. Chromatogr., 174: 379-384.
UNGER, P.D., SALERNO, A.J., NESS, W.C., & FRIEDMAN, M.A.
(1980) Tissue distribution and excretion of
2,4[143]-toluenediamine in the mouse. J. Toxicol. environ.
Health, 6: 107-114.
US EPA (1980) Materials balance for 2,4-diaminotoluene.
Level 1. Preliminary, Washington DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/13:
79-016).
US ITC (1977) Synthetic organic chemicals: US production and
sales, 1975, Washington DC, US International Trade Commission,
pp. 22, 36, 42, 49, 60, 62, 65, 74 (US ITC Publication 804).
US ITC (1982) Preliminary report on US production of
selected synthetic organic chemicals. Preliminary totals 1981,
Washington DC, US International Trade Commission (SOC Series
C/P-82-1).
US ITC (1985) Preliminary report on US production of
selected synthetic organic chemicals. November, December, and
cummulative totals, 1984, Washington DC, US International
Trade Commission (SOC Series C/P-85.1).
VENITT, S. (1978) Mutagenicity of hair dyes: some more
evidence and the problems of its interpretation. Mutat. Res.,
53: 278-279.
VON OETTINGEN, W.F. (1941) The aromatic amino and nitro
compounds: their toxicity and potential dangers. A review of
the literature, Washington DC, Division of Industrial Hygiene,
National Institute of Health, pp. 55-63.
WAGNER, J.C. (1975) Linear compartment models. In:
Fundamentals of clinical pharmacokinetics, Hamilton, Illinois,
Drug Intelligence Publications, pp. 57-63.
WARING, R.H. & PHEASANT, A.E. (1976) Some phenolic metabolites
of 2,4-diaminotoluene in the rabbit, rat and guinea-pig.
Xenobiotica, 6: 257-262.
WEISBROD, D. & STEPHAN, U. (1983) [Studies of the toxic,
methaemoglobin-producing and erythrocyte-damaging effects of
diaminotoluene after a single administration.] Z. gesamte
Hyg., 29: 395-397 (in German).
WEISBURGER, E.K., RUSSFIELD, A.B., HOMBURGER, F., WEISBURGER,
J.H., BOGER, E., VAN DONGEN, C.G., & CHU, K.C. (1978)
Testing of twenty-one environmental aromatic amines or
derivatives for long-term toxicity or carcinogenicity.
J. environ. Pathol. Toxicol., 2(2): 325-356.
WILLEBOORDSE, F., QUICK, Q., & BISHOP, E. (1968) Direct gas
chromatographic analysis of isomeric diaminotoluenes. Anal.
Chem., 40: 1455-1458.
WILLIAM, R.T. (1971) The metabolism of certain drugs and
food chemicals in man. Ann. N.Y. Acad. Sci., 179: 141-154.
WILLIAMS, G.M. (1977) Detection of chemical carcinogens by
unscheduled DNA synthesis in rat liver primary cell cultures.
Cancer Res., 37: 1845-1851.
WILLIAMS, G.M. (1978) Further improvements in the hepatocyte
primary culture DNA repair test for carcinogens: detection of
carcinogenic biphenyl derivatives. Cancer Lett., 4: 69-75.
WILLIAMS, G.M. & LASPIA, M.F. (1979) The detection of
various nitrosamines in the hepatocyte primary culture/DNA
repair test. Cancer Lett., 66: 199-206.
Commercial grades of diaminotoluenes are available; however,
the most commonly marketed diaminotoluenes are: (a) "crude"
diaminotoluenes-mixture, containing all 6 isomers (Table 1); (b)
Meta-diaminotoluene (Meta-DAT), containing approximately 80% 2,4-
and 20% 2,6-isomers (also produced in smaller amounts as 65:35
mixture); and (c) Ortho-diaminotoluene (Ortho-DAT), consisting of
approximately 40% 2,3- and 60% 3,4-isomers. All commercial grades
contain traces of the other isomers; therefore, diaminotoluenes and
their mixtures are reviewed together in this document.
Most of the common and trade names for commercial
diaminotoluenes are listed in Table 2.
Table 2. Diaminotoluenes synonyms and trade names
----------------------------------------------------------------------------
A. Commercial mixtures
I. Meta-Diaminotoluenes
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names benzenediamine,ar-methyl (RTECS: TDB);
diaminotoluene (RTECS: TDB);
phenylenediamine,ar-methyl- (TDB);
Meta-diaminotoluene; Meta-toluene-
diamine (MTD);
toluene-ar,ar-diamine (8CI) (CAS: TDB);
toluenediamine (RTECS: TDB, DOT);
tolylenediamine (RTECS, TDB)
Common name diaminotoluene; toluenediamine; TDA
II. Ortho-Diaminotoluene
Chemical abstract name benzenediamine,ar-methyl- (9CI)
Other chemical names o-TDA
Common name Ortho-toluenediamine; OTD
B. Diaminotoluene isomers
I. 2,3-isomer
Chemical abstract name 1,2-benzenediamine,3-methyl- (9CI)
Other chemical names toluene-2,3-diamine (8CI) (CAS: TDB);
1-methyl-1,2,3-phenylenediamine (TDB);
1,2-diamino-3-methylbenzene (TDB);
2,3-diaminotoluene (TDB*);
2,3-toluylenediamine (TDB);
2,3-tolylenediamine (TDB);
3-methyl-o-phenylenediamine (TDB);
3-methyl-1,2-phenylenediamine (TDB)
Common names 2,3-TDA
II. 2,4-isomer
Chemical abstract name 1,3-benzenediamine,4-methyl- (9CI)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
II. 2,4-isomer (contd.)
Other chemical names m-toluenediamine (RTECS);
m-toluylendiamin (Czech, RTECS: TDB);
m-toluylenediamine (RTECS: TDB);
m-tolyenediamine (RTECS: TDB);
m-tolylenediamine (RTECS);
Meta-toluylene diamine (RTECS: TDB);
toluene-2,4-diamine (8CI) (CAS, RTECS:
TDB*);
tolylene-2,4-diamine (RTECS: TDB);
1,3-diamino-4-methylbenzene (RTECS: TDB);
2,4-diamino-1-methylbenzene (RTECS: TDB);
2,4-diamino-1-toluene (RTECS: TDB);
2,4-diaminotoluen (Czech, RTECS: TDB);
2,4-diaminotoluene (MESH, RTECS: TDB);
2,4-diaminotoluol (RTECS: TDB);
2,4-tolamine (RTECS: TDB);
2,4-toluenediamine (MESH, RTECS: TDB);
2,4-toluylenediamine (DOT, RTECS: TDB);
2,4-tolylenediamine (RTECS: TDB);
3-amino- p-toluidine (RTECS: TDB);
4- m-tolylenediamine (RTECS: TDB);
4-methyl- m-phenylenediamine (RTECS: TDB);
4-methyl-1,3-benzenediamine (RTECS: TDB);
5-amino- o-toluidine (RTECS: TDB)
Common names TDA; MTD; 2,4-TDA (CAS, RTECS: TDB)
Trade names Azogen Developer H; Benzofur MT;
C.I. Oxidation Base (RTECS);
C.I. Oxidation Base 20;
C.I. Oxidation Base 35 (RTECS);
C.I. Oxidation Base 200;
Developer B (RTECS: TDB); Developer DB
(RTECS: TDB);
Developer DBJ (RTECS: TDB); Developer H;
Developer MC (RTECS: TDB); Developer MT
(RTECS: TDB);
Developer MT-CF (RTECS: TDB);
Developer MTD (RTECS; TDB);
Developer T (RTECS: TDB); Developer 14;
Eucanine GB (RTECS: TDB); Fouramine;
Fouramine J (RTECS: TDB); Fourrine M
(RTECS: TDB);
Fourrine 94 (RTECS: TDB);
Lekutherm-Haerter VP-KU 6546;
Nako TMT (RTECS: TDB); NCI-C02302 (RTECS:
TDB);
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
Trade names (contd.) Pelagol J (RTECS: TDB); Pelagol Grey J
(RTECS: TDB);
Pontamine Developer TN (RTECS: TDB);
Renel MD (RTECS: TDB); Tertral G;
Zoba GKE (RTECS: TDB);
Zogen Developer H (RTECS: TDB).
Colour index number 76035
III. 2,5-isomer
Chemical abstract name 1,4-benzenediamine,2-methyl-(9CI)
Other chemical names p-toluenediamine (MESH, RTECS: TDB);
p-toluylendiamine (RTECS: TDB);
P,m-tolylenediamine (RTECS: TDB);
para-meta-tolylenediamine;
para-toluenediamine;
para-toluylenediamine;
para-tolylenediamine;
toluene-2,5-diamine (8CI) (CAS, RTECS:
TDB);
toluylene-2,5-diamine (RTECS: TDB);
2-methyl- p-phenylenediamine (RTECS: TDB);
2-methyl-1,4-benzenediamine (RTECS: TDB*);
2,5-diaminotoluene (MESH, RTECS: TDB);
2,5-toluenediamine;
4-amino-2-methylaniline (RTECS: TDB)
Common name 2,5-TDA
Trade names Oxidation Base 4 (as sulfate)
Colour index number C.I. 76042 (RTECS); 76043 (as sulfate)
IV. 2,6-isomer
Chemical abstract name 1,3-benzenediamine,2-methyl- (9CI)
Other chemical names m-phenylenediamine-2-methyl;
toluene-2,6-diamine (8CI) (CAS, RTECS:
TDB);
tolylene-2,6-diamine;
1,3-benzenediamine;
2-methyl- m-phenylenediamine (TDB);
2-methyl-1,3-benzenediamine (TDB);
2-methyl-1,3-phenylenediamine (TDB);
2,6-diamino-1-methylbenzene (TDB);
2,6-diaminotoluene (MESH, RTECS: TDB*);
2,6-toluenediamine;
2,6-toluylenediamine (RTECS: TDB);
2,6-tolylenediamine (RTECS: TDB)
----------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
IV. 2,6-isomer (contd.)
Common names 2,6-TDA
V. 3,4-isomer
Chemical abstract names 1,2-benzenediamine,4-methyl- (9CI)
Other chemical names o-toluenediamine;
toluene-3,4-diamine (8CI) (CAS, RTECS:
TDB);
1,2-benzenediamine,4-methyl- (RTECS: TDB);
1,2-diamino-4-methylbenzene (TDB);
3,4-diamino-1-methylbenzene (TDB);
3,4-diaminotoluene (RTECS: TDB);
3,4-toluenediamine;
3,4-toluylenediamine (RTECS);
3,4-tolylenediamine (RTECS: TDB);
4-methyl- o-phenylenediamine (TDB);
4-methyl-1,2-benzenediamine (TDB);
4-methyl-1,2-diaminobenzene (TDB);
4-methyl-1,2-phenylenediamine (TDB)
Common name 3,4-TDA
VI. 3,5-isomer
Chemical abstract name 1,3-benzenediamine,5-methyl- (9CI)
Other chemical names 3,5-diaminotoluene;
3,5-toluenediamine
Common name 3,5-TDA
----------------------------------------------------------------------------
2.2. Physical and Chemical Properties
Diaminotoluenes are colourless crystals that are freely soluble
in hot water, alcohol, ether, and hot benzene. Some of the
physical properties of the 6 isomers are listed in Table 3 (Buist,
1970; CRC, 1975). Diaminotoluenes are oxidized readily in neutral
or alkaline solution to form dark-coloured products and tars. The
oxidation products have not been fully characterized. When heated,
diaminotoluenes emit toxic fumes of nitrogen oxides.
The composition and physical properties of the commercial
mixtures vary considerably. Some of the physical properties of the
2 most widely-used commercial mixtures are summarized in Table 4.
Meta- and Ortho-diaminotoluenes are weakly basic and react with
mineral acids to form water-soluble amine salts. These salts are
more resistant to oxidation than the parent amine.
Table 3. Physical properties of the diaminotoluene isomers
-------------------------------------------------------------------
Property Diaminotoluene isomers
2,3- 2,4- 2,5- 2,6- 3,4- 3,5-
-------------------------------------------------------------------
Melting point (°C) 63-64 99 64 105 88.5 -
Boiling point (°C) 255 280 273-274 289b 265 283-285
(subl)
Vapour pressurea (kPa)
at 150 °C 1.20 1.47 - 2.13 - -
at 160 °C 1.87 2.27 - 3.33 - -
at 180 °C 2.67 4.80 - 7.60 - -
-------------------------------------------------------------------
a To convert kPa to mmHg, divide by 0.133.
b Obtained by extrapolation from vapour pressure-temperature data
and Antoine constants. From: Willeboordse et al. (1968).
Table 4. Physical and chemical properties of commercial grades of
diaminotoluene
----------------------------------------------------------------------
Meta-DAT Ortho-DAT
(80:20, 2,4-/2,6-isomers) (60:40, 3,4-/2,3-isomers)
----------------------------------------------------------------------
Appearance solid, light yellow to tan; light grey to purple
darkens on storage and solid
exposure to air
Odour slight ammonia-like slight ammonia-like
Melting range 80 - 90 °C (176 - 194 °F) 40 - 50 °C (104 - 122 °F)
Boiling point 283 °C (541 °F) at 760 mmHg > 250 °C (> 480 °F)
Flash point 140 °C (284 °F) > 110 °C (> 230 °F)
Autoignition 450 °C (842 °F) 540 °C (1005 °F)
temperature
Vapour 0.34 x 10-3 mmHg at 37.8 °C 2.23 mmHg at 100 °C
pressure 1 mmHg at 106.5 °C 27.8 mmHg at 140 °C
100 mmHg at 212 °C 43.5 mmHg at 160 °C
Specific - 1.045 at 100 °C
gravity
Density 0.086 kg/litre at 105 °C -
Solubility in hot water, alcohol, in hot water, alcohol,
ether and many polar ether and many polar
organic solvents organic solvents
----------------------------------------------------------------------
2.3. Conversion Factors
1 ppm in air = 5 mg/m3 at 25 °C and 760 mmHg.
2.4. Analytical Methods
Analytical methods for the determination of diaminotoluenes in
water, air, different consumer products, and biological fluids are
listed in Table 5.
Diaminotoluenes may be analysed as free bases by reversed phase
high-performance liquid chromatography using both ultraviolet (UV)
and electrochemical detection (Purnell & Warwick, 1981; Purnell et
al., 1982; Nieminen et al., 1983). Gas chromatographic methods
usually involve derivatization to facilitate separation and
increase sensitivity (Olufsen, 1979; Skarping et al., 1983a,b).
Detection limits in air samples range from 0.1 to 10 µg/m3.
Diaminotoluenes and their derivatives have been studied in
blood, urine, and liver cytosol preparations using thin-layer
chromatography and gas chromatography/mass spectrometry (GC/MS)
(Kiese & Rauscher, 1968; Kiese et al., 1968; Glinsukon et al.,
1975; Waring & Pheasant, 1976). A high-performance liquid
chromatographic method for the determination of diaminotoluenes in
urine and plasma has been described by Unger & Friedman (1979).
Table 5. Analytical methods for the determination of diaminotoluenes
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Water high-performance liquid chrom- 2,4-, 2,5-, 2,6-, 0.2 - 0.7 ng Riggin & Howard
atography with ultraviolet and 3,4-isomers (1983)
electrochemical detection
Air gas-liquid chromatography/ 2,4-isomer 3 µg/m3 Becher (1981)
nitrogen-phosphorus detector
on glass capillary columns
high-performance liquid chrom- 0.1 - Purnell et al.
atography with ultraviolet 10 µg/m3 (1982)
and electrochemical detection
high-performance liquid chrom- 2,4-isomer - Nieminen et al.
atography with ultraviolet (1983)
detection
gas-liquid chromatography with 2,4- and 2,6-isomers ~ 0.1 - Skarping et al.
electron capture detection on 0.4 pg (1983a)
glass capillary column
gas-liquid chromatography/ 2,4- and 2,6-isomers 10 - 20 pg Skarping et al.
nitrogen-phosphorus detector amine (1983b)
on glass capillary columns
Hair dyes gas-liquid chromatography/ 2,5-isomer 5 mg/litre Choudhary (1980)
flame ionization detector
thin-layer chromatography 2,4-, 2,5-, and 0.2 mg/litre Kottemann (1966)
3,4-isomer
high-performance liquid chrom- 2,4-, 2,5-, 0.5 mg/litre Johansson et al.
atography/ultraviolet detection and 2,6-isomers (1981)
high-performance liquid chrom- 2,4-, 2,5-, - Liem & Rooselaar
atography/ultraviolet detection and 3,4-isomers (1981)
------------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------------
Matrix Analytical procedure Determination Detection Reference
limit
------------------------------------------------------------------------------------------------------
Hair dyes high-performance liquid chrom- 2,4- and 2,6-isomers 0.1 µg/litre Snyder et al.
(contd.) atography/ultraviolet detection (1982)
gas-liquid chromatography/mass
spectrometry
Polyurethane thin-layer chromatography/ 2,4- and 2,6-isomers 1 µg/g Guthrie &
foams fluorimetry McKinney (1977)
Biological high-performance liquid chrom- 2,4- and 2,6-isomers 2 mg/litre Unger & Friedman
tissues and atography/ultraviolet detection (1979)
fluids
Isomeric nuclear magnetic resonance all isomers - Mathias (1966)
mixtures spectrometry
thin-layer chromatography all isomers - Macke (1968)
gas-liquid chromatography/ all isomers - Boufford (1968)
flame ionization detector
gas-liquid chromatography/ all isomers - Willeboordse et
thermal detector al. (1968)
infra-red spectroscopy 2,4- and 2,6-isomers - Biernacka et al.
(1974)
------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Natural Occurrence
Diaminotoluenes are not known to occur as natural products.
3.2. Production
Currently, diaminotoluenes are produced commercially through
the catalytic hydrogenation of dinitrotoluenes. This procedure,
economic only for large-scale production, is used in the
manufacture of toluene diisocyanates. At dye plants, diamino-
toluenes are produced by the reaction of hydrochloric acid on
dinitrotoluenes, in the presence of an iron catalyst (Austin,
1974).
Most diaminotoluenes produced are used on site by the
manufacturer; therefore, published production figures do not
adequately reflect the true world production of diaminotoluenes.
Between 1972 and 1976, the average annual production of
diaminotoluenes in the USA was 89 x 106 kg, ranging from 76 x 106
kg in 1972 to 105 x 106 kg in 1976 (US ITC, 1977). Thereafter, the
production was estimated from the known production of toluene
diisocyanates ranging between 305 x 106 kg annually in the period
1977 - 81, and 360 x 106 kg in 1984 (US ITC, 1982, 1985).
Up to 1978, an estimated 180 - 200 x 106 kg of 2,4-DAT was
produced annually in western Europe (IARC, 1978). During
1971 - 75, the the annual production of 2,4-DAT in Japan was
approximately 210 x 103 kg; the compound was neither imported nor
exported (IARC, l978). However, in 1981, the production of 2,4-DAT
in Japan was estimated to have declined to 50 x 103 kg (CIC Japan,
1983).
3.3. Uses
Diaminotoluenes are used extensively within the chemical
industry as intermediates in the manufacture of widely different
commercial products (Table 6). Minor applications of diamino-
toluene isomers include their use as raw materials, co-reactants,
and curing agents. Toluene diisocyanates represent the largest
end-use accounting for more than 90% of the total annual production
of diaminotoluenes, largely a mixture of 2,4- and 2,6-isomers
(Backus, 1974; Milligan & Gilbert, 1978).
Diaminotoluenes are intermediates in the synthesis of dyes used
for textiles, furs, leathers, biological stains and indicators,
spirit varnishes and wood stains, and pigments. Previously used in
hair-dyes, 2,4-DAT was removed from use by many countries after it
was found to be a hepatocarcinogen in rats (Ito et al., 1969).
Meta-DAT is used to produce diethyltoluenediamine (DETDA) for the
manufacture of certain urethane elastomers (Milligan & Gilbert,
1978). Ortho-DAT is used to produce mercaptotoluimidazole (MTI)
and its zinc salt, both of which are used primarily as specialty
antioxidants in nitrile rubber elastomers (Gan et al., 1975).
Table 6. End-use application(s) of individual
diaminotoluene isomers
------------------------------------------------------
Application 2,3- 2,6- 2,3- 3,4- 2,5-
------------------------------------------------------
Toluene diisocyanate X X
(> 90% of total use of
diaminotoluenes)
Urethane co-reactants X X X X
(DAT-initiated polyols)
DETDAa X X
DYESb Xd X d
Tolyltriazole X X
Epoxy curing X X X
Mercaptotoluimidazole X X
Photographic developer X
------------------------------------------------------
a DETDA = Diethyltoluenediamine.
b DYES = Fur, leather, biological stains, indicators,
textiles, hair, spirit varnishes, wood stains, and
pigments.
c Use in hair dyes and cosmetics prohibited in USA
since 1971.
d Forbidden in Italy - 1978.
3.4. Release into the Environment, Distribution, and Transformation
Data are lacking on the extent of the global release of
diaminotoluenes, as well as their transport, distribution, and
degradation within the environment.
Releases of 2,4-DAT into the environment have been estimated in
the USA, the largest contribution being over 6 x 106 kg dumped in
authorized landfills. Releases of 1.4 x 106 kg were estimated to
occur from the production of diaminotoluenes, and 0.3 x 106 kg
during dye production and usage; unknown quantities of DAT may
derive from the hydrolysis of TDI released into the environment.
Information on the transport, distribution, and degradation of
DAT isomers under conditions approaching those found in natural
bodies of water have not been reported in the literature. However,
a bench-scale treatability study for 2,4-DAT using acclimated
sludge from a treatment plant showed that the isomers are
degradable. The observed total organic carbon removal was 45% in
4 h (Matsui et al., 1975).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental Levels
No information was available in the literature reviewed from
which environmental levels could be calculated. Two properties of
the diaminotoluenes are relevant to this problem. Since the vapour
pressure is low (Tables 4 and 5), the risk of contaminating the
environment through evaporation is minimal. However, air emissions
from inappropriately operated plants may pose a hazard. Since the
chemical is soluble in water, the potential for exposure through
water contamination is of concern. No data are available on levels
of diaminotoluenes in surface and groundwater, in soil, and/or air.
4.2. General Population Exposure
No information is available on the exposure of the general
population to diaminotoluenes.
4.3. Occupational Exposure
Filatova et al. (1970) reported concentrations of diamino-
toluenes in manufacturing plants of up to 0.2 mg/m3, with
occasional excursions up to 11 mg/m3.
The results of studies conducted in 3 plants manufacturing
diaminotoluenes in the USA showed that the work-place ambient air
levels ranged from 0.005 to 0.44 mg/m3 (NIOSH, 1980, 1981, 1982).
The highest level of diaminotoluenes (0.44 mg/m3) was found in the
filter room at one plant (NIOSH, 1980). A level of 0.39 mg
diaminotoluenes/m3 was measured in a sample taken at the breathing
zone of an operator in a second plant (NIOSH, 1981). All values
were calculated as time-weighted averages.
5. KINETICS AND METABOLISM
5.1. Studies on Experimental Animals
5.1.1. Absorption and retention
Skin penetration by test materials varied amoung species
(monkeys, swine), and was affected by vehicle and site of
application. In one study, [14C]-2,4-DAT (4 µg/cm2), dissolved in
acetone, methanol, or a skin lotion, was applied to 3 - 15 cm2 of
the ventral forearm, abdomen, or back of 3 - 6 monkeys/group (9
groups). The material was removed after 24 h by washing with soap
and water. The greatest absorption (53.8 ± 15.4%) resulted when
[14C]-2,4-DAT in acetone was applied to the abdominal skin of
monkeys (Marzulli et al., 1981). The permeability of diamino-
toluenes across the epidermis was highly dependent on the
formulation used. When 1.4 g of 2,5-DAT was applied in a gel
to the abdominal skin of dogs for a contact period of 3 h, 2.9%
(40 mg) was absorbed. Addition of hydrogen peroxide, similar to
the formulation used in hair dye, reduced the amount absorbed
to < 0.21% (Kiese et al., 1968) or < 0.13% (Hruby, 1977). The
hair in the exposed area retained 4% of the 14C activity, 5 days
after application (Hruby, 1977).
Hruby (1977) studied the absorption of [14C]-2,5-DAT following
oral and subcutaneous single-dose administrations to rats. Five
days following subcutaneous injection of 3 - 5 mg [14C]-2,5-DAT (in
water), 6.9% of the dose was found in the total-body homogenate and
1.7% remained at the injection site. Five days after oral (gavage)
administration of 10 mg [14C]-2,5-DAT (in water), the rat gastro-
intestinal tract retained 1.4% of the applied radioactivity and
1.2% was found in the total body homogenate (Hruby, 1977).
No studies on uptake after inhalation were found.
5.1.2. Distribution and reaction with body components
The distribution of diaminotoluenes and their reaction with
body components have been investigated, mainly after
intraperitoneal injection of radioactive labelled compounds. No
data on the distribution of diaminotoluenes and reaction with
tissues after inhalation or oral ingestion were found in published
reports.
Distribution of [14C]-2,4-DAT after intraperitoneal injection
was rapid, and the peak concentration in rat and mouse blood and
plasma occurred in 1 h, then decreased rapidly for 7 h. On a
comparative basis, all tissue concentrations of bound 14C were
considerably lower in the male NIH-Swiss mice than in the male
Fischer rats (Grantham et al., 1980).
Tissue distribution of [Me-14C]-2,4-DAT hydrochloride was
studied in male B6C3F1 mice given a single intraperitoneal
injection (1 µCi, 0.667 mg/kg body weight) (Unger et al., 1980).
The highest concentrations, 1/2 h after dosing, were found in the
kidneys, gonads, epididymis, lungs, muscle, and blood. One hour
after dosing, the liver contained the greatest amount, accounting
for nearly 12% of the dose. The concentration in the adrenal
glands exceeded that in the kidney, 1 and 2 h after dosing. High
concentrations of radioactivity were also observed in the
gastrointestinal tract.
Four hours after an intraperitoneal injection of 100 mg (0.8
mmol/kg, ring-labelled [3H]-2,4-DAT) in male Wistar rats, 0.3 nmol
was found covalently bound per mg liver protein. A similar degree
of binding was seen in the kidneys. Subcellular fractionation of
the liver showed that most of the bound material was in the
microsomal fraction (Dybing et al., 1978). No significant binding
to DNA in vitro or in vivo could be demonstrated using [3H]-2,4-
DAT, whereas it was found to bind covalently to hepatic RNA in
vivo. These findings were confirmed by Aune et al. (1979).
5.1.3. Metabolism
Glinsukon et al. (1975, 1976) found that the 2,4-isomer was
selectively N-acetylated at the p-amino group by liver cytosol
prepared from hamsters, guinea-pigs, rabbits, mice, and rats. The
cytosol from liver, kidney, intestinal mucosa, and lung of hamsters
and rabbits was studied for N-acetyl transferase activity using
2,4-DAT and 4-acetylamino-2-amino-toluene as substrates. All
tissues showed marked species differences in enzyme activity.
Tissues with high N-acetyl transferase levels, such as liver,
could produce both 4-acetylamino-2-aminotoluene and 2,4-
diacetylaminotoluene (Glinsukon et al., 1975). There were also sex
differences in the N-acetylation capacity of the liver cytosol.
After a single ip injection of 2,4-DAT (77 mg/kg body weight)
in male rats, 69.4% of the dose was eliminated in the urine and
faeces after 24 h as a complex mixture of metabolites, indicating
both free and conjugated derivatives. The major urinary
metabolites identified were 4-acetylamino-2-aminotoluene, 2,4-
diacetylaminotoluene, and 4-acetylamino-2-aminobenzoic acid. In
mice, oxidation of the methyl group to a benzoic acid was the major
reaction and the major urinary metabolites in mice were 4-
acetylamino-2-aminobenzoic, 4-acetylamino-2-aminotoluene, and 2,4-
diacetylaminobenzoic acid (Grantham et al., 1980). Waring &
Pheasant (1976) investigated the metabolism of 2,4-DAT in female
rabbits, rats, and guinea-pigs to determine whether the isomer gave
rise to hydroxylamines or aminophenols, which might account for the
observed toxic and carcinogenic effects. After oral administration
(gavage) of 2,4-DAT (50 mg/kg body weight), phenolic metabolites
were excreted in the urine. When free and conjugated metabolites
were combined, 5-hydroxy-2,4-DAT was the major metabolite in all 3
species (Table 7).
Table 7. Excretion of metabolites after dosing with 2,4-diamino-
toluene
-------------------------------------------------------------------
Metabolite Percentage dose excreteda
Rabbit Rat Guinea-pig
-------------------------------------------------------------------
2,4-DAT trace 1.3 trace
3-hydroxy-2,4-DAT 10 8 trace
5-hydroxy-2,4-DAT 22 12 9
6-hydroxy-2,4-DAT ( m-aminophenol) trace 5 trace
3-hydroxy-4-acetylamino-2-aminotoluene 10 18 trace
5-hydroxy-4-acetylamino-2-aminotoluene 6 14 17
glucuronide I, 3-hydroxy-DAT 10 16 15
glucuronide II, 5 hydroxy-DAT 32 12 46
glucuronide III, 6-hydroxy-DAT 2 6 4
unidentified phenolic compounds 0 trace trace
-------------------------------------------------------------------
a Results are given as percentage dose, average of 10 studies,
standard deviation 6.4% for metabolites 1 - 5, and 12.8% for
metabolites 6 - 8. Animals were dosed orally at 50 mg/kg; urine
was collected for 48 h.
From: Waring & Pheasant (1976).
The levels of methaemoglobin found in the rabbit, rat, and
guinea-pig correlated well with the total urinary excretion of
aminophenol. The methaemoglobin levels reached a peak 6 - 12 h
after the administration of 2,4-DAT and then slowly declined. The
highest levels of aminophenols and of methaemoglobin were found in
the rabbit (Waring & Pheasant, 1976).
5.1.4. Excretion
During a 24-h period of dermal contact with [14C]-2,4-DAT, 14C
urinary excretion in monkeys reached a peak at 8 - 12 h (Marzulli
et al., 1981).
Data from studies on the rat, rabbit, mouse, guinea-pig, and
dog exposed to diaminotoluenes (cutaneous, subcutaneous,
intravenous, intraperitoneal, or oral by gavage) showed fast
elimination rates (Kiese et al., 1968; Waring & Pheasant, 1976;
Hruby, 1977; Grantham et al., 1980; Unger et al., 1980). The
elimination of radioactivity from various tissues in rodents
followed a well-defined biphasic pattern. Rapid elimination over
7 h was followed by a rather slow decline in the isotopic contents
of tissues (Grantham et al., 1980; Unger et al., 1980). The half-
lives of tissue elimination during the fast phase were 0.89, 0.43,
and 1.51 h for male mouse liver, kidneys, and blood, respectively.
During the slow phase of elimination, the half-lives for liver,
kidneys, and blood in male mice were 11.7, 9.1, and 12.6 h,
respectively. The half-lives of elimination of radioactivity,
during the slow phase, were greater for muscle (23.9 h) and skin
(29.2 h) than for any other tissue (Wagner, 1975; Unger et al.,
1980).
The primary route of elimination in rodents was via the kidneys
during the first hour after exposure. However, after 2 h, the
predominant route shifted from urinary to faecal, probably a
reflection of biliary excretion. Only 1.25% of the administered
radioactivity had been exhaled after 24 h (Unger et al., 1980). On
a comparative basis, faecal elimination was greater in rats than in
mice, but the rate of urinary excretion was more rapid in mice than
in rats. Approximately 90% of a dose was eliminated in the urine
of mice in 24 h compared with 74% in the urine of rats (Grantham et
al., 1980). Complete elimination was accomplished in 2 days in
mice, while rats required 6 days.
Male and female rats, injected subcutaneously with 3 - 5 mg
[14C]-2,5-DAT hydrochloride, eliminated 65% of the dose in the
urine and 5% in faeces after 24 h. The same pattern of elimination
was found after oral administration of 10 mg of the labelled
compound (Hruby, 1977).
When beagle dogs were intravenously injected with a dose of
224 mg, infused over 3 h, the total amounts of radioactivity
eliminated in the urine and faeces were 60% and 19%, respectively.
After 4 days, elimination mainly occurred within the first 24 h.
After a skin application of 1.4 g [14C]-2,4-DAT for 3 h (in 50 ml
of a dye formulation), only 0.092 and 0.84% of the dose were
eliminated, respectively, in the urine and faeces of beagle dogs
over 4 days, reflecting the inhibitory effect of the dye
formulation on the absorption of the 2,4-DAT (Hruby, 1977). In
another study on dogs, about 40 mg 2,5-diaminotoluene was absorbed
through the skin from a gel containing 1.4 g of the material. The
addition of hydrogen peroxide to the gel reduced the amount
absorbed to less than 3 mg. The amount excreted unchanged in the
urine was 60 - 70 µg (Kiese et al., 1968).
5.2. Human Studies
As only limited information is available on the absorption,
distribution, metabolism, and excretion of diaminotoluenes in human
beings, these aspects are discussed together, rather than in
separate sections.
Although the high boiling point of diaminotoluenes makes
absorption through the lungs unlikely under normal working
conditions, inhalation may occur when hot vapours escape from
stills. Possible inhalation and dermal exposure to dusts may occur
if diaminotoluenes are handled in a less than optimal manner.
Since diaminotoluenes are soluble in water, absorption from the
gastrointestinal tract could occur following ingestion. However,
no data were found on the kinetics and metabolism of diamino-
toluenes after oral or inhalation exposures.
Skin penetration by [14C]-2,4-DAT was measured in human beings
(Marzulli et al., 1981). When 4 µg [14C]-2,4-DAT in acetone/cm2
was applied to the skin of the forearm, the highest absorption of
the chemical (23.7 % ± 16.1% of the applied dose) resulted after
24 h of dermal contact. Urinary excretion reached a peak after
4 - 8 h of skin contact. In a study by Kiese & Rauscher (1968),
the hair of 5 human subjects was dyed (40 min) with a formula
containing 2.5 g 2,5-DAT; absorption of approximately 0.2% of the
applied material occurred. No data were given on the retention and
distribution of diaminotoluenes after this dermal contact.
Data from studies on 6 volunteers (3 males and 3 females)
showed that, after subcutaneous injection of 5.54 mg 2,5-DAT, 47.6%
of the dose was excreted in the urine as N,N'-diacetyl-2,5-DAT.
The rate of excretion was highest during the first 24 h, and only a
trace appeared in the urine excreted on the third day, in one
study. When the compound was applied as a hair dye (40 min), the
highest rate of excretion was observed during a period of 5 - 8 h
after application. On average, a total amount of 3.7 mg N,N'-
diacetyl-2,5-DAT (i.e., 0.09% of the applied dose) was calculated
to have been excreted in urine taken over 2 days from 5 subjects
(Kiese & Rauscher, 1968).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Little information is available on the effects of diamino-
toluenes on animal populations found in the environment. The
effects of 2,4-DAT at concentrations ranging from 1 to 1000
mg/litre were observed for Daphnia ( Daphnia magna Straus),
ostracoda, guppies ( Lebistes reticulatus Peters), and channel
seaweed (Scenedesmus obliquus). Daphnia was the most sensitive
species; 5 mg/litre was lethal in 5 - 10 days, and prolonged
exposure to 2 mg/litre caused a reduction in the number of
offspring produced. A concentration of 20 mg/litre was not lethal
for ostracods after 10 days, but 50 mg/litre was lethal in 5 - 8
days. Fish survived for 10 days at 200 mg/litre, but a
concentration of 500 mg/litre was lethal in 2 - 3 days. The algae
tested were the most resistant, surviving for 10 days at a
concentration of 1000 mg/litre (Smirnova et al., 1967).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
The early literature (1881 - 1939) contains several reports on
toxicological manifestations associated with the administration of
diaminotoluenes in experimental animals. Most of the papers are
difficult to interpret and to use in the assessment of chemical
hazards, because massive doses of chemicals of unknown purity and
isomeric composition were used, and because of the experimental
designs chosen. The toxic effects were characterized by icterus,
haemoglobinuria, disposition of haemosiderin in the spleen, bone
marrow, and liver, respiratory and generalized central nervous
system (CNS) depression, pulmonary and cerebral oedema, and
increased bile acids in the liver, blood, and/or urine of exposed
animals (Von Oettingen, 1941).
7.1. Single Exposures
Diaminotoluenes are considered to be dermal and eye irritants.
In studies on rabbits, 12.5 mg 2,5-DAT or 500 mg 2,4-DAT caused
skin irritation, defined as erythema and oedema, after 24 h of
dermal contact. Instillation of 100 µg of the 2,4-isomer into the
rabbit eye caused severe eye irritation within 24 h. Data showing
the extent of the acute toxicity of diaminotoluenes in various
laboratory animals are summarized in Tables 8 and 9.
The acute toxic effects of diaminotoluenes were characterized
by marked central nervous system depression during exposure (e.g.,
decreased locomotor activity, piloerection, ptosis, ataxia,
tremors) and production of methaemoglobin, 6 - 8 h after exposure.
Duodenal and glandular mucosal damage in the stomach were
observed in fed, unrestrained rats, 24 h following a single
subcutaneous dose of 3,4-DAT. The optimal ulcerogenic dose of 3,4-
DAT (i.e., the dose causing a low mortality and a maximal incidence
of duodenal damage within 24 h), was 350 mg/kg body weight (Perkins
& Green, 1975).
7.2. Short-Term Exposures
When guinea-pigs were treated with 1 - 10% 2,5-DAT (24 h/day
for 5 days, 2 days without treatment, followed by exposure for
another 5 days), sensitization was obtained in 35% of treated
animals (Schäfer et al., 1978).
Both the 2,4- and 3,4-isomers caused severe icterus in rats.
In male and female rats, 3,4-DAT (unlike the 2,4-isomer), given
orally or parenterally, produced a high incidence of perforating
duodenal ulcers within a few days (Selye, 1973). These effects
were obtained in animals allowed to move freely with access to food
and water during the period of observation. A dose of 500 mg
diaminotoluenes/kg body weight was administered in 2 ml water,
twice daily.
Table 8. Lethality of diaminotoluenes
---------------------------------------------------------------------------
Species Exposure LD50 Reference
route (mg/kg
body
weight)
---------------------------------------------------------------------------
Ortho-DAT (2,3-, 3,4- mix)
Rat oral 810 Carpenter et al. (1974)
Rabbit dermal 1120 Carpenter et al. (1974)
Meta-DAT (2,4-, 2,6- mix)
Rat oral 300 Izmerov et al. (1982)
Rat (male) oral 270 Weisbrod & Stephan (1983)
Mouse (male) oral 350 Weisbrod & Stephan (1983)
Rat (male) ip 230a Weisbrod & Stephan (1983)
Mouse (male) ip 240b Weisbrod & Stephan (1983)
Rat (male) iv 350 Weisbrod & Stephan (1983)
Mouse (male) iv 90-105 Weisbrod & Stephan (1983)
Rat dermal 1200 Izmerov et al. (1982)
2,4-DAT (technical grade)
Fischer rat (male) ip 325 Grantham et al. (1980)
NIH-Swiss mouse (male) ip 480 Grantham et al. (1980)
HaM/ICR mouse (male) ip 80 Weisburger et al. (1978)
HaM/ICR mouse (female) ip 90 Weisburger et al. (1978)
---------------------------------------------------------------------------
a A methaemoglobin level of 8.4% was observed, 6 h after ip administration.
b A methaemoglobin level of 7.8% was observed, 6 h after ip treatment.
Note: No published data on the acute toxicity of the 2,3-isomer were
available.
The effects of 2,4-DAT on the liver microsomal mixed-function
oxidase system, DT-diaphorase, and epoxide hydrolase were reported
by Dent & Graichen (1982). Following oral treatment with 2,4-DAT
at 70 mg/kg body weight per day for 5 days, the activities of
microsomal cytochrome P-450-dependent enzymes were depressed, while
epoxide hydrolase activity was markedly elevated (3 - 8 times
control) in male F-344 rats. Under these experimental conditions,
an increase in the liver to body weight ratio (3.2 - 4%), and in
the liver microsomal protein concentration (19.3 - 27.4 g/kg) were
induced by 2,4-DAT.
7.3. Long-Term Exposure
Oral administration of 2,4-DAT (see Table 11, section 7.6, for
doses) for 79 - 103 weeks accelerated the appearance of renal
toxicity in male F-344 rats, associated with a high incidence of
secondary hyperparathyroidism (NCI, 1979). The chronic renal
disease reported was believed to have decreased the longevity of
the treated rats, either directly or through inhibition of the
clearance of toxic metabolites (Cardy, 1979).
Table 9. Summary of some single-dose studies
--------------------------------------------------------------------------
Species Route of Dose Effects Reference
exposure (mg/kg
body
weight)
--------------------------------------------------------------------------
2,4-DAT (technical grade)
Wistar rat oral 50 produced metHba; highest Waring &
(male) amounts (5 - 6%) were Pheasant (1976)
found 6 - 8 h after
exposure
> 50 toxic
Sprague oral 500 developed icterus and Selye (1973)
Dawley rat death
(male and
female)
NZW rabbit oral 50 MetHb reached 18 - 20% Waring &
level 6 - 8 h after Pheasant (1976)
application
> 50 toxic
Dunkin- oral 50 MetHb reached 3 - 4% level Waring &
Harvey 6 - 8 h after application Pheasant (1976)
guinea-pig
> 50 toxic
3,4-DAT (97% pure)
Sprague subcut- 125-500 discrete, non-perforated Perkins &
Dawley rat aneous duodenal lesions were Green (1975)
(female) observed immediately
distal to the gastroduo-
denal junction 24 h
following the administra-
tion of a single dose
--------------------------------------------------------------------------
a metHb = methaemoglobin.
No studies were found on the effects of diaminotoluenes on the
nervous system or the immune system after long-term exposure.
7.4. Reproduction and Teratogenicity
7.4.1. Reproduction
There are 2 studies on experimental animals that evaluate the
reproductive toxicity of diaminotoluenes. Soares & Lock (1980)
administered 2,4-DAT orally or ip at 40 mg/kg body weight for
2 days to DBA/2J male mice. Forty-eight hours after treatment,
mating trials were conducted for 8 weeks. There were no treatment-
related effects on sperm morphology or fertility, as measured by
this dominant lethal assay. However, in male Sprague Dawley rats,
long-term exposure to 2,4-DAT in the feed impaired reproductive
performance and capacity (Thysen et al., 1985a,b). Dietary levels
of 0.03% 2,4-DAT for 10 weeks (~ 15 mg/kg body weight per day)
decreased fertility and exerted an inhibitory effect on sperm
production in male rats. Eleven weeks after treatment, the sperm
count remained significantly depressed ( P < 0.001), suggesting
irreversible damage to the germinal components in the testes. Data
from hormone analyses at the end of the 10 weeks of exposure, and
at the end of 11 weeks after treatment, showed a significant
decrease in serum-testosterone and an elevation of serum-luteinizing
hormone concentrations, which were associated with a reduction in
seminal vesicle weight. Histological changes found in the
reproductive organs from treated males were correlated with these
physiological changes. At a lower dose (0.01% or ~ 5 mg/kg body
weight), 2,4-DAT did not cause any of these toxic responses.
7.4.2. Teratogenicity
Studies on the teratogenic potential of diaminotoluenes are
summarized in Table 10. Skin application of 2,4-DAT induced a low
incidence of skeletal changes in rats (Burnett et al., 1976). Oral
or intraperitoneal administration of this isomer did not produce
any effects on the fertility or reproductive performance of male
mice (Soares & Lock, 1980). Subcutaneous or intraperitoneal
injection of 2,5-DAT in mice on day 8 of gestation, at levels of 50
or 75 mg/kg body weight, caused crainiofacial malformation and
fused or distorted thoracic vertebrae associated with the absence
of, or fused, ribs (Inouye & Murakami, 1976, 1977). However, 2,5-
DAT sulfate, at levels of 16 - 64 mg/kg body weight per day
administered subcutaneously on days 6 - 15 of gestation, did not
cause any malformations in mice or rats (Marks et al., 1981).
The results of oral administration of 2,6-DAT to rats and
rabbits, at doses of between 10 and 300 mg/kg body weight
(Knickerbocker et al., 1980), showed that, in rats, doses of
between 100 and 300 mg/kg body weight increased the occurrence of
incomplete vertebrae and that the highest dose resulted in missing
sternebrae and incomplete closure of the skull. A no-observed-
adverse-effect level of 10 mg/kg body weight was reported in rats.
No skeletal or soft-tissue abnormalities were observed in the
offspring of rabbits, but, using fetal toxicity indices, a no-
observed-adverse-effect level of 30 mg/kg body weight was reported.
Becci et al. (1983) administered Ortho-DAT (2,3-, 3,4-isomer
mixture) by gavage to rats and rabbits (Table 10). Reduced body
weight during gestation was noted at 300 mg/kg body weight in rats
and 100 mg/kg body weight in rabbits. An increased incidence of
several skeletal variations in the fetuses was noted, probably due,
in part, to the maternal toxicity. The no-observed-adverse-effect
level in both rats and rabbits was 30 mg/kg body weight.
Table 10. Teratogenicity studies with diaminotoluenes
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,4- Charles skin 2 ml/kg body weight skeletal changes seen in 6/169 Burnett et al.
(3% in River/CD on days 1, 4, 7, 10, live fetuses ( P > 0.05) (1976)
hair-dye rat 13, 16, and 19 of
formula) (female) gestation
2,5- Charles skin 2 ml/kg body weight no increase in abnormalities in Burnett et al.
(sulfate) River/CD on days 1, 4, 7, 10, treated groups (1976)
(3% in rat 13, 16, and 19 of
hair-dye gestation
formula)
2,5- JCL:ddn subcutaneous 50 mg/kg body weight In groups treated sc or ip on day Inouye & Murakami
dihydro- mice or intraper- on one day of days 8 of gestation, there was evidence (1976, 1977)
chloride (female) itoneal 7 - 14 of gestation of craniofacial malformation:
single dose or 75 mg/kg on day exencephaly, prosoposchisis, and
8 of gestation or hair lip with cleft palate, and
50 mg/kg on day 8 of high incidence of skeletal
gestation malformation: fused or distorted
thoracic vertebrae associated with
absence of, or fused, ribs; no
such malformed fetuses were found
in groups treated on days 10 - 14
of gestation; only a very low
incidence of vertebral and rib
anomalies followed treatment on
day 7 or 9; maternal toxicity was
reported at 75 mg/kg but not at
50 mg/kg
2,5- CD-1 mice subcutaneous 16, 32, 48, or 64 no teratogenic effects were noted; Marks et al.
(sulfate) mg/kg body weight maternal toxicity was evident at (1981)
per day on days 48 and 64 mg/kg; reduced fetal
6 - 15 of gestation weight was noted at > 32 mg/kg
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
2,5- rat oral (gavage) 10, 50, or 80 mg/kg maternal toxicity and embryo- Spengler et al.
(sulfate) body weight per toxicity evident at 80 mg/kg; no (1986)
day on day 15 of effects observed at lower doses
gestation
rabbit oral (gavage) 10, 25, or 50 mg/kg no effects observed
body weight per day
on days 6 - 18 of
gestation
2,6- Sprague oral (gavage) 10, 30, 100, or 300 no effects on pregnancy, number Knickerbocker et
Dawley mg/kg body weight of live fetuses, and resorption al. (1980)
rat per day on days sites/dam; 300 mg/kg produced
6 - 15 of gestation smaller body weight gain in the
dams; 30 - 300 mg/kg produced
increased haemorrhagic abdomens
in the fetuses; 100 and 300 mg/kg
increased the occurrence of
incomplete vertebrae, and 300
mg/kg showed missing sternebrae
and incomplete skull closure in
the fetuses; the no-observed-
adverse-effect dose was 10 mg/kg
per day
2,6 Dutch oral (gavage) 3, 10, 30, or 100 100 mg/kg per day reduced dam Knickerbocker et
belted mg/kg body weight weights, increased resorptions, al. (1980)
rabbit per day on days decreased fetal weights, and
(female) 6 - 18 of gestation neonatal survival;
oral (gavage) 3, 10, 30, or 100 there were no differences in Knickerbocker et
mg/kg body weight skeletal or soft-tissue al. (1980)
per day on days abnormalities between treated
6 - 18 of gestation animals and controls; the no-
observed-adverse-effect dose was
30 mg/kg per day
-----------------------------------------------------------------------------------------------------------------
Table 10. (contd.)
-----------------------------------------------------------------------------------------------------------------
DAT Species Route of Dose and duration Effects Reference
isomer administration
-----------------------------------------------------------------------------------------------------------------
o-DAT Sprague oral 10, 30, 100, or 300 maternal toxicity was indicated Becci et al.
(2,3-, Dawley mg/kg body weight at 300 mg/kg per day by reduced (1983)
3,4- rat per day on days body weight gain during gestation;
isomer 6 - 15 of gestation No significant differences in
mix) numbers of live fetuses,
implantation or resorption sites;
fetal body weight was reduced at
the highest dose ( P < 0.05); no
evidence of teratogenic effects
or effects on dams at doses < 30
mg/kg; no skeletal or soft-tissue
malformations that could be
related to treatment; however,
increased incidence of missing
sternebrae at 300 mg/kg per day
and incomplete ossified vertebrae
at 100 and 300 mg/kg per day were
noted compared with controls
o-DAT Dutch oral 3, 10, 30, or 100 maternal toxicity at 100 mg/kg per Becci et al.
(2,3-, belted mg/kg body weight day elicited by reduced body (1983)
3,4- rabbit per day on days weight gain during pregnancy; no
isomer 6 - 18 of gestation significant difference in the
mix) number of implantations; at 100
mg/kg per day, fetal body weight
was reduced and the number of
resorption sites was increased;
no skeletal or soft-tissue
malformations that could be
related to treatment were noted
-----------------------------------------------------------------------------------------------------------------
7.5. Mutagenicity and Related End-Points
7.5.1. DNA damage
At concentrations of 1 x 10-4 mol/litre and below, 2,4-DAT, but
not 2,6-DAT, induced unscheduled DNA synthesis in primary cultures
of rat hepatocytes (Bermudez et al., 1979). 2,4-DAT produced a
significant elevation in unscheduled DNA synthesis at 2 and 12 h in
the in vivo/in vitro hepatocyte DNA repair assay (Mirsalis &
Butterworth, 1982; Mirsalis et al., 1982). 2,5-DAT produced a
positive response in a DNA-repair assay in rat hepatocytes and a
weak positive response in hamster hepatocytes at 10-5 mol, the
highest concentration that was not toxic to the cells that were
tested (Kornbrust & Barfknecht, 1984).
Shooter & Venitt (1979) used continuous administration of 2,4-
DAT in the drinking-water to determine whether phosphotriesters
could be detected in the DNA of the liver of treated rats.
Positive results were obtained at 10 mg/litre. A low, but
significant, level of these lesions was produced. Shooter &
Venitt's studies on rodents indicated that methyl- and
ethylphosphotriesters persist for many weeks in the DNA of certain
organs (notably liver, kidney, and lung) and that such lesions are
not eliminated by DNA repair.
The results of studies by Greene et al. (1981) showed that
2,4-, 2,5-, 2,6-, and 3,4-isomers significantly inhibited the
incorporation of [125I]-iododeoxyuridine into mouse testicular DNA
and demonstrated dose-response characteristics. The 2,4-, 2,5-,
and 3,4-isomers were capable of reaching the testes and of passing
target cell membranes at this site. They concluded that the 3
isomers may present a genetic health hazard for an intact animal.
The inhibition induced by 2,6-DAT might have been caused by a
chemically-induced decrease in body temperature (Greene et al.,
1981).
DNA damage was not found in human cultured fibroblasts after
exposure to 100 µmol 2,4-DAT alone (3.5 ± 1.5 increase in percent
single-strand DNA). When the cells were incubated in the presence
of 1 mg ram seminal vesicle microsomes/ml and 100 µmol arachidonic
acid, a significant increase in the fraction of single-strand DNA
(21.3 ± 3.7, P < 0.001) was found in cells exposed to 2,4-DAT.
DNA strand breaks were not induced when prostaglandin synthase
(PGS) was inhibited by adding indomethacin (100 µmol) or
acetylsalicylic acid (1 mmol) (Nordenskjöld et al., 1984). These
results, which suggest that 2,4-DAT may be activated by PGS to form
products that cause DNA damage in cultured human fibroblasts, are
in agreement with the findings of Rahimtula et al. (1982).
7.5.2. Mutation
Several studies have shown that 2,4-, 2,6-, and 2,5-isomers can
induce reverse mutations in Salmonella typhimurium strains TA 1538
and TA 98, in the presence of various metabolic activation systems
(McCann et al., 1975; Dybing & Thorgeirsson, 1977; Dybing et al.,
1977; Pienta et al., 1977b; Cinkotai et al., 1978; Aune et al.,
1979; Shahin et al., 1980). However, Mori et al. (1982) showed
that 2,4-DAT was inactive for strains TA 98 and TA 100 at doses
ranging from 5 to 1000 µg/plate. While 2,3-DAT is inactive in S.
typhimurium (Florin et al., 1980), its homologue 3,4-DAT showed a
marginal response in strains TA 98 and TA 1538 (Greene et al.,
1979).
2,4-DAT was shown to be a weak mutagen in Drosophila
melanogaster, inducing sex-linked recessive lethals when fed to
adult males at a concentration of 15.2 mmol (Blijleven, 1977;
Venitt, 1978). In a study reported by Fahmy & Fahmy (1977), 2,4-
DAT was injected around the testes of adult male Drosophila at
doses ranging from 5 to 20 mmol. Mutagenicity was measured at the
various stages of spermatogenesis, both on the X-chromosome and RNA
genes. The overall induced frequency of X-recessives was extremely
low. It was also observed that mutation yield was not dose-related
and that it was maximal in the earliest progeny fraction,
suggesting a greater toxicity for mature sperm.
2,4-DAT was mutagenic in L5178Y mouse lymphoma cells and CHO-
AT3-2 cells (Matheson & Creasy, 1976; Coppinger et al., 1984).
Mutagenic activity was observed in L5178Y cells, only in the
absence of exogenous metabolic activation, but was observed in CHO-
AT3-2 cells both with and without activation.
The in vivo mutagenic activity of 2,4-DAT was studied in
DBA/2J male mice by the dominant lethal assay, sperm abnormality
assay, and the recessive spot test. Mice were administered the
compound by intraperitoneal injection and orally by gavage (2 daily
doses of 40 mg/kg body weight), just before mating (Soares & Lock,
1980). No induction of dominant lethals was noted, nor was any
increase in abnormal sperm or recessive spots reported.
No dominant lethals were induced in Charles River rats injected
intraperitoneally, 3 times weekly for 8 weeks, with 20 mg 2,5-
DAT/kg body weight, before mating (Burnett et al., 1977).
7.5.3. Cell transformation
Several studies have shown that the 2,4-, 2,5-, 2,6-, and 3,4-
isomers can induce morphological transformations in Syrian golden
hamster embryo cells (Pienta et al., 1977a; Greene & Friedman,
1980). Each isomer chemically transformed secondary hamster embryo
cells, but none were active in more than 50% of the 5 or 6 separate
tests performed on each isomer (Greene & Freidman, 1980).
7.5.4. Chromosomal effects
Cytogenetic preparations were made from the bone marrow of male
mice, 30 and 48 h after intraperitoneal injection of 2 daily doses
of 2,4-DAT at 40 mg/kg body weight. The treatment did not induce
any obvious chromosome breaks (Soares & Lock, 1980).
2,5-DAT did not induce micronucleated cells in bone marrow
after oral administration of 120 mg/kg body weight to male and
female rats, in 2 doses separated by an interval of 24 h (Hossack &
Richardson, 1977).
7.6. Carcinogenicity
Several long-term studies on the carcinogenic potential of the
2,4-, 2,5-, and 2,6-DAT isomers have been published. The
experimental designs used in these studies are summarized in Table
11. The experimental designs used in 2 studies on the carcinogenic
effects of hair dye formulations are also given in Table 11.
Although the early studies of Umeda (1955) and Ito et al.
(1969) used protocols that generated data of minimal use for a
hazard evaluation, they did produce qualitative information showing
that 2,4-DAT was carcinogenic for rats. These studies have been
extended to the 2,5-, 2,6-, as well as 2,4-DATs using more animals
per study and well-defined protocols (NCI, 1978, 1979, 1980;
Weisburger et al., 1978).
Groups of 25 male Charles River/CD rats were administered 2,4-
DAT in the diet at time-weighted levels of 300 and 625 mg/kg for 18
months. Similarly, groups of 25 male and female CD-1 mice were
given diets containing 500 and 1000 mg 2,4-DAT/kg for 18 months
(Weisburger et al., 1978). The rats and mice used in these studies
had a high incidence of spontaneous tumours. However, there was a
statistically significant increase in subcutaneous fibromas in male
rats and hepatocellular carcinomas and vascular tumours in male and
female mice compared with controls.
Studies by the US National Cancer Institute on the
carcinogenicity of 2,4-DAT in rats and mice (NCI, 1979) confirmed
the report by Weisburger et al. (1978). Administration of time-
weighted average doses of 79 and 176 mg 2,4-DAT/kg diet to groups
of 50 male and 50 female Fisher 344 rats, for 103 weeks, led to a
severe depression in body weight gain, high mortality, and a dose-
related development of hepatocellular carcinomas or neoplastic
nodules in treated rats of both sexes. In addition, NCI reported
that carcinomas and adenomas of the mammary gland occurred in
female rats at incidences that were dose related and significantly
greater than those in the controls in both the high- and low-dose
groups. Groups of 50 male and 50 female B6C3F1 mice were similarly
administered 2,4-DAT at 100 or 200 mg/kg diet, for 101 weeks. In
male mice, tumour incidence was not significantly increased
compared with that in the control animals. However, the incidence
of hepatocellular carcinomas in female mice in both treated groups
was dose related and significantly higher than that in the
controls. Numbers of lymphomas were also higher in low-dose female
mice. On the basis of these results, it was concluded that 2,4-DAT
was carcinogenic for Fisher 344 rats of both sexes and for female
B6C3F1 mice (NCI, 1979).
Table 11. Experimental designs of carcinogenicity studies on diaminotoluenes
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,4- rat (male and female) 20 subcutaneous; 2 mg in 0.5 ml Umeda (1955)
propylene glycol weekly;
total of 28 injections; 452
days
2,4- Wistar rat (male) 12/12 (6) oral (diet); 0.6 and 1 g/kg; Ito et al.
36 weeks (1969)
2,4- Charles River/CD rat 25/25 (25) oral (diet); 500 and 1000 Weisburger et
(male) mg/kg for 4 months and 250 al. (1978)
Charles River mouse 25/25 (25) and 500 mg/kg for 14 months;
(male) oral (diet); 500 and 1000
Charles River mouse 25/25 (25) mg/kg for 18 months
(female)
2,5- Fisher 344 rat (male) 50/50 (50/25) oral (diet); 600 and 2000 NCI (1978)
Fisher 344 rat (female) 50/50 (50/25) mg/kg for 78 weeks + 31
weeks observation
B6C3F1 mouse (male) 50/50 (50/50) oral (diet); 600 and 2000
B6C3F1 mouse (female) 50/50 (50/50) mg/kg for 78 weeks + 19
weeks observation
2,4- Fisher 344 rat (male) 50/50 (20) oral (diet); 125 and 250 NCI (1979)
Fisher 344 rat (female) 50/50 (20) mg/kg for 40 weeks reduced
to 50 and 100 mg/kg for 63
weeks (time-weighted-average
79 and 176 mg/kg); high-dose
males killed after 79 weeks
and high-dose females after
84 weeks
2,4 B6C3F1 mouse (male) 50/50 (20) oral (diet); 100 and 200 NCI (1979)
B6C3F1 mouse (female) 50/50 (20) mg/kg for 101 weeks
---------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------
Isomer Species (sex) Initial size of Route of administration/ Reference
high-/low-dose dose and duration
groups (control)
---------------------------------------------------------------------------------------------------------
2,6- Fisher 344 rat (male) 50/50 (50) oral (diet) 250 and 500 NCI (1980)
Fisher 344 rat (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
B6C3F1 mouse (male) 50/50 (50) oral (diet); 250 and 500
B6C3F1 mouse (female) 50/50 (50) mg/kg for 103 weeks plus 1
week observation
2,5- Sprague Dawley rat (male) 50 (50) dermal application twice Kinkel &
(formulations Sprague Dawley rat 50 (50) weekly of 0.5 g of synthetic Holzmann (1973)
with 6% (female) formulation containing 4%
hydrogen 2,5-DAT mixed with equal
peroxide volume of 6% H202; treated 2
added) years
2,5- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(equal mixture male and ml weekly of formulation to (1975)
female) which equal volume of 6%
H202 had been added;
treatment period, 18 months
2,5- + 2,4- Swiss Webster mouse 100 (250) dermal application of 0.05 Burnett et al.
(formulations (equal mixture male and ml weekly of formulation to (1975)
with 6% female) which equal volume of 6%
hydrogen H202 had been added;
peroxide treatment period, 18 months
added) (same control group as
above study)
---------------------------------------------------------------------------------------------------------
The carcinogenic potential of 2,5-DAT and 2,6-DAT for rats and
mice was determined by the US National Cancer Institute. After
administration of 2,5-DAT at 600 and 2000 mg/kg feed to groups of
50 male and 50 female Fisher 344 rats and 50 male and 50 female
B6C3F1 mice, for 78 weeks, there was not sufficient evidence to
demonstrate the carcinogenicity of 2,5-DAT (NCI, 1978). However,
the study had been curtailed and this reduced the potential of the
test for detecting carcinogenicity.
Using a similar protocol, 2,6-DAT was incorporated at levels of
250 and 500 mg/kg into the diet of groups of 50 male and 50 female
Fisher 344 rats, and B6C3F1 mice for 103 weeks (NCI, 1980). There
was some question of whether mice of either sex received a maximum
tolerated dose, but the dose given to rats appeared to be at the
maximum tolerated level. As reported by NCI (1980), islet-cell
adenomas of the pancreas and neoplastic nodules or carcinomas of
the liver occurred in male rats in dose-related trends that were
significant using the Cochran-Armitage test, but not using the
Fisher exact test. In the low-dose male mice, NCI reported that
the incidence of lymphomas was greater than that in the controls.
However, the incidence was not significant when the Bonferroni
criterion of multiple comparison was used. Similarly, the
occurrence of hepatocellular carcinomas in female mice was dose
related, but not significant by the Fisher exact test, when the
incidence in the high-dose groups was compared with that in the
controls. Under the conditions of the bioassay, it was concluded
that 2,6-DAT was not carcinogenic for male and female F344 rats or
for male and female B6C3F1 mice (NCI, l980). Summaries of the 3
NCI bioassays have been published by Cardy (1979), Reuber (1979),
and Sontag (1981).
Using the protocols outlined in Table 11, no evidence of
carcinogenicity was obtained when hair-dye formulations containing
2,5- and 2,5- plus 2,4-DAT were painted on the skin of rats and
mice (Kinkel & Holzmann, 1973; Burnett et al., 1975). Given the
duration of exposure, the amounts of diaminotoluenes placed on the
skin, and the use of hydrogen peroxide prior to administration,
such negative results would be expected in studies on the formula
containing 2,4-DAT (Burnett et al., 1975). These studies have
shown a low order of dermal toxicity for these hair dyes, even
after long-term exposure. However, no definitive statement can be
made on the carcinogenic potential of 2,4- and 2,5-DAT after dermal
administration.
8. EFFECTS ON MAN
8.1. Single and Short-Term Exposures
In human beings, as in animals, diaminotoluenes are considered
to be irritants for the mucous membranes and skin, and to lead to
conjunctivitis and corneal opacities. When solutions come into
contact with skin, they can cause irritation, severe dermatitis,
and blistering (Von Oettingen, 1941). In case of the inhalation of
fumes, coughing, dyspnoea, and respiratory distress can result. No
data are available for evaluating the sensitizing potential of
diaminotoluenes. In the case of ingestion of massive amounts,
nausea, vomiting, and diarrhoea would occur, with the possible
production of methaemoglobinaemia. No cases of human poisoning
from short-term exposures to 2,4-DAT have been documented in the
published literature.
8.2. Long-Term Occupational Exposure - Epidemiological Studies
Filatova et al. (1970) investigated the physiological and
biochemical status of workers in a plant manufacturing
diaminotoluenes. Fifty-two of the 59 workers (58 males and 1
female) had worked in the plant for > 2 1/2 years. Seventeen
workers were between 30 and 45 years of age and 42 workers were
under 30 years old. In general, all workers had equal exposure to
the toxic chemicals used at the plant, namely, dinitrotoluene,
diaminotoluenes, methanol, and o-dichlorobenzene. There were a
few complaints of headache (2 cases), excess coughing (2 cases),
stomach pains (2 cases), and chest pain (4 cases); however, the
majority of the workers did not exhibit any exposure-related
adverse effects. Nevertheless, the investigators concluded that
the 10 workers exhibiting the symptoms listed were indeed affected
by their exposure to the complex of chemicals at this factory. It
is impossible to delineate the role played by the diaminotoluenes
in the production of these adverse effects.
The US National Institute for Occupational Safety and Health
(NIOSH) evaluated the reproductive health of workers in 3 plants
manufacturing diaminotoluenes (NIOSH, 1980, 1981, 1982). Exposure
usually involved both DAT and dinitrotoluene (DNT). All 3 surveys
were conducted in response to requests from employees or their
unions. The reason for the first request was the workers' belief
that their wives were suffering increased rates of spontaneous
abortion. The other 2 studies were prompted by the publicity given
to the first study.
In 2 of the studies, environmental hygiene sampling took place
(section 4.2) and workers were invited to volunteer for a medical
examination, to complete a reproductive history questionnaire, and
to provide semen and blood samples. Semen samples were analysed
for volume, sperm count, and sperm morphology. Blood samples were
analysed for various markers of renal and hepatic function, neither
of which showed any significant inter-group differences in any of
the studies. Wives of workers were given a more detailed
reproductive history questionnaire.
In the first study (NIOSH, 1980), there were 44 volunteers, 30
of whom provided usable semen specimens. The total potential study
population was not given. Of the 30, 9 were exposed, 9 were
controls, and 12 were in an intermediate category. The rate of
spontaneous abortions was higher among the wives of exposed workers
(6/18 pregnancies while the husband was exposed) compared with the
controls (4/23 pregnancies), with 6/28 for the intermediate group.
The small number of congenital malformations was not exposure
related. The sperm count for the exposed (median = 49 million) was
significantly ( P < 0.03) lower than that for the control group
(median = 121 million); however, the latter figure was unusually
high. The exposed group showed a significant reduction in the
proportion of the large morphological type. The second study
involved only a reproductive history questionnaire and the
reporting of hygiene data by the company. Thirty-five out of 41
workers in DAT- and DNT-production areas were interviewed. The
rates of congenital abnormalities or spontaneous abortion did not
significantly differ between exposure groups. Where the husband
was employed in DNT production, 1 out of 9 pregnancies ended in a
spontaneous abortion; the equivalent data for DAT was 1 miscarriage
out of 14 pregnancies.
In the third study, 50 volunteers were examined, 41 of whom
provided semen specimens. The total eligible population was not
given, though it was reported that 25 workers were regularly
exposed, 15 of whom participated in the study. There were no
significant differences in sperm count morphology between exposure
groups, but the miscarriage rate was reported to be significantly
( P < 0.05) higher for the wives of workers in the DAT-exposed area
of the plant, where 6 out of 15 pregnancies ended in miscarriage
compared with 1 out of 7 for the wives of DNT-exposed workers, and
3 out of 38 for the wives of unexposed workers.
The ranges of levels of reported exposures overlapped between
the 3 studies and were all within the OSHA recommended standard of
1.5 mg/m3 for dinitrotoluenes (NIOSH, 1982). The first study might
have been expected to show an excess, because it was provoked by a
cluster of miscarriages. All the studies were of limited size and
subject to some risk of selection bias, because the population was
restricted to those who volunteered. Also, in all 3 studies, crude
figures, with no adjustment for age, were presented. However, in
the 2 follow-up studies, where apparently neither of the
populations held a prior belief that there was an excess
miscarriage rate, it is significant that an apparent excess of
miscarriages was found in the wives of DAT-exposed workers.
An epidemiological assessment of the reproduction hazards for
males after occupational exposure to diaminotoluenes and
dinitrotoluenes was carried out by Hamill et al. (1982). Reported
occupational exposures were similar to those reported by NIOSH
(1980, 1981, 1982) and were generally well within the OSHA
recommended level of 1.5 mg/m3 for dinitrotoluenes. Examination of
84 workers and 119 unexposed subjects consisted of semen analysis,
blood testing, medical examination, and an interview. Seventy-two
percent of non-vasectomized exposed workers provided semen samples.
These groups of workers were defined by exposure intensity,
frequency, and recency, and compared with controls. Although no
significant differences in miscarriage rates were reported between
exposure categories, the categories were as defined at the time of
the study, not at the time of pregnancy. Fertility rates were
reported to be unaffected by exposure, but no figures were given.
There were no statistically significant differences in semen
analysis, sperm count and morphology, and FSH levels, between the 3
exposure groups and unexposed workers. The authors concluded that
the results of their study suggested that no detectable
reproductive effects existed among male workers exposed to
dinitrotoluenes and diaminotoluenes.
Levine et al. (1985) reported an analysis of the fertility of
workers exposed to DNT and DAT. The approach taken consisted of
workers completing reproductive history questionnaires and of
observed births being compared with expected births for the married
workers. Expected births were derived from US birth rates by age,
calendar year, and parity, and the ratio of observed to expected
was expressed as a standardized fertility ratio (SFR). Populations
of 137, 207, and 235 persons, respectively, were studied and were
largely, but not exclusively, male. It is not clear whether any of
the plants were the same as those described above. Comparisons of
SFR between different exposure categories, both for the whole
population and also among individuals who spent at least part of
their reproductive life exposed, did not reveal any significant
effects on SFR between exposure groups. The authors estimated that
the power of this study to detect a 50% reduction in fertility
would have been 90%. In the third NIOSH study, the miscarriage
rate for wives of DAT-exposed workers was 6.8 times higher than
that for wives of unexposed workers (rates given as miscarriages
per 100 person-years), but the fertility rate was only 0.8 times
lower (ratio of rates of live births per 100 person-years). Thus,
overall fertility may not be a sensitive index of adverse
reproductive outcome.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1. Evaluation of Human Health Risks
9.1.1. General considerations
There are insufficient data on the effects of diaminotoluenes
on human beings to carry out a detailed hazard assessment or risk
evaluation. However, absorption and metabolic studies in human
beings have indicated that diaminotoluenes are rapidly absorbed,
metabolized, and excreted in the urine in a manner similar to that
found in experimental animals. Therefore, the risk evaluation that
follows is based largely on data from animals, supported by data
from human studies, where available.
9.1.2. Assessment of exposure
Diaminotoluenes can be absorbed through the skin and
gastrointestinal tract, and by inhalation. Given the properties of
this class of chemicals, the major route of human exposure is
dermal, in the work-place, with a possibility of the inhalation of
fumes during heating. Exposure through ingestion is minimal,
except in case of accidents.
No data exist on general ambient levels of diaminotoluenes in
air, water, and food. Bioaccumulation of diaminotoluenes in the
food-chain should not occur. Levels in the work-place air of up to
0.44 mg/m3, with occasional excursions up to 11 mg/m3, have been
reported.
9.1.3. Single and short-term exposures
Diaminotoluenes are classed as toxic, highly irritant
chemicals. The oral LD50 for animals is between 270 and 350 mg/kg
body weight. Dermal contact has been shown to cause irritation,
severe dermatitis, blistering, and possible skin sensitization.
Single oral doses of diaminotoluenes of 50 mg/kg body weight have
led to methaemoglobinaemia in rats, rabbits, and guinea-pigs. Eye
contact with diaminotoluenes has led to conjunctivitis and corneal
opacities. In case of inhalation of fumes, coughing, dyspnoea, and
respiratory distress can result.
9.1.4. Long-term exposure
9.1.4.1. Carcinogenicity and mutagenicity
No epidemiological data are available on the incidence of
cancer in human beings after exposure to diaminotoluenes.
Several studies using 2,4-DAT have been carried out on
experimental animals and, in each, the isomer was shown to be
carcinogenic for rats and mice. In the most recent study, doses
of, or greater than, 79 mg/kg diet led to an increase in
hepatocellular carcinomas or neoplastic nodules in rats; there was
an increase in hepatocellular carcinomas and lymphomas in female
mice at doses exceeding 100 mg/kg diet.
Using a similar protocol, the US NCI concluded that 2,6-DAT was
not carcinogenic for rats and mice after administration of up to
500 mg/kg diet for 103 weeks. It should be noted that
hepatocellular carcinomas, neoplastic nodules, and lymphomas were
detected, as in the bioassay for 2,4-DAT, however, they were
considered not significant after detailed statistical analyses.
There was no evidence of carcinogenicity in mice and rats after
administration of 2,5-DAT at levels of up to 2000 mg/kg diet, for
78 weeks. However, the short duration of the study reduced the
potential of the test for detecting carcinogenicity. No evidence
of carcinogenicity was noted after the dermal application of hair-
dye formulations containing 2,5-DAT (following application of
hydrogen peroxide) or a mixture of 2,5-DAT and 2,4-DAT with
hydrogen peroxide.
Positive mutagenic activity was noted in S. typhimurium when
2,4-, 2,5-, 2,6-, and 3,4-DAT were tested. In addition, DAT
isomers were mutagenic in mammalian cells in vitro. Significant
DNA damage was produced by 2,4-DAT in human cultured fibroblasts,
only after activation by prostoglandin synthase. The isomer 2,4-
DAT was weakly mutagenic in Drosophila melanogaster and induced
unscheduled DNA synthesis in primary rat hepatocytes in vitro.
The 2,4- and 2,5-isomers were inactive in in vivo mammalian
mutagenicity assays. Micronuclei and dominant lethals were not
produced by 2,5-DAT, and 2,4-DAT did not produce chromosomal
breaks, dominant lethals, abnormal sperm morphology, or recessive
spots.
It has been shown that 2,4-, 2,5-, and 2,6-DAT can inhibit DNA
synthesis in the testes after ip injection of high doses. On the
basis of this study, 2,4-DAT may pose a genetic hazard in addition
to its potential to cause adverse effects on reproduction.
9.1.4.2. Reproduction and teratogenicity
The results of limited studies on the reproduction hazards for
male workers exposed to diaminotoluenes are equivocal. In surveys
of reproductive outcome in 3 plants, an excess of spontaneous
abortions among the wives of male workers exposed to DAT and
dinitrotoluene was reported in 2 surveys, though these excesses
were based on small numbers, and not all workers in the plants
participated in the studies. In 1 out of the 3 plants studied,
some adverse effects on spermatogenesis were suggested. Analysis
of the overall fertility of workers in 3 other production plants
did not reveal any adverse effects from exposure to DAT.
In a study on animals fed 2,4-DAT, there was a significant and
persistent decrease in the sperm count.
Embryotoxicity was observed in animal studies after oral and
dermal doses exceeding 30 mg/kg body weight for the 2,3-and 3,4-
isomers and 10 mg/kg body weight for 2,6-DAT.
Skeletal changes were noted after dermal application of a hair-
dye formula containing 3% 2,4-DAT at 2 ml/kg body weight.
9.2. Evaluation of Effects on the Environment
Information is lacking concerning levels of diaminotoluenes in
the environment, and their transport, bioconcentration,
biotransformation, and biodegradation.
A few data indicate that diaminotoluenes may be hazardous for
aquatic organisms. No data on the effects of diaminotoluenes on
other non-mammalian targets in the environment could be found.
9.3. Conclusions
Diaminotoluenes are highly irritating to the skin and eyes and
the fumes are irritating to the respiratory tract. They are
readily absorbed through the skin. Methaemoglobinaemia may occur
in exposed individuals. Renal toxicity after oral administration
of 2,4-DAT has been reported in experimental animals. 2,4-DAT has
been shown to be carcinogenic for animals, but there is inadequate
evidence to evaluate the carcinogenic potential of 2,5- and 2,6-
diaminotoluene. All 3 of these isomers have been shown to be
mutagenic. Limited data are available concerning a reproductive
hazard for male workers handling DATs. DATs have been shown to
impair spermatogenesis in experimental animals and to be both
embryotoxic and teratogenic.
10. RECOMMENDATIONS
1. Monitoring should be undertaken to determine the sources,
levels, and fate of diaminotoluenes in the environment.
Ecotoxicity data should be collected.
2. For a better evaluation of occupational exposure and effects,
studies on the uptake, kinetics, and metabolism of DAT and the
relevant routes of exposure are important to provide a sound
basis for biological monitoring.
3. To assist in the development of appropriate health
surveillance systems, a systematic evaluation of the toxicity
of diaminotoluenes should be carried out to compliment
available data on carcinogenicity and reproductive effects.
4. Additional data should be obtained on human morbidity and
mortality related to exposure to diaminotoluenes, with
particular emphasis on carcinogenic, teratogenic, and
reproductive end-points.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1978) evaluated the data on the carcinogenicity of
diaminotoluenes and concluded that there was sufficient evidence of
the carcinogenicity of 2,4-diaminotoluene in experimental animals.
An evaluation of additional data by IARC (1986) further supported
this conclusion.
In the absence of case reports or epidemiological studies,
there was inadequate data to assess the carcinogenicity of
diaminotoluenes for human beings (IARC, 1978).
REFERENCES
AUNE, T., NELSON, S.D., & DYBING, E. (1979) Mutagenicity and
irreversible binding of the hepatocarcinogen 2,4-diaminotoluene.
Chem.-biol. Interact., 25(1): 23-24.
AUSTIN, G.T. (1974) Industrially significant organic chemicals.
Part 9. Chem. Eng., 11: 96-100.
BACKUS, J.K. (1974) Urethanes. In: Considine, D.M., ed.
Chemical and process technology encyclopedia, New York, McGraw
Hill Book Co., pp. 1121-1125.
BECCI, P.J., REAGAN, E.L., KNICKERBOCKER, M.J., BARBEE, S.J.,
& WEDIG, J.H. (1983) Teratogenesis study of o-toluenediamine
in rats and rabbits. Toxicol. appl. Pharmacol., 71: 323-329.
BECHER, G. (198l) Glass capillary columns in the gas
chromatographic separation of aromatic amines. 2. Application
of samples from workplace atmospheres using nitrogen-selective
detection. J. Chromatogr., 211(1): 103-111.
BERMUDEZ, E. & BUTTERWORTH, B.E. (1979) Analysis of the
activities of 2,4-diaminotoluene and 2,4- and 2,6-dinitrotoluene
in the primary hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 1: 168-169.
BERMUDEZ, E., TILLERY, D., & BUTTERWORTH, B.E. (1979) Effect
of 2,4-diaminotoluene and isomers of dinitrotoluene on
unscheduled DNA synthesis in primary rat hepatocytes. Environ.
Mutagen., 1: 39l-398.
BIERNACKA, T., SEKOWSKA, B., & MICHONSKA, J. (1974) [Determination
of 2,4- and 2,6-diaminotoluene in technical products by infra-red
spectroscopy.] Chem. Anal. (Warsaw), 19: 6l9-632 (in Polish).
BLIJLEVEN, W.G.H. (1977) Mutagenicity of four hair dyes in
Drosophila melanogaster. Mutat. Res., 48: 181-186.
BOUFFORD, C.E. (l968) Determination of isomeric diaminotoluenes
by direct gas liquid chromatography. J. Gas Chromatogr., 6:
438-440.
BUIST, J.M. (1970) Isocyanates in industry. Proc. R. Soc. Med.,
63: 365-366.
BURNETT, C., LANMAN, B., GIOVACCHINI, R., WOLCOTT, G., & SCALA, R.
(1975) Long-term toxicity studies on oxidation hair dyes.
Food Cosmet. Toxicol., 13(3): 353-357.
BURNETT, C., GOLDENTHAL, E.I., HARRIS, S.B., WAZETER, F.X.,
STRAUSBURG, J., KAPP, R., & VOELKER, R. (1976) Teratology
and percutaneous toxicity studies on hair dyes. J. Toxicol.
environ. Health, 1: 1027-1040.
BURNETT, C., LOEHR, R., & CORBETT, J. (1977) Dominant lethal
mutagenicity study on hair dyes. J. Toxicol. environ. Health,
2: 657-662.
CARDY, R.H. (1979) Carcinogenicity and chronic toxicity of
2,4-toluenediamine in F344 rats. J. Natl Cancer Inst., 62:
1107-1116.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F., Jr (1974)
Range-finding toxicity data. Toxicol. appl. Pharmacol., 28:
313-319.
CHOUDHARY, G. (1980) Gas-liquid chromatographic determination
of toxic diamines in permanent hair dyes. J. Chromatogr., 193:
277-284.
CIC JAPAN (1983) Commercial industrial chemicals, Tokyo,
Chemical Daily Co., Ltd.
CINKOTAI, K.I., JONES, C.C., TOPHAM, J.C., & WATKINS, P.A. (1978)
Experience with mutagenic tests as indicators of carcinogenic
activity. Mutat. Res., 53: 167-168.
COPPINGER, W.J., BRENNAN, S.A., CARVER, J.H., & THOMPSON, E.D.
(1984) Locus specificity of mutagenicity of 2,4-diaminotoluene in
both L5l78Y mouse lymphoma and AT3-2 chinese hamster ovary cells.
Mutat. Res., 135: 115-123.
CRC (1975) CRC handbook of chemistry and physics, 56th ed.,
Cleveland, Ohio, CRC Press.
DENT, J.G. & GRAICHEN, M.E. (1982) Effect of hepatocarcinogens
on epoxide hydrolase and other xenobiotic metabolizing enzymes.
Carcinogenesis, 3(7): 733-738.
DYBING, E. & THORGEIRSSON, S.S. (1977) Metabolic activation
of 2,4-diaminoanisole, a hair dye component. 1. Role of
cytochrome P-450 metabolism in mutagenicity in vitro. Biochem.
Pharmacol., 26: 729-734.
DYBING, E., AUNE, T., & SODERLUND, E.J. (1977) Use of the
Salmonella mutagenicity test in drug metabolism studies. Acta
pharmacol. toxicol., 41: 31.
DYBING, E., AUNE, T., & NELSON, S. (1978) Covalent binding
of 2,4-diaminoanisole and 2,4-diaminotoluene in vivo. Toxicol.
Aspects Food Saf. Arch. Toxicol., Suppl. 1: 213-217.
FAHMY, M.J. & FAHMY, O.G. (1977) Mutagenicity of hair dye
components relative to the carcinogens benzidine in Drosophila
melanogaster. Mutat. Res., 56: 31-38.
FILATOVA, V.S., TUBINA, A.Ya., SHARONOVA, Z.V., GOLOVA, I.A.,
FILINA, V.I., & DOROFEEVA, E.D. (1970) [Hygienic aspects of
work and health of workers in the production of toluylene-
diamine.] Gig. i Sanit., 35(2): 16-30 (in Russian with English
summary).
GAN, L.H., BLAIS, P., CARLSSON, D.J., SUPRUNCHUCK, T., & WILES, D.M.
(1975) Physicochemical characterization of some fully aromatic
polyamides. J. appl. polym. Sci., 19: 69-82.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., WEISBURGER, E.K., &
ROLLER, P.P. (1975) Enzymic N-acetylation of 2,4-toluenediamine
by liver cytosols from various species. Xenobiotica, 5(8): 475-483.
GLINSUKON, T., BENJAMIN, T., GRANTHAM, P.H., LEWIS, N.L., &
WEISBURGER, E.K. (1976) N-acetylation as a route of 2,4-
toluenediamine metabolism by hamster liver cytosol. Biochem.
Pharmacol., 25: 95-97.
GRANTHAM, P.H., MOHAN, L., BENJAMIN, T., ROLLER, P.P., MILLER, J.R.,
& WEISBURGER, E.K. (1980) Comparison of the metabolism of 2,4-
toluenediamine in rats and mice. J. environ. Pathol. Toxicol.,
3(1-2): 149-l66.
GREENE, E.J. & FRIEDMAN, M.A. (1980) In vitro cell transformation
screening of 4 toluene diamine isomers. Mutat. Res., 79: 363-375.
GREENE, E.J., FRIEDMAN, M.A., & SHERROD, J.A. (1979) In vitro
mutagenicity and cell transformation testing of four toluene
diamine isomers. Environ. Mutagen., 1: 194.
GREENE, E.J., SALERNO, A.J., & FRIEDMAN, M.A. (1981) Effect
of 4 toluene diamine isomers on murine testicular DNA synthesis.
Mutat. Res., 91: 75-79.
GUTHRIE, J.L. & MCKINNEY, R.W. (1977) Determination of 2,4-
and 2,6-diaminotoluene in flexible urethane foams. Anal.
Chem., 49: 1676-1680.
HAMILL, V.V., STEINBERGER, E., LEVINE, R.J., RODRIGUEZ-RIGAU,
L.J., LEMESHOW, S., & AVRUNIN, J.S. (1982) The epidemiologic
assessment of male reproductive hazard from occupational
exposure to TDA and DNT. J. occup. Med., 24: 982-993.
HOSSACK, D.J.N. & RICHARDSON, J.C. (1977) Examination of the
potential mutagenicity of hair dye constituents using the
micronucleus test. Experientia (Basel), 33: 377-378.
HRUBY, R. (1977) The absorption of p-toluenediamine by the
skin of rats and dogs. Food Cosmet. Toxicol., 15: 595-599.
IARC (1978) 2,4-Diaminotoluene. In: Some aromatic amines and
related nitro compounds: hair dyes, colouring agents, and
miscellaneous industrial compounds, Lyons, International
Agency for Research on Cancer, pp. 83-95 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 16).
IARC (1986) Some chemicals used in plastics and elastomers,
Lyons, International Agency for Research on Cancer, p. 304
(Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 39).
INOUYE, M. & MURAKAMI, U. (1976) Teratogenicity of 2,5-
diaminotoluene, a hair dye component in mice. Teratology, 14:
241-242.
INOUYE, M. & MURAKAMI, U. (1977) Teratogenicity of 2,5-
diaminotoluene, a hair dye constituent in mice. Food Cosmet.
Toxicol., 15: 447-451.
ITO, N., HIASA, Y., KONISHI, Y., & MARUGAMI, M. (1969) The
development of carcinoma in liver of rats treated with
m-toluylenediamine and the synergistic and antagonistic
effects with other chemicals. Cancer Res., 29: 1137-1145.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982)
Toxicometric paramaters of industrial toxic chemicals under
single exposure, Moscow, USSR/SCST/USSR Commission for UNEP,
Centre of International Projects.
JOHANSSON, K., RAPPE, C., LINDBERG, W., & NYGREN, M. (1981)
Determination of aromatic diamines in hair dyes using liquid
chromatography. In: Egan, H., Fishbein, L., O'Neil, I.K.,
Castegnaro, M., & Bartsch, H., ed. Environmental carcinogens
selected methods of analysis. Volume 4: Some aromatic amines
and azo dyes in the general and industrial environment. Lyons,
International Agency for Research on Cancer (IARC Publications
No. 40).
KIESE, M. & RAUSCHER, E. (1968) The absorbtion of p-toluene-
diamine through human skin in hair dyeing. Toxicol. appl.
Pharmacol., 13: 325-331.
KIESE, M., RACHOR, M., & RAUSCHER, E. (1968) The absorption
of some phenylenediamines through the skin of dogs. Toxicol.
appl. Pharmacol., 12: 495-507.
KINKEL, H.J. & HOLZMANN, S. (1973) Study of long-term
percutaneous toxicity and carcinogenicity of hair dyes
(oxidizing dyes) in rats. Food Cosmet. Toxicol., 11: 641-648.
KNICKERBOCKER, M., RE, T.A., PARENT, R.A., & WEDING, J.H.
(1980) Teratogenic evaluation of ortho-toluenediamine
(o-TDA) in Sprague Dawley rats and Dutch Belted rabbits.
Toxicologist, 19: A.89.
KORNBRUST, D.J. & BARFKNECHT, T.R. (1984) Comparison of 7
azo dyes and their reduction products in the rat and hamster
hepatocyte primary culture/DNA-repair assays. Mutat. Res.,
136: 255-266.
KOTTEMANN, C.M. (1966) Two dimensional thin-layer
chromatographic procedure for the identification of dye
intermediates in arylamine oxidation hair dyes. J. Assoc. Off.
Anal. Chem., 49(5): 954-959.
LEVINE, R.J., CORSO, D.D., & BLUNDEN, P.B. (1985) Fertility
of workers exposed to dinitrotoluene and toluenediamine at
three chemical plants. In: Rickert, D.E., ed. Toxicity of
nitroaromatic compounds, Washington DC, Hemisphere.
LIEM, D.H. & ROOSELAAR, J. (1981) HPLC of oxidation hair
colours. Mitt. Geb. Lebensm. Hyg., 72: 164-176.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975)
Detection of carcinogens as mutagens in the Salmonella microsome
test: Assay of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72:
5135-5139.
MACKE, G.F. (1968) Use of tetracyanoethylene as a thin-layer
chromatographic spray reagent. J. Chromatogr., 36: 537-539.
MARKS, T.A., GUPTA, B.N., LEDOUX, T.A., & STAPLES, R.E.
(1981) Teratogenic evaluation of 2-nitro-p-phenyl-enediamine,
4-nitro-o-phenylendiamine, and 2,5-toluenediamine sulfate in
the mouse. Teratology, 24: 253-265.
MARZULLI, F.N., ANJO, D.M., & MAIBACH, H.I. (1981) In vivo
skin penetration studies of 2,4-toluenediamine, 2,4-diamino-
anisole, 2-nitro-p-phenylenediamine, p-dioxane and n-nitro-
sodiethanolamine in cosmetics. Food Cosmet. Toxicol., 19: 743.
MATHESON, D. & CREASY, B. (1976) Use of the L5178Y (TK+/-)
mouse lymphoma cell line coupled with an in vitro microsomal
enzyme activation system to study chemical promutagens. Mutat.
Res., 38: 400-401.
MATHIAS, A. (1966) Analysis of diaminotoluene isomer
mixtures by nuclear magnetic resonance spectrometry. Anal.
Chem., 38: 1931-1932.
MATSUI, S., MURAKAMI, T., SASAKI, T., HIROSE, Y., & IGUMA, Y.
(1975) Activated sludge degradability of organic substances
in the wastewater of the Kashima petroleum and petrochemical
industrial complex in Japan. Prog. Water Technol., 7:645-659.
MILLIGAN, B. & GILBERT, K.E. (1978) Diaminotoluenes. In:
Kirk-Othmer encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 1, pp. 321-329.
MIRSALIS, J.C. & BUTTERWORTH, B.E. (1982) Induction of
unscheduled DNA synthesis in rat hepatocytes following in vivo
treatment with dinitrotoluene. Carcinogenesis, 3: 241-245.
MIRSALIS, J.C., TYSON, C.M., & BUTTERWORTH, B.E. (1982)
Detection of genotoxic carcinogens in the in vivo/in vitro
hepatocyte DNA repair assay. Environ. Mutagen., 4: 553-562.
MORI, M.A., MIYAHARA, T., TANIGUCHI, K., HASEGAWA, K., KOZUKA,
H., MIYAGOSHI, M., & NAGAYAMA, T. (1982) Mutagenicity of
2,4-dinitrotoluene and its metabolites in Salmonella typhimurium.
Toxicol. Lett., 13: 1-5.
NCI (1978) Bioassay of 2,5-toluenediamine sulfate for
possible carcinogenicity, Bethesda, Maryland, National Cancer
Institute (NCI Carcinogenesis Technical Report Series No. 126;
DHEW Publication No. (NIH) 78-1381).
NCI (1979) Bioassay of 2,4-diaminotoluene for Possible
Carcinogenicity, Bethesda, Maryland, National Cancer Institute
(NCI Carcinogenesis Technical Report Series No. 162).
NCI (1980) Bioassay of 2,6-toluenediamine dihydrochloride
for possible carcinogenicity, Bethesda, Maryland, National
Cancer Institute (NCI Carcinogenesis Technical Report Series
No. 200; NTP No. 80-20; NIH Publication No. 80-1756).
NIEMINEN, E.H., SAARINAN, L.H., & LAAKSO, J.T. (1983)
Simultaneous determination of aromatic isocyanates and some
carcinogenic amines in the work atmosphere by reversed-phase
high-performance liquid chromatography. J. liq. Chromatogr.,
6: 453-469.
NIOSH (1978) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Service, Center for Disease Control,
Vol. 4 (Publication No. 78-175).
NIOSH (1980) Health hazard evaluation determination report
HE 79-113-728, Brandenburg, Kentucky, Olin Chemical Company,
and Cincinnati, Ohio, National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1981) Interim Report No. 1. HETA 81-118,
New Martinsville, West Virginia, Mobay Chemical Corporation,
and Cincinatti, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NIOSH (1982) Interim report No. 1. HETA 81-295-1155,
Moundsville, West Virginia, Allied Chemical Company, and
Cincinnati, Ohio, US National Institute for Occupational
Safety and Health, US Department of Health and Human Services,
Center for Disease Control.
NORDENSKJOLD, M., ANDERSSON, B., RAHIMTULA, A., & MOLDEUS, P.
(1984) Prostaglandin synthesis-catalyzed metabolic activation
of some aromatic amines to genotoxic products. Mutat. Res.,
127: 107-112.
OLUFSEN, B. (1979) Glass capillary columns in the gas
chromatographic separation of aromatic amines. I. J. Chromatogr.,
179: 97-103.
OSHA (1983) Code of federal regulations, Washington DC, US
Occupational Safety and Health Administration, pp. 660-664
(29CFR, 19101000, Table Z-1).
PERKINS, W.E. & GREEN, T.J. (1975) Effect of 3,4-toluenediamine
on output from in situ rat brunner's glands pouches (38941).
Proc. Soc. Exp. Biol. Med., 149: 991-994.
PIENTA, R.J., POILEY, J.A., & LEBHERZ, W.B., III (1977a)
Morphological transformation of early passage golden Syrian
hamster embryo cells derived from cryopreserved primary
cultures as a reliable in vitro bioassay for identifying
diverse carcinogens. Int. J. Cancer, 19: 642-655.
PIENTA, R.J., SHAH, M.J., LEBHERZ, W.B., & ANDREWS, A.W.
(1977b) Correlation of bacterial mutagenicity and hamster
cell transformation with mutagenicity induced by 2,4-
toluenediamine. Cancer Lett., 3: 45-52.
PURNELL, C.J. & WARWICK, C.J. (1981) Application of electro-
chemical detection in high-performance liquid chromatography
to the measurement of toxic substances in air. Anal. Proc.,
18: 151-154.
PURNELL, C.J., BAGON, D.A., & WARWICK, C.J. (1982) The
determination of organic contaminant concentrations in
workplace atmospheres by high-performance liquid chromatography.
Pergamon Ser. environ. Sci., 7: 203-219.
RAHIMTULA, A., MOLDEUS, P., ANDERSSON, B., & NORDENSKJOLD, M.
(1982) Prostaglandin synthetase catalyzed DNA strand breaks
by aromatic amines. In: Prostaglandins and related lipids,
New York, Alan R. Liss, Vol. 2, pp. 159-162.
REUBER, M.D. (1979) Carcinomas of the liver in female mice
fed toluene-2,4-diamine. Gann, 70: 453-457.
RIGGIN, R.M. & HOWARD, C.C. (1983) High performance liquid
chromatographic determination of phenylenediamines in aqueous
environmental samples. J. liq. Chromatogr., 6: 1897-1905.
SCHAFER, U. METZ, J., PEVNY, I., & ROCKL, H. (1978)
[Attempts of sensitizing guinea pigs with five different
derivates of para-substituted benzene.] Arch dermatol. Res.,
216: 153-161 (in German).
SELYE, H. (1973) Production of perforating duodenal ulcers
by 3,4-toluenediamine in the rat. Proc. Soc. Exp. Biol. Med.,
142: 1192-1194.
SHAHIN, M.M., BUGAUT, A., & KALOPISSIS, G. (1980) Structure-
activity relationship within a series of m-diaminobenzene
derivatives. Mutat. Res., 78: 25-31.
SHOOTER, K.V. & VENITT, S. (1979) Phosphotriesters in DNA:
non-repairable lesions as markers for the early detection of
chemical carcinogens in intact animals. Mutat. Res., 64: 106.
SKARPING, G., RENMAN, L., & SMITH, B.E.F. (1983a) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. I. Determination of
aromatic amines as perfluoro-fatty acid amines using electron-
capture detection. J. Chromatogr., 267: 315-327.
SKARPING, G., RENMAN, L., & DALENE, M. (1983b) Trace
analysis of amines and isocyanates using glass capillary gas
chromatography and selective detection. II Determination of
aromatic amines as perfluoro-fatty acid amines using nitrogen-
selective detection. J. Chromatogr., 270: 207-218.
SMIRNOVA, A.N., TOROPOVA, L.A., & DOBROVOL'SKAYA, V.V.
(1967) [Effect of toluylenediamine and ortho-toluidine on
aquatic organisms.] Vodosnarzh Kanaltz Giurotekh, 5: 17-23 (in
Russian) (EPA translation).
SNYDER, R.C. & BREDER, C.V. (1982) High performance liquid
chromatographic determination of 2,4- and 2,6-toluenediamine
in aqueous extracts. J. Chromatogr., 236: 429-440.
SNYDER, R.C., BRUMLEY, W.C., BREDER, C.V., & FAZIO, T.
(1982) Gas chromatographic and gas chromatographic-mass
spectrometric confirmation of 2,4- and 2,6-toluenediamine
determined by liquid chromatography in aqueous extracts. J.
Assoc. Off. Anal. Chem., 65(6): 1388-1394.
SOARES, E.R. & LOCK, L.F. (1980) Lack of an indication of
mutagenic effects of dinitrotoluenes and diaminotoluenes in
mice. Environ. Mutagen., 2: 111-124.
SONTAG, J.M. (1981) Carcinogenicity of substituted-benzene-
diamines (phenylenediamines) in rats and mice. J. Natl Cancer
Inst., 66: 591-602.
SPENGLER, J., OSTERBURG, I., & KORTE, R. (1986) Abstract:
Teratogenic evaluation of p-toluenediamine sulphate.
Resorcinal and p-aminophenol in rats and rabbits. Teratology,
33(2): 31.
THYSEN, B., BLOCH, E., & VARMA, S.K. (1985a) Reproductive
toxicity of 2,4-toluenediamine in the rat. 2. Spermatogenic
and hormonal effects. J. Toxicol. environ. Health, 16: 763-769.
THYSEN, B., VARMA, S.K., & BLOCH, E (1985b) Reproductive
toxicity of 2,4-toluenediamine in the rat. 1. Effect on male
fertility. J. Toxicol. environ. Health, 16: 753-761.
UMEDA, M. (1955) Production of rat sarcoma by injections of
propylene glycol solution of m-toluylenediamine. Gann, 46:
597-606.
UNGER, P.D. & FRIEDMAN, M.A. (1979) High-performance liquid
chromatography of 2,6- and 2,4-diaminotoluene, and its
application to the determination of 2,4-diaminotoluene in
urine and plasma. J. Chromatogr., 174: 379-384.
UNGER, P.D., SALERNO, A.J., NESS, W.C., & FRIEDMAN, M.A.
(1980) Tissue distribution and excretion of
2,4[143]-toluenediamine in the mouse. J. Toxicol. environ.
Health, 6: 107-114.
US EPA (1980) Materials balance for 2,4-diaminotoluene.
Level 1. Preliminary, Washington DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/13:
79-016).
US ITC (1977) Synthetic organic chemicals: US production and
sales, 1975, Washington DC, US International Trade Commission,
pp. 22, 36, 42, 49, 60, 62, 65, 74 (US ITC Publication 804).
US ITC (1982) Preliminary report on US production of
selected synthetic organic chemicals. Preliminary totals 1981,
Washington DC, US International Trade Commission (SOC Series
C/P-82-1).
US ITC (1985) Preliminary report on US production of
selected synthetic organic chemicals. November, December, and
cummulative totals, 1984, Washington DC, US International
Trade Commission (SOC Series C/P-85.1).
VENITT, S. (1978) Mutagenicity of hair dyes: some more
evidence and the problems of its interpretation. Mutat. Res.,
53: 278-279.
VON OETTINGEN, W.F. (1941) The aromatic amino and nitro
compounds: their toxicity and potential dangers. A review of
the literature, Washington DC, Division of Industrial Hygiene,
National Institute of Health, pp. 55-63.
WAGNER, J.C. (1975) Linear compartment models. In:
Fundamentals of clinical pharmacokinetics, Hamilton, Illinois,
Drug Intelligence Publications, pp. 57-63.
WARING, R.H. & PHEASANT, A.E. (1976) Some phenolic metabolites
of 2,4-diaminotoluene in the rabbit, rat and guinea-pig.
Xenobiotica, 6: 257-262.
WEISBROD, D. & STEPHAN, U. (1983) [Studies of the toxic,
methaemoglobin-producing and erythrocyte-damaging effects of
diaminotoluene after a single administration.] Z. gesamte
Hyg., 29: 395-397 (in German).
WEISBURGER, E.K., RUSSFIELD, A.B., HOMBURGER, F., WEISBURGER,
J.H., BOGER, E., VAN DONGEN, C.G., & CHU, K.C. (1978)
Testing of twenty-one environmental aromatic amines or
derivatives for long-term toxicity or carcinogenicity.
J. environ. Pathol. Toxicol., 2(2): 325-356.
WILLEBOORDSE, F., QUICK, Q., & BISHOP, E. (1968) Direct gas
chromatographic analysis of isomeric diaminotoluenes. Anal.
Chem., 40: 1455-1458.
WILLIAM, R.T. (1971) The metabolism of certain drugs and
food chemicals in man. Ann. N.Y. Acad. Sci., 179: 141-154.
WILLIAMS, G.M. (1977) Detection of chemical carcinogens by
unscheduled DNA synthesis in rat liver primary cell cultures.
Cancer Res., 37: 1845-1851.
WILLIAMS, G.M. (1978) Further improvements in the hepatocyte
primary culture DNA repair test for carcinogens: detection of
carcinogenic biphenyl derivatives. Cancer Lett., 4: 69-75.
WILLIAMS, G.M. & LASPIA, M.F. (1979) The detection of
various nitrosamines in the hepatocyte primary culture/DNA
repair test. Cancer Lett., 66: 199-206.
