
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 73
PHOSPHINE AND SELECTED METAL PHOSPHIDES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1988
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154273 X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PHOSPHINE AND SELECTED METAL PHOSPHIDES
1. SUMMARY
1.1. Properties, analysis, and occurrence
1.2. Effects on organisms in the environment
1.3. Effects on animals
1.4. Effects on man
1.5. Evaluation
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity, physical and chemical properties
2.1.1. Phosphine
2.1.1.1 Identity
2.1.1.2 Physical and chemical properties
2.1.1.3 Conversion factors
2.1.2. Metal phosphides
2.2. Analytical methods
2.2.1. Gaseous phosphine
2.2.1.1 Direct-indicating methods
2.2.1.2 Absorptive or adsorptive sampling and analysis
2.2.1.3 Continuous methods
2.2.2. Residues
2.2.3. Metal phosphides
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 World production figures
3.2.1.2 Manufacturing processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Soil
4.1.3. Aquatic environment
4.1.4. Vegetation, wildlife, and entry into the food chain
4.2. Biotransformation
4.3. Ultimate fate
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air, water, and soil
5.1.2. Food and feed
5.1.2.1 Residue values
5.1.2.2 Factors affecting residue levels
5.1.3. Tobacco and consumer products
5.1.4. Terrestrial and aquatic organisms
5.2. General population exposure
5.2.1. Access to phosphine and phosphides
5.2.2. Residue exposure
5.2.3. Subgroups at special risk
5.3. Occupational exposure during manufacture, formulation, or use
6. KINETICS AND METABOLISM
6.1. Insects
6.2. Mammals
6.2.1. Absorption
6.2.1.1 Inhalation
6.2.1.2 Dermal
6.2.1.3 Oral
6.3. Distribution
6.4. Metabolic transformation
6.5. Elimination and excretion
6.6. Retention and turnover
6.7. Reaction with body components
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.3. Terrestrial organisms
7.3.1. Insects and mites
7.3.2. Birds
7.3.3. Mammals
7.3.3.1 Non-target species
7.3.3.2 Rodents
7.4. Plants
7.4.1. Harvested plants
7.4.2. Viable seeds and grain
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Inhalation studies on phosphine
8.1.2. Inhalation studies on zinc phosphide
8.1.3. Oral studies on metal phosphides
8.1.4. Dermal and other studies on metal phosphides
8.2. Short-term exposures
8.2.1. Inhalation exposure to phosphine
8.2.2. Oral exposure to metal phosphides
8.2.3. Dermal and other exposures
8.3. Skin and eye irritation; sensitization
8.4. Long-term exposure
8.5. Reproduction, mutagenicity, and carcinogenicity
8.6. Factors modifying toxicity; toxicity of metabolites
8.7. Mechanisms of toxicity - mode of action
9. EFFECTS ON MAN
9.1. Organoleptic effects
9.2. General population exposure
9.2.1. Phosphine
9.2.2. Metal phosphides
9.3. Occupational exposure
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
10.3. Conclusions
11. RECOMMENDATIONS
11.1. Gaps in knowledge
11.2. Preventive measures
11.2.1. Management
11.2.2. Treatment of poisoning
11.2.2.1 Inhalation of phosphine
11.2.2.2 Ingestion of metal phosphides
11.2.3. Leaks, spillages, residues, and empty containers
11.2.3.1 Phosphine
11.2.3.2 Aluminium and magnesium phosphides
and their formulated preparations
11.2.3.3 Zinc phosphide and preparations
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON PHOSPHINE AND SELECTED METAL PHOSPHIDES
Members
Professor E.A. Bababunmi, Laboratory of Biomembrane Research,
Department of Biochemistry, University of Ibadan, College
of Medicine, Ibadan, Nigeria
Dr L. Badaeva, All Union Scientific Research Institute of
Hygiene and Toxicology of Pesticides, Polymers and
Plastics, Kiev, USSR
Dr J.R. Jackson, Medicine and Environmental Health, Monsanto
Europe, Brussels, Belgium (Rapporteur)
Dr C.N. Ong, Department of Social Medicine and Public Health,
National University of Singapore, Kent Ridge, Singapore
Dr N.R. Price, United Kingdom Ministry of Agriculture,
Fisheries and Food, Slough Laboratory, Berkshire, United
Kingdom
Dr V.A. Rao, Department of Toxicology, Haffkine Institute,
Acharya Donde Marg, Bombay, India (Vice-Chairman)
Dr Ch. Reichmuth, Stored Products Protection Institute, Federal
Biological Research Centre for Agriculture and Forestry,
Berlin (West)
Dr F.G.R. Reyes, School of Food Engineering, State University of
Campinas, UNICAMP, Campinas, Sao Paulo, Brazil (Chairman)
Secretariat
Dr E.M. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
Dr J.K. Chipman, Department of Biochemistry, University of
Birmingham, Birmingham, United Kingdom (Temporary Adviser)
Dr Thomas K.-W. Ng, Office of Occupational Health, World
Health Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to
the Manager of the International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland, in order that
they may be included in corrigenda, which will appear in
subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained
from the International Register of Potentially Toxic Chemicals,
Palais des Nations, 1211 Geneva 10, Switzerland (Telephone no.
988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR PHOSPHINE AND SELECTED METAL PHOSPHIDES
A WHO Task Group on Environmental Health Criteria for
Phosphine and Selected Metal Phosphides met in Geneva on 17-21
November 1986. Dr E.M. Smith opened the meeting on behalf of
the Director-General. The Task Group reviewed and revised the
draft criteria document and made an evaluation of the risks for
human health and the environment from exposure to phosphine and
metal phosphides.
The original draft of this document was prepared by DR J.R.
JACKSON, Department of Medicine and Health Science, Monsanto
Europe, Brussels, Belgium.
The contributions of all who helped in the preparation and
finalization of this document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Effects.
1. SUMMARY
1.1. Properties, Analysis, and Occurrence
Phosphine, or hydrogen phosphide, is a colourless gas which
is odourless when pure, but the technical product usually has a
foul odour, described as "fishy" or "garlicky", because of the
presence of substituted phosphines and diphosphine (P2H4).
Other impurities may be methane, arsine, hydrogen, and nitrogen.
For fumigation, it is produced at the site by hydrolysis of a
metal phosphide and supplied in cylinders either as pure phos-
phine or diluted with nitrogen. Aluminium, magnesium (trimag-
nesium diphosphide), and zinc (trizinc diphosphide) phosphides
are the most commonly used metal phosphides for this purpose.
Phosphine is flammable and explosive in air and can
autoignite at ambient temperatures. It is slightly soluble in
water and soluble in most organic solvents. Metal phosphides
are usually powders of various colours, which hydrolyse in acids
to yield phosphine and metal salts. Aluminium and magnesium
phosphides hydrolyse in water.
Phosphine in air can be detected by the discoloration of
silver nitrate or indicator papers impregnated with mercury (II)
chloride, and can be measured using indicator tubes or by flame
photometry, infrared spectroscopy, mass spectrometry, or gas
chromatography. Samples can be taken on solid adsorbents and
desorbed for analysis. Various classical analytical techniques
are available in which phosphine is trapped by mercury (II)
salts.
Phosphine residues in foods can be measured by nitrogen
purging and trapping the phosphine. Phosphide residues can be
included in the analysis by extraction with silver nitrate or
sulfuric acid and measuring the chromophore or the phosphine
contents of the head-space gas, respectively.
Metal phosphides are most easily determined after hydrolysis
to phosphine. A method has been described in which magnesium
phosphide is converted to magnesium pyrophosphate, which is then
weighed.
Phosphine may occur naturally in the anaerobic degradation
of phosphorus-containing organic matter, such as in the
production of marsh gas. Naturally-occurring phosphides are
extremely rare. None have been reported in the earth's crust
but an iron-nickel phosphide (schreibersite) is found in all
iron meteorites.
Phosphine is manufactured by the hydrolysis of metal
phosphides, by the electrolysis of phosphorus in the presence of
hydrogen, and by a phosphorus-steam reaction. It is produced
incidentally by the hydrolysis of impurities in calcium carbide
(in the production of ethyne (acetylene)), in ferrosilicon and
spheroidal graphite iron, and in various metallurgical
operations. Metal phosphides can be prepared by the reduction
of phosphates, by a direct reaction between the metal and
phosphorus vapour or amorphous phosphorus, or by an exchange
reaction between the metal and another metal phosphide.
Phosphine is used in the synthesis of organophosphines and
organic phosphonium derivatives and as a dopant in the manu-
facture of semiconductors in the electronics industry. Formul-
ations of aluminium or magnesium phosphide are available for
fumigation in pest control. Zinc phosphide is used as a rodent-
icide in the form of a powder or a paste containing 2.5 or 5% of
zinc phosphide, which is incorporated in bait at 1 part in 10.
Phosphine in air reacts with HO x radicals and is removed by
this mechanism with a half time of 5 - 28 h depending on the
conditions. Phosphine in air is slowly absorbed by soil at a
rate that is dependent on surface effects and the permeability
of the soil matrix, and is slower in wet conditions. Zinc
phosphide undergoes negligible hydrolysis in a variety of
surface and ocean waters over a period of 11 days, but is
oxidized within about five weeks in soils with a 50% or more
saturated moisture content. Significant hydrolysis only occurs
at a pH value of 4 or less. Aluminium and magnesium phosphides
are rapidly hydrolysed in neutral moist conditions. Phosphine
and zinc, magnesium, and aluminium phosphides are inherently
degradable and non-persistent in the environment. The ultimate
fate for the phosphides is inorganic phosphate, water, and
metallic compounds.
Phosphine is normally undetectable in air, water, or soil.
Residues in fumigated foods depend on the technique of fumiga-
tion, but are normally low after aeration, except where a metal
phosphide fails to react completely. In general, residues are
below the WHO/FAO recommended levels of 0.1 mg/kg (PH3) for raw
cereals and below 0.01 mg/kg (PH3) for other stored products.
1.2. Effects on Organisms in the Environment
The antimicrobial activity of phosphine varies depending on
the microbial species, and the type and moisture content of the
product being fumigated. Many microorganisms survive fumigation
at exposures (concentration x time) that are effective against
arthropods.
The few studies available indicate that phosphine has little
effect on growing plants (e.g., sugar cane and lettuce) at
effective pesticidal doses. Germination of seeds was unaffected
by their fumigation with phosphine or by prior fumigation of the
soil in which they were planted, except when the moisture
content of the seed exceeded 20%. Lettuce sustained substan-
tially less damage after fumigation with phosphine than with 5
other fumigants.
The few data available on the effects of phosphine and
phosphides on aquatic organisms suggest that, despite its low
solubility, phosphine in solution can be acutely toxic.
Generally, insects, a principal target, are susceptible,
though the susceptibility at different stages of the life-cycle
varies and diapausing larvae are particularly tolerant. The
threshold for adverse effects of phosphine to Drosophila
melanogaster is about 1.4 mg/m3 (1 ppm), which is similar to the
threshold for acute inhalation effects in mammals. Resistant
strains of insects exist and are sometimes difficult to control.
The mechanism of resistance may have a metabolic basis that
persists throughout all stages of metamorphosis, and in some
cases has been shown to be a process of active exclusion of
phosphine by energy-dependent processes. In general, mites are
less sensitive than insects.
Wild birds and mammals are similarly susceptible to both
phosphine and phosphides. Zinc phosphide formulations vary in
acceptability as baits, and the relative efficacy of different
commercial preparations may be a function of this. Aversion to
zinc phosphide at 0.05% in the diet was demonstrated in Indian
gerbils. Census studies have shown that, with appropriate use
of bait or the application of aluminium phosphide to the
entrance of active burrows, elimination of most or nearly all
target species can be achieved with a single application.
Ingested zinc phosphide is detectable in the intestine and liver
of poisoned animals, but not in the muscle tissue. Poisoned
animals are not toxic to carrion eaters.
1.3. Effects on Animals
In mammals, phosphine is readily absorbed by inhalation.
Aluminium or magnesium phosphide powder, if inhaled, releases
phosphine for absorption on contact with the moist respiratory
epithelium. Zinc phosphide would not hydrolyse rapidly in the
respiratory tract but might be absorbed as such and hydrolyse in
the tissues. The acute dermal LD50 for zinc phosphide in
rabbits is in the range of 2000 - 5000 mg/kg body weight,
suggesting little dermal absorption. Gastrointestinal absorption
of phosphine produced by the hydrolysis of ingested phosphide is
likely and the absorption of zinc phosphide itself and its
transport to the liver, where it can be detected for many hours,
has been demonstrated in the rat and in a case of fatal human
poisoning. Information regarding the distribution of phosphine
in the body has been derived from the clinical syndromes of
poisoning, which indicate that it reaches the central nervous
system, liver, and kidney.
Absorbed phosphide is hydrolysed to phosphine or oxidized to
the salts of the oxyacids of phosphorus. Phosphine is both
slowly oxidized to oxyacids and excreted unchanged in the
expired air. Hypophosphite is the principal urinary excretion
product. Following an oral dose of zinc phosphide, phosphine in
the expired air had disappeared after 12 h and clinical symptoms
lasted only a few hours. This suggests that phosphine is
eliminated fairly rapidly. On the other hand, the phosphide
contents of the liver were higher after daily dosing with zinc
phosphide than after a single dose, suggesting that liver
phosphide is not completely eliminated within 24 h.
Studies by the inhalation route indicate that both the
concentration and duration of exposure are important deter-
minants of acute lethality and that different mammalian species
are essentially similar in susceptibility. The 4-h LC50 of
phosphine in rats is about 15 mg/m3. The oral LD50 value of zinc
phosphide in wild Norwegian rats is 40.5 mg/kg body weight.
Results of short-term administration indicate that the
effects of phosphine exposure cumulate with daily exposure so
that after 6 days pretreatment, the survival time at a concen-
tration of 681 mg/m3 was reduced to one-third of its value in
animals without previous exposure. Clinical features of liver
and kidney dysfunction were observed and all the parenchymatous
organs were affected by congestion and oedema. Neurohisto-
logical changes were seen in rats and less markedly in guinea-
pigs and cats. Changes in various serum enzyme levels at very
low levels of exposure over a period of 1.5 months have been
reported. Short-term feeding studies on female rats administered
zinc phosphide resulted in mortality at concentrations of 200
and 500 mg/kg diet, but biological effects, qualitatively
similar to those at higher doses, were seen at the lowest
dietary concentration of 50 mg/kg.
There are no studies relating to long-term effects, carcino-
genicity, or mutagenicity. There is no information regarding
factors modifying toxicity in vertebrates or the toxicity of any
metabolites. Information on biochemical effects is insufficient
to explain the mechanisms of toxicity, in either animals or
plants.
1.4. Effects on Man
Because the odour of phosphine depends on impurities which
may be removed by purification or adsorption, odour cannot be
relied on for warning of toxic concentrations.
Ingestion of phosphides may cause nausea, vomiting,
diarrhoea, retrosternal and abdominal pain, tightness in the
chest and coughing, headache and dizziness. In more severe
cases this may progress to cardiovascular collapse, pulmonary
oedema, cyanosis and respiratory failure. Pericarditis, renal
failure, and hepatic damage including jaundice, may develop
later.
Symptoms may be delayed and death may occur up to one week
after poisoning. Pathological findings include fatty
degeneration and necrosis of the liver and pulmonary hyperaemia
and oedema.
Inhalation of phosphine or phosphide may cause severe
pulmonary irritation. Mild exposure may cause only mucous
membrane irritation, with initial symptoms mimicking an upper
respiratory tract infection. Other symptoms may include nausea,
vomiting, diarrhoea, headache, fatigue, and coughing, whilst
more severe symptoms may include ataxia, paraesthesia, intention
tremor, diplopia, and jaundice. Very severe cases may progress
to acute pulmonary oedema, cardiac arrhythmias, convulsions, and
coma. Renal damage and leukopenia may also occur. Exposure to
1400 mg/m3 (1000 ppm) for 30 min may be fatal.
Death, which may be sudden, usually occurs within four days
but may be delayed for one to two weeks. Post-mortem
examinations have revealed focal myocardial infiltration and
necrosis, pulmonary oedema and widespread small vessel injury.
There is no evidence for cumulative effects from intermittent
low-level exposure averaging 14 mg/m3 (10 ppm) or less.
Chronic poisoning from inhalation or ingestion may cause
toothache, swelling of the jaw, necrosis of the mandible (phossy
jaw), weight loss, weakness, anaemia, and spontaneous
fractures.
Laboratory findings may include abnormal liver function
tests, acidosis, increased blood urea and bilirubin, haematuria,
and proteinuria. Other diagnostic studies should include
electrocardiogram, sputum, and differential white blood cell
count.
Occasional cases of accidental exposure of the general
population to phosphine have occurred in the region of
fumigation operations and on board ships carrying cargoes
capable of releasing phosphine. There have been many cases of
accidental or suicidal ingestion of phosphide pesticides.
Lethal doses vary, but most fatal cases have ingested more than
20 g zinc phosphide, and most of those who recovered had
ingested less than 20 g phosphide. Pulmonary oedema and
congestion and necrosis of the liver and kidneys are the
principal pathological features in fatal cases. There have been
occasional cases of fatal occupational exposure to phosphine,
some of which have involved repeated exposures.
Various reports of adverse effects of occupational exposure
at normal operational levels have been published, but in no case
has the description of exposure or the control group been
adequate to draw definite conclusions regarding the possibility
of the adverse effects of phosphine at the higher current
occupational exposure limits.
1.5. Evaluation
Phosphine and metal phosphides are toxic. They have a very
limited distribution in the environment. Proper standards and
procedures in their use prevent harmful effects. No significant
global effects on the environment have resulted from the use of
phosphine or phosphides.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity, Physical and Chemical Properties
2.1.1. Phosphine
2.1.1.1 Identity
Chemical structure: H
|
P-H
|
H
Molecular formula: PH3
Common synonyms: hydrogen phosphide, phosphorus
trihydride, phosphoretted hydrogen,
phosphane
CAS registry number: 7803-51-2
2.1.1.2 Physical and chemical properties
Pure phosphine is a colourless gas at ambient temperature
and pressure. Its relative molecular mass is 34.
The principal physical properties of phosphine are given in
Table 1 (Lowe, 1971). The chemistry of phosphine has been
extensively reviewed (Fluck, 1973).
Phosphine is odourless when pure, at least up to a concen-
tration of 282 mg/m3 (200 ppm), which is a highly toxic level.
The odour of technical phosphine depends on the presence of
odoriferous impurities and their concentrations and the odour
threshold is usually in the range 0.14 - 7 mg/m3 (Netherlands,
1984). The odour factors can easily be removed from phosphine
(Dumas & Bond, 1974).
Pure phosphine has an autoignition temperature of 38 °C but,
because of the presence of other phosphorus hydrides
(particularly diphosphine) as impurities, the technical product
often ignites spontaneously at room temperature (ACGIH, 1986).
Phosphine forms explosive mixtures with air at concentrations
greater than 1.8%.
Phosphine has intense ultraviolet absorption in the 185 -
250 nm (1850 - 2500 Å) region. It dissociates to phosphorus and
hydrogen in contact with hot surfaces in the absence of oxygen
(Lowe, 1971).
Oxidation of phosphine involves a branching chain reaction.
In air, the upper and lower explosion limits depend on the
temperature, pressure, and proportions of phosphine, oxygen,
inert gases and water vapour present, and also on the ultra-
violet irradiation. In aqueous solutions, oxidation of
phosphine results in the production of hypophosphorous acid.
Table 1. Physical properties of phosphine and some phosphidesa
------------------------------------------------------------------------------
Phosphine Trizinc Aluminium Magnesium
diphosphide phosphide phosphide
------------------------------------------------------------------------------
Formula PH3 Zn3P2 AlP Mg3P2
Physical state gas solid solid solid
Colour none grey grey/yellow grey
Melting point (°C) -133.5 sublimes > 1350b > 750
Boiling point (°C) -87.4 - - -
Spontaneous ignition
temperature (°C) 38d - - -
Lower explosive 1.8% - - -
limitc,e
Upper explosive unknown - - -
limitc
Vapour density (air = 1) 1.17 - - -
Density (temp °C) 4.55 (13) 2.85 (25) 2.1
------------------------------------------------------------------------------
a From: Lowe (1971) and Wilson (1971).
b Some authorities give a temperature in excess of 2000 °C.
c In air, depending on presence of other gases and ultraviolet
irradiation.
d The spontaneous ignition behaviour of phosphine is very unpredictable
and though this figure is quoted, its validity will depend on the
conditions of measurement.
e From: Anon. (1936).
An important reaction of phosphine is with metals,
especially with copper or copper-containing alloys, which causes
severe corrosion. The reaction is enhanced in the presence of
ammonia (which is given off with phosphine during the
decomposition of some fumigation tablets or pellets) and in the
presence of moisture and salt. Copper-containing equipment,
especially electrical apparatus, may be severely damaged by
exposure to phosphine during fumigation.
Bond et al. (1984) reported a systematic study on the
corrosion of metals by phosphine using a specially developed
method in which the reaction of phosphine was determined by its
depletion from an enclosed space. The authors confirmed the
positive effects of phosphine concentration, oxygen and relative
humidity on the rate of corrosion. The rate of reaction with
copper exceeded by a factor of ten or more the rates of reaction
with brass, silver, steel, aluminium, galvanized steel (various
galvanizing processes), fine gold, nickel, lead solder,
platinum, and iron powder. Eighteen carat gold jewellery
reacted at one-eighth of the rate of copper. The corrosion of
copper appears to be similar to that produced by phosphoric acid
and can be reduced by coating the copper with a saturated
solution of sodium carbonate (1/3 rate) or, more effectively,
with a saturated solution of potassium dichromate (1/44 rate).
The type of polymeric spray used to moisture-proof ignition
systems on internal combustion engines was also effective (1/7
rate).
Table 2. Solubility of phosphine in water and organic solvents
---------------------------------------------------------
Temperature (°C) Solubilitya
---------------------------------------------------------
Water 17 0.26
24 0.24
Acetic acid 20 3.19
Acetone 22.4 4.45
Aniline 22 2.8
Benzene 22 7.26
Carbon disulphide 21 10.25
Carbon tetrachloride 20.5 4.19
Cyclohexane 18.5 7.47
Toluene 22.5 7.15
Trifluoroacetic acid 26 15.9
---------------------------------------------------------
a Solubility is measured by the volume of phosphine
(measured at the temperature of the study and at 1
atmosphere pressure) dissolved in one volume of solvent
under a partial pressure of 1 atmosphere. From: Lowe
(1971).
Phosphine dissolves in water to form a neutral solution.
Solubility is little affected by the pH. Dissolved phosphine
reacts with hydrogen ions to form the phosphonium ion - (PH4)+.
Its solubility in water at different temperatures and in various
organic solvents is given in Table 2.
The technical product can contain impurities including
higher phosphines (e.g., up to 5% diphosphine, P2H4) and
substituted phosphines, which are responsible for the
characteristic foul odour of phosphine which is often described
as "fishy" or "garlicky" (Fluck, 1976) (section 9.1). Depending
on the method of manufacture, other impurities can be methane,
arsine, hydrogen, and nitrogen. Phosphine is often produced
directly, when required, by the hydrolysis of metal phosphides
or phosphonium iodide; it may also be supplied compressed in
cylinders either pure or in various concentrations in nitrogen.
2.1.1.3 Conversion factors
At 20 °C, 1 ppm = 1.41 mg/m3; 1 mg/m3 = 0.71 ppm.
1 ppm (as P) = 1.1 ppm (as PH3).
2.1.2. Metal phosphides
The metal phosphides dealt with in this document are trizinc
diphosphide (zinc phosphide), aluminium phosphide, and tri-
magnesium diphosphide (magnesium phosphide).
The principal physical and chemical properties of zinc,
magnesium, and aluminium phosphides are given in Table 1
(Wilson, 1971).
Many metals have one or more phosphides that can be
synthesized and have been characterized, but few are of
commercial importance. The properties of tricalcium diphosphide
(Ca3P2) are similar to those of trimagnesium diphosphide.
Tricalcium diphosphide also reacts with excess white phosphorus
to form calcium monophosphide (CaP) which, in turn, reacts with
water to form diphosphine by the reaction 2CaP + 4H2O = 2Ca(OH)2
+ P2H4. Zinc diphosphide is formed when phosphorus vapour in
nitrogen is passed over zinc at 700 °C. Trizinc diphosphide
(Zn3P2, 1314-84-7, relative molecular mass = 258.1), commonly
referred to as zinc phosphide, is used as a rodenticide and
fumigant because of its reaction with acid to release phosphine.
As a rodenticide, the acid in the stomach causes the hydrolysis.
For fumigation, the acid has to be supplied. Since they
hydrolyse in neutral moist conditions, aluminium or magnesium
phosphide are preferred as fumigants.
Aluminium phosphide (AlP, 20859-73-8, relative molecular
mass = 57.96) reacts with water to release phosphine. This
reaction may be incomplete, possibly owing to the formation of a
protective layer of aluminium hydroxide on the surface.
Aluminium phosphide has been used as a rodenticide and a
fumigant. It is the only compound of aluminium and phosphorus
(Wilson, 1971; Van Wazer, 1982).
Trimagnesium diphosphide (Mg3P2, 12057-74-8, relative
molecular mass = 134.87), commonly known as magnesium phosphide,
is used as a pesticide and fumigant.
Zinc phosphide is available in bulk, typically to a
specification of at least 80% Zn3P2, and as pastes containing 5%
or 2.5% for use as a rodenticide by mixing in bait. Aluminium
and magnesium phosphides are available as a number of commercial
formulations. Aluminium phosphide formulations usually contain
approximately 57% active ingredient and those of magnesium
phosphide 34% active ingredient. Some registered trade names
and presentations are:
Aluminium phosphide
Alutal
Celphide (tablets)
Celphine (tablets)
Celphos (tablets)
Delicia Gastoxin
Detia Gas-Ex-B (bags)
Detia Gas-Ex-P (pellets)
Detia Gas-Ex-T (tablets)
"L" fume (tablets)
Phosfume (pellets and tablets)
Phostek (pellets and tablets)
Phostoxin (pellets, tablets, "prepacs", rounds, strips)
Quickfos (pellets and tablets)
Zedesa (bags, pellets, tablets)
Magnesium phosphide
Detiaphos (pellets)
Mag-disc (plates)
Magtoxin (pellets, tablets, rounds)
Phostoxin (strip) formulations are designed to produce a
controlled release of phosphine to achieve efficient fumigation
with low operator risks. Some include other ingredients
designed to reduce fire hazards. Depending on the storage
conditions, the composition of metal phosphides and their
formulations may change over time by hydrolysis and oxidation.
Metal phosphides are analysed by assaying the phosphine
liberated by acid hydrolysis. After this preparatory step, the
procedure is the same as for gaseous phosphine (Bontoyan, 1981;
Terzic, 1981; Worthing & Walker, 1983).
2.2. Analytical Methods
2.2.1. Gaseous phosphine
Work-place air monitoring and fumigation control demand a
measurement range from about 0.04 µg/m3 to more than the lower
explosion limit of about 25 000 mg/m3. Thus, methods covering
concentrations differing by six orders of magnitude are
required.
Techniques are available that: (a) directly indicate the
concentration in a grab sample or time-weighted average sample;
(b) adsorb or absorb phosphine from a known volume of air for
subsequent analysis directly or by desorption and gas analysis;
and (c) give a continuous record of time-dependent concentra-
tions. Some methods reviewed by Verstuyft (1978) are given in
Table 3.
Table 3. Methods of sampling and analysisa
---------------------------------------------------------------------------------------------------------
Method Range Efficiency Interference
ppm mg/m3
---------------------------------------------------------------------------------------------------------
Sampling
Silver nitrate (0.1 N) impregnated paper 0.05 - 8.0 9.07 - 11.3 90%
Ethanolic mercuric chloride 0.05 - 3.0 0.07 - 4.2 NH3
Acidic potassium permanganate (0.1 N) impinger 0.01 - 0.05 100% H3S
Silver diethyldithiocarbamate (0.5%) bubbler 0.6 - 18 0.85 - 25 54 - 86.2% H2S,AsH3, SbH3
Mercuric chloride (0.5%) aqueous bubbler 10 - 28 14 - 28 AsH3
Toluene impinger 41.5%
Mercuric chloride (0.1%) conductance cell 0.05 - 2.5 0.07 - 3.5 88.0% SO2, H2S, AsH3,
SbH3
Silver nitrate impregnated silica gel 0.05 - 4.1 0.07 - 5.8 95% H2A, AsH3
Auric chloride impregnated silica gel 0.01 - 1000 0.014 - 1 400 100% AsH3, SbH3
Ethanolic mercuric chloride (0.1%) 0.0006 88 - 100% AsH3,SO2,HCN,
H2S
Mercuric cyanide impregnated silica gel 0.014 - 1.18 0.02 - 1.7 80%
---------------------------------------------------------------------------------------------------------
Table 3 (contd.)
-------------------------------------------------------------------------------
Methodb Sensitivity
-------------------------------------------------------------------------------
Analysis
Bendix phosphorus method/sulfur monitor ns
HNU photoionization detector 0.1 - 100 ppm 0.14 - 140 mg/m3
Gas chromatography - FID. 3% TCP ns
Gas chromatography - Therm. 4.5% QF-1 2 ppt 3 pg/m3
Gas chromatography - FPD. 3% Carbowax 0.5 ppt 0.7 pg/m3
Gas chromatography - Coulson 500 ppt 0.7 µg/m3
Gas chromatography - TC. porous beads 10 ppm 14 mg/m3
Gas chromatography - Therm. 30% Apiezon 50 ppb 70 µg/m3
Gas chromatography - FID. PPD. beta-ioni- 20 ppb 30 µg/m3
zation Poropak Q
Colorimetric - ammonium molybdate 720 nm 10 mg/litre
Colorimetric ammonium molybdate 880 nm 10 mg/litre
Colorimetric silver DDC 465 nm 0.05 ppm 70 µg/m3
Colorimetric silver sulfide 470 nm 0.02 ppm 30 µg/m3
-------------------------------------------------------------------------------
a From: Verstuyft (1978).
b DDC - diethyldithiocarbamate.
FID = flame ionization detector.
FPD = flame photometric detector.
Therm = thermal detector.
ns = not stated.
2.2.1.1 Direct-indicating methods
Phosphine can be detected by filter papers impregnated with
a mixture of silver nitrate and mercury (II) chloride. The
method can be made semi-quantitative by appropriate configura-
tion and measurement of stain-length (Brandon, 1983) or colour
comparison (Hughes & Jones, 1963). Kashi & Muthu (1975)
described a simple and sensitive method for detecting phosphine,
using paper strips impregnated with dimethyl yellow, cresol red,
and mercury (II) chloride in methanol. It is claimed that
these paper strips have a better shelf-life and are more
sensitive than paper strips impregnated with dimethyl yellow
alone, or cresol red plus mercury (II) chloride. The strips
need not be kept in air-tight containers or under controlled
conditions of temperature and humidity.
Direct-indicating detector tubes are commercially available
for spot sampling (using syringes and grab-samplers) and
personal monitoring of occupational exposure (using portable
pumps). Leesch (1982) compared the accuracy of various pump (5)
and detector tube (7) combinations. Means of measured values
for test concentrations varied from 59 to 256% of actual
concentrations over the whole range of pump/tube combinations.
Pumps and tubes from the same manufacturer gave mean values
varying from 85 to 214% of the actual values. Measurements at
low levels of phosphine in the range 2.8 - 7 mg/m3 (2 - 5 ppm)
with matched pumps and tubes from the same manufacturers
resulted in mean values of 133%, 130%, and 128%. Using tubes
from three manufacturer's and the pump from only one
manufacturer gave mean values of 120%, 101%, and 90%. In most
cases, the techniques over-estimated the concentrations.
Clearly, inaccuracies of individual measurements are greater
than those of these mean values and care is required in the
choice of pumps and detector tubes, in their calibration, and in
the interpretation of results obtained with them.
Other direct-indicating tubes of lower sensitivity are
available for the measurement of the higher phosphine
concentrations used in fumigation. Classical analytical
techniques, such as oxidation to phosphate and the formation of
the phosphomolybdate complex have been specially applied to
fumigants (Kao, 1981). Shaheen et al. (1983) recently described
the use of indicator tubes as passive dosimeters to record
concentration x time product integrals. The stain-length was
closely, but not linearly, related to the concentration x time
product in the range 0 - 1400 mg x days/m3.
2.2.1.2 Absorptive or adsorptive sampling and analysis
Gas chromatography is the most sensitive method for the
determination of the phosphine content of air samples.
Usually, samples are desorbed from a solid absorbent coated with
mercury (II) cyanide, although samples taken in syringes, gas
bags, or tonometers can be used. Microcoulometric and thermionic
detectors have detection limits of 5000 and 20 pg, respectively.
The limit for flame photometric and argon and helium beta
ionization detectors is 5 pg and that for mass spectrometry,
1 ng. Photoionization detection is also commonly used. Flame
photometry combines both sensitivity and stability (Verstuyft,
1978).
Using a gas chromatograph equipped with a nitrogen/
phosphorus-selective detector, Vinsjansen & Thrane (1978) were
able to detect phosphine in the environment at a concentration
of 0.04 µg/m3.
Other techniques for estimating phosphine in air involve
entrapping phosphine by adsorption or reaction with subsequent
analysis of the desorbed or reacted sample. In a classical
method (Furman, 1962), air containing phosphine is reacted with
mercury (II) chloride, followed by the addition of potassium
iodide and then excess standard iodine solution. Back-titration
of the excess iodine with thiosulfate is used to quantify the
phosphine.
Of the solid sorbents, acid-washed charcoal and silica gels
coated with silver nitrate and potassium permanganate
effectively collect phosphine but quantitative release has not
been achieved. Mercury (II) cyanide on silica gel collects
phosphine quantitatively and holds 80% of the phosphine over 2
weeks storage. The phosphine is released for gas chromatographic
analysis by treatment with alkaline sodium borohydride solution
(Barrett & Dillon, 1977) or is oxidized (using a hot acid
permanganate solution) to phosphate, which is measured using the
phosphomolybdate colorimetric technique (US NIOSH, 1979). It
should be noted that many commercially available sorbents are
unsuitable (Dumas & Bond, 1981).
Liquid impingers/bubblers containing a variety of solutions
can be used to collect and react phosphine for quantification by
colorimetry/spectrophotometry, by conductance, or by potentio-
metric titration. Classical colorimetric techniques are the
development of the red-orange complex with silver diethyldithio-
carbamate, which can be measured at 465 nm, or the oxidation by
permanganate to phosphate which is reacted with a solution of
ammonium molybdate in concentrated sulfuric acid, extracted with
toluene-isobutanol, reduced with tin (II) chloride and measured
as the phosphomolybdate complex at 625 nm (US NIOSH, 1979). The
first method suffers from arsine interference and the second
also measures any phosphorus species that are oxidized to
phosphate by oxalic acid/permanganate treatment.
The quantity of phosphine bubbled through a solution of
mercury (II) chloride and reacting:
PH3 + 3HgCl2 = P(HgCl)3 + 3HCl
can be measured by the change in electrical conductivity using a
conductance cell or by potentiometric titration of the HCl with
NaOH (Verstuyft, 1978).
2.2.1.3 Continuous methods
There are directly indicating continuous samplers in which
phosphine-containing gas is passed through a paper tape
impregnated with a silver nitrate-containing mixture, which
develops a colour related to the phosphine concentration. The
tape can subsequently be passed through a reader-recorder to
produce a concentration versus time plot (McMahon & Fiorese,
1983). Other continuous monitors draw air at a metered rate
through detectors using either flame photometry or photo-
ionization. The detection limit of infrared spectroscopy is
about 0.4 mg/m3 (0.3 ppm) but this is barely sensitive enough
for monitoring occupational exposure (Webley et al., 1981).
Continuous direct estimation of phosphine can also be made using
the property of chemiluminescence in ozone and measuring the
emission at 550 - 560 nm using a photomultiplier tube (Boubal et
al., 1981). A portable quadrupole mass spectrometer has been
used (Arnold & Robbiano, 1974).
2.2.2. Residues
Fumigated foodstuffs may contain gaseous phosphine (adsorbed
and interstitial) and residual aluminium or magnesium phosphide.
Different techniques for the determination of residues may
measure the phosphine or the phosphide residues.
Interstitial and adsorbed phosphine can be purged by
nitrogen and trapped in reagents for classical analysis or on
adsorbents for chromatographic analysis (Dumas, 1978; Nowicki,
1978; Saeed & Abu-Tabanja, 1984).
Total phosphine and phosphide is measured by extraction of
the fumigated stored product with silver nitrate (Rangaswamy,
1984; Rangaswamy & Muthu, 1985), or with sulfuric acid (Nowicki,
1978; Saeed & Abu-Tabanja, 1984). The silver nitrate forms a
chromophore, which is measured spectrophotometrically at 400 nm.
Following sulfuric acid extraction, headspace gas can be
analysed spectrophotometrically for phosphine.
Saeed & Abu-Tabanja (1984) reported substantial differences
between the purge-and-trap method and the sulfuric acid method.
Some of these differences may have been due to the presence of
powdered aluminium phosphide in the sample, but some may have
been due to incomplete desorption during purging. The sulfuric
acid method was preferred because it also measures the capacity
of the product to release phosphine and this is of more biolo-
gical significance than the measurement of free phosphine only.
Robinson (1972) reported the measurement by neutron
activation analysis of phosphorus-containing residues in filter
paper fumigated with PhostoxinR and demonstrated a net gain in
phosphorus content.
Robison & Hilton (1971) described the estimation of
phosphine/phosphide residues in zinc phosphide-treated sugarcane
by extraction in sealed flasks into a mixture of aqueous acid
and toluene followed by the gas chromatographic analysis of
toluene.
2.2.3. Metal phosphides
Hydrolysis of metal phosphides with acids yields phosphine,
which can be measured by any of the methods already described.
Terzic (1981) has described a method in which the evolved
phosphine is absorbed in silver nitrate to form silver
phosphide, which is oxidized to silver phosphate, precipitated
as magnesium ammonium phosphate, and converted by ignition to
magnesium pyrophosphate, which is weighed. A single conversion
factor applied to this weight gives the quantity of phosphide in
the sample.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Phosphine is extremely rare in nature. It occurs transiently
in marsh gas and other sites of anaerobic degradation of
phosphorus-containing matter, and the equilibrium of reactions
in which it participates favour its oxidation (Ciba, 1978).
Although phosphorus could be expected to occur naturally as a
phosphide, the only phosphide in the earth's crust is found in
iron meteorites as the mineral schreibersite (Fe,Ni)3P, in which
cobalt and copper may also be found (Van Wazer, 1961).
3.2. Man-Made Sources
3.2.1. Production levels and processes
Apart from natural sources, atmospheric phosphine results
from emissions and effluents from industrial processes and from
the use of phosphides as rodenticides and fumigants. Unexpected
focal release of phosphine may occur due to the action of water
on phosphides present as impurities in some industrial
materials. Amounts of phosphate arising from atmospheric
phosphine are insignificant in comparison with the amounts of
phosphate added to the environment from sewage, agricultural
run-off, and industrial and urban effluents.
3.2.1.1 World production figures
It is difficult to quantify the production of phosphine,
since much of it is manufactured and used in relation to another
process. Though some phosphine is supplied in cylinders, it is
often produced, as and when required, by hydrolysis of a metal
phosphide. Phosphine is also produced as a by-product or evolved
incidentally in various industrial processes.
A main use of phosphine is as a dopant in the electronics
industry. A total of 42 electronics companies used 6 million
litres of phosphine gas mixtures in various concentrations in
1979, probably equivalent to some 300 000 litres of pure
phosphine (LaDou, 1983). In 1981, 90% of speciality gases for
the electronics industry were produced by six manufacturers
(LaDou, 1983).The annual growth rate for the use of dopants by
the electronics industry has been estimated to be 30% per annum
(SRI, 1982). By contrast, volumes more than twice this may be
produced and used as a chemical intermediate in a single plant,
and even greater amounts are used for fumigation of stored
products. For example, in the Federal Republic of Germany in
1975, 37 000 kg (approximately 28 million litres) of phosphine
were used for fumigation, though it decreased to 10 000 kg
(approximately 7.5 million litres) in 1977 as a result of the
introduction of alternatives (Noack & Reichmuth, 1982a).
3.2.1.2 Manufacturing processes
Phosphine is manufactured by the hydrolysis of aluminium
phosphide or magnesium-aluminium phosphide, or by the electro-
lysis of phosphorus in the presence of nascent hydrogen (Boenig
et al., 1982). It is formed as a co-product in the manufacture
of hypophosphites by the reaction of white phosphorus with
alkali where conditions can be established so that the
phosphorus-steam reaction yields phosphine. Phosphine is
produced as a by-product in the manufacture of acetylene,
through the hydrolysis of calcium carbide, if this contains
calcium phosphide as an impurity. It is also evolved from
phosphorus furnaces (Al'zhanov et al., 1983), impurities in
ferrosilicon alloy (Anon., 1956; Lutzmann et al., 1963),
machining of spheroidal graphite iron (Bowker, 1958; Mathew,
1961), steel pickling (Vdovenko et al., 1984), and other
metallurgical operations (Habashi & Ismail, 1975). Zinc
phosphide has been prepared by reducing trizinc phosphate with
hydrogen at 600° C, by passing phosphorus vapour over zinc at
400 °C, or by direct reaction between amorphous phosphorus and
powdered zinc under pressure or heat. Aluminium phosphide and
magnesium phosphide are produced in similar ways or by exchange
reactions between aluminium or magnesium and a heavy-metal
phosphide (Wilson, 1971).
3.2.2. Uses
A ternary compound, magnesium aluminium phosphide, in which
aluminium and magnesium are present in the ratio by weight 1:3,
is used in sea flares and as an intermediate for the local
formation of phosphine by hydrolysis.
As a chemical intermediate, phosphine is used in the
synthesis of organophosphines and organic phosphonium
derivatives. Alkyl phosphines may be made by additional
reactions across an olefinic double bond. Using Grignard
reactions, both aryl and alkyl phosphines can be made from the
appropriate alkyl or aryl halide and PCl3. Organophosphines are
used in oil-additive and pharmaceutical applications. Phos-
phonium compounds are made by the addition of P-H bonds across a
carbonyl function with acid catalysis. For example, the reaction
of phosphine with formaldehyde, in the presence of hydrochloric
or sulfuric acid, forms the corresponding salt of tetrakis-
(hydroxymethyl)-phosphonium which is employed in the manufacture
of polymers used in the flame-retardant treatment of cotton
fabrics.
As a fumigant in pest control, phosphine is invariably
produced at the site of fumigation by the hydrolysis of a
phosphide, usually of aluminium or magnesium. As a dopant in
the electronics industry, phosphine is used in high purity at
low concentrations in nitrogen.
Zinc phosphide is used principally in the form of a 2.5% or
5% paste as a rodenticide incorporated in food (10%) as a bait
(WHO/FAO, 1976). There are other formulations in which powdered
zinc phosphide is incorporated, possibly with a zinc salt to
inhibit phosphine formation before use, and histamine, or a
histamine releaser, to stimulate acid secretion (and therefore
phosphine production) in the stomach of rodents taking the bait.
One formulation includes a binder and is encapsulated in a form
that enables it to be included with grain as bait (Degesch GmbH,
1978). Aluminium and magnesium phosphides are used in powder
form or in formulations as a source of phosphine for the
fumigation of storage facilities and stored products, to prevent
spoilage by a wide range of pests. In general, for the
satisfactory hydrolysis of the phosphide, the material being
fumigated should have a moisture content of 10% or more.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
4.1.1. Air
Frank & Rippen (1986) studied the disappearance of phosphine
from air released following the aeration of fumigated premises.
The most important chemical reactions are with the HO x radi-
cals, which are usually present in abundance in the atmosphere
due to the reaction of ozone with water, a reaction enhanced by
impurities such as NOx:
H2O + O3 +2 e - -> 2 HO x + 02
The following reactions of HO x and phosphine may occur:
PH3 + HO x -> H2O + PH2 x
PH3 + HO x -> HOP x H3
This reaction is dependent on phosphine concentration and is
very rapid with a reaction rate constant at room temperature of
about 1.5 x 10-11 cm3/mol x sec (Fritz et al., 1982; Becker et
al., 1984). Direct reactions with ozone are quantitatively
unimportant, since reaction with HO x occurs before PH3 can
reach the ozone-rich upper atmosphere. With the usual concentra-
tions of HO x , the half-life of phosphine in air is about 28 h.
Sunshine increases the HO x concentration and may reduce the
half-life to less than 5 h. Direct photolysis cannot be expected
with phosphine (Calvert & Pitts, 1966). The eventual oxidation
product of phosphine will be phosphorus oxyacids and inorganic
phosphate, which will be deposited and contribute to the
nutritive environment of soils and surface waters.
Hilton & Robison (1972) studied the disappearance of
phosphine from dry tubes sealed by a rubber membrane and from
similar tubes containing a small quantity of water. Phosphine
completely disappeared from the dry tubes in 40 days, but the
presence of water in the others reduced the rate of disappear-
ance. However, possible losses through, or by adsorption on,
the membrane were not quantified and no information was given
regarding illumination of the tubes.
4.1.2. Soil
Hilton & Robison (1972) introduced phosphine at 1.4 g/m3
(1000 ppm) (as P) in the headspace of tubes containing 3 types
of soil at 5 moisture levels, 0%, 25%, 50%, 75%, and 100%
saturation. It was not stated whether the soils had been
sterilized. Phosphine disappeared within 18 days from all air-
dried soils, whereas up to 40 days was necessary for disappear-
ance from moisture-saturated soils. Quantities of phosphorus
recoverable as phosphate from the soils after incubation for 40
days varied widely with different soil types and reached about
70% of total phosphine in a slightly acidic soil, containing
12% - 15% organic matter content at 25% moisture saturation.
Variation in phosphate recovery probably reflected rates of
diffusion of phosphine into the soil matrix as a function of
moisture content, as well as differences in the efficiency of
different soils with different moisture contents as oxidizing
substrates for phosphine. Clearly, in time, soils are able to
entrap the phosphine in the air in contact with them and oxidize
it to orthophosphate.
Hilton & Robison (1972) studied the fate of zinc phosphide
and phosphine in the soil-water environment. The results of
preliminary experiments showed that phosphine was undetectable
in headspace gases over soil containing zinc phosphide at
1.4 mg/m3 and 14 mg/m3 (1 and 10 ppm) (as PH3). Zinc phosphide
was added at 1000 ppm (as P) to each of 3 types of soil at 0%,
25%, 50%, 75%, and 100% water saturation in sealed bottles which
were incubated at 27 - 28 °C for up to 34 days. Headspace gases
were sampled periodically and the appearance and disappearance
of phosphine followed. At the end of the phosphine evolution
period, acetic acid was added to the samples to hydrolyse
phosphide to phosphine with minimum conversion to phosphate.
The headspace was analysed for phosphine after heating for 3 h
and cooling. It was then flushed with nitrogen to remove
phosphine, and sulfuric acid was added to the soil to extract
the phosphate. The amount of phosphine in the headspace
increased with increasing moisture content up to 50%. The data
were sufficient to estimate the extent of hydrolysis of zinc
phosphide to phosphine. After 34 days of incubation, residual
phosphide was detectable only in the dry (about 35% of original)
and 25% saturated (about 10% of original) soils. Over 80% of
the phosphide phosphorus was recovered as phosphate, except in
the case of dry soil samples.
4.1.3. Aquatic environment
The hydrolysis of zinc phosphide was negligible in a variety
of surface waters, tap water, and ocean water over periods of up
to 11 days. Only in a buffered solution at pH 4 did significant
hydrolysis take place (Hilton & Robison, 1972). The authors
concluded that zinc phosphide released or carried into streams
or ocean water would not decompose rapidly. Bottom or suspended
sediments would be likely to decompose zinc phosphide with the
formation of phosphine or phosphoric acid under anaerobic and
aerobic conditions, respectively.
4.1.4. Vegetation, wildlife, and entry into the food chain
Robison & Hilton (1971) studied phosphine residues in
sugarcane in vitro using 32PH3. About 30% of the 32PH3 reacted
irreversibly to form water-soluble compounds of phosphorus while
another 10% remained irreversibly bound to the fibre. It was
suggested that the water-soluble compounds were phosphorus
oxyacids and a portion of the acids may have formed insoluble
iron or aluminium salts in the fibre.
Effects on viable seeds, (section 6.4.3) suggest little
effect on plant metabolism.
The only significant exposure of wildlife to phosphine or
metal phosphides is through their use as pesticides; domestic
animals may also be accidentally exposed in this way. Zinc
phosphide baits are known to be highly palatable for rodents.
Acceptability of bait by non-target species depends on the
presentation (Janda, 1973) and the precise location in relation
to natural feeding sites (Chentsova, 1972). Persistence of
phosphine or phosphides in the carcasses of poisoned animals is
low and their carcasses are not toxic when eaten by other
animals (Kozhemyakin et al., 1971; Schitoskey, 1975).
Various studies have been undertaken on the effects of
feeding fumigated diet to experimental animals. Kadkol &
Jayaraj (1967) reported the effects of feeding phosphine-
fumigated rice to albino rats for 12 weeks. The only dose-
related effects were slight increases in liver and kidney
weights in male rats. Hackenberg (1972) reported a study on the
effects on Wistar rats (30 male and 30 female in both test and
control groups) of a standard laboratory chow treated for 72 h
with PhostoxinR uniformly distributed in the food at 48 mg/kg
for the first 16 weeks and thereafter at 90 mg/kg (corresponding
to 10 times the recommended treatment level). Following
treatment, food was mixed and aerated for 1 h before determina-
ation of phosphine, which was found to be in the region of
1 mg/kg food, including any residues of pellets. The methods of
administration and storage of food were probably responsible for
further losses of phosphine after the aeration period. No
differences attributable to diet were observed in behaviour,
development, body weights, food consumption, or in blood and
urine composition (at any stage in the study), and in gross or
microscopic pathology between test and control animals.
Cabrol Telle et al. (1985) undertook a similar study of the
effects of feeding a phosphine-fumigated test diet to 30 Sprague
Dawley rats of both sexes for 2 years, with a control group fed
an identical but non-fumigated control diet. The test diet had
been fumigated by storage in phosphine at 2820 mg/m3 (2000 ppm)
for at least 6 months. Just before consumption, it was aerated
for 48 h and supplemented with a balanced vitamin preparation
(also added to the non-fumigated control diet). The average
residue level in the fumigated diet was less than 5 mg/kg.
There were no differences between test and control groups
attributable to the consumption of a fumigated diet in terms of
weight gain, food consumption, plasma chemistry, haematology,
urinalysis, behaviour, growth, survival, organ weights, histo-
pathology, tumour incidence, or carcass analysis.
It is unlikely, therefore, that the use of phosphine or
phosphides results in food residues that are of any toxico-
logical significance.
4.2. Biotransformation
There have been no studies on the biotransformation of
phosphine or metal phosphides. Energy considerations suggest
that phosphine is liable to be oxidized to phosphate in
biological systems (Ciba, 1978). There is no suggestion of
bioaccumulation or biomagnification.
4.3. Ultimate Fate
The ultimate fate of phosphine and the phosphide moiety of
metal phosphides is the formation of phosphate.
Disposal of phosphide-bearing wastes is regulated in many
countries. Effluent phosphine is usually burned and the
resultant gases can be scrubbed to remove phosphorus oxides and
oxy-acids before discharge into the atmosphere. Zinc phosphide
formulations and baits in some countries are regulated as
hazardous wastes, for instance when their concentration exceeds
10% (US EPA, 1983). Deep burial is the preferred method of
disposal.
IRPTC (1985) gives the following recommendations:
Aluminium phosphide
"Allow it to react slowly with moisture out in the open,
taking precautions to see that the poisonous gas (phosphine) is
dissipated"a
Phosphine
"Surplus gas or leaking cylinders can be vented slowly to
air in a safe, open area or gas can be burnt off through a
suitable burner in a fume cupboard".
----------------------------
a It should be added that precautions should be taken to
ensure that the area is inaccessible to children and ani-
mals. The IRPTC comments "Phosphine gas creates no permanent
environmental hazard because it is eventually converted to
harmless phosphoric acid and water". An alternative is
admixture with an inert dry diluent and burning at a temper-
ature greater than 1000 °C with effluent gas scrubbing.
These recommendations also apply to magnesium phosphide.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air, water, and soil
Phosphine and metal phosphides have only been detected in
the general environment in relation to the recent use of metal
phosphides in pest control and in relation to a number of
industrial activities.
Arendt et al. (1979) studied open-air concentrations in the
vicinity of a silo at successive stages during fumigation with
phosphine and aeration. Within 20 m of the silo wall, concen-
trations reached a peak of 3 mg/m3 (2.15 ppm) during the
fumigation phase and averaged 0.37 mg/m3 (0.26 ppm) during the
aeration phase, but the maximum value after 3 h of aeration was
0.06 mg/m3 (0.04 ppm). At distances greater than 20 m from the
silo, the concentration was at all times less than 0.05 mg/m3
(0.035 ppm). Measurements at 7 locations from < 5 to 60 m from
the silo, during aeration, showed that the highest concentration
20 - 30 min after the start of aeration was 0.1 mg/m3 (0.08
ppm), while other values were about half of this or less. Seven
hours after the commencement of aeration, concentrations were
uniformly less than 0.014 mg/m3 (0.01 ppm).
Reichmuth et al. (1981) reported similar results. They
found that open air concentrations immediately adjacent to the
outer walls of buildings under phosphine fumigation reached
280 mg/m3 (200 ppm (v/v)), but at a distance of > 10 m from the
buildings, all concentrations except one were less than
0.14 mg/m3 (0.1 ppm (v/v)). However, there are isolated reports
of persons in the neighbourhood of fumigated stores being
affected by phosphine (Gessner, 1937; Hallerman & Primela, 1959;
Sayvetz, 1982); in the 2 earlier reports, effects were fatal
(section 9.2).
These results indicate that fumigation operations contribute
only locally and transiently to atmospheric phosphine exposure.
The volumes released in industrial operations (section 3.2.1.2)
are much smaller and are therefore of less significance in
relation to atmospheric concentrations, except at the immediate
work station.
5.1.2. Food and feed
5.1.2.1 Residue values
Because of the techniques of analysis used, residues of
phosphine or metal phosphides are usually measured together and
reported as total phosphine or phosphorus. The results indicate
that residues in fumigated foods are negligible at 0.01 mg/kg
(0.01 ppm) or less (Dieterich et al., 1967). Urga (1983)
reported residue levels in a variety of stored grains or pulses
(minimum moisture content 7.6%) that had been fumigated by
inserting PhostoxinR at 3 tablets per tonne under PVC sheeting
for 3 - 8 days, followed by a minimum of 2 months' aeration.
Phosphine was measured by bromine oxidation and the phospho-
molybdate colorimetric method. Average phosphine residues
measured as phosphorus in different stored grains or pulses are
shown in Table 4.
Table 4. Average PH3 residues (as phosphorus)a
---------------------------------------------------
Number of mg/kg
samples average (range)
---------------------------------------------------
Wheat 15 0.05 (0.01-0.08)
Chick-peas 9 0.07 (0.02-0.12)
Barley 3 0.11
Maize 12 0.06 (0.01-0.11)
Sorghum 3 0.11
---------------------------------------------------
a From: Urga (1983).
Cabrol Telle et al. (1985) (section 4.1.4) found that
residue levels after prolonged fumigation at 2820 mg/m3
(2000 ppm) were on average 0.005 mg/kg (0.005 ppm), but the
phosphine was apparently not produced from mixing a metal
phosphide in the diet. Hackenberg (1972) mixed PhostoxinR
pellets with basic diet and determined residues included
hydrolysis of unreacted phosphide to phosphine. Residues were
generally under 0.75 mg PH3/kg feed. Since the fumigation was
at 10 times the recommended rate, this result is comparable with
a residue level of less than 0.1 mg/kg (0.1 ppm) at normal
doses. Samples of 2 of the batches of feed contained exception-
ally high levels of 1.3 and 7.5 mg PH3/kg feed, respectively,
presumably as a result of residual phosphide in the samples
selected. Singh et al. (1983) fumigated pulses with PhosfumeR
tablets at the recommended dosage (2 tablets/tonne) and also at
2 and 4 times the recommended dosage. After fumigation for 5
days, phosphine residues decreased exponentially with a half-
life that depended on the type of pulse and the fumigation dose;
the half-lives varied from 0.66 to 1.33 days. In each case,
residues fell below the limit of detection of 0.001 mg/kg
(0.001 ppm) in less than 3 days at normal fumigation rates, and
in less than 6 days at 4-fold fumigation rates.
Breyer (1973) described field studies using magnesium and
aluminium phosphide formulations as the source of phosphine for
the treatment of wheat. After 12 days exposure at 17 °C with a
concentration of 6 g phosphine/tonne, the wheat contained
0.009 mg phosphine/kg in the case of magnesium phosphide and
0.012 mg/kg for aluminium phosphide.
Residues in wheat, millet, milled rice, soya beans, and
azuki beans were studied after exposure to 5640 mg phosphine/m3
(4000 ppm) at 25 °C (Sato & Suwanai, 1974). After 12 days of
fumigation, the residue levels were 0.46, 1.16, 0.34, 0.18, and
0.24 mg phosphine/kg, respectively.
When wheat was fumigated with phosphine at 5 mg
phosphine/kg, the residue level after 4 days of aeration was
0.2 µg/kg (0.2 ppb); after 220 days of aeration, the level
dropped to 0.004 µg/kg (0.004 ppb) (Dumas, 1986).
Phosphine levels in Iraqi dates fell rapidly within 24 h
after fumigation, but residues persisted for at least 9 days.
Higher residue levels were found with storage at low
temperature. For example, more than 1 mg phosphine/kg (1 ppm)
could still be detected after 10 days in dates stored at 4 °C
(Alomar & Al-Bassomy, 1984).
5.1.2.2 Factors affecting residue levels
The factors affecting phosphine residues in hazel nuts, soya
beans, and wheat that had been fumigated at 20.5 mg
phosphine/litre, for periods of 1 and 14 days, were studied
(Noack et al., 1984a). Fumigated food was then aerated for 5
weeks in a layer about 20 mm deep in a wire basket, open to the
air at 70% relative humidity and at temperatures of -18 °C,
+5 °C, +20 °C, and +35 °C. Residue levels were studied as a
function of fumigation duration and aeration temperature and
duration. Residues decreased approximately exponentially with
time, more rapidly at higher temperatures and with the shorter
fumigation period. Residues were much more persistent in hazel
nuts than in wheat, with intermediate levels in soya beans.
Temperature dependence was much less marked with hazel nuts than
with the other 2 foods. Residue levels immediately at the end
of fumigation were not much higher after 14 days of fumigation
than after 1 day, presumably because equilibrium was achieved
within the shorter fumigation period. Concentrations in wheat
and soya beans after 3 days aeration or more were about 2.5
times higher when fumigated for 14 days than when fumigated for
1 day. For hazel nuts, the corresponding ratio was about 5.
Times for phosphine residues to reach 0.1 mg/kg (0.1 ppm) and
0.01 mg/kg (0.01 ppm) are given in Table 5 (Noack et al., 1983,
1984a).
The shorter the period of fumigation, the lower the dose,
and the higher the storage temperature, the more rapid is the
decomposition of phosphine. Crops with high contents of fat and
protein appear to retain a higher level of phosphine than those
with a high water content. The residue level for hazel nuts
fumigated at 20.5 mg phosphine/litre for 14 days was 15.2 mg/kg,
whereas for wheat similarly fumigated only 0.6 mg phosphine/kg
was detected (Noack et al., 1983).
A further study described in the same report showed that
after fumigation for 10 days (5.6 mg phosphine/litre) and active
ventilation at 8, 50, or 100 litres/h for 12 days during
aeration, residue levels were independent of airflow.
Table 5. Approximate time necessary for phosphine residues to reach
0.14 mg/kg and 0.01 mg/kg in different stored products at
various storage temperatures and durations of fumigationa
-------------------------------------------------------------
Food type Fumigation Target Time to reach residue
(20.5 mg residue level (days)
pH3/litre) level Temperature (°C)
duration mg/kg -18 +5 +20 +35
(days)
-------------------------------------------------------------
Hazel nuts 1 0.1 35+ 35+ 35+ 20
0.0 35+ 35+ 35+ 35+
14 0.1 35+ 35+ 35+ 35+
0.01 35+ 35+ 35+ 35+
Soya beans 1 0.1 35+ 10 4 3
0.01 35+ 35+ 9 6
14 0.1 35+ 35+ 18 5
0.01 35+ 35+ 35+ 16
Wheat 1 0.1 20 < 1 < 1 < 1
0.01 35+ 25 4 3
14 0.1 35+ 15 7 2
0.01 35+ 35+ 28 8
-------------------------------------------------------------
a Adapted from: Noack et al. (1984a).
The results indicate that the decrease in phosphine residues
is limited by the rate of diffusion within the food substance,
the rate of desorption from its surface, or chemical decompo-
sition, rather than by interstitial diffusion; the rate-limiting
process is temperature dependent.
Noack et al. (1984a,b) were able to describe the behaviour
of phosphine residues in semi-empirical mathematical models
which were in good agreement with experimental data. In a
further study (Noack & Wohlgemuth, 1985), the effects of non-
constant phosphine concentrations on fumigation residues were
examined. There was a correlation between the final residues
and the concentration of phosphine used but during fumigation
concentrations rose to a peak then declined. Residues in
samples of foodstuffs removed from fumigation at a particular
concentration in the rising phase decreased more rapidly than in
samples removed from fumigation at the same concentration in
the declining phase. Residue levels in samples of food fumigated
at constant concentration reached a maximum later than the time
at which the maximum fumigation concentration had been achieved.
These results could be explained on the basis of the sorption
and diffusion of phosphine and the authors concluded that
residues are minimized by fumigation at low concentrations (a
few hundred mg/m3) over a long period (2 - 3 weeks).
Robison & Hilton (1971) measured phosphine residues in
sugarcane harvested from fields treated 4 times with zinc
phosphide as a rodenticide at 5.6, 11.2, or 56 kg/ha. Sugarcane
was analysed for phosphine after 7 and 110 days using extraction
by aqueous acid and toluene and gas chromatographic analysis.
In general, residue levels were less than 0.01 mg/kg (0.01 ppm)
and in no case did the levels exceed 0.1 mg/kg (0.1 ppm). At
harvest, 110 days after application, sugarcane from a wet
location did not contain any residues of phosphine, whereas that
from a dry location still contained up to 0.032 mg/kg
(0.032 ppm) (highest application).
In a study by Robinson & Bond (1970), 32P-labelled phosphine
derived from labelled aluminium phosphide was used to
investigate the presence of phosphorus residues after treatment
of wheat and flour. The radioactive residue in wheat and flour
could not be removed by thorough aeration or by heating at
baking temperature. It was shown to be largely water soluble
and paper chromatography identified the main products as
hypophosphite and phosphite. It was concluded that the oxidation
of phosphine to the lower oxyacids of phosphorus was mainly a
surface phenomenon and that, in the normal course of air
oxidation, all residues would eventually appear as ortho-
phosphate. Deposition of oxidation products of phosphine also
occurred on glass and other surfaces.
Laboratory studies by Disney & Fowler (1972a) showed that
wheat exposed to phosphine levels (3 mg/litre) several times
higher than those normally used, at 25 °C for 5 days (10%
moisture content) and 14 days (19.7% moisture content),
contained residue levels varying from 1.7 mg/m3 (1.2 ppm) in the
first case to 25.9 mg/m3 (18.4 ppm) in the second, demonstrating
the important effect of water content as well as exposure period
in determining residues. Autoradiography of sections of whole
grain showed that most of the radioactivity was present in the
outer layers and crease, and that 70% of the residues were
extractable with hot water. Tkachuk (1971, 1972) showed that,
in wheat, flax, and rapeseed, approximately 50% of the phosphine
formed non-phosphine residues, which were distributed in wheat
in the bran (85%), endosperm (14%), and germ fractions. Eleven
percent of the residues were water soluble and appeared to be
hypophosphite and pyrophosphate. The remainder may have included
insoluble aluminium salts. It was suggested that this did not
represent the behaviour of non-radioactive phosphine, but
further studies confirmed the isotopic studies (Disney & Fowler,
1972b; WHO/FAO, 1972). The adsorbed non-phosphide residues were
oxyacids of phosphorus and of no toxicological significance
(Robinson, 1972; US EPA, 1986).
Some national and international standards and recommenda-
tions for phosphine residues in food and feeds are given in
Table 6.
Table 6. Various national and international standards for phosphine
residues in food
------------------------------------------------------------------------------
Organization Commodity Level of
(reference) phosphine
ppm mg/kg
------------------------------------------------------------------------------
Joint FAO/WHO Food Standards Programme Codes Alimentarius Commission
Codex Alimentarius Maximum Residue Limits (MRL)
(1986)
Cereal grains 0.1
Flour and other milled cereal 0.01
products, dried foods, fruit
and vegetables, spices, cocoa
beans, nuts, peanuts and
breakfast cereals
Canada
cited in Raw cereals, soy beans, 0.1 0.1
US EPA (1986) processed foods, animal feeds
India
Government of Whole food grains 0.05 0.05
India: Department
of Health (1976)
(cited by Smugh Milled food grains 0.01 0.01
et al. (1983))
USA
US EPA (1985a) Tolerances for residues 0.1 0.1
applicable to either aluminium
or magnesium phosphide:
almonds, barley, cashews,
cocoa beans, coffee beans,
corn, cottonseed, dates,
filberts, millet, nuts (Brazil,
pistachio), oats, peanuts,
pecans, popcorn, rice, rye,
flower seed, sesame seed,
sorghum, soybeans, sunflower
seed
Tolerances for residues of 0.1 0.1
aluminium phosphide:
vegetables (seed and pod,
except soybeans)
Walnuts, wheat 0.01 0.01
Tolerances for residues of 0.01 0.01
magnesium phosphide:
avocados, bananas, Chinese
cabbage, egg-plants,
endive (escavole)
grapefruit, kumquats, lemons,
lettuce, limes, mangoes, mush-
rooms, oranges, papayas,
Table 6. (contd.)
------------------------------------------------------------------------------
Organization Commodity Level of
(reference) phosphine
ppm mg/kg
------------------------------------------------------------------------------
peppers, persimons, pimentos,
plantains, salsify tops,
tangelos, tangerines, tomatoes
USSR
Kagan (1985) Cereals 0.01 0.01
------------------------------------------------------------------------------
5.1.3. Tobacco and consumer products
Residues of phosphine in fumigated tobacco were reported to
be not greater than 8.3 µg/kg (8.3 ppb) (Childs et al., 1969; Kuhn
et al., 1971). Experimental fumigation of tobacco with 32P-
phosphine similarly led to residual 32P-phosphorus of less than
5 mg/kg (5 ppm) (Underwood, 1972). However, this may not
qualitatively simulate the residues found in practice since the
32P-phosphorus contained in tobacco was predominantly in the
form of phosphate (which is a normal constituent). This
contrasts with the results of a similar study (Kuhn et al.,
1971) where the majority of residual 32P-phosphorus was as
phosphine and approximately 25% was non-volatile. In
Underwood's study, fumigated tobacco was made into cigarettes
that were smoked in a smoking machine and the ash, mainstream
smoke, and butt analysed for 32P. The 32P was found to be
confined to the ash as phosphate. Winks (1970) reported that
tablet and pellet residues from tobacco fumigated with
PhostoxinR contained 3.4% and 2.9%, respectively, of unreacted
aluminium phosphide. According to subjective evaluation of the
fumigated leaf after aeration for 2 days there were no off-
odours or the odour of phosphine.
5.1.4. Terrestrial and aquatic organisms
Phosphine or phosphides have only been reported in
terrestrial or aquatic organisms due to deliberate or accidental
administration of phosphine or phosphides.
Muscle tissue of rabbits and chickens poisoned with zinc
phosphide did not contain detectable residues (Kozhemyakin et
al., 1971; Bubien et al., 1974). Intestines and liver may
contain phosphides (Bubien et al., 1974; Meredith, 1981) but the
whole carcass of a kangaroo rat killed by a dose of zinc
phosphide was not toxic to the kit fox, despite the fact that
the amount ingested by the rat was 3 times the amount expected
to be lethal for the fox, on a body weight and LD50 of zinc
phosphide for the fox (Schitoskey, 1975). Rats, cats, and mice
fed for 30 days on meat from the carcasses of rabbits and
chickens killed with zinc phosphide did not show any specific
toxic effects (Kozhemyakin et al., 1971).
5.2. General Population Exposure
5.2.1. Access to phosphine and phosphides
Zinc phosphide pastes, though legally restricted in many
countries, are available without restriction for use as
rodenticides in others, and there are many reports of their
ingestion in suicide cases. Magnesium and aluminium phosphide
formulations are also restricted and not normally available to
the general public. In most countries, there is strict control
of fumigation to prevent public exposure to phosphine, and
guidelines for safe fumigation are available. These measures,
combined with negligible ambient air concentrations, effectively
limit exposure.
5.2.2. Residue exposure
Residue levels in fumigated foods are generally regulated at
0.1 mg/kg (0.1 ppm) or sometimes 0.01 mg/kg (0.01 ppm). Even
among populations whose diet is mainly derived from stored pro-
ducts, the daily intake would be unlikely to exceed 0.1 mg/day,
even if the phosphine or phosphides survived cooking. Under
most circumstances daily intakes would be several orders of
magnitude lower than this, because of lower residues, the small
fractions of the diet that have been fumigated, and losses of
fumigant in storage and food preparation.
5.2.3. Subgroups at special risk
No subgroups of the general population have been identified
to be at special risk from phosphine or phosphides except for
children, who might find and eat bait containing phosphides.
5.3. Occupational Exposure During Manufacture, Formulation, or Use
Occupational exposure can be divided into 4 general
categories: (a) workers producing phosphine and phosphides; (b)
workers in operations that can release phosphine, e.g., welding,
metallurgy, semi-conductors (c) fumigators and pest-control
operatives; and (d) transport workers, e.g., drivers, seamen.
Both the exposure patterns and the potential for control of
exposure differ from case to case.
Exposure to phosphine and phosphorus oxides, which occurs
during the manufacture of metal phosphides, varies according to
the method of manufacture. High levels of exposure may occur in
the direct methods involving the reaction of red phosphorus with
powdered metal. Freshly produced powdered phosphides evolve
phosphine at fairly high rates initially, producing warehouse
concentrations ranging from 0.4 to 1.6 mg/m3 (0.3 - 1.13 ppm).
Concentrations of 2.5 and 4 mg/m3 (1.8 and 2.9 ppm), necessit-
ating the use of personal respiratory protection, have been
reported in production areas (Jackson & Elias, in press).
The use of zinc phosphide in the preparation of poisoned
bait would not be associated with significant phosphine
exposure, because of the stability of zinc phosphide in neutral
media. There is no literature regarding occupational exposure
to phosphine during the formulation of preparations of aluminium
phosphide for use in fumigation. Jones et al. (1964) reported
that atmospheric levels to which operatives were exposed while
adding tablets of formulated aluminium phosphide to wheat were
undetectable. Levels encountered when stores were re-entered for
loading or turning were much higher, ranging from 18 to 35 mg/m3
(13 - 25 ppm). In the USA, an estimated one million workers are
at risk of inadvertent exposure. Ten thousand of these workers
are engaged in the grain freight trades where accidental
exposure is a considerable hazard (US NIOSH, 1977).
Exposure to phosphine has also been described in the
operation of acetylene generators (Harger & Spolyar, 1958);
exposure to a level of 11 mg/m3 for up to 2 h per day has been
estimated. Exposure to phosphine can also occur in the
production of phosphorus (Beloskurskaya, 1978), in the
conversion of white phosphorus to red phosphorus where levels of
up to 0.5 mg/m3 (0.35 ppm) were found (Jackson & Elias, in
press), and also in steel pickling (Vdovenko et al., 1984).
The carriage of ferrosilicon as a badly ventilated cargo,
particularly in barges, can release phosphine accidentally by
the action of water on calcium phosphide, one of the impurities
present. The transport of ferrosilicon requires precautionary
measures to prevent dangerous emissions of phosphine (Hunter,
1978). High ship-board concentrations of 1.4 - 3 mg/m3 (1 -
2 ppm) in living quarters and about 8.5 mg/m3 (6 ppm) in the
hold were reported in a vessel transporting ferrosilicon
(Lutzmann, 1963) and a case of fatal poisoning in the same
environment was reported by Ziemer, (1963). Jones et al.
(1964) reported phosphine concentrations of 5 - 13 mg/m3 (3.7 -
9 ppm) in the holds of ships transporting fumigated wheat.
Phosphine fumigation of agricultural commodities on board ship
during transit is common practice in some parts of the world.
Countries that import grain normally accept fumigation en route
and some nations require such treatment (Davis, 1986). Guide-
lines for safe fumigation practice have been established by the
United Nations International Maritime Organization (IMO, 1980)
and include the regular monitoring of air in the living quarters
to ensure that there is no hazard for crew members from the
accumulation of phosphine.
Many metals contain phosphorus in small amounts, and
phosphine can be generated in a variety of metallurgical
processes. Cole & Bennett (1950) reported the presence of about
4 mg phosphine/m3 in the immediate vicinity of magnesium powder
after its manufacture from bulk metal containing 0.0038 -
0.0093% phosphorus. Ferrosilicon alloys in contact with water
form phosphine in amounts that vary with the silicon content
(which correlates with the phosphide levels) so that alloys
containing 30 - 60% silicon can release 10 - 230 litres
phosphine per tonne (Delomenie, 1933). Concentrations of
phosphine close to the tool cutting edge in the machining of
spheroidal graphite iron were reported by Mathew (1961) to vary
between 0.01 and 6.5 mg/m3 (0.01 and 4.6 ppm). Concentrations
in the breathing zone ranged from 0.01 to 1.3 mg/m3 (0.01 -
0.95 ppm) and the average was 0.9 mg/m3 (0.65 ppm). Earlier,
Bowker (1958) had reported similar results and found that
phosphine concentrations fell from 8.5 mg/m3 (6 ppm) at a
distance of 1 cm from the tool to slightly over 1 mg/m3
(0.8 ppm) at 8 cm from the tool.
Although phosphine is used extensively in semi-conductor
manufacture, there are no published figures for occupational
exposure in this industry. Since installations are invariably
modern and phosphine is used under precise and strictly
controlled conditions, frequently diluted with inert gases,
exposure of workers is not likely to be high. There are no
published data relating to exposure to phosphine in the
synthesis of organophosphine or phosphonium derivatives.
Some recommended occupational exposure limits for phosphine
in various countries are given in Table 7.
Table 7. Occupational exposure limits for phosphine in various countries
------------------------------------------------------------------------------
Country Legal mg/m3 Comments Source
------------------------------------------------------------------------------
Australia Reca 0.4 TLV TWAc Approved occupational health
guide threshold limit values
(1983)
Belgium Recb 0.4 TLV Threshold limit values (1978)
Bulgaria Reg 0.1 MPCc Official journal, 88 (1971)
Czechoslovakia Reg 0.1 MAC TWA Hygienicke predpisy
0.2 MAC Ceiling Ministerstva zdravotnictvi
value CSR/Hygienic regulations of
Ministry of Health of CSR, 58
(1985)
Uprava No. 7/1985 Vest. Mz SSR
Reg. V Ciast.24/1985 ZB/
Regulation of Ministry of Health
of SSR No. 7/1985 Reg. In
Section 24/1985 ZB
Finland Reg 0.1 MPC TWA Luftfoereningar paa Arbets-
platsen (Air pollutants at the
workplace) (1982)
German Reg 0.1 TWA Maximale zulässige
Democratic 0.3 STELc Konzentrationen Gesundheits-
Republic gefärdender Stoffe in der Luft
am Arbeitsplatz (Maximum
allowable concentrations of
noxious substances in the
Atmosphere (1983)
Table 7 (contd).
------------------------------------------------------------------------------
Country Legal mg/m3 Comments Source
------------------------------------------------------------------------------
Germany, Rec 0.15 8-h TWA Deutsche Forschungsgemein-
Federal 0.3 5-min STEL schaft, "Maximale Arbeits-
Republic of platzkonzentrationen",
xxi, 16, (1985)
German Association for
Research, "Maximum Workplace
Concentrations",
xxi, 16, (1985)
Italy Rec 0.4 8-h TWA Valori limitati ponderati
(Threshold limit values) (1978)
Hungary 0.1 ILO (1980)
Netherlands Rec 0.4 TWA Nationale MAC-Lijst
1.5 STEL (National MAC-List) (1985)
Poland Reg 0.1 Ceiling Ordinance of the Minister of
value Labour, Wages & Social Affairs
22 Dec. (1982)
Romania Reg 0.2 TWA Ordinance of the Ministry
0.5 Ceiling of Health, 60 (1975)
value
Sweden Reg 0.4 1-day TWA Arbetarskyddsstyrelsens
Foerfattiningssamling, (1984,
1985) 10 (1984)
Switzerland Reg 0.15 TWA Zulässige werte in Arbeitsplatz
(permitted values in the
workplace (1984))
United Rec 0.4 8-h TWA Health and Safety Executive
Kingdom 1.0 10-min TWA EH40/85 (1985)
USA Rec 0.4 TWA ACGIH (1986)
1.0 STEL ACGIH (1986)
US CFR (1981)
USSR Reg 0.1 Ceiling Gosndarstrennyi Standart
value SSSR/State Standard of USSR,
12.1.005 (1976)
Yugoslavia Reg 0.1 MAC TWA Ordinance, 24-3698/1 (1971)
------------------------------------------------------------------------------
a Rec. = Recommendation.
b Reg. = Registered regulatory requirement.
c TLV = Threshold limit value.
TWA = Time-weighted average.
MPC = Maximum permitted concentration.
MAC = Maximum allowable concentration.
STEL = Short-term exposure limit.
6. KINETICS AND METABOLISM
6.1. Insects
Uptake of phosphine by insects is rapid in the presence of
oxygen, but little absorption occurs in low or zero oxygen
atmospheres, and the insecticide potential is thus reduced.
Over 100 mg phosphine/kg body weight may be absorbed by insects
at high dosage rates, and some insects continue to absorb
phosphine for long periods, even after knock down (Bond et al.,
1969). Phosphine taken up by insects is not removed by ventila-
tion of volatile phosphine derivatives or phosphine itself but
is apparently excreted slowly (Price et al., 1983). Most of the
32P, derived from 32PH3, taken up by insects is found in the
soluble fraction of the cells (Robinson & Bond, 1970); in
deproteinized tissue extracts, the radiolabel is present mainly
as hypophosphite and orthophosphate (Price et al., 1982).
6.2. Mammals
There have been no formal studies of the toxicokinetics of
phosphine and metal phosphides in mammals.
6.2.1. Absorption
6.2.1.1 Inhalation
Because systemic toxic effects are detectable after short
exposures to very low atmospheric concentrations of phosphine,
inhaled phosphine is generally considered to be readily absorbed
through the lungs. Hydrolysis suggests that aluminium or
magnesium phosphides deposited on the moist surfaces of the
respiratory tract would release absorbable phosphine but zinc
phosphide, which hydrolyses significantly only under acid
conditions, would be stable for some time. However, the
transfer of a proportion of inhaled zinc phosphide to the
intestinal tract by the lung particulate clearance mechanisms
would permit hydrolysis to phosphine by gastric acid as well as
absorption of the zinc phosphide. The lungs also absorb
particulates and, as it is known that zinc phosphide is absorbed
intact from the gut (Meredith, 1981), inhaled zinc phosphide
dust might be absorbed directly via the respiratory tract and
then hydrolysed in the tissues.
6.2.1.2 Dermal
An acute dermal LD50 for zinc phosphide in rabbits (2000 -
5000 mg/kg body weight) has been reported, but no details are
available. Hydrolysis of aluminium and magnesium phosphides on
the skin would lead to the evolution of gaseous phosphine, which
could then be absorbed by inhalation, but this is unlikely with
zinc phosphide, and the LD50 result suggests toxicity by dermal
absorption (US EPA, 1983). In general, dermal absorption of
phosphine and metal phosphides is insignificant.
6.2.1.3 Oral
The oral route is not relevant to the absorption of gaseous
phosphine. Ingestion of zinc phosphide results in detectable
amounts of acid-hydrolysable phosphide in the liver of rats
(Curry et al., 1959; Meredith, 1981). Human ingestion of
tablets containing aluminium phosphide yielded evidence of acid-
hydrolysable phosphide in blood and liver (Chan et al., 1983).
These results indicate that metal phosphides can be absorbed
directly. Meredith (1981) showed that when zinc phosphide was
administered to rats by gavage in corn oil, the fraction
recovered as phosphine from the air of the metabolic chamber
increased with the dose administered up to about 25% at a dose
of 4 mg/rat (approximately 20 mg/kg). The possibility that this
phosphine derived directly from the alimentary canal and not via
systemic absorption and subsequent exhalation could not be
excluded. For comparison, in vitro hydrolysis of zinc phosphide
for 12 h yielded 7.1% as phosphine at pH 4 and 38.8% at pH 2
and, unless there is some special enzymic mechanism, acidic
gastric conditions afford the only opportunity for such a level
of hydrolysis. The efficacy of zinc phosphide as a rodenticide
depends on the absorption of phosphide or phosphine after oral
administration. Meredith (1981) also showed that about 4 times
as much phosphide was recoverable from the liver when zinc
phosphide was administered in corn oil rather than in water,
suggesting greater absorption of unhydrolysed material.
McGirr (1953) speculated that commercially available zinc
phosphide might consist of 2 fractions that are attacked at
different rates in the gastrointestinal tract. This was based
on the fact that phosphine is produced rapidly and yet zinc
phosphide is recoverable from the liver of poisoned animals.
However, this did not consider the possibility that storage in
fat might lead to a fraction of the zinc phosphide being more
slowly hydrolysed and thus more readily absorbed unchanged
(Meredith, 1981).
6.3. Distribution
Inhaled phosphine produces neurological and hepatic symptoms
suggesting that it reaches the nervous system and liver (Childs
& Coates, 1971). Ingested phosphides have been shown to reach
the liver and blood in rats and human beings (Curry et al.,
1959; Meredith, 1981; Chan et al., 1983). On the other hand,
muscle tissue of animals poisoned with supralethal doses of zinc
phosphide did not contain detectable levels of phosphine or
phosphide and did not produce toxic effects when fed to test
animals (Kozhemyakin et al., 1971; Bubien et al., 1974). Curry
et al. (1959) reported the presence of acid-hydrolysable
phosphide in the kidney as well as in the liver of a fatal case
of zinc phosphide poisoning.
6.4. Metabolic Transformation
Metal phosphides are hydrolysed to phosphine and the corres-
ponding metal cation (Van Wazer, 1982). In rats, phosphine that
is not excreted in the expired air is oxidized and appears in
the urine, chiefly as hypophosphite and phosphite (Curry et al.,
1959; Meredith, 1981). Meredith (1981) also reported an
unidentified metabolite, detectable by paper chromatography and
distinct from pyrophosphate and metaphosphate. The fact that
(a) phosphine is incompletely oxidized; and (b) the proportion
of an administered dose that is eliminated as expired phosphine
increases with the dose suggests that the oxidative pathway is
slow.
6.5. Elimination and Excretion
Meredith (1981) administered zinc phosphide suspended in
corn oil, by gavage, to Wistar rats (body weight approximately
200 g) and measured the phosphine concentrations in a metabolic
chamber over the following 12 h. After doses of 0.5, 1, 2, 3,
and 4 mg, the proportions of the administered doses as phosphine
in air were 1.5%, 1.7%, 3.2%, 15.6%, and 23.5%, respectively,
but some or much of this could have been derived from faeces or
intestinal gas rather than by absorption and exhalation. Paper
chromatographic estimation of hypophosphite and phosphite in the
urine of rats dosed with zinc phosphide showed gradients
positively correlated with dose, but the proportion of the
administered dose excreted was not quantified. Hypophosphite is
the principal urinary excretion product (Curry et al., 1959).
6.6. Retention and Turnover
Virtually all the phosphine in the air of metabolic chambers
housing Wistar rats, dosed orally with zinc phosphide, had
disappeared after 12 h (Meredith, 1981), and this period
corresponds with the duration of symptoms following sub-lethal
doses of zinc phosphide. The same author determined the acid-
hydrolysable phosphide in the liver of single rats given a diet
containing 15 mg zinc phosphide/kg diet. The liver of a rat fed
for 15 days contained nearly twice as much as that of a rat fed
for 7 days. However, this limited study cannot be considered to
provide evidence for accumulation of metal phosphides.
6.7. Reaction with Body Components
Phosphine reacts with some haem- and copper-containing
proteins in vitro. Insect cytochrome c oxidase is reduced and
not reoxidizable in air (Rajak, 1971). Mammalian haemoglobin
does not react with phosphine in the absence of oxygen, but
oxyhaemoglobin is converted through Fe3+-containing compounds to
a verdichromogen-like material (Trimborn & Klimmer, 1962). The
nature of the reaction between phosphine and these proteins is
uncertain, but oxyhaemoglobin is denatured and a variety of
enzymes are inhibited by reaction with phosphine.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
Ruschel & Da Costa (1966) treated seeds of French bean
( Phaseolum vulgaris L.) with aluminium phosphide, using 3
PhostoxinR tablets/m3. Treated and untreated control seeds,
inoculated with a pure culture of the nitrogen-fixing bacterium
Rhizobium phaseoli, were sown in pots containing sandy soil
(pH 4.5) and maintained under greenhouse conditions. Plants,
sampled during the flowering period, showed no differences in
the numbers of nodules formed by the bacteria.
Natarajan & Bagyaraj (1984) reported laboratory studies on
the effects of phosphine fumigation (100 mg/litre for 15 days at
an unspecified temperature) on the microbial load (fungi,
bacteria, and actinomycetes) of blackgram and fieldbean seeds
with different moisture contents. Total microbial populations
were estimated using the dilution plate technique. Results are
given in Table 8. It was concluded that phosphine was effective
on actinomycetes. The effects of phosphine on each type of
microorganism depended on the seed type and the moisture
content.
Table 8. Microbial loada per g seedsb
---------------------------------------------------------------
Moisture Fungi Bacteria Actinomycetes
level (%) (x100) (x1000) (x100)
---------------------------------------------------------------
Blackgram
Control 8 11.54 25.86 0.41
12.5 51.96 267.20 0.30
15 61.92 358.80 0.35
Phosphine- 8 .95 13.89 0.33
fumigated 12.5 1.16 34.23 0.06
15 .88 69.71 0.00
Fieldbean
Control 8.6 1.80 21.86 0.82
12.5 3.79 35.77 0.38
15 5.29 67.68 0.27
Phosphine- 8.6 1.32 6.17 0.22
fumigated 12.5 1.02 12.13 0.29
15 .60 18.10 0.20
---------------------------------------------------------------
a Microbial counts after 15 days fumigation at 100 mg
phosphine/litre.
b From: Natarajan & Bagyaraj (1984).
Lück et al. (1984) reported the effects of phosphine
fumigation over 6 days with variable concentrations equivalent
to at least 85 mg x h/litre on microorganisms in a variety of
dairy products. There were no differences in bacterial, yeast,
or mould counts between fumigated products and non-fumigated
control products handled similarly but without fumigation.
The data of Rolulich & Menser (1970) (section 6.4.3),
indicate that phosphine damages and inhibits the respiration and
growth of microflora in moist stored wheat grains.
7.2. Aquatic Organisms
An LC50 for phosphine for the frog from a 30-min exposure
was reported to be 0.56 mg/litre. The LC50 for a 15-min
exposure was 0.84 mg/litre (WHO/FAO, 1980). The aluminium
phosphide formulation known as Detia PelletsR is reported to be
highly toxic for the bluegill sunfish, with a 96-h LC50 of
0.178 mg/m3 (0.126 ppm) (Freyberg, 1979).
7.3. Terrestrial Organisms
7.3.1. Insects and mites
Insects are a major target organism of phosphine as a
pesticide.
In practice, fumigation is carried out by the distributed
insertion of formulated aluminium phosphide tablets into the
bulk of the stored product, so that the phosphine evolved by
hydrolysis with the moisture content of the product percolates
the bulk and is retained by the gas-tight design of the store or
the use of an impermeable covering. Typically, the phosphine
concentration rises to a maximum and then decays over a longer
period. The phosphine dose is often described in terms of the
duration of exposure and estimate of concentrations or by the
concentration x time product. This is calculated from the area
under the curve of concentration versus time Ct x dt or, approx-
imately, sigma (Ct x It), where Ct is the concentration at time t
and It is the interval over which Ct is a sufficiently accurate
measure of the actual concentration.
Because the susceptibility to phosphine of different
developmental stages varies, it is possible that some indivi-
duals will be at a less susceptible stage at the time of the
peak phosphine concentration and may survive fumigation.
Moreover, acclimatization may occur with rising concentrations.
The dose or concentration that is lethal for a particular
species is therefore a complicated function of the concentration
and its time-course (Reichmuth, 1985). Laboratory fumigation at
constant concentrations is not a sound basis for the calculation
of doses for practical fumigation (Reichmuth, 1986). Moreover,
the effects of any concentration x time product will be
influenced by the temperature and other aspects of the gaseous
environment, such as the partial pressure of oxygen which may
fall to a low level in stores of metabolically active pulses.
It has been shown that insects are tolerant to phosphine in a
nitrogen- or oxygen-deficient atmosphere (Kashi, 1981a,b). By
influencing the rate of hydrolysis of the phosphide, the
relative humidity or moisture content affects the concentra-
tion x time course and thus independently influences the
toxicity for pests.
In spite of the complexity of these relationships, the
concentration x time product is frequently calculated as an
index of the dose. However, though the concentration x time
product is related to mortality with some insecticides, there is
a deviation with phosphine. Longer exposures are much more
effective in achieving control than shorter ones with the
equivalent concentration x time product (Hole et al., 1976;
Kashi, 1982; Winks, 1984, 1985). Winks (1985) suggested that
part of the explanation of the relative tolerance to high
dosages was narcosis, which reduced absorption to sub-lethal
doses, but his experimental data neither confirmed nor refuted
his hypothesis.
In addition to these uncertainties in determining the
effectiveness of fumigation, there is considerable variation in
susceptibility to phosphine among target organisms. Grain mites
are tolerant (Bowley & Bell, 1981) to concentration x time
products 2 or 3 orders of magnitude greater than those effective
for many insect species. Hole (1981) reported that 3 strains of
Sitophilus granarius and Rhyzopertha dominica were relatively
tolerant compared with 3 other species of Coleoptera. The data
also indicated considerable variation between strains within a
species (Table 9).
Adu & Muthu (1985) reported a 6-fold variation in the LC95
values for different life cycle stages of Callosobruchus
chinensis L. with the susceptibility of developmental stages
decreasing in the order: larva > adult > pupa > egg. However,
Bell et al. (1984) found that the eggs of Trogoderma granarium
were much more susceptible than the larva and Winks (1981)
found that the early pupae of Tribolium castaneum were substan-
tially more tolerant than the larvae.
Larvae of many species can enter a state of suppressed
development known as diapause, so that the insects can survive
under unfavourable conditions. Cox et al. (1984) found that
diapause increased tolerance to phosphine in the larvae of
Ephestia kühniella. Diapausing larvae of Trogoderma
granarium survived a 5-day exposure to a concentration x time
product of 164 mg x h/litre, while laboratory stock larvae were
killed by a 4-day exposure to 120 mg x h/litre (Bell et al.,
1984).
Table 9. Concentration x time products of phosphine for 100% mortality and
maximum survived doses (MSD) for various numbers of strains of 7
species of stored-product Coleopteraa
------------------------------------------------------------------------------
Species Number Stage Temper- RH LDhi(mort %) MSDb
ature (%) (mg x h/ (mg x h/
(°C) litre) litre)
------------------------------------------------------------------------------
Sitophilus granarius 8 all 25 70 46 (100) 22
3 all 25 70 > 46 (100) 46
Sitophilus zeamais 5 all 25 70 46 (100) 22
5 all 25 70 > 46 (100) 46
Sitophilus oryzae 1 all 25 70 22 (100) 9
6 all 25 70 46 (100) 22
4 all 25 70 > 46 (100) 46
Rhyzopertha dominica 5 all 25 70 9 (100) < 9
4 all 25 70 22 (100) 9
1 all 25 70 46 (100) 22
Oryzaephilus 7 all 25 70 > 4 (100) > 4
surinamensis
Tribolium castaneum 17 all 25 70 9 (100) 4
9 all 25 70 > 9 (100) 9
Tribolium confusum 3 all 25 70 > 22 (100) > 22
1 all 25 70 > 9 (100) > 9
------------------------------------------------------------------------------
a From: Hole (1981).
b Maximum phosphine concentrations were about 0.28 mg/litre and the dose
varied with the duration of the exposure period. In many cases, 100%
lethality was not achieved at the highest dose used.
A further variant in the response of insects to phosphine
has been the development of resistant strains (Champ & Dyte,
1976). Attia (1984) found that phosphine resistance in Tribolium
castaneum and Rhyzopertha dominica was sometimes coincident
with, but not otherwise related to, resistance to 6 other
insecticides. The phosphine-resistant strains had resistance
factors of about 5 and 10, respectively. Nakakita & Winks
(1981) reported that ratios of LC50 and LC99.9 values for
various stages of resistant and susceptible strains of Tribolium
castaneum varied from 0.1 to about 6. In general, all stages of
a resistant species have a higher tolerance than the equivalent
stage of the susceptible strain, suggesting a metabolic
difference persisting through metamorphosis. Price (1984)
reported that adult insects of a resistant strain absorbed much
less radioactive phosphine than their susceptible counterparts
and retained normal respiratory activity. Living resistant
insects absorbed less phosphine than dead insects, and the
author concluded that the active exclusion of phosphine as a
possible mechanism for resistance was supported by the data.
Noack & Reichmuth (1981) determined a toxic threshold value
for phosphine in Drosophila melanogaster of about 1.4 mg/m3
(1 ppm) in air, using the criterion of a statistically just-
significant increase in mortality rate. The value obtained is
comparable to the threshold for toxicity in human beings and
other mammals.
Lethal doses of phosphine for a variety of stored-product
pests are given in Tables 9, 10, and 11. Childs (1972) reported
that all stages of the cigarette beetle were killed by fumiga-
tion with phosphine at concentrations that never exceeded
500 ppm (< 33.6 mg x h/litre) for 48 h, in field tests using
transport containers at an unspecified temperature and
humidity.
Winks (1970) inoculated tobacco bales with the tobacco
beetle Lasioderma serricorne and studied the mortality after
phosphine fumigation (20 tablets PhostoxinR/28 m3). Tempera-
tures ranged from 11 to 25 °C and the relative humidity from 65
to 82%. Peak concentrations were about 300 mg/m3. Mortality
was assessed after a 7-day recovery period, and the number of
progeny from eggs laid during fumigation was assessed 8 weeks
after fumigation. Mortality was 100% and the number of progeny
was 0.2% compared with unfumigated controls.
7.3.2. Birds
Ikeda (1971) studied the lethality of zinc phosphide for the
quail and reported an oral LD50 of 35 mg/kg body weight; there
was a reduction in egg laying at 3.5 mg/kg. Shivanandappa
(1979) found that the acute oral LD50 and LD90 of zinc phosphide
in poultry were 25 and 31 mg/kg body weight, respectively.
Treatment of chickens with encapsulated doses of 14, 21, 31.5,
or 47.2 mg/kg body weight, daily for 4 weeks, resulted in deaths
at all doses. Mortality was 12% at the lowest dose and 100% at
the highest dose, where death occurred within 6 - 18 h of
administration of the first dose. Hill et al. (1975) studied
the effects of zinc phosphide administered in the diet for 5
days to mallard ducks. The dosing period was followed by 3 days
of untreated feed. The zinc phosphide concentration of
1285 mg/kg diet was calculated to produce 50% mortality. Though
the acute oral LD50 for most avian species is generally in the
range of 20 - 100 mg zinc phosphide/kg body weight, it has been
reported that chickens fed 12 - 16 mg/kg body weight displayed
toxic symptoms, including reduced red-cell counts, reduced
haemoglobin concentration, and leukocytosis, 1 - 1.5 h after
dosing (Kozhemyakin et al., 1971). Baxland & Gordon (1945)
administered oral doses of zinc phosphide ranging from 15 to
400 mg/kg body weight to single domestic hens of 2 species. All
the birds receiving more than 30 mg/kg body weight died while
those receiving 20 mg/kg or less survived.
Table 10. Concentration x time products for high and 50% lethality for
different stages of common insect pests under specified conditions
---------------------------------------------------------------------------------------------------------
Species Stage Temper- Relative Time LDhi(mort %) LD50 Reference
ature humidity (h) (mg x h/litre) (mg x
(°C) (%) h/litre)
---------------------------------------------------------------------------------------------------------
Trogoderma DPa larva 20 60 120 75.0 (100) - Bell et al. (1984)
egg 20 60 72 50.0 (100) - Bell et al. (1984)
egg 20 60 48 22.6 (100) - Bell et al. (1984)
Callosobruchus egg 25-27 70-75 24 3.127 (95) 0.0708 Adu & Muthu (1985)
chinensis L. larva 25-27 70-75 24 0.556 (95) 0.1622 Adu & Muthu (1985)
pupa 25-27 70-75 24 2.542 (95) 0.684 Adu & Muthu (1985)
adult 25-27 70-75 24 0.823 (95) 0.240 Adu & Muthu (1985)
Rhyzopertha adult 27 65 20 0.5 (99.9) 0.126 Attia & Greening (1981)
dominica
Tribolium adult 27 65 20 0.32 (99.9) 0.166 Attia & Greening (1981)
castaneum
Tribolium adult 27 65 20 0.58 (99.9) 0.26 Attia & Greening (1981)
confusum
Ephestia DP larva 10 70 < 20 79.6b (100) 29-42 Cox et al. (1984)
Tribolium Sc 15-day larva 25 ns 6 0.52 (99.9) 0.17 Nakakita & Winks (1981)
castaneum Rd 15-day larva 25 ns 6 2.57 (99.9) 0.34 Nakakita & Winks (1981)
S 20-day larva 25 ns 6 0.29 (99.9) 0.13 Nakakita & Winks (1981)
R 20-day larva 25 ns 6 0.74 (99.9) 0.18 Nakakita & Winks (1981)
S pre-pupa 25 ns 6 0.74 (99.9) 0.17 Nakakita & Winks (1981)
R pre-pupa 25 ns 6 4.22 (99.9) 0.33 Nakakita & Winks (1981)
S early pupa 25 ns 6 11.94 (99.9) 4.2 Nakakita & Winks (1981)
R early pupa 25 ns 6 - - Nakakita & Winks (1981)
S mid-pupa 25 ns 6 - 0.69 Nakakita & Winks (1981)
R mid-pupa 25 ns 6 - 21.0 Nakakita & Winks (1981)
S late pupa 25 ns 6 1.12 (99.9) 0.09 Nakakita & Winks (1981)
R late pupa 25 ns 6 0.80 (99.9) 0.438 Nakakita & Winks (1981)
Table 10. (contd.)
---------------------------------------------------------------------------------------------------------
Species Stage Temper- Relative Time LDhi(mort %) LD50 Reference
ature humidity (h) (mg x h/litre) (mg x
(°C) (%) h/litre)
---------------------------------------------------------------------------------------------------------
Trichoplusia egg 24 ns 2 0.56 (100) - Leesch (1984)
ni Hübner larva 24 ns 0.67 0.28 (100) - Leesch (1984)
pupa 24 ns 2 0.56 (100) - Leesch (1984)
---------------------------------------------------------------------------------------------------------
a Diapausing.
b Approximate values.
c Sensitive strain.
d Resistant strain.
ns = not stated.
Table 11. Concentration x time products for 100% mortality and maximum
survived doses (MSD) for three species of grain-infesting mitea
---------------------------------------------------------------------
Species Stage Temper- Relative Time LDhi(mort %) MSD
ature humidity (days) (mg x h/ (mg x h/
(°C) (%) litre) litre)
---------------------------------------------------------------------
Tyrophagus adult 10 60-70 21 450 (100) 190
longior
Acurus siro adult 10 60-70 14 310 (100) 150
Glycyphagus adult 10 60-70 14 310 (100) 150
destructor
---------------------------------------------------------------------
a From: Bowley & Bell (1981).
Klimmer (1969) exposed 3 turkeys to phosphine at a concen-
tration of 211 mg/m3 and 6 hens at 224 mg/m3 in an acute
inhalation study. The turkeys exhibited apathy, restlessness,
dyspnoea, and tonic-clonic convulsions, and died after 68, 74,
and 80 min, respectively. When examined, organs were congested
with oxygenated blood. Hens exhibited tonic-clonic convulsions
and died after an average of 59 min (range, 50 - 64 min). Their
organs were also congested with oxygenated blood.
7.3.3. Mammals
7.3.3.1 Non-target species
The acute oral LD50 of zinc phosphide in the kit fox was
calculated to be 93 mg/kg body weight (Schitoskey, 1975). Dogs
(which may also be a target species) were reported to be killed
by a dose of 100 mg zinc phosphide/kg body weight when fasted
for 24 h, but not when fed (Aminzhanov, 1972).
The acute toxicity of zinc phosphide for large domestic
animals was reported by Fitzpatrick et al. (1955). Single doses
were administered by capsule, stomach tube, or mixed with the
feed. Deaths occurred as shown in Table 12.
McGirr (1953) quoted unpublished work by Fitzpatrick
indicating that the dose of zinc phosphide producing toxic
effects in cats and dogs lies between 20 and 40 mg/kg body
weight.
Table 12. Mortality of domestic mammals given single oral doses
of zinc phosphide by capsule, stomach tube, or mixed with the feed
Dose (mg/kg Mortality
body weight) Cow Goat Sheep Pig Total
20 0/1 1/2 - 0/3 1/6
30 - - 0/1 0/3 0/4
40 1/1 0/1 1/2 1/2 3/6
50 - - - 0/1 0/1
60 - 1/1 1/1 1/2 3/4
7.3.3.2 Rodents
The results of acceptance studies of 4 commercially avail-
able wax baits containing zinc phosphide on Norwegian rats and
house mice were reported by Marshall (1981). Each animal was
offered the bait and a palatable non-toxic alternative. While
one bait achieved an acceptance of 48.8% with 100% mortality in
rats and 38.5% acceptance with 100% mortality in mice, the other
baits achieved less than 6.5% acceptance and mortality rates did
not exceed 50%. Gill & Redfern (1983) reported 1 day "no-
choice" feeding tests, using standard procedures, which studied
the effects of zinc phosphide administered to Shaw's gerbil
(Meriones shawi) at 10, 20, 30, 40, or 50 g/kg diet (EPPO,
1975). There was very little reduction in average food consump-
tion at 40 and 50 g/kg diet and none at 10, 20, and 30 g/kg
diet. Zinc phosphide at 10 g/kg resulted in 20% mortality but
dietary concentrations of 20 - 50 g/kg resulted in 70 - 100%
mortality. In palatability tests, consumption of both poisoned
(zinc phosphide at 20 or 50 g/kg) and plain baits was very low,
presumably because the poison acted rapidly and interfered with
feeding. Survival of 8/10 animals given zinc phosphide bait
(20 g/kg) and 2/10 given 50 g/kg may indicate that these animals
could detect the poison and avoided consuming a lethal dose.
Similar results were reported at doses of 40 and 50 g/kg for the
golden hamster ( Mesocricetus auratus Waterhouse) (Bradfield &
Gill, 1984). Sridhara (1983), in a study on the Indian gerbil
( Tatera indica cuvieri Waterhouse), reported that phosphide
incorporated in ragi (Eleusine coracana), a preferred food, at
0.5 g/kg induced aversion to this and enhanced the consumption
of maize (Zea mais) offered as an alternative. Administra-
tion of sub-lethally baited food on the fifth day only of the
study also induced aversion to the same food baited with a
different poison, and to a new food substituted for the
preferred food. However, it was not reported how long the
animals that had been given the poisoned preferred food on day 5
exhibited subsequent aversion to the same unpoisoned food. This
makes it difficult to evaluate the study.
Field census studies have been carried out to assess the
effects of rodenticide use. Traps are set and the rodent
population assessed by the number of each species trapped per
100 traps per 24 h. The rodenticide is administered and the
traps again set and the numbers captured are compared with those
of the earlier study to yield a percentage figure for rodent
control. Advani (1983) reported 90 - 95% overall control both
in winter (wheat, vegetable, and oil-seed crops) and summer
(millet crop) with zinc phosphide administered, after pre-
baiting, at 20 g/kg for one day at the entrance to active
burrows and the administration of aluminium phosphide at
1.5 g/burrow followed by sealing with moist earth. Chopra (1984)
undertook a similar study on aluminium phosphide in rice and
wheat fields with 3 species in which the following percentage
control rates were found (Table 13).
Table 13. Control rates (%) for 3 species in 2 crops
achieved by aluminium phosphidea
-------------------------------------------------------
Species Rice fields Wheat fields
-------------------------------------------------------
Rattus meltada 85.88 75
Bandicota bengalensis 63.33 50
Mus species 100 91.8
-------------------------------------------------------
a From: Chopra (1983).
Zinc phosphide dose-response data were reported for Shaw's
gerbil (Meriones shawi) (Gill & Redfern, 1983) and the golden
hamster (Mesocricetus auratus Waterhouse) (Bradfield & Gill,
1984). Zinc phosphide was administered in no-choice feeding
tests at 10, 20, 30, 40, or 50 g/kg diet according to a standard
protocol (EPPO, 1975). Their mortality data showed an irregular
relationship for the gerbil (100% mortality at 20 g/kg diet but
90% at 50 g/kg diet), probably as a result of small study
numbers. For the hamster, there was a dose-related mortality
with 100% at 50 g/kg diet.
An LD50 of zinc phosphide for the black-tail prairie dog has
been reported to be 18 mg/kg body weight (Tietjen, 1976).
7.4. Plants
The threshold concentration of phosphine in air for a
harmful effect on growing lettuce (chosen as a representative,
highly sensitive species) was determined by Noack & Reichmuth
(1982b) to be between 3 and 8 mg/m3. There were no adverse
effects on the germination of watercress seeds in soil that had
been treated for 3 days with air containing either 20 or 1400 mg
phosphine/m3; in fact, the high phosphine concentration
stimulated the growth of watercress plants.
7.4.1. Harvested plants
Leesch (1984) studied the damage to harvested fresh iceberg
lettuce 14 days after fumigation with various fumigants,
including phosphine, at 4.4 °C without air circulation and at
24 °C both with and without air circulation. Three lettuce
heads were exposed to phosphine at each of 3 concentrations
(0.28, 0.56, and 0.83 mg/litre) for each of 3 periods (16, 24,
and 48 h). Lettuce heads were then stored at 4.4 °C in a sealed
bag made of 1 mm polythene. An ordinal 5-point scale was used
to rate damage to the lettuce heads and an index calculated by
dividing the sum of the scores for each of the 3 lettuces by the
sum of the scores of 3 unfumigated control lettuces. There was
no clear dose-effect relationship between the concentra-
tion x time product and the damage index. Damage was slight to
moderate after fumigation at 24 °C with circulation, but without
circulation (both at 4.4 and 24 °C) damage was generally rated
as "none", though one excursion into the "slight" category
occurred. This level of damage was small compared with the
damage resulting from 5 other fumigants. Only acetaldehyde
produced similar slight damage. However, the comparisons are
not clear because of the arithmetic manipulation of ordinal
scales.
7.4.2. Viable seeds and grain
Natarajan & Bagyaraj (1984) studied the effects of phosphine
on the viability of blackgram and fieldbean seeds and found that
it did not have any effects on seed germination.
Effects on viable seeds of leguminous plants were studied by
Singh et al. (1983) who reported the effects on stored pulses of
phosphine fumigation for 5 days. Phosphine residues at normal
fumigation levels (2 tablets of PhosfumeR per tonne) decreased
to below the detection limit of 1.5 µg/kg after 3 or 4 days
aeration. Standard germination tests carried out immediately at
the end of fumigation revealed no impairment of germination,
even with pulses fumigated at 4 times the recommended dose.
These results suggest that stored pulses are not adversely
affected by exposure to phosphine or formulated aluminium
phosphide.
Ahmad (1976) studied seeds of 11 edible legumes and measured
the moisture content and the germination rate of control seeds
and of seeds fumigated for 7 days at 21 ± 5 °C using PhostoxinR
at a rate equivalent to approximately 3 tablets/m3, about 4
times the recommended dosage. After fumigation, test seeds were
aerated for 3 days. No differences in germination rates were
observed.
Zutshi (1966) cited earlier work on the effects of phosphine
fumigation on seed germination and reported the results of germ-
ination tests on paddy, wheat, maize, bhindi, brinjal, tomato,
and onion seeds. Three lots of each type of seed were fumigated
with PhostoxinR at doses equivalent to 12 or 18 mg/kg for 7 days
followed by 24 h aeration. After 30 days air-tight storage, one
lot was given a second fumigation for 7 days, and a second lot
was stored for 90 days in air-tight jars. In no case was there
any reduction in germination rate.
Kamel et al. (1973, 1974) reported that one variety of wheat
(Giza 155) was susceptible to phosphine fumigation (PhostoxinR,
2.5 tablets/m3) over 72 h with a germination rate that fell
rapidly with the number of fumigations.
The effects of phosphine on the biochemical reactions in
wheat grain of different moisture contents, which had been
fumigated with 10 mg phosphine/litre at 20 °C for 5 days, were
studied (Rohrlich & Menser, 1970). Germination of fumigated
grain with a 30% moisture content was totally inhibited, it was
reduced with a 25% moisture content, and was unaltered with a
20% content. Phosphine reduced the respiration of wheat partly
by damaging the microflora present. Activity of glutamate
decarboxylase was reduced by phosphine, when the moisture
content was 18% or more. The activity of glutamate-oxaloacetate
transaminase was not significantly changed compared with that in
untreated grain. The enzymes hexokinase, aldolase, glycer-
aldehyde-3-phosphate dehydrogenase, and pyruvate kinase had
nearly constant activity throughout all the studies. Alcohol
dehydrogenase activity was reduced to zero within 7 days as a
result of phosphine exposure of the grain at a moisture content
of more than 24%. Catalase activity in wheat (moisture content
about 15%) was reduced by about 20% after a 2-week exposure to
phosphine fumigation. Phosphine treatment markedly inhibited
respiration and growth of microorganisms in wheat with a
moisture content up to 20%, thereby protecting the grain from
microbial damage. In grain with a moisture content of between
18 and 27%, the amount of adenosine triphosphate (ATP) was
reduced by phosphine fumigation, but adenosine diphosphate (ADP)
was not, indicating that the respiratory activity in treated
grain was markedly reduced.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
This section deals with studies of effects on experimental
animals. Studies related to the field use of phosphine and
phosphides as a fumigant or rodenticide are discussed in
section 6.
8.1. Single Exposures
8.1.1. Inhalation studies on phosphine
The effects of phosphine on a kitten, a puppy, rabbits
and a guinea-pig exposed to concentrations that proved fatal in
a matter of hours were described in 1890 (Schulz, 1890).
Meissner (1924) reported observations on rabbits in which expo-
sure to 70 mg phosphine/m3 (50 ppm) for 10 min did not produce
any symptoms, but exposure to 140 mg/m3 (100 ppm) was fatal in
2.5 - 3 h, and 700 mg/m3 (500 ppm) was fatal in 25 - 30 min.
Rats survived exposure to 80 and 800 mg/m3 for 4 and 1 h, res-
pectively; cats survived exposure to 240 mg/m3 for 2 h; guinea-
pigs did not survive 2 h exposure to 400 mg/m3 (Rebmann, 1933).
Klimmer (1969) investigated the effects of single inhalation
exposures on cats, rabbits, rats, and guinea-pigs. The concen-
trations varied from 35 to 564 mg/m3. The symptoms described
were similar to those described in section 8.2.1 for short-term
studies. Death was attributed to respiratory paralysis followed
by cardiac arrest.
The Pesticide Registration Standards for both aluminium
phosphide and magnesium phosphide (US EPA, 1981, 1982) tabulate
the survival times for all these studies and there is a clear
relationship, independent of species, between concentration and
survival time. This is illustrated in Fig 1. The relationship
approximates to C x t1.43 = 200 000, where C is the concentra-
tion of phosphine measured in mg/m3 and t is the time to death
in minutes.
Waritz & Brown (1975) determined a 4-h LC50 (95% confidence
limits) for phosphine in male Charles River-CD rats of 15 (11 -
21) mg/m3 (11 (8.1 - 15) ppm). Using CFT-Wistar female rats,
Muthu et al. (1980) calculated LC50 and LC95 values for
phosphine, produced by hydrolysis of aluminium phosphide, on the
basis of concentration x time products. The LC50 (95% confidence
limits) was 220 (180 - 270) mg x h/m3, corresponding to a 4-h
LC50 of 55 mg/m3. However, the exposure durations were longer
than 4 h, and there is evidence that the concentration x time
product is not a good index of lethality in insects. The LC95
was 420 (260 - 670) mg x h/m3. These values were obtained at
27 + 2 °C, whereas an LC50 value of 360 mg x h/m3 and an LC95 of
490 mg x h/m3 were obtained at about 26 °C.
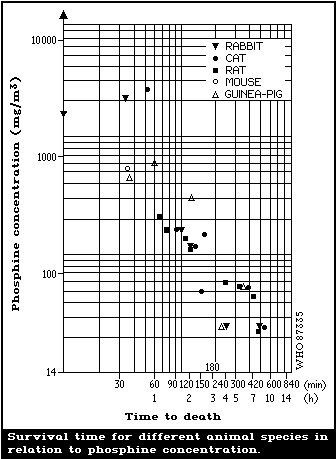 Neubert & Hoffmeister (1960) investigated the effects of
short-term exposures ranging from 460 to 2150 mg phosphine/m3 on
cellular respiration in rats. The oxidation of alpha-keto-
glutarate in liver cells was inhibited, but not oxidative
phosphorylation. Alpha-ketoglutarate oxidation was also reduced
in heart muscle, and phosphorylation was disrupted. Survival of
groups of 8 rats at various concentrations of diphosphine-free
phosphine was studied with the following results (Table 14).
Table 14. Survival times of 8 rats in different concentrations of
phosphine. Concentration x time products calculated from
the mid-point of the range of survival timesa
---------------------------------------------------
Concentration Survival Concentration x time
(mg/m3) (min) product (mg x min/m3)
---------------------------------------------------
4640 16 - 20 83 520
1000 50 - 70 60 000
320 150 - 200 56 000
100 400 - 600 50 000
---------------------------------------------------
a From: Neubert & Hoffmeister (1960).
If the use of the mid-point of the range of survival times
is reasonable, these results indicate that the relationship
between concentration and survival time is not simply
reciprocal, and that the index of t is greater than 1, but less
than the value of 1.43 derived from the data quoted by US EPA
(1981, 1982).
Klimmer (1969) determined lethal concentration x time pro-
ducts for rats and calculated, for example, 19 000 mg x min/m3
for a concentration of 287 mg/m3, but these results do not agree
with those calculated from the data of US EPA (1981, 1982) nor
with those of Neubert & Hoffmeister (1960). The value of the
concentration x time product, as a measure of phosphine dose in
mammals, is therefore open to question, as it is in insects.
8.1.2. Inhalation studies on zinc phosphide
The US National Pest Control Association submitted a value
of 19.6 mg/litre for an inhalation LC50 of 10% zinc phosphide
powder in rats. It was not stated whether the value was for
pure zinc phosphide or the 10% dilution and there was no
indication of exposure time (US EPA, 1983).
8.1.3. Oral studies on metal phosphides
In a study on 35 rats of both sexes administered doses of
20, 40, 50, or 80 mg/kg, Dieke & Richter (1946) reported an LD50
for zinc phosphide of 40.5 ± 2.9 mg/kg body weight for wild
Norway rats. Schitoskey (1975) reported an LD50 for the kit fox
of 93 mg zinc phosphide/kg body weight. Aminzhanov (1972)
reported that 100 mg/kg body weight of zinc phosphide was fatal
for starved dogs but not for dogs that were fed. Ikimanova
(1977) reported increased blood- and urinary-porphyrin concen-
trations in rats dosed with zinc phosphide. Other authorities
refer to 27 mg/kg body weight for an acute oral LD50 value for
94% pure zinc phosphide in rats (US EPA, 1983).
8.1.4. Dermal and other studies on metal phosphides
An acute dermal LD50 of 2 000 - 5 000 mg/kg body weight for
zinc phosphide (94% Zn3P2) in rabbits was submitted by the US
National Pest Control Association (US EPA, 1983).
8.2. Short-term Exposures
8.2.1. Inhalation exposure to phosphine
Jokote (1904) determined cumulative survival times in inter-
mittent phosphine exposure as shown in Table 15.
Table 15. Survival time for rabbits, cats, and guinea-pigs at 3
concentrations of phosphinea
-----------------------------------------------------------
Concentration of phosphine (mg/m3)
14 35 140
-----------------------------------------------------------
Survival time (h) 16 - 30 8.5 - 12 2.5 - 3.5
(rabbits, cats, and
guinea-pigs)
-----------------------------------------------------------
a From: Jokote (1904).
Miller (1940) exposed rabbits and guinea-pigs for 4 h per
day to various concentrations of phosphine. At 28 mg/m3 (20
ppm), both rabbits and guinea-pigs died during or after the
second exposure. At 14 mg/m3 (10 ppm), rabbits survived 7 - 14
successive exposures, but at 12 mg/m3 (8.3 ppm), only 4 or 5
exposures. In another study in the series, 2 rabbits that had
had five 4-h exposures to phosphine at 7 mg/m3 were accidentally
exposed to 20 mg/m3 (14 ppm) on the sixth day. Both rabbits
died during this exposure. The authors concluded that
pretreatment with sub-lethal concentrations of phosphine reduced
resistance to near-lethal concentrations. At low concentrations
(up to 14 mg/m3), animals displayed no signs until about 1/2 h
before death when they exhibited diminished reactivity, became
stuporose with shallow respiration, and died in coma.
Occasionally, animals died following exposure, with symptoms of
pulmonary oedema. At 28 mg/m3 or more, all animals exhibited
signs of respiratory irritation and died of pulmonary oedema.
Pathological examination of the lungs revealed bronchiolitis and
atelectasis of the lungs. There was no evidence of haemolysis,
but all organs were hyperaemic. The liver showed fatty
infiltration and there was cloudy swelling of kidney tubular
cells. After prolonged exposure, lungs frequently contained a
brown pigment that did not stain with Prussian Blue and
therefore was not iron-containing.
Neubert & Hoffmeister (1960) exposed female Wistar and E-B
(inbred strain) rats in a 121-litre chamber repeatedly for 6
days to 681 mg/m3 phosphine for 10 - 20 min per day. On the
seventh day, they were exposed until they died (22 - 35 min).
The concentration x time product for lethality after 6 days
pretreatment (about 20 mg x min/litre) was about one third of
that for a first exposure (48 - 87 mg x min/litre) and the
authors concluded that the effects of exposure were cumulative,
rather than that the phosphine accumulated in the body.
Klimmer (1969) exposed cats, guinea-pigs and rats to
phosphine concentrations of 1.4 and 3.5 mg/m3 (1 and 2.5 ppm)
for 4 - 6 h per day, 6 days a week, for a total of more than
800 h over a 24-week period without any clinical, laboratory, or
pathological evidence of effects. The liver function in the
cats, measured by bromosulphthalein (BSP) excretion, was normal.
In cats (3), guinea-pigs (3), and rats (10) exposed to phosphine
at 7 mg/m3 (5 ppm) for 6 - 8 h per day, the cats became
apathetic, showing anorexia after exposure, but exhibiting
thirst. Later, they developed unsteadiness, vomiting,
agitation, dyspnoea, and apnoea, before cardiac arrest after a
total of 35.5 - 45.5 h of exposure. The haemoglobin concen-
tration and erythrocyte count were reduced by 10 - 20% compared
with initial values, there was proteinuria and delayed BSP
excretion. Post-mortem examination revealed congestion of all
organs, with oedema and focal emphysema and red pigmentation of
the lungs. The guinea-pigs exhibited signs that were similar
to, but more marked than, those in the cats, and 2 animals had
asphyxial convulsions. Blood tests showed no deviations from
normal values, but the blood contained a brown coloration that
was not due to methaemoglobin, since spectroscopy revealed the
absorption bands of oxyhaemoglobin only. The guinea-pigs died
on the sixth day after 24 h - 32 h cumulative exposure, and
post-mortem examination revealed pulmonary oedema and red
discolouration and congestion of other organs. The rats died
after 27 - 36 h cumulative exposure. All exhibited congestion
of organs, 3 had pulmonary oedema and 7 had proteinuria. A
second series of exposures under slightly changed exposure
conditions at a different time of year produced qualitatively
similar results. In both series, neurohistological studies in
rats showed widening of the perivascular spaces, vacuolation of
the nuclei of ganglion cells, a reduction in the Purkinje cells,
and a glial reaction. Similar, but less marked, changes without
glial reaction were seen in the cats and guinea-pigs. There was
no control group in these studies, and certain observations in
exposed animals were dismissed on the grounds that they had
previously been seen in unexposed animals.
Waritz & Brown (1975) exposed Charles River-CD rats to
phosphine for 4 h per day, for 12 days, at a concentration of
5.5 mg/m3 (0.163 µmol/litre). The rate of weight gain was
reduced during the exposure period. Although the authors stated
that the rate returned to normal during a 14-day post-exposure
observation period, their data indicate a uniformly reduced rate
of weight gain throughout the exposure and recovery phases.
Post-mortem examination of rats, sacrificed both at the end of
the exposure period and at the end of the recovery period,
revealed no gross or microscopic abnormalities.
The effects of a 1.5-month exposure of white rats to
phosphine at concentrations of 0.05, 0.2, 1.5, and 8 mg/m3 were
reported by Pazynich et al. (1984). There were changes in blood
cholinesterase, peroxidase, and catalase activity and in
phagocyte behaviour. The magnitude of the changes was large,
but not generally dose-related. On the basis of their results,
the authors recommended mean exposure limits for urban air for
exposure durations of 24 h, one month, and one year of 0.004,
0.0015, and 0.001 mg/m3, respectively, with a ceiling value of
0.01 mg/m3, and this recommendation has been adopted in the
USSR.
In a study of the effects of phosphine on rats by inhalation
at 0.1 mg/m3 and 0.05 mg/m3, Atchabarov et al. (1984) found a
reduction in total plasma protein without a change in the
relative proportions of the various fractions, a modest but
significant reduction in the glycoprotein A fraction with some
changes in the relative proportions of the sub-fractions,
increased plasma bile acids, and a marked increase in
seromucoids. Liver glycogen, lipids, and cytochrome oxidase
levels were reduced. Biochemical changes were similar to those
in a control group treated with hydrogen fluoride at a known
toxic level.
8.2.2. Oral exposure to metal phosphides
Bai et al. (1980) reported a 13 week feeding study in female
weanling albino rats. Zinc phosphide was mixed with the diet at
0 (control), 50 mg/kg (50 ppm), 100 mg/kg (100 ppm), 200 mg/kg
(200 ppm), and 500 mg/kg (500 ppm) w/w. Deaths occurred at the
2 higher dosage regimes in 1/12 and 10/12 animals, respectively.
Food intake and weight increase were reduced and dose-dependent
depilation occurred at all dosages. The relative weights of
liver, heart, brain, and thyroid were increased at 200 and
500 mg/m3 and the serum zinc and liver alkaline phosphatase
levels were increased at the highest dose. There was a dose-
dependent reduction in haemoglobin concentration, red cell
count, and haematocrit.
The WHO/FAO Data Sheet on Pesticides (No. 24) which deals
with zinc phosphide (WHO/FAO, 1976) cites a study in which
300 mg zinc phosphide/kg administered to rats produced 6/6
deaths in the second week while 200 mg/kg produced reduced
weight gain and 2/6 deaths. Histopathological studies revealed
liver damage in the peripheral and central lobular areas and the
lungs were congested with haemorrhage or exudate in the alveolar
spaces.
8.2.3. Dermal and other exposures
No reports are available on short-term studies involving the
dermal or other routes.
8.3. Skin and Eye Irritation; Sensitization
Zinc phosphide powder moistened with saline produced mild
skin irritation in rabbits (Draize method) (Rao, 1986).a
8.4. Long-Term Exposure
No long-term studies on phosphine or metal phosphides
exposure have been reported.
8.5. Reproductive, Mutagenicity, and Carcinogenicity
There have been no long-term reproductive, mutagenicity, or
carcinogenicity studies on phosphine or metal phosphides.
---------------------------
a Personal communication to the IPCS Task Group on Phosphine
and Metal Phosphides.
8.6. Factors Modifying Toxicity; Toxicity of Metabolites
Aminzhanov (1972) reported that 100 mg zinc phosphide/kg
body weight was lethal for dogs starved for 24 h beforehand but
not for dogs that were fed.
Phosphine is not a powerful reducing agent and its toxic
effects are unlikely to be associated with chemical reduction.
The recent discovery that phosphites and phosphorous acid have a
fungicidal activity (Bompeix & Saindreman, 1984) suggests that
metabolites of phosphine have biological effects and therefore
might contribute to its toxicity. However, this contribution
can only be small, since phosphine is only slowly oxidized to
hypophosphites and phosphites and a maximum of about 40% of the
phosphide dose is recovered as these metabolites, even with
small doses. Moreover, the toxicity of phosphites is much less
than that of phosphides (Schulz, 1887). The contribution of
toxicity of contaminants of phosphine such as diphosphides is
uncertain.
8.7. Mechanisms of Toxicity - Mode of Action
Studies on isolated rat liver showed that mitochondrial
oxygen uptake was inhibited by phosphine (Nakakita et al., 1971)
and that this effect was due to the reaction of phosphine with
cytochrome c and cytochrome c oxidase (Kashi & Chefurka, 1976).
Although this inhibitory in vitro effect was also shown in
insects, it was found that insects severely poisoned with
phosphine did not suffer any inhibition of their cytochrome
system (Price & Dance, 1983). In the same report, phosphine was
found to inhibit insect catalase though this appeared to be an
indirect effect and might have been a result of phosphine
toxicity rather than a cause.
There have not been any systematic studies on the mechanism
of phosphine toxicity. Various effects on intermediary
metabolism have been described. Ikimanova (1977) reported
increased blood- and urinary-porphyrin concentrations, which
were related to the dose of zinc phosphide and the duration of
treatment. In a study on rabbits, Minchev & Dimitrov (1970)
reported changes in serum glutamic-pyruvic and glutamic-
oxaloacetic transaminases, leucine aminopeptidase, aldolase,
alkaline phosphatase and albumin in the first 24 h of zinc
phosphide poisoning. Dysfunction of hepatic fat metabolism was
also observed. Loss of cell viability and cell membrane
integrity accounted for the raised hepatic enzymes, the
bronchiolitic effect, the cloudy swelling of renal tubular cells
and the occasionally reported haemorrhagic lesions in the
myocardium. There is no adequate explanation for the fact that
phosphine does not cause the haemolysis characteristic of
arsine.
9. EFFECTS ON MAN
9.1. Organoleptic Effects
The odour of phosphine depends on the impurities it con-
tains; phosphine of high purity has no odour, even at 280 mg/m3
(Dumas & Bond, 1974; Fluck, 1976). Phosphine prepared conven-
tionally, without purification, has a fishy or garlic-like odour
attributed to impurities. These may be adsorbed by stored pro-
ducts during fumigation with resultant loss of odour, even
though phosphine remains at toxic concentrations (Bond & Dumas,
1967). Amoore & Hautala (1983) reviewed the literature on odour
thresholds and stated that the geometric mean of all the 6
reported values for phosphine was 0.71 mg/m3 (0.51 ppm). Fluck
(1976) reported that thresholds for phosphine at which 50% or
more of 10 persons could positively identify the odour asso-
ciated with phosphine differed according to the source of the
phosphine (Table 16).
Table 16. Odour thresholds for phosphinea
--------------------------------------------------------------
Source Odour thresholds for
PH3 (mg/m3 converted
from ppm)
--------------------------------------------------------------
Technical aluminium phosphide + H2SO4 0.14 - 0.28
Phosphorium iodide + aqueous hydroxide 2.8
Phostoxin + H2SO4 0.014 - 0.028
(5 Å molecular seived) 1.4 - 2.8
Phostoxin + H2SO4
--------------------------------------------------------------
a From: Fluck (1976)
Amoore & Hautala (1983) classified phosphine in safety class
D in their classification because 20 - 50% of attentive persons
can detect the threshold limit value (TLV) (0.42 mg/m3) by
smell. However, the smell of phosphine cannot be relied on to
warn of toxic concentrations.
9.2. General Population Exposure
9.2.1. Phosphine
There is negligible exposure of the general population to
phosphine. Gessner (1937) described an incident in which the 12
inhabitants of an apartment house developed nausea and one died
when phosphine was emitted from an adjacent warehouse containing
bags of aluminium phosphide, which became damp. Some passengers
on ships and barges carrying cargoes of ferrosilicon or grain
under fumigation have also been poisoned by phosphine (Harger &
Spolyar, 1958; Netherlands, 1984). Effects were similar to
acute occupational poisoning.
Hallerman & Ribilla (1959) reported the deaths of 2 adults
and a child living in a dwelling with a party wall to a granary
being fumigated. Symptoms were non-specific and insidious
initially and illustrate the risks of sustained exposures to
relatively low concentrations (a few mg phosphine/m3); it was
estimated that the concentration in the bedroom reached
1.2 mg/m3. At autopsy, there was congestion of all organs.
Pulmonary oedema and focal emphysema were found in the lungs and
there was vacuolation in the liver.
9.2.2. Metal phosphides
Zinc phosphide baits and formulated aluminium phosphide
pellets are widely used. Occasional accidental, or more usually
suicidal, exposure to metal phosphides may be encountered.
Ingestion, the only easily toxic route, has almost always been
suicidal and the effects acute (Stephenson, 1967).
Stephenson (1967) reviewed 20 cases of acute zinc phosphide
poisoning by ingestion (including one treated by himself) in
which the approximate dose was recorded. Ten cases were fatal
and the doses ranged from 4.5 to 180 g; 6 cases had ingested
20 g or more. In the 10 non-fatal cases, the doses ranged from
0.5 to 50 g with 7/10 ingesting less than 20 g. In the case
treated by the author, clinical features included metabolic
acidosis, hypocalcaemic tetany, methaemalbuminaemia, and reduced
blood coagulation (thrombotest 28% of normal). Post-mortem
findings included blood in all the serous cavities, pulmonary
congestion and oedema, haemorrhagic changes in the intestinal
epithelium, centrilobular congestion and necrosis and yellow
discoloration of the liver, and patchy necrosis of the proximal
convoluted tubules of the kidneys.
An unsuccessful suicidal attempt was described by Zipf
(1967). A 25-year-old man ingested 6 tablets of PhostoxinR in
water (= 6 g phosphine). Immediate symptoms were severe retro-
sternal pain, a generalized burning sensation, and vomiting.
There was circulatory collapse necessitating resuscitation and
he was also treated by gastric lavage with potassium perman-
ganate and magnesium sulfate. Subsequently, symptoms of cardiac,
cerebral, and hepatic dysfunction appeared and there was severe
renal failure requiring haemodialysis.
9.3. Occupational Exposure
Cases of acute phosphine poisoning reported in the litera-
ture were reviewed by Harger & Spolyar (1958). Fifty-nine cases
with 26 deaths had been recorded since 1900 (including Gessner's
(1937) non-occupational cases). In 6 out of 11 papers, cargoes
of ferrosilicon were cited as the source of phosphine and, in
these cases, the victims were passengers or crew members of the
ships or barges concerned. Other cases involved the exposure of
welders to calcium carbide and raw acetylene and of submariners
to sodium phosphide. The most common autopsy finding was
congestion of the lungs with marked oedema. The authors added a
case of their own in which a 16-year-old youth operating
acetylene generators died, probably as a result of phosphine
exposure at a level of about 11 mg/m3 (8 ppm) for 1 - 2 h daily
over a period of 6 weeks. Arsine and hydrogen sulfide exposures
were considered to have been too low to have accounted for the
death. Autopsy revealed acute pulmonary oedema. Other cases
have been described, all of which exhibited similar features
(Ziemer, 1963; Furuno et al., 1976; & Netherlands, 1984). Three
groups of symptoms of acute phosphine poisoning were described
by Childs & Coates (1971): (a) nervous symptoms including
headache, vertigo, tremors, and unsteady gait, progressing in
severe cases to convulsions, coma, and death; (b) gastro-
intestinal symptoms include loss of appetite, thirst, nausea and
vomiting, diarrhoea, and severe epigastric pain; and (c) respir-
atory symptoms including a feeling of pressure and pain in the
chest as well as shortness of breath. In addition, a sharp fall
in blood pressure was described as a characteristic, but seldom-
mentioned symptom. Chronic effects include anaemia, bronchitis,
and gastrointestinal, speech, and motor disturbances, but these
are by no means general.
The toxic effects of phosphine are summarized in Table 17.
The IDLH (Immediately Dangerous to Life or Health) level is
282 mg/m3 (200 ppm) (US EPA, 1985b).
Verga & Belloni (1958) described a case of purpura ascribed
to poisoning by phosphine. The platelet count was reduced to
60 000/mm3; the red-cell count was also low at 3.1 x 106/mm3 and
the haemoglobin concentration (Sahli) was 55%. With recovery,
both the red-cell and thrombocyte counts increased to 4.8 x 106
and 210 000/mm3, respectively, at the end of the observation
period. The electrolysis of phosphate solutions being applied
to metals as a corrosion inhibitor was considered to have
produced phosphine, but there was no measurement of the level of
exposure and it is possible that the exposure also included
arsine, which would account for the anaemia with a hyperplastic
erythroid series in the marrow biopsy. While haemolysis is
recognized as a complication of arsine poisoning, purpura and
thrombocytopenia are not widely recognized features of
phosphine poisoning, though Borodin et al. (1983) described
changes related to haemolysis (prolongation of clotting time and
reduced plasminogen levels) in workers exposed to a variety of
biocides and disinfectants, including zinc phosphide. However,
these changes could not be related specifically to exposure to
phosphine.
Table 17. Toxic effects of phosphinea
------------------------------------------------------------
Effect Concentration
mg/m3 ppm
------------------------------------------------------------
Rapidly fatal 2800 2000
Death after 1/2 - 1 h 560 - 840 400 - 600
Dangerous to life after 1/2 - 1 h 400 - 600 290 - 430
Serious effect after 1/2 - 1 h 140 - 260 100 - 190
No serious effects after 1/2 - 1 h 10 7
------------------------------------------------------------
a From: Childs & Coates (1971).
In 1978, Beloskurskaya et al. published the findings of a
study on 206 phosphorus workers, which revealed 57 cases of
toxic hepatitis. In 49 of these cases, there was stated to be no
other apparent cause than occupational exposure to phosphorus,
phosphine, or phosphorus oxides. No measurements of exposure
were reported. Diagnostic criteria included a history of
hypochondrial pain, clinical hepatomegaly, and abnormal liver
function tests and 131I-Rose Bengal scanning and clearance
studies. Forty-six of the 57 cases had additional symptoms,
such as the astheno-vegetative syndrome, chronic gastritis,
impotence, toxic encephalopathy, toxic cardiomyopathy, and
respiratory disease. There was no control group and no informa-
tion on alcohol consumption was provided.
In a later study, Wilson et al. (1980) described the
symptoms of acute poisoning by phosphine as headache, fatigue,
nausea, vomiting, jaundice, paraethesia, ataxia, tremor,
diplopia, myocardial infiltration with necrosis, pulmonary
oedema, and myocardial and peripheral muscle damage. Laboratory
results indicated that there had been urinary tract involvement
(occult blood) and levels of several liver enzymes were
elevated. Furthermore, Roaldsnes (1982) reported that metal-
workers at a large shipyard in Norway, drilling deep holes in
spheroidal graphite iron, became ill during work. The symptoms
were mostly nausea, dizziness, chest tightness, dyspepsia, and
disturbances of smell and taste. Measurement of the phosphine
concentration in the workers' breathing zone (with Dräger tubes)
showed a phosphine concentration of about 1.4 mg/m3 (1 ppm).
After installing local exhaust ventilation on the drilling
machines, there were no longer any measurable amounts of
phosphine, and there were no complaints from the workers. When
the local exhaust ventilation was removed for technical reasons
5 years later, illness among the workers recurred. Measurement
of phosphine levels just above the machines, showed concentra-
tions of up to 56 - 70 mg/m3 (40 - 50 ppm). When the local
exhaust ventilation was re-installed, the phosphine concentra-
tions dropped to unmeasurable amounts, and no further cases of
illness were reported. Addition of copper sulfate solution to
the cutting oil effectively removed the phosphine gas, but
resulted in problems of corrosion.
Jackson & Elias (in press) studied liver function and
gastrointestinal symptoms in: (a) workers exposed to white
phosphorus at up to 5 times the occupational exposure limit and
also to some phosphine (0 - 0.5 mg/m3); (b) workers exposed to
phosphine alone (at up to 2.8 mg/m3, depending on the efficacy
and use of air-line breathing apparatus); and (c) gastro-
intestinal symptoms in a group of workers who were not exposed
to either compound, but who worked the same shift pattern.
Complaints to the factory medical department of gastrointestinal
symptoms in the phosphine-exposed group, in the phosphorus
exposed-group, and in the unexposed group were 1.51, 0.61, and
0.24 per man per year, respectively. However, the alcohol
consumption in the phosphine- and phosphorus-exposed groups
differed (289 and 154 ml per week, respectively). The alcohol
consumption was not estimated in the control group. None of the
conventional liver function tests reflected the differences in
occupational exposure or alcohol intake. The phosphine-exposed
group did have a significantly raised post-prandial bile acid
concentration, but whether this reflected a difference in phos-
phine exposure or alcohol consumption could not be determined.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Phosphine and metal phosphides are only focally distributed
in the environment and exposure of large numbers of the general
population is unlikely. The sources and uses of phosphine and
metal phosphides are sufficiently well established for safety
techniques to be generally known, though it is disturbing that
shipboard poisonings are still occurring when the first case was
reported over half a century ago. The extensive world-wide use
of metal phosphides as fumigant sources and as rodenticides
creates the hazard of accidental poisoning in children and
others and provides a means of suicide, unless the supply and
use of these materials is appropriately regulated. There is no
evidence of danger to the population from residues of phosphine
in fumigated foods or from residues of phosphides, if adequate
fumigation and aeration techniques are used. Permitted limits
for residues are in the range of 0.01 - 0.1 mg total phosphine/
kg. There do not appear to be any deleterious effects on food
quality as a result of adequately undertaken fumigation with
phosphine.
Published data on occupational exposure to phosphine in most
industries are not sufficient to evaluate the success of control
measures in relation to occupational exposure limits. There is
also some disagreement regarding these limits and some data
suggest that an occupational exposure limit of 0.4 mg
phosphine/m3 is above the threshold for biological effects with
long-term exposure. However, there are no studies adequate to
settle this question definitively.
Major accidental release of stored phosphine presents a
serious explosion/fire hazard and an acute toxic hazard for man
and animals.
10.2. Evaluation of Effects on the Environment
Only very small amounts of phosphine and phosphides occur in
the environment as a result of natural processes and those
resulting from human activity are not persistent.
Phosphine and phosphides are introduced into the environment
for the control of pests, for which they are particularly
suitable, because of their efficacy, lack of persistence, and
harmless decomposition products.
Careful positioning of bait and the low toxicity of
carcasses for scavengers of poisoned animal targets minimize the
effects of rodenticidal metal phosphides on non-target species.
Phosphine and phosphides are oxidized in the environment and the
final product, phosphate, is normally widely present in the
natural environment. Phosphine and phosphides are only used
where the natural environment is already modified by human
activity and their environmental impact must be viewed in this
context.
Since resistance to phosphine in insects does not appear to
reverse in the absence of selective pressures, it is
particularly important to achieve effective doses of phosphine
by using adequate treatment rates and good practices. In
practice, the development of resistance is more of a problem
than the effects on non-target species. Major accidental
release of stored phosphine would not result in long-term
environmental consequences.
10.3. Conclusions
In general, there is no risk to the public from the use of
phosphine or phosphides, from emissions from fumigated products
or spaces, from alloys, industrial processes, or residues in
food, provided that proper fumigation, transport, and industrial
practices are used.
While phosphine and metal phosphides are toxic, their
extensive use in occupational settings has resulted in the
establishment of good practice guidelines. If these are
followed, there is a low order of risk for human health.
However, there is some doubt about the completeness of
protection afforded by the higher occupational exposure limit
(0.4 mg phosphine/m3).
There is no risk of important environmental effects from
phosphine or metal phosphides.
11. RECOMMENDATIONS
11.1. Gaps in Knowledge
1. The principal area related to human health where information
is inadequate is concerned with occupational exposure levels and
their safety. There is a need for an adequate study of well-
being and organ function in exposed and control populations,
with a complete description of phosphine exposure, concurrent
exposures to other chemicals (which should be low), alcohol
consumption, and smoking habits. The establishment of exposure
levels that are free from any detectable effects would help to
determine agreed occupational exposure limits.
2. It would be of assistance, in this regard, to undertake
laboratory studies to identify biological markers that are most
indicative of phosphine effects and reflect the suggested short-
term cumulative effects of repeated phosphine exposure.
3. Further research on the mechanisms of action of phosphine
and phosphides will assist in the design of good fumigation
practice and fumigation schedules, and minimize the occurrence
of resistance in target organisms and associated problems.
4. It has been suggested that zinc phosphide may be relatively
persistent in aquatic sediments and there is a general lack of
aquatic toxicological data. There is a need for research on the
levels and persistence of zinc phosphide in sediments and for
further studies of its effects on aquatic organisms.
11.2. Preventive Measures
11.2.1. Management
The most important factor in the safe handling of phosphine
and metal phosphides, and in their formulation, is proper work
practices.
Management should identify these, provide training for the
operatives, and ensure that the practices are carried out.
Personal protection measures recommended to reduce the
likelihood of absorption of phosphide preparations include the
wearing of:
(a) synthetic rubber gloves;
(b) rubber boots;
(c) lightweight impervious overalls; and
(d) suitable eye protection.
Adequate washing facilities should be available at all times
during handling. Eating, drinking, and smoking should be
prohibited during handling, and before washing after handling.
The means to measure concentrations of phosphine in air should
be available and used to check atmospheric concentrations. When
necessary, respiratory protective equipment should be worn. In
fumigation, each operator or other person liable to be exposed
to the gas must be provided with an efficient means of respira-
tory protection. Persons exposed to magnesium phosphide or
aluminium phosphide powders (or other readily hydrolysed
phosphides), which may give rise to airborne dust should be
protected by respiratory protective equipment, effective against
gaseous phosphine, since hydrolysis of dust in the filter of a
dust mask or respirator may give rise to high phosphine
exposure.
11.2.2. Treatment of poisoning
11.2.2.1 Inhalation of phosphine
(a) First Aid
Remove from exposure, keep at rest. Rescuers should follow
full safety procedures.
If the patient is unconscious, place in semi-prone recovery
position or otherwise maintain the airway.
If the patient is conscious but has difficulty in breathing,
treat in a seated position and give oxygen if available.
Otherwise, allow the patient to recline with the legs slightly
elevated.
If breathing stops, immediately ventilate the patient
artificially (mouth-to-mouth/nose or mechanically with oxygen,
if available).
If the heart stops, begin cardiopulmonary resuscitation
(CPR).
(b) Medical Treatment
1. Give oxygen by mask if required; perform baseline chest X-
ray and examine chest. Treat shock conventionally.
2. All patients should be observed for 48 - 72 h, as onset of
pulmonary oedema may be delayed.
3. If respiratory distress is severe, give steroids (methyl-
prednisolone 30 mg/kg, or equivalent, intramuscularly),
preferably within 4 h of exposure.
4. If pulmonary oedema occurs, treat by positive and expiratory
pressure ventilation. Antibiotics should only be given if a
secondary infection is present.
5. Treat any fits conventionally; give general supportive care
with particular attention to fluid balance.
6. Some sources of exposure to phosphine are likely to lead
also to exposure to arsine, which has haemolytic effects.
Monitor for haemoglobinaemia, haemoglobinuria, unconjugated
hyperbilirubinaemia, and renal failure.
In general, recovery is rapid following removal from
exposure, but renal damage and leukopenia may occur after
several days.
11.2.2.2 Ingestion of metal phosphides
(a) First aid
Do not give milk, fats, or saline emetics by mouth.
Give oxygen if there is respiratory distress.
If first aiders are medically authorized to do so, and the
patient is conscious, induce vomiting.
After 20 min (or after vomiting), administer activated
charcoal (50 g in water by mouth) if available.
Obtain medical attention as soon as possible: preferably
send immediately to hospital.
(b) Medical treatment
1. Consider tracheal intubation and gastric lavage with 2%
sodium bicarbonate solution (to limit hydrolysis of zinc
phosphide).
2. Activated charcoal or medicinal liquid paraffin may limit
absorption of phosphine and zinc phosphide, respectively,
and may be administered by mouth or stomach tube.
3. Monitor and support vital functions, particularly hepatic
and renal function. Treat shock conventionally.
5. Hepatic and renal failure should be treated in specialist
centres.
11.2.3. Leaks, spillages, residues, and empty containers
11.2.3.1 Phosphine
Small leaks and residues of compressed gas can be discharged
slowly to the atmosphere in the open air. Larger quantities
should be burned using an appropriate burner.
11.2.3.2 Aluminium and magnesium phosphides and their formulated
preparations
Spillages and residues in containers will evolve phosphine
for several days by reaction with atmospheric moisture. Respir-
atory protective equipment will be required by those dealing
with them. Even if atmospheric concentrations of phosphine are
initially low, they may rise as residues are disturbed.
Moderate quantities should be collected in a secure place to
protect the public and wildlife, and left in the open air and
kept moist until hydrolysis is complete.
Larger amounts should be removed to a deep pit, at an
approved site, until phosphine evolution is complete. Then they
can be buried.
Residues at the site of spillage should be washed away in a
large quantity of water and the area kept secure and aerated
until checked for zero gas concentration.
Combustible packages can be incinerated at high temperature
(> 1000 °C) using proper facilities. Containers should NOT be
cleaned for re-use, but should be disposed of by deep burial, at
an approved site, well away from habitation and where there is
no danger of contamination of water sources.
11.2.3.3 Zinc phosphide and preparations
Zinc phosphide hydrolyses only slowly. Nevertheless,
phosphine may be evolved and respiratory protective equipment
should be available.
Spillages and residues in containers should be incinerated
at high temperatures (> 1000 °C) or crushed and buried below the
topsoil at an appropriate site. Contaminated surface material
should be treated similarly or else the area should be secured
from public access until the gas concentration is zero.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Phosphine was evaluated in 1971 by WHO and FAO, as a
pesticide residue in food (WHO/FAO, 1972). A tolerance of
0.1 mg/kg (0.1 ppm) in raw cereals was confirmed. It was
recommended that the tolerance of 0.01 mg/kg (0.01 ppm) in
flour, other milled cereal products, breakfast cereals, dried
vegetables, and spices be confirmed and extended to include
nuts, groundnuts, dried fruit, cocoa beans, and other similar
foods, known to be fumigated with phosphine.
The use of zinc phosphide as a rodenticide in public health
was reviewed by WHO in 1972. It was concluded that it was a
generally effective compound and, while highly toxic to domestic
fowl, its safety record was good. The use of zinc phosphide was
endorsed (WHO, 1973).
REFERENCES
ACGIH (1986) Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists.
ADU, O.O. & MUTHU, M. (1985) The relative toxicity of seven
fumigants to life cycle stages of Callosobruchus chinensis L.
Insect Sci. Appl., 6(1): 75-78.
ADVANI, R. (1983) A comparative trial of zinc phosphide,
aluminium phosphide and RH-787 against field mixed population of
rodents in crop ecosystem of the Indian desert. Ztg angew.
Zool.,70(2): 153-168.
AHMAD, M. (1976) Effect of phosphine fumigation on the
germination of edible legume seeds. J. stored Prod. Res., 12:
211-212.
AL-OMAR, M.A. & AL-BASSOMY, M. (1984) Persistence of phosphine
gas in fumigated Irafir dates. J. food Saf., 6: 253-260.
AL'ZHANOV, S., MOLDABEKOV, Sh.M., ERSHOV, V.A., & POPOV, A.P.
(1983) [ Use of copper and iron sulfates in cleaning furnace
gas from phosphorus production,] Moscow, Academy of Sciences of
the USSR, Institute of Scientific Information (VINITI) (Document
4802-83) (in Russian).
AMINZHANOV, M. (1972) [Use of zinc phosphide in controlling
stray dogs.] In: Azimov, Sh.A., Shevchenko, N.Kh., & Nurmatov,
R.Sh., ed. [ Proceedings of the 7th Conference of Young Uzbek
Agricultural Scientists,] Tashkent, Uzbeksii Nauchno-Issled.
Veterinary Institute (in Russian).
AMOORE, J.E. & HAUTALA, E. (1983) Odour as an aid to chemical
safety: odour thresholds compared with threshold limit values
and volatilities for 214 industrial chemicals in air and water
dilution. J. appl. Toxicol., 3(6): 272.
ANON. (1936) [Trials to establish whether the Delicia grain
weevil fumigation procedure is safe to apply and free from the
risk of fire and explosion.] Gutachten chim. tech. Reichsanst.,
8(4): 5 (in German).
ANON. (1956) Ferro-silicon explosion risks. Chem. Trade J.
Chem. Eng., 16 Nov.: 1180.
ARENDT, G., FARWIK, W., HAAG, F., KAUFMANN, G., KOHL, E.-G.,
PRUGGMAYER, D., & WAGNER, W. (1979) [Environmental aspects of
pest control. Fumigation of grain and food stores.] Lebensmit-
teltechnik, 5: 2-8 (in German).
ARNOLD, J.T. & ROBBIANO, P. (1974) Portable vapour surveil-
lance system, Springfield, Virginia, National Technical
Information Service (NTIS Report No. AD-782 844).
ATCHABAROV, B.A., AITBAYEV, T.KH., & AITBEMBETOV, B.N. (1984)
[Comparison of the effects of hydrogen fluoride and phosphine on
the organism with various exposure levels.] In:[ Health care in
Kazakhstan. Part 1,] pp. 28-31 (in Russian).
ATTIA, F.I. (1984) Multiple cross-resistance characteristics
in phosphine-resistant strains of Rhizopertha dominica and
Tribolium castaneum. In: Ripp, B., ed. Controlled atmosphere
and fumigation in grain storages, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 49-53 (Developments in
Agricultural Engineering No. 5).
ATTIA, F.I. & GREENING, H.G. (1981) Survey of resistance to
phosphine in coleopterous pests of grain and stored products in
New South Wales. Gen. appl. Entomol., 13: 93-97.
BAI, K.M., KRISHNAKUMARI, M.K., RAMESH, H.P., SHIVANANDAPPA, T.,
& MAJUMDER, S.K. (1980) Short-term toxicity study of zinc
phosphide in Albino rats (Rattus norvegicus). Indian J. exp.
Biol., 18(8): 854-857.
BARRETT, W.J. & DILLON, H.K. (1978) Development of methods
for the determination of elemental phosphorus and phosphine in
air, Cincinnati, Ohio, National Institute of Occupational Safety
and Health, 102 pp (DHEW (NIOSH) Publication No. 78-177).
BECKER, K.H., BIEHL, H.M., BRUCKMANN, P., FINK, E.H., FUHR, F.,
KLOPFER, W., ZELLNER, R., & ZETSCH, C. (1984) Methods for the
ecotoxicological evaluation of chemicals, photochemical degra-
dation in the gas phase. VI. OH x reaction rate constants and
tropospheric lifetimes of selected environmental chemicals,
Jülich, Kernforschungsanlage (Projektträgerschaft Umweltchemi-
kalien Report 1980-83, No. 279).
BELL, C.H., WILSON, S.M., & BANKS, H.J. (1984) Studies on the
toxicity of phosphine to tolerant stages of Trogoderma granarium
everts (Coleoptera: Dermestidae). J. stored Prod. Res., 20(2):
111-117.
BELOSKURSKAYA, G.I., PARASKEVOPULOS, YA.G., & SHLYGINA, O.E.
(1978) [On the clinical and functional condition of the liver
in patients with chronic phosphorus poisoning.] Tr. Inst. Kraev.
Patol. Akad. Nauk Kazakhskoi SSR, 36: 25-30 (in Russian).
BLAXLAND, J.D. & GORDON, R.F. (1945) Zinc phosphide poisoning
in poultry. Vet. J., 101: 108-110.
BOENIG, I.A., CRUTCHFIELD, M.M., & HEITSCH, C.W. (1982)
Phosphorus compounds. In: Kirk-Othmer, ed. Encyclopaedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol.
17, pp. 490-539.
BOMPEIX, G. & SAINDRENAN, P. (1984) In vitro antifungal
activity of Fosetyl Al and phosphorous acid on Phytophthera
species. Fruits, 39(12): 777-786.
BOND, E.J. & DUMAS, T. (1967) Loss of warning odour from
phosphine. J. stored Prod. Res., 3: 389-392.
BOND, E.J., ROBINSON, J.R., & BUCKLAND, C.T. (1969) The toxic
action of phosphine. Absorption and symptoms of poisoning in
insects. J. stored Prod. Res., 5: 289-298.
BOND, E.J., DUMAS, T., & HOBBS, S. (1984) Corrosion of metals
by the fumigant phosphine. J. stored Prod. Res., 20(2): 57-63.
BONTOYAN, W.R. (1981) Report on pesticide formulations: herbi-
cides, fungicides and miscellaneous. J. Assoc. Off. Anal. Chem.,
64(2): 355.
BORODIN, A., KLIMEK, M., & ZAKL, K. (1983) [Blood clotting and
fibrinolysis systems in workers of a disinfection, disinfesta-
tion and deratization employment (sic).] Med. Pr., 34(4): 313
(in Polish).
BOUBAL, M., LOUISE, J., BELOT, D., & POLITI, J.M. (1981)
Analyse directe et continue de traces d'hydrures gazeux à très
basses teneurs, Paris, Institut National de la Proprieté
Industrielle, Sociéte d'Air Liquide (Document No. 2 506 459)
(Demande de Brevet d'Invention No. 81 10316).
BOWKER, J.R. (1958) The liberation of phosphine in the
machining of spheroidal graphite iron. In: Proceedings of the
British Cast Iron Research Association, 8 November, p. 50.
BOWLEY, C.R. & BELL, C.H. (1981) The toxicity of twelve
fumigants to three species of mites infesting grain. J. stored
Prod. Res., 17: 83-87.
BRADFIELD, A.A.G. & GILL, J.E. (1984) Laboratory trials of
five rodenticides for the control of Mesocricetus auratus
Waterhouse. J. Hyg. (Lond.), 93: 389-394.
BRANDON, R.W. (1983) The use of chemically impregnated paper
tapes for toxic gas detection and monitoring. Anal. Chem. Symp.
Ser., 17: 726-31.
BREYER, D. (1973) [Studies on hydrogen phosphide residues in
grain after treatment with fumigation preparations in powder
form.] Mühle Mischfuttertech., 110: 699-700 in German).
BUBIEN, Z., WARTENBERG, L., & ROSZYK, E. (1974) [Content of
zinc in hen carcasses poisoned with zinc phosphide.] Zesz. Nauk.
Akad. Roln Wroclawiu Weter., 31: 173-180 (in Polish).
CABROL TELLE, A.M. & DE SAINT BLANQUAT, G. (1984) In vitro
digestibility of starch, casein and triolein after fumigation
with phosphine. Sci. Aliments, 4(Hors Ser. 3): 287-291.
CABROL TELLE, A.M., DE SAINT BLANQUAT, G., DERACHE, R, HOLLANDS,
E., PERIQUET, B., & THOUVENOT, J.-P. (1985) Nutritional and
toxicological effects of long-term ingestion of phosphine-
fumigated diet by the rat. Food Chem. Toxicol., 23(11): 1001-
1009.
CALVERT, J.G. & PITTS, J.N., Jr (1966) Photochemistry, New
York, John Wiley and Sons.
CHAMP, B.R. & DYTE, C.E. (1976) Report of the FAO global
survey of pesticide susceptibility of stored grain pests, Rome,
Food and Agriculture Organization of the United Nations, pp. 1-
257 (FAO Plant Production and Protection Paper No. 5).
CHAN, L.T.F., CROWLEY, R.J., DELLIOU, D., & GEYER, R. (1983)
Phosphine analysis in post-mortem specimens following ingestion
of aluminium phosphide. J. anal. Toxicol., 7: 165-167.
CHENTSOVA, N.YU. (1972) [Danger to poultry during scattering
of zinc phosphide-poisoned grain to control rodents in forest
plantings.] In: Voronova, L.D., ed. [ Effects of pesticides on
wild animals,] Moscow, pp. 90-102 (in Russian).
CHILDS, A.F. & COATES, H. (1971) The toxicity of phosphorus
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3, pp. 1437-1440.
CHILDS, D.P. (1972) Tobacco consignment fumigated in
containers while in transit. Container News, October.
CHILDS, D.P., OVERBY, J.E., & NIFFENEGGER, D. (1969) Phosphine
fumigation of flue-cured tobacco warehouses for the control of
the cigarette beetle. Tobacco, 168(21): 20-25.
CHOPRA, G. (1984) Field efficacy of aluminium phosphide
against rodents. Indian J. agric. Sci., 54(3): 204-205.
CIBA (1978) Phosphorus in the environment: its chemistry and
biochemistry, Amsterdam, Oxford, New York, Elsevier North
Holland (Ciba Foundation Symposium 57 - new series).
CODEX ALIMENTARIUS (1986) Codex maximum limits for pesticide
residues, 2nd ed., Rome, Food and Agriculture Organization of
the United Nations (CAC/Vol. XIII-Ed.2).
COLE, R.J. & BENNETT, W.H. (1950) An investigation of the
possibility of the occurrence of phosphine and acetylene in the
atmosphere of factories for the powdering of magnesium. J. Soc.
Chem. Ind., 69: 29-30.
CORBRIDGE, D.E.C. (1974) The structural chemistry of
phosphorus, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 268-269.
COX, P.D., BELL, C.H., PEARSON, J., & BEIREN, M.A., (1984) The
effect of diapause on the tolerance of larvae of Ephestia
küehniella to methyl bromide and phosphine. J. stored Prod.
Res., 20(4): 215-219.
CURRY, A.S., PRICE, D.E., & TRYHORN, F.G. (1959) Absorption of
zinc phosphide particles. Nature (Lond.), 184(4686): 642-643.
DAVIS, R. (1986) Fumigation of ships. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 18-21 March, London, Foreign and
Commonwealth Office, Overseas Development Administration,
Tropical Development and Research Institute, pp. 24-34.
DELOMENIE, H. (1933) Gases evolved from ferrosilicons under
the influence of water. J. Pharm. Chim., 18: 289-292, 532
DIEKE, S.H. & RICHTER, C.P. (1946) Comparative assays of
rodenticides on wild Norway rats. Public Health Rep., 61: 672-
679.
DIETERICH, W.H., MAYR, G., HILD, K., SULLIVAN, J.B., & MURPHY,
J. (1967) Hydrogen phosphide as a fumigant for foods, feeds
and processed food products. Residue Rev., 19: 135-149.
DISNEY, R.W. & FOWLER, K.S. (1972a) Residues in cereals
exposed to hydrogen phosphide. In: Radiotracer studies on
chemical residues in food and agriculture. Proceedings of a
Combined Panel and Research Coordination Meeting, Vienna,
Austria, 25-29 October 1971, Vienna, International Atomic
Energy Agency, pp. 99-101.
DISNEY, R.W. & FOWLER, K.S. (1972b) The possibility of isotope
exchange or other interfering reactions occurring during the
determination of residues from fumigation with 32P-labelled
phosphine. In: Radiotracer studies on chemical residues in food
and agriculture. Proceedings of a Combined Panel and Research
Coordination Meeting, Vienna, Austria, 25-29 October 1971,
Vienna, International Atomic Energy Agency, pp. 87-88.
DUMAS, T. (1964) Determination of phosphine in air by gas
chromatography. J. agric. food Chem., 12(3): 257-258.
DUMAS, T. (1978) Modified gas chromatographic determination of
phosphine. J. Assoc. Off. Anal. Chem., 61(1): 5-7.
DUMAS, T. (1980) Phosphine sorption and desorption by stored
wheat and corn. J. agric. food Chem., 28: 337-339.
DUMAS, T. & BOND, E.J. (1974) Separation of phosphine from
odour-producing impurities. J. stored Prod. Res., 10: 67-68.
DUMAS, T. & BOND, E.J. (1981) Method of trapping low levels of
phosphine at ambient temperature for gas-chromatographic
analysis. J. Chromatogr., 206(2): 384-386.
EPPO (1975) Guidelines for the development and biological
evaluation of rodenticides, Paris, European and Mediterranean
Plant Protection Organisation (Bulletin No. 5(1)).
FITZPATRICK, R.J., MCGIRR, J.L., & PAPWORTH, D.S. (1955) The
toxicity of rodenticides. Vet. Rec., 67: 142-145.
FLUCK, E. (1973) The chemistry of phosphine. Fortschr. chim
Forsch., 35: 1-64.
FLUCK, E. (1976) The odor threshold of phosphine. J. Air
Pollut. Control Assoc., 26(8): 795.
FRANK, R. & RIPPEN, G. (1986) [ Content of phosphine in the
atmosphere,] Frankfurt am Main, Batelle-Institut e.v. (in
German).
FREYBERG, W. (1979) Acute toxicity of Detia R pellets to
bluegill (Lepomis macrochirus ), Washington DC, US Environmental
Protection Agency (Unpublished report).
FRITZ, B., LORENZ, K., STEINERT, W., & ZELLNER, R. (1982)
Laboratory kinetic investigations of the tropospheric oxidation
of selected industrial emissions. In: Versino, B. & Ott, H., ed.
Physico-chemical behaviour of atmospheric pollutants,
Dordrecht, Reidel, pp. 192-202.
FURMAN, N.H., ed. (1962) Standard methods of chemical
analysis, 6th ed., Princeton, New Jersey, D. van Nostrand
Company Inc., Vol. 1, pp. 821-823.
FURUNO, J., SUGAWARA, N., & IKEDA, H. (1976) A fatal case
poisoned by hydrogen phosphide and the simultaneous separation
of inorganic gases by gas chromatography. Bull. Yamaguchi med.
Sch., 23(1-2): 49-58.
GESSNER, O. (1937) Fatal phosphine poisoning from "Delicia"
grain weevil fumigant (aluminium phosphide). Samml. Vergiftungs-
fällen, 8: B-79.
GILL, J.E. & REDFERN, R. (1983) Laboratory tests of seven
rodenticides for the control of Meriones shawi. J. Hyg. (Lond.),
91: 351-357.
HABASHI, F. & ISMAIL, M.I. (1975) Health hazards and pollution
in the metallurgical industry due to phosphine and arsine. CIM
Bull., August: 99-103.
HACKENBERG, U. (1972) Chronic ingestion by rats of standard
diet treated with aluminium phosphide. Toxicol. appl.
Pharmacol., 23: 147-158.
HALLERMANN, W. & PRIBILLA, O. (1959) [Fatal poisoning with
phosphine.] Arch. Toxikol., 17(4): 219-242 (in German).
HARGER, R.N. & SPOLYAR, L.W. (1958) Toxicity of phosphine with
a possible fatality from this poison. Am. Med. Assoc. Arch.
Ind. Health, 18: 497-504.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds.
Wildlife, 191: 1-61.
HILTON, H.W. & ROBISON, W.H. (1972) Fate of zinc phosphide and
phosphine in the soil-water environment. J. agric. food Chem.,
20(6): 1209-1213.
HOLE, B.D. (1981) Variation in tolerance of seven species of
stored product Coleoptera to methyl bromide and phosphine in
strains from twenty-nine countries. Bull. entomol. Res., 71(2):
299-306.
HOLE, B.D., BELL, C.H., MILLS, K.A., & GOODSHIP, G. (1976) The
toxicity of phosphine to all developmental stages of thirteen
species of stored product beetles. J. stored Prod. Res., 12:
235-244.
HUGHES, J.G. & JONES, A.T. (1963) Estimation of phosphine in
air. Ind. Hyg. J., March-April: 164-167
HUNTER, D. (1978) The diseases of occupations, 6th ed.,
London, Hodder and Stoughton.
IKEDA, S. (1971) [Effect of zinc phosphide on Coturnix coturnix
japonica.] Ringyo Shikenjo Kenkyu Hokuko, 238: 141-148 (in
Japanese).
IKIMANOVA, G.K. (1977) [Porphyrin metabolism in experimental
phosphorus poisoning.] Zdravookhr. Kaz., 1: 39-42 (in Russian).
IMO (1980) Recommendations on the safe use of pesticides in
ships, London, International Maritime Organization (UN IMO
Circular No. 298, revised).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, 303 pp (Data
Profile Series No. 5).
JACKSON, J.R. & ELIAS, E. (1984) Hepatic function and exposure
to phosphine and white phosphorus. In: Proceedings of the 12th
International Congress on Occupational Health in the Chemical
Industry, Dublin, Ireland, 9-14 September 1984, pp. 219-228.
JANDA, J. (1973) Danger of rodenticides containing zinc
phosphide to wild life. Agrochemia, 13(3): 85-86.
JOKOTE, Ch. (1904) [Studies on hydrogen phosphine.] Arch. Hyg.
Bakteriol., 49: 275-306 (in German).
JONES, A.T., JONES, R.C., & LONGLEY, E.O. (1964) Environmental
and clinical aspects of bulk wheat fumigation with aluminium
phosphide. Am. Ind. Hyg. Assoc. J., 25: 376-379.
KADKOL, S.B. & JAYARAJ, P. (1967) Effect of phosphine-
fumigated rice on the growth of albino rats. Rep. Cent. Food
Technol. Res. Inst. (Mysore), 2: 6.
KAGAN, YU.S. (1985) Principles of pesticide toxicology,
Moscow, Centre of International Projects, GKNT, 176 pp.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1973)
The phytotoxic effect of repeated fumigation of certain cereal
crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 7: 57-62.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1974)
The phytotoxic effect of carbon bisulphide, methyl bromide and
hydrogen phosphide on the germination of seeds of certain cereal
field crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 8: 75-80.
KAO, C. (1981) Determination of aluminium phosphide in
fumigants. J. food Sci., 46: 1632-1634.
KASHI, K.P. (1975) A mixed indicator strip for phosphine
detection. Pestic. Sci., 6: 511-514.
KASHI, K.P. (1981a) Toxicity of phosphine to five species of
stored-product insects in atmospheres of air and nitrogen.
Pestic. Sci., 12: 116-122.
KASHI, K.P. (1981b) Response of five species of stored-product
insects to phosphine in oxygen-deficient atmospheres. Pestic.
Sci., 12: 111-115.
KASHI, K.P. (1982) Dose-mortality responses of five species of
stored-products insects to phosphine. Int. Pest Control,
March/April: 46-49.
KASHI, K.P. & CHEFURKA, W. (1976) The effect of phosphine of
absorption and circular dichroic spectra of cytochrome-c and
cytochrome-c oxidase. Pestic. Biochem. Physiol., 6: 350-362.
KASHI, K.P. & MUTHU, M. (1975) A mixed indicator strip for
phosphine detection. Pestic. Sci., 6: 511-514.
KIM, H.H., KIM, K.H., & HONG, S.S. (1977) Effect of pesticides
on exocrine pancreas and liver. Yonse Uidea Nonmunjip, 10(2):
170-180.
KLIMMER, O.R. (1969) [Contribution on poisoning by phosphine.
On the question of sub-chronic phosphine intoxication.] Arch.
Toxikol., 24: 164 (in German).
KOZHEMYAKIN, N.G., BUTYAGIN, V.N., BASHMURIN, A.F., & POTAPENKO,
M.I. (1971) [Veterinary-sanitary evaluation of the carcasses
and organs of animals which had to be killed in relation to
their contamination with zinc phosphide.] Sb. Rab. Leningrad
Vet. Inst., 32: 231-236 (in Russian).
KUHN, H., MAREK, J., & REIF, H. (1971) [Fumigation of tobacco
with phosphine.] Fachliche Mitt. Austria Tabakwerke AG, 12: 191-
203 (in German).
LADOU, J. (1983) Potential occupational health hazards in the
microelectronics industry. Scand. J. Work. environ. Health, 9:
42-46.
LEESCH, J.G. (1982) Accuracy of different sampling pumps and
detector tube combinations to determine phosphine
concentrations. J. econ. Entomol., 75: 899-905.
LEESCH, J.G. (1984) Fumigation of lettuce: efficacy and phyto-
toxicity. J. econ. Entomol., 77(1): 142-150.
LOWE, E.J. (1971) Phosphorus hydrides and phosphonium
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3.
LUCK, H. & ALLEMANN, A. (1984) Control of insect infestation
during storage of dairy products. Fumigation with hydrogen
phosphide. S. Afr. J. dairy Technol., 16(3): 103-107.
LUTZMANN, L., KEINITZ, M., & KLOSTERKOTTER, W. (1963)
[Emission of phosphine during transport of ferrosilicon.] Med.
Welt, 58: 114-116 (in German).
MCGIRR, J.L. (1953) Poisoning of livestock by the newer
rodenticides, insecticides and weed killers. In: Proceedings of
the XVth International Veterinary Congress, Stockholm, 9-15
August, Part 1, Vol. 1, pp. 479-480.
MCMAHON, R. & FORESE, F.F. (1983) Hydride gas detecting tape,
Glenview, Illinois, MDA Scientific Inc. (UK Patent Application
GB 2 108 662A).
MARSHALL, E.F. (1981) Efficacy variations of rodent baits with
the same active ingredient. Pest Control, January: 22-23.
MATHEW, G.G. (1961) The production of phosphine while
machining spheroidal graphite iron. Ann. occup. Hyg., 4: 19-35.
MEISSNER, R. (1924) [The toxicology of hydrogen phosphide.] Z.
gesamte Med., 42: 267 (in German).
MEREDITH, C. (1981) Toxicological studies on zinc phosphide,
Birmingham, University of Birmingham, Department of Bio-
chemistry (M.Sc. Thesis).
MINCHEV, T. & DIMITROV, D. (1970) [Early changes in the serum
of rabbits poisoned with zinc phosphide.] In: Vasilev, R.A., ed.
[ Nauch. Tr. Sud. Med. Deontol.,] Sofia, Meditsina i
Fizkultura, pp. 33-38 (in Bulgarian).
MUELLER, W. (1940) [Toxicity of phosphine (animal
experiments). Part 1. Acute and subacute toxicity.] Arch. exp.
Pathol. Pharmakol., 195: 184-193 (in German).
MUTHU, M., KRISHNAKUMARI, M.K., MURALIDHARA, V., & MAJUMDER,
S.K. (1980) A study on the acute inhalation toxicity of
phosphine to albino rats. Bull. environ. Contam. Toxicol., 24:
404-410.
NAKAKITA, H. & WINKS, R.G. (1981) Phosphine resistance in
immature stages of a laboratory selected strain of Tribolium
castaneum Herbst (Coleoptera: Tenebrionidae). J. stored Prod.
Res., 17: 43-52.
NAKAKITA, H., KATSUMATA, Y., & OZAWA, T. (1971) The effect of
phosphine on the respiration of rat liver mitochondria. J.
Biochem., 69: 589-593.
NATARAJAN, T. & BAGYARAJ, D.J. (1984) Fumigation effect on
microflora and viability of blackgram and fieldbean seeds.
Pesticides, 18(4): 40-42.
NAUS, A. (1982) [Olfactory thresholds of some industrial
compounds.] Prac. Lek., 34(6-7): 217-218 (in Polish).
NETHERLANDS, WERKGROEP VAN DESKUNDIGEN VAN DE NATIONALE MAC-
COMMISSIE. (1984) [ Report on the TLV of phosphine,] 2nd ed.,
Voorburg, Working Group of the National TLV Committee, 27 pp
(Report No. 1/80) (in Dutch).
NEUBERT, D. & HOFFMEISTER, I. (1960) Changes in intermediary
metabolism following exposure to hydrogen phosphide. Naunyn-
Schmiedeberg's Arch. exp. Pathol. Pharmakol., 239: 219-233.
NOACK, S., VON & REICHMUTH, C. (1981) [Determination of limits
for injuring animals and plants by phosphine and methyl bromide.
I. Studies on Drosophila melanogaster. Anz. schädlingskd.
Pflanzenschutz Umweltschutz, 54: 23-27 (in German).
NOACK, S., VON & REICHMUTH, C. (1982a) [Quantities of
phosphine, methyl bromide and hydrocyanic acid used in stored
products protection in the FRG from 1975-1977.] Nachrichtenbl.
Dtsch. Pflanzenschutzd., 34(2)S: 17-21 (in German).
NOACK, S., VON & REICHMUTH, C. (1982b) [Determination of
limits for injuring animals and plants by phosphine or methyl
bromide. II. Studies on water cress and lettuce Lactuca sativa
capitata.] Anz. schädlingskd. Pflanzenschutz. Umweltschutz,
55: 57-59 (in German).
NOACK, S., VON & WOHLGEMUTH, R. (1985) [Phosphine residues in
hazelnuts, soybeans and wheat after fumigation with non-constant
concentrations.] Z. Lebensm. Unters. Forsch., 180: 101-108 (in
German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1983)
[Relationship of PH3 residues after fumigation to concentration,
time of exposure and length of storage.] Z. Lebensm. Unters.
Forsch., 177: 87-93 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984a)
[Decomposition of phosphine in treated foods related to storage
temperature and aeration.] Z. Lebensm. Unters. Forsch., 178: 31-
37 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984b) [A
half-empirical mathematical model for calculation of PH3-
residues in hazelnuts fumigated with phosphine.] Z. Lebensm.
Unters. Forsch., 178: 97-103 (in German).
NOWICKI, T.W. (1978) Gas-liquid chromatography and flame
photometric detection of phosphine in wheat. J. Assoc. Off.
Anal. Chem., 61(4): 829-836.
PAZYNICH, V.M., MAZUR, I.A., & PODLOZNYI, A.V. (1984)
[Experimental substantiation and prediction of maximum
atmospheric phosphine concentrations differentiated by time.]
Gig. i Sanit., 1984(1): 13-15 (in Russian).
PRICE, N.R. (1984) Active exclusion of phosphine as a
mechanism of resistance in Rhyzopertha dominica F (Coleoptera:
Bostrychidae). J. stored Prod. Res., 20(3): 163-168.
PRICE, N.R. & DANCE, S.J. (1983) Some biochemical aspects of
phosphine action and resistance in three species of stored
product beetles. Comp. Biochem. Physiol., 76C(2): 277-281.
PRICE, N.R., MILLS, K.A., & HUMPHRIES, L.A. (1982) Phosphine
toxicity and catalase activity in susceptible and resistant
strains of the lesser grain borer (Rhyzopertha dominica).
Comp. Biochem. Physiol., 73C: 411-413.
RANGASWAMY, J.R. (1984) Simple spectrophotometric method for
determination of phosphine residues in wheat. J. Assoc. Off.
Anal. Chem., 67(1): 117-122.
RANGASWAMY, J.R. & MUTHU, M. (1985) Spectrophotometric
determination of phosphine residues in rice. J. Assoc. Off.
Anal. Chem., 68(2): 205-208.
REBMANN, Z. (1933) [On a new pesticide (Fosfolon).] Gesund-
heitstech. Städtehyg. ver. Städtische Tiefbau, 5: 280-284 (in
German).
REICHMUTH, C., ed. (1985) [Response of the granary weevil
Sitophilus granarius L. (Coleoptera: Curculionidae) to
gradually increasing and decreasing phosphine concentrations.]
Anz. schädlingskd. Pflanzenschutz Umweltschutz, 58(1): 10-16
(in German).
REICHMUTH, C., ed. (1986) The significance of changing
concentrations in toxicity of phosphine. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 8-21 March, London, Overseas Development
Administration, Tropical Development and Research Institute.
pp. 88-98.
REICHMUTH, C., NOACK, S., & WREDE, A. (1981) [Emission of
phosphine caused by fumigation for stored products protection.]
Nachrichtenbl. Dtsch. Pflanzenschutzd., 33(9): 132-136 (in
German).
ROALDSNES, J. (1982) [Phosphine poisoning due to machining of
ductile iron.] Norsk. bedv. h. tj., 3: 12-16 (in Norwegian).
ROBINSON, J.R. (1972) Residues containing phosphorus following
phosphine treatment: measurement by neutron activation. J.
stored Prod. Res., 8: 19-26
ROBINSON, J.R. & BOND, E.J. (1970) The toxic action of
phosphine. Studies with 32PH3: terminal residues in biological
materials. J. stored Prod. Res., 6: 133-146.
ROBISON, W.H. & HILTON, H.W. (1971) Gas chromatography of
phosphine derived from zinc phosphide in sugarcane. J. agric.
food Chem., 19(5): 875-878.
ROHRLICH, M. & MEUSER, F. (1970) [Studies on grain fumigated
with hydrogen phosphide. III. Communication: biochemical aspects
of phosphine fumigation.] Getreide Mehl, 20(1): 1-8 (in
German).
RUSCHEL, A.P. & DA COSTA, W.F. (1966) [Symbiotic fixing of
atmospheric nitrogen in the french bean ( Phaseolus vulgaris L).
III. Effect of some insecticides and fungicides.] Pesqui.
Agropecu. Bras., 1: 147-149 (in Portugese).
SAEED, T. & ABU-TABANJA, R. (1984) Determination of phosphine:
comparison of rates of desorption by purge-and-trap method and
by sulfuric acid treatment. J. Assoc. Off. Anal. Chem., 68(1):
61-64.
SATO, K. & SUWANAI, M. (1974) Absorption of hydrogen phosphide
to cereal products. Appl. Entomol. Zool., 9(3): 127-132.
SAYVETZ, T.A. (1982) Possible phosphine inhalation related to
warehouse fumigation, Richmond, Virginia, Virginia State Health
Department, Office of Health Protection and Environmental
Management, 6 p.
SCHITOSKEY, F. (1975) Primary and secondary hazards of three
rodenticides to kit fox. J. wildl. Manage., 39(2): 416-418.
SCHULZ, H. (1887) [Correction concerning the toxicity of
phosphorus compounds containing oxygen.] Arch. exp. Pathol., 23:
150-151 (in German).
SCHULZ, H. (1890) [Concerning phosphine.] Arch. exp. Pathol.
Pharmakol., 27: 314-335 (in German).
SHAHEEN, D.G., BRAGG, L.A., STANOVICK, R.P., & SULLIVAN, J.B.
(1983) Phosphine detector tubes as passive dosimeters for use
with metal phosphide fumigants. Tob. Int., 185(20): 36-39.
SHIVANANDAPPA, T., RAMESH, H.P., & KRISHNAKUMARI, M.K. (1979)
Rodenticidal poisoning of non-target animals: acute oral
toxicity of zinc phosphide to poultry. Bull. environ. Contam.
Toxicol., 23(4-5): 452-455.
SINGH, K.N., SRIVASTAVA, B.P., & NATH, G. (1983) Phosphine
residues and its effect on the germination of stored pulses.
Indian J. Entomol., 45(1): 71-80.
SRI (1982) Electronic chemicals, Menlo Park, California,
Standford Research International, pp. 40-41.
SRIDHARA, S. (1983) Rodenticide induced bait aversion and
neophobia in Tatera indica cuvieri. Z. angew. Zool., 70(4): 429-
440.
STEPHENSON, J.B.P. (1967) Zinc phosphide poisoning. Arch.
environ. Health, 15: 83-88.
TERZIC, Lj. (1981) Quantitative method for the determination
of zinc phosphide in technical products. Acta vet. (Beograd),
31(5-6): 279-288.
TIETJEN, H.P. (1976) Zinc phosphide: its development as a
control agent for black-tailed prairie dogs, Washington DC, US
Department of the Interior, Fish and Wildlife Research Centre
(Special Scientific Report Wildlife No. 195).
TKACHUK, R. (1971) Phosphine fumigation of wheat - residue
formation. Cereal Sci. Today, 16: 307.
TKACHUK, R. (1972) Phosphorus residues in wheat due to
phosphine fumigation. Cereal Chem., 49: 258-267.
TRIMBORN, H. & KLIMMER, O. (1962) [Experimental studies on
chemical changes in blood pigments caused by hydrogen
phosphide in vitro.] Arch. int. Pharmacodyn., 137(3-4): 331-
347 (in German).
UNDERWOOD, J.G. (1972) The radiochemical analysis of phosphine
residues in fumigated tobaccos. Tob. Sci., October 13: 47-49.
URGA, K. (1983) Methyl bromide and phosphine residues in
cereal grains and pulses. Ethiop. med. J., 21: 79-83.
US CFR (1981) Code of federal regulations, 29(1910): 673.
US EPA (1981) Pesticide registration standard: aluminium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-137514).
US EPA (1982) Pesticide registration standard: magnesium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-195777).
US EPA (1983) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 48(37): 7714-
7716.
US EPA (1984) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 49(92): 19922-
19923.
US EPA (1985a) Aluminium phosphide/magnesium phosphide:
tolerances for residues. Code Fed. Reg., 40: 180, 225, 291-292,
333, 375.
US EPA (1985b) EPA chemical profile: phosphine, Washington DC,
US Environmental Protection Agency.
US EPA (1986) Guidance for the re-registration of pesticide
products containing aluminium phosphide, February 1986. An
amendment to the registration standard issued in October 1981,
Washington DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (PB 84-241405).
US NIOSH (1977) National occupational hazard survey,
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, Vol. 3 (Publication No. 78-114).
US NIOSH (1979) Ten NIOSH analytical methods: phosphine,
Cincinnati, Ohio, National Institute for Occupational Safety
and Health, Division of Physical Sciences and Engineering, 42
pp.
VAN WAZER, J.R., ed. (1961) Phosphorus and its compounds:
technology, biological functions and applications, New York,
Interscience Publishers Inc., Vol. II, p. 957.
VAN WAZER, J.R. (1982) Phosphorus and the phosphides. In:
Kirk-Othmer, ed. Encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 17, pp. 473-489.
VDOVENKO, I.D., VAKULENKO, L.I., YAKOVLEVA, L.A., SHEDENKA,
L.I., BERESLAVSKAYA, A.N., & YATSENKO, V.S. (1984) [Effect of
surfactants on phosphine formation in steel pickling.] Ukr.
Khim. Zh., 50(4): 418-421 (in Russian).
VERGA, L. & BELLONI, F. (1958) [Over a case of purpura due to
empoisonment of phosphine (a haemato-clinical and histochemical
study.] Rass. Med. ind. Ig. Lav., 27(3): 230-241 (in Italian).
VERSTUYFT, A.W. (1978) Sampling and analytical methods for
phosphine - a review. Am. Ind. Hyg. Assoc. J., 39: 431-437.
VINSJANSEN, A. & THRANE, K.E. (1978) Gas-chromatographic
determination of phosphine in ambient air. Analyst, 103: 1195-
1198.
WARITZ, R.S. & BROWN, R.M. (1975) Acute and subacute
inhalation toxicities of phosphine, phenylphosphine and
triphenylphosphine. Am. Ind. Hyg. Assoc. J., 36: 452-458.
WEBLEY, D.J., HARRIS, A.H., & KILMINSTER, K. (1981) The use of
a portable gas analyser to measure phosphine concentrations in
experimental fumigations in the tropics. Int. pest Control,
March/April: 47-50.
WILSON, A. (1971) The metal phosphides. In: Mellor's compre-
hensive treatise on inorganic and theoretical chemistry, White
Plains, New York, Longman, Vol. VIII, Suppl. III.
WILSON, R., LOVEJOY, F.H., JAEGER, R.J., & LANDRIGAN, P.L.
(1980) Acute phosphine poisoning aboard a grain freighter.
Epidemiologic, clinical and pathological findings. J. Am. Med.
Assoc., 244(2): 148-150.
WINKS, R.G. (1970) Phosphine fumigation of farm-stored
tobacco. Queensland agric. J., November.
WINKS, R.G. (1984) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): time as a dosage factor. J.
stored Prod. Res., 20(1): 45-56.
WINKS, R.G. (1985) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): phosphine-induced narcosis. J.
stored Prod. Res., 21(1): 25-29.
WHO (1973) WHO Technical Report Series, No. 513 (Twentieth
Report of the Expert Committee on Insecticides), Geneva, World
Health Organization, p. 39.
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food, Geneva, World Health Organization, pp. 289-295 (WHO
Pesticide Residue Series, No. 1).
WHO/FAO (1976) Data sheet on pesticides No. 24: zinc
phosphide, Geneva, World Health Organization (VBC/DS/77.24).
WHO/FAO (1980) Data sheet on pesticides No. 46: phosphine,
Geneva, World Health Organization (VBC/DS/80.46).
WORTHING, C.R. & WALKER, S.B., ed. (1983) The pesticide manual:
a world compendium, 7th ed., London, British Crop Protection
Council.
ZIEMER, U. (1963) [Fatal hydrogen phosphide poisoning on a
freighter.] Zbl. Arbeitsmed., 13: 38-39 (in German).
ZIPF, K.E., ARNDT, TH., & HEINTZ, R. (1967) [Clinical
observation of a case of Phostoxin poisoning.] Arch. Toxikol.,
22(4): 209-222 (in German).
ZUTSHI, M.K. (1966) Effect of aluminium phosphide (Phostoxin)
on the germination of certain cereal and vegetable seeds. Bull.
grain Technol., 4(4): 197-199.
Neubert & Hoffmeister (1960) investigated the effects of
short-term exposures ranging from 460 to 2150 mg phosphine/m3 on
cellular respiration in rats. The oxidation of alpha-keto-
glutarate in liver cells was inhibited, but not oxidative
phosphorylation. Alpha-ketoglutarate oxidation was also reduced
in heart muscle, and phosphorylation was disrupted. Survival of
groups of 8 rats at various concentrations of diphosphine-free
phosphine was studied with the following results (Table 14).
Table 14. Survival times of 8 rats in different concentrations of
phosphine. Concentration x time products calculated from
the mid-point of the range of survival timesa
---------------------------------------------------
Concentration Survival Concentration x time
(mg/m3) (min) product (mg x min/m3)
---------------------------------------------------
4640 16 - 20 83 520
1000 50 - 70 60 000
320 150 - 200 56 000
100 400 - 600 50 000
---------------------------------------------------
a From: Neubert & Hoffmeister (1960).
If the use of the mid-point of the range of survival times
is reasonable, these results indicate that the relationship
between concentration and survival time is not simply
reciprocal, and that the index of t is greater than 1, but less
than the value of 1.43 derived from the data quoted by US EPA
(1981, 1982).
Klimmer (1969) determined lethal concentration x time pro-
ducts for rats and calculated, for example, 19 000 mg x min/m3
for a concentration of 287 mg/m3, but these results do not agree
with those calculated from the data of US EPA (1981, 1982) nor
with those of Neubert & Hoffmeister (1960). The value of the
concentration x time product, as a measure of phosphine dose in
mammals, is therefore open to question, as it is in insects.
8.1.2. Inhalation studies on zinc phosphide
The US National Pest Control Association submitted a value
of 19.6 mg/litre for an inhalation LC50 of 10% zinc phosphide
powder in rats. It was not stated whether the value was for
pure zinc phosphide or the 10% dilution and there was no
indication of exposure time (US EPA, 1983).
8.1.3. Oral studies on metal phosphides
In a study on 35 rats of both sexes administered doses of
20, 40, 50, or 80 mg/kg, Dieke & Richter (1946) reported an LD50
for zinc phosphide of 40.5 ± 2.9 mg/kg body weight for wild
Norway rats. Schitoskey (1975) reported an LD50 for the kit fox
of 93 mg zinc phosphide/kg body weight. Aminzhanov (1972)
reported that 100 mg/kg body weight of zinc phosphide was fatal
for starved dogs but not for dogs that were fed. Ikimanova
(1977) reported increased blood- and urinary-porphyrin concen-
trations in rats dosed with zinc phosphide. Other authorities
refer to 27 mg/kg body weight for an acute oral LD50 value for
94% pure zinc phosphide in rats (US EPA, 1983).
8.1.4. Dermal and other studies on metal phosphides
An acute dermal LD50 of 2 000 - 5 000 mg/kg body weight for
zinc phosphide (94% Zn3P2) in rabbits was submitted by the US
National Pest Control Association (US EPA, 1983).
8.2. Short-term Exposures
8.2.1. Inhalation exposure to phosphine
Jokote (1904) determined cumulative survival times in inter-
mittent phosphine exposure as shown in Table 15.
Table 15. Survival time for rabbits, cats, and guinea-pigs at 3
concentrations of phosphinea
-----------------------------------------------------------
Concentration of phosphine (mg/m3)
14 35 140
-----------------------------------------------------------
Survival time (h) 16 - 30 8.5 - 12 2.5 - 3.5
(rabbits, cats, and
guinea-pigs)
-----------------------------------------------------------
a From: Jokote (1904).
Miller (1940) exposed rabbits and guinea-pigs for 4 h per
day to various concentrations of phosphine. At 28 mg/m3 (20
ppm), both rabbits and guinea-pigs died during or after the
second exposure. At 14 mg/m3 (10 ppm), rabbits survived 7 - 14
successive exposures, but at 12 mg/m3 (8.3 ppm), only 4 or 5
exposures. In another study in the series, 2 rabbits that had
had five 4-h exposures to phosphine at 7 mg/m3 were accidentally
exposed to 20 mg/m3 (14 ppm) on the sixth day. Both rabbits
died during this exposure. The authors concluded that
pretreatment with sub-lethal concentrations of phosphine reduced
resistance to near-lethal concentrations. At low concentrations
(up to 14 mg/m3), animals displayed no signs until about 1/2 h
before death when they exhibited diminished reactivity, became
stuporose with shallow respiration, and died in coma.
Occasionally, animals died following exposure, with symptoms of
pulmonary oedema. At 28 mg/m3 or more, all animals exhibited
signs of respiratory irritation and died of pulmonary oedema.
Pathological examination of the lungs revealed bronchiolitis and
atelectasis of the lungs. There was no evidence of haemolysis,
but all organs were hyperaemic. The liver showed fatty
infiltration and there was cloudy swelling of kidney tubular
cells. After prolonged exposure, lungs frequently contained a
brown pigment that did not stain with Prussian Blue and
therefore was not iron-containing.
Neubert & Hoffmeister (1960) exposed female Wistar and E-B
(inbred strain) rats in a 121-litre chamber repeatedly for 6
days to 681 mg/m3 phosphine for 10 - 20 min per day. On the
seventh day, they were exposed until they died (22 - 35 min).
The concentration x time product for lethality after 6 days
pretreatment (about 20 mg x min/litre) was about one third of
that for a first exposure (48 - 87 mg x min/litre) and the
authors concluded that the effects of exposure were cumulative,
rather than that the phosphine accumulated in the body.
Klimmer (1969) exposed cats, guinea-pigs and rats to
phosphine concentrations of 1.4 and 3.5 mg/m3 (1 and 2.5 ppm)
for 4 - 6 h per day, 6 days a week, for a total of more than
800 h over a 24-week period without any clinical, laboratory, or
pathological evidence of effects. The liver function in the
cats, measured by bromosulphthalein (BSP) excretion, was normal.
In cats (3), guinea-pigs (3), and rats (10) exposed to phosphine
at 7 mg/m3 (5 ppm) for 6 - 8 h per day, the cats became
apathetic, showing anorexia after exposure, but exhibiting
thirst. Later, they developed unsteadiness, vomiting,
agitation, dyspnoea, and apnoea, before cardiac arrest after a
total of 35.5 - 45.5 h of exposure. The haemoglobin concen-
tration and erythrocyte count were reduced by 10 - 20% compared
with initial values, there was proteinuria and delayed BSP
excretion. Post-mortem examination revealed congestion of all
organs, with oedema and focal emphysema and red pigmentation of
the lungs. The guinea-pigs exhibited signs that were similar
to, but more marked than, those in the cats, and 2 animals had
asphyxial convulsions. Blood tests showed no deviations from
normal values, but the blood contained a brown coloration that
was not due to methaemoglobin, since spectroscopy revealed the
absorption bands of oxyhaemoglobin only. The guinea-pigs died
on the sixth day after 24 h - 32 h cumulative exposure, and
post-mortem examination revealed pulmonary oedema and red
discolouration and congestion of other organs. The rats died
after 27 - 36 h cumulative exposure. All exhibited congestion
of organs, 3 had pulmonary oedema and 7 had proteinuria. A
second series of exposures under slightly changed exposure
conditions at a different time of year produced qualitatively
similar results. In both series, neurohistological studies in
rats showed widening of the perivascular spaces, vacuolation of
the nuclei of ganglion cells, a reduction in the Purkinje cells,
and a glial reaction. Similar, but less marked, changes without
glial reaction were seen in the cats and guinea-pigs. There was
no control group in these studies, and certain observations in
exposed animals were dismissed on the grounds that they had
previously been seen in unexposed animals.
Waritz & Brown (1975) exposed Charles River-CD rats to
phosphine for 4 h per day, for 12 days, at a concentration of
5.5 mg/m3 (0.163 µmol/litre). The rate of weight gain was
reduced during the exposure period. Although the authors stated
that the rate returned to normal during a 14-day post-exposure
observation period, their data indicate a uniformly reduced rate
of weight gain throughout the exposure and recovery phases.
Post-mortem examination of rats, sacrificed both at the end of
the exposure period and at the end of the recovery period,
revealed no gross or microscopic abnormalities.
The effects of a 1.5-month exposure of white rats to
phosphine at concentrations of 0.05, 0.2, 1.5, and 8 mg/m3 were
reported by Pazynich et al. (1984). There were changes in blood
cholinesterase, peroxidase, and catalase activity and in
phagocyte behaviour. The magnitude of the changes was large,
but not generally dose-related. On the basis of their results,
the authors recommended mean exposure limits for urban air for
exposure durations of 24 h, one month, and one year of 0.004,
0.0015, and 0.001 mg/m3, respectively, with a ceiling value of
0.01 mg/m3, and this recommendation has been adopted in the
USSR.
In a study of the effects of phosphine on rats by inhalation
at 0.1 mg/m3 and 0.05 mg/m3, Atchabarov et al. (1984) found a
reduction in total plasma protein without a change in the
relative proportions of the various fractions, a modest but
significant reduction in the glycoprotein A fraction with some
changes in the relative proportions of the sub-fractions,
increased plasma bile acids, and a marked increase in
seromucoids. Liver glycogen, lipids, and cytochrome oxidase
levels were reduced. Biochemical changes were similar to those
in a control group treated with hydrogen fluoride at a known
toxic level.
8.2.2. Oral exposure to metal phosphides
Bai et al. (1980) reported a 13 week feeding study in female
weanling albino rats. Zinc phosphide was mixed with the diet at
0 (control), 50 mg/kg (50 ppm), 100 mg/kg (100 ppm), 200 mg/kg
(200 ppm), and 500 mg/kg (500 ppm) w/w. Deaths occurred at the
2 higher dosage regimes in 1/12 and 10/12 animals, respectively.
Food intake and weight increase were reduced and dose-dependent
depilation occurred at all dosages. The relative weights of
liver, heart, brain, and thyroid were increased at 200 and
500 mg/m3 and the serum zinc and liver alkaline phosphatase
levels were increased at the highest dose. There was a dose-
dependent reduction in haemoglobin concentration, red cell
count, and haematocrit.
The WHO/FAO Data Sheet on Pesticides (No. 24) which deals
with zinc phosphide (WHO/FAO, 1976) cites a study in which
300 mg zinc phosphide/kg administered to rats produced 6/6
deaths in the second week while 200 mg/kg produced reduced
weight gain and 2/6 deaths. Histopathological studies revealed
liver damage in the peripheral and central lobular areas and the
lungs were congested with haemorrhage or exudate in the alveolar
spaces.
8.2.3. Dermal and other exposures
No reports are available on short-term studies involving the
dermal or other routes.
8.3. Skin and Eye Irritation; Sensitization
Zinc phosphide powder moistened with saline produced mild
skin irritation in rabbits (Draize method) (Rao, 1986).a
8.4. Long-Term Exposure
No long-term studies on phosphine or metal phosphides
exposure have been reported.
8.5. Reproductive, Mutagenicity, and Carcinogenicity
There have been no long-term reproductive, mutagenicity, or
carcinogenicity studies on phosphine or metal phosphides.
---------------------------
a Personal communication to the IPCS Task Group on Phosphine
and Metal Phosphides.
8.6. Factors Modifying Toxicity; Toxicity of Metabolites
Aminzhanov (1972) reported that 100 mg zinc phosphide/kg
body weight was lethal for dogs starved for 24 h beforehand but
not for dogs that were fed.
Phosphine is not a powerful reducing agent and its toxic
effects are unlikely to be associated with chemical reduction.
The recent discovery that phosphites and phosphorous acid have a
fungicidal activity (Bompeix & Saindreman, 1984) suggests that
metabolites of phosphine have biological effects and therefore
might contribute to its toxicity. However, this contribution
can only be small, since phosphine is only slowly oxidized to
hypophosphites and phosphites and a maximum of about 40% of the
phosphide dose is recovered as these metabolites, even with
small doses. Moreover, the toxicity of phosphites is much less
than that of phosphides (Schulz, 1887). The contribution of
toxicity of contaminants of phosphine such as diphosphides is
uncertain.
8.7. Mechanisms of Toxicity - Mode of Action
Studies on isolated rat liver showed that mitochondrial
oxygen uptake was inhibited by phosphine (Nakakita et al., 1971)
and that this effect was due to the reaction of phosphine with
cytochrome c and cytochrome c oxidase (Kashi & Chefurka, 1976).
Although this inhibitory in vitro effect was also shown in
insects, it was found that insects severely poisoned with
phosphine did not suffer any inhibition of their cytochrome
system (Price & Dance, 1983). In the same report, phosphine was
found to inhibit insect catalase though this appeared to be an
indirect effect and might have been a result of phosphine
toxicity rather than a cause.
There have not been any systematic studies on the mechanism
of phosphine toxicity. Various effects on intermediary
metabolism have been described. Ikimanova (1977) reported
increased blood- and urinary-porphyrin concentrations, which
were related to the dose of zinc phosphide and the duration of
treatment. In a study on rabbits, Minchev & Dimitrov (1970)
reported changes in serum glutamic-pyruvic and glutamic-
oxaloacetic transaminases, leucine aminopeptidase, aldolase,
alkaline phosphatase and albumin in the first 24 h of zinc
phosphide poisoning. Dysfunction of hepatic fat metabolism was
also observed. Loss of cell viability and cell membrane
integrity accounted for the raised hepatic enzymes, the
bronchiolitic effect, the cloudy swelling of renal tubular cells
and the occasionally reported haemorrhagic lesions in the
myocardium. There is no adequate explanation for the fact that
phosphine does not cause the haemolysis characteristic of
arsine.
9. EFFECTS ON MAN
9.1. Organoleptic Effects
The odour of phosphine depends on the impurities it con-
tains; phosphine of high purity has no odour, even at 280 mg/m3
(Dumas & Bond, 1974; Fluck, 1976). Phosphine prepared conven-
tionally, without purification, has a fishy or garlic-like odour
attributed to impurities. These may be adsorbed by stored pro-
ducts during fumigation with resultant loss of odour, even
though phosphine remains at toxic concentrations (Bond & Dumas,
1967). Amoore & Hautala (1983) reviewed the literature on odour
thresholds and stated that the geometric mean of all the 6
reported values for phosphine was 0.71 mg/m3 (0.51 ppm). Fluck
(1976) reported that thresholds for phosphine at which 50% or
more of 10 persons could positively identify the odour asso-
ciated with phosphine differed according to the source of the
phosphine (Table 16).
Table 16. Odour thresholds for phosphinea
--------------------------------------------------------------
Source Odour thresholds for
PH3 (mg/m3 converted
from ppm)
--------------------------------------------------------------
Technical aluminium phosphide + H2SO4 0.14 - 0.28
Phosphorium iodide + aqueous hydroxide 2.8
Phostoxin + H2SO4 0.014 - 0.028
(5 Å molecular seived) 1.4 - 2.8
Phostoxin + H2SO4
--------------------------------------------------------------
a From: Fluck (1976)
Amoore & Hautala (1983) classified phosphine in safety class
D in their classification because 20 - 50% of attentive persons
can detect the threshold limit value (TLV) (0.42 mg/m3) by
smell. However, the smell of phosphine cannot be relied on to
warn of toxic concentrations.
9.2. General Population Exposure
9.2.1. Phosphine
There is negligible exposure of the general population to
phosphine. Gessner (1937) described an incident in which the 12
inhabitants of an apartment house developed nausea and one died
when phosphine was emitted from an adjacent warehouse containing
bags of aluminium phosphide, which became damp. Some passengers
on ships and barges carrying cargoes of ferrosilicon or grain
under fumigation have also been poisoned by phosphine (Harger &
Spolyar, 1958; Netherlands, 1984). Effects were similar to
acute occupational poisoning.
Hallerman & Ribilla (1959) reported the deaths of 2 adults
and a child living in a dwelling with a party wall to a granary
being fumigated. Symptoms were non-specific and insidious
initially and illustrate the risks of sustained exposures to
relatively low concentrations (a few mg phosphine/m3); it was
estimated that the concentration in the bedroom reached
1.2 mg/m3. At autopsy, there was congestion of all organs.
Pulmonary oedema and focal emphysema were found in the lungs and
there was vacuolation in the liver.
9.2.2. Metal phosphides
Zinc phosphide baits and formulated aluminium phosphide
pellets are widely used. Occasional accidental, or more usually
suicidal, exposure to metal phosphides may be encountered.
Ingestion, the only easily toxic route, has almost always been
suicidal and the effects acute (Stephenson, 1967).
Stephenson (1967) reviewed 20 cases of acute zinc phosphide
poisoning by ingestion (including one treated by himself) in
which the approximate dose was recorded. Ten cases were fatal
and the doses ranged from 4.5 to 180 g; 6 cases had ingested
20 g or more. In the 10 non-fatal cases, the doses ranged from
0.5 to 50 g with 7/10 ingesting less than 20 g. In the case
treated by the author, clinical features included metabolic
acidosis, hypocalcaemic tetany, methaemalbuminaemia, and reduced
blood coagulation (thrombotest 28% of normal). Post-mortem
findings included blood in all the serous cavities, pulmonary
congestion and oedema, haemorrhagic changes in the intestinal
epithelium, centrilobular congestion and necrosis and yellow
discoloration of the liver, and patchy necrosis of the proximal
convoluted tubules of the kidneys.
An unsuccessful suicidal attempt was described by Zipf
(1967). A 25-year-old man ingested 6 tablets of PhostoxinR in
water (= 6 g phosphine). Immediate symptoms were severe retro-
sternal pain, a generalized burning sensation, and vomiting.
There was circulatory collapse necessitating resuscitation and
he was also treated by gastric lavage with potassium perman-
ganate and magnesium sulfate. Subsequently, symptoms of cardiac,
cerebral, and hepatic dysfunction appeared and there was severe
renal failure requiring haemodialysis.
9.3. Occupational Exposure
Cases of acute phosphine poisoning reported in the litera-
ture were reviewed by Harger & Spolyar (1958). Fifty-nine cases
with 26 deaths had been recorded since 1900 (including Gessner's
(1937) non-occupational cases). In 6 out of 11 papers, cargoes
of ferrosilicon were cited as the source of phosphine and, in
these cases, the victims were passengers or crew members of the
ships or barges concerned. Other cases involved the exposure of
welders to calcium carbide and raw acetylene and of submariners
to sodium phosphide. The most common autopsy finding was
congestion of the lungs with marked oedema. The authors added a
case of their own in which a 16-year-old youth operating
acetylene generators died, probably as a result of phosphine
exposure at a level of about 11 mg/m3 (8 ppm) for 1 - 2 h daily
over a period of 6 weeks. Arsine and hydrogen sulfide exposures
were considered to have been too low to have accounted for the
death. Autopsy revealed acute pulmonary oedema. Other cases
have been described, all of which exhibited similar features
(Ziemer, 1963; Furuno et al., 1976; & Netherlands, 1984). Three
groups of symptoms of acute phosphine poisoning were described
by Childs & Coates (1971): (a) nervous symptoms including
headache, vertigo, tremors, and unsteady gait, progressing in
severe cases to convulsions, coma, and death; (b) gastro-
intestinal symptoms include loss of appetite, thirst, nausea and
vomiting, diarrhoea, and severe epigastric pain; and (c) respir-
atory symptoms including a feeling of pressure and pain in the
chest as well as shortness of breath. In addition, a sharp fall
in blood pressure was described as a characteristic, but seldom-
mentioned symptom. Chronic effects include anaemia, bronchitis,
and gastrointestinal, speech, and motor disturbances, but these
are by no means general.
The toxic effects of phosphine are summarized in Table 17.
The IDLH (Immediately Dangerous to Life or Health) level is
282 mg/m3 (200 ppm) (US EPA, 1985b).
Verga & Belloni (1958) described a case of purpura ascribed
to poisoning by phosphine. The platelet count was reduced to
60 000/mm3; the red-cell count was also low at 3.1 x 106/mm3 and
the haemoglobin concentration (Sahli) was 55%. With recovery,
both the red-cell and thrombocyte counts increased to 4.8 x 106
and 210 000/mm3, respectively, at the end of the observation
period. The electrolysis of phosphate solutions being applied
to metals as a corrosion inhibitor was considered to have
produced phosphine, but there was no measurement of the level of
exposure and it is possible that the exposure also included
arsine, which would account for the anaemia with a hyperplastic
erythroid series in the marrow biopsy. While haemolysis is
recognized as a complication of arsine poisoning, purpura and
thrombocytopenia are not widely recognized features of
phosphine poisoning, though Borodin et al. (1983) described
changes related to haemolysis (prolongation of clotting time and
reduced plasminogen levels) in workers exposed to a variety of
biocides and disinfectants, including zinc phosphide. However,
these changes could not be related specifically to exposure to
phosphine.
Table 17. Toxic effects of phosphinea
------------------------------------------------------------
Effect Concentration
mg/m3 ppm
------------------------------------------------------------
Rapidly fatal 2800 2000
Death after 1/2 - 1 h 560 - 840 400 - 600
Dangerous to life after 1/2 - 1 h 400 - 600 290 - 430
Serious effect after 1/2 - 1 h 140 - 260 100 - 190
No serious effects after 1/2 - 1 h 10 7
------------------------------------------------------------
a From: Childs & Coates (1971).
In 1978, Beloskurskaya et al. published the findings of a
study on 206 phosphorus workers, which revealed 57 cases of
toxic hepatitis. In 49 of these cases, there was stated to be no
other apparent cause than occupational exposure to phosphorus,
phosphine, or phosphorus oxides. No measurements of exposure
were reported. Diagnostic criteria included a history of
hypochondrial pain, clinical hepatomegaly, and abnormal liver
function tests and 131I-Rose Bengal scanning and clearance
studies. Forty-six of the 57 cases had additional symptoms,
such as the astheno-vegetative syndrome, chronic gastritis,
impotence, toxic encephalopathy, toxic cardiomyopathy, and
respiratory disease. There was no control group and no informa-
tion on alcohol consumption was provided.
In a later study, Wilson et al. (1980) described the
symptoms of acute poisoning by phosphine as headache, fatigue,
nausea, vomiting, jaundice, paraethesia, ataxia, tremor,
diplopia, myocardial infiltration with necrosis, pulmonary
oedema, and myocardial and peripheral muscle damage. Laboratory
results indicated that there had been urinary tract involvement
(occult blood) and levels of several liver enzymes were
elevated. Furthermore, Roaldsnes (1982) reported that metal-
workers at a large shipyard in Norway, drilling deep holes in
spheroidal graphite iron, became ill during work. The symptoms
were mostly nausea, dizziness, chest tightness, dyspepsia, and
disturbances of smell and taste. Measurement of the phosphine
concentration in the workers' breathing zone (with Dräger tubes)
showed a phosphine concentration of about 1.4 mg/m3 (1 ppm).
After installing local exhaust ventilation on the drilling
machines, there were no longer any measurable amounts of
phosphine, and there were no complaints from the workers. When
the local exhaust ventilation was removed for technical reasons
5 years later, illness among the workers recurred. Measurement
of phosphine levels just above the machines, showed concentra-
tions of up to 56 - 70 mg/m3 (40 - 50 ppm). When the local
exhaust ventilation was re-installed, the phosphine concentra-
tions dropped to unmeasurable amounts, and no further cases of
illness were reported. Addition of copper sulfate solution to
the cutting oil effectively removed the phosphine gas, but
resulted in problems of corrosion.
Jackson & Elias (in press) studied liver function and
gastrointestinal symptoms in: (a) workers exposed to white
phosphorus at up to 5 times the occupational exposure limit and
also to some phosphine (0 - 0.5 mg/m3); (b) workers exposed to
phosphine alone (at up to 2.8 mg/m3, depending on the efficacy
and use of air-line breathing apparatus); and (c) gastro-
intestinal symptoms in a group of workers who were not exposed
to either compound, but who worked the same shift pattern.
Complaints to the factory medical department of gastrointestinal
symptoms in the phosphine-exposed group, in the phosphorus
exposed-group, and in the unexposed group were 1.51, 0.61, and
0.24 per man per year, respectively. However, the alcohol
consumption in the phosphine- and phosphorus-exposed groups
differed (289 and 154 ml per week, respectively). The alcohol
consumption was not estimated in the control group. None of the
conventional liver function tests reflected the differences in
occupational exposure or alcohol intake. The phosphine-exposed
group did have a significantly raised post-prandial bile acid
concentration, but whether this reflected a difference in phos-
phine exposure or alcohol consumption could not be determined.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Phosphine and metal phosphides are only focally distributed
in the environment and exposure of large numbers of the general
population is unlikely. The sources and uses of phosphine and
metal phosphides are sufficiently well established for safety
techniques to be generally known, though it is disturbing that
shipboard poisonings are still occurring when the first case was
reported over half a century ago. The extensive world-wide use
of metal phosphides as fumigant sources and as rodenticides
creates the hazard of accidental poisoning in children and
others and provides a means of suicide, unless the supply and
use of these materials is appropriately regulated. There is no
evidence of danger to the population from residues of phosphine
in fumigated foods or from residues of phosphides, if adequate
fumigation and aeration techniques are used. Permitted limits
for residues are in the range of 0.01 - 0.1 mg total phosphine/
kg. There do not appear to be any deleterious effects on food
quality as a result of adequately undertaken fumigation with
phosphine.
Published data on occupational exposure to phosphine in most
industries are not sufficient to evaluate the success of control
measures in relation to occupational exposure limits. There is
also some disagreement regarding these limits and some data
suggest that an occupational exposure limit of 0.4 mg
phosphine/m3 is above the threshold for biological effects with
long-term exposure. However, there are no studies adequate to
settle this question definitively.
Major accidental release of stored phosphine presents a
serious explosion/fire hazard and an acute toxic hazard for man
and animals.
10.2. Evaluation of Effects on the Environment
Only very small amounts of phosphine and phosphides occur in
the environment as a result of natural processes and those
resulting from human activity are not persistent.
Phosphine and phosphides are introduced into the environment
for the control of pests, for which they are particularly
suitable, because of their efficacy, lack of persistence, and
harmless decomposition products.
Careful positioning of bait and the low toxicity of
carcasses for scavengers of poisoned animal targets minimize the
effects of rodenticidal metal phosphides on non-target species.
Phosphine and phosphides are oxidized in the environment and the
final product, phosphate, is normally widely present in the
natural environment. Phosphine and phosphides are only used
where the natural environment is already modified by human
activity and their environmental impact must be viewed in this
context.
Since resistance to phosphine in insects does not appear to
reverse in the absence of selective pressures, it is
particularly important to achieve effective doses of phosphine
by using adequate treatment rates and good practices. In
practice, the development of resistance is more of a problem
than the effects on non-target species. Major accidental
release of stored phosphine would not result in long-term
environmental consequences.
10.3. Conclusions
In general, there is no risk to the public from the use of
phosphine or phosphides, from emissions from fumigated products
or spaces, from alloys, industrial processes, or residues in
food, provided that proper fumigation, transport, and industrial
practices are used.
While phosphine and metal phosphides are toxic, their
extensive use in occupational settings has resulted in the
establishment of good practice guidelines. If these are
followed, there is a low order of risk for human health.
However, there is some doubt about the completeness of
protection afforded by the higher occupational exposure limit
(0.4 mg phosphine/m3).
There is no risk of important environmental effects from
phosphine or metal phosphides.
11. RECOMMENDATIONS
11.1. Gaps in Knowledge
1. The principal area related to human health where information
is inadequate is concerned with occupational exposure levels and
their safety. There is a need for an adequate study of well-
being and organ function in exposed and control populations,
with a complete description of phosphine exposure, concurrent
exposures to other chemicals (which should be low), alcohol
consumption, and smoking habits. The establishment of exposure
levels that are free from any detectable effects would help to
determine agreed occupational exposure limits.
2. It would be of assistance, in this regard, to undertake
laboratory studies to identify biological markers that are most
indicative of phosphine effects and reflect the suggested short-
term cumulative effects of repeated phosphine exposure.
3. Further research on the mechanisms of action of phosphine
and phosphides will assist in the design of good fumigation
practice and fumigation schedules, and minimize the occurrence
of resistance in target organisms and associated problems.
4. It has been suggested that zinc phosphide may be relatively
persistent in aquatic sediments and there is a general lack of
aquatic toxicological data. There is a need for research on the
levels and persistence of zinc phosphide in sediments and for
further studies of its effects on aquatic organisms.
11.2. Preventive Measures
11.2.1. Management
The most important factor in the safe handling of phosphine
and metal phosphides, and in their formulation, is proper work
practices.
Management should identify these, provide training for the
operatives, and ensure that the practices are carried out.
Personal protection measures recommended to reduce the
likelihood of absorption of phosphide preparations include the
wearing of:
(a) synthetic rubber gloves;
(b) rubber boots;
(c) lightweight impervious overalls; and
(d) suitable eye protection.
Adequate washing facilities should be available at all times
during handling. Eating, drinking, and smoking should be
prohibited during handling, and before washing after handling.
The means to measure concentrations of phosphine in air should
be available and used to check atmospheric concentrations. When
necessary, respiratory protective equipment should be worn. In
fumigation, each operator or other person liable to be exposed
to the gas must be provided with an efficient means of respira-
tory protection. Persons exposed to magnesium phosphide or
aluminium phosphide powders (or other readily hydrolysed
phosphides), which may give rise to airborne dust should be
protected by respiratory protective equipment, effective against
gaseous phosphine, since hydrolysis of dust in the filter of a
dust mask or respirator may give rise to high phosphine
exposure.
11.2.2. Treatment of poisoning
11.2.2.1 Inhalation of phosphine
(a) First Aid
Remove from exposure, keep at rest. Rescuers should follow
full safety procedures.
If the patient is unconscious, place in semi-prone recovery
position or otherwise maintain the airway.
If the patient is conscious but has difficulty in breathing,
treat in a seated position and give oxygen if available.
Otherwise, allow the patient to recline with the legs slightly
elevated.
If breathing stops, immediately ventilate the patient
artificially (mouth-to-mouth/nose or mechanically with oxygen,
if available).
If the heart stops, begin cardiopulmonary resuscitation
(CPR).
(b) Medical Treatment
1. Give oxygen by mask if required; perform baseline chest X-
ray and examine chest. Treat shock conventionally.
2. All patients should be observed for 48 - 72 h, as onset of
pulmonary oedema may be delayed.
3. If respiratory distress is severe, give steroids (methyl-
prednisolone 30 mg/kg, or equivalent, intramuscularly),
preferably within 4 h of exposure.
4. If pulmonary oedema occurs, treat by positive and expiratory
pressure ventilation. Antibiotics should only be given if a
secondary infection is present.
5. Treat any fits conventionally; give general supportive care
with particular attention to fluid balance.
6. Some sources of exposure to phosphine are likely to lead
also to exposure to arsine, which has haemolytic effects.
Monitor for haemoglobinaemia, haemoglobinuria, unconjugated
hyperbilirubinaemia, and renal failure.
In general, recovery is rapid following removal from
exposure, but renal damage and leukopenia may occur after
several days.
11.2.2.2 Ingestion of metal phosphides
(a) First aid
Do not give milk, fats, or saline emetics by mouth.
Give oxygen if there is respiratory distress.
If first aiders are medically authorized to do so, and the
patient is conscious, induce vomiting.
After 20 min (or after vomiting), administer activated
charcoal (50 g in water by mouth) if available.
Obtain medical attention as soon as possible: preferably
send immediately to hospital.
(b) Medical treatment
1. Consider tracheal intubation and gastric lavage with 2%
sodium bicarbonate solution (to limit hydrolysis of zinc
phosphide).
2. Activated charcoal or medicinal liquid paraffin may limit
absorption of phosphine and zinc phosphide, respectively,
and may be administered by mouth or stomach tube.
3. Monitor and support vital functions, particularly hepatic
and renal function. Treat shock conventionally.
5. Hepatic and renal failure should be treated in specialist
centres.
11.2.3. Leaks, spillages, residues, and empty containers
11.2.3.1 Phosphine
Small leaks and residues of compressed gas can be discharged
slowly to the atmosphere in the open air. Larger quantities
should be burned using an appropriate burner.
11.2.3.2 Aluminium and magnesium phosphides and their formulated
preparations
Spillages and residues in containers will evolve phosphine
for several days by reaction with atmospheric moisture. Respir-
atory protective equipment will be required by those dealing
with them. Even if atmospheric concentrations of phosphine are
initially low, they may rise as residues are disturbed.
Moderate quantities should be collected in a secure place to
protect the public and wildlife, and left in the open air and
kept moist until hydrolysis is complete.
Larger amounts should be removed to a deep pit, at an
approved site, until phosphine evolution is complete. Then they
can be buried.
Residues at the site of spillage should be washed away in a
large quantity of water and the area kept secure and aerated
until checked for zero gas concentration.
Combustible packages can be incinerated at high temperature
(> 1000 °C) using proper facilities. Containers should NOT be
cleaned for re-use, but should be disposed of by deep burial, at
an approved site, well away from habitation and where there is
no danger of contamination of water sources.
11.2.3.3 Zinc phosphide and preparations
Zinc phosphide hydrolyses only slowly. Nevertheless,
phosphine may be evolved and respiratory protective equipment
should be available.
Spillages and residues in containers should be incinerated
at high temperatures (> 1000 °C) or crushed and buried below the
topsoil at an appropriate site. Contaminated surface material
should be treated similarly or else the area should be secured
from public access until the gas concentration is zero.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Phosphine was evaluated in 1971 by WHO and FAO, as a
pesticide residue in food (WHO/FAO, 1972). A tolerance of
0.1 mg/kg (0.1 ppm) in raw cereals was confirmed. It was
recommended that the tolerance of 0.01 mg/kg (0.01 ppm) in
flour, other milled cereal products, breakfast cereals, dried
vegetables, and spices be confirmed and extended to include
nuts, groundnuts, dried fruit, cocoa beans, and other similar
foods, known to be fumigated with phosphine.
The use of zinc phosphide as a rodenticide in public health
was reviewed by WHO in 1972. It was concluded that it was a
generally effective compound and, while highly toxic to domestic
fowl, its safety record was good. The use of zinc phosphide was
endorsed (WHO, 1973).
REFERENCES
ACGIH (1986) Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists.
ADU, O.O. & MUTHU, M. (1985) The relative toxicity of seven
fumigants to life cycle stages of Callosobruchus chinensis L.
Insect Sci. Appl., 6(1): 75-78.
ADVANI, R. (1983) A comparative trial of zinc phosphide,
aluminium phosphide and RH-787 against field mixed population of
rodents in crop ecosystem of the Indian desert. Ztg angew.
Zool.,70(2): 153-168.
AHMAD, M. (1976) Effect of phosphine fumigation on the
germination of edible legume seeds. J. stored Prod. Res., 12:
211-212.
AL-OMAR, M.A. & AL-BASSOMY, M. (1984) Persistence of phosphine
gas in fumigated Irafir dates. J. food Saf., 6: 253-260.
AL'ZHANOV, S., MOLDABEKOV, Sh.M., ERSHOV, V.A., & POPOV, A.P.
(1983) [ Use of copper and iron sulfates in cleaning furnace
gas from phosphorus production,] Moscow, Academy of Sciences of
the USSR, Institute of Scientific Information (VINITI) (Document
4802-83) (in Russian).
AMINZHANOV, M. (1972) [Use of zinc phosphide in controlling
stray dogs.] In: Azimov, Sh.A., Shevchenko, N.Kh., & Nurmatov,
R.Sh., ed. [ Proceedings of the 7th Conference of Young Uzbek
Agricultural Scientists,] Tashkent, Uzbeksii Nauchno-Issled.
Veterinary Institute (in Russian).
AMOORE, J.E. & HAUTALA, E. (1983) Odour as an aid to chemical
safety: odour thresholds compared with threshold limit values
and volatilities for 214 industrial chemicals in air and water
dilution. J. appl. Toxicol., 3(6): 272.
ANON. (1936) [Trials to establish whether the Delicia grain
weevil fumigation procedure is safe to apply and free from the
risk of fire and explosion.] Gutachten chim. tech. Reichsanst.,
8(4): 5 (in German).
ANON. (1956) Ferro-silicon explosion risks. Chem. Trade J.
Chem. Eng., 16 Nov.: 1180.
ARENDT, G., FARWIK, W., HAAG, F., KAUFMANN, G., KOHL, E.-G.,
PRUGGMAYER, D., & WAGNER, W. (1979) [Environmental aspects of
pest control. Fumigation of grain and food stores.] Lebensmit-
teltechnik, 5: 2-8 (in German).
ARNOLD, J.T. & ROBBIANO, P. (1974) Portable vapour surveil-
lance system, Springfield, Virginia, National Technical
Information Service (NTIS Report No. AD-782 844).
ATCHABAROV, B.A., AITBAYEV, T.KH., & AITBEMBETOV, B.N. (1984)
[Comparison of the effects of hydrogen fluoride and phosphine on
the organism with various exposure levels.] In:[ Health care in
Kazakhstan. Part 1,] pp. 28-31 (in Russian).
ATTIA, F.I. (1984) Multiple cross-resistance characteristics
in phosphine-resistant strains of Rhizopertha dominica and
Tribolium castaneum. In: Ripp, B., ed. Controlled atmosphere
and fumigation in grain storages, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 49-53 (Developments in
Agricultural Engineering No. 5).
ATTIA, F.I. & GREENING, H.G. (1981) Survey of resistance to
phosphine in coleopterous pests of grain and stored products in
New South Wales. Gen. appl. Entomol., 13: 93-97.
BAI, K.M., KRISHNAKUMARI, M.K., RAMESH, H.P., SHIVANANDAPPA, T.,
& MAJUMDER, S.K. (1980) Short-term toxicity study of zinc
phosphide in Albino rats (Rattus norvegicus). Indian J. exp.
Biol., 18(8): 854-857.
BARRETT, W.J. & DILLON, H.K. (1978) Development of methods
for the determination of elemental phosphorus and phosphine in
air, Cincinnati, Ohio, National Institute of Occupational Safety
and Health, 102 pp (DHEW (NIOSH) Publication No. 78-177).
BECKER, K.H., BIEHL, H.M., BRUCKMANN, P., FINK, E.H., FUHR, F.,
KLOPFER, W., ZELLNER, R., & ZETSCH, C. (1984) Methods for the
ecotoxicological evaluation of chemicals, photochemical degra-
dation in the gas phase. VI. OH x reaction rate constants and
tropospheric lifetimes of selected environmental chemicals,
Jülich, Kernforschungsanlage (Projektträgerschaft Umweltchemi-
kalien Report 1980-83, No. 279).
BELL, C.H., WILSON, S.M., & BANKS, H.J. (1984) Studies on the
toxicity of phosphine to tolerant stages of Trogoderma granarium
everts (Coleoptera: Dermestidae). J. stored Prod. Res., 20(2):
111-117.
BELOSKURSKAYA, G.I., PARASKEVOPULOS, YA.G., & SHLYGINA, O.E.
(1978) [On the clinical and functional condition of the liver
in patients with chronic phosphorus poisoning.] Tr. Inst. Kraev.
Patol. Akad. Nauk Kazakhskoi SSR, 36: 25-30 (in Russian).
BLAXLAND, J.D. & GORDON, R.F. (1945) Zinc phosphide poisoning
in poultry. Vet. J., 101: 108-110.
BOENIG, I.A., CRUTCHFIELD, M.M., & HEITSCH, C.W. (1982)
Phosphorus compounds. In: Kirk-Othmer, ed. Encyclopaedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol.
17, pp. 490-539.
BOMPEIX, G. & SAINDRENAN, P. (1984) In vitro antifungal
activity of Fosetyl Al and phosphorous acid on Phytophthera
species. Fruits, 39(12): 777-786.
BOND, E.J. & DUMAS, T. (1967) Loss of warning odour from
phosphine. J. stored Prod. Res., 3: 389-392.
BOND, E.J., ROBINSON, J.R., & BUCKLAND, C.T. (1969) The toxic
action of phosphine. Absorption and symptoms of poisoning in
insects. J. stored Prod. Res., 5: 289-298.
BOND, E.J., DUMAS, T., & HOBBS, S. (1984) Corrosion of metals
by the fumigant phosphine. J. stored Prod. Res., 20(2): 57-63.
BONTOYAN, W.R. (1981) Report on pesticide formulations: herbi-
cides, fungicides and miscellaneous. J. Assoc. Off. Anal. Chem.,
64(2): 355.
BORODIN, A., KLIMEK, M., & ZAKL, K. (1983) [Blood clotting and
fibrinolysis systems in workers of a disinfection, disinfesta-
tion and deratization employment (sic).] Med. Pr., 34(4): 313
(in Polish).
BOUBAL, M., LOUISE, J., BELOT, D., & POLITI, J.M. (1981)
Analyse directe et continue de traces d'hydrures gazeux à très
basses teneurs, Paris, Institut National de la Proprieté
Industrielle, Sociéte d'Air Liquide (Document No. 2 506 459)
(Demande de Brevet d'Invention No. 81 10316).
BOWKER, J.R. (1958) The liberation of phosphine in the
machining of spheroidal graphite iron. In: Proceedings of the
British Cast Iron Research Association, 8 November, p. 50.
BOWLEY, C.R. & BELL, C.H. (1981) The toxicity of twelve
fumigants to three species of mites infesting grain. J. stored
Prod. Res., 17: 83-87.
BRADFIELD, A.A.G. & GILL, J.E. (1984) Laboratory trials of
five rodenticides for the control of Mesocricetus auratus
Waterhouse. J. Hyg. (Lond.), 93: 389-394.
BRANDON, R.W. (1983) The use of chemically impregnated paper
tapes for toxic gas detection and monitoring. Anal. Chem. Symp.
Ser., 17: 726-31.
BREYER, D. (1973) [Studies on hydrogen phosphide residues in
grain after treatment with fumigation preparations in powder
form.] Mühle Mischfuttertech., 110: 699-700 in German).
BUBIEN, Z., WARTENBERG, L., & ROSZYK, E. (1974) [Content of
zinc in hen carcasses poisoned with zinc phosphide.] Zesz. Nauk.
Akad. Roln Wroclawiu Weter., 31: 173-180 (in Polish).
CABROL TELLE, A.M. & DE SAINT BLANQUAT, G. (1984) In vitro
digestibility of starch, casein and triolein after fumigation
with phosphine. Sci. Aliments, 4(Hors Ser. 3): 287-291.
CABROL TELLE, A.M., DE SAINT BLANQUAT, G., DERACHE, R, HOLLANDS,
E., PERIQUET, B., & THOUVENOT, J.-P. (1985) Nutritional and
toxicological effects of long-term ingestion of phosphine-
fumigated diet by the rat. Food Chem. Toxicol., 23(11): 1001-
1009.
CALVERT, J.G. & PITTS, J.N., Jr (1966) Photochemistry, New
York, John Wiley and Sons.
CHAMP, B.R. & DYTE, C.E. (1976) Report of the FAO global
survey of pesticide susceptibility of stored grain pests, Rome,
Food and Agriculture Organization of the United Nations, pp. 1-
257 (FAO Plant Production and Protection Paper No. 5).
CHAN, L.T.F., CROWLEY, R.J., DELLIOU, D., & GEYER, R. (1983)
Phosphine analysis in post-mortem specimens following ingestion
of aluminium phosphide. J. anal. Toxicol., 7: 165-167.
CHENTSOVA, N.YU. (1972) [Danger to poultry during scattering
of zinc phosphide-poisoned grain to control rodents in forest
plantings.] In: Voronova, L.D., ed. [ Effects of pesticides on
wild animals,] Moscow, pp. 90-102 (in Russian).
CHILDS, A.F. & COATES, H. (1971) The toxicity of phosphorus
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3, pp. 1437-1440.
CHILDS, D.P. (1972) Tobacco consignment fumigated in
containers while in transit. Container News, October.
CHILDS, D.P., OVERBY, J.E., & NIFFENEGGER, D. (1969) Phosphine
fumigation of flue-cured tobacco warehouses for the control of
the cigarette beetle. Tobacco, 168(21): 20-25.
CHOPRA, G. (1984) Field efficacy of aluminium phosphide
against rodents. Indian J. agric. Sci., 54(3): 204-205.
CIBA (1978) Phosphorus in the environment: its chemistry and
biochemistry, Amsterdam, Oxford, New York, Elsevier North
Holland (Ciba Foundation Symposium 57 - new series).
CODEX ALIMENTARIUS (1986) Codex maximum limits for pesticide
residues, 2nd ed., Rome, Food and Agriculture Organization of
the United Nations (CAC/Vol. XIII-Ed.2).
COLE, R.J. & BENNETT, W.H. (1950) An investigation of the
possibility of the occurrence of phosphine and acetylene in the
atmosphere of factories for the powdering of magnesium. J. Soc.
Chem. Ind., 69: 29-30.
CORBRIDGE, D.E.C. (1974) The structural chemistry of
phosphorus, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 268-269.
COX, P.D., BELL, C.H., PEARSON, J., & BEIREN, M.A., (1984) The
effect of diapause on the tolerance of larvae of Ephestia
küehniella to methyl bromide and phosphine. J. stored Prod.
Res., 20(4): 215-219.
CURRY, A.S., PRICE, D.E., & TRYHORN, F.G. (1959) Absorption of
zinc phosphide particles. Nature (Lond.), 184(4686): 642-643.
DAVIS, R. (1986) Fumigation of ships. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 18-21 March, London, Foreign and
Commonwealth Office, Overseas Development Administration,
Tropical Development and Research Institute, pp. 24-34.
DELOMENIE, H. (1933) Gases evolved from ferrosilicons under
the influence of water. J. Pharm. Chim., 18: 289-292, 532
DIEKE, S.H. & RICHTER, C.P. (1946) Comparative assays of
rodenticides on wild Norway rats. Public Health Rep., 61: 672-
679.
DIETERICH, W.H., MAYR, G., HILD, K., SULLIVAN, J.B., & MURPHY,
J. (1967) Hydrogen phosphide as a fumigant for foods, feeds
and processed food products. Residue Rev., 19: 135-149.
DISNEY, R.W. & FOWLER, K.S. (1972a) Residues in cereals
exposed to hydrogen phosphide. In: Radiotracer studies on
chemical residues in food and agriculture. Proceedings of a
Combined Panel and Research Coordination Meeting, Vienna,
Austria, 25-29 October 1971, Vienna, International Atomic
Energy Agency, pp. 99-101.
DISNEY, R.W. & FOWLER, K.S. (1972b) The possibility of isotope
exchange or other interfering reactions occurring during the
determination of residues from fumigation with 32P-labelled
phosphine. In: Radiotracer studies on chemical residues in food
and agriculture. Proceedings of a Combined Panel and Research
Coordination Meeting, Vienna, Austria, 25-29 October 1971,
Vienna, International Atomic Energy Agency, pp. 87-88.
DUMAS, T. (1964) Determination of phosphine in air by gas
chromatography. J. agric. food Chem., 12(3): 257-258.
DUMAS, T. (1978) Modified gas chromatographic determination of
phosphine. J. Assoc. Off. Anal. Chem., 61(1): 5-7.
DUMAS, T. (1980) Phosphine sorption and desorption by stored
wheat and corn. J. agric. food Chem., 28: 337-339.
DUMAS, T. & BOND, E.J. (1974) Separation of phosphine from
odour-producing impurities. J. stored Prod. Res., 10: 67-68.
DUMAS, T. & BOND, E.J. (1981) Method of trapping low levels of
phosphine at ambient temperature for gas-chromatographic
analysis. J. Chromatogr., 206(2): 384-386.
EPPO (1975) Guidelines for the development and biological
evaluation of rodenticides, Paris, European and Mediterranean
Plant Protection Organisation (Bulletin No. 5(1)).
FITZPATRICK, R.J., MCGIRR, J.L., & PAPWORTH, D.S. (1955) The
toxicity of rodenticides. Vet. Rec., 67: 142-145.
FLUCK, E. (1973) The chemistry of phosphine. Fortschr. chim
Forsch., 35: 1-64.
FLUCK, E. (1976) The odor threshold of phosphine. J. Air
Pollut. Control Assoc., 26(8): 795.
FRANK, R. & RIPPEN, G. (1986) [ Content of phosphine in the
atmosphere,] Frankfurt am Main, Batelle-Institut e.v. (in
German).
FREYBERG, W. (1979) Acute toxicity of Detia R pellets to
bluegill (Lepomis macrochirus ), Washington DC, US Environmental
Protection Agency (Unpublished report).
FRITZ, B., LORENZ, K., STEINERT, W., & ZELLNER, R. (1982)
Laboratory kinetic investigations of the tropospheric oxidation
of selected industrial emissions. In: Versino, B. & Ott, H., ed.
Physico-chemical behaviour of atmospheric pollutants,
Dordrecht, Reidel, pp. 192-202.
FURMAN, N.H., ed. (1962) Standard methods of chemical
analysis, 6th ed., Princeton, New Jersey, D. van Nostrand
Company Inc., Vol. 1, pp. 821-823.
FURUNO, J., SUGAWARA, N., & IKEDA, H. (1976) A fatal case
poisoned by hydrogen phosphide and the simultaneous separation
of inorganic gases by gas chromatography. Bull. Yamaguchi med.
Sch., 23(1-2): 49-58.
GESSNER, O. (1937) Fatal phosphine poisoning from "Delicia"
grain weevil fumigant (aluminium phosphide). Samml. Vergiftungs-
fällen, 8: B-79.
GILL, J.E. & REDFERN, R. (1983) Laboratory tests of seven
rodenticides for the control of Meriones shawi. J. Hyg. (Lond.),
91: 351-357.
HABASHI, F. & ISMAIL, M.I. (1975) Health hazards and pollution
in the metallurgical industry due to phosphine and arsine. CIM
Bull., August: 99-103.
HACKENBERG, U. (1972) Chronic ingestion by rats of standard
diet treated with aluminium phosphide. Toxicol. appl.
Pharmacol., 23: 147-158.
HALLERMANN, W. & PRIBILLA, O. (1959) [Fatal poisoning with
phosphine.] Arch. Toxikol., 17(4): 219-242 (in German).
HARGER, R.N. & SPOLYAR, L.W. (1958) Toxicity of phosphine with
a possible fatality from this poison. Am. Med. Assoc. Arch.
Ind. Health, 18: 497-504.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds.
Wildlife, 191: 1-61.
HILTON, H.W. & ROBISON, W.H. (1972) Fate of zinc phosphide and
phosphine in the soil-water environment. J. agric. food Chem.,
20(6): 1209-1213.
HOLE, B.D. (1981) Variation in tolerance of seven species of
stored product Coleoptera to methyl bromide and phosphine in
strains from twenty-nine countries. Bull. entomol. Res., 71(2):
299-306.
HOLE, B.D., BELL, C.H., MILLS, K.A., & GOODSHIP, G. (1976) The
toxicity of phosphine to all developmental stages of thirteen
species of stored product beetles. J. stored Prod. Res., 12:
235-244.
HUGHES, J.G. & JONES, A.T. (1963) Estimation of phosphine in
air. Ind. Hyg. J., March-April: 164-167
HUNTER, D. (1978) The diseases of occupations, 6th ed.,
London, Hodder and Stoughton.
IKEDA, S. (1971) [Effect of zinc phosphide on Coturnix coturnix
japonica.] Ringyo Shikenjo Kenkyu Hokuko, 238: 141-148 (in
Japanese).
IKIMANOVA, G.K. (1977) [Porphyrin metabolism in experimental
phosphorus poisoning.] Zdravookhr. Kaz., 1: 39-42 (in Russian).
IMO (1980) Recommendations on the safe use of pesticides in
ships, London, International Maritime Organization (UN IMO
Circular No. 298, revised).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, 303 pp (Data
Profile Series No. 5).
JACKSON, J.R. & ELIAS, E. (1984) Hepatic function and exposure
to phosphine and white phosphorus. In: Proceedings of the 12th
International Congress on Occupational Health in the Chemical
Industry, Dublin, Ireland, 9-14 September 1984, pp. 219-228.
JANDA, J. (1973) Danger of rodenticides containing zinc
phosphide to wild life. Agrochemia, 13(3): 85-86.
JOKOTE, Ch. (1904) [Studies on hydrogen phosphine.] Arch. Hyg.
Bakteriol., 49: 275-306 (in German).
JONES, A.T., JONES, R.C., & LONGLEY, E.O. (1964) Environmental
and clinical aspects of bulk wheat fumigation with aluminium
phosphide. Am. Ind. Hyg. Assoc. J., 25: 376-379.
KADKOL, S.B. & JAYARAJ, P. (1967) Effect of phosphine-
fumigated rice on the growth of albino rats. Rep. Cent. Food
Technol. Res. Inst. (Mysore), 2: 6.
KAGAN, YU.S. (1985) Principles of pesticide toxicology,
Moscow, Centre of International Projects, GKNT, 176 pp.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1973)
The phytotoxic effect of repeated fumigation of certain cereal
crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 7: 57-62.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1974)
The phytotoxic effect of carbon bisulphide, methyl bromide and
hydrogen phosphide on the germination of seeds of certain cereal
field crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 8: 75-80.
KAO, C. (1981) Determination of aluminium phosphide in
fumigants. J. food Sci., 46: 1632-1634.
KASHI, K.P. (1975) A mixed indicator strip for phosphine
detection. Pestic. Sci., 6: 511-514.
KASHI, K.P. (1981a) Toxicity of phosphine to five species of
stored-product insects in atmospheres of air and nitrogen.
Pestic. Sci., 12: 116-122.
KASHI, K.P. (1981b) Response of five species of stored-product
insects to phosphine in oxygen-deficient atmospheres. Pestic.
Sci., 12: 111-115.
KASHI, K.P. (1982) Dose-mortality responses of five species of
stored-products insects to phosphine. Int. Pest Control,
March/April: 46-49.
KASHI, K.P. & CHEFURKA, W. (1976) The effect of phosphine of
absorption and circular dichroic spectra of cytochrome-c and
cytochrome-c oxidase. Pestic. Biochem. Physiol., 6: 350-362.
KASHI, K.P. & MUTHU, M. (1975) A mixed indicator strip for
phosphine detection. Pestic. Sci., 6: 511-514.
KIM, H.H., KIM, K.H., & HONG, S.S. (1977) Effect of pesticides
on exocrine pancreas and liver. Yonse Uidea Nonmunjip, 10(2):
170-180.
KLIMMER, O.R. (1969) [Contribution on poisoning by phosphine.
On the question of sub-chronic phosphine intoxication.] Arch.
Toxikol., 24: 164 (in German).
KOZHEMYAKIN, N.G., BUTYAGIN, V.N., BASHMURIN, A.F., & POTAPENKO,
M.I. (1971) [Veterinary-sanitary evaluation of the carcasses
and organs of animals which had to be killed in relation to
their contamination with zinc phosphide.] Sb. Rab. Leningrad
Vet. Inst., 32: 231-236 (in Russian).
KUHN, H., MAREK, J., & REIF, H. (1971) [Fumigation of tobacco
with phosphine.] Fachliche Mitt. Austria Tabakwerke AG, 12: 191-
203 (in German).
LADOU, J. (1983) Potential occupational health hazards in the
microelectronics industry. Scand. J. Work. environ. Health, 9:
42-46.
LEESCH, J.G. (1982) Accuracy of different sampling pumps and
detector tube combinations to determine phosphine
concentrations. J. econ. Entomol., 75: 899-905.
LEESCH, J.G. (1984) Fumigation of lettuce: efficacy and phyto-
toxicity. J. econ. Entomol., 77(1): 142-150.
LOWE, E.J. (1971) Phosphorus hydrides and phosphonium
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3.
LUCK, H. & ALLEMANN, A. (1984) Control of insect infestation
during storage of dairy products. Fumigation with hydrogen
phosphide. S. Afr. J. dairy Technol., 16(3): 103-107.
LUTZMANN, L., KEINITZ, M., & KLOSTERKOTTER, W. (1963)
[Emission of phosphine during transport of ferrosilicon.] Med.
Welt, 58: 114-116 (in German).
MCGIRR, J.L. (1953) Poisoning of livestock by the newer
rodenticides, insecticides and weed killers. In: Proceedings of
the XVth International Veterinary Congress, Stockholm, 9-15
August, Part 1, Vol. 1, pp. 479-480.
MCMAHON, R. & FORESE, F.F. (1983) Hydride gas detecting tape,
Glenview, Illinois, MDA Scientific Inc. (UK Patent Application
GB 2 108 662A).
MARSHALL, E.F. (1981) Efficacy variations of rodent baits with
the same active ingredient. Pest Control, January: 22-23.
MATHEW, G.G. (1961) The production of phosphine while
machining spheroidal graphite iron. Ann. occup. Hyg., 4: 19-35.
MEISSNER, R. (1924) [The toxicology of hydrogen phosphide.] Z.
gesamte Med., 42: 267 (in German).
MEREDITH, C. (1981) Toxicological studies on zinc phosphide,
Birmingham, University of Birmingham, Department of Bio-
chemistry (M.Sc. Thesis).
MINCHEV, T. & DIMITROV, D. (1970) [Early changes in the serum
of rabbits poisoned with zinc phosphide.] In: Vasilev, R.A., ed.
[ Nauch. Tr. Sud. Med. Deontol.,] Sofia, Meditsina i
Fizkultura, pp. 33-38 (in Bulgarian).
MUELLER, W. (1940) [Toxicity of phosphine (animal
experiments). Part 1. Acute and subacute toxicity.] Arch. exp.
Pathol. Pharmakol., 195: 184-193 (in German).
MUTHU, M., KRISHNAKUMARI, M.K., MURALIDHARA, V., & MAJUMDER,
S.K. (1980) A study on the acute inhalation toxicity of
phosphine to albino rats. Bull. environ. Contam. Toxicol., 24:
404-410.
NAKAKITA, H. & WINKS, R.G. (1981) Phosphine resistance in
immature stages of a laboratory selected strain of Tribolium
castaneum Herbst (Coleoptera: Tenebrionidae). J. stored Prod.
Res., 17: 43-52.
NAKAKITA, H., KATSUMATA, Y., & OZAWA, T. (1971) The effect of
phosphine on the respiration of rat liver mitochondria. J.
Biochem., 69: 589-593.
NATARAJAN, T. & BAGYARAJ, D.J. (1984) Fumigation effect on
microflora and viability of blackgram and fieldbean seeds.
Pesticides, 18(4): 40-42.
NAUS, A. (1982) [Olfactory thresholds of some industrial
compounds.] Prac. Lek., 34(6-7): 217-218 (in Polish).
NETHERLANDS, WERKGROEP VAN DESKUNDIGEN VAN DE NATIONALE MAC-
COMMISSIE. (1984) [ Report on the TLV of phosphine,] 2nd ed.,
Voorburg, Working Group of the National TLV Committee, 27 pp
(Report No. 1/80) (in Dutch).
NEUBERT, D. & HOFFMEISTER, I. (1960) Changes in intermediary
metabolism following exposure to hydrogen phosphide. Naunyn-
Schmiedeberg's Arch. exp. Pathol. Pharmakol., 239: 219-233.
NOACK, S., VON & REICHMUTH, C. (1981) [Determination of limits
for injuring animals and plants by phosphine and methyl bromide.
I. Studies on Drosophila melanogaster. Anz. schädlingskd.
Pflanzenschutz Umweltschutz, 54: 23-27 (in German).
NOACK, S., VON & REICHMUTH, C. (1982a) [Quantities of
phosphine, methyl bromide and hydrocyanic acid used in stored
products protection in the FRG from 1975-1977.] Nachrichtenbl.
Dtsch. Pflanzenschutzd., 34(2)S: 17-21 (in German).
NOACK, S., VON & REICHMUTH, C. (1982b) [Determination of
limits for injuring animals and plants by phosphine or methyl
bromide. II. Studies on water cress and lettuce Lactuca sativa
capitata.] Anz. schädlingskd. Pflanzenschutz. Umweltschutz,
55: 57-59 (in German).
NOACK, S., VON & WOHLGEMUTH, R. (1985) [Phosphine residues in
hazelnuts, soybeans and wheat after fumigation with non-constant
concentrations.] Z. Lebensm. Unters. Forsch., 180: 101-108 (in
German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1983)
[Relationship of PH3 residues after fumigation to concentration,
time of exposure and length of storage.] Z. Lebensm. Unters.
Forsch., 177: 87-93 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984a)
[Decomposition of phosphine in treated foods related to storage
temperature and aeration.] Z. Lebensm. Unters. Forsch., 178: 31-
37 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984b) [A
half-empirical mathematical model for calculation of PH3-
residues in hazelnuts fumigated with phosphine.] Z. Lebensm.
Unters. Forsch., 178: 97-103 (in German).
NOWICKI, T.W. (1978) Gas-liquid chromatography and flame
photometric detection of phosphine in wheat. J. Assoc. Off.
Anal. Chem., 61(4): 829-836.
PAZYNICH, V.M., MAZUR, I.A., & PODLOZNYI, A.V. (1984)
[Experimental substantiation and prediction of maximum
atmospheric phosphine concentrations differentiated by time.]
Gig. i Sanit., 1984(1): 13-15 (in Russian).
PRICE, N.R. (1984) Active exclusion of phosphine as a
mechanism of resistance in Rhyzopertha dominica F (Coleoptera:
Bostrychidae). J. stored Prod. Res., 20(3): 163-168.
PRICE, N.R. & DANCE, S.J. (1983) Some biochemical aspects of
phosphine action and resistance in three species of stored
product beetles. Comp. Biochem. Physiol., 76C(2): 277-281.
PRICE, N.R., MILLS, K.A., & HUMPHRIES, L.A. (1982) Phosphine
toxicity and catalase activity in susceptible and resistant
strains of the lesser grain borer (Rhyzopertha dominica).
Comp. Biochem. Physiol., 73C: 411-413.
RANGASWAMY, J.R. (1984) Simple spectrophotometric method for
determination of phosphine residues in wheat. J. Assoc. Off.
Anal. Chem., 67(1): 117-122.
RANGASWAMY, J.R. & MUTHU, M. (1985) Spectrophotometric
determination of phosphine residues in rice. J. Assoc. Off.
Anal. Chem., 68(2): 205-208.
REBMANN, Z. (1933) [On a new pesticide (Fosfolon).] Gesund-
heitstech. Städtehyg. ver. Städtische Tiefbau, 5: 280-284 (in
German).
REICHMUTH, C., ed. (1985) [Response of the granary weevil
Sitophilus granarius L. (Coleoptera: Curculionidae) to
gradually increasing and decreasing phosphine concentrations.]
Anz. schädlingskd. Pflanzenschutz Umweltschutz, 58(1): 10-16
(in German).
REICHMUTH, C., ed. (1986) The significance of changing
concentrations in toxicity of phosphine. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 8-21 March, London, Overseas Development
Administration, Tropical Development and Research Institute.
pp. 88-98.
REICHMUTH, C., NOACK, S., & WREDE, A. (1981) [Emission of
phosphine caused by fumigation for stored products protection.]
Nachrichtenbl. Dtsch. Pflanzenschutzd., 33(9): 132-136 (in
German).
ROALDSNES, J. (1982) [Phosphine poisoning due to machining of
ductile iron.] Norsk. bedv. h. tj., 3: 12-16 (in Norwegian).
ROBINSON, J.R. (1972) Residues containing phosphorus following
phosphine treatment: measurement by neutron activation. J.
stored Prod. Res., 8: 19-26
ROBINSON, J.R. & BOND, E.J. (1970) The toxic action of
phosphine. Studies with 32PH3: terminal residues in biological
materials. J. stored Prod. Res., 6: 133-146.
ROBISON, W.H. & HILTON, H.W. (1971) Gas chromatography of
phosphine derived from zinc phosphide in sugarcane. J. agric.
food Chem., 19(5): 875-878.
ROHRLICH, M. & MEUSER, F. (1970) [Studies on grain fumigated
with hydrogen phosphide. III. Communication: biochemical aspects
of phosphine fumigation.] Getreide Mehl, 20(1): 1-8 (in
German).
RUSCHEL, A.P. & DA COSTA, W.F. (1966) [Symbiotic fixing of
atmospheric nitrogen in the french bean ( Phaseolus vulgaris L).
III. Effect of some insecticides and fungicides.] Pesqui.
Agropecu. Bras., 1: 147-149 (in Portugese).
SAEED, T. & ABU-TABANJA, R. (1984) Determination of phosphine:
comparison of rates of desorption by purge-and-trap method and
by sulfuric acid treatment. J. Assoc. Off. Anal. Chem., 68(1):
61-64.
SATO, K. & SUWANAI, M. (1974) Absorption of hydrogen phosphide
to cereal products. Appl. Entomol. Zool., 9(3): 127-132.
SAYVETZ, T.A. (1982) Possible phosphine inhalation related to
warehouse fumigation, Richmond, Virginia, Virginia State Health
Department, Office of Health Protection and Environmental
Management, 6 p.
SCHITOSKEY, F. (1975) Primary and secondary hazards of three
rodenticides to kit fox. J. wildl. Manage., 39(2): 416-418.
SCHULZ, H. (1887) [Correction concerning the toxicity of
phosphorus compounds containing oxygen.] Arch. exp. Pathol., 23:
150-151 (in German).
SCHULZ, H. (1890) [Concerning phosphine.] Arch. exp. Pathol.
Pharmakol., 27: 314-335 (in German).
SHAHEEN, D.G., BRAGG, L.A., STANOVICK, R.P., & SULLIVAN, J.B.
(1983) Phosphine detector tubes as passive dosimeters for use
with metal phosphide fumigants. Tob. Int., 185(20): 36-39.
SHIVANANDAPPA, T., RAMESH, H.P., & KRISHNAKUMARI, M.K. (1979)
Rodenticidal poisoning of non-target animals: acute oral
toxicity of zinc phosphide to poultry. Bull. environ. Contam.
Toxicol., 23(4-5): 452-455.
SINGH, K.N., SRIVASTAVA, B.P., & NATH, G. (1983) Phosphine
residues and its effect on the germination of stored pulses.
Indian J. Entomol., 45(1): 71-80.
SRI (1982) Electronic chemicals, Menlo Park, California,
Standford Research International, pp. 40-41.
SRIDHARA, S. (1983) Rodenticide induced bait aversion and
neophobia in Tatera indica cuvieri. Z. angew. Zool., 70(4): 429-
440.
STEPHENSON, J.B.P. (1967) Zinc phosphide poisoning. Arch.
environ. Health, 15: 83-88.
TERZIC, Lj. (1981) Quantitative method for the determination
of zinc phosphide in technical products. Acta vet. (Beograd),
31(5-6): 279-288.
TIETJEN, H.P. (1976) Zinc phosphide: its development as a
control agent for black-tailed prairie dogs, Washington DC, US
Department of the Interior, Fish and Wildlife Research Centre
(Special Scientific Report Wildlife No. 195).
TKACHUK, R. (1971) Phosphine fumigation of wheat - residue
formation. Cereal Sci. Today, 16: 307.
TKACHUK, R. (1972) Phosphorus residues in wheat due to
phosphine fumigation. Cereal Chem., 49: 258-267.
TRIMBORN, H. & KLIMMER, O. (1962) [Experimental studies on
chemical changes in blood pigments caused by hydrogen
phosphide in vitro.] Arch. int. Pharmacodyn., 137(3-4): 331-
347 (in German).
UNDERWOOD, J.G. (1972) The radiochemical analysis of phosphine
residues in fumigated tobaccos. Tob. Sci., October 13: 47-49.
URGA, K. (1983) Methyl bromide and phosphine residues in
cereal grains and pulses. Ethiop. med. J., 21: 79-83.
US CFR (1981) Code of federal regulations, 29(1910): 673.
US EPA (1981) Pesticide registration standard: aluminium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-137514).
US EPA (1982) Pesticide registration standard: magnesium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-195777).
US EPA (1983) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 48(37): 7714-
7716.
US EPA (1984) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 49(92): 19922-
19923.
US EPA (1985a) Aluminium phosphide/magnesium phosphide:
tolerances for residues. Code Fed. Reg., 40: 180, 225, 291-292,
333, 375.
US EPA (1985b) EPA chemical profile: phosphine, Washington DC,
US Environmental Protection Agency.
US EPA (1986) Guidance for the re-registration of pesticide
products containing aluminium phosphide, February 1986. An
amendment to the registration standard issued in October 1981,
Washington DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (PB 84-241405).
US NIOSH (1977) National occupational hazard survey,
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, Vol. 3 (Publication No. 78-114).
US NIOSH (1979) Ten NIOSH analytical methods: phosphine,
Cincinnati, Ohio, National Institute for Occupational Safety
and Health, Division of Physical Sciences and Engineering, 42
pp.
VAN WAZER, J.R., ed. (1961) Phosphorus and its compounds:
technology, biological functions and applications, New York,
Interscience Publishers Inc., Vol. II, p. 957.
VAN WAZER, J.R. (1982) Phosphorus and the phosphides. In:
Kirk-Othmer, ed. Encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 17, pp. 473-489.
VDOVENKO, I.D., VAKULENKO, L.I., YAKOVLEVA, L.A., SHEDENKA,
L.I., BERESLAVSKAYA, A.N., & YATSENKO, V.S. (1984) [Effect of
surfactants on phosphine formation in steel pickling.] Ukr.
Khim. Zh., 50(4): 418-421 (in Russian).
VERGA, L. & BELLONI, F. (1958) [Over a case of purpura due to
empoisonment of phosphine (a haemato-clinical and histochemical
study.] Rass. Med. ind. Ig. Lav., 27(3): 230-241 (in Italian).
VERSTUYFT, A.W. (1978) Sampling and analytical methods for
phosphine - a review. Am. Ind. Hyg. Assoc. J., 39: 431-437.
VINSJANSEN, A. & THRANE, K.E. (1978) Gas-chromatographic
determination of phosphine in ambient air. Analyst, 103: 1195-
1198.
WARITZ, R.S. & BROWN, R.M. (1975) Acute and subacute
inhalation toxicities of phosphine, phenylphosphine and
triphenylphosphine. Am. Ind. Hyg. Assoc. J., 36: 452-458.
WEBLEY, D.J., HARRIS, A.H., & KILMINSTER, K. (1981) The use of
a portable gas analyser to measure phosphine concentrations in
experimental fumigations in the tropics. Int. pest Control,
March/April: 47-50.
WILSON, A. (1971) The metal phosphides. In: Mellor's compre-
hensive treatise on inorganic and theoretical chemistry, White
Plains, New York, Longman, Vol. VIII, Suppl. III.
WILSON, R., LOVEJOY, F.H., JAEGER, R.J., & LANDRIGAN, P.L.
(1980) Acute phosphine poisoning aboard a grain freighter.
Epidemiologic, clinical and pathological findings. J. Am. Med.
Assoc., 244(2): 148-150.
WINKS, R.G. (1970) Phosphine fumigation of farm-stored
tobacco. Queensland agric. J., November.
WINKS, R.G. (1984) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): time as a dosage factor. J.
stored Prod. Res., 20(1): 45-56.
WINKS, R.G. (1985) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): phosphine-induced narcosis. J.
stored Prod. Res., 21(1): 25-29.
WHO (1973) WHO Technical Report Series, No. 513 (Twentieth
Report of the Expert Committee on Insecticides), Geneva, World
Health Organization, p. 39.
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food, Geneva, World Health Organization, pp. 289-295 (WHO
Pesticide Residue Series, No. 1).
WHO/FAO (1976) Data sheet on pesticides No. 24: zinc
phosphide, Geneva, World Health Organization (VBC/DS/77.24).
WHO/FAO (1980) Data sheet on pesticides No. 46: phosphine,
Geneva, World Health Organization (VBC/DS/80.46).
WORTHING, C.R. & WALKER, S.B., ed. (1983) The pesticide manual:
a world compendium, 7th ed., London, British Crop Protection
Council.
ZIEMER, U. (1963) [Fatal hydrogen phosphide poisoning on a
freighter.] Zbl. Arbeitsmed., 13: 38-39 (in German).
ZIPF, K.E., ARNDT, TH., & HEINTZ, R. (1967) [Clinical
observation of a case of Phostoxin poisoning.] Arch. Toxikol.,
22(4): 209-222 (in German).
ZUTSHI, M.K. (1966) Effect of aluminium phosphide (Phostoxin)
on the germination of certain cereal and vegetable seeds. Bull.
grain Technol., 4(4): 197-199.
See Also:
Toxicological Abbreviations
 Neubert & Hoffmeister (1960) investigated the effects of
short-term exposures ranging from 460 to 2150 mg phosphine/m3 on
cellular respiration in rats. The oxidation of alpha-keto-
glutarate in liver cells was inhibited, but not oxidative
phosphorylation. Alpha-ketoglutarate oxidation was also reduced
in heart muscle, and phosphorylation was disrupted. Survival of
groups of 8 rats at various concentrations of diphosphine-free
phosphine was studied with the following results (Table 14).
Table 14. Survival times of 8 rats in different concentrations of
phosphine. Concentration x time products calculated from
the mid-point of the range of survival timesa
---------------------------------------------------
Concentration Survival Concentration x time
(mg/m3) (min) product (mg x min/m3)
---------------------------------------------------
4640 16 - 20 83 520
1000 50 - 70 60 000
320 150 - 200 56 000
100 400 - 600 50 000
---------------------------------------------------
a From: Neubert & Hoffmeister (1960).
If the use of the mid-point of the range of survival times
is reasonable, these results indicate that the relationship
between concentration and survival time is not simply
reciprocal, and that the index of t is greater than 1, but less
than the value of 1.43 derived from the data quoted by US EPA
(1981, 1982).
Klimmer (1969) determined lethal concentration x time pro-
ducts for rats and calculated, for example, 19 000 mg x min/m3
for a concentration of 287 mg/m3, but these results do not agree
with those calculated from the data of US EPA (1981, 1982) nor
with those of Neubert & Hoffmeister (1960). The value of the
concentration x time product, as a measure of phosphine dose in
mammals, is therefore open to question, as it is in insects.
8.1.2. Inhalation studies on zinc phosphide
The US National Pest Control Association submitted a value
of 19.6 mg/litre for an inhalation LC50 of 10% zinc phosphide
powder in rats. It was not stated whether the value was for
pure zinc phosphide or the 10% dilution and there was no
indication of exposure time (US EPA, 1983).
8.1.3. Oral studies on metal phosphides
In a study on 35 rats of both sexes administered doses of
20, 40, 50, or 80 mg/kg, Dieke & Richter (1946) reported an LD50
for zinc phosphide of 40.5 ± 2.9 mg/kg body weight for wild
Norway rats. Schitoskey (1975) reported an LD50 for the kit fox
of 93 mg zinc phosphide/kg body weight. Aminzhanov (1972)
reported that 100 mg/kg body weight of zinc phosphide was fatal
for starved dogs but not for dogs that were fed. Ikimanova
(1977) reported increased blood- and urinary-porphyrin concen-
trations in rats dosed with zinc phosphide. Other authorities
refer to 27 mg/kg body weight for an acute oral LD50 value for
94% pure zinc phosphide in rats (US EPA, 1983).
8.1.4. Dermal and other studies on metal phosphides
An acute dermal LD50 of 2 000 - 5 000 mg/kg body weight for
zinc phosphide (94% Zn3P2) in rabbits was submitted by the US
National Pest Control Association (US EPA, 1983).
8.2. Short-term Exposures
8.2.1. Inhalation exposure to phosphine
Jokote (1904) determined cumulative survival times in inter-
mittent phosphine exposure as shown in Table 15.
Table 15. Survival time for rabbits, cats, and guinea-pigs at 3
concentrations of phosphinea
-----------------------------------------------------------
Concentration of phosphine (mg/m3)
14 35 140
-----------------------------------------------------------
Survival time (h) 16 - 30 8.5 - 12 2.5 - 3.5
(rabbits, cats, and
guinea-pigs)
-----------------------------------------------------------
a From: Jokote (1904).
Miller (1940) exposed rabbits and guinea-pigs for 4 h per
day to various concentrations of phosphine. At 28 mg/m3 (20
ppm), both rabbits and guinea-pigs died during or after the
second exposure. At 14 mg/m3 (10 ppm), rabbits survived 7 - 14
successive exposures, but at 12 mg/m3 (8.3 ppm), only 4 or 5
exposures. In another study in the series, 2 rabbits that had
had five 4-h exposures to phosphine at 7 mg/m3 were accidentally
exposed to 20 mg/m3 (14 ppm) on the sixth day. Both rabbits
died during this exposure. The authors concluded that
pretreatment with sub-lethal concentrations of phosphine reduced
resistance to near-lethal concentrations. At low concentrations
(up to 14 mg/m3), animals displayed no signs until about 1/2 h
before death when they exhibited diminished reactivity, became
stuporose with shallow respiration, and died in coma.
Occasionally, animals died following exposure, with symptoms of
pulmonary oedema. At 28 mg/m3 or more, all animals exhibited
signs of respiratory irritation and died of pulmonary oedema.
Pathological examination of the lungs revealed bronchiolitis and
atelectasis of the lungs. There was no evidence of haemolysis,
but all organs were hyperaemic. The liver showed fatty
infiltration and there was cloudy swelling of kidney tubular
cells. After prolonged exposure, lungs frequently contained a
brown pigment that did not stain with Prussian Blue and
therefore was not iron-containing.
Neubert & Hoffmeister (1960) exposed female Wistar and E-B
(inbred strain) rats in a 121-litre chamber repeatedly for 6
days to 681 mg/m3 phosphine for 10 - 20 min per day. On the
seventh day, they were exposed until they died (22 - 35 min).
The concentration x time product for lethality after 6 days
pretreatment (about 20 mg x min/litre) was about one third of
that for a first exposure (48 - 87 mg x min/litre) and the
authors concluded that the effects of exposure were cumulative,
rather than that the phosphine accumulated in the body.
Klimmer (1969) exposed cats, guinea-pigs and rats to
phosphine concentrations of 1.4 and 3.5 mg/m3 (1 and 2.5 ppm)
for 4 - 6 h per day, 6 days a week, for a total of more than
800 h over a 24-week period without any clinical, laboratory, or
pathological evidence of effects. The liver function in the
cats, measured by bromosulphthalein (BSP) excretion, was normal.
In cats (3), guinea-pigs (3), and rats (10) exposed to phosphine
at 7 mg/m3 (5 ppm) for 6 - 8 h per day, the cats became
apathetic, showing anorexia after exposure, but exhibiting
thirst. Later, they developed unsteadiness, vomiting,
agitation, dyspnoea, and apnoea, before cardiac arrest after a
total of 35.5 - 45.5 h of exposure. The haemoglobin concen-
tration and erythrocyte count were reduced by 10 - 20% compared
with initial values, there was proteinuria and delayed BSP
excretion. Post-mortem examination revealed congestion of all
organs, with oedema and focal emphysema and red pigmentation of
the lungs. The guinea-pigs exhibited signs that were similar
to, but more marked than, those in the cats, and 2 animals had
asphyxial convulsions. Blood tests showed no deviations from
normal values, but the blood contained a brown coloration that
was not due to methaemoglobin, since spectroscopy revealed the
absorption bands of oxyhaemoglobin only. The guinea-pigs died
on the sixth day after 24 h - 32 h cumulative exposure, and
post-mortem examination revealed pulmonary oedema and red
discolouration and congestion of other organs. The rats died
after 27 - 36 h cumulative exposure. All exhibited congestion
of organs, 3 had pulmonary oedema and 7 had proteinuria. A
second series of exposures under slightly changed exposure
conditions at a different time of year produced qualitatively
similar results. In both series, neurohistological studies in
rats showed widening of the perivascular spaces, vacuolation of
the nuclei of ganglion cells, a reduction in the Purkinje cells,
and a glial reaction. Similar, but less marked, changes without
glial reaction were seen in the cats and guinea-pigs. There was
no control group in these studies, and certain observations in
exposed animals were dismissed on the grounds that they had
previously been seen in unexposed animals.
Waritz & Brown (1975) exposed Charles River-CD rats to
phosphine for 4 h per day, for 12 days, at a concentration of
5.5 mg/m3 (0.163 µmol/litre). The rate of weight gain was
reduced during the exposure period. Although the authors stated
that the rate returned to normal during a 14-day post-exposure
observation period, their data indicate a uniformly reduced rate
of weight gain throughout the exposure and recovery phases.
Post-mortem examination of rats, sacrificed both at the end of
the exposure period and at the end of the recovery period,
revealed no gross or microscopic abnormalities.
The effects of a 1.5-month exposure of white rats to
phosphine at concentrations of 0.05, 0.2, 1.5, and 8 mg/m3 were
reported by Pazynich et al. (1984). There were changes in blood
cholinesterase, peroxidase, and catalase activity and in
phagocyte behaviour. The magnitude of the changes was large,
but not generally dose-related. On the basis of their results,
the authors recommended mean exposure limits for urban air for
exposure durations of 24 h, one month, and one year of 0.004,
0.0015, and 0.001 mg/m3, respectively, with a ceiling value of
0.01 mg/m3, and this recommendation has been adopted in the
USSR.
In a study of the effects of phosphine on rats by inhalation
at 0.1 mg/m3 and 0.05 mg/m3, Atchabarov et al. (1984) found a
reduction in total plasma protein without a change in the
relative proportions of the various fractions, a modest but
significant reduction in the glycoprotein A fraction with some
changes in the relative proportions of the sub-fractions,
increased plasma bile acids, and a marked increase in
seromucoids. Liver glycogen, lipids, and cytochrome oxidase
levels were reduced. Biochemical changes were similar to those
in a control group treated with hydrogen fluoride at a known
toxic level.
8.2.2. Oral exposure to metal phosphides
Bai et al. (1980) reported a 13 week feeding study in female
weanling albino rats. Zinc phosphide was mixed with the diet at
0 (control), 50 mg/kg (50 ppm), 100 mg/kg (100 ppm), 200 mg/kg
(200 ppm), and 500 mg/kg (500 ppm) w/w. Deaths occurred at the
2 higher dosage regimes in 1/12 and 10/12 animals, respectively.
Food intake and weight increase were reduced and dose-dependent
depilation occurred at all dosages. The relative weights of
liver, heart, brain, and thyroid were increased at 200 and
500 mg/m3 and the serum zinc and liver alkaline phosphatase
levels were increased at the highest dose. There was a dose-
dependent reduction in haemoglobin concentration, red cell
count, and haematocrit.
The WHO/FAO Data Sheet on Pesticides (No. 24) which deals
with zinc phosphide (WHO/FAO, 1976) cites a study in which
300 mg zinc phosphide/kg administered to rats produced 6/6
deaths in the second week while 200 mg/kg produced reduced
weight gain and 2/6 deaths. Histopathological studies revealed
liver damage in the peripheral and central lobular areas and the
lungs were congested with haemorrhage or exudate in the alveolar
spaces.
8.2.3. Dermal and other exposures
No reports are available on short-term studies involving the
dermal or other routes.
8.3. Skin and Eye Irritation; Sensitization
Zinc phosphide powder moistened with saline produced mild
skin irritation in rabbits (Draize method) (Rao, 1986).a
8.4. Long-Term Exposure
No long-term studies on phosphine or metal phosphides
exposure have been reported.
8.5. Reproductive, Mutagenicity, and Carcinogenicity
There have been no long-term reproductive, mutagenicity, or
carcinogenicity studies on phosphine or metal phosphides.
---------------------------
a Personal communication to the IPCS Task Group on Phosphine
and Metal Phosphides.
8.6. Factors Modifying Toxicity; Toxicity of Metabolites
Aminzhanov (1972) reported that 100 mg zinc phosphide/kg
body weight was lethal for dogs starved for 24 h beforehand but
not for dogs that were fed.
Phosphine is not a powerful reducing agent and its toxic
effects are unlikely to be associated with chemical reduction.
The recent discovery that phosphites and phosphorous acid have a
fungicidal activity (Bompeix & Saindreman, 1984) suggests that
metabolites of phosphine have biological effects and therefore
might contribute to its toxicity. However, this contribution
can only be small, since phosphine is only slowly oxidized to
hypophosphites and phosphites and a maximum of about 40% of the
phosphide dose is recovered as these metabolites, even with
small doses. Moreover, the toxicity of phosphites is much less
than that of phosphides (Schulz, 1887). The contribution of
toxicity of contaminants of phosphine such as diphosphides is
uncertain.
8.7. Mechanisms of Toxicity - Mode of Action
Studies on isolated rat liver showed that mitochondrial
oxygen uptake was inhibited by phosphine (Nakakita et al., 1971)
and that this effect was due to the reaction of phosphine with
cytochrome c and cytochrome c oxidase (Kashi & Chefurka, 1976).
Although this inhibitory in vitro effect was also shown in
insects, it was found that insects severely poisoned with
phosphine did not suffer any inhibition of their cytochrome
system (Price & Dance, 1983). In the same report, phosphine was
found to inhibit insect catalase though this appeared to be an
indirect effect and might have been a result of phosphine
toxicity rather than a cause.
There have not been any systematic studies on the mechanism
of phosphine toxicity. Various effects on intermediary
metabolism have been described. Ikimanova (1977) reported
increased blood- and urinary-porphyrin concentrations, which
were related to the dose of zinc phosphide and the duration of
treatment. In a study on rabbits, Minchev & Dimitrov (1970)
reported changes in serum glutamic-pyruvic and glutamic-
oxaloacetic transaminases, leucine aminopeptidase, aldolase,
alkaline phosphatase and albumin in the first 24 h of zinc
phosphide poisoning. Dysfunction of hepatic fat metabolism was
also observed. Loss of cell viability and cell membrane
integrity accounted for the raised hepatic enzymes, the
bronchiolitic effect, the cloudy swelling of renal tubular cells
and the occasionally reported haemorrhagic lesions in the
myocardium. There is no adequate explanation for the fact that
phosphine does not cause the haemolysis characteristic of
arsine.
9. EFFECTS ON MAN
9.1. Organoleptic Effects
The odour of phosphine depends on the impurities it con-
tains; phosphine of high purity has no odour, even at 280 mg/m3
(Dumas & Bond, 1974; Fluck, 1976). Phosphine prepared conven-
tionally, without purification, has a fishy or garlic-like odour
attributed to impurities. These may be adsorbed by stored pro-
ducts during fumigation with resultant loss of odour, even
though phosphine remains at toxic concentrations (Bond & Dumas,
1967). Amoore & Hautala (1983) reviewed the literature on odour
thresholds and stated that the geometric mean of all the 6
reported values for phosphine was 0.71 mg/m3 (0.51 ppm). Fluck
(1976) reported that thresholds for phosphine at which 50% or
more of 10 persons could positively identify the odour asso-
ciated with phosphine differed according to the source of the
phosphine (Table 16).
Table 16. Odour thresholds for phosphinea
--------------------------------------------------------------
Source Odour thresholds for
PH3 (mg/m3 converted
from ppm)
--------------------------------------------------------------
Technical aluminium phosphide + H2SO4 0.14 - 0.28
Phosphorium iodide + aqueous hydroxide 2.8
Phostoxin + H2SO4 0.014 - 0.028
(5 Å molecular seived) 1.4 - 2.8
Phostoxin + H2SO4
--------------------------------------------------------------
a From: Fluck (1976)
Amoore & Hautala (1983) classified phosphine in safety class
D in their classification because 20 - 50% of attentive persons
can detect the threshold limit value (TLV) (0.42 mg/m3) by
smell. However, the smell of phosphine cannot be relied on to
warn of toxic concentrations.
9.2. General Population Exposure
9.2.1. Phosphine
There is negligible exposure of the general population to
phosphine. Gessner (1937) described an incident in which the 12
inhabitants of an apartment house developed nausea and one died
when phosphine was emitted from an adjacent warehouse containing
bags of aluminium phosphide, which became damp. Some passengers
on ships and barges carrying cargoes of ferrosilicon or grain
under fumigation have also been poisoned by phosphine (Harger &
Spolyar, 1958; Netherlands, 1984). Effects were similar to
acute occupational poisoning.
Hallerman & Ribilla (1959) reported the deaths of 2 adults
and a child living in a dwelling with a party wall to a granary
being fumigated. Symptoms were non-specific and insidious
initially and illustrate the risks of sustained exposures to
relatively low concentrations (a few mg phosphine/m3); it was
estimated that the concentration in the bedroom reached
1.2 mg/m3. At autopsy, there was congestion of all organs.
Pulmonary oedema and focal emphysema were found in the lungs and
there was vacuolation in the liver.
9.2.2. Metal phosphides
Zinc phosphide baits and formulated aluminium phosphide
pellets are widely used. Occasional accidental, or more usually
suicidal, exposure to metal phosphides may be encountered.
Ingestion, the only easily toxic route, has almost always been
suicidal and the effects acute (Stephenson, 1967).
Stephenson (1967) reviewed 20 cases of acute zinc phosphide
poisoning by ingestion (including one treated by himself) in
which the approximate dose was recorded. Ten cases were fatal
and the doses ranged from 4.5 to 180 g; 6 cases had ingested
20 g or more. In the 10 non-fatal cases, the doses ranged from
0.5 to 50 g with 7/10 ingesting less than 20 g. In the case
treated by the author, clinical features included metabolic
acidosis, hypocalcaemic tetany, methaemalbuminaemia, and reduced
blood coagulation (thrombotest 28% of normal). Post-mortem
findings included blood in all the serous cavities, pulmonary
congestion and oedema, haemorrhagic changes in the intestinal
epithelium, centrilobular congestion and necrosis and yellow
discoloration of the liver, and patchy necrosis of the proximal
convoluted tubules of the kidneys.
An unsuccessful suicidal attempt was described by Zipf
(1967). A 25-year-old man ingested 6 tablets of PhostoxinR in
water (= 6 g phosphine). Immediate symptoms were severe retro-
sternal pain, a generalized burning sensation, and vomiting.
There was circulatory collapse necessitating resuscitation and
he was also treated by gastric lavage with potassium perman-
ganate and magnesium sulfate. Subsequently, symptoms of cardiac,
cerebral, and hepatic dysfunction appeared and there was severe
renal failure requiring haemodialysis.
9.3. Occupational Exposure
Cases of acute phosphine poisoning reported in the litera-
ture were reviewed by Harger & Spolyar (1958). Fifty-nine cases
with 26 deaths had been recorded since 1900 (including Gessner's
(1937) non-occupational cases). In 6 out of 11 papers, cargoes
of ferrosilicon were cited as the source of phosphine and, in
these cases, the victims were passengers or crew members of the
ships or barges concerned. Other cases involved the exposure of
welders to calcium carbide and raw acetylene and of submariners
to sodium phosphide. The most common autopsy finding was
congestion of the lungs with marked oedema. The authors added a
case of their own in which a 16-year-old youth operating
acetylene generators died, probably as a result of phosphine
exposure at a level of about 11 mg/m3 (8 ppm) for 1 - 2 h daily
over a period of 6 weeks. Arsine and hydrogen sulfide exposures
were considered to have been too low to have accounted for the
death. Autopsy revealed acute pulmonary oedema. Other cases
have been described, all of which exhibited similar features
(Ziemer, 1963; Furuno et al., 1976; & Netherlands, 1984). Three
groups of symptoms of acute phosphine poisoning were described
by Childs & Coates (1971): (a) nervous symptoms including
headache, vertigo, tremors, and unsteady gait, progressing in
severe cases to convulsions, coma, and death; (b) gastro-
intestinal symptoms include loss of appetite, thirst, nausea and
vomiting, diarrhoea, and severe epigastric pain; and (c) respir-
atory symptoms including a feeling of pressure and pain in the
chest as well as shortness of breath. In addition, a sharp fall
in blood pressure was described as a characteristic, but seldom-
mentioned symptom. Chronic effects include anaemia, bronchitis,
and gastrointestinal, speech, and motor disturbances, but these
are by no means general.
The toxic effects of phosphine are summarized in Table 17.
The IDLH (Immediately Dangerous to Life or Health) level is
282 mg/m3 (200 ppm) (US EPA, 1985b).
Verga & Belloni (1958) described a case of purpura ascribed
to poisoning by phosphine. The platelet count was reduced to
60 000/mm3; the red-cell count was also low at 3.1 x 106/mm3 and
the haemoglobin concentration (Sahli) was 55%. With recovery,
both the red-cell and thrombocyte counts increased to 4.8 x 106
and 210 000/mm3, respectively, at the end of the observation
period. The electrolysis of phosphate solutions being applied
to metals as a corrosion inhibitor was considered to have
produced phosphine, but there was no measurement of the level of
exposure and it is possible that the exposure also included
arsine, which would account for the anaemia with a hyperplastic
erythroid series in the marrow biopsy. While haemolysis is
recognized as a complication of arsine poisoning, purpura and
thrombocytopenia are not widely recognized features of
phosphine poisoning, though Borodin et al. (1983) described
changes related to haemolysis (prolongation of clotting time and
reduced plasminogen levels) in workers exposed to a variety of
biocides and disinfectants, including zinc phosphide. However,
these changes could not be related specifically to exposure to
phosphine.
Table 17. Toxic effects of phosphinea
------------------------------------------------------------
Effect Concentration
mg/m3 ppm
------------------------------------------------------------
Rapidly fatal 2800 2000
Death after 1/2 - 1 h 560 - 840 400 - 600
Dangerous to life after 1/2 - 1 h 400 - 600 290 - 430
Serious effect after 1/2 - 1 h 140 - 260 100 - 190
No serious effects after 1/2 - 1 h 10 7
------------------------------------------------------------
a From: Childs & Coates (1971).
In 1978, Beloskurskaya et al. published the findings of a
study on 206 phosphorus workers, which revealed 57 cases of
toxic hepatitis. In 49 of these cases, there was stated to be no
other apparent cause than occupational exposure to phosphorus,
phosphine, or phosphorus oxides. No measurements of exposure
were reported. Diagnostic criteria included a history of
hypochondrial pain, clinical hepatomegaly, and abnormal liver
function tests and 131I-Rose Bengal scanning and clearance
studies. Forty-six of the 57 cases had additional symptoms,
such as the astheno-vegetative syndrome, chronic gastritis,
impotence, toxic encephalopathy, toxic cardiomyopathy, and
respiratory disease. There was no control group and no informa-
tion on alcohol consumption was provided.
In a later study, Wilson et al. (1980) described the
symptoms of acute poisoning by phosphine as headache, fatigue,
nausea, vomiting, jaundice, paraethesia, ataxia, tremor,
diplopia, myocardial infiltration with necrosis, pulmonary
oedema, and myocardial and peripheral muscle damage. Laboratory
results indicated that there had been urinary tract involvement
(occult blood) and levels of several liver enzymes were
elevated. Furthermore, Roaldsnes (1982) reported that metal-
workers at a large shipyard in Norway, drilling deep holes in
spheroidal graphite iron, became ill during work. The symptoms
were mostly nausea, dizziness, chest tightness, dyspepsia, and
disturbances of smell and taste. Measurement of the phosphine
concentration in the workers' breathing zone (with Dräger tubes)
showed a phosphine concentration of about 1.4 mg/m3 (1 ppm).
After installing local exhaust ventilation on the drilling
machines, there were no longer any measurable amounts of
phosphine, and there were no complaints from the workers. When
the local exhaust ventilation was removed for technical reasons
5 years later, illness among the workers recurred. Measurement
of phosphine levels just above the machines, showed concentra-
tions of up to 56 - 70 mg/m3 (40 - 50 ppm). When the local
exhaust ventilation was re-installed, the phosphine concentra-
tions dropped to unmeasurable amounts, and no further cases of
illness were reported. Addition of copper sulfate solution to
the cutting oil effectively removed the phosphine gas, but
resulted in problems of corrosion.
Jackson & Elias (in press) studied liver function and
gastrointestinal symptoms in: (a) workers exposed to white
phosphorus at up to 5 times the occupational exposure limit and
also to some phosphine (0 - 0.5 mg/m3); (b) workers exposed to
phosphine alone (at up to 2.8 mg/m3, depending on the efficacy
and use of air-line breathing apparatus); and (c) gastro-
intestinal symptoms in a group of workers who were not exposed
to either compound, but who worked the same shift pattern.
Complaints to the factory medical department of gastrointestinal
symptoms in the phosphine-exposed group, in the phosphorus
exposed-group, and in the unexposed group were 1.51, 0.61, and
0.24 per man per year, respectively. However, the alcohol
consumption in the phosphine- and phosphorus-exposed groups
differed (289 and 154 ml per week, respectively). The alcohol
consumption was not estimated in the control group. None of the
conventional liver function tests reflected the differences in
occupational exposure or alcohol intake. The phosphine-exposed
group did have a significantly raised post-prandial bile acid
concentration, but whether this reflected a difference in phos-
phine exposure or alcohol consumption could not be determined.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Phosphine and metal phosphides are only focally distributed
in the environment and exposure of large numbers of the general
population is unlikely. The sources and uses of phosphine and
metal phosphides are sufficiently well established for safety
techniques to be generally known, though it is disturbing that
shipboard poisonings are still occurring when the first case was
reported over half a century ago. The extensive world-wide use
of metal phosphides as fumigant sources and as rodenticides
creates the hazard of accidental poisoning in children and
others and provides a means of suicide, unless the supply and
use of these materials is appropriately regulated. There is no
evidence of danger to the population from residues of phosphine
in fumigated foods or from residues of phosphides, if adequate
fumigation and aeration techniques are used. Permitted limits
for residues are in the range of 0.01 - 0.1 mg total phosphine/
kg. There do not appear to be any deleterious effects on food
quality as a result of adequately undertaken fumigation with
phosphine.
Published data on occupational exposure to phosphine in most
industries are not sufficient to evaluate the success of control
measures in relation to occupational exposure limits. There is
also some disagreement regarding these limits and some data
suggest that an occupational exposure limit of 0.4 mg
phosphine/m3 is above the threshold for biological effects with
long-term exposure. However, there are no studies adequate to
settle this question definitively.
Major accidental release of stored phosphine presents a
serious explosion/fire hazard and an acute toxic hazard for man
and animals.
10.2. Evaluation of Effects on the Environment
Only very small amounts of phosphine and phosphides occur in
the environment as a result of natural processes and those
resulting from human activity are not persistent.
Phosphine and phosphides are introduced into the environment
for the control of pests, for which they are particularly
suitable, because of their efficacy, lack of persistence, and
harmless decomposition products.
Careful positioning of bait and the low toxicity of
carcasses for scavengers of poisoned animal targets minimize the
effects of rodenticidal metal phosphides on non-target species.
Phosphine and phosphides are oxidized in the environment and the
final product, phosphate, is normally widely present in the
natural environment. Phosphine and phosphides are only used
where the natural environment is already modified by human
activity and their environmental impact must be viewed in this
context.
Since resistance to phosphine in insects does not appear to
reverse in the absence of selective pressures, it is
particularly important to achieve effective doses of phosphine
by using adequate treatment rates and good practices. In
practice, the development of resistance is more of a problem
than the effects on non-target species. Major accidental
release of stored phosphine would not result in long-term
environmental consequences.
10.3. Conclusions
In general, there is no risk to the public from the use of
phosphine or phosphides, from emissions from fumigated products
or spaces, from alloys, industrial processes, or residues in
food, provided that proper fumigation, transport, and industrial
practices are used.
While phosphine and metal phosphides are toxic, their
extensive use in occupational settings has resulted in the
establishment of good practice guidelines. If these are
followed, there is a low order of risk for human health.
However, there is some doubt about the completeness of
protection afforded by the higher occupational exposure limit
(0.4 mg phosphine/m3).
There is no risk of important environmental effects from
phosphine or metal phosphides.
11. RECOMMENDATIONS
11.1. Gaps in Knowledge
1. The principal area related to human health where information
is inadequate is concerned with occupational exposure levels and
their safety. There is a need for an adequate study of well-
being and organ function in exposed and control populations,
with a complete description of phosphine exposure, concurrent
exposures to other chemicals (which should be low), alcohol
consumption, and smoking habits. The establishment of exposure
levels that are free from any detectable effects would help to
determine agreed occupational exposure limits.
2. It would be of assistance, in this regard, to undertake
laboratory studies to identify biological markers that are most
indicative of phosphine effects and reflect the suggested short-
term cumulative effects of repeated phosphine exposure.
3. Further research on the mechanisms of action of phosphine
and phosphides will assist in the design of good fumigation
practice and fumigation schedules, and minimize the occurrence
of resistance in target organisms and associated problems.
4. It has been suggested that zinc phosphide may be relatively
persistent in aquatic sediments and there is a general lack of
aquatic toxicological data. There is a need for research on the
levels and persistence of zinc phosphide in sediments and for
further studies of its effects on aquatic organisms.
11.2. Preventive Measures
11.2.1. Management
The most important factor in the safe handling of phosphine
and metal phosphides, and in their formulation, is proper work
practices.
Management should identify these, provide training for the
operatives, and ensure that the practices are carried out.
Personal protection measures recommended to reduce the
likelihood of absorption of phosphide preparations include the
wearing of:
(a) synthetic rubber gloves;
(b) rubber boots;
(c) lightweight impervious overalls; and
(d) suitable eye protection.
Adequate washing facilities should be available at all times
during handling. Eating, drinking, and smoking should be
prohibited during handling, and before washing after handling.
The means to measure concentrations of phosphine in air should
be available and used to check atmospheric concentrations. When
necessary, respiratory protective equipment should be worn. In
fumigation, each operator or other person liable to be exposed
to the gas must be provided with an efficient means of respira-
tory protection. Persons exposed to magnesium phosphide or
aluminium phosphide powders (or other readily hydrolysed
phosphides), which may give rise to airborne dust should be
protected by respiratory protective equipment, effective against
gaseous phosphine, since hydrolysis of dust in the filter of a
dust mask or respirator may give rise to high phosphine
exposure.
11.2.2. Treatment of poisoning
11.2.2.1 Inhalation of phosphine
(a) First Aid
Remove from exposure, keep at rest. Rescuers should follow
full safety procedures.
If the patient is unconscious, place in semi-prone recovery
position or otherwise maintain the airway.
If the patient is conscious but has difficulty in breathing,
treat in a seated position and give oxygen if available.
Otherwise, allow the patient to recline with the legs slightly
elevated.
If breathing stops, immediately ventilate the patient
artificially (mouth-to-mouth/nose or mechanically with oxygen,
if available).
If the heart stops, begin cardiopulmonary resuscitation
(CPR).
(b) Medical Treatment
1. Give oxygen by mask if required; perform baseline chest X-
ray and examine chest. Treat shock conventionally.
2. All patients should be observed for 48 - 72 h, as onset of
pulmonary oedema may be delayed.
3. If respiratory distress is severe, give steroids (methyl-
prednisolone 30 mg/kg, or equivalent, intramuscularly),
preferably within 4 h of exposure.
4. If pulmonary oedema occurs, treat by positive and expiratory
pressure ventilation. Antibiotics should only be given if a
secondary infection is present.
5. Treat any fits conventionally; give general supportive care
with particular attention to fluid balance.
6. Some sources of exposure to phosphine are likely to lead
also to exposure to arsine, which has haemolytic effects.
Monitor for haemoglobinaemia, haemoglobinuria, unconjugated
hyperbilirubinaemia, and renal failure.
In general, recovery is rapid following removal from
exposure, but renal damage and leukopenia may occur after
several days.
11.2.2.2 Ingestion of metal phosphides
(a) First aid
Do not give milk, fats, or saline emetics by mouth.
Give oxygen if there is respiratory distress.
If first aiders are medically authorized to do so, and the
patient is conscious, induce vomiting.
After 20 min (or after vomiting), administer activated
charcoal (50 g in water by mouth) if available.
Obtain medical attention as soon as possible: preferably
send immediately to hospital.
(b) Medical treatment
1. Consider tracheal intubation and gastric lavage with 2%
sodium bicarbonate solution (to limit hydrolysis of zinc
phosphide).
2. Activated charcoal or medicinal liquid paraffin may limit
absorption of phosphine and zinc phosphide, respectively,
and may be administered by mouth or stomach tube.
3. Monitor and support vital functions, particularly hepatic
and renal function. Treat shock conventionally.
5. Hepatic and renal failure should be treated in specialist
centres.
11.2.3. Leaks, spillages, residues, and empty containers
11.2.3.1 Phosphine
Small leaks and residues of compressed gas can be discharged
slowly to the atmosphere in the open air. Larger quantities
should be burned using an appropriate burner.
11.2.3.2 Aluminium and magnesium phosphides and their formulated
preparations
Spillages and residues in containers will evolve phosphine
for several days by reaction with atmospheric moisture. Respir-
atory protective equipment will be required by those dealing
with them. Even if atmospheric concentrations of phosphine are
initially low, they may rise as residues are disturbed.
Moderate quantities should be collected in a secure place to
protect the public and wildlife, and left in the open air and
kept moist until hydrolysis is complete.
Larger amounts should be removed to a deep pit, at an
approved site, until phosphine evolution is complete. Then they
can be buried.
Residues at the site of spillage should be washed away in a
large quantity of water and the area kept secure and aerated
until checked for zero gas concentration.
Combustible packages can be incinerated at high temperature
(> 1000 °C) using proper facilities. Containers should NOT be
cleaned for re-use, but should be disposed of by deep burial, at
an approved site, well away from habitation and where there is
no danger of contamination of water sources.
11.2.3.3 Zinc phosphide and preparations
Zinc phosphide hydrolyses only slowly. Nevertheless,
phosphine may be evolved and respiratory protective equipment
should be available.
Spillages and residues in containers should be incinerated
at high temperatures (> 1000 °C) or crushed and buried below the
topsoil at an appropriate site. Contaminated surface material
should be treated similarly or else the area should be secured
from public access until the gas concentration is zero.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Phosphine was evaluated in 1971 by WHO and FAO, as a
pesticide residue in food (WHO/FAO, 1972). A tolerance of
0.1 mg/kg (0.1 ppm) in raw cereals was confirmed. It was
recommended that the tolerance of 0.01 mg/kg (0.01 ppm) in
flour, other milled cereal products, breakfast cereals, dried
vegetables, and spices be confirmed and extended to include
nuts, groundnuts, dried fruit, cocoa beans, and other similar
foods, known to be fumigated with phosphine.
The use of zinc phosphide as a rodenticide in public health
was reviewed by WHO in 1972. It was concluded that it was a
generally effective compound and, while highly toxic to domestic
fowl, its safety record was good. The use of zinc phosphide was
endorsed (WHO, 1973).
REFERENCES
ACGIH (1986) Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists.
ADU, O.O. & MUTHU, M. (1985) The relative toxicity of seven
fumigants to life cycle stages of Callosobruchus chinensis L.
Insect Sci. Appl., 6(1): 75-78.
ADVANI, R. (1983) A comparative trial of zinc phosphide,
aluminium phosphide and RH-787 against field mixed population of
rodents in crop ecosystem of the Indian desert. Ztg angew.
Zool.,70(2): 153-168.
AHMAD, M. (1976) Effect of phosphine fumigation on the
germination of edible legume seeds. J. stored Prod. Res., 12:
211-212.
AL-OMAR, M.A. & AL-BASSOMY, M. (1984) Persistence of phosphine
gas in fumigated Irafir dates. J. food Saf., 6: 253-260.
AL'ZHANOV, S., MOLDABEKOV, Sh.M., ERSHOV, V.A., & POPOV, A.P.
(1983) [ Use of copper and iron sulfates in cleaning furnace
gas from phosphorus production,] Moscow, Academy of Sciences of
the USSR, Institute of Scientific Information (VINITI) (Document
4802-83) (in Russian).
AMINZHANOV, M. (1972) [Use of zinc phosphide in controlling
stray dogs.] In: Azimov, Sh.A., Shevchenko, N.Kh., & Nurmatov,
R.Sh., ed. [ Proceedings of the 7th Conference of Young Uzbek
Agricultural Scientists,] Tashkent, Uzbeksii Nauchno-Issled.
Veterinary Institute (in Russian).
AMOORE, J.E. & HAUTALA, E. (1983) Odour as an aid to chemical
safety: odour thresholds compared with threshold limit values
and volatilities for 214 industrial chemicals in air and water
dilution. J. appl. Toxicol., 3(6): 272.
ANON. (1936) [Trials to establish whether the Delicia grain
weevil fumigation procedure is safe to apply and free from the
risk of fire and explosion.] Gutachten chim. tech. Reichsanst.,
8(4): 5 (in German).
ANON. (1956) Ferro-silicon explosion risks. Chem. Trade J.
Chem. Eng., 16 Nov.: 1180.
ARENDT, G., FARWIK, W., HAAG, F., KAUFMANN, G., KOHL, E.-G.,
PRUGGMAYER, D., & WAGNER, W. (1979) [Environmental aspects of
pest control. Fumigation of grain and food stores.] Lebensmit-
teltechnik, 5: 2-8 (in German).
ARNOLD, J.T. & ROBBIANO, P. (1974) Portable vapour surveil-
lance system, Springfield, Virginia, National Technical
Information Service (NTIS Report No. AD-782 844).
ATCHABAROV, B.A., AITBAYEV, T.KH., & AITBEMBETOV, B.N. (1984)
[Comparison of the effects of hydrogen fluoride and phosphine on
the organism with various exposure levels.] In:[ Health care in
Kazakhstan. Part 1,] pp. 28-31 (in Russian).
ATTIA, F.I. (1984) Multiple cross-resistance characteristics
in phosphine-resistant strains of Rhizopertha dominica and
Tribolium castaneum. In: Ripp, B., ed. Controlled atmosphere
and fumigation in grain storages, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 49-53 (Developments in
Agricultural Engineering No. 5).
ATTIA, F.I. & GREENING, H.G. (1981) Survey of resistance to
phosphine in coleopterous pests of grain and stored products in
New South Wales. Gen. appl. Entomol., 13: 93-97.
BAI, K.M., KRISHNAKUMARI, M.K., RAMESH, H.P., SHIVANANDAPPA, T.,
& MAJUMDER, S.K. (1980) Short-term toxicity study of zinc
phosphide in Albino rats (Rattus norvegicus). Indian J. exp.
Biol., 18(8): 854-857.
BARRETT, W.J. & DILLON, H.K. (1978) Development of methods
for the determination of elemental phosphorus and phosphine in
air, Cincinnati, Ohio, National Institute of Occupational Safety
and Health, 102 pp (DHEW (NIOSH) Publication No. 78-177).
BECKER, K.H., BIEHL, H.M., BRUCKMANN, P., FINK, E.H., FUHR, F.,
KLOPFER, W., ZELLNER, R., & ZETSCH, C. (1984) Methods for the
ecotoxicological evaluation of chemicals, photochemical degra-
dation in the gas phase. VI. OH x reaction rate constants and
tropospheric lifetimes of selected environmental chemicals,
Jülich, Kernforschungsanlage (Projektträgerschaft Umweltchemi-
kalien Report 1980-83, No. 279).
BELL, C.H., WILSON, S.M., & BANKS, H.J. (1984) Studies on the
toxicity of phosphine to tolerant stages of Trogoderma granarium
everts (Coleoptera: Dermestidae). J. stored Prod. Res., 20(2):
111-117.
BELOSKURSKAYA, G.I., PARASKEVOPULOS, YA.G., & SHLYGINA, O.E.
(1978) [On the clinical and functional condition of the liver
in patients with chronic phosphorus poisoning.] Tr. Inst. Kraev.
Patol. Akad. Nauk Kazakhskoi SSR, 36: 25-30 (in Russian).
BLAXLAND, J.D. & GORDON, R.F. (1945) Zinc phosphide poisoning
in poultry. Vet. J., 101: 108-110.
BOENIG, I.A., CRUTCHFIELD, M.M., & HEITSCH, C.W. (1982)
Phosphorus compounds. In: Kirk-Othmer, ed. Encyclopaedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol.
17, pp. 490-539.
BOMPEIX, G. & SAINDRENAN, P. (1984) In vitro antifungal
activity of Fosetyl Al and phosphorous acid on Phytophthera
species. Fruits, 39(12): 777-786.
BOND, E.J. & DUMAS, T. (1967) Loss of warning odour from
phosphine. J. stored Prod. Res., 3: 389-392.
BOND, E.J., ROBINSON, J.R., & BUCKLAND, C.T. (1969) The toxic
action of phosphine. Absorption and symptoms of poisoning in
insects. J. stored Prod. Res., 5: 289-298.
BOND, E.J., DUMAS, T., & HOBBS, S. (1984) Corrosion of metals
by the fumigant phosphine. J. stored Prod. Res., 20(2): 57-63.
BONTOYAN, W.R. (1981) Report on pesticide formulations: herbi-
cides, fungicides and miscellaneous. J. Assoc. Off. Anal. Chem.,
64(2): 355.
BORODIN, A., KLIMEK, M., & ZAKL, K. (1983) [Blood clotting and
fibrinolysis systems in workers of a disinfection, disinfesta-
tion and deratization employment (sic).] Med. Pr., 34(4): 313
(in Polish).
BOUBAL, M., LOUISE, J., BELOT, D., & POLITI, J.M. (1981)
Analyse directe et continue de traces d'hydrures gazeux à très
basses teneurs, Paris, Institut National de la Proprieté
Industrielle, Sociéte d'Air Liquide (Document No. 2 506 459)
(Demande de Brevet d'Invention No. 81 10316).
BOWKER, J.R. (1958) The liberation of phosphine in the
machining of spheroidal graphite iron. In: Proceedings of the
British Cast Iron Research Association, 8 November, p. 50.
BOWLEY, C.R. & BELL, C.H. (1981) The toxicity of twelve
fumigants to three species of mites infesting grain. J. stored
Prod. Res., 17: 83-87.
BRADFIELD, A.A.G. & GILL, J.E. (1984) Laboratory trials of
five rodenticides for the control of Mesocricetus auratus
Waterhouse. J. Hyg. (Lond.), 93: 389-394.
BRANDON, R.W. (1983) The use of chemically impregnated paper
tapes for toxic gas detection and monitoring. Anal. Chem. Symp.
Ser., 17: 726-31.
BREYER, D. (1973) [Studies on hydrogen phosphide residues in
grain after treatment with fumigation preparations in powder
form.] Mühle Mischfuttertech., 110: 699-700 in German).
BUBIEN, Z., WARTENBERG, L., & ROSZYK, E. (1974) [Content of
zinc in hen carcasses poisoned with zinc phosphide.] Zesz. Nauk.
Akad. Roln Wroclawiu Weter., 31: 173-180 (in Polish).
CABROL TELLE, A.M. & DE SAINT BLANQUAT, G. (1984) In vitro
digestibility of starch, casein and triolein after fumigation
with phosphine. Sci. Aliments, 4(Hors Ser. 3): 287-291.
CABROL TELLE, A.M., DE SAINT BLANQUAT, G., DERACHE, R, HOLLANDS,
E., PERIQUET, B., & THOUVENOT, J.-P. (1985) Nutritional and
toxicological effects of long-term ingestion of phosphine-
fumigated diet by the rat. Food Chem. Toxicol., 23(11): 1001-
1009.
CALVERT, J.G. & PITTS, J.N., Jr (1966) Photochemistry, New
York, John Wiley and Sons.
CHAMP, B.R. & DYTE, C.E. (1976) Report of the FAO global
survey of pesticide susceptibility of stored grain pests, Rome,
Food and Agriculture Organization of the United Nations, pp. 1-
257 (FAO Plant Production and Protection Paper No. 5).
CHAN, L.T.F., CROWLEY, R.J., DELLIOU, D., & GEYER, R. (1983)
Phosphine analysis in post-mortem specimens following ingestion
of aluminium phosphide. J. anal. Toxicol., 7: 165-167.
CHENTSOVA, N.YU. (1972) [Danger to poultry during scattering
of zinc phosphide-poisoned grain to control rodents in forest
plantings.] In: Voronova, L.D., ed. [ Effects of pesticides on
wild animals,] Moscow, pp. 90-102 (in Russian).
CHILDS, A.F. & COATES, H. (1971) The toxicity of phosphorus
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3, pp. 1437-1440.
CHILDS, D.P. (1972) Tobacco consignment fumigated in
containers while in transit. Container News, October.
CHILDS, D.P., OVERBY, J.E., & NIFFENEGGER, D. (1969) Phosphine
fumigation of flue-cured tobacco warehouses for the control of
the cigarette beetle. Tobacco, 168(21): 20-25.
CHOPRA, G. (1984) Field efficacy of aluminium phosphide
against rodents. Indian J. agric. Sci., 54(3): 204-205.
CIBA (1978) Phosphorus in the environment: its chemistry and
biochemistry, Amsterdam, Oxford, New York, Elsevier North
Holland (Ciba Foundation Symposium 57 - new series).
CODEX ALIMENTARIUS (1986) Codex maximum limits for pesticide
residues, 2nd ed., Rome, Food and Agriculture Organization of
the United Nations (CAC/Vol. XIII-Ed.2).
COLE, R.J. & BENNETT, W.H. (1950) An investigation of the
possibility of the occurrence of phosphine and acetylene in the
atmosphere of factories for the powdering of magnesium. J. Soc.
Chem. Ind., 69: 29-30.
CORBRIDGE, D.E.C. (1974) The structural chemistry of
phosphorus, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 268-269.
COX, P.D., BELL, C.H., PEARSON, J., & BEIREN, M.A., (1984) The
effect of diapause on the tolerance of larvae of Ephestia
küehniella to methyl bromide and phosphine. J. stored Prod.
Res., 20(4): 215-219.
CURRY, A.S., PRICE, D.E., & TRYHORN, F.G. (1959) Absorption of
zinc phosphide particles. Nature (Lond.), 184(4686): 642-643.
DAVIS, R. (1986) Fumigation of ships. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 18-21 March, London, Foreign and
Commonwealth Office, Overseas Development Administration,
Tropical Development and Research Institute, pp. 24-34.
DELOMENIE, H. (1933) Gases evolved from ferrosilicons under
the influence of water. J. Pharm. Chim., 18: 289-292, 532
DIEKE, S.H. & RICHTER, C.P. (1946) Comparative assays of
rodenticides on wild Norway rats. Public Health Rep., 61: 672-
679.
DIETERICH, W.H., MAYR, G., HILD, K., SULLIVAN, J.B., & MURPHY,
J. (1967) Hydrogen phosphide as a fumigant for foods, feeds
and processed food products. Residue Rev., 19: 135-149.
DISNEY, R.W. & FOWLER, K.S. (1972a) Residues in cereals
exposed to hydrogen phosphide. In: Radiotracer studies on
chemical residues in food and agriculture. Proceedings of a
Combined Panel and Research Coordination Meeting, Vienna,
Austria, 25-29 October 1971, Vienna, International Atomic
Energy Agency, pp. 99-101.
DISNEY, R.W. & FOWLER, K.S. (1972b) The possibility of isotope
exchange or other interfering reactions occurring during the
determination of residues from fumigation with 32P-labelled
phosphine. In: Radiotracer studies on chemical residues in food
and agriculture. Proceedings of a Combined Panel and Research
Coordination Meeting, Vienna, Austria, 25-29 October 1971,
Vienna, International Atomic Energy Agency, pp. 87-88.
DUMAS, T. (1964) Determination of phosphine in air by gas
chromatography. J. agric. food Chem., 12(3): 257-258.
DUMAS, T. (1978) Modified gas chromatographic determination of
phosphine. J. Assoc. Off. Anal. Chem., 61(1): 5-7.
DUMAS, T. (1980) Phosphine sorption and desorption by stored
wheat and corn. J. agric. food Chem., 28: 337-339.
DUMAS, T. & BOND, E.J. (1974) Separation of phosphine from
odour-producing impurities. J. stored Prod. Res., 10: 67-68.
DUMAS, T. & BOND, E.J. (1981) Method of trapping low levels of
phosphine at ambient temperature for gas-chromatographic
analysis. J. Chromatogr., 206(2): 384-386.
EPPO (1975) Guidelines for the development and biological
evaluation of rodenticides, Paris, European and Mediterranean
Plant Protection Organisation (Bulletin No. 5(1)).
FITZPATRICK, R.J., MCGIRR, J.L., & PAPWORTH, D.S. (1955) The
toxicity of rodenticides. Vet. Rec., 67: 142-145.
FLUCK, E. (1973) The chemistry of phosphine. Fortschr. chim
Forsch., 35: 1-64.
FLUCK, E. (1976) The odor threshold of phosphine. J. Air
Pollut. Control Assoc., 26(8): 795.
FRANK, R. & RIPPEN, G. (1986) [ Content of phosphine in the
atmosphere,] Frankfurt am Main, Batelle-Institut e.v. (in
German).
FREYBERG, W. (1979) Acute toxicity of Detia R pellets to
bluegill (Lepomis macrochirus ), Washington DC, US Environmental
Protection Agency (Unpublished report).
FRITZ, B., LORENZ, K., STEINERT, W., & ZELLNER, R. (1982)
Laboratory kinetic investigations of the tropospheric oxidation
of selected industrial emissions. In: Versino, B. & Ott, H., ed.
Physico-chemical behaviour of atmospheric pollutants,
Dordrecht, Reidel, pp. 192-202.
FURMAN, N.H., ed. (1962) Standard methods of chemical
analysis, 6th ed., Princeton, New Jersey, D. van Nostrand
Company Inc., Vol. 1, pp. 821-823.
FURUNO, J., SUGAWARA, N., & IKEDA, H. (1976) A fatal case
poisoned by hydrogen phosphide and the simultaneous separation
of inorganic gases by gas chromatography. Bull. Yamaguchi med.
Sch., 23(1-2): 49-58.
GESSNER, O. (1937) Fatal phosphine poisoning from "Delicia"
grain weevil fumigant (aluminium phosphide). Samml. Vergiftungs-
fällen, 8: B-79.
GILL, J.E. & REDFERN, R. (1983) Laboratory tests of seven
rodenticides for the control of Meriones shawi. J. Hyg. (Lond.),
91: 351-357.
HABASHI, F. & ISMAIL, M.I. (1975) Health hazards and pollution
in the metallurgical industry due to phosphine and arsine. CIM
Bull., August: 99-103.
HACKENBERG, U. (1972) Chronic ingestion by rats of standard
diet treated with aluminium phosphide. Toxicol. appl.
Pharmacol., 23: 147-158.
HALLERMANN, W. & PRIBILLA, O. (1959) [Fatal poisoning with
phosphine.] Arch. Toxikol., 17(4): 219-242 (in German).
HARGER, R.N. & SPOLYAR, L.W. (1958) Toxicity of phosphine with
a possible fatality from this poison. Am. Med. Assoc. Arch.
Ind. Health, 18: 497-504.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds.
Wildlife, 191: 1-61.
HILTON, H.W. & ROBISON, W.H. (1972) Fate of zinc phosphide and
phosphine in the soil-water environment. J. agric. food Chem.,
20(6): 1209-1213.
HOLE, B.D. (1981) Variation in tolerance of seven species of
stored product Coleoptera to methyl bromide and phosphine in
strains from twenty-nine countries. Bull. entomol. Res., 71(2):
299-306.
HOLE, B.D., BELL, C.H., MILLS, K.A., & GOODSHIP, G. (1976) The
toxicity of phosphine to all developmental stages of thirteen
species of stored product beetles. J. stored Prod. Res., 12:
235-244.
HUGHES, J.G. & JONES, A.T. (1963) Estimation of phosphine in
air. Ind. Hyg. J., March-April: 164-167
HUNTER, D. (1978) The diseases of occupations, 6th ed.,
London, Hodder and Stoughton.
IKEDA, S. (1971) [Effect of zinc phosphide on Coturnix coturnix
japonica.] Ringyo Shikenjo Kenkyu Hokuko, 238: 141-148 (in
Japanese).
IKIMANOVA, G.K. (1977) [Porphyrin metabolism in experimental
phosphorus poisoning.] Zdravookhr. Kaz., 1: 39-42 (in Russian).
IMO (1980) Recommendations on the safe use of pesticides in
ships, London, International Maritime Organization (UN IMO
Circular No. 298, revised).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, 303 pp (Data
Profile Series No. 5).
JACKSON, J.R. & ELIAS, E. (1984) Hepatic function and exposure
to phosphine and white phosphorus. In: Proceedings of the 12th
International Congress on Occupational Health in the Chemical
Industry, Dublin, Ireland, 9-14 September 1984, pp. 219-228.
JANDA, J. (1973) Danger of rodenticides containing zinc
phosphide to wild life. Agrochemia, 13(3): 85-86.
JOKOTE, Ch. (1904) [Studies on hydrogen phosphine.] Arch. Hyg.
Bakteriol., 49: 275-306 (in German).
JONES, A.T., JONES, R.C., & LONGLEY, E.O. (1964) Environmental
and clinical aspects of bulk wheat fumigation with aluminium
phosphide. Am. Ind. Hyg. Assoc. J., 25: 376-379.
KADKOL, S.B. & JAYARAJ, P. (1967) Effect of phosphine-
fumigated rice on the growth of albino rats. Rep. Cent. Food
Technol. Res. Inst. (Mysore), 2: 6.
KAGAN, YU.S. (1985) Principles of pesticide toxicology,
Moscow, Centre of International Projects, GKNT, 176 pp.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1973)
The phytotoxic effect of repeated fumigation of certain cereal
crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 7: 57-62.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1974)
The phytotoxic effect of carbon bisulphide, methyl bromide and
hydrogen phosphide on the germination of seeds of certain cereal
field crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 8: 75-80.
KAO, C. (1981) Determination of aluminium phosphide in
fumigants. J. food Sci., 46: 1632-1634.
KASHI, K.P. (1975) A mixed indicator strip for phosphine
detection. Pestic. Sci., 6: 511-514.
KASHI, K.P. (1981a) Toxicity of phosphine to five species of
stored-product insects in atmospheres of air and nitrogen.
Pestic. Sci., 12: 116-122.
KASHI, K.P. (1981b) Response of five species of stored-product
insects to phosphine in oxygen-deficient atmospheres. Pestic.
Sci., 12: 111-115.
KASHI, K.P. (1982) Dose-mortality responses of five species of
stored-products insects to phosphine. Int. Pest Control,
March/April: 46-49.
KASHI, K.P. & CHEFURKA, W. (1976) The effect of phosphine of
absorption and circular dichroic spectra of cytochrome-c and
cytochrome-c oxidase. Pestic. Biochem. Physiol., 6: 350-362.
KASHI, K.P. & MUTHU, M. (1975) A mixed indicator strip for
phosphine detection. Pestic. Sci., 6: 511-514.
KIM, H.H., KIM, K.H., & HONG, S.S. (1977) Effect of pesticides
on exocrine pancreas and liver. Yonse Uidea Nonmunjip, 10(2):
170-180.
KLIMMER, O.R. (1969) [Contribution on poisoning by phosphine.
On the question of sub-chronic phosphine intoxication.] Arch.
Toxikol., 24: 164 (in German).
KOZHEMYAKIN, N.G., BUTYAGIN, V.N., BASHMURIN, A.F., & POTAPENKO,
M.I. (1971) [Veterinary-sanitary evaluation of the carcasses
and organs of animals which had to be killed in relation to
their contamination with zinc phosphide.] Sb. Rab. Leningrad
Vet. Inst., 32: 231-236 (in Russian).
KUHN, H., MAREK, J., & REIF, H. (1971) [Fumigation of tobacco
with phosphine.] Fachliche Mitt. Austria Tabakwerke AG, 12: 191-
203 (in German).
LADOU, J. (1983) Potential occupational health hazards in the
microelectronics industry. Scand. J. Work. environ. Health, 9:
42-46.
LEESCH, J.G. (1982) Accuracy of different sampling pumps and
detector tube combinations to determine phosphine
concentrations. J. econ. Entomol., 75: 899-905.
LEESCH, J.G. (1984) Fumigation of lettuce: efficacy and phyto-
toxicity. J. econ. Entomol., 77(1): 142-150.
LOWE, E.J. (1971) Phosphorus hydrides and phosphonium
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3.
LUCK, H. & ALLEMANN, A. (1984) Control of insect infestation
during storage of dairy products. Fumigation with hydrogen
phosphide. S. Afr. J. dairy Technol., 16(3): 103-107.
LUTZMANN, L., KEINITZ, M., & KLOSTERKOTTER, W. (1963)
[Emission of phosphine during transport of ferrosilicon.] Med.
Welt, 58: 114-116 (in German).
MCGIRR, J.L. (1953) Poisoning of livestock by the newer
rodenticides, insecticides and weed killers. In: Proceedings of
the XVth International Veterinary Congress, Stockholm, 9-15
August, Part 1, Vol. 1, pp. 479-480.
MCMAHON, R. & FORESE, F.F. (1983) Hydride gas detecting tape,
Glenview, Illinois, MDA Scientific Inc. (UK Patent Application
GB 2 108 662A).
MARSHALL, E.F. (1981) Efficacy variations of rodent baits with
the same active ingredient. Pest Control, January: 22-23.
MATHEW, G.G. (1961) The production of phosphine while
machining spheroidal graphite iron. Ann. occup. Hyg., 4: 19-35.
MEISSNER, R. (1924) [The toxicology of hydrogen phosphide.] Z.
gesamte Med., 42: 267 (in German).
MEREDITH, C. (1981) Toxicological studies on zinc phosphide,
Birmingham, University of Birmingham, Department of Bio-
chemistry (M.Sc. Thesis).
MINCHEV, T. & DIMITROV, D. (1970) [Early changes in the serum
of rabbits poisoned with zinc phosphide.] In: Vasilev, R.A., ed.
[ Nauch. Tr. Sud. Med. Deontol.,] Sofia, Meditsina i
Fizkultura, pp. 33-38 (in Bulgarian).
MUELLER, W. (1940) [Toxicity of phosphine (animal
experiments). Part 1. Acute and subacute toxicity.] Arch. exp.
Pathol. Pharmakol., 195: 184-193 (in German).
MUTHU, M., KRISHNAKUMARI, M.K., MURALIDHARA, V., & MAJUMDER,
S.K. (1980) A study on the acute inhalation toxicity of
phosphine to albino rats. Bull. environ. Contam. Toxicol., 24:
404-410.
NAKAKITA, H. & WINKS, R.G. (1981) Phosphine resistance in
immature stages of a laboratory selected strain of Tribolium
castaneum Herbst (Coleoptera: Tenebrionidae). J. stored Prod.
Res., 17: 43-52.
NAKAKITA, H., KATSUMATA, Y., & OZAWA, T. (1971) The effect of
phosphine on the respiration of rat liver mitochondria. J.
Biochem., 69: 589-593.
NATARAJAN, T. & BAGYARAJ, D.J. (1984) Fumigation effect on
microflora and viability of blackgram and fieldbean seeds.
Pesticides, 18(4): 40-42.
NAUS, A. (1982) [Olfactory thresholds of some industrial
compounds.] Prac. Lek., 34(6-7): 217-218 (in Polish).
NETHERLANDS, WERKGROEP VAN DESKUNDIGEN VAN DE NATIONALE MAC-
COMMISSIE. (1984) [ Report on the TLV of phosphine,] 2nd ed.,
Voorburg, Working Group of the National TLV Committee, 27 pp
(Report No. 1/80) (in Dutch).
NEUBERT, D. & HOFFMEISTER, I. (1960) Changes in intermediary
metabolism following exposure to hydrogen phosphide. Naunyn-
Schmiedeberg's Arch. exp. Pathol. Pharmakol., 239: 219-233.
NOACK, S., VON & REICHMUTH, C. (1981) [Determination of limits
for injuring animals and plants by phosphine and methyl bromide.
I. Studies on Drosophila melanogaster. Anz. schädlingskd.
Pflanzenschutz Umweltschutz, 54: 23-27 (in German).
NOACK, S., VON & REICHMUTH, C. (1982a) [Quantities of
phosphine, methyl bromide and hydrocyanic acid used in stored
products protection in the FRG from 1975-1977.] Nachrichtenbl.
Dtsch. Pflanzenschutzd., 34(2)S: 17-21 (in German).
NOACK, S., VON & REICHMUTH, C. (1982b) [Determination of
limits for injuring animals and plants by phosphine or methyl
bromide. II. Studies on water cress and lettuce Lactuca sativa
capitata.] Anz. schädlingskd. Pflanzenschutz. Umweltschutz,
55: 57-59 (in German).
NOACK, S., VON & WOHLGEMUTH, R. (1985) [Phosphine residues in
hazelnuts, soybeans and wheat after fumigation with non-constant
concentrations.] Z. Lebensm. Unters. Forsch., 180: 101-108 (in
German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1983)
[Relationship of PH3 residues after fumigation to concentration,
time of exposure and length of storage.] Z. Lebensm. Unters.
Forsch., 177: 87-93 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984a)
[Decomposition of phosphine in treated foods related to storage
temperature and aeration.] Z. Lebensm. Unters. Forsch., 178: 31-
37 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984b) [A
half-empirical mathematical model for calculation of PH3-
residues in hazelnuts fumigated with phosphine.] Z. Lebensm.
Unters. Forsch., 178: 97-103 (in German).
NOWICKI, T.W. (1978) Gas-liquid chromatography and flame
photometric detection of phosphine in wheat. J. Assoc. Off.
Anal. Chem., 61(4): 829-836.
PAZYNICH, V.M., MAZUR, I.A., & PODLOZNYI, A.V. (1984)
[Experimental substantiation and prediction of maximum
atmospheric phosphine concentrations differentiated by time.]
Gig. i Sanit., 1984(1): 13-15 (in Russian).
PRICE, N.R. (1984) Active exclusion of phosphine as a
mechanism of resistance in Rhyzopertha dominica F (Coleoptera:
Bostrychidae). J. stored Prod. Res., 20(3): 163-168.
PRICE, N.R. & DANCE, S.J. (1983) Some biochemical aspects of
phosphine action and resistance in three species of stored
product beetles. Comp. Biochem. Physiol., 76C(2): 277-281.
PRICE, N.R., MILLS, K.A., & HUMPHRIES, L.A. (1982) Phosphine
toxicity and catalase activity in susceptible and resistant
strains of the lesser grain borer (Rhyzopertha dominica).
Comp. Biochem. Physiol., 73C: 411-413.
RANGASWAMY, J.R. (1984) Simple spectrophotometric method for
determination of phosphine residues in wheat. J. Assoc. Off.
Anal. Chem., 67(1): 117-122.
RANGASWAMY, J.R. & MUTHU, M. (1985) Spectrophotometric
determination of phosphine residues in rice. J. Assoc. Off.
Anal. Chem., 68(2): 205-208.
REBMANN, Z. (1933) [On a new pesticide (Fosfolon).] Gesund-
heitstech. Städtehyg. ver. Städtische Tiefbau, 5: 280-284 (in
German).
REICHMUTH, C., ed. (1985) [Response of the granary weevil
Sitophilus granarius L. (Coleoptera: Curculionidae) to
gradually increasing and decreasing phosphine concentrations.]
Anz. schädlingskd. Pflanzenschutz Umweltschutz, 58(1): 10-16
(in German).
REICHMUTH, C., ed. (1986) The significance of changing
concentrations in toxicity of phosphine. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 8-21 March, London, Overseas Development
Administration, Tropical Development and Research Institute.
pp. 88-98.
REICHMUTH, C., NOACK, S., & WREDE, A. (1981) [Emission of
phosphine caused by fumigation for stored products protection.]
Nachrichtenbl. Dtsch. Pflanzenschutzd., 33(9): 132-136 (in
German).
ROALDSNES, J. (1982) [Phosphine poisoning due to machining of
ductile iron.] Norsk. bedv. h. tj., 3: 12-16 (in Norwegian).
ROBINSON, J.R. (1972) Residues containing phosphorus following
phosphine treatment: measurement by neutron activation. J.
stored Prod. Res., 8: 19-26
ROBINSON, J.R. & BOND, E.J. (1970) The toxic action of
phosphine. Studies with 32PH3: terminal residues in biological
materials. J. stored Prod. Res., 6: 133-146.
ROBISON, W.H. & HILTON, H.W. (1971) Gas chromatography of
phosphine derived from zinc phosphide in sugarcane. J. agric.
food Chem., 19(5): 875-878.
ROHRLICH, M. & MEUSER, F. (1970) [Studies on grain fumigated
with hydrogen phosphide. III. Communication: biochemical aspects
of phosphine fumigation.] Getreide Mehl, 20(1): 1-8 (in
German).
RUSCHEL, A.P. & DA COSTA, W.F. (1966) [Symbiotic fixing of
atmospheric nitrogen in the french bean ( Phaseolus vulgaris L).
III. Effect of some insecticides and fungicides.] Pesqui.
Agropecu. Bras., 1: 147-149 (in Portugese).
SAEED, T. & ABU-TABANJA, R. (1984) Determination of phosphine:
comparison of rates of desorption by purge-and-trap method and
by sulfuric acid treatment. J. Assoc. Off. Anal. Chem., 68(1):
61-64.
SATO, K. & SUWANAI, M. (1974) Absorption of hydrogen phosphide
to cereal products. Appl. Entomol. Zool., 9(3): 127-132.
SAYVETZ, T.A. (1982) Possible phosphine inhalation related to
warehouse fumigation, Richmond, Virginia, Virginia State Health
Department, Office of Health Protection and Environmental
Management, 6 p.
SCHITOSKEY, F. (1975) Primary and secondary hazards of three
rodenticides to kit fox. J. wildl. Manage., 39(2): 416-418.
SCHULZ, H. (1887) [Correction concerning the toxicity of
phosphorus compounds containing oxygen.] Arch. exp. Pathol., 23:
150-151 (in German).
SCHULZ, H. (1890) [Concerning phosphine.] Arch. exp. Pathol.
Pharmakol., 27: 314-335 (in German).
SHAHEEN, D.G., BRAGG, L.A., STANOVICK, R.P., & SULLIVAN, J.B.
(1983) Phosphine detector tubes as passive dosimeters for use
with metal phosphide fumigants. Tob. Int., 185(20): 36-39.
SHIVANANDAPPA, T., RAMESH, H.P., & KRISHNAKUMARI, M.K. (1979)
Rodenticidal poisoning of non-target animals: acute oral
toxicity of zinc phosphide to poultry. Bull. environ. Contam.
Toxicol., 23(4-5): 452-455.
SINGH, K.N., SRIVASTAVA, B.P., & NATH, G. (1983) Phosphine
residues and its effect on the germination of stored pulses.
Indian J. Entomol., 45(1): 71-80.
SRI (1982) Electronic chemicals, Menlo Park, California,
Standford Research International, pp. 40-41.
SRIDHARA, S. (1983) Rodenticide induced bait aversion and
neophobia in Tatera indica cuvieri. Z. angew. Zool., 70(4): 429-
440.
STEPHENSON, J.B.P. (1967) Zinc phosphide poisoning. Arch.
environ. Health, 15: 83-88.
TERZIC, Lj. (1981) Quantitative method for the determination
of zinc phosphide in technical products. Acta vet. (Beograd),
31(5-6): 279-288.
TIETJEN, H.P. (1976) Zinc phosphide: its development as a
control agent for black-tailed prairie dogs, Washington DC, US
Department of the Interior, Fish and Wildlife Research Centre
(Special Scientific Report Wildlife No. 195).
TKACHUK, R. (1971) Phosphine fumigation of wheat - residue
formation. Cereal Sci. Today, 16: 307.
TKACHUK, R. (1972) Phosphorus residues in wheat due to
phosphine fumigation. Cereal Chem., 49: 258-267.
TRIMBORN, H. & KLIMMER, O. (1962) [Experimental studies on
chemical changes in blood pigments caused by hydrogen
phosphide in vitro.] Arch. int. Pharmacodyn., 137(3-4): 331-
347 (in German).
UNDERWOOD, J.G. (1972) The radiochemical analysis of phosphine
residues in fumigated tobaccos. Tob. Sci., October 13: 47-49.
URGA, K. (1983) Methyl bromide and phosphine residues in
cereal grains and pulses. Ethiop. med. J., 21: 79-83.
US CFR (1981) Code of federal regulations, 29(1910): 673.
US EPA (1981) Pesticide registration standard: aluminium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-137514).
US EPA (1982) Pesticide registration standard: magnesium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-195777).
US EPA (1983) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 48(37): 7714-
7716.
US EPA (1984) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 49(92): 19922-
19923.
US EPA (1985a) Aluminium phosphide/magnesium phosphide:
tolerances for residues. Code Fed. Reg., 40: 180, 225, 291-292,
333, 375.
US EPA (1985b) EPA chemical profile: phosphine, Washington DC,
US Environmental Protection Agency.
US EPA (1986) Guidance for the re-registration of pesticide
products containing aluminium phosphide, February 1986. An
amendment to the registration standard issued in October 1981,
Washington DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (PB 84-241405).
US NIOSH (1977) National occupational hazard survey,
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, Vol. 3 (Publication No. 78-114).
US NIOSH (1979) Ten NIOSH analytical methods: phosphine,
Cincinnati, Ohio, National Institute for Occupational Safety
and Health, Division of Physical Sciences and Engineering, 42
pp.
VAN WAZER, J.R., ed. (1961) Phosphorus and its compounds:
technology, biological functions and applications, New York,
Interscience Publishers Inc., Vol. II, p. 957.
VAN WAZER, J.R. (1982) Phosphorus and the phosphides. In:
Kirk-Othmer, ed. Encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 17, pp. 473-489.
VDOVENKO, I.D., VAKULENKO, L.I., YAKOVLEVA, L.A., SHEDENKA,
L.I., BERESLAVSKAYA, A.N., & YATSENKO, V.S. (1984) [Effect of
surfactants on phosphine formation in steel pickling.] Ukr.
Khim. Zh., 50(4): 418-421 (in Russian).
VERGA, L. & BELLONI, F. (1958) [Over a case of purpura due to
empoisonment of phosphine (a haemato-clinical and histochemical
study.] Rass. Med. ind. Ig. Lav., 27(3): 230-241 (in Italian).
VERSTUYFT, A.W. (1978) Sampling and analytical methods for
phosphine - a review. Am. Ind. Hyg. Assoc. J., 39: 431-437.
VINSJANSEN, A. & THRANE, K.E. (1978) Gas-chromatographic
determination of phosphine in ambient air. Analyst, 103: 1195-
1198.
WARITZ, R.S. & BROWN, R.M. (1975) Acute and subacute
inhalation toxicities of phosphine, phenylphosphine and
triphenylphosphine. Am. Ind. Hyg. Assoc. J., 36: 452-458.
WEBLEY, D.J., HARRIS, A.H., & KILMINSTER, K. (1981) The use of
a portable gas analyser to measure phosphine concentrations in
experimental fumigations in the tropics. Int. pest Control,
March/April: 47-50.
WILSON, A. (1971) The metal phosphides. In: Mellor's compre-
hensive treatise on inorganic and theoretical chemistry, White
Plains, New York, Longman, Vol. VIII, Suppl. III.
WILSON, R., LOVEJOY, F.H., JAEGER, R.J., & LANDRIGAN, P.L.
(1980) Acute phosphine poisoning aboard a grain freighter.
Epidemiologic, clinical and pathological findings. J. Am. Med.
Assoc., 244(2): 148-150.
WINKS, R.G. (1970) Phosphine fumigation of farm-stored
tobacco. Queensland agric. J., November.
WINKS, R.G. (1984) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): time as a dosage factor. J.
stored Prod. Res., 20(1): 45-56.
WINKS, R.G. (1985) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): phosphine-induced narcosis. J.
stored Prod. Res., 21(1): 25-29.
WHO (1973) WHO Technical Report Series, No. 513 (Twentieth
Report of the Expert Committee on Insecticides), Geneva, World
Health Organization, p. 39.
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food, Geneva, World Health Organization, pp. 289-295 (WHO
Pesticide Residue Series, No. 1).
WHO/FAO (1976) Data sheet on pesticides No. 24: zinc
phosphide, Geneva, World Health Organization (VBC/DS/77.24).
WHO/FAO (1980) Data sheet on pesticides No. 46: phosphine,
Geneva, World Health Organization (VBC/DS/80.46).
WORTHING, C.R. & WALKER, S.B., ed. (1983) The pesticide manual:
a world compendium, 7th ed., London, British Crop Protection
Council.
ZIEMER, U. (1963) [Fatal hydrogen phosphide poisoning on a
freighter.] Zbl. Arbeitsmed., 13: 38-39 (in German).
ZIPF, K.E., ARNDT, TH., & HEINTZ, R. (1967) [Clinical
observation of a case of Phostoxin poisoning.] Arch. Toxikol.,
22(4): 209-222 (in German).
ZUTSHI, M.K. (1966) Effect of aluminium phosphide (Phostoxin)
on the germination of certain cereal and vegetable seeds. Bull.
grain Technol., 4(4): 197-199.
Neubert & Hoffmeister (1960) investigated the effects of
short-term exposures ranging from 460 to 2150 mg phosphine/m3 on
cellular respiration in rats. The oxidation of alpha-keto-
glutarate in liver cells was inhibited, but not oxidative
phosphorylation. Alpha-ketoglutarate oxidation was also reduced
in heart muscle, and phosphorylation was disrupted. Survival of
groups of 8 rats at various concentrations of diphosphine-free
phosphine was studied with the following results (Table 14).
Table 14. Survival times of 8 rats in different concentrations of
phosphine. Concentration x time products calculated from
the mid-point of the range of survival timesa
---------------------------------------------------
Concentration Survival Concentration x time
(mg/m3) (min) product (mg x min/m3)
---------------------------------------------------
4640 16 - 20 83 520
1000 50 - 70 60 000
320 150 - 200 56 000
100 400 - 600 50 000
---------------------------------------------------
a From: Neubert & Hoffmeister (1960).
If the use of the mid-point of the range of survival times
is reasonable, these results indicate that the relationship
between concentration and survival time is not simply
reciprocal, and that the index of t is greater than 1, but less
than the value of 1.43 derived from the data quoted by US EPA
(1981, 1982).
Klimmer (1969) determined lethal concentration x time pro-
ducts for rats and calculated, for example, 19 000 mg x min/m3
for a concentration of 287 mg/m3, but these results do not agree
with those calculated from the data of US EPA (1981, 1982) nor
with those of Neubert & Hoffmeister (1960). The value of the
concentration x time product, as a measure of phosphine dose in
mammals, is therefore open to question, as it is in insects.
8.1.2. Inhalation studies on zinc phosphide
The US National Pest Control Association submitted a value
of 19.6 mg/litre for an inhalation LC50 of 10% zinc phosphide
powder in rats. It was not stated whether the value was for
pure zinc phosphide or the 10% dilution and there was no
indication of exposure time (US EPA, 1983).
8.1.3. Oral studies on metal phosphides
In a study on 35 rats of both sexes administered doses of
20, 40, 50, or 80 mg/kg, Dieke & Richter (1946) reported an LD50
for zinc phosphide of 40.5 ± 2.9 mg/kg body weight for wild
Norway rats. Schitoskey (1975) reported an LD50 for the kit fox
of 93 mg zinc phosphide/kg body weight. Aminzhanov (1972)
reported that 100 mg/kg body weight of zinc phosphide was fatal
for starved dogs but not for dogs that were fed. Ikimanova
(1977) reported increased blood- and urinary-porphyrin concen-
trations in rats dosed with zinc phosphide. Other authorities
refer to 27 mg/kg body weight for an acute oral LD50 value for
94% pure zinc phosphide in rats (US EPA, 1983).
8.1.4. Dermal and other studies on metal phosphides
An acute dermal LD50 of 2 000 - 5 000 mg/kg body weight for
zinc phosphide (94% Zn3P2) in rabbits was submitted by the US
National Pest Control Association (US EPA, 1983).
8.2. Short-term Exposures
8.2.1. Inhalation exposure to phosphine
Jokote (1904) determined cumulative survival times in inter-
mittent phosphine exposure as shown in Table 15.
Table 15. Survival time for rabbits, cats, and guinea-pigs at 3
concentrations of phosphinea
-----------------------------------------------------------
Concentration of phosphine (mg/m3)
14 35 140
-----------------------------------------------------------
Survival time (h) 16 - 30 8.5 - 12 2.5 - 3.5
(rabbits, cats, and
guinea-pigs)
-----------------------------------------------------------
a From: Jokote (1904).
Miller (1940) exposed rabbits and guinea-pigs for 4 h per
day to various concentrations of phosphine. At 28 mg/m3 (20
ppm), both rabbits and guinea-pigs died during or after the
second exposure. At 14 mg/m3 (10 ppm), rabbits survived 7 - 14
successive exposures, but at 12 mg/m3 (8.3 ppm), only 4 or 5
exposures. In another study in the series, 2 rabbits that had
had five 4-h exposures to phosphine at 7 mg/m3 were accidentally
exposed to 20 mg/m3 (14 ppm) on the sixth day. Both rabbits
died during this exposure. The authors concluded that
pretreatment with sub-lethal concentrations of phosphine reduced
resistance to near-lethal concentrations. At low concentrations
(up to 14 mg/m3), animals displayed no signs until about 1/2 h
before death when they exhibited diminished reactivity, became
stuporose with shallow respiration, and died in coma.
Occasionally, animals died following exposure, with symptoms of
pulmonary oedema. At 28 mg/m3 or more, all animals exhibited
signs of respiratory irritation and died of pulmonary oedema.
Pathological examination of the lungs revealed bronchiolitis and
atelectasis of the lungs. There was no evidence of haemolysis,
but all organs were hyperaemic. The liver showed fatty
infiltration and there was cloudy swelling of kidney tubular
cells. After prolonged exposure, lungs frequently contained a
brown pigment that did not stain with Prussian Blue and
therefore was not iron-containing.
Neubert & Hoffmeister (1960) exposed female Wistar and E-B
(inbred strain) rats in a 121-litre chamber repeatedly for 6
days to 681 mg/m3 phosphine for 10 - 20 min per day. On the
seventh day, they were exposed until they died (22 - 35 min).
The concentration x time product for lethality after 6 days
pretreatment (about 20 mg x min/litre) was about one third of
that for a first exposure (48 - 87 mg x min/litre) and the
authors concluded that the effects of exposure were cumulative,
rather than that the phosphine accumulated in the body.
Klimmer (1969) exposed cats, guinea-pigs and rats to
phosphine concentrations of 1.4 and 3.5 mg/m3 (1 and 2.5 ppm)
for 4 - 6 h per day, 6 days a week, for a total of more than
800 h over a 24-week period without any clinical, laboratory, or
pathological evidence of effects. The liver function in the
cats, measured by bromosulphthalein (BSP) excretion, was normal.
In cats (3), guinea-pigs (3), and rats (10) exposed to phosphine
at 7 mg/m3 (5 ppm) for 6 - 8 h per day, the cats became
apathetic, showing anorexia after exposure, but exhibiting
thirst. Later, they developed unsteadiness, vomiting,
agitation, dyspnoea, and apnoea, before cardiac arrest after a
total of 35.5 - 45.5 h of exposure. The haemoglobin concen-
tration and erythrocyte count were reduced by 10 - 20% compared
with initial values, there was proteinuria and delayed BSP
excretion. Post-mortem examination revealed congestion of all
organs, with oedema and focal emphysema and red pigmentation of
the lungs. The guinea-pigs exhibited signs that were similar
to, but more marked than, those in the cats, and 2 animals had
asphyxial convulsions. Blood tests showed no deviations from
normal values, but the blood contained a brown coloration that
was not due to methaemoglobin, since spectroscopy revealed the
absorption bands of oxyhaemoglobin only. The guinea-pigs died
on the sixth day after 24 h - 32 h cumulative exposure, and
post-mortem examination revealed pulmonary oedema and red
discolouration and congestion of other organs. The rats died
after 27 - 36 h cumulative exposure. All exhibited congestion
of organs, 3 had pulmonary oedema and 7 had proteinuria. A
second series of exposures under slightly changed exposure
conditions at a different time of year produced qualitatively
similar results. In both series, neurohistological studies in
rats showed widening of the perivascular spaces, vacuolation of
the nuclei of ganglion cells, a reduction in the Purkinje cells,
and a glial reaction. Similar, but less marked, changes without
glial reaction were seen in the cats and guinea-pigs. There was
no control group in these studies, and certain observations in
exposed animals were dismissed on the grounds that they had
previously been seen in unexposed animals.
Waritz & Brown (1975) exposed Charles River-CD rats to
phosphine for 4 h per day, for 12 days, at a concentration of
5.5 mg/m3 (0.163 µmol/litre). The rate of weight gain was
reduced during the exposure period. Although the authors stated
that the rate returned to normal during a 14-day post-exposure
observation period, their data indicate a uniformly reduced rate
of weight gain throughout the exposure and recovery phases.
Post-mortem examination of rats, sacrificed both at the end of
the exposure period and at the end of the recovery period,
revealed no gross or microscopic abnormalities.
The effects of a 1.5-month exposure of white rats to
phosphine at concentrations of 0.05, 0.2, 1.5, and 8 mg/m3 were
reported by Pazynich et al. (1984). There were changes in blood
cholinesterase, peroxidase, and catalase activity and in
phagocyte behaviour. The magnitude of the changes was large,
but not generally dose-related. On the basis of their results,
the authors recommended mean exposure limits for urban air for
exposure durations of 24 h, one month, and one year of 0.004,
0.0015, and 0.001 mg/m3, respectively, with a ceiling value of
0.01 mg/m3, and this recommendation has been adopted in the
USSR.
In a study of the effects of phosphine on rats by inhalation
at 0.1 mg/m3 and 0.05 mg/m3, Atchabarov et al. (1984) found a
reduction in total plasma protein without a change in the
relative proportions of the various fractions, a modest but
significant reduction in the glycoprotein A fraction with some
changes in the relative proportions of the sub-fractions,
increased plasma bile acids, and a marked increase in
seromucoids. Liver glycogen, lipids, and cytochrome oxidase
levels were reduced. Biochemical changes were similar to those
in a control group treated with hydrogen fluoride at a known
toxic level.
8.2.2. Oral exposure to metal phosphides
Bai et al. (1980) reported a 13 week feeding study in female
weanling albino rats. Zinc phosphide was mixed with the diet at
0 (control), 50 mg/kg (50 ppm), 100 mg/kg (100 ppm), 200 mg/kg
(200 ppm), and 500 mg/kg (500 ppm) w/w. Deaths occurred at the
2 higher dosage regimes in 1/12 and 10/12 animals, respectively.
Food intake and weight increase were reduced and dose-dependent
depilation occurred at all dosages. The relative weights of
liver, heart, brain, and thyroid were increased at 200 and
500 mg/m3 and the serum zinc and liver alkaline phosphatase
levels were increased at the highest dose. There was a dose-
dependent reduction in haemoglobin concentration, red cell
count, and haematocrit.
The WHO/FAO Data Sheet on Pesticides (No. 24) which deals
with zinc phosphide (WHO/FAO, 1976) cites a study in which
300 mg zinc phosphide/kg administered to rats produced 6/6
deaths in the second week while 200 mg/kg produced reduced
weight gain and 2/6 deaths. Histopathological studies revealed
liver damage in the peripheral and central lobular areas and the
lungs were congested with haemorrhage or exudate in the alveolar
spaces.
8.2.3. Dermal and other exposures
No reports are available on short-term studies involving the
dermal or other routes.
8.3. Skin and Eye Irritation; Sensitization
Zinc phosphide powder moistened with saline produced mild
skin irritation in rabbits (Draize method) (Rao, 1986).a
8.4. Long-Term Exposure
No long-term studies on phosphine or metal phosphides
exposure have been reported.
8.5. Reproductive, Mutagenicity, and Carcinogenicity
There have been no long-term reproductive, mutagenicity, or
carcinogenicity studies on phosphine or metal phosphides.
---------------------------
a Personal communication to the IPCS Task Group on Phosphine
and Metal Phosphides.
8.6. Factors Modifying Toxicity; Toxicity of Metabolites
Aminzhanov (1972) reported that 100 mg zinc phosphide/kg
body weight was lethal for dogs starved for 24 h beforehand but
not for dogs that were fed.
Phosphine is not a powerful reducing agent and its toxic
effects are unlikely to be associated with chemical reduction.
The recent discovery that phosphites and phosphorous acid have a
fungicidal activity (Bompeix & Saindreman, 1984) suggests that
metabolites of phosphine have biological effects and therefore
might contribute to its toxicity. However, this contribution
can only be small, since phosphine is only slowly oxidized to
hypophosphites and phosphites and a maximum of about 40% of the
phosphide dose is recovered as these metabolites, even with
small doses. Moreover, the toxicity of phosphites is much less
than that of phosphides (Schulz, 1887). The contribution of
toxicity of contaminants of phosphine such as diphosphides is
uncertain.
8.7. Mechanisms of Toxicity - Mode of Action
Studies on isolated rat liver showed that mitochondrial
oxygen uptake was inhibited by phosphine (Nakakita et al., 1971)
and that this effect was due to the reaction of phosphine with
cytochrome c and cytochrome c oxidase (Kashi & Chefurka, 1976).
Although this inhibitory in vitro effect was also shown in
insects, it was found that insects severely poisoned with
phosphine did not suffer any inhibition of their cytochrome
system (Price & Dance, 1983). In the same report, phosphine was
found to inhibit insect catalase though this appeared to be an
indirect effect and might have been a result of phosphine
toxicity rather than a cause.
There have not been any systematic studies on the mechanism
of phosphine toxicity. Various effects on intermediary
metabolism have been described. Ikimanova (1977) reported
increased blood- and urinary-porphyrin concentrations, which
were related to the dose of zinc phosphide and the duration of
treatment. In a study on rabbits, Minchev & Dimitrov (1970)
reported changes in serum glutamic-pyruvic and glutamic-
oxaloacetic transaminases, leucine aminopeptidase, aldolase,
alkaline phosphatase and albumin in the first 24 h of zinc
phosphide poisoning. Dysfunction of hepatic fat metabolism was
also observed. Loss of cell viability and cell membrane
integrity accounted for the raised hepatic enzymes, the
bronchiolitic effect, the cloudy swelling of renal tubular cells
and the occasionally reported haemorrhagic lesions in the
myocardium. There is no adequate explanation for the fact that
phosphine does not cause the haemolysis characteristic of
arsine.
9. EFFECTS ON MAN
9.1. Organoleptic Effects
The odour of phosphine depends on the impurities it con-
tains; phosphine of high purity has no odour, even at 280 mg/m3
(Dumas & Bond, 1974; Fluck, 1976). Phosphine prepared conven-
tionally, without purification, has a fishy or garlic-like odour
attributed to impurities. These may be adsorbed by stored pro-
ducts during fumigation with resultant loss of odour, even
though phosphine remains at toxic concentrations (Bond & Dumas,
1967). Amoore & Hautala (1983) reviewed the literature on odour
thresholds and stated that the geometric mean of all the 6
reported values for phosphine was 0.71 mg/m3 (0.51 ppm). Fluck
(1976) reported that thresholds for phosphine at which 50% or
more of 10 persons could positively identify the odour asso-
ciated with phosphine differed according to the source of the
phosphine (Table 16).
Table 16. Odour thresholds for phosphinea
--------------------------------------------------------------
Source Odour thresholds for
PH3 (mg/m3 converted
from ppm)
--------------------------------------------------------------
Technical aluminium phosphide + H2SO4 0.14 - 0.28
Phosphorium iodide + aqueous hydroxide 2.8
Phostoxin + H2SO4 0.014 - 0.028
(5 Å molecular seived) 1.4 - 2.8
Phostoxin + H2SO4
--------------------------------------------------------------
a From: Fluck (1976)
Amoore & Hautala (1983) classified phosphine in safety class
D in their classification because 20 - 50% of attentive persons
can detect the threshold limit value (TLV) (0.42 mg/m3) by
smell. However, the smell of phosphine cannot be relied on to
warn of toxic concentrations.
9.2. General Population Exposure
9.2.1. Phosphine
There is negligible exposure of the general population to
phosphine. Gessner (1937) described an incident in which the 12
inhabitants of an apartment house developed nausea and one died
when phosphine was emitted from an adjacent warehouse containing
bags of aluminium phosphide, which became damp. Some passengers
on ships and barges carrying cargoes of ferrosilicon or grain
under fumigation have also been poisoned by phosphine (Harger &
Spolyar, 1958; Netherlands, 1984). Effects were similar to
acute occupational poisoning.
Hallerman & Ribilla (1959) reported the deaths of 2 adults
and a child living in a dwelling with a party wall to a granary
being fumigated. Symptoms were non-specific and insidious
initially and illustrate the risks of sustained exposures to
relatively low concentrations (a few mg phosphine/m3); it was
estimated that the concentration in the bedroom reached
1.2 mg/m3. At autopsy, there was congestion of all organs.
Pulmonary oedema and focal emphysema were found in the lungs and
there was vacuolation in the liver.
9.2.2. Metal phosphides
Zinc phosphide baits and formulated aluminium phosphide
pellets are widely used. Occasional accidental, or more usually
suicidal, exposure to metal phosphides may be encountered.
Ingestion, the only easily toxic route, has almost always been
suicidal and the effects acute (Stephenson, 1967).
Stephenson (1967) reviewed 20 cases of acute zinc phosphide
poisoning by ingestion (including one treated by himself) in
which the approximate dose was recorded. Ten cases were fatal
and the doses ranged from 4.5 to 180 g; 6 cases had ingested
20 g or more. In the 10 non-fatal cases, the doses ranged from
0.5 to 50 g with 7/10 ingesting less than 20 g. In the case
treated by the author, clinical features included metabolic
acidosis, hypocalcaemic tetany, methaemalbuminaemia, and reduced
blood coagulation (thrombotest 28% of normal). Post-mortem
findings included blood in all the serous cavities, pulmonary
congestion and oedema, haemorrhagic changes in the intestinal
epithelium, centrilobular congestion and necrosis and yellow
discoloration of the liver, and patchy necrosis of the proximal
convoluted tubules of the kidneys.
An unsuccessful suicidal attempt was described by Zipf
(1967). A 25-year-old man ingested 6 tablets of PhostoxinR in
water (= 6 g phosphine). Immediate symptoms were severe retro-
sternal pain, a generalized burning sensation, and vomiting.
There was circulatory collapse necessitating resuscitation and
he was also treated by gastric lavage with potassium perman-
ganate and magnesium sulfate. Subsequently, symptoms of cardiac,
cerebral, and hepatic dysfunction appeared and there was severe
renal failure requiring haemodialysis.
9.3. Occupational Exposure
Cases of acute phosphine poisoning reported in the litera-
ture were reviewed by Harger & Spolyar (1958). Fifty-nine cases
with 26 deaths had been recorded since 1900 (including Gessner's
(1937) non-occupational cases). In 6 out of 11 papers, cargoes
of ferrosilicon were cited as the source of phosphine and, in
these cases, the victims were passengers or crew members of the
ships or barges concerned. Other cases involved the exposure of
welders to calcium carbide and raw acetylene and of submariners
to sodium phosphide. The most common autopsy finding was
congestion of the lungs with marked oedema. The authors added a
case of their own in which a 16-year-old youth operating
acetylene generators died, probably as a result of phosphine
exposure at a level of about 11 mg/m3 (8 ppm) for 1 - 2 h daily
over a period of 6 weeks. Arsine and hydrogen sulfide exposures
were considered to have been too low to have accounted for the
death. Autopsy revealed acute pulmonary oedema. Other cases
have been described, all of which exhibited similar features
(Ziemer, 1963; Furuno et al., 1976; & Netherlands, 1984). Three
groups of symptoms of acute phosphine poisoning were described
by Childs & Coates (1971): (a) nervous symptoms including
headache, vertigo, tremors, and unsteady gait, progressing in
severe cases to convulsions, coma, and death; (b) gastro-
intestinal symptoms include loss of appetite, thirst, nausea and
vomiting, diarrhoea, and severe epigastric pain; and (c) respir-
atory symptoms including a feeling of pressure and pain in the
chest as well as shortness of breath. In addition, a sharp fall
in blood pressure was described as a characteristic, but seldom-
mentioned symptom. Chronic effects include anaemia, bronchitis,
and gastrointestinal, speech, and motor disturbances, but these
are by no means general.
The toxic effects of phosphine are summarized in Table 17.
The IDLH (Immediately Dangerous to Life or Health) level is
282 mg/m3 (200 ppm) (US EPA, 1985b).
Verga & Belloni (1958) described a case of purpura ascribed
to poisoning by phosphine. The platelet count was reduced to
60 000/mm3; the red-cell count was also low at 3.1 x 106/mm3 and
the haemoglobin concentration (Sahli) was 55%. With recovery,
both the red-cell and thrombocyte counts increased to 4.8 x 106
and 210 000/mm3, respectively, at the end of the observation
period. The electrolysis of phosphate solutions being applied
to metals as a corrosion inhibitor was considered to have
produced phosphine, but there was no measurement of the level of
exposure and it is possible that the exposure also included
arsine, which would account for the anaemia with a hyperplastic
erythroid series in the marrow biopsy. While haemolysis is
recognized as a complication of arsine poisoning, purpura and
thrombocytopenia are not widely recognized features of
phosphine poisoning, though Borodin et al. (1983) described
changes related to haemolysis (prolongation of clotting time and
reduced plasminogen levels) in workers exposed to a variety of
biocides and disinfectants, including zinc phosphide. However,
these changes could not be related specifically to exposure to
phosphine.
Table 17. Toxic effects of phosphinea
------------------------------------------------------------
Effect Concentration
mg/m3 ppm
------------------------------------------------------------
Rapidly fatal 2800 2000
Death after 1/2 - 1 h 560 - 840 400 - 600
Dangerous to life after 1/2 - 1 h 400 - 600 290 - 430
Serious effect after 1/2 - 1 h 140 - 260 100 - 190
No serious effects after 1/2 - 1 h 10 7
------------------------------------------------------------
a From: Childs & Coates (1971).
In 1978, Beloskurskaya et al. published the findings of a
study on 206 phosphorus workers, which revealed 57 cases of
toxic hepatitis. In 49 of these cases, there was stated to be no
other apparent cause than occupational exposure to phosphorus,
phosphine, or phosphorus oxides. No measurements of exposure
were reported. Diagnostic criteria included a history of
hypochondrial pain, clinical hepatomegaly, and abnormal liver
function tests and 131I-Rose Bengal scanning and clearance
studies. Forty-six of the 57 cases had additional symptoms,
such as the astheno-vegetative syndrome, chronic gastritis,
impotence, toxic encephalopathy, toxic cardiomyopathy, and
respiratory disease. There was no control group and no informa-
tion on alcohol consumption was provided.
In a later study, Wilson et al. (1980) described the
symptoms of acute poisoning by phosphine as headache, fatigue,
nausea, vomiting, jaundice, paraethesia, ataxia, tremor,
diplopia, myocardial infiltration with necrosis, pulmonary
oedema, and myocardial and peripheral muscle damage. Laboratory
results indicated that there had been urinary tract involvement
(occult blood) and levels of several liver enzymes were
elevated. Furthermore, Roaldsnes (1982) reported that metal-
workers at a large shipyard in Norway, drilling deep holes in
spheroidal graphite iron, became ill during work. The symptoms
were mostly nausea, dizziness, chest tightness, dyspepsia, and
disturbances of smell and taste. Measurement of the phosphine
concentration in the workers' breathing zone (with Dräger tubes)
showed a phosphine concentration of about 1.4 mg/m3 (1 ppm).
After installing local exhaust ventilation on the drilling
machines, there were no longer any measurable amounts of
phosphine, and there were no complaints from the workers. When
the local exhaust ventilation was removed for technical reasons
5 years later, illness among the workers recurred. Measurement
of phosphine levels just above the machines, showed concentra-
tions of up to 56 - 70 mg/m3 (40 - 50 ppm). When the local
exhaust ventilation was re-installed, the phosphine concentra-
tions dropped to unmeasurable amounts, and no further cases of
illness were reported. Addition of copper sulfate solution to
the cutting oil effectively removed the phosphine gas, but
resulted in problems of corrosion.
Jackson & Elias (in press) studied liver function and
gastrointestinal symptoms in: (a) workers exposed to white
phosphorus at up to 5 times the occupational exposure limit and
also to some phosphine (0 - 0.5 mg/m3); (b) workers exposed to
phosphine alone (at up to 2.8 mg/m3, depending on the efficacy
and use of air-line breathing apparatus); and (c) gastro-
intestinal symptoms in a group of workers who were not exposed
to either compound, but who worked the same shift pattern.
Complaints to the factory medical department of gastrointestinal
symptoms in the phosphine-exposed group, in the phosphorus
exposed-group, and in the unexposed group were 1.51, 0.61, and
0.24 per man per year, respectively. However, the alcohol
consumption in the phosphine- and phosphorus-exposed groups
differed (289 and 154 ml per week, respectively). The alcohol
consumption was not estimated in the control group. None of the
conventional liver function tests reflected the differences in
occupational exposure or alcohol intake. The phosphine-exposed
group did have a significantly raised post-prandial bile acid
concentration, but whether this reflected a difference in phos-
phine exposure or alcohol consumption could not be determined.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Phosphine and metal phosphides are only focally distributed
in the environment and exposure of large numbers of the general
population is unlikely. The sources and uses of phosphine and
metal phosphides are sufficiently well established for safety
techniques to be generally known, though it is disturbing that
shipboard poisonings are still occurring when the first case was
reported over half a century ago. The extensive world-wide use
of metal phosphides as fumigant sources and as rodenticides
creates the hazard of accidental poisoning in children and
others and provides a means of suicide, unless the supply and
use of these materials is appropriately regulated. There is no
evidence of danger to the population from residues of phosphine
in fumigated foods or from residues of phosphides, if adequate
fumigation and aeration techniques are used. Permitted limits
for residues are in the range of 0.01 - 0.1 mg total phosphine/
kg. There do not appear to be any deleterious effects on food
quality as a result of adequately undertaken fumigation with
phosphine.
Published data on occupational exposure to phosphine in most
industries are not sufficient to evaluate the success of control
measures in relation to occupational exposure limits. There is
also some disagreement regarding these limits and some data
suggest that an occupational exposure limit of 0.4 mg
phosphine/m3 is above the threshold for biological effects with
long-term exposure. However, there are no studies adequate to
settle this question definitively.
Major accidental release of stored phosphine presents a
serious explosion/fire hazard and an acute toxic hazard for man
and animals.
10.2. Evaluation of Effects on the Environment
Only very small amounts of phosphine and phosphides occur in
the environment as a result of natural processes and those
resulting from human activity are not persistent.
Phosphine and phosphides are introduced into the environment
for the control of pests, for which they are particularly
suitable, because of their efficacy, lack of persistence, and
harmless decomposition products.
Careful positioning of bait and the low toxicity of
carcasses for scavengers of poisoned animal targets minimize the
effects of rodenticidal metal phosphides on non-target species.
Phosphine and phosphides are oxidized in the environment and the
final product, phosphate, is normally widely present in the
natural environment. Phosphine and phosphides are only used
where the natural environment is already modified by human
activity and their environmental impact must be viewed in this
context.
Since resistance to phosphine in insects does not appear to
reverse in the absence of selective pressures, it is
particularly important to achieve effective doses of phosphine
by using adequate treatment rates and good practices. In
practice, the development of resistance is more of a problem
than the effects on non-target species. Major accidental
release of stored phosphine would not result in long-term
environmental consequences.
10.3. Conclusions
In general, there is no risk to the public from the use of
phosphine or phosphides, from emissions from fumigated products
or spaces, from alloys, industrial processes, or residues in
food, provided that proper fumigation, transport, and industrial
practices are used.
While phosphine and metal phosphides are toxic, their
extensive use in occupational settings has resulted in the
establishment of good practice guidelines. If these are
followed, there is a low order of risk for human health.
However, there is some doubt about the completeness of
protection afforded by the higher occupational exposure limit
(0.4 mg phosphine/m3).
There is no risk of important environmental effects from
phosphine or metal phosphides.
11. RECOMMENDATIONS
11.1. Gaps in Knowledge
1. The principal area related to human health where information
is inadequate is concerned with occupational exposure levels and
their safety. There is a need for an adequate study of well-
being and organ function in exposed and control populations,
with a complete description of phosphine exposure, concurrent
exposures to other chemicals (which should be low), alcohol
consumption, and smoking habits. The establishment of exposure
levels that are free from any detectable effects would help to
determine agreed occupational exposure limits.
2. It would be of assistance, in this regard, to undertake
laboratory studies to identify biological markers that are most
indicative of phosphine effects and reflect the suggested short-
term cumulative effects of repeated phosphine exposure.
3. Further research on the mechanisms of action of phosphine
and phosphides will assist in the design of good fumigation
practice and fumigation schedules, and minimize the occurrence
of resistance in target organisms and associated problems.
4. It has been suggested that zinc phosphide may be relatively
persistent in aquatic sediments and there is a general lack of
aquatic toxicological data. There is a need for research on the
levels and persistence of zinc phosphide in sediments and for
further studies of its effects on aquatic organisms.
11.2. Preventive Measures
11.2.1. Management
The most important factor in the safe handling of phosphine
and metal phosphides, and in their formulation, is proper work
practices.
Management should identify these, provide training for the
operatives, and ensure that the practices are carried out.
Personal protection measures recommended to reduce the
likelihood of absorption of phosphide preparations include the
wearing of:
(a) synthetic rubber gloves;
(b) rubber boots;
(c) lightweight impervious overalls; and
(d) suitable eye protection.
Adequate washing facilities should be available at all times
during handling. Eating, drinking, and smoking should be
prohibited during handling, and before washing after handling.
The means to measure concentrations of phosphine in air should
be available and used to check atmospheric concentrations. When
necessary, respiratory protective equipment should be worn. In
fumigation, each operator or other person liable to be exposed
to the gas must be provided with an efficient means of respira-
tory protection. Persons exposed to magnesium phosphide or
aluminium phosphide powders (or other readily hydrolysed
phosphides), which may give rise to airborne dust should be
protected by respiratory protective equipment, effective against
gaseous phosphine, since hydrolysis of dust in the filter of a
dust mask or respirator may give rise to high phosphine
exposure.
11.2.2. Treatment of poisoning
11.2.2.1 Inhalation of phosphine
(a) First Aid
Remove from exposure, keep at rest. Rescuers should follow
full safety procedures.
If the patient is unconscious, place in semi-prone recovery
position or otherwise maintain the airway.
If the patient is conscious but has difficulty in breathing,
treat in a seated position and give oxygen if available.
Otherwise, allow the patient to recline with the legs slightly
elevated.
If breathing stops, immediately ventilate the patient
artificially (mouth-to-mouth/nose or mechanically with oxygen,
if available).
If the heart stops, begin cardiopulmonary resuscitation
(CPR).
(b) Medical Treatment
1. Give oxygen by mask if required; perform baseline chest X-
ray and examine chest. Treat shock conventionally.
2. All patients should be observed for 48 - 72 h, as onset of
pulmonary oedema may be delayed.
3. If respiratory distress is severe, give steroids (methyl-
prednisolone 30 mg/kg, or equivalent, intramuscularly),
preferably within 4 h of exposure.
4. If pulmonary oedema occurs, treat by positive and expiratory
pressure ventilation. Antibiotics should only be given if a
secondary infection is present.
5. Treat any fits conventionally; give general supportive care
with particular attention to fluid balance.
6. Some sources of exposure to phosphine are likely to lead
also to exposure to arsine, which has haemolytic effects.
Monitor for haemoglobinaemia, haemoglobinuria, unconjugated
hyperbilirubinaemia, and renal failure.
In general, recovery is rapid following removal from
exposure, but renal damage and leukopenia may occur after
several days.
11.2.2.2 Ingestion of metal phosphides
(a) First aid
Do not give milk, fats, or saline emetics by mouth.
Give oxygen if there is respiratory distress.
If first aiders are medically authorized to do so, and the
patient is conscious, induce vomiting.
After 20 min (or after vomiting), administer activated
charcoal (50 g in water by mouth) if available.
Obtain medical attention as soon as possible: preferably
send immediately to hospital.
(b) Medical treatment
1. Consider tracheal intubation and gastric lavage with 2%
sodium bicarbonate solution (to limit hydrolysis of zinc
phosphide).
2. Activated charcoal or medicinal liquid paraffin may limit
absorption of phosphine and zinc phosphide, respectively,
and may be administered by mouth or stomach tube.
3. Monitor and support vital functions, particularly hepatic
and renal function. Treat shock conventionally.
5. Hepatic and renal failure should be treated in specialist
centres.
11.2.3. Leaks, spillages, residues, and empty containers
11.2.3.1 Phosphine
Small leaks and residues of compressed gas can be discharged
slowly to the atmosphere in the open air. Larger quantities
should be burned using an appropriate burner.
11.2.3.2 Aluminium and magnesium phosphides and their formulated
preparations
Spillages and residues in containers will evolve phosphine
for several days by reaction with atmospheric moisture. Respir-
atory protective equipment will be required by those dealing
with them. Even if atmospheric concentrations of phosphine are
initially low, they may rise as residues are disturbed.
Moderate quantities should be collected in a secure place to
protect the public and wildlife, and left in the open air and
kept moist until hydrolysis is complete.
Larger amounts should be removed to a deep pit, at an
approved site, until phosphine evolution is complete. Then they
can be buried.
Residues at the site of spillage should be washed away in a
large quantity of water and the area kept secure and aerated
until checked for zero gas concentration.
Combustible packages can be incinerated at high temperature
(> 1000 °C) using proper facilities. Containers should NOT be
cleaned for re-use, but should be disposed of by deep burial, at
an approved site, well away from habitation and where there is
no danger of contamination of water sources.
11.2.3.3 Zinc phosphide and preparations
Zinc phosphide hydrolyses only slowly. Nevertheless,
phosphine may be evolved and respiratory protective equipment
should be available.
Spillages and residues in containers should be incinerated
at high temperatures (> 1000 °C) or crushed and buried below the
topsoil at an appropriate site. Contaminated surface material
should be treated similarly or else the area should be secured
from public access until the gas concentration is zero.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Phosphine was evaluated in 1971 by WHO and FAO, as a
pesticide residue in food (WHO/FAO, 1972). A tolerance of
0.1 mg/kg (0.1 ppm) in raw cereals was confirmed. It was
recommended that the tolerance of 0.01 mg/kg (0.01 ppm) in
flour, other milled cereal products, breakfast cereals, dried
vegetables, and spices be confirmed and extended to include
nuts, groundnuts, dried fruit, cocoa beans, and other similar
foods, known to be fumigated with phosphine.
The use of zinc phosphide as a rodenticide in public health
was reviewed by WHO in 1972. It was concluded that it was a
generally effective compound and, while highly toxic to domestic
fowl, its safety record was good. The use of zinc phosphide was
endorsed (WHO, 1973).
REFERENCES
ACGIH (1986) Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists.
ADU, O.O. & MUTHU, M. (1985) The relative toxicity of seven
fumigants to life cycle stages of Callosobruchus chinensis L.
Insect Sci. Appl., 6(1): 75-78.
ADVANI, R. (1983) A comparative trial of zinc phosphide,
aluminium phosphide and RH-787 against field mixed population of
rodents in crop ecosystem of the Indian desert. Ztg angew.
Zool.,70(2): 153-168.
AHMAD, M. (1976) Effect of phosphine fumigation on the
germination of edible legume seeds. J. stored Prod. Res., 12:
211-212.
AL-OMAR, M.A. & AL-BASSOMY, M. (1984) Persistence of phosphine
gas in fumigated Irafir dates. J. food Saf., 6: 253-260.
AL'ZHANOV, S., MOLDABEKOV, Sh.M., ERSHOV, V.A., & POPOV, A.P.
(1983) [ Use of copper and iron sulfates in cleaning furnace
gas from phosphorus production,] Moscow, Academy of Sciences of
the USSR, Institute of Scientific Information (VINITI) (Document
4802-83) (in Russian).
AMINZHANOV, M. (1972) [Use of zinc phosphide in controlling
stray dogs.] In: Azimov, Sh.A., Shevchenko, N.Kh., & Nurmatov,
R.Sh., ed. [ Proceedings of the 7th Conference of Young Uzbek
Agricultural Scientists,] Tashkent, Uzbeksii Nauchno-Issled.
Veterinary Institute (in Russian).
AMOORE, J.E. & HAUTALA, E. (1983) Odour as an aid to chemical
safety: odour thresholds compared with threshold limit values
and volatilities for 214 industrial chemicals in air and water
dilution. J. appl. Toxicol., 3(6): 272.
ANON. (1936) [Trials to establish whether the Delicia grain
weevil fumigation procedure is safe to apply and free from the
risk of fire and explosion.] Gutachten chim. tech. Reichsanst.,
8(4): 5 (in German).
ANON. (1956) Ferro-silicon explosion risks. Chem. Trade J.
Chem. Eng., 16 Nov.: 1180.
ARENDT, G., FARWIK, W., HAAG, F., KAUFMANN, G., KOHL, E.-G.,
PRUGGMAYER, D., & WAGNER, W. (1979) [Environmental aspects of
pest control. Fumigation of grain and food stores.] Lebensmit-
teltechnik, 5: 2-8 (in German).
ARNOLD, J.T. & ROBBIANO, P. (1974) Portable vapour surveil-
lance system, Springfield, Virginia, National Technical
Information Service (NTIS Report No. AD-782 844).
ATCHABAROV, B.A., AITBAYEV, T.KH., & AITBEMBETOV, B.N. (1984)
[Comparison of the effects of hydrogen fluoride and phosphine on
the organism with various exposure levels.] In:[ Health care in
Kazakhstan. Part 1,] pp. 28-31 (in Russian).
ATTIA, F.I. (1984) Multiple cross-resistance characteristics
in phosphine-resistant strains of Rhizopertha dominica and
Tribolium castaneum. In: Ripp, B., ed. Controlled atmosphere
and fumigation in grain storages, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 49-53 (Developments in
Agricultural Engineering No. 5).
ATTIA, F.I. & GREENING, H.G. (1981) Survey of resistance to
phosphine in coleopterous pests of grain and stored products in
New South Wales. Gen. appl. Entomol., 13: 93-97.
BAI, K.M., KRISHNAKUMARI, M.K., RAMESH, H.P., SHIVANANDAPPA, T.,
& MAJUMDER, S.K. (1980) Short-term toxicity study of zinc
phosphide in Albino rats (Rattus norvegicus). Indian J. exp.
Biol., 18(8): 854-857.
BARRETT, W.J. & DILLON, H.K. (1978) Development of methods
for the determination of elemental phosphorus and phosphine in
air, Cincinnati, Ohio, National Institute of Occupational Safety
and Health, 102 pp (DHEW (NIOSH) Publication No. 78-177).
BECKER, K.H., BIEHL, H.M., BRUCKMANN, P., FINK, E.H., FUHR, F.,
KLOPFER, W., ZELLNER, R., & ZETSCH, C. (1984) Methods for the
ecotoxicological evaluation of chemicals, photochemical degra-
dation in the gas phase. VI. OH x reaction rate constants and
tropospheric lifetimes of selected environmental chemicals,
Jülich, Kernforschungsanlage (Projektträgerschaft Umweltchemi-
kalien Report 1980-83, No. 279).
BELL, C.H., WILSON, S.M., & BANKS, H.J. (1984) Studies on the
toxicity of phosphine to tolerant stages of Trogoderma granarium
everts (Coleoptera: Dermestidae). J. stored Prod. Res., 20(2):
111-117.
BELOSKURSKAYA, G.I., PARASKEVOPULOS, YA.G., & SHLYGINA, O.E.
(1978) [On the clinical and functional condition of the liver
in patients with chronic phosphorus poisoning.] Tr. Inst. Kraev.
Patol. Akad. Nauk Kazakhskoi SSR, 36: 25-30 (in Russian).
BLAXLAND, J.D. & GORDON, R.F. (1945) Zinc phosphide poisoning
in poultry. Vet. J., 101: 108-110.
BOENIG, I.A., CRUTCHFIELD, M.M., & HEITSCH, C.W. (1982)
Phosphorus compounds. In: Kirk-Othmer, ed. Encyclopaedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol.
17, pp. 490-539.
BOMPEIX, G. & SAINDRENAN, P. (1984) In vitro antifungal
activity of Fosetyl Al and phosphorous acid on Phytophthera
species. Fruits, 39(12): 777-786.
BOND, E.J. & DUMAS, T. (1967) Loss of warning odour from
phosphine. J. stored Prod. Res., 3: 389-392.
BOND, E.J., ROBINSON, J.R., & BUCKLAND, C.T. (1969) The toxic
action of phosphine. Absorption and symptoms of poisoning in
insects. J. stored Prod. Res., 5: 289-298.
BOND, E.J., DUMAS, T., & HOBBS, S. (1984) Corrosion of metals
by the fumigant phosphine. J. stored Prod. Res., 20(2): 57-63.
BONTOYAN, W.R. (1981) Report on pesticide formulations: herbi-
cides, fungicides and miscellaneous. J. Assoc. Off. Anal. Chem.,
64(2): 355.
BORODIN, A., KLIMEK, M., & ZAKL, K. (1983) [Blood clotting and
fibrinolysis systems in workers of a disinfection, disinfesta-
tion and deratization employment (sic).] Med. Pr., 34(4): 313
(in Polish).
BOUBAL, M., LOUISE, J., BELOT, D., & POLITI, J.M. (1981)
Analyse directe et continue de traces d'hydrures gazeux à très
basses teneurs, Paris, Institut National de la Proprieté
Industrielle, Sociéte d'Air Liquide (Document No. 2 506 459)
(Demande de Brevet d'Invention No. 81 10316).
BOWKER, J.R. (1958) The liberation of phosphine in the
machining of spheroidal graphite iron. In: Proceedings of the
British Cast Iron Research Association, 8 November, p. 50.
BOWLEY, C.R. & BELL, C.H. (1981) The toxicity of twelve
fumigants to three species of mites infesting grain. J. stored
Prod. Res., 17: 83-87.
BRADFIELD, A.A.G. & GILL, J.E. (1984) Laboratory trials of
five rodenticides for the control of Mesocricetus auratus
Waterhouse. J. Hyg. (Lond.), 93: 389-394.
BRANDON, R.W. (1983) The use of chemically impregnated paper
tapes for toxic gas detection and monitoring. Anal. Chem. Symp.
Ser., 17: 726-31.
BREYER, D. (1973) [Studies on hydrogen phosphide residues in
grain after treatment with fumigation preparations in powder
form.] Mühle Mischfuttertech., 110: 699-700 in German).
BUBIEN, Z., WARTENBERG, L., & ROSZYK, E. (1974) [Content of
zinc in hen carcasses poisoned with zinc phosphide.] Zesz. Nauk.
Akad. Roln Wroclawiu Weter., 31: 173-180 (in Polish).
CABROL TELLE, A.M. & DE SAINT BLANQUAT, G. (1984) In vitro
digestibility of starch, casein and triolein after fumigation
with phosphine. Sci. Aliments, 4(Hors Ser. 3): 287-291.
CABROL TELLE, A.M., DE SAINT BLANQUAT, G., DERACHE, R, HOLLANDS,
E., PERIQUET, B., & THOUVENOT, J.-P. (1985) Nutritional and
toxicological effects of long-term ingestion of phosphine-
fumigated diet by the rat. Food Chem. Toxicol., 23(11): 1001-
1009.
CALVERT, J.G. & PITTS, J.N., Jr (1966) Photochemistry, New
York, John Wiley and Sons.
CHAMP, B.R. & DYTE, C.E. (1976) Report of the FAO global
survey of pesticide susceptibility of stored grain pests, Rome,
Food and Agriculture Organization of the United Nations, pp. 1-
257 (FAO Plant Production and Protection Paper No. 5).
CHAN, L.T.F., CROWLEY, R.J., DELLIOU, D., & GEYER, R. (1983)
Phosphine analysis in post-mortem specimens following ingestion
of aluminium phosphide. J. anal. Toxicol., 7: 165-167.
CHENTSOVA, N.YU. (1972) [Danger to poultry during scattering
of zinc phosphide-poisoned grain to control rodents in forest
plantings.] In: Voronova, L.D., ed. [ Effects of pesticides on
wild animals,] Moscow, pp. 90-102 (in Russian).
CHILDS, A.F. & COATES, H. (1971) The toxicity of phosphorus
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3, pp. 1437-1440.
CHILDS, D.P. (1972) Tobacco consignment fumigated in
containers while in transit. Container News, October.
CHILDS, D.P., OVERBY, J.E., & NIFFENEGGER, D. (1969) Phosphine
fumigation of flue-cured tobacco warehouses for the control of
the cigarette beetle. Tobacco, 168(21): 20-25.
CHOPRA, G. (1984) Field efficacy of aluminium phosphide
against rodents. Indian J. agric. Sci., 54(3): 204-205.
CIBA (1978) Phosphorus in the environment: its chemistry and
biochemistry, Amsterdam, Oxford, New York, Elsevier North
Holland (Ciba Foundation Symposium 57 - new series).
CODEX ALIMENTARIUS (1986) Codex maximum limits for pesticide
residues, 2nd ed., Rome, Food and Agriculture Organization of
the United Nations (CAC/Vol. XIII-Ed.2).
COLE, R.J. & BENNETT, W.H. (1950) An investigation of the
possibility of the occurrence of phosphine and acetylene in the
atmosphere of factories for the powdering of magnesium. J. Soc.
Chem. Ind., 69: 29-30.
CORBRIDGE, D.E.C. (1974) The structural chemistry of
phosphorus, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 268-269.
COX, P.D., BELL, C.H., PEARSON, J., & BEIREN, M.A., (1984) The
effect of diapause on the tolerance of larvae of Ephestia
küehniella to methyl bromide and phosphine. J. stored Prod.
Res., 20(4): 215-219.
CURRY, A.S., PRICE, D.E., & TRYHORN, F.G. (1959) Absorption of
zinc phosphide particles. Nature (Lond.), 184(4686): 642-643.
DAVIS, R. (1986) Fumigation of ships. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 18-21 March, London, Foreign and
Commonwealth Office, Overseas Development Administration,
Tropical Development and Research Institute, pp. 24-34.
DELOMENIE, H. (1933) Gases evolved from ferrosilicons under
the influence of water. J. Pharm. Chim., 18: 289-292, 532
DIEKE, S.H. & RICHTER, C.P. (1946) Comparative assays of
rodenticides on wild Norway rats. Public Health Rep., 61: 672-
679.
DIETERICH, W.H., MAYR, G., HILD, K., SULLIVAN, J.B., & MURPHY,
J. (1967) Hydrogen phosphide as a fumigant for foods, feeds
and processed food products. Residue Rev., 19: 135-149.
DISNEY, R.W. & FOWLER, K.S. (1972a) Residues in cereals
exposed to hydrogen phosphide. In: Radiotracer studies on
chemical residues in food and agriculture. Proceedings of a
Combined Panel and Research Coordination Meeting, Vienna,
Austria, 25-29 October 1971, Vienna, International Atomic
Energy Agency, pp. 99-101.
DISNEY, R.W. & FOWLER, K.S. (1972b) The possibility of isotope
exchange or other interfering reactions occurring during the
determination of residues from fumigation with 32P-labelled
phosphine. In: Radiotracer studies on chemical residues in food
and agriculture. Proceedings of a Combined Panel and Research
Coordination Meeting, Vienna, Austria, 25-29 October 1971,
Vienna, International Atomic Energy Agency, pp. 87-88.
DUMAS, T. (1964) Determination of phosphine in air by gas
chromatography. J. agric. food Chem., 12(3): 257-258.
DUMAS, T. (1978) Modified gas chromatographic determination of
phosphine. J. Assoc. Off. Anal. Chem., 61(1): 5-7.
DUMAS, T. (1980) Phosphine sorption and desorption by stored
wheat and corn. J. agric. food Chem., 28: 337-339.
DUMAS, T. & BOND, E.J. (1974) Separation of phosphine from
odour-producing impurities. J. stored Prod. Res., 10: 67-68.
DUMAS, T. & BOND, E.J. (1981) Method of trapping low levels of
phosphine at ambient temperature for gas-chromatographic
analysis. J. Chromatogr., 206(2): 384-386.
EPPO (1975) Guidelines for the development and biological
evaluation of rodenticides, Paris, European and Mediterranean
Plant Protection Organisation (Bulletin No. 5(1)).
FITZPATRICK, R.J., MCGIRR, J.L., & PAPWORTH, D.S. (1955) The
toxicity of rodenticides. Vet. Rec., 67: 142-145.
FLUCK, E. (1973) The chemistry of phosphine. Fortschr. chim
Forsch., 35: 1-64.
FLUCK, E. (1976) The odor threshold of phosphine. J. Air
Pollut. Control Assoc., 26(8): 795.
FRANK, R. & RIPPEN, G. (1986) [ Content of phosphine in the
atmosphere,] Frankfurt am Main, Batelle-Institut e.v. (in
German).
FREYBERG, W. (1979) Acute toxicity of Detia R pellets to
bluegill (Lepomis macrochirus ), Washington DC, US Environmental
Protection Agency (Unpublished report).
FRITZ, B., LORENZ, K., STEINERT, W., & ZELLNER, R. (1982)
Laboratory kinetic investigations of the tropospheric oxidation
of selected industrial emissions. In: Versino, B. & Ott, H., ed.
Physico-chemical behaviour of atmospheric pollutants,
Dordrecht, Reidel, pp. 192-202.
FURMAN, N.H., ed. (1962) Standard methods of chemical
analysis, 6th ed., Princeton, New Jersey, D. van Nostrand
Company Inc., Vol. 1, pp. 821-823.
FURUNO, J., SUGAWARA, N., & IKEDA, H. (1976) A fatal case
poisoned by hydrogen phosphide and the simultaneous separation
of inorganic gases by gas chromatography. Bull. Yamaguchi med.
Sch., 23(1-2): 49-58.
GESSNER, O. (1937) Fatal phosphine poisoning from "Delicia"
grain weevil fumigant (aluminium phosphide). Samml. Vergiftungs-
fällen, 8: B-79.
GILL, J.E. & REDFERN, R. (1983) Laboratory tests of seven
rodenticides for the control of Meriones shawi. J. Hyg. (Lond.),
91: 351-357.
HABASHI, F. & ISMAIL, M.I. (1975) Health hazards and pollution
in the metallurgical industry due to phosphine and arsine. CIM
Bull., August: 99-103.
HACKENBERG, U. (1972) Chronic ingestion by rats of standard
diet treated with aluminium phosphide. Toxicol. appl.
Pharmacol., 23: 147-158.
HALLERMANN, W. & PRIBILLA, O. (1959) [Fatal poisoning with
phosphine.] Arch. Toxikol., 17(4): 219-242 (in German).
HARGER, R.N. & SPOLYAR, L.W. (1958) Toxicity of phosphine with
a possible fatality from this poison. Am. Med. Assoc. Arch.
Ind. Health, 18: 497-504.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds.
Wildlife, 191: 1-61.
HILTON, H.W. & ROBISON, W.H. (1972) Fate of zinc phosphide and
phosphine in the soil-water environment. J. agric. food Chem.,
20(6): 1209-1213.
HOLE, B.D. (1981) Variation in tolerance of seven species of
stored product Coleoptera to methyl bromide and phosphine in
strains from twenty-nine countries. Bull. entomol. Res., 71(2):
299-306.
HOLE, B.D., BELL, C.H., MILLS, K.A., & GOODSHIP, G. (1976) The
toxicity of phosphine to all developmental stages of thirteen
species of stored product beetles. J. stored Prod. Res., 12:
235-244.
HUGHES, J.G. & JONES, A.T. (1963) Estimation of phosphine in
air. Ind. Hyg. J., March-April: 164-167
HUNTER, D. (1978) The diseases of occupations, 6th ed.,
London, Hodder and Stoughton.
IKEDA, S. (1971) [Effect of zinc phosphide on Coturnix coturnix
japonica.] Ringyo Shikenjo Kenkyu Hokuko, 238: 141-148 (in
Japanese).
IKIMANOVA, G.K. (1977) [Porphyrin metabolism in experimental
phosphorus poisoning.] Zdravookhr. Kaz., 1: 39-42 (in Russian).
IMO (1980) Recommendations on the safe use of pesticides in
ships, London, International Maritime Organization (UN IMO
Circular No. 298, revised).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, 303 pp (Data
Profile Series No. 5).
JACKSON, J.R. & ELIAS, E. (1984) Hepatic function and exposure
to phosphine and white phosphorus. In: Proceedings of the 12th
International Congress on Occupational Health in the Chemical
Industry, Dublin, Ireland, 9-14 September 1984, pp. 219-228.
JANDA, J. (1973) Danger of rodenticides containing zinc
phosphide to wild life. Agrochemia, 13(3): 85-86.
JOKOTE, Ch. (1904) [Studies on hydrogen phosphine.] Arch. Hyg.
Bakteriol., 49: 275-306 (in German).
JONES, A.T., JONES, R.C., & LONGLEY, E.O. (1964) Environmental
and clinical aspects of bulk wheat fumigation with aluminium
phosphide. Am. Ind. Hyg. Assoc. J., 25: 376-379.
KADKOL, S.B. & JAYARAJ, P. (1967) Effect of phosphine-
fumigated rice on the growth of albino rats. Rep. Cent. Food
Technol. Res. Inst. (Mysore), 2: 6.
KAGAN, YU.S. (1985) Principles of pesticide toxicology,
Moscow, Centre of International Projects, GKNT, 176 pp.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1973)
The phytotoxic effect of repeated fumigation of certain cereal
crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 7: 57-62.
KAMEL, A.H., FAM, E.Z., MAHDY, M.T., & SHELTAWY, E.M. (1974)
The phytotoxic effect of carbon bisulphide, methyl bromide and
hydrogen phosphide on the germination of seeds of certain cereal
field crops. Bull. Entomol. Soc. Egypt (Econ. Ser.), 8: 75-80.
KAO, C. (1981) Determination of aluminium phosphide in
fumigants. J. food Sci., 46: 1632-1634.
KASHI, K.P. (1975) A mixed indicator strip for phosphine
detection. Pestic. Sci., 6: 511-514.
KASHI, K.P. (1981a) Toxicity of phosphine to five species of
stored-product insects in atmospheres of air and nitrogen.
Pestic. Sci., 12: 116-122.
KASHI, K.P. (1981b) Response of five species of stored-product
insects to phosphine in oxygen-deficient atmospheres. Pestic.
Sci., 12: 111-115.
KASHI, K.P. (1982) Dose-mortality responses of five species of
stored-products insects to phosphine. Int. Pest Control,
March/April: 46-49.
KASHI, K.P. & CHEFURKA, W. (1976) The effect of phosphine of
absorption and circular dichroic spectra of cytochrome-c and
cytochrome-c oxidase. Pestic. Biochem. Physiol., 6: 350-362.
KASHI, K.P. & MUTHU, M. (1975) A mixed indicator strip for
phosphine detection. Pestic. Sci., 6: 511-514.
KIM, H.H., KIM, K.H., & HONG, S.S. (1977) Effect of pesticides
on exocrine pancreas and liver. Yonse Uidea Nonmunjip, 10(2):
170-180.
KLIMMER, O.R. (1969) [Contribution on poisoning by phosphine.
On the question of sub-chronic phosphine intoxication.] Arch.
Toxikol., 24: 164 (in German).
KOZHEMYAKIN, N.G., BUTYAGIN, V.N., BASHMURIN, A.F., & POTAPENKO,
M.I. (1971) [Veterinary-sanitary evaluation of the carcasses
and organs of animals which had to be killed in relation to
their contamination with zinc phosphide.] Sb. Rab. Leningrad
Vet. Inst., 32: 231-236 (in Russian).
KUHN, H., MAREK, J., & REIF, H. (1971) [Fumigation of tobacco
with phosphine.] Fachliche Mitt. Austria Tabakwerke AG, 12: 191-
203 (in German).
LADOU, J. (1983) Potential occupational health hazards in the
microelectronics industry. Scand. J. Work. environ. Health, 9:
42-46.
LEESCH, J.G. (1982) Accuracy of different sampling pumps and
detector tube combinations to determine phosphine
concentrations. J. econ. Entomol., 75: 899-905.
LEESCH, J.G. (1984) Fumigation of lettuce: efficacy and phyto-
toxicity. J. econ. Entomol., 77(1): 142-150.
LOWE, E.J. (1971) Phosphorus hydrides and phosphonium
compounds. In: Mellor's comprehensive treatise on inorganic and
theoretical chemistry, White Plains, New York, Longman, Vol. 8,
Suppl. 3.
LUCK, H. & ALLEMANN, A. (1984) Control of insect infestation
during storage of dairy products. Fumigation with hydrogen
phosphide. S. Afr. J. dairy Technol., 16(3): 103-107.
LUTZMANN, L., KEINITZ, M., & KLOSTERKOTTER, W. (1963)
[Emission of phosphine during transport of ferrosilicon.] Med.
Welt, 58: 114-116 (in German).
MCGIRR, J.L. (1953) Poisoning of livestock by the newer
rodenticides, insecticides and weed killers. In: Proceedings of
the XVth International Veterinary Congress, Stockholm, 9-15
August, Part 1, Vol. 1, pp. 479-480.
MCMAHON, R. & FORESE, F.F. (1983) Hydride gas detecting tape,
Glenview, Illinois, MDA Scientific Inc. (UK Patent Application
GB 2 108 662A).
MARSHALL, E.F. (1981) Efficacy variations of rodent baits with
the same active ingredient. Pest Control, January: 22-23.
MATHEW, G.G. (1961) The production of phosphine while
machining spheroidal graphite iron. Ann. occup. Hyg., 4: 19-35.
MEISSNER, R. (1924) [The toxicology of hydrogen phosphide.] Z.
gesamte Med., 42: 267 (in German).
MEREDITH, C. (1981) Toxicological studies on zinc phosphide,
Birmingham, University of Birmingham, Department of Bio-
chemistry (M.Sc. Thesis).
MINCHEV, T. & DIMITROV, D. (1970) [Early changes in the serum
of rabbits poisoned with zinc phosphide.] In: Vasilev, R.A., ed.
[ Nauch. Tr. Sud. Med. Deontol.,] Sofia, Meditsina i
Fizkultura, pp. 33-38 (in Bulgarian).
MUELLER, W. (1940) [Toxicity of phosphine (animal
experiments). Part 1. Acute and subacute toxicity.] Arch. exp.
Pathol. Pharmakol., 195: 184-193 (in German).
MUTHU, M., KRISHNAKUMARI, M.K., MURALIDHARA, V., & MAJUMDER,
S.K. (1980) A study on the acute inhalation toxicity of
phosphine to albino rats. Bull. environ. Contam. Toxicol., 24:
404-410.
NAKAKITA, H. & WINKS, R.G. (1981) Phosphine resistance in
immature stages of a laboratory selected strain of Tribolium
castaneum Herbst (Coleoptera: Tenebrionidae). J. stored Prod.
Res., 17: 43-52.
NAKAKITA, H., KATSUMATA, Y., & OZAWA, T. (1971) The effect of
phosphine on the respiration of rat liver mitochondria. J.
Biochem., 69: 589-593.
NATARAJAN, T. & BAGYARAJ, D.J. (1984) Fumigation effect on
microflora and viability of blackgram and fieldbean seeds.
Pesticides, 18(4): 40-42.
NAUS, A. (1982) [Olfactory thresholds of some industrial
compounds.] Prac. Lek., 34(6-7): 217-218 (in Polish).
NETHERLANDS, WERKGROEP VAN DESKUNDIGEN VAN DE NATIONALE MAC-
COMMISSIE. (1984) [ Report on the TLV of phosphine,] 2nd ed.,
Voorburg, Working Group of the National TLV Committee, 27 pp
(Report No. 1/80) (in Dutch).
NEUBERT, D. & HOFFMEISTER, I. (1960) Changes in intermediary
metabolism following exposure to hydrogen phosphide. Naunyn-
Schmiedeberg's Arch. exp. Pathol. Pharmakol., 239: 219-233.
NOACK, S., VON & REICHMUTH, C. (1981) [Determination of limits
for injuring animals and plants by phosphine and methyl bromide.
I. Studies on Drosophila melanogaster. Anz. schädlingskd.
Pflanzenschutz Umweltschutz, 54: 23-27 (in German).
NOACK, S., VON & REICHMUTH, C. (1982a) [Quantities of
phosphine, methyl bromide and hydrocyanic acid used in stored
products protection in the FRG from 1975-1977.] Nachrichtenbl.
Dtsch. Pflanzenschutzd., 34(2)S: 17-21 (in German).
NOACK, S., VON & REICHMUTH, C. (1982b) [Determination of
limits for injuring animals and plants by phosphine or methyl
bromide. II. Studies on water cress and lettuce Lactuca sativa
capitata.] Anz. schädlingskd. Pflanzenschutz. Umweltschutz,
55: 57-59 (in German).
NOACK, S., VON & WOHLGEMUTH, R. (1985) [Phosphine residues in
hazelnuts, soybeans and wheat after fumigation with non-constant
concentrations.] Z. Lebensm. Unters. Forsch., 180: 101-108 (in
German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1983)
[Relationship of PH3 residues after fumigation to concentration,
time of exposure and length of storage.] Z. Lebensm. Unters.
Forsch., 177: 87-93 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984a)
[Decomposition of phosphine in treated foods related to storage
temperature and aeration.] Z. Lebensm. Unters. Forsch., 178: 31-
37 (in German).
NOACK, S., VON, REICHMUTH, C., & WOHLGEMUTH, R. (1984b) [A
half-empirical mathematical model for calculation of PH3-
residues in hazelnuts fumigated with phosphine.] Z. Lebensm.
Unters. Forsch., 178: 97-103 (in German).
NOWICKI, T.W. (1978) Gas-liquid chromatography and flame
photometric detection of phosphine in wheat. J. Assoc. Off.
Anal. Chem., 61(4): 829-836.
PAZYNICH, V.M., MAZUR, I.A., & PODLOZNYI, A.V. (1984)
[Experimental substantiation and prediction of maximum
atmospheric phosphine concentrations differentiated by time.]
Gig. i Sanit., 1984(1): 13-15 (in Russian).
PRICE, N.R. (1984) Active exclusion of phosphine as a
mechanism of resistance in Rhyzopertha dominica F (Coleoptera:
Bostrychidae). J. stored Prod. Res., 20(3): 163-168.
PRICE, N.R. & DANCE, S.J. (1983) Some biochemical aspects of
phosphine action and resistance in three species of stored
product beetles. Comp. Biochem. Physiol., 76C(2): 277-281.
PRICE, N.R., MILLS, K.A., & HUMPHRIES, L.A. (1982) Phosphine
toxicity and catalase activity in susceptible and resistant
strains of the lesser grain borer (Rhyzopertha dominica).
Comp. Biochem. Physiol., 73C: 411-413.
RANGASWAMY, J.R. (1984) Simple spectrophotometric method for
determination of phosphine residues in wheat. J. Assoc. Off.
Anal. Chem., 67(1): 117-122.
RANGASWAMY, J.R. & MUTHU, M. (1985) Spectrophotometric
determination of phosphine residues in rice. J. Assoc. Off.
Anal. Chem., 68(2): 205-208.
REBMANN, Z. (1933) [On a new pesticide (Fosfolon).] Gesund-
heitstech. Städtehyg. ver. Städtische Tiefbau, 5: 280-284 (in
German).
REICHMUTH, C., ed. (1985) [Response of the granary weevil
Sitophilus granarius L. (Coleoptera: Curculionidae) to
gradually increasing and decreasing phosphine concentrations.]
Anz. schädlingskd. Pflanzenschutz Umweltschutz, 58(1): 10-16
(in German).
REICHMUTH, C., ed. (1986) The significance of changing
concentrations in toxicity of phosphine. In: Proceedings of the
GASGA Seminar on Fumigation Technology in Developing Countries,
Slough, United Kingdom, 8-21 March, London, Overseas Development
Administration, Tropical Development and Research Institute.
pp. 88-98.
REICHMUTH, C., NOACK, S., & WREDE, A. (1981) [Emission of
phosphine caused by fumigation for stored products protection.]
Nachrichtenbl. Dtsch. Pflanzenschutzd., 33(9): 132-136 (in
German).
ROALDSNES, J. (1982) [Phosphine poisoning due to machining of
ductile iron.] Norsk. bedv. h. tj., 3: 12-16 (in Norwegian).
ROBINSON, J.R. (1972) Residues containing phosphorus following
phosphine treatment: measurement by neutron activation. J.
stored Prod. Res., 8: 19-26
ROBINSON, J.R. & BOND, E.J. (1970) The toxic action of
phosphine. Studies with 32PH3: terminal residues in biological
materials. J. stored Prod. Res., 6: 133-146.
ROBISON, W.H. & HILTON, H.W. (1971) Gas chromatography of
phosphine derived from zinc phosphide in sugarcane. J. agric.
food Chem., 19(5): 875-878.
ROHRLICH, M. & MEUSER, F. (1970) [Studies on grain fumigated
with hydrogen phosphide. III. Communication: biochemical aspects
of phosphine fumigation.] Getreide Mehl, 20(1): 1-8 (in
German).
RUSCHEL, A.P. & DA COSTA, W.F. (1966) [Symbiotic fixing of
atmospheric nitrogen in the french bean ( Phaseolus vulgaris L).
III. Effect of some insecticides and fungicides.] Pesqui.
Agropecu. Bras., 1: 147-149 (in Portugese).
SAEED, T. & ABU-TABANJA, R. (1984) Determination of phosphine:
comparison of rates of desorption by purge-and-trap method and
by sulfuric acid treatment. J. Assoc. Off. Anal. Chem., 68(1):
61-64.
SATO, K. & SUWANAI, M. (1974) Absorption of hydrogen phosphide
to cereal products. Appl. Entomol. Zool., 9(3): 127-132.
SAYVETZ, T.A. (1982) Possible phosphine inhalation related to
warehouse fumigation, Richmond, Virginia, Virginia State Health
Department, Office of Health Protection and Environmental
Management, 6 p.
SCHITOSKEY, F. (1975) Primary and secondary hazards of three
rodenticides to kit fox. J. wildl. Manage., 39(2): 416-418.
SCHULZ, H. (1887) [Correction concerning the toxicity of
phosphorus compounds containing oxygen.] Arch. exp. Pathol., 23:
150-151 (in German).
SCHULZ, H. (1890) [Concerning phosphine.] Arch. exp. Pathol.
Pharmakol., 27: 314-335 (in German).
SHAHEEN, D.G., BRAGG, L.A., STANOVICK, R.P., & SULLIVAN, J.B.
(1983) Phosphine detector tubes as passive dosimeters for use
with metal phosphide fumigants. Tob. Int., 185(20): 36-39.
SHIVANANDAPPA, T., RAMESH, H.P., & KRISHNAKUMARI, M.K. (1979)
Rodenticidal poisoning of non-target animals: acute oral
toxicity of zinc phosphide to poultry. Bull. environ. Contam.
Toxicol., 23(4-5): 452-455.
SINGH, K.N., SRIVASTAVA, B.P., & NATH, G. (1983) Phosphine
residues and its effect on the germination of stored pulses.
Indian J. Entomol., 45(1): 71-80.
SRI (1982) Electronic chemicals, Menlo Park, California,
Standford Research International, pp. 40-41.
SRIDHARA, S. (1983) Rodenticide induced bait aversion and
neophobia in Tatera indica cuvieri. Z. angew. Zool., 70(4): 429-
440.
STEPHENSON, J.B.P. (1967) Zinc phosphide poisoning. Arch.
environ. Health, 15: 83-88.
TERZIC, Lj. (1981) Quantitative method for the determination
of zinc phosphide in technical products. Acta vet. (Beograd),
31(5-6): 279-288.
TIETJEN, H.P. (1976) Zinc phosphide: its development as a
control agent for black-tailed prairie dogs, Washington DC, US
Department of the Interior, Fish and Wildlife Research Centre
(Special Scientific Report Wildlife No. 195).
TKACHUK, R. (1971) Phosphine fumigation of wheat - residue
formation. Cereal Sci. Today, 16: 307.
TKACHUK, R. (1972) Phosphorus residues in wheat due to
phosphine fumigation. Cereal Chem., 49: 258-267.
TRIMBORN, H. & KLIMMER, O. (1962) [Experimental studies on
chemical changes in blood pigments caused by hydrogen
phosphide in vitro.] Arch. int. Pharmacodyn., 137(3-4): 331-
347 (in German).
UNDERWOOD, J.G. (1972) The radiochemical analysis of phosphine
residues in fumigated tobaccos. Tob. Sci., October 13: 47-49.
URGA, K. (1983) Methyl bromide and phosphine residues in
cereal grains and pulses. Ethiop. med. J., 21: 79-83.
US CFR (1981) Code of federal regulations, 29(1910): 673.
US EPA (1981) Pesticide registration standard: aluminium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-137514).
US EPA (1982) Pesticide registration standard: magnesium
phosphide, Washington DC, US Environmental Protection Agency (PB
82-195777).
US EPA (1983) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 48(37): 7714-
7716.
US EPA (1984) Hazardous waste management system: identifica-
tion and listing of hazardous waste. Fed. Reg., 49(92): 19922-
19923.
US EPA (1985a) Aluminium phosphide/magnesium phosphide:
tolerances for residues. Code Fed. Reg., 40: 180, 225, 291-292,
333, 375.
US EPA (1985b) EPA chemical profile: phosphine, Washington DC,
US Environmental Protection Agency.
US EPA (1986) Guidance for the re-registration of pesticide
products containing aluminium phosphide, February 1986. An
amendment to the registration standard issued in October 1981,
Washington DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances (PB 84-241405).
US NIOSH (1977) National occupational hazard survey,
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, Vol. 3 (Publication No. 78-114).
US NIOSH (1979) Ten NIOSH analytical methods: phosphine,
Cincinnati, Ohio, National Institute for Occupational Safety
and Health, Division of Physical Sciences and Engineering, 42
pp.
VAN WAZER, J.R., ed. (1961) Phosphorus and its compounds:
technology, biological functions and applications, New York,
Interscience Publishers Inc., Vol. II, p. 957.
VAN WAZER, J.R. (1982) Phosphorus and the phosphides. In:
Kirk-Othmer, ed. Encyclopaedia of chemical technology, 3rd ed.,
New York, John Wiley and Sons, Vol. 17, pp. 473-489.
VDOVENKO, I.D., VAKULENKO, L.I., YAKOVLEVA, L.A., SHEDENKA,
L.I., BERESLAVSKAYA, A.N., & YATSENKO, V.S. (1984) [Effect of
surfactants on phosphine formation in steel pickling.] Ukr.
Khim. Zh., 50(4): 418-421 (in Russian).
VERGA, L. & BELLONI, F. (1958) [Over a case of purpura due to
empoisonment of phosphine (a haemato-clinical and histochemical
study.] Rass. Med. ind. Ig. Lav., 27(3): 230-241 (in Italian).
VERSTUYFT, A.W. (1978) Sampling and analytical methods for
phosphine - a review. Am. Ind. Hyg. Assoc. J., 39: 431-437.
VINSJANSEN, A. & THRANE, K.E. (1978) Gas-chromatographic
determination of phosphine in ambient air. Analyst, 103: 1195-
1198.
WARITZ, R.S. & BROWN, R.M. (1975) Acute and subacute
inhalation toxicities of phosphine, phenylphosphine and
triphenylphosphine. Am. Ind. Hyg. Assoc. J., 36: 452-458.
WEBLEY, D.J., HARRIS, A.H., & KILMINSTER, K. (1981) The use of
a portable gas analyser to measure phosphine concentrations in
experimental fumigations in the tropics. Int. pest Control,
March/April: 47-50.
WILSON, A. (1971) The metal phosphides. In: Mellor's compre-
hensive treatise on inorganic and theoretical chemistry, White
Plains, New York, Longman, Vol. VIII, Suppl. III.
WILSON, R., LOVEJOY, F.H., JAEGER, R.J., & LANDRIGAN, P.L.
(1980) Acute phosphine poisoning aboard a grain freighter.
Epidemiologic, clinical and pathological findings. J. Am. Med.
Assoc., 244(2): 148-150.
WINKS, R.G. (1970) Phosphine fumigation of farm-stored
tobacco. Queensland agric. J., November.
WINKS, R.G. (1984) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): time as a dosage factor. J.
stored Prod. Res., 20(1): 45-56.
WINKS, R.G. (1985) The toxicity of phosphine to adults of
Tribolium castaneum (Herbst): phosphine-induced narcosis. J.
stored Prod. Res., 21(1): 25-29.
WHO (1973) WHO Technical Report Series, No. 513 (Twentieth
Report of the Expert Committee on Insecticides), Geneva, World
Health Organization, p. 39.
WHO/FAO (1972) 1971 Evaluations of some pesticide residues in
food, Geneva, World Health Organization, pp. 289-295 (WHO
Pesticide Residue Series, No. 1).
WHO/FAO (1976) Data sheet on pesticides No. 24: zinc
phosphide, Geneva, World Health Organization (VBC/DS/77.24).
WHO/FAO (1980) Data sheet on pesticides No. 46: phosphine,
Geneva, World Health Organization (VBC/DS/80.46).
WORTHING, C.R. & WALKER, S.B., ed. (1983) The pesticide manual:
a world compendium, 7th ed., London, British Crop Protection
Council.
ZIEMER, U. (1963) [Fatal hydrogen phosphide poisoning on a
freighter.] Zbl. Arbeitsmed., 13: 38-39 (in German).
ZIPF, K.E., ARNDT, TH., & HEINTZ, R. (1967) [Clinical
observation of a case of Phostoxin poisoning.] Arch. Toxikol.,
22(4): 209-222 (in German).
ZUTSHI, M.K. (1966) Effect of aluminium phosphide (Phostoxin)
on the germination of certain cereal and vegetable seeds. Bull.
grain Technol., 4(4): 197-199.
