
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 64
CARBAMATE PESTICIDES: A GENERAL INTRODUCTION
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154264 0
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1986
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CARBAMATE PESTICIDES: A GENERAL
INTRODUCTION
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General
1.1.2. Properties, uses, and analytical methods
1.1.3. Sources, environmental transport and distribution
1.1.4. Environmental levels and exposures
1.1.5. Effects on organisms in the environment
1.1.6. Kinetics and metabolism
1.1.7. Mechanism of toxicity
1.1.8. Effects on experimental animals and in vitro
test systems
1.1.9. Mutagenicity and related end-points
1.1.10. Carcinogenicity
1.1.11. Effects on man
1.1.12. Previous evaluations by international bodies
1.2. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels, processes, and uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Vegetation and wildlife
4.1.5. Entry into the food chain
4.2. Biotransformation
4.2.1. Microbial degradation
4.2.2. Photodegradation
4.2.3. Photodecomposition in the aquatic environment
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Exposure of the general population
5.1.1. Food and drinking-water
6. METABOLISM AND MODE OF ACTION
6.1. Mode of action
6.1.1. Neuropathy target esterase
6.2. Metabolism
6.2.1. Absorption
6.2.2. Distribution
6.2.3. Metabolic transformation
6.2.3.1 Biotransformation mechanisms
6.2.3.2 Oxidation
6.2.3.3 Hydrolysis
6.3.2.4 Conjugation
6.2.3.5 Examples of biotransformation
of carbamates
6.3. Elimination and excretion in expired air, faeces, and
urine
6.3.1. Man
6.3.2. Laboratory animals
6.4. Metabolism in plants
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic Organisms
7.2.1. Field studies
7.3. Terrestrial Organisms
7.3.1. Effects on soil fauna
7.3.2. Wildlife
7.3.3. Bees
7.4. Earthworm and mite populations
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Oral
8.1.2. Dermal
8.1.3. Inhalation
8.2. Short- and long-term exposures
8.2.1. Oral
8.2.1.1 Further information on short-
and long-term toxicity
8.2.2. Dermal
8.3. Skin and eye irritation; sensitization
8.3.1. Skin irritation
8.3.2. Eye irritation
8.3.3. Skin sensitization
8.4. Inhalation
8.5. Reproduction, embryotoxicity, and teratogenicity
8.5.1. Reproduction
8.5.2. Endocrine system
8.5.3. Embryotoxicity and teratogenicity
8.6. Mutagenicity and related end-points
8.7. Carcinogenicity
8.7.1. General
8.8. Special studies
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Acute toxicity: poisoning incidents
9.1.2. Effects of short- and long-term exposure
9.1.3. Controlled human studies
9.2. Occupational exposure
9.2.1. Acute toxicity: poisoning incidents
9.2.2. Effects of short- and long-term exposure
9.2.3. Epidemiological studies
9.3. Signs and symptoms of acute intoxication by carbamates
9.3.1. Biochemical methods for measurement of effects
9.4. Treatment of acute poisoning by carbamate insecticides
9.4.1. Minimizing the absorption
9.4.2. General supportive treatment
9.4.3. Specific pharmacological treatment
9.4.3.1 Atropine
9.4.3.2 Oxime reactivators
9.4.3.3 Diazepam
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
ANNEX I NAMES AND STRUCTURES AND SOME PHYSICAL AND CHEMICAL
PROPERTIES OF CARBAMATE PESTICIDES
ANNEX II SUMMARY OF THE SHORT- AND LONG-TERM TOXICITY STUDIES
THAT WERE USED TO ESTABLISH THE ACCEPTABLE DAILY INTAKES
FOR HUMAN BEINGS FOR CARBAMATE COMPOUNDS
ANNEX III CARBAMATES: JMPR REVIEWS, ACCEPTABLE DAILY INTAKES,
EVALUATION BY IARC, CLASSIFICATION BY HAZARD, FAO/WHO
DATA SHEETS, IRPTC DATA PROFILE AND LEGAL FILE
ANNEX IV ABBREVIATIONS
WHO TASK GROUP ON CARBAMATE PESTICIDES
Members
Dr D. Ecobichon, Department of Pharmacology and Therapeutics,
McGill University, Montreal, Quebec, Canada
Dr A.H. El-Sebae, Department of Pesticide Chemistry, Faculty
of Agriculture, University of Alexandria, Alexandria, Egypt
Dr L. Ivanova-Chemishanska, Institute of Hygiene and Occupational
Health, Medical Academy, Sofia, Bulgaria (Vice-Chairman)
Dr M.K. Johnson, Toxicology Unit, Medical Research Council
Laboratories, Carshalton, Surrey, United Kingdom
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr M. Lotti, Institute of Occupational Health, Padua, Italy
Dr L. Martson, All-Union Scientific Research Institute of the
Hygiene and Toxicology of Pesticides, Polymers, and
Plastics (VNIIGINTOX), Kiev, USSRa
Dr U.G. Oleru, College of Medicine, University of Lagos,
Lagos, Nigeria.
Dr W.O. Phoon, Department of Social Medicine and Public
Health, National University of Singapore, Outram Hill,
Republic of Singapore (Chairman)
Dr A.F. Rahde, Ministry of Public Health, Porto Alegre, Brazil
Dr E. Reiner, Institute for Medical Research and Occupational
Health, Zagreb, Yugoslavia
Dr J. Sekizawa, National Institute of Hygienic Sciences,
Tokyo, Japan
Observers
Mr R.J. Lacoste, International Group of National Associations
of Pesticide Manufacturers (GIFAP), Brussels, Belgium
Secretariat
Mrs B. Bender, United Nations Environment Programme,
International Register of Potentially Toxic Chemicals,
Geneva, Switzerland
Dr J.R.P. Cabral, Unit of Mechanisms of Carcinogenesis,
International Agency for Research on Cancer, Lyons, France
---------------------------------------------------------------------------
a Invited, but unable to attend.
Secretariat (contd.)
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
Dr G.J. Van Esch, Bilthoven, The Netherlands (Temporary
Adviser) (Rapporteur)
Dr C. Xintaras, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
Detailed data profiles and legal files for most of the
carbamate pesticides can be obtained from the International
Register of Potentially Toxic Chemicals, Palais des Nations, 1211
Geneva 10, Switzerland (Telephone no. 988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR CARBAMATE PESTICIDES
A WHO Task Group on Environmental Health Criteria for Carbamate
Pesticides met in Geneva from 30 September to 4 October 1985. Dr
K.W. Jager opened the meeting on behalf of the Director-General.
The Task Group reviewed and finalized the draft criteria document.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General
The carbamates discussed in this publication are those mainly
used in agriculture, as insecticides, fungicides, herbicides,
nematocides, or sprout inhibitors. In addition, they are used as
biocides for industrial or other applications and in household
products. A potential use is in public health vector control.
Thus, these chemicals are part of the large group of synthetic
pesticides that have been developed, produced, and used on a large
scale in the last 40 years.
The general formula of the carbamates is:
O
||
R1NH - C - OR2
where R1 and R2 are alkyl or aryl groups.
More than 50 carbamates are known, and it is clear that it is
not within the scope of this introduction to include all the
information about each compound. However, all the different
aspects of the different classes of carbamates are touched on,
making use of available publications and reports of studies and
using well known carbamates, such as carbaryl, benomyl, and a few
others, as examples.
Thiocarbamates and dithiocarbamates have not been included,
because these groups of compounds have a different mode of action
and will be dealt with in a separate publication.
1.1.2. Properties, uses, and analytical methods
Three classes of carbamate pesticides are known. The carbamate
ester derivatives, used as insecticides (and nematocides), are
generally stable and have a low vapour pressure and low water
solubility. The carbamate herbicides (and sprout inhibitors) have
the general structure R1NHC(O)OR2, in which R1 and R2 are aromatic
and/or aliphatic moieties. Carbamate fungicides contain a
benzimidazole group.
The only physical or chemical properties given in this
publication are the relative molecular mass, vapour pressure, and
water solubility.
Analytical methods for a number of important carbamates have
been tabulated.
1.1.3. Sources, environmental transport and distribution
The synthesis and commercialization of the carbamate pesticides
has been in progress since the 1950s. The benzimidazole fungicides
were introduced on the market in about 1970.
In general, the vapour pressure of the carbamates is low;
nevertheless, they will evaporate or sublimate slowly at normal
temperature, which may lead to volatilization of carbamates from
water and soil. However, distribution via air will be a minor
factor. The aqueous environment will be an important route of
transport for highly-soluble carbamates. The light absorption
characteristics of carbamates contribute to their rapid
decomposition (by photodegradation or photodecomposition) under
aqueous conditions. Thus, the hazards of long-term contamination
with carbamates seem small. Carbamate insecticides are mainly
applied on the plants, and can reach the soil, while carbamate
nematocides and herbicides are applied directly to the soil.
Several factors influence the biodegradation of carbamates in soil,
such as volatility, soil type, soil moisture, adsorption, pH,
temperature, and photodecomposition. Because the different
carbamates have different properties, it is clear that each should
be evaluated on its own merits, and no extrapolation of results can
be made from one carbamate to another. One carbamate may be easily
decomposed, while another may be strongly adsorbed on soil. Some
leach out easily and may reach groundwater. In these processes,
the soil type and water solubility are of great importance.
Furthermore, it should be recognized that this not only concerns
the parent compound but also the breakdown products or metabolites.
Environmental conditions that favour the growth and activity of
microorganisms also favour the degradation of carbamates. The
first step in the metabolic degradation of carbamates in soil is
hydrolysis. The hydrolysis products will be further metabolized in
the soil-plant system.
Plants can metabolize carbamates in which arylhydroxylation and
conjugation, or hydrolytic breakdown are the main routes of
detoxification. The results of a number of studies suggest that
carbamates are exclusively distributed via the apoplastic system in
plants.
Carbamates are metabolized by microorganisms, plants, and
animals or broken down in water and soil.
1.1.4. Environmental levels and exposures
From the available data, it appears that bioaccumulation in the
different species and different food chains will only take place to
a slight extent. Certain carbamates may reach groundwater and as a
consequence may find their way into drinking-water. The few
studies available indicate that exposure of the general population
is low. However, this should be confirmed by market-basket and/or
total-diet studies.
1.1.5. Effects on organisms in the environment
Soil microorganisms are capable of metabolizing (hydrolysing)
carbamates and can easily adapt themselves to metabolize the
different types of carbamates. Nevertheless, carbamates and their
metabolites can, at high dose levels, affect the microflora and
cause changes that may be of importance in soil productivity.
Although carbamates are not very stable under aquatic conditions,
and will not persist long in this environment, some bioaccumulate
in fish, mainly because the metabolism is slow in fish. Other
carbamates are metabolized rapidly and no accumulation occurs.
Some carbamates are highly toxic for invertebrates and fish, others
much less so. In certain cases, the use of toxic carbamates may
cause a significant reduction in non-target organisms. Carbamates
are toxic for worms and other organisms living in the soil.
Although a great reduction in the earthworm population may occur
when applying carbamates to the soil, numbers will return to
normal, because of the rather rapid breakdown of these compounds.
In general, the toxicity of carbamates for wildlife is low, but
exceptions exist. This means that, in order to judge the impact of
carbamates on the organisms in the environment, the information on
the individual carbamates should be referred to. As a rule, birds
are not very sensitive to carbamates while bees are extremely
sensitive.
1.1.6. Kinetics and metabolism
The metabolic fate of carbamates is basically the same in
plants, insects, and mammals. Carbamates are usually easily
absorbed through the skin, mucous membranes, and respiratory and
gastrointestinal tracts, but there are exceptions. Generally, the
metabolites are less toxic than the parent compounds. However, in
certain cases, the metabolites are just as toxic or even more toxic
than the parent carbamate. In most mammals, the metabolites are
mainly excreted rather rapidly in the urine. The dog seems to be
different in this respect. Accumulation takes place in certain
cases, but is of minor importance because of the rapid metabolism.
The first step in the metabolism of carbamates is hydrolysis to
carbamic acid, which decomposes to carbon dioxide (CO2) and the
corresponding amine.
The mechanism of hydrolysis is different for N -methyl and
N -dimethyl derivatives. The N -methyl carbamates pass through an
isocyanate intermediate, whereas in the hydrolysis of N -
dimethylcarbamates, an addition product with a hydroxyl ion is
formed yielding the alcohol and N -dimethyl substituted acid. The
rate of hydrolysis by esterases is faster in mammals than in plants
and insects.
Apart from hydrolysis, oxidation also takes place including:
hydroxylation of the aromatic ring, O -dealkylation, N -methyl
hydroxylation, N -dealkylation, oxidation of aliphatic side chains,
and sulfoxidation to the corresponding sulfone. Oxidation is
associated with the mixed-function oxidase (MFO) enzymes.
Conjugation leads to the formation of O - and N -glucuronides,
sulfates, and mercapturic acid derivatives in mammals. Glycosides
and phosphates are conjugation products more common in plants.
The metabolism of a number of carbamates is discussed in the
text.
Little information is available on the distribution of
carbamates in the various organs and tissues in mammals following
exposure by inhalation or the oral route. The organs in which
residues have been reported are the liver, kidneys, brain, fat, and
muscle. The half-life in the rat is of the order of 3 - 8 h. From
the limited data available, it seems that the excretion of
carbamates via urine is also rapid in man, and that the metabolic
pathways in man are the same as those in the rat.
1.1.7. Mechanism of toxicity
Carbamates are effective insecticides by virtue of their
ability to inhibit acetylcholinesterase (AChE) (EC 3.1.1.7) in the
nervous system. They can also inhibit other esterases.
The carbamylation of the enzyme is unstable, and the
regeneration of AChE is relatively rapid compared with that from a
phosphorylated enzyme. Thus, carbamate pesticides are less
dangerous with regard to human exposure than organophosphorus
pesticides. The ratio between the dose required to produce death
and the dose required to produce minimum symptoms of poisoning is
substantially larger for carbamate compounds than for
organophosphorus compounds.
Because of their chemical structure, carbamates do not cause
delayed neuropathy.
1.1.8. Effects on experimental animals and in vitro test systems
The acute toxicity of the different carbamates ranges from
highly toxic to only slightly toxic or practically non-toxic. The
LD50 for the rat ranges from less than 1 mg/kg to over 5000 mg/kg
body weight. For certain methyl carbamates, the LD50 is 20 or more
times the corresponding ED50. This means that, in general, an
early indication of poisoning can be obtained before a lethal dose
is absorbed.
A dose-effect relationship exists between the dose, the
severity of symptoms, and the degree of cholinesterase (ChE)
inhibition. Because most carbamates have a low volatility,
inhalation studies are mainly carried out using a dust or mist. In
these studies, the toxicity is highly dependent on the size of the
particles or droplets and, therefore, difficult to evaluate.
The acute dermal toxicity of carbamates is generally low to
moderate; an exception is aldicarb, which is highly toxic. It
should be noted that data are available for only a limited number
of substances.
Carbamates produce slight to moderate skin and eye irritation,
depending on the vehicle used, duration of contact, and on whether
the substance is applied to the abraided or intact skin. From the
available data, it cannot be excluded that some of the carbamates
will have a slight to moderate sensitization potential.
Short- and long-term toxicity studies have been carried out.
Some carbamates are very toxic and others are less toxic in long-
term studies. From these studies, it is evident that, apart from
the anticholinesterase activity, the following changes can be
found: an influence on the haemopoietic system, an influence on the
functioning of, and, at higher dosages, degeneration of, the liver
and kidneys, and degeneration of testes. These abnormalities in
the different organ systems depend on the animal strain and on the
chemical structure of the carbamate. A clear influence on the
nervous system, functional as well as histological, was found,
particularly in non-laboratory animals such as pigs.
For many years, long-term toxicity data on carbamates have been
evaluated by the FAO/WHO Joint Meeting on Pesticide Residues
(JMPR), and a number of ADIs for carbamates have been established.
In Annex II and III, the no-observed-adverse-effect levels and the
ADIs are summarized.
A considerable number of reproduction and teratogenicity
studies have been carried out with different carbamates and various
animal species. Different types of abnormalities were found, i.e.,
increase in mortality, disturbance of the endocrine system, and
effects on the hypophysis and its gonadotrophic function. These
effects were mainly seen at high dose levels. Generally, the fetal
effects included an increase in mortality, decreased weight gain in
the first weeks after birth, and induction of early embryonic
death. All these effects can be summarized as embryotoxic effects.
Certain carbamates also induce teratogenic effects, mainly at high
dose levels applied by stomach tube. When the same dose level was
administered with the diet, no effects were seen.
1.1.9. Mutagenicity and related end-points
The well-known carbamates have been tested for their mutagenic
activity in different test systems. Some induce mutagenic effects,
others are negative. In general, the methyl carbamates are
negative in mammalian tests, while compounds such as carbendazim,
benomyl, and the 2 thiophanate derivatives showed a positive effect
with very high dose levels in certain systems. The benzimidazole
moiety may act as a base analogue for DNA and as a spindle poison.
They are antimitotic agents and cause mitotic arrest, mitotic
delay, and a low incidence of chromosome damage. Sometimes, the
results are contradictory or cannot be reproduced, but positive
results for point mutation and chromosome aberrations are well
documented. These benzimidazole derivatives can be considered as
weak mutagenic compounds.
1.1.10. Carcinogenicity
Ethyl carbamate (urethane) is a well-known carcinogen, and it
seems that its chemical structure is optimal for such an effect.
Any change in the molecule seems to decrease the carcinogenic
potency, particularly when the ethyl group is replaced by larger
side chains. Alkyl groups on the nitrogen also reduce this
activity.
However, no clear indications of carcinogenic effects have been
found in the available long-term carcinogenicity studies with
different carbamates.
The carcinogenicity studies with benzimidazole derivatives
showed either positive or equivocal results. Added to the fact
that certain mutagenicity studies also give positive results, it
cannot be excluded that these compounds may have carcinogenic or
promotor properties. It should be kept in mind that the dose
levels in most tests were of the order of 50 and 500 mg/kg body
weight.
Carbamate pesticides may be converted to N -nitroso compounds.
This was demonstrated in a great number of in vivo nitrosation
studies in which high levels of the carbamates were administered to
animals in combination with high levels of nitrite. These N -
nitroso compounds have to be considered as mutagenic and
carcinogenic. However, the amount of nitroso compounds that can be
expected to result from dietary intake of carbamate pesticide
residues is negligible in comparison with nitroso-precursors that
occur naturally in food and drinking-water.
1.1.11. Effects on man
Health hazards for man occur mainly from occupational over-
exposure to carbamate insecticides resulting in poisoning
characterized by cholinergic symptoms caused by inhibition of the
enzyme AChE. Various cases of intoxication have been described.
Most of them were spraymen applying insecticides inside houses in
the tropics to control mosquito vectors of malaria, or plant
protection workers. The main routes of exposure are inhalation and
skin.
From controlled human studies, it is clear that poisoning
symptoms can be seen a few minutes after exposure, and can last for
a few hours. Thereafter, recovery starts and within hours, the
symptoms disappear, and the ChE activity in erythrocytes and plasma
returns to normal, because the carbamate is rather rapidly
metabolized and the metabolites excreted. The appearance of these
metabolites in the urine may be used for biological monitoring.
Apart from the symptoms indicative of ChE poisoning, other signs
and symptoms induced by certain carbamates have been described,
such as skin and eye irritation, hyperpigmentation, and influence
on the function of testes (slight increase of sperm abnormalities).
These signs and symptoms were found in a few studies and should be
confirmed before it can be stated that they were induced by
carbamates. Epidemiological studies with persons primarily exposed
to carbamates are not available.
1.1.12. Previous evaluations by international bodies
Previous evaluations of individual carbamates by the Joint
FAO/WHO Meeting on Pesticide Residues (JMPR) and the International
Agency for Research on Cancer (IARC) are summarized in Annex III.
The WHO recommended classification of pesticides by hazard is
included, and the availability is indicated of WHO/FAO Data Sheets
and the IRPTC data profile and legal file on the substance.
1.2. Recommendations
1. More up-to-date information is necessary on the world-wide
production and uses of the different carbamates.
2. Except for the well-known carbamates, such as carbaryl,
benomyl, and carbendazim, more information is required on
environmental pathways, concentrations, and distribution.
3. More information is necessary on the occurrence and fate of the
carbamates in surface water, soil, and groundwater, and their
impact on plants, invertebrates, and mammals.
4. Further studies are necessary on the occurrence of carbamates
in the different food chains (bioaccumulation), and in food and
drinking-water (market-basket or total-diet studies), in order to
estimate the daily exposure of the human population.
5. More information is needed, for certain carbamates, on the
acute and long-term toxicity in aquatic and terrestrial organisms.
6. In general, data on the mode of action and metabolism are
available; however, more knowledge is needed on the distribution of
the carbamates in organs and tissues in mammals.
7. For many carbamates, information concerning long-term toxicity,
mutagenicity, carcinogenicity, reproduction, and teratogenicity is
still needed. This is the reason why acceptable daily intakes have
not been established for two-thirds of the carbamates.
8. Apart from a number of studies on human volunteers and a number
of accidents with well-known carbamates, information on the effects
of human exposure to carbamates is missing. There are no
epidemiological studies. More information should be collected to
evaluate the health risk in cases of human exposure to carbamates.
In particular, more information is needed to elucidate the
mechanisms of the effects of benzimidazole carbamates on the
testes.
9. More data are needed on the use of oximes in the therapy of
poisoning, especially in light of the fact that there are
unconfirmed reports that oximes might potentiate carbamate
toxicity.
10. Further work should be done to develop more adequate
analytical methods (i.e., faster procedures and simpler equipment)
to determine carbamate residues in biological material (urine,
blood, and food).
11. Information is required concerning the changes in toxicity due
to impurities that can arise in pesticides as a consequence of
different manufacturing processes, formulating practices, and
improper storage.
12. Users should be encouraged to be aware of the necessity to
establish safe re-entry periods according to local conditions.
Recommendations for further work have also been described in
previous monographs on carbamates published in the WHO Pesticide
Residues Series and in the FAO Plant Production and Protection
Papers, as a result of the Joint FAO/WHO Meetings on Pesticide
Residues (JMPR).
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Carbamates are N -substituted esters of carbamic acid. Their
general formula is:
O
||
R1NH - C - OR2
where R2 is an aromatic or aliphatic moiety. Three main classes of
carbamate pesticides are known:
(a) carbamate insecticides; R1 is a methyl group;
(b) carbamate herbicides; R1 is an aromatic moiety; and
(c) carbamate fungicides; R1 is a benzimidazole moiety.
2.2 Physical and Chemical Properties
In general, simple esters or N -substituted derivatives of
carbamic acid are unstable compounds, especially under alkaline
conditions. Decomposition takes place, and the parent alcohol,
phenol, ammonia, amine, and carbon dioxide are formed.
The salts and esters of substituted carbamic acid are more
stable than carbamic acid. This enhanced stability is the basis
for the synthesis of many derivatives that are biologically active
pesticides.
Carbamate ester derivatives are crystalline solids of low
vapour pressure with variable, but usually low, water solubility.
They are moderately soluble in solvents such as benzene, toluene,
xylene, chloroform, dichloromethane, and 1,2-dichloroethane. In
general, they are poorly soluble in nonpolar organic solvents such
as petroleum hydrocarbons but highly soluble in polar organic
solvents such as methanol, ethanol, acetone, dimethylformamide,
etc.
The carbamate derivatives with herbicidal action (such as
pyrolan and dimetilan) are substantially more stable to alkaline
hydrolysis than the methyl carbamate derivatives (carbaryl and
propoxur), which have an insecticidal action. For example, the
half-life of carbaryl is 15 min at pH 10 compared with 10 days at
pH 7. However, pyrolan and dimetilan do not hydrolyse in the pH
range of 4 - 10 (Aly & El Dib, 1972). Instability with alkali is of
use for decontamination and clean-up. Vassilieff & Ecobichon
(1982) showed that, in acid fresh water, which is characteristic of
the lakes and streams in heavily forested Canada, aminocarb would
be rather stable and would persist long enough to be bioaccumulated
by various trophic levels of food chains.
The carbamate fungicides carbendazim, benomyl, and thiophanates
are related. Carbendazim and benomyl are derivatives of
benzimidazole. Carbendazim is slowly hydrolysed by alkali to 2-
aminobenzimidazole, but it is stable as acid-forming water-soluble
salts (Sypesteijn et al., 1977). Benomyl breaks down to methyl 2-
benzimidazole carbamate (MBC) in water. Benomyl is rather unstable
in common solvents (White et al., 1973; Chiba & Doornbos, 1974).
The names, chemical structure, and pesticidal activity of the
principal carbamates are presented in Table 1, and the CAS number,
chemical name, common names, molecular formula, relative molecular
mass, and selected chemical and physical properties are summarized
in Annex I. It should be noted that it is not within the scope of
this general introduction to describe all the physical and chemical
properties of each pesticide in detail.
2.3 Analytical Methods
Analysis for pesticide residues consists of sampling the
contaminated environmental material or matrix, extracting the
pesticide residue, removing interfering substances from the
extract, and identifying and quantifying the pesticide contaminant.
The manner in which the matrix material is sampled, stored, and
handled can affect the results; samples should be truly
representative, and their handling and storage must not further
contaminate or degrade the contaminant to be measured. Many
detection methods are available, and the one chosen depends on the
physical and chemical properties of the contaminant as well as on
the equipment available.
A detailed review of all the analytical procedures to determine
carbamates in the different matrices is beyond the scope of this
document. However, for better understanding of the validity of the
data, a brief summary of some of the analytical procedures for
different carbamates is included in Table 2.
Enzymatic methods to determine erythrocyte- and plasma-ChE
activity are used as monitors of exposure and systemic absorption
of organophosphorus compounds and carbamate pesticides. In the case
of carbamates, the inhibition may not be easily detected because of
the rapid reversibility of the carbamate-enzyme inhibition
reaction.
Conditions and time of storage must be carefully controlled
before measurement of activity. Methods must be chosen that allow
a short time for hydrolysis of the substrate (Ellman et al., 1961;
Voss & Schuler, 1967; Wilhelm & Reiner, 1973; Wilhelm et al., 1973;
Reiner et al., 1974; Abd-Elroaf et al., 1977; Izmirova, 1980).
A colorimetric screening method has been described, to estimate
Unden(R), carbofuran, and carbaryl in the air of the manufacturing
and formulating plants, as well as in washings from body surfaces,
hands, and other contaminated surfaces (Izmirova, 1980; Izmirova &
Izmirov, unpublished report, 1985)a.
---------------------------------------------------------------------------
a Pharmatest-cholinesterase reactive papers for determination
of cholinesterase activity (ChEA) of serum or plasma.
Table 1. Relationship of chemical structure and pesticidal activity of carbamates
---------------------------------------------------------------------------------------------------------
Pesticidal Chemical structure Common or other names
activity
---------------------------------------------------------------------------------------------------------
Insecticide O aldoxycarb, allyxycarb, aminocarb, BPMC, bendiocarb,
|| bufencarb, butacarb, carbanolate, carbaryl, carbofuran,
CH3-NH-C-O-aryl cloethocarb, dimetilan, dioxacarb, ethiofencarb, forme-
tanate, hoppcide, isoprocarb, trimethacarb, MPMC,
methiocarb, metolcarb, mexacarbate, pirimicarb,
promacyl, promecarb, propoxur, MTMC, XMC, xylylcarb
O aldicarb, methomyl, oxamyl, thiofanox, thiodicarb
||
CH3-NH-C-O- N-alkyl
Herbicide O asulam, barban, carbetamide, chlorbufam, desmedipham,
|| phenmedipham, swep
aryl-NH-C-O-alkyl
O dichlormate, karbutilate, terbucarb
||
alkyl-NH-C-O-aryl
Herbicide O propham, chlorpropham
and sprout ||
inhibitors aryl-NH-C-O-alkyl
Fungicide O benomyl, carbendazim, thiophanate-methyl,
|| thiophanate-ethyl
aryl-NH-C-O-alkyl
---------------------------------------------------------------------------------------------------------
Table 2. Analytical methods for carbamate pesticide residues
---------------------------------------------------------------------------------------------------------
Chemical Sample type Extraction and clean-up Method of Detectionb Reference
detectiona limit
---------------------------------------------------------------------------------------------------------
aldicarb cotton seed, acetone-water-peracetic acid GLC/SFPD 0.01 mg/kg Romine (1973)
fruit/ extraction evaporation,
vegetables absorption on Florisil column,
elution with acetone-ether
asulam crops acetone extraction, absorption colorimetry 0.05 mg/kg Brockelsby &
on Florisil column, elution, Muggleton (1973)
reaction with chromophore
benomyl soils, fruit/ acidic methanol extraction and HPLC/UV 0.05 mg/kg Bleidner et al.
vegetables, ethyl acetate extraction, (1978)
tissues conversion to carbendazim
soils acetone-ammonium chloride UV Austin & Briggs
extraction and solvent (1976)
partition
soils, fruit/ review of methods including 0.05 - Baker & Hoodless
vegetables, those for carbendazim and 3.0 mg/kg (1974)
tissues thiophanate-methyl
carbendazim water reverse phase and adsorption HPLC, variable Austin et al.
systems wavelength (1976)
UV-detector
carbaryl urine acid hydrolysis, benzene GLC/ECD 0.02 Shafik et al.
extraction, reaction with mg/litre (1971)
chloroacetic anhydride,
absorption on silica gel column
and elution with benzene-hexane
carbaryl plant tissue extraction, hydrolysis, and colorimetry 0.1 mg/kg Stansbury &
reaction with chromophore Miskus (1964)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Sample type Extraction and clean-up Method of Detectionb Reference
detectiona limit
---------------------------------------------------------------------------------------------------------
methiocarb fruit and acetone extraction of GLC/SFPD 0.01 mg/kg Bowman & Beroza
vegetables carbamates and free phenols, (1969)
partition on silica gel column,
hydrolysis of conjugated phenols
methomyl ethyl acetate extraction, GLC/SFPD 0.02 mg/kg Leitch & Pease
hexane extraction, chloroform (1973)
solution, hydrolysis and ethyl
acetate extraction
phenmedipham plant hydrolysis, distillation- colorimetry 0.05 mg/kg Kossmann & Jenny
tissues extraction, and reaction (1973)
with chromophore
propoxur plant and acetone-chloroform extraction, GLC/ECD 0.1 mg/kg Anderson (1973)
animal absorption on Florisil column,
tissues chloro form elution, solution
milk in benzene, and reaction with 0.01 mg/kg
trichloroacetyl chloride
thiofanox soil acetone extraction, oxidation GLC/SFPD Chin et al.
with hydrogen peroxide, (1975)
absorption on Florisil column
and elution with chloroform
and diethyl ether, solution
benzene
---------------------------------------------------------------------------------------------------------
a GLC = gas-liquid chromatography.
ECD = electron-capture detector.
SFPD = sulfur (sensitive) flame photometric detector.
HPLC = high-performance liquid chromatography.
UV = ultraviolet.
b Limit of detection is the sensitivity of the method; however, each method may not be able to measure
all the contaminants originally present in the sample, i.e., the recovery rates for spiked
samples < 100%.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural Occurrence
Methyl carbamates are related to a naturally-occurring
carbamate alkaloid, physostigmine, isolated from the calabar bean
(Physostigma venenosum) in 1864.
Physostigmine (or eserine) has a pronounced cholinergic action
(Still & Herrett, 1975).
3.2 Man-Made Sources
Carbamate pesticides have been produced and in commercial use
since the 1950s.
Benomyl and carbendazim, belonging to the benzimidazole group
of fungicides, came on the market around 1970.
3.2.1 Production levels, processes, and uses
The carbamates included in this review are those mainly used in
agriculture. They are some of the many synthetic organic
pesticides that have been produced on a large scale. Additional
uses are as biocides for industrial or other commercial
applications and in household products; a potential use is in
public health vector control.
Global consumption of the carbamate insecticides and herbicides
over the period 1974-82 is summarized in Table 3. The data are not
complete but give an estimate of the magnitude of the consumption
and distribution of the compounds throughout the world. The
reported global consumption is between 20 000 and 35 000
tonnes/year. The herbicidal carbamates, an integral part of
industrialized agriculture, are used mainly in North and Central
America, and Europe, with little reported use in Africa, Asia, and
South America. However, in the period mentioned, carbamate
insecticides were substantially used in Asia (FAO, 1985).
Table 3. Global consumption of carbamate insecticides
(in 100 kg)a
-----------------------------------------------------------
Region 1974-76 1981 1982 1983
-----------------------------------------------------------
Africa
Sierra Leone 25
Sudan 1590
Zimbabwe 4837
North/Central America
Bermuda
Canada 7911
Cuba 7333
El Salvador 507
Mexico 25 367 21 630 21 380 17 210
Montserrat 2 2
USA 125 000 115 000
South America
Argentina 2960 2700
Guyana 36
Suriname 369
Uruguay 63 74 70 179
Asia
Brunei 7 12 29
Cyprus 20 124 21
Hong Kong 100 125 64 102
India 33 447 32 160 23 730
Israel 1333 2160 2580 2270
Japan 27 770
Jordan 2000 16 745
Korea Republic 7618 19 270 17 716
Kuwait 2
Oman 134 6 16
Pakistan 298 3511 1136
Saudi Arabia 63
Turkey 635 666
Europe
Austria 213 184 153 86
Czechoslovakia 1490 569 712
Denmark 60 307 440
Finland 7
Greece 8767
Hungary 5854 1539 3396 11 650
Italy 28 014 28 284 23 041
Norway 5 5 10
Poland 12 353 3325 4365 4977
Portugal 512 216 200
Sweden 94 130
Switzerland 133 140 120
-----------------------------------------------------------
a From: FAO (1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and Distribution Between Media
4.1.1 Air
In general, the vapour pressure of carbamates is rather low.
Some of them may sublimate slowly at room temperature, and this may
also explain their loss from soil surfaces (Gray, 1971).
4.1.2 Water
The aqueous environment is an important factor in transport.
Carbamates may enter surface water from industrial wastes,
accidental spillage, and dumping. However, the hazard of this is
limited by their rapid decomposition under aqueous conditions.
Thus, while long-term contamination by this type of compound is
unlikely, adverse effects on aquatic animals may result from direct
addition or from run-off shortly after application.
4.1.3 Soil
Reviews of the action of herbicidal carbamates in the soil have
been made by Gray (1971) and Ashton & Crafts (1973).
Herbicidal carbamates are often applied directly to the soil,
whereas insecticidal carbamates, generally applied to plants, reach
the soil either directly or indirectly. Several factors are
involved in the degradation of carbamates in the soil. These
include volatility, leaching, soil moisture, absorption, pH,
temperature, photodecomposition, microbial degradation, and soil
type (Ogle & Warren, 1954).
Different soil types possess different binding capabilities.
In general, carbamate insecticides are not very persistent in the
soil. However, the fungicide carbendazim is very persistent, with
a half-life of about one year.
Because of the many factors involved and the fact that many
carbamates have different properties, it is clear that results with
one soil type and one carbamate cannot be extrapolated to others.
In general, the water solubility of carbamates is rather low
and may explain the relative immobility of the carbamate herbicides
in the soil with regard to leaching and diffusion. When applied to
the soil, some chloropropham (CIPC) can be lost by vaporization or
sublimation, but otherwise it is tightly adsorbed to certain soils
(Ogle & Warren, 1954). It was found by De Rose (1951) that
chloropropham (CIPC) persisted in the soil about twice as long as
propham (IPC). The disappearance of propham from the soil took
about 24 days, while chloropropham persisted at least 48 days.
Water can displace the carbamates from adsorption sites and cause
them to be lost by volatilization (Parochetti & Warren, 1966).
The degradation of the carbamate herbicides in the environment
is summarized in Fig. 1, using chloropropham as a representative
example of the group.
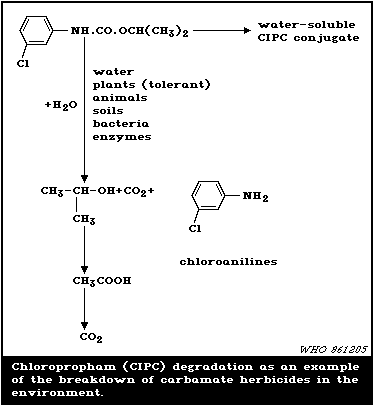 Soil run-off and leaching characteristics of benomyl and its
metabolites were studied in the greenhouse and laboratory using
14C-labelled materials on soil and turf plots and on soil thin-
layer chromatographic plates (Rhodes & Long, 1974). The studies
showed that benomyl and its metabolites are immobile in soil and do
not leach or move from the site of application.
The first step in the metabolic degradation of these carbamates
in soil is hydrolysis (Sonawane & Knowles, 1971). Simple esters are
hydrolysed to the parent (unstable) acid and alcohol. The general
reaction of carbamates and the breakdown of carbamates in soils are
described in detail in the review of Still & Herrett (1975).
Chloropropham, barban, and swep are hydrolysed to their respective
anilines (Still & Herrett, 1975). In alkaline soil, phenmedipharm
converts hydrolytically to methyl-3-hydroxyphenyl-carbamate, which
in turn hydrolyses to 3-aminophenol (Sonawane & Knowles, 1971).
The substituted anilines that are formed are further metabolized by
oxidation processes (Kaufman & Blake, 1973; Still & Herrett, 1975).
Methomyl and oxamyl degrade rapidly with carbon dioxide as the
end product (Harvey & Pease, 1973; Harvey & Han, 1978b).
In soils, the predominant metabolic pathway is cleavage of the
carbamate bond to yield the alcohol and amino moieties, which may
be further metabolized by the soil-plant system.
Williams et al. (1976a,b) found that the degradation of
carbofuran in British Columbia was slower than expected. Soils
showed rather high residues of this compound (up to approximately 4
mg/kg soil), and there was some evidence of build-up where
treatments were repeated for two successive years. While
carbofuran has a half-life of up to 50 weeks in neutral or acid
soils, it degrades rapidly in alkaline soils.
4.1.4 Vegetation and wildlife
Plant metabolism of carbanilate herbicides has been shown to
involve aryl hydroxylation and conjugation besides hydro-lytic
breakdown. Consequently, they are readily transformed from
lyophilic compounds to hydrophylic metabolites, which contain the
intact carbamoyl group. These polar products are not translocated
but remain in the plant at the site of formation. All available
data indicate that the hydroxylated carbamate metabolites are
detoxification products (Still & Herrett, 1975). In studies on
chloropropham in oat shoots, Still & Rusness (1977) found that the
phenolic metabolites were converted to an S -cysteinyl conjugate,
i.e., S -cysteinyl-hydroxychloropropham.
A large number of studies on the absorption and translocation
of carbamate herbicides have been carried out to study the fate of
these compounds in plants. The results suggest that plant leaf
surfaces are a barrier to the absorption of carbamates. Roots,
however, absorb the herbicides to a much greater extent, and the
carbamate moves to all plant parts. Thus, it is suggested that
carbamates are exclusively distributed via the apoplastic system.
The carrier that is used plays an important role in these
absorption studies.
Many studies are available concerning the actual entry of
carbamates into the plant, their hydrolysis, aromatic ring
hydroxylation, hydrolytic breakdown, and oxidation of aliphatic
groups. Other types of metabolic reactions in different plant
species following different methods of application have also been
described (Baldwin et al., 1954; Abdel-Wahab et al., 1966;
Prendeville et al., 1968; Knowles & Sonawane, 1972; Still &
Mansager, 1972, 1973a,b, 1975; Wiedmann et al., 1976; Guardigli et
al., 1977).
For the metabolic pathway in wildlife, see sections 6 and 7.
4.1.5 Entry into the food chain
Carbamates are metabolized or broken down in soil, plants, and
animals. The questions of the environmental impact and the
significance of the metabolites with respect to persistence and
bioaccumulation in certain species and in the food chain have still
to be answered. For the moment, it appears that most, if not all,
of the known carbamate metabolites are biodegraded rapidly and are
less toxic for the environment than the parent molecules (Still &
Herrett, 1975).
4.2 Biotransformation
4.2.1 Microbial degradation
The carbamates are readily degraded by soil microorganisms in
most soils (Gray, 1971). Environmental conditions that favour the
growth and activity of microorganisms also favour degradation (Ogle
& Warren, 1954). The residual herbicidal activity of both barban
and propham persists longer in sterile soil than in non-sterile
soil.
Kaufman & Kearney (1965) and Kaufman & Blake (1973) isolated
(by soil-enrichment techniques) and identified a soil microorganism
capable of degrading carbamates such as chloropropham. They
suggested that hydrolysis was a major degradation pathway in soils.
Each of the soil microorganisms demonstrated a different range of
substrate specificity, but all were capable of degrading and
dehalogenating a variety of pesticides (production of 3-
chloroaniline and subsequent liberation of free chloride ion).
These studies illustrated the tremendous adaptability of the
soil microbial populations in altering the persistence and
character of the different foreign compounds. Studies carried out
by Clark & Wright (1970) confirmed that cultures of Arthrobacter
sp. and Achromobacter sp. were able to convert phenyl carbamates
to the corresponding aniline compounds.
Suzuki & Takeda (1976) studied the metabolism of dimetilan in
Aspergillus niger van Tieghem. Together with hydrolysis,
oxidation of the alkyl side chain appeared the most important
modification (detoxification) process. Williams et al. (1976b)
found that carbofuran was rapidly degraded in soils containing high
levels of actinomycetes.
4.2.2 Photodegradation
N -methyl carbamates absorb radiation available in the solar
region (lambda = 300 nm) and hence, would be expected to undergo
photo-oxidation as well as metabolic degradation. Addison et al.
(1974) studied the fate of various N -methyl carbamates when
solutions were sprayed on bean foliage and exposed to sunlight or
artificial light (lambda = 254 nm). It is not entirely clear
whether the products they observed resulted solely from a
photochemical reaction or from absorption followed by enzymatic
attack (Addison et al., 1973, 1974).
4.2.3 Photodecomposition in the aquatic environment
Carbamate insecticides in water are subject to photo-
decomposition under the effects of ultraviolet radiation (UVR).
The pH of the aqueous medium was found to be an important factor in
relationship to the rates of photolysis of carbaryl and propoxur,
which were slow at low pH values and tended to increase with
increaseing pH value. However, the decomposition of, for instance,
dimetilan, was not affected by the pH of the irradiated medium.
The primary effect of the UVR appears to be cleavage of the ester
bond resulting in the production of the phenol or heterocyclic enol
of the carbamate esters tested. The hydrolysis products produced
were further photodecomposed to other unidentified degradation
products. Carbaryl produced 5 degradation products, one of which
was identified as 1-naphthol. It is assumed that, apart from the
cleavage of the ester bond, changes at other positions in the
molecule are produced by UVR. However, the intact carbamate ester
group is retained, and consequently the ChE-inhibiting activity
(Crosby et al., 1965; Crosby 1969; Aly & El Dib, 1972).
Similar results were reported for the irradiation products of
other carbamate esters such as aminocarb, mexacarbate, and a number
of analogues (Eberle & Gunther, 1965; Abdel-Wahab F & Casida,
1967).
When the half-life for photodecomposition was studied in a
number of carbamate insecticides, the results suggested that the
light absorption characteristics of the insecticide influenced the
extent of its photodecomposition by a specific light source.
However, the extent of photodecomposition was not the same under
different conditions of irradiation (presence of solvents) and
wave-length (Crosby et al., 1965; Eberle & Gunther, 1965).
Thus, it seems reasonable to suggest that photodecomposition
may account for some loss of carbamate insecticides in clear
surface waters exposed to sunlight for a long period. However,
photolysis may be a minor factor in the decomposition of these
compounds in highly-turbid waters, where the penetration of light
will be greatly reduced (Aly & El-Dib, 1971, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Exposure of the General Population
5.1.1 Food and drinking-water
From a market-basket study conducted during 1977-80 by the
Finnish National Board of Trade and Consumer Interests, it became
apparent that the residues of benomyl in food had increased.
During the period 1974-76, the average benomyl intake was about 0.2
mg/person per year from both domestic and imported foods. During
the period 1977-80, average intake was 9 mg/year. A later more
accurate study showed an intake of 14 mg/person per year (Finnish
National Board of Health, 1982).
Contamination of groundwater and drinking-water sources by
aldicarb, a carbamate that is used as an insecticide and as a
nematocide, was reported from the USA in 1979 and 1980-81; levels
even higher than 0.075 mg/litre were found (Rothschild et al.,
1982; Zaki et al., 1982). In the Federal Republic of Germany,
detectable amounts of up to 0.001 mg aldicarb/litre were found in a
few samples of drinking-water (Federal Republic of Germany,
personal communication, 1985)a.
---------------------------------------------------------------------------
a Letter to the IPCS from the Ministry of the Interior of
the Federal Republic of Germany, Bonn, Federal Republic of
Germany, 28 May, 1985.
6. METABOLISM AND MODE OF ACTION
Most carbamates are active inhibitors of AChE and they do not
require metabolic activation. However, some carbamates, such as
the benzimidazole carbamates, do not have anticholinesterase
activity. Carbamates undergo metabolism, and the metabolites are
generally less toxic than the parent compound; some exceptions
will be discussed later. The metabolism of carbamates is basically
the same in mammals, insects, and plants, and the toxic effects of
carbamates are similar in mammals and insects. Carbamates do not
accumulate in the mammalian body, but are rapidly excreted, mainly
via the urine.
6.1 Mode of Action
Carbamates are effective insecticides by virtue of their
ability to inhibit AChE in the nervous system. AChE catalyses the
hydrolysis of the neurotransmitter acetylcholine (ACh) to choline
and acetic acid.
ACh is the synaptic mediator of nerve impulses in the nervous
system of mammals and insects:
(a) as a neurotransmitter in the brain of mammals, and in
the central nervous system of insects;
(b) as a pre-ganglionic neurotransmitter in the autonomic
nervous system of mammals;
(c) in post-ganglionic nerve endings of the autonomic
nervous system; and
(d) at the neuromuscular junction of skeletal muscle.
Carbamates, like organophosphates, can inhibit esterases that
have serine in their catalytic centre; these are called serine-
esterases or beta-esterases. Although the inhibition of serine-
esterases other than AChE is not significant for the toxicity of
the compounds, it may have significance for the potentiation of
toxicity of other compounds after long-term low-level exposure
(Sakai & Matsumura, 1968, 1971; Aldridge & Magos, 1978).
To understand the mechanism of toxicity, it is necessary to
examine the events that take place at the neuromuscular junction.
When a muscle is innervated, as shown in Fig. 2, a nerve impulse
moving down a neuron reaches the nerve ending where ACh, which is
stored in vesicles at the nerve endings, is released into the
junction. Within 2 - 3 ms, ACh impinges on the receptor side of
the muscle.
Soil run-off and leaching characteristics of benomyl and its
metabolites were studied in the greenhouse and laboratory using
14C-labelled materials on soil and turf plots and on soil thin-
layer chromatographic plates (Rhodes & Long, 1974). The studies
showed that benomyl and its metabolites are immobile in soil and do
not leach or move from the site of application.
The first step in the metabolic degradation of these carbamates
in soil is hydrolysis (Sonawane & Knowles, 1971). Simple esters are
hydrolysed to the parent (unstable) acid and alcohol. The general
reaction of carbamates and the breakdown of carbamates in soils are
described in detail in the review of Still & Herrett (1975).
Chloropropham, barban, and swep are hydrolysed to their respective
anilines (Still & Herrett, 1975). In alkaline soil, phenmedipharm
converts hydrolytically to methyl-3-hydroxyphenyl-carbamate, which
in turn hydrolyses to 3-aminophenol (Sonawane & Knowles, 1971).
The substituted anilines that are formed are further metabolized by
oxidation processes (Kaufman & Blake, 1973; Still & Herrett, 1975).
Methomyl and oxamyl degrade rapidly with carbon dioxide as the
end product (Harvey & Pease, 1973; Harvey & Han, 1978b).
In soils, the predominant metabolic pathway is cleavage of the
carbamate bond to yield the alcohol and amino moieties, which may
be further metabolized by the soil-plant system.
Williams et al. (1976a,b) found that the degradation of
carbofuran in British Columbia was slower than expected. Soils
showed rather high residues of this compound (up to approximately 4
mg/kg soil), and there was some evidence of build-up where
treatments were repeated for two successive years. While
carbofuran has a half-life of up to 50 weeks in neutral or acid
soils, it degrades rapidly in alkaline soils.
4.1.4 Vegetation and wildlife
Plant metabolism of carbanilate herbicides has been shown to
involve aryl hydroxylation and conjugation besides hydro-lytic
breakdown. Consequently, they are readily transformed from
lyophilic compounds to hydrophylic metabolites, which contain the
intact carbamoyl group. These polar products are not translocated
but remain in the plant at the site of formation. All available
data indicate that the hydroxylated carbamate metabolites are
detoxification products (Still & Herrett, 1975). In studies on
chloropropham in oat shoots, Still & Rusness (1977) found that the
phenolic metabolites were converted to an S -cysteinyl conjugate,
i.e., S -cysteinyl-hydroxychloropropham.
A large number of studies on the absorption and translocation
of carbamate herbicides have been carried out to study the fate of
these compounds in plants. The results suggest that plant leaf
surfaces are a barrier to the absorption of carbamates. Roots,
however, absorb the herbicides to a much greater extent, and the
carbamate moves to all plant parts. Thus, it is suggested that
carbamates are exclusively distributed via the apoplastic system.
The carrier that is used plays an important role in these
absorption studies.
Many studies are available concerning the actual entry of
carbamates into the plant, their hydrolysis, aromatic ring
hydroxylation, hydrolytic breakdown, and oxidation of aliphatic
groups. Other types of metabolic reactions in different plant
species following different methods of application have also been
described (Baldwin et al., 1954; Abdel-Wahab et al., 1966;
Prendeville et al., 1968; Knowles & Sonawane, 1972; Still &
Mansager, 1972, 1973a,b, 1975; Wiedmann et al., 1976; Guardigli et
al., 1977).
For the metabolic pathway in wildlife, see sections 6 and 7.
4.1.5 Entry into the food chain
Carbamates are metabolized or broken down in soil, plants, and
animals. The questions of the environmental impact and the
significance of the metabolites with respect to persistence and
bioaccumulation in certain species and in the food chain have still
to be answered. For the moment, it appears that most, if not all,
of the known carbamate metabolites are biodegraded rapidly and are
less toxic for the environment than the parent molecules (Still &
Herrett, 1975).
4.2 Biotransformation
4.2.1 Microbial degradation
The carbamates are readily degraded by soil microorganisms in
most soils (Gray, 1971). Environmental conditions that favour the
growth and activity of microorganisms also favour degradation (Ogle
& Warren, 1954). The residual herbicidal activity of both barban
and propham persists longer in sterile soil than in non-sterile
soil.
Kaufman & Kearney (1965) and Kaufman & Blake (1973) isolated
(by soil-enrichment techniques) and identified a soil microorganism
capable of degrading carbamates such as chloropropham. They
suggested that hydrolysis was a major degradation pathway in soils.
Each of the soil microorganisms demonstrated a different range of
substrate specificity, but all were capable of degrading and
dehalogenating a variety of pesticides (production of 3-
chloroaniline and subsequent liberation of free chloride ion).
These studies illustrated the tremendous adaptability of the
soil microbial populations in altering the persistence and
character of the different foreign compounds. Studies carried out
by Clark & Wright (1970) confirmed that cultures of Arthrobacter
sp. and Achromobacter sp. were able to convert phenyl carbamates
to the corresponding aniline compounds.
Suzuki & Takeda (1976) studied the metabolism of dimetilan in
Aspergillus niger van Tieghem. Together with hydrolysis,
oxidation of the alkyl side chain appeared the most important
modification (detoxification) process. Williams et al. (1976b)
found that carbofuran was rapidly degraded in soils containing high
levels of actinomycetes.
4.2.2 Photodegradation
N -methyl carbamates absorb radiation available in the solar
region (lambda = 300 nm) and hence, would be expected to undergo
photo-oxidation as well as metabolic degradation. Addison et al.
(1974) studied the fate of various N -methyl carbamates when
solutions were sprayed on bean foliage and exposed to sunlight or
artificial light (lambda = 254 nm). It is not entirely clear
whether the products they observed resulted solely from a
photochemical reaction or from absorption followed by enzymatic
attack (Addison et al., 1973, 1974).
4.2.3 Photodecomposition in the aquatic environment
Carbamate insecticides in water are subject to photo-
decomposition under the effects of ultraviolet radiation (UVR).
The pH of the aqueous medium was found to be an important factor in
relationship to the rates of photolysis of carbaryl and propoxur,
which were slow at low pH values and tended to increase with
increaseing pH value. However, the decomposition of, for instance,
dimetilan, was not affected by the pH of the irradiated medium.
The primary effect of the UVR appears to be cleavage of the ester
bond resulting in the production of the phenol or heterocyclic enol
of the carbamate esters tested. The hydrolysis products produced
were further photodecomposed to other unidentified degradation
products. Carbaryl produced 5 degradation products, one of which
was identified as 1-naphthol. It is assumed that, apart from the
cleavage of the ester bond, changes at other positions in the
molecule are produced by UVR. However, the intact carbamate ester
group is retained, and consequently the ChE-inhibiting activity
(Crosby et al., 1965; Crosby 1969; Aly & El Dib, 1972).
Similar results were reported for the irradiation products of
other carbamate esters such as aminocarb, mexacarbate, and a number
of analogues (Eberle & Gunther, 1965; Abdel-Wahab F & Casida,
1967).
When the half-life for photodecomposition was studied in a
number of carbamate insecticides, the results suggested that the
light absorption characteristics of the insecticide influenced the
extent of its photodecomposition by a specific light source.
However, the extent of photodecomposition was not the same under
different conditions of irradiation (presence of solvents) and
wave-length (Crosby et al., 1965; Eberle & Gunther, 1965).
Thus, it seems reasonable to suggest that photodecomposition
may account for some loss of carbamate insecticides in clear
surface waters exposed to sunlight for a long period. However,
photolysis may be a minor factor in the decomposition of these
compounds in highly-turbid waters, where the penetration of light
will be greatly reduced (Aly & El-Dib, 1971, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Exposure of the General Population
5.1.1 Food and drinking-water
From a market-basket study conducted during 1977-80 by the
Finnish National Board of Trade and Consumer Interests, it became
apparent that the residues of benomyl in food had increased.
During the period 1974-76, the average benomyl intake was about 0.2
mg/person per year from both domestic and imported foods. During
the period 1977-80, average intake was 9 mg/year. A later more
accurate study showed an intake of 14 mg/person per year (Finnish
National Board of Health, 1982).
Contamination of groundwater and drinking-water sources by
aldicarb, a carbamate that is used as an insecticide and as a
nematocide, was reported from the USA in 1979 and 1980-81; levels
even higher than 0.075 mg/litre were found (Rothschild et al.,
1982; Zaki et al., 1982). In the Federal Republic of Germany,
detectable amounts of up to 0.001 mg aldicarb/litre were found in a
few samples of drinking-water (Federal Republic of Germany,
personal communication, 1985)a.
---------------------------------------------------------------------------
a Letter to the IPCS from the Ministry of the Interior of
the Federal Republic of Germany, Bonn, Federal Republic of
Germany, 28 May, 1985.
6. METABOLISM AND MODE OF ACTION
Most carbamates are active inhibitors of AChE and they do not
require metabolic activation. However, some carbamates, such as
the benzimidazole carbamates, do not have anticholinesterase
activity. Carbamates undergo metabolism, and the metabolites are
generally less toxic than the parent compound; some exceptions
will be discussed later. The metabolism of carbamates is basically
the same in mammals, insects, and plants, and the toxic effects of
carbamates are similar in mammals and insects. Carbamates do not
accumulate in the mammalian body, but are rapidly excreted, mainly
via the urine.
6.1 Mode of Action
Carbamates are effective insecticides by virtue of their
ability to inhibit AChE in the nervous system. AChE catalyses the
hydrolysis of the neurotransmitter acetylcholine (ACh) to choline
and acetic acid.
ACh is the synaptic mediator of nerve impulses in the nervous
system of mammals and insects:
(a) as a neurotransmitter in the brain of mammals, and in
the central nervous system of insects;
(b) as a pre-ganglionic neurotransmitter in the autonomic
nervous system of mammals;
(c) in post-ganglionic nerve endings of the autonomic
nervous system; and
(d) at the neuromuscular junction of skeletal muscle.
Carbamates, like organophosphates, can inhibit esterases that
have serine in their catalytic centre; these are called serine-
esterases or beta-esterases. Although the inhibition of serine-
esterases other than AChE is not significant for the toxicity of
the compounds, it may have significance for the potentiation of
toxicity of other compounds after long-term low-level exposure
(Sakai & Matsumura, 1968, 1971; Aldridge & Magos, 1978).
To understand the mechanism of toxicity, it is necessary to
examine the events that take place at the neuromuscular junction.
When a muscle is innervated, as shown in Fig. 2, a nerve impulse
moving down a neuron reaches the nerve ending where ACh, which is
stored in vesicles at the nerve endings, is released into the
junction. Within 2 - 3 ms, ACh impinges on the receptor side of
the muscle.
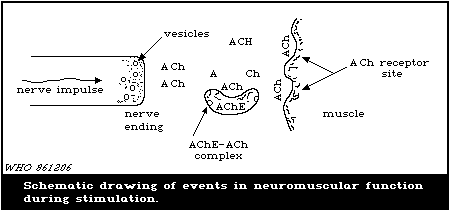 AChE then hydrolytically converts the ACh into choline and
acetic acid, which results in a decrease in the concentration of
ACh and cessation of muscle contraction. When AChE is inhibited by
a carbamate ester, it can no longer hydrolyse the ACh. Thus, the
ACh concentration remains high in the junction giving rise to
continuous stimulation of the muscle which, in turn, leads to
exhaustion and tetany.
Thus, inhibition of AChE by carbamate esters, causes toxic
effects in animals and human beings that result in a variety of
poisoning symptoms and eventually culminate in respiratory failure
and death.
The mechanism of inhibition of AChE by a carbamate ester can be
formulated as follows (Reiner & Aldridge, 1967; Aldridge & Reiner,
1972):
O O
|| k1 ||
AChE + RO-C-NHCH3 '=========' [AChE x RO-C-NHCH3] (enzyme-carbamate
k-1 | complex)
| kc
\/
O
kr ||
AChE + CH3NH2 + CO2 <------ AChE-C-NHCH3 + RO-
H2O
(carbamylated enzyme)
where: k1 = second order rate constant for formation of
complex;
k-1 = first order rate constant for breakdown of
complex to starting materials;
kc = first order rate constant for carbamylation of
the enzyme;
kr = first order rate constant for hydrolysis of the
carbamylated enzyme.
The site for carbamylation of the enzyme is the hydroxyl moiety of
the serine amino acid. The rate of regeneration of the
carbamylated enzyme to AChE (kr) is relatively rapid compared with
that of an enzyme that has been inhibited (phosphorylated) by an
organophosphorus pesticide (Reiner & Aldridge, 1967; Reiner, 1971).
Thus, human exposure to the carbamate pesticides is less dangerous
than exposure to organophosphorus pesticides, because the ratio
between the dose required to produce mortality and the dose
required to produce minimum poisoning symptoms is, in general,
substantially larger for carbamate compounds than for
organophosphorus compounds (Goldberg et al., 1963; Vandekar, 1965;
Vandekar et al., 1971). An individual experiencing symptoms of
poisoning from a carbamate is more likely to recover on termination
of exposure and with appropriate medical treatment than an
individual poisoned by an organophosphorus compound.
The spontaneous reactivation of various carbamylated ChEs
expressed as half-life, at pH 7.0 - 7.4 and 25 °C ranged between 2
and 240 min for AChE and between 2 and 17 min for serum-ChE. This
instability of the carbamylated enzyme affects the determination of
the inhibitory power of carbamates, the recovery after poisoning,
and the determination of inhibition of blood-ChEs (Reiner, 1971).
6.1.1 Neuropathy target esterase
This esterase, formerly known as neurotoxic esterase (NTE), is
the target for the initiation of delayed neuropathy caused by some
organophosphorus esters. Both organophosphorylation of NTE and a
subsequent "aging" reaction of the inhibited enzyme are required to
initiate neuropathy. Certain N -aryl carbamates can also inhibit
NTE, but the chemistry of such carbamates is such that no "aging"
reaction is possible. These carbamate inhibitors do not initiate
delayed neuropathy in vivo but can actually prevent the neuropathic
effects of a challenge dose of an organophosphate given while the
tissue NTE is carbamylated (Johnson, 1970). No delayed neuropathic
effects have been observed in tests on carbamate pesticides
according to protocols that detect neuropathic organophosphates,
and this agrees with the mechanism described above.
6.2 Metabolism
The metabolic fate of carbamates is basically the same in
plants, insects, and mammals.
Carbamates can penetrate the skin, mucous membranes,
respiratory tract, and gastrointestinal tract of mammals.
6.2.1 Absorption
In vivo studies have shown that carbamates are almost
completely absorbed during normal transit through the gastro-
intestinal tract.
According to absorption studies on rats, the dermal absorption
of radioactive labelled benomyl is slow. After absorption, rapid
metabolism and elimination via urine took place. After 1 h, small
amounts of the major metabolites of benomyl, 5-hydroxy-2-
benzimidazole carbamate (5-HBC), and methyl 2-benzimidazole
carbamate (MBC), could be detected in urine, confirming the rapid
metabolism of benomyl. No radioactivity was found in any body
tissues sampled 24 h after application (FAO/WHO, 1985a).
6.2.2 Distribution
Ahdaya et al. (1981) studied the absorption and distribution of
carbamates. They found that, in mice, all carbamates were rapidly
distributed to the tissues and organs. The half-life values of
penetration ranged from 8 to 17 min for carbamates.
No residues of benomyl or its degradation products were
detected (level of sensitivity 0.02 mg/kg) in eggs from chickens
fed 5 mg benomyl/kg diet. Only 5-HBC (0.03 - 0.06 mg/kg) was found
in eggs from chickens fed 25 mg benomyl/kg for four weeks. No
residues of benomyl or MBC were found in milk (< 0.02 mg/litre)
from dairy cows fed benomyl for 32 days at dietary levels of 0, 2,
10, and 50 mg/kg. At the highest dietary level, the combined
residue of 5-HBC plus 5-hydroxy-2-benzimidazole carbamate (4-HBC)
in the milk was approximately 0.1 mg/litre. These metabolic
studies appear to indicate that benomyl and its metabolites do not
accumulate in animal tissues or animal products (Gardiner et al.,
1974; FAO/WHO, 1985a).
A rat was administered a diet containing 2500 mg non-labelled
benomyl/kg. Twelve days later, the animal received an intragastric
intubation of 2-14C-benomyl (7.7 mg). Urine and faeces were
collected, and organs were analysed for radioactivity. The
elimination was rather rapid and mainly via the urine. After 72 h,
0.2% of the administered dose was found in the liver, while < 0.01%
was present in all the other organs and the carcass. The same
results were found when a rat was administered 2-14C-MBC
(Gardiner et al., 1974)
A male beagle dog fed a diet containing non-labelled benomyl at
2500 mg/kg, and administered 30.8 mg 2-14C-benomyl by capsule 7
days later, showed a completely different excretion pattern. After
3 days, residues were found only in the liver (0.31% of the
administered dose); the levels in the other organs were < 0.01%
(Gardiner et al., 1974).
Rats administered an oral dose of 14C-labelled propham or
chloropropham (labelled in the chain or in the ring), showed
radioactivity in all tissues, with the highest concentration in
the kidneys. The average biological half-life of 14C from both
compounds in most organs was short, ranging from 3 to 8 h.
However, in brain, fat, and muscle, the half-life was about twice
this value (Fang et al., 1974).
6.2.3 Metabolic transformation
6.2.3.1 Biotransformation mechanisms
Carbamate pesticides are transformed metabolically by a variety
of chemical reactions into more water-soluble molecules with
increased polar properties. The initial step, usually oxidative in
nature, introduces a functional hydroxyl group that serves as a
site for secondary conjugative reactions to yield products that
can be excreted via the urine and/or faeces. In some cases, the
oxygenated metabolites, such as 5-hydroxypropoxur and 5-hydroxy-
carbaryl, are known to be toxic and to possess anticholinesterase
activity (Fig. 3) (Oonnithan & Casida, 1968; Black et al., 1973).
This undoubtedly contributes to the overall toxicity of the parent
compound (Black et al., 1973).
6.2.3.2 Oxidation
The principal route of metabolism of insecticidal carbamate
esters is oxidative and is generally associated with the mixed-
function oxidase (MFO) enzymes, which are present in several
tissues. Examples of the sites of oxidative attack on a
hypothetical methyl carbamate are given in Fig. 4.
Depending on the functional groups in the molecule, a variety
of reactions catalysed by these enzymes may occur (Fukuto, 1972),
as shown in Fig. 4. Typical oxidative reactions include: (a)
hydroxylation of aromatic rings, or epoxidation; (b) O -
dealkylation; (c) N -methyl hydroxylation; (d) N -dealkylation; (e)
hydroxylation and subsequent oxidation of aliphatic side chains;
and (f) thioether oxidation to sulfoxides and sulfones.
Because of the variety of different groups present in carbamate
insecticides, the metabolism of these compounds is often complex.
Carbaryl, for example, is a relatively simple compound, yet it is
metabolized to at least 15 different compounds in mammals through a
variety of oxidative and hydrolytic reactions (Leeling & Casida,
1966). A study showed that, as well as the organic solvent-soluble
unconjugated metabolites, the urine from carbaryl-treated rabbits
contained at least 4 or 5 other water-soluble metabolites. These
are probably conjugates of the hydroxylated products of carbaryl,
i.e., glucuronides or sulfates.
AChE then hydrolytically converts the ACh into choline and
acetic acid, which results in a decrease in the concentration of
ACh and cessation of muscle contraction. When AChE is inhibited by
a carbamate ester, it can no longer hydrolyse the ACh. Thus, the
ACh concentration remains high in the junction giving rise to
continuous stimulation of the muscle which, in turn, leads to
exhaustion and tetany.
Thus, inhibition of AChE by carbamate esters, causes toxic
effects in animals and human beings that result in a variety of
poisoning symptoms and eventually culminate in respiratory failure
and death.
The mechanism of inhibition of AChE by a carbamate ester can be
formulated as follows (Reiner & Aldridge, 1967; Aldridge & Reiner,
1972):
O O
|| k1 ||
AChE + RO-C-NHCH3 '=========' [AChE x RO-C-NHCH3] (enzyme-carbamate
k-1 | complex)
| kc
\/
O
kr ||
AChE + CH3NH2 + CO2 <------ AChE-C-NHCH3 + RO-
H2O
(carbamylated enzyme)
where: k1 = second order rate constant for formation of
complex;
k-1 = first order rate constant for breakdown of
complex to starting materials;
kc = first order rate constant for carbamylation of
the enzyme;
kr = first order rate constant for hydrolysis of the
carbamylated enzyme.
The site for carbamylation of the enzyme is the hydroxyl moiety of
the serine amino acid. The rate of regeneration of the
carbamylated enzyme to AChE (kr) is relatively rapid compared with
that of an enzyme that has been inhibited (phosphorylated) by an
organophosphorus pesticide (Reiner & Aldridge, 1967; Reiner, 1971).
Thus, human exposure to the carbamate pesticides is less dangerous
than exposure to organophosphorus pesticides, because the ratio
between the dose required to produce mortality and the dose
required to produce minimum poisoning symptoms is, in general,
substantially larger for carbamate compounds than for
organophosphorus compounds (Goldberg et al., 1963; Vandekar, 1965;
Vandekar et al., 1971). An individual experiencing symptoms of
poisoning from a carbamate is more likely to recover on termination
of exposure and with appropriate medical treatment than an
individual poisoned by an organophosphorus compound.
The spontaneous reactivation of various carbamylated ChEs
expressed as half-life, at pH 7.0 - 7.4 and 25 °C ranged between 2
and 240 min for AChE and between 2 and 17 min for serum-ChE. This
instability of the carbamylated enzyme affects the determination of
the inhibitory power of carbamates, the recovery after poisoning,
and the determination of inhibition of blood-ChEs (Reiner, 1971).
6.1.1 Neuropathy target esterase
This esterase, formerly known as neurotoxic esterase (NTE), is
the target for the initiation of delayed neuropathy caused by some
organophosphorus esters. Both organophosphorylation of NTE and a
subsequent "aging" reaction of the inhibited enzyme are required to
initiate neuropathy. Certain N -aryl carbamates can also inhibit
NTE, but the chemistry of such carbamates is such that no "aging"
reaction is possible. These carbamate inhibitors do not initiate
delayed neuropathy in vivo but can actually prevent the neuropathic
effects of a challenge dose of an organophosphate given while the
tissue NTE is carbamylated (Johnson, 1970). No delayed neuropathic
effects have been observed in tests on carbamate pesticides
according to protocols that detect neuropathic organophosphates,
and this agrees with the mechanism described above.
6.2 Metabolism
The metabolic fate of carbamates is basically the same in
plants, insects, and mammals.
Carbamates can penetrate the skin, mucous membranes,
respiratory tract, and gastrointestinal tract of mammals.
6.2.1 Absorption
In vivo studies have shown that carbamates are almost
completely absorbed during normal transit through the gastro-
intestinal tract.
According to absorption studies on rats, the dermal absorption
of radioactive labelled benomyl is slow. After absorption, rapid
metabolism and elimination via urine took place. After 1 h, small
amounts of the major metabolites of benomyl, 5-hydroxy-2-
benzimidazole carbamate (5-HBC), and methyl 2-benzimidazole
carbamate (MBC), could be detected in urine, confirming the rapid
metabolism of benomyl. No radioactivity was found in any body
tissues sampled 24 h after application (FAO/WHO, 1985a).
6.2.2 Distribution
Ahdaya et al. (1981) studied the absorption and distribution of
carbamates. They found that, in mice, all carbamates were rapidly
distributed to the tissues and organs. The half-life values of
penetration ranged from 8 to 17 min for carbamates.
No residues of benomyl or its degradation products were
detected (level of sensitivity 0.02 mg/kg) in eggs from chickens
fed 5 mg benomyl/kg diet. Only 5-HBC (0.03 - 0.06 mg/kg) was found
in eggs from chickens fed 25 mg benomyl/kg for four weeks. No
residues of benomyl or MBC were found in milk (< 0.02 mg/litre)
from dairy cows fed benomyl for 32 days at dietary levels of 0, 2,
10, and 50 mg/kg. At the highest dietary level, the combined
residue of 5-HBC plus 5-hydroxy-2-benzimidazole carbamate (4-HBC)
in the milk was approximately 0.1 mg/litre. These metabolic
studies appear to indicate that benomyl and its metabolites do not
accumulate in animal tissues or animal products (Gardiner et al.,
1974; FAO/WHO, 1985a).
A rat was administered a diet containing 2500 mg non-labelled
benomyl/kg. Twelve days later, the animal received an intragastric
intubation of 2-14C-benomyl (7.7 mg). Urine and faeces were
collected, and organs were analysed for radioactivity. The
elimination was rather rapid and mainly via the urine. After 72 h,
0.2% of the administered dose was found in the liver, while < 0.01%
was present in all the other organs and the carcass. The same
results were found when a rat was administered 2-14C-MBC
(Gardiner et al., 1974)
A male beagle dog fed a diet containing non-labelled benomyl at
2500 mg/kg, and administered 30.8 mg 2-14C-benomyl by capsule 7
days later, showed a completely different excretion pattern. After
3 days, residues were found only in the liver (0.31% of the
administered dose); the levels in the other organs were < 0.01%
(Gardiner et al., 1974).
Rats administered an oral dose of 14C-labelled propham or
chloropropham (labelled in the chain or in the ring), showed
radioactivity in all tissues, with the highest concentration in
the kidneys. The average biological half-life of 14C from both
compounds in most organs was short, ranging from 3 to 8 h.
However, in brain, fat, and muscle, the half-life was about twice
this value (Fang et al., 1974).
6.2.3 Metabolic transformation
6.2.3.1 Biotransformation mechanisms
Carbamate pesticides are transformed metabolically by a variety
of chemical reactions into more water-soluble molecules with
increased polar properties. The initial step, usually oxidative in
nature, introduces a functional hydroxyl group that serves as a
site for secondary conjugative reactions to yield products that
can be excreted via the urine and/or faeces. In some cases, the
oxygenated metabolites, such as 5-hydroxypropoxur and 5-hydroxy-
carbaryl, are known to be toxic and to possess anticholinesterase
activity (Fig. 3) (Oonnithan & Casida, 1968; Black et al., 1973).
This undoubtedly contributes to the overall toxicity of the parent
compound (Black et al., 1973).
6.2.3.2 Oxidation
The principal route of metabolism of insecticidal carbamate
esters is oxidative and is generally associated with the mixed-
function oxidase (MFO) enzymes, which are present in several
tissues. Examples of the sites of oxidative attack on a
hypothetical methyl carbamate are given in Fig. 4.
Depending on the functional groups in the molecule, a variety
of reactions catalysed by these enzymes may occur (Fukuto, 1972),
as shown in Fig. 4. Typical oxidative reactions include: (a)
hydroxylation of aromatic rings, or epoxidation; (b) O -
dealkylation; (c) N -methyl hydroxylation; (d) N -dealkylation; (e)
hydroxylation and subsequent oxidation of aliphatic side chains;
and (f) thioether oxidation to sulfoxides and sulfones.
Because of the variety of different groups present in carbamate
insecticides, the metabolism of these compounds is often complex.
Carbaryl, for example, is a relatively simple compound, yet it is
metabolized to at least 15 different compounds in mammals through a
variety of oxidative and hydrolytic reactions (Leeling & Casida,
1966). A study showed that, as well as the organic solvent-soluble
unconjugated metabolites, the urine from carbaryl-treated rabbits
contained at least 4 or 5 other water-soluble metabolites. These
are probably conjugates of the hydroxylated products of carbaryl,
i.e., glucuronides or sulfates.
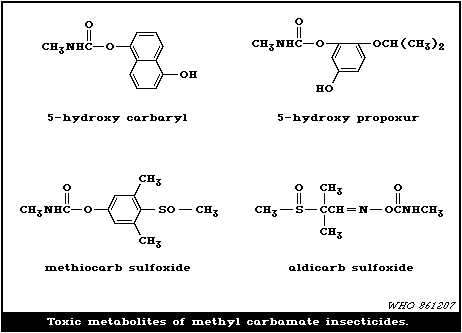
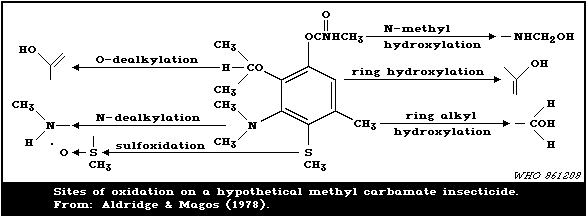 6.2.3.3 Hydrolysis
Carbamates are hydrolysed either spontaneously or by esterases
(Aldridge & Reiner, 1972), yielding, as final products, an amine,
carbon dioxide (CO2), and an alcohol or phenol:
R1HN-C(O)OR2 + H2O ---> R1NH2 + CO2 + R2OH
The mechanism of hydrolysis is different for N -methyl and
N -dimethyl carbamates.
In general, the rates of hydrolysis of carbamates by esterases
are faster in mammals than in plants and insects, though there are
exceptions. The differences in enzymatic rates of hydrolysis
depend on the structure of the carbamate and on the particular
esterase.
Hydrolysis of the carbamates is catalysed by a group of enzymes
known as A-esterases or arylesterases (EC 3.1.1.2). It has been
established that enzymatic hydrolysis occurs both in vitro and in
vivo, but, to date, it has not been determined to what extent the
in vivo hydrolysis contributes to the detoxification of the
chemical.
The hydrolytic activity of plasma-albumin has also been
indicated (Augustinsson & Casida 1959; Casida & Augustinsson, 1959;
Reiner & Skrinjaric-Spoljar, 1968).
6.2.3.4 Conjugation
The conversion to conjugated compounds of the hydroxy products
produced by the drug-metabolizing enzyme systems is an important
reaction that leads to the formation of water-soluble compounds
such as O - and N -glucuronides, sulfates, and mercapturic acid,
which can be eliminated via the urine or faeces.
6.2.3.5 Examples of biotransformation of carbamates
A few examples of the biotransformation of carbamates are given
in Fig. 5.
Aldicarb is unusual in that it does not contain an aromatic
ring, and it is also a carbamate ester of an oxime. Knaak et al.
(1966) studied its metabolism in rats. The metabolic reactions of
aldicarb are similar to the oxidative and hydrolytic pathways found
in the thioether-containing organophosphorous esters. The
thioether moiety is rapidly oxidized to the oxime-sulfoxide; the
oxime-sulfoxide is oxidized much more slowly to the oxime-sulfone.
A large number of metabolites resulting from the hydrolysis of the
carbamate moiety of the oxidative metabolites of aldicarb have been
identified.
6.2.3.3 Hydrolysis
Carbamates are hydrolysed either spontaneously or by esterases
(Aldridge & Reiner, 1972), yielding, as final products, an amine,
carbon dioxide (CO2), and an alcohol or phenol:
R1HN-C(O)OR2 + H2O ---> R1NH2 + CO2 + R2OH
The mechanism of hydrolysis is different for N -methyl and
N -dimethyl carbamates.
In general, the rates of hydrolysis of carbamates by esterases
are faster in mammals than in plants and insects, though there are
exceptions. The differences in enzymatic rates of hydrolysis
depend on the structure of the carbamate and on the particular
esterase.
Hydrolysis of the carbamates is catalysed by a group of enzymes
known as A-esterases or arylesterases (EC 3.1.1.2). It has been
established that enzymatic hydrolysis occurs both in vitro and in
vivo, but, to date, it has not been determined to what extent the
in vivo hydrolysis contributes to the detoxification of the
chemical.
The hydrolytic activity of plasma-albumin has also been
indicated (Augustinsson & Casida 1959; Casida & Augustinsson, 1959;
Reiner & Skrinjaric-Spoljar, 1968).
6.2.3.4 Conjugation
The conversion to conjugated compounds of the hydroxy products
produced by the drug-metabolizing enzyme systems is an important
reaction that leads to the formation of water-soluble compounds
such as O - and N -glucuronides, sulfates, and mercapturic acid,
which can be eliminated via the urine or faeces.
6.2.3.5 Examples of biotransformation of carbamates
A few examples of the biotransformation of carbamates are given
in Fig. 5.
Aldicarb is unusual in that it does not contain an aromatic
ring, and it is also a carbamate ester of an oxime. Knaak et al.
(1966) studied its metabolism in rats. The metabolic reactions of
aldicarb are similar to the oxidative and hydrolytic pathways found
in the thioether-containing organophosphorous esters. The
thioether moiety is rapidly oxidized to the oxime-sulfoxide; the
oxime-sulfoxide is oxidized much more slowly to the oxime-sulfone.
A large number of metabolites resulting from the hydrolysis of the
carbamate moiety of the oxidative metabolites of aldicarb have been
identified.

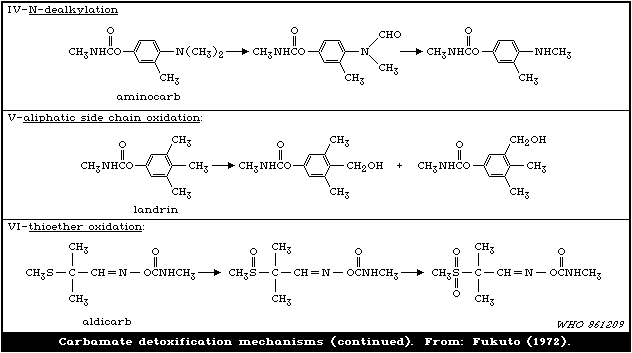 The oxime-sulfoxide was found to be the major hydrolytic
metabolite in plants and insects. Other research workers recovered
the nitrile sulfoxide, in greater quantities, both compounds
arising from cleavage of the carbamate moiety of aldicarb sulfoxide
(Metcalf et al., 1966; Fukuto, 1972).
Propham and chlorpropham have been studied in a number of
animal species. Both compounds are rather rapidly metabolized.
The major metabolites recovered from the animals were 4-
hydroxypropham and 4-hydroxychloropropham, respectively. Both were
excreted as the sulfate and/or glucuronide. Depending on the animal
species, hydroxylation of propham also occurred in the 2- and 3-
position, and even 3,4-dihydroxypropham was identified. Oxidation
of the 1-carbon of the isopropyl moiety of chlorpropham resulted in
the formation of the monohydroxy product, which was converted to
the 1,3-hydroxy and 1-carboxy derivative. Hydrolysis of
chloropropham also occurred to a significant extent, since the
other major metabolites found were conjugates of 3-chloro-4-
hydroxy- and 5-chloro-2-hydroxy-acetanilide. Chlorpropham is more
susceptible to hydrolytic degradation than propham, or, conversely,
propham is more susceptible to oxidative degradation than
chlorpropham (Bobik et al., 1972; Paulson et al., 1973; Fang et
al., 1974).
Phenmedipham and desmedipham were hydrolysed mainly into
methyl- N or ethyl- N -(3 hydroxyphenyl) carbamates. Both compounds
were subsequently hydrolysed to 3-aminophenol. Furthermore, the
aminophenol was N -acetylated, and the phenolic hydroxyl moiety was
conjugated with the sulfate or glucuronide (Sonawane & Knowles,
1972).
Carbaryl is rapidly hydroxylated or hydrolysed and thereafter
conjugated and eliminated from a number of animal species,
principally in the urine as glucuronides or sulfates. Depending on
the animal species, at least 8 water-soluble metabolites were
found. Dorough & Casida (1964) and Fukuto (1972) studied the
biotransformation of carbaryl in animals. It was found that
hydroxylation took place, and the following metabolites were
identified: 1-naphthyl N -hydroxymethylcarbamate (and as a result
of ring hydroxylation) 4-hydroxy-1-naphthyl- N -methylcarbamate,
5-hydroxy-1-naphthyl- N -methyl-carbamate, and 5,6-dihydroxy-1-
naphthylmethylcarbamate.
Certain metabolites appeared to be the hydrolytic products of
carbamates with modified ring structures, such as 1-hydroxy-5,6-
dihydro-naphthalene and 1-naphthol. The latter compound is found
in the urine as 1-naphthyl glucuronide and/or 1-naphthyl sulfate.
Sullivan et al. (1972) identified one of these metabolites, 5,6-
dihydroxy carbaryl glucuronide, in the urine of rats. Most
metabolites of carbaryl are eliminated in a similar pattern in
human beings, rats, guinea-pigs, and sheep, but in a different way
in dogs (FAO/WHO, 1968b).
The metabolism of benomyl has been studied in the mouse, rat,
rabbit, dog, sheep, and cow and is qualitatively the same in all
animal species studied.
The basic route of benomyl metabolism involves hydroxylation
to 5-hydroxy-2-benzimidazolecarbamate (5-HBC). This is the main
metabolite and conjugates (glucuronides or sulfates) of the
compound are usually eliminated via the urine, bile, and faeces
(Douch, 1973; Gardiner et al., 1974). In contrast, dogs treated
orally with 14C-benomyl eliminated only 16% of the radioactivity in
the urine and 83% in the faeces. The principal metabolites in the
faeces were carbendazim and 5-hydroxycarbendazim. In addition, 4-
hydroxycarbendazim was identified as a metabolite in the urine,
faeces, and milk of a cow treated with benomyl (Fig. 6).
The oxime-sulfoxide was found to be the major hydrolytic
metabolite in plants and insects. Other research workers recovered
the nitrile sulfoxide, in greater quantities, both compounds
arising from cleavage of the carbamate moiety of aldicarb sulfoxide
(Metcalf et al., 1966; Fukuto, 1972).
Propham and chlorpropham have been studied in a number of
animal species. Both compounds are rather rapidly metabolized.
The major metabolites recovered from the animals were 4-
hydroxypropham and 4-hydroxychloropropham, respectively. Both were
excreted as the sulfate and/or glucuronide. Depending on the animal
species, hydroxylation of propham also occurred in the 2- and 3-
position, and even 3,4-dihydroxypropham was identified. Oxidation
of the 1-carbon of the isopropyl moiety of chlorpropham resulted in
the formation of the monohydroxy product, which was converted to
the 1,3-hydroxy and 1-carboxy derivative. Hydrolysis of
chloropropham also occurred to a significant extent, since the
other major metabolites found were conjugates of 3-chloro-4-
hydroxy- and 5-chloro-2-hydroxy-acetanilide. Chlorpropham is more
susceptible to hydrolytic degradation than propham, or, conversely,
propham is more susceptible to oxidative degradation than
chlorpropham (Bobik et al., 1972; Paulson et al., 1973; Fang et
al., 1974).
Phenmedipham and desmedipham were hydrolysed mainly into
methyl- N or ethyl- N -(3 hydroxyphenyl) carbamates. Both compounds
were subsequently hydrolysed to 3-aminophenol. Furthermore, the
aminophenol was N -acetylated, and the phenolic hydroxyl moiety was
conjugated with the sulfate or glucuronide (Sonawane & Knowles,
1972).
Carbaryl is rapidly hydroxylated or hydrolysed and thereafter
conjugated and eliminated from a number of animal species,
principally in the urine as glucuronides or sulfates. Depending on
the animal species, at least 8 water-soluble metabolites were
found. Dorough & Casida (1964) and Fukuto (1972) studied the
biotransformation of carbaryl in animals. It was found that
hydroxylation took place, and the following metabolites were
identified: 1-naphthyl N -hydroxymethylcarbamate (and as a result
of ring hydroxylation) 4-hydroxy-1-naphthyl- N -methylcarbamate,
5-hydroxy-1-naphthyl- N -methyl-carbamate, and 5,6-dihydroxy-1-
naphthylmethylcarbamate.
Certain metabolites appeared to be the hydrolytic products of
carbamates with modified ring structures, such as 1-hydroxy-5,6-
dihydro-naphthalene and 1-naphthol. The latter compound is found
in the urine as 1-naphthyl glucuronide and/or 1-naphthyl sulfate.
Sullivan et al. (1972) identified one of these metabolites, 5,6-
dihydroxy carbaryl glucuronide, in the urine of rats. Most
metabolites of carbaryl are eliminated in a similar pattern in
human beings, rats, guinea-pigs, and sheep, but in a different way
in dogs (FAO/WHO, 1968b).
The metabolism of benomyl has been studied in the mouse, rat,
rabbit, dog, sheep, and cow and is qualitatively the same in all
animal species studied.
The basic route of benomyl metabolism involves hydroxylation
to 5-hydroxy-2-benzimidazolecarbamate (5-HBC). This is the main
metabolite and conjugates (glucuronides or sulfates) of the
compound are usually eliminated via the urine, bile, and faeces
(Douch, 1973; Gardiner et al., 1974). In contrast, dogs treated
orally with 14C-benomyl eliminated only 16% of the radioactivity in
the urine and 83% in the faeces. The principal metabolites in the
faeces were carbendazim and 5-hydroxycarbendazim. In addition, 4-
hydroxycarbendazim was identified as a metabolite in the urine,
faeces, and milk of a cow treated with benomyl (Fig. 6).
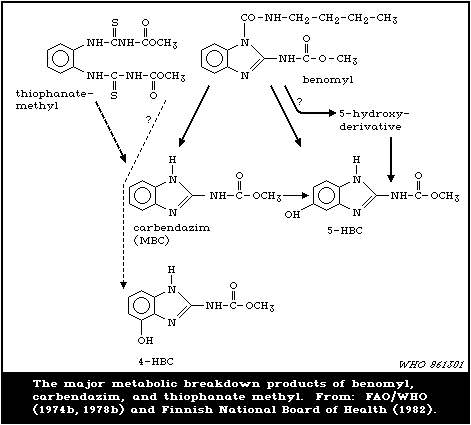 Heybroek et al. (1984) studied the metabolism of [ring-14C]
asulam in the rat. Most of the radioactivity (61 -74%)
administered orally or intravenously was excreted in the urine in
24 h as unchanged asulam, 8 - 14% as N 4-acetylasulam, and up to
2.5% as N 4-acetylsulfanilamide. Small amounts of radioactivity
were found in faeces.
These examples illustrate the different metabolic pathways of
biotransformation. For more details, readers should refer to
Fukuto (1972) who has reviewed the metabolism of carbaryl,
mexacarbamate, aminocarb, formetanate, aldicarb, methiocarb,
carbofuran, MPMC, trimethacarb carbanolate, propoxur, and
dimetilan. Harvey & Han (1978a) have described the metabolism of
oxamyl and Harvey et al. (1973), of methomyl in the rat. The
metabolism of pirimicarb was extensively described in FAO/WHO
(1977b). Furthermore, information on the metabolism is provided in
the handbooks on carbamates, and the different monographs of the
Joint FAO/WHO Meetings on Pesticide Residues.
6.3 Elimination and Excretion in Expired Air, Faeces, and Urine
6.3.1 Man
In a study on human volunteers, 2 men were dosed orally with
carbaryl in gelatin capsules at 2.0 mg/kg body weight.
Chromatographic analyses of a 4-h urine sample revealed 1-naphthyl
glucuronide (15%), 1-naphthyl sulfate (8%), and 4-(methylcarbamoyl-
oxy)-1-naphthyl glucuronide (4%). In addition, 1-naphthyl
methylimidocarbonate O -glucuronide was identified by fluorometry
(FAO/WHO, 1968b). During the first 8 h, the urinary excretion of
these metabolites constituted 12 - 15% of the carbaryl
administered. Small amounts of urinary metabolites were detected
by fluorometric analysis on the second day, but not afterwards
(Knaak et al., 1968). In another study, 1-naphthyl glucuronide, 1-
naphthyl sulfate, and other unidentified metabolites were found in
24-h urine samples from men exposed to carbaryl dust during a
packaging operation in a factory (details not available) (Knaak et
al., 1965).
The determination of 1-naphthol or its sulfate or glucuronide
in the urine of persons occupationally exposed to carbaryl is one
method of biological monitoring for this exposure (FAO/WHO, 1982b).
Dawson et al. (1964) investigated the metabolism and excretion
of propoxur in 3 male volunteers orally administered a dose of 50
mg. Within 8 - 10 h, 27% of the propoxur appeared in the urine as
its metabolite 2-isopropoxyphenol, indicating that propoxur is
rapidly absorbed and hydrolysed in human beings.
The absorption and fate of benomyl in animals following oral
administration of the compound varies with species. According to a
study that involved one rat and one dog administered 2-14C-benomyl
by stomach tube, the rat eliminated about 86% of a single dose via
the urine and 13% via faeces. The dog eliminated only 16% of a
similar benomyl dose via the urine, while almost 83% was detected
in faeces. No data are available concerning the possible roles of
the enterohepatic circulation and biliary excretion of benomyl or
its metabolites (Gardiner et al., 1974).
6.3.2 Laboratory animals
In animals, oxidation of carbamates often, but not always,
results in detoxification. Oxidative metabolism generally leads to
products of greater polarity and water solubility, and these can be
more readily eliminated through the urine and faeces than the
parent compound.
In the rat, benomyl is mainly excreted via the urine (78.9% of
the given dose) as 5-HBC conjugates. In the dog, 99% of the dose
administered was eliminated within 72 h. The major route of
elimination was via the faeces in which benomyl and/or MBC and 5-
HBC were detected. On the basis of studies on the rat, dog, dairy
cow, and chicken, neither benomyl nor its metabolites tend to
accumulate in animal tissues (Gardiner et al., 1974).
According to FAO/WHO (1974b), feeding of 14C- or 35S-labelled
thiophanate-methyl to rats, mice, and dogs resulted in 80 - 100%
recovery of the label in the faeces and urine within 96 h.
Unmetabolized thiophanate-methyl has been reported to be the major
component of faecal elimination. The minor part consisted of 4-
hydroxy-thiophanate-methyl, dimethyl-4,4- O -phenylenebisallophanate.
MBC and 5-hydroxy-MBC were also detected.
Following application of carbendazim to rats and mice by
intragastric intubation, almost all metabolites in the urine were
conjugated as sulfate esters. 5-HBC was released as the only
metabolite extractable from water. A higher proportion of polar
compounds was found in the urine of mice than in that of rats.
Polarity was caused by the phenolic hydroxyl group (FAO/WHO,
1985a).
The excretion of 14C-labelled propham and chlorpropham was
investigated in female rats after a single oral dose. The average
3-day urinary excretion of radioactivity was between 56 and 85%,
depending on the position of labelling: viz chain or ring
labelling. With chain 14C-chlorpropham, an average of 35% of the
administered radioactivity appeared in the respired air, compared
with only 5% for chain 14C-propham (Fang et al., 1974).
6.4 Metabolism in Plants
Studies on the metabolism and fate of carbamate pesticides in
plants are of great importance for assessing the human hazards from
the consumption of fruit and vegetables containing residues of the
applied pesticides and metabolites.
Generally, the metabolism of carbamates in plants follows
another route of detoxification. The hydroxylated carbamates
produced by enzymes are conjugated either with amino acids (for
instance cysteine) or as glycosides and phosphates, which are
stored as terminal metabolites (Still & Rusness, 1977; Aldridge &
Magos, 1978).
The toxicological properties of plant conjugates in mammals or
other animals are not generally known. Insecticidal methyl
carbamate esters are vulnerable to hydrolytic cleavage, which
results in detoxified products. Detoxification of carbamate esters
in plants by hydrolysis occurs to a lesser extent than
detoxification by oxidative metabolism; nevertheless, hydrolytic
cleavage of the ester moiety is a significant detoxification
mechanism. For instance, in sugar beets treated with desmedipham,
the major metabolites were ethyl-3-hydroxy phenyl carbamate and 3-
aminophenol (Knowles & Sonawane, 1972). Carbetamide is hydrolysed
to aniline (Guardigli et al., 1977). Aromatic ring hydroxylation
appears to be the predominant metabolic reaction that carbamate
herbicides undergo in plants. The major metabolites isolated from
soybean plants, after uptake of chlorpropham, were glucoside
conjugates of 2-hydroxy- and 4-hydroxypropham. Similar results
were obtained with propham (Still & Mansager, 1973a,b, 1975).
There is evidence that the ring hydroxylation varies with the
plant species. In other carbamates, for instance in the case of
barban, ring hydroxylation did not appear to take place, and polar
metabolites were produced, which were hydrolysed into
chloroanilines (Still & Mansager, 1972).
Furthermore, oxidation of aliphatic groups takes place in
carbamate herbicides (Wiedmann et al., 1976). Still & Herrett
(1975) demonstrated N -methyl hydroxylation for dichlormate, a
metabolite that is either conjugated or loses the hydroxymethyl
moiety to produce N -demethylated dichlormate.
Thiophanate-methyl is mainly metabolized into carbendazim (MBC)
and, to a less extent, into 2-aminobenzimidazole (2AB). A few other
metabolites have also been found (FAO/WHO, 1974b).
The metabolic pathway for carbaryl in plants is identical,
whether the compound is injected into the stem or applied to the
leaf surface. After entering the plant, carbaryl undergoes
biotransformation to its primary metabolites, which are similar to
the ones formed in animals. These hydroxylated metabolites, which
are less toxic than carbaryl itself, are conjugated by plants to
form water-soluble glycosides. Injection of carbaryl into the
bean plant produced water-soluble metabolites attributable to
hydroxylation of the ring or N -methyl group followed by
conjugation, mainly as glycosides (Kuhr, 1968; FAO/WHO, 1970b;
Fukuto, 1972).
The metabolism of aldicarb in plants is the same as that in
mammals. In the cotton plant, the thioether moiety is rapidly
oxidized to the sulfoxide, and the latter is slowly transformed to
the sulfone. The sulfoxide is rather stable and can be present in
cotton plants, even as long as 2 months after treatment. The total
metabolites present can be as high as 80% (Kuhr, 1968). Because of
their persistence in plants, and their even higher anticholinesterase
activity compared with aldicarb itself, the sulfoxide and sulfone
should be taken into account when considering residue tolerances.
The major metabolite isolated from bean plants, 28 days after
treatment with carbofuran, was a conjugate of 3-hydroxy-carbofuran,
which remained in the aqueous phase after extraction with an
organic solvent. The conjugate represented 55% of the total 14C-
labelled residues present in the plant. 3-Hydroxy-carbofuran is
highly toxic for mammals (LD50 in the rat 7 mg/kg body weight).
The conjugate may also be toxic, particularly taking into account
the acid conditions of the mammalian stomach under which the
conjugate will be hydrolysed (FAO/WHO, 1977b, 1980b).
Harvey et al. (1978) studied the metabolism of oxamyl in plants
(tobacco, alfalfa, peanuts, potatoes, apples, oranges, and
tomatoes). The major route of degradation involved hydrolysis to
the corresponding oximino compound, which in turn became conjugated
with glucose. Further metabolism resulted in the loss of one of
the N' -methyl groups and/or addition of other glucose units to the
sugar moiety of the original conjugate. Total breakdown into
normal natural products has been demonstrated.
In a study by Harvey & Reiser (1973), methomyl rapidly degraded
to carbon dioxide and acetonitrile, which volatilized from the
plant tissues. The half-life for methomyl was of the order of 3 -
6 days. The remainder of the compound had been reincorporated into
natural plant components.
The metabolic pathways for a number of carbamate pesticides are
described in more detail in Fukuto (1972).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
As described in section 4.2.1, soil microorganisms are capable
of hydrolysing carbamates. Furthermore, it seems that
microorganisms can easily adapt themselves to metabolize the
different types of carbamates (Aly & El-Dib, 1972). Nevertheless,
carbamates and their metabolites can affect the microflora and
cause changes that may have an important impact on the maintenance
of the soil productivity (Filip, 1974).
7.2 Aquatic Organisms
In general, carbamates are not very stable under aquatic
conditions (section 4). The solubility in water differs
considerably for the different carbamates (Annex I). Furthermore,
photodecomposition takes place and microorganisms are able to
degrade these compounds. By these processes, the carbamate is
broken down (oxidation, hydrolysis) into other compounds. Some
will be less toxic, others even more toxic than the parent
compound. Carbaryl will be hydrolysed into 1-naphthol, which is
just as toxic (Armstrong & Millemann, 1974a). It seems unlikely
that most of the carbamates will persist long in the environment,
but there are exceptions, such as dimetilan and carbendazim.
Many data are available on the acute toxicity of the carbamates
in several fresh- and salt-water fish and other organisms, some of
which are listed in Tables 4 and 5.
When carbamates are deliberately added to an aquatic system,
caution is necessary since, with high concentrations, a number of
species are at risk. For example, while applications of carbaryl
of 0.93 kg/ha were effective in controlling the ghost shrimp
(Callianassa californiensis), an oyster pest, they also caused a
reduction in the population of juvenile clams (Armstrong &
Millemann, 1974a).
In a similar study (Armstrong & Millemann, 1974b), carbaryl
and its hydrolytic product, 1-naphthol, were examined for other
effects on the mussel (Mytilus edulis). An age dependence was
noted; the most sensitive stage (appearance of the first polar body
shortly after fertilization) had EC50s of 5.3 and 5.2 mg/litre for
carbaryl and 1-naphthol, respectively. Effects of the compounds
on development were characterized by disjunction of blastomeres and
asynchronous and unaligned cleavages. Carbaryl, at a single dose
of 0.1 mg/litre, also disrupted normal schooling behaviour in
juvenile Menidia medidia; the disruptive effects were attributed
to the 1-naphthol rather than to the parent carbamate. These
effects were reversed within 3 days of placing the fish in clean
water (Weis & Weis, 1974). Hansen (1969) studied the capacity of
sheepshead minnows (Cyprinodon variegatus) to avoid carbaryl. The
fish did not avoid carbaryl in concentrations of 0.1 - 10 mg/litre
water.
Table 4. Acute aquatic toxicity (LC50 in mg/litre)a
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Aldicarb fish Rainbow trout (Salmo gairdneri) 0.5 12 0.56
fish Bluegill (Lepomis machrochirus) 1.3 24 0.05
Aminocarb fish Rainbow trout (Salmo gairdneri) 1.5 12 13.5b
fish Bluegill (Lepomis machrochirus) 2.0 20 3.1b
crustacea Daphnia (Daphnia magna) first instar 21 0.01 - 0.1c
(48-h EC50)
fish Rainbow trout (Salmo gairdneri) 1.5 10 0.13c
fish Bluegill (Lepomis machrochirus) 0.6 20 0.1c
insect Midge (Chironomus plumosus) fourth instar 20 0.27c
Benomyl fish Rainbow trout (Salmo gairdneri) 1.2 12 0.17d
fish Bluegill (Lepomis machrochirus) 0.9 22 0.85d
fish Rainbow trout (Salmo gairdneri) 1.0 12 0.31e
fish Bluegill (Lepomis machrochirus) 0.6 22 1.2e
Benomyl fish Rainbow trout (Salmo gairdneri) 0.2 12 0.37f
metabolite
MBC
Bufencarb crustacea Scud (Gammarus fasciatus) mature 15 0.001
fish Goldfish (Carassius auratus) 1.0 18 0.29
Carbaryl fish Rainbow trout (Salmo gairdneri) 1.5 12 1.95d
fish Bluegill (Lepomis machrochirus) 1.2 18 6.76d
crustacea Daphnia (Daphnia pulex) first instar 16 0.0064d
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature 21 0.026d
----------------------------------------------------------------------------------------------------
Table 4. (contd.)
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Carbofuran fish Rainbow trout (Salmo gairdneri) 1.5 12 0.38d
fish Bluegill (Lepomis machrochirus) 0.8 18 0.24e
Dichlormate fish Rainbow trout (Salmo gairdneri) 0.8 12 4.9
Methiocarb fish Rainbow trout (Salmo gairdneri) 1.3 12 0.80
fish Bluegill (Lepomis machrochirus) 1.0 24 0.21
Methomyl fish Rainbow trout (Salmo gairdneri) 1.1 12 1.60b
fish Bluegill (Lepomis machrochirus) 0.9 20 1.05b
crustacea Daphnia (Daphina magna) first instar 21 0.009b
(48-h EC50)
Mexacarbate fish Rainbow trout (Salmo gairdneri) 1.0 11 12.0g
fish Bluegill (Lepomis machrochirus) 0.7 12 22.9g
crustacea Daphina (Daphina pulex) first instar 15 0.010g
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature - 0.04g
Trimethacarb fish Rainbow trout (Salmo gairdneri) 1.2 12 1.0
fish Bluegill (Lepomis machrochirus) 0.9 18 11.6
----------------------------------------------------------------------------------------------------
a From: Johnson & Finley (1980).
b Technical material, 95 - 98%.
c Liquid formulation, 17%.
d Technical material, 99%.
e Wettable powder, 50%.
f Methyl-2-benzimidazole (MBC), 99%.
g Technical material, 90 - 95%.
Note: It should be recognized that the LC50 and EC50 values only give an indication of the
toxicity and that the toxicity for these organisms may be appreciably changed by variations
in temperature, pH, oxygen content, and water hardness. A 10-fold increase in the toxicity
may be found. Also, the life stage of the organisms is an important factor in estimating
the toxicity of the compound.
Table 5. Acute toxicity of carbamate pesticides for some aquatic organisms
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
Benomyl 7.5 12 11 (wettable - 14 Yoshida & Nishiuchi
powder) (1972)
BPMC 16 10 ~ 40 1.7 5.0 0.32 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Carbaryl 13 10 ~ 40 2.8 2.8 0.05 Yoshida & Nishiuchi
(1972)
Carbendazim > 40 - 40 - > 40 Yoshida & Nishiuchi
(1976)
CPMC 10 ~ 40 10 ~ 40 5.6 7.0 0.1 ~ 0.5 Yoshida & Nishiuchi
(emulsifiable (1972); Nishiuchi
concentrate) (1974)
Isoprocarb 10 ~ 40 10 ~ 40 5.9 3.0 0.30 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Mecarbam 0.70 0.68 0.35 0.055 0.03 Yoshida & Nishiuchi
granule (1972); Nishiuchi
(1974)
Methomyl 2.8 2.7 0.87 1.0 0.045 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
MPMC 10 ~ 40 10 ~ 40 12 7.3 0.07 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
MTMC 10 ~ 40 10 ~ 40 27 13 0.35 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Pirimicarb > 40 - 40 - 0.048 Yoshida & Nishiuchi
(1976)
Promecarb 2.7 - 3.3 - 0.020 Yoshida & Nishiuchi
(1976)
Terbam 0.92 - 2.2 - 0.025 Yoshida & Nishiuchi
(1976)
Thiophanate > 40 > 40 > 40 - > 40 Yoshida & Nishiuchi
(1972)
Thiophanate 11 > 40 11 (wettable - > 40 Yoshida & Nishiuchi
-methyl powder) (1972)
XMC > 40 > 40 33 25 0.055 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
a BPMC = 1-sec-butylphenylmethyl carbamate
CPMC = 1-chlorophenylmethyl carbamate
MPMC = 3,4-xylylmethyl carbamate
MTMC = 4-tolylmethyl carbamate
Terbam = 4-tert-butylphenylmethyl carbamate
XMC = 3,5-xylylmethyl carbamate
Note: Test methods are officially recognized methods based on the Notification of the
Ministry of Agriculture, Forestry, and Fisheries of Japan.
Carbamates can have adverse effects on algae. Stadnyk et al.
(1971) reported that carbaryl at a concentration of 0.1 mg/litre
caused an increase in cell numbers and in the biomass of the green
algae Scenedesmus quadricaudata.
Motsuage fish (Motsugo pseudoras bora parva) were reported to
accumulate carbaryl and BPMC during a 30-day exposure to a
concentration of 0.6 - 1.2 mg/litre. Carbaryl uptake was greatest.
However, this compound underwent more rapid metabolism and
excretion than BPMC. Metabolism of BPMC was slow and introduced
permanent spinal curvature of the backbone in about 30% of the fish
exposed, an effect seen with the organophosphorus insecticide
diazinon (Kanazawa, 1975).
A residue study indicated that over a 28-day period at the
highest exposure level of 0.75 mg methomyl/litre, no accumulation
of the carbamate took place. During the entire exposure period,
the values ranged from 0.36 to 0.45 mg/kg. After a 3-day withdrawal
period, no methomyl (< 0.02 mg/kg) was detected in the fish
(Kaplan & Sherman, 1977).
The results summarized in Table 4 are representative of the
toxicity of the different carbamates for fish and invertebrate
species. In general, the LC50s of carbamates for different fish
species range from approximately 0.1 to 10 mg/litre. Invertebrates
are usually more sensitive. The EC50 values for Daphnids and
other species are mainly below 0.1 mg/litre. Some carbamates seem
to be very toxic (< 0.01 mg/litre) for these invertebrates.
7.2.1 Field studies
Little information is available concerning the fate and
persistence of carbamates in the aquatic environment. The presence
of these carbamates in surface waters may have an effect on water
organisms.
Quraishi (1972) studied the persistence of aldicarb. Field
water treated in the laboratory at 100 mg aldicarb/litre resulted
in residues of aldicarb and its metabolites of 0.4 mg/litre after
11 months. Water was stored at 16 - 20 °C and exposed for
approximately 507 h to sunlight.
Data concerning the fate of aminocarb have been reviewed by
Vassilieff & Ecobichon (1982). A half-life of 28.5 days in pond
water was found under normal environmental conditions. The
principal metabolite appeared to be the phenol, though the
methylamino and formylamino analogues were also observed.
Harvey & Pease (1973) found that, under field conditions in
Delaware, Florida, and North Carolina, methomyl broke down almost
completely within 1 month; most of it was lost from the soil by
volatilization, presumably as carbon dioxide. Small amounts
extracted from the soil consisted of methomyl, S -methyl N -
hydroxythioacetimidate, and some polar compounds. A run-off study,
under farm conditions, showed that the compound did not move into
untreated areas with run-off water.
7.3 Terrestrial Organisms
7.3.1 Effects on soil fauna
Thiophanate-methyl and carbendazim have been shown to be
equally as toxic through contact as benomyl. Earthworms (Lumbricus
terrestris) immersed for 1 min in a 0.6% aqueous suspension of
these compounds died in 14 days. Worms in pots containing soil
drenched at a rate of 0.78 g/m2 died within 18 days (Wright &
Stringer, 1973).
7.3.2 Wildlife
The LD50 of benomyl for mallard ducks and quail is > 5000
mg/kg body weight. The cumulative toxicity of formetanate (7 days)
is approximately 6800 mg/kg body weight for ducks, and > 4640 mg/kg
body weight for quail and for pheasant (Aldridge & Magos, 1978).
Lethal and sub-lethal doses of aldicarb, methiocarb, oxamyl,
pirimicarb, and thiofanox administered to Japanese quail produced
significant inhibition of plasma- and brain-ChE and influenced the
activity of other enzymes, such as alpha-naphthyl acetate esterase,
glutamate dehydrogenase, and glutamate oxaloacetic transaminase.
With sub-lethal dose levels, plasma-ChE activity recovered within a
few days (Westlake et al., 1981).
An 8-day dietary LC50 for the Peking duck was 1890 mg/kg
feed; for bobwhite quail, the dietary LC50 was 3680 mg/kg feed
(Kaplan & Sherman, 1977).
Bobwhites (Colinus virginianus) were provided diets containing
sublethal levels of carbaryl (237 or 1235 mg/kg), for 7 days, or
carbofuran (26 mg/kg), for 14 days. Food intake, body weight, and
the locomotor activity of adult bobwhites were normal.
Administration of a diet containing 131 mg carbofuran/kg resulted
in reductions in food intake, body weight, and locomotor activity
(Robel et al., 1982).
7.3.3 Bees
An undesirable effect caused by the use of carbamate
insecticides has been the mortality of honey bees, a species that
exhibits a high sensitivity to these compounds. Because of the
agricultural and ecological importance of bees, thorough
comparative toxicity studies have been carried out on a large
number of compounds to identify those with the widest margin of
safety (Atkins et al., 1973).
Abdel-Aal & Fahmy (1977) compared the effects of aldicarb,
methomyl, N -desmethylmethomyl, carbaryl, and carbofuran on the
honey bee (Apis mellifera) and found that all of the compounds
were extremely toxic.
7.4 Earthworm and Mite Populations
Studies by Stringer & Wright (1973) and Wright & Stringer
(1973) showed that earthworm populations in apple orchard plots
sprayed with either benomyl or thiophanate-methyl were reduced. In
particular, Lumbricus terrestris, an important species in the
ecology of the apple orchard, was virtually eliminated. Captive
worms would not feed on leaf material that had been sprayed at 1.75
µg/cm2 with benomyl, methyl benzimidazol-2-yl carbamate (MBC), or
thiophanate-methyl, and feeding was significantly reduced at a
level of 0.87 µg/cm2. All the worms were killed following contact
with benomyl as an aqueous suspension or soil drench (7.75 kg/ha).
It was suggested by the authors that the toxicity of benomyl
might be due to the anticholinesterase activity of the carbamate
moiety. Effects such as lethargic movements, muscular paralysis,
and death support this supposition.
The spraying of benomyl and thiophanate-methyl in orchards
reduced the number and biomass of all earthworm species combined
and also of each individual species (Stringer & Lyons, 1974). It
was observed that over 90% reduction in earthworm populations
occurred in pastures treated with benomyl. It was suggested that
these changes were reversible, and that the populations of
earthworms would return to normal a few years after the termination
of the treatment (Tomlin & Gore, 1974).
The carbamate insecticide most commonly used in the soil is
carbaryl; it is used mainly in woodlands and orchards where the
formation and fertility of the soil are important.
Stegeman & LeRoy (1964) applied carbaryl at 1.3, 11.2, and 56
kg ai/ha to a red pine plantation and mixed hardwood stands, and,
though the lowest dose did not have any effects on mite and
Collembola populations, the other 2 dose levels considerably
decreased the numbers of mites and Collembolla. Populations began
to recover after 2 months, but Collembola were more sensitive and
recovered more slowly. There seems to be little likelihood of
long-term effects of carbaryl on mite populations (Edwards &
Thompson, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
Short- and long-term toxicity studies with carbamates have been
carried out for a period of 20 - 30 years. It is clear that the
production of these carbamates has improved during this period and
that, consequently, purer products have become available. The use
of less pure substances in earlier toxicity studies could explain
the conflicting results between these and later studies.
8.1.1. Oral
Acute oral and dermal toxicity data on animals, for most of the
carbamate pesticides, are given in Table 6. The WHO Recommended
Classification of Pesticides by Hazard is also cited. This
classification is based primarily on the acute oral and dermal
toxicity of the technical material for the rat (WHO, 1984). The
oral LD50s range from less than 1 mg/kg body weight for the highly-
toxic aldicarb to over 5000 mg/kg body weight for the non-
insecticidal carbamates such as phenmedipham, carbendazim, propham,
and benomyl.
A dose-effect relationship is generally noticed between the
administered dose, the severity of effects, and the amount of ChE
inhibition. A correlation also exists between the duration of
symptoms and the in vivo persistence of the compound.
In general, the toxicological effects produced by carbaryl are
typical of those produced by methyl carbamates. The effects of
carbaryl on a number of different animal species were studied by
Carpenter et al. (1961). Rats given a single oral dose of 560
mg/kg body weight showed 42% and 30% inhibition of erythrocyte- and
brain-ChE, respectively, within 1/2 h of treatment; only about 5%
inhibition of plasma-ChE was observed. All ChE levels were almost
normal after 24 h. In studies on rats administered single oral
doses ranging from 5 to 50 mg/kg body weight, a decrease in ChE
activity occurred within 5 min. Recovery was quite rapid
(Krechniak & Foss, 1982).
In 4 dogs, marginal inhibition of erythrocyte-ChE activity was
seen after treatment with a single oral dose of 375 mg carbaryl/kg
body weight. After 30 min, signs of poisoning were observed
including salivation, lachrymation, constriction of pupils,
urination, defaecation, increase in respiratory rate, muscular
twitching, tremors, and mild convulsions. The animals appeared to
have completely recovered the following day. These signs of
poisoning are classic of overstimulation of the parasympathetic
nervous system (Carpenter et al., 1961).
Vassilieff & Ecobichon (1983) studied the influence of a single
dose of 25 mg aminocarb/kg body weight on the activity of
erythrocyte- and brain-AChE, plasma-ChE, and hepatic
carboxylesterases in rats. Significant inhibition of all the
esterases was observed, 30 min or more after the administration of
aminocarb. This severe, but transient, inhibition of the tissue
esterases had recovered after 24 h.
Table 6. Acute oral and dermal toxicity data for a number of
carbamate pesticides
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
aldicarb 0.9 > 10.0 (rabbit) IA
aldoxycarb 26.8 700 - 1400 -
allyxycarb 90 - 99 500 -
aminocarb 30 - 40 275 IB
asulam > 4000 > 1200 -
barban 1376 - 1429 > 1600 -
> 20 000 (rabbit)
BPMC 623 - 657 > 5000 -
bendiocarb 40 - 156 566 - 600 II
benomyl > 10 000 > 10 000 (rabbit) 0
> 1000 (dog)
bufencarb 87 680 (rabbit) -
butacarb NA NA -
carbanolate NA NA -
carbaryl approximately > 4000 II
500 - 600 > 2000 (rabbit)
carbendazim > 15 000 > 2000 0
> 2500 (dog) > 10 000 (rabbit)
> 10 000 (quail)
carbetamide 10 000 > 2000 -
1250 (mouse) > 500 (rabbit)
1000 (dog)
carbofuran 6 - 14 3400c (rabbit) IB
15 - 19 (dog)
chlorbufam 2500 NA -
chlorpropham 5000 - 8000 10 200d O
5000 (rabbit) 2000 (dog)
cloethocarb 35.4 4000 -
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
desmedipham > 10 250 2000 - 10 000 -
2025e (rabbit)
dimetilan 64 > 2000 -
60 - 65 (mouse)
dichlormate NA NA
dioxacarb 72 approximately -
3000 1950
(rabbit)
ethiofencarb 411 - 499 > 1150 II
224 - 256 (mouse)
155 (quail)
formetanate 21, 18 (mouse) > 5600
19 (dog) > 10 200 (rabbit)
hoppicide NA NA -
isoprocarb 403 - 485 > 500
487 - 512 (mouse)
ca 500 (rabbit)
karbutilate 3000 > 15 400 -
methiocarb 100 350 - 400 II
40 (guinea-pig)
methomyl 17 - 24 > 5000 (rabbit) IB
28 (hen)
metolcarb 498 - 580 > 2000 -
mexacarbate 15 - 63 > 500 -
MPMC 380 > 1500 (mouse) -
MTMC 268 (mouse) 6000 -
oxamyl 5.4 710 (rabbit) IB
4.2 (quail)
phenmedipham > 8000 > 4000 -
> 4000 (dog)
> 3000 (chicken)
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
pirimicarb 147 (101 - 210) > 500 (rabbit) II
107 (mouse)
100 - 200 (dog)
25 - 50 (poultry)
promacyl 1220 > 4000 -
2000 - 4000 (mouse)
4000 - 8000 (hen)
promecarb 61 - 90 > 1000 -
> 1000 (rabbit)
propham 9000 6800f (rabbit) O
3000 (mouse)
propoxur 80 - 191 1000 - 2400 II
100 - 109 (mouse)
40 (guinea-pig)
swep NA NA -
terbucarb NA NA -
thiodicarb NA NA -
thiofanox 8.5 39 (rabbit) IB
43 (quail)
thiophanate > 15 000 > 15 000 -
-ethyl
thiophanate 6640 - 7500 > 10 000 O
-methyl
trimethacarb NA NA -
xylylcarb 325 - 375 > 1000 -
XMC 542 - -
445 (rabbit)
---------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
b From: WHO (1984). See this reference for classification of
organophosphates not mentioned in this annex.
The hazard referred to in this Classification is the acute risk for
health (that is, the risk of single or multiple exposures over a
relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the
directions for handling by the manufacturer or in accordance with the
Table 6 (contd.)
rules laid down for storage and transportation by competent
international bodies.
Classification relates to the technical material, and not to the
formulated product:
Class IA = Extremely hazardous. Class III = Slightly hazardous.
Class IB = Highly hazardous. Class O = unlikely to present
Class II = Moderately hazardous. acute hazard in normal use.
c With 75% water-dispersible powder.
d With 50% emulsifiable concentrate formulation.
e With 16% formulation.
f With 25% emulsifiable concentrate formulation.
NA = Not available.
Note: References are the reports of the different WHO/FAO Joint
meetings on pesticides residues (Annex III), Kaplan & Sherman
(1977), and Worthing & Walker (1983).
8.1.2 Dermal
Acute dermal toxicity values for most carbamates are mainly
above 500 mg/kg body weight with the exception of, for instance,
aldicarb, which is highly toxic.
8.1.3 Inhalation
Exposure to carbaryl (50% wettable powder, average particle
size, 15 µm) at a mean concentration of 390 mg/m3 (range 344 - 722
mg/m3), for 4 h, produced nasal and ocular irritation in 6 guinea-
pigs. When another group of guinea-pigs inhaled a mean of 230 mg
carbaryl/m3 (average particle size, 5 µm), for 4 h, no clear
effects were observed. Single exposures of dogs ranging from 2 to
5 h to a concentration of 75 mg carbaryl/m3 (average particle size,
5 µm) caused clinical signs of ChE inhibition (Carpenter et al.,
1961). Cats exposed to 80 mg carbaryl/m3 for 6 h showed
salivation, excitation, respiratory difficulties, and 39 - 55%
inhibition of ChE activity in the serum and 53 - 71% in the
erythrocytes. A single exposure to a level of 20 mg/m3 for 6 h did
not induce signs of intoxication, but serum- and erythrocyte-ChE
activities were slightly inhibited (Yakim, 1967).
8.2 Short- and Long-Term Exposures
A number of short-term studies have been carried out with
different carbamates and a variety of laboratory animal species.
In the context of this introduction, it is not possible to mention
all the reports available. Details on the short- and long-term
toxicities of the individual carbamates will be provided in future
Environmental Health Criteria. Most carbamates have been discussed
by the Joint FAO/WHO Meetings on Pesticide Residues and monographs
have been published (see reference list). Furthermore, the
International Agency for Research on Cancer has published a
monograph in which a number of carbamates have been evaluated
(IARC, 1976).
In the context of this introduction, a number of examples are
given to illustrate dose-response relationships and/or target
organs or tissues for a number of carbamates.
8.2.1 Oral
Short-term tests on rats or dogs exposed to repeated oral doses
of carbamate insecticides or fungicides (such as carbaryl,
propoxur, formetanate, carbofuran, and benomyl), revealed
inhibition of plasma (serum)-, erythrocyte-, and brain-ChE
(Carpenter et al., 1961; Ivanova-Chemishanska & Antov, 1976;
Krechniak & Foss, 1982).
(a) Pirimcarb
In a study on 4 dogs (2 males and 2 females) treated with 25 or
50 mg pirimicarb/kg body weight per day, for 110 weeks, 2 of the
animals developed anaemia. Haemoglobin content was decreased,
methaemoglobin was present, and the reticulocyte percentage was
significantly elevated. Levels of unconjugated bilirubin in serum
were increased. The 2 other animals were unaffected. There was
some indication that pirimicarb was able to induce the production
of immune IgG antibodies (Jackson et al., 1977).
(b) Carbaryl
Kidney alterations, such as cytoplasmic vacuolization, were
observed in the proximal tubules in short-term studies on monkeys
and rats adminstered carbaryl at dose levels of up to 600 or 300
mg/kg body weight, respectively (Coulston, 1967; cited in FAO/WHO,
1970b).
Two pigs from one litter were fed a ration containing carbaryl
at the rate of 150 mg/kg body weight for 72 and 83 days,
respectively. Three pigs in the same litter were fed 150 mg/kg
body weight for 4 weeks and, thereafter, 300 mg/kg body weight, the
total feeding period being 46 or 85 days. Two other pigs were
maintained on the basal ration, as controls. The clinical syndrome
of chronic intoxication was characterized by progressive
myasthenia, incoordination, ataxia, tremors, and chronic muscular
contractions terminating in paralysis and death. The lesions were
confined to the central nervous system and skeletal muscle.
Moderate to severe oedema of the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord was associated with
vascular degenerative changes, consistent with a vasogenic oedema
(Smalley et al., 1969). Spontaneous recovery from the effects of
sub-lethal doses occurred in about 8 h, indicating the ready
reversibility of the carbaryl-enzyme reaction (Carpenter et al.,
1961).
Carbaryl was administered to dogs at dose levels of 0, 0.45,
1.8, or 7.2 mg/kg body weight per day, for 5 days/week, for one
year. Cloudy swelling of the proximal convoluted and loop tubules
of the kidneys was found in the group given 7.2 mg/kg body weight.
There were no abnormalities in the composition of the blood, and no
ChE inhibition was found. It should be noted that the dose levels
tested were low (Carpenter et al., 1961).
In a long-term (2-year) toxicity study on CF-N rats, levels of
0, 50, 100, 200, or 400 mg carbaryl/kg diet (equivalent to 0, 2.5,
5, 10, or 20 mg/kg body weight) were tested. The highest dose
level produced cloudy swelling in the tubules of the kidneys
(Carpenter et al., 1961).
(c) Carbendazim
Groups of ChR-CD male rats (6/dose) were intubated with 200,
3400, or 5000 mg carbendazim/kg body weight per day, 5 times per
week, for 2 weeks. In all groups, the absolute and relative
weights of the kidneys were lower, and adverse effects on the
testes and reduction or absence of sperm in the epididymides were
found (FAO/WHO, 1985a).
(d) Benomyl
In a 2-year toxicity study, Charles River rats were
administered dietary levels of benomyl of 0, 100, 500, or 2500
mg/kg (equivalent to 0, 5, 25, or 125 mg/kg body weight). No
increase in mortality and no other compound-related effects on
haematology, urinalysis, organ weight, or histology were found
(Haskell Laboratories, 1969 cited in FAO/WHO, 1985a).
In another 2-year study, dogs were administered diets
containing 0, 100, 500, or 2500 mg benomyl/kg (equivalent to 0,
2.5, 12.5, and 62.5 mg/kg body weight). Liver cirrhosis, bile duct
proliferation, and testicular degeneration were found at the
highest dose level; however, 100 and 500 mg/kg diet did not show
any clear effects (Haskell Laboratories, 1970 cited in FAO/WHO,
1985a).
(e) Thiophanate-methyl
An oral toxicity study in which thiophanate-methyl at dose
levels of 0, 2, 10, 50, or 250 mg/kg body weight (in capsules) was
administered to beagle dogs (8 or 10 animals per group) was
conducted for 24 months. Retardation of growth was seen in both
sexes in the 250 mg/kg group. Both male and female dogs in the 50
and 250 mg groups showed a significant and dose-related increase in
the weight of the thyroid. However, there were no changes in the
serum-protein-bound iodine (PBI) levels and no histological
differences in the thyroid were found between the control group and
the 2 highest dose groups. No other abnormalities were observed
(Hashimoto & Fukuda, 1972 cited in FAO/WHO, 1974b).
(f) Methomyl
Groups of rats were given diets containing 0, 10, 50, 125
(increased to 500), or 250 mg methomyl/kg for 90 days. Weight gain
in animals administered 250 and 500 mg/kg was slightly suppressed.
No clear changes in the different parameters including plasma- and
erythrocyte-ChE activity were found (Kaplan & Sherman, 1977).
The same authors carried out studies over 90 days and 2 years
on beagle dogs. Dietary levels of 0, 50, 100, and 400 mg
methomyl/kg did not result in any nutritional, clinical,
haematological, urinary, biochemical, or pathological changes. In
the 2-year feeding study, histopathological alterations were seen
in kidneys and spleen of animals receiving 400 and 1000 mg
methomyl/kg diet. Histopathological changes were seen in the liver
and bone marrow in animals administered 1000 mg/kg diet (Kaplan &
Sherman, 1977).
The above-mentioned studies give some indication that
carbamates may have different mechanisms of action on target organs
that are related to substituents other than the carbamate ester
moiety (such as the imidazole moiety).
Apart from the effects on ChE activity, effects have been found
on the liver, kidneys, thyroid, and testes and also on the
activities of other enzyme systems such as ATPase, Gl-6-ase, LDH,
GPT, Alk-Pase, etc. (Haskell Laboratories, 1968 cited in FAO/WHO,
1985a; Ivanova-Chemishankska & Antov, 1976).
8.2.1.1 Further information on short- and long-term toxicity
It is not the purpose of this introductory document to provide
all the available data on long-term toxicity. However, these long-
term studies are important for the establishment of the no-
observed-adverse-effect level and consequently the Acceptable Daily
Intake (ADI) for man. Thus, it is necessary to indicate the
studies that were considered adequate and used by the different
FAO/WHO Joint Meetings on Pesticide Residues. The International
Agency for Research on Cancer has also evaluated long-term/
carcinogenicity studies of a number of carbamates. The results of
these international evaluations are summarized in Annex II and III.
From these Annexes, it is clear that aldicarb is the most toxic
of the carbamates considered; the established ADI for this compound
is 0.001 mg/kg body weight.
The ADIs for the other carbamates may vary from 0.01 to 0.1
mg/kg body weight. These quantities are equivalent to a daily
intake for man (60 kg body weight) of approximately 0.6 - 6 mg.
8.2.2 Dermal
(a) Pirimicarb
Daily dermal applications of 500 mg pirimicarb/kg body weight
to the skin of rabbits for 24 h over a 14-day period did not
produce any signs of intoxication (FAO/WHO, 1977b).
(b) Methomyl
Methomyl applied to the intact skin of rabbits at a repeated
rate of 200 mg/kg per day caused mild signs of intoxication such as
nasal discharge, wheezing, and transient diarrhoea. All animals
survived 15 applications. However, when applied on the abraided
skin, much more serious systemic signs were seen, such as nasal
discharge, salivation, tremors, poor coordination, and abdominal
hypertonia; a few animals died (Kaplan & Sherman, 1977).
(c) Carbaryl
Application of carbaryl at 500 mg/kg body weight to the skin of
6 rabbits resulted in 39% inhibition of serum-ChE activity and 36%
inhibition of red cell-ChE activity during the first 24 h following
treatment. ChE activity returned to initial levels in 72 h (Yakim,
1967).
8.3 Skin and Eye Irritation; Sensitization
8.3.1 Skin irritation
Rabbits exposed to a single dermal application of carbaryl at a
concentration of 100 g/litre in acetone did not show any skin
irritation (Carpenter et al., 1961).
8.3.2 Eye irritation
Undiluted carbaryl or solutions of different concentrations
were applied to the eyes of rabbits. Technical carbaryl applied in
excess as a 100 g/litre suspension in propylene glycol produced
mild injury. A 25% aqueous suspension of a microfine material did
not cause any injuries; 50 mg of the dust caused only traces of
corneal necrosis (Carpenter et al., 1961).
8.3.3 Skin sensitization
(a) Carbaryl
Groups of albino guinea-pigs were given 8 intracutaneous
injections (3 per week) of 0.1 ml of a 0.1% dispersion of carbaryl
in 3.3% propylene glycol. After a 3-week incubation period,
followed by a challenge dose, no clear sensitization reaction was
noticed (Carpenter et al., 1961).
(b) Benomyl and thiophanate-methyl
It has been shown that benomyl and thiophanate-methyl can cause
slight reversible skin irritation and weak sensitization in guinea-
pigs (FAO/WHO, 1974, 1985a).
8.4 Inhalation
(a) Carbaryl
The inhalation toxicity of carbamates for mammals has not been
widely studied.
The effects of acute inhalation exposure to carbaryl dust have
been examined in rats (10 mg/m3), guinea-pigs (390 mg/m3), and dogs
(75 mg/m3) (Carpenter et al., 1961) (section 8.1.3). No treatment-
associated, gross or microscopic lesions were seen in guinea-pigs
and dogs, even though the exposure was sufficient to cause ChE
inhibition. Haemorrhagic areas in the lung were seen in guinea-
pigs at the highest level tested (390 mg/m3).
Four cats exposed to carbaryl in air at 63 mg/m3 for 6 h per
day for 1 month developed signs of cholinergic stimulation during
the first 2 h of exposure each day. Periodic salivation was noted.
One cat died with marked signs of poisoning on day 20. There was
already a significant decrease in serum- and erythrocyte-ChE
activity after the first exposure. Exposure to 40 mg/m3 for 6 h
per day for 2 months was reported to impair conditioned reflexes in
cats. On certain days, the ChE activity dropped to below 50% of
pre-exposure values. Exposure to concentrations of 16 mg/m3, for 6
h per day, for 4 months did not result in any signs of toxicity
(Yakim, 1967). Carpenter et al. (1961) did not find any increase in
mortality or "grossly-visible injury" when rats were exposed to a
mean concentration of 10 mg/m3 (range 5 - 20 mg/m3), for 7 h per
day, 5 days/week, over 90 days.
(b) Propoxur
Male and female rats were exposed to propoxur aerosols at
average concentrations of 0, 5.7, 10.7, or 31.7 mg/m3, for 6 h
daily, 5 days/week, over a period of 12 weeks. Depression of
plasma-, erythrocyte-, and brain-ChE activity was observed at the
highest dose level (Kimmerle & Iyatomi, 1976).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
A considerable number of reproduction and teratogenicity
studies have been carried out on different carbamates in several
animal species. A number of these will be used to show the state
of the art giving the well-known carbamates, such as carbaryl,
benomyl, carbendazim, and thiophanate-methyl, as examples.
For more details concerning different carbamates, the reader is
referred to the reports of the Joint FAO/WHO Meetings on Pesticide
Residues and, in the future, to the Environmental Health Criteria
Documents on the individual carbamates.
8.5.1 Reproduction
(a) Methomyl
A 3-generation reproduction study with methomyl was carried out
on rats. Dose levels of 0, 50, or 100 mg/kg diet were administered
for 3 months. There was no evidence from the different indices of
reproduction, lactation, and weanling body weights to suggest that
methomyl affected reproduction or lactation performance in the
litters of three generations (Kaplan & Sherman, 1977).
(b) Benomyl
The effects of benomyl on the gonads and reproductive function
were studied in a 3-generation study on rats. Pronounced effects,
such as a decrease in fertility and decreased activities of a
number of enzymes in the testes were found in rats treated orally
with 1000 mg/kg body weight (the only dose level tested), 5
days/week, for 4 months. Besides the effects mentioned, a
decreased gestation index was also found, which may indicate early
embryonic death due to the induction of a dominant lethal gene in
the sperm cells. An increase in mortality and decreased viablility
and fertility were found in the F1 - F3 generations (not exposed to
the carbamate) (Ivanova-Chemishanska & Antov, 1980; Ivanova-
Chemishanska et al., 1980). In a second 3-generation rat
reproduction study with benomyl, no differences in reproduction or
lactation were observed among control and test groups of animals at
the highest dietary level of 2500 mg/kg diet. No pathological
changes were found in tissues from weanling pups of the F3b litter
(Sherman et al., 1975).
(c) Carbendazim
From 3 multigeneration reproduction studies on rats, in which
carbendazim was tested at dose levels of up to 10 000 mg/kg diet,
no apparent adverse effects on reproduction were found at levels of
up to 2000 mg/kg diet. A reduction in average litter weights was
observed at dietary levels of 5000 and 10 000 mg/kg (FAO/WHO,
1985a).
(d) Carbaryl
In 3-generation rat studies, carbaryl added to the diet to
provide daily doses of up to 10 mg/kg body weight, did not result
in any statistically-significant dose-related effects on fertility,
gestation, viability of pups, or lactation (Weil et al., 1972).
In other studies by Weil et al. (1972, 1973), groups of rats
received carbaryl in the diet at daily doses of 0, 3, 7, 25, or 100
mg/kg body weight by intubation, for 5 days/week, or 0 or 100 mg/kg
body weight per day in the diet. Significant effects were found
on reproduction (mortality and reduced fertility) in the group
receiving 100 mg/kg. A group receiving 200 mg/kg diet did not show
any effects.
The influence of carbaryl at dose levels of 2 or 5 mg/kg body
weight on the reproductive function of rats was studied by
Shtenberg & Otovan (1971). Both dose levels produced adverse
effects on the testes and ovaries (atrophic, dystrophic and
necrotic changes) and changed the gonadotropic function of the
hypophysis. The changes observed increased progressively over the
generations. The fecundity of test females fell, the number of
still-born offspring increased, and there was a high death rate
among young animals.
Carbaryl was fed at dietary levels of 0, 2000, 5000, or 10 000
mg/kg feed to Osborne-Mendel (10 males and 10 females) rats for 3
generations. At the highest dose level, fertility was impaired and
no litters were produced from the second mating of the second
generation. Marked effects from birth to day 4 were manifested as
seen in the survival index. Dose-related decreases in average
litter size, number of live-born progeny, number of survivors to
day 4, and number weaned were observed.
In a comparative study on gerbils, dietary levels of 0, (40
animals) 2000, 4000, 6000 (each 30 animals), and 10 000 mg
carbaryl/kg (18 animals) were tested for 3 generations. No litters
were produced from the second mating of the third generation, at
the highest dose level. Gerbils appeared to be more sensitive than
rats since significant decreases were found consistently at lower
dietary levels, but the effects were not clearly dose-related.
Effects in both species at 2000 mg/kg diet were still marginal but
significant (Collins et al., 1971).
(e) Thiophanate-methyl
Thiophanate-methyl was tested in a 3-generation reproduction
study on rats at dose levels of 0, 40, 160, or 640 mg/kg diet. The
highest dose level affected growth, but no effects of the compound
on reproduction parameters were apparent (FAO/WHO, 1974b).
(f) Carbofuran
A 3-generation reproduction study was conducted on rats (8
males and 16 females per group), with dietary levels of 0, 1, 10,
or 100 mg carbofuran/kg diet. The highest dose level resulted in
decreased weight gain in the parents and a marked reduction in
survival of the young. Lower levels did not have any effects.
Follow-up studies with lower dose levels showed that 20 mg/kg diet
induced adverse effects on reproduction variables, noted after the
first generation (McCarthy et al., 1971; FAO/WHO, 1977b, 1981b).
8.5.2 Endocrine system
(a) Carbaryl
Short-term studies on male and female rats were carried out to
study the functioning of the reproductive system under the
influence of carbaryl. The dose levels ranged from 2 to 300 mg
carbaryl/kg body weight and were administered over periods of 3 -
12 months; in one study, 4 generations were bred. Different
criteria were studied, such as reduction of sperm motility,
duration of sperm survival, inhibition of spermatogenesis and
oögenesis, estrous cycle, induction of degeneration of the germinal
epithelium, and the activities of different enzymes in the testes
and ovaries. Shtenberg & Rybakova (1968) found marked functional
and morphological changes in the endocrine organs of rats
administered carbaryl at daily doses of 7, 14, or 70 mg/kg body
weight for up to 12 months. It was considered by the authors that
the effects of carbaryl on the testes and ovaries might be
explained by the observed increase in hypophyseal gonadotropic
function. A direct effect on the testes and ovaries cannot be
excluded.
Orlova & Zhalbe (1968) studied the effects of carbaryl on white
rats. Dose levels of 2.5 or 5 mg/kg body weight were administered.
Disturbances in the enzymatic activity in the testes and ovaries,
inhibition of spermatogenesis and oögenesis, and decreased
fertility were observed in the P generation, and a decrease in
viability, in the F1 generation.
Shtenberg & Otovan (1971) studied this effect of carbaryl in
more detail in a 4-generation study. The dose levels studied were
0, 2, and 5 mg/kg body weight, and were applied for 6 months in
each generation. The disturbances in the function of the testes
and ovaries described above were found at both 2 and 5 mg/kg
(Vashakidze, 1965, 1967; Shtenberg & Rybakova, 1968; Shtenberg &
Otovan, 1971; Shtenberg et al., 1973). However, the studies
reporting adverse effects on spermatogenesis in male rats were not
confirmed in studies on mice with carbaryl by Dikshith et al.
(1976) or in reproductive performance studies on rats.
(b) Benomyl
A study on the short-term toxicity of benomyl carried out by
Torchinsky et al. (1976) showed that the testis of Wistar rats was
the organ most affected. When administered over a 12-month period,
a dose of 62.5 mg/kg body weight showed borderline effects; 12.5
mg/kg body weight did not show an effect. A recent study on
Sprague Dawley rats receiving 10 daily treatments of 0, 200, or 400
mg benomyl/kg body weight, by gavage, showed a significant decrease
in the total epididymal sperm counts and sperm concentrations in
the vas deferens at both levels. At the higher dose level, slight
to severe hypospermatocytogenesis and generalized
hypospermatogenesis were observed (Carter & Laskey, 1982; FAO/WHO,
1985a). Pastushenko (1983) studied the gonadotoxic effects of
benomyl in rats exposed to dose levels of 0.5, 1.2, 5.0, or 12.4
mg/m3 air. The 2 highest dose levels showed dose-related changes
in the gonads, i.e., disturbances of the morphological and
functional states. At 1.2 mg/m3, only morphological changes were
observed. No effects were found with 0.5 mg/m3.
(c) Carbendazim
When Sprague Dawley rats were fed diets containing 0, 2000,
4000, or 8000 mg carbendazim/kg diet for 28 days, degeneration of
testicular tissue was observed at 4000 and 8000 mg/kg diet.
Spermatogenesis and oögenesis were affected at these feeding
levels. Changes were also found in the organ weights relative to
body or heart weight, changes in the liver being observed at 2000
mg/kg diet (FAO/WHO, 1974b).
No effects on testicular weights and morphology were found in a
study on groups of ChR-CD rats fed carbendazim in the diet for 90
days at dose levels of 0, 100, 500, or 2500 mg/kg (FAO/WHO, 1985a).
8.5.3 Embryotoxicity and teratogencity
A number of carbamate esters have been tested for
teratogenicity with the chicken embryo bioassay (Marliac, 1964;
Ghadiri & Greenwood, 1966; Khera, 1966; Ghadiri et al., 1967;
Rybakova, 1968; Olefir & Vinogradova, 1968; Proctor et al., 1976;
Moscioni et al., 1977). However, this test is not considered of
relevance in testing for teratogenicity, and the results will not
be discussed in this document.
(a) Propoxur
Roszkowski (1982) studied the effects of propoxur on
intrauterine development and on the state of ossification of rat
fetuses. An embryotoxic effect was found at a dose of 20% of the
LD50, administered on days 7 - 19 of the gestation period; 11.9% of
anomalies occurred in the fetuses of treated animals compared with
6.3% in the controls. Decreased weight was also found in the
fetuses. Ossification was disturbed, but this was not related to
the period of gestation during which the substance was
administered. A decrease in the concentrations of calcium and
magnesium was found in the serum of mothers whose fetuses had shown
bone anomalies.
(b) Benomyl
In a teratogenicity study, pregnant ChR-CD rats were fed a diet
containing 0, 100, 500, 2500, or 5000 mg benomyl/kg from day 6 to
day 15 of gestation; no effects were found on the outcome of
pregnancy or embryonal development (Sherman et al., 1975).
Hazleton Laboratories (1968) cited in FAO/WHO (1985a) reported
similar negative results for rabbits, at dose levels up to 500 mg
benomyl/kg diet administered on days 8 - 16 of gestation.
Torchinsky (1973) studied the influence of carbaryl (106 mg/kg
body weight, daily, from day 1 to day 20 of pregnancy) and benomyl
(single dose of 145 or 250 mg/kg body weight on day 12 of
pregnancy) in relation to the protein content of the diet. The
animals were kept on diets containing 5, 10, 19, or 35% of protein
either for one month prior to mating and during the entire
pregnancy or only during pregnancy. The teratogenic and embryotoxic
actions of benomyl and carbaryl did not differ in groups where the
females received diets containing 5, 10, or 19% protein from the
beginning of pregnancy. An increased post-implantational lethality
for the embryos was noted in females kept on a diet with 5% protein
from one month before conception. When these animals were exposed
to carbaryl, the weight of 20-day-old fetuses declined
considerably, yet the severity of the teratogenic action of benomyl
did not increase under these conditions. The teratogenic action of
benomyl and the embryotoxic action of carbaryl were less severe in
animals fed diets containing 10% of protein from one month before
conception than in the groups kept on diets containing 19 or 35%
protein. The results of a few other studies have indicated that
benomyl causes embryotoxic and teratogenic effects in both rats and
mice when administered orally (by gavage) at dose levels at 62.5
mg/kg body weight (rat) and 100 mg/kg body weight (mouse) or more.
At lower dose levels (31.2 mg for the rat and 50 mg/kg for the
mouse), no teratogenic effects were observed. A broad spectrum of
malformations such as exencephaly, hydrocephaly, and
meningocephaly, and disturbance of the CNS were found (Ruzicska et
al., 1975; Petrova-Vergieva, 1979; Kavlock et al., 1982).
(c) Carbaryl
A number of teratogenicity studies carried out with carbaryl
gave contradictory results. Weil et al. (1972) administered up to
500 mg carbaryl/kg body weight to rats. No teratogenic effects or
effects on fertility or gestation were seen at this high dose
level; however, the mean body weight gain of the females during
pregnancy was decreased. Many pups died before weaning. No effects
were found with administration of carbaryl at 100 mg/kg body weight
(Weil et al., 1972).
Oral administration of carbaryl to pregnant rabbits and
hamsters at doses of up to 250 mg/kg body weight, by intubation,
did not cause any teratogenic effects or dose-related fetal
mortality (Weil et al., 1973).
Dougherty et al. (1975), cited in FAO/WHO (1974b), did not
observe any teratogenic effects in monkeys given doses of carbaryl
of 0, 0.2, 2, or 20 mg/kg body weight from day 18 to day 40 of
gestation. This study indicated that even the highest dose level
did not induce a reproductive hazard.
Vashakidze (1965, 1967) found reduced reproduction and abnormal
fetal changes with intubated dose levels of 100 mg/kg body weight
and higher. A level of 50 mg/kg produced negative results.
Carbaryl, administered to guinea-pigs orally during organogenesis
(10 consecutive days between days 11 and 20 of gestation) at 300
mg/kg body weight, induced increased maternal and fetal mortality
and offspring with axial skeletal defects, such as cervical
vertebrae fusion. No abnormal fetal changes were produced in
hamsters at the sub-lethal levels of 125 and 250 mg/kg body weight.
In rabbits, levels ranging from 50 to 200 mg/kg body weight did not
produce any effects on mortality or morbidity, and no abnormal
fetal changes were seen (Robens, 1969).
Smalley et al. (1968) conducted studies on pregnant beagle dogs
fed a diet containing 0, 3.125, 6.25, 12.5, 25, or 50 mg
carbaryl/kg body weight from mating to the termination of weaning.
Effects observed included a number of animals with dystocia
(difficult births) due to atonic uterine musculature, an apparent
contraceptive effect at the highest dose level, and teratogenic
effects (different types of severe defects) at all but the lowest
dose level.
The effects of carbaryl on rat reproduction and guinea-pig
teratology were compared by Weil et al. (1973). A 3-generation
reproduction study on rats included groups receiving carbaryl at
maximum daily doses of 200 mg/kg diet. Other groups concurrently
received daily oral intubation doses as high as 100 mg/kg body
weight. In the guinea-pig teratology study, maximum levels of 300
mg/kg diet or 200 mg/kg body weight (intubation) were given. The
concurrent administration of carbaryl by daily oral administration
or by dietary inclusion did not result in either teratological or
mutagenic effects in the rat or teratological effects in the
guinea-pig, at the maximum dosage levels. However, the groups
receiving maximum levels orally showed severe toxic effects in both
studies compared with little or no effect with oral administration
of 25 mg/kg in rats and 100 mg/kg in guinea-pigs.
The authors concluded that the severe effects found in rats by
Shtenberg & Otovan (1971) following repeated oral intubation of
carbaryl at 2 mg/kg body weight could not be confirmed, even at the
maximum oral intubation level of 100 mg/kg body weight.
(d) Methomyl
When rabbits were fed dietary levels of 0, 50, or 100 mg
methomyl/kg on days 8 - 16 of gestation, no skeletal or visceral
abnormalities were seen in the fetuses (Kaplan & Sherman, 1977).
(e) Carbofuran
Teratogenicity studies have been carried out on albino rats and
albino rabbits with carbofuran. In rats, dose levels of 0, 0.1,
0.3, or 1.0 mg/kg body weight were administered throughout
gestation (days 6 - 15 of pregnancy) and, in rabbits, levels of 0,
0.2, 0.6, or 2.0 mg carbofuran/kg body weight were administered
from day 6 to day 18 of gestation. In both animal species, the
highest dose level showed clear signs of poisoning. However,
pregnancy, viability, fetal body weights, and all the other
examinations did not show any effects. All the progeny were
normal. No teratogenic effects were seen in rats or rabbits
(McCarthy et al., 1971; FAO/WHO, 1981b).
(f) Carbendazim
Carbendazim has been shown to be embryotoxic at dose levels of
approximately 19 mg/kg body weight or more. This effect was not
seen at 10 mg/kg body weight (Delatour & Richard, 1976).
8.6 Mutagenicity and Related End-Points
There is little evidence, if any, to suggest that methyl
carbamate compounds are mutagenic. An evaluation of the mutagenic
potential of carbaryl, methomyl, propoxur, carbofuran, and
pirimicarb, using a variety of microorganisms as indicator strains,
including strains of Salmonella typhimurium, Escherichia coli,
Saccharomyces cerevisiae, and Bacillus subtilus, produced
negative results. These studies showed that carbamate compounds
have little potential for presumptive gene mutations in higher
animals.
Anderson et al. (1972) examined barban, chloropropham, propham,
and swep for their ability to induce point mutations in 8
histidine-requiring strains of S. typhimurium. None showed induced
mutations. This study was carried out in the absence of any
metabolic system.
The chemical structure of benomyl probably enables it to act
both as a base analogue for DNA (benzimidazole ring incorporates
into nucleic acids instead of guanine) and as a spindle poison
(carbamate groups) (Seiler, 1975, 1976b; FAO/WHO, 1985a). The
binding of benomyl to microtubular proteins disturbs the mitotic
spindle, thus causing malsegregation of chromosomes during the
mitotic anaphase (Morris, 1980, cited by Finnish National Board of
Health Toxicology Group, 1982). The results of several studies
suggest that benomyl is a strong antimitotic agent which, even in
low concentrations, induces non-disjunction of single chromosomes
leading to aneuploidy (FAO/WHO, 1985a).
The induction of low-frequency forward mutations by carbendazim
in S. typhimurium strain LT-2 was reported by Seiler (1972).
Reversions reported to occur in S. typhimurium strains His G46 and
TA 1530 were attributed to base-substitution mutations caused by
carbendazim.
Another study was made with the WP2 uvrA repair-deficient
strain of E. coli and the TA 1535 repair-deficient strain of
S. typhimurium. Mutagenic activity was detected for benomyl in the
former, but not in the latter or in TA 1538 (Kappas et al., 1976).
No effects were found in the CM-611 strain of E. coli, deficient
in either the excision repair pathway or the lex A+-dependent
misrepair pathway. In studies carried out recently, benomyl was
negative in all S. typhimurium strains used, even though, in some
of the repeats, significant increases in the number of revertants
were observed in TA 1535 strain. Carbendazim was clearly mutagenic
in the TA 98 strain with metabolic activation (S-9 mix) and
slightly mutagenic in the TA 1535, TA 1537, and TA 100 strains. A
positive result for carbendazim in the TA 1537 strain was observed,
also without metabolic activation (Haskell Laboratories, cited by
the Finnish National Board of Health, 1982; FAO/WHO, 1985a).
There is evidence that carbendazim and related compounds are
antimitotic agents and cause mitotic arrest, mitotic delay, and a
low incidence of chromosome damage in SPF Wistar rats and mammalian
cell lines. The mutagenic effects of carbendazim in bacteria are
probably due to base substitutions, resulting from the
incorporation of small quantities of benzimidazole into nucleic
acids. The effects on mammalian cells in vivo and in vitro
indicate that carbendazim causes chromosome damage, which may
result from this type of mutagenic activity, but the effects on
mitosis suggest that, in higher organisms, the main genetic effect
is to cause non-disjunction (Styles & Garner, 1974).
An abnormal number of micronucleated erythrocytes have been
observed in the bone marrow of mice treated orally with carbendazim
at 100 mg or more/kg body weight. No effects were seen at 50
mg/kg. Benomyl at 1000 mg/kg body weight produced a slight
increase in micronucleated erythrocytes.
Carbendazim did not induce chromosome breakage in Chinese
hamster bone-marrow cells at oral doses as high as 1000 mg/kg body
weight (Seiler, 1976a). It was concluded that carbendazim is not
a chromosome-breaking agent, and its micronucleic action is
attributable to antimitotic activity caused by inhibition of
spindle formation or spindle function.
An evaluation of the mutagenic properties of benomyl has been
completed and reported by the US Environmental Protection Agency
Pesticide Genotoxicology Program. Benomyl increased the mutation
frequency in a concentration-related manner in L 51784 mouse
lymphoma cells and induced sister chromatid exchange in Chinese
hamster ovary (CHO) cells. Benomyl was also positive at all dose
levels tested in the in vivo micronucleus test with mice. The
results of the spot test conducted on mice support the idea that
carbendazim (oral application of 100 - 300 mg/kg body weight to the
mother on the 10th day following conception) may be mutagenic in
mammalian cells due to a direct action on DNA (Fahrig & Seiler,
1979).
A dominant lethal study to evaluate the potential mutagenic
effects in the reproductive cycle of the male mouse was conducted
with propoxur at dose levels of 0, 2.5, or 5 mg/kg body weight.
For a period of 6 weeks, 3 virgin females were exposed to each male
at weekly intervals and reproduction indices recorded. The
administration of propoxur proved to have a transient effect on the
reproductive capability of the males but there was no sign of early
resorption indicating a mutagenic effect (FAO/WHO, 1974b).
DNA-repair tests (with both isolated rat and mouse hepatocytes)
were conducted using benomyl and carbendazim. All tests showed
negative results for the induction of DNA repair (Haskell
Laboratories, 1981a,b cited in FAO/WHO, 1985a).
The Finnish National Board of Health (1982) summarized the
mutagenicity data on benomyl, carbendazim, and thiophanate-methyl
(Table 7) and concluded that the results were sometimes
contradictory. However, positive mutagenicity results from point
mutation tests and chromosome aberration tests are well documented,
especially in higher organisms, and it can be concluded that
carbendazim fungicides should be considered as weak mutagenic
compounds.
Without going into detail, it is known that a large number of N
-nitroso derivatives of methyl carbamates are potent mutagens. The
N -nitroso derivatives of aldicarb, propoxur, carbofuran,
trimethacarb, and methomyl caused numerous single-strand breaks in
normal human skin cells. The data suggested that human cellular
DNA in vivo was irreversibly altered resulting in numerous alkali-
sensitive bonds (Blevins et al., 1977).
A similar result was obtained with N -nitroso carbaryl. This
compound showed strong mutagenic activity in 2 bacterial systems,
E. coli and Haemophilus influenzae. Using S. typhimurium, N -
nitroso carbaryl acted as a potent base-pair substition mutagen and
showed a relatively mild frameshift activity. The parent compound,
carbaryl, gave negative results (Regan et al., 1976; FAO/WHO,
1982b).
Uchiyama et al. (1975) tested the nitroso derivatives of BPMC,
propoxur, hoppcide, isoprocarb, MPMC, and MTMC, in the back
mutation test with E. coli B/r WP2 try- and in the rec-assay
method with B. subtilus strains. The N -nitroso derivatives of
all these N -methylcarbamates showed potent mutagenic activity.
Table 7. A summary of the mutagenicity data on benomyl, carbendazim (MBC), and
thiophanate-methyl
----------------------------------------------------------------------------------------
Point mutations Genetic change
Chromosomal aberrations Non-disjunction
----------------------------------------------------------------------------------------
Positive results + + +
Salmonella typhimurium rat fetus
(Seiler, 1972) (Ruzicska et al., 1975)
Salmonella typhimurium rat bone marrow rat bone marrow
(Haskell Laboratories, 1982 (Styles & Garner, 1974) (Styles & Garner, 1974)
cited in FAO/WHO, 1985a)
mouse bone marrow
(Seiler, 1976a)
Escherichia coli Aspergillus nidulans
(Kappas et al., 1976) (Kappas et al., 1974;
Bignami et al., 1977)
Fusarium oxysporum
(Dassenoy & Meyer, 1973)
Mouse/spot-test Chinese hamster ovary (SCE)
(Fahrig & Seiler, 1979) (SRI International, 1980,
cited in FAO/WHO, 1985a)
micronucleus in vivo /mouse
(SRI International, 1980,
cited in FAO/WHO, 1985a)
Negative results - - -
Streptomyces coelicolor rat bone marrow dominant lethal/rat
(Carere et al., 1978) (Ruzicska et al., 1975) (Sherman et al., 1975)
Salmonella typhimurium Chinese hamster bone marrow dominant lethal/mice
(Fiscor et al., 1978) (Seiler, 1976a) (Makita et al., 1973)
(Carere et al., 1978)
Salmonella typhimurium lymphocyte culture-cytotoxicity
(Haskell Laboratories, 1981; obs.
cited in FAO/WHO, 1985a) (Lamb et al., 1980)
Aspergillus nidulans benomyl-workers
(Bignami et al., 1977) (Ruzicska et al., 1975)
Drosophila /recessive lethal hepatocyte culture/DNA-repair
- sterility obs. rat, mouse
(Lamb & Lilly, 1980) (Haskell Laboratories, 1981,
cited in FAO/WHO, 1985a)
human lymphocyte culture
(Gupta & Legator, 1975,
cited in FAO/WHO, 1985a)
----------------------------------------------------------------------------------------
From: The Finnish National Board of Health (1982) FAO/WHO (1985a).
8.7 Carcinogenicity
8.7.1 General
The mechanism of the carcinogenic action of ethylcarbamate
(urethane) has not yet been established, but its structure seems to
be optimal. All changes seem to reduce activity, particularly when
the ethyl group is replaced with larger side chains. The addition
of alkyl groups to the nitrogen also reduces activity (Mirvish,
1968). It is not so clear why urethane is carcinogenic, whether it
is the proximal toxin or whether it has to be converted to an
active metabolite. The low carcinogenic potential of esters of N -
substituted carbamic acids is possible.
In 1976, a Working Group of the International Agency for
Research on Cancer (IARC, 1976) reviewed the available data on a
number of carbamate pesticides included carbaryl, propham,
chloropropham, and mexacarbate.
Carbaryl was tested in a number of carcinogenicity tests some
of which were not relevant or were of inadequate design. The rat
study (40 per group), in which carbaryl was administered at dose
levels of 0, 50, 100, 200, or 400 mg/kg diet for 2 years, did not
give any indication of an increase in tumour incidence (Carpenter
et al., 1961; Innes et al., 1969; Andrianova & Alekseev, 1970;
IARC, 1976).
Groups of 48 male and 48 female CD-1 mice were given 0, 100, or
400 mg carbaryl/kg diet. After 80 weeks, the tumour incidence
between the groups was similar (Mellon Institute, 1963 cited in
FAO/WHO, 1965b).
Mexacarbate was administered orally to 2 strains of mice. The
design of this study was inadequate (only a small number of
animals), but an increased incidence of lung adenomas was shown in
both strains, together with a slight increase in liver tumours in a
number of males of one strain. According to IARC (1976), it was
not possible to make an evaluation of the carcinogenicity of
mexacarbate on the basis of this study by Innes et al. (1969).
Propham and chlorpropham were administered via the oral route
in a number of carcinogenicity tests on mice, rats, and hamsters.
One or both compounds were also tested on mice and/or rats via the
subcutaneous, intraperitoneal, or intrapleural route. The design
of some of these studies was inadequate. Propham and chloropropham
were tested for initiation properties, because the ethyl carbamates
are related to the well-known carcinogen ethylcarbamate (urethane),
which is an initiator of skin tumours. There was no evidence that
propham and/or chloropropham were carcinogenic after oral,
subcutaneous, intraperitoneal, and intrapleural application.
However, these two compounds acted as weak initiators in the two-
stage carcinogenicity studies on mice. A second study by the same
authors did not confirm the results (Van Esch et al., 1958;
FAO/WHO, 1965b; Innes et al., 1969; Van Esch & Kroes, 1972; IARC,
1976).
Long-term studies on rats did not provide any evidence of
carcinogenic activity when propoxur was fed in the diet for 2 years
at levels of 0, 250, 750, 2000, or 6000 mg/kg diet. Increased liver
weight was found at the highest dose level (6000 mg/kg) in males
and in the three highest dose levels in females. This liver change
was not reflected in liver function tests, clinical chemical
examinations, or histological changes (FAO/WHO, 1974b). Similarly,
long-term and 2-year studies on rats, dogs, mice given carbofuran
or pirimicarb did not indicate any carcinogenic potential. The
dose levels tested were 175 mg/kg diet for rats and 1.8 mg/kg body
weight for dogs for pirimicarb, 100 mg/kg diet for rats, and 500
mg/kg diet for mice (24 months in mice) for carbofuran (FAO/WHO,
1977b, 1981b). On the basis of a variety of test protocols, these
carbamate compounds did not show any significant carcinogenic
potential.
Benomyl was tested for carcinogenicity in CD-1 mice, at dose
levels of 0, 500, 1500, or 5000 mg/kg diet (approximately
equivalent to 0, 50, 150, or 500 mg/kg body weight). A significant
dose-dependent increase in the incidence of primary hepatocellular
adenomas and carcinomas in female mice was seen. Furthermore,
histopathological changes occurred in a number of organs (Haskell
Laboratories, 1981 cited in FAO/WHO, 1985a).
Low dose levels of thiophanate-methyl (0 - 640 mg/kg diet,
equivalent to approximately 0 - 64 mg/kg body weight) were tested
for carcinogenicity in ICR-SCL mice. This study showed that
ingestion of 640 mg thiophanate-methyl in the diet by a mouse
strain susceptible to various tumours did not affect carcinogenic
activity. In another study on rats, dose levels of 0, 10, 40, 160,
or 640 mg/kg diet did not have any apparent effects on the
occurrence of tumours; the only significant effects appeared to be
decreased growth at the highest dose levels (Hashimoto & Fukuda,
1972, cited in FAO/WHO, 1974b).
The influence on the testes of ChR-CD rats of carbendazim
administered at dose levels of 0 - 10 000 mg/kg diet was studied
over a period of 2 years. However, the incidence of testicular
changes was so high in the two control groups that no opinion could
be given on the possible influence of carbendazim on the testes
(Haskell Laboratories, 1977 cited in FAO/WHO, 1985a).
Carbendazim at dose levels of 0, 150, 300, or 1000 mg/kg diet
(this dose level was increased to 2000 mg/kg at week 4 and to 5000
mg/kg at week 8 for the remainder of the study) was administered to
SPF-Swiss mice. The duration of the study was 80 weeks. At the
highest dose level, neoplastic nodules were found in the livers of
female mice. In males, there was an increased incidence of
hepatoblastomas (CIVO/TNO, 1976 cited in FAO/WHO, 1985a).
A 2-year feeding study on CD-1 mice was conducted using
carbendazim at dose levels of 0, 500, 1500, or 7500 mg/kg diet
(only females completed the 2-year period, the 7500 mg/kg level for
males was decreased to 3750 mg/kg after the first 15 months of the
study and the final sacrifice of this group was antedated by 7
months). A significant increase in hepato-cellular carcinomas
occurred at 1500 mg and 7500 mg/kg in female mice and at 1500 mg/kg
in male mice (at the highest dose level for males, there were too
few survivors to judge the effects) (Haskell Laboratories, 1982;
FAO/WHO, 1985a).
Other studies demonstrated that carbamates in the presence of
nitrite could be converted to N -nitroso derivatives, which may be
(partly) responsible for the "carcinogenic activity of the
carbamates".
Studies in which carbendazim was administered to Swiss mice,
twice, at 250 mg/kg body weight, intragastrically, weekly, in
combination with 5000 mg sodium nitrite/litre drinking-water caused
a slight increase in the incidence of lymphosarcomas (Börzsönyi &
Csik, 1975; Börzsönyi & Pinter, 1977).
The results of an in vitro study of Beraud et al. (1979)
(IARC, 1976) suggested that nitrosocarbaryl could be produced in
rat gastric juice from carbaryl.
The proposal that carbamate pesticides may be converted to N -
nitroso compounds under the acid conditions of the stomach is based
on the results of in vivo nitrosation studies with secondary
amines and other compounds in the presence of nitrite (originating,
for instance, from certain vegetables and present in saliva)
(Tannenbaum et al., 1974). Elespuru & Lijinsky (1973), Eisenbrand
et al. (1974, 1975a,b), Elespuru et al. (1974), Ungerer et al.
(1974), Quarles & Tennant (1975), Lijinsky & Taylor (1976), and
Oliver (1981) have shown that certain pesticides, such as carbaryl
and dithiocarbamates, can be nitrosated in vivo.
As pointed out by IARC (1976), the extrapolation of findings in
experimental animals to man is complicated by many factors. It is
relatively easy to show that N -nitroso derivatives can be formed
and that these are mutagenic and/or carcinogenic. However, the
crucial information is the quantity produced in man under the
prevailing conditions. The concentration of both reactants,
differences in pH, the influence of competing reactions, and the
presence of accelerators and inhibitors are all important. In
addition to these difficulties in defining potential human
exposure, the susceptibility of man has to be compared with that of
experimental animals. General considerations on N -nitrosatable
pesticides are described in IARC (1983).
8.8. Special Studies
Adequate characterization of the neurobehavioral effects of
carbamates is still incomplete or not available. In general, when
given in single doses the ChE-inhibiting carbamates decrease
activity and schedule-controlled behaviour, interfere with
avoidance and escape performance, and decrease the amplitude of a
variety of electrophysiological measures. The possible
interference with learning and memory by the carbamates has been of
interest because of the hypothesized role of the cholinergic system
in these functions. However, the evidence that these compounds
interfere with learning and memory is equivocal (Miller, 1982).
Carbamates interfere with shock-motivated behaviour including
discrete-trial and one-way avoidances as well as shock-induced
suppression. Furthermore, these compounds decrease responsiveness
to shock (Sideroff & Santolucito, 1972).
The effects of the ChE-inhibiting carbamates have been
evaluated in man. However, these studies have been mainly
concerned with correlating ChE inhibition and overt symptoms. It is
assumed that, if the clinical indicators (such as ChE levels) are
normal after pesticide exposure, or if symptoms associated with
cholinergic overstimulation are absent, then no risk is involved
(Miller, 1982).
9. EFFECTS ON MAN
The health hazards from overexposure to carbamate insecticides
occur primarily as a result of the inhibition of the enzyme ChE.
ChE inhibition leads to the accumulation of endogenous ACh and
other choline esters in the organism. This produces signs and
symptoms as specified in section 9.3.
9.1 General Population Exposure
9.1.1 Acute toxicity: poisoning incidents
Carbamate pesticides have occasionally been used in cases of
suicide. Fatal poisonings have occurred with carbaryl, propoxur,
and mexacarbate. Farago (1969) described a case involving a 39-
year-old man who drank 1/2 litre of carbaryl. He entered the
hospital 1 1/2 h later and received gastric lavage, but developed
serious respiratory depression. His condition improved somewhat
after 4 injections of atropine at 1/2-h intervals. Three hours
after the carbaryl ingestion, 250 mg of 2-PAM intravenous was
administered. His lung condition rapidly worsened and he died.
Administration of 2-PAM is contraindicated for cases of carbaryl
poisoning, as it may increase toxicity rather than reactivate the
inhibited enzyme and, in this case, it was concluded that the fatal
outcome, though unavoidable, was hastened by 2-PAM administration.
Reich & Welke (1966) reported a fatal case of mexacarbate
poisoning involving a 17-year-old nursery employee who ingested
approximately 55 g mexacarbate in the form of a 22% formulation.
The young man, found unconscious, had pinpoint pupils and an
irregular heartbeat. Emergency procedures at the hospital briefly
restored regular heart rhythm, but brachycardia developed later
with recurrent heart failure. The patient died about 4 - 4 1/2 h
after the ingestion of the mexacarbate.
Two severe cases of propoxur poisoning following ingestion of
150 - 200 ml of commercial Baygon formulation were described by
Bomirska & Winiarska (1972). One individual responded to treatment
and recovered but the other died after failing to respond to
treatment with atropine and 2-PAM and toxogonin given
intravenously. A normal ChE suggested spontaneous reactivation of
this enzyme.
9.1.2 Effects of short- and long-term exposure
Propoxur was tested for its effectiveness in controlling the
mosquito vectors of malaria in Iran. The insecticide was sprayed
inside houses in a 300 km2 area in which 11 000 people lived in 33
villages. Sixty-nine out of the 11 000 inhabitants complained of
mild cholinergic symptoms, when they entered their houses during
the spraying, contrary to instructions. The symptoms were
headache, nausea, sweating, and vomiting. The symptoms subsided in
2 - 3 h. Similar experiences were reported in other villages
sprayed with propoxur or with carbaryl (Vandekar et al., 1968).
9.1.3 Controlled human studies
Human volunteers tolerated single oral doses of aqueous
solutions of aldicarb at doses of 0.025, 0.05, or 0.1 mg/kg body
weight, with only mild depression of whole blood-ChE in all cases.
Only the persons receiving 0.1 mg/kg presented symptoms of acute
cholinergic stress (i.e., nausea, sweating, pinpoint pupils,
salivation) (FAO/WHO, 1982b).
Groups of male volunteers (5 or 6 per group) were administered
daily doses of 0.06 and 0.13 mg carbaryl/kg body weight, orally,
for 6 weeks. No subjective or objective changes were observed,
except a slight reversible decrease in the ability of the kidneys
to reabsorb aminoacids in the group at the highest dose level
(Wills et al., 1968).
Thirty-eight urban volunteers were monitored for carbaryl
exposure during the summer. All volunteers were involved in the
application of carbaryl incidental to their employment or leisure
activities. Exposure of clothing, skin, with and without gloves,
were measured. The maximum dermal exposure recorded was 2.86 mg/kg
per h, the maximum air concentration was 0.28 mg/m3. Only a small
mean decrease in serum- and erythrocyte-AChE activity was found
(Gold et al., 1982). Comparable results were obtained by Leavitt et
al. (1982) in a study on 2 groups of professional pesticide spray
operators with higher dermal exposure, spraying trees with
carbaryl.
A 42-year-old male volunteer took a single oral dose of 135 mg
propoxur (1.5 mg/kg body weight) about 2 h after breakfast.
Plasma- and erythrocyte-ChE activities were determined every 15 min
for the first 2 h after ingestion and then every hour thereafter.
Pulse and blood pressure were recorded. A rapid drop in
erythrocyte-ChE activity was observed, reaching the lowest level of
27% of normal within 20 min. ChE activity gradually recovered,
reaching normal levels about 2 h after ingestion. Plasma-ChE
activity was not affected. In another study (single dose of 0.36
mg/kg body weight), the lowest erythrocyte-ChE activity occurred
within 10 min together with short-lasting stomach discomfort,
blurred vision, nausea, and facial perspiration. Twenty min after
ingestion, the volunteer's pulse rate and blood pressure were
increased. Pronounced nausea accompanied by repeated vomiting and
profuse sweating occurred until approximately 45 min after the
ingestion of propoxur. From then on, poisoning symptoms
diminished, blood pressure and pulse returned to pre-ingestion
values. The disappearance within about 2 h of the poisoning
symptoms was in line with the return of normal erythrocyte-ChE
activity. Forty-five percent of the ingested dose of propoxur was
excreted as the metabolite, 2-(1-methylethyl)phenol, within 24 h of
ingestion (Vandekar et al., 1971; FAO/WHO, 1974b).
In another study, 5 doses of propoxur, at 0.15 or 0.20 mg/kg
body weight, were administered to volunteers at 1/2-h intervals.
In this study, a symptomless depression of erythrocyte-ChE to 60%
of normal was observed over the course of the dosing. The enzyme
activity recovered to normal levels soon after termination of the
dosing. From the above studies, it was concluded by the authors
that an oral dose of 0.36 mg propoxur/kg body weight may be
sufficient to induce initial cholinergic symptoms, while larger
doses may be tolerated without symptoms if the administration is
gradually (divided doses) (Vandekar et al., 1971).
9.2 Occupational Exposure
9.2.1 Acute toxicity: poisoning incidents
Tobin (1970) reported 3 cases of persons poisoned with
carbofuran. Two were formulation plant employees, preparing 10%
granules and the third was an entomologist who began to feel
uncomfortable while weighing a 50% water-dispersible powder
formulation. The 2 formulators developed similar symptoms
indicative of carbamate poisoning: profuse sweating, weakness,
blurred vision, and nausea. Both were taken to their respective
physicians about 3 h after the onset of symptoms. One physician
administered atropine; the other, noting the regression of the
symptoms, did not administer atropine. The patient who received
the atropine (0.02 g im) was fully recovered in 30 min. The
untreated patient recovered over the course of 2 - 3 h. The third
case, the entomologist experienced mild discomfort that regressed
without atropine administration in 4 - 6 h.
Richardson & Batteese (1973) described the case of a spray-
plane co-pilot overexposed to mexacarbate during an spraying
operation. The cause of the overexposure was a pin-hole leak in a
high pressure pump line, which emitted a fine aerosol of the
mexacarbate formulation into the fuselage. The co-pilot developed
the classical signs of ChE poisoning. Toxic symptoms progressed to
paralysis of the extremities before he could be treated. In
hospital, atropine sulfate was immediately administered
intravenously followed by a second injection subcutaneously 40 min
later. The symptoms subsided quickly. ChE activity in blood drawn
after the first atropine injection was severely depressed, but
returned to normal after 3 days.
Minor and transient cholinergic symptoms were experienced by
spraymen applying propoxur formulations inside houses for testing
this insecticide against mosquito vectors of malaria in villages in
El Salvador (Quinby & Babione, 1966) and Iran (Vandekar et al.,
1968; Montazemi, 1969). A pronounced fall in blood-ChE activity
during the work and a distinct recovery after exposure ceased was
established as a daily pattern of activity of this enzyme,
erythrocyte-ChE being more sensitive to propoxur than plasma-ChE.
No cumulative inhibition of ChE during the 6-week exposure was
seen. The investigators noted that the exposed spraymen and a few
inhabitants recovered rapidly when removed from the source of
exposure and when treated with atropine.
Vandekar et al. (1968) commented that most spraymen who became
poisoned had heavy skin contamination, and had not cleaned their
skin sufficiently after spraying. The inhabitants entered the
house while it was being sprayed. Moreover, it was noted that
respirators did not offer protection against intoxication under
these circumstances and that dermal absorption accounted for most
of the absorbed dose.
No adverse health effects due to carbaryl exposure (except for
one case of skin rash) were observed in a study conducted by the
WHO Insecticide Testing Unit in Southern Nigeria. Ten men (out of
105 sprayers) spraying over a single 6-h period and 95 villagers
who resided in the sprayed houses, were examined. The sprayers
wore overalls, hats, and rubber boots during the entire 6 h of
spraying, but wore masks for the first hour only. Plasma-ChE
activity as well as urinary metabolites of carbaryl were determined
before and after spraying. A slight (average 5%) reduction in
plasma-ChE activity was reported for all 10 sprayers, the day after
the carbaryl application. Inhabitants of sprayed houses showed no
inhibition of plasma-ChE activity, 1 week after the spraying. A
slight increase in 1-naphthol was found in the urine of the
sprayers only on the sixth day after the exposure (Vandekar, 1965).
In a study on 19 agricultural workers in the USSR, whole blood-
ChE activity was measured before and after 4 to 6-h exposures to
airborne carbaryl for 3 - 4 days. Significant ChE inhibition was
found in men exposed to a mean airborne carbaryl concentration of
up to 4 mg/m3. No objective signs of ill health were observed. No
changes were measured at 0.7 mg/m3 (Yakim, 1967).
A case of acute intoxication of a woman with a total dose of 60
mg carbofuran was described by Izmirova et al. (1981). Slight ChE
inhibition was found, but within 72 h the patient recovered
completely.
The above reports identify systemic ChE poisoning as the major
hazard associated with occupational exposure to carbamate
pesticides. However, other publications indicate that certain of
these compounds can also irritate the exterior membranes of the
body. Tobin (1970) described 2 cases of field workers that
illustrate direct effects on the eye, (irritation and blurred
vision) during carbofuran spraying operations. These 2 persons did
not developed symptoms of systemic poisoning.
Vandekar (1965) reported skin rash in a sprayman accidentally
splashed with a carbaryl formulation. Although carbamate compounds
have not generally been implicated as the cause of dermatitis or
allergic skin reactions, Vandekar (1965) suggested that certain
individuals could develop this after unusually heavy exposure.
9.2.2 Effects of short- and long-term exposure
Little is known about the short- and long-term effects of
occupational exposure to carbamate pesticides. The few reports
that discuss this aspect do not implicate long-term exposure to the
carbamate compounds as a cause of human disease. Vandekar et al.
(1968) did not observe any evidence of cumulative toxicity among
spraymen applying propoxur formulations during a 6-week operation.
No long-term biochemical changes were reported among New Jersey
farmers and spraymen compared with a minimally exposed control
group. It was noted that significantly higher incidences of
hearing and eye problems, chronic cough, dizziness, headaches, and
hypertension occurred in the occupationally exposed group.
However, the various pesticides to which this group was exposed
were not tabulated (Kwalick, 1971).
Chromosome studies on the blood cultures of 20 workers engaged
in the production of benomyl did not reveal any increased frequency
of structural chromosome aberrations (Ruzicska et al., 1975).
In a survey of occupationally acquired disease in workers at a
pesticide plant, 11 out of 102 workers had been hospitalized for
illness related to chemical exposure. The commonest cause of
hospitalization was intoxication with the ChE inhibitor methomyl.
On clinical evaluation, 5 out of 11 packaging workers, with the
highest exposure to methomyl had experienced blurred vision or
pupillary constriction (Morse & Baker, 1979).
9.2.3 Epidemiological studies
Epidemiological studies on persons occupationally exposed
exclusively (or at least primarily) to carbamate pesticides are not
available. Such studies are needed before the long-term effects of
this class of pesticides on human beings can be adequately
evaluated.
9.3 Signs and Symptoms of Acute Intoxication by Carbamates
The clinical picture of carbamates intoxication results from
accumulation of ACh at nerve endings. Extensive description of
the syndrome is given in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980). The signs and symptoms can be
categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases,
diaphragm and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by the respiratory failure sometimes
leading to pulmonary oedema due to the combination of the above-
mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure;
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to light; pulse rate is not of
diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ;
on the other hand, since changes in the conduction
and excitability of the heart might be
life-threatening, monitoring should be performed.
(c) confirmation of diagnosis is made by measurement of
AChE in RBC or plasma-pseudoChE (dibucaine number
also, to rule out genetic deficiencies). In view of
the rapid reversibility, particular care should be
taken to use analytical procedures that include
immediate analysis and a careful control of sample
dilutions (section 2.3). Chemical analysis of body
fluids (gastric lavage, blood, urine) should be
performed for the identification of the chemical(s).
9.3.1 Biochemical methods for measurement of effects
AChE present in human erythrocytes is the same as the enzymes
present in the target synapses. Measurements of AChE in RBC is
therefore taken to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the carbamate has equal access to blood and the
synapses, and that AChE inhibition by carbamates is reversible.
In the case of acute poisoning, a high inhibition of RBC-AChE
is pathognomonic, but, in the follow-up of the intoxication, it
might not be correlated with the severity of symptoms. However,
monitoring of pre- and post-exposure levels of AChE in RBC gives a
good measure of the effect of an exposure (Kaloyanova, 1976). In
cases when the pre-exposure AChE level is not known (as in
accidental poisoning), reference can be made to a mean population
AChE activity. Blood-plasma contains a related enzyme called ChE or
pseudo-ChE, which contributes to the whole blood enzymatic
activity; the extent of the contribution of plasma-ChE will depend
on which substrate was used and at what concentration. PseudoChE
has no known physiological function, but can be inhibited
selectively by some carbamates without causing a toxic response.
The sensitivities of AChE and pseudoChE to inhibitors differ so
that measurements of the ability of whole blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many field conditions, procedures using whole blood are more
practical than those using separated erythrocytes.
9.4 Treatment of Acute Poisoning by Carbamate Insecticides
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977; Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
9.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in the cleaning of the skin area where
venupuncture is performed. Blood might be contaminated with
carbamates and therefore inaccurate measures of ChE inhibition
might result. Extensive eye irrigation with water or saline should
also be performed. In the case of ingestion, vomiting can be
induced, if the patient is conscious, by the administration of
ipecacuanha syrup (10 - 30 ml) followed by 200 ml of water.
However, this treatment is contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
the addition of bicarbonate solution or activated charcoal) can
also be performed, particularly in unconscious patients, taking
care to prevent aspiration of fluids into the lungs (i.e., only
after a tracheal tube has been put in place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
9.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
9.4.3 Specific pharmacological treatment
9.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.).
9.4.3.2 Oxime reactivators
There is no rational basis for using these drugs. Furthermore,
some unconfirmed reports suggest an increased toxicity of
carbamates when oximes have been administered.
9.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of
artificial respiration procedures.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
For more than 20 years, the Joint FAO/WHO Meetings on Pesticide
Residues (JMPR) and the International Agency for Research on Cancer
(IARC) have been evaluating the toxicity and carcinogenicity data
on the different carbamates. As part of the JMPR meeting, the FAO
deals with the use and application of these pesticides and
proposes residue limits. Data and conclusions of the meetings were
initially published in the WHO Technical Report Series but, since
1977, they have been published regularly in the FAO Plant
Production and Protection Papers.
It is not possible to review completely the results of the
discussions on the approximately 50 carbamates mentioned in these
documents. However, Annex III contains a list of the JMPR meetings
in which carbamates have been evaluated together with appropriate
references. Other information available in Annex III indicates
whether IARC evaluations and WHO/FAO Data Sheets are available, and
whether an IRPTC data profile and legal file exist.
REFERENCES
ABDEL-AAL, Y.A.I. & FAHMY, M.A.H. (1977) Molecular
modification of carbamates in relation to their selective
toxicity. In: Proceedings of the British Crop Protection
Conference on Pests and Diseases, Brighton, Nov. 1977, British
Crop Protection Council, Croydon, pp. 155-159.
ABD-ELROAF, T.K., FAHMY, M.A.H., FUKUTO, T.R., & EL-SEBAE,
A.H. (1977) Anticholinesterase activity and toxicity of
substituted phenyl methylcarbamates to honey bee. J. econ.
Entomol., 70(1): 78-82.
ABDEL-WAHAB, A.M., KUHR, R.J., & CASIDA, J.E. (1966) Fate of
14C-carbonyl-labelled aryl methylcarbamate insecticide
chemicals in and on bean plants. J. agric. food Chem., 14:
290-298.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1973) The
photochemical reactions of carbamates. III. The solution
photochemistry of metacrate, 3-methylphenyl- N -methyl-
carbamate. Int. J. environ. anal. Chem., 3: 73-79.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1974) The solution
photochemistry of metacil (4-dimethyl-amino- m -tolyl- N -methyl
carbamate) and Landrin (3,4,5-trimethylphenyl- N -methyl-
carbamate). Bull. environ. Contam. Toxicol., 11: 250-255.
AHDAYA, S.M., MONROE, R.J., & GUTHRIE, F.E. (1981)
Absorption and distribution of intubated insecticides in
fasted mice. Pestic. Biochem. Physiol., 16(1): 38-46.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarba-
mates, and dithiocarbamates, Luxembourg, Commission of the
European Communities.
ALDRIDGE, W.N. & REINER, E. (1972) Enzyme inhibitors as
substrates. Interaction of esterases with esters of
organophosphorus and carbamic acids, Amsterdam, Oxford, New
York, Elsevier Science Publishers, 328 pp.
ALY, O.M. & EL-DIB, M.A. (1971) Photodecomposition of some
carbamate insecticides in aquatic environments. In: Faust,
S.D. & Hunter, J.V., ed. Organic compounds in aquatic
environments, New York, Marcel Dekker, pp. 469-493.
ALY, O.M. & EL-DIB, M.A. (1972) Studies of the persistence
of some carbamate insecticides in the aquatic environment. In:
Fate of organic compounds in the aquatic environment. III.
Advances in chemistry series, Washington DC, American Chemical
Society, pp. 210-243.
ANDERSON, C.A. (1973) Baygon. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7, pp.
163-178.
ANDERSON, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDRIANOVA, M.M. & ALEKSEEV, I.V. (1970) [On the carcino-
genous properties of the pesticides sevine, maneb, ciram and
cineb.] Vopr. Pitan., 29(6): 71-74 (in Russian, with English
summary).
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974a) Effects of the
insecticide carbaryl on clams and some other intertidal mud
flat animals. J. Fish. Res. Board Can., 31: 466-470.
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974b) Effects of the
insecticide Sevin and its first hydrolytic product 1-naphthol
on some early developmental stages of the bay mussel Mytilus
edulis. Mar. Biol., 28: 11-15.
ASHTON, F.M. & CRAFTS, A.S. (1973) Carbamates. In: Mode of
action of herbicides, New York, Wiley Interscience, pp.
200-220.
ATKINS, E.L., GREYWOOD, E.A., & MCDONALD, R.L. (1973)
Toxicity of pesticides and other agricultural chemicals to
honey bees. Laboratory studies, Riverside, USA, University of
California, Agriculture Extension Service.
AUGUSTINSSON, K.B. & CASIDA, J.E. (1959) Enzymic hydrolysis
of N,N -dimethylcarbamoyl fluoride. Biochem. Pharmacol., 3:
60-67.
AUSTIN D.J. & BRIGGS, G.G. (1976) A new extraction method
for benomyl residues in soil and its application in movement
and persistence studies. Pestic. Sci., 7: 201-210.
AUSTIN, D.J., LORD, K.A., & WILLIAMS, I.H. (1976) High
pressure liquid chromatography of benzimidazoles. Pestic.
Sci., 7: 211-222.
BAKER, P.B. & HOODLESS, R.A. (1974) Analytical methods for
the detection and determination of residues of systemic
fungicides. Pestic. Sci., 5: 465-472.
BALDWIN, R.E., FREED, V.H., & FANG, S.C. (1954) Absorption
and translocation of carbon-14-applied as O -isopropyl
N -phenylcarbamate in Avena and Zea. J. agric. food Chem.,
2(8): 428-430.
BERAUD, M., PIPY, B., DERACHE, R., & GAILLARD, D. (1979)
Formation d'un cancérigène le N -nitrosocarbaryl par
interactions entre un insecticide de la série des carbamates
le carbaryl et le nitrite de sodium dans le suc gastrique de
rat. Food Cosmet. Toxicol., 17: 579-583.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of
pesticides in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BLACK, A.L., CHIU, Y.C., FAHMY, M.A.H., & FUKUTO, T.R.
(1973) Selective toxicity of N -sulfenylated derivatives of
insecticidal methylcarbamate esters. J. agric. food Chem., 21:
743-747.
BLEIDNER, W.E., MORALES, R., & HOLT, R.F. (1978) Benomyl.
In: Zweig, G. & Sherma, J., ed. Analytical methods for
pesticides and plant growth regulators, New York, London,
Academic Press, pp. 157-171.
BLEVINS, R.D., LYINSKY, W., & REGAN, J.D. (1977) Nitrosated
methylcarbamate insecticides. Effect on the DNA of human
cells. Mutat. Res., 44: 1-7.
BOBIK, A., HOLDER, G.M., & RYAN, A.J. (1972) Excretory and
metabolic studies of isopropyl N -(3-chlorophenyl)carbamate in
the rat. Food Cosmet. Toxicol., 10: 163-170.
BOMIRSKA, T. & WINIARSKA, A. (1972) [Toxicology of
carbamates in the light of intoxication with Baygon
insecticide.] Pol. Tyg. Lek., 27: 1448-1450 (in Polish).
BORZSONYI, M. & CSIK, M. (1975) Induction of malignant
lymphomas in Swiss mice by N -nitroso compounds formed in vivo.
Int. J. Cancer, 15: 830-838.
BORZSONYI, M. & PINTER, A. (1977) The carcinogenicity of
N -nitroso compounds formed endogenously in mice from
benzimidazole carbamate pesticides. Neoplasma, 24: 119-122.
BOWMAN, M.C. & BEROZA, M. (1969) Determination of Mesurol
and five of its metabolites in apples, pears and corn by gas
chromatography. J. Assoc. Off. Anal. Chem., 52: 1054-1063.
BROCKELSBY, C.H. & MUGGLETON, D.F. (1973) Asulam. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 497-508.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARPENTER, C.P., WEIL, C.S., PALM, P.E., WOODSIDE, M.W., NAIR,
J.H., & SMYTH, H.F. (1961) Mammalian toxicity of 1-naphthyl
N -methylcarbamate (Sevin insecticide). J. agric. food Chem.,
9: 30-39.
CARTER, S.D. & LASKEY, J.W. (1982) Effect of benomyl on
reproduction in the male rat. Toxicol. Lett., 11: 87-94.
CASIDA, J.E. & AUGUSTINSSON, K.B. (1959) Reaction of plasma
albumin with 1-naphthyl N -methylcarbamate and certain other
esters. Biochem. Biophys. Acta, 36: 411-426.
CHIBA, M. & DOORNBOS, F. (1974) Instability of benomyl in
various conditions. Bull. environ. Contam. Toxicol., 11:
273-274.
CHIN, W.T., DUANE, W.C., SZALKOWSKI, M.B., & STALLARD, D.E.
(1975) Gas-chromatographic determination of thiofanox
residues in soil, plants, and water. J. agric. food Chem.,
23(5): 963-966.
CLARK, C.G. & WRIGHT, S.J.L. (1970) Degradation of the
herbicide isopropyl N -phenylcarbamate by Arthrobacter and
Achromobacter sp. from soil. Soil Biol. Biochem., 2: 217.
COLLINS, T.F.X., HANSEN, W.H., & KEELER, H.V. (1971) The
effect of carbaryl (Sevin) on reproduction of the rat and the
gerbil. Toxicol. appl. Pharmacol., 19: 202-216.
CROSBY, D.G., LEITIS, E., & WINTERLIN, W.L. (1965)
Photodecomposition of carbamate insecticides. J. agric. food
Chem., 13(3): 204-207.
DASSENOY, B. & MEYER, J.A. (1973) Mutagenic effect of
benomyl on Fusarium oxysporum. Mutat. Res., 21: 119-120.
DAWSON, J.A., HEATH, D.F., ROSE, J.A., THAIN, E.M., & WARD,
J.B. (1964) The excretion by humans of the phenol derived in
vivo from 2-isopropoxyphenyl- N -methylcarbamate. Bull. World
Health Org., 30: 127-134.
DELATOUR, P. & RICHARD, Y. (1976) Propriétés embryotoxiques
et antimitotiques en série benzimidazole. Therapie, 31(4):
505-515.
DE ROSE, H.R. (1951) Crabgrass inhibition with O -isopropyl-
N -(3-chlorophenyl)carbamate. Agron. J., 43: 139-142.
DIKSHITH, T.S.S., GUPTA, P.K., GAUR, J.S., DATTA, K.K., &
MATHUR, A.K. (1976) Ninety-day toxicity of carbaryl in male
rats. Environ. Res., 12: 161-170.
DOROUGH, H.W. & CASIDA, J.E. (1964) Nature of certain
carbamate metabolites of the insecticide Sevin. J. agric. food
Chem., 12(4): 294-304.
DOUCH, P.G.C. (1973) The metabolism of benomyl fungicide in
animals. Xenobiotica, 3: 367-380.
EBERLE, D.O. & GUNTHER, F.A. (1965) Chromatographic,
spectrometric, and irradiation behaviour of five carbamate
insecticides. J. Assoc. Off. Agric. Chem., 48:(5) 927-937.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the
soil fauna. Residue Rev., 45: 1-79.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N -nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under stimulated gastric conditions and in vivo in the
rat stomach. Food Cosmet. Toxicol., 12: 229-232.
EISENBRAND, G., IVANKOVIC, S., PREUSSMANN, R., SCHMAHL, D., &
WIESSLER, M. (1975a) Some recent results on the chemistry,
formation and biological activity of N -nitroso compounds. Gaun
Monogr. Cancer Res., 17: 133-143.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1975b) The
reaction of nitrite with pesticides. II. Formation, chemical
properties, and carcinogenic activity of the N -nitroso
derivative of N -methyl-1-naphthylcarbamate (carbaryl). Food
Cosmet. Toxicol., 13: 365-367.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ELESPURU, R.K., LIJINSKY, W., & SETLOW, J.K. (1974)
Nitrosocarbaryl as a potent mutagen of environmental
significance. Nature (Lond.), 247: 386-387.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V. Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
FANG, S.C., FALLIN, E., MONTGOMERY, M.L., & FREED, V.H.
(1974) Metabolic studies of 14C-labelled propham and
chlorpropham in the female rat. Pestic. Biochem. Physiol., 4:
1-11.
FAHRIG, R. & SEILER, J.P. (1979) Dose and effect of
methyl-2-benzimidazolylcarbamate in the "mammalian spot test",
an in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-biol. Interact., 26: 115-120.
FAO (1985) Production yearbook, 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23.64).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Agricultural Studies No. 73; WHO Technical
Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391.
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
10 Rev).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Suppl.).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Suppl.).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Suppl.).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1985a) 1983 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985c) 1984 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Pesticide residues in food. Report of the
1985 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (in press) 1985 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations.
FARAGO, A. (1969) [Fatal suicidal poisoning with Sevin
(1-naphthyl- N -methyl carbamate).] Arch. Toxikol., 24: 309-315
(in German).
FICSOR, G., BORDAS, S., & STEWART, S.J. (1978) Mutagenicity
testing of benomyl methyl-2-benzimidazole carbamate,
streptozotocin, and N -methyl- N' -nitro- N' -nitrosoguanidine in
Salmonella typhimurium in vitro and in rodent host-mediated
assays. Mutat. Res., 51: 151-164.
FINNISH NATIONAL BOARD OF HEALTH TOXICOLOGY GROUP (1982) The
toxicity of the benzimidazol type fungicides: benomyl,
carbendazim, and thiophanate-methyl (second report), Helsinki,
Finnish National Board of Health.
FILIP, Z. (1974) [Pesticides in powder form.] Vesmir, 53:
243-244 (in Czech).
FUKUTO, T.R. (1972) Metabolism of carbamate insecticides.
Drug Metab. Rev., 1: 117-150.
GARDINER, J.A., KIRKLAND, J.J., KLOPPING, H.L., & SHERMAN, H.
(1974) Fate of benomyl in animals. J. agric. food Chem.,
22(3): 419-427.
GHADIRI M. & GREENWOOD, D.A. (1966) Toxicity and biologic
effects of malathion, phosdrin, and sevin in the chick embryo.
Toxicol. appl. Pharmacol., 8: 342.
GHADIRI M., GREENWOOD, D.A., & BINNS, W. (1967) Feeding of
malathion and carbaryl to laying hens and roosters. Toxicol.
appl. Pharmacol., 10: 392.
GOLD, R.E., LEAVITT, J.R.C., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of urban applications to carbaryl. Arch. environ.
Contam. Toxicol., 11: 63-67.
GOLDBERG, M.E., JOHNSON, H.E., KNAAK, J.B., & SMYTH, H.F., Jr
(1963) Psychopharmacological effects of reversible
cholinesterase inhibition induced by N -methyl-3-isopropyl-
phenyl carbamate (compound 10854). J. Pharm. exp. Ther., 141:
244-252.
GRAY, R.A. (1971) Behaviour, persistence, and degradation of
carbamate and thiocarbamate herbicides in the environment. In:
Proceedings of the California Weed Control Conference, 18-20
January 1971, Mountain View, California, Stauffer Western
Research Centre, pp. 128-143.
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1977) Determination
of carbetamid residues and its aniline metabolite. J. agric.
food Chem., 20: 348-350.
GUPTA, A.K. & LEGATOR, M.S. (1975) Chromosome aberrations in
cultured human leukocytes after treatment with the fungicide
benlate. In: Proceedings of a Symposium on Mutagenic
Carcinogenic and Teratogenic Chemistry, New Delhi, India,
Department of Atomic Energy, pp. 95-103.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained
sheepshead minnows. Trans. Am. Fish. Soc., 98: 426-429.
HARVEY, J., Jr & HAN, J.C.Y. (1978a) Metabolism of oxamyl
and selected metabolites in the rat. J. agric. food Chem.,
26(4): 902-910.
HARVEY, J., Jr & HAN, J.C.Y. (1978b) Decomposition of oxamyl
in soil and water. J. agric. food Chem., 26(3): 536-541.
HARVEY, J., Jr & PEASE, H.L. (1973) Decomposition of
methomyl in soil. J. agric. food Chem., 21(5): 784-786.
HARVEY, J., Jr & REISER, R.W. (1973) Metabolism of methomyl
in tobacco, corn, and cabbage. J. agric. food Chem., 21(5):
775-783.
HARVEY, J., Jr, JELINEK, A.G., & SHERMAN, H. (1973)
Metabolism of methomyl in the rat. J. agric. food Chem.,
21(5): 769-775.
HARVEY, J., Jr, HAN, J.C.Y., & REISER, R.W. (1978)
Metabolism of oxamyl in plants. J. agric. food Chem., 26(3):
529-536.
HEYBROEK, W.M.H., MUGGLETON, D.F., & PARKE, D.V. (1984)
Metabolism of the carbamate herbicide asulam in the rat.
Xenobiotica, 14:(3) 235-247.
IARC (1976) Some carbamates, thiocarbarmates, and
carbazides, Lyons, International Agency for Research on Cancer
(IARC Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 12).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 30;
Appendix 359-406).
INNES, J.R.M., ULLAND, B.M., VALLERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, J., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1976) Changes in the
activity of some enzymes under the action of benomyl. Hig. i
Zdraveopazvane, Sofia, 19(5): 431-435 (in Bulgarian, with
English summary).
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads, reproduction, and generations of white rats under the
effect of some pesticides. Arch. Toxicol., 4(Suppl.): 459-462.
IVANOVA-CHEMISHANSKA, L., ANTOV, G., MIHAILOVA, A., & HINKOVA,
L. (1980) Study on the toxicology of benomyl. In: The Fourth
International Congress of Pesticide Chemistry, Zurich,
Switzerland, 24-28 July, 1978, pp. 11-559.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorous pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application, Amsterdam, Oxford, New
York, Elsevier Scientific Publishers, pp. 169-172 (Studies in
Environmental Science 7).
IZMIROVA, N., MILCEVA, V., MONOV, A., & KALOYANOVA, F.
(1981) [Acute carbofuran intoxication.] Hig. i
zdraveopazvane, 24(5): 445-448 (in Bulgarian, with English
summary).
JACKSON, J.A., CHART, I.S., SANDERSON, J.H., & GARNER, R.
(1977) Pirimicarb induced immune haemolytic anaemia in dogs.
Scand. J. Haematol., 19: 360-366.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of brain "neurotoxic esterase" and the development of delayed
neurotoxicity in hens. Biochem. J., 120: 523-531.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute
toxicity of chemicals to fish and aquatic invertebrates,
Washington DC, US Fish and Wildlife Service (Resource
Publication No. 137).
KAGAN, J.S. (1977) [The toxicity of organophosphorus
pesticides,] Moscow (in Russian).
KALOYANOVA, F. (1976) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Symposium on
Environmental Monitoring, 14-19 September, 1975, Las Vegas,
Nevada, Vol. 1, pp. 1-4, New York, Institute of Electrical &
Electronics Engineers, Inc.
KANAZAWA, J. (1975) Uptake and excretion of organophosphorus
and carbamate insecticides by fresh-water fish Motsugo
pseudoras bora parva. Bull. environ. Contam. Toxicol., 14:
346-352.
KAPLAN, A.M. & SHERMAN, H. (1977) Toxicity studies with
methyl- N -methylamino-carbonyl-oxyethanimidothioate. Toxicol.
appl. Pharmacol., 40: 1-17.
KAPPAS, A., GEORGOPOULOS, S.G., & HASTIE, A.C. (1974) On the
genetic activity of benzimidazole and thiophanate fungicides
on diploid Aspergillus nidulans. Mutat. Res., 26: 17-27.
KAPPAS, A., GREEN, M.H.L., BRIDGES, B.A., ROGERS, A.M., &
MURIEL, W.J. (1976) Benomyl: a novel type of base analogue
mutagen. Mutat. Res., 40: 379-382.
KAVLOCK, R.J., CHERNOFF, N., GRAY, L.E., Jr, GRAY, J.A., &
WHITEHOUSE, D. (1982) Teratogenic effects of benomyl in the
Wistar rat and CD-1 mouse, with emphasis on the route of
administration. Toxicol. appl. Pharmacol., 62: 44-54.
KAUFMAN, D.D. & BLAKE, J. (1973) Microbial degradation of
several acetamide, acrylamide, carbamate, toluidine, and urea
pesticides. Soil Biol. Biochem., 5: 297-308.
KAUFMAN, D.D. & KEARNEY, P.C. (1965) Microbial degradation
of isopropyl- N -(3-chlorophenyl carbamate and 2-chloroethyl- N -
(3-chlorophenyl carbamate. Appl. Microbiol., 13(3): 443-446.
KHERA, K.S. (1966) Toxic and teratogenic effects of
insecticides in duck and chick embryos. Toxicol. appl.
Pharmacol., 8: 345.
KIMMERLE, G. & IYATOMI, A. (1976) Toxicity of propoxur to
rats by subacute inhalation. Jpn J. ind. Health, 18: 375-382.
KNAAK, J.B., TALLANT, M.J., BARTLEY, W.Y., & SULLIVAN, L.J.
(1965) The metabolism of carbaryl in the rat, guinea pig and
man. J. agric. food Chem., 13(4): 537-543.
KNAAK, J.B., TALLANT, M.J., & SULLIVAN, L.J. (1966) The
metabolism of 2-methyl-2-(methylthio)propionaldehyde- O -
methylcarbamoyl)oxime in the rat. J. agric. food Chem., 14(6):
573-578.
KNAAK, J.B., TALLANT, M.J., KOZBELT, S.J., & SULLIVAN, L.J.
(1968) Metabolism of carbaryl in man, monkey, pig, and sheep.
J. agric. food Chem., 16(3): 465-470.
KNOWLES, C.O. & SONAWANE, B.R. (1972) Ethyl m- hydroxy-
carbanilate carbanilate (EP-475) metabolism in sugar beets.
Bull. environ. Contam. Toxicol., 8: 73-76.
KOSSMANN, K. & JENNY, N.A. (1973) Phenmedipham. In: Sherma,
J. & Zweig, G., ed. Analytical methods for pesticides and
plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 611-623.
KRECHNIAK, J. & FOSS, W. (1982) Cholinesterase activity in
rats treated with propoxur. Bull. environ. Contam. Toxicol.,
29(5): 599-604.
KUHR, R.J. (1968) Metabolism of methylcarbamate insecticide
chemicals in plants. J. Sci. Food Agric. 44, (Suppl.): 43-49.
KWALICK, D.S. (1971) Physician's pesticides primer. J. Med.
Soc. New Jersey, 68:(5) 375-377.
LAMB, M.J. & LILLY, L.J. (1980) An investigation of some
genetic toxicological effects of the fungicide benomyl.
Toxicology, 17: 83-95.
LEAVITT, J.R.C., GOLD, R.E., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of professional pesticide applicators to carbaryl.
Arch. environ. Contam. Toxicol., 11: 57-62.
LEELING, N.C. & CASIDA, J.E. (1966) Metabolites of carbaryl
(1-naphthyl methylcarmate) in mammals and enzymatic systems
for their formation. J. agric. food Chem., 14: 281-290.
LEITCH, R.E. & PEASE, H.L. (1973) Lannate-methomyl. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 331-338.
LYINSKY, W. & TAYLOR, H.W. (1976) Carcinogenesis in Sprague
Dawley rats of N -nitroso- N -alkylcarbamate esters. Cancer
Lett., 1: 275-279.
MAKITA, T., HASHIMOTO, Y., & NOGUCHI, T. (1973) Mutagenic,
cytogenetic, and teratogenic studies on thiophanate-methyl.
Toxicol. appl. Pharmacol., 24: 206-215.
MARLIAC, J.P. (1964) Toxicity and teratogenic effects of 12
pesticides in the chick embryo. Fed. Proc. Fed. Am. Soc. Exp.
Biol., 23: 105-126
MCCARTHY, J.F., FANCHER, O.E., KENNEDY, G.L., KEPLINGER, M.L.,
& CALANDRA, J.C. (1971) Reproduction and teratology studies
with the insecticide carbofuran. Toxicol. appl. Pharmacology,
19: 370.
METCALF, R.L., FUKUTO, T.R., COLLINS, C., BORCK, K., BURK J.,
REYNOLDS, H.T., & OSMAN, M.F. (1966) Metabolism in plant and
insect. Metabolism of 2-methyl-2-(methylthio)-propionaldehyde
O -methylcarbamoyl-oxime in plant and insect. J. agric. food
Chem., 14: 579-584.
MILLER, D.B. (1982) Neurotoxicity of the pesticidal
carbamates. Neurobehav. Toxicol. Teratol., 4: 779-787.
MIRVISH, H.S.S. (1968) The carcinogenic action and
metabolism of urethan and N -hydroxyurethan. Adv. Cancer Res.,
11: 1-42.
MONTAZEMI, K. (1969) Toxicological studies of Baygon
insecticide in Shabankareh area, Iran. Trop. geogr. Med., 21:
186-190.
MORSE, D.L. & BAKER, E.L., Jr (1979) Propanil-chloracne and
methomyl toxicity in workers of a pesticide manufacturing
plant. Clin. Tox., 15(1): 13-21.
MOSCIONI, A.D., ENGEL, J.L., & CASIDA, J.E. (1977)
Kynurenine formamidase inhibition as a possible mechanism for
certain teratogenic effect of organophosphorus and
methylcarbamate insecticides in chicken embryos. Biochem.
Pharmacol., 26: 2251-2258.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisonning due to organophosphate insecticies: acute and
chronic manifestations. Am. J. Med., 50: 475-492.
NATIONAL INSTITUTE OF HYGIENIC SCIENCES (1985) Letter to the
International Programme on Chemical Safety, 1.10.85, Tokyo,
Japan.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22: 16-18 (in
Japanese).
OGLE, R.E. & WARREN, G.F. (1954) Fate and activity of
herbicides in soils. Weeds, 3: 257-273.
OLEFIR, A.I. & VINOGRADOVA, V.Kh. (1968) [A study of the
embryotropic effect of the pesticides sevine and cyram.]
Vrach. Delo., 11: 103-106 (in Russian).
OLIVER, J.E. (1981) Pesticide-derived nitrosoamines.
Occurrence and environmental fate. In: Scanlan, R.A. &
Tannenbaum, S.T., ed. N-nitroso compounds, Washington DC,
American Chemical Society (ACS Symposium Series 174).
OONNITHAN, E.S. & CASIDA, J.E. (1968) Oxidation of methyl
and dimethylcarbamate insecticide chemicals by microsomal
enzymes and anticholinesterase activity of the metabolites. J.
agric. food Chem., 16: 28-44.
ORLOVA, N.V. & ZHALBE, E.P. (1968) Experimental material
contributive to the profile of maximal permissible amounts of
Sevin in food products. Vopr. Pitan., 27: 49-55 (in Russian).
PAROCHETTI, J.V. & WARREN, G.F. (1966) Vapour losses of IPC
and CIPC. Weeds, 14; 281-285.
PASTUSHENKO, T.B. (1983) [Study on the ganodotropic effects
of methyl N -2-benzimidazol carbamate aiming at evaluating its
effective concentrations.] Gig. i Sanit., 7: 83-85 (in
Russian).
PAULSON, G.D., JACOBSEN, A.M., ZAYLSKIE, R.G., & FEIL, V.J.
(1973) Isolation and identification of propham isopropyl-
carbanilate metabolites from the rat and goat. J. agric. food
Chem., 21: 804-811.
PETROVA-VERGIEVA, T. (1979) [Changes in the intrauterine
development of experimental animals under fundazol effect.]
Hig. Zdraveopaz., 22: 487-495 (in Bulgarian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization.
(Unpublished report VBC/84.889).
PRENDEVILLE, G.N., ESHEL, Y., JAMES, C.S., WAREN, G.F.E., &
SCHREIBER, M.M. (1968) Movement and metabolism of CIPC in
resistent and susceptible species. Weed Sci., 16: 432-435.
PROCTOR, N.H., MOSCIONI, A.D., & CASIDA, J.E. (1976) Chicken
embryo NAD levels lowered by teratogenic organophophorus and
methylcarbamate insecticides. Biochem. Pharmacol., 25: 757-762.
QUARLES, J.M. & TENNANT, R.W. (1975) Effects of nitroso-
carbaryl on BALB/3T3 cells. Cancer Res., 35: 2637-2645.
QUINBY, G.E. & BABIONE, R.W. (1966) Toxicological
observations on the use of Baygon (2-isopropoxyphenyl
N -methylcarbamate) as a malarial control insecticide in El
Salvador. Ind. Med. Surg., 35: 596-597.
QURAISHI, M.S. (1972) Edaphic and water relationships of
aldicarb and its metabolites. Can. Entomol., 104(3): 1191-1196.
REGAN, J.D., SETLOW, R.B., FRANCIS, A.A., & LYINSKY, W.
(1976) Nitrosocarbaryl: its effect on human DNA. Mutat. Res.,
38: 293-301.
REICH, G.A. & WELKE, J.O. (1966) Death due to a pesticide.
New Engl. J. Med., 274: 1432.
REINER, E. (1971) Spontaneous reactivation of phosphorylated
and carbamylated cholinesterases. Bull. World Health Org., 44:
109-112.
REINER, E. & ALDRIDGE, W.N. (1967) Effect of pH on
inhibition and spontaneous reactivation of acetylcholin-
esterase treated with esters of phosphoric acids and of
carbamic acids. Biochem. J., 105: 171-179.
REINER, E. & SKRINJARIC-SPOLJAR, M. (1968) Hydrolysis of
some monomethylcarbamates in human sera. Croat. Chem. Acta,
40: 87-90.
REINER, E., BUNTIC, A., TRDAK, M., & SIMEON, V. (1974)
Effect of temperature on the activity of human blood
cholinesterases. Arch. Toxicol., 32: 347-350.
RHODES, R.C. & LONG, J.D. (1974) Run-off and mobility
studies on benomyl in soils and turf. Bull. environ. Contam.
Toxicol., 12(4): 385-393.
RICHARDSON, E.M. & BATTEESE, R.I., Jr (1973) An incident of
Zectran poisoning. J. Maine Med. Assoc., 64: 158-159.
ROBEL, R.J., FELTHOUSEN, R.W., & DAYTON, A.D. (1982) Effects
of carbamates on Bobwhite food intake body weight and
locomotor activity. Arch. environ. Contam. Toxicol., 11:
611-615.
ROBENS, J.F. (1979) Teratologic Studies of Carbaryl,
Diazinon, Norea, Disulfiram, and Thiram in small laboratory
animals. Tox. Appl. Pharmacol., 15: 152-163.
ROMINE, R.R. (1973) Aldicarb. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7,
pp. 147-162.
ROSZKOWSKI, P. (1982) [Effect of the preparation propoxur on
intrauterine development and on the state of bone formation in
the progeny of the rat.] Ginek. Pol., 53(4): 201-207 (in
Polish).
ROTHSCHILD, E.R., MANSER, R.J., & ANDERSON, M.P. (1982)
Investigation of aldicarb in groundwater in selected areas of
the central sand plain of Wisconsin. Groundwater, 20(4):
437-445.
RUZICSKA, P., PETER, S., & CZEIZEL, A. (1975) Studies on the
chromosomal mutagenic effect of benomyl in rat and humans.
Mutat. Res., 29: 201.
RYBAKOVA, M.N. (1967) [Comparative toxic effects of sevin
and DDT used in the treatment of food crops.] Vopr. Pitan.,
26: 9-15 (in Russian).
RYBAKOVA, M.N. (1968) [Effect of some pesticides on the
hypophysis and its gonadotropic functions.] Gig. i Sanit.,
33(11): 27-31 (in Russian).
SAKAI, K. & MATSUMURA, F. (1968) Esterases of mouse brain
active in hydrolysing organophosphate and carbamate
insecticides. J. agric. food Chem., 16(5): 803-807.
SAKAI, K. & MATSUMURA, F. (1971) Degradation of certain
organophosphate and carbamate insecticides by human brain
esterases. Toxicol. appl. Pharmacol., 19(4): 660-666.
SEILER, J.P. (1972) The mutagenicity of benzimidazole and
benzimidazole derivatives. I. Forward and reverse mutations in
Salmonella typhimurium caused by benzimidazole and some of its
derivatives. Mutat. Res., 15: 273-276.
SEILER, J.P. (1975) Toxicology and genetic effects of
benzimidazole compounds. Mutat. Res., 32: 151-168.
SEILER, J.P. (1976a) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40: 339-347.
SEILER, J.P. (1976b) Inhibition of testicular DNA synthesis
by chemical mutagens and carcinogens. Preliminary result in
the validation of a novel short-term test. Mutat. Res., 46:
305-310.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method
for the determination of 1-naphthol in urine. Bull. environ.
Contam. Toxicol., 6: 34-39.
SHERMAN, H., CULIK, R., & JACKSON, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. appl.
Pharmacol., 32: 305-315.
SHTENBERG, A.I. & OZHOVAN, M.V. (1971) [Influence of low
Sevin doses on the reproductive function of animals in a
number of generations.] Vopr. Pitan., 30(1): 42-49 (in
Russian, with English summary).
SHTENBERG, A.I. & RYBAKOVA, M.N. (1968) Effect of carbaryl
on the neuroendocrine system of rats. Food Cosmet. Toxicol.,
6: 461-467.
SHTENBERG, A.I., ORLOVA, N.V., & TORCHINSKY, A.M. (1973)
[The action of different pesticides of different chemical
structure on the gonads and embryogenesis of experimental
animals.] Gig. i Sanit., 38(8): 16-20 (in Russian).
SIDEROFF, S.T. & SANTOLUCITO, J.A. (1972) Behavioural and
physiological effects of cholinesterase inhibitor carbaryl
(1-naphthyl methylcarbamate). Physiol. Behav., 9: 459-462.
SMALLEY, H.E., CURTIS, J.M., & EARL, F.L. (1968) Teratogenic
action of carbaryl in beagle dogs. Toxicol. appl. Pharmacol.,
13: 392-403.
SMALLEY, H.E., O'HARA, P.J., BRIDGES, C.H., & RADELEFF, R.D.
(1969) The effects of chronic carbaryl administration on the
neuromuscular system of swine. Toxicol. appl. Pharmacol., 14:
409-419.
SONAWANE, B.R. & KNOWLES, C.O. (1971) Phenmedipham and
m -aminophenol decomposition in alkaline soil. Bull. environ.
Contam. Toxicol., 6: 322-327.
SONAWANE, B.R. & KNOWLES, C.O. (1972) Comparative metabolism
of two carbanilate herbicides (EP-475 and phenmedipham) in
rats. Pestic. Biochem. Physiol., 1: 472-482.
STADNYK, L., CAMPELL, R.S., & JOHNSON, B.T. (1971) Pesticide
effect on growth and 14C assimilation in a fresh-water alga.
Bull. environ. Contam. Toxicol., 6: 1-8.
STANSBURY, H.A., Jr & MISKUS, R. (1964) In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators,
and food additives, New York, London, Academic Press, Vol. 2,
pp. 437-450.
STEGEMAN LE ROY, C. (1964) The effects of the carbamate
insecticide carbaryl upon forest soil mites and Collembola. J.
econ. Entomol., 57(6): 803-808.
STRINGER, A. & LYONS, C.H. (1974) The effects of benomyl and
thiophanate-methyl on earth worm populations; Apple Orchards
Pestic. Sci., 5: 189-195.
STILL, G.G. & HERRETT, R.A. (1975) Methylcarbamates,
carbanilates, and acylanilides. In: Kearney, P.C. & Kaufman,
D.D., ed. Herbicides, chemistry, degradation, and mode of
action. New York, Marcel Dekker, Vol. 2, pp. 609-664.
STILL, G.G. & MANSAGER, E.R. (1972) Metabolism of 4-chloro-
2-butynyl-3-chloro-carbanilate by soybean plants. J. agric.
food Chem., 20: 402-406.
STILL, G.G. & MANSAGER, E.R. (1973a) Soybean shoot
metabolism of isopropyl 3-chlorocarbanilate ortho and para
arylhydroxylation. Pestic. Biochem. Physiol., 3:: 87-95.
STILL, G.G. & MANSAGER, E.R. (1973b) Metabolism of isopropyl
carbanilate by soybean plants. Pestic. Biochem. Physiol., 3:
289-299.
STILL, G.G. & MANSAGER, E.R. (1975) Alfalfa metabolism of
propham. Pestic. Biochem. Physiol., 5: 515-522.
STILL, G.G. & RUSNESS, D.G. (1977) S -cysteinyl-hydroxychlor-
propham: formation of the S -cysteinyl conjugate of isopropyl-
3'-chloro-4'-hydroxy-carbanilate in oat (Avena savita L).
Pestic. Biochem. Physiol., 7: 210-219.
STRINGER, A. & WRIGHT, M.A. (1973) The effect of benomyl and
some related compounds on Lumbricus terrestris and other
earthworms. Pestic. Sci., 4: 165-170.
STYLES, J.A. & GARNER, R. (1974) Benzimidazole carbamate
methylester evaluation of its effect in vivo and in vitro.
Mutat. Res., 26:: 177-187.
SULLIVAN, L.J., ELDRIDGE, J.M., KNAAK, J.B., & TALLANT, M.J.
(1972) 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide as a
significant metabolite of carbaryl in the rat. J. agric. food
Chem., 20(5): 980-985.
SUZUKI, T. & TAKEDA, M. (1976) Microbial metabolism of
N -methylcarbamate insecticide I. Metabolism of O -sec-
butylphenyl N -methylcarbamate by Aspergillus niger van
Tieghem. Chem. Pharm. Bull., 24(9): 1967-1975.
SYPESTEYN, A.K., DEKHUYZEN, H.M., & VONK, J.W. (1977)
Biological conversion of fungicides in plants and
microorganisms, In: Siegel, M.R. & Sisler, H.D., ed.
Antifungal compounds, New York, Marcel Dekker, Vol. 2,
pp. 91-147.
TANNENBAUM, S.R., INSKEY, A.J., WEISMAN, M., & BISHOP, W.
(1974) Nitrite in human. Its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co., pp.
100-119.
TOBIN, J.S. (1970) Carbofuran a new carbamate insecticide.
J. occup. Med., 12: 16-19.
TOMLIN, A.D. & GORE, F.L. (1974) Effects of six insecticides
and a fungicide on the numbers of biomass of earthworms in
pasture. Bull. environ. Contam. Toxicol., 12(4): 487-492.
TORCHINSKY, A.M. (1973) [Significance of diets with
different protein content in manifestations of teratogenic and
embryotoxic action of some pesticides.] Vopr. Pitan., 3: 76-80
(in Russian).
TORCHINSKY, A.M., ORLOVA, N.V., SMIRNOVA, E.V., & MAKAROVA,
L.F. (1976) [Data for toxico-hygienic characteristics of the
pesticide Benlate of carbamate group.] Gig. i Sanit., 2: 35-39
(in Russian, with English summary).
UCHIYAMA, M., TAKEDA, M., SUZUKI, T., & YOSHIKAWA, K. (1975)
Mutagenicity of nitroso derivatives of N -methylcarbamate
insecticides in microbiological method. Bull. environ. Contam.
Toxicol., 14: 389-394.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) [The
reaction of nitrite with pesticides.] Z. Krebsforsch., 81:
217-224 (in German, with English summary).
VANDEKAR, M. (1965) Observations on toxicity of carbaryl,
folithion, and 3-isopropylphenyl- N -methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. World Health
Org., 33: 107-115.
VANDEKAR, M., HEDAYAT, S., PLESTINA, R., & AHMADY, G. (1968)
A study of the safety of O -isopropoxyphenyl methylcarbamate in
an operational field-trial in Iran. Bull. World Health Org.,
38: 609-623.
VANDEKAR, M., PLESTINA, R., & WILHELM, K. (1971) Toxicity of
carbamates for mammals. Bull. World Health Org., 44: 241-249.
VAN ESCH, G.J. & KROES, R. (1972) Long-term toxicity studies
of chlorpropham and propham in mice and hamsters. Food Cosmet.
Toxicol., 10: 373-381.
VAN ESCH, G.J., VAN GENDEREN, H., & VINK, H.H. (1958) The
production of skin tumours in mice by oral treatment with
urethane, isopropyl- N -phenylcarbamate, or isopropyl- N -chloro-
phenyl carbamate in combination with skin painting with croton
oil and Tween 60. Br. J. Cancer, 12: 355-362.
VASHAKIDZE, V.I. (1965) [Some questions of the harmful
action of sevin on the reproductive function of experimental
animals.] Soobshch. Akad. Nauk Gruz. SSR, 39: 471-478 (in
Russian).
VASHAKIDZE, V.I. (1967) [Mechanism of action of pesticides
(granosana, sevin, donoc) on the reproductive cycle of
experimental animal.] Soobshch. Akad. Nauk Gruz. SSR, 48:
219-224 (in Russian).
VASSILIEFF, I. & ECOBICHON, D.J. (1982) Stability of
Matacil(R) in aqueous media as measured by changes in
anticholinesterase potency. Bull. environ. Contam. Toxicol.,
29: 366-370.
VASSILIEFF, I. & ECOBICHON, D.J. (1983) Acute toxicity of
aminocarb in male rats and inhibition of tissue esterases.
Bull. environ. Contam. Toxicol., 31: 326-330.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. Vol. 1. Toxicity profiles, Boca Raton,
Florida, CRC Press, Inc., 232 p..
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticide
reference index, JMPR 1961-84, Geneva, World Health
Organization (Unpublished report).
VOSS, G. & SCHULER, J. (1967) A fast and simple procedure
for routine determination of plasma ChE activity. Bull.
environ. Contam. Toxicol., 2(6): 357-363.
WHO (1984) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-85, Geneva, World
Health Organization (Unpublished report VBC/84.2).
WHO/FAO (1984) Data sheets on pesticides, Geneva, World
Health Organization.
WEIL, C.S., WOODSIDE, M.D., CARPENTER, C.P., & SMYTH, H.F.,
Jr (1972) Current studies of tests of carbaryl for
reproductive and teratogenic effects. Toxicol. appl.
Pharmacol., 21: 390-404.
WEIL, C.S., WOODSIDE, M.D., BERNARD, J.B., CONDRA, N.I., KING,
J.M., & CARPENTER, C.P., (1973) Comparative effect of
carbaryl on rat reproduction and guinea-pig teratology when
fed either in the diet or by stomach intubation. Toxicol.
appl. Pharmacol., 26: 621-638.
WEIS, P. & WEIS, J.S. (1974) Schooling behaviour of Menidia
menidia in the presence of insecticide Sevin (carbaryl). Mar.
Biol., 28: 261-263.
WESTLAKE, G.E., BUNYAN, P.J., MARTIN, A.D., STANLEY, P.I., &
STEED, L.C. (1981) Carbamate poisoning. Effect of selected
carbamate pesticides on plasma enzymes and brain esterases of
Japanese quail (Coturnix coturnix japonica). J. agric. food
Chem., 29(4): 779-785.
WHITE, E.R., BOSE, E.A., OGAWA, J.M., MANJI, B.T., & KILGORA,
W.W. (1973) Thermal and base-catalysed hydrolysis products
of the systemic fungicide benomyl. J. agric. food Chem., 21:
616-618.
WIEDMANN, J.L.M., ECKE, G.G., & STILL, G.G. (1976) Synthesis
and isolation of 1-hydroxy-2-propyl-3-chloro-carbanilate from
soybean plants treated with isopropyl-3-chlorocarbanilate J.
agric. food Chem., 24: 588-592.
WILHELM, K. & REINER, E. (1973) Effect of sample storage on
human blood cholinesterase activity after inhibition by
carbamates. Bull. World Health Org., 48: 235-238.
WILHELM, K., VANDEKAR, M., & REINER, E. (1973) Comparison of
methods of measuring cholinesterase inhibition by carbamates.
Bull. World Health Org., 48: 41-44.
WILLIAMS, I.H., BROWN, M.J., & WHITEHEAD, P. (1976a)
Persistence of carbofuran residues in some British Columbia
soils. Bull. environ. Contam. Toxicol., 15(2): 242-243.
WILLIAMS, I.H., PEPIN, H.S., & BROWN, M.J. (1976b)
Degradation of carbofuran by soil microorganisms. Bull.
environ. Contam. Toxicol., 15(2): 244-249.
WILLS, J.H., JAMESON, E. & COULSTON, F. (1968) Effects of
oral doses of carbaryl on man. Clin. Toxicol., 1(3): 265-271.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., London, British Crop Protection
Council.
WRIGHT, M.A. & STRINGER, A. (1973) The toxicity of
thiabendazole, benomyl, methyl-benzimidazol-2-yl-carbamate,
and thiophanate-methyl to the earthworm Lumbricus terrestris.
Pestic. Sci., 4: 431-432.
WYROBEK, A.J., WATCHMAKER, G., GORDON, L., WONG, K., MOORE,
D.I., & WHORTON (1981) Sperm shape abnormalities in carbaryl
exposed employees. Environ. Health Perspect., 40: 255-265.
YAKIM, V.S. (1967) [Data for substantiating the maximum
permissible concentration of sevine in the air of working
zones.] Gig. i Sanit., 32: 29-33 (in Russian).
YOSHIDA, K. & NISHIUCHI, Y. (1972) [Toxicity of pesticides
to some water organisms.] Bull. agric. Chem. Inspect. Stn.
(Japan), 12: 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) [Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn.
(Japan),. 6: 65-69 (in Japanese).
ZAKI, M.H., MORAN, D., & HARRIS, D. (1982) Pesticides in
groundwater: the aldicarb story in Suffolk Country, New York.
Am. J. public Health, 72(12): 1391-1395.
Annex I. Names and structures and some physical and chemical properties of carbamate pesticides
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aldicarb Temik propanal, 2-methyl- C7H14N2O2S 190.29 13 mPa 0.6%
2-(methylthio)-,
O -[(methylamino)
carbonyl]oxime
(116-06-3)
Heybroek et al. (1984) studied the metabolism of [ring-14C]
asulam in the rat. Most of the radioactivity (61 -74%)
administered orally or intravenously was excreted in the urine in
24 h as unchanged asulam, 8 - 14% as N 4-acetylasulam, and up to
2.5% as N 4-acetylsulfanilamide. Small amounts of radioactivity
were found in faeces.
These examples illustrate the different metabolic pathways of
biotransformation. For more details, readers should refer to
Fukuto (1972) who has reviewed the metabolism of carbaryl,
mexacarbamate, aminocarb, formetanate, aldicarb, methiocarb,
carbofuran, MPMC, trimethacarb carbanolate, propoxur, and
dimetilan. Harvey & Han (1978a) have described the metabolism of
oxamyl and Harvey et al. (1973), of methomyl in the rat. The
metabolism of pirimicarb was extensively described in FAO/WHO
(1977b). Furthermore, information on the metabolism is provided in
the handbooks on carbamates, and the different monographs of the
Joint FAO/WHO Meetings on Pesticide Residues.
6.3 Elimination and Excretion in Expired Air, Faeces, and Urine
6.3.1 Man
In a study on human volunteers, 2 men were dosed orally with
carbaryl in gelatin capsules at 2.0 mg/kg body weight.
Chromatographic analyses of a 4-h urine sample revealed 1-naphthyl
glucuronide (15%), 1-naphthyl sulfate (8%), and 4-(methylcarbamoyl-
oxy)-1-naphthyl glucuronide (4%). In addition, 1-naphthyl
methylimidocarbonate O -glucuronide was identified by fluorometry
(FAO/WHO, 1968b). During the first 8 h, the urinary excretion of
these metabolites constituted 12 - 15% of the carbaryl
administered. Small amounts of urinary metabolites were detected
by fluorometric analysis on the second day, but not afterwards
(Knaak et al., 1968). In another study, 1-naphthyl glucuronide, 1-
naphthyl sulfate, and other unidentified metabolites were found in
24-h urine samples from men exposed to carbaryl dust during a
packaging operation in a factory (details not available) (Knaak et
al., 1965).
The determination of 1-naphthol or its sulfate or glucuronide
in the urine of persons occupationally exposed to carbaryl is one
method of biological monitoring for this exposure (FAO/WHO, 1982b).
Dawson et al. (1964) investigated the metabolism and excretion
of propoxur in 3 male volunteers orally administered a dose of 50
mg. Within 8 - 10 h, 27% of the propoxur appeared in the urine as
its metabolite 2-isopropoxyphenol, indicating that propoxur is
rapidly absorbed and hydrolysed in human beings.
The absorption and fate of benomyl in animals following oral
administration of the compound varies with species. According to a
study that involved one rat and one dog administered 2-14C-benomyl
by stomach tube, the rat eliminated about 86% of a single dose via
the urine and 13% via faeces. The dog eliminated only 16% of a
similar benomyl dose via the urine, while almost 83% was detected
in faeces. No data are available concerning the possible roles of
the enterohepatic circulation and biliary excretion of benomyl or
its metabolites (Gardiner et al., 1974).
6.3.2 Laboratory animals
In animals, oxidation of carbamates often, but not always,
results in detoxification. Oxidative metabolism generally leads to
products of greater polarity and water solubility, and these can be
more readily eliminated through the urine and faeces than the
parent compound.
In the rat, benomyl is mainly excreted via the urine (78.9% of
the given dose) as 5-HBC conjugates. In the dog, 99% of the dose
administered was eliminated within 72 h. The major route of
elimination was via the faeces in which benomyl and/or MBC and 5-
HBC were detected. On the basis of studies on the rat, dog, dairy
cow, and chicken, neither benomyl nor its metabolites tend to
accumulate in animal tissues (Gardiner et al., 1974).
According to FAO/WHO (1974b), feeding of 14C- or 35S-labelled
thiophanate-methyl to rats, mice, and dogs resulted in 80 - 100%
recovery of the label in the faeces and urine within 96 h.
Unmetabolized thiophanate-methyl has been reported to be the major
component of faecal elimination. The minor part consisted of 4-
hydroxy-thiophanate-methyl, dimethyl-4,4- O -phenylenebisallophanate.
MBC and 5-hydroxy-MBC were also detected.
Following application of carbendazim to rats and mice by
intragastric intubation, almost all metabolites in the urine were
conjugated as sulfate esters. 5-HBC was released as the only
metabolite extractable from water. A higher proportion of polar
compounds was found in the urine of mice than in that of rats.
Polarity was caused by the phenolic hydroxyl group (FAO/WHO,
1985a).
The excretion of 14C-labelled propham and chlorpropham was
investigated in female rats after a single oral dose. The average
3-day urinary excretion of radioactivity was between 56 and 85%,
depending on the position of labelling: viz chain or ring
labelling. With chain 14C-chlorpropham, an average of 35% of the
administered radioactivity appeared in the respired air, compared
with only 5% for chain 14C-propham (Fang et al., 1974).
6.4 Metabolism in Plants
Studies on the metabolism and fate of carbamate pesticides in
plants are of great importance for assessing the human hazards from
the consumption of fruit and vegetables containing residues of the
applied pesticides and metabolites.
Generally, the metabolism of carbamates in plants follows
another route of detoxification. The hydroxylated carbamates
produced by enzymes are conjugated either with amino acids (for
instance cysteine) or as glycosides and phosphates, which are
stored as terminal metabolites (Still & Rusness, 1977; Aldridge &
Magos, 1978).
The toxicological properties of plant conjugates in mammals or
other animals are not generally known. Insecticidal methyl
carbamate esters are vulnerable to hydrolytic cleavage, which
results in detoxified products. Detoxification of carbamate esters
in plants by hydrolysis occurs to a lesser extent than
detoxification by oxidative metabolism; nevertheless, hydrolytic
cleavage of the ester moiety is a significant detoxification
mechanism. For instance, in sugar beets treated with desmedipham,
the major metabolites were ethyl-3-hydroxy phenyl carbamate and 3-
aminophenol (Knowles & Sonawane, 1972). Carbetamide is hydrolysed
to aniline (Guardigli et al., 1977). Aromatic ring hydroxylation
appears to be the predominant metabolic reaction that carbamate
herbicides undergo in plants. The major metabolites isolated from
soybean plants, after uptake of chlorpropham, were glucoside
conjugates of 2-hydroxy- and 4-hydroxypropham. Similar results
were obtained with propham (Still & Mansager, 1973a,b, 1975).
There is evidence that the ring hydroxylation varies with the
plant species. In other carbamates, for instance in the case of
barban, ring hydroxylation did not appear to take place, and polar
metabolites were produced, which were hydrolysed into
chloroanilines (Still & Mansager, 1972).
Furthermore, oxidation of aliphatic groups takes place in
carbamate herbicides (Wiedmann et al., 1976). Still & Herrett
(1975) demonstrated N -methyl hydroxylation for dichlormate, a
metabolite that is either conjugated or loses the hydroxymethyl
moiety to produce N -demethylated dichlormate.
Thiophanate-methyl is mainly metabolized into carbendazim (MBC)
and, to a less extent, into 2-aminobenzimidazole (2AB). A few other
metabolites have also been found (FAO/WHO, 1974b).
The metabolic pathway for carbaryl in plants is identical,
whether the compound is injected into the stem or applied to the
leaf surface. After entering the plant, carbaryl undergoes
biotransformation to its primary metabolites, which are similar to
the ones formed in animals. These hydroxylated metabolites, which
are less toxic than carbaryl itself, are conjugated by plants to
form water-soluble glycosides. Injection of carbaryl into the
bean plant produced water-soluble metabolites attributable to
hydroxylation of the ring or N -methyl group followed by
conjugation, mainly as glycosides (Kuhr, 1968; FAO/WHO, 1970b;
Fukuto, 1972).
The metabolism of aldicarb in plants is the same as that in
mammals. In the cotton plant, the thioether moiety is rapidly
oxidized to the sulfoxide, and the latter is slowly transformed to
the sulfone. The sulfoxide is rather stable and can be present in
cotton plants, even as long as 2 months after treatment. The total
metabolites present can be as high as 80% (Kuhr, 1968). Because of
their persistence in plants, and their even higher anticholinesterase
activity compared with aldicarb itself, the sulfoxide and sulfone
should be taken into account when considering residue tolerances.
The major metabolite isolated from bean plants, 28 days after
treatment with carbofuran, was a conjugate of 3-hydroxy-carbofuran,
which remained in the aqueous phase after extraction with an
organic solvent. The conjugate represented 55% of the total 14C-
labelled residues present in the plant. 3-Hydroxy-carbofuran is
highly toxic for mammals (LD50 in the rat 7 mg/kg body weight).
The conjugate may also be toxic, particularly taking into account
the acid conditions of the mammalian stomach under which the
conjugate will be hydrolysed (FAO/WHO, 1977b, 1980b).
Harvey et al. (1978) studied the metabolism of oxamyl in plants
(tobacco, alfalfa, peanuts, potatoes, apples, oranges, and
tomatoes). The major route of degradation involved hydrolysis to
the corresponding oximino compound, which in turn became conjugated
with glucose. Further metabolism resulted in the loss of one of
the N' -methyl groups and/or addition of other glucose units to the
sugar moiety of the original conjugate. Total breakdown into
normal natural products has been demonstrated.
In a study by Harvey & Reiser (1973), methomyl rapidly degraded
to carbon dioxide and acetonitrile, which volatilized from the
plant tissues. The half-life for methomyl was of the order of 3 -
6 days. The remainder of the compound had been reincorporated into
natural plant components.
The metabolic pathways for a number of carbamate pesticides are
described in more detail in Fukuto (1972).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
As described in section 4.2.1, soil microorganisms are capable
of hydrolysing carbamates. Furthermore, it seems that
microorganisms can easily adapt themselves to metabolize the
different types of carbamates (Aly & El-Dib, 1972). Nevertheless,
carbamates and their metabolites can affect the microflora and
cause changes that may have an important impact on the maintenance
of the soil productivity (Filip, 1974).
7.2 Aquatic Organisms
In general, carbamates are not very stable under aquatic
conditions (section 4). The solubility in water differs
considerably for the different carbamates (Annex I). Furthermore,
photodecomposition takes place and microorganisms are able to
degrade these compounds. By these processes, the carbamate is
broken down (oxidation, hydrolysis) into other compounds. Some
will be less toxic, others even more toxic than the parent
compound. Carbaryl will be hydrolysed into 1-naphthol, which is
just as toxic (Armstrong & Millemann, 1974a). It seems unlikely
that most of the carbamates will persist long in the environment,
but there are exceptions, such as dimetilan and carbendazim.
Many data are available on the acute toxicity of the carbamates
in several fresh- and salt-water fish and other organisms, some of
which are listed in Tables 4 and 5.
When carbamates are deliberately added to an aquatic system,
caution is necessary since, with high concentrations, a number of
species are at risk. For example, while applications of carbaryl
of 0.93 kg/ha were effective in controlling the ghost shrimp
(Callianassa californiensis), an oyster pest, they also caused a
reduction in the population of juvenile clams (Armstrong &
Millemann, 1974a).
In a similar study (Armstrong & Millemann, 1974b), carbaryl
and its hydrolytic product, 1-naphthol, were examined for other
effects on the mussel (Mytilus edulis). An age dependence was
noted; the most sensitive stage (appearance of the first polar body
shortly after fertilization) had EC50s of 5.3 and 5.2 mg/litre for
carbaryl and 1-naphthol, respectively. Effects of the compounds
on development were characterized by disjunction of blastomeres and
asynchronous and unaligned cleavages. Carbaryl, at a single dose
of 0.1 mg/litre, also disrupted normal schooling behaviour in
juvenile Menidia medidia; the disruptive effects were attributed
to the 1-naphthol rather than to the parent carbamate. These
effects were reversed within 3 days of placing the fish in clean
water (Weis & Weis, 1974). Hansen (1969) studied the capacity of
sheepshead minnows (Cyprinodon variegatus) to avoid carbaryl. The
fish did not avoid carbaryl in concentrations of 0.1 - 10 mg/litre
water.
Table 4. Acute aquatic toxicity (LC50 in mg/litre)a
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Aldicarb fish Rainbow trout (Salmo gairdneri) 0.5 12 0.56
fish Bluegill (Lepomis machrochirus) 1.3 24 0.05
Aminocarb fish Rainbow trout (Salmo gairdneri) 1.5 12 13.5b
fish Bluegill (Lepomis machrochirus) 2.0 20 3.1b
crustacea Daphnia (Daphnia magna) first instar 21 0.01 - 0.1c
(48-h EC50)
fish Rainbow trout (Salmo gairdneri) 1.5 10 0.13c
fish Bluegill (Lepomis machrochirus) 0.6 20 0.1c
insect Midge (Chironomus plumosus) fourth instar 20 0.27c
Benomyl fish Rainbow trout (Salmo gairdneri) 1.2 12 0.17d
fish Bluegill (Lepomis machrochirus) 0.9 22 0.85d
fish Rainbow trout (Salmo gairdneri) 1.0 12 0.31e
fish Bluegill (Lepomis machrochirus) 0.6 22 1.2e
Benomyl fish Rainbow trout (Salmo gairdneri) 0.2 12 0.37f
metabolite
MBC
Bufencarb crustacea Scud (Gammarus fasciatus) mature 15 0.001
fish Goldfish (Carassius auratus) 1.0 18 0.29
Carbaryl fish Rainbow trout (Salmo gairdneri) 1.5 12 1.95d
fish Bluegill (Lepomis machrochirus) 1.2 18 6.76d
crustacea Daphnia (Daphnia pulex) first instar 16 0.0064d
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature 21 0.026d
----------------------------------------------------------------------------------------------------
Table 4. (contd.)
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Carbofuran fish Rainbow trout (Salmo gairdneri) 1.5 12 0.38d
fish Bluegill (Lepomis machrochirus) 0.8 18 0.24e
Dichlormate fish Rainbow trout (Salmo gairdneri) 0.8 12 4.9
Methiocarb fish Rainbow trout (Salmo gairdneri) 1.3 12 0.80
fish Bluegill (Lepomis machrochirus) 1.0 24 0.21
Methomyl fish Rainbow trout (Salmo gairdneri) 1.1 12 1.60b
fish Bluegill (Lepomis machrochirus) 0.9 20 1.05b
crustacea Daphnia (Daphina magna) first instar 21 0.009b
(48-h EC50)
Mexacarbate fish Rainbow trout (Salmo gairdneri) 1.0 11 12.0g
fish Bluegill (Lepomis machrochirus) 0.7 12 22.9g
crustacea Daphina (Daphina pulex) first instar 15 0.010g
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature - 0.04g
Trimethacarb fish Rainbow trout (Salmo gairdneri) 1.2 12 1.0
fish Bluegill (Lepomis machrochirus) 0.9 18 11.6
----------------------------------------------------------------------------------------------------
a From: Johnson & Finley (1980).
b Technical material, 95 - 98%.
c Liquid formulation, 17%.
d Technical material, 99%.
e Wettable powder, 50%.
f Methyl-2-benzimidazole (MBC), 99%.
g Technical material, 90 - 95%.
Note: It should be recognized that the LC50 and EC50 values only give an indication of the
toxicity and that the toxicity for these organisms may be appreciably changed by variations
in temperature, pH, oxygen content, and water hardness. A 10-fold increase in the toxicity
may be found. Also, the life stage of the organisms is an important factor in estimating
the toxicity of the compound.
Table 5. Acute toxicity of carbamate pesticides for some aquatic organisms
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
Benomyl 7.5 12 11 (wettable - 14 Yoshida & Nishiuchi
powder) (1972)
BPMC 16 10 ~ 40 1.7 5.0 0.32 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Carbaryl 13 10 ~ 40 2.8 2.8 0.05 Yoshida & Nishiuchi
(1972)
Carbendazim > 40 - 40 - > 40 Yoshida & Nishiuchi
(1976)
CPMC 10 ~ 40 10 ~ 40 5.6 7.0 0.1 ~ 0.5 Yoshida & Nishiuchi
(emulsifiable (1972); Nishiuchi
concentrate) (1974)
Isoprocarb 10 ~ 40 10 ~ 40 5.9 3.0 0.30 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Mecarbam 0.70 0.68 0.35 0.055 0.03 Yoshida & Nishiuchi
granule (1972); Nishiuchi
(1974)
Methomyl 2.8 2.7 0.87 1.0 0.045 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
MPMC 10 ~ 40 10 ~ 40 12 7.3 0.07 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
MTMC 10 ~ 40 10 ~ 40 27 13 0.35 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Pirimicarb > 40 - 40 - 0.048 Yoshida & Nishiuchi
(1976)
Promecarb 2.7 - 3.3 - 0.020 Yoshida & Nishiuchi
(1976)
Terbam 0.92 - 2.2 - 0.025 Yoshida & Nishiuchi
(1976)
Thiophanate > 40 > 40 > 40 - > 40 Yoshida & Nishiuchi
(1972)
Thiophanate 11 > 40 11 (wettable - > 40 Yoshida & Nishiuchi
-methyl powder) (1972)
XMC > 40 > 40 33 25 0.055 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
a BPMC = 1-sec-butylphenylmethyl carbamate
CPMC = 1-chlorophenylmethyl carbamate
MPMC = 3,4-xylylmethyl carbamate
MTMC = 4-tolylmethyl carbamate
Terbam = 4-tert-butylphenylmethyl carbamate
XMC = 3,5-xylylmethyl carbamate
Note: Test methods are officially recognized methods based on the Notification of the
Ministry of Agriculture, Forestry, and Fisheries of Japan.
Carbamates can have adverse effects on algae. Stadnyk et al.
(1971) reported that carbaryl at a concentration of 0.1 mg/litre
caused an increase in cell numbers and in the biomass of the green
algae Scenedesmus quadricaudata.
Motsuage fish (Motsugo pseudoras bora parva) were reported to
accumulate carbaryl and BPMC during a 30-day exposure to a
concentration of 0.6 - 1.2 mg/litre. Carbaryl uptake was greatest.
However, this compound underwent more rapid metabolism and
excretion than BPMC. Metabolism of BPMC was slow and introduced
permanent spinal curvature of the backbone in about 30% of the fish
exposed, an effect seen with the organophosphorus insecticide
diazinon (Kanazawa, 1975).
A residue study indicated that over a 28-day period at the
highest exposure level of 0.75 mg methomyl/litre, no accumulation
of the carbamate took place. During the entire exposure period,
the values ranged from 0.36 to 0.45 mg/kg. After a 3-day withdrawal
period, no methomyl (< 0.02 mg/kg) was detected in the fish
(Kaplan & Sherman, 1977).
The results summarized in Table 4 are representative of the
toxicity of the different carbamates for fish and invertebrate
species. In general, the LC50s of carbamates for different fish
species range from approximately 0.1 to 10 mg/litre. Invertebrates
are usually more sensitive. The EC50 values for Daphnids and
other species are mainly below 0.1 mg/litre. Some carbamates seem
to be very toxic (< 0.01 mg/litre) for these invertebrates.
7.2.1 Field studies
Little information is available concerning the fate and
persistence of carbamates in the aquatic environment. The presence
of these carbamates in surface waters may have an effect on water
organisms.
Quraishi (1972) studied the persistence of aldicarb. Field
water treated in the laboratory at 100 mg aldicarb/litre resulted
in residues of aldicarb and its metabolites of 0.4 mg/litre after
11 months. Water was stored at 16 - 20 °C and exposed for
approximately 507 h to sunlight.
Data concerning the fate of aminocarb have been reviewed by
Vassilieff & Ecobichon (1982). A half-life of 28.5 days in pond
water was found under normal environmental conditions. The
principal metabolite appeared to be the phenol, though the
methylamino and formylamino analogues were also observed.
Harvey & Pease (1973) found that, under field conditions in
Delaware, Florida, and North Carolina, methomyl broke down almost
completely within 1 month; most of it was lost from the soil by
volatilization, presumably as carbon dioxide. Small amounts
extracted from the soil consisted of methomyl, S -methyl N -
hydroxythioacetimidate, and some polar compounds. A run-off study,
under farm conditions, showed that the compound did not move into
untreated areas with run-off water.
7.3 Terrestrial Organisms
7.3.1 Effects on soil fauna
Thiophanate-methyl and carbendazim have been shown to be
equally as toxic through contact as benomyl. Earthworms (Lumbricus
terrestris) immersed for 1 min in a 0.6% aqueous suspension of
these compounds died in 14 days. Worms in pots containing soil
drenched at a rate of 0.78 g/m2 died within 18 days (Wright &
Stringer, 1973).
7.3.2 Wildlife
The LD50 of benomyl for mallard ducks and quail is > 5000
mg/kg body weight. The cumulative toxicity of formetanate (7 days)
is approximately 6800 mg/kg body weight for ducks, and > 4640 mg/kg
body weight for quail and for pheasant (Aldridge & Magos, 1978).
Lethal and sub-lethal doses of aldicarb, methiocarb, oxamyl,
pirimicarb, and thiofanox administered to Japanese quail produced
significant inhibition of plasma- and brain-ChE and influenced the
activity of other enzymes, such as alpha-naphthyl acetate esterase,
glutamate dehydrogenase, and glutamate oxaloacetic transaminase.
With sub-lethal dose levels, plasma-ChE activity recovered within a
few days (Westlake et al., 1981).
An 8-day dietary LC50 for the Peking duck was 1890 mg/kg
feed; for bobwhite quail, the dietary LC50 was 3680 mg/kg feed
(Kaplan & Sherman, 1977).
Bobwhites (Colinus virginianus) were provided diets containing
sublethal levels of carbaryl (237 or 1235 mg/kg), for 7 days, or
carbofuran (26 mg/kg), for 14 days. Food intake, body weight, and
the locomotor activity of adult bobwhites were normal.
Administration of a diet containing 131 mg carbofuran/kg resulted
in reductions in food intake, body weight, and locomotor activity
(Robel et al., 1982).
7.3.3 Bees
An undesirable effect caused by the use of carbamate
insecticides has been the mortality of honey bees, a species that
exhibits a high sensitivity to these compounds. Because of the
agricultural and ecological importance of bees, thorough
comparative toxicity studies have been carried out on a large
number of compounds to identify those with the widest margin of
safety (Atkins et al., 1973).
Abdel-Aal & Fahmy (1977) compared the effects of aldicarb,
methomyl, N -desmethylmethomyl, carbaryl, and carbofuran on the
honey bee (Apis mellifera) and found that all of the compounds
were extremely toxic.
7.4 Earthworm and Mite Populations
Studies by Stringer & Wright (1973) and Wright & Stringer
(1973) showed that earthworm populations in apple orchard plots
sprayed with either benomyl or thiophanate-methyl were reduced. In
particular, Lumbricus terrestris, an important species in the
ecology of the apple orchard, was virtually eliminated. Captive
worms would not feed on leaf material that had been sprayed at 1.75
µg/cm2 with benomyl, methyl benzimidazol-2-yl carbamate (MBC), or
thiophanate-methyl, and feeding was significantly reduced at a
level of 0.87 µg/cm2. All the worms were killed following contact
with benomyl as an aqueous suspension or soil drench (7.75 kg/ha).
It was suggested by the authors that the toxicity of benomyl
might be due to the anticholinesterase activity of the carbamate
moiety. Effects such as lethargic movements, muscular paralysis,
and death support this supposition.
The spraying of benomyl and thiophanate-methyl in orchards
reduced the number and biomass of all earthworm species combined
and also of each individual species (Stringer & Lyons, 1974). It
was observed that over 90% reduction in earthworm populations
occurred in pastures treated with benomyl. It was suggested that
these changes were reversible, and that the populations of
earthworms would return to normal a few years after the termination
of the treatment (Tomlin & Gore, 1974).
The carbamate insecticide most commonly used in the soil is
carbaryl; it is used mainly in woodlands and orchards where the
formation and fertility of the soil are important.
Stegeman & LeRoy (1964) applied carbaryl at 1.3, 11.2, and 56
kg ai/ha to a red pine plantation and mixed hardwood stands, and,
though the lowest dose did not have any effects on mite and
Collembola populations, the other 2 dose levels considerably
decreased the numbers of mites and Collembolla. Populations began
to recover after 2 months, but Collembola were more sensitive and
recovered more slowly. There seems to be little likelihood of
long-term effects of carbaryl on mite populations (Edwards &
Thompson, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
Short- and long-term toxicity studies with carbamates have been
carried out for a period of 20 - 30 years. It is clear that the
production of these carbamates has improved during this period and
that, consequently, purer products have become available. The use
of less pure substances in earlier toxicity studies could explain
the conflicting results between these and later studies.
8.1.1. Oral
Acute oral and dermal toxicity data on animals, for most of the
carbamate pesticides, are given in Table 6. The WHO Recommended
Classification of Pesticides by Hazard is also cited. This
classification is based primarily on the acute oral and dermal
toxicity of the technical material for the rat (WHO, 1984). The
oral LD50s range from less than 1 mg/kg body weight for the highly-
toxic aldicarb to over 5000 mg/kg body weight for the non-
insecticidal carbamates such as phenmedipham, carbendazim, propham,
and benomyl.
A dose-effect relationship is generally noticed between the
administered dose, the severity of effects, and the amount of ChE
inhibition. A correlation also exists between the duration of
symptoms and the in vivo persistence of the compound.
In general, the toxicological effects produced by carbaryl are
typical of those produced by methyl carbamates. The effects of
carbaryl on a number of different animal species were studied by
Carpenter et al. (1961). Rats given a single oral dose of 560
mg/kg body weight showed 42% and 30% inhibition of erythrocyte- and
brain-ChE, respectively, within 1/2 h of treatment; only about 5%
inhibition of plasma-ChE was observed. All ChE levels were almost
normal after 24 h. In studies on rats administered single oral
doses ranging from 5 to 50 mg/kg body weight, a decrease in ChE
activity occurred within 5 min. Recovery was quite rapid
(Krechniak & Foss, 1982).
In 4 dogs, marginal inhibition of erythrocyte-ChE activity was
seen after treatment with a single oral dose of 375 mg carbaryl/kg
body weight. After 30 min, signs of poisoning were observed
including salivation, lachrymation, constriction of pupils,
urination, defaecation, increase in respiratory rate, muscular
twitching, tremors, and mild convulsions. The animals appeared to
have completely recovered the following day. These signs of
poisoning are classic of overstimulation of the parasympathetic
nervous system (Carpenter et al., 1961).
Vassilieff & Ecobichon (1983) studied the influence of a single
dose of 25 mg aminocarb/kg body weight on the activity of
erythrocyte- and brain-AChE, plasma-ChE, and hepatic
carboxylesterases in rats. Significant inhibition of all the
esterases was observed, 30 min or more after the administration of
aminocarb. This severe, but transient, inhibition of the tissue
esterases had recovered after 24 h.
Table 6. Acute oral and dermal toxicity data for a number of
carbamate pesticides
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
aldicarb 0.9 > 10.0 (rabbit) IA
aldoxycarb 26.8 700 - 1400 -
allyxycarb 90 - 99 500 -
aminocarb 30 - 40 275 IB
asulam > 4000 > 1200 -
barban 1376 - 1429 > 1600 -
> 20 000 (rabbit)
BPMC 623 - 657 > 5000 -
bendiocarb 40 - 156 566 - 600 II
benomyl > 10 000 > 10 000 (rabbit) 0
> 1000 (dog)
bufencarb 87 680 (rabbit) -
butacarb NA NA -
carbanolate NA NA -
carbaryl approximately > 4000 II
500 - 600 > 2000 (rabbit)
carbendazim > 15 000 > 2000 0
> 2500 (dog) > 10 000 (rabbit)
> 10 000 (quail)
carbetamide 10 000 > 2000 -
1250 (mouse) > 500 (rabbit)
1000 (dog)
carbofuran 6 - 14 3400c (rabbit) IB
15 - 19 (dog)
chlorbufam 2500 NA -
chlorpropham 5000 - 8000 10 200d O
5000 (rabbit) 2000 (dog)
cloethocarb 35.4 4000 -
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
desmedipham > 10 250 2000 - 10 000 -
2025e (rabbit)
dimetilan 64 > 2000 -
60 - 65 (mouse)
dichlormate NA NA
dioxacarb 72 approximately -
3000 1950
(rabbit)
ethiofencarb 411 - 499 > 1150 II
224 - 256 (mouse)
155 (quail)
formetanate 21, 18 (mouse) > 5600
19 (dog) > 10 200 (rabbit)
hoppicide NA NA -
isoprocarb 403 - 485 > 500
487 - 512 (mouse)
ca 500 (rabbit)
karbutilate 3000 > 15 400 -
methiocarb 100 350 - 400 II
40 (guinea-pig)
methomyl 17 - 24 > 5000 (rabbit) IB
28 (hen)
metolcarb 498 - 580 > 2000 -
mexacarbate 15 - 63 > 500 -
MPMC 380 > 1500 (mouse) -
MTMC 268 (mouse) 6000 -
oxamyl 5.4 710 (rabbit) IB
4.2 (quail)
phenmedipham > 8000 > 4000 -
> 4000 (dog)
> 3000 (chicken)
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
pirimicarb 147 (101 - 210) > 500 (rabbit) II
107 (mouse)
100 - 200 (dog)
25 - 50 (poultry)
promacyl 1220 > 4000 -
2000 - 4000 (mouse)
4000 - 8000 (hen)
promecarb 61 - 90 > 1000 -
> 1000 (rabbit)
propham 9000 6800f (rabbit) O
3000 (mouse)
propoxur 80 - 191 1000 - 2400 II
100 - 109 (mouse)
40 (guinea-pig)
swep NA NA -
terbucarb NA NA -
thiodicarb NA NA -
thiofanox 8.5 39 (rabbit) IB
43 (quail)
thiophanate > 15 000 > 15 000 -
-ethyl
thiophanate 6640 - 7500 > 10 000 O
-methyl
trimethacarb NA NA -
xylylcarb 325 - 375 > 1000 -
XMC 542 - -
445 (rabbit)
---------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
b From: WHO (1984). See this reference for classification of
organophosphates not mentioned in this annex.
The hazard referred to in this Classification is the acute risk for
health (that is, the risk of single or multiple exposures over a
relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the
directions for handling by the manufacturer or in accordance with the
Table 6 (contd.)
rules laid down for storage and transportation by competent
international bodies.
Classification relates to the technical material, and not to the
formulated product:
Class IA = Extremely hazardous. Class III = Slightly hazardous.
Class IB = Highly hazardous. Class O = unlikely to present
Class II = Moderately hazardous. acute hazard in normal use.
c With 75% water-dispersible powder.
d With 50% emulsifiable concentrate formulation.
e With 16% formulation.
f With 25% emulsifiable concentrate formulation.
NA = Not available.
Note: References are the reports of the different WHO/FAO Joint
meetings on pesticides residues (Annex III), Kaplan & Sherman
(1977), and Worthing & Walker (1983).
8.1.2 Dermal
Acute dermal toxicity values for most carbamates are mainly
above 500 mg/kg body weight with the exception of, for instance,
aldicarb, which is highly toxic.
8.1.3 Inhalation
Exposure to carbaryl (50% wettable powder, average particle
size, 15 µm) at a mean concentration of 390 mg/m3 (range 344 - 722
mg/m3), for 4 h, produced nasal and ocular irritation in 6 guinea-
pigs. When another group of guinea-pigs inhaled a mean of 230 mg
carbaryl/m3 (average particle size, 5 µm), for 4 h, no clear
effects were observed. Single exposures of dogs ranging from 2 to
5 h to a concentration of 75 mg carbaryl/m3 (average particle size,
5 µm) caused clinical signs of ChE inhibition (Carpenter et al.,
1961). Cats exposed to 80 mg carbaryl/m3 for 6 h showed
salivation, excitation, respiratory difficulties, and 39 - 55%
inhibition of ChE activity in the serum and 53 - 71% in the
erythrocytes. A single exposure to a level of 20 mg/m3 for 6 h did
not induce signs of intoxication, but serum- and erythrocyte-ChE
activities were slightly inhibited (Yakim, 1967).
8.2 Short- and Long-Term Exposures
A number of short-term studies have been carried out with
different carbamates and a variety of laboratory animal species.
In the context of this introduction, it is not possible to mention
all the reports available. Details on the short- and long-term
toxicities of the individual carbamates will be provided in future
Environmental Health Criteria. Most carbamates have been discussed
by the Joint FAO/WHO Meetings on Pesticide Residues and monographs
have been published (see reference list). Furthermore, the
International Agency for Research on Cancer has published a
monograph in which a number of carbamates have been evaluated
(IARC, 1976).
In the context of this introduction, a number of examples are
given to illustrate dose-response relationships and/or target
organs or tissues for a number of carbamates.
8.2.1 Oral
Short-term tests on rats or dogs exposed to repeated oral doses
of carbamate insecticides or fungicides (such as carbaryl,
propoxur, formetanate, carbofuran, and benomyl), revealed
inhibition of plasma (serum)-, erythrocyte-, and brain-ChE
(Carpenter et al., 1961; Ivanova-Chemishanska & Antov, 1976;
Krechniak & Foss, 1982).
(a) Pirimcarb
In a study on 4 dogs (2 males and 2 females) treated with 25 or
50 mg pirimicarb/kg body weight per day, for 110 weeks, 2 of the
animals developed anaemia. Haemoglobin content was decreased,
methaemoglobin was present, and the reticulocyte percentage was
significantly elevated. Levels of unconjugated bilirubin in serum
were increased. The 2 other animals were unaffected. There was
some indication that pirimicarb was able to induce the production
of immune IgG antibodies (Jackson et al., 1977).
(b) Carbaryl
Kidney alterations, such as cytoplasmic vacuolization, were
observed in the proximal tubules in short-term studies on monkeys
and rats adminstered carbaryl at dose levels of up to 600 or 300
mg/kg body weight, respectively (Coulston, 1967; cited in FAO/WHO,
1970b).
Two pigs from one litter were fed a ration containing carbaryl
at the rate of 150 mg/kg body weight for 72 and 83 days,
respectively. Three pigs in the same litter were fed 150 mg/kg
body weight for 4 weeks and, thereafter, 300 mg/kg body weight, the
total feeding period being 46 or 85 days. Two other pigs were
maintained on the basal ration, as controls. The clinical syndrome
of chronic intoxication was characterized by progressive
myasthenia, incoordination, ataxia, tremors, and chronic muscular
contractions terminating in paralysis and death. The lesions were
confined to the central nervous system and skeletal muscle.
Moderate to severe oedema of the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord was associated with
vascular degenerative changes, consistent with a vasogenic oedema
(Smalley et al., 1969). Spontaneous recovery from the effects of
sub-lethal doses occurred in about 8 h, indicating the ready
reversibility of the carbaryl-enzyme reaction (Carpenter et al.,
1961).
Carbaryl was administered to dogs at dose levels of 0, 0.45,
1.8, or 7.2 mg/kg body weight per day, for 5 days/week, for one
year. Cloudy swelling of the proximal convoluted and loop tubules
of the kidneys was found in the group given 7.2 mg/kg body weight.
There were no abnormalities in the composition of the blood, and no
ChE inhibition was found. It should be noted that the dose levels
tested were low (Carpenter et al., 1961).
In a long-term (2-year) toxicity study on CF-N rats, levels of
0, 50, 100, 200, or 400 mg carbaryl/kg diet (equivalent to 0, 2.5,
5, 10, or 20 mg/kg body weight) were tested. The highest dose
level produced cloudy swelling in the tubules of the kidneys
(Carpenter et al., 1961).
(c) Carbendazim
Groups of ChR-CD male rats (6/dose) were intubated with 200,
3400, or 5000 mg carbendazim/kg body weight per day, 5 times per
week, for 2 weeks. In all groups, the absolute and relative
weights of the kidneys were lower, and adverse effects on the
testes and reduction or absence of sperm in the epididymides were
found (FAO/WHO, 1985a).
(d) Benomyl
In a 2-year toxicity study, Charles River rats were
administered dietary levels of benomyl of 0, 100, 500, or 2500
mg/kg (equivalent to 0, 5, 25, or 125 mg/kg body weight). No
increase in mortality and no other compound-related effects on
haematology, urinalysis, organ weight, or histology were found
(Haskell Laboratories, 1969 cited in FAO/WHO, 1985a).
In another 2-year study, dogs were administered diets
containing 0, 100, 500, or 2500 mg benomyl/kg (equivalent to 0,
2.5, 12.5, and 62.5 mg/kg body weight). Liver cirrhosis, bile duct
proliferation, and testicular degeneration were found at the
highest dose level; however, 100 and 500 mg/kg diet did not show
any clear effects (Haskell Laboratories, 1970 cited in FAO/WHO,
1985a).
(e) Thiophanate-methyl
An oral toxicity study in which thiophanate-methyl at dose
levels of 0, 2, 10, 50, or 250 mg/kg body weight (in capsules) was
administered to beagle dogs (8 or 10 animals per group) was
conducted for 24 months. Retardation of growth was seen in both
sexes in the 250 mg/kg group. Both male and female dogs in the 50
and 250 mg groups showed a significant and dose-related increase in
the weight of the thyroid. However, there were no changes in the
serum-protein-bound iodine (PBI) levels and no histological
differences in the thyroid were found between the control group and
the 2 highest dose groups. No other abnormalities were observed
(Hashimoto & Fukuda, 1972 cited in FAO/WHO, 1974b).
(f) Methomyl
Groups of rats were given diets containing 0, 10, 50, 125
(increased to 500), or 250 mg methomyl/kg for 90 days. Weight gain
in animals administered 250 and 500 mg/kg was slightly suppressed.
No clear changes in the different parameters including plasma- and
erythrocyte-ChE activity were found (Kaplan & Sherman, 1977).
The same authors carried out studies over 90 days and 2 years
on beagle dogs. Dietary levels of 0, 50, 100, and 400 mg
methomyl/kg did not result in any nutritional, clinical,
haematological, urinary, biochemical, or pathological changes. In
the 2-year feeding study, histopathological alterations were seen
in kidneys and spleen of animals receiving 400 and 1000 mg
methomyl/kg diet. Histopathological changes were seen in the liver
and bone marrow in animals administered 1000 mg/kg diet (Kaplan &
Sherman, 1977).
The above-mentioned studies give some indication that
carbamates may have different mechanisms of action on target organs
that are related to substituents other than the carbamate ester
moiety (such as the imidazole moiety).
Apart from the effects on ChE activity, effects have been found
on the liver, kidneys, thyroid, and testes and also on the
activities of other enzyme systems such as ATPase, Gl-6-ase, LDH,
GPT, Alk-Pase, etc. (Haskell Laboratories, 1968 cited in FAO/WHO,
1985a; Ivanova-Chemishankska & Antov, 1976).
8.2.1.1 Further information on short- and long-term toxicity
It is not the purpose of this introductory document to provide
all the available data on long-term toxicity. However, these long-
term studies are important for the establishment of the no-
observed-adverse-effect level and consequently the Acceptable Daily
Intake (ADI) for man. Thus, it is necessary to indicate the
studies that were considered adequate and used by the different
FAO/WHO Joint Meetings on Pesticide Residues. The International
Agency for Research on Cancer has also evaluated long-term/
carcinogenicity studies of a number of carbamates. The results of
these international evaluations are summarized in Annex II and III.
From these Annexes, it is clear that aldicarb is the most toxic
of the carbamates considered; the established ADI for this compound
is 0.001 mg/kg body weight.
The ADIs for the other carbamates may vary from 0.01 to 0.1
mg/kg body weight. These quantities are equivalent to a daily
intake for man (60 kg body weight) of approximately 0.6 - 6 mg.
8.2.2 Dermal
(a) Pirimicarb
Daily dermal applications of 500 mg pirimicarb/kg body weight
to the skin of rabbits for 24 h over a 14-day period did not
produce any signs of intoxication (FAO/WHO, 1977b).
(b) Methomyl
Methomyl applied to the intact skin of rabbits at a repeated
rate of 200 mg/kg per day caused mild signs of intoxication such as
nasal discharge, wheezing, and transient diarrhoea. All animals
survived 15 applications. However, when applied on the abraided
skin, much more serious systemic signs were seen, such as nasal
discharge, salivation, tremors, poor coordination, and abdominal
hypertonia; a few animals died (Kaplan & Sherman, 1977).
(c) Carbaryl
Application of carbaryl at 500 mg/kg body weight to the skin of
6 rabbits resulted in 39% inhibition of serum-ChE activity and 36%
inhibition of red cell-ChE activity during the first 24 h following
treatment. ChE activity returned to initial levels in 72 h (Yakim,
1967).
8.3 Skin and Eye Irritation; Sensitization
8.3.1 Skin irritation
Rabbits exposed to a single dermal application of carbaryl at a
concentration of 100 g/litre in acetone did not show any skin
irritation (Carpenter et al., 1961).
8.3.2 Eye irritation
Undiluted carbaryl or solutions of different concentrations
were applied to the eyes of rabbits. Technical carbaryl applied in
excess as a 100 g/litre suspension in propylene glycol produced
mild injury. A 25% aqueous suspension of a microfine material did
not cause any injuries; 50 mg of the dust caused only traces of
corneal necrosis (Carpenter et al., 1961).
8.3.3 Skin sensitization
(a) Carbaryl
Groups of albino guinea-pigs were given 8 intracutaneous
injections (3 per week) of 0.1 ml of a 0.1% dispersion of carbaryl
in 3.3% propylene glycol. After a 3-week incubation period,
followed by a challenge dose, no clear sensitization reaction was
noticed (Carpenter et al., 1961).
(b) Benomyl and thiophanate-methyl
It has been shown that benomyl and thiophanate-methyl can cause
slight reversible skin irritation and weak sensitization in guinea-
pigs (FAO/WHO, 1974, 1985a).
8.4 Inhalation
(a) Carbaryl
The inhalation toxicity of carbamates for mammals has not been
widely studied.
The effects of acute inhalation exposure to carbaryl dust have
been examined in rats (10 mg/m3), guinea-pigs (390 mg/m3), and dogs
(75 mg/m3) (Carpenter et al., 1961) (section 8.1.3). No treatment-
associated, gross or microscopic lesions were seen in guinea-pigs
and dogs, even though the exposure was sufficient to cause ChE
inhibition. Haemorrhagic areas in the lung were seen in guinea-
pigs at the highest level tested (390 mg/m3).
Four cats exposed to carbaryl in air at 63 mg/m3 for 6 h per
day for 1 month developed signs of cholinergic stimulation during
the first 2 h of exposure each day. Periodic salivation was noted.
One cat died with marked signs of poisoning on day 20. There was
already a significant decrease in serum- and erythrocyte-ChE
activity after the first exposure. Exposure to 40 mg/m3 for 6 h
per day for 2 months was reported to impair conditioned reflexes in
cats. On certain days, the ChE activity dropped to below 50% of
pre-exposure values. Exposure to concentrations of 16 mg/m3, for 6
h per day, for 4 months did not result in any signs of toxicity
(Yakim, 1967). Carpenter et al. (1961) did not find any increase in
mortality or "grossly-visible injury" when rats were exposed to a
mean concentration of 10 mg/m3 (range 5 - 20 mg/m3), for 7 h per
day, 5 days/week, over 90 days.
(b) Propoxur
Male and female rats were exposed to propoxur aerosols at
average concentrations of 0, 5.7, 10.7, or 31.7 mg/m3, for 6 h
daily, 5 days/week, over a period of 12 weeks. Depression of
plasma-, erythrocyte-, and brain-ChE activity was observed at the
highest dose level (Kimmerle & Iyatomi, 1976).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
A considerable number of reproduction and teratogenicity
studies have been carried out on different carbamates in several
animal species. A number of these will be used to show the state
of the art giving the well-known carbamates, such as carbaryl,
benomyl, carbendazim, and thiophanate-methyl, as examples.
For more details concerning different carbamates, the reader is
referred to the reports of the Joint FAO/WHO Meetings on Pesticide
Residues and, in the future, to the Environmental Health Criteria
Documents on the individual carbamates.
8.5.1 Reproduction
(a) Methomyl
A 3-generation reproduction study with methomyl was carried out
on rats. Dose levels of 0, 50, or 100 mg/kg diet were administered
for 3 months. There was no evidence from the different indices of
reproduction, lactation, and weanling body weights to suggest that
methomyl affected reproduction or lactation performance in the
litters of three generations (Kaplan & Sherman, 1977).
(b) Benomyl
The effects of benomyl on the gonads and reproductive function
were studied in a 3-generation study on rats. Pronounced effects,
such as a decrease in fertility and decreased activities of a
number of enzymes in the testes were found in rats treated orally
with 1000 mg/kg body weight (the only dose level tested), 5
days/week, for 4 months. Besides the effects mentioned, a
decreased gestation index was also found, which may indicate early
embryonic death due to the induction of a dominant lethal gene in
the sperm cells. An increase in mortality and decreased viablility
and fertility were found in the F1 - F3 generations (not exposed to
the carbamate) (Ivanova-Chemishanska & Antov, 1980; Ivanova-
Chemishanska et al., 1980). In a second 3-generation rat
reproduction study with benomyl, no differences in reproduction or
lactation were observed among control and test groups of animals at
the highest dietary level of 2500 mg/kg diet. No pathological
changes were found in tissues from weanling pups of the F3b litter
(Sherman et al., 1975).
(c) Carbendazim
From 3 multigeneration reproduction studies on rats, in which
carbendazim was tested at dose levels of up to 10 000 mg/kg diet,
no apparent adverse effects on reproduction were found at levels of
up to 2000 mg/kg diet. A reduction in average litter weights was
observed at dietary levels of 5000 and 10 000 mg/kg (FAO/WHO,
1985a).
(d) Carbaryl
In 3-generation rat studies, carbaryl added to the diet to
provide daily doses of up to 10 mg/kg body weight, did not result
in any statistically-significant dose-related effects on fertility,
gestation, viability of pups, or lactation (Weil et al., 1972).
In other studies by Weil et al. (1972, 1973), groups of rats
received carbaryl in the diet at daily doses of 0, 3, 7, 25, or 100
mg/kg body weight by intubation, for 5 days/week, or 0 or 100 mg/kg
body weight per day in the diet. Significant effects were found
on reproduction (mortality and reduced fertility) in the group
receiving 100 mg/kg. A group receiving 200 mg/kg diet did not show
any effects.
The influence of carbaryl at dose levels of 2 or 5 mg/kg body
weight on the reproductive function of rats was studied by
Shtenberg & Otovan (1971). Both dose levels produced adverse
effects on the testes and ovaries (atrophic, dystrophic and
necrotic changes) and changed the gonadotropic function of the
hypophysis. The changes observed increased progressively over the
generations. The fecundity of test females fell, the number of
still-born offspring increased, and there was a high death rate
among young animals.
Carbaryl was fed at dietary levels of 0, 2000, 5000, or 10 000
mg/kg feed to Osborne-Mendel (10 males and 10 females) rats for 3
generations. At the highest dose level, fertility was impaired and
no litters were produced from the second mating of the second
generation. Marked effects from birth to day 4 were manifested as
seen in the survival index. Dose-related decreases in average
litter size, number of live-born progeny, number of survivors to
day 4, and number weaned were observed.
In a comparative study on gerbils, dietary levels of 0, (40
animals) 2000, 4000, 6000 (each 30 animals), and 10 000 mg
carbaryl/kg (18 animals) were tested for 3 generations. No litters
were produced from the second mating of the third generation, at
the highest dose level. Gerbils appeared to be more sensitive than
rats since significant decreases were found consistently at lower
dietary levels, but the effects were not clearly dose-related.
Effects in both species at 2000 mg/kg diet were still marginal but
significant (Collins et al., 1971).
(e) Thiophanate-methyl
Thiophanate-methyl was tested in a 3-generation reproduction
study on rats at dose levels of 0, 40, 160, or 640 mg/kg diet. The
highest dose level affected growth, but no effects of the compound
on reproduction parameters were apparent (FAO/WHO, 1974b).
(f) Carbofuran
A 3-generation reproduction study was conducted on rats (8
males and 16 females per group), with dietary levels of 0, 1, 10,
or 100 mg carbofuran/kg diet. The highest dose level resulted in
decreased weight gain in the parents and a marked reduction in
survival of the young. Lower levels did not have any effects.
Follow-up studies with lower dose levels showed that 20 mg/kg diet
induced adverse effects on reproduction variables, noted after the
first generation (McCarthy et al., 1971; FAO/WHO, 1977b, 1981b).
8.5.2 Endocrine system
(a) Carbaryl
Short-term studies on male and female rats were carried out to
study the functioning of the reproductive system under the
influence of carbaryl. The dose levels ranged from 2 to 300 mg
carbaryl/kg body weight and were administered over periods of 3 -
12 months; in one study, 4 generations were bred. Different
criteria were studied, such as reduction of sperm motility,
duration of sperm survival, inhibition of spermatogenesis and
oögenesis, estrous cycle, induction of degeneration of the germinal
epithelium, and the activities of different enzymes in the testes
and ovaries. Shtenberg & Rybakova (1968) found marked functional
and morphological changes in the endocrine organs of rats
administered carbaryl at daily doses of 7, 14, or 70 mg/kg body
weight for up to 12 months. It was considered by the authors that
the effects of carbaryl on the testes and ovaries might be
explained by the observed increase in hypophyseal gonadotropic
function. A direct effect on the testes and ovaries cannot be
excluded.
Orlova & Zhalbe (1968) studied the effects of carbaryl on white
rats. Dose levels of 2.5 or 5 mg/kg body weight were administered.
Disturbances in the enzymatic activity in the testes and ovaries,
inhibition of spermatogenesis and oögenesis, and decreased
fertility were observed in the P generation, and a decrease in
viability, in the F1 generation.
Shtenberg & Otovan (1971) studied this effect of carbaryl in
more detail in a 4-generation study. The dose levels studied were
0, 2, and 5 mg/kg body weight, and were applied for 6 months in
each generation. The disturbances in the function of the testes
and ovaries described above were found at both 2 and 5 mg/kg
(Vashakidze, 1965, 1967; Shtenberg & Rybakova, 1968; Shtenberg &
Otovan, 1971; Shtenberg et al., 1973). However, the studies
reporting adverse effects on spermatogenesis in male rats were not
confirmed in studies on mice with carbaryl by Dikshith et al.
(1976) or in reproductive performance studies on rats.
(b) Benomyl
A study on the short-term toxicity of benomyl carried out by
Torchinsky et al. (1976) showed that the testis of Wistar rats was
the organ most affected. When administered over a 12-month period,
a dose of 62.5 mg/kg body weight showed borderline effects; 12.5
mg/kg body weight did not show an effect. A recent study on
Sprague Dawley rats receiving 10 daily treatments of 0, 200, or 400
mg benomyl/kg body weight, by gavage, showed a significant decrease
in the total epididymal sperm counts and sperm concentrations in
the vas deferens at both levels. At the higher dose level, slight
to severe hypospermatocytogenesis and generalized
hypospermatogenesis were observed (Carter & Laskey, 1982; FAO/WHO,
1985a). Pastushenko (1983) studied the gonadotoxic effects of
benomyl in rats exposed to dose levels of 0.5, 1.2, 5.0, or 12.4
mg/m3 air. The 2 highest dose levels showed dose-related changes
in the gonads, i.e., disturbances of the morphological and
functional states. At 1.2 mg/m3, only morphological changes were
observed. No effects were found with 0.5 mg/m3.
(c) Carbendazim
When Sprague Dawley rats were fed diets containing 0, 2000,
4000, or 8000 mg carbendazim/kg diet for 28 days, degeneration of
testicular tissue was observed at 4000 and 8000 mg/kg diet.
Spermatogenesis and oögenesis were affected at these feeding
levels. Changes were also found in the organ weights relative to
body or heart weight, changes in the liver being observed at 2000
mg/kg diet (FAO/WHO, 1974b).
No effects on testicular weights and morphology were found in a
study on groups of ChR-CD rats fed carbendazim in the diet for 90
days at dose levels of 0, 100, 500, or 2500 mg/kg (FAO/WHO, 1985a).
8.5.3 Embryotoxicity and teratogencity
A number of carbamate esters have been tested for
teratogenicity with the chicken embryo bioassay (Marliac, 1964;
Ghadiri & Greenwood, 1966; Khera, 1966; Ghadiri et al., 1967;
Rybakova, 1968; Olefir & Vinogradova, 1968; Proctor et al., 1976;
Moscioni et al., 1977). However, this test is not considered of
relevance in testing for teratogenicity, and the results will not
be discussed in this document.
(a) Propoxur
Roszkowski (1982) studied the effects of propoxur on
intrauterine development and on the state of ossification of rat
fetuses. An embryotoxic effect was found at a dose of 20% of the
LD50, administered on days 7 - 19 of the gestation period; 11.9% of
anomalies occurred in the fetuses of treated animals compared with
6.3% in the controls. Decreased weight was also found in the
fetuses. Ossification was disturbed, but this was not related to
the period of gestation during which the substance was
administered. A decrease in the concentrations of calcium and
magnesium was found in the serum of mothers whose fetuses had shown
bone anomalies.
(b) Benomyl
In a teratogenicity study, pregnant ChR-CD rats were fed a diet
containing 0, 100, 500, 2500, or 5000 mg benomyl/kg from day 6 to
day 15 of gestation; no effects were found on the outcome of
pregnancy or embryonal development (Sherman et al., 1975).
Hazleton Laboratories (1968) cited in FAO/WHO (1985a) reported
similar negative results for rabbits, at dose levels up to 500 mg
benomyl/kg diet administered on days 8 - 16 of gestation.
Torchinsky (1973) studied the influence of carbaryl (106 mg/kg
body weight, daily, from day 1 to day 20 of pregnancy) and benomyl
(single dose of 145 or 250 mg/kg body weight on day 12 of
pregnancy) in relation to the protein content of the diet. The
animals were kept on diets containing 5, 10, 19, or 35% of protein
either for one month prior to mating and during the entire
pregnancy or only during pregnancy. The teratogenic and embryotoxic
actions of benomyl and carbaryl did not differ in groups where the
females received diets containing 5, 10, or 19% protein from the
beginning of pregnancy. An increased post-implantational lethality
for the embryos was noted in females kept on a diet with 5% protein
from one month before conception. When these animals were exposed
to carbaryl, the weight of 20-day-old fetuses declined
considerably, yet the severity of the teratogenic action of benomyl
did not increase under these conditions. The teratogenic action of
benomyl and the embryotoxic action of carbaryl were less severe in
animals fed diets containing 10% of protein from one month before
conception than in the groups kept on diets containing 19 or 35%
protein. The results of a few other studies have indicated that
benomyl causes embryotoxic and teratogenic effects in both rats and
mice when administered orally (by gavage) at dose levels at 62.5
mg/kg body weight (rat) and 100 mg/kg body weight (mouse) or more.
At lower dose levels (31.2 mg for the rat and 50 mg/kg for the
mouse), no teratogenic effects were observed. A broad spectrum of
malformations such as exencephaly, hydrocephaly, and
meningocephaly, and disturbance of the CNS were found (Ruzicska et
al., 1975; Petrova-Vergieva, 1979; Kavlock et al., 1982).
(c) Carbaryl
A number of teratogenicity studies carried out with carbaryl
gave contradictory results. Weil et al. (1972) administered up to
500 mg carbaryl/kg body weight to rats. No teratogenic effects or
effects on fertility or gestation were seen at this high dose
level; however, the mean body weight gain of the females during
pregnancy was decreased. Many pups died before weaning. No effects
were found with administration of carbaryl at 100 mg/kg body weight
(Weil et al., 1972).
Oral administration of carbaryl to pregnant rabbits and
hamsters at doses of up to 250 mg/kg body weight, by intubation,
did not cause any teratogenic effects or dose-related fetal
mortality (Weil et al., 1973).
Dougherty et al. (1975), cited in FAO/WHO (1974b), did not
observe any teratogenic effects in monkeys given doses of carbaryl
of 0, 0.2, 2, or 20 mg/kg body weight from day 18 to day 40 of
gestation. This study indicated that even the highest dose level
did not induce a reproductive hazard.
Vashakidze (1965, 1967) found reduced reproduction and abnormal
fetal changes with intubated dose levels of 100 mg/kg body weight
and higher. A level of 50 mg/kg produced negative results.
Carbaryl, administered to guinea-pigs orally during organogenesis
(10 consecutive days between days 11 and 20 of gestation) at 300
mg/kg body weight, induced increased maternal and fetal mortality
and offspring with axial skeletal defects, such as cervical
vertebrae fusion. No abnormal fetal changes were produced in
hamsters at the sub-lethal levels of 125 and 250 mg/kg body weight.
In rabbits, levels ranging from 50 to 200 mg/kg body weight did not
produce any effects on mortality or morbidity, and no abnormal
fetal changes were seen (Robens, 1969).
Smalley et al. (1968) conducted studies on pregnant beagle dogs
fed a diet containing 0, 3.125, 6.25, 12.5, 25, or 50 mg
carbaryl/kg body weight from mating to the termination of weaning.
Effects observed included a number of animals with dystocia
(difficult births) due to atonic uterine musculature, an apparent
contraceptive effect at the highest dose level, and teratogenic
effects (different types of severe defects) at all but the lowest
dose level.
The effects of carbaryl on rat reproduction and guinea-pig
teratology were compared by Weil et al. (1973). A 3-generation
reproduction study on rats included groups receiving carbaryl at
maximum daily doses of 200 mg/kg diet. Other groups concurrently
received daily oral intubation doses as high as 100 mg/kg body
weight. In the guinea-pig teratology study, maximum levels of 300
mg/kg diet or 200 mg/kg body weight (intubation) were given. The
concurrent administration of carbaryl by daily oral administration
or by dietary inclusion did not result in either teratological or
mutagenic effects in the rat or teratological effects in the
guinea-pig, at the maximum dosage levels. However, the groups
receiving maximum levels orally showed severe toxic effects in both
studies compared with little or no effect with oral administration
of 25 mg/kg in rats and 100 mg/kg in guinea-pigs.
The authors concluded that the severe effects found in rats by
Shtenberg & Otovan (1971) following repeated oral intubation of
carbaryl at 2 mg/kg body weight could not be confirmed, even at the
maximum oral intubation level of 100 mg/kg body weight.
(d) Methomyl
When rabbits were fed dietary levels of 0, 50, or 100 mg
methomyl/kg on days 8 - 16 of gestation, no skeletal or visceral
abnormalities were seen in the fetuses (Kaplan & Sherman, 1977).
(e) Carbofuran
Teratogenicity studies have been carried out on albino rats and
albino rabbits with carbofuran. In rats, dose levels of 0, 0.1,
0.3, or 1.0 mg/kg body weight were administered throughout
gestation (days 6 - 15 of pregnancy) and, in rabbits, levels of 0,
0.2, 0.6, or 2.0 mg carbofuran/kg body weight were administered
from day 6 to day 18 of gestation. In both animal species, the
highest dose level showed clear signs of poisoning. However,
pregnancy, viability, fetal body weights, and all the other
examinations did not show any effects. All the progeny were
normal. No teratogenic effects were seen in rats or rabbits
(McCarthy et al., 1971; FAO/WHO, 1981b).
(f) Carbendazim
Carbendazim has been shown to be embryotoxic at dose levels of
approximately 19 mg/kg body weight or more. This effect was not
seen at 10 mg/kg body weight (Delatour & Richard, 1976).
8.6 Mutagenicity and Related End-Points
There is little evidence, if any, to suggest that methyl
carbamate compounds are mutagenic. An evaluation of the mutagenic
potential of carbaryl, methomyl, propoxur, carbofuran, and
pirimicarb, using a variety of microorganisms as indicator strains,
including strains of Salmonella typhimurium, Escherichia coli,
Saccharomyces cerevisiae, and Bacillus subtilus, produced
negative results. These studies showed that carbamate compounds
have little potential for presumptive gene mutations in higher
animals.
Anderson et al. (1972) examined barban, chloropropham, propham,
and swep for their ability to induce point mutations in 8
histidine-requiring strains of S. typhimurium. None showed induced
mutations. This study was carried out in the absence of any
metabolic system.
The chemical structure of benomyl probably enables it to act
both as a base analogue for DNA (benzimidazole ring incorporates
into nucleic acids instead of guanine) and as a spindle poison
(carbamate groups) (Seiler, 1975, 1976b; FAO/WHO, 1985a). The
binding of benomyl to microtubular proteins disturbs the mitotic
spindle, thus causing malsegregation of chromosomes during the
mitotic anaphase (Morris, 1980, cited by Finnish National Board of
Health Toxicology Group, 1982). The results of several studies
suggest that benomyl is a strong antimitotic agent which, even in
low concentrations, induces non-disjunction of single chromosomes
leading to aneuploidy (FAO/WHO, 1985a).
The induction of low-frequency forward mutations by carbendazim
in S. typhimurium strain LT-2 was reported by Seiler (1972).
Reversions reported to occur in S. typhimurium strains His G46 and
TA 1530 were attributed to base-substitution mutations caused by
carbendazim.
Another study was made with the WP2 uvrA repair-deficient
strain of E. coli and the TA 1535 repair-deficient strain of
S. typhimurium. Mutagenic activity was detected for benomyl in the
former, but not in the latter or in TA 1538 (Kappas et al., 1976).
No effects were found in the CM-611 strain of E. coli, deficient
in either the excision repair pathway or the lex A+-dependent
misrepair pathway. In studies carried out recently, benomyl was
negative in all S. typhimurium strains used, even though, in some
of the repeats, significant increases in the number of revertants
were observed in TA 1535 strain. Carbendazim was clearly mutagenic
in the TA 98 strain with metabolic activation (S-9 mix) and
slightly mutagenic in the TA 1535, TA 1537, and TA 100 strains. A
positive result for carbendazim in the TA 1537 strain was observed,
also without metabolic activation (Haskell Laboratories, cited by
the Finnish National Board of Health, 1982; FAO/WHO, 1985a).
There is evidence that carbendazim and related compounds are
antimitotic agents and cause mitotic arrest, mitotic delay, and a
low incidence of chromosome damage in SPF Wistar rats and mammalian
cell lines. The mutagenic effects of carbendazim in bacteria are
probably due to base substitutions, resulting from the
incorporation of small quantities of benzimidazole into nucleic
acids. The effects on mammalian cells in vivo and in vitro
indicate that carbendazim causes chromosome damage, which may
result from this type of mutagenic activity, but the effects on
mitosis suggest that, in higher organisms, the main genetic effect
is to cause non-disjunction (Styles & Garner, 1974).
An abnormal number of micronucleated erythrocytes have been
observed in the bone marrow of mice treated orally with carbendazim
at 100 mg or more/kg body weight. No effects were seen at 50
mg/kg. Benomyl at 1000 mg/kg body weight produced a slight
increase in micronucleated erythrocytes.
Carbendazim did not induce chromosome breakage in Chinese
hamster bone-marrow cells at oral doses as high as 1000 mg/kg body
weight (Seiler, 1976a). It was concluded that carbendazim is not
a chromosome-breaking agent, and its micronucleic action is
attributable to antimitotic activity caused by inhibition of
spindle formation or spindle function.
An evaluation of the mutagenic properties of benomyl has been
completed and reported by the US Environmental Protection Agency
Pesticide Genotoxicology Program. Benomyl increased the mutation
frequency in a concentration-related manner in L 51784 mouse
lymphoma cells and induced sister chromatid exchange in Chinese
hamster ovary (CHO) cells. Benomyl was also positive at all dose
levels tested in the in vivo micronucleus test with mice. The
results of the spot test conducted on mice support the idea that
carbendazim (oral application of 100 - 300 mg/kg body weight to the
mother on the 10th day following conception) may be mutagenic in
mammalian cells due to a direct action on DNA (Fahrig & Seiler,
1979).
A dominant lethal study to evaluate the potential mutagenic
effects in the reproductive cycle of the male mouse was conducted
with propoxur at dose levels of 0, 2.5, or 5 mg/kg body weight.
For a period of 6 weeks, 3 virgin females were exposed to each male
at weekly intervals and reproduction indices recorded. The
administration of propoxur proved to have a transient effect on the
reproductive capability of the males but there was no sign of early
resorption indicating a mutagenic effect (FAO/WHO, 1974b).
DNA-repair tests (with both isolated rat and mouse hepatocytes)
were conducted using benomyl and carbendazim. All tests showed
negative results for the induction of DNA repair (Haskell
Laboratories, 1981a,b cited in FAO/WHO, 1985a).
The Finnish National Board of Health (1982) summarized the
mutagenicity data on benomyl, carbendazim, and thiophanate-methyl
(Table 7) and concluded that the results were sometimes
contradictory. However, positive mutagenicity results from point
mutation tests and chromosome aberration tests are well documented,
especially in higher organisms, and it can be concluded that
carbendazim fungicides should be considered as weak mutagenic
compounds.
Without going into detail, it is known that a large number of N
-nitroso derivatives of methyl carbamates are potent mutagens. The
N -nitroso derivatives of aldicarb, propoxur, carbofuran,
trimethacarb, and methomyl caused numerous single-strand breaks in
normal human skin cells. The data suggested that human cellular
DNA in vivo was irreversibly altered resulting in numerous alkali-
sensitive bonds (Blevins et al., 1977).
A similar result was obtained with N -nitroso carbaryl. This
compound showed strong mutagenic activity in 2 bacterial systems,
E. coli and Haemophilus influenzae. Using S. typhimurium, N -
nitroso carbaryl acted as a potent base-pair substition mutagen and
showed a relatively mild frameshift activity. The parent compound,
carbaryl, gave negative results (Regan et al., 1976; FAO/WHO,
1982b).
Uchiyama et al. (1975) tested the nitroso derivatives of BPMC,
propoxur, hoppcide, isoprocarb, MPMC, and MTMC, in the back
mutation test with E. coli B/r WP2 try- and in the rec-assay
method with B. subtilus strains. The N -nitroso derivatives of
all these N -methylcarbamates showed potent mutagenic activity.
Table 7. A summary of the mutagenicity data on benomyl, carbendazim (MBC), and
thiophanate-methyl
----------------------------------------------------------------------------------------
Point mutations Genetic change
Chromosomal aberrations Non-disjunction
----------------------------------------------------------------------------------------
Positive results + + +
Salmonella typhimurium rat fetus
(Seiler, 1972) (Ruzicska et al., 1975)
Salmonella typhimurium rat bone marrow rat bone marrow
(Haskell Laboratories, 1982 (Styles & Garner, 1974) (Styles & Garner, 1974)
cited in FAO/WHO, 1985a)
mouse bone marrow
(Seiler, 1976a)
Escherichia coli Aspergillus nidulans
(Kappas et al., 1976) (Kappas et al., 1974;
Bignami et al., 1977)
Fusarium oxysporum
(Dassenoy & Meyer, 1973)
Mouse/spot-test Chinese hamster ovary (SCE)
(Fahrig & Seiler, 1979) (SRI International, 1980,
cited in FAO/WHO, 1985a)
micronucleus in vivo /mouse
(SRI International, 1980,
cited in FAO/WHO, 1985a)
Negative results - - -
Streptomyces coelicolor rat bone marrow dominant lethal/rat
(Carere et al., 1978) (Ruzicska et al., 1975) (Sherman et al., 1975)
Salmonella typhimurium Chinese hamster bone marrow dominant lethal/mice
(Fiscor et al., 1978) (Seiler, 1976a) (Makita et al., 1973)
(Carere et al., 1978)
Salmonella typhimurium lymphocyte culture-cytotoxicity
(Haskell Laboratories, 1981; obs.
cited in FAO/WHO, 1985a) (Lamb et al., 1980)
Aspergillus nidulans benomyl-workers
(Bignami et al., 1977) (Ruzicska et al., 1975)
Drosophila /recessive lethal hepatocyte culture/DNA-repair
- sterility obs. rat, mouse
(Lamb & Lilly, 1980) (Haskell Laboratories, 1981,
cited in FAO/WHO, 1985a)
human lymphocyte culture
(Gupta & Legator, 1975,
cited in FAO/WHO, 1985a)
----------------------------------------------------------------------------------------
From: The Finnish National Board of Health (1982) FAO/WHO (1985a).
8.7 Carcinogenicity
8.7.1 General
The mechanism of the carcinogenic action of ethylcarbamate
(urethane) has not yet been established, but its structure seems to
be optimal. All changes seem to reduce activity, particularly when
the ethyl group is replaced with larger side chains. The addition
of alkyl groups to the nitrogen also reduces activity (Mirvish,
1968). It is not so clear why urethane is carcinogenic, whether it
is the proximal toxin or whether it has to be converted to an
active metabolite. The low carcinogenic potential of esters of N -
substituted carbamic acids is possible.
In 1976, a Working Group of the International Agency for
Research on Cancer (IARC, 1976) reviewed the available data on a
number of carbamate pesticides included carbaryl, propham,
chloropropham, and mexacarbate.
Carbaryl was tested in a number of carcinogenicity tests some
of which were not relevant or were of inadequate design. The rat
study (40 per group), in which carbaryl was administered at dose
levels of 0, 50, 100, 200, or 400 mg/kg diet for 2 years, did not
give any indication of an increase in tumour incidence (Carpenter
et al., 1961; Innes et al., 1969; Andrianova & Alekseev, 1970;
IARC, 1976).
Groups of 48 male and 48 female CD-1 mice were given 0, 100, or
400 mg carbaryl/kg diet. After 80 weeks, the tumour incidence
between the groups was similar (Mellon Institute, 1963 cited in
FAO/WHO, 1965b).
Mexacarbate was administered orally to 2 strains of mice. The
design of this study was inadequate (only a small number of
animals), but an increased incidence of lung adenomas was shown in
both strains, together with a slight increase in liver tumours in a
number of males of one strain. According to IARC (1976), it was
not possible to make an evaluation of the carcinogenicity of
mexacarbate on the basis of this study by Innes et al. (1969).
Propham and chlorpropham were administered via the oral route
in a number of carcinogenicity tests on mice, rats, and hamsters.
One or both compounds were also tested on mice and/or rats via the
subcutaneous, intraperitoneal, or intrapleural route. The design
of some of these studies was inadequate. Propham and chloropropham
were tested for initiation properties, because the ethyl carbamates
are related to the well-known carcinogen ethylcarbamate (urethane),
which is an initiator of skin tumours. There was no evidence that
propham and/or chloropropham were carcinogenic after oral,
subcutaneous, intraperitoneal, and intrapleural application.
However, these two compounds acted as weak initiators in the two-
stage carcinogenicity studies on mice. A second study by the same
authors did not confirm the results (Van Esch et al., 1958;
FAO/WHO, 1965b; Innes et al., 1969; Van Esch & Kroes, 1972; IARC,
1976).
Long-term studies on rats did not provide any evidence of
carcinogenic activity when propoxur was fed in the diet for 2 years
at levels of 0, 250, 750, 2000, or 6000 mg/kg diet. Increased liver
weight was found at the highest dose level (6000 mg/kg) in males
and in the three highest dose levels in females. This liver change
was not reflected in liver function tests, clinical chemical
examinations, or histological changes (FAO/WHO, 1974b). Similarly,
long-term and 2-year studies on rats, dogs, mice given carbofuran
or pirimicarb did not indicate any carcinogenic potential. The
dose levels tested were 175 mg/kg diet for rats and 1.8 mg/kg body
weight for dogs for pirimicarb, 100 mg/kg diet for rats, and 500
mg/kg diet for mice (24 months in mice) for carbofuran (FAO/WHO,
1977b, 1981b). On the basis of a variety of test protocols, these
carbamate compounds did not show any significant carcinogenic
potential.
Benomyl was tested for carcinogenicity in CD-1 mice, at dose
levels of 0, 500, 1500, or 5000 mg/kg diet (approximately
equivalent to 0, 50, 150, or 500 mg/kg body weight). A significant
dose-dependent increase in the incidence of primary hepatocellular
adenomas and carcinomas in female mice was seen. Furthermore,
histopathological changes occurred in a number of organs (Haskell
Laboratories, 1981 cited in FAO/WHO, 1985a).
Low dose levels of thiophanate-methyl (0 - 640 mg/kg diet,
equivalent to approximately 0 - 64 mg/kg body weight) were tested
for carcinogenicity in ICR-SCL mice. This study showed that
ingestion of 640 mg thiophanate-methyl in the diet by a mouse
strain susceptible to various tumours did not affect carcinogenic
activity. In another study on rats, dose levels of 0, 10, 40, 160,
or 640 mg/kg diet did not have any apparent effects on the
occurrence of tumours; the only significant effects appeared to be
decreased growth at the highest dose levels (Hashimoto & Fukuda,
1972, cited in FAO/WHO, 1974b).
The influence on the testes of ChR-CD rats of carbendazim
administered at dose levels of 0 - 10 000 mg/kg diet was studied
over a period of 2 years. However, the incidence of testicular
changes was so high in the two control groups that no opinion could
be given on the possible influence of carbendazim on the testes
(Haskell Laboratories, 1977 cited in FAO/WHO, 1985a).
Carbendazim at dose levels of 0, 150, 300, or 1000 mg/kg diet
(this dose level was increased to 2000 mg/kg at week 4 and to 5000
mg/kg at week 8 for the remainder of the study) was administered to
SPF-Swiss mice. The duration of the study was 80 weeks. At the
highest dose level, neoplastic nodules were found in the livers of
female mice. In males, there was an increased incidence of
hepatoblastomas (CIVO/TNO, 1976 cited in FAO/WHO, 1985a).
A 2-year feeding study on CD-1 mice was conducted using
carbendazim at dose levels of 0, 500, 1500, or 7500 mg/kg diet
(only females completed the 2-year period, the 7500 mg/kg level for
males was decreased to 3750 mg/kg after the first 15 months of the
study and the final sacrifice of this group was antedated by 7
months). A significant increase in hepato-cellular carcinomas
occurred at 1500 mg and 7500 mg/kg in female mice and at 1500 mg/kg
in male mice (at the highest dose level for males, there were too
few survivors to judge the effects) (Haskell Laboratories, 1982;
FAO/WHO, 1985a).
Other studies demonstrated that carbamates in the presence of
nitrite could be converted to N -nitroso derivatives, which may be
(partly) responsible for the "carcinogenic activity of the
carbamates".
Studies in which carbendazim was administered to Swiss mice,
twice, at 250 mg/kg body weight, intragastrically, weekly, in
combination with 5000 mg sodium nitrite/litre drinking-water caused
a slight increase in the incidence of lymphosarcomas (Börzsönyi &
Csik, 1975; Börzsönyi & Pinter, 1977).
The results of an in vitro study of Beraud et al. (1979)
(IARC, 1976) suggested that nitrosocarbaryl could be produced in
rat gastric juice from carbaryl.
The proposal that carbamate pesticides may be converted to N -
nitroso compounds under the acid conditions of the stomach is based
on the results of in vivo nitrosation studies with secondary
amines and other compounds in the presence of nitrite (originating,
for instance, from certain vegetables and present in saliva)
(Tannenbaum et al., 1974). Elespuru & Lijinsky (1973), Eisenbrand
et al. (1974, 1975a,b), Elespuru et al. (1974), Ungerer et al.
(1974), Quarles & Tennant (1975), Lijinsky & Taylor (1976), and
Oliver (1981) have shown that certain pesticides, such as carbaryl
and dithiocarbamates, can be nitrosated in vivo.
As pointed out by IARC (1976), the extrapolation of findings in
experimental animals to man is complicated by many factors. It is
relatively easy to show that N -nitroso derivatives can be formed
and that these are mutagenic and/or carcinogenic. However, the
crucial information is the quantity produced in man under the
prevailing conditions. The concentration of both reactants,
differences in pH, the influence of competing reactions, and the
presence of accelerators and inhibitors are all important. In
addition to these difficulties in defining potential human
exposure, the susceptibility of man has to be compared with that of
experimental animals. General considerations on N -nitrosatable
pesticides are described in IARC (1983).
8.8. Special Studies
Adequate characterization of the neurobehavioral effects of
carbamates is still incomplete or not available. In general, when
given in single doses the ChE-inhibiting carbamates decrease
activity and schedule-controlled behaviour, interfere with
avoidance and escape performance, and decrease the amplitude of a
variety of electrophysiological measures. The possible
interference with learning and memory by the carbamates has been of
interest because of the hypothesized role of the cholinergic system
in these functions. However, the evidence that these compounds
interfere with learning and memory is equivocal (Miller, 1982).
Carbamates interfere with shock-motivated behaviour including
discrete-trial and one-way avoidances as well as shock-induced
suppression. Furthermore, these compounds decrease responsiveness
to shock (Sideroff & Santolucito, 1972).
The effects of the ChE-inhibiting carbamates have been
evaluated in man. However, these studies have been mainly
concerned with correlating ChE inhibition and overt symptoms. It is
assumed that, if the clinical indicators (such as ChE levels) are
normal after pesticide exposure, or if symptoms associated with
cholinergic overstimulation are absent, then no risk is involved
(Miller, 1982).
9. EFFECTS ON MAN
The health hazards from overexposure to carbamate insecticides
occur primarily as a result of the inhibition of the enzyme ChE.
ChE inhibition leads to the accumulation of endogenous ACh and
other choline esters in the organism. This produces signs and
symptoms as specified in section 9.3.
9.1 General Population Exposure
9.1.1 Acute toxicity: poisoning incidents
Carbamate pesticides have occasionally been used in cases of
suicide. Fatal poisonings have occurred with carbaryl, propoxur,
and mexacarbate. Farago (1969) described a case involving a 39-
year-old man who drank 1/2 litre of carbaryl. He entered the
hospital 1 1/2 h later and received gastric lavage, but developed
serious respiratory depression. His condition improved somewhat
after 4 injections of atropine at 1/2-h intervals. Three hours
after the carbaryl ingestion, 250 mg of 2-PAM intravenous was
administered. His lung condition rapidly worsened and he died.
Administration of 2-PAM is contraindicated for cases of carbaryl
poisoning, as it may increase toxicity rather than reactivate the
inhibited enzyme and, in this case, it was concluded that the fatal
outcome, though unavoidable, was hastened by 2-PAM administration.
Reich & Welke (1966) reported a fatal case of mexacarbate
poisoning involving a 17-year-old nursery employee who ingested
approximately 55 g mexacarbate in the form of a 22% formulation.
The young man, found unconscious, had pinpoint pupils and an
irregular heartbeat. Emergency procedures at the hospital briefly
restored regular heart rhythm, but brachycardia developed later
with recurrent heart failure. The patient died about 4 - 4 1/2 h
after the ingestion of the mexacarbate.
Two severe cases of propoxur poisoning following ingestion of
150 - 200 ml of commercial Baygon formulation were described by
Bomirska & Winiarska (1972). One individual responded to treatment
and recovered but the other died after failing to respond to
treatment with atropine and 2-PAM and toxogonin given
intravenously. A normal ChE suggested spontaneous reactivation of
this enzyme.
9.1.2 Effects of short- and long-term exposure
Propoxur was tested for its effectiveness in controlling the
mosquito vectors of malaria in Iran. The insecticide was sprayed
inside houses in a 300 km2 area in which 11 000 people lived in 33
villages. Sixty-nine out of the 11 000 inhabitants complained of
mild cholinergic symptoms, when they entered their houses during
the spraying, contrary to instructions. The symptoms were
headache, nausea, sweating, and vomiting. The symptoms subsided in
2 - 3 h. Similar experiences were reported in other villages
sprayed with propoxur or with carbaryl (Vandekar et al., 1968).
9.1.3 Controlled human studies
Human volunteers tolerated single oral doses of aqueous
solutions of aldicarb at doses of 0.025, 0.05, or 0.1 mg/kg body
weight, with only mild depression of whole blood-ChE in all cases.
Only the persons receiving 0.1 mg/kg presented symptoms of acute
cholinergic stress (i.e., nausea, sweating, pinpoint pupils,
salivation) (FAO/WHO, 1982b).
Groups of male volunteers (5 or 6 per group) were administered
daily doses of 0.06 and 0.13 mg carbaryl/kg body weight, orally,
for 6 weeks. No subjective or objective changes were observed,
except a slight reversible decrease in the ability of the kidneys
to reabsorb aminoacids in the group at the highest dose level
(Wills et al., 1968).
Thirty-eight urban volunteers were monitored for carbaryl
exposure during the summer. All volunteers were involved in the
application of carbaryl incidental to their employment or leisure
activities. Exposure of clothing, skin, with and without gloves,
were measured. The maximum dermal exposure recorded was 2.86 mg/kg
per h, the maximum air concentration was 0.28 mg/m3. Only a small
mean decrease in serum- and erythrocyte-AChE activity was found
(Gold et al., 1982). Comparable results were obtained by Leavitt et
al. (1982) in a study on 2 groups of professional pesticide spray
operators with higher dermal exposure, spraying trees with
carbaryl.
A 42-year-old male volunteer took a single oral dose of 135 mg
propoxur (1.5 mg/kg body weight) about 2 h after breakfast.
Plasma- and erythrocyte-ChE activities were determined every 15 min
for the first 2 h after ingestion and then every hour thereafter.
Pulse and blood pressure were recorded. A rapid drop in
erythrocyte-ChE activity was observed, reaching the lowest level of
27% of normal within 20 min. ChE activity gradually recovered,
reaching normal levels about 2 h after ingestion. Plasma-ChE
activity was not affected. In another study (single dose of 0.36
mg/kg body weight), the lowest erythrocyte-ChE activity occurred
within 10 min together with short-lasting stomach discomfort,
blurred vision, nausea, and facial perspiration. Twenty min after
ingestion, the volunteer's pulse rate and blood pressure were
increased. Pronounced nausea accompanied by repeated vomiting and
profuse sweating occurred until approximately 45 min after the
ingestion of propoxur. From then on, poisoning symptoms
diminished, blood pressure and pulse returned to pre-ingestion
values. The disappearance within about 2 h of the poisoning
symptoms was in line with the return of normal erythrocyte-ChE
activity. Forty-five percent of the ingested dose of propoxur was
excreted as the metabolite, 2-(1-methylethyl)phenol, within 24 h of
ingestion (Vandekar et al., 1971; FAO/WHO, 1974b).
In another study, 5 doses of propoxur, at 0.15 or 0.20 mg/kg
body weight, were administered to volunteers at 1/2-h intervals.
In this study, a symptomless depression of erythrocyte-ChE to 60%
of normal was observed over the course of the dosing. The enzyme
activity recovered to normal levels soon after termination of the
dosing. From the above studies, it was concluded by the authors
that an oral dose of 0.36 mg propoxur/kg body weight may be
sufficient to induce initial cholinergic symptoms, while larger
doses may be tolerated without symptoms if the administration is
gradually (divided doses) (Vandekar et al., 1971).
9.2 Occupational Exposure
9.2.1 Acute toxicity: poisoning incidents
Tobin (1970) reported 3 cases of persons poisoned with
carbofuran. Two were formulation plant employees, preparing 10%
granules and the third was an entomologist who began to feel
uncomfortable while weighing a 50% water-dispersible powder
formulation. The 2 formulators developed similar symptoms
indicative of carbamate poisoning: profuse sweating, weakness,
blurred vision, and nausea. Both were taken to their respective
physicians about 3 h after the onset of symptoms. One physician
administered atropine; the other, noting the regression of the
symptoms, did not administer atropine. The patient who received
the atropine (0.02 g im) was fully recovered in 30 min. The
untreated patient recovered over the course of 2 - 3 h. The third
case, the entomologist experienced mild discomfort that regressed
without atropine administration in 4 - 6 h.
Richardson & Batteese (1973) described the case of a spray-
plane co-pilot overexposed to mexacarbate during an spraying
operation. The cause of the overexposure was a pin-hole leak in a
high pressure pump line, which emitted a fine aerosol of the
mexacarbate formulation into the fuselage. The co-pilot developed
the classical signs of ChE poisoning. Toxic symptoms progressed to
paralysis of the extremities before he could be treated. In
hospital, atropine sulfate was immediately administered
intravenously followed by a second injection subcutaneously 40 min
later. The symptoms subsided quickly. ChE activity in blood drawn
after the first atropine injection was severely depressed, but
returned to normal after 3 days.
Minor and transient cholinergic symptoms were experienced by
spraymen applying propoxur formulations inside houses for testing
this insecticide against mosquito vectors of malaria in villages in
El Salvador (Quinby & Babione, 1966) and Iran (Vandekar et al.,
1968; Montazemi, 1969). A pronounced fall in blood-ChE activity
during the work and a distinct recovery after exposure ceased was
established as a daily pattern of activity of this enzyme,
erythrocyte-ChE being more sensitive to propoxur than plasma-ChE.
No cumulative inhibition of ChE during the 6-week exposure was
seen. The investigators noted that the exposed spraymen and a few
inhabitants recovered rapidly when removed from the source of
exposure and when treated with atropine.
Vandekar et al. (1968) commented that most spraymen who became
poisoned had heavy skin contamination, and had not cleaned their
skin sufficiently after spraying. The inhabitants entered the
house while it was being sprayed. Moreover, it was noted that
respirators did not offer protection against intoxication under
these circumstances and that dermal absorption accounted for most
of the absorbed dose.
No adverse health effects due to carbaryl exposure (except for
one case of skin rash) were observed in a study conducted by the
WHO Insecticide Testing Unit in Southern Nigeria. Ten men (out of
105 sprayers) spraying over a single 6-h period and 95 villagers
who resided in the sprayed houses, were examined. The sprayers
wore overalls, hats, and rubber boots during the entire 6 h of
spraying, but wore masks for the first hour only. Plasma-ChE
activity as well as urinary metabolites of carbaryl were determined
before and after spraying. A slight (average 5%) reduction in
plasma-ChE activity was reported for all 10 sprayers, the day after
the carbaryl application. Inhabitants of sprayed houses showed no
inhibition of plasma-ChE activity, 1 week after the spraying. A
slight increase in 1-naphthol was found in the urine of the
sprayers only on the sixth day after the exposure (Vandekar, 1965).
In a study on 19 agricultural workers in the USSR, whole blood-
ChE activity was measured before and after 4 to 6-h exposures to
airborne carbaryl for 3 - 4 days. Significant ChE inhibition was
found in men exposed to a mean airborne carbaryl concentration of
up to 4 mg/m3. No objective signs of ill health were observed. No
changes were measured at 0.7 mg/m3 (Yakim, 1967).
A case of acute intoxication of a woman with a total dose of 60
mg carbofuran was described by Izmirova et al. (1981). Slight ChE
inhibition was found, but within 72 h the patient recovered
completely.
The above reports identify systemic ChE poisoning as the major
hazard associated with occupational exposure to carbamate
pesticides. However, other publications indicate that certain of
these compounds can also irritate the exterior membranes of the
body. Tobin (1970) described 2 cases of field workers that
illustrate direct effects on the eye, (irritation and blurred
vision) during carbofuran spraying operations. These 2 persons did
not developed symptoms of systemic poisoning.
Vandekar (1965) reported skin rash in a sprayman accidentally
splashed with a carbaryl formulation. Although carbamate compounds
have not generally been implicated as the cause of dermatitis or
allergic skin reactions, Vandekar (1965) suggested that certain
individuals could develop this after unusually heavy exposure.
9.2.2 Effects of short- and long-term exposure
Little is known about the short- and long-term effects of
occupational exposure to carbamate pesticides. The few reports
that discuss this aspect do not implicate long-term exposure to the
carbamate compounds as a cause of human disease. Vandekar et al.
(1968) did not observe any evidence of cumulative toxicity among
spraymen applying propoxur formulations during a 6-week operation.
No long-term biochemical changes were reported among New Jersey
farmers and spraymen compared with a minimally exposed control
group. It was noted that significantly higher incidences of
hearing and eye problems, chronic cough, dizziness, headaches, and
hypertension occurred in the occupationally exposed group.
However, the various pesticides to which this group was exposed
were not tabulated (Kwalick, 1971).
Chromosome studies on the blood cultures of 20 workers engaged
in the production of benomyl did not reveal any increased frequency
of structural chromosome aberrations (Ruzicska et al., 1975).
In a survey of occupationally acquired disease in workers at a
pesticide plant, 11 out of 102 workers had been hospitalized for
illness related to chemical exposure. The commonest cause of
hospitalization was intoxication with the ChE inhibitor methomyl.
On clinical evaluation, 5 out of 11 packaging workers, with the
highest exposure to methomyl had experienced blurred vision or
pupillary constriction (Morse & Baker, 1979).
9.2.3 Epidemiological studies
Epidemiological studies on persons occupationally exposed
exclusively (or at least primarily) to carbamate pesticides are not
available. Such studies are needed before the long-term effects of
this class of pesticides on human beings can be adequately
evaluated.
9.3 Signs and Symptoms of Acute Intoxication by Carbamates
The clinical picture of carbamates intoxication results from
accumulation of ACh at nerve endings. Extensive description of
the syndrome is given in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980). The signs and symptoms can be
categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases,
diaphragm and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by the respiratory failure sometimes
leading to pulmonary oedema due to the combination of the above-
mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure;
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to light; pulse rate is not of
diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ;
on the other hand, since changes in the conduction
and excitability of the heart might be
life-threatening, monitoring should be performed.
(c) confirmation of diagnosis is made by measurement of
AChE in RBC or plasma-pseudoChE (dibucaine number
also, to rule out genetic deficiencies). In view of
the rapid reversibility, particular care should be
taken to use analytical procedures that include
immediate analysis and a careful control of sample
dilutions (section 2.3). Chemical analysis of body
fluids (gastric lavage, blood, urine) should be
performed for the identification of the chemical(s).
9.3.1 Biochemical methods for measurement of effects
AChE present in human erythrocytes is the same as the enzymes
present in the target synapses. Measurements of AChE in RBC is
therefore taken to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the carbamate has equal access to blood and the
synapses, and that AChE inhibition by carbamates is reversible.
In the case of acute poisoning, a high inhibition of RBC-AChE
is pathognomonic, but, in the follow-up of the intoxication, it
might not be correlated with the severity of symptoms. However,
monitoring of pre- and post-exposure levels of AChE in RBC gives a
good measure of the effect of an exposure (Kaloyanova, 1976). In
cases when the pre-exposure AChE level is not known (as in
accidental poisoning), reference can be made to a mean population
AChE activity. Blood-plasma contains a related enzyme called ChE or
pseudo-ChE, which contributes to the whole blood enzymatic
activity; the extent of the contribution of plasma-ChE will depend
on which substrate was used and at what concentration. PseudoChE
has no known physiological function, but can be inhibited
selectively by some carbamates without causing a toxic response.
The sensitivities of AChE and pseudoChE to inhibitors differ so
that measurements of the ability of whole blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many field conditions, procedures using whole blood are more
practical than those using separated erythrocytes.
9.4 Treatment of Acute Poisoning by Carbamate Insecticides
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977; Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
9.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in the cleaning of the skin area where
venupuncture is performed. Blood might be contaminated with
carbamates and therefore inaccurate measures of ChE inhibition
might result. Extensive eye irrigation with water or saline should
also be performed. In the case of ingestion, vomiting can be
induced, if the patient is conscious, by the administration of
ipecacuanha syrup (10 - 30 ml) followed by 200 ml of water.
However, this treatment is contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
the addition of bicarbonate solution or activated charcoal) can
also be performed, particularly in unconscious patients, taking
care to prevent aspiration of fluids into the lungs (i.e., only
after a tracheal tube has been put in place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
9.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
9.4.3 Specific pharmacological treatment
9.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.).
9.4.3.2 Oxime reactivators
There is no rational basis for using these drugs. Furthermore,
some unconfirmed reports suggest an increased toxicity of
carbamates when oximes have been administered.
9.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of
artificial respiration procedures.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
For more than 20 years, the Joint FAO/WHO Meetings on Pesticide
Residues (JMPR) and the International Agency for Research on Cancer
(IARC) have been evaluating the toxicity and carcinogenicity data
on the different carbamates. As part of the JMPR meeting, the FAO
deals with the use and application of these pesticides and
proposes residue limits. Data and conclusions of the meetings were
initially published in the WHO Technical Report Series but, since
1977, they have been published regularly in the FAO Plant
Production and Protection Papers.
It is not possible to review completely the results of the
discussions on the approximately 50 carbamates mentioned in these
documents. However, Annex III contains a list of the JMPR meetings
in which carbamates have been evaluated together with appropriate
references. Other information available in Annex III indicates
whether IARC evaluations and WHO/FAO Data Sheets are available, and
whether an IRPTC data profile and legal file exist.
REFERENCES
ABDEL-AAL, Y.A.I. & FAHMY, M.A.H. (1977) Molecular
modification of carbamates in relation to their selective
toxicity. In: Proceedings of the British Crop Protection
Conference on Pests and Diseases, Brighton, Nov. 1977, British
Crop Protection Council, Croydon, pp. 155-159.
ABD-ELROAF, T.K., FAHMY, M.A.H., FUKUTO, T.R., & EL-SEBAE,
A.H. (1977) Anticholinesterase activity and toxicity of
substituted phenyl methylcarbamates to honey bee. J. econ.
Entomol., 70(1): 78-82.
ABDEL-WAHAB, A.M., KUHR, R.J., & CASIDA, J.E. (1966) Fate of
14C-carbonyl-labelled aryl methylcarbamate insecticide
chemicals in and on bean plants. J. agric. food Chem., 14:
290-298.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1973) The
photochemical reactions of carbamates. III. The solution
photochemistry of metacrate, 3-methylphenyl- N -methyl-
carbamate. Int. J. environ. anal. Chem., 3: 73-79.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1974) The solution
photochemistry of metacil (4-dimethyl-amino- m -tolyl- N -methyl
carbamate) and Landrin (3,4,5-trimethylphenyl- N -methyl-
carbamate). Bull. environ. Contam. Toxicol., 11: 250-255.
AHDAYA, S.M., MONROE, R.J., & GUTHRIE, F.E. (1981)
Absorption and distribution of intubated insecticides in
fasted mice. Pestic. Biochem. Physiol., 16(1): 38-46.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarba-
mates, and dithiocarbamates, Luxembourg, Commission of the
European Communities.
ALDRIDGE, W.N. & REINER, E. (1972) Enzyme inhibitors as
substrates. Interaction of esterases with esters of
organophosphorus and carbamic acids, Amsterdam, Oxford, New
York, Elsevier Science Publishers, 328 pp.
ALY, O.M. & EL-DIB, M.A. (1971) Photodecomposition of some
carbamate insecticides in aquatic environments. In: Faust,
S.D. & Hunter, J.V., ed. Organic compounds in aquatic
environments, New York, Marcel Dekker, pp. 469-493.
ALY, O.M. & EL-DIB, M.A. (1972) Studies of the persistence
of some carbamate insecticides in the aquatic environment. In:
Fate of organic compounds in the aquatic environment. III.
Advances in chemistry series, Washington DC, American Chemical
Society, pp. 210-243.
ANDERSON, C.A. (1973) Baygon. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7, pp.
163-178.
ANDERSON, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDRIANOVA, M.M. & ALEKSEEV, I.V. (1970) [On the carcino-
genous properties of the pesticides sevine, maneb, ciram and
cineb.] Vopr. Pitan., 29(6): 71-74 (in Russian, with English
summary).
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974a) Effects of the
insecticide carbaryl on clams and some other intertidal mud
flat animals. J. Fish. Res. Board Can., 31: 466-470.
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974b) Effects of the
insecticide Sevin and its first hydrolytic product 1-naphthol
on some early developmental stages of the bay mussel Mytilus
edulis. Mar. Biol., 28: 11-15.
ASHTON, F.M. & CRAFTS, A.S. (1973) Carbamates. In: Mode of
action of herbicides, New York, Wiley Interscience, pp.
200-220.
ATKINS, E.L., GREYWOOD, E.A., & MCDONALD, R.L. (1973)
Toxicity of pesticides and other agricultural chemicals to
honey bees. Laboratory studies, Riverside, USA, University of
California, Agriculture Extension Service.
AUGUSTINSSON, K.B. & CASIDA, J.E. (1959) Enzymic hydrolysis
of N,N -dimethylcarbamoyl fluoride. Biochem. Pharmacol., 3:
60-67.
AUSTIN D.J. & BRIGGS, G.G. (1976) A new extraction method
for benomyl residues in soil and its application in movement
and persistence studies. Pestic. Sci., 7: 201-210.
AUSTIN, D.J., LORD, K.A., & WILLIAMS, I.H. (1976) High
pressure liquid chromatography of benzimidazoles. Pestic.
Sci., 7: 211-222.
BAKER, P.B. & HOODLESS, R.A. (1974) Analytical methods for
the detection and determination of residues of systemic
fungicides. Pestic. Sci., 5: 465-472.
BALDWIN, R.E., FREED, V.H., & FANG, S.C. (1954) Absorption
and translocation of carbon-14-applied as O -isopropyl
N -phenylcarbamate in Avena and Zea. J. agric. food Chem.,
2(8): 428-430.
BERAUD, M., PIPY, B., DERACHE, R., & GAILLARD, D. (1979)
Formation d'un cancérigène le N -nitrosocarbaryl par
interactions entre un insecticide de la série des carbamates
le carbaryl et le nitrite de sodium dans le suc gastrique de
rat. Food Cosmet. Toxicol., 17: 579-583.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of
pesticides in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BLACK, A.L., CHIU, Y.C., FAHMY, M.A.H., & FUKUTO, T.R.
(1973) Selective toxicity of N -sulfenylated derivatives of
insecticidal methylcarbamate esters. J. agric. food Chem., 21:
743-747.
BLEIDNER, W.E., MORALES, R., & HOLT, R.F. (1978) Benomyl.
In: Zweig, G. & Sherma, J., ed. Analytical methods for
pesticides and plant growth regulators, New York, London,
Academic Press, pp. 157-171.
BLEVINS, R.D., LYINSKY, W., & REGAN, J.D. (1977) Nitrosated
methylcarbamate insecticides. Effect on the DNA of human
cells. Mutat. Res., 44: 1-7.
BOBIK, A., HOLDER, G.M., & RYAN, A.J. (1972) Excretory and
metabolic studies of isopropyl N -(3-chlorophenyl)carbamate in
the rat. Food Cosmet. Toxicol., 10: 163-170.
BOMIRSKA, T. & WINIARSKA, A. (1972) [Toxicology of
carbamates in the light of intoxication with Baygon
insecticide.] Pol. Tyg. Lek., 27: 1448-1450 (in Polish).
BORZSONYI, M. & CSIK, M. (1975) Induction of malignant
lymphomas in Swiss mice by N -nitroso compounds formed in vivo.
Int. J. Cancer, 15: 830-838.
BORZSONYI, M. & PINTER, A. (1977) The carcinogenicity of
N -nitroso compounds formed endogenously in mice from
benzimidazole carbamate pesticides. Neoplasma, 24: 119-122.
BOWMAN, M.C. & BEROZA, M. (1969) Determination of Mesurol
and five of its metabolites in apples, pears and corn by gas
chromatography. J. Assoc. Off. Anal. Chem., 52: 1054-1063.
BROCKELSBY, C.H. & MUGGLETON, D.F. (1973) Asulam. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 497-508.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARPENTER, C.P., WEIL, C.S., PALM, P.E., WOODSIDE, M.W., NAIR,
J.H., & SMYTH, H.F. (1961) Mammalian toxicity of 1-naphthyl
N -methylcarbamate (Sevin insecticide). J. agric. food Chem.,
9: 30-39.
CARTER, S.D. & LASKEY, J.W. (1982) Effect of benomyl on
reproduction in the male rat. Toxicol. Lett., 11: 87-94.
CASIDA, J.E. & AUGUSTINSSON, K.B. (1959) Reaction of plasma
albumin with 1-naphthyl N -methylcarbamate and certain other
esters. Biochem. Biophys. Acta, 36: 411-426.
CHIBA, M. & DOORNBOS, F. (1974) Instability of benomyl in
various conditions. Bull. environ. Contam. Toxicol., 11:
273-274.
CHIN, W.T., DUANE, W.C., SZALKOWSKI, M.B., & STALLARD, D.E.
(1975) Gas-chromatographic determination of thiofanox
residues in soil, plants, and water. J. agric. food Chem.,
23(5): 963-966.
CLARK, C.G. & WRIGHT, S.J.L. (1970) Degradation of the
herbicide isopropyl N -phenylcarbamate by Arthrobacter and
Achromobacter sp. from soil. Soil Biol. Biochem., 2: 217.
COLLINS, T.F.X., HANSEN, W.H., & KEELER, H.V. (1971) The
effect of carbaryl (Sevin) on reproduction of the rat and the
gerbil. Toxicol. appl. Pharmacol., 19: 202-216.
CROSBY, D.G., LEITIS, E., & WINTERLIN, W.L. (1965)
Photodecomposition of carbamate insecticides. J. agric. food
Chem., 13(3): 204-207.
DASSENOY, B. & MEYER, J.A. (1973) Mutagenic effect of
benomyl on Fusarium oxysporum. Mutat. Res., 21: 119-120.
DAWSON, J.A., HEATH, D.F., ROSE, J.A., THAIN, E.M., & WARD,
J.B. (1964) The excretion by humans of the phenol derived in
vivo from 2-isopropoxyphenyl- N -methylcarbamate. Bull. World
Health Org., 30: 127-134.
DELATOUR, P. & RICHARD, Y. (1976) Propriétés embryotoxiques
et antimitotiques en série benzimidazole. Therapie, 31(4):
505-515.
DE ROSE, H.R. (1951) Crabgrass inhibition with O -isopropyl-
N -(3-chlorophenyl)carbamate. Agron. J., 43: 139-142.
DIKSHITH, T.S.S., GUPTA, P.K., GAUR, J.S., DATTA, K.K., &
MATHUR, A.K. (1976) Ninety-day toxicity of carbaryl in male
rats. Environ. Res., 12: 161-170.
DOROUGH, H.W. & CASIDA, J.E. (1964) Nature of certain
carbamate metabolites of the insecticide Sevin. J. agric. food
Chem., 12(4): 294-304.
DOUCH, P.G.C. (1973) The metabolism of benomyl fungicide in
animals. Xenobiotica, 3: 367-380.
EBERLE, D.O. & GUNTHER, F.A. (1965) Chromatographic,
spectrometric, and irradiation behaviour of five carbamate
insecticides. J. Assoc. Off. Agric. Chem., 48:(5) 927-937.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the
soil fauna. Residue Rev., 45: 1-79.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N -nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under stimulated gastric conditions and in vivo in the
rat stomach. Food Cosmet. Toxicol., 12: 229-232.
EISENBRAND, G., IVANKOVIC, S., PREUSSMANN, R., SCHMAHL, D., &
WIESSLER, M. (1975a) Some recent results on the chemistry,
formation and biological activity of N -nitroso compounds. Gaun
Monogr. Cancer Res., 17: 133-143.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1975b) The
reaction of nitrite with pesticides. II. Formation, chemical
properties, and carcinogenic activity of the N -nitroso
derivative of N -methyl-1-naphthylcarbamate (carbaryl). Food
Cosmet. Toxicol., 13: 365-367.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ELESPURU, R.K., LIJINSKY, W., & SETLOW, J.K. (1974)
Nitrosocarbaryl as a potent mutagen of environmental
significance. Nature (Lond.), 247: 386-387.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V. Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
FANG, S.C., FALLIN, E., MONTGOMERY, M.L., & FREED, V.H.
(1974) Metabolic studies of 14C-labelled propham and
chlorpropham in the female rat. Pestic. Biochem. Physiol., 4:
1-11.
FAHRIG, R. & SEILER, J.P. (1979) Dose and effect of
methyl-2-benzimidazolylcarbamate in the "mammalian spot test",
an in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-biol. Interact., 26: 115-120.
FAO (1985) Production yearbook, 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23.64).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Agricultural Studies No. 73; WHO Technical
Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391.
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
10 Rev).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Suppl.).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Suppl.).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Suppl.).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1985a) 1983 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985c) 1984 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Pesticide residues in food. Report of the
1985 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (in press) 1985 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations.
FARAGO, A. (1969) [Fatal suicidal poisoning with Sevin
(1-naphthyl- N -methyl carbamate).] Arch. Toxikol., 24: 309-315
(in German).
FICSOR, G., BORDAS, S., & STEWART, S.J. (1978) Mutagenicity
testing of benomyl methyl-2-benzimidazole carbamate,
streptozotocin, and N -methyl- N' -nitro- N' -nitrosoguanidine in
Salmonella typhimurium in vitro and in rodent host-mediated
assays. Mutat. Res., 51: 151-164.
FINNISH NATIONAL BOARD OF HEALTH TOXICOLOGY GROUP (1982) The
toxicity of the benzimidazol type fungicides: benomyl,
carbendazim, and thiophanate-methyl (second report), Helsinki,
Finnish National Board of Health.
FILIP, Z. (1974) [Pesticides in powder form.] Vesmir, 53:
243-244 (in Czech).
FUKUTO, T.R. (1972) Metabolism of carbamate insecticides.
Drug Metab. Rev., 1: 117-150.
GARDINER, J.A., KIRKLAND, J.J., KLOPPING, H.L., & SHERMAN, H.
(1974) Fate of benomyl in animals. J. agric. food Chem.,
22(3): 419-427.
GHADIRI M. & GREENWOOD, D.A. (1966) Toxicity and biologic
effects of malathion, phosdrin, and sevin in the chick embryo.
Toxicol. appl. Pharmacol., 8: 342.
GHADIRI M., GREENWOOD, D.A., & BINNS, W. (1967) Feeding of
malathion and carbaryl to laying hens and roosters. Toxicol.
appl. Pharmacol., 10: 392.
GOLD, R.E., LEAVITT, J.R.C., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of urban applications to carbaryl. Arch. environ.
Contam. Toxicol., 11: 63-67.
GOLDBERG, M.E., JOHNSON, H.E., KNAAK, J.B., & SMYTH, H.F., Jr
(1963) Psychopharmacological effects of reversible
cholinesterase inhibition induced by N -methyl-3-isopropyl-
phenyl carbamate (compound 10854). J. Pharm. exp. Ther., 141:
244-252.
GRAY, R.A. (1971) Behaviour, persistence, and degradation of
carbamate and thiocarbamate herbicides in the environment. In:
Proceedings of the California Weed Control Conference, 18-20
January 1971, Mountain View, California, Stauffer Western
Research Centre, pp. 128-143.
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1977) Determination
of carbetamid residues and its aniline metabolite. J. agric.
food Chem., 20: 348-350.
GUPTA, A.K. & LEGATOR, M.S. (1975) Chromosome aberrations in
cultured human leukocytes after treatment with the fungicide
benlate. In: Proceedings of a Symposium on Mutagenic
Carcinogenic and Teratogenic Chemistry, New Delhi, India,
Department of Atomic Energy, pp. 95-103.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained
sheepshead minnows. Trans. Am. Fish. Soc., 98: 426-429.
HARVEY, J., Jr & HAN, J.C.Y. (1978a) Metabolism of oxamyl
and selected metabolites in the rat. J. agric. food Chem.,
26(4): 902-910.
HARVEY, J., Jr & HAN, J.C.Y. (1978b) Decomposition of oxamyl
in soil and water. J. agric. food Chem., 26(3): 536-541.
HARVEY, J., Jr & PEASE, H.L. (1973) Decomposition of
methomyl in soil. J. agric. food Chem., 21(5): 784-786.
HARVEY, J., Jr & REISER, R.W. (1973) Metabolism of methomyl
in tobacco, corn, and cabbage. J. agric. food Chem., 21(5):
775-783.
HARVEY, J., Jr, JELINEK, A.G., & SHERMAN, H. (1973)
Metabolism of methomyl in the rat. J. agric. food Chem.,
21(5): 769-775.
HARVEY, J., Jr, HAN, J.C.Y., & REISER, R.W. (1978)
Metabolism of oxamyl in plants. J. agric. food Chem., 26(3):
529-536.
HEYBROEK, W.M.H., MUGGLETON, D.F., & PARKE, D.V. (1984)
Metabolism of the carbamate herbicide asulam in the rat.
Xenobiotica, 14:(3) 235-247.
IARC (1976) Some carbamates, thiocarbarmates, and
carbazides, Lyons, International Agency for Research on Cancer
(IARC Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 12).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 30;
Appendix 359-406).
INNES, J.R.M., ULLAND, B.M., VALLERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, J., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1976) Changes in the
activity of some enzymes under the action of benomyl. Hig. i
Zdraveopazvane, Sofia, 19(5): 431-435 (in Bulgarian, with
English summary).
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads, reproduction, and generations of white rats under the
effect of some pesticides. Arch. Toxicol., 4(Suppl.): 459-462.
IVANOVA-CHEMISHANSKA, L., ANTOV, G., MIHAILOVA, A., & HINKOVA,
L. (1980) Study on the toxicology of benomyl. In: The Fourth
International Congress of Pesticide Chemistry, Zurich,
Switzerland, 24-28 July, 1978, pp. 11-559.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorous pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application, Amsterdam, Oxford, New
York, Elsevier Scientific Publishers, pp. 169-172 (Studies in
Environmental Science 7).
IZMIROVA, N., MILCEVA, V., MONOV, A., & KALOYANOVA, F.
(1981) [Acute carbofuran intoxication.] Hig. i
zdraveopazvane, 24(5): 445-448 (in Bulgarian, with English
summary).
JACKSON, J.A., CHART, I.S., SANDERSON, J.H., & GARNER, R.
(1977) Pirimicarb induced immune haemolytic anaemia in dogs.
Scand. J. Haematol., 19: 360-366.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of brain "neurotoxic esterase" and the development of delayed
neurotoxicity in hens. Biochem. J., 120: 523-531.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute
toxicity of chemicals to fish and aquatic invertebrates,
Washington DC, US Fish and Wildlife Service (Resource
Publication No. 137).
KAGAN, J.S. (1977) [The toxicity of organophosphorus
pesticides,] Moscow (in Russian).
KALOYANOVA, F. (1976) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Symposium on
Environmental Monitoring, 14-19 September, 1975, Las Vegas,
Nevada, Vol. 1, pp. 1-4, New York, Institute of Electrical &
Electronics Engineers, Inc.
KANAZAWA, J. (1975) Uptake and excretion of organophosphorus
and carbamate insecticides by fresh-water fish Motsugo
pseudoras bora parva. Bull. environ. Contam. Toxicol., 14:
346-352.
KAPLAN, A.M. & SHERMAN, H. (1977) Toxicity studies with
methyl- N -methylamino-carbonyl-oxyethanimidothioate. Toxicol.
appl. Pharmacol., 40: 1-17.
KAPPAS, A., GEORGOPOULOS, S.G., & HASTIE, A.C. (1974) On the
genetic activity of benzimidazole and thiophanate fungicides
on diploid Aspergillus nidulans. Mutat. Res., 26: 17-27.
KAPPAS, A., GREEN, M.H.L., BRIDGES, B.A., ROGERS, A.M., &
MURIEL, W.J. (1976) Benomyl: a novel type of base analogue
mutagen. Mutat. Res., 40: 379-382.
KAVLOCK, R.J., CHERNOFF, N., GRAY, L.E., Jr, GRAY, J.A., &
WHITEHOUSE, D. (1982) Teratogenic effects of benomyl in the
Wistar rat and CD-1 mouse, with emphasis on the route of
administration. Toxicol. appl. Pharmacol., 62: 44-54.
KAUFMAN, D.D. & BLAKE, J. (1973) Microbial degradation of
several acetamide, acrylamide, carbamate, toluidine, and urea
pesticides. Soil Biol. Biochem., 5: 297-308.
KAUFMAN, D.D. & KEARNEY, P.C. (1965) Microbial degradation
of isopropyl- N -(3-chlorophenyl carbamate and 2-chloroethyl- N -
(3-chlorophenyl carbamate. Appl. Microbiol., 13(3): 443-446.
KHERA, K.S. (1966) Toxic and teratogenic effects of
insecticides in duck and chick embryos. Toxicol. appl.
Pharmacol., 8: 345.
KIMMERLE, G. & IYATOMI, A. (1976) Toxicity of propoxur to
rats by subacute inhalation. Jpn J. ind. Health, 18: 375-382.
KNAAK, J.B., TALLANT, M.J., BARTLEY, W.Y., & SULLIVAN, L.J.
(1965) The metabolism of carbaryl in the rat, guinea pig and
man. J. agric. food Chem., 13(4): 537-543.
KNAAK, J.B., TALLANT, M.J., & SULLIVAN, L.J. (1966) The
metabolism of 2-methyl-2-(methylthio)propionaldehyde- O -
methylcarbamoyl)oxime in the rat. J. agric. food Chem., 14(6):
573-578.
KNAAK, J.B., TALLANT, M.J., KOZBELT, S.J., & SULLIVAN, L.J.
(1968) Metabolism of carbaryl in man, monkey, pig, and sheep.
J. agric. food Chem., 16(3): 465-470.
KNOWLES, C.O. & SONAWANE, B.R. (1972) Ethyl m- hydroxy-
carbanilate carbanilate (EP-475) metabolism in sugar beets.
Bull. environ. Contam. Toxicol., 8: 73-76.
KOSSMANN, K. & JENNY, N.A. (1973) Phenmedipham. In: Sherma,
J. & Zweig, G., ed. Analytical methods for pesticides and
plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 611-623.
KRECHNIAK, J. & FOSS, W. (1982) Cholinesterase activity in
rats treated with propoxur. Bull. environ. Contam. Toxicol.,
29(5): 599-604.
KUHR, R.J. (1968) Metabolism of methylcarbamate insecticide
chemicals in plants. J. Sci. Food Agric. 44, (Suppl.): 43-49.
KWALICK, D.S. (1971) Physician's pesticides primer. J. Med.
Soc. New Jersey, 68:(5) 375-377.
LAMB, M.J. & LILLY, L.J. (1980) An investigation of some
genetic toxicological effects of the fungicide benomyl.
Toxicology, 17: 83-95.
LEAVITT, J.R.C., GOLD, R.E., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of professional pesticide applicators to carbaryl.
Arch. environ. Contam. Toxicol., 11: 57-62.
LEELING, N.C. & CASIDA, J.E. (1966) Metabolites of carbaryl
(1-naphthyl methylcarmate) in mammals and enzymatic systems
for their formation. J. agric. food Chem., 14: 281-290.
LEITCH, R.E. & PEASE, H.L. (1973) Lannate-methomyl. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 331-338.
LYINSKY, W. & TAYLOR, H.W. (1976) Carcinogenesis in Sprague
Dawley rats of N -nitroso- N -alkylcarbamate esters. Cancer
Lett., 1: 275-279.
MAKITA, T., HASHIMOTO, Y., & NOGUCHI, T. (1973) Mutagenic,
cytogenetic, and teratogenic studies on thiophanate-methyl.
Toxicol. appl. Pharmacol., 24: 206-215.
MARLIAC, J.P. (1964) Toxicity and teratogenic effects of 12
pesticides in the chick embryo. Fed. Proc. Fed. Am. Soc. Exp.
Biol., 23: 105-126
MCCARTHY, J.F., FANCHER, O.E., KENNEDY, G.L., KEPLINGER, M.L.,
& CALANDRA, J.C. (1971) Reproduction and teratology studies
with the insecticide carbofuran. Toxicol. appl. Pharmacology,
19: 370.
METCALF, R.L., FUKUTO, T.R., COLLINS, C., BORCK, K., BURK J.,
REYNOLDS, H.T., & OSMAN, M.F. (1966) Metabolism in plant and
insect. Metabolism of 2-methyl-2-(methylthio)-propionaldehyde
O -methylcarbamoyl-oxime in plant and insect. J. agric. food
Chem., 14: 579-584.
MILLER, D.B. (1982) Neurotoxicity of the pesticidal
carbamates. Neurobehav. Toxicol. Teratol., 4: 779-787.
MIRVISH, H.S.S. (1968) The carcinogenic action and
metabolism of urethan and N -hydroxyurethan. Adv. Cancer Res.,
11: 1-42.
MONTAZEMI, K. (1969) Toxicological studies of Baygon
insecticide in Shabankareh area, Iran. Trop. geogr. Med., 21:
186-190.
MORSE, D.L. & BAKER, E.L., Jr (1979) Propanil-chloracne and
methomyl toxicity in workers of a pesticide manufacturing
plant. Clin. Tox., 15(1): 13-21.
MOSCIONI, A.D., ENGEL, J.L., & CASIDA, J.E. (1977)
Kynurenine formamidase inhibition as a possible mechanism for
certain teratogenic effect of organophosphorus and
methylcarbamate insecticides in chicken embryos. Biochem.
Pharmacol., 26: 2251-2258.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisonning due to organophosphate insecticies: acute and
chronic manifestations. Am. J. Med., 50: 475-492.
NATIONAL INSTITUTE OF HYGIENIC SCIENCES (1985) Letter to the
International Programme on Chemical Safety, 1.10.85, Tokyo,
Japan.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22: 16-18 (in
Japanese).
OGLE, R.E. & WARREN, G.F. (1954) Fate and activity of
herbicides in soils. Weeds, 3: 257-273.
OLEFIR, A.I. & VINOGRADOVA, V.Kh. (1968) [A study of the
embryotropic effect of the pesticides sevine and cyram.]
Vrach. Delo., 11: 103-106 (in Russian).
OLIVER, J.E. (1981) Pesticide-derived nitrosoamines.
Occurrence and environmental fate. In: Scanlan, R.A. &
Tannenbaum, S.T., ed. N-nitroso compounds, Washington DC,
American Chemical Society (ACS Symposium Series 174).
OONNITHAN, E.S. & CASIDA, J.E. (1968) Oxidation of methyl
and dimethylcarbamate insecticide chemicals by microsomal
enzymes and anticholinesterase activity of the metabolites. J.
agric. food Chem., 16: 28-44.
ORLOVA, N.V. & ZHALBE, E.P. (1968) Experimental material
contributive to the profile of maximal permissible amounts of
Sevin in food products. Vopr. Pitan., 27: 49-55 (in Russian).
PAROCHETTI, J.V. & WARREN, G.F. (1966) Vapour losses of IPC
and CIPC. Weeds, 14; 281-285.
PASTUSHENKO, T.B. (1983) [Study on the ganodotropic effects
of methyl N -2-benzimidazol carbamate aiming at evaluating its
effective concentrations.] Gig. i Sanit., 7: 83-85 (in
Russian).
PAULSON, G.D., JACOBSEN, A.M., ZAYLSKIE, R.G., & FEIL, V.J.
(1973) Isolation and identification of propham isopropyl-
carbanilate metabolites from the rat and goat. J. agric. food
Chem., 21: 804-811.
PETROVA-VERGIEVA, T. (1979) [Changes in the intrauterine
development of experimental animals under fundazol effect.]
Hig. Zdraveopaz., 22: 487-495 (in Bulgarian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization.
(Unpublished report VBC/84.889).
PRENDEVILLE, G.N., ESHEL, Y., JAMES, C.S., WAREN, G.F.E., &
SCHREIBER, M.M. (1968) Movement and metabolism of CIPC in
resistent and susceptible species. Weed Sci., 16: 432-435.
PROCTOR, N.H., MOSCIONI, A.D., & CASIDA, J.E. (1976) Chicken
embryo NAD levels lowered by teratogenic organophophorus and
methylcarbamate insecticides. Biochem. Pharmacol., 25: 757-762.
QUARLES, J.M. & TENNANT, R.W. (1975) Effects of nitroso-
carbaryl on BALB/3T3 cells. Cancer Res., 35: 2637-2645.
QUINBY, G.E. & BABIONE, R.W. (1966) Toxicological
observations on the use of Baygon (2-isopropoxyphenyl
N -methylcarbamate) as a malarial control insecticide in El
Salvador. Ind. Med. Surg., 35: 596-597.
QURAISHI, M.S. (1972) Edaphic and water relationships of
aldicarb and its metabolites. Can. Entomol., 104(3): 1191-1196.
REGAN, J.D., SETLOW, R.B., FRANCIS, A.A., & LYINSKY, W.
(1976) Nitrosocarbaryl: its effect on human DNA. Mutat. Res.,
38: 293-301.
REICH, G.A. & WELKE, J.O. (1966) Death due to a pesticide.
New Engl. J. Med., 274: 1432.
REINER, E. (1971) Spontaneous reactivation of phosphorylated
and carbamylated cholinesterases. Bull. World Health Org., 44:
109-112.
REINER, E. & ALDRIDGE, W.N. (1967) Effect of pH on
inhibition and spontaneous reactivation of acetylcholin-
esterase treated with esters of phosphoric acids and of
carbamic acids. Biochem. J., 105: 171-179.
REINER, E. & SKRINJARIC-SPOLJAR, M. (1968) Hydrolysis of
some monomethylcarbamates in human sera. Croat. Chem. Acta,
40: 87-90.
REINER, E., BUNTIC, A., TRDAK, M., & SIMEON, V. (1974)
Effect of temperature on the activity of human blood
cholinesterases. Arch. Toxicol., 32: 347-350.
RHODES, R.C. & LONG, J.D. (1974) Run-off and mobility
studies on benomyl in soils and turf. Bull. environ. Contam.
Toxicol., 12(4): 385-393.
RICHARDSON, E.M. & BATTEESE, R.I., Jr (1973) An incident of
Zectran poisoning. J. Maine Med. Assoc., 64: 158-159.
ROBEL, R.J., FELTHOUSEN, R.W., & DAYTON, A.D. (1982) Effects
of carbamates on Bobwhite food intake body weight and
locomotor activity. Arch. environ. Contam. Toxicol., 11:
611-615.
ROBENS, J.F. (1979) Teratologic Studies of Carbaryl,
Diazinon, Norea, Disulfiram, and Thiram in small laboratory
animals. Tox. Appl. Pharmacol., 15: 152-163.
ROMINE, R.R. (1973) Aldicarb. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7,
pp. 147-162.
ROSZKOWSKI, P. (1982) [Effect of the preparation propoxur on
intrauterine development and on the state of bone formation in
the progeny of the rat.] Ginek. Pol., 53(4): 201-207 (in
Polish).
ROTHSCHILD, E.R., MANSER, R.J., & ANDERSON, M.P. (1982)
Investigation of aldicarb in groundwater in selected areas of
the central sand plain of Wisconsin. Groundwater, 20(4):
437-445.
RUZICSKA, P., PETER, S., & CZEIZEL, A. (1975) Studies on the
chromosomal mutagenic effect of benomyl in rat and humans.
Mutat. Res., 29: 201.
RYBAKOVA, M.N. (1967) [Comparative toxic effects of sevin
and DDT used in the treatment of food crops.] Vopr. Pitan.,
26: 9-15 (in Russian).
RYBAKOVA, M.N. (1968) [Effect of some pesticides on the
hypophysis and its gonadotropic functions.] Gig. i Sanit.,
33(11): 27-31 (in Russian).
SAKAI, K. & MATSUMURA, F. (1968) Esterases of mouse brain
active in hydrolysing organophosphate and carbamate
insecticides. J. agric. food Chem., 16(5): 803-807.
SAKAI, K. & MATSUMURA, F. (1971) Degradation of certain
organophosphate and carbamate insecticides by human brain
esterases. Toxicol. appl. Pharmacol., 19(4): 660-666.
SEILER, J.P. (1972) The mutagenicity of benzimidazole and
benzimidazole derivatives. I. Forward and reverse mutations in
Salmonella typhimurium caused by benzimidazole and some of its
derivatives. Mutat. Res., 15: 273-276.
SEILER, J.P. (1975) Toxicology and genetic effects of
benzimidazole compounds. Mutat. Res., 32: 151-168.
SEILER, J.P. (1976a) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40: 339-347.
SEILER, J.P. (1976b) Inhibition of testicular DNA synthesis
by chemical mutagens and carcinogens. Preliminary result in
the validation of a novel short-term test. Mutat. Res., 46:
305-310.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method
for the determination of 1-naphthol in urine. Bull. environ.
Contam. Toxicol., 6: 34-39.
SHERMAN, H., CULIK, R., & JACKSON, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. appl.
Pharmacol., 32: 305-315.
SHTENBERG, A.I. & OZHOVAN, M.V. (1971) [Influence of low
Sevin doses on the reproductive function of animals in a
number of generations.] Vopr. Pitan., 30(1): 42-49 (in
Russian, with English summary).
SHTENBERG, A.I. & RYBAKOVA, M.N. (1968) Effect of carbaryl
on the neuroendocrine system of rats. Food Cosmet. Toxicol.,
6: 461-467.
SHTENBERG, A.I., ORLOVA, N.V., & TORCHINSKY, A.M. (1973)
[The action of different pesticides of different chemical
structure on the gonads and embryogenesis of experimental
animals.] Gig. i Sanit., 38(8): 16-20 (in Russian).
SIDEROFF, S.T. & SANTOLUCITO, J.A. (1972) Behavioural and
physiological effects of cholinesterase inhibitor carbaryl
(1-naphthyl methylcarbamate). Physiol. Behav., 9: 459-462.
SMALLEY, H.E., CURTIS, J.M., & EARL, F.L. (1968) Teratogenic
action of carbaryl in beagle dogs. Toxicol. appl. Pharmacol.,
13: 392-403.
SMALLEY, H.E., O'HARA, P.J., BRIDGES, C.H., & RADELEFF, R.D.
(1969) The effects of chronic carbaryl administration on the
neuromuscular system of swine. Toxicol. appl. Pharmacol., 14:
409-419.
SONAWANE, B.R. & KNOWLES, C.O. (1971) Phenmedipham and
m -aminophenol decomposition in alkaline soil. Bull. environ.
Contam. Toxicol., 6: 322-327.
SONAWANE, B.R. & KNOWLES, C.O. (1972) Comparative metabolism
of two carbanilate herbicides (EP-475 and phenmedipham) in
rats. Pestic. Biochem. Physiol., 1: 472-482.
STADNYK, L., CAMPELL, R.S., & JOHNSON, B.T. (1971) Pesticide
effect on growth and 14C assimilation in a fresh-water alga.
Bull. environ. Contam. Toxicol., 6: 1-8.
STANSBURY, H.A., Jr & MISKUS, R. (1964) In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators,
and food additives, New York, London, Academic Press, Vol. 2,
pp. 437-450.
STEGEMAN LE ROY, C. (1964) The effects of the carbamate
insecticide carbaryl upon forest soil mites and Collembola. J.
econ. Entomol., 57(6): 803-808.
STRINGER, A. & LYONS, C.H. (1974) The effects of benomyl and
thiophanate-methyl on earth worm populations; Apple Orchards
Pestic. Sci., 5: 189-195.
STILL, G.G. & HERRETT, R.A. (1975) Methylcarbamates,
carbanilates, and acylanilides. In: Kearney, P.C. & Kaufman,
D.D., ed. Herbicides, chemistry, degradation, and mode of
action. New York, Marcel Dekker, Vol. 2, pp. 609-664.
STILL, G.G. & MANSAGER, E.R. (1972) Metabolism of 4-chloro-
2-butynyl-3-chloro-carbanilate by soybean plants. J. agric.
food Chem., 20: 402-406.
STILL, G.G. & MANSAGER, E.R. (1973a) Soybean shoot
metabolism of isopropyl 3-chlorocarbanilate ortho and para
arylhydroxylation. Pestic. Biochem. Physiol., 3:: 87-95.
STILL, G.G. & MANSAGER, E.R. (1973b) Metabolism of isopropyl
carbanilate by soybean plants. Pestic. Biochem. Physiol., 3:
289-299.
STILL, G.G. & MANSAGER, E.R. (1975) Alfalfa metabolism of
propham. Pestic. Biochem. Physiol., 5: 515-522.
STILL, G.G. & RUSNESS, D.G. (1977) S -cysteinyl-hydroxychlor-
propham: formation of the S -cysteinyl conjugate of isopropyl-
3'-chloro-4'-hydroxy-carbanilate in oat (Avena savita L).
Pestic. Biochem. Physiol., 7: 210-219.
STRINGER, A. & WRIGHT, M.A. (1973) The effect of benomyl and
some related compounds on Lumbricus terrestris and other
earthworms. Pestic. Sci., 4: 165-170.
STYLES, J.A. & GARNER, R. (1974) Benzimidazole carbamate
methylester evaluation of its effect in vivo and in vitro.
Mutat. Res., 26:: 177-187.
SULLIVAN, L.J., ELDRIDGE, J.M., KNAAK, J.B., & TALLANT, M.J.
(1972) 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide as a
significant metabolite of carbaryl in the rat. J. agric. food
Chem., 20(5): 980-985.
SUZUKI, T. & TAKEDA, M. (1976) Microbial metabolism of
N -methylcarbamate insecticide I. Metabolism of O -sec-
butylphenyl N -methylcarbamate by Aspergillus niger van
Tieghem. Chem. Pharm. Bull., 24(9): 1967-1975.
SYPESTEYN, A.K., DEKHUYZEN, H.M., & VONK, J.W. (1977)
Biological conversion of fungicides in plants and
microorganisms, In: Siegel, M.R. & Sisler, H.D., ed.
Antifungal compounds, New York, Marcel Dekker, Vol. 2,
pp. 91-147.
TANNENBAUM, S.R., INSKEY, A.J., WEISMAN, M., & BISHOP, W.
(1974) Nitrite in human. Its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co., pp.
100-119.
TOBIN, J.S. (1970) Carbofuran a new carbamate insecticide.
J. occup. Med., 12: 16-19.
TOMLIN, A.D. & GORE, F.L. (1974) Effects of six insecticides
and a fungicide on the numbers of biomass of earthworms in
pasture. Bull. environ. Contam. Toxicol., 12(4): 487-492.
TORCHINSKY, A.M. (1973) [Significance of diets with
different protein content in manifestations of teratogenic and
embryotoxic action of some pesticides.] Vopr. Pitan., 3: 76-80
(in Russian).
TORCHINSKY, A.M., ORLOVA, N.V., SMIRNOVA, E.V., & MAKAROVA,
L.F. (1976) [Data for toxico-hygienic characteristics of the
pesticide Benlate of carbamate group.] Gig. i Sanit., 2: 35-39
(in Russian, with English summary).
UCHIYAMA, M., TAKEDA, M., SUZUKI, T., & YOSHIKAWA, K. (1975)
Mutagenicity of nitroso derivatives of N -methylcarbamate
insecticides in microbiological method. Bull. environ. Contam.
Toxicol., 14: 389-394.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) [The
reaction of nitrite with pesticides.] Z. Krebsforsch., 81:
217-224 (in German, with English summary).
VANDEKAR, M. (1965) Observations on toxicity of carbaryl,
folithion, and 3-isopropylphenyl- N -methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. World Health
Org., 33: 107-115.
VANDEKAR, M., HEDAYAT, S., PLESTINA, R., & AHMADY, G. (1968)
A study of the safety of O -isopropoxyphenyl methylcarbamate in
an operational field-trial in Iran. Bull. World Health Org.,
38: 609-623.
VANDEKAR, M., PLESTINA, R., & WILHELM, K. (1971) Toxicity of
carbamates for mammals. Bull. World Health Org., 44: 241-249.
VAN ESCH, G.J. & KROES, R. (1972) Long-term toxicity studies
of chlorpropham and propham in mice and hamsters. Food Cosmet.
Toxicol., 10: 373-381.
VAN ESCH, G.J., VAN GENDEREN, H., & VINK, H.H. (1958) The
production of skin tumours in mice by oral treatment with
urethane, isopropyl- N -phenylcarbamate, or isopropyl- N -chloro-
phenyl carbamate in combination with skin painting with croton
oil and Tween 60. Br. J. Cancer, 12: 355-362.
VASHAKIDZE, V.I. (1965) [Some questions of the harmful
action of sevin on the reproductive function of experimental
animals.] Soobshch. Akad. Nauk Gruz. SSR, 39: 471-478 (in
Russian).
VASHAKIDZE, V.I. (1967) [Mechanism of action of pesticides
(granosana, sevin, donoc) on the reproductive cycle of
experimental animal.] Soobshch. Akad. Nauk Gruz. SSR, 48:
219-224 (in Russian).
VASSILIEFF, I. & ECOBICHON, D.J. (1982) Stability of
Matacil(R) in aqueous media as measured by changes in
anticholinesterase potency. Bull. environ. Contam. Toxicol.,
29: 366-370.
VASSILIEFF, I. & ECOBICHON, D.J. (1983) Acute toxicity of
aminocarb in male rats and inhibition of tissue esterases.
Bull. environ. Contam. Toxicol., 31: 326-330.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. Vol. 1. Toxicity profiles, Boca Raton,
Florida, CRC Press, Inc., 232 p..
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticide
reference index, JMPR 1961-84, Geneva, World Health
Organization (Unpublished report).
VOSS, G. & SCHULER, J. (1967) A fast and simple procedure
for routine determination of plasma ChE activity. Bull.
environ. Contam. Toxicol., 2(6): 357-363.
WHO (1984) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-85, Geneva, World
Health Organization (Unpublished report VBC/84.2).
WHO/FAO (1984) Data sheets on pesticides, Geneva, World
Health Organization.
WEIL, C.S., WOODSIDE, M.D., CARPENTER, C.P., & SMYTH, H.F.,
Jr (1972) Current studies of tests of carbaryl for
reproductive and teratogenic effects. Toxicol. appl.
Pharmacol., 21: 390-404.
WEIL, C.S., WOODSIDE, M.D., BERNARD, J.B., CONDRA, N.I., KING,
J.M., & CARPENTER, C.P., (1973) Comparative effect of
carbaryl on rat reproduction and guinea-pig teratology when
fed either in the diet or by stomach intubation. Toxicol.
appl. Pharmacol., 26: 621-638.
WEIS, P. & WEIS, J.S. (1974) Schooling behaviour of Menidia
menidia in the presence of insecticide Sevin (carbaryl). Mar.
Biol., 28: 261-263.
WESTLAKE, G.E., BUNYAN, P.J., MARTIN, A.D., STANLEY, P.I., &
STEED, L.C. (1981) Carbamate poisoning. Effect of selected
carbamate pesticides on plasma enzymes and brain esterases of
Japanese quail (Coturnix coturnix japonica). J. agric. food
Chem., 29(4): 779-785.
WHITE, E.R., BOSE, E.A., OGAWA, J.M., MANJI, B.T., & KILGORA,
W.W. (1973) Thermal and base-catalysed hydrolysis products
of the systemic fungicide benomyl. J. agric. food Chem., 21:
616-618.
WIEDMANN, J.L.M., ECKE, G.G., & STILL, G.G. (1976) Synthesis
and isolation of 1-hydroxy-2-propyl-3-chloro-carbanilate from
soybean plants treated with isopropyl-3-chlorocarbanilate J.
agric. food Chem., 24: 588-592.
WILHELM, K. & REINER, E. (1973) Effect of sample storage on
human blood cholinesterase activity after inhibition by
carbamates. Bull. World Health Org., 48: 235-238.
WILHELM, K., VANDEKAR, M., & REINER, E. (1973) Comparison of
methods of measuring cholinesterase inhibition by carbamates.
Bull. World Health Org., 48: 41-44.
WILLIAMS, I.H., BROWN, M.J., & WHITEHEAD, P. (1976a)
Persistence of carbofuran residues in some British Columbia
soils. Bull. environ. Contam. Toxicol., 15(2): 242-243.
WILLIAMS, I.H., PEPIN, H.S., & BROWN, M.J. (1976b)
Degradation of carbofuran by soil microorganisms. Bull.
environ. Contam. Toxicol., 15(2): 244-249.
WILLS, J.H., JAMESON, E. & COULSTON, F. (1968) Effects of
oral doses of carbaryl on man. Clin. Toxicol., 1(3): 265-271.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., London, British Crop Protection
Council.
WRIGHT, M.A. & STRINGER, A. (1973) The toxicity of
thiabendazole, benomyl, methyl-benzimidazol-2-yl-carbamate,
and thiophanate-methyl to the earthworm Lumbricus terrestris.
Pestic. Sci., 4: 431-432.
WYROBEK, A.J., WATCHMAKER, G., GORDON, L., WONG, K., MOORE,
D.I., & WHORTON (1981) Sperm shape abnormalities in carbaryl
exposed employees. Environ. Health Perspect., 40: 255-265.
YAKIM, V.S. (1967) [Data for substantiating the maximum
permissible concentration of sevine in the air of working
zones.] Gig. i Sanit., 32: 29-33 (in Russian).
YOSHIDA, K. & NISHIUCHI, Y. (1972) [Toxicity of pesticides
to some water organisms.] Bull. agric. Chem. Inspect. Stn.
(Japan), 12: 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) [Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn.
(Japan),. 6: 65-69 (in Japanese).
ZAKI, M.H., MORAN, D., & HARRIS, D. (1982) Pesticides in
groundwater: the aldicarb story in Suffolk Country, New York.
Am. J. public Health, 72(12): 1391-1395.
Annex I. Names and structures and some physical and chemical properties of carbamate pesticides
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aldicarb Temik propanal, 2-methyl- C7H14N2O2S 190.29 13 mPa 0.6%
2-(methylthio)-,
O -[(methylamino)
carbonyl]oxime
(116-06-3)
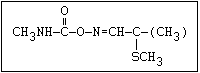 aldoxycarb Standak 2-methyl-2-(methyl- C7H14N2O4S 222.3 12 mPa 0.9%
sulfonyl)-propanal
O -[(methylamino)-
carbonyl]oxime
(1646-88-4)
aldoxycarb Standak 2-methyl-2-(methyl- C7H14N2O4S 222.3 12 mPa 0.9%
sulfonyl)-propanal
O -[(methylamino)-
carbonyl]oxime
(1646-88-4)
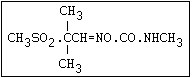 allyxycarb Hydrol phenol, 4-(di-2-pro- C16H22N2O2 274.4
APC phenylamino)-3,5-
dimethyl, methyl
carbamate
(6392-46-7)
allyxycarb Hydrol phenol, 4-(di-2-pro- C16H22N2O2 274.4
APC phenylamino)-3,5-
dimethyl, methyl
carbamate
(6392-46-7)
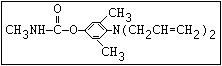 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aminocarb Matacil phenol, 4-(dimethyl- C11H16N2O2 208.29 non- slightly
amino)-3-methyl-, volatile
methyl carbamate
(2032-59-9)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aminocarb Matacil phenol, 4-(dimethyl- C11H16N2O2 208.29 non- slightly
amino)-3-methyl-, volatile
methyl carbamate
(2032-59-9)
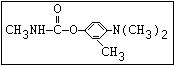 asulam Asulox carbamic acid, C8H10N2O4S 230.26 - 0.5%
Asilan [(4,-aminophenyl)-
sulfonyl], methyl
ester
(3337-71-1)
asulam Asulox carbamic acid, C8H10N2O4S 230.26 - 0.5%
Asilan [(4,-aminophenyl)-
sulfonyl], methyl
ester
(3337-71-1)
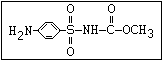 barban CBN carbamic acid, C11H9Cl2NO2 258.11 50 µPa 0.0011%
Carbyne 3-chlorophenyl-,
Chlorinat 4-chloro-2-butynyl
ester
(101-27-9)
barban CBN carbamic acid, C11H9Cl2NO2 258.11 50 µPa 0.0011%
Carbyne 3-chlorophenyl-,
Chlorinat 4-chloro-2-butynyl
ester
(101-27-9)
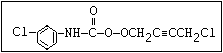 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
BPMC Bay-Bassa phenol, 2-(1-methyl- C12H17NO2 207.3 48 mPab 0.061%c
Baycarb propyl)-,methyl
Sumibas carbamate
(3766-81-2)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
BPMC Bay-Bassa phenol, 2-(1-methyl- C12H17NO2 207.3 48 mPab 0.061%c
Baycarb propyl)-,methyl
Sumibas carbamate
(3766-81-2)
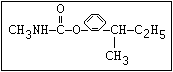 bendiocarb Ficam 1,3-benzodioxol- C11H13NO4 223.25 660 µPa 0.004%
Garvox 4-yl, 2,2-dimethyl,
Multimet methyl carbamate
Tattoo (22781-23-3)
Seedox
bendiocarb Ficam 1,3-benzodioxol- C11H13NO4 223.25 660 µPa 0.004%
Garvox 4-yl, 2,2-dimethyl,
Multimet methyl carbamate
Tattoo (22781-23-3)
Seedox
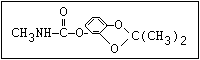 benomyl Arilate carbamic acid,[1- C14H18N4O3 290.36 non- ~0.0002%
Benlate [(butylamino)- volatile
Tersan carbonyl]-1H-
benzimidazole-2-yl]-,
methyl ester
(17804-35-2)
benomyl Arilate carbamic acid,[1- C14H18N4O3 290.36 non- ~0.0002%
Benlate [(butylamino)- volatile
Tersan carbonyl]-1H-
benzimidazole-2-yl]-,
methyl ester
(17804-35-2)
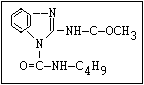 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
bufencarb Bux mixture of phenol, C13H19NO2 221.33 4.0 mPac < 0.005%
Metalkamate 3-(1-methylbutyl)-
phenyl)-, and
3-(1-ethylpropyl-
phenyl)-, methyl
carbamates
(8065-36-9)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
bufencarb Bux mixture of phenol, C13H19NO2 221.33 4.0 mPac < 0.005%
Metalkamate 3-(1-methylbutyl)-
phenyl)-, and
3-(1-ethylpropyl-
phenyl)-, methyl
carbamates
(8065-36-9)
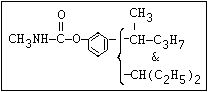 butacarb phenol, 3,5-bis C16H25NO2 263
(1,1-dimethylethyl)-,
methyl carbamate
(2655-19-8)
butacarb phenol, 3,5-bis C16H25NO2 263
(1,1-dimethylethyl)-,
methyl carbamate
(2655-19-8)
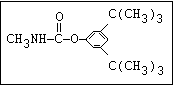 carbanolate Banol phenol, 2-chloro-4, C10H12ClNO2 213.68
SOK 5-dimethyl-, methyl
carbamate
(671-04-5)
carbanolate Banol phenol, 2-chloro-4, C10H12ClNO2 213.68
SOK 5-dimethyl-, methyl
carbamate
(671-04-5)
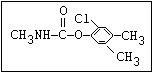 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbaryl Sevin 1-naphthalenol C12H11NO2 201.24 < 665 0.012%c
(Sevkul) methyl carbamate mPaf
(Carbicide) (63-25-2)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbaryl Sevin 1-naphthalenol C12H11NO2 201.24 < 665 0.012%c
(Sevkul) methyl carbamate mPaf
(Carbicide) (63-25-2)
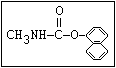 carbendazim Bavistin carbamic acid, C9H9N3O2 191.18 < 100 0.0028%c
BCM/MBC -1H-benzimidazole- nPab (pH 4.8)
Derosal 2-yl-, methyl ester
(10605-21-7)
carbendazim Bavistin carbamic acid, C9H9N3O2 191.18 < 100 0.0028%c
BCM/MBC -1H-benzimidazole- nPab (pH 4.8)
Derosal 2-yl-, methyl ester
(10605-21-7)
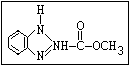 carbetamide Legurame propanamide, N -ethyl C12H16N2O3 236.30 negligible 0.35%b
Carbetamex -2-[[(phenyl-amino) at 20°C
carbonyl]oxy]-, (R)-
(16118-49-3)
carbetamide Legurame propanamide, N -ethyl C12H16N2O3 236.30 negligible 0.35%b
Carbetamex -2-[[(phenyl-amino) at 20°C
carbonyl]oxy]-, (R)-
(16118-49-3)
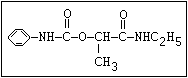 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbofuran Furadan 7-benzofuranol, C12H15NO3 221.28 2.7 mPag 0.07%
Yaltox 2,3-dihydro-2,2-
Curater dimethyl-,
methyl carbamate
(1563-66-2)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbofuran Furadan 7-benzofuranol, C12H15NO3 221.28 2.7 mPag 0.07%
Yaltox 2,3-dihydro-2,2-
Curater dimethyl-,
methyl carbamate
(1563-66-2)
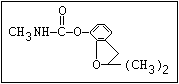 chlorbufam - 1-methylprop-2ynyl- C11H10ClNO2 223.7 2.1 mPab 0.054%b
3-chlorocarbanilate
chlorbufam - 1-methylprop-2ynyl- C11H10ClNO2 223.7 2.1 mPab 0.054%b
3-chlorocarbanilate
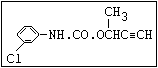 cloethocarb - 2-(2-chloro-1-a C11H14ClNO4 259.7 - 0.13%b
methoxyethoxy)-
phenylmethyl
carbamate
cloethocarb - 2-(2-chloro-1-a C11H14ClNO4 259.7 - 0.13%b
methoxyethoxy)-
phenylmethyl
carbamate
 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
chlorpropham Elbanil carbamic acid, C10H12ClNO2 213.68 - 0.009%
Furloe 3-chlorophenyl-,
Prevenol 1-methylethyl ester
Chloro IPC (101-21-3)
(CIPC)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
chlorpropham Elbanil carbamic acid, C10H12ClNO2 213.68 - 0.009%
Furloe 3-chlorophenyl-,
Prevenol 1-methylethyl ester
Chloro IPC (101-21-3)
(CIPC)
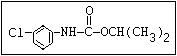 desmedipham Betanex carbamic acid, [3- C16H16N2O4 300.34
[[(phenylamino)-
carbonyl]oxy]phenyl]-,
ethyl ester
(13684-56-6)
desmedipham Betanex carbamic acid, [3- C16H16N2O4 300.34
[[(phenylamino)-
carbonyl]oxy]phenyl]-,
ethyl ester
(13684-56-6)
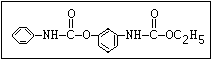 dimetilan Snip carbamic acid, C10H16N4O3 240.3 readily
dimethyl-,
1-[(dimethylamino)
carbonyl]-5-methyl-
1H-pyrazol-3-yl ester
(644-64-4)
dimetilan Snip carbamic acid, C10H16N4O3 240.3 readily
dimethyl-,
1-[(dimethylamino)
carbonyl]-5-methyl-
1H-pyrazol-3-yl ester
(644-64-4)
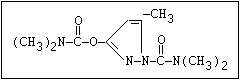 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
dichlormate benzenemethanol, C9H9Cl2NO2 199 0.017%
2,3-dichloro-,
methyl carbamate
(62046-37-1)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
dichlormate benzenemethanol, C9H9Cl2NO2 199 0.017%
2,3-dichloro-,
methyl carbamate
(62046-37-1)
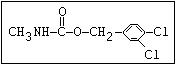 dioxacarb Famid phenol, 2-(1,3-di- C11H13NO4 223.25 40 µPac 0.6%c
Elecron oxolan-2-yl)-,
methyl carbamate
(6988-21-2)
dioxacarb Famid phenol, 2-(1,3-di- C11H13NO4 223.25 40 µPac 0.6%c
Elecron oxolan-2-yl)-,
methyl carbamate
(6988-21-2)
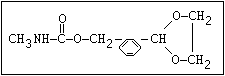 ethiofencarb Croneton phenol, 2-[(ethyl- C11H15NO2S 225.33 13 mPac 0.182%b
thio)methyl]-,
methyl carbamate
(29973-13-5)
ethiofencarb Croneton phenol, 2-[(ethyl- C11H15NO2S 225.33 13 mPac 0.182%b
thio)methyl]-,
methyl carbamate
(29973-13-5)
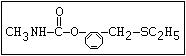 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
etrofol hopcide phenol, 2-chloro-, C8H8ClNO2 185.62 -
CPMC methyl carbamate
(3942-54-9)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
etrofol hopcide phenol, 2-chloro-, C8H8ClNO2 185.62 -
CPMC methyl carbamate
(3942-54-9)
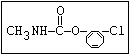 formetanate Dicarzol methanimidamide, C11H15N3O2 257.75 27 µPa 0.63%
Carzol N,N -dimethyl- N' -
[3-[[(methylamino)
carbonyl]oxy]-
phenyl]-
(22259-30-9)
formetanate Dicarzol methanimidamide, C11H15N3O2 257.75 27 µPa 0.63%
Carzol N,N -dimethyl- N' -
[3-[[(methylamino)
carbonyl]oxy]-
phenyl]-
(22259-30-9)
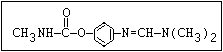 isoprocarb Etrofolan phenol, 2-(1-methyl- C11H15NO2 193.27 - insoluble
Carbamat IPM ethyl)methyl
Hytox carbamate
Mipcin (2631-40-5)
MIPC
isoprocarb Etrofolan phenol, 2-(1-methyl- C11H15NO2 193.27 - insoluble
Carbamat IPM ethyl)methyl
Hytox carbamate
Mipcin (2631-40-5)
MIPC
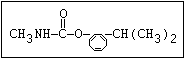 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
karbutilate Tandex carbamic acid, C14H21N3O3 279.38 6.0 nPab 0.033%b
(1,1-dimethylethyl)-,
3-[[(dimethylamino)
carbonyl]-amino]
phenyl ester
(4849-23-5)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
karbutilate Tandex carbamic acid, C14H21N3O3 279.38 6.0 nPab 0.033%b
(1,1-dimethylethyl)-,
3-[[(dimethylamino)
carbonyl]-amino]
phenyl ester
(4849-23-5)
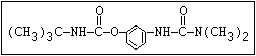 MPMC Meobal phenol, 3,4-di- C10H13NO2 179.24
methyl-,
methyl carbamate
(2425-10-7)
MPMC Meobal phenol, 3,4-di- C10H13NO2 179.24
methyl-,
methyl carbamate
(2425-10-7)
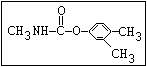 methiocarb Draza phenol, 3,5-di- C11H15NO2S 225.33 15 mPah 0.003%b
Mesurol methyl-4-(methyl-
thio)-, methyl
carbamate
(2032-65-7)
methiocarb Draza phenol, 3,5-di- C11H15NO2S 225.33 15 mPah 0.003%b
Mesurol methyl-4-(methyl-
thio)-, methyl
carbamate
(2032-65-7)
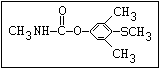 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
methomyl Lannate ethanimidothioic C5H10N2O2S 162.23 5.8%
Methavin acid, N -[[(methyl-
amino)carbonyl]oxy]-,
methyl ester
(16752-77-5)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
methomyl Lannate ethanimidothioic C5H10N2O2S 162.23 5.8%
Methavin acid, N -[[(methyl-
amino)carbonyl]oxy]-,
methyl ester
(16752-77-5)
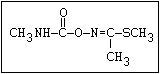 metolcarb - m -tolyl methyla C9H11NO2 165.2 145 mPab 0.26%c
carbamate
metolcarb - m -tolyl methyla C9H11NO2 165.2 145 mPab 0.26%c
carbamate
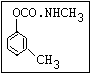 mexacarbate Zectran phenol, 4-dimethyl- C12H18N2O2 222.32
amino)-3,5-dimethyl-,
methyl carbamate
(ester)
(315-18-4)
mexacarbate Zectran phenol, 4-dimethyl- C12H18N2O2 222.32
amino)-3,5-dimethyl-,
methyl carbamate
(ester)
(315-18-4)
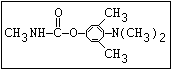 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
oxamyl Vydate ethanimidothioic C7H13N3O3S 219.29 31 mPa 28.0%
acid, 2-(dimethyl-
amino)- N -[[(methyl-
amino)carbonyl]-oxy]-
2-oxo-, methyl ester
(23135-22-0)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
oxamyl Vydate ethanimidothioic C7H13N3O3S 219.29 31 mPa 28.0%
acid, 2-(dimethyl-
amino)- N -[[(methyl-
amino)carbonyl]-oxy]-
2-oxo-, methyl ester
(23135-22-0)
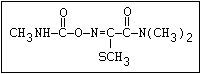 phenmedipham Betanal carbamic acid, C16H16N2O4 300.34 - .0.0005%
(3-methylphenyl)-,
3-[(methoxycarbonyl)
amino]phenyl ester
(13684-63-4)
phenmedipham Betanal carbamic acid, C16H16N2O4 300.34 - .0.0005%
(3-methylphenyl)-,
3-[(methoxycarbonyl)
amino]phenyl ester
(13684-63-4)
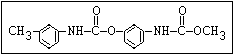 pirimicarb Aphox carbamic acid, C11H18N4O2 238.33 4.0 mPac 0.27%
Fernos dimethyl-, 2-(di-
Pirimor methylamino)-5,
Rapid 6-dimethyl-4-pyrimi-
dinyl ester
(23103-98-2)
pirimicarb Aphox carbamic acid, C11H18N4O2 238.33 4.0 mPac 0.27%
Fernos dimethyl-, 2-(di-
Pirimor methylamino)-5,
Rapid 6-dimethyl-4-pyrimi-
dinyl ester
(23103-98-2)
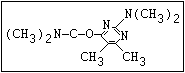 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
promacyl Promicide 5-methyl- m -cumenyla C16H23NO3 277.4 400 Pai slightly
butyryl(methyl)
carbamate
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
promacyl Promicide 5-methyl- m -cumenyla C16H23NO3 277.4 400 Pai slightly
butyryl(methyl)
carbamate
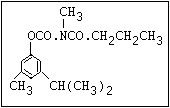 promecarb Carbamult phenol, 3-methyl-5- C12H17NO2 207.3 0.0092%
Minacide (1-methylethyl)-,
methyl carbamate
(2631-37-0)
promecarb Carbamult phenol, 3-methyl-5- C12H17NO2 207.3 0.0092%
Minacide (1-methylethyl)-,
methyl carbamate
(2631-37-0)
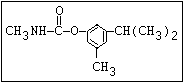 propham Banhoe carbamic acid, C10H13NO2 179.24 - 0.025%
Tuberit phenyl-, 1-methyl-
IPC ethyl ester
(122-42-9)
propham Banhoe carbamic acid, C10H13NO2 179.24 - 0.025%
Tuberit phenyl-, 1-methyl-
IPC ethyl ester
(122-42-9)
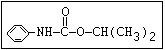 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
propoxur Baygon phenol, 2-(1-methyl C11H15NO3 209.27 6.5 x 0.2%
Blattanex ethoxy)-, methyl 10-6
Sendran carbamate mm Hg
Suncide (114-26-1) (20 °C)
Unden
Tendex
Arprocarb
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
propoxur Baygon phenol, 2-(1-methyl C11H15NO3 209.27 6.5 x 0.2%
Blattanex ethoxy)-, methyl 10-6
Sendran carbamate mm Hg
Suncide (114-26-1) (20 °C)
Unden
Tendex
Arprocarb
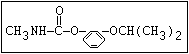 swep carbamic acid, 3,4- C8H7Cl2NO2 220.06
dichlorophenyl-,
methyl ester
(1918-18-9)
swep carbamic acid, 3,4- C8H7Cl2NO2 220.06
dichlorophenyl-,
methyl ester
(1918-18-9)
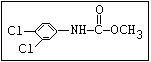 terbucarb Azac 2,6-di-ter-butyl- C17H27NO2 277.4 0.0067%
Azar p -tolyl methyl
carbamate
thiodicarb Larvin ethanimidothioic C10H18N4O4S3 354.3
acid, N,N' -[thiobis
(methylimino)-
carbonyloxy]
bis-dimethyl-ester
(59669-26-0)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
thiofanox Dacamox 2-butanone, 3,3-di- C9H18N2O2S 218.35 22.6 mPa 0.52%d
methyl-l-(methyl-
amino)-, O -[(methyl-
amino)- carbonyl]oxime
(39196-18-4)
terbucarb Azac 2,6-di-ter-butyl- C17H27NO2 277.4 0.0067%
Azar p -tolyl methyl
carbamate
thiodicarb Larvin ethanimidothioic C10H18N4O4S3 354.3
acid, N,N' -[thiobis
(methylimino)-
carbonyloxy]
bis-dimethyl-ester
(59669-26-0)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
thiofanox Dacamox 2-butanone, 3,3-di- C9H18N2O2S 218.35 22.6 mPa 0.52%d
methyl-l-(methyl-
amino)-, O -[(methyl-
amino)- carbonyl]oxime
(39196-18-4)
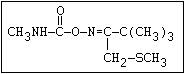 thiophanate Topsin carbamic acid, C14H18N4O4S2 370.48 - insoluble
ethyl Cercobin [1,2-phenylenebis
Enovit (iminocarbonothioyl)]
bis-, diethyl ester
(23564-06-9)
thiophanate Topsin carbamic acid, C14H18N4O4S2 370.48 - insoluble
ethyl Cercobin [1,2-phenylenebis
Enovit (iminocarbonothioyl)]
bis-, diethyl ester
(23564-06-9)
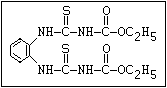 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
thiophanate Topsin-M carbamic acid, C12H14N4O4S2 342.42 - slightly
methyl Cercobin-M [1,2-phenylenebis-
Enovit-M iminocarbonothioyl)]
Fungo bis-, dimethyl ester
Mildothane (23564-05-8)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
thiophanate Topsin-M carbamic acid, C12H14N4O4S2 342.42 - slightly
methyl Cercobin-M [1,2-phenylenebis-
Enovit-M iminocarbonothioyl)]
Fungo bis-, dimethyl ester
Mildothane (23564-05-8)
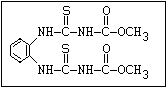 trimethacarb Broot 4:1 mixture of C11H15NO2 193.24
Landrin phenol, 3,4,5-tri-
methyl-, methyl-
carbamate and phenol,
2,3,5-trimethyl-
methyl carbamate
(2686-99-9 and
2655-15-4)
MTMC Tsumacide carbamic acid, C9H11NO2 165.21
methyl-, 3-methyl-
phenyl ester
(1129-41-5)
trimethacarb Broot 4:1 mixture of C11H15NO2 193.24
Landrin phenol, 3,4,5-tri-
methyl-, methyl-
carbamate and phenol,
2,3,5-trimethyl-
methyl carbamate
(2686-99-9 and
2655-15-4)
MTMC Tsumacide carbamic acid, C9H11NO2 165.21
methyl-, 3-methyl-
phenyl ester
(1129-41-5)
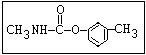 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
xylylcarb - 3,4-xylylmethyla C10H13NO2 179.2 70 mPab 0.058%b
carbamate
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
xylylcarb - 3,4-xylylmethyla C10H13NO2 179.2 70 mPab 0.058%b
carbamate
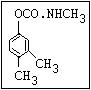 XMC Macbal 3,5 xylylmethyla
Cosban carbamate C10H13NO2 179.2 insoluble
--------------------------------------------------------------------------------------------
a IUPAC name.
b At 20 °C.
c At 30 °C.
d At 22 °C.
e From: Worthing & Walker (1983) (given in the reference list of the main document).
f At 26 °C.
g At 33 °C.
h At 60 °C.
i At 149 °C.
Annex II. Summary of the short- and long-term toxicity studies that were used to establish
the acceptable daily intakes for human beings for carbamate compounds
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Aldicarb 2 years 2.5 0.125 FAO/WHO (1983b)
0.25 (dog)
Aminocarb 2 years 100 FAO/WHO (1980b)
Bendiocarb 0.38
0.25 (dog) FAO/WHO (1985a)
Benomyl 2 years 2500 125 FAO/WHO (1983b,
30 (based on teratology) 1985a)
Carbaryl 2 years 200 10 FAO/WHO (1985a)
6 weeks 0.06 (man)
80 weeks 400 (mice) 60 (mice)
1 year 1.8 (dog)
Carbendazim 2 years 500 25 FAO/WHO (1986b)
2 years 100 (dog) 2.5
Carbofuran 2 years 20 1.0 FAO/WHO (1981b)
2 years 20 (mice) 2.5
Chloropropham 2 years 2000 FAO/WHO (1965b)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Ethiofencarb 90 days 500 10 FAO/WHO (1983b)
1000 (dog) 25
Methiocarb 1 year 25 1.3 FAO/WHO (1985a)
5 (dog) 0.125
Methomyl 22 months 100 5 FAO/WHO (1978b)
Oxamyl 14 days 100 (dog) 2.5 FAO/WHO (1985a)
Pirimicarb 2 years 175 9 FAO/WHO (1983b)
2 years 1.8 (dog)
17 weeks 2 (monkey)
Propoxur 2 years 250 12.5 FAO/WHO (1983b)
2 years (dogs)
Thiophanate 2 years 160 8 FAO/WHO (1976b, 1978b)
-methyl 2 years 50 (dog)
2 years 160 23 (mice)
---------------------------------------------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
References are to reports of WHO/FAO Joint Meeting on Pesticide Residues. Vettorazzi (1979) gives a
summary of these reports up to and including 1977.
Note: The references to Annex II are given in the reference list of the main document.
Annex III. Carbamates: JMPR reviews, ADIs, Evaluation by IARC, Classification by Hazard,
FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Aldicarb 1982 0-0.005 1983b + + IA No. 53 (1982)
1983a
1979 0-0.001 1980b
1980a
Aminocarb 1979 no ADI 1980b IB
1980a
1978 no ADI 1979b
1979a
1984 0-0.004 1985b
Bendiocarb 1982 0-0.002 1983b + + II No. 52 (1982)
(temporary) (rev. 1)
Benomyl 1983 0.02 1984 - + No. 87 (in
1985a 0 (preparation)
1978 no ADI 1979a
1975 no ADI 1976a
1976a
1973 no ADI 1974a
1974b
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbaryl 1984i 0-0.01 1985b
1979i 0-0.01 1980b + + II No. 3 (1978)
1980a (rev. 1)
1977i 0-0.01 1978b
1978a
1976i 0-0.01 1977b Vol. 12,
page 37
1977a
1975i 0-0.01 1976b
1976a
1973 0-0.01 1974b
1974a
1971i 0-0.01 1972a
(temporary)
1970i 0-0.01 1971b
(temporary)
1971a
1969 0-0.01 1970b
(temporary)
1970a
1968i 0-0.02 1969b
1969a
1967 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbendazim 1985 0-0.01 1986b
1983 0-0.01 1984 0 No. 89 (in
1985a (preparation)
1978 no ADI 1979a
1979b
1977 no ADI 1978a
1978b
1976 no ADI 1977a
1977b
1973 no ADI 1974a
1974b
Carbofuran 1982 0-0.01 1983a + + IB
1980 0-0.01 1981b
1981a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Chlor- 1965 no ADI 1965b Vol. 12, 0
propham page 55
1965a
1963 no ADI 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Ethio- 1983i 0-0.1 1984a II
fencarb 1982 0-0.1 1983b
1983a
1981i 0-0.1 1982b
(temporary)
1982a
1980i 0-0.1 1981b
(temporary)
1981a
1978i 0-0.1 1979b
(temporary)
1979a
1977 0-0.1 1978b
(temporary)
Methiocarb 1985 0-0.001 1986b
1984 0-0.001 1985b II
1983 0.001 1984
1985a
1981 0-0.001 1982b
1982a
Methomyl 1978 no ADI 1979b + + IB No. 55 (1983)
1979a
1977i no ADI 1978b
1978a
1976i no ADI 1977b
1977a
1975i no ADI 1976b
1976a
Mexacarbate Vol. 12,
page 237
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Oxamyl 1985 0-0.03 1986b
1984 0-0.03 1985b
1983i 0-0.01 1984 + + IB No. 54 (1983)
(temporary)
1980 0-0.01 1981b
(temporary)
1981a
0-0.0014
Pirimicarb 1982 0-0.02 1983b + + II
1983a
1981i 0-0.01 1982b
(temporary)
1982a
1979i 0-0.01 1980b
(temporary)
1980a
1978 0-0.01 1979b
(temporary)
1979a
1976 0-0.004 1977b
(temporary)
1977a
Propham 1965 no ADI 1965a Vol. 12 + + 0
1965b Page 189
1963 no ADI 1964a
Propoxur 1983 0-0.02 1984 + + II No. 25 (1976)
1981i 0-0.02 1982b
1982a
1978i 0-0.02 1979a
1977i 0-0.02 1978a
1973 0-0.02 1974b
1974a
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Thiodicarb 1985 0-0.1 1986b
(temporary)
Thiofanox 1978i no ADI 1979a IB
Thio- 1978i 0-0.08 1979a + 0
phanate- 1977 0-0.08 1978b
methyl 1978a
1975 0-0.08 1976b
1976a
1973 0-0.08 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in this
annex.
Annex III (contd.)
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling by the
manufacturer or in accordance with the rules laid down for storage and transportation by competent
international bodies. Classification relates to the technical material, and not to the formulated
product:
-----------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
-----------------------------------------------------------------------------------
1A Extremely hazardous 5 or less 20 or less 10 or less 40 or less
1B Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal use
-----------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex IV. Abbreviations
2-AB 2-aminobenzimidazole
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
EEG electroencephalogram
EMG electromyogram
FAO Food and Agriculture Organization of the United
Nations
4-HBC 4-hydroxy-2-benzimidazole carbamate
5-HBC 5-hydroxy-2-benzimidazole carbamate
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety (World
Health Organization)
IRPTC International Register of Potentially Toxic Chemicals
(United Nations Environment Programme)
iv intravenous
JMPR Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and a WHO Expert
Group on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NTE neuropathy target esterase (formerly neurotoxic
esterase)
PBI protein-bound iodine
pseudoChE pseudocholinesterase
RBC red blood cells
sc subcutaneous
UVR ultraviolet radiation
XMC Macbal 3,5 xylylmethyla
Cosban carbamate C10H13NO2 179.2 insoluble
--------------------------------------------------------------------------------------------
a IUPAC name.
b At 20 °C.
c At 30 °C.
d At 22 °C.
e From: Worthing & Walker (1983) (given in the reference list of the main document).
f At 26 °C.
g At 33 °C.
h At 60 °C.
i At 149 °C.
Annex II. Summary of the short- and long-term toxicity studies that were used to establish
the acceptable daily intakes for human beings for carbamate compounds
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Aldicarb 2 years 2.5 0.125 FAO/WHO (1983b)
0.25 (dog)
Aminocarb 2 years 100 FAO/WHO (1980b)
Bendiocarb 0.38
0.25 (dog) FAO/WHO (1985a)
Benomyl 2 years 2500 125 FAO/WHO (1983b,
30 (based on teratology) 1985a)
Carbaryl 2 years 200 10 FAO/WHO (1985a)
6 weeks 0.06 (man)
80 weeks 400 (mice) 60 (mice)
1 year 1.8 (dog)
Carbendazim 2 years 500 25 FAO/WHO (1986b)
2 years 100 (dog) 2.5
Carbofuran 2 years 20 1.0 FAO/WHO (1981b)
2 years 20 (mice) 2.5
Chloropropham 2 years 2000 FAO/WHO (1965b)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Ethiofencarb 90 days 500 10 FAO/WHO (1983b)
1000 (dog) 25
Methiocarb 1 year 25 1.3 FAO/WHO (1985a)
5 (dog) 0.125
Methomyl 22 months 100 5 FAO/WHO (1978b)
Oxamyl 14 days 100 (dog) 2.5 FAO/WHO (1985a)
Pirimicarb 2 years 175 9 FAO/WHO (1983b)
2 years 1.8 (dog)
17 weeks 2 (monkey)
Propoxur 2 years 250 12.5 FAO/WHO (1983b)
2 years (dogs)
Thiophanate 2 years 160 8 FAO/WHO (1976b, 1978b)
-methyl 2 years 50 (dog)
2 years 160 23 (mice)
---------------------------------------------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
References are to reports of WHO/FAO Joint Meeting on Pesticide Residues. Vettorazzi (1979) gives a
summary of these reports up to and including 1977.
Note: The references to Annex II are given in the reference list of the main document.
Annex III. Carbamates: JMPR reviews, ADIs, Evaluation by IARC, Classification by Hazard,
FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Aldicarb 1982 0-0.005 1983b + + IA No. 53 (1982)
1983a
1979 0-0.001 1980b
1980a
Aminocarb 1979 no ADI 1980b IB
1980a
1978 no ADI 1979b
1979a
1984 0-0.004 1985b
Bendiocarb 1982 0-0.002 1983b + + II No. 52 (1982)
(temporary) (rev. 1)
Benomyl 1983 0.02 1984 - + No. 87 (in
1985a 0 (preparation)
1978 no ADI 1979a
1975 no ADI 1976a
1976a
1973 no ADI 1974a
1974b
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbaryl 1984i 0-0.01 1985b
1979i 0-0.01 1980b + + II No. 3 (1978)
1980a (rev. 1)
1977i 0-0.01 1978b
1978a
1976i 0-0.01 1977b Vol. 12,
page 37
1977a
1975i 0-0.01 1976b
1976a
1973 0-0.01 1974b
1974a
1971i 0-0.01 1972a
(temporary)
1970i 0-0.01 1971b
(temporary)
1971a
1969 0-0.01 1970b
(temporary)
1970a
1968i 0-0.02 1969b
1969a
1967 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbendazim 1985 0-0.01 1986b
1983 0-0.01 1984 0 No. 89 (in
1985a (preparation)
1978 no ADI 1979a
1979b
1977 no ADI 1978a
1978b
1976 no ADI 1977a
1977b
1973 no ADI 1974a
1974b
Carbofuran 1982 0-0.01 1983a + + IB
1980 0-0.01 1981b
1981a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Chlor- 1965 no ADI 1965b Vol. 12, 0
propham page 55
1965a
1963 no ADI 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Ethio- 1983i 0-0.1 1984a II
fencarb 1982 0-0.1 1983b
1983a
1981i 0-0.1 1982b
(temporary)
1982a
1980i 0-0.1 1981b
(temporary)
1981a
1978i 0-0.1 1979b
(temporary)
1979a
1977 0-0.1 1978b
(temporary)
Methiocarb 1985 0-0.001 1986b
1984 0-0.001 1985b II
1983 0.001 1984
1985a
1981 0-0.001 1982b
1982a
Methomyl 1978 no ADI 1979b + + IB No. 55 (1983)
1979a
1977i no ADI 1978b
1978a
1976i no ADI 1977b
1977a
1975i no ADI 1976b
1976a
Mexacarbate Vol. 12,
page 237
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Oxamyl 1985 0-0.03 1986b
1984 0-0.03 1985b
1983i 0-0.01 1984 + + IB No. 54 (1983)
(temporary)
1980 0-0.01 1981b
(temporary)
1981a
0-0.0014
Pirimicarb 1982 0-0.02 1983b + + II
1983a
1981i 0-0.01 1982b
(temporary)
1982a
1979i 0-0.01 1980b
(temporary)
1980a
1978 0-0.01 1979b
(temporary)
1979a
1976 0-0.004 1977b
(temporary)
1977a
Propham 1965 no ADI 1965a Vol. 12 + + 0
1965b Page 189
1963 no ADI 1964a
Propoxur 1983 0-0.02 1984 + + II No. 25 (1976)
1981i 0-0.02 1982b
1982a
1978i 0-0.02 1979a
1977i 0-0.02 1978a
1973 0-0.02 1974b
1974a
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Thiodicarb 1985 0-0.1 1986b
(temporary)
Thiofanox 1978i no ADI 1979a IB
Thio- 1978i 0-0.08 1979a + 0
phanate- 1977 0-0.08 1978b
methyl 1978a
1975 0-0.08 1976b
1976a
1973 0-0.08 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in this
annex.
Annex III (contd.)
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling by the
manufacturer or in accordance with the rules laid down for storage and transportation by competent
international bodies. Classification relates to the technical material, and not to the formulated
product:
-----------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
-----------------------------------------------------------------------------------
1A Extremely hazardous 5 or less 20 or less 10 or less 40 or less
1B Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal use
-----------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex IV. Abbreviations
2-AB 2-aminobenzimidazole
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
EEG electroencephalogram
EMG electromyogram
FAO Food and Agriculture Organization of the United
Nations
4-HBC 4-hydroxy-2-benzimidazole carbamate
5-HBC 5-hydroxy-2-benzimidazole carbamate
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety (World
Health Organization)
IRPTC International Register of Potentially Toxic Chemicals
(United Nations Environment Programme)
iv intravenous
JMPR Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and a WHO Expert
Group on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NTE neuropathy target esterase (formerly neurotoxic
esterase)
PBI protein-bound iodine
pseudoChE pseudocholinesterase
RBC red blood cells
sc subcutaneous
UVR ultraviolet radiation
 Soil run-off and leaching characteristics of benomyl and its
metabolites were studied in the greenhouse and laboratory using
14C-labelled materials on soil and turf plots and on soil thin-
layer chromatographic plates (Rhodes & Long, 1974). The studies
showed that benomyl and its metabolites are immobile in soil and do
not leach or move from the site of application.
The first step in the metabolic degradation of these carbamates
in soil is hydrolysis (Sonawane & Knowles, 1971). Simple esters are
hydrolysed to the parent (unstable) acid and alcohol. The general
reaction of carbamates and the breakdown of carbamates in soils are
described in detail in the review of Still & Herrett (1975).
Chloropropham, barban, and swep are hydrolysed to their respective
anilines (Still & Herrett, 1975). In alkaline soil, phenmedipharm
converts hydrolytically to methyl-3-hydroxyphenyl-carbamate, which
in turn hydrolyses to 3-aminophenol (Sonawane & Knowles, 1971).
The substituted anilines that are formed are further metabolized by
oxidation processes (Kaufman & Blake, 1973; Still & Herrett, 1975).
Methomyl and oxamyl degrade rapidly with carbon dioxide as the
end product (Harvey & Pease, 1973; Harvey & Han, 1978b).
In soils, the predominant metabolic pathway is cleavage of the
carbamate bond to yield the alcohol and amino moieties, which may
be further metabolized by the soil-plant system.
Williams et al. (1976a,b) found that the degradation of
carbofuran in British Columbia was slower than expected. Soils
showed rather high residues of this compound (up to approximately 4
mg/kg soil), and there was some evidence of build-up where
treatments were repeated for two successive years. While
carbofuran has a half-life of up to 50 weeks in neutral or acid
soils, it degrades rapidly in alkaline soils.
4.1.4 Vegetation and wildlife
Plant metabolism of carbanilate herbicides has been shown to
involve aryl hydroxylation and conjugation besides hydro-lytic
breakdown. Consequently, they are readily transformed from
lyophilic compounds to hydrophylic metabolites, which contain the
intact carbamoyl group. These polar products are not translocated
but remain in the plant at the site of formation. All available
data indicate that the hydroxylated carbamate metabolites are
detoxification products (Still & Herrett, 1975). In studies on
chloropropham in oat shoots, Still & Rusness (1977) found that the
phenolic metabolites were converted to an S -cysteinyl conjugate,
i.e., S -cysteinyl-hydroxychloropropham.
A large number of studies on the absorption and translocation
of carbamate herbicides have been carried out to study the fate of
these compounds in plants. The results suggest that plant leaf
surfaces are a barrier to the absorption of carbamates. Roots,
however, absorb the herbicides to a much greater extent, and the
carbamate moves to all plant parts. Thus, it is suggested that
carbamates are exclusively distributed via the apoplastic system.
The carrier that is used plays an important role in these
absorption studies.
Many studies are available concerning the actual entry of
carbamates into the plant, their hydrolysis, aromatic ring
hydroxylation, hydrolytic breakdown, and oxidation of aliphatic
groups. Other types of metabolic reactions in different plant
species following different methods of application have also been
described (Baldwin et al., 1954; Abdel-Wahab et al., 1966;
Prendeville et al., 1968; Knowles & Sonawane, 1972; Still &
Mansager, 1972, 1973a,b, 1975; Wiedmann et al., 1976; Guardigli et
al., 1977).
For the metabolic pathway in wildlife, see sections 6 and 7.
4.1.5 Entry into the food chain
Carbamates are metabolized or broken down in soil, plants, and
animals. The questions of the environmental impact and the
significance of the metabolites with respect to persistence and
bioaccumulation in certain species and in the food chain have still
to be answered. For the moment, it appears that most, if not all,
of the known carbamate metabolites are biodegraded rapidly and are
less toxic for the environment than the parent molecules (Still &
Herrett, 1975).
4.2 Biotransformation
4.2.1 Microbial degradation
The carbamates are readily degraded by soil microorganisms in
most soils (Gray, 1971). Environmental conditions that favour the
growth and activity of microorganisms also favour degradation (Ogle
& Warren, 1954). The residual herbicidal activity of both barban
and propham persists longer in sterile soil than in non-sterile
soil.
Kaufman & Kearney (1965) and Kaufman & Blake (1973) isolated
(by soil-enrichment techniques) and identified a soil microorganism
capable of degrading carbamates such as chloropropham. They
suggested that hydrolysis was a major degradation pathway in soils.
Each of the soil microorganisms demonstrated a different range of
substrate specificity, but all were capable of degrading and
dehalogenating a variety of pesticides (production of 3-
chloroaniline and subsequent liberation of free chloride ion).
These studies illustrated the tremendous adaptability of the
soil microbial populations in altering the persistence and
character of the different foreign compounds. Studies carried out
by Clark & Wright (1970) confirmed that cultures of Arthrobacter
sp. and Achromobacter sp. were able to convert phenyl carbamates
to the corresponding aniline compounds.
Suzuki & Takeda (1976) studied the metabolism of dimetilan in
Aspergillus niger van Tieghem. Together with hydrolysis,
oxidation of the alkyl side chain appeared the most important
modification (detoxification) process. Williams et al. (1976b)
found that carbofuran was rapidly degraded in soils containing high
levels of actinomycetes.
4.2.2 Photodegradation
N -methyl carbamates absorb radiation available in the solar
region (lambda = 300 nm) and hence, would be expected to undergo
photo-oxidation as well as metabolic degradation. Addison et al.
(1974) studied the fate of various N -methyl carbamates when
solutions were sprayed on bean foliage and exposed to sunlight or
artificial light (lambda = 254 nm). It is not entirely clear
whether the products they observed resulted solely from a
photochemical reaction or from absorption followed by enzymatic
attack (Addison et al., 1973, 1974).
4.2.3 Photodecomposition in the aquatic environment
Carbamate insecticides in water are subject to photo-
decomposition under the effects of ultraviolet radiation (UVR).
The pH of the aqueous medium was found to be an important factor in
relationship to the rates of photolysis of carbaryl and propoxur,
which were slow at low pH values and tended to increase with
increaseing pH value. However, the decomposition of, for instance,
dimetilan, was not affected by the pH of the irradiated medium.
The primary effect of the UVR appears to be cleavage of the ester
bond resulting in the production of the phenol or heterocyclic enol
of the carbamate esters tested. The hydrolysis products produced
were further photodecomposed to other unidentified degradation
products. Carbaryl produced 5 degradation products, one of which
was identified as 1-naphthol. It is assumed that, apart from the
cleavage of the ester bond, changes at other positions in the
molecule are produced by UVR. However, the intact carbamate ester
group is retained, and consequently the ChE-inhibiting activity
(Crosby et al., 1965; Crosby 1969; Aly & El Dib, 1972).
Similar results were reported for the irradiation products of
other carbamate esters such as aminocarb, mexacarbate, and a number
of analogues (Eberle & Gunther, 1965; Abdel-Wahab F & Casida,
1967).
When the half-life for photodecomposition was studied in a
number of carbamate insecticides, the results suggested that the
light absorption characteristics of the insecticide influenced the
extent of its photodecomposition by a specific light source.
However, the extent of photodecomposition was not the same under
different conditions of irradiation (presence of solvents) and
wave-length (Crosby et al., 1965; Eberle & Gunther, 1965).
Thus, it seems reasonable to suggest that photodecomposition
may account for some loss of carbamate insecticides in clear
surface waters exposed to sunlight for a long period. However,
photolysis may be a minor factor in the decomposition of these
compounds in highly-turbid waters, where the penetration of light
will be greatly reduced (Aly & El-Dib, 1971, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Exposure of the General Population
5.1.1 Food and drinking-water
From a market-basket study conducted during 1977-80 by the
Finnish National Board of Trade and Consumer Interests, it became
apparent that the residues of benomyl in food had increased.
During the period 1974-76, the average benomyl intake was about 0.2
mg/person per year from both domestic and imported foods. During
the period 1977-80, average intake was 9 mg/year. A later more
accurate study showed an intake of 14 mg/person per year (Finnish
National Board of Health, 1982).
Contamination of groundwater and drinking-water sources by
aldicarb, a carbamate that is used as an insecticide and as a
nematocide, was reported from the USA in 1979 and 1980-81; levels
even higher than 0.075 mg/litre were found (Rothschild et al.,
1982; Zaki et al., 1982). In the Federal Republic of Germany,
detectable amounts of up to 0.001 mg aldicarb/litre were found in a
few samples of drinking-water (Federal Republic of Germany,
personal communication, 1985)a.
---------------------------------------------------------------------------
a Letter to the IPCS from the Ministry of the Interior of
the Federal Republic of Germany, Bonn, Federal Republic of
Germany, 28 May, 1985.
6. METABOLISM AND MODE OF ACTION
Most carbamates are active inhibitors of AChE and they do not
require metabolic activation. However, some carbamates, such as
the benzimidazole carbamates, do not have anticholinesterase
activity. Carbamates undergo metabolism, and the metabolites are
generally less toxic than the parent compound; some exceptions
will be discussed later. The metabolism of carbamates is basically
the same in mammals, insects, and plants, and the toxic effects of
carbamates are similar in mammals and insects. Carbamates do not
accumulate in the mammalian body, but are rapidly excreted, mainly
via the urine.
6.1 Mode of Action
Carbamates are effective insecticides by virtue of their
ability to inhibit AChE in the nervous system. AChE catalyses the
hydrolysis of the neurotransmitter acetylcholine (ACh) to choline
and acetic acid.
ACh is the synaptic mediator of nerve impulses in the nervous
system of mammals and insects:
(a) as a neurotransmitter in the brain of mammals, and in
the central nervous system of insects;
(b) as a pre-ganglionic neurotransmitter in the autonomic
nervous system of mammals;
(c) in post-ganglionic nerve endings of the autonomic
nervous system; and
(d) at the neuromuscular junction of skeletal muscle.
Carbamates, like organophosphates, can inhibit esterases that
have serine in their catalytic centre; these are called serine-
esterases or beta-esterases. Although the inhibition of serine-
esterases other than AChE is not significant for the toxicity of
the compounds, it may have significance for the potentiation of
toxicity of other compounds after long-term low-level exposure
(Sakai & Matsumura, 1968, 1971; Aldridge & Magos, 1978).
To understand the mechanism of toxicity, it is necessary to
examine the events that take place at the neuromuscular junction.
When a muscle is innervated, as shown in Fig. 2, a nerve impulse
moving down a neuron reaches the nerve ending where ACh, which is
stored in vesicles at the nerve endings, is released into the
junction. Within 2 - 3 ms, ACh impinges on the receptor side of
the muscle.
Soil run-off and leaching characteristics of benomyl and its
metabolites were studied in the greenhouse and laboratory using
14C-labelled materials on soil and turf plots and on soil thin-
layer chromatographic plates (Rhodes & Long, 1974). The studies
showed that benomyl and its metabolites are immobile in soil and do
not leach or move from the site of application.
The first step in the metabolic degradation of these carbamates
in soil is hydrolysis (Sonawane & Knowles, 1971). Simple esters are
hydrolysed to the parent (unstable) acid and alcohol. The general
reaction of carbamates and the breakdown of carbamates in soils are
described in detail in the review of Still & Herrett (1975).
Chloropropham, barban, and swep are hydrolysed to their respective
anilines (Still & Herrett, 1975). In alkaline soil, phenmedipharm
converts hydrolytically to methyl-3-hydroxyphenyl-carbamate, which
in turn hydrolyses to 3-aminophenol (Sonawane & Knowles, 1971).
The substituted anilines that are formed are further metabolized by
oxidation processes (Kaufman & Blake, 1973; Still & Herrett, 1975).
Methomyl and oxamyl degrade rapidly with carbon dioxide as the
end product (Harvey & Pease, 1973; Harvey & Han, 1978b).
In soils, the predominant metabolic pathway is cleavage of the
carbamate bond to yield the alcohol and amino moieties, which may
be further metabolized by the soil-plant system.
Williams et al. (1976a,b) found that the degradation of
carbofuran in British Columbia was slower than expected. Soils
showed rather high residues of this compound (up to approximately 4
mg/kg soil), and there was some evidence of build-up where
treatments were repeated for two successive years. While
carbofuran has a half-life of up to 50 weeks in neutral or acid
soils, it degrades rapidly in alkaline soils.
4.1.4 Vegetation and wildlife
Plant metabolism of carbanilate herbicides has been shown to
involve aryl hydroxylation and conjugation besides hydro-lytic
breakdown. Consequently, they are readily transformed from
lyophilic compounds to hydrophylic metabolites, which contain the
intact carbamoyl group. These polar products are not translocated
but remain in the plant at the site of formation. All available
data indicate that the hydroxylated carbamate metabolites are
detoxification products (Still & Herrett, 1975). In studies on
chloropropham in oat shoots, Still & Rusness (1977) found that the
phenolic metabolites were converted to an S -cysteinyl conjugate,
i.e., S -cysteinyl-hydroxychloropropham.
A large number of studies on the absorption and translocation
of carbamate herbicides have been carried out to study the fate of
these compounds in plants. The results suggest that plant leaf
surfaces are a barrier to the absorption of carbamates. Roots,
however, absorb the herbicides to a much greater extent, and the
carbamate moves to all plant parts. Thus, it is suggested that
carbamates are exclusively distributed via the apoplastic system.
The carrier that is used plays an important role in these
absorption studies.
Many studies are available concerning the actual entry of
carbamates into the plant, their hydrolysis, aromatic ring
hydroxylation, hydrolytic breakdown, and oxidation of aliphatic
groups. Other types of metabolic reactions in different plant
species following different methods of application have also been
described (Baldwin et al., 1954; Abdel-Wahab et al., 1966;
Prendeville et al., 1968; Knowles & Sonawane, 1972; Still &
Mansager, 1972, 1973a,b, 1975; Wiedmann et al., 1976; Guardigli et
al., 1977).
For the metabolic pathway in wildlife, see sections 6 and 7.
4.1.5 Entry into the food chain
Carbamates are metabolized or broken down in soil, plants, and
animals. The questions of the environmental impact and the
significance of the metabolites with respect to persistence and
bioaccumulation in certain species and in the food chain have still
to be answered. For the moment, it appears that most, if not all,
of the known carbamate metabolites are biodegraded rapidly and are
less toxic for the environment than the parent molecules (Still &
Herrett, 1975).
4.2 Biotransformation
4.2.1 Microbial degradation
The carbamates are readily degraded by soil microorganisms in
most soils (Gray, 1971). Environmental conditions that favour the
growth and activity of microorganisms also favour degradation (Ogle
& Warren, 1954). The residual herbicidal activity of both barban
and propham persists longer in sterile soil than in non-sterile
soil.
Kaufman & Kearney (1965) and Kaufman & Blake (1973) isolated
(by soil-enrichment techniques) and identified a soil microorganism
capable of degrading carbamates such as chloropropham. They
suggested that hydrolysis was a major degradation pathway in soils.
Each of the soil microorganisms demonstrated a different range of
substrate specificity, but all were capable of degrading and
dehalogenating a variety of pesticides (production of 3-
chloroaniline and subsequent liberation of free chloride ion).
These studies illustrated the tremendous adaptability of the
soil microbial populations in altering the persistence and
character of the different foreign compounds. Studies carried out
by Clark & Wright (1970) confirmed that cultures of Arthrobacter
sp. and Achromobacter sp. were able to convert phenyl carbamates
to the corresponding aniline compounds.
Suzuki & Takeda (1976) studied the metabolism of dimetilan in
Aspergillus niger van Tieghem. Together with hydrolysis,
oxidation of the alkyl side chain appeared the most important
modification (detoxification) process. Williams et al. (1976b)
found that carbofuran was rapidly degraded in soils containing high
levels of actinomycetes.
4.2.2 Photodegradation
N -methyl carbamates absorb radiation available in the solar
region (lambda = 300 nm) and hence, would be expected to undergo
photo-oxidation as well as metabolic degradation. Addison et al.
(1974) studied the fate of various N -methyl carbamates when
solutions were sprayed on bean foliage and exposed to sunlight or
artificial light (lambda = 254 nm). It is not entirely clear
whether the products they observed resulted solely from a
photochemical reaction or from absorption followed by enzymatic
attack (Addison et al., 1973, 1974).
4.2.3 Photodecomposition in the aquatic environment
Carbamate insecticides in water are subject to photo-
decomposition under the effects of ultraviolet radiation (UVR).
The pH of the aqueous medium was found to be an important factor in
relationship to the rates of photolysis of carbaryl and propoxur,
which were slow at low pH values and tended to increase with
increaseing pH value. However, the decomposition of, for instance,
dimetilan, was not affected by the pH of the irradiated medium.
The primary effect of the UVR appears to be cleavage of the ester
bond resulting in the production of the phenol or heterocyclic enol
of the carbamate esters tested. The hydrolysis products produced
were further photodecomposed to other unidentified degradation
products. Carbaryl produced 5 degradation products, one of which
was identified as 1-naphthol. It is assumed that, apart from the
cleavage of the ester bond, changes at other positions in the
molecule are produced by UVR. However, the intact carbamate ester
group is retained, and consequently the ChE-inhibiting activity
(Crosby et al., 1965; Crosby 1969; Aly & El Dib, 1972).
Similar results were reported for the irradiation products of
other carbamate esters such as aminocarb, mexacarbate, and a number
of analogues (Eberle & Gunther, 1965; Abdel-Wahab F & Casida,
1967).
When the half-life for photodecomposition was studied in a
number of carbamate insecticides, the results suggested that the
light absorption characteristics of the insecticide influenced the
extent of its photodecomposition by a specific light source.
However, the extent of photodecomposition was not the same under
different conditions of irradiation (presence of solvents) and
wave-length (Crosby et al., 1965; Eberle & Gunther, 1965).
Thus, it seems reasonable to suggest that photodecomposition
may account for some loss of carbamate insecticides in clear
surface waters exposed to sunlight for a long period. However,
photolysis may be a minor factor in the decomposition of these
compounds in highly-turbid waters, where the penetration of light
will be greatly reduced (Aly & El-Dib, 1971, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Exposure of the General Population
5.1.1 Food and drinking-water
From a market-basket study conducted during 1977-80 by the
Finnish National Board of Trade and Consumer Interests, it became
apparent that the residues of benomyl in food had increased.
During the period 1974-76, the average benomyl intake was about 0.2
mg/person per year from both domestic and imported foods. During
the period 1977-80, average intake was 9 mg/year. A later more
accurate study showed an intake of 14 mg/person per year (Finnish
National Board of Health, 1982).
Contamination of groundwater and drinking-water sources by
aldicarb, a carbamate that is used as an insecticide and as a
nematocide, was reported from the USA in 1979 and 1980-81; levels
even higher than 0.075 mg/litre were found (Rothschild et al.,
1982; Zaki et al., 1982). In the Federal Republic of Germany,
detectable amounts of up to 0.001 mg aldicarb/litre were found in a
few samples of drinking-water (Federal Republic of Germany,
personal communication, 1985)a.
---------------------------------------------------------------------------
a Letter to the IPCS from the Ministry of the Interior of
the Federal Republic of Germany, Bonn, Federal Republic of
Germany, 28 May, 1985.
6. METABOLISM AND MODE OF ACTION
Most carbamates are active inhibitors of AChE and they do not
require metabolic activation. However, some carbamates, such as
the benzimidazole carbamates, do not have anticholinesterase
activity. Carbamates undergo metabolism, and the metabolites are
generally less toxic than the parent compound; some exceptions
will be discussed later. The metabolism of carbamates is basically
the same in mammals, insects, and plants, and the toxic effects of
carbamates are similar in mammals and insects. Carbamates do not
accumulate in the mammalian body, but are rapidly excreted, mainly
via the urine.
6.1 Mode of Action
Carbamates are effective insecticides by virtue of their
ability to inhibit AChE in the nervous system. AChE catalyses the
hydrolysis of the neurotransmitter acetylcholine (ACh) to choline
and acetic acid.
ACh is the synaptic mediator of nerve impulses in the nervous
system of mammals and insects:
(a) as a neurotransmitter in the brain of mammals, and in
the central nervous system of insects;
(b) as a pre-ganglionic neurotransmitter in the autonomic
nervous system of mammals;
(c) in post-ganglionic nerve endings of the autonomic
nervous system; and
(d) at the neuromuscular junction of skeletal muscle.
Carbamates, like organophosphates, can inhibit esterases that
have serine in their catalytic centre; these are called serine-
esterases or beta-esterases. Although the inhibition of serine-
esterases other than AChE is not significant for the toxicity of
the compounds, it may have significance for the potentiation of
toxicity of other compounds after long-term low-level exposure
(Sakai & Matsumura, 1968, 1971; Aldridge & Magos, 1978).
To understand the mechanism of toxicity, it is necessary to
examine the events that take place at the neuromuscular junction.
When a muscle is innervated, as shown in Fig. 2, a nerve impulse
moving down a neuron reaches the nerve ending where ACh, which is
stored in vesicles at the nerve endings, is released into the
junction. Within 2 - 3 ms, ACh impinges on the receptor side of
the muscle.
 AChE then hydrolytically converts the ACh into choline and
acetic acid, which results in a decrease in the concentration of
ACh and cessation of muscle contraction. When AChE is inhibited by
a carbamate ester, it can no longer hydrolyse the ACh. Thus, the
ACh concentration remains high in the junction giving rise to
continuous stimulation of the muscle which, in turn, leads to
exhaustion and tetany.
Thus, inhibition of AChE by carbamate esters, causes toxic
effects in animals and human beings that result in a variety of
poisoning symptoms and eventually culminate in respiratory failure
and death.
The mechanism of inhibition of AChE by a carbamate ester can be
formulated as follows (Reiner & Aldridge, 1967; Aldridge & Reiner,
1972):
O O
|| k1 ||
AChE + RO-C-NHCH3 '=========' [AChE x RO-C-NHCH3] (enzyme-carbamate
k-1 | complex)
| kc
\/
O
kr ||
AChE + CH3NH2 + CO2 <------ AChE-C-NHCH3 + RO-
H2O
(carbamylated enzyme)
where: k1 = second order rate constant for formation of
complex;
k-1 = first order rate constant for breakdown of
complex to starting materials;
kc = first order rate constant for carbamylation of
the enzyme;
kr = first order rate constant for hydrolysis of the
carbamylated enzyme.
The site for carbamylation of the enzyme is the hydroxyl moiety of
the serine amino acid. The rate of regeneration of the
carbamylated enzyme to AChE (kr) is relatively rapid compared with
that of an enzyme that has been inhibited (phosphorylated) by an
organophosphorus pesticide (Reiner & Aldridge, 1967; Reiner, 1971).
Thus, human exposure to the carbamate pesticides is less dangerous
than exposure to organophosphorus pesticides, because the ratio
between the dose required to produce mortality and the dose
required to produce minimum poisoning symptoms is, in general,
substantially larger for carbamate compounds than for
organophosphorus compounds (Goldberg et al., 1963; Vandekar, 1965;
Vandekar et al., 1971). An individual experiencing symptoms of
poisoning from a carbamate is more likely to recover on termination
of exposure and with appropriate medical treatment than an
individual poisoned by an organophosphorus compound.
The spontaneous reactivation of various carbamylated ChEs
expressed as half-life, at pH 7.0 - 7.4 and 25 °C ranged between 2
and 240 min for AChE and between 2 and 17 min for serum-ChE. This
instability of the carbamylated enzyme affects the determination of
the inhibitory power of carbamates, the recovery after poisoning,
and the determination of inhibition of blood-ChEs (Reiner, 1971).
6.1.1 Neuropathy target esterase
This esterase, formerly known as neurotoxic esterase (NTE), is
the target for the initiation of delayed neuropathy caused by some
organophosphorus esters. Both organophosphorylation of NTE and a
subsequent "aging" reaction of the inhibited enzyme are required to
initiate neuropathy. Certain N -aryl carbamates can also inhibit
NTE, but the chemistry of such carbamates is such that no "aging"
reaction is possible. These carbamate inhibitors do not initiate
delayed neuropathy in vivo but can actually prevent the neuropathic
effects of a challenge dose of an organophosphate given while the
tissue NTE is carbamylated (Johnson, 1970). No delayed neuropathic
effects have been observed in tests on carbamate pesticides
according to protocols that detect neuropathic organophosphates,
and this agrees with the mechanism described above.
6.2 Metabolism
The metabolic fate of carbamates is basically the same in
plants, insects, and mammals.
Carbamates can penetrate the skin, mucous membranes,
respiratory tract, and gastrointestinal tract of mammals.
6.2.1 Absorption
In vivo studies have shown that carbamates are almost
completely absorbed during normal transit through the gastro-
intestinal tract.
According to absorption studies on rats, the dermal absorption
of radioactive labelled benomyl is slow. After absorption, rapid
metabolism and elimination via urine took place. After 1 h, small
amounts of the major metabolites of benomyl, 5-hydroxy-2-
benzimidazole carbamate (5-HBC), and methyl 2-benzimidazole
carbamate (MBC), could be detected in urine, confirming the rapid
metabolism of benomyl. No radioactivity was found in any body
tissues sampled 24 h after application (FAO/WHO, 1985a).
6.2.2 Distribution
Ahdaya et al. (1981) studied the absorption and distribution of
carbamates. They found that, in mice, all carbamates were rapidly
distributed to the tissues and organs. The half-life values of
penetration ranged from 8 to 17 min for carbamates.
No residues of benomyl or its degradation products were
detected (level of sensitivity 0.02 mg/kg) in eggs from chickens
fed 5 mg benomyl/kg diet. Only 5-HBC (0.03 - 0.06 mg/kg) was found
in eggs from chickens fed 25 mg benomyl/kg for four weeks. No
residues of benomyl or MBC were found in milk (< 0.02 mg/litre)
from dairy cows fed benomyl for 32 days at dietary levels of 0, 2,
10, and 50 mg/kg. At the highest dietary level, the combined
residue of 5-HBC plus 5-hydroxy-2-benzimidazole carbamate (4-HBC)
in the milk was approximately 0.1 mg/litre. These metabolic
studies appear to indicate that benomyl and its metabolites do not
accumulate in animal tissues or animal products (Gardiner et al.,
1974; FAO/WHO, 1985a).
A rat was administered a diet containing 2500 mg non-labelled
benomyl/kg. Twelve days later, the animal received an intragastric
intubation of 2-14C-benomyl (7.7 mg). Urine and faeces were
collected, and organs were analysed for radioactivity. The
elimination was rather rapid and mainly via the urine. After 72 h,
0.2% of the administered dose was found in the liver, while < 0.01%
was present in all the other organs and the carcass. The same
results were found when a rat was administered 2-14C-MBC
(Gardiner et al., 1974)
A male beagle dog fed a diet containing non-labelled benomyl at
2500 mg/kg, and administered 30.8 mg 2-14C-benomyl by capsule 7
days later, showed a completely different excretion pattern. After
3 days, residues were found only in the liver (0.31% of the
administered dose); the levels in the other organs were < 0.01%
(Gardiner et al., 1974).
Rats administered an oral dose of 14C-labelled propham or
chloropropham (labelled in the chain or in the ring), showed
radioactivity in all tissues, with the highest concentration in
the kidneys. The average biological half-life of 14C from both
compounds in most organs was short, ranging from 3 to 8 h.
However, in brain, fat, and muscle, the half-life was about twice
this value (Fang et al., 1974).
6.2.3 Metabolic transformation
6.2.3.1 Biotransformation mechanisms
Carbamate pesticides are transformed metabolically by a variety
of chemical reactions into more water-soluble molecules with
increased polar properties. The initial step, usually oxidative in
nature, introduces a functional hydroxyl group that serves as a
site for secondary conjugative reactions to yield products that
can be excreted via the urine and/or faeces. In some cases, the
oxygenated metabolites, such as 5-hydroxypropoxur and 5-hydroxy-
carbaryl, are known to be toxic and to possess anticholinesterase
activity (Fig. 3) (Oonnithan & Casida, 1968; Black et al., 1973).
This undoubtedly contributes to the overall toxicity of the parent
compound (Black et al., 1973).
6.2.3.2 Oxidation
The principal route of metabolism of insecticidal carbamate
esters is oxidative and is generally associated with the mixed-
function oxidase (MFO) enzymes, which are present in several
tissues. Examples of the sites of oxidative attack on a
hypothetical methyl carbamate are given in Fig. 4.
Depending on the functional groups in the molecule, a variety
of reactions catalysed by these enzymes may occur (Fukuto, 1972),
as shown in Fig. 4. Typical oxidative reactions include: (a)
hydroxylation of aromatic rings, or epoxidation; (b) O -
dealkylation; (c) N -methyl hydroxylation; (d) N -dealkylation; (e)
hydroxylation and subsequent oxidation of aliphatic side chains;
and (f) thioether oxidation to sulfoxides and sulfones.
Because of the variety of different groups present in carbamate
insecticides, the metabolism of these compounds is often complex.
Carbaryl, for example, is a relatively simple compound, yet it is
metabolized to at least 15 different compounds in mammals through a
variety of oxidative and hydrolytic reactions (Leeling & Casida,
1966). A study showed that, as well as the organic solvent-soluble
unconjugated metabolites, the urine from carbaryl-treated rabbits
contained at least 4 or 5 other water-soluble metabolites. These
are probably conjugates of the hydroxylated products of carbaryl,
i.e., glucuronides or sulfates.
AChE then hydrolytically converts the ACh into choline and
acetic acid, which results in a decrease in the concentration of
ACh and cessation of muscle contraction. When AChE is inhibited by
a carbamate ester, it can no longer hydrolyse the ACh. Thus, the
ACh concentration remains high in the junction giving rise to
continuous stimulation of the muscle which, in turn, leads to
exhaustion and tetany.
Thus, inhibition of AChE by carbamate esters, causes toxic
effects in animals and human beings that result in a variety of
poisoning symptoms and eventually culminate in respiratory failure
and death.
The mechanism of inhibition of AChE by a carbamate ester can be
formulated as follows (Reiner & Aldridge, 1967; Aldridge & Reiner,
1972):
O O
|| k1 ||
AChE + RO-C-NHCH3 '=========' [AChE x RO-C-NHCH3] (enzyme-carbamate
k-1 | complex)
| kc
\/
O
kr ||
AChE + CH3NH2 + CO2 <------ AChE-C-NHCH3 + RO-
H2O
(carbamylated enzyme)
where: k1 = second order rate constant for formation of
complex;
k-1 = first order rate constant for breakdown of
complex to starting materials;
kc = first order rate constant for carbamylation of
the enzyme;
kr = first order rate constant for hydrolysis of the
carbamylated enzyme.
The site for carbamylation of the enzyme is the hydroxyl moiety of
the serine amino acid. The rate of regeneration of the
carbamylated enzyme to AChE (kr) is relatively rapid compared with
that of an enzyme that has been inhibited (phosphorylated) by an
organophosphorus pesticide (Reiner & Aldridge, 1967; Reiner, 1971).
Thus, human exposure to the carbamate pesticides is less dangerous
than exposure to organophosphorus pesticides, because the ratio
between the dose required to produce mortality and the dose
required to produce minimum poisoning symptoms is, in general,
substantially larger for carbamate compounds than for
organophosphorus compounds (Goldberg et al., 1963; Vandekar, 1965;
Vandekar et al., 1971). An individual experiencing symptoms of
poisoning from a carbamate is more likely to recover on termination
of exposure and with appropriate medical treatment than an
individual poisoned by an organophosphorus compound.
The spontaneous reactivation of various carbamylated ChEs
expressed as half-life, at pH 7.0 - 7.4 and 25 °C ranged between 2
and 240 min for AChE and between 2 and 17 min for serum-ChE. This
instability of the carbamylated enzyme affects the determination of
the inhibitory power of carbamates, the recovery after poisoning,
and the determination of inhibition of blood-ChEs (Reiner, 1971).
6.1.1 Neuropathy target esterase
This esterase, formerly known as neurotoxic esterase (NTE), is
the target for the initiation of delayed neuropathy caused by some
organophosphorus esters. Both organophosphorylation of NTE and a
subsequent "aging" reaction of the inhibited enzyme are required to
initiate neuropathy. Certain N -aryl carbamates can also inhibit
NTE, but the chemistry of such carbamates is such that no "aging"
reaction is possible. These carbamate inhibitors do not initiate
delayed neuropathy in vivo but can actually prevent the neuropathic
effects of a challenge dose of an organophosphate given while the
tissue NTE is carbamylated (Johnson, 1970). No delayed neuropathic
effects have been observed in tests on carbamate pesticides
according to protocols that detect neuropathic organophosphates,
and this agrees with the mechanism described above.
6.2 Metabolism
The metabolic fate of carbamates is basically the same in
plants, insects, and mammals.
Carbamates can penetrate the skin, mucous membranes,
respiratory tract, and gastrointestinal tract of mammals.
6.2.1 Absorption
In vivo studies have shown that carbamates are almost
completely absorbed during normal transit through the gastro-
intestinal tract.
According to absorption studies on rats, the dermal absorption
of radioactive labelled benomyl is slow. After absorption, rapid
metabolism and elimination via urine took place. After 1 h, small
amounts of the major metabolites of benomyl, 5-hydroxy-2-
benzimidazole carbamate (5-HBC), and methyl 2-benzimidazole
carbamate (MBC), could be detected in urine, confirming the rapid
metabolism of benomyl. No radioactivity was found in any body
tissues sampled 24 h after application (FAO/WHO, 1985a).
6.2.2 Distribution
Ahdaya et al. (1981) studied the absorption and distribution of
carbamates. They found that, in mice, all carbamates were rapidly
distributed to the tissues and organs. The half-life values of
penetration ranged from 8 to 17 min for carbamates.
No residues of benomyl or its degradation products were
detected (level of sensitivity 0.02 mg/kg) in eggs from chickens
fed 5 mg benomyl/kg diet. Only 5-HBC (0.03 - 0.06 mg/kg) was found
in eggs from chickens fed 25 mg benomyl/kg for four weeks. No
residues of benomyl or MBC were found in milk (< 0.02 mg/litre)
from dairy cows fed benomyl for 32 days at dietary levels of 0, 2,
10, and 50 mg/kg. At the highest dietary level, the combined
residue of 5-HBC plus 5-hydroxy-2-benzimidazole carbamate (4-HBC)
in the milk was approximately 0.1 mg/litre. These metabolic
studies appear to indicate that benomyl and its metabolites do not
accumulate in animal tissues or animal products (Gardiner et al.,
1974; FAO/WHO, 1985a).
A rat was administered a diet containing 2500 mg non-labelled
benomyl/kg. Twelve days later, the animal received an intragastric
intubation of 2-14C-benomyl (7.7 mg). Urine and faeces were
collected, and organs were analysed for radioactivity. The
elimination was rather rapid and mainly via the urine. After 72 h,
0.2% of the administered dose was found in the liver, while < 0.01%
was present in all the other organs and the carcass. The same
results were found when a rat was administered 2-14C-MBC
(Gardiner et al., 1974)
A male beagle dog fed a diet containing non-labelled benomyl at
2500 mg/kg, and administered 30.8 mg 2-14C-benomyl by capsule 7
days later, showed a completely different excretion pattern. After
3 days, residues were found only in the liver (0.31% of the
administered dose); the levels in the other organs were < 0.01%
(Gardiner et al., 1974).
Rats administered an oral dose of 14C-labelled propham or
chloropropham (labelled in the chain or in the ring), showed
radioactivity in all tissues, with the highest concentration in
the kidneys. The average biological half-life of 14C from both
compounds in most organs was short, ranging from 3 to 8 h.
However, in brain, fat, and muscle, the half-life was about twice
this value (Fang et al., 1974).
6.2.3 Metabolic transformation
6.2.3.1 Biotransformation mechanisms
Carbamate pesticides are transformed metabolically by a variety
of chemical reactions into more water-soluble molecules with
increased polar properties. The initial step, usually oxidative in
nature, introduces a functional hydroxyl group that serves as a
site for secondary conjugative reactions to yield products that
can be excreted via the urine and/or faeces. In some cases, the
oxygenated metabolites, such as 5-hydroxypropoxur and 5-hydroxy-
carbaryl, are known to be toxic and to possess anticholinesterase
activity (Fig. 3) (Oonnithan & Casida, 1968; Black et al., 1973).
This undoubtedly contributes to the overall toxicity of the parent
compound (Black et al., 1973).
6.2.3.2 Oxidation
The principal route of metabolism of insecticidal carbamate
esters is oxidative and is generally associated with the mixed-
function oxidase (MFO) enzymes, which are present in several
tissues. Examples of the sites of oxidative attack on a
hypothetical methyl carbamate are given in Fig. 4.
Depending on the functional groups in the molecule, a variety
of reactions catalysed by these enzymes may occur (Fukuto, 1972),
as shown in Fig. 4. Typical oxidative reactions include: (a)
hydroxylation of aromatic rings, or epoxidation; (b) O -
dealkylation; (c) N -methyl hydroxylation; (d) N -dealkylation; (e)
hydroxylation and subsequent oxidation of aliphatic side chains;
and (f) thioether oxidation to sulfoxides and sulfones.
Because of the variety of different groups present in carbamate
insecticides, the metabolism of these compounds is often complex.
Carbaryl, for example, is a relatively simple compound, yet it is
metabolized to at least 15 different compounds in mammals through a
variety of oxidative and hydrolytic reactions (Leeling & Casida,
1966). A study showed that, as well as the organic solvent-soluble
unconjugated metabolites, the urine from carbaryl-treated rabbits
contained at least 4 or 5 other water-soluble metabolites. These
are probably conjugates of the hydroxylated products of carbaryl,
i.e., glucuronides or sulfates.

 6.2.3.3 Hydrolysis
Carbamates are hydrolysed either spontaneously or by esterases
(Aldridge & Reiner, 1972), yielding, as final products, an amine,
carbon dioxide (CO2), and an alcohol or phenol:
R1HN-C(O)OR2 + H2O ---> R1NH2 + CO2 + R2OH
The mechanism of hydrolysis is different for N -methyl and
N -dimethyl carbamates.
In general, the rates of hydrolysis of carbamates by esterases
are faster in mammals than in plants and insects, though there are
exceptions. The differences in enzymatic rates of hydrolysis
depend on the structure of the carbamate and on the particular
esterase.
Hydrolysis of the carbamates is catalysed by a group of enzymes
known as A-esterases or arylesterases (EC 3.1.1.2). It has been
established that enzymatic hydrolysis occurs both in vitro and in
vivo, but, to date, it has not been determined to what extent the
in vivo hydrolysis contributes to the detoxification of the
chemical.
The hydrolytic activity of plasma-albumin has also been
indicated (Augustinsson & Casida 1959; Casida & Augustinsson, 1959;
Reiner & Skrinjaric-Spoljar, 1968).
6.2.3.4 Conjugation
The conversion to conjugated compounds of the hydroxy products
produced by the drug-metabolizing enzyme systems is an important
reaction that leads to the formation of water-soluble compounds
such as O - and N -glucuronides, sulfates, and mercapturic acid,
which can be eliminated via the urine or faeces.
6.2.3.5 Examples of biotransformation of carbamates
A few examples of the biotransformation of carbamates are given
in Fig. 5.
Aldicarb is unusual in that it does not contain an aromatic
ring, and it is also a carbamate ester of an oxime. Knaak et al.
(1966) studied its metabolism in rats. The metabolic reactions of
aldicarb are similar to the oxidative and hydrolytic pathways found
in the thioether-containing organophosphorous esters. The
thioether moiety is rapidly oxidized to the oxime-sulfoxide; the
oxime-sulfoxide is oxidized much more slowly to the oxime-sulfone.
A large number of metabolites resulting from the hydrolysis of the
carbamate moiety of the oxidative metabolites of aldicarb have been
identified.
6.2.3.3 Hydrolysis
Carbamates are hydrolysed either spontaneously or by esterases
(Aldridge & Reiner, 1972), yielding, as final products, an amine,
carbon dioxide (CO2), and an alcohol or phenol:
R1HN-C(O)OR2 + H2O ---> R1NH2 + CO2 + R2OH
The mechanism of hydrolysis is different for N -methyl and
N -dimethyl carbamates.
In general, the rates of hydrolysis of carbamates by esterases
are faster in mammals than in plants and insects, though there are
exceptions. The differences in enzymatic rates of hydrolysis
depend on the structure of the carbamate and on the particular
esterase.
Hydrolysis of the carbamates is catalysed by a group of enzymes
known as A-esterases or arylesterases (EC 3.1.1.2). It has been
established that enzymatic hydrolysis occurs both in vitro and in
vivo, but, to date, it has not been determined to what extent the
in vivo hydrolysis contributes to the detoxification of the
chemical.
The hydrolytic activity of plasma-albumin has also been
indicated (Augustinsson & Casida 1959; Casida & Augustinsson, 1959;
Reiner & Skrinjaric-Spoljar, 1968).
6.2.3.4 Conjugation
The conversion to conjugated compounds of the hydroxy products
produced by the drug-metabolizing enzyme systems is an important
reaction that leads to the formation of water-soluble compounds
such as O - and N -glucuronides, sulfates, and mercapturic acid,
which can be eliminated via the urine or faeces.
6.2.3.5 Examples of biotransformation of carbamates
A few examples of the biotransformation of carbamates are given
in Fig. 5.
Aldicarb is unusual in that it does not contain an aromatic
ring, and it is also a carbamate ester of an oxime. Knaak et al.
(1966) studied its metabolism in rats. The metabolic reactions of
aldicarb are similar to the oxidative and hydrolytic pathways found
in the thioether-containing organophosphorous esters. The
thioether moiety is rapidly oxidized to the oxime-sulfoxide; the
oxime-sulfoxide is oxidized much more slowly to the oxime-sulfone.
A large number of metabolites resulting from the hydrolysis of the
carbamate moiety of the oxidative metabolites of aldicarb have been
identified.

 The oxime-sulfoxide was found to be the major hydrolytic
metabolite in plants and insects. Other research workers recovered
the nitrile sulfoxide, in greater quantities, both compounds
arising from cleavage of the carbamate moiety of aldicarb sulfoxide
(Metcalf et al., 1966; Fukuto, 1972).
Propham and chlorpropham have been studied in a number of
animal species. Both compounds are rather rapidly metabolized.
The major metabolites recovered from the animals were 4-
hydroxypropham and 4-hydroxychloropropham, respectively. Both were
excreted as the sulfate and/or glucuronide. Depending on the animal
species, hydroxylation of propham also occurred in the 2- and 3-
position, and even 3,4-dihydroxypropham was identified. Oxidation
of the 1-carbon of the isopropyl moiety of chlorpropham resulted in
the formation of the monohydroxy product, which was converted to
the 1,3-hydroxy and 1-carboxy derivative. Hydrolysis of
chloropropham also occurred to a significant extent, since the
other major metabolites found were conjugates of 3-chloro-4-
hydroxy- and 5-chloro-2-hydroxy-acetanilide. Chlorpropham is more
susceptible to hydrolytic degradation than propham, or, conversely,
propham is more susceptible to oxidative degradation than
chlorpropham (Bobik et al., 1972; Paulson et al., 1973; Fang et
al., 1974).
Phenmedipham and desmedipham were hydrolysed mainly into
methyl- N or ethyl- N -(3 hydroxyphenyl) carbamates. Both compounds
were subsequently hydrolysed to 3-aminophenol. Furthermore, the
aminophenol was N -acetylated, and the phenolic hydroxyl moiety was
conjugated with the sulfate or glucuronide (Sonawane & Knowles,
1972).
Carbaryl is rapidly hydroxylated or hydrolysed and thereafter
conjugated and eliminated from a number of animal species,
principally in the urine as glucuronides or sulfates. Depending on
the animal species, at least 8 water-soluble metabolites were
found. Dorough & Casida (1964) and Fukuto (1972) studied the
biotransformation of carbaryl in animals. It was found that
hydroxylation took place, and the following metabolites were
identified: 1-naphthyl N -hydroxymethylcarbamate (and as a result
of ring hydroxylation) 4-hydroxy-1-naphthyl- N -methylcarbamate,
5-hydroxy-1-naphthyl- N -methyl-carbamate, and 5,6-dihydroxy-1-
naphthylmethylcarbamate.
Certain metabolites appeared to be the hydrolytic products of
carbamates with modified ring structures, such as 1-hydroxy-5,6-
dihydro-naphthalene and 1-naphthol. The latter compound is found
in the urine as 1-naphthyl glucuronide and/or 1-naphthyl sulfate.
Sullivan et al. (1972) identified one of these metabolites, 5,6-
dihydroxy carbaryl glucuronide, in the urine of rats. Most
metabolites of carbaryl are eliminated in a similar pattern in
human beings, rats, guinea-pigs, and sheep, but in a different way
in dogs (FAO/WHO, 1968b).
The metabolism of benomyl has been studied in the mouse, rat,
rabbit, dog, sheep, and cow and is qualitatively the same in all
animal species studied.
The basic route of benomyl metabolism involves hydroxylation
to 5-hydroxy-2-benzimidazolecarbamate (5-HBC). This is the main
metabolite and conjugates (glucuronides or sulfates) of the
compound are usually eliminated via the urine, bile, and faeces
(Douch, 1973; Gardiner et al., 1974). In contrast, dogs treated
orally with 14C-benomyl eliminated only 16% of the radioactivity in
the urine and 83% in the faeces. The principal metabolites in the
faeces were carbendazim and 5-hydroxycarbendazim. In addition, 4-
hydroxycarbendazim was identified as a metabolite in the urine,
faeces, and milk of a cow treated with benomyl (Fig. 6).
The oxime-sulfoxide was found to be the major hydrolytic
metabolite in plants and insects. Other research workers recovered
the nitrile sulfoxide, in greater quantities, both compounds
arising from cleavage of the carbamate moiety of aldicarb sulfoxide
(Metcalf et al., 1966; Fukuto, 1972).
Propham and chlorpropham have been studied in a number of
animal species. Both compounds are rather rapidly metabolized.
The major metabolites recovered from the animals were 4-
hydroxypropham and 4-hydroxychloropropham, respectively. Both were
excreted as the sulfate and/or glucuronide. Depending on the animal
species, hydroxylation of propham also occurred in the 2- and 3-
position, and even 3,4-dihydroxypropham was identified. Oxidation
of the 1-carbon of the isopropyl moiety of chlorpropham resulted in
the formation of the monohydroxy product, which was converted to
the 1,3-hydroxy and 1-carboxy derivative. Hydrolysis of
chloropropham also occurred to a significant extent, since the
other major metabolites found were conjugates of 3-chloro-4-
hydroxy- and 5-chloro-2-hydroxy-acetanilide. Chlorpropham is more
susceptible to hydrolytic degradation than propham, or, conversely,
propham is more susceptible to oxidative degradation than
chlorpropham (Bobik et al., 1972; Paulson et al., 1973; Fang et
al., 1974).
Phenmedipham and desmedipham were hydrolysed mainly into
methyl- N or ethyl- N -(3 hydroxyphenyl) carbamates. Both compounds
were subsequently hydrolysed to 3-aminophenol. Furthermore, the
aminophenol was N -acetylated, and the phenolic hydroxyl moiety was
conjugated with the sulfate or glucuronide (Sonawane & Knowles,
1972).
Carbaryl is rapidly hydroxylated or hydrolysed and thereafter
conjugated and eliminated from a number of animal species,
principally in the urine as glucuronides or sulfates. Depending on
the animal species, at least 8 water-soluble metabolites were
found. Dorough & Casida (1964) and Fukuto (1972) studied the
biotransformation of carbaryl in animals. It was found that
hydroxylation took place, and the following metabolites were
identified: 1-naphthyl N -hydroxymethylcarbamate (and as a result
of ring hydroxylation) 4-hydroxy-1-naphthyl- N -methylcarbamate,
5-hydroxy-1-naphthyl- N -methyl-carbamate, and 5,6-dihydroxy-1-
naphthylmethylcarbamate.
Certain metabolites appeared to be the hydrolytic products of
carbamates with modified ring structures, such as 1-hydroxy-5,6-
dihydro-naphthalene and 1-naphthol. The latter compound is found
in the urine as 1-naphthyl glucuronide and/or 1-naphthyl sulfate.
Sullivan et al. (1972) identified one of these metabolites, 5,6-
dihydroxy carbaryl glucuronide, in the urine of rats. Most
metabolites of carbaryl are eliminated in a similar pattern in
human beings, rats, guinea-pigs, and sheep, but in a different way
in dogs (FAO/WHO, 1968b).
The metabolism of benomyl has been studied in the mouse, rat,
rabbit, dog, sheep, and cow and is qualitatively the same in all
animal species studied.
The basic route of benomyl metabolism involves hydroxylation
to 5-hydroxy-2-benzimidazolecarbamate (5-HBC). This is the main
metabolite and conjugates (glucuronides or sulfates) of the
compound are usually eliminated via the urine, bile, and faeces
(Douch, 1973; Gardiner et al., 1974). In contrast, dogs treated
orally with 14C-benomyl eliminated only 16% of the radioactivity in
the urine and 83% in the faeces. The principal metabolites in the
faeces were carbendazim and 5-hydroxycarbendazim. In addition, 4-
hydroxycarbendazim was identified as a metabolite in the urine,
faeces, and milk of a cow treated with benomyl (Fig. 6).
 Heybroek et al. (1984) studied the metabolism of [ring-14C]
asulam in the rat. Most of the radioactivity (61 -74%)
administered orally or intravenously was excreted in the urine in
24 h as unchanged asulam, 8 - 14% as N 4-acetylasulam, and up to
2.5% as N 4-acetylsulfanilamide. Small amounts of radioactivity
were found in faeces.
These examples illustrate the different metabolic pathways of
biotransformation. For more details, readers should refer to
Fukuto (1972) who has reviewed the metabolism of carbaryl,
mexacarbamate, aminocarb, formetanate, aldicarb, methiocarb,
carbofuran, MPMC, trimethacarb carbanolate, propoxur, and
dimetilan. Harvey & Han (1978a) have described the metabolism of
oxamyl and Harvey et al. (1973), of methomyl in the rat. The
metabolism of pirimicarb was extensively described in FAO/WHO
(1977b). Furthermore, information on the metabolism is provided in
the handbooks on carbamates, and the different monographs of the
Joint FAO/WHO Meetings on Pesticide Residues.
6.3 Elimination and Excretion in Expired Air, Faeces, and Urine
6.3.1 Man
In a study on human volunteers, 2 men were dosed orally with
carbaryl in gelatin capsules at 2.0 mg/kg body weight.
Chromatographic analyses of a 4-h urine sample revealed 1-naphthyl
glucuronide (15%), 1-naphthyl sulfate (8%), and 4-(methylcarbamoyl-
oxy)-1-naphthyl glucuronide (4%). In addition, 1-naphthyl
methylimidocarbonate O -glucuronide was identified by fluorometry
(FAO/WHO, 1968b). During the first 8 h, the urinary excretion of
these metabolites constituted 12 - 15% of the carbaryl
administered. Small amounts of urinary metabolites were detected
by fluorometric analysis on the second day, but not afterwards
(Knaak et al., 1968). In another study, 1-naphthyl glucuronide, 1-
naphthyl sulfate, and other unidentified metabolites were found in
24-h urine samples from men exposed to carbaryl dust during a
packaging operation in a factory (details not available) (Knaak et
al., 1965).
The determination of 1-naphthol or its sulfate or glucuronide
in the urine of persons occupationally exposed to carbaryl is one
method of biological monitoring for this exposure (FAO/WHO, 1982b).
Dawson et al. (1964) investigated the metabolism and excretion
of propoxur in 3 male volunteers orally administered a dose of 50
mg. Within 8 - 10 h, 27% of the propoxur appeared in the urine as
its metabolite 2-isopropoxyphenol, indicating that propoxur is
rapidly absorbed and hydrolysed in human beings.
The absorption and fate of benomyl in animals following oral
administration of the compound varies with species. According to a
study that involved one rat and one dog administered 2-14C-benomyl
by stomach tube, the rat eliminated about 86% of a single dose via
the urine and 13% via faeces. The dog eliminated only 16% of a
similar benomyl dose via the urine, while almost 83% was detected
in faeces. No data are available concerning the possible roles of
the enterohepatic circulation and biliary excretion of benomyl or
its metabolites (Gardiner et al., 1974).
6.3.2 Laboratory animals
In animals, oxidation of carbamates often, but not always,
results in detoxification. Oxidative metabolism generally leads to
products of greater polarity and water solubility, and these can be
more readily eliminated through the urine and faeces than the
parent compound.
In the rat, benomyl is mainly excreted via the urine (78.9% of
the given dose) as 5-HBC conjugates. In the dog, 99% of the dose
administered was eliminated within 72 h. The major route of
elimination was via the faeces in which benomyl and/or MBC and 5-
HBC were detected. On the basis of studies on the rat, dog, dairy
cow, and chicken, neither benomyl nor its metabolites tend to
accumulate in animal tissues (Gardiner et al., 1974).
According to FAO/WHO (1974b), feeding of 14C- or 35S-labelled
thiophanate-methyl to rats, mice, and dogs resulted in 80 - 100%
recovery of the label in the faeces and urine within 96 h.
Unmetabolized thiophanate-methyl has been reported to be the major
component of faecal elimination. The minor part consisted of 4-
hydroxy-thiophanate-methyl, dimethyl-4,4- O -phenylenebisallophanate.
MBC and 5-hydroxy-MBC were also detected.
Following application of carbendazim to rats and mice by
intragastric intubation, almost all metabolites in the urine were
conjugated as sulfate esters. 5-HBC was released as the only
metabolite extractable from water. A higher proportion of polar
compounds was found in the urine of mice than in that of rats.
Polarity was caused by the phenolic hydroxyl group (FAO/WHO,
1985a).
The excretion of 14C-labelled propham and chlorpropham was
investigated in female rats after a single oral dose. The average
3-day urinary excretion of radioactivity was between 56 and 85%,
depending on the position of labelling: viz chain or ring
labelling. With chain 14C-chlorpropham, an average of 35% of the
administered radioactivity appeared in the respired air, compared
with only 5% for chain 14C-propham (Fang et al., 1974).
6.4 Metabolism in Plants
Studies on the metabolism and fate of carbamate pesticides in
plants are of great importance for assessing the human hazards from
the consumption of fruit and vegetables containing residues of the
applied pesticides and metabolites.
Generally, the metabolism of carbamates in plants follows
another route of detoxification. The hydroxylated carbamates
produced by enzymes are conjugated either with amino acids (for
instance cysteine) or as glycosides and phosphates, which are
stored as terminal metabolites (Still & Rusness, 1977; Aldridge &
Magos, 1978).
The toxicological properties of plant conjugates in mammals or
other animals are not generally known. Insecticidal methyl
carbamate esters are vulnerable to hydrolytic cleavage, which
results in detoxified products. Detoxification of carbamate esters
in plants by hydrolysis occurs to a lesser extent than
detoxification by oxidative metabolism; nevertheless, hydrolytic
cleavage of the ester moiety is a significant detoxification
mechanism. For instance, in sugar beets treated with desmedipham,
the major metabolites were ethyl-3-hydroxy phenyl carbamate and 3-
aminophenol (Knowles & Sonawane, 1972). Carbetamide is hydrolysed
to aniline (Guardigli et al., 1977). Aromatic ring hydroxylation
appears to be the predominant metabolic reaction that carbamate
herbicides undergo in plants. The major metabolites isolated from
soybean plants, after uptake of chlorpropham, were glucoside
conjugates of 2-hydroxy- and 4-hydroxypropham. Similar results
were obtained with propham (Still & Mansager, 1973a,b, 1975).
There is evidence that the ring hydroxylation varies with the
plant species. In other carbamates, for instance in the case of
barban, ring hydroxylation did not appear to take place, and polar
metabolites were produced, which were hydrolysed into
chloroanilines (Still & Mansager, 1972).
Furthermore, oxidation of aliphatic groups takes place in
carbamate herbicides (Wiedmann et al., 1976). Still & Herrett
(1975) demonstrated N -methyl hydroxylation for dichlormate, a
metabolite that is either conjugated or loses the hydroxymethyl
moiety to produce N -demethylated dichlormate.
Thiophanate-methyl is mainly metabolized into carbendazim (MBC)
and, to a less extent, into 2-aminobenzimidazole (2AB). A few other
metabolites have also been found (FAO/WHO, 1974b).
The metabolic pathway for carbaryl in plants is identical,
whether the compound is injected into the stem or applied to the
leaf surface. After entering the plant, carbaryl undergoes
biotransformation to its primary metabolites, which are similar to
the ones formed in animals. These hydroxylated metabolites, which
are less toxic than carbaryl itself, are conjugated by plants to
form water-soluble glycosides. Injection of carbaryl into the
bean plant produced water-soluble metabolites attributable to
hydroxylation of the ring or N -methyl group followed by
conjugation, mainly as glycosides (Kuhr, 1968; FAO/WHO, 1970b;
Fukuto, 1972).
The metabolism of aldicarb in plants is the same as that in
mammals. In the cotton plant, the thioether moiety is rapidly
oxidized to the sulfoxide, and the latter is slowly transformed to
the sulfone. The sulfoxide is rather stable and can be present in
cotton plants, even as long as 2 months after treatment. The total
metabolites present can be as high as 80% (Kuhr, 1968). Because of
their persistence in plants, and their even higher anticholinesterase
activity compared with aldicarb itself, the sulfoxide and sulfone
should be taken into account when considering residue tolerances.
The major metabolite isolated from bean plants, 28 days after
treatment with carbofuran, was a conjugate of 3-hydroxy-carbofuran,
which remained in the aqueous phase after extraction with an
organic solvent. The conjugate represented 55% of the total 14C-
labelled residues present in the plant. 3-Hydroxy-carbofuran is
highly toxic for mammals (LD50 in the rat 7 mg/kg body weight).
The conjugate may also be toxic, particularly taking into account
the acid conditions of the mammalian stomach under which the
conjugate will be hydrolysed (FAO/WHO, 1977b, 1980b).
Harvey et al. (1978) studied the metabolism of oxamyl in plants
(tobacco, alfalfa, peanuts, potatoes, apples, oranges, and
tomatoes). The major route of degradation involved hydrolysis to
the corresponding oximino compound, which in turn became conjugated
with glucose. Further metabolism resulted in the loss of one of
the N' -methyl groups and/or addition of other glucose units to the
sugar moiety of the original conjugate. Total breakdown into
normal natural products has been demonstrated.
In a study by Harvey & Reiser (1973), methomyl rapidly degraded
to carbon dioxide and acetonitrile, which volatilized from the
plant tissues. The half-life for methomyl was of the order of 3 -
6 days. The remainder of the compound had been reincorporated into
natural plant components.
The metabolic pathways for a number of carbamate pesticides are
described in more detail in Fukuto (1972).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
As described in section 4.2.1, soil microorganisms are capable
of hydrolysing carbamates. Furthermore, it seems that
microorganisms can easily adapt themselves to metabolize the
different types of carbamates (Aly & El-Dib, 1972). Nevertheless,
carbamates and their metabolites can affect the microflora and
cause changes that may have an important impact on the maintenance
of the soil productivity (Filip, 1974).
7.2 Aquatic Organisms
In general, carbamates are not very stable under aquatic
conditions (section 4). The solubility in water differs
considerably for the different carbamates (Annex I). Furthermore,
photodecomposition takes place and microorganisms are able to
degrade these compounds. By these processes, the carbamate is
broken down (oxidation, hydrolysis) into other compounds. Some
will be less toxic, others even more toxic than the parent
compound. Carbaryl will be hydrolysed into 1-naphthol, which is
just as toxic (Armstrong & Millemann, 1974a). It seems unlikely
that most of the carbamates will persist long in the environment,
but there are exceptions, such as dimetilan and carbendazim.
Many data are available on the acute toxicity of the carbamates
in several fresh- and salt-water fish and other organisms, some of
which are listed in Tables 4 and 5.
When carbamates are deliberately added to an aquatic system,
caution is necessary since, with high concentrations, a number of
species are at risk. For example, while applications of carbaryl
of 0.93 kg/ha were effective in controlling the ghost shrimp
(Callianassa californiensis), an oyster pest, they also caused a
reduction in the population of juvenile clams (Armstrong &
Millemann, 1974a).
In a similar study (Armstrong & Millemann, 1974b), carbaryl
and its hydrolytic product, 1-naphthol, were examined for other
effects on the mussel (Mytilus edulis). An age dependence was
noted; the most sensitive stage (appearance of the first polar body
shortly after fertilization) had EC50s of 5.3 and 5.2 mg/litre for
carbaryl and 1-naphthol, respectively. Effects of the compounds
on development were characterized by disjunction of blastomeres and
asynchronous and unaligned cleavages. Carbaryl, at a single dose
of 0.1 mg/litre, also disrupted normal schooling behaviour in
juvenile Menidia medidia; the disruptive effects were attributed
to the 1-naphthol rather than to the parent carbamate. These
effects were reversed within 3 days of placing the fish in clean
water (Weis & Weis, 1974). Hansen (1969) studied the capacity of
sheepshead minnows (Cyprinodon variegatus) to avoid carbaryl. The
fish did not avoid carbaryl in concentrations of 0.1 - 10 mg/litre
water.
Table 4. Acute aquatic toxicity (LC50 in mg/litre)a
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Aldicarb fish Rainbow trout (Salmo gairdneri) 0.5 12 0.56
fish Bluegill (Lepomis machrochirus) 1.3 24 0.05
Aminocarb fish Rainbow trout (Salmo gairdneri) 1.5 12 13.5b
fish Bluegill (Lepomis machrochirus) 2.0 20 3.1b
crustacea Daphnia (Daphnia magna) first instar 21 0.01 - 0.1c
(48-h EC50)
fish Rainbow trout (Salmo gairdneri) 1.5 10 0.13c
fish Bluegill (Lepomis machrochirus) 0.6 20 0.1c
insect Midge (Chironomus plumosus) fourth instar 20 0.27c
Benomyl fish Rainbow trout (Salmo gairdneri) 1.2 12 0.17d
fish Bluegill (Lepomis machrochirus) 0.9 22 0.85d
fish Rainbow trout (Salmo gairdneri) 1.0 12 0.31e
fish Bluegill (Lepomis machrochirus) 0.6 22 1.2e
Benomyl fish Rainbow trout (Salmo gairdneri) 0.2 12 0.37f
metabolite
MBC
Bufencarb crustacea Scud (Gammarus fasciatus) mature 15 0.001
fish Goldfish (Carassius auratus) 1.0 18 0.29
Carbaryl fish Rainbow trout (Salmo gairdneri) 1.5 12 1.95d
fish Bluegill (Lepomis machrochirus) 1.2 18 6.76d
crustacea Daphnia (Daphnia pulex) first instar 16 0.0064d
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature 21 0.026d
----------------------------------------------------------------------------------------------------
Table 4. (contd.)
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Carbofuran fish Rainbow trout (Salmo gairdneri) 1.5 12 0.38d
fish Bluegill (Lepomis machrochirus) 0.8 18 0.24e
Dichlormate fish Rainbow trout (Salmo gairdneri) 0.8 12 4.9
Methiocarb fish Rainbow trout (Salmo gairdneri) 1.3 12 0.80
fish Bluegill (Lepomis machrochirus) 1.0 24 0.21
Methomyl fish Rainbow trout (Salmo gairdneri) 1.1 12 1.60b
fish Bluegill (Lepomis machrochirus) 0.9 20 1.05b
crustacea Daphnia (Daphina magna) first instar 21 0.009b
(48-h EC50)
Mexacarbate fish Rainbow trout (Salmo gairdneri) 1.0 11 12.0g
fish Bluegill (Lepomis machrochirus) 0.7 12 22.9g
crustacea Daphina (Daphina pulex) first instar 15 0.010g
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature - 0.04g
Trimethacarb fish Rainbow trout (Salmo gairdneri) 1.2 12 1.0
fish Bluegill (Lepomis machrochirus) 0.9 18 11.6
----------------------------------------------------------------------------------------------------
a From: Johnson & Finley (1980).
b Technical material, 95 - 98%.
c Liquid formulation, 17%.
d Technical material, 99%.
e Wettable powder, 50%.
f Methyl-2-benzimidazole (MBC), 99%.
g Technical material, 90 - 95%.
Note: It should be recognized that the LC50 and EC50 values only give an indication of the
toxicity and that the toxicity for these organisms may be appreciably changed by variations
in temperature, pH, oxygen content, and water hardness. A 10-fold increase in the toxicity
may be found. Also, the life stage of the organisms is an important factor in estimating
the toxicity of the compound.
Table 5. Acute toxicity of carbamate pesticides for some aquatic organisms
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
Benomyl 7.5 12 11 (wettable - 14 Yoshida & Nishiuchi
powder) (1972)
BPMC 16 10 ~ 40 1.7 5.0 0.32 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Carbaryl 13 10 ~ 40 2.8 2.8 0.05 Yoshida & Nishiuchi
(1972)
Carbendazim > 40 - 40 - > 40 Yoshida & Nishiuchi
(1976)
CPMC 10 ~ 40 10 ~ 40 5.6 7.0 0.1 ~ 0.5 Yoshida & Nishiuchi
(emulsifiable (1972); Nishiuchi
concentrate) (1974)
Isoprocarb 10 ~ 40 10 ~ 40 5.9 3.0 0.30 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Mecarbam 0.70 0.68 0.35 0.055 0.03 Yoshida & Nishiuchi
granule (1972); Nishiuchi
(1974)
Methomyl 2.8 2.7 0.87 1.0 0.045 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
MPMC 10 ~ 40 10 ~ 40 12 7.3 0.07 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
MTMC 10 ~ 40 10 ~ 40 27 13 0.35 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Pirimicarb > 40 - 40 - 0.048 Yoshida & Nishiuchi
(1976)
Promecarb 2.7 - 3.3 - 0.020 Yoshida & Nishiuchi
(1976)
Terbam 0.92 - 2.2 - 0.025 Yoshida & Nishiuchi
(1976)
Thiophanate > 40 > 40 > 40 - > 40 Yoshida & Nishiuchi
(1972)
Thiophanate 11 > 40 11 (wettable - > 40 Yoshida & Nishiuchi
-methyl powder) (1972)
XMC > 40 > 40 33 25 0.055 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
a BPMC = 1-sec-butylphenylmethyl carbamate
CPMC = 1-chlorophenylmethyl carbamate
MPMC = 3,4-xylylmethyl carbamate
MTMC = 4-tolylmethyl carbamate
Terbam = 4-tert-butylphenylmethyl carbamate
XMC = 3,5-xylylmethyl carbamate
Note: Test methods are officially recognized methods based on the Notification of the
Ministry of Agriculture, Forestry, and Fisheries of Japan.
Carbamates can have adverse effects on algae. Stadnyk et al.
(1971) reported that carbaryl at a concentration of 0.1 mg/litre
caused an increase in cell numbers and in the biomass of the green
algae Scenedesmus quadricaudata.
Motsuage fish (Motsugo pseudoras bora parva) were reported to
accumulate carbaryl and BPMC during a 30-day exposure to a
concentration of 0.6 - 1.2 mg/litre. Carbaryl uptake was greatest.
However, this compound underwent more rapid metabolism and
excretion than BPMC. Metabolism of BPMC was slow and introduced
permanent spinal curvature of the backbone in about 30% of the fish
exposed, an effect seen with the organophosphorus insecticide
diazinon (Kanazawa, 1975).
A residue study indicated that over a 28-day period at the
highest exposure level of 0.75 mg methomyl/litre, no accumulation
of the carbamate took place. During the entire exposure period,
the values ranged from 0.36 to 0.45 mg/kg. After a 3-day withdrawal
period, no methomyl (< 0.02 mg/kg) was detected in the fish
(Kaplan & Sherman, 1977).
The results summarized in Table 4 are representative of the
toxicity of the different carbamates for fish and invertebrate
species. In general, the LC50s of carbamates for different fish
species range from approximately 0.1 to 10 mg/litre. Invertebrates
are usually more sensitive. The EC50 values for Daphnids and
other species are mainly below 0.1 mg/litre. Some carbamates seem
to be very toxic (< 0.01 mg/litre) for these invertebrates.
7.2.1 Field studies
Little information is available concerning the fate and
persistence of carbamates in the aquatic environment. The presence
of these carbamates in surface waters may have an effect on water
organisms.
Quraishi (1972) studied the persistence of aldicarb. Field
water treated in the laboratory at 100 mg aldicarb/litre resulted
in residues of aldicarb and its metabolites of 0.4 mg/litre after
11 months. Water was stored at 16 - 20 °C and exposed for
approximately 507 h to sunlight.
Data concerning the fate of aminocarb have been reviewed by
Vassilieff & Ecobichon (1982). A half-life of 28.5 days in pond
water was found under normal environmental conditions. The
principal metabolite appeared to be the phenol, though the
methylamino and formylamino analogues were also observed.
Harvey & Pease (1973) found that, under field conditions in
Delaware, Florida, and North Carolina, methomyl broke down almost
completely within 1 month; most of it was lost from the soil by
volatilization, presumably as carbon dioxide. Small amounts
extracted from the soil consisted of methomyl, S -methyl N -
hydroxythioacetimidate, and some polar compounds. A run-off study,
under farm conditions, showed that the compound did not move into
untreated areas with run-off water.
7.3 Terrestrial Organisms
7.3.1 Effects on soil fauna
Thiophanate-methyl and carbendazim have been shown to be
equally as toxic through contact as benomyl. Earthworms (Lumbricus
terrestris) immersed for 1 min in a 0.6% aqueous suspension of
these compounds died in 14 days. Worms in pots containing soil
drenched at a rate of 0.78 g/m2 died within 18 days (Wright &
Stringer, 1973).
7.3.2 Wildlife
The LD50 of benomyl for mallard ducks and quail is > 5000
mg/kg body weight. The cumulative toxicity of formetanate (7 days)
is approximately 6800 mg/kg body weight for ducks, and > 4640 mg/kg
body weight for quail and for pheasant (Aldridge & Magos, 1978).
Lethal and sub-lethal doses of aldicarb, methiocarb, oxamyl,
pirimicarb, and thiofanox administered to Japanese quail produced
significant inhibition of plasma- and brain-ChE and influenced the
activity of other enzymes, such as alpha-naphthyl acetate esterase,
glutamate dehydrogenase, and glutamate oxaloacetic transaminase.
With sub-lethal dose levels, plasma-ChE activity recovered within a
few days (Westlake et al., 1981).
An 8-day dietary LC50 for the Peking duck was 1890 mg/kg
feed; for bobwhite quail, the dietary LC50 was 3680 mg/kg feed
(Kaplan & Sherman, 1977).
Bobwhites (Colinus virginianus) were provided diets containing
sublethal levels of carbaryl (237 or 1235 mg/kg), for 7 days, or
carbofuran (26 mg/kg), for 14 days. Food intake, body weight, and
the locomotor activity of adult bobwhites were normal.
Administration of a diet containing 131 mg carbofuran/kg resulted
in reductions in food intake, body weight, and locomotor activity
(Robel et al., 1982).
7.3.3 Bees
An undesirable effect caused by the use of carbamate
insecticides has been the mortality of honey bees, a species that
exhibits a high sensitivity to these compounds. Because of the
agricultural and ecological importance of bees, thorough
comparative toxicity studies have been carried out on a large
number of compounds to identify those with the widest margin of
safety (Atkins et al., 1973).
Abdel-Aal & Fahmy (1977) compared the effects of aldicarb,
methomyl, N -desmethylmethomyl, carbaryl, and carbofuran on the
honey bee (Apis mellifera) and found that all of the compounds
were extremely toxic.
7.4 Earthworm and Mite Populations
Studies by Stringer & Wright (1973) and Wright & Stringer
(1973) showed that earthworm populations in apple orchard plots
sprayed with either benomyl or thiophanate-methyl were reduced. In
particular, Lumbricus terrestris, an important species in the
ecology of the apple orchard, was virtually eliminated. Captive
worms would not feed on leaf material that had been sprayed at 1.75
µg/cm2 with benomyl, methyl benzimidazol-2-yl carbamate (MBC), or
thiophanate-methyl, and feeding was significantly reduced at a
level of 0.87 µg/cm2. All the worms were killed following contact
with benomyl as an aqueous suspension or soil drench (7.75 kg/ha).
It was suggested by the authors that the toxicity of benomyl
might be due to the anticholinesterase activity of the carbamate
moiety. Effects such as lethargic movements, muscular paralysis,
and death support this supposition.
The spraying of benomyl and thiophanate-methyl in orchards
reduced the number and biomass of all earthworm species combined
and also of each individual species (Stringer & Lyons, 1974). It
was observed that over 90% reduction in earthworm populations
occurred in pastures treated with benomyl. It was suggested that
these changes were reversible, and that the populations of
earthworms would return to normal a few years after the termination
of the treatment (Tomlin & Gore, 1974).
The carbamate insecticide most commonly used in the soil is
carbaryl; it is used mainly in woodlands and orchards where the
formation and fertility of the soil are important.
Stegeman & LeRoy (1964) applied carbaryl at 1.3, 11.2, and 56
kg ai/ha to a red pine plantation and mixed hardwood stands, and,
though the lowest dose did not have any effects on mite and
Collembola populations, the other 2 dose levels considerably
decreased the numbers of mites and Collembolla. Populations began
to recover after 2 months, but Collembola were more sensitive and
recovered more slowly. There seems to be little likelihood of
long-term effects of carbaryl on mite populations (Edwards &
Thompson, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
Short- and long-term toxicity studies with carbamates have been
carried out for a period of 20 - 30 years. It is clear that the
production of these carbamates has improved during this period and
that, consequently, purer products have become available. The use
of less pure substances in earlier toxicity studies could explain
the conflicting results between these and later studies.
8.1.1. Oral
Acute oral and dermal toxicity data on animals, for most of the
carbamate pesticides, are given in Table 6. The WHO Recommended
Classification of Pesticides by Hazard is also cited. This
classification is based primarily on the acute oral and dermal
toxicity of the technical material for the rat (WHO, 1984). The
oral LD50s range from less than 1 mg/kg body weight for the highly-
toxic aldicarb to over 5000 mg/kg body weight for the non-
insecticidal carbamates such as phenmedipham, carbendazim, propham,
and benomyl.
A dose-effect relationship is generally noticed between the
administered dose, the severity of effects, and the amount of ChE
inhibition. A correlation also exists between the duration of
symptoms and the in vivo persistence of the compound.
In general, the toxicological effects produced by carbaryl are
typical of those produced by methyl carbamates. The effects of
carbaryl on a number of different animal species were studied by
Carpenter et al. (1961). Rats given a single oral dose of 560
mg/kg body weight showed 42% and 30% inhibition of erythrocyte- and
brain-ChE, respectively, within 1/2 h of treatment; only about 5%
inhibition of plasma-ChE was observed. All ChE levels were almost
normal after 24 h. In studies on rats administered single oral
doses ranging from 5 to 50 mg/kg body weight, a decrease in ChE
activity occurred within 5 min. Recovery was quite rapid
(Krechniak & Foss, 1982).
In 4 dogs, marginal inhibition of erythrocyte-ChE activity was
seen after treatment with a single oral dose of 375 mg carbaryl/kg
body weight. After 30 min, signs of poisoning were observed
including salivation, lachrymation, constriction of pupils,
urination, defaecation, increase in respiratory rate, muscular
twitching, tremors, and mild convulsions. The animals appeared to
have completely recovered the following day. These signs of
poisoning are classic of overstimulation of the parasympathetic
nervous system (Carpenter et al., 1961).
Vassilieff & Ecobichon (1983) studied the influence of a single
dose of 25 mg aminocarb/kg body weight on the activity of
erythrocyte- and brain-AChE, plasma-ChE, and hepatic
carboxylesterases in rats. Significant inhibition of all the
esterases was observed, 30 min or more after the administration of
aminocarb. This severe, but transient, inhibition of the tissue
esterases had recovered after 24 h.
Table 6. Acute oral and dermal toxicity data for a number of
carbamate pesticides
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
aldicarb 0.9 > 10.0 (rabbit) IA
aldoxycarb 26.8 700 - 1400 -
allyxycarb 90 - 99 500 -
aminocarb 30 - 40 275 IB
asulam > 4000 > 1200 -
barban 1376 - 1429 > 1600 -
> 20 000 (rabbit)
BPMC 623 - 657 > 5000 -
bendiocarb 40 - 156 566 - 600 II
benomyl > 10 000 > 10 000 (rabbit) 0
> 1000 (dog)
bufencarb 87 680 (rabbit) -
butacarb NA NA -
carbanolate NA NA -
carbaryl approximately > 4000 II
500 - 600 > 2000 (rabbit)
carbendazim > 15 000 > 2000 0
> 2500 (dog) > 10 000 (rabbit)
> 10 000 (quail)
carbetamide 10 000 > 2000 -
1250 (mouse) > 500 (rabbit)
1000 (dog)
carbofuran 6 - 14 3400c (rabbit) IB
15 - 19 (dog)
chlorbufam 2500 NA -
chlorpropham 5000 - 8000 10 200d O
5000 (rabbit) 2000 (dog)
cloethocarb 35.4 4000 -
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
desmedipham > 10 250 2000 - 10 000 -
2025e (rabbit)
dimetilan 64 > 2000 -
60 - 65 (mouse)
dichlormate NA NA
dioxacarb 72 approximately -
3000 1950
(rabbit)
ethiofencarb 411 - 499 > 1150 II
224 - 256 (mouse)
155 (quail)
formetanate 21, 18 (mouse) > 5600
19 (dog) > 10 200 (rabbit)
hoppicide NA NA -
isoprocarb 403 - 485 > 500
487 - 512 (mouse)
ca 500 (rabbit)
karbutilate 3000 > 15 400 -
methiocarb 100 350 - 400 II
40 (guinea-pig)
methomyl 17 - 24 > 5000 (rabbit) IB
28 (hen)
metolcarb 498 - 580 > 2000 -
mexacarbate 15 - 63 > 500 -
MPMC 380 > 1500 (mouse) -
MTMC 268 (mouse) 6000 -
oxamyl 5.4 710 (rabbit) IB
4.2 (quail)
phenmedipham > 8000 > 4000 -
> 4000 (dog)
> 3000 (chicken)
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
pirimicarb 147 (101 - 210) > 500 (rabbit) II
107 (mouse)
100 - 200 (dog)
25 - 50 (poultry)
promacyl 1220 > 4000 -
2000 - 4000 (mouse)
4000 - 8000 (hen)
promecarb 61 - 90 > 1000 -
> 1000 (rabbit)
propham 9000 6800f (rabbit) O
3000 (mouse)
propoxur 80 - 191 1000 - 2400 II
100 - 109 (mouse)
40 (guinea-pig)
swep NA NA -
terbucarb NA NA -
thiodicarb NA NA -
thiofanox 8.5 39 (rabbit) IB
43 (quail)
thiophanate > 15 000 > 15 000 -
-ethyl
thiophanate 6640 - 7500 > 10 000 O
-methyl
trimethacarb NA NA -
xylylcarb 325 - 375 > 1000 -
XMC 542 - -
445 (rabbit)
---------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
b From: WHO (1984). See this reference for classification of
organophosphates not mentioned in this annex.
The hazard referred to in this Classification is the acute risk for
health (that is, the risk of single or multiple exposures over a
relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the
directions for handling by the manufacturer or in accordance with the
Table 6 (contd.)
rules laid down for storage and transportation by competent
international bodies.
Classification relates to the technical material, and not to the
formulated product:
Class IA = Extremely hazardous. Class III = Slightly hazardous.
Class IB = Highly hazardous. Class O = unlikely to present
Class II = Moderately hazardous. acute hazard in normal use.
c With 75% water-dispersible powder.
d With 50% emulsifiable concentrate formulation.
e With 16% formulation.
f With 25% emulsifiable concentrate formulation.
NA = Not available.
Note: References are the reports of the different WHO/FAO Joint
meetings on pesticides residues (Annex III), Kaplan & Sherman
(1977), and Worthing & Walker (1983).
8.1.2 Dermal
Acute dermal toxicity values for most carbamates are mainly
above 500 mg/kg body weight with the exception of, for instance,
aldicarb, which is highly toxic.
8.1.3 Inhalation
Exposure to carbaryl (50% wettable powder, average particle
size, 15 µm) at a mean concentration of 390 mg/m3 (range 344 - 722
mg/m3), for 4 h, produced nasal and ocular irritation in 6 guinea-
pigs. When another group of guinea-pigs inhaled a mean of 230 mg
carbaryl/m3 (average particle size, 5 µm), for 4 h, no clear
effects were observed. Single exposures of dogs ranging from 2 to
5 h to a concentration of 75 mg carbaryl/m3 (average particle size,
5 µm) caused clinical signs of ChE inhibition (Carpenter et al.,
1961). Cats exposed to 80 mg carbaryl/m3 for 6 h showed
salivation, excitation, respiratory difficulties, and 39 - 55%
inhibition of ChE activity in the serum and 53 - 71% in the
erythrocytes. A single exposure to a level of 20 mg/m3 for 6 h did
not induce signs of intoxication, but serum- and erythrocyte-ChE
activities were slightly inhibited (Yakim, 1967).
8.2 Short- and Long-Term Exposures
A number of short-term studies have been carried out with
different carbamates and a variety of laboratory animal species.
In the context of this introduction, it is not possible to mention
all the reports available. Details on the short- and long-term
toxicities of the individual carbamates will be provided in future
Environmental Health Criteria. Most carbamates have been discussed
by the Joint FAO/WHO Meetings on Pesticide Residues and monographs
have been published (see reference list). Furthermore, the
International Agency for Research on Cancer has published a
monograph in which a number of carbamates have been evaluated
(IARC, 1976).
In the context of this introduction, a number of examples are
given to illustrate dose-response relationships and/or target
organs or tissues for a number of carbamates.
8.2.1 Oral
Short-term tests on rats or dogs exposed to repeated oral doses
of carbamate insecticides or fungicides (such as carbaryl,
propoxur, formetanate, carbofuran, and benomyl), revealed
inhibition of plasma (serum)-, erythrocyte-, and brain-ChE
(Carpenter et al., 1961; Ivanova-Chemishanska & Antov, 1976;
Krechniak & Foss, 1982).
(a) Pirimcarb
In a study on 4 dogs (2 males and 2 females) treated with 25 or
50 mg pirimicarb/kg body weight per day, for 110 weeks, 2 of the
animals developed anaemia. Haemoglobin content was decreased,
methaemoglobin was present, and the reticulocyte percentage was
significantly elevated. Levels of unconjugated bilirubin in serum
were increased. The 2 other animals were unaffected. There was
some indication that pirimicarb was able to induce the production
of immune IgG antibodies (Jackson et al., 1977).
(b) Carbaryl
Kidney alterations, such as cytoplasmic vacuolization, were
observed in the proximal tubules in short-term studies on monkeys
and rats adminstered carbaryl at dose levels of up to 600 or 300
mg/kg body weight, respectively (Coulston, 1967; cited in FAO/WHO,
1970b).
Two pigs from one litter were fed a ration containing carbaryl
at the rate of 150 mg/kg body weight for 72 and 83 days,
respectively. Three pigs in the same litter were fed 150 mg/kg
body weight for 4 weeks and, thereafter, 300 mg/kg body weight, the
total feeding period being 46 or 85 days. Two other pigs were
maintained on the basal ration, as controls. The clinical syndrome
of chronic intoxication was characterized by progressive
myasthenia, incoordination, ataxia, tremors, and chronic muscular
contractions terminating in paralysis and death. The lesions were
confined to the central nervous system and skeletal muscle.
Moderate to severe oedema of the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord was associated with
vascular degenerative changes, consistent with a vasogenic oedema
(Smalley et al., 1969). Spontaneous recovery from the effects of
sub-lethal doses occurred in about 8 h, indicating the ready
reversibility of the carbaryl-enzyme reaction (Carpenter et al.,
1961).
Carbaryl was administered to dogs at dose levels of 0, 0.45,
1.8, or 7.2 mg/kg body weight per day, for 5 days/week, for one
year. Cloudy swelling of the proximal convoluted and loop tubules
of the kidneys was found in the group given 7.2 mg/kg body weight.
There were no abnormalities in the composition of the blood, and no
ChE inhibition was found. It should be noted that the dose levels
tested were low (Carpenter et al., 1961).
In a long-term (2-year) toxicity study on CF-N rats, levels of
0, 50, 100, 200, or 400 mg carbaryl/kg diet (equivalent to 0, 2.5,
5, 10, or 20 mg/kg body weight) were tested. The highest dose
level produced cloudy swelling in the tubules of the kidneys
(Carpenter et al., 1961).
(c) Carbendazim
Groups of ChR-CD male rats (6/dose) were intubated with 200,
3400, or 5000 mg carbendazim/kg body weight per day, 5 times per
week, for 2 weeks. In all groups, the absolute and relative
weights of the kidneys were lower, and adverse effects on the
testes and reduction or absence of sperm in the epididymides were
found (FAO/WHO, 1985a).
(d) Benomyl
In a 2-year toxicity study, Charles River rats were
administered dietary levels of benomyl of 0, 100, 500, or 2500
mg/kg (equivalent to 0, 5, 25, or 125 mg/kg body weight). No
increase in mortality and no other compound-related effects on
haematology, urinalysis, organ weight, or histology were found
(Haskell Laboratories, 1969 cited in FAO/WHO, 1985a).
In another 2-year study, dogs were administered diets
containing 0, 100, 500, or 2500 mg benomyl/kg (equivalent to 0,
2.5, 12.5, and 62.5 mg/kg body weight). Liver cirrhosis, bile duct
proliferation, and testicular degeneration were found at the
highest dose level; however, 100 and 500 mg/kg diet did not show
any clear effects (Haskell Laboratories, 1970 cited in FAO/WHO,
1985a).
(e) Thiophanate-methyl
An oral toxicity study in which thiophanate-methyl at dose
levels of 0, 2, 10, 50, or 250 mg/kg body weight (in capsules) was
administered to beagle dogs (8 or 10 animals per group) was
conducted for 24 months. Retardation of growth was seen in both
sexes in the 250 mg/kg group. Both male and female dogs in the 50
and 250 mg groups showed a significant and dose-related increase in
the weight of the thyroid. However, there were no changes in the
serum-protein-bound iodine (PBI) levels and no histological
differences in the thyroid were found between the control group and
the 2 highest dose groups. No other abnormalities were observed
(Hashimoto & Fukuda, 1972 cited in FAO/WHO, 1974b).
(f) Methomyl
Groups of rats were given diets containing 0, 10, 50, 125
(increased to 500), or 250 mg methomyl/kg for 90 days. Weight gain
in animals administered 250 and 500 mg/kg was slightly suppressed.
No clear changes in the different parameters including plasma- and
erythrocyte-ChE activity were found (Kaplan & Sherman, 1977).
The same authors carried out studies over 90 days and 2 years
on beagle dogs. Dietary levels of 0, 50, 100, and 400 mg
methomyl/kg did not result in any nutritional, clinical,
haematological, urinary, biochemical, or pathological changes. In
the 2-year feeding study, histopathological alterations were seen
in kidneys and spleen of animals receiving 400 and 1000 mg
methomyl/kg diet. Histopathological changes were seen in the liver
and bone marrow in animals administered 1000 mg/kg diet (Kaplan &
Sherman, 1977).
The above-mentioned studies give some indication that
carbamates may have different mechanisms of action on target organs
that are related to substituents other than the carbamate ester
moiety (such as the imidazole moiety).
Apart from the effects on ChE activity, effects have been found
on the liver, kidneys, thyroid, and testes and also on the
activities of other enzyme systems such as ATPase, Gl-6-ase, LDH,
GPT, Alk-Pase, etc. (Haskell Laboratories, 1968 cited in FAO/WHO,
1985a; Ivanova-Chemishankska & Antov, 1976).
8.2.1.1 Further information on short- and long-term toxicity
It is not the purpose of this introductory document to provide
all the available data on long-term toxicity. However, these long-
term studies are important for the establishment of the no-
observed-adverse-effect level and consequently the Acceptable Daily
Intake (ADI) for man. Thus, it is necessary to indicate the
studies that were considered adequate and used by the different
FAO/WHO Joint Meetings on Pesticide Residues. The International
Agency for Research on Cancer has also evaluated long-term/
carcinogenicity studies of a number of carbamates. The results of
these international evaluations are summarized in Annex II and III.
From these Annexes, it is clear that aldicarb is the most toxic
of the carbamates considered; the established ADI for this compound
is 0.001 mg/kg body weight.
The ADIs for the other carbamates may vary from 0.01 to 0.1
mg/kg body weight. These quantities are equivalent to a daily
intake for man (60 kg body weight) of approximately 0.6 - 6 mg.
8.2.2 Dermal
(a) Pirimicarb
Daily dermal applications of 500 mg pirimicarb/kg body weight
to the skin of rabbits for 24 h over a 14-day period did not
produce any signs of intoxication (FAO/WHO, 1977b).
(b) Methomyl
Methomyl applied to the intact skin of rabbits at a repeated
rate of 200 mg/kg per day caused mild signs of intoxication such as
nasal discharge, wheezing, and transient diarrhoea. All animals
survived 15 applications. However, when applied on the abraided
skin, much more serious systemic signs were seen, such as nasal
discharge, salivation, tremors, poor coordination, and abdominal
hypertonia; a few animals died (Kaplan & Sherman, 1977).
(c) Carbaryl
Application of carbaryl at 500 mg/kg body weight to the skin of
6 rabbits resulted in 39% inhibition of serum-ChE activity and 36%
inhibition of red cell-ChE activity during the first 24 h following
treatment. ChE activity returned to initial levels in 72 h (Yakim,
1967).
8.3 Skin and Eye Irritation; Sensitization
8.3.1 Skin irritation
Rabbits exposed to a single dermal application of carbaryl at a
concentration of 100 g/litre in acetone did not show any skin
irritation (Carpenter et al., 1961).
8.3.2 Eye irritation
Undiluted carbaryl or solutions of different concentrations
were applied to the eyes of rabbits. Technical carbaryl applied in
excess as a 100 g/litre suspension in propylene glycol produced
mild injury. A 25% aqueous suspension of a microfine material did
not cause any injuries; 50 mg of the dust caused only traces of
corneal necrosis (Carpenter et al., 1961).
8.3.3 Skin sensitization
(a) Carbaryl
Groups of albino guinea-pigs were given 8 intracutaneous
injections (3 per week) of 0.1 ml of a 0.1% dispersion of carbaryl
in 3.3% propylene glycol. After a 3-week incubation period,
followed by a challenge dose, no clear sensitization reaction was
noticed (Carpenter et al., 1961).
(b) Benomyl and thiophanate-methyl
It has been shown that benomyl and thiophanate-methyl can cause
slight reversible skin irritation and weak sensitization in guinea-
pigs (FAO/WHO, 1974, 1985a).
8.4 Inhalation
(a) Carbaryl
The inhalation toxicity of carbamates for mammals has not been
widely studied.
The effects of acute inhalation exposure to carbaryl dust have
been examined in rats (10 mg/m3), guinea-pigs (390 mg/m3), and dogs
(75 mg/m3) (Carpenter et al., 1961) (section 8.1.3). No treatment-
associated, gross or microscopic lesions were seen in guinea-pigs
and dogs, even though the exposure was sufficient to cause ChE
inhibition. Haemorrhagic areas in the lung were seen in guinea-
pigs at the highest level tested (390 mg/m3).
Four cats exposed to carbaryl in air at 63 mg/m3 for 6 h per
day for 1 month developed signs of cholinergic stimulation during
the first 2 h of exposure each day. Periodic salivation was noted.
One cat died with marked signs of poisoning on day 20. There was
already a significant decrease in serum- and erythrocyte-ChE
activity after the first exposure. Exposure to 40 mg/m3 for 6 h
per day for 2 months was reported to impair conditioned reflexes in
cats. On certain days, the ChE activity dropped to below 50% of
pre-exposure values. Exposure to concentrations of 16 mg/m3, for 6
h per day, for 4 months did not result in any signs of toxicity
(Yakim, 1967). Carpenter et al. (1961) did not find any increase in
mortality or "grossly-visible injury" when rats were exposed to a
mean concentration of 10 mg/m3 (range 5 - 20 mg/m3), for 7 h per
day, 5 days/week, over 90 days.
(b) Propoxur
Male and female rats were exposed to propoxur aerosols at
average concentrations of 0, 5.7, 10.7, or 31.7 mg/m3, for 6 h
daily, 5 days/week, over a period of 12 weeks. Depression of
plasma-, erythrocyte-, and brain-ChE activity was observed at the
highest dose level (Kimmerle & Iyatomi, 1976).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
A considerable number of reproduction and teratogenicity
studies have been carried out on different carbamates in several
animal species. A number of these will be used to show the state
of the art giving the well-known carbamates, such as carbaryl,
benomyl, carbendazim, and thiophanate-methyl, as examples.
For more details concerning different carbamates, the reader is
referred to the reports of the Joint FAO/WHO Meetings on Pesticide
Residues and, in the future, to the Environmental Health Criteria
Documents on the individual carbamates.
8.5.1 Reproduction
(a) Methomyl
A 3-generation reproduction study with methomyl was carried out
on rats. Dose levels of 0, 50, or 100 mg/kg diet were administered
for 3 months. There was no evidence from the different indices of
reproduction, lactation, and weanling body weights to suggest that
methomyl affected reproduction or lactation performance in the
litters of three generations (Kaplan & Sherman, 1977).
(b) Benomyl
The effects of benomyl on the gonads and reproductive function
were studied in a 3-generation study on rats. Pronounced effects,
such as a decrease in fertility and decreased activities of a
number of enzymes in the testes were found in rats treated orally
with 1000 mg/kg body weight (the only dose level tested), 5
days/week, for 4 months. Besides the effects mentioned, a
decreased gestation index was also found, which may indicate early
embryonic death due to the induction of a dominant lethal gene in
the sperm cells. An increase in mortality and decreased viablility
and fertility were found in the F1 - F3 generations (not exposed to
the carbamate) (Ivanova-Chemishanska & Antov, 1980; Ivanova-
Chemishanska et al., 1980). In a second 3-generation rat
reproduction study with benomyl, no differences in reproduction or
lactation were observed among control and test groups of animals at
the highest dietary level of 2500 mg/kg diet. No pathological
changes were found in tissues from weanling pups of the F3b litter
(Sherman et al., 1975).
(c) Carbendazim
From 3 multigeneration reproduction studies on rats, in which
carbendazim was tested at dose levels of up to 10 000 mg/kg diet,
no apparent adverse effects on reproduction were found at levels of
up to 2000 mg/kg diet. A reduction in average litter weights was
observed at dietary levels of 5000 and 10 000 mg/kg (FAO/WHO,
1985a).
(d) Carbaryl
In 3-generation rat studies, carbaryl added to the diet to
provide daily doses of up to 10 mg/kg body weight, did not result
in any statistically-significant dose-related effects on fertility,
gestation, viability of pups, or lactation (Weil et al., 1972).
In other studies by Weil et al. (1972, 1973), groups of rats
received carbaryl in the diet at daily doses of 0, 3, 7, 25, or 100
mg/kg body weight by intubation, for 5 days/week, or 0 or 100 mg/kg
body weight per day in the diet. Significant effects were found
on reproduction (mortality and reduced fertility) in the group
receiving 100 mg/kg. A group receiving 200 mg/kg diet did not show
any effects.
The influence of carbaryl at dose levels of 2 or 5 mg/kg body
weight on the reproductive function of rats was studied by
Shtenberg & Otovan (1971). Both dose levels produced adverse
effects on the testes and ovaries (atrophic, dystrophic and
necrotic changes) and changed the gonadotropic function of the
hypophysis. The changes observed increased progressively over the
generations. The fecundity of test females fell, the number of
still-born offspring increased, and there was a high death rate
among young animals.
Carbaryl was fed at dietary levels of 0, 2000, 5000, or 10 000
mg/kg feed to Osborne-Mendel (10 males and 10 females) rats for 3
generations. At the highest dose level, fertility was impaired and
no litters were produced from the second mating of the second
generation. Marked effects from birth to day 4 were manifested as
seen in the survival index. Dose-related decreases in average
litter size, number of live-born progeny, number of survivors to
day 4, and number weaned were observed.
In a comparative study on gerbils, dietary levels of 0, (40
animals) 2000, 4000, 6000 (each 30 animals), and 10 000 mg
carbaryl/kg (18 animals) were tested for 3 generations. No litters
were produced from the second mating of the third generation, at
the highest dose level. Gerbils appeared to be more sensitive than
rats since significant decreases were found consistently at lower
dietary levels, but the effects were not clearly dose-related.
Effects in both species at 2000 mg/kg diet were still marginal but
significant (Collins et al., 1971).
(e) Thiophanate-methyl
Thiophanate-methyl was tested in a 3-generation reproduction
study on rats at dose levels of 0, 40, 160, or 640 mg/kg diet. The
highest dose level affected growth, but no effects of the compound
on reproduction parameters were apparent (FAO/WHO, 1974b).
(f) Carbofuran
A 3-generation reproduction study was conducted on rats (8
males and 16 females per group), with dietary levels of 0, 1, 10,
or 100 mg carbofuran/kg diet. The highest dose level resulted in
decreased weight gain in the parents and a marked reduction in
survival of the young. Lower levels did not have any effects.
Follow-up studies with lower dose levels showed that 20 mg/kg diet
induced adverse effects on reproduction variables, noted after the
first generation (McCarthy et al., 1971; FAO/WHO, 1977b, 1981b).
8.5.2 Endocrine system
(a) Carbaryl
Short-term studies on male and female rats were carried out to
study the functioning of the reproductive system under the
influence of carbaryl. The dose levels ranged from 2 to 300 mg
carbaryl/kg body weight and were administered over periods of 3 -
12 months; in one study, 4 generations were bred. Different
criteria were studied, such as reduction of sperm motility,
duration of sperm survival, inhibition of spermatogenesis and
oögenesis, estrous cycle, induction of degeneration of the germinal
epithelium, and the activities of different enzymes in the testes
and ovaries. Shtenberg & Rybakova (1968) found marked functional
and morphological changes in the endocrine organs of rats
administered carbaryl at daily doses of 7, 14, or 70 mg/kg body
weight for up to 12 months. It was considered by the authors that
the effects of carbaryl on the testes and ovaries might be
explained by the observed increase in hypophyseal gonadotropic
function. A direct effect on the testes and ovaries cannot be
excluded.
Orlova & Zhalbe (1968) studied the effects of carbaryl on white
rats. Dose levels of 2.5 or 5 mg/kg body weight were administered.
Disturbances in the enzymatic activity in the testes and ovaries,
inhibition of spermatogenesis and oögenesis, and decreased
fertility were observed in the P generation, and a decrease in
viability, in the F1 generation.
Shtenberg & Otovan (1971) studied this effect of carbaryl in
more detail in a 4-generation study. The dose levels studied were
0, 2, and 5 mg/kg body weight, and were applied for 6 months in
each generation. The disturbances in the function of the testes
and ovaries described above were found at both 2 and 5 mg/kg
(Vashakidze, 1965, 1967; Shtenberg & Rybakova, 1968; Shtenberg &
Otovan, 1971; Shtenberg et al., 1973). However, the studies
reporting adverse effects on spermatogenesis in male rats were not
confirmed in studies on mice with carbaryl by Dikshith et al.
(1976) or in reproductive performance studies on rats.
(b) Benomyl
A study on the short-term toxicity of benomyl carried out by
Torchinsky et al. (1976) showed that the testis of Wistar rats was
the organ most affected. When administered over a 12-month period,
a dose of 62.5 mg/kg body weight showed borderline effects; 12.5
mg/kg body weight did not show an effect. A recent study on
Sprague Dawley rats receiving 10 daily treatments of 0, 200, or 400
mg benomyl/kg body weight, by gavage, showed a significant decrease
in the total epididymal sperm counts and sperm concentrations in
the vas deferens at both levels. At the higher dose level, slight
to severe hypospermatocytogenesis and generalized
hypospermatogenesis were observed (Carter & Laskey, 1982; FAO/WHO,
1985a). Pastushenko (1983) studied the gonadotoxic effects of
benomyl in rats exposed to dose levels of 0.5, 1.2, 5.0, or 12.4
mg/m3 air. The 2 highest dose levels showed dose-related changes
in the gonads, i.e., disturbances of the morphological and
functional states. At 1.2 mg/m3, only morphological changes were
observed. No effects were found with 0.5 mg/m3.
(c) Carbendazim
When Sprague Dawley rats were fed diets containing 0, 2000,
4000, or 8000 mg carbendazim/kg diet for 28 days, degeneration of
testicular tissue was observed at 4000 and 8000 mg/kg diet.
Spermatogenesis and oögenesis were affected at these feeding
levels. Changes were also found in the organ weights relative to
body or heart weight, changes in the liver being observed at 2000
mg/kg diet (FAO/WHO, 1974b).
No effects on testicular weights and morphology were found in a
study on groups of ChR-CD rats fed carbendazim in the diet for 90
days at dose levels of 0, 100, 500, or 2500 mg/kg (FAO/WHO, 1985a).
8.5.3 Embryotoxicity and teratogencity
A number of carbamate esters have been tested for
teratogenicity with the chicken embryo bioassay (Marliac, 1964;
Ghadiri & Greenwood, 1966; Khera, 1966; Ghadiri et al., 1967;
Rybakova, 1968; Olefir & Vinogradova, 1968; Proctor et al., 1976;
Moscioni et al., 1977). However, this test is not considered of
relevance in testing for teratogenicity, and the results will not
be discussed in this document.
(a) Propoxur
Roszkowski (1982) studied the effects of propoxur on
intrauterine development and on the state of ossification of rat
fetuses. An embryotoxic effect was found at a dose of 20% of the
LD50, administered on days 7 - 19 of the gestation period; 11.9% of
anomalies occurred in the fetuses of treated animals compared with
6.3% in the controls. Decreased weight was also found in the
fetuses. Ossification was disturbed, but this was not related to
the period of gestation during which the substance was
administered. A decrease in the concentrations of calcium and
magnesium was found in the serum of mothers whose fetuses had shown
bone anomalies.
(b) Benomyl
In a teratogenicity study, pregnant ChR-CD rats were fed a diet
containing 0, 100, 500, 2500, or 5000 mg benomyl/kg from day 6 to
day 15 of gestation; no effects were found on the outcome of
pregnancy or embryonal development (Sherman et al., 1975).
Hazleton Laboratories (1968) cited in FAO/WHO (1985a) reported
similar negative results for rabbits, at dose levels up to 500 mg
benomyl/kg diet administered on days 8 - 16 of gestation.
Torchinsky (1973) studied the influence of carbaryl (106 mg/kg
body weight, daily, from day 1 to day 20 of pregnancy) and benomyl
(single dose of 145 or 250 mg/kg body weight on day 12 of
pregnancy) in relation to the protein content of the diet. The
animals were kept on diets containing 5, 10, 19, or 35% of protein
either for one month prior to mating and during the entire
pregnancy or only during pregnancy. The teratogenic and embryotoxic
actions of benomyl and carbaryl did not differ in groups where the
females received diets containing 5, 10, or 19% protein from the
beginning of pregnancy. An increased post-implantational lethality
for the embryos was noted in females kept on a diet with 5% protein
from one month before conception. When these animals were exposed
to carbaryl, the weight of 20-day-old fetuses declined
considerably, yet the severity of the teratogenic action of benomyl
did not increase under these conditions. The teratogenic action of
benomyl and the embryotoxic action of carbaryl were less severe in
animals fed diets containing 10% of protein from one month before
conception than in the groups kept on diets containing 19 or 35%
protein. The results of a few other studies have indicated that
benomyl causes embryotoxic and teratogenic effects in both rats and
mice when administered orally (by gavage) at dose levels at 62.5
mg/kg body weight (rat) and 100 mg/kg body weight (mouse) or more.
At lower dose levels (31.2 mg for the rat and 50 mg/kg for the
mouse), no teratogenic effects were observed. A broad spectrum of
malformations such as exencephaly, hydrocephaly, and
meningocephaly, and disturbance of the CNS were found (Ruzicska et
al., 1975; Petrova-Vergieva, 1979; Kavlock et al., 1982).
(c) Carbaryl
A number of teratogenicity studies carried out with carbaryl
gave contradictory results. Weil et al. (1972) administered up to
500 mg carbaryl/kg body weight to rats. No teratogenic effects or
effects on fertility or gestation were seen at this high dose
level; however, the mean body weight gain of the females during
pregnancy was decreased. Many pups died before weaning. No effects
were found with administration of carbaryl at 100 mg/kg body weight
(Weil et al., 1972).
Oral administration of carbaryl to pregnant rabbits and
hamsters at doses of up to 250 mg/kg body weight, by intubation,
did not cause any teratogenic effects or dose-related fetal
mortality (Weil et al., 1973).
Dougherty et al. (1975), cited in FAO/WHO (1974b), did not
observe any teratogenic effects in monkeys given doses of carbaryl
of 0, 0.2, 2, or 20 mg/kg body weight from day 18 to day 40 of
gestation. This study indicated that even the highest dose level
did not induce a reproductive hazard.
Vashakidze (1965, 1967) found reduced reproduction and abnormal
fetal changes with intubated dose levels of 100 mg/kg body weight
and higher. A level of 50 mg/kg produced negative results.
Carbaryl, administered to guinea-pigs orally during organogenesis
(10 consecutive days between days 11 and 20 of gestation) at 300
mg/kg body weight, induced increased maternal and fetal mortality
and offspring with axial skeletal defects, such as cervical
vertebrae fusion. No abnormal fetal changes were produced in
hamsters at the sub-lethal levels of 125 and 250 mg/kg body weight.
In rabbits, levels ranging from 50 to 200 mg/kg body weight did not
produce any effects on mortality or morbidity, and no abnormal
fetal changes were seen (Robens, 1969).
Smalley et al. (1968) conducted studies on pregnant beagle dogs
fed a diet containing 0, 3.125, 6.25, 12.5, 25, or 50 mg
carbaryl/kg body weight from mating to the termination of weaning.
Effects observed included a number of animals with dystocia
(difficult births) due to atonic uterine musculature, an apparent
contraceptive effect at the highest dose level, and teratogenic
effects (different types of severe defects) at all but the lowest
dose level.
The effects of carbaryl on rat reproduction and guinea-pig
teratology were compared by Weil et al. (1973). A 3-generation
reproduction study on rats included groups receiving carbaryl at
maximum daily doses of 200 mg/kg diet. Other groups concurrently
received daily oral intubation doses as high as 100 mg/kg body
weight. In the guinea-pig teratology study, maximum levels of 300
mg/kg diet or 200 mg/kg body weight (intubation) were given. The
concurrent administration of carbaryl by daily oral administration
or by dietary inclusion did not result in either teratological or
mutagenic effects in the rat or teratological effects in the
guinea-pig, at the maximum dosage levels. However, the groups
receiving maximum levels orally showed severe toxic effects in both
studies compared with little or no effect with oral administration
of 25 mg/kg in rats and 100 mg/kg in guinea-pigs.
The authors concluded that the severe effects found in rats by
Shtenberg & Otovan (1971) following repeated oral intubation of
carbaryl at 2 mg/kg body weight could not be confirmed, even at the
maximum oral intubation level of 100 mg/kg body weight.
(d) Methomyl
When rabbits were fed dietary levels of 0, 50, or 100 mg
methomyl/kg on days 8 - 16 of gestation, no skeletal or visceral
abnormalities were seen in the fetuses (Kaplan & Sherman, 1977).
(e) Carbofuran
Teratogenicity studies have been carried out on albino rats and
albino rabbits with carbofuran. In rats, dose levels of 0, 0.1,
0.3, or 1.0 mg/kg body weight were administered throughout
gestation (days 6 - 15 of pregnancy) and, in rabbits, levels of 0,
0.2, 0.6, or 2.0 mg carbofuran/kg body weight were administered
from day 6 to day 18 of gestation. In both animal species, the
highest dose level showed clear signs of poisoning. However,
pregnancy, viability, fetal body weights, and all the other
examinations did not show any effects. All the progeny were
normal. No teratogenic effects were seen in rats or rabbits
(McCarthy et al., 1971; FAO/WHO, 1981b).
(f) Carbendazim
Carbendazim has been shown to be embryotoxic at dose levels of
approximately 19 mg/kg body weight or more. This effect was not
seen at 10 mg/kg body weight (Delatour & Richard, 1976).
8.6 Mutagenicity and Related End-Points
There is little evidence, if any, to suggest that methyl
carbamate compounds are mutagenic. An evaluation of the mutagenic
potential of carbaryl, methomyl, propoxur, carbofuran, and
pirimicarb, using a variety of microorganisms as indicator strains,
including strains of Salmonella typhimurium, Escherichia coli,
Saccharomyces cerevisiae, and Bacillus subtilus, produced
negative results. These studies showed that carbamate compounds
have little potential for presumptive gene mutations in higher
animals.
Anderson et al. (1972) examined barban, chloropropham, propham,
and swep for their ability to induce point mutations in 8
histidine-requiring strains of S. typhimurium. None showed induced
mutations. This study was carried out in the absence of any
metabolic system.
The chemical structure of benomyl probably enables it to act
both as a base analogue for DNA (benzimidazole ring incorporates
into nucleic acids instead of guanine) and as a spindle poison
(carbamate groups) (Seiler, 1975, 1976b; FAO/WHO, 1985a). The
binding of benomyl to microtubular proteins disturbs the mitotic
spindle, thus causing malsegregation of chromosomes during the
mitotic anaphase (Morris, 1980, cited by Finnish National Board of
Health Toxicology Group, 1982). The results of several studies
suggest that benomyl is a strong antimitotic agent which, even in
low concentrations, induces non-disjunction of single chromosomes
leading to aneuploidy (FAO/WHO, 1985a).
The induction of low-frequency forward mutations by carbendazim
in S. typhimurium strain LT-2 was reported by Seiler (1972).
Reversions reported to occur in S. typhimurium strains His G46 and
TA 1530 were attributed to base-substitution mutations caused by
carbendazim.
Another study was made with the WP2 uvrA repair-deficient
strain of E. coli and the TA 1535 repair-deficient strain of
S. typhimurium. Mutagenic activity was detected for benomyl in the
former, but not in the latter or in TA 1538 (Kappas et al., 1976).
No effects were found in the CM-611 strain of E. coli, deficient
in either the excision repair pathway or the lex A+-dependent
misrepair pathway. In studies carried out recently, benomyl was
negative in all S. typhimurium strains used, even though, in some
of the repeats, significant increases in the number of revertants
were observed in TA 1535 strain. Carbendazim was clearly mutagenic
in the TA 98 strain with metabolic activation (S-9 mix) and
slightly mutagenic in the TA 1535, TA 1537, and TA 100 strains. A
positive result for carbendazim in the TA 1537 strain was observed,
also without metabolic activation (Haskell Laboratories, cited by
the Finnish National Board of Health, 1982; FAO/WHO, 1985a).
There is evidence that carbendazim and related compounds are
antimitotic agents and cause mitotic arrest, mitotic delay, and a
low incidence of chromosome damage in SPF Wistar rats and mammalian
cell lines. The mutagenic effects of carbendazim in bacteria are
probably due to base substitutions, resulting from the
incorporation of small quantities of benzimidazole into nucleic
acids. The effects on mammalian cells in vivo and in vitro
indicate that carbendazim causes chromosome damage, which may
result from this type of mutagenic activity, but the effects on
mitosis suggest that, in higher organisms, the main genetic effect
is to cause non-disjunction (Styles & Garner, 1974).
An abnormal number of micronucleated erythrocytes have been
observed in the bone marrow of mice treated orally with carbendazim
at 100 mg or more/kg body weight. No effects were seen at 50
mg/kg. Benomyl at 1000 mg/kg body weight produced a slight
increase in micronucleated erythrocytes.
Carbendazim did not induce chromosome breakage in Chinese
hamster bone-marrow cells at oral doses as high as 1000 mg/kg body
weight (Seiler, 1976a). It was concluded that carbendazim is not
a chromosome-breaking agent, and its micronucleic action is
attributable to antimitotic activity caused by inhibition of
spindle formation or spindle function.
An evaluation of the mutagenic properties of benomyl has been
completed and reported by the US Environmental Protection Agency
Pesticide Genotoxicology Program. Benomyl increased the mutation
frequency in a concentration-related manner in L 51784 mouse
lymphoma cells and induced sister chromatid exchange in Chinese
hamster ovary (CHO) cells. Benomyl was also positive at all dose
levels tested in the in vivo micronucleus test with mice. The
results of the spot test conducted on mice support the idea that
carbendazim (oral application of 100 - 300 mg/kg body weight to the
mother on the 10th day following conception) may be mutagenic in
mammalian cells due to a direct action on DNA (Fahrig & Seiler,
1979).
A dominant lethal study to evaluate the potential mutagenic
effects in the reproductive cycle of the male mouse was conducted
with propoxur at dose levels of 0, 2.5, or 5 mg/kg body weight.
For a period of 6 weeks, 3 virgin females were exposed to each male
at weekly intervals and reproduction indices recorded. The
administration of propoxur proved to have a transient effect on the
reproductive capability of the males but there was no sign of early
resorption indicating a mutagenic effect (FAO/WHO, 1974b).
DNA-repair tests (with both isolated rat and mouse hepatocytes)
were conducted using benomyl and carbendazim. All tests showed
negative results for the induction of DNA repair (Haskell
Laboratories, 1981a,b cited in FAO/WHO, 1985a).
The Finnish National Board of Health (1982) summarized the
mutagenicity data on benomyl, carbendazim, and thiophanate-methyl
(Table 7) and concluded that the results were sometimes
contradictory. However, positive mutagenicity results from point
mutation tests and chromosome aberration tests are well documented,
especially in higher organisms, and it can be concluded that
carbendazim fungicides should be considered as weak mutagenic
compounds.
Without going into detail, it is known that a large number of N
-nitroso derivatives of methyl carbamates are potent mutagens. The
N -nitroso derivatives of aldicarb, propoxur, carbofuran,
trimethacarb, and methomyl caused numerous single-strand breaks in
normal human skin cells. The data suggested that human cellular
DNA in vivo was irreversibly altered resulting in numerous alkali-
sensitive bonds (Blevins et al., 1977).
A similar result was obtained with N -nitroso carbaryl. This
compound showed strong mutagenic activity in 2 bacterial systems,
E. coli and Haemophilus influenzae. Using S. typhimurium, N -
nitroso carbaryl acted as a potent base-pair substition mutagen and
showed a relatively mild frameshift activity. The parent compound,
carbaryl, gave negative results (Regan et al., 1976; FAO/WHO,
1982b).
Uchiyama et al. (1975) tested the nitroso derivatives of BPMC,
propoxur, hoppcide, isoprocarb, MPMC, and MTMC, in the back
mutation test with E. coli B/r WP2 try- and in the rec-assay
method with B. subtilus strains. The N -nitroso derivatives of
all these N -methylcarbamates showed potent mutagenic activity.
Table 7. A summary of the mutagenicity data on benomyl, carbendazim (MBC), and
thiophanate-methyl
----------------------------------------------------------------------------------------
Point mutations Genetic change
Chromosomal aberrations Non-disjunction
----------------------------------------------------------------------------------------
Positive results + + +
Salmonella typhimurium rat fetus
(Seiler, 1972) (Ruzicska et al., 1975)
Salmonella typhimurium rat bone marrow rat bone marrow
(Haskell Laboratories, 1982 (Styles & Garner, 1974) (Styles & Garner, 1974)
cited in FAO/WHO, 1985a)
mouse bone marrow
(Seiler, 1976a)
Escherichia coli Aspergillus nidulans
(Kappas et al., 1976) (Kappas et al., 1974;
Bignami et al., 1977)
Fusarium oxysporum
(Dassenoy & Meyer, 1973)
Mouse/spot-test Chinese hamster ovary (SCE)
(Fahrig & Seiler, 1979) (SRI International, 1980,
cited in FAO/WHO, 1985a)
micronucleus in vivo /mouse
(SRI International, 1980,
cited in FAO/WHO, 1985a)
Negative results - - -
Streptomyces coelicolor rat bone marrow dominant lethal/rat
(Carere et al., 1978) (Ruzicska et al., 1975) (Sherman et al., 1975)
Salmonella typhimurium Chinese hamster bone marrow dominant lethal/mice
(Fiscor et al., 1978) (Seiler, 1976a) (Makita et al., 1973)
(Carere et al., 1978)
Salmonella typhimurium lymphocyte culture-cytotoxicity
(Haskell Laboratories, 1981; obs.
cited in FAO/WHO, 1985a) (Lamb et al., 1980)
Aspergillus nidulans benomyl-workers
(Bignami et al., 1977) (Ruzicska et al., 1975)
Drosophila /recessive lethal hepatocyte culture/DNA-repair
- sterility obs. rat, mouse
(Lamb & Lilly, 1980) (Haskell Laboratories, 1981,
cited in FAO/WHO, 1985a)
human lymphocyte culture
(Gupta & Legator, 1975,
cited in FAO/WHO, 1985a)
----------------------------------------------------------------------------------------
From: The Finnish National Board of Health (1982) FAO/WHO (1985a).
8.7 Carcinogenicity
8.7.1 General
The mechanism of the carcinogenic action of ethylcarbamate
(urethane) has not yet been established, but its structure seems to
be optimal. All changes seem to reduce activity, particularly when
the ethyl group is replaced with larger side chains. The addition
of alkyl groups to the nitrogen also reduces activity (Mirvish,
1968). It is not so clear why urethane is carcinogenic, whether it
is the proximal toxin or whether it has to be converted to an
active metabolite. The low carcinogenic potential of esters of N -
substituted carbamic acids is possible.
In 1976, a Working Group of the International Agency for
Research on Cancer (IARC, 1976) reviewed the available data on a
number of carbamate pesticides included carbaryl, propham,
chloropropham, and mexacarbate.
Carbaryl was tested in a number of carcinogenicity tests some
of which were not relevant or were of inadequate design. The rat
study (40 per group), in which carbaryl was administered at dose
levels of 0, 50, 100, 200, or 400 mg/kg diet for 2 years, did not
give any indication of an increase in tumour incidence (Carpenter
et al., 1961; Innes et al., 1969; Andrianova & Alekseev, 1970;
IARC, 1976).
Groups of 48 male and 48 female CD-1 mice were given 0, 100, or
400 mg carbaryl/kg diet. After 80 weeks, the tumour incidence
between the groups was similar (Mellon Institute, 1963 cited in
FAO/WHO, 1965b).
Mexacarbate was administered orally to 2 strains of mice. The
design of this study was inadequate (only a small number of
animals), but an increased incidence of lung adenomas was shown in
both strains, together with a slight increase in liver tumours in a
number of males of one strain. According to IARC (1976), it was
not possible to make an evaluation of the carcinogenicity of
mexacarbate on the basis of this study by Innes et al. (1969).
Propham and chlorpropham were administered via the oral route
in a number of carcinogenicity tests on mice, rats, and hamsters.
One or both compounds were also tested on mice and/or rats via the
subcutaneous, intraperitoneal, or intrapleural route. The design
of some of these studies was inadequate. Propham and chloropropham
were tested for initiation properties, because the ethyl carbamates
are related to the well-known carcinogen ethylcarbamate (urethane),
which is an initiator of skin tumours. There was no evidence that
propham and/or chloropropham were carcinogenic after oral,
subcutaneous, intraperitoneal, and intrapleural application.
However, these two compounds acted as weak initiators in the two-
stage carcinogenicity studies on mice. A second study by the same
authors did not confirm the results (Van Esch et al., 1958;
FAO/WHO, 1965b; Innes et al., 1969; Van Esch & Kroes, 1972; IARC,
1976).
Long-term studies on rats did not provide any evidence of
carcinogenic activity when propoxur was fed in the diet for 2 years
at levels of 0, 250, 750, 2000, or 6000 mg/kg diet. Increased liver
weight was found at the highest dose level (6000 mg/kg) in males
and in the three highest dose levels in females. This liver change
was not reflected in liver function tests, clinical chemical
examinations, or histological changes (FAO/WHO, 1974b). Similarly,
long-term and 2-year studies on rats, dogs, mice given carbofuran
or pirimicarb did not indicate any carcinogenic potential. The
dose levels tested were 175 mg/kg diet for rats and 1.8 mg/kg body
weight for dogs for pirimicarb, 100 mg/kg diet for rats, and 500
mg/kg diet for mice (24 months in mice) for carbofuran (FAO/WHO,
1977b, 1981b). On the basis of a variety of test protocols, these
carbamate compounds did not show any significant carcinogenic
potential.
Benomyl was tested for carcinogenicity in CD-1 mice, at dose
levels of 0, 500, 1500, or 5000 mg/kg diet (approximately
equivalent to 0, 50, 150, or 500 mg/kg body weight). A significant
dose-dependent increase in the incidence of primary hepatocellular
adenomas and carcinomas in female mice was seen. Furthermore,
histopathological changes occurred in a number of organs (Haskell
Laboratories, 1981 cited in FAO/WHO, 1985a).
Low dose levels of thiophanate-methyl (0 - 640 mg/kg diet,
equivalent to approximately 0 - 64 mg/kg body weight) were tested
for carcinogenicity in ICR-SCL mice. This study showed that
ingestion of 640 mg thiophanate-methyl in the diet by a mouse
strain susceptible to various tumours did not affect carcinogenic
activity. In another study on rats, dose levels of 0, 10, 40, 160,
or 640 mg/kg diet did not have any apparent effects on the
occurrence of tumours; the only significant effects appeared to be
decreased growth at the highest dose levels (Hashimoto & Fukuda,
1972, cited in FAO/WHO, 1974b).
The influence on the testes of ChR-CD rats of carbendazim
administered at dose levels of 0 - 10 000 mg/kg diet was studied
over a period of 2 years. However, the incidence of testicular
changes was so high in the two control groups that no opinion could
be given on the possible influence of carbendazim on the testes
(Haskell Laboratories, 1977 cited in FAO/WHO, 1985a).
Carbendazim at dose levels of 0, 150, 300, or 1000 mg/kg diet
(this dose level was increased to 2000 mg/kg at week 4 and to 5000
mg/kg at week 8 for the remainder of the study) was administered to
SPF-Swiss mice. The duration of the study was 80 weeks. At the
highest dose level, neoplastic nodules were found in the livers of
female mice. In males, there was an increased incidence of
hepatoblastomas (CIVO/TNO, 1976 cited in FAO/WHO, 1985a).
A 2-year feeding study on CD-1 mice was conducted using
carbendazim at dose levels of 0, 500, 1500, or 7500 mg/kg diet
(only females completed the 2-year period, the 7500 mg/kg level for
males was decreased to 3750 mg/kg after the first 15 months of the
study and the final sacrifice of this group was antedated by 7
months). A significant increase in hepato-cellular carcinomas
occurred at 1500 mg and 7500 mg/kg in female mice and at 1500 mg/kg
in male mice (at the highest dose level for males, there were too
few survivors to judge the effects) (Haskell Laboratories, 1982;
FAO/WHO, 1985a).
Other studies demonstrated that carbamates in the presence of
nitrite could be converted to N -nitroso derivatives, which may be
(partly) responsible for the "carcinogenic activity of the
carbamates".
Studies in which carbendazim was administered to Swiss mice,
twice, at 250 mg/kg body weight, intragastrically, weekly, in
combination with 5000 mg sodium nitrite/litre drinking-water caused
a slight increase in the incidence of lymphosarcomas (Börzsönyi &
Csik, 1975; Börzsönyi & Pinter, 1977).
The results of an in vitro study of Beraud et al. (1979)
(IARC, 1976) suggested that nitrosocarbaryl could be produced in
rat gastric juice from carbaryl.
The proposal that carbamate pesticides may be converted to N -
nitroso compounds under the acid conditions of the stomach is based
on the results of in vivo nitrosation studies with secondary
amines and other compounds in the presence of nitrite (originating,
for instance, from certain vegetables and present in saliva)
(Tannenbaum et al., 1974). Elespuru & Lijinsky (1973), Eisenbrand
et al. (1974, 1975a,b), Elespuru et al. (1974), Ungerer et al.
(1974), Quarles & Tennant (1975), Lijinsky & Taylor (1976), and
Oliver (1981) have shown that certain pesticides, such as carbaryl
and dithiocarbamates, can be nitrosated in vivo.
As pointed out by IARC (1976), the extrapolation of findings in
experimental animals to man is complicated by many factors. It is
relatively easy to show that N -nitroso derivatives can be formed
and that these are mutagenic and/or carcinogenic. However, the
crucial information is the quantity produced in man under the
prevailing conditions. The concentration of both reactants,
differences in pH, the influence of competing reactions, and the
presence of accelerators and inhibitors are all important. In
addition to these difficulties in defining potential human
exposure, the susceptibility of man has to be compared with that of
experimental animals. General considerations on N -nitrosatable
pesticides are described in IARC (1983).
8.8. Special Studies
Adequate characterization of the neurobehavioral effects of
carbamates is still incomplete or not available. In general, when
given in single doses the ChE-inhibiting carbamates decrease
activity and schedule-controlled behaviour, interfere with
avoidance and escape performance, and decrease the amplitude of a
variety of electrophysiological measures. The possible
interference with learning and memory by the carbamates has been of
interest because of the hypothesized role of the cholinergic system
in these functions. However, the evidence that these compounds
interfere with learning and memory is equivocal (Miller, 1982).
Carbamates interfere with shock-motivated behaviour including
discrete-trial and one-way avoidances as well as shock-induced
suppression. Furthermore, these compounds decrease responsiveness
to shock (Sideroff & Santolucito, 1972).
The effects of the ChE-inhibiting carbamates have been
evaluated in man. However, these studies have been mainly
concerned with correlating ChE inhibition and overt symptoms. It is
assumed that, if the clinical indicators (such as ChE levels) are
normal after pesticide exposure, or if symptoms associated with
cholinergic overstimulation are absent, then no risk is involved
(Miller, 1982).
9. EFFECTS ON MAN
The health hazards from overexposure to carbamate insecticides
occur primarily as a result of the inhibition of the enzyme ChE.
ChE inhibition leads to the accumulation of endogenous ACh and
other choline esters in the organism. This produces signs and
symptoms as specified in section 9.3.
9.1 General Population Exposure
9.1.1 Acute toxicity: poisoning incidents
Carbamate pesticides have occasionally been used in cases of
suicide. Fatal poisonings have occurred with carbaryl, propoxur,
and mexacarbate. Farago (1969) described a case involving a 39-
year-old man who drank 1/2 litre of carbaryl. He entered the
hospital 1 1/2 h later and received gastric lavage, but developed
serious respiratory depression. His condition improved somewhat
after 4 injections of atropine at 1/2-h intervals. Three hours
after the carbaryl ingestion, 250 mg of 2-PAM intravenous was
administered. His lung condition rapidly worsened and he died.
Administration of 2-PAM is contraindicated for cases of carbaryl
poisoning, as it may increase toxicity rather than reactivate the
inhibited enzyme and, in this case, it was concluded that the fatal
outcome, though unavoidable, was hastened by 2-PAM administration.
Reich & Welke (1966) reported a fatal case of mexacarbate
poisoning involving a 17-year-old nursery employee who ingested
approximately 55 g mexacarbate in the form of a 22% formulation.
The young man, found unconscious, had pinpoint pupils and an
irregular heartbeat. Emergency procedures at the hospital briefly
restored regular heart rhythm, but brachycardia developed later
with recurrent heart failure. The patient died about 4 - 4 1/2 h
after the ingestion of the mexacarbate.
Two severe cases of propoxur poisoning following ingestion of
150 - 200 ml of commercial Baygon formulation were described by
Bomirska & Winiarska (1972). One individual responded to treatment
and recovered but the other died after failing to respond to
treatment with atropine and 2-PAM and toxogonin given
intravenously. A normal ChE suggested spontaneous reactivation of
this enzyme.
9.1.2 Effects of short- and long-term exposure
Propoxur was tested for its effectiveness in controlling the
mosquito vectors of malaria in Iran. The insecticide was sprayed
inside houses in a 300 km2 area in which 11 000 people lived in 33
villages. Sixty-nine out of the 11 000 inhabitants complained of
mild cholinergic symptoms, when they entered their houses during
the spraying, contrary to instructions. The symptoms were
headache, nausea, sweating, and vomiting. The symptoms subsided in
2 - 3 h. Similar experiences were reported in other villages
sprayed with propoxur or with carbaryl (Vandekar et al., 1968).
9.1.3 Controlled human studies
Human volunteers tolerated single oral doses of aqueous
solutions of aldicarb at doses of 0.025, 0.05, or 0.1 mg/kg body
weight, with only mild depression of whole blood-ChE in all cases.
Only the persons receiving 0.1 mg/kg presented symptoms of acute
cholinergic stress (i.e., nausea, sweating, pinpoint pupils,
salivation) (FAO/WHO, 1982b).
Groups of male volunteers (5 or 6 per group) were administered
daily doses of 0.06 and 0.13 mg carbaryl/kg body weight, orally,
for 6 weeks. No subjective or objective changes were observed,
except a slight reversible decrease in the ability of the kidneys
to reabsorb aminoacids in the group at the highest dose level
(Wills et al., 1968).
Thirty-eight urban volunteers were monitored for carbaryl
exposure during the summer. All volunteers were involved in the
application of carbaryl incidental to their employment or leisure
activities. Exposure of clothing, skin, with and without gloves,
were measured. The maximum dermal exposure recorded was 2.86 mg/kg
per h, the maximum air concentration was 0.28 mg/m3. Only a small
mean decrease in serum- and erythrocyte-AChE activity was found
(Gold et al., 1982). Comparable results were obtained by Leavitt et
al. (1982) in a study on 2 groups of professional pesticide spray
operators with higher dermal exposure, spraying trees with
carbaryl.
A 42-year-old male volunteer took a single oral dose of 135 mg
propoxur (1.5 mg/kg body weight) about 2 h after breakfast.
Plasma- and erythrocyte-ChE activities were determined every 15 min
for the first 2 h after ingestion and then every hour thereafter.
Pulse and blood pressure were recorded. A rapid drop in
erythrocyte-ChE activity was observed, reaching the lowest level of
27% of normal within 20 min. ChE activity gradually recovered,
reaching normal levels about 2 h after ingestion. Plasma-ChE
activity was not affected. In another study (single dose of 0.36
mg/kg body weight), the lowest erythrocyte-ChE activity occurred
within 10 min together with short-lasting stomach discomfort,
blurred vision, nausea, and facial perspiration. Twenty min after
ingestion, the volunteer's pulse rate and blood pressure were
increased. Pronounced nausea accompanied by repeated vomiting and
profuse sweating occurred until approximately 45 min after the
ingestion of propoxur. From then on, poisoning symptoms
diminished, blood pressure and pulse returned to pre-ingestion
values. The disappearance within about 2 h of the poisoning
symptoms was in line with the return of normal erythrocyte-ChE
activity. Forty-five percent of the ingested dose of propoxur was
excreted as the metabolite, 2-(1-methylethyl)phenol, within 24 h of
ingestion (Vandekar et al., 1971; FAO/WHO, 1974b).
In another study, 5 doses of propoxur, at 0.15 or 0.20 mg/kg
body weight, were administered to volunteers at 1/2-h intervals.
In this study, a symptomless depression of erythrocyte-ChE to 60%
of normal was observed over the course of the dosing. The enzyme
activity recovered to normal levels soon after termination of the
dosing. From the above studies, it was concluded by the authors
that an oral dose of 0.36 mg propoxur/kg body weight may be
sufficient to induce initial cholinergic symptoms, while larger
doses may be tolerated without symptoms if the administration is
gradually (divided doses) (Vandekar et al., 1971).
9.2 Occupational Exposure
9.2.1 Acute toxicity: poisoning incidents
Tobin (1970) reported 3 cases of persons poisoned with
carbofuran. Two were formulation plant employees, preparing 10%
granules and the third was an entomologist who began to feel
uncomfortable while weighing a 50% water-dispersible powder
formulation. The 2 formulators developed similar symptoms
indicative of carbamate poisoning: profuse sweating, weakness,
blurred vision, and nausea. Both were taken to their respective
physicians about 3 h after the onset of symptoms. One physician
administered atropine; the other, noting the regression of the
symptoms, did not administer atropine. The patient who received
the atropine (0.02 g im) was fully recovered in 30 min. The
untreated patient recovered over the course of 2 - 3 h. The third
case, the entomologist experienced mild discomfort that regressed
without atropine administration in 4 - 6 h.
Richardson & Batteese (1973) described the case of a spray-
plane co-pilot overexposed to mexacarbate during an spraying
operation. The cause of the overexposure was a pin-hole leak in a
high pressure pump line, which emitted a fine aerosol of the
mexacarbate formulation into the fuselage. The co-pilot developed
the classical signs of ChE poisoning. Toxic symptoms progressed to
paralysis of the extremities before he could be treated. In
hospital, atropine sulfate was immediately administered
intravenously followed by a second injection subcutaneously 40 min
later. The symptoms subsided quickly. ChE activity in blood drawn
after the first atropine injection was severely depressed, but
returned to normal after 3 days.
Minor and transient cholinergic symptoms were experienced by
spraymen applying propoxur formulations inside houses for testing
this insecticide against mosquito vectors of malaria in villages in
El Salvador (Quinby & Babione, 1966) and Iran (Vandekar et al.,
1968; Montazemi, 1969). A pronounced fall in blood-ChE activity
during the work and a distinct recovery after exposure ceased was
established as a daily pattern of activity of this enzyme,
erythrocyte-ChE being more sensitive to propoxur than plasma-ChE.
No cumulative inhibition of ChE during the 6-week exposure was
seen. The investigators noted that the exposed spraymen and a few
inhabitants recovered rapidly when removed from the source of
exposure and when treated with atropine.
Vandekar et al. (1968) commented that most spraymen who became
poisoned had heavy skin contamination, and had not cleaned their
skin sufficiently after spraying. The inhabitants entered the
house while it was being sprayed. Moreover, it was noted that
respirators did not offer protection against intoxication under
these circumstances and that dermal absorption accounted for most
of the absorbed dose.
No adverse health effects due to carbaryl exposure (except for
one case of skin rash) were observed in a study conducted by the
WHO Insecticide Testing Unit in Southern Nigeria. Ten men (out of
105 sprayers) spraying over a single 6-h period and 95 villagers
who resided in the sprayed houses, were examined. The sprayers
wore overalls, hats, and rubber boots during the entire 6 h of
spraying, but wore masks for the first hour only. Plasma-ChE
activity as well as urinary metabolites of carbaryl were determined
before and after spraying. A slight (average 5%) reduction in
plasma-ChE activity was reported for all 10 sprayers, the day after
the carbaryl application. Inhabitants of sprayed houses showed no
inhibition of plasma-ChE activity, 1 week after the spraying. A
slight increase in 1-naphthol was found in the urine of the
sprayers only on the sixth day after the exposure (Vandekar, 1965).
In a study on 19 agricultural workers in the USSR, whole blood-
ChE activity was measured before and after 4 to 6-h exposures to
airborne carbaryl for 3 - 4 days. Significant ChE inhibition was
found in men exposed to a mean airborne carbaryl concentration of
up to 4 mg/m3. No objective signs of ill health were observed. No
changes were measured at 0.7 mg/m3 (Yakim, 1967).
A case of acute intoxication of a woman with a total dose of 60
mg carbofuran was described by Izmirova et al. (1981). Slight ChE
inhibition was found, but within 72 h the patient recovered
completely.
The above reports identify systemic ChE poisoning as the major
hazard associated with occupational exposure to carbamate
pesticides. However, other publications indicate that certain of
these compounds can also irritate the exterior membranes of the
body. Tobin (1970) described 2 cases of field workers that
illustrate direct effects on the eye, (irritation and blurred
vision) during carbofuran spraying operations. These 2 persons did
not developed symptoms of systemic poisoning.
Vandekar (1965) reported skin rash in a sprayman accidentally
splashed with a carbaryl formulation. Although carbamate compounds
have not generally been implicated as the cause of dermatitis or
allergic skin reactions, Vandekar (1965) suggested that certain
individuals could develop this after unusually heavy exposure.
9.2.2 Effects of short- and long-term exposure
Little is known about the short- and long-term effects of
occupational exposure to carbamate pesticides. The few reports
that discuss this aspect do not implicate long-term exposure to the
carbamate compounds as a cause of human disease. Vandekar et al.
(1968) did not observe any evidence of cumulative toxicity among
spraymen applying propoxur formulations during a 6-week operation.
No long-term biochemical changes were reported among New Jersey
farmers and spraymen compared with a minimally exposed control
group. It was noted that significantly higher incidences of
hearing and eye problems, chronic cough, dizziness, headaches, and
hypertension occurred in the occupationally exposed group.
However, the various pesticides to which this group was exposed
were not tabulated (Kwalick, 1971).
Chromosome studies on the blood cultures of 20 workers engaged
in the production of benomyl did not reveal any increased frequency
of structural chromosome aberrations (Ruzicska et al., 1975).
In a survey of occupationally acquired disease in workers at a
pesticide plant, 11 out of 102 workers had been hospitalized for
illness related to chemical exposure. The commonest cause of
hospitalization was intoxication with the ChE inhibitor methomyl.
On clinical evaluation, 5 out of 11 packaging workers, with the
highest exposure to methomyl had experienced blurred vision or
pupillary constriction (Morse & Baker, 1979).
9.2.3 Epidemiological studies
Epidemiological studies on persons occupationally exposed
exclusively (or at least primarily) to carbamate pesticides are not
available. Such studies are needed before the long-term effects of
this class of pesticides on human beings can be adequately
evaluated.
9.3 Signs and Symptoms of Acute Intoxication by Carbamates
The clinical picture of carbamates intoxication results from
accumulation of ACh at nerve endings. Extensive description of
the syndrome is given in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980). The signs and symptoms can be
categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases,
diaphragm and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by the respiratory failure sometimes
leading to pulmonary oedema due to the combination of the above-
mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure;
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to light; pulse rate is not of
diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ;
on the other hand, since changes in the conduction
and excitability of the heart might be
life-threatening, monitoring should be performed.
(c) confirmation of diagnosis is made by measurement of
AChE in RBC or plasma-pseudoChE (dibucaine number
also, to rule out genetic deficiencies). In view of
the rapid reversibility, particular care should be
taken to use analytical procedures that include
immediate analysis and a careful control of sample
dilutions (section 2.3). Chemical analysis of body
fluids (gastric lavage, blood, urine) should be
performed for the identification of the chemical(s).
9.3.1 Biochemical methods for measurement of effects
AChE present in human erythrocytes is the same as the enzymes
present in the target synapses. Measurements of AChE in RBC is
therefore taken to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the carbamate has equal access to blood and the
synapses, and that AChE inhibition by carbamates is reversible.
In the case of acute poisoning, a high inhibition of RBC-AChE
is pathognomonic, but, in the follow-up of the intoxication, it
might not be correlated with the severity of symptoms. However,
monitoring of pre- and post-exposure levels of AChE in RBC gives a
good measure of the effect of an exposure (Kaloyanova, 1976). In
cases when the pre-exposure AChE level is not known (as in
accidental poisoning), reference can be made to a mean population
AChE activity. Blood-plasma contains a related enzyme called ChE or
pseudo-ChE, which contributes to the whole blood enzymatic
activity; the extent of the contribution of plasma-ChE will depend
on which substrate was used and at what concentration. PseudoChE
has no known physiological function, but can be inhibited
selectively by some carbamates without causing a toxic response.
The sensitivities of AChE and pseudoChE to inhibitors differ so
that measurements of the ability of whole blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many field conditions, procedures using whole blood are more
practical than those using separated erythrocytes.
9.4 Treatment of Acute Poisoning by Carbamate Insecticides
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977; Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
9.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in the cleaning of the skin area where
venupuncture is performed. Blood might be contaminated with
carbamates and therefore inaccurate measures of ChE inhibition
might result. Extensive eye irrigation with water or saline should
also be performed. In the case of ingestion, vomiting can be
induced, if the patient is conscious, by the administration of
ipecacuanha syrup (10 - 30 ml) followed by 200 ml of water.
However, this treatment is contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
the addition of bicarbonate solution or activated charcoal) can
also be performed, particularly in unconscious patients, taking
care to prevent aspiration of fluids into the lungs (i.e., only
after a tracheal tube has been put in place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
9.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
9.4.3 Specific pharmacological treatment
9.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.).
9.4.3.2 Oxime reactivators
There is no rational basis for using these drugs. Furthermore,
some unconfirmed reports suggest an increased toxicity of
carbamates when oximes have been administered.
9.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of
artificial respiration procedures.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
For more than 20 years, the Joint FAO/WHO Meetings on Pesticide
Residues (JMPR) and the International Agency for Research on Cancer
(IARC) have been evaluating the toxicity and carcinogenicity data
on the different carbamates. As part of the JMPR meeting, the FAO
deals with the use and application of these pesticides and
proposes residue limits. Data and conclusions of the meetings were
initially published in the WHO Technical Report Series but, since
1977, they have been published regularly in the FAO Plant
Production and Protection Papers.
It is not possible to review completely the results of the
discussions on the approximately 50 carbamates mentioned in these
documents. However, Annex III contains a list of the JMPR meetings
in which carbamates have been evaluated together with appropriate
references. Other information available in Annex III indicates
whether IARC evaluations and WHO/FAO Data Sheets are available, and
whether an IRPTC data profile and legal file exist.
REFERENCES
ABDEL-AAL, Y.A.I. & FAHMY, M.A.H. (1977) Molecular
modification of carbamates in relation to their selective
toxicity. In: Proceedings of the British Crop Protection
Conference on Pests and Diseases, Brighton, Nov. 1977, British
Crop Protection Council, Croydon, pp. 155-159.
ABD-ELROAF, T.K., FAHMY, M.A.H., FUKUTO, T.R., & EL-SEBAE,
A.H. (1977) Anticholinesterase activity and toxicity of
substituted phenyl methylcarbamates to honey bee. J. econ.
Entomol., 70(1): 78-82.
ABDEL-WAHAB, A.M., KUHR, R.J., & CASIDA, J.E. (1966) Fate of
14C-carbonyl-labelled aryl methylcarbamate insecticide
chemicals in and on bean plants. J. agric. food Chem., 14:
290-298.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1973) The
photochemical reactions of carbamates. III. The solution
photochemistry of metacrate, 3-methylphenyl- N -methyl-
carbamate. Int. J. environ. anal. Chem., 3: 73-79.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1974) The solution
photochemistry of metacil (4-dimethyl-amino- m -tolyl- N -methyl
carbamate) and Landrin (3,4,5-trimethylphenyl- N -methyl-
carbamate). Bull. environ. Contam. Toxicol., 11: 250-255.
AHDAYA, S.M., MONROE, R.J., & GUTHRIE, F.E. (1981)
Absorption and distribution of intubated insecticides in
fasted mice. Pestic. Biochem. Physiol., 16(1): 38-46.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarba-
mates, and dithiocarbamates, Luxembourg, Commission of the
European Communities.
ALDRIDGE, W.N. & REINER, E. (1972) Enzyme inhibitors as
substrates. Interaction of esterases with esters of
organophosphorus and carbamic acids, Amsterdam, Oxford, New
York, Elsevier Science Publishers, 328 pp.
ALY, O.M. & EL-DIB, M.A. (1971) Photodecomposition of some
carbamate insecticides in aquatic environments. In: Faust,
S.D. & Hunter, J.V., ed. Organic compounds in aquatic
environments, New York, Marcel Dekker, pp. 469-493.
ALY, O.M. & EL-DIB, M.A. (1972) Studies of the persistence
of some carbamate insecticides in the aquatic environment. In:
Fate of organic compounds in the aquatic environment. III.
Advances in chemistry series, Washington DC, American Chemical
Society, pp. 210-243.
ANDERSON, C.A. (1973) Baygon. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7, pp.
163-178.
ANDERSON, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDRIANOVA, M.M. & ALEKSEEV, I.V. (1970) [On the carcino-
genous properties of the pesticides sevine, maneb, ciram and
cineb.] Vopr. Pitan., 29(6): 71-74 (in Russian, with English
summary).
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974a) Effects of the
insecticide carbaryl on clams and some other intertidal mud
flat animals. J. Fish. Res. Board Can., 31: 466-470.
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974b) Effects of the
insecticide Sevin and its first hydrolytic product 1-naphthol
on some early developmental stages of the bay mussel Mytilus
edulis. Mar. Biol., 28: 11-15.
ASHTON, F.M. & CRAFTS, A.S. (1973) Carbamates. In: Mode of
action of herbicides, New York, Wiley Interscience, pp.
200-220.
ATKINS, E.L., GREYWOOD, E.A., & MCDONALD, R.L. (1973)
Toxicity of pesticides and other agricultural chemicals to
honey bees. Laboratory studies, Riverside, USA, University of
California, Agriculture Extension Service.
AUGUSTINSSON, K.B. & CASIDA, J.E. (1959) Enzymic hydrolysis
of N,N -dimethylcarbamoyl fluoride. Biochem. Pharmacol., 3:
60-67.
AUSTIN D.J. & BRIGGS, G.G. (1976) A new extraction method
for benomyl residues in soil and its application in movement
and persistence studies. Pestic. Sci., 7: 201-210.
AUSTIN, D.J., LORD, K.A., & WILLIAMS, I.H. (1976) High
pressure liquid chromatography of benzimidazoles. Pestic.
Sci., 7: 211-222.
BAKER, P.B. & HOODLESS, R.A. (1974) Analytical methods for
the detection and determination of residues of systemic
fungicides. Pestic. Sci., 5: 465-472.
BALDWIN, R.E., FREED, V.H., & FANG, S.C. (1954) Absorption
and translocation of carbon-14-applied as O -isopropyl
N -phenylcarbamate in Avena and Zea. J. agric. food Chem.,
2(8): 428-430.
BERAUD, M., PIPY, B., DERACHE, R., & GAILLARD, D. (1979)
Formation d'un cancérigène le N -nitrosocarbaryl par
interactions entre un insecticide de la série des carbamates
le carbaryl et le nitrite de sodium dans le suc gastrique de
rat. Food Cosmet. Toxicol., 17: 579-583.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of
pesticides in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BLACK, A.L., CHIU, Y.C., FAHMY, M.A.H., & FUKUTO, T.R.
(1973) Selective toxicity of N -sulfenylated derivatives of
insecticidal methylcarbamate esters. J. agric. food Chem., 21:
743-747.
BLEIDNER, W.E., MORALES, R., & HOLT, R.F. (1978) Benomyl.
In: Zweig, G. & Sherma, J., ed. Analytical methods for
pesticides and plant growth regulators, New York, London,
Academic Press, pp. 157-171.
BLEVINS, R.D., LYINSKY, W., & REGAN, J.D. (1977) Nitrosated
methylcarbamate insecticides. Effect on the DNA of human
cells. Mutat. Res., 44: 1-7.
BOBIK, A., HOLDER, G.M., & RYAN, A.J. (1972) Excretory and
metabolic studies of isopropyl N -(3-chlorophenyl)carbamate in
the rat. Food Cosmet. Toxicol., 10: 163-170.
BOMIRSKA, T. & WINIARSKA, A. (1972) [Toxicology of
carbamates in the light of intoxication with Baygon
insecticide.] Pol. Tyg. Lek., 27: 1448-1450 (in Polish).
BORZSONYI, M. & CSIK, M. (1975) Induction of malignant
lymphomas in Swiss mice by N -nitroso compounds formed in vivo.
Int. J. Cancer, 15: 830-838.
BORZSONYI, M. & PINTER, A. (1977) The carcinogenicity of
N -nitroso compounds formed endogenously in mice from
benzimidazole carbamate pesticides. Neoplasma, 24: 119-122.
BOWMAN, M.C. & BEROZA, M. (1969) Determination of Mesurol
and five of its metabolites in apples, pears and corn by gas
chromatography. J. Assoc. Off. Anal. Chem., 52: 1054-1063.
BROCKELSBY, C.H. & MUGGLETON, D.F. (1973) Asulam. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 497-508.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARPENTER, C.P., WEIL, C.S., PALM, P.E., WOODSIDE, M.W., NAIR,
J.H., & SMYTH, H.F. (1961) Mammalian toxicity of 1-naphthyl
N -methylcarbamate (Sevin insecticide). J. agric. food Chem.,
9: 30-39.
CARTER, S.D. & LASKEY, J.W. (1982) Effect of benomyl on
reproduction in the male rat. Toxicol. Lett., 11: 87-94.
CASIDA, J.E. & AUGUSTINSSON, K.B. (1959) Reaction of plasma
albumin with 1-naphthyl N -methylcarbamate and certain other
esters. Biochem. Biophys. Acta, 36: 411-426.
CHIBA, M. & DOORNBOS, F. (1974) Instability of benomyl in
various conditions. Bull. environ. Contam. Toxicol., 11:
273-274.
CHIN, W.T., DUANE, W.C., SZALKOWSKI, M.B., & STALLARD, D.E.
(1975) Gas-chromatographic determination of thiofanox
residues in soil, plants, and water. J. agric. food Chem.,
23(5): 963-966.
CLARK, C.G. & WRIGHT, S.J.L. (1970) Degradation of the
herbicide isopropyl N -phenylcarbamate by Arthrobacter and
Achromobacter sp. from soil. Soil Biol. Biochem., 2: 217.
COLLINS, T.F.X., HANSEN, W.H., & KEELER, H.V. (1971) The
effect of carbaryl (Sevin) on reproduction of the rat and the
gerbil. Toxicol. appl. Pharmacol., 19: 202-216.
CROSBY, D.G., LEITIS, E., & WINTERLIN, W.L. (1965)
Photodecomposition of carbamate insecticides. J. agric. food
Chem., 13(3): 204-207.
DASSENOY, B. & MEYER, J.A. (1973) Mutagenic effect of
benomyl on Fusarium oxysporum. Mutat. Res., 21: 119-120.
DAWSON, J.A., HEATH, D.F., ROSE, J.A., THAIN, E.M., & WARD,
J.B. (1964) The excretion by humans of the phenol derived in
vivo from 2-isopropoxyphenyl- N -methylcarbamate. Bull. World
Health Org., 30: 127-134.
DELATOUR, P. & RICHARD, Y. (1976) Propriétés embryotoxiques
et antimitotiques en série benzimidazole. Therapie, 31(4):
505-515.
DE ROSE, H.R. (1951) Crabgrass inhibition with O -isopropyl-
N -(3-chlorophenyl)carbamate. Agron. J., 43: 139-142.
DIKSHITH, T.S.S., GUPTA, P.K., GAUR, J.S., DATTA, K.K., &
MATHUR, A.K. (1976) Ninety-day toxicity of carbaryl in male
rats. Environ. Res., 12: 161-170.
DOROUGH, H.W. & CASIDA, J.E. (1964) Nature of certain
carbamate metabolites of the insecticide Sevin. J. agric. food
Chem., 12(4): 294-304.
DOUCH, P.G.C. (1973) The metabolism of benomyl fungicide in
animals. Xenobiotica, 3: 367-380.
EBERLE, D.O. & GUNTHER, F.A. (1965) Chromatographic,
spectrometric, and irradiation behaviour of five carbamate
insecticides. J. Assoc. Off. Agric. Chem., 48:(5) 927-937.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the
soil fauna. Residue Rev., 45: 1-79.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N -nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under stimulated gastric conditions and in vivo in the
rat stomach. Food Cosmet. Toxicol., 12: 229-232.
EISENBRAND, G., IVANKOVIC, S., PREUSSMANN, R., SCHMAHL, D., &
WIESSLER, M. (1975a) Some recent results on the chemistry,
formation and biological activity of N -nitroso compounds. Gaun
Monogr. Cancer Res., 17: 133-143.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1975b) The
reaction of nitrite with pesticides. II. Formation, chemical
properties, and carcinogenic activity of the N -nitroso
derivative of N -methyl-1-naphthylcarbamate (carbaryl). Food
Cosmet. Toxicol., 13: 365-367.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ELESPURU, R.K., LIJINSKY, W., & SETLOW, J.K. (1974)
Nitrosocarbaryl as a potent mutagen of environmental
significance. Nature (Lond.), 247: 386-387.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V. Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
FANG, S.C., FALLIN, E., MONTGOMERY, M.L., & FREED, V.H.
(1974) Metabolic studies of 14C-labelled propham and
chlorpropham in the female rat. Pestic. Biochem. Physiol., 4:
1-11.
FAHRIG, R. & SEILER, J.P. (1979) Dose and effect of
methyl-2-benzimidazolylcarbamate in the "mammalian spot test",
an in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-biol. Interact., 26: 115-120.
FAO (1985) Production yearbook, 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23.64).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Agricultural Studies No. 73; WHO Technical
Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391.
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
10 Rev).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Suppl.).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Suppl.).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Suppl.).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1985a) 1983 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985c) 1984 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Pesticide residues in food. Report of the
1985 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (in press) 1985 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations.
FARAGO, A. (1969) [Fatal suicidal poisoning with Sevin
(1-naphthyl- N -methyl carbamate).] Arch. Toxikol., 24: 309-315
(in German).
FICSOR, G., BORDAS, S., & STEWART, S.J. (1978) Mutagenicity
testing of benomyl methyl-2-benzimidazole carbamate,
streptozotocin, and N -methyl- N' -nitro- N' -nitrosoguanidine in
Salmonella typhimurium in vitro and in rodent host-mediated
assays. Mutat. Res., 51: 151-164.
FINNISH NATIONAL BOARD OF HEALTH TOXICOLOGY GROUP (1982) The
toxicity of the benzimidazol type fungicides: benomyl,
carbendazim, and thiophanate-methyl (second report), Helsinki,
Finnish National Board of Health.
FILIP, Z. (1974) [Pesticides in powder form.] Vesmir, 53:
243-244 (in Czech).
FUKUTO, T.R. (1972) Metabolism of carbamate insecticides.
Drug Metab. Rev., 1: 117-150.
GARDINER, J.A., KIRKLAND, J.J., KLOPPING, H.L., & SHERMAN, H.
(1974) Fate of benomyl in animals. J. agric. food Chem.,
22(3): 419-427.
GHADIRI M. & GREENWOOD, D.A. (1966) Toxicity and biologic
effects of malathion, phosdrin, and sevin in the chick embryo.
Toxicol. appl. Pharmacol., 8: 342.
GHADIRI M., GREENWOOD, D.A., & BINNS, W. (1967) Feeding of
malathion and carbaryl to laying hens and roosters. Toxicol.
appl. Pharmacol., 10: 392.
GOLD, R.E., LEAVITT, J.R.C., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of urban applications to carbaryl. Arch. environ.
Contam. Toxicol., 11: 63-67.
GOLDBERG, M.E., JOHNSON, H.E., KNAAK, J.B., & SMYTH, H.F., Jr
(1963) Psychopharmacological effects of reversible
cholinesterase inhibition induced by N -methyl-3-isopropyl-
phenyl carbamate (compound 10854). J. Pharm. exp. Ther., 141:
244-252.
GRAY, R.A. (1971) Behaviour, persistence, and degradation of
carbamate and thiocarbamate herbicides in the environment. In:
Proceedings of the California Weed Control Conference, 18-20
January 1971, Mountain View, California, Stauffer Western
Research Centre, pp. 128-143.
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1977) Determination
of carbetamid residues and its aniline metabolite. J. agric.
food Chem., 20: 348-350.
GUPTA, A.K. & LEGATOR, M.S. (1975) Chromosome aberrations in
cultured human leukocytes after treatment with the fungicide
benlate. In: Proceedings of a Symposium on Mutagenic
Carcinogenic and Teratogenic Chemistry, New Delhi, India,
Department of Atomic Energy, pp. 95-103.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained
sheepshead minnows. Trans. Am. Fish. Soc., 98: 426-429.
HARVEY, J., Jr & HAN, J.C.Y. (1978a) Metabolism of oxamyl
and selected metabolites in the rat. J. agric. food Chem.,
26(4): 902-910.
HARVEY, J., Jr & HAN, J.C.Y. (1978b) Decomposition of oxamyl
in soil and water. J. agric. food Chem., 26(3): 536-541.
HARVEY, J., Jr & PEASE, H.L. (1973) Decomposition of
methomyl in soil. J. agric. food Chem., 21(5): 784-786.
HARVEY, J., Jr & REISER, R.W. (1973) Metabolism of methomyl
in tobacco, corn, and cabbage. J. agric. food Chem., 21(5):
775-783.
HARVEY, J., Jr, JELINEK, A.G., & SHERMAN, H. (1973)
Metabolism of methomyl in the rat. J. agric. food Chem.,
21(5): 769-775.
HARVEY, J., Jr, HAN, J.C.Y., & REISER, R.W. (1978)
Metabolism of oxamyl in plants. J. agric. food Chem., 26(3):
529-536.
HEYBROEK, W.M.H., MUGGLETON, D.F., & PARKE, D.V. (1984)
Metabolism of the carbamate herbicide asulam in the rat.
Xenobiotica, 14:(3) 235-247.
IARC (1976) Some carbamates, thiocarbarmates, and
carbazides, Lyons, International Agency for Research on Cancer
(IARC Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 12).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 30;
Appendix 359-406).
INNES, J.R.M., ULLAND, B.M., VALLERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, J., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1976) Changes in the
activity of some enzymes under the action of benomyl. Hig. i
Zdraveopazvane, Sofia, 19(5): 431-435 (in Bulgarian, with
English summary).
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads, reproduction, and generations of white rats under the
effect of some pesticides. Arch. Toxicol., 4(Suppl.): 459-462.
IVANOVA-CHEMISHANSKA, L., ANTOV, G., MIHAILOVA, A., & HINKOVA,
L. (1980) Study on the toxicology of benomyl. In: The Fourth
International Congress of Pesticide Chemistry, Zurich,
Switzerland, 24-28 July, 1978, pp. 11-559.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorous pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application, Amsterdam, Oxford, New
York, Elsevier Scientific Publishers, pp. 169-172 (Studies in
Environmental Science 7).
IZMIROVA, N., MILCEVA, V., MONOV, A., & KALOYANOVA, F.
(1981) [Acute carbofuran intoxication.] Hig. i
zdraveopazvane, 24(5): 445-448 (in Bulgarian, with English
summary).
JACKSON, J.A., CHART, I.S., SANDERSON, J.H., & GARNER, R.
(1977) Pirimicarb induced immune haemolytic anaemia in dogs.
Scand. J. Haematol., 19: 360-366.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of brain "neurotoxic esterase" and the development of delayed
neurotoxicity in hens. Biochem. J., 120: 523-531.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute
toxicity of chemicals to fish and aquatic invertebrates,
Washington DC, US Fish and Wildlife Service (Resource
Publication No. 137).
KAGAN, J.S. (1977) [The toxicity of organophosphorus
pesticides,] Moscow (in Russian).
KALOYANOVA, F. (1976) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Symposium on
Environmental Monitoring, 14-19 September, 1975, Las Vegas,
Nevada, Vol. 1, pp. 1-4, New York, Institute of Electrical &
Electronics Engineers, Inc.
KANAZAWA, J. (1975) Uptake and excretion of organophosphorus
and carbamate insecticides by fresh-water fish Motsugo
pseudoras bora parva. Bull. environ. Contam. Toxicol., 14:
346-352.
KAPLAN, A.M. & SHERMAN, H. (1977) Toxicity studies with
methyl- N -methylamino-carbonyl-oxyethanimidothioate. Toxicol.
appl. Pharmacol., 40: 1-17.
KAPPAS, A., GEORGOPOULOS, S.G., & HASTIE, A.C. (1974) On the
genetic activity of benzimidazole and thiophanate fungicides
on diploid Aspergillus nidulans. Mutat. Res., 26: 17-27.
KAPPAS, A., GREEN, M.H.L., BRIDGES, B.A., ROGERS, A.M., &
MURIEL, W.J. (1976) Benomyl: a novel type of base analogue
mutagen. Mutat. Res., 40: 379-382.
KAVLOCK, R.J., CHERNOFF, N., GRAY, L.E., Jr, GRAY, J.A., &
WHITEHOUSE, D. (1982) Teratogenic effects of benomyl in the
Wistar rat and CD-1 mouse, with emphasis on the route of
administration. Toxicol. appl. Pharmacol., 62: 44-54.
KAUFMAN, D.D. & BLAKE, J. (1973) Microbial degradation of
several acetamide, acrylamide, carbamate, toluidine, and urea
pesticides. Soil Biol. Biochem., 5: 297-308.
KAUFMAN, D.D. & KEARNEY, P.C. (1965) Microbial degradation
of isopropyl- N -(3-chlorophenyl carbamate and 2-chloroethyl- N -
(3-chlorophenyl carbamate. Appl. Microbiol., 13(3): 443-446.
KHERA, K.S. (1966) Toxic and teratogenic effects of
insecticides in duck and chick embryos. Toxicol. appl.
Pharmacol., 8: 345.
KIMMERLE, G. & IYATOMI, A. (1976) Toxicity of propoxur to
rats by subacute inhalation. Jpn J. ind. Health, 18: 375-382.
KNAAK, J.B., TALLANT, M.J., BARTLEY, W.Y., & SULLIVAN, L.J.
(1965) The metabolism of carbaryl in the rat, guinea pig and
man. J. agric. food Chem., 13(4): 537-543.
KNAAK, J.B., TALLANT, M.J., & SULLIVAN, L.J. (1966) The
metabolism of 2-methyl-2-(methylthio)propionaldehyde- O -
methylcarbamoyl)oxime in the rat. J. agric. food Chem., 14(6):
573-578.
KNAAK, J.B., TALLANT, M.J., KOZBELT, S.J., & SULLIVAN, L.J.
(1968) Metabolism of carbaryl in man, monkey, pig, and sheep.
J. agric. food Chem., 16(3): 465-470.
KNOWLES, C.O. & SONAWANE, B.R. (1972) Ethyl m- hydroxy-
carbanilate carbanilate (EP-475) metabolism in sugar beets.
Bull. environ. Contam. Toxicol., 8: 73-76.
KOSSMANN, K. & JENNY, N.A. (1973) Phenmedipham. In: Sherma,
J. & Zweig, G., ed. Analytical methods for pesticides and
plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 611-623.
KRECHNIAK, J. & FOSS, W. (1982) Cholinesterase activity in
rats treated with propoxur. Bull. environ. Contam. Toxicol.,
29(5): 599-604.
KUHR, R.J. (1968) Metabolism of methylcarbamate insecticide
chemicals in plants. J. Sci. Food Agric. 44, (Suppl.): 43-49.
KWALICK, D.S. (1971) Physician's pesticides primer. J. Med.
Soc. New Jersey, 68:(5) 375-377.
LAMB, M.J. & LILLY, L.J. (1980) An investigation of some
genetic toxicological effects of the fungicide benomyl.
Toxicology, 17: 83-95.
LEAVITT, J.R.C., GOLD, R.E., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of professional pesticide applicators to carbaryl.
Arch. environ. Contam. Toxicol., 11: 57-62.
LEELING, N.C. & CASIDA, J.E. (1966) Metabolites of carbaryl
(1-naphthyl methylcarmate) in mammals and enzymatic systems
for their formation. J. agric. food Chem., 14: 281-290.
LEITCH, R.E. & PEASE, H.L. (1973) Lannate-methomyl. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 331-338.
LYINSKY, W. & TAYLOR, H.W. (1976) Carcinogenesis in Sprague
Dawley rats of N -nitroso- N -alkylcarbamate esters. Cancer
Lett., 1: 275-279.
MAKITA, T., HASHIMOTO, Y., & NOGUCHI, T. (1973) Mutagenic,
cytogenetic, and teratogenic studies on thiophanate-methyl.
Toxicol. appl. Pharmacol., 24: 206-215.
MARLIAC, J.P. (1964) Toxicity and teratogenic effects of 12
pesticides in the chick embryo. Fed. Proc. Fed. Am. Soc. Exp.
Biol., 23: 105-126
MCCARTHY, J.F., FANCHER, O.E., KENNEDY, G.L., KEPLINGER, M.L.,
& CALANDRA, J.C. (1971) Reproduction and teratology studies
with the insecticide carbofuran. Toxicol. appl. Pharmacology,
19: 370.
METCALF, R.L., FUKUTO, T.R., COLLINS, C., BORCK, K., BURK J.,
REYNOLDS, H.T., & OSMAN, M.F. (1966) Metabolism in plant and
insect. Metabolism of 2-methyl-2-(methylthio)-propionaldehyde
O -methylcarbamoyl-oxime in plant and insect. J. agric. food
Chem., 14: 579-584.
MILLER, D.B. (1982) Neurotoxicity of the pesticidal
carbamates. Neurobehav. Toxicol. Teratol., 4: 779-787.
MIRVISH, H.S.S. (1968) The carcinogenic action and
metabolism of urethan and N -hydroxyurethan. Adv. Cancer Res.,
11: 1-42.
MONTAZEMI, K. (1969) Toxicological studies of Baygon
insecticide in Shabankareh area, Iran. Trop. geogr. Med., 21:
186-190.
MORSE, D.L. & BAKER, E.L., Jr (1979) Propanil-chloracne and
methomyl toxicity in workers of a pesticide manufacturing
plant. Clin. Tox., 15(1): 13-21.
MOSCIONI, A.D., ENGEL, J.L., & CASIDA, J.E. (1977)
Kynurenine formamidase inhibition as a possible mechanism for
certain teratogenic effect of organophosphorus and
methylcarbamate insecticides in chicken embryos. Biochem.
Pharmacol., 26: 2251-2258.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisonning due to organophosphate insecticies: acute and
chronic manifestations. Am. J. Med., 50: 475-492.
NATIONAL INSTITUTE OF HYGIENIC SCIENCES (1985) Letter to the
International Programme on Chemical Safety, 1.10.85, Tokyo,
Japan.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22: 16-18 (in
Japanese).
OGLE, R.E. & WARREN, G.F. (1954) Fate and activity of
herbicides in soils. Weeds, 3: 257-273.
OLEFIR, A.I. & VINOGRADOVA, V.Kh. (1968) [A study of the
embryotropic effect of the pesticides sevine and cyram.]
Vrach. Delo., 11: 103-106 (in Russian).
OLIVER, J.E. (1981) Pesticide-derived nitrosoamines.
Occurrence and environmental fate. In: Scanlan, R.A. &
Tannenbaum, S.T., ed. N-nitroso compounds, Washington DC,
American Chemical Society (ACS Symposium Series 174).
OONNITHAN, E.S. & CASIDA, J.E. (1968) Oxidation of methyl
and dimethylcarbamate insecticide chemicals by microsomal
enzymes and anticholinesterase activity of the metabolites. J.
agric. food Chem., 16: 28-44.
ORLOVA, N.V. & ZHALBE, E.P. (1968) Experimental material
contributive to the profile of maximal permissible amounts of
Sevin in food products. Vopr. Pitan., 27: 49-55 (in Russian).
PAROCHETTI, J.V. & WARREN, G.F. (1966) Vapour losses of IPC
and CIPC. Weeds, 14; 281-285.
PASTUSHENKO, T.B. (1983) [Study on the ganodotropic effects
of methyl N -2-benzimidazol carbamate aiming at evaluating its
effective concentrations.] Gig. i Sanit., 7: 83-85 (in
Russian).
PAULSON, G.D., JACOBSEN, A.M., ZAYLSKIE, R.G., & FEIL, V.J.
(1973) Isolation and identification of propham isopropyl-
carbanilate metabolites from the rat and goat. J. agric. food
Chem., 21: 804-811.
PETROVA-VERGIEVA, T. (1979) [Changes in the intrauterine
development of experimental animals under fundazol effect.]
Hig. Zdraveopaz., 22: 487-495 (in Bulgarian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization.
(Unpublished report VBC/84.889).
PRENDEVILLE, G.N., ESHEL, Y., JAMES, C.S., WAREN, G.F.E., &
SCHREIBER, M.M. (1968) Movement and metabolism of CIPC in
resistent and susceptible species. Weed Sci., 16: 432-435.
PROCTOR, N.H., MOSCIONI, A.D., & CASIDA, J.E. (1976) Chicken
embryo NAD levels lowered by teratogenic organophophorus and
methylcarbamate insecticides. Biochem. Pharmacol., 25: 757-762.
QUARLES, J.M. & TENNANT, R.W. (1975) Effects of nitroso-
carbaryl on BALB/3T3 cells. Cancer Res., 35: 2637-2645.
QUINBY, G.E. & BABIONE, R.W. (1966) Toxicological
observations on the use of Baygon (2-isopropoxyphenyl
N -methylcarbamate) as a malarial control insecticide in El
Salvador. Ind. Med. Surg., 35: 596-597.
QURAISHI, M.S. (1972) Edaphic and water relationships of
aldicarb and its metabolites. Can. Entomol., 104(3): 1191-1196.
REGAN, J.D., SETLOW, R.B., FRANCIS, A.A., & LYINSKY, W.
(1976) Nitrosocarbaryl: its effect on human DNA. Mutat. Res.,
38: 293-301.
REICH, G.A. & WELKE, J.O. (1966) Death due to a pesticide.
New Engl. J. Med., 274: 1432.
REINER, E. (1971) Spontaneous reactivation of phosphorylated
and carbamylated cholinesterases. Bull. World Health Org., 44:
109-112.
REINER, E. & ALDRIDGE, W.N. (1967) Effect of pH on
inhibition and spontaneous reactivation of acetylcholin-
esterase treated with esters of phosphoric acids and of
carbamic acids. Biochem. J., 105: 171-179.
REINER, E. & SKRINJARIC-SPOLJAR, M. (1968) Hydrolysis of
some monomethylcarbamates in human sera. Croat. Chem. Acta,
40: 87-90.
REINER, E., BUNTIC, A., TRDAK, M., & SIMEON, V. (1974)
Effect of temperature on the activity of human blood
cholinesterases. Arch. Toxicol., 32: 347-350.
RHODES, R.C. & LONG, J.D. (1974) Run-off and mobility
studies on benomyl in soils and turf. Bull. environ. Contam.
Toxicol., 12(4): 385-393.
RICHARDSON, E.M. & BATTEESE, R.I., Jr (1973) An incident of
Zectran poisoning. J. Maine Med. Assoc., 64: 158-159.
ROBEL, R.J., FELTHOUSEN, R.W., & DAYTON, A.D. (1982) Effects
of carbamates on Bobwhite food intake body weight and
locomotor activity. Arch. environ. Contam. Toxicol., 11:
611-615.
ROBENS, J.F. (1979) Teratologic Studies of Carbaryl,
Diazinon, Norea, Disulfiram, and Thiram in small laboratory
animals. Tox. Appl. Pharmacol., 15: 152-163.
ROMINE, R.R. (1973) Aldicarb. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7,
pp. 147-162.
ROSZKOWSKI, P. (1982) [Effect of the preparation propoxur on
intrauterine development and on the state of bone formation in
the progeny of the rat.] Ginek. Pol., 53(4): 201-207 (in
Polish).
ROTHSCHILD, E.R., MANSER, R.J., & ANDERSON, M.P. (1982)
Investigation of aldicarb in groundwater in selected areas of
the central sand plain of Wisconsin. Groundwater, 20(4):
437-445.
RUZICSKA, P., PETER, S., & CZEIZEL, A. (1975) Studies on the
chromosomal mutagenic effect of benomyl in rat and humans.
Mutat. Res., 29: 201.
RYBAKOVA, M.N. (1967) [Comparative toxic effects of sevin
and DDT used in the treatment of food crops.] Vopr. Pitan.,
26: 9-15 (in Russian).
RYBAKOVA, M.N. (1968) [Effect of some pesticides on the
hypophysis and its gonadotropic functions.] Gig. i Sanit.,
33(11): 27-31 (in Russian).
SAKAI, K. & MATSUMURA, F. (1968) Esterases of mouse brain
active in hydrolysing organophosphate and carbamate
insecticides. J. agric. food Chem., 16(5): 803-807.
SAKAI, K. & MATSUMURA, F. (1971) Degradation of certain
organophosphate and carbamate insecticides by human brain
esterases. Toxicol. appl. Pharmacol., 19(4): 660-666.
SEILER, J.P. (1972) The mutagenicity of benzimidazole and
benzimidazole derivatives. I. Forward and reverse mutations in
Salmonella typhimurium caused by benzimidazole and some of its
derivatives. Mutat. Res., 15: 273-276.
SEILER, J.P. (1975) Toxicology and genetic effects of
benzimidazole compounds. Mutat. Res., 32: 151-168.
SEILER, J.P. (1976a) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40: 339-347.
SEILER, J.P. (1976b) Inhibition of testicular DNA synthesis
by chemical mutagens and carcinogens. Preliminary result in
the validation of a novel short-term test. Mutat. Res., 46:
305-310.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method
for the determination of 1-naphthol in urine. Bull. environ.
Contam. Toxicol., 6: 34-39.
SHERMAN, H., CULIK, R., & JACKSON, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. appl.
Pharmacol., 32: 305-315.
SHTENBERG, A.I. & OZHOVAN, M.V. (1971) [Influence of low
Sevin doses on the reproductive function of animals in a
number of generations.] Vopr. Pitan., 30(1): 42-49 (in
Russian, with English summary).
SHTENBERG, A.I. & RYBAKOVA, M.N. (1968) Effect of carbaryl
on the neuroendocrine system of rats. Food Cosmet. Toxicol.,
6: 461-467.
SHTENBERG, A.I., ORLOVA, N.V., & TORCHINSKY, A.M. (1973)
[The action of different pesticides of different chemical
structure on the gonads and embryogenesis of experimental
animals.] Gig. i Sanit., 38(8): 16-20 (in Russian).
SIDEROFF, S.T. & SANTOLUCITO, J.A. (1972) Behavioural and
physiological effects of cholinesterase inhibitor carbaryl
(1-naphthyl methylcarbamate). Physiol. Behav., 9: 459-462.
SMALLEY, H.E., CURTIS, J.M., & EARL, F.L. (1968) Teratogenic
action of carbaryl in beagle dogs. Toxicol. appl. Pharmacol.,
13: 392-403.
SMALLEY, H.E., O'HARA, P.J., BRIDGES, C.H., & RADELEFF, R.D.
(1969) The effects of chronic carbaryl administration on the
neuromuscular system of swine. Toxicol. appl. Pharmacol., 14:
409-419.
SONAWANE, B.R. & KNOWLES, C.O. (1971) Phenmedipham and
m -aminophenol decomposition in alkaline soil. Bull. environ.
Contam. Toxicol., 6: 322-327.
SONAWANE, B.R. & KNOWLES, C.O. (1972) Comparative metabolism
of two carbanilate herbicides (EP-475 and phenmedipham) in
rats. Pestic. Biochem. Physiol., 1: 472-482.
STADNYK, L., CAMPELL, R.S., & JOHNSON, B.T. (1971) Pesticide
effect on growth and 14C assimilation in a fresh-water alga.
Bull. environ. Contam. Toxicol., 6: 1-8.
STANSBURY, H.A., Jr & MISKUS, R. (1964) In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators,
and food additives, New York, London, Academic Press, Vol. 2,
pp. 437-450.
STEGEMAN LE ROY, C. (1964) The effects of the carbamate
insecticide carbaryl upon forest soil mites and Collembola. J.
econ. Entomol., 57(6): 803-808.
STRINGER, A. & LYONS, C.H. (1974) The effects of benomyl and
thiophanate-methyl on earth worm populations; Apple Orchards
Pestic. Sci., 5: 189-195.
STILL, G.G. & HERRETT, R.A. (1975) Methylcarbamates,
carbanilates, and acylanilides. In: Kearney, P.C. & Kaufman,
D.D., ed. Herbicides, chemistry, degradation, and mode of
action. New York, Marcel Dekker, Vol. 2, pp. 609-664.
STILL, G.G. & MANSAGER, E.R. (1972) Metabolism of 4-chloro-
2-butynyl-3-chloro-carbanilate by soybean plants. J. agric.
food Chem., 20: 402-406.
STILL, G.G. & MANSAGER, E.R. (1973a) Soybean shoot
metabolism of isopropyl 3-chlorocarbanilate ortho and para
arylhydroxylation. Pestic. Biochem. Physiol., 3:: 87-95.
STILL, G.G. & MANSAGER, E.R. (1973b) Metabolism of isopropyl
carbanilate by soybean plants. Pestic. Biochem. Physiol., 3:
289-299.
STILL, G.G. & MANSAGER, E.R. (1975) Alfalfa metabolism of
propham. Pestic. Biochem. Physiol., 5: 515-522.
STILL, G.G. & RUSNESS, D.G. (1977) S -cysteinyl-hydroxychlor-
propham: formation of the S -cysteinyl conjugate of isopropyl-
3'-chloro-4'-hydroxy-carbanilate in oat (Avena savita L).
Pestic. Biochem. Physiol., 7: 210-219.
STRINGER, A. & WRIGHT, M.A. (1973) The effect of benomyl and
some related compounds on Lumbricus terrestris and other
earthworms. Pestic. Sci., 4: 165-170.
STYLES, J.A. & GARNER, R. (1974) Benzimidazole carbamate
methylester evaluation of its effect in vivo and in vitro.
Mutat. Res., 26:: 177-187.
SULLIVAN, L.J., ELDRIDGE, J.M., KNAAK, J.B., & TALLANT, M.J.
(1972) 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide as a
significant metabolite of carbaryl in the rat. J. agric. food
Chem., 20(5): 980-985.
SUZUKI, T. & TAKEDA, M. (1976) Microbial metabolism of
N -methylcarbamate insecticide I. Metabolism of O -sec-
butylphenyl N -methylcarbamate by Aspergillus niger van
Tieghem. Chem. Pharm. Bull., 24(9): 1967-1975.
SYPESTEYN, A.K., DEKHUYZEN, H.M., & VONK, J.W. (1977)
Biological conversion of fungicides in plants and
microorganisms, In: Siegel, M.R. & Sisler, H.D., ed.
Antifungal compounds, New York, Marcel Dekker, Vol. 2,
pp. 91-147.
TANNENBAUM, S.R., INSKEY, A.J., WEISMAN, M., & BISHOP, W.
(1974) Nitrite in human. Its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co., pp.
100-119.
TOBIN, J.S. (1970) Carbofuran a new carbamate insecticide.
J. occup. Med., 12: 16-19.
TOMLIN, A.D. & GORE, F.L. (1974) Effects of six insecticides
and a fungicide on the numbers of biomass of earthworms in
pasture. Bull. environ. Contam. Toxicol., 12(4): 487-492.
TORCHINSKY, A.M. (1973) [Significance of diets with
different protein content in manifestations of teratogenic and
embryotoxic action of some pesticides.] Vopr. Pitan., 3: 76-80
(in Russian).
TORCHINSKY, A.M., ORLOVA, N.V., SMIRNOVA, E.V., & MAKAROVA,
L.F. (1976) [Data for toxico-hygienic characteristics of the
pesticide Benlate of carbamate group.] Gig. i Sanit., 2: 35-39
(in Russian, with English summary).
UCHIYAMA, M., TAKEDA, M., SUZUKI, T., & YOSHIKAWA, K. (1975)
Mutagenicity of nitroso derivatives of N -methylcarbamate
insecticides in microbiological method. Bull. environ. Contam.
Toxicol., 14: 389-394.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) [The
reaction of nitrite with pesticides.] Z. Krebsforsch., 81:
217-224 (in German, with English summary).
VANDEKAR, M. (1965) Observations on toxicity of carbaryl,
folithion, and 3-isopropylphenyl- N -methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. World Health
Org., 33: 107-115.
VANDEKAR, M., HEDAYAT, S., PLESTINA, R., & AHMADY, G. (1968)
A study of the safety of O -isopropoxyphenyl methylcarbamate in
an operational field-trial in Iran. Bull. World Health Org.,
38: 609-623.
VANDEKAR, M., PLESTINA, R., & WILHELM, K. (1971) Toxicity of
carbamates for mammals. Bull. World Health Org., 44: 241-249.
VAN ESCH, G.J. & KROES, R. (1972) Long-term toxicity studies
of chlorpropham and propham in mice and hamsters. Food Cosmet.
Toxicol., 10: 373-381.
VAN ESCH, G.J., VAN GENDEREN, H., & VINK, H.H. (1958) The
production of skin tumours in mice by oral treatment with
urethane, isopropyl- N -phenylcarbamate, or isopropyl- N -chloro-
phenyl carbamate in combination with skin painting with croton
oil and Tween 60. Br. J. Cancer, 12: 355-362.
VASHAKIDZE, V.I. (1965) [Some questions of the harmful
action of sevin on the reproductive function of experimental
animals.] Soobshch. Akad. Nauk Gruz. SSR, 39: 471-478 (in
Russian).
VASHAKIDZE, V.I. (1967) [Mechanism of action of pesticides
(granosana, sevin, donoc) on the reproductive cycle of
experimental animal.] Soobshch. Akad. Nauk Gruz. SSR, 48:
219-224 (in Russian).
VASSILIEFF, I. & ECOBICHON, D.J. (1982) Stability of
Matacil(R) in aqueous media as measured by changes in
anticholinesterase potency. Bull. environ. Contam. Toxicol.,
29: 366-370.
VASSILIEFF, I. & ECOBICHON, D.J. (1983) Acute toxicity of
aminocarb in male rats and inhibition of tissue esterases.
Bull. environ. Contam. Toxicol., 31: 326-330.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. Vol. 1. Toxicity profiles, Boca Raton,
Florida, CRC Press, Inc., 232 p..
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticide
reference index, JMPR 1961-84, Geneva, World Health
Organization (Unpublished report).
VOSS, G. & SCHULER, J. (1967) A fast and simple procedure
for routine determination of plasma ChE activity. Bull.
environ. Contam. Toxicol., 2(6): 357-363.
WHO (1984) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-85, Geneva, World
Health Organization (Unpublished report VBC/84.2).
WHO/FAO (1984) Data sheets on pesticides, Geneva, World
Health Organization.
WEIL, C.S., WOODSIDE, M.D., CARPENTER, C.P., & SMYTH, H.F.,
Jr (1972) Current studies of tests of carbaryl for
reproductive and teratogenic effects. Toxicol. appl.
Pharmacol., 21: 390-404.
WEIL, C.S., WOODSIDE, M.D., BERNARD, J.B., CONDRA, N.I., KING,
J.M., & CARPENTER, C.P., (1973) Comparative effect of
carbaryl on rat reproduction and guinea-pig teratology when
fed either in the diet or by stomach intubation. Toxicol.
appl. Pharmacol., 26: 621-638.
WEIS, P. & WEIS, J.S. (1974) Schooling behaviour of Menidia
menidia in the presence of insecticide Sevin (carbaryl). Mar.
Biol., 28: 261-263.
WESTLAKE, G.E., BUNYAN, P.J., MARTIN, A.D., STANLEY, P.I., &
STEED, L.C. (1981) Carbamate poisoning. Effect of selected
carbamate pesticides on plasma enzymes and brain esterases of
Japanese quail (Coturnix coturnix japonica). J. agric. food
Chem., 29(4): 779-785.
WHITE, E.R., BOSE, E.A., OGAWA, J.M., MANJI, B.T., & KILGORA,
W.W. (1973) Thermal and base-catalysed hydrolysis products
of the systemic fungicide benomyl. J. agric. food Chem., 21:
616-618.
WIEDMANN, J.L.M., ECKE, G.G., & STILL, G.G. (1976) Synthesis
and isolation of 1-hydroxy-2-propyl-3-chloro-carbanilate from
soybean plants treated with isopropyl-3-chlorocarbanilate J.
agric. food Chem., 24: 588-592.
WILHELM, K. & REINER, E. (1973) Effect of sample storage on
human blood cholinesterase activity after inhibition by
carbamates. Bull. World Health Org., 48: 235-238.
WILHELM, K., VANDEKAR, M., & REINER, E. (1973) Comparison of
methods of measuring cholinesterase inhibition by carbamates.
Bull. World Health Org., 48: 41-44.
WILLIAMS, I.H., BROWN, M.J., & WHITEHEAD, P. (1976a)
Persistence of carbofuran residues in some British Columbia
soils. Bull. environ. Contam. Toxicol., 15(2): 242-243.
WILLIAMS, I.H., PEPIN, H.S., & BROWN, M.J. (1976b)
Degradation of carbofuran by soil microorganisms. Bull.
environ. Contam. Toxicol., 15(2): 244-249.
WILLS, J.H., JAMESON, E. & COULSTON, F. (1968) Effects of
oral doses of carbaryl on man. Clin. Toxicol., 1(3): 265-271.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., London, British Crop Protection
Council.
WRIGHT, M.A. & STRINGER, A. (1973) The toxicity of
thiabendazole, benomyl, methyl-benzimidazol-2-yl-carbamate,
and thiophanate-methyl to the earthworm Lumbricus terrestris.
Pestic. Sci., 4: 431-432.
WYROBEK, A.J., WATCHMAKER, G., GORDON, L., WONG, K., MOORE,
D.I., & WHORTON (1981) Sperm shape abnormalities in carbaryl
exposed employees. Environ. Health Perspect., 40: 255-265.
YAKIM, V.S. (1967) [Data for substantiating the maximum
permissible concentration of sevine in the air of working
zones.] Gig. i Sanit., 32: 29-33 (in Russian).
YOSHIDA, K. & NISHIUCHI, Y. (1972) [Toxicity of pesticides
to some water organisms.] Bull. agric. Chem. Inspect. Stn.
(Japan), 12: 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) [Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn.
(Japan),. 6: 65-69 (in Japanese).
ZAKI, M.H., MORAN, D., & HARRIS, D. (1982) Pesticides in
groundwater: the aldicarb story in Suffolk Country, New York.
Am. J. public Health, 72(12): 1391-1395.
Annex I. Names and structures and some physical and chemical properties of carbamate pesticides
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aldicarb Temik propanal, 2-methyl- C7H14N2O2S 190.29 13 mPa 0.6%
2-(methylthio)-,
O -[(methylamino)
carbonyl]oxime
(
Heybroek et al. (1984) studied the metabolism of [ring-14C]
asulam in the rat. Most of the radioactivity (61 -74%)
administered orally or intravenously was excreted in the urine in
24 h as unchanged asulam, 8 - 14% as N 4-acetylasulam, and up to
2.5% as N 4-acetylsulfanilamide. Small amounts of radioactivity
were found in faeces.
These examples illustrate the different metabolic pathways of
biotransformation. For more details, readers should refer to
Fukuto (1972) who has reviewed the metabolism of carbaryl,
mexacarbamate, aminocarb, formetanate, aldicarb, methiocarb,
carbofuran, MPMC, trimethacarb carbanolate, propoxur, and
dimetilan. Harvey & Han (1978a) have described the metabolism of
oxamyl and Harvey et al. (1973), of methomyl in the rat. The
metabolism of pirimicarb was extensively described in FAO/WHO
(1977b). Furthermore, information on the metabolism is provided in
the handbooks on carbamates, and the different monographs of the
Joint FAO/WHO Meetings on Pesticide Residues.
6.3 Elimination and Excretion in Expired Air, Faeces, and Urine
6.3.1 Man
In a study on human volunteers, 2 men were dosed orally with
carbaryl in gelatin capsules at 2.0 mg/kg body weight.
Chromatographic analyses of a 4-h urine sample revealed 1-naphthyl
glucuronide (15%), 1-naphthyl sulfate (8%), and 4-(methylcarbamoyl-
oxy)-1-naphthyl glucuronide (4%). In addition, 1-naphthyl
methylimidocarbonate O -glucuronide was identified by fluorometry
(FAO/WHO, 1968b). During the first 8 h, the urinary excretion of
these metabolites constituted 12 - 15% of the carbaryl
administered. Small amounts of urinary metabolites were detected
by fluorometric analysis on the second day, but not afterwards
(Knaak et al., 1968). In another study, 1-naphthyl glucuronide, 1-
naphthyl sulfate, and other unidentified metabolites were found in
24-h urine samples from men exposed to carbaryl dust during a
packaging operation in a factory (details not available) (Knaak et
al., 1965).
The determination of 1-naphthol or its sulfate or glucuronide
in the urine of persons occupationally exposed to carbaryl is one
method of biological monitoring for this exposure (FAO/WHO, 1982b).
Dawson et al. (1964) investigated the metabolism and excretion
of propoxur in 3 male volunteers orally administered a dose of 50
mg. Within 8 - 10 h, 27% of the propoxur appeared in the urine as
its metabolite 2-isopropoxyphenol, indicating that propoxur is
rapidly absorbed and hydrolysed in human beings.
The absorption and fate of benomyl in animals following oral
administration of the compound varies with species. According to a
study that involved one rat and one dog administered 2-14C-benomyl
by stomach tube, the rat eliminated about 86% of a single dose via
the urine and 13% via faeces. The dog eliminated only 16% of a
similar benomyl dose via the urine, while almost 83% was detected
in faeces. No data are available concerning the possible roles of
the enterohepatic circulation and biliary excretion of benomyl or
its metabolites (Gardiner et al., 1974).
6.3.2 Laboratory animals
In animals, oxidation of carbamates often, but not always,
results in detoxification. Oxidative metabolism generally leads to
products of greater polarity and water solubility, and these can be
more readily eliminated through the urine and faeces than the
parent compound.
In the rat, benomyl is mainly excreted via the urine (78.9% of
the given dose) as 5-HBC conjugates. In the dog, 99% of the dose
administered was eliminated within 72 h. The major route of
elimination was via the faeces in which benomyl and/or MBC and 5-
HBC were detected. On the basis of studies on the rat, dog, dairy
cow, and chicken, neither benomyl nor its metabolites tend to
accumulate in animal tissues (Gardiner et al., 1974).
According to FAO/WHO (1974b), feeding of 14C- or 35S-labelled
thiophanate-methyl to rats, mice, and dogs resulted in 80 - 100%
recovery of the label in the faeces and urine within 96 h.
Unmetabolized thiophanate-methyl has been reported to be the major
component of faecal elimination. The minor part consisted of 4-
hydroxy-thiophanate-methyl, dimethyl-4,4- O -phenylenebisallophanate.
MBC and 5-hydroxy-MBC were also detected.
Following application of carbendazim to rats and mice by
intragastric intubation, almost all metabolites in the urine were
conjugated as sulfate esters. 5-HBC was released as the only
metabolite extractable from water. A higher proportion of polar
compounds was found in the urine of mice than in that of rats.
Polarity was caused by the phenolic hydroxyl group (FAO/WHO,
1985a).
The excretion of 14C-labelled propham and chlorpropham was
investigated in female rats after a single oral dose. The average
3-day urinary excretion of radioactivity was between 56 and 85%,
depending on the position of labelling: viz chain or ring
labelling. With chain 14C-chlorpropham, an average of 35% of the
administered radioactivity appeared in the respired air, compared
with only 5% for chain 14C-propham (Fang et al., 1974).
6.4 Metabolism in Plants
Studies on the metabolism and fate of carbamate pesticides in
plants are of great importance for assessing the human hazards from
the consumption of fruit and vegetables containing residues of the
applied pesticides and metabolites.
Generally, the metabolism of carbamates in plants follows
another route of detoxification. The hydroxylated carbamates
produced by enzymes are conjugated either with amino acids (for
instance cysteine) or as glycosides and phosphates, which are
stored as terminal metabolites (Still & Rusness, 1977; Aldridge &
Magos, 1978).
The toxicological properties of plant conjugates in mammals or
other animals are not generally known. Insecticidal methyl
carbamate esters are vulnerable to hydrolytic cleavage, which
results in detoxified products. Detoxification of carbamate esters
in plants by hydrolysis occurs to a lesser extent than
detoxification by oxidative metabolism; nevertheless, hydrolytic
cleavage of the ester moiety is a significant detoxification
mechanism. For instance, in sugar beets treated with desmedipham,
the major metabolites were ethyl-3-hydroxy phenyl carbamate and 3-
aminophenol (Knowles & Sonawane, 1972). Carbetamide is hydrolysed
to aniline (Guardigli et al., 1977). Aromatic ring hydroxylation
appears to be the predominant metabolic reaction that carbamate
herbicides undergo in plants. The major metabolites isolated from
soybean plants, after uptake of chlorpropham, were glucoside
conjugates of 2-hydroxy- and 4-hydroxypropham. Similar results
were obtained with propham (Still & Mansager, 1973a,b, 1975).
There is evidence that the ring hydroxylation varies with the
plant species. In other carbamates, for instance in the case of
barban, ring hydroxylation did not appear to take place, and polar
metabolites were produced, which were hydrolysed into
chloroanilines (Still & Mansager, 1972).
Furthermore, oxidation of aliphatic groups takes place in
carbamate herbicides (Wiedmann et al., 1976). Still & Herrett
(1975) demonstrated N -methyl hydroxylation for dichlormate, a
metabolite that is either conjugated or loses the hydroxymethyl
moiety to produce N -demethylated dichlormate.
Thiophanate-methyl is mainly metabolized into carbendazim (MBC)
and, to a less extent, into 2-aminobenzimidazole (2AB). A few other
metabolites have also been found (FAO/WHO, 1974b).
The metabolic pathway for carbaryl in plants is identical,
whether the compound is injected into the stem or applied to the
leaf surface. After entering the plant, carbaryl undergoes
biotransformation to its primary metabolites, which are similar to
the ones formed in animals. These hydroxylated metabolites, which
are less toxic than carbaryl itself, are conjugated by plants to
form water-soluble glycosides. Injection of carbaryl into the
bean plant produced water-soluble metabolites attributable to
hydroxylation of the ring or N -methyl group followed by
conjugation, mainly as glycosides (Kuhr, 1968; FAO/WHO, 1970b;
Fukuto, 1972).
The metabolism of aldicarb in plants is the same as that in
mammals. In the cotton plant, the thioether moiety is rapidly
oxidized to the sulfoxide, and the latter is slowly transformed to
the sulfone. The sulfoxide is rather stable and can be present in
cotton plants, even as long as 2 months after treatment. The total
metabolites present can be as high as 80% (Kuhr, 1968). Because of
their persistence in plants, and their even higher anticholinesterase
activity compared with aldicarb itself, the sulfoxide and sulfone
should be taken into account when considering residue tolerances.
The major metabolite isolated from bean plants, 28 days after
treatment with carbofuran, was a conjugate of 3-hydroxy-carbofuran,
which remained in the aqueous phase after extraction with an
organic solvent. The conjugate represented 55% of the total 14C-
labelled residues present in the plant. 3-Hydroxy-carbofuran is
highly toxic for mammals (LD50 in the rat 7 mg/kg body weight).
The conjugate may also be toxic, particularly taking into account
the acid conditions of the mammalian stomach under which the
conjugate will be hydrolysed (FAO/WHO, 1977b, 1980b).
Harvey et al. (1978) studied the metabolism of oxamyl in plants
(tobacco, alfalfa, peanuts, potatoes, apples, oranges, and
tomatoes). The major route of degradation involved hydrolysis to
the corresponding oximino compound, which in turn became conjugated
with glucose. Further metabolism resulted in the loss of one of
the N' -methyl groups and/or addition of other glucose units to the
sugar moiety of the original conjugate. Total breakdown into
normal natural products has been demonstrated.
In a study by Harvey & Reiser (1973), methomyl rapidly degraded
to carbon dioxide and acetonitrile, which volatilized from the
plant tissues. The half-life for methomyl was of the order of 3 -
6 days. The remainder of the compound had been reincorporated into
natural plant components.
The metabolic pathways for a number of carbamate pesticides are
described in more detail in Fukuto (1972).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
As described in section 4.2.1, soil microorganisms are capable
of hydrolysing carbamates. Furthermore, it seems that
microorganisms can easily adapt themselves to metabolize the
different types of carbamates (Aly & El-Dib, 1972). Nevertheless,
carbamates and their metabolites can affect the microflora and
cause changes that may have an important impact on the maintenance
of the soil productivity (Filip, 1974).
7.2 Aquatic Organisms
In general, carbamates are not very stable under aquatic
conditions (section 4). The solubility in water differs
considerably for the different carbamates (Annex I). Furthermore,
photodecomposition takes place and microorganisms are able to
degrade these compounds. By these processes, the carbamate is
broken down (oxidation, hydrolysis) into other compounds. Some
will be less toxic, others even more toxic than the parent
compound. Carbaryl will be hydrolysed into 1-naphthol, which is
just as toxic (Armstrong & Millemann, 1974a). It seems unlikely
that most of the carbamates will persist long in the environment,
but there are exceptions, such as dimetilan and carbendazim.
Many data are available on the acute toxicity of the carbamates
in several fresh- and salt-water fish and other organisms, some of
which are listed in Tables 4 and 5.
When carbamates are deliberately added to an aquatic system,
caution is necessary since, with high concentrations, a number of
species are at risk. For example, while applications of carbaryl
of 0.93 kg/ha were effective in controlling the ghost shrimp
(Callianassa californiensis), an oyster pest, they also caused a
reduction in the population of juvenile clams (Armstrong &
Millemann, 1974a).
In a similar study (Armstrong & Millemann, 1974b), carbaryl
and its hydrolytic product, 1-naphthol, were examined for other
effects on the mussel (Mytilus edulis). An age dependence was
noted; the most sensitive stage (appearance of the first polar body
shortly after fertilization) had EC50s of 5.3 and 5.2 mg/litre for
carbaryl and 1-naphthol, respectively. Effects of the compounds
on development were characterized by disjunction of blastomeres and
asynchronous and unaligned cleavages. Carbaryl, at a single dose
of 0.1 mg/litre, also disrupted normal schooling behaviour in
juvenile Menidia medidia; the disruptive effects were attributed
to the 1-naphthol rather than to the parent carbamate. These
effects were reversed within 3 days of placing the fish in clean
water (Weis & Weis, 1974). Hansen (1969) studied the capacity of
sheepshead minnows (Cyprinodon variegatus) to avoid carbaryl. The
fish did not avoid carbaryl in concentrations of 0.1 - 10 mg/litre
water.
Table 4. Acute aquatic toxicity (LC50 in mg/litre)a
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Aldicarb fish Rainbow trout (Salmo gairdneri) 0.5 12 0.56
fish Bluegill (Lepomis machrochirus) 1.3 24 0.05
Aminocarb fish Rainbow trout (Salmo gairdneri) 1.5 12 13.5b
fish Bluegill (Lepomis machrochirus) 2.0 20 3.1b
crustacea Daphnia (Daphnia magna) first instar 21 0.01 - 0.1c
(48-h EC50)
fish Rainbow trout (Salmo gairdneri) 1.5 10 0.13c
fish Bluegill (Lepomis machrochirus) 0.6 20 0.1c
insect Midge (Chironomus plumosus) fourth instar 20 0.27c
Benomyl fish Rainbow trout (Salmo gairdneri) 1.2 12 0.17d
fish Bluegill (Lepomis machrochirus) 0.9 22 0.85d
fish Rainbow trout (Salmo gairdneri) 1.0 12 0.31e
fish Bluegill (Lepomis machrochirus) 0.6 22 1.2e
Benomyl fish Rainbow trout (Salmo gairdneri) 0.2 12 0.37f
metabolite
MBC
Bufencarb crustacea Scud (Gammarus fasciatus) mature 15 0.001
fish Goldfish (Carassius auratus) 1.0 18 0.29
Carbaryl fish Rainbow trout (Salmo gairdneri) 1.5 12 1.95d
fish Bluegill (Lepomis machrochirus) 1.2 18 6.76d
crustacea Daphnia (Daphnia pulex) first instar 16 0.0064d
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature 21 0.026d
----------------------------------------------------------------------------------------------------
Table 4. (contd.)
----------------------------------------------------------------------------------------------------
Compound Type of Name of organism Stage or Temp- Concentration
organism weight (g) erature (mg/litre)
(°C) (after 96 h)
----------------------------------------------------------------------------------------------------
Carbofuran fish Rainbow trout (Salmo gairdneri) 1.5 12 0.38d
fish Bluegill (Lepomis machrochirus) 0.8 18 0.24e
Dichlormate fish Rainbow trout (Salmo gairdneri) 0.8 12 4.9
Methiocarb fish Rainbow trout (Salmo gairdneri) 1.3 12 0.80
fish Bluegill (Lepomis machrochirus) 1.0 24 0.21
Methomyl fish Rainbow trout (Salmo gairdneri) 1.1 12 1.60b
fish Bluegill (Lepomis machrochirus) 0.9 20 1.05b
crustacea Daphnia (Daphina magna) first instar 21 0.009b
(48-h EC50)
Mexacarbate fish Rainbow trout (Salmo gairdneri) 1.0 11 12.0g
fish Bluegill (Lepomis machrochirus) 0.7 12 22.9g
crustacea Daphina (Daphina pulex) first instar 15 0.010g
(48-h EC50)
crustacea Scud (Gammarus fasciatus) mature - 0.04g
Trimethacarb fish Rainbow trout (Salmo gairdneri) 1.2 12 1.0
fish Bluegill (Lepomis machrochirus) 0.9 18 11.6
----------------------------------------------------------------------------------------------------
a From: Johnson & Finley (1980).
b Technical material, 95 - 98%.
c Liquid formulation, 17%.
d Technical material, 99%.
e Wettable powder, 50%.
f Methyl-2-benzimidazole (MBC), 99%.
g Technical material, 90 - 95%.
Note: It should be recognized that the LC50 and EC50 values only give an indication of the
toxicity and that the toxicity for these organisms may be appreciably changed by variations
in temperature, pH, oxygen content, and water hardness. A 10-fold increase in the toxicity
may be found. Also, the life stage of the organisms is an important factor in estimating
the toxicity of the compound.
Table 5. Acute toxicity of carbamate pesticides for some aquatic organisms
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
Benomyl 7.5 12 11 (wettable - 14 Yoshida & Nishiuchi
powder) (1972)
BPMC 16 10 ~ 40 1.7 5.0 0.32 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Carbaryl 13 10 ~ 40 2.8 2.8 0.05 Yoshida & Nishiuchi
(1972)
Carbendazim > 40 - 40 - > 40 Yoshida & Nishiuchi
(1976)
CPMC 10 ~ 40 10 ~ 40 5.6 7.0 0.1 ~ 0.5 Yoshida & Nishiuchi
(emulsifiable (1972); Nishiuchi
concentrate) (1974)
Isoprocarb 10 ~ 40 10 ~ 40 5.9 3.0 0.30 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Mecarbam 0.70 0.68 0.35 0.055 0.03 Yoshida & Nishiuchi
granule (1972); Nishiuchi
(1974)
Methomyl 2.8 2.7 0.87 1.0 0.045 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
Table 5. (contd.)
------------------------------------------------------------------------------------------------
Pesticidea TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldifsh Killifish Guppy Water flea
(Cyprinus (Carassius ( Fundulus ( Lebistes ( Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
------------------------------------------------------------------------------------------------
MPMC 10 ~ 40 10 ~ 40 12 7.3 0.07 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
MTMC 10 ~ 40 10 ~ 40 27 13 0.35 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
Pirimicarb > 40 - 40 - 0.048 Yoshida & Nishiuchi
(1976)
Promecarb 2.7 - 3.3 - 0.020 Yoshida & Nishiuchi
(1976)
Terbam 0.92 - 2.2 - 0.025 Yoshida & Nishiuchi
(1976)
Thiophanate > 40 > 40 > 40 - > 40 Yoshida & Nishiuchi
(1972)
Thiophanate 11 > 40 11 (wettable - > 40 Yoshida & Nishiuchi
-methyl powder) (1972)
XMC > 40 > 40 33 25 0.055 Yoshida & Nishiuchi
(1972); Nishiuchi
(1974)
------------------------------------------------------------------------------------------------
a BPMC = 1-sec-butylphenylmethyl carbamate
CPMC = 1-chlorophenylmethyl carbamate
MPMC = 3,4-xylylmethyl carbamate
MTMC = 4-tolylmethyl carbamate
Terbam = 4-tert-butylphenylmethyl carbamate
XMC = 3,5-xylylmethyl carbamate
Note: Test methods are officially recognized methods based on the Notification of the
Ministry of Agriculture, Forestry, and Fisheries of Japan.
Carbamates can have adverse effects on algae. Stadnyk et al.
(1971) reported that carbaryl at a concentration of 0.1 mg/litre
caused an increase in cell numbers and in the biomass of the green
algae Scenedesmus quadricaudata.
Motsuage fish (Motsugo pseudoras bora parva) were reported to
accumulate carbaryl and BPMC during a 30-day exposure to a
concentration of 0.6 - 1.2 mg/litre. Carbaryl uptake was greatest.
However, this compound underwent more rapid metabolism and
excretion than BPMC. Metabolism of BPMC was slow and introduced
permanent spinal curvature of the backbone in about 30% of the fish
exposed, an effect seen with the organophosphorus insecticide
diazinon (Kanazawa, 1975).
A residue study indicated that over a 28-day period at the
highest exposure level of 0.75 mg methomyl/litre, no accumulation
of the carbamate took place. During the entire exposure period,
the values ranged from 0.36 to 0.45 mg/kg. After a 3-day withdrawal
period, no methomyl (< 0.02 mg/kg) was detected in the fish
(Kaplan & Sherman, 1977).
The results summarized in Table 4 are representative of the
toxicity of the different carbamates for fish and invertebrate
species. In general, the LC50s of carbamates for different fish
species range from approximately 0.1 to 10 mg/litre. Invertebrates
are usually more sensitive. The EC50 values for Daphnids and
other species are mainly below 0.1 mg/litre. Some carbamates seem
to be very toxic (< 0.01 mg/litre) for these invertebrates.
7.2.1 Field studies
Little information is available concerning the fate and
persistence of carbamates in the aquatic environment. The presence
of these carbamates in surface waters may have an effect on water
organisms.
Quraishi (1972) studied the persistence of aldicarb. Field
water treated in the laboratory at 100 mg aldicarb/litre resulted
in residues of aldicarb and its metabolites of 0.4 mg/litre after
11 months. Water was stored at 16 - 20 °C and exposed for
approximately 507 h to sunlight.
Data concerning the fate of aminocarb have been reviewed by
Vassilieff & Ecobichon (1982). A half-life of 28.5 days in pond
water was found under normal environmental conditions. The
principal metabolite appeared to be the phenol, though the
methylamino and formylamino analogues were also observed.
Harvey & Pease (1973) found that, under field conditions in
Delaware, Florida, and North Carolina, methomyl broke down almost
completely within 1 month; most of it was lost from the soil by
volatilization, presumably as carbon dioxide. Small amounts
extracted from the soil consisted of methomyl, S -methyl N -
hydroxythioacetimidate, and some polar compounds. A run-off study,
under farm conditions, showed that the compound did not move into
untreated areas with run-off water.
7.3 Terrestrial Organisms
7.3.1 Effects on soil fauna
Thiophanate-methyl and carbendazim have been shown to be
equally as toxic through contact as benomyl. Earthworms (Lumbricus
terrestris) immersed for 1 min in a 0.6% aqueous suspension of
these compounds died in 14 days. Worms in pots containing soil
drenched at a rate of 0.78 g/m2 died within 18 days (Wright &
Stringer, 1973).
7.3.2 Wildlife
The LD50 of benomyl for mallard ducks and quail is > 5000
mg/kg body weight. The cumulative toxicity of formetanate (7 days)
is approximately 6800 mg/kg body weight for ducks, and > 4640 mg/kg
body weight for quail and for pheasant (Aldridge & Magos, 1978).
Lethal and sub-lethal doses of aldicarb, methiocarb, oxamyl,
pirimicarb, and thiofanox administered to Japanese quail produced
significant inhibition of plasma- and brain-ChE and influenced the
activity of other enzymes, such as alpha-naphthyl acetate esterase,
glutamate dehydrogenase, and glutamate oxaloacetic transaminase.
With sub-lethal dose levels, plasma-ChE activity recovered within a
few days (Westlake et al., 1981).
An 8-day dietary LC50 for the Peking duck was 1890 mg/kg
feed; for bobwhite quail, the dietary LC50 was 3680 mg/kg feed
(Kaplan & Sherman, 1977).
Bobwhites (Colinus virginianus) were provided diets containing
sublethal levels of carbaryl (237 or 1235 mg/kg), for 7 days, or
carbofuran (26 mg/kg), for 14 days. Food intake, body weight, and
the locomotor activity of adult bobwhites were normal.
Administration of a diet containing 131 mg carbofuran/kg resulted
in reductions in food intake, body weight, and locomotor activity
(Robel et al., 1982).
7.3.3 Bees
An undesirable effect caused by the use of carbamate
insecticides has been the mortality of honey bees, a species that
exhibits a high sensitivity to these compounds. Because of the
agricultural and ecological importance of bees, thorough
comparative toxicity studies have been carried out on a large
number of compounds to identify those with the widest margin of
safety (Atkins et al., 1973).
Abdel-Aal & Fahmy (1977) compared the effects of aldicarb,
methomyl, N -desmethylmethomyl, carbaryl, and carbofuran on the
honey bee (Apis mellifera) and found that all of the compounds
were extremely toxic.
7.4 Earthworm and Mite Populations
Studies by Stringer & Wright (1973) and Wright & Stringer
(1973) showed that earthworm populations in apple orchard plots
sprayed with either benomyl or thiophanate-methyl were reduced. In
particular, Lumbricus terrestris, an important species in the
ecology of the apple orchard, was virtually eliminated. Captive
worms would not feed on leaf material that had been sprayed at 1.75
µg/cm2 with benomyl, methyl benzimidazol-2-yl carbamate (MBC), or
thiophanate-methyl, and feeding was significantly reduced at a
level of 0.87 µg/cm2. All the worms were killed following contact
with benomyl as an aqueous suspension or soil drench (7.75 kg/ha).
It was suggested by the authors that the toxicity of benomyl
might be due to the anticholinesterase activity of the carbamate
moiety. Effects such as lethargic movements, muscular paralysis,
and death support this supposition.
The spraying of benomyl and thiophanate-methyl in orchards
reduced the number and biomass of all earthworm species combined
and also of each individual species (Stringer & Lyons, 1974). It
was observed that over 90% reduction in earthworm populations
occurred in pastures treated with benomyl. It was suggested that
these changes were reversible, and that the populations of
earthworms would return to normal a few years after the termination
of the treatment (Tomlin & Gore, 1974).
The carbamate insecticide most commonly used in the soil is
carbaryl; it is used mainly in woodlands and orchards where the
formation and fertility of the soil are important.
Stegeman & LeRoy (1964) applied carbaryl at 1.3, 11.2, and 56
kg ai/ha to a red pine plantation and mixed hardwood stands, and,
though the lowest dose did not have any effects on mite and
Collembola populations, the other 2 dose levels considerably
decreased the numbers of mites and Collembolla. Populations began
to recover after 2 months, but Collembola were more sensitive and
recovered more slowly. There seems to be little likelihood of
long-term effects of carbaryl on mite populations (Edwards &
Thompson, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
Short- and long-term toxicity studies with carbamates have been
carried out for a period of 20 - 30 years. It is clear that the
production of these carbamates has improved during this period and
that, consequently, purer products have become available. The use
of less pure substances in earlier toxicity studies could explain
the conflicting results between these and later studies.
8.1.1. Oral
Acute oral and dermal toxicity data on animals, for most of the
carbamate pesticides, are given in Table 6. The WHO Recommended
Classification of Pesticides by Hazard is also cited. This
classification is based primarily on the acute oral and dermal
toxicity of the technical material for the rat (WHO, 1984). The
oral LD50s range from less than 1 mg/kg body weight for the highly-
toxic aldicarb to over 5000 mg/kg body weight for the non-
insecticidal carbamates such as phenmedipham, carbendazim, propham,
and benomyl.
A dose-effect relationship is generally noticed between the
administered dose, the severity of effects, and the amount of ChE
inhibition. A correlation also exists between the duration of
symptoms and the in vivo persistence of the compound.
In general, the toxicological effects produced by carbaryl are
typical of those produced by methyl carbamates. The effects of
carbaryl on a number of different animal species were studied by
Carpenter et al. (1961). Rats given a single oral dose of 560
mg/kg body weight showed 42% and 30% inhibition of erythrocyte- and
brain-ChE, respectively, within 1/2 h of treatment; only about 5%
inhibition of plasma-ChE was observed. All ChE levels were almost
normal after 24 h. In studies on rats administered single oral
doses ranging from 5 to 50 mg/kg body weight, a decrease in ChE
activity occurred within 5 min. Recovery was quite rapid
(Krechniak & Foss, 1982).
In 4 dogs, marginal inhibition of erythrocyte-ChE activity was
seen after treatment with a single oral dose of 375 mg carbaryl/kg
body weight. After 30 min, signs of poisoning were observed
including salivation, lachrymation, constriction of pupils,
urination, defaecation, increase in respiratory rate, muscular
twitching, tremors, and mild convulsions. The animals appeared to
have completely recovered the following day. These signs of
poisoning are classic of overstimulation of the parasympathetic
nervous system (Carpenter et al., 1961).
Vassilieff & Ecobichon (1983) studied the influence of a single
dose of 25 mg aminocarb/kg body weight on the activity of
erythrocyte- and brain-AChE, plasma-ChE, and hepatic
carboxylesterases in rats. Significant inhibition of all the
esterases was observed, 30 min or more after the administration of
aminocarb. This severe, but transient, inhibition of the tissue
esterases had recovered after 24 h.
Table 6. Acute oral and dermal toxicity data for a number of
carbamate pesticides
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
aldicarb 0.9 > 10.0 (rabbit) IA
aldoxycarb 26.8 700 - 1400 -
allyxycarb 90 - 99 500 -
aminocarb 30 - 40 275 IB
asulam > 4000 > 1200 -
barban 1376 - 1429 > 1600 -
> 20 000 (rabbit)
BPMC 623 - 657 > 5000 -
bendiocarb 40 - 156 566 - 600 II
benomyl > 10 000 > 10 000 (rabbit) 0
> 1000 (dog)
bufencarb 87 680 (rabbit) -
butacarb NA NA -
carbanolate NA NA -
carbaryl approximately > 4000 II
500 - 600 > 2000 (rabbit)
carbendazim > 15 000 > 2000 0
> 2500 (dog) > 10 000 (rabbit)
> 10 000 (quail)
carbetamide 10 000 > 2000 -
1250 (mouse) > 500 (rabbit)
1000 (dog)
carbofuran 6 - 14 3400c (rabbit) IB
15 - 19 (dog)
chlorbufam 2500 NA -
chlorpropham 5000 - 8000 10 200d O
5000 (rabbit) 2000 (dog)
cloethocarb 35.4 4000 -
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
desmedipham > 10 250 2000 - 10 000 -
2025e (rabbit)
dimetilan 64 > 2000 -
60 - 65 (mouse)
dichlormate NA NA
dioxacarb 72 approximately -
3000 1950
(rabbit)
ethiofencarb 411 - 499 > 1150 II
224 - 256 (mouse)
155 (quail)
formetanate 21, 18 (mouse) > 5600
19 (dog) > 10 200 (rabbit)
hoppicide NA NA -
isoprocarb 403 - 485 > 500
487 - 512 (mouse)
ca 500 (rabbit)
karbutilate 3000 > 15 400 -
methiocarb 100 350 - 400 II
40 (guinea-pig)
methomyl 17 - 24 > 5000 (rabbit) IB
28 (hen)
metolcarb 498 - 580 > 2000 -
mexacarbate 15 - 63 > 500 -
MPMC 380 > 1500 (mouse) -
MTMC 268 (mouse) 6000 -
oxamyl 5.4 710 (rabbit) IB
4.2 (quail)
phenmedipham > 8000 > 4000 -
> 4000 (dog)
> 3000 (chicken)
---------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------
Carbamate LD50 (mg/kg body weight)a WHO Recommended
oral dermal Classification
of Pesticides
by Hazardb
---------------------------------------------------------------------
pirimicarb 147 (101 - 210) > 500 (rabbit) II
107 (mouse)
100 - 200 (dog)
25 - 50 (poultry)
promacyl 1220 > 4000 -
2000 - 4000 (mouse)
4000 - 8000 (hen)
promecarb 61 - 90 > 1000 -
> 1000 (rabbit)
propham 9000 6800f (rabbit) O
3000 (mouse)
propoxur 80 - 191 1000 - 2400 II
100 - 109 (mouse)
40 (guinea-pig)
swep NA NA -
terbucarb NA NA -
thiodicarb NA NA -
thiofanox 8.5 39 (rabbit) IB
43 (quail)
thiophanate > 15 000 > 15 000 -
-ethyl
thiophanate 6640 - 7500 > 10 000 O
-methyl
trimethacarb NA NA -
xylylcarb 325 - 375 > 1000 -
XMC 542 - -
445 (rabbit)
---------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
b From: WHO (1984). See this reference for classification of
organophosphates not mentioned in this annex.
The hazard referred to in this Classification is the acute risk for
health (that is, the risk of single or multiple exposures over a
relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the
directions for handling by the manufacturer or in accordance with the
Table 6 (contd.)
rules laid down for storage and transportation by competent
international bodies.
Classification relates to the technical material, and not to the
formulated product:
Class IA = Extremely hazardous. Class III = Slightly hazardous.
Class IB = Highly hazardous. Class O = unlikely to present
Class II = Moderately hazardous. acute hazard in normal use.
c With 75% water-dispersible powder.
d With 50% emulsifiable concentrate formulation.
e With 16% formulation.
f With 25% emulsifiable concentrate formulation.
NA = Not available.
Note: References are the reports of the different WHO/FAO Joint
meetings on pesticides residues (Annex III), Kaplan & Sherman
(1977), and Worthing & Walker (1983).
8.1.2 Dermal
Acute dermal toxicity values for most carbamates are mainly
above 500 mg/kg body weight with the exception of, for instance,
aldicarb, which is highly toxic.
8.1.3 Inhalation
Exposure to carbaryl (50% wettable powder, average particle
size, 15 µm) at a mean concentration of 390 mg/m3 (range 344 - 722
mg/m3), for 4 h, produced nasal and ocular irritation in 6 guinea-
pigs. When another group of guinea-pigs inhaled a mean of 230 mg
carbaryl/m3 (average particle size, 5 µm), for 4 h, no clear
effects were observed. Single exposures of dogs ranging from 2 to
5 h to a concentration of 75 mg carbaryl/m3 (average particle size,
5 µm) caused clinical signs of ChE inhibition (Carpenter et al.,
1961). Cats exposed to 80 mg carbaryl/m3 for 6 h showed
salivation, excitation, respiratory difficulties, and 39 - 55%
inhibition of ChE activity in the serum and 53 - 71% in the
erythrocytes. A single exposure to a level of 20 mg/m3 for 6 h did
not induce signs of intoxication, but serum- and erythrocyte-ChE
activities were slightly inhibited (Yakim, 1967).
8.2 Short- and Long-Term Exposures
A number of short-term studies have been carried out with
different carbamates and a variety of laboratory animal species.
In the context of this introduction, it is not possible to mention
all the reports available. Details on the short- and long-term
toxicities of the individual carbamates will be provided in future
Environmental Health Criteria. Most carbamates have been discussed
by the Joint FAO/WHO Meetings on Pesticide Residues and monographs
have been published (see reference list). Furthermore, the
International Agency for Research on Cancer has published a
monograph in which a number of carbamates have been evaluated
(IARC, 1976).
In the context of this introduction, a number of examples are
given to illustrate dose-response relationships and/or target
organs or tissues for a number of carbamates.
8.2.1 Oral
Short-term tests on rats or dogs exposed to repeated oral doses
of carbamate insecticides or fungicides (such as carbaryl,
propoxur, formetanate, carbofuran, and benomyl), revealed
inhibition of plasma (serum)-, erythrocyte-, and brain-ChE
(Carpenter et al., 1961; Ivanova-Chemishanska & Antov, 1976;
Krechniak & Foss, 1982).
(a) Pirimcarb
In a study on 4 dogs (2 males and 2 females) treated with 25 or
50 mg pirimicarb/kg body weight per day, for 110 weeks, 2 of the
animals developed anaemia. Haemoglobin content was decreased,
methaemoglobin was present, and the reticulocyte percentage was
significantly elevated. Levels of unconjugated bilirubin in serum
were increased. The 2 other animals were unaffected. There was
some indication that pirimicarb was able to induce the production
of immune IgG antibodies (Jackson et al., 1977).
(b) Carbaryl
Kidney alterations, such as cytoplasmic vacuolization, were
observed in the proximal tubules in short-term studies on monkeys
and rats adminstered carbaryl at dose levels of up to 600 or 300
mg/kg body weight, respectively (Coulston, 1967; cited in FAO/WHO,
1970b).
Two pigs from one litter were fed a ration containing carbaryl
at the rate of 150 mg/kg body weight for 72 and 83 days,
respectively. Three pigs in the same litter were fed 150 mg/kg
body weight for 4 weeks and, thereafter, 300 mg/kg body weight, the
total feeding period being 46 or 85 days. Two other pigs were
maintained on the basal ration, as controls. The clinical syndrome
of chronic intoxication was characterized by progressive
myasthenia, incoordination, ataxia, tremors, and chronic muscular
contractions terminating in paralysis and death. The lesions were
confined to the central nervous system and skeletal muscle.
Moderate to severe oedema of the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord was associated with
vascular degenerative changes, consistent with a vasogenic oedema
(Smalley et al., 1969). Spontaneous recovery from the effects of
sub-lethal doses occurred in about 8 h, indicating the ready
reversibility of the carbaryl-enzyme reaction (Carpenter et al.,
1961).
Carbaryl was administered to dogs at dose levels of 0, 0.45,
1.8, or 7.2 mg/kg body weight per day, for 5 days/week, for one
year. Cloudy swelling of the proximal convoluted and loop tubules
of the kidneys was found in the group given 7.2 mg/kg body weight.
There were no abnormalities in the composition of the blood, and no
ChE inhibition was found. It should be noted that the dose levels
tested were low (Carpenter et al., 1961).
In a long-term (2-year) toxicity study on CF-N rats, levels of
0, 50, 100, 200, or 400 mg carbaryl/kg diet (equivalent to 0, 2.5,
5, 10, or 20 mg/kg body weight) were tested. The highest dose
level produced cloudy swelling in the tubules of the kidneys
(Carpenter et al., 1961).
(c) Carbendazim
Groups of ChR-CD male rats (6/dose) were intubated with 200,
3400, or 5000 mg carbendazim/kg body weight per day, 5 times per
week, for 2 weeks. In all groups, the absolute and relative
weights of the kidneys were lower, and adverse effects on the
testes and reduction or absence of sperm in the epididymides were
found (FAO/WHO, 1985a).
(d) Benomyl
In a 2-year toxicity study, Charles River rats were
administered dietary levels of benomyl of 0, 100, 500, or 2500
mg/kg (equivalent to 0, 5, 25, or 125 mg/kg body weight). No
increase in mortality and no other compound-related effects on
haematology, urinalysis, organ weight, or histology were found
(Haskell Laboratories, 1969 cited in FAO/WHO, 1985a).
In another 2-year study, dogs were administered diets
containing 0, 100, 500, or 2500 mg benomyl/kg (equivalent to 0,
2.5, 12.5, and 62.5 mg/kg body weight). Liver cirrhosis, bile duct
proliferation, and testicular degeneration were found at the
highest dose level; however, 100 and 500 mg/kg diet did not show
any clear effects (Haskell Laboratories, 1970 cited in FAO/WHO,
1985a).
(e) Thiophanate-methyl
An oral toxicity study in which thiophanate-methyl at dose
levels of 0, 2, 10, 50, or 250 mg/kg body weight (in capsules) was
administered to beagle dogs (8 or 10 animals per group) was
conducted for 24 months. Retardation of growth was seen in both
sexes in the 250 mg/kg group. Both male and female dogs in the 50
and 250 mg groups showed a significant and dose-related increase in
the weight of the thyroid. However, there were no changes in the
serum-protein-bound iodine (PBI) levels and no histological
differences in the thyroid were found between the control group and
the 2 highest dose groups. No other abnormalities were observed
(Hashimoto & Fukuda, 1972 cited in FAO/WHO, 1974b).
(f) Methomyl
Groups of rats were given diets containing 0, 10, 50, 125
(increased to 500), or 250 mg methomyl/kg for 90 days. Weight gain
in animals administered 250 and 500 mg/kg was slightly suppressed.
No clear changes in the different parameters including plasma- and
erythrocyte-ChE activity were found (Kaplan & Sherman, 1977).
The same authors carried out studies over 90 days and 2 years
on beagle dogs. Dietary levels of 0, 50, 100, and 400 mg
methomyl/kg did not result in any nutritional, clinical,
haematological, urinary, biochemical, or pathological changes. In
the 2-year feeding study, histopathological alterations were seen
in kidneys and spleen of animals receiving 400 and 1000 mg
methomyl/kg diet. Histopathological changes were seen in the liver
and bone marrow in animals administered 1000 mg/kg diet (Kaplan &
Sherman, 1977).
The above-mentioned studies give some indication that
carbamates may have different mechanisms of action on target organs
that are related to substituents other than the carbamate ester
moiety (such as the imidazole moiety).
Apart from the effects on ChE activity, effects have been found
on the liver, kidneys, thyroid, and testes and also on the
activities of other enzyme systems such as ATPase, Gl-6-ase, LDH,
GPT, Alk-Pase, etc. (Haskell Laboratories, 1968 cited in FAO/WHO,
1985a; Ivanova-Chemishankska & Antov, 1976).
8.2.1.1 Further information on short- and long-term toxicity
It is not the purpose of this introductory document to provide
all the available data on long-term toxicity. However, these long-
term studies are important for the establishment of the no-
observed-adverse-effect level and consequently the Acceptable Daily
Intake (ADI) for man. Thus, it is necessary to indicate the
studies that were considered adequate and used by the different
FAO/WHO Joint Meetings on Pesticide Residues. The International
Agency for Research on Cancer has also evaluated long-term/
carcinogenicity studies of a number of carbamates. The results of
these international evaluations are summarized in Annex II and III.
From these Annexes, it is clear that aldicarb is the most toxic
of the carbamates considered; the established ADI for this compound
is 0.001 mg/kg body weight.
The ADIs for the other carbamates may vary from 0.01 to 0.1
mg/kg body weight. These quantities are equivalent to a daily
intake for man (60 kg body weight) of approximately 0.6 - 6 mg.
8.2.2 Dermal
(a) Pirimicarb
Daily dermal applications of 500 mg pirimicarb/kg body weight
to the skin of rabbits for 24 h over a 14-day period did not
produce any signs of intoxication (FAO/WHO, 1977b).
(b) Methomyl
Methomyl applied to the intact skin of rabbits at a repeated
rate of 200 mg/kg per day caused mild signs of intoxication such as
nasal discharge, wheezing, and transient diarrhoea. All animals
survived 15 applications. However, when applied on the abraided
skin, much more serious systemic signs were seen, such as nasal
discharge, salivation, tremors, poor coordination, and abdominal
hypertonia; a few animals died (Kaplan & Sherman, 1977).
(c) Carbaryl
Application of carbaryl at 500 mg/kg body weight to the skin of
6 rabbits resulted in 39% inhibition of serum-ChE activity and 36%
inhibition of red cell-ChE activity during the first 24 h following
treatment. ChE activity returned to initial levels in 72 h (Yakim,
1967).
8.3 Skin and Eye Irritation; Sensitization
8.3.1 Skin irritation
Rabbits exposed to a single dermal application of carbaryl at a
concentration of 100 g/litre in acetone did not show any skin
irritation (Carpenter et al., 1961).
8.3.2 Eye irritation
Undiluted carbaryl or solutions of different concentrations
were applied to the eyes of rabbits. Technical carbaryl applied in
excess as a 100 g/litre suspension in propylene glycol produced
mild injury. A 25% aqueous suspension of a microfine material did
not cause any injuries; 50 mg of the dust caused only traces of
corneal necrosis (Carpenter et al., 1961).
8.3.3 Skin sensitization
(a) Carbaryl
Groups of albino guinea-pigs were given 8 intracutaneous
injections (3 per week) of 0.1 ml of a 0.1% dispersion of carbaryl
in 3.3% propylene glycol. After a 3-week incubation period,
followed by a challenge dose, no clear sensitization reaction was
noticed (Carpenter et al., 1961).
(b) Benomyl and thiophanate-methyl
It has been shown that benomyl and thiophanate-methyl can cause
slight reversible skin irritation and weak sensitization in guinea-
pigs (FAO/WHO, 1974, 1985a).
8.4 Inhalation
(a) Carbaryl
The inhalation toxicity of carbamates for mammals has not been
widely studied.
The effects of acute inhalation exposure to carbaryl dust have
been examined in rats (10 mg/m3), guinea-pigs (390 mg/m3), and dogs
(75 mg/m3) (Carpenter et al., 1961) (section 8.1.3). No treatment-
associated, gross or microscopic lesions were seen in guinea-pigs
and dogs, even though the exposure was sufficient to cause ChE
inhibition. Haemorrhagic areas in the lung were seen in guinea-
pigs at the highest level tested (390 mg/m3).
Four cats exposed to carbaryl in air at 63 mg/m3 for 6 h per
day for 1 month developed signs of cholinergic stimulation during
the first 2 h of exposure each day. Periodic salivation was noted.
One cat died with marked signs of poisoning on day 20. There was
already a significant decrease in serum- and erythrocyte-ChE
activity after the first exposure. Exposure to 40 mg/m3 for 6 h
per day for 2 months was reported to impair conditioned reflexes in
cats. On certain days, the ChE activity dropped to below 50% of
pre-exposure values. Exposure to concentrations of 16 mg/m3, for 6
h per day, for 4 months did not result in any signs of toxicity
(Yakim, 1967). Carpenter et al. (1961) did not find any increase in
mortality or "grossly-visible injury" when rats were exposed to a
mean concentration of 10 mg/m3 (range 5 - 20 mg/m3), for 7 h per
day, 5 days/week, over 90 days.
(b) Propoxur
Male and female rats were exposed to propoxur aerosols at
average concentrations of 0, 5.7, 10.7, or 31.7 mg/m3, for 6 h
daily, 5 days/week, over a period of 12 weeks. Depression of
plasma-, erythrocyte-, and brain-ChE activity was observed at the
highest dose level (Kimmerle & Iyatomi, 1976).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
A considerable number of reproduction and teratogenicity
studies have been carried out on different carbamates in several
animal species. A number of these will be used to show the state
of the art giving the well-known carbamates, such as carbaryl,
benomyl, carbendazim, and thiophanate-methyl, as examples.
For more details concerning different carbamates, the reader is
referred to the reports of the Joint FAO/WHO Meetings on Pesticide
Residues and, in the future, to the Environmental Health Criteria
Documents on the individual carbamates.
8.5.1 Reproduction
(a) Methomyl
A 3-generation reproduction study with methomyl was carried out
on rats. Dose levels of 0, 50, or 100 mg/kg diet were administered
for 3 months. There was no evidence from the different indices of
reproduction, lactation, and weanling body weights to suggest that
methomyl affected reproduction or lactation performance in the
litters of three generations (Kaplan & Sherman, 1977).
(b) Benomyl
The effects of benomyl on the gonads and reproductive function
were studied in a 3-generation study on rats. Pronounced effects,
such as a decrease in fertility and decreased activities of a
number of enzymes in the testes were found in rats treated orally
with 1000 mg/kg body weight (the only dose level tested), 5
days/week, for 4 months. Besides the effects mentioned, a
decreased gestation index was also found, which may indicate early
embryonic death due to the induction of a dominant lethal gene in
the sperm cells. An increase in mortality and decreased viablility
and fertility were found in the F1 - F3 generations (not exposed to
the carbamate) (Ivanova-Chemishanska & Antov, 1980; Ivanova-
Chemishanska et al., 1980). In a second 3-generation rat
reproduction study with benomyl, no differences in reproduction or
lactation were observed among control and test groups of animals at
the highest dietary level of 2500 mg/kg diet. No pathological
changes were found in tissues from weanling pups of the F3b litter
(Sherman et al., 1975).
(c) Carbendazim
From 3 multigeneration reproduction studies on rats, in which
carbendazim was tested at dose levels of up to 10 000 mg/kg diet,
no apparent adverse effects on reproduction were found at levels of
up to 2000 mg/kg diet. A reduction in average litter weights was
observed at dietary levels of 5000 and 10 000 mg/kg (FAO/WHO,
1985a).
(d) Carbaryl
In 3-generation rat studies, carbaryl added to the diet to
provide daily doses of up to 10 mg/kg body weight, did not result
in any statistically-significant dose-related effects on fertility,
gestation, viability of pups, or lactation (Weil et al., 1972).
In other studies by Weil et al. (1972, 1973), groups of rats
received carbaryl in the diet at daily doses of 0, 3, 7, 25, or 100
mg/kg body weight by intubation, for 5 days/week, or 0 or 100 mg/kg
body weight per day in the diet. Significant effects were found
on reproduction (mortality and reduced fertility) in the group
receiving 100 mg/kg. A group receiving 200 mg/kg diet did not show
any effects.
The influence of carbaryl at dose levels of 2 or 5 mg/kg body
weight on the reproductive function of rats was studied by
Shtenberg & Otovan (1971). Both dose levels produced adverse
effects on the testes and ovaries (atrophic, dystrophic and
necrotic changes) and changed the gonadotropic function of the
hypophysis. The changes observed increased progressively over the
generations. The fecundity of test females fell, the number of
still-born offspring increased, and there was a high death rate
among young animals.
Carbaryl was fed at dietary levels of 0, 2000, 5000, or 10 000
mg/kg feed to Osborne-Mendel (10 males and 10 females) rats for 3
generations. At the highest dose level, fertility was impaired and
no litters were produced from the second mating of the second
generation. Marked effects from birth to day 4 were manifested as
seen in the survival index. Dose-related decreases in average
litter size, number of live-born progeny, number of survivors to
day 4, and number weaned were observed.
In a comparative study on gerbils, dietary levels of 0, (40
animals) 2000, 4000, 6000 (each 30 animals), and 10 000 mg
carbaryl/kg (18 animals) were tested for 3 generations. No litters
were produced from the second mating of the third generation, at
the highest dose level. Gerbils appeared to be more sensitive than
rats since significant decreases were found consistently at lower
dietary levels, but the effects were not clearly dose-related.
Effects in both species at 2000 mg/kg diet were still marginal but
significant (Collins et al., 1971).
(e) Thiophanate-methyl
Thiophanate-methyl was tested in a 3-generation reproduction
study on rats at dose levels of 0, 40, 160, or 640 mg/kg diet. The
highest dose level affected growth, but no effects of the compound
on reproduction parameters were apparent (FAO/WHO, 1974b).
(f) Carbofuran
A 3-generation reproduction study was conducted on rats (8
males and 16 females per group), with dietary levels of 0, 1, 10,
or 100 mg carbofuran/kg diet. The highest dose level resulted in
decreased weight gain in the parents and a marked reduction in
survival of the young. Lower levels did not have any effects.
Follow-up studies with lower dose levels showed that 20 mg/kg diet
induced adverse effects on reproduction variables, noted after the
first generation (McCarthy et al., 1971; FAO/WHO, 1977b, 1981b).
8.5.2 Endocrine system
(a) Carbaryl
Short-term studies on male and female rats were carried out to
study the functioning of the reproductive system under the
influence of carbaryl. The dose levels ranged from 2 to 300 mg
carbaryl/kg body weight and were administered over periods of 3 -
12 months; in one study, 4 generations were bred. Different
criteria were studied, such as reduction of sperm motility,
duration of sperm survival, inhibition of spermatogenesis and
oögenesis, estrous cycle, induction of degeneration of the germinal
epithelium, and the activities of different enzymes in the testes
and ovaries. Shtenberg & Rybakova (1968) found marked functional
and morphological changes in the endocrine organs of rats
administered carbaryl at daily doses of 7, 14, or 70 mg/kg body
weight for up to 12 months. It was considered by the authors that
the effects of carbaryl on the testes and ovaries might be
explained by the observed increase in hypophyseal gonadotropic
function. A direct effect on the testes and ovaries cannot be
excluded.
Orlova & Zhalbe (1968) studied the effects of carbaryl on white
rats. Dose levels of 2.5 or 5 mg/kg body weight were administered.
Disturbances in the enzymatic activity in the testes and ovaries,
inhibition of spermatogenesis and oögenesis, and decreased
fertility were observed in the P generation, and a decrease in
viability, in the F1 generation.
Shtenberg & Otovan (1971) studied this effect of carbaryl in
more detail in a 4-generation study. The dose levels studied were
0, 2, and 5 mg/kg body weight, and were applied for 6 months in
each generation. The disturbances in the function of the testes
and ovaries described above were found at both 2 and 5 mg/kg
(Vashakidze, 1965, 1967; Shtenberg & Rybakova, 1968; Shtenberg &
Otovan, 1971; Shtenberg et al., 1973). However, the studies
reporting adverse effects on spermatogenesis in male rats were not
confirmed in studies on mice with carbaryl by Dikshith et al.
(1976) or in reproductive performance studies on rats.
(b) Benomyl
A study on the short-term toxicity of benomyl carried out by
Torchinsky et al. (1976) showed that the testis of Wistar rats was
the organ most affected. When administered over a 12-month period,
a dose of 62.5 mg/kg body weight showed borderline effects; 12.5
mg/kg body weight did not show an effect. A recent study on
Sprague Dawley rats receiving 10 daily treatments of 0, 200, or 400
mg benomyl/kg body weight, by gavage, showed a significant decrease
in the total epididymal sperm counts and sperm concentrations in
the vas deferens at both levels. At the higher dose level, slight
to severe hypospermatocytogenesis and generalized
hypospermatogenesis were observed (Carter & Laskey, 1982; FAO/WHO,
1985a). Pastushenko (1983) studied the gonadotoxic effects of
benomyl in rats exposed to dose levels of 0.5, 1.2, 5.0, or 12.4
mg/m3 air. The 2 highest dose levels showed dose-related changes
in the gonads, i.e., disturbances of the morphological and
functional states. At 1.2 mg/m3, only morphological changes were
observed. No effects were found with 0.5 mg/m3.
(c) Carbendazim
When Sprague Dawley rats were fed diets containing 0, 2000,
4000, or 8000 mg carbendazim/kg diet for 28 days, degeneration of
testicular tissue was observed at 4000 and 8000 mg/kg diet.
Spermatogenesis and oögenesis were affected at these feeding
levels. Changes were also found in the organ weights relative to
body or heart weight, changes in the liver being observed at 2000
mg/kg diet (FAO/WHO, 1974b).
No effects on testicular weights and morphology were found in a
study on groups of ChR-CD rats fed carbendazim in the diet for 90
days at dose levels of 0, 100, 500, or 2500 mg/kg (FAO/WHO, 1985a).
8.5.3 Embryotoxicity and teratogencity
A number of carbamate esters have been tested for
teratogenicity with the chicken embryo bioassay (Marliac, 1964;
Ghadiri & Greenwood, 1966; Khera, 1966; Ghadiri et al., 1967;
Rybakova, 1968; Olefir & Vinogradova, 1968; Proctor et al., 1976;
Moscioni et al., 1977). However, this test is not considered of
relevance in testing for teratogenicity, and the results will not
be discussed in this document.
(a) Propoxur
Roszkowski (1982) studied the effects of propoxur on
intrauterine development and on the state of ossification of rat
fetuses. An embryotoxic effect was found at a dose of 20% of the
LD50, administered on days 7 - 19 of the gestation period; 11.9% of
anomalies occurred in the fetuses of treated animals compared with
6.3% in the controls. Decreased weight was also found in the
fetuses. Ossification was disturbed, but this was not related to
the period of gestation during which the substance was
administered. A decrease in the concentrations of calcium and
magnesium was found in the serum of mothers whose fetuses had shown
bone anomalies.
(b) Benomyl
In a teratogenicity study, pregnant ChR-CD rats were fed a diet
containing 0, 100, 500, 2500, or 5000 mg benomyl/kg from day 6 to
day 15 of gestation; no effects were found on the outcome of
pregnancy or embryonal development (Sherman et al., 1975).
Hazleton Laboratories (1968) cited in FAO/WHO (1985a) reported
similar negative results for rabbits, at dose levels up to 500 mg
benomyl/kg diet administered on days 8 - 16 of gestation.
Torchinsky (1973) studied the influence of carbaryl (106 mg/kg
body weight, daily, from day 1 to day 20 of pregnancy) and benomyl
(single dose of 145 or 250 mg/kg body weight on day 12 of
pregnancy) in relation to the protein content of the diet. The
animals were kept on diets containing 5, 10, 19, or 35% of protein
either for one month prior to mating and during the entire
pregnancy or only during pregnancy. The teratogenic and embryotoxic
actions of benomyl and carbaryl did not differ in groups where the
females received diets containing 5, 10, or 19% protein from the
beginning of pregnancy. An increased post-implantational lethality
for the embryos was noted in females kept on a diet with 5% protein
from one month before conception. When these animals were exposed
to carbaryl, the weight of 20-day-old fetuses declined
considerably, yet the severity of the teratogenic action of benomyl
did not increase under these conditions. The teratogenic action of
benomyl and the embryotoxic action of carbaryl were less severe in
animals fed diets containing 10% of protein from one month before
conception than in the groups kept on diets containing 19 or 35%
protein. The results of a few other studies have indicated that
benomyl causes embryotoxic and teratogenic effects in both rats and
mice when administered orally (by gavage) at dose levels at 62.5
mg/kg body weight (rat) and 100 mg/kg body weight (mouse) or more.
At lower dose levels (31.2 mg for the rat and 50 mg/kg for the
mouse), no teratogenic effects were observed. A broad spectrum of
malformations such as exencephaly, hydrocephaly, and
meningocephaly, and disturbance of the CNS were found (Ruzicska et
al., 1975; Petrova-Vergieva, 1979; Kavlock et al., 1982).
(c) Carbaryl
A number of teratogenicity studies carried out with carbaryl
gave contradictory results. Weil et al. (1972) administered up to
500 mg carbaryl/kg body weight to rats. No teratogenic effects or
effects on fertility or gestation were seen at this high dose
level; however, the mean body weight gain of the females during
pregnancy was decreased. Many pups died before weaning. No effects
were found with administration of carbaryl at 100 mg/kg body weight
(Weil et al., 1972).
Oral administration of carbaryl to pregnant rabbits and
hamsters at doses of up to 250 mg/kg body weight, by intubation,
did not cause any teratogenic effects or dose-related fetal
mortality (Weil et al., 1973).
Dougherty et al. (1975), cited in FAO/WHO (1974b), did not
observe any teratogenic effects in monkeys given doses of carbaryl
of 0, 0.2, 2, or 20 mg/kg body weight from day 18 to day 40 of
gestation. This study indicated that even the highest dose level
did not induce a reproductive hazard.
Vashakidze (1965, 1967) found reduced reproduction and abnormal
fetal changes with intubated dose levels of 100 mg/kg body weight
and higher. A level of 50 mg/kg produced negative results.
Carbaryl, administered to guinea-pigs orally during organogenesis
(10 consecutive days between days 11 and 20 of gestation) at 300
mg/kg body weight, induced increased maternal and fetal mortality
and offspring with axial skeletal defects, such as cervical
vertebrae fusion. No abnormal fetal changes were produced in
hamsters at the sub-lethal levels of 125 and 250 mg/kg body weight.
In rabbits, levels ranging from 50 to 200 mg/kg body weight did not
produce any effects on mortality or morbidity, and no abnormal
fetal changes were seen (Robens, 1969).
Smalley et al. (1968) conducted studies on pregnant beagle dogs
fed a diet containing 0, 3.125, 6.25, 12.5, 25, or 50 mg
carbaryl/kg body weight from mating to the termination of weaning.
Effects observed included a number of animals with dystocia
(difficult births) due to atonic uterine musculature, an apparent
contraceptive effect at the highest dose level, and teratogenic
effects (different types of severe defects) at all but the lowest
dose level.
The effects of carbaryl on rat reproduction and guinea-pig
teratology were compared by Weil et al. (1973). A 3-generation
reproduction study on rats included groups receiving carbaryl at
maximum daily doses of 200 mg/kg diet. Other groups concurrently
received daily oral intubation doses as high as 100 mg/kg body
weight. In the guinea-pig teratology study, maximum levels of 300
mg/kg diet or 200 mg/kg body weight (intubation) were given. The
concurrent administration of carbaryl by daily oral administration
or by dietary inclusion did not result in either teratological or
mutagenic effects in the rat or teratological effects in the
guinea-pig, at the maximum dosage levels. However, the groups
receiving maximum levels orally showed severe toxic effects in both
studies compared with little or no effect with oral administration
of 25 mg/kg in rats and 100 mg/kg in guinea-pigs.
The authors concluded that the severe effects found in rats by
Shtenberg & Otovan (1971) following repeated oral intubation of
carbaryl at 2 mg/kg body weight could not be confirmed, even at the
maximum oral intubation level of 100 mg/kg body weight.
(d) Methomyl
When rabbits were fed dietary levels of 0, 50, or 100 mg
methomyl/kg on days 8 - 16 of gestation, no skeletal or visceral
abnormalities were seen in the fetuses (Kaplan & Sherman, 1977).
(e) Carbofuran
Teratogenicity studies have been carried out on albino rats and
albino rabbits with carbofuran. In rats, dose levels of 0, 0.1,
0.3, or 1.0 mg/kg body weight were administered throughout
gestation (days 6 - 15 of pregnancy) and, in rabbits, levels of 0,
0.2, 0.6, or 2.0 mg carbofuran/kg body weight were administered
from day 6 to day 18 of gestation. In both animal species, the
highest dose level showed clear signs of poisoning. However,
pregnancy, viability, fetal body weights, and all the other
examinations did not show any effects. All the progeny were
normal. No teratogenic effects were seen in rats or rabbits
(McCarthy et al., 1971; FAO/WHO, 1981b).
(f) Carbendazim
Carbendazim has been shown to be embryotoxic at dose levels of
approximately 19 mg/kg body weight or more. This effect was not
seen at 10 mg/kg body weight (Delatour & Richard, 1976).
8.6 Mutagenicity and Related End-Points
There is little evidence, if any, to suggest that methyl
carbamate compounds are mutagenic. An evaluation of the mutagenic
potential of carbaryl, methomyl, propoxur, carbofuran, and
pirimicarb, using a variety of microorganisms as indicator strains,
including strains of Salmonella typhimurium, Escherichia coli,
Saccharomyces cerevisiae, and Bacillus subtilus, produced
negative results. These studies showed that carbamate compounds
have little potential for presumptive gene mutations in higher
animals.
Anderson et al. (1972) examined barban, chloropropham, propham,
and swep for their ability to induce point mutations in 8
histidine-requiring strains of S. typhimurium. None showed induced
mutations. This study was carried out in the absence of any
metabolic system.
The chemical structure of benomyl probably enables it to act
both as a base analogue for DNA (benzimidazole ring incorporates
into nucleic acids instead of guanine) and as a spindle poison
(carbamate groups) (Seiler, 1975, 1976b; FAO/WHO, 1985a). The
binding of benomyl to microtubular proteins disturbs the mitotic
spindle, thus causing malsegregation of chromosomes during the
mitotic anaphase (Morris, 1980, cited by Finnish National Board of
Health Toxicology Group, 1982). The results of several studies
suggest that benomyl is a strong antimitotic agent which, even in
low concentrations, induces non-disjunction of single chromosomes
leading to aneuploidy (FAO/WHO, 1985a).
The induction of low-frequency forward mutations by carbendazim
in S. typhimurium strain LT-2 was reported by Seiler (1972).
Reversions reported to occur in S. typhimurium strains His G46 and
TA 1530 were attributed to base-substitution mutations caused by
carbendazim.
Another study was made with the WP2 uvrA repair-deficient
strain of E. coli and the TA 1535 repair-deficient strain of
S. typhimurium. Mutagenic activity was detected for benomyl in the
former, but not in the latter or in TA 1538 (Kappas et al., 1976).
No effects were found in the CM-611 strain of E. coli, deficient
in either the excision repair pathway or the lex A+-dependent
misrepair pathway. In studies carried out recently, benomyl was
negative in all S. typhimurium strains used, even though, in some
of the repeats, significant increases in the number of revertants
were observed in TA 1535 strain. Carbendazim was clearly mutagenic
in the TA 98 strain with metabolic activation (S-9 mix) and
slightly mutagenic in the TA 1535, TA 1537, and TA 100 strains. A
positive result for carbendazim in the TA 1537 strain was observed,
also without metabolic activation (Haskell Laboratories, cited by
the Finnish National Board of Health, 1982; FAO/WHO, 1985a).
There is evidence that carbendazim and related compounds are
antimitotic agents and cause mitotic arrest, mitotic delay, and a
low incidence of chromosome damage in SPF Wistar rats and mammalian
cell lines. The mutagenic effects of carbendazim in bacteria are
probably due to base substitutions, resulting from the
incorporation of small quantities of benzimidazole into nucleic
acids. The effects on mammalian cells in vivo and in vitro
indicate that carbendazim causes chromosome damage, which may
result from this type of mutagenic activity, but the effects on
mitosis suggest that, in higher organisms, the main genetic effect
is to cause non-disjunction (Styles & Garner, 1974).
An abnormal number of micronucleated erythrocytes have been
observed in the bone marrow of mice treated orally with carbendazim
at 100 mg or more/kg body weight. No effects were seen at 50
mg/kg. Benomyl at 1000 mg/kg body weight produced a slight
increase in micronucleated erythrocytes.
Carbendazim did not induce chromosome breakage in Chinese
hamster bone-marrow cells at oral doses as high as 1000 mg/kg body
weight (Seiler, 1976a). It was concluded that carbendazim is not
a chromosome-breaking agent, and its micronucleic action is
attributable to antimitotic activity caused by inhibition of
spindle formation or spindle function.
An evaluation of the mutagenic properties of benomyl has been
completed and reported by the US Environmental Protection Agency
Pesticide Genotoxicology Program. Benomyl increased the mutation
frequency in a concentration-related manner in L 51784 mouse
lymphoma cells and induced sister chromatid exchange in Chinese
hamster ovary (CHO) cells. Benomyl was also positive at all dose
levels tested in the in vivo micronucleus test with mice. The
results of the spot test conducted on mice support the idea that
carbendazim (oral application of 100 - 300 mg/kg body weight to the
mother on the 10th day following conception) may be mutagenic in
mammalian cells due to a direct action on DNA (Fahrig & Seiler,
1979).
A dominant lethal study to evaluate the potential mutagenic
effects in the reproductive cycle of the male mouse was conducted
with propoxur at dose levels of 0, 2.5, or 5 mg/kg body weight.
For a period of 6 weeks, 3 virgin females were exposed to each male
at weekly intervals and reproduction indices recorded. The
administration of propoxur proved to have a transient effect on the
reproductive capability of the males but there was no sign of early
resorption indicating a mutagenic effect (FAO/WHO, 1974b).
DNA-repair tests (with both isolated rat and mouse hepatocytes)
were conducted using benomyl and carbendazim. All tests showed
negative results for the induction of DNA repair (Haskell
Laboratories, 1981a,b cited in FAO/WHO, 1985a).
The Finnish National Board of Health (1982) summarized the
mutagenicity data on benomyl, carbendazim, and thiophanate-methyl
(Table 7) and concluded that the results were sometimes
contradictory. However, positive mutagenicity results from point
mutation tests and chromosome aberration tests are well documented,
especially in higher organisms, and it can be concluded that
carbendazim fungicides should be considered as weak mutagenic
compounds.
Without going into detail, it is known that a large number of N
-nitroso derivatives of methyl carbamates are potent mutagens. The
N -nitroso derivatives of aldicarb, propoxur, carbofuran,
trimethacarb, and methomyl caused numerous single-strand breaks in
normal human skin cells. The data suggested that human cellular
DNA in vivo was irreversibly altered resulting in numerous alkali-
sensitive bonds (Blevins et al., 1977).
A similar result was obtained with N -nitroso carbaryl. This
compound showed strong mutagenic activity in 2 bacterial systems,
E. coli and Haemophilus influenzae. Using S. typhimurium, N -
nitroso carbaryl acted as a potent base-pair substition mutagen and
showed a relatively mild frameshift activity. The parent compound,
carbaryl, gave negative results (Regan et al., 1976; FAO/WHO,
1982b).
Uchiyama et al. (1975) tested the nitroso derivatives of BPMC,
propoxur, hoppcide, isoprocarb, MPMC, and MTMC, in the back
mutation test with E. coli B/r WP2 try- and in the rec-assay
method with B. subtilus strains. The N -nitroso derivatives of
all these N -methylcarbamates showed potent mutagenic activity.
Table 7. A summary of the mutagenicity data on benomyl, carbendazim (MBC), and
thiophanate-methyl
----------------------------------------------------------------------------------------
Point mutations Genetic change
Chromosomal aberrations Non-disjunction
----------------------------------------------------------------------------------------
Positive results + + +
Salmonella typhimurium rat fetus
(Seiler, 1972) (Ruzicska et al., 1975)
Salmonella typhimurium rat bone marrow rat bone marrow
(Haskell Laboratories, 1982 (Styles & Garner, 1974) (Styles & Garner, 1974)
cited in FAO/WHO, 1985a)
mouse bone marrow
(Seiler, 1976a)
Escherichia coli Aspergillus nidulans
(Kappas et al., 1976) (Kappas et al., 1974;
Bignami et al., 1977)
Fusarium oxysporum
(Dassenoy & Meyer, 1973)
Mouse/spot-test Chinese hamster ovary (SCE)
(Fahrig & Seiler, 1979) (SRI International, 1980,
cited in FAO/WHO, 1985a)
micronucleus in vivo /mouse
(SRI International, 1980,
cited in FAO/WHO, 1985a)
Negative results - - -
Streptomyces coelicolor rat bone marrow dominant lethal/rat
(Carere et al., 1978) (Ruzicska et al., 1975) (Sherman et al., 1975)
Salmonella typhimurium Chinese hamster bone marrow dominant lethal/mice
(Fiscor et al., 1978) (Seiler, 1976a) (Makita et al., 1973)
(Carere et al., 1978)
Salmonella typhimurium lymphocyte culture-cytotoxicity
(Haskell Laboratories, 1981; obs.
cited in FAO/WHO, 1985a) (Lamb et al., 1980)
Aspergillus nidulans benomyl-workers
(Bignami et al., 1977) (Ruzicska et al., 1975)
Drosophila /recessive lethal hepatocyte culture/DNA-repair
- sterility obs. rat, mouse
(Lamb & Lilly, 1980) (Haskell Laboratories, 1981,
cited in FAO/WHO, 1985a)
human lymphocyte culture
(Gupta & Legator, 1975,
cited in FAO/WHO, 1985a)
----------------------------------------------------------------------------------------
From: The Finnish National Board of Health (1982) FAO/WHO (1985a).
8.7 Carcinogenicity
8.7.1 General
The mechanism of the carcinogenic action of ethylcarbamate
(urethane) has not yet been established, but its structure seems to
be optimal. All changes seem to reduce activity, particularly when
the ethyl group is replaced with larger side chains. The addition
of alkyl groups to the nitrogen also reduces activity (Mirvish,
1968). It is not so clear why urethane is carcinogenic, whether it
is the proximal toxin or whether it has to be converted to an
active metabolite. The low carcinogenic potential of esters of N -
substituted carbamic acids is possible.
In 1976, a Working Group of the International Agency for
Research on Cancer (IARC, 1976) reviewed the available data on a
number of carbamate pesticides included carbaryl, propham,
chloropropham, and mexacarbate.
Carbaryl was tested in a number of carcinogenicity tests some
of which were not relevant or were of inadequate design. The rat
study (40 per group), in which carbaryl was administered at dose
levels of 0, 50, 100, 200, or 400 mg/kg diet for 2 years, did not
give any indication of an increase in tumour incidence (Carpenter
et al., 1961; Innes et al., 1969; Andrianova & Alekseev, 1970;
IARC, 1976).
Groups of 48 male and 48 female CD-1 mice were given 0, 100, or
400 mg carbaryl/kg diet. After 80 weeks, the tumour incidence
between the groups was similar (Mellon Institute, 1963 cited in
FAO/WHO, 1965b).
Mexacarbate was administered orally to 2 strains of mice. The
design of this study was inadequate (only a small number of
animals), but an increased incidence of lung adenomas was shown in
both strains, together with a slight increase in liver tumours in a
number of males of one strain. According to IARC (1976), it was
not possible to make an evaluation of the carcinogenicity of
mexacarbate on the basis of this study by Innes et al. (1969).
Propham and chlorpropham were administered via the oral route
in a number of carcinogenicity tests on mice, rats, and hamsters.
One or both compounds were also tested on mice and/or rats via the
subcutaneous, intraperitoneal, or intrapleural route. The design
of some of these studies was inadequate. Propham and chloropropham
were tested for initiation properties, because the ethyl carbamates
are related to the well-known carcinogen ethylcarbamate (urethane),
which is an initiator of skin tumours. There was no evidence that
propham and/or chloropropham were carcinogenic after oral,
subcutaneous, intraperitoneal, and intrapleural application.
However, these two compounds acted as weak initiators in the two-
stage carcinogenicity studies on mice. A second study by the same
authors did not confirm the results (Van Esch et al., 1958;
FAO/WHO, 1965b; Innes et al., 1969; Van Esch & Kroes, 1972; IARC,
1976).
Long-term studies on rats did not provide any evidence of
carcinogenic activity when propoxur was fed in the diet for 2 years
at levels of 0, 250, 750, 2000, or 6000 mg/kg diet. Increased liver
weight was found at the highest dose level (6000 mg/kg) in males
and in the three highest dose levels in females. This liver change
was not reflected in liver function tests, clinical chemical
examinations, or histological changes (FAO/WHO, 1974b). Similarly,
long-term and 2-year studies on rats, dogs, mice given carbofuran
or pirimicarb did not indicate any carcinogenic potential. The
dose levels tested were 175 mg/kg diet for rats and 1.8 mg/kg body
weight for dogs for pirimicarb, 100 mg/kg diet for rats, and 500
mg/kg diet for mice (24 months in mice) for carbofuran (FAO/WHO,
1977b, 1981b). On the basis of a variety of test protocols, these
carbamate compounds did not show any significant carcinogenic
potential.
Benomyl was tested for carcinogenicity in CD-1 mice, at dose
levels of 0, 500, 1500, or 5000 mg/kg diet (approximately
equivalent to 0, 50, 150, or 500 mg/kg body weight). A significant
dose-dependent increase in the incidence of primary hepatocellular
adenomas and carcinomas in female mice was seen. Furthermore,
histopathological changes occurred in a number of organs (Haskell
Laboratories, 1981 cited in FAO/WHO, 1985a).
Low dose levels of thiophanate-methyl (0 - 640 mg/kg diet,
equivalent to approximately 0 - 64 mg/kg body weight) were tested
for carcinogenicity in ICR-SCL mice. This study showed that
ingestion of 640 mg thiophanate-methyl in the diet by a mouse
strain susceptible to various tumours did not affect carcinogenic
activity. In another study on rats, dose levels of 0, 10, 40, 160,
or 640 mg/kg diet did not have any apparent effects on the
occurrence of tumours; the only significant effects appeared to be
decreased growth at the highest dose levels (Hashimoto & Fukuda,
1972, cited in FAO/WHO, 1974b).
The influence on the testes of ChR-CD rats of carbendazim
administered at dose levels of 0 - 10 000 mg/kg diet was studied
over a period of 2 years. However, the incidence of testicular
changes was so high in the two control groups that no opinion could
be given on the possible influence of carbendazim on the testes
(Haskell Laboratories, 1977 cited in FAO/WHO, 1985a).
Carbendazim at dose levels of 0, 150, 300, or 1000 mg/kg diet
(this dose level was increased to 2000 mg/kg at week 4 and to 5000
mg/kg at week 8 for the remainder of the study) was administered to
SPF-Swiss mice. The duration of the study was 80 weeks. At the
highest dose level, neoplastic nodules were found in the livers of
female mice. In males, there was an increased incidence of
hepatoblastomas (CIVO/TNO, 1976 cited in FAO/WHO, 1985a).
A 2-year feeding study on CD-1 mice was conducted using
carbendazim at dose levels of 0, 500, 1500, or 7500 mg/kg diet
(only females completed the 2-year period, the 7500 mg/kg level for
males was decreased to 3750 mg/kg after the first 15 months of the
study and the final sacrifice of this group was antedated by 7
months). A significant increase in hepato-cellular carcinomas
occurred at 1500 mg and 7500 mg/kg in female mice and at 1500 mg/kg
in male mice (at the highest dose level for males, there were too
few survivors to judge the effects) (Haskell Laboratories, 1982;
FAO/WHO, 1985a).
Other studies demonstrated that carbamates in the presence of
nitrite could be converted to N -nitroso derivatives, which may be
(partly) responsible for the "carcinogenic activity of the
carbamates".
Studies in which carbendazim was administered to Swiss mice,
twice, at 250 mg/kg body weight, intragastrically, weekly, in
combination with 5000 mg sodium nitrite/litre drinking-water caused
a slight increase in the incidence of lymphosarcomas (Börzsönyi &
Csik, 1975; Börzsönyi & Pinter, 1977).
The results of an in vitro study of Beraud et al. (1979)
(IARC, 1976) suggested that nitrosocarbaryl could be produced in
rat gastric juice from carbaryl.
The proposal that carbamate pesticides may be converted to N -
nitroso compounds under the acid conditions of the stomach is based
on the results of in vivo nitrosation studies with secondary
amines and other compounds in the presence of nitrite (originating,
for instance, from certain vegetables and present in saliva)
(Tannenbaum et al., 1974). Elespuru & Lijinsky (1973), Eisenbrand
et al. (1974, 1975a,b), Elespuru et al. (1974), Ungerer et al.
(1974), Quarles & Tennant (1975), Lijinsky & Taylor (1976), and
Oliver (1981) have shown that certain pesticides, such as carbaryl
and dithiocarbamates, can be nitrosated in vivo.
As pointed out by IARC (1976), the extrapolation of findings in
experimental animals to man is complicated by many factors. It is
relatively easy to show that N -nitroso derivatives can be formed
and that these are mutagenic and/or carcinogenic. However, the
crucial information is the quantity produced in man under the
prevailing conditions. The concentration of both reactants,
differences in pH, the influence of competing reactions, and the
presence of accelerators and inhibitors are all important. In
addition to these difficulties in defining potential human
exposure, the susceptibility of man has to be compared with that of
experimental animals. General considerations on N -nitrosatable
pesticides are described in IARC (1983).
8.8. Special Studies
Adequate characterization of the neurobehavioral effects of
carbamates is still incomplete or not available. In general, when
given in single doses the ChE-inhibiting carbamates decrease
activity and schedule-controlled behaviour, interfere with
avoidance and escape performance, and decrease the amplitude of a
variety of electrophysiological measures. The possible
interference with learning and memory by the carbamates has been of
interest because of the hypothesized role of the cholinergic system
in these functions. However, the evidence that these compounds
interfere with learning and memory is equivocal (Miller, 1982).
Carbamates interfere with shock-motivated behaviour including
discrete-trial and one-way avoidances as well as shock-induced
suppression. Furthermore, these compounds decrease responsiveness
to shock (Sideroff & Santolucito, 1972).
The effects of the ChE-inhibiting carbamates have been
evaluated in man. However, these studies have been mainly
concerned with correlating ChE inhibition and overt symptoms. It is
assumed that, if the clinical indicators (such as ChE levels) are
normal after pesticide exposure, or if symptoms associated with
cholinergic overstimulation are absent, then no risk is involved
(Miller, 1982).
9. EFFECTS ON MAN
The health hazards from overexposure to carbamate insecticides
occur primarily as a result of the inhibition of the enzyme ChE.
ChE inhibition leads to the accumulation of endogenous ACh and
other choline esters in the organism. This produces signs and
symptoms as specified in section 9.3.
9.1 General Population Exposure
9.1.1 Acute toxicity: poisoning incidents
Carbamate pesticides have occasionally been used in cases of
suicide. Fatal poisonings have occurred with carbaryl, propoxur,
and mexacarbate. Farago (1969) described a case involving a 39-
year-old man who drank 1/2 litre of carbaryl. He entered the
hospital 1 1/2 h later and received gastric lavage, but developed
serious respiratory depression. His condition improved somewhat
after 4 injections of atropine at 1/2-h intervals. Three hours
after the carbaryl ingestion, 250 mg of 2-PAM intravenous was
administered. His lung condition rapidly worsened and he died.
Administration of 2-PAM is contraindicated for cases of carbaryl
poisoning, as it may increase toxicity rather than reactivate the
inhibited enzyme and, in this case, it was concluded that the fatal
outcome, though unavoidable, was hastened by 2-PAM administration.
Reich & Welke (1966) reported a fatal case of mexacarbate
poisoning involving a 17-year-old nursery employee who ingested
approximately 55 g mexacarbate in the form of a 22% formulation.
The young man, found unconscious, had pinpoint pupils and an
irregular heartbeat. Emergency procedures at the hospital briefly
restored regular heart rhythm, but brachycardia developed later
with recurrent heart failure. The patient died about 4 - 4 1/2 h
after the ingestion of the mexacarbate.
Two severe cases of propoxur poisoning following ingestion of
150 - 200 ml of commercial Baygon formulation were described by
Bomirska & Winiarska (1972). One individual responded to treatment
and recovered but the other died after failing to respond to
treatment with atropine and 2-PAM and toxogonin given
intravenously. A normal ChE suggested spontaneous reactivation of
this enzyme.
9.1.2 Effects of short- and long-term exposure
Propoxur was tested for its effectiveness in controlling the
mosquito vectors of malaria in Iran. The insecticide was sprayed
inside houses in a 300 km2 area in which 11 000 people lived in 33
villages. Sixty-nine out of the 11 000 inhabitants complained of
mild cholinergic symptoms, when they entered their houses during
the spraying, contrary to instructions. The symptoms were
headache, nausea, sweating, and vomiting. The symptoms subsided in
2 - 3 h. Similar experiences were reported in other villages
sprayed with propoxur or with carbaryl (Vandekar et al., 1968).
9.1.3 Controlled human studies
Human volunteers tolerated single oral doses of aqueous
solutions of aldicarb at doses of 0.025, 0.05, or 0.1 mg/kg body
weight, with only mild depression of whole blood-ChE in all cases.
Only the persons receiving 0.1 mg/kg presented symptoms of acute
cholinergic stress (i.e., nausea, sweating, pinpoint pupils,
salivation) (FAO/WHO, 1982b).
Groups of male volunteers (5 or 6 per group) were administered
daily doses of 0.06 and 0.13 mg carbaryl/kg body weight, orally,
for 6 weeks. No subjective or objective changes were observed,
except a slight reversible decrease in the ability of the kidneys
to reabsorb aminoacids in the group at the highest dose level
(Wills et al., 1968).
Thirty-eight urban volunteers were monitored for carbaryl
exposure during the summer. All volunteers were involved in the
application of carbaryl incidental to their employment or leisure
activities. Exposure of clothing, skin, with and without gloves,
were measured. The maximum dermal exposure recorded was 2.86 mg/kg
per h, the maximum air concentration was 0.28 mg/m3. Only a small
mean decrease in serum- and erythrocyte-AChE activity was found
(Gold et al., 1982). Comparable results were obtained by Leavitt et
al. (1982) in a study on 2 groups of professional pesticide spray
operators with higher dermal exposure, spraying trees with
carbaryl.
A 42-year-old male volunteer took a single oral dose of 135 mg
propoxur (1.5 mg/kg body weight) about 2 h after breakfast.
Plasma- and erythrocyte-ChE activities were determined every 15 min
for the first 2 h after ingestion and then every hour thereafter.
Pulse and blood pressure were recorded. A rapid drop in
erythrocyte-ChE activity was observed, reaching the lowest level of
27% of normal within 20 min. ChE activity gradually recovered,
reaching normal levels about 2 h after ingestion. Plasma-ChE
activity was not affected. In another study (single dose of 0.36
mg/kg body weight), the lowest erythrocyte-ChE activity occurred
within 10 min together with short-lasting stomach discomfort,
blurred vision, nausea, and facial perspiration. Twenty min after
ingestion, the volunteer's pulse rate and blood pressure were
increased. Pronounced nausea accompanied by repeated vomiting and
profuse sweating occurred until approximately 45 min after the
ingestion of propoxur. From then on, poisoning symptoms
diminished, blood pressure and pulse returned to pre-ingestion
values. The disappearance within about 2 h of the poisoning
symptoms was in line with the return of normal erythrocyte-ChE
activity. Forty-five percent of the ingested dose of propoxur was
excreted as the metabolite, 2-(1-methylethyl)phenol, within 24 h of
ingestion (Vandekar et al., 1971; FAO/WHO, 1974b).
In another study, 5 doses of propoxur, at 0.15 or 0.20 mg/kg
body weight, were administered to volunteers at 1/2-h intervals.
In this study, a symptomless depression of erythrocyte-ChE to 60%
of normal was observed over the course of the dosing. The enzyme
activity recovered to normal levels soon after termination of the
dosing. From the above studies, it was concluded by the authors
that an oral dose of 0.36 mg propoxur/kg body weight may be
sufficient to induce initial cholinergic symptoms, while larger
doses may be tolerated without symptoms if the administration is
gradually (divided doses) (Vandekar et al., 1971).
9.2 Occupational Exposure
9.2.1 Acute toxicity: poisoning incidents
Tobin (1970) reported 3 cases of persons poisoned with
carbofuran. Two were formulation plant employees, preparing 10%
granules and the third was an entomologist who began to feel
uncomfortable while weighing a 50% water-dispersible powder
formulation. The 2 formulators developed similar symptoms
indicative of carbamate poisoning: profuse sweating, weakness,
blurred vision, and nausea. Both were taken to their respective
physicians about 3 h after the onset of symptoms. One physician
administered atropine; the other, noting the regression of the
symptoms, did not administer atropine. The patient who received
the atropine (0.02 g im) was fully recovered in 30 min. The
untreated patient recovered over the course of 2 - 3 h. The third
case, the entomologist experienced mild discomfort that regressed
without atropine administration in 4 - 6 h.
Richardson & Batteese (1973) described the case of a spray-
plane co-pilot overexposed to mexacarbate during an spraying
operation. The cause of the overexposure was a pin-hole leak in a
high pressure pump line, which emitted a fine aerosol of the
mexacarbate formulation into the fuselage. The co-pilot developed
the classical signs of ChE poisoning. Toxic symptoms progressed to
paralysis of the extremities before he could be treated. In
hospital, atropine sulfate was immediately administered
intravenously followed by a second injection subcutaneously 40 min
later. The symptoms subsided quickly. ChE activity in blood drawn
after the first atropine injection was severely depressed, but
returned to normal after 3 days.
Minor and transient cholinergic symptoms were experienced by
spraymen applying propoxur formulations inside houses for testing
this insecticide against mosquito vectors of malaria in villages in
El Salvador (Quinby & Babione, 1966) and Iran (Vandekar et al.,
1968; Montazemi, 1969). A pronounced fall in blood-ChE activity
during the work and a distinct recovery after exposure ceased was
established as a daily pattern of activity of this enzyme,
erythrocyte-ChE being more sensitive to propoxur than plasma-ChE.
No cumulative inhibition of ChE during the 6-week exposure was
seen. The investigators noted that the exposed spraymen and a few
inhabitants recovered rapidly when removed from the source of
exposure and when treated with atropine.
Vandekar et al. (1968) commented that most spraymen who became
poisoned had heavy skin contamination, and had not cleaned their
skin sufficiently after spraying. The inhabitants entered the
house while it was being sprayed. Moreover, it was noted that
respirators did not offer protection against intoxication under
these circumstances and that dermal absorption accounted for most
of the absorbed dose.
No adverse health effects due to carbaryl exposure (except for
one case of skin rash) were observed in a study conducted by the
WHO Insecticide Testing Unit in Southern Nigeria. Ten men (out of
105 sprayers) spraying over a single 6-h period and 95 villagers
who resided in the sprayed houses, were examined. The sprayers
wore overalls, hats, and rubber boots during the entire 6 h of
spraying, but wore masks for the first hour only. Plasma-ChE
activity as well as urinary metabolites of carbaryl were determined
before and after spraying. A slight (average 5%) reduction in
plasma-ChE activity was reported for all 10 sprayers, the day after
the carbaryl application. Inhabitants of sprayed houses showed no
inhibition of plasma-ChE activity, 1 week after the spraying. A
slight increase in 1-naphthol was found in the urine of the
sprayers only on the sixth day after the exposure (Vandekar, 1965).
In a study on 19 agricultural workers in the USSR, whole blood-
ChE activity was measured before and after 4 to 6-h exposures to
airborne carbaryl for 3 - 4 days. Significant ChE inhibition was
found in men exposed to a mean airborne carbaryl concentration of
up to 4 mg/m3. No objective signs of ill health were observed. No
changes were measured at 0.7 mg/m3 (Yakim, 1967).
A case of acute intoxication of a woman with a total dose of 60
mg carbofuran was described by Izmirova et al. (1981). Slight ChE
inhibition was found, but within 72 h the patient recovered
completely.
The above reports identify systemic ChE poisoning as the major
hazard associated with occupational exposure to carbamate
pesticides. However, other publications indicate that certain of
these compounds can also irritate the exterior membranes of the
body. Tobin (1970) described 2 cases of field workers that
illustrate direct effects on the eye, (irritation and blurred
vision) during carbofuran spraying operations. These 2 persons did
not developed symptoms of systemic poisoning.
Vandekar (1965) reported skin rash in a sprayman accidentally
splashed with a carbaryl formulation. Although carbamate compounds
have not generally been implicated as the cause of dermatitis or
allergic skin reactions, Vandekar (1965) suggested that certain
individuals could develop this after unusually heavy exposure.
9.2.2 Effects of short- and long-term exposure
Little is known about the short- and long-term effects of
occupational exposure to carbamate pesticides. The few reports
that discuss this aspect do not implicate long-term exposure to the
carbamate compounds as a cause of human disease. Vandekar et al.
(1968) did not observe any evidence of cumulative toxicity among
spraymen applying propoxur formulations during a 6-week operation.
No long-term biochemical changes were reported among New Jersey
farmers and spraymen compared with a minimally exposed control
group. It was noted that significantly higher incidences of
hearing and eye problems, chronic cough, dizziness, headaches, and
hypertension occurred in the occupationally exposed group.
However, the various pesticides to which this group was exposed
were not tabulated (Kwalick, 1971).
Chromosome studies on the blood cultures of 20 workers engaged
in the production of benomyl did not reveal any increased frequency
of structural chromosome aberrations (Ruzicska et al., 1975).
In a survey of occupationally acquired disease in workers at a
pesticide plant, 11 out of 102 workers had been hospitalized for
illness related to chemical exposure. The commonest cause of
hospitalization was intoxication with the ChE inhibitor methomyl.
On clinical evaluation, 5 out of 11 packaging workers, with the
highest exposure to methomyl had experienced blurred vision or
pupillary constriction (Morse & Baker, 1979).
9.2.3 Epidemiological studies
Epidemiological studies on persons occupationally exposed
exclusively (or at least primarily) to carbamate pesticides are not
available. Such studies are needed before the long-term effects of
this class of pesticides on human beings can be adequately
evaluated.
9.3 Signs and Symptoms of Acute Intoxication by Carbamates
The clinical picture of carbamates intoxication results from
accumulation of ACh at nerve endings. Extensive description of
the syndrome is given in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980). The signs and symptoms can be
categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases,
diaphragm and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by the respiratory failure sometimes
leading to pulmonary oedema due to the combination of the above-
mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure;
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to light; pulse rate is not of
diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ;
on the other hand, since changes in the conduction
and excitability of the heart might be
life-threatening, monitoring should be performed.
(c) confirmation of diagnosis is made by measurement of
AChE in RBC or plasma-pseudoChE (dibucaine number
also, to rule out genetic deficiencies). In view of
the rapid reversibility, particular care should be
taken to use analytical procedures that include
immediate analysis and a careful control of sample
dilutions (section 2.3). Chemical analysis of body
fluids (gastric lavage, blood, urine) should be
performed for the identification of the chemical(s).
9.3.1 Biochemical methods for measurement of effects
AChE present in human erythrocytes is the same as the enzymes
present in the target synapses. Measurements of AChE in RBC is
therefore taken to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the carbamate has equal access to blood and the
synapses, and that AChE inhibition by carbamates is reversible.
In the case of acute poisoning, a high inhibition of RBC-AChE
is pathognomonic, but, in the follow-up of the intoxication, it
might not be correlated with the severity of symptoms. However,
monitoring of pre- and post-exposure levels of AChE in RBC gives a
good measure of the effect of an exposure (Kaloyanova, 1976). In
cases when the pre-exposure AChE level is not known (as in
accidental poisoning), reference can be made to a mean population
AChE activity. Blood-plasma contains a related enzyme called ChE or
pseudo-ChE, which contributes to the whole blood enzymatic
activity; the extent of the contribution of plasma-ChE will depend
on which substrate was used and at what concentration. PseudoChE
has no known physiological function, but can be inhibited
selectively by some carbamates without causing a toxic response.
The sensitivities of AChE and pseudoChE to inhibitors differ so
that measurements of the ability of whole blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many field conditions, procedures using whole blood are more
practical than those using separated erythrocytes.
9.4 Treatment of Acute Poisoning by Carbamate Insecticides
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977; Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
9.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in the cleaning of the skin area where
venupuncture is performed. Blood might be contaminated with
carbamates and therefore inaccurate measures of ChE inhibition
might result. Extensive eye irrigation with water or saline should
also be performed. In the case of ingestion, vomiting can be
induced, if the patient is conscious, by the administration of
ipecacuanha syrup (10 - 30 ml) followed by 200 ml of water.
However, this treatment is contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
the addition of bicarbonate solution or activated charcoal) can
also be performed, particularly in unconscious patients, taking
care to prevent aspiration of fluids into the lungs (i.e., only
after a tracheal tube has been put in place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
9.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
9.4.3 Specific pharmacological treatment
9.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.).
9.4.3.2 Oxime reactivators
There is no rational basis for using these drugs. Furthermore,
some unconfirmed reports suggest an increased toxicity of
carbamates when oximes have been administered.
9.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of
artificial respiration procedures.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
For more than 20 years, the Joint FAO/WHO Meetings on Pesticide
Residues (JMPR) and the International Agency for Research on Cancer
(IARC) have been evaluating the toxicity and carcinogenicity data
on the different carbamates. As part of the JMPR meeting, the FAO
deals with the use and application of these pesticides and
proposes residue limits. Data and conclusions of the meetings were
initially published in the WHO Technical Report Series but, since
1977, they have been published regularly in the FAO Plant
Production and Protection Papers.
It is not possible to review completely the results of the
discussions on the approximately 50 carbamates mentioned in these
documents. However, Annex III contains a list of the JMPR meetings
in which carbamates have been evaluated together with appropriate
references. Other information available in Annex III indicates
whether IARC evaluations and WHO/FAO Data Sheets are available, and
whether an IRPTC data profile and legal file exist.
REFERENCES
ABDEL-AAL, Y.A.I. & FAHMY, M.A.H. (1977) Molecular
modification of carbamates in relation to their selective
toxicity. In: Proceedings of the British Crop Protection
Conference on Pests and Diseases, Brighton, Nov. 1977, British
Crop Protection Council, Croydon, pp. 155-159.
ABD-ELROAF, T.K., FAHMY, M.A.H., FUKUTO, T.R., & EL-SEBAE,
A.H. (1977) Anticholinesterase activity and toxicity of
substituted phenyl methylcarbamates to honey bee. J. econ.
Entomol., 70(1): 78-82.
ABDEL-WAHAB, A.M., KUHR, R.J., & CASIDA, J.E. (1966) Fate of
14C-carbonyl-labelled aryl methylcarbamate insecticide
chemicals in and on bean plants. J. agric. food Chem., 14:
290-298.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1973) The
photochemical reactions of carbamates. III. The solution
photochemistry of metacrate, 3-methylphenyl- N -methyl-
carbamate. Int. J. environ. anal. Chem., 3: 73-79.
ADDISON, J.B., SILK, P.J., & UNGER, I. (1974) The solution
photochemistry of metacil (4-dimethyl-amino- m -tolyl- N -methyl
carbamate) and Landrin (3,4,5-trimethylphenyl- N -methyl-
carbamate). Bull. environ. Contam. Toxicol., 11: 250-255.
AHDAYA, S.M., MONROE, R.J., & GUTHRIE, F.E. (1981)
Absorption and distribution of intubated insecticides in
fasted mice. Pestic. Biochem. Physiol., 16(1): 38-46.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarba-
mates, and dithiocarbamates, Luxembourg, Commission of the
European Communities.
ALDRIDGE, W.N. & REINER, E. (1972) Enzyme inhibitors as
substrates. Interaction of esterases with esters of
organophosphorus and carbamic acids, Amsterdam, Oxford, New
York, Elsevier Science Publishers, 328 pp.
ALY, O.M. & EL-DIB, M.A. (1971) Photodecomposition of some
carbamate insecticides in aquatic environments. In: Faust,
S.D. & Hunter, J.V., ed. Organic compounds in aquatic
environments, New York, Marcel Dekker, pp. 469-493.
ALY, O.M. & EL-DIB, M.A. (1972) Studies of the persistence
of some carbamate insecticides in the aquatic environment. In:
Fate of organic compounds in the aquatic environment. III.
Advances in chemistry series, Washington DC, American Chemical
Society, pp. 210-243.
ANDERSON, C.A. (1973) Baygon. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7, pp.
163-178.
ANDERSON, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972)
Evaluation of herbicides for possible mutagenic properties.
J. agric. food Chem., 20: 649-656.
ANDRIANOVA, M.M. & ALEKSEEV, I.V. (1970) [On the carcino-
genous properties of the pesticides sevine, maneb, ciram and
cineb.] Vopr. Pitan., 29(6): 71-74 (in Russian, with English
summary).
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974a) Effects of the
insecticide carbaryl on clams and some other intertidal mud
flat animals. J. Fish. Res. Board Can., 31: 466-470.
ARMSTRONG, D.A. & MILLEMANN, R.E. (1974b) Effects of the
insecticide Sevin and its first hydrolytic product 1-naphthol
on some early developmental stages of the bay mussel Mytilus
edulis. Mar. Biol., 28: 11-15.
ASHTON, F.M. & CRAFTS, A.S. (1973) Carbamates. In: Mode of
action of herbicides, New York, Wiley Interscience, pp.
200-220.
ATKINS, E.L., GREYWOOD, E.A., & MCDONALD, R.L. (1973)
Toxicity of pesticides and other agricultural chemicals to
honey bees. Laboratory studies, Riverside, USA, University of
California, Agriculture Extension Service.
AUGUSTINSSON, K.B. & CASIDA, J.E. (1959) Enzymic hydrolysis
of N,N -dimethylcarbamoyl fluoride. Biochem. Pharmacol., 3:
60-67.
AUSTIN D.J. & BRIGGS, G.G. (1976) A new extraction method
for benomyl residues in soil and its application in movement
and persistence studies. Pestic. Sci., 7: 201-210.
AUSTIN, D.J., LORD, K.A., & WILLIAMS, I.H. (1976) High
pressure liquid chromatography of benzimidazoles. Pestic.
Sci., 7: 211-222.
BAKER, P.B. & HOODLESS, R.A. (1974) Analytical methods for
the detection and determination of residues of systemic
fungicides. Pestic. Sci., 5: 465-472.
BALDWIN, R.E., FREED, V.H., & FANG, S.C. (1954) Absorption
and translocation of carbon-14-applied as O -isopropyl
N -phenylcarbamate in Avena and Zea. J. agric. food Chem.,
2(8): 428-430.
BERAUD, M., PIPY, B., DERACHE, R., & GAILLARD, D. (1979)
Formation d'un cancérigène le N -nitrosocarbaryl par
interactions entre un insecticide de la série des carbamates
le carbaryl et le nitrite de sodium dans le suc gastrique de
rat. Food Cosmet. Toxicol., 17: 579-583.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of
pesticides in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BLACK, A.L., CHIU, Y.C., FAHMY, M.A.H., & FUKUTO, T.R.
(1973) Selective toxicity of N -sulfenylated derivatives of
insecticidal methylcarbamate esters. J. agric. food Chem., 21:
743-747.
BLEIDNER, W.E., MORALES, R., & HOLT, R.F. (1978) Benomyl.
In: Zweig, G. & Sherma, J., ed. Analytical methods for
pesticides and plant growth regulators, New York, London,
Academic Press, pp. 157-171.
BLEVINS, R.D., LYINSKY, W., & REGAN, J.D. (1977) Nitrosated
methylcarbamate insecticides. Effect on the DNA of human
cells. Mutat. Res., 44: 1-7.
BOBIK, A., HOLDER, G.M., & RYAN, A.J. (1972) Excretory and
metabolic studies of isopropyl N -(3-chlorophenyl)carbamate in
the rat. Food Cosmet. Toxicol., 10: 163-170.
BOMIRSKA, T. & WINIARSKA, A. (1972) [Toxicology of
carbamates in the light of intoxication with Baygon
insecticide.] Pol. Tyg. Lek., 27: 1448-1450 (in Polish).
BORZSONYI, M. & CSIK, M. (1975) Induction of malignant
lymphomas in Swiss mice by N -nitroso compounds formed in vivo.
Int. J. Cancer, 15: 830-838.
BORZSONYI, M. & PINTER, A. (1977) The carcinogenicity of
N -nitroso compounds formed endogenously in mice from
benzimidazole carbamate pesticides. Neoplasma, 24: 119-122.
BOWMAN, M.C. & BEROZA, M. (1969) Determination of Mesurol
and five of its metabolites in apples, pears and corn by gas
chromatography. J. Assoc. Off. Anal. Chem., 52: 1054-1063.
BROCKELSBY, C.H. & MUGGLETON, D.F. (1973) Asulam. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 497-508.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARPENTER, C.P., WEIL, C.S., PALM, P.E., WOODSIDE, M.W., NAIR,
J.H., & SMYTH, H.F. (1961) Mammalian toxicity of 1-naphthyl
N -methylcarbamate (Sevin insecticide). J. agric. food Chem.,
9: 30-39.
CARTER, S.D. & LASKEY, J.W. (1982) Effect of benomyl on
reproduction in the male rat. Toxicol. Lett., 11: 87-94.
CASIDA, J.E. & AUGUSTINSSON, K.B. (1959) Reaction of plasma
albumin with 1-naphthyl N -methylcarbamate and certain other
esters. Biochem. Biophys. Acta, 36: 411-426.
CHIBA, M. & DOORNBOS, F. (1974) Instability of benomyl in
various conditions. Bull. environ. Contam. Toxicol., 11:
273-274.
CHIN, W.T., DUANE, W.C., SZALKOWSKI, M.B., & STALLARD, D.E.
(1975) Gas-chromatographic determination of thiofanox
residues in soil, plants, and water. J. agric. food Chem.,
23(5): 963-966.
CLARK, C.G. & WRIGHT, S.J.L. (1970) Degradation of the
herbicide isopropyl N -phenylcarbamate by Arthrobacter and
Achromobacter sp. from soil. Soil Biol. Biochem., 2: 217.
COLLINS, T.F.X., HANSEN, W.H., & KEELER, H.V. (1971) The
effect of carbaryl (Sevin) on reproduction of the rat and the
gerbil. Toxicol. appl. Pharmacol., 19: 202-216.
CROSBY, D.G., LEITIS, E., & WINTERLIN, W.L. (1965)
Photodecomposition of carbamate insecticides. J. agric. food
Chem., 13(3): 204-207.
DASSENOY, B. & MEYER, J.A. (1973) Mutagenic effect of
benomyl on Fusarium oxysporum. Mutat. Res., 21: 119-120.
DAWSON, J.A., HEATH, D.F., ROSE, J.A., THAIN, E.M., & WARD,
J.B. (1964) The excretion by humans of the phenol derived in
vivo from 2-isopropoxyphenyl- N -methylcarbamate. Bull. World
Health Org., 30: 127-134.
DELATOUR, P. & RICHARD, Y. (1976) Propriétés embryotoxiques
et antimitotiques en série benzimidazole. Therapie, 31(4):
505-515.
DE ROSE, H.R. (1951) Crabgrass inhibition with O -isopropyl-
N -(3-chlorophenyl)carbamate. Agron. J., 43: 139-142.
DIKSHITH, T.S.S., GUPTA, P.K., GAUR, J.S., DATTA, K.K., &
MATHUR, A.K. (1976) Ninety-day toxicity of carbaryl in male
rats. Environ. Res., 12: 161-170.
DOROUGH, H.W. & CASIDA, J.E. (1964) Nature of certain
carbamate metabolites of the insecticide Sevin. J. agric. food
Chem., 12(4): 294-304.
DOUCH, P.G.C. (1973) The metabolism of benomyl fungicide in
animals. Xenobiotica, 3: 367-380.
EBERLE, D.O. & GUNTHER, F.A. (1965) Chromatographic,
spectrometric, and irradiation behaviour of five carbamate
insecticides. J. Assoc. Off. Agric. Chem., 48:(5) 927-937.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the
soil fauna. Residue Rev., 45: 1-79.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N -nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under stimulated gastric conditions and in vivo in the
rat stomach. Food Cosmet. Toxicol., 12: 229-232.
EISENBRAND, G., IVANKOVIC, S., PREUSSMANN, R., SCHMAHL, D., &
WIESSLER, M. (1975a) Some recent results on the chemistry,
formation and biological activity of N -nitroso compounds. Gaun
Monogr. Cancer Res., 17: 133-143.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1975b) The
reaction of nitrite with pesticides. II. Formation, chemical
properties, and carcinogenic activity of the N -nitroso
derivative of N -methyl-1-naphthylcarbamate (carbaryl). Food
Cosmet. Toxicol., 13: 365-367.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ELESPURU, R.K., LIJINSKY, W., & SETLOW, J.K. (1974)
Nitrosocarbaryl as a potent mutagen of environmental
significance. Nature (Lond.), 247: 386-387.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V. Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
FANG, S.C., FALLIN, E., MONTGOMERY, M.L., & FREED, V.H.
(1974) Metabolic studies of 14C-labelled propham and
chlorpropham in the female rat. Pestic. Biochem. Physiol., 4:
1-11.
FAHRIG, R. & SEILER, J.P. (1979) Dose and effect of
methyl-2-benzimidazolylcarbamate in the "mammalian spot test",
an in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-biol. Interact., 26: 115-120.
FAO (1985) Production yearbook, 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23.64).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Agricultural Studies No. 73; WHO Technical
Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391.
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
10 Rev).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Suppl.).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Suppl.).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Suppl.).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1985a) 1983 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985c) 1984 Evaluation of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Pesticide residues in food. Report of the
1985 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (in press) 1985 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations.
FARAGO, A. (1969) [Fatal suicidal poisoning with Sevin
(1-naphthyl- N -methyl carbamate).] Arch. Toxikol., 24: 309-315
(in German).
FICSOR, G., BORDAS, S., & STEWART, S.J. (1978) Mutagenicity
testing of benomyl methyl-2-benzimidazole carbamate,
streptozotocin, and N -methyl- N' -nitro- N' -nitrosoguanidine in
Salmonella typhimurium in vitro and in rodent host-mediated
assays. Mutat. Res., 51: 151-164.
FINNISH NATIONAL BOARD OF HEALTH TOXICOLOGY GROUP (1982) The
toxicity of the benzimidazol type fungicides: benomyl,
carbendazim, and thiophanate-methyl (second report), Helsinki,
Finnish National Board of Health.
FILIP, Z. (1974) [Pesticides in powder form.] Vesmir, 53:
243-244 (in Czech).
FUKUTO, T.R. (1972) Metabolism of carbamate insecticides.
Drug Metab. Rev., 1: 117-150.
GARDINER, J.A., KIRKLAND, J.J., KLOPPING, H.L., & SHERMAN, H.
(1974) Fate of benomyl in animals. J. agric. food Chem.,
22(3): 419-427.
GHADIRI M. & GREENWOOD, D.A. (1966) Toxicity and biologic
effects of malathion, phosdrin, and sevin in the chick embryo.
Toxicol. appl. Pharmacol., 8: 342.
GHADIRI M., GREENWOOD, D.A., & BINNS, W. (1967) Feeding of
malathion and carbaryl to laying hens and roosters. Toxicol.
appl. Pharmacol., 10: 392.
GOLD, R.E., LEAVITT, J.R.C., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of urban applications to carbaryl. Arch. environ.
Contam. Toxicol., 11: 63-67.
GOLDBERG, M.E., JOHNSON, H.E., KNAAK, J.B., & SMYTH, H.F., Jr
(1963) Psychopharmacological effects of reversible
cholinesterase inhibition induced by N -methyl-3-isopropyl-
phenyl carbamate (compound 10854). J. Pharm. exp. Ther., 141:
244-252.
GRAY, R.A. (1971) Behaviour, persistence, and degradation of
carbamate and thiocarbamate herbicides in the environment. In:
Proceedings of the California Weed Control Conference, 18-20
January 1971, Mountain View, California, Stauffer Western
Research Centre, pp. 128-143.
GUARDIGLI, A., CHOW, W., & LEFAR, M.S. (1977) Determination
of carbetamid residues and its aniline metabolite. J. agric.
food Chem., 20: 348-350.
GUPTA, A.K. & LEGATOR, M.S. (1975) Chromosome aberrations in
cultured human leukocytes after treatment with the fungicide
benlate. In: Proceedings of a Symposium on Mutagenic
Carcinogenic and Teratogenic Chemistry, New Delhi, India,
Department of Atomic Energy, pp. 95-103.
HANSEN, D.J. (1969) Avoidance of pesticides by untrained
sheepshead minnows. Trans. Am. Fish. Soc., 98: 426-429.
HARVEY, J., Jr & HAN, J.C.Y. (1978a) Metabolism of oxamyl
and selected metabolites in the rat. J. agric. food Chem.,
26(4): 902-910.
HARVEY, J., Jr & HAN, J.C.Y. (1978b) Decomposition of oxamyl
in soil and water. J. agric. food Chem., 26(3): 536-541.
HARVEY, J., Jr & PEASE, H.L. (1973) Decomposition of
methomyl in soil. J. agric. food Chem., 21(5): 784-786.
HARVEY, J., Jr & REISER, R.W. (1973) Metabolism of methomyl
in tobacco, corn, and cabbage. J. agric. food Chem., 21(5):
775-783.
HARVEY, J., Jr, JELINEK, A.G., & SHERMAN, H. (1973)
Metabolism of methomyl in the rat. J. agric. food Chem.,
21(5): 769-775.
HARVEY, J., Jr, HAN, J.C.Y., & REISER, R.W. (1978)
Metabolism of oxamyl in plants. J. agric. food Chem., 26(3):
529-536.
HEYBROEK, W.M.H., MUGGLETON, D.F., & PARKE, D.V. (1984)
Metabolism of the carbamate herbicide asulam in the rat.
Xenobiotica, 14:(3) 235-247.
IARC (1976) Some carbamates, thiocarbarmates, and
carbazides, Lyons, International Agency for Research on Cancer
(IARC Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 12).
IARC (1983) Miscellaneous pesticides, Lyons, International
Agency for Research on Cancer (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 30;
Appendix 359-406).
INNES, J.R.M., ULLAND, B.M., VALLERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, J., & PETERS, J.
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J. Natl Cancer
Inst., 42: 1101-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1976) Changes in the
activity of some enzymes under the action of benomyl. Hig. i
Zdraveopazvane, Sofia, 19(5): 431-435 (in Bulgarian, with
English summary).
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads, reproduction, and generations of white rats under the
effect of some pesticides. Arch. Toxicol., 4(Suppl.): 459-462.
IVANOVA-CHEMISHANSKA, L., ANTOV, G., MIHAILOVA, A., & HINKOVA,
L. (1980) Study on the toxicology of benomyl. In: The Fourth
International Congress of Pesticide Chemistry, Zurich,
Switzerland, 24-28 July, 1978, pp. 11-559.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorous pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application, Amsterdam, Oxford, New
York, Elsevier Scientific Publishers, pp. 169-172 (Studies in
Environmental Science 7).
IZMIROVA, N., MILCEVA, V., MONOV, A., & KALOYANOVA, F.
(1981) [Acute carbofuran intoxication.] Hig. i
zdraveopazvane, 24(5): 445-448 (in Bulgarian, with English
summary).
JACKSON, J.A., CHART, I.S., SANDERSON, J.H., & GARNER, R.
(1977) Pirimicarb induced immune haemolytic anaemia in dogs.
Scand. J. Haematol., 19: 360-366.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of brain "neurotoxic esterase" and the development of delayed
neurotoxicity in hens. Biochem. J., 120: 523-531.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute
toxicity of chemicals to fish and aquatic invertebrates,
Washington DC, US Fish and Wildlife Service (Resource
Publication No. 137).
KAGAN, J.S. (1977) [The toxicity of organophosphorus
pesticides,] Moscow (in Russian).
KALOYANOVA, F. (1976) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Symposium on
Environmental Monitoring, 14-19 September, 1975, Las Vegas,
Nevada, Vol. 1, pp. 1-4, New York, Institute of Electrical &
Electronics Engineers, Inc.
KANAZAWA, J. (1975) Uptake and excretion of organophosphorus
and carbamate insecticides by fresh-water fish Motsugo
pseudoras bora parva. Bull. environ. Contam. Toxicol., 14:
346-352.
KAPLAN, A.M. & SHERMAN, H. (1977) Toxicity studies with
methyl- N -methylamino-carbonyl-oxyethanimidothioate. Toxicol.
appl. Pharmacol., 40: 1-17.
KAPPAS, A., GEORGOPOULOS, S.G., & HASTIE, A.C. (1974) On the
genetic activity of benzimidazole and thiophanate fungicides
on diploid Aspergillus nidulans. Mutat. Res., 26: 17-27.
KAPPAS, A., GREEN, M.H.L., BRIDGES, B.A., ROGERS, A.M., &
MURIEL, W.J. (1976) Benomyl: a novel type of base analogue
mutagen. Mutat. Res., 40: 379-382.
KAVLOCK, R.J., CHERNOFF, N., GRAY, L.E., Jr, GRAY, J.A., &
WHITEHOUSE, D. (1982) Teratogenic effects of benomyl in the
Wistar rat and CD-1 mouse, with emphasis on the route of
administration. Toxicol. appl. Pharmacol., 62: 44-54.
KAUFMAN, D.D. & BLAKE, J. (1973) Microbial degradation of
several acetamide, acrylamide, carbamate, toluidine, and urea
pesticides. Soil Biol. Biochem., 5: 297-308.
KAUFMAN, D.D. & KEARNEY, P.C. (1965) Microbial degradation
of isopropyl- N -(3-chlorophenyl carbamate and 2-chloroethyl- N -
(3-chlorophenyl carbamate. Appl. Microbiol., 13(3): 443-446.
KHERA, K.S. (1966) Toxic and teratogenic effects of
insecticides in duck and chick embryos. Toxicol. appl.
Pharmacol., 8: 345.
KIMMERLE, G. & IYATOMI, A. (1976) Toxicity of propoxur to
rats by subacute inhalation. Jpn J. ind. Health, 18: 375-382.
KNAAK, J.B., TALLANT, M.J., BARTLEY, W.Y., & SULLIVAN, L.J.
(1965) The metabolism of carbaryl in the rat, guinea pig and
man. J. agric. food Chem., 13(4): 537-543.
KNAAK, J.B., TALLANT, M.J., & SULLIVAN, L.J. (1966) The
metabolism of 2-methyl-2-(methylthio)propionaldehyde- O -
methylcarbamoyl)oxime in the rat. J. agric. food Chem., 14(6):
573-578.
KNAAK, J.B., TALLANT, M.J., KOZBELT, S.J., & SULLIVAN, L.J.
(1968) Metabolism of carbaryl in man, monkey, pig, and sheep.
J. agric. food Chem., 16(3): 465-470.
KNOWLES, C.O. & SONAWANE, B.R. (1972) Ethyl m- hydroxy-
carbanilate carbanilate (EP-475) metabolism in sugar beets.
Bull. environ. Contam. Toxicol., 8: 73-76.
KOSSMANN, K. & JENNY, N.A. (1973) Phenmedipham. In: Sherma,
J. & Zweig, G., ed. Analytical methods for pesticides and
plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 611-623.
KRECHNIAK, J. & FOSS, W. (1982) Cholinesterase activity in
rats treated with propoxur. Bull. environ. Contam. Toxicol.,
29(5): 599-604.
KUHR, R.J. (1968) Metabolism of methylcarbamate insecticide
chemicals in plants. J. Sci. Food Agric. 44, (Suppl.): 43-49.
KWALICK, D.S. (1971) Physician's pesticides primer. J. Med.
Soc. New Jersey, 68:(5) 375-377.
LAMB, M.J. & LILLY, L.J. (1980) An investigation of some
genetic toxicological effects of the fungicide benomyl.
Toxicology, 17: 83-95.
LEAVITT, J.R.C., GOLD, R.E., HOLCSLAW, T., & TUPY, D. (1982)
Exposure of professional pesticide applicators to carbaryl.
Arch. environ. Contam. Toxicol., 11: 57-62.
LEELING, N.C. & CASIDA, J.E. (1966) Metabolites of carbaryl
(1-naphthyl methylcarmate) in mammals and enzymatic systems
for their formation. J. agric. food Chem., 14: 281-290.
LEITCH, R.E. & PEASE, H.L. (1973) Lannate-methomyl. In:
Sherma, J. & Zweig, G., ed. Analytical methods for pesticides
and plant growth regulators, New York, London, Academic Press,
Vol. 7, pp. 331-338.
LYINSKY, W. & TAYLOR, H.W. (1976) Carcinogenesis in Sprague
Dawley rats of N -nitroso- N -alkylcarbamate esters. Cancer
Lett., 1: 275-279.
MAKITA, T., HASHIMOTO, Y., & NOGUCHI, T. (1973) Mutagenic,
cytogenetic, and teratogenic studies on thiophanate-methyl.
Toxicol. appl. Pharmacol., 24: 206-215.
MARLIAC, J.P. (1964) Toxicity and teratogenic effects of 12
pesticides in the chick embryo. Fed. Proc. Fed. Am. Soc. Exp.
Biol., 23: 105-126
MCCARTHY, J.F., FANCHER, O.E., KENNEDY, G.L., KEPLINGER, M.L.,
& CALANDRA, J.C. (1971) Reproduction and teratology studies
with the insecticide carbofuran. Toxicol. appl. Pharmacology,
19: 370.
METCALF, R.L., FUKUTO, T.R., COLLINS, C., BORCK, K., BURK J.,
REYNOLDS, H.T., & OSMAN, M.F. (1966) Metabolism in plant and
insect. Metabolism of 2-methyl-2-(methylthio)-propionaldehyde
O -methylcarbamoyl-oxime in plant and insect. J. agric. food
Chem., 14: 579-584.
MILLER, D.B. (1982) Neurotoxicity of the pesticidal
carbamates. Neurobehav. Toxicol. Teratol., 4: 779-787.
MIRVISH, H.S.S. (1968) The carcinogenic action and
metabolism of urethan and N -hydroxyurethan. Adv. Cancer Res.,
11: 1-42.
MONTAZEMI, K. (1969) Toxicological studies of Baygon
insecticide in Shabankareh area, Iran. Trop. geogr. Med., 21:
186-190.
MORSE, D.L. & BAKER, E.L., Jr (1979) Propanil-chloracne and
methomyl toxicity in workers of a pesticide manufacturing
plant. Clin. Tox., 15(1): 13-21.
MOSCIONI, A.D., ENGEL, J.L., & CASIDA, J.E. (1977)
Kynurenine formamidase inhibition as a possible mechanism for
certain teratogenic effect of organophosphorus and
methylcarbamate insecticides in chicken embryos. Biochem.
Pharmacol., 26: 2251-2258.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisonning due to organophosphate insecticies: acute and
chronic manifestations. Am. J. Med., 50: 475-492.
NATIONAL INSTITUTE OF HYGIENIC SCIENCES (1985) Letter to the
International Programme on Chemical Safety, 1.10.85, Tokyo,
Japan.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22: 16-18 (in
Japanese).
OGLE, R.E. & WARREN, G.F. (1954) Fate and activity of
herbicides in soils. Weeds, 3: 257-273.
OLEFIR, A.I. & VINOGRADOVA, V.Kh. (1968) [A study of the
embryotropic effect of the pesticides sevine and cyram.]
Vrach. Delo., 11: 103-106 (in Russian).
OLIVER, J.E. (1981) Pesticide-derived nitrosoamines.
Occurrence and environmental fate. In: Scanlan, R.A. &
Tannenbaum, S.T., ed. N-nitroso compounds, Washington DC,
American Chemical Society (ACS Symposium Series 174).
OONNITHAN, E.S. & CASIDA, J.E. (1968) Oxidation of methyl
and dimethylcarbamate insecticide chemicals by microsomal
enzymes and anticholinesterase activity of the metabolites. J.
agric. food Chem., 16: 28-44.
ORLOVA, N.V. & ZHALBE, E.P. (1968) Experimental material
contributive to the profile of maximal permissible amounts of
Sevin in food products. Vopr. Pitan., 27: 49-55 (in Russian).
PAROCHETTI, J.V. & WARREN, G.F. (1966) Vapour losses of IPC
and CIPC. Weeds, 14; 281-285.
PASTUSHENKO, T.B. (1983) [Study on the ganodotropic effects
of methyl N -2-benzimidazol carbamate aiming at evaluating its
effective concentrations.] Gig. i Sanit., 7: 83-85 (in
Russian).
PAULSON, G.D., JACOBSEN, A.M., ZAYLSKIE, R.G., & FEIL, V.J.
(1973) Isolation and identification of propham isopropyl-
carbanilate metabolites from the rat and goat. J. agric. food
Chem., 21: 804-811.
PETROVA-VERGIEVA, T. (1979) [Changes in the intrauterine
development of experimental animals under fundazol effect.]
Hig. Zdraveopaz., 22: 487-495 (in Bulgarian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization.
(Unpublished report VBC/84.889).
PRENDEVILLE, G.N., ESHEL, Y., JAMES, C.S., WAREN, G.F.E., &
SCHREIBER, M.M. (1968) Movement and metabolism of CIPC in
resistent and susceptible species. Weed Sci., 16: 432-435.
PROCTOR, N.H., MOSCIONI, A.D., & CASIDA, J.E. (1976) Chicken
embryo NAD levels lowered by teratogenic organophophorus and
methylcarbamate insecticides. Biochem. Pharmacol., 25: 757-762.
QUARLES, J.M. & TENNANT, R.W. (1975) Effects of nitroso-
carbaryl on BALB/3T3 cells. Cancer Res., 35: 2637-2645.
QUINBY, G.E. & BABIONE, R.W. (1966) Toxicological
observations on the use of Baygon (2-isopropoxyphenyl
N -methylcarbamate) as a malarial control insecticide in El
Salvador. Ind. Med. Surg., 35: 596-597.
QURAISHI, M.S. (1972) Edaphic and water relationships of
aldicarb and its metabolites. Can. Entomol., 104(3): 1191-1196.
REGAN, J.D., SETLOW, R.B., FRANCIS, A.A., & LYINSKY, W.
(1976) Nitrosocarbaryl: its effect on human DNA. Mutat. Res.,
38: 293-301.
REICH, G.A. & WELKE, J.O. (1966) Death due to a pesticide.
New Engl. J. Med., 274: 1432.
REINER, E. (1971) Spontaneous reactivation of phosphorylated
and carbamylated cholinesterases. Bull. World Health Org., 44:
109-112.
REINER, E. & ALDRIDGE, W.N. (1967) Effect of pH on
inhibition and spontaneous reactivation of acetylcholin-
esterase treated with esters of phosphoric acids and of
carbamic acids. Biochem. J., 105: 171-179.
REINER, E. & SKRINJARIC-SPOLJAR, M. (1968) Hydrolysis of
some monomethylcarbamates in human sera. Croat. Chem. Acta,
40: 87-90.
REINER, E., BUNTIC, A., TRDAK, M., & SIMEON, V. (1974)
Effect of temperature on the activity of human blood
cholinesterases. Arch. Toxicol., 32: 347-350.
RHODES, R.C. & LONG, J.D. (1974) Run-off and mobility
studies on benomyl in soils and turf. Bull. environ. Contam.
Toxicol., 12(4): 385-393.
RICHARDSON, E.M. & BATTEESE, R.I., Jr (1973) An incident of
Zectran poisoning. J. Maine Med. Assoc., 64: 158-159.
ROBEL, R.J., FELTHOUSEN, R.W., & DAYTON, A.D. (1982) Effects
of carbamates on Bobwhite food intake body weight and
locomotor activity. Arch. environ. Contam. Toxicol., 11:
611-615.
ROBENS, J.F. (1979) Teratologic Studies of Carbaryl,
Diazinon, Norea, Disulfiram, and Thiram in small laboratory
animals. Tox. Appl. Pharmacol., 15: 152-163.
ROMINE, R.R. (1973) Aldicarb. In: Sherma, J. & Zweig, G.,
ed. Analytical methods for pesticides and plant growth
regulators, New York, London, Academic Press, Vol. 7,
pp. 147-162.
ROSZKOWSKI, P. (1982) [Effect of the preparation propoxur on
intrauterine development and on the state of bone formation in
the progeny of the rat.] Ginek. Pol., 53(4): 201-207 (in
Polish).
ROTHSCHILD, E.R., MANSER, R.J., & ANDERSON, M.P. (1982)
Investigation of aldicarb in groundwater in selected areas of
the central sand plain of Wisconsin. Groundwater, 20(4):
437-445.
RUZICSKA, P., PETER, S., & CZEIZEL, A. (1975) Studies on the
chromosomal mutagenic effect of benomyl in rat and humans.
Mutat. Res., 29: 201.
RYBAKOVA, M.N. (1967) [Comparative toxic effects of sevin
and DDT used in the treatment of food crops.] Vopr. Pitan.,
26: 9-15 (in Russian).
RYBAKOVA, M.N. (1968) [Effect of some pesticides on the
hypophysis and its gonadotropic functions.] Gig. i Sanit.,
33(11): 27-31 (in Russian).
SAKAI, K. & MATSUMURA, F. (1968) Esterases of mouse brain
active in hydrolysing organophosphate and carbamate
insecticides. J. agric. food Chem., 16(5): 803-807.
SAKAI, K. & MATSUMURA, F. (1971) Degradation of certain
organophosphate and carbamate insecticides by human brain
esterases. Toxicol. appl. Pharmacol., 19(4): 660-666.
SEILER, J.P. (1972) The mutagenicity of benzimidazole and
benzimidazole derivatives. I. Forward and reverse mutations in
Salmonella typhimurium caused by benzimidazole and some of its
derivatives. Mutat. Res., 15: 273-276.
SEILER, J.P. (1975) Toxicology and genetic effects of
benzimidazole compounds. Mutat. Res., 32: 151-168.
SEILER, J.P. (1976a) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40: 339-347.
SEILER, J.P. (1976b) Inhibition of testicular DNA synthesis
by chemical mutagens and carcinogens. Preliminary result in
the validation of a novel short-term test. Mutat. Res., 46:
305-310.
SHAFIK, M.T., SULLIVAN, H.C., & ENOS, H.F. (1971) A method
for the determination of 1-naphthol in urine. Bull. environ.
Contam. Toxicol., 6: 34-39.
SHERMAN, H., CULIK, R., & JACKSON, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. appl.
Pharmacol., 32: 305-315.
SHTENBERG, A.I. & OZHOVAN, M.V. (1971) [Influence of low
Sevin doses on the reproductive function of animals in a
number of generations.] Vopr. Pitan., 30(1): 42-49 (in
Russian, with English summary).
SHTENBERG, A.I. & RYBAKOVA, M.N. (1968) Effect of carbaryl
on the neuroendocrine system of rats. Food Cosmet. Toxicol.,
6: 461-467.
SHTENBERG, A.I., ORLOVA, N.V., & TORCHINSKY, A.M. (1973)
[The action of different pesticides of different chemical
structure on the gonads and embryogenesis of experimental
animals.] Gig. i Sanit., 38(8): 16-20 (in Russian).
SIDEROFF, S.T. & SANTOLUCITO, J.A. (1972) Behavioural and
physiological effects of cholinesterase inhibitor carbaryl
(1-naphthyl methylcarbamate). Physiol. Behav., 9: 459-462.
SMALLEY, H.E., CURTIS, J.M., & EARL, F.L. (1968) Teratogenic
action of carbaryl in beagle dogs. Toxicol. appl. Pharmacol.,
13: 392-403.
SMALLEY, H.E., O'HARA, P.J., BRIDGES, C.H., & RADELEFF, R.D.
(1969) The effects of chronic carbaryl administration on the
neuromuscular system of swine. Toxicol. appl. Pharmacol., 14:
409-419.
SONAWANE, B.R. & KNOWLES, C.O. (1971) Phenmedipham and
m -aminophenol decomposition in alkaline soil. Bull. environ.
Contam. Toxicol., 6: 322-327.
SONAWANE, B.R. & KNOWLES, C.O. (1972) Comparative metabolism
of two carbanilate herbicides (EP-475 and phenmedipham) in
rats. Pestic. Biochem. Physiol., 1: 472-482.
STADNYK, L., CAMPELL, R.S., & JOHNSON, B.T. (1971) Pesticide
effect on growth and 14C assimilation in a fresh-water alga.
Bull. environ. Contam. Toxicol., 6: 1-8.
STANSBURY, H.A., Jr & MISKUS, R. (1964) In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators,
and food additives, New York, London, Academic Press, Vol. 2,
pp. 437-450.
STEGEMAN LE ROY, C. (1964) The effects of the carbamate
insecticide carbaryl upon forest soil mites and Collembola. J.
econ. Entomol., 57(6): 803-808.
STRINGER, A. & LYONS, C.H. (1974) The effects of benomyl and
thiophanate-methyl on earth worm populations; Apple Orchards
Pestic. Sci., 5: 189-195.
STILL, G.G. & HERRETT, R.A. (1975) Methylcarbamates,
carbanilates, and acylanilides. In: Kearney, P.C. & Kaufman,
D.D., ed. Herbicides, chemistry, degradation, and mode of
action. New York, Marcel Dekker, Vol. 2, pp. 609-664.
STILL, G.G. & MANSAGER, E.R. (1972) Metabolism of 4-chloro-
2-butynyl-3-chloro-carbanilate by soybean plants. J. agric.
food Chem., 20: 402-406.
STILL, G.G. & MANSAGER, E.R. (1973a) Soybean shoot
metabolism of isopropyl 3-chlorocarbanilate ortho and para
arylhydroxylation. Pestic. Biochem. Physiol., 3:: 87-95.
STILL, G.G. & MANSAGER, E.R. (1973b) Metabolism of isopropyl
carbanilate by soybean plants. Pestic. Biochem. Physiol., 3:
289-299.
STILL, G.G. & MANSAGER, E.R. (1975) Alfalfa metabolism of
propham. Pestic. Biochem. Physiol., 5: 515-522.
STILL, G.G. & RUSNESS, D.G. (1977) S -cysteinyl-hydroxychlor-
propham: formation of the S -cysteinyl conjugate of isopropyl-
3'-chloro-4'-hydroxy-carbanilate in oat (Avena savita L).
Pestic. Biochem. Physiol., 7: 210-219.
STRINGER, A. & WRIGHT, M.A. (1973) The effect of benomyl and
some related compounds on Lumbricus terrestris and other
earthworms. Pestic. Sci., 4: 165-170.
STYLES, J.A. & GARNER, R. (1974) Benzimidazole carbamate
methylester evaluation of its effect in vivo and in vitro.
Mutat. Res., 26:: 177-187.
SULLIVAN, L.J., ELDRIDGE, J.M., KNAAK, J.B., & TALLANT, M.J.
(1972) 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide as a
significant metabolite of carbaryl in the rat. J. agric. food
Chem., 20(5): 980-985.
SUZUKI, T. & TAKEDA, M. (1976) Microbial metabolism of
N -methylcarbamate insecticide I. Metabolism of O -sec-
butylphenyl N -methylcarbamate by Aspergillus niger van
Tieghem. Chem. Pharm. Bull., 24(9): 1967-1975.
SYPESTEYN, A.K., DEKHUYZEN, H.M., & VONK, J.W. (1977)
Biological conversion of fungicides in plants and
microorganisms, In: Siegel, M.R. & Sisler, H.D., ed.
Antifungal compounds, New York, Marcel Dekker, Vol. 2,
pp. 91-147.
TANNENBAUM, S.R., INSKEY, A.J., WEISMAN, M., & BISHOP, W.
(1974) Nitrite in human. Its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co., pp.
100-119.
TOBIN, J.S. (1970) Carbofuran a new carbamate insecticide.
J. occup. Med., 12: 16-19.
TOMLIN, A.D. & GORE, F.L. (1974) Effects of six insecticides
and a fungicide on the numbers of biomass of earthworms in
pasture. Bull. environ. Contam. Toxicol., 12(4): 487-492.
TORCHINSKY, A.M. (1973) [Significance of diets with
different protein content in manifestations of teratogenic and
embryotoxic action of some pesticides.] Vopr. Pitan., 3: 76-80
(in Russian).
TORCHINSKY, A.M., ORLOVA, N.V., SMIRNOVA, E.V., & MAKAROVA,
L.F. (1976) [Data for toxico-hygienic characteristics of the
pesticide Benlate of carbamate group.] Gig. i Sanit., 2: 35-39
(in Russian, with English summary).
UCHIYAMA, M., TAKEDA, M., SUZUKI, T., & YOSHIKAWA, K. (1975)
Mutagenicity of nitroso derivatives of N -methylcarbamate
insecticides in microbiological method. Bull. environ. Contam.
Toxicol., 14: 389-394.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) [The
reaction of nitrite with pesticides.] Z. Krebsforsch., 81:
217-224 (in German, with English summary).
VANDEKAR, M. (1965) Observations on toxicity of carbaryl,
folithion, and 3-isopropylphenyl- N -methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. World Health
Org., 33: 107-115.
VANDEKAR, M., HEDAYAT, S., PLESTINA, R., & AHMADY, G. (1968)
A study of the safety of O -isopropoxyphenyl methylcarbamate in
an operational field-trial in Iran. Bull. World Health Org.,
38: 609-623.
VANDEKAR, M., PLESTINA, R., & WILHELM, K. (1971) Toxicity of
carbamates for mammals. Bull. World Health Org., 44: 241-249.
VAN ESCH, G.J. & KROES, R. (1972) Long-term toxicity studies
of chlorpropham and propham in mice and hamsters. Food Cosmet.
Toxicol., 10: 373-381.
VAN ESCH, G.J., VAN GENDEREN, H., & VINK, H.H. (1958) The
production of skin tumours in mice by oral treatment with
urethane, isopropyl- N -phenylcarbamate, or isopropyl- N -chloro-
phenyl carbamate in combination with skin painting with croton
oil and Tween 60. Br. J. Cancer, 12: 355-362.
VASHAKIDZE, V.I. (1965) [Some questions of the harmful
action of sevin on the reproductive function of experimental
animals.] Soobshch. Akad. Nauk Gruz. SSR, 39: 471-478 (in
Russian).
VASHAKIDZE, V.I. (1967) [Mechanism of action of pesticides
(granosana, sevin, donoc) on the reproductive cycle of
experimental animal.] Soobshch. Akad. Nauk Gruz. SSR, 48:
219-224 (in Russian).
VASSILIEFF, I. & ECOBICHON, D.J. (1982) Stability of
Matacil(R) in aqueous media as measured by changes in
anticholinesterase potency. Bull. environ. Contam. Toxicol.,
29: 366-370.
VASSILIEFF, I. & ECOBICHON, D.J. (1983) Acute toxicity of
aminocarb in male rats and inhibition of tissue esterases.
Bull. environ. Contam. Toxicol., 31: 326-330.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. Vol. 1. Toxicity profiles, Boca Raton,
Florida, CRC Press, Inc., 232 p..
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticide
reference index, JMPR 1961-84, Geneva, World Health
Organization (Unpublished report).
VOSS, G. & SCHULER, J. (1967) A fast and simple procedure
for routine determination of plasma ChE activity. Bull.
environ. Contam. Toxicol., 2(6): 357-363.
WHO (1984) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-85, Geneva, World
Health Organization (Unpublished report VBC/84.2).
WHO/FAO (1984) Data sheets on pesticides, Geneva, World
Health Organization.
WEIL, C.S., WOODSIDE, M.D., CARPENTER, C.P., & SMYTH, H.F.,
Jr (1972) Current studies of tests of carbaryl for
reproductive and teratogenic effects. Toxicol. appl.
Pharmacol., 21: 390-404.
WEIL, C.S., WOODSIDE, M.D., BERNARD, J.B., CONDRA, N.I., KING,
J.M., & CARPENTER, C.P., (1973) Comparative effect of
carbaryl on rat reproduction and guinea-pig teratology when
fed either in the diet or by stomach intubation. Toxicol.
appl. Pharmacol., 26: 621-638.
WEIS, P. & WEIS, J.S. (1974) Schooling behaviour of Menidia
menidia in the presence of insecticide Sevin (carbaryl). Mar.
Biol., 28: 261-263.
WESTLAKE, G.E., BUNYAN, P.J., MARTIN, A.D., STANLEY, P.I., &
STEED, L.C. (1981) Carbamate poisoning. Effect of selected
carbamate pesticides on plasma enzymes and brain esterases of
Japanese quail (Coturnix coturnix japonica). J. agric. food
Chem., 29(4): 779-785.
WHITE, E.R., BOSE, E.A., OGAWA, J.M., MANJI, B.T., & KILGORA,
W.W. (1973) Thermal and base-catalysed hydrolysis products
of the systemic fungicide benomyl. J. agric. food Chem., 21:
616-618.
WIEDMANN, J.L.M., ECKE, G.G., & STILL, G.G. (1976) Synthesis
and isolation of 1-hydroxy-2-propyl-3-chloro-carbanilate from
soybean plants treated with isopropyl-3-chlorocarbanilate J.
agric. food Chem., 24: 588-592.
WILHELM, K. & REINER, E. (1973) Effect of sample storage on
human blood cholinesterase activity after inhibition by
carbamates. Bull. World Health Org., 48: 235-238.
WILHELM, K., VANDEKAR, M., & REINER, E. (1973) Comparison of
methods of measuring cholinesterase inhibition by carbamates.
Bull. World Health Org., 48: 41-44.
WILLIAMS, I.H., BROWN, M.J., & WHITEHEAD, P. (1976a)
Persistence of carbofuran residues in some British Columbia
soils. Bull. environ. Contam. Toxicol., 15(2): 242-243.
WILLIAMS, I.H., PEPIN, H.S., & BROWN, M.J. (1976b)
Degradation of carbofuran by soil microorganisms. Bull.
environ. Contam. Toxicol., 15(2): 244-249.
WILLS, J.H., JAMESON, E. & COULSTON, F. (1968) Effects of
oral doses of carbaryl on man. Clin. Toxicol., 1(3): 265-271.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., London, British Crop Protection
Council.
WRIGHT, M.A. & STRINGER, A. (1973) The toxicity of
thiabendazole, benomyl, methyl-benzimidazol-2-yl-carbamate,
and thiophanate-methyl to the earthworm Lumbricus terrestris.
Pestic. Sci., 4: 431-432.
WYROBEK, A.J., WATCHMAKER, G., GORDON, L., WONG, K., MOORE,
D.I., & WHORTON (1981) Sperm shape abnormalities in carbaryl
exposed employees. Environ. Health Perspect., 40: 255-265.
YAKIM, V.S. (1967) [Data for substantiating the maximum
permissible concentration of sevine in the air of working
zones.] Gig. i Sanit., 32: 29-33 (in Russian).
YOSHIDA, K. & NISHIUCHI, Y. (1972) [Toxicity of pesticides
to some water organisms.] Bull. agric. Chem. Inspect. Stn.
(Japan), 12: 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) [Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn.
(Japan),. 6: 65-69 (in Japanese).
ZAKI, M.H., MORAN, D., & HARRIS, D. (1982) Pesticides in
groundwater: the aldicarb story in Suffolk Country, New York.
Am. J. public Health, 72(12): 1391-1395.
Annex I. Names and structures and some physical and chemical properties of carbamate pesticides
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aldicarb Temik propanal, 2-methyl- C7H14N2O2S 190.29 13 mPa 0.6%
2-(methylthio)-,
O -[(methylamino)
carbonyl]oxime
(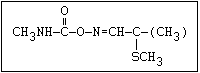 aldoxycarb Standak 2-methyl-2-(methyl- C7H14N2O4S 222.3 12 mPa 0.9%
sulfonyl)-propanal
O -[(methylamino)-
carbonyl]oxime
(
aldoxycarb Standak 2-methyl-2-(methyl- C7H14N2O4S 222.3 12 mPa 0.9%
sulfonyl)-propanal
O -[(methylamino)-
carbonyl]oxime
( allyxycarb Hydrol phenol, 4-(di-2-pro- C16H22N2O2 274.4
APC phenylamino)-3,5-
dimethyl, methyl
carbamate
(
allyxycarb Hydrol phenol, 4-(di-2-pro- C16H22N2O2 274.4
APC phenylamino)-3,5-
dimethyl, methyl
carbamate
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aminocarb Matacil phenol, 4-(dimethyl- C11H16N2O2 208.29 non- slightly
amino)-3-methyl-, volatile
methyl carbamate
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
aminocarb Matacil phenol, 4-(dimethyl- C11H16N2O2 208.29 non- slightly
amino)-3-methyl-, volatile
methyl carbamate
( asulam Asulox carbamic acid, C8H10N2O4S 230.26 - 0.5%
Asilan [(4,-aminophenyl)-
sulfonyl], methyl
ester
(
asulam Asulox carbamic acid, C8H10N2O4S 230.26 - 0.5%
Asilan [(4,-aminophenyl)-
sulfonyl], methyl
ester
( barban CBN carbamic acid, C11H9Cl2NO2 258.11 50 µPa 0.0011%
Carbyne 3-chlorophenyl-,
Chlorinat 4-chloro-2-butynyl
ester
(
barban CBN carbamic acid, C11H9Cl2NO2 258.11 50 µPa 0.0011%
Carbyne 3-chlorophenyl-,
Chlorinat 4-chloro-2-butynyl
ester
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
BPMC Bay-Bassa phenol, 2-(1-methyl- C12H17NO2 207.3 48 mPab 0.061%c
Baycarb propyl)-,methyl
Sumibas carbamate
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
BPMC Bay-Bassa phenol, 2-(1-methyl- C12H17NO2 207.3 48 mPab 0.061%c
Baycarb propyl)-,methyl
Sumibas carbamate
( bendiocarb Ficam 1,3-benzodioxol- C11H13NO4 223.25 660 µPa 0.004%
Garvox 4-yl, 2,2-dimethyl,
Multimet methyl carbamate
Tattoo (
bendiocarb Ficam 1,3-benzodioxol- C11H13NO4 223.25 660 µPa 0.004%
Garvox 4-yl, 2,2-dimethyl,
Multimet methyl carbamate
Tattoo ( benomyl Arilate carbamic acid,[1- C14H18N4O3 290.36 non- ~0.0002%
Benlate [(butylamino)- volatile
Tersan carbonyl]-1H-
benzimidazole-2-yl]-,
methyl ester
(
benomyl Arilate carbamic acid,[1- C14H18N4O3 290.36 non- ~0.0002%
Benlate [(butylamino)- volatile
Tersan carbonyl]-1H-
benzimidazole-2-yl]-,
methyl ester
(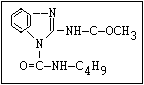 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
bufencarb Bux mixture of phenol, C13H19NO2 221.33 4.0 mPac < 0.005%
Metalkamate 3-(1-methylbutyl)-
phenyl)-, and
3-(1-ethylpropyl-
phenyl)-, methyl
carbamates
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
bufencarb Bux mixture of phenol, C13H19NO2 221.33 4.0 mPac < 0.005%
Metalkamate 3-(1-methylbutyl)-
phenyl)-, and
3-(1-ethylpropyl-
phenyl)-, methyl
carbamates
( butacarb phenol, 3,5-bis C16H25NO2 263
(1,1-dimethylethyl)-,
methyl carbamate
(
butacarb phenol, 3,5-bis C16H25NO2 263
(1,1-dimethylethyl)-,
methyl carbamate
( carbanolate Banol phenol, 2-chloro-4, C10H12ClNO2 213.68
SOK 5-dimethyl-, methyl
carbamate
(
carbanolate Banol phenol, 2-chloro-4, C10H12ClNO2 213.68
SOK 5-dimethyl-, methyl
carbamate
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbaryl Sevin 1-naphthalenol C12H11NO2 201.24 < 665 0.012%c
(Sevkul) methyl carbamate mPaf
(Carbicide) (
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbaryl Sevin 1-naphthalenol C12H11NO2 201.24 < 665 0.012%c
(Sevkul) methyl carbamate mPaf
(Carbicide) ( carbendazim Bavistin carbamic acid, C9H9N3O2 191.18 < 100 0.0028%c
BCM/MBC -1H-benzimidazole- nPab (pH 4.8)
Derosal 2-yl-, methyl ester
(
carbendazim Bavistin carbamic acid, C9H9N3O2 191.18 < 100 0.0028%c
BCM/MBC -1H-benzimidazole- nPab (pH 4.8)
Derosal 2-yl-, methyl ester
( carbetamide Legurame propanamide, N -ethyl C12H16N2O3 236.30 negligible 0.35%b
Carbetamex -2-[[(phenyl-amino) at 20°C
carbonyl]oxy]-, (R)-
(
carbetamide Legurame propanamide, N -ethyl C12H16N2O3 236.30 negligible 0.35%b
Carbetamex -2-[[(phenyl-amino) at 20°C
carbonyl]oxy]-, (R)-
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbofuran Furadan 7-benzofuranol, C12H15NO3 221.28 2.7 mPag 0.07%
Yaltox 2,3-dihydro-2,2-
Curater dimethyl-,
methyl carbamate
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
carbofuran Furadan 7-benzofuranol, C12H15NO3 221.28 2.7 mPag 0.07%
Yaltox 2,3-dihydro-2,2-
Curater dimethyl-,
methyl carbamate
( chlorbufam - 1-methylprop-2ynyl- C11H10ClNO2 223.7 2.1 mPab 0.054%b
3-chlorocarbanilate
chlorbufam - 1-methylprop-2ynyl- C11H10ClNO2 223.7 2.1 mPab 0.054%b
3-chlorocarbanilate
 cloethocarb - 2-(2-chloro-1-a C11H14ClNO4 259.7 - 0.13%b
methoxyethoxy)-
phenylmethyl
carbamate
cloethocarb - 2-(2-chloro-1-a C11H14ClNO4 259.7 - 0.13%b
methoxyethoxy)-
phenylmethyl
carbamate
 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
chlorpropham Elbanil carbamic acid, C10H12ClNO2 213.68 - 0.009%
Furloe 3-chlorophenyl-,
Prevenol 1-methylethyl ester
Chloro IPC (
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
chlorpropham Elbanil carbamic acid, C10H12ClNO2 213.68 - 0.009%
Furloe 3-chlorophenyl-,
Prevenol 1-methylethyl ester
Chloro IPC ( desmedipham Betanex carbamic acid, [3- C16H16N2O4 300.34
[[(phenylamino)-
carbonyl]oxy]phenyl]-,
ethyl ester
(13684-56-6)
desmedipham Betanex carbamic acid, [3- C16H16N2O4 300.34
[[(phenylamino)-
carbonyl]oxy]phenyl]-,
ethyl ester
(13684-56-6)
 dimetilan Snip carbamic acid, C10H16N4O3 240.3 readily
dimethyl-,
1-[(dimethylamino)
carbonyl]-5-methyl-
1H-pyrazol-3-yl ester
(
dimetilan Snip carbamic acid, C10H16N4O3 240.3 readily
dimethyl-,
1-[(dimethylamino)
carbonyl]-5-methyl-
1H-pyrazol-3-yl ester
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
dichlormate benzenemethanol, C9H9Cl2NO2 199 0.017%
2,3-dichloro-,
methyl carbamate
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
dichlormate benzenemethanol, C9H9Cl2NO2 199 0.017%
2,3-dichloro-,
methyl carbamate
(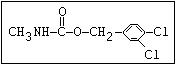 dioxacarb Famid phenol, 2-(1,3-di- C11H13NO4 223.25 40 µPac 0.6%c
Elecron oxolan-2-yl)-,
methyl carbamate
(
dioxacarb Famid phenol, 2-(1,3-di- C11H13NO4 223.25 40 µPac 0.6%c
Elecron oxolan-2-yl)-,
methyl carbamate
( ethiofencarb Croneton phenol, 2-[(ethyl- C11H15NO2S 225.33 13 mPac 0.182%b
thio)methyl]-,
methyl carbamate
(
ethiofencarb Croneton phenol, 2-[(ethyl- C11H15NO2S 225.33 13 mPac 0.182%b
thio)methyl]-,
methyl carbamate
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
etrofol hopcide phenol, 2-chloro-, C8H8ClNO2 185.62 -
CPMC methyl carbamate
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
etrofol hopcide phenol, 2-chloro-, C8H8ClNO2 185.62 -
CPMC methyl carbamate
( formetanate Dicarzol methanimidamide, C11H15N3O2 257.75 27 µPa 0.63%
Carzol N,N -dimethyl- N' -
[3-[[(methylamino)
carbonyl]oxy]-
phenyl]-
(
formetanate Dicarzol methanimidamide, C11H15N3O2 257.75 27 µPa 0.63%
Carzol N,N -dimethyl- N' -
[3-[[(methylamino)
carbonyl]oxy]-
phenyl]-
(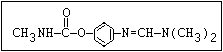 isoprocarb Etrofolan phenol, 2-(1-methyl- C11H15NO2 193.27 - insoluble
Carbamat IPM ethyl)methyl
Hytox carbamate
Mipcin (
isoprocarb Etrofolan phenol, 2-(1-methyl- C11H15NO2 193.27 - insoluble
Carbamat IPM ethyl)methyl
Hytox carbamate
Mipcin ( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
karbutilate Tandex carbamic acid, C14H21N3O3 279.38 6.0 nPab 0.033%b
(1,1-dimethylethyl)-,
3-[[(dimethylamino)
carbonyl]-amino]
phenyl ester
(4849-23-5)
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
karbutilate Tandex carbamic acid, C14H21N3O3 279.38 6.0 nPab 0.033%b
(1,1-dimethylethyl)-,
3-[[(dimethylamino)
carbonyl]-amino]
phenyl ester
(4849-23-5)
 MPMC Meobal phenol, 3,4-di- C10H13NO2 179.24
methyl-,
methyl carbamate
(
MPMC Meobal phenol, 3,4-di- C10H13NO2 179.24
methyl-,
methyl carbamate
( methiocarb Draza phenol, 3,5-di- C11H15NO2S 225.33 15 mPah 0.003%b
Mesurol methyl-4-(methyl-
thio)-, methyl
carbamate
(
methiocarb Draza phenol, 3,5-di- C11H15NO2S 225.33 15 mPah 0.003%b
Mesurol methyl-4-(methyl-
thio)-, methyl
carbamate
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
methomyl Lannate ethanimidothioic C5H10N2O2S 162.23 5.8%
Methavin acid, N -[[(methyl-
amino)carbonyl]oxy]-,
methyl ester
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
methomyl Lannate ethanimidothioic C5H10N2O2S 162.23 5.8%
Methavin acid, N -[[(methyl-
amino)carbonyl]oxy]-,
methyl ester
( metolcarb - m -tolyl methyla C9H11NO2 165.2 145 mPab 0.26%c
carbamate
metolcarb - m -tolyl methyla C9H11NO2 165.2 145 mPab 0.26%c
carbamate
 mexacarbate Zectran phenol, 4-dimethyl- C12H18N2O2 222.32
amino)-3,5-dimethyl-,
methyl carbamate
(ester)
(
mexacarbate Zectran phenol, 4-dimethyl- C12H18N2O2 222.32
amino)-3,5-dimethyl-,
methyl carbamate
(ester)
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
oxamyl Vydate ethanimidothioic C7H13N3O3S 219.29 31 mPa 28.0%
acid, 2-(dimethyl-
amino)- N -[[(methyl-
amino)carbonyl]-oxy]-
2-oxo-, methyl ester
(
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
oxamyl Vydate ethanimidothioic C7H13N3O3S 219.29 31 mPa 28.0%
acid, 2-(dimethyl-
amino)- N -[[(methyl-
amino)carbonyl]-oxy]-
2-oxo-, methyl ester
( phenmedipham Betanal carbamic acid, C16H16N2O4 300.34 - .0.0005%
(3-methylphenyl)-,
3-[(methoxycarbonyl)
amino]phenyl ester
(
phenmedipham Betanal carbamic acid, C16H16N2O4 300.34 - .0.0005%
(3-methylphenyl)-,
3-[(methoxycarbonyl)
amino]phenyl ester
( pirimicarb Aphox carbamic acid, C11H18N4O2 238.33 4.0 mPac 0.27%
Fernos dimethyl-, 2-(di-
Pirimor methylamino)-5,
Rapid 6-dimethyl-4-pyrimi-
dinyl ester
(
pirimicarb Aphox carbamic acid, C11H18N4O2 238.33 4.0 mPac 0.27%
Fernos dimethyl-, 2-(di-
Pirimor methylamino)-5,
Rapid 6-dimethyl-4-pyrimi-
dinyl ester
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
promacyl Promicide 5-methyl- m -cumenyla C16H23NO3 277.4 400 Pai slightly
butyryl(methyl)
carbamate
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
promacyl Promicide 5-methyl- m -cumenyla C16H23NO3 277.4 400 Pai slightly
butyryl(methyl)
carbamate
 promecarb Carbamult phenol, 3-methyl-5- C12H17NO2 207.3 0.0092%
Minacide (1-methylethyl)-,
methyl carbamate
(
promecarb Carbamult phenol, 3-methyl-5- C12H17NO2 207.3 0.0092%
Minacide (1-methylethyl)-,
methyl carbamate
( propham Banhoe carbamic acid, C10H13NO2 179.24 - 0.025%
Tuberit phenyl-, 1-methyl-
IPC ethyl ester
(
propham Banhoe carbamic acid, C10H13NO2 179.24 - 0.025%
Tuberit phenyl-, 1-methyl-
IPC ethyl ester
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
propoxur Baygon phenol, 2-(1-methyl C11H15NO3 209.27 6.5 x 0.2%
Blattanex ethoxy)-, methyl 10-6
Sendran carbamate mm Hg
Suncide (
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
propoxur Baygon phenol, 2-(1-methyl C11H15NO3 209.27 6.5 x 0.2%
Blattanex ethoxy)-, methyl 10-6
Sendran carbamate mm Hg
Suncide ( swep carbamic acid, 3,4- C8H7Cl2NO2 220.06
dichlorophenyl-,
methyl ester
(
swep carbamic acid, 3,4- C8H7Cl2NO2 220.06
dichlorophenyl-,
methyl ester
( terbucarb Azac 2,6-di-ter-butyl- C17H27NO2 277.4 0.0067%
Azar p -tolyl methyl
carbamate
thiodicarb Larvin ethanimidothioic C10H18N4O4S3 354.3
acid, N,N' -[thiobis
(methylimino)-
carbonyloxy]
bis-dimethyl-ester
(
terbucarb Azac 2,6-di-ter-butyl- C17H27NO2 277.4 0.0067%
Azar p -tolyl methyl
carbamate
thiodicarb Larvin ethanimidothioic C10H18N4O4S3 354.3
acid, N,N' -[thiobis
(methylimino)-
carbonyloxy]
bis-dimethyl-ester
(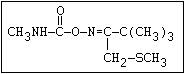 thiophanate Topsin carbamic acid, C14H18N4O4S2 370.48 - insoluble
ethyl Cercobin [1,2-phenylenebis
Enovit (iminocarbonothioyl)]
bis-, diethyl ester
(
thiophanate Topsin carbamic acid, C14H18N4O4S2 370.48 - insoluble
ethyl Cercobin [1,2-phenylenebis
Enovit (iminocarbonothioyl)]
bis-, diethyl ester
(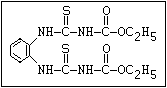 --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
thiophanate Topsin-M carbamic acid, C12H14N4O4S2 342.42 - slightly
methyl Cercobin-M [1,2-phenylenebis-
Enovit-M iminocarbonothioyl)]
Fungo bis-, dimethyl ester
Mildothane (
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
thiophanate Topsin-M carbamic acid, C12H14N4O4S2 342.42 - slightly
methyl Cercobin-M [1,2-phenylenebis-
Enovit-M iminocarbonothioyl)]
Fungo bis-, dimethyl ester
Mildothane (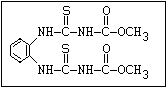 trimethacarb Broot 4:1 mixture of C11H15NO2 193.24
Landrin phenol, 3,4,5-tri-
methyl-, methyl-
carbamate and phenol,
2,3,5-trimethyl-
methyl carbamate
(
trimethacarb Broot 4:1 mixture of C11H15NO2 193.24
Landrin phenol, 3,4,5-tri-
methyl-, methyl-
carbamate and phenol,
2,3,5-trimethyl-
methyl carbamate
( --------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
xylylcarb - 3,4-xylylmethyla C10H13NO2 179.2 70 mPab 0.058%b
carbamate
--------------------------------------------------------------------------------------------
Annex I. (contd.)
--------------------------------------------------------------------------------------------
Common name Trade/other CAS chemical name/ Molecular Relative Vapour Water
name CAS registry number formula molecular pressure solubility
mass (25 °C) (25 °C)
--------------------------------------------------------------------------------------------
xylylcarb - 3,4-xylylmethyla C10H13NO2 179.2 70 mPab 0.058%b
carbamate
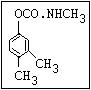 XMC Macbal 3,5 xylylmethyla
Cosban carbamate C10H13NO2 179.2 insoluble
--------------------------------------------------------------------------------------------
a IUPAC name.
b At 20 °C.
c At 30 °C.
d At 22 °C.
e From: Worthing & Walker (1983) (given in the reference list of the main document).
f At 26 °C.
g At 33 °C.
h At 60 °C.
i At 149 °C.
Annex II. Summary of the short- and long-term toxicity studies that were used to establish
the acceptable daily intakes for human beings for carbamate compounds
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Aldicarb 2 years 2.5 0.125 FAO/WHO (1983b)
0.25 (dog)
Aminocarb 2 years 100 FAO/WHO (1980b)
Bendiocarb 0.38
0.25 (dog) FAO/WHO (1985a)
Benomyl 2 years 2500 125 FAO/WHO (1983b,
30 (based on teratology) 1985a)
Carbaryl 2 years 200 10 FAO/WHO (1985a)
6 weeks 0.06 (man)
80 weeks 400 (mice) 60 (mice)
1 year 1.8 (dog)
Carbendazim 2 years 500 25 FAO/WHO (1986b)
2 years 100 (dog) 2.5
Carbofuran 2 years 20 1.0 FAO/WHO (1981b)
2 years 20 (mice) 2.5
Chloropropham 2 years 2000 FAO/WHO (1965b)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Ethiofencarb 90 days 500 10 FAO/WHO (1983b)
1000 (dog) 25
Methiocarb 1 year 25 1.3 FAO/WHO (1985a)
5 (dog) 0.125
Methomyl 22 months 100 5 FAO/WHO (1978b)
Oxamyl 14 days 100 (dog) 2.5 FAO/WHO (1985a)
Pirimicarb 2 years 175 9 FAO/WHO (1983b)
2 years 1.8 (dog)
17 weeks 2 (monkey)
Propoxur 2 years 250 12.5 FAO/WHO (1983b)
2 years (dogs)
Thiophanate 2 years 160 8 FAO/WHO (1976b, 1978b)
-methyl 2 years 50 (dog)
2 years 160 23 (mice)
---------------------------------------------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
References are to reports of WHO/FAO Joint Meeting on Pesticide Residues. Vettorazzi (1979) gives a
summary of these reports up to and including 1977.
Note: The references to Annex II are given in the reference list of the main document.
Annex III. Carbamates: JMPR reviews, ADIs, Evaluation by IARC, Classification by Hazard,
FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Aldicarb 1982 0-0.005 1983b + + IA No. 53 (1982)
1983a
1979 0-0.001 1980b
1980a
Aminocarb 1979 no ADI 1980b IB
1980a
1978 no ADI 1979b
1979a
1984 0-0.004 1985b
Bendiocarb 1982 0-0.002 1983b + + II No. 52 (1982)
(temporary) (rev. 1)
Benomyl 1983 0.02 1984 - + No. 87 (in
1985a 0 (preparation)
1978 no ADI 1979a
1975 no ADI 1976a
1976a
1973 no ADI 1974a
1974b
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbaryl 1984i 0-0.01 1985b
1979i 0-0.01 1980b + + II No. 3 (1978)
1980a (rev. 1)
1977i 0-0.01 1978b
1978a
1976i 0-0.01 1977b Vol. 12,
page 37
1977a
1975i 0-0.01 1976b
1976a
1973 0-0.01 1974b
1974a
1971i 0-0.01 1972a
(temporary)
1970i 0-0.01 1971b
(temporary)
1971a
1969 0-0.01 1970b
(temporary)
1970a
1968i 0-0.02 1969b
1969a
1967 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbendazim 1985 0-0.01 1986b
1983 0-0.01 1984 0 No. 89 (in
1985a (preparation)
1978 no ADI 1979a
1979b
1977 no ADI 1978a
1978b
1976 no ADI 1977a
1977b
1973 no ADI 1974a
1974b
Carbofuran 1982 0-0.01 1983a + + IB
1980 0-0.01 1981b
1981a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Chlor- 1965 no ADI 1965b Vol. 12, 0
propham page 55
1965a
1963 no ADI 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Ethio- 1983i 0-0.1 1984a II
fencarb 1982 0-0.1 1983b
1983a
1981i 0-0.1 1982b
(temporary)
1982a
1980i 0-0.1 1981b
(temporary)
1981a
1978i 0-0.1 1979b
(temporary)
1979a
1977 0-0.1 1978b
(temporary)
Methiocarb 1985 0-0.001 1986b
1984 0-0.001 1985b II
1983 0.001 1984
1985a
1981 0-0.001 1982b
1982a
Methomyl 1978 no ADI 1979b + + IB No. 55 (1983)
1979a
1977i no ADI 1978b
1978a
1976i no ADI 1977b
1977a
1975i no ADI 1976b
1976a
Mexacarbate Vol. 12,
page 237
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Oxamyl 1985 0-0.03 1986b
1984 0-0.03 1985b
1983i 0-0.01 1984 + + IB No. 54 (1983)
(temporary)
1980 0-0.01 1981b
(temporary)
1981a
0-0.0014
Pirimicarb 1982 0-0.02 1983b + + II
1983a
1981i 0-0.01 1982b
(temporary)
1982a
1979i 0-0.01 1980b
(temporary)
1980a
1978 0-0.01 1979b
(temporary)
1979a
1976 0-0.004 1977b
(temporary)
1977a
Propham 1965 no ADI 1965a Vol. 12 + + 0
1965b Page 189
1963 no ADI 1964a
Propoxur 1983 0-0.02 1984 + + II No. 25 (1976)
1981i 0-0.02 1982b
1982a
1978i 0-0.02 1979a
1977i 0-0.02 1978a
1973 0-0.02 1974b
1974a
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Thiodicarb 1985 0-0.1 1986b
(temporary)
Thiofanox 1978i no ADI 1979a IB
Thio- 1978i 0-0.08 1979a + 0
phanate- 1977 0-0.08 1978b
methyl 1978a
1975 0-0.08 1976b
1976a
1973 0-0.08 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in this
annex.
Annex III (contd.)
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling by the
manufacturer or in accordance with the rules laid down for storage and transportation by competent
international bodies. Classification relates to the technical material, and not to the formulated
product:
-----------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
-----------------------------------------------------------------------------------
1A Extremely hazardous 5 or less 20 or less 10 or less 40 or less
1B Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal use
-----------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex IV. Abbreviations
2-AB 2-aminobenzimidazole
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
EEG electroencephalogram
EMG electromyogram
FAO Food and Agriculture Organization of the United
Nations
4-HBC 4-hydroxy-2-benzimidazole carbamate
5-HBC 5-hydroxy-2-benzimidazole carbamate
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety (World
Health Organization)
IRPTC International Register of Potentially Toxic Chemicals
(United Nations Environment Programme)
iv intravenous
JMPR Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and a WHO Expert
Group on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NTE neuropathy target esterase (formerly neurotoxic
esterase)
PBI protein-bound iodine
pseudoChE pseudocholinesterase
RBC red blood cells
sc subcutaneous
UVR ultraviolet radiation
XMC Macbal 3,5 xylylmethyla
Cosban carbamate C10H13NO2 179.2 insoluble
--------------------------------------------------------------------------------------------
a IUPAC name.
b At 20 °C.
c At 30 °C.
d At 22 °C.
e From: Worthing & Walker (1983) (given in the reference list of the main document).
f At 26 °C.
g At 33 °C.
h At 60 °C.
i At 149 °C.
Annex II. Summary of the short- and long-term toxicity studies that were used to establish
the acceptable daily intakes for human beings for carbamate compounds
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Aldicarb 2 years 2.5 0.125 FAO/WHO (1983b)
0.25 (dog)
Aminocarb 2 years 100 FAO/WHO (1980b)
Bendiocarb 0.38
0.25 (dog) FAO/WHO (1985a)
Benomyl 2 years 2500 125 FAO/WHO (1983b,
30 (based on teratology) 1985a)
Carbaryl 2 years 200 10 FAO/WHO (1985a)
6 weeks 0.06 (man)
80 weeks 400 (mice) 60 (mice)
1 year 1.8 (dog)
Carbendazim 2 years 500 25 FAO/WHO (1986b)
2 years 100 (dog) 2.5
Carbofuran 2 years 20 1.0 FAO/WHO (1981b)
2 years 20 (mice) 2.5
Chloropropham 2 years 2000 FAO/WHO (1965b)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Carbamate Duration No-observed-adverse-effect level in ratsa Reference
of test mg/kg diet mg/kg body weight
---------------------------------------------------------------------------------------------------------
Ethiofencarb 90 days 500 10 FAO/WHO (1983b)
1000 (dog) 25
Methiocarb 1 year 25 1.3 FAO/WHO (1985a)
5 (dog) 0.125
Methomyl 22 months 100 5 FAO/WHO (1978b)
Oxamyl 14 days 100 (dog) 2.5 FAO/WHO (1985a)
Pirimicarb 2 years 175 9 FAO/WHO (1983b)
2 years 1.8 (dog)
17 weeks 2 (monkey)
Propoxur 2 years 250 12.5 FAO/WHO (1983b)
2 years (dogs)
Thiophanate 2 years 160 8 FAO/WHO (1976b, 1978b)
-methyl 2 years 50 (dog)
2 years 160 23 (mice)
---------------------------------------------------------------------------------------------------------
a Data given for rats, unless otherwise stated.
References are to reports of WHO/FAO Joint Meeting on Pesticide Residues. Vettorazzi (1979) gives a
summary of these reports up to and including 1977.
Note: The references to Annex II are given in the reference list of the main document.
Annex III. Carbamates: JMPR reviews, ADIs, Evaluation by IARC, Classification by Hazard,
FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Aldicarb 1982 0-0.005 1983b + + IA No. 53 (1982)
1983a
1979 0-0.001 1980b
1980a
Aminocarb 1979 no ADI 1980b IB
1980a
1978 no ADI 1979b
1979a
1984 0-0.004 1985b
Bendiocarb 1982 0-0.002 1983b + + II No. 52 (1982)
(temporary) (rev. 1)
Benomyl 1983 0.02 1984 - + No. 87 (in
1985a 0 (preparation)
1978 no ADI 1979a
1975 no ADI 1976a
1976a
1973 no ADI 1974a
1974b
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbaryl 1984i 0-0.01 1985b
1979i 0-0.01 1980b + + II No. 3 (1978)
1980a (rev. 1)
1977i 0-0.01 1978b
1978a
1976i 0-0.01 1977b Vol. 12,
page 37
1977a
1975i 0-0.01 1976b
1976a
1973 0-0.01 1974b
1974a
1971i 0-0.01 1972a
(temporary)
1970i 0-0.01 1971b
(temporary)
1971a
1969 0-0.01 1970b
(temporary)
1970a
1968i 0-0.02 1969b
1969a
1967 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Carbendazim 1985 0-0.01 1986b
1983 0-0.01 1984 0 No. 89 (in
1985a (preparation)
1978 no ADI 1979a
1979b
1977 no ADI 1978a
1978b
1976 no ADI 1977a
1977b
1973 no ADI 1974a
1974b
Carbofuran 1982 0-0.01 1983a + + IB
1980 0-0.01 1981b
1981a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Chlor- 1965 no ADI 1965b Vol. 12, 0
propham page 55
1965a
1963 no ADI 1964
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Ethio- 1983i 0-0.1 1984a II
fencarb 1982 0-0.1 1983b
1983a
1981i 0-0.1 1982b
(temporary)
1982a
1980i 0-0.1 1981b
(temporary)
1981a
1978i 0-0.1 1979b
(temporary)
1979a
1977 0-0.1 1978b
(temporary)
Methiocarb 1985 0-0.001 1986b
1984 0-0.001 1985b II
1983 0.001 1984
1985a
1981 0-0.001 1982b
1982a
Methomyl 1978 no ADI 1979b + + IB No. 55 (1983)
1979a
1977i no ADI 1978b
1978a
1976i no ADI 1977b
1977a
1975i no ADI 1976b
1976a
Mexacarbate Vol. 12,
page 237
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Oxamyl 1985 0-0.03 1986b
1984 0-0.03 1985b
1983i 0-0.01 1984 + + IB No. 54 (1983)
(temporary)
1980 0-0.01 1981b
(temporary)
1981a
0-0.0014
Pirimicarb 1982 0-0.02 1983b + + II
1983a
1981i 0-0.01 1982b
(temporary)
1982a
1979i 0-0.01 1980b
(temporary)
1980a
1978 0-0.01 1979b
(temporary)
1979a
1976 0-0.004 1977b
(temporary)
1977a
Propham 1965 no ADI 1965a Vol. 12 + + 0
1965b Page 189
1963 no ADI 1964a
Propoxur 1983 0-0.02 1984 + + II No. 25 (1976)
1981i 0-0.02 1982b
1982a
1978i 0-0.02 1979a
1977i 0-0.02 1978a
1973 0-0.02 1974b
1974a
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc: Evaluation of IRPTCe: mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: genicity Profile filef of pesticides
FAO/WHO by hazardg
---------------------------------------------------------------------------------------------------------
Thiodicarb 1985 0-0.1 1986b
(temporary)
Thiofanox 1978i no ADI 1979a IB
Thio- 1978i 0-0.08 1979a + 0
phanate- 1977 0-0.08 1978b
methyl 1978a
1975 0-0.08 1976b
1976a
1973 0-0.08 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in this
annex.
Annex III (contd.)
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling by the
manufacturer or in accordance with the rules laid down for storage and transportation by competent
international bodies. Classification relates to the technical material, and not to the formulated
product:
-----------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
-----------------------------------------------------------------------------------
1A Extremely hazardous 5 or less 20 or less 10 or less 40 or less
1B Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal use
-----------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex IV. Abbreviations
2-AB 2-aminobenzimidazole
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
EEG electroencephalogram
EMG electromyogram
FAO Food and Agriculture Organization of the United
Nations
4-HBC 4-hydroxy-2-benzimidazole carbamate
5-HBC 5-hydroxy-2-benzimidazole carbamate
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety (World
Health Organization)
IRPTC International Register of Potentially Toxic Chemicals
(United Nations Environment Programme)
iv intravenous
JMPR Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and a WHO Expert
Group on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NTE neuropathy target esterase (formerly neurotoxic
esterase)
PBI protein-bound iodine
pseudoChE pseudocholinesterase
RBC red blood cells
sc subcutaneous
UVR ultraviolet radiation
