
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 63
ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154263 2
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1986
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ORGANOPHOSPHOROUS INSECTICIDES -
A GENERAL INTRODUCTION
PREFACE
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General
1.1.2. Properties and analytical methods
1.1.3. Sources; environmental transport and distribution
1.1.4. Environmental levels and exposure
1.1.5. Effects on organisms in the environment
1.1.6. Metabolism
1.1.7. Mode of action
1.1.8. Effects on experimental animals and in vitro
test systems
1.1.9. Effects on human beings
1.1.10. Therapy of poisoning
1.2. Recommendations
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Chemical and physical properties
2.1.1. Effects of light
2.1.2. Effects of solutes and solvents
2.2. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION, EXPOSURE LEVELS
3.1. Sources of pollution
3.2. Environmental transport and distribution
3.2.1. Distribution in air and water
3.2.2. Distribution in food
3.3. Bioaccumulation and degradation in the environment
3.4. Exposure levels
3.4.1. Exposure of the general population
3.4.2. Occupational exposure
4. METABOLISM AND MODE OF ACTION
4.1. Uptake
4.1.1. Dermal uptake
4.1.2. Gastrointestinal tract
4.1.3. Inhalation
4.2. Distribution and storage
4.2.1. Experimental animal studies on distribution
and storage
4.3. Biotransformation
4.3.1. Mixed-function oxidases (MFOs)
4.3.1.1 Oxidative desulfuration
4.3.1.2 Oxidative N - dealkylation
4.3.1.3 Oxidative O -dealkylation
4.3.1.4 Oxidative de-arylation
4.3.1.5 Thioether oxidation
4.3.1.6 Side-chain oxidation
4.3.2. Hydrolases
4.3.3. Transferases
4.3.3.1 Transferases handling primary metabolites
4.3.4. Tissue binding
4.4. Elimination
4.5. Mode of action
4.5.1. Inhibition of esterases
4.5.2. Possible alkylation of biological macromolecules
5. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
5.1. Aquatic organisms
6. EFFECTS ON ANIMALS
6.1. Effects on the nervous system
6.1.1. Effects attributed to interaction with esterases
6.1.1.1 Cholinergic effects
6.1.1.2 Delayed neuropathic effects
6.1.2. Behavioural and other effects on the nervous system
6.2. Other effects
6.2.1. Mutagenic and carcinogenic effects
6.2.2. Teratogenic effects
6.2.3. Effects on the immune system
6.2.4. Effects on tissue carboxyesterases
6.2.5. Sundry other effects of organophosphorus
pesticides
6.2.5.1 Effects on hormones
6.2.5.2 Effects on the reproductive system
6.2.5.3 Effects on the retina
6.2.5.4 Porphyric effect
6.2.5.5 Lipid metabolism
6.2.5.6 Effects causing delayed deaths
6.2.5.7 Selective inhibition of thermogenesis
6.3. Factors influencing organophosphorus insecticide toxicity
6.3.1. Dosage-effect
6.3.2. Age and sex
6.3.3. Nutrition
6.3.4. Effects of impurities and of storage
6.3.4.1 Impurities toxic in their own right
6.3.4.2 Impurities potentiating the toxicity
of the major ingredient
6.3.5. Effects of other pesticides and of drugs
6.3.6. Species
6.3.7. Other factors
6.4. Acquisition of tolerance to organophosphorus
insecticides
6.5. Therapy of experimental organophosphorus poisoning
6.5.1. Palliation
6.5.2. Antagonism of effects of ACh
6.5.3. Reactivation of inhibited AChE
6.5.4. Efficacy of therapy
7. EFFECTS ON MAN
7.1. Acute cholinergic poisoning
7.1.1. Methods for assessing absorption and effects of
organophosphorus insecticides
7.1.1.1 Analysis of urine as a means of monitoring
exposed populations
7.1.1.2 Biochemical methods for the measurement of
effects
7.1.1.3 Electrophysiological methods for the study
of effects
7.1.2. Monitoring studies
7.1.3. Retrospective studies of populations exposed to
organophosphorus pesticides: acute and long-term
exposure
7.2. Other effects on the nervous and neuromuscular system due
to acute or long-term exposure
7.2.1. Delayed neuropathic effects
7.2.2. Behavioural effects
7.3. Effects on other organs and systems
7.4. Treatment of organophosphate insecticide poisoning in man
7.4.1. Minimizing the absorption
7.4.2. General supportive treatment
7.4.3. Specific pharmacological treatment
7.4.3.1 Atropine
7.4.3.2 Oxime reactivators
7.4.3.3 Diazepam
7.4.3.4 Notes on the recommended treatment
REFERENCES
ANNEX I: NAMES AND STRUCTURES OF SELECTED ORGANOPHOSPHORUS
PESTICIDES
ANNEX II: ORGANOPHOSPHORUS INSECTICIDES: JMPR REVIEWS, ADIs,
EVALUATION BY IARC, CLASSIFICATION BY HAZARD, FAO/WHO
DATA SHEETS, IRPTC DATA PROFILE, AND LEGAL FILE
ANNEX III: LD50s AND NO-OBSERVED-ADVERSE-EFFECT LEVELS IN ANIMALS
ANNEX IV: ABBREVIATIONS USED IN THE DOCUMENT
WHO TASK GROUP ON ORGANOPHOSPHOROUS INSECTICIDES
Members
Dr D. Ecobichon, Department of Pharmacology and Therapeutics,
McGill University, Montreal, Quebec, Canada
Dr A.H. El-Sebae, Department of Pesticide Chemistry, Faculty
of Agriculture, University of Alexandria, Alexandria, Egypt
Dr L. Ivanova-Chemishanska, Institute of Hygiene and Occupational
Health, Medical Academy, Sofia, Bulgaria (Vice-Chairman)
Dr M.K. Johnson, Toxicology Unit, Medical Research Council
Laboratories, Carshalton, Surrey, United Kingdom (Rapporteur)
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr M. Lotti, Institute of Occupational Health, Padua, Italy
Dr L. Martson, All-Union Scientific Research Institute of the
Hygiene and Toxicology of Pesticides, Polymers, and
Plastics (VNIIGINTOX), Kiev, USSRa
Dr U.G. Oleru, College of Medicine, University of Lagos,
Lagos, Nigeria
Dr W.O. Phoon, Department of Social Medicine and Public
Health, National University of Singapore, Outram Hill,
Republic of Singapore (Chairman)
Dr E. Reiner, Institute for Medical Research and Occupational
Health, Zagreb, Yugoslavia
Dr A.F. Rahde, Ministry of Public Health, Porto Alegre, Brazil
Dr J. Sekizawa, National Institute of Hygienic Sciences,
Tokyo, Japan
Observers
Mr R.J. Lacoste, International Group of National Associations
of Pesticide Manufacturers (GIFAP), Brussels, Belgium
Dr W.O. Phoon, International Commission on Occupational
Health, Geneva, Switzerland
Secretariat
Mme B. Bender, United Nations Environment Programme,
International Register of Potentially Toxic Chemicals,
Geneva, Switzerland
Secretariat (contd.)
Dr J.R.P. Cabral, Unit of Mechanisms of Carcinogenesis,
International Agency for Research on Cancer, Lyons, France
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)
Dr G.J. Van Esch, Bilthoven, The Netherlands (Temporary
Adviser)
Dr C. Xintaras, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
---------------------------------------------------------------------------
a Invited, but unable to attend.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
Detailed data profiles and legal files for most of the
organophosphorus insecticides can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR ORGANOPHOSPHORUS INSECTICIDES
A WHO Task Group on Environmental Health Criteria for
Organophosphorus Insecticides was held in Geneva on 30 September -
4 October 1985. Dr K.W. Jager opened the meeting on behalf of the
Director-General. The Task Group reviewed and finalized the draft
criteria document.
The drafts of this document were prepared by DR M.K. JOHNSON of
the UNITED KINGDOM MEDICAL RESEARCH COUNCIL.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
PREFACE
Following the Second World War, organochlorine pesticides made
a major contribution to improvements in agricultural output and in
the control of disease vectors. While the persistence of these
compounds after application was of considerable benefit to the
user, it also introduced problems. As these problems became more
widely appreciated, insect pest control began to rely more on the
anticholinesterase organophosphorus and carbamate ester pesticides.
A large number of such esters have been introduced on the market,
and a much greater number have been screened for pesticidal
activity. Unlike many environmental pollutants, pesticides are
deliberately added to the environment and are devised to be lethal
agents.
It would not be possible to review the class of organo-
phosphorus insecticides in one document, because they are so
numerous (more than 100) and cover a wide range of toxicity.
However, because they have many properties in common, it was
decided to prepare Organophosphorus Insecticides - A General
Introduction to provide background information for brief
Environmental Health Criteria documents on specific organo-
phosphorus insectides.
In addition to the literature cited in the text, much useful
information has been obtained from the following works of
reference: CEC (1977), Kagan (1977, 1985), Hayes (1982), Medved &
Kagan (1983), Worthing (1983), Mel'nikov et al. (1985), and Farm
Chemicals Handbook (1985).
For the purposes of this document, the word "insecticide" is
used more broadly than the strict zoological classification of
insects. Many have some selectivity for particular classes of
pests (mites, aphids, etc.) but, with 2 exceptions, all the
compounds covered in the introductory document exert their primary
effect by inhibiting the vital acetylcholinesterase of the nervous
system.
Non-ester organophosphorus compounds, having herbicidal
activity are not considered, but the herbicide amiprophos ( O -
ethyl- O -4-methyl-6-nitrophenyl N- isopropyl phosphoramidothioate)
and defoliants related to DEF ( S,S,S -tri- n -butylphosphoro-
trithioate) are included because they, in common with
organophosphorus insecticides, are esters and possess the ability
to inhibit tissue esterases and can cause cholinergic and/or
delayed neuropathic responses.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. General
At least 100 organophosphorus insecticides have been reviewed
by WHO for consideration as agents for the control of disease
vectors. A large number have been reviewed by the FAO/WHO Joint
Meetings on Pesticide Residues. Unlike many compounds scrutinized
by the IPCS, these compounds are designed to be toxic for certain
pests and are added deliberately to the environment. However, they
have a wide range of acute toxicity for experimental animals, and
it is impossible to review the whole class in a single
comprehensive document. Thus, the purpose of this document is to
give a framework of information and understanding with suitable
illustrations that will provide the background to brief
Environmental Health Criteria documents on specific insecticides.
For the purposes of this document, the word "insecticide" is
used in a broad sense and covers miticides, acaricides, etc. A few
organophosphorus insecticide compounds, with a toxicological mode
of action similar to that of the insecticides, are mentioned,
though they are intended for use as herbicides.
1.1.2. Properties and analytical methods
Organophosphorus insecticides are normally esters, amides, or
thiol derivatives of phosphoric, phosphonic, phosphorothioic, or
phosphonothioic acids. Most are only sightly soluble in water and
have a high oil-to-water partition coefficient and low vapour
pressure.
Physical and chemical data are not given in this introduction
but may be obtained from other sources including other WHO
publications, IRPTC profiles, and the handbooks included in the
list of references. A principal source for analytical methods is
provided by the Codex Alimentarius Commission of the Joint FAO/WHO
Food Standards Programme.
1.1.3. Sources; environmental transport and distribution
While there has been a considerable increase in the annual use
of organophosphorus insecticides for crop protection since 1970,
the overall increase has been less since the early 1980s. However,
new uses and formulations have been introduced. In particular,
parathion and malathion are widely used. Only a few of the less
hazardous organophosphorus insecticides have been evaluated for
disease vector control, and these contribute a very small
percentage to total usage.
With the exception of dichlorvos, most organophosphorus
insecticides are of comparatively low volatility. Dispersion of
spray droplets by wind is possible, but, in general, only small
amounts are likely to be distributed in this way.
The principal route of degradation in the environment seems to
be hydrolysis. In soil and the aqueous environment, the survival
time and the possibility of distribution in water may be influenced
by light intensity and pH. Most organophosphorus insecticides are
more stable in the pH range that may be encountered in the
environment (pH: 3 - 6), than at neutral pH. The influence of
microbiological factors in the degradation of these insecticides in
soil and water may be considerable. Different climatic conditions,
especially temperature and humidity, before, during, and after
spraying may influence the survival time markedly.
1.1.4. Environmental levels and exposure
Apart from occupationally exposed workers or populations
exposed as a result of disease-vector control programmes, marked
exposure of the general population is not expected. While exposure
via foodstuffs is sometimes monitored and controlled, there is
little information about exposure via groundwater, which may reach
drinking-water.
1.1.5. Effects on organisms in the environment
Only a little information is available on the toxicity of
organophosphorus insecticides for fish and aquatic insects. The
mechanism of toxicity has not been shown to be necessarily an
anticholinesterase effect. Lethal concentrations derived from 48-h
exposures in clean laboratory water may be artificially low
compared with exposure in environmental waters.
1.1.6. Metabolism
The metabolic fate of organophosphorus insecticides is
basically the same in insects, animals, and plants. Uptake in
animals and insects may occur through the skin, respiratory system,
or gastrointestinal tract. While uptake of active ingredient
through the skin from powdered or granulated formulations may be
relatively inefficient, the presence of aqueous dispersing agents
or organic solvents in a spray concentrate or formulation may
greatly enhance uptake. Although the actual exposure of the
respiratory system may not be as high as the exposure of skin in
unprotected persons, the efficiency of absorption might be high.
Metabolism occurs principally by oxidation, hydrolysis by
esterases, and by transfer of portions of the molecule to
glutathione. Oxidation of organophosphorus insecticides may result
in more or less toxic products. In general, phosphorothioates are
not directly toxic but require oxidative metabolism to the proximal
toxin. Most mammals have more efficient hydrolytic enzymes than
insects and, therefore, are often more efficient in their
detoxification processes. Birds usually have lower esterase
activity than mammals. The glutathione transferase reactions
produce products, that are, in most cases, of low toxicity.
Hydrolytic and transferase reactions affect both the thioates and
their oxons. Numerous conjugation reactions follow the primary
metabolic processes, and elimination of the phosphorus-containing
residue may be via the urine or faeces. Some bound residues remain
in exposed animals. Binding seems to be to proteins, principally,
and the turnover appears to be related to the half-life of these
proteins. There are limited data showing that incorporation of
residues into DNA occurs only in trace amounts and not by direct
alkylation, such as might be believed to be associated with genetic
damage.
1.1.7. Mode of Action
Organophosphorus insecticides exert their acute effects in both
insects and mammals by inhibiting acetylcholinesterase (AChE) in
the nervous system with subsequent accumulation of toxic levels of
acetylcholine (ACh), which is a neurotransmitter. In many cases,
the organophosphorylated enzyme is fairly stable, so that recovery
from intoxication may be slow.
Because of the greater stability of organophosphorylated AChE
compared with carbamylated enzyme, the ratio of the dose of an
organophosphorus insecticide required to produce mortality and that
which produces minimum symptoms of poisoning is substantially less
than the same ratio for carbamate insecticides. Reactivation of
inhibited enzyme may occur spontaneously, rates of reactivation
depending on the species and the tissue, as well as on the chemical
group attached to the enzyme. In particular, in most mammals,
dimethylphosphorylated AChE undergoes substantial spontaneous
reactivation within one day, which facilitates recovery from
intoxication. Reactivation of inhibited AChE may be induced by
some oxime reagents, and this fact provides opportunities for
therapy. Response to reactivating agents declines with time, and
this process is called "aging" of the inhibited enzyme.
Delayed neuropathy is initiated by attack on a nervous tissue
esterase distinct from AChE. The target has esterase activity and
is called neuropathy target esterase (formerly neurotoxic esterase
(NTE)). The disorder develops not because of loss of esterase
activity but because of some overall change brought about in the
protein molecule resulting from the process of aging of inhibited
NTE: catalytic activity of NTE reappears in the nervous tissue,
even during the period of development of neuropathy. Some
organophosphinates, sulfonyl fluorides, and carbamates may inhibit
NTE and act as protective agents, covering the target with
molecules that cannot engage in the aging reaction. The
structure/activity relationships for inhibitors of NTE differ from
those for AChE, so that pesticides designed as inhibitors of AChE
may be less effective as inhibitors of NTE, and may have low
neuropathic potential.
The rate of reaction of one chosen organophosphorus insecticide
with AChE was many orders higher than its rate of alkylation of the
test nucleophile, 4-nitrobenzylpyridine. On this limited data, it
seems unlikely that alkylation of biological macromolecules by
organophosphorus insecticides would occur in mammals.
1.1.8. Effects on experimental animals and in vitro test systems
The acute toxicity of organophosphorus insecticides is due to
their anticholinesterase action. The oral and dermal LD50s for
many compounds are listed in Annex III. It cannot be over-
emphasized that these numbers are not precise, and substantially
different values may be reported from different sources, even when
the factors of species, age, and sex have been standardized. These
LD50 values range from less than 10 mg/kg body weight to more than
3000 mg/kg for the oral route and, for most compounds, are
significantly higher for the dermal route.
For single exposures, a dose-effect relationship exists between
the dose and the severity of symptoms, and, also, the degree of
AchE inhibition in nervous tissue. The inhibition of blood-AChE
may not be similar to that in nervous tissue. Effects on plasma-
pseudocholinesterase (pseudoChE) are dose-related but are not
correlated with intensity of symptoms. For some insecticides,
pseudocholinesterase is more sensitive to inhibition than AChE,
but, for others, the converse is true.
The majority of organophosphorus insecticides do not cause
delayed neuropathy in test animals at doses up to the LD50. When a
dose is above the LD50 but is given in conjunction with therapy
against anticholinesterase effects, more compounds have been shown
to cause clinical neuropathy and, for others, substantial, but sub-
threshold, effects on NTE have been shown. For other compounds,
only slight effects on NTE have been shown, even at doses much
above the normally lethal dose. The results of dose-response
studies have shown that at least 70% inhibition of NTE in the brain
and spinal cord is required for initiation of delayed neuropathy in
adult hens, the usual test species. This threshold is not so
clearly defined for other species, and laboratory rodents do not
display clinical signs of neuropathy after a single dose. No
marked change in the threshold level of inhibition has been shown
between adult hens of different strains, but more information is
required.
Short- and long-term toxicity studies have been carried out.
While typical cholinergic intoxication only occurs when nervous
tissue AChE is substantially inhibited, the converse may not be
true in cases of long-term exposure because of the development of
tolerance, which is believed to be due, in part, to changes in some
cholinergic receptors. NTE appears to be synthesized continuously.
Consequently, continuous administration of an organophosphorus
compound does not necessarily lead to a continuous increase in the
level of inhibited NTE; the level may tend to reach equilibrium
below the threshold required to initiate neuropathy. With
continuous administration of neuropathic organophosphorus compounds
for up to 90 days, a peak level of about 50% inhibition of NTE must
be maintained to initiate neuropathy.
Acceptable daily intakes (ADIs) have been established as a
result of the evaluation of data by the FAO/WHO Joint Meetings on
Pesticide Residues (JMPR) (Annex II). ADIs are derived from
measurements or estimates of the highest dietary level that does
not cause significant changes in any measured variable, the most
sensitive of which is usually the AChE or pseudoChE activity in
blood. For no-observed-adverse-effect levels, see Annex III.
A variety of behavioural changes have been seen in response to
single or long-term dosing, but, in nearly all of the cases
reported, there was concomitant inhibition of AChE, though not
necessarily up to levels associated with typical signs of
poisoning; dose-response relationships have not always been
established. So far, behavioural tests have not proved adequate to
screen for organophosphate intoxication.
Effects on tissue carboxyesterases may be caused by some
organophosphorus insecticides at doses below those affecting AChE
or ChE. Apart from the delayed neuropathic effect arising from the
inhibition and aging of NTE, inhibition of other carboxyesterases
is not known to have any direct toxic effects. However, prior
inhibition of carboxyesterases may potentiate the toxicity for
mammals of pesticides, such as malathion and most pyrethroids,
which are normally detoxified by tissue esterases.
Various organophosphorus pesticides have been reported to show
positive responses in in vitro mutagenicity tests, but full
experimental details of the tests and control conditions have not
always been available. It can be concluded that some agents are
weakly mutagenic in vitro. Six organophosphorous pesticides have
been evaluated for mutagenic and carcinogenic potential by the
International Agency for Research on Cancer (IARC). In several
cases, the conclusion was that acceptable tests had been performed
with no evidence of carcinogenic potential, while, in others, the
conclusion was that there was "limited evidence consisting of very
small effects above the control background levels in lifetime
studies".
Many organophosphorus insecticides are embryotoxic at doses
that are toxic for the mother. Teratogenic effects have been
reported for trichlorphon in pigs, but few teratogenic effects have
been reported for other compounds.
Some deficiency in immune responses has been reported in
animals dosed with quantities of organophosphorus insecticides that
depressed AChE levels, but not at doses that did not affect AChE.
Several other toxic effects have been claimed after single or
repeated doses of individual compounds, but these effects have not
been reported for a range of the insecticides. Tissues and systems
reported to have been affected include the retina, lung, and
reproductive system.
Differences in toxic dose, but not in the mode of toxicity,
have been reported in animals according to species, age, sex, and
nutritional state. All these factors influence the status of a
variety of metabolizing enzymes in the body, but there is no steady
observable general trend towards increased or decreased toxicity in
response to variations in these variables.
Impurities may be found in either technical grade or formulated
organophosphorus insecticides. The impurities arise during the
synthesis or storage of technical or formulated material. The
levels of impurities may differ according to the route of synthesis
chosen, the formulating ingredients added, or the storage
conditions. Impurities may be toxic in their own right, toxic as
potentiators that block the metabolic degradation of the major
toxic ingredient, or not toxic.
1.1.9. Effects on human beings
Signs and symptoms of acute intoxication by organophosphorus
insecticides include muscarinic, nicotinic, and central nervous
system (CNS) manifestations. Symptoms may develop rapidly, or
there may be a delay of several hours after exposure before they
become evident. The delay tends to be longer in the case of more
lipophilic compounds, which also require metabolic activation.
Symptoms may increase in severity for more than one day and may
last for several days. In severe cases, respiratory failure is a
dominant effect.
In mild cases, or where the compound is disposed of rapidly,
symptoms may regress quite quickly, though depressed blood-ChE
levels may take several weeks to return to normal levels. There
appear to be few long-term effects after acute intoxication, though
weakness and fatigue may persist for several months.
Several methods are available for measuring exposure to, and
effects of, organophosphorus insecticides, and the combined use of
all methods is valuable, both in diagnosis of poisoning and in
determination of exposure. Standard methods for measuring dermal
exposure have been described in technical reports of WHO.
Determination of urinary metabolites provides an indication of
exposure, and analysis of serial samples is more valuable than a
single sample. Methods for the determination of residues are
available, and the Report of the Codex Alimentarius Commission of
FAO/WHO provides details and critical assessment of methods. In
general, it is not possible to relate the concentration of urinary
metabolites to the level of intoxication, though some guidelines
may be developed in connection with the controlled use of any
single organophosphorus pesticide.
Levels of erythrocyte- or whole blood-AChE are a satisfactory
guide to the level of acute intoxication. Plasma or serum levels
of pseudoChE are only useful as indicators of exposure. It is
essential that skin is cleansed carefully before taking blood
samples for analysis. Both enzymes are measurable with good
accuracy using a standard kit suitable for field work and
purchaseable from the World Health Organization; paper tests for
screening purposes have been described. Depression of AChE or
pseudoChE below about 75% of pre-exposure levels is generally
accepted as indicating that a hazard exists, and that workers
should be removed from all contact with the specific insecticide
until the levels recover. Signs of poisoning do not usually appear
until blood levels of AChE are below 50%, while severe poisoning is
usually associated with depression to below 30%. While measurement
of AChE is useful in preventive work and in diagnosis, measuring
the levels of blood-AChE as intoxication or therapy progress is of
less value. Electromyographic (EMG) monitoring of occupationally
exposed workers has been reported to be valuable in assessing
hazard, but there is some dispute, and no certain characteristic
change in EMG has been agreed. Further work under controlled
exposure conditions with parallel chemical, biochemical, and
clinical monitoring is desirable.
In all cases of intoxication, labels from containers should be
preserved, but these may be misleading. Whenever possible, a
sample of the incriminating agent should be stored carefully, and
tissue samples should be taken to aid in the identification of the
active agent.
Delayed neuropathies in occupationally exposed workers have
been reported for only a few of the many currently used
organophosphorus insecticides. For one pesticide, methamidophos,
the syndrome has not been reproduced in experimental animals. There
is no specific treatment for neuropathy, though physiotherapy may
limit the degree of muscle wasting that follows denervation. In
mild cases, some slow improvement can occur, but, in more severe
cases, the defects are permanent. The NTE of human tissue appears
to be similar to the NTE of experimental animals, and
extrapolations from results of laboratory tests in animals may be
of value. Samples of blood lymphocytes provide an accessible
source of NTE for monitoring purposes, though there is some
uncertainty about the stability of NTE in stored lymphocytes.
Continuous long-term exposure to high levels of
organophosphorus insecticides may precipitate typical cholinergic
symptoms, though most of the compounds do not accumulate
extensively in the body. Removal from exposure until AChE levels
return to pre-exposure levels appears to be an adequate health
precaution. There is no clear evidence of adverse effects on
health from long-term exposure to organophosphorus insecticides at
levels that do not affect AChE.
There is limited anecdotal evidence of behavioural effects
arising from long-term, or occasionally even a single, exposure to
one or other organophosphorus insecticide. The reports are
difficult to evaluate and are often complicated by the presence of
other factors, such as endogenous disorders and exposure to other
chemicals.
1.1.10. Therapy of poisoning
Therapy of AChE poisoning by organophosphates may be graded
according to the severity of intoxication. Effective therapy for
most compounds appears to consist of co-administration of atropine
with an oxime reactivating agent plus diazepam. Useful physical
measures include the maintenance of clear airways plus artificial
respiration. Efficacy of oximes may decline as the inhibited AChE
ages. Oxime therapy may continue to be effective in reactivating
AChE, freshly inhibited by inhibitor released from storage in body
depots, long after the bulk of the inhibited enzyme has aged.
There is no known therapy for severe delayed neuropathy. Mild
neuropathies tend to regress, presumably due to some regeneration
or adaptation of peripheral nerves.
1.2. Recommendations
Recommendations for further work on individual organophosphorus
insecticides have been made in the Monographs published in the
Technical Report Series of the JMPR and in some reports from IARC.
Apart from these, some general and specific recommendations are:
1. More up-to-date information should be obtained on the world-
wide production and uses of organophosphorus pesticides.
2. Information is needed on environmental pathways,
concentrations, and distribution of organophosphorus pesticides.
3. There is a need for more information on the occurrence and fate
of organophosphorus insecticides in surface water, soil, and
groundwater, and on their impact on plants, invertebrates, and
mammals.
4. Further studies are necessary on the occurrence of
organophosphorus insecticides in the different food chains
(bioaccumulation) and in the food and drinking-water of man (market
basket or total-diet studies), in order to estimate the daily
exposure of the population.
5. More information should be obtained on the acute and long-term
toxicity of certain organophosphorus insecticides for aquatic and
terrestrial organisms.
6. Apart from a number of studies on human volunteers and a number
of accidents, there is little information on the effects of human
exposure to organophosphorus insecticides. More information should
be collected to evaluate the risks of human exposure to these
compounds.
7. Further work should be done to develop more adequate analytical
methods (i.e., faster procedures and simpler equipment) to
determine organophosphorus residues in biological material (urine,
blood), and also in food. In this connection, the work of the
Codex Alimentarius Commission is noted. Also, further work is
required to develop less hazardous reagents for these analyses.
8. Exposure and health variables in workers exposed occupationally
to only one organophosphorus insecticide at a time should be
carefully monitored. This is an essential background to the more
complex problem of assessment of workers exposed to a variety of
pesticides. Procedures should include validation of methodology of
chemical, biochemical, behavioural, and electrophysiological tests
and should demonstrate the variation in results in both pre-
exposure and post-exposure situations. Adequate groups of matched
controls should also be studied.
9. Measurements of NTE responses in toxicity tests on hens should
be evaluated. The validity and variability of such tests should
be established. Further studies are needed to establish whether
NTE in lymphocytes and/or platelets should be measured in people
exposed to certain organophosphorus insecticides.
10. Enzymes that hydrolyse organophosphates play a role in
detoxifying some organophosphorus insecticides. Further studies
are required to establish whether the activity of these enzymes in
plasma is a good guide to the total hydrolytic capacity of the
whole body.
11. Liasion between National Poison Control Centres and experts
studying the effects of organophosphorus insecticides should be
improved. Preservation of blood, urine, and gastric lavage fluids
might assure the identity of an intoxicating agent. Also,
preservation of autopsied nervous tissue from fatal cases may
facilitate laboratory studies on the dose-response of human nervous
tissue NTE. Such studies may indicate the threshold of NTE
inhibition that might be expected to initiate delayed neuropathy in
man.
12. Information should be obtained concerning the changes in
toxicity due to impurities that can arise in pesticides as a
consequence of different manufacturing processes, the use of
formulating ingredients, and improper storage.
13. Consideration should be given to possible conflicts of
therapeutic procedures recommended for the treatment of poisoning
by other classes of pesticide when dealing with severe intoxication
by mixtures of such compounds with organophosphorus insecticides.
14. Users should be encouraged to be aware of the necessity to
establish a safe re-entry period according to local conditions.
2. PROPERTIES AND ANALYTICAL METHODS
2.1 Chemical and Physical Properties
Various structures of organophosphorus insecticides are
illustrated in Table 1. The compounds are normally esters, amides,
or thiol derivatives of phosphoric or phosphonic acid:
R1 O (or S)
\ ||
P -- X
/
R2
where R1 and R2 are usually simple alkyl or aryl groups, both of
which may be bonded directly to phosphorus (in phosphinates), or
linked via -O-, or -S- (in phosphates), or R1 may be bonded
directly and R2 , bonded via one of the above groups (phosphonates).
In phosphoramidates, carbon is linked to phosphorus through an -NH
group. The group X can be any one of a wide variety of substituted
and branched aliphatic, aromatic, or heterocyclic groups linked to
phosphorus via a bond of some lability (usually -O- or -S-) and is
referred to as the leaving group. The double-bonded atom may be
oxygen or sulfur and related compounds would, for example, be
called phosphates or phosphorothioates (the nomenclature
"thiophosphate" or "thionophosphate" is now less used).
The P=O form of a thioate ester may be referred to as the oxon,
and this is often incorporated in the trivial name (e.g., parathion
is the parent P=S compound of paraoxon).
The variations in the phosphorus group for the insecticides
that have been developed, are shown in Table 1 together with the
common or other names for some pesticides falling into this
classification. The complete structure and names for all the
organophosphorus compounds mentioned are listed in Annex I. It can
be seen that, in terms of numbers of commercial compounds, there
are 3 main groups: phosphates (without a sulfur atom),
phosphorothioates (with one sulfur atom), and phosphorodithioate
(with 2 sulfur atoms). Since the P=S form is intrinsically more
stable, many insecticides are manufactured in this form which can
be converted to the biologically active oxon in tissues. The
manner of this conversion is discussed in section 4.
Specific biotransformation of substituent groups in R1 , R2 , and
X may occur, and this is also considered later. Cleavage of the
direct carbon-to-phosphorus bonds of phosphonates and phosphinates
may occur to a small extent in the final stages of biodegradation,
but is probably insignificant as far as biological effects are
concerned.
Table 1. Variations in the chemical structure of organophosphorus insecticides
---------------------------------------------------------------------------------------------------------
Type of phosphorus group Outline of structure Common or other name
---------------------------------------------------------------------------------------------------------
Phosphate O chlorfenvinphos, crotoxyphos, dichlorvos,
|| dicrotophos, heptenphos, mevinphos, monocroto-
(R-O)2-P-O-X phos, naled, phosphamidon, TEPP, tetrachlor-
vinphos, triazophos
O -alkyl phosphorothioate O amiton, demeton-S-methyl, omethoate, oxydemeton-
|| methyl, phoxim, vamidothion
(R-O)2-P-S-X
S azothoate, bromophos, bromophos-ethyl, chlor-
|| pyriphos, chlorpyriphos-methyl, coumaphos, dia-
(R-O)2-P-O-X zinon, dichlofenthion, fenchlorphos, fenitro-
thion, fenthion, iodofenphos, parathion, para-
thion-methyl, pyrazophos, pyrimiphos-ethyl,
pyrimiphos-methyl, sulfotep, temephos, thionazin
Phosphorodithioate S amidithion, azinophos-ethyl, azinophos-methyl,
|| dimethoate, dioxathion, disulfoton, ethion,
(R-O)2-P-S-X formothion, malathion, mecarbam, menazon, meth-
idathion, morphothion, phenthoate, phorate,
phosalone, phosmet, prothoate, thiometon
S- alkyl phosphorothioate profenofos, trifenofos
R O
\ ||
S ||
\||
P-O-X
/
O
/
R
S- alkyl phosphorodithioate S prothiofos, sulprofos
R-S ||
\||
P-O-X
/
R-O
Phosphoramidate O cruformate, fenamiphos, fosthietan
||
(R-O)2-P-NR2
---------------------------------------------------------------------------------------------------------
Table 1. (contd.)
---------------------------------------------------------------------------------------------------------
Type of phosphorus group Outline of structure Common or other name
---------------------------------------------------------------------------------------------------------
Phosphorotriamidate O triamiphos
||
R2N-P-N
|
NR2
Phosphorothioamidate O methamidophos
||
R-O-P-NR2
|
S-alkyl
S isofenphos
||
(R-O)2-P-NR2
Phosphonate O butonate, trichlorfon
RO ||
\||
P-O-X
/
R
Phosphonothioate S EPN, trichlornat, leptophos, cyanofenphos
R-O ||
\||
P-O-X
/
R
---------------------------------------------------------------------------------------------------------
In order to be useful, these compounds must be reasonably
stable at neutral pH, since many are formulated as concentrates in
oil, in water-miscible solvents such as ethylene glycol monomethyl
ether, or are absorbed on to inert granules for application
directly or after dispersion in water. However, nearly all are
rapidly hydrolysed by alkali and many are also unstable at pH
levels below 2. Phosphoramidates are hydrolysed in an acid-
catalysed reaction, even at pH 4 - 5, and, since acid is produced,
decomposition tends to accelerate due to autocatalysis.
Oxidation of phosphorothioates to phosphates (-P=S --> -P=O)
is potentially dangerous, since the phosphates are more volatile
and are directly toxic agents. This can occur by oxidation of
stored products at elevated temperatures. The enzymatic catalysis
of this reaction is considered in section 4.
Various uncatalysed isomerizations are reported to occur under
forcing conditions of heating at over 100 °C for many hours in the
laboratory (Dauterman, 1971). Also, an isomerization associated
with considerable toxic hazard has been observed during the storage
of some formulations of malathion, particularly under warm humid
climatic conditions:
S O
| ||
(CH3O)2-P-SR ---> CH3S-P-SR
|
OCH3
The S- methyl derivatives, formed from malathion by this
isomerization, potentiate the toxicity of malathion markedly
(section 6.3.4). The isomerization reaction is not completely
understood, but it has been shown to be catalysed by dimethyl
formamide under laboratory conditions (Eto & Ohkawa, 1970). It is
not clear whether all alkyl phosphorothioates are subject to this
reaction, but it probably occurs most readily with the methyl
esters and may be influenced by the formulating agents. The hazard
resulting from isomerization will depend, not only on the extent of
the reaction and the intrinsic toxicity of the product, but also on
the manner of metabolic disposal of the parent compound (see
discussion in section 7).
Besides the various effects of heat and air noted above, both
light and solvent may influence the stability of these
organophosphorus compounds.
2.1.1 Effects of light
Parathion was one of the first organophosphorus compounds in
which the anticholinesterase activity, as measured in vitro, was
shown experimentally to increase during exposure to ultraviolet
radiation (UVR) and sunlight. However, the acute toxicity of
parathion decreased under UVR, although the in vitro
anticholinesterase activity increased as the result of the
formation of more polar products; the metabolites were identified
as paraoxon and the S- ethyl and S -phenyl isomers of parathion,
together with unknown products (Dauterman, 1971). This study
showed that UVR is able to oxidize as well as isomerize parathion.
When parathion-methyl was given the same UVR treatment, only the
methyl homologue of paraoxon was found. In a similar study, in
which EPN was exposed to UVR, the oxygen analogue of EPN and
p -nitrophenol were found together with unidentified resins,
also indicating cleavage of the P-O-aryl bond. Studies with 7
organophosphorus pesticides containing sulfur in a thioether group
indicated that exposure to UVR (254 nm) resulted in a variety of
oxidation products. With phorate, disulfoton, and thiometon, the
corresponding sulfoxides and sulfones were identified as products
of UVR. With thiometon, evidence of oxidation of the thiono sulfur
was also obtained. In all 7 cases, the oxidation products were
more acutely toxic than the parent compound. Exposure of a
carbethoxy analogue of mevinphos to UVR results in another type of
photoisomerization. Starting with either the cis- or the trans-
isomer, or a mixture of the isomers, and exposing the compounds to
UVR, results in a mixture of approximately 30% of the cis- and 70%
of the trans-isomer; in all cases, the trans-isomer was
predominant. When chlorpyrifos is exposed to UVR or sunlight, it
undergoes hydrolysis in the presence of water to liberate 3,5,6-
trichlor-2-pyridinol, which then undergoes complete
photodechlorination with the formation of diols, triols, and
tetraols.
2.1.2 Effects of solutes and solvents
The hydrolysis of organophosphorus compounds is influenced by
solutes, e.g., some amino acids, hydroxylammonium derivatives;
metal ions such as Cu++ act as catalysts.
Solvents used in formulating organophosphorus compounds to
obtain properties that will increase the chances of contact between
the insecticide and the target organism, influence their stability.
It has been found that dimethoate in certain hydroxylic solvents,
particularly 2-alkoxyethanols, increased in toxicity on storage
(Casida & Sanderson, 1963). The acute oral LD50 for rats decreased
from 150 - 250 mg/kg body weight to 30 -40 mg/kg, after 7 months
storage at normal temperatures. Studies indicated that many
reaction products were formed in the presence of methylcellosolve.
The degradation involved hydrolysis of the amide bond, hydrolysis
of ester groups, and loss of the thiono group. The most toxic
fraction was identified as dimethoate with probably one, but
possibly both, of the methyl groups replaced by 2-methoxyethyl
groups. No evidence was obtained for the formation of pyro-
phosphates. In the same study, the toxicity of a few other
phosphorothioate compounds was also found to increase in the
presence of 2-methoxyethanol.
Another type of reaction occurs when organophosphorus compounds
containing a sulfide group (R-S-R) are stored undiluted or in an
aqueous solution. Heath & Vandekar (1957) observed that a 1%
solution of demeton- S -methyl increased in toxicity spontaneously
at 35 °C during the course of one day. This increase was found to
be due to the formation of a transalkylated sulfonium derivative,
the toxicity of which was more than 1000 times that of the parent
compound. A similar reaction has also been shown to take place
with demeton-O (phosphorothioic acid, O,O -diethyl O -[2-
(ethylthio)-ethyl] ether:
S
||
(C2H5O)2-P-OC2H4-SC2H5
Samples of demeton-S-methyl that have been stored for a few months
may contain up to 4% of the sulfonium compound. The
transalkylation reaction is extremely rapid with demetonmethyl, but
slower with demeton.
2.2 Analytical Methods
Procedures consist of sampling, extraction, clean-up of
extract, and determination of compounds. Different procedures are
required for the lipophilic alkali-labile parent pesticides and for
residues that may be mainly stable non-lipophilic hydrolysis
products. Procedures for the determination of pesticide residues
(not only organophosphorus insecticides) are discussed in the
Report of a Joint FAO/WHO Course (Ambrus & Greenhalgh, 1984).
Separation and clean-up usually involve partition between solvents
and chromatography. Detection may be by partially specific colour
reagents or by enzyme inhibition tests applied to spots on thin-
layer chromatographic plates (Stefanac et al., 1976) or by
formation of volatile derivatives suitable for detection by gas
chromatography (Shafik et al., 1973). Diazopentane has been
recommended as a reagent that is less toxic and less difficult to
handle than diazomethane which is often used, but not all workers
regard the modification as satisfactory (Drevenkar et al., 1979).
The hazard of using the volatile and highly carcinogenic
diazomethane as a laboratory reagent should be recognized.
Methods for the the determination of residues of many
individual pesticides are given by the Codex Alimentarius
Commission (1984).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION, EXPOSURE LEVELS
3.1 Sources of Pollution
Organophosphorus pesticides are mainly used in crop protection.
The world-wide consumption of these compounds from 1974-83 is
shown in Table 2. Only parathion and malathion can be shown
separately from the other organophosphorus pesticides. The
information is incomplete since, for example, the USA and some
other countries and regions do not report figures for every year.
However, comparison of the figures given on a yearly basis gives an
idea of the magnitude of the consumption and distribution of the
organophosphorus pesticides throughout the world.
All organophosphorus pesticides are subject to degradation by
hydrolysis yielding water-soluble products that are believed to be
non-toxic at all practical concentrations. The toxic hazard is
therefore essentially short-term in contrast to that of the
persistent organochlorine pesticides, though the half-life at
neutral pH may vary from a few hours for dichlorvos to weeks for
parathion. At the pH of slightly acidic soils (pH 4 - 5), these
half-lives will be extended many-fold. However, constituents of
soil and of river water may themselves catalyse degradation.
3.2 Environmental Transport and Distribution
3.2.1 Distribution in air and water
With the exception of dichlorvos, most organophosphorus
pesticides are of comparatively low volatility. Aerial sprays of
dispersions of organophosphates may be spread by wind, but no
evidence of contamination beyond limits of 1 - 2 km from the
spraying source has been noted.
Three sources of entry into water are possible. One is from
industrial waste or effluent discharged directly into water. A
second is by seepage from buried toxic wastes into water supplies.
Neither of these should be tolerated, since prior treatment of the
waste with alkali (or acid in cases such as diazinon) followed by
neutralization can destroy the toxic agent. Contamination of
running water directly or from run-off during spraying operations
can occur. No studies on the degradation of organophosphorus
pesticides in running water have been noted. In static water, in a
simulated aquatic environment, there is evidence of the
contributions of light, suspended particulates, and bacteria to
degradation. Thus, the degradation of fenitrothion in lake water
under illumination occurred with a half-life of about 2 days,
compared with 50 days in the dark (Greenhalgh et al., 1980).
Furthermore, Drevenkar et al. (1976) concluded that, though
temperature and pH were major factors controlling the rate of
hydrolysis of dichlorvos in water, large differences in the half-
life of this pesticide in different river waters must be attributed
to microbiological factors.
Table 2. Consumption of organophosphorus insecticides (in 100 kg)a
-----------------------------------------------------------------------------------------------------------------
Country Parathion Malathion Other organophosphorus
insecticides
1974 1981 1982 1983 1974 1981 1982 1983 1974 1981 1982 1983
-76 -76 -76
-----------------------------------------------------------------------------------------------------------------
Africa
Burundi 3
Egypt 397 3573 2080 54 267 7200
Gambia 120 1000 477 350
Madagascar 2
Mauritania 50 107
Niger 694 263 151 170 45
Rwanda 1 2 3
Sierra Leone 40
South Africa 24 083
Sudan 6787
Swaziland 17
Zimbabwe 215 450 91 10 3018
North/Central America
Bermuda 7 2
Canada 238 2398 11 519
Cuba 22 667
El Salvador 4000 50 357
Guatemala 7704 1010
Honduras 391 414
Mexico 46 000 50 000 48 000 48 000 3452 12 000 18 000 5000 21 812 54 520 46 350 48 300
Mont Serrat 1 1 1 1
USA 115 000 110 000 15 000 15 000 190 000 175 000
South America
Argentina 1650 4750 2280 2350 2993 8340 15 560
Guyana 52 60
Surinam 28 712
Uruguay 10 105 78 179 45 91 47 201 415 344 735
-----------------------------------------------------------------------------------------------------------------
Table 2. (contd.)
-----------------------------------------------------------------------------------------------------------------
Country Parathion Malathion Other organophosphorus
insecticides
1974 1981 1982 1983 1974 1981 1982 1983 1974 1981 1982 1983
-76 -76 -76
-----------------------------------------------------------------------------------------------------------------
Asia
Bahrain 5
Bangladesh 620 649
Brunei 8 6 20
Burma 34 317
Cyprus 222 1782 842 89 255 212 132 534 591
Hong Kong 1000 414 604 536
India 9657 20 920 30 300 15 640 6800 8000 14 927 40 740 53 510
Israel 8557 11 280 7550 5860
Japan 1800 1000 128 880
Jordan 5000 4500 103 736 40 382
Korea Rep. 711 1426 1601 2759 726 337 24 522 28 224 28 615
Kuwait 4 4
Oman 350 240 830 498 108
Pakistan 982 530 324 90 2381 675 7929 8998 7460
Philippines 4800 630 310 80
Saudi Arabia 55
Sri Lanka 590 1690 20
Turkey 6480 1750 1837 1939 550 577 20 764 11 000 11 550
United Arab 34
Emirates
Europe
Austria 129 201 156 150 839 798 992 993
Czecho- 20 130 44 187 3370 4812 4892
slavakia
Denmark 1581 2578 2334 206 110 108 656 847 1123
Finland 55 74 407
Greece 2873 2837 6493
Hungary 30 599 17 325 11 301 10 190 2711 1584 1598 3130 66 624 77 361 66 848 44 606
Iceland 2 2 2 3 3 2
Italy 23 147 24 231 18 591 8997 6068 5524 87 204 149 558 144 956
Malta 350 250
Norway 196 205 202 212
Poland 530 707 1062 1038 6730 5099 12 246 13 549
Portugal 301 509 643 109 144 227 854 818 880
Sweden 3020 74 60 1029 1264
Switzerland 800 850 800
----------------------------------------------------------------------------------------------------------------
a From: FAO (1984).
3.2.2. Distribution in food
Exposure of food materials to organophosphorus pesticides
occurs chiefly at the crop-growing stage. The scale and frequency
of application varies enormously. Thus, one or 2 applications may
be adequate for pest control in temperate climates, while as many
as 50 applications in one peach-growing season have been reported
for a hot and humid region (Wicker et al., 1979). The amount
remaining on the crop at harvest depends chiefly on the interval
between application and harvest and on the effects of rainfall,
which can wash the active agent off and also provide a milieu for
hydrolysis. Thus, under exceedingly hot and dry conditions, very
high residues of paraoxon were found on citrus plants that had been
sprayed with parathion, 28 days previously: these levels accounted
for the poisoning of several orange pickers, who were working in
the grove at this time, after what is normally an acceptable safe
interval from the time of spraying (Spear et al., 1977). It seems
that both excessive photo-oxidative formation of paraoxon and
absence of hydrolysis or wash-off accounted for the toxic level of
paraoxon. Post-harvest levels of these organophosphorus pesticides
in food appear to decline steadily: this loss is thought to be
principally due to hydrolysis. Dichlorvos, malathion, or
pirimiphos-methyl may be applied to stored grain for the control of
some pests.
For each of the organophosphorus pesticides covered by the
Joint FAO/WHO Meetings, considerable detail is available on the
rate of decline of residues on a wide variety of crops, under
different climatic conditions.
3.3 Bioaccumulation and Degradation in the Environment
While storage in the fat of an organism or animal may reduce
the rate of clearance from that individual, it is unlikely that
significant amounts of an organophosphorus pesticide stored in one
organism could survive the hydrolytic processes of consumption and
digestion to be stored successively by higher members of the food-
chain. Direct poisoning of consumers of sprayed food or pest-
contaminated carcasses can occur, of course.
Degradation in the environment involves both hydrolysis and
oxidation to mono- or di-substituted phosphoric or phosphonic acids
or their thio analogues. There is no evidence that these products
are toxic to any significant extent. If aerial oxidation of a
phosphorothioate precedes hydrolysis, then the product will be a
toxic anticholinesterase, so that hazard due to exposure may
increase for a few days in a dry atmosphere after spraying (section
2.1). Occasionally, other reactions that can be regarded as
chemical degradation yield a more toxic product. Thus, leptophos
(phosphonothioic acid, phenyl-, O -(4-bromo-2,5-dichloro-phenyl)
O -methyl ester) is converted to its desbromo analogue in sunlight,
and the product is considerably more active than the parent in
causing delayed neuropathy (Johnson, 1975b; Sanborn et al., 1977).
Further degradation of the acids to inorganic phosphate is not well
documented, but bacterial cleavage of the carbon-phosphorus bond of
a phosphonate has been reported (Daughton et al., 1979). Whatever
the precise means of degradation, it is clear that residues of most
organophosphorus pesticides are rapidly lost from food crops and
are usually barely detectable 4 weeks after application, though the
exact rate of loss depends on the weather conditions. For a few
organophosphorus pesticides, such as leptophos and fenamiphos, the
residual life is longer (El-Sebae, personal communication, 1985).
Fenamiphos is claimed by its manufacturers to have a residual
activity in soil of "several months" (Bayer, 1971).
3.4 Exposure Levels
3.4.1 Exposure of the general population
Exposure of the general population may occur through the
consumption of foodstuffs treated incorrectly with pesticides or
harvested prematurely before residues have declined to acceptable
levels, from contact with treated areas, or from domestic use.
Exposure of limited populations during disease vector control
is considered below. Significant exposure of the general
population should be unlikely, since the use of these compounds
for crop protection under "good agriculture practice" does not
leave residues that are considered harmful in food. At annual
meetings of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide
Residues, data accumulated on new and older compounds over the
years are reviewed, and maximum residue limits (MRLs) in various
foods and acceptable daily intakes (ADIs) for the individual
compounds established. The ADIs for the compounds discussed in
this review are given in Annex II.
3.4.2 Occupational exposure
Exposure of factory workers during the undisturbed synthesis of
pesticides is probably negligible, since the processes are carried
out in closed vessels. However, the formulation and dispensing of
formulated pesticides may cause considerable contamination of
workers. The whole range of workers associated with pesticide-
treatment of crops or premises is also liable to exposure as are
both workers and segments of the population during disease vector
control procedures.
Exposure may be via the inhalation, dermal, or oral route.
Dermal contact is the most important route of exposure for
pesticide workers. Durham & Wolfe (1962) described and evaluated
procedures for the use of air samples, pads attached to exposed
body surfaces, and washes, in the direct measurement of the dermal
and respiratory exposure of workers to pesticides. Good methods
are not available for measuring oral exposure. The extent of
exposure depends on disciplined hygiene among workers. Provided
smoking, eating, and drinking in the work area are forbidden, and
these activities are only engaged in after workers have washed
thoroughly, oral intake should be negligible. Exposure by other
routes depends on the amount of protective clothing worn, and, on
the physical state of the pesticide. The majority of
organophosphorus pesticides are liquids having different vapour
pressures at room temperature (i.e., dichlorvos is much more
volatile than malathion); thus, hazard due to inhalation of vapour
varies from compound to compound. The vapour pressure of the
active agent is reduced on dilution with solvent, emulsifier, etc.,
so that the inhalation hazard is reduced, but these additives may
facilitate adsorption of spilled material through the skin. The
likelihood of acute poisoning occurring among process workers seems
greatest when dealing with liquid formulations. It was impossible
to judge the comparative contributions via the dermal and
respiratory routes in the case of poisoning with demeton- S -methyl
reported by Vale & Scott (1974), but it was noted that the area
where intoxication occurred was an unventilated cubicle. The
routine use of gas masks or bottled air respirators may be
necessary, when concentrated liquid pesticides are dispensed. In
studies on several powder-formulated pesticides, Wolfe et al.
(1978) showed that potential dermal exposure markedly exceeded
respiratory exposure; thus, the mean exposure to parathion for the
most contaminated group of workers in a formulation plant was 184
mg/h of work activity for dermal contamination and 0.03 mg/h for
respiratory exposure, the highest values being 33.5 and 33.8 mg/h,
respectively, for one individual. The actual uptake as a result of
such exposures is harder to quantify and may vary according to the
mode of formulation of the pesticide as well as to the
lipophilicity and volatility of the compound and the area of
exposed skin. Using the calculations of Durham & Wolfe (1962), the
highest exposure noted above would have represented 25% of the
toxic dose, had it all been absorbed. However, these authors
calculated that even with contamination by liquid parathion
formulations (which presumably enter more easily through contact
with the skin), the amount absorbed by orchard spraymen was only a
mean 1.23% (0.40 - 1.95% range) of the measured potential dermal
exposure (Durham et al., 1972). The mean dermal and respiratory
exposures of the spraymen were 19 and 0.02 mg/h, respectively,
which was markedly lower than those in the formulating plant. In
view of the inefficiency of absorption, it is perhaps less
surprising that ChE changes were negligible, though total urinary
4-nitrophenol excretion was significant, when a volunteer was
totally covered with 2% parathion dust and enclosed in a rubber
suit for 7 h, spent alternatively in the sun and shade (Hayes et
al., 1964).
Similarly, a 2-h exposure to 48% parathion emulsifiable
concentrate swabbed on the right hand and forearm of a volunteer to
the point of run-off did not cause any change in erythrocyte- or
plasma-ChEs and an average of 10 µg 4-nitrophenol/h was excreted
during the following 24 h. Hayes (1971) stated that absorption of
parathion was tolerated without illness and with little or no
reduction in ChE activity, as long as the concentration of
4-nitrophenol in the urine did not rise above 60 - 80 µg/h (2 ppm),
assuming an average urine excretion of 30 - 40 ml/h.
Studies on orchard spray workers (Wolfe et al., 1967) showed
that, as in the formulation plant, the potential exposure of a
worker without special protective clothing was largely dermal, for
instance, 19.4 mg parathion/h by dermal exposure and 0.02 mg/h
respiratory. This is about 3 times less than the mean exposure of
some formulator/baggers (see above). However, the respiratory
exposure was increased 4-fold, when applying dusts compared with
dilute spray, and 10-fold, when using aerosols of concentrated
pesticide (not necessarily organophosphates). Thus, in the last
case, the respiratory route could be highly important when the
efficiency of absorption is allowed for.
The droplet size in pesticide sprays is influenced by the spray
machinery and a recent study compared potential dermal exposure
during mixing and loading with that during spraying with various
machines. Knapsack spraying seemed to cause much greater dermal
exposure in operators than electrostatic spraying (British
Agrochemical Association, 1983).
Significant exposure of workers may occur when they enter a
previously sprayed crop area for the purposes of further
cultivation or hand-harvesting. The re-entry concept was first
discussed by Milby et al. (1964) in relation to the prevention of
illness. The extent of exposure depends on many factors, including
the physical properties of the pesticide and its biodegradability,
the crop, the nature of the proposed worker operation, and, also,
on the local weather; thus, marked regional differences may occur.
Procedures for determining foliar residues and their dissipation
rates were described by Gunther et al. (1973, 1974), and the topic
was further reviewed by Knaak (1980). Kahn (1979) provided an
outline guide to the procedures and factors to be considered when
performing field studies to establish safe re-entry intervals in
relation to organophosphorus pesticides. One such study was
described by Guthrie et al. (1974). Kahn (1979) cited the US EPA
(1975) in its Registration Procedures as requiring "data necessary
to determine required intervals between pesticide application and
safe re-entry". Re-entry periods appropriate to local conditions
for some pesticides and crops have been reported by Knaak (1980)
and by Kaloyanova-Simeonova & Izmirova-Mosheva (1983).
The situation for workers entering fields that have been
sprayed with some organophosphorus esters differs from that of
workers exposed to dieldrin, for example. In the case of the
former, the oxidation products generated by the action of light and
air may be far more toxic for man than the applied pesticide
(section 2.1), so that the residues on the crops may be more
hazardous for a few days after application than at the time of
application. Degradation is fairly rapid, but clearly a balance of
effects between activation and degradation must be taken into
account, initially.
A "Standard Protocol for Field Surveys of Exposure to
Pesticides" has been published by the World Health Organization
(WHO, 1982).
4. METABOLISM AND MODE OF ACTION
4.1 Uptake
Most organophosphorus pesticides are not ionized and are very
lipophilic. Thus, inhaled or swallowed material will be easily
taken up.
4.1.1 Dermal uptake
Many accidental acute poisonings have occurred following
spillage of pesticide on skin and clothing. The extent of uptake
will depend on persistence time (related to volatility, clothing,
coverage, and thoroughness of washing after exposure), and also on
the presence of solvents and emulsifiers that may facilitate
uptake. However, the evidence concerning parathion, quoted in
section 3.4.2, suggests that dermal absorption is not an efficient
process, under normal working conditions. Experimental
determinations of dermal toxicity depend on the conditions
employed, particularly on whether the treated skin is covered or
not, and on how long the application is left before cleansing.
These are frequently not stated in toxicological reports. With
this limitation in mind, the comparison can be made for the
toxicity of omethoate in rats by 2 routes: the dermal LD50 is 860 -
1020 mg/kg body weight and the oral LD50 is 25 - 28 mg/kg body
weight (FAO/WHO, 1979b). In contrast, uptake through the skin can
be very efficient for more lipophilic agents and, since they avoid
the first-pass metabolic disposal in the liver, agents such as DEF
and EPN may be at least as toxic by the dermal route as by the oral
route in laboratory tests.
4.1.2 Gastrointestinal tract
In rats, the uptake of most of the organophosphorus pesticides
reviewed seems to be rapid and efficient under test conditions
usually involving a dose well below the LD50.
However, the question that does not appear to have been
answered is whether this is true with large doses of low-toxicity
compounds. Thus, the LD50 of bromophos, for rats, is > 3 g/kg
body weight (FAO/WHO, 1973b), but it is not clear whether this low
toxicity is in part a reflection of failure to absorb the majority
of the dose above some unknown threshold. In absorption studies,
using radiolabelled bromophos at a dose of 10 mg/kg body weight,
approximately 96% of the radiolabel was absorbed and excreted in
the urine within 24 h of oral dosing. There is evidence of
comparatively inefficient absorption in hens administered large
doses of very insoluble organophosphorus pesticides with a high
relative molecular mass, such as haloxon [phosphoric acid, 3-
chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl bis-(2-chloroethyl)
ester]:
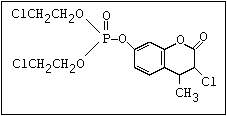 or leptophos [phosphonothioic acid, phenyl-, O -(4-bromo-2,5-
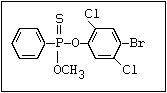
dichlorophenyl) O -methyl ester]:
or leptophos [phosphonothioic acid, phenyl-, O -(4-bromo-2,5-
dichlorophenyl) O -methyl ester]:
 Thus, divided doses may exert a greater toxic effect than the same
amount given as a single large dose (section 6.1.2).
The question of the bioavailability of preparations given by
the oral route needs to be considered. It must be taken into
account when discussing the results obtained for LD50s, and it is
certainly important when considering the toxicity of pesticides
residues. From a chemical point of view, these residues can be
described as parent compounds, free metabolites, and their
conjugates (Kaufman, 1976). The bioavailability of these
fractions and, thus, their toxic potential are not the same
(Dorough, 1976; Marshall & Dorough, 1977). In general, bound
residues appear to have a lower bioavailability and lower toxicity.
This was discussed by Rico & Burgat-Sacaze (1984), and demonstrated
for some pesticides residues by Marshall & Dorough (1977).
4.1.3 Inhalation
Total urinary output of 4-nitrophenol was compared in workers
spraying parathion, who either breathed a pure air supply but did
not wear protective clothing, or who wore total protective
clothing, but did not have any respiratory protection (Durham et
al., 1972). Output derived from the respiratory source compared
with that derived from the dermal source was 1.2% in one test and
12% in another. Since the total exposures by the dermal and
respiratory routes were in the proportion of 1000:1, and the
efficiency of dermal absorption was 1 - 2%, it follows that the
efficiency of absorption by the respiratory route was higher than
20% and could well have been complete.
4.2 Distribution and Storage
The intrinsically reactive chemical nature of organophosphorus
pesticides means that any that enter the body are immediately
liable to a number of biotransformations and reactions with tissue
constituents (particularly tissue proteins carrying esterase active
sites), so that the tracing of radiolabelled material alone does
not give any clue to the distribution of the unchanged parent
compound. It is possible to determine the rate of disposal of
metabolites and thereby to estimate an approximate half-life of
pesticide in the body. Such numbers may be helpful in estimating
safe intervals between successive low exposures, under working
conditions. However, although the half-life of organophosphorus
pesticides and their inhibitory metabolites in vivo is
comparatively short, at least one case of poisoning demonstrated
that significant amounts remained in the body for several weeks
after an acute crisis. Ecobichon et al. (1977) reported a case of
poisoning by fenitrothion. After an effective treatment period
with atropine and an oxime reactivator, leading to 2 days without
symptoms or any therapy, symptoms of nausea and diarrhoea recurred
associated with a decline in the previously restored blood-ChE
levels. These symptoms were controlled by further administration
of oxime, which led to a prompt restoration of the enzyme to a
near-normal level. Further recurrence of symptoms was reported, at
intervals, especially associated with periods of mobilization of
adipose tissue. The conclusion is that the treatment was reversing
the recent inhibition of AChE by a compound that had been stored in
the body and was entering the circulation over a period of many
days.
4.2.1 Experimental animal studies on distribution and storage
In view of the inherent instability of organophosphorus
insecticides, storage in human tissue is not anticipated to be
prolonged (unlike the situation for DDT); population studies,
including analyses of cadavers, do not seem to have been carried
out and would be a pointless exercise. Experimental animal studies
have shown that most of a radiolabelled dose is rapidly excreted in
expired air, urine, and faeces. Thus, it was reported that from 67
to 100% of the administered radioactivity was recovered within 1
week in the combined urine and faeces of cows, rats, and a goat,
given various doses of 32 P-dichlorvos; no organosoluble
radioactivity, which might include unchanged dichlorvos, was
detected after the first 2 h (Blair et al., 1975), though 14 C in
alkyl groups may enter the general metabolic pool and be
incorporated into tissues. Also, phosphorylated proteins are
presumably replaced only by resynthesis and this is a comparatively
slow process with enzymes, such as erythrocyte- and brain-AChE,
typically returning to pre-exposure levels over a period of a few
weeks after irreversible phosphorylation.
In a case of human poisoning by dichlorofenthion, steadily
decreasing concentrations of the pesticide were found in serial fat
biopsy samples up to 48 days after intoxication: the decline
matched a return of blood-ChE levels towards normal, and recovery
of health. Indirect evidence for the short-term storage of
significant amounts of lipophilic organophosphorus compounds was
the return of cholinergic poisoning signs a day or two after
discontinuing oxime therapy in a woman who had been accidentally
poisoned with fenitrothion (Ecobichon et al., 1977). Reinstatement
of therapy led to rapid amelioration of signs and the cycle was
repeated at intervals up to the 15th day after intoxication, after
which her health improved slowly.
4.3 Biotransformation
Alternative metabolic pathways, often available in animals and
man, are listed below, with examples. Most general studies of
pathways have been made on phosphates and their thioate analogues.
Although the ultimate fate of phosphonate pesticides has been
determined, the pathways are usually presumed on the basis of
phosphate studies, which indicate that cleavage of the phosphoric-
carbon bond is limited in mammalian systems. A summary is given
below; further details can be found in Dauterman (1971), Eto
(1974), CEC (1977), and in the annual reports of the Joint FAO/WHO
Meetings on Pesticide Residues in Food (Annex II).
Biotransformation reactions can be divided into three distinct
classes. The former are reactions involving (a) mixed-function
oxidases; (b) hydrolases; and (c) transferases. There is also a
miscellaneous group of unrelated reactions. Binding of
organophosphorus insecticide oxons to tissue is also a significant
biotransformation reaction.
4.3.1 Mixed-function oxidases (MFOs)
Many apparently unrelated substrates can be oxidized by mixed-
function oxidase (MFO) systems associated typically with liver
endoplasmic reticulum, but present also in some other tissues such
as intestine, lung, and kidney. Within the liver, there appears to
be a family of MFOs, possibly with some enzymes in common, but
utilizing slightly different cytochromes of which cytochrome P-450
is the best known. The MFO activity in the liver can vary greatly
according to the nutritional and hormonal state of the animal and
also according to stimuli arising from the ingestion of some
foreign compounds (section 6.3).
4.3.1.1 Oxidative desulfuration
The reaction (Fig. 1) is essentially the activation of the
precursor phosphorothioate to the directly inhibitory phosphate
ester, which is responsible for the inhibition of AChE and for
subsequent toxic effects. There is no evidence of inhibition of
AChE by phosphorothioates occurring under normal situations,
without prior conversion to the phosphate; reports of the
inhibitory power of technical grades of phosphorothioates in vitro
are meaningless, since the activity is almost certainly caused by
traces of the oxon, the activity of which is several orders
greater.
Thus, divided doses may exert a greater toxic effect than the same
amount given as a single large dose (section 6.1.2).
The question of the bioavailability of preparations given by
the oral route needs to be considered. It must be taken into
account when discussing the results obtained for LD50s, and it is
certainly important when considering the toxicity of pesticides
residues. From a chemical point of view, these residues can be
described as parent compounds, free metabolites, and their
conjugates (Kaufman, 1976). The bioavailability of these
fractions and, thus, their toxic potential are not the same
(Dorough, 1976; Marshall & Dorough, 1977). In general, bound
residues appear to have a lower bioavailability and lower toxicity.
This was discussed by Rico & Burgat-Sacaze (1984), and demonstrated
for some pesticides residues by Marshall & Dorough (1977).
4.1.3 Inhalation
Total urinary output of 4-nitrophenol was compared in workers
spraying parathion, who either breathed a pure air supply but did
not wear protective clothing, or who wore total protective
clothing, but did not have any respiratory protection (Durham et
al., 1972). Output derived from the respiratory source compared
with that derived from the dermal source was 1.2% in one test and
12% in another. Since the total exposures by the dermal and
respiratory routes were in the proportion of 1000:1, and the
efficiency of dermal absorption was 1 - 2%, it follows that the
efficiency of absorption by the respiratory route was higher than
20% and could well have been complete.
4.2 Distribution and Storage
The intrinsically reactive chemical nature of organophosphorus
pesticides means that any that enter the body are immediately
liable to a number of biotransformations and reactions with tissue
constituents (particularly tissue proteins carrying esterase active
sites), so that the tracing of radiolabelled material alone does
not give any clue to the distribution of the unchanged parent
compound. It is possible to determine the rate of disposal of
metabolites and thereby to estimate an approximate half-life of
pesticide in the body. Such numbers may be helpful in estimating
safe intervals between successive low exposures, under working
conditions. However, although the half-life of organophosphorus
pesticides and their inhibitory metabolites in vivo is
comparatively short, at least one case of poisoning demonstrated
that significant amounts remained in the body for several weeks
after an acute crisis. Ecobichon et al. (1977) reported a case of
poisoning by fenitrothion. After an effective treatment period
with atropine and an oxime reactivator, leading to 2 days without
symptoms or any therapy, symptoms of nausea and diarrhoea recurred
associated with a decline in the previously restored blood-ChE
levels. These symptoms were controlled by further administration
of oxime, which led to a prompt restoration of the enzyme to a
near-normal level. Further recurrence of symptoms was reported, at
intervals, especially associated with periods of mobilization of
adipose tissue. The conclusion is that the treatment was reversing
the recent inhibition of AChE by a compound that had been stored in
the body and was entering the circulation over a period of many
days.
4.2.1 Experimental animal studies on distribution and storage
In view of the inherent instability of organophosphorus
insecticides, storage in human tissue is not anticipated to be
prolonged (unlike the situation for DDT); population studies,
including analyses of cadavers, do not seem to have been carried
out and would be a pointless exercise. Experimental animal studies
have shown that most of a radiolabelled dose is rapidly excreted in
expired air, urine, and faeces. Thus, it was reported that from 67
to 100% of the administered radioactivity was recovered within 1
week in the combined urine and faeces of cows, rats, and a goat,
given various doses of 32 P-dichlorvos; no organosoluble
radioactivity, which might include unchanged dichlorvos, was
detected after the first 2 h (Blair et al., 1975), though 14 C in
alkyl groups may enter the general metabolic pool and be
incorporated into tissues. Also, phosphorylated proteins are
presumably replaced only by resynthesis and this is a comparatively
slow process with enzymes, such as erythrocyte- and brain-AChE,
typically returning to pre-exposure levels over a period of a few
weeks after irreversible phosphorylation.
In a case of human poisoning by dichlorofenthion, steadily
decreasing concentrations of the pesticide were found in serial fat
biopsy samples up to 48 days after intoxication: the decline
matched a return of blood-ChE levels towards normal, and recovery
of health. Indirect evidence for the short-term storage of
significant amounts of lipophilic organophosphorus compounds was
the return of cholinergic poisoning signs a day or two after
discontinuing oxime therapy in a woman who had been accidentally
poisoned with fenitrothion (Ecobichon et al., 1977). Reinstatement
of therapy led to rapid amelioration of signs and the cycle was
repeated at intervals up to the 15th day after intoxication, after
which her health improved slowly.
4.3 Biotransformation
Alternative metabolic pathways, often available in animals and
man, are listed below, with examples. Most general studies of
pathways have been made on phosphates and their thioate analogues.
Although the ultimate fate of phosphonate pesticides has been
determined, the pathways are usually presumed on the basis of
phosphate studies, which indicate that cleavage of the phosphoric-
carbon bond is limited in mammalian systems. A summary is given
below; further details can be found in Dauterman (1971), Eto
(1974), CEC (1977), and in the annual reports of the Joint FAO/WHO
Meetings on Pesticide Residues in Food (Annex II).
Biotransformation reactions can be divided into three distinct
classes. The former are reactions involving (a) mixed-function
oxidases; (b) hydrolases; and (c) transferases. There is also a
miscellaneous group of unrelated reactions. Binding of
organophosphorus insecticide oxons to tissue is also a significant
biotransformation reaction.
4.3.1 Mixed-function oxidases (MFOs)
Many apparently unrelated substrates can be oxidized by mixed-
function oxidase (MFO) systems associated typically with liver
endoplasmic reticulum, but present also in some other tissues such
as intestine, lung, and kidney. Within the liver, there appears to
be a family of MFOs, possibly with some enzymes in common, but
utilizing slightly different cytochromes of which cytochrome P-450
is the best known. The MFO activity in the liver can vary greatly
according to the nutritional and hormonal state of the animal and
also according to stimuli arising from the ingestion of some
foreign compounds (section 6.3).
4.3.1.1 Oxidative desulfuration
The reaction (Fig. 1) is essentially the activation of the
precursor phosphorothioate to the directly inhibitory phosphate
ester, which is responsible for the inhibition of AChE and for
subsequent toxic effects. There is no evidence of inhibition of
AChE by phosphorothioates occurring under normal situations,
without prior conversion to the phosphate; reports of the
inhibitory power of technical grades of phosphorothioates in vitro
are meaningless, since the activity is almost certainly caused by
traces of the oxon, the activity of which is several orders
greater.
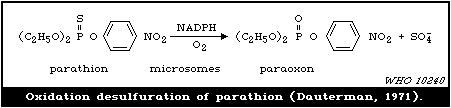 4.3.1.2 Oxidative N -dealkylation
This reaction may be associated with the metabolic activation
of a non-inhibitory precursor, such as schradan, or with
transformation of one inhibitor to another (Fig. 2).
4.3.1.2 Oxidative N -dealkylation
This reaction may be associated with the metabolic activation
of a non-inhibitory precursor, such as schradan, or with
transformation of one inhibitor to another (Fig. 2).
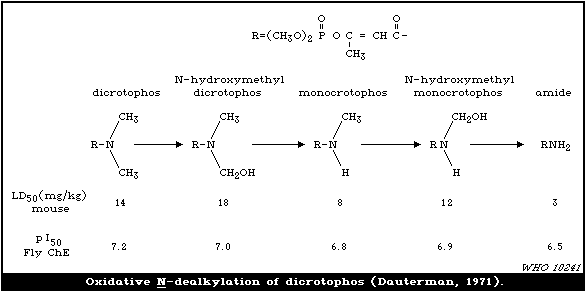 4.3.1.3 Oxidative O -dealkylation
The conversion of triesters to diester is a detoxication
process and was once considered to be mediated only by hydrolytic
enzymes (phosphoryl phosphatases or A-esterases). However, a
reaction requiring liver microsomes, NADPH, and oxygen (the typical
MFO system) deethylates chlorofenvinphos with the production of
acetaldehyde, probably as shown in Fig. 3. It seems that various
phosphates, but not phosphorothioates, are metabolized by this
route in mammals.
4.3.1.3 Oxidative O -dealkylation
The conversion of triesters to diester is a detoxication
process and was once considered to be mediated only by hydrolytic
enzymes (phosphoryl phosphatases or A-esterases). However, a
reaction requiring liver microsomes, NADPH, and oxygen (the typical
MFO system) deethylates chlorofenvinphos with the production of
acetaldehyde, probably as shown in Fig. 3. It seems that various
phosphates, but not phosphorothioates, are metabolized by this
route in mammals.
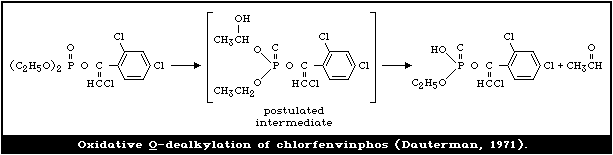 4.3.1.4 Oxidative de-arylation
Liver MFOs from rat or rabbit can cleave the acid-anhydride
bond coupling phosphorus to the phenolic group in parathion and
analogues (Nakatsugawa et al., 1968), and the same system may also
be responsible for the cleavage of diazinon (Yang et al., 1971).
In contrast to O -dealkylation noted above, this reaction appears
to deal only with phosphorothioates and not phosphates.
4.3.1.5 Thioether oxidation
Oxidation of sulfur in the phosphorus-sulfur-carbon moiety of
demeton-S or or dimethoate and omethoate has not been reported, but
oxidation is known of carbon-sulfur-carbon moieties with the
formation of sulfoxides and sulfones that are more active AChE
inhibitors than the parent compound and remain in circulation for a
comparatively long time (Fig. 4).
4.3.1.4 Oxidative de-arylation
Liver MFOs from rat or rabbit can cleave the acid-anhydride
bond coupling phosphorus to the phenolic group in parathion and
analogues (Nakatsugawa et al., 1968), and the same system may also
be responsible for the cleavage of diazinon (Yang et al., 1971).
In contrast to O -dealkylation noted above, this reaction appears
to deal only with phosphorothioates and not phosphates.
4.3.1.5 Thioether oxidation
Oxidation of sulfur in the phosphorus-sulfur-carbon moiety of
demeton-S or or dimethoate and omethoate has not been reported, but
oxidation is known of carbon-sulfur-carbon moieties with the
formation of sulfoxides and sulfones that are more active AChE
inhibitors than the parent compound and remain in circulation for a
comparatively long time (Fig. 4).
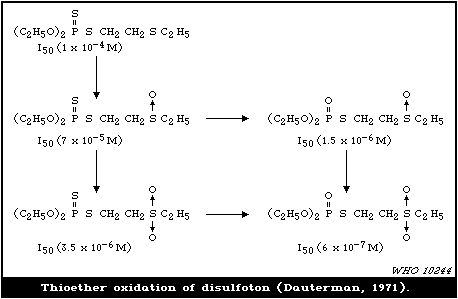 4.3.1.6 Side-chain oxidation
Stepwise oxidation of simple alkyl groups to hydroxy-, oxo-, or
carboxy-derivatives is a well-known process in the metabolism of
many compounds, apart from the organophosphorus compounds. The
conversion of fenitrothion to the water-soluble, 3-carboxy
derivative (Fig. 5) can account for the comparatively low mammalian
toxicity of this pesticide compared with that of the homologous
methyl parathion (rat oral LD50s of about 600 - 800 and 10 - 25
mg/kg body weight, respectively).
4.3.1.6 Side-chain oxidation
Stepwise oxidation of simple alkyl groups to hydroxy-, oxo-, or
carboxy-derivatives is a well-known process in the metabolism of
many compounds, apart from the organophosphorus compounds. The
conversion of fenitrothion to the water-soluble, 3-carboxy
derivative (Fig. 5) can account for the comparatively low mammalian
toxicity of this pesticide compared with that of the homologous
methyl parathion (rat oral LD50s of about 600 - 800 and 10 - 25
mg/kg body weight, respectively).
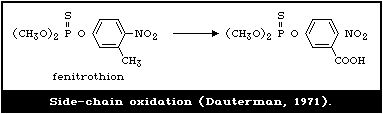 4.3.2 Hydrolases
Hydrolysis of the acid anhydride type ester bond of the leaving
group in pesticidal triesters is well known. The monobasic
diesters and their derivatives are the major urinary metabolites of
organophosphorus insecticides (Fig. 6). The enzymes commonly known
as A-esterases or phosphoryl phosphatases are widespread in
mammalian tissues, such as liver, plasma, intestine, etc., though
they are less abundant in many birds and may not be present in some
insects (Brealey et al., 1980). These enzymes are sometimes
referred to as DFPase or paraoxonase according to the substrate
used, but it does not mean that the enzymes are specific only for a
given organophosphorus compound. Although plasma contains enzymes
that can distinguish between closely related structures such as
paraoxon and 4-nitrophenyl, ethyl, or propylphosphonate, the
enzymes are not totally specific (Becker & Barbaro, 1964); the same
is probably true of A-esterase in other tissues.
4.3.2 Hydrolases
Hydrolysis of the acid anhydride type ester bond of the leaving
group in pesticidal triesters is well known. The monobasic
diesters and their derivatives are the major urinary metabolites of
organophosphorus insecticides (Fig. 6). The enzymes commonly known
as A-esterases or phosphoryl phosphatases are widespread in
mammalian tissues, such as liver, plasma, intestine, etc., though
they are less abundant in many birds and may not be present in some
insects (Brealey et al., 1980). These enzymes are sometimes
referred to as DFPase or paraoxonase according to the substrate
used, but it does not mean that the enzymes are specific only for a
given organophosphorus compound. Although plasma contains enzymes
that can distinguish between closely related structures such as
paraoxon and 4-nitrophenyl, ethyl, or propylphosphonate, the
enzymes are not totally specific (Becker & Barbaro, 1964); the same
is probably true of A-esterase in other tissues.
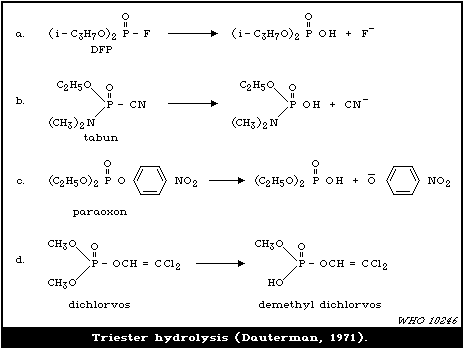 Hydrolysis of carboxylic acid ester bonds and carboxyl-amide
bonds in organophosphorus insecticides may be catalysed by
carboxylesterases (or B-esterases), which again occur widely in
mammalian tissues. Malathion, which contains 2 carboxylic ester
bonds, is the best-known organophosphorus pesticide that is
hydrolysed in this way (Fig. 7). The importance of this metabolic
route is shown by the fact that the rat oral LD50 for pure
malathion can be reduced from 10 000 to 100 mg/kg body weight, when
the tissue carboxyesterases are inhibited: this profound
potentiation is discussed further in section 5.3.
The hydrolysis of carboxylamide bonds such as in dimethoate is
catalysed by a liver enzyme (Chen & Dauterman, 1971) (Fig. 7).
Although apparently distinct from liver carboxyesterase, it too is
inhibited by its oxygen analogue (omethoate) and also by other
amide-containing phosphates such as dicrotophos.
Hydrolysis of carboxylic acid ester bonds and carboxyl-amide
bonds in organophosphorus insecticides may be catalysed by
carboxylesterases (or B-esterases), which again occur widely in
mammalian tissues. Malathion, which contains 2 carboxylic ester
bonds, is the best-known organophosphorus pesticide that is
hydrolysed in this way (Fig. 7). The importance of this metabolic
route is shown by the fact that the rat oral LD50 for pure
malathion can be reduced from 10 000 to 100 mg/kg body weight, when
the tissue carboxyesterases are inhibited: this profound
potentiation is discussed further in section 5.3.
The hydrolysis of carboxylamide bonds such as in dimethoate is
catalysed by a liver enzyme (Chen & Dauterman, 1971) (Fig. 7).
Although apparently distinct from liver carboxyesterase, it too is
inhibited by its oxygen analogue (omethoate) and also by other
amide-containing phosphates such as dicrotophos.
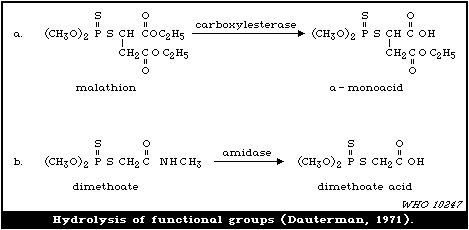 4.3.3 Transferases
The only transferase reaction that is known to deal with the
intact pesticidal organophosphorus triesters involves glutathione,
which is a required substrate for a number of transferase enzymes
present in liver and some other tissues. The enzymes have limited
but overlapping specificity so that the glutathione transferase
responsible for demethylating methyl paraoxon is distinct from that
which conjugates the 4-nitrophenol group in parathion. Activity in
the liver is greatest with methyl esters, but no evidence has been
found of methyl phosphonates undergoing this reaction (Dauterman,
1971).
4.3.3.1 Transferases handling primary metabolites
Reactions involving the conjugation of carboxylic acids,
alcohols, phenols, and amino, imino, and sulfydryl groups are well-
known and applicable to compounds carrying such groups, formed
after the oxidation, hydrolysis, etc. of an organophosphorus
pesticide. Such conjugation reactions aid in the elimination of
primary degradation products, which are usually devoid of
anticholinesterase activity, though they may cause other toxic
effects if they accumulate in the body.
4.3.4 Tissue binding
It is well-known that active metabolites of most
organophosphorus insecticides react covalently to some extent with
tissue esterases other than AChE. Since few of these esterases
appear vital to health (section 6.2.4), the binding reaction may be
considered a detoxification process. Although the catalytic
activity of these esterases is high, the actual quantity of such
sites is comparatively small. Crude measurements using 32 P-
labelled diisopropyl phosphorofluoridate (an agent reacting with
most organophosphorus-sensitive esterases) suggest that 100 - 150
µg bind per kg body weight of an adult hen injected with about the
LD50 dose (Johnson, M.K., personal communication, 1985). Binding
was principally in liver and muscle. The quantity bound would not
be expected to be much greater whatever the LD50 of an administered
organophosphorus insecticide. Thus, it is a significant proportion
of the total dose only for a very toxic compound such as paraoxon
(Lauwerys & Murphy, 1969) but not for compounds with much higher
LD50s. However, the number of binding sites may, in some cases, be
very significant compared with the quantity of circulating
anticholinesterase oxon that has avoided other metabolic disposal
processes. Molecules of oxon bound to these non-vial sites are
prevented from attacking the vital sites such as AChE or NTE
(section 6.1). Binding sites can therefore be considered an
important second line of defence against intoxication.
The specific problem of tissue binding that leads to
potentiation of the toxicity of malathion and other pesticides
containing carboxylester bonds is discussed in section 6.2.4.
4.4 Elimination
There is no evidence of prolonged storage of organophosphorus
compounds in the body, but the process of elimination can be
subdivided roughly according to the speed of the reactions
involved. Most organophosphorus pesticides are degraded quickly by
the metabolic reactions listed in section 4.3, and the elimination
of the products, mostly in the urine with lesser amounts in faeces
and expired air, is not delayed, so that rates of excretion usually
reach a peak within 2 days and decline quite rapidly. That they do
not almost immediately fall to zero is due to storage in fat and
covalent binding. As indicated in section 4.2, the former process
preserves toxic material, which is slowly released into the
circulation and which is active and is metabolized in the same way
as the bulk of the dose received. Covalent binding involves
phosphorylation of proteins, probably esterases having active
sites, including serine, which are mechanistically related to AChE.
The consequences of such phosphorylation depend on the esterase
involved, but many seem to be of only minor importance to the
continuing health of animals, and temporary inhibition may not be
expressed in physiological defects. The special case of neuropathy
target esterase, which is phosphorylated only by agents capable of
causing delayed neuropathy, is considered later (section 6.1.1.2).
4.5 Mode of Action
4.5.1 Inhibition of esterases
The primary biochemical effect associated with toxicity caused
by organophosphorus pesticides is inhibition of AChE. The normal
function of AChE is to terminate neurotransmission due to ACh that
has been liberated at cholinergic nerve endings in response to
nervous stimuli. Loss of AChE activity may lead to a range of
effects resulting from excessive nervous stimulation and
culminating in respiratory failure and death (section 6.1). The
chemistry of inhibition of AChE and of many other esterases (e.g.,
NTE and liver carboxyesterases, which are discussed elsewhere) by
these chemicals is similar and is given in schematic form in Fig.
8. Following the formation of a Michaelis complex (reaction 1), a
specific serine residue in the protein is phosphorylated with loss
of the leaving group X (reaction 2). Two further reactions are
possible: reaction 3 (reactivation) may occur spontaneously at a
rate that is dependent on the nature of the attached group and on
the protein and is also dependent on the influence of pH and of
added nucleophilic reagents, such as oximes, which may catalyse
reactivation. Reaction 4 ("aging") involves cleavage of an R-O-P-
bond with the loss of R and the formation of a charged mono-
substituted phosphoric acid residue still attached to protein. The
reaction is called "aging" because it is time-dependent, and the
product is no longer responsive to nucleophilic reactivating agents
such as some oximes. Since therapy of organophosphorus compound
poisoning is, in part, dependent on the reactivating power of
oximes (sections 6, 7), understanding of the "aging" reaction is
important. PseudoChE, which is present in blood-plasma and nervous
tissue but has no known physiological function, is inhibited by
organophosphorus compounds in a similar way to AChE, but the
specificity of the 2 enzymes is different. Though no toxic effect
arises as a result of inhibition of pseudoChE, measures of its
inhibition can be made for monitoring purposes (section 7.1.1.2).
4.5.2 Possible alkylation of biological macromolecules
It has been shown, under laboratory conditions, that some
organophosphates could react with and alkylate the reagent
4-nitrobenzylpyridine (Preussmann et al., 1969). The study was
interpreted to imply that the in vivo alkylating potential of some
pesticides was similar to that of the known mutagens dimethyl
sulfate and methyl methanesulfonate. Furthermore, Löfroth et al.,
(1969) derived a substrate constant (a logarithmic measure of
alkylating ability) of 0.75 for dichlorvos, which is intermediate
between those known for methyl and ethyl methanesulfonates.
Concern over the possible mutagenic and carcinogenic potential of
organophosphorus compounds on the basis of the above data was
misplaced, since alternative reactions were not considered.
Compared with the carbon atom of the alkyl group, the phosphorus
atom is markedly more electron-deficient and susceptible to attack
by nucleophiles. Analysis by Bedford & Robinson (1972) of the data
of Löfroth et al. (1969) revealed that the proposed rates of
alkylation by hard nucleophiles were probably combined rates of
phosphorylation and alkylation, and that phosphorylation was the
totally dominant reaction in the case of the hydroxide ion. The
comparison with known mutagens was therefore inappropriate. Two
factors detract further from the toxicological significance of the
alkylation studies. The first is that mammalian tissues (plasma,
liver, etc.) contain active enzymes that catalyse the
phosphorylation of water by the organophosphorus esters. Viewed
inversely, these enzymes (often called A-esterases) catalyse the
hydrolysis of the organophosphorus esters, thereby rapidly reducing
circulating levels of hazardous material. Secondly, the
comparative rate of reaction of most of these pesticides with AChE
is many orders greater than their rate of alkylation of the typical
nucleophile 4-nitrobenzylpyridine: for dichlorvos, the ratio of
rates was 1 x 107 in favour of the inhibitory phosphorylation of
AChE (Aldridge & Johnson, 1977). It follows that, at low exposure
levels, in vivo phosphorylation of AChE and other esterases will
be the dominant reaction with negligible uncatalysed alkylation of
genetic material. Indeed, no such alkylation has been detected in
sensitive in vivo studies designed to check this point (Wooder et
al., 1977). Some catalysed alkylations of glutathione by
organophosphorus compounds are known to occur in vivo (section 4),
but these are essentially detoxification reactions. The topic of
alkylation and the possible mutagenic or carcinogenic consequences
is discussed further in section 6.2.
4.3.3 Transferases
The only transferase reaction that is known to deal with the
intact pesticidal organophosphorus triesters involves glutathione,
which is a required substrate for a number of transferase enzymes
present in liver and some other tissues. The enzymes have limited
but overlapping specificity so that the glutathione transferase
responsible for demethylating methyl paraoxon is distinct from that
which conjugates the 4-nitrophenol group in parathion. Activity in
the liver is greatest with methyl esters, but no evidence has been
found of methyl phosphonates undergoing this reaction (Dauterman,
1971).
4.3.3.1 Transferases handling primary metabolites
Reactions involving the conjugation of carboxylic acids,
alcohols, phenols, and amino, imino, and sulfydryl groups are well-
known and applicable to compounds carrying such groups, formed
after the oxidation, hydrolysis, etc. of an organophosphorus
pesticide. Such conjugation reactions aid in the elimination of
primary degradation products, which are usually devoid of
anticholinesterase activity, though they may cause other toxic
effects if they accumulate in the body.
4.3.4 Tissue binding
It is well-known that active metabolites of most
organophosphorus insecticides react covalently to some extent with
tissue esterases other than AChE. Since few of these esterases
appear vital to health (section 6.2.4), the binding reaction may be
considered a detoxification process. Although the catalytic
activity of these esterases is high, the actual quantity of such
sites is comparatively small. Crude measurements using 32 P-
labelled diisopropyl phosphorofluoridate (an agent reacting with
most organophosphorus-sensitive esterases) suggest that 100 - 150
µg bind per kg body weight of an adult hen injected with about the
LD50 dose (Johnson, M.K., personal communication, 1985). Binding
was principally in liver and muscle. The quantity bound would not
be expected to be much greater whatever the LD50 of an administered
organophosphorus insecticide. Thus, it is a significant proportion
of the total dose only for a very toxic compound such as paraoxon
(Lauwerys & Murphy, 1969) but not for compounds with much higher
LD50s. However, the number of binding sites may, in some cases, be
very significant compared with the quantity of circulating
anticholinesterase oxon that has avoided other metabolic disposal
processes. Molecules of oxon bound to these non-vial sites are
prevented from attacking the vital sites such as AChE or NTE
(section 6.1). Binding sites can therefore be considered an
important second line of defence against intoxication.
The specific problem of tissue binding that leads to
potentiation of the toxicity of malathion and other pesticides
containing carboxylester bonds is discussed in section 6.2.4.
4.4 Elimination
There is no evidence of prolonged storage of organophosphorus
compounds in the body, but the process of elimination can be
subdivided roughly according to the speed of the reactions
involved. Most organophosphorus pesticides are degraded quickly by
the metabolic reactions listed in section 4.3, and the elimination
of the products, mostly in the urine with lesser amounts in faeces
and expired air, is not delayed, so that rates of excretion usually
reach a peak within 2 days and decline quite rapidly. That they do
not almost immediately fall to zero is due to storage in fat and
covalent binding. As indicated in section 4.2, the former process
preserves toxic material, which is slowly released into the
circulation and which is active and is metabolized in the same way
as the bulk of the dose received. Covalent binding involves
phosphorylation of proteins, probably esterases having active
sites, including serine, which are mechanistically related to AChE.
The consequences of such phosphorylation depend on the esterase
involved, but many seem to be of only minor importance to the
continuing health of animals, and temporary inhibition may not be
expressed in physiological defects. The special case of neuropathy
target esterase, which is phosphorylated only by agents capable of
causing delayed neuropathy, is considered later (section 6.1.1.2).
4.5 Mode of Action
4.5.1 Inhibition of esterases
The primary biochemical effect associated with toxicity caused
by organophosphorus pesticides is inhibition of AChE. The normal
function of AChE is to terminate neurotransmission due to ACh that
has been liberated at cholinergic nerve endings in response to
nervous stimuli. Loss of AChE activity may lead to a range of
effects resulting from excessive nervous stimulation and
culminating in respiratory failure and death (section 6.1). The
chemistry of inhibition of AChE and of many other esterases (e.g.,
NTE and liver carboxyesterases, which are discussed elsewhere) by
these chemicals is similar and is given in schematic form in Fig.
8. Following the formation of a Michaelis complex (reaction 1), a
specific serine residue in the protein is phosphorylated with loss
of the leaving group X (reaction 2). Two further reactions are
possible: reaction 3 (reactivation) may occur spontaneously at a
rate that is dependent on the nature of the attached group and on
the protein and is also dependent on the influence of pH and of
added nucleophilic reagents, such as oximes, which may catalyse
reactivation. Reaction 4 ("aging") involves cleavage of an R-O-P-
bond with the loss of R and the formation of a charged mono-
substituted phosphoric acid residue still attached to protein. The
reaction is called "aging" because it is time-dependent, and the
product is no longer responsive to nucleophilic reactivating agents
such as some oximes. Since therapy of organophosphorus compound
poisoning is, in part, dependent on the reactivating power of
oximes (sections 6, 7), understanding of the "aging" reaction is
important. PseudoChE, which is present in blood-plasma and nervous
tissue but has no known physiological function, is inhibited by
organophosphorus compounds in a similar way to AChE, but the
specificity of the 2 enzymes is different. Though no toxic effect
arises as a result of inhibition of pseudoChE, measures of its
inhibition can be made for monitoring purposes (section 7.1.1.2).
4.5.2 Possible alkylation of biological macromolecules
It has been shown, under laboratory conditions, that some
organophosphates could react with and alkylate the reagent
4-nitrobenzylpyridine (Preussmann et al., 1969). The study was
interpreted to imply that the in vivo alkylating potential of some
pesticides was similar to that of the known mutagens dimethyl
sulfate and methyl methanesulfonate. Furthermore, Löfroth et al.,
(1969) derived a substrate constant (a logarithmic measure of
alkylating ability) of 0.75 for dichlorvos, which is intermediate
between those known for methyl and ethyl methanesulfonates.
Concern over the possible mutagenic and carcinogenic potential of
organophosphorus compounds on the basis of the above data was
misplaced, since alternative reactions were not considered.
Compared with the carbon atom of the alkyl group, the phosphorus
atom is markedly more electron-deficient and susceptible to attack
by nucleophiles. Analysis by Bedford & Robinson (1972) of the data
of Löfroth et al. (1969) revealed that the proposed rates of
alkylation by hard nucleophiles were probably combined rates of
phosphorylation and alkylation, and that phosphorylation was the
totally dominant reaction in the case of the hydroxide ion. The
comparison with known mutagens was therefore inappropriate. Two
factors detract further from the toxicological significance of the
alkylation studies. The first is that mammalian tissues (plasma,
liver, etc.) contain active enzymes that catalyse the
phosphorylation of water by the organophosphorus esters. Viewed
inversely, these enzymes (often called A-esterases) catalyse the
hydrolysis of the organophosphorus esters, thereby rapidly reducing
circulating levels of hazardous material. Secondly, the
comparative rate of reaction of most of these pesticides with AChE
is many orders greater than their rate of alkylation of the typical
nucleophile 4-nitrobenzylpyridine: for dichlorvos, the ratio of
rates was 1 x 107 in favour of the inhibitory phosphorylation of
AChE (Aldridge & Johnson, 1977). It follows that, at low exposure
levels, in vivo phosphorylation of AChE and other esterases will
be the dominant reaction with negligible uncatalysed alkylation of
genetic material. Indeed, no such alkylation has been detected in
sensitive in vivo studies designed to check this point (Wooder et
al., 1977). Some catalysed alkylations of glutathione by
organophosphorus compounds are known to occur in vivo (section 4),
but these are essentially detoxification reactions. The topic of
alkylation and the possible mutagenic or carcinogenic consequences
is discussed further in section 6.2.
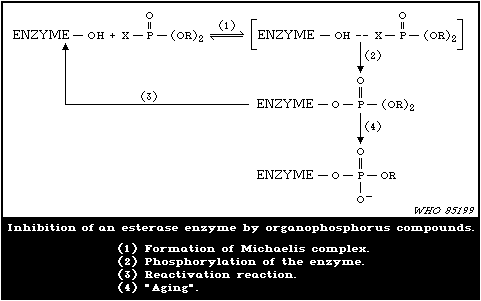 5. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
5.1 Aquatic Organisms
Organophosphorus insecticides are not very stable in aqueous
media. However, accidental leaching may occur from treated areas
into rivers and lakes where they may exert toxic effects on aquatic
organisms before degradation is complete. In clean water in the
laboratory, toxic effects were seen in several aquatic organisms
when they were exposed to concentrations of organophosphorus
insecticides ranging from 0.01 to 1 mg/litre for 48 h (Nishiuchi,
1981). However, lethal concentrations derived from 48-h exposures
in clear laboratory water may be artificially low compared with the
concentrations that would be effective in true environmental
waters.
One pesticide (phenthoate) appeared to be more toxic for
aquatic insects ("median tolerable limit" = 9 - 75 µg/kg)
(Nishiuchi, 1981) than for one species of fresh-water fish exposed
under apparently similar conditions (20% mortality caused by 263
µg/kg) (Jash & Bhattacharya, 1982).
Accidental release of pesticides in lakes, rivers, and bays
sometimes caused massive death of fish and many of the compounds
were strongly toxic for small aquatic organisms such as Daphnia, as
shown in Table 3.
Table 3. Acute toxicity of organophosphorus pesticides for some aquatic organisms
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Acephate > 40 - > 40 - > 40 Nishiuchi (1974)
Calvinphos > 40 - > 40 - 0.0042 Nishiuchi (1974)
Chlorfenvinphos 0.27 0.34 0.23 0.12 0.011 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Chlorpyrifos 0.13 0.20 0.47 0.74 0.0050 Yoshida &
emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Chlorpyrifos 2.1 - 3.4 - 0.017 Yoshida &
-methyl Nishiuchi (1976)
Cyanofenphos 1.2 1.3 6.3 15 0.0085 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Cyanophos 15 10 ~ 40 28 18 0.34 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Dialifos 1.3 - 0.80 - 0.027 Yoshida &
Nishiuchi (1976)
Dichlofenthion 5.1 10 ~ 40 1.4 10 0.005 Yoshida &
(emulsifiable (dust Nishiuchi (1972);
concentrate) formulation) Nishiuchi (1974)
Diazinon 3.2 5.1 5.3 4.1 0.08 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Dichlorvos > 40 10 ~ 40 18 - 2.8 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Dimethoate > 40 > 40 > 40 - 10 ~ 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Dimethylvinphos 5.6 - 1.5 - 0.010 Yoshida &
Nishiuchi (1976)
Dioxathion 10 ~ 40 10 ~ 40 1.4 - 0.007 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Disulfoton 8.7 10 ~ 40 21 0.37 0.07 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Edifenphos 2.5 1.8 1.8 - 0.27 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
EPN 0.20 0.32 0.50 0.085 0.0017 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Ethion 1.2 1.1 5.5 - 0.005 Yoshida &
Nishiuchi (1972)
Fenitrothion 8.2 3.4 7.0 0.75 0.050 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Fenthion 3.3 1.9 2.5 2.3 0.070 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Formothion 15 10 ~ 40 10 ~ 40 - 5.8 Yoshida &
Nishiuchi (1972)
IBP 10 ~ 40 12 7.2 - 2.3 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
Leptophos > 40 10 ~ 40 8.5 - 0.002 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Malathion 23 7.8 0.75 1.4 0.030 Yoshida &
Nishiuchi (1972)
Menazon > 40 > 40 > 40 100 10 ~ 40 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Methidathion 2.5 2.3 0.034 0.22 0.007 Yoshida &
(emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Naled 1.3 1.2 28 2.3 0.005 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972);
concentrate) concentrate) Nishiuchi (1974)
Parathion 4.5 1.7 2.9 - 0.0050 Yoshida &
Nishiuchi (1972)
Parathion-methyl 7.5 10 ~ 40 12 - 0.00050 Yoshida &
Nishiuchi (1972)
Phenkapton 2.0 3.8 3.5 - 0.008 Yoshida &
Nishiuchi (1972)
Phenthoate 2.5 2.4 0.17 0.20 0.008 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Phosalone 1.2 1.2 0.35 - 0.05 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Phosmet 5.3 4.7 1.8 1.0 0.025 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Pirimiphos-methyl 1.8 - 3.0 - 0.018 Yoshida &
Nishiuchi (1976)
Propaphos 4.8 - 4.1 - 0.0063 Yoshida &
Nishiuchi (1976)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Propoxur 10 ~ 40 10 ~ 40 10 ~ 40 - 0.37 Yoshida &
Nishiuchi (1972)
Prothiophos 9.5 - 10 - 0.13 Yoshida &
Nishiuchi (1976)
Temivinphos 0.58 - 0.48 - 0.0080 Yoshida &
Nishiuchi (1976)
TEPP 5.6 10 ~ 40 4.8 - 10 ~ 40 Yoshida &
(liquid (liquid (liquid (liquid Nishiuchi (1972)
formulation) formulation) formulation) formulation)
Tetrachlorvinphos 4.3 3.9 4.2 - 0.0035 Yoshida &
(wettable Nishiuchi (1972)
powder)
Thiometon 7.5 10 ~ 40 10 ~ 40 - 5.5 Yoshida &
Nishiuchi (1972)
Trichlorphon 28 10 ~ 40 25 12 0.005 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Vamidothion > 40 > 40 > 40 - > 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Note: Test methods are officially recognized methods based on the Notification of the Ministry of
Agriculture, Forestry and Fishery of Japan, as described in the separate papers.
6. EFFECTS ON ANIMALS
Insecticides are designed as lethal agents. Although they may
be designed to be less toxic for animals than for insects, all
organophosphorus insecticides present a toxic hazard to some
extent. Values for the oral and dermal LD50s in rat, shown for
different compounds in Annex III, range from less than 10 to more
than 3000 mg/kg body weight. The dose-response line for
organophosphorus insecticides is usually steeper than that for
carbamates, though both kill by their anticholinesterase action.
The reason for the difference lies in the faster rate of
spontaneous reactivation of carbamylated AChE compared with
phosphorylated AChE.
By the time the 1984 Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) had ended, the toxicology of 57 organophosphorus
pesticides had been reviewed (Vettorazzi, 1984). Usually, the
compounds had been reviewed several times as additional information
became available. The compounds, with the years of the JMPR
review, and the acceptable daily intake (ADI) advised are listed
Annex II. It also lists the year of review by IARC. Moreover, it
gives the WHO recommended classification by acute toxic hazard
(WHO, 1984a) and an indication on whether WHO/FAO issued a "Data
Sheet on Chemical Pesticides" on this substance.
Details of the tests and of the results are recorded in the
appropriate published evaluations from the FAO/WHO Joint Committee
Meetings, which have been summarized and commented on by Vettorazzi
(1979).
It should be realized that, while reports on toxicological
tests may give some indication of the purity of the sample as
percentage content of the major active ingredient, there is seldom
any indication of the nature or quantity of the impurities or of
whether the impurities may significantly influence toxicity. It
cannot be emphasized too strongly that measurements such as acute
LD50s are not absolute values.
The LD50 values for one typical compound, fenitrothion, in
various species when administered by various routes are listed in
Table 4 (FAO/WHO, 1970b, 1975b). From this Table, it is clear that
there are marked differences in the LD50 depending on species and
route of administration. Variability in LD50 values for the same
route and species is often considered to be due to the vehicule in
which the pesticide is applied, which may influence its uptake into
the body. However, it is also possible that such differences are
real and can throw light on mechanisms of toxicity and/or of
detoxication, thus making more intelligent assessment of toxicity
possible. It can be argued that the presence of the very toxic
impurities in some formulations of diazinon (section 6.3.4) could
have been deduced much earlier from differences in LD50 data.
Likewise, the Pakistani poisonings due to strong potentiators in
stored malathion (Baker et al., 1978) (section 6.3.4) might have
been prevented, if more questions had been asked about the range of
LD50 values given in the literature for malathion.
Table 4. Comparison of acute toxicity data for
fenitrothion listed in evaluation reports of
joint FAO/WHO meetingsa
----------------------------------------------------
Animal Sex Route LD50 (mg/kg
body weight)
----------------------------------------------------
1970 Report
Mouse M oral 1336
Mouse F oral 1416
Mouse M ip 115
Mouse F ip 110
Mouse iv 220
Rat M oral 740
Rat F oral 570
Rat M ip 135
Rat F ip 160
Rat iv 33
Guinea-pig M oral 500
Guinea-pig oral 1850
Guinea-pig M ip 110
Guinea-pig iv 112
Cat oral 142
1975 Report
Mouse M oral 1030
Mouse F oral 1040
Rat M oral 330
Rat F oral 800
Ring-neck pheasant oral 34.5
Mallard duck oral 2550
Dog oral MLDb 681 mg/kg
Rat M oral 940
Rat F oral 600
----------------------------------------------------
a From: FAO/WHO (1970b, 1975b).
b MLD = minimum lethal dose.
6.1 Effects on the Nervous System
6.1.1 Effects attributed to interaction with esterases
6.1.1.1 Cholinergic effects
(a) Acute toxicity
If non-ester herbicidal compounds are excluded, then the acute
toxicity of all other organophosphorus pesticides shares the basic
mechanism outlined in section 4.5.1, involving inhibition of AChE,
accumulation of ACh, and over-stimulation of some central
cholinergic neurons and of the sympathetic and parasympathetic
nervous systems. Signs and symptoms of poisoning are described
fully in section 7.1. Death is caused by respiratory failure due
to a combination of blocking of the respiratory centre,
bronchospasm, and paralysis of the respiratory muscles.
(b) Chronic toxicity
The cholinergic effects brought about by repeated
administration of less than a single fatal dose are similar in type
to the acute single-dose effects and are discussed in section
6.3.1. For the majority of these compounds, long-term feeding
tests have been performed to establish the no-observed-adverse-
effect levels. In every case, except bromophos-ethyl, the most
sensitive indicator of an effect was depression of ChE activity in
plasma or erythrocytes. The phenomenon of tolerance to repeated
doses of anticholinesterase compounds is covered in sections 6.3.1
and 6.4. For bromophos-ethyl, it has been reported that the
urinary excretion of ascorbic acid and dehydroascorbic acid was
slightly increased in beagle dogs given 0.39 mg/kg body weight
daily, for 18 weeks, at which dose, depression of serum-ChE was not
significant (FAO/WHO, 1973b).
6.1.1.2 Delayed neuropathic effects
Delayed neuropathy has occurred occasionally in man and
experimental animals after intoxication with a variety of
organophosphorus esters. The subject has been reviewed by Johnson
(1975a,b, 1980, 1982a) and by Davis & Richardson (1980). An
account suitable for physicians is given by Lotti et al. (1984).
Delayed neuropathy is not inevitably associated with intoxication
by organophosphorus pesticides (Johnson, 1982a; Soliman et al.,
1982). Improvements in the therapy of acute poisoning (section
7.4) mean that higher doses of some organophosphorus insecticides
can now be tolerated without fatal consequences. However, many
organophosphorus pesticides that might, theoretically, cause
neuropathy, would only do so at a dose far above the lethal dose.
(a) Characteristics
Regardless of the severity of anticholinesterase effects, there
is a delay after intoxication before neuropathic signs and symptoms
appear. In the adult hen, which is the test species of choice,
this delay is 8 - 14 days, while in man, the delay may be up to 4
weeks after acute exposure. The first symptoms are often sensory
with tingling and burning sensations in the limb extremities
followed by weakness in the lower limbs and ataxia. This
progresses to paralysis, which, in severe cases, affects the upper
limbs also. Children and young animals are less severely affected
than adults, but recovery is slow and seldom complete in adults;
with the passage of time, the clinical picture changes from a
flaccid to a spastic type of paralysis. Cats, hens, and a number
of larger species are affected by a single dose. Repeated dosing
does not reduce the delay in onset to less than 8 days from the
first dose. Baboons, monkeys, and marmosets do not respond easily
to single doses of several typical neuropathic esters, and it is
difficult to produce typical delayed neurotoxic effects in rodents,
even by repeated dosing. In early histological examinations,
methods were used that showed mainly degeneration of the fatty
myelin sheath surrounding long nerve axons and names such as
"Organophosphate Demyelinating Disease" are still erroneously used,
in spite of later work that showed that the nerve axon itself was
primarily affected and damage to the myelin sheath was secondary
(Cavanagh, 1954; Bouldin & Cavanagh, 1979). The preparation of
tissues and identification of lesions are described by Bradley
(1976), Bickford & Sprague (1984), and by Prentice & Roberts (1984)
in the Workshop Report edited by Cranmer & Hixson (1984). This
publication also covers many aspects of mechanism and testing.
(b) Mechanism
The first essential step in the initiation of the delayed
neuropathic effect of an organophosphate is phosphorylation of a
target protein in the nervous system. The protein has esteratic
enzyme activity. The phosphorylation, which was originally studied
radiochemically, can be monitored conveniently as progressive
inhibition of the activity of this enzyme, which is now referred to
as Neuropathy Target Esterase (NTE or Neurotoxic Esterase)
(Johnson, 1980, 1982a). The second, and equally essential, step is
the transformation of the phosphorylated NTE to a modified form:
one of the remaining ester bonds of the inhibitor molecule attached
to the NTE active site undergoes a biochemical cleavage leaving an
ionized acidic residue bound to the protein: this residue is
negatively charged and the reaction is referred to as "aging".
Both inhibition and aging of inhibited NTE are essential to
initiate neuropathy, but the role of the negative charge in the
initiation of axonal degeneration is not known. The process of
"aging" of inhibited NTE has some analogy with the better-known
"aging" of inhibited AChE. However, the analogy does not last
above the level of enzyme inhibition. Acute toxicity arises
directly from the loss of catalytic activity of AChE, leading to
accumulation of excess physiological substrate. Mere loss of
catalytic activity of NTE (without aging) does not initiate
neuropathy (see below). There is no evidence of a deleterious
accumulation of a physiological substrate for NTE or lack of
hydrolysis products after inhibition in vivo, and the effect of
the negative charge may be focused on some quite separate process.
When NTE has been inhibited by a suitable phosphate,
phosphonate, or phosphoramidate, aging is always possible, and this
process has been shown to occur rapidly with a wide variety of
neurotoxic esters and hen NTE. However, after inhibition by
phosphinates (which contain 2 phosphorus-carbon bonds) or by
sulfonyl fluorides, no hydrolysable bonds remain in the attached
inhibitor molecule. Thus, aging is not possible, and these
compounds are not neuropathic in vivo. Whenever hen NTE can be
phosphinylated or sulphonated in vivo, the birds become resistant
to challenge doses of typical neuropathic esters, because the
2-step initiation process has been blocked halfway. Similarly,
carbamates do not form aged inhibited enzymes and are either
totally without effect (anti-cholinesterase carbamates are poor
inhibitors of NTE) or inhibit the enzyme, but do not age. Thus,
they protect the hen in the same way as the phosphinates. Now that
this mechanism is understood, it is obvious that carbamates need
not be subjected to tests that were designed to detect delayed
neuropathic potential in organophosphorus anticholinesterase
pesticides.
The events that follow inhibition and aging of NTE are not
known, but it is clear that, in adult hens, detectable delayed
neuropathic events (clinical or histological) are never seen after
a single dose/exposure of an organophosphorus ester, unless there
is at least 70% (probably 80 - 90%) inhibition of the NTE in the
brain and spinal cord soon after dosing (4 - 40 h). Owing to the
synthesis of fresh protein, this inhibition declines markedly
during the 8 to 14-day delay period, and there is no correlation
between neuropathy and NTE inhibition measured at the time that
clinical signs reach their peak. NTE has been found in the nervous
tissue of all mammals and birds examined and can be inhibited, even
in species such as the rat, in which there is no obvious clinical
neuropathic response to a single dose. It has been shown recently
that, when single doses of certain neuropathic organophosphorus
compounds are given to rats, degenerative lesions develop in their
peripheral nerves and spinal cord. Although these lesions are
similar to those in ataxic hens, they are not accompanied by
clinical signs. The dose-response of NTE inhibition and the
neurological damage in rats are well correlated, and, as in hens,
the lesions are prevented by protectively predosing the rats with
sulfonyl fluoride (Padilla & Veronesi, 1985; Veronesi & Padilla, in
press). Human NTE has been examined in vitro (Lotti & Johnson,
1978), and its response to inhibitors is similar to that of the hen
enzyme (I50s differed by not more than 4-fold). What is not known
is the numerical value of the threshold of inhibition of NTE in man
that is associated with clinical neuropathy. However, it is known
that numerous people treated only with atropine for poisoning by
trichlorfon have survived and then developed neuropathy (Johnson,
1981a). In contrast, it is very difficult to produce neuropathy
with trichlorfon in the hen, without enormous doses coupled with
prophylaxis as well as therapy for severe anticholinesterase
effects. It seems, therefore, that in the case of this pesticide,
the chances of a severely poisoned man developing neuropathy rather
than dying are greater than those of the hen. It might be deduced
that the threshold level of NTE inhibition for neuropathic effects
in man is somewhat less than the 70% value for hen, and caution
should be applied in the extrapolation from hen tests. This
argument is merely a comparison of the two hazards, death and
neuropathy: it does not give any guide to the relative dose
required to intoxicate man and hen.
(c) Delayed neurotoxicity testing
Two distinct procedures are currently practiced by testers, and
each depends on the anticipated hazard. In acute tests, it is
recommended that hens surviving an LD50 test (preferably a test in
which the therapeutic use of atropine raised the LD50) should be
observed for three weeks for abnormalities of gait and then be
re-dosed and watched for a further three weeks, after which a
thorough histological examination of the distal ends of neurons in
the spinal cord and peripheral nerve should be performed. Long-
term neurotoxicity tests require feeding hens for up to 90 days
with doses in the diet ranging up to those causing obvious adverse
cholinergic effects; evaluation is by the same criteria as for the
acute test. It may be argued that long-term tests should be
omitted, in view of the greater speed and simplicity of the acute
test in obtaining yes/no answers and the fact that no pesticidal
compound has ever been found to give a positive clinical response
in feeding trials when negative in the 2-massive-doses test. The
practice in the United Kingdom has been to voluntarily exclude from
agricultural use any compound shown to be neuropathic in acute
tests. Guidelines for the performance of acute and long-term
delayed neurotoxicity tests are available (United Kingdom PSPS,
1979; US EPA, 1982; OECD, 1983). The OECD Guidelines point out
that acute tests would be improved if they were accompanied by
assays of NTE inhibition in the brain and spinal cord, one and two
days after dosing. Results of assays would provide graded dose-
response relationships instead of the present all-or-none scoring.
Some examples are given in Johnson (1975b). Particularly useful are
data such as those obtained with malathion (Johnson, 1981b), which
showed negligible (< 10%) inhibition of NTE at the LD50 dose in
hens. These data indicate a different order of safety compared
with that of some other compounds which caused 50 - 60% inhibition,
though there was no visible clinical response. A way to integrate
the NTE test efficiently into delayed neurotoxicity test protocols
has been suggested by Johnson (1984). However, further discussion
to improve this method is required.
(d) Delayed neurotoxic response to long-term feeding
For some neuropathic compounds, the potency of a single dose
may be matched or even exceeded by the effect of the same amount
spread over a few days. In particular, compounds such as TOCP or
leptophos, which are very poorly soluble in water and are required
in a very large single dose to be effective, may well be absorbed
with greater efficiency in a divided lower-dose regime. However,
the results of various studies (Johnson, 1982a) have shown that, as
the dose is reduced further, there is a clear cut-off point below
which there does not appear to be any cumulative effect. Thus,
TOCP in the diet of hens at 400 mg/kg produced neuropathy in 21
days, while a level of 100 mg/kg diet did not cause any detectable
clinical or histological damage after 140 days (Barnes, 1975).
Monitoring of the NTE response to continuous feeding of non-
neurotoxic regimes of 2 organophosphates (0.125 mg DFP/kg for 5
days per week, over 4 weeks, or 2.5 mg mono-2-cresyl diphenyl
phosphate/kg, daily, for 10 weeks) showed that NTE levels in the
brain and spinal cord were depressed within 2 - 3 weeks to about 45
- 55% of normal and remained unchanged thereafter; the equilibrium
is presumbably the result of daily synthesis matching daily
inhibition. These doses did not cause detectable neuropathy during
either the feeding period or the 3 ensuing weeks (Lotti & Johnson,
1980). On the basis of these and other studies, Johnson (1982a)
concluded that the hen nervous system might tolerate the
biochemical defect of prolonged inhibition of NTE to about 50%
normal level brought about by long-term dosing but could not
tolerate the brief 80 - 90% inhibition that follows a single larger
dose. If long-term feeding tests in hens are required, then weekly
measurements of the level of NTE inhibition should provide valuable
predictive information, early in the test (Johnson, 1984). Since
it is necessary to kill birds for tissue sampling, more birds may
be necessary, at least in the early stages.
(e) Results of delayed neurotoxicity testing
No pesticidal organophosphorus compounds, giving negative
results in massive-dose tests, have caused delayed neuropathy in
long-term feeding studies. With the exception of trichlorfon,
where 2 doses, 3 days apart, were necessary (Johnson 1970, 1981a),
the massive-dose tests have been single doses given with
prophylaxis and therapy (eserine + atropine and atropine + oxime -
repeated if necessary) to ensure that doses above LD50 could be
examined. In Table 5, known pesticidal compounds are listed
according to the doses known to produce marked-to-severe neuropathy
in the majority of birds tested. Administration by either the oral
or dermal route may be effective.
The review by Johnson (1975b) is believed to list all
pesticidal and non-pesticidal compounds, or their derived oxons,
tested and reported on, up to that time. The JMPR Reports were not
included as a literature source. NTE responses are given where
these have been measured. Johnson (1982a) lists a number of
pesticides for which both clinical and NTE tests had been reported
since 1975 and notes that carbophenthion, cyanophos, diazinon,
fenitrothion, malathion, methyl parathion, omethoate, and parathion
can positively be declared non-neuropathic on the basis of
negligible NTE inhibition responses, while chlorpyrifos,
methamidophos, and salithion gave intermediate NTE responses
without clinical expression at the doses tested. Isofenphos
( o -ethyl- o -2-iso-propoxycarbonylphenyl isopropylphosphor-
amidothioate) has been shown by NTE assays and clinical tests to
cause delayed neuropathy in hens at about 20 x LD50 (Wilson et al.,
1984), while the insecticidal synergist o-n -propyl- o -(2-propynyl)
phenylphosphonate was neuropathic by both criteria at about the
LD50 (Soliman, 1982).
Table 5. Organophosphorus pesticides causing delayed neuropathy
in hens after a single dose
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
mipafox 25 im Bidstrup et al.
N,N -diisopropylphosphoro- (1953)
diamidic fluoride
haloxon 1000 oral Malone (1964)
3-chloro-4-methyl-2-oxo-
2H-1-benzopyran-7-yl-
bis-(2-chloroethyl) phosphate
EPN 40-80 sc Witter & Gaines
(1963)
trichlornat 310 oral Johnson (1975b)
ethyl 2,4,5-trichlorophenyl 300-400 oral Johnson (1975b)
phenylphosphonate
ethyl 2,4-dichlorophenyl > 2000 oral Abou-Donia et al.
phenylphosphonothioate (1979)
leptophos 400-500 oral Abou-Donia et al.
(1974); Johnson
(1975b)
desbromoleptophos 60 oral Johnson (1975b)
S,S,S -tributyl phosphoro- 1110 sc Johnson (1970)
trithioate (DEF)
cyanofenphos > 100 oral Ohkawa et al.
(1980)
isofenphos 100 oral Wilson et al.
O -ethyl O -2-isopropoxy- (1984)
carbonylphenyl isopropyl-
phosphoramidothioate
O-n- propyl O -(2-propynyl) 400 oral Soliman (1982)
phenylphosphonate
dichlorvosb 100 scc Caroldi & Lotti
(1981)
amiprophos 600 oralc,d Huang et al.
O -ethyl O -4-methyl-6- (1979)
nitrophenyl
N- isopropylphos-
phoramidothionate
Table 5 (contd.)
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
coumaphos 50 oralc Abou-Donia et al.
500 dermal (1982)
chlorpyrifos 150 oral Lotti et al.
(1986)
salithion 120 oral El-Sebae et al.
(1981)
--------------------------------------------------------------
a Dose needed to cause marked-to-severe neuropathy in the
majority of birds tested.
b 50% formulation in hydrocarbons: dose calculated as active
ingredient.
c Mild neuropathy only at maximum tolerated dose.
d Test performed in cockerels.
The statement by Namba et al. (1971) that chlorpyrifos produced
neuropathy in hens seems to have been without foundation and may
arise from the misreading of a report by Gaines (1969), which
mentioned that chlorpyrifos caused a rapid onset short-term
weakness (sometimes called paralytic effect) similar to that caused
by malathion. However, one recent case of human poisoning and
laboratory tests with doses well above the unprotected LD50 have
shown neuropathic effects from this pesticide (Lotti & Moretto, in
press).
(f) Structure/activity relationships
As noted above, not all organophosphorus pesticides cause
delayed neuropathy. In vivo tests and target-enzyme studies
listed by Johnson (1975b) can be condensed according to a number of
factors as listed by Johnson (1980, 1982a):
(1) Factors that increase delayed neurotoxicity potential more
than acute toxicity are:
(a) choice of phosphonates or phosphoramidates rather
than analogous phosphates;
(b) increase in chain-length or hydrophobicity of R1 and
R2 ; and
(c) a leaving group X, which does not sterically hinder
approach to the active site of NTE;
(2) Factors that decrease the comparative potential are:
(a) the converse of (1) a, b, and c;
(b) choice of R or X groups that are very bulky
(naphthyloxy) or non-planar;
(c) choice of a nitrophenyl group at X (a steric effect?);
(d) choice of comparatively more hydrophilic X groups
(oximes or heterocyclics); and
(e) choice of thioether linkages at X.
Considering these factors, it can be seen why malathion and
diazinon are both far below the neurotoxicity hazard line, why, in
its homologous series, only dichlorvos is not neurotoxic at the
LD50, why EPN, a phosphonothioate with a hydrophobic phenyl group
at R1 is neurotoxic, even with a 4-nitrophenyl leaving group, and
why it is not surprising that other phenylphosphonothioates such as
desbromoleptophos, or cyanofenphos are also neurotoxic. The
apparent non-neurotoxicity of the ethyl analogue of leptophos
(Hollingshaus et al., 1979) seems to contradict (1-b) above, but
the dominant factor seems to be the problem of absorption of this
very poorly soluble compound after oral dosage (Hansen & Hansen,
1985).
6.1.2 Behavioural and other effects on the nervous system
The problems of interpreting behavioural changes in relation to
inhibition of AChE and the time-dependent changes in these
variables have been discussed by Bignami (1976) and Bignami et al.
(1975). It appears that some learned responses may be acquired
more quickly by rats recovering from an inhibitory dose of, e.g.,
DFP (1 mg/kg body weight). This may be a sign of changed
inhibitory responses in learning pathways, but it is difficult to
say whether it can be classified as a toxic response.
Numerous research workers have reported changes at doses that
affect levels of AChE, but without overt signs of intoxication.
For example, Kaloyanova-Simeonova (1961) noted that small doses of
chlorthion (5 or 10 mg/kg body weight in the rat) intensified
conditioned motor reflexes and depressed them at higher doses: ChE
levels were depressed in all cases and were restored more slowly
than the return to normal reflex activity. Reiter et al. (1975)
did not find any effects on performance of learned visual
discrimination tasks by monkeys at doses of parathion of 0.5 mg/kg
body weight, which depressed blood levels of AChE by about 25% and
those of pseudoChE by about 35%. Doses causing 40% or more
inhibition of AChE were associated with decreased responses, which
returned to normal faster than the recovery of AChE levels. A very
sensitive response to the nerve agent soman (pinacolyl
methylphosphonofluoridate) was reported in one out of several
behavioural tests by Wolthuis & Vanwersch (1984). In an open-field
test, a number of performance variables were affected by a dose of
only 3% of the LD50. Effects were also seen with doses of 4 - 6%
LD50 of 2 carbamates but not below 30% LD50 of the pesticide, TEPP,
or of another nerve agent, sarin (isopropyl methylphosphono-
fluoridate). Unfortunately, the effects on ChE levels were not
measured. There is no obvious reason for the contrasting effects
of TEPP and sarin, on the one hand, and of soman and physostigmine,
on the other. The authors noted that, judged by poisoning signs at
near-lethal doses, soman exerted a greater proportion of its
effects centrally than the other 2 organophosphorus esters. The
fact that changes may be seen in some, but not all behavioural
responses at a certain dose emphasizes the statement by Revzin
(1983) that no single behavioural or neurophysiological test can
give a definite conclusion about organophosphate toxicity.
There appears to be only one other report in the literature of
a behavioural change in animals at doses less than those that
inhibit ChEs (Desi et al., 1971). Behavioural and EEG changes were
noted in rats fed bromophos at 500 or 100 mg/kg diet for 6 weeks,
doses that also caused ChE inhibition, but, at 30 and 10 mg/kg,
there were no effects on ChE, though some behavioural changes were
still observed. It is not clear whether undosed animals were
handled in precisely the same fashion as the dosed.
However, in contrast to the above, Desi (1983) reported tests
applied after 3 months of daily consumption of diet containing
various percentages of the LD50 of bromophos. The lowest
concentration that produced changes in a behavioural test (maze
running) and in EEG (both the complex EEG and computer-analysed
segments of the EEG) was about 0.26% of LD50, daily, but this also
caused significant changes in erythrocyte-AChE and plasma-ChE.
Among 6 organophosphorus pesticides assessed by these procedures,
none produced changes greater than that caused by the vehicle alone
without also causing effects on ChE. This conclusion differs from
that written by the author.
Analysis of the EEG records of a small group of rhesus monkeys
has been carried out both before, and one year after, intoxication
with the potent anticholinesterase agent sarin (isopropyl
methylphosphonofluoridate) (Duffy & Burchfield, 1980). Three
animals received a single "large dose" (5 µg/kg body weight iv),
and 3 others received a series of 10 injections of 1 µg sarin/kg
body weight im at weekly intervals: there were 10 controls. The
"large dose" animals had generalized convulsions and were
maintained on Gallamine relaxant with artificial respiration;
small-dose animals were considered in pilot studies to be near the
threshold of poisoning, but showed few overt signs; they did not
receive relaxant or artificial respiration. Twenty-four hours
after a "large dose", marked differences were seen in the EEG
frequency spectrum, which is not surprising. However, one year
after the dose, there was still a small increase in the percentage
energy in the beta-2 region of the spectrum and the change was said
to be statistically significant. Some changes in the beta region
were detectable in all 6 dosed monkeys one year after; no
significant changes were seen in the 10 controls. However,
apparently the changes were only seen under some lighting
conditions and were small compared with differences between the
frequency spectra of the only 2 controls for which data were shown.
The statistical treatment of the acquired data seems valid, but the
actual values measured one year after dosing are obviously well
within the normal range. No indication was given of how much
variation can be caused in the pattern of undosed animals by
variations in the conditions of handling and observation, or what
the range is for apparently identically treated normal animals. The
doubts pertaining to the toxicological significance of these
measurements apply also to some human studies using the same
technique (section 7.2.2).
Effects of cholinergic agents on the visual system have been
monitored by Revzin (1980). In urethane-anaesthetised pigeons with
implanted electrodes, various changes in the response of specific
neurones of the optic tectum and of the hippocampus were noted
after doses both of mevinphos and of atropine. The author claims
that the procedure was sensitive in detecting effects in the
absence of detectable peripheral parasympathetic signs. However,
the lowest effective dose of mevinphos was only one-third of that
(0.15 mg/kg body weight) which produced parasympathetic signs that
would certainly be associated with substantial inhibition of AChE.
Thus, the claim to sensitivity of this complex procedure in
surgically modified birds seems excessive.
Some possible effects of anticholinesterases on non-
cholinesterase targets were considered by O'Neill (1981). No clear
effects seem definable at concentrations lower than those that
inhibit AChE and some are probably secondary to stimulation of non-
cholinergic nerves with cholinergic innervation. However, an
endopeptidase that can hydrolyse putative transmitter peptides in
the nervous system is known to be inhibited by di-isopropyl
phosphorofluoridate at a concentration similar to that which
inhibits AChE (Kato et al., 1980). Thus, some involvement of non-
cholinergic pathways in the causing of CNS effects in
organophosphate poisoning cannot be excluded. Moreover, some
anticholinesterases also exert effects directly at the cholinergic
receptor as well as by the inhibition of AChE (Karczmar & Ohta,
1981).
6.2 Other Effects
A variety of histological lesions has been described at autopsy
in animals severely poisoned with organophosphate pesticides.
However, very few effects are described in the absence of obvious
poisoning or at doses that do not markedly inhibit the ChEs. In
the following sections, the evidence is reviewed for effects not
obviously attributable to inhibtion of either AChE or NTE.
6.2.1 Mutagenic and carcinogenic effects
The proposed theoretical basis for believing that dimethyl or
diethyl phosphate pesticides might be mutagenic or carcinogenic
has already been discussed (section 4.5.2). This basis was shown
to be defective by Bedford & Robinson (1972). No alkylation was
detected in N7 of guanine in RNA and DNA of liver of animals that
were exposed for 12 h to working concentrations of dichlorvos of
64 g/m3 (0.064 g/litre air) (Wooder et al., 1977); these authors
contrasted their data with earlier published work showing modest
alkylation of guanine in suspensions of cells exposed to very high
solution concentrations of dichlorvos. In vivo, the acute
anticholinesterase effects limit the circulating concentration
that can be tolerated by any mammal and there is also strong
preference for phosphorylation of biological scavenger molecules
rather than for alkylation built into the organophosphorus
pesticide molecules.
It has frequently been suggested that dichlorvos has
carcinogenic potential because of observed mutagenic effects in in
vitro test systems. The IARC (1979) Working Group accepted
extensive data showing no evidence for the mutagenicity of
dichlorvos in mammals. FAO/WHO (1978b) accepted animal studies
showing no dose-related carcinogenic effects in life-time studies
carried out at doses that depressed blood-ChE levels.
In a 78-week feeding test on groups of 50 male and 50 female
mice (B6 C3 F1 hybrid), no dose-related effects were seen when the
diet contained about 600 or 300 mg dichlorvos/kg, respectively.
The IARC Working Group evaluating this study noted that a few
oesophageal tumours were seen in treated mice. It appears that
this fact influenced their verdict that "the available data do not
allow an evaluation of the carcinogenicity of dichlorvos to be
made" (IARC, 1979).
An IARC Monograph (1983) included evaluations of 5 widely-used
organophosphate pesticides (malathion, methyl parathion, parathion,
tetrachlorvinphos, and trichlorfon). In several cases, the
conclusions were that acceptable tests had been performed with no
evidence of carcinogenic effects or of mutagenic effects in
mammals. For others, the conclusions were of "limited evidence"
consisting of very small effects above the control background
levels in life-time studies. None of these compounds was judged to
be a strong mammalian mutagen or carcinogen and the same statement
is true for all organosphosphorus pesticides that have been
evaluated by FAO/WHO Working Parties or by other authorities.
However, recent published results show that controversies do occur
when evaluating the outcome of carcinogenicity studies. Huff et
al. (1985) reevaluated the pathology of the original studies by
the US National Cancer Institute in 2 different strains of rats.
Histopathological reexamination confirmed the earlier conclusions
that malathion and malaoxon were not carcinogenic. These
conclusions differed from those of Reuber (1985) who evaluated the
same studies and rated both compounds as carcinogenic.
6.2.2 Teratogenic effects
Defects in the development of fertilized hen eggs, injected
with various organophosphates, are known, but many of these are
associated with the inhibition of the enzyme kynurine formamidase
and a depression of NAD levels at a critical period of development
(Seifert & Casida, 1980). This pathway is not critical in mammals,
and no equivalent effects are known. If teratogenicity is taken to
mean induction of malformations in live offspring without decrease
in number of births (i.e., no embryotoxicity), then for the vast
majority of organophosphorus pesticides, no adverse effects of
continuous feeding of organophosphates on pre- or postpartum
mortality have been reported, nor have embryonic defects been
proved, except at doses that significantly retarded growth in the
mother (Vergieva, 1983). Single high doses causing significant
toxic effects in mothers may be deleterious: a number of these
toxicity-linked effects have been summarized by Seifert & Casida
(1980). Kimbrough & Gaines (1968) reported deaths, and that
resorptions were increased in pregnant rats given a single high
dose of parathion or diazinon on the 11th day of gestation.
However, these effects were associated with significant toxic
effects on the mothers. Similarly, trichlorfon at very high doses
(400 mg/kg per day with the dose divided into 3 spaced aliquots)
and given on days 6 - 15 of gestation, produced defects in
offspring: each dose produced cholinergic symptoms; no effects were
seen in rats, mice, or hamsters when the daily dose was 200 mg/kg
(Staples & Goulding, 1979). However, a specific defect consisting
of hypoplasia of the cerebellum in offspring was noted, both in
field cases and experimental tests, 8 pregnant pigs were dosed once
or twice with neguvon (a veterinary grade of trichlorfon) between
days 55 and 70 of pregnancy (Knox et al., 1978). Doses were 50 -
60 mg/kg body weight, which caused maximum inhibition of
erythrocyte-ChE levels of 40 -80%, without overt signs of
poisoning. The hypoplasia was accompanied by severe ataxia and
tremors, while voice and vision appeared unaffected. No defects
were seen in the offspring of over 100 control sows housed with the
dosed animals, and serological and virological tests did not show
anything incriminating. A genuine teratogenic effect of moderate
doses of trichlorfon commonly used in veterinary practice has been
demonstrated in the pregnant pig. However, it may be that the
herds tested were unusually susceptible, since (apparently)
congenital tremor had been known in litters borne over many years
in the area where the trichlorfon-induced effects were seen.
6.2.3 Effects on the immune system
In a review, Zackov (1983) stated that "most ... organo-
phosphorus pesticides elicit autoimmune reactions and suppress the
production of antibodies against vaccines". No evidence was given
and it is not clear whether the statement was claiming specific
effects or referred to doses that are sufficient to produce a range
of toxic effects.
Shtenberg et al. (1974) claimed that oral doses of methyl-
nitrophos (fenitrothion) or chlorphos (trichlorfon) at 5 or 7 mg/kg
body weight per day, for an unspecified period, suppressed
haemaglutinin levels in rats immunized against sheep red blood
cells; these doses would have significantly inhibited ChEs and were
said to be more effective in rats fed a protein-deficient diet.
Dandliker et al. (1979) reported a depression in antibody titre in
rats in the 6 - 7 weeks of an immunization procedure that commenced
either one day before or one day after (both statements are made)
an oral dose of half of the LD50 of parathion. The immunization
consisted of weekly intramuscular doses of 400 µg fluorescein-
labelled ovalbumin with Freunds Complete Adjuvant. The confusion
concerning the order of intoxication and primary immunization is
very important when such a high dose was given, since cholinergic
symptoms, fluid loss, imbalance, and general debility would have
been marked. Administration to mice of 0.1 x LD50 of parathion for
8 days led to a 10% loss in body weight. Immunization on the 9th
day showed a statistically-significant (34%) reduction in the
number of antibody plaque-forming cells derived from the spleen, 4
days after immunization, but the reduction was insignificant (10%
only) when immunization was carried out on day 10, though several
animals in the group died at this point due to accumulated toxic
effects (Wiltrout et al., 1978).
Some decreased antibody titres "proportional" to AChE
inhibition were noted in response to prolonged doses of malathion
or dichlorvos (0.025 - 0.4 x LD50 given 5 days per week for 6
weeks) (Desi et al., 1978). Clearly, none of the above responses
can be dissociated from the general cholinergic intoxicating
effects of the pesticides. There do not appear to be any reports
of studies on the immunological status of healthy animals receiving
doses causing little or no depression in ChE levels.
6.2.4 Effects on tissue carboxyesterases
A variety of carboxyesterases abound in serum, liver,
intestine, and other tissues (section 4.4). Although inhibition of
one specific carboxyesterase (NTE) has toxic sequelae (section
6.1.1.2), no direct deleterious effects of inhibiting other
carboxyesterases have been demonstrated. However, they may
contribute markedly to the metabolic disposal of malathion and
certain other organophosphorus pesticides, so that inhibition of
tissue carboxyesterases may potentiate the toxicity of such
pesticides (section 6.3.5). The structure/activity relationships
for inhibition of these enzymes by organophosphorus compounds
inevitably differ from those for inhibition of AChE. For EPN and
fenchlorphos, both serum- and liver-carboxyesterases of rats were
markedly more sensitive than brain- and serum-AChE; in other cases,
the liver enzymes, but not those in serum, were more sensitive (Su
et al., 1971).
6.2.5 Sundry other effects of organophosphorus pesticides
Very few effects other than those described in the sections
above have been noted, except those arising from ill-health due to
severe anticholinesterase effects. Thus, impaired growth rate is
commonly associated with a rapid depression in AChE levels to less
than 50%, but much lower levels can be tolerated without ill-
effects, if the depression is brought about over several weeks, and
then maintained for up to a year (section 6.3.1).
Various changes in glucose metabolism, in serum enzymes, and in
other clinical chemical variables have been reported after single,
acute, or repeated doses of various pesticides at from one-tenth to
one-quarter of the LD50, daily (Dimov & Kaloyanova, 1967; Enan et
al., 1982).
A reversible, mild muscle-necrotising effect could be detected
histologically in the diaphragm muscles of rats, 24 h after a dose
of paraoxon, parathion, or other anticholinesterases, sufficient
to cause marked fasciculations (Fenichel et al., 1972; Dettbarn,
1984). The damage appeared to be a function of the prolonged
cholinergic stimulation of the muscle, since it was entirely
prevented by doses of atropine, which prevent fasciculations, or by
alpha-bungarotoxin applied directly to the myoneural junction
(Salpeter et al., 1979).
Several adverse effects attributed only to certain
organophosphorus esters are listed below. The list may be of value
in promoting more careful examination of intoxicated animals or
human beings.
6.2.5.1 Effects on hormones
Changes in the diurnal pattern of plasma-ACTH and adrenal
levels of some related enzymes have been reported in rats
maintained with dichlorvos in the drinking-water at 2 mg/litre:
blood-ChE levels were not affected (Civen et al., 1980). However,
the weight gain of the treated animals was only half that of the
controls, and the fluid consumption was increased by 20%. These
deleterious effects could be due to diarrhoea and imbalance of
fluids with inevitable repercussions on hormonal levels, etc.;
preferential effects of the dichlorvos on intestinal esterases
might be the primary effect.
6.2.5.2 Effects on the reproductive system
Damaged seminiferous tubules were reported in mice given either
a single dose of about half of the LD50 of dichlorvos or 18 doses
of about one-tenth of the LD50 (Krause & Homola, 1974). However,
the doses were high, the percentage increases seem small, and the
number of samples taken was very small; thus, no statistical
evaluation is possible. Amiprophos has been reported to cause some
gonadotrophic effects in adult cockerels (Huang et al., 1979), but
details are few.
6.2.5.3 Effects on the retina
Fenthion administered intramuscularly at about one-quarter of
the LD50 (50 mg/kg body weight), every 4 days for one year
(solvent, if any, not stated), affected the electroretinogram in 2
strains of rats (Wistar and black Long-Evans) within 3 months and
abolished it after one year (Imai, 1977). Similar studies involving
the administration of either fenitrothion or ethylthiometon to
beagle dogs for 5 days per week for 2 years, were reported by
Ishikawa & Miyata (1980). Doses that depressed plasma-ChE levels
to about 30% of normal, throughout the period, led to changes in
optical function after 13 months continuous exposure and to
morphological changes in the ciliary muscle at termination.
6.2.5.4 Porphyric effect
Daily application of technical (85%) diazinon (20 or 40 mg/kg
body weight) to the skin of Dark Agouti rats just above the tail
produced a 4-fold increase in faecal porphyrins, after 8 - 12 weeks
(Bleakley et al., 1979). There was no increase in urinary
porphyrins and no effect when diazinon was given in food (about
8 mg per day to rats, initially weighing 180 g). The pattern of
porphyrins excretion was said to be indistinguishable from
porphyria cutanea tarda. However, the increase in excreted
porphyrins in classical porphyria cutanea tarda may be as much as
1000-fold, with massive amounts in the urine as well as in the
faeces. The authors failed to reproduce even this small effect in
rats when they used more pure (97%) diazinon (Nichol et al., 1982).
However, they also found that an impurity in stored technical
material, isodiazinon (diethyl 2-isopropyl-6-methyl-4- S -
pyrimidinyl phosphorothioate), was very effective in causing
porphyrin accumulation, when added to cultures of chick
hepatocytes; confusingly, however, the accumulated porphyrin was
coproporphyrin rather than the expected protoporphyrin. Although
human poisoning with diazinon is not uncommon, there has only been
one report implicating technical diazinon in a few cases of
porphyria cutanea tarda in occupationally-exposed workers (Bopp &
Kasminsky, 1975). Further experimental animal studies seem
warranted. No reports have been found of porphyria connected with
exposure to other organophosphorus pesticides.
6.2.5.5 Lipid metabolism
Organophosphate esters can inhibit the activities of some
triglyceridases and lipases in vitro and in vivo. However, no
repercussions from such inhibition were found when appropriate
parameters were measured in rats fed for one year with either of 2
pesticides that caused marked (60 - 80%) depression of blood-ChE.
Thus, male or female rats were fed either a normal diet or a diet
enriched sufficiently with fat to increase aortic fatty acids by
170%. No changes occurred in: the hormone-sensitive lipase and
lipoprotein lipase in adipose tissue; the free and total fatty
acids and total glycerol and total cholesterol in serum; and the
total fatty acids, cholesterol, and glycerol in the aorta (Buchet
et al., l977). The pesticides were chlorpyriphos (100 mg/kg diet)
and triamiphos (10 mg/kg) and the findings were contrary to those
from a preliminary study by the same workers.
6.2.5.6 Effects causing delayed deaths
Although not pesticidal agents themselves, two of the
phosphorothiolate impurities found in technical malathion and some
analogues of these impurities caused delayed effects, which were
lethal for rats at doses below the cholinergic LD50 (Aldridge et
al., 1979; Mallipudi et al., 1979; Verschoyle et al., 1980). The
compounds were O,O,S -trimethyl phosphorothioate (I), O,S,S -
trimethyl phosphorodithioate (II), and the ethyl analogues (III and
IV). After an oral LD50 dose (as low as 26 mg/kg body weight for
II), all 4 compounds produced cholinergic responses that lasted
less than 24 h and deaths at this time were only seen with IV:
doses 2 - 6 x LD50 were needed to cause cholinergic deaths with I -
III. Rats dosed at, or near, the LD50 recovered from the initial
cholinergic effects, but by day 3, they had lost weight and were
panting with laboured respiration; deaths occurred 3 - 6 days after
dosing. However, survivors appeared normal, 10 days after dosing.
Death was due to pulmonary insufficiency associated with
progressive cell proliferation (Dinsdale et al., 1982; Imamura et
al., 1983; Aldridge & Nemery, 1984), and combined therapy with
atropine and oxime was ineffective. The biochemical mechanism of
these effects is not fully known, but it is probable that the
proximal toxin is produced in the lung by oxidative attack on the
alkylthio moiety of the compounds (Aldridge et al., 1985). The
activities of brain-AChE and plasma-ChE and carboxylesterase, which
were partially inhibited during the first day after dosing,
increased thereafter and were at least 50% of the levels of
activity in the controls at the time of death. The margin between
delayed death LD50 and cholinergic LD50 was markedly less with the
triethyl, than with trimethyl compounds. Such effects were seen
with S,S,S -trimethyl phosphorotrithiolate but not with the higher
analogue ethoprophos ( O -ethyl S,S -di- n -propyl
phosphorodithiolate) or with methamidophos (Verschoyle & Cabral,
1982).
A different form of delayed acute toxicity was reported to
occur 4 days after large oral doses of DEF in hens. The effect was
not seen after a single dose, sufficient to cause delayed
neurotoxicity, was given subcutaneously (Johnson, 1970) or dermally
(Abou-Donia et al., 1980). The effect was distinct from both
cholinergic and delayed neuropathic effects and is attributed to
the acute toxicity of n- butyl mercaptan produced by the degradation
of DEF in the gastrointestinal tract.
6.2.5.7 Selective inhibition of thermogenesis
The defoliant DEF ( S,S,S -tri- n -butyl phosphorotrithiolate)
acted as an anticholinesterase at high doses, but, at lower doses
(60 - 200 mg/kg in rats and mice), it caused a profound fall in
body temperature (as much as 10 °C over a few h), without marked
sedation; deaths occurred mostly after the depression had persisted
for a day (Ray, 1980). The effect was different from the smaller
atropine-sensitive changes due to some cholinomimetics and was due
to blocking of cold-induced thermogenesis without affecting heat
conservation mechanisms. The effect seems unique to the DEF
chemical structure. Ray & Cunningham (1985) demonstrated that the
effect was a selective action on a central thermogenic control
mechanism rather than on peripheral thermogenic processes and that
it was probably due to a metabolite of DEF rather than to the
parent compound.
6.3 Factors Influencing Organophosphorus Insecticide Toxicity
6.3.1 Dosage-effect
The lethal effects of organophosphorus insecticides are due to
severe cholinergic effects arising from excessive inhibition of
AChE. With few exceptions, the AChE activity of the tissues is
inhibited soon after the administration of acutely toxic doses of
all anti-AChE agents. This is true, not only for compounds that do
not require metabolic conversion to anti-AChE agents, but also for
most phosphorothioates, phosphorodithioates, and
phosphorodiamidates that are oxidized by the liver to metabolites
with anti-AChE activity. In general, the duration of action of
most anti-AChE agents is relatively short, as evidenced by
considerable reversal of the inhibition within a few days. For
this reason, a 10-day observation period is sufficient for acute
LD50 measurements on all anti-AChE agents that have been studied,
and 2 days suffice for most.
The data in Table 6 give a comparison of the maximal amount of
inhibition of the ChE activities in the brain, submaxillary glands,
and serum of rats for several compounds, all of which were given at
dose levels equivalent to 5/8 of the LD50. The time at which
maximal inhibition occurred and the period required for complete
reversal of the inhibition are also presented. From these
examples, it can be seen that, in general, equivalent fractions of
the LD50 of various anti-ChE compounds produce similar levels of
inhibition of ChEs, though the LD50 values for the various
compounds differ considerably. The time at which maximal
inhibition of ChEs occurs varies from 15 min to 3 h after
administration. In some cases, the AChE activity of the brain and
parasympathetic nervous system, as indicated by the submaxillary
gland, and the non-specific ChE of the serum are inhibited to the
same extent by a particular compound. However, notable exceptions
are OMPA, which is converted in the liver to a very labile
inhibitor that never reaches the brain, and Guthion, which does not
inhibit non-specific ChE.
Differences between the responsiveness of rat brain- and serum-
ChE have been reported also in the case of dietary administration
of various organophosphorus insecticides (Su et al., 1971). Thus,
a level of only 40 mg EPN/kg fed for 1 week reduced brain-ChE to
50%, while 125 mg/kg was needed to achieve the effect in serum; the
sensitivity was reversed for fenchlorphos, while the sensitivities
of the 2 tissues were similar for demeton. It is not clear whether
these differences reflect differences in access of the compounds to
their targets, differences between the tissue AChEs, or the fact
that pseudoChE is present as well as AChE in the serum of rats, so
that assay of the hydrolysis of ACh using serum measures both
enzymes, whereas, in the brain, the activity is about 90% specific.
Marked differences in the rate of reversal of the inhibitory
effects on ChEs of different compounds, in vivo, are shown in Table
6. In view of the variable duration of action of various anti-ChE
agents, the performance of assays on the tissues of animals at
intervals after acutely toxic doses provides a great deal of useful
information regarding the toxicity of these compounds. The
transition from a tolerable to a lethal dose (either acute or
chronic) often occurs within a 2 to 4-fold range. This is not
surprising, since the AChE of nervous tissue and effector organs
must be inhibited by 50 - 80% before pharmacological effects can be
seen (Holmstedt, 1959). The increment in dose to raise inhibition
to 90% with associated deaths is not very great.
Table 6. Onset and duration of the anticholinesterese action
of some organophosphorus compounds in ratsa
-----------------------------------------------------------------------
Compound Maximum inhibition of cholinesterase (%)
Dose Brain Serum Sub- Time to Time to
(mg/kg maxillary maximum complete
body gland inhib- reversal
weight) tion (h) (h)
-----------------------------------------------------------------------
Iso-Systox 1.0 85 80 75 3.0 120
(demeton- S )b
Disyston 1.25 75 85 75 3.0 120
(disulfoton)
Guthion 3.5 60 0 50 0.5 24
(azinophosmethyl)
Dipterex 140.0 85 82 85 0.25 6
(trichlorfon)
Octamethyl 5.0 0 85 88 2.0 144
pyrophosphor-
tetramide (OMPA)c
-----------------------------------------------------------------------
a From: DuBois (1963).
b Phosphorothioic acid, O -[2-(ethylthio)ethyl] O,O -diethyl ester.
c Octamethylpyrophosphoramide.
The general correlation of inhibition of ChE with symptoms of
poisoning is also seen with repeated dosing with organo-phosphates,
but details vary greatly. It is always true that a prerequisite of
death is profound inhibition of AChE in the central and/or
peripheral nerves, but the tolerable maximum inhibition increases
when this level is reached, stepwise, over a period of 2 - 4 weeks
or more. Thus, when commercial Systox (containing about equal
amounts of the O - and S -isomers of demeton) was fed to rats at
20 mg/kg diet, there were no signs of poisoning, though, at the end of
16 weeks, the brain- and whole blood-ChE activities were 26 and 28%
of normal, respectively (Barnes & Denz, 1954). At 50 mg/kg, 3 out
of 12 rats died, but all others in the group improved after initial
marked signs of poisoning during the first month, their food intake
increased above normal (and therefore their actual dose increased),
and their growth rate became normal. This improvement was not due
to any marked increase in ability to detoxify the agent, since, at
the end of 16 weeks, these apparently healthy rats had only 7 - 8%
of normal AChE activity in the brain and blood. This level would
be associated with fatalities if brought about by a single dose;
indeed, at the end of the study, the animals were consuming daily a
dose equivalent to 96% of the single-dose LD50. In a similar way,
Barnes & Denz (1951) found that parathion in the diet at 100 or
75 mg/kg was lethal for the majority of rats in large groups within
3 - 4 weeks, while results with 50 mg/kg were variable (26/72 deaths
in one trial and 3/36 in a later trial) and no deaths attributable
to poisoning occurred in groups fed 20 or 10 mg/kg. The rats
surviving 50 mg/kg showed clinical signs of intoxication (notably
fasciculations) and ate less, initially: they ate normally after 3
weeks, but failed to gain weight as rapidly as the controls.
However, signs of poisoning decreased in severity and frequency
during the third month and seldom reappeared during the remainder
of a year's feeding. This pattern of response and of adaptation is
typical for all anticholinesterase pesticides. When monitoring of
enzymes is carried out in parallel with feeding, the level of
activity often rises to a steady state after an initial decline.
Factors determining the fraction of an LD50 dose that is tolerable
include:
(a) Speed of absorption, of subsequent metabolic activation,
and of elimination of the compound. Thus, for demeton- S -
methyl, much of the sulfoxide metabolite from a single dose
will still be circulating on the following day, though the
parent compound may not linger. In such a case, lethal
concentrations will build up more easily than with, say,
trichlorfon, which is converted to the inhibitory dichlorvos,
both of which are rapidly eliminated;
(b) The net rate of formation of a stable form of
inhibited AChE arising from the 3 reactions of inhibition,
reactivation, and aging described in section 4.5. The
relationship of the chemical structure of an oxon inhibitor to
rates of these reactions is complex and also varies between
species. AChEs from the rat have not been purified and
subjected to extensive kinetic study in vitro. In most
studies, crystallized (not 100% pure) bovine erythrocyte-AChE
has been used. Rates of spontaneous reactivation and aging for
this enzyme inhibited with dimethoxy, diethoxy, and ethoxy,
ethanthio-substituted phosphates are shown in Table 7.
Although data derived in this way cannot be transposed directly to
in vivo situations, they are consistent with the well-known fact
that, after poisoning by a sub-lethal dose of some dimethyl
phosphates, recovery with the disappearance of symptoms is complete
within a few h. The value of k+3 for erythrocyte-ChE taken from
rats dosed in vivo with dimethyl phosphate, was reported to be 57
x 10-4 (a half-life of inhibited enzyme of 2 h). One day after
such a sub-lethal dose, most of the rat AChE will again be in the
uninhibited form with a small fraction in the aged inhibited form.
In contrast, not more than half of the inhibited enzyme would be
expected to be reactivated in one day after poisoning with diethyl
phosphate and a substantial proportion of the inhibited enzyme
would be aged, so that recovery to 100% activity would be a very
slow process, depending on the synthesis of fresh enzyme. It
follows that markedly different outcomes would be expected from
repeated intoxication with doses of dimethyl phosphate and diethyl
phosphate which both caused an initial response of, say, 50%
inhibition, the former would be less hazardous than the latter.
This interpretation concurs with the fact that rats can survive
daily doses of 25% of the LD50 of trichlorfon, but only about 12%
of the LD50 of parathion (DuBois, 1963). DuBois (1963) also
pointed out that ChE inhibition mounted steadily as a result of
daily doses of OMPA and that toxic effects were seen when
inhibition reached about 70%. It is believed that no spontaneous
reactivation of ChE occurs after inhibition by phosphoramidates,
which makes such compounds intrinsically undesirable as pesticides.
Table 7. Rates of spontaneous reactivation (k+3 ) and of
aging (k+4 ) of bovine erythrocyte cholinesterase after
inhibition in vitro a
---------------------------------------------------------
O Rate constants x 104
|| R1 (per min) at pH 7.4
|| / and 37 °C
Enz-O-P substituents
\
R2
R1 R2 k+3 k+4
---------------------------------------------------------
-OCH3 -OCH3 115 14
-OCH3 -SCH3 1170 95
-OC2 H5 -OC2 H5 2.0 2.2
-OC2 H5 -SC2 H5 270 32
---------------------------------------------------------
a From: Clothier et al. (1981).
Dose-effect relationships for delayed neurotoxicity have been
listed (Johnson, 1975b). It has been noted that, as for
cholinergic effects, levels of long-term dosing can be found that
are detectable by a biochemical response at the primary target but
have no clinically- or histologically-observable correlate. The
threshold of the tolerable response is probably a permanent
inhibition or 40 - 50% of NTE (Johnson, 1982a).
6.3.2 Age and sex
It is well-known that the microsomal MFOs and other drug-
metabolizing enzymes are present at comparatively low levels in
neonatal animals, but activity develops to approximately the adult
level early in maturation. Since MFOs are involved in both the
activation and degradation of many organophosphorus pesticides
(section 4.3), the likely net result in terms of LD50 is hard to
predict. One-day-old rats were 9 times more susceptible to
malathion than 17-day-old animals (Mendoza, 1976). The toxicity of
methyl parathion and of parathion for rats decreased from birth
through the developmental period: the decrease was best correlated
with the increasing capacity of the animals to metabolize the
oxygen analogues by both oxidative and hydrolytic pathways (Benke &
Murphy, 1975). The LD50 (ip) of trichlorfon in adult male rats was
reported to be 250 mg/kg body weight, compared with 190 mg/kg in
male weanlings (FAO/WHO, 1972b). Whether this is a significant
difference is not clear. Liver MFO activity fluctuates according
to the hormonal status of female animals. LD50 values quoted for
males and females often differ, but these values generally arise
from different laboratory animals subjected to many variable
factors (including the purity of the test sample). Among several
representative pesticides surveyed for this review, only parathion
showed a marked and apparently real difference in LD50s between the
sexes, the oral LD50 in male rats being 5 - 30 mg/kg body weight,
depending on the solvent, compared with 1.8 - 5 mg/kg in females
(FAO/WHO, 1964).
6.3.3 Nutrition
It is well-known that liver MFO activity can be manipulated by
administering a diet severely deficient in protein. The
consequences can be dramatic in terms of the toxicity of compounds,
such as carbon tetrachloride, which undergo a single
biotransformation step leading to a directly toxic product.
However, as with the age and sex factors discussed above (section
6.3.2), several metabolic steps may be affected. No clear-cut
effects seem to have been reported. Thus, the acute toxicity of
diazinon is greater (up to 2-fold) in rats maintained on a diet,
either very low (4%), or very high (81%) in protein compared with a
standard (29%) protein diet (FAO/WHO, 1971b). A similar increase
in toxicity is seen with naled ( O,O -dimethyl O -1,2-dibromo-2,2-
dichloroethyl phos-phate) (Kaloyanova & Tasheva, 1983). Boyd
(1969) reported that, while increases in toxicity were 2-3 fold for
diazinon, malathion, and demeton, the increase in parathion
toxicity was 7.6 fold, in malnourished rats. Whether the effects
at such extremes were directly due to changes in the
biotransformation of the agent or to the animals becoming generally
unhealthy is not clear.
6.3.4 Effects of impurities and of storage
Insecticides are manufactured and formulated in various ways
and in many countries. There may be significant differences in
these procedures and in the conditions of storage of formulated
products. These factors can influence the nature and extent of
impurities present in the material that is ultimately applied.
Impurities in a pesticide may be of very low toxicity (the
majority), may be toxic in their own right (more or less toxic than
the major component), or they may be potentiators of the toxicity
of another component.
6.3.4.1 Impurities toxic in their own right
(a) Non-anticholinesterase effects
The most dramatic example of an impurity exerting an effect
different from that of the principal component does not come from
the realm of organophosphorus pesticides. The potent toxicant
2,3,7,8-tetrachlorodibenzodioxin (TCDD) may be present in the
herbicide 2,4,5-trichlorophenoxyacetic acid so that, even at a few
mg/kg, the effects of the impurity may dominate the toxicological
response. An analogous non-cholinergic response due to impurities
is not known in anticholinesterase pesticides. Questions have been
asked about the possible mutagenic effects of trimethyl phosphate,
which may be present at a few percent in some technical
preparations of dimethyl phosphates, but the possible exposure in
vivo is limited by the main anticholinesterase response to the
pesticide, and there appears to be no evidence for mutagenic
effects in mammals of any organophosphorus pesticide (section
6.2.1).
(b) Anticholinesterase effects
LD50 values for technical preparations of diazinon have varied
over an unusually wide range. The oral LD50 for the rat was
reported to be 76 - 108 mg/kg body weight in 1964 (FAO/WHO, 1964)
and 250 - 466 mg/kg in 1971 (FAO/WHO, 1971b). A major contributing
factor, according to the latter report, was the presence of highly
toxic pyrophosphates in earlier samples; it was implied that the
impurities had been produced during storage and eliminated by
stabilization (detail unspecified) of formulated material. It
seems likely that the pyrophosphate concerned in this improvement
was monothiono-TEPP with an oral LD50 in mice of about 4 mg/kg body
weight (Margot & Gysen, 1957). Both the sulfotepp and monothiono-
TEPP content of an emulsifiable concentrate of diazinon and its
toxicity increased rapidly when it was stored in tinned-steel
containers instead of in inert-lined aluminium ones (Soliman et
al., 1982). However, in 1979, it was reported that sulfotepp was
also present in many standard and formulated preparations of
diazinon at concentrations ranging from 0.2 to 0.8% and that the
percentage was unrelated to the age of the sample (Meier et al.,
1979). It seems likely that this impurity was formed during the
synthesis of diazinon using diethyl phosphorothiochloridate.
Sulfotepp was 60 - 80 times more toxic than diazinon for the rat,
so that at least one-third of the toxicity of typical diazinon
might be attributed to the impurity. This calculation is probably
an underestimate, since it seems that metabolic disposal of an
impurity is often slowed markedly by competition from the major
component. It can be said that there may be "reverse potentiation"
of the toxicity of the impurity by the major component (diazinon).
Formation of pyrophosphates is implicit in the mode of
synthesis of the many organophosphorus pesticides with a
phosphorochloridate or phosphorothionochloridate as a precursor.
TEPP or its methyl analogue would be unlikely to survive much
aqueous washing during production, but the mixed mono- or disulfo
analogues may well survive, unless deliberately eliminated. It
seems likely that sulfotepp is generally present in parathion
(Diggory, 1977); however, since both the parent and the impurity
have similar LD50s, pure and impure preparations do not differ
significantly. It is a curious fact that the lower the true
toxicity of a pesticide, the more marked may be the effect of an
impurity in changing its toxicity. It might be very rewarding
both to analyse more thoroughly and to reexamine the toxicities of
low-toxicity pesticides such as bromophos which is said to have an
LD50 in various mammals of 3 - 8 g/kg body weight (FAO/WHO, 1973b);
even this low toxicity might be attributable to the impurities
rather than to the pure compound. An analogous situation certainly
pertains concerning the potentiation of malathion (see below).
6.3.4.2 Impurities potentiating the toxicity of the major
ingredient
There does not appear to be any indication that the MFO status
of animals is different after administration of technical grade
organophosphorus insecticides compared with pure. Alterations of
the MFO status of animals because of diet, sex, drugs, etc.,
discussed in sections 6.3.2, 6.3.3, and 6.3.5, are unpredictable in
their effect on the LD50. In contrast, inhibition of the esteratic
capacity of mammals increases the toxicity of pesticides that
depend principally on tissue esterases in their metabolism (section
4.3). Malathion is a notable example of an organophosphorus
pesticide in which the toxicity is enhanced when tissue esterases
are inhibited. Until comparatively recently, such inhibition was
only known in situations where an unrelated organophosphorus ester
was administered to test animals a short time before the malathion.
However, it is now known that several impurities present in most
samples of malathion prepared for use as pesticides, are capable of
inhibiting tissue carboxylesterases. Some of these impurities act
very rapidly and so prevent the normal metabolism of malathion and
potentiate its toxicity. This enhanced toxicity has been expressed
in man. Several hundred spray workers were intoxicated while
spraying certain formulations of malathion in Pakistan, and 5 died
(Baker et al., 1978). Isomalathion and several trimethyl
phosphorothiolates are found in most commercial preparations of
malathion, but the levels depend markedly on the formulation and
on storage conditions. The potentiating power of small amounts of
these impurities are shown in Table 8. Examination of samples of
formulated malathion, known to be unusually toxic, showed a fair
correlation of toxicity only with the percentage content of
isomalathion (Aldridge et al., 1979; Miles et al., 1979). However,
the correlation was imperfect when a large number of samples were
examined and the addition of known further amounts of pure
isomalathion to the formulated samples caused more than the
expected potentiation (Aldridge et al., 1979). The authors
concluded that, although isomalathion contributed the main effect,
there was also significant potentiation by other agents present in
the samples; the chief candidate was O,S,S -phosphorodithioate.
Most unformulated samples of technical grade malathion seem to
have an LD50 for rats in the range of 1500 - 2000 mg/kg body weight.
Such material contains some potentiating impurities (Pellegrini &
Santi, 1972; Umetsu et al., 1977), but has proved acceptable as a
basis for formulated insecticides with little toxic hazard.
However, it is now clear that some formulated samples increase
markedly in both their impurity content and toxicity, when they are
stored at elevated temperatures. Not only are temperature and time
important, but also the formulating agents (Table 9), and almost
half of the malathion lost from Formulation C is apparently
transformed to isomalathion with a massive potentiation, whereas
the increase in toxicity is less marked in Formulations A and B, in
which a much smaller proportion of lost malathion is converted to
isomalathion.
Table 8. Potentiation of acute oral toxicity for rats by impurities
added to malathion
------------------------------------------------------------------------
Compound Amount Potentiation Purified Reference
added added ratio found malathion
(%) used (LD50
mg/kg body
weight)
------------------------------------------------------------------------
isomalathion 0.4 3 10 700 Aldridge et al.
0.6 5 (1979)
2 12
8 25
0.05 3 12 500 Umetsu et al.
0.1 4 (1977)
0.5 6
2 10
O,S,S -trimethyl 0.15 3 10 700 Aldridge et al.
phosphorodithioate 0.3 4 (1979)
0.5 8
1.0 13
2.0 20
0.05 4 12 500 Umetsu et al.
0.2 6 (1977)
0.5 7
0.035 2 8000 Pellegrini &
0.1 3 Santi (1972)
0.2 4
0.5 7
O,O,S -trimethyl 0.3 2 10 700 Aldridge et al.
phosphorothioate 1.3 4 (1979)
0.2 3 12 500 Umetsu et al.
1 4 (1977)
0.2 3 8000 Pellegrini &
0.5 4 Santi (1972)
O,O,S -trimethyl 1.5 2 10 700 Aldridge et al.
phosphorodithioate 5 5 (1979)
1 4 12 500 Umetsu et al.
5 5 (1977)
3.5 2 8000 Pellegrini &
4.5 3 Santi (1972)
------------------------------------------------------------------------
There is much evidence that potentiation of malathion by
extraneous compounds is associated with the inhibition of
carboxylesterases. Using malathion as specific substrate, Talcott
et al. (1979b) showed that isomalathion and O,S,S -trimethyl
phosphorodithioate were potent inhibitors of rat liver and plasma
malathion carboxylesterase, in vitro and in vivo; a partially-
purified sample of carboxylesterase from human liver was also
sensitive to isomalathion (Talcott et al., 1979a).
The same hazard exists with impure samples of phenthoate as for
malathion. Pellegrini & Santi (1972) showed that technical samples
containing 61 - 91% of the principal ester had LD50s (rat oral) of
78 - 243 mg/kg body weight, while a purified preparation (98.5%)
had an LD50 of 4700 mg/kg, though its toxicity for insects
increased approximately in proportion to the purity. The principal
potentiating impurities were the S -methyl isomer and the identical
trimethyl phosphorothiolates found in malathion. For phenthoate,
as for malathion, the vulnerability to potentiation lies in the
presence of the hydrolysable ethoxycarbonyl ester bond which, in
pure samples, is the key to low mammalian toxicity.
Table 9. Effects of formulating agents and of storage
time and temperature on composition and toxicity of
malathion (50% wdp)a
----------------------------------------------------------
Storage conditions Composition (%) Oral LD50
Temperature Time Malathion Isomalation (rat)
(°C) (days)
----------------------------------------------------------
Formulation A
0 48.0 0.38 2800
38 60 45.5 0.37 2230
90 44.2 0.49 1740
55 6 47.6 0.30 2520
13 46.4 0.37 1760
90 1 40.9 0.69 950
Formulation B
0 48.8 0.18 2540
38 60 47.2 0.79 1130
90 46.5 0.81 1330
55 6 45.2 0.67 1200
13 43.6 0.55 1170
90 1 39.9 0.32 1900
Formulation C
0 50.6 0.61 2660
38 90 44.9 3.7 590
55 6 46.2 3.4 535
13 43.7 3.5 555
----------------------------------------------------------
a From: Miles et al. (1979).
The presence of the S -methyl isomer (0.32%) in a commercial
fenitrothion formulation was shown by Miles et al. (1979). The
concentration increased to > 1% during accelerated storage tests,
but there was no concomitant increase in the toxicity of the
formulation. This observation on a compound not heavily dependent
on esterases for the primary step of detoxication, points to the
need to consider biochemical mechanisms in assessing possible
hazards. There need be no general concern about the presence of
small amounts of isomers in organophosphorus pesticides. The
presence of isoparathion in parathion is well-known, but there is
no suggestion of any marked change in toxicity brought about by
this impurity.
Some organophosphorus pesticides contain carboxylamide bonds
rather than carboxyester. These include dimethoate, dicrotophos,
monocrotophos, phosphamidon, and acephate. There appears to be
little evidence that impurities in commercial formulations of any
of the above pesticides markedly alter the toxicity, apart from a
small (1.6x) decrease in the mammalian toxicity of acephate, after
storage for 6 months at 40 °C; the insecticidal activity of the
compound was unchanged (Umetsu et al., 1977). During the storage
period, the concentration of various impurities changed, but no
relationship between these changes and altered toxicity was
obvious.
6.3.5 Effects of other pesticides and of drugs
All organophosphorus and carbamate insecticides exert their
acute toxic action by attack on the AChE. Thus, it follows that
exposure to more than one such pesticide will usually produce at
least an additive effect. Besides this simple effect, other
pesticides or drugs may also influence the toxicity of an
individual organophosphorus pesticide by interfering with its
metabolism, activation, and disposal.
Not all organophosphorus pesticides and probably no carbamate
pesticides inhibit tissue carboxylesterase to potentiate malathion
in the manner discussed in section 6.3.4. However, like all other
enzymes, the carboxylesterases have their own structure-activity
pattern: this has not been worked out in a systematic fashion. It
is clear that, with some compounds, profound inhibition of liver
carboxylesterase can be achieved without inhibition of AChE
sufficient to cause signs of poisoning. This class includes many
thioalkyl esters such as the trimethyl esters and also S,S,S -tri- n
-butyl phosphorotrithioate (DEF), which are potent potentiators of
malathion toxicity via carboxylesterase inhibition. EPN is a
phenylphosphorothioate insecticide that also acts in this way.
Tables 10 and 11 show examples in which measurements of effects on
tissue carboxylesterases and AChE are of value in predicting the
potentiation of malathion toxicity (Murphy, 1969).
Table 10. Comparison of enzyme inhibition caused by
several pesticidesa
---------------------------------------------------------
Insecticide Dietary concentration (mg/kg) resulting in
(period) 40 - 60% inhibition of:
Red cell- Liver- Plasma-
cholinesterase malathionase malathionase
---------------------------------------------------------
Parathion 3 5 5
(7 days)
Fenchlorphos 500 30 30
(7 days)
Malathion 500 100 500
(30 days)
---------------------------------------------------------
a From: Murphy (1969).
Table 11. Effect of feeding fenchlorphos on in vivo
anticholinesterase activity of malathiona
-------------------------------------------------------
Fenchlorphos Malathion Inhibition of brain
concentration challenge dose AChE 1 h after
in diet (mg/kg) (mg/kg ip) challenge (%)
-------------------------------------------------------
0 200 13
30 0 1
30 200 61
-------------------------------------------------------
a From: Murphy (1969).
Potentiation of the toxicity of organophosphate compounds for
mammals not containing a carboxylester function, does not appear to
be a significant hazard, though it is possible that potentiation of
some carboxylamide pesticides by an analogous inhibition of tissue
amidases may occur; certainly, EPN potentiates the toxicity of
dimethoate (El-Sebae, 1980). Potentiation of the toxicity of
organophosphorus pesticides for insects by inhibition of MFO
activity is well-known, and many potent synergists are used in
agriculture for this purpose (Wilkinson, 1971), but much less has
been reported concerning similar effects in mammals. This may
reflect the greater versatility of mammals compared with insects in
disposing of organophosphorus esters (section 4.3). Competition
for one metabolic route within the animal often does not greatly
alter its total capacity to deal with a foreign compound.
Keplinger & Deichmann (1967) combined pairs of various pesticides
in proportion to their oral LD50s, determined individually, and
then measured the oral toxicity of the mixtures in rats or mice.
They calculated a ratio of expected LD50/observed LD50 where
"expected LD50" was the sum of half the LD50 value of each of the 2
constituents. Their study included 7 chlorinated hydrocarbons,
the carbamate carbaryl, and 5 organophosphates including diazinon,
malathion, and parathion. With a "no-effect" ratio of 1.0, they
considered measured ratios greater than 1.75 or less than 0.57 as
probably significant of real effects. In a number of cases, less
than additive effects were noted for combinations of a chlorinated
hydrocarbon and an organophosphate, e.g., aldrin + diazinon (0.55),
DDT + malathion (0.54), toxaphene + carbonylfenthion (0.54): this
might well be expected if the mode of toxicity were different and
metabolic pathways were not markedly altered. The only cases of
potentiation involving organophosphates were also not surprising.
A triple combination of parathion, malathion, and chlordane had a
ratio of 1.99. This effect was probably due to simple potentiation
of malathion by parathion, since chlordane alone did not potentiate
either organophosphorus compound. Mixtures of Aramite (sulfurous
acid 2-chloroethyl 2-[4-(1-1-dimethylethyl)-phenoxyl-1-methylethyl
ester) with several organophosphates in mice had ratios of 1.86 -
2.14. However, since the LD50 of Aramite in mice is high (2000
mg/kg body weight), it may be that the 500 mg/kg administered in a
mixture was absorbed more efficiently and was therefore more
effective proportionately than the much higher LD50 dose of Aramite
alone.
As noted above, aldrin had little effect on the toxicity of
organophosphorus insecticides, when administered at the same time.
However, a number of chlorinated hydrocarbons (aldrin, DDT,
chlordane, etc.) are well-known as stimulators of MFO activity in
(principally) the mammalian liver. This activity increased
markedly during a period of a few days after dosage, and
pretreatment of mice with aldrin (16 mg/kg body weight), 4 days
before a challenge dose of 6 different organophosphates, markedly
reduced (up to 5-fold) the toxicity of each (Murphy, 1969).
Similar results were obtained with other classes of MFO inducers
such as phenobarbital. Murphy points out that the mechanism of
these effects probably includes stimulation of liver
carboxylesterase as well as MFO.
From the observations above, it appears that simple mixing of
an organophosphorus insecticide with a chlorinated hydrocarbon is
unlikely to adversely influence the acute toxicity for mammals, as
expressed by LD50 value. However, administration of DDE at 55
mg/kg diet to adult male Japanese quail led to an increasing
susceptibility to challenge doses of parathion (2.5 mg/kg)
administered orally; mortality in these birds increased from 0 to
30% after 1 week of feeding and 60% after 3 weeks (Ludke, 1977).
The problem of potentiation by some organophosphates of the
toxicity of pesticides containing carboxyl ester bonds may be
significant. This has been demonstrated for malathion in animals
(section 6.3.4) and in man (section 7.1.3). It may also be a
problem for pyrethroid insecticides for most of which degradation
by mammalian carboxyesterases is a significant detoxification
pathway (Miyamoto, 1976).
6.3.6 Species
No clear ranking of species sensitivity to organophosphorus
pesticides as a class can be given. A general impression is that
mice, hamsters, and guinea-pigs may be more sensitive than rats,
with respect to a number of compounds, but the converse is seldom
true. However, most available data have been produced with little
reference to conditions of husbandry, diet, hormonal status, etc.,
so that only very marked differences, which do not usually seem to
exist, would emerge. Birds tend to be more sensitive to
organophosphorus pesticides (Schafer, 1972) and amphibians less
sensitive than mammals. It has been suggested, but not confirmed,
that these differences might be due to differences in the activity
of enzymes in species that hydrolyse organophosphorus compounds and
thereby contribute to detoxification.
The toxicity of several organophosphorus pesticides for
different species seems to be inversely related to the activity of
the plasma A esterase, which degrades the pesticide oxon. Such
activity is considerably lower in birds than in mammals (Machin et
al., 1978). When 14 avian species were compared with 5 mammalian,
the average plasma activity against pirimiphos-methyl oxon was 170
times less, and that against paraoxon, at least 13 times less
(Brealey et al., 1980). Differences in liver microsomal oxidative
activity involved in the metabolism of several organophosphorus
pesticides by mammals and birds were less profound, though fish
liver was less active (Miyamoto & Ohkawa, 1978).
A multitude of factors contribute to the great difference
between the oral toxicity of chlorofenvinphos for the rat (10 mg/kg
body weight) and that for the dog (> 5000 mg/kg). These include
efficiency of absorption, at least 2 metabolic detoxification
processes, the rate of uptake by the brain, and a 7-fold difference
in the sensitivity of the brain AChE to this compound in the 2
species (Hutson & Hathway, 1967; Donninger, 1971).
Adult hens, cats, dogs, and larger farm animals are all
susceptible to organophosphorus delayed neurotoxicants (Davis &
Richardson, 1980; Johnson, 1982a). There is no clear ranking of
dose-sensitivity, though hens seem to be most uniformly responsive.
The full clinical response is not easily seen in laboratory
primates and rodents, though morphological damage may be detected
(section 6.1.1.2).
6.3.7 Other factors
The effects of solvents on chemical stability and isomerization
reactions were noted in section 4.5. The effects of formulation
agents on stability and, therefore, on the toxicity of malathion
have also been noted previously (section 6.3.4). It is likely that
percutaneous absorption will be greater for liquid formulations
than for powders but that powders may adhere longer thereby
enhancing an effect, if proper hygiene is not observed. A number
of examples are quoted by El-Sebae (1980), in which formulated
pesticides were more toxic than the technical preparation (Table
12). In many cases, this is due to the fact that the solvent in
the formulation facilitates the uptake of the pesticide into the
body. The toxicity of other components of the formulation may play
a role (especially in the case of pesticides of very low toxicity,
such as tetrachlorvinphos) (Table 12), as well as potentiation.
El-Sebae noted that, in some cases, the I50 for inhibition of ChEs
in vitro by these compounds differed. This could be relevant in
the case of the directly-active oxon-type pesticide tetrachlorvinphos,
but in cases where the test was performed with a thioate, the
anticholinesterase activity would depend almost entirely on trace
oxon impurities, which are often destroyed rapidly in vivo and
contribute little to toxicity compared with the bulk of oxon
produced metabolically.
Table 12. Comparative toxicity for mice of
some technical and formulated
organophosphorus pesticidesa
--------------------------------------------
Pesticide 24-h oral LD50
(mg/kg body weight)
Technical Formulated
--------------------------------------------
phosfolanb 12 11
chlorpyriphos 140 60
leptophos 162 83
tetrachlorvinphos 5000 1800
--------------------------------------------
a From: El-Sebae (1980).
b (diethoxyphosphinothioyl)dithioimidocarbonic
acid, cyclic ethylene ester.
6.4 Acquisition of Tolerance to Organophosphorus Insecticides
This topic has been discussed in section 6.3.1, where it was
noted that when AChE levels were reduced progressively over a
number of days or weeks, animals showed cholinergic signs of
poisoning which, in animals that survived, decreased in severity
and sometimes disappeared completely, though ChE inhibition was
maintained. This phenomenon is separate from the fact that
permanent inhibition of 30 - 50% is ineffective in producing
measurable symptoms. The basis for acquired tolerance is not fully
known, though a "down regulation" in the muscarinic ACh receptor is
thought to be a contributary cause. This involves both reduced
sensitivity and reduced numbers of receptors (Costa et al., 1982).
6.5 Therapy of Experimental Organophosphorus Poisoning
The understanding of the mechanism of acute toxicity of
organophosphorus pesticides has provided the basis for rational
therapy. The effects of inhibition of AChE, as described in
section 6.1.1, are common to all organophosphorus pesticides
intoxications. However, the speed of onset and the rate of unaided
recovery from sub-lethal doses vary greatly, depending on the
chemical nature of the pesticide, the route of exposure, and on
whether this exposure was a sudden overwhelming dose or a drawn-
out process.
Factors leading to a slow onset of symptoms include:
(a) Slow absorption or metabolic activation: this is often
associated with extremely low solubility and therefore
with the presence of large hydrophobic groups in the
ester molecule; pesticides such as haloxon, chlorpyrifos,
and leptophos are of this type;
(b) Persistence in the system of a comparatively stable
inhibitor of ChEs. This could be as a low concentration
of an active inhibitor such as demeton- S -methyl
sulfoxide or high concentrations of a weak inhibitor such
as methamidophos.
Factors leading to rapid clearance of symptoms include:
(a) Rapid clearance of the pesticide and its active agents,
as with trichlorfon and dichlorvos; rapid clearance
occurs also with nerve agents such as soman and sarin,
which have been much used in studies on therapy in
experimental animals.
(b) A slow rate of aging of inhibited AChE giving opportunity
for reactivation (spontaneous or induced) to occur;
diethyl phosphorylated AChE ages more slowly than
dimethyl or diisopropyl.
(c) Rapid spontaneous reactivation of inhibited AChE, such as
occurs after inhibition by all dimethyl or bis-2-
chloroethyl phosphates. In this case, the possibility of
reinhibition by a persistent compound will affect the
picture.
The factors noted above, which influence speed of onset and
remission of effects, influence the prognosis for response to
therapy but do not markedly alter the nature of optimal treatment.
Maximum benefit comes from combined treatment with an
anticholinergic drug (usually atropine) plus a reactivator of
inhibited ChE (an oxime) with diazepam, and also artificial
respiration. The effects of the components will be discussed
individually below.
6.5.1 Palliation
Artificial respiration alone can be very effective in
maintaining life, since the primary cause of death in
organophosphorus poisoning is respiratory failure (section 6.1.1).
Such treatment gains time for the processes, natural or imposed,
that lead to the return of sufficient ChE activity to maintain
life.
6.5.2 Antagonism of effects of ACh
Atropine antagonizes many of the peripheral muscarinic effects
of excess ACh and also some central effects. However, there was no
correlation between the peripheral anticholinergic activity of a
range of atropine-like drugs and their capacity when used alone (or
in conjunction with oximes) (section 6.5.3) to protect against the
lethal effects of sarin (Coleman et. al., 1962; Brimblecombe et.
al., 1970). Moreover, the ranking of the therapeutic efficacy of
atropine analogues varied according to the test species (rat,
mouse, or guinea-pig), and all effects were small (protective ratio
< 1.5) in mice and guinea-pigs, but much larger and more variable
(1.2 - 9.3) in rats. The ranking changed yet further when the drug
was combined with oxime P2S (see below), though the protective
ratio with some compounds rose to 18 - 24 in guinea-pigs, 9 - 80 in
rats, but only 1.8 - 6.3 in mice. These confusing effects are now
thought to be at least partially due to an anticonvulsant effect
contributed variously by the atropine analogues. Anticonvulsants
often supplement the effects of atropine or of combined
atropine/oxime therapy (see below). In particular, diazepam
(valium) is known both to raise the LD50 and speed recovery in some
cases (Johnson & Wilcox, 1975). These authors implied that the
mechanism might be partly direct antagonism to some central effects
of ACh and partly indirect. When diazepam is included in the
therapeutic package, there appears to be little evidence that
alternative anticholinergic drugs to the well-proved atropine are
superior (Green et al., 1977). It has been reported that, in
prophylaxis against the toxicity of DFP in mice, the protection
factor was 28 when atropine and obidoxime were used but 180 when
Dexetimide (a drug with strong central anticholinergic activity)
was substituted for atropine. The particular advantage claimed for
this drug was that, in the therapy of rabbits intoxicated with up
to 60 x LD50 of paraoxon or 80 x LD50 of DFP, one single
intravenous injection of Dexetimide (8 - 16 mg/kg body weight) was
effective in conjunction with obidoxime, whereas repeated doses of
atropine were necessary (Bertram et al., 1977). Dexetimide is used
for the treatment of parkinsonism and is available commercially.
However, Dexetimide has not been evaluated in conjunction with
diazepam or compared with atropine plus diazepam, and no details
were given of the hazards of its use and side-effects in control
animals.
6.5.3 Reactivation of inhibited AChE
As indicated in section 4.5.1, inhibited ChEs can be
reactivated in vitro by treatment with appropriate nucleophilic
agents, of which salts of the oxime N- methylpyridinium-2-aldoxime
are the most commonly used (the chloride is known as pralidoxime
and the methanesulfonate as P2S). In some countries, obidoxime
(ToxogoninR ), which is a bis-quaternary oxime, is recommended at
slightly different doses than pralidoxime, but the mode of action
is similar. The scope for further improvement in the design of
therapeutic oximes is discussed by Gray (1984).
In vivo, there are 2 limitations to the benefits to be
obtained from the use of these agents:
(a) Access
The quaternary oximes are thought not to cross the blood-brain
barrier easily (Taylor, 1980). However, some experimental
work, summarized by Lotti & Becker (1982b), suggests that there
may be limited access, and this may have a significant, albeit
small, effect in reversing inhibition of AChE to improve the
clinical state. Other more direct beneficial action of the
oximes directly at synapses in the medullary respiratory centre
cannot be ruled out. The prompt improvement in the level of
consciousness observed and in the EEG of an intoxicated child
when iv infusion of 2-PAM was commenced (Lotti & Becker, 1982b)
also seemed to indicate that there was some access to important
brain regions. It has been claimed that obidoxime (25 mg/kg
body weight) injected intraperitoneally in rats, 5 min after a
dose of armin, was effective in reactivating 33% of the
inhibited AChE of the ponto-medullary region, which contains
the centre for control of respiration (Vasic et al., 1977).
However, there were no controls appropriate to disprove the
alternative explanation that the oxime had altered the
circulating level of armin (by direct destructive interaction
or otherwise), which, although interesting, is unlikely to be
relevant to therapy instituted later after dosing, nor is such
destruction-protection unique to toxogonin.
(b) Aging of inhibited AChE
As noted in section 4.5.1, inhibited AChE is converted by a
time-dependent reaction to a form resistant to reactivators.
Thus, oxime therapy becomes less effective with time after
poisoning. The rate of aging of dialkyl-phosphorylated AChE is
Me > IsoPr > Et (O'Brien, 1967), but little has been published
on the rate of aging when phosphonyl groups derived from
pesticides are attached to the enzyme. When one or both of the
residual alkyl groups are attached to phosphorus through sulfur
rather than oxygen, the rates of both spontaneous reactivation
and of aging of inhibited bovine erythrocyte AChE are markedly
increased (Clothier et al., 1981). Ethoprophos is one of the
newer pesticides that contain such a residual alkylthio group;
no published studies are known of therapy after poisoning with
this or related pesticides.
6.5.4 Efficacy of therapy
As reported above, the combination of atropine plus oxime is
far more effective in most cases than the mere summed effects.
This is because the peripheral neuromuscular junctions
(particularly the diaphragm) and the sympathetic ganglia, where
oximes reactivate AChE, are nicotinic and are unaffected by
atropine, so that separate aspects of intoxication are treated by
the two agents. There is speculation that some oximes may exert a
protective effect by acting as depolarizing agents at the
neuromuscular junction. This may also account for the slight
therapeutic effects of some analogues of toxogonin which do not
have any nucleophilic oxime group or reactivating power (Schoene &
Oldiges, 1973). Diazepam, also, is ineffective, except in
combination with atropine and oxime.
Persistence of the toxic agent may interfere with successful
therapy. Thus, single doses of atropine + oxime were of only
marginal efficacy in altering the LD50 of isofenphos in rats
(FAO/WHO, 1982b). However, when therapeutic doses were given
repeatedly at about 12-h intervals, for 2 - 3 days, the LD50 for
rats was raised about 4-fold; for hens, the increase was 15-fold
(Wilson et al., 1984). It appears that the failure of one-shot
therapy in this case was due to persistence of the toxic agent
rather than to a different mode of intoxication or to formation of
an inhibited form of AChE that resisted reactivation. The same
situation may pertain for profenofos intoxication, which appears
not to respond well to therapy (El-Sebae, personal communication,
1985).
7. EFFECTS ON MAN
7.1 Acute Cholinergic Poisoning
The clinical picture of organophosphorus intoxication results
from accumulation of ACh at nerve endings. The syndrome is
described in detail in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980; HMSO, 1983; Plestina, 1984). The
symptoms can be summarized in three groups as follows:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles and, in more severe
cases, of diaphragm and respiratory muscles; and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, restlessness, and anxiety;
- mental confusion, convulsions, and coma; and
- depression of the respiratory centre.
All these symptoms can occur in different combinations and can
vary in time of onset, sequence, and duration, depending on the
chemical, dose, and route of exposure. Mild poisoning might
include muscarinic and nicotinic signs only. Severe cases always
show central nervous system involvement; the clinical picture is
dominated by respiratory failure, sometimes leading to pulmonary
oedema, due to the combination of the above-mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure; and
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to the light. Pulse rate is not
of diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ.
On the other hand, since changes in the conduction
and excitability of the heart might be life-
threatening, monitoring should be performed.
Confirmation of diagnosis is made by measurement of AChE in RBC
or plasma-pseudoChE, and, also, of the dibucaine number (to rule
out genetic deficiencies).
Measurements of blood-ChE during therapy are also useful in
assessing the treatment with oximes, though there might not be a
correlation between the severity of symptoms and the degree of ChE
inhibition: comparison should be made with pre-exposure levels,
wherever possible.
Chemical analysis of body fluids (urine, blood, gastric lavage)
should be made in order to identify the compounds that caused
poisoning.
7.1.1 Methods for assessing absorption and effects of
organophosphorus insecticides
As well as assessments of general health and behaviour, the
study of the effects of this class of pesticides is favoured
compared with that of some other classes since the basic
biochemical mechanisms (inhibition of esterases) are known for the
major toxic effects. Biochemical and neuro-physiological
techniques, relevant to the principal effects of all the compounds,
have been established. Identification of the monobasic acid type
of urinary metabolite, which is commonly produced, is an indicator
of exposure rather than of an effect, but it seems appropriate to
outline the technique in this sub-section. Wherever possible, test
findings should be compared with pre-exposure measurements on the
same individual.
7.1.1.1 Analysis of urine as a means of monitoring exposed
populations
As previously discussed in section 4, organophosphorus
pesticides may undergo hydrolysis in vivo to yield substituted
phosphoric acids that are subsequently excreted in urine. Advances
in gas chromatography and combined gas chromatography/mass
spectrometry (CG/MS) have made it possible to analyse the urine of
exposed persons for the presence of appropriate metabolites. It is
usually necessary to preserve the sample by the addition of
chloroform, to concentrate or extract the metabolite(s), and to
convert them to suitably-volatile derivatives that can be detected
by GC. Obviously, access to a well-equipped analytical laboratory,
capable of the quick processing of samples, is a necessary factor
if monitoring by urine analysis is proposed. However, in some
cases, simpler and sensitive colorimetric tests are available for
screening the urine of exposed persons. Thus, 4-nitrophenol can
be measured directly in the urine of workers exposed to parathion
(Wolfe et al., 1970).
Consideration of the concentration of metabolite(s) in the
urine can be helpful in determining patterns of exposure, and these
concentrations can be calibrated against the effects on AChE for a
particular pesticide. However, the time-course and peak of
excretion of metabolites appears to vary according to dose (Bradway
et al., 1977), so that serial sampling and analyses of urine are
desirable. Levels of metabolite alone cannot be considered a guide
to hazard. This is obvious when it is realized that pesticides
that have very different toxicities may yield identical acidic
metabolites. Thus, the level of metabolites in urine, after
exposure to sufficient amounts of the very toxic parathion-methyl
to depress blood-AChE to 50%, will be much lower than that of the
identical metabolites, following exposure to the related
fenitrothion, which is about 40 times less toxic.
7.1.1.2 Biochemical methods for the measurement of effects
AChE is present in human erythrocytes (RBC) and is the same as
the enzyme present in the target synapses. Thus, levels of AChE in
RBC are assumed to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the organophosphate has equal access to blood and
synapses. In the case of acute poisoning, a high inhibition of
RBC-AChE is pathognomonic, but, in the follow-up of the
intoxication, it might not be correlated with the severity of
symptoms. In the case of repeated exposures, additional
difficulties in interpretation arise from possible development of
tolerance. However, monitoring of pre- and post-exposure levels of
AChE in RBC gives a good measure of the effects of an exposure
(Kaloyanova, 1975). In cases where the pre-exposure AChE level is
not known (as in accidental poisoning), reference can be made to a
mean population AChE activity. Blood-plasma contains a related
enzyme called ChE or pseudoChE, which contributes to the whole-
blood enzymatic activity; the contribution of plasma-ChE in assays
of AChE will depend on the type and concentration of the substrate
used. PseudoChE has no known physiological function and can be
inhibited selectively by some compounds without causing a toxic
response. The sensitivities of AChE and ChE to inhibitors differ,
so that measurements of the ability of whole-blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many kinds of field conditions, procedures using whole blood, are
more practical than those using separated erythrocytes. Quite
commonly, pseudoChE is more sensitive to inhibitors. Thus, if
separation of plasma and erythrocytes is possible, prior to assay,
an indication of exposure can be obtained by assay of pseudoChE
only. Examples of selected organophosphorus insecticides, arranged
according to their ability to inhibit preferentially either plasma
or red cell-ChE in man, are given in Table 13 (Hayes, 1982).
Table 13. Selected organophosphorus insecticides arranged
according to their ability to inhibit either plasma- or
red cell-cholinesterase in mana
---------------------------------------------------------
Plasma enzyme more inhibited RBC enzyme more inhibited
---------------------------------------------------------
Chlorpyrifos Dimefox
Demeton Mevinphos
Diazinon Parathion
dichlorvos Parathion-methyl
malathion
Mipafox
Trichlorphon
---------------------------------------------------------
a Modified from: Hayes (1982).
ChE assay procedures vary greatly in sophistication, but the
most satisfactory is that based on the procedure of Ellman et al.
(1961). A field method and kit for whole blood- and plasma-ChE
determination have been developed (WHO, 1984b). Quick methods exist
for the determination of ChE in serum using paper tests (Izmirova,
1980) and for the colorimetric determination of ChE in whole blood
(Tintometer): these may be useful in the differential diagnosis of
organophosphate poisoning. Interpretation of the test results is
discussed in section 7.1.2.
The potential for delayed neuropathic response to an
organophosphorus ester can be predicted by the assay of the
esteratic activity of the target protein (NTE), in autopsy brain
samples from dosed adult hens (section 6.1.2). It has been shown
(Dudek et al., 1979; Richardson & Dudek, 1983) that a low level of
similar enzyme activity resides in lymphocytes and that there may
be correlations under some circumstances between neurotoxic dose
and available lymphocyte enzyme activity. The possibility of
monitoring exposed individuals by means of human lymphocyte or
platelet NTE activity is being explored (Lotti et al., 1983;
Bertocin et al., 1985; Maroni & Bleeker, 1986).
7.1.1.3 Electrophysiological methods for the study of effects
Electromyographic (EMG) studies using non-invasive surface
electrodes have been claimed to give sensitive indications of
exposure to organophosphorus pesticides, even in situations where
blood-ChE activity has returned to normal levels (Jager et al.,
1970; Roberts, 1976). The method requires electro-physiological
equipment and a very skilled practitioner. There is still
considerable doubt about the validity of some published studies.
Reproducibility is known to be very sensitive to local factors such
as temperature of the skin, and conflicting results have been
published, some of which show small increases and some, small
decreases in the ampli-tude of evoked muscle action potential, in
response to nerve stimulation. These findings have been reviewed
by LeQuesne & Maxwell (1981), who noted that changes that have been
reported tended not to be dose-related. In addition, they
evaluated the technique under controlled circumstances. In a
treatment to eradicate parasitic schistosomes, 55 children were
dosed orally with trichlorfon, 3 times, at 2-weekly intervals, at
doses that measurably depressed blood-ChE (mean 50%), but were not
enough to cause overt toxic effects, apart from mild cramps and
diarrhoea in a few cases. Only 3 children showed a significant
alteration in electromyographic response. Shortly after the last
(and highest) dose of 10 mg/kg body weight, 3 children developed
repetitive activity recorded over the thenar muscles following
supramaximal stimulation of the median nerve at the wrist. The
activity consisted of a small potential at the end of the main
muscle response and was characterized by being abolished by a
second stimulus 30 or 80 milliseconds after the first, or by
maximum voluntary contraction for 10 seconds; the amplitude of the
response to the second stimulus was not reduced. These
characteristics are necessary criteria that distinguish (these)
dose-related responses from pre-existing natural (and
idiosyncratic) responses, which can otherwise confuse EMG studies
in a population. Changes in amplitude measured on 52 control
subjects (mean 13.8 ħ 2.5 SD), on 2 occasions (2 weeks apart),
ranged from +5 to -3 mV. Thus, EMG does not appear to give a
highly sensitive measure of exposure to an ingested
organophosphorus compound.
7.1.2 Monitoring studies
Measurement of whole blood-AChE is the most widely adopted
method for monitoring the effects of occupational exposure to
organophosphorus insecticides. Physiological variations in blood-
ChE levels occur in a healthy person and are seen among a
population. It has been estimated that the coefficient of
variation for AChE activity in samples from an individual is 8 -
11%, and that a decrease of 23% below pre-exposure level may,
therefore, be considered significant. If the average of several
pre-exposure values were available, then a decrease of 17% would be
significant. It has been recommended that, if measured activity is
reduced by 30% or more of the pre-exposure value, AChE measurements
should be repeated at appropriate intervals to confirm the results.
Depressions of AChE or ChE in excess of 20 - 25% are considered
diagnostic of exposure but not, necessarily, indicative of hazard.
Depressions of 30 - 50% or more are considered indicators for
removal of an exposed individual from further contact with
pesticides until levels return to normal. Work procedures and
hygiene should also be checked (Zielhuis, 1972; WHO, 1975; CEC,
1977; Kaloyanova et al., 1979; Plestina, 1984).
Urinary metabolites have been monitored as a means of comparing
the efficiency of absorption of a pesticide by different routes.
The reports of the annual Joint FAO/WHO Working Parties,
mentioned previously, contain summaries of numerous controlled
exposure studies. No cases appear to be known of significant
clinical effects in man in the absence of depression of plasma- or
erythrocyte-ChE levels. No-observed-adverse-effect levels have
been calculated on this basis, where the data are available, or
have been estimated for man by extrapolation of the available data
for exposed animals.
7.1.3 Retrospective studies of populations exposed to
organophosphorus pesticides: acute and long-term exposure
Many thousands of cases of acute poisoning by organophosphorus
pesticides have been recorded (Namba et al., 1971). The majority
have been due to parathion and methyl parathion. Thus, Namba (1974)
in a discussion of the relative toxicities of parathion and
malathion, quoting Japanese Government statistics for the 7 years
1958-62, 1966, and 1967, stated that there were 3311 accidental or
occupational poisonings due to parathion including 188 deaths,
while for malathion, the numbers were 63 and 10, respectively. He
noted that the difference was not due to the restricted use of
malathion.
In the context of this general introduction, no descriptions
and breakdown will be given of retrospective studies of populations
exposed to organophosphorus pesticides. Such figures are relevant
to individual substances and will be given in the appropriate
Environmental Health Criteria.
It is generally thought that the only long-term effects
attributable to overt or subclinical acute intoxication with
organophosphorus compounds, or to prolonged low-level exposure,
are behavioural (rather doubtful), and delayed-onset neuropathy in
the case of certain compounds; these are dealt with in section 7.2.
>7.2 Other Effects on the Nervous and Neuromuscular System Due to
Acute or Long-Term Exposure
7.2.1 Delayed neuropathic effects
The characteristics of this disorder are given in section
6.1.2. The agents most commonly causing delayed neuropathy in man
are triaryl phosphate esters used in, e.g., hydraulic fluids; these
do not have any AChE activity and are not pesticides. In Table 14,
organophosphorus pesticides are listed for which there is
reasonable evidence that they have caused delayed neuropathy in
man.
Table 14. Organophosphorus pesticides reported to cause
delayed neuropathy in man
--------------------------------------------------------
Pesticide Number Reference
of cases
--------------------------------------------------------
mipafox 2 Bidstrup et al. (1953)
leptophos 8 Xintaras et al. (1978);
FAO/WHO (1979b)
methamidophos 9 Senanayake & Johnson
(1982)
trichlorphon many Shiraishi et al. (1977);
Hierons & Johnson (1978);
Johnson (1981a)
trichlornat 2 Jedrzejowska et al.
(1980); Willems (1981)
EPN 3a Xintaras & Burg (1980)
Chlorpyrifos 1 Lotti & Moretto (1986)
--------------------------------------------------------
a Moderate effects only and possibly other
etiological factors.
The cases with mipafox involved a single occupational exposure
to a compound that was developed before delayed neuropathy was a
recognized hazard. The cases with EPN and leptophos arose through
repeated occupational exposure with inadequate precautions.
Apparently cholinergic effects were often experienced, but not at
the level of severe poisoning. A few cases with methamidophos and
trichlorfon involved substantial occupational exposure, which
caused severe acute poisoning prior to the development of
neuropathy, but the majority of cases involved accidental or
deliberate ingestion of quantities that might well have been fatal
but for medical intervention. The fact that most of the cases
listed are due to exposure to phosphonates or phosphoramidates is
in line with the structure-activity relationships listed in section
6.1.2.
The value of measuring the neuropathy target esterase of human
lymphocytes as a predictive monitor was proposed by Dudek et al.
(1979) and Richardson & Dudek (1983). Lotti et al. (1983) reported
occupationally related changes in the lymphocyte NTE in spraymen
during seasonal spraying of DEF, but overt neuropathy was absent.
In a case of self-poisoning by a mixture of pesticides including
chlorpyrifos (about 20 g diluted in petroleum distillates),
Osterloh et al. (1983) noted that signs of cholinergic poisoning
were limited and they attributed death to the effects of
chlorophenoxyacetic acids. Brain esterases were not inhibited at
autopsy, but erythrocyte and peripheral nerve AChE levels were
about 22% of normal and nerve NTE was about 30%. This substantial
inhibition of NTE suggested that treated survivors of severe
poisoning by chlorpyrifos might well develop delayed neuropathy.
This prediction was confirmed in a recent case of self-intoxication
with chlorpyrifos (estimated dose 300 mg/kg body weight) in which
very low levels of lymphocyte-NTE were found, 30 days after
intoxication and after recovery from very severe cholinergic
effects. Typical moderate polyneuropathy developed in the
following days (Lotti & Moretto, 1985).
Allegations have been made against a few other organophosphorus
insecticides including malathion, omethoate, and parathion, though
many accidental and intentional poisonings by these agents have not
had any neuropathic sequelae. Experimental evidence against a
neuropathic potential in these compounds is strong (section 6.1.2).
However, in view of the serious paralytic effects involved, the
evidence adducing that these pesticides were the causal agents is
reviewed below.
(a) Malathion
Two alleged cases can be discounted. Petry (1958) reported a
case in which a physician contaminated himself frequently with
chlorinated hydrocarbon pesticides during day-long gardening
activities, about once per week, over a 10-year period. In 1954,
he commenced using 6% malathion in a "hose-on" device for garden
pest control and, in 1955, he commenced using 50% malathion in a
hand spray, both indoors and outside. He soon developed signs of
chronic anticholinesterase poisoning (generalized weakness and
tremor, irritability, difficulty in focusing). He eventually
collapsed and his general condition improved in hospital. However,
he continued to experience generalized weakness and particular
weakness in the right shoulder girdle, right serratus anterior, and
both peroneal muscle groups, and these symptoms persisted to some
extent for over a year. The distribution of these symptoms of
deficient muscle performance is quite atypical for delayed
neuropathy and seems more likely to be due to prolonged moderate
cholinergic insult from malathion precipitating weakness, anorexia,
and weight loss, which then precipitated further ill-health as
previously-stored chlorinated hydrocarbons was mobilized from
degraded fat stores. DDT at 23 mg/kg plus a high level of organic
chloride were found in a subcutaneous fat biopsy. A muscle-
necrotising effect, due to prolonged cholinergic stimulation, as
described in section 6.2.6, is also possible.
A separate case report of ascending paralysis following
malathion intoxication (Healy, 1959) concerned an 18-month-old
child exposed daily for 6 weeks to malathion from a garden spray.
Contamination was dermal and also by inhalation and ingestion,
leading ultimately to a cholinergic crisis and a prolonged period
of weakness including extensive flaccid paralysis for several days.
The condition responded to atropine and rest within 4 weeks. In
spite of the author's conclusion that this was a "demyelinating"
(i.e., delayed neuropathic) disorder, the picture is typical of
prolonged cholinergic insult responding to atropine and the
comparatively slow clearance of accumulated pesticide with
recovery from excessive nerve-muscle stimulation.
(b) Omethoate
A typical delayed neuropathy followed ingestion of an
organophosphorus pesticide with suicidal intent (Curtes et al.,
1979). Identification of the actual pesticide ingested was
doubtful depending on a later hearsay report concerning a bottle in
a garden shed, the contents of which were not analysed. Lotti et
al. (1981) assayed both the AChE and the NTE activities in
autopsied brain after a fatal intoxication with omethoate and found
that, even at the fatal dose, the NTE levels were normal while the
AChE activity was highly inhibited. Considering this data together
with evidence of similar non-inhibition in experimental hens at up
to 8 x the unprotected LD50, the authors concluded that, though the
neuropathy was typical, it was likely that the toxic agent was some
other organophosphorus compound more liable to cause neuropathy,
such as trichlorfon or trichlornat (Table 13).
(c) Parathion
Only two cases of permanent incapacity have been attributed to
parathion, though thousands of parathion poisonings are known (see
earlier). Petry (1951) attributed a case of neuropathy to the
aftermath of several occupational incidents of cholinergic
poisoning, but the history is entirely atypical in that symptoms
developed only 4 months, rather than 2 - 4 weeks, after the last
exposure. Causation by parathion is therefore very unlikely. A
farmer deliberately ingested parathion at a dose estimated to be at
least 150 g (perhaps 500 x the estimated human lethal dose) in 600
ml of methanol. Vigorous therapy preserved his life, though he
remained in a deep coma for 7 weeks. On recovery from this
experience, he was found to be suffering from flaccid paralysis of
both legs and weakness of both hands with muscle atrophy (De Jager
et al., 1981; 1982); partial recovery occurred during one year.
Lotti & Becker (1982b) have discussed the complicating factors of
the potentially supra-lethal dose of methanol and of the long coma.
However, the clinical picture is not unlike a true moderate
organophosphorus-induced neuropathy. At such a colossal dose, the
possibility of ingesting a significant amount of a neuropathic
impurity in the parathion must be recognized. This could be ethyl
bis-(4-nitrophenyl) phosphorothioate; small but significant
amounts of the appropriate oxon are present in some samples of
paraoxon made from diethyl phosphorochloridate (Johnson, 1982b),
and this oxon is a potent inhibitor of NTE.
(d) Other organophosphorus pesticides
Besides the atypical case attributed to malathion, Petry (1958)
described another case in which symptoms persisted after a
cholinergic crisis that followed severe intermittent exposures over
3 seasons to a variety of insecticides including parathion, EPN,
DDT, dieldrin, and lead arsenate. Some of the persistent symptoms
might be compatible with a mild peripheral neuropathy. Among the
agents used, lead arsenate and dieldrin would be expected to
contribute damage to the nervous system and EPN at about the LD50
level causes neuropathy in hens and man (Tables 4, 13).
A case of slow-onset profound weakness with complete recovery
within 3 months following contamination of an agricultural worker
with the cotton defoliant, merphos ( S,S,S -tri- n -butyl
phosphorothioite), was thought by the author to be of the delayed
neuropathy type (Fisher, 1977). However, the clinical picture
showed signs that are not seen in acute organophosphate
intoxication (influenza-like onset of the syndrome and high level
of protein in the spinal fluid). These signs are, however,
characteristic of a Guillan-Barré syndrome, which might have been
coincidental to the merphos exposure only 4 days previously. It is
possible that, later, a mild organophosphorus neuropathic effect
was superimposed on the Guillan-Barré effect, since merphos can
produce neuropathy in experimental animals (Johnson, 1970, 1975b).
Recovery from very mild neuropathies is usually complete.
7.2.2 Behavioural effects
Although many epidemiological studies have been carried out,
few controlled studies on man have been reported. It is generally
recognized that there are behavioural and psychic changes during
overt clinical poisoning by organophosphorus insecticides and that
these may take several months to regress (Karczmar, 1984).
However, there is no information to suggest that effects occur at
exposure levels that do not either alter ChE levels or produce
physical symptoms. Levin & Rodnitsky (1976) have reviewed the
literature, including their own work, on different aspects of
behaviour as affected by organophosphates. Much was based on
generalized complaints from workers occupationally exposed to many
agricultural chemicals (and probably also to automobile fuels and
lubricants and to alcohol). In summary, they found that, in human
subjects sufficiently exposed to organophosphates to depress
plasma- or erythrocyte-ChEs, some or all of the following
behavioural variables might be impaired. In cognition: vigilance,
information processing and psychomotor speed, and memory; in
speech: both performance and perception; in psychic state:
increased tendencies to depression, anxiety, and irritability; and
in EEG records: a tendency to faster frequencies and higher
voltages. They also concluded that the EEG abnormalities were
positively related to the level of AChE inhibition during the
initial stages of inhibition. Concerning studies on asymptomatic
workers at risk from repeated exposure to organophosphorus
pesticides, they considered that the evidence was equivocal for the
presence of less severe or latent forms of any behavioural
abnormalities. However, Duffy & Burchfield (1980) claimed that
changes in the EEG of individuals accidentally exposed to the
nerve-agent sarin could be detected twelve months after the
exposure. This study was a sequel to the study on monkeys
described in section 6.1.2, and the analyses of EEGs was performed
as noted there. They claimed significant differences (very small
differences analysed by complex statistical procedures) between the
group of sarin-exposed workers and controls, particularly in the
region of beta-rhythm (but the comment on the spread among normal
monkeys should be noted). However, the authors were unable to pick
out sarin-exposed individuals on the basis of the EEG. They also
found (small) increased amounts of REM-sleep in the exposed
workers. Without controlled exposure and serial monitoring of
effects in individuals, little can be deduced from these apparent
marginal changes.
7.3 Effects on Other Organs and Systems
Very few effects, other than those described in sections 7.1
and 7.2, have been noted, except those arising from ill health due
to severe anticholinesterase effects.
Several adverse effects attributed to only one organophosphorus
ester will be listed under the individual substances.
7.4 Treatment of Organophosphate Insecticide Poisoning in Man
All cases of organophosphorus poisoning should be dealt with as
an emergency and the patient sent to hospital as quickly as
possible. Although symptoms may develop rapidly, delay in onset or
a steady increase in severity may be seen up to 48 h after
ingestion of some formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major references
(Kagan 1977; Taylor 1980; HMSO, 1983; Plestina 1984) and will also
be included in the IPCS Health and Safety Guides to be prepared for
selected organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
7.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in cleaning the skin area where venupuncture
is performed. Blood might be contaminated with direct-acting
organophosphorus esters, and, therefore, inaccurate measures of ChE
inhibition might result. Extensive eye irrigation with water or
saline should also be performed. In the case of ingestion, vomiting
might be induced, if the patient is conscious, by the
administration of ipecacuanha syrup (10 - 30 ml) followed by 200 ml
water. This treatment is, however, contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also be
performed, particularly in unconscious patients, taking care to
prevent aspiration of fluids into the lungs (i.e., only after a
tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
7.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
7.4.3 Specific pharmacological treatment
7.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given at
15 to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be necessary
in extreme cases and total daily doses up to several hundred mg may
be necessary during the first few days of treatment.
7.4.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs (section 6.5.3). However,
if absorption, distribution, and metabolism are thought to be
delayed for any reasons, oximes can be administered for several
days after intoxication. Effective treatment with oximes reduces
the required dose of atropine. Pralidoxime is the most widely
available oxime. A dose of 1 g pralidoxime can be given either im
or iv and repeated 2 - 3 times per day or, in extreme cases, more
often. If possible, blood samples should be taken for AChE
determinations before and during treatment. Skin should be
carefully cleansed before sampling. Results of the assays should
influence the decision whether to continue oxime therapy after the
first 2 days.
The possible beneficial effects of oxime therapy on CNS-derived
symptoms is discussed in section 6.5.3.
7.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to counteract
some aspects of CNS-derived symptoms, which are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required (Vale & Scott, 1974). Other centrally acting
drugs and drugs that may depress respiration are not recommended in
the absence of artificial respiration procedures.
7.4.3.4 Notes on the recommended treatment
(a) Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
(b) Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinisation. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
(c) Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be
taken into, and then released from, fat depots over a period of
many days. It is therefore quite incorrect to abandon oxime
treatment after 1 - 2 days on the supposition that all inhibited
enzyme will be aged. Ecobichon et al. (1977) noted prompt
improvement in both condition and blood-ChEs in response to
pralidoxime given on the 11th - 15th days after major symptoms of
poisoning appeared due to extended exposure to fenitrothion (a
dimethyl phosphate with a short half-life for aging of inhibited
AChE).
(d) Dosage of atropine and oxime
The recommended doses above pertain to exposures, usual for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in
error, while drunk. Therapeutic dosages were progressively
increased up to 6 mg atropine iv every 15 min together with
continuous iv infusion of pralidoxime chloride at 0.5 g/h for 72 h,
from days 3 to 6 after intoxication. After considerable
improvement, the patient relapsed and further aggressive therapy
was given at a declining rate from days 10 to 16 (atropine) and to
day 23 (oxime), respectively. In total, 92 g of pralidoxime
chloride and 3912 mg of atropine were given and the patient was
discharged on the thirty-third day with no apparent sequelae.
REFERENCES
ABOU-DONIA, M.B., OTHMAN, M.A., TANTAWAY, G., ZAKI KHALIL, A.,
& SHAWER, M.F. (1974) Neurotoxic effect of leptophos.
Experientia (Basel), 30 : 63-64.
ABOU-DONIA, M.B., GRAHAM, D.G., & KOMEIL, A.A. (1979)
Delayed neurotoxicity of O -ethyl O -(2,4-dichlorophenyl)phenyl-
phosphonothioate: effects of a single oral dose on hens.
Toxicol. appl. Pharmacol., 49 : 293-303.
ABOU-DONIA, M.B., GRAHAM, D.G., TIMMONS, P.R., & REICHERT,
B.L. (1980) Late acute, delayed neurotoxic, and cholinergic
effects of S,S,S -tributyl phosphorotrithioate on the hen:
effect of route of administration. Neurotoxicology, 1 : 425-447.
ABOU-DONIA, M.B., MAKKAWY, H-A., & GRAHAM, D.G. (1982)
Coumaphos: delayed neurotoxic effect following dermal
administration in hens. J. Toxicol. environ. Health, 10 : 87-99.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and
structure activity relationships providing a high safety
factor for anti-cholinesterase insecticides. In: Proceedings
of the British Crop Protection Conference, Brighton, November
1977, Croydon, British Crop Protection Council, pp. 721-729.
ALDRDIGE, W.N. & NEMERY, B. (1984) Toxicology of
trialkylphosphorothioates with particular reference to lung
toxicity. Fund. appl. Toxicol., 4 : 5215-5223.
ALDRIDGE, W.N., MILES, J.W., MOUNT, D.L., & VERSCHOYLE, R.D.
(1979) The toxicological properties of impurities in
malathion. Arch. Toxicol., 42 : 95-106.
ALDRIDGE, W.N., DINSDALE, D., NEMERY, B., & VERSCHOYLE, R.D.
(1985) Some aspects of the toxicology of trimethyl and
triethyl phosphorothioates. Fund. appl. Toxicol., 5 : 47-60.
AMBRUS, A. & GREENHALGH, R., ed. (1984) Pesticide residue
analysis. Proceedings of a joint FAO/WHO course, Eger,
Hungary, 13-26 April, 1983, Copenhagen, World Health
Organization (Health Aspects of Chemical Safety, Interim
Document No. 14).
BAKER, E.L., Jr, ZACK, M., MILES, J.W., ALDERMAN, L.,
MCWILSON, W., DOBBIN, R.D., MILLER, S., & TEETERS, W.R.
(1978) Epidemiologic malathion poisoning in Pakistan malaria
workers. Lancet, 7 January : 31.
BARNES, J.M. (1975) Assessing hazard from prolonged and
repeated exposure to low doses of toxic substances. Br. med.
Bull., 31 : 196-200.
BARNES, J.M. & DENZ, F.A. (1951) The chronic toxicity of
p- nitrophenyl diethyl thiophosphate (E. 605): a long-term
feeding experiment with rats. J. Hyg., 49 : 430-441.
BARNES, J.M. & DENZ, F.A. (1954) The reaction of rats to
diets containing octamethyl pyrophosphoramide (schradan) and
O,O- diethyl S- ethylmercaptoethanol thiophosphate ("systox").
Br. J. ind. Med., 11 : 11-19.
BAYER (1971) Nemacur, Leverkusen, Bayer Chemical Company
(Technical Information Leaflet No. E.1-781/26734).
BECKER, E.L. & BARBARO, J.F. (1964) The enzymatic hydrolysis
of p- nitrophenyl ethylphosphonates by mammalian plasmas.
Biochem. Pharmacol., 13 : 1219-1227.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating
properties of organophosphates. Xenobiotica, 2 : 307-337.
BENKE, G.M. & MURPHY, S.D. (1975) The influence of age on
the toxicity and metabolism of methyl parathion and parathion
in male and female rats. Toxicol. appl. Pharmacol., 31 :
254-269.
BERTOCIN, D., RUSSOLO, A., CAROLDI, S., & LOTTI, M. (1985)
Neurotoxic esterase in human lympocytes. Arch. environ.
Health, 40 : 139-144..
BERTRAM, U., KASTEN, A., LULLMANN, H., & ZIEGLER, A. (1977)
Improved treatment of organophosphate intoxication by use of
scopolamine or dexetimide. Experientia (Basel), 33 : 1196-1197.
BICKFORD, A.A. & SPRAGUE, G.L. (1984) The significance of
background neurologic lesions in acute delayed neurotoxicity
studies: a comparison of neurohistopathologic lesions induced
in commercial hens by tri- O -tolyl phosphate (TOCP) with those
observed in negative control hens. In: Cranmer, J.M. & Hixson,
J.E., ed. Delayed neurotoxicity. Proceedings of the Delayed
Neurotoxicity Workshop, Urbana-Champaign, Illinois, 27-30
June, 1982, Little Rock, Intox Press.
BIDSTRUP, P.L., BONNEL, J.A., & BECKETT, A.G. (1953)
Paralysis following poisoning by a new organic phosphorus
insecticide (mipafox). Br. med. J., 1 : 1068-1072.
BIGNAMI, G. (1976) Behavioural pharmacology and toxicology.
Ann. Rev. Pharm. Toxicol., 16 : 329-366.
BIGNAMI, G., ROSIC, H., MICHALEK, H., MILOSEVIC, M., & GATTI,
G.L. (1975) Behavioural toxicity of anticholinesterase
agents. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Publishing Company, pp. 155-215.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The
distribution of dichlorvos in the tissues of mammals after its
inhalation or intravenous administration. Toxicol. appl.
Pharmacol., 31 : 243-253.
BLEAKLEY, P., NICHOL, A.W., & COLLINS, A.G. (1979) Diazinon
and porphyria cutanea tarda. Med. J. Aust., 1 : 314-315.
BOPP, C. & KOSMINSKY, B. (1975) [Hepato-dermal toxic
prophyria.] Med. Cut. I.L.N., 4 : 271-280 (in Portugese).
BOULDIN, T.W. & CAVANAGH, J.B. (1979) Organophosphorus
neuropathy. A teased-fiber study of the spatio-temporal spread
of axonal degeneration. Am. J. Pathol., 94 (2): 241-252.
BOYD, E.M. (1969) Dietary protein and pesticide toxicity in
male weaning rats. WHO Bull., 40 : 801-805.
BRADLEY, W.A. (1976) The pathology of delayed neurotoxicity
due to organophosphates. In: Baron, R.L., ed. Pesticide-
induced delayed neurotoxicity, Washington, US Environmental
Protection Agency, pp. 84-101 (EPA No. 600/1-76-025).
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison
of cholinesterase activity, residue levels, and urinary
metabolite excretion of rats exposed to organophosphorus
pesticides. J. agric. food Chem., 25 : 1353-1358.
BREALEY, C., WALKER, C.H., & BALDWIN, B.C. (1980) A-esterase
activities in relation to the differential toxicity of
pirimiphos-methyl to birds and mammals. Pestic. Sci., 11 :
546-554.
BRIMBLECOMBE, R.W., GREEN, D.M., STRATTON, J.A., & THOMPSON,
P.B.J. (1970) The protective actions of some anticholinergic
drugs in sarin poisoning. Br. J. Pharmacol., 39 : 822-830.
BRITISH AGROCHEMICAL ASSOCIATION (1983) Spray operator
safety study, London, British Agrochemical Association, pp.
1-9.
BUCHET, J.P., LAUWERYS, R., & ROELS, H. (1977) Long-term
exposure to organophosphorus pesticides and lipid metabolism
in the rat. Bull. environ. Contam. Toxicol., 17 : 175-183.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused
by a single massive dose of dichlorvos to adult hens. Toxicol.
Lett., 9 : 157-159.
CASIDA, J.E. & SANDERSON, D.M. (1963) Reaction of certain
phosphorothionate insecticides with alcohols and potentiation
by breakdown products. J. agric. food Chem., 11 : 91-96.
CAVANAGH, J.B. (1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system: an experimental study in
hens. J. Neurol. Neurosurg. Psychiatr., 17 : 163-172.
CEC (1977) Organophosphorus pesticides. Criteria
(dose/effect relationships) for organophosphorus pesticides,
Oxford, New York, Pergamon Press, 199 pp (Report of a Working
Group of Experts prepared for the Commission of the European
Communities, Directorate-General for Social Affairs, Health
and Safety Directorate).
CHEN, P.R.S. & DAUTERMAN, W.C. (1971) Studies on the
toxicity of dimethoate analogues and their hydrolysis by sheep
liver amidase. Pestic. Biochem. Physiol., 1 : 340-348.
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN,
R.J. (1980) Effects of low-level administration of
dichlorvos on adrenocorticotrophic hormone secretion, adrenal
cholesteryl ester, and steroid metabolism. Biochem.
Pharmacol., 29 : 635-641.
CLOTHIER, B., JOHNSON, M.K., & REINER, E. (1981) Interaction
of some trialkyl phosphorothiolates with acetylcholinesterase:
characterization of inhibition, aging, and reactivation.
Biochem. Biophys. Acta, 660 : 306-316.
CODEX ALIMENTARIUS COMMISSION (1984) Codex Alimentarius
Commission guide to Codex recommendations concerning pesticide
residues. VIII. Recommendations for methods of analysis of
pesticide residues, Rome, FAO/WHO (CAC/PR 8-1984).
COLEMAN, I.W., LITTLE, P.E., & BANNARD, R.A.B. (1962)
Cholinolytics in the treatment of anticholinesterase
poisoning. I. The effectiveness of certain cholinolytics in
combination with an oxime for treatment of sarin poisoning.
Can. J. Biochem. Physiol., 40 : 815-826.
COSTA, L.G., SCHWAB, B.W., & MURPHY, S.D. (1982) Tolerance
to anticholinesterase compounds in mammals. Toxicology, 25 :
79-97.
CRANMER, J.M. & HIXSON, J.E., ed. (1984) Delayed
neurotoxicity. Proceedings of the Delayed Neurotoxicity
Workshop, Urbana-Champaign, Illinois, 27-30 June, 1982, Little
Rock, Intox Press.
CURTES, J.P., DEVELAY, P., & HUBERT, J.P. (1979) Late
peripheral neuropathy due to an acute voluntary intoxication
by organo-phosporic compounds. In: International Congress on
Neurotoxicology, Varese, Italy, 27-30 September, 1979, Oxford,
Pergamon Press.
DANDLIKER, W.B., HICKS, A.N., LEVINSON, S.A., STEWART, K., &
BRAWN, R.J. (1979) Effects of pesticides on the immune
response, La Jolla, California, Scripps Clinic and Research
Foundation (EPA 600/1-79-039).
DAUGHTON, C.G., COOK, A.M., & ALEXANDER, M. (1979) Bacterial
and conversion of alkylphosphonates to natural products via
carbon-phosphorus bond cleavage. J. agric. food Chem., 27 :
1375-1382.
DAUTERMAN, W.C. (1971) Biological and nonbiological
modification of organophosphorus compounds. WHO Bull., 44 :
133-150.
DAVIES, J.E., BARQUET, A., FREED, V.H., HAGUE, R., MORGADE,
C., SONNEBORN, R.E., & VACLAVEK, C. (1975) Human pesticide
poisonings by a fat-soluble organophosphate insecticide. Arch.
environ. Health, 30 : 608-613.
DAVIS, C.S. & RICHARDSON, R.J. (1980) In: Spencer, P.S. &
Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 527-544.
DE JAGER, A.E.J., VAN WEERDON, T.W., HOUTOFF, H.J., & DE
MONCHY, J.G.R. (1981) Polyneuropathy after massive exposure
to parathion. Neurology, 31 : 603-605.
DE JAGER, A.E.J., VAN WEERDON, T.W., & MONCHY, J.G.R. (1982)
Reply to letter. Neurology, 32 : 218.
DESI, I. (1983) Neurotoxicological investigation of
pesticides in animal experiments. Neurobehav. Toxicol.
Teratol., 5 : 503-515.
DESI, I., FARKAS, I., SIMON, G., & CZIELESZKY, V. (1971)
[Investigation of the neurotoxic action of the phosphorus
compound bromophos.] Int. Arch. Arbeitsmed., 28 : 203-222 (in
German).
DESI, I., VARGA, L., & FARKAS, I. (1978) Studies on the
immunosuppressive effect of organochlorine and organo-
phosphoric pesticides in subacute experiments. J. Hyg.
Epidemiol. Microbiol. Immunol., 22 : 115-122.
DETTBARN, W.D. (1984) Pesticide-induced muscle necrosis:
mechanisms and prevention. Fund. appl. Toxicol., 4 : 18-26.
DHSS (1983) Pesticide poisoning. Notes for the guidance of
medical practitioners, London, Department of Health and Social
Security, pp. 41-47.
DIGGORY, H.J.P., LANDRIGEN, P.J., LATIMER, K.P., ELLINGTON,
A.C., KIMBROUGH, R.D., LIDDLE, J.D., CLINE, R.E., & SMERK,
A.L. (1977) Fatal parathion poisoning caused by contamination
of flour in international commerce. Am. J. Epidemiol.,
106 : 145-153.
DIMOV, G. & KALOYANOVA, F. (1967) Carbohydrate metabolism
disorders in the liver and muscles in acute parathion
poisonings. C R Acad. Bulg. Sci., 20 : 1007-1009.
DINSDALE, D., VERSCHOYLE, R.D., & CABRAL, J.R.P. (1982)
Cellular responses to trialkylphosphorothioate-induced injury
in rat lung. Arch. Toxicol., 51 : 79-89.
DONNINGER, C. (1971) Species specificity of phosphate
triester anticholinesterases. WHO Bull., 44 : 265-268.
DOROUGH, H.W. (1976) Biological activity of pesticide
conjugate. In: Kaufman, D.D., ed. Bound and conjugated
pesticide residues, Washington DC, American Chemical Society,
pp. 11-34 (ACS Symposium Series No. 29).
DREVENKAR, V., FINK, K., STIPCEVIC, M., & TOKALCEVIC, B.
(1976) The fate of pesticides in aquatic environment. II.
Hydrolysis of dichlorvos in model system and in river water.
Arh. hig. rada, 27 : 297-305.
DREVENKAR, V., STIPCEVIC, M., STENGL, B., & STEFANAC, Z.
(1979) Gas chromatographic determination of alkali metal
O,O- diethylphosphorodithioate present in tract amounts.
Microchim. Acta, 1 : 385-394.
DUBOIS, K.P. (1963) Toxicological evaluation of the anti-
cholinesterase agents. In: Koelle, G.B., ed. Handbook on
experimental pharmacology, Berlin, Springer-Verlag, Vol. 15,
pp. 833-857.
DUDEK, B.R., BARTH, M., GEPHART, J., HUGGINS, J., & RICHARD-
SON, R.J. (1979) Correlation of brain and lymphocyte neuro-
toxic esterase inhibition in the adult hen following dosing
with neurotoxic compounds. Toxicol. appl. Pharmacol., 48 : A198.
DUFFY, F.H. & BURCHFIELD, J.L. (1980) Long-term effects of
the organophosphate sarin on EEGs in monkeys and humans.
Neurotoxicology, 1 : 667-689.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the
exposure of workers to pesticides. WHO Bull., 26 : 75-91.
DURHAM, W.F., WOLFE, H.R., & ELLIOTT, J.W. (1972) Absorption
and excretion of parathion by spraymen. Arch. environ. Health,
24 : 381-387.
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S.
(1977) Acute fenitrothion poisoning. Can. Med. Assoc. J.,
116 : 377-379.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7 : 88-95.
EL-SEBAE, A.H. (1980) Biochemical challenges in future
toxicological research. J. environ. Sci. Health, B15 : 689-721.
EL-SEBAE, A.H., SOLIMAN, S.A., AHMED, N.S., & CURLEY, A.
(1981) Biochemical interaction of six op delayed
neurotoxicants with several neurotargets. J. environ. Sci.
Health, B16 (4): 465-474.
ENAN, E.E., EL-SEBAE, A.H., ENAN, O.H., & EL-FIKI, S. (1982)
In vivo interaction of some organophosphorus insecticides with
different biochemical targets in white rats. J. environ. Sci.
Health, B17 : 549-570.
ETO, M. (1974) Organophosphorus pesticides: organic and
biological chemistry, Boca Raton, Florida, CRC Press.
ETO, M. & OHKAWA, H. (1970) Alkylation reaction of
organophosphorus pesticides: its chemical and biochemical
significance. In: Biochemical toxicology of insecticides, New
York, London, Academic Press, pp. 93-104.
FAO (1984) Production yearbook, Rome, Food and Agriculture
Organization of the United Nations, Vol. 38.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23 (1964)).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report, No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report, No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues (FAO Agricultural Studies No.
73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party and the WHO Expert
Committee, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1971/M/9/1;
WHO Pesticide Residues Series No. 1).
FAO/WHO (1973a) Pesticide residues in food. Report of the
1972 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 90; WHO Technical Report Series No. 525).
FAO/WHO (1973b) 1972 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1972/M/9/1;
WHO Pesticide Residues Series No. 2).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection
Paper 10 Rev).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide Residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide Residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide Residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide Residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986) Pesticide Residues in food. Report of the
1985 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 68).
FAO/WHO (in press) 1985 Evaluations of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper).
FARM CHEMICALS HANDBOOK (1985) Willoughby, Ohio, Meister
Publishing Company.
FENICHEL, G.M., KIBLER, W.B., OLSON, B.A., & DETTBARN, W.D.
(1972) Chronic inhibition of cholinesterase as a cause of
myopathy. Neurology, 22 : 1026-1033.
FISHER, J.R. (1977) Guillain-Barré syndrome following
organophosphate poisoning. J. Am. Med. Assoc., 238 : 1950-1951.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14 : 514-534.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health
hazards on use of dichlorvos (DDVP), especially in resin
strips. Res. Rev., 44 : 115-157.
GRAY, A.P. (1984) Design and structure-activity relationships
of antidotes to organophosphorus anticholinesterase agents.
Drug. Metab. Rev., 15 (3): 557-589.
GREEN, D.M., MUIR, A.W., STRATTON, J.A., & INCH, T.D. (1977)
Dual mechanism of the antidotal action of atropine-like drugs
in poisoning by organophosphorus anticholinesterase. J. Pharm.
Pharmacol., 29 . 62-64.
GREENHALGH, R., DHAWAN, K.L., & WEINBERGER, P. (1980)
Hydrolysis of fenitrothion in model and natural aquatic
systems. J. agric. food Chem., 28 : 102-105.
GROB, D. (1963) Anticholinesterase intoxication in man and
its treatment. In: Koelle, G.B., ed. Handbook of experimental
pharmacology, Berlin, Springer-Verlag, Vol. 15, pp. 989-1027.
GUNTHER, F.A., WESTLAKE, W.E., BARKELY, J.H., WINTERLIN, W., &
LANGBEHN, L. (1973) Establishing dislodgeable pesticide
residues on leaf surfaces. Bull. environ. Contam. Toxicol., 9 :
243-250.
GUNTHER, F.A., WESTLAKE, W.E., & BARKELY, J.H. (1974) Worker
environment research. II. Sampling and processing techniques
for determining dislodgeable pesticide residues on leaf
surfaces. Bull. environ. Contam. Toxicol., 12 : 641-645.
HANSEN, L.G. & HANSEN, T.K. (1985) Intravenous dosing
abolishes differences in delayed neurotoxicity potency between
leptophos and desbromoleptophos. Pestic. Biochem. Physiol.,
24 : 136.
HAYES, G.R., FUNCKES, A.J., & HARTWELL, W.V. (1964) Dermal
exposure of human volunteers to parathion. Arch. environ.
Health, 8 : 829-833.
HAYES, W.J., Jr (1971) Studies on exposure during the use of
anticholinesterase pesticides. WHO Bull., 44 : 277-288.
HAYES, W.J., Jr (1975) Diagnosis and treatment of poisoning.
In: Toxicology of pesticides, Baltimore, Maryland, Williams
and Wilkins Company, pp. 409-417.
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, 561 pp.
HEALY, J.K. (1959) Ascending paralysis following malathion
intoxication: a case report. Med. J. Aust., 46 : 765-767.
HEATH, D.F. & VANDEKAR, M. (1957) Some spontaneous reactions
of O,O- dimethyl S- ethylthioethyl phosphorothiolate and related
compounds in water and on storage, and their effects on the
toxicological properties of the compounds. Biochem. J., 67 :
187-202.
HIERONS, R. & JOHNSON, M.K. (1978) Clinical and
toxicological investigations of a case of delayed neuropathy
in man after acute poisoning by an organophosphorus pesticide.
Arch. Toxicol., 40 : 279-284.
HOLLINGSHAUS, J.G., ABU-EL-HAJ, S., & FUKUTO, T.R. (1979)
Delayed neurotoxicity of O -alkyl O -aryl phenylphosphonothioate
analogues related to leptophos administered orally to the hen.
J. agric. food Chem., 27 : 1197-1201.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus
cholinesterase inhibitors. Pharmacol. Rev., 11 : 567-688.
HUANG, X.S., SHU, W.A., ZU, W.C., LAI, M.T., TSIN, Z.A., &
KWOK, C.S. (1979) [Toxicity studies of the herbicide
Amiprophos.] Chekiang Univ. Sch. Med. Bull., 8 : 63-66 (in
Chinese).
HUFF, J.E., BATES, R., EUSTIS, S.L., HASEMAN, J.K., &
MCCONNELL, E.E. (1985) Malathion and malaoxon: histo-
pathology reexamination of the National Cancer Institute's
Carcinogenesis Studies. Environ. Res., 37 : 154-173.
HUTSON, D.H. & HATHWAY, D.E. (1967) Toxic effects of
chlorofenvinphos in dogs and rats. Biochem. Pharmacol., 16 :
949-962.
IARC (1979) Dichlorvos. In: Some halogenated hydrocarbons,
Lyons, International Agency for Research on Cancer, pp. 97-127
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 20).
IARC (1983) Miscellaneous chemicals, Lyons, International
Agency for Research on Cancer (Monographs on the Carcinogenic
Risk of Chemicals to Humans, Vol. 30).
IMAI, H. (1977) Experimental pigmentary degeneration of the
retina caused by an organophosphorus pesticide (fenthion).
Acta soc. ophthalmol., 81 : 925-932 (Abstract 245, Exerp. Med.
(1979), 9 : 53).
IMAMURA, T., GANDY, J., FUKUTO, T.R., & TALBOT, P. (1983) An
impurity of malathion alters the morphology of rat lung
bronchiolar epithelium. Toxicology, 26 : 73-79.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISHIKAWA, S. & MIYATA, M. (1980) Development of myopia
following chronic organophosphate pesticide intoxication: an
epidemiological and experimental study. In: Merigan, W.H. &
Weiss, B., ed. Neurotoxicity of the visual system, New York,
Raven Press, pp. 233-254.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorus pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application. Proceedings of the
Fifth International Workshop of the Scientific Committee on
Pesticides of the International Association on Occupational
Health, The Hague, The Netherlands, 9-11 October, 1979,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 169-172.
JAGER, K.W., ROBERTS, D.V., & WILSON, A. (1970) Neuro-
muscular function in pesticide workers. Br. J. ind. Med., 27 :
273-278.
JASH, N.B. & BHATTACHARYA, S. (1982) Neurotoxicity of
phenthoate to a non-target species. In: Proceedings of the 5th
International Congress on Pesticide Chemicals, Kyoto, 29
August, 1982 (Abstract No. V1a-15).
JEDRZEJOWSKA, H., ROWINSKA-MARCINSKA, K., & HOPPE, B. (1980)
Neuropathy due to phytosol (agritox): report of a case. Acta
neuropathol., 49 : 163-168.
JOHNSON, D.D. & WILCOX, W.C. (1975) Studies on the mechanism
of the protective and antidotal actions of diazepam in
organophosphate poisoning. Eur. J. Pharmacol., 34 : 127-132.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of the brain "neurotoxic esterase" and the development of
delayed neurotoxicity in hens. Biochem. J., 120 : 523-531.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. Crit. Rev.
Toxicol., 3 : 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing
delayed neurotoxic effects: mechanism of action and structure/
activity studies. Arch. Toxicol., 34 : 259-288.
JOHNSON, M.K. (1977) Improved assay of neurotoxic esterase
for screening organophosphates for delayed neurotoxicity
potential. Arch. Toxicol., 37 : 113-115.
JOHNSON, M.K. (1980) Delayed neurotoxicity induced by
organophosphorus compounds: areas of understanding and
ignorance. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard
evaluation, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 27-38.
JOHNSON, M.K. (1981a) Do trichlorphon and/or dichlorvos
cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol. Scand., 49 (Suppl. 5): 87-98.
JOHNSON, M.K. (1981b) Grading of non-neurotoxicity of
organophosphorus pesticides and plasticisers by in vivo/in
vitro assay of neurotoxic esterase. In: Proceedings of the
European Society of Toxicologists, Dublin, 17-19 August
(Abstract No. 66).
JOHNSON, M.K. (1982a) The target for initiation of delayed
neurotoxicity by organophosphorus esters: biochemical studies
and toxicological applications. In: Hodgson, E., Bend, J.R., &
Phillip, R.M., ed. Reviews in biochemistry and toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 4, pp. 141-212.
JOHNSON, M.K. (1982b) Check your paraoxon and parathion for
neurotoxic impurities. Vet. hum. Toxicol., 24 (3): 220a.
JOHNSON, M.K. (1984) Delayed neurotoxicity tests of
organophosphorus esters: a proposed protocol integrating
neuropathy target esterase (NTE) assays with behaviour and
histopathology tests to obtain more information more quickly
from fewer animals. In: El-Sebae, A.H., ed. Proceedings of the
International Conference on Environmental Hazards of
Agrochemicals in Developing Countries, Alexandria, Egypt, 8-12
November, 1983, Alexandria, University of Alexandria, Vol. 1,
pp. 474-493.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus
pesticides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269
(in Russian).
KAGAN, JU.S. (1985) [Principles of toxicology,] Moscow,
United Nations Environment Programme (in Russian).
KAHN, E. (1979) Outline guide for performance of field
studies to establish safe reentry intervals for
organophosphorus pesticides. Residues Rev., 70 : 27-43.
KALOYANOVA-SIMEONOVA, F. (1961) Effet du chlorthion sur les
réflexes moteurs conditionnés des rats albinos. Act. nerv.
super., 3 : 284-288.
KALOYANOVA, F. (1979) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Conference on
Environmental Sensing and Assessment, Las Vegas, Nevada,
14-19 September, 1975, Vol. 1.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J.,
ed. IUPAC pesticide chemistry - Human welfare and the
environment, Oxford, New York, Pergamon Press, pp. 237-238.
KALOYANOVA, F. & TASHEVA, M. (1983) Effect of protein
malnutrition on toxicity of pesticides. In: Miyamoto, J. &
Kearney, P.C., ed. IUPAC pesticide chemistry, human welfare,
and the environment, Oxford, New York, Pergamon Press,
pp. 527-529.
KALOYANOVA, F., BENCHEV, I., GHEORGHIEV, G., IZMIROVA, N., &
RISOV, N. (1979) Pesticides and persistent substances.
Biological specimen collection. In: Berlin, A., Wolff, A.H., &
Hosegawa, J., ed. Biological specimens used for the assessment
of human exposure to environmental pollutants, Sophia,
Martinus N. Publishers, pp. 231-242.
KARCZMAR, A.G. (1984) Acute and long-lasting actions of
organophosphorus agents. Fundam. appl. Toxicol., 4 : S1-S7.
KARCZMAR, A.G. & OHTA, Y. (1981) Neuromyopharmacology as
related to anticholinesterase action. Fundam. appl. Toxicol.,
1 : 135-142.
KATO, T., NAKANO, T., KOJIMA, K., NAGATSU, T., & SAKAKIBARA,
S. (1980) Changes in propyl endopeptidase during maturation
of the rat brain and hydrolysis of substance P by the purified
enzyme, J. Neurochem., 35 : 527-535.
KAUFMANN, D.D., ed. (1976) Bound and conjugated pesticide
residues, Washington DC, American Chemical Society, pp. 1-10
(ACS Symposium Series No. 29).
KEPLINGER, M.L. & DEICHMANN, W.B. (1967) Acute toxicity of
combinations of pesticides. Toxicol. appl. Pharmacol., 10 :
586-595.
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic
phosphorus compounds and alkylating agents on the rat fetus.
Arch. environ. Health, 16 : 805-808.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides: Techniques for establishing safe levels of foliar
residues. Residues Rev., 75 : 81-96.
KNOX, B., ASKAA, J., BASSE, A., BITSCH, V., ESKILDSEN, M.,
MANDRUP, M., OTTOSEN, H.E., OVERBY, E., PEDERSEN, K.B., &
RASMUSSEN, F. (1978) Congenital ataxia and tremor with
cerebellar hypoplasia in piglets born by sows treated with
NeguvonR vet (metrifonate, trichlorfon) during pregnancy.
Nord. Vet. Med., 30 : 538-545.
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the
seminiferous epithelium and the Leydig cells of the rat testis
after the application of dichlorvos. Bull. environ. Contam.
Toxicol., 11 (5): 429-433.
LAUWERYS, R.L. & MURPHY, S.D. (1969) Interaction between
paraoxon and tri- o- tolyl phosphate in rats. Toxicol. appl.
Pharmacol., 14 : 348-357.
LE QUESNE, P.M. & MAXWELL, I.C. (1981) Effect of metrifonate
on neuromuscular transmission. Acta pharmacol. toxicol.
Scand., 49 (Suppl. 5): 99-104.
LEVIN, H.S. & RODNITZKY, R.L. (1976) Behavioural effects of
organophosphate pesticides in man. Clin. Toxicol., 9 (3):
391-405.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating
properties of 2,2-dichlorovinyl dimethyl phosphate: a
disregarded hazard. Environ. Mutat. Soc. Newslett., 2 : 21-27.
LOTTI, M. & BECKER, C.E. (1982a) Treatment of acute
organophosphate poisoning: evidence of a direct effect on
central nervous system by 2-PAM (pyridine-2-aldoxime methyl
chloride). J. Toxicol. clin. Toxicol., 19 : 121-127.
LOTTI, M. & BECKER, C.E. (1982b) Letter: polyneuropathy and
exposure to parathion. Neurology, 32 : 317.
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of
organophosphorus pesticides: predictions can be based on in
vitro studies with hen and human enzymes. Arch. Toxicol., 41 :
215-221.
LOTTI, M. & JOHNSON, M.K. (1980) Repeated small doses of
neurotoxic organophosphate: monitoring of neurotoxic esterase
in brain and spinal cord. Arch. Toxicol., 45 : 263-271.
LOTTI, M. & MORRETTO, A. (1986) Inhibition of lymphocyte
neuropathy target esterase predicts the development of
organophosphate polyneuropathy in man. Hum. Toxicol., 5 : 114.
LOTTI, M., FERRARA, S.D., CAROLDI, S., & SINIGAGLIA, F.
(1981) Enzyme studies with human hen autopsy tissue suggest
omethoate does not cause delayed neuropathy in man. Arch.
Toxicol., 48 : 265-270.
LOTTI, M., BECKER, C.E., AMINOFF, M.J., WOODROW, J.E., SEIBER,
J.N., TALCOTT, R.E., & RICHARDSON, R.J. (1983) Occupational
exposure to the cotton defoliants DEF and MERPHOS. J. occup.
Med., 25 : 517-522.
LOTTI, M., BECKER, C.E., & AMINOFF, M.J. (1984) Organo-
phosphate polyneuropathy: pathogenesis and prevention.
Neurology, 34 : 658-662.
LOTTI, M., BERTONCIN, D., & MORETTO, A. (1986) Organo-
phosphate induced delayed polyneuropathy (OPIDP) by chlor-
pyrifos in man and hens. Toxicologist, 6 : 22 (Abstract No. 86).
LUDKE, J.L. (1977) DDE increases the toxicity of parathion
to corturnix quail. Pestic. Biochem. Physiol., 1 : 28-33.
MACHIN, A.P., ANDERSON, P.H., HOWELLS, L.C., & DUNDY, D.E.
(1978) Enzymic degradation of phosphorothionate oxons in the
plasma of species of varying susceptibility to poisoning. In:
Proceedings of the 4th IUPAC International Congress on
Pesticides and Chemicals, Zurich, 24-28 July, 1978 (Abstract
V.620).
MALLIPUDI, N.M., UMETSU, N., TOIA, R.F., TALCOTT, R.E., &
FUKUTO, T.R. (1979) Toxicity of O,O,S -trimethyl and triethyl
phosphorothioate to the rat. J. agric. food Chem., 27 : 463-466.
MALONE, J.C. (1964) Toxicity of haloxon. Res. vet. Sci., 5 :
17-31.
MAQUIS, J.K. (1985) Non-cholinergic mechanisms of
insecticide toxicity. Trends pharmacol. Sci., 6 (2): 59-60.
MARGOT, A. & GYSIN, H. (1957) [Diazinon: its decomposition
product and their properties.] Helv. Chim. Acta, 40 (162):
1562-1573 (in German).
MARONI, M. & BLEEKER, M.L. (1986) Neuropathy target esterase
in human lymphocytes and platelets. J. appl. Toxicol., 6 : 1-7.
MARSHALL, T.C. & DOROUGH, H.W (1977) Bioavailability in rats
of bound and conjugated plant carbamate insecticide residues.
J. agric. food Chem., 25 (5): 1003-1009.
MEDVED, L.I. & KAGAN, JU.S. (1983) Pesticides, organophos-
phorus. In: Parmeggiani, L., ed. Encyclopaedia of occupational
health and safety, 3rd ed., Geneva, International Labour
Office, pp. 1637-1646.
MEIER, E.P., DENNIS, W.H., ROSENCRANCE, A.B., RANDALL, W.F.,
COOPER, W.J., & WARNER, M.C. (1979) Sulfotepp: a toxic
impurity in formulations of diazinon. Bull. environ. Contam.
Toxicol., 23 : 158-164.
MEL'NIKOV, N.N., ET AL. (1985) [Pesticide handbook,] Moscow,
Himija Press (in Russian).
MENDOZA, C.E. (1976) Toxicity and effects of malathion on
esterases of suckling albino rats. Toxicol. appl. Pharmacol.,
35 : 229-238.
MILBY, T.H., OTTOBONI, F., & MITCHELL, H.W. (1964) Parathion
residue poisoning among orchard workers. J. Am. Med. Assoc.,
189 : 351-358.
MILES, J.W., MOUNT, D.L., STAIGER, M.A., & TEETERS, W.R.
(1979) S- methyl isomer content of stored malathion and
fenitrothion water-dispersible powders and its relationship to
toxicity. J. agric. food Chem., 27 : 421-425.
MIYAMOTO, J. (1976) Degradation, metabolism, and toxicity of
synthetic pyrethroids. Environ. Health Perspect., 14 : 15-28.
MIYAMOTO, J. & OHKAWA, H. (1978) Oxidative processes in
pesticide transformation. In: Proceedings of the 4th IUPAC
International Congress on Pesticides and Chemicals, Zurich,
24-28 July, 1978.
MURPHY, S.D. (1969) Mechanisms of pesticide interactions in
vertebrates. Res. Rev., 25 : 201-221.
NAKATSUGAWA, T., TOLMAN, N.M., & DHAM, P.A. (1968)
Degradation and activation of parathion analogues by
microsomal enzymes. Biochem. Pharmacol., 17 : 1517-1528.
NAMBA, T. (1974) Relative toxicity of malathion (contd). New
Engl. J. Med., 290 : 347.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisoning due to organophosphate insecticides: acute and
chronic manifestations. Am. J. Med., 50 : 475-492.
NICHOL, A.W., ELSBURY, S., ELDER, G.H., JACKSON, A.H., &
NAGARAJA RAO, K.R. (1982) Separation of impurities in
diazinon preparations and their effect on porthyrin
biosynthesis in tissue culture. Biochem. Pharmacol., 31 :
1033-1038.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22 : 16-18 (in
Japanese).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitaikagaku ecol. Chem., 4 : 31-46 (in Japanese).
O'BRIEN, R.C. (1967) Insecticides: action and metabolism,
New York, Academic Press, pp. 32-54.
OECD (1983) Acute delayed neurotoxicity of organophosphorus
substances: subchronic delayed neurotoxicity of organo-
phosphorus substances: 90-day study, Paris, Organization of
Economic Cooperation and Development (OECD Guidelines for the
Testing of Chemicals, Nos. 418, 419).
OHKAWA, H., OSHITA, H., & MIYAMOTO, J. (1980) Comparison of
inhibitory activity of various organophosphorus compounds
against acetylcholinesterase and neurotoxic esterase of hens
with respect to delayed neurotoxicity. Biochem. Pharmacol.,
29 : 2721-2727.
O'NEIL, J.J. (1981) Non-cholinesterase effects of
anticholinesterases, Fundam. appl. Toxicol., 1 : 154-160.
OSTERLOH, J., LOTTI, M.D., & POND, S.M. (1983) Toxicological
studies in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos.
J. anal. Toxicol., 7 : 125-129.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78 : 78-87.
PELLEGRINI, G. & SANTI, R. (1972) Potentiation of toxicity
of organophosphorus compounds containing carboxylic ester
functions toward warm-blooded animals by some organophosphorus
impurities. J. agric. food Chem., 20 : 944-950.
PETRY, C.S. (1958) Organic phosphate insecticide poisoning.
Am. J. Med., 24 : 467-470.
PETRY, H. (1951) [Polyneuritis from E 605.] Zentralbl.
Arbeitsmed. Arbeitssch., 1 : 86-89 (in German).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PRENTICE, D.E. & ROBERTS, N.L. (1984) Acute delayed
neurotoxicity in hens dosed with tri-ortho-cresyl phosphate
(TOCP): correlation between clinical ataxia and neuropatho-
logical findings. In: Cranmer, J.M. & Hixson, J.E., ed.,
Delayed neurotoxicity. Proceedings of the Delayed Neuro-
toxicity Workshop, Urbana-Champaign, Illinois, 27-30 June,
1982, Little Rock, Intox Press.
PREUSSMANN, R., SCHNEIDER, H., & EPPLE, F. (1969)
[Investigation of effects of alkylating agents. II. The
investigation of different classes of alkylating agents by
means of a modified colour reaction with 4-(4-nitro-benzyl)
pyridine (NBP).] Arzneimittelforschung, 7 : 1059-1073 (in
German).
RAY, D.E. (1980) Selective inhibition of thermogenesis by
tributyl S,S,S -phosphorotrithioate (DEF). Br. J. Pharmacol.,
69 : 257-264.
RAY, D.E. & CUNNINGHAM, V.J. (1985) Hypothermia produced by
S,S,S -phosphortrithioate (DEF). Arch. Toxicol., 56 : 279-282.
REITER, L.W., TALENS, G.M., & WOOLEY, D.E. (1975) Parathion
administration in the monkey: time course of inhibition and
recovery of blood cholinesterases and visual discrimination
performance. Toxicol. appl. Pharmacol., 33 : 1-13.
REUBER, M.D. (1985) Carcinogenicity and toxicity of
malathion and malaoxon. Environ. Res., 37 : 119-153.
REVZIN, A.M. (1980) Effects of organophosphate pesticides
and alcohol on visual mechanisms. In: Merigan, W.H. & Weiss,
B., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 255-268.
REVZIN, A.M. (1983) Neurophysiological and behavioural
assessment of pesticide toxicity. In: Miyamoto, J. & Kearney,
P.C., ed. Pesticide chemistry: human welfare and the
environment, Oxford, New York, Pergamon Press, Vol. 3, pp.
419-424.
RICHARDSON, R.J. & DUDEK, B.R. (1983) Neurotoxic esterase:
characterization and potential for a protective screen for
exposure to neuropathic organophosphates. In: Miyamoto, J.,
ed. Proceedings of the IUPAC Pesticide Chemistry Congress on
Human Welfare and the Environment, Oxford, New York, Pergamon
Press, pp. 491-495.
RICO, A.G. & BURGAT-SACAZE, V. (1984) Toxicological
significance of covalently-bound residues. Food Add. Contam.,
1 (2): 157-161.
ROBERTS, D.V. (1976) EMG voltage and motor nerve conduction
velocity in organophosphorus pesticide factory workers. Int.
Arch. occup. environ. Health, 36 : 267-274.
SALPETER, M.M., KASPRZAK, H., FENG, H., & FERTUCK, H. (1979)
Endplates after esterase inactivation in vivo: correlation
between esterase concentration, functional response, and fine
structure. J. Neurocytol., 8 : 95-115.
SANBORN, J.R., METCALF, R.L., & HANSEN, L.G. (1977) The
neurotoxicity of O -2(2,5-dichlorophenyl) O -methyl phenylphos-
phonothioate, an impurity and photoproduct of leptophos
(PhosveR ) insecticide. Pestic. Biochem. Physiol., 7 : 142-145.
SCHAFER, E.W. (1972) The acute oral toxicity of 369
pesticidal, pharmaceutical and other chemicals to wild birds.
Toxicol. appl. Pharmacol., 21 : 315-330.
SCHOENE, J. & OLDIGES, H. (1973) [Antidotal action of
pyridinium salts for tabun and sarin poisoning in vivo and in
vitro. ] Arch. int. Pharmacodyn. Ther., 204 : 110-123 (in
German).
SEIFERT, J. & CASIDA, J.E. (1980) Mechanisms of terato-
genesis induced by organophosphorus and methylcarbamate
insecticides. In: Progress in pesticide biochemistry,
Berkeley, California, University of California.
SENANAYAKE, N. & JOHNSON, M.K. (1982) Acute polyneuropathy
following poisoning by a new organophosphate insecticide: a
preliminary report. New Eng. J. Med., 306 : 155-157.
SHAFIK, T., BRADWAY, D.E., ENOS, E.F., & YOBS, A.R. (1973)
Human exposure to organophosphorus pesticides. A modified
procedure for the gas-liquid chromatographic analysis of alkyl
phosphate metabolites in urine. J. agric. food Chem., 21 (4):
625.
SHIRAISHI, S., GOTO, I., YAMASHITA, Y., ONISHI, A., & NAGAO,
H. (1977) [Dipterex polyneuropathy.] Neurol. Med. (Japan),
6 : 34-38 (in Japanese with English summary).
SHTENBERG, A.I., KHOVAEVA, L.A., & ZAVARZINA, M.V. (1974)
Effect of chlorophos and methyl nitrophos on the immune
reactions of the organism against the background of
protein-deficient nutrition. Vopr. Pitan., 8 (4): 35-42.
SOLIMAN, S.A. (1982) Delayed neuropathy in hen by the
insecticide synergist O-n- propyl O- (2-propynyl)phenylphos-
phonate (NIA 16388) and other phenylphosphonate esters. J.
Toxicol. environ. Health, 10 : 907-920.
SOLIMAN, S.A., FARMER, J., & CURLEY, A. (1982) Is delayed
neuropathy a property of all organophosphorus compounds?
Toxicology, 23 : 267-279.
SOLIMAN, S.A., SOVOCOOL, G.W., CURLEY, A., AHMED, N.S.,
EL-FIKI, S., & EL-SEBAE, A.H. (1982) Two acute human
poisoning cases resulting from exposure to diazinon
transformation products in Egypt. Arch. environ. Health, 37 :
207-212.
SPEAR, R.C., POPENDORF, W.J., SPENCER, W.F., & MILBY, T.H.
(1977) Worker poisonings due to paraoxon residues. J. occup.
Med., 19 : 411-414.
STAPLES, R.E. & GOULDING, E.H. (1979) Dipterex
teratogenicity in the rat, hamster, and mouse when given by
gavage. Environ. Health Perspect., 30 : 105-113.
STEFANAC, Z., STENGL, B., & VASILIC, Z. (1976) Quantitative
determination of organophosphorus pesticides by thin-layer
densitometry. J. Chromatogr., 124 : 127-133.
SU, M.-Q., KINOSHITA, F.K., FRAWLEY, J.P., & DUBOIS, K.P.
(1971) Comparative inhibition of aliesterases and
cholinesterase in rats fed eighteen organophosphorus
insecticides. Toxicol. appl. Pharmacol., 20 : 241-249.
TALCOTT, R.E., DENK, H., & MALLIPUDI, N.M. (1979a) Malathion
carboxylesterase activity in human liver and its inactivation
by isomalathion. Toxicol. appl. Pharmacol., 49 : 373-376.
TALCOTT, R.E., MALLIPUDI, N.M., UMETSU, N., & FUKUTO, T.R.
(1979b) Inactivation of esterases by impurities isolated from
technical malathion. Toxicol. appl. Pharmacol., 49 : 107-112.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co.,
pp. 100-119.
UMETSU, N., GROSE, F.H., ALLAHYARI, R., ABU-EL-HAJ, S., &
FUKUTO, T.R. (1977) Effect of impurities on the mammalian
toxicity of technical malathion and acephate. J. agric. food
Chem., 25 : 946-953.
UNITED KINGDOM PSPS (1979) Guidance on toxicity data
requirements, London, MAFF, United Kingdom Pesticide Safety
Precautions Scheme, 3 pp (Working Document B5).
US EPA (1975) Registration, re-registration, and
classification procedures. Fed. Reg., 40 (129): 28276-28277.
US EPA (1982) Acute delayed neurotoxicity of organo-
phosphorus substances, Washington DC, US Environmental
Protection Agency, Section 81-7, pp. 62-65 (Pesticide
Assessment Guidelines, Subdivision F, Hazard Evaluation: human
and domestic animals) (EPA 540/9-82-025).
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning.
Guy's Hosp. Rep., 123 : 13-25.
VASIC, B.V., MILOSEVIC, M.P., & TERZIC, M.R. (1977) Acetyl-
choline content and cholinesterase activity in the ponto-
medullary region of brain in rats treated with armin and
obidoxime. Biochem. Pharmacol., 26 : 601-602.
VELSICOL (1977) Phosvel (technical leptophos) data for
regulatory authorities, Chicago, Illinois, Velsicol Chemical
Corporation.
VERGIEVA, T. (1983) Embryotoxicity and teratogenicity of
pesticides. In: Kaloyanova, F. & Tarkowski, S., ed. Toxicology
of pesticides, Copenhagen, World Health Organization, pp.
67-77 (Health Aspects of Chemical Safety, Interim Document
No. 9).
VERONESI, B. & PADILLA, S. (1985) Phenylmethylsulfonyl
fluoride protects rats from mipafox-induced delayed
neuropathy. Toxicol. appl. Pharmacol., 81 (2): 258-264.
VERSCHOYLE, R.D. & CABRAL, J.R.P. (1982) Investigation of
the acute toxicity of some trimethyl and triethyl
phosphorthioates with particular reference to those causing
lung damage. Arch. Toxicol., 51 : 221-231.
VERSCHOYLE, R.D., ALDRIDGE, W.N., & CABRAL, J.R.P. (1980)
Toxicology of trimethyl and triethyl phosphorothioates. In:
Holmstedt, B., Lauwerys, R., Mercier, M., & Roberfroid, M.,
ed. Mechanisms of toxicity and hazard evaluation, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 631-634.
VETTORAZZI, G. (1979) International regulatory aspects of
pesticide chemicals, Vol. 1 & 2, Boca Raton, Florida, CRC
Press, 232 and 214 pp.
VETTORAZZI, G. & VAN DEN HURK, G. (1984) Pesticides
reference index: JMPR-IARC-IPCS-IRPTC-VBC: 1961-1984, Geneva,
World Health Organization (Unpublished report).
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977)
Severe organophosphate poisoning complicated by alcohol and
terpentine ingestion. Arch. environ. Health, 32 : 203-205.
WHO (1975) Early detection of health impairment in
occupational exposure to health hazards, Geneva, World Health
Organization (Technical Report Series No. 571).
WHO (1979) Third report of the WHO Expert Committee on
vector biology and control, Geneva, World Health Organization,
44 pp (WHO Technical Report Series No. 634).
WHO (1982) Field surveys of exposure to pesticides -
standard protocol, Geneva, World Health Organization
(Unpublished report VBC/82.1).
WHO (1984a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-1985, Geneva,
World Health Organization (Unpublished report VBC/84.2).
WHO (1984b) Spectrophotometric kit for measuring
cholinesterase activity, Geneva, World Health Organization
(Unpublished report VBC/84.888).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World
Health Organization (VBC).
WICKER, G.W., WILLIAMS, W.A., & GUTHRIE, F.E. (1979)
Exposure of field workers to organophosphorus insecticides:
sweet corn and peaches. Arch. environ. Contam. Toxicol., 8 :
175-182.
WILKINSON, C.F. (1971) Effects of synergists on the
metabolism and toxicity of anticholinesterases. WHO Bull., 44 :
171-190.
WILLEMS, J.L. (1981) Poisoning by organophosphate
insecticides: analysis of 53 human cases with regard to
management and drug treatment. Acta med. milit. Belg., 134 :
7-14.
WILLS, J.H. (1963) [Pharmacological antagonists of the
anticholinesterase agents.] In: Koelle, G.B., ed. [Handbook of
experimental pharmacology,] Berlin, Springer-Verlag, Vol. 15,
pp. 883-920 (in German).
WILSON, B.W., HOOPER, M., CHOW, E., HIGGINS, R.J., & KNAAK,
J.B. (1984) Antidotes and neuropathic potential of
isofenphos. Bull. environ. Contam. Toxicol., 33 : 386-394.
WILTROUT, R.W., ERGEGOVICH, C.D., & CEGLOWSKI, W.S. (1978)
Humoral immunity in mice following oral administration of
selected pesticides. Arch. environ. Contam. Toxicol., 20 :
423-431.
WITTER, R.F. & GAINES, T.B. (1963) Relationship between
depression of the brain or plasma cholinesterase and paralysis
in chickens caused by certain organic phosphorus compounds.
Biochem. Pharmacol., 12 : 1377-1386.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1967) Exposure
of workers to pesticides. Arch. environ. Health, 14 : 622-633.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1970) Urinary
excretion of insecticide metabolites. Arch. environ. Health,
21 : 711-716.
WOLFE, H.R., STAIFF, D.C., & ARMSTRONG, J.F. (1978) Exposure
of pesticide formulating plant workers to parathion. Bull.
environ. Contam. Toxicol., 20 : 340-343.
WOLTHUIS, O.L. & VANWERSCH, R.A.P. (1984) Behavioural
changes in the rat after low doses of cholinesterase
inhibitors. Toxicol. appl. Pharmacol., 4 : S195-S208.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) In vivo
alkylation studies with dichlorvos at practical use
concentrations. Chem.-biol. Interact., 19 : 25-46.
WORTHING, C.R., ed. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council,
655 pp.
XINTARAS, C. & BURG, J.R. (1980) Screening and prevention of
human neurotoxic outbreaks: issues and problems. In: Spencer,
P.S. & Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams & Wilkins
Company, pp. 663-674.
XINTARAS, C., BURG, J.R., TANAKA, S., LEE, S.T., JOHNSON,
B.L., COTTRILL, C.A., & BENDER, J. (1978) Occupational
exposure to leptophos and other chemicals, Cincinnati, Ohio,
US Department of Health and Welfare, National Institue of
Occupational Safety and Health (US DHEW (NIOSH) Publication
No. 78-136).
YANG, R.S.H., HODGSON, E., & DAUTERMAN, W.C. (1971)
Metabolism in vitro of diazinon and diazoxon in rat liver. J.
agric. food Chem., 19 : 10-13.
YOSHIDA, K. & NISHIUCHI, Y. (1972) Toxicity of pesticides to
some water organisms.] Bull. agric. Chem. Inspect. Stn
(Japan), 12 : 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn
(Japan), 16 : 65-69 (in Japanese).
ZACKOV, K. (1983) Immunotoxicology of pesticides. In:
Kaloyanova, F. & Tarkowski, S., ed. Toxicology of pesticides,
Copenhagen, World Health Organization, pp. 75-88 (Health
Aspects of Chemical Safety, Interim Document No. 9).
ZIELHUIS, R.L. (1972) Epidemiological toxicology of
pesticide exposure: report of an international workshop. Arch.
environ. Health, 25 : 399-405.
Annex I. Names and structures of selected organophosphorus pesticides
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other formula molecular Registry
name mass number
-------------------------------------------------------------------------------------------
acephate Orthene phosphoramidothioic C4 H10 NO3 PS 183.18 30560-19-1
Ortran acid, acetyl-, O,S-
dimetyl ester
5. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
5.1 Aquatic Organisms
Organophosphorus insecticides are not very stable in aqueous
media. However, accidental leaching may occur from treated areas
into rivers and lakes where they may exert toxic effects on aquatic
organisms before degradation is complete. In clean water in the
laboratory, toxic effects were seen in several aquatic organisms
when they were exposed to concentrations of organophosphorus
insecticides ranging from 0.01 to 1 mg/litre for 48 h (Nishiuchi,
1981). However, lethal concentrations derived from 48-h exposures
in clear laboratory water may be artificially low compared with the
concentrations that would be effective in true environmental
waters.
One pesticide (phenthoate) appeared to be more toxic for
aquatic insects ("median tolerable limit" = 9 - 75 µg/kg)
(Nishiuchi, 1981) than for one species of fresh-water fish exposed
under apparently similar conditions (20% mortality caused by 263
µg/kg) (Jash & Bhattacharya, 1982).
Accidental release of pesticides in lakes, rivers, and bays
sometimes caused massive death of fish and many of the compounds
were strongly toxic for small aquatic organisms such as Daphnia, as
shown in Table 3.
Table 3. Acute toxicity of organophosphorus pesticides for some aquatic organisms
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Acephate > 40 - > 40 - > 40 Nishiuchi (1974)
Calvinphos > 40 - > 40 - 0.0042 Nishiuchi (1974)
Chlorfenvinphos 0.27 0.34 0.23 0.12 0.011 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Chlorpyrifos 0.13 0.20 0.47 0.74 0.0050 Yoshida &
emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Chlorpyrifos 2.1 - 3.4 - 0.017 Yoshida &
-methyl Nishiuchi (1976)
Cyanofenphos 1.2 1.3 6.3 15 0.0085 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Cyanophos 15 10 ~ 40 28 18 0.34 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Dialifos 1.3 - 0.80 - 0.027 Yoshida &
Nishiuchi (1976)
Dichlofenthion 5.1 10 ~ 40 1.4 10 0.005 Yoshida &
(emulsifiable (dust Nishiuchi (1972);
concentrate) formulation) Nishiuchi (1974)
Diazinon 3.2 5.1 5.3 4.1 0.08 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Dichlorvos > 40 10 ~ 40 18 - 2.8 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Dimethoate > 40 > 40 > 40 - 10 ~ 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Dimethylvinphos 5.6 - 1.5 - 0.010 Yoshida &
Nishiuchi (1976)
Dioxathion 10 ~ 40 10 ~ 40 1.4 - 0.007 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Disulfoton 8.7 10 ~ 40 21 0.37 0.07 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Edifenphos 2.5 1.8 1.8 - 0.27 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
EPN 0.20 0.32 0.50 0.085 0.0017 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Ethion 1.2 1.1 5.5 - 0.005 Yoshida &
Nishiuchi (1972)
Fenitrothion 8.2 3.4 7.0 0.75 0.050 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Fenthion 3.3 1.9 2.5 2.3 0.070 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Formothion 15 10 ~ 40 10 ~ 40 - 5.8 Yoshida &
Nishiuchi (1972)
IBP 10 ~ 40 12 7.2 - 2.3 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
Leptophos > 40 10 ~ 40 8.5 - 0.002 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Malathion 23 7.8 0.75 1.4 0.030 Yoshida &
Nishiuchi (1972)
Menazon > 40 > 40 > 40 100 10 ~ 40 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Methidathion 2.5 2.3 0.034 0.22 0.007 Yoshida &
(emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Naled 1.3 1.2 28 2.3 0.005 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972);
concentrate) concentrate) Nishiuchi (1974)
Parathion 4.5 1.7 2.9 - 0.0050 Yoshida &
Nishiuchi (1972)
Parathion-methyl 7.5 10 ~ 40 12 - 0.00050 Yoshida &
Nishiuchi (1972)
Phenkapton 2.0 3.8 3.5 - 0.008 Yoshida &
Nishiuchi (1972)
Phenthoate 2.5 2.4 0.17 0.20 0.008 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Phosalone 1.2 1.2 0.35 - 0.05 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Phosmet 5.3 4.7 1.8 1.0 0.025 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Pirimiphos-methyl 1.8 - 3.0 - 0.018 Yoshida &
Nishiuchi (1976)
Propaphos 4.8 - 4.1 - 0.0063 Yoshida &
Nishiuchi (1976)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Propoxur 10 ~ 40 10 ~ 40 10 ~ 40 - 0.37 Yoshida &
Nishiuchi (1972)
Prothiophos 9.5 - 10 - 0.13 Yoshida &
Nishiuchi (1976)
Temivinphos 0.58 - 0.48 - 0.0080 Yoshida &
Nishiuchi (1976)
TEPP 5.6 10 ~ 40 4.8 - 10 ~ 40 Yoshida &
(liquid (liquid (liquid (liquid Nishiuchi (1972)
formulation) formulation) formulation) formulation)
Tetrachlorvinphos 4.3 3.9 4.2 - 0.0035 Yoshida &
(wettable Nishiuchi (1972)
powder)
Thiometon 7.5 10 ~ 40 10 ~ 40 - 5.5 Yoshida &
Nishiuchi (1972)
Trichlorphon 28 10 ~ 40 25 12 0.005 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Vamidothion > 40 > 40 > 40 - > 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Note: Test methods are officially recognized methods based on the Notification of the Ministry of
Agriculture, Forestry and Fishery of Japan, as described in the separate papers.
6. EFFECTS ON ANIMALS
Insecticides are designed as lethal agents. Although they may
be designed to be less toxic for animals than for insects, all
organophosphorus insecticides present a toxic hazard to some
extent. Values for the oral and dermal LD50s in rat, shown for
different compounds in Annex III, range from less than 10 to more
than 3000 mg/kg body weight. The dose-response line for
organophosphorus insecticides is usually steeper than that for
carbamates, though both kill by their anticholinesterase action.
The reason for the difference lies in the faster rate of
spontaneous reactivation of carbamylated AChE compared with
phosphorylated AChE.
By the time the 1984 Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) had ended, the toxicology of 57 organophosphorus
pesticides had been reviewed (Vettorazzi, 1984). Usually, the
compounds had been reviewed several times as additional information
became available. The compounds, with the years of the JMPR
review, and the acceptable daily intake (ADI) advised are listed
Annex II. It also lists the year of review by IARC. Moreover, it
gives the WHO recommended classification by acute toxic hazard
(WHO, 1984a) and an indication on whether WHO/FAO issued a "Data
Sheet on Chemical Pesticides" on this substance.
Details of the tests and of the results are recorded in the
appropriate published evaluations from the FAO/WHO Joint Committee
Meetings, which have been summarized and commented on by Vettorazzi
(1979).
It should be realized that, while reports on toxicological
tests may give some indication of the purity of the sample as
percentage content of the major active ingredient, there is seldom
any indication of the nature or quantity of the impurities or of
whether the impurities may significantly influence toxicity. It
cannot be emphasized too strongly that measurements such as acute
LD50s are not absolute values.
The LD50 values for one typical compound, fenitrothion, in
various species when administered by various routes are listed in
Table 4 (FAO/WHO, 1970b, 1975b). From this Table, it is clear that
there are marked differences in the LD50 depending on species and
route of administration. Variability in LD50 values for the same
route and species is often considered to be due to the vehicule in
which the pesticide is applied, which may influence its uptake into
the body. However, it is also possible that such differences are
real and can throw light on mechanisms of toxicity and/or of
detoxication, thus making more intelligent assessment of toxicity
possible. It can be argued that the presence of the very toxic
impurities in some formulations of diazinon (section 6.3.4) could
have been deduced much earlier from differences in LD50 data.
Likewise, the Pakistani poisonings due to strong potentiators in
stored malathion (Baker et al., 1978) (section 6.3.4) might have
been prevented, if more questions had been asked about the range of
LD50 values given in the literature for malathion.
Table 4. Comparison of acute toxicity data for
fenitrothion listed in evaluation reports of
joint FAO/WHO meetingsa
----------------------------------------------------
Animal Sex Route LD50 (mg/kg
body weight)
----------------------------------------------------
1970 Report
Mouse M oral 1336
Mouse F oral 1416
Mouse M ip 115
Mouse F ip 110
Mouse iv 220
Rat M oral 740
Rat F oral 570
Rat M ip 135
Rat F ip 160
Rat iv 33
Guinea-pig M oral 500
Guinea-pig oral 1850
Guinea-pig M ip 110
Guinea-pig iv 112
Cat oral 142
1975 Report
Mouse M oral 1030
Mouse F oral 1040
Rat M oral 330
Rat F oral 800
Ring-neck pheasant oral 34.5
Mallard duck oral 2550
Dog oral MLDb 681 mg/kg
Rat M oral 940
Rat F oral 600
----------------------------------------------------
a From: FAO/WHO (1970b, 1975b).
b MLD = minimum lethal dose.
6.1 Effects on the Nervous System
6.1.1 Effects attributed to interaction with esterases
6.1.1.1 Cholinergic effects
(a) Acute toxicity
If non-ester herbicidal compounds are excluded, then the acute
toxicity of all other organophosphorus pesticides shares the basic
mechanism outlined in section 4.5.1, involving inhibition of AChE,
accumulation of ACh, and over-stimulation of some central
cholinergic neurons and of the sympathetic and parasympathetic
nervous systems. Signs and symptoms of poisoning are described
fully in section 7.1. Death is caused by respiratory failure due
to a combination of blocking of the respiratory centre,
bronchospasm, and paralysis of the respiratory muscles.
(b) Chronic toxicity
The cholinergic effects brought about by repeated
administration of less than a single fatal dose are similar in type
to the acute single-dose effects and are discussed in section
6.3.1. For the majority of these compounds, long-term feeding
tests have been performed to establish the no-observed-adverse-
effect levels. In every case, except bromophos-ethyl, the most
sensitive indicator of an effect was depression of ChE activity in
plasma or erythrocytes. The phenomenon of tolerance to repeated
doses of anticholinesterase compounds is covered in sections 6.3.1
and 6.4. For bromophos-ethyl, it has been reported that the
urinary excretion of ascorbic acid and dehydroascorbic acid was
slightly increased in beagle dogs given 0.39 mg/kg body weight
daily, for 18 weeks, at which dose, depression of serum-ChE was not
significant (FAO/WHO, 1973b).
6.1.1.2 Delayed neuropathic effects
Delayed neuropathy has occurred occasionally in man and
experimental animals after intoxication with a variety of
organophosphorus esters. The subject has been reviewed by Johnson
(1975a,b, 1980, 1982a) and by Davis & Richardson (1980). An
account suitable for physicians is given by Lotti et al. (1984).
Delayed neuropathy is not inevitably associated with intoxication
by organophosphorus pesticides (Johnson, 1982a; Soliman et al.,
1982). Improvements in the therapy of acute poisoning (section
7.4) mean that higher doses of some organophosphorus insecticides
can now be tolerated without fatal consequences. However, many
organophosphorus pesticides that might, theoretically, cause
neuropathy, would only do so at a dose far above the lethal dose.
(a) Characteristics
Regardless of the severity of anticholinesterase effects, there
is a delay after intoxication before neuropathic signs and symptoms
appear. In the adult hen, which is the test species of choice,
this delay is 8 - 14 days, while in man, the delay may be up to 4
weeks after acute exposure. The first symptoms are often sensory
with tingling and burning sensations in the limb extremities
followed by weakness in the lower limbs and ataxia. This
progresses to paralysis, which, in severe cases, affects the upper
limbs also. Children and young animals are less severely affected
than adults, but recovery is slow and seldom complete in adults;
with the passage of time, the clinical picture changes from a
flaccid to a spastic type of paralysis. Cats, hens, and a number
of larger species are affected by a single dose. Repeated dosing
does not reduce the delay in onset to less than 8 days from the
first dose. Baboons, monkeys, and marmosets do not respond easily
to single doses of several typical neuropathic esters, and it is
difficult to produce typical delayed neurotoxic effects in rodents,
even by repeated dosing. In early histological examinations,
methods were used that showed mainly degeneration of the fatty
myelin sheath surrounding long nerve axons and names such as
"Organophosphate Demyelinating Disease" are still erroneously used,
in spite of later work that showed that the nerve axon itself was
primarily affected and damage to the myelin sheath was secondary
(Cavanagh, 1954; Bouldin & Cavanagh, 1979). The preparation of
tissues and identification of lesions are described by Bradley
(1976), Bickford & Sprague (1984), and by Prentice & Roberts (1984)
in the Workshop Report edited by Cranmer & Hixson (1984). This
publication also covers many aspects of mechanism and testing.
(b) Mechanism
The first essential step in the initiation of the delayed
neuropathic effect of an organophosphate is phosphorylation of a
target protein in the nervous system. The protein has esteratic
enzyme activity. The phosphorylation, which was originally studied
radiochemically, can be monitored conveniently as progressive
inhibition of the activity of this enzyme, which is now referred to
as Neuropathy Target Esterase (NTE or Neurotoxic Esterase)
(Johnson, 1980, 1982a). The second, and equally essential, step is
the transformation of the phosphorylated NTE to a modified form:
one of the remaining ester bonds of the inhibitor molecule attached
to the NTE active site undergoes a biochemical cleavage leaving an
ionized acidic residue bound to the protein: this residue is
negatively charged and the reaction is referred to as "aging".
Both inhibition and aging of inhibited NTE are essential to
initiate neuropathy, but the role of the negative charge in the
initiation of axonal degeneration is not known. The process of
"aging" of inhibited NTE has some analogy with the better-known
"aging" of inhibited AChE. However, the analogy does not last
above the level of enzyme inhibition. Acute toxicity arises
directly from the loss of catalytic activity of AChE, leading to
accumulation of excess physiological substrate. Mere loss of
catalytic activity of NTE (without aging) does not initiate
neuropathy (see below). There is no evidence of a deleterious
accumulation of a physiological substrate for NTE or lack of
hydrolysis products after inhibition in vivo, and the effect of
the negative charge may be focused on some quite separate process.
When NTE has been inhibited by a suitable phosphate,
phosphonate, or phosphoramidate, aging is always possible, and this
process has been shown to occur rapidly with a wide variety of
neurotoxic esters and hen NTE. However, after inhibition by
phosphinates (which contain 2 phosphorus-carbon bonds) or by
sulfonyl fluorides, no hydrolysable bonds remain in the attached
inhibitor molecule. Thus, aging is not possible, and these
compounds are not neuropathic in vivo. Whenever hen NTE can be
phosphinylated or sulphonated in vivo, the birds become resistant
to challenge doses of typical neuropathic esters, because the
2-step initiation process has been blocked halfway. Similarly,
carbamates do not form aged inhibited enzymes and are either
totally without effect (anti-cholinesterase carbamates are poor
inhibitors of NTE) or inhibit the enzyme, but do not age. Thus,
they protect the hen in the same way as the phosphinates. Now that
this mechanism is understood, it is obvious that carbamates need
not be subjected to tests that were designed to detect delayed
neuropathic potential in organophosphorus anticholinesterase
pesticides.
The events that follow inhibition and aging of NTE are not
known, but it is clear that, in adult hens, detectable delayed
neuropathic events (clinical or histological) are never seen after
a single dose/exposure of an organophosphorus ester, unless there
is at least 70% (probably 80 - 90%) inhibition of the NTE in the
brain and spinal cord soon after dosing (4 - 40 h). Owing to the
synthesis of fresh protein, this inhibition declines markedly
during the 8 to 14-day delay period, and there is no correlation
between neuropathy and NTE inhibition measured at the time that
clinical signs reach their peak. NTE has been found in the nervous
tissue of all mammals and birds examined and can be inhibited, even
in species such as the rat, in which there is no obvious clinical
neuropathic response to a single dose. It has been shown recently
that, when single doses of certain neuropathic organophosphorus
compounds are given to rats, degenerative lesions develop in their
peripheral nerves and spinal cord. Although these lesions are
similar to those in ataxic hens, they are not accompanied by
clinical signs. The dose-response of NTE inhibition and the
neurological damage in rats are well correlated, and, as in hens,
the lesions are prevented by protectively predosing the rats with
sulfonyl fluoride (Padilla & Veronesi, 1985; Veronesi & Padilla, in
press). Human NTE has been examined in vitro (Lotti & Johnson,
1978), and its response to inhibitors is similar to that of the hen
enzyme (I50s differed by not more than 4-fold). What is not known
is the numerical value of the threshold of inhibition of NTE in man
that is associated with clinical neuropathy. However, it is known
that numerous people treated only with atropine for poisoning by
trichlorfon have survived and then developed neuropathy (Johnson,
1981a). In contrast, it is very difficult to produce neuropathy
with trichlorfon in the hen, without enormous doses coupled with
prophylaxis as well as therapy for severe anticholinesterase
effects. It seems, therefore, that in the case of this pesticide,
the chances of a severely poisoned man developing neuropathy rather
than dying are greater than those of the hen. It might be deduced
that the threshold level of NTE inhibition for neuropathic effects
in man is somewhat less than the 70% value for hen, and caution
should be applied in the extrapolation from hen tests. This
argument is merely a comparison of the two hazards, death and
neuropathy: it does not give any guide to the relative dose
required to intoxicate man and hen.
(c) Delayed neurotoxicity testing
Two distinct procedures are currently practiced by testers, and
each depends on the anticipated hazard. In acute tests, it is
recommended that hens surviving an LD50 test (preferably a test in
which the therapeutic use of atropine raised the LD50) should be
observed for three weeks for abnormalities of gait and then be
re-dosed and watched for a further three weeks, after which a
thorough histological examination of the distal ends of neurons in
the spinal cord and peripheral nerve should be performed. Long-
term neurotoxicity tests require feeding hens for up to 90 days
with doses in the diet ranging up to those causing obvious adverse
cholinergic effects; evaluation is by the same criteria as for the
acute test. It may be argued that long-term tests should be
omitted, in view of the greater speed and simplicity of the acute
test in obtaining yes/no answers and the fact that no pesticidal
compound has ever been found to give a positive clinical response
in feeding trials when negative in the 2-massive-doses test. The
practice in the United Kingdom has been to voluntarily exclude from
agricultural use any compound shown to be neuropathic in acute
tests. Guidelines for the performance of acute and long-term
delayed neurotoxicity tests are available (United Kingdom PSPS,
1979; US EPA, 1982; OECD, 1983). The OECD Guidelines point out
that acute tests would be improved if they were accompanied by
assays of NTE inhibition in the brain and spinal cord, one and two
days after dosing. Results of assays would provide graded dose-
response relationships instead of the present all-or-none scoring.
Some examples are given in Johnson (1975b). Particularly useful are
data such as those obtained with malathion (Johnson, 1981b), which
showed negligible (< 10%) inhibition of NTE at the LD50 dose in
hens. These data indicate a different order of safety compared
with that of some other compounds which caused 50 - 60% inhibition,
though there was no visible clinical response. A way to integrate
the NTE test efficiently into delayed neurotoxicity test protocols
has been suggested by Johnson (1984). However, further discussion
to improve this method is required.
(d) Delayed neurotoxic response to long-term feeding
For some neuropathic compounds, the potency of a single dose
may be matched or even exceeded by the effect of the same amount
spread over a few days. In particular, compounds such as TOCP or
leptophos, which are very poorly soluble in water and are required
in a very large single dose to be effective, may well be absorbed
with greater efficiency in a divided lower-dose regime. However,
the results of various studies (Johnson, 1982a) have shown that, as
the dose is reduced further, there is a clear cut-off point below
which there does not appear to be any cumulative effect. Thus,
TOCP in the diet of hens at 400 mg/kg produced neuropathy in 21
days, while a level of 100 mg/kg diet did not cause any detectable
clinical or histological damage after 140 days (Barnes, 1975).
Monitoring of the NTE response to continuous feeding of non-
neurotoxic regimes of 2 organophosphates (0.125 mg DFP/kg for 5
days per week, over 4 weeks, or 2.5 mg mono-2-cresyl diphenyl
phosphate/kg, daily, for 10 weeks) showed that NTE levels in the
brain and spinal cord were depressed within 2 - 3 weeks to about 45
- 55% of normal and remained unchanged thereafter; the equilibrium
is presumbably the result of daily synthesis matching daily
inhibition. These doses did not cause detectable neuropathy during
either the feeding period or the 3 ensuing weeks (Lotti & Johnson,
1980). On the basis of these and other studies, Johnson (1982a)
concluded that the hen nervous system might tolerate the
biochemical defect of prolonged inhibition of NTE to about 50%
normal level brought about by long-term dosing but could not
tolerate the brief 80 - 90% inhibition that follows a single larger
dose. If long-term feeding tests in hens are required, then weekly
measurements of the level of NTE inhibition should provide valuable
predictive information, early in the test (Johnson, 1984). Since
it is necessary to kill birds for tissue sampling, more birds may
be necessary, at least in the early stages.
(e) Results of delayed neurotoxicity testing
No pesticidal organophosphorus compounds, giving negative
results in massive-dose tests, have caused delayed neuropathy in
long-term feeding studies. With the exception of trichlorfon,
where 2 doses, 3 days apart, were necessary (Johnson 1970, 1981a),
the massive-dose tests have been single doses given with
prophylaxis and therapy (eserine + atropine and atropine + oxime -
repeated if necessary) to ensure that doses above LD50 could be
examined. In Table 5, known pesticidal compounds are listed
according to the doses known to produce marked-to-severe neuropathy
in the majority of birds tested. Administration by either the oral
or dermal route may be effective.
The review by Johnson (1975b) is believed to list all
pesticidal and non-pesticidal compounds, or their derived oxons,
tested and reported on, up to that time. The JMPR Reports were not
included as a literature source. NTE responses are given where
these have been measured. Johnson (1982a) lists a number of
pesticides for which both clinical and NTE tests had been reported
since 1975 and notes that carbophenthion, cyanophos, diazinon,
fenitrothion, malathion, methyl parathion, omethoate, and parathion
can positively be declared non-neuropathic on the basis of
negligible NTE inhibition responses, while chlorpyrifos,
methamidophos, and salithion gave intermediate NTE responses
without clinical expression at the doses tested. Isofenphos
( o -ethyl- o -2-iso-propoxycarbonylphenyl isopropylphosphor-
amidothioate) has been shown by NTE assays and clinical tests to
cause delayed neuropathy in hens at about 20 x LD50 (Wilson et al.,
1984), while the insecticidal synergist o-n -propyl- o -(2-propynyl)
phenylphosphonate was neuropathic by both criteria at about the
LD50 (Soliman, 1982).
Table 5. Organophosphorus pesticides causing delayed neuropathy
in hens after a single dose
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
mipafox 25 im Bidstrup et al.
N,N -diisopropylphosphoro- (1953)
diamidic fluoride
haloxon 1000 oral Malone (1964)
3-chloro-4-methyl-2-oxo-
2H-1-benzopyran-7-yl-
bis-(2-chloroethyl) phosphate
EPN 40-80 sc Witter & Gaines
(1963)
trichlornat 310 oral Johnson (1975b)
ethyl 2,4,5-trichlorophenyl 300-400 oral Johnson (1975b)
phenylphosphonate
ethyl 2,4-dichlorophenyl > 2000 oral Abou-Donia et al.
phenylphosphonothioate (1979)
leptophos 400-500 oral Abou-Donia et al.
(1974); Johnson
(1975b)
desbromoleptophos 60 oral Johnson (1975b)
S,S,S -tributyl phosphoro- 1110 sc Johnson (1970)
trithioate (DEF)
cyanofenphos > 100 oral Ohkawa et al.
(1980)
isofenphos 100 oral Wilson et al.
O -ethyl O -2-isopropoxy- (1984)
carbonylphenyl isopropyl-
phosphoramidothioate
O-n- propyl O -(2-propynyl) 400 oral Soliman (1982)
phenylphosphonate
dichlorvosb 100 scc Caroldi & Lotti
(1981)
amiprophos 600 oralc,d Huang et al.
O -ethyl O -4-methyl-6- (1979)
nitrophenyl
N- isopropylphos-
phoramidothionate
Table 5 (contd.)
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
coumaphos 50 oralc Abou-Donia et al.
500 dermal (1982)
chlorpyrifos 150 oral Lotti et al.
(1986)
salithion 120 oral El-Sebae et al.
(1981)
--------------------------------------------------------------
a Dose needed to cause marked-to-severe neuropathy in the
majority of birds tested.
b 50% formulation in hydrocarbons: dose calculated as active
ingredient.
c Mild neuropathy only at maximum tolerated dose.
d Test performed in cockerels.
The statement by Namba et al. (1971) that chlorpyrifos produced
neuropathy in hens seems to have been without foundation and may
arise from the misreading of a report by Gaines (1969), which
mentioned that chlorpyrifos caused a rapid onset short-term
weakness (sometimes called paralytic effect) similar to that caused
by malathion. However, one recent case of human poisoning and
laboratory tests with doses well above the unprotected LD50 have
shown neuropathic effects from this pesticide (Lotti & Moretto, in
press).
(f) Structure/activity relationships
As noted above, not all organophosphorus pesticides cause
delayed neuropathy. In vivo tests and target-enzyme studies
listed by Johnson (1975b) can be condensed according to a number of
factors as listed by Johnson (1980, 1982a):
(1) Factors that increase delayed neurotoxicity potential more
than acute toxicity are:
(a) choice of phosphonates or phosphoramidates rather
than analogous phosphates;
(b) increase in chain-length or hydrophobicity of R1 and
R2 ; and
(c) a leaving group X, which does not sterically hinder
approach to the active site of NTE;
(2) Factors that decrease the comparative potential are:
(a) the converse of (1) a, b, and c;
(b) choice of R or X groups that are very bulky
(naphthyloxy) or non-planar;
(c) choice of a nitrophenyl group at X (a steric effect?);
(d) choice of comparatively more hydrophilic X groups
(oximes or heterocyclics); and
(e) choice of thioether linkages at X.
Considering these factors, it can be seen why malathion and
diazinon are both far below the neurotoxicity hazard line, why, in
its homologous series, only dichlorvos is not neurotoxic at the
LD50, why EPN, a phosphonothioate with a hydrophobic phenyl group
at R1 is neurotoxic, even with a 4-nitrophenyl leaving group, and
why it is not surprising that other phenylphosphonothioates such as
desbromoleptophos, or cyanofenphos are also neurotoxic. The
apparent non-neurotoxicity of the ethyl analogue of leptophos
(Hollingshaus et al., 1979) seems to contradict (1-b) above, but
the dominant factor seems to be the problem of absorption of this
very poorly soluble compound after oral dosage (Hansen & Hansen,
1985).
6.1.2 Behavioural and other effects on the nervous system
The problems of interpreting behavioural changes in relation to
inhibition of AChE and the time-dependent changes in these
variables have been discussed by Bignami (1976) and Bignami et al.
(1975). It appears that some learned responses may be acquired
more quickly by rats recovering from an inhibitory dose of, e.g.,
DFP (1 mg/kg body weight). This may be a sign of changed
inhibitory responses in learning pathways, but it is difficult to
say whether it can be classified as a toxic response.
Numerous research workers have reported changes at doses that
affect levels of AChE, but without overt signs of intoxication.
For example, Kaloyanova-Simeonova (1961) noted that small doses of
chlorthion (5 or 10 mg/kg body weight in the rat) intensified
conditioned motor reflexes and depressed them at higher doses: ChE
levels were depressed in all cases and were restored more slowly
than the return to normal reflex activity. Reiter et al. (1975)
did not find any effects on performance of learned visual
discrimination tasks by monkeys at doses of parathion of 0.5 mg/kg
body weight, which depressed blood levels of AChE by about 25% and
those of pseudoChE by about 35%. Doses causing 40% or more
inhibition of AChE were associated with decreased responses, which
returned to normal faster than the recovery of AChE levels. A very
sensitive response to the nerve agent soman (pinacolyl
methylphosphonofluoridate) was reported in one out of several
behavioural tests by Wolthuis & Vanwersch (1984). In an open-field
test, a number of performance variables were affected by a dose of
only 3% of the LD50. Effects were also seen with doses of 4 - 6%
LD50 of 2 carbamates but not below 30% LD50 of the pesticide, TEPP,
or of another nerve agent, sarin (isopropyl methylphosphono-
fluoridate). Unfortunately, the effects on ChE levels were not
measured. There is no obvious reason for the contrasting effects
of TEPP and sarin, on the one hand, and of soman and physostigmine,
on the other. The authors noted that, judged by poisoning signs at
near-lethal doses, soman exerted a greater proportion of its
effects centrally than the other 2 organophosphorus esters. The
fact that changes may be seen in some, but not all behavioural
responses at a certain dose emphasizes the statement by Revzin
(1983) that no single behavioural or neurophysiological test can
give a definite conclusion about organophosphate toxicity.
There appears to be only one other report in the literature of
a behavioural change in animals at doses less than those that
inhibit ChEs (Desi et al., 1971). Behavioural and EEG changes were
noted in rats fed bromophos at 500 or 100 mg/kg diet for 6 weeks,
doses that also caused ChE inhibition, but, at 30 and 10 mg/kg,
there were no effects on ChE, though some behavioural changes were
still observed. It is not clear whether undosed animals were
handled in precisely the same fashion as the dosed.
However, in contrast to the above, Desi (1983) reported tests
applied after 3 months of daily consumption of diet containing
various percentages of the LD50 of bromophos. The lowest
concentration that produced changes in a behavioural test (maze
running) and in EEG (both the complex EEG and computer-analysed
segments of the EEG) was about 0.26% of LD50, daily, but this also
caused significant changes in erythrocyte-AChE and plasma-ChE.
Among 6 organophosphorus pesticides assessed by these procedures,
none produced changes greater than that caused by the vehicle alone
without also causing effects on ChE. This conclusion differs from
that written by the author.
Analysis of the EEG records of a small group of rhesus monkeys
has been carried out both before, and one year after, intoxication
with the potent anticholinesterase agent sarin (isopropyl
methylphosphonofluoridate) (Duffy & Burchfield, 1980). Three
animals received a single "large dose" (5 µg/kg body weight iv),
and 3 others received a series of 10 injections of 1 µg sarin/kg
body weight im at weekly intervals: there were 10 controls. The
"large dose" animals had generalized convulsions and were
maintained on Gallamine relaxant with artificial respiration;
small-dose animals were considered in pilot studies to be near the
threshold of poisoning, but showed few overt signs; they did not
receive relaxant or artificial respiration. Twenty-four hours
after a "large dose", marked differences were seen in the EEG
frequency spectrum, which is not surprising. However, one year
after the dose, there was still a small increase in the percentage
energy in the beta-2 region of the spectrum and the change was said
to be statistically significant. Some changes in the beta region
were detectable in all 6 dosed monkeys one year after; no
significant changes were seen in the 10 controls. However,
apparently the changes were only seen under some lighting
conditions and were small compared with differences between the
frequency spectra of the only 2 controls for which data were shown.
The statistical treatment of the acquired data seems valid, but the
actual values measured one year after dosing are obviously well
within the normal range. No indication was given of how much
variation can be caused in the pattern of undosed animals by
variations in the conditions of handling and observation, or what
the range is for apparently identically treated normal animals. The
doubts pertaining to the toxicological significance of these
measurements apply also to some human studies using the same
technique (section 7.2.2).
Effects of cholinergic agents on the visual system have been
monitored by Revzin (1980). In urethane-anaesthetised pigeons with
implanted electrodes, various changes in the response of specific
neurones of the optic tectum and of the hippocampus were noted
after doses both of mevinphos and of atropine. The author claims
that the procedure was sensitive in detecting effects in the
absence of detectable peripheral parasympathetic signs. However,
the lowest effective dose of mevinphos was only one-third of that
(0.15 mg/kg body weight) which produced parasympathetic signs that
would certainly be associated with substantial inhibition of AChE.
Thus, the claim to sensitivity of this complex procedure in
surgically modified birds seems excessive.
Some possible effects of anticholinesterases on non-
cholinesterase targets were considered by O'Neill (1981). No clear
effects seem definable at concentrations lower than those that
inhibit AChE and some are probably secondary to stimulation of non-
cholinergic nerves with cholinergic innervation. However, an
endopeptidase that can hydrolyse putative transmitter peptides in
the nervous system is known to be inhibited by di-isopropyl
phosphorofluoridate at a concentration similar to that which
inhibits AChE (Kato et al., 1980). Thus, some involvement of non-
cholinergic pathways in the causing of CNS effects in
organophosphate poisoning cannot be excluded. Moreover, some
anticholinesterases also exert effects directly at the cholinergic
receptor as well as by the inhibition of AChE (Karczmar & Ohta,
1981).
6.2 Other Effects
A variety of histological lesions has been described at autopsy
in animals severely poisoned with organophosphate pesticides.
However, very few effects are described in the absence of obvious
poisoning or at doses that do not markedly inhibit the ChEs. In
the following sections, the evidence is reviewed for effects not
obviously attributable to inhibtion of either AChE or NTE.
6.2.1 Mutagenic and carcinogenic effects
The proposed theoretical basis for believing that dimethyl or
diethyl phosphate pesticides might be mutagenic or carcinogenic
has already been discussed (section 4.5.2). This basis was shown
to be defective by Bedford & Robinson (1972). No alkylation was
detected in N7 of guanine in RNA and DNA of liver of animals that
were exposed for 12 h to working concentrations of dichlorvos of
64 g/m3 (0.064 g/litre air) (Wooder et al., 1977); these authors
contrasted their data with earlier published work showing modest
alkylation of guanine in suspensions of cells exposed to very high
solution concentrations of dichlorvos. In vivo, the acute
anticholinesterase effects limit the circulating concentration
that can be tolerated by any mammal and there is also strong
preference for phosphorylation of biological scavenger molecules
rather than for alkylation built into the organophosphorus
pesticide molecules.
It has frequently been suggested that dichlorvos has
carcinogenic potential because of observed mutagenic effects in in
vitro test systems. The IARC (1979) Working Group accepted
extensive data showing no evidence for the mutagenicity of
dichlorvos in mammals. FAO/WHO (1978b) accepted animal studies
showing no dose-related carcinogenic effects in life-time studies
carried out at doses that depressed blood-ChE levels.
In a 78-week feeding test on groups of 50 male and 50 female
mice (B6 C3 F1 hybrid), no dose-related effects were seen when the
diet contained about 600 or 300 mg dichlorvos/kg, respectively.
The IARC Working Group evaluating this study noted that a few
oesophageal tumours were seen in treated mice. It appears that
this fact influenced their verdict that "the available data do not
allow an evaluation of the carcinogenicity of dichlorvos to be
made" (IARC, 1979).
An IARC Monograph (1983) included evaluations of 5 widely-used
organophosphate pesticides (malathion, methyl parathion, parathion,
tetrachlorvinphos, and trichlorfon). In several cases, the
conclusions were that acceptable tests had been performed with no
evidence of carcinogenic effects or of mutagenic effects in
mammals. For others, the conclusions were of "limited evidence"
consisting of very small effects above the control background
levels in life-time studies. None of these compounds was judged to
be a strong mammalian mutagen or carcinogen and the same statement
is true for all organosphosphorus pesticides that have been
evaluated by FAO/WHO Working Parties or by other authorities.
However, recent published results show that controversies do occur
when evaluating the outcome of carcinogenicity studies. Huff et
al. (1985) reevaluated the pathology of the original studies by
the US National Cancer Institute in 2 different strains of rats.
Histopathological reexamination confirmed the earlier conclusions
that malathion and malaoxon were not carcinogenic. These
conclusions differed from those of Reuber (1985) who evaluated the
same studies and rated both compounds as carcinogenic.
6.2.2 Teratogenic effects
Defects in the development of fertilized hen eggs, injected
with various organophosphates, are known, but many of these are
associated with the inhibition of the enzyme kynurine formamidase
and a depression of NAD levels at a critical period of development
(Seifert & Casida, 1980). This pathway is not critical in mammals,
and no equivalent effects are known. If teratogenicity is taken to
mean induction of malformations in live offspring without decrease
in number of births (i.e., no embryotoxicity), then for the vast
majority of organophosphorus pesticides, no adverse effects of
continuous feeding of organophosphates on pre- or postpartum
mortality have been reported, nor have embryonic defects been
proved, except at doses that significantly retarded growth in the
mother (Vergieva, 1983). Single high doses causing significant
toxic effects in mothers may be deleterious: a number of these
toxicity-linked effects have been summarized by Seifert & Casida
(1980). Kimbrough & Gaines (1968) reported deaths, and that
resorptions were increased in pregnant rats given a single high
dose of parathion or diazinon on the 11th day of gestation.
However, these effects were associated with significant toxic
effects on the mothers. Similarly, trichlorfon at very high doses
(400 mg/kg per day with the dose divided into 3 spaced aliquots)
and given on days 6 - 15 of gestation, produced defects in
offspring: each dose produced cholinergic symptoms; no effects were
seen in rats, mice, or hamsters when the daily dose was 200 mg/kg
(Staples & Goulding, 1979). However, a specific defect consisting
of hypoplasia of the cerebellum in offspring was noted, both in
field cases and experimental tests, 8 pregnant pigs were dosed once
or twice with neguvon (a veterinary grade of trichlorfon) between
days 55 and 70 of pregnancy (Knox et al., 1978). Doses were 50 -
60 mg/kg body weight, which caused maximum inhibition of
erythrocyte-ChE levels of 40 -80%, without overt signs of
poisoning. The hypoplasia was accompanied by severe ataxia and
tremors, while voice and vision appeared unaffected. No defects
were seen in the offspring of over 100 control sows housed with the
dosed animals, and serological and virological tests did not show
anything incriminating. A genuine teratogenic effect of moderate
doses of trichlorfon commonly used in veterinary practice has been
demonstrated in the pregnant pig. However, it may be that the
herds tested were unusually susceptible, since (apparently)
congenital tremor had been known in litters borne over many years
in the area where the trichlorfon-induced effects were seen.
6.2.3 Effects on the immune system
In a review, Zackov (1983) stated that "most ... organo-
phosphorus pesticides elicit autoimmune reactions and suppress the
production of antibodies against vaccines". No evidence was given
and it is not clear whether the statement was claiming specific
effects or referred to doses that are sufficient to produce a range
of toxic effects.
Shtenberg et al. (1974) claimed that oral doses of methyl-
nitrophos (fenitrothion) or chlorphos (trichlorfon) at 5 or 7 mg/kg
body weight per day, for an unspecified period, suppressed
haemaglutinin levels in rats immunized against sheep red blood
cells; these doses would have significantly inhibited ChEs and were
said to be more effective in rats fed a protein-deficient diet.
Dandliker et al. (1979) reported a depression in antibody titre in
rats in the 6 - 7 weeks of an immunization procedure that commenced
either one day before or one day after (both statements are made)
an oral dose of half of the LD50 of parathion. The immunization
consisted of weekly intramuscular doses of 400 µg fluorescein-
labelled ovalbumin with Freunds Complete Adjuvant. The confusion
concerning the order of intoxication and primary immunization is
very important when such a high dose was given, since cholinergic
symptoms, fluid loss, imbalance, and general debility would have
been marked. Administration to mice of 0.1 x LD50 of parathion for
8 days led to a 10% loss in body weight. Immunization on the 9th
day showed a statistically-significant (34%) reduction in the
number of antibody plaque-forming cells derived from the spleen, 4
days after immunization, but the reduction was insignificant (10%
only) when immunization was carried out on day 10, though several
animals in the group died at this point due to accumulated toxic
effects (Wiltrout et al., 1978).
Some decreased antibody titres "proportional" to AChE
inhibition were noted in response to prolonged doses of malathion
or dichlorvos (0.025 - 0.4 x LD50 given 5 days per week for 6
weeks) (Desi et al., 1978). Clearly, none of the above responses
can be dissociated from the general cholinergic intoxicating
effects of the pesticides. There do not appear to be any reports
of studies on the immunological status of healthy animals receiving
doses causing little or no depression in ChE levels.
6.2.4 Effects on tissue carboxyesterases
A variety of carboxyesterases abound in serum, liver,
intestine, and other tissues (section 4.4). Although inhibition of
one specific carboxyesterase (NTE) has toxic sequelae (section
6.1.1.2), no direct deleterious effects of inhibiting other
carboxyesterases have been demonstrated. However, they may
contribute markedly to the metabolic disposal of malathion and
certain other organophosphorus pesticides, so that inhibition of
tissue carboxyesterases may potentiate the toxicity of such
pesticides (section 6.3.5). The structure/activity relationships
for inhibition of these enzymes by organophosphorus compounds
inevitably differ from those for inhibition of AChE. For EPN and
fenchlorphos, both serum- and liver-carboxyesterases of rats were
markedly more sensitive than brain- and serum-AChE; in other cases,
the liver enzymes, but not those in serum, were more sensitive (Su
et al., 1971).
6.2.5 Sundry other effects of organophosphorus pesticides
Very few effects other than those described in the sections
above have been noted, except those arising from ill-health due to
severe anticholinesterase effects. Thus, impaired growth rate is
commonly associated with a rapid depression in AChE levels to less
than 50%, but much lower levels can be tolerated without ill-
effects, if the depression is brought about over several weeks, and
then maintained for up to a year (section 6.3.1).
Various changes in glucose metabolism, in serum enzymes, and in
other clinical chemical variables have been reported after single,
acute, or repeated doses of various pesticides at from one-tenth to
one-quarter of the LD50, daily (Dimov & Kaloyanova, 1967; Enan et
al., 1982).
A reversible, mild muscle-necrotising effect could be detected
histologically in the diaphragm muscles of rats, 24 h after a dose
of paraoxon, parathion, or other anticholinesterases, sufficient
to cause marked fasciculations (Fenichel et al., 1972; Dettbarn,
1984). The damage appeared to be a function of the prolonged
cholinergic stimulation of the muscle, since it was entirely
prevented by doses of atropine, which prevent fasciculations, or by
alpha-bungarotoxin applied directly to the myoneural junction
(Salpeter et al., 1979).
Several adverse effects attributed only to certain
organophosphorus esters are listed below. The list may be of value
in promoting more careful examination of intoxicated animals or
human beings.
6.2.5.1 Effects on hormones
Changes in the diurnal pattern of plasma-ACTH and adrenal
levels of some related enzymes have been reported in rats
maintained with dichlorvos in the drinking-water at 2 mg/litre:
blood-ChE levels were not affected (Civen et al., 1980). However,
the weight gain of the treated animals was only half that of the
controls, and the fluid consumption was increased by 20%. These
deleterious effects could be due to diarrhoea and imbalance of
fluids with inevitable repercussions on hormonal levels, etc.;
preferential effects of the dichlorvos on intestinal esterases
might be the primary effect.
6.2.5.2 Effects on the reproductive system
Damaged seminiferous tubules were reported in mice given either
a single dose of about half of the LD50 of dichlorvos or 18 doses
of about one-tenth of the LD50 (Krause & Homola, 1974). However,
the doses were high, the percentage increases seem small, and the
number of samples taken was very small; thus, no statistical
evaluation is possible. Amiprophos has been reported to cause some
gonadotrophic effects in adult cockerels (Huang et al., 1979), but
details are few.
6.2.5.3 Effects on the retina
Fenthion administered intramuscularly at about one-quarter of
the LD50 (50 mg/kg body weight), every 4 days for one year
(solvent, if any, not stated), affected the electroretinogram in 2
strains of rats (Wistar and black Long-Evans) within 3 months and
abolished it after one year (Imai, 1977). Similar studies involving
the administration of either fenitrothion or ethylthiometon to
beagle dogs for 5 days per week for 2 years, were reported by
Ishikawa & Miyata (1980). Doses that depressed plasma-ChE levels
to about 30% of normal, throughout the period, led to changes in
optical function after 13 months continuous exposure and to
morphological changes in the ciliary muscle at termination.
6.2.5.4 Porphyric effect
Daily application of technical (85%) diazinon (20 or 40 mg/kg
body weight) to the skin of Dark Agouti rats just above the tail
produced a 4-fold increase in faecal porphyrins, after 8 - 12 weeks
(Bleakley et al., 1979). There was no increase in urinary
porphyrins and no effect when diazinon was given in food (about
8 mg per day to rats, initially weighing 180 g). The pattern of
porphyrins excretion was said to be indistinguishable from
porphyria cutanea tarda. However, the increase in excreted
porphyrins in classical porphyria cutanea tarda may be as much as
1000-fold, with massive amounts in the urine as well as in the
faeces. The authors failed to reproduce even this small effect in
rats when they used more pure (97%) diazinon (Nichol et al., 1982).
However, they also found that an impurity in stored technical
material, isodiazinon (diethyl 2-isopropyl-6-methyl-4- S -
pyrimidinyl phosphorothioate), was very effective in causing
porphyrin accumulation, when added to cultures of chick
hepatocytes; confusingly, however, the accumulated porphyrin was
coproporphyrin rather than the expected protoporphyrin. Although
human poisoning with diazinon is not uncommon, there has only been
one report implicating technical diazinon in a few cases of
porphyria cutanea tarda in occupationally-exposed workers (Bopp &
Kasminsky, 1975). Further experimental animal studies seem
warranted. No reports have been found of porphyria connected with
exposure to other organophosphorus pesticides.
6.2.5.5 Lipid metabolism
Organophosphate esters can inhibit the activities of some
triglyceridases and lipases in vitro and in vivo. However, no
repercussions from such inhibition were found when appropriate
parameters were measured in rats fed for one year with either of 2
pesticides that caused marked (60 - 80%) depression of blood-ChE.
Thus, male or female rats were fed either a normal diet or a diet
enriched sufficiently with fat to increase aortic fatty acids by
170%. No changes occurred in: the hormone-sensitive lipase and
lipoprotein lipase in adipose tissue; the free and total fatty
acids and total glycerol and total cholesterol in serum; and the
total fatty acids, cholesterol, and glycerol in the aorta (Buchet
et al., l977). The pesticides were chlorpyriphos (100 mg/kg diet)
and triamiphos (10 mg/kg) and the findings were contrary to those
from a preliminary study by the same workers.
6.2.5.6 Effects causing delayed deaths
Although not pesticidal agents themselves, two of the
phosphorothiolate impurities found in technical malathion and some
analogues of these impurities caused delayed effects, which were
lethal for rats at doses below the cholinergic LD50 (Aldridge et
al., 1979; Mallipudi et al., 1979; Verschoyle et al., 1980). The
compounds were O,O,S -trimethyl phosphorothioate (I), O,S,S -
trimethyl phosphorodithioate (II), and the ethyl analogues (III and
IV). After an oral LD50 dose (as low as 26 mg/kg body weight for
II), all 4 compounds produced cholinergic responses that lasted
less than 24 h and deaths at this time were only seen with IV:
doses 2 - 6 x LD50 were needed to cause cholinergic deaths with I -
III. Rats dosed at, or near, the LD50 recovered from the initial
cholinergic effects, but by day 3, they had lost weight and were
panting with laboured respiration; deaths occurred 3 - 6 days after
dosing. However, survivors appeared normal, 10 days after dosing.
Death was due to pulmonary insufficiency associated with
progressive cell proliferation (Dinsdale et al., 1982; Imamura et
al., 1983; Aldridge & Nemery, 1984), and combined therapy with
atropine and oxime was ineffective. The biochemical mechanism of
these effects is not fully known, but it is probable that the
proximal toxin is produced in the lung by oxidative attack on the
alkylthio moiety of the compounds (Aldridge et al., 1985). The
activities of brain-AChE and plasma-ChE and carboxylesterase, which
were partially inhibited during the first day after dosing,
increased thereafter and were at least 50% of the levels of
activity in the controls at the time of death. The margin between
delayed death LD50 and cholinergic LD50 was markedly less with the
triethyl, than with trimethyl compounds. Such effects were seen
with S,S,S -trimethyl phosphorotrithiolate but not with the higher
analogue ethoprophos ( O -ethyl S,S -di- n -propyl
phosphorodithiolate) or with methamidophos (Verschoyle & Cabral,
1982).
A different form of delayed acute toxicity was reported to
occur 4 days after large oral doses of DEF in hens. The effect was
not seen after a single dose, sufficient to cause delayed
neurotoxicity, was given subcutaneously (Johnson, 1970) or dermally
(Abou-Donia et al., 1980). The effect was distinct from both
cholinergic and delayed neuropathic effects and is attributed to
the acute toxicity of n- butyl mercaptan produced by the degradation
of DEF in the gastrointestinal tract.
6.2.5.7 Selective inhibition of thermogenesis
The defoliant DEF ( S,S,S -tri- n -butyl phosphorotrithiolate)
acted as an anticholinesterase at high doses, but, at lower doses
(60 - 200 mg/kg in rats and mice), it caused a profound fall in
body temperature (as much as 10 °C over a few h), without marked
sedation; deaths occurred mostly after the depression had persisted
for a day (Ray, 1980). The effect was different from the smaller
atropine-sensitive changes due to some cholinomimetics and was due
to blocking of cold-induced thermogenesis without affecting heat
conservation mechanisms. The effect seems unique to the DEF
chemical structure. Ray & Cunningham (1985) demonstrated that the
effect was a selective action on a central thermogenic control
mechanism rather than on peripheral thermogenic processes and that
it was probably due to a metabolite of DEF rather than to the
parent compound.
6.3 Factors Influencing Organophosphorus Insecticide Toxicity
6.3.1 Dosage-effect
The lethal effects of organophosphorus insecticides are due to
severe cholinergic effects arising from excessive inhibition of
AChE. With few exceptions, the AChE activity of the tissues is
inhibited soon after the administration of acutely toxic doses of
all anti-AChE agents. This is true, not only for compounds that do
not require metabolic conversion to anti-AChE agents, but also for
most phosphorothioates, phosphorodithioates, and
phosphorodiamidates that are oxidized by the liver to metabolites
with anti-AChE activity. In general, the duration of action of
most anti-AChE agents is relatively short, as evidenced by
considerable reversal of the inhibition within a few days. For
this reason, a 10-day observation period is sufficient for acute
LD50 measurements on all anti-AChE agents that have been studied,
and 2 days suffice for most.
The data in Table 6 give a comparison of the maximal amount of
inhibition of the ChE activities in the brain, submaxillary glands,
and serum of rats for several compounds, all of which were given at
dose levels equivalent to 5/8 of the LD50. The time at which
maximal inhibition occurred and the period required for complete
reversal of the inhibition are also presented. From these
examples, it can be seen that, in general, equivalent fractions of
the LD50 of various anti-ChE compounds produce similar levels of
inhibition of ChEs, though the LD50 values for the various
compounds differ considerably. The time at which maximal
inhibition of ChEs occurs varies from 15 min to 3 h after
administration. In some cases, the AChE activity of the brain and
parasympathetic nervous system, as indicated by the submaxillary
gland, and the non-specific ChE of the serum are inhibited to the
same extent by a particular compound. However, notable exceptions
are OMPA, which is converted in the liver to a very labile
inhibitor that never reaches the brain, and Guthion, which does not
inhibit non-specific ChE.
Differences between the responsiveness of rat brain- and serum-
ChE have been reported also in the case of dietary administration
of various organophosphorus insecticides (Su et al., 1971). Thus,
a level of only 40 mg EPN/kg fed for 1 week reduced brain-ChE to
50%, while 125 mg/kg was needed to achieve the effect in serum; the
sensitivity was reversed for fenchlorphos, while the sensitivities
of the 2 tissues were similar for demeton. It is not clear whether
these differences reflect differences in access of the compounds to
their targets, differences between the tissue AChEs, or the fact
that pseudoChE is present as well as AChE in the serum of rats, so
that assay of the hydrolysis of ACh using serum measures both
enzymes, whereas, in the brain, the activity is about 90% specific.
Marked differences in the rate of reversal of the inhibitory
effects on ChEs of different compounds, in vivo, are shown in Table
6. In view of the variable duration of action of various anti-ChE
agents, the performance of assays on the tissues of animals at
intervals after acutely toxic doses provides a great deal of useful
information regarding the toxicity of these compounds. The
transition from a tolerable to a lethal dose (either acute or
chronic) often occurs within a 2 to 4-fold range. This is not
surprising, since the AChE of nervous tissue and effector organs
must be inhibited by 50 - 80% before pharmacological effects can be
seen (Holmstedt, 1959). The increment in dose to raise inhibition
to 90% with associated deaths is not very great.
Table 6. Onset and duration of the anticholinesterese action
of some organophosphorus compounds in ratsa
-----------------------------------------------------------------------
Compound Maximum inhibition of cholinesterase (%)
Dose Brain Serum Sub- Time to Time to
(mg/kg maxillary maximum complete
body gland inhib- reversal
weight) tion (h) (h)
-----------------------------------------------------------------------
Iso-Systox 1.0 85 80 75 3.0 120
(demeton- S )b
Disyston 1.25 75 85 75 3.0 120
(disulfoton)
Guthion 3.5 60 0 50 0.5 24
(azinophosmethyl)
Dipterex 140.0 85 82 85 0.25 6
(trichlorfon)
Octamethyl 5.0 0 85 88 2.0 144
pyrophosphor-
tetramide (OMPA)c
-----------------------------------------------------------------------
a From: DuBois (1963).
b Phosphorothioic acid, O -[2-(ethylthio)ethyl] O,O -diethyl ester.
c Octamethylpyrophosphoramide.
The general correlation of inhibition of ChE with symptoms of
poisoning is also seen with repeated dosing with organo-phosphates,
but details vary greatly. It is always true that a prerequisite of
death is profound inhibition of AChE in the central and/or
peripheral nerves, but the tolerable maximum inhibition increases
when this level is reached, stepwise, over a period of 2 - 4 weeks
or more. Thus, when commercial Systox (containing about equal
amounts of the O - and S -isomers of demeton) was fed to rats at
20 mg/kg diet, there were no signs of poisoning, though, at the end of
16 weeks, the brain- and whole blood-ChE activities were 26 and 28%
of normal, respectively (Barnes & Denz, 1954). At 50 mg/kg, 3 out
of 12 rats died, but all others in the group improved after initial
marked signs of poisoning during the first month, their food intake
increased above normal (and therefore their actual dose increased),
and their growth rate became normal. This improvement was not due
to any marked increase in ability to detoxify the agent, since, at
the end of 16 weeks, these apparently healthy rats had only 7 - 8%
of normal AChE activity in the brain and blood. This level would
be associated with fatalities if brought about by a single dose;
indeed, at the end of the study, the animals were consuming daily a
dose equivalent to 96% of the single-dose LD50. In a similar way,
Barnes & Denz (1951) found that parathion in the diet at 100 or
75 mg/kg was lethal for the majority of rats in large groups within
3 - 4 weeks, while results with 50 mg/kg were variable (26/72 deaths
in one trial and 3/36 in a later trial) and no deaths attributable
to poisoning occurred in groups fed 20 or 10 mg/kg. The rats
surviving 50 mg/kg showed clinical signs of intoxication (notably
fasciculations) and ate less, initially: they ate normally after 3
weeks, but failed to gain weight as rapidly as the controls.
However, signs of poisoning decreased in severity and frequency
during the third month and seldom reappeared during the remainder
of a year's feeding. This pattern of response and of adaptation is
typical for all anticholinesterase pesticides. When monitoring of
enzymes is carried out in parallel with feeding, the level of
activity often rises to a steady state after an initial decline.
Factors determining the fraction of an LD50 dose that is tolerable
include:
(a) Speed of absorption, of subsequent metabolic activation,
and of elimination of the compound. Thus, for demeton- S -
methyl, much of the sulfoxide metabolite from a single dose
will still be circulating on the following day, though the
parent compound may not linger. In such a case, lethal
concentrations will build up more easily than with, say,
trichlorfon, which is converted to the inhibitory dichlorvos,
both of which are rapidly eliminated;
(b) The net rate of formation of a stable form of
inhibited AChE arising from the 3 reactions of inhibition,
reactivation, and aging described in section 4.5. The
relationship of the chemical structure of an oxon inhibitor to
rates of these reactions is complex and also varies between
species. AChEs from the rat have not been purified and
subjected to extensive kinetic study in vitro. In most
studies, crystallized (not 100% pure) bovine erythrocyte-AChE
has been used. Rates of spontaneous reactivation and aging for
this enzyme inhibited with dimethoxy, diethoxy, and ethoxy,
ethanthio-substituted phosphates are shown in Table 7.
Although data derived in this way cannot be transposed directly to
in vivo situations, they are consistent with the well-known fact
that, after poisoning by a sub-lethal dose of some dimethyl
phosphates, recovery with the disappearance of symptoms is complete
within a few h. The value of k+3 for erythrocyte-ChE taken from
rats dosed in vivo with dimethyl phosphate, was reported to be 57
x 10-4 (a half-life of inhibited enzyme of 2 h). One day after
such a sub-lethal dose, most of the rat AChE will again be in the
uninhibited form with a small fraction in the aged inhibited form.
In contrast, not more than half of the inhibited enzyme would be
expected to be reactivated in one day after poisoning with diethyl
phosphate and a substantial proportion of the inhibited enzyme
would be aged, so that recovery to 100% activity would be a very
slow process, depending on the synthesis of fresh enzyme. It
follows that markedly different outcomes would be expected from
repeated intoxication with doses of dimethyl phosphate and diethyl
phosphate which both caused an initial response of, say, 50%
inhibition, the former would be less hazardous than the latter.
This interpretation concurs with the fact that rats can survive
daily doses of 25% of the LD50 of trichlorfon, but only about 12%
of the LD50 of parathion (DuBois, 1963). DuBois (1963) also
pointed out that ChE inhibition mounted steadily as a result of
daily doses of OMPA and that toxic effects were seen when
inhibition reached about 70%. It is believed that no spontaneous
reactivation of ChE occurs after inhibition by phosphoramidates,
which makes such compounds intrinsically undesirable as pesticides.
Table 7. Rates of spontaneous reactivation (k+3 ) and of
aging (k+4 ) of bovine erythrocyte cholinesterase after
inhibition in vitro a
---------------------------------------------------------
O Rate constants x 104
|| R1 (per min) at pH 7.4
|| / and 37 °C
Enz-O-P substituents
\
R2
R1 R2 k+3 k+4
---------------------------------------------------------
-OCH3 -OCH3 115 14
-OCH3 -SCH3 1170 95
-OC2 H5 -OC2 H5 2.0 2.2
-OC2 H5 -SC2 H5 270 32
---------------------------------------------------------
a From: Clothier et al. (1981).
Dose-effect relationships for delayed neurotoxicity have been
listed (Johnson, 1975b). It has been noted that, as for
cholinergic effects, levels of long-term dosing can be found that
are detectable by a biochemical response at the primary target but
have no clinically- or histologically-observable correlate. The
threshold of the tolerable response is probably a permanent
inhibition or 40 - 50% of NTE (Johnson, 1982a).
6.3.2 Age and sex
It is well-known that the microsomal MFOs and other drug-
metabolizing enzymes are present at comparatively low levels in
neonatal animals, but activity develops to approximately the adult
level early in maturation. Since MFOs are involved in both the
activation and degradation of many organophosphorus pesticides
(section 4.3), the likely net result in terms of LD50 is hard to
predict. One-day-old rats were 9 times more susceptible to
malathion than 17-day-old animals (Mendoza, 1976). The toxicity of
methyl parathion and of parathion for rats decreased from birth
through the developmental period: the decrease was best correlated
with the increasing capacity of the animals to metabolize the
oxygen analogues by both oxidative and hydrolytic pathways (Benke &
Murphy, 1975). The LD50 (ip) of trichlorfon in adult male rats was
reported to be 250 mg/kg body weight, compared with 190 mg/kg in
male weanlings (FAO/WHO, 1972b). Whether this is a significant
difference is not clear. Liver MFO activity fluctuates according
to the hormonal status of female animals. LD50 values quoted for
males and females often differ, but these values generally arise
from different laboratory animals subjected to many variable
factors (including the purity of the test sample). Among several
representative pesticides surveyed for this review, only parathion
showed a marked and apparently real difference in LD50s between the
sexes, the oral LD50 in male rats being 5 - 30 mg/kg body weight,
depending on the solvent, compared with 1.8 - 5 mg/kg in females
(FAO/WHO, 1964).
6.3.3 Nutrition
It is well-known that liver MFO activity can be manipulated by
administering a diet severely deficient in protein. The
consequences can be dramatic in terms of the toxicity of compounds,
such as carbon tetrachloride, which undergo a single
biotransformation step leading to a directly toxic product.
However, as with the age and sex factors discussed above (section
6.3.2), several metabolic steps may be affected. No clear-cut
effects seem to have been reported. Thus, the acute toxicity of
diazinon is greater (up to 2-fold) in rats maintained on a diet,
either very low (4%), or very high (81%) in protein compared with a
standard (29%) protein diet (FAO/WHO, 1971b). A similar increase
in toxicity is seen with naled ( O,O -dimethyl O -1,2-dibromo-2,2-
dichloroethyl phos-phate) (Kaloyanova & Tasheva, 1983). Boyd
(1969) reported that, while increases in toxicity were 2-3 fold for
diazinon, malathion, and demeton, the increase in parathion
toxicity was 7.6 fold, in malnourished rats. Whether the effects
at such extremes were directly due to changes in the
biotransformation of the agent or to the animals becoming generally
unhealthy is not clear.
6.3.4 Effects of impurities and of storage
Insecticides are manufactured and formulated in various ways
and in many countries. There may be significant differences in
these procedures and in the conditions of storage of formulated
products. These factors can influence the nature and extent of
impurities present in the material that is ultimately applied.
Impurities in a pesticide may be of very low toxicity (the
majority), may be toxic in their own right (more or less toxic than
the major component), or they may be potentiators of the toxicity
of another component.
6.3.4.1 Impurities toxic in their own right
(a) Non-anticholinesterase effects
The most dramatic example of an impurity exerting an effect
different from that of the principal component does not come from
the realm of organophosphorus pesticides. The potent toxicant
2,3,7,8-tetrachlorodibenzodioxin (TCDD) may be present in the
herbicide 2,4,5-trichlorophenoxyacetic acid so that, even at a few
mg/kg, the effects of the impurity may dominate the toxicological
response. An analogous non-cholinergic response due to impurities
is not known in anticholinesterase pesticides. Questions have been
asked about the possible mutagenic effects of trimethyl phosphate,
which may be present at a few percent in some technical
preparations of dimethyl phosphates, but the possible exposure in
vivo is limited by the main anticholinesterase response to the
pesticide, and there appears to be no evidence for mutagenic
effects in mammals of any organophosphorus pesticide (section
6.2.1).
(b) Anticholinesterase effects
LD50 values for technical preparations of diazinon have varied
over an unusually wide range. The oral LD50 for the rat was
reported to be 76 - 108 mg/kg body weight in 1964 (FAO/WHO, 1964)
and 250 - 466 mg/kg in 1971 (FAO/WHO, 1971b). A major contributing
factor, according to the latter report, was the presence of highly
toxic pyrophosphates in earlier samples; it was implied that the
impurities had been produced during storage and eliminated by
stabilization (detail unspecified) of formulated material. It
seems likely that the pyrophosphate concerned in this improvement
was monothiono-TEPP with an oral LD50 in mice of about 4 mg/kg body
weight (Margot & Gysen, 1957). Both the sulfotepp and monothiono-
TEPP content of an emulsifiable concentrate of diazinon and its
toxicity increased rapidly when it was stored in tinned-steel
containers instead of in inert-lined aluminium ones (Soliman et
al., 1982). However, in 1979, it was reported that sulfotepp was
also present in many standard and formulated preparations of
diazinon at concentrations ranging from 0.2 to 0.8% and that the
percentage was unrelated to the age of the sample (Meier et al.,
1979). It seems likely that this impurity was formed during the
synthesis of diazinon using diethyl phosphorothiochloridate.
Sulfotepp was 60 - 80 times more toxic than diazinon for the rat,
so that at least one-third of the toxicity of typical diazinon
might be attributed to the impurity. This calculation is probably
an underestimate, since it seems that metabolic disposal of an
impurity is often slowed markedly by competition from the major
component. It can be said that there may be "reverse potentiation"
of the toxicity of the impurity by the major component (diazinon).
Formation of pyrophosphates is implicit in the mode of
synthesis of the many organophosphorus pesticides with a
phosphorochloridate or phosphorothionochloridate as a precursor.
TEPP or its methyl analogue would be unlikely to survive much
aqueous washing during production, but the mixed mono- or disulfo
analogues may well survive, unless deliberately eliminated. It
seems likely that sulfotepp is generally present in parathion
(Diggory, 1977); however, since both the parent and the impurity
have similar LD50s, pure and impure preparations do not differ
significantly. It is a curious fact that the lower the true
toxicity of a pesticide, the more marked may be the effect of an
impurity in changing its toxicity. It might be very rewarding
both to analyse more thoroughly and to reexamine the toxicities of
low-toxicity pesticides such as bromophos which is said to have an
LD50 in various mammals of 3 - 8 g/kg body weight (FAO/WHO, 1973b);
even this low toxicity might be attributable to the impurities
rather than to the pure compound. An analogous situation certainly
pertains concerning the potentiation of malathion (see below).
6.3.4.2 Impurities potentiating the toxicity of the major
ingredient
There does not appear to be any indication that the MFO status
of animals is different after administration of technical grade
organophosphorus insecticides compared with pure. Alterations of
the MFO status of animals because of diet, sex, drugs, etc.,
discussed in sections 6.3.2, 6.3.3, and 6.3.5, are unpredictable in
their effect on the LD50. In contrast, inhibition of the esteratic
capacity of mammals increases the toxicity of pesticides that
depend principally on tissue esterases in their metabolism (section
4.3). Malathion is a notable example of an organophosphorus
pesticide in which the toxicity is enhanced when tissue esterases
are inhibited. Until comparatively recently, such inhibition was
only known in situations where an unrelated organophosphorus ester
was administered to test animals a short time before the malathion.
However, it is now known that several impurities present in most
samples of malathion prepared for use as pesticides, are capable of
inhibiting tissue carboxylesterases. Some of these impurities act
very rapidly and so prevent the normal metabolism of malathion and
potentiate its toxicity. This enhanced toxicity has been expressed
in man. Several hundred spray workers were intoxicated while
spraying certain formulations of malathion in Pakistan, and 5 died
(Baker et al., 1978). Isomalathion and several trimethyl
phosphorothiolates are found in most commercial preparations of
malathion, but the levels depend markedly on the formulation and
on storage conditions. The potentiating power of small amounts of
these impurities are shown in Table 8. Examination of samples of
formulated malathion, known to be unusually toxic, showed a fair
correlation of toxicity only with the percentage content of
isomalathion (Aldridge et al., 1979; Miles et al., 1979). However,
the correlation was imperfect when a large number of samples were
examined and the addition of known further amounts of pure
isomalathion to the formulated samples caused more than the
expected potentiation (Aldridge et al., 1979). The authors
concluded that, although isomalathion contributed the main effect,
there was also significant potentiation by other agents present in
the samples; the chief candidate was O,S,S -phosphorodithioate.
Most unformulated samples of technical grade malathion seem to
have an LD50 for rats in the range of 1500 - 2000 mg/kg body weight.
Such material contains some potentiating impurities (Pellegrini &
Santi, 1972; Umetsu et al., 1977), but has proved acceptable as a
basis for formulated insecticides with little toxic hazard.
However, it is now clear that some formulated samples increase
markedly in both their impurity content and toxicity, when they are
stored at elevated temperatures. Not only are temperature and time
important, but also the formulating agents (Table 9), and almost
half of the malathion lost from Formulation C is apparently
transformed to isomalathion with a massive potentiation, whereas
the increase in toxicity is less marked in Formulations A and B, in
which a much smaller proportion of lost malathion is converted to
isomalathion.
Table 8. Potentiation of acute oral toxicity for rats by impurities
added to malathion
------------------------------------------------------------------------
Compound Amount Potentiation Purified Reference
added added ratio found malathion
(%) used (LD50
mg/kg body
weight)
------------------------------------------------------------------------
isomalathion 0.4 3 10 700 Aldridge et al.
0.6 5 (1979)
2 12
8 25
0.05 3 12 500 Umetsu et al.
0.1 4 (1977)
0.5 6
2 10
O,S,S -trimethyl 0.15 3 10 700 Aldridge et al.
phosphorodithioate 0.3 4 (1979)
0.5 8
1.0 13
2.0 20
0.05 4 12 500 Umetsu et al.
0.2 6 (1977)
0.5 7
0.035 2 8000 Pellegrini &
0.1 3 Santi (1972)
0.2 4
0.5 7
O,O,S -trimethyl 0.3 2 10 700 Aldridge et al.
phosphorothioate 1.3 4 (1979)
0.2 3 12 500 Umetsu et al.
1 4 (1977)
0.2 3 8000 Pellegrini &
0.5 4 Santi (1972)
O,O,S -trimethyl 1.5 2 10 700 Aldridge et al.
phosphorodithioate 5 5 (1979)
1 4 12 500 Umetsu et al.
5 5 (1977)
3.5 2 8000 Pellegrini &
4.5 3 Santi (1972)
------------------------------------------------------------------------
There is much evidence that potentiation of malathion by
extraneous compounds is associated with the inhibition of
carboxylesterases. Using malathion as specific substrate, Talcott
et al. (1979b) showed that isomalathion and O,S,S -trimethyl
phosphorodithioate were potent inhibitors of rat liver and plasma
malathion carboxylesterase, in vitro and in vivo; a partially-
purified sample of carboxylesterase from human liver was also
sensitive to isomalathion (Talcott et al., 1979a).
The same hazard exists with impure samples of phenthoate as for
malathion. Pellegrini & Santi (1972) showed that technical samples
containing 61 - 91% of the principal ester had LD50s (rat oral) of
78 - 243 mg/kg body weight, while a purified preparation (98.5%)
had an LD50 of 4700 mg/kg, though its toxicity for insects
increased approximately in proportion to the purity. The principal
potentiating impurities were the S -methyl isomer and the identical
trimethyl phosphorothiolates found in malathion. For phenthoate,
as for malathion, the vulnerability to potentiation lies in the
presence of the hydrolysable ethoxycarbonyl ester bond which, in
pure samples, is the key to low mammalian toxicity.
Table 9. Effects of formulating agents and of storage
time and temperature on composition and toxicity of
malathion (50% wdp)a
----------------------------------------------------------
Storage conditions Composition (%) Oral LD50
Temperature Time Malathion Isomalation (rat)
(°C) (days)
----------------------------------------------------------
Formulation A
0 48.0 0.38 2800
38 60 45.5 0.37 2230
90 44.2 0.49 1740
55 6 47.6 0.30 2520
13 46.4 0.37 1760
90 1 40.9 0.69 950
Formulation B
0 48.8 0.18 2540
38 60 47.2 0.79 1130
90 46.5 0.81 1330
55 6 45.2 0.67 1200
13 43.6 0.55 1170
90 1 39.9 0.32 1900
Formulation C
0 50.6 0.61 2660
38 90 44.9 3.7 590
55 6 46.2 3.4 535
13 43.7 3.5 555
----------------------------------------------------------
a From: Miles et al. (1979).
The presence of the S -methyl isomer (0.32%) in a commercial
fenitrothion formulation was shown by Miles et al. (1979). The
concentration increased to > 1% during accelerated storage tests,
but there was no concomitant increase in the toxicity of the
formulation. This observation on a compound not heavily dependent
on esterases for the primary step of detoxication, points to the
need to consider biochemical mechanisms in assessing possible
hazards. There need be no general concern about the presence of
small amounts of isomers in organophosphorus pesticides. The
presence of isoparathion in parathion is well-known, but there is
no suggestion of any marked change in toxicity brought about by
this impurity.
Some organophosphorus pesticides contain carboxylamide bonds
rather than carboxyester. These include dimethoate, dicrotophos,
monocrotophos, phosphamidon, and acephate. There appears to be
little evidence that impurities in commercial formulations of any
of the above pesticides markedly alter the toxicity, apart from a
small (1.6x) decrease in the mammalian toxicity of acephate, after
storage for 6 months at 40 °C; the insecticidal activity of the
compound was unchanged (Umetsu et al., 1977). During the storage
period, the concentration of various impurities changed, but no
relationship between these changes and altered toxicity was
obvious.
6.3.5 Effects of other pesticides and of drugs
All organophosphorus and carbamate insecticides exert their
acute toxic action by attack on the AChE. Thus, it follows that
exposure to more than one such pesticide will usually produce at
least an additive effect. Besides this simple effect, other
pesticides or drugs may also influence the toxicity of an
individual organophosphorus pesticide by interfering with its
metabolism, activation, and disposal.
Not all organophosphorus pesticides and probably no carbamate
pesticides inhibit tissue carboxylesterase to potentiate malathion
in the manner discussed in section 6.3.4. However, like all other
enzymes, the carboxylesterases have their own structure-activity
pattern: this has not been worked out in a systematic fashion. It
is clear that, with some compounds, profound inhibition of liver
carboxylesterase can be achieved without inhibition of AChE
sufficient to cause signs of poisoning. This class includes many
thioalkyl esters such as the trimethyl esters and also S,S,S -tri- n
-butyl phosphorotrithioate (DEF), which are potent potentiators of
malathion toxicity via carboxylesterase inhibition. EPN is a
phenylphosphorothioate insecticide that also acts in this way.
Tables 10 and 11 show examples in which measurements of effects on
tissue carboxylesterases and AChE are of value in predicting the
potentiation of malathion toxicity (Murphy, 1969).
Table 10. Comparison of enzyme inhibition caused by
several pesticidesa
---------------------------------------------------------
Insecticide Dietary concentration (mg/kg) resulting in
(period) 40 - 60% inhibition of:
Red cell- Liver- Plasma-
cholinesterase malathionase malathionase
---------------------------------------------------------
Parathion 3 5 5
(7 days)
Fenchlorphos 500 30 30
(7 days)
Malathion 500 100 500
(30 days)
---------------------------------------------------------
a From: Murphy (1969).
Table 11. Effect of feeding fenchlorphos on in vivo
anticholinesterase activity of malathiona
-------------------------------------------------------
Fenchlorphos Malathion Inhibition of brain
concentration challenge dose AChE 1 h after
in diet (mg/kg) (mg/kg ip) challenge (%)
-------------------------------------------------------
0 200 13
30 0 1
30 200 61
-------------------------------------------------------
a From: Murphy (1969).
Potentiation of the toxicity of organophosphate compounds for
mammals not containing a carboxylester function, does not appear to
be a significant hazard, though it is possible that potentiation of
some carboxylamide pesticides by an analogous inhibition of tissue
amidases may occur; certainly, EPN potentiates the toxicity of
dimethoate (El-Sebae, 1980). Potentiation of the toxicity of
organophosphorus pesticides for insects by inhibition of MFO
activity is well-known, and many potent synergists are used in
agriculture for this purpose (Wilkinson, 1971), but much less has
been reported concerning similar effects in mammals. This may
reflect the greater versatility of mammals compared with insects in
disposing of organophosphorus esters (section 4.3). Competition
for one metabolic route within the animal often does not greatly
alter its total capacity to deal with a foreign compound.
Keplinger & Deichmann (1967) combined pairs of various pesticides
in proportion to their oral LD50s, determined individually, and
then measured the oral toxicity of the mixtures in rats or mice.
They calculated a ratio of expected LD50/observed LD50 where
"expected LD50" was the sum of half the LD50 value of each of the 2
constituents. Their study included 7 chlorinated hydrocarbons,
the carbamate carbaryl, and 5 organophosphates including diazinon,
malathion, and parathion. With a "no-effect" ratio of 1.0, they
considered measured ratios greater than 1.75 or less than 0.57 as
probably significant of real effects. In a number of cases, less
than additive effects were noted for combinations of a chlorinated
hydrocarbon and an organophosphate, e.g., aldrin + diazinon (0.55),
DDT + malathion (0.54), toxaphene + carbonylfenthion (0.54): this
might well be expected if the mode of toxicity were different and
metabolic pathways were not markedly altered. The only cases of
potentiation involving organophosphates were also not surprising.
A triple combination of parathion, malathion, and chlordane had a
ratio of 1.99. This effect was probably due to simple potentiation
of malathion by parathion, since chlordane alone did not potentiate
either organophosphorus compound. Mixtures of Aramite (sulfurous
acid 2-chloroethyl 2-[4-(1-1-dimethylethyl)-phenoxyl-1-methylethyl
ester) with several organophosphates in mice had ratios of 1.86 -
2.14. However, since the LD50 of Aramite in mice is high (2000
mg/kg body weight), it may be that the 500 mg/kg administered in a
mixture was absorbed more efficiently and was therefore more
effective proportionately than the much higher LD50 dose of Aramite
alone.
As noted above, aldrin had little effect on the toxicity of
organophosphorus insecticides, when administered at the same time.
However, a number of chlorinated hydrocarbons (aldrin, DDT,
chlordane, etc.) are well-known as stimulators of MFO activity in
(principally) the mammalian liver. This activity increased
markedly during a period of a few days after dosage, and
pretreatment of mice with aldrin (16 mg/kg body weight), 4 days
before a challenge dose of 6 different organophosphates, markedly
reduced (up to 5-fold) the toxicity of each (Murphy, 1969).
Similar results were obtained with other classes of MFO inducers
such as phenobarbital. Murphy points out that the mechanism of
these effects probably includes stimulation of liver
carboxylesterase as well as MFO.
From the observations above, it appears that simple mixing of
an organophosphorus insecticide with a chlorinated hydrocarbon is
unlikely to adversely influence the acute toxicity for mammals, as
expressed by LD50 value. However, administration of DDE at 55
mg/kg diet to adult male Japanese quail led to an increasing
susceptibility to challenge doses of parathion (2.5 mg/kg)
administered orally; mortality in these birds increased from 0 to
30% after 1 week of feeding and 60% after 3 weeks (Ludke, 1977).
The problem of potentiation by some organophosphates of the
toxicity of pesticides containing carboxyl ester bonds may be
significant. This has been demonstrated for malathion in animals
(section 6.3.4) and in man (section 7.1.3). It may also be a
problem for pyrethroid insecticides for most of which degradation
by mammalian carboxyesterases is a significant detoxification
pathway (Miyamoto, 1976).
6.3.6 Species
No clear ranking of species sensitivity to organophosphorus
pesticides as a class can be given. A general impression is that
mice, hamsters, and guinea-pigs may be more sensitive than rats,
with respect to a number of compounds, but the converse is seldom
true. However, most available data have been produced with little
reference to conditions of husbandry, diet, hormonal status, etc.,
so that only very marked differences, which do not usually seem to
exist, would emerge. Birds tend to be more sensitive to
organophosphorus pesticides (Schafer, 1972) and amphibians less
sensitive than mammals. It has been suggested, but not confirmed,
that these differences might be due to differences in the activity
of enzymes in species that hydrolyse organophosphorus compounds and
thereby contribute to detoxification.
The toxicity of several organophosphorus pesticides for
different species seems to be inversely related to the activity of
the plasma A esterase, which degrades the pesticide oxon. Such
activity is considerably lower in birds than in mammals (Machin et
al., 1978). When 14 avian species were compared with 5 mammalian,
the average plasma activity against pirimiphos-methyl oxon was 170
times less, and that against paraoxon, at least 13 times less
(Brealey et al., 1980). Differences in liver microsomal oxidative
activity involved in the metabolism of several organophosphorus
pesticides by mammals and birds were less profound, though fish
liver was less active (Miyamoto & Ohkawa, 1978).
A multitude of factors contribute to the great difference
between the oral toxicity of chlorofenvinphos for the rat (10 mg/kg
body weight) and that for the dog (> 5000 mg/kg). These include
efficiency of absorption, at least 2 metabolic detoxification
processes, the rate of uptake by the brain, and a 7-fold difference
in the sensitivity of the brain AChE to this compound in the 2
species (Hutson & Hathway, 1967; Donninger, 1971).
Adult hens, cats, dogs, and larger farm animals are all
susceptible to organophosphorus delayed neurotoxicants (Davis &
Richardson, 1980; Johnson, 1982a). There is no clear ranking of
dose-sensitivity, though hens seem to be most uniformly responsive.
The full clinical response is not easily seen in laboratory
primates and rodents, though morphological damage may be detected
(section 6.1.1.2).
6.3.7 Other factors
The effects of solvents on chemical stability and isomerization
reactions were noted in section 4.5. The effects of formulation
agents on stability and, therefore, on the toxicity of malathion
have also been noted previously (section 6.3.4). It is likely that
percutaneous absorption will be greater for liquid formulations
than for powders but that powders may adhere longer thereby
enhancing an effect, if proper hygiene is not observed. A number
of examples are quoted by El-Sebae (1980), in which formulated
pesticides were more toxic than the technical preparation (Table
12). In many cases, this is due to the fact that the solvent in
the formulation facilitates the uptake of the pesticide into the
body. The toxicity of other components of the formulation may play
a role (especially in the case of pesticides of very low toxicity,
such as tetrachlorvinphos) (Table 12), as well as potentiation.
El-Sebae noted that, in some cases, the I50 for inhibition of ChEs
in vitro by these compounds differed. This could be relevant in
the case of the directly-active oxon-type pesticide tetrachlorvinphos,
but in cases where the test was performed with a thioate, the
anticholinesterase activity would depend almost entirely on trace
oxon impurities, which are often destroyed rapidly in vivo and
contribute little to toxicity compared with the bulk of oxon
produced metabolically.
Table 12. Comparative toxicity for mice of
some technical and formulated
organophosphorus pesticidesa
--------------------------------------------
Pesticide 24-h oral LD50
(mg/kg body weight)
Technical Formulated
--------------------------------------------
phosfolanb 12 11
chlorpyriphos 140 60
leptophos 162 83
tetrachlorvinphos 5000 1800
--------------------------------------------
a From: El-Sebae (1980).
b (diethoxyphosphinothioyl)dithioimidocarbonic
acid, cyclic ethylene ester.
6.4 Acquisition of Tolerance to Organophosphorus Insecticides
This topic has been discussed in section 6.3.1, where it was
noted that when AChE levels were reduced progressively over a
number of days or weeks, animals showed cholinergic signs of
poisoning which, in animals that survived, decreased in severity
and sometimes disappeared completely, though ChE inhibition was
maintained. This phenomenon is separate from the fact that
permanent inhibition of 30 - 50% is ineffective in producing
measurable symptoms. The basis for acquired tolerance is not fully
known, though a "down regulation" in the muscarinic ACh receptor is
thought to be a contributary cause. This involves both reduced
sensitivity and reduced numbers of receptors (Costa et al., 1982).
6.5 Therapy of Experimental Organophosphorus Poisoning
The understanding of the mechanism of acute toxicity of
organophosphorus pesticides has provided the basis for rational
therapy. The effects of inhibition of AChE, as described in
section 6.1.1, are common to all organophosphorus pesticides
intoxications. However, the speed of onset and the rate of unaided
recovery from sub-lethal doses vary greatly, depending on the
chemical nature of the pesticide, the route of exposure, and on
whether this exposure was a sudden overwhelming dose or a drawn-
out process.
Factors leading to a slow onset of symptoms include:
(a) Slow absorption or metabolic activation: this is often
associated with extremely low solubility and therefore
with the presence of large hydrophobic groups in the
ester molecule; pesticides such as haloxon, chlorpyrifos,
and leptophos are of this type;
(b) Persistence in the system of a comparatively stable
inhibitor of ChEs. This could be as a low concentration
of an active inhibitor such as demeton- S -methyl
sulfoxide or high concentrations of a weak inhibitor such
as methamidophos.
Factors leading to rapid clearance of symptoms include:
(a) Rapid clearance of the pesticide and its active agents,
as with trichlorfon and dichlorvos; rapid clearance
occurs also with nerve agents such as soman and sarin,
which have been much used in studies on therapy in
experimental animals.
(b) A slow rate of aging of inhibited AChE giving opportunity
for reactivation (spontaneous or induced) to occur;
diethyl phosphorylated AChE ages more slowly than
dimethyl or diisopropyl.
(c) Rapid spontaneous reactivation of inhibited AChE, such as
occurs after inhibition by all dimethyl or bis-2-
chloroethyl phosphates. In this case, the possibility of
reinhibition by a persistent compound will affect the
picture.
The factors noted above, which influence speed of onset and
remission of effects, influence the prognosis for response to
therapy but do not markedly alter the nature of optimal treatment.
Maximum benefit comes from combined treatment with an
anticholinergic drug (usually atropine) plus a reactivator of
inhibited ChE (an oxime) with diazepam, and also artificial
respiration. The effects of the components will be discussed
individually below.
6.5.1 Palliation
Artificial respiration alone can be very effective in
maintaining life, since the primary cause of death in
organophosphorus poisoning is respiratory failure (section 6.1.1).
Such treatment gains time for the processes, natural or imposed,
that lead to the return of sufficient ChE activity to maintain
life.
6.5.2 Antagonism of effects of ACh
Atropine antagonizes many of the peripheral muscarinic effects
of excess ACh and also some central effects. However, there was no
correlation between the peripheral anticholinergic activity of a
range of atropine-like drugs and their capacity when used alone (or
in conjunction with oximes) (section 6.5.3) to protect against the
lethal effects of sarin (Coleman et. al., 1962; Brimblecombe et.
al., 1970). Moreover, the ranking of the therapeutic efficacy of
atropine analogues varied according to the test species (rat,
mouse, or guinea-pig), and all effects were small (protective ratio
< 1.5) in mice and guinea-pigs, but much larger and more variable
(1.2 - 9.3) in rats. The ranking changed yet further when the drug
was combined with oxime P2S (see below), though the protective
ratio with some compounds rose to 18 - 24 in guinea-pigs, 9 - 80 in
rats, but only 1.8 - 6.3 in mice. These confusing effects are now
thought to be at least partially due to an anticonvulsant effect
contributed variously by the atropine analogues. Anticonvulsants
often supplement the effects of atropine or of combined
atropine/oxime therapy (see below). In particular, diazepam
(valium) is known both to raise the LD50 and speed recovery in some
cases (Johnson & Wilcox, 1975). These authors implied that the
mechanism might be partly direct antagonism to some central effects
of ACh and partly indirect. When diazepam is included in the
therapeutic package, there appears to be little evidence that
alternative anticholinergic drugs to the well-proved atropine are
superior (Green et al., 1977). It has been reported that, in
prophylaxis against the toxicity of DFP in mice, the protection
factor was 28 when atropine and obidoxime were used but 180 when
Dexetimide (a drug with strong central anticholinergic activity)
was substituted for atropine. The particular advantage claimed for
this drug was that, in the therapy of rabbits intoxicated with up
to 60 x LD50 of paraoxon or 80 x LD50 of DFP, one single
intravenous injection of Dexetimide (8 - 16 mg/kg body weight) was
effective in conjunction with obidoxime, whereas repeated doses of
atropine were necessary (Bertram et al., 1977). Dexetimide is used
for the treatment of parkinsonism and is available commercially.
However, Dexetimide has not been evaluated in conjunction with
diazepam or compared with atropine plus diazepam, and no details
were given of the hazards of its use and side-effects in control
animals.
6.5.3 Reactivation of inhibited AChE
As indicated in section 4.5.1, inhibited ChEs can be
reactivated in vitro by treatment with appropriate nucleophilic
agents, of which salts of the oxime N- methylpyridinium-2-aldoxime
are the most commonly used (the chloride is known as pralidoxime
and the methanesulfonate as P2S). In some countries, obidoxime
(ToxogoninR ), which is a bis-quaternary oxime, is recommended at
slightly different doses than pralidoxime, but the mode of action
is similar. The scope for further improvement in the design of
therapeutic oximes is discussed by Gray (1984).
In vivo, there are 2 limitations to the benefits to be
obtained from the use of these agents:
(a) Access
The quaternary oximes are thought not to cross the blood-brain
barrier easily (Taylor, 1980). However, some experimental
work, summarized by Lotti & Becker (1982b), suggests that there
may be limited access, and this may have a significant, albeit
small, effect in reversing inhibition of AChE to improve the
clinical state. Other more direct beneficial action of the
oximes directly at synapses in the medullary respiratory centre
cannot be ruled out. The prompt improvement in the level of
consciousness observed and in the EEG of an intoxicated child
when iv infusion of 2-PAM was commenced (Lotti & Becker, 1982b)
also seemed to indicate that there was some access to important
brain regions. It has been claimed that obidoxime (25 mg/kg
body weight) injected intraperitoneally in rats, 5 min after a
dose of armin, was effective in reactivating 33% of the
inhibited AChE of the ponto-medullary region, which contains
the centre for control of respiration (Vasic et al., 1977).
However, there were no controls appropriate to disprove the
alternative explanation that the oxime had altered the
circulating level of armin (by direct destructive interaction
or otherwise), which, although interesting, is unlikely to be
relevant to therapy instituted later after dosing, nor is such
destruction-protection unique to toxogonin.
(b) Aging of inhibited AChE
As noted in section 4.5.1, inhibited AChE is converted by a
time-dependent reaction to a form resistant to reactivators.
Thus, oxime therapy becomes less effective with time after
poisoning. The rate of aging of dialkyl-phosphorylated AChE is
Me > IsoPr > Et (O'Brien, 1967), but little has been published
on the rate of aging when phosphonyl groups derived from
pesticides are attached to the enzyme. When one or both of the
residual alkyl groups are attached to phosphorus through sulfur
rather than oxygen, the rates of both spontaneous reactivation
and of aging of inhibited bovine erythrocyte AChE are markedly
increased (Clothier et al., 1981). Ethoprophos is one of the
newer pesticides that contain such a residual alkylthio group;
no published studies are known of therapy after poisoning with
this or related pesticides.
6.5.4 Efficacy of therapy
As reported above, the combination of atropine plus oxime is
far more effective in most cases than the mere summed effects.
This is because the peripheral neuromuscular junctions
(particularly the diaphragm) and the sympathetic ganglia, where
oximes reactivate AChE, are nicotinic and are unaffected by
atropine, so that separate aspects of intoxication are treated by
the two agents. There is speculation that some oximes may exert a
protective effect by acting as depolarizing agents at the
neuromuscular junction. This may also account for the slight
therapeutic effects of some analogues of toxogonin which do not
have any nucleophilic oxime group or reactivating power (Schoene &
Oldiges, 1973). Diazepam, also, is ineffective, except in
combination with atropine and oxime.
Persistence of the toxic agent may interfere with successful
therapy. Thus, single doses of atropine + oxime were of only
marginal efficacy in altering the LD50 of isofenphos in rats
(FAO/WHO, 1982b). However, when therapeutic doses were given
repeatedly at about 12-h intervals, for 2 - 3 days, the LD50 for
rats was raised about 4-fold; for hens, the increase was 15-fold
(Wilson et al., 1984). It appears that the failure of one-shot
therapy in this case was due to persistence of the toxic agent
rather than to a different mode of intoxication or to formation of
an inhibited form of AChE that resisted reactivation. The same
situation may pertain for profenofos intoxication, which appears
not to respond well to therapy (El-Sebae, personal communication,
1985).
7. EFFECTS ON MAN
7.1 Acute Cholinergic Poisoning
The clinical picture of organophosphorus intoxication results
from accumulation of ACh at nerve endings. The syndrome is
described in detail in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980; HMSO, 1983; Plestina, 1984). The
symptoms can be summarized in three groups as follows:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles and, in more severe
cases, of diaphragm and respiratory muscles; and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, restlessness, and anxiety;
- mental confusion, convulsions, and coma; and
- depression of the respiratory centre.
All these symptoms can occur in different combinations and can
vary in time of onset, sequence, and duration, depending on the
chemical, dose, and route of exposure. Mild poisoning might
include muscarinic and nicotinic signs only. Severe cases always
show central nervous system involvement; the clinical picture is
dominated by respiratory failure, sometimes leading to pulmonary
oedema, due to the combination of the above-mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure; and
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to the light. Pulse rate is not
of diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ.
On the other hand, since changes in the conduction
and excitability of the heart might be life-
threatening, monitoring should be performed.
Confirmation of diagnosis is made by measurement of AChE in RBC
or plasma-pseudoChE, and, also, of the dibucaine number (to rule
out genetic deficiencies).
Measurements of blood-ChE during therapy are also useful in
assessing the treatment with oximes, though there might not be a
correlation between the severity of symptoms and the degree of ChE
inhibition: comparison should be made with pre-exposure levels,
wherever possible.
Chemical analysis of body fluids (urine, blood, gastric lavage)
should be made in order to identify the compounds that caused
poisoning.
7.1.1 Methods for assessing absorption and effects of
organophosphorus insecticides
As well as assessments of general health and behaviour, the
study of the effects of this class of pesticides is favoured
compared with that of some other classes since the basic
biochemical mechanisms (inhibition of esterases) are known for the
major toxic effects. Biochemical and neuro-physiological
techniques, relevant to the principal effects of all the compounds,
have been established. Identification of the monobasic acid type
of urinary metabolite, which is commonly produced, is an indicator
of exposure rather than of an effect, but it seems appropriate to
outline the technique in this sub-section. Wherever possible, test
findings should be compared with pre-exposure measurements on the
same individual.
7.1.1.1 Analysis of urine as a means of monitoring exposed
populations
As previously discussed in section 4, organophosphorus
pesticides may undergo hydrolysis in vivo to yield substituted
phosphoric acids that are subsequently excreted in urine. Advances
in gas chromatography and combined gas chromatography/mass
spectrometry (CG/MS) have made it possible to analyse the urine of
exposed persons for the presence of appropriate metabolites. It is
usually necessary to preserve the sample by the addition of
chloroform, to concentrate or extract the metabolite(s), and to
convert them to suitably-volatile derivatives that can be detected
by GC. Obviously, access to a well-equipped analytical laboratory,
capable of the quick processing of samples, is a necessary factor
if monitoring by urine analysis is proposed. However, in some
cases, simpler and sensitive colorimetric tests are available for
screening the urine of exposed persons. Thus, 4-nitrophenol can
be measured directly in the urine of workers exposed to parathion
(Wolfe et al., 1970).
Consideration of the concentration of metabolite(s) in the
urine can be helpful in determining patterns of exposure, and these
concentrations can be calibrated against the effects on AChE for a
particular pesticide. However, the time-course and peak of
excretion of metabolites appears to vary according to dose (Bradway
et al., 1977), so that serial sampling and analyses of urine are
desirable. Levels of metabolite alone cannot be considered a guide
to hazard. This is obvious when it is realized that pesticides
that have very different toxicities may yield identical acidic
metabolites. Thus, the level of metabolites in urine, after
exposure to sufficient amounts of the very toxic parathion-methyl
to depress blood-AChE to 50%, will be much lower than that of the
identical metabolites, following exposure to the related
fenitrothion, which is about 40 times less toxic.
7.1.1.2 Biochemical methods for the measurement of effects
AChE is present in human erythrocytes (RBC) and is the same as
the enzyme present in the target synapses. Thus, levels of AChE in
RBC are assumed to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the organophosphate has equal access to blood and
synapses. In the case of acute poisoning, a high inhibition of
RBC-AChE is pathognomonic, but, in the follow-up of the
intoxication, it might not be correlated with the severity of
symptoms. In the case of repeated exposures, additional
difficulties in interpretation arise from possible development of
tolerance. However, monitoring of pre- and post-exposure levels of
AChE in RBC gives a good measure of the effects of an exposure
(Kaloyanova, 1975). In cases where the pre-exposure AChE level is
not known (as in accidental poisoning), reference can be made to a
mean population AChE activity. Blood-plasma contains a related
enzyme called ChE or pseudoChE, which contributes to the whole-
blood enzymatic activity; the contribution of plasma-ChE in assays
of AChE will depend on the type and concentration of the substrate
used. PseudoChE has no known physiological function and can be
inhibited selectively by some compounds without causing a toxic
response. The sensitivities of AChE and ChE to inhibitors differ,
so that measurements of the ability of whole-blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many kinds of field conditions, procedures using whole blood, are
more practical than those using separated erythrocytes. Quite
commonly, pseudoChE is more sensitive to inhibitors. Thus, if
separation of plasma and erythrocytes is possible, prior to assay,
an indication of exposure can be obtained by assay of pseudoChE
only. Examples of selected organophosphorus insecticides, arranged
according to their ability to inhibit preferentially either plasma
or red cell-ChE in man, are given in Table 13 (Hayes, 1982).
Table 13. Selected organophosphorus insecticides arranged
according to their ability to inhibit either plasma- or
red cell-cholinesterase in mana
---------------------------------------------------------
Plasma enzyme more inhibited RBC enzyme more inhibited
---------------------------------------------------------
Chlorpyrifos Dimefox
Demeton Mevinphos
Diazinon Parathion
dichlorvos Parathion-methyl
malathion
Mipafox
Trichlorphon
---------------------------------------------------------
a Modified from: Hayes (1982).
ChE assay procedures vary greatly in sophistication, but the
most satisfactory is that based on the procedure of Ellman et al.
(1961). A field method and kit for whole blood- and plasma-ChE
determination have been developed (WHO, 1984b). Quick methods exist
for the determination of ChE in serum using paper tests (Izmirova,
1980) and for the colorimetric determination of ChE in whole blood
(Tintometer): these may be useful in the differential diagnosis of
organophosphate poisoning. Interpretation of the test results is
discussed in section 7.1.2.
The potential for delayed neuropathic response to an
organophosphorus ester can be predicted by the assay of the
esteratic activity of the target protein (NTE), in autopsy brain
samples from dosed adult hens (section 6.1.2). It has been shown
(Dudek et al., 1979; Richardson & Dudek, 1983) that a low level of
similar enzyme activity resides in lymphocytes and that there may
be correlations under some circumstances between neurotoxic dose
and available lymphocyte enzyme activity. The possibility of
monitoring exposed individuals by means of human lymphocyte or
platelet NTE activity is being explored (Lotti et al., 1983;
Bertocin et al., 1985; Maroni & Bleeker, 1986).
7.1.1.3 Electrophysiological methods for the study of effects
Electromyographic (EMG) studies using non-invasive surface
electrodes have been claimed to give sensitive indications of
exposure to organophosphorus pesticides, even in situations where
blood-ChE activity has returned to normal levels (Jager et al.,
1970; Roberts, 1976). The method requires electro-physiological
equipment and a very skilled practitioner. There is still
considerable doubt about the validity of some published studies.
Reproducibility is known to be very sensitive to local factors such
as temperature of the skin, and conflicting results have been
published, some of which show small increases and some, small
decreases in the ampli-tude of evoked muscle action potential, in
response to nerve stimulation. These findings have been reviewed
by LeQuesne & Maxwell (1981), who noted that changes that have been
reported tended not to be dose-related. In addition, they
evaluated the technique under controlled circumstances. In a
treatment to eradicate parasitic schistosomes, 55 children were
dosed orally with trichlorfon, 3 times, at 2-weekly intervals, at
doses that measurably depressed blood-ChE (mean 50%), but were not
enough to cause overt toxic effects, apart from mild cramps and
diarrhoea in a few cases. Only 3 children showed a significant
alteration in electromyographic response. Shortly after the last
(and highest) dose of 10 mg/kg body weight, 3 children developed
repetitive activity recorded over the thenar muscles following
supramaximal stimulation of the median nerve at the wrist. The
activity consisted of a small potential at the end of the main
muscle response and was characterized by being abolished by a
second stimulus 30 or 80 milliseconds after the first, or by
maximum voluntary contraction for 10 seconds; the amplitude of the
response to the second stimulus was not reduced. These
characteristics are necessary criteria that distinguish (these)
dose-related responses from pre-existing natural (and
idiosyncratic) responses, which can otherwise confuse EMG studies
in a population. Changes in amplitude measured on 52 control
subjects (mean 13.8 ħ 2.5 SD), on 2 occasions (2 weeks apart),
ranged from +5 to -3 mV. Thus, EMG does not appear to give a
highly sensitive measure of exposure to an ingested
organophosphorus compound.
7.1.2 Monitoring studies
Measurement of whole blood-AChE is the most widely adopted
method for monitoring the effects of occupational exposure to
organophosphorus insecticides. Physiological variations in blood-
ChE levels occur in a healthy person and are seen among a
population. It has been estimated that the coefficient of
variation for AChE activity in samples from an individual is 8 -
11%, and that a decrease of 23% below pre-exposure level may,
therefore, be considered significant. If the average of several
pre-exposure values were available, then a decrease of 17% would be
significant. It has been recommended that, if measured activity is
reduced by 30% or more of the pre-exposure value, AChE measurements
should be repeated at appropriate intervals to confirm the results.
Depressions of AChE or ChE in excess of 20 - 25% are considered
diagnostic of exposure but not, necessarily, indicative of hazard.
Depressions of 30 - 50% or more are considered indicators for
removal of an exposed individual from further contact with
pesticides until levels return to normal. Work procedures and
hygiene should also be checked (Zielhuis, 1972; WHO, 1975; CEC,
1977; Kaloyanova et al., 1979; Plestina, 1984).
Urinary metabolites have been monitored as a means of comparing
the efficiency of absorption of a pesticide by different routes.
The reports of the annual Joint FAO/WHO Working Parties,
mentioned previously, contain summaries of numerous controlled
exposure studies. No cases appear to be known of significant
clinical effects in man in the absence of depression of plasma- or
erythrocyte-ChE levels. No-observed-adverse-effect levels have
been calculated on this basis, where the data are available, or
have been estimated for man by extrapolation of the available data
for exposed animals.
7.1.3 Retrospective studies of populations exposed to
organophosphorus pesticides: acute and long-term exposure
Many thousands of cases of acute poisoning by organophosphorus
pesticides have been recorded (Namba et al., 1971). The majority
have been due to parathion and methyl parathion. Thus, Namba (1974)
in a discussion of the relative toxicities of parathion and
malathion, quoting Japanese Government statistics for the 7 years
1958-62, 1966, and 1967, stated that there were 3311 accidental or
occupational poisonings due to parathion including 188 deaths,
while for malathion, the numbers were 63 and 10, respectively. He
noted that the difference was not due to the restricted use of
malathion.
In the context of this general introduction, no descriptions
and breakdown will be given of retrospective studies of populations
exposed to organophosphorus pesticides. Such figures are relevant
to individual substances and will be given in the appropriate
Environmental Health Criteria.
It is generally thought that the only long-term effects
attributable to overt or subclinical acute intoxication with
organophosphorus compounds, or to prolonged low-level exposure,
are behavioural (rather doubtful), and delayed-onset neuropathy in
the case of certain compounds; these are dealt with in section 7.2.
>7.2 Other Effects on the Nervous and Neuromuscular System Due to
Acute or Long-Term Exposure
7.2.1 Delayed neuropathic effects
The characteristics of this disorder are given in section
6.1.2. The agents most commonly causing delayed neuropathy in man
are triaryl phosphate esters used in, e.g., hydraulic fluids; these
do not have any AChE activity and are not pesticides. In Table 14,
organophosphorus pesticides are listed for which there is
reasonable evidence that they have caused delayed neuropathy in
man.
Table 14. Organophosphorus pesticides reported to cause
delayed neuropathy in man
--------------------------------------------------------
Pesticide Number Reference
of cases
--------------------------------------------------------
mipafox 2 Bidstrup et al. (1953)
leptophos 8 Xintaras et al. (1978);
FAO/WHO (1979b)
methamidophos 9 Senanayake & Johnson
(1982)
trichlorphon many Shiraishi et al. (1977);
Hierons & Johnson (1978);
Johnson (1981a)
trichlornat 2 Jedrzejowska et al.
(1980); Willems (1981)
EPN 3a Xintaras & Burg (1980)
Chlorpyrifos 1 Lotti & Moretto (1986)
--------------------------------------------------------
a Moderate effects only and possibly other
etiological factors.
The cases with mipafox involved a single occupational exposure
to a compound that was developed before delayed neuropathy was a
recognized hazard. The cases with EPN and leptophos arose through
repeated occupational exposure with inadequate precautions.
Apparently cholinergic effects were often experienced, but not at
the level of severe poisoning. A few cases with methamidophos and
trichlorfon involved substantial occupational exposure, which
caused severe acute poisoning prior to the development of
neuropathy, but the majority of cases involved accidental or
deliberate ingestion of quantities that might well have been fatal
but for medical intervention. The fact that most of the cases
listed are due to exposure to phosphonates or phosphoramidates is
in line with the structure-activity relationships listed in section
6.1.2.
The value of measuring the neuropathy target esterase of human
lymphocytes as a predictive monitor was proposed by Dudek et al.
(1979) and Richardson & Dudek (1983). Lotti et al. (1983) reported
occupationally related changes in the lymphocyte NTE in spraymen
during seasonal spraying of DEF, but overt neuropathy was absent.
In a case of self-poisoning by a mixture of pesticides including
chlorpyrifos (about 20 g diluted in petroleum distillates),
Osterloh et al. (1983) noted that signs of cholinergic poisoning
were limited and they attributed death to the effects of
chlorophenoxyacetic acids. Brain esterases were not inhibited at
autopsy, but erythrocyte and peripheral nerve AChE levels were
about 22% of normal and nerve NTE was about 30%. This substantial
inhibition of NTE suggested that treated survivors of severe
poisoning by chlorpyrifos might well develop delayed neuropathy.
This prediction was confirmed in a recent case of self-intoxication
with chlorpyrifos (estimated dose 300 mg/kg body weight) in which
very low levels of lymphocyte-NTE were found, 30 days after
intoxication and after recovery from very severe cholinergic
effects. Typical moderate polyneuropathy developed in the
following days (Lotti & Moretto, 1985).
Allegations have been made against a few other organophosphorus
insecticides including malathion, omethoate, and parathion, though
many accidental and intentional poisonings by these agents have not
had any neuropathic sequelae. Experimental evidence against a
neuropathic potential in these compounds is strong (section 6.1.2).
However, in view of the serious paralytic effects involved, the
evidence adducing that these pesticides were the causal agents is
reviewed below.
(a) Malathion
Two alleged cases can be discounted. Petry (1958) reported a
case in which a physician contaminated himself frequently with
chlorinated hydrocarbon pesticides during day-long gardening
activities, about once per week, over a 10-year period. In 1954,
he commenced using 6% malathion in a "hose-on" device for garden
pest control and, in 1955, he commenced using 50% malathion in a
hand spray, both indoors and outside. He soon developed signs of
chronic anticholinesterase poisoning (generalized weakness and
tremor, irritability, difficulty in focusing). He eventually
collapsed and his general condition improved in hospital. However,
he continued to experience generalized weakness and particular
weakness in the right shoulder girdle, right serratus anterior, and
both peroneal muscle groups, and these symptoms persisted to some
extent for over a year. The distribution of these symptoms of
deficient muscle performance is quite atypical for delayed
neuropathy and seems more likely to be due to prolonged moderate
cholinergic insult from malathion precipitating weakness, anorexia,
and weight loss, which then precipitated further ill-health as
previously-stored chlorinated hydrocarbons was mobilized from
degraded fat stores. DDT at 23 mg/kg plus a high level of organic
chloride were found in a subcutaneous fat biopsy. A muscle-
necrotising effect, due to prolonged cholinergic stimulation, as
described in section 6.2.6, is also possible.
A separate case report of ascending paralysis following
malathion intoxication (Healy, 1959) concerned an 18-month-old
child exposed daily for 6 weeks to malathion from a garden spray.
Contamination was dermal and also by inhalation and ingestion,
leading ultimately to a cholinergic crisis and a prolonged period
of weakness including extensive flaccid paralysis for several days.
The condition responded to atropine and rest within 4 weeks. In
spite of the author's conclusion that this was a "demyelinating"
(i.e., delayed neuropathic) disorder, the picture is typical of
prolonged cholinergic insult responding to atropine and the
comparatively slow clearance of accumulated pesticide with
recovery from excessive nerve-muscle stimulation.
(b) Omethoate
A typical delayed neuropathy followed ingestion of an
organophosphorus pesticide with suicidal intent (Curtes et al.,
1979). Identification of the actual pesticide ingested was
doubtful depending on a later hearsay report concerning a bottle in
a garden shed, the contents of which were not analysed. Lotti et
al. (1981) assayed both the AChE and the NTE activities in
autopsied brain after a fatal intoxication with omethoate and found
that, even at the fatal dose, the NTE levels were normal while the
AChE activity was highly inhibited. Considering this data together
with evidence of similar non-inhibition in experimental hens at up
to 8 x the unprotected LD50, the authors concluded that, though the
neuropathy was typical, it was likely that the toxic agent was some
other organophosphorus compound more liable to cause neuropathy,
such as trichlorfon or trichlornat (Table 13).
(c) Parathion
Only two cases of permanent incapacity have been attributed to
parathion, though thousands of parathion poisonings are known (see
earlier). Petry (1951) attributed a case of neuropathy to the
aftermath of several occupational incidents of cholinergic
poisoning, but the history is entirely atypical in that symptoms
developed only 4 months, rather than 2 - 4 weeks, after the last
exposure. Causation by parathion is therefore very unlikely. A
farmer deliberately ingested parathion at a dose estimated to be at
least 150 g (perhaps 500 x the estimated human lethal dose) in 600
ml of methanol. Vigorous therapy preserved his life, though he
remained in a deep coma for 7 weeks. On recovery from this
experience, he was found to be suffering from flaccid paralysis of
both legs and weakness of both hands with muscle atrophy (De Jager
et al., 1981; 1982); partial recovery occurred during one year.
Lotti & Becker (1982b) have discussed the complicating factors of
the potentially supra-lethal dose of methanol and of the long coma.
However, the clinical picture is not unlike a true moderate
organophosphorus-induced neuropathy. At such a colossal dose, the
possibility of ingesting a significant amount of a neuropathic
impurity in the parathion must be recognized. This could be ethyl
bis-(4-nitrophenyl) phosphorothioate; small but significant
amounts of the appropriate oxon are present in some samples of
paraoxon made from diethyl phosphorochloridate (Johnson, 1982b),
and this oxon is a potent inhibitor of NTE.
(d) Other organophosphorus pesticides
Besides the atypical case attributed to malathion, Petry (1958)
described another case in which symptoms persisted after a
cholinergic crisis that followed severe intermittent exposures over
3 seasons to a variety of insecticides including parathion, EPN,
DDT, dieldrin, and lead arsenate. Some of the persistent symptoms
might be compatible with a mild peripheral neuropathy. Among the
agents used, lead arsenate and dieldrin would be expected to
contribute damage to the nervous system and EPN at about the LD50
level causes neuropathy in hens and man (Tables 4, 13).
A case of slow-onset profound weakness with complete recovery
within 3 months following contamination of an agricultural worker
with the cotton defoliant, merphos ( S,S,S -tri- n -butyl
phosphorothioite), was thought by the author to be of the delayed
neuropathy type (Fisher, 1977). However, the clinical picture
showed signs that are not seen in acute organophosphate
intoxication (influenza-like onset of the syndrome and high level
of protein in the spinal fluid). These signs are, however,
characteristic of a Guillan-Barré syndrome, which might have been
coincidental to the merphos exposure only 4 days previously. It is
possible that, later, a mild organophosphorus neuropathic effect
was superimposed on the Guillan-Barré effect, since merphos can
produce neuropathy in experimental animals (Johnson, 1970, 1975b).
Recovery from very mild neuropathies is usually complete.
7.2.2 Behavioural effects
Although many epidemiological studies have been carried out,
few controlled studies on man have been reported. It is generally
recognized that there are behavioural and psychic changes during
overt clinical poisoning by organophosphorus insecticides and that
these may take several months to regress (Karczmar, 1984).
However, there is no information to suggest that effects occur at
exposure levels that do not either alter ChE levels or produce
physical symptoms. Levin & Rodnitsky (1976) have reviewed the
literature, including their own work, on different aspects of
behaviour as affected by organophosphates. Much was based on
generalized complaints from workers occupationally exposed to many
agricultural chemicals (and probably also to automobile fuels and
lubricants and to alcohol). In summary, they found that, in human
subjects sufficiently exposed to organophosphates to depress
plasma- or erythrocyte-ChEs, some or all of the following
behavioural variables might be impaired. In cognition: vigilance,
information processing and psychomotor speed, and memory; in
speech: both performance and perception; in psychic state:
increased tendencies to depression, anxiety, and irritability; and
in EEG records: a tendency to faster frequencies and higher
voltages. They also concluded that the EEG abnormalities were
positively related to the level of AChE inhibition during the
initial stages of inhibition. Concerning studies on asymptomatic
workers at risk from repeated exposure to organophosphorus
pesticides, they considered that the evidence was equivocal for the
presence of less severe or latent forms of any behavioural
abnormalities. However, Duffy & Burchfield (1980) claimed that
changes in the EEG of individuals accidentally exposed to the
nerve-agent sarin could be detected twelve months after the
exposure. This study was a sequel to the study on monkeys
described in section 6.1.2, and the analyses of EEGs was performed
as noted there. They claimed significant differences (very small
differences analysed by complex statistical procedures) between the
group of sarin-exposed workers and controls, particularly in the
region of beta-rhythm (but the comment on the spread among normal
monkeys should be noted). However, the authors were unable to pick
out sarin-exposed individuals on the basis of the EEG. They also
found (small) increased amounts of REM-sleep in the exposed
workers. Without controlled exposure and serial monitoring of
effects in individuals, little can be deduced from these apparent
marginal changes.
7.3 Effects on Other Organs and Systems
Very few effects, other than those described in sections 7.1
and 7.2, have been noted, except those arising from ill health due
to severe anticholinesterase effects.
Several adverse effects attributed to only one organophosphorus
ester will be listed under the individual substances.
7.4 Treatment of Organophosphate Insecticide Poisoning in Man
All cases of organophosphorus poisoning should be dealt with as
an emergency and the patient sent to hospital as quickly as
possible. Although symptoms may develop rapidly, delay in onset or
a steady increase in severity may be seen up to 48 h after
ingestion of some formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major references
(Kagan 1977; Taylor 1980; HMSO, 1983; Plestina 1984) and will also
be included in the IPCS Health and Safety Guides to be prepared for
selected organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
7.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in cleaning the skin area where venupuncture
is performed. Blood might be contaminated with direct-acting
organophosphorus esters, and, therefore, inaccurate measures of ChE
inhibition might result. Extensive eye irrigation with water or
saline should also be performed. In the case of ingestion, vomiting
might be induced, if the patient is conscious, by the
administration of ipecacuanha syrup (10 - 30 ml) followed by 200 ml
water. This treatment is, however, contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also be
performed, particularly in unconscious patients, taking care to
prevent aspiration of fluids into the lungs (i.e., only after a
tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
7.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
7.4.3 Specific pharmacological treatment
7.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given at
15 to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be necessary
in extreme cases and total daily doses up to several hundred mg may
be necessary during the first few days of treatment.
7.4.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs (section 6.5.3). However,
if absorption, distribution, and metabolism are thought to be
delayed for any reasons, oximes can be administered for several
days after intoxication. Effective treatment with oximes reduces
the required dose of atropine. Pralidoxime is the most widely
available oxime. A dose of 1 g pralidoxime can be given either im
or iv and repeated 2 - 3 times per day or, in extreme cases, more
often. If possible, blood samples should be taken for AChE
determinations before and during treatment. Skin should be
carefully cleansed before sampling. Results of the assays should
influence the decision whether to continue oxime therapy after the
first 2 days.
The possible beneficial effects of oxime therapy on CNS-derived
symptoms is discussed in section 6.5.3.
7.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to counteract
some aspects of CNS-derived symptoms, which are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required (Vale & Scott, 1974). Other centrally acting
drugs and drugs that may depress respiration are not recommended in
the absence of artificial respiration procedures.
7.4.3.4 Notes on the recommended treatment
(a) Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
(b) Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinisation. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
(c) Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be
taken into, and then released from, fat depots over a period of
many days. It is therefore quite incorrect to abandon oxime
treatment after 1 - 2 days on the supposition that all inhibited
enzyme will be aged. Ecobichon et al. (1977) noted prompt
improvement in both condition and blood-ChEs in response to
pralidoxime given on the 11th - 15th days after major symptoms of
poisoning appeared due to extended exposure to fenitrothion (a
dimethyl phosphate with a short half-life for aging of inhibited
AChE).
(d) Dosage of atropine and oxime
The recommended doses above pertain to exposures, usual for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in
error, while drunk. Therapeutic dosages were progressively
increased up to 6 mg atropine iv every 15 min together with
continuous iv infusion of pralidoxime chloride at 0.5 g/h for 72 h,
from days 3 to 6 after intoxication. After considerable
improvement, the patient relapsed and further aggressive therapy
was given at a declining rate from days 10 to 16 (atropine) and to
day 23 (oxime), respectively. In total, 92 g of pralidoxime
chloride and 3912 mg of atropine were given and the patient was
discharged on the thirty-third day with no apparent sequelae.
REFERENCES
ABOU-DONIA, M.B., OTHMAN, M.A., TANTAWAY, G., ZAKI KHALIL, A.,
& SHAWER, M.F. (1974) Neurotoxic effect of leptophos.
Experientia (Basel), 30 : 63-64.
ABOU-DONIA, M.B., GRAHAM, D.G., & KOMEIL, A.A. (1979)
Delayed neurotoxicity of O -ethyl O -(2,4-dichlorophenyl)phenyl-
phosphonothioate: effects of a single oral dose on hens.
Toxicol. appl. Pharmacol., 49 : 293-303.
ABOU-DONIA, M.B., GRAHAM, D.G., TIMMONS, P.R., & REICHERT,
B.L. (1980) Late acute, delayed neurotoxic, and cholinergic
effects of S,S,S -tributyl phosphorotrithioate on the hen:
effect of route of administration. Neurotoxicology, 1 : 425-447.
ABOU-DONIA, M.B., MAKKAWY, H-A., & GRAHAM, D.G. (1982)
Coumaphos: delayed neurotoxic effect following dermal
administration in hens. J. Toxicol. environ. Health, 10 : 87-99.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and
structure activity relationships providing a high safety
factor for anti-cholinesterase insecticides. In: Proceedings
of the British Crop Protection Conference, Brighton, November
1977, Croydon, British Crop Protection Council, pp. 721-729.
ALDRDIGE, W.N. & NEMERY, B. (1984) Toxicology of
trialkylphosphorothioates with particular reference to lung
toxicity. Fund. appl. Toxicol., 4 : 5215-5223.
ALDRIDGE, W.N., MILES, J.W., MOUNT, D.L., & VERSCHOYLE, R.D.
(1979) The toxicological properties of impurities in
malathion. Arch. Toxicol., 42 : 95-106.
ALDRIDGE, W.N., DINSDALE, D., NEMERY, B., & VERSCHOYLE, R.D.
(1985) Some aspects of the toxicology of trimethyl and
triethyl phosphorothioates. Fund. appl. Toxicol., 5 : 47-60.
AMBRUS, A. & GREENHALGH, R., ed. (1984) Pesticide residue
analysis. Proceedings of a joint FAO/WHO course, Eger,
Hungary, 13-26 April, 1983, Copenhagen, World Health
Organization (Health Aspects of Chemical Safety, Interim
Document No. 14).
BAKER, E.L., Jr, ZACK, M., MILES, J.W., ALDERMAN, L.,
MCWILSON, W., DOBBIN, R.D., MILLER, S., & TEETERS, W.R.
(1978) Epidemiologic malathion poisoning in Pakistan malaria
workers. Lancet, 7 January : 31.
BARNES, J.M. (1975) Assessing hazard from prolonged and
repeated exposure to low doses of toxic substances. Br. med.
Bull., 31 : 196-200.
BARNES, J.M. & DENZ, F.A. (1951) The chronic toxicity of
p- nitrophenyl diethyl thiophosphate (E. 605): a long-term
feeding experiment with rats. J. Hyg., 49 : 430-441.
BARNES, J.M. & DENZ, F.A. (1954) The reaction of rats to
diets containing octamethyl pyrophosphoramide (schradan) and
O,O- diethyl S- ethylmercaptoethanol thiophosphate ("systox").
Br. J. ind. Med., 11 : 11-19.
BAYER (1971) Nemacur, Leverkusen, Bayer Chemical Company
(Technical Information Leaflet No. E.1-781/26734).
BECKER, E.L. & BARBARO, J.F. (1964) The enzymatic hydrolysis
of p- nitrophenyl ethylphosphonates by mammalian plasmas.
Biochem. Pharmacol., 13 : 1219-1227.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating
properties of organophosphates. Xenobiotica, 2 : 307-337.
BENKE, G.M. & MURPHY, S.D. (1975) The influence of age on
the toxicity and metabolism of methyl parathion and parathion
in male and female rats. Toxicol. appl. Pharmacol., 31 :
254-269.
BERTOCIN, D., RUSSOLO, A., CAROLDI, S., & LOTTI, M. (1985)
Neurotoxic esterase in human lympocytes. Arch. environ.
Health, 40 : 139-144..
BERTRAM, U., KASTEN, A., LULLMANN, H., & ZIEGLER, A. (1977)
Improved treatment of organophosphate intoxication by use of
scopolamine or dexetimide. Experientia (Basel), 33 : 1196-1197.
BICKFORD, A.A. & SPRAGUE, G.L. (1984) The significance of
background neurologic lesions in acute delayed neurotoxicity
studies: a comparison of neurohistopathologic lesions induced
in commercial hens by tri- O -tolyl phosphate (TOCP) with those
observed in negative control hens. In: Cranmer, J.M. & Hixson,
J.E., ed. Delayed neurotoxicity. Proceedings of the Delayed
Neurotoxicity Workshop, Urbana-Champaign, Illinois, 27-30
June, 1982, Little Rock, Intox Press.
BIDSTRUP, P.L., BONNEL, J.A., & BECKETT, A.G. (1953)
Paralysis following poisoning by a new organic phosphorus
insecticide (mipafox). Br. med. J., 1 : 1068-1072.
BIGNAMI, G. (1976) Behavioural pharmacology and toxicology.
Ann. Rev. Pharm. Toxicol., 16 : 329-366.
BIGNAMI, G., ROSIC, H., MICHALEK, H., MILOSEVIC, M., & GATTI,
G.L. (1975) Behavioural toxicity of anticholinesterase
agents. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Publishing Company, pp. 155-215.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The
distribution of dichlorvos in the tissues of mammals after its
inhalation or intravenous administration. Toxicol. appl.
Pharmacol., 31 : 243-253.
BLEAKLEY, P., NICHOL, A.W., & COLLINS, A.G. (1979) Diazinon
and porphyria cutanea tarda. Med. J. Aust., 1 : 314-315.
BOPP, C. & KOSMINSKY, B. (1975) [Hepato-dermal toxic
prophyria.] Med. Cut. I.L.N., 4 : 271-280 (in Portugese).
BOULDIN, T.W. & CAVANAGH, J.B. (1979) Organophosphorus
neuropathy. A teased-fiber study of the spatio-temporal spread
of axonal degeneration. Am. J. Pathol., 94 (2): 241-252.
BOYD, E.M. (1969) Dietary protein and pesticide toxicity in
male weaning rats. WHO Bull., 40 : 801-805.
BRADLEY, W.A. (1976) The pathology of delayed neurotoxicity
due to organophosphates. In: Baron, R.L., ed. Pesticide-
induced delayed neurotoxicity, Washington, US Environmental
Protection Agency, pp. 84-101 (EPA No. 600/1-76-025).
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison
of cholinesterase activity, residue levels, and urinary
metabolite excretion of rats exposed to organophosphorus
pesticides. J. agric. food Chem., 25 : 1353-1358.
BREALEY, C., WALKER, C.H., & BALDWIN, B.C. (1980) A-esterase
activities in relation to the differential toxicity of
pirimiphos-methyl to birds and mammals. Pestic. Sci., 11 :
546-554.
BRIMBLECOMBE, R.W., GREEN, D.M., STRATTON, J.A., & THOMPSON,
P.B.J. (1970) The protective actions of some anticholinergic
drugs in sarin poisoning. Br. J. Pharmacol., 39 : 822-830.
BRITISH AGROCHEMICAL ASSOCIATION (1983) Spray operator
safety study, London, British Agrochemical Association, pp.
1-9.
BUCHET, J.P., LAUWERYS, R., & ROELS, H. (1977) Long-term
exposure to organophosphorus pesticides and lipid metabolism
in the rat. Bull. environ. Contam. Toxicol., 17 : 175-183.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused
by a single massive dose of dichlorvos to adult hens. Toxicol.
Lett., 9 : 157-159.
CASIDA, J.E. & SANDERSON, D.M. (1963) Reaction of certain
phosphorothionate insecticides with alcohols and potentiation
by breakdown products. J. agric. food Chem., 11 : 91-96.
CAVANAGH, J.B. (1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system: an experimental study in
hens. J. Neurol. Neurosurg. Psychiatr., 17 : 163-172.
CEC (1977) Organophosphorus pesticides. Criteria
(dose/effect relationships) for organophosphorus pesticides,
Oxford, New York, Pergamon Press, 199 pp (Report of a Working
Group of Experts prepared for the Commission of the European
Communities, Directorate-General for Social Affairs, Health
and Safety Directorate).
CHEN, P.R.S. & DAUTERMAN, W.C. (1971) Studies on the
toxicity of dimethoate analogues and their hydrolysis by sheep
liver amidase. Pestic. Biochem. Physiol., 1 : 340-348.
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN,
R.J. (1980) Effects of low-level administration of
dichlorvos on adrenocorticotrophic hormone secretion, adrenal
cholesteryl ester, and steroid metabolism. Biochem.
Pharmacol., 29 : 635-641.
CLOTHIER, B., JOHNSON, M.K., & REINER, E. (1981) Interaction
of some trialkyl phosphorothiolates with acetylcholinesterase:
characterization of inhibition, aging, and reactivation.
Biochem. Biophys. Acta, 660 : 306-316.
CODEX ALIMENTARIUS COMMISSION (1984) Codex Alimentarius
Commission guide to Codex recommendations concerning pesticide
residues. VIII. Recommendations for methods of analysis of
pesticide residues, Rome, FAO/WHO (CAC/PR 8-1984).
COLEMAN, I.W., LITTLE, P.E., & BANNARD, R.A.B. (1962)
Cholinolytics in the treatment of anticholinesterase
poisoning. I. The effectiveness of certain cholinolytics in
combination with an oxime for treatment of sarin poisoning.
Can. J. Biochem. Physiol., 40 : 815-826.
COSTA, L.G., SCHWAB, B.W., & MURPHY, S.D. (1982) Tolerance
to anticholinesterase compounds in mammals. Toxicology, 25 :
79-97.
CRANMER, J.M. & HIXSON, J.E., ed. (1984) Delayed
neurotoxicity. Proceedings of the Delayed Neurotoxicity
Workshop, Urbana-Champaign, Illinois, 27-30 June, 1982, Little
Rock, Intox Press.
CURTES, J.P., DEVELAY, P., & HUBERT, J.P. (1979) Late
peripheral neuropathy due to an acute voluntary intoxication
by organo-phosporic compounds. In: International Congress on
Neurotoxicology, Varese, Italy, 27-30 September, 1979, Oxford,
Pergamon Press.
DANDLIKER, W.B., HICKS, A.N., LEVINSON, S.A., STEWART, K., &
BRAWN, R.J. (1979) Effects of pesticides on the immune
response, La Jolla, California, Scripps Clinic and Research
Foundation (EPA 600/1-79-039).
DAUGHTON, C.G., COOK, A.M., & ALEXANDER, M. (1979) Bacterial
and conversion of alkylphosphonates to natural products via
carbon-phosphorus bond cleavage. J. agric. food Chem., 27 :
1375-1382.
DAUTERMAN, W.C. (1971) Biological and nonbiological
modification of organophosphorus compounds. WHO Bull., 44 :
133-150.
DAVIES, J.E., BARQUET, A., FREED, V.H., HAGUE, R., MORGADE,
C., SONNEBORN, R.E., & VACLAVEK, C. (1975) Human pesticide
poisonings by a fat-soluble organophosphate insecticide. Arch.
environ. Health, 30 : 608-613.
DAVIS, C.S. & RICHARDSON, R.J. (1980) In: Spencer, P.S. &
Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 527-544.
DE JAGER, A.E.J., VAN WEERDON, T.W., HOUTOFF, H.J., & DE
MONCHY, J.G.R. (1981) Polyneuropathy after massive exposure
to parathion. Neurology, 31 : 603-605.
DE JAGER, A.E.J., VAN WEERDON, T.W., & MONCHY, J.G.R. (1982)
Reply to letter. Neurology, 32 : 218.
DESI, I. (1983) Neurotoxicological investigation of
pesticides in animal experiments. Neurobehav. Toxicol.
Teratol., 5 : 503-515.
DESI, I., FARKAS, I., SIMON, G., & CZIELESZKY, V. (1971)
[Investigation of the neurotoxic action of the phosphorus
compound bromophos.] Int. Arch. Arbeitsmed., 28 : 203-222 (in
German).
DESI, I., VARGA, L., & FARKAS, I. (1978) Studies on the
immunosuppressive effect of organochlorine and organo-
phosphoric pesticides in subacute experiments. J. Hyg.
Epidemiol. Microbiol. Immunol., 22 : 115-122.
DETTBARN, W.D. (1984) Pesticide-induced muscle necrosis:
mechanisms and prevention. Fund. appl. Toxicol., 4 : 18-26.
DHSS (1983) Pesticide poisoning. Notes for the guidance of
medical practitioners, London, Department of Health and Social
Security, pp. 41-47.
DIGGORY, H.J.P., LANDRIGEN, P.J., LATIMER, K.P., ELLINGTON,
A.C., KIMBROUGH, R.D., LIDDLE, J.D., CLINE, R.E., & SMERK,
A.L. (1977) Fatal parathion poisoning caused by contamination
of flour in international commerce. Am. J. Epidemiol.,
106 : 145-153.
DIMOV, G. & KALOYANOVA, F. (1967) Carbohydrate metabolism
disorders in the liver and muscles in acute parathion
poisonings. C R Acad. Bulg. Sci., 20 : 1007-1009.
DINSDALE, D., VERSCHOYLE, R.D., & CABRAL, J.R.P. (1982)
Cellular responses to trialkylphosphorothioate-induced injury
in rat lung. Arch. Toxicol., 51 : 79-89.
DONNINGER, C. (1971) Species specificity of phosphate
triester anticholinesterases. WHO Bull., 44 : 265-268.
DOROUGH, H.W. (1976) Biological activity of pesticide
conjugate. In: Kaufman, D.D., ed. Bound and conjugated
pesticide residues, Washington DC, American Chemical Society,
pp. 11-34 (ACS Symposium Series No. 29).
DREVENKAR, V., FINK, K., STIPCEVIC, M., & TOKALCEVIC, B.
(1976) The fate of pesticides in aquatic environment. II.
Hydrolysis of dichlorvos in model system and in river water.
Arh. hig. rada, 27 : 297-305.
DREVENKAR, V., STIPCEVIC, M., STENGL, B., & STEFANAC, Z.
(1979) Gas chromatographic determination of alkali metal
O,O- diethylphosphorodithioate present in tract amounts.
Microchim. Acta, 1 : 385-394.
DUBOIS, K.P. (1963) Toxicological evaluation of the anti-
cholinesterase agents. In: Koelle, G.B., ed. Handbook on
experimental pharmacology, Berlin, Springer-Verlag, Vol. 15,
pp. 833-857.
DUDEK, B.R., BARTH, M., GEPHART, J., HUGGINS, J., & RICHARD-
SON, R.J. (1979) Correlation of brain and lymphocyte neuro-
toxic esterase inhibition in the adult hen following dosing
with neurotoxic compounds. Toxicol. appl. Pharmacol., 48 : A198.
DUFFY, F.H. & BURCHFIELD, J.L. (1980) Long-term effects of
the organophosphate sarin on EEGs in monkeys and humans.
Neurotoxicology, 1 : 667-689.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the
exposure of workers to pesticides. WHO Bull., 26 : 75-91.
DURHAM, W.F., WOLFE, H.R., & ELLIOTT, J.W. (1972) Absorption
and excretion of parathion by spraymen. Arch. environ. Health,
24 : 381-387.
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S.
(1977) Acute fenitrothion poisoning. Can. Med. Assoc. J.,
116 : 377-379.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7 : 88-95.
EL-SEBAE, A.H. (1980) Biochemical challenges in future
toxicological research. J. environ. Sci. Health, B15 : 689-721.
EL-SEBAE, A.H., SOLIMAN, S.A., AHMED, N.S., & CURLEY, A.
(1981) Biochemical interaction of six op delayed
neurotoxicants with several neurotargets. J. environ. Sci.
Health, B16 (4): 465-474.
ENAN, E.E., EL-SEBAE, A.H., ENAN, O.H., & EL-FIKI, S. (1982)
In vivo interaction of some organophosphorus insecticides with
different biochemical targets in white rats. J. environ. Sci.
Health, B17 : 549-570.
ETO, M. (1974) Organophosphorus pesticides: organic and
biological chemistry, Boca Raton, Florida, CRC Press.
ETO, M. & OHKAWA, H. (1970) Alkylation reaction of
organophosphorus pesticides: its chemical and biochemical
significance. In: Biochemical toxicology of insecticides, New
York, London, Academic Press, pp. 93-104.
FAO (1984) Production yearbook, Rome, Food and Agriculture
Organization of the United Nations, Vol. 38.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23 (1964)).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report, No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report, No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues (FAO Agricultural Studies No.
73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party and the WHO Expert
Committee, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1971/M/9/1;
WHO Pesticide Residues Series No. 1).
FAO/WHO (1973a) Pesticide residues in food. Report of the
1972 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 90; WHO Technical Report Series No. 525).
FAO/WHO (1973b) 1972 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1972/M/9/1;
WHO Pesticide Residues Series No. 2).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection
Paper 10 Rev).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide Residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide Residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide Residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide Residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986) Pesticide Residues in food. Report of the
1985 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 68).
FAO/WHO (in press) 1985 Evaluations of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper).
FARM CHEMICALS HANDBOOK (1985) Willoughby, Ohio, Meister
Publishing Company.
FENICHEL, G.M., KIBLER, W.B., OLSON, B.A., & DETTBARN, W.D.
(1972) Chronic inhibition of cholinesterase as a cause of
myopathy. Neurology, 22 : 1026-1033.
FISHER, J.R. (1977) Guillain-Barré syndrome following
organophosphate poisoning. J. Am. Med. Assoc., 238 : 1950-1951.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14 : 514-534.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health
hazards on use of dichlorvos (DDVP), especially in resin
strips. Res. Rev., 44 : 115-157.
GRAY, A.P. (1984) Design and structure-activity relationships
of antidotes to organophosphorus anticholinesterase agents.
Drug. Metab. Rev., 15 (3): 557-589.
GREEN, D.M., MUIR, A.W., STRATTON, J.A., & INCH, T.D. (1977)
Dual mechanism of the antidotal action of atropine-like drugs
in poisoning by organophosphorus anticholinesterase. J. Pharm.
Pharmacol., 29 . 62-64.
GREENHALGH, R., DHAWAN, K.L., & WEINBERGER, P. (1980)
Hydrolysis of fenitrothion in model and natural aquatic
systems. J. agric. food Chem., 28 : 102-105.
GROB, D. (1963) Anticholinesterase intoxication in man and
its treatment. In: Koelle, G.B., ed. Handbook of experimental
pharmacology, Berlin, Springer-Verlag, Vol. 15, pp. 989-1027.
GUNTHER, F.A., WESTLAKE, W.E., BARKELY, J.H., WINTERLIN, W., &
LANGBEHN, L. (1973) Establishing dislodgeable pesticide
residues on leaf surfaces. Bull. environ. Contam. Toxicol., 9 :
243-250.
GUNTHER, F.A., WESTLAKE, W.E., & BARKELY, J.H. (1974) Worker
environment research. II. Sampling and processing techniques
for determining dislodgeable pesticide residues on leaf
surfaces. Bull. environ. Contam. Toxicol., 12 : 641-645.
HANSEN, L.G. & HANSEN, T.K. (1985) Intravenous dosing
abolishes differences in delayed neurotoxicity potency between
leptophos and desbromoleptophos. Pestic. Biochem. Physiol.,
24 : 136.
HAYES, G.R., FUNCKES, A.J., & HARTWELL, W.V. (1964) Dermal
exposure of human volunteers to parathion. Arch. environ.
Health, 8 : 829-833.
HAYES, W.J., Jr (1971) Studies on exposure during the use of
anticholinesterase pesticides. WHO Bull., 44 : 277-288.
HAYES, W.J., Jr (1975) Diagnosis and treatment of poisoning.
In: Toxicology of pesticides, Baltimore, Maryland, Williams
and Wilkins Company, pp. 409-417.
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, 561 pp.
HEALY, J.K. (1959) Ascending paralysis following malathion
intoxication: a case report. Med. J. Aust., 46 : 765-767.
HEATH, D.F. & VANDEKAR, M. (1957) Some spontaneous reactions
of O,O- dimethyl S- ethylthioethyl phosphorothiolate and related
compounds in water and on storage, and their effects on the
toxicological properties of the compounds. Biochem. J., 67 :
187-202.
HIERONS, R. & JOHNSON, M.K. (1978) Clinical and
toxicological investigations of a case of delayed neuropathy
in man after acute poisoning by an organophosphorus pesticide.
Arch. Toxicol., 40 : 279-284.
HOLLINGSHAUS, J.G., ABU-EL-HAJ, S., & FUKUTO, T.R. (1979)
Delayed neurotoxicity of O -alkyl O -aryl phenylphosphonothioate
analogues related to leptophos administered orally to the hen.
J. agric. food Chem., 27 : 1197-1201.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus
cholinesterase inhibitors. Pharmacol. Rev., 11 : 567-688.
HUANG, X.S., SHU, W.A., ZU, W.C., LAI, M.T., TSIN, Z.A., &
KWOK, C.S. (1979) [Toxicity studies of the herbicide
Amiprophos.] Chekiang Univ. Sch. Med. Bull., 8 : 63-66 (in
Chinese).
HUFF, J.E., BATES, R., EUSTIS, S.L., HASEMAN, J.K., &
MCCONNELL, E.E. (1985) Malathion and malaoxon: histo-
pathology reexamination of the National Cancer Institute's
Carcinogenesis Studies. Environ. Res., 37 : 154-173.
HUTSON, D.H. & HATHWAY, D.E. (1967) Toxic effects of
chlorofenvinphos in dogs and rats. Biochem. Pharmacol., 16 :
949-962.
IARC (1979) Dichlorvos. In: Some halogenated hydrocarbons,
Lyons, International Agency for Research on Cancer, pp. 97-127
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 20).
IARC (1983) Miscellaneous chemicals, Lyons, International
Agency for Research on Cancer (Monographs on the Carcinogenic
Risk of Chemicals to Humans, Vol. 30).
IMAI, H. (1977) Experimental pigmentary degeneration of the
retina caused by an organophosphorus pesticide (fenthion).
Acta soc. ophthalmol., 81 : 925-932 (Abstract 245, Exerp. Med.
(1979), 9 : 53).
IMAMURA, T., GANDY, J., FUKUTO, T.R., & TALBOT, P. (1983) An
impurity of malathion alters the morphology of rat lung
bronchiolar epithelium. Toxicology, 26 : 73-79.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISHIKAWA, S. & MIYATA, M. (1980) Development of myopia
following chronic organophosphate pesticide intoxication: an
epidemiological and experimental study. In: Merigan, W.H. &
Weiss, B., ed. Neurotoxicity of the visual system, New York,
Raven Press, pp. 233-254.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorus pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application. Proceedings of the
Fifth International Workshop of the Scientific Committee on
Pesticides of the International Association on Occupational
Health, The Hague, The Netherlands, 9-11 October, 1979,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 169-172.
JAGER, K.W., ROBERTS, D.V., & WILSON, A. (1970) Neuro-
muscular function in pesticide workers. Br. J. ind. Med., 27 :
273-278.
JASH, N.B. & BHATTACHARYA, S. (1982) Neurotoxicity of
phenthoate to a non-target species. In: Proceedings of the 5th
International Congress on Pesticide Chemicals, Kyoto, 29
August, 1982 (Abstract No. V1a-15).
JEDRZEJOWSKA, H., ROWINSKA-MARCINSKA, K., & HOPPE, B. (1980)
Neuropathy due to phytosol (agritox): report of a case. Acta
neuropathol., 49 : 163-168.
JOHNSON, D.D. & WILCOX, W.C. (1975) Studies on the mechanism
of the protective and antidotal actions of diazepam in
organophosphate poisoning. Eur. J. Pharmacol., 34 : 127-132.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of the brain "neurotoxic esterase" and the development of
delayed neurotoxicity in hens. Biochem. J., 120 : 523-531.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. Crit. Rev.
Toxicol., 3 : 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing
delayed neurotoxic effects: mechanism of action and structure/
activity studies. Arch. Toxicol., 34 : 259-288.
JOHNSON, M.K. (1977) Improved assay of neurotoxic esterase
for screening organophosphates for delayed neurotoxicity
potential. Arch. Toxicol., 37 : 113-115.
JOHNSON, M.K. (1980) Delayed neurotoxicity induced by
organophosphorus compounds: areas of understanding and
ignorance. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard
evaluation, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 27-38.
JOHNSON, M.K. (1981a) Do trichlorphon and/or dichlorvos
cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol. Scand., 49 (Suppl. 5): 87-98.
JOHNSON, M.K. (1981b) Grading of non-neurotoxicity of
organophosphorus pesticides and plasticisers by in vivo/in
vitro assay of neurotoxic esterase. In: Proceedings of the
European Society of Toxicologists, Dublin, 17-19 August
(Abstract No. 66).
JOHNSON, M.K. (1982a) The target for initiation of delayed
neurotoxicity by organophosphorus esters: biochemical studies
and toxicological applications. In: Hodgson, E., Bend, J.R., &
Phillip, R.M., ed. Reviews in biochemistry and toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 4, pp. 141-212.
JOHNSON, M.K. (1982b) Check your paraoxon and parathion for
neurotoxic impurities. Vet. hum. Toxicol., 24 (3): 220a.
JOHNSON, M.K. (1984) Delayed neurotoxicity tests of
organophosphorus esters: a proposed protocol integrating
neuropathy target esterase (NTE) assays with behaviour and
histopathology tests to obtain more information more quickly
from fewer animals. In: El-Sebae, A.H., ed. Proceedings of the
International Conference on Environmental Hazards of
Agrochemicals in Developing Countries, Alexandria, Egypt, 8-12
November, 1983, Alexandria, University of Alexandria, Vol. 1,
pp. 474-493.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus
pesticides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269
(in Russian).
KAGAN, JU.S. (1985) [Principles of toxicology,] Moscow,
United Nations Environment Programme (in Russian).
KAHN, E. (1979) Outline guide for performance of field
studies to establish safe reentry intervals for
organophosphorus pesticides. Residues Rev., 70 : 27-43.
KALOYANOVA-SIMEONOVA, F. (1961) Effet du chlorthion sur les
réflexes moteurs conditionnés des rats albinos. Act. nerv.
super., 3 : 284-288.
KALOYANOVA, F. (1979) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Conference on
Environmental Sensing and Assessment, Las Vegas, Nevada,
14-19 September, 1975, Vol. 1.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J.,
ed. IUPAC pesticide chemistry - Human welfare and the
environment, Oxford, New York, Pergamon Press, pp. 237-238.
KALOYANOVA, F. & TASHEVA, M. (1983) Effect of protein
malnutrition on toxicity of pesticides. In: Miyamoto, J. &
Kearney, P.C., ed. IUPAC pesticide chemistry, human welfare,
and the environment, Oxford, New York, Pergamon Press,
pp. 527-529.
KALOYANOVA, F., BENCHEV, I., GHEORGHIEV, G., IZMIROVA, N., &
RISOV, N. (1979) Pesticides and persistent substances.
Biological specimen collection. In: Berlin, A., Wolff, A.H., &
Hosegawa, J., ed. Biological specimens used for the assessment
of human exposure to environmental pollutants, Sophia,
Martinus N. Publishers, pp. 231-242.
KARCZMAR, A.G. (1984) Acute and long-lasting actions of
organophosphorus agents. Fundam. appl. Toxicol., 4 : S1-S7.
KARCZMAR, A.G. & OHTA, Y. (1981) Neuromyopharmacology as
related to anticholinesterase action. Fundam. appl. Toxicol.,
1 : 135-142.
KATO, T., NAKANO, T., KOJIMA, K., NAGATSU, T., & SAKAKIBARA,
S. (1980) Changes in propyl endopeptidase during maturation
of the rat brain and hydrolysis of substance P by the purified
enzyme, J. Neurochem., 35 : 527-535.
KAUFMANN, D.D., ed. (1976) Bound and conjugated pesticide
residues, Washington DC, American Chemical Society, pp. 1-10
(ACS Symposium Series No. 29).
KEPLINGER, M.L. & DEICHMANN, W.B. (1967) Acute toxicity of
combinations of pesticides. Toxicol. appl. Pharmacol., 10 :
586-595.
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic
phosphorus compounds and alkylating agents on the rat fetus.
Arch. environ. Health, 16 : 805-808.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides: Techniques for establishing safe levels of foliar
residues. Residues Rev., 75 : 81-96.
KNOX, B., ASKAA, J., BASSE, A., BITSCH, V., ESKILDSEN, M.,
MANDRUP, M., OTTOSEN, H.E., OVERBY, E., PEDERSEN, K.B., &
RASMUSSEN, F. (1978) Congenital ataxia and tremor with
cerebellar hypoplasia in piglets born by sows treated with
NeguvonR vet (metrifonate, trichlorfon) during pregnancy.
Nord. Vet. Med., 30 : 538-545.
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the
seminiferous epithelium and the Leydig cells of the rat testis
after the application of dichlorvos. Bull. environ. Contam.
Toxicol., 11 (5): 429-433.
LAUWERYS, R.L. & MURPHY, S.D. (1969) Interaction between
paraoxon and tri- o- tolyl phosphate in rats. Toxicol. appl.
Pharmacol., 14 : 348-357.
LE QUESNE, P.M. & MAXWELL, I.C. (1981) Effect of metrifonate
on neuromuscular transmission. Acta pharmacol. toxicol.
Scand., 49 (Suppl. 5): 99-104.
LEVIN, H.S. & RODNITZKY, R.L. (1976) Behavioural effects of
organophosphate pesticides in man. Clin. Toxicol., 9 (3):
391-405.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating
properties of 2,2-dichlorovinyl dimethyl phosphate: a
disregarded hazard. Environ. Mutat. Soc. Newslett., 2 : 21-27.
LOTTI, M. & BECKER, C.E. (1982a) Treatment of acute
organophosphate poisoning: evidence of a direct effect on
central nervous system by 2-PAM (pyridine-2-aldoxime methyl
chloride). J. Toxicol. clin. Toxicol., 19 : 121-127.
LOTTI, M. & BECKER, C.E. (1982b) Letter: polyneuropathy and
exposure to parathion. Neurology, 32 : 317.
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of
organophosphorus pesticides: predictions can be based on in
vitro studies with hen and human enzymes. Arch. Toxicol., 41 :
215-221.
LOTTI, M. & JOHNSON, M.K. (1980) Repeated small doses of
neurotoxic organophosphate: monitoring of neurotoxic esterase
in brain and spinal cord. Arch. Toxicol., 45 : 263-271.
LOTTI, M. & MORRETTO, A. (1986) Inhibition of lymphocyte
neuropathy target esterase predicts the development of
organophosphate polyneuropathy in man. Hum. Toxicol., 5 : 114.
LOTTI, M., FERRARA, S.D., CAROLDI, S., & SINIGAGLIA, F.
(1981) Enzyme studies with human hen autopsy tissue suggest
omethoate does not cause delayed neuropathy in man. Arch.
Toxicol., 48 : 265-270.
LOTTI, M., BECKER, C.E., AMINOFF, M.J., WOODROW, J.E., SEIBER,
J.N., TALCOTT, R.E., & RICHARDSON, R.J. (1983) Occupational
exposure to the cotton defoliants DEF and MERPHOS. J. occup.
Med., 25 : 517-522.
LOTTI, M., BECKER, C.E., & AMINOFF, M.J. (1984) Organo-
phosphate polyneuropathy: pathogenesis and prevention.
Neurology, 34 : 658-662.
LOTTI, M., BERTONCIN, D., & MORETTO, A. (1986) Organo-
phosphate induced delayed polyneuropathy (OPIDP) by chlor-
pyrifos in man and hens. Toxicologist, 6 : 22 (Abstract No. 86).
LUDKE, J.L. (1977) DDE increases the toxicity of parathion
to corturnix quail. Pestic. Biochem. Physiol., 1 : 28-33.
MACHIN, A.P., ANDERSON, P.H., HOWELLS, L.C., & DUNDY, D.E.
(1978) Enzymic degradation of phosphorothionate oxons in the
plasma of species of varying susceptibility to poisoning. In:
Proceedings of the 4th IUPAC International Congress on
Pesticides and Chemicals, Zurich, 24-28 July, 1978 (Abstract
V.620).
MALLIPUDI, N.M., UMETSU, N., TOIA, R.F., TALCOTT, R.E., &
FUKUTO, T.R. (1979) Toxicity of O,O,S -trimethyl and triethyl
phosphorothioate to the rat. J. agric. food Chem., 27 : 463-466.
MALONE, J.C. (1964) Toxicity of haloxon. Res. vet. Sci., 5 :
17-31.
MAQUIS, J.K. (1985) Non-cholinergic mechanisms of
insecticide toxicity. Trends pharmacol. Sci., 6 (2): 59-60.
MARGOT, A. & GYSIN, H. (1957) [Diazinon: its decomposition
product and their properties.] Helv. Chim. Acta, 40 (162):
1562-1573 (in German).
MARONI, M. & BLEEKER, M.L. (1986) Neuropathy target esterase
in human lymphocytes and platelets. J. appl. Toxicol., 6 : 1-7.
MARSHALL, T.C. & DOROUGH, H.W (1977) Bioavailability in rats
of bound and conjugated plant carbamate insecticide residues.
J. agric. food Chem., 25 (5): 1003-1009.
MEDVED, L.I. & KAGAN, JU.S. (1983) Pesticides, organophos-
phorus. In: Parmeggiani, L., ed. Encyclopaedia of occupational
health and safety, 3rd ed., Geneva, International Labour
Office, pp. 1637-1646.
MEIER, E.P., DENNIS, W.H., ROSENCRANCE, A.B., RANDALL, W.F.,
COOPER, W.J., & WARNER, M.C. (1979) Sulfotepp: a toxic
impurity in formulations of diazinon. Bull. environ. Contam.
Toxicol., 23 : 158-164.
MEL'NIKOV, N.N., ET AL. (1985) [Pesticide handbook,] Moscow,
Himija Press (in Russian).
MENDOZA, C.E. (1976) Toxicity and effects of malathion on
esterases of suckling albino rats. Toxicol. appl. Pharmacol.,
35 : 229-238.
MILBY, T.H., OTTOBONI, F., & MITCHELL, H.W. (1964) Parathion
residue poisoning among orchard workers. J. Am. Med. Assoc.,
189 : 351-358.
MILES, J.W., MOUNT, D.L., STAIGER, M.A., & TEETERS, W.R.
(1979) S- methyl isomer content of stored malathion and
fenitrothion water-dispersible powders and its relationship to
toxicity. J. agric. food Chem., 27 : 421-425.
MIYAMOTO, J. (1976) Degradation, metabolism, and toxicity of
synthetic pyrethroids. Environ. Health Perspect., 14 : 15-28.
MIYAMOTO, J. & OHKAWA, H. (1978) Oxidative processes in
pesticide transformation. In: Proceedings of the 4th IUPAC
International Congress on Pesticides and Chemicals, Zurich,
24-28 July, 1978.
MURPHY, S.D. (1969) Mechanisms of pesticide interactions in
vertebrates. Res. Rev., 25 : 201-221.
NAKATSUGAWA, T., TOLMAN, N.M., & DHAM, P.A. (1968)
Degradation and activation of parathion analogues by
microsomal enzymes. Biochem. Pharmacol., 17 : 1517-1528.
NAMBA, T. (1974) Relative toxicity of malathion (contd). New
Engl. J. Med., 290 : 347.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisoning due to organophosphate insecticides: acute and
chronic manifestations. Am. J. Med., 50 : 475-492.
NICHOL, A.W., ELSBURY, S., ELDER, G.H., JACKSON, A.H., &
NAGARAJA RAO, K.R. (1982) Separation of impurities in
diazinon preparations and their effect on porthyrin
biosynthesis in tissue culture. Biochem. Pharmacol., 31 :
1033-1038.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22 : 16-18 (in
Japanese).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitaikagaku ecol. Chem., 4 : 31-46 (in Japanese).
O'BRIEN, R.C. (1967) Insecticides: action and metabolism,
New York, Academic Press, pp. 32-54.
OECD (1983) Acute delayed neurotoxicity of organophosphorus
substances: subchronic delayed neurotoxicity of organo-
phosphorus substances: 90-day study, Paris, Organization of
Economic Cooperation and Development (OECD Guidelines for the
Testing of Chemicals, Nos. 418, 419).
OHKAWA, H., OSHITA, H., & MIYAMOTO, J. (1980) Comparison of
inhibitory activity of various organophosphorus compounds
against acetylcholinesterase and neurotoxic esterase of hens
with respect to delayed neurotoxicity. Biochem. Pharmacol.,
29 : 2721-2727.
O'NEIL, J.J. (1981) Non-cholinesterase effects of
anticholinesterases, Fundam. appl. Toxicol., 1 : 154-160.
OSTERLOH, J., LOTTI, M.D., & POND, S.M. (1983) Toxicological
studies in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos.
J. anal. Toxicol., 7 : 125-129.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78 : 78-87.
PELLEGRINI, G. & SANTI, R. (1972) Potentiation of toxicity
of organophosphorus compounds containing carboxylic ester
functions toward warm-blooded animals by some organophosphorus
impurities. J. agric. food Chem., 20 : 944-950.
PETRY, C.S. (1958) Organic phosphate insecticide poisoning.
Am. J. Med., 24 : 467-470.
PETRY, H. (1951) [Polyneuritis from E 605.] Zentralbl.
Arbeitsmed. Arbeitssch., 1 : 86-89 (in German).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PRENTICE, D.E. & ROBERTS, N.L. (1984) Acute delayed
neurotoxicity in hens dosed with tri-ortho-cresyl phosphate
(TOCP): correlation between clinical ataxia and neuropatho-
logical findings. In: Cranmer, J.M. & Hixson, J.E., ed.,
Delayed neurotoxicity. Proceedings of the Delayed Neuro-
toxicity Workshop, Urbana-Champaign, Illinois, 27-30 June,
1982, Little Rock, Intox Press.
PREUSSMANN, R., SCHNEIDER, H., & EPPLE, F. (1969)
[Investigation of effects of alkylating agents. II. The
investigation of different classes of alkylating agents by
means of a modified colour reaction with 4-(4-nitro-benzyl)
pyridine (NBP).] Arzneimittelforschung, 7 : 1059-1073 (in
German).
RAY, D.E. (1980) Selective inhibition of thermogenesis by
tributyl S,S,S -phosphorotrithioate (DEF). Br. J. Pharmacol.,
69 : 257-264.
RAY, D.E. & CUNNINGHAM, V.J. (1985) Hypothermia produced by
S,S,S -phosphortrithioate (DEF). Arch. Toxicol., 56 : 279-282.
REITER, L.W., TALENS, G.M., & WOOLEY, D.E. (1975) Parathion
administration in the monkey: time course of inhibition and
recovery of blood cholinesterases and visual discrimination
performance. Toxicol. appl. Pharmacol., 33 : 1-13.
REUBER, M.D. (1985) Carcinogenicity and toxicity of
malathion and malaoxon. Environ. Res., 37 : 119-153.
REVZIN, A.M. (1980) Effects of organophosphate pesticides
and alcohol on visual mechanisms. In: Merigan, W.H. & Weiss,
B., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 255-268.
REVZIN, A.M. (1983) Neurophysiological and behavioural
assessment of pesticide toxicity. In: Miyamoto, J. & Kearney,
P.C., ed. Pesticide chemistry: human welfare and the
environment, Oxford, New York, Pergamon Press, Vol. 3, pp.
419-424.
RICHARDSON, R.J. & DUDEK, B.R. (1983) Neurotoxic esterase:
characterization and potential for a protective screen for
exposure to neuropathic organophosphates. In: Miyamoto, J.,
ed. Proceedings of the IUPAC Pesticide Chemistry Congress on
Human Welfare and the Environment, Oxford, New York, Pergamon
Press, pp. 491-495.
RICO, A.G. & BURGAT-SACAZE, V. (1984) Toxicological
significance of covalently-bound residues. Food Add. Contam.,
1 (2): 157-161.
ROBERTS, D.V. (1976) EMG voltage and motor nerve conduction
velocity in organophosphorus pesticide factory workers. Int.
Arch. occup. environ. Health, 36 : 267-274.
SALPETER, M.M., KASPRZAK, H., FENG, H., & FERTUCK, H. (1979)
Endplates after esterase inactivation in vivo: correlation
between esterase concentration, functional response, and fine
structure. J. Neurocytol., 8 : 95-115.
SANBORN, J.R., METCALF, R.L., & HANSEN, L.G. (1977) The
neurotoxicity of O -2(2,5-dichlorophenyl) O -methyl phenylphos-
phonothioate, an impurity and photoproduct of leptophos
(PhosveR ) insecticide. Pestic. Biochem. Physiol., 7 : 142-145.
SCHAFER, E.W. (1972) The acute oral toxicity of 369
pesticidal, pharmaceutical and other chemicals to wild birds.
Toxicol. appl. Pharmacol., 21 : 315-330.
SCHOENE, J. & OLDIGES, H. (1973) [Antidotal action of
pyridinium salts for tabun and sarin poisoning in vivo and in
vitro. ] Arch. int. Pharmacodyn. Ther., 204 : 110-123 (in
German).
SEIFERT, J. & CASIDA, J.E. (1980) Mechanisms of terato-
genesis induced by organophosphorus and methylcarbamate
insecticides. In: Progress in pesticide biochemistry,
Berkeley, California, University of California.
SENANAYAKE, N. & JOHNSON, M.K. (1982) Acute polyneuropathy
following poisoning by a new organophosphate insecticide: a
preliminary report. New Eng. J. Med., 306 : 155-157.
SHAFIK, T., BRADWAY, D.E., ENOS, E.F., & YOBS, A.R. (1973)
Human exposure to organophosphorus pesticides. A modified
procedure for the gas-liquid chromatographic analysis of alkyl
phosphate metabolites in urine. J. agric. food Chem., 21 (4):
625.
SHIRAISHI, S., GOTO, I., YAMASHITA, Y., ONISHI, A., & NAGAO,
H. (1977) [Dipterex polyneuropathy.] Neurol. Med. (Japan),
6 : 34-38 (in Japanese with English summary).
SHTENBERG, A.I., KHOVAEVA, L.A., & ZAVARZINA, M.V. (1974)
Effect of chlorophos and methyl nitrophos on the immune
reactions of the organism against the background of
protein-deficient nutrition. Vopr. Pitan., 8 (4): 35-42.
SOLIMAN, S.A. (1982) Delayed neuropathy in hen by the
insecticide synergist O-n- propyl O- (2-propynyl)phenylphos-
phonate (NIA 16388) and other phenylphosphonate esters. J.
Toxicol. environ. Health, 10 : 907-920.
SOLIMAN, S.A., FARMER, J., & CURLEY, A. (1982) Is delayed
neuropathy a property of all organophosphorus compounds?
Toxicology, 23 : 267-279.
SOLIMAN, S.A., SOVOCOOL, G.W., CURLEY, A., AHMED, N.S.,
EL-FIKI, S., & EL-SEBAE, A.H. (1982) Two acute human
poisoning cases resulting from exposure to diazinon
transformation products in Egypt. Arch. environ. Health, 37 :
207-212.
SPEAR, R.C., POPENDORF, W.J., SPENCER, W.F., & MILBY, T.H.
(1977) Worker poisonings due to paraoxon residues. J. occup.
Med., 19 : 411-414.
STAPLES, R.E. & GOULDING, E.H. (1979) Dipterex
teratogenicity in the rat, hamster, and mouse when given by
gavage. Environ. Health Perspect., 30 : 105-113.
STEFANAC, Z., STENGL, B., & VASILIC, Z. (1976) Quantitative
determination of organophosphorus pesticides by thin-layer
densitometry. J. Chromatogr., 124 : 127-133.
SU, M.-Q., KINOSHITA, F.K., FRAWLEY, J.P., & DUBOIS, K.P.
(1971) Comparative inhibition of aliesterases and
cholinesterase in rats fed eighteen organophosphorus
insecticides. Toxicol. appl. Pharmacol., 20 : 241-249.
TALCOTT, R.E., DENK, H., & MALLIPUDI, N.M. (1979a) Malathion
carboxylesterase activity in human liver and its inactivation
by isomalathion. Toxicol. appl. Pharmacol., 49 : 373-376.
TALCOTT, R.E., MALLIPUDI, N.M., UMETSU, N., & FUKUTO, T.R.
(1979b) Inactivation of esterases by impurities isolated from
technical malathion. Toxicol. appl. Pharmacol., 49 : 107-112.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co.,
pp. 100-119.
UMETSU, N., GROSE, F.H., ALLAHYARI, R., ABU-EL-HAJ, S., &
FUKUTO, T.R. (1977) Effect of impurities on the mammalian
toxicity of technical malathion and acephate. J. agric. food
Chem., 25 : 946-953.
UNITED KINGDOM PSPS (1979) Guidance on toxicity data
requirements, London, MAFF, United Kingdom Pesticide Safety
Precautions Scheme, 3 pp (Working Document B5).
US EPA (1975) Registration, re-registration, and
classification procedures. Fed. Reg., 40 (129): 28276-28277.
US EPA (1982) Acute delayed neurotoxicity of organo-
phosphorus substances, Washington DC, US Environmental
Protection Agency, Section 81-7, pp. 62-65 (Pesticide
Assessment Guidelines, Subdivision F, Hazard Evaluation: human
and domestic animals) (EPA 540/9-82-025).
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning.
Guy's Hosp. Rep., 123 : 13-25.
VASIC, B.V., MILOSEVIC, M.P., & TERZIC, M.R. (1977) Acetyl-
choline content and cholinesterase activity in the ponto-
medullary region of brain in rats treated with armin and
obidoxime. Biochem. Pharmacol., 26 : 601-602.
VELSICOL (1977) Phosvel (technical leptophos) data for
regulatory authorities, Chicago, Illinois, Velsicol Chemical
Corporation.
VERGIEVA, T. (1983) Embryotoxicity and teratogenicity of
pesticides. In: Kaloyanova, F. & Tarkowski, S., ed. Toxicology
of pesticides, Copenhagen, World Health Organization, pp.
67-77 (Health Aspects of Chemical Safety, Interim Document
No. 9).
VERONESI, B. & PADILLA, S. (1985) Phenylmethylsulfonyl
fluoride protects rats from mipafox-induced delayed
neuropathy. Toxicol. appl. Pharmacol., 81 (2): 258-264.
VERSCHOYLE, R.D. & CABRAL, J.R.P. (1982) Investigation of
the acute toxicity of some trimethyl and triethyl
phosphorthioates with particular reference to those causing
lung damage. Arch. Toxicol., 51 : 221-231.
VERSCHOYLE, R.D., ALDRIDGE, W.N., & CABRAL, J.R.P. (1980)
Toxicology of trimethyl and triethyl phosphorothioates. In:
Holmstedt, B., Lauwerys, R., Mercier, M., & Roberfroid, M.,
ed. Mechanisms of toxicity and hazard evaluation, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 631-634.
VETTORAZZI, G. (1979) International regulatory aspects of
pesticide chemicals, Vol. 1 & 2, Boca Raton, Florida, CRC
Press, 232 and 214 pp.
VETTORAZZI, G. & VAN DEN HURK, G. (1984) Pesticides
reference index: JMPR-IARC-IPCS-IRPTC-VBC: 1961-1984, Geneva,
World Health Organization (Unpublished report).
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977)
Severe organophosphate poisoning complicated by alcohol and
terpentine ingestion. Arch. environ. Health, 32 : 203-205.
WHO (1975) Early detection of health impairment in
occupational exposure to health hazards, Geneva, World Health
Organization (Technical Report Series No. 571).
WHO (1979) Third report of the WHO Expert Committee on
vector biology and control, Geneva, World Health Organization,
44 pp (WHO Technical Report Series No. 634).
WHO (1982) Field surveys of exposure to pesticides -
standard protocol, Geneva, World Health Organization
(Unpublished report VBC/82.1).
WHO (1984a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-1985, Geneva,
World Health Organization (Unpublished report VBC/84.2).
WHO (1984b) Spectrophotometric kit for measuring
cholinesterase activity, Geneva, World Health Organization
(Unpublished report VBC/84.888).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World
Health Organization (VBC).
WICKER, G.W., WILLIAMS, W.A., & GUTHRIE, F.E. (1979)
Exposure of field workers to organophosphorus insecticides:
sweet corn and peaches. Arch. environ. Contam. Toxicol., 8 :
175-182.
WILKINSON, C.F. (1971) Effects of synergists on the
metabolism and toxicity of anticholinesterases. WHO Bull., 44 :
171-190.
WILLEMS, J.L. (1981) Poisoning by organophosphate
insecticides: analysis of 53 human cases with regard to
management and drug treatment. Acta med. milit. Belg., 134 :
7-14.
WILLS, J.H. (1963) [Pharmacological antagonists of the
anticholinesterase agents.] In: Koelle, G.B., ed. [Handbook of
experimental pharmacology,] Berlin, Springer-Verlag, Vol. 15,
pp. 883-920 (in German).
WILSON, B.W., HOOPER, M., CHOW, E., HIGGINS, R.J., & KNAAK,
J.B. (1984) Antidotes and neuropathic potential of
isofenphos. Bull. environ. Contam. Toxicol., 33 : 386-394.
WILTROUT, R.W., ERGEGOVICH, C.D., & CEGLOWSKI, W.S. (1978)
Humoral immunity in mice following oral administration of
selected pesticides. Arch. environ. Contam. Toxicol., 20 :
423-431.
WITTER, R.F. & GAINES, T.B. (1963) Relationship between
depression of the brain or plasma cholinesterase and paralysis
in chickens caused by certain organic phosphorus compounds.
Biochem. Pharmacol., 12 : 1377-1386.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1967) Exposure
of workers to pesticides. Arch. environ. Health, 14 : 622-633.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1970) Urinary
excretion of insecticide metabolites. Arch. environ. Health,
21 : 711-716.
WOLFE, H.R., STAIFF, D.C., & ARMSTRONG, J.F. (1978) Exposure
of pesticide formulating plant workers to parathion. Bull.
environ. Contam. Toxicol., 20 : 340-343.
WOLTHUIS, O.L. & VANWERSCH, R.A.P. (1984) Behavioural
changes in the rat after low doses of cholinesterase
inhibitors. Toxicol. appl. Pharmacol., 4 : S195-S208.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) In vivo
alkylation studies with dichlorvos at practical use
concentrations. Chem.-biol. Interact., 19 : 25-46.
WORTHING, C.R., ed. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council,
655 pp.
XINTARAS, C. & BURG, J.R. (1980) Screening and prevention of
human neurotoxic outbreaks: issues and problems. In: Spencer,
P.S. & Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams & Wilkins
Company, pp. 663-674.
XINTARAS, C., BURG, J.R., TANAKA, S., LEE, S.T., JOHNSON,
B.L., COTTRILL, C.A., & BENDER, J. (1978) Occupational
exposure to leptophos and other chemicals, Cincinnati, Ohio,
US Department of Health and Welfare, National Institue of
Occupational Safety and Health (US DHEW (NIOSH) Publication
No. 78-136).
YANG, R.S.H., HODGSON, E., & DAUTERMAN, W.C. (1971)
Metabolism in vitro of diazinon and diazoxon in rat liver. J.
agric. food Chem., 19 : 10-13.
YOSHIDA, K. & NISHIUCHI, Y. (1972) Toxicity of pesticides to
some water organisms.] Bull. agric. Chem. Inspect. Stn
(Japan), 12 : 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn
(Japan), 16 : 65-69 (in Japanese).
ZACKOV, K. (1983) Immunotoxicology of pesticides. In:
Kaloyanova, F. & Tarkowski, S., ed. Toxicology of pesticides,
Copenhagen, World Health Organization, pp. 75-88 (Health
Aspects of Chemical Safety, Interim Document No. 9).
ZIELHUIS, R.L. (1972) Epidemiological toxicology of
pesticide exposure: report of an international workshop. Arch.
environ. Health, 25 : 399-405.
Annex I. Names and structures of selected organophosphorus pesticides
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other formula molecular Registry
name mass number
-------------------------------------------------------------------------------------------
acephate Orthene phosphoramidothioic C4 H10 NO3 PS 183.18 30560-19-1
Ortran acid, acetyl-, O,S-
dimetyl ester
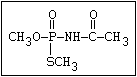 amidithion Thiocron phosphorodithioic C7 H16 NO4 PS2 273.33 919-76-6
acid, O,O -dimethyl
S -[2-[(2-methoxy-
ethyl)amino]-2-oxo-
ethyl/ester
amidithion Thiocron phosphorodithioic C7 H16 NO4 PS2 273.33 919-76-6
acid, O,O -dimethyl
S -[2-[(2-methoxy-
ethyl)amino]-2-oxo-
ethyl/ester
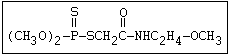 amiton Citram phosphorothioic C10 H24 NO3 PS 269.38 78-53-5
Inferno acid, S -[2-(diethyl-
Metramac amino)ethyl] O,O -di-
Tetram ethyl ester
amiton Citram phosphorothioic C10 H24 NO3 PS 269.38 78-53-5
Inferno acid, S -[2-(diethyl-
Metramac amino)ethyl] O,O -di-
Tetram ethyl ester
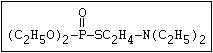 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
azinophos- Cotnion- phosphorodithioic C12 H16 N3 O3 PS2 345.4 2642-71-9
ethyl ethyl acid, O,O -diethyl-
Gusathion S -[[4-oxo-1,2-benzo-
Ethyl, triazin-3(4H)-yl]
Guthion methyl] ester
ethyl,
Trazotion
(Russian)
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
azinophos- Cotnion- phosphorodithioic C12 H16 N3 O3 PS2 345.4 2642-71-9
ethyl ethyl acid, O,O -diethyl-
Gusathion S -[[4-oxo-1,2-benzo-
Ethyl, triazin-3(4H)-yl]
Guthion methyl] ester
ethyl,
Trazotion
(Russian)
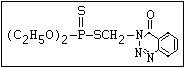 azinophos- Cotnion- phosphorodithiotic C10 H12 N3 O3 PS2 317.34 86-50-0
methyl methyl acid, O,O -dimethyl-
gusathion S -[[4-oxo-1,2,3-
guthion benzotriazin-3(4H)-
metiltr- yl]methyl] ester
azotion
azinophos- Cotnion- phosphorodithiotic C10 H12 N3 O3 PS2 317.34 86-50-0
methyl methyl acid, O,O -dimethyl-
gusathion S -[[4-oxo-1,2,3-
guthion benzotriazin-3(4H)-
metiltr- yl]methyl] ester
azotion
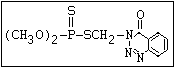 azothoate alamos phosphorothiotic C14 H14 ClN2 O3 PS 356.78 5834-96-8
acid, O -[4-[(4-
chlorophenyl)azo]
phenyl] O,O -dimethyl
ester
azothoate alamos phosphorothiotic C14 H14 ClN2 O3 PS 356.78 5834-96-8
acid, O -[4-[(4-
chlorophenyl)azo]
phenyl] O,O -dimethyl
ester
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
bromophos netal phosphorothioic C8 H8 BrCl2 O3 PS 366.0 2104-96-3
nexion acid, O -(4-bromo-
2,5-dichlorophenyl)
O,O -dimethyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
bromophos netal phosphorothioic C8 H8 BrCl2 O3 PS 366.0 2104-96-3
nexion acid, O -(4-bromo-
2,5-dichlorophenyl)
O,O -dimethyl ester
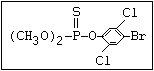 bromophos- nexagan phosphorothioic C10 H12 BrCl2 O3 PS 394.06 4824-78-6
ethyl acid, O -(4-bromo-
2,5-dichlorophenyl)
O,O -diethyl ester
bromophos- nexagan phosphorothioic C10 H12 BrCl2 O3 PS 394.06 4824-78-6
ethyl acid, O -(4-bromo-
2,5-dichlorophenyl)
O,O -diethyl ester
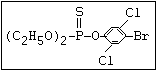 butonate tribufon butanoic acid, C8 H14 Cl3 O5 P 327.54 126-22-7
2,2,2-trichloro-1-
(dimethoxyphosphi-
nyl) ethyl ester
butonate tribufon butanoic acid, C8 H14 Cl3 O5 P 327.54 126-22-7
2,2,2-trichloro-1-
(dimethoxyphosphi-
nyl) ethyl ester
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
chlorfen- birlane phosphoric acid, C12 H14 Cl3 O4 P 359.58 2701-86-2
vinphos sapecron 2-chloro-1-(2,4-
supona dichlorophenyl)
ethenyl dimethyl
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
chlorfen- birlane phosphoric acid, C12 H14 Cl3 O4 P 359.58 2701-86-2
vinphos sapecron 2-chloro-1-(2,4-
supona dichlorophenyl)
ethenyl dimethyl
ester
 chlorpyri- dursban phosphorothioic C9 H11 Cl3 NO3 PS 350.59 2921-88-2
fos lorsban acid, O,O -diethyl
O -(3,5,6-trichloro-
2-pyridinyl) ester
chlorpyri- dursban phosphorothioic C9 H11 Cl3 NO3 PS 350.59 2921-88-2
fos lorsban acid, O,O -diethyl
O -(3,5,6-trichloro-
2-pyridinyl) ester
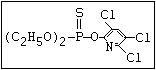 chlorpyri- fospirate phosphorothioic C7 H7 Cl3 NO3 PS 322.53 5598-13-0
fos reldan acid, O,O -dimethyl
methyl zertell O -(33,5,6-trichloro-
2-pyridinyl) ester
chlorpyri- fospirate phosphorothioic C7 H7 Cl3 NO3 PS 322.53 5598-13-0
fos reldan acid, O,O -dimethyl
methyl zertell O -(33,5,6-trichloro-
2-pyridinyl) ester
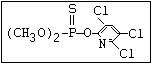 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
coumaphos agridip phosphorothioic C14 H16 Cl05 PS 326.78 56-72-4
asunthol acid, O -(3-chloro-
co-ral 4-methyl-2-oxo-2H-
meldane 1-benzopyran-7-yl)
muscatox O,O -diethyl ester
resistox
suntol
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
coumaphos agridip phosphorothioic C14 H16 Cl05 PS 326.78 56-72-4
asunthol acid, O -(3-chloro-
co-ral 4-methyl-2-oxo-2H-
meldane 1-benzopyran-7-yl)
muscatox O,O -diethyl ester
resistox
suntol
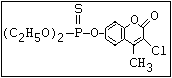 crotoxy- ciodrin 2-butenoic acid, C14 H19 O6 P 314.3 7700-17-6
phos 3-[(dimethoxyphos-
phinyl)oxy]-,
1-phenylethyl ester
crotoxy- ciodrin 2-butenoic acid, C14 H19 O6 P 314.3 7700-17-6
phos 3-[(dimethoxyphos-
phinyl)oxy]-,
1-phenylethyl ester
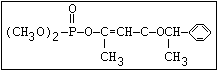 crufomate montrel phosphoramidic C12 H19 ClNO3 P 291.74 299-86-5
ruelene acid, methyl-,
2-chloro-4(1,1-
dimethylethyl)-
phenyl methyl ester
crufomate montrel phosphoramidic C12 H19 ClNO3 P 291.74 299-86-5
ruelene acid, methyl-,
2-chloro-4(1,1-
dimethylethyl)-
phenyl methyl ester
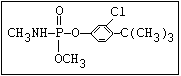 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
demeton- metasystox phosporothioic C6 H15 O3 PS2 230.3 919-86-8
S -methyl methyl acid, S -[2-(ethyl-
isosystox thio)ethyl] O,O -
dimethyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
demeton- metasystox phosporothioic C6 H15 O3 PS2 230.3 919-86-8
S -methyl methyl acid, S -[2-(ethyl-
isosystox thio)ethyl] O,O -
dimethyl ester
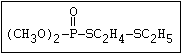 diazinon basudin phosphorothioc C12 H21 N2 O3 PS 304.38 333-41-5
dazzel acid, O,O -diethyl
diazajet O -[6-methyl-2-(1-
diazide methylethyl)-4-
diazol pyrimidinyl] ester
gardentox
nucidol
diazinon basudin phosphorothioc C12 H21 N2 O3 PS 304.38 333-41-5
dazzel acid, O,O -diethyl
diazajet O -[6-methyl-2-(1-
diazide methylethyl)-4-
diazol pyrimidinyl] ester
gardentox
nucidol
 dichlo- ECP phosphorothioic C10 H13 Cl2 O3 PS 315.16 97-17-6
fenthion hexa-nema acid, O -(2,4-di-
mobilawn chlorophenyl) O,O -
nemacide diethyl ester
dichlo- ECP phosphorothioic C10 H13 Cl2 O3 PS 315.16 97-17-6
fenthion hexa-nema acid, O -(2,4-di-
mobilawn chlorophenyl) O,O -
nemacide diethyl ester
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dichlorves atgard phosphoric acid, C4 H7 Cl2 O4 P 220.98 62-73-7
canogard 2,2-dichloro-
cekusan ethenyl dimethyl
DDVP ester
dedevap
equigard
herkal
marvex
nuvan
task
vapona
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dichlorves atgard phosphoric acid, C4 H7 Cl2 O4 P 220.98 62-73-7
canogard 2,2-dichloro-
cekusan ethenyl dimethyl
DDVP ester
dedevap
equigard
herkal
marvex
nuvan
task
vapona
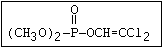 dicroto- bidrin phosphoric acid, C8 H16 NO5 P 237.22 141-66-2
phos carbicron 3-(dimethylamino)-
ektafos 1-methyl-3-oxo-1-
propenyl dimethyl
ester
dicroto- bidrin phosphoric acid, C8 H16 NO5 P 237.22 141-66-2
phos carbicron 3-(dimethylamino)-
ektafos 1-methyl-3-oxo-1-
propenyl dimethyl
ester
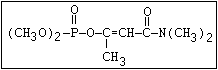 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dimethoate cygon phosphorodithioic C5 H12 NO3 PS2 229.27 60-51-5
daphene acid, O,O -dimethyl
dimeton S -[2-(methylamine)-
ferkethion 2-exoethyl] ester
fortion
fosfamid
fosfotox
lurgo
perfektion
rebelate
rogor
roxion
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dimethoate cygon phosphorodithioic C5 H12 NO3 PS2 229.27 60-51-5
daphene acid, O,O -dimethyl
dimeton S -[2-(methylamine)-
ferkethion 2-exoethyl] ester
fortion
fosfamid
fosfotox
lurgo
perfektion
rebelate
rogor
roxion
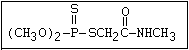 dioxathion delnav phosphorodithioic C12 H26 O6 P2 S4 456.56 78-34-2
kavadel acid, S-S' -1,4-
navadel diexane-2,3-diyl
ruphos O,O,O',O' -tetra-
ethyl ester
dioxathion delnav phosphorodithioic C12 H26 O6 P2 S4 456.56 78-34-2
kavadel acid, S-S' -1,4-
navadel diexane-2,3-diyl
ruphos O,O,O',O' -tetra-
ethyl ester
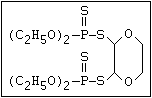 disulfoton dimaz phosphorodithioic C8 H19 O2 PS3 274.42 298-04-4
disyston acid, O,O -diethyl
disystox S -[2-(ethylthio)
frumin ethyl] ester
solvirex
disulfoton dimaz phosphorodithioic C8 H19 O2 PS3 274.42 298-04-4
disyston acid, O,O -diethyl
disystox S -[2-(ethylthio)
frumin ethyl] ester
solvirex
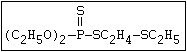 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
EPN phosphonothioic C14 H14 NO4 PS 323.32 2104-64-5
acid, phenyl- O -
ethyl O -(4-nitro-
phenyl) ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
EPN phosphonothioic C14 H14 NO4 PS 323.32 2104-64-5
acid, phenyl- O -
ethyl O -(4-nitro-
phenyl) ester
 ethion bladan phosphorodithioic C9 H22 O4 P2 S4 384.49 22756-17-8
fosfono 50 acid, S,S' -methy-
nialate lene O,O,O',O'-
redocid tetramethyl ester
seprathion
ethion bladan phosphorodithioic C9 H22 O4 P2 S4 384.49 22756-17-8
fosfono 50 acid, S,S' -methy-
nialate lene O,O,O',O'-
redocid tetramethyl ester
seprathion
 fenamiphos nemacur phosphoramidic C13 H22 NO3 PS 272.34 22224-92-6
acid, (1-methyl-
ethyl)-ethyl 3-
methyl-4-(methyl-
thio)phenyl ester
fenamiphos nemacur phosphoramidic C13 H22 NO3 PS 272.34 22224-92-6
acid, (1-methyl-
ethyl)-ethyl 3-
methyl-4-(methyl-
thio)phenyl ester
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fenchlor- ectoral phosphorothioic C8 H8 Cl3 O3 PS 321.54 299-84-3
phos etrolene acid, O,O -dimethyl
korlane O -(2,4,5-trichloro-
nanchlor phenyl) ester
nankor
trolene
viozene
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fenchlor- ectoral phosphorothioic C8 H8 Cl3 O3 PS 321.54 299-84-3
phos etrolene acid, O,O -dimethyl
korlane O -(2,4,5-trichloro-
nanchlor phenyl) ester
nankor
trolene
viozene
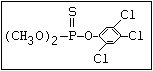 fenitro- accothion phosphorothioic C9 H12 NO5 PS 277.25 122-12-5
thion cyfen acid, O,O -dimethyl
cytel O -(3-methyl-4-
felithion nitrophenyl) pester
metathion
nitrophos
nevathion
sumithion
fenitro- accothion phosphorothioic C9 H12 NO5 PS 277.25 122-12-5
thion cyfen acid, O,O -dimethyl
cytel O -(3-methyl-4-
felithion nitrophenyl) pester
metathion
nitrophos
nevathion
sumithion
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fensulfo- dasanit phosphorothioic C11 H17 O4 PS2 308.37 4824-78-6
thion terracur-P acid, O,O -diethyl
O -[4-(methyl-
sulfinyl)phenyl]
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fensulfo- dasanit phosphorothioic C11 H17 O4 PS2 308.37 4824-78-6
thion terracur-P acid, O,O -diethyl
O -[4-(methyl-
sulfinyl)phenyl]
ester
 fenthion baycid phosphorothioic C10 H15 O3 PS2 278.34 55-38-9
baytex acid, O,O -dimethyl
entex O -[3-methyl-4-
lebaycid (methylthio)phenyl]
mercaptophos ester
queletox
tiguvon
fenthion baycid phosphorothioic C10 H15 O3 PS2 278.34 55-38-9
baytex acid, O,O -dimethyl
entex O -[3-methyl-4-
lebaycid (methylthio)phenyl]
mercaptophos ester
queletox
tiguvon
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fonofos Dyfonate O -ethyl S -phenyl C10 H15 OPS2 246.3 944-22-9
(RS)-ethyl-phospho-
nodithioate
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fonofos Dyfonate O -ethyl S -phenyl C10 H15 OPS2 246.3 944-22-9
(RS)-ethyl-phospho-
nodithioate
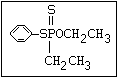 formothion aflix phosphorodithioic C6 H12 NO4 PS2 257.28 2540-32-1
anthio acid, S -[2-(formyl-
methylamino)-2-oxo-
ethyl] O,O -dimethyl
ester
formothion aflix phosphorodithioic C6 H12 NO4 PS2 257.28 2540-32-1
anthio acid, S -[2-(formyl-
methylamino)-2-oxo-
ethyl] O,O -dimethyl
ester
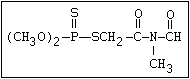 fosthietan phosphoramidic C6 H12 NO3 PS2 241. 21548-32-3
acid, 1,3-
dithietan-2-
ylidene-, diethyl
ester
fosthietan phosphoramidic C6 H12 NO3 PS2 241. 21548-32-3
acid, 1,3-
dithietan-2-
ylidene-, diethyl
ester
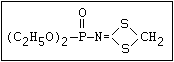 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
hepteno- hostaquick phosphoric acid, C9 H12 ClO4 P 250.63 23560-59-0
phos ragadan 7-chlorobicyclo-
[3.2.0]hepta-2,6-
dien-6-yl dimethyl
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
hepteno- hostaquick phosphoric acid, C9 H12 ClO4 P 250.63 23560-59-0
phos ragadan 7-chlorobicyclo-
[3.2.0]hepta-2,6-
dien-6-yl dimethyl
ester
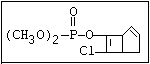 idofenphos alfacron phosphorothioic C8 H8 Cl2 IO3 PS 412.99 18181-70-9
nuvanol-N acid, O -(2,5-
dichloro-4-
iodophenyl) O,O -
dimethyl ester
idofenphos alfacron phosphorothioic C8 H8 Cl2 IO3 PS 412.99 18181-70-9
nuvanol-N acid, O -(2,5-
dichloro-4-
iodophenyl) O,O -
dimethyl ester
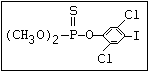 isofenphos oftanol benzoic acid, 2- C15 H24 NO4 PS 345.43 25311-71-1
[[ethoxy-[(1-
methyl-ethyl)amino]
phosphinothioyl]-
oxy]-, 1-methyl-
ethyl ester
isofenphos oftanol benzoic acid, 2- C15 H24 NO4 PS 345.43 25311-71-1
[[ethoxy-[(1-
methyl-ethyl)amino]
phosphinothioyl]-
oxy]-, 1-methyl-
ethyl ester
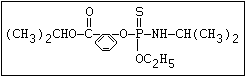 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
leptophos Phosvel O -(4-bromo-2,5-di- C13 H10 BrCl2 O2 PS 412.1 21609-90-5
Abar chlorophenyl) O -
methyl phenylphos-
phonothioate
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
leptophos Phosvel O -(4-bromo-2,5-di- C13 H10 BrCl2 O2 PS 412.1 21609-90-5
Abar chlorophenyl) O -
methyl phenylphos-
phonothioate
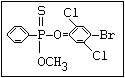 malathion carbetox butanedioic acid, C10 H19 O6 PS2 330.38 121-75-5
carbefos [(dimethoxyphos-
chemation phinothioyl)thio]-,
cythion diethyl ester
emmatos
fyfanon
kypfos
sadafos
zithiol
malathion carbetox butanedioic acid, C10 H19 O6 PS2 330.38 121-75-5
carbefos [(dimethoxyphos-
chemation phinothioyl)thio]-,
cythion diethyl ester
emmatos
fyfanon
kypfos
sadafos
zithiol
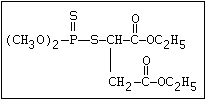 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
mecarbam afos carbamic acid, C10 H20 NO5 PS2 329.4 2595-54-2
muratox [[[(diethoxyphos-
pestan phinothioyl)thio]-
acetyl]methyl]-,
ethyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
mecarbam afos carbamic acid, C10 H20 NO5 PS2 329.4 2595-54-2
muratox [[[(diethoxyphos-
pestan phinothioyl)thio]-
acetyl]methyl]-,
ethyl ester
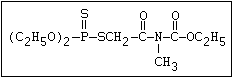 menazon azidithion phosphorodithioic C6 H12 N5 O2 PS2 281.32 78-57-9
saphizon acid, S -[(4,6-di-
saphos amino-1,3,5-
sayfor triazin-2-yl)-
syphos methyl] O,O -
dimethyl ester
menazon azidithion phosphorodithioic C6 H12 N5 O2 PS2 281.32 78-57-9
saphizon acid, S -[(4,6-di-
saphos amino-1,3,5-
sayfor triazin-2-yl)-
syphos methyl] O,O -
dimethyl ester
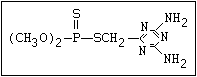 mephosfolan Cytrolane diethyl(4-methyl-1, C8 H16 NO3 PS2 269.3 950-10-7
3-dithiolan-2-
ylidene)phosphor
amidate
mephosfolan Cytrolane diethyl(4-methyl-1, C8 H16 NO3 PS2 269.3 950-10-7
3-dithiolan-2-
ylidene)phosphor
amidate
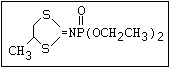 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
methamido- hamidop phosphoramidothioic C2 H8 NO2 PS 141.14 10265-92-6
phos monitor acid, O,S -dimethyl
tamaron ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
methamido- hamidop phosphoramidothioic C2 H8 NO2 PS 141.14 10265-92-6
phos monitor acid, O,S -dimethyl
tamaron ester
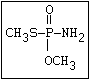 methida- supracide phosphorodithioic C6 H11 N2 O4 PS2 302.34 950-37-8
thion ultracide acid, S -[(5-
methoxy-2-oxo-1,3,
4-thiadiazol-3(2 H)-
yl)-methyl] O,O -
dimethyl ester
methida- supracide phosphorodithioic C6 H11 N2 O4 PS2 302.34 950-37-8
thion ultracide acid, S -[(5-
methoxy-2-oxo-1,3,
4-thiadiazol-3(2 H)-
yl)-methyl] O,O -
dimethyl ester
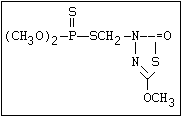 mevinphos gestid 2-butenoic acid, C7 H13 O6 P 224.17 7786-34-7
menite 3-[(dimethoxyphos-
phosdrin phinyl)oxy]-,
phosfene methyl ester
mevinphos gestid 2-butenoic acid, C7 H13 O6 P 224.17 7786-34-7
menite 3-[(dimethoxyphos-
phosdrin phinyl)oxy]-,
phosfene methyl ester
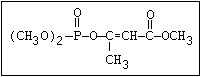 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
monocro- azodrin phosphoric acid, C7 H14 NO5 P 223.19 2157-98-4
tophos monocron dimethyl 1-methyl-
nuvacron 3-(methylamino)-3-
oxo-1-propenyl
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
monocro- azodrin phosphoric acid, C7 H14 NO5 P 223.19 2157-98-4
tophos monocron dimethyl 1-methyl-
nuvacron 3-(methylamino)-3-
oxo-1-propenyl
ester
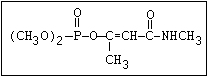 morpho- ekatin phosphorodithioic C8 H16 NO4 PS2 285.34 144-41-2
thion morphotox acid, O,O -dimethyl
S -[2-(4-morpho-
linyl)-2-oxoethyl]
ester
morpho- ekatin phosphorodithioic C8 H16 NO4 PS2 285.34 144-41-2
thion morphotox acid, O,O -dimethyl
S -[2-(4-morpho-
linyl)-2-oxoethyl]
ester
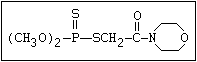 naled arthodibrom phosphoric acid, C4 H7 Br2 Cl2 O4 P 380.8 300-76-5
bromex 1,2-dibromo-2,2-
dibrom dichloroethyl
dimethyl ester
naled arthodibrom phosphoric acid, C4 H7 Br2 Cl2 O4 P 380.8 300-76-5
bromex 1,2-dibromo-2,2-
dibrom dichloroethyl
dimethyl ester
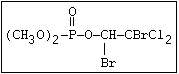 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
omethoate dimethoxon phosphorothioic C5 H10 NO4 PS 213.21 1113-02-6
folimat acid, O,O -dimethyl
S -[2-(methylamino)-
2-oxoethyl] ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
omethoate dimethoxon phosphorothioic C5 H10 NO4 PS 213.21 1113-02-6
folimat acid, O,O -dimethyl
S -[2-(methylamino)-
2-oxoethyl] ester
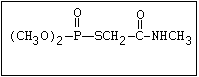 oxydeme- phosphorothioic C6 H15 O4 PS2 246.3 301-12-2
ton- acid, S -[2-(ethyl-
methyl sulfinyl)ethyl]
O,O -dimethyl ester
oxydeme- phosphorothioic C6 H15 O4 PS2 246.3 301-12-2
ton- acid, S -[2-(ethyl-
methyl sulfinyl)ethyl]
O,O -dimethyl ester
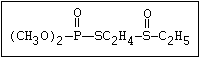 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
parathion alkron phosphorothioic C10 H14 NO5 PS 291.28 56-38-2
alleron acid, O,O -diethyl
cerothion O -(4-nitrophenyl)
danthion ester
ekatox
folidol
fosfex
kypthion
niran
stathion
sulphos
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
parathion alkron phosphorothioic C10 H14 NO5 PS 291.28 56-38-2
alleron acid, O,O -diethyl
cerothion O -(4-nitrophenyl)
danthion ester
ekatox
folidol
fosfex
kypthion
niran
stathion
sulphos
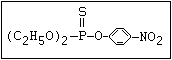 parathion- amofos phosphorothioic C8 H10 NO5 PS 263.22 298-00-0
methyl dalf acid, O,O -dimethyl
metafos O -(4-nitrophenyl)
metaphor ester
matron
nitrox
tekwaisa
thiophenit
vofatox
parathion- amofos phosphorothioic C8 H10 NO5 PS 263.22 298-00-0
methyl dalf acid, O,O -dimethyl
metafos O -(4-nitrophenyl)
metaphor ester
matron
nitrox
tekwaisa
thiophenit
vofatox
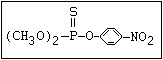 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phenthoate pap benzeneacetic acid, C12 H17 O4 PS2 320.38 2597-03-2
papthion alpha-[(dimethoxy-
tanone phosphinothioyl)-
cidial thio]-, ethyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phenthoate pap benzeneacetic acid, C12 H17 O4 PS2 320.38 2597-03-2
papthion alpha-[(dimethoxy-
tanone phosphinothioyl)-
cidial thio]-, ethyl ester
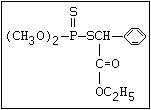 phorate granutox phosphorodithioic C7 H17 O2P S3 260.39 298-02-2
rampart acid, O,O -diethyl
thimet S -[(ethylthio)methyl]
vegfru ester
phorate granutox phosphorodithioic C7 H17 O2P S3 260.39 298-02-2
rampart acid, O,O -diethyl
thimet S -[(ethylthio)methyl]
vegfru ester
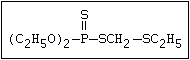 phosalone azofene phosphordithioic C12 H15 ClNO4 PS2 367.82 2310-17-0
benzphos acid, S -[(6-chloro-
rubitox 2-oxo-3(2 H)- benzoxa-
zolone zolyl)methyl] O,O -
diethyl ester
phosalone azofene phosphordithioic C12 H15 ClNO4 PS2 367.82 2310-17-0
benzphos acid, S -[(6-chloro-
rubitox 2-oxo-3(2 H)- benzoxa-
zolone zolyl)methyl] O,O -
diethyl ester
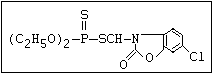 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phosmet decemthion phosphorodithioic C11 H12 NO2 PS2 317.33 732-11-6
appa acid, S -[(1,3-di-
ftalophos hydro-1,3-dioxo-2H-
imidan isoindol-2-yl)methyl]
prolate O,O -diethyl ester
smidan
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phosmet decemthion phosphorodithioic C11 H12 NO2 PS2 317.33 732-11-6
appa acid, S -[(1,3-di-
ftalophos hydro-1,3-dioxo-2H-
imidan isoindol-2-yl)methyl]
prolate O,O -diethyl ester
smidan
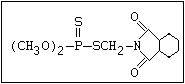 phospha- dimeron phosphoric acid, C10 H19 ClNO5 P 299.72 13171-21-6
midon famfos 2-chloro-3-(diethyl-
amino)-1-methyl-3-
oxo-1-propenyl di-
ethyl ester
phospha- dimeron phosphoric acid, C10 H19 ClNO5 P 299.72 13171-21-6
midon famfos 2-chloro-3-(diethyl-
amino)-1-methyl-3-
oxo-1-propenyl di-
ethyl ester
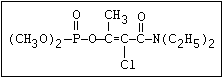 phosfolan Cyolane P,P-diethyl cyclic- C7 H14 NO3 PS2 255.3 947-02-4
Cyolan ethylene ester of
Cyalane phosphonodithiomido-
Cylan carbonic acid
phosfolan Cyolane P,P-diethyl cyclic- C7 H14 NO3 PS2 255.3 947-02-4
Cyolan ethylene ester of
Cyalane phosphonodithiomido-
Cylan carbonic acid
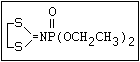 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phoxim baythion 3,5-dioxa-6-aza- C12 H15 N2 O3 PS 298.32 14816-18-3
valexon 4-phosphaocta-6-ene-
volaton S -nitrile, 4-ethoxy-
7-phenyl, 4-sulfide
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phoxim baythion 3,5-dioxa-6-aza- C12 H15 N2 O3 PS 298.32 14816-18-3
valexon 4-phosphaocta-6-ene-
volaton S -nitrile, 4-ethoxy-
7-phenyl, 4-sulfide
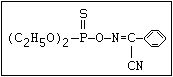 profenofos Curacron O -(4-bromo-2-chloro- C11 H15 BrCl03 PS 373.6 41198-08-7
Selecron phenyl) O -ethyl S -
propyl phosphoro-
thioate
profenofos Curacron O -(4-bromo-2-chloro- C11 H15 BrCl03 PS 373.6 41198-08-7
Selecron phenyl) O -ethyl S -
propyl phosphoro-
thioate
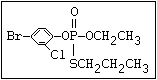 prothiofos Tokuthion dichlorophenyl O - C11 H15 Cl2 O2 PS2 345.2 34643-46-4
ethyl S- propyl phos-
phorodithioate
prothiofos Tokuthion dichlorophenyl O - C11 H15 Cl2 O2 PS2 345.2 34643-46-4
ethyl S- propyl phos-
phorodithioate
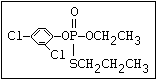 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
prothoate fal phosphorodithioic C9 H20 NO3 PS2 285.39 2275-18-5
fostion acid, O,O -diethyl
oleofac S -[2-[1-methylethyl)-
telefos amino]-2-oxo-ethyl]
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
prothoate fal phosphorodithioic C9 H20 NO3 PS2 285.39 2275-18-5
fostion acid, O,O -diethyl
oleofac S -[2-[1-methylethyl)-
telefos amino]-2-oxo-ethyl]
ester
 pyrimiphos- fernex phosphorothioic C13 H24 N3 O3 PS 302.46 23505-41-1
ethyl primieid acid, O -[2-(diethyl-
primotec amino)-6-methyl-4-
pyrimidinyl] O,O -
diethyl ester
pyrimiphos- fernex phosphorothioic C13 H24 N3 O3 PS 302.46 23505-41-1
ethyl primieid acid, O -[2-(diethyl-
primotec amino)-6-methyl-4-
pyrimidinyl] O,O -
diethyl ester
 pyrimiphos- actellic phosphorothioic C11 H20 N3 O3 PS 274.4 29232-93-7
methyl actellifog acid, O -[2-(diethyl-
blex amino)-6-methyl-4-
silosan pyrimidinyl] O,O -
dimethyl ester
pyrimiphos- actellic phosphorothioic C11 H20 N3 O3 PS 274.4 29232-93-7
methyl actellifog acid, O -[2-(diethyl-
blex amino)-6-methyl-4-
silosan pyrimidinyl] O,O -
dimethyl ester
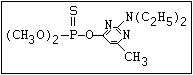 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
pyrazophos afugan pyrazolo[1,5a]pyri- C14 H20 N3 O5 PS 373.4 13457-18-6
curamil midine-6-carboxylic
acid, 2-[(diethoxyd-
phosphinothioyl)oxy]-
5-methyl-, ethyl
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
pyrazophos afugan pyrazolo[1,5a]pyri- C14 H20 N3 O5 PS 373.4 13457-18-6
curamil midine-6-carboxylic
acid, 2-[(diethoxyd-
phosphinothioyl)oxy]-
5-methyl-, ethyl
ester
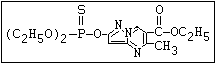 sulfotep bladafume thiodiphosphoric C8 H20 O5 P2 S2 322.34 3689-24-5
dithiofos acid, tetraetyl
dithione ester
dithiotep
sulfotep bladafume thiodiphosphoric C8 H20 O5 P2 S2 322.34 3689-24-5
dithiofos acid, tetraetyl
dithione ester
dithiotep
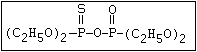 sulprofos Bolstar O -ethyl O -[4- C12 H19 O2 PS3 322.4 35400-43-2
(methylthio) phenyl]
phenyl] S -propyl
phosphorodithioate
sulprofos Bolstar O -ethyl O -[4- C12 H19 O2 PS3 322.4 35400-43-2
(methylthio) phenyl]
phenyl] S -propyl
phosphorodithioate
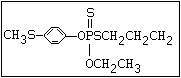 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
temephos abate phosphorothioic C16 H20 O6 P2 S3 466.48 3383-96-8
abathion acid, O,O' -(thio-
biothion di-4,1-phenylene)
difethos O,O,O',O' -tetra-
nimitox methyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
temephos abate phosphorothioic C16 H20 O6 P2 S3 466.48 3383-96-8
abathion acid, O,O' -(thio-
biothion di-4,1-phenylene)
difethos O,O,O',O' -tetra-
nimitox methyl ester
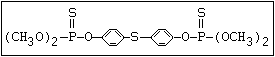 TEPP bladan diphosphoric acid, C8 H20 O7 P2 290.22 107-49-3
bladex tetraethyl ester
fosuex
grisol
hexamite
lirohex
mortopal
nifos
TEPP bladan diphosphoric acid, C8 H20 O7 P2 290.22 107-49-3
bladex tetraethyl ester
fosuex
grisol
hexamite
lirohex
mortopal
nifos
 tetrachlor- appex phosphoric acid, C10 H9 Cl4 O4 P 365.96 961-11-5
vinphos gardcide 2-chloro-1-(2,4,5-
gardona trichlorophenyl)-
rabon ethenyl dimethyl
stirofos ester
tetrachlor- appex phosphoric acid, C10 H9 Cl4 O4 P 365.96 961-11-5
vinphos gardcide 2-chloro-1-(2,4,5-
gardona trichlorophenyl)-
rabon ethenyl dimethyl
stirofos ester
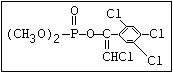 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
thiometon ekatin phosphorodithioic C6 H15 O2 PS3 246.36 640-15-3
intrathion acid, S -[2-(ethyl-
thio)ethyl] O,O -
dimethyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
thiometon ekatin phosphorodithioic C6 H15 O2 PS3 246.36 640-15-3
intrathion acid, S -[2-(ethyl-
thio)ethyl] O,O -
dimethyl ester
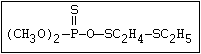 thionazin cynem phosphorothioic C6 H13 N2 O3 PS 248.26 297-97-2
nemafos acid, O,O -diethyl
zinophos O -pyrazin-2-yl ester
thionazin cynem phosphorothioic C6 H13 N2 O3 PS 248.26 297-97-2
nemafos acid, O,O -diethyl
zinophos O -pyrazin-2-yl ester
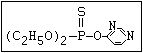 triamiphos wepsin phosphonic diamide, C12 H19 N6 CP 294.34 1031-47-6
P -(5-amino-3-phenyl-
1 H- 1,2,4-triazol-1-
yl)- N,N,N' -tetra-
methyl
triamiphos wepsin phosphonic diamide, C12 H19 N6 CP 294.34 1031-47-6
P -(5-amino-3-phenyl-
1 H- 1,2,4-triazol-1-
yl)- N,N,N' -tetra-
methyl
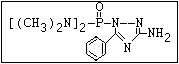 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
triazophos Hostathion O,O -diethyl O -(1H- C12 H16 N3 O3 PS 313.3 24017-47-8
HOE 2960 1.2.4-triazol-3-yl)
phosphorothioate
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
triazophos Hostathion O,O -diethyl O -(1H- C12 H16 N3 O3 PS 313.3 24017-47-8
HOE 2960 1.2.4-triazol-3-yl)
phosphorothioate
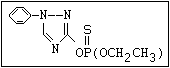 trichlor- anthion phosphonic acid, C4 H8 Cl3 O4 P 257.44 52-68-6
fon bovinox (2,2,2-trichloro-
briton 1-hydroxyethyl)-,
cehuion dimethyl ester
clorofos
ciclosom
danex
dipterex
dylox
metrifonate
proxol
trichlor- anthion phosphonic acid, C4 H8 Cl3 O4 P 257.44 52-68-6
fon bovinox (2,2,2-trichloro-
briton 1-hydroxyethyl)-,
cehuion dimethyl ester
clorofos
ciclosom
danex
dipterex
dylox
metrifonate
proxol
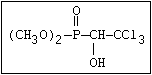 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
trichlor- agrisil phosphonothioic C10 H12 Cl3 O2 PS 333.6 327-98-0
nat agritox acid, ethyl-, O -
ethyl O -(2,4,5-
trichlorophenyl)
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
trichlor- agrisil phosphonothioic C10 H12 Cl3 O2 PS 333.6 327-98-0
nat agritox acid, ethyl-, O -
ethyl O -(2,4,5-
trichlorophenyl)
ester
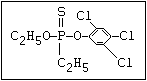 trifenofos RH 218 O -ethyl- S -propyl- O- C11 H14 Cl3 O3 PS 363.6 38524-82-2
(2,4,6-tri-chloro-
phenyl) phosphoro
thioate
trifenofos RH 218 O -ethyl- S -propyl- O- C11 H14 Cl3 O3 PS 363.6 38524-82-2
(2,4,6-tri-chloro-
phenyl) phosphoro
thioate
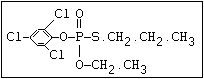 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
vamidothion kilyal phosphorothioic C8 H18 NO4 PS2 287.36 2275-23-2
trucidor acid, O,O -dimethyl
vamidoate S -[2-[[1-methyl-2-
(methylamino)-2-
oxoethyl]thio]ethyl]
ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
vamidothion kilyal phosphorothioic C8 H18 NO4 PS2 287.36 2275-23-2
trucidor acid, O,O -dimethyl
vamidoate S -[2-[[1-methyl-2-
(methylamino)-2-
oxoethyl]thio]ethyl]
ester
 -------------------------------------------------------------------------------------------
Annex II. Organophosphorus pesticides: JMPR reviews, ADIs, Evaluation by IARC, Classification by
Hazard, FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Acephate 1984 0-0.0005 1985a
(temporary)
1982 0-0.003 1983b + III
(temporary)
1983a
1981i 0-0.02 1982b
1982a
1979i 0-0.02 1980b
1980a
1978 0-0.02 1979a
1976 0-0.02 1977b
1977a
Azinphos 1983i no ADI 1984a + +
-ethyl 1973 no ADI 1974b IB
1974a
Azinphos 1974i 1975b + + IB
-methyl 1975a
1973 0-0.0025 1974b
1974a
1972i 0-0.0025 1973b
1973a
1968 0-0.0025 1969b
1969a
1965 1965b
0-0.0025 1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Bromophos 1984i 0-0.04 1985b
1982i 0-0.04 1983b + + III
1983a
1978i 0-0.04 1979a
1977 0-0.04 1978b
1978a
1975i 0-0.006 1976b
(temporary) 1976a
1972 0-0.006 1973b
(temporary) 1973a
Bromophos 1978i 0-0.003 1979a + + IB
-ethyl 1977i 0-0.003 1978b
1978a
1975 0-0.003 1976b
1976a
1972 0-0.003 1973b
(temporary) 1973a
Carbophen- 1983i 0-0.0005 1984a + + IB
othion 1980 0-0.0005 1981b
1981a
1979 0-0.0005 1980b
1980a
1977 0-0.0002 1978b
(temporary) 1978a
1976 temporary 1977b
ADI withdrawn
1977a
1972 0-0.005 1973b
(temporary)
1973a
Chlorfen- 1971 0-0.002 1972b + + IA
vinphos 1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Chlorpy- 1983i 0-0.01 1984a + + II No. 18
rifos 1982 0-0.01 1983b (1975)
1983a
1981i 0-0.001 1982b
1982a
1977 0-0.001 1978b
1978a
1975i 0-0.0015 1976b
1976a
1974i 0-0.0015 1975b
1975a
1972 0-0.0015 1973b
1973a
Chlorpy- 1979i 0-0.01 1980b + + No. 33
rifos- 1980a (1978)
methyl 1975 0-0.01 1976b II
1976a
Chlorthion 1965 no ADI 1965b
1965a -
1963 no ADI 1964
Coumaphos 1983i ADI withdrawn 1984a + + IA
1980i ADI Withdrawn 1981b
1981a
1978i 0-0.005 1979b
(temporary)
1979a
1975i 0-0.005 1976b
(temporary)
1976a
1972i 0-0.0005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1968 0-0.0005 1969b
(temporary)
1969a
Crufomate 1972i 0-0.1 1973b III
1973a
1968 0-0.1 1969b
1969a
Cyano- 1983 ADI withdrawn 1984a II
fenphos 1982i 0-0.001 1983b
(temporary)
1983a
1980 0-0.001 1981b
(temporary)
1981a
1978i 0-0.005 1979a
(temporary)
Cyano- 1975 0-0.005 1976b
fenphos (temporary)
1976a
Demeton 1983i no ADI 1984a + +
(see also 1982 ADI withdrawn 1983a
disulfoton) 1975 0-0.005 1976b IA No. 60
(in prep)
1976a
1967i 0-0.0025 1968b
1968a
1965 0-0.0025 1965b
1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- S - 1984 no ADI 1985b
methyl and 1983i no ADI 1984a + + IB
Related 1982 ADI withdrawn 1983a
Compounds 1979i 0-0.005 (the 1980b
(see also total demeton-
oxydemeton- S -methyl, 1980a IB
methyl for demeton- S -
1963 to 1968 methyl sulfo-
evaluations) xide and deme-
ton- S -methyl
sulfone not
to exceed
this figure)
1973 0-0.005 (the 1974b
total demeton 1974a
- S -methyl,
demeton- S -
methyl sulf-
oxide and
demeton- S -
methyl sulfone
not to exceed
this figure)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- 1984 no ADI 1985b
S -methyl 1983i no ADI 1984a
sulfoxide 1982 ADI 1983a IB
(see withdrawn
oxydemeton-
methyl for
1963 to 1968
evaluation)
(see demeton-
S -methyl and
related
compounds
after 1968)
Dialifos 1982 ADI 1983a II
withdrawn
1978i 0-0.003 1979a
1976 0-0.003 1977b
1977a
Diazinon 1979i 0-0.002 1983a + + II No. 45/1979
1980a
1975i 0-0.002 1976b
1976a
1970 0-0.002 1971b
1971a
1968i 0-0.002 1969b
1969a
1967i 0-0.002 1968b
1968a
1966 0-0.002 1969b
1967a
1965 0-0.002 1965b
1965a
1963 no ADI 1964 +
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Dichlorvos 1977 0-0.004 1978b Vol 20 + + IB No. 2 (1978)
p.97 (Rev. 1)
1978a
1974i 0-0.004 1975b
1975a
1970 0-0.004 1971b
1971a
1969i 0-0.004 1970b
1970a
1967 1968b
0-0.004 1968a
1966 1967b
0-0.004 1967a
1965 no ADI 1965b
1965a
Dimethoate 1984 0-0.002 1985b
(temporary)
1983i 0-0.02 1984a + + No. 42
1978i 0-0.02 1979a (1980)
1977i 0-0.02 1978b Vol. 15 II
page 177
1970i 0-0.02 1971b
1971a
1967 0-0.02 1968b
1968a
1966i 0-0.004 1967b
1967a
1965 0-0.004 1965b
1965a
1963 0-0.004 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Disulfoton 1984i 0-0.002 1985b
(see also 1981i 0-0.002 1982b + + No. 60 (in
demeton) prep.)
1982a
1979i 0-0.002 1980b IA
1980a
1978i 0-0.002 1979a
1975 0-0.002 1976b
1976a
1973 0-0.001 1974b
(temporary)
1974a
Edifenphos 1981 0-0.003 1982b + IB
1982a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Ethion 1985 0-0.0005 1986b
(temporary)
1983i 0-0.001 1984a + + II
(temporary)
1982 0-0.001 1983b
(temporary)
1983a
1975i 0-0.005 1976b
1976a
1972 0-0.005 1973b
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1970i 0-0.00125 1971b
1971a
1969i 0-0.00125 1970b
1970a
1968 0-0.00125 1969b
1969a
Ethoprophos 1983 no ADI 1984a IA No. 70 (in
preparation)
Etrimfos 1982 0-0.003 1983b + + II
1983a
1980 0-0.003 1981b
(temporary)
1981a
Fenamiphos 1985 0-0.0003 1986b
(temporary)
1980i 0-0.0006 1981b IA
1981a
1978i 0-0.0006 1979b
R30-17
1977i 0-0.0006 1978b
1978a
1974 0-0.0006 1975b
1975a
Fenclorphos 1983i 0-0.01 1984a + + II
1972i 0-0.01 1973b
1973a
1968 0-0.01 1969b
1969a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenitro- 1984 0-0.003 1985a
thion 1983i 0-0.001 1974a + + No. 30
(temporary) (1977)
1982 0-0.001 1983b
(temporary)
1983a II
1979i 0-0.005 1980b
1980a
1977 0-0.005 1978b
1978a
1976i 0-0.005 1977b
1977a
1974 0-0.005 1975b
1975a
1969 0-0.001 1970b
(temporary)
1970a
Fensulf- 1983i 0-0.0003 1984a + +
othion 1982 0-0.0003 1983b
1983a IA No. 44
1972 0-0.0003 1973b (1980)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenthion 1983i 0-0.001 1984a + No. 23
1980 0-0.001 1981b (1976)
1981a IB
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1977i 0-0.0005 1978b
(temporary)
1978a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
Formothion 1978i 0-0.02 1979a + + II
1973 0-0.02 1974b
1974a
1972i no ADI 1973b
1973a
1969 no ADI 1970b
1970a
Isophenphos 1984i 0-0.0005 1985a
1982 0-0.0005 1983b IB
(temporary)
1983a
1981 0-0.0005 1982b
(temporary)
1982a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Iodophenphos O No. 43
(1980)
Leptophos 1978i ADI 1979b + + No. 38
withdrawn 1979a (1979)
1976i 0-0.001 1977a IA
(temporary)
1975 0-0.001 1976b
(temporary)
1976a
1974 No ADI 1975b
1975a
Malathion 1984 0-0.02 1985b
1977i 0-0.02 1978b Vol. 30 + + No. 29
page 103 (1977)
1978a
1975i 0-0.02 1976b III
1976a
1973i 0-0.02 1974b
1974a
1970i 0-0.02 1971b
1971a
1969i 0-0.02 1970b
1970a
1968i 0-0.02 1969b
1969a
1967i 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Mecarbam 1985 0-0.0005 1986b
(temporary)
1983 0-0.001 1984a IB
(temporary)
1980 0-0.001 1981b
(temporary)
1981a
Methacrifos 1982 0-0.0003 1983b -
(temporary)
1983a
1980 0-0.0003 1981b
(temporary)
1981a
Methamido- 1985 0-0.0006 1986b
phos 1984i 0-0.0004 1985a
(temporary)
1982 0-0.0004 1983b + + IB
(temporary)
1983a
1981i 0-0.002 1982b
1982a
1979i 0-0.002 1980b
1980a
1976 0-0.002 1977b
1977a
Methida- 1979i 0-0.005 1980b + + IB
thion 1980a
1977i 0-0.005 1978a
1975 0-0.005 1976b
1976a
1972 0-0.005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe> : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Methyl parathion
(see parathionmethyl)
Mevinphos 1972 0-0.0015 1973b + + IA No. 14
1973a (1975)
1965 no ADI 1965b +
1965a
1963 no ADI 1964
Monocroto- 1975 0-0.0006 1976b + + IB
phos 1976a
1972 0-0.0003 1973b
1973a
Omethoate 1985 0-0.0003 1986b
1984i 0-0.0005 1985a
(temporary)
1981 0-0.0005 1982b IB
(temporary)
1982a
1980i 0-0.0005 1981b
(temporary)
1981a
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Oxydemeton- 1968i ADI 1969b
methyl withdrawn 1969a IB
(referred in 1967 0-0.0025 1968b
1963 and 1965 1968a
reports as 1965 0-0.0025 1965b
demeton- S - 1965a
methyl 1963 0-0.0025 1964
sulfoxide)
(see
demeton- S -
methyl and
related comp-
ounds for
evaluations
after 1968)
Parathion 1984i 0-0.005 1985a
1970i 0-0.005 1971b Vol. 30 + + IA No. 6 (1978)
page 153 (Rev. 1)
1971a
1969i 0-0.005 1970b
1970a
1967 0-0.005 1968b
1968a
1965 0-0.005 1965b
1965a
1963 0-0.005 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Parathion- 1984 0-0.02 1985b
methyl
(evaluated 1982 0-0.001 1983b vol. page + + IA No. 7 (1978)
under methyl (temporary) 30-131 (Rev. 1)
parathion 1983a
in 1963 and 1980 0-0.001 1981b
1965) (temporary)
1981a
1979 0-0.001 1980b
(temporary)
1980a
1978i 0-0.001 1979b
(temporary)
1979a
1975 0-0.001 1976b
(temporary)
1976a
1972i 0-0.001 1973b
(temporary)
1973a
1968 0-0.001 1969b
(temporary)
1969a
1965 0-0.01 1965b
1965a
1963 0-0.01 1964
Phenthoate 1984 0-0.003 1985b
1981i 0-0.001 1982b + + II No. 48
(temporary) (1983)
1982a
1980 0-0.001 1981b
(temporary)
1981a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phorate 1985 0-0.0002 1986b
1984i 0-0.0002 1985a
(temporary)
1983 0-0.0002 1984a + + IA No. 75 (in
(temporary) preparation)
1982 0-0.0002 1983b
(temporary)
1983a
1977 No ADI 1978b
established
1978a
Phosalone 1976i 0-0.006 1977b + + II
1977a
1975i 0-0.006 1976b
1976a
1972 0-0.006 1973b
1973a
Phosmet 1984i 0-0.02 1985b
1981i 0-0.02 1982b + + II
1982a
1979 0-0.02 1980b
1980a
1978 0-0.005 1979b
(temporary)
1979a
1977 -corrigenda 1978b
to 1976
evaluations -
1977i no ADI 1978a
1976i no ADI 1977b
1977a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phosphamidon 1985 0-0.0005 1986b
1982 0-0.001 1983b + + IA No. 74 (in
(temporary) preparation)
1983a
1974i 0-0.01 1975b
1975a
1972i 0-0.001 1973b
1973a
1969i 0-0.001 1970b
1970a
1968 0-0.001 1969b
1969a
1966 0-0.001 1967b
1967a
1965 no ADI 1965b
1965a
1963 no ADI 1964
Phoxim 1984 0-0.001 1985b
1983i 0-0.0005 1984a No. 31 (1978)
(temporary)
1982 0-0.0005 1983b
(temporary)
1983a II
Pirimiphos- 1983 0-0.01 1984a No. 49 (1983)
methyl 1979i 0-0.01 1980b
1980a
1977i 0-0.01 1978b III
1978a
1976 0-0.01 1977b
1977a
1974 0-0.005 1975b
(temporary)
1975a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Temephos + + 0 No. 8 (1978)
(Rev. 1)
Thiometon 1979 0-0.003 1980b + + IB
1980a
1976i 0-0.005 1977b
(temporary)
1977a
1973 0-0.005 1974b
(temporary)
1974a
1969 no ADI 1970b
1970a
Triazophos 1983i 0-0.0002 1984a IB
(temporary)
1982 0-0.0002 1983b
(temporary)
1983a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Trichlorfon 1978 0-0.01 1979b + + III No. 27 (1977)
1979a
1975 0-0.005 1976b Vol. 30
(temporary) page 207
1976a
1971 0-0.01 1972b
(temporary)
1972a
Trichloronat 1971 no ADI 1972b + + IA
1972a
Vamidothion 1985 0-0.0003 1986b
(temporary)
1982 0-0.0003 1983b + + IB
(temporary)
1983a
1973 no ADI 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in
this annex.
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling
by the manufacturer or in accordance with the rules laid down for storage and transportation
by competent international bodies.
Classification relates to the technical material, and not to the formulated
product:
--------------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
--------------------------------------------------------------------------------------
IA Extremely hazardous 5 or less 20 or less 10 or less 40 or less
IB Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal
use
--------------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex III. LD50s and no-observed-adverse-effect levels in animals
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Azinophos- 16.4 2.5 0.125 2 years FAO/WHO (1974b)
methyl 80 (guinea-pig) 5 (dog) 0.125 (dog) 2 years
Bromophos- 71 - 127 1366 (rabbit) 0.78 2 years FAO/WHO (1976b)
ethyl 10 (dog) 0.26 (dog) 2 years
225 - 550 (mice)
Bromophos 3750 - 7700 20 (dog) 0.5 (dog) 1 year FAO/WHO (1978b)
2829 - 5850 0.4 (man) 4 weeks
(mice)
720 (rabbit)
Carbopheno- 32.3 1270 (rabbit) 3 0.15 3 generations FAO/WHO (1980b)
thion 0.02 (dog) 3 months
0.01 (man) 1 month
Chlorfen- 10 - 39 31 - 108, 1 0.05 3 months FAO/WHO (1972b)
vinphos 117 - 200 (mice) 417 - 4700 1 dog 0.05 (dog) 16 weeks
(rabbit)
300 - 1000
(rabbit)
> 12 000 (dog)
Chlorpyr- 135 - 163 approximately 0.1 2 years FAO/WHO (1982b)
ifos 2000
500 (guinea-pig) (rabbit) 0.1 (dog) 90 days
32 (chicken) 0.1 (man) 1 month
1000 - 2000
(rabbit)
Crufomate 770 - 950 40 2 2 year FAO/WHO (1969b)
400 - 600 40 (dog) 1 dog 75 days
(rabbit)
Demeton 2.5 - 12
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Demeton- S - 57 - 106 302
methyl 110 (guinea-pig)
Diazinon 300 - 850 > 2150 2 0.1 90 days FAO/WHO (1971b)
0.02 (dog) 31 days
0.05 (monkey) 2 years
0.02 (man) 37 days
Dichlorvos 56 - 108 75 - 210 0.033 (man) 28 days FAO/WHO (1977b)
5 0.25 15 weeks
Dimethoate 320 - 380 0.2 (man) 57 days FAO/WHO (1985b)
15 (pheasant)
40 (duck)
Dioxathion 43 235 3 0.15 13 weeks FAO/WHO (1969b)
0.075 (dog) 90 days
0.075 (man) 28 days
Disulfoton 2.6 - 8.6 ca 20 1 0.05 2 years FAO/WHO (1976b)
1 (dog) 0.025 (dog) 12 weeks
0.075 (man) 30 days
Ethion 24.4 - 208 915 3 0.15 13 weeks FAO/WHO (1986b)
(rabbit) 0.125 (dog) 90 days
Fenamiphos 15.3 - 19.4 500 3 0.17 2 years FAO/WHO (1986b)
10 (dog) 1 (dog) 0.025 (dog) 2 years
75 - 100
(guinea-pig)
12 (hen)
Fenchlor- 1740 2000 0.5 2 years FAO/WHO (1969b)
phos 1 (dog) 2 years
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Fenitro- 250 - 500 > 3000 5 0.25 34 weeks FAO/WHO (1985b)
thion 870 (mice) (mice) 10 (dog) 0.3 (dog) 12 months
Fenthion 190 - 315 330 - 500 3 0.15 2 years FAO/WH0 (1981b)
3 (dog) 0.09 2 years
0.07 (monkey) 1 year
0.02 (man) -
Formothion 365 - 500 > 1000 20 1 2 years FAO/WHO (1974b)
40 (dog) 1 (dog) 2 years
Malathion 2800 4100 100 5 2 years FAO/WHO (1967b)
(rabbit) 0.2 (man) 88 days
Methida- 25 - 54 1546 - 1663 4 0.2 104 weeks FAO/WHO (1976b)
thion 25 - 20 (mice) 0.25 (monkey) 23 months
0.11 (man) 6 weeks
Mevinphos 3 - 12 1 - 90 0.37 0.02 2 years FAO/WHO (1973b)
7 - 18 (mice) 16 - 34 0.025 (dog) 2 years
(rabbit) 0.014 (man) 30 days
Monocroto- 14 - 23 336 0.5 0.025 12 weeks FAO/WHO (1973b)
phos (rabbit) 0.5 (dog) 0.0125 (dog) 13 weeks
Omethoate ca 50 700 1 0.05 3 months FAO/WHO (1986b)
0.025 (dog) 12 months
Parathion 3.6 - 13 6.8 - 21 0.05 (man) 3 weeks FAO/WHO (1967b)
Parathion- 2 0.1 2 years FAO/WHO (1985b)
methyl 14 - 24 67 0.3 (man) 30 days
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Phosalone 120 - 170 1500 25 1.25 2 years FAO/WHO (1973b)
180 (mice) > 1000 25 (dog) 0.625 2 years
(rabbit)
380 (guinea-pig)
290 (pheasant)
Phospha- 17 - 30 374 - 530 2 0.1 12 weeks FAO/WHO (1986)
midon 0.5 (dog) 90 days
Pirimiphos- 2050 > 2000 10 0.5 2 years FAO/WHO (1977b)
methyl (rabbit) 5 (mouse) 0.5 (mouse) 80 weeks
1180 (mice) 0.25 (man) 28 days
1000 - 2000
(guinea-pig)
1150 - 2300
(rabbit)
30 - 60 (hen)
Thiometon 120 - 130 > 1000 2.5 0.12 2 years FAO/WHO (1980b)
6 (dog) 0.5 (dog) 2 years
Trichlorfon 560 - 630 > 2000 50 2.5 2 years FAO/WHO (1979b)
50 (dog) 1.25 (dog) 4 years (dog)
---------------------------------------------------------------------------------------------------------
a From: Worthing (1983).
N.B. This reference is listed in the reference list of the main document.
Annex IV. Abbreviations used in the document
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
DDE dichlorodiphenyldichloroethylene
DDT dichlorodiphenyltrichloroethane
DEF S,S,S -tributyl phosphorotrithioate
DFP di-isopropyl fluorophosphate
EEG electroencephalogram
EMG electromyography
EPN o -ethyl- O -(4-nitrophenyl)phenylphosphonothioate
FAO Food and Agricultural Organization (United Nations)
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety
(World Health Organization)
IRPTC International Register of Potentially Toxic
Chemicals (United Nations Environment Programme)
iv intravenous
JMPR FAO/WHO Joint Meeting on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NAD nicotinamide-adenine-dinucleotide
NADPH nicotinamide-adenine-dinucleotide phosphate
(reduced form)
NTE neuropathy target esterase (formerly
neurotoxic esterase)
OMPA octamethylpyrophosphorictetramide
2-PAM pyridine-2-aldoxime methyl chloride
pseudoChE pseudocholinesterase
sc subcutaneous
TCDD 2,3,7,8-tetrachlorodibenzo-1,4-dioxin
TEPP tetraethyl pyrophosphate
TOCP tri- o -cresyl phosphate
UVR ultraviolet radiation
-------------------------------------------------------------------------------------------
Annex II. Organophosphorus pesticides: JMPR reviews, ADIs, Evaluation by IARC, Classification by
Hazard, FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Acephate 1984 0-0.0005 1985a
(temporary)
1982 0-0.003 1983b + III
(temporary)
1983a
1981i 0-0.02 1982b
1982a
1979i 0-0.02 1980b
1980a
1978 0-0.02 1979a
1976 0-0.02 1977b
1977a
Azinphos 1983i no ADI 1984a + +
-ethyl 1973 no ADI 1974b IB
1974a
Azinphos 1974i 1975b + + IB
-methyl 1975a
1973 0-0.0025 1974b
1974a
1972i 0-0.0025 1973b
1973a
1968 0-0.0025 1969b
1969a
1965 1965b
0-0.0025 1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Bromophos 1984i 0-0.04 1985b
1982i 0-0.04 1983b + + III
1983a
1978i 0-0.04 1979a
1977 0-0.04 1978b
1978a
1975i 0-0.006 1976b
(temporary) 1976a
1972 0-0.006 1973b
(temporary) 1973a
Bromophos 1978i 0-0.003 1979a + + IB
-ethyl 1977i 0-0.003 1978b
1978a
1975 0-0.003 1976b
1976a
1972 0-0.003 1973b
(temporary) 1973a
Carbophen- 1983i 0-0.0005 1984a + + IB
othion 1980 0-0.0005 1981b
1981a
1979 0-0.0005 1980b
1980a
1977 0-0.0002 1978b
(temporary) 1978a
1976 temporary 1977b
ADI withdrawn
1977a
1972 0-0.005 1973b
(temporary)
1973a
Chlorfen- 1971 0-0.002 1972b + + IA
vinphos 1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Chlorpy- 1983i 0-0.01 1984a + + II No. 18
rifos 1982 0-0.01 1983b (1975)
1983a
1981i 0-0.001 1982b
1982a
1977 0-0.001 1978b
1978a
1975i 0-0.0015 1976b
1976a
1974i 0-0.0015 1975b
1975a
1972 0-0.0015 1973b
1973a
Chlorpy- 1979i 0-0.01 1980b + + No. 33
rifos- 1980a (1978)
methyl 1975 0-0.01 1976b II
1976a
Chlorthion 1965 no ADI 1965b
1965a -
1963 no ADI 1964
Coumaphos 1983i ADI withdrawn 1984a + + IA
1980i ADI Withdrawn 1981b
1981a
1978i 0-0.005 1979b
(temporary)
1979a
1975i 0-0.005 1976b
(temporary)
1976a
1972i 0-0.0005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1968 0-0.0005 1969b
(temporary)
1969a
Crufomate 1972i 0-0.1 1973b III
1973a
1968 0-0.1 1969b
1969a
Cyano- 1983 ADI withdrawn 1984a II
fenphos 1982i 0-0.001 1983b
(temporary)
1983a
1980 0-0.001 1981b
(temporary)
1981a
1978i 0-0.005 1979a
(temporary)
Cyano- 1975 0-0.005 1976b
fenphos (temporary)
1976a
Demeton 1983i no ADI 1984a + +
(see also 1982 ADI withdrawn 1983a
disulfoton) 1975 0-0.005 1976b IA No. 60
(in prep)
1976a
1967i 0-0.0025 1968b
1968a
1965 0-0.0025 1965b
1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- S - 1984 no ADI 1985b
methyl and 1983i no ADI 1984a + + IB
Related 1982 ADI withdrawn 1983a
Compounds 1979i 0-0.005 (the 1980b
(see also total demeton-
oxydemeton- S -methyl, 1980a IB
methyl for demeton- S -
1963 to 1968 methyl sulfo-
evaluations) xide and deme-
ton- S -methyl
sulfone not
to exceed
this figure)
1973 0-0.005 (the 1974b
total demeton 1974a
- S -methyl,
demeton- S -
methyl sulf-
oxide and
demeton- S -
methyl sulfone
not to exceed
this figure)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- 1984 no ADI 1985b
S -methyl 1983i no ADI 1984a
sulfoxide 1982 ADI 1983a IB
(see withdrawn
oxydemeton-
methyl for
1963 to 1968
evaluation)
(see demeton-
S -methyl and
related
compounds
after 1968)
Dialifos 1982 ADI 1983a II
withdrawn
1978i 0-0.003 1979a
1976 0-0.003 1977b
1977a
Diazinon 1979i 0-0.002 1983a + + II No. 45/1979
1980a
1975i 0-0.002 1976b
1976a
1970 0-0.002 1971b
1971a
1968i 0-0.002 1969b
1969a
1967i 0-0.002 1968b
1968a
1966 0-0.002 1969b
1967a
1965 0-0.002 1965b
1965a
1963 no ADI 1964 +
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Dichlorvos 1977 0-0.004 1978b Vol 20 + + IB No. 2 (1978)
p.97 (Rev. 1)
1978a
1974i 0-0.004 1975b
1975a
1970 0-0.004 1971b
1971a
1969i 0-0.004 1970b
1970a
1967 1968b
0-0.004 1968a
1966 1967b
0-0.004 1967a
1965 no ADI 1965b
1965a
Dimethoate 1984 0-0.002 1985b
(temporary)
1983i 0-0.02 1984a + + No. 42
1978i 0-0.02 1979a (1980)
1977i 0-0.02 1978b Vol. 15 II
page 177
1970i 0-0.02 1971b
1971a
1967 0-0.02 1968b
1968a
1966i 0-0.004 1967b
1967a
1965 0-0.004 1965b
1965a
1963 0-0.004 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Disulfoton 1984i 0-0.002 1985b
(see also 1981i 0-0.002 1982b + + No. 60 (in
demeton) prep.)
1982a
1979i 0-0.002 1980b IA
1980a
1978i 0-0.002 1979a
1975 0-0.002 1976b
1976a
1973 0-0.001 1974b
(temporary)
1974a
Edifenphos 1981 0-0.003 1982b + IB
1982a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Ethion 1985 0-0.0005 1986b
(temporary)
1983i 0-0.001 1984a + + II
(temporary)
1982 0-0.001 1983b
(temporary)
1983a
1975i 0-0.005 1976b
1976a
1972 0-0.005 1973b
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1970i 0-0.00125 1971b
1971a
1969i 0-0.00125 1970b
1970a
1968 0-0.00125 1969b
1969a
Ethoprophos 1983 no ADI 1984a IA No. 70 (in
preparation)
Etrimfos 1982 0-0.003 1983b + + II
1983a
1980 0-0.003 1981b
(temporary)
1981a
Fenamiphos 1985 0-0.0003 1986b
(temporary)
1980i 0-0.0006 1981b IA
1981a
1978i 0-0.0006 1979b
R30-17
1977i 0-0.0006 1978b
1978a
1974 0-0.0006 1975b
1975a
Fenclorphos 1983i 0-0.01 1984a + + II
1972i 0-0.01 1973b
1973a
1968 0-0.01 1969b
1969a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenitro- 1984 0-0.003 1985a
thion 1983i 0-0.001 1974a + + No. 30
(temporary) (1977)
1982 0-0.001 1983b
(temporary)
1983a II
1979i 0-0.005 1980b
1980a
1977 0-0.005 1978b
1978a
1976i 0-0.005 1977b
1977a
1974 0-0.005 1975b
1975a
1969 0-0.001 1970b
(temporary)
1970a
Fensulf- 1983i 0-0.0003 1984a + +
othion 1982 0-0.0003 1983b
1983a IA No. 44
1972 0-0.0003 1973b (1980)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenthion 1983i 0-0.001 1984a + No. 23
1980 0-0.001 1981b (1976)
1981a IB
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1977i 0-0.0005 1978b
(temporary)
1978a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
Formothion 1978i 0-0.02 1979a + + II
1973 0-0.02 1974b
1974a
1972i no ADI 1973b
1973a
1969 no ADI 1970b
1970a
Isophenphos 1984i 0-0.0005 1985a
1982 0-0.0005 1983b IB
(temporary)
1983a
1981 0-0.0005 1982b
(temporary)
1982a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Iodophenphos O No. 43
(1980)
Leptophos 1978i ADI 1979b + + No. 38
withdrawn 1979a (1979)
1976i 0-0.001 1977a IA
(temporary)
1975 0-0.001 1976b
(temporary)
1976a
1974 No ADI 1975b
1975a
Malathion 1984 0-0.02 1985b
1977i 0-0.02 1978b Vol. 30 + + No. 29
page 103 (1977)
1978a
1975i 0-0.02 1976b III
1976a
1973i 0-0.02 1974b
1974a
1970i 0-0.02 1971b
1971a
1969i 0-0.02 1970b
1970a
1968i 0-0.02 1969b
1969a
1967i 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Mecarbam 1985 0-0.0005 1986b
(temporary)
1983 0-0.001 1984a IB
(temporary)
1980 0-0.001 1981b
(temporary)
1981a
Methacrifos 1982 0-0.0003 1983b -
(temporary)
1983a
1980 0-0.0003 1981b
(temporary)
1981a
Methamido- 1985 0-0.0006 1986b
phos 1984i 0-0.0004 1985a
(temporary)
1982 0-0.0004 1983b + + IB
(temporary)
1983a
1981i 0-0.002 1982b
1982a
1979i 0-0.002 1980b
1980a
1976 0-0.002 1977b
1977a
Methida- 1979i 0-0.005 1980b + + IB
thion 1980a
1977i 0-0.005 1978a
1975 0-0.005 1976b
1976a
1972 0-0.005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe> : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Methyl parathion
(see parathionmethyl)
Mevinphos 1972 0-0.0015 1973b + + IA No. 14
1973a (1975)
1965 no ADI 1965b +
1965a
1963 no ADI 1964
Monocroto- 1975 0-0.0006 1976b + + IB
phos 1976a
1972 0-0.0003 1973b
1973a
Omethoate 1985 0-0.0003 1986b
1984i 0-0.0005 1985a
(temporary)
1981 0-0.0005 1982b IB
(temporary)
1982a
1980i 0-0.0005 1981b
(temporary)
1981a
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Oxydemeton- 1968i ADI 1969b
methyl withdrawn 1969a IB
(referred in 1967 0-0.0025 1968b
1963 and 1965 1968a
reports as 1965 0-0.0025 1965b
demeton- S - 1965a
methyl 1963 0-0.0025 1964
sulfoxide)
(see
demeton- S -
methyl and
related comp-
ounds for
evaluations
after 1968)
Parathion 1984i 0-0.005 1985a
1970i 0-0.005 1971b Vol. 30 + + IA No. 6 (1978)
page 153 (Rev. 1)
1971a
1969i 0-0.005 1970b
1970a
1967 0-0.005 1968b
1968a
1965 0-0.005 1965b
1965a
1963 0-0.005 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Parathion- 1984 0-0.02 1985b
methyl
(evaluated 1982 0-0.001 1983b vol. page + + IA No. 7 (1978)
under methyl (temporary) 30-131 (Rev. 1)
parathion 1983a
in 1963 and 1980 0-0.001 1981b
1965) (temporary)
1981a
1979 0-0.001 1980b
(temporary)
1980a
1978i 0-0.001 1979b
(temporary)
1979a
1975 0-0.001 1976b
(temporary)
1976a
1972i 0-0.001 1973b
(temporary)
1973a
1968 0-0.001 1969b
(temporary)
1969a
1965 0-0.01 1965b
1965a
1963 0-0.01 1964
Phenthoate 1984 0-0.003 1985b
1981i 0-0.001 1982b + + II No. 48
(temporary) (1983)
1982a
1980 0-0.001 1981b
(temporary)
1981a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phorate 1985 0-0.0002 1986b
1984i 0-0.0002 1985a
(temporary)
1983 0-0.0002 1984a + + IA No. 75 (in
(temporary) preparation)
1982 0-0.0002 1983b
(temporary)
1983a
1977 No ADI 1978b
established
1978a
Phosalone 1976i 0-0.006 1977b + + II
1977a
1975i 0-0.006 1976b
1976a
1972 0-0.006 1973b
1973a
Phosmet 1984i 0-0.02 1985b
1981i 0-0.02 1982b + + II
1982a
1979 0-0.02 1980b
1980a
1978 0-0.005 1979b
(temporary)
1979a
1977 -corrigenda 1978b
to 1976
evaluations -
1977i no ADI 1978a
1976i no ADI 1977b
1977a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phosphamidon 1985 0-0.0005 1986b
1982 0-0.001 1983b + + IA No. 74 (in
(temporary) preparation)
1983a
1974i 0-0.01 1975b
1975a
1972i 0-0.001 1973b
1973a
1969i 0-0.001 1970b
1970a
1968 0-0.001 1969b
1969a
1966 0-0.001 1967b
1967a
1965 no ADI 1965b
1965a
1963 no ADI 1964
Phoxim 1984 0-0.001 1985b
1983i 0-0.0005 1984a No. 31 (1978)
(temporary)
1982 0-0.0005 1983b
(temporary)
1983a II
Pirimiphos- 1983 0-0.01 1984a No. 49 (1983)
methyl 1979i 0-0.01 1980b
1980a
1977i 0-0.01 1978b III
1978a
1976 0-0.01 1977b
1977a
1974 0-0.005 1975b
(temporary)
1975a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Temephos + + 0 No. 8 (1978)
(Rev. 1)
Thiometon 1979 0-0.003 1980b + + IB
1980a
1976i 0-0.005 1977b
(temporary)
1977a
1973 0-0.005 1974b
(temporary)
1974a
1969 no ADI 1970b
1970a
Triazophos 1983i 0-0.0002 1984a IB
(temporary)
1982 0-0.0002 1983b
(temporary)
1983a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Trichlorfon 1978 0-0.01 1979b + + III No. 27 (1977)
1979a
1975 0-0.005 1976b Vol. 30
(temporary) page 207
1976a
1971 0-0.01 1972b
(temporary)
1972a
Trichloronat 1971 no ADI 1972b + + IA
1972a
Vamidothion 1985 0-0.0003 1986b
(temporary)
1982 0-0.0003 1983b + + IB
(temporary)
1983a
1973 no ADI 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in
this annex.
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling
by the manufacturer or in accordance with the rules laid down for storage and transportation
by competent international bodies.
Classification relates to the technical material, and not to the formulated
product:
--------------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
--------------------------------------------------------------------------------------
IA Extremely hazardous 5 or less 20 or less 10 or less 40 or less
IB Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal
use
--------------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex III. LD50s and no-observed-adverse-effect levels in animals
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Azinophos- 16.4 2.5 0.125 2 years FAO/WHO (1974b)
methyl 80 (guinea-pig) 5 (dog) 0.125 (dog) 2 years
Bromophos- 71 - 127 1366 (rabbit) 0.78 2 years FAO/WHO (1976b)
ethyl 10 (dog) 0.26 (dog) 2 years
225 - 550 (mice)
Bromophos 3750 - 7700 20 (dog) 0.5 (dog) 1 year FAO/WHO (1978b)
2829 - 5850 0.4 (man) 4 weeks
(mice)
720 (rabbit)
Carbopheno- 32.3 1270 (rabbit) 3 0.15 3 generations FAO/WHO (1980b)
thion 0.02 (dog) 3 months
0.01 (man) 1 month
Chlorfen- 10 - 39 31 - 108, 1 0.05 3 months FAO/WHO (1972b)
vinphos 117 - 200 (mice) 417 - 4700 1 dog 0.05 (dog) 16 weeks
(rabbit)
300 - 1000
(rabbit)
> 12 000 (dog)
Chlorpyr- 135 - 163 approximately 0.1 2 years FAO/WHO (1982b)
ifos 2000
500 (guinea-pig) (rabbit) 0.1 (dog) 90 days
32 (chicken) 0.1 (man) 1 month
1000 - 2000
(rabbit)
Crufomate 770 - 950 40 2 2 year FAO/WHO (1969b)
400 - 600 40 (dog) 1 dog 75 days
(rabbit)
Demeton 2.5 - 12
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Demeton- S - 57 - 106 302
methyl 110 (guinea-pig)
Diazinon 300 - 850 > 2150 2 0.1 90 days FAO/WHO (1971b)
0.02 (dog) 31 days
0.05 (monkey) 2 years
0.02 (man) 37 days
Dichlorvos 56 - 108 75 - 210 0.033 (man) 28 days FAO/WHO (1977b)
5 0.25 15 weeks
Dimethoate 320 - 380 0.2 (man) 57 days FAO/WHO (1985b)
15 (pheasant)
40 (duck)
Dioxathion 43 235 3 0.15 13 weeks FAO/WHO (1969b)
0.075 (dog) 90 days
0.075 (man) 28 days
Disulfoton 2.6 - 8.6 ca 20 1 0.05 2 years FAO/WHO (1976b)
1 (dog) 0.025 (dog) 12 weeks
0.075 (man) 30 days
Ethion 24.4 - 208 915 3 0.15 13 weeks FAO/WHO (1986b)
(rabbit) 0.125 (dog) 90 days
Fenamiphos 15.3 - 19.4 500 3 0.17 2 years FAO/WHO (1986b)
10 (dog) 1 (dog) 0.025 (dog) 2 years
75 - 100
(guinea-pig)
12 (hen)
Fenchlor- 1740 2000 0.5 2 years FAO/WHO (1969b)
phos 1 (dog) 2 years
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Fenitro- 250 - 500 > 3000 5 0.25 34 weeks FAO/WHO (1985b)
thion 870 (mice) (mice) 10 (dog) 0.3 (dog) 12 months
Fenthion 190 - 315 330 - 500 3 0.15 2 years FAO/WH0 (1981b)
3 (dog) 0.09 2 years
0.07 (monkey) 1 year
0.02 (man) -
Formothion 365 - 500 > 1000 20 1 2 years FAO/WHO (1974b)
40 (dog) 1 (dog) 2 years
Malathion 2800 4100 100 5 2 years FAO/WHO (1967b)
(rabbit) 0.2 (man) 88 days
Methida- 25 - 54 1546 - 1663 4 0.2 104 weeks FAO/WHO (1976b)
thion 25 - 20 (mice) 0.25 (monkey) 23 months
0.11 (man) 6 weeks
Mevinphos 3 - 12 1 - 90 0.37 0.02 2 years FAO/WHO (1973b)
7 - 18 (mice) 16 - 34 0.025 (dog) 2 years
(rabbit) 0.014 (man) 30 days
Monocroto- 14 - 23 336 0.5 0.025 12 weeks FAO/WHO (1973b)
phos (rabbit) 0.5 (dog) 0.0125 (dog) 13 weeks
Omethoate ca 50 700 1 0.05 3 months FAO/WHO (1986b)
0.025 (dog) 12 months
Parathion 3.6 - 13 6.8 - 21 0.05 (man) 3 weeks FAO/WHO (1967b)
Parathion- 2 0.1 2 years FAO/WHO (1985b)
methyl 14 - 24 67 0.3 (man) 30 days
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Phosalone 120 - 170 1500 25 1.25 2 years FAO/WHO (1973b)
180 (mice) > 1000 25 (dog) 0.625 2 years
(rabbit)
380 (guinea-pig)
290 (pheasant)
Phospha- 17 - 30 374 - 530 2 0.1 12 weeks FAO/WHO (1986)
midon 0.5 (dog) 90 days
Pirimiphos- 2050 > 2000 10 0.5 2 years FAO/WHO (1977b)
methyl (rabbit) 5 (mouse) 0.5 (mouse) 80 weeks
1180 (mice) 0.25 (man) 28 days
1000 - 2000
(guinea-pig)
1150 - 2300
(rabbit)
30 - 60 (hen)
Thiometon 120 - 130 > 1000 2.5 0.12 2 years FAO/WHO (1980b)
6 (dog) 0.5 (dog) 2 years
Trichlorfon 560 - 630 > 2000 50 2.5 2 years FAO/WHO (1979b)
50 (dog) 1.25 (dog) 4 years (dog)
---------------------------------------------------------------------------------------------------------
a From: Worthing (1983).
N.B. This reference is listed in the reference list of the main document.
Annex IV. Abbreviations used in the document
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
DDE dichlorodiphenyldichloroethylene
DDT dichlorodiphenyltrichloroethane
DEF S,S,S -tributyl phosphorotrithioate
DFP di-isopropyl fluorophosphate
EEG electroencephalogram
EMG electromyography
EPN o -ethyl- O -(4-nitrophenyl)phenylphosphonothioate
FAO Food and Agricultural Organization (United Nations)
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety
(World Health Organization)
IRPTC International Register of Potentially Toxic
Chemicals (United Nations Environment Programme)
iv intravenous
JMPR FAO/WHO Joint Meeting on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NAD nicotinamide-adenine-dinucleotide
NADPH nicotinamide-adenine-dinucleotide phosphate
(reduced form)
NTE neuropathy target esterase (formerly
neurotoxic esterase)
OMPA octamethylpyrophosphorictetramide
2-PAM pyridine-2-aldoxime methyl chloride
pseudoChE pseudocholinesterase
sc subcutaneous
TCDD 2,3,7,8-tetrachlorodibenzo-1,4-dioxin
TEPP tetraethyl pyrophosphate
TOCP tri- o -cresyl phosphate
UVR ultraviolet radiation
 or leptophos [phosphonothioic acid, phenyl-, O -(4-bromo-2,5-
dichlorophenyl) O -methyl ester]:
or leptophos [phosphonothioic acid, phenyl-, O -(4-bromo-2,5-
dichlorophenyl) O -methyl ester]:
 Thus, divided doses may exert a greater toxic effect than the same
amount given as a single large dose (section 6.1.2).
The question of the bioavailability of preparations given by
the oral route needs to be considered. It must be taken into
account when discussing the results obtained for LD50s, and it is
certainly important when considering the toxicity of pesticides
residues. From a chemical point of view, these residues can be
described as parent compounds, free metabolites, and their
conjugates (Kaufman, 1976). The bioavailability of these
fractions and, thus, their toxic potential are not the same
(Dorough, 1976; Marshall & Dorough, 1977). In general, bound
residues appear to have a lower bioavailability and lower toxicity.
This was discussed by Rico & Burgat-Sacaze (1984), and demonstrated
for some pesticides residues by Marshall & Dorough (1977).
4.1.3 Inhalation
Total urinary output of 4-nitrophenol was compared in workers
spraying parathion, who either breathed a pure air supply but did
not wear protective clothing, or who wore total protective
clothing, but did not have any respiratory protection (Durham et
al., 1972). Output derived from the respiratory source compared
with that derived from the dermal source was 1.2% in one test and
12% in another. Since the total exposures by the dermal and
respiratory routes were in the proportion of 1000:1, and the
efficiency of dermal absorption was 1 - 2%, it follows that the
efficiency of absorption by the respiratory route was higher than
20% and could well have been complete.
4.2 Distribution and Storage
The intrinsically reactive chemical nature of organophosphorus
pesticides means that any that enter the body are immediately
liable to a number of biotransformations and reactions with tissue
constituents (particularly tissue proteins carrying esterase active
sites), so that the tracing of radiolabelled material alone does
not give any clue to the distribution of the unchanged parent
compound. It is possible to determine the rate of disposal of
metabolites and thereby to estimate an approximate half-life of
pesticide in the body. Such numbers may be helpful in estimating
safe intervals between successive low exposures, under working
conditions. However, although the half-life of organophosphorus
pesticides and their inhibitory metabolites in vivo is
comparatively short, at least one case of poisoning demonstrated
that significant amounts remained in the body for several weeks
after an acute crisis. Ecobichon et al. (1977) reported a case of
poisoning by fenitrothion. After an effective treatment period
with atropine and an oxime reactivator, leading to 2 days without
symptoms or any therapy, symptoms of nausea and diarrhoea recurred
associated with a decline in the previously restored blood-ChE
levels. These symptoms were controlled by further administration
of oxime, which led to a prompt restoration of the enzyme to a
near-normal level. Further recurrence of symptoms was reported, at
intervals, especially associated with periods of mobilization of
adipose tissue. The conclusion is that the treatment was reversing
the recent inhibition of AChE by a compound that had been stored in
the body and was entering the circulation over a period of many
days.
4.2.1 Experimental animal studies on distribution and storage
In view of the inherent instability of organophosphorus
insecticides, storage in human tissue is not anticipated to be
prolonged (unlike the situation for DDT); population studies,
including analyses of cadavers, do not seem to have been carried
out and would be a pointless exercise. Experimental animal studies
have shown that most of a radiolabelled dose is rapidly excreted in
expired air, urine, and faeces. Thus, it was reported that from 67
to 100% of the administered radioactivity was recovered within 1
week in the combined urine and faeces of cows, rats, and a goat,
given various doses of 32 P-dichlorvos; no organosoluble
radioactivity, which might include unchanged dichlorvos, was
detected after the first 2 h (Blair et al., 1975), though 14 C in
alkyl groups may enter the general metabolic pool and be
incorporated into tissues. Also, phosphorylated proteins are
presumably replaced only by resynthesis and this is a comparatively
slow process with enzymes, such as erythrocyte- and brain-AChE,
typically returning to pre-exposure levels over a period of a few
weeks after irreversible phosphorylation.
In a case of human poisoning by dichlorofenthion, steadily
decreasing concentrations of the pesticide were found in serial fat
biopsy samples up to 48 days after intoxication: the decline
matched a return of blood-ChE levels towards normal, and recovery
of health. Indirect evidence for the short-term storage of
significant amounts of lipophilic organophosphorus compounds was
the return of cholinergic poisoning signs a day or two after
discontinuing oxime therapy in a woman who had been accidentally
poisoned with fenitrothion (Ecobichon et al., 1977). Reinstatement
of therapy led to rapid amelioration of signs and the cycle was
repeated at intervals up to the 15th day after intoxication, after
which her health improved slowly.
4.3 Biotransformation
Alternative metabolic pathways, often available in animals and
man, are listed below, with examples. Most general studies of
pathways have been made on phosphates and their thioate analogues.
Although the ultimate fate of phosphonate pesticides has been
determined, the pathways are usually presumed on the basis of
phosphate studies, which indicate that cleavage of the phosphoric-
carbon bond is limited in mammalian systems. A summary is given
below; further details can be found in Dauterman (1971), Eto
(1974), CEC (1977), and in the annual reports of the Joint FAO/WHO
Meetings on Pesticide Residues in Food (Annex II).
Biotransformation reactions can be divided into three distinct
classes. The former are reactions involving (a) mixed-function
oxidases; (b) hydrolases; and (c) transferases. There is also a
miscellaneous group of unrelated reactions. Binding of
organophosphorus insecticide oxons to tissue is also a significant
biotransformation reaction.
4.3.1 Mixed-function oxidases (MFOs)
Many apparently unrelated substrates can be oxidized by mixed-
function oxidase (MFO) systems associated typically with liver
endoplasmic reticulum, but present also in some other tissues such
as intestine, lung, and kidney. Within the liver, there appears to
be a family of MFOs, possibly with some enzymes in common, but
utilizing slightly different cytochromes of which cytochrome P-450
is the best known. The MFO activity in the liver can vary greatly
according to the nutritional and hormonal state of the animal and
also according to stimuli arising from the ingestion of some
foreign compounds (section 6.3).
4.3.1.1 Oxidative desulfuration
The reaction (Fig. 1) is essentially the activation of the
precursor phosphorothioate to the directly inhibitory phosphate
ester, which is responsible for the inhibition of AChE and for
subsequent toxic effects. There is no evidence of inhibition of
AChE by phosphorothioates occurring under normal situations,
without prior conversion to the phosphate; reports of the
inhibitory power of technical grades of phosphorothioates in vitro
are meaningless, since the activity is almost certainly caused by
traces of the oxon, the activity of which is several orders
greater.
Thus, divided doses may exert a greater toxic effect than the same
amount given as a single large dose (section 6.1.2).
The question of the bioavailability of preparations given by
the oral route needs to be considered. It must be taken into
account when discussing the results obtained for LD50s, and it is
certainly important when considering the toxicity of pesticides
residues. From a chemical point of view, these residues can be
described as parent compounds, free metabolites, and their
conjugates (Kaufman, 1976). The bioavailability of these
fractions and, thus, their toxic potential are not the same
(Dorough, 1976; Marshall & Dorough, 1977). In general, bound
residues appear to have a lower bioavailability and lower toxicity.
This was discussed by Rico & Burgat-Sacaze (1984), and demonstrated
for some pesticides residues by Marshall & Dorough (1977).
4.1.3 Inhalation
Total urinary output of 4-nitrophenol was compared in workers
spraying parathion, who either breathed a pure air supply but did
not wear protective clothing, or who wore total protective
clothing, but did not have any respiratory protection (Durham et
al., 1972). Output derived from the respiratory source compared
with that derived from the dermal source was 1.2% in one test and
12% in another. Since the total exposures by the dermal and
respiratory routes were in the proportion of 1000:1, and the
efficiency of dermal absorption was 1 - 2%, it follows that the
efficiency of absorption by the respiratory route was higher than
20% and could well have been complete.
4.2 Distribution and Storage
The intrinsically reactive chemical nature of organophosphorus
pesticides means that any that enter the body are immediately
liable to a number of biotransformations and reactions with tissue
constituents (particularly tissue proteins carrying esterase active
sites), so that the tracing of radiolabelled material alone does
not give any clue to the distribution of the unchanged parent
compound. It is possible to determine the rate of disposal of
metabolites and thereby to estimate an approximate half-life of
pesticide in the body. Such numbers may be helpful in estimating
safe intervals between successive low exposures, under working
conditions. However, although the half-life of organophosphorus
pesticides and their inhibitory metabolites in vivo is
comparatively short, at least one case of poisoning demonstrated
that significant amounts remained in the body for several weeks
after an acute crisis. Ecobichon et al. (1977) reported a case of
poisoning by fenitrothion. After an effective treatment period
with atropine and an oxime reactivator, leading to 2 days without
symptoms or any therapy, symptoms of nausea and diarrhoea recurred
associated with a decline in the previously restored blood-ChE
levels. These symptoms were controlled by further administration
of oxime, which led to a prompt restoration of the enzyme to a
near-normal level. Further recurrence of symptoms was reported, at
intervals, especially associated with periods of mobilization of
adipose tissue. The conclusion is that the treatment was reversing
the recent inhibition of AChE by a compound that had been stored in
the body and was entering the circulation over a period of many
days.
4.2.1 Experimental animal studies on distribution and storage
In view of the inherent instability of organophosphorus
insecticides, storage in human tissue is not anticipated to be
prolonged (unlike the situation for DDT); population studies,
including analyses of cadavers, do not seem to have been carried
out and would be a pointless exercise. Experimental animal studies
have shown that most of a radiolabelled dose is rapidly excreted in
expired air, urine, and faeces. Thus, it was reported that from 67
to 100% of the administered radioactivity was recovered within 1
week in the combined urine and faeces of cows, rats, and a goat,
given various doses of 32 P-dichlorvos; no organosoluble
radioactivity, which might include unchanged dichlorvos, was
detected after the first 2 h (Blair et al., 1975), though 14 C in
alkyl groups may enter the general metabolic pool and be
incorporated into tissues. Also, phosphorylated proteins are
presumably replaced only by resynthesis and this is a comparatively
slow process with enzymes, such as erythrocyte- and brain-AChE,
typically returning to pre-exposure levels over a period of a few
weeks after irreversible phosphorylation.
In a case of human poisoning by dichlorofenthion, steadily
decreasing concentrations of the pesticide were found in serial fat
biopsy samples up to 48 days after intoxication: the decline
matched a return of blood-ChE levels towards normal, and recovery
of health. Indirect evidence for the short-term storage of
significant amounts of lipophilic organophosphorus compounds was
the return of cholinergic poisoning signs a day or two after
discontinuing oxime therapy in a woman who had been accidentally
poisoned with fenitrothion (Ecobichon et al., 1977). Reinstatement
of therapy led to rapid amelioration of signs and the cycle was
repeated at intervals up to the 15th day after intoxication, after
which her health improved slowly.
4.3 Biotransformation
Alternative metabolic pathways, often available in animals and
man, are listed below, with examples. Most general studies of
pathways have been made on phosphates and their thioate analogues.
Although the ultimate fate of phosphonate pesticides has been
determined, the pathways are usually presumed on the basis of
phosphate studies, which indicate that cleavage of the phosphoric-
carbon bond is limited in mammalian systems. A summary is given
below; further details can be found in Dauterman (1971), Eto
(1974), CEC (1977), and in the annual reports of the Joint FAO/WHO
Meetings on Pesticide Residues in Food (Annex II).
Biotransformation reactions can be divided into three distinct
classes. The former are reactions involving (a) mixed-function
oxidases; (b) hydrolases; and (c) transferases. There is also a
miscellaneous group of unrelated reactions. Binding of
organophosphorus insecticide oxons to tissue is also a significant
biotransformation reaction.
4.3.1 Mixed-function oxidases (MFOs)
Many apparently unrelated substrates can be oxidized by mixed-
function oxidase (MFO) systems associated typically with liver
endoplasmic reticulum, but present also in some other tissues such
as intestine, lung, and kidney. Within the liver, there appears to
be a family of MFOs, possibly with some enzymes in common, but
utilizing slightly different cytochromes of which cytochrome P-450
is the best known. The MFO activity in the liver can vary greatly
according to the nutritional and hormonal state of the animal and
also according to stimuli arising from the ingestion of some
foreign compounds (section 6.3).
4.3.1.1 Oxidative desulfuration
The reaction (Fig. 1) is essentially the activation of the
precursor phosphorothioate to the directly inhibitory phosphate
ester, which is responsible for the inhibition of AChE and for
subsequent toxic effects. There is no evidence of inhibition of
AChE by phosphorothioates occurring under normal situations,
without prior conversion to the phosphate; reports of the
inhibitory power of technical grades of phosphorothioates in vitro
are meaningless, since the activity is almost certainly caused by
traces of the oxon, the activity of which is several orders
greater.
 4.3.1.2 Oxidative N -dealkylation
This reaction may be associated with the metabolic activation
of a non-inhibitory precursor, such as schradan, or with
transformation of one inhibitor to another (Fig. 2).
4.3.1.2 Oxidative N -dealkylation
This reaction may be associated with the metabolic activation
of a non-inhibitory precursor, such as schradan, or with
transformation of one inhibitor to another (Fig. 2).
 4.3.1.3 Oxidative O -dealkylation
The conversion of triesters to diester is a detoxication
process and was once considered to be mediated only by hydrolytic
enzymes (phosphoryl phosphatases or A-esterases). However, a
reaction requiring liver microsomes, NADPH, and oxygen (the typical
MFO system) deethylates chlorofenvinphos with the production of
acetaldehyde, probably as shown in Fig. 3. It seems that various
phosphates, but not phosphorothioates, are metabolized by this
route in mammals.
4.3.1.3 Oxidative O -dealkylation
The conversion of triesters to diester is a detoxication
process and was once considered to be mediated only by hydrolytic
enzymes (phosphoryl phosphatases or A-esterases). However, a
reaction requiring liver microsomes, NADPH, and oxygen (the typical
MFO system) deethylates chlorofenvinphos with the production of
acetaldehyde, probably as shown in Fig. 3. It seems that various
phosphates, but not phosphorothioates, are metabolized by this
route in mammals.
 4.3.1.4 Oxidative de-arylation
Liver MFOs from rat or rabbit can cleave the acid-anhydride
bond coupling phosphorus to the phenolic group in parathion and
analogues (Nakatsugawa et al., 1968), and the same system may also
be responsible for the cleavage of diazinon (Yang et al., 1971).
In contrast to O -dealkylation noted above, this reaction appears
to deal only with phosphorothioates and not phosphates.
4.3.1.5 Thioether oxidation
Oxidation of sulfur in the phosphorus-sulfur-carbon moiety of
demeton-S or or dimethoate and omethoate has not been reported, but
oxidation is known of carbon-sulfur-carbon moieties with the
formation of sulfoxides and sulfones that are more active AChE
inhibitors than the parent compound and remain in circulation for a
comparatively long time (Fig. 4).
4.3.1.4 Oxidative de-arylation
Liver MFOs from rat or rabbit can cleave the acid-anhydride
bond coupling phosphorus to the phenolic group in parathion and
analogues (Nakatsugawa et al., 1968), and the same system may also
be responsible for the cleavage of diazinon (Yang et al., 1971).
In contrast to O -dealkylation noted above, this reaction appears
to deal only with phosphorothioates and not phosphates.
4.3.1.5 Thioether oxidation
Oxidation of sulfur in the phosphorus-sulfur-carbon moiety of
demeton-S or or dimethoate and omethoate has not been reported, but
oxidation is known of carbon-sulfur-carbon moieties with the
formation of sulfoxides and sulfones that are more active AChE
inhibitors than the parent compound and remain in circulation for a
comparatively long time (Fig. 4).
 4.3.1.6 Side-chain oxidation
Stepwise oxidation of simple alkyl groups to hydroxy-, oxo-, or
carboxy-derivatives is a well-known process in the metabolism of
many compounds, apart from the organophosphorus compounds. The
conversion of fenitrothion to the water-soluble, 3-carboxy
derivative (Fig. 5) can account for the comparatively low mammalian
toxicity of this pesticide compared with that of the homologous
methyl parathion (rat oral LD50s of about 600 - 800 and 10 - 25
mg/kg body weight, respectively).
4.3.1.6 Side-chain oxidation
Stepwise oxidation of simple alkyl groups to hydroxy-, oxo-, or
carboxy-derivatives is a well-known process in the metabolism of
many compounds, apart from the organophosphorus compounds. The
conversion of fenitrothion to the water-soluble, 3-carboxy
derivative (Fig. 5) can account for the comparatively low mammalian
toxicity of this pesticide compared with that of the homologous
methyl parathion (rat oral LD50s of about 600 - 800 and 10 - 25
mg/kg body weight, respectively).
 4.3.2 Hydrolases
Hydrolysis of the acid anhydride type ester bond of the leaving
group in pesticidal triesters is well known. The monobasic
diesters and their derivatives are the major urinary metabolites of
organophosphorus insecticides (Fig. 6). The enzymes commonly known
as A-esterases or phosphoryl phosphatases are widespread in
mammalian tissues, such as liver, plasma, intestine, etc., though
they are less abundant in many birds and may not be present in some
insects (Brealey et al., 1980). These enzymes are sometimes
referred to as DFPase or paraoxonase according to the substrate
used, but it does not mean that the enzymes are specific only for a
given organophosphorus compound. Although plasma contains enzymes
that can distinguish between closely related structures such as
paraoxon and 4-nitrophenyl, ethyl, or propylphosphonate, the
enzymes are not totally specific (Becker & Barbaro, 1964); the same
is probably true of A-esterase in other tissues.
4.3.2 Hydrolases
Hydrolysis of the acid anhydride type ester bond of the leaving
group in pesticidal triesters is well known. The monobasic
diesters and their derivatives are the major urinary metabolites of
organophosphorus insecticides (Fig. 6). The enzymes commonly known
as A-esterases or phosphoryl phosphatases are widespread in
mammalian tissues, such as liver, plasma, intestine, etc., though
they are less abundant in many birds and may not be present in some
insects (Brealey et al., 1980). These enzymes are sometimes
referred to as DFPase or paraoxonase according to the substrate
used, but it does not mean that the enzymes are specific only for a
given organophosphorus compound. Although plasma contains enzymes
that can distinguish between closely related structures such as
paraoxon and 4-nitrophenyl, ethyl, or propylphosphonate, the
enzymes are not totally specific (Becker & Barbaro, 1964); the same
is probably true of A-esterase in other tissues.
 Hydrolysis of carboxylic acid ester bonds and carboxyl-amide
bonds in organophosphorus insecticides may be catalysed by
carboxylesterases (or B-esterases), which again occur widely in
mammalian tissues. Malathion, which contains 2 carboxylic ester
bonds, is the best-known organophosphorus pesticide that is
hydrolysed in this way (Fig. 7). The importance of this metabolic
route is shown by the fact that the rat oral LD50 for pure
malathion can be reduced from 10 000 to 100 mg/kg body weight, when
the tissue carboxyesterases are inhibited: this profound
potentiation is discussed further in section 5.3.
The hydrolysis of carboxylamide bonds such as in dimethoate is
catalysed by a liver enzyme (Chen & Dauterman, 1971) (Fig. 7).
Although apparently distinct from liver carboxyesterase, it too is
inhibited by its oxygen analogue (omethoate) and also by other
amide-containing phosphates such as dicrotophos.
Hydrolysis of carboxylic acid ester bonds and carboxyl-amide
bonds in organophosphorus insecticides may be catalysed by
carboxylesterases (or B-esterases), which again occur widely in
mammalian tissues. Malathion, which contains 2 carboxylic ester
bonds, is the best-known organophosphorus pesticide that is
hydrolysed in this way (Fig. 7). The importance of this metabolic
route is shown by the fact that the rat oral LD50 for pure
malathion can be reduced from 10 000 to 100 mg/kg body weight, when
the tissue carboxyesterases are inhibited: this profound
potentiation is discussed further in section 5.3.
The hydrolysis of carboxylamide bonds such as in dimethoate is
catalysed by a liver enzyme (Chen & Dauterman, 1971) (Fig. 7).
Although apparently distinct from liver carboxyesterase, it too is
inhibited by its oxygen analogue (omethoate) and also by other
amide-containing phosphates such as dicrotophos.
 4.3.3 Transferases
The only transferase reaction that is known to deal with the
intact pesticidal organophosphorus triesters involves glutathione,
which is a required substrate for a number of transferase enzymes
present in liver and some other tissues. The enzymes have limited
but overlapping specificity so that the glutathione transferase
responsible for demethylating methyl paraoxon is distinct from that
which conjugates the 4-nitrophenol group in parathion. Activity in
the liver is greatest with methyl esters, but no evidence has been
found of methyl phosphonates undergoing this reaction (Dauterman,
1971).
4.3.3.1 Transferases handling primary metabolites
Reactions involving the conjugation of carboxylic acids,
alcohols, phenols, and amino, imino, and sulfydryl groups are well-
known and applicable to compounds carrying such groups, formed
after the oxidation, hydrolysis, etc. of an organophosphorus
pesticide. Such conjugation reactions aid in the elimination of
primary degradation products, which are usually devoid of
anticholinesterase activity, though they may cause other toxic
effects if they accumulate in the body.
4.3.4 Tissue binding
It is well-known that active metabolites of most
organophosphorus insecticides react covalently to some extent with
tissue esterases other than AChE. Since few of these esterases
appear vital to health (section 6.2.4), the binding reaction may be
considered a detoxification process. Although the catalytic
activity of these esterases is high, the actual quantity of such
sites is comparatively small. Crude measurements using 32 P-
labelled diisopropyl phosphorofluoridate (an agent reacting with
most organophosphorus-sensitive esterases) suggest that 100 - 150
µg bind per kg body weight of an adult hen injected with about the
LD50 dose (Johnson, M.K., personal communication, 1985). Binding
was principally in liver and muscle. The quantity bound would not
be expected to be much greater whatever the LD50 of an administered
organophosphorus insecticide. Thus, it is a significant proportion
of the total dose only for a very toxic compound such as paraoxon
(Lauwerys & Murphy, 1969) but not for compounds with much higher
LD50s. However, the number of binding sites may, in some cases, be
very significant compared with the quantity of circulating
anticholinesterase oxon that has avoided other metabolic disposal
processes. Molecules of oxon bound to these non-vial sites are
prevented from attacking the vital sites such as AChE or NTE
(section 6.1). Binding sites can therefore be considered an
important second line of defence against intoxication.
The specific problem of tissue binding that leads to
potentiation of the toxicity of malathion and other pesticides
containing carboxylester bonds is discussed in section 6.2.4.
4.4 Elimination
There is no evidence of prolonged storage of organophosphorus
compounds in the body, but the process of elimination can be
subdivided roughly according to the speed of the reactions
involved. Most organophosphorus pesticides are degraded quickly by
the metabolic reactions listed in section 4.3, and the elimination
of the products, mostly in the urine with lesser amounts in faeces
and expired air, is not delayed, so that rates of excretion usually
reach a peak within 2 days and decline quite rapidly. That they do
not almost immediately fall to zero is due to storage in fat and
covalent binding. As indicated in section 4.2, the former process
preserves toxic material, which is slowly released into the
circulation and which is active and is metabolized in the same way
as the bulk of the dose received. Covalent binding involves
phosphorylation of proteins, probably esterases having active
sites, including serine, which are mechanistically related to AChE.
The consequences of such phosphorylation depend on the esterase
involved, but many seem to be of only minor importance to the
continuing health of animals, and temporary inhibition may not be
expressed in physiological defects. The special case of neuropathy
target esterase, which is phosphorylated only by agents capable of
causing delayed neuropathy, is considered later (section 6.1.1.2).
4.5 Mode of Action
4.5.1 Inhibition of esterases
The primary biochemical effect associated with toxicity caused
by organophosphorus pesticides is inhibition of AChE. The normal
function of AChE is to terminate neurotransmission due to ACh that
has been liberated at cholinergic nerve endings in response to
nervous stimuli. Loss of AChE activity may lead to a range of
effects resulting from excessive nervous stimulation and
culminating in respiratory failure and death (section 6.1). The
chemistry of inhibition of AChE and of many other esterases (e.g.,
NTE and liver carboxyesterases, which are discussed elsewhere) by
these chemicals is similar and is given in schematic form in Fig.
8. Following the formation of a Michaelis complex (reaction 1), a
specific serine residue in the protein is phosphorylated with loss
of the leaving group X (reaction 2). Two further reactions are
possible: reaction 3 (reactivation) may occur spontaneously at a
rate that is dependent on the nature of the attached group and on
the protein and is also dependent on the influence of pH and of
added nucleophilic reagents, such as oximes, which may catalyse
reactivation. Reaction 4 ("aging") involves cleavage of an R-O-P-
bond with the loss of R and the formation of a charged mono-
substituted phosphoric acid residue still attached to protein. The
reaction is called "aging" because it is time-dependent, and the
product is no longer responsive to nucleophilic reactivating agents
such as some oximes. Since therapy of organophosphorus compound
poisoning is, in part, dependent on the reactivating power of
oximes (sections 6, 7), understanding of the "aging" reaction is
important. PseudoChE, which is present in blood-plasma and nervous
tissue but has no known physiological function, is inhibited by
organophosphorus compounds in a similar way to AChE, but the
specificity of the 2 enzymes is different. Though no toxic effect
arises as a result of inhibition of pseudoChE, measures of its
inhibition can be made for monitoring purposes (section 7.1.1.2).
4.5.2 Possible alkylation of biological macromolecules
It has been shown, under laboratory conditions, that some
organophosphates could react with and alkylate the reagent
4-nitrobenzylpyridine (Preussmann et al., 1969). The study was
interpreted to imply that the in vivo alkylating potential of some
pesticides was similar to that of the known mutagens dimethyl
sulfate and methyl methanesulfonate. Furthermore, Löfroth et al.,
(1969) derived a substrate constant (a logarithmic measure of
alkylating ability) of 0.75 for dichlorvos, which is intermediate
between those known for methyl and ethyl methanesulfonates.
Concern over the possible mutagenic and carcinogenic potential of
organophosphorus compounds on the basis of the above data was
misplaced, since alternative reactions were not considered.
Compared with the carbon atom of the alkyl group, the phosphorus
atom is markedly more electron-deficient and susceptible to attack
by nucleophiles. Analysis by Bedford & Robinson (1972) of the data
of Löfroth et al. (1969) revealed that the proposed rates of
alkylation by hard nucleophiles were probably combined rates of
phosphorylation and alkylation, and that phosphorylation was the
totally dominant reaction in the case of the hydroxide ion. The
comparison with known mutagens was therefore inappropriate. Two
factors detract further from the toxicological significance of the
alkylation studies. The first is that mammalian tissues (plasma,
liver, etc.) contain active enzymes that catalyse the
phosphorylation of water by the organophosphorus esters. Viewed
inversely, these enzymes (often called A-esterases) catalyse the
hydrolysis of the organophosphorus esters, thereby rapidly reducing
circulating levels of hazardous material. Secondly, the
comparative rate of reaction of most of these pesticides with AChE
is many orders greater than their rate of alkylation of the typical
nucleophile 4-nitrobenzylpyridine: for dichlorvos, the ratio of
rates was 1 x 107 in favour of the inhibitory phosphorylation of
AChE (Aldridge & Johnson, 1977). It follows that, at low exposure
levels, in vivo phosphorylation of AChE and other esterases will
be the dominant reaction with negligible uncatalysed alkylation of
genetic material. Indeed, no such alkylation has been detected in
sensitive in vivo studies designed to check this point (Wooder et
al., 1977). Some catalysed alkylations of glutathione by
organophosphorus compounds are known to occur in vivo (section 4),
but these are essentially detoxification reactions. The topic of
alkylation and the possible mutagenic or carcinogenic consequences
is discussed further in section 6.2.
4.3.3 Transferases
The only transferase reaction that is known to deal with the
intact pesticidal organophosphorus triesters involves glutathione,
which is a required substrate for a number of transferase enzymes
present in liver and some other tissues. The enzymes have limited
but overlapping specificity so that the glutathione transferase
responsible for demethylating methyl paraoxon is distinct from that
which conjugates the 4-nitrophenol group in parathion. Activity in
the liver is greatest with methyl esters, but no evidence has been
found of methyl phosphonates undergoing this reaction (Dauterman,
1971).
4.3.3.1 Transferases handling primary metabolites
Reactions involving the conjugation of carboxylic acids,
alcohols, phenols, and amino, imino, and sulfydryl groups are well-
known and applicable to compounds carrying such groups, formed
after the oxidation, hydrolysis, etc. of an organophosphorus
pesticide. Such conjugation reactions aid in the elimination of
primary degradation products, which are usually devoid of
anticholinesterase activity, though they may cause other toxic
effects if they accumulate in the body.
4.3.4 Tissue binding
It is well-known that active metabolites of most
organophosphorus insecticides react covalently to some extent with
tissue esterases other than AChE. Since few of these esterases
appear vital to health (section 6.2.4), the binding reaction may be
considered a detoxification process. Although the catalytic
activity of these esterases is high, the actual quantity of such
sites is comparatively small. Crude measurements using 32 P-
labelled diisopropyl phosphorofluoridate (an agent reacting with
most organophosphorus-sensitive esterases) suggest that 100 - 150
µg bind per kg body weight of an adult hen injected with about the
LD50 dose (Johnson, M.K., personal communication, 1985). Binding
was principally in liver and muscle. The quantity bound would not
be expected to be much greater whatever the LD50 of an administered
organophosphorus insecticide. Thus, it is a significant proportion
of the total dose only for a very toxic compound such as paraoxon
(Lauwerys & Murphy, 1969) but not for compounds with much higher
LD50s. However, the number of binding sites may, in some cases, be
very significant compared with the quantity of circulating
anticholinesterase oxon that has avoided other metabolic disposal
processes. Molecules of oxon bound to these non-vial sites are
prevented from attacking the vital sites such as AChE or NTE
(section 6.1). Binding sites can therefore be considered an
important second line of defence against intoxication.
The specific problem of tissue binding that leads to
potentiation of the toxicity of malathion and other pesticides
containing carboxylester bonds is discussed in section 6.2.4.
4.4 Elimination
There is no evidence of prolonged storage of organophosphorus
compounds in the body, but the process of elimination can be
subdivided roughly according to the speed of the reactions
involved. Most organophosphorus pesticides are degraded quickly by
the metabolic reactions listed in section 4.3, and the elimination
of the products, mostly in the urine with lesser amounts in faeces
and expired air, is not delayed, so that rates of excretion usually
reach a peak within 2 days and decline quite rapidly. That they do
not almost immediately fall to zero is due to storage in fat and
covalent binding. As indicated in section 4.2, the former process
preserves toxic material, which is slowly released into the
circulation and which is active and is metabolized in the same way
as the bulk of the dose received. Covalent binding involves
phosphorylation of proteins, probably esterases having active
sites, including serine, which are mechanistically related to AChE.
The consequences of such phosphorylation depend on the esterase
involved, but many seem to be of only minor importance to the
continuing health of animals, and temporary inhibition may not be
expressed in physiological defects. The special case of neuropathy
target esterase, which is phosphorylated only by agents capable of
causing delayed neuropathy, is considered later (section 6.1.1.2).
4.5 Mode of Action
4.5.1 Inhibition of esterases
The primary biochemical effect associated with toxicity caused
by organophosphorus pesticides is inhibition of AChE. The normal
function of AChE is to terminate neurotransmission due to ACh that
has been liberated at cholinergic nerve endings in response to
nervous stimuli. Loss of AChE activity may lead to a range of
effects resulting from excessive nervous stimulation and
culminating in respiratory failure and death (section 6.1). The
chemistry of inhibition of AChE and of many other esterases (e.g.,
NTE and liver carboxyesterases, which are discussed elsewhere) by
these chemicals is similar and is given in schematic form in Fig.
8. Following the formation of a Michaelis complex (reaction 1), a
specific serine residue in the protein is phosphorylated with loss
of the leaving group X (reaction 2). Two further reactions are
possible: reaction 3 (reactivation) may occur spontaneously at a
rate that is dependent on the nature of the attached group and on
the protein and is also dependent on the influence of pH and of
added nucleophilic reagents, such as oximes, which may catalyse
reactivation. Reaction 4 ("aging") involves cleavage of an R-O-P-
bond with the loss of R and the formation of a charged mono-
substituted phosphoric acid residue still attached to protein. The
reaction is called "aging" because it is time-dependent, and the
product is no longer responsive to nucleophilic reactivating agents
such as some oximes. Since therapy of organophosphorus compound
poisoning is, in part, dependent on the reactivating power of
oximes (sections 6, 7), understanding of the "aging" reaction is
important. PseudoChE, which is present in blood-plasma and nervous
tissue but has no known physiological function, is inhibited by
organophosphorus compounds in a similar way to AChE, but the
specificity of the 2 enzymes is different. Though no toxic effect
arises as a result of inhibition of pseudoChE, measures of its
inhibition can be made for monitoring purposes (section 7.1.1.2).
4.5.2 Possible alkylation of biological macromolecules
It has been shown, under laboratory conditions, that some
organophosphates could react with and alkylate the reagent
4-nitrobenzylpyridine (Preussmann et al., 1969). The study was
interpreted to imply that the in vivo alkylating potential of some
pesticides was similar to that of the known mutagens dimethyl
sulfate and methyl methanesulfonate. Furthermore, Löfroth et al.,
(1969) derived a substrate constant (a logarithmic measure of
alkylating ability) of 0.75 for dichlorvos, which is intermediate
between those known for methyl and ethyl methanesulfonates.
Concern over the possible mutagenic and carcinogenic potential of
organophosphorus compounds on the basis of the above data was
misplaced, since alternative reactions were not considered.
Compared with the carbon atom of the alkyl group, the phosphorus
atom is markedly more electron-deficient and susceptible to attack
by nucleophiles. Analysis by Bedford & Robinson (1972) of the data
of Löfroth et al. (1969) revealed that the proposed rates of
alkylation by hard nucleophiles were probably combined rates of
phosphorylation and alkylation, and that phosphorylation was the
totally dominant reaction in the case of the hydroxide ion. The
comparison with known mutagens was therefore inappropriate. Two
factors detract further from the toxicological significance of the
alkylation studies. The first is that mammalian tissues (plasma,
liver, etc.) contain active enzymes that catalyse the
phosphorylation of water by the organophosphorus esters. Viewed
inversely, these enzymes (often called A-esterases) catalyse the
hydrolysis of the organophosphorus esters, thereby rapidly reducing
circulating levels of hazardous material. Secondly, the
comparative rate of reaction of most of these pesticides with AChE
is many orders greater than their rate of alkylation of the typical
nucleophile 4-nitrobenzylpyridine: for dichlorvos, the ratio of
rates was 1 x 107 in favour of the inhibitory phosphorylation of
AChE (Aldridge & Johnson, 1977). It follows that, at low exposure
levels, in vivo phosphorylation of AChE and other esterases will
be the dominant reaction with negligible uncatalysed alkylation of
genetic material. Indeed, no such alkylation has been detected in
sensitive in vivo studies designed to check this point (Wooder et
al., 1977). Some catalysed alkylations of glutathione by
organophosphorus compounds are known to occur in vivo (section 4),
but these are essentially detoxification reactions. The topic of
alkylation and the possible mutagenic or carcinogenic consequences
is discussed further in section 6.2.
 5. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
5.1 Aquatic Organisms
Organophosphorus insecticides are not very stable in aqueous
media. However, accidental leaching may occur from treated areas
into rivers and lakes where they may exert toxic effects on aquatic
organisms before degradation is complete. In clean water in the
laboratory, toxic effects were seen in several aquatic organisms
when they were exposed to concentrations of organophosphorus
insecticides ranging from 0.01 to 1 mg/litre for 48 h (Nishiuchi,
1981). However, lethal concentrations derived from 48-h exposures
in clear laboratory water may be artificially low compared with the
concentrations that would be effective in true environmental
waters.
One pesticide (phenthoate) appeared to be more toxic for
aquatic insects ("median tolerable limit" = 9 - 75 µg/kg)
(Nishiuchi, 1981) than for one species of fresh-water fish exposed
under apparently similar conditions (20% mortality caused by 263
µg/kg) (Jash & Bhattacharya, 1982).
Accidental release of pesticides in lakes, rivers, and bays
sometimes caused massive death of fish and many of the compounds
were strongly toxic for small aquatic organisms such as Daphnia, as
shown in Table 3.
Table 3. Acute toxicity of organophosphorus pesticides for some aquatic organisms
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Acephate > 40 - > 40 - > 40 Nishiuchi (1974)
Calvinphos > 40 - > 40 - 0.0042 Nishiuchi (1974)
Chlorfenvinphos 0.27 0.34 0.23 0.12 0.011 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Chlorpyrifos 0.13 0.20 0.47 0.74 0.0050 Yoshida &
emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Chlorpyrifos 2.1 - 3.4 - 0.017 Yoshida &
-methyl Nishiuchi (1976)
Cyanofenphos 1.2 1.3 6.3 15 0.0085 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Cyanophos 15 10 ~ 40 28 18 0.34 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Dialifos 1.3 - 0.80 - 0.027 Yoshida &
Nishiuchi (1976)
Dichlofenthion 5.1 10 ~ 40 1.4 10 0.005 Yoshida &
(emulsifiable (dust Nishiuchi (1972);
concentrate) formulation) Nishiuchi (1974)
Diazinon 3.2 5.1 5.3 4.1 0.08 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Dichlorvos > 40 10 ~ 40 18 - 2.8 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Dimethoate > 40 > 40 > 40 - 10 ~ 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Dimethylvinphos 5.6 - 1.5 - 0.010 Yoshida &
Nishiuchi (1976)
Dioxathion 10 ~ 40 10 ~ 40 1.4 - 0.007 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Disulfoton 8.7 10 ~ 40 21 0.37 0.07 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Edifenphos 2.5 1.8 1.8 - 0.27 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
EPN 0.20 0.32 0.50 0.085 0.0017 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Ethion 1.2 1.1 5.5 - 0.005 Yoshida &
Nishiuchi (1972)
Fenitrothion 8.2 3.4 7.0 0.75 0.050 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Fenthion 3.3 1.9 2.5 2.3 0.070 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Formothion 15 10 ~ 40 10 ~ 40 - 5.8 Yoshida &
Nishiuchi (1972)
IBP 10 ~ 40 12 7.2 - 2.3 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
Leptophos > 40 10 ~ 40 8.5 - 0.002 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Malathion 23 7.8 0.75 1.4 0.030 Yoshida &
Nishiuchi (1972)
Menazon > 40 > 40 > 40 100 10 ~ 40 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Methidathion 2.5 2.3 0.034 0.22 0.007 Yoshida &
(emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Naled 1.3 1.2 28 2.3 0.005 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972);
concentrate) concentrate) Nishiuchi (1974)
Parathion 4.5 1.7 2.9 - 0.0050 Yoshida &
Nishiuchi (1972)
Parathion-methyl 7.5 10 ~ 40 12 - 0.00050 Yoshida &
Nishiuchi (1972)
Phenkapton 2.0 3.8 3.5 - 0.008 Yoshida &
Nishiuchi (1972)
Phenthoate 2.5 2.4 0.17 0.20 0.008 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Phosalone 1.2 1.2 0.35 - 0.05 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Phosmet 5.3 4.7 1.8 1.0 0.025 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Pirimiphos-methyl 1.8 - 3.0 - 0.018 Yoshida &
Nishiuchi (1976)
Propaphos 4.8 - 4.1 - 0.0063 Yoshida &
Nishiuchi (1976)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Propoxur 10 ~ 40 10 ~ 40 10 ~ 40 - 0.37 Yoshida &
Nishiuchi (1972)
Prothiophos 9.5 - 10 - 0.13 Yoshida &
Nishiuchi (1976)
Temivinphos 0.58 - 0.48 - 0.0080 Yoshida &
Nishiuchi (1976)
TEPP 5.6 10 ~ 40 4.8 - 10 ~ 40 Yoshida &
(liquid (liquid (liquid (liquid Nishiuchi (1972)
formulation) formulation) formulation) formulation)
Tetrachlorvinphos 4.3 3.9 4.2 - 0.0035 Yoshida &
(wettable Nishiuchi (1972)
powder)
Thiometon 7.5 10 ~ 40 10 ~ 40 - 5.5 Yoshida &
Nishiuchi (1972)
Trichlorphon 28 10 ~ 40 25 12 0.005 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Vamidothion > 40 > 40 > 40 - > 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Note: Test methods are officially recognized methods based on the Notification of the Ministry of
Agriculture, Forestry and Fishery of Japan, as described in the separate papers.
6. EFFECTS ON ANIMALS
Insecticides are designed as lethal agents. Although they may
be designed to be less toxic for animals than for insects, all
organophosphorus insecticides present a toxic hazard to some
extent. Values for the oral and dermal LD50s in rat, shown for
different compounds in Annex III, range from less than 10 to more
than 3000 mg/kg body weight. The dose-response line for
organophosphorus insecticides is usually steeper than that for
carbamates, though both kill by their anticholinesterase action.
The reason for the difference lies in the faster rate of
spontaneous reactivation of carbamylated AChE compared with
phosphorylated AChE.
By the time the 1984 Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) had ended, the toxicology of 57 organophosphorus
pesticides had been reviewed (Vettorazzi, 1984). Usually, the
compounds had been reviewed several times as additional information
became available. The compounds, with the years of the JMPR
review, and the acceptable daily intake (ADI) advised are listed
Annex II. It also lists the year of review by IARC. Moreover, it
gives the WHO recommended classification by acute toxic hazard
(WHO, 1984a) and an indication on whether WHO/FAO issued a "Data
Sheet on Chemical Pesticides" on this substance.
Details of the tests and of the results are recorded in the
appropriate published evaluations from the FAO/WHO Joint Committee
Meetings, which have been summarized and commented on by Vettorazzi
(1979).
It should be realized that, while reports on toxicological
tests may give some indication of the purity of the sample as
percentage content of the major active ingredient, there is seldom
any indication of the nature or quantity of the impurities or of
whether the impurities may significantly influence toxicity. It
cannot be emphasized too strongly that measurements such as acute
LD50s are not absolute values.
The LD50 values for one typical compound, fenitrothion, in
various species when administered by various routes are listed in
Table 4 (FAO/WHO, 1970b, 1975b). From this Table, it is clear that
there are marked differences in the LD50 depending on species and
route of administration. Variability in LD50 values for the same
route and species is often considered to be due to the vehicule in
which the pesticide is applied, which may influence its uptake into
the body. However, it is also possible that such differences are
real and can throw light on mechanisms of toxicity and/or of
detoxication, thus making more intelligent assessment of toxicity
possible. It can be argued that the presence of the very toxic
impurities in some formulations of diazinon (section 6.3.4) could
have been deduced much earlier from differences in LD50 data.
Likewise, the Pakistani poisonings due to strong potentiators in
stored malathion (Baker et al., 1978) (section 6.3.4) might have
been prevented, if more questions had been asked about the range of
LD50 values given in the literature for malathion.
Table 4. Comparison of acute toxicity data for
fenitrothion listed in evaluation reports of
joint FAO/WHO meetingsa
----------------------------------------------------
Animal Sex Route LD50 (mg/kg
body weight)
----------------------------------------------------
1970 Report
Mouse M oral 1336
Mouse F oral 1416
Mouse M ip 115
Mouse F ip 110
Mouse iv 220
Rat M oral 740
Rat F oral 570
Rat M ip 135
Rat F ip 160
Rat iv 33
Guinea-pig M oral 500
Guinea-pig oral 1850
Guinea-pig M ip 110
Guinea-pig iv 112
Cat oral 142
1975 Report
Mouse M oral 1030
Mouse F oral 1040
Rat M oral 330
Rat F oral 800
Ring-neck pheasant oral 34.5
Mallard duck oral 2550
Dog oral MLDb 681 mg/kg
Rat M oral 940
Rat F oral 600
----------------------------------------------------
a From: FAO/WHO (1970b, 1975b).
b MLD = minimum lethal dose.
6.1 Effects on the Nervous System
6.1.1 Effects attributed to interaction with esterases
6.1.1.1 Cholinergic effects
(a) Acute toxicity
If non-ester herbicidal compounds are excluded, then the acute
toxicity of all other organophosphorus pesticides shares the basic
mechanism outlined in section 4.5.1, involving inhibition of AChE,
accumulation of ACh, and over-stimulation of some central
cholinergic neurons and of the sympathetic and parasympathetic
nervous systems. Signs and symptoms of poisoning are described
fully in section 7.1. Death is caused by respiratory failure due
to a combination of blocking of the respiratory centre,
bronchospasm, and paralysis of the respiratory muscles.
(b) Chronic toxicity
The cholinergic effects brought about by repeated
administration of less than a single fatal dose are similar in type
to the acute single-dose effects and are discussed in section
6.3.1. For the majority of these compounds, long-term feeding
tests have been performed to establish the no-observed-adverse-
effect levels. In every case, except bromophos-ethyl, the most
sensitive indicator of an effect was depression of ChE activity in
plasma or erythrocytes. The phenomenon of tolerance to repeated
doses of anticholinesterase compounds is covered in sections 6.3.1
and 6.4. For bromophos-ethyl, it has been reported that the
urinary excretion of ascorbic acid and dehydroascorbic acid was
slightly increased in beagle dogs given 0.39 mg/kg body weight
daily, for 18 weeks, at which dose, depression of serum-ChE was not
significant (FAO/WHO, 1973b).
6.1.1.2 Delayed neuropathic effects
Delayed neuropathy has occurred occasionally in man and
experimental animals after intoxication with a variety of
organophosphorus esters. The subject has been reviewed by Johnson
(1975a,b, 1980, 1982a) and by Davis & Richardson (1980). An
account suitable for physicians is given by Lotti et al. (1984).
Delayed neuropathy is not inevitably associated with intoxication
by organophosphorus pesticides (Johnson, 1982a; Soliman et al.,
1982). Improvements in the therapy of acute poisoning (section
7.4) mean that higher doses of some organophosphorus insecticides
can now be tolerated without fatal consequences. However, many
organophosphorus pesticides that might, theoretically, cause
neuropathy, would only do so at a dose far above the lethal dose.
(a) Characteristics
Regardless of the severity of anticholinesterase effects, there
is a delay after intoxication before neuropathic signs and symptoms
appear. In the adult hen, which is the test species of choice,
this delay is 8 - 14 days, while in man, the delay may be up to 4
weeks after acute exposure. The first symptoms are often sensory
with tingling and burning sensations in the limb extremities
followed by weakness in the lower limbs and ataxia. This
progresses to paralysis, which, in severe cases, affects the upper
limbs also. Children and young animals are less severely affected
than adults, but recovery is slow and seldom complete in adults;
with the passage of time, the clinical picture changes from a
flaccid to a spastic type of paralysis. Cats, hens, and a number
of larger species are affected by a single dose. Repeated dosing
does not reduce the delay in onset to less than 8 days from the
first dose. Baboons, monkeys, and marmosets do not respond easily
to single doses of several typical neuropathic esters, and it is
difficult to produce typical delayed neurotoxic effects in rodents,
even by repeated dosing. In early histological examinations,
methods were used that showed mainly degeneration of the fatty
myelin sheath surrounding long nerve axons and names such as
"Organophosphate Demyelinating Disease" are still erroneously used,
in spite of later work that showed that the nerve axon itself was
primarily affected and damage to the myelin sheath was secondary
(Cavanagh, 1954; Bouldin & Cavanagh, 1979). The preparation of
tissues and identification of lesions are described by Bradley
(1976), Bickford & Sprague (1984), and by Prentice & Roberts (1984)
in the Workshop Report edited by Cranmer & Hixson (1984). This
publication also covers many aspects of mechanism and testing.
(b) Mechanism
The first essential step in the initiation of the delayed
neuropathic effect of an organophosphate is phosphorylation of a
target protein in the nervous system. The protein has esteratic
enzyme activity. The phosphorylation, which was originally studied
radiochemically, can be monitored conveniently as progressive
inhibition of the activity of this enzyme, which is now referred to
as Neuropathy Target Esterase (NTE or Neurotoxic Esterase)
(Johnson, 1980, 1982a). The second, and equally essential, step is
the transformation of the phosphorylated NTE to a modified form:
one of the remaining ester bonds of the inhibitor molecule attached
to the NTE active site undergoes a biochemical cleavage leaving an
ionized acidic residue bound to the protein: this residue is
negatively charged and the reaction is referred to as "aging".
Both inhibition and aging of inhibited NTE are essential to
initiate neuropathy, but the role of the negative charge in the
initiation of axonal degeneration is not known. The process of
"aging" of inhibited NTE has some analogy with the better-known
"aging" of inhibited AChE. However, the analogy does not last
above the level of enzyme inhibition. Acute toxicity arises
directly from the loss of catalytic activity of AChE, leading to
accumulation of excess physiological substrate. Mere loss of
catalytic activity of NTE (without aging) does not initiate
neuropathy (see below). There is no evidence of a deleterious
accumulation of a physiological substrate for NTE or lack of
hydrolysis products after inhibition in vivo, and the effect of
the negative charge may be focused on some quite separate process.
When NTE has been inhibited by a suitable phosphate,
phosphonate, or phosphoramidate, aging is always possible, and this
process has been shown to occur rapidly with a wide variety of
neurotoxic esters and hen NTE. However, after inhibition by
phosphinates (which contain 2 phosphorus-carbon bonds) or by
sulfonyl fluorides, no hydrolysable bonds remain in the attached
inhibitor molecule. Thus, aging is not possible, and these
compounds are not neuropathic in vivo. Whenever hen NTE can be
phosphinylated or sulphonated in vivo, the birds become resistant
to challenge doses of typical neuropathic esters, because the
2-step initiation process has been blocked halfway. Similarly,
carbamates do not form aged inhibited enzymes and are either
totally without effect (anti-cholinesterase carbamates are poor
inhibitors of NTE) or inhibit the enzyme, but do not age. Thus,
they protect the hen in the same way as the phosphinates. Now that
this mechanism is understood, it is obvious that carbamates need
not be subjected to tests that were designed to detect delayed
neuropathic potential in organophosphorus anticholinesterase
pesticides.
The events that follow inhibition and aging of NTE are not
known, but it is clear that, in adult hens, detectable delayed
neuropathic events (clinical or histological) are never seen after
a single dose/exposure of an organophosphorus ester, unless there
is at least 70% (probably 80 - 90%) inhibition of the NTE in the
brain and spinal cord soon after dosing (4 - 40 h). Owing to the
synthesis of fresh protein, this inhibition declines markedly
during the 8 to 14-day delay period, and there is no correlation
between neuropathy and NTE inhibition measured at the time that
clinical signs reach their peak. NTE has been found in the nervous
tissue of all mammals and birds examined and can be inhibited, even
in species such as the rat, in which there is no obvious clinical
neuropathic response to a single dose. It has been shown recently
that, when single doses of certain neuropathic organophosphorus
compounds are given to rats, degenerative lesions develop in their
peripheral nerves and spinal cord. Although these lesions are
similar to those in ataxic hens, they are not accompanied by
clinical signs. The dose-response of NTE inhibition and the
neurological damage in rats are well correlated, and, as in hens,
the lesions are prevented by protectively predosing the rats with
sulfonyl fluoride (Padilla & Veronesi, 1985; Veronesi & Padilla, in
press). Human NTE has been examined in vitro (Lotti & Johnson,
1978), and its response to inhibitors is similar to that of the hen
enzyme (I50s differed by not more than 4-fold). What is not known
is the numerical value of the threshold of inhibition of NTE in man
that is associated with clinical neuropathy. However, it is known
that numerous people treated only with atropine for poisoning by
trichlorfon have survived and then developed neuropathy (Johnson,
1981a). In contrast, it is very difficult to produce neuropathy
with trichlorfon in the hen, without enormous doses coupled with
prophylaxis as well as therapy for severe anticholinesterase
effects. It seems, therefore, that in the case of this pesticide,
the chances of a severely poisoned man developing neuropathy rather
than dying are greater than those of the hen. It might be deduced
that the threshold level of NTE inhibition for neuropathic effects
in man is somewhat less than the 70% value for hen, and caution
should be applied in the extrapolation from hen tests. This
argument is merely a comparison of the two hazards, death and
neuropathy: it does not give any guide to the relative dose
required to intoxicate man and hen.
(c) Delayed neurotoxicity testing
Two distinct procedures are currently practiced by testers, and
each depends on the anticipated hazard. In acute tests, it is
recommended that hens surviving an LD50 test (preferably a test in
which the therapeutic use of atropine raised the LD50) should be
observed for three weeks for abnormalities of gait and then be
re-dosed and watched for a further three weeks, after which a
thorough histological examination of the distal ends of neurons in
the spinal cord and peripheral nerve should be performed. Long-
term neurotoxicity tests require feeding hens for up to 90 days
with doses in the diet ranging up to those causing obvious adverse
cholinergic effects; evaluation is by the same criteria as for the
acute test. It may be argued that long-term tests should be
omitted, in view of the greater speed and simplicity of the acute
test in obtaining yes/no answers and the fact that no pesticidal
compound has ever been found to give a positive clinical response
in feeding trials when negative in the 2-massive-doses test. The
practice in the United Kingdom has been to voluntarily exclude from
agricultural use any compound shown to be neuropathic in acute
tests. Guidelines for the performance of acute and long-term
delayed neurotoxicity tests are available (United Kingdom PSPS,
1979; US EPA, 1982; OECD, 1983). The OECD Guidelines point out
that acute tests would be improved if they were accompanied by
assays of NTE inhibition in the brain and spinal cord, one and two
days after dosing. Results of assays would provide graded dose-
response relationships instead of the present all-or-none scoring.
Some examples are given in Johnson (1975b). Particularly useful are
data such as those obtained with malathion (Johnson, 1981b), which
showed negligible (< 10%) inhibition of NTE at the LD50 dose in
hens. These data indicate a different order of safety compared
with that of some other compounds which caused 50 - 60% inhibition,
though there was no visible clinical response. A way to integrate
the NTE test efficiently into delayed neurotoxicity test protocols
has been suggested by Johnson (1984). However, further discussion
to improve this method is required.
(d) Delayed neurotoxic response to long-term feeding
For some neuropathic compounds, the potency of a single dose
may be matched or even exceeded by the effect of the same amount
spread over a few days. In particular, compounds such as TOCP or
leptophos, which are very poorly soluble in water and are required
in a very large single dose to be effective, may well be absorbed
with greater efficiency in a divided lower-dose regime. However,
the results of various studies (Johnson, 1982a) have shown that, as
the dose is reduced further, there is a clear cut-off point below
which there does not appear to be any cumulative effect. Thus,
TOCP in the diet of hens at 400 mg/kg produced neuropathy in 21
days, while a level of 100 mg/kg diet did not cause any detectable
clinical or histological damage after 140 days (Barnes, 1975).
Monitoring of the NTE response to continuous feeding of non-
neurotoxic regimes of 2 organophosphates (0.125 mg DFP/kg for 5
days per week, over 4 weeks, or 2.5 mg mono-2-cresyl diphenyl
phosphate/kg, daily, for 10 weeks) showed that NTE levels in the
brain and spinal cord were depressed within 2 - 3 weeks to about 45
- 55% of normal and remained unchanged thereafter; the equilibrium
is presumbably the result of daily synthesis matching daily
inhibition. These doses did not cause detectable neuropathy during
either the feeding period or the 3 ensuing weeks (Lotti & Johnson,
1980). On the basis of these and other studies, Johnson (1982a)
concluded that the hen nervous system might tolerate the
biochemical defect of prolonged inhibition of NTE to about 50%
normal level brought about by long-term dosing but could not
tolerate the brief 80 - 90% inhibition that follows a single larger
dose. If long-term feeding tests in hens are required, then weekly
measurements of the level of NTE inhibition should provide valuable
predictive information, early in the test (Johnson, 1984). Since
it is necessary to kill birds for tissue sampling, more birds may
be necessary, at least in the early stages.
(e) Results of delayed neurotoxicity testing
No pesticidal organophosphorus compounds, giving negative
results in massive-dose tests, have caused delayed neuropathy in
long-term feeding studies. With the exception of trichlorfon,
where 2 doses, 3 days apart, were necessary (Johnson 1970, 1981a),
the massive-dose tests have been single doses given with
prophylaxis and therapy (eserine + atropine and atropine + oxime -
repeated if necessary) to ensure that doses above LD50 could be
examined. In Table 5, known pesticidal compounds are listed
according to the doses known to produce marked-to-severe neuropathy
in the majority of birds tested. Administration by either the oral
or dermal route may be effective.
The review by Johnson (1975b) is believed to list all
pesticidal and non-pesticidal compounds, or their derived oxons,
tested and reported on, up to that time. The JMPR Reports were not
included as a literature source. NTE responses are given where
these have been measured. Johnson (1982a) lists a number of
pesticides for which both clinical and NTE tests had been reported
since 1975 and notes that carbophenthion, cyanophos, diazinon,
fenitrothion, malathion, methyl parathion, omethoate, and parathion
can positively be declared non-neuropathic on the basis of
negligible NTE inhibition responses, while chlorpyrifos,
methamidophos, and salithion gave intermediate NTE responses
without clinical expression at the doses tested. Isofenphos
( o -ethyl- o -2-iso-propoxycarbonylphenyl isopropylphosphor-
amidothioate) has been shown by NTE assays and clinical tests to
cause delayed neuropathy in hens at about 20 x LD50 (Wilson et al.,
1984), while the insecticidal synergist o-n -propyl- o -(2-propynyl)
phenylphosphonate was neuropathic by both criteria at about the
LD50 (Soliman, 1982).
Table 5. Organophosphorus pesticides causing delayed neuropathy
in hens after a single dose
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
mipafox 25 im Bidstrup et al.
N,N -diisopropylphosphoro- (1953)
diamidic fluoride
haloxon 1000 oral Malone (1964)
3-chloro-4-methyl-2-oxo-
2H-1-benzopyran-7-yl-
bis-(2-chloroethyl) phosphate
EPN 40-80 sc Witter & Gaines
(1963)
trichlornat 310 oral Johnson (1975b)
ethyl 2,4,5-trichlorophenyl 300-400 oral Johnson (1975b)
phenylphosphonate
ethyl 2,4-dichlorophenyl > 2000 oral Abou-Donia et al.
phenylphosphonothioate (1979)
leptophos 400-500 oral Abou-Donia et al.
(1974); Johnson
(1975b)
desbromoleptophos 60 oral Johnson (1975b)
S,S,S -tributyl phosphoro- 1110 sc Johnson (1970)
trithioate (DEF)
cyanofenphos > 100 oral Ohkawa et al.
(1980)
isofenphos 100 oral Wilson et al.
O -ethyl O -2-isopropoxy- (1984)
carbonylphenyl isopropyl-
phosphoramidothioate
O-n- propyl O -(2-propynyl) 400 oral Soliman (1982)
phenylphosphonate
dichlorvosb 100 scc Caroldi & Lotti
(1981)
amiprophos 600 oralc,d Huang et al.
O -ethyl O -4-methyl-6- (1979)
nitrophenyl
N- isopropylphos-
phoramidothionate
Table 5 (contd.)
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
coumaphos 50 oralc Abou-Donia et al.
500 dermal (1982)
chlorpyrifos 150 oral Lotti et al.
(1986)
salithion 120 oral El-Sebae et al.
(1981)
--------------------------------------------------------------
a Dose needed to cause marked-to-severe neuropathy in the
majority of birds tested.
b 50% formulation in hydrocarbons: dose calculated as active
ingredient.
c Mild neuropathy only at maximum tolerated dose.
d Test performed in cockerels.
The statement by Namba et al. (1971) that chlorpyrifos produced
neuropathy in hens seems to have been without foundation and may
arise from the misreading of a report by Gaines (1969), which
mentioned that chlorpyrifos caused a rapid onset short-term
weakness (sometimes called paralytic effect) similar to that caused
by malathion. However, one recent case of human poisoning and
laboratory tests with doses well above the unprotected LD50 have
shown neuropathic effects from this pesticide (Lotti & Moretto, in
press).
(f) Structure/activity relationships
As noted above, not all organophosphorus pesticides cause
delayed neuropathy. In vivo tests and target-enzyme studies
listed by Johnson (1975b) can be condensed according to a number of
factors as listed by Johnson (1980, 1982a):
(1) Factors that increase delayed neurotoxicity potential more
than acute toxicity are:
(a) choice of phosphonates or phosphoramidates rather
than analogous phosphates;
(b) increase in chain-length or hydrophobicity of R1 and
R2 ; and
(c) a leaving group X, which does not sterically hinder
approach to the active site of NTE;
(2) Factors that decrease the comparative potential are:
(a) the converse of (1) a, b, and c;
(b) choice of R or X groups that are very bulky
(naphthyloxy) or non-planar;
(c) choice of a nitrophenyl group at X (a steric effect?);
(d) choice of comparatively more hydrophilic X groups
(oximes or heterocyclics); and
(e) choice of thioether linkages at X.
Considering these factors, it can be seen why malathion and
diazinon are both far below the neurotoxicity hazard line, why, in
its homologous series, only dichlorvos is not neurotoxic at the
LD50, why EPN, a phosphonothioate with a hydrophobic phenyl group
at R1 is neurotoxic, even with a 4-nitrophenyl leaving group, and
why it is not surprising that other phenylphosphonothioates such as
desbromoleptophos, or cyanofenphos are also neurotoxic. The
apparent non-neurotoxicity of the ethyl analogue of leptophos
(Hollingshaus et al., 1979) seems to contradict (1-b) above, but
the dominant factor seems to be the problem of absorption of this
very poorly soluble compound after oral dosage (Hansen & Hansen,
1985).
6.1.2 Behavioural and other effects on the nervous system
The problems of interpreting behavioural changes in relation to
inhibition of AChE and the time-dependent changes in these
variables have been discussed by Bignami (1976) and Bignami et al.
(1975). It appears that some learned responses may be acquired
more quickly by rats recovering from an inhibitory dose of, e.g.,
DFP (1 mg/kg body weight). This may be a sign of changed
inhibitory responses in learning pathways, but it is difficult to
say whether it can be classified as a toxic response.
Numerous research workers have reported changes at doses that
affect levels of AChE, but without overt signs of intoxication.
For example, Kaloyanova-Simeonova (1961) noted that small doses of
chlorthion (5 or 10 mg/kg body weight in the rat) intensified
conditioned motor reflexes and depressed them at higher doses: ChE
levels were depressed in all cases and were restored more slowly
than the return to normal reflex activity. Reiter et al. (1975)
did not find any effects on performance of learned visual
discrimination tasks by monkeys at doses of parathion of 0.5 mg/kg
body weight, which depressed blood levels of AChE by about 25% and
those of pseudoChE by about 35%. Doses causing 40% or more
inhibition of AChE were associated with decreased responses, which
returned to normal faster than the recovery of AChE levels. A very
sensitive response to the nerve agent soman (pinacolyl
methylphosphonofluoridate) was reported in one out of several
behavioural tests by Wolthuis & Vanwersch (1984). In an open-field
test, a number of performance variables were affected by a dose of
only 3% of the LD50. Effects were also seen with doses of 4 - 6%
LD50 of 2 carbamates but not below 30% LD50 of the pesticide, TEPP,
or of another nerve agent, sarin (isopropyl methylphosphono-
fluoridate). Unfortunately, the effects on ChE levels were not
measured. There is no obvious reason for the contrasting effects
of TEPP and sarin, on the one hand, and of soman and physostigmine,
on the other. The authors noted that, judged by poisoning signs at
near-lethal doses, soman exerted a greater proportion of its
effects centrally than the other 2 organophosphorus esters. The
fact that changes may be seen in some, but not all behavioural
responses at a certain dose emphasizes the statement by Revzin
(1983) that no single behavioural or neurophysiological test can
give a definite conclusion about organophosphate toxicity.
There appears to be only one other report in the literature of
a behavioural change in animals at doses less than those that
inhibit ChEs (Desi et al., 1971). Behavioural and EEG changes were
noted in rats fed bromophos at 500 or 100 mg/kg diet for 6 weeks,
doses that also caused ChE inhibition, but, at 30 and 10 mg/kg,
there were no effects on ChE, though some behavioural changes were
still observed. It is not clear whether undosed animals were
handled in precisely the same fashion as the dosed.
However, in contrast to the above, Desi (1983) reported tests
applied after 3 months of daily consumption of diet containing
various percentages of the LD50 of bromophos. The lowest
concentration that produced changes in a behavioural test (maze
running) and in EEG (both the complex EEG and computer-analysed
segments of the EEG) was about 0.26% of LD50, daily, but this also
caused significant changes in erythrocyte-AChE and plasma-ChE.
Among 6 organophosphorus pesticides assessed by these procedures,
none produced changes greater than that caused by the vehicle alone
without also causing effects on ChE. This conclusion differs from
that written by the author.
Analysis of the EEG records of a small group of rhesus monkeys
has been carried out both before, and one year after, intoxication
with the potent anticholinesterase agent sarin (isopropyl
methylphosphonofluoridate) (Duffy & Burchfield, 1980). Three
animals received a single "large dose" (5 µg/kg body weight iv),
and 3 others received a series of 10 injections of 1 µg sarin/kg
body weight im at weekly intervals: there were 10 controls. The
"large dose" animals had generalized convulsions and were
maintained on Gallamine relaxant with artificial respiration;
small-dose animals were considered in pilot studies to be near the
threshold of poisoning, but showed few overt signs; they did not
receive relaxant or artificial respiration. Twenty-four hours
after a "large dose", marked differences were seen in the EEG
frequency spectrum, which is not surprising. However, one year
after the dose, there was still a small increase in the percentage
energy in the beta-2 region of the spectrum and the change was said
to be statistically significant. Some changes in the beta region
were detectable in all 6 dosed monkeys one year after; no
significant changes were seen in the 10 controls. However,
apparently the changes were only seen under some lighting
conditions and were small compared with differences between the
frequency spectra of the only 2 controls for which data were shown.
The statistical treatment of the acquired data seems valid, but the
actual values measured one year after dosing are obviously well
within the normal range. No indication was given of how much
variation can be caused in the pattern of undosed animals by
variations in the conditions of handling and observation, or what
the range is for apparently identically treated normal animals. The
doubts pertaining to the toxicological significance of these
measurements apply also to some human studies using the same
technique (section 7.2.2).
Effects of cholinergic agents on the visual system have been
monitored by Revzin (1980). In urethane-anaesthetised pigeons with
implanted electrodes, various changes in the response of specific
neurones of the optic tectum and of the hippocampus were noted
after doses both of mevinphos and of atropine. The author claims
that the procedure was sensitive in detecting effects in the
absence of detectable peripheral parasympathetic signs. However,
the lowest effective dose of mevinphos was only one-third of that
(0.15 mg/kg body weight) which produced parasympathetic signs that
would certainly be associated with substantial inhibition of AChE.
Thus, the claim to sensitivity of this complex procedure in
surgically modified birds seems excessive.
Some possible effects of anticholinesterases on non-
cholinesterase targets were considered by O'Neill (1981). No clear
effects seem definable at concentrations lower than those that
inhibit AChE and some are probably secondary to stimulation of non-
cholinergic nerves with cholinergic innervation. However, an
endopeptidase that can hydrolyse putative transmitter peptides in
the nervous system is known to be inhibited by di-isopropyl
phosphorofluoridate at a concentration similar to that which
inhibits AChE (Kato et al., 1980). Thus, some involvement of non-
cholinergic pathways in the causing of CNS effects in
organophosphate poisoning cannot be excluded. Moreover, some
anticholinesterases also exert effects directly at the cholinergic
receptor as well as by the inhibition of AChE (Karczmar & Ohta,
1981).
6.2 Other Effects
A variety of histological lesions has been described at autopsy
in animals severely poisoned with organophosphate pesticides.
However, very few effects are described in the absence of obvious
poisoning or at doses that do not markedly inhibit the ChEs. In
the following sections, the evidence is reviewed for effects not
obviously attributable to inhibtion of either AChE or NTE.
6.2.1 Mutagenic and carcinogenic effects
The proposed theoretical basis for believing that dimethyl or
diethyl phosphate pesticides might be mutagenic or carcinogenic
has already been discussed (section 4.5.2). This basis was shown
to be defective by Bedford & Robinson (1972). No alkylation was
detected in N7 of guanine in RNA and DNA of liver of animals that
were exposed for 12 h to working concentrations of dichlorvos of
64 g/m3 (0.064 g/litre air) (Wooder et al., 1977); these authors
contrasted their data with earlier published work showing modest
alkylation of guanine in suspensions of cells exposed to very high
solution concentrations of dichlorvos. In vivo, the acute
anticholinesterase effects limit the circulating concentration
that can be tolerated by any mammal and there is also strong
preference for phosphorylation of biological scavenger molecules
rather than for alkylation built into the organophosphorus
pesticide molecules.
It has frequently been suggested that dichlorvos has
carcinogenic potential because of observed mutagenic effects in in
vitro test systems. The IARC (1979) Working Group accepted
extensive data showing no evidence for the mutagenicity of
dichlorvos in mammals. FAO/WHO (1978b) accepted animal studies
showing no dose-related carcinogenic effects in life-time studies
carried out at doses that depressed blood-ChE levels.
In a 78-week feeding test on groups of 50 male and 50 female
mice (B6 C3 F1 hybrid), no dose-related effects were seen when the
diet contained about 600 or 300 mg dichlorvos/kg, respectively.
The IARC Working Group evaluating this study noted that a few
oesophageal tumours were seen in treated mice. It appears that
this fact influenced their verdict that "the available data do not
allow an evaluation of the carcinogenicity of dichlorvos to be
made" (IARC, 1979).
An IARC Monograph (1983) included evaluations of 5 widely-used
organophosphate pesticides (malathion, methyl parathion, parathion,
tetrachlorvinphos, and trichlorfon). In several cases, the
conclusions were that acceptable tests had been performed with no
evidence of carcinogenic effects or of mutagenic effects in
mammals. For others, the conclusions were of "limited evidence"
consisting of very small effects above the control background
levels in life-time studies. None of these compounds was judged to
be a strong mammalian mutagen or carcinogen and the same statement
is true for all organosphosphorus pesticides that have been
evaluated by FAO/WHO Working Parties or by other authorities.
However, recent published results show that controversies do occur
when evaluating the outcome of carcinogenicity studies. Huff et
al. (1985) reevaluated the pathology of the original studies by
the US National Cancer Institute in 2 different strains of rats.
Histopathological reexamination confirmed the earlier conclusions
that malathion and malaoxon were not carcinogenic. These
conclusions differed from those of Reuber (1985) who evaluated the
same studies and rated both compounds as carcinogenic.
6.2.2 Teratogenic effects
Defects in the development of fertilized hen eggs, injected
with various organophosphates, are known, but many of these are
associated with the inhibition of the enzyme kynurine formamidase
and a depression of NAD levels at a critical period of development
(Seifert & Casida, 1980). This pathway is not critical in mammals,
and no equivalent effects are known. If teratogenicity is taken to
mean induction of malformations in live offspring without decrease
in number of births (i.e., no embryotoxicity), then for the vast
majority of organophosphorus pesticides, no adverse effects of
continuous feeding of organophosphates on pre- or postpartum
mortality have been reported, nor have embryonic defects been
proved, except at doses that significantly retarded growth in the
mother (Vergieva, 1983). Single high doses causing significant
toxic effects in mothers may be deleterious: a number of these
toxicity-linked effects have been summarized by Seifert & Casida
(1980). Kimbrough & Gaines (1968) reported deaths, and that
resorptions were increased in pregnant rats given a single high
dose of parathion or diazinon on the 11th day of gestation.
However, these effects were associated with significant toxic
effects on the mothers. Similarly, trichlorfon at very high doses
(400 mg/kg per day with the dose divided into 3 spaced aliquots)
and given on days 6 - 15 of gestation, produced defects in
offspring: each dose produced cholinergic symptoms; no effects were
seen in rats, mice, or hamsters when the daily dose was 200 mg/kg
(Staples & Goulding, 1979). However, a specific defect consisting
of hypoplasia of the cerebellum in offspring was noted, both in
field cases and experimental tests, 8 pregnant pigs were dosed once
or twice with neguvon (a veterinary grade of trichlorfon) between
days 55 and 70 of pregnancy (Knox et al., 1978). Doses were 50 -
60 mg/kg body weight, which caused maximum inhibition of
erythrocyte-ChE levels of 40 -80%, without overt signs of
poisoning. The hypoplasia was accompanied by severe ataxia and
tremors, while voice and vision appeared unaffected. No defects
were seen in the offspring of over 100 control sows housed with the
dosed animals, and serological and virological tests did not show
anything incriminating. A genuine teratogenic effect of moderate
doses of trichlorfon commonly used in veterinary practice has been
demonstrated in the pregnant pig. However, it may be that the
herds tested were unusually susceptible, since (apparently)
congenital tremor had been known in litters borne over many years
in the area where the trichlorfon-induced effects were seen.
6.2.3 Effects on the immune system
In a review, Zackov (1983) stated that "most ... organo-
phosphorus pesticides elicit autoimmune reactions and suppress the
production of antibodies against vaccines". No evidence was given
and it is not clear whether the statement was claiming specific
effects or referred to doses that are sufficient to produce a range
of toxic effects.
Shtenberg et al. (1974) claimed that oral doses of methyl-
nitrophos (fenitrothion) or chlorphos (trichlorfon) at 5 or 7 mg/kg
body weight per day, for an unspecified period, suppressed
haemaglutinin levels in rats immunized against sheep red blood
cells; these doses would have significantly inhibited ChEs and were
said to be more effective in rats fed a protein-deficient diet.
Dandliker et al. (1979) reported a depression in antibody titre in
rats in the 6 - 7 weeks of an immunization procedure that commenced
either one day before or one day after (both statements are made)
an oral dose of half of the LD50 of parathion. The immunization
consisted of weekly intramuscular doses of 400 µg fluorescein-
labelled ovalbumin with Freunds Complete Adjuvant. The confusion
concerning the order of intoxication and primary immunization is
very important when such a high dose was given, since cholinergic
symptoms, fluid loss, imbalance, and general debility would have
been marked. Administration to mice of 0.1 x LD50 of parathion for
8 days led to a 10% loss in body weight. Immunization on the 9th
day showed a statistically-significant (34%) reduction in the
number of antibody plaque-forming cells derived from the spleen, 4
days after immunization, but the reduction was insignificant (10%
only) when immunization was carried out on day 10, though several
animals in the group died at this point due to accumulated toxic
effects (Wiltrout et al., 1978).
Some decreased antibody titres "proportional" to AChE
inhibition were noted in response to prolonged doses of malathion
or dichlorvos (0.025 - 0.4 x LD50 given 5 days per week for 6
weeks) (Desi et al., 1978). Clearly, none of the above responses
can be dissociated from the general cholinergic intoxicating
effects of the pesticides. There do not appear to be any reports
of studies on the immunological status of healthy animals receiving
doses causing little or no depression in ChE levels.
6.2.4 Effects on tissue carboxyesterases
A variety of carboxyesterases abound in serum, liver,
intestine, and other tissues (section 4.4). Although inhibition of
one specific carboxyesterase (NTE) has toxic sequelae (section
6.1.1.2), no direct deleterious effects of inhibiting other
carboxyesterases have been demonstrated. However, they may
contribute markedly to the metabolic disposal of malathion and
certain other organophosphorus pesticides, so that inhibition of
tissue carboxyesterases may potentiate the toxicity of such
pesticides (section 6.3.5). The structure/activity relationships
for inhibition of these enzymes by organophosphorus compounds
inevitably differ from those for inhibition of AChE. For EPN and
fenchlorphos, both serum- and liver-carboxyesterases of rats were
markedly more sensitive than brain- and serum-AChE; in other cases,
the liver enzymes, but not those in serum, were more sensitive (Su
et al., 1971).
6.2.5 Sundry other effects of organophosphorus pesticides
Very few effects other than those described in the sections
above have been noted, except those arising from ill-health due to
severe anticholinesterase effects. Thus, impaired growth rate is
commonly associated with a rapid depression in AChE levels to less
than 50%, but much lower levels can be tolerated without ill-
effects, if the depression is brought about over several weeks, and
then maintained for up to a year (section 6.3.1).
Various changes in glucose metabolism, in serum enzymes, and in
other clinical chemical variables have been reported after single,
acute, or repeated doses of various pesticides at from one-tenth to
one-quarter of the LD50, daily (Dimov & Kaloyanova, 1967; Enan et
al., 1982).
A reversible, mild muscle-necrotising effect could be detected
histologically in the diaphragm muscles of rats, 24 h after a dose
of paraoxon, parathion, or other anticholinesterases, sufficient
to cause marked fasciculations (Fenichel et al., 1972; Dettbarn,
1984). The damage appeared to be a function of the prolonged
cholinergic stimulation of the muscle, since it was entirely
prevented by doses of atropine, which prevent fasciculations, or by
alpha-bungarotoxin applied directly to the myoneural junction
(Salpeter et al., 1979).
Several adverse effects attributed only to certain
organophosphorus esters are listed below. The list may be of value
in promoting more careful examination of intoxicated animals or
human beings.
6.2.5.1 Effects on hormones
Changes in the diurnal pattern of plasma-ACTH and adrenal
levels of some related enzymes have been reported in rats
maintained with dichlorvos in the drinking-water at 2 mg/litre:
blood-ChE levels were not affected (Civen et al., 1980). However,
the weight gain of the treated animals was only half that of the
controls, and the fluid consumption was increased by 20%. These
deleterious effects could be due to diarrhoea and imbalance of
fluids with inevitable repercussions on hormonal levels, etc.;
preferential effects of the dichlorvos on intestinal esterases
might be the primary effect.
6.2.5.2 Effects on the reproductive system
Damaged seminiferous tubules were reported in mice given either
a single dose of about half of the LD50 of dichlorvos or 18 doses
of about one-tenth of the LD50 (Krause & Homola, 1974). However,
the doses were high, the percentage increases seem small, and the
number of samples taken was very small; thus, no statistical
evaluation is possible. Amiprophos has been reported to cause some
gonadotrophic effects in adult cockerels (Huang et al., 1979), but
details are few.
6.2.5.3 Effects on the retina
Fenthion administered intramuscularly at about one-quarter of
the LD50 (50 mg/kg body weight), every 4 days for one year
(solvent, if any, not stated), affected the electroretinogram in 2
strains of rats (Wistar and black Long-Evans) within 3 months and
abolished it after one year (Imai, 1977). Similar studies involving
the administration of either fenitrothion or ethylthiometon to
beagle dogs for 5 days per week for 2 years, were reported by
Ishikawa & Miyata (1980). Doses that depressed plasma-ChE levels
to about 30% of normal, throughout the period, led to changes in
optical function after 13 months continuous exposure and to
morphological changes in the ciliary muscle at termination.
6.2.5.4 Porphyric effect
Daily application of technical (85%) diazinon (20 or 40 mg/kg
body weight) to the skin of Dark Agouti rats just above the tail
produced a 4-fold increase in faecal porphyrins, after 8 - 12 weeks
(Bleakley et al., 1979). There was no increase in urinary
porphyrins and no effect when diazinon was given in food (about
8 mg per day to rats, initially weighing 180 g). The pattern of
porphyrins excretion was said to be indistinguishable from
porphyria cutanea tarda. However, the increase in excreted
porphyrins in classical porphyria cutanea tarda may be as much as
1000-fold, with massive amounts in the urine as well as in the
faeces. The authors failed to reproduce even this small effect in
rats when they used more pure (97%) diazinon (Nichol et al., 1982).
However, they also found that an impurity in stored technical
material, isodiazinon (diethyl 2-isopropyl-6-methyl-4- S -
pyrimidinyl phosphorothioate), was very effective in causing
porphyrin accumulation, when added to cultures of chick
hepatocytes; confusingly, however, the accumulated porphyrin was
coproporphyrin rather than the expected protoporphyrin. Although
human poisoning with diazinon is not uncommon, there has only been
one report implicating technical diazinon in a few cases of
porphyria cutanea tarda in occupationally-exposed workers (Bopp &
Kasminsky, 1975). Further experimental animal studies seem
warranted. No reports have been found of porphyria connected with
exposure to other organophosphorus pesticides.
6.2.5.5 Lipid metabolism
Organophosphate esters can inhibit the activities of some
triglyceridases and lipases in vitro and in vivo. However, no
repercussions from such inhibition were found when appropriate
parameters were measured in rats fed for one year with either of 2
pesticides that caused marked (60 - 80%) depression of blood-ChE.
Thus, male or female rats were fed either a normal diet or a diet
enriched sufficiently with fat to increase aortic fatty acids by
170%. No changes occurred in: the hormone-sensitive lipase and
lipoprotein lipase in adipose tissue; the free and total fatty
acids and total glycerol and total cholesterol in serum; and the
total fatty acids, cholesterol, and glycerol in the aorta (Buchet
et al., l977). The pesticides were chlorpyriphos (100 mg/kg diet)
and triamiphos (10 mg/kg) and the findings were contrary to those
from a preliminary study by the same workers.
6.2.5.6 Effects causing delayed deaths
Although not pesticidal agents themselves, two of the
phosphorothiolate impurities found in technical malathion and some
analogues of these impurities caused delayed effects, which were
lethal for rats at doses below the cholinergic LD50 (Aldridge et
al., 1979; Mallipudi et al., 1979; Verschoyle et al., 1980). The
compounds were O,O,S -trimethyl phosphorothioate (I), O,S,S -
trimethyl phosphorodithioate (II), and the ethyl analogues (III and
IV). After an oral LD50 dose (as low as 26 mg/kg body weight for
II), all 4 compounds produced cholinergic responses that lasted
less than 24 h and deaths at this time were only seen with IV:
doses 2 - 6 x LD50 were needed to cause cholinergic deaths with I -
III. Rats dosed at, or near, the LD50 recovered from the initial
cholinergic effects, but by day 3, they had lost weight and were
panting with laboured respiration; deaths occurred 3 - 6 days after
dosing. However, survivors appeared normal, 10 days after dosing.
Death was due to pulmonary insufficiency associated with
progressive cell proliferation (Dinsdale et al., 1982; Imamura et
al., 1983; Aldridge & Nemery, 1984), and combined therapy with
atropine and oxime was ineffective. The biochemical mechanism of
these effects is not fully known, but it is probable that the
proximal toxin is produced in the lung by oxidative attack on the
alkylthio moiety of the compounds (Aldridge et al., 1985). The
activities of brain-AChE and plasma-ChE and carboxylesterase, which
were partially inhibited during the first day after dosing,
increased thereafter and were at least 50% of the levels of
activity in the controls at the time of death. The margin between
delayed death LD50 and cholinergic LD50 was markedly less with the
triethyl, than with trimethyl compounds. Such effects were seen
with S,S,S -trimethyl phosphorotrithiolate but not with the higher
analogue ethoprophos ( O -ethyl S,S -di- n -propyl
phosphorodithiolate) or with methamidophos (Verschoyle & Cabral,
1982).
A different form of delayed acute toxicity was reported to
occur 4 days after large oral doses of DEF in hens. The effect was
not seen after a single dose, sufficient to cause delayed
neurotoxicity, was given subcutaneously (Johnson, 1970) or dermally
(Abou-Donia et al., 1980). The effect was distinct from both
cholinergic and delayed neuropathic effects and is attributed to
the acute toxicity of n- butyl mercaptan produced by the degradation
of DEF in the gastrointestinal tract.
6.2.5.7 Selective inhibition of thermogenesis
The defoliant DEF ( S,S,S -tri- n -butyl phosphorotrithiolate)
acted as an anticholinesterase at high doses, but, at lower doses
(60 - 200 mg/kg in rats and mice), it caused a profound fall in
body temperature (as much as 10 °C over a few h), without marked
sedation; deaths occurred mostly after the depression had persisted
for a day (Ray, 1980). The effect was different from the smaller
atropine-sensitive changes due to some cholinomimetics and was due
to blocking of cold-induced thermogenesis without affecting heat
conservation mechanisms. The effect seems unique to the DEF
chemical structure. Ray & Cunningham (1985) demonstrated that the
effect was a selective action on a central thermogenic control
mechanism rather than on peripheral thermogenic processes and that
it was probably due to a metabolite of DEF rather than to the
parent compound.
6.3 Factors Influencing Organophosphorus Insecticide Toxicity
6.3.1 Dosage-effect
The lethal effects of organophosphorus insecticides are due to
severe cholinergic effects arising from excessive inhibition of
AChE. With few exceptions, the AChE activity of the tissues is
inhibited soon after the administration of acutely toxic doses of
all anti-AChE agents. This is true, not only for compounds that do
not require metabolic conversion to anti-AChE agents, but also for
most phosphorothioates, phosphorodithioates, and
phosphorodiamidates that are oxidized by the liver to metabolites
with anti-AChE activity. In general, the duration of action of
most anti-AChE agents is relatively short, as evidenced by
considerable reversal of the inhibition within a few days. For
this reason, a 10-day observation period is sufficient for acute
LD50 measurements on all anti-AChE agents that have been studied,
and 2 days suffice for most.
The data in Table 6 give a comparison of the maximal amount of
inhibition of the ChE activities in the brain, submaxillary glands,
and serum of rats for several compounds, all of which were given at
dose levels equivalent to 5/8 of the LD50. The time at which
maximal inhibition occurred and the period required for complete
reversal of the inhibition are also presented. From these
examples, it can be seen that, in general, equivalent fractions of
the LD50 of various anti-ChE compounds produce similar levels of
inhibition of ChEs, though the LD50 values for the various
compounds differ considerably. The time at which maximal
inhibition of ChEs occurs varies from 15 min to 3 h after
administration. In some cases, the AChE activity of the brain and
parasympathetic nervous system, as indicated by the submaxillary
gland, and the non-specific ChE of the serum are inhibited to the
same extent by a particular compound. However, notable exceptions
are OMPA, which is converted in the liver to a very labile
inhibitor that never reaches the brain, and Guthion, which does not
inhibit non-specific ChE.
Differences between the responsiveness of rat brain- and serum-
ChE have been reported also in the case of dietary administration
of various organophosphorus insecticides (Su et al., 1971). Thus,
a level of only 40 mg EPN/kg fed for 1 week reduced brain-ChE to
50%, while 125 mg/kg was needed to achieve the effect in serum; the
sensitivity was reversed for fenchlorphos, while the sensitivities
of the 2 tissues were similar for demeton. It is not clear whether
these differences reflect differences in access of the compounds to
their targets, differences between the tissue AChEs, or the fact
that pseudoChE is present as well as AChE in the serum of rats, so
that assay of the hydrolysis of ACh using serum measures both
enzymes, whereas, in the brain, the activity is about 90% specific.
Marked differences in the rate of reversal of the inhibitory
effects on ChEs of different compounds, in vivo, are shown in Table
6. In view of the variable duration of action of various anti-ChE
agents, the performance of assays on the tissues of animals at
intervals after acutely toxic doses provides a great deal of useful
information regarding the toxicity of these compounds. The
transition from a tolerable to a lethal dose (either acute or
chronic) often occurs within a 2 to 4-fold range. This is not
surprising, since the AChE of nervous tissue and effector organs
must be inhibited by 50 - 80% before pharmacological effects can be
seen (Holmstedt, 1959). The increment in dose to raise inhibition
to 90% with associated deaths is not very great.
Table 6. Onset and duration of the anticholinesterese action
of some organophosphorus compounds in ratsa
-----------------------------------------------------------------------
Compound Maximum inhibition of cholinesterase (%)
Dose Brain Serum Sub- Time to Time to
(mg/kg maxillary maximum complete
body gland inhib- reversal
weight) tion (h) (h)
-----------------------------------------------------------------------
Iso-Systox 1.0 85 80 75 3.0 120
(demeton- S )b
Disyston 1.25 75 85 75 3.0 120
(disulfoton)
Guthion 3.5 60 0 50 0.5 24
(azinophosmethyl)
Dipterex 140.0 85 82 85 0.25 6
(trichlorfon)
Octamethyl 5.0 0 85 88 2.0 144
pyrophosphor-
tetramide (OMPA)c
-----------------------------------------------------------------------
a From: DuBois (1963).
b Phosphorothioic acid, O -[2-(ethylthio)ethyl] O,O -diethyl ester.
c Octamethylpyrophosphoramide.
The general correlation of inhibition of ChE with symptoms of
poisoning is also seen with repeated dosing with organo-phosphates,
but details vary greatly. It is always true that a prerequisite of
death is profound inhibition of AChE in the central and/or
peripheral nerves, but the tolerable maximum inhibition increases
when this level is reached, stepwise, over a period of 2 - 4 weeks
or more. Thus, when commercial Systox (containing about equal
amounts of the O - and S -isomers of demeton) was fed to rats at
20 mg/kg diet, there were no signs of poisoning, though, at the end of
16 weeks, the brain- and whole blood-ChE activities were 26 and 28%
of normal, respectively (Barnes & Denz, 1954). At 50 mg/kg, 3 out
of 12 rats died, but all others in the group improved after initial
marked signs of poisoning during the first month, their food intake
increased above normal (and therefore their actual dose increased),
and their growth rate became normal. This improvement was not due
to any marked increase in ability to detoxify the agent, since, at
the end of 16 weeks, these apparently healthy rats had only 7 - 8%
of normal AChE activity in the brain and blood. This level would
be associated with fatalities if brought about by a single dose;
indeed, at the end of the study, the animals were consuming daily a
dose equivalent to 96% of the single-dose LD50. In a similar way,
Barnes & Denz (1951) found that parathion in the diet at 100 or
75 mg/kg was lethal for the majority of rats in large groups within
3 - 4 weeks, while results with 50 mg/kg were variable (26/72 deaths
in one trial and 3/36 in a later trial) and no deaths attributable
to poisoning occurred in groups fed 20 or 10 mg/kg. The rats
surviving 50 mg/kg showed clinical signs of intoxication (notably
fasciculations) and ate less, initially: they ate normally after 3
weeks, but failed to gain weight as rapidly as the controls.
However, signs of poisoning decreased in severity and frequency
during the third month and seldom reappeared during the remainder
of a year's feeding. This pattern of response and of adaptation is
typical for all anticholinesterase pesticides. When monitoring of
enzymes is carried out in parallel with feeding, the level of
activity often rises to a steady state after an initial decline.
Factors determining the fraction of an LD50 dose that is tolerable
include:
(a) Speed of absorption, of subsequent metabolic activation,
and of elimination of the compound. Thus, for demeton- S -
methyl, much of the sulfoxide metabolite from a single dose
will still be circulating on the following day, though the
parent compound may not linger. In such a case, lethal
concentrations will build up more easily than with, say,
trichlorfon, which is converted to the inhibitory dichlorvos,
both of which are rapidly eliminated;
(b) The net rate of formation of a stable form of
inhibited AChE arising from the 3 reactions of inhibition,
reactivation, and aging described in section 4.5. The
relationship of the chemical structure of an oxon inhibitor to
rates of these reactions is complex and also varies between
species. AChEs from the rat have not been purified and
subjected to extensive kinetic study in vitro. In most
studies, crystallized (not 100% pure) bovine erythrocyte-AChE
has been used. Rates of spontaneous reactivation and aging for
this enzyme inhibited with dimethoxy, diethoxy, and ethoxy,
ethanthio-substituted phosphates are shown in Table 7.
Although data derived in this way cannot be transposed directly to
in vivo situations, they are consistent with the well-known fact
that, after poisoning by a sub-lethal dose of some dimethyl
phosphates, recovery with the disappearance of symptoms is complete
within a few h. The value of k+3 for erythrocyte-ChE taken from
rats dosed in vivo with dimethyl phosphate, was reported to be 57
x 10-4 (a half-life of inhibited enzyme of 2 h). One day after
such a sub-lethal dose, most of the rat AChE will again be in the
uninhibited form with a small fraction in the aged inhibited form.
In contrast, not more than half of the inhibited enzyme would be
expected to be reactivated in one day after poisoning with diethyl
phosphate and a substantial proportion of the inhibited enzyme
would be aged, so that recovery to 100% activity would be a very
slow process, depending on the synthesis of fresh enzyme. It
follows that markedly different outcomes would be expected from
repeated intoxication with doses of dimethyl phosphate and diethyl
phosphate which both caused an initial response of, say, 50%
inhibition, the former would be less hazardous than the latter.
This interpretation concurs with the fact that rats can survive
daily doses of 25% of the LD50 of trichlorfon, but only about 12%
of the LD50 of parathion (DuBois, 1963). DuBois (1963) also
pointed out that ChE inhibition mounted steadily as a result of
daily doses of OMPA and that toxic effects were seen when
inhibition reached about 70%. It is believed that no spontaneous
reactivation of ChE occurs after inhibition by phosphoramidates,
which makes such compounds intrinsically undesirable as pesticides.
Table 7. Rates of spontaneous reactivation (k+3 ) and of
aging (k+4 ) of bovine erythrocyte cholinesterase after
inhibition in vitro a
---------------------------------------------------------
O Rate constants x 104
|| R1 (per min) at pH 7.4
|| / and 37 °C
Enz-O-P substituents
\
R2
R1 R2 k+3 k+4
---------------------------------------------------------
-OCH3 -OCH3 115 14
-OCH3 -SCH3 1170 95
-OC2 H5 -OC2 H5 2.0 2.2
-OC2 H5 -SC2 H5 270 32
---------------------------------------------------------
a From: Clothier et al. (1981).
Dose-effect relationships for delayed neurotoxicity have been
listed (Johnson, 1975b). It has been noted that, as for
cholinergic effects, levels of long-term dosing can be found that
are detectable by a biochemical response at the primary target but
have no clinically- or histologically-observable correlate. The
threshold of the tolerable response is probably a permanent
inhibition or 40 - 50% of NTE (Johnson, 1982a).
6.3.2 Age and sex
It is well-known that the microsomal MFOs and other drug-
metabolizing enzymes are present at comparatively low levels in
neonatal animals, but activity develops to approximately the adult
level early in maturation. Since MFOs are involved in both the
activation and degradation of many organophosphorus pesticides
(section 4.3), the likely net result in terms of LD50 is hard to
predict. One-day-old rats were 9 times more susceptible to
malathion than 17-day-old animals (Mendoza, 1976). The toxicity of
methyl parathion and of parathion for rats decreased from birth
through the developmental period: the decrease was best correlated
with the increasing capacity of the animals to metabolize the
oxygen analogues by both oxidative and hydrolytic pathways (Benke &
Murphy, 1975). The LD50 (ip) of trichlorfon in adult male rats was
reported to be 250 mg/kg body weight, compared with 190 mg/kg in
male weanlings (FAO/WHO, 1972b). Whether this is a significant
difference is not clear. Liver MFO activity fluctuates according
to the hormonal status of female animals. LD50 values quoted for
males and females often differ, but these values generally arise
from different laboratory animals subjected to many variable
factors (including the purity of the test sample). Among several
representative pesticides surveyed for this review, only parathion
showed a marked and apparently real difference in LD50s between the
sexes, the oral LD50 in male rats being 5 - 30 mg/kg body weight,
depending on the solvent, compared with 1.8 - 5 mg/kg in females
(FAO/WHO, 1964).
6.3.3 Nutrition
It is well-known that liver MFO activity can be manipulated by
administering a diet severely deficient in protein. The
consequences can be dramatic in terms of the toxicity of compounds,
such as carbon tetrachloride, which undergo a single
biotransformation step leading to a directly toxic product.
However, as with the age and sex factors discussed above (section
6.3.2), several metabolic steps may be affected. No clear-cut
effects seem to have been reported. Thus, the acute toxicity of
diazinon is greater (up to 2-fold) in rats maintained on a diet,
either very low (4%), or very high (81%) in protein compared with a
standard (29%) protein diet (FAO/WHO, 1971b). A similar increase
in toxicity is seen with naled ( O,O -dimethyl O -1,2-dibromo-2,2-
dichloroethyl phos-phate) (Kaloyanova & Tasheva, 1983). Boyd
(1969) reported that, while increases in toxicity were 2-3 fold for
diazinon, malathion, and demeton, the increase in parathion
toxicity was 7.6 fold, in malnourished rats. Whether the effects
at such extremes were directly due to changes in the
biotransformation of the agent or to the animals becoming generally
unhealthy is not clear.
6.3.4 Effects of impurities and of storage
Insecticides are manufactured and formulated in various ways
and in many countries. There may be significant differences in
these procedures and in the conditions of storage of formulated
products. These factors can influence the nature and extent of
impurities present in the material that is ultimately applied.
Impurities in a pesticide may be of very low toxicity (the
majority), may be toxic in their own right (more or less toxic than
the major component), or they may be potentiators of the toxicity
of another component.
6.3.4.1 Impurities toxic in their own right
(a) Non-anticholinesterase effects
The most dramatic example of an impurity exerting an effect
different from that of the principal component does not come from
the realm of organophosphorus pesticides. The potent toxicant
2,3,7,8-tetrachlorodibenzodioxin (TCDD) may be present in the
herbicide 2,4,5-trichlorophenoxyacetic acid so that, even at a few
mg/kg, the effects of the impurity may dominate the toxicological
response. An analogous non-cholinergic response due to impurities
is not known in anticholinesterase pesticides. Questions have been
asked about the possible mutagenic effects of trimethyl phosphate,
which may be present at a few percent in some technical
preparations of dimethyl phosphates, but the possible exposure in
vivo is limited by the main anticholinesterase response to the
pesticide, and there appears to be no evidence for mutagenic
effects in mammals of any organophosphorus pesticide (section
6.2.1).
(b) Anticholinesterase effects
LD50 values for technical preparations of diazinon have varied
over an unusually wide range. The oral LD50 for the rat was
reported to be 76 - 108 mg/kg body weight in 1964 (FAO/WHO, 1964)
and 250 - 466 mg/kg in 1971 (FAO/WHO, 1971b). A major contributing
factor, according to the latter report, was the presence of highly
toxic pyrophosphates in earlier samples; it was implied that the
impurities had been produced during storage and eliminated by
stabilization (detail unspecified) of formulated material. It
seems likely that the pyrophosphate concerned in this improvement
was monothiono-TEPP with an oral LD50 in mice of about 4 mg/kg body
weight (Margot & Gysen, 1957). Both the sulfotepp and monothiono-
TEPP content of an emulsifiable concentrate of diazinon and its
toxicity increased rapidly when it was stored in tinned-steel
containers instead of in inert-lined aluminium ones (Soliman et
al., 1982). However, in 1979, it was reported that sulfotepp was
also present in many standard and formulated preparations of
diazinon at concentrations ranging from 0.2 to 0.8% and that the
percentage was unrelated to the age of the sample (Meier et al.,
1979). It seems likely that this impurity was formed during the
synthesis of diazinon using diethyl phosphorothiochloridate.
Sulfotepp was 60 - 80 times more toxic than diazinon for the rat,
so that at least one-third of the toxicity of typical diazinon
might be attributed to the impurity. This calculation is probably
an underestimate, since it seems that metabolic disposal of an
impurity is often slowed markedly by competition from the major
component. It can be said that there may be "reverse potentiation"
of the toxicity of the impurity by the major component (diazinon).
Formation of pyrophosphates is implicit in the mode of
synthesis of the many organophosphorus pesticides with a
phosphorochloridate or phosphorothionochloridate as a precursor.
TEPP or its methyl analogue would be unlikely to survive much
aqueous washing during production, but the mixed mono- or disulfo
analogues may well survive, unless deliberately eliminated. It
seems likely that sulfotepp is generally present in parathion
(Diggory, 1977); however, since both the parent and the impurity
have similar LD50s, pure and impure preparations do not differ
significantly. It is a curious fact that the lower the true
toxicity of a pesticide, the more marked may be the effect of an
impurity in changing its toxicity. It might be very rewarding
both to analyse more thoroughly and to reexamine the toxicities of
low-toxicity pesticides such as bromophos which is said to have an
LD50 in various mammals of 3 - 8 g/kg body weight (FAO/WHO, 1973b);
even this low toxicity might be attributable to the impurities
rather than to the pure compound. An analogous situation certainly
pertains concerning the potentiation of malathion (see below).
6.3.4.2 Impurities potentiating the toxicity of the major
ingredient
There does not appear to be any indication that the MFO status
of animals is different after administration of technical grade
organophosphorus insecticides compared with pure. Alterations of
the MFO status of animals because of diet, sex, drugs, etc.,
discussed in sections 6.3.2, 6.3.3, and 6.3.5, are unpredictable in
their effect on the LD50. In contrast, inhibition of the esteratic
capacity of mammals increases the toxicity of pesticides that
depend principally on tissue esterases in their metabolism (section
4.3). Malathion is a notable example of an organophosphorus
pesticide in which the toxicity is enhanced when tissue esterases
are inhibited. Until comparatively recently, such inhibition was
only known in situations where an unrelated organophosphorus ester
was administered to test animals a short time before the malathion.
However, it is now known that several impurities present in most
samples of malathion prepared for use as pesticides, are capable of
inhibiting tissue carboxylesterases. Some of these impurities act
very rapidly and so prevent the normal metabolism of malathion and
potentiate its toxicity. This enhanced toxicity has been expressed
in man. Several hundred spray workers were intoxicated while
spraying certain formulations of malathion in Pakistan, and 5 died
(Baker et al., 1978). Isomalathion and several trimethyl
phosphorothiolates are found in most commercial preparations of
malathion, but the levels depend markedly on the formulation and
on storage conditions. The potentiating power of small amounts of
these impurities are shown in Table 8. Examination of samples of
formulated malathion, known to be unusually toxic, showed a fair
correlation of toxicity only with the percentage content of
isomalathion (Aldridge et al., 1979; Miles et al., 1979). However,
the correlation was imperfect when a large number of samples were
examined and the addition of known further amounts of pure
isomalathion to the formulated samples caused more than the
expected potentiation (Aldridge et al., 1979). The authors
concluded that, although isomalathion contributed the main effect,
there was also significant potentiation by other agents present in
the samples; the chief candidate was O,S,S -phosphorodithioate.
Most unformulated samples of technical grade malathion seem to
have an LD50 for rats in the range of 1500 - 2000 mg/kg body weight.
Such material contains some potentiating impurities (Pellegrini &
Santi, 1972; Umetsu et al., 1977), but has proved acceptable as a
basis for formulated insecticides with little toxic hazard.
However, it is now clear that some formulated samples increase
markedly in both their impurity content and toxicity, when they are
stored at elevated temperatures. Not only are temperature and time
important, but also the formulating agents (Table 9), and almost
half of the malathion lost from Formulation C is apparently
transformed to isomalathion with a massive potentiation, whereas
the increase in toxicity is less marked in Formulations A and B, in
which a much smaller proportion of lost malathion is converted to
isomalathion.
Table 8. Potentiation of acute oral toxicity for rats by impurities
added to malathion
------------------------------------------------------------------------
Compound Amount Potentiation Purified Reference
added added ratio found malathion
(%) used (LD50
mg/kg body
weight)
------------------------------------------------------------------------
isomalathion 0.4 3 10 700 Aldridge et al.
0.6 5 (1979)
2 12
8 25
0.05 3 12 500 Umetsu et al.
0.1 4 (1977)
0.5 6
2 10
O,S,S -trimethyl 0.15 3 10 700 Aldridge et al.
phosphorodithioate 0.3 4 (1979)
0.5 8
1.0 13
2.0 20
0.05 4 12 500 Umetsu et al.
0.2 6 (1977)
0.5 7
0.035 2 8000 Pellegrini &
0.1 3 Santi (1972)
0.2 4
0.5 7
O,O,S -trimethyl 0.3 2 10 700 Aldridge et al.
phosphorothioate 1.3 4 (1979)
0.2 3 12 500 Umetsu et al.
1 4 (1977)
0.2 3 8000 Pellegrini &
0.5 4 Santi (1972)
O,O,S -trimethyl 1.5 2 10 700 Aldridge et al.
phosphorodithioate 5 5 (1979)
1 4 12 500 Umetsu et al.
5 5 (1977)
3.5 2 8000 Pellegrini &
4.5 3 Santi (1972)
------------------------------------------------------------------------
There is much evidence that potentiation of malathion by
extraneous compounds is associated with the inhibition of
carboxylesterases. Using malathion as specific substrate, Talcott
et al. (1979b) showed that isomalathion and O,S,S -trimethyl
phosphorodithioate were potent inhibitors of rat liver and plasma
malathion carboxylesterase, in vitro and in vivo; a partially-
purified sample of carboxylesterase from human liver was also
sensitive to isomalathion (Talcott et al., 1979a).
The same hazard exists with impure samples of phenthoate as for
malathion. Pellegrini & Santi (1972) showed that technical samples
containing 61 - 91% of the principal ester had LD50s (rat oral) of
78 - 243 mg/kg body weight, while a purified preparation (98.5%)
had an LD50 of 4700 mg/kg, though its toxicity for insects
increased approximately in proportion to the purity. The principal
potentiating impurities were the S -methyl isomer and the identical
trimethyl phosphorothiolates found in malathion. For phenthoate,
as for malathion, the vulnerability to potentiation lies in the
presence of the hydrolysable ethoxycarbonyl ester bond which, in
pure samples, is the key to low mammalian toxicity.
Table 9. Effects of formulating agents and of storage
time and temperature on composition and toxicity of
malathion (50% wdp)a
----------------------------------------------------------
Storage conditions Composition (%) Oral LD50
Temperature Time Malathion Isomalation (rat)
(°C) (days)
----------------------------------------------------------
Formulation A
0 48.0 0.38 2800
38 60 45.5 0.37 2230
90 44.2 0.49 1740
55 6 47.6 0.30 2520
13 46.4 0.37 1760
90 1 40.9 0.69 950
Formulation B
0 48.8 0.18 2540
38 60 47.2 0.79 1130
90 46.5 0.81 1330
55 6 45.2 0.67 1200
13 43.6 0.55 1170
90 1 39.9 0.32 1900
Formulation C
0 50.6 0.61 2660
38 90 44.9 3.7 590
55 6 46.2 3.4 535
13 43.7 3.5 555
----------------------------------------------------------
a From: Miles et al. (1979).
The presence of the S -methyl isomer (0.32%) in a commercial
fenitrothion formulation was shown by Miles et al. (1979). The
concentration increased to > 1% during accelerated storage tests,
but there was no concomitant increase in the toxicity of the
formulation. This observation on a compound not heavily dependent
on esterases for the primary step of detoxication, points to the
need to consider biochemical mechanisms in assessing possible
hazards. There need be no general concern about the presence of
small amounts of isomers in organophosphorus pesticides. The
presence of isoparathion in parathion is well-known, but there is
no suggestion of any marked change in toxicity brought about by
this impurity.
Some organophosphorus pesticides contain carboxylamide bonds
rather than carboxyester. These include dimethoate, dicrotophos,
monocrotophos, phosphamidon, and acephate. There appears to be
little evidence that impurities in commercial formulations of any
of the above pesticides markedly alter the toxicity, apart from a
small (1.6x) decrease in the mammalian toxicity of acephate, after
storage for 6 months at 40 °C; the insecticidal activity of the
compound was unchanged (Umetsu et al., 1977). During the storage
period, the concentration of various impurities changed, but no
relationship between these changes and altered toxicity was
obvious.
6.3.5 Effects of other pesticides and of drugs
All organophosphorus and carbamate insecticides exert their
acute toxic action by attack on the AChE. Thus, it follows that
exposure to more than one such pesticide will usually produce at
least an additive effect. Besides this simple effect, other
pesticides or drugs may also influence the toxicity of an
individual organophosphorus pesticide by interfering with its
metabolism, activation, and disposal.
Not all organophosphorus pesticides and probably no carbamate
pesticides inhibit tissue carboxylesterase to potentiate malathion
in the manner discussed in section 6.3.4. However, like all other
enzymes, the carboxylesterases have their own structure-activity
pattern: this has not been worked out in a systematic fashion. It
is clear that, with some compounds, profound inhibition of liver
carboxylesterase can be achieved without inhibition of AChE
sufficient to cause signs of poisoning. This class includes many
thioalkyl esters such as the trimethyl esters and also S,S,S -tri- n
-butyl phosphorotrithioate (DEF), which are potent potentiators of
malathion toxicity via carboxylesterase inhibition. EPN is a
phenylphosphorothioate insecticide that also acts in this way.
Tables 10 and 11 show examples in which measurements of effects on
tissue carboxylesterases and AChE are of value in predicting the
potentiation of malathion toxicity (Murphy, 1969).
Table 10. Comparison of enzyme inhibition caused by
several pesticidesa
---------------------------------------------------------
Insecticide Dietary concentration (mg/kg) resulting in
(period) 40 - 60% inhibition of:
Red cell- Liver- Plasma-
cholinesterase malathionase malathionase
---------------------------------------------------------
Parathion 3 5 5
(7 days)
Fenchlorphos 500 30 30
(7 days)
Malathion 500 100 500
(30 days)
---------------------------------------------------------
a From: Murphy (1969).
Table 11. Effect of feeding fenchlorphos on in vivo
anticholinesterase activity of malathiona
-------------------------------------------------------
Fenchlorphos Malathion Inhibition of brain
concentration challenge dose AChE 1 h after
in diet (mg/kg) (mg/kg ip) challenge (%)
-------------------------------------------------------
0 200 13
30 0 1
30 200 61
-------------------------------------------------------
a From: Murphy (1969).
Potentiation of the toxicity of organophosphate compounds for
mammals not containing a carboxylester function, does not appear to
be a significant hazard, though it is possible that potentiation of
some carboxylamide pesticides by an analogous inhibition of tissue
amidases may occur; certainly, EPN potentiates the toxicity of
dimethoate (El-Sebae, 1980). Potentiation of the toxicity of
organophosphorus pesticides for insects by inhibition of MFO
activity is well-known, and many potent synergists are used in
agriculture for this purpose (Wilkinson, 1971), but much less has
been reported concerning similar effects in mammals. This may
reflect the greater versatility of mammals compared with insects in
disposing of organophosphorus esters (section 4.3). Competition
for one metabolic route within the animal often does not greatly
alter its total capacity to deal with a foreign compound.
Keplinger & Deichmann (1967) combined pairs of various pesticides
in proportion to their oral LD50s, determined individually, and
then measured the oral toxicity of the mixtures in rats or mice.
They calculated a ratio of expected LD50/observed LD50 where
"expected LD50" was the sum of half the LD50 value of each of the 2
constituents. Their study included 7 chlorinated hydrocarbons,
the carbamate carbaryl, and 5 organophosphates including diazinon,
malathion, and parathion. With a "no-effect" ratio of 1.0, they
considered measured ratios greater than 1.75 or less than 0.57 as
probably significant of real effects. In a number of cases, less
than additive effects were noted for combinations of a chlorinated
hydrocarbon and an organophosphate, e.g., aldrin + diazinon (0.55),
DDT + malathion (0.54), toxaphene + carbonylfenthion (0.54): this
might well be expected if the mode of toxicity were different and
metabolic pathways were not markedly altered. The only cases of
potentiation involving organophosphates were also not surprising.
A triple combination of parathion, malathion, and chlordane had a
ratio of 1.99. This effect was probably due to simple potentiation
of malathion by parathion, since chlordane alone did not potentiate
either organophosphorus compound. Mixtures of Aramite (sulfurous
acid 2-chloroethyl 2-[4-(1-1-dimethylethyl)-phenoxyl-1-methylethyl
ester) with several organophosphates in mice had ratios of 1.86 -
2.14. However, since the LD50 of Aramite in mice is high (2000
mg/kg body weight), it may be that the 500 mg/kg administered in a
mixture was absorbed more efficiently and was therefore more
effective proportionately than the much higher LD50 dose of Aramite
alone.
As noted above, aldrin had little effect on the toxicity of
organophosphorus insecticides, when administered at the same time.
However, a number of chlorinated hydrocarbons (aldrin, DDT,
chlordane, etc.) are well-known as stimulators of MFO activity in
(principally) the mammalian liver. This activity increased
markedly during a period of a few days after dosage, and
pretreatment of mice with aldrin (16 mg/kg body weight), 4 days
before a challenge dose of 6 different organophosphates, markedly
reduced (up to 5-fold) the toxicity of each (Murphy, 1969).
Similar results were obtained with other classes of MFO inducers
such as phenobarbital. Murphy points out that the mechanism of
these effects probably includes stimulation of liver
carboxylesterase as well as MFO.
From the observations above, it appears that simple mixing of
an organophosphorus insecticide with a chlorinated hydrocarbon is
unlikely to adversely influence the acute toxicity for mammals, as
expressed by LD50 value. However, administration of DDE at 55
mg/kg diet to adult male Japanese quail led to an increasing
susceptibility to challenge doses of parathion (2.5 mg/kg)
administered orally; mortality in these birds increased from 0 to
30% after 1 week of feeding and 60% after 3 weeks (Ludke, 1977).
The problem of potentiation by some organophosphates of the
toxicity of pesticides containing carboxyl ester bonds may be
significant. This has been demonstrated for malathion in animals
(section 6.3.4) and in man (section 7.1.3). It may also be a
problem for pyrethroid insecticides for most of which degradation
by mammalian carboxyesterases is a significant detoxification
pathway (Miyamoto, 1976).
6.3.6 Species
No clear ranking of species sensitivity to organophosphorus
pesticides as a class can be given. A general impression is that
mice, hamsters, and guinea-pigs may be more sensitive than rats,
with respect to a number of compounds, but the converse is seldom
true. However, most available data have been produced with little
reference to conditions of husbandry, diet, hormonal status, etc.,
so that only very marked differences, which do not usually seem to
exist, would emerge. Birds tend to be more sensitive to
organophosphorus pesticides (Schafer, 1972) and amphibians less
sensitive than mammals. It has been suggested, but not confirmed,
that these differences might be due to differences in the activity
of enzymes in species that hydrolyse organophosphorus compounds and
thereby contribute to detoxification.
The toxicity of several organophosphorus pesticides for
different species seems to be inversely related to the activity of
the plasma A esterase, which degrades the pesticide oxon. Such
activity is considerably lower in birds than in mammals (Machin et
al., 1978). When 14 avian species were compared with 5 mammalian,
the average plasma activity against pirimiphos-methyl oxon was 170
times less, and that against paraoxon, at least 13 times less
(Brealey et al., 1980). Differences in liver microsomal oxidative
activity involved in the metabolism of several organophosphorus
pesticides by mammals and birds were less profound, though fish
liver was less active (Miyamoto & Ohkawa, 1978).
A multitude of factors contribute to the great difference
between the oral toxicity of chlorofenvinphos for the rat (10 mg/kg
body weight) and that for the dog (> 5000 mg/kg). These include
efficiency of absorption, at least 2 metabolic detoxification
processes, the rate of uptake by the brain, and a 7-fold difference
in the sensitivity of the brain AChE to this compound in the 2
species (Hutson & Hathway, 1967; Donninger, 1971).
Adult hens, cats, dogs, and larger farm animals are all
susceptible to organophosphorus delayed neurotoxicants (Davis &
Richardson, 1980; Johnson, 1982a). There is no clear ranking of
dose-sensitivity, though hens seem to be most uniformly responsive.
The full clinical response is not easily seen in laboratory
primates and rodents, though morphological damage may be detected
(section 6.1.1.2).
6.3.7 Other factors
The effects of solvents on chemical stability and isomerization
reactions were noted in section 4.5. The effects of formulation
agents on stability and, therefore, on the toxicity of malathion
have also been noted previously (section 6.3.4). It is likely that
percutaneous absorption will be greater for liquid formulations
than for powders but that powders may adhere longer thereby
enhancing an effect, if proper hygiene is not observed. A number
of examples are quoted by El-Sebae (1980), in which formulated
pesticides were more toxic than the technical preparation (Table
12). In many cases, this is due to the fact that the solvent in
the formulation facilitates the uptake of the pesticide into the
body. The toxicity of other components of the formulation may play
a role (especially in the case of pesticides of very low toxicity,
such as tetrachlorvinphos) (Table 12), as well as potentiation.
El-Sebae noted that, in some cases, the I50 for inhibition of ChEs
in vitro by these compounds differed. This could be relevant in
the case of the directly-active oxon-type pesticide tetrachlorvinphos,
but in cases where the test was performed with a thioate, the
anticholinesterase activity would depend almost entirely on trace
oxon impurities, which are often destroyed rapidly in vivo and
contribute little to toxicity compared with the bulk of oxon
produced metabolically.
Table 12. Comparative toxicity for mice of
some technical and formulated
organophosphorus pesticidesa
--------------------------------------------
Pesticide 24-h oral LD50
(mg/kg body weight)
Technical Formulated
--------------------------------------------
phosfolanb 12 11
chlorpyriphos 140 60
leptophos 162 83
tetrachlorvinphos 5000 1800
--------------------------------------------
a From: El-Sebae (1980).
b (diethoxyphosphinothioyl)dithioimidocarbonic
acid, cyclic ethylene ester.
6.4 Acquisition of Tolerance to Organophosphorus Insecticides
This topic has been discussed in section 6.3.1, where it was
noted that when AChE levels were reduced progressively over a
number of days or weeks, animals showed cholinergic signs of
poisoning which, in animals that survived, decreased in severity
and sometimes disappeared completely, though ChE inhibition was
maintained. This phenomenon is separate from the fact that
permanent inhibition of 30 - 50% is ineffective in producing
measurable symptoms. The basis for acquired tolerance is not fully
known, though a "down regulation" in the muscarinic ACh receptor is
thought to be a contributary cause. This involves both reduced
sensitivity and reduced numbers of receptors (Costa et al., 1982).
6.5 Therapy of Experimental Organophosphorus Poisoning
The understanding of the mechanism of acute toxicity of
organophosphorus pesticides has provided the basis for rational
therapy. The effects of inhibition of AChE, as described in
section 6.1.1, are common to all organophosphorus pesticides
intoxications. However, the speed of onset and the rate of unaided
recovery from sub-lethal doses vary greatly, depending on the
chemical nature of the pesticide, the route of exposure, and on
whether this exposure was a sudden overwhelming dose or a drawn-
out process.
Factors leading to a slow onset of symptoms include:
(a) Slow absorption or metabolic activation: this is often
associated with extremely low solubility and therefore
with the presence of large hydrophobic groups in the
ester molecule; pesticides such as haloxon, chlorpyrifos,
and leptophos are of this type;
(b) Persistence in the system of a comparatively stable
inhibitor of ChEs. This could be as a low concentration
of an active inhibitor such as demeton- S -methyl
sulfoxide or high concentrations of a weak inhibitor such
as methamidophos.
Factors leading to rapid clearance of symptoms include:
(a) Rapid clearance of the pesticide and its active agents,
as with trichlorfon and dichlorvos; rapid clearance
occurs also with nerve agents such as soman and sarin,
which have been much used in studies on therapy in
experimental animals.
(b) A slow rate of aging of inhibited AChE giving opportunity
for reactivation (spontaneous or induced) to occur;
diethyl phosphorylated AChE ages more slowly than
dimethyl or diisopropyl.
(c) Rapid spontaneous reactivation of inhibited AChE, such as
occurs after inhibition by all dimethyl or bis-2-
chloroethyl phosphates. In this case, the possibility of
reinhibition by a persistent compound will affect the
picture.
The factors noted above, which influence speed of onset and
remission of effects, influence the prognosis for response to
therapy but do not markedly alter the nature of optimal treatment.
Maximum benefit comes from combined treatment with an
anticholinergic drug (usually atropine) plus a reactivator of
inhibited ChE (an oxime) with diazepam, and also artificial
respiration. The effects of the components will be discussed
individually below.
6.5.1 Palliation
Artificial respiration alone can be very effective in
maintaining life, since the primary cause of death in
organophosphorus poisoning is respiratory failure (section 6.1.1).
Such treatment gains time for the processes, natural or imposed,
that lead to the return of sufficient ChE activity to maintain
life.
6.5.2 Antagonism of effects of ACh
Atropine antagonizes many of the peripheral muscarinic effects
of excess ACh and also some central effects. However, there was no
correlation between the peripheral anticholinergic activity of a
range of atropine-like drugs and their capacity when used alone (or
in conjunction with oximes) (section 6.5.3) to protect against the
lethal effects of sarin (Coleman et. al., 1962; Brimblecombe et.
al., 1970). Moreover, the ranking of the therapeutic efficacy of
atropine analogues varied according to the test species (rat,
mouse, or guinea-pig), and all effects were small (protective ratio
< 1.5) in mice and guinea-pigs, but much larger and more variable
(1.2 - 9.3) in rats. The ranking changed yet further when the drug
was combined with oxime P2S (see below), though the protective
ratio with some compounds rose to 18 - 24 in guinea-pigs, 9 - 80 in
rats, but only 1.8 - 6.3 in mice. These confusing effects are now
thought to be at least partially due to an anticonvulsant effect
contributed variously by the atropine analogues. Anticonvulsants
often supplement the effects of atropine or of combined
atropine/oxime therapy (see below). In particular, diazepam
(valium) is known both to raise the LD50 and speed recovery in some
cases (Johnson & Wilcox, 1975). These authors implied that the
mechanism might be partly direct antagonism to some central effects
of ACh and partly indirect. When diazepam is included in the
therapeutic package, there appears to be little evidence that
alternative anticholinergic drugs to the well-proved atropine are
superior (Green et al., 1977). It has been reported that, in
prophylaxis against the toxicity of DFP in mice, the protection
factor was 28 when atropine and obidoxime were used but 180 when
Dexetimide (a drug with strong central anticholinergic activity)
was substituted for atropine. The particular advantage claimed for
this drug was that, in the therapy of rabbits intoxicated with up
to 60 x LD50 of paraoxon or 80 x LD50 of DFP, one single
intravenous injection of Dexetimide (8 - 16 mg/kg body weight) was
effective in conjunction with obidoxime, whereas repeated doses of
atropine were necessary (Bertram et al., 1977). Dexetimide is used
for the treatment of parkinsonism and is available commercially.
However, Dexetimide has not been evaluated in conjunction with
diazepam or compared with atropine plus diazepam, and no details
were given of the hazards of its use and side-effects in control
animals.
6.5.3 Reactivation of inhibited AChE
As indicated in section 4.5.1, inhibited ChEs can be
reactivated in vitro by treatment with appropriate nucleophilic
agents, of which salts of the oxime N- methylpyridinium-2-aldoxime
are the most commonly used (the chloride is known as pralidoxime
and the methanesulfonate as P2S). In some countries, obidoxime
(ToxogoninR ), which is a bis-quaternary oxime, is recommended at
slightly different doses than pralidoxime, but the mode of action
is similar. The scope for further improvement in the design of
therapeutic oximes is discussed by Gray (1984).
In vivo, there are 2 limitations to the benefits to be
obtained from the use of these agents:
(a) Access
The quaternary oximes are thought not to cross the blood-brain
barrier easily (Taylor, 1980). However, some experimental
work, summarized by Lotti & Becker (1982b), suggests that there
may be limited access, and this may have a significant, albeit
small, effect in reversing inhibition of AChE to improve the
clinical state. Other more direct beneficial action of the
oximes directly at synapses in the medullary respiratory centre
cannot be ruled out. The prompt improvement in the level of
consciousness observed and in the EEG of an intoxicated child
when iv infusion of 2-PAM was commenced (Lotti & Becker, 1982b)
also seemed to indicate that there was some access to important
brain regions. It has been claimed that obidoxime (25 mg/kg
body weight) injected intraperitoneally in rats, 5 min after a
dose of armin, was effective in reactivating 33% of the
inhibited AChE of the ponto-medullary region, which contains
the centre for control of respiration (Vasic et al., 1977).
However, there were no controls appropriate to disprove the
alternative explanation that the oxime had altered the
circulating level of armin (by direct destructive interaction
or otherwise), which, although interesting, is unlikely to be
relevant to therapy instituted later after dosing, nor is such
destruction-protection unique to toxogonin.
(b) Aging of inhibited AChE
As noted in section 4.5.1, inhibited AChE is converted by a
time-dependent reaction to a form resistant to reactivators.
Thus, oxime therapy becomes less effective with time after
poisoning. The rate of aging of dialkyl-phosphorylated AChE is
Me > IsoPr > Et (O'Brien, 1967), but little has been published
on the rate of aging when phosphonyl groups derived from
pesticides are attached to the enzyme. When one or both of the
residual alkyl groups are attached to phosphorus through sulfur
rather than oxygen, the rates of both spontaneous reactivation
and of aging of inhibited bovine erythrocyte AChE are markedly
increased (Clothier et al., 1981). Ethoprophos is one of the
newer pesticides that contain such a residual alkylthio group;
no published studies are known of therapy after poisoning with
this or related pesticides.
6.5.4 Efficacy of therapy
As reported above, the combination of atropine plus oxime is
far more effective in most cases than the mere summed effects.
This is because the peripheral neuromuscular junctions
(particularly the diaphragm) and the sympathetic ganglia, where
oximes reactivate AChE, are nicotinic and are unaffected by
atropine, so that separate aspects of intoxication are treated by
the two agents. There is speculation that some oximes may exert a
protective effect by acting as depolarizing agents at the
neuromuscular junction. This may also account for the slight
therapeutic effects of some analogues of toxogonin which do not
have any nucleophilic oxime group or reactivating power (Schoene &
Oldiges, 1973). Diazepam, also, is ineffective, except in
combination with atropine and oxime.
Persistence of the toxic agent may interfere with successful
therapy. Thus, single doses of atropine + oxime were of only
marginal efficacy in altering the LD50 of isofenphos in rats
(FAO/WHO, 1982b). However, when therapeutic doses were given
repeatedly at about 12-h intervals, for 2 - 3 days, the LD50 for
rats was raised about 4-fold; for hens, the increase was 15-fold
(Wilson et al., 1984). It appears that the failure of one-shot
therapy in this case was due to persistence of the toxic agent
rather than to a different mode of intoxication or to formation of
an inhibited form of AChE that resisted reactivation. The same
situation may pertain for profenofos intoxication, which appears
not to respond well to therapy (El-Sebae, personal communication,
1985).
7. EFFECTS ON MAN
7.1 Acute Cholinergic Poisoning
The clinical picture of organophosphorus intoxication results
from accumulation of ACh at nerve endings. The syndrome is
described in detail in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980; HMSO, 1983; Plestina, 1984). The
symptoms can be summarized in three groups as follows:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles and, in more severe
cases, of diaphragm and respiratory muscles; and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, restlessness, and anxiety;
- mental confusion, convulsions, and coma; and
- depression of the respiratory centre.
All these symptoms can occur in different combinations and can
vary in time of onset, sequence, and duration, depending on the
chemical, dose, and route of exposure. Mild poisoning might
include muscarinic and nicotinic signs only. Severe cases always
show central nervous system involvement; the clinical picture is
dominated by respiratory failure, sometimes leading to pulmonary
oedema, due to the combination of the above-mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure; and
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to the light. Pulse rate is not
of diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ.
On the other hand, since changes in the conduction
and excitability of the heart might be life-
threatening, monitoring should be performed.
Confirmation of diagnosis is made by measurement of AChE in RBC
or plasma-pseudoChE, and, also, of the dibucaine number (to rule
out genetic deficiencies).
Measurements of blood-ChE during therapy are also useful in
assessing the treatment with oximes, though there might not be a
correlation between the severity of symptoms and the degree of ChE
inhibition: comparison should be made with pre-exposure levels,
wherever possible.
Chemical analysis of body fluids (urine, blood, gastric lavage)
should be made in order to identify the compounds that caused
poisoning.
7.1.1 Methods for assessing absorption and effects of
organophosphorus insecticides
As well as assessments of general health and behaviour, the
study of the effects of this class of pesticides is favoured
compared with that of some other classes since the basic
biochemical mechanisms (inhibition of esterases) are known for the
major toxic effects. Biochemical and neuro-physiological
techniques, relevant to the principal effects of all the compounds,
have been established. Identification of the monobasic acid type
of urinary metabolite, which is commonly produced, is an indicator
of exposure rather than of an effect, but it seems appropriate to
outline the technique in this sub-section. Wherever possible, test
findings should be compared with pre-exposure measurements on the
same individual.
7.1.1.1 Analysis of urine as a means of monitoring exposed
populations
As previously discussed in section 4, organophosphorus
pesticides may undergo hydrolysis in vivo to yield substituted
phosphoric acids that are subsequently excreted in urine. Advances
in gas chromatography and combined gas chromatography/mass
spectrometry (CG/MS) have made it possible to analyse the urine of
exposed persons for the presence of appropriate metabolites. It is
usually necessary to preserve the sample by the addition of
chloroform, to concentrate or extract the metabolite(s), and to
convert them to suitably-volatile derivatives that can be detected
by GC. Obviously, access to a well-equipped analytical laboratory,
capable of the quick processing of samples, is a necessary factor
if monitoring by urine analysis is proposed. However, in some
cases, simpler and sensitive colorimetric tests are available for
screening the urine of exposed persons. Thus, 4-nitrophenol can
be measured directly in the urine of workers exposed to parathion
(Wolfe et al., 1970).
Consideration of the concentration of metabolite(s) in the
urine can be helpful in determining patterns of exposure, and these
concentrations can be calibrated against the effects on AChE for a
particular pesticide. However, the time-course and peak of
excretion of metabolites appears to vary according to dose (Bradway
et al., 1977), so that serial sampling and analyses of urine are
desirable. Levels of metabolite alone cannot be considered a guide
to hazard. This is obvious when it is realized that pesticides
that have very different toxicities may yield identical acidic
metabolites. Thus, the level of metabolites in urine, after
exposure to sufficient amounts of the very toxic parathion-methyl
to depress blood-AChE to 50%, will be much lower than that of the
identical metabolites, following exposure to the related
fenitrothion, which is about 40 times less toxic.
7.1.1.2 Biochemical methods for the measurement of effects
AChE is present in human erythrocytes (RBC) and is the same as
the enzyme present in the target synapses. Thus, levels of AChE in
RBC are assumed to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the organophosphate has equal access to blood and
synapses. In the case of acute poisoning, a high inhibition of
RBC-AChE is pathognomonic, but, in the follow-up of the
intoxication, it might not be correlated with the severity of
symptoms. In the case of repeated exposures, additional
difficulties in interpretation arise from possible development of
tolerance. However, monitoring of pre- and post-exposure levels of
AChE in RBC gives a good measure of the effects of an exposure
(Kaloyanova, 1975). In cases where the pre-exposure AChE level is
not known (as in accidental poisoning), reference can be made to a
mean population AChE activity. Blood-plasma contains a related
enzyme called ChE or pseudoChE, which contributes to the whole-
blood enzymatic activity; the contribution of plasma-ChE in assays
of AChE will depend on the type and concentration of the substrate
used. PseudoChE has no known physiological function and can be
inhibited selectively by some compounds without causing a toxic
response. The sensitivities of AChE and ChE to inhibitors differ,
so that measurements of the ability of whole-blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many kinds of field conditions, procedures using whole blood, are
more practical than those using separated erythrocytes. Quite
commonly, pseudoChE is more sensitive to inhibitors. Thus, if
separation of plasma and erythrocytes is possible, prior to assay,
an indication of exposure can be obtained by assay of pseudoChE
only. Examples of selected organophosphorus insecticides, arranged
according to their ability to inhibit preferentially either plasma
or red cell-ChE in man, are given in Table 13 (Hayes, 1982).
Table 13. Selected organophosphorus insecticides arranged
according to their ability to inhibit either plasma- or
red cell-cholinesterase in mana
---------------------------------------------------------
Plasma enzyme more inhibited RBC enzyme more inhibited
---------------------------------------------------------
Chlorpyrifos Dimefox
Demeton Mevinphos
Diazinon Parathion
dichlorvos Parathion-methyl
malathion
Mipafox
Trichlorphon
---------------------------------------------------------
a Modified from: Hayes (1982).
ChE assay procedures vary greatly in sophistication, but the
most satisfactory is that based on the procedure of Ellman et al.
(1961). A field method and kit for whole blood- and plasma-ChE
determination have been developed (WHO, 1984b). Quick methods exist
for the determination of ChE in serum using paper tests (Izmirova,
1980) and for the colorimetric determination of ChE in whole blood
(Tintometer): these may be useful in the differential diagnosis of
organophosphate poisoning. Interpretation of the test results is
discussed in section 7.1.2.
The potential for delayed neuropathic response to an
organophosphorus ester can be predicted by the assay of the
esteratic activity of the target protein (NTE), in autopsy brain
samples from dosed adult hens (section 6.1.2). It has been shown
(Dudek et al., 1979; Richardson & Dudek, 1983) that a low level of
similar enzyme activity resides in lymphocytes and that there may
be correlations under some circumstances between neurotoxic dose
and available lymphocyte enzyme activity. The possibility of
monitoring exposed individuals by means of human lymphocyte or
platelet NTE activity is being explored (Lotti et al., 1983;
Bertocin et al., 1985; Maroni & Bleeker, 1986).
7.1.1.3 Electrophysiological methods for the study of effects
Electromyographic (EMG) studies using non-invasive surface
electrodes have been claimed to give sensitive indications of
exposure to organophosphorus pesticides, even in situations where
blood-ChE activity has returned to normal levels (Jager et al.,
1970; Roberts, 1976). The method requires electro-physiological
equipment and a very skilled practitioner. There is still
considerable doubt about the validity of some published studies.
Reproducibility is known to be very sensitive to local factors such
as temperature of the skin, and conflicting results have been
published, some of which show small increases and some, small
decreases in the ampli-tude of evoked muscle action potential, in
response to nerve stimulation. These findings have been reviewed
by LeQuesne & Maxwell (1981), who noted that changes that have been
reported tended not to be dose-related. In addition, they
evaluated the technique under controlled circumstances. In a
treatment to eradicate parasitic schistosomes, 55 children were
dosed orally with trichlorfon, 3 times, at 2-weekly intervals, at
doses that measurably depressed blood-ChE (mean 50%), but were not
enough to cause overt toxic effects, apart from mild cramps and
diarrhoea in a few cases. Only 3 children showed a significant
alteration in electromyographic response. Shortly after the last
(and highest) dose of 10 mg/kg body weight, 3 children developed
repetitive activity recorded over the thenar muscles following
supramaximal stimulation of the median nerve at the wrist. The
activity consisted of a small potential at the end of the main
muscle response and was characterized by being abolished by a
second stimulus 30 or 80 milliseconds after the first, or by
maximum voluntary contraction for 10 seconds; the amplitude of the
response to the second stimulus was not reduced. These
characteristics are necessary criteria that distinguish (these)
dose-related responses from pre-existing natural (and
idiosyncratic) responses, which can otherwise confuse EMG studies
in a population. Changes in amplitude measured on 52 control
subjects (mean 13.8 ħ 2.5 SD), on 2 occasions (2 weeks apart),
ranged from +5 to -3 mV. Thus, EMG does not appear to give a
highly sensitive measure of exposure to an ingested
organophosphorus compound.
7.1.2 Monitoring studies
Measurement of whole blood-AChE is the most widely adopted
method for monitoring the effects of occupational exposure to
organophosphorus insecticides. Physiological variations in blood-
ChE levels occur in a healthy person and are seen among a
population. It has been estimated that the coefficient of
variation for AChE activity in samples from an individual is 8 -
11%, and that a decrease of 23% below pre-exposure level may,
therefore, be considered significant. If the average of several
pre-exposure values were available, then a decrease of 17% would be
significant. It has been recommended that, if measured activity is
reduced by 30% or more of the pre-exposure value, AChE measurements
should be repeated at appropriate intervals to confirm the results.
Depressions of AChE or ChE in excess of 20 - 25% are considered
diagnostic of exposure but not, necessarily, indicative of hazard.
Depressions of 30 - 50% or more are considered indicators for
removal of an exposed individual from further contact with
pesticides until levels return to normal. Work procedures and
hygiene should also be checked (Zielhuis, 1972; WHO, 1975; CEC,
1977; Kaloyanova et al., 1979; Plestina, 1984).
Urinary metabolites have been monitored as a means of comparing
the efficiency of absorption of a pesticide by different routes.
The reports of the annual Joint FAO/WHO Working Parties,
mentioned previously, contain summaries of numerous controlled
exposure studies. No cases appear to be known of significant
clinical effects in man in the absence of depression of plasma- or
erythrocyte-ChE levels. No-observed-adverse-effect levels have
been calculated on this basis, where the data are available, or
have been estimated for man by extrapolation of the available data
for exposed animals.
7.1.3 Retrospective studies of populations exposed to
organophosphorus pesticides: acute and long-term exposure
Many thousands of cases of acute poisoning by organophosphorus
pesticides have been recorded (Namba et al., 1971). The majority
have been due to parathion and methyl parathion. Thus, Namba (1974)
in a discussion of the relative toxicities of parathion and
malathion, quoting Japanese Government statistics for the 7 years
1958-62, 1966, and 1967, stated that there were 3311 accidental or
occupational poisonings due to parathion including 188 deaths,
while for malathion, the numbers were 63 and 10, respectively. He
noted that the difference was not due to the restricted use of
malathion.
In the context of this general introduction, no descriptions
and breakdown will be given of retrospective studies of populations
exposed to organophosphorus pesticides. Such figures are relevant
to individual substances and will be given in the appropriate
Environmental Health Criteria.
It is generally thought that the only long-term effects
attributable to overt or subclinical acute intoxication with
organophosphorus compounds, or to prolonged low-level exposure,
are behavioural (rather doubtful), and delayed-onset neuropathy in
the case of certain compounds; these are dealt with in section 7.2.
>7.2 Other Effects on the Nervous and Neuromuscular System Due to
Acute or Long-Term Exposure
7.2.1 Delayed neuropathic effects
The characteristics of this disorder are given in section
6.1.2. The agents most commonly causing delayed neuropathy in man
are triaryl phosphate esters used in, e.g., hydraulic fluids; these
do not have any AChE activity and are not pesticides. In Table 14,
organophosphorus pesticides are listed for which there is
reasonable evidence that they have caused delayed neuropathy in
man.
Table 14. Organophosphorus pesticides reported to cause
delayed neuropathy in man
--------------------------------------------------------
Pesticide Number Reference
of cases
--------------------------------------------------------
mipafox 2 Bidstrup et al. (1953)
leptophos 8 Xintaras et al. (1978);
FAO/WHO (1979b)
methamidophos 9 Senanayake & Johnson
(1982)
trichlorphon many Shiraishi et al. (1977);
Hierons & Johnson (1978);
Johnson (1981a)
trichlornat 2 Jedrzejowska et al.
(1980); Willems (1981)
EPN 3a Xintaras & Burg (1980)
Chlorpyrifos 1 Lotti & Moretto (1986)
--------------------------------------------------------
a Moderate effects only and possibly other
etiological factors.
The cases with mipafox involved a single occupational exposure
to a compound that was developed before delayed neuropathy was a
recognized hazard. The cases with EPN and leptophos arose through
repeated occupational exposure with inadequate precautions.
Apparently cholinergic effects were often experienced, but not at
the level of severe poisoning. A few cases with methamidophos and
trichlorfon involved substantial occupational exposure, which
caused severe acute poisoning prior to the development of
neuropathy, but the majority of cases involved accidental or
deliberate ingestion of quantities that might well have been fatal
but for medical intervention. The fact that most of the cases
listed are due to exposure to phosphonates or phosphoramidates is
in line with the structure-activity relationships listed in section
6.1.2.
The value of measuring the neuropathy target esterase of human
lymphocytes as a predictive monitor was proposed by Dudek et al.
(1979) and Richardson & Dudek (1983). Lotti et al. (1983) reported
occupationally related changes in the lymphocyte NTE in spraymen
during seasonal spraying of DEF, but overt neuropathy was absent.
In a case of self-poisoning by a mixture of pesticides including
chlorpyrifos (about 20 g diluted in petroleum distillates),
Osterloh et al. (1983) noted that signs of cholinergic poisoning
were limited and they attributed death to the effects of
chlorophenoxyacetic acids. Brain esterases were not inhibited at
autopsy, but erythrocyte and peripheral nerve AChE levels were
about 22% of normal and nerve NTE was about 30%. This substantial
inhibition of NTE suggested that treated survivors of severe
poisoning by chlorpyrifos might well develop delayed neuropathy.
This prediction was confirmed in a recent case of self-intoxication
with chlorpyrifos (estimated dose 300 mg/kg body weight) in which
very low levels of lymphocyte-NTE were found, 30 days after
intoxication and after recovery from very severe cholinergic
effects. Typical moderate polyneuropathy developed in the
following days (Lotti & Moretto, 1985).
Allegations have been made against a few other organophosphorus
insecticides including malathion, omethoate, and parathion, though
many accidental and intentional poisonings by these agents have not
had any neuropathic sequelae. Experimental evidence against a
neuropathic potential in these compounds is strong (section 6.1.2).
However, in view of the serious paralytic effects involved, the
evidence adducing that these pesticides were the causal agents is
reviewed below.
(a) Malathion
Two alleged cases can be discounted. Petry (1958) reported a
case in which a physician contaminated himself frequently with
chlorinated hydrocarbon pesticides during day-long gardening
activities, about once per week, over a 10-year period. In 1954,
he commenced using 6% malathion in a "hose-on" device for garden
pest control and, in 1955, he commenced using 50% malathion in a
hand spray, both indoors and outside. He soon developed signs of
chronic anticholinesterase poisoning (generalized weakness and
tremor, irritability, difficulty in focusing). He eventually
collapsed and his general condition improved in hospital. However,
he continued to experience generalized weakness and particular
weakness in the right shoulder girdle, right serratus anterior, and
both peroneal muscle groups, and these symptoms persisted to some
extent for over a year. The distribution of these symptoms of
deficient muscle performance is quite atypical for delayed
neuropathy and seems more likely to be due to prolonged moderate
cholinergic insult from malathion precipitating weakness, anorexia,
and weight loss, which then precipitated further ill-health as
previously-stored chlorinated hydrocarbons was mobilized from
degraded fat stores. DDT at 23 mg/kg plus a high level of organic
chloride were found in a subcutaneous fat biopsy. A muscle-
necrotising effect, due to prolonged cholinergic stimulation, as
described in section 6.2.6, is also possible.
A separate case report of ascending paralysis following
malathion intoxication (Healy, 1959) concerned an 18-month-old
child exposed daily for 6 weeks to malathion from a garden spray.
Contamination was dermal and also by inhalation and ingestion,
leading ultimately to a cholinergic crisis and a prolonged period
of weakness including extensive flaccid paralysis for several days.
The condition responded to atropine and rest within 4 weeks. In
spite of the author's conclusion that this was a "demyelinating"
(i.e., delayed neuropathic) disorder, the picture is typical of
prolonged cholinergic insult responding to atropine and the
comparatively slow clearance of accumulated pesticide with
recovery from excessive nerve-muscle stimulation.
(b) Omethoate
A typical delayed neuropathy followed ingestion of an
organophosphorus pesticide with suicidal intent (Curtes et al.,
1979). Identification of the actual pesticide ingested was
doubtful depending on a later hearsay report concerning a bottle in
a garden shed, the contents of which were not analysed. Lotti et
al. (1981) assayed both the AChE and the NTE activities in
autopsied brain after a fatal intoxication with omethoate and found
that, even at the fatal dose, the NTE levels were normal while the
AChE activity was highly inhibited. Considering this data together
with evidence of similar non-inhibition in experimental hens at up
to 8 x the unprotected LD50, the authors concluded that, though the
neuropathy was typical, it was likely that the toxic agent was some
other organophosphorus compound more liable to cause neuropathy,
such as trichlorfon or trichlornat (Table 13).
(c) Parathion
Only two cases of permanent incapacity have been attributed to
parathion, though thousands of parathion poisonings are known (see
earlier). Petry (1951) attributed a case of neuropathy to the
aftermath of several occupational incidents of cholinergic
poisoning, but the history is entirely atypical in that symptoms
developed only 4 months, rather than 2 - 4 weeks, after the last
exposure. Causation by parathion is therefore very unlikely. A
farmer deliberately ingested parathion at a dose estimated to be at
least 150 g (perhaps 500 x the estimated human lethal dose) in 600
ml of methanol. Vigorous therapy preserved his life, though he
remained in a deep coma for 7 weeks. On recovery from this
experience, he was found to be suffering from flaccid paralysis of
both legs and weakness of both hands with muscle atrophy (De Jager
et al., 1981; 1982); partial recovery occurred during one year.
Lotti & Becker (1982b) have discussed the complicating factors of
the potentially supra-lethal dose of methanol and of the long coma.
However, the clinical picture is not unlike a true moderate
organophosphorus-induced neuropathy. At such a colossal dose, the
possibility of ingesting a significant amount of a neuropathic
impurity in the parathion must be recognized. This could be ethyl
bis-(4-nitrophenyl) phosphorothioate; small but significant
amounts of the appropriate oxon are present in some samples of
paraoxon made from diethyl phosphorochloridate (Johnson, 1982b),
and this oxon is a potent inhibitor of NTE.
(d) Other organophosphorus pesticides
Besides the atypical case attributed to malathion, Petry (1958)
described another case in which symptoms persisted after a
cholinergic crisis that followed severe intermittent exposures over
3 seasons to a variety of insecticides including parathion, EPN,
DDT, dieldrin, and lead arsenate. Some of the persistent symptoms
might be compatible with a mild peripheral neuropathy. Among the
agents used, lead arsenate and dieldrin would be expected to
contribute damage to the nervous system and EPN at about the LD50
level causes neuropathy in hens and man (Tables 4, 13).
A case of slow-onset profound weakness with complete recovery
within 3 months following contamination of an agricultural worker
with the cotton defoliant, merphos ( S,S,S -tri- n -butyl
phosphorothioite), was thought by the author to be of the delayed
neuropathy type (Fisher, 1977). However, the clinical picture
showed signs that are not seen in acute organophosphate
intoxication (influenza-like onset of the syndrome and high level
of protein in the spinal fluid). These signs are, however,
characteristic of a Guillan-Barré syndrome, which might have been
coincidental to the merphos exposure only 4 days previously. It is
possible that, later, a mild organophosphorus neuropathic effect
was superimposed on the Guillan-Barré effect, since merphos can
produce neuropathy in experimental animals (Johnson, 1970, 1975b).
Recovery from very mild neuropathies is usually complete.
7.2.2 Behavioural effects
Although many epidemiological studies have been carried out,
few controlled studies on man have been reported. It is generally
recognized that there are behavioural and psychic changes during
overt clinical poisoning by organophosphorus insecticides and that
these may take several months to regress (Karczmar, 1984).
However, there is no information to suggest that effects occur at
exposure levels that do not either alter ChE levels or produce
physical symptoms. Levin & Rodnitsky (1976) have reviewed the
literature, including their own work, on different aspects of
behaviour as affected by organophosphates. Much was based on
generalized complaints from workers occupationally exposed to many
agricultural chemicals (and probably also to automobile fuels and
lubricants and to alcohol). In summary, they found that, in human
subjects sufficiently exposed to organophosphates to depress
plasma- or erythrocyte-ChEs, some or all of the following
behavioural variables might be impaired. In cognition: vigilance,
information processing and psychomotor speed, and memory; in
speech: both performance and perception; in psychic state:
increased tendencies to depression, anxiety, and irritability; and
in EEG records: a tendency to faster frequencies and higher
voltages. They also concluded that the EEG abnormalities were
positively related to the level of AChE inhibition during the
initial stages of inhibition. Concerning studies on asymptomatic
workers at risk from repeated exposure to organophosphorus
pesticides, they considered that the evidence was equivocal for the
presence of less severe or latent forms of any behavioural
abnormalities. However, Duffy & Burchfield (1980) claimed that
changes in the EEG of individuals accidentally exposed to the
nerve-agent sarin could be detected twelve months after the
exposure. This study was a sequel to the study on monkeys
described in section 6.1.2, and the analyses of EEGs was performed
as noted there. They claimed significant differences (very small
differences analysed by complex statistical procedures) between the
group of sarin-exposed workers and controls, particularly in the
region of beta-rhythm (but the comment on the spread among normal
monkeys should be noted). However, the authors were unable to pick
out sarin-exposed individuals on the basis of the EEG. They also
found (small) increased amounts of REM-sleep in the exposed
workers. Without controlled exposure and serial monitoring of
effects in individuals, little can be deduced from these apparent
marginal changes.
7.3 Effects on Other Organs and Systems
Very few effects, other than those described in sections 7.1
and 7.2, have been noted, except those arising from ill health due
to severe anticholinesterase effects.
Several adverse effects attributed to only one organophosphorus
ester will be listed under the individual substances.
7.4 Treatment of Organophosphate Insecticide Poisoning in Man
All cases of organophosphorus poisoning should be dealt with as
an emergency and the patient sent to hospital as quickly as
possible. Although symptoms may develop rapidly, delay in onset or
a steady increase in severity may be seen up to 48 h after
ingestion of some formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major references
(Kagan 1977; Taylor 1980; HMSO, 1983; Plestina 1984) and will also
be included in the IPCS Health and Safety Guides to be prepared for
selected organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
7.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in cleaning the skin area where venupuncture
is performed. Blood might be contaminated with direct-acting
organophosphorus esters, and, therefore, inaccurate measures of ChE
inhibition might result. Extensive eye irrigation with water or
saline should also be performed. In the case of ingestion, vomiting
might be induced, if the patient is conscious, by the
administration of ipecacuanha syrup (10 - 30 ml) followed by 200 ml
water. This treatment is, however, contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also be
performed, particularly in unconscious patients, taking care to
prevent aspiration of fluids into the lungs (i.e., only after a
tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
7.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
7.4.3 Specific pharmacological treatment
7.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given at
15 to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be necessary
in extreme cases and total daily doses up to several hundred mg may
be necessary during the first few days of treatment.
7.4.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs (section 6.5.3). However,
if absorption, distribution, and metabolism are thought to be
delayed for any reasons, oximes can be administered for several
days after intoxication. Effective treatment with oximes reduces
the required dose of atropine. Pralidoxime is the most widely
available oxime. A dose of 1 g pralidoxime can be given either im
or iv and repeated 2 - 3 times per day or, in extreme cases, more
often. If possible, blood samples should be taken for AChE
determinations before and during treatment. Skin should be
carefully cleansed before sampling. Results of the assays should
influence the decision whether to continue oxime therapy after the
first 2 days.
The possible beneficial effects of oxime therapy on CNS-derived
symptoms is discussed in section 6.5.3.
7.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to counteract
some aspects of CNS-derived symptoms, which are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required (Vale & Scott, 1974). Other centrally acting
drugs and drugs that may depress respiration are not recommended in
the absence of artificial respiration procedures.
7.4.3.4 Notes on the recommended treatment
(a) Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
(b) Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinisation. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
(c) Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be
taken into, and then released from, fat depots over a period of
many days. It is therefore quite incorrect to abandon oxime
treatment after 1 - 2 days on the supposition that all inhibited
enzyme will be aged. Ecobichon et al. (1977) noted prompt
improvement in both condition and blood-ChEs in response to
pralidoxime given on the 11th - 15th days after major symptoms of
poisoning appeared due to extended exposure to fenitrothion (a
dimethyl phosphate with a short half-life for aging of inhibited
AChE).
(d) Dosage of atropine and oxime
The recommended doses above pertain to exposures, usual for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in
error, while drunk. Therapeutic dosages were progressively
increased up to 6 mg atropine iv every 15 min together with
continuous iv infusion of pralidoxime chloride at 0.5 g/h for 72 h,
from days 3 to 6 after intoxication. After considerable
improvement, the patient relapsed and further aggressive therapy
was given at a declining rate from days 10 to 16 (atropine) and to
day 23 (oxime), respectively. In total, 92 g of pralidoxime
chloride and 3912 mg of atropine were given and the patient was
discharged on the thirty-third day with no apparent sequelae.
REFERENCES
ABOU-DONIA, M.B., OTHMAN, M.A., TANTAWAY, G., ZAKI KHALIL, A.,
& SHAWER, M.F. (1974) Neurotoxic effect of leptophos.
Experientia (Basel), 30 : 63-64.
ABOU-DONIA, M.B., GRAHAM, D.G., & KOMEIL, A.A. (1979)
Delayed neurotoxicity of O -ethyl O -(2,4-dichlorophenyl)phenyl-
phosphonothioate: effects of a single oral dose on hens.
Toxicol. appl. Pharmacol., 49 : 293-303.
ABOU-DONIA, M.B., GRAHAM, D.G., TIMMONS, P.R., & REICHERT,
B.L. (1980) Late acute, delayed neurotoxic, and cholinergic
effects of S,S,S -tributyl phosphorotrithioate on the hen:
effect of route of administration. Neurotoxicology, 1 : 425-447.
ABOU-DONIA, M.B., MAKKAWY, H-A., & GRAHAM, D.G. (1982)
Coumaphos: delayed neurotoxic effect following dermal
administration in hens. J. Toxicol. environ. Health, 10 : 87-99.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and
structure activity relationships providing a high safety
factor for anti-cholinesterase insecticides. In: Proceedings
of the British Crop Protection Conference, Brighton, November
1977, Croydon, British Crop Protection Council, pp. 721-729.
ALDRDIGE, W.N. & NEMERY, B. (1984) Toxicology of
trialkylphosphorothioates with particular reference to lung
toxicity. Fund. appl. Toxicol., 4 : 5215-5223.
ALDRIDGE, W.N., MILES, J.W., MOUNT, D.L., & VERSCHOYLE, R.D.
(1979) The toxicological properties of impurities in
malathion. Arch. Toxicol., 42 : 95-106.
ALDRIDGE, W.N., DINSDALE, D., NEMERY, B., & VERSCHOYLE, R.D.
(1985) Some aspects of the toxicology of trimethyl and
triethyl phosphorothioates. Fund. appl. Toxicol., 5 : 47-60.
AMBRUS, A. & GREENHALGH, R., ed. (1984) Pesticide residue
analysis. Proceedings of a joint FAO/WHO course, Eger,
Hungary, 13-26 April, 1983, Copenhagen, World Health
Organization (Health Aspects of Chemical Safety, Interim
Document No. 14).
BAKER, E.L., Jr, ZACK, M., MILES, J.W., ALDERMAN, L.,
MCWILSON, W., DOBBIN, R.D., MILLER, S., & TEETERS, W.R.
(1978) Epidemiologic malathion poisoning in Pakistan malaria
workers. Lancet, 7 January : 31.
BARNES, J.M. (1975) Assessing hazard from prolonged and
repeated exposure to low doses of toxic substances. Br. med.
Bull., 31 : 196-200.
BARNES, J.M. & DENZ, F.A. (1951) The chronic toxicity of
p- nitrophenyl diethyl thiophosphate (E. 605): a long-term
feeding experiment with rats. J. Hyg., 49 : 430-441.
BARNES, J.M. & DENZ, F.A. (1954) The reaction of rats to
diets containing octamethyl pyrophosphoramide (schradan) and
O,O- diethyl S- ethylmercaptoethanol thiophosphate ("systox").
Br. J. ind. Med., 11 : 11-19.
BAYER (1971) Nemacur, Leverkusen, Bayer Chemical Company
(Technical Information Leaflet No. E.1-781/26734).
BECKER, E.L. & BARBARO, J.F. (1964) The enzymatic hydrolysis
of p- nitrophenyl ethylphosphonates by mammalian plasmas.
Biochem. Pharmacol., 13 : 1219-1227.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating
properties of organophosphates. Xenobiotica, 2 : 307-337.
BENKE, G.M. & MURPHY, S.D. (1975) The influence of age on
the toxicity and metabolism of methyl parathion and parathion
in male and female rats. Toxicol. appl. Pharmacol., 31 :
254-269.
BERTOCIN, D., RUSSOLO, A., CAROLDI, S., & LOTTI, M. (1985)
Neurotoxic esterase in human lympocytes. Arch. environ.
Health, 40 : 139-144..
BERTRAM, U., KASTEN, A., LULLMANN, H., & ZIEGLER, A. (1977)
Improved treatment of organophosphate intoxication by use of
scopolamine or dexetimide. Experientia (Basel), 33 : 1196-1197.
BICKFORD, A.A. & SPRAGUE, G.L. (1984) The significance of
background neurologic lesions in acute delayed neurotoxicity
studies: a comparison of neurohistopathologic lesions induced
in commercial hens by tri- O -tolyl phosphate (TOCP) with those
observed in negative control hens. In: Cranmer, J.M. & Hixson,
J.E., ed. Delayed neurotoxicity. Proceedings of the Delayed
Neurotoxicity Workshop, Urbana-Champaign, Illinois, 27-30
June, 1982, Little Rock, Intox Press.
BIDSTRUP, P.L., BONNEL, J.A., & BECKETT, A.G. (1953)
Paralysis following poisoning by a new organic phosphorus
insecticide (mipafox). Br. med. J., 1 : 1068-1072.
BIGNAMI, G. (1976) Behavioural pharmacology and toxicology.
Ann. Rev. Pharm. Toxicol., 16 : 329-366.
BIGNAMI, G., ROSIC, H., MICHALEK, H., MILOSEVIC, M., & GATTI,
G.L. (1975) Behavioural toxicity of anticholinesterase
agents. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Publishing Company, pp. 155-215.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The
distribution of dichlorvos in the tissues of mammals after its
inhalation or intravenous administration. Toxicol. appl.
Pharmacol., 31 : 243-253.
BLEAKLEY, P., NICHOL, A.W., & COLLINS, A.G. (1979) Diazinon
and porphyria cutanea tarda. Med. J. Aust., 1 : 314-315.
BOPP, C. & KOSMINSKY, B. (1975) [Hepato-dermal toxic
prophyria.] Med. Cut. I.L.N., 4 : 271-280 (in Portugese).
BOULDIN, T.W. & CAVANAGH, J.B. (1979) Organophosphorus
neuropathy. A teased-fiber study of the spatio-temporal spread
of axonal degeneration. Am. J. Pathol., 94 (2): 241-252.
BOYD, E.M. (1969) Dietary protein and pesticide toxicity in
male weaning rats. WHO Bull., 40 : 801-805.
BRADLEY, W.A. (1976) The pathology of delayed neurotoxicity
due to organophosphates. In: Baron, R.L., ed. Pesticide-
induced delayed neurotoxicity, Washington, US Environmental
Protection Agency, pp. 84-101 (EPA No. 600/1-76-025).
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison
of cholinesterase activity, residue levels, and urinary
metabolite excretion of rats exposed to organophosphorus
pesticides. J. agric. food Chem., 25 : 1353-1358.
BREALEY, C., WALKER, C.H., & BALDWIN, B.C. (1980) A-esterase
activities in relation to the differential toxicity of
pirimiphos-methyl to birds and mammals. Pestic. Sci., 11 :
546-554.
BRIMBLECOMBE, R.W., GREEN, D.M., STRATTON, J.A., & THOMPSON,
P.B.J. (1970) The protective actions of some anticholinergic
drugs in sarin poisoning. Br. J. Pharmacol., 39 : 822-830.
BRITISH AGROCHEMICAL ASSOCIATION (1983) Spray operator
safety study, London, British Agrochemical Association, pp.
1-9.
BUCHET, J.P., LAUWERYS, R., & ROELS, H. (1977) Long-term
exposure to organophosphorus pesticides and lipid metabolism
in the rat. Bull. environ. Contam. Toxicol., 17 : 175-183.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused
by a single massive dose of dichlorvos to adult hens. Toxicol.
Lett., 9 : 157-159.
CASIDA, J.E. & SANDERSON, D.M. (1963) Reaction of certain
phosphorothionate insecticides with alcohols and potentiation
by breakdown products. J. agric. food Chem., 11 : 91-96.
CAVANAGH, J.B. (1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system: an experimental study in
hens. J. Neurol. Neurosurg. Psychiatr., 17 : 163-172.
CEC (1977) Organophosphorus pesticides. Criteria
(dose/effect relationships) for organophosphorus pesticides,
Oxford, New York, Pergamon Press, 199 pp (Report of a Working
Group of Experts prepared for the Commission of the European
Communities, Directorate-General for Social Affairs, Health
and Safety Directorate).
CHEN, P.R.S. & DAUTERMAN, W.C. (1971) Studies on the
toxicity of dimethoate analogues and their hydrolysis by sheep
liver amidase. Pestic. Biochem. Physiol., 1 : 340-348.
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN,
R.J. (1980) Effects of low-level administration of
dichlorvos on adrenocorticotrophic hormone secretion, adrenal
cholesteryl ester, and steroid metabolism. Biochem.
Pharmacol., 29 : 635-641.
CLOTHIER, B., JOHNSON, M.K., & REINER, E. (1981) Interaction
of some trialkyl phosphorothiolates with acetylcholinesterase:
characterization of inhibition, aging, and reactivation.
Biochem. Biophys. Acta, 660 : 306-316.
CODEX ALIMENTARIUS COMMISSION (1984) Codex Alimentarius
Commission guide to Codex recommendations concerning pesticide
residues. VIII. Recommendations for methods of analysis of
pesticide residues, Rome, FAO/WHO (CAC/PR 8-1984).
COLEMAN, I.W., LITTLE, P.E., & BANNARD, R.A.B. (1962)
Cholinolytics in the treatment of anticholinesterase
poisoning. I. The effectiveness of certain cholinolytics in
combination with an oxime for treatment of sarin poisoning.
Can. J. Biochem. Physiol., 40 : 815-826.
COSTA, L.G., SCHWAB, B.W., & MURPHY, S.D. (1982) Tolerance
to anticholinesterase compounds in mammals. Toxicology, 25 :
79-97.
CRANMER, J.M. & HIXSON, J.E., ed. (1984) Delayed
neurotoxicity. Proceedings of the Delayed Neurotoxicity
Workshop, Urbana-Champaign, Illinois, 27-30 June, 1982, Little
Rock, Intox Press.
CURTES, J.P., DEVELAY, P., & HUBERT, J.P. (1979) Late
peripheral neuropathy due to an acute voluntary intoxication
by organo-phosporic compounds. In: International Congress on
Neurotoxicology, Varese, Italy, 27-30 September, 1979, Oxford,
Pergamon Press.
DANDLIKER, W.B., HICKS, A.N., LEVINSON, S.A., STEWART, K., &
BRAWN, R.J. (1979) Effects of pesticides on the immune
response, La Jolla, California, Scripps Clinic and Research
Foundation (EPA 600/1-79-039).
DAUGHTON, C.G., COOK, A.M., & ALEXANDER, M. (1979) Bacterial
and conversion of alkylphosphonates to natural products via
carbon-phosphorus bond cleavage. J. agric. food Chem., 27 :
1375-1382.
DAUTERMAN, W.C. (1971) Biological and nonbiological
modification of organophosphorus compounds. WHO Bull., 44 :
133-150.
DAVIES, J.E., BARQUET, A., FREED, V.H., HAGUE, R., MORGADE,
C., SONNEBORN, R.E., & VACLAVEK, C. (1975) Human pesticide
poisonings by a fat-soluble organophosphate insecticide. Arch.
environ. Health, 30 : 608-613.
DAVIS, C.S. & RICHARDSON, R.J. (1980) In: Spencer, P.S. &
Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 527-544.
DE JAGER, A.E.J., VAN WEERDON, T.W., HOUTOFF, H.J., & DE
MONCHY, J.G.R. (1981) Polyneuropathy after massive exposure
to parathion. Neurology, 31 : 603-605.
DE JAGER, A.E.J., VAN WEERDON, T.W., & MONCHY, J.G.R. (1982)
Reply to letter. Neurology, 32 : 218.
DESI, I. (1983) Neurotoxicological investigation of
pesticides in animal experiments. Neurobehav. Toxicol.
Teratol., 5 : 503-515.
DESI, I., FARKAS, I., SIMON, G., & CZIELESZKY, V. (1971)
[Investigation of the neurotoxic action of the phosphorus
compound bromophos.] Int. Arch. Arbeitsmed., 28 : 203-222 (in
German).
DESI, I., VARGA, L., & FARKAS, I. (1978) Studies on the
immunosuppressive effect of organochlorine and organo-
phosphoric pesticides in subacute experiments. J. Hyg.
Epidemiol. Microbiol. Immunol., 22 : 115-122.
DETTBARN, W.D. (1984) Pesticide-induced muscle necrosis:
mechanisms and prevention. Fund. appl. Toxicol., 4 : 18-26.
DHSS (1983) Pesticide poisoning. Notes for the guidance of
medical practitioners, London, Department of Health and Social
Security, pp. 41-47.
DIGGORY, H.J.P., LANDRIGEN, P.J., LATIMER, K.P., ELLINGTON,
A.C., KIMBROUGH, R.D., LIDDLE, J.D., CLINE, R.E., & SMERK,
A.L. (1977) Fatal parathion poisoning caused by contamination
of flour in international commerce. Am. J. Epidemiol.,
106 : 145-153.
DIMOV, G. & KALOYANOVA, F. (1967) Carbohydrate metabolism
disorders in the liver and muscles in acute parathion
poisonings. C R Acad. Bulg. Sci., 20 : 1007-1009.
DINSDALE, D., VERSCHOYLE, R.D., & CABRAL, J.R.P. (1982)
Cellular responses to trialkylphosphorothioate-induced injury
in rat lung. Arch. Toxicol., 51 : 79-89.
DONNINGER, C. (1971) Species specificity of phosphate
triester anticholinesterases. WHO Bull., 44 : 265-268.
DOROUGH, H.W. (1976) Biological activity of pesticide
conjugate. In: Kaufman, D.D., ed. Bound and conjugated
pesticide residues, Washington DC, American Chemical Society,
pp. 11-34 (ACS Symposium Series No. 29).
DREVENKAR, V., FINK, K., STIPCEVIC, M., & TOKALCEVIC, B.
(1976) The fate of pesticides in aquatic environment. II.
Hydrolysis of dichlorvos in model system and in river water.
Arh. hig. rada, 27 : 297-305.
DREVENKAR, V., STIPCEVIC, M., STENGL, B., & STEFANAC, Z.
(1979) Gas chromatographic determination of alkali metal
O,O- diethylphosphorodithioate present in tract amounts.
Microchim. Acta, 1 : 385-394.
DUBOIS, K.P. (1963) Toxicological evaluation of the anti-
cholinesterase agents. In: Koelle, G.B., ed. Handbook on
experimental pharmacology, Berlin, Springer-Verlag, Vol. 15,
pp. 833-857.
DUDEK, B.R., BARTH, M., GEPHART, J., HUGGINS, J., & RICHARD-
SON, R.J. (1979) Correlation of brain and lymphocyte neuro-
toxic esterase inhibition in the adult hen following dosing
with neurotoxic compounds. Toxicol. appl. Pharmacol., 48 : A198.
DUFFY, F.H. & BURCHFIELD, J.L. (1980) Long-term effects of
the organophosphate sarin on EEGs in monkeys and humans.
Neurotoxicology, 1 : 667-689.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the
exposure of workers to pesticides. WHO Bull., 26 : 75-91.
DURHAM, W.F., WOLFE, H.R., & ELLIOTT, J.W. (1972) Absorption
and excretion of parathion by spraymen. Arch. environ. Health,
24 : 381-387.
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S.
(1977) Acute fenitrothion poisoning. Can. Med. Assoc. J.,
116 : 377-379.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7 : 88-95.
EL-SEBAE, A.H. (1980) Biochemical challenges in future
toxicological research. J. environ. Sci. Health, B15 : 689-721.
EL-SEBAE, A.H., SOLIMAN, S.A., AHMED, N.S., & CURLEY, A.
(1981) Biochemical interaction of six op delayed
neurotoxicants with several neurotargets. J. environ. Sci.
Health, B16 (4): 465-474.
ENAN, E.E., EL-SEBAE, A.H., ENAN, O.H., & EL-FIKI, S. (1982)
In vivo interaction of some organophosphorus insecticides with
different biochemical targets in white rats. J. environ. Sci.
Health, B17 : 549-570.
ETO, M. (1974) Organophosphorus pesticides: organic and
biological chemistry, Boca Raton, Florida, CRC Press.
ETO, M. & OHKAWA, H. (1970) Alkylation reaction of
organophosphorus pesticides: its chemical and biochemical
significance. In: Biochemical toxicology of insecticides, New
York, London, Academic Press, pp. 93-104.
FAO (1984) Production yearbook, Rome, Food and Agriculture
Organization of the United Nations, Vol. 38.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23 (1964)).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report, No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report, No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues (FAO Agricultural Studies No.
73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party and the WHO Expert
Committee, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1971/M/9/1;
WHO Pesticide Residues Series No. 1).
FAO/WHO (1973a) Pesticide residues in food. Report of the
1972 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 90; WHO Technical Report Series No. 525).
FAO/WHO (1973b) 1972 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1972/M/9/1;
WHO Pesticide Residues Series No. 2).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection
Paper 10 Rev).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide Residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide Residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide Residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide Residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986) Pesticide Residues in food. Report of the
1985 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 68).
FAO/WHO (in press) 1985 Evaluations of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper).
FARM CHEMICALS HANDBOOK (1985) Willoughby, Ohio, Meister
Publishing Company.
FENICHEL, G.M., KIBLER, W.B., OLSON, B.A., & DETTBARN, W.D.
(1972) Chronic inhibition of cholinesterase as a cause of
myopathy. Neurology, 22 : 1026-1033.
FISHER, J.R. (1977) Guillain-Barré syndrome following
organophosphate poisoning. J. Am. Med. Assoc., 238 : 1950-1951.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14 : 514-534.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health
hazards on use of dichlorvos (DDVP), especially in resin
strips. Res. Rev., 44 : 115-157.
GRAY, A.P. (1984) Design and structure-activity relationships
of antidotes to organophosphorus anticholinesterase agents.
Drug. Metab. Rev., 15 (3): 557-589.
GREEN, D.M., MUIR, A.W., STRATTON, J.A., & INCH, T.D. (1977)
Dual mechanism of the antidotal action of atropine-like drugs
in poisoning by organophosphorus anticholinesterase. J. Pharm.
Pharmacol., 29 . 62-64.
GREENHALGH, R., DHAWAN, K.L., & WEINBERGER, P. (1980)
Hydrolysis of fenitrothion in model and natural aquatic
systems. J. agric. food Chem., 28 : 102-105.
GROB, D. (1963) Anticholinesterase intoxication in man and
its treatment. In: Koelle, G.B., ed. Handbook of experimental
pharmacology, Berlin, Springer-Verlag, Vol. 15, pp. 989-1027.
GUNTHER, F.A., WESTLAKE, W.E., BARKELY, J.H., WINTERLIN, W., &
LANGBEHN, L. (1973) Establishing dislodgeable pesticide
residues on leaf surfaces. Bull. environ. Contam. Toxicol., 9 :
243-250.
GUNTHER, F.A., WESTLAKE, W.E., & BARKELY, J.H. (1974) Worker
environment research. II. Sampling and processing techniques
for determining dislodgeable pesticide residues on leaf
surfaces. Bull. environ. Contam. Toxicol., 12 : 641-645.
HANSEN, L.G. & HANSEN, T.K. (1985) Intravenous dosing
abolishes differences in delayed neurotoxicity potency between
leptophos and desbromoleptophos. Pestic. Biochem. Physiol.,
24 : 136.
HAYES, G.R., FUNCKES, A.J., & HARTWELL, W.V. (1964) Dermal
exposure of human volunteers to parathion. Arch. environ.
Health, 8 : 829-833.
HAYES, W.J., Jr (1971) Studies on exposure during the use of
anticholinesterase pesticides. WHO Bull., 44 : 277-288.
HAYES, W.J., Jr (1975) Diagnosis and treatment of poisoning.
In: Toxicology of pesticides, Baltimore, Maryland, Williams
and Wilkins Company, pp. 409-417.
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, 561 pp.
HEALY, J.K. (1959) Ascending paralysis following malathion
intoxication: a case report. Med. J. Aust., 46 : 765-767.
HEATH, D.F. & VANDEKAR, M. (1957) Some spontaneous reactions
of O,O- dimethyl S- ethylthioethyl phosphorothiolate and related
compounds in water and on storage, and their effects on the
toxicological properties of the compounds. Biochem. J., 67 :
187-202.
HIERONS, R. & JOHNSON, M.K. (1978) Clinical and
toxicological investigations of a case of delayed neuropathy
in man after acute poisoning by an organophosphorus pesticide.
Arch. Toxicol., 40 : 279-284.
HOLLINGSHAUS, J.G., ABU-EL-HAJ, S., & FUKUTO, T.R. (1979)
Delayed neurotoxicity of O -alkyl O -aryl phenylphosphonothioate
analogues related to leptophos administered orally to the hen.
J. agric. food Chem., 27 : 1197-1201.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus
cholinesterase inhibitors. Pharmacol. Rev., 11 : 567-688.
HUANG, X.S., SHU, W.A., ZU, W.C., LAI, M.T., TSIN, Z.A., &
KWOK, C.S. (1979) [Toxicity studies of the herbicide
Amiprophos.] Chekiang Univ. Sch. Med. Bull., 8 : 63-66 (in
Chinese).
HUFF, J.E., BATES, R., EUSTIS, S.L., HASEMAN, J.K., &
MCCONNELL, E.E. (1985) Malathion and malaoxon: histo-
pathology reexamination of the National Cancer Institute's
Carcinogenesis Studies. Environ. Res., 37 : 154-173.
HUTSON, D.H. & HATHWAY, D.E. (1967) Toxic effects of
chlorofenvinphos in dogs and rats. Biochem. Pharmacol., 16 :
949-962.
IARC (1979) Dichlorvos. In: Some halogenated hydrocarbons,
Lyons, International Agency for Research on Cancer, pp. 97-127
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 20).
IARC (1983) Miscellaneous chemicals, Lyons, International
Agency for Research on Cancer (Monographs on the Carcinogenic
Risk of Chemicals to Humans, Vol. 30).
IMAI, H. (1977) Experimental pigmentary degeneration of the
retina caused by an organophosphorus pesticide (fenthion).
Acta soc. ophthalmol., 81 : 925-932 (Abstract 245, Exerp. Med.
(1979), 9 : 53).
IMAMURA, T., GANDY, J., FUKUTO, T.R., & TALBOT, P. (1983) An
impurity of malathion alters the morphology of rat lung
bronchiolar epithelium. Toxicology, 26 : 73-79.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISHIKAWA, S. & MIYATA, M. (1980) Development of myopia
following chronic organophosphate pesticide intoxication: an
epidemiological and experimental study. In: Merigan, W.H. &
Weiss, B., ed. Neurotoxicity of the visual system, New York,
Raven Press, pp. 233-254.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorus pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application. Proceedings of the
Fifth International Workshop of the Scientific Committee on
Pesticides of the International Association on Occupational
Health, The Hague, The Netherlands, 9-11 October, 1979,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 169-172.
JAGER, K.W., ROBERTS, D.V., & WILSON, A. (1970) Neuro-
muscular function in pesticide workers. Br. J. ind. Med., 27 :
273-278.
JASH, N.B. & BHATTACHARYA, S. (1982) Neurotoxicity of
phenthoate to a non-target species. In: Proceedings of the 5th
International Congress on Pesticide Chemicals, Kyoto, 29
August, 1982 (Abstract No. V1a-15).
JEDRZEJOWSKA, H., ROWINSKA-MARCINSKA, K., & HOPPE, B. (1980)
Neuropathy due to phytosol (agritox): report of a case. Acta
neuropathol., 49 : 163-168.
JOHNSON, D.D. & WILCOX, W.C. (1975) Studies on the mechanism
of the protective and antidotal actions of diazepam in
organophosphate poisoning. Eur. J. Pharmacol., 34 : 127-132.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of the brain "neurotoxic esterase" and the development of
delayed neurotoxicity in hens. Biochem. J., 120 : 523-531.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. Crit. Rev.
Toxicol., 3 : 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing
delayed neurotoxic effects: mechanism of action and structure/
activity studies. Arch. Toxicol., 34 : 259-288.
JOHNSON, M.K. (1977) Improved assay of neurotoxic esterase
for screening organophosphates for delayed neurotoxicity
potential. Arch. Toxicol., 37 : 113-115.
JOHNSON, M.K. (1980) Delayed neurotoxicity induced by
organophosphorus compounds: areas of understanding and
ignorance. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard
evaluation, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 27-38.
JOHNSON, M.K. (1981a) Do trichlorphon and/or dichlorvos
cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol. Scand., 49 (Suppl. 5): 87-98.
JOHNSON, M.K. (1981b) Grading of non-neurotoxicity of
organophosphorus pesticides and plasticisers by in vivo/in
vitro assay of neurotoxic esterase. In: Proceedings of the
European Society of Toxicologists, Dublin, 17-19 August
(Abstract No. 66).
JOHNSON, M.K. (1982a) The target for initiation of delayed
neurotoxicity by organophosphorus esters: biochemical studies
and toxicological applications. In: Hodgson, E., Bend, J.R., &
Phillip, R.M., ed. Reviews in biochemistry and toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 4, pp. 141-212.
JOHNSON, M.K. (1982b) Check your paraoxon and parathion for
neurotoxic impurities. Vet. hum. Toxicol., 24 (3): 220a.
JOHNSON, M.K. (1984) Delayed neurotoxicity tests of
organophosphorus esters: a proposed protocol integrating
neuropathy target esterase (NTE) assays with behaviour and
histopathology tests to obtain more information more quickly
from fewer animals. In: El-Sebae, A.H., ed. Proceedings of the
International Conference on Environmental Hazards of
Agrochemicals in Developing Countries, Alexandria, Egypt, 8-12
November, 1983, Alexandria, University of Alexandria, Vol. 1,
pp. 474-493.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus
pesticides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269
(in Russian).
KAGAN, JU.S. (1985) [Principles of toxicology,] Moscow,
United Nations Environment Programme (in Russian).
KAHN, E. (1979) Outline guide for performance of field
studies to establish safe reentry intervals for
organophosphorus pesticides. Residues Rev., 70 : 27-43.
KALOYANOVA-SIMEONOVA, F. (1961) Effet du chlorthion sur les
réflexes moteurs conditionnés des rats albinos. Act. nerv.
super., 3 : 284-288.
KALOYANOVA, F. (1979) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Conference on
Environmental Sensing and Assessment, Las Vegas, Nevada,
14-19 September, 1975, Vol. 1.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J.,
ed. IUPAC pesticide chemistry - Human welfare and the
environment, Oxford, New York, Pergamon Press, pp. 237-238.
KALOYANOVA, F. & TASHEVA, M. (1983) Effect of protein
malnutrition on toxicity of pesticides. In: Miyamoto, J. &
Kearney, P.C., ed. IUPAC pesticide chemistry, human welfare,
and the environment, Oxford, New York, Pergamon Press,
pp. 527-529.
KALOYANOVA, F., BENCHEV, I., GHEORGHIEV, G., IZMIROVA, N., &
RISOV, N. (1979) Pesticides and persistent substances.
Biological specimen collection. In: Berlin, A., Wolff, A.H., &
Hosegawa, J., ed. Biological specimens used for the assessment
of human exposure to environmental pollutants, Sophia,
Martinus N. Publishers, pp. 231-242.
KARCZMAR, A.G. (1984) Acute and long-lasting actions of
organophosphorus agents. Fundam. appl. Toxicol., 4 : S1-S7.
KARCZMAR, A.G. & OHTA, Y. (1981) Neuromyopharmacology as
related to anticholinesterase action. Fundam. appl. Toxicol.,
1 : 135-142.
KATO, T., NAKANO, T., KOJIMA, K., NAGATSU, T., & SAKAKIBARA,
S. (1980) Changes in propyl endopeptidase during maturation
of the rat brain and hydrolysis of substance P by the purified
enzyme, J. Neurochem., 35 : 527-535.
KAUFMANN, D.D., ed. (1976) Bound and conjugated pesticide
residues, Washington DC, American Chemical Society, pp. 1-10
(ACS Symposium Series No. 29).
KEPLINGER, M.L. & DEICHMANN, W.B. (1967) Acute toxicity of
combinations of pesticides. Toxicol. appl. Pharmacol., 10 :
586-595.
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic
phosphorus compounds and alkylating agents on the rat fetus.
Arch. environ. Health, 16 : 805-808.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides: Techniques for establishing safe levels of foliar
residues. Residues Rev., 75 : 81-96.
KNOX, B., ASKAA, J., BASSE, A., BITSCH, V., ESKILDSEN, M.,
MANDRUP, M., OTTOSEN, H.E., OVERBY, E., PEDERSEN, K.B., &
RASMUSSEN, F. (1978) Congenital ataxia and tremor with
cerebellar hypoplasia in piglets born by sows treated with
NeguvonR vet (metrifonate, trichlorfon) during pregnancy.
Nord. Vet. Med., 30 : 538-545.
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the
seminiferous epithelium and the Leydig cells of the rat testis
after the application of dichlorvos. Bull. environ. Contam.
Toxicol., 11 (5): 429-433.
LAUWERYS, R.L. & MURPHY, S.D. (1969) Interaction between
paraoxon and tri- o- tolyl phosphate in rats. Toxicol. appl.
Pharmacol., 14 : 348-357.
LE QUESNE, P.M. & MAXWELL, I.C. (1981) Effect of metrifonate
on neuromuscular transmission. Acta pharmacol. toxicol.
Scand., 49 (Suppl. 5): 99-104.
LEVIN, H.S. & RODNITZKY, R.L. (1976) Behavioural effects of
organophosphate pesticides in man. Clin. Toxicol., 9 (3):
391-405.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating
properties of 2,2-dichlorovinyl dimethyl phosphate: a
disregarded hazard. Environ. Mutat. Soc. Newslett., 2 : 21-27.
LOTTI, M. & BECKER, C.E. (1982a) Treatment of acute
organophosphate poisoning: evidence of a direct effect on
central nervous system by 2-PAM (pyridine-2-aldoxime methyl
chloride). J. Toxicol. clin. Toxicol., 19 : 121-127.
LOTTI, M. & BECKER, C.E. (1982b) Letter: polyneuropathy and
exposure to parathion. Neurology, 32 : 317.
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of
organophosphorus pesticides: predictions can be based on in
vitro studies with hen and human enzymes. Arch. Toxicol., 41 :
215-221.
LOTTI, M. & JOHNSON, M.K. (1980) Repeated small doses of
neurotoxic organophosphate: monitoring of neurotoxic esterase
in brain and spinal cord. Arch. Toxicol., 45 : 263-271.
LOTTI, M. & MORRETTO, A. (1986) Inhibition of lymphocyte
neuropathy target esterase predicts the development of
organophosphate polyneuropathy in man. Hum. Toxicol., 5 : 114.
LOTTI, M., FERRARA, S.D., CAROLDI, S., & SINIGAGLIA, F.
(1981) Enzyme studies with human hen autopsy tissue suggest
omethoate does not cause delayed neuropathy in man. Arch.
Toxicol., 48 : 265-270.
LOTTI, M., BECKER, C.E., AMINOFF, M.J., WOODROW, J.E., SEIBER,
J.N., TALCOTT, R.E., & RICHARDSON, R.J. (1983) Occupational
exposure to the cotton defoliants DEF and MERPHOS. J. occup.
Med., 25 : 517-522.
LOTTI, M., BECKER, C.E., & AMINOFF, M.J. (1984) Organo-
phosphate polyneuropathy: pathogenesis and prevention.
Neurology, 34 : 658-662.
LOTTI, M., BERTONCIN, D., & MORETTO, A. (1986) Organo-
phosphate induced delayed polyneuropathy (OPIDP) by chlor-
pyrifos in man and hens. Toxicologist, 6 : 22 (Abstract No. 86).
LUDKE, J.L. (1977) DDE increases the toxicity of parathion
to corturnix quail. Pestic. Biochem. Physiol., 1 : 28-33.
MACHIN, A.P., ANDERSON, P.H., HOWELLS, L.C., & DUNDY, D.E.
(1978) Enzymic degradation of phosphorothionate oxons in the
plasma of species of varying susceptibility to poisoning. In:
Proceedings of the 4th IUPAC International Congress on
Pesticides and Chemicals, Zurich, 24-28 July, 1978 (Abstract
V.620).
MALLIPUDI, N.M., UMETSU, N., TOIA, R.F., TALCOTT, R.E., &
FUKUTO, T.R. (1979) Toxicity of O,O,S -trimethyl and triethyl
phosphorothioate to the rat. J. agric. food Chem., 27 : 463-466.
MALONE, J.C. (1964) Toxicity of haloxon. Res. vet. Sci., 5 :
17-31.
MAQUIS, J.K. (1985) Non-cholinergic mechanisms of
insecticide toxicity. Trends pharmacol. Sci., 6 (2): 59-60.
MARGOT, A. & GYSIN, H. (1957) [Diazinon: its decomposition
product and their properties.] Helv. Chim. Acta, 40 (162):
1562-1573 (in German).
MARONI, M. & BLEEKER, M.L. (1986) Neuropathy target esterase
in human lymphocytes and platelets. J. appl. Toxicol., 6 : 1-7.
MARSHALL, T.C. & DOROUGH, H.W (1977) Bioavailability in rats
of bound and conjugated plant carbamate insecticide residues.
J. agric. food Chem., 25 (5): 1003-1009.
MEDVED, L.I. & KAGAN, JU.S. (1983) Pesticides, organophos-
phorus. In: Parmeggiani, L., ed. Encyclopaedia of occupational
health and safety, 3rd ed., Geneva, International Labour
Office, pp. 1637-1646.
MEIER, E.P., DENNIS, W.H., ROSENCRANCE, A.B., RANDALL, W.F.,
COOPER, W.J., & WARNER, M.C. (1979) Sulfotepp: a toxic
impurity in formulations of diazinon. Bull. environ. Contam.
Toxicol., 23 : 158-164.
MEL'NIKOV, N.N., ET AL. (1985) [Pesticide handbook,] Moscow,
Himija Press (in Russian).
MENDOZA, C.E. (1976) Toxicity and effects of malathion on
esterases of suckling albino rats. Toxicol. appl. Pharmacol.,
35 : 229-238.
MILBY, T.H., OTTOBONI, F., & MITCHELL, H.W. (1964) Parathion
residue poisoning among orchard workers. J. Am. Med. Assoc.,
189 : 351-358.
MILES, J.W., MOUNT, D.L., STAIGER, M.A., & TEETERS, W.R.
(1979) S- methyl isomer content of stored malathion and
fenitrothion water-dispersible powders and its relationship to
toxicity. J. agric. food Chem., 27 : 421-425.
MIYAMOTO, J. (1976) Degradation, metabolism, and toxicity of
synthetic pyrethroids. Environ. Health Perspect., 14 : 15-28.
MIYAMOTO, J. & OHKAWA, H. (1978) Oxidative processes in
pesticide transformation. In: Proceedings of the 4th IUPAC
International Congress on Pesticides and Chemicals, Zurich,
24-28 July, 1978.
MURPHY, S.D. (1969) Mechanisms of pesticide interactions in
vertebrates. Res. Rev., 25 : 201-221.
NAKATSUGAWA, T., TOLMAN, N.M., & DHAM, P.A. (1968)
Degradation and activation of parathion analogues by
microsomal enzymes. Biochem. Pharmacol., 17 : 1517-1528.
NAMBA, T. (1974) Relative toxicity of malathion (contd). New
Engl. J. Med., 290 : 347.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisoning due to organophosphate insecticides: acute and
chronic manifestations. Am. J. Med., 50 : 475-492.
NICHOL, A.W., ELSBURY, S., ELDER, G.H., JACKSON, A.H., &
NAGARAJA RAO, K.R. (1982) Separation of impurities in
diazinon preparations and their effect on porthyrin
biosynthesis in tissue culture. Biochem. Pharmacol., 31 :
1033-1038.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22 : 16-18 (in
Japanese).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitaikagaku ecol. Chem., 4 : 31-46 (in Japanese).
O'BRIEN, R.C. (1967) Insecticides: action and metabolism,
New York, Academic Press, pp. 32-54.
OECD (1983) Acute delayed neurotoxicity of organophosphorus
substances: subchronic delayed neurotoxicity of organo-
phosphorus substances: 90-day study, Paris, Organization of
Economic Cooperation and Development (OECD Guidelines for the
Testing of Chemicals, Nos. 418, 419).
OHKAWA, H., OSHITA, H., & MIYAMOTO, J. (1980) Comparison of
inhibitory activity of various organophosphorus compounds
against acetylcholinesterase and neurotoxic esterase of hens
with respect to delayed neurotoxicity. Biochem. Pharmacol.,
29 : 2721-2727.
O'NEIL, J.J. (1981) Non-cholinesterase effects of
anticholinesterases, Fundam. appl. Toxicol., 1 : 154-160.
OSTERLOH, J., LOTTI, M.D., & POND, S.M. (1983) Toxicological
studies in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos.
J. anal. Toxicol., 7 : 125-129.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78 : 78-87.
PELLEGRINI, G. & SANTI, R. (1972) Potentiation of toxicity
of organophosphorus compounds containing carboxylic ester
functions toward warm-blooded animals by some organophosphorus
impurities. J. agric. food Chem., 20 : 944-950.
PETRY, C.S. (1958) Organic phosphate insecticide poisoning.
Am. J. Med., 24 : 467-470.
PETRY, H. (1951) [Polyneuritis from E 605.] Zentralbl.
Arbeitsmed. Arbeitssch., 1 : 86-89 (in German).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PRENTICE, D.E. & ROBERTS, N.L. (1984) Acute delayed
neurotoxicity in hens dosed with tri-ortho-cresyl phosphate
(TOCP): correlation between clinical ataxia and neuropatho-
logical findings. In: Cranmer, J.M. & Hixson, J.E., ed.,
Delayed neurotoxicity. Proceedings of the Delayed Neuro-
toxicity Workshop, Urbana-Champaign, Illinois, 27-30 June,
1982, Little Rock, Intox Press.
PREUSSMANN, R., SCHNEIDER, H., & EPPLE, F. (1969)
[Investigation of effects of alkylating agents. II. The
investigation of different classes of alkylating agents by
means of a modified colour reaction with 4-(4-nitro-benzyl)
pyridine (NBP).] Arzneimittelforschung, 7 : 1059-1073 (in
German).
RAY, D.E. (1980) Selective inhibition of thermogenesis by
tributyl S,S,S -phosphorotrithioate (DEF). Br. J. Pharmacol.,
69 : 257-264.
RAY, D.E. & CUNNINGHAM, V.J. (1985) Hypothermia produced by
S,S,S -phosphortrithioate (DEF). Arch. Toxicol., 56 : 279-282.
REITER, L.W., TALENS, G.M., & WOOLEY, D.E. (1975) Parathion
administration in the monkey: time course of inhibition and
recovery of blood cholinesterases and visual discrimination
performance. Toxicol. appl. Pharmacol., 33 : 1-13.
REUBER, M.D. (1985) Carcinogenicity and toxicity of
malathion and malaoxon. Environ. Res., 37 : 119-153.
REVZIN, A.M. (1980) Effects of organophosphate pesticides
and alcohol on visual mechanisms. In: Merigan, W.H. & Weiss,
B., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 255-268.
REVZIN, A.M. (1983) Neurophysiological and behavioural
assessment of pesticide toxicity. In: Miyamoto, J. & Kearney,
P.C., ed. Pesticide chemistry: human welfare and the
environment, Oxford, New York, Pergamon Press, Vol. 3, pp.
419-424.
RICHARDSON, R.J. & DUDEK, B.R. (1983) Neurotoxic esterase:
characterization and potential for a protective screen for
exposure to neuropathic organophosphates. In: Miyamoto, J.,
ed. Proceedings of the IUPAC Pesticide Chemistry Congress on
Human Welfare and the Environment, Oxford, New York, Pergamon
Press, pp. 491-495.
RICO, A.G. & BURGAT-SACAZE, V. (1984) Toxicological
significance of covalently-bound residues. Food Add. Contam.,
1 (2): 157-161.
ROBERTS, D.V. (1976) EMG voltage and motor nerve conduction
velocity in organophosphorus pesticide factory workers. Int.
Arch. occup. environ. Health, 36 : 267-274.
SALPETER, M.M., KASPRZAK, H., FENG, H., & FERTUCK, H. (1979)
Endplates after esterase inactivation in vivo: correlation
between esterase concentration, functional response, and fine
structure. J. Neurocytol., 8 : 95-115.
SANBORN, J.R., METCALF, R.L., & HANSEN, L.G. (1977) The
neurotoxicity of O -2(2,5-dichlorophenyl) O -methyl phenylphos-
phonothioate, an impurity and photoproduct of leptophos
(PhosveR ) insecticide. Pestic. Biochem. Physiol., 7 : 142-145.
SCHAFER, E.W. (1972) The acute oral toxicity of 369
pesticidal, pharmaceutical and other chemicals to wild birds.
Toxicol. appl. Pharmacol., 21 : 315-330.
SCHOENE, J. & OLDIGES, H. (1973) [Antidotal action of
pyridinium salts for tabun and sarin poisoning in vivo and in
vitro. ] Arch. int. Pharmacodyn. Ther., 204 : 110-123 (in
German).
SEIFERT, J. & CASIDA, J.E. (1980) Mechanisms of terato-
genesis induced by organophosphorus and methylcarbamate
insecticides. In: Progress in pesticide biochemistry,
Berkeley, California, University of California.
SENANAYAKE, N. & JOHNSON, M.K. (1982) Acute polyneuropathy
following poisoning by a new organophosphate insecticide: a
preliminary report. New Eng. J. Med., 306 : 155-157.
SHAFIK, T., BRADWAY, D.E., ENOS, E.F., & YOBS, A.R. (1973)
Human exposure to organophosphorus pesticides. A modified
procedure for the gas-liquid chromatographic analysis of alkyl
phosphate metabolites in urine. J. agric. food Chem., 21 (4):
625.
SHIRAISHI, S., GOTO, I., YAMASHITA, Y., ONISHI, A., & NAGAO,
H. (1977) [Dipterex polyneuropathy.] Neurol. Med. (Japan),
6 : 34-38 (in Japanese with English summary).
SHTENBERG, A.I., KHOVAEVA, L.A., & ZAVARZINA, M.V. (1974)
Effect of chlorophos and methyl nitrophos on the immune
reactions of the organism against the background of
protein-deficient nutrition. Vopr. Pitan., 8 (4): 35-42.
SOLIMAN, S.A. (1982) Delayed neuropathy in hen by the
insecticide synergist O-n- propyl O- (2-propynyl)phenylphos-
phonate (NIA 16388) and other phenylphosphonate esters. J.
Toxicol. environ. Health, 10 : 907-920.
SOLIMAN, S.A., FARMER, J., & CURLEY, A. (1982) Is delayed
neuropathy a property of all organophosphorus compounds?
Toxicology, 23 : 267-279.
SOLIMAN, S.A., SOVOCOOL, G.W., CURLEY, A., AHMED, N.S.,
EL-FIKI, S., & EL-SEBAE, A.H. (1982) Two acute human
poisoning cases resulting from exposure to diazinon
transformation products in Egypt. Arch. environ. Health, 37 :
207-212.
SPEAR, R.C., POPENDORF, W.J., SPENCER, W.F., & MILBY, T.H.
(1977) Worker poisonings due to paraoxon residues. J. occup.
Med., 19 : 411-414.
STAPLES, R.E. & GOULDING, E.H. (1979) Dipterex
teratogenicity in the rat, hamster, and mouse when given by
gavage. Environ. Health Perspect., 30 : 105-113.
STEFANAC, Z., STENGL, B., & VASILIC, Z. (1976) Quantitative
determination of organophosphorus pesticides by thin-layer
densitometry. J. Chromatogr., 124 : 127-133.
SU, M.-Q., KINOSHITA, F.K., FRAWLEY, J.P., & DUBOIS, K.P.
(1971) Comparative inhibition of aliesterases and
cholinesterase in rats fed eighteen organophosphorus
insecticides. Toxicol. appl. Pharmacol., 20 : 241-249.
TALCOTT, R.E., DENK, H., & MALLIPUDI, N.M. (1979a) Malathion
carboxylesterase activity in human liver and its inactivation
by isomalathion. Toxicol. appl. Pharmacol., 49 : 373-376.
TALCOTT, R.E., MALLIPUDI, N.M., UMETSU, N., & FUKUTO, T.R.
(1979b) Inactivation of esterases by impurities isolated from
technical malathion. Toxicol. appl. Pharmacol., 49 : 107-112.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co.,
pp. 100-119.
UMETSU, N., GROSE, F.H., ALLAHYARI, R., ABU-EL-HAJ, S., &
FUKUTO, T.R. (1977) Effect of impurities on the mammalian
toxicity of technical malathion and acephate. J. agric. food
Chem., 25 : 946-953.
UNITED KINGDOM PSPS (1979) Guidance on toxicity data
requirements, London, MAFF, United Kingdom Pesticide Safety
Precautions Scheme, 3 pp (Working Document B5).
US EPA (1975) Registration, re-registration, and
classification procedures. Fed. Reg., 40 (129): 28276-28277.
US EPA (1982) Acute delayed neurotoxicity of organo-
phosphorus substances, Washington DC, US Environmental
Protection Agency, Section 81-7, pp. 62-65 (Pesticide
Assessment Guidelines, Subdivision F, Hazard Evaluation: human
and domestic animals) (EPA 540/9-82-025).
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning.
Guy's Hosp. Rep., 123 : 13-25.
VASIC, B.V., MILOSEVIC, M.P., & TERZIC, M.R. (1977) Acetyl-
choline content and cholinesterase activity in the ponto-
medullary region of brain in rats treated with armin and
obidoxime. Biochem. Pharmacol., 26 : 601-602.
VELSICOL (1977) Phosvel (technical leptophos) data for
regulatory authorities, Chicago, Illinois, Velsicol Chemical
Corporation.
VERGIEVA, T. (1983) Embryotoxicity and teratogenicity of
pesticides. In: Kaloyanova, F. & Tarkowski, S., ed. Toxicology
of pesticides, Copenhagen, World Health Organization, pp.
67-77 (Health Aspects of Chemical Safety, Interim Document
No. 9).
VERONESI, B. & PADILLA, S. (1985) Phenylmethylsulfonyl
fluoride protects rats from mipafox-induced delayed
neuropathy. Toxicol. appl. Pharmacol., 81 (2): 258-264.
VERSCHOYLE, R.D. & CABRAL, J.R.P. (1982) Investigation of
the acute toxicity of some trimethyl and triethyl
phosphorthioates with particular reference to those causing
lung damage. Arch. Toxicol., 51 : 221-231.
VERSCHOYLE, R.D., ALDRIDGE, W.N., & CABRAL, J.R.P. (1980)
Toxicology of trimethyl and triethyl phosphorothioates. In:
Holmstedt, B., Lauwerys, R., Mercier, M., & Roberfroid, M.,
ed. Mechanisms of toxicity and hazard evaluation, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 631-634.
VETTORAZZI, G. (1979) International regulatory aspects of
pesticide chemicals, Vol. 1 & 2, Boca Raton, Florida, CRC
Press, 232 and 214 pp.
VETTORAZZI, G. & VAN DEN HURK, G. (1984) Pesticides
reference index: JMPR-IARC-IPCS-IRPTC-VBC: 1961-1984, Geneva,
World Health Organization (Unpublished report).
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977)
Severe organophosphate poisoning complicated by alcohol and
terpentine ingestion. Arch. environ. Health, 32 : 203-205.
WHO (1975) Early detection of health impairment in
occupational exposure to health hazards, Geneva, World Health
Organization (Technical Report Series No. 571).
WHO (1979) Third report of the WHO Expert Committee on
vector biology and control, Geneva, World Health Organization,
44 pp (WHO Technical Report Series No. 634).
WHO (1982) Field surveys of exposure to pesticides -
standard protocol, Geneva, World Health Organization
(Unpublished report VBC/82.1).
WHO (1984a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-1985, Geneva,
World Health Organization (Unpublished report VBC/84.2).
WHO (1984b) Spectrophotometric kit for measuring
cholinesterase activity, Geneva, World Health Organization
(Unpublished report VBC/84.888).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World
Health Organization (VBC).
WICKER, G.W., WILLIAMS, W.A., & GUTHRIE, F.E. (1979)
Exposure of field workers to organophosphorus insecticides:
sweet corn and peaches. Arch. environ. Contam. Toxicol., 8 :
175-182.
WILKINSON, C.F. (1971) Effects of synergists on the
metabolism and toxicity of anticholinesterases. WHO Bull., 44 :
171-190.
WILLEMS, J.L. (1981) Poisoning by organophosphate
insecticides: analysis of 53 human cases with regard to
management and drug treatment. Acta med. milit. Belg., 134 :
7-14.
WILLS, J.H. (1963) [Pharmacological antagonists of the
anticholinesterase agents.] In: Koelle, G.B., ed. [Handbook of
experimental pharmacology,] Berlin, Springer-Verlag, Vol. 15,
pp. 883-920 (in German).
WILSON, B.W., HOOPER, M., CHOW, E., HIGGINS, R.J., & KNAAK,
J.B. (1984) Antidotes and neuropathic potential of
isofenphos. Bull. environ. Contam. Toxicol., 33 : 386-394.
WILTROUT, R.W., ERGEGOVICH, C.D., & CEGLOWSKI, W.S. (1978)
Humoral immunity in mice following oral administration of
selected pesticides. Arch. environ. Contam. Toxicol., 20 :
423-431.
WITTER, R.F. & GAINES, T.B. (1963) Relationship between
depression of the brain or plasma cholinesterase and paralysis
in chickens caused by certain organic phosphorus compounds.
Biochem. Pharmacol., 12 : 1377-1386.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1967) Exposure
of workers to pesticides. Arch. environ. Health, 14 : 622-633.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1970) Urinary
excretion of insecticide metabolites. Arch. environ. Health,
21 : 711-716.
WOLFE, H.R., STAIFF, D.C., & ARMSTRONG, J.F. (1978) Exposure
of pesticide formulating plant workers to parathion. Bull.
environ. Contam. Toxicol., 20 : 340-343.
WOLTHUIS, O.L. & VANWERSCH, R.A.P. (1984) Behavioural
changes in the rat after low doses of cholinesterase
inhibitors. Toxicol. appl. Pharmacol., 4 : S195-S208.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) In vivo
alkylation studies with dichlorvos at practical use
concentrations. Chem.-biol. Interact., 19 : 25-46.
WORTHING, C.R., ed. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council,
655 pp.
XINTARAS, C. & BURG, J.R. (1980) Screening and prevention of
human neurotoxic outbreaks: issues and problems. In: Spencer,
P.S. & Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams & Wilkins
Company, pp. 663-674.
XINTARAS, C., BURG, J.R., TANAKA, S., LEE, S.T., JOHNSON,
B.L., COTTRILL, C.A., & BENDER, J. (1978) Occupational
exposure to leptophos and other chemicals, Cincinnati, Ohio,
US Department of Health and Welfare, National Institue of
Occupational Safety and Health (US DHEW (NIOSH) Publication
No. 78-136).
YANG, R.S.H., HODGSON, E., & DAUTERMAN, W.C. (1971)
Metabolism in vitro of diazinon and diazoxon in rat liver. J.
agric. food Chem., 19 : 10-13.
YOSHIDA, K. & NISHIUCHI, Y. (1972) Toxicity of pesticides to
some water organisms.] Bull. agric. Chem. Inspect. Stn
(Japan), 12 : 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn
(Japan), 16 : 65-69 (in Japanese).
ZACKOV, K. (1983) Immunotoxicology of pesticides. In:
Kaloyanova, F. & Tarkowski, S., ed. Toxicology of pesticides,
Copenhagen, World Health Organization, pp. 75-88 (Health
Aspects of Chemical Safety, Interim Document No. 9).
ZIELHUIS, R.L. (1972) Epidemiological toxicology of
pesticide exposure: report of an international workshop. Arch.
environ. Health, 25 : 399-405.
Annex I. Names and structures of selected organophosphorus pesticides
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other formula molecular Registry
name mass number
-------------------------------------------------------------------------------------------
acephate Orthene phosphoramidothioic C4 H10 NO3 PS 183.18
5. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
5.1 Aquatic Organisms
Organophosphorus insecticides are not very stable in aqueous
media. However, accidental leaching may occur from treated areas
into rivers and lakes where they may exert toxic effects on aquatic
organisms before degradation is complete. In clean water in the
laboratory, toxic effects were seen in several aquatic organisms
when they were exposed to concentrations of organophosphorus
insecticides ranging from 0.01 to 1 mg/litre for 48 h (Nishiuchi,
1981). However, lethal concentrations derived from 48-h exposures
in clear laboratory water may be artificially low compared with the
concentrations that would be effective in true environmental
waters.
One pesticide (phenthoate) appeared to be more toxic for
aquatic insects ("median tolerable limit" = 9 - 75 µg/kg)
(Nishiuchi, 1981) than for one species of fresh-water fish exposed
under apparently similar conditions (20% mortality caused by 263
µg/kg) (Jash & Bhattacharya, 1982).
Accidental release of pesticides in lakes, rivers, and bays
sometimes caused massive death of fish and many of the compounds
were strongly toxic for small aquatic organisms such as Daphnia, as
shown in Table 3.
Table 3. Acute toxicity of organophosphorus pesticides for some aquatic organisms
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Acephate > 40 - > 40 - > 40 Nishiuchi (1974)
Calvinphos > 40 - > 40 - 0.0042 Nishiuchi (1974)
Chlorfenvinphos 0.27 0.34 0.23 0.12 0.011 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Chlorpyrifos 0.13 0.20 0.47 0.74 0.0050 Yoshida &
emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Chlorpyrifos 2.1 - 3.4 - 0.017 Yoshida &
-methyl Nishiuchi (1976)
Cyanofenphos 1.2 1.3 6.3 15 0.0085 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Cyanophos 15 10 ~ 40 28 18 0.34 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Dialifos 1.3 - 0.80 - 0.027 Yoshida &
Nishiuchi (1976)
Dichlofenthion 5.1 10 ~ 40 1.4 10 0.005 Yoshida &
(emulsifiable (dust Nishiuchi (1972);
concentrate) formulation) Nishiuchi (1974)
Diazinon 3.2 5.1 5.3 4.1 0.08 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Dichlorvos > 40 10 ~ 40 18 - 2.8 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Dimethoate > 40 > 40 > 40 - 10 ~ 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Dimethylvinphos 5.6 - 1.5 - 0.010 Yoshida &
Nishiuchi (1976)
Dioxathion 10 ~ 40 10 ~ 40 1.4 - 0.007 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Disulfoton 8.7 10 ~ 40 21 0.37 0.07 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Edifenphos 2.5 1.8 1.8 - 0.27 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
EPN 0.20 0.32 0.50 0.085 0.0017 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Ethion 1.2 1.1 5.5 - 0.005 Yoshida &
Nishiuchi (1972)
Fenitrothion 8.2 3.4 7.0 0.75 0.050 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Fenthion 3.3 1.9 2.5 2.3 0.070 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Formothion 15 10 ~ 40 10 ~ 40 - 5.8 Yoshida &
Nishiuchi (1972)
IBP 10 ~ 40 12 7.2 - 2.3 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972)
concentrate) concentrate)
Leptophos > 40 10 ~ 40 8.5 - 0.002 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Malathion 23 7.8 0.75 1.4 0.030 Yoshida &
Nishiuchi (1972)
Menazon > 40 > 40 > 40 100 10 ~ 40 Yoshida &
(wettable Nishiuchi (1972);
powder) Nishiuchi (1974)
Methidathion 2.5 2.3 0.034 0.22 0.007 Yoshida &
(emulsifiable Nishiuchi (1972);
concentrate) Nishiuchi (1974)
Naled 1.3 1.2 28 2.3 0.005 Yoshida &
(emulsifiable (emulsifiable Nishiuchi (1972);
concentrate) concentrate) Nishiuchi (1974)
Parathion 4.5 1.7 2.9 - 0.0050 Yoshida &
Nishiuchi (1972)
Parathion-methyl 7.5 10 ~ 40 12 - 0.00050 Yoshida &
Nishiuchi (1972)
Phenkapton 2.0 3.8 3.5 - 0.008 Yoshida &
Nishiuchi (1972)
Phenthoate 2.5 2.4 0.17 0.20 0.008 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Phosalone 1.2 1.2 0.35 - 0.05 Yoshida &
(emulsifiable Nishiuchi (1972)
concentrate)
Phosmet 5.3 4.7 1.8 1.0 0.025 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Pirimiphos-methyl 1.8 - 3.0 - 0.018 Yoshida &
Nishiuchi (1976)
Propaphos 4.8 - 4.1 - 0.0063 Yoshida &
Nishiuchi (1976)
---------------------------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Pesticide TLMs for organisms at indicated time (mg/litre) Reference
Carp Goldfish Killifish Guppy Water flea
( Cyprinus (Carassius (Fundulus (Lebistes (Daphnia
carpio auratus) sp) reticulatus pulex
Linné) Peters) Leydig)
48 h 48 h 48 h 48 h 3 h
---------------------------------------------------------------------------------------------------------
Propoxur 10 ~ 40 10 ~ 40 10 ~ 40 - 0.37 Yoshida &
Nishiuchi (1972)
Prothiophos 9.5 - 10 - 0.13 Yoshida &
Nishiuchi (1976)
Temivinphos 0.58 - 0.48 - 0.0080 Yoshida &
Nishiuchi (1976)
TEPP 5.6 10 ~ 40 4.8 - 10 ~ 40 Yoshida &
(liquid (liquid (liquid (liquid Nishiuchi (1972)
formulation) formulation) formulation) formulation)
Tetrachlorvinphos 4.3 3.9 4.2 - 0.0035 Yoshida &
(wettable Nishiuchi (1972)
powder)
Thiometon 7.5 10 ~ 40 10 ~ 40 - 5.5 Yoshida &
Nishiuchi (1972)
Trichlorphon 28 10 ~ 40 25 12 0.005 Yoshida &
Nishiuchi (1972);
Nishiuchi (1974)
Vamidothion > 40 > 40 > 40 - > 40 Yoshida &
Nishiuchi (1972)
---------------------------------------------------------------------------------------------------------
Note: Test methods are officially recognized methods based on the Notification of the Ministry of
Agriculture, Forestry and Fishery of Japan, as described in the separate papers.
6. EFFECTS ON ANIMALS
Insecticides are designed as lethal agents. Although they may
be designed to be less toxic for animals than for insects, all
organophosphorus insecticides present a toxic hazard to some
extent. Values for the oral and dermal LD50s in rat, shown for
different compounds in Annex III, range from less than 10 to more
than 3000 mg/kg body weight. The dose-response line for
organophosphorus insecticides is usually steeper than that for
carbamates, though both kill by their anticholinesterase action.
The reason for the difference lies in the faster rate of
spontaneous reactivation of carbamylated AChE compared with
phosphorylated AChE.
By the time the 1984 Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) had ended, the toxicology of 57 organophosphorus
pesticides had been reviewed (Vettorazzi, 1984). Usually, the
compounds had been reviewed several times as additional information
became available. The compounds, with the years of the JMPR
review, and the acceptable daily intake (ADI) advised are listed
Annex II. It also lists the year of review by IARC. Moreover, it
gives the WHO recommended classification by acute toxic hazard
(WHO, 1984a) and an indication on whether WHO/FAO issued a "Data
Sheet on Chemical Pesticides" on this substance.
Details of the tests and of the results are recorded in the
appropriate published evaluations from the FAO/WHO Joint Committee
Meetings, which have been summarized and commented on by Vettorazzi
(1979).
It should be realized that, while reports on toxicological
tests may give some indication of the purity of the sample as
percentage content of the major active ingredient, there is seldom
any indication of the nature or quantity of the impurities or of
whether the impurities may significantly influence toxicity. It
cannot be emphasized too strongly that measurements such as acute
LD50s are not absolute values.
The LD50 values for one typical compound, fenitrothion, in
various species when administered by various routes are listed in
Table 4 (FAO/WHO, 1970b, 1975b). From this Table, it is clear that
there are marked differences in the LD50 depending on species and
route of administration. Variability in LD50 values for the same
route and species is often considered to be due to the vehicule in
which the pesticide is applied, which may influence its uptake into
the body. However, it is also possible that such differences are
real and can throw light on mechanisms of toxicity and/or of
detoxication, thus making more intelligent assessment of toxicity
possible. It can be argued that the presence of the very toxic
impurities in some formulations of diazinon (section 6.3.4) could
have been deduced much earlier from differences in LD50 data.
Likewise, the Pakistani poisonings due to strong potentiators in
stored malathion (Baker et al., 1978) (section 6.3.4) might have
been prevented, if more questions had been asked about the range of
LD50 values given in the literature for malathion.
Table 4. Comparison of acute toxicity data for
fenitrothion listed in evaluation reports of
joint FAO/WHO meetingsa
----------------------------------------------------
Animal Sex Route LD50 (mg/kg
body weight)
----------------------------------------------------
1970 Report
Mouse M oral 1336
Mouse F oral 1416
Mouse M ip 115
Mouse F ip 110
Mouse iv 220
Rat M oral 740
Rat F oral 570
Rat M ip 135
Rat F ip 160
Rat iv 33
Guinea-pig M oral 500
Guinea-pig oral 1850
Guinea-pig M ip 110
Guinea-pig iv 112
Cat oral 142
1975 Report
Mouse M oral 1030
Mouse F oral 1040
Rat M oral 330
Rat F oral 800
Ring-neck pheasant oral 34.5
Mallard duck oral 2550
Dog oral MLDb 681 mg/kg
Rat M oral 940
Rat F oral 600
----------------------------------------------------
a From: FAO/WHO (1970b, 1975b).
b MLD = minimum lethal dose.
6.1 Effects on the Nervous System
6.1.1 Effects attributed to interaction with esterases
6.1.1.1 Cholinergic effects
(a) Acute toxicity
If non-ester herbicidal compounds are excluded, then the acute
toxicity of all other organophosphorus pesticides shares the basic
mechanism outlined in section 4.5.1, involving inhibition of AChE,
accumulation of ACh, and over-stimulation of some central
cholinergic neurons and of the sympathetic and parasympathetic
nervous systems. Signs and symptoms of poisoning are described
fully in section 7.1. Death is caused by respiratory failure due
to a combination of blocking of the respiratory centre,
bronchospasm, and paralysis of the respiratory muscles.
(b) Chronic toxicity
The cholinergic effects brought about by repeated
administration of less than a single fatal dose are similar in type
to the acute single-dose effects and are discussed in section
6.3.1. For the majority of these compounds, long-term feeding
tests have been performed to establish the no-observed-adverse-
effect levels. In every case, except bromophos-ethyl, the most
sensitive indicator of an effect was depression of ChE activity in
plasma or erythrocytes. The phenomenon of tolerance to repeated
doses of anticholinesterase compounds is covered in sections 6.3.1
and 6.4. For bromophos-ethyl, it has been reported that the
urinary excretion of ascorbic acid and dehydroascorbic acid was
slightly increased in beagle dogs given 0.39 mg/kg body weight
daily, for 18 weeks, at which dose, depression of serum-ChE was not
significant (FAO/WHO, 1973b).
6.1.1.2 Delayed neuropathic effects
Delayed neuropathy has occurred occasionally in man and
experimental animals after intoxication with a variety of
organophosphorus esters. The subject has been reviewed by Johnson
(1975a,b, 1980, 1982a) and by Davis & Richardson (1980). An
account suitable for physicians is given by Lotti et al. (1984).
Delayed neuropathy is not inevitably associated with intoxication
by organophosphorus pesticides (Johnson, 1982a; Soliman et al.,
1982). Improvements in the therapy of acute poisoning (section
7.4) mean that higher doses of some organophosphorus insecticides
can now be tolerated without fatal consequences. However, many
organophosphorus pesticides that might, theoretically, cause
neuropathy, would only do so at a dose far above the lethal dose.
(a) Characteristics
Regardless of the severity of anticholinesterase effects, there
is a delay after intoxication before neuropathic signs and symptoms
appear. In the adult hen, which is the test species of choice,
this delay is 8 - 14 days, while in man, the delay may be up to 4
weeks after acute exposure. The first symptoms are often sensory
with tingling and burning sensations in the limb extremities
followed by weakness in the lower limbs and ataxia. This
progresses to paralysis, which, in severe cases, affects the upper
limbs also. Children and young animals are less severely affected
than adults, but recovery is slow and seldom complete in adults;
with the passage of time, the clinical picture changes from a
flaccid to a spastic type of paralysis. Cats, hens, and a number
of larger species are affected by a single dose. Repeated dosing
does not reduce the delay in onset to less than 8 days from the
first dose. Baboons, monkeys, and marmosets do not respond easily
to single doses of several typical neuropathic esters, and it is
difficult to produce typical delayed neurotoxic effects in rodents,
even by repeated dosing. In early histological examinations,
methods were used that showed mainly degeneration of the fatty
myelin sheath surrounding long nerve axons and names such as
"Organophosphate Demyelinating Disease" are still erroneously used,
in spite of later work that showed that the nerve axon itself was
primarily affected and damage to the myelin sheath was secondary
(Cavanagh, 1954; Bouldin & Cavanagh, 1979). The preparation of
tissues and identification of lesions are described by Bradley
(1976), Bickford & Sprague (1984), and by Prentice & Roberts (1984)
in the Workshop Report edited by Cranmer & Hixson (1984). This
publication also covers many aspects of mechanism and testing.
(b) Mechanism
The first essential step in the initiation of the delayed
neuropathic effect of an organophosphate is phosphorylation of a
target protein in the nervous system. The protein has esteratic
enzyme activity. The phosphorylation, which was originally studied
radiochemically, can be monitored conveniently as progressive
inhibition of the activity of this enzyme, which is now referred to
as Neuropathy Target Esterase (NTE or Neurotoxic Esterase)
(Johnson, 1980, 1982a). The second, and equally essential, step is
the transformation of the phosphorylated NTE to a modified form:
one of the remaining ester bonds of the inhibitor molecule attached
to the NTE active site undergoes a biochemical cleavage leaving an
ionized acidic residue bound to the protein: this residue is
negatively charged and the reaction is referred to as "aging".
Both inhibition and aging of inhibited NTE are essential to
initiate neuropathy, but the role of the negative charge in the
initiation of axonal degeneration is not known. The process of
"aging" of inhibited NTE has some analogy with the better-known
"aging" of inhibited AChE. However, the analogy does not last
above the level of enzyme inhibition. Acute toxicity arises
directly from the loss of catalytic activity of AChE, leading to
accumulation of excess physiological substrate. Mere loss of
catalytic activity of NTE (without aging) does not initiate
neuropathy (see below). There is no evidence of a deleterious
accumulation of a physiological substrate for NTE or lack of
hydrolysis products after inhibition in vivo, and the effect of
the negative charge may be focused on some quite separate process.
When NTE has been inhibited by a suitable phosphate,
phosphonate, or phosphoramidate, aging is always possible, and this
process has been shown to occur rapidly with a wide variety of
neurotoxic esters and hen NTE. However, after inhibition by
phosphinates (which contain 2 phosphorus-carbon bonds) or by
sulfonyl fluorides, no hydrolysable bonds remain in the attached
inhibitor molecule. Thus, aging is not possible, and these
compounds are not neuropathic in vivo. Whenever hen NTE can be
phosphinylated or sulphonated in vivo, the birds become resistant
to challenge doses of typical neuropathic esters, because the
2-step initiation process has been blocked halfway. Similarly,
carbamates do not form aged inhibited enzymes and are either
totally without effect (anti-cholinesterase carbamates are poor
inhibitors of NTE) or inhibit the enzyme, but do not age. Thus,
they protect the hen in the same way as the phosphinates. Now that
this mechanism is understood, it is obvious that carbamates need
not be subjected to tests that were designed to detect delayed
neuropathic potential in organophosphorus anticholinesterase
pesticides.
The events that follow inhibition and aging of NTE are not
known, but it is clear that, in adult hens, detectable delayed
neuropathic events (clinical or histological) are never seen after
a single dose/exposure of an organophosphorus ester, unless there
is at least 70% (probably 80 - 90%) inhibition of the NTE in the
brain and spinal cord soon after dosing (4 - 40 h). Owing to the
synthesis of fresh protein, this inhibition declines markedly
during the 8 to 14-day delay period, and there is no correlation
between neuropathy and NTE inhibition measured at the time that
clinical signs reach their peak. NTE has been found in the nervous
tissue of all mammals and birds examined and can be inhibited, even
in species such as the rat, in which there is no obvious clinical
neuropathic response to a single dose. It has been shown recently
that, when single doses of certain neuropathic organophosphorus
compounds are given to rats, degenerative lesions develop in their
peripheral nerves and spinal cord. Although these lesions are
similar to those in ataxic hens, they are not accompanied by
clinical signs. The dose-response of NTE inhibition and the
neurological damage in rats are well correlated, and, as in hens,
the lesions are prevented by protectively predosing the rats with
sulfonyl fluoride (Padilla & Veronesi, 1985; Veronesi & Padilla, in
press). Human NTE has been examined in vitro (Lotti & Johnson,
1978), and its response to inhibitors is similar to that of the hen
enzyme (I50s differed by not more than 4-fold). What is not known
is the numerical value of the threshold of inhibition of NTE in man
that is associated with clinical neuropathy. However, it is known
that numerous people treated only with atropine for poisoning by
trichlorfon have survived and then developed neuropathy (Johnson,
1981a). In contrast, it is very difficult to produce neuropathy
with trichlorfon in the hen, without enormous doses coupled with
prophylaxis as well as therapy for severe anticholinesterase
effects. It seems, therefore, that in the case of this pesticide,
the chances of a severely poisoned man developing neuropathy rather
than dying are greater than those of the hen. It might be deduced
that the threshold level of NTE inhibition for neuropathic effects
in man is somewhat less than the 70% value for hen, and caution
should be applied in the extrapolation from hen tests. This
argument is merely a comparison of the two hazards, death and
neuropathy: it does not give any guide to the relative dose
required to intoxicate man and hen.
(c) Delayed neurotoxicity testing
Two distinct procedures are currently practiced by testers, and
each depends on the anticipated hazard. In acute tests, it is
recommended that hens surviving an LD50 test (preferably a test in
which the therapeutic use of atropine raised the LD50) should be
observed for three weeks for abnormalities of gait and then be
re-dosed and watched for a further three weeks, after which a
thorough histological examination of the distal ends of neurons in
the spinal cord and peripheral nerve should be performed. Long-
term neurotoxicity tests require feeding hens for up to 90 days
with doses in the diet ranging up to those causing obvious adverse
cholinergic effects; evaluation is by the same criteria as for the
acute test. It may be argued that long-term tests should be
omitted, in view of the greater speed and simplicity of the acute
test in obtaining yes/no answers and the fact that no pesticidal
compound has ever been found to give a positive clinical response
in feeding trials when negative in the 2-massive-doses test. The
practice in the United Kingdom has been to voluntarily exclude from
agricultural use any compound shown to be neuropathic in acute
tests. Guidelines for the performance of acute and long-term
delayed neurotoxicity tests are available (United Kingdom PSPS,
1979; US EPA, 1982; OECD, 1983). The OECD Guidelines point out
that acute tests would be improved if they were accompanied by
assays of NTE inhibition in the brain and spinal cord, one and two
days after dosing. Results of assays would provide graded dose-
response relationships instead of the present all-or-none scoring.
Some examples are given in Johnson (1975b). Particularly useful are
data such as those obtained with malathion (Johnson, 1981b), which
showed negligible (< 10%) inhibition of NTE at the LD50 dose in
hens. These data indicate a different order of safety compared
with that of some other compounds which caused 50 - 60% inhibition,
though there was no visible clinical response. A way to integrate
the NTE test efficiently into delayed neurotoxicity test protocols
has been suggested by Johnson (1984). However, further discussion
to improve this method is required.
(d) Delayed neurotoxic response to long-term feeding
For some neuropathic compounds, the potency of a single dose
may be matched or even exceeded by the effect of the same amount
spread over a few days. In particular, compounds such as TOCP or
leptophos, which are very poorly soluble in water and are required
in a very large single dose to be effective, may well be absorbed
with greater efficiency in a divided lower-dose regime. However,
the results of various studies (Johnson, 1982a) have shown that, as
the dose is reduced further, there is a clear cut-off point below
which there does not appear to be any cumulative effect. Thus,
TOCP in the diet of hens at 400 mg/kg produced neuropathy in 21
days, while a level of 100 mg/kg diet did not cause any detectable
clinical or histological damage after 140 days (Barnes, 1975).
Monitoring of the NTE response to continuous feeding of non-
neurotoxic regimes of 2 organophosphates (0.125 mg DFP/kg for 5
days per week, over 4 weeks, or 2.5 mg mono-2-cresyl diphenyl
phosphate/kg, daily, for 10 weeks) showed that NTE levels in the
brain and spinal cord were depressed within 2 - 3 weeks to about 45
- 55% of normal and remained unchanged thereafter; the equilibrium
is presumbably the result of daily synthesis matching daily
inhibition. These doses did not cause detectable neuropathy during
either the feeding period or the 3 ensuing weeks (Lotti & Johnson,
1980). On the basis of these and other studies, Johnson (1982a)
concluded that the hen nervous system might tolerate the
biochemical defect of prolonged inhibition of NTE to about 50%
normal level brought about by long-term dosing but could not
tolerate the brief 80 - 90% inhibition that follows a single larger
dose. If long-term feeding tests in hens are required, then weekly
measurements of the level of NTE inhibition should provide valuable
predictive information, early in the test (Johnson, 1984). Since
it is necessary to kill birds for tissue sampling, more birds may
be necessary, at least in the early stages.
(e) Results of delayed neurotoxicity testing
No pesticidal organophosphorus compounds, giving negative
results in massive-dose tests, have caused delayed neuropathy in
long-term feeding studies. With the exception of trichlorfon,
where 2 doses, 3 days apart, were necessary (Johnson 1970, 1981a),
the massive-dose tests have been single doses given with
prophylaxis and therapy (eserine + atropine and atropine + oxime -
repeated if necessary) to ensure that doses above LD50 could be
examined. In Table 5, known pesticidal compounds are listed
according to the doses known to produce marked-to-severe neuropathy
in the majority of birds tested. Administration by either the oral
or dermal route may be effective.
The review by Johnson (1975b) is believed to list all
pesticidal and non-pesticidal compounds, or their derived oxons,
tested and reported on, up to that time. The JMPR Reports were not
included as a literature source. NTE responses are given where
these have been measured. Johnson (1982a) lists a number of
pesticides for which both clinical and NTE tests had been reported
since 1975 and notes that carbophenthion, cyanophos, diazinon,
fenitrothion, malathion, methyl parathion, omethoate, and parathion
can positively be declared non-neuropathic on the basis of
negligible NTE inhibition responses, while chlorpyrifos,
methamidophos, and salithion gave intermediate NTE responses
without clinical expression at the doses tested. Isofenphos
( o -ethyl- o -2-iso-propoxycarbonylphenyl isopropylphosphor-
amidothioate) has been shown by NTE assays and clinical tests to
cause delayed neuropathy in hens at about 20 x LD50 (Wilson et al.,
1984), while the insecticidal synergist o-n -propyl- o -(2-propynyl)
phenylphosphonate was neuropathic by both criteria at about the
LD50 (Soliman, 1982).
Table 5. Organophosphorus pesticides causing delayed neuropathy
in hens after a single dose
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
mipafox 25 im Bidstrup et al.
N,N -diisopropylphosphoro- (1953)
diamidic fluoride
haloxon 1000 oral Malone (1964)
3-chloro-4-methyl-2-oxo-
2H-1-benzopyran-7-yl-
bis-(2-chloroethyl) phosphate
EPN 40-80 sc Witter & Gaines
(1963)
trichlornat 310 oral Johnson (1975b)
ethyl 2,4,5-trichlorophenyl 300-400 oral Johnson (1975b)
phenylphosphonate
ethyl 2,4-dichlorophenyl > 2000 oral Abou-Donia et al.
phenylphosphonothioate (1979)
leptophos 400-500 oral Abou-Donia et al.
(1974); Johnson
(1975b)
desbromoleptophos 60 oral Johnson (1975b)
S,S,S -tributyl phosphoro- 1110 sc Johnson (1970)
trithioate (DEF)
cyanofenphos > 100 oral Ohkawa et al.
(1980)
isofenphos 100 oral Wilson et al.
O -ethyl O -2-isopropoxy- (1984)
carbonylphenyl isopropyl-
phosphoramidothioate
O-n- propyl O -(2-propynyl) 400 oral Soliman (1982)
phenylphosphonate
dichlorvosb 100 scc Caroldi & Lotti
(1981)
amiprophos 600 oralc,d Huang et al.
O -ethyl O -4-methyl-6- (1979)
nitrophenyl
N- isopropylphos-
phoramidothionate
Table 5 (contd.)
--------------------------------------------------------------
Compound Dose (mg/kg) Reference
and route
--------------------------------------------------------------
coumaphos 50 oralc Abou-Donia et al.
500 dermal (1982)
chlorpyrifos 150 oral Lotti et al.
(1986)
salithion 120 oral El-Sebae et al.
(1981)
--------------------------------------------------------------
a Dose needed to cause marked-to-severe neuropathy in the
majority of birds tested.
b 50% formulation in hydrocarbons: dose calculated as active
ingredient.
c Mild neuropathy only at maximum tolerated dose.
d Test performed in cockerels.
The statement by Namba et al. (1971) that chlorpyrifos produced
neuropathy in hens seems to have been without foundation and may
arise from the misreading of a report by Gaines (1969), which
mentioned that chlorpyrifos caused a rapid onset short-term
weakness (sometimes called paralytic effect) similar to that caused
by malathion. However, one recent case of human poisoning and
laboratory tests with doses well above the unprotected LD50 have
shown neuropathic effects from this pesticide (Lotti & Moretto, in
press).
(f) Structure/activity relationships
As noted above, not all organophosphorus pesticides cause
delayed neuropathy. In vivo tests and target-enzyme studies
listed by Johnson (1975b) can be condensed according to a number of
factors as listed by Johnson (1980, 1982a):
(1) Factors that increase delayed neurotoxicity potential more
than acute toxicity are:
(a) choice of phosphonates or phosphoramidates rather
than analogous phosphates;
(b) increase in chain-length or hydrophobicity of R1 and
R2 ; and
(c) a leaving group X, which does not sterically hinder
approach to the active site of NTE;
(2) Factors that decrease the comparative potential are:
(a) the converse of (1) a, b, and c;
(b) choice of R or X groups that are very bulky
(naphthyloxy) or non-planar;
(c) choice of a nitrophenyl group at X (a steric effect?);
(d) choice of comparatively more hydrophilic X groups
(oximes or heterocyclics); and
(e) choice of thioether linkages at X.
Considering these factors, it can be seen why malathion and
diazinon are both far below the neurotoxicity hazard line, why, in
its homologous series, only dichlorvos is not neurotoxic at the
LD50, why EPN, a phosphonothioate with a hydrophobic phenyl group
at R1 is neurotoxic, even with a 4-nitrophenyl leaving group, and
why it is not surprising that other phenylphosphonothioates such as
desbromoleptophos, or cyanofenphos are also neurotoxic. The
apparent non-neurotoxicity of the ethyl analogue of leptophos
(Hollingshaus et al., 1979) seems to contradict (1-b) above, but
the dominant factor seems to be the problem of absorption of this
very poorly soluble compound after oral dosage (Hansen & Hansen,
1985).
6.1.2 Behavioural and other effects on the nervous system
The problems of interpreting behavioural changes in relation to
inhibition of AChE and the time-dependent changes in these
variables have been discussed by Bignami (1976) and Bignami et al.
(1975). It appears that some learned responses may be acquired
more quickly by rats recovering from an inhibitory dose of, e.g.,
DFP (1 mg/kg body weight). This may be a sign of changed
inhibitory responses in learning pathways, but it is difficult to
say whether it can be classified as a toxic response.
Numerous research workers have reported changes at doses that
affect levels of AChE, but without overt signs of intoxication.
For example, Kaloyanova-Simeonova (1961) noted that small doses of
chlorthion (5 or 10 mg/kg body weight in the rat) intensified
conditioned motor reflexes and depressed them at higher doses: ChE
levels were depressed in all cases and were restored more slowly
than the return to normal reflex activity. Reiter et al. (1975)
did not find any effects on performance of learned visual
discrimination tasks by monkeys at doses of parathion of 0.5 mg/kg
body weight, which depressed blood levels of AChE by about 25% and
those of pseudoChE by about 35%. Doses causing 40% or more
inhibition of AChE were associated with decreased responses, which
returned to normal faster than the recovery of AChE levels. A very
sensitive response to the nerve agent soman (pinacolyl
methylphosphonofluoridate) was reported in one out of several
behavioural tests by Wolthuis & Vanwersch (1984). In an open-field
test, a number of performance variables were affected by a dose of
only 3% of the LD50. Effects were also seen with doses of 4 - 6%
LD50 of 2 carbamates but not below 30% LD50 of the pesticide, TEPP,
or of another nerve agent, sarin (isopropyl methylphosphono-
fluoridate). Unfortunately, the effects on ChE levels were not
measured. There is no obvious reason for the contrasting effects
of TEPP and sarin, on the one hand, and of soman and physostigmine,
on the other. The authors noted that, judged by poisoning signs at
near-lethal doses, soman exerted a greater proportion of its
effects centrally than the other 2 organophosphorus esters. The
fact that changes may be seen in some, but not all behavioural
responses at a certain dose emphasizes the statement by Revzin
(1983) that no single behavioural or neurophysiological test can
give a definite conclusion about organophosphate toxicity.
There appears to be only one other report in the literature of
a behavioural change in animals at doses less than those that
inhibit ChEs (Desi et al., 1971). Behavioural and EEG changes were
noted in rats fed bromophos at 500 or 100 mg/kg diet for 6 weeks,
doses that also caused ChE inhibition, but, at 30 and 10 mg/kg,
there were no effects on ChE, though some behavioural changes were
still observed. It is not clear whether undosed animals were
handled in precisely the same fashion as the dosed.
However, in contrast to the above, Desi (1983) reported tests
applied after 3 months of daily consumption of diet containing
various percentages of the LD50 of bromophos. The lowest
concentration that produced changes in a behavioural test (maze
running) and in EEG (both the complex EEG and computer-analysed
segments of the EEG) was about 0.26% of LD50, daily, but this also
caused significant changes in erythrocyte-AChE and plasma-ChE.
Among 6 organophosphorus pesticides assessed by these procedures,
none produced changes greater than that caused by the vehicle alone
without also causing effects on ChE. This conclusion differs from
that written by the author.
Analysis of the EEG records of a small group of rhesus monkeys
has been carried out both before, and one year after, intoxication
with the potent anticholinesterase agent sarin (isopropyl
methylphosphonofluoridate) (Duffy & Burchfield, 1980). Three
animals received a single "large dose" (5 µg/kg body weight iv),
and 3 others received a series of 10 injections of 1 µg sarin/kg
body weight im at weekly intervals: there were 10 controls. The
"large dose" animals had generalized convulsions and were
maintained on Gallamine relaxant with artificial respiration;
small-dose animals were considered in pilot studies to be near the
threshold of poisoning, but showed few overt signs; they did not
receive relaxant or artificial respiration. Twenty-four hours
after a "large dose", marked differences were seen in the EEG
frequency spectrum, which is not surprising. However, one year
after the dose, there was still a small increase in the percentage
energy in the beta-2 region of the spectrum and the change was said
to be statistically significant. Some changes in the beta region
were detectable in all 6 dosed monkeys one year after; no
significant changes were seen in the 10 controls. However,
apparently the changes were only seen under some lighting
conditions and were small compared with differences between the
frequency spectra of the only 2 controls for which data were shown.
The statistical treatment of the acquired data seems valid, but the
actual values measured one year after dosing are obviously well
within the normal range. No indication was given of how much
variation can be caused in the pattern of undosed animals by
variations in the conditions of handling and observation, or what
the range is for apparently identically treated normal animals. The
doubts pertaining to the toxicological significance of these
measurements apply also to some human studies using the same
technique (section 7.2.2).
Effects of cholinergic agents on the visual system have been
monitored by Revzin (1980). In urethane-anaesthetised pigeons with
implanted electrodes, various changes in the response of specific
neurones of the optic tectum and of the hippocampus were noted
after doses both of mevinphos and of atropine. The author claims
that the procedure was sensitive in detecting effects in the
absence of detectable peripheral parasympathetic signs. However,
the lowest effective dose of mevinphos was only one-third of that
(0.15 mg/kg body weight) which produced parasympathetic signs that
would certainly be associated with substantial inhibition of AChE.
Thus, the claim to sensitivity of this complex procedure in
surgically modified birds seems excessive.
Some possible effects of anticholinesterases on non-
cholinesterase targets were considered by O'Neill (1981). No clear
effects seem definable at concentrations lower than those that
inhibit AChE and some are probably secondary to stimulation of non-
cholinergic nerves with cholinergic innervation. However, an
endopeptidase that can hydrolyse putative transmitter peptides in
the nervous system is known to be inhibited by di-isopropyl
phosphorofluoridate at a concentration similar to that which
inhibits AChE (Kato et al., 1980). Thus, some involvement of non-
cholinergic pathways in the causing of CNS effects in
organophosphate poisoning cannot be excluded. Moreover, some
anticholinesterases also exert effects directly at the cholinergic
receptor as well as by the inhibition of AChE (Karczmar & Ohta,
1981).
6.2 Other Effects
A variety of histological lesions has been described at autopsy
in animals severely poisoned with organophosphate pesticides.
However, very few effects are described in the absence of obvious
poisoning or at doses that do not markedly inhibit the ChEs. In
the following sections, the evidence is reviewed for effects not
obviously attributable to inhibtion of either AChE or NTE.
6.2.1 Mutagenic and carcinogenic effects
The proposed theoretical basis for believing that dimethyl or
diethyl phosphate pesticides might be mutagenic or carcinogenic
has already been discussed (section 4.5.2). This basis was shown
to be defective by Bedford & Robinson (1972). No alkylation was
detected in N7 of guanine in RNA and DNA of liver of animals that
were exposed for 12 h to working concentrations of dichlorvos of
64 g/m3 (0.064 g/litre air) (Wooder et al., 1977); these authors
contrasted their data with earlier published work showing modest
alkylation of guanine in suspensions of cells exposed to very high
solution concentrations of dichlorvos. In vivo, the acute
anticholinesterase effects limit the circulating concentration
that can be tolerated by any mammal and there is also strong
preference for phosphorylation of biological scavenger molecules
rather than for alkylation built into the organophosphorus
pesticide molecules.
It has frequently been suggested that dichlorvos has
carcinogenic potential because of observed mutagenic effects in in
vitro test systems. The IARC (1979) Working Group accepted
extensive data showing no evidence for the mutagenicity of
dichlorvos in mammals. FAO/WHO (1978b) accepted animal studies
showing no dose-related carcinogenic effects in life-time studies
carried out at doses that depressed blood-ChE levels.
In a 78-week feeding test on groups of 50 male and 50 female
mice (B6 C3 F1 hybrid), no dose-related effects were seen when the
diet contained about 600 or 300 mg dichlorvos/kg, respectively.
The IARC Working Group evaluating this study noted that a few
oesophageal tumours were seen in treated mice. It appears that
this fact influenced their verdict that "the available data do not
allow an evaluation of the carcinogenicity of dichlorvos to be
made" (IARC, 1979).
An IARC Monograph (1983) included evaluations of 5 widely-used
organophosphate pesticides (malathion, methyl parathion, parathion,
tetrachlorvinphos, and trichlorfon). In several cases, the
conclusions were that acceptable tests had been performed with no
evidence of carcinogenic effects or of mutagenic effects in
mammals. For others, the conclusions were of "limited evidence"
consisting of very small effects above the control background
levels in life-time studies. None of these compounds was judged to
be a strong mammalian mutagen or carcinogen and the same statement
is true for all organosphosphorus pesticides that have been
evaluated by FAO/WHO Working Parties or by other authorities.
However, recent published results show that controversies do occur
when evaluating the outcome of carcinogenicity studies. Huff et
al. (1985) reevaluated the pathology of the original studies by
the US National Cancer Institute in 2 different strains of rats.
Histopathological reexamination confirmed the earlier conclusions
that malathion and malaoxon were not carcinogenic. These
conclusions differed from those of Reuber (1985) who evaluated the
same studies and rated both compounds as carcinogenic.
6.2.2 Teratogenic effects
Defects in the development of fertilized hen eggs, injected
with various organophosphates, are known, but many of these are
associated with the inhibition of the enzyme kynurine formamidase
and a depression of NAD levels at a critical period of development
(Seifert & Casida, 1980). This pathway is not critical in mammals,
and no equivalent effects are known. If teratogenicity is taken to
mean induction of malformations in live offspring without decrease
in number of births (i.e., no embryotoxicity), then for the vast
majority of organophosphorus pesticides, no adverse effects of
continuous feeding of organophosphates on pre- or postpartum
mortality have been reported, nor have embryonic defects been
proved, except at doses that significantly retarded growth in the
mother (Vergieva, 1983). Single high doses causing significant
toxic effects in mothers may be deleterious: a number of these
toxicity-linked effects have been summarized by Seifert & Casida
(1980). Kimbrough & Gaines (1968) reported deaths, and that
resorptions were increased in pregnant rats given a single high
dose of parathion or diazinon on the 11th day of gestation.
However, these effects were associated with significant toxic
effects on the mothers. Similarly, trichlorfon at very high doses
(400 mg/kg per day with the dose divided into 3 spaced aliquots)
and given on days 6 - 15 of gestation, produced defects in
offspring: each dose produced cholinergic symptoms; no effects were
seen in rats, mice, or hamsters when the daily dose was 200 mg/kg
(Staples & Goulding, 1979). However, a specific defect consisting
of hypoplasia of the cerebellum in offspring was noted, both in
field cases and experimental tests, 8 pregnant pigs were dosed once
or twice with neguvon (a veterinary grade of trichlorfon) between
days 55 and 70 of pregnancy (Knox et al., 1978). Doses were 50 -
60 mg/kg body weight, which caused maximum inhibition of
erythrocyte-ChE levels of 40 -80%, without overt signs of
poisoning. The hypoplasia was accompanied by severe ataxia and
tremors, while voice and vision appeared unaffected. No defects
were seen in the offspring of over 100 control sows housed with the
dosed animals, and serological and virological tests did not show
anything incriminating. A genuine teratogenic effect of moderate
doses of trichlorfon commonly used in veterinary practice has been
demonstrated in the pregnant pig. However, it may be that the
herds tested were unusually susceptible, since (apparently)
congenital tremor had been known in litters borne over many years
in the area where the trichlorfon-induced effects were seen.
6.2.3 Effects on the immune system
In a review, Zackov (1983) stated that "most ... organo-
phosphorus pesticides elicit autoimmune reactions and suppress the
production of antibodies against vaccines". No evidence was given
and it is not clear whether the statement was claiming specific
effects or referred to doses that are sufficient to produce a range
of toxic effects.
Shtenberg et al. (1974) claimed that oral doses of methyl-
nitrophos (fenitrothion) or chlorphos (trichlorfon) at 5 or 7 mg/kg
body weight per day, for an unspecified period, suppressed
haemaglutinin levels in rats immunized against sheep red blood
cells; these doses would have significantly inhibited ChEs and were
said to be more effective in rats fed a protein-deficient diet.
Dandliker et al. (1979) reported a depression in antibody titre in
rats in the 6 - 7 weeks of an immunization procedure that commenced
either one day before or one day after (both statements are made)
an oral dose of half of the LD50 of parathion. The immunization
consisted of weekly intramuscular doses of 400 µg fluorescein-
labelled ovalbumin with Freunds Complete Adjuvant. The confusion
concerning the order of intoxication and primary immunization is
very important when such a high dose was given, since cholinergic
symptoms, fluid loss, imbalance, and general debility would have
been marked. Administration to mice of 0.1 x LD50 of parathion for
8 days led to a 10% loss in body weight. Immunization on the 9th
day showed a statistically-significant (34%) reduction in the
number of antibody plaque-forming cells derived from the spleen, 4
days after immunization, but the reduction was insignificant (10%
only) when immunization was carried out on day 10, though several
animals in the group died at this point due to accumulated toxic
effects (Wiltrout et al., 1978).
Some decreased antibody titres "proportional" to AChE
inhibition were noted in response to prolonged doses of malathion
or dichlorvos (0.025 - 0.4 x LD50 given 5 days per week for 6
weeks) (Desi et al., 1978). Clearly, none of the above responses
can be dissociated from the general cholinergic intoxicating
effects of the pesticides. There do not appear to be any reports
of studies on the immunological status of healthy animals receiving
doses causing little or no depression in ChE levels.
6.2.4 Effects on tissue carboxyesterases
A variety of carboxyesterases abound in serum, liver,
intestine, and other tissues (section 4.4). Although inhibition of
one specific carboxyesterase (NTE) has toxic sequelae (section
6.1.1.2), no direct deleterious effects of inhibiting other
carboxyesterases have been demonstrated. However, they may
contribute markedly to the metabolic disposal of malathion and
certain other organophosphorus pesticides, so that inhibition of
tissue carboxyesterases may potentiate the toxicity of such
pesticides (section 6.3.5). The structure/activity relationships
for inhibition of these enzymes by organophosphorus compounds
inevitably differ from those for inhibition of AChE. For EPN and
fenchlorphos, both serum- and liver-carboxyesterases of rats were
markedly more sensitive than brain- and serum-AChE; in other cases,
the liver enzymes, but not those in serum, were more sensitive (Su
et al., 1971).
6.2.5 Sundry other effects of organophosphorus pesticides
Very few effects other than those described in the sections
above have been noted, except those arising from ill-health due to
severe anticholinesterase effects. Thus, impaired growth rate is
commonly associated with a rapid depression in AChE levels to less
than 50%, but much lower levels can be tolerated without ill-
effects, if the depression is brought about over several weeks, and
then maintained for up to a year (section 6.3.1).
Various changes in glucose metabolism, in serum enzymes, and in
other clinical chemical variables have been reported after single,
acute, or repeated doses of various pesticides at from one-tenth to
one-quarter of the LD50, daily (Dimov & Kaloyanova, 1967; Enan et
al., 1982).
A reversible, mild muscle-necrotising effect could be detected
histologically in the diaphragm muscles of rats, 24 h after a dose
of paraoxon, parathion, or other anticholinesterases, sufficient
to cause marked fasciculations (Fenichel et al., 1972; Dettbarn,
1984). The damage appeared to be a function of the prolonged
cholinergic stimulation of the muscle, since it was entirely
prevented by doses of atropine, which prevent fasciculations, or by
alpha-bungarotoxin applied directly to the myoneural junction
(Salpeter et al., 1979).
Several adverse effects attributed only to certain
organophosphorus esters are listed below. The list may be of value
in promoting more careful examination of intoxicated animals or
human beings.
6.2.5.1 Effects on hormones
Changes in the diurnal pattern of plasma-ACTH and adrenal
levels of some related enzymes have been reported in rats
maintained with dichlorvos in the drinking-water at 2 mg/litre:
blood-ChE levels were not affected (Civen et al., 1980). However,
the weight gain of the treated animals was only half that of the
controls, and the fluid consumption was increased by 20%. These
deleterious effects could be due to diarrhoea and imbalance of
fluids with inevitable repercussions on hormonal levels, etc.;
preferential effects of the dichlorvos on intestinal esterases
might be the primary effect.
6.2.5.2 Effects on the reproductive system
Damaged seminiferous tubules were reported in mice given either
a single dose of about half of the LD50 of dichlorvos or 18 doses
of about one-tenth of the LD50 (Krause & Homola, 1974). However,
the doses were high, the percentage increases seem small, and the
number of samples taken was very small; thus, no statistical
evaluation is possible. Amiprophos has been reported to cause some
gonadotrophic effects in adult cockerels (Huang et al., 1979), but
details are few.
6.2.5.3 Effects on the retina
Fenthion administered intramuscularly at about one-quarter of
the LD50 (50 mg/kg body weight), every 4 days for one year
(solvent, if any, not stated), affected the electroretinogram in 2
strains of rats (Wistar and black Long-Evans) within 3 months and
abolished it after one year (Imai, 1977). Similar studies involving
the administration of either fenitrothion or ethylthiometon to
beagle dogs for 5 days per week for 2 years, were reported by
Ishikawa & Miyata (1980). Doses that depressed plasma-ChE levels
to about 30% of normal, throughout the period, led to changes in
optical function after 13 months continuous exposure and to
morphological changes in the ciliary muscle at termination.
6.2.5.4 Porphyric effect
Daily application of technical (85%) diazinon (20 or 40 mg/kg
body weight) to the skin of Dark Agouti rats just above the tail
produced a 4-fold increase in faecal porphyrins, after 8 - 12 weeks
(Bleakley et al., 1979). There was no increase in urinary
porphyrins and no effect when diazinon was given in food (about
8 mg per day to rats, initially weighing 180 g). The pattern of
porphyrins excretion was said to be indistinguishable from
porphyria cutanea tarda. However, the increase in excreted
porphyrins in classical porphyria cutanea tarda may be as much as
1000-fold, with massive amounts in the urine as well as in the
faeces. The authors failed to reproduce even this small effect in
rats when they used more pure (97%) diazinon (Nichol et al., 1982).
However, they also found that an impurity in stored technical
material, isodiazinon (diethyl 2-isopropyl-6-methyl-4- S -
pyrimidinyl phosphorothioate), was very effective in causing
porphyrin accumulation, when added to cultures of chick
hepatocytes; confusingly, however, the accumulated porphyrin was
coproporphyrin rather than the expected protoporphyrin. Although
human poisoning with diazinon is not uncommon, there has only been
one report implicating technical diazinon in a few cases of
porphyria cutanea tarda in occupationally-exposed workers (Bopp &
Kasminsky, 1975). Further experimental animal studies seem
warranted. No reports have been found of porphyria connected with
exposure to other organophosphorus pesticides.
6.2.5.5 Lipid metabolism
Organophosphate esters can inhibit the activities of some
triglyceridases and lipases in vitro and in vivo. However, no
repercussions from such inhibition were found when appropriate
parameters were measured in rats fed for one year with either of 2
pesticides that caused marked (60 - 80%) depression of blood-ChE.
Thus, male or female rats were fed either a normal diet or a diet
enriched sufficiently with fat to increase aortic fatty acids by
170%. No changes occurred in: the hormone-sensitive lipase and
lipoprotein lipase in adipose tissue; the free and total fatty
acids and total glycerol and total cholesterol in serum; and the
total fatty acids, cholesterol, and glycerol in the aorta (Buchet
et al., l977). The pesticides were chlorpyriphos (100 mg/kg diet)
and triamiphos (10 mg/kg) and the findings were contrary to those
from a preliminary study by the same workers.
6.2.5.6 Effects causing delayed deaths
Although not pesticidal agents themselves, two of the
phosphorothiolate impurities found in technical malathion and some
analogues of these impurities caused delayed effects, which were
lethal for rats at doses below the cholinergic LD50 (Aldridge et
al., 1979; Mallipudi et al., 1979; Verschoyle et al., 1980). The
compounds were O,O,S -trimethyl phosphorothioate (I), O,S,S -
trimethyl phosphorodithioate (II), and the ethyl analogues (III and
IV). After an oral LD50 dose (as low as 26 mg/kg body weight for
II), all 4 compounds produced cholinergic responses that lasted
less than 24 h and deaths at this time were only seen with IV:
doses 2 - 6 x LD50 were needed to cause cholinergic deaths with I -
III. Rats dosed at, or near, the LD50 recovered from the initial
cholinergic effects, but by day 3, they had lost weight and were
panting with laboured respiration; deaths occurred 3 - 6 days after
dosing. However, survivors appeared normal, 10 days after dosing.
Death was due to pulmonary insufficiency associated with
progressive cell proliferation (Dinsdale et al., 1982; Imamura et
al., 1983; Aldridge & Nemery, 1984), and combined therapy with
atropine and oxime was ineffective. The biochemical mechanism of
these effects is not fully known, but it is probable that the
proximal toxin is produced in the lung by oxidative attack on the
alkylthio moiety of the compounds (Aldridge et al., 1985). The
activities of brain-AChE and plasma-ChE and carboxylesterase, which
were partially inhibited during the first day after dosing,
increased thereafter and were at least 50% of the levels of
activity in the controls at the time of death. The margin between
delayed death LD50 and cholinergic LD50 was markedly less with the
triethyl, than with trimethyl compounds. Such effects were seen
with S,S,S -trimethyl phosphorotrithiolate but not with the higher
analogue ethoprophos ( O -ethyl S,S -di- n -propyl
phosphorodithiolate) or with methamidophos (Verschoyle & Cabral,
1982).
A different form of delayed acute toxicity was reported to
occur 4 days after large oral doses of DEF in hens. The effect was
not seen after a single dose, sufficient to cause delayed
neurotoxicity, was given subcutaneously (Johnson, 1970) or dermally
(Abou-Donia et al., 1980). The effect was distinct from both
cholinergic and delayed neuropathic effects and is attributed to
the acute toxicity of n- butyl mercaptan produced by the degradation
of DEF in the gastrointestinal tract.
6.2.5.7 Selective inhibition of thermogenesis
The defoliant DEF ( S,S,S -tri- n -butyl phosphorotrithiolate)
acted as an anticholinesterase at high doses, but, at lower doses
(60 - 200 mg/kg in rats and mice), it caused a profound fall in
body temperature (as much as 10 °C over a few h), without marked
sedation; deaths occurred mostly after the depression had persisted
for a day (Ray, 1980). The effect was different from the smaller
atropine-sensitive changes due to some cholinomimetics and was due
to blocking of cold-induced thermogenesis without affecting heat
conservation mechanisms. The effect seems unique to the DEF
chemical structure. Ray & Cunningham (1985) demonstrated that the
effect was a selective action on a central thermogenic control
mechanism rather than on peripheral thermogenic processes and that
it was probably due to a metabolite of DEF rather than to the
parent compound.
6.3 Factors Influencing Organophosphorus Insecticide Toxicity
6.3.1 Dosage-effect
The lethal effects of organophosphorus insecticides are due to
severe cholinergic effects arising from excessive inhibition of
AChE. With few exceptions, the AChE activity of the tissues is
inhibited soon after the administration of acutely toxic doses of
all anti-AChE agents. This is true, not only for compounds that do
not require metabolic conversion to anti-AChE agents, but also for
most phosphorothioates, phosphorodithioates, and
phosphorodiamidates that are oxidized by the liver to metabolites
with anti-AChE activity. In general, the duration of action of
most anti-AChE agents is relatively short, as evidenced by
considerable reversal of the inhibition within a few days. For
this reason, a 10-day observation period is sufficient for acute
LD50 measurements on all anti-AChE agents that have been studied,
and 2 days suffice for most.
The data in Table 6 give a comparison of the maximal amount of
inhibition of the ChE activities in the brain, submaxillary glands,
and serum of rats for several compounds, all of which were given at
dose levels equivalent to 5/8 of the LD50. The time at which
maximal inhibition occurred and the period required for complete
reversal of the inhibition are also presented. From these
examples, it can be seen that, in general, equivalent fractions of
the LD50 of various anti-ChE compounds produce similar levels of
inhibition of ChEs, though the LD50 values for the various
compounds differ considerably. The time at which maximal
inhibition of ChEs occurs varies from 15 min to 3 h after
administration. In some cases, the AChE activity of the brain and
parasympathetic nervous system, as indicated by the submaxillary
gland, and the non-specific ChE of the serum are inhibited to the
same extent by a particular compound. However, notable exceptions
are OMPA, which is converted in the liver to a very labile
inhibitor that never reaches the brain, and Guthion, which does not
inhibit non-specific ChE.
Differences between the responsiveness of rat brain- and serum-
ChE have been reported also in the case of dietary administration
of various organophosphorus insecticides (Su et al., 1971). Thus,
a level of only 40 mg EPN/kg fed for 1 week reduced brain-ChE to
50%, while 125 mg/kg was needed to achieve the effect in serum; the
sensitivity was reversed for fenchlorphos, while the sensitivities
of the 2 tissues were similar for demeton. It is not clear whether
these differences reflect differences in access of the compounds to
their targets, differences between the tissue AChEs, or the fact
that pseudoChE is present as well as AChE in the serum of rats, so
that assay of the hydrolysis of ACh using serum measures both
enzymes, whereas, in the brain, the activity is about 90% specific.
Marked differences in the rate of reversal of the inhibitory
effects on ChEs of different compounds, in vivo, are shown in Table
6. In view of the variable duration of action of various anti-ChE
agents, the performance of assays on the tissues of animals at
intervals after acutely toxic doses provides a great deal of useful
information regarding the toxicity of these compounds. The
transition from a tolerable to a lethal dose (either acute or
chronic) often occurs within a 2 to 4-fold range. This is not
surprising, since the AChE of nervous tissue and effector organs
must be inhibited by 50 - 80% before pharmacological effects can be
seen (Holmstedt, 1959). The increment in dose to raise inhibition
to 90% with associated deaths is not very great.
Table 6. Onset and duration of the anticholinesterese action
of some organophosphorus compounds in ratsa
-----------------------------------------------------------------------
Compound Maximum inhibition of cholinesterase (%)
Dose Brain Serum Sub- Time to Time to
(mg/kg maxillary maximum complete
body gland inhib- reversal
weight) tion (h) (h)
-----------------------------------------------------------------------
Iso-Systox 1.0 85 80 75 3.0 120
(demeton- S )b
Disyston 1.25 75 85 75 3.0 120
(disulfoton)
Guthion 3.5 60 0 50 0.5 24
(azinophosmethyl)
Dipterex 140.0 85 82 85 0.25 6
(trichlorfon)
Octamethyl 5.0 0 85 88 2.0 144
pyrophosphor-
tetramide (OMPA)c
-----------------------------------------------------------------------
a From: DuBois (1963).
b Phosphorothioic acid, O -[2-(ethylthio)ethyl] O,O -diethyl ester.
c Octamethylpyrophosphoramide.
The general correlation of inhibition of ChE with symptoms of
poisoning is also seen with repeated dosing with organo-phosphates,
but details vary greatly. It is always true that a prerequisite of
death is profound inhibition of AChE in the central and/or
peripheral nerves, but the tolerable maximum inhibition increases
when this level is reached, stepwise, over a period of 2 - 4 weeks
or more. Thus, when commercial Systox (containing about equal
amounts of the O - and S -isomers of demeton) was fed to rats at
20 mg/kg diet, there were no signs of poisoning, though, at the end of
16 weeks, the brain- and whole blood-ChE activities were 26 and 28%
of normal, respectively (Barnes & Denz, 1954). At 50 mg/kg, 3 out
of 12 rats died, but all others in the group improved after initial
marked signs of poisoning during the first month, their food intake
increased above normal (and therefore their actual dose increased),
and their growth rate became normal. This improvement was not due
to any marked increase in ability to detoxify the agent, since, at
the end of 16 weeks, these apparently healthy rats had only 7 - 8%
of normal AChE activity in the brain and blood. This level would
be associated with fatalities if brought about by a single dose;
indeed, at the end of the study, the animals were consuming daily a
dose equivalent to 96% of the single-dose LD50. In a similar way,
Barnes & Denz (1951) found that parathion in the diet at 100 or
75 mg/kg was lethal for the majority of rats in large groups within
3 - 4 weeks, while results with 50 mg/kg were variable (26/72 deaths
in one trial and 3/36 in a later trial) and no deaths attributable
to poisoning occurred in groups fed 20 or 10 mg/kg. The rats
surviving 50 mg/kg showed clinical signs of intoxication (notably
fasciculations) and ate less, initially: they ate normally after 3
weeks, but failed to gain weight as rapidly as the controls.
However, signs of poisoning decreased in severity and frequency
during the third month and seldom reappeared during the remainder
of a year's feeding. This pattern of response and of adaptation is
typical for all anticholinesterase pesticides. When monitoring of
enzymes is carried out in parallel with feeding, the level of
activity often rises to a steady state after an initial decline.
Factors determining the fraction of an LD50 dose that is tolerable
include:
(a) Speed of absorption, of subsequent metabolic activation,
and of elimination of the compound. Thus, for demeton- S -
methyl, much of the sulfoxide metabolite from a single dose
will still be circulating on the following day, though the
parent compound may not linger. In such a case, lethal
concentrations will build up more easily than with, say,
trichlorfon, which is converted to the inhibitory dichlorvos,
both of which are rapidly eliminated;
(b) The net rate of formation of a stable form of
inhibited AChE arising from the 3 reactions of inhibition,
reactivation, and aging described in section 4.5. The
relationship of the chemical structure of an oxon inhibitor to
rates of these reactions is complex and also varies between
species. AChEs from the rat have not been purified and
subjected to extensive kinetic study in vitro. In most
studies, crystallized (not 100% pure) bovine erythrocyte-AChE
has been used. Rates of spontaneous reactivation and aging for
this enzyme inhibited with dimethoxy, diethoxy, and ethoxy,
ethanthio-substituted phosphates are shown in Table 7.
Although data derived in this way cannot be transposed directly to
in vivo situations, they are consistent with the well-known fact
that, after poisoning by a sub-lethal dose of some dimethyl
phosphates, recovery with the disappearance of symptoms is complete
within a few h. The value of k+3 for erythrocyte-ChE taken from
rats dosed in vivo with dimethyl phosphate, was reported to be 57
x 10-4 (a half-life of inhibited enzyme of 2 h). One day after
such a sub-lethal dose, most of the rat AChE will again be in the
uninhibited form with a small fraction in the aged inhibited form.
In contrast, not more than half of the inhibited enzyme would be
expected to be reactivated in one day after poisoning with diethyl
phosphate and a substantial proportion of the inhibited enzyme
would be aged, so that recovery to 100% activity would be a very
slow process, depending on the synthesis of fresh enzyme. It
follows that markedly different outcomes would be expected from
repeated intoxication with doses of dimethyl phosphate and diethyl
phosphate which both caused an initial response of, say, 50%
inhibition, the former would be less hazardous than the latter.
This interpretation concurs with the fact that rats can survive
daily doses of 25% of the LD50 of trichlorfon, but only about 12%
of the LD50 of parathion (DuBois, 1963). DuBois (1963) also
pointed out that ChE inhibition mounted steadily as a result of
daily doses of OMPA and that toxic effects were seen when
inhibition reached about 70%. It is believed that no spontaneous
reactivation of ChE occurs after inhibition by phosphoramidates,
which makes such compounds intrinsically undesirable as pesticides.
Table 7. Rates of spontaneous reactivation (k+3 ) and of
aging (k+4 ) of bovine erythrocyte cholinesterase after
inhibition in vitro a
---------------------------------------------------------
O Rate constants x 104
|| R1 (per min) at pH 7.4
|| / and 37 °C
Enz-O-P substituents
\
R2
R1 R2 k+3 k+4
---------------------------------------------------------
-OCH3 -OCH3 115 14
-OCH3 -SCH3 1170 95
-OC2 H5 -OC2 H5 2.0 2.2
-OC2 H5 -SC2 H5 270 32
---------------------------------------------------------
a From: Clothier et al. (1981).
Dose-effect relationships for delayed neurotoxicity have been
listed (Johnson, 1975b). It has been noted that, as for
cholinergic effects, levels of long-term dosing can be found that
are detectable by a biochemical response at the primary target but
have no clinically- or histologically-observable correlate. The
threshold of the tolerable response is probably a permanent
inhibition or 40 - 50% of NTE (Johnson, 1982a).
6.3.2 Age and sex
It is well-known that the microsomal MFOs and other drug-
metabolizing enzymes are present at comparatively low levels in
neonatal animals, but activity develops to approximately the adult
level early in maturation. Since MFOs are involved in both the
activation and degradation of many organophosphorus pesticides
(section 4.3), the likely net result in terms of LD50 is hard to
predict. One-day-old rats were 9 times more susceptible to
malathion than 17-day-old animals (Mendoza, 1976). The toxicity of
methyl parathion and of parathion for rats decreased from birth
through the developmental period: the decrease was best correlated
with the increasing capacity of the animals to metabolize the
oxygen analogues by both oxidative and hydrolytic pathways (Benke &
Murphy, 1975). The LD50 (ip) of trichlorfon in adult male rats was
reported to be 250 mg/kg body weight, compared with 190 mg/kg in
male weanlings (FAO/WHO, 1972b). Whether this is a significant
difference is not clear. Liver MFO activity fluctuates according
to the hormonal status of female animals. LD50 values quoted for
males and females often differ, but these values generally arise
from different laboratory animals subjected to many variable
factors (including the purity of the test sample). Among several
representative pesticides surveyed for this review, only parathion
showed a marked and apparently real difference in LD50s between the
sexes, the oral LD50 in male rats being 5 - 30 mg/kg body weight,
depending on the solvent, compared with 1.8 - 5 mg/kg in females
(FAO/WHO, 1964).
6.3.3 Nutrition
It is well-known that liver MFO activity can be manipulated by
administering a diet severely deficient in protein. The
consequences can be dramatic in terms of the toxicity of compounds,
such as carbon tetrachloride, which undergo a single
biotransformation step leading to a directly toxic product.
However, as with the age and sex factors discussed above (section
6.3.2), several metabolic steps may be affected. No clear-cut
effects seem to have been reported. Thus, the acute toxicity of
diazinon is greater (up to 2-fold) in rats maintained on a diet,
either very low (4%), or very high (81%) in protein compared with a
standard (29%) protein diet (FAO/WHO, 1971b). A similar increase
in toxicity is seen with naled ( O,O -dimethyl O -1,2-dibromo-2,2-
dichloroethyl phos-phate) (Kaloyanova & Tasheva, 1983). Boyd
(1969) reported that, while increases in toxicity were 2-3 fold for
diazinon, malathion, and demeton, the increase in parathion
toxicity was 7.6 fold, in malnourished rats. Whether the effects
at such extremes were directly due to changes in the
biotransformation of the agent or to the animals becoming generally
unhealthy is not clear.
6.3.4 Effects of impurities and of storage
Insecticides are manufactured and formulated in various ways
and in many countries. There may be significant differences in
these procedures and in the conditions of storage of formulated
products. These factors can influence the nature and extent of
impurities present in the material that is ultimately applied.
Impurities in a pesticide may be of very low toxicity (the
majority), may be toxic in their own right (more or less toxic than
the major component), or they may be potentiators of the toxicity
of another component.
6.3.4.1 Impurities toxic in their own right
(a) Non-anticholinesterase effects
The most dramatic example of an impurity exerting an effect
different from that of the principal component does not come from
the realm of organophosphorus pesticides. The potent toxicant
2,3,7,8-tetrachlorodibenzodioxin (TCDD) may be present in the
herbicide 2,4,5-trichlorophenoxyacetic acid so that, even at a few
mg/kg, the effects of the impurity may dominate the toxicological
response. An analogous non-cholinergic response due to impurities
is not known in anticholinesterase pesticides. Questions have been
asked about the possible mutagenic effects of trimethyl phosphate,
which may be present at a few percent in some technical
preparations of dimethyl phosphates, but the possible exposure in
vivo is limited by the main anticholinesterase response to the
pesticide, and there appears to be no evidence for mutagenic
effects in mammals of any organophosphorus pesticide (section
6.2.1).
(b) Anticholinesterase effects
LD50 values for technical preparations of diazinon have varied
over an unusually wide range. The oral LD50 for the rat was
reported to be 76 - 108 mg/kg body weight in 1964 (FAO/WHO, 1964)
and 250 - 466 mg/kg in 1971 (FAO/WHO, 1971b). A major contributing
factor, according to the latter report, was the presence of highly
toxic pyrophosphates in earlier samples; it was implied that the
impurities had been produced during storage and eliminated by
stabilization (detail unspecified) of formulated material. It
seems likely that the pyrophosphate concerned in this improvement
was monothiono-TEPP with an oral LD50 in mice of about 4 mg/kg body
weight (Margot & Gysen, 1957). Both the sulfotepp and monothiono-
TEPP content of an emulsifiable concentrate of diazinon and its
toxicity increased rapidly when it was stored in tinned-steel
containers instead of in inert-lined aluminium ones (Soliman et
al., 1982). However, in 1979, it was reported that sulfotepp was
also present in many standard and formulated preparations of
diazinon at concentrations ranging from 0.2 to 0.8% and that the
percentage was unrelated to the age of the sample (Meier et al.,
1979). It seems likely that this impurity was formed during the
synthesis of diazinon using diethyl phosphorothiochloridate.
Sulfotepp was 60 - 80 times more toxic than diazinon for the rat,
so that at least one-third of the toxicity of typical diazinon
might be attributed to the impurity. This calculation is probably
an underestimate, since it seems that metabolic disposal of an
impurity is often slowed markedly by competition from the major
component. It can be said that there may be "reverse potentiation"
of the toxicity of the impurity by the major component (diazinon).
Formation of pyrophosphates is implicit in the mode of
synthesis of the many organophosphorus pesticides with a
phosphorochloridate or phosphorothionochloridate as a precursor.
TEPP or its methyl analogue would be unlikely to survive much
aqueous washing during production, but the mixed mono- or disulfo
analogues may well survive, unless deliberately eliminated. It
seems likely that sulfotepp is generally present in parathion
(Diggory, 1977); however, since both the parent and the impurity
have similar LD50s, pure and impure preparations do not differ
significantly. It is a curious fact that the lower the true
toxicity of a pesticide, the more marked may be the effect of an
impurity in changing its toxicity. It might be very rewarding
both to analyse more thoroughly and to reexamine the toxicities of
low-toxicity pesticides such as bromophos which is said to have an
LD50 in various mammals of 3 - 8 g/kg body weight (FAO/WHO, 1973b);
even this low toxicity might be attributable to the impurities
rather than to the pure compound. An analogous situation certainly
pertains concerning the potentiation of malathion (see below).
6.3.4.2 Impurities potentiating the toxicity of the major
ingredient
There does not appear to be any indication that the MFO status
of animals is different after administration of technical grade
organophosphorus insecticides compared with pure. Alterations of
the MFO status of animals because of diet, sex, drugs, etc.,
discussed in sections 6.3.2, 6.3.3, and 6.3.5, are unpredictable in
their effect on the LD50. In contrast, inhibition of the esteratic
capacity of mammals increases the toxicity of pesticides that
depend principally on tissue esterases in their metabolism (section
4.3). Malathion is a notable example of an organophosphorus
pesticide in which the toxicity is enhanced when tissue esterases
are inhibited. Until comparatively recently, such inhibition was
only known in situations where an unrelated organophosphorus ester
was administered to test animals a short time before the malathion.
However, it is now known that several impurities present in most
samples of malathion prepared for use as pesticides, are capable of
inhibiting tissue carboxylesterases. Some of these impurities act
very rapidly and so prevent the normal metabolism of malathion and
potentiate its toxicity. This enhanced toxicity has been expressed
in man. Several hundred spray workers were intoxicated while
spraying certain formulations of malathion in Pakistan, and 5 died
(Baker et al., 1978). Isomalathion and several trimethyl
phosphorothiolates are found in most commercial preparations of
malathion, but the levels depend markedly on the formulation and
on storage conditions. The potentiating power of small amounts of
these impurities are shown in Table 8. Examination of samples of
formulated malathion, known to be unusually toxic, showed a fair
correlation of toxicity only with the percentage content of
isomalathion (Aldridge et al., 1979; Miles et al., 1979). However,
the correlation was imperfect when a large number of samples were
examined and the addition of known further amounts of pure
isomalathion to the formulated samples caused more than the
expected potentiation (Aldridge et al., 1979). The authors
concluded that, although isomalathion contributed the main effect,
there was also significant potentiation by other agents present in
the samples; the chief candidate was O,S,S -phosphorodithioate.
Most unformulated samples of technical grade malathion seem to
have an LD50 for rats in the range of 1500 - 2000 mg/kg body weight.
Such material contains some potentiating impurities (Pellegrini &
Santi, 1972; Umetsu et al., 1977), but has proved acceptable as a
basis for formulated insecticides with little toxic hazard.
However, it is now clear that some formulated samples increase
markedly in both their impurity content and toxicity, when they are
stored at elevated temperatures. Not only are temperature and time
important, but also the formulating agents (Table 9), and almost
half of the malathion lost from Formulation C is apparently
transformed to isomalathion with a massive potentiation, whereas
the increase in toxicity is less marked in Formulations A and B, in
which a much smaller proportion of lost malathion is converted to
isomalathion.
Table 8. Potentiation of acute oral toxicity for rats by impurities
added to malathion
------------------------------------------------------------------------
Compound Amount Potentiation Purified Reference
added added ratio found malathion
(%) used (LD50
mg/kg body
weight)
------------------------------------------------------------------------
isomalathion 0.4 3 10 700 Aldridge et al.
0.6 5 (1979)
2 12
8 25
0.05 3 12 500 Umetsu et al.
0.1 4 (1977)
0.5 6
2 10
O,S,S -trimethyl 0.15 3 10 700 Aldridge et al.
phosphorodithioate 0.3 4 (1979)
0.5 8
1.0 13
2.0 20
0.05 4 12 500 Umetsu et al.
0.2 6 (1977)
0.5 7
0.035 2 8000 Pellegrini &
0.1 3 Santi (1972)
0.2 4
0.5 7
O,O,S -trimethyl 0.3 2 10 700 Aldridge et al.
phosphorothioate 1.3 4 (1979)
0.2 3 12 500 Umetsu et al.
1 4 (1977)
0.2 3 8000 Pellegrini &
0.5 4 Santi (1972)
O,O,S -trimethyl 1.5 2 10 700 Aldridge et al.
phosphorodithioate 5 5 (1979)
1 4 12 500 Umetsu et al.
5 5 (1977)
3.5 2 8000 Pellegrini &
4.5 3 Santi (1972)
------------------------------------------------------------------------
There is much evidence that potentiation of malathion by
extraneous compounds is associated with the inhibition of
carboxylesterases. Using malathion as specific substrate, Talcott
et al. (1979b) showed that isomalathion and O,S,S -trimethyl
phosphorodithioate were potent inhibitors of rat liver and plasma
malathion carboxylesterase, in vitro and in vivo; a partially-
purified sample of carboxylesterase from human liver was also
sensitive to isomalathion (Talcott et al., 1979a).
The same hazard exists with impure samples of phenthoate as for
malathion. Pellegrini & Santi (1972) showed that technical samples
containing 61 - 91% of the principal ester had LD50s (rat oral) of
78 - 243 mg/kg body weight, while a purified preparation (98.5%)
had an LD50 of 4700 mg/kg, though its toxicity for insects
increased approximately in proportion to the purity. The principal
potentiating impurities were the S -methyl isomer and the identical
trimethyl phosphorothiolates found in malathion. For phenthoate,
as for malathion, the vulnerability to potentiation lies in the
presence of the hydrolysable ethoxycarbonyl ester bond which, in
pure samples, is the key to low mammalian toxicity.
Table 9. Effects of formulating agents and of storage
time and temperature on composition and toxicity of
malathion (50% wdp)a
----------------------------------------------------------
Storage conditions Composition (%) Oral LD50
Temperature Time Malathion Isomalation (rat)
(°C) (days)
----------------------------------------------------------
Formulation A
0 48.0 0.38 2800
38 60 45.5 0.37 2230
90 44.2 0.49 1740
55 6 47.6 0.30 2520
13 46.4 0.37 1760
90 1 40.9 0.69 950
Formulation B
0 48.8 0.18 2540
38 60 47.2 0.79 1130
90 46.5 0.81 1330
55 6 45.2 0.67 1200
13 43.6 0.55 1170
90 1 39.9 0.32 1900
Formulation C
0 50.6 0.61 2660
38 90 44.9 3.7 590
55 6 46.2 3.4 535
13 43.7 3.5 555
----------------------------------------------------------
a From: Miles et al. (1979).
The presence of the S -methyl isomer (0.32%) in a commercial
fenitrothion formulation was shown by Miles et al. (1979). The
concentration increased to > 1% during accelerated storage tests,
but there was no concomitant increase in the toxicity of the
formulation. This observation on a compound not heavily dependent
on esterases for the primary step of detoxication, points to the
need to consider biochemical mechanisms in assessing possible
hazards. There need be no general concern about the presence of
small amounts of isomers in organophosphorus pesticides. The
presence of isoparathion in parathion is well-known, but there is
no suggestion of any marked change in toxicity brought about by
this impurity.
Some organophosphorus pesticides contain carboxylamide bonds
rather than carboxyester. These include dimethoate, dicrotophos,
monocrotophos, phosphamidon, and acephate. There appears to be
little evidence that impurities in commercial formulations of any
of the above pesticides markedly alter the toxicity, apart from a
small (1.6x) decrease in the mammalian toxicity of acephate, after
storage for 6 months at 40 °C; the insecticidal activity of the
compound was unchanged (Umetsu et al., 1977). During the storage
period, the concentration of various impurities changed, but no
relationship between these changes and altered toxicity was
obvious.
6.3.5 Effects of other pesticides and of drugs
All organophosphorus and carbamate insecticides exert their
acute toxic action by attack on the AChE. Thus, it follows that
exposure to more than one such pesticide will usually produce at
least an additive effect. Besides this simple effect, other
pesticides or drugs may also influence the toxicity of an
individual organophosphorus pesticide by interfering with its
metabolism, activation, and disposal.
Not all organophosphorus pesticides and probably no carbamate
pesticides inhibit tissue carboxylesterase to potentiate malathion
in the manner discussed in section 6.3.4. However, like all other
enzymes, the carboxylesterases have their own structure-activity
pattern: this has not been worked out in a systematic fashion. It
is clear that, with some compounds, profound inhibition of liver
carboxylesterase can be achieved without inhibition of AChE
sufficient to cause signs of poisoning. This class includes many
thioalkyl esters such as the trimethyl esters and also S,S,S -tri- n
-butyl phosphorotrithioate (DEF), which are potent potentiators of
malathion toxicity via carboxylesterase inhibition. EPN is a
phenylphosphorothioate insecticide that also acts in this way.
Tables 10 and 11 show examples in which measurements of effects on
tissue carboxylesterases and AChE are of value in predicting the
potentiation of malathion toxicity (Murphy, 1969).
Table 10. Comparison of enzyme inhibition caused by
several pesticidesa
---------------------------------------------------------
Insecticide Dietary concentration (mg/kg) resulting in
(period) 40 - 60% inhibition of:
Red cell- Liver- Plasma-
cholinesterase malathionase malathionase
---------------------------------------------------------
Parathion 3 5 5
(7 days)
Fenchlorphos 500 30 30
(7 days)
Malathion 500 100 500
(30 days)
---------------------------------------------------------
a From: Murphy (1969).
Table 11. Effect of feeding fenchlorphos on in vivo
anticholinesterase activity of malathiona
-------------------------------------------------------
Fenchlorphos Malathion Inhibition of brain
concentration challenge dose AChE 1 h after
in diet (mg/kg) (mg/kg ip) challenge (%)
-------------------------------------------------------
0 200 13
30 0 1
30 200 61
-------------------------------------------------------
a From: Murphy (1969).
Potentiation of the toxicity of organophosphate compounds for
mammals not containing a carboxylester function, does not appear to
be a significant hazard, though it is possible that potentiation of
some carboxylamide pesticides by an analogous inhibition of tissue
amidases may occur; certainly, EPN potentiates the toxicity of
dimethoate (El-Sebae, 1980). Potentiation of the toxicity of
organophosphorus pesticides for insects by inhibition of MFO
activity is well-known, and many potent synergists are used in
agriculture for this purpose (Wilkinson, 1971), but much less has
been reported concerning similar effects in mammals. This may
reflect the greater versatility of mammals compared with insects in
disposing of organophosphorus esters (section 4.3). Competition
for one metabolic route within the animal often does not greatly
alter its total capacity to deal with a foreign compound.
Keplinger & Deichmann (1967) combined pairs of various pesticides
in proportion to their oral LD50s, determined individually, and
then measured the oral toxicity of the mixtures in rats or mice.
They calculated a ratio of expected LD50/observed LD50 where
"expected LD50" was the sum of half the LD50 value of each of the 2
constituents. Their study included 7 chlorinated hydrocarbons,
the carbamate carbaryl, and 5 organophosphates including diazinon,
malathion, and parathion. With a "no-effect" ratio of 1.0, they
considered measured ratios greater than 1.75 or less than 0.57 as
probably significant of real effects. In a number of cases, less
than additive effects were noted for combinations of a chlorinated
hydrocarbon and an organophosphate, e.g., aldrin + diazinon (0.55),
DDT + malathion (0.54), toxaphene + carbonylfenthion (0.54): this
might well be expected if the mode of toxicity were different and
metabolic pathways were not markedly altered. The only cases of
potentiation involving organophosphates were also not surprising.
A triple combination of parathion, malathion, and chlordane had a
ratio of 1.99. This effect was probably due to simple potentiation
of malathion by parathion, since chlordane alone did not potentiate
either organophosphorus compound. Mixtures of Aramite (sulfurous
acid 2-chloroethyl 2-[4-(1-1-dimethylethyl)-phenoxyl-1-methylethyl
ester) with several organophosphates in mice had ratios of 1.86 -
2.14. However, since the LD50 of Aramite in mice is high (2000
mg/kg body weight), it may be that the 500 mg/kg administered in a
mixture was absorbed more efficiently and was therefore more
effective proportionately than the much higher LD50 dose of Aramite
alone.
As noted above, aldrin had little effect on the toxicity of
organophosphorus insecticides, when administered at the same time.
However, a number of chlorinated hydrocarbons (aldrin, DDT,
chlordane, etc.) are well-known as stimulators of MFO activity in
(principally) the mammalian liver. This activity increased
markedly during a period of a few days after dosage, and
pretreatment of mice with aldrin (16 mg/kg body weight), 4 days
before a challenge dose of 6 different organophosphates, markedly
reduced (up to 5-fold) the toxicity of each (Murphy, 1969).
Similar results were obtained with other classes of MFO inducers
such as phenobarbital. Murphy points out that the mechanism of
these effects probably includes stimulation of liver
carboxylesterase as well as MFO.
From the observations above, it appears that simple mixing of
an organophosphorus insecticide with a chlorinated hydrocarbon is
unlikely to adversely influence the acute toxicity for mammals, as
expressed by LD50 value. However, administration of DDE at 55
mg/kg diet to adult male Japanese quail led to an increasing
susceptibility to challenge doses of parathion (2.5 mg/kg)
administered orally; mortality in these birds increased from 0 to
30% after 1 week of feeding and 60% after 3 weeks (Ludke, 1977).
The problem of potentiation by some organophosphates of the
toxicity of pesticides containing carboxyl ester bonds may be
significant. This has been demonstrated for malathion in animals
(section 6.3.4) and in man (section 7.1.3). It may also be a
problem for pyrethroid insecticides for most of which degradation
by mammalian carboxyesterases is a significant detoxification
pathway (Miyamoto, 1976).
6.3.6 Species
No clear ranking of species sensitivity to organophosphorus
pesticides as a class can be given. A general impression is that
mice, hamsters, and guinea-pigs may be more sensitive than rats,
with respect to a number of compounds, but the converse is seldom
true. However, most available data have been produced with little
reference to conditions of husbandry, diet, hormonal status, etc.,
so that only very marked differences, which do not usually seem to
exist, would emerge. Birds tend to be more sensitive to
organophosphorus pesticides (Schafer, 1972) and amphibians less
sensitive than mammals. It has been suggested, but not confirmed,
that these differences might be due to differences in the activity
of enzymes in species that hydrolyse organophosphorus compounds and
thereby contribute to detoxification.
The toxicity of several organophosphorus pesticides for
different species seems to be inversely related to the activity of
the plasma A esterase, which degrades the pesticide oxon. Such
activity is considerably lower in birds than in mammals (Machin et
al., 1978). When 14 avian species were compared with 5 mammalian,
the average plasma activity against pirimiphos-methyl oxon was 170
times less, and that against paraoxon, at least 13 times less
(Brealey et al., 1980). Differences in liver microsomal oxidative
activity involved in the metabolism of several organophosphorus
pesticides by mammals and birds were less profound, though fish
liver was less active (Miyamoto & Ohkawa, 1978).
A multitude of factors contribute to the great difference
between the oral toxicity of chlorofenvinphos for the rat (10 mg/kg
body weight) and that for the dog (> 5000 mg/kg). These include
efficiency of absorption, at least 2 metabolic detoxification
processes, the rate of uptake by the brain, and a 7-fold difference
in the sensitivity of the brain AChE to this compound in the 2
species (Hutson & Hathway, 1967; Donninger, 1971).
Adult hens, cats, dogs, and larger farm animals are all
susceptible to organophosphorus delayed neurotoxicants (Davis &
Richardson, 1980; Johnson, 1982a). There is no clear ranking of
dose-sensitivity, though hens seem to be most uniformly responsive.
The full clinical response is not easily seen in laboratory
primates and rodents, though morphological damage may be detected
(section 6.1.1.2).
6.3.7 Other factors
The effects of solvents on chemical stability and isomerization
reactions were noted in section 4.5. The effects of formulation
agents on stability and, therefore, on the toxicity of malathion
have also been noted previously (section 6.3.4). It is likely that
percutaneous absorption will be greater for liquid formulations
than for powders but that powders may adhere longer thereby
enhancing an effect, if proper hygiene is not observed. A number
of examples are quoted by El-Sebae (1980), in which formulated
pesticides were more toxic than the technical preparation (Table
12). In many cases, this is due to the fact that the solvent in
the formulation facilitates the uptake of the pesticide into the
body. The toxicity of other components of the formulation may play
a role (especially in the case of pesticides of very low toxicity,
such as tetrachlorvinphos) (Table 12), as well as potentiation.
El-Sebae noted that, in some cases, the I50 for inhibition of ChEs
in vitro by these compounds differed. This could be relevant in
the case of the directly-active oxon-type pesticide tetrachlorvinphos,
but in cases where the test was performed with a thioate, the
anticholinesterase activity would depend almost entirely on trace
oxon impurities, which are often destroyed rapidly in vivo and
contribute little to toxicity compared with the bulk of oxon
produced metabolically.
Table 12. Comparative toxicity for mice of
some technical and formulated
organophosphorus pesticidesa
--------------------------------------------
Pesticide 24-h oral LD50
(mg/kg body weight)
Technical Formulated
--------------------------------------------
phosfolanb 12 11
chlorpyriphos 140 60
leptophos 162 83
tetrachlorvinphos 5000 1800
--------------------------------------------
a From: El-Sebae (1980).
b (diethoxyphosphinothioyl)dithioimidocarbonic
acid, cyclic ethylene ester.
6.4 Acquisition of Tolerance to Organophosphorus Insecticides
This topic has been discussed in section 6.3.1, where it was
noted that when AChE levels were reduced progressively over a
number of days or weeks, animals showed cholinergic signs of
poisoning which, in animals that survived, decreased in severity
and sometimes disappeared completely, though ChE inhibition was
maintained. This phenomenon is separate from the fact that
permanent inhibition of 30 - 50% is ineffective in producing
measurable symptoms. The basis for acquired tolerance is not fully
known, though a "down regulation" in the muscarinic ACh receptor is
thought to be a contributary cause. This involves both reduced
sensitivity and reduced numbers of receptors (Costa et al., 1982).
6.5 Therapy of Experimental Organophosphorus Poisoning
The understanding of the mechanism of acute toxicity of
organophosphorus pesticides has provided the basis for rational
therapy. The effects of inhibition of AChE, as described in
section 6.1.1, are common to all organophosphorus pesticides
intoxications. However, the speed of onset and the rate of unaided
recovery from sub-lethal doses vary greatly, depending on the
chemical nature of the pesticide, the route of exposure, and on
whether this exposure was a sudden overwhelming dose or a drawn-
out process.
Factors leading to a slow onset of symptoms include:
(a) Slow absorption or metabolic activation: this is often
associated with extremely low solubility and therefore
with the presence of large hydrophobic groups in the
ester molecule; pesticides such as haloxon, chlorpyrifos,
and leptophos are of this type;
(b) Persistence in the system of a comparatively stable
inhibitor of ChEs. This could be as a low concentration
of an active inhibitor such as demeton- S -methyl
sulfoxide or high concentrations of a weak inhibitor such
as methamidophos.
Factors leading to rapid clearance of symptoms include:
(a) Rapid clearance of the pesticide and its active agents,
as with trichlorfon and dichlorvos; rapid clearance
occurs also with nerve agents such as soman and sarin,
which have been much used in studies on therapy in
experimental animals.
(b) A slow rate of aging of inhibited AChE giving opportunity
for reactivation (spontaneous or induced) to occur;
diethyl phosphorylated AChE ages more slowly than
dimethyl or diisopropyl.
(c) Rapid spontaneous reactivation of inhibited AChE, such as
occurs after inhibition by all dimethyl or bis-2-
chloroethyl phosphates. In this case, the possibility of
reinhibition by a persistent compound will affect the
picture.
The factors noted above, which influence speed of onset and
remission of effects, influence the prognosis for response to
therapy but do not markedly alter the nature of optimal treatment.
Maximum benefit comes from combined treatment with an
anticholinergic drug (usually atropine) plus a reactivator of
inhibited ChE (an oxime) with diazepam, and also artificial
respiration. The effects of the components will be discussed
individually below.
6.5.1 Palliation
Artificial respiration alone can be very effective in
maintaining life, since the primary cause of death in
organophosphorus poisoning is respiratory failure (section 6.1.1).
Such treatment gains time for the processes, natural or imposed,
that lead to the return of sufficient ChE activity to maintain
life.
6.5.2 Antagonism of effects of ACh
Atropine antagonizes many of the peripheral muscarinic effects
of excess ACh and also some central effects. However, there was no
correlation between the peripheral anticholinergic activity of a
range of atropine-like drugs and their capacity when used alone (or
in conjunction with oximes) (section 6.5.3) to protect against the
lethal effects of sarin (Coleman et. al., 1962; Brimblecombe et.
al., 1970). Moreover, the ranking of the therapeutic efficacy of
atropine analogues varied according to the test species (rat,
mouse, or guinea-pig), and all effects were small (protective ratio
< 1.5) in mice and guinea-pigs, but much larger and more variable
(1.2 - 9.3) in rats. The ranking changed yet further when the drug
was combined with oxime P2S (see below), though the protective
ratio with some compounds rose to 18 - 24 in guinea-pigs, 9 - 80 in
rats, but only 1.8 - 6.3 in mice. These confusing effects are now
thought to be at least partially due to an anticonvulsant effect
contributed variously by the atropine analogues. Anticonvulsants
often supplement the effects of atropine or of combined
atropine/oxime therapy (see below). In particular, diazepam
(valium) is known both to raise the LD50 and speed recovery in some
cases (Johnson & Wilcox, 1975). These authors implied that the
mechanism might be partly direct antagonism to some central effects
of ACh and partly indirect. When diazepam is included in the
therapeutic package, there appears to be little evidence that
alternative anticholinergic drugs to the well-proved atropine are
superior (Green et al., 1977). It has been reported that, in
prophylaxis against the toxicity of DFP in mice, the protection
factor was 28 when atropine and obidoxime were used but 180 when
Dexetimide (a drug with strong central anticholinergic activity)
was substituted for atropine. The particular advantage claimed for
this drug was that, in the therapy of rabbits intoxicated with up
to 60 x LD50 of paraoxon or 80 x LD50 of DFP, one single
intravenous injection of Dexetimide (8 - 16 mg/kg body weight) was
effective in conjunction with obidoxime, whereas repeated doses of
atropine were necessary (Bertram et al., 1977). Dexetimide is used
for the treatment of parkinsonism and is available commercially.
However, Dexetimide has not been evaluated in conjunction with
diazepam or compared with atropine plus diazepam, and no details
were given of the hazards of its use and side-effects in control
animals.
6.5.3 Reactivation of inhibited AChE
As indicated in section 4.5.1, inhibited ChEs can be
reactivated in vitro by treatment with appropriate nucleophilic
agents, of which salts of the oxime N- methylpyridinium-2-aldoxime
are the most commonly used (the chloride is known as pralidoxime
and the methanesulfonate as P2S). In some countries, obidoxime
(ToxogoninR ), which is a bis-quaternary oxime, is recommended at
slightly different doses than pralidoxime, but the mode of action
is similar. The scope for further improvement in the design of
therapeutic oximes is discussed by Gray (1984).
In vivo, there are 2 limitations to the benefits to be
obtained from the use of these agents:
(a) Access
The quaternary oximes are thought not to cross the blood-brain
barrier easily (Taylor, 1980). However, some experimental
work, summarized by Lotti & Becker (1982b), suggests that there
may be limited access, and this may have a significant, albeit
small, effect in reversing inhibition of AChE to improve the
clinical state. Other more direct beneficial action of the
oximes directly at synapses in the medullary respiratory centre
cannot be ruled out. The prompt improvement in the level of
consciousness observed and in the EEG of an intoxicated child
when iv infusion of 2-PAM was commenced (Lotti & Becker, 1982b)
also seemed to indicate that there was some access to important
brain regions. It has been claimed that obidoxime (25 mg/kg
body weight) injected intraperitoneally in rats, 5 min after a
dose of armin, was effective in reactivating 33% of the
inhibited AChE of the ponto-medullary region, which contains
the centre for control of respiration (Vasic et al., 1977).
However, there were no controls appropriate to disprove the
alternative explanation that the oxime had altered the
circulating level of armin (by direct destructive interaction
or otherwise), which, although interesting, is unlikely to be
relevant to therapy instituted later after dosing, nor is such
destruction-protection unique to toxogonin.
(b) Aging of inhibited AChE
As noted in section 4.5.1, inhibited AChE is converted by a
time-dependent reaction to a form resistant to reactivators.
Thus, oxime therapy becomes less effective with time after
poisoning. The rate of aging of dialkyl-phosphorylated AChE is
Me > IsoPr > Et (O'Brien, 1967), but little has been published
on the rate of aging when phosphonyl groups derived from
pesticides are attached to the enzyme. When one or both of the
residual alkyl groups are attached to phosphorus through sulfur
rather than oxygen, the rates of both spontaneous reactivation
and of aging of inhibited bovine erythrocyte AChE are markedly
increased (Clothier et al., 1981). Ethoprophos is one of the
newer pesticides that contain such a residual alkylthio group;
no published studies are known of therapy after poisoning with
this or related pesticides.
6.5.4 Efficacy of therapy
As reported above, the combination of atropine plus oxime is
far more effective in most cases than the mere summed effects.
This is because the peripheral neuromuscular junctions
(particularly the diaphragm) and the sympathetic ganglia, where
oximes reactivate AChE, are nicotinic and are unaffected by
atropine, so that separate aspects of intoxication are treated by
the two agents. There is speculation that some oximes may exert a
protective effect by acting as depolarizing agents at the
neuromuscular junction. This may also account for the slight
therapeutic effects of some analogues of toxogonin which do not
have any nucleophilic oxime group or reactivating power (Schoene &
Oldiges, 1973). Diazepam, also, is ineffective, except in
combination with atropine and oxime.
Persistence of the toxic agent may interfere with successful
therapy. Thus, single doses of atropine + oxime were of only
marginal efficacy in altering the LD50 of isofenphos in rats
(FAO/WHO, 1982b). However, when therapeutic doses were given
repeatedly at about 12-h intervals, for 2 - 3 days, the LD50 for
rats was raised about 4-fold; for hens, the increase was 15-fold
(Wilson et al., 1984). It appears that the failure of one-shot
therapy in this case was due to persistence of the toxic agent
rather than to a different mode of intoxication or to formation of
an inhibited form of AChE that resisted reactivation. The same
situation may pertain for profenofos intoxication, which appears
not to respond well to therapy (El-Sebae, personal communication,
1985).
7. EFFECTS ON MAN
7.1 Acute Cholinergic Poisoning
The clinical picture of organophosphorus intoxication results
from accumulation of ACh at nerve endings. The syndrome is
described in detail in several major references (Namba et al.,
1971; Kagan, 1977; Taylor, 1980; HMSO, 1983; Plestina, 1984). The
symptoms can be summarized in three groups as follows:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lachrymation;
- pinpoint pupils, bronchoconstriction, abdominal
cramps (vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles and, in more severe
cases, of diaphragm and respiratory muscles; and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, restlessness, and anxiety;
- mental confusion, convulsions, and coma; and
- depression of the respiratory centre.
All these symptoms can occur in different combinations and can
vary in time of onset, sequence, and duration, depending on the
chemical, dose, and route of exposure. Mild poisoning might
include muscarinic and nicotinic signs only. Severe cases always
show central nervous system involvement; the clinical picture is
dominated by respiratory failure, sometimes leading to pulmonary
oedema, due to the combination of the above-mentioned symptoms.
Clinical diagnosis is relatively easy and is based on:
(a) medical history and circumstances of exposure; and
(b) presence of several of the above-mentioned symptoms,
in particular, bronchoconstriction and pinpoint
pupils not reactive to the light. Pulse rate is not
of diagnostic value, because the AChE effects on the
heart reflect the complex innervation of this organ.
On the other hand, since changes in the conduction
and excitability of the heart might be life-
threatening, monitoring should be performed.
Confirmation of diagnosis is made by measurement of AChE in RBC
or plasma-pseudoChE, and, also, of the dibucaine number (to rule
out genetic deficiencies).
Measurements of blood-ChE during therapy are also useful in
assessing the treatment with oximes, though there might not be a
correlation between the severity of symptoms and the degree of ChE
inhibition: comparison should be made with pre-exposure levels,
wherever possible.
Chemical analysis of body fluids (urine, blood, gastric lavage)
should be made in order to identify the compounds that caused
poisoning.
7.1.1 Methods for assessing absorption and effects of
organophosphorus insecticides
As well as assessments of general health and behaviour, the
study of the effects of this class of pesticides is favoured
compared with that of some other classes since the basic
biochemical mechanisms (inhibition of esterases) are known for the
major toxic effects. Biochemical and neuro-physiological
techniques, relevant to the principal effects of all the compounds,
have been established. Identification of the monobasic acid type
of urinary metabolite, which is commonly produced, is an indicator
of exposure rather than of an effect, but it seems appropriate to
outline the technique in this sub-section. Wherever possible, test
findings should be compared with pre-exposure measurements on the
same individual.
7.1.1.1 Analysis of urine as a means of monitoring exposed
populations
As previously discussed in section 4, organophosphorus
pesticides may undergo hydrolysis in vivo to yield substituted
phosphoric acids that are subsequently excreted in urine. Advances
in gas chromatography and combined gas chromatography/mass
spectrometry (CG/MS) have made it possible to analyse the urine of
exposed persons for the presence of appropriate metabolites. It is
usually necessary to preserve the sample by the addition of
chloroform, to concentrate or extract the metabolite(s), and to
convert them to suitably-volatile derivatives that can be detected
by GC. Obviously, access to a well-equipped analytical laboratory,
capable of the quick processing of samples, is a necessary factor
if monitoring by urine analysis is proposed. However, in some
cases, simpler and sensitive colorimetric tests are available for
screening the urine of exposed persons. Thus, 4-nitrophenol can
be measured directly in the urine of workers exposed to parathion
(Wolfe et al., 1970).
Consideration of the concentration of metabolite(s) in the
urine can be helpful in determining patterns of exposure, and these
concentrations can be calibrated against the effects on AChE for a
particular pesticide. However, the time-course and peak of
excretion of metabolites appears to vary according to dose (Bradway
et al., 1977), so that serial sampling and analyses of urine are
desirable. Levels of metabolite alone cannot be considered a guide
to hazard. This is obvious when it is realized that pesticides
that have very different toxicities may yield identical acidic
metabolites. Thus, the level of metabolites in urine, after
exposure to sufficient amounts of the very toxic parathion-methyl
to depress blood-AChE to 50%, will be much lower than that of the
identical metabolites, following exposure to the related
fenitrothion, which is about 40 times less toxic.
7.1.1.2 Biochemical methods for the measurement of effects
AChE is present in human erythrocytes (RBC) and is the same as
the enzyme present in the target synapses. Thus, levels of AChE in
RBC are assumed to mirror the effects in the target organs.
However, it must be borne in mind that this assumption is only
correct when the organophosphate has equal access to blood and
synapses. In the case of acute poisoning, a high inhibition of
RBC-AChE is pathognomonic, but, in the follow-up of the
intoxication, it might not be correlated with the severity of
symptoms. In the case of repeated exposures, additional
difficulties in interpretation arise from possible development of
tolerance. However, monitoring of pre- and post-exposure levels of
AChE in RBC gives a good measure of the effects of an exposure
(Kaloyanova, 1975). In cases where the pre-exposure AChE level is
not known (as in accidental poisoning), reference can be made to a
mean population AChE activity. Blood-plasma contains a related
enzyme called ChE or pseudoChE, which contributes to the whole-
blood enzymatic activity; the contribution of plasma-ChE in assays
of AChE will depend on the type and concentration of the substrate
used. PseudoChE has no known physiological function and can be
inhibited selectively by some compounds without causing a toxic
response. The sensitivities of AChE and ChE to inhibitors differ,
so that measurements of the ability of whole-blood samples to
hydrolyse the usual analytical substrates give only an approximate
estimate of the activity of the erythrocyte-AChE. However, under
many kinds of field conditions, procedures using whole blood, are
more practical than those using separated erythrocytes. Quite
commonly, pseudoChE is more sensitive to inhibitors. Thus, if
separation of plasma and erythrocytes is possible, prior to assay,
an indication of exposure can be obtained by assay of pseudoChE
only. Examples of selected organophosphorus insecticides, arranged
according to their ability to inhibit preferentially either plasma
or red cell-ChE in man, are given in Table 13 (Hayes, 1982).
Table 13. Selected organophosphorus insecticides arranged
according to their ability to inhibit either plasma- or
red cell-cholinesterase in mana
---------------------------------------------------------
Plasma enzyme more inhibited RBC enzyme more inhibited
---------------------------------------------------------
Chlorpyrifos Dimefox
Demeton Mevinphos
Diazinon Parathion
dichlorvos Parathion-methyl
malathion
Mipafox
Trichlorphon
---------------------------------------------------------
a Modified from: Hayes (1982).
ChE assay procedures vary greatly in sophistication, but the
most satisfactory is that based on the procedure of Ellman et al.
(1961). A field method and kit for whole blood- and plasma-ChE
determination have been developed (WHO, 1984b). Quick methods exist
for the determination of ChE in serum using paper tests (Izmirova,
1980) and for the colorimetric determination of ChE in whole blood
(Tintometer): these may be useful in the differential diagnosis of
organophosphate poisoning. Interpretation of the test results is
discussed in section 7.1.2.
The potential for delayed neuropathic response to an
organophosphorus ester can be predicted by the assay of the
esteratic activity of the target protein (NTE), in autopsy brain
samples from dosed adult hens (section 6.1.2). It has been shown
(Dudek et al., 1979; Richardson & Dudek, 1983) that a low level of
similar enzyme activity resides in lymphocytes and that there may
be correlations under some circumstances between neurotoxic dose
and available lymphocyte enzyme activity. The possibility of
monitoring exposed individuals by means of human lymphocyte or
platelet NTE activity is being explored (Lotti et al., 1983;
Bertocin et al., 1985; Maroni & Bleeker, 1986).
7.1.1.3 Electrophysiological methods for the study of effects
Electromyographic (EMG) studies using non-invasive surface
electrodes have been claimed to give sensitive indications of
exposure to organophosphorus pesticides, even in situations where
blood-ChE activity has returned to normal levels (Jager et al.,
1970; Roberts, 1976). The method requires electro-physiological
equipment and a very skilled practitioner. There is still
considerable doubt about the validity of some published studies.
Reproducibility is known to be very sensitive to local factors such
as temperature of the skin, and conflicting results have been
published, some of which show small increases and some, small
decreases in the ampli-tude of evoked muscle action potential, in
response to nerve stimulation. These findings have been reviewed
by LeQuesne & Maxwell (1981), who noted that changes that have been
reported tended not to be dose-related. In addition, they
evaluated the technique under controlled circumstances. In a
treatment to eradicate parasitic schistosomes, 55 children were
dosed orally with trichlorfon, 3 times, at 2-weekly intervals, at
doses that measurably depressed blood-ChE (mean 50%), but were not
enough to cause overt toxic effects, apart from mild cramps and
diarrhoea in a few cases. Only 3 children showed a significant
alteration in electromyographic response. Shortly after the last
(and highest) dose of 10 mg/kg body weight, 3 children developed
repetitive activity recorded over the thenar muscles following
supramaximal stimulation of the median nerve at the wrist. The
activity consisted of a small potential at the end of the main
muscle response and was characterized by being abolished by a
second stimulus 30 or 80 milliseconds after the first, or by
maximum voluntary contraction for 10 seconds; the amplitude of the
response to the second stimulus was not reduced. These
characteristics are necessary criteria that distinguish (these)
dose-related responses from pre-existing natural (and
idiosyncratic) responses, which can otherwise confuse EMG studies
in a population. Changes in amplitude measured on 52 control
subjects (mean 13.8 ħ 2.5 SD), on 2 occasions (2 weeks apart),
ranged from +5 to -3 mV. Thus, EMG does not appear to give a
highly sensitive measure of exposure to an ingested
organophosphorus compound.
7.1.2 Monitoring studies
Measurement of whole blood-AChE is the most widely adopted
method for monitoring the effects of occupational exposure to
organophosphorus insecticides. Physiological variations in blood-
ChE levels occur in a healthy person and are seen among a
population. It has been estimated that the coefficient of
variation for AChE activity in samples from an individual is 8 -
11%, and that a decrease of 23% below pre-exposure level may,
therefore, be considered significant. If the average of several
pre-exposure values were available, then a decrease of 17% would be
significant. It has been recommended that, if measured activity is
reduced by 30% or more of the pre-exposure value, AChE measurements
should be repeated at appropriate intervals to confirm the results.
Depressions of AChE or ChE in excess of 20 - 25% are considered
diagnostic of exposure but not, necessarily, indicative of hazard.
Depressions of 30 - 50% or more are considered indicators for
removal of an exposed individual from further contact with
pesticides until levels return to normal. Work procedures and
hygiene should also be checked (Zielhuis, 1972; WHO, 1975; CEC,
1977; Kaloyanova et al., 1979; Plestina, 1984).
Urinary metabolites have been monitored as a means of comparing
the efficiency of absorption of a pesticide by different routes.
The reports of the annual Joint FAO/WHO Working Parties,
mentioned previously, contain summaries of numerous controlled
exposure studies. No cases appear to be known of significant
clinical effects in man in the absence of depression of plasma- or
erythrocyte-ChE levels. No-observed-adverse-effect levels have
been calculated on this basis, where the data are available, or
have been estimated for man by extrapolation of the available data
for exposed animals.
7.1.3 Retrospective studies of populations exposed to
organophosphorus pesticides: acute and long-term exposure
Many thousands of cases of acute poisoning by organophosphorus
pesticides have been recorded (Namba et al., 1971). The majority
have been due to parathion and methyl parathion. Thus, Namba (1974)
in a discussion of the relative toxicities of parathion and
malathion, quoting Japanese Government statistics for the 7 years
1958-62, 1966, and 1967, stated that there were 3311 accidental or
occupational poisonings due to parathion including 188 deaths,
while for malathion, the numbers were 63 and 10, respectively. He
noted that the difference was not due to the restricted use of
malathion.
In the context of this general introduction, no descriptions
and breakdown will be given of retrospective studies of populations
exposed to organophosphorus pesticides. Such figures are relevant
to individual substances and will be given in the appropriate
Environmental Health Criteria.
It is generally thought that the only long-term effects
attributable to overt or subclinical acute intoxication with
organophosphorus compounds, or to prolonged low-level exposure,
are behavioural (rather doubtful), and delayed-onset neuropathy in
the case of certain compounds; these are dealt with in section 7.2.
>7.2 Other Effects on the Nervous and Neuromuscular System Due to
Acute or Long-Term Exposure
7.2.1 Delayed neuropathic effects
The characteristics of this disorder are given in section
6.1.2. The agents most commonly causing delayed neuropathy in man
are triaryl phosphate esters used in, e.g., hydraulic fluids; these
do not have any AChE activity and are not pesticides. In Table 14,
organophosphorus pesticides are listed for which there is
reasonable evidence that they have caused delayed neuropathy in
man.
Table 14. Organophosphorus pesticides reported to cause
delayed neuropathy in man
--------------------------------------------------------
Pesticide Number Reference
of cases
--------------------------------------------------------
mipafox 2 Bidstrup et al. (1953)
leptophos 8 Xintaras et al. (1978);
FAO/WHO (1979b)
methamidophos 9 Senanayake & Johnson
(1982)
trichlorphon many Shiraishi et al. (1977);
Hierons & Johnson (1978);
Johnson (1981a)
trichlornat 2 Jedrzejowska et al.
(1980); Willems (1981)
EPN 3a Xintaras & Burg (1980)
Chlorpyrifos 1 Lotti & Moretto (1986)
--------------------------------------------------------
a Moderate effects only and possibly other
etiological factors.
The cases with mipafox involved a single occupational exposure
to a compound that was developed before delayed neuropathy was a
recognized hazard. The cases with EPN and leptophos arose through
repeated occupational exposure with inadequate precautions.
Apparently cholinergic effects were often experienced, but not at
the level of severe poisoning. A few cases with methamidophos and
trichlorfon involved substantial occupational exposure, which
caused severe acute poisoning prior to the development of
neuropathy, but the majority of cases involved accidental or
deliberate ingestion of quantities that might well have been fatal
but for medical intervention. The fact that most of the cases
listed are due to exposure to phosphonates or phosphoramidates is
in line with the structure-activity relationships listed in section
6.1.2.
The value of measuring the neuropathy target esterase of human
lymphocytes as a predictive monitor was proposed by Dudek et al.
(1979) and Richardson & Dudek (1983). Lotti et al. (1983) reported
occupationally related changes in the lymphocyte NTE in spraymen
during seasonal spraying of DEF, but overt neuropathy was absent.
In a case of self-poisoning by a mixture of pesticides including
chlorpyrifos (about 20 g diluted in petroleum distillates),
Osterloh et al. (1983) noted that signs of cholinergic poisoning
were limited and they attributed death to the effects of
chlorophenoxyacetic acids. Brain esterases were not inhibited at
autopsy, but erythrocyte and peripheral nerve AChE levels were
about 22% of normal and nerve NTE was about 30%. This substantial
inhibition of NTE suggested that treated survivors of severe
poisoning by chlorpyrifos might well develop delayed neuropathy.
This prediction was confirmed in a recent case of self-intoxication
with chlorpyrifos (estimated dose 300 mg/kg body weight) in which
very low levels of lymphocyte-NTE were found, 30 days after
intoxication and after recovery from very severe cholinergic
effects. Typical moderate polyneuropathy developed in the
following days (Lotti & Moretto, 1985).
Allegations have been made against a few other organophosphorus
insecticides including malathion, omethoate, and parathion, though
many accidental and intentional poisonings by these agents have not
had any neuropathic sequelae. Experimental evidence against a
neuropathic potential in these compounds is strong (section 6.1.2).
However, in view of the serious paralytic effects involved, the
evidence adducing that these pesticides were the causal agents is
reviewed below.
(a) Malathion
Two alleged cases can be discounted. Petry (1958) reported a
case in which a physician contaminated himself frequently with
chlorinated hydrocarbon pesticides during day-long gardening
activities, about once per week, over a 10-year period. In 1954,
he commenced using 6% malathion in a "hose-on" device for garden
pest control and, in 1955, he commenced using 50% malathion in a
hand spray, both indoors and outside. He soon developed signs of
chronic anticholinesterase poisoning (generalized weakness and
tremor, irritability, difficulty in focusing). He eventually
collapsed and his general condition improved in hospital. However,
he continued to experience generalized weakness and particular
weakness in the right shoulder girdle, right serratus anterior, and
both peroneal muscle groups, and these symptoms persisted to some
extent for over a year. The distribution of these symptoms of
deficient muscle performance is quite atypical for delayed
neuropathy and seems more likely to be due to prolonged moderate
cholinergic insult from malathion precipitating weakness, anorexia,
and weight loss, which then precipitated further ill-health as
previously-stored chlorinated hydrocarbons was mobilized from
degraded fat stores. DDT at 23 mg/kg plus a high level of organic
chloride were found in a subcutaneous fat biopsy. A muscle-
necrotising effect, due to prolonged cholinergic stimulation, as
described in section 6.2.6, is also possible.
A separate case report of ascending paralysis following
malathion intoxication (Healy, 1959) concerned an 18-month-old
child exposed daily for 6 weeks to malathion from a garden spray.
Contamination was dermal and also by inhalation and ingestion,
leading ultimately to a cholinergic crisis and a prolonged period
of weakness including extensive flaccid paralysis for several days.
The condition responded to atropine and rest within 4 weeks. In
spite of the author's conclusion that this was a "demyelinating"
(i.e., delayed neuropathic) disorder, the picture is typical of
prolonged cholinergic insult responding to atropine and the
comparatively slow clearance of accumulated pesticide with
recovery from excessive nerve-muscle stimulation.
(b) Omethoate
A typical delayed neuropathy followed ingestion of an
organophosphorus pesticide with suicidal intent (Curtes et al.,
1979). Identification of the actual pesticide ingested was
doubtful depending on a later hearsay report concerning a bottle in
a garden shed, the contents of which were not analysed. Lotti et
al. (1981) assayed both the AChE and the NTE activities in
autopsied brain after a fatal intoxication with omethoate and found
that, even at the fatal dose, the NTE levels were normal while the
AChE activity was highly inhibited. Considering this data together
with evidence of similar non-inhibition in experimental hens at up
to 8 x the unprotected LD50, the authors concluded that, though the
neuropathy was typical, it was likely that the toxic agent was some
other organophosphorus compound more liable to cause neuropathy,
such as trichlorfon or trichlornat (Table 13).
(c) Parathion
Only two cases of permanent incapacity have been attributed to
parathion, though thousands of parathion poisonings are known (see
earlier). Petry (1951) attributed a case of neuropathy to the
aftermath of several occupational incidents of cholinergic
poisoning, but the history is entirely atypical in that symptoms
developed only 4 months, rather than 2 - 4 weeks, after the last
exposure. Causation by parathion is therefore very unlikely. A
farmer deliberately ingested parathion at a dose estimated to be at
least 150 g (perhaps 500 x the estimated human lethal dose) in 600
ml of methanol. Vigorous therapy preserved his life, though he
remained in a deep coma for 7 weeks. On recovery from this
experience, he was found to be suffering from flaccid paralysis of
both legs and weakness of both hands with muscle atrophy (De Jager
et al., 1981; 1982); partial recovery occurred during one year.
Lotti & Becker (1982b) have discussed the complicating factors of
the potentially supra-lethal dose of methanol and of the long coma.
However, the clinical picture is not unlike a true moderate
organophosphorus-induced neuropathy. At such a colossal dose, the
possibility of ingesting a significant amount of a neuropathic
impurity in the parathion must be recognized. This could be ethyl
bis-(4-nitrophenyl) phosphorothioate; small but significant
amounts of the appropriate oxon are present in some samples of
paraoxon made from diethyl phosphorochloridate (Johnson, 1982b),
and this oxon is a potent inhibitor of NTE.
(d) Other organophosphorus pesticides
Besides the atypical case attributed to malathion, Petry (1958)
described another case in which symptoms persisted after a
cholinergic crisis that followed severe intermittent exposures over
3 seasons to a variety of insecticides including parathion, EPN,
DDT, dieldrin, and lead arsenate. Some of the persistent symptoms
might be compatible with a mild peripheral neuropathy. Among the
agents used, lead arsenate and dieldrin would be expected to
contribute damage to the nervous system and EPN at about the LD50
level causes neuropathy in hens and man (Tables 4, 13).
A case of slow-onset profound weakness with complete recovery
within 3 months following contamination of an agricultural worker
with the cotton defoliant, merphos ( S,S,S -tri- n -butyl
phosphorothioite), was thought by the author to be of the delayed
neuropathy type (Fisher, 1977). However, the clinical picture
showed signs that are not seen in acute organophosphate
intoxication (influenza-like onset of the syndrome and high level
of protein in the spinal fluid). These signs are, however,
characteristic of a Guillan-Barré syndrome, which might have been
coincidental to the merphos exposure only 4 days previously. It is
possible that, later, a mild organophosphorus neuropathic effect
was superimposed on the Guillan-Barré effect, since merphos can
produce neuropathy in experimental animals (Johnson, 1970, 1975b).
Recovery from very mild neuropathies is usually complete.
7.2.2 Behavioural effects
Although many epidemiological studies have been carried out,
few controlled studies on man have been reported. It is generally
recognized that there are behavioural and psychic changes during
overt clinical poisoning by organophosphorus insecticides and that
these may take several months to regress (Karczmar, 1984).
However, there is no information to suggest that effects occur at
exposure levels that do not either alter ChE levels or produce
physical symptoms. Levin & Rodnitsky (1976) have reviewed the
literature, including their own work, on different aspects of
behaviour as affected by organophosphates. Much was based on
generalized complaints from workers occupationally exposed to many
agricultural chemicals (and probably also to automobile fuels and
lubricants and to alcohol). In summary, they found that, in human
subjects sufficiently exposed to organophosphates to depress
plasma- or erythrocyte-ChEs, some or all of the following
behavioural variables might be impaired. In cognition: vigilance,
information processing and psychomotor speed, and memory; in
speech: both performance and perception; in psychic state:
increased tendencies to depression, anxiety, and irritability; and
in EEG records: a tendency to faster frequencies and higher
voltages. They also concluded that the EEG abnormalities were
positively related to the level of AChE inhibition during the
initial stages of inhibition. Concerning studies on asymptomatic
workers at risk from repeated exposure to organophosphorus
pesticides, they considered that the evidence was equivocal for the
presence of less severe or latent forms of any behavioural
abnormalities. However, Duffy & Burchfield (1980) claimed that
changes in the EEG of individuals accidentally exposed to the
nerve-agent sarin could be detected twelve months after the
exposure. This study was a sequel to the study on monkeys
described in section 6.1.2, and the analyses of EEGs was performed
as noted there. They claimed significant differences (very small
differences analysed by complex statistical procedures) between the
group of sarin-exposed workers and controls, particularly in the
region of beta-rhythm (but the comment on the spread among normal
monkeys should be noted). However, the authors were unable to pick
out sarin-exposed individuals on the basis of the EEG. They also
found (small) increased amounts of REM-sleep in the exposed
workers. Without controlled exposure and serial monitoring of
effects in individuals, little can be deduced from these apparent
marginal changes.
7.3 Effects on Other Organs and Systems
Very few effects, other than those described in sections 7.1
and 7.2, have been noted, except those arising from ill health due
to severe anticholinesterase effects.
Several adverse effects attributed to only one organophosphorus
ester will be listed under the individual substances.
7.4 Treatment of Organophosphate Insecticide Poisoning in Man
All cases of organophosphorus poisoning should be dealt with as
an emergency and the patient sent to hospital as quickly as
possible. Although symptoms may develop rapidly, delay in onset or
a steady increase in severity may be seen up to 48 h after
ingestion of some formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major references
(Kagan 1977; Taylor 1980; HMSO, 1983; Plestina 1984) and will also
be included in the IPCS Health and Safety Guides to be prepared for
selected organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
7.4.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular
care should be taken in cleaning the skin area where venupuncture
is performed. Blood might be contaminated with direct-acting
organophosphorus esters, and, therefore, inaccurate measures of ChE
inhibition might result. Extensive eye irrigation with water or
saline should also be performed. In the case of ingestion, vomiting
might be induced, if the patient is conscious, by the
administration of ipecacuanha syrup (10 - 30 ml) followed by 200 ml
water. This treatment is, however, contraindicated in the case of
pesticides dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also be
performed, particularly in unconscious patients, taking care to
prevent aspiration of fluids into the lungs (i.e., only after a
tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further
analysis (i.e., detection of toxicologically relevant impurities).
A purge to remove the ingested compound can be administered.
7.4.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as
long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
7.4.3 Specific pharmacological treatment
7.4.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given at
15 to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the
patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be necessary
in extreme cases and total daily doses up to several hundred mg may
be necessary during the first few days of treatment.
7.4.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs (section 6.5.3). However,
if absorption, distribution, and metabolism are thought to be
delayed for any reasons, oximes can be administered for several
days after intoxication. Effective treatment with oximes reduces
the required dose of atropine. Pralidoxime is the most widely
available oxime. A dose of 1 g pralidoxime can be given either im
or iv and repeated 2 - 3 times per day or, in extreme cases, more
often. If possible, blood samples should be taken for AChE
determinations before and during treatment. Skin should be
carefully cleansed before sampling. Results of the assays should
influence the decision whether to continue oxime therapy after the
first 2 days.
The possible beneficial effects of oxime therapy on CNS-derived
symptoms is discussed in section 6.5.3.
7.4.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to counteract
some aspects of CNS-derived symptoms, which are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required (Vale & Scott, 1974). Other centrally acting
drugs and drugs that may depress respiration are not recommended in
the absence of artificial respiration procedures.
7.4.3.4 Notes on the recommended treatment
(a) Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
(b) Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinisation. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
(c) Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be
taken into, and then released from, fat depots over a period of
many days. It is therefore quite incorrect to abandon oxime
treatment after 1 - 2 days on the supposition that all inhibited
enzyme will be aged. Ecobichon et al. (1977) noted prompt
improvement in both condition and blood-ChEs in response to
pralidoxime given on the 11th - 15th days after major symptoms of
poisoning appeared due to extended exposure to fenitrothion (a
dimethyl phosphate with a short half-life for aging of inhibited
AChE).
(d) Dosage of atropine and oxime
The recommended doses above pertain to exposures, usual for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in
error, while drunk. Therapeutic dosages were progressively
increased up to 6 mg atropine iv every 15 min together with
continuous iv infusion of pralidoxime chloride at 0.5 g/h for 72 h,
from days 3 to 6 after intoxication. After considerable
improvement, the patient relapsed and further aggressive therapy
was given at a declining rate from days 10 to 16 (atropine) and to
day 23 (oxime), respectively. In total, 92 g of pralidoxime
chloride and 3912 mg of atropine were given and the patient was
discharged on the thirty-third day with no apparent sequelae.
REFERENCES
ABOU-DONIA, M.B., OTHMAN, M.A., TANTAWAY, G., ZAKI KHALIL, A.,
& SHAWER, M.F. (1974) Neurotoxic effect of leptophos.
Experientia (Basel), 30 : 63-64.
ABOU-DONIA, M.B., GRAHAM, D.G., & KOMEIL, A.A. (1979)
Delayed neurotoxicity of O -ethyl O -(2,4-dichlorophenyl)phenyl-
phosphonothioate: effects of a single oral dose on hens.
Toxicol. appl. Pharmacol., 49 : 293-303.
ABOU-DONIA, M.B., GRAHAM, D.G., TIMMONS, P.R., & REICHERT,
B.L. (1980) Late acute, delayed neurotoxic, and cholinergic
effects of S,S,S -tributyl phosphorotrithioate on the hen:
effect of route of administration. Neurotoxicology, 1 : 425-447.
ABOU-DONIA, M.B., MAKKAWY, H-A., & GRAHAM, D.G. (1982)
Coumaphos: delayed neurotoxic effect following dermal
administration in hens. J. Toxicol. environ. Health, 10 : 87-99.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and
structure activity relationships providing a high safety
factor for anti-cholinesterase insecticides. In: Proceedings
of the British Crop Protection Conference, Brighton, November
1977, Croydon, British Crop Protection Council, pp. 721-729.
ALDRDIGE, W.N. & NEMERY, B. (1984) Toxicology of
trialkylphosphorothioates with particular reference to lung
toxicity. Fund. appl. Toxicol., 4 : 5215-5223.
ALDRIDGE, W.N., MILES, J.W., MOUNT, D.L., & VERSCHOYLE, R.D.
(1979) The toxicological properties of impurities in
malathion. Arch. Toxicol., 42 : 95-106.
ALDRIDGE, W.N., DINSDALE, D., NEMERY, B., & VERSCHOYLE, R.D.
(1985) Some aspects of the toxicology of trimethyl and
triethyl phosphorothioates. Fund. appl. Toxicol., 5 : 47-60.
AMBRUS, A. & GREENHALGH, R., ed. (1984) Pesticide residue
analysis. Proceedings of a joint FAO/WHO course, Eger,
Hungary, 13-26 April, 1983, Copenhagen, World Health
Organization (Health Aspects of Chemical Safety, Interim
Document No. 14).
BAKER, E.L., Jr, ZACK, M., MILES, J.W., ALDERMAN, L.,
MCWILSON, W., DOBBIN, R.D., MILLER, S., & TEETERS, W.R.
(1978) Epidemiologic malathion poisoning in Pakistan malaria
workers. Lancet, 7 January : 31.
BARNES, J.M. (1975) Assessing hazard from prolonged and
repeated exposure to low doses of toxic substances. Br. med.
Bull., 31 : 196-200.
BARNES, J.M. & DENZ, F.A. (1951) The chronic toxicity of
p- nitrophenyl diethyl thiophosphate (E. 605): a long-term
feeding experiment with rats. J. Hyg., 49 : 430-441.
BARNES, J.M. & DENZ, F.A. (1954) The reaction of rats to
diets containing octamethyl pyrophosphoramide (schradan) and
O,O- diethyl S- ethylmercaptoethanol thiophosphate ("systox").
Br. J. ind. Med., 11 : 11-19.
BAYER (1971) Nemacur, Leverkusen, Bayer Chemical Company
(Technical Information Leaflet No. E.1-781/26734).
BECKER, E.L. & BARBARO, J.F. (1964) The enzymatic hydrolysis
of p- nitrophenyl ethylphosphonates by mammalian plasmas.
Biochem. Pharmacol., 13 : 1219-1227.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating
properties of organophosphates. Xenobiotica, 2 : 307-337.
BENKE, G.M. & MURPHY, S.D. (1975) The influence of age on
the toxicity and metabolism of methyl parathion and parathion
in male and female rats. Toxicol. appl. Pharmacol., 31 :
254-269.
BERTOCIN, D., RUSSOLO, A., CAROLDI, S., & LOTTI, M. (1985)
Neurotoxic esterase in human lympocytes. Arch. environ.
Health, 40 : 139-144..
BERTRAM, U., KASTEN, A., LULLMANN, H., & ZIEGLER, A. (1977)
Improved treatment of organophosphate intoxication by use of
scopolamine or dexetimide. Experientia (Basel), 33 : 1196-1197.
BICKFORD, A.A. & SPRAGUE, G.L. (1984) The significance of
background neurologic lesions in acute delayed neurotoxicity
studies: a comparison of neurohistopathologic lesions induced
in commercial hens by tri- O -tolyl phosphate (TOCP) with those
observed in negative control hens. In: Cranmer, J.M. & Hixson,
J.E., ed. Delayed neurotoxicity. Proceedings of the Delayed
Neurotoxicity Workshop, Urbana-Champaign, Illinois, 27-30
June, 1982, Little Rock, Intox Press.
BIDSTRUP, P.L., BONNEL, J.A., & BECKETT, A.G. (1953)
Paralysis following poisoning by a new organic phosphorus
insecticide (mipafox). Br. med. J., 1 : 1068-1072.
BIGNAMI, G. (1976) Behavioural pharmacology and toxicology.
Ann. Rev. Pharm. Toxicol., 16 : 329-366.
BIGNAMI, G., ROSIC, H., MICHALEK, H., MILOSEVIC, M., & GATTI,
G.L. (1975) Behavioural toxicity of anticholinesterase
agents. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Publishing Company, pp. 155-215.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The
distribution of dichlorvos in the tissues of mammals after its
inhalation or intravenous administration. Toxicol. appl.
Pharmacol., 31 : 243-253.
BLEAKLEY, P., NICHOL, A.W., & COLLINS, A.G. (1979) Diazinon
and porphyria cutanea tarda. Med. J. Aust., 1 : 314-315.
BOPP, C. & KOSMINSKY, B. (1975) [Hepato-dermal toxic
prophyria.] Med. Cut. I.L.N., 4 : 271-280 (in Portugese).
BOULDIN, T.W. & CAVANAGH, J.B. (1979) Organophosphorus
neuropathy. A teased-fiber study of the spatio-temporal spread
of axonal degeneration. Am. J. Pathol., 94 (2): 241-252.
BOYD, E.M. (1969) Dietary protein and pesticide toxicity in
male weaning rats. WHO Bull., 40 : 801-805.
BRADLEY, W.A. (1976) The pathology of delayed neurotoxicity
due to organophosphates. In: Baron, R.L., ed. Pesticide-
induced delayed neurotoxicity, Washington, US Environmental
Protection Agency, pp. 84-101 (EPA No. 600/1-76-025).
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison
of cholinesterase activity, residue levels, and urinary
metabolite excretion of rats exposed to organophosphorus
pesticides. J. agric. food Chem., 25 : 1353-1358.
BREALEY, C., WALKER, C.H., & BALDWIN, B.C. (1980) A-esterase
activities in relation to the differential toxicity of
pirimiphos-methyl to birds and mammals. Pestic. Sci., 11 :
546-554.
BRIMBLECOMBE, R.W., GREEN, D.M., STRATTON, J.A., & THOMPSON,
P.B.J. (1970) The protective actions of some anticholinergic
drugs in sarin poisoning. Br. J. Pharmacol., 39 : 822-830.
BRITISH AGROCHEMICAL ASSOCIATION (1983) Spray operator
safety study, London, British Agrochemical Association, pp.
1-9.
BUCHET, J.P., LAUWERYS, R., & ROELS, H. (1977) Long-term
exposure to organophosphorus pesticides and lipid metabolism
in the rat. Bull. environ. Contam. Toxicol., 17 : 175-183.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused
by a single massive dose of dichlorvos to adult hens. Toxicol.
Lett., 9 : 157-159.
CASIDA, J.E. & SANDERSON, D.M. (1963) Reaction of certain
phosphorothionate insecticides with alcohols and potentiation
by breakdown products. J. agric. food Chem., 11 : 91-96.
CAVANAGH, J.B. (1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system: an experimental study in
hens. J. Neurol. Neurosurg. Psychiatr., 17 : 163-172.
CEC (1977) Organophosphorus pesticides. Criteria
(dose/effect relationships) for organophosphorus pesticides,
Oxford, New York, Pergamon Press, 199 pp (Report of a Working
Group of Experts prepared for the Commission of the European
Communities, Directorate-General for Social Affairs, Health
and Safety Directorate).
CHEN, P.R.S. & DAUTERMAN, W.C. (1971) Studies on the
toxicity of dimethoate analogues and their hydrolysis by sheep
liver amidase. Pestic. Biochem. Physiol., 1 : 340-348.
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN,
R.J. (1980) Effects of low-level administration of
dichlorvos on adrenocorticotrophic hormone secretion, adrenal
cholesteryl ester, and steroid metabolism. Biochem.
Pharmacol., 29 : 635-641.
CLOTHIER, B., JOHNSON, M.K., & REINER, E. (1981) Interaction
of some trialkyl phosphorothiolates with acetylcholinesterase:
characterization of inhibition, aging, and reactivation.
Biochem. Biophys. Acta, 660 : 306-316.
CODEX ALIMENTARIUS COMMISSION (1984) Codex Alimentarius
Commission guide to Codex recommendations concerning pesticide
residues. VIII. Recommendations for methods of analysis of
pesticide residues, Rome, FAO/WHO (CAC/PR 8-1984).
COLEMAN, I.W., LITTLE, P.E., & BANNARD, R.A.B. (1962)
Cholinolytics in the treatment of anticholinesterase
poisoning. I. The effectiveness of certain cholinolytics in
combination with an oxime for treatment of sarin poisoning.
Can. J. Biochem. Physiol., 40 : 815-826.
COSTA, L.G., SCHWAB, B.W., & MURPHY, S.D. (1982) Tolerance
to anticholinesterase compounds in mammals. Toxicology, 25 :
79-97.
CRANMER, J.M. & HIXSON, J.E., ed. (1984) Delayed
neurotoxicity. Proceedings of the Delayed Neurotoxicity
Workshop, Urbana-Champaign, Illinois, 27-30 June, 1982, Little
Rock, Intox Press.
CURTES, J.P., DEVELAY, P., & HUBERT, J.P. (1979) Late
peripheral neuropathy due to an acute voluntary intoxication
by organo-phosporic compounds. In: International Congress on
Neurotoxicology, Varese, Italy, 27-30 September, 1979, Oxford,
Pergamon Press.
DANDLIKER, W.B., HICKS, A.N., LEVINSON, S.A., STEWART, K., &
BRAWN, R.J. (1979) Effects of pesticides on the immune
response, La Jolla, California, Scripps Clinic and Research
Foundation (EPA 600/1-79-039).
DAUGHTON, C.G., COOK, A.M., & ALEXANDER, M. (1979) Bacterial
and conversion of alkylphosphonates to natural products via
carbon-phosphorus bond cleavage. J. agric. food Chem., 27 :
1375-1382.
DAUTERMAN, W.C. (1971) Biological and nonbiological
modification of organophosphorus compounds. WHO Bull., 44 :
133-150.
DAVIES, J.E., BARQUET, A., FREED, V.H., HAGUE, R., MORGADE,
C., SONNEBORN, R.E., & VACLAVEK, C. (1975) Human pesticide
poisonings by a fat-soluble organophosphate insecticide. Arch.
environ. Health, 30 : 608-613.
DAVIS, C.S. & RICHARDSON, R.J. (1980) In: Spencer, P.S. &
Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 527-544.
DE JAGER, A.E.J., VAN WEERDON, T.W., HOUTOFF, H.J., & DE
MONCHY, J.G.R. (1981) Polyneuropathy after massive exposure
to parathion. Neurology, 31 : 603-605.
DE JAGER, A.E.J., VAN WEERDON, T.W., & MONCHY, J.G.R. (1982)
Reply to letter. Neurology, 32 : 218.
DESI, I. (1983) Neurotoxicological investigation of
pesticides in animal experiments. Neurobehav. Toxicol.
Teratol., 5 : 503-515.
DESI, I., FARKAS, I., SIMON, G., & CZIELESZKY, V. (1971)
[Investigation of the neurotoxic action of the phosphorus
compound bromophos.] Int. Arch. Arbeitsmed., 28 : 203-222 (in
German).
DESI, I., VARGA, L., & FARKAS, I. (1978) Studies on the
immunosuppressive effect of organochlorine and organo-
phosphoric pesticides in subacute experiments. J. Hyg.
Epidemiol. Microbiol. Immunol., 22 : 115-122.
DETTBARN, W.D. (1984) Pesticide-induced muscle necrosis:
mechanisms and prevention. Fund. appl. Toxicol., 4 : 18-26.
DHSS (1983) Pesticide poisoning. Notes for the guidance of
medical practitioners, London, Department of Health and Social
Security, pp. 41-47.
DIGGORY, H.J.P., LANDRIGEN, P.J., LATIMER, K.P., ELLINGTON,
A.C., KIMBROUGH, R.D., LIDDLE, J.D., CLINE, R.E., & SMERK,
A.L. (1977) Fatal parathion poisoning caused by contamination
of flour in international commerce. Am. J. Epidemiol.,
106 : 145-153.
DIMOV, G. & KALOYANOVA, F. (1967) Carbohydrate metabolism
disorders in the liver and muscles in acute parathion
poisonings. C R Acad. Bulg. Sci., 20 : 1007-1009.
DINSDALE, D., VERSCHOYLE, R.D., & CABRAL, J.R.P. (1982)
Cellular responses to trialkylphosphorothioate-induced injury
in rat lung. Arch. Toxicol., 51 : 79-89.
DONNINGER, C. (1971) Species specificity of phosphate
triester anticholinesterases. WHO Bull., 44 : 265-268.
DOROUGH, H.W. (1976) Biological activity of pesticide
conjugate. In: Kaufman, D.D., ed. Bound and conjugated
pesticide residues, Washington DC, American Chemical Society,
pp. 11-34 (ACS Symposium Series No. 29).
DREVENKAR, V., FINK, K., STIPCEVIC, M., & TOKALCEVIC, B.
(1976) The fate of pesticides in aquatic environment. II.
Hydrolysis of dichlorvos in model system and in river water.
Arh. hig. rada, 27 : 297-305.
DREVENKAR, V., STIPCEVIC, M., STENGL, B., & STEFANAC, Z.
(1979) Gas chromatographic determination of alkali metal
O,O- diethylphosphorodithioate present in tract amounts.
Microchim. Acta, 1 : 385-394.
DUBOIS, K.P. (1963) Toxicological evaluation of the anti-
cholinesterase agents. In: Koelle, G.B., ed. Handbook on
experimental pharmacology, Berlin, Springer-Verlag, Vol. 15,
pp. 833-857.
DUDEK, B.R., BARTH, M., GEPHART, J., HUGGINS, J., & RICHARD-
SON, R.J. (1979) Correlation of brain and lymphocyte neuro-
toxic esterase inhibition in the adult hen following dosing
with neurotoxic compounds. Toxicol. appl. Pharmacol., 48 : A198.
DUFFY, F.H. & BURCHFIELD, J.L. (1980) Long-term effects of
the organophosphate sarin on EEGs in monkeys and humans.
Neurotoxicology, 1 : 667-689.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the
exposure of workers to pesticides. WHO Bull., 26 : 75-91.
DURHAM, W.F., WOLFE, H.R., & ELLIOTT, J.W. (1972) Absorption
and excretion of parathion by spraymen. Arch. environ. Health,
24 : 381-387.
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S.
(1977) Acute fenitrothion poisoning. Can. Med. Assoc. J.,
116 : 377-379.
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7 : 88-95.
EL-SEBAE, A.H. (1980) Biochemical challenges in future
toxicological research. J. environ. Sci. Health, B15 : 689-721.
EL-SEBAE, A.H., SOLIMAN, S.A., AHMED, N.S., & CURLEY, A.
(1981) Biochemical interaction of six op delayed
neurotoxicants with several neurotargets. J. environ. Sci.
Health, B16 (4): 465-474.
ENAN, E.E., EL-SEBAE, A.H., ENAN, O.H., & EL-FIKI, S. (1982)
In vivo interaction of some organophosphorus insecticides with
different biochemical targets in white rats. J. environ. Sci.
Health, B17 : 549-570.
ETO, M. (1974) Organophosphorus pesticides: organic and
biological chemistry, Boca Raton, Florida, CRC Press.
ETO, M. & OHKAWA, H. (1970) Alkylation reaction of
organophosphorus pesticides: its chemical and biochemical
significance. In: Biochemical toxicology of insecticides, New
York, London, Academic Press, pp. 93-104.
FAO (1984) Production yearbook, Rome, Food and Agriculture
Organization of the United Nations, Vol. 38.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1963/13; WHO/Food
Add./23 (1964)).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the
FAO Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report, No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO
Meeting Report, No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of
the FAO Working Party on Pesticide Residues and the WHO Expert
Committee on Pesticide Residues (FAO Agricultural Studies No.
73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO: PL/CP/15; WHO
Food Add./67.32).
FAO/WHO (1968a) Pesticide residues in food. Report of the
1967 Joint Meeting of the FAO Working Party and the WHO Expert
Committee, Geneva, World Health Organization (FAO Meeting
Report No. PL:1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the
1968 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 78; WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues
in food, Geneva, World Health Organization (FAO PL:1968/M/9/1;
WHO Food Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the
1969 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Group on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (FAO Report No.
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the
1970 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1979/M/12/1;
WHO Food Add./71.42).
FAO/WHO (1972a) Pesticide residues in food. Report of the
1971 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 88; WHO Technical Report Series No. 502).
FAO/WHO (1972b) 1971 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1971/M/9/1;
WHO Pesticide Residues Series No. 1).
FAO/WHO (1973a) Pesticide residues in food. Report of the
1972 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 90; WHO Technical Report Series No. 525).
FAO/WHO (1973b) 1972 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1972/M/9/1;
WHO Pesticide Residues Series No. 2).
FAO/WHO (1974a) Pesticide residues in food. Report of the
1973 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 92; WHO Technical Report Series No. 545).
FAO/WHO (1974b) 1973 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1973/M/9/1;
WHO Pesticide Residues Series No. 3).
FAO/WHO (1975a) Pesticide residues in food. Report of the
1974 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Agricultural
Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the
1975 Joint Meeting of the FAO Working Party of Experts on
Pesticide Residues and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Plant
Production and Protection Series No. 1; WHO Technical Report
Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues
in food, Geneva, World Health Organization (AGP 1975/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1977a) Pesticide residues in food. Report of the
1976 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Geneva, World Health Organization (FAO
Food and Nutrition Series No. 9; FAO Plant Production and
Protection Series No. 8; WHO Technical Report Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (AGP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the
1977 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection
Paper 10 Rev).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1979a) Pesticide residues in food. Report of the
1978 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 15).
FAO/WHO (1979b) 1978 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup).
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the
1980 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide Residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide Residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide Residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide Residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986) Pesticide Residues in food. Report of the
1985 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 68).
FAO/WHO (in press) 1985 Evaluations of some pesticide
residues in food, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper).
FARM CHEMICALS HANDBOOK (1985) Willoughby, Ohio, Meister
Publishing Company.
FENICHEL, G.M., KIBLER, W.B., OLSON, B.A., & DETTBARN, W.D.
(1972) Chronic inhibition of cholinesterase as a cause of
myopathy. Neurology, 22 : 1026-1033.
FISHER, J.R. (1977) Guillain-Barré syndrome following
organophosphate poisoning. J. Am. Med. Assoc., 238 : 1950-1951.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14 : 514-534.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health
hazards on use of dichlorvos (DDVP), especially in resin
strips. Res. Rev., 44 : 115-157.
GRAY, A.P. (1984) Design and structure-activity relationships
of antidotes to organophosphorus anticholinesterase agents.
Drug. Metab. Rev., 15 (3): 557-589.
GREEN, D.M., MUIR, A.W., STRATTON, J.A., & INCH, T.D. (1977)
Dual mechanism of the antidotal action of atropine-like drugs
in poisoning by organophosphorus anticholinesterase. J. Pharm.
Pharmacol., 29 . 62-64.
GREENHALGH, R., DHAWAN, K.L., & WEINBERGER, P. (1980)
Hydrolysis of fenitrothion in model and natural aquatic
systems. J. agric. food Chem., 28 : 102-105.
GROB, D. (1963) Anticholinesterase intoxication in man and
its treatment. In: Koelle, G.B., ed. Handbook of experimental
pharmacology, Berlin, Springer-Verlag, Vol. 15, pp. 989-1027.
GUNTHER, F.A., WESTLAKE, W.E., BARKELY, J.H., WINTERLIN, W., &
LANGBEHN, L. (1973) Establishing dislodgeable pesticide
residues on leaf surfaces. Bull. environ. Contam. Toxicol., 9 :
243-250.
GUNTHER, F.A., WESTLAKE, W.E., & BARKELY, J.H. (1974) Worker
environment research. II. Sampling and processing techniques
for determining dislodgeable pesticide residues on leaf
surfaces. Bull. environ. Contam. Toxicol., 12 : 641-645.
HANSEN, L.G. & HANSEN, T.K. (1985) Intravenous dosing
abolishes differences in delayed neurotoxicity potency between
leptophos and desbromoleptophos. Pestic. Biochem. Physiol.,
24 : 136.
HAYES, G.R., FUNCKES, A.J., & HARTWELL, W.V. (1964) Dermal
exposure of human volunteers to parathion. Arch. environ.
Health, 8 : 829-833.
HAYES, W.J., Jr (1971) Studies on exposure during the use of
anticholinesterase pesticides. WHO Bull., 44 : 277-288.
HAYES, W.J., Jr (1975) Diagnosis and treatment of poisoning.
In: Toxicology of pesticides, Baltimore, Maryland, Williams
and Wilkins Company, pp. 409-417.
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, 561 pp.
HEALY, J.K. (1959) Ascending paralysis following malathion
intoxication: a case report. Med. J. Aust., 46 : 765-767.
HEATH, D.F. & VANDEKAR, M. (1957) Some spontaneous reactions
of O,O- dimethyl S- ethylthioethyl phosphorothiolate and related
compounds in water and on storage, and their effects on the
toxicological properties of the compounds. Biochem. J., 67 :
187-202.
HIERONS, R. & JOHNSON, M.K. (1978) Clinical and
toxicological investigations of a case of delayed neuropathy
in man after acute poisoning by an organophosphorus pesticide.
Arch. Toxicol., 40 : 279-284.
HOLLINGSHAUS, J.G., ABU-EL-HAJ, S., & FUKUTO, T.R. (1979)
Delayed neurotoxicity of O -alkyl O -aryl phenylphosphonothioate
analogues related to leptophos administered orally to the hen.
J. agric. food Chem., 27 : 1197-1201.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus
cholinesterase inhibitors. Pharmacol. Rev., 11 : 567-688.
HUANG, X.S., SHU, W.A., ZU, W.C., LAI, M.T., TSIN, Z.A., &
KWOK, C.S. (1979) [Toxicity studies of the herbicide
Amiprophos.] Chekiang Univ. Sch. Med. Bull., 8 : 63-66 (in
Chinese).
HUFF, J.E., BATES, R., EUSTIS, S.L., HASEMAN, J.K., &
MCCONNELL, E.E. (1985) Malathion and malaoxon: histo-
pathology reexamination of the National Cancer Institute's
Carcinogenesis Studies. Environ. Res., 37 : 154-173.
HUTSON, D.H. & HATHWAY, D.E. (1967) Toxic effects of
chlorofenvinphos in dogs and rats. Biochem. Pharmacol., 16 :
949-962.
IARC (1979) Dichlorvos. In: Some halogenated hydrocarbons,
Lyons, International Agency for Research on Cancer, pp. 97-127
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Vol. 20).
IARC (1983) Miscellaneous chemicals, Lyons, International
Agency for Research on Cancer (Monographs on the Carcinogenic
Risk of Chemicals to Humans, Vol. 30).
IMAI, H. (1977) Experimental pigmentary degeneration of the
retina caused by an organophosphorus pesticide (fenthion).
Acta soc. ophthalmol., 81 : 925-932 (Abstract 245, Exerp. Med.
(1979), 9 : 53).
IMAMURA, T., GANDY, J., FUKUTO, T.R., & TALBOT, P. (1983) An
impurity of malathion alters the morphology of rat lung
bronchiolar epithelium. Toxicology, 26 : 73-79.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISHIKAWA, S. & MIYATA, M. (1980) Development of myopia
following chronic organophosphate pesticide intoxication: an
epidemiological and experimental study. In: Merigan, W.H. &
Weiss, B., ed. Neurotoxicity of the visual system, New York,
Raven Press, pp. 233-254.
IZMIROVA, N. (1980) Methods for determination of exposure of
agricultural workers to organophosphorus pesticides. In:
Tordoir, W.F. & van Heemstra-Lequin, E.A.H., ed. Field worker
exposure during pesticide application. Proceedings of the
Fifth International Workshop of the Scientific Committee on
Pesticides of the International Association on Occupational
Health, The Hague, The Netherlands, 9-11 October, 1979,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp. 169-172.
JAGER, K.W., ROBERTS, D.V., & WILSON, A. (1970) Neuro-
muscular function in pesticide workers. Br. J. ind. Med., 27 :
273-278.
JASH, N.B. & BHATTACHARYA, S. (1982) Neurotoxicity of
phenthoate to a non-target species. In: Proceedings of the 5th
International Congress on Pesticide Chemicals, Kyoto, 29
August, 1982 (Abstract No. V1a-15).
JEDRZEJOWSKA, H., ROWINSKA-MARCINSKA, K., & HOPPE, B. (1980)
Neuropathy due to phytosol (agritox): report of a case. Acta
neuropathol., 49 : 163-168.
JOHNSON, D.D. & WILCOX, W.C. (1975) Studies on the mechanism
of the protective and antidotal actions of diazepam in
organophosphate poisoning. Eur. J. Pharmacol., 34 : 127-132.
JOHNSON, M.K. (1970) Organophosphorus and other inhibitors
of the brain "neurotoxic esterase" and the development of
delayed neurotoxicity in hens. Biochem. J., 120 : 523-531.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. Crit. Rev.
Toxicol., 3 : 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing
delayed neurotoxic effects: mechanism of action and structure/
activity studies. Arch. Toxicol., 34 : 259-288.
JOHNSON, M.K. (1977) Improved assay of neurotoxic esterase
for screening organophosphates for delayed neurotoxicity
potential. Arch. Toxicol., 37 : 113-115.
JOHNSON, M.K. (1980) Delayed neurotoxicity induced by
organophosphorus compounds: areas of understanding and
ignorance. In: Holmstedt, B., Lauwerys, R., Mercier, M., &
Roberfroid, M., ed. Mechanisms of toxicity and hazard
evaluation, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 27-38.
JOHNSON, M.K. (1981a) Do trichlorphon and/or dichlorvos
cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol. Scand., 49 (Suppl. 5): 87-98.
JOHNSON, M.K. (1981b) Grading of non-neurotoxicity of
organophosphorus pesticides and plasticisers by in vivo/in
vitro assay of neurotoxic esterase. In: Proceedings of the
European Society of Toxicologists, Dublin, 17-19 August
(Abstract No. 66).
JOHNSON, M.K. (1982a) The target for initiation of delayed
neurotoxicity by organophosphorus esters: biochemical studies
and toxicological applications. In: Hodgson, E., Bend, J.R., &
Phillip, R.M., ed. Reviews in biochemistry and toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 4, pp. 141-212.
JOHNSON, M.K. (1982b) Check your paraoxon and parathion for
neurotoxic impurities. Vet. hum. Toxicol., 24 (3): 220a.
JOHNSON, M.K. (1984) Delayed neurotoxicity tests of
organophosphorus esters: a proposed protocol integrating
neuropathy target esterase (NTE) assays with behaviour and
histopathology tests to obtain more information more quickly
from fewer animals. In: El-Sebae, A.H., ed. Proceedings of the
International Conference on Environmental Hazards of
Agrochemicals in Developing Countries, Alexandria, Egypt, 8-12
November, 1983, Alexandria, University of Alexandria, Vol. 1,
pp. 474-493.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus
pesticides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269
(in Russian).
KAGAN, JU.S. (1985) [Principles of toxicology,] Moscow,
United Nations Environment Programme (in Russian).
KAHN, E. (1979) Outline guide for performance of field
studies to establish safe reentry intervals for
organophosphorus pesticides. Residues Rev., 70 : 27-43.
KALOYANOVA-SIMEONOVA, F. (1961) Effet du chlorthion sur les
réflexes moteurs conditionnés des rats albinos. Act. nerv.
super., 3 : 284-288.
KALOYANOVA, F. (1979) Cholinesterase activity as a
biochemical indicator for monitoring exposure to certain
pesticides. In: Proceedings of the International Conference on
Environmental Sensing and Assessment, Las Vegas, Nevada,
14-19 September, 1975, Vol. 1.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J.,
ed. IUPAC pesticide chemistry - Human welfare and the
environment, Oxford, New York, Pergamon Press, pp. 237-238.
KALOYANOVA, F. & TASHEVA, M. (1983) Effect of protein
malnutrition on toxicity of pesticides. In: Miyamoto, J. &
Kearney, P.C., ed. IUPAC pesticide chemistry, human welfare,
and the environment, Oxford, New York, Pergamon Press,
pp. 527-529.
KALOYANOVA, F., BENCHEV, I., GHEORGHIEV, G., IZMIROVA, N., &
RISOV, N. (1979) Pesticides and persistent substances.
Biological specimen collection. In: Berlin, A., Wolff, A.H., &
Hosegawa, J., ed. Biological specimens used for the assessment
of human exposure to environmental pollutants, Sophia,
Martinus N. Publishers, pp. 231-242.
KARCZMAR, A.G. (1984) Acute and long-lasting actions of
organophosphorus agents. Fundam. appl. Toxicol., 4 : S1-S7.
KARCZMAR, A.G. & OHTA, Y. (1981) Neuromyopharmacology as
related to anticholinesterase action. Fundam. appl. Toxicol.,
1 : 135-142.
KATO, T., NAKANO, T., KOJIMA, K., NAGATSU, T., & SAKAKIBARA,
S. (1980) Changes in propyl endopeptidase during maturation
of the rat brain and hydrolysis of substance P by the purified
enzyme, J. Neurochem., 35 : 527-535.
KAUFMANN, D.D., ed. (1976) Bound and conjugated pesticide
residues, Washington DC, American Chemical Society, pp. 1-10
(ACS Symposium Series No. 29).
KEPLINGER, M.L. & DEICHMANN, W.B. (1967) Acute toxicity of
combinations of pesticides. Toxicol. appl. Pharmacol., 10 :
586-595.
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic
phosphorus compounds and alkylating agents on the rat fetus.
Arch. environ. Health, 16 : 805-808.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides: Techniques for establishing safe levels of foliar
residues. Residues Rev., 75 : 81-96.
KNOX, B., ASKAA, J., BASSE, A., BITSCH, V., ESKILDSEN, M.,
MANDRUP, M., OTTOSEN, H.E., OVERBY, E., PEDERSEN, K.B., &
RASMUSSEN, F. (1978) Congenital ataxia and tremor with
cerebellar hypoplasia in piglets born by sows treated with
NeguvonR vet (metrifonate, trichlorfon) during pregnancy.
Nord. Vet. Med., 30 : 538-545.
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the
seminiferous epithelium and the Leydig cells of the rat testis
after the application of dichlorvos. Bull. environ. Contam.
Toxicol., 11 (5): 429-433.
LAUWERYS, R.L. & MURPHY, S.D. (1969) Interaction between
paraoxon and tri- o- tolyl phosphate in rats. Toxicol. appl.
Pharmacol., 14 : 348-357.
LE QUESNE, P.M. & MAXWELL, I.C. (1981) Effect of metrifonate
on neuromuscular transmission. Acta pharmacol. toxicol.
Scand., 49 (Suppl. 5): 99-104.
LEVIN, H.S. & RODNITZKY, R.L. (1976) Behavioural effects of
organophosphate pesticides in man. Clin. Toxicol., 9 (3):
391-405.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating
properties of 2,2-dichlorovinyl dimethyl phosphate: a
disregarded hazard. Environ. Mutat. Soc. Newslett., 2 : 21-27.
LOTTI, M. & BECKER, C.E. (1982a) Treatment of acute
organophosphate poisoning: evidence of a direct effect on
central nervous system by 2-PAM (pyridine-2-aldoxime methyl
chloride). J. Toxicol. clin. Toxicol., 19 : 121-127.
LOTTI, M. & BECKER, C.E. (1982b) Letter: polyneuropathy and
exposure to parathion. Neurology, 32 : 317.
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of
organophosphorus pesticides: predictions can be based on in
vitro studies with hen and human enzymes. Arch. Toxicol., 41 :
215-221.
LOTTI, M. & JOHNSON, M.K. (1980) Repeated small doses of
neurotoxic organophosphate: monitoring of neurotoxic esterase
in brain and spinal cord. Arch. Toxicol., 45 : 263-271.
LOTTI, M. & MORRETTO, A. (1986) Inhibition of lymphocyte
neuropathy target esterase predicts the development of
organophosphate polyneuropathy in man. Hum. Toxicol., 5 : 114.
LOTTI, M., FERRARA, S.D., CAROLDI, S., & SINIGAGLIA, F.
(1981) Enzyme studies with human hen autopsy tissue suggest
omethoate does not cause delayed neuropathy in man. Arch.
Toxicol., 48 : 265-270.
LOTTI, M., BECKER, C.E., AMINOFF, M.J., WOODROW, J.E., SEIBER,
J.N., TALCOTT, R.E., & RICHARDSON, R.J. (1983) Occupational
exposure to the cotton defoliants DEF and MERPHOS. J. occup.
Med., 25 : 517-522.
LOTTI, M., BECKER, C.E., & AMINOFF, M.J. (1984) Organo-
phosphate polyneuropathy: pathogenesis and prevention.
Neurology, 34 : 658-662.
LOTTI, M., BERTONCIN, D., & MORETTO, A. (1986) Organo-
phosphate induced delayed polyneuropathy (OPIDP) by chlor-
pyrifos in man and hens. Toxicologist, 6 : 22 (Abstract No. 86).
LUDKE, J.L. (1977) DDE increases the toxicity of parathion
to corturnix quail. Pestic. Biochem. Physiol., 1 : 28-33.
MACHIN, A.P., ANDERSON, P.H., HOWELLS, L.C., & DUNDY, D.E.
(1978) Enzymic degradation of phosphorothionate oxons in the
plasma of species of varying susceptibility to poisoning. In:
Proceedings of the 4th IUPAC International Congress on
Pesticides and Chemicals, Zurich, 24-28 July, 1978 (Abstract
V.620).
MALLIPUDI, N.M., UMETSU, N., TOIA, R.F., TALCOTT, R.E., &
FUKUTO, T.R. (1979) Toxicity of O,O,S -trimethyl and triethyl
phosphorothioate to the rat. J. agric. food Chem., 27 : 463-466.
MALONE, J.C. (1964) Toxicity of haloxon. Res. vet. Sci., 5 :
17-31.
MAQUIS, J.K. (1985) Non-cholinergic mechanisms of
insecticide toxicity. Trends pharmacol. Sci., 6 (2): 59-60.
MARGOT, A. & GYSIN, H. (1957) [Diazinon: its decomposition
product and their properties.] Helv. Chim. Acta, 40 (162):
1562-1573 (in German).
MARONI, M. & BLEEKER, M.L. (1986) Neuropathy target esterase
in human lymphocytes and platelets. J. appl. Toxicol., 6 : 1-7.
MARSHALL, T.C. & DOROUGH, H.W (1977) Bioavailability in rats
of bound and conjugated plant carbamate insecticide residues.
J. agric. food Chem., 25 (5): 1003-1009.
MEDVED, L.I. & KAGAN, JU.S. (1983) Pesticides, organophos-
phorus. In: Parmeggiani, L., ed. Encyclopaedia of occupational
health and safety, 3rd ed., Geneva, International Labour
Office, pp. 1637-1646.
MEIER, E.P., DENNIS, W.H., ROSENCRANCE, A.B., RANDALL, W.F.,
COOPER, W.J., & WARNER, M.C. (1979) Sulfotepp: a toxic
impurity in formulations of diazinon. Bull. environ. Contam.
Toxicol., 23 : 158-164.
MEL'NIKOV, N.N., ET AL. (1985) [Pesticide handbook,] Moscow,
Himija Press (in Russian).
MENDOZA, C.E. (1976) Toxicity and effects of malathion on
esterases of suckling albino rats. Toxicol. appl. Pharmacol.,
35 : 229-238.
MILBY, T.H., OTTOBONI, F., & MITCHELL, H.W. (1964) Parathion
residue poisoning among orchard workers. J. Am. Med. Assoc.,
189 : 351-358.
MILES, J.W., MOUNT, D.L., STAIGER, M.A., & TEETERS, W.R.
(1979) S- methyl isomer content of stored malathion and
fenitrothion water-dispersible powders and its relationship to
toxicity. J. agric. food Chem., 27 : 421-425.
MIYAMOTO, J. (1976) Degradation, metabolism, and toxicity of
synthetic pyrethroids. Environ. Health Perspect., 14 : 15-28.
MIYAMOTO, J. & OHKAWA, H. (1978) Oxidative processes in
pesticide transformation. In: Proceedings of the 4th IUPAC
International Congress on Pesticides and Chemicals, Zurich,
24-28 July, 1978.
MURPHY, S.D. (1969) Mechanisms of pesticide interactions in
vertebrates. Res. Rev., 25 : 201-221.
NAKATSUGAWA, T., TOLMAN, N.M., & DHAM, P.A. (1968)
Degradation and activation of parathion analogues by
microsomal enzymes. Biochem. Pharmacol., 17 : 1517-1528.
NAMBA, T. (1974) Relative toxicity of malathion (contd). New
Engl. J. Med., 290 : 347.
NAMBA, T., NOLTE, C.T., JACKREL, G., & GROB, D. (1971)
Poisoning due to organophosphate insecticides: acute and
chronic manifestations. Am. J. Med., 50 : 475-492.
NICHOL, A.W., ELSBURY, S., ELDER, G.H., JACKSON, A.H., &
NAGARAJA RAO, K.R. (1982) Separation of impurities in
diazinon preparations and their effect on porthyrin
biosynthesis in tissue culture. Biochem. Pharmacol., 31 :
1033-1038.
NISHIUCHI, Y. (1974) [Toxicity of pesticides formulations to
some fresh-water organisms. XXIII.] Aquiculture, 22 : 16-18 (in
Japanese).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitaikagaku ecol. Chem., 4 : 31-46 (in Japanese).
O'BRIEN, R.C. (1967) Insecticides: action and metabolism,
New York, Academic Press, pp. 32-54.
OECD (1983) Acute delayed neurotoxicity of organophosphorus
substances: subchronic delayed neurotoxicity of organo-
phosphorus substances: 90-day study, Paris, Organization of
Economic Cooperation and Development (OECD Guidelines for the
Testing of Chemicals, Nos. 418, 419).
OHKAWA, H., OSHITA, H., & MIYAMOTO, J. (1980) Comparison of
inhibitory activity of various organophosphorus compounds
against acetylcholinesterase and neurotoxic esterase of hens
with respect to delayed neurotoxicity. Biochem. Pharmacol.,
29 : 2721-2727.
O'NEIL, J.J. (1981) Non-cholinesterase effects of
anticholinesterases, Fundam. appl. Toxicol., 1 : 154-160.
OSTERLOH, J., LOTTI, M.D., & POND, S.M. (1983) Toxicological
studies in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos.
J. anal. Toxicol., 7 : 125-129.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78 : 78-87.
PELLEGRINI, G. & SANTI, R. (1972) Potentiation of toxicity
of organophosphorus compounds containing carboxylic ester
functions toward warm-blooded animals by some organophosphorus
impurities. J. agric. food Chem., 20 : 944-950.
PETRY, C.S. (1958) Organic phosphate insecticide poisoning.
Am. J. Med., 24 : 467-470.
PETRY, H. (1951) [Polyneuritis from E 605.] Zentralbl.
Arbeitsmed. Arbeitssch., 1 : 86-89 (in German).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PRENTICE, D.E. & ROBERTS, N.L. (1984) Acute delayed
neurotoxicity in hens dosed with tri-ortho-cresyl phosphate
(TOCP): correlation between clinical ataxia and neuropatho-
logical findings. In: Cranmer, J.M. & Hixson, J.E., ed.,
Delayed neurotoxicity. Proceedings of the Delayed Neuro-
toxicity Workshop, Urbana-Champaign, Illinois, 27-30 June,
1982, Little Rock, Intox Press.
PREUSSMANN, R., SCHNEIDER, H., & EPPLE, F. (1969)
[Investigation of effects of alkylating agents. II. The
investigation of different classes of alkylating agents by
means of a modified colour reaction with 4-(4-nitro-benzyl)
pyridine (NBP).] Arzneimittelforschung, 7 : 1059-1073 (in
German).
RAY, D.E. (1980) Selective inhibition of thermogenesis by
tributyl S,S,S -phosphorotrithioate (DEF). Br. J. Pharmacol.,
69 : 257-264.
RAY, D.E. & CUNNINGHAM, V.J. (1985) Hypothermia produced by
S,S,S -phosphortrithioate (DEF). Arch. Toxicol., 56 : 279-282.
REITER, L.W., TALENS, G.M., & WOOLEY, D.E. (1975) Parathion
administration in the monkey: time course of inhibition and
recovery of blood cholinesterases and visual discrimination
performance. Toxicol. appl. Pharmacol., 33 : 1-13.
REUBER, M.D. (1985) Carcinogenicity and toxicity of
malathion and malaoxon. Environ. Res., 37 : 119-153.
REVZIN, A.M. (1980) Effects of organophosphate pesticides
and alcohol on visual mechanisms. In: Merigan, W.H. & Weiss,
B., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 255-268.
REVZIN, A.M. (1983) Neurophysiological and behavioural
assessment of pesticide toxicity. In: Miyamoto, J. & Kearney,
P.C., ed. Pesticide chemistry: human welfare and the
environment, Oxford, New York, Pergamon Press, Vol. 3, pp.
419-424.
RICHARDSON, R.J. & DUDEK, B.R. (1983) Neurotoxic esterase:
characterization and potential for a protective screen for
exposure to neuropathic organophosphates. In: Miyamoto, J.,
ed. Proceedings of the IUPAC Pesticide Chemistry Congress on
Human Welfare and the Environment, Oxford, New York, Pergamon
Press, pp. 491-495.
RICO, A.G. & BURGAT-SACAZE, V. (1984) Toxicological
significance of covalently-bound residues. Food Add. Contam.,
1 (2): 157-161.
ROBERTS, D.V. (1976) EMG voltage and motor nerve conduction
velocity in organophosphorus pesticide factory workers. Int.
Arch. occup. environ. Health, 36 : 267-274.
SALPETER, M.M., KASPRZAK, H., FENG, H., & FERTUCK, H. (1979)
Endplates after esterase inactivation in vivo: correlation
between esterase concentration, functional response, and fine
structure. J. Neurocytol., 8 : 95-115.
SANBORN, J.R., METCALF, R.L., & HANSEN, L.G. (1977) The
neurotoxicity of O -2(2,5-dichlorophenyl) O -methyl phenylphos-
phonothioate, an impurity and photoproduct of leptophos
(PhosveR ) insecticide. Pestic. Biochem. Physiol., 7 : 142-145.
SCHAFER, E.W. (1972) The acute oral toxicity of 369
pesticidal, pharmaceutical and other chemicals to wild birds.
Toxicol. appl. Pharmacol., 21 : 315-330.
SCHOENE, J. & OLDIGES, H. (1973) [Antidotal action of
pyridinium salts for tabun and sarin poisoning in vivo and in
vitro. ] Arch. int. Pharmacodyn. Ther., 204 : 110-123 (in
German).
SEIFERT, J. & CASIDA, J.E. (1980) Mechanisms of terato-
genesis induced by organophosphorus and methylcarbamate
insecticides. In: Progress in pesticide biochemistry,
Berkeley, California, University of California.
SENANAYAKE, N. & JOHNSON, M.K. (1982) Acute polyneuropathy
following poisoning by a new organophosphate insecticide: a
preliminary report. New Eng. J. Med., 306 : 155-157.
SHAFIK, T., BRADWAY, D.E., ENOS, E.F., & YOBS, A.R. (1973)
Human exposure to organophosphorus pesticides. A modified
procedure for the gas-liquid chromatographic analysis of alkyl
phosphate metabolites in urine. J. agric. food Chem., 21 (4):
625.
SHIRAISHI, S., GOTO, I., YAMASHITA, Y., ONISHI, A., & NAGAO,
H. (1977) [Dipterex polyneuropathy.] Neurol. Med. (Japan),
6 : 34-38 (in Japanese with English summary).
SHTENBERG, A.I., KHOVAEVA, L.A., & ZAVARZINA, M.V. (1974)
Effect of chlorophos and methyl nitrophos on the immune
reactions of the organism against the background of
protein-deficient nutrition. Vopr. Pitan., 8 (4): 35-42.
SOLIMAN, S.A. (1982) Delayed neuropathy in hen by the
insecticide synergist O-n- propyl O- (2-propynyl)phenylphos-
phonate (NIA 16388) and other phenylphosphonate esters. J.
Toxicol. environ. Health, 10 : 907-920.
SOLIMAN, S.A., FARMER, J., & CURLEY, A. (1982) Is delayed
neuropathy a property of all organophosphorus compounds?
Toxicology, 23 : 267-279.
SOLIMAN, S.A., SOVOCOOL, G.W., CURLEY, A., AHMED, N.S.,
EL-FIKI, S., & EL-SEBAE, A.H. (1982) Two acute human
poisoning cases resulting from exposure to diazinon
transformation products in Egypt. Arch. environ. Health, 37 :
207-212.
SPEAR, R.C., POPENDORF, W.J., SPENCER, W.F., & MILBY, T.H.
(1977) Worker poisonings due to paraoxon residues. J. occup.
Med., 19 : 411-414.
STAPLES, R.E. & GOULDING, E.H. (1979) Dipterex
teratogenicity in the rat, hamster, and mouse when given by
gavage. Environ. Health Perspect., 30 : 105-113.
STEFANAC, Z., STENGL, B., & VASILIC, Z. (1976) Quantitative
determination of organophosphorus pesticides by thin-layer
densitometry. J. Chromatogr., 124 : 127-133.
SU, M.-Q., KINOSHITA, F.K., FRAWLEY, J.P., & DUBOIS, K.P.
(1971) Comparative inhibition of aliesterases and
cholinesterase in rats fed eighteen organophosphorus
insecticides. Toxicol. appl. Pharmacol., 20 : 241-249.
TALCOTT, R.E., DENK, H., & MALLIPUDI, N.M. (1979a) Malathion
carboxylesterase activity in human liver and its inactivation
by isomalathion. Toxicol. appl. Pharmacol., 49 : 373-376.
TALCOTT, R.E., MALLIPUDI, N.M., UMETSU, N., & FUKUTO, T.R.
(1979b) Inactivation of esterases by impurities isolated from
technical malathion. Toxicol. appl. Pharmacol., 49 : 107-112.
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman,
L.S. & Gilman, A., ed. The pharmacological basis of
therapeutics, 6th ed., New York, Macmillan Publishing Co.,
pp. 100-119.
UMETSU, N., GROSE, F.H., ALLAHYARI, R., ABU-EL-HAJ, S., &
FUKUTO, T.R. (1977) Effect of impurities on the mammalian
toxicity of technical malathion and acephate. J. agric. food
Chem., 25 : 946-953.
UNITED KINGDOM PSPS (1979) Guidance on toxicity data
requirements, London, MAFF, United Kingdom Pesticide Safety
Precautions Scheme, 3 pp (Working Document B5).
US EPA (1975) Registration, re-registration, and
classification procedures. Fed. Reg., 40 (129): 28276-28277.
US EPA (1982) Acute delayed neurotoxicity of organo-
phosphorus substances, Washington DC, US Environmental
Protection Agency, Section 81-7, pp. 62-65 (Pesticide
Assessment Guidelines, Subdivision F, Hazard Evaluation: human
and domestic animals) (EPA 540/9-82-025).
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning.
Guy's Hosp. Rep., 123 : 13-25.
VASIC, B.V., MILOSEVIC, M.P., & TERZIC, M.R. (1977) Acetyl-
choline content and cholinesterase activity in the ponto-
medullary region of brain in rats treated with armin and
obidoxime. Biochem. Pharmacol., 26 : 601-602.
VELSICOL (1977) Phosvel (technical leptophos) data for
regulatory authorities, Chicago, Illinois, Velsicol Chemical
Corporation.
VERGIEVA, T. (1983) Embryotoxicity and teratogenicity of
pesticides. In: Kaloyanova, F. & Tarkowski, S., ed. Toxicology
of pesticides, Copenhagen, World Health Organization, pp.
67-77 (Health Aspects of Chemical Safety, Interim Document
No. 9).
VERONESI, B. & PADILLA, S. (1985) Phenylmethylsulfonyl
fluoride protects rats from mipafox-induced delayed
neuropathy. Toxicol. appl. Pharmacol., 81 (2): 258-264.
VERSCHOYLE, R.D. & CABRAL, J.R.P. (1982) Investigation of
the acute toxicity of some trimethyl and triethyl
phosphorthioates with particular reference to those causing
lung damage. Arch. Toxicol., 51 : 221-231.
VERSCHOYLE, R.D., ALDRIDGE, W.N., & CABRAL, J.R.P. (1980)
Toxicology of trimethyl and triethyl phosphorothioates. In:
Holmstedt, B., Lauwerys, R., Mercier, M., & Roberfroid, M.,
ed. Mechanisms of toxicity and hazard evaluation, Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp. 631-634.
VETTORAZZI, G. (1979) International regulatory aspects of
pesticide chemicals, Vol. 1 & 2, Boca Raton, Florida, CRC
Press, 232 and 214 pp.
VETTORAZZI, G. & VAN DEN HURK, G. (1984) Pesticides
reference index: JMPR-IARC-IPCS-IRPTC-VBC: 1961-1984, Geneva,
World Health Organization (Unpublished report).
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977)
Severe organophosphate poisoning complicated by alcohol and
terpentine ingestion. Arch. environ. Health, 32 : 203-205.
WHO (1975) Early detection of health impairment in
occupational exposure to health hazards, Geneva, World Health
Organization (Technical Report Series No. 571).
WHO (1979) Third report of the WHO Expert Committee on
vector biology and control, Geneva, World Health Organization,
44 pp (WHO Technical Report Series No. 634).
WHO (1982) Field surveys of exposure to pesticides -
standard protocol, Geneva, World Health Organization
(Unpublished report VBC/82.1).
WHO (1984a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1984-1985, Geneva,
World Health Organization (Unpublished report VBC/84.2).
WHO (1984b) Spectrophotometric kit for measuring
cholinesterase activity, Geneva, World Health Organization
(Unpublished report VBC/84.888).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World
Health Organization (VBC).
WICKER, G.W., WILLIAMS, W.A., & GUTHRIE, F.E. (1979)
Exposure of field workers to organophosphorus insecticides:
sweet corn and peaches. Arch. environ. Contam. Toxicol., 8 :
175-182.
WILKINSON, C.F. (1971) Effects of synergists on the
metabolism and toxicity of anticholinesterases. WHO Bull., 44 :
171-190.
WILLEMS, J.L. (1981) Poisoning by organophosphate
insecticides: analysis of 53 human cases with regard to
management and drug treatment. Acta med. milit. Belg., 134 :
7-14.
WILLS, J.H. (1963) [Pharmacological antagonists of the
anticholinesterase agents.] In: Koelle, G.B., ed. [Handbook of
experimental pharmacology,] Berlin, Springer-Verlag, Vol. 15,
pp. 883-920 (in German).
WILSON, B.W., HOOPER, M., CHOW, E., HIGGINS, R.J., & KNAAK,
J.B. (1984) Antidotes and neuropathic potential of
isofenphos. Bull. environ. Contam. Toxicol., 33 : 386-394.
WILTROUT, R.W., ERGEGOVICH, C.D., & CEGLOWSKI, W.S. (1978)
Humoral immunity in mice following oral administration of
selected pesticides. Arch. environ. Contam. Toxicol., 20 :
423-431.
WITTER, R.F. & GAINES, T.B. (1963) Relationship between
depression of the brain or plasma cholinesterase and paralysis
in chickens caused by certain organic phosphorus compounds.
Biochem. Pharmacol., 12 : 1377-1386.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1967) Exposure
of workers to pesticides. Arch. environ. Health, 14 : 622-633.
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1970) Urinary
excretion of insecticide metabolites. Arch. environ. Health,
21 : 711-716.
WOLFE, H.R., STAIFF, D.C., & ARMSTRONG, J.F. (1978) Exposure
of pesticide formulating plant workers to parathion. Bull.
environ. Contam. Toxicol., 20 : 340-343.
WOLTHUIS, O.L. & VANWERSCH, R.A.P. (1984) Behavioural
changes in the rat after low doses of cholinesterase
inhibitors. Toxicol. appl. Pharmacol., 4 : S195-S208.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) In vivo
alkylation studies with dichlorvos at practical use
concentrations. Chem.-biol. Interact., 19 : 25-46.
WORTHING, C.R., ed. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council,
655 pp.
XINTARAS, C. & BURG, J.R. (1980) Screening and prevention of
human neurotoxic outbreaks: issues and problems. In: Spencer,
P.S. & Schaumburg, H.H., ed. Clinical and experimental
neurotoxicology, Baltimore, Maryland, Williams & Wilkins
Company, pp. 663-674.
XINTARAS, C., BURG, J.R., TANAKA, S., LEE, S.T., JOHNSON,
B.L., COTTRILL, C.A., & BENDER, J. (1978) Occupational
exposure to leptophos and other chemicals, Cincinnati, Ohio,
US Department of Health and Welfare, National Institue of
Occupational Safety and Health (US DHEW (NIOSH) Publication
No. 78-136).
YANG, R.S.H., HODGSON, E., & DAUTERMAN, W.C. (1971)
Metabolism in vitro of diazinon and diazoxon in rat liver. J.
agric. food Chem., 19 : 10-13.
YOSHIDA, K. & NISHIUCHI, Y. (1972) Toxicity of pesticides to
some water organisms.] Bull. agric. Chem. Inspect. Stn
(Japan), 12 : 122-128 (in Japanese).
YOSHIDA, K. & NISHIUCHI, Y. (1976) Toxicity of pesticides to
some aquatic animals.] Bull. agric. Chem. Inspect. Stn
(Japan), 16 : 65-69 (in Japanese).
ZACKOV, K. (1983) Immunotoxicology of pesticides. In:
Kaloyanova, F. & Tarkowski, S., ed. Toxicology of pesticides,
Copenhagen, World Health Organization, pp. 75-88 (Health
Aspects of Chemical Safety, Interim Document No. 9).
ZIELHUIS, R.L. (1972) Epidemiological toxicology of
pesticide exposure: report of an international workshop. Arch.
environ. Health, 25 : 399-405.
Annex I. Names and structures of selected organophosphorus pesticides
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other formula molecular Registry
name mass number
-------------------------------------------------------------------------------------------
acephate Orthene phosphoramidothioic C4 H10 NO3 PS 183.18  amidithion Thiocron phosphorodithioic C7 H16 NO4 PS2 273.33
amidithion Thiocron phosphorodithioic C7 H16 NO4 PS2 273.33  amiton Citram phosphorothioic C10 H24 NO3 PS 269.38
amiton Citram phosphorothioic C10 H24 NO3 PS 269.38  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
azinophos- Cotnion- phosphorodithioic C12 H16 N3 O3 PS2 345.4
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
azinophos- Cotnion- phosphorodithioic C12 H16 N3 O3 PS2 345.4  azinophos- Cotnion- phosphorodithiotic C10 H12 N3 O3 PS2 317.34
azinophos- Cotnion- phosphorodithiotic C10 H12 N3 O3 PS2 317.34 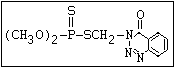 azothoate alamos phosphorothiotic C14 H14 ClN2 O3 PS 356.78
azothoate alamos phosphorothiotic C14 H14 ClN2 O3 PS 356.78  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
bromophos netal phosphorothioic C8 H8 BrCl2 O3 PS 366.0
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
bromophos netal phosphorothioic C8 H8 BrCl2 O3 PS 366.0  bromophos- nexagan phosphorothioic C10 H12 BrCl2 O3 PS 394.06
bromophos- nexagan phosphorothioic C10 H12 BrCl2 O3 PS 394.06  butonate tribufon butanoic acid, C8 H14 Cl3 O5 P 327.54
butonate tribufon butanoic acid, C8 H14 Cl3 O5 P 327.54  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
chlorfen- birlane phosphoric acid, C12 H14 Cl3 O4 P 359.58
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
chlorfen- birlane phosphoric acid, C12 H14 Cl3 O4 P 359.58  chlorpyri- dursban phosphorothioic C9 H11 Cl3 NO3 PS 350.59
chlorpyri- dursban phosphorothioic C9 H11 Cl3 NO3 PS 350.59  chlorpyri- fospirate phosphorothioic C7 H7 Cl3 NO3 PS 322.53
chlorpyri- fospirate phosphorothioic C7 H7 Cl3 NO3 PS 322.53  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
coumaphos agridip phosphorothioic C14 H16 Cl05 PS 326.78
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
coumaphos agridip phosphorothioic C14 H16 Cl05 PS 326.78  crotoxy- ciodrin 2-butenoic acid, C14 H19 O6 P 314.3
crotoxy- ciodrin 2-butenoic acid, C14 H19 O6 P 314.3  crufomate montrel phosphoramidic C12 H19 ClNO3 P 291.74
crufomate montrel phosphoramidic C12 H19 ClNO3 P 291.74  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
demeton- metasystox phosporothioic C6 H15 O3 PS2 230.3
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
demeton- metasystox phosporothioic C6 H15 O3 PS2 230.3 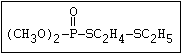 diazinon basudin phosphorothioc C12 H21 N2 O3 PS 304.38
diazinon basudin phosphorothioc C12 H21 N2 O3 PS 304.38  dichlo- ECP phosphorothioic C10 H13 Cl2 O3 PS 315.16
dichlo- ECP phosphorothioic C10 H13 Cl2 O3 PS 315.16  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dichlorves atgard phosphoric acid, C4 H7 Cl2 O4 P 220.98
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dichlorves atgard phosphoric acid, C4 H7 Cl2 O4 P 220.98  dicroto- bidrin phosphoric acid, C8 H16 NO5 P 237.22
dicroto- bidrin phosphoric acid, C8 H16 NO5 P 237.22  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dimethoate cygon phosphorodithioic C5 H12 NO3 PS2 229.27
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
dimethoate cygon phosphorodithioic C5 H12 NO3 PS2 229.27  dioxathion delnav phosphorodithioic C12 H26 O6 P2 S4 456.56
dioxathion delnav phosphorodithioic C12 H26 O6 P2 S4 456.56  disulfoton dimaz phosphorodithioic C8 H19 O2 PS3 274.42
disulfoton dimaz phosphorodithioic C8 H19 O2 PS3 274.42  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
EPN phosphonothioic C14 H14 NO4 PS 323.32
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
EPN phosphonothioic C14 H14 NO4 PS 323.32  ethion bladan phosphorodithioic C9 H22 O4 P2 S4 384.49
ethion bladan phosphorodithioic C9 H22 O4 P2 S4 384.49  fenamiphos nemacur phosphoramidic C13 H22 NO3 PS 272.34
fenamiphos nemacur phosphoramidic C13 H22 NO3 PS 272.34  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fenchlor- ectoral phosphorothioic C8 H8 Cl3 O3 PS 321.54
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fenchlor- ectoral phosphorothioic C8 H8 Cl3 O3 PS 321.54  fenitro- accothion phosphorothioic C9 H12 NO5 PS 277.25 122-12-5
thion cyfen acid, O,O -dimethyl
cytel O -(3-methyl-4-
felithion nitrophenyl) pester
metathion
nitrophos
nevathion
sumithion
fenitro- accothion phosphorothioic C9 H12 NO5 PS 277.25 122-12-5
thion cyfen acid, O,O -dimethyl
cytel O -(3-methyl-4-
felithion nitrophenyl) pester
metathion
nitrophos
nevathion
sumithion
 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fensulfo- dasanit phosphorothioic C11 H17 O4 PS2 308.37
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fensulfo- dasanit phosphorothioic C11 H17 O4 PS2 308.37  fenthion baycid phosphorothioic C10 H15 O3 PS2 278.34
fenthion baycid phosphorothioic C10 H15 O3 PS2 278.34  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fonofos Dyfonate O -ethyl S -phenyl C10 H15 OPS2 246.3
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
fonofos Dyfonate O -ethyl S -phenyl C10 H15 OPS2 246.3  formothion aflix phosphorodithioic C6 H12 NO4 PS2 257.28
formothion aflix phosphorodithioic C6 H12 NO4 PS2 257.28 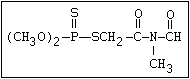 fosthietan phosphoramidic C6 H12 NO3 PS2 241.
fosthietan phosphoramidic C6 H12 NO3 PS2 241.  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
hepteno- hostaquick phosphoric acid, C9 H12 ClO4 P 250.63
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
hepteno- hostaquick phosphoric acid, C9 H12 ClO4 P 250.63  idofenphos alfacron phosphorothioic C8 H8 Cl2 IO3 PS 412.99
idofenphos alfacron phosphorothioic C8 H8 Cl2 IO3 PS 412.99  isofenphos oftanol benzoic acid, 2- C15 H24 NO4 PS 345.43
isofenphos oftanol benzoic acid, 2- C15 H24 NO4 PS 345.43  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
leptophos Phosvel O -(4-bromo-2,5-di- C13 H10 BrCl2 O2 PS 412.1
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
leptophos Phosvel O -(4-bromo-2,5-di- C13 H10 BrCl2 O2 PS 412.1  malathion carbetox butanedioic acid, C10 H19 O6 PS2 330.38
malathion carbetox butanedioic acid, C10 H19 O6 PS2 330.38  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
mecarbam afos carbamic acid, C10 H20 NO5 PS2 329.4
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
mecarbam afos carbamic acid, C10 H20 NO5 PS2 329.4 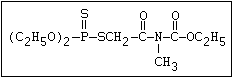 menazon azidithion phosphorodithioic C6 H12 N5 O2 PS2 281.32
menazon azidithion phosphorodithioic C6 H12 N5 O2 PS2 281.32  mephosfolan Cytrolane diethyl(4-methyl-1, C8 H16 NO3 PS2 269.3
mephosfolan Cytrolane diethyl(4-methyl-1, C8 H16 NO3 PS2 269.3  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
methamido- hamidop phosphoramidothioic C2 H8 NO2 PS 141.14
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
methamido- hamidop phosphoramidothioic C2 H8 NO2 PS 141.14 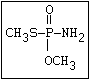 methida- supracide phosphorodithioic C6 H11 N2 O4 PS2 302.34
methida- supracide phosphorodithioic C6 H11 N2 O4 PS2 302.34 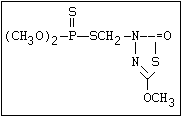 mevinphos gestid 2-butenoic acid, C7 H13 O6 P 224.17
mevinphos gestid 2-butenoic acid, C7 H13 O6 P 224.17 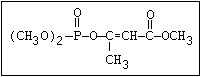 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
monocro- azodrin phosphoric acid, C7 H14 NO5 P 223.19
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
monocro- azodrin phosphoric acid, C7 H14 NO5 P 223.19  morpho- ekatin phosphorodithioic C8 H16 NO4 PS2 285.34
morpho- ekatin phosphorodithioic C8 H16 NO4 PS2 285.34 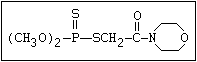 naled arthodibrom phosphoric acid, C4 H7 Br2 Cl2 O4 P 380.8
naled arthodibrom phosphoric acid, C4 H7 Br2 Cl2 O4 P 380.8  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
omethoate dimethoxon phosphorothioic C5 H10 NO4 PS 213.21
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
omethoate dimethoxon phosphorothioic C5 H10 NO4 PS 213.21  oxydeme- phosphorothioic C6 H15 O4 PS2 246.3
oxydeme- phosphorothioic C6 H15 O4 PS2 246.3  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
parathion alkron phosphorothioic C10 H14 NO5 PS 291.28
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
parathion alkron phosphorothioic C10 H14 NO5 PS 291.28  parathion- amofos phosphorothioic C8 H10 NO5 PS 263.22
parathion- amofos phosphorothioic C8 H10 NO5 PS 263.22 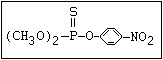 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phenthoate pap benzeneacetic acid, C12 H17 O4 PS2 320.38 2597-03-2
papthion alpha-[(dimethoxy-
tanone phosphinothioyl)-
cidial thio]-, ethyl ester
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phenthoate pap benzeneacetic acid, C12 H17 O4 PS2 320.38 2597-03-2
papthion alpha-[(dimethoxy-
tanone phosphinothioyl)-
cidial thio]-, ethyl ester
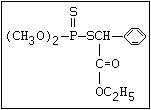 phorate granutox phosphorodithioic C7 H17 O2P S3 260.39
phorate granutox phosphorodithioic C7 H17 O2P S3 260.39  phosalone azofene phosphordithioic C12 H15 ClNO4 PS2 367.82
phosalone azofene phosphordithioic C12 H15 ClNO4 PS2 367.82  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phosmet decemthion phosphorodithioic C11 H12 NO2 PS2 317.33
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phosmet decemthion phosphorodithioic C11 H12 NO2 PS2 317.33 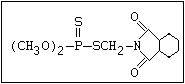 phospha- dimeron phosphoric acid, C10 H19 ClNO5 P 299.72
phospha- dimeron phosphoric acid, C10 H19 ClNO5 P 299.72  phosfolan Cyolane P,P-diethyl cyclic- C7 H14 NO3 PS2 255.3
phosfolan Cyolane P,P-diethyl cyclic- C7 H14 NO3 PS2 255.3 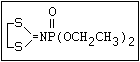 -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phoxim baythion 3,5-dioxa-6-aza- C12 H15 N2 O3 PS 298.32
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
phoxim baythion 3,5-dioxa-6-aza- C12 H15 N2 O3 PS 298.32  profenofos Curacron O -(4-bromo-2-chloro- C11 H15 BrCl03 PS 373.6
profenofos Curacron O -(4-bromo-2-chloro- C11 H15 BrCl03 PS 373.6  prothiofos Tokuthion dichlorophenyl O - C11 H15 Cl2 O2 PS2 345.2
prothiofos Tokuthion dichlorophenyl O - C11 H15 Cl2 O2 PS2 345.2  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
prothoate fal phosphorodithioic C9 H20 NO3 PS2 285.39
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
prothoate fal phosphorodithioic C9 H20 NO3 PS2 285.39  pyrimiphos- fernex phosphorothioic C13 H24 N3 O3 PS 302.46
pyrimiphos- fernex phosphorothioic C13 H24 N3 O3 PS 302.46  pyrimiphos- actellic phosphorothioic C11 H20 N3 O3 PS 274.4
pyrimiphos- actellic phosphorothioic C11 H20 N3 O3 PS 274.4  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
pyrazophos afugan pyrazolo[1,5a]pyri- C14 H20 N3 O5 PS 373.4
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
pyrazophos afugan pyrazolo[1,5a]pyri- C14 H20 N3 O5 PS 373.4  sulfotep bladafume thiodiphosphoric C8 H20 O5 P2 S2 322.34
sulfotep bladafume thiodiphosphoric C8 H20 O5 P2 S2 322.34 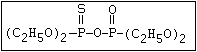 sulprofos Bolstar O -ethyl O -[4- C12 H19 O2 PS3 322.4
sulprofos Bolstar O -ethyl O -[4- C12 H19 O2 PS3 322.4  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
temephos abate phosphorothioic C16 H20 O6 P2 S3 466.48
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
temephos abate phosphorothioic C16 H20 O6 P2 S3 466.48  TEPP bladan diphosphoric acid, C8 H20 O7 P2 290.22
TEPP bladan diphosphoric acid, C8 H20 O7 P2 290.22  tetrachlor- appex phosphoric acid, C10 H9 Cl4 O4 P 365.96
tetrachlor- appex phosphoric acid, C10 H9 Cl4 O4 P 365.96  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
thiometon ekatin phosphorodithioic C6 H15 O2 PS3 246.36
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
thiometon ekatin phosphorodithioic C6 H15 O2 PS3 246.36 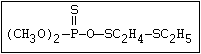 thionazin cynem phosphorothioic C6 H13 N2 O3 PS 248.26
thionazin cynem phosphorothioic C6 H13 N2 O3 PS 248.26  triamiphos wepsin phosphonic diamide, C12 H19 N6 CP 294.34
triamiphos wepsin phosphonic diamide, C12 H19 N6 CP 294.34  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
triazophos Hostathion O,O -diethyl O -(1H- C12 H16 N3 O3 PS 313.3
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
triazophos Hostathion O,O -diethyl O -(1H- C12 H16 N3 O3 PS 313.3  trichlor- anthion phosphonic acid, C4 H8 Cl3 O4 P 257.44
trichlor- anthion phosphonic acid, C4 H8 Cl3 O4 P 257.44  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
trichlor- agrisil phosphonothioic C10 H12 Cl3 O2 PS 333.6
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
trichlor- agrisil phosphonothioic C10 H12 Cl3 O2 PS 333.6  trifenofos RH 218 O -ethyl- S -propyl- O- C11 H14 Cl3 O3 PS 363.6
trifenofos RH 218 O -ethyl- S -propyl- O- C11 H14 Cl3 O3 PS 363.6  -------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
vamidothion kilyal phosphorothioic C8 H18 NO4 PS2 287.36
-------------------------------------------------------------------------------------------
Annex I. (contd.)
-------------------------------------------------------------------------------------------
Common name Trade or CAS chemical name Molecular Relative CAS
other name formula molecular Registry
mass number
-------------------------------------------------------------------------------------------
vamidothion kilyal phosphorothioic C8 H18 NO4 PS2 287.36  -------------------------------------------------------------------------------------------
Annex II. Organophosphorus pesticides: JMPR reviews, ADIs, Evaluation by IARC, Classification by
Hazard, FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Acephate 1984 0-0.0005 1985a
(temporary)
1982 0-0.003 1983b + III
(temporary)
1983a
1981i 0-0.02 1982b
1982a
1979i 0-0.02 1980b
1980a
1978 0-0.02 1979a
1976 0-0.02 1977b
1977a
Azinphos 1983i no ADI 1984a + +
-ethyl 1973 no ADI 1974b IB
1974a
Azinphos 1974i 1975b + + IB
-methyl 1975a
1973 0-0.0025 1974b
1974a
1972i 0-0.0025 1973b
1973a
1968 0-0.0025 1969b
1969a
1965 1965b
0-0.0025 1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Bromophos 1984i 0-0.04 1985b
1982i 0-0.04 1983b + + III
1983a
1978i 0-0.04 1979a
1977 0-0.04 1978b
1978a
1975i 0-0.006 1976b
(temporary) 1976a
1972 0-0.006 1973b
(temporary) 1973a
Bromophos 1978i 0-0.003 1979a + + IB
-ethyl 1977i 0-0.003 1978b
1978a
1975 0-0.003 1976b
1976a
1972 0-0.003 1973b
(temporary) 1973a
Carbophen- 1983i 0-0.0005 1984a + + IB
othion 1980 0-0.0005 1981b
1981a
1979 0-0.0005 1980b
1980a
1977 0-0.0002 1978b
(temporary) 1978a
1976 temporary 1977b
ADI withdrawn
1977a
1972 0-0.005 1973b
(temporary)
1973a
Chlorfen- 1971 0-0.002 1972b + + IA
vinphos 1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Chlorpy- 1983i 0-0.01 1984a + + II No. 18
rifos 1982 0-0.01 1983b (1975)
1983a
1981i 0-0.001 1982b
1982a
1977 0-0.001 1978b
1978a
1975i 0-0.0015 1976b
1976a
1974i 0-0.0015 1975b
1975a
1972 0-0.0015 1973b
1973a
Chlorpy- 1979i 0-0.01 1980b + + No. 33
rifos- 1980a (1978)
methyl 1975 0-0.01 1976b II
1976a
Chlorthion 1965 no ADI 1965b
1965a -
1963 no ADI 1964
Coumaphos 1983i ADI withdrawn 1984a + + IA
1980i ADI Withdrawn 1981b
1981a
1978i 0-0.005 1979b
(temporary)
1979a
1975i 0-0.005 1976b
(temporary)
1976a
1972i 0-0.0005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1968 0-0.0005 1969b
(temporary)
1969a
Crufomate 1972i 0-0.1 1973b III
1973a
1968 0-0.1 1969b
1969a
Cyano- 1983 ADI withdrawn 1984a II
fenphos 1982i 0-0.001 1983b
(temporary)
1983a
1980 0-0.001 1981b
(temporary)
1981a
1978i 0-0.005 1979a
(temporary)
Cyano- 1975 0-0.005 1976b
fenphos (temporary)
1976a
Demeton 1983i no ADI 1984a + +
(see also 1982 ADI withdrawn 1983a
disulfoton) 1975 0-0.005 1976b IA No. 60
(in prep)
1976a
1967i 0-0.0025 1968b
1968a
1965 0-0.0025 1965b
1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- S - 1984 no ADI 1985b
methyl and 1983i no ADI 1984a + + IB
Related 1982 ADI withdrawn 1983a
Compounds 1979i 0-0.005 (the 1980b
(see also total demeton-
oxydemeton- S -methyl, 1980a IB
methyl for demeton- S -
1963 to 1968 methyl sulfo-
evaluations) xide and deme-
ton- S -methyl
sulfone not
to exceed
this figure)
1973 0-0.005 (the 1974b
total demeton 1974a
- S -methyl,
demeton- S -
methyl sulf-
oxide and
demeton- S -
methyl sulfone
not to exceed
this figure)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- 1984 no ADI 1985b
S -methyl 1983i no ADI 1984a
sulfoxide 1982 ADI 1983a IB
(see withdrawn
oxydemeton-
methyl for
1963 to 1968
evaluation)
(see demeton-
S -methyl and
related
compounds
after 1968)
Dialifos 1982 ADI 1983a II
withdrawn
1978i 0-0.003 1979a
1976 0-0.003 1977b
1977a
Diazinon 1979i 0-0.002 1983a + + II No. 45/1979
1980a
1975i 0-0.002 1976b
1976a
1970 0-0.002 1971b
1971a
1968i 0-0.002 1969b
1969a
1967i 0-0.002 1968b
1968a
1966 0-0.002 1969b
1967a
1965 0-0.002 1965b
1965a
1963 no ADI 1964 +
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Dichlorvos 1977 0-0.004 1978b Vol 20 + + IB No. 2 (1978)
p.97 (Rev. 1)
1978a
1974i 0-0.004 1975b
1975a
1970 0-0.004 1971b
1971a
1969i 0-0.004 1970b
1970a
1967 1968b
0-0.004 1968a
1966 1967b
0-0.004 1967a
1965 no ADI 1965b
1965a
Dimethoate 1984 0-0.002 1985b
(temporary)
1983i 0-0.02 1984a + + No. 42
1978i 0-0.02 1979a (1980)
1977i 0-0.02 1978b Vol. 15 II
page 177
1970i 0-0.02 1971b
1971a
1967 0-0.02 1968b
1968a
1966i 0-0.004 1967b
1967a
1965 0-0.004 1965b
1965a
1963 0-0.004 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Disulfoton 1984i 0-0.002 1985b
(see also 1981i 0-0.002 1982b + + No. 60 (in
demeton) prep.)
1982a
1979i 0-0.002 1980b IA
1980a
1978i 0-0.002 1979a
1975 0-0.002 1976b
1976a
1973 0-0.001 1974b
(temporary)
1974a
Edifenphos 1981 0-0.003 1982b + IB
1982a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Ethion 1985 0-0.0005 1986b
(temporary)
1983i 0-0.001 1984a + + II
(temporary)
1982 0-0.001 1983b
(temporary)
1983a
1975i 0-0.005 1976b
1976a
1972 0-0.005 1973b
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1970i 0-0.00125 1971b
1971a
1969i 0-0.00125 1970b
1970a
1968 0-0.00125 1969b
1969a
Ethoprophos 1983 no ADI 1984a IA No. 70 (in
preparation)
Etrimfos 1982 0-0.003 1983b + + II
1983a
1980 0-0.003 1981b
(temporary)
1981a
Fenamiphos 1985 0-0.0003 1986b
(temporary)
1980i 0-0.0006 1981b IA
1981a
1978i 0-0.0006 1979b
R30-17
1977i 0-0.0006 1978b
1978a
1974 0-0.0006 1975b
1975a
Fenclorphos 1983i 0-0.01 1984a + + II
1972i 0-0.01 1973b
1973a
1968 0-0.01 1969b
1969a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenitro- 1984 0-0.003 1985a
thion 1983i 0-0.001 1974a + + No. 30
(temporary) (1977)
1982 0-0.001 1983b
(temporary)
1983a II
1979i 0-0.005 1980b
1980a
1977 0-0.005 1978b
1978a
1976i 0-0.005 1977b
1977a
1974 0-0.005 1975b
1975a
1969 0-0.001 1970b
(temporary)
1970a
Fensulf- 1983i 0-0.0003 1984a + +
othion 1982 0-0.0003 1983b
1983a IA No. 44
1972 0-0.0003 1973b (1980)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenthion 1983i 0-0.001 1984a + No. 23
1980 0-0.001 1981b (1976)
1981a IB
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1977i 0-0.0005 1978b
(temporary)
1978a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
Formothion 1978i 0-0.02 1979a + + II
1973 0-0.02 1974b
1974a
1972i no ADI 1973b
1973a
1969 no ADI 1970b
1970a
Isophenphos 1984i 0-0.0005 1985a
1982 0-0.0005 1983b IB
(temporary)
1983a
1981 0-0.0005 1982b
(temporary)
1982a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Iodophenphos O No. 43
(1980)
Leptophos 1978i ADI 1979b + + No. 38
withdrawn 1979a (1979)
1976i 0-0.001 1977a IA
(temporary)
1975 0-0.001 1976b
(temporary)
1976a
1974 No ADI 1975b
1975a
Malathion 1984 0-0.02 1985b
1977i 0-0.02 1978b Vol. 30 + + No. 29
page 103 (1977)
1978a
1975i 0-0.02 1976b III
1976a
1973i 0-0.02 1974b
1974a
1970i 0-0.02 1971b
1971a
1969i 0-0.02 1970b
1970a
1968i 0-0.02 1969b
1969a
1967i 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Mecarbam 1985 0-0.0005 1986b
(temporary)
1983 0-0.001 1984a IB
(temporary)
1980 0-0.001 1981b
(temporary)
1981a
Methacrifos 1982 0-0.0003 1983b -
(temporary)
1983a
1980 0-0.0003 1981b
(temporary)
1981a
Methamido- 1985 0-0.0006 1986b
phos 1984i 0-0.0004 1985a
(temporary)
1982 0-0.0004 1983b + + IB
(temporary)
1983a
1981i 0-0.002 1982b
1982a
1979i 0-0.002 1980b
1980a
1976 0-0.002 1977b
1977a
Methida- 1979i 0-0.005 1980b + + IB
thion 1980a
1977i 0-0.005 1978a
1975 0-0.005 1976b
1976a
1972 0-0.005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe> : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Methyl parathion
(see parathionmethyl)
Mevinphos 1972 0-0.0015 1973b + + IA No. 14
1973a (1975)
1965 no ADI 1965b +
1965a
1963 no ADI 1964
Monocroto- 1975 0-0.0006 1976b + + IB
phos 1976a
1972 0-0.0003 1973b
1973a
Omethoate 1985 0-0.0003 1986b
1984i 0-0.0005 1985a
(temporary)
1981 0-0.0005 1982b IB
(temporary)
1982a
1980i 0-0.0005 1981b
(temporary)
1981a
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Oxydemeton- 1968i ADI 1969b
methyl withdrawn 1969a IB
(referred in 1967 0-0.0025 1968b
1963 and 1965 1968a
reports as 1965 0-0.0025 1965b
demeton- S - 1965a
methyl 1963 0-0.0025 1964
sulfoxide)
(see
demeton- S -
methyl and
related comp-
ounds for
evaluations
after 1968)
Parathion 1984i 0-0.005 1985a
1970i 0-0.005 1971b Vol. 30 + + IA No. 6 (1978)
page 153 (Rev. 1)
1971a
1969i 0-0.005 1970b
1970a
1967 0-0.005 1968b
1968a
1965 0-0.005 1965b
1965a
1963 0-0.005 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Parathion- 1984 0-0.02 1985b
methyl
(evaluated 1982 0-0.001 1983b vol. page + + IA No. 7 (1978)
under methyl (temporary) 30-131 (Rev. 1)
parathion 1983a
in 1963 and 1980 0-0.001 1981b
1965) (temporary)
1981a
1979 0-0.001 1980b
(temporary)
1980a
1978i 0-0.001 1979b
(temporary)
1979a
1975 0-0.001 1976b
(temporary)
1976a
1972i 0-0.001 1973b
(temporary)
1973a
1968 0-0.001 1969b
(temporary)
1969a
1965 0-0.01 1965b
1965a
1963 0-0.01 1964
Phenthoate 1984 0-0.003 1985b
1981i 0-0.001 1982b + + II No. 48
(temporary) (1983)
1982a
1980 0-0.001 1981b
(temporary)
1981a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phorate 1985 0-0.0002 1986b
1984i 0-0.0002 1985a
(temporary)
1983 0-0.0002 1984a + + IA No. 75 (in
(temporary) preparation)
1982 0-0.0002 1983b
(temporary)
1983a
1977 No ADI 1978b
established
1978a
Phosalone 1976i 0-0.006 1977b + + II
1977a
1975i 0-0.006 1976b
1976a
1972 0-0.006 1973b
1973a
Phosmet 1984i 0-0.02 1985b
1981i 0-0.02 1982b + + II
1982a
1979 0-0.02 1980b
1980a
1978 0-0.005 1979b
(temporary)
1979a
1977 -corrigenda 1978b
to 1976
evaluations -
1977i no ADI 1978a
1976i no ADI 1977b
1977a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phosphamidon 1985 0-0.0005 1986b
1982 0-0.001 1983b + + IA No. 74 (in
(temporary) preparation)
1983a
1974i 0-0.01 1975b
1975a
1972i 0-0.001 1973b
1973a
1969i 0-0.001 1970b
1970a
1968 0-0.001 1969b
1969a
1966 0-0.001 1967b
1967a
1965 no ADI 1965b
1965a
1963 no ADI 1964
Phoxim 1984 0-0.001 1985b
1983i 0-0.0005 1984a No. 31 (1978)
(temporary)
1982 0-0.0005 1983b
(temporary)
1983a II
Pirimiphos- 1983 0-0.01 1984a No. 49 (1983)
methyl 1979i 0-0.01 1980b
1980a
1977i 0-0.01 1978b III
1978a
1976 0-0.01 1977b
1977a
1974 0-0.005 1975b
(temporary)
1975a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Temephos + + 0 No. 8 (1978)
(Rev. 1)
Thiometon 1979 0-0.003 1980b + + IB
1980a
1976i 0-0.005 1977b
(temporary)
1977a
1973 0-0.005 1974b
(temporary)
1974a
1969 no ADI 1970b
1970a
Triazophos 1983i 0-0.0002 1984a IB
(temporary)
1982 0-0.0002 1983b
(temporary)
1983a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Trichlorfon 1978 0-0.01 1979b + + III No. 27 (1977)
1979a
1975 0-0.005 1976b Vol. 30
(temporary) page 207
1976a
1971 0-0.01 1972b
(temporary)
1972a
Trichloronat 1971 no ADI 1972b + + IA
1972a
Vamidothion 1985 0-0.0003 1986b
(temporary)
1982 0-0.0003 1983b + + IB
(temporary)
1983a
1973 no ADI 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in
this annex.
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling
by the manufacturer or in accordance with the rules laid down for storage and transportation
by competent international bodies.
Classification relates to the technical material, and not to the formulated
product:
--------------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
--------------------------------------------------------------------------------------
IA Extremely hazardous 5 or less 20 or less 10 or less 40 or less
IB Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal
use
--------------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex III. LD50s and no-observed-adverse-effect levels in animals
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Azinophos- 16.4 2.5 0.125 2 years FAO/WHO (1974b)
methyl 80 (guinea-pig) 5 (dog) 0.125 (dog) 2 years
Bromophos- 71 - 127 1366 (rabbit) 0.78 2 years FAO/WHO (1976b)
ethyl 10 (dog) 0.26 (dog) 2 years
225 - 550 (mice)
Bromophos 3750 - 7700 20 (dog) 0.5 (dog) 1 year FAO/WHO (1978b)
2829 - 5850 0.4 (man) 4 weeks
(mice)
720 (rabbit)
Carbopheno- 32.3 1270 (rabbit) 3 0.15 3 generations FAO/WHO (1980b)
thion 0.02 (dog) 3 months
0.01 (man) 1 month
Chlorfen- 10 - 39 31 - 108, 1 0.05 3 months FAO/WHO (1972b)
vinphos 117 - 200 (mice) 417 - 4700 1 dog 0.05 (dog) 16 weeks
(rabbit)
300 - 1000
(rabbit)
> 12 000 (dog)
Chlorpyr- 135 - 163 approximately 0.1 2 years FAO/WHO (1982b)
ifos 2000
500 (guinea-pig) (rabbit) 0.1 (dog) 90 days
32 (chicken) 0.1 (man) 1 month
1000 - 2000
(rabbit)
Crufomate 770 - 950 40 2 2 year FAO/WHO (1969b)
400 - 600 40 (dog) 1 dog 75 days
(rabbit)
Demeton 2.5 - 12
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Demeton- S - 57 - 106 302
methyl 110 (guinea-pig)
Diazinon 300 - 850 > 2150 2 0.1 90 days FAO/WHO (1971b)
0.02 (dog) 31 days
0.05 (monkey) 2 years
0.02 (man) 37 days
Dichlorvos 56 - 108 75 - 210 0.033 (man) 28 days FAO/WHO (1977b)
5 0.25 15 weeks
Dimethoate 320 - 380 0.2 (man) 57 days FAO/WHO (1985b)
15 (pheasant)
40 (duck)
Dioxathion 43 235 3 0.15 13 weeks FAO/WHO (1969b)
0.075 (dog) 90 days
0.075 (man) 28 days
Disulfoton 2.6 - 8.6 ca 20 1 0.05 2 years FAO/WHO (1976b)
1 (dog) 0.025 (dog) 12 weeks
0.075 (man) 30 days
Ethion 24.4 - 208 915 3 0.15 13 weeks FAO/WHO (1986b)
(rabbit) 0.125 (dog) 90 days
Fenamiphos 15.3 - 19.4 500 3 0.17 2 years FAO/WHO (1986b)
10 (dog) 1 (dog) 0.025 (dog) 2 years
75 - 100
(guinea-pig)
12 (hen)
Fenchlor- 1740 2000 0.5 2 years FAO/WHO (1969b)
phos 1 (dog) 2 years
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Fenitro- 250 - 500 > 3000 5 0.25 34 weeks FAO/WHO (1985b)
thion 870 (mice) (mice) 10 (dog) 0.3 (dog) 12 months
Fenthion 190 - 315 330 - 500 3 0.15 2 years FAO/WH0 (1981b)
3 (dog) 0.09 2 years
0.07 (monkey) 1 year
0.02 (man) -
Formothion 365 - 500 > 1000 20 1 2 years FAO/WHO (1974b)
40 (dog) 1 (dog) 2 years
Malathion 2800 4100 100 5 2 years FAO/WHO (1967b)
(rabbit) 0.2 (man) 88 days
Methida- 25 - 54 1546 - 1663 4 0.2 104 weeks FAO/WHO (1976b)
thion 25 - 20 (mice) 0.25 (monkey) 23 months
0.11 (man) 6 weeks
Mevinphos 3 - 12 1 - 90 0.37 0.02 2 years FAO/WHO (1973b)
7 - 18 (mice) 16 - 34 0.025 (dog) 2 years
(rabbit) 0.014 (man) 30 days
Monocroto- 14 - 23 336 0.5 0.025 12 weeks FAO/WHO (1973b)
phos (rabbit) 0.5 (dog) 0.0125 (dog) 13 weeks
Omethoate ca 50 700 1 0.05 3 months FAO/WHO (1986b)
0.025 (dog) 12 months
Parathion 3.6 - 13 6.8 - 21 0.05 (man) 3 weeks FAO/WHO (1967b)
Parathion- 2 0.1 2 years FAO/WHO (1985b)
methyl 14 - 24 67 0.3 (man) 30 days
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Phosalone 120 - 170 1500 25 1.25 2 years FAO/WHO (1973b)
180 (mice) > 1000 25 (dog) 0.625 2 years
(rabbit)
380 (guinea-pig)
290 (pheasant)
Phospha- 17 - 30 374 - 530 2 0.1 12 weeks FAO/WHO (1986)
midon 0.5 (dog) 90 days
Pirimiphos- 2050 > 2000 10 0.5 2 years FAO/WHO (1977b)
methyl (rabbit) 5 (mouse) 0.5 (mouse) 80 weeks
1180 (mice) 0.25 (man) 28 days
1000 - 2000
(guinea-pig)
1150 - 2300
(rabbit)
30 - 60 (hen)
Thiometon 120 - 130 > 1000 2.5 0.12 2 years FAO/WHO (1980b)
6 (dog) 0.5 (dog) 2 years
Trichlorfon 560 - 630 > 2000 50 2.5 2 years FAO/WHO (1979b)
50 (dog) 1.25 (dog) 4 years (dog)
---------------------------------------------------------------------------------------------------------
a From: Worthing (1983).
N.B. This reference is listed in the reference list of the main document.
Annex IV. Abbreviations used in the document
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
DDE dichlorodiphenyldichloroethylene
DDT dichlorodiphenyltrichloroethane
DEF S,S,S -tributyl phosphorotrithioate
DFP di-isopropyl fluorophosphate
EEG electroencephalogram
EMG electromyography
EPN o -ethyl- O -(4-nitrophenyl)phenylphosphonothioate
FAO Food and Agricultural Organization (United Nations)
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety
(World Health Organization)
IRPTC International Register of Potentially Toxic
Chemicals (United Nations Environment Programme)
iv intravenous
JMPR FAO/WHO Joint Meeting on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NAD nicotinamide-adenine-dinucleotide
NADPH nicotinamide-adenine-dinucleotide phosphate
(reduced form)
NTE neuropathy target esterase (formerly
neurotoxic esterase)
OMPA octamethylpyrophosphorictetramide
2-PAM pyridine-2-aldoxime methyl chloride
pseudoChE pseudocholinesterase
sc subcutaneous
TCDD 2,3,7,8-tetrachlorodibenzo-1,4-dioxin
TEPP tetraethyl pyrophosphate
TOCP tri- o -cresyl phosphate
UVR ultraviolet radiation
-------------------------------------------------------------------------------------------
Annex II. Organophosphorus pesticides: JMPR reviews, ADIs, Evaluation by IARC, Classification by
Hazard, FAO/WHO Data Sheets, IRPTC Data Profile and Legal Filea
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Acephate 1984 0-0.0005 1985a
(temporary)
1982 0-0.003 1983b + III
(temporary)
1983a
1981i 0-0.02 1982b
1982a
1979i 0-0.02 1980b
1980a
1978 0-0.02 1979a
1976 0-0.02 1977b
1977a
Azinphos 1983i no ADI 1984a + +
-ethyl 1973 no ADI 1974b IB
1974a
Azinphos 1974i 1975b + + IB
-methyl 1975a
1973 0-0.0025 1974b
1974a
1972i 0-0.0025 1973b
1973a
1968 0-0.0025 1969b
1969a
1965 1965b
0-0.0025 1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Bromophos 1984i 0-0.04 1985b
1982i 0-0.04 1983b + + III
1983a
1978i 0-0.04 1979a
1977 0-0.04 1978b
1978a
1975i 0-0.006 1976b
(temporary) 1976a
1972 0-0.006 1973b
(temporary) 1973a
Bromophos 1978i 0-0.003 1979a + + IB
-ethyl 1977i 0-0.003 1978b
1978a
1975 0-0.003 1976b
1976a
1972 0-0.003 1973b
(temporary) 1973a
Carbophen- 1983i 0-0.0005 1984a + + IB
othion 1980 0-0.0005 1981b
1981a
1979 0-0.0005 1980b
1980a
1977 0-0.0002 1978b
(temporary) 1978a
1976 temporary 1977b
ADI withdrawn
1977a
1972 0-0.005 1973b
(temporary)
1973a
Chlorfen- 1971 0-0.002 1972b + + IA
vinphos 1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Chlorpy- 1983i 0-0.01 1984a + + II No. 18
rifos 1982 0-0.01 1983b (1975)
1983a
1981i 0-0.001 1982b
1982a
1977 0-0.001 1978b
1978a
1975i 0-0.0015 1976b
1976a
1974i 0-0.0015 1975b
1975a
1972 0-0.0015 1973b
1973a
Chlorpy- 1979i 0-0.01 1980b + + No. 33
rifos- 1980a (1978)
methyl 1975 0-0.01 1976b II
1976a
Chlorthion 1965 no ADI 1965b
1965a -
1963 no ADI 1964
Coumaphos 1983i ADI withdrawn 1984a + + IA
1980i ADI Withdrawn 1981b
1981a
1978i 0-0.005 1979b
(temporary)
1979a
1975i 0-0.005 1976b
(temporary)
1976a
1972i 0-0.0005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1968 0-0.0005 1969b
(temporary)
1969a
Crufomate 1972i 0-0.1 1973b III
1973a
1968 0-0.1 1969b
1969a
Cyano- 1983 ADI withdrawn 1984a II
fenphos 1982i 0-0.001 1983b
(temporary)
1983a
1980 0-0.001 1981b
(temporary)
1981a
1978i 0-0.005 1979a
(temporary)
Cyano- 1975 0-0.005 1976b
fenphos (temporary)
1976a
Demeton 1983i no ADI 1984a + +
(see also 1982 ADI withdrawn 1983a
disulfoton) 1975 0-0.005 1976b IA No. 60
(in prep)
1976a
1967i 0-0.0025 1968b
1968a
1965 0-0.0025 1965b
1965a
1963 0-0.0025 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- S - 1984 no ADI 1985b
methyl and 1983i no ADI 1984a + + IB
Related 1982 ADI withdrawn 1983a
Compounds 1979i 0-0.005 (the 1980b
(see also total demeton-
oxydemeton- S -methyl, 1980a IB
methyl for demeton- S -
1963 to 1968 methyl sulfo-
evaluations) xide and deme-
ton- S -methyl
sulfone not
to exceed
this figure)
1973 0-0.005 (the 1974b
total demeton 1974a
- S -methyl,
demeton- S -
methyl sulf-
oxide and
demeton- S -
methyl sulfone
not to exceed
this figure)
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Demeton- 1984 no ADI 1985b
S -methyl 1983i no ADI 1984a
sulfoxide 1982 ADI 1983a IB
(see withdrawn
oxydemeton-
methyl for
1963 to 1968
evaluation)
(see demeton-
S -methyl and
related
compounds
after 1968)
Dialifos 1982 ADI 1983a II
withdrawn
1978i 0-0.003 1979a
1976 0-0.003 1977b
1977a
Diazinon 1979i 0-0.002 1983a + + II No. 45/1979
1980a
1975i 0-0.002 1976b
1976a
1970 0-0.002 1971b
1971a
1968i 0-0.002 1969b
1969a
1967i 0-0.002 1968b
1968a
1966 0-0.002 1969b
1967a
1965 0-0.002 1965b
1965a
1963 no ADI 1964 +
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Dichlorvos 1977 0-0.004 1978b Vol 20 + + IB No. 2 (1978)
p.97 (Rev. 1)
1978a
1974i 0-0.004 1975b
1975a
1970 0-0.004 1971b
1971a
1969i 0-0.004 1970b
1970a
1967 1968b
0-0.004 1968a
1966 1967b
0-0.004 1967a
1965 no ADI 1965b
1965a
Dimethoate 1984 0-0.002 1985b
(temporary)
1983i 0-0.02 1984a + + No. 42
1978i 0-0.02 1979a (1980)
1977i 0-0.02 1978b Vol. 15 II
page 177
1970i 0-0.02 1971b
1971a
1967 0-0.02 1968b
1968a
1966i 0-0.004 1967b
1967a
1965 0-0.004 1965b
1965a
1963 0-0.004 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Disulfoton 1984i 0-0.002 1985b
(see also 1981i 0-0.002 1982b + + No. 60 (in
demeton) prep.)
1982a
1979i 0-0.002 1980b IA
1980a
1978i 0-0.002 1979a
1975 0-0.002 1976b
1976a
1973 0-0.001 1974b
(temporary)
1974a
Edifenphos 1981 0-0.003 1982b + IB
1982a
1979 0-0.003 1980b
(temporary)
1980a
1976 0-0.003 1977b
(temporary)
1977a
Ethion 1985 0-0.0005 1986b
(temporary)
1983i 0-0.001 1984a + + II
(temporary)
1982 0-0.001 1983b
(temporary)
1983a
1975i 0-0.005 1976b
1976a
1972 0-0.005 1973b
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
1970i 0-0.00125 1971b
1971a
1969i 0-0.00125 1970b
1970a
1968 0-0.00125 1969b
1969a
Ethoprophos 1983 no ADI 1984a IA No. 70 (in
preparation)
Etrimfos 1982 0-0.003 1983b + + II
1983a
1980 0-0.003 1981b
(temporary)
1981a
Fenamiphos 1985 0-0.0003 1986b
(temporary)
1980i 0-0.0006 1981b IA
1981a
1978i 0-0.0006 1979b
R30-17
1977i 0-0.0006 1978b
1978a
1974 0-0.0006 1975b
1975a
Fenclorphos 1983i 0-0.01 1984a + + II
1972i 0-0.01 1973b
1973a
1968 0-0.01 1969b
1969a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenitro- 1984 0-0.003 1985a
thion 1983i 0-0.001 1974a + + No. 30
(temporary) (1977)
1982 0-0.001 1983b
(temporary)
1983a II
1979i 0-0.005 1980b
1980a
1977 0-0.005 1978b
1978a
1976i 0-0.005 1977b
1977a
1974 0-0.005 1975b
1975a
1969 0-0.001 1970b
(temporary)
1970a
Fensulf- 1983i 0-0.0003 1984a + +
othion 1982 0-0.0003 1983b
1983a IA No. 44
1972 0-0.0003 1973b (1980)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Fenthion 1983i 0-0.001 1984a + No. 23
1980 0-0.001 1981b (1976)
1981a IB
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1977i 0-0.0005 1978b
(temporary)
1978a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
Formothion 1978i 0-0.02 1979a + + II
1973 0-0.02 1974b
1974a
1972i no ADI 1973b
1973a
1969 no ADI 1970b
1970a
Isophenphos 1984i 0-0.0005 1985a
1982 0-0.0005 1983b IB
(temporary)
1983a
1981 0-0.0005 1982b
(temporary)
1982a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Iodophenphos O No. 43
(1980)
Leptophos 1978i ADI 1979b + + No. 38
withdrawn 1979a (1979)
1976i 0-0.001 1977a IA
(temporary)
1975 0-0.001 1976b
(temporary)
1976a
1974 No ADI 1975b
1975a
Malathion 1984 0-0.02 1985b
1977i 0-0.02 1978b Vol. 30 + + No. 29
page 103 (1977)
1978a
1975i 0-0.02 1976b III
1976a
1973i 0-0.02 1974b
1974a
1970i 0-0.02 1971b
1971a
1969i 0-0.02 1970b
1970a
1968i 0-0.02 1969b
1969a
1967i 0-0.02 1968b
1968a
1966 0-0.02 1967b
1967a
1965 0-0.02 1965b
1965a
1963 0-0.02 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Mecarbam 1985 0-0.0005 1986b
(temporary)
1983 0-0.001 1984a IB
(temporary)
1980 0-0.001 1981b
(temporary)
1981a
Methacrifos 1982 0-0.0003 1983b -
(temporary)
1983a
1980 0-0.0003 1981b
(temporary)
1981a
Methamido- 1985 0-0.0006 1986b
phos 1984i 0-0.0004 1985a
(temporary)
1982 0-0.0004 1983b + + IB
(temporary)
1983a
1981i 0-0.002 1982b
1982a
1979i 0-0.002 1980b
1980a
1976 0-0.002 1977b
1977a
Methida- 1979i 0-0.005 1980b + + IB
thion 1980a
1977i 0-0.005 1978a
1975 0-0.005 1976b
1976a
1972 0-0.005 1973b
(temporary)
1973a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe> : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Methyl parathion
(see parathionmethyl)
Mevinphos 1972 0-0.0015 1973b + + IA No. 14
1973a (1975)
1965 no ADI 1965b +
1965a
1963 no ADI 1964
Monocroto- 1975 0-0.0006 1976b + + IB
phos 1976a
1972 0-0.0003 1973b
1973a
Omethoate 1985 0-0.0003 1986b
1984i 0-0.0005 1985a
(temporary)
1981 0-0.0005 1982b IB
(temporary)
1982a
1980i 0-0.0005 1981b
(temporary)
1981a
1979 0-0.0005 1980b
(temporary)
1980a
1978 0-0.0005 1979b
(temporary)
1979a
1975 0-0.0005 1976b
(temporary)
1976a
1971 0-0.0005 1972b
(temporary)
1972a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Oxydemeton- 1968i ADI 1969b
methyl withdrawn 1969a IB
(referred in 1967 0-0.0025 1968b
1963 and 1965 1968a
reports as 1965 0-0.0025 1965b
demeton- S - 1965a
methyl 1963 0-0.0025 1964
sulfoxide)
(see
demeton- S -
methyl and
related comp-
ounds for
evaluations
after 1968)
Parathion 1984i 0-0.005 1985a
1970i 0-0.005 1971b Vol. 30 + + IA No. 6 (1978)
page 153 (Rev. 1)
1971a
1969i 0-0.005 1970b
1970a
1967 0-0.005 1968b
1968a
1965 0-0.005 1965b
1965a
1963 0-0.005 1964
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Parathion- 1984 0-0.02 1985b
methyl
(evaluated 1982 0-0.001 1983b vol. page + + IA No. 7 (1978)
under methyl (temporary) 30-131 (Rev. 1)
parathion 1983a
in 1963 and 1980 0-0.001 1981b
1965) (temporary)
1981a
1979 0-0.001 1980b
(temporary)
1980a
1978i 0-0.001 1979b
(temporary)
1979a
1975 0-0.001 1976b
(temporary)
1976a
1972i 0-0.001 1973b
(temporary)
1973a
1968 0-0.001 1969b
(temporary)
1969a
1965 0-0.01 1965b
1965a
1963 0-0.01 1964
Phenthoate 1984 0-0.003 1985b
1981i 0-0.001 1982b + + II No. 48
(temporary) (1983)
1982a
1980 0-0.001 1981b
(temporary)
1981a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phorate 1985 0-0.0002 1986b
1984i 0-0.0002 1985a
(temporary)
1983 0-0.0002 1984a + + IA No. 75 (in
(temporary) preparation)
1982 0-0.0002 1983b
(temporary)
1983a
1977 No ADI 1978b
established
1978a
Phosalone 1976i 0-0.006 1977b + + II
1977a
1975i 0-0.006 1976b
1976a
1972 0-0.006 1973b
1973a
Phosmet 1984i 0-0.02 1985b
1981i 0-0.02 1982b + + II
1982a
1979 0-0.02 1980b
1980a
1978 0-0.005 1979b
(temporary)
1979a
1977 -corrigenda 1978b
to 1976
evaluations -
1977i no ADI 1978a
1976i no ADI 1977b
1977a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Phosphamidon 1985 0-0.0005 1986b
1982 0-0.001 1983b + + IA No. 74 (in
(temporary) preparation)
1983a
1974i 0-0.01 1975b
1975a
1972i 0-0.001 1973b
1973a
1969i 0-0.001 1970b
1970a
1968 0-0.001 1969b
1969a
1966 0-0.001 1967b
1967a
1965 no ADI 1965b
1965a
1963 no ADI 1964
Phoxim 1984 0-0.001 1985b
1983i 0-0.0005 1984a No. 31 (1978)
(temporary)
1982 0-0.0005 1983b
(temporary)
1983a II
Pirimiphos- 1983 0-0.01 1984a No. 49 (1983)
methyl 1979i 0-0.01 1980b
1980a
1977i 0-0.01 1978b III
1978a
1976 0-0.01 1977b
1977a
1974 0-0.005 1975b
(temporary)
1975a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Temephos + + 0 No. 8 (1978)
(Rev. 1)
Thiometon 1979 0-0.003 1980b + + IB
1980a
1976i 0-0.005 1977b
(temporary)
1977a
1973 0-0.005 1974b
(temporary)
1974a
1969 no ADI 1970b
1970a
Triazophos 1983i 0-0.0002 1984a IB
(temporary)
1982 0-0.0002 1983b
(temporary)
1983a
---------------------------------------------------------------------------------------------------------
Annex II. (contd.)
---------------------------------------------------------------------------------------------------------
Compound Year of ADIb Evaluation IARCd Availability WHO recom- FAO/WHO Data
JMPR (mg/kg body by JMPRc : Evaluation of IRPTCe : mended clas- Sheets on
meeting weight) Published of Carcino- Data Legal sification Pesticidesh
in: FAO/WHO genicity Profile filef of pesticides
by hazardg
---------------------------------------------------------------------------------------------------------
Trichlorfon 1978 0-0.01 1979b + + III No. 27 (1977)
1979a
1975 0-0.005 1976b Vol. 30
(temporary) page 207
1976a
1971 0-0.01 1972b
(temporary)
1972a
Trichloronat 1971 no ADI 1972b + + IA
1972a
Vamidothion 1985 0-0.0003 1986b
(temporary)
1982 0-0.0003 1983b + + IB
(temporary)
1983a
1973 no ADI 1974b
1974a
---------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register of Potentially Toxic Chemicals (UNEP, Geneva).
f From: IRPTC (1983).
g From: WHO (1984a). See this reference for classification of organophosphates not mentioned in
this annex.
The hazard referred to in this Classification is the acute risk for health (that is, the risk of
single or multiple exposures over a relatively short period of time) that might be encountered
accidentally by any person handling the product in accordance with the directions for handling
by the manufacturer or in accordance with the rules laid down for storage and transportation
by competent international bodies.
Classification relates to the technical material, and not to the formulated
product:
--------------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
--------------------------------------------------------------------------------------
IA Extremely hazardous 5 or less 20 or less 10 or less 40 or less
IB Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 Over 4000
O Unlikely to present
acute hazard in normal
use
--------------------------------------------------------------------------------------
h WHO/FAO Data Sheets on Pesticides with number and year of appearance.
i No toxicological evaluation - residues only.
N.B. References to Annex II are listed in the reference list of the main document.
Annex III. LD50s and no-observed-adverse-effect levels in animals
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Azinophos- 16.4 2.5 0.125 2 years FAO/WHO (1974b)
methyl 80 (guinea-pig) 5 (dog) 0.125 (dog) 2 years
Bromophos- 71 - 127 1366 (rabbit) 0.78 2 years FAO/WHO (1976b)
ethyl 10 (dog) 0.26 (dog) 2 years
225 - 550 (mice)
Bromophos 3750 - 7700 20 (dog) 0.5 (dog) 1 year FAO/WHO (1978b)
2829 - 5850 0.4 (man) 4 weeks
(mice)
720 (rabbit)
Carbopheno- 32.3 1270 (rabbit) 3 0.15 3 generations FAO/WHO (1980b)
thion 0.02 (dog) 3 months
0.01 (man) 1 month
Chlorfen- 10 - 39 31 - 108, 1 0.05 3 months FAO/WHO (1972b)
vinphos 117 - 200 (mice) 417 - 4700 1 dog 0.05 (dog) 16 weeks
(rabbit)
300 - 1000
(rabbit)
> 12 000 (dog)
Chlorpyr- 135 - 163 approximately 0.1 2 years FAO/WHO (1982b)
ifos 2000
500 (guinea-pig) (rabbit) 0.1 (dog) 90 days
32 (chicken) 0.1 (man) 1 month
1000 - 2000
(rabbit)
Crufomate 770 - 950 40 2 2 year FAO/WHO (1969b)
400 - 600 40 (dog) 1 dog 75 days
(rabbit)
Demeton 2.5 - 12
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Demeton- S - 57 - 106 302
methyl 110 (guinea-pig)
Diazinon 300 - 850 > 2150 2 0.1 90 days FAO/WHO (1971b)
0.02 (dog) 31 days
0.05 (monkey) 2 years
0.02 (man) 37 days
Dichlorvos 56 - 108 75 - 210 0.033 (man) 28 days FAO/WHO (1977b)
5 0.25 15 weeks
Dimethoate 320 - 380 0.2 (man) 57 days FAO/WHO (1985b)
15 (pheasant)
40 (duck)
Dioxathion 43 235 3 0.15 13 weeks FAO/WHO (1969b)
0.075 (dog) 90 days
0.075 (man) 28 days
Disulfoton 2.6 - 8.6 ca 20 1 0.05 2 years FAO/WHO (1976b)
1 (dog) 0.025 (dog) 12 weeks
0.075 (man) 30 days
Ethion 24.4 - 208 915 3 0.15 13 weeks FAO/WHO (1986b)
(rabbit) 0.125 (dog) 90 days
Fenamiphos 15.3 - 19.4 500 3 0.17 2 years FAO/WHO (1986b)
10 (dog) 1 (dog) 0.025 (dog) 2 years
75 - 100
(guinea-pig)
12 (hen)
Fenchlor- 1740 2000 0.5 2 years FAO/WHO (1969b)
phos 1 (dog) 2 years
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Fenitro- 250 - 500 > 3000 5 0.25 34 weeks FAO/WHO (1985b)
thion 870 (mice) (mice) 10 (dog) 0.3 (dog) 12 months
Fenthion 190 - 315 330 - 500 3 0.15 2 years FAO/WH0 (1981b)
3 (dog) 0.09 2 years
0.07 (monkey) 1 year
0.02 (man) -
Formothion 365 - 500 > 1000 20 1 2 years FAO/WHO (1974b)
40 (dog) 1 (dog) 2 years
Malathion 2800 4100 100 5 2 years FAO/WHO (1967b)
(rabbit) 0.2 (man) 88 days
Methida- 25 - 54 1546 - 1663 4 0.2 104 weeks FAO/WHO (1976b)
thion 25 - 20 (mice) 0.25 (monkey) 23 months
0.11 (man) 6 weeks
Mevinphos 3 - 12 1 - 90 0.37 0.02 2 years FAO/WHO (1973b)
7 - 18 (mice) 16 - 34 0.025 (dog) 2 years
(rabbit) 0.014 (man) 30 days
Monocroto- 14 - 23 336 0.5 0.025 12 weeks FAO/WHO (1973b)
phos (rabbit) 0.5 (dog) 0.0125 (dog) 13 weeks
Omethoate ca 50 700 1 0.05 3 months FAO/WHO (1986b)
0.025 (dog) 12 months
Parathion 3.6 - 13 6.8 - 21 0.05 (man) 3 weeks FAO/WHO (1967b)
Parathion- 2 0.1 2 years FAO/WHO (1985b)
methyl 14 - 24 67 0.3 (man) 30 days
---------------------------------------------------------------------------------------------------------
Annex III. (contd.)
---------------------------------------------------------------------------------------------------------
Chemical Acute LD50 No-observed-adverse-effect level in Reference
(mg/kg body weight)a animals (rats unless otherwise stated)
Oral Dermal (mg/kg (mg/kg body Duration
diet) weight) of test
---------------------------------------------------------------------------------------------------------
Phosalone 120 - 170 1500 25 1.25 2 years FAO/WHO (1973b)
180 (mice) > 1000 25 (dog) 0.625 2 years
(rabbit)
380 (guinea-pig)
290 (pheasant)
Phospha- 17 - 30 374 - 530 2 0.1 12 weeks FAO/WHO (1986)
midon 0.5 (dog) 90 days
Pirimiphos- 2050 > 2000 10 0.5 2 years FAO/WHO (1977b)
methyl (rabbit) 5 (mouse) 0.5 (mouse) 80 weeks
1180 (mice) 0.25 (man) 28 days
1000 - 2000
(guinea-pig)
1150 - 2300
(rabbit)
30 - 60 (hen)
Thiometon 120 - 130 > 1000 2.5 0.12 2 years FAO/WHO (1980b)
6 (dog) 0.5 (dog) 2 years
Trichlorfon 560 - 630 > 2000 50 2.5 2 years FAO/WHO (1979b)
50 (dog) 1.25 (dog) 4 years (dog)
---------------------------------------------------------------------------------------------------------
a From: Worthing (1983).
N.B. This reference is listed in the reference list of the main document.
Annex IV. Abbreviations used in the document
ACh acetylcholine
AChE acetylcholinesterase
ACTH adrenocorticotropic hormone
ADI acceptable daily intake
ChE cholinesterase
CNS central nervous system
DDE dichlorodiphenyldichloroethylene
DDT dichlorodiphenyltrichloroethane
DEF S,S,S -tributyl phosphorotrithioate
DFP di-isopropyl fluorophosphate
EEG electroencephalogram
EMG electromyography
EPN o -ethyl- O -(4-nitrophenyl)phenylphosphonothioate
FAO Food and Agricultural Organization (United Nations)
IARC International Agency for Research on Cancer
im intramuscular
IPCS International Programme on Chemical Safety
(World Health Organization)
IRPTC International Register of Potentially Toxic
Chemicals (United Nations Environment Programme)
iv intravenous
JMPR FAO/WHO Joint Meeting on Pesticide Residues
MFO mixed-function oxidase
MLD minimum lethal dose
MRL maximum residue limit
NAD nicotinamide-adenine-dinucleotide
NADPH nicotinamide-adenine-dinucleotide phosphate
(reduced form)
NTE neuropathy target esterase (formerly
neurotoxic esterase)
OMPA octamethylpyrophosphorictetramide
2-PAM pyridine-2-aldoxime methyl chloride
pseudoChE pseudocholinesterase
sc subcutaneous
TCDD 2,3,7,8-tetrachlorodibenzo-1,4-dioxin
TEPP tetraethyl pyrophosphate
TOCP tri- o -cresyl phosphate
UVR ultraviolet radiation
