
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 67
TETRADIFON
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154267 5
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1986
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TETRADIFON
1. SUMMARY AND CONCLUSIONS
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental levels
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Food
4.1.5. Other products
4.1.6. Terrestrial and aquatic organisms
4.2. General population exposure
4.3. Occupational exposure
5. KINETICS AND METABOLISM
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.2. Terrestrial organisms
6.3. Microorganisms
6.4. Bioaccumulation
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Toxicity
7.1.2. Skin Irritation
7.1.3. Eye irritation
7.2. Short-term exposures
7.3. Long-term exposures
7.4. Effects on reproduction
7.5. Mutagenicity
7.6. Carcinogenicity
8. EFFECTS ON MAN
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON THE
ENVIRONMENT
9.1. Evaluation of health risks for man
9.2. Evaluation of effects on the environment
9.3. Conclusions
10. RECOMMENDATIONS
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
ANNEX EXTRACT FROM HEALTH AND SAFETY GUIDE INCLUDING
INTERNATIONAL CHEMICAL SAFETY CARD
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
ORGANOCHLORINE PESTICIDES
Members
Dr L. Albert, Environmental Pollution Programme, National Institute
of Biological Resource Research, Xalapa, Mexico (Vice-
Chairman)b
Dr E. Astolfi, Faculty of Medicine of Buenos Aires, Buenos Aires,
Argentinaa
Dr I. Desi, Department of Environmental Hygienic Toxicology,
National Institute of Hygiene, Budapest, Hungary (Vice-
Chairman)a
Dr R. Drew, Department of Clinical Pharmacology, Flinders
University of South Australia, Bedford Park, South
Australiaa
Dr Y. Hayashi, Pathology Division, National Institute of
Hygienic Sciences, Tokyo, Japanb
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, Indiaa
Dr R. Kimbrough, Center for Environmental Health, Centers for
Disease Control, Atlanta, Georgia, USA (Rapporteur)b
Dr A.N. Mohammed, University of Calabar, Calabar, Nigeriaa
Mr Y.T. Mosuro, Federal Ministry of Health, Food, and Drug
Administration and Laboratory Services, Oshodi, Nigeriab
Dr Y. Osman, Occupational Health Department, Ministry of
Health, Khartoum, Sudanb
Dr O.E. Paynter, Office of Pesticide Programs, US Environmental
Protection Agency, Washington DC, USAa
Dr W.O. Phoon, Department of Social Medicine and Public
Health, Faculty of Medicine, University of Singapore,
Singapore (Chairman)a
Dr L. Rosival, Centre of Hygiene, Research, Institute of
Preventive Medicine, Bratislava, Czechoslovakia (Chairman)b
Dr Sakdiprayoon Deema, Ministry of Agriculture and Cooperatives,
Bangkok, Thailandb
Dr F.W. van der Kreek, Ministry of Welfare, Health, and
Culture, Leidschendam, Netherlandsb
Dr D. Wassermann, Department of Occupational Health, The
Hebrew University, Hadassah Medical School, Jerusalem,
Israela
Dr Xue Shou Zheng, School of Public Health, Shanghai Medical
University, Shanghai, Chinab
Representatives of Other Organizations
Dr A. Berlin, Health and Safety Directorate, Commission of the
European Communities, Luxembourgb
Dr V.E.F. Solman, International Union for Conservation of
Nature and Natural Resources (IUCN), Ottawa, Ontario,
Canadaa
Observers
Dr H. Kaufmann, International Group of National Associations
of Agrochemical Manufacturers (GIFAP), Brussels, Belgiuma
Dr A.A. van Kolfschoten, International Group of National
Associations of Agrochemical Manufacturers (GIFAP),
Brussels, Belgiumb
Secretariat
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
(Temporary Advisor)a,b
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerlanda,b
Dr K.W. Jager, Division of Environmental Health, International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland (Secretary)a,b
Dr A. Pelfrene, Vector Biology and Control, Insecticides
Development and Safe Use, World Health Organization,
Geneva, Switzerlandb
Dr T. Vermeire, National Institute for Public Health and
Environmental Hygiene, Bilthoven, Netherlands (Temporary
Adviser)b
Dr D.C. Villeneuve, Health Protection Branch, Department of
National Health and Welfare, Tunney's Pasture, Ottawa,
Ontario, Canada (Temporary Advisor) (Rapporteur)a
Mr J.D. Wilbourn, International Agency for Research on Cancer,
Lyons, Francea
--------------------------------------------------------------------------
a Present at first Task Group meeting.
b Present at second Task Group meeting.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR TETRADIFON
A WHO Task Group on Environmental Health Criteria for
Organochlorine Pesticides other than DDT (Endosulfan, Quintozene,
Tecnazene, Tetradifon) was held at the Health Protection Branch,
Department of National Health and Welfare Ottawa from 28 May - 1
June, 1984. The meeting was opened by Dr E. Somers, Director-
General, Environmental Health Directorate, and Dr K.W. Jager
welcomed the participants on behalf of the three co-sponsoring
organizations of the IPCS (UNEP/ILO/WHO). The Task Group reviewed
and revised the draft criteria document and concluded that the data
available were so sparse that no proper evaluation could be made of
the potential hazard of tetradifon for the general population,
exposed workers, or the environment. The Task Group recommended
that a preliminary hazard assessment should be circulated to all
IPCS and IRPTC national focal points with a request for further
information.
A second WHO Task Group was convened in Geneva 9-13 December
1985 to review and revise an amended draft and to make an
evaluation of the risks of tetradifon on human health and the
environment.
The initial drafts of the tetradifon document were prepared by
DR D.C. VILLENEUVE of Canada and DR S. DOBSON of the United
Kingdom.
The proprietary data mentioned in this document were made
available to the Central Unit by Duphar BV for use at the Task
Group.
The present draft was prepared by the IPCS Secretariat,
updating the preliminary hazard assessment with new information
received in more than 50 replies.
Selected sections from the Health and Safety Guide on
Tetradifon, published by the World Health Organization, are
included as an Annex.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the costs of printing.
1. SUMMARY AND CONCLUSIONS
Technical tetradifon (1,2,4-trichloro-5-(4-chlorophenyl)-
sulfonyl benzene) is a white crystalline solid that is more than
94% pure. It is used in formulation as an acaricide. The method of
choice for its determination is gas-liquid chromatography with
electron capture detection.
Tetradifon is persistent and only slightly mobile in soils.
The compound degrades more rapidly with increasingly aerobic
conditions. Both the parent compound and its initial degradation
product in soil, adsorb on soil particles and resist leaching.
The short-term toxicity of tetradifon is low for birds,
moderate for fish, and moderate to high for aquatic crustacea. It
is relatively non-toxic for bees. Long-term studies are not
available for these organisms. Tetradifon does not bioaccumulate
significantly in fish, which metabolize and eliminate the compound
rapidly. No adverse effects on terrestrial plants have been
reported and tetradifon did not have any effects on cultures of an
aquatic unicellular alga (Chlorella pyrenoidova).
Exposure of the general population is mainly through food,
but, with recommended application rates, residues in food are
virtually absent. Occupational exposure levels for workers
spraying crops at recommended use levels have been estimated to be
far below toxic levels, based on oral and dermal LD50s for rats.
In the rat, most (70%) of a single, orally-administered dose
of tetradifon was excreted via the bile in the faeces within 48 h.
Part of the remainder was distributed in all organs and tissues.
The results of one study suggest that tetradifon and its
metabolites are rapidly excreted from the body. On continued
dosing in beef cattle, tetradifon was detected in adipose tissue.
It is not known whether tetradifon is excreted in milk.
The oral LD50 for technical tetradifon in the rat ranges from
5000 to 14 700 mg/kg body weight. Tetradifon has been classified
in the category of technical products that are unlikely to present
acute hazards if used as recommended. The formulated product may
be more toxic, depending on the other components of the
formulation. The toxicity may also vary with the purity of the
product. In the past, both chlorinated dibenzodioxins and 2,4,5-T
have been identified in samples of tetradifon. It has been shown
that, with regular strict quality control procedures, levels of
these contaminants are below the limits of detection.
In a 90-day study on rats, 50 mg/kg tetradifon in the diet was
a no-observed-adverse-effect level. At higher dose levels,
induction of microsomal liver enzymes occurred, with increased
liver weight and increased endoplasmic reticulum. At 200 mg/kg
diet, histological changes were noted in the thyroid. In a 2-year
study on rats, induction of microsomal enzymes and increased liver
weight were noted at a dietary level of 1200 mg/kg.
No effects on reproduction were found in a 2-generation
reproduction study on rats administered tetradifon at 0, 40, 200,
or 1000 mg/kg diet. In a 90-day study on the F2b generation, the
only effects of dietary tetradifon were a lower body weight gain
and an increased dose-related incidence of dilated renal pelvis.
In a 1-year study on dogs, enlarged livers were seen at
dietary concentrations of 5000 mg technical tetradifon/kg. Small
infarct-like spots in the outer cortical layer of the kidneys were
reported at a dietary level of 5000 mg/kg and in one of 4 dogs
administered 1000 mg/kg diet. Serum-alkaline phosphatase values
were slightly elevated at 5000 mg/kg diet. The no-observed-adverse-
effect level in this study was suggested to be in the range of 500
- 1000 mg/kg diet, which is roughly equivalent to a daily intake of
12.5 - 25 mg/kg body weight.
Tetradifon was negative in short-term in vitro tests for
mutagenicity.
Negative results were obtained in a screening test for
carcinogenicity in mice, but the test was considered to be
inadequate for the evaluation of the carcinogenicity of this
compound.
No adverse health effects from exposure to tetradifon have
been reported in man.
Although the information available for this evaluation of
tetradifon is incomplete and not always up to present-day
standards, there are no indications, at present, that the normal
recommended use of tested tetradifon products as an acaricide
causes any health or safety hazards for the general population,
exposed workers, or the environment.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
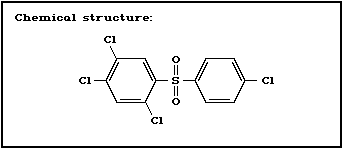 Molecular formula: C12H6C14O2S
CAS chemical name: 1,2,4-trichloro-5-[(4-chlorophenyl)-
sulfonyl]-benzene
Synonyms: 4-chlorophenyl-2,4,5-trichlorophenyl sulfone,
2,4,4',5-tetrachlorodiphenyl sulfone
Common trade names: Akaritox, Aredion, Duphar 23737, ENT 23737,
FMC 5488, Mition, NIA 5488, Polacaritox,
Roztoczol, Roztozol, Tedion V18,
Tetradichlone (a complete list of trade names
is available from IRPTC)
CAS registry number: 116-29-0
2.2. Physical and Chemical Properties
Some physical and chemical properties of tetradifon are given
in Table 1. The solubility of tetradifon in different organic
solvents at room temperature varies from 10 g/litre (in kerosene
and methanol) to 255 g/litre (in chloroform) (Van Rossum et al.,
1978).
Tetradifon is manufactured by a Friedel-Craft's reaction
between 2,4,5-trichlorophenylsulfonyl chloride and
monochlorobenzene in the presence of anhydrous aluminium chloride
or ferric chloride (Van Rossum et al., 1978), or by Sandmeyer
diazotization (Windholz et al., 1983). The technical product is
more than 94% pure, but since production processes may vary, the
type and quality of impurities present may differ in commercial
products from different companies. In addition, the limits of
detection for different chlorinated dibenzodioxins, in commercial
products vary. In a study by Woolson et. al. (1972), the limit of
detection for chlorinated dibenzodioxins was equal to or less than
0.5 mg/kg. The di-, tri-, tetra-, and hexachlorinated
dibenzodioxins were not separated from each other; the authors
stated that higher chlorinated dibenzodioxins were found in some
samples of tetradifon, but no specific information was given.
Table 1. Some physical and chemical properties
of tetradifona
-------------------------------------------------
Physical state crystalline solid
Colour slightly yellow
Relative molecular mass 356.04
Melting point 148 - 149 °C
Vapour pressure (20 °C) 0.32 x 10-10 kPa
Octanol/water partition 4.61
coefficient
Solubility in water (10 °C) 0.05 %
(20 °C) 0.08 %
(50 °C) 0.34 %
-------------------------------------------------
a From: Van Rossum et al. (1978) and Duphar
BV, personal communication (1982).
Impurities including 2-chlorophenyl-2,4,5-trichlorophenyl
sulfone up to a maximum level of 5 g/kg and 2,4,5-trichloro-
phenoxyacetic acid (2,4,5-T) have been reported by Matano et al.
(1971) and trace amounts of highly-chlorinated dibenzodioxins, but
not 2,3,7,8-TCDD by Woolson et al. (1972). It has been shown that
regular strict quality control procedures maintain the total sum of
all isomeric tetrachlorodibenzofurans in technical tetradifon at
less than 10 µg/kg (detection limit) (Duphar BV, 1985).
Furthermore, analysis of concentrated mother liquids revealed that
the concentrations of 2,3,7,8-tetrachlorodibenzofuran, the sum of
all isomeric tetrachlorodibenzo- p -dioxins, and 2,3,7,8-tetra-
chlorodibenzo- p -dioxin were all lower than 1 µg/kg, and that
2,4,5-tri-chlorophenoxyacetic acid (2,4,5-T) was not present as an
impurity (Duphar BV, 1985).
Tetradifon is resistant to hydrolysis by acid and alkali and
is non-corrosive (Worthing, 1979). Particle size has a strong
influence on its biological effectiveness in that small particles
show a better rain resistance than larger ones (Maas, 1979). In
1954, tetradifon was introduced in the Netherlands as a non-
systemic acaricide, which was toxic for the eggs (ovicide) and all
non-adult stages of a wide range of phytophagous mites. It is
used, particularly in mixtures, in horticulture, including domestic
greenhouses, mainly on top-fruit, vegetables, ornamentals, hops,
cotton, and sugarcane, and forestry.
Tetradifon remained stable to ultraviolet radiation (UVR) for
12 h at 50 - 60 °C (Duphar BV, 1985).
2.3. Analytical Methods
Several methods have been used for the extraction of
tetradifon residues from different tissues. Burke & Mills (1963)
introduced a method involving microcoulometric gas-liquid
chromatography (GLC). Mitchell (1976) used GLC with electron
capture detection to study the tetradifon contents of apple and
cucumber samples. According to Van Rossum et al. (1978), the
recovery of tetradifon from crop samples using the GLC method was
85 - 100%. They also reported that tetradifon could be extracted
from soil samples with ether and then detected directly by GLC with
electron capture detection. The limit of detection in soil samples
was 0.02 mg/kg.
Analytical methods are based on total organic chlorine, column
chromatography, and UV spectrophotometric detection (Zweig &
Sherma, 1972; Suzuki et al., 1973, 1974; Burke, 1976; Bontoyan,
1979). Zweig & Sherma (1972) mentioned a recovery of 95 - 102%
from vegetables. Gas chromatography is recommended by CIPAC
(Henriet et al., 1983) for the analysis of formulations and
technical material.
3. SOURCES, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
Tetradifon is manufactured in the Netherlands and in Italy and
formulated in several countries. The uses of tetradifon in
selected countries are shown in Table 2.
Table 2. The uses of tetradifon in selected countriesa
---------------------------------------------------------------
Country Quantity Year Uses
---------------------------------------------------------------
Colombia 2786 kg 1982 agricultural acaricide
2073 kg 1981 recommended in the growth
2343 kg 1980 of cotton, fruits, and
other products
Mexico 1000 kg 1981 on strawberries, tomatoes,
grapes, cucumber, apples,
and citrus fruits
Sweden 400 kg 1981 agricultural and
horticultural use
Thailand 42 100 kg 1984
14 600 kg 1983
14 175 kg 1982 acaricide
12 237 kg 1981 acaricide
1620 kg 1980 acaricide
216 kg 1979 acaricide
1000 kg 1978 acaricide
208 kg 1977 acaricide
272 kg 1976 acaricide
United Kingdom 320 kg/year 1975-79 acaricide
USA 1983 used prior to 1983;
registration voluntarily
cancelled in 1983 by
producers
---------------------------------------------------------------
a Information received from national contact points of
IRPTC (1984).
Responses to a questionnaire received from 49 countries around
the world indicated the continuing registration and use of
tetradifon in 37 countries. Nine developing countries stated that
tetradifon was not used, and 3 countries (China, the Federal
Republic of Germany, and the USA) stated that registration of
tetradifon had been discontinued for reasons other than its
toxicity. Thirteen of these countries taken together used
approximately 200 tonnes of tetradifon per year during the years
1982-84 (information received from national contact points of the
IPCS and IRPTC, 1985).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Only limited information is available on tetradifon as an
environmental pollutant. Its water solubility is low, and a study
by Yaron et al. (1974) showed that there was only restricted
leaching from soil by rain.
4.1. Environmental Levels
4.1.1. Air
No data are available on tetradifon levels in air.
4.1.2. Water
No data are available on tetradifon levels in water.
4.1.3. Soil
Only minute amounts of tetradifon were found in the deep
layers of soil from an irrigated potato field sprayed at a rate of
10 kg/ha. Tetradifon persisted throughout the irrigation season.
Transport of tetradifon into the soil was not affected by the
amount of water applied (Yaron et al., 1974).
In a degradation experiment, sandy loam soil was incubated
aerobically for 106 weeks. Approximately 70% of the added
tetradifon was recovered unchanged and 20% was recovered as
extractable metabolites (Borst et al., 1983). Four metabolite
fractions were identified. One chlorine atom appeared to be
substituted by more polar groups (-SOCH3, -SO2CH3, -SO3H, -SCH3)
(Borst et al., 1983; Willems et al., 1983). In a water/sandy loam
hydro-soil system, tetradifon had a half-life of 36 weeks. Almost
all of the tetradifon was retained in the soil phase. Under more
aerobic conditions, degradation was more rapid, 31% of the added
tetradifon being recovered after 32 weeks (Willems & Nimmo, 1981).
The movement of tetradifon and partially degraded tetradifon in
overlying loam soil was also investigated. The results showed that
neither tetradifon nor its degradation products are leachable
compounds (Willems & Smit, 1982).
4.1.4. Food
In a study on the fate of insecticides in an irrigated field,
Yaron et al. (1974) found a tetradifon concentration of 250 mg/kg
(wet weight) in the peel of potato tubers. This was due to the
direct contact of the pesticide with the tuber. No tetradifon was
found in the tuber pulp or in the leaves of the potato plant; the
amounts of pesticide applied in this study were much greater (2
applications of 10 kg pesticide/ha soil) than those normally used.
Radioactive tetradifon in a solution prepared from either a
wettable powder (WP) or a miscible oil (MO) was sprayed on 2 apple
trees. Changes in radioactivity in and on the leaves were followed
for 4 months. Externally, the greater part of the activity was
lost from the leaves within the first few days. Uptake into the
leaves reached a maximum in approximately 1 day equalling 8% of
initial activity with the WP and 30% with the MO. More than a
month after spraying, intact tetradifon could still be found on and
in the leaves. The level in the ripe apples was less than 0.2 mg/kg
(Halberstadt, 1958).
Cassil & Fullmer (1958) sprayed fruit-bearing apple, pear,
peach, lemon, and orange trees with either 500 or 1500 g of 25%
tedion wettable powder per 455 litres of water, 32 days before
harvest. Pears and peaches were sprayed at 0.56 litre/m2, oranges
and lemons at 1.16 litres/m2, and apples were sprayed manually to
runoff. Maximum residue levels on the surface of the fruits
sprayed at 500 and 1500 g varied from 1.5 to 3.6 and from 3.4 to
7.0 mg/kg, respectively. Tetradifon residues diminished largely as
a result of fruit growth and not through decomposition.
In a second study, Cassil & Fullmer (1958) followed tetradifon
residue levels on similarly-sprayed oranges for 100 days. As in
the first study, they concluded that tetradifon is very stable when
exposed to weathering at high summer temperatures.
For many years, the US Food and Drug Administration has
examined more than 10 000 food samples yearly using methods with
which it is possible to determine residues of tetradifon. Residues
have been found infrequently and have been limited primarily to
fresh fruits. From 1964 to 1969, 1607 fruit samples were examined
and tetradifon residues ranging from trace to 2 mg/kg were found in
only 2.05% of the fruits; from 1970 to 1976, 1201 fruit samples
were examined and similar tetradifon residues were found in only
1.42% of the samples. On the basis of the total number of fruit
samples examined, the average residue of tetradifon in fruits was
0.007 mg/kg for 1964-69 and 0.004 mg/kg for 1970-76. The only
other foods in which tetradifon residues were found during these
years were one sample of cereal by-products, one sample of fish,
and one sample of leafy vegetables. Findings have been less
frequent in recent years and have primarily been found in produce
imported from Mexico (US FDA, personal communication, 1984).
In New Zealand, apples from 32 growers were checked for
tetradifon residues. The maximum level found was 0.7 mg/kg
(average 0.11 mg/kg) (Department of Health, Wellington, personal
communication, 1984).
No studies are available from other countries.
4.1.5. Other products
When tetradifon was sprayed on a tea plantation at 0.5 g/litre
and 750 - 1000 litres/ha, initial deposits on leaves were in the
range of 12.6 - 13.6 mg/kg. After 10 days, residues were < 5
mg/kg (Rajukkannu et al., 1981). No other studies were available
for review.
4.1.6. Terrestrial and aquatic organisms
In pregnant beef cattle fed apple pomace containing about 0.07
- 0.53 mg tetradifon/kg and 0.53 - 8.33 mg total DDT/kg, for 160
days, tetradifon accumulated in depot fat at a rate similar to
those of DDT and DDD, but only 29% as fast as DDE. On day 160, the
concentrations in the extracted fat were 0.16 mg tetradifon/kg and
2.56 mg total DDT/kg (Rumsey et al., 1977). The depletion of these
two compounds following cessation of exposure was not investigated.
Thus, this study does not give any information on the persistence
of tetradifon in cattle.
Tetradifon was not detected (detection level: 0.05 mg/kg) in
the tissues of 750 fish samples collected from 11 major lakes and
rivers in Alberta, Canada (Chovelon et al., 1984).
4.2. General Population Exposure
In a market-monitoring programme conducted in California and
Arizona, during 1967, the maximum tetradifon residue found on
citrus fruits (whole fruit) was 0.3 mg/kg (Gunther, 1969). The
results of market-basket studies in the USA during 1966-67, which
included both domestic and imported food, showed that tetradifon
was virtually absent (USDA, 1968). In a market-basket study in
Spain in 1971-72, tetradifon was not detected in 97% of fruit
samples and in 87% of vegetable samples (Carrasco et al., 1976).
In the USA in the years 1975-76, tetradifon was not detected
in foods including unfortified infant and toddler total diet
samples from 10 cities in the USA sampled between August 1975 and
July 1976 (Johnson et al., 1981a,b). Furthermore, there have been
no findings of tetradifon in FDA total diet ("market basket")
studies from 1976 to the present time (US FDA, personal
communication, 1984).
While most measurements in food were negative, no information
was available on the use patterns in the countries where the food
was monitored.
4.3. Occupational Exposure
Following the analyses of dermal exposure pads or hand rinses
and of respirator pads, an estimate of the levels of dermal and
respiratory exposure that sprayers would potentially incur was made
by Wolfe et al. (1967, 1972). The values derived were 36.4 mg/h
for dermal exposure and 0.07 mg/h for inhalation (Durham & Wolfe,
1962; Wolfe et al., 1967). No information on the risks of combined
dermal and inhalation exposure is available for formulators or
workers manufacturing the product.
5. KINETICS AND METABOLISM
In a preliminary study, a few rats were fed 10 mg 35S-
tetradifon/rat per day for 10 days and then examined. The highest
percentages of radioactivity during the 10 days were found in the
faeces and urine, followed by fat, gastrointestinal tissue, liver,
and muscles (Halberstadt, 1958). One rat was given 10 mg 35S-
tetradifon suspended in peanut oil/water, by stomach tube, and
killed 48 h later. Seventy-one percent of the dose was eliminated
with the faeces, 4% with urine, and 7% was recovered from the
gastrointestinal tract. The remaining 18% was present in very
small quantities in all organs and tissues. Only 20 - 40% of the
activity in organs was associated with unchanged tetradifon
(Halberstadt, 1958). The Task Group realized that results from
single animals are difficult to interpret, however, no other data
were available.
In another study (De Lange et al., 1975), rats received a
single oral dose of approximately 1 mg labelled tetradifon. The
elimination of radioactivity in urine and faeces was measured in 6
rats, for 96 h. Collection of urine and faeces was continued for
168 h in 3 more rats. The elimination of radioactivity in these
rats was low in urine (2 - 4%) and high in faeces. Total
recoveries were about 75% of the dose after 96 h and 87% after 168
h; the carcass contained about 11% at 96 h. When the bile duct was
ligated in 3 rats, excretion via the urine increased and was equal
to about two-thirds of the dose. By cannulating the bile duct in 3
rats, the biliary excretion of 1 mg tetradifon was determined to be
30 - 60% of the dose. This means that up to two-thirds of the dose
was absorbed.
The distribution of tetradifon in intact rats was studied (De
Lange et al., 1975). The radioactivity in the fatty tissues was
found to exceed the plasma level by a factor of 50 at 96 h; the
level in the lung was about 15 times higher than the plasma level.
The continuing excretion of radioactive material after 96 h
suggests depletion of these depots.
Unchanged tetradifon was not detected in any of the urine and
bile samples. The chromatographic determination of metabolite
patterns in urine suggested that the principal metabolites could be
chlorinated benzenesulfonic acids. However, another pattern seems
to be present in the bile.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic Organisms
The LC50s for tetradifon in 2 species of crustacea and 3
species of fish are summarized in Table 3. The compound is
moderately toxic for fish, and moderately to highly toxic for
crustacea. The guppy (Lebistes reticulatus) showed signs of
intoxication when exposed to tetradifon at 1 mg/litre for 5 h but
recovered completely after being transferred to untreated water
(Adlung, 1957). Guppies, 3-4 weeks old, did not show any signs of
intoxication when exposed to suspensions containing 2 mg technical
tetradifon/litre for 96 h (Gijswijt, 1984a).
Waterfleas (Daphnia magna) were exposed to water suspensions
of tetradifon of 0.2 or 2.0 mg/litre for 48 h (both concentrations
exceed the water solubility of tetradifon). No toxic effects were
observed (Gijswijt, 1984b).
6.2. Terrestrial Organisms
Sherman & Sanchez (1968) did not find any toxic effects on
plant growth or gross leaf pathology, when tetradifon was sprayed
on papaya plants, once a week for 3 weeks, at a concentration of
1.97 g/litre.
Tetradifon is relatively non-toxic for birds. Hill et al.
(1975) studied its toxicity for 4 species: bobwhite quail, ring-
necked pheasant, mallard (all at 10 days of age), and 12-day-old
Japanese quail. Tetradifon was included in food at concentrations
of up to 5000 mg/kg and fed to the birds for 5 days. The mortality
rate was estimated at 8 days. Bobwhite quail showed a 10%
mortality rate at 5000 mg tetradifon/kg diet, but no deaths
occurred in any other species. The LC50 for birds is therefore
greater than 5000 mg/kg diet. No long-term studies on birds were
available for review. Beran (1970) reported an oral LD50 for bees
of 1600 µg/bee and an LD50 of 160 µg/bee for tetradifon when
applied topically. This second LD50, expressed as a surface
deposit, was stated to be equivalent to an application rate of 25
kg tetradifon active ingredient/ha. Tetradifon, at aqueous
concentrations of 0.2 - 1.6%, applied externally to bees was not
toxic over a 24-h period (Roger, 1968). A topical LD50 of > 1250
µg/bee was reported by Lippold (1960). Roger (1968) fed Tedion V18
emulsifiable concentrate in honey syrup to honey bees and reported
an oral LD50 of 176 µg/bee (of the formulation). Müeller (1959)
reported the oral LD50 of tetradifon for bees to be between 50 and
100 µg/bee. No contact activity of tetradifon was detected. A
field trial with a spray concentration of up to 4 g/litre did not
produce any effects in honey bees. Tetradifon was classified by
Anderson & Atkins (1968) as "relatively non-toxic" for bees (LD50
greater than 11 µg/bee). A higher toxicity of tetradifon for bees,
with an oral LD100 over 12 h of 1.01 µg/bee, several orders of
magnitude below other reported values was reported in a series of
studies by Arzone & Vidano (1974) and Vidano & Arzone (1975). No
explanation for this discrepancy is given.
Table 3. Toxicity of tetradifon for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Age/ Grade Temp pH Parameter Concentration Reference
weight (°C) (µg/litre)
---------------------------------------------------------------------------------------------------------
Scud 2 months technical 23.8 7.1 24-h LC50 370 (280-500) Sanders (1969)
(Gammarus lacustris) 2 months technical 23.8 7.1 48-h LC50 140 (100-200) Sanders (1969)
2 months technical 23.8 7.1 96-h LC50 110 (80-150) Sanders (1969)
21 96-h LC50 111 (82-150) Johnson & Finley (1980)
(Gammarus fasciatus)
Rainbow trout 1.1 g technical 12 96-h LC50 1200 Johnson & Finley (1980)
(Salmo gairdneri) (949-1600)
Rainbow trout 96 h LCO 10 Sterner et al. (1978)
(Salmo gairdneri)
Channel catfish 0.3 g technical 18 96-h LC50 2100 Johnson & Finley (1980)
(Ictalurus punctatus) (1150-3830)
Bluegill 0.8 g technical 24 96-h LC50 880 (664-1166) Johnson & Finley (1980)
(Lepomis macrochirus)
---------------------------------------------------------------------------------------------------------
Addition of tetradifon to insecticides increases their
toxicity for bees (synergism) (Johansen, 1983).
In studies on other insects, Lippold (1960) reported LD50
values for topically applied tetradifon (acetone solution) as >
9000 µg/insect for the Mexican bean beetle (Epilachna varivertis);
> 4300 µg/insect for the milkweed bug (Oncopeltus fasciatus), and
> 6900 µg/insect for plum curculio (Conotrachelus nenuphar).
6.3. Microorganisms
The growth of cultures of the fresh water green alga Chlorella
pyrenoidova in a suspension of tetradifon at 2 mg/litre for 96 h
did not differ significantly from that of controls (Gijswijt,
1984c).
6.4. Bioaccumulation
In a study to examine uptake and loss of tetradifon, guppies
(Lebistes reticulatus) were exposed to water containing 15 µg 14C-
labelled tetradifon/litre, for 14 days, and then transferred to
clean water for a further 7 days. Fish were sampled at regular
intervals throughout the study and whole body extracts
(acetonitrile and methanol under reflux) were made. Tetradifon was
determined in these extracts using high-performance liquid
chromatography (HPLC). Maximum tetradifon residues were found on
the third day and represented a bioconcentration factor of 200
times the water concentration. The residues declined over the
remainder of the study, in spite of continued exposure to
tetradifon. The decline was rapid and stabilized at between 15 and
45X water concentrations between days 7 and 14. Seventeen, 21, and
28 days after transfer to clear water, tetradifon could not be
detected in fish extracts. The results are explained by rapid
metabolism and excretion of tetradifon after an initial lag period
(Willems & de Winter, 1985). These results confirm that tetradifon
does not bioaccumulate significantly in fish.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single Exposure
7.1.1. Toxicity
Data on the acute toxicity of tetradifon in experimental
animals are summarized in Table 4.
Table 4. Acute toxicity of tetradifon
------------------------------------------------------------------
Animal Route LD50 (mg/kg Reference
body weight)
------------------------------------------------------------------
rat oral 5000 - 14 700 Bordas (1968); Jones
et al. (1968)
rat oral 566a Ben-Dyke et al. (1970)
dog oral 2000 Hendriksen (1956)
rabbit dermal > 10 000 Ben-Dyke et al. (1970);
US NIOSH (1977)
mouse intraperitoneal 75 Ishida & Shirakawa
(1969)
mouse subcutaneous 1953 (TDLo)b Bionetics (1973)
------------------------------------------------------------------
a Tedion V18 emulsifiable concentrate, containing 8% tetradifon
(weight/volume) was tested. LD50 only in part related to
tetradifon.
b TDLo = lowest toxic dose.
Ishikawa et al. (1978) examined the effects of tetradifon on
plasma-alpha-lipoprotein cholesterol in male Sprague Dawley rats
following an intraperitoneal injection of 40 mg/kg body weight. No
effects were observed with regard to alpha-lipoprotein cholesterol,
plasma-triglycerides, and weight gain. Treatment with tetradifon
resulted in slightly elevated levels of total plasma-cholesterol, 7
days after treatment, with a return to threshold levels by day 21.
7.1.2. Skin irritation
A total of 0.5 g technical tetradifon was applied to the back
of the rabbit under an occlusive dressing. No skin irritation was
noted (Koopman 1985a). However, the tetradifon formulation Tedion
EC-8 was slightly irritating to rabbit skin (0.5 ml of the
concentrate, which contains 80 g/litre in xylene) (Koopman 1985b).
7.1.3. Eye irritation
Application of 100 mg technical tetradifon in the rabbit's eye
produced slight irritation (Koopman, (1985c); Tedion EC-8 was
moderately irritating at a dose of 0.1 ml of the concentrate
containing 80 g tetradifon/litre in xylene (Koopman 1985d).
7.2. Short-Term Exposures
Street et al. (1971) reported tetradifon to be fairly
effective in rats in reducing dieldrin storage and hexobarbital
sleeping time, and increasing the oxidative metabolism of O -
ethyl- O -( p -nitrophenyl)-phenylphosphonothioate (EPN). The
compound was added to the diet at a concentration of 50 mg/kg and,
depending on the enzymatic test, exposures ranged from 10 to 15
days.
When tetradifon was tested in rats (7 days at 200 mg/kg body
weight) and fish (7 days at 2 mg/litre water), no clinical changes
were seen. The main biochemical alterations were increased values
for some enzymes (Zamfir et al., 1972).
A good correlation was found between no-observed-adverse-
effect levels based on the induction of liver microsomal enzymes in
male rats and no-observed-adverse-effect levels based on
histopathological changes, for 13 organochlorine pesticides.
Tetradifon was one of the least active substances in this series.
The lowest dose level that induced an effect was 50 mg/kg diet for
2 weeks (Den Tonkelaar & Van Esch 1974).
Verschuuren et al. (1973) conducted a study on rats in which 5
structurally-related acaricides were compared. Groups of 10 male
and 10 female rats were fed these substances in the diet at 0, 50,
200, 1000, or 3000 mg/kg for 90 days. In the case of tetradifon, a
dietary level of 50 mg/kg was a no-observed-adverse-effect level.
There was no growth retardation at any level. Histological changes
in the thyroid were noted at 200 mg/kg diet; liver weight was
increased at 1000 mg/kg with the appearance of SER whorls,
consisting of smooth endoplasmic reticulum.
Niepolomski et al. (1972) administered doses of 0, 30, 90,
270, 810, or 2430 mg tetradifon/kg body weight, by gavage, to
groups of 10 male and 10 female Wistar rats, for 90 days. Only the
30 mg/kg level did not produce any effects. From 90 mg/kg onwards,
dose-related pathomorphological changes in liver, kidney, and lung
and impairment of lipid metabolism were seen.
Technical tetradifon was administered to groups of 4 dogs at
dietary levels of 0, 500, 1000, and 5000 mg/kg, for 1 year (Keller,
1959). No effects of tetradifon treatment were seen on behaviour,
general health, and haematological and most biochemical parameters.
Serum-alkaline phosphatase values were slightly elevated at 5000
mg/kg diet. Gross autopsy at the end of the study revealed
enlarged livers at 5000 mg/kg diet and small grey infarct-like
spots in the outer cortical layer of the kidneys in one dog at the
dietary level 1000 mg/kg and 2 dogs at the 5000 mg/kg dietary
level. The gross findings were not described at the microscopic
level. Histopathology did not reveal any compound-related changes.
The no-observed-adverse-effect level was suggested to be in the
range of 500 - 1000 mg/kg feed.
7.3. Long-Term Exposures
Two 2-year studies were conducted with tetradifon as long ago
as 1955 (Duphar BV, 1960). Both chemically pure and commercial
grade tetradifon were tested in groups of 15 male and 15 female
rats at concentrations of 0, 30, 100, 300, 1200, 5000, or 20 000
mg/kg in the diet. No dose-related changes were found in body
weight, haemoglobin, white blood cell and red blood cell
concentrations, or differential blood count.
Pathology and histological examination of liver, kidney,
spleen, heart, lung, stomach, small intestine, colon, thyroid,
adrenal, testis/ovary, and bone marrow after 24 months did not show
any dose-related effects at levels of 300 mg/kg or less in either
groups. At 1200 mg/kg upwards, degenerative changes developed in
the liver and kidney in both groups.
In the studies available for review, the numbers of animals
used, the tests, and the reports were limited compared with
present-day standards. However, review of these studies suggests a
no-observed-adverse-effect level for short-term and long-term
exposure of rats of between 2.5 and 15 mg/kg body weight.
7.4. Effects on Reproduction
A 2-generation reproduction study with tetradifon (98.4% pure)
was carried out on Charles River Sprague Dawley rats (25 per sex
per group) at dose levels 0, 40, 200, or 1000 mg/kg diet. The
parents in both generations were fed the appropriate diets for at
least 9 weeks and then subjected to 2 subsequent matings. No
differences attributable to tetradifon administration were noted in
parental body weight, food or water consumption data, survival
rates, pregnancy rates, implantation efficiencies, or parturition
indices. The first F1 generation offspring were killed and
examined at weaning. One-fifth of the second F1 litters was
evaluated for teratogenic effects after Caesarian section, one
fifth was born naturally and examined 3 weeks postnatally, and
three-fifths of the litters were used to produce the F2 litters.
The first F2 litters were killed and examined at weaning. Half of
the second set of F2 litters (F2a) were delivered by Caesarian
section and used for teratological evaluation. The other half of
the second set of F2 litters (F2b) was evaluated after 3 months of
postnatal treatment at dietary levels of 40, 200, and 1000 mg
tetradifon/kg, after weaning. Evaluation of the survival indices,
sex ratios, and body weights of the fetuses taken by Caesarian
section, and the offspring examined at 3 weeks postnatally, did not
reveal any compound-related differences between the control and
treated groups. In the F2b litters examined 3 months postnatally,
body weight gains were lower than control values in all treated
groups; there was no correlation with dose. However, in the same
set of litters, there was a dose-dependent increase in the
incidence of dilated renal pelvis (7.8%, 11.9%, and 26.7%,
respectively, in 37.5, 42.9, and 87.5% of the litters) compared
with the control value (6.6% in 40.0% of the litters). The
difference was statistically significant only at the highest dose.
These phenomena were not seen in the offspring examined at weaning.
Moreover, in the F1 offspring evaluated at weaning, dilated renal
pelvis was seen in 10.8% of the control pups and 19.4% of the low-
dose group but not in the middle- and high-dose groups. The
significance of the findings is not clear.
7.5. Mutagenicity
Tetradifon was not among the compounds that responded
positively in tests using two strains of Bacillus subtilis, two
strains of Escherichia coli, and four strains of Salmonella
typhimurium. However, it must be noted that no metabolic
activation was attempted with tetradifon and also that Salmonella
TA 98 and TA 100, two of the most sensitive strains, were not
included in the early studies of this group (Shirasu et al., 1976).
The same group (Moriya et al., 1983) reported negative results for
tetradifon in a bacterial reversion assay with S. typhimurium TA
100, TA 98, TA 1535, TA 1537, TA 1538 and E. coli WP2 hcm.
Tetradifon was weakly positive in a sister chromatid exchange
assay in human lymphocyte cultures at a 10-4 molar concentration,
but inactive at 10-5 or 10-6 molar concentration (Sobti et al.,
1983).
Cultures of human lymphocytes treated with tetradifon did not
show any significant increase in the proportion of metaphase
figures containing chromosome abberrations, compared with the
concurrent solvent controls. Thus, tetradifon did not show any
evidence of mutagenic potential in this in vitro cytogenetic assay
(Allen, 1985).
7.6. Carcinogenicity
Innes et al. (1969) tested many pesticides in a special
screening test. Small groups of mice were given tetradifon at 100
mg/kg body weight by intubation on the 7th - 28th day of age,
followed by 260 mg/kg diet for approximately 18 months. Tetradifon
was reported not to produce a significant increase in the incidence
of tumours. No further details were given and this study was not
considered adequate for the evaluation of the carcinogenicity of
tetradifon. No other studies were available for review.
8. EFFECTS ON MAN
No adverse health effects on human beings from exposure to
tetradifon have been reported.
In photo-patch tests on 51 patients, Horiuchi & Ando (1978)
found tetradifon to be one of the least active compounds of 29
pesticides tested.
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON
THE ENVIRONMENT
9.1. Evaluation of Health Risks For Man
The oral LD50 in rats ranged from 5000 - 14 700 mg/kg body
weight. In the WHO Classification of Pesticides by Hazard,
tetradifon was included in the category of technical products
unlikely to present acute hazards in normal use (WHO, 1984). The
formulated product may be more toxic, depending on other components
of the formulation.
In experimental animals, most orally administered tetradifon
is eliminated rapidly with the faeces.
A no-observed-adverse-effect level of 50 mg/kg diet was
reported in a 90-day study on rats. At higher dose levels, the
liver increased in weight, and induction of microsomal liver
enzymes occurred. At levels exceeding 200 mg/kg, there was an
increase in thyroid weight with histological changes. In 2-year
studies on rats, dietary levels of 1200 mg/kg or more caused
degenerative changes in the liver and kidneys.
No reproductive or teratogenic effects were found in a 2-
generation reproduction study on rats. However, in a 90-day
dietary study on the F2b offspring, there was a dose-related
increased incidence of dilated renal pelvis.
In several short-term in vitro tests adopted to detect somatic
mutagenicity, tetradifon showed a negative response. No information
on the mutagenic effects on germ cells is available at present.
Adequate carcinogenicity studies are not available.
The general population is mainly exposed through food, but
market-basket studies have shown that, at normal application rates
of tetradifon as an acaricide, residues are virtually absent from
food.
Under normal application conditions, it has been estimated
that occupational exposure levels would be less than 0.01% of the
dermal toxic dose per hour. No adverse health effects from
exposure to tetradifon have been reported in man.
9.2. Evaluation of Effects on the Environment
Tetradifon is not toxic for Chlorella pyrenoidova. A single
study reported the absence of toxic effects on papaya plants. The
short-term toxicity of the compound is low for birds, moderate for
fish, and moderate to high for aquatic crustacea. Tetradifon is
relatively non-toxic for honey bees and its toxicity for other
insects is low. However, it may synergize with insecticides to
increase their insecticidal potency. Tetradifon does not
bioaccumulate significantly in fish. No long-term toxicity data
are available and, therefore, more subtle hazards cannot be
adequately evaluated. On the basis of the data available,
tetradifon does not present a short-term threat for the
environment.
9.3. Conclusions
Notwithstanding the fact that the information available for
this evaluation of tetradifon is incomplete and not always up to
present-day standards, there are no indications, at present, that
the normal recommended use of tested tetradifon products as an
acaricide causes any health or safety hazards for the general
population, exposed workers, or the environment.
10. RECOMMENDATIONS
1. More information is needed on metabolism, on the effects on
reproduction, and on long-term toxic effects including
carcinogenicity.
2. It is advised that the purity of the products registered and
used be ascertained, since the contamination of the product by some
chlorinated compounds may increase its toxicity.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the "Guidelines to the Use of the WHO Recommended
Classification of Pesticides by Hazard" (WHO, 1984), tetradifon is
classified in the list of technical products unlikely to present an
acute hazard in normal use.
REFERENCES
ADLUNG, K.G. (1957) [The toxicity of insecticides and
acaricides in fish.] Naturwissenschaften, 44: 471-472 (in
German).
ALLEN, J.A. (1985) Tetradifon, metaphase, chromosome analysis
of human lymphocytes cultured in vitro, Weesp, Netherlands,
Duphar BV (Unpublished Proprietary Report No. 56645/78/85).
ANDERSON, L.D. & ATKINS, E.L. (1968) Pesticide usage in
relation to bee-keeping. Ann. Rev. Entomol., 13: 213-238.
ARZONE, A. & VIDANO, C. (1974) [Verification of the action
on honey bees of pesticides declared harmless to useful
insects.] Ann. fac. Sci. Agr. Univ. Stud. Torino, 9: 171-182
(in Italian with English summary).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest Control, 7-9:
119-127.
BERAN, F. (1970) [Our present knowledge on the toxicity and
hazard to bees of our pesticides.] Gesunde Pflanzen, 22 (2):
21-31 (in German).
BIONETICS RESEARCH LABORATORIES (1973) Evaluation of
carcinogenic, teratogenic, and mutagenic activities of
selected pesticides and industrial chemicals. II. Teratogenic
study in mice and rats, Bethesda, Maryland, Bionetics Research
Laboratories (Report No. NCI-DCCP-C6-1973-1-2).
BONTOYAN, W.R. (1979) Report on the twenty-second annual
meeting of the Collaborative International Pesticides
Analytical Council (CIPAC) J. Assoc. Off. Anal. Chem., 62:
431-432.
BORDAS, S., ed. (1968) [Dangerous pesticides,] 6th ed.,
Budapest, Mezogazdasagi Kiado, pp. 1-720 (in Hungarian).
BORST, A.J.M, KLEIN G., JOUSTRA, K.D., SCHERPENISSE, P.M., &
WILLEMS, A.G.M. (1983) Identification of tetradifon
degradation products in sandy loam soil, Weesp, Netherlands,
Duphar BV (Unpublished Proprietary Report No. 56630/183/82).
BURKE, J.A. (1976) Report on chlorinated pesticides. J.
Assoc. Off. Anal. Chem., 59: 338-340.
BURKE, J.A. & HOLSWADE, W. (1966) A gas chromatographic
column for pesticide residue analysis: retention times and
response data. J. Assoc. Off. Agric. Chem., 47: 374-385.
BURKE, J. & MILLS, P.A. (1963) Microcoulometric gas
chromatographic determination of thiodan and Tedion in green
vegetables. J. Assoc. Off. Agric. Chem., 46: 177-182.
CARRASCO, J.M., CUNAT, P., MARTINEZ, M., & PRIMO, E. (1976)
Pesticide residues in total diet samples, Spain 1971-72.
Pestic. Monit. J., 10(1): 18-23.
CASSIL, C.C. & FULLMER, O.H. (1958) Persistence of Tedion
residues on fruits. J. agric. food Chem., 6(12): 908-910.
CHOVELON, A., GEORGE, L., GULAYETS, C., HOYANO, Y.,
MCGUINNESS, E., MOORE, J., RAMAMOORTHY, S., RAMAMOORTHY, SUB.,
SINGER, P., SMILEY, K., & WHEATLEY, A. (1984) Pesticide and
PCB levels in fish from Alberta (Canada). Chemosphere, 13(1):
19-32.
DE LANGE, N., VINCENT, W.R., & POST, L.C. (1975) Disposition
of 35 S-tetradifon in the rat, Weesp, Netherlands, Duphar BV,
(Unpublished Proprietary Report No. 56654/20/75).
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels
of organochlorine pesticides based on induction of microsomal
liver enzymes in short-term toxicity experiments. Toxicology,
2: 371-380.
DUPHAR BV (1960) Investigation of the toxicity of Tedion
V18, Weesp, Netherlands, Duphar BV (Unpublished Proprietary
Report No. 77.260)
DUPHAR BV (1985) Proprietary data made available to the Task
Group.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the
exposure of workers to pesticides, Geneva, World Health
Organization, pp. 75-91 (WHO Bulletin No. 26).
GIJSWIJT, M.J. (1984a) The acute toxicity of tetradifon to
Lebistes reticulatus (Guppy), Weesp, Netherlands, Duphar BV
(Unpublished Proprietory Report No. 56635/11/84).
GIJSWIJT, M.J. (1984b) The acute toxicity of tetradifon to
Daphnia magna, Weesp, Netherlands, Duphar BV (Unpublished
Proprietary Report No. 56635/21/84).
GIJSWIJT, M.J. (1984c) Tetradifon and algal growth, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report No.
56635/23/84).
GUNTHER, F.A. (1969) Insecticide residues in California
citrus fruits and products. Residue Rev., 28: 1-119.
HALBERSTADT, J. (1958) Some experiments with radioactive
preparations of 2,4,5,4'-tetrachlorodiphenylsulfone, a new
acaricide. Meded. Landbouwhogeschool Gent., 23: 788-794.
HANYU, I. & TSUJI, T. (1974) [Results of investigation on
the effects of agricultural chemicals, especially organo-
phosphorous pesticides on farmers health.] Koshu Eisei Joho,
4: 20 (in Japanese).
HENDRIKSEN, TH.W.J. (1956) Investigation into the toxicity
of 2,4,5,4'-tetrachlorodiphenylsulfone (Duphar Tedion V18),
Weesp, Netherlands, Duphar BV (Unpublished Proprietary Report
No. 6748/4/56).
HENRIET, J., LOVETT, J.F., MARTIJN, A., & POULSEN, H.H.
(1983) Analysis of technical and formulated pesticides,
Cambridge, England, Heffers Printers Ltd, pp. 1901 (CIPAC
Handbook Vol. 1B).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D.
(1975) Lethal dietary toxicities of environmental pollutants
to birds, Washington DC, US Fish and Wildlife Service, 61 pp
(Special Scientific Report No. 191).
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., ed. Handbook of
toxicology, Philadelphia, Pennsylvania, W.B. Saunders Company.
HORIUCHI, N. & ANDO, Y. (1978) Photosensitivity caused by
pesticides. In: Proceedings of the 7th International Congress
on Rural Medicine, Salt Lake City, 1978, Oakdale, Utah,
Institute of Agricultural Medicine, pp. 279-284.
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J.
(1969) Bioassay pesticides and industrial chemicals for
tumourigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme (UNEP), Vols. 1 and II.
ISHIDA, K. & SHIRAKAWA, K. (1969) [Histopathological studies
of liver and kidney of mice injected with lethal doses of
pesticides.] Niigata Norin Kenkyu, 21: 183-201 (in Japanese).
ISHIKAWA, T.T., MCNEELY, S., STEINER, P.M., GLUECK, C.J.,
MELLIES, M., GARTSIDE, P.S., & MCMILLIN, C. (1978) Effects
on chlorinated hydrocarbons on plasma alpha-lipoprotein
cholesterol in rats. Metabolism, 27: 89-96.
JOHANSEN, C.A. (1983) Pesticides and bees. Environ.
Entomol., 12(5): 1513-1518.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute
toxicity to fish and aquatic invertebrates, Washington DC, US
Fish and Wildlife Service, 75 pp (Research Publications
No. 137).
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981a) Pesticide, heavy metal, and other chemical residues
in adult total diet samples. XII. August 1975 - July 1976.
Pestic. Monit. J., 15: 54-71.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b) Food
and feed: pesticide, heavy metal, and other chemical residues
in infant and toddler total diet samples. II. August 1975 -
July 1976. Pestic. Monit. J., 15(1): 39-50.
JONES, K.H., SANDERSON, D.M., & NOAKES, D.N. (1968) Acute
toxicity data for pesticides (1968). World Rev. Pestic.
Control, 7(3): 135-143.
KADIR, H.A. & KNOWLES, C.O. (1981) Inhibition of rat brain
monoamineoxidase by insecticides, acaricides, and related
compounds. Gen. Pharmacol., 12: 239-247.
KELLER, J.G. (1959) Tedion technical: repeated oral
administration to dogs (Report to Niagara Chemical Division -
Hazleton Laboratories, Falls Church, Virginia).
KOOPMAN, T.S.M. (1985a) Primary irritation study of
tetradifon technical to the skin of the male rabbit, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report
No. 56645/71/85).
KOOPMAN, T.S.M. (1985b) Primary irritation study of Tedion
EC-8 to the skin of the male rabbit, Weesp, Netherlands,
Duphar BV (Unpublished Proprietary Report No. 56645/74/85).
KOOPMAN, T.S.M. (1985c) Primary irritation study of
tetradifon technical to the eye of the male rabbit, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report
No. 56645/72/85).
KOOPMAN, T.S.M. (1985d) Primary irritation study of Tedion
EC-8 to the eye of the male rabbit, Weesp, Netherlands, Duphar
BV (Unpublished Proprietary Report No. 56645/75/85).
LIPPOLD, P.C. (1960) Tedion: tests with insects (Internal
Report FMC Niagara Chemical Division, Middleport, New York).
MAAS, W. (1979) Influence of particle size on pesticidal
activity. Adv. Pest. Sci., 3: 772-779.
MATANO, O., YUKIMOTO, M., NISHIJIMA, O., & KASHIWA, T.
(1971) [Chemical and biological determination of 2,4,5-T in
tetradifon formulations.] Noyaku Kensasho Hokoku, 11: 32-36
(in Japanese).
MITCHELL, L.R. (1976) Collaborative study of the determina-
tion of endosulfan, endosulfan sulfate, tetrasul, and
tetradifon residues in fresh fruits and vegetables. J. Assoc.
Off. Anal. Chem., 59: 209-212.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on
pesticides in bacterial reversion assay systems. Mutat. Res.,
116: 185-216.
MUELLER, H. (1959) [Testing of toxicity for bees: Tedion V18
emulsion,] (Internal Report from the Federal Biological
Institute, Braunschweig, Federal Republic of Germany) (in
German).
NIEPOLOMSKI, W., SZEZUREK, Z., KOLODZIEJCZYK, A., & KITA, K.
(1972) [Morphological studies upon shortened chronic akaritox
toxicity in rats.] Med. Prac., 23: 577-584 (in Polish).
NIOSH (1977) Registry of toxic effects of chemical
substances, Cincinnati, Ohio, US National Institute of
Occupational Safety and Health, pp. 883.
RAJUKKANNU, K., BALASUBRAMANIAN, M., & VASUDEVAN, P. (1981)
Residues of dicofol and tetradifon in tea leaves. Plant Crops,
9: 124-125.
ROGER, S. (1968) Essais de toxicite a l'egard de l'abeille
de produit Tedion V-18 emulsion 8 (Unpublished Report of the
"Station de Recherche sur l'abeille et les insectes sociaux",
Bures sur Yvette, France).
RUMSEY, T.S., BOVAREL, K.P., FONTENOT, J.P., OLTJEN, R.R., &
PRIODE, B.M. (1977) Supplementation of apple pomace with
non-protein nitrogen for gestating beef cows. IV. Pesticide
accumulation in cows. J. anim. Sci., 46: 543-550.
RUTTER, H.A. (1976) A two-generation reproduction study on
rats. Tedion. Final report, Vienna, Virginia, Hazleton
Laboratories (Project No. 681-109) (Proprietary data for
product registration purposes made available by Duphar BV to
the IPCS).
RUTTER, H.A. (1982) A two-generation reproduction study on
rats. Tedion. Addendum to final report, Vienna, Virginia,
Hazleton Laboratories (Project No. 681-110) (Proprietary data
for product registration purposes made available by Duphar BV
to the IPCS).
SANDERS, O. (1969) Toxicity of pesticides to the crustacean
Gammarus lacustris, Washington DC, US Fish and Wildlife
Service, Bureau of Sport, Fish and Wildlife, 18 pp (Technical
Paper No. 25).
SHERMAN, M. & SANCHEZ, F.F. (1968) Further studies on the
toxicity of insecticides and acaricides to the papaya. Hawaii
Agric. Exp. Stn Univ. Hawaii. Tech. Bull., 74: 5-63.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening on pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H.,
TERAMOTO, S., & KADA, T. (1977) Mutagenicity screening on
pesticides and modification products: a basis of carcino-
genicity evaluation. In: Hiatt & Watson, ed. Origins of human
cancer. A. Incidence of cancer in humans, Cold Springs
Harbour, New York, Cold Springs Harbour Laboratories.
SOBTI, R.C., KRISHAN, A., & DAVIES, J. (1983) Cytokinetic
and cytogenetic effect of agricultural chemicals on human
lymphoid cells in vitro. II. Organochlorine pesticides. Arch.
Toxicol., 52: 221-231.
STERNER W., KORN, W.D., & PFENNIG, K.D. (1978) [Testing the
acute toxicity of technical tetradifon for fresh-water fish,]
Hanover, Federal Republic of Germany, International Bio
Research (Unpublished Report No. 1-8-210-78).
STREET, J.C., URRY, F.M., WAGSTAFF, D.J., & BLAU, S.E.
(1971) Induction by different inducers: structure-activity
relationships among DDT analogues. In: Proceedings of the 2nd
International IUPAC Congress of Pesticide Chemistry, Israel,
22-26 February 1971, New York, Gordon and Breach Science
Publishers, pp. 237-256.
SUZUKI, K., MIYASHITA, K., NAGAYOSHI, H., & KASHIWA, T.
(1973) Separation and identification of 20 pesticides in
mixtures. Agric. Biol. Chem., 37: 1959-1962.
SUZUKI, K., NAGAYOSHI, H., & KASHIWA, T. (1974) The systemic
separation and identification of pesticides in the first
division. Agric. Biol. Chem., 38: 279-285.
US FDA (1968) The regulation of pesticides in the United
States, Washington DC, US Department of Agriculture, US
Department of Health, Education and Welfare, Food and Drug
Administration.
VAN DIJK, L.P., WIESE, I.H., & MULLEN, J.E.C. (1982)
Management and determination of pesticide resides in South
Africa. Residue Rev., 82: 38-121.
VAN KOLFSCHOTEN, A.A. (1982) A two-generation reproduction
study in rats. Tedion. Further evaluation of report No.
681-109 and Addendum 681-110, Vienna, Virginia, Hazleton
Laboratories (Duphar Report No. 56645/30/82) (Proprietary data
for product registration purposes made available by Duphar BV
to the IPCS).
VAN ROSSUM, A., DEWILDE, P.C., DEBOER, F.G., & KORVER, P.K.
(1978) Tetradifon. In: Zweig, G. & Sherma, J., ed. Analytical
methods for pesticides and plant growth regulators, New York,
Academic Press, pp. 119-126.
VAN ROSSUM, B., MARTIJN, A., & LAUNER, J.E. (1981) Gas-liquid
chromotographic determination of tetradifon technical and
formulations: collaborative study J. Assoc. Off. Anal. Chem.,
64: 829-832.
VERSCHUUREN, H.G., KROES, R., & DEN TONKELAAR, E.M. (1973)
Toxicity studies on tetrasul. III. Short-term comparative
studies in rats with tetrasul and structurally-related
acaricides. Toxicology, 1: 113-123.
VIDANO, C. & ARZONE A. (1975) [Research and considerations on
pesticides demonstrated to be harmless for bees.] Apicot.
Mod., 66(5): 163-167 (in Italian).
WHO (1984) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-1985, Geneva,
World Health Organization (Unpublished Report VBC/84.2).
WILLEMS, A.G.M. & DE WINTER, M.L., (1985) Bioaccummulation
of tetradifon by fish, Weesp, Netherlands, Duphar BV
(Unpublished Proprietary Report No. 56637/77/1985).
WILLEMS, A.G.M. & NIMMO, W.B. (1981) The degradation of
14 C-tetradifon in a water/hydro soil system, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report
No. 56635/41/81).
WILLEMS, A.G.M. & SMIT J. (1982) Movement of tetradifon and
its degradation products in soil, Weesp, Netherlands, Duphar
BV (Unpublished Proprietary Report No. 56635/28/82).
WINDHOLZ, M., BUDAVARI, S., BLUMETTI, R.F., & OTTERBEIN, E.S.,
ed. (1983) The Merck Index, 10th ed., Rahway, New Jersey,
Merck and Co., Inc.
WOLFE, H.R. (1976) Field exposure to airborne pesticides.
In: Lee, R.E., ed. Air pollution from pesticides and
agricultural processes, Florida, CRC Press, pp. 137-161.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1967) Exposure
of workers to pesticides. Arch. environ. Health, 14: 622-633.
WOLFE, H.R., ARMSTRONG, J.F., STAIFF, D.C., & COMWER, S.W.
(1972) Exposure of spraymen to pesticides. A rch. environ.
Health, 25: 29-31.
WOOLSON, E.A., THOMAS, R.F., & ENSOR, P.D.J. (1972) Survey
of polychlorodibenzo- p-dioxin content in selected pesticides.
J. agric. food Chem., 20: 351-354.
WORTHING, C.R. (1979) The pesticide manual, 6th ed.,
Croydon, British Crop Protection Council (BCPC Publications).
YARON, B., BICLORAI, H., & KLIZER, L. (1974) Fate of
insecticides in an irrigated field: azinphosmethyl and
tetradifon cases. J. environ. Qual., 3: 413-417.
ZAMFIR, G., RAILEANU, L., AVADANII, R., & DRAGOMIRESCU, A.
(1972) [Biochemical changes due to sublethal doses of
tetradifon.] Igiena, 21: 665-670 (in Rumanian).
ZWEIG, G. & SHERMA, J. (1972) Tedion (tetradifon). In:
Zweig, G. & Sherma, J., ed. Analytical methods for pesticides
and plant growth regulators. Gas chromatographic analysis, New
York, Academic Press, Vol. 6, pp. 488-492.
ANNEX
EXTRACT FROM HEALTH AND SAFETY GUIDE INCLUDING INTERNATIONAL
CHEMICAL SAFETY CARD
A1. HEALTH HAZARDS FOR MAN; PREVENTION AND PROTECTION; EMERGENCY
ACTION
A1.1 Main Hazards for Man; Prevention and Protection; First Aid
The toxicity of technical tetradifon for man is thought to be
low, and no adverse health effects from exposure to tetradifon have
been reported. The toxicity and hazard of a formulation may
largely depend on the vehicle used.
A1.1.1 Prevention and Protection
In spite of the low toxicity and hazard of tetradifon, the
following precautions should be observed during handling and use in
order to reduce the risk of accidental contamination:
(a) Avoid contact with the skin and eyes. If eyes become
contaminated, flush with water. If irritation
persists, obtain medical attention.
(b) Wash hands and any exposed skin before eating,
drinking, smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable
powder formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear
protective PVC or neoprene gloves.
(f) When handling leaking containers or when dealing with
leaks and spills, wear overalls and PVC or neoprene
gloves and boots. If overalls become contaminated,
change and wash them thoroughly before re-use.
(g) Store products in original containers out of reach of
children and away from food and feeding stuffs.
A1.1.2 First aid
Poisoning by tetradifon is unlikely unless there has been
gross (negligent) exposure or intentional ingestion. In cases of
overexposure, apply routine first aid measures.
If material has been spilled on the skin, immediately remove
the patient from the source of the contamination, remove all
contaminated clothing, and wash affected areas with soap and
running water. If material is in the eyes, flush with clean water
for at least 15 min. Keep patient prone and quiet. Start
artificial respiration immediately if patient is not breathing.
Never give anything by mouth to an unconscious person.
In serious cases, medical attention should be sought.
A1.2 Advice to Physicians
The human toxicity of tetradifon is believed to be low. There
is no specific antidote. Treat symptomatically when required. In
cases of ingestion, gastric lavage may be indicated.
A1.3 Explosion and Fire Hazards and Precautions
Technical tetradifon is not highly flammable, but liquid
formulations may be, depending on the solvent used.
Fight small fires with CO2, dry powder, or alcohol resistant
foam. Confine the use of water spray to cooling of unaffected
stock only, thus avoiding the accumulation of polluted run-off from
the site.
Fire service personnel should be advised that self-contained
breathing apparatus may be required, because noxious fumes may be
generated through a fire.
A1.4 Storage and Transport Precautions
All products should be stored in secure buildings, out of
reach of children and animals, and also comply with any local
transport regulations. Containers should be sound and well
labelled.
A1.5 Spillage/Disposal Procedures
Keep spectators away from any leakage. Prevent contamination
of other goods or cargo, or nearby vegetation and waterways.
Absorb spillage of liquid products with sawdust or sand, sweep
up and place in separate container.
Empty any product remaining in damaged or leaking containers
into a clean empty container, which should be suitably labelled.
Sweep up any spilt powder with damp sawdust taking care not to
raise a dust cloud. Place in separate container for subsequent
disposal.
Contaminated absorbents, used containers, surplus product,
etc., should be burnt in an incinerator designed for pesticide
disposal. When no incinerator is available, bury in an approved
dump or in an area where there is no risk of contamination of
ground or surface water. Comply with any local legislation
applying to waste disposal.
A1.6 International Chemical Safety Card
This card should be easily available to all health workers
concerned and to all users of tetradifon. It should be displayed
at, or near, entrances to areas with potential exposure to
tetradifon, on processing equipment, and on containers. The sheet
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
A2. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
No effects on the environment have been reported for
tetradifon.
A2.1 Precautionary Measures to Protect the Environment
Do not contaminate ponds, waterways, and ditches with product
or used containers. Puncture empty containers.
A3. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from
the International Register of Potentially Toxic Chemicals (IRPTC)
legal file.
The file contains regulatory data on chemicals from 12
countries and recommendations issued by 6 international
organizations.
The reader must be aware that regulatory decisions about
chemicals taken in a certain country can only be fully understood
in the framework of the legislation of that country. A full
reference to the original national document from which the
information was extracted can be obtained from the IRPTC.
When no effective date appears in the IRPTC legal file, the
publication year of the national document from which the data are
taken is mentioned; where appropriate, this is indicated by (r).
Sample International Chemical Safety Card for Tetradifon (technical)
(2,4,5,4'-tetrachlorodiphenylsulfone; C12H6Cl4O2S)
---------------------------------------------------------------------------------------------------------
Physical properties Other characteristics
---------------------------------------------------------------------------------------------------------
Relative molecular mass 356.04 slightly yellow crystalline solid;
Melting point 148 - 149 °C very stable non-corrosive substance;
Water solubility (20 °C) 0.08% may emit toxic fumes when heated
Density (20 °C) 1.515 to decomposition
Octanol/water partition coefficient 4.61
Vapour pressure (20 °C) 0.32 x 10-10 kPa
---------------------------------------------------------------------------------------------------------
Hazard/symptom Prevention and protection First aid
---------------------------------------------------------------------------------------------------------
Skin: mild irritation neoprene gloves, face shield remove contaminated clothing;
wash with plenty of water
Eyes: marginal irritant safety goggles, face shield flush with clean water for
at least 15 min
Inhalation: irritation of upper local exhaust ventilation; fresh air
respiratory tract wear a dust mask
Ingestion: none observed unlikely professional hazard gastric lavage may be indicated
---------------------------------------------------------------------------------------------------------
Spillage Storage Fire and explosion
---------------------------------------------------------------------------------------------------------
Collect spillage in closed container store cool and dry in Fire: not flammable under normal
or dust bin bag; in the case of original packing conditions
liquid, first use absorbant material; Explosion: none
clean up with water Fire extinguishing agents: foam, CO2,
dry chemical
---------------------------------------------------------------------------------------------------------
Waste disposal
---------------------------------------------------------------------------------------------------------
Should be burnt in an incinerator
designed for pesticide disposal
---------------------------------------------------------------------------------------------------------
A3.1 Exposure Limit Values
Some exposure limit values are given in Table A.1.
A3.2 Specific Regulatory Actions
In Czechoslovakia (effective date: 1981) and the United
Kingdom (1983 (r)), the substance is approved as a pesticide or
acaricide, and specified uses, limitations, and safety precautions
are listed. In the USSR, the substance is approved as an
insecticide for agricultural use and application; dose, mode, and
treatment frequency are specified (effective date: 1982). In
Sweden, the substance is an active ingredient in pesticide
formulations that are registered at the products control board and
therefore may be marketed and used. The formulations may be sold
only to persons authorized to use such formulations (1984 (r)).
A3.3 Transport and Labelling
The United Nations Committee of Experts on the Transportation
of Dangerous Goods classifies tetradifon as a poisonous substance
(Class 6.1) presenting minor danger for packing purposes when the
active ingredient constitutes 25 - 100% of the formulation (1982
(r)). The recommended label is:
Molecular formula: C12H6C14O2S
CAS chemical name: 1,2,4-trichloro-5-[(4-chlorophenyl)-
sulfonyl]-benzene
Synonyms: 4-chlorophenyl-2,4,5-trichlorophenyl sulfone,
2,4,4',5-tetrachlorodiphenyl sulfone
Common trade names: Akaritox, Aredion, Duphar 23737, ENT 23737,
FMC 5488, Mition, NIA 5488, Polacaritox,
Roztoczol, Roztozol, Tedion V18,
Tetradichlone (a complete list of trade names
is available from IRPTC)
CAS registry number: 116-29-0
2.2. Physical and Chemical Properties
Some physical and chemical properties of tetradifon are given
in Table 1. The solubility of tetradifon in different organic
solvents at room temperature varies from 10 g/litre (in kerosene
and methanol) to 255 g/litre (in chloroform) (Van Rossum et al.,
1978).
Tetradifon is manufactured by a Friedel-Craft's reaction
between 2,4,5-trichlorophenylsulfonyl chloride and
monochlorobenzene in the presence of anhydrous aluminium chloride
or ferric chloride (Van Rossum et al., 1978), or by Sandmeyer
diazotization (Windholz et al., 1983). The technical product is
more than 94% pure, but since production processes may vary, the
type and quality of impurities present may differ in commercial
products from different companies. In addition, the limits of
detection for different chlorinated dibenzodioxins, in commercial
products vary. In a study by Woolson et. al. (1972), the limit of
detection for chlorinated dibenzodioxins was equal to or less than
0.5 mg/kg. The di-, tri-, tetra-, and hexachlorinated
dibenzodioxins were not separated from each other; the authors
stated that higher chlorinated dibenzodioxins were found in some
samples of tetradifon, but no specific information was given.
Table 1. Some physical and chemical properties
of tetradifona
-------------------------------------------------
Physical state crystalline solid
Colour slightly yellow
Relative molecular mass 356.04
Melting point 148 - 149 °C
Vapour pressure (20 °C) 0.32 x 10-10 kPa
Octanol/water partition 4.61
coefficient
Solubility in water (10 °C) 0.05 %
(20 °C) 0.08 %
(50 °C) 0.34 %
-------------------------------------------------
a From: Van Rossum et al. (1978) and Duphar
BV, personal communication (1982).
Impurities including 2-chlorophenyl-2,4,5-trichlorophenyl
sulfone up to a maximum level of 5 g/kg and 2,4,5-trichloro-
phenoxyacetic acid (2,4,5-T) have been reported by Matano et al.
(1971) and trace amounts of highly-chlorinated dibenzodioxins, but
not 2,3,7,8-TCDD by Woolson et al. (1972). It has been shown that
regular strict quality control procedures maintain the total sum of
all isomeric tetrachlorodibenzofurans in technical tetradifon at
less than 10 µg/kg (detection limit) (Duphar BV, 1985).
Furthermore, analysis of concentrated mother liquids revealed that
the concentrations of 2,3,7,8-tetrachlorodibenzofuran, the sum of
all isomeric tetrachlorodibenzo- p -dioxins, and 2,3,7,8-tetra-
chlorodibenzo- p -dioxin were all lower than 1 µg/kg, and that
2,4,5-tri-chlorophenoxyacetic acid (2,4,5-T) was not present as an
impurity (Duphar BV, 1985).
Tetradifon is resistant to hydrolysis by acid and alkali and
is non-corrosive (Worthing, 1979). Particle size has a strong
influence on its biological effectiveness in that small particles
show a better rain resistance than larger ones (Maas, 1979). In
1954, tetradifon was introduced in the Netherlands as a non-
systemic acaricide, which was toxic for the eggs (ovicide) and all
non-adult stages of a wide range of phytophagous mites. It is
used, particularly in mixtures, in horticulture, including domestic
greenhouses, mainly on top-fruit, vegetables, ornamentals, hops,
cotton, and sugarcane, and forestry.
Tetradifon remained stable to ultraviolet radiation (UVR) for
12 h at 50 - 60 °C (Duphar BV, 1985).
2.3. Analytical Methods
Several methods have been used for the extraction of
tetradifon residues from different tissues. Burke & Mills (1963)
introduced a method involving microcoulometric gas-liquid
chromatography (GLC). Mitchell (1976) used GLC with electron
capture detection to study the tetradifon contents of apple and
cucumber samples. According to Van Rossum et al. (1978), the
recovery of tetradifon from crop samples using the GLC method was
85 - 100%. They also reported that tetradifon could be extracted
from soil samples with ether and then detected directly by GLC with
electron capture detection. The limit of detection in soil samples
was 0.02 mg/kg.
Analytical methods are based on total organic chlorine, column
chromatography, and UV spectrophotometric detection (Zweig &
Sherma, 1972; Suzuki et al., 1973, 1974; Burke, 1976; Bontoyan,
1979). Zweig & Sherma (1972) mentioned a recovery of 95 - 102%
from vegetables. Gas chromatography is recommended by CIPAC
(Henriet et al., 1983) for the analysis of formulations and
technical material.
3. SOURCES, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
Tetradifon is manufactured in the Netherlands and in Italy and
formulated in several countries. The uses of tetradifon in
selected countries are shown in Table 2.
Table 2. The uses of tetradifon in selected countriesa
---------------------------------------------------------------
Country Quantity Year Uses
---------------------------------------------------------------
Colombia 2786 kg 1982 agricultural acaricide
2073 kg 1981 recommended in the growth
2343 kg 1980 of cotton, fruits, and
other products
Mexico 1000 kg 1981 on strawberries, tomatoes,
grapes, cucumber, apples,
and citrus fruits
Sweden 400 kg 1981 agricultural and
horticultural use
Thailand 42 100 kg 1984
14 600 kg 1983
14 175 kg 1982 acaricide
12 237 kg 1981 acaricide
1620 kg 1980 acaricide
216 kg 1979 acaricide
1000 kg 1978 acaricide
208 kg 1977 acaricide
272 kg 1976 acaricide
United Kingdom 320 kg/year 1975-79 acaricide
USA 1983 used prior to 1983;
registration voluntarily
cancelled in 1983 by
producers
---------------------------------------------------------------
a Information received from national contact points of
IRPTC (1984).
Responses to a questionnaire received from 49 countries around
the world indicated the continuing registration and use of
tetradifon in 37 countries. Nine developing countries stated that
tetradifon was not used, and 3 countries (China, the Federal
Republic of Germany, and the USA) stated that registration of
tetradifon had been discontinued for reasons other than its
toxicity. Thirteen of these countries taken together used
approximately 200 tonnes of tetradifon per year during the years
1982-84 (information received from national contact points of the
IPCS and IRPTC, 1985).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Only limited information is available on tetradifon as an
environmental pollutant. Its water solubility is low, and a study
by Yaron et al. (1974) showed that there was only restricted
leaching from soil by rain.
4.1. Environmental Levels
4.1.1. Air
No data are available on tetradifon levels in air.
4.1.2. Water
No data are available on tetradifon levels in water.
4.1.3. Soil
Only minute amounts of tetradifon were found in the deep
layers of soil from an irrigated potato field sprayed at a rate of
10 kg/ha. Tetradifon persisted throughout the irrigation season.
Transport of tetradifon into the soil was not affected by the
amount of water applied (Yaron et al., 1974).
In a degradation experiment, sandy loam soil was incubated
aerobically for 106 weeks. Approximately 70% of the added
tetradifon was recovered unchanged and 20% was recovered as
extractable metabolites (Borst et al., 1983). Four metabolite
fractions were identified. One chlorine atom appeared to be
substituted by more polar groups (-SOCH3, -SO2CH3, -SO3H, -SCH3)
(Borst et al., 1983; Willems et al., 1983). In a water/sandy loam
hydro-soil system, tetradifon had a half-life of 36 weeks. Almost
all of the tetradifon was retained in the soil phase. Under more
aerobic conditions, degradation was more rapid, 31% of the added
tetradifon being recovered after 32 weeks (Willems & Nimmo, 1981).
The movement of tetradifon and partially degraded tetradifon in
overlying loam soil was also investigated. The results showed that
neither tetradifon nor its degradation products are leachable
compounds (Willems & Smit, 1982).
4.1.4. Food
In a study on the fate of insecticides in an irrigated field,
Yaron et al. (1974) found a tetradifon concentration of 250 mg/kg
(wet weight) in the peel of potato tubers. This was due to the
direct contact of the pesticide with the tuber. No tetradifon was
found in the tuber pulp or in the leaves of the potato plant; the
amounts of pesticide applied in this study were much greater (2
applications of 10 kg pesticide/ha soil) than those normally used.
Radioactive tetradifon in a solution prepared from either a
wettable powder (WP) or a miscible oil (MO) was sprayed on 2 apple
trees. Changes in radioactivity in and on the leaves were followed
for 4 months. Externally, the greater part of the activity was
lost from the leaves within the first few days. Uptake into the
leaves reached a maximum in approximately 1 day equalling 8% of
initial activity with the WP and 30% with the MO. More than a
month after spraying, intact tetradifon could still be found on and
in the leaves. The level in the ripe apples was less than 0.2 mg/kg
(Halberstadt, 1958).
Cassil & Fullmer (1958) sprayed fruit-bearing apple, pear,
peach, lemon, and orange trees with either 500 or 1500 g of 25%
tedion wettable powder per 455 litres of water, 32 days before
harvest. Pears and peaches were sprayed at 0.56 litre/m2, oranges
and lemons at 1.16 litres/m2, and apples were sprayed manually to
runoff. Maximum residue levels on the surface of the fruits
sprayed at 500 and 1500 g varied from 1.5 to 3.6 and from 3.4 to
7.0 mg/kg, respectively. Tetradifon residues diminished largely as
a result of fruit growth and not through decomposition.
In a second study, Cassil & Fullmer (1958) followed tetradifon
residue levels on similarly-sprayed oranges for 100 days. As in
the first study, they concluded that tetradifon is very stable when
exposed to weathering at high summer temperatures.
For many years, the US Food and Drug Administration has
examined more than 10 000 food samples yearly using methods with
which it is possible to determine residues of tetradifon. Residues
have been found infrequently and have been limited primarily to
fresh fruits. From 1964 to 1969, 1607 fruit samples were examined
and tetradifon residues ranging from trace to 2 mg/kg were found in
only 2.05% of the fruits; from 1970 to 1976, 1201 fruit samples
were examined and similar tetradifon residues were found in only
1.42% of the samples. On the basis of the total number of fruit
samples examined, the average residue of tetradifon in fruits was
0.007 mg/kg for 1964-69 and 0.004 mg/kg for 1970-76. The only
other foods in which tetradifon residues were found during these
years were one sample of cereal by-products, one sample of fish,
and one sample of leafy vegetables. Findings have been less
frequent in recent years and have primarily been found in produce
imported from Mexico (US FDA, personal communication, 1984).
In New Zealand, apples from 32 growers were checked for
tetradifon residues. The maximum level found was 0.7 mg/kg
(average 0.11 mg/kg) (Department of Health, Wellington, personal
communication, 1984).
No studies are available from other countries.
4.1.5. Other products
When tetradifon was sprayed on a tea plantation at 0.5 g/litre
and 750 - 1000 litres/ha, initial deposits on leaves were in the
range of 12.6 - 13.6 mg/kg. After 10 days, residues were < 5
mg/kg (Rajukkannu et al., 1981). No other studies were available
for review.
4.1.6. Terrestrial and aquatic organisms
In pregnant beef cattle fed apple pomace containing about 0.07
- 0.53 mg tetradifon/kg and 0.53 - 8.33 mg total DDT/kg, for 160
days, tetradifon accumulated in depot fat at a rate similar to
those of DDT and DDD, but only 29% as fast as DDE. On day 160, the
concentrations in the extracted fat were 0.16 mg tetradifon/kg and
2.56 mg total DDT/kg (Rumsey et al., 1977). The depletion of these
two compounds following cessation of exposure was not investigated.
Thus, this study does not give any information on the persistence
of tetradifon in cattle.
Tetradifon was not detected (detection level: 0.05 mg/kg) in
the tissues of 750 fish samples collected from 11 major lakes and
rivers in Alberta, Canada (Chovelon et al., 1984).
4.2. General Population Exposure
In a market-monitoring programme conducted in California and
Arizona, during 1967, the maximum tetradifon residue found on
citrus fruits (whole fruit) was 0.3 mg/kg (Gunther, 1969). The
results of market-basket studies in the USA during 1966-67, which
included both domestic and imported food, showed that tetradifon
was virtually absent (USDA, 1968). In a market-basket study in
Spain in 1971-72, tetradifon was not detected in 97% of fruit
samples and in 87% of vegetable samples (Carrasco et al., 1976).
In the USA in the years 1975-76, tetradifon was not detected
in foods including unfortified infant and toddler total diet
samples from 10 cities in the USA sampled between August 1975 and
July 1976 (Johnson et al., 1981a,b). Furthermore, there have been
no findings of tetradifon in FDA total diet ("market basket")
studies from 1976 to the present time (US FDA, personal
communication, 1984).
While most measurements in food were negative, no information
was available on the use patterns in the countries where the food
was monitored.
4.3. Occupational Exposure
Following the analyses of dermal exposure pads or hand rinses
and of respirator pads, an estimate of the levels of dermal and
respiratory exposure that sprayers would potentially incur was made
by Wolfe et al. (1967, 1972). The values derived were 36.4 mg/h
for dermal exposure and 0.07 mg/h for inhalation (Durham & Wolfe,
1962; Wolfe et al., 1967). No information on the risks of combined
dermal and inhalation exposure is available for formulators or
workers manufacturing the product.
5. KINETICS AND METABOLISM
In a preliminary study, a few rats were fed 10 mg 35S-
tetradifon/rat per day for 10 days and then examined. The highest
percentages of radioactivity during the 10 days were found in the
faeces and urine, followed by fat, gastrointestinal tissue, liver,
and muscles (Halberstadt, 1958). One rat was given 10 mg 35S-
tetradifon suspended in peanut oil/water, by stomach tube, and
killed 48 h later. Seventy-one percent of the dose was eliminated
with the faeces, 4% with urine, and 7% was recovered from the
gastrointestinal tract. The remaining 18% was present in very
small quantities in all organs and tissues. Only 20 - 40% of the
activity in organs was associated with unchanged tetradifon
(Halberstadt, 1958). The Task Group realized that results from
single animals are difficult to interpret, however, no other data
were available.
In another study (De Lange et al., 1975), rats received a
single oral dose of approximately 1 mg labelled tetradifon. The
elimination of radioactivity in urine and faeces was measured in 6
rats, for 96 h. Collection of urine and faeces was continued for
168 h in 3 more rats. The elimination of radioactivity in these
rats was low in urine (2 - 4%) and high in faeces. Total
recoveries were about 75% of the dose after 96 h and 87% after 168
h; the carcass contained about 11% at 96 h. When the bile duct was
ligated in 3 rats, excretion via the urine increased and was equal
to about two-thirds of the dose. By cannulating the bile duct in 3
rats, the biliary excretion of 1 mg tetradifon was determined to be
30 - 60% of the dose. This means that up to two-thirds of the dose
was absorbed.
The distribution of tetradifon in intact rats was studied (De
Lange et al., 1975). The radioactivity in the fatty tissues was
found to exceed the plasma level by a factor of 50 at 96 h; the
level in the lung was about 15 times higher than the plasma level.
The continuing excretion of radioactive material after 96 h
suggests depletion of these depots.
Unchanged tetradifon was not detected in any of the urine and
bile samples. The chromatographic determination of metabolite
patterns in urine suggested that the principal metabolites could be
chlorinated benzenesulfonic acids. However, another pattern seems
to be present in the bile.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic Organisms
The LC50s for tetradifon in 2 species of crustacea and 3
species of fish are summarized in Table 3. The compound is
moderately toxic for fish, and moderately to highly toxic for
crustacea. The guppy (Lebistes reticulatus) showed signs of
intoxication when exposed to tetradifon at 1 mg/litre for 5 h but
recovered completely after being transferred to untreated water
(Adlung, 1957). Guppies, 3-4 weeks old, did not show any signs of
intoxication when exposed to suspensions containing 2 mg technical
tetradifon/litre for 96 h (Gijswijt, 1984a).
Waterfleas (Daphnia magna) were exposed to water suspensions
of tetradifon of 0.2 or 2.0 mg/litre for 48 h (both concentrations
exceed the water solubility of tetradifon). No toxic effects were
observed (Gijswijt, 1984b).
6.2. Terrestrial Organisms
Sherman & Sanchez (1968) did not find any toxic effects on
plant growth or gross leaf pathology, when tetradifon was sprayed
on papaya plants, once a week for 3 weeks, at a concentration of
1.97 g/litre.
Tetradifon is relatively non-toxic for birds. Hill et al.
(1975) studied its toxicity for 4 species: bobwhite quail, ring-
necked pheasant, mallard (all at 10 days of age), and 12-day-old
Japanese quail. Tetradifon was included in food at concentrations
of up to 5000 mg/kg and fed to the birds for 5 days. The mortality
rate was estimated at 8 days. Bobwhite quail showed a 10%
mortality rate at 5000 mg tetradifon/kg diet, but no deaths
occurred in any other species. The LC50 for birds is therefore
greater than 5000 mg/kg diet. No long-term studies on birds were
available for review. Beran (1970) reported an oral LD50 for bees
of 1600 µg/bee and an LD50 of 160 µg/bee for tetradifon when
applied topically. This second LD50, expressed as a surface
deposit, was stated to be equivalent to an application rate of 25
kg tetradifon active ingredient/ha. Tetradifon, at aqueous
concentrations of 0.2 - 1.6%, applied externally to bees was not
toxic over a 24-h period (Roger, 1968). A topical LD50 of > 1250
µg/bee was reported by Lippold (1960). Roger (1968) fed Tedion V18
emulsifiable concentrate in honey syrup to honey bees and reported
an oral LD50 of 176 µg/bee (of the formulation). Müeller (1959)
reported the oral LD50 of tetradifon for bees to be between 50 and
100 µg/bee. No contact activity of tetradifon was detected. A
field trial with a spray concentration of up to 4 g/litre did not
produce any effects in honey bees. Tetradifon was classified by
Anderson & Atkins (1968) as "relatively non-toxic" for bees (LD50
greater than 11 µg/bee). A higher toxicity of tetradifon for bees,
with an oral LD100 over 12 h of 1.01 µg/bee, several orders of
magnitude below other reported values was reported in a series of
studies by Arzone & Vidano (1974) and Vidano & Arzone (1975). No
explanation for this discrepancy is given.
Table 3. Toxicity of tetradifon for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Age/ Grade Temp pH Parameter Concentration Reference
weight (°C) (µg/litre)
---------------------------------------------------------------------------------------------------------
Scud 2 months technical 23.8 7.1 24-h LC50 370 (280-500) Sanders (1969)
(Gammarus lacustris) 2 months technical 23.8 7.1 48-h LC50 140 (100-200) Sanders (1969)
2 months technical 23.8 7.1 96-h LC50 110 (80-150) Sanders (1969)
21 96-h LC50 111 (82-150) Johnson & Finley (1980)
(Gammarus fasciatus)
Rainbow trout 1.1 g technical 12 96-h LC50 1200 Johnson & Finley (1980)
(Salmo gairdneri) (949-1600)
Rainbow trout 96 h LCO 10 Sterner et al. (1978)
(Salmo gairdneri)
Channel catfish 0.3 g technical 18 96-h LC50 2100 Johnson & Finley (1980)
(Ictalurus punctatus) (1150-3830)
Bluegill 0.8 g technical 24 96-h LC50 880 (664-1166) Johnson & Finley (1980)
(Lepomis macrochirus)
---------------------------------------------------------------------------------------------------------
Addition of tetradifon to insecticides increases their
toxicity for bees (synergism) (Johansen, 1983).
In studies on other insects, Lippold (1960) reported LD50
values for topically applied tetradifon (acetone solution) as >
9000 µg/insect for the Mexican bean beetle (Epilachna varivertis);
> 4300 µg/insect for the milkweed bug (Oncopeltus fasciatus), and
> 6900 µg/insect for plum curculio (Conotrachelus nenuphar).
6.3. Microorganisms
The growth of cultures of the fresh water green alga Chlorella
pyrenoidova in a suspension of tetradifon at 2 mg/litre for 96 h
did not differ significantly from that of controls (Gijswijt,
1984c).
6.4. Bioaccumulation
In a study to examine uptake and loss of tetradifon, guppies
(Lebistes reticulatus) were exposed to water containing 15 µg 14C-
labelled tetradifon/litre, for 14 days, and then transferred to
clean water for a further 7 days. Fish were sampled at regular
intervals throughout the study and whole body extracts
(acetonitrile and methanol under reflux) were made. Tetradifon was
determined in these extracts using high-performance liquid
chromatography (HPLC). Maximum tetradifon residues were found on
the third day and represented a bioconcentration factor of 200
times the water concentration. The residues declined over the
remainder of the study, in spite of continued exposure to
tetradifon. The decline was rapid and stabilized at between 15 and
45X water concentrations between days 7 and 14. Seventeen, 21, and
28 days after transfer to clear water, tetradifon could not be
detected in fish extracts. The results are explained by rapid
metabolism and excretion of tetradifon after an initial lag period
(Willems & de Winter, 1985). These results confirm that tetradifon
does not bioaccumulate significantly in fish.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single Exposure
7.1.1. Toxicity
Data on the acute toxicity of tetradifon in experimental
animals are summarized in Table 4.
Table 4. Acute toxicity of tetradifon
------------------------------------------------------------------
Animal Route LD50 (mg/kg Reference
body weight)
------------------------------------------------------------------
rat oral 5000 - 14 700 Bordas (1968); Jones
et al. (1968)
rat oral 566a Ben-Dyke et al. (1970)
dog oral 2000 Hendriksen (1956)
rabbit dermal > 10 000 Ben-Dyke et al. (1970);
US NIOSH (1977)
mouse intraperitoneal 75 Ishida & Shirakawa
(1969)
mouse subcutaneous 1953 (TDLo)b Bionetics (1973)
------------------------------------------------------------------
a Tedion V18 emulsifiable concentrate, containing 8% tetradifon
(weight/volume) was tested. LD50 only in part related to
tetradifon.
b TDLo = lowest toxic dose.
Ishikawa et al. (1978) examined the effects of tetradifon on
plasma-alpha-lipoprotein cholesterol in male Sprague Dawley rats
following an intraperitoneal injection of 40 mg/kg body weight. No
effects were observed with regard to alpha-lipoprotein cholesterol,
plasma-triglycerides, and weight gain. Treatment with tetradifon
resulted in slightly elevated levels of total plasma-cholesterol, 7
days after treatment, with a return to threshold levels by day 21.
7.1.2. Skin irritation
A total of 0.5 g technical tetradifon was applied to the back
of the rabbit under an occlusive dressing. No skin irritation was
noted (Koopman 1985a). However, the tetradifon formulation Tedion
EC-8 was slightly irritating to rabbit skin (0.5 ml of the
concentrate, which contains 80 g/litre in xylene) (Koopman 1985b).
7.1.3. Eye irritation
Application of 100 mg technical tetradifon in the rabbit's eye
produced slight irritation (Koopman, (1985c); Tedion EC-8 was
moderately irritating at a dose of 0.1 ml of the concentrate
containing 80 g tetradifon/litre in xylene (Koopman 1985d).
7.2. Short-Term Exposures
Street et al. (1971) reported tetradifon to be fairly
effective in rats in reducing dieldrin storage and hexobarbital
sleeping time, and increasing the oxidative metabolism of O -
ethyl- O -( p -nitrophenyl)-phenylphosphonothioate (EPN). The
compound was added to the diet at a concentration of 50 mg/kg and,
depending on the enzymatic test, exposures ranged from 10 to 15
days.
When tetradifon was tested in rats (7 days at 200 mg/kg body
weight) and fish (7 days at 2 mg/litre water), no clinical changes
were seen. The main biochemical alterations were increased values
for some enzymes (Zamfir et al., 1972).
A good correlation was found between no-observed-adverse-
effect levels based on the induction of liver microsomal enzymes in
male rats and no-observed-adverse-effect levels based on
histopathological changes, for 13 organochlorine pesticides.
Tetradifon was one of the least active substances in this series.
The lowest dose level that induced an effect was 50 mg/kg diet for
2 weeks (Den Tonkelaar & Van Esch 1974).
Verschuuren et al. (1973) conducted a study on rats in which 5
structurally-related acaricides were compared. Groups of 10 male
and 10 female rats were fed these substances in the diet at 0, 50,
200, 1000, or 3000 mg/kg for 90 days. In the case of tetradifon, a
dietary level of 50 mg/kg was a no-observed-adverse-effect level.
There was no growth retardation at any level. Histological changes
in the thyroid were noted at 200 mg/kg diet; liver weight was
increased at 1000 mg/kg with the appearance of SER whorls,
consisting of smooth endoplasmic reticulum.
Niepolomski et al. (1972) administered doses of 0, 30, 90,
270, 810, or 2430 mg tetradifon/kg body weight, by gavage, to
groups of 10 male and 10 female Wistar rats, for 90 days. Only the
30 mg/kg level did not produce any effects. From 90 mg/kg onwards,
dose-related pathomorphological changes in liver, kidney, and lung
and impairment of lipid metabolism were seen.
Technical tetradifon was administered to groups of 4 dogs at
dietary levels of 0, 500, 1000, and 5000 mg/kg, for 1 year (Keller,
1959). No effects of tetradifon treatment were seen on behaviour,
general health, and haematological and most biochemical parameters.
Serum-alkaline phosphatase values were slightly elevated at 5000
mg/kg diet. Gross autopsy at the end of the study revealed
enlarged livers at 5000 mg/kg diet and small grey infarct-like
spots in the outer cortical layer of the kidneys in one dog at the
dietary level 1000 mg/kg and 2 dogs at the 5000 mg/kg dietary
level. The gross findings were not described at the microscopic
level. Histopathology did not reveal any compound-related changes.
The no-observed-adverse-effect level was suggested to be in the
range of 500 - 1000 mg/kg feed.
7.3. Long-Term Exposures
Two 2-year studies were conducted with tetradifon as long ago
as 1955 (Duphar BV, 1960). Both chemically pure and commercial
grade tetradifon were tested in groups of 15 male and 15 female
rats at concentrations of 0, 30, 100, 300, 1200, 5000, or 20 000
mg/kg in the diet. No dose-related changes were found in body
weight, haemoglobin, white blood cell and red blood cell
concentrations, or differential blood count.
Pathology and histological examination of liver, kidney,
spleen, heart, lung, stomach, small intestine, colon, thyroid,
adrenal, testis/ovary, and bone marrow after 24 months did not show
any dose-related effects at levels of 300 mg/kg or less in either
groups. At 1200 mg/kg upwards, degenerative changes developed in
the liver and kidney in both groups.
In the studies available for review, the numbers of animals
used, the tests, and the reports were limited compared with
present-day standards. However, review of these studies suggests a
no-observed-adverse-effect level for short-term and long-term
exposure of rats of between 2.5 and 15 mg/kg body weight.
7.4. Effects on Reproduction
A 2-generation reproduction study with tetradifon (98.4% pure)
was carried out on Charles River Sprague Dawley rats (25 per sex
per group) at dose levels 0, 40, 200, or 1000 mg/kg diet. The
parents in both generations were fed the appropriate diets for at
least 9 weeks and then subjected to 2 subsequent matings. No
differences attributable to tetradifon administration were noted in
parental body weight, food or water consumption data, survival
rates, pregnancy rates, implantation efficiencies, or parturition
indices. The first F1 generation offspring were killed and
examined at weaning. One-fifth of the second F1 litters was
evaluated for teratogenic effects after Caesarian section, one
fifth was born naturally and examined 3 weeks postnatally, and
three-fifths of the litters were used to produce the F2 litters.
The first F2 litters were killed and examined at weaning. Half of
the second set of F2 litters (F2a) were delivered by Caesarian
section and used for teratological evaluation. The other half of
the second set of F2 litters (F2b) was evaluated after 3 months of
postnatal treatment at dietary levels of 40, 200, and 1000 mg
tetradifon/kg, after weaning. Evaluation of the survival indices,
sex ratios, and body weights of the fetuses taken by Caesarian
section, and the offspring examined at 3 weeks postnatally, did not
reveal any compound-related differences between the control and
treated groups. In the F2b litters examined 3 months postnatally,
body weight gains were lower than control values in all treated
groups; there was no correlation with dose. However, in the same
set of litters, there was a dose-dependent increase in the
incidence of dilated renal pelvis (7.8%, 11.9%, and 26.7%,
respectively, in 37.5, 42.9, and 87.5% of the litters) compared
with the control value (6.6% in 40.0% of the litters). The
difference was statistically significant only at the highest dose.
These phenomena were not seen in the offspring examined at weaning.
Moreover, in the F1 offspring evaluated at weaning, dilated renal
pelvis was seen in 10.8% of the control pups and 19.4% of the low-
dose group but not in the middle- and high-dose groups. The
significance of the findings is not clear.
7.5. Mutagenicity
Tetradifon was not among the compounds that responded
positively in tests using two strains of Bacillus subtilis, two
strains of Escherichia coli, and four strains of Salmonella
typhimurium. However, it must be noted that no metabolic
activation was attempted with tetradifon and also that Salmonella
TA 98 and TA 100, two of the most sensitive strains, were not
included in the early studies of this group (Shirasu et al., 1976).
The same group (Moriya et al., 1983) reported negative results for
tetradifon in a bacterial reversion assay with S. typhimurium TA
100, TA 98, TA 1535, TA 1537, TA 1538 and E. coli WP2 hcm.
Tetradifon was weakly positive in a sister chromatid exchange
assay in human lymphocyte cultures at a 10-4 molar concentration,
but inactive at 10-5 or 10-6 molar concentration (Sobti et al.,
1983).
Cultures of human lymphocytes treated with tetradifon did not
show any significant increase in the proportion of metaphase
figures containing chromosome abberrations, compared with the
concurrent solvent controls. Thus, tetradifon did not show any
evidence of mutagenic potential in this in vitro cytogenetic assay
(Allen, 1985).
7.6. Carcinogenicity
Innes et al. (1969) tested many pesticides in a special
screening test. Small groups of mice were given tetradifon at 100
mg/kg body weight by intubation on the 7th - 28th day of age,
followed by 260 mg/kg diet for approximately 18 months. Tetradifon
was reported not to produce a significant increase in the incidence
of tumours. No further details were given and this study was not
considered adequate for the evaluation of the carcinogenicity of
tetradifon. No other studies were available for review.
8. EFFECTS ON MAN
No adverse health effects on human beings from exposure to
tetradifon have been reported.
In photo-patch tests on 51 patients, Horiuchi & Ando (1978)
found tetradifon to be one of the least active compounds of 29
pesticides tested.
9. EVALUATION OF RISKS FOR HUMAN HEALTH AND EFFECTS ON
THE ENVIRONMENT
9.1. Evaluation of Health Risks For Man
The oral LD50 in rats ranged from 5000 - 14 700 mg/kg body
weight. In the WHO Classification of Pesticides by Hazard,
tetradifon was included in the category of technical products
unlikely to present acute hazards in normal use (WHO, 1984). The
formulated product may be more toxic, depending on other components
of the formulation.
In experimental animals, most orally administered tetradifon
is eliminated rapidly with the faeces.
A no-observed-adverse-effect level of 50 mg/kg diet was
reported in a 90-day study on rats. At higher dose levels, the
liver increased in weight, and induction of microsomal liver
enzymes occurred. At levels exceeding 200 mg/kg, there was an
increase in thyroid weight with histological changes. In 2-year
studies on rats, dietary levels of 1200 mg/kg or more caused
degenerative changes in the liver and kidneys.
No reproductive or teratogenic effects were found in a 2-
generation reproduction study on rats. However, in a 90-day
dietary study on the F2b offspring, there was a dose-related
increased incidence of dilated renal pelvis.
In several short-term in vitro tests adopted to detect somatic
mutagenicity, tetradifon showed a negative response. No information
on the mutagenic effects on germ cells is available at present.
Adequate carcinogenicity studies are not available.
The general population is mainly exposed through food, but
market-basket studies have shown that, at normal application rates
of tetradifon as an acaricide, residues are virtually absent from
food.
Under normal application conditions, it has been estimated
that occupational exposure levels would be less than 0.01% of the
dermal toxic dose per hour. No adverse health effects from
exposure to tetradifon have been reported in man.
9.2. Evaluation of Effects on the Environment
Tetradifon is not toxic for Chlorella pyrenoidova. A single
study reported the absence of toxic effects on papaya plants. The
short-term toxicity of the compound is low for birds, moderate for
fish, and moderate to high for aquatic crustacea. Tetradifon is
relatively non-toxic for honey bees and its toxicity for other
insects is low. However, it may synergize with insecticides to
increase their insecticidal potency. Tetradifon does not
bioaccumulate significantly in fish. No long-term toxicity data
are available and, therefore, more subtle hazards cannot be
adequately evaluated. On the basis of the data available,
tetradifon does not present a short-term threat for the
environment.
9.3. Conclusions
Notwithstanding the fact that the information available for
this evaluation of tetradifon is incomplete and not always up to
present-day standards, there are no indications, at present, that
the normal recommended use of tested tetradifon products as an
acaricide causes any health or safety hazards for the general
population, exposed workers, or the environment.
10. RECOMMENDATIONS
1. More information is needed on metabolism, on the effects on
reproduction, and on long-term toxic effects including
carcinogenicity.
2. It is advised that the purity of the products registered and
used be ascertained, since the contamination of the product by some
chlorinated compounds may increase its toxicity.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the "Guidelines to the Use of the WHO Recommended
Classification of Pesticides by Hazard" (WHO, 1984), tetradifon is
classified in the list of technical products unlikely to present an
acute hazard in normal use.
REFERENCES
ADLUNG, K.G. (1957) [The toxicity of insecticides and
acaricides in fish.] Naturwissenschaften, 44: 471-472 (in
German).
ALLEN, J.A. (1985) Tetradifon, metaphase, chromosome analysis
of human lymphocytes cultured in vitro, Weesp, Netherlands,
Duphar BV (Unpublished Proprietary Report No. 56645/78/85).
ANDERSON, L.D. & ATKINS, E.L. (1968) Pesticide usage in
relation to bee-keeping. Ann. Rev. Entomol., 13: 213-238.
ARZONE, A. & VIDANO, C. (1974) [Verification of the action
on honey bees of pesticides declared harmless to useful
insects.] Ann. fac. Sci. Agr. Univ. Stud. Torino, 9: 171-182
(in Italian with English summary).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest Control, 7-9:
119-127.
BERAN, F. (1970) [Our present knowledge on the toxicity and
hazard to bees of our pesticides.] Gesunde Pflanzen, 22 (2):
21-31 (in German).
BIONETICS RESEARCH LABORATORIES (1973) Evaluation of
carcinogenic, teratogenic, and mutagenic activities of
selected pesticides and industrial chemicals. II. Teratogenic
study in mice and rats, Bethesda, Maryland, Bionetics Research
Laboratories (Report No. NCI-DCCP-C6-1973-1-2).
BONTOYAN, W.R. (1979) Report on the twenty-second annual
meeting of the Collaborative International Pesticides
Analytical Council (CIPAC) J. Assoc. Off. Anal. Chem., 62:
431-432.
BORDAS, S., ed. (1968) [Dangerous pesticides,] 6th ed.,
Budapest, Mezogazdasagi Kiado, pp. 1-720 (in Hungarian).
BORST, A.J.M, KLEIN G., JOUSTRA, K.D., SCHERPENISSE, P.M., &
WILLEMS, A.G.M. (1983) Identification of tetradifon
degradation products in sandy loam soil, Weesp, Netherlands,
Duphar BV (Unpublished Proprietary Report No. 56630/183/82).
BURKE, J.A. (1976) Report on chlorinated pesticides. J.
Assoc. Off. Anal. Chem., 59: 338-340.
BURKE, J.A. & HOLSWADE, W. (1966) A gas chromatographic
column for pesticide residue analysis: retention times and
response data. J. Assoc. Off. Agric. Chem., 47: 374-385.
BURKE, J. & MILLS, P.A. (1963) Microcoulometric gas
chromatographic determination of thiodan and Tedion in green
vegetables. J. Assoc. Off. Agric. Chem., 46: 177-182.
CARRASCO, J.M., CUNAT, P., MARTINEZ, M., & PRIMO, E. (1976)
Pesticide residues in total diet samples, Spain 1971-72.
Pestic. Monit. J., 10(1): 18-23.
CASSIL, C.C. & FULLMER, O.H. (1958) Persistence of Tedion
residues on fruits. J. agric. food Chem., 6(12): 908-910.
CHOVELON, A., GEORGE, L., GULAYETS, C., HOYANO, Y.,
MCGUINNESS, E., MOORE, J., RAMAMOORTHY, S., RAMAMOORTHY, SUB.,
SINGER, P., SMILEY, K., & WHEATLEY, A. (1984) Pesticide and
PCB levels in fish from Alberta (Canada). Chemosphere, 13(1):
19-32.
DE LANGE, N., VINCENT, W.R., & POST, L.C. (1975) Disposition
of 35 S-tetradifon in the rat, Weesp, Netherlands, Duphar BV,
(Unpublished Proprietary Report No. 56654/20/75).
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels
of organochlorine pesticides based on induction of microsomal
liver enzymes in short-term toxicity experiments. Toxicology,
2: 371-380.
DUPHAR BV (1960) Investigation of the toxicity of Tedion
V18, Weesp, Netherlands, Duphar BV (Unpublished Proprietary
Report No. 77.260)
DUPHAR BV (1985) Proprietary data made available to the Task
Group.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the
exposure of workers to pesticides, Geneva, World Health
Organization, pp. 75-91 (WHO Bulletin No. 26).
GIJSWIJT, M.J. (1984a) The acute toxicity of tetradifon to
Lebistes reticulatus (Guppy), Weesp, Netherlands, Duphar BV
(Unpublished Proprietory Report No. 56635/11/84).
GIJSWIJT, M.J. (1984b) The acute toxicity of tetradifon to
Daphnia magna, Weesp, Netherlands, Duphar BV (Unpublished
Proprietary Report No. 56635/21/84).
GIJSWIJT, M.J. (1984c) Tetradifon and algal growth, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report No.
56635/23/84).
GUNTHER, F.A. (1969) Insecticide residues in California
citrus fruits and products. Residue Rev., 28: 1-119.
HALBERSTADT, J. (1958) Some experiments with radioactive
preparations of 2,4,5,4'-tetrachlorodiphenylsulfone, a new
acaricide. Meded. Landbouwhogeschool Gent., 23: 788-794.
HANYU, I. & TSUJI, T. (1974) [Results of investigation on
the effects of agricultural chemicals, especially organo-
phosphorous pesticides on farmers health.] Koshu Eisei Joho,
4: 20 (in Japanese).
HENDRIKSEN, TH.W.J. (1956) Investigation into the toxicity
of 2,4,5,4'-tetrachlorodiphenylsulfone (Duphar Tedion V18),
Weesp, Netherlands, Duphar BV (Unpublished Proprietary Report
No. 6748/4/56).
HENRIET, J., LOVETT, J.F., MARTIJN, A., & POULSEN, H.H.
(1983) Analysis of technical and formulated pesticides,
Cambridge, England, Heffers Printers Ltd, pp. 1901 (CIPAC
Handbook Vol. 1B).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D.
(1975) Lethal dietary toxicities of environmental pollutants
to birds, Washington DC, US Fish and Wildlife Service, 61 pp
(Special Scientific Report No. 191).
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., ed. Handbook of
toxicology, Philadelphia, Pennsylvania, W.B. Saunders Company.
HORIUCHI, N. & ANDO, Y. (1978) Photosensitivity caused by
pesticides. In: Proceedings of the 7th International Congress
on Rural Medicine, Salt Lake City, 1978, Oakdale, Utah,
Institute of Agricultural Medicine, pp. 279-284.
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J.
(1969) Bioassay pesticides and industrial chemicals for
tumourigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme (UNEP), Vols. 1 and II.
ISHIDA, K. & SHIRAKAWA, K. (1969) [Histopathological studies
of liver and kidney of mice injected with lethal doses of
pesticides.] Niigata Norin Kenkyu, 21: 183-201 (in Japanese).
ISHIKAWA, T.T., MCNEELY, S., STEINER, P.M., GLUECK, C.J.,
MELLIES, M., GARTSIDE, P.S., & MCMILLIN, C. (1978) Effects
on chlorinated hydrocarbons on plasma alpha-lipoprotein
cholesterol in rats. Metabolism, 27: 89-96.
JOHANSEN, C.A. (1983) Pesticides and bees. Environ.
Entomol., 12(5): 1513-1518.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute
toxicity to fish and aquatic invertebrates, Washington DC, US
Fish and Wildlife Service, 75 pp (Research Publications
No. 137).
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981a) Pesticide, heavy metal, and other chemical residues
in adult total diet samples. XII. August 1975 - July 1976.
Pestic. Monit. J., 15: 54-71.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b) Food
and feed: pesticide, heavy metal, and other chemical residues
in infant and toddler total diet samples. II. August 1975 -
July 1976. Pestic. Monit. J., 15(1): 39-50.
JONES, K.H., SANDERSON, D.M., & NOAKES, D.N. (1968) Acute
toxicity data for pesticides (1968). World Rev. Pestic.
Control, 7(3): 135-143.
KADIR, H.A. & KNOWLES, C.O. (1981) Inhibition of rat brain
monoamineoxidase by insecticides, acaricides, and related
compounds. Gen. Pharmacol., 12: 239-247.
KELLER, J.G. (1959) Tedion technical: repeated oral
administration to dogs (Report to Niagara Chemical Division -
Hazleton Laboratories, Falls Church, Virginia).
KOOPMAN, T.S.M. (1985a) Primary irritation study of
tetradifon technical to the skin of the male rabbit, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report
No. 56645/71/85).
KOOPMAN, T.S.M. (1985b) Primary irritation study of Tedion
EC-8 to the skin of the male rabbit, Weesp, Netherlands,
Duphar BV (Unpublished Proprietary Report No. 56645/74/85).
KOOPMAN, T.S.M. (1985c) Primary irritation study of
tetradifon technical to the eye of the male rabbit, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report
No. 56645/72/85).
KOOPMAN, T.S.M. (1985d) Primary irritation study of Tedion
EC-8 to the eye of the male rabbit, Weesp, Netherlands, Duphar
BV (Unpublished Proprietary Report No. 56645/75/85).
LIPPOLD, P.C. (1960) Tedion: tests with insects (Internal
Report FMC Niagara Chemical Division, Middleport, New York).
MAAS, W. (1979) Influence of particle size on pesticidal
activity. Adv. Pest. Sci., 3: 772-779.
MATANO, O., YUKIMOTO, M., NISHIJIMA, O., & KASHIWA, T.
(1971) [Chemical and biological determination of 2,4,5-T in
tetradifon formulations.] Noyaku Kensasho Hokoku, 11: 32-36
(in Japanese).
MITCHELL, L.R. (1976) Collaborative study of the determina-
tion of endosulfan, endosulfan sulfate, tetrasul, and
tetradifon residues in fresh fruits and vegetables. J. Assoc.
Off. Anal. Chem., 59: 209-212.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on
pesticides in bacterial reversion assay systems. Mutat. Res.,
116: 185-216.
MUELLER, H. (1959) [Testing of toxicity for bees: Tedion V18
emulsion,] (Internal Report from the Federal Biological
Institute, Braunschweig, Federal Republic of Germany) (in
German).
NIEPOLOMSKI, W., SZEZUREK, Z., KOLODZIEJCZYK, A., & KITA, K.
(1972) [Morphological studies upon shortened chronic akaritox
toxicity in rats.] Med. Prac., 23: 577-584 (in Polish).
NIOSH (1977) Registry of toxic effects of chemical
substances, Cincinnati, Ohio, US National Institute of
Occupational Safety and Health, pp. 883.
RAJUKKANNU, K., BALASUBRAMANIAN, M., & VASUDEVAN, P. (1981)
Residues of dicofol and tetradifon in tea leaves. Plant Crops,
9: 124-125.
ROGER, S. (1968) Essais de toxicite a l'egard de l'abeille
de produit Tedion V-18 emulsion 8 (Unpublished Report of the
"Station de Recherche sur l'abeille et les insectes sociaux",
Bures sur Yvette, France).
RUMSEY, T.S., BOVAREL, K.P., FONTENOT, J.P., OLTJEN, R.R., &
PRIODE, B.M. (1977) Supplementation of apple pomace with
non-protein nitrogen for gestating beef cows. IV. Pesticide
accumulation in cows. J. anim. Sci., 46: 543-550.
RUTTER, H.A. (1976) A two-generation reproduction study on
rats. Tedion. Final report, Vienna, Virginia, Hazleton
Laboratories (Project No. 681-109) (Proprietary data for
product registration purposes made available by Duphar BV to
the IPCS).
RUTTER, H.A. (1982) A two-generation reproduction study on
rats. Tedion. Addendum to final report, Vienna, Virginia,
Hazleton Laboratories (Project No. 681-110) (Proprietary data
for product registration purposes made available by Duphar BV
to the IPCS).
SANDERS, O. (1969) Toxicity of pesticides to the crustacean
Gammarus lacustris, Washington DC, US Fish and Wildlife
Service, Bureau of Sport, Fish and Wildlife, 18 pp (Technical
Paper No. 25).
SHERMAN, M. & SANCHEZ, F.F. (1968) Further studies on the
toxicity of insecticides and acaricides to the papaya. Hawaii
Agric. Exp. Stn Univ. Hawaii. Tech. Bull., 74: 5-63.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening on pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H.,
TERAMOTO, S., & KADA, T. (1977) Mutagenicity screening on
pesticides and modification products: a basis of carcino-
genicity evaluation. In: Hiatt & Watson, ed. Origins of human
cancer. A. Incidence of cancer in humans, Cold Springs
Harbour, New York, Cold Springs Harbour Laboratories.
SOBTI, R.C., KRISHAN, A., & DAVIES, J. (1983) Cytokinetic
and cytogenetic effect of agricultural chemicals on human
lymphoid cells in vitro. II. Organochlorine pesticides. Arch.
Toxicol., 52: 221-231.
STERNER W., KORN, W.D., & PFENNIG, K.D. (1978) [Testing the
acute toxicity of technical tetradifon for fresh-water fish,]
Hanover, Federal Republic of Germany, International Bio
Research (Unpublished Report No. 1-8-210-78).
STREET, J.C., URRY, F.M., WAGSTAFF, D.J., & BLAU, S.E.
(1971) Induction by different inducers: structure-activity
relationships among DDT analogues. In: Proceedings of the 2nd
International IUPAC Congress of Pesticide Chemistry, Israel,
22-26 February 1971, New York, Gordon and Breach Science
Publishers, pp. 237-256.
SUZUKI, K., MIYASHITA, K., NAGAYOSHI, H., & KASHIWA, T.
(1973) Separation and identification of 20 pesticides in
mixtures. Agric. Biol. Chem., 37: 1959-1962.
SUZUKI, K., NAGAYOSHI, H., & KASHIWA, T. (1974) The systemic
separation and identification of pesticides in the first
division. Agric. Biol. Chem., 38: 279-285.
US FDA (1968) The regulation of pesticides in the United
States, Washington DC, US Department of Agriculture, US
Department of Health, Education and Welfare, Food and Drug
Administration.
VAN DIJK, L.P., WIESE, I.H., & MULLEN, J.E.C. (1982)
Management and determination of pesticide resides in South
Africa. Residue Rev., 82: 38-121.
VAN KOLFSCHOTEN, A.A. (1982) A two-generation reproduction
study in rats. Tedion. Further evaluation of report No.
681-109 and Addendum 681-110, Vienna, Virginia, Hazleton
Laboratories (Duphar Report No. 56645/30/82) (Proprietary data
for product registration purposes made available by Duphar BV
to the IPCS).
VAN ROSSUM, A., DEWILDE, P.C., DEBOER, F.G., & KORVER, P.K.
(1978) Tetradifon. In: Zweig, G. & Sherma, J., ed. Analytical
methods for pesticides and plant growth regulators, New York,
Academic Press, pp. 119-126.
VAN ROSSUM, B., MARTIJN, A., & LAUNER, J.E. (1981) Gas-liquid
chromotographic determination of tetradifon technical and
formulations: collaborative study J. Assoc. Off. Anal. Chem.,
64: 829-832.
VERSCHUUREN, H.G., KROES, R., & DEN TONKELAAR, E.M. (1973)
Toxicity studies on tetrasul. III. Short-term comparative
studies in rats with tetrasul and structurally-related
acaricides. Toxicology, 1: 113-123.
VIDANO, C. & ARZONE A. (1975) [Research and considerations on
pesticides demonstrated to be harmless for bees.] Apicot.
Mod., 66(5): 163-167 (in Italian).
WHO (1984) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-1985, Geneva,
World Health Organization (Unpublished Report VBC/84.2).
WILLEMS, A.G.M. & DE WINTER, M.L., (1985) Bioaccummulation
of tetradifon by fish, Weesp, Netherlands, Duphar BV
(Unpublished Proprietary Report No. 56637/77/1985).
WILLEMS, A.G.M. & NIMMO, W.B. (1981) The degradation of
14 C-tetradifon in a water/hydro soil system, Weesp,
Netherlands, Duphar BV (Unpublished Proprietary Report
No. 56635/41/81).
WILLEMS, A.G.M. & SMIT J. (1982) Movement of tetradifon and
its degradation products in soil, Weesp, Netherlands, Duphar
BV (Unpublished Proprietary Report No. 56635/28/82).
WINDHOLZ, M., BUDAVARI, S., BLUMETTI, R.F., & OTTERBEIN, E.S.,
ed. (1983) The Merck Index, 10th ed., Rahway, New Jersey,
Merck and Co., Inc.
WOLFE, H.R. (1976) Field exposure to airborne pesticides.
In: Lee, R.E., ed. Air pollution from pesticides and
agricultural processes, Florida, CRC Press, pp. 137-161.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1967) Exposure
of workers to pesticides. Arch. environ. Health, 14: 622-633.
WOLFE, H.R., ARMSTRONG, J.F., STAIFF, D.C., & COMWER, S.W.
(1972) Exposure of spraymen to pesticides. A rch. environ.
Health, 25: 29-31.
WOOLSON, E.A., THOMAS, R.F., & ENSOR, P.D.J. (1972) Survey
of polychlorodibenzo- p-dioxin content in selected pesticides.
J. agric. food Chem., 20: 351-354.
WORTHING, C.R. (1979) The pesticide manual, 6th ed.,
Croydon, British Crop Protection Council (BCPC Publications).
YARON, B., BICLORAI, H., & KLIZER, L. (1974) Fate of
insecticides in an irrigated field: azinphosmethyl and
tetradifon cases. J. environ. Qual., 3: 413-417.
ZAMFIR, G., RAILEANU, L., AVADANII, R., & DRAGOMIRESCU, A.
(1972) [Biochemical changes due to sublethal doses of
tetradifon.] Igiena, 21: 665-670 (in Rumanian).
ZWEIG, G. & SHERMA, J. (1972) Tedion (tetradifon). In:
Zweig, G. & Sherma, J., ed. Analytical methods for pesticides
and plant growth regulators. Gas chromatographic analysis, New
York, Academic Press, Vol. 6, pp. 488-492.
ANNEX
EXTRACT FROM HEALTH AND SAFETY GUIDE INCLUDING INTERNATIONAL
CHEMICAL SAFETY CARD
A1. HEALTH HAZARDS FOR MAN; PREVENTION AND PROTECTION; EMERGENCY
ACTION
A1.1 Main Hazards for Man; Prevention and Protection; First Aid
The toxicity of technical tetradifon for man is thought to be
low, and no adverse health effects from exposure to tetradifon have
been reported. The toxicity and hazard of a formulation may
largely depend on the vehicle used.
A1.1.1 Prevention and Protection
In spite of the low toxicity and hazard of tetradifon, the
following precautions should be observed during handling and use in
order to reduce the risk of accidental contamination:
(a) Avoid contact with the skin and eyes. If eyes become
contaminated, flush with water. If irritation
persists, obtain medical attention.
(b) Wash hands and any exposed skin before eating,
drinking, smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable
powder formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear
protective PVC or neoprene gloves.
(f) When handling leaking containers or when dealing with
leaks and spills, wear overalls and PVC or neoprene
gloves and boots. If overalls become contaminated,
change and wash them thoroughly before re-use.
(g) Store products in original containers out of reach of
children and away from food and feeding stuffs.
A1.1.2 First aid
Poisoning by tetradifon is unlikely unless there has been
gross (negligent) exposure or intentional ingestion. In cases of
overexposure, apply routine first aid measures.
If material has been spilled on the skin, immediately remove
the patient from the source of the contamination, remove all
contaminated clothing, and wash affected areas with soap and
running water. If material is in the eyes, flush with clean water
for at least 15 min. Keep patient prone and quiet. Start
artificial respiration immediately if patient is not breathing.
Never give anything by mouth to an unconscious person.
In serious cases, medical attention should be sought.
A1.2 Advice to Physicians
The human toxicity of tetradifon is believed to be low. There
is no specific antidote. Treat symptomatically when required. In
cases of ingestion, gastric lavage may be indicated.
A1.3 Explosion and Fire Hazards and Precautions
Technical tetradifon is not highly flammable, but liquid
formulations may be, depending on the solvent used.
Fight small fires with CO2, dry powder, or alcohol resistant
foam. Confine the use of water spray to cooling of unaffected
stock only, thus avoiding the accumulation of polluted run-off from
the site.
Fire service personnel should be advised that self-contained
breathing apparatus may be required, because noxious fumes may be
generated through a fire.
A1.4 Storage and Transport Precautions
All products should be stored in secure buildings, out of
reach of children and animals, and also comply with any local
transport regulations. Containers should be sound and well
labelled.
A1.5 Spillage/Disposal Procedures
Keep spectators away from any leakage. Prevent contamination
of other goods or cargo, or nearby vegetation and waterways.
Absorb spillage of liquid products with sawdust or sand, sweep
up and place in separate container.
Empty any product remaining in damaged or leaking containers
into a clean empty container, which should be suitably labelled.
Sweep up any spilt powder with damp sawdust taking care not to
raise a dust cloud. Place in separate container for subsequent
disposal.
Contaminated absorbents, used containers, surplus product,
etc., should be burnt in an incinerator designed for pesticide
disposal. When no incinerator is available, bury in an approved
dump or in an area where there is no risk of contamination of
ground or surface water. Comply with any local legislation
applying to waste disposal.
A1.6 International Chemical Safety Card
This card should be easily available to all health workers
concerned and to all users of tetradifon. It should be displayed
at, or near, entrances to areas with potential exposure to
tetradifon, on processing equipment, and on containers. The sheet
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
A2. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
No effects on the environment have been reported for
tetradifon.
A2.1 Precautionary Measures to Protect the Environment
Do not contaminate ponds, waterways, and ditches with product
or used containers. Puncture empty containers.
A3. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from
the International Register of Potentially Toxic Chemicals (IRPTC)
legal file.
The file contains regulatory data on chemicals from 12
countries and recommendations issued by 6 international
organizations.
The reader must be aware that regulatory decisions about
chemicals taken in a certain country can only be fully understood
in the framework of the legislation of that country. A full
reference to the original national document from which the
information was extracted can be obtained from the IRPTC.
When no effective date appears in the IRPTC legal file, the
publication year of the national document from which the data are
taken is mentioned; where appropriate, this is indicated by (r).
Sample International Chemical Safety Card for Tetradifon (technical)
(2,4,5,4'-tetrachlorodiphenylsulfone; C12H6Cl4O2S)
---------------------------------------------------------------------------------------------------------
Physical properties Other characteristics
---------------------------------------------------------------------------------------------------------
Relative molecular mass 356.04 slightly yellow crystalline solid;
Melting point 148 - 149 °C very stable non-corrosive substance;
Water solubility (20 °C) 0.08% may emit toxic fumes when heated
Density (20 °C) 1.515 to decomposition
Octanol/water partition coefficient 4.61
Vapour pressure (20 °C) 0.32 x 10-10 kPa
---------------------------------------------------------------------------------------------------------
Hazard/symptom Prevention and protection First aid
---------------------------------------------------------------------------------------------------------
Skin: mild irritation neoprene gloves, face shield remove contaminated clothing;
wash with plenty of water
Eyes: marginal irritant safety goggles, face shield flush with clean water for
at least 15 min
Inhalation: irritation of upper local exhaust ventilation; fresh air
respiratory tract wear a dust mask
Ingestion: none observed unlikely professional hazard gastric lavage may be indicated
---------------------------------------------------------------------------------------------------------
Spillage Storage Fire and explosion
---------------------------------------------------------------------------------------------------------
Collect spillage in closed container store cool and dry in Fire: not flammable under normal
or dust bin bag; in the case of original packing conditions
liquid, first use absorbant material; Explosion: none
clean up with water Fire extinguishing agents: foam, CO2,
dry chemical
---------------------------------------------------------------------------------------------------------
Waste disposal
---------------------------------------------------------------------------------------------------------
Should be burnt in an incinerator
designed for pesticide disposal
---------------------------------------------------------------------------------------------------------
A3.1 Exposure Limit Values
Some exposure limit values are given in Table A.1.
A3.2 Specific Regulatory Actions
In Czechoslovakia (effective date: 1981) and the United
Kingdom (1983 (r)), the substance is approved as a pesticide or
acaricide, and specified uses, limitations, and safety precautions
are listed. In the USSR, the substance is approved as an
insecticide for agricultural use and application; dose, mode, and
treatment frequency are specified (effective date: 1982). In
Sweden, the substance is an active ingredient in pesticide
formulations that are registered at the products control board and
therefore may be marketed and used. The formulations may be sold
only to persons authorized to use such formulations (1984 (r)).
A3.3 Transport and Labelling
The United Nations Committee of Experts on the Transportation
of Dangerous Goods classifies tetradifon as a poisonous substance
(Class 6.1) presenting minor danger for packing purposes when the
active ingredient constitutes 25 - 100% of the formulation (1982
(r)). The recommended label is:
 Table A.1. Some exposure limit values
---------------------------------------------------------------------------------------------------------
Medium Specification Country Exposure limit description Value Effective
(mg/kg) date
---------------------------------------------------------------------------------------------------------
Food plant Argentina maximum residue limit 1 - 5 1969
meat, milk 0 1969
mint 100 1969
hops 30 1969
dried hops 120 1969
Food plant (specified) Brazil acceptable limit (safety 1 - 5 1981
interval: 14 days)
Food plant (specified) Germany, Federal maximum residue limit 1.5 1978
Republic of
plant (general) maximum residue limit 0.05 1978
Food food products Kenya maximum limit 1 - 100 1978 (r)
Food fruit, vegetables Sweden maximum acceptable concentration 2 1983
Food food products USA residue tolerance 8 - 120 1981 (r)
(specified)
raw agricultural acceptable residue limit 0 - 100 1981 (r)
products
Food food products USSR maximum residue limit 0.1 - 1983
(specified) 0.7
---------------------------------------------------------------------------------------------------------
Table A.1. Some exposure limit values
---------------------------------------------------------------------------------------------------------
Medium Specification Country Exposure limit description Value Effective
(mg/kg) date
---------------------------------------------------------------------------------------------------------
Food plant Argentina maximum residue limit 1 - 5 1969
meat, milk 0 1969
mint 100 1969
hops 30 1969
dried hops 120 1969
Food plant (specified) Brazil acceptable limit (safety 1 - 5 1981
interval: 14 days)
Food plant (specified) Germany, Federal maximum residue limit 1.5 1978
Republic of
plant (general) maximum residue limit 0.05 1978
Food food products Kenya maximum limit 1 - 100 1978 (r)
Food fruit, vegetables Sweden maximum acceptable concentration 2 1983
Food food products USA residue tolerance 8 - 120 1981 (r)
(specified)
raw agricultural acceptable residue limit 0 - 100 1981 (r)
products
Food food products USSR maximum residue limit 0.1 - 1983
(specified) 0.7
---------------------------------------------------------------------------------------------------------
 Molecular formula: C12H6C14O2S
CAS chemical name: 1,2,4-trichloro-5-[(4-chlorophenyl)-
sulfonyl]-benzene
Synonyms: 4-chlorophenyl-2,4,5-trichlorophenyl sulfone,
2,4,4',5-tetrachlorodiphenyl sulfone
Common trade names: Akaritox, Aredion, Duphar 23737, ENT 23737,
FMC 5488, Mition, NIA 5488, Polacaritox,
Roztoczol, Roztozol, Tedion V18,
Tetradichlone (a complete list of trade names
is available from IRPTC)
CAS registry number:
Molecular formula: C12H6C14O2S
CAS chemical name: 1,2,4-trichloro-5-[(4-chlorophenyl)-
sulfonyl]-benzene
Synonyms: 4-chlorophenyl-2,4,5-trichlorophenyl sulfone,
2,4,4',5-tetrachlorodiphenyl sulfone
Common trade names: Akaritox, Aredion, Duphar 23737, ENT 23737,
FMC 5488, Mition, NIA 5488, Polacaritox,
Roztoczol, Roztozol, Tedion V18,
Tetradichlone (a complete list of trade names
is available from IRPTC)
CAS registry number:  Table A.1. Some exposure limit values
---------------------------------------------------------------------------------------------------------
Medium Specification Country Exposure limit description Value Effective
(mg/kg) date
---------------------------------------------------------------------------------------------------------
Food plant Argentina maximum residue limit 1 - 5 1969
meat, milk 0 1969
mint 100 1969
hops 30 1969
dried hops 120 1969
Food plant (specified) Brazil acceptable limit (safety 1 - 5 1981
interval: 14 days)
Food plant (specified) Germany, Federal maximum residue limit 1.5 1978
Republic of
plant (general) maximum residue limit 0.05 1978
Food food products Kenya maximum limit 1 - 100 1978 (r)
Food fruit, vegetables Sweden maximum acceptable concentration 2 1983
Food food products USA residue tolerance 8 - 120 1981 (r)
(specified)
raw agricultural acceptable residue limit 0 - 100 1981 (r)
products
Food food products USSR maximum residue limit 0.1 - 1983
(specified) 0.7
---------------------------------------------------------------------------------------------------------
Table A.1. Some exposure limit values
---------------------------------------------------------------------------------------------------------
Medium Specification Country Exposure limit description Value Effective
(mg/kg) date
---------------------------------------------------------------------------------------------------------
Food plant Argentina maximum residue limit 1 - 5 1969
meat, milk 0 1969
mint 100 1969
hops 30 1969
dried hops 120 1969
Food plant (specified) Brazil acceptable limit (safety 1 - 5 1981
interval: 14 days)
Food plant (specified) Germany, Federal maximum residue limit 1.5 1978
Republic of
plant (general) maximum residue limit 0.05 1978
Food food products Kenya maximum limit 1 - 100 1978 (r)
Food fruit, vegetables Sweden maximum acceptable concentration 2 1983
Food food products USA residue tolerance 8 - 120 1981 (r)
(specified)
raw agricultural acceptable residue limit 0 - 100 1981 (r)
products
Food food products USSR maximum residue limit 0.1 - 1983
(specified) 0.7
---------------------------------------------------------------------------------------------------------
